User login
Medicare proposes direct payments to PAs, telehealth expansion
It also intends to change the approach to payments for office visits and for coaching programs for diabetes prevention.
The Centers for Medicare & Medicaid Services recently posted its proposed 2022 physician fee schedule. Running to more than 1,700 pages, the draft rule contains myriad other changes in how the giant federal health program pays for medical care, including revisions to its approach to evaluation and management (E/M) services, which represent many office visits. In addition, Medicare is seeking to increase participation in a program intended to prevent people from developing diabetes.
Physician groups posted quick complaints about a proposed 3.75% reduction to the conversion factor because of budget neutrality requirements. The cut reinstates a reduction Congress prevented in late 2020.
In a statement, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, called the draft rule a “mixed bag for physician practices.” Mr. Gilberg said the MGMA will seek congressional intervention to avert the cut for services in 2022.
In keeping with a provision Congress included in a massive spending bill enacted in December, Medicare will let PAs directly bill, as nurse practitioners already can. In a press release, CMS on July 13 described this as a move likely to expand access to care and reduce administrative burden. In 2020, the American Academy of PAs praised the inclusion in the spending bill of the provision allowing its members to directly bill Medicare.
In the draft rule, CMS also intends to remove certain geographic restrictions regarding use of telehealth services for diagnosis, evaluation, and treatment of mental health disorders. CMS also is proposing to allow payment to eligible clinicians for certain mental health and behavioral health services to patients via audio-only telephone calls. These services would include counseling and therapy services provided through opioid treatment programs.
“These changes would be particularly helpful for those in areas with poor broadband infrastructure and among people with Medicare who are not capable of, or do not consent to the use of, devices that permit a two-way, audio/video interaction for their health care visits,” CMS said in a statement.
Slimmer Medicare enrollees, bigger payments for coaches?
CMS is seeking to draw more participants to the Medicare Diabetes Prevention Program (MDPP). This program includes organizations that provide structured, coach-led sessions in community and health care settings to help people lose weight and exercise more. During the COVID-19 public health emergency, CMS waived an enrollment fee for new suppliers of services in MDPP. CMS now is proposing to waive this fee for all organizations that submit an application to enroll in Medicare as an MDPP supplier on or after Jan. 1, 2022.
Another proposed change in MDPP services is a restructuring of payments so that organizations involved in coaching would receive larger payments when their participants reach milestones for attendance and for becoming slimmer.
“We propose to increase performance payments for MDPP beneficiary achievement of the 5% weight-loss goal, as well as continued attendance during each core maintenance interval,” CMS said in a statement.
Medicare remains engaged in a review of its payments for E/M services. In the draft rule, CMS is proposing a number of refinements to current policies for split, or shared, E/M visits, critical care services, and services furnished by teaching physicians involving residents. The intention of these changes is to “better reflect the current practice of medicine, the evolving role of nonphysician practitioners as members of the medical team, and to clarify conditions of payment that must be met to bill Medicare for these services,” CMS said.
A version of this article first appeared on Medscape.com.
It also intends to change the approach to payments for office visits and for coaching programs for diabetes prevention.
The Centers for Medicare & Medicaid Services recently posted its proposed 2022 physician fee schedule. Running to more than 1,700 pages, the draft rule contains myriad other changes in how the giant federal health program pays for medical care, including revisions to its approach to evaluation and management (E/M) services, which represent many office visits. In addition, Medicare is seeking to increase participation in a program intended to prevent people from developing diabetes.
Physician groups posted quick complaints about a proposed 3.75% reduction to the conversion factor because of budget neutrality requirements. The cut reinstates a reduction Congress prevented in late 2020.
In a statement, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, called the draft rule a “mixed bag for physician practices.” Mr. Gilberg said the MGMA will seek congressional intervention to avert the cut for services in 2022.
In keeping with a provision Congress included in a massive spending bill enacted in December, Medicare will let PAs directly bill, as nurse practitioners already can. In a press release, CMS on July 13 described this as a move likely to expand access to care and reduce administrative burden. In 2020, the American Academy of PAs praised the inclusion in the spending bill of the provision allowing its members to directly bill Medicare.
In the draft rule, CMS also intends to remove certain geographic restrictions regarding use of telehealth services for diagnosis, evaluation, and treatment of mental health disorders. CMS also is proposing to allow payment to eligible clinicians for certain mental health and behavioral health services to patients via audio-only telephone calls. These services would include counseling and therapy services provided through opioid treatment programs.
“These changes would be particularly helpful for those in areas with poor broadband infrastructure and among people with Medicare who are not capable of, or do not consent to the use of, devices that permit a two-way, audio/video interaction for their health care visits,” CMS said in a statement.
Slimmer Medicare enrollees, bigger payments for coaches?
CMS is seeking to draw more participants to the Medicare Diabetes Prevention Program (MDPP). This program includes organizations that provide structured, coach-led sessions in community and health care settings to help people lose weight and exercise more. During the COVID-19 public health emergency, CMS waived an enrollment fee for new suppliers of services in MDPP. CMS now is proposing to waive this fee for all organizations that submit an application to enroll in Medicare as an MDPP supplier on or after Jan. 1, 2022.
Another proposed change in MDPP services is a restructuring of payments so that organizations involved in coaching would receive larger payments when their participants reach milestones for attendance and for becoming slimmer.
“We propose to increase performance payments for MDPP beneficiary achievement of the 5% weight-loss goal, as well as continued attendance during each core maintenance interval,” CMS said in a statement.
Medicare remains engaged in a review of its payments for E/M services. In the draft rule, CMS is proposing a number of refinements to current policies for split, or shared, E/M visits, critical care services, and services furnished by teaching physicians involving residents. The intention of these changes is to “better reflect the current practice of medicine, the evolving role of nonphysician practitioners as members of the medical team, and to clarify conditions of payment that must be met to bill Medicare for these services,” CMS said.
A version of this article first appeared on Medscape.com.
It also intends to change the approach to payments for office visits and for coaching programs for diabetes prevention.
The Centers for Medicare & Medicaid Services recently posted its proposed 2022 physician fee schedule. Running to more than 1,700 pages, the draft rule contains myriad other changes in how the giant federal health program pays for medical care, including revisions to its approach to evaluation and management (E/M) services, which represent many office visits. In addition, Medicare is seeking to increase participation in a program intended to prevent people from developing diabetes.
Physician groups posted quick complaints about a proposed 3.75% reduction to the conversion factor because of budget neutrality requirements. The cut reinstates a reduction Congress prevented in late 2020.
In a statement, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, called the draft rule a “mixed bag for physician practices.” Mr. Gilberg said the MGMA will seek congressional intervention to avert the cut for services in 2022.
In keeping with a provision Congress included in a massive spending bill enacted in December, Medicare will let PAs directly bill, as nurse practitioners already can. In a press release, CMS on July 13 described this as a move likely to expand access to care and reduce administrative burden. In 2020, the American Academy of PAs praised the inclusion in the spending bill of the provision allowing its members to directly bill Medicare.
In the draft rule, CMS also intends to remove certain geographic restrictions regarding use of telehealth services for diagnosis, evaluation, and treatment of mental health disorders. CMS also is proposing to allow payment to eligible clinicians for certain mental health and behavioral health services to patients via audio-only telephone calls. These services would include counseling and therapy services provided through opioid treatment programs.
“These changes would be particularly helpful for those in areas with poor broadband infrastructure and among people with Medicare who are not capable of, or do not consent to the use of, devices that permit a two-way, audio/video interaction for their health care visits,” CMS said in a statement.
Slimmer Medicare enrollees, bigger payments for coaches?
CMS is seeking to draw more participants to the Medicare Diabetes Prevention Program (MDPP). This program includes organizations that provide structured, coach-led sessions in community and health care settings to help people lose weight and exercise more. During the COVID-19 public health emergency, CMS waived an enrollment fee for new suppliers of services in MDPP. CMS now is proposing to waive this fee for all organizations that submit an application to enroll in Medicare as an MDPP supplier on or after Jan. 1, 2022.
Another proposed change in MDPP services is a restructuring of payments so that organizations involved in coaching would receive larger payments when their participants reach milestones for attendance and for becoming slimmer.
“We propose to increase performance payments for MDPP beneficiary achievement of the 5% weight-loss goal, as well as continued attendance during each core maintenance interval,” CMS said in a statement.
Medicare remains engaged in a review of its payments for E/M services. In the draft rule, CMS is proposing a number of refinements to current policies for split, or shared, E/M visits, critical care services, and services furnished by teaching physicians involving residents. The intention of these changes is to “better reflect the current practice of medicine, the evolving role of nonphysician practitioners as members of the medical team, and to clarify conditions of payment that must be met to bill Medicare for these services,” CMS said.
A version of this article first appeared on Medscape.com.
NIAID advances universal flu vaccine candidate into phase 1 trial
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
More and more doctors abandoning private practice
according to a new report.
These patterns likely reflect broader trends toward consolidation in health care, with both insurance companies and hospitals also having grown in size in recent years.
The latest biennial analysis of doctors’ practices by the American Medical Association showed an acceleration of a trend away from private practice, defined as a practice wholly owned by physicians. The 2020 results found less than half – 49.1 % – of doctors involved in patient care worked in a private practice, the AMA said in a report released in May 2021.
This marked the first time private practice was not the dominant approach since the AMA analysis began in 2012. What’s more, the trend appears to be gaining steam, with a drop of almost 5 percentage points from 54.0% in private practice in 2018. The percent of doctors in private practice declined at a slower rate in previous AMA surveys, slipping to 55.8% in 2016 from 56.8% in 2018 and 60.1% in 2012.
Employment and ownership structures have become so varied that no single approach or size of organization “can or should be considered the typical physician practice,” the report noted.
The AMA, for example, added to its 2020 benchmark survey an option to identify private equity organizations as employers. The survey found 4% of doctors involved in patient care worked in practices owned by these kinds of firms. Other options include practices wholly or jointly owned by hospital and health systems and insurers, as well as direct employment and contracting.
There are signs that the shift away from smaller private practices will continue, with younger doctors appearing more likely to seek employment.
The survey found 42% of doctors ages 55 and older were employed by someone else, compared with 51.2% of doctors ages 40-54 and 70% of physicians under the age of 40.
The AMA surveyed 3,500 U.S. doctors through the 2020 Physician Practice Benchmark Survey. The survey was conducted from September to October 2020, roughly 6 months into the COVID-19 pandemic, and therefore may not reflect its full impact.
“Physician practices were hit hard by the economic impact of the early pandemic as patient volume and revenues shrank while medical supply expenses spiked. The impact of these economic forces on physician practice arrangements is ongoing and may not be fully realized for some time,” AMA President Susan R. Bailey, MD, said in a statement.
In a survey released in 2020 by McKinsey & Company, 53% of independent doctors reported that they were worried about their practices surviving the stresses of the pandemic, this news organization reported.
Challenging environment
It’s not just money leading to the shift away from private practice, according to a 2020 report from the American Hospital Association, titled “Evolving Physician-Practice Ownership Models.”
Many recent graduates of medical schools have significant debt and are more likely to opt for employment, which offers more financial stability and work-life balance, the report said.
Doctors also need to keep up with expectations of their patients that have been shaped by advances in other sectors, like banking, the AHA noted. People are used to working on their own schedules, and want to make appointments through apps, get test results rapidly and on their mobile devices, and communicate with their providers virtually.
“It is challenging to meet these expectations and make the necessary technology investments as a solo or small group practice,” the AHA report said.
Hospitals face competition for doctors from insurers, which have been looking in some cases to directly employ more physicians, the AHA also noted. The report cites insurance giant UnitedHealth Group’s Optum unit as the most visible example of this trend.
On a January call about corporate earnings, David Wichmann, then chief executive of UnitedHealth, spoke about the firm’s “aim to reinvent health care delivery,” including efforts to have its own primary and multispecialty care practices.
“OptumCare entered 2021 with over 50,000 physicians and 1,400 clinics,” Mr. Wichmann said. “Over the course of this year, we expect to grow our employed and affiliated physicians by at least 10,000. This work of building local physician-led systems of care continues to be central to our mission. “
UnitedHealth’s new CEO is Andrew Witty, who had led the Optum unit.
Attractions of larger groups
Older doctors – those 55 years and up – were significantly more likely to work in small practices than those younger than 40, the 2020 survey found. Results showed 40.9% of doctors under 40 worked in practices of 10 or fewer colleagues, compared with 61.4% of those age 55 and older.
The large difference between age groups suggests that attrition is one reason for the shift in practice size. Retiring doctors who leave small practices are not being replaced on a one-for-one basis by younger doctors, AMA said. The same reason also appears to be a factor in the shift in practice ownership to larger systems.
Doctors in larger group practices can count on a stable business model, with a better ability to survive disruptive market trends, including those of a more extreme nature, like COVID-19, said Fred Horton, president of AMGA Consulting.
AMGA Consulting is a wholly-owned subsidiary of AMGA, formerly called American Medical Group Association. Its more than 400 members include well-known multispecialty groups and health care systems such as the Mayo Clinic, Cleveland Clinic, Geisinger, the Permanente Medical Group, and Intermountain Healthcare as well as many smaller physician practices.
Mr. Horton, who holds a master’s degree in health administration, said some doctors may want to participate in alternative payment programs offered by insurers, who are seeking to shift away from the fee-for-service model
“Larger organizations can dedicate more resources to continuous quality improvement,” Mr. Horton said. “This is especially important for physicians who are taking on risk-based contracts, as quality can directly impact how much they earn.”
For one oncologist, it was turning to alternative payment methods that helped him keep his private practice afloat.
Kashyap Patel, MD, chief executive of the Carolina Blood and Cancer Care Associates in Rock Hill, S.C., said he maintained the independence of his practice amid pressure from a large health system, which had been buying medical groups in the area. That began to interfere with referrals of patients from other doctors, which are key for cancer specialists, said Dr. Patel, who also is president of the Community Oncology Alliance.
In response, Dr. Patel worked with Blue Cross Blue Shield of South Carolina on an arrangement where his practice sought certifications from the National Committee for Quality Assurance to get better rates.
The effort has allowed Dr. Patel’s clinic to focus more on preventing hospitalizations and visits to the emergency room he said.
In Dr. Patel’s view, his patients benefit from his efforts to remain in independent practice. A switch to ownership by a large health care organization would have put them at risk for higher medical bills, jeopardizing their access to treatment, he said. The reason? Hospitals can charge more for services provided by doctors they employ.
“Nothing would change. I would be the same. The building would be the same, but the cost would go up,” Dr. Patel said.
For its part, the AHA has repeatedly challenged arguments that acquisitions and mergers result in higher costs for patients.
Instead, the AHA has raised alarms about consolidation of health insurers, a concern it shares with AMA. In a 2020 report examining competition among insurers, AMA noted doctors working in small practices can be put at a disadvantage if mergers and acquisitions leave an insurer with too much market power.
“Under antitrust law, independent physicians cannot negotiate collectively with health
Insurers,” the AMA said in the report. “This imbalance in relative size leaves most physicians with a weak bargaining position relative to commercial payers.”
AMA’s research on the effects of insurers’ wielding significant market clout has been used in effort to thwart mergers in this industry.
‘Dramatic restructuring’
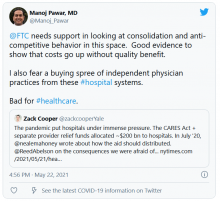
The Federal Trade Commission also has taken note of the trends discussed in the new AMA report, saying that “U.S. physician markets are undergoing a dramatic restructuring.”
The FTC in January announced a study of the impact of the consolidation of doctors groups and health care facilities. FTC is seeking data for inpatient, outpatient, and doctors services in 15 states from 2015 through 2020. To gather this data, the commission has issued orders to six major insurers – Aetna, Anthem, Florida Blue, Cigna, Health Care Service Corporation and United Healthcare.
The FTC is concerned that acquired practices may have to alter their referral patterns to favor their affiliated hospital system over competing hospital systems. But FTC staff also said it might be that these acquisitions result in efficiencies, such as enhanced coordination of care between doctors and hospitals “that outweigh potential competitive harms.”
The research project will likely take several years to complete because of its scope, the FTC said. For that reason, the FTC said its Bureau of Economics will release a series of research papers examining different aspects of this inquiry rather than a single paper containing all of the analyses.
Private equity ‘roll-ups’
On the day the FTC announced the study of the impact of doctors groups, one of the panel’s commissioners argued for a closer look at how private equity firms make their purchases.
In a Jan. 15 tweet, FTC Commissioner Rohit Chopra said his agency needs to challenge their “roll-ups of small physician practices” as well as clinics and labs. This is a practice of using a series of acquisitions too small to trigger the federal threshold for a serious look from the FTC and Department of Justice. (The threshold for 2021 stands around the $92 million mark. This benchmark is known as Hart-Scott-Rodino notification after a 1976 law that set a reporting standard.)
Mr. Chopra attached to his Jan. 15 tweet a 2020 statement in which he called for stepped-up scrutiny of private-equity firms’ acquisitions of doctors’ practices. Mr. Chopra noted that private-equity firms have been buying practices focused on anesthesiology and emergency medicine, fields which triggered consumer complaints about surprise billing for emergency care.
“Given trends in today’s markets, it is critical that the FTC find new ways to ensure the agency has a rigorous, data-driven approach to market monitoring and enforcement,” Mr. Chopra wrote.
A version of this article first appeared on WebMD.com.
according to a new report.
These patterns likely reflect broader trends toward consolidation in health care, with both insurance companies and hospitals also having grown in size in recent years.
The latest biennial analysis of doctors’ practices by the American Medical Association showed an acceleration of a trend away from private practice, defined as a practice wholly owned by physicians. The 2020 results found less than half – 49.1 % – of doctors involved in patient care worked in a private practice, the AMA said in a report released in May 2021.
This marked the first time private practice was not the dominant approach since the AMA analysis began in 2012. What’s more, the trend appears to be gaining steam, with a drop of almost 5 percentage points from 54.0% in private practice in 2018. The percent of doctors in private practice declined at a slower rate in previous AMA surveys, slipping to 55.8% in 2016 from 56.8% in 2018 and 60.1% in 2012.
Employment and ownership structures have become so varied that no single approach or size of organization “can or should be considered the typical physician practice,” the report noted.
The AMA, for example, added to its 2020 benchmark survey an option to identify private equity organizations as employers. The survey found 4% of doctors involved in patient care worked in practices owned by these kinds of firms. Other options include practices wholly or jointly owned by hospital and health systems and insurers, as well as direct employment and contracting.
There are signs that the shift away from smaller private practices will continue, with younger doctors appearing more likely to seek employment.
The survey found 42% of doctors ages 55 and older were employed by someone else, compared with 51.2% of doctors ages 40-54 and 70% of physicians under the age of 40.
The AMA surveyed 3,500 U.S. doctors through the 2020 Physician Practice Benchmark Survey. The survey was conducted from September to October 2020, roughly 6 months into the COVID-19 pandemic, and therefore may not reflect its full impact.
“Physician practices were hit hard by the economic impact of the early pandemic as patient volume and revenues shrank while medical supply expenses spiked. The impact of these economic forces on physician practice arrangements is ongoing and may not be fully realized for some time,” AMA President Susan R. Bailey, MD, said in a statement.
In a survey released in 2020 by McKinsey & Company, 53% of independent doctors reported that they were worried about their practices surviving the stresses of the pandemic, this news organization reported.
Challenging environment
It’s not just money leading to the shift away from private practice, according to a 2020 report from the American Hospital Association, titled “Evolving Physician-Practice Ownership Models.”
Many recent graduates of medical schools have significant debt and are more likely to opt for employment, which offers more financial stability and work-life balance, the report said.
Doctors also need to keep up with expectations of their patients that have been shaped by advances in other sectors, like banking, the AHA noted. People are used to working on their own schedules, and want to make appointments through apps, get test results rapidly and on their mobile devices, and communicate with their providers virtually.
“It is challenging to meet these expectations and make the necessary technology investments as a solo or small group practice,” the AHA report said.
Hospitals face competition for doctors from insurers, which have been looking in some cases to directly employ more physicians, the AHA also noted. The report cites insurance giant UnitedHealth Group’s Optum unit as the most visible example of this trend.
On a January call about corporate earnings, David Wichmann, then chief executive of UnitedHealth, spoke about the firm’s “aim to reinvent health care delivery,” including efforts to have its own primary and multispecialty care practices.
“OptumCare entered 2021 with over 50,000 physicians and 1,400 clinics,” Mr. Wichmann said. “Over the course of this year, we expect to grow our employed and affiliated physicians by at least 10,000. This work of building local physician-led systems of care continues to be central to our mission. “
UnitedHealth’s new CEO is Andrew Witty, who had led the Optum unit.
Attractions of larger groups
Older doctors – those 55 years and up – were significantly more likely to work in small practices than those younger than 40, the 2020 survey found. Results showed 40.9% of doctors under 40 worked in practices of 10 or fewer colleagues, compared with 61.4% of those age 55 and older.
The large difference between age groups suggests that attrition is one reason for the shift in practice size. Retiring doctors who leave small practices are not being replaced on a one-for-one basis by younger doctors, AMA said. The same reason also appears to be a factor in the shift in practice ownership to larger systems.
Doctors in larger group practices can count on a stable business model, with a better ability to survive disruptive market trends, including those of a more extreme nature, like COVID-19, said Fred Horton, president of AMGA Consulting.
AMGA Consulting is a wholly-owned subsidiary of AMGA, formerly called American Medical Group Association. Its more than 400 members include well-known multispecialty groups and health care systems such as the Mayo Clinic, Cleveland Clinic, Geisinger, the Permanente Medical Group, and Intermountain Healthcare as well as many smaller physician practices.
Mr. Horton, who holds a master’s degree in health administration, said some doctors may want to participate in alternative payment programs offered by insurers, who are seeking to shift away from the fee-for-service model
“Larger organizations can dedicate more resources to continuous quality improvement,” Mr. Horton said. “This is especially important for physicians who are taking on risk-based contracts, as quality can directly impact how much they earn.”
For one oncologist, it was turning to alternative payment methods that helped him keep his private practice afloat.
Kashyap Patel, MD, chief executive of the Carolina Blood and Cancer Care Associates in Rock Hill, S.C., said he maintained the independence of his practice amid pressure from a large health system, which had been buying medical groups in the area. That began to interfere with referrals of patients from other doctors, which are key for cancer specialists, said Dr. Patel, who also is president of the Community Oncology Alliance.
In response, Dr. Patel worked with Blue Cross Blue Shield of South Carolina on an arrangement where his practice sought certifications from the National Committee for Quality Assurance to get better rates.
The effort has allowed Dr. Patel’s clinic to focus more on preventing hospitalizations and visits to the emergency room he said.
In Dr. Patel’s view, his patients benefit from his efforts to remain in independent practice. A switch to ownership by a large health care organization would have put them at risk for higher medical bills, jeopardizing their access to treatment, he said. The reason? Hospitals can charge more for services provided by doctors they employ.
“Nothing would change. I would be the same. The building would be the same, but the cost would go up,” Dr. Patel said.
For its part, the AHA has repeatedly challenged arguments that acquisitions and mergers result in higher costs for patients.
Instead, the AHA has raised alarms about consolidation of health insurers, a concern it shares with AMA. In a 2020 report examining competition among insurers, AMA noted doctors working in small practices can be put at a disadvantage if mergers and acquisitions leave an insurer with too much market power.
“Under antitrust law, independent physicians cannot negotiate collectively with health
Insurers,” the AMA said in the report. “This imbalance in relative size leaves most physicians with a weak bargaining position relative to commercial payers.”
AMA’s research on the effects of insurers’ wielding significant market clout has been used in effort to thwart mergers in this industry.
‘Dramatic restructuring’
The Federal Trade Commission also has taken note of the trends discussed in the new AMA report, saying that “U.S. physician markets are undergoing a dramatic restructuring.”
The FTC in January announced a study of the impact of the consolidation of doctors groups and health care facilities. FTC is seeking data for inpatient, outpatient, and doctors services in 15 states from 2015 through 2020. To gather this data, the commission has issued orders to six major insurers – Aetna, Anthem, Florida Blue, Cigna, Health Care Service Corporation and United Healthcare.
The FTC is concerned that acquired practices may have to alter their referral patterns to favor their affiliated hospital system over competing hospital systems. But FTC staff also said it might be that these acquisitions result in efficiencies, such as enhanced coordination of care between doctors and hospitals “that outweigh potential competitive harms.”
The research project will likely take several years to complete because of its scope, the FTC said. For that reason, the FTC said its Bureau of Economics will release a series of research papers examining different aspects of this inquiry rather than a single paper containing all of the analyses.
Private equity ‘roll-ups’
On the day the FTC announced the study of the impact of doctors groups, one of the panel’s commissioners argued for a closer look at how private equity firms make their purchases.
In a Jan. 15 tweet, FTC Commissioner Rohit Chopra said his agency needs to challenge their “roll-ups of small physician practices” as well as clinics and labs. This is a practice of using a series of acquisitions too small to trigger the federal threshold for a serious look from the FTC and Department of Justice. (The threshold for 2021 stands around the $92 million mark. This benchmark is known as Hart-Scott-Rodino notification after a 1976 law that set a reporting standard.)
Mr. Chopra attached to his Jan. 15 tweet a 2020 statement in which he called for stepped-up scrutiny of private-equity firms’ acquisitions of doctors’ practices. Mr. Chopra noted that private-equity firms have been buying practices focused on anesthesiology and emergency medicine, fields which triggered consumer complaints about surprise billing for emergency care.
“Given trends in today’s markets, it is critical that the FTC find new ways to ensure the agency has a rigorous, data-driven approach to market monitoring and enforcement,” Mr. Chopra wrote.
A version of this article first appeared on WebMD.com.
according to a new report.
These patterns likely reflect broader trends toward consolidation in health care, with both insurance companies and hospitals also having grown in size in recent years.
The latest biennial analysis of doctors’ practices by the American Medical Association showed an acceleration of a trend away from private practice, defined as a practice wholly owned by physicians. The 2020 results found less than half – 49.1 % – of doctors involved in patient care worked in a private practice, the AMA said in a report released in May 2021.
This marked the first time private practice was not the dominant approach since the AMA analysis began in 2012. What’s more, the trend appears to be gaining steam, with a drop of almost 5 percentage points from 54.0% in private practice in 2018. The percent of doctors in private practice declined at a slower rate in previous AMA surveys, slipping to 55.8% in 2016 from 56.8% in 2018 and 60.1% in 2012.
Employment and ownership structures have become so varied that no single approach or size of organization “can or should be considered the typical physician practice,” the report noted.
The AMA, for example, added to its 2020 benchmark survey an option to identify private equity organizations as employers. The survey found 4% of doctors involved in patient care worked in practices owned by these kinds of firms. Other options include practices wholly or jointly owned by hospital and health systems and insurers, as well as direct employment and contracting.
There are signs that the shift away from smaller private practices will continue, with younger doctors appearing more likely to seek employment.
The survey found 42% of doctors ages 55 and older were employed by someone else, compared with 51.2% of doctors ages 40-54 and 70% of physicians under the age of 40.
The AMA surveyed 3,500 U.S. doctors through the 2020 Physician Practice Benchmark Survey. The survey was conducted from September to October 2020, roughly 6 months into the COVID-19 pandemic, and therefore may not reflect its full impact.
“Physician practices were hit hard by the economic impact of the early pandemic as patient volume and revenues shrank while medical supply expenses spiked. The impact of these economic forces on physician practice arrangements is ongoing and may not be fully realized for some time,” AMA President Susan R. Bailey, MD, said in a statement.
In a survey released in 2020 by McKinsey & Company, 53% of independent doctors reported that they were worried about their practices surviving the stresses of the pandemic, this news organization reported.
Challenging environment
It’s not just money leading to the shift away from private practice, according to a 2020 report from the American Hospital Association, titled “Evolving Physician-Practice Ownership Models.”
Many recent graduates of medical schools have significant debt and are more likely to opt for employment, which offers more financial stability and work-life balance, the report said.
Doctors also need to keep up with expectations of their patients that have been shaped by advances in other sectors, like banking, the AHA noted. People are used to working on their own schedules, and want to make appointments through apps, get test results rapidly and on their mobile devices, and communicate with their providers virtually.
“It is challenging to meet these expectations and make the necessary technology investments as a solo or small group practice,” the AHA report said.
Hospitals face competition for doctors from insurers, which have been looking in some cases to directly employ more physicians, the AHA also noted. The report cites insurance giant UnitedHealth Group’s Optum unit as the most visible example of this trend.
On a January call about corporate earnings, David Wichmann, then chief executive of UnitedHealth, spoke about the firm’s “aim to reinvent health care delivery,” including efforts to have its own primary and multispecialty care practices.
“OptumCare entered 2021 with over 50,000 physicians and 1,400 clinics,” Mr. Wichmann said. “Over the course of this year, we expect to grow our employed and affiliated physicians by at least 10,000. This work of building local physician-led systems of care continues to be central to our mission. “
UnitedHealth’s new CEO is Andrew Witty, who had led the Optum unit.
Attractions of larger groups
Older doctors – those 55 years and up – were significantly more likely to work in small practices than those younger than 40, the 2020 survey found. Results showed 40.9% of doctors under 40 worked in practices of 10 or fewer colleagues, compared with 61.4% of those age 55 and older.
The large difference between age groups suggests that attrition is one reason for the shift in practice size. Retiring doctors who leave small practices are not being replaced on a one-for-one basis by younger doctors, AMA said. The same reason also appears to be a factor in the shift in practice ownership to larger systems.
Doctors in larger group practices can count on a stable business model, with a better ability to survive disruptive market trends, including those of a more extreme nature, like COVID-19, said Fred Horton, president of AMGA Consulting.
AMGA Consulting is a wholly-owned subsidiary of AMGA, formerly called American Medical Group Association. Its more than 400 members include well-known multispecialty groups and health care systems such as the Mayo Clinic, Cleveland Clinic, Geisinger, the Permanente Medical Group, and Intermountain Healthcare as well as many smaller physician practices.
Mr. Horton, who holds a master’s degree in health administration, said some doctors may want to participate in alternative payment programs offered by insurers, who are seeking to shift away from the fee-for-service model
“Larger organizations can dedicate more resources to continuous quality improvement,” Mr. Horton said. “This is especially important for physicians who are taking on risk-based contracts, as quality can directly impact how much they earn.”
For one oncologist, it was turning to alternative payment methods that helped him keep his private practice afloat.
Kashyap Patel, MD, chief executive of the Carolina Blood and Cancer Care Associates in Rock Hill, S.C., said he maintained the independence of his practice amid pressure from a large health system, which had been buying medical groups in the area. That began to interfere with referrals of patients from other doctors, which are key for cancer specialists, said Dr. Patel, who also is president of the Community Oncology Alliance.
In response, Dr. Patel worked with Blue Cross Blue Shield of South Carolina on an arrangement where his practice sought certifications from the National Committee for Quality Assurance to get better rates.
The effort has allowed Dr. Patel’s clinic to focus more on preventing hospitalizations and visits to the emergency room he said.
In Dr. Patel’s view, his patients benefit from his efforts to remain in independent practice. A switch to ownership by a large health care organization would have put them at risk for higher medical bills, jeopardizing their access to treatment, he said. The reason? Hospitals can charge more for services provided by doctors they employ.
“Nothing would change. I would be the same. The building would be the same, but the cost would go up,” Dr. Patel said.
For its part, the AHA has repeatedly challenged arguments that acquisitions and mergers result in higher costs for patients.
Instead, the AHA has raised alarms about consolidation of health insurers, a concern it shares with AMA. In a 2020 report examining competition among insurers, AMA noted doctors working in small practices can be put at a disadvantage if mergers and acquisitions leave an insurer with too much market power.
“Under antitrust law, independent physicians cannot negotiate collectively with health
Insurers,” the AMA said in the report. “This imbalance in relative size leaves most physicians with a weak bargaining position relative to commercial payers.”
AMA’s research on the effects of insurers’ wielding significant market clout has been used in effort to thwart mergers in this industry.
‘Dramatic restructuring’
The Federal Trade Commission also has taken note of the trends discussed in the new AMA report, saying that “U.S. physician markets are undergoing a dramatic restructuring.”
The FTC in January announced a study of the impact of the consolidation of doctors groups and health care facilities. FTC is seeking data for inpatient, outpatient, and doctors services in 15 states from 2015 through 2020. To gather this data, the commission has issued orders to six major insurers – Aetna, Anthem, Florida Blue, Cigna, Health Care Service Corporation and United Healthcare.
The FTC is concerned that acquired practices may have to alter their referral patterns to favor their affiliated hospital system over competing hospital systems. But FTC staff also said it might be that these acquisitions result in efficiencies, such as enhanced coordination of care between doctors and hospitals “that outweigh potential competitive harms.”
The research project will likely take several years to complete because of its scope, the FTC said. For that reason, the FTC said its Bureau of Economics will release a series of research papers examining different aspects of this inquiry rather than a single paper containing all of the analyses.
Private equity ‘roll-ups’
On the day the FTC announced the study of the impact of doctors groups, one of the panel’s commissioners argued for a closer look at how private equity firms make their purchases.
In a Jan. 15 tweet, FTC Commissioner Rohit Chopra said his agency needs to challenge their “roll-ups of small physician practices” as well as clinics and labs. This is a practice of using a series of acquisitions too small to trigger the federal threshold for a serious look from the FTC and Department of Justice. (The threshold for 2021 stands around the $92 million mark. This benchmark is known as Hart-Scott-Rodino notification after a 1976 law that set a reporting standard.)
Mr. Chopra attached to his Jan. 15 tweet a 2020 statement in which he called for stepped-up scrutiny of private-equity firms’ acquisitions of doctors’ practices. Mr. Chopra noted that private-equity firms have been buying practices focused on anesthesiology and emergency medicine, fields which triggered consumer complaints about surprise billing for emergency care.
“Given trends in today’s markets, it is critical that the FTC find new ways to ensure the agency has a rigorous, data-driven approach to market monitoring and enforcement,” Mr. Chopra wrote.
A version of this article first appeared on WebMD.com.
Bill seeks to streamline prior authorization in Medicare Advantage plans
A group of bipartisan lawmakers intends to compel insurers to streamline prior authorization processes for Medicare Advantage plans, including a bid to end the use of faxes and develop systems that can allow for real-time decisions.
Rep. Suzan DelBene (D-Wash.); Rep. Mike Kelly (R-Pa.); Rep. Ami Bera, MD (D-Calif.); and Rep. Larry Bucshon, MD, (R-Ind.) on May 13 introduced a bill that would task federal officials with refining standards regarding prior authorization for Medicare Advantage. Titled the Improving Seniors’ Timely Access to Care Act of 2021, the bill would direct the Department of Health & Human Services to create rules intended to make prior authorization more transparent and speedy for the insurer-run Medicare plans. Known as Medicare Advantage, these plans cover about 24.1 million people of the 62 million enrolled in the giant federal health program, according to the nonprofit Kaiser Family Foundation.
These revamped prior authorization systems could not rely on faxes nor could they employ proprietary payer portals that did not meet HHS’ standards, says the text of the bill released by Rep. DelBene. Insurers would also have to report to the Centers for Medicare & Medicaid Services about the extent of their use of prior authorization and the rate of approvals or denials. The bill seeks to encourage plans to adopt prior authorization programs that adhere to evidence-based medical guidelines in consultation with physicians.
There were several reasons for focusing on Medicare Advantage plans, although prior authorization concerns extend more broadly in the U.S. health care system, said Susan Bailey, MD, president of the American Medical Association.
There’s an ample body of research about issues seen in the Medicare Advantage plans. Dr. Bailey also said that, in her experience, Medicare Advantage plans have had some of the most restrictive policies. And, by starting with Medicare Advantage, there’s a potential for a ripple effect in the industry, easing this issue when physicians work with other insurers as well.
“When Medicare adopts a policy whether it be a payment policy or a coverage policy, private insurers typically follow along,” she said.
Strong support among health care groups
There’s strong support for streamlining prior authorization both in the medical community and in Congress.
The bill has the support of about 70 health care organizations, including the AMA and the American Academy of Family Physicians, according to its sponsors. As of May 17, the bill had attracted the backing of 97 members of the House of Representatives, roughly evenly split among Democrats and Republicans.
Rep. DelBene’s previous version of this bill, the Improving Seniors’ Timely Access to Care Act of 2019, attracted 143 Democratic cosponsors and 137 Republican ones, or more than half of the members of the House. This bill was not completed during the previous session of Congress (January 2019–January 2021) because of the more urgent needs of pandemic response, said Rep. Bucshon, who practiced cardiothoracic surgery before joining Congress.
“It wasn’t quite on the radar as much as it might have been if we didn’t have COVID,” Rep. Bucshon said.
Rep. Bucshon added that he expects strong Senate support for a companion measure of the House bill, which could make the difference for efforts to pass it this year.
Insurers have become more aggressive over time in denying payments through prior authorization systems for services that physicians say their patients need, according to Rep. Bucshon. There may be some “bad actors” in medicine who would order unnecessary procedures, Rep. Bucshon allowed, but in most cases, the cumbersome prior authorization processes only put a hurdle for patients seeking needed treatments, he said.
“The premise is that it controls health care costs but actually what it does is it helps insurance company’s bottom line,” Rep. Bucshon said.
In a prepared statement, former Pennsylvania representative Allyson Y. Schwartz, now CEO of the Better Medicare Alliance, said her group had spoken with sponsors of this legislation and appreciates “their receptiveness to feedback in this process.”
“Prior authorization ensures beneficiaries receive clinically appropriate care and reduces exposures to duplicative and unnecessary services,” Ms. Schwartz said. “We share an interest in ensuring prior authorization works as smoothly and effectively as possible for beneficiaries while protecting its essential function of facilitating safe, evidenced-based care.”
The Better Medicare Alliance said its funders include UnitedHealth, Humana, and CVS Health/Aetna, which run Advantage plans. The group also lists as its partners many medical organizations.
“Rationing care by hassling”
Like Rep. Bucshon, Dr. Bailey sees a different motivation in insurers’ persistence in keeping the prior authorization process cumbersome.
Phone calls and faxes remain the key methods for handling prior authorization for medical services, according to the results of a survey done by the AMA in December. Phone calls were always or often required for prior authorization for medical services (59%), with faxes the second-most common approach (46%), followed by health plans’ online portals (39%), electronic health records and practice management systems (29%), and email or U.S. mail (26%), according to the AMA’s report on the survey.
“It seems like every step in the process is designed to make the patient less likely to get the therapy that the doctor thinks that the patient needs,” Dr. Bailey said. “It’s almost like rationing care by hassling the patient and the physician.”
The findings of an investigation by HHS’ internal watchdog unit appear to support Dr. Bailey’s view, showing that insurer-run Medicare plans had a pattern of often walking back their initial rejections.
In 2018, the Office of the Inspector General for HHS reported that Medicare Advantage organizations (MAOs) overturned 75% of their own denials during 2014-16. In addition, independent reviewers within the appeals process overturned additional denials in favor of patients and clinicians, OIG said.
“The high number of overturned denials raises concerns that some Medicare Advantage beneficiaries and providers were initially denied services and payments that should have been provided,” the OIG said in the report. “This is especially concerning because beneficiaries and providers rarely used the appeals process, which is designed to ensure access to care and payment.”
During 2014-2016, patients and clinicians appealed only 1% of denials to the first level of appeal, OIG said. In the report, the watchdog group noted that CMS audits had highlighted “widespread and persistent MAO performance problems related to denials of care and payment.” In 2015, for example, CMS cited 56% of audited contracts for making inappropriate denials.
Dr. Bailey also said in an interview that she routinely encounters problems with prior authorization in her own practice as an allergist and immunologist in Fort Worth, Tex.
In late May, for example, a Medicare Advantage plan made a patient whose chronic asthma had been stable for years change to a new inhaler that resulted in him developing a yeast infection in his mouth, Dr. Bailey said.
“We treated the yeast infection, made some changes in the way he uses his inhaler, so hopefully he would tolerate it better,” Dr. Bailey said. “He had a reaction to the medication to treat the yeast infection and ended up in the hospital. How is that helping anyone? It certainly hasn’t helped my patient.”
Dr. Bailey said insurers have also asked to seek prior authorization to prescribe medications that have been generic for years and have used the process to challenge her on cases of what seem to be common sense in medical practice. This included a bid to have Dr. Bailey prescribe a medication in pill form for a 6-month-old baby who had no teeth.
“Every doctor has got absurd stories like that, but unfortunately, every doctor is going to have tragic stories where prior authorization has resulted in death and harm to the patients,” Dr. Bailey said.
Some physicians leave it to the patient to try to overcome insurers’ decisions on prior authorization, seeing this task as falling outside of their duties, Dr. Bailey said.
“I don’t do that. I fight. I spend a lot of time fighting. I don’t like to lose. I don’t like my patients to lose, so I will go to the mat for them,” Dr. Bailey said. “But I’m blessed to be in a specialty where I’ve got loads more control over my schedule than many other specialties do.”
A version of this article first appeared on Medscape.com.
A group of bipartisan lawmakers intends to compel insurers to streamline prior authorization processes for Medicare Advantage plans, including a bid to end the use of faxes and develop systems that can allow for real-time decisions.
Rep. Suzan DelBene (D-Wash.); Rep. Mike Kelly (R-Pa.); Rep. Ami Bera, MD (D-Calif.); and Rep. Larry Bucshon, MD, (R-Ind.) on May 13 introduced a bill that would task federal officials with refining standards regarding prior authorization for Medicare Advantage. Titled the Improving Seniors’ Timely Access to Care Act of 2021, the bill would direct the Department of Health & Human Services to create rules intended to make prior authorization more transparent and speedy for the insurer-run Medicare plans. Known as Medicare Advantage, these plans cover about 24.1 million people of the 62 million enrolled in the giant federal health program, according to the nonprofit Kaiser Family Foundation.
These revamped prior authorization systems could not rely on faxes nor could they employ proprietary payer portals that did not meet HHS’ standards, says the text of the bill released by Rep. DelBene. Insurers would also have to report to the Centers for Medicare & Medicaid Services about the extent of their use of prior authorization and the rate of approvals or denials. The bill seeks to encourage plans to adopt prior authorization programs that adhere to evidence-based medical guidelines in consultation with physicians.
There were several reasons for focusing on Medicare Advantage plans, although prior authorization concerns extend more broadly in the U.S. health care system, said Susan Bailey, MD, president of the American Medical Association.
There’s an ample body of research about issues seen in the Medicare Advantage plans. Dr. Bailey also said that, in her experience, Medicare Advantage plans have had some of the most restrictive policies. And, by starting with Medicare Advantage, there’s a potential for a ripple effect in the industry, easing this issue when physicians work with other insurers as well.
“When Medicare adopts a policy whether it be a payment policy or a coverage policy, private insurers typically follow along,” she said.
Strong support among health care groups
There’s strong support for streamlining prior authorization both in the medical community and in Congress.
The bill has the support of about 70 health care organizations, including the AMA and the American Academy of Family Physicians, according to its sponsors. As of May 17, the bill had attracted the backing of 97 members of the House of Representatives, roughly evenly split among Democrats and Republicans.
Rep. DelBene’s previous version of this bill, the Improving Seniors’ Timely Access to Care Act of 2019, attracted 143 Democratic cosponsors and 137 Republican ones, or more than half of the members of the House. This bill was not completed during the previous session of Congress (January 2019–January 2021) because of the more urgent needs of pandemic response, said Rep. Bucshon, who practiced cardiothoracic surgery before joining Congress.
“It wasn’t quite on the radar as much as it might have been if we didn’t have COVID,” Rep. Bucshon said.
Rep. Bucshon added that he expects strong Senate support for a companion measure of the House bill, which could make the difference for efforts to pass it this year.
Insurers have become more aggressive over time in denying payments through prior authorization systems for services that physicians say their patients need, according to Rep. Bucshon. There may be some “bad actors” in medicine who would order unnecessary procedures, Rep. Bucshon allowed, but in most cases, the cumbersome prior authorization processes only put a hurdle for patients seeking needed treatments, he said.
“The premise is that it controls health care costs but actually what it does is it helps insurance company’s bottom line,” Rep. Bucshon said.
In a prepared statement, former Pennsylvania representative Allyson Y. Schwartz, now CEO of the Better Medicare Alliance, said her group had spoken with sponsors of this legislation and appreciates “their receptiveness to feedback in this process.”
“Prior authorization ensures beneficiaries receive clinically appropriate care and reduces exposures to duplicative and unnecessary services,” Ms. Schwartz said. “We share an interest in ensuring prior authorization works as smoothly and effectively as possible for beneficiaries while protecting its essential function of facilitating safe, evidenced-based care.”
The Better Medicare Alliance said its funders include UnitedHealth, Humana, and CVS Health/Aetna, which run Advantage plans. The group also lists as its partners many medical organizations.
“Rationing care by hassling”
Like Rep. Bucshon, Dr. Bailey sees a different motivation in insurers’ persistence in keeping the prior authorization process cumbersome.
Phone calls and faxes remain the key methods for handling prior authorization for medical services, according to the results of a survey done by the AMA in December. Phone calls were always or often required for prior authorization for medical services (59%), with faxes the second-most common approach (46%), followed by health plans’ online portals (39%), electronic health records and practice management systems (29%), and email or U.S. mail (26%), according to the AMA’s report on the survey.
“It seems like every step in the process is designed to make the patient less likely to get the therapy that the doctor thinks that the patient needs,” Dr. Bailey said. “It’s almost like rationing care by hassling the patient and the physician.”
The findings of an investigation by HHS’ internal watchdog unit appear to support Dr. Bailey’s view, showing that insurer-run Medicare plans had a pattern of often walking back their initial rejections.
In 2018, the Office of the Inspector General for HHS reported that Medicare Advantage organizations (MAOs) overturned 75% of their own denials during 2014-16. In addition, independent reviewers within the appeals process overturned additional denials in favor of patients and clinicians, OIG said.
“The high number of overturned denials raises concerns that some Medicare Advantage beneficiaries and providers were initially denied services and payments that should have been provided,” the OIG said in the report. “This is especially concerning because beneficiaries and providers rarely used the appeals process, which is designed to ensure access to care and payment.”
During 2014-2016, patients and clinicians appealed only 1% of denials to the first level of appeal, OIG said. In the report, the watchdog group noted that CMS audits had highlighted “widespread and persistent MAO performance problems related to denials of care and payment.” In 2015, for example, CMS cited 56% of audited contracts for making inappropriate denials.
Dr. Bailey also said in an interview that she routinely encounters problems with prior authorization in her own practice as an allergist and immunologist in Fort Worth, Tex.
In late May, for example, a Medicare Advantage plan made a patient whose chronic asthma had been stable for years change to a new inhaler that resulted in him developing a yeast infection in his mouth, Dr. Bailey said.
“We treated the yeast infection, made some changes in the way he uses his inhaler, so hopefully he would tolerate it better,” Dr. Bailey said. “He had a reaction to the medication to treat the yeast infection and ended up in the hospital. How is that helping anyone? It certainly hasn’t helped my patient.”
Dr. Bailey said insurers have also asked to seek prior authorization to prescribe medications that have been generic for years and have used the process to challenge her on cases of what seem to be common sense in medical practice. This included a bid to have Dr. Bailey prescribe a medication in pill form for a 6-month-old baby who had no teeth.
“Every doctor has got absurd stories like that, but unfortunately, every doctor is going to have tragic stories where prior authorization has resulted in death and harm to the patients,” Dr. Bailey said.
Some physicians leave it to the patient to try to overcome insurers’ decisions on prior authorization, seeing this task as falling outside of their duties, Dr. Bailey said.
“I don’t do that. I fight. I spend a lot of time fighting. I don’t like to lose. I don’t like my patients to lose, so I will go to the mat for them,” Dr. Bailey said. “But I’m blessed to be in a specialty where I’ve got loads more control over my schedule than many other specialties do.”
A version of this article first appeared on Medscape.com.
A group of bipartisan lawmakers intends to compel insurers to streamline prior authorization processes for Medicare Advantage plans, including a bid to end the use of faxes and develop systems that can allow for real-time decisions.
Rep. Suzan DelBene (D-Wash.); Rep. Mike Kelly (R-Pa.); Rep. Ami Bera, MD (D-Calif.); and Rep. Larry Bucshon, MD, (R-Ind.) on May 13 introduced a bill that would task federal officials with refining standards regarding prior authorization for Medicare Advantage. Titled the Improving Seniors’ Timely Access to Care Act of 2021, the bill would direct the Department of Health & Human Services to create rules intended to make prior authorization more transparent and speedy for the insurer-run Medicare plans. Known as Medicare Advantage, these plans cover about 24.1 million people of the 62 million enrolled in the giant federal health program, according to the nonprofit Kaiser Family Foundation.
These revamped prior authorization systems could not rely on faxes nor could they employ proprietary payer portals that did not meet HHS’ standards, says the text of the bill released by Rep. DelBene. Insurers would also have to report to the Centers for Medicare & Medicaid Services about the extent of their use of prior authorization and the rate of approvals or denials. The bill seeks to encourage plans to adopt prior authorization programs that adhere to evidence-based medical guidelines in consultation with physicians.
There were several reasons for focusing on Medicare Advantage plans, although prior authorization concerns extend more broadly in the U.S. health care system, said Susan Bailey, MD, president of the American Medical Association.
There’s an ample body of research about issues seen in the Medicare Advantage plans. Dr. Bailey also said that, in her experience, Medicare Advantage plans have had some of the most restrictive policies. And, by starting with Medicare Advantage, there’s a potential for a ripple effect in the industry, easing this issue when physicians work with other insurers as well.
“When Medicare adopts a policy whether it be a payment policy or a coverage policy, private insurers typically follow along,” she said.
Strong support among health care groups
There’s strong support for streamlining prior authorization both in the medical community and in Congress.
The bill has the support of about 70 health care organizations, including the AMA and the American Academy of Family Physicians, according to its sponsors. As of May 17, the bill had attracted the backing of 97 members of the House of Representatives, roughly evenly split among Democrats and Republicans.
Rep. DelBene’s previous version of this bill, the Improving Seniors’ Timely Access to Care Act of 2019, attracted 143 Democratic cosponsors and 137 Republican ones, or more than half of the members of the House. This bill was not completed during the previous session of Congress (January 2019–January 2021) because of the more urgent needs of pandemic response, said Rep. Bucshon, who practiced cardiothoracic surgery before joining Congress.
“It wasn’t quite on the radar as much as it might have been if we didn’t have COVID,” Rep. Bucshon said.
Rep. Bucshon added that he expects strong Senate support for a companion measure of the House bill, which could make the difference for efforts to pass it this year.
Insurers have become more aggressive over time in denying payments through prior authorization systems for services that physicians say their patients need, according to Rep. Bucshon. There may be some “bad actors” in medicine who would order unnecessary procedures, Rep. Bucshon allowed, but in most cases, the cumbersome prior authorization processes only put a hurdle for patients seeking needed treatments, he said.
“The premise is that it controls health care costs but actually what it does is it helps insurance company’s bottom line,” Rep. Bucshon said.
In a prepared statement, former Pennsylvania representative Allyson Y. Schwartz, now CEO of the Better Medicare Alliance, said her group had spoken with sponsors of this legislation and appreciates “their receptiveness to feedback in this process.”
“Prior authorization ensures beneficiaries receive clinically appropriate care and reduces exposures to duplicative and unnecessary services,” Ms. Schwartz said. “We share an interest in ensuring prior authorization works as smoothly and effectively as possible for beneficiaries while protecting its essential function of facilitating safe, evidenced-based care.”
The Better Medicare Alliance said its funders include UnitedHealth, Humana, and CVS Health/Aetna, which run Advantage plans. The group also lists as its partners many medical organizations.
“Rationing care by hassling”
Like Rep. Bucshon, Dr. Bailey sees a different motivation in insurers’ persistence in keeping the prior authorization process cumbersome.
Phone calls and faxes remain the key methods for handling prior authorization for medical services, according to the results of a survey done by the AMA in December. Phone calls were always or often required for prior authorization for medical services (59%), with faxes the second-most common approach (46%), followed by health plans’ online portals (39%), electronic health records and practice management systems (29%), and email or U.S. mail (26%), according to the AMA’s report on the survey.
“It seems like every step in the process is designed to make the patient less likely to get the therapy that the doctor thinks that the patient needs,” Dr. Bailey said. “It’s almost like rationing care by hassling the patient and the physician.”
The findings of an investigation by HHS’ internal watchdog unit appear to support Dr. Bailey’s view, showing that insurer-run Medicare plans had a pattern of often walking back their initial rejections.
In 2018, the Office of the Inspector General for HHS reported that Medicare Advantage organizations (MAOs) overturned 75% of their own denials during 2014-16. In addition, independent reviewers within the appeals process overturned additional denials in favor of patients and clinicians, OIG said.
“The high number of overturned denials raises concerns that some Medicare Advantage beneficiaries and providers were initially denied services and payments that should have been provided,” the OIG said in the report. “This is especially concerning because beneficiaries and providers rarely used the appeals process, which is designed to ensure access to care and payment.”
During 2014-2016, patients and clinicians appealed only 1% of denials to the first level of appeal, OIG said. In the report, the watchdog group noted that CMS audits had highlighted “widespread and persistent MAO performance problems related to denials of care and payment.” In 2015, for example, CMS cited 56% of audited contracts for making inappropriate denials.
Dr. Bailey also said in an interview that she routinely encounters problems with prior authorization in her own practice as an allergist and immunologist in Fort Worth, Tex.
In late May, for example, a Medicare Advantage plan made a patient whose chronic asthma had been stable for years change to a new inhaler that resulted in him developing a yeast infection in his mouth, Dr. Bailey said.
“We treated the yeast infection, made some changes in the way he uses his inhaler, so hopefully he would tolerate it better,” Dr. Bailey said. “He had a reaction to the medication to treat the yeast infection and ended up in the hospital. How is that helping anyone? It certainly hasn’t helped my patient.”
Dr. Bailey said insurers have also asked to seek prior authorization to prescribe medications that have been generic for years and have used the process to challenge her on cases of what seem to be common sense in medical practice. This included a bid to have Dr. Bailey prescribe a medication in pill form for a 6-month-old baby who had no teeth.
“Every doctor has got absurd stories like that, but unfortunately, every doctor is going to have tragic stories where prior authorization has resulted in death and harm to the patients,” Dr. Bailey said.
Some physicians leave it to the patient to try to overcome insurers’ decisions on prior authorization, seeing this task as falling outside of their duties, Dr. Bailey said.
“I don’t do that. I fight. I spend a lot of time fighting. I don’t like to lose. I don’t like my patients to lose, so I will go to the mat for them,” Dr. Bailey said. “But I’m blessed to be in a specialty where I’ve got loads more control over my schedule than many other specialties do.”
A version of this article first appeared on Medscape.com.
CDC recommends use of Pfizer’s COVID vaccine in 12- to 15-year-olds
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
FDA panel narrowly backs avacopan approval
A panel of federal advisers on May 6 lent support to the ChemoCentryx bid for approval of avacopan for a rare and serious autoimmune condition. But they also flagged concerns about both the evidence supporting claims of a benefit for this experimental drug and its safety.
At a meeting of the Food and Drug Administration’s Arthritis Advisory Committee, panelists voted 10-8 on a question of whether the risk-benefit profile of avacopan is adequate to support approval.
ChemoCentryx is seeking approval of avacopan for antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis in the subtypes of granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
Regardless of their vote on this approval question, the panelists shared an interest in avacopan’s potential to reduce glucocorticoid use among some patients with ANCA-associated vasculitis, also called AAV. Mara L. Becker, MD, MSCE, the chair of the FDA’s panel, was among the panelists who said they reluctantly voted no.
“It pains me because I really want more steroid-sparing” medicines, said Dr. Becker of Duke University, Durham, N.C., who cited a need to gather more data on avacopan.
Margrit Wiesendanger, MD, PhD, of the Icahn School of Medicine at Mount Sinai, New York, who was among the panelists voting yes, spoke of a need for caution if the FDA approves avacopan.
“Judicious use of this new medication will be warranted and perhaps additional guidance could be given to rheumatologists to help them decide for whom this medication is best,” she said.
Panelists had spoken earlier of avacopan as a possible alternative medicine for people with AAV who have conditions that make glucocorticoids riskier for them, such as those who have diabetes.
Close votes on safety profile, efficacy
The panel also voted 10-8 on a question about whether the safety profile of avacopan is adequate to support approval of avacopan for the treatment of adult patients with AAV.
In addition, the panel voted 9-9 on a question about whether efficacy data support approval of avacopan for the treatment of adult patients with AAV.
The FDA considers the recommendations of its advisory panels, but is not bound by them.
The FDA staff clearly expressed the view that ChemoCentryx fell short with the evidence presented for avacopan approval. Shares of San Carlos, Calif.–based ChemoCentryx dropped sharply from a May 3 closing price of $48.82 to a May 4 closing price of $26.63 after the FDA released the staff’s review of avacopan.
In a briefing prepared for the meeting, FDA staff detailed concerns about the evidence ChemoCentryx is using to seek approval. While acknowledging a need for new treatments for AAV as a rare condition, FDA staff honed in on what they described flaws in the testing of this experimental medicine, which is a small-molecule antagonist of the receptor of C5a, an end product of the complement cascade that acts as a potent neutrophil chemoattractant and agonist.
The FDA usually requires two phase 3 studies for approval of a new medicine but will do so with a single trial in cases of exceptional need, the agency staff said. But in these cases, the bar rises for the evidence provided from that single trial.
Difficulties in interpretation of complex study design
In the case of avacopan, though, the data from the key avacopan trial, Study CL010_168, known as ADVOCATE, there were substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.
In the briefing document, the FDA staff noted that it had “communicated many of the concerns” about ChemoCentryx’s research earlier to the company.
“Complexities of the study design, as detailed in the briefing document, raise questions about the interpretability of the data to define a clinically meaningful benefit of avacopan and its role in the management of AAV,” the FDA staff wrote.
“We acknowledge that AAV is a rare and serious disease associated with high morbidity and increased mortality. It is also a disease with high unmet need for new therapies. However, FDA wants to ensure that new products have a defined context of use, i.e., how a product would be used, and a favorable benefit-risk assessment for patients,” the staff added.
In addition, there were differences in the assessments performed by investigators and the adjudication committee, most frequently related to the attribution of persistent vasculitis, the FDA staff noted.
Statistical analyses of the primary endpoint using investigators’ estimates “resulted in more conservative estimates of treatment effect, e.g., statistical significance for superiority would no longer be demonstrated,” the FDA staff noted. “While the prespecified analysis used the Adjudicator assessments, the assessment based on the Investigators, experienced in management of vasculitis, may better reflect real-world use.”
Imbalances in use of glucocorticoids and maintenance therapy
Also among the complications in assessing the ADVOCATE trial data were the glucocorticoids taken by patients in the study, the FDA staff said.
In the avacopan arm of the trial, 86% of patients received non–study-supplied glucocorticoids. In addition, more avacopan‐treated patients experienced adverse events and serious adverse events within the hepatobiliary system leading to discontinuation.
Subgroups given different treatments represented another challenge in interpreting ADVOCATE results for the FDA staff.
At week 26, the proportion of patients in disease remission in the avacopan group (72.3%) was noninferior to the prednisone group (70.1%), the FDA staff said in the briefing document.
But at week 52, a disparity was observed between subgroups that had received rituximab and cyclophosphamide (intravenous and oral) induction treatment. The estimated risk difference for disease remission at week 52 was 15.0% (95% CI, 2.2%-27.7%) in the subgroup receiving induction with rituximab and 3.3% (95% CI, –14.8% to 21.4%) in the cyclophosphamide plus maintenance azathioprine subgroup, the agency’s staff said.
“Based on the data, there is no evidence of clinically meaningful treatment effect in the cyclophosphamide induction subgroup,” the FDA staff wrote. “Further, the treatment comparison in the complementary rituximab induction subgroup may not be considered meaningful because these patients did not receive maintenance therapy, i.e., due to undertreating of patients, the effect observed in the rituximab subgroup may not represent a clinically meaningful treatment effect, compared to standard of care.”
Rachel L. Glaser, MD, clinical team leader in FDA’s division of rheumatology and transplant medicine, reiterated these concerns to the advisory committee at the May 6 meeting.
“Throughout the development program, FDA advised the applicant that a noninferiority comparison would not be sufficient to show that avacopan can replaced glucocorticoids as it would be difficult to establish whether avacopan is effective or whether an effect was due to the rituximab or cyclophosphamide administered to both treatment arms,” she said.
In its briefing for the meeting, ChemoCentryx noted the limits of treatments now available for AAV. It also emphasized the toll of the condition, ranging from skin manifestations to glomerulonephritis to life-threatening pulmonary hemorrhage. If untreated, 80% of patients with GPA or MPA die within 2 years of disease onset, ChemoCentryx said in its briefing materials for the meeting.
The side effects of glucocorticoids were well known to the FDA panelists and the ChemoCentryx presenters. Witnesses at an open public hearing told their own stories of depression, anxiety, and irritability caused by these medicines.
During the ChemoCentryx presentation, a presenter for the company, Peter Merkel, MD, MPH, of the University of Pennsylvania, Philadelphia, said avacopan would provide patients with AAV with an alternative allowing them “to go on a much lower glucocorticoids regimen.”
A similar view was presented in a February 2021 editorial in the New England Journal of Medicine, titled “Avacopan – Time to Replace Glucocorticoids?” Written by Kenneth J. Warrington, MD, of the Mayo Clinic, Rochester, Minn., the opinion article called the ADVOCATE trial “a milestone in the treatment of ANCA-associated vasculitis; complement inhibition with avacopan has glucocorticoid-sparing effects and results in superior disease control.”
Dr. Warrington reported no conflicts in connection with his editorial nor payments from ChemoCentryx. He did report grants from other firms such as Eli Lilly.
Julia Lewis, MD, of Vanderbilt University, Nashville, Tenn., was among the more skeptical members of the FDA panel. She was among the “nays” in all three voting questions put to the panel. Still, she said there were signs of “clinically meaningful benefit” in the data presented, but noted that the nonstudy use of glucocorticoids made it difficult to interpret the ADVOCATE results.
Dr. Lewis noted that the FDA usually requires two studies for a drug approval, particularly with a compound not yet cleared for any use. While ANCA-associated vasculitis is rare, it would be possible to recruit patients for another trial of avacopan, adding to the results reported already for avacopan from ADVOCATE, she said.
“Were there to be another study, this would certainly be a supportive study and maybe qualify as two studies,” she said.
A panel of federal advisers on May 6 lent support to the ChemoCentryx bid for approval of avacopan for a rare and serious autoimmune condition. But they also flagged concerns about both the evidence supporting claims of a benefit for this experimental drug and its safety.
At a meeting of the Food and Drug Administration’s Arthritis Advisory Committee, panelists voted 10-8 on a question of whether the risk-benefit profile of avacopan is adequate to support approval.
ChemoCentryx is seeking approval of avacopan for antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis in the subtypes of granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
Regardless of their vote on this approval question, the panelists shared an interest in avacopan’s potential to reduce glucocorticoid use among some patients with ANCA-associated vasculitis, also called AAV. Mara L. Becker, MD, MSCE, the chair of the FDA’s panel, was among the panelists who said they reluctantly voted no.
“It pains me because I really want more steroid-sparing” medicines, said Dr. Becker of Duke University, Durham, N.C., who cited a need to gather more data on avacopan.
Margrit Wiesendanger, MD, PhD, of the Icahn School of Medicine at Mount Sinai, New York, who was among the panelists voting yes, spoke of a need for caution if the FDA approves avacopan.
“Judicious use of this new medication will be warranted and perhaps additional guidance could be given to rheumatologists to help them decide for whom this medication is best,” she said.
Panelists had spoken earlier of avacopan as a possible alternative medicine for people with AAV who have conditions that make glucocorticoids riskier for them, such as those who have diabetes.
Close votes on safety profile, efficacy
The panel also voted 10-8 on a question about whether the safety profile of avacopan is adequate to support approval of avacopan for the treatment of adult patients with AAV.
In addition, the panel voted 9-9 on a question about whether efficacy data support approval of avacopan for the treatment of adult patients with AAV.
The FDA considers the recommendations of its advisory panels, but is not bound by them.
The FDA staff clearly expressed the view that ChemoCentryx fell short with the evidence presented for avacopan approval. Shares of San Carlos, Calif.–based ChemoCentryx dropped sharply from a May 3 closing price of $48.82 to a May 4 closing price of $26.63 after the FDA released the staff’s review of avacopan.
In a briefing prepared for the meeting, FDA staff detailed concerns about the evidence ChemoCentryx is using to seek approval. While acknowledging a need for new treatments for AAV as a rare condition, FDA staff honed in on what they described flaws in the testing of this experimental medicine, which is a small-molecule antagonist of the receptor of C5a, an end product of the complement cascade that acts as a potent neutrophil chemoattractant and agonist.
The FDA usually requires two phase 3 studies for approval of a new medicine but will do so with a single trial in cases of exceptional need, the agency staff said. But in these cases, the bar rises for the evidence provided from that single trial.
Difficulties in interpretation of complex study design
In the case of avacopan, though, the data from the key avacopan trial, Study CL010_168, known as ADVOCATE, there were substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.
In the briefing document, the FDA staff noted that it had “communicated many of the concerns” about ChemoCentryx’s research earlier to the company.
“Complexities of the study design, as detailed in the briefing document, raise questions about the interpretability of the data to define a clinically meaningful benefit of avacopan and its role in the management of AAV,” the FDA staff wrote.
“We acknowledge that AAV is a rare and serious disease associated with high morbidity and increased mortality. It is also a disease with high unmet need for new therapies. However, FDA wants to ensure that new products have a defined context of use, i.e., how a product would be used, and a favorable benefit-risk assessment for patients,” the staff added.
In addition, there were differences in the assessments performed by investigators and the adjudication committee, most frequently related to the attribution of persistent vasculitis, the FDA staff noted.
Statistical analyses of the primary endpoint using investigators’ estimates “resulted in more conservative estimates of treatment effect, e.g., statistical significance for superiority would no longer be demonstrated,” the FDA staff noted. “While the prespecified analysis used the Adjudicator assessments, the assessment based on the Investigators, experienced in management of vasculitis, may better reflect real-world use.”
Imbalances in use of glucocorticoids and maintenance therapy
Also among the complications in assessing the ADVOCATE trial data were the glucocorticoids taken by patients in the study, the FDA staff said.
In the avacopan arm of the trial, 86% of patients received non–study-supplied glucocorticoids. In addition, more avacopan‐treated patients experienced adverse events and serious adverse events within the hepatobiliary system leading to discontinuation.
Subgroups given different treatments represented another challenge in interpreting ADVOCATE results for the FDA staff.
At week 26, the proportion of patients in disease remission in the avacopan group (72.3%) was noninferior to the prednisone group (70.1%), the FDA staff said in the briefing document.
But at week 52, a disparity was observed between subgroups that had received rituximab and cyclophosphamide (intravenous and oral) induction treatment. The estimated risk difference for disease remission at week 52 was 15.0% (95% CI, 2.2%-27.7%) in the subgroup receiving induction with rituximab and 3.3% (95% CI, –14.8% to 21.4%) in the cyclophosphamide plus maintenance azathioprine subgroup, the agency’s staff said.
“Based on the data, there is no evidence of clinically meaningful treatment effect in the cyclophosphamide induction subgroup,” the FDA staff wrote. “Further, the treatment comparison in the complementary rituximab induction subgroup may not be considered meaningful because these patients did not receive maintenance therapy, i.e., due to undertreating of patients, the effect observed in the rituximab subgroup may not represent a clinically meaningful treatment effect, compared to standard of care.”
Rachel L. Glaser, MD, clinical team leader in FDA’s division of rheumatology and transplant medicine, reiterated these concerns to the advisory committee at the May 6 meeting.
“Throughout the development program, FDA advised the applicant that a noninferiority comparison would not be sufficient to show that avacopan can replaced glucocorticoids as it would be difficult to establish whether avacopan is effective or whether an effect was due to the rituximab or cyclophosphamide administered to both treatment arms,” she said.
In its briefing for the meeting, ChemoCentryx noted the limits of treatments now available for AAV. It also emphasized the toll of the condition, ranging from skin manifestations to glomerulonephritis to life-threatening pulmonary hemorrhage. If untreated, 80% of patients with GPA or MPA die within 2 years of disease onset, ChemoCentryx said in its briefing materials for the meeting.
The side effects of glucocorticoids were well known to the FDA panelists and the ChemoCentryx presenters. Witnesses at an open public hearing told their own stories of depression, anxiety, and irritability caused by these medicines.
During the ChemoCentryx presentation, a presenter for the company, Peter Merkel, MD, MPH, of the University of Pennsylvania, Philadelphia, said avacopan would provide patients with AAV with an alternative allowing them “to go on a much lower glucocorticoids regimen.”
A similar view was presented in a February 2021 editorial in the New England Journal of Medicine, titled “Avacopan – Time to Replace Glucocorticoids?” Written by Kenneth J. Warrington, MD, of the Mayo Clinic, Rochester, Minn., the opinion article called the ADVOCATE trial “a milestone in the treatment of ANCA-associated vasculitis; complement inhibition with avacopan has glucocorticoid-sparing effects and results in superior disease control.”
Dr. Warrington reported no conflicts in connection with his editorial nor payments from ChemoCentryx. He did report grants from other firms such as Eli Lilly.
Julia Lewis, MD, of Vanderbilt University, Nashville, Tenn., was among the more skeptical members of the FDA panel. She was among the “nays” in all three voting questions put to the panel. Still, she said there were signs of “clinically meaningful benefit” in the data presented, but noted that the nonstudy use of glucocorticoids made it difficult to interpret the ADVOCATE results.
Dr. Lewis noted that the FDA usually requires two studies for a drug approval, particularly with a compound not yet cleared for any use. While ANCA-associated vasculitis is rare, it would be possible to recruit patients for another trial of avacopan, adding to the results reported already for avacopan from ADVOCATE, she said.
“Were there to be another study, this would certainly be a supportive study and maybe qualify as two studies,” she said.
A panel of federal advisers on May 6 lent support to the ChemoCentryx bid for approval of avacopan for a rare and serious autoimmune condition. But they also flagged concerns about both the evidence supporting claims of a benefit for this experimental drug and its safety.
At a meeting of the Food and Drug Administration’s Arthritis Advisory Committee, panelists voted 10-8 on a question of whether the risk-benefit profile of avacopan is adequate to support approval.
ChemoCentryx is seeking approval of avacopan for antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis in the subtypes of granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
Regardless of their vote on this approval question, the panelists shared an interest in avacopan’s potential to reduce glucocorticoid use among some patients with ANCA-associated vasculitis, also called AAV. Mara L. Becker, MD, MSCE, the chair of the FDA’s panel, was among the panelists who said they reluctantly voted no.
“It pains me because I really want more steroid-sparing” medicines, said Dr. Becker of Duke University, Durham, N.C., who cited a need to gather more data on avacopan.
Margrit Wiesendanger, MD, PhD, of the Icahn School of Medicine at Mount Sinai, New York, who was among the panelists voting yes, spoke of a need for caution if the FDA approves avacopan.
“Judicious use of this new medication will be warranted and perhaps additional guidance could be given to rheumatologists to help them decide for whom this medication is best,” she said.
Panelists had spoken earlier of avacopan as a possible alternative medicine for people with AAV who have conditions that make glucocorticoids riskier for them, such as those who have diabetes.
Close votes on safety profile, efficacy
The panel also voted 10-8 on a question about whether the safety profile of avacopan is adequate to support approval of avacopan for the treatment of adult patients with AAV.
In addition, the panel voted 9-9 on a question about whether efficacy data support approval of avacopan for the treatment of adult patients with AAV.
The FDA considers the recommendations of its advisory panels, but is not bound by them.
The FDA staff clearly expressed the view that ChemoCentryx fell short with the evidence presented for avacopan approval. Shares of San Carlos, Calif.–based ChemoCentryx dropped sharply from a May 3 closing price of $48.82 to a May 4 closing price of $26.63 after the FDA released the staff’s review of avacopan.
In a briefing prepared for the meeting, FDA staff detailed concerns about the evidence ChemoCentryx is using to seek approval. While acknowledging a need for new treatments for AAV as a rare condition, FDA staff honed in on what they described flaws in the testing of this experimental medicine, which is a small-molecule antagonist of the receptor of C5a, an end product of the complement cascade that acts as a potent neutrophil chemoattractant and agonist.
The FDA usually requires two phase 3 studies for approval of a new medicine but will do so with a single trial in cases of exceptional need, the agency staff said. But in these cases, the bar rises for the evidence provided from that single trial.
Difficulties in interpretation of complex study design
In the case of avacopan, though, the data from the key avacopan trial, Study CL010_168, known as ADVOCATE, there were substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.
In the briefing document, the FDA staff noted that it had “communicated many of the concerns” about ChemoCentryx’s research earlier to the company.
“Complexities of the study design, as detailed in the briefing document, raise questions about the interpretability of the data to define a clinically meaningful benefit of avacopan and its role in the management of AAV,” the FDA staff wrote.
“We acknowledge that AAV is a rare and serious disease associated with high morbidity and increased mortality. It is also a disease with high unmet need for new therapies. However, FDA wants to ensure that new products have a defined context of use, i.e., how a product would be used, and a favorable benefit-risk assessment for patients,” the staff added.
In addition, there were differences in the assessments performed by investigators and the adjudication committee, most frequently related to the attribution of persistent vasculitis, the FDA staff noted.
Statistical analyses of the primary endpoint using investigators’ estimates “resulted in more conservative estimates of treatment effect, e.g., statistical significance for superiority would no longer be demonstrated,” the FDA staff noted. “While the prespecified analysis used the Adjudicator assessments, the assessment based on the Investigators, experienced in management of vasculitis, may better reflect real-world use.”
Imbalances in use of glucocorticoids and maintenance therapy
Also among the complications in assessing the ADVOCATE trial data were the glucocorticoids taken by patients in the study, the FDA staff said.
In the avacopan arm of the trial, 86% of patients received non–study-supplied glucocorticoids. In addition, more avacopan‐treated patients experienced adverse events and serious adverse events within the hepatobiliary system leading to discontinuation.
Subgroups given different treatments represented another challenge in interpreting ADVOCATE results for the FDA staff.
At week 26, the proportion of patients in disease remission in the avacopan group (72.3%) was noninferior to the prednisone group (70.1%), the FDA staff said in the briefing document.
But at week 52, a disparity was observed between subgroups that had received rituximab and cyclophosphamide (intravenous and oral) induction treatment. The estimated risk difference for disease remission at week 52 was 15.0% (95% CI, 2.2%-27.7%) in the subgroup receiving induction with rituximab and 3.3% (95% CI, –14.8% to 21.4%) in the cyclophosphamide plus maintenance azathioprine subgroup, the agency’s staff said.
“Based on the data, there is no evidence of clinically meaningful treatment effect in the cyclophosphamide induction subgroup,” the FDA staff wrote. “Further, the treatment comparison in the complementary rituximab induction subgroup may not be considered meaningful because these patients did not receive maintenance therapy, i.e., due to undertreating of patients, the effect observed in the rituximab subgroup may not represent a clinically meaningful treatment effect, compared to standard of care.”
Rachel L. Glaser, MD, clinical team leader in FDA’s division of rheumatology and transplant medicine, reiterated these concerns to the advisory committee at the May 6 meeting.
“Throughout the development program, FDA advised the applicant that a noninferiority comparison would not be sufficient to show that avacopan can replaced glucocorticoids as it would be difficult to establish whether avacopan is effective or whether an effect was due to the rituximab or cyclophosphamide administered to both treatment arms,” she said.
In its briefing for the meeting, ChemoCentryx noted the limits of treatments now available for AAV. It also emphasized the toll of the condition, ranging from skin manifestations to glomerulonephritis to life-threatening pulmonary hemorrhage. If untreated, 80% of patients with GPA or MPA die within 2 years of disease onset, ChemoCentryx said in its briefing materials for the meeting.
The side effects of glucocorticoids were well known to the FDA panelists and the ChemoCentryx presenters. Witnesses at an open public hearing told their own stories of depression, anxiety, and irritability caused by these medicines.
During the ChemoCentryx presentation, a presenter for the company, Peter Merkel, MD, MPH, of the University of Pennsylvania, Philadelphia, said avacopan would provide patients with AAV with an alternative allowing them “to go on a much lower glucocorticoids regimen.”
A similar view was presented in a February 2021 editorial in the New England Journal of Medicine, titled “Avacopan – Time to Replace Glucocorticoids?” Written by Kenneth J. Warrington, MD, of the Mayo Clinic, Rochester, Minn., the opinion article called the ADVOCATE trial “a milestone in the treatment of ANCA-associated vasculitis; complement inhibition with avacopan has glucocorticoid-sparing effects and results in superior disease control.”
Dr. Warrington reported no conflicts in connection with his editorial nor payments from ChemoCentryx. He did report grants from other firms such as Eli Lilly.
Julia Lewis, MD, of Vanderbilt University, Nashville, Tenn., was among the more skeptical members of the FDA panel. She was among the “nays” in all three voting questions put to the panel. Still, she said there were signs of “clinically meaningful benefit” in the data presented, but noted that the nonstudy use of glucocorticoids made it difficult to interpret the ADVOCATE results.
Dr. Lewis noted that the FDA usually requires two studies for a drug approval, particularly with a compound not yet cleared for any use. While ANCA-associated vasculitis is rare, it would be possible to recruit patients for another trial of avacopan, adding to the results reported already for avacopan from ADVOCATE, she said.
“Were there to be another study, this would certainly be a supportive study and maybe qualify as two studies,” she said.
FDA panel votes against 2 cancer indications but backs 4 of 6
Federal advisers have supported the efforts of pharmaceutical companies in four of six cases in which these firms are fighting to maintain cancer indications for approved drugs. The advisers voted against the companies in two cases.
The staff of the Food and Drug Administration will now consider these votes as they decide what to do regarding the six cases of what they have termed “dangling” accelerated approvals.
“One of the reasons I think we’re convening today is to prevent these accelerated approvals from dangling ad infinitum,” commented one of the members of the advisory panel.
In these cases, companies have been unable to prove the expected benefits that led the FDA to grant accelerated approvals for these indications.
These accelerated approvals, which are often based on surrogate endpoints, such as overall response rates, are granted on the condition that further findings show a clinical benefit – such as in progression-free survival or overall survival – in larger trials.
The FDA tasked its Oncologic Drugs Advisory Committee (ODAC) with conducting the review of the six accelerated approvals for cancer indications at a 3-day meeting (April 27-29).
These reviews were only for specific cancer indications and will not lead to the removal of drugs from the market. These drugs have already been approved for several cancer indications. For example, one of the drugs that was reviewed, pembrolizumab (Keytruda), is approved in the United States for 28 indications.
The FDA is facing growing pains in its efforts to manage the rapidly changing landscape for these immune checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials, owing in part to the agency’s willingness to accept surrogate markers for accelerated approvals. Although some companies have struggled with these, others have built strong cases for the use of their checkpoint inhibitors for these indications.
The ODAC panelists, for example, noted the emergence of nivolumab (Opdivo) as an option for patients with gastric cancer as a reason for seeking to withdraw an indication for pembrolizumab (Keytruda) for this disease.
Just weeks before the meeting, on April 16, the FDA approved nivolumab plus chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. This was a full approval based on data showing an overall survival benefit from a phase 3 trial.
Votes by indication
On April 29, the last day of the meeting, the ODAC panel voted 6-2 against maintaining pembrolizumab’s indication as monotherapy for an advanced form of gastric cancer. This was an accelerated approval (granted in 2017) that was based on overall response rates from an open-label trial.
That last day of the meeting also saw another negative vote. On April 29, the ODAC panel voted 5-4 against maintaining an indication for nivolumab in patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib (Nexavar).
This accelerated approval for nivolumab was granted in 2017. The FDA said it had requested ODAC’s feedback on this indication because of the recent full approval of another checkpoint inhibitor for HCC, atezolizumab (Tecentriq), in combination with bevacizumab (Avastin) for patients with unresectable or metastatic diseases who have not received prior systemic therapy. This full approval (in May 2020) was based on an overall survival benefit.
There was one last vote on the third day of the meeting, and it was positive. The ODAC panel voted 8-0 in favor of maintaining the indication for the use of pembrolizumab as monotherapy for patients with HCC who have previously been treated with sorafenib.
The FDA altered the composition of the ODAC panel during the week, adding members in some cases who had expertise in particular cancers. That led to different totals for the week’s ODAC votes, as shown in the tallies summarized below.
On the first day of the meeting (April 27), the ODAC panel voted 7-2 in favor of maintaining a breast cancer indication for atezolizumab (Tecentriq). This covered use of the immunotherapy in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1.
The second day of the meeting (April 28) also saw two positive votes. The ODAC panel voted 10-1 for maintaining the indication for atezolizumab for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial. The panel also voted 5-3 for maintaining the indication for pembrolizumab in patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1.
The FDA is not bound to follow the voting and recommendations of its advisory panels, but it usually does so.
Managing shifts in treatment
In both of the cases in which ODAC voted against maintaining indications, Richard Pazdur, MD, the FDA’s top regulator for cancer medicines, jumped into the debate. Dr. Pazdur countered arguments put forward by representatives of the manufacturers as they sought to maintain indications for their drugs.
Merck officials and representatives argued for pembrolizumab, saying that maintaining the gastric cancer indication might help patients whose disease has progressed despite earlier treatment.
Dr. Pazdur emphasized that the agency would help Merck and physicians to have access to pembrolizumab for these patients even if this one indication were to be withdrawn. But Dr. Pazdur and ODAC members also noted the recent shift in the landscape for gastric cancer, with the recent approval of a new indication for nivolumab.
“I want to emphasize to the patient community out there [that] we firmly believe in the role of checkpoint inhibitors in this disease,” Dr. Pazdur said during the discussion of the indication for pembrolizumab for gastric cancer. “We have to be cognizant of what is the appropriate setting for that, and it currently is in the first line.”
Dr. Pazdur noted that two studies had failed to confirm the expected benefit from pembrolizumab for patients with more advanced disease. Still, if “small numbers” of patients with advanced disease wanted access to Merck’s drug, the FDA and the company could accommodate them. The FDA could delay the removal of the gastric indication to allow patients to continue receiving it. The FDA also could work with physicians on other routes to provide the medicine, such as through single-patient investigational new drug applications or an expanded access program.
“Or Merck can alternatively give the drug gratis to patients,” Dr. Pazdur said.
#ProjectFacilitate for expanded access
One of Merck’s speakers at the ODAC meeting, Peter Enzinger, MD, of the Dana-Farber Cancer Institute, Boston, objected to Dr. Pazdur’s plan.
A loss of the gastric indication for pembrolizumab would result in patients with advanced cancer missing out on a chance to try this therapy. Some patients will not have had a chance to try a checkpoint inhibitor earlier in their treatment, and a loss of the indication would cost them that opportunity, he said.
“An expanded-access program sounds very nice, but the reality is that our patients are incredibly sick and that weeks matter,” Dr. Enzinger said, citing administrative hurdles as a barrier to treatment.
“Our patients just don’t have the time for that, and therefore I don’t think an expanded access program is the way to go,” Dr. Enzinger said.
Dr. Pazdur responded to these objections by highlighting an initiative called Project Facilitate at the FDA’s Oncology Center for Excellence. During the meeting, Dr. Pazdur’s division used its @FDAOncology Twitter handle to draw attention to this project.
ODAC panelist Diane Reidy-Lagunes, MD, of Memorial Sloan Kettering Cancer Center, New York, said she had struggled with this vote. She was one of the two panelists to vote in favor of keeping the indication.
“This is also incredibly hard for me. I actually changed it at the last minute,” she said of her vote.
But Dr. Reidy-Lagunes said she was concerned that some patients with advanced disease might not be able to get a checkpoint inhibitor.
“With disparities in healthcare and differences in the way that patients are treated throughout our country, I was nervous that they may not be able to get treated,” she said, noting that she shared her fellow panelists’ doubts about use of pembrolizumab as third-line treatment, owing to negative results in trials.
ODAC member David Mitchell, who served as a consumer representative, also said he found the vote on the gastric indication for pembrolizumab to be a difficult decision.
“As a patient with incurable cancer who’s now being given all three major classes of drugs to treat my disease in combination, these issues really cut close to home,” Mr. Mitchell said.
He said the expectation that the FDA’s expanded access program could help patients with advanced disease try pembrolizumab helped him decide to vote with the 6-2 majority against maintaining this gastric cancer approval.
His vote was based on “the changing treatment landscape.” There is general agreement that the patients in question should receive checkpoint inhibitors as first-line treatment, not third-line treatment, Mr. Mitchell said. The FDA should delay a withdrawal of the approval for pembrolizumab in this case and should allow a transition for those who missed out on treatment with a checkpoint inhibitor earlier in the disease course, he suggested.
“To protect the safety and well-being of patients, we have to base decisions on data,” Mr. Mitchell said. “The data don’t support maintaining the indication” for pembrolizumab.
Close split on nivolumab
In contrast to the 6-2 vote against maintaining the pembrolizumab indication, the ODAC panel split more closely, 5-4, on the question of maintaining an indication for the use as monotherapy of nivolumab in HCC.
ODAC panelist Philip C. Hoffman, MD, of the University of Chicago was among those who supported keeping the indication.
“There’s still an unmet need for second-line immunotherapy because there will always be some patients who are poor candidates for bevacizumab or who are not tolerating or responding to sorafenib,” he said.
ODAC panelist Mark A. Lewis, MD, of Intermountain Healthcare, Salt Lake City, said he voted “no” in part because he doubted that Bristol-Myers Squibb would be able to soon produce data for nivolumab that was needed to support this indication.
A version of this article first appeared on Medscape.com.
Federal advisers have supported the efforts of pharmaceutical companies in four of six cases in which these firms are fighting to maintain cancer indications for approved drugs. The advisers voted against the companies in two cases.
The staff of the Food and Drug Administration will now consider these votes as they decide what to do regarding the six cases of what they have termed “dangling” accelerated approvals.
“One of the reasons I think we’re convening today is to prevent these accelerated approvals from dangling ad infinitum,” commented one of the members of the advisory panel.
In these cases, companies have been unable to prove the expected benefits that led the FDA to grant accelerated approvals for these indications.
These accelerated approvals, which are often based on surrogate endpoints, such as overall response rates, are granted on the condition that further findings show a clinical benefit – such as in progression-free survival or overall survival – in larger trials.
The FDA tasked its Oncologic Drugs Advisory Committee (ODAC) with conducting the review of the six accelerated approvals for cancer indications at a 3-day meeting (April 27-29).
These reviews were only for specific cancer indications and will not lead to the removal of drugs from the market. These drugs have already been approved for several cancer indications. For example, one of the drugs that was reviewed, pembrolizumab (Keytruda), is approved in the United States for 28 indications.
The FDA is facing growing pains in its efforts to manage the rapidly changing landscape for these immune checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials, owing in part to the agency’s willingness to accept surrogate markers for accelerated approvals. Although some companies have struggled with these, others have built strong cases for the use of their checkpoint inhibitors for these indications.
The ODAC panelists, for example, noted the emergence of nivolumab (Opdivo) as an option for patients with gastric cancer as a reason for seeking to withdraw an indication for pembrolizumab (Keytruda) for this disease.
Just weeks before the meeting, on April 16, the FDA approved nivolumab plus chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. This was a full approval based on data showing an overall survival benefit from a phase 3 trial.
Votes by indication
On April 29, the last day of the meeting, the ODAC panel voted 6-2 against maintaining pembrolizumab’s indication as monotherapy for an advanced form of gastric cancer. This was an accelerated approval (granted in 2017) that was based on overall response rates from an open-label trial.
That last day of the meeting also saw another negative vote. On April 29, the ODAC panel voted 5-4 against maintaining an indication for nivolumab in patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib (Nexavar).
This accelerated approval for nivolumab was granted in 2017. The FDA said it had requested ODAC’s feedback on this indication because of the recent full approval of another checkpoint inhibitor for HCC, atezolizumab (Tecentriq), in combination with bevacizumab (Avastin) for patients with unresectable or metastatic diseases who have not received prior systemic therapy. This full approval (in May 2020) was based on an overall survival benefit.
There was one last vote on the third day of the meeting, and it was positive. The ODAC panel voted 8-0 in favor of maintaining the indication for the use of pembrolizumab as monotherapy for patients with HCC who have previously been treated with sorafenib.
The FDA altered the composition of the ODAC panel during the week, adding members in some cases who had expertise in particular cancers. That led to different totals for the week’s ODAC votes, as shown in the tallies summarized below.
On the first day of the meeting (April 27), the ODAC panel voted 7-2 in favor of maintaining a breast cancer indication for atezolizumab (Tecentriq). This covered use of the immunotherapy in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1.
The second day of the meeting (April 28) also saw two positive votes. The ODAC panel voted 10-1 for maintaining the indication for atezolizumab for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial. The panel also voted 5-3 for maintaining the indication for pembrolizumab in patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1.
The FDA is not bound to follow the voting and recommendations of its advisory panels, but it usually does so.
Managing shifts in treatment
In both of the cases in which ODAC voted against maintaining indications, Richard Pazdur, MD, the FDA’s top regulator for cancer medicines, jumped into the debate. Dr. Pazdur countered arguments put forward by representatives of the manufacturers as they sought to maintain indications for their drugs.
Merck officials and representatives argued for pembrolizumab, saying that maintaining the gastric cancer indication might help patients whose disease has progressed despite earlier treatment.
Dr. Pazdur emphasized that the agency would help Merck and physicians to have access to pembrolizumab for these patients even if this one indication were to be withdrawn. But Dr. Pazdur and ODAC members also noted the recent shift in the landscape for gastric cancer, with the recent approval of a new indication for nivolumab.
“I want to emphasize to the patient community out there [that] we firmly believe in the role of checkpoint inhibitors in this disease,” Dr. Pazdur said during the discussion of the indication for pembrolizumab for gastric cancer. “We have to be cognizant of what is the appropriate setting for that, and it currently is in the first line.”
Dr. Pazdur noted that two studies had failed to confirm the expected benefit from pembrolizumab for patients with more advanced disease. Still, if “small numbers” of patients with advanced disease wanted access to Merck’s drug, the FDA and the company could accommodate them. The FDA could delay the removal of the gastric indication to allow patients to continue receiving it. The FDA also could work with physicians on other routes to provide the medicine, such as through single-patient investigational new drug applications or an expanded access program.
“Or Merck can alternatively give the drug gratis to patients,” Dr. Pazdur said.
#ProjectFacilitate for expanded access
One of Merck’s speakers at the ODAC meeting, Peter Enzinger, MD, of the Dana-Farber Cancer Institute, Boston, objected to Dr. Pazdur’s plan.
A loss of the gastric indication for pembrolizumab would result in patients with advanced cancer missing out on a chance to try this therapy. Some patients will not have had a chance to try a checkpoint inhibitor earlier in their treatment, and a loss of the indication would cost them that opportunity, he said.
“An expanded-access program sounds very nice, but the reality is that our patients are incredibly sick and that weeks matter,” Dr. Enzinger said, citing administrative hurdles as a barrier to treatment.
“Our patients just don’t have the time for that, and therefore I don’t think an expanded access program is the way to go,” Dr. Enzinger said.
Dr. Pazdur responded to these objections by highlighting an initiative called Project Facilitate at the FDA’s Oncology Center for Excellence. During the meeting, Dr. Pazdur’s division used its @FDAOncology Twitter handle to draw attention to this project.
ODAC panelist Diane Reidy-Lagunes, MD, of Memorial Sloan Kettering Cancer Center, New York, said she had struggled with this vote. She was one of the two panelists to vote in favor of keeping the indication.
“This is also incredibly hard for me. I actually changed it at the last minute,” she said of her vote.
But Dr. Reidy-Lagunes said she was concerned that some patients with advanced disease might not be able to get a checkpoint inhibitor.
“With disparities in healthcare and differences in the way that patients are treated throughout our country, I was nervous that they may not be able to get treated,” she said, noting that she shared her fellow panelists’ doubts about use of pembrolizumab as third-line treatment, owing to negative results in trials.
ODAC member David Mitchell, who served as a consumer representative, also said he found the vote on the gastric indication for pembrolizumab to be a difficult decision.
“As a patient with incurable cancer who’s now being given all three major classes of drugs to treat my disease in combination, these issues really cut close to home,” Mr. Mitchell said.
He said the expectation that the FDA’s expanded access program could help patients with advanced disease try pembrolizumab helped him decide to vote with the 6-2 majority against maintaining this gastric cancer approval.
His vote was based on “the changing treatment landscape.” There is general agreement that the patients in question should receive checkpoint inhibitors as first-line treatment, not third-line treatment, Mr. Mitchell said. The FDA should delay a withdrawal of the approval for pembrolizumab in this case and should allow a transition for those who missed out on treatment with a checkpoint inhibitor earlier in the disease course, he suggested.
“To protect the safety and well-being of patients, we have to base decisions on data,” Mr. Mitchell said. “The data don’t support maintaining the indication” for pembrolizumab.
Close split on nivolumab
In contrast to the 6-2 vote against maintaining the pembrolizumab indication, the ODAC panel split more closely, 5-4, on the question of maintaining an indication for the use as monotherapy of nivolumab in HCC.
ODAC panelist Philip C. Hoffman, MD, of the University of Chicago was among those who supported keeping the indication.
“There’s still an unmet need for second-line immunotherapy because there will always be some patients who are poor candidates for bevacizumab or who are not tolerating or responding to sorafenib,” he said.
ODAC panelist Mark A. Lewis, MD, of Intermountain Healthcare, Salt Lake City, said he voted “no” in part because he doubted that Bristol-Myers Squibb would be able to soon produce data for nivolumab that was needed to support this indication.
A version of this article first appeared on Medscape.com.
Federal advisers have supported the efforts of pharmaceutical companies in four of six cases in which these firms are fighting to maintain cancer indications for approved drugs. The advisers voted against the companies in two cases.
The staff of the Food and Drug Administration will now consider these votes as they decide what to do regarding the six cases of what they have termed “dangling” accelerated approvals.
“One of the reasons I think we’re convening today is to prevent these accelerated approvals from dangling ad infinitum,” commented one of the members of the advisory panel.
In these cases, companies have been unable to prove the expected benefits that led the FDA to grant accelerated approvals for these indications.
These accelerated approvals, which are often based on surrogate endpoints, such as overall response rates, are granted on the condition that further findings show a clinical benefit – such as in progression-free survival or overall survival – in larger trials.
The FDA tasked its Oncologic Drugs Advisory Committee (ODAC) with conducting the review of the six accelerated approvals for cancer indications at a 3-day meeting (April 27-29).
These reviews were only for specific cancer indications and will not lead to the removal of drugs from the market. These drugs have already been approved for several cancer indications. For example, one of the drugs that was reviewed, pembrolizumab (Keytruda), is approved in the United States for 28 indications.
The FDA is facing growing pains in its efforts to manage the rapidly changing landscape for these immune checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials, owing in part to the agency’s willingness to accept surrogate markers for accelerated approvals. Although some companies have struggled with these, others have built strong cases for the use of their checkpoint inhibitors for these indications.
The ODAC panelists, for example, noted the emergence of nivolumab (Opdivo) as an option for patients with gastric cancer as a reason for seeking to withdraw an indication for pembrolizumab (Keytruda) for this disease.
Just weeks before the meeting, on April 16, the FDA approved nivolumab plus chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. This was a full approval based on data showing an overall survival benefit from a phase 3 trial.
Votes by indication
On April 29, the last day of the meeting, the ODAC panel voted 6-2 against maintaining pembrolizumab’s indication as monotherapy for an advanced form of gastric cancer. This was an accelerated approval (granted in 2017) that was based on overall response rates from an open-label trial.
That last day of the meeting also saw another negative vote. On April 29, the ODAC panel voted 5-4 against maintaining an indication for nivolumab in patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib (Nexavar).
This accelerated approval for nivolumab was granted in 2017. The FDA said it had requested ODAC’s feedback on this indication because of the recent full approval of another checkpoint inhibitor for HCC, atezolizumab (Tecentriq), in combination with bevacizumab (Avastin) for patients with unresectable or metastatic diseases who have not received prior systemic therapy. This full approval (in May 2020) was based on an overall survival benefit.
There was one last vote on the third day of the meeting, and it was positive. The ODAC panel voted 8-0 in favor of maintaining the indication for the use of pembrolizumab as monotherapy for patients with HCC who have previously been treated with sorafenib.
The FDA altered the composition of the ODAC panel during the week, adding members in some cases who had expertise in particular cancers. That led to different totals for the week’s ODAC votes, as shown in the tallies summarized below.
On the first day of the meeting (April 27), the ODAC panel voted 7-2 in favor of maintaining a breast cancer indication for atezolizumab (Tecentriq). This covered use of the immunotherapy in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1.
The second day of the meeting (April 28) also saw two positive votes. The ODAC panel voted 10-1 for maintaining the indication for atezolizumab for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial. The panel also voted 5-3 for maintaining the indication for pembrolizumab in patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1.
The FDA is not bound to follow the voting and recommendations of its advisory panels, but it usually does so.
Managing shifts in treatment
In both of the cases in which ODAC voted against maintaining indications, Richard Pazdur, MD, the FDA’s top regulator for cancer medicines, jumped into the debate. Dr. Pazdur countered arguments put forward by representatives of the manufacturers as they sought to maintain indications for their drugs.
Merck officials and representatives argued for pembrolizumab, saying that maintaining the gastric cancer indication might help patients whose disease has progressed despite earlier treatment.
Dr. Pazdur emphasized that the agency would help Merck and physicians to have access to pembrolizumab for these patients even if this one indication were to be withdrawn. But Dr. Pazdur and ODAC members also noted the recent shift in the landscape for gastric cancer, with the recent approval of a new indication for nivolumab.
“I want to emphasize to the patient community out there [that] we firmly believe in the role of checkpoint inhibitors in this disease,” Dr. Pazdur said during the discussion of the indication for pembrolizumab for gastric cancer. “We have to be cognizant of what is the appropriate setting for that, and it currently is in the first line.”
Dr. Pazdur noted that two studies had failed to confirm the expected benefit from pembrolizumab for patients with more advanced disease. Still, if “small numbers” of patients with advanced disease wanted access to Merck’s drug, the FDA and the company could accommodate them. The FDA could delay the removal of the gastric indication to allow patients to continue receiving it. The FDA also could work with physicians on other routes to provide the medicine, such as through single-patient investigational new drug applications or an expanded access program.
“Or Merck can alternatively give the drug gratis to patients,” Dr. Pazdur said.
#ProjectFacilitate for expanded access
One of Merck’s speakers at the ODAC meeting, Peter Enzinger, MD, of the Dana-Farber Cancer Institute, Boston, objected to Dr. Pazdur’s plan.
A loss of the gastric indication for pembrolizumab would result in patients with advanced cancer missing out on a chance to try this therapy. Some patients will not have had a chance to try a checkpoint inhibitor earlier in their treatment, and a loss of the indication would cost them that opportunity, he said.
“An expanded-access program sounds very nice, but the reality is that our patients are incredibly sick and that weeks matter,” Dr. Enzinger said, citing administrative hurdles as a barrier to treatment.
“Our patients just don’t have the time for that, and therefore I don’t think an expanded access program is the way to go,” Dr. Enzinger said.
Dr. Pazdur responded to these objections by highlighting an initiative called Project Facilitate at the FDA’s Oncology Center for Excellence. During the meeting, Dr. Pazdur’s division used its @FDAOncology Twitter handle to draw attention to this project.
ODAC panelist Diane Reidy-Lagunes, MD, of Memorial Sloan Kettering Cancer Center, New York, said she had struggled with this vote. She was one of the two panelists to vote in favor of keeping the indication.
“This is also incredibly hard for me. I actually changed it at the last minute,” she said of her vote.
But Dr. Reidy-Lagunes said she was concerned that some patients with advanced disease might not be able to get a checkpoint inhibitor.
“With disparities in healthcare and differences in the way that patients are treated throughout our country, I was nervous that they may not be able to get treated,” she said, noting that she shared her fellow panelists’ doubts about use of pembrolizumab as third-line treatment, owing to negative results in trials.
ODAC member David Mitchell, who served as a consumer representative, also said he found the vote on the gastric indication for pembrolizumab to be a difficult decision.
“As a patient with incurable cancer who’s now being given all three major classes of drugs to treat my disease in combination, these issues really cut close to home,” Mr. Mitchell said.
He said the expectation that the FDA’s expanded access program could help patients with advanced disease try pembrolizumab helped him decide to vote with the 6-2 majority against maintaining this gastric cancer approval.
His vote was based on “the changing treatment landscape.” There is general agreement that the patients in question should receive checkpoint inhibitors as first-line treatment, not third-line treatment, Mr. Mitchell said. The FDA should delay a withdrawal of the approval for pembrolizumab in this case and should allow a transition for those who missed out on treatment with a checkpoint inhibitor earlier in the disease course, he suggested.
“To protect the safety and well-being of patients, we have to base decisions on data,” Mr. Mitchell said. “The data don’t support maintaining the indication” for pembrolizumab.
Close split on nivolumab
In contrast to the 6-2 vote against maintaining the pembrolizumab indication, the ODAC panel split more closely, 5-4, on the question of maintaining an indication for the use as monotherapy of nivolumab in HCC.
ODAC panelist Philip C. Hoffman, MD, of the University of Chicago was among those who supported keeping the indication.
“There’s still an unmet need for second-line immunotherapy because there will always be some patients who are poor candidates for bevacizumab or who are not tolerating or responding to sorafenib,” he said.
ODAC panelist Mark A. Lewis, MD, of Intermountain Healthcare, Salt Lake City, said he voted “no” in part because he doubted that Bristol-Myers Squibb would be able to soon produce data for nivolumab that was needed to support this indication.
A version of this article first appeared on Medscape.com.
To stay: Two more cancer indications with ‘dangling approvals’
Two more cancer indications that had been granted accelerated approval by the Food and Drug Administration are going to stay in place, at least for now. This was the verdict after the second day of a historic 3-day meeting (April 27-29) and follows a similar verdict from day 1.
Federal advisers so far have supported the idea of maintaining conditional approvals of some cancer indications for a number of immune checkpoint inhibitors, despite poor results in studies that were meant to confirm the benefit of these medicines for certain patients.
On the second day (April 28) of the FDA meeting, the Oncologic Drugs Advisory Committee (ODAC) supported the views of pharmaceutical companies in two more cases of what top agency staff call “dangling accelerated approvals.”
ODAC voted 10-1 in favor of maintaining the indication for atezolizumab (Tecentriq) for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial.
ODAC also voted 5-3 that day in favor of maintaining accelerated approval for pembrolizumab (Keytruda) for first-line cisplatin- and carboplatin-ineligible patients with advanced/metastatic urothelial carcinoma.
The FDA often follows the advice of its panels, but it is not bound to do so. If the FDA were to decide to strip the indications in question from these PD-1 medicines, such decisions would not remove these drugs from the market. The three drugs have already been approved for a number of other cancer indications.
Off-label prescribing is not uncommon in oncology, but a loss of an approved indication would affect reimbursement for these medicines, Scot Ebbinghaus, MD, vice president of oncology clinical research at Merck (the manufacturer of pembrolizumab), told ODAC members during a discussion of the possible consequences of removing the indications in question.
“Access to those treatments may end up being substantially limited, and really the best way to ensure that there’s access is to maintain FDA approval,” Dr. Ebbinghaus said.
Another participant at the meeting asked the panel and the FDA to consider the burden on patients in paying for medicines that have not yet proved to be beneficial.
Diana Zuckerman, PhD, of the nonprofit National Center for Health Research, noted that the ODAC panel included physicians who see cancer patients.
“You’re used to trying different types of treatments in hopes that something will work,” she said. “Shouldn’t cancer patients be eligible for free treatment in clinical trials instead of paying for treatment that isn’t proven to work?”
Rapid development of PD-1 drugs
Top officials at the FDA framed the challenges with accelerated approvals for immunotherapy drugs in an April 21 article in The New England Journal of Medicine. Over the course of about 6 years, the FDA approved six of these PD-1 or PD-L1 drugs for more than 75 indications in oncology, wrote Richard Pazdur, MD, and Julia A. Beaver, MD, of the FDA.
“Development of drugs in this class occurred more rapidly than that in any other therapeutic area in history,” they wrote.
In 10 cases, the required follow-up trials did not confirm the expected benefit, and yet marketing authorization for these drugs continued, leading Dr. Pazdur and Dr. Beaver to dub these “dangling” accelerated approvals. Four of these indications were voluntarily withdrawn. For the other six indications, the FDA sought feedback from ODAC during the 3-day meeting. Over the first 2 days of the meeting, ODAC recommended that three of these cancer indications remain. Three more will be considered on the last day of the meeting.
A version of this article first appeared on Medscape.com.
Two more cancer indications that had been granted accelerated approval by the Food and Drug Administration are going to stay in place, at least for now. This was the verdict after the second day of a historic 3-day meeting (April 27-29) and follows a similar verdict from day 1.
Federal advisers so far have supported the idea of maintaining conditional approvals of some cancer indications for a number of immune checkpoint inhibitors, despite poor results in studies that were meant to confirm the benefit of these medicines for certain patients.
On the second day (April 28) of the FDA meeting, the Oncologic Drugs Advisory Committee (ODAC) supported the views of pharmaceutical companies in two more cases of what top agency staff call “dangling accelerated approvals.”
ODAC voted 10-1 in favor of maintaining the indication for atezolizumab (Tecentriq) for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial.
ODAC also voted 5-3 that day in favor of maintaining accelerated approval for pembrolizumab (Keytruda) for first-line cisplatin- and carboplatin-ineligible patients with advanced/metastatic urothelial carcinoma.
The FDA often follows the advice of its panels, but it is not bound to do so. If the FDA were to decide to strip the indications in question from these PD-1 medicines, such decisions would not remove these drugs from the market. The three drugs have already been approved for a number of other cancer indications.
Off-label prescribing is not uncommon in oncology, but a loss of an approved indication would affect reimbursement for these medicines, Scot Ebbinghaus, MD, vice president of oncology clinical research at Merck (the manufacturer of pembrolizumab), told ODAC members during a discussion of the possible consequences of removing the indications in question.
“Access to those treatments may end up being substantially limited, and really the best way to ensure that there’s access is to maintain FDA approval,” Dr. Ebbinghaus said.
Another participant at the meeting asked the panel and the FDA to consider the burden on patients in paying for medicines that have not yet proved to be beneficial.
Diana Zuckerman, PhD, of the nonprofit National Center for Health Research, noted that the ODAC panel included physicians who see cancer patients.
“You’re used to trying different types of treatments in hopes that something will work,” she said. “Shouldn’t cancer patients be eligible for free treatment in clinical trials instead of paying for treatment that isn’t proven to work?”
Rapid development of PD-1 drugs
Top officials at the FDA framed the challenges with accelerated approvals for immunotherapy drugs in an April 21 article in The New England Journal of Medicine. Over the course of about 6 years, the FDA approved six of these PD-1 or PD-L1 drugs for more than 75 indications in oncology, wrote Richard Pazdur, MD, and Julia A. Beaver, MD, of the FDA.
“Development of drugs in this class occurred more rapidly than that in any other therapeutic area in history,” they wrote.
In 10 cases, the required follow-up trials did not confirm the expected benefit, and yet marketing authorization for these drugs continued, leading Dr. Pazdur and Dr. Beaver to dub these “dangling” accelerated approvals. Four of these indications were voluntarily withdrawn. For the other six indications, the FDA sought feedback from ODAC during the 3-day meeting. Over the first 2 days of the meeting, ODAC recommended that three of these cancer indications remain. Three more will be considered on the last day of the meeting.
A version of this article first appeared on Medscape.com.
Two more cancer indications that had been granted accelerated approval by the Food and Drug Administration are going to stay in place, at least for now. This was the verdict after the second day of a historic 3-day meeting (April 27-29) and follows a similar verdict from day 1.
Federal advisers so far have supported the idea of maintaining conditional approvals of some cancer indications for a number of immune checkpoint inhibitors, despite poor results in studies that were meant to confirm the benefit of these medicines for certain patients.
On the second day (April 28) of the FDA meeting, the Oncologic Drugs Advisory Committee (ODAC) supported the views of pharmaceutical companies in two more cases of what top agency staff call “dangling accelerated approvals.”
ODAC voted 10-1 in favor of maintaining the indication for atezolizumab (Tecentriq) for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial.
ODAC also voted 5-3 that day in favor of maintaining accelerated approval for pembrolizumab (Keytruda) for first-line cisplatin- and carboplatin-ineligible patients with advanced/metastatic urothelial carcinoma.
The FDA often follows the advice of its panels, but it is not bound to do so. If the FDA were to decide to strip the indications in question from these PD-1 medicines, such decisions would not remove these drugs from the market. The three drugs have already been approved for a number of other cancer indications.
Off-label prescribing is not uncommon in oncology, but a loss of an approved indication would affect reimbursement for these medicines, Scot Ebbinghaus, MD, vice president of oncology clinical research at Merck (the manufacturer of pembrolizumab), told ODAC members during a discussion of the possible consequences of removing the indications in question.
“Access to those treatments may end up being substantially limited, and really the best way to ensure that there’s access is to maintain FDA approval,” Dr. Ebbinghaus said.
Another participant at the meeting asked the panel and the FDA to consider the burden on patients in paying for medicines that have not yet proved to be beneficial.
Diana Zuckerman, PhD, of the nonprofit National Center for Health Research, noted that the ODAC panel included physicians who see cancer patients.
“You’re used to trying different types of treatments in hopes that something will work,” she said. “Shouldn’t cancer patients be eligible for free treatment in clinical trials instead of paying for treatment that isn’t proven to work?”
Rapid development of PD-1 drugs
Top officials at the FDA framed the challenges with accelerated approvals for immunotherapy drugs in an April 21 article in The New England Journal of Medicine. Over the course of about 6 years, the FDA approved six of these PD-1 or PD-L1 drugs for more than 75 indications in oncology, wrote Richard Pazdur, MD, and Julia A. Beaver, MD, of the FDA.
“Development of drugs in this class occurred more rapidly than that in any other therapeutic area in history,” they wrote.
In 10 cases, the required follow-up trials did not confirm the expected benefit, and yet marketing authorization for these drugs continued, leading Dr. Pazdur and Dr. Beaver to dub these “dangling” accelerated approvals. Four of these indications were voluntarily withdrawn. For the other six indications, the FDA sought feedback from ODAC during the 3-day meeting. Over the first 2 days of the meeting, ODAC recommended that three of these cancer indications remain. Three more will be considered on the last day of the meeting.
A version of this article first appeared on Medscape.com.
FDA panel backs atezolizumab for mTNBC – at least for now
On the first day of a historic 3-day meeting about drugs that were granted an accelerated approval by the Food and Drug Administration for cancer indications, the first approval to come under discussion is staying in place, at least for now.
Members of the FDA’s Oncologic Drugs Advisory Committee voted 7-2 in favor of keeping in place the indication for atezolizumab (Tecentriq) for use in a certain form of breast cancer. At the same time, the committee urged the manufacturer, Genentech, to do the research needed to prove the medicine works for these patients.
The specific indication is for atezolizumab as part of a combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) whose tumors are PD-L1 positive.
The FDA granted accelerated approval in 2019 for this use of atezolizumab, expecting Genentech to produce more extensive evidence of this benefit. But so far, Genentech has not produced the data proving to the FDA that atezolizumab provides the expected benefit.
The drug was already available for use in bladder cancer, having been granted a full approval for this indication in 2016.
Other accelerated approvals withdrawn
This week’s 3-day ODAC meeting is part of the FDA’s broader reconsideration of what it has described as “dangling accelerated approvals.”
Earlier discussions between the FDA and drugmakers have already triggered four voluntary withdrawals of cancer indications with these accelerated approvals, noted Julia A. Beaver, MD, and Richard Pazdur, MD, two of the FDA’s top regulators of oncology medicine, in an April 21 perspective article in the New England Journal of Medicine.
“The small percentage of drugs whose clinical benefit is ultimately not confirmed should be viewed not as a failure of accelerated approval but rather as an expected trade-off in expediting drug development that benefits patients with severe or life-threatening diseases,” Dr. Beaver and Dr. Pazdur wrote.
But making these calls can be tough. On the first day of the meeting, even ODAC panelists who backed Genentech’s bid to maintain an mTNBC indication for atezolizumab expressed discomfort with this choice.
The FDA granted the accelerated approval for use of this drug in March 2019 based on improved progression-free survival from the IMpassion130 trial. But the drug fell short in subsequent efforts to confirm the results seen in that study. The confirmatory IMpassion131 trial failed to meet the primary endpoint, the FDA staff noted in briefing materials for the ODAC meeting.
ODAC panelist Stan Lipkowitz, MD, PhD, of the National Cancer Institute, said he expected this vote had been a tough one for all members serving on ODAC that day.
“In some ways, the purist in me said I should have voted no. But when I looked at the data, there are a couple of things that struck me,” said Dr. Lipkowitz, who is the chief of the Women’s Malignancies Branch at NCI’s Center for Cancer Research. “First of all, the landscape hasn’t changed. There’s really no therapy in the first line for triple-negative metastatic that is shown to improve survival.”
Dr. Lipkowitz emphasized that Genentech needs to continue to try to prove atezolizumab works in this setting.
“There needs to be confirmatory study,” Dr. Lipkowitz concluded.
ODAC panelist Matthew Ellis, MD, PhD, of Baylor College of Medicine, Houston, said he also understood the difficult outlook for women fighting this cancer, but he voted against maintaining the approval.
“It’s not that I don’t feel the tragedy of these women,” said Dr. Ellis, citing his own decades of clinical experience.
“I just think that the data are the data,” Dr. Ellis said, adding that, in his view, “the only correct interpretation” of the evidence supported a vote against allowing the indication to stay.
The FDA considers the recommendations of its advisory committees but is not bound by them.
In a statement issued after the vote, Genentech said it intends to work with the FDA to determine the next steps for this indication of atezolizumab because “the clinically meaningful benefit demonstrated in the IMpassion130 study remains.”
The ODAC meeting continues for 2 more days, and will consider five more cancer indications that have been granted an accelerated approval.
A version of this article first appeared on Medscape.com.
On the first day of a historic 3-day meeting about drugs that were granted an accelerated approval by the Food and Drug Administration for cancer indications, the first approval to come under discussion is staying in place, at least for now.
Members of the FDA’s Oncologic Drugs Advisory Committee voted 7-2 in favor of keeping in place the indication for atezolizumab (Tecentriq) for use in a certain form of breast cancer. At the same time, the committee urged the manufacturer, Genentech, to do the research needed to prove the medicine works for these patients.
The specific indication is for atezolizumab as part of a combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) whose tumors are PD-L1 positive.
The FDA granted accelerated approval in 2019 for this use of atezolizumab, expecting Genentech to produce more extensive evidence of this benefit. But so far, Genentech has not produced the data proving to the FDA that atezolizumab provides the expected benefit.
The drug was already available for use in bladder cancer, having been granted a full approval for this indication in 2016.
Other accelerated approvals withdrawn
This week’s 3-day ODAC meeting is part of the FDA’s broader reconsideration of what it has described as “dangling accelerated approvals.”
Earlier discussions between the FDA and drugmakers have already triggered four voluntary withdrawals of cancer indications with these accelerated approvals, noted Julia A. Beaver, MD, and Richard Pazdur, MD, two of the FDA’s top regulators of oncology medicine, in an April 21 perspective article in the New England Journal of Medicine.
“The small percentage of drugs whose clinical benefit is ultimately not confirmed should be viewed not as a failure of accelerated approval but rather as an expected trade-off in expediting drug development that benefits patients with severe or life-threatening diseases,” Dr. Beaver and Dr. Pazdur wrote.
But making these calls can be tough. On the first day of the meeting, even ODAC panelists who backed Genentech’s bid to maintain an mTNBC indication for atezolizumab expressed discomfort with this choice.
The FDA granted the accelerated approval for use of this drug in March 2019 based on improved progression-free survival from the IMpassion130 trial. But the drug fell short in subsequent efforts to confirm the results seen in that study. The confirmatory IMpassion131 trial failed to meet the primary endpoint, the FDA staff noted in briefing materials for the ODAC meeting.
ODAC panelist Stan Lipkowitz, MD, PhD, of the National Cancer Institute, said he expected this vote had been a tough one for all members serving on ODAC that day.
“In some ways, the purist in me said I should have voted no. But when I looked at the data, there are a couple of things that struck me,” said Dr. Lipkowitz, who is the chief of the Women’s Malignancies Branch at NCI’s Center for Cancer Research. “First of all, the landscape hasn’t changed. There’s really no therapy in the first line for triple-negative metastatic that is shown to improve survival.”
Dr. Lipkowitz emphasized that Genentech needs to continue to try to prove atezolizumab works in this setting.
“There needs to be confirmatory study,” Dr. Lipkowitz concluded.
ODAC panelist Matthew Ellis, MD, PhD, of Baylor College of Medicine, Houston, said he also understood the difficult outlook for women fighting this cancer, but he voted against maintaining the approval.
“It’s not that I don’t feel the tragedy of these women,” said Dr. Ellis, citing his own decades of clinical experience.
“I just think that the data are the data,” Dr. Ellis said, adding that, in his view, “the only correct interpretation” of the evidence supported a vote against allowing the indication to stay.
The FDA considers the recommendations of its advisory committees but is not bound by them.
In a statement issued after the vote, Genentech said it intends to work with the FDA to determine the next steps for this indication of atezolizumab because “the clinically meaningful benefit demonstrated in the IMpassion130 study remains.”
The ODAC meeting continues for 2 more days, and will consider five more cancer indications that have been granted an accelerated approval.
A version of this article first appeared on Medscape.com.
On the first day of a historic 3-day meeting about drugs that were granted an accelerated approval by the Food and Drug Administration for cancer indications, the first approval to come under discussion is staying in place, at least for now.
Members of the FDA’s Oncologic Drugs Advisory Committee voted 7-2 in favor of keeping in place the indication for atezolizumab (Tecentriq) for use in a certain form of breast cancer. At the same time, the committee urged the manufacturer, Genentech, to do the research needed to prove the medicine works for these patients.
The specific indication is for atezolizumab as part of a combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) whose tumors are PD-L1 positive.
The FDA granted accelerated approval in 2019 for this use of atezolizumab, expecting Genentech to produce more extensive evidence of this benefit. But so far, Genentech has not produced the data proving to the FDA that atezolizumab provides the expected benefit.
The drug was already available for use in bladder cancer, having been granted a full approval for this indication in 2016.
Other accelerated approvals withdrawn
This week’s 3-day ODAC meeting is part of the FDA’s broader reconsideration of what it has described as “dangling accelerated approvals.”
Earlier discussions between the FDA and drugmakers have already triggered four voluntary withdrawals of cancer indications with these accelerated approvals, noted Julia A. Beaver, MD, and Richard Pazdur, MD, two of the FDA’s top regulators of oncology medicine, in an April 21 perspective article in the New England Journal of Medicine.
“The small percentage of drugs whose clinical benefit is ultimately not confirmed should be viewed not as a failure of accelerated approval but rather as an expected trade-off in expediting drug development that benefits patients with severe or life-threatening diseases,” Dr. Beaver and Dr. Pazdur wrote.
But making these calls can be tough. On the first day of the meeting, even ODAC panelists who backed Genentech’s bid to maintain an mTNBC indication for atezolizumab expressed discomfort with this choice.
The FDA granted the accelerated approval for use of this drug in March 2019 based on improved progression-free survival from the IMpassion130 trial. But the drug fell short in subsequent efforts to confirm the results seen in that study. The confirmatory IMpassion131 trial failed to meet the primary endpoint, the FDA staff noted in briefing materials for the ODAC meeting.
ODAC panelist Stan Lipkowitz, MD, PhD, of the National Cancer Institute, said he expected this vote had been a tough one for all members serving on ODAC that day.
“In some ways, the purist in me said I should have voted no. But when I looked at the data, there are a couple of things that struck me,” said Dr. Lipkowitz, who is the chief of the Women’s Malignancies Branch at NCI’s Center for Cancer Research. “First of all, the landscape hasn’t changed. There’s really no therapy in the first line for triple-negative metastatic that is shown to improve survival.”
Dr. Lipkowitz emphasized that Genentech needs to continue to try to prove atezolizumab works in this setting.
“There needs to be confirmatory study,” Dr. Lipkowitz concluded.
ODAC panelist Matthew Ellis, MD, PhD, of Baylor College of Medicine, Houston, said he also understood the difficult outlook for women fighting this cancer, but he voted against maintaining the approval.
“It’s not that I don’t feel the tragedy of these women,” said Dr. Ellis, citing his own decades of clinical experience.
“I just think that the data are the data,” Dr. Ellis said, adding that, in his view, “the only correct interpretation” of the evidence supported a vote against allowing the indication to stay.
The FDA considers the recommendations of its advisory committees but is not bound by them.
In a statement issued after the vote, Genentech said it intends to work with the FDA to determine the next steps for this indication of atezolizumab because “the clinically meaningful benefit demonstrated in the IMpassion130 study remains.”
The ODAC meeting continues for 2 more days, and will consider five more cancer indications that have been granted an accelerated approval.
A version of this article first appeared on Medscape.com.
FDA scrutinizes cancer therapies granted accelerated approval
U.S. regulators are stepping up scrutiny of therapies that were granted an accelerated approval to treat cancers on the basis of surrogate endpoints but have failed to show clinical or survival benefits upon more extensive testing.
At issue are a number of cancer indications for immunotherapies. Four have already been withdrawn (voluntarily by the manufacturer), and six more will be reviewed at an upcoming meeting.
In recent years, the US Food and Drug Administration has granted accelerated approvals to oncology medicines on the basis of evidence that suggests a benefit for patients. Examples of such evidence relate to response rates and estimates of tumor shrinkage. But these approvals are granted on the condition that the manufacturer conducts larger clinical trials that show clinical benefit, including benefit in overall survival.
Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, has argued that the point of these conditional approvals is to find acceptable surrogate markers to allow people with “desperate illnesses” to have access to potentially helpful drugs while work continues to determine the drug’s actual benefit to patients.
Oncologists are now questioning whether the FDA has become too lenient in its approach, Daniel A. Goldstein, MD, a senior physician in medical oncology and internal medicine at the Rabin Medical Center, Petah Tikva, Israel, told this news organization.
“The main two things you want from a cancer drug is to live longer and live a higher quality of life,” said Goldstein. “But these endpoints that they’ve been using over the past few years are not really giving us confidence that these drugs are actually going to help to live longer or better.”
Dr. Pazdur said the FDA will consider withdrawing its accelerated approvals when results of further studies do not confirm expected benefit for patients.
“This is like the pendulum has swung as far as it was going to swing and now is on the backswing,” said Dr. Goldstein, also of the department of health policy and management at the University of North Carolina at Chapel Hill. “You could call this a watershed moment.”
Although there’s near universal interest in allowing people with advanced cancer access to promising medicines, there’s also rising concern about exposing patients needlessly to costly drugs with potentially tough side effects. That may prompt a shift in the standards U.S. regulators apply to cancer medicines, Dr. Goldstein said.
Indications withdrawn and under review
In a meeting scheduled for April 27-29, the FDA’s Oncologic Drugs Advisory Committee will review indications granted through the accelerated approval process for three immunotherapies: pembrolizumab (Keytruda), atezolizumab (Tecentriq), and nivolumab (Opdivo).
It is part of an industry-wide evaluation of accelerated approvals for cancer indications in which confirmatory trials did not confirm clinical benefit, the FDA noted.
The process has already led to voluntary withdrawals of four cancer indications by the manufacturers, including one indication each for pembrolizumab, atezolizumab, and nivolumab, and one for durvalumab (Imfinzi).
All of these immunotherapies are approved for numerous cancer indications, and they all remain on the market. It is only the U.S. approvals for particular cancer indications that have been withdrawn.
In the past, olaratumab (Lartruvo) was withdrawn from the market altogether. The FDA granted accelerated approval of the drug for soft tissue sarcoma, but clinical benefit was not confirmed in a phase 3 trial.
Issue highlighted by Dr. Prasad and Dr. Gyawali
In recent years, much of the attention on accelerated approvals was spurred by the work of a few researchers, particularly Vinay Prasad, MD, MPH, associate professor in the department of epidemiology and biostatistics, University of California, San Francisco, and Bishal Gyawali, MD, PhD, from Queen’s University Cancer Research Institute, Kingston, Ont. (Both are regular contributors to the oncology section of this news organization.)
Dr. Goldstein made this point in a tweet about the FDA’s announcement of the April ODAC meetings:
“Well done to @oncology_bg and @VPrasadMDMPH among others for highlighting in their papers that the FDA wasn’t properly evaluating accelerated approval drugs.
FDA have listened.
And I thought that the impact of academia was limited!”
Dr. Prasad has made the case for closer scrutiny of accelerated approvals in a number of journal articles and in his 2020 book, “Malignant: How Bad Policy and Bad Evidence Harm People with Cancer,” published by Johns Hopkins University Press.
The book includes highlights of a 2016 article published in Mayo Clinic Proceedings that focused on surrogate endpoints used for FDA approvals. In the article, Dr. Prasad and his coauthor report that they did not find formal analyses of the strength of the surrogate-survival correlation in 14 of 25 cases of accelerated approvals (56%) and in 11 of 30 traditional approvals (37%).
“Our results were concerning. They imply that many surrogates are based on little more than a gut feeling. You might rationalize that and argue a gut feeling is the same as ‘reasonably likely to predict,’ but no reasonable person could think a gut feeling means established,” Dr. Prasad writes in his book. “Our result suggests the FDA is using surrogate endpoints far beyond what may be fair or reasonable.”
Dr. Gyawali has argued that the process by which the FDA assesses cancer drugs for approvals has undergone a profound shift. He has most recently remarked on this in an October 2020 commentary on Medscape.
“Until the recent floodgate of approvals based on response rates from single-arm trials, the majority of cancer therapy decisions were supported by evidence generated from randomized controlled trials (RCTs),” Dr. Gyawali wrote. “The evidence base to support clinical decisions in managing therapeutic side effects has been comparatively sparse.”
Accelerated approval to improve access
The FDA has struggled for about 2 decades with questions of where to set the bar on evidence for promising cancer drugs.
The agency’s accelerated approval program for drugs began in 1992. During the first decade, the focus was largely on medicines related to HIV.
In the early 2000s, oncology drugs began to dominate the program.
Dr. Pazdur has presided over the FDA’s marked changes regarding the use of surrogate markers when weighing whether to allow sales of cancer medicines. Formerly a professor at the University of Texas MD Anderson Cancer Center, Houston, Dr. Pazdur joined the FDA as director of the Division of Oncology Drug Products in 1999.
Soon after his appointment, he had to field inquiries from pharmaceutical companies about how much evidence they needed to receive accelerated approvals.
Early on, he publicly expressed impatience about the drugmakers’ approach. “The purpose of accelerated approval was not accelerated drug company profits,” Dr. Padzur said at a 2004 ODAC meeting.
Rather, the point is to allow access to potentially helpful drugs while work continues to determine their actual benefit to patients, he explained.
“It wasn’t a license to do less, less, less, and less to a point now that we may be getting companies that are coming in” intent on determining the minimum evidence the FDA will take, Dr. Pazdur said. “It shouldn’t be what is the lowest. It is what is a sufficient amount to give patients and physicians a real understanding of what their drug will do.”
In a 2016 interview with The New York Times, Dr. Pazdur said that his views on cancer drug approvals have evolved with time. He described himself as being “on a jihad to streamline the review process and get things out the door faster.”
“I have evolved from regulator to regulator-advocate,” Dr. Pazdur told the newspaper.
His attitude reflected his personal experience in losing his wife to ovarian cancer in 2015, as well as shifts in science and law. In 2012, Congress passed a law that gave the FDA new resources to speed medicines for life-threatening diseases to market. In addition, advances in genetics appeared to be making some medications more effective and easier to test, Dr. Pazdur said in The New York Times interview.
Withdrawals seen as sign of success
Since the program’s inception, only 6% of accelerated approvals for oncology indications have been withdrawn, the FDA said.
It would be a sign that the program is working if the April meetings lead to further withdrawals of indications that have been granted accelerated approval, Julie R. Gralow, MD, chief medical officer of the American Society of Clinical Oncology, said in an interview with this news organization.
“It shouldn’t be seen as a failure,” Dr. Gralow said.
In her own practice at the Fred Hutchinson Cancer Research Center, Seattle, she has seen the value of emerging therapies for patients fighting advanced cancers. During her 25 years of clinical practice in an academic setting, she has gained access to drugs through single-patient investigative new drug applications.
However, this path is not an option for many patients who undergo treatment in facilities other than academic centers, she commented. She noted that the accelerated approval process is a way to expand access to emerging medicines, but she sees a need for caution in the use of drugs that have been given only this conditional approval. She emphasizes that such drugs may be suitable only for certain patients.
“I would say that, for metastatic patients, patients with incurable disease, we are willing to take some risk,” Dr. Gralow said. “We don’t have other options. They can’t wait the years that it would take to get a drug approved.”
One such patient is David Mitchell, who serves as the consumer representative on ODAC. He told this news organization that he is taking three drugs for multiple myeloma that received accelerated approvals: pomalidomide, bortezomib, and daratumumab.
“I want the FDA to have the option to approve drugs in an accelerated pathway, because as a patient taking three drugs granted accelerated approval, I’m benefiting – I’ve lived the benefit,” Mr. Mitchell said, “and I want other patients to have the opportunity to have that benefit.”
He believes that the FDA’s approach regarding accelerated approvals serves to get potentially beneficial medicines to patients who have few options and also fulfills the FDA’s mandate to protect the public from treatments that have little benefit but can cause harm.
Accelerated approval also offers needed flexibility to drugmakers as they develop more specifically targeted drugs for diseases that affect relatively few people, such as multiple myeloma, he said. “As the targeting of your therapies gets tighter and for smaller groups of patients, you have a harder time following the traditional model,” such as conducting large, double-blind, placebo-controlled trials that may indicate increased overall survival, he said.
“To me, this is the way the FDA intended it to work,” he added. “It’s going to offer the accelerated approval based on a surrogate endpoint for a safe drug, but it’s going to require the confirmatory trial, and if the confirmatory trial fails, it will pull the drug off the market.”
Some medicines that have received accelerated approvals may ultimately be found not to benefit patients, Mr. Mitchell acknowledged. But people in his situation, whose disease has progressed despite treatments, may want to take that risk, he added.
Four cancer indications recently withdrawn voluntarily by the manufacturer
- December 2020: Nivolumab for the treatment of patients with metastatic small cell lung cancer with progression after platinum-based chemotherapy and at least one other line of therapy (Bristol Myers Squibb).
- February 2021: Durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy (AstraZeneca).
- March 2021: Pembrolizumab for the treatment of patients with metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (Merck).
- March 2021: Atezolizumab for treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing atezolizumab chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (Genentech).
Six cancer indications under review at the April 2021 ODAC meeting
- Atezolizumab indicated in combination with protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test.
- Atezolizumab indicated for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (Combined Positive Score ≥1), as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
- Pembrolizumab indicated for the treatment of patients with who have been previously treated with .
- Nivolumab indicated as a single agent for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
A version of this article first appeared on Medscape.com.
U.S. regulators are stepping up scrutiny of therapies that were granted an accelerated approval to treat cancers on the basis of surrogate endpoints but have failed to show clinical or survival benefits upon more extensive testing.
At issue are a number of cancer indications for immunotherapies. Four have already been withdrawn (voluntarily by the manufacturer), and six more will be reviewed at an upcoming meeting.
In recent years, the US Food and Drug Administration has granted accelerated approvals to oncology medicines on the basis of evidence that suggests a benefit for patients. Examples of such evidence relate to response rates and estimates of tumor shrinkage. But these approvals are granted on the condition that the manufacturer conducts larger clinical trials that show clinical benefit, including benefit in overall survival.
Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, has argued that the point of these conditional approvals is to find acceptable surrogate markers to allow people with “desperate illnesses” to have access to potentially helpful drugs while work continues to determine the drug’s actual benefit to patients.
Oncologists are now questioning whether the FDA has become too lenient in its approach, Daniel A. Goldstein, MD, a senior physician in medical oncology and internal medicine at the Rabin Medical Center, Petah Tikva, Israel, told this news organization.
“The main two things you want from a cancer drug is to live longer and live a higher quality of life,” said Goldstein. “But these endpoints that they’ve been using over the past few years are not really giving us confidence that these drugs are actually going to help to live longer or better.”
Dr. Pazdur said the FDA will consider withdrawing its accelerated approvals when results of further studies do not confirm expected benefit for patients.
“This is like the pendulum has swung as far as it was going to swing and now is on the backswing,” said Dr. Goldstein, also of the department of health policy and management at the University of North Carolina at Chapel Hill. “You could call this a watershed moment.”
Although there’s near universal interest in allowing people with advanced cancer access to promising medicines, there’s also rising concern about exposing patients needlessly to costly drugs with potentially tough side effects. That may prompt a shift in the standards U.S. regulators apply to cancer medicines, Dr. Goldstein said.
Indications withdrawn and under review
In a meeting scheduled for April 27-29, the FDA’s Oncologic Drugs Advisory Committee will review indications granted through the accelerated approval process for three immunotherapies: pembrolizumab (Keytruda), atezolizumab (Tecentriq), and nivolumab (Opdivo).
It is part of an industry-wide evaluation of accelerated approvals for cancer indications in which confirmatory trials did not confirm clinical benefit, the FDA noted.
The process has already led to voluntary withdrawals of four cancer indications by the manufacturers, including one indication each for pembrolizumab, atezolizumab, and nivolumab, and one for durvalumab (Imfinzi).
All of these immunotherapies are approved for numerous cancer indications, and they all remain on the market. It is only the U.S. approvals for particular cancer indications that have been withdrawn.
In the past, olaratumab (Lartruvo) was withdrawn from the market altogether. The FDA granted accelerated approval of the drug for soft tissue sarcoma, but clinical benefit was not confirmed in a phase 3 trial.
Issue highlighted by Dr. Prasad and Dr. Gyawali
In recent years, much of the attention on accelerated approvals was spurred by the work of a few researchers, particularly Vinay Prasad, MD, MPH, associate professor in the department of epidemiology and biostatistics, University of California, San Francisco, and Bishal Gyawali, MD, PhD, from Queen’s University Cancer Research Institute, Kingston, Ont. (Both are regular contributors to the oncology section of this news organization.)
Dr. Goldstein made this point in a tweet about the FDA’s announcement of the April ODAC meetings:
“Well done to @oncology_bg and @VPrasadMDMPH among others for highlighting in their papers that the FDA wasn’t properly evaluating accelerated approval drugs.
FDA have listened.
And I thought that the impact of academia was limited!”
Dr. Prasad has made the case for closer scrutiny of accelerated approvals in a number of journal articles and in his 2020 book, “Malignant: How Bad Policy and Bad Evidence Harm People with Cancer,” published by Johns Hopkins University Press.
The book includes highlights of a 2016 article published in Mayo Clinic Proceedings that focused on surrogate endpoints used for FDA approvals. In the article, Dr. Prasad and his coauthor report that they did not find formal analyses of the strength of the surrogate-survival correlation in 14 of 25 cases of accelerated approvals (56%) and in 11 of 30 traditional approvals (37%).
“Our results were concerning. They imply that many surrogates are based on little more than a gut feeling. You might rationalize that and argue a gut feeling is the same as ‘reasonably likely to predict,’ but no reasonable person could think a gut feeling means established,” Dr. Prasad writes in his book. “Our result suggests the FDA is using surrogate endpoints far beyond what may be fair or reasonable.”
Dr. Gyawali has argued that the process by which the FDA assesses cancer drugs for approvals has undergone a profound shift. He has most recently remarked on this in an October 2020 commentary on Medscape.
“Until the recent floodgate of approvals based on response rates from single-arm trials, the majority of cancer therapy decisions were supported by evidence generated from randomized controlled trials (RCTs),” Dr. Gyawali wrote. “The evidence base to support clinical decisions in managing therapeutic side effects has been comparatively sparse.”
Accelerated approval to improve access
The FDA has struggled for about 2 decades with questions of where to set the bar on evidence for promising cancer drugs.
The agency’s accelerated approval program for drugs began in 1992. During the first decade, the focus was largely on medicines related to HIV.
In the early 2000s, oncology drugs began to dominate the program.
Dr. Pazdur has presided over the FDA’s marked changes regarding the use of surrogate markers when weighing whether to allow sales of cancer medicines. Formerly a professor at the University of Texas MD Anderson Cancer Center, Houston, Dr. Pazdur joined the FDA as director of the Division of Oncology Drug Products in 1999.
Soon after his appointment, he had to field inquiries from pharmaceutical companies about how much evidence they needed to receive accelerated approvals.
Early on, he publicly expressed impatience about the drugmakers’ approach. “The purpose of accelerated approval was not accelerated drug company profits,” Dr. Padzur said at a 2004 ODAC meeting.
Rather, the point is to allow access to potentially helpful drugs while work continues to determine their actual benefit to patients, he explained.
“It wasn’t a license to do less, less, less, and less to a point now that we may be getting companies that are coming in” intent on determining the minimum evidence the FDA will take, Dr. Pazdur said. “It shouldn’t be what is the lowest. It is what is a sufficient amount to give patients and physicians a real understanding of what their drug will do.”
In a 2016 interview with The New York Times, Dr. Pazdur said that his views on cancer drug approvals have evolved with time. He described himself as being “on a jihad to streamline the review process and get things out the door faster.”
“I have evolved from regulator to regulator-advocate,” Dr. Pazdur told the newspaper.
His attitude reflected his personal experience in losing his wife to ovarian cancer in 2015, as well as shifts in science and law. In 2012, Congress passed a law that gave the FDA new resources to speed medicines for life-threatening diseases to market. In addition, advances in genetics appeared to be making some medications more effective and easier to test, Dr. Pazdur said in The New York Times interview.
Withdrawals seen as sign of success
Since the program’s inception, only 6% of accelerated approvals for oncology indications have been withdrawn, the FDA said.
It would be a sign that the program is working if the April meetings lead to further withdrawals of indications that have been granted accelerated approval, Julie R. Gralow, MD, chief medical officer of the American Society of Clinical Oncology, said in an interview with this news organization.
“It shouldn’t be seen as a failure,” Dr. Gralow said.
In her own practice at the Fred Hutchinson Cancer Research Center, Seattle, she has seen the value of emerging therapies for patients fighting advanced cancers. During her 25 years of clinical practice in an academic setting, she has gained access to drugs through single-patient investigative new drug applications.
However, this path is not an option for many patients who undergo treatment in facilities other than academic centers, she commented. She noted that the accelerated approval process is a way to expand access to emerging medicines, but she sees a need for caution in the use of drugs that have been given only this conditional approval. She emphasizes that such drugs may be suitable only for certain patients.
“I would say that, for metastatic patients, patients with incurable disease, we are willing to take some risk,” Dr. Gralow said. “We don’t have other options. They can’t wait the years that it would take to get a drug approved.”
One such patient is David Mitchell, who serves as the consumer representative on ODAC. He told this news organization that he is taking three drugs for multiple myeloma that received accelerated approvals: pomalidomide, bortezomib, and daratumumab.
“I want the FDA to have the option to approve drugs in an accelerated pathway, because as a patient taking three drugs granted accelerated approval, I’m benefiting – I’ve lived the benefit,” Mr. Mitchell said, “and I want other patients to have the opportunity to have that benefit.”
He believes that the FDA’s approach regarding accelerated approvals serves to get potentially beneficial medicines to patients who have few options and also fulfills the FDA’s mandate to protect the public from treatments that have little benefit but can cause harm.
Accelerated approval also offers needed flexibility to drugmakers as they develop more specifically targeted drugs for diseases that affect relatively few people, such as multiple myeloma, he said. “As the targeting of your therapies gets tighter and for smaller groups of patients, you have a harder time following the traditional model,” such as conducting large, double-blind, placebo-controlled trials that may indicate increased overall survival, he said.
“To me, this is the way the FDA intended it to work,” he added. “It’s going to offer the accelerated approval based on a surrogate endpoint for a safe drug, but it’s going to require the confirmatory trial, and if the confirmatory trial fails, it will pull the drug off the market.”
Some medicines that have received accelerated approvals may ultimately be found not to benefit patients, Mr. Mitchell acknowledged. But people in his situation, whose disease has progressed despite treatments, may want to take that risk, he added.
Four cancer indications recently withdrawn voluntarily by the manufacturer
- December 2020: Nivolumab for the treatment of patients with metastatic small cell lung cancer with progression after platinum-based chemotherapy and at least one other line of therapy (Bristol Myers Squibb).
- February 2021: Durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy (AstraZeneca).
- March 2021: Pembrolizumab for the treatment of patients with metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (Merck).
- March 2021: Atezolizumab for treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing atezolizumab chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (Genentech).
Six cancer indications under review at the April 2021 ODAC meeting
- Atezolizumab indicated in combination with protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test.
- Atezolizumab indicated for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (Combined Positive Score ≥1), as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
- Pembrolizumab indicated for the treatment of patients with who have been previously treated with .
- Nivolumab indicated as a single agent for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
A version of this article first appeared on Medscape.com.
U.S. regulators are stepping up scrutiny of therapies that were granted an accelerated approval to treat cancers on the basis of surrogate endpoints but have failed to show clinical or survival benefits upon more extensive testing.
At issue are a number of cancer indications for immunotherapies. Four have already been withdrawn (voluntarily by the manufacturer), and six more will be reviewed at an upcoming meeting.
In recent years, the US Food and Drug Administration has granted accelerated approvals to oncology medicines on the basis of evidence that suggests a benefit for patients. Examples of such evidence relate to response rates and estimates of tumor shrinkage. But these approvals are granted on the condition that the manufacturer conducts larger clinical trials that show clinical benefit, including benefit in overall survival.
Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, has argued that the point of these conditional approvals is to find acceptable surrogate markers to allow people with “desperate illnesses” to have access to potentially helpful drugs while work continues to determine the drug’s actual benefit to patients.
Oncologists are now questioning whether the FDA has become too lenient in its approach, Daniel A. Goldstein, MD, a senior physician in medical oncology and internal medicine at the Rabin Medical Center, Petah Tikva, Israel, told this news organization.
“The main two things you want from a cancer drug is to live longer and live a higher quality of life,” said Goldstein. “But these endpoints that they’ve been using over the past few years are not really giving us confidence that these drugs are actually going to help to live longer or better.”
Dr. Pazdur said the FDA will consider withdrawing its accelerated approvals when results of further studies do not confirm expected benefit for patients.
“This is like the pendulum has swung as far as it was going to swing and now is on the backswing,” said Dr. Goldstein, also of the department of health policy and management at the University of North Carolina at Chapel Hill. “You could call this a watershed moment.”
Although there’s near universal interest in allowing people with advanced cancer access to promising medicines, there’s also rising concern about exposing patients needlessly to costly drugs with potentially tough side effects. That may prompt a shift in the standards U.S. regulators apply to cancer medicines, Dr. Goldstein said.
Indications withdrawn and under review
In a meeting scheduled for April 27-29, the FDA’s Oncologic Drugs Advisory Committee will review indications granted through the accelerated approval process for three immunotherapies: pembrolizumab (Keytruda), atezolizumab (Tecentriq), and nivolumab (Opdivo).
It is part of an industry-wide evaluation of accelerated approvals for cancer indications in which confirmatory trials did not confirm clinical benefit, the FDA noted.
The process has already led to voluntary withdrawals of four cancer indications by the manufacturers, including one indication each for pembrolizumab, atezolizumab, and nivolumab, and one for durvalumab (Imfinzi).
All of these immunotherapies are approved for numerous cancer indications, and they all remain on the market. It is only the U.S. approvals for particular cancer indications that have been withdrawn.
In the past, olaratumab (Lartruvo) was withdrawn from the market altogether. The FDA granted accelerated approval of the drug for soft tissue sarcoma, but clinical benefit was not confirmed in a phase 3 trial.
Issue highlighted by Dr. Prasad and Dr. Gyawali
In recent years, much of the attention on accelerated approvals was spurred by the work of a few researchers, particularly Vinay Prasad, MD, MPH, associate professor in the department of epidemiology and biostatistics, University of California, San Francisco, and Bishal Gyawali, MD, PhD, from Queen’s University Cancer Research Institute, Kingston, Ont. (Both are regular contributors to the oncology section of this news organization.)
Dr. Goldstein made this point in a tweet about the FDA’s announcement of the April ODAC meetings:
“Well done to @oncology_bg and @VPrasadMDMPH among others for highlighting in their papers that the FDA wasn’t properly evaluating accelerated approval drugs.
FDA have listened.
And I thought that the impact of academia was limited!”
Dr. Prasad has made the case for closer scrutiny of accelerated approvals in a number of journal articles and in his 2020 book, “Malignant: How Bad Policy and Bad Evidence Harm People with Cancer,” published by Johns Hopkins University Press.
The book includes highlights of a 2016 article published in Mayo Clinic Proceedings that focused on surrogate endpoints used for FDA approvals. In the article, Dr. Prasad and his coauthor report that they did not find formal analyses of the strength of the surrogate-survival correlation in 14 of 25 cases of accelerated approvals (56%) and in 11 of 30 traditional approvals (37%).
“Our results were concerning. They imply that many surrogates are based on little more than a gut feeling. You might rationalize that and argue a gut feeling is the same as ‘reasonably likely to predict,’ but no reasonable person could think a gut feeling means established,” Dr. Prasad writes in his book. “Our result suggests the FDA is using surrogate endpoints far beyond what may be fair or reasonable.”
Dr. Gyawali has argued that the process by which the FDA assesses cancer drugs for approvals has undergone a profound shift. He has most recently remarked on this in an October 2020 commentary on Medscape.
“Until the recent floodgate of approvals based on response rates from single-arm trials, the majority of cancer therapy decisions were supported by evidence generated from randomized controlled trials (RCTs),” Dr. Gyawali wrote. “The evidence base to support clinical decisions in managing therapeutic side effects has been comparatively sparse.”
Accelerated approval to improve access
The FDA has struggled for about 2 decades with questions of where to set the bar on evidence for promising cancer drugs.
The agency’s accelerated approval program for drugs began in 1992. During the first decade, the focus was largely on medicines related to HIV.
In the early 2000s, oncology drugs began to dominate the program.
Dr. Pazdur has presided over the FDA’s marked changes regarding the use of surrogate markers when weighing whether to allow sales of cancer medicines. Formerly a professor at the University of Texas MD Anderson Cancer Center, Houston, Dr. Pazdur joined the FDA as director of the Division of Oncology Drug Products in 1999.
Soon after his appointment, he had to field inquiries from pharmaceutical companies about how much evidence they needed to receive accelerated approvals.
Early on, he publicly expressed impatience about the drugmakers’ approach. “The purpose of accelerated approval was not accelerated drug company profits,” Dr. Padzur said at a 2004 ODAC meeting.
Rather, the point is to allow access to potentially helpful drugs while work continues to determine their actual benefit to patients, he explained.
“It wasn’t a license to do less, less, less, and less to a point now that we may be getting companies that are coming in” intent on determining the minimum evidence the FDA will take, Dr. Pazdur said. “It shouldn’t be what is the lowest. It is what is a sufficient amount to give patients and physicians a real understanding of what their drug will do.”
In a 2016 interview with The New York Times, Dr. Pazdur said that his views on cancer drug approvals have evolved with time. He described himself as being “on a jihad to streamline the review process and get things out the door faster.”
“I have evolved from regulator to regulator-advocate,” Dr. Pazdur told the newspaper.
His attitude reflected his personal experience in losing his wife to ovarian cancer in 2015, as well as shifts in science and law. In 2012, Congress passed a law that gave the FDA new resources to speed medicines for life-threatening diseases to market. In addition, advances in genetics appeared to be making some medications more effective and easier to test, Dr. Pazdur said in The New York Times interview.
Withdrawals seen as sign of success
Since the program’s inception, only 6% of accelerated approvals for oncology indications have been withdrawn, the FDA said.
It would be a sign that the program is working if the April meetings lead to further withdrawals of indications that have been granted accelerated approval, Julie R. Gralow, MD, chief medical officer of the American Society of Clinical Oncology, said in an interview with this news organization.
“It shouldn’t be seen as a failure,” Dr. Gralow said.
In her own practice at the Fred Hutchinson Cancer Research Center, Seattle, she has seen the value of emerging therapies for patients fighting advanced cancers. During her 25 years of clinical practice in an academic setting, she has gained access to drugs through single-patient investigative new drug applications.
However, this path is not an option for many patients who undergo treatment in facilities other than academic centers, she commented. She noted that the accelerated approval process is a way to expand access to emerging medicines, but she sees a need for caution in the use of drugs that have been given only this conditional approval. She emphasizes that such drugs may be suitable only for certain patients.
“I would say that, for metastatic patients, patients with incurable disease, we are willing to take some risk,” Dr. Gralow said. “We don’t have other options. They can’t wait the years that it would take to get a drug approved.”
One such patient is David Mitchell, who serves as the consumer representative on ODAC. He told this news organization that he is taking three drugs for multiple myeloma that received accelerated approvals: pomalidomide, bortezomib, and daratumumab.
“I want the FDA to have the option to approve drugs in an accelerated pathway, because as a patient taking three drugs granted accelerated approval, I’m benefiting – I’ve lived the benefit,” Mr. Mitchell said, “and I want other patients to have the opportunity to have that benefit.”
He believes that the FDA’s approach regarding accelerated approvals serves to get potentially beneficial medicines to patients who have few options and also fulfills the FDA’s mandate to protect the public from treatments that have little benefit but can cause harm.
Accelerated approval also offers needed flexibility to drugmakers as they develop more specifically targeted drugs for diseases that affect relatively few people, such as multiple myeloma, he said. “As the targeting of your therapies gets tighter and for smaller groups of patients, you have a harder time following the traditional model,” such as conducting large, double-blind, placebo-controlled trials that may indicate increased overall survival, he said.
“To me, this is the way the FDA intended it to work,” he added. “It’s going to offer the accelerated approval based on a surrogate endpoint for a safe drug, but it’s going to require the confirmatory trial, and if the confirmatory trial fails, it will pull the drug off the market.”
Some medicines that have received accelerated approvals may ultimately be found not to benefit patients, Mr. Mitchell acknowledged. But people in his situation, whose disease has progressed despite treatments, may want to take that risk, he added.
Four cancer indications recently withdrawn voluntarily by the manufacturer
- December 2020: Nivolumab for the treatment of patients with metastatic small cell lung cancer with progression after platinum-based chemotherapy and at least one other line of therapy (Bristol Myers Squibb).
- February 2021: Durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed during or following platinum-based chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy (AstraZeneca).
- March 2021: Pembrolizumab for the treatment of patients with metastatic small cell lung cancer with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (Merck).
- March 2021: Atezolizumab for treatment of patients with locally advanced or metastatic urothelial carcinoma who experience disease progression during or following platinum-containing atezolizumab chemotherapy or disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy (Genentech).
Six cancer indications under review at the April 2021 ODAC meeting
- Atezolizumab indicated in combination with protein-bound for the treatment of adults with unresectable locally advanced or metastatic triple-negative whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test.
- Atezolizumab indicated for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy.
- Pembrolizumab indicated for the treatment of patients with recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (Combined Positive Score ≥1), as determined by an FDA-approved test, with disease progression on or after two or more prior lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy.
- Pembrolizumab indicated for the treatment of patients with who have been previously treated with .
- Nivolumab indicated as a single agent for the treatment of patients with hepatocellular carcinoma who have been previously treated with sorafenib.
A version of this article first appeared on Medscape.com.