User login
Recommendations on the Use of Ultrasound Guidance for Adult Lumbar Puncture: A Position Statement of the Society of Hospital Medicine
Approximately 400,000 lumbar punctures (LPs) are performed in the United States annually for either diagnostic workup or therapeutic relief.1 Lumbar punctures are increasingly being performed in the United States, with an estimated 97,000 LPs performed on Medicare fee-for-service beneficiaries in 2011 alone, which is an increase of approximately 4,000 LPs in the same population from 1991.2 Approximately 273,612 LPs were performed on hospitalized patients in the United States in 2010,1 and the inpatient hospital setting is the most common site for LPs.2,3
Many LPs are referred to radiologists who have access to imaging guidance to aid with needle insertion.2 However, referrals to radiology delay performance of LPs, and delayed diagnosis of acute bacterial meningitis, the most common yet serious condition for which LPs are performed, is associated with increased morbidity and mortality.4-8 Furthermore, although initiating empiric antibiotic treatment for suspected acute bacterial meningitis is recommended in some cases, doing so routinely can cause false-negative cerebrospinal fluid (CSF) culture results, complicating decisions about de-escalation and duration of antibiotics that could have been safely avoided by promptly performing an LP.9
Delaying the performance of LP has been associated with increased mortality.10 Demonstration of proficiency in performance of lumbar puncture is considered a core competency for hospitalists,11 and with the increasing availability of point-of-care ultrasound, hospitalists can use ultrasound to guide performance of LPs at the bedside.12 However, 30% of patients requiring LP in emergency departments have difficult-to-palpate lumbar spine landmarks,13 and lumbar puncture performed based on palpation of landmarks alone has been reported to fail or be traumatic in 28% of patients.14 Use of ultrasound guidance for lumbar puncture has been shown in randomized controlled trials to improve procedural success rates, while reducing the time to successful LP, needle passes, patient pain scores, and risk of a traumatic LP.15-17
The purpose of this position statement is to review the literature and present consensus-based recommendations on the performance of ultrasound-guided LP in adult patients. This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP. Similar to previously published Society of Hospital Medicine (SHM) position statements,12,18,19 this document presents recommendations with supporting evidence for the clinical outcomes, techniques, and training for using ultrasound guidance for LP. A manuscript describing the technique of ultrasound guidance for LPs has been previously published by some of the authors of this position statement.20
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the six working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. Updated searches were conducted in November 2016, January 2018, and October 2018. The search strings are included in Appendix 3. All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide LP were selected. In addition, the following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. Moreover, studies focusing on the use of ultrasound guidance for spinal nerve root injections, regional anesthesia, and assessment of lumbar spine anatomy alone were excluded. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided LP were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into the draft recommendations.
The Research and Development (RAND) Appropriateness Method that required panel judgment and consensus was used.21 The 27 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (REDCap™) in February 2018 and April 2018 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale. The three zones of the 9-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” A strong recommendation required 80% of the votes within one integer of the median, following the RAND rules. Disagreement was defined as >30% of panelists voting outside of the zone of the median.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording of each recommendation (Table 2). The revised consensus-based recommendations underwent internal and external reviews by POCUS experts from different subspecialties. The final review of this position statement was performed by members of the SHM POCUS Task Force, SHM Education Committee, and SHM Executive Committee. The SHM Executive Committee endorsed this position statement in June 2018 before submission to the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 4,389 references were pooled from four different sources: a search by a certified medical librarian in December 2015 (3,212 citations) that was updated in November 2016 (380 citations), January 2018 (282 citations), and October 2018 (274 citations); working group members’ personal bibliographies and searches (31 citations); and a search focusing on ultrasound-guided LP training (210 citations). A total of 232 full-text articles were reviewed, and the final selection included 77 articles that were abstracted into a data table and incorporated into the draft recommendations. Details of the literature search strategy are presented in Appendix 3.
RECOMMENDATIONS
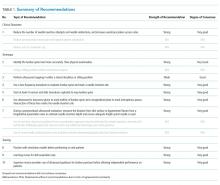
Four domains (clinical outcomes, technique, training, and knowledge gaps) with 16 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation were drafted citing supporting evidence. After two rounds of panel voting, five recommendations did not achieve agreement based on the RAND rules, one recommendation was combined with another recommendation during peer review, and 10 statements received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Nine statements were approved as strong recommendations, and one was approved as a conditional recommendation. Therefore, the final recommendation count was 10. The strength of the recommendation and degree of consensus for each recommendation are summarized in Table 1.
Terminology
LP is a procedure in which a spinal needle is introduced into the subarachnoid space for the purpose of collecting CSF for diagnostic evaluation and/or therapeutic relief.
Throughout this document, the phrases “ultrasound-guided” and “ultrasound guidance” refer to the use of ultrasound to mark a needle insertion site immediately before performing the procedure. This is also known as static ultrasound guidance. Real-time or dynamic ultrasound guidance refers to direct visualization of the needle tip as it traverses through the skin and soft tissues to reach the ligamentum flavum. Any reference to real-time ultrasound guidance is explicitly stated.
Clinical outcomes
1) When ultrasound equipment is available, along with providers who are appropriately trained to use it, we recommend that ultrasound guidance should be used for site selection of LPs to reduce the number of needle insertion attempts and needle redirections and increase the overall procedure success rates, especially in patients who are obese or have difficult-to-palpate landmarks.
Rationale. LPs have historically been performed by selecting a needle insertion site based on palpation of anatomical landmarks. However, an estimated 30% of patients requiring LP in emergency departments have lumbar spine landmarks that are difficult to palpate, most commonly due to obesity.13 Furthermore, lumbar puncture performed based on palpation of landmarks alone has been reported to fail in 28% of patients.14
Ultrasound can be used at the bedside to elucidate the lumbar spine anatomy to guide performance of LP or epidural catheterization. Since the early 2000s, randomized studies comparing the use of ultrasound guidance (ultrasound-guided) versus anatomical landmarks (landmark-guided) to map the lumbar spine for epidural catheterization have emerged. It is important to recognize that the exact same ultrasound technique is used for site marking of LP, epidural catheterization, and spinal anesthesia—the key difference is how deep the needle tip is inserted. Therefore, data from these three ultrasound-guided procedures are often pooled. Currently, at least 33 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP, epidural catheterization, or spinal anesthesia have been published.22-49 We present three meta-analyses below that pooled data primarily from randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP or spinal anesthesia.
In 2013, Shaikh et al. published the first meta-analysis with 14 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP (n = 5) or epidural catheterization (n = 9). The pooled data showed that use of ultrasound guidance decreased the proportion of failed procedures (risk ratio 0.21, 95% CI 0.10-0.43) with an absolute risk reduction of 6.3% (95% CI 4.1%-8.4%) and a number needed to treat of 16 (95% CI 12-25) to prevent one failed procedure. In addition, the use of ultrasound reduced the mean number of attempts by 0.44 (95% CI 0.24-0.64) and reduced the mean number of needle redirections by 1.00 (95% CI 0.75-1.24). The reduction in risk of a failed procedure was similar for LPs (risk ratio 0.19 [95% CI 0.07-0.56]) and epidural catheterizations (risk ratio 0.23 [95% CI 0.09-0.60]).16
A similar meta-analysis published by Perlas et al. in 2016 included a total of 31 studies, both randomized controlled and cohort studies, evaluating the use of ultrasound guidance for LP, spinal anesthesia, and epidural catheterization.50 The goal of this systematic review and meta-analysis was to establish clinical practice recommendations. The authors concluded (1) the data consistently suggest that ultrasound is more accurate than palpation for lumbar interspace identification, (2) ultrasound allows accurate measurement of the needle insertion depth to reach the epidural space with a mean difference of <3 mm compared with the actual needle insertion depth, and (3) ultrasound increases the efficacy of lumbar epidural or spinal anesthesia by decreasing the mean number of needle passes for success by 0.75 (95% CI 0.44-1.07) and reducing the risk of a failed procedure (risk ratio 0.51 [95% CI 0.32-0.80]), both in patients with normal surface anatomy and in those with technically difficult surface anatomy due to obesity, scoliosis, or previous spine surgery.
Compared to the two earlier meta-analyses that included studies of both LP and spinal anesthesia procedures, the meta-analysis conducted by Gottlieb et al. in 2018 pooled data from 12 randomized controlled studies of ultrasound guidance for LPs only. For the primary outcome, pooled data from both adult and pediatric studies demonstrated higher procedural success rates with ultrasound-guided vs landmark-guided LPs (90% vs 81%) with an odds ratio of 2.1 (95% CI 0.66-7.44) in favor of ultrasound; however, there were no statistically significant differences when the adult and pediatric subgroups were analyzed separately, probably due to underpowering. For the secondary outcomes, data from the adult subgroup showed that use of ultrasound guidance was associated with fewer traumatic LPs (OR 0.28, 95% CI 0.14-0.59), shorter time to procedural success (adjusted mean difference –3.03 minutes, 95% CI –3.54 to –2.52), fewer number of needle passes (adjusted mean difference –0.81 passes, 95% CI –1.57 to –0.05), and lower patient pain scores (adjusted mean difference –2.53, 95% CI –3.89 to –1.17).
At least 12 randomized controlled studies have been published comparing the use of ultrasound guidance vs landmarks for the performance of LP or spinal anesthesia in adult patients, which were not included in the abovementioned meta-analyses. These individual studies demonstrated similar benefits of using ultrasound guidance: reduced needle insertion attempts, reduced needle redirections, and increased overall procedural success rates.17,31,37,40,41,43-49
It is important to recognize that four randomized controlled studies did not demonstrate any benefits of ultrasound guidance on the number of attempts or procedural success rates,23,33,41,51 and three of these studies were included in the abovementioned meta-analyses.23,33,51 Limitations of these negative studies include potential selection bias, inadequate sample sizes, and varying levels of operator skills in procedures, ultrasound guidance, or both. One study included emergency medicine residents as operators with varying degrees of ultrasound skills, and more importantly, patient enrollment occurred by convenience sampling, which may have introduced selection bias. Furthermore, most of the patients were not obese (median BMI of 27 kg/m2), and it is unclear why 10 years lapsed from data collection until publication.33 Another study with three experienced anesthesiologists as operators performing spinal anesthesia enrolled only patients who were not obese (mean BMI of 29 kg/m2) and had easily palpable bony landmarks—two patient characteristics associated with the least benefit of using ultrasound guidance in other studies.23 Another negative study had one experienced anesthesiologist marking obstetric patients with ultrasound, but junior residents performing the actual procedure in the absence of the anesthesiologist who had marked the patient.41
In general, the greatest benefit of using ultrasound guidance for LP has been demonstrated in obese patients.24,32,34,35,52,53 Benefits have been shown in specific obese patient populations, including obstetric,31,54,55 orthopedic,24,56,57 and emergency department patients.30
By increasing the procedural success rates with the use of ultrasound at the bedside, fewer patients may be referred to interventional radiology for fluoroscopic-guided LP, decreasing the patient exposure to ionizing radiation. A randomized study (n = 112) that compared site marking with ultrasound guidance versus fluoroscopic guidance for epidural steroid injections found the two techniques to be equivalent with respect to mean procedure time, number of needle insertion attempts, or needle passes.58 Another randomized study found that the performance time of ultrasound guidance was two minutes shorter (P < .05) than fluoroscopic guidance.59
Techniques
2) We recommend that ultrasound should be used to more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients.
Rationale. Traditionally, an imaginary line connecting the iliac crests (intercristal line, Tuffier’s line, or Jacoby’s line) was considered to identify the L4 vertebra or the L4-L5 interspinous space in the midline; however, studies have revealed this traditional landmark to be much less accurate than previously thought. In general, palpating the iliac crests to mark the intercristal line identifies an interspinous space that is one space cephalad (ie, the L2-L3 interspinous space) but can range from L1-L2 to L4-L5.46,60-64 If an LP is inadvertently performed in the L1-L2 interspinous space, the risk of spinal cord injury is higher than that when performed in a more distal interspinous space.
A study by Margarido et al. with 45 patients with a mean BMI of 30 kg/m2 found that the intercristal line was located above the L4-L5 interspinous space in 100% of patients. More importantly, the intercristal line was above L2-L3 in 36% of patients and above L1-L2 in 4% of patients. It is important to note that patients with scoliosis or previous spine surgery were excluded from this study, and all examinations were performed by two experienced anesthesiologists with patients in a sitting position—all factors that would favor accurate palpation and marking of the iliac crests.60
In a study of nonobese patients (mean BMI 28 kg/m2) undergoing spinal anesthesia, Duniec et al. compared the lumbar level identified by palpation versus ultrasound and found discordance between the two techniques in 36% of patients; 18% were one space too cephalad, 16% were one space too caudal, and 2% were off by two interspinous spaces.61 Another study found discordance in 64% of patients (mean BMI 28 kg/m2) when comparing the interspinous level where spinal anesthesia had been performed by palpation versus a post-procedural ultrasound examination. This study revealed that the interspinous space was more cephalad in 50% of patients with 6% of punctures performed in the L1-L2 interspace.62 A similar study compared the accuracy of palpation vs ultrasound to identify the L3-L4 interspinous space in obese (mean BMI 34 kg/m2) versus nonobese (mean BMI 27 kg/m2) patients. This study found marking a space above L3-L4 in 51% of obese and 40% of nonobese patients and marking of the L1-L2 interspace in 7% of obese and 4% of nonobese patients.64
A study comparing palpation vs ultrasound found that 68% of obese patients with a BMI of >30 kg/m2 had difficult-to-palpate lumbar spine landmarks, but with the use of ultrasound, landmarks were identified in 76% of all patients, including obese and nonobese, with difficult-to-palpate landmarks.65
3) We suggest using ultrasound for selecting and marking a needle insertion site just before performing LPs in either a lateral decubitus or sitting position. The patient should remain in the same position after marking the needle insertion site.
Rationale. Ultrasound mapping of the lumbar spine can be performed in either a lateral decubitus or sitting position. Selecting and marking a needle insertion site should be performed at the bedside just before performing the procedure. The patient must remain in the same position in the interim between marking and inserting the needle, as a slight change in position can alter the needle trajectory, lowering the LP success rate. Although performing LPs in a lateral decubitus position has the advantage of accurately measuring the opening pressure, misalignment of the shoulder and pelvic girdles and bowing of the bed in a lateral decubitus position may lower LP success rates.
One randomized study comparing ultrasound-guided spinal anesthesia in a lateral decubitus versus sitting position found no difference in the number of needle insertion attempts or measurement of the skin-dura distance; however, the needle insertion depth was 0.73 cm greater in a lateral decubitus vs sitting position (P = .002).66 Procedural success rates of LP with ultrasound guidance have not been directly compared in a sitting versus lateral decubitus position, although the overall procedural success rates were higher in one study that allowed the operator to choose either sitting or lateral decubitus position when ultrasound was used.32
4) We recommend that a low-frequency transducer, preferably a curvilinear array transducer, should be used to evaluate the lumbar spine and mark a needle insertion site in most patients. A high-frequency linear array transducer may be used in nonobese patients.
Rationale. Low-frequency transducers emit sound waves that penetrate deep tissues, allowing visualization of bones and ligaments of the lumbar spine. A high-frequency linear transducer offers better resolution but shallower penetration to approximately 6-9 cm, limiting its use for site marking in overweight and obese patients. In obese patients, the ligamentum flavum is often deeper than 6 cm, which requires a low-frequency transducer to be visualized.
Most of the randomized controlled studies demonstrating benefits of using ultrasound guidance compared with landmark guidance for performance of LP, epidural anesthesia, or spinal anesthesia have used a low-frequency, curvilinear transducer.22,24,26-28,31,34-36,39,43-45,67 Two randomized controlled trials used a high-frequency linear transducer for site marking of lumbar procedures.30,32,37 Using a high-frequency linear transducer has been described in real-time, ultrasound-guided LPs, the advantage being better needle visualization with a linear transducer.29 Detection of blood vessels by color flow Doppler may be another advantage of using a high-frequency linear transducer, although a study by Grau et al. showed that use of color flow Doppler with a low-frequency curvilinear transducer permitted visualization of interspinous vessels as small as 0.5 mm in size.68
5) We recommend that ultrasound should be used to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad, sequentially identifying the lumbar spine interspaces.Rationale. Although no studies have directly compared different ultrasound scanning protocols to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad to sequentially identify the lumbar interspinous spaces is the most commonly described technique in studies demonstrating improved clinical outcomes with the use of ultrasound.24,31,34,37,39,40,45,56,57,67 Because the sacrum can be easily recognized, identifying it first is most beneficial in patients with few or no palpable landmarks.
All five lumbar spinous processes and interspinous spaces can be mapped from the sacrum using either a midline or a paramedian approach, and the widest interspinous space can be selected. In a midline approach, either a transverse or a longitudinal view is obtained. The transducer is centered on the sacrum and slid cephalad from L5 to L1 to identify each spinous process and interspinous space. In a paramedian approach, longitudinal paramedian views are obtained from the L5–sacrum interspace to the L1–L2 interspace, and each interspinous space is identified as the transducer is slid cephalad. Both these approaches are effective for mapping the lumbar spine. Whether the entire lumbar spine is mapped, and whether a midline or a paramedian approach is utilized, will depend on the operator’s preference.
6) We recommend that ultrasound should be used in a transverse plane to mark the midline of the lumbar spine and a longitudinal plane to mark the interspinous spaces. The intersection of these two lines marks the needle insertion site.
Rationale. The most common technique described in comparative studies of ultrasound vs landmarks includes visualization of the lumbar spine in two planes, a transverse plane to identify the midline and a longitudinal plane to identify the interspinous spaces. The majority of randomized controlled studies that demonstrated a reduction in the number of needle insertion attempts and an increase in the procedural success rates have used this technique (see Clinical Outcomes).22,24,28,32,35-37,43,44 Marking the midline and interspinous space(s) for LP may be performed in any order, starting with either the transverse or longitudinal plane first.
The midline of the spine is marked by placing the transducer in a transverse plane over the lumbar spine, centering over the spinous processes that have a distinct hyperechoic tip and a prominent acoustic shadow deep to the bone, and drawing a line perpendicular to the center of the transducer delineating the midline. The midline should be marked over a minimum of two or three spinous processes.
To identify the interspinous spaces, the transducer is aligned longitudinally over the midline. The transducer is slid along the midline to identify the widest interspinous space. Once the transducer is centered over the widest interspinous space, a line perpendicular to the center of the transducer is drawn to mark the interspinous space. The intersection of the lines marking the spinal midline and the selected interspinous space identifies the needle entry point.
To visualize the ligamentum flavum from a paramedian view, the transducer is oriented longitudinally over the midline, slid approximately 1 cm laterally, and tilted approximately 15 degrees aiming the ultrasound beam toward the midline. The skin–ligamentum flavum distance is most reliably measured from a paramedian view. Alternatively, in some patients, the ligamentum flavum may be visualized in the midline and the depth can be measured.
7) We recommend that ultrasound should be used during a preprocedural evaluation to measure the distance from the skin surface to the ligamentum flavum from a longitudinal paramedian view to estimate the needle insertion depth and ensure that a spinal needle of adequate length is used.
Rationale. The distance from the skin to the ligamentum flavum can be measured using ultrasound during preprocedural planning. Knowing the depth to the ligamentum flavum preprocedurally allows the operator to procure a spinal needle of adequate length, anticipate the insertion depth before CSF can be obtained, determine the depth to which a local anesthetic will need to be injected, and decide whether the anticipated difficulty of the procedure warrants referral to or consultation with another specialist.
The skin–ligamentum flavum distance can be measured from a transverse midline view or a longitudinal paramedian view. A longitudinal paramedian view provides an unobstructed view of the ligamentum flavum due to less shadowing from bony structures compared with a midline view. Several studies have demonstrated a strong correlation between the skin–ligamentum flavum distance measured by ultrasound and the actual needle insertion depth in both midline and paramedian views.28,34,36,53,54,57,69,70
A meta-analysis that included 13 comparative studies evaluating the correlation between ultrasound-measured depth and actual needle insertion depth to reach the epidural or intrathecal space consistently demonstrated a strong correlation between the measured and actual depth.50 A few studies have reported near-perfect Pearson correlation coefficients of 0.98.55,71,72 The pooled correlation was 0.91 (95% CI 0.87-0.94). All studies measured the depth from the skin to the ventral side of the ligamentum flavum or the intrathecal space from either a longitudinal paramedian view (n = 4) or a transverse midline view (n = 9). Eight of the more recent studies evaluated the accuracy of the ultrasound measurements and found the depth measurements by ultrasound to be accurate within 1-13 mm of the actual needle insertion depth, with seven of the eight studies reporting a mean difference of ≤3 mm.50
Measurement of the distance between the skin and the ligamentum flavum generally underestimates the needle insertion depth. One study reported that measurement of the skin–ligamentum flavum distance underestimates the needle insertion depth by 7.6 mm to obtain CSF, whereas measurement of the skin–posterior longitudinal ligament distance overestimates the needle insertion depth by 2.5 mm.57 A well-accepted contributor to underestimation of the depth measurements using ultrasound is compression of the skin and soft tissues by the transducer, and therefore, pressure on the skin must be released before freezing an image and measuring the depth to the subarachnoid space.
Training
8) We recommend that novices should undergo simulation-based training, where available, before attempting ultrasound-guided LPs on actual patients.
Rationale. Similar to training for other bedside procedures, dedicated training sessions, including didactics, supervised practice on patients, and simulation-based practice, should be considered when teaching novices to perform ultrasound-guided LP. Simulation-based training facilitates acquisition of knowledge and skills to perform invasive bedside procedures, including LP.73 Simulation-based training has been commonly incorporated into procedure training for trainees using an immersive experience, such as a “boot camp,”74-77 or a standardized curriculum,78,79 and has demonstrated improvements in post-course procedural knowledge, technical skills, and operator confidence. Two of these studies included training in the use of ultrasound guidance for LP. These studies showed that simulation-based practice improved skill acquisition and confidence.80,81 Simulation using novel computer software may improve skill acquisition in the use of ultrasound guidance for LP.82
9) We recommend that training in ultrasound-guided LPs should be adapted based on prior ultrasound experience, as learning curves will vary.Rationale. The learning curve to achieve competency in the use of ultrasound guidance for LP has not been well studied. The rate of attaining competency in identifying lumbar spine structures using ultrasound will vary by provider based on prior skills in ultrasound-guided procedures.83 Thus, providers with prior ultrasound experience may require less training than those without such experience to achieve competency. However, extensive experience in performing landmark-guided LPs does not necessarily translate into rapid acquisition of skills to perform the procedure with ultrasound guidance. A study of practicing anesthesiologists with no prior ultrasound experience demonstrated that 20 supervised trials of ultrasound-guided spinal anesthesia were insufficient to achieve competency.84 Although minimums may be a necessary step to gain competence, using them as a sole means to define competence does not account for variable learning curves.12 Based on a national survey of 21 hospitalist procedure experts, the mean current vs suggested minimums for initial and ongoing hospital privileging for LPs were 1.8 vs 6.9 and 2.2 vs 4.6 annually in one report.85
A fundamental question that needs to be answered is how to define competency in the use of ultrasound guidance for LP, including the specific skills and knowledge that must be mastered. At a minimum, providers must be able to identify lumbar spinous processes and distinguish them from the sacrum, identify the lumbar interspinous spaces and their corresponding levels, and estimate the depth from the skin to the ligamentum flavum from the midline and paramedian planes. Novice operators may benefit from practicing lumbar spine mapping of nonobese patients using a high-frequency linear transducer that generates high-resolution images and facilitates recognition of lumbar spine structures.
10) We recommend that novice providers should be supervised when performing ultrasound-guided LPs before performing the procedure independently on patients.
Rationale: Demonstration of competency in the use of ultrasound to identify lumbar spine anatomy should be achieved before routinely performing the procedure independently on patients.18 All providers will require a variable period of supervised practice to demonstrate the appropriate technique, followed by a period of unsupervised practice before competency is achieved. Supervised practice with guidance and feedback has been shown to significantly improve providers’ ability to delineate lumbar spine anatomy.86
KNOWLEDGE GAPS
The process of producing these guidelines revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for LP.
First, it is unclear whether the use of ultrasound guidance for LP reduces postprocedural back pain and whether it improves patient satisfaction. Several studies have evaluated postprocedural back pain28,30,32,33,52 and patient satisfaction28,29,33,51 with the use of ultrasound guidance, but these studies have found inconsistent results. Some of these results were probably due to insufficient statistical power or confounding variables. Furthermore, benefits have been demonstrated in certain subgroups, such as overweight patients or those with anatomical abnormalities, as was found in two studies.52,87 Use of ultrasound guidance for spinal anesthesia has been shown to reduce postprocedural headache28 and improve patient satisfaction51, although similar benefit has not been demonstrated in patients undergoing LP.
Second, the effect of using ultrasound guidance on the frequency of traumatic LPs is an area of uncertainty. A “traumatic tap” is defined as an inadvertent puncture of an epidural vein during passage of the spinal needle through the dura. It remains difficult to discern in these studies whether red blood cells detected in the CSF resulted from puncture of an epidural vein or from needle trauma of the skin and soft tissues. Despite this uncertainty, at least seven randomized controlled studies have assessed the effect of ultrasound guidance on traumatic LPs. The meta-analysis by Shaikh et al. included five randomized controlled studies that assessed the effect of ultrasound guidance on the reporting of traumatic taps. The study found a reduced risk of traumatic taps (risk ratio 0.27 [95% CI 0.11-0.67]), an absolute risk reduction of 5.9% (95% CI 2.3%-9.5%), and a number needed to treat of 17 (95% CI 11-44) to prevent one traumatic tap.16 Similarly, the meta-analysis by Gottlieb et al. showed a lower risk of traumatic taps among adults undergoing LP with ultrasound guidance in five randomized controlled studies with an odds ratio of 0.28 (95% CI 0.14-0.59). The meta-analysis by Gottlieb et al. included two adult studies that were not included by Shaikh et al.
Third, several important questions about the technique of ultrasound-guided LP remain unanswered. In addition to the static technique, a dynamic technique with real-time needle tracking has been described to perform ultrasound-guided LP, epidural catheterization, and spinal anesthesia. A pilot study by Grau et al. found that ultrasound used either statically or dynamically had fewer insertion attempts and needle redirections than use of landmarks alone.29 Three other pilot studies showed successful spinal anesthesia in almost all patients88-90 and one large study demonstrated successful spinal anesthesia with real-time ultrasound guidance in 97 of 100 patients with a median of three needle passes.91 Furthermore, a few industry-sponsored studies with small numbers of patients have described the use of novel needle tracking systems that facilitate needle visualization during real-time ultrasound-guided LP.92,93 However, to our knowledge, no comparative studies of static versus dynamic guidance using novel needle tracking systems in human subjects have been published, and any potential role for these novel needle tracking systems has not yet been defined.
Finally, the effects of using ultrasound guidance on clinical decision-making, timeliness, and cost-effectiveness of LP have not yet been explored but could have important clinical practice implications.
CONCLUSION
Randomized controlled trials have demonstrated that using ultrasound guidance for LPs can reduce the number of needle insertion attempts and needle redirections and increase the overall procedural success rates. Ultrasound can more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients, although the greatest benefit of using ultrasound guidance for LPs has been shown in obese patients.
Ultrasound permits assessment of the interspinous space width and measurement of the ligamentum flavum depth to select an optimal needle insertion site and adequate length spinal needle. Although the use of real-time ultrasound guidance has been described, the use of static ultrasound guidance for LP site marking remains the standard technique.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators from Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Paul Mayo, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclosures
The authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1).
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023.
2. Kroll H, Duszak R, Jr, Nsiah E, et al. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. AJR Am J Roentgenol. 2015;204(1):15-19. https://doi.org/10.2214/AJR.14.12622.
3. Vickers A, Donnelly JP, Moore JX, Wang HE. 263EMF epidemiology of lumbar punctures in hospitalized patients in United States. Ann Emerg Med. 2017;70(4):S104. https://doi.org/10.1016/j.annemergmed.2017.07.241.
4. Køster-Rasmussen R, Korshin A, Meyer CN. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008;57(6):449-454. https://doi.org/10.1016/j.jinf.2008.09.033.
5. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129(11):862-869. https://doi.org/10.7326/0003-4819-129-11_Part_1-199812010-00004.
6. Lepur D, Barsić B. Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection. 2007;35(4):225-231. https://doi.org/10.1007/s15010-007-6202-0.
7. Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98(4):291-298. https://doi.org/10.1093/qjmed/hci047.
8. Auburtin M, Wolff M, Charpentier J, et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit Care Med. 2006;34(11):2758-2765. https://doi.org/10.1097/01.CCM.0000239434.26669.65.
9. Michael B, Menezes BF, Cunniffe J, et al. Effect of delayed lumbar punctures on the diagnosis of acute bacterial meningitis in adults. Emerg Med J. 2010;27(6):433-438. https://doi.org/10.1136/emj.2009.075598.
10. Glimåker M, Johansson B, Grindborg Ö, et al. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis. 2015;60(8):1162-1169. https://doi.org/10.1093/cid/civ011.
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 Revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715.
12. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079.
13. Shah KH, McGillicuddy D, Spear J, Edlow JA. Predicting difficult and traumatic lumbar punctures. Am J Emerg Med. 2007;25(6):608-611. https://doi.org/10.1016/j.ajem.2006.11.025.
14. Williams P, Tait G, Wijeratne T. Success rate of elective lumbar puncture at a major Melbourne neurology unit. Surg Neurol Int. 2018;9:12. https://doi.org/10.4103/sni.sni_426_17.
15. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2018;26(1). https://doi.org/10.1111/acem.13558.
16. Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ. 2013;346:f1720. https://doi.org/10.1136/bmj.f1720.
17. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
18. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
19. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
20. Soni NJ, Franco-Sadud R, Schnobrich D, et al. Ultrasound guidance for lumbar puncture. Neurol Clin Pract. 2016;6(4):358-368. https://doi.org/10.1212/CPJ.0000000000000265.
21. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The Rand/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand Corp; 2001.
22. Abdelhamid SA, Mansour MA. Ultrasound-guided intrathecal anesthesia: does scanning help? Egypt J Anaesth. 2013;29(4):389-394. https://doi.org/10.1016/j.egja.2013.06.003.
23. Ansari T, Yousef A, El Gamassy A, Fayez M. Ultrasound-guided spinal anaesthesia in obstetrics: is there an advantage over the landmark technique in patients with easily palpable spines? Int J Obstet Anesth. 2014;23(3):213-216. https://doi.org/10.1016/j.ijoa.2014.03.001.
24. Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115(1):94-101. https://doi.org/10.1097/ALN.0b013e31821a8ad4.
25. Cho YC, Koo DH, Oh SK, et al. Comparison of ultrasound-assisted lumbar puncture with lumbar puncture using palpation of landmarks in aged patients in an emergency center. J Korean Soc Emerg Med. 2009;20(3):304.
26. Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45(6):766-771. https://doi.org/10.1034/j.1399-6576.2001.045006766.x.
27. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Ultrasound imaging facilitates localization of the epidural space during combined spinal and epidural anesthesia. Reg Anesth Pain Med. 2001;26(1):64-67. https://doi.org/10.1053/rapm.2001.19633.
28. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth. 2002;14(3):169-175. https://doi.org/10.1016/S0952-8180(01)00378-6.
29. Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2004;21(1):25-31. https://doi.org/10.1017/S026502150400105X.
30. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
31. Nassar M, Abdelazim IA. Pre-puncture ultrasound guided epidural insertion before vaginal delivery. J Clin Monit Comput. 2015;29(5):573-577. https://doi.org/10.1007/s10877-014-9634-y.
32. Nomura JT, Leech SJ, Shenbagamurthi S, et al. A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med. 2007;26(10):1341-1348. https://doi.org/10.7863/jum.2007.26.10.1341.
33. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
34. Sahin T, Balaban O, Sahin L, Solak M, Toker K. A randomized controlled trial of preinsertion ultrasound guidance for spinal anaesthesia in pregnancy: outcomes among obese and lean parturients: ultrasound for spinal anesthesia in pregnancy. J Anesth. 2014;28(3):413-419. https://doi.org/10.1007/s00540-013-1726-1.
35. Wang Q, Yin C, Wang TL. Ultrasound facilitates identification of combined spinal-epidural puncture in obese parturients. Chin Med J (Engl). 2012;125(21):3840-3843. PubMed
36. Vallejo MC, Phelps AL, Singh S, Orebaugh SL, Sah N. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19(4):373-378. https://doi.org/10.1016/j.ijoa.2010.04.002.
37. Darrieutort-Laffite C, Bart G, Planche L, et al. Usefulness of a pre-procedure ultrasound scanning of the lumbar spine before epidural injection in patients with a presumed difficult puncture: a randomized controlled trial. Joint Bone Spine. 2015;82(5):356-361. https://doi.org/10.1016/j.jbspin.2015.02.001.
38. Vosko MR, Brunner C, Schreiber S. Lumbar puncture with ultrasound study (lupus study)-international prospective randomized multicentre trial. Int J Stroke. 2017;12(1):22. https://doi.org/10.1055/s-0037-1606991.
39. Urfalioğlu A, Bilal B, Öksüz G, et al. Comparison of the landmark and ultrasound methods in cesarean sections performed under spinal anesthesia on obese pregnants. J Matern Fetal Neonatal Med. 2017;30(9):1051-1056. https://doi.org/10.1080/14767058.2016.1199677.
40. Tawfik MM, Atallah MM, Elkharboutly WS, Allakkany NS, Abdelkhalek M. Does preprocedural ultrasound increase the first-pass success rate of epidural catheterization before cesarean delivery? A randomized controlled trial. Anesth Analg. 2017;124(3):851-856. https://doi.org/10.1213/ANE.0000000000001325.
41. Turkstra TP, Marmai KL, Armstrong KP, Kumar K, Singh SI. Preprocedural ultrasound assessment does not improve trainee performance of spinal anesthesia for obstetrical patients: a randomized controlled trial. J Clin Anesth. 2017;37:21-24. https://doi.org/10.1016/j.jclinane.2016.10.034.
42. Chong SE, Mohd Nikman A, Saedah A, et al. Real-time ultrasound-guided paramedian spinal anaesthesia: evaluation of the efficacy and the success rate of single needle pass. Br J Anaesth. 2017;118(5):799-801. https://doi.org/10.1093/bja/aex108.
43. Creaney M, Mullane D, Casby C, Tan T. Ultrasound to identify the lumbar space in women with impalpable bony landmarks presenting for elective caesarean delivery under spinal anaesthesia: a randomised trial. Int J Obstet Anesth. 2016;28:12-16. https://doi.org/10.1016/j.ijoa.2016.07.007.
44. Ekinci M, Alici HA, Ahiskalioglu A, et al. The use of ultrasound in planned cesarean delivery under spinal anesthesia for patients having nonprominent anatomic landmarks. J Clin Anesth. 2017;37:82-85. https://doi.org/10.1016/j.jclinane.2016.10.014.
45. Perna P, Gioia A, Ragazzi R, Volta CA, Innamorato M. Can pre-procedure neuroaxial ultrasound improve the identification of the potential epidural space when compared with anatomical landmarks? A prospective randomized study. Minerva Anestesiol. 2017;83(1):41-49. https://doi.org/10.23736/S0375-9393.16.11399-9.
46. Chin A, Crooke B, Heywood L, et al. A randomised controlled trial comparing needle movements during combined spinal-epidural anaesthesia with and without ultrasound assistance. Anaesthesia. 2018;73(4):466-473. https://doi.org/10.1111/anae.14206.
47. Dhanger S, Vinayagam S, Vaidhyanathan B, Rajesh IJ, Tripathy DK. Comparison of landmark versus pre-procedural ultrasonography-assisted midline approach for identification of subarachnoid space in elective caesarean section: a randomised controlled trial. Indian J Anaesth. 2018;62(4):280-284. https://doi.org/10.4103/ija.IJA_488_17.
48. Evans DP, Tozer J, Joyce M, Vitto MJ. Comparison of ultrasound-guided and landmark-based lumbar punctures in inexperienced resident physicians. J Ultrasound Med. 2019;38(3):613-620. https://doi.org/10.1002/jum.14728.
49. Srinivasan KK, Leo AM, Iohom G, Loughnane F, Lee PJ. Pre-procedure ultrasound-guided paramedian spinal anaesthesia at L5-S1: is this better than landmark-guided midline approach? A randomised controlled trial. Indian J Anaesth. 2018;62(1):53-60. https://doi.org/10.4103/ija.IJA_448_17.
50. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
51. Lim YC, Choo CY, Tan KT. A randomised controlled trial of ultrasound-assisted spinal anaesthesia. Anaesth Intensive Care. 2014;42(2):191-198. https://doi.org/10.1177/0310057X1404200205.
52. Honarbakhsh S, Osman C, Teo JTH, Gabriel C. Ultrasound-guided lumbar puncture as a diagnostic aid to reduce number of attempts and complication rates. Ultrasound. 2013;21(4):170-175. https://doi.org/10.1177/1742271X13504332.
53. Sahota JS, Carvalho JC, Balki M, Fanning N, Arzola C. Ultrasound estimates for midline epidural punctures in the obese parturient: paramedian sagittal oblique is comparable to transverse median plane. Anesth Analg. 2013;116(4):829-835. https://doi.org/10.1213/ANE.0b013e31827f55f0.
54. Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108(6):1876-1881. https://doi.org/10.1213/ane.0b013e3181a323f6.
55. Wallace DH, Currie JM, Gilstrap LC, Santos R. Indirect sonographic guidance for epidural anesthesia in obese pregnant patients. Reg Anesth. 1992;17(4):233-236. PubMed
56. Srinivasan KK, Iohom G, Loughnane F, Lee PJ. Conventional landmark-guided midline versus preprocedure ultrasound-guided paramedian techniques in spinal anesthesia. Anesth Analg. 2015;21(4):1089-1096. https://doi.org/10.1213/ANE.0000000000000911.
57. Chin KJ, Perlas A, Singh M, et al. An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can J Anaesth. 2009;56(9):643-650. https://doi.org/10.1007/s12630-009-9132-8.
58. Evansa I, Logina I, Vanags I, Borgeat A. Ultrasound versus fluoroscopic-guided epidural steroid injections in patients with degenerative spinal diseases: a randomised study. Eur J Anaesthesiol. 2015;32(4):262-268. https://doi.org/10.1097/EJA.0000000000000103.
59. Park Y, Lee JH, Park KD, et al. Ultrasound-guided vs fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: a prospective, randomized, single-blind clinical study. Am J Phys Med Rehabil. 2013;92(7):575-586. https://doi.org/10.1097/PHM.0b013e318292356b.
60. Margarido CB, Mikhael R, Arzola C, Balki M, Carvalho JC. The intercristal line determined by palpation is not a reliable anatomical landmark for neuraxial anesthesia. Can J Anaesth. 2011;58(3):262-266. https://doi.org/10.1007/s12630-010-9432-z.
61. Duniec L, Nowakowski P, Kosson D, Łazowski T. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther. 2013;45(1):1-6. https://doi.org/10.5603/AIT.2013.0001.
62. Schlotterbeck H, Schaeffer R, Dow WA, et al. Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia. Br J Anaesth. 2008;100(2):230-234. https://doi.org/10.1093/bja/aem371.
63. Whitty R, Moore M, Macarthur A. Identification of the lumbar interspinous spaces: palpation versus ultrasound. Anesth Analg. 2008;106(2):538-540, table of contents. https://doi.org/10.1213/ane.0b013e31816069d9.
64. Locks Gde F, Almeida MC, Pereira AA. Use of the ultrasound to determine the level of lumbar puncture in pregnant women. Rev Bras Anestesiol. 2010;60(1):13-19. https://doi.org/10.1016/S0034-7094(10)70002-7.
65. Stiffler KA, Jwayyed S, Wilber ST, Robinson A. The use of ultrasound to identify pertinent landmarks for lumbar puncture. Am J Emerg Med. 2007;25(3):331-334. https://doi.org/10.1016/j.ajem.2006.07.010.
66. Gulay U, Meltem T, Nadir SS, Aysin A. Ultrasound-guided evaluation of the lumbar subarachnoid space in lateral and sitting positions in pregnant patients to receive elective cesarean operation. Pak J Med Sci. 2015;31(1):76-81. https://doi.org/10.12669/pjms.311.5647.
67. Kawaguchi R, Yamauchi M, Sugino S, Yamakage M. Ultrasound-aided ipsilateral-dominant epidural block for total hip arthroplasty: a randomised controlled single-blind study. Eur J Anaesthesiol. 2011;28(2):137-140. https://doi.org/10.1097/EJA.0b013e3283423457.
68. Grau T, Leipold RW, Horter J, Martin E, Motsch J. Colour Doppler imaging of the interspinous and epidural space. Eur J Anaesthesiol. 2001;18(11):706-712. https://doi.org/10.1097/00003643-200111000-00002.
69. Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 2007;104(5):1188-92, tables of contents. https://doi.org/10.1213/01.ane.0000250912.66057.41.
70. Chauhan AK, Bhatia R, Agrawal S. Lumbar epidural depth using transverse ultrasound scan and its correlation with loss of resistance technique: a prospective observational study in Indian population. Saudi J Anaesth. 2018;12(2):279-282. https://doi.org/10.4103/sja.SJA_679_17.
71. Gnaho A, Nguyen V, Villevielle T, et al. Assessing the depth of the subarachnoid space by ultrasound. Rev Bras Anestesiol. 2012;62(4):520-530. https://doi.org/10.1016/S0034-7094(12)70150-2.
72. Cork RC, Kryc JJ, Vaughan RW. Ultrasonic localization of the lumbar epidural space. Anesthesiology. 1980;52(6):513-516. https://doi.org/10.1097/00000542-198006000-00013.
73. Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. https://doi.org/10.1212/WNL.0b013e31825dd39d.
74. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. https://doi.org/10.3109/0142159X.2010.509412.
75. Wayne DB, Cohen ER, Singer BD, et al. Progress toward improving medical school graduates’ skills via a “boot camp” curriculum. Simul Healthc. 2014;9(1):33-39. https://doi.org/10.1097/SIH.0000000000000001.
76. Cohen ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern boot camp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a.
77. Martin R, Gannon D, Riggle J, et al. A comprehensive workshop using simulation to train internal medicine residents in bedside procedures performed by internists. Chest. 2012;142(4):545A. https://doi.org/10.1378/chest.1390093.
78. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
79. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1.
80. Restrepo CG, Baker MD, Pruitt CM, Gullett JP, Pigott DC. Ability of pediatric emergency medicine physicians to identify anatomic landmarks with the assistance of ultrasound prior to lumbar puncture in a simulated obese model. Pediatr Emerg Care. 2015;31(1):15-19. https://doi.org/10.1097/PEC.0000000000000330.
81. VanderWielen BA, Harris R, Galgon RE, VanderWielen LM, Schroeder KM. Teaching sonoanatomy to anesthesia faculty and residents: utility of hands-on gel phantom and instructional video training models. J Clin Anesth. 2015;27(3):188-194. https://doi.org/10.1016/j.jclinane.2014.07.007.
82. Keri Z, Sydor D, Ungi T, et al. Computerized training system for ultrasound-guided lumbar puncture on abnormal spine models: a randomized controlled trial. Can J Anaesth. 2015;62(7):777-784. https://doi.org/10.1007/s12630-015-0367-2.
83. Deacon AJ, Melhuishi NS, Terblanche NC. CUSUM method for construction of trainee spinal ultrasound learning curves following standardised teaching. Anaesth Intensive Care. 2014;42(4):480-486. https://doi.org/10.1177/0310057X1404200409.
84. Margarido CB, Arzola C, Balki M, Carvalho JC. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anaesth. 2010;57(2):120-126. https://doi.org/10.1007/s12630-009-9219-2.
85. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
86. Terblanche NC, Arzola C, Wills KE, et al. Standardised training program in spinal ultrasound for epidural insertion: protocol driven versus non-protocol driven teaching approach. Anaesth Intensive Care. 2014;42(4):460-466. https://doi.org/10.1177/0310057X1404200406.
87. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
88. Karmakar MK, Li X, Ho AM, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth. 2009;102(6):845-854. https://doi.org/10.1093/bja/aep079.
89. Tran D, Kamani AA, Al-Attas E, et al. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anaesth. 2010;57(4):313-321. https://doi.org/10.1007/s12630-009-9252-1.
90. Liu Y, Qian W, Ke XJ, Mei W. Real-time ultrasound-guided spinal anesthesia using a new paramedian transverse approach. Curr Med Sci. 2018;38(5):910-913. https://doi.org/10.1007/s11596-018-1961-7.
91. Conroy PH, Luyet C, McCartney CJ, McHardy PG. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol Res Pract. 2013;2013:525818. https://doi.org/10.1155/2013/525818.
92. Brinkmann S, Tang R, Sawka A, Vaghadia H. Single-operator real-time ultrasound-guided spinal injection using SonixGPS™: a case series. Can J Anaesth. 2013;60(9):896-901. https://doi.org/10.1007/s12630-013-9984-9.
93. Niazi AU, Chin KJ, Jin R, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS ultrasound guidance system: a feasibility study. Acta Anaesthesiol Scand. 2014;58(7):875-881. https://doi.org/10.1111/aas.12353.
Approximately 400,000 lumbar punctures (LPs) are performed in the United States annually for either diagnostic workup or therapeutic relief.1 Lumbar punctures are increasingly being performed in the United States, with an estimated 97,000 LPs performed on Medicare fee-for-service beneficiaries in 2011 alone, which is an increase of approximately 4,000 LPs in the same population from 1991.2 Approximately 273,612 LPs were performed on hospitalized patients in the United States in 2010,1 and the inpatient hospital setting is the most common site for LPs.2,3
Many LPs are referred to radiologists who have access to imaging guidance to aid with needle insertion.2 However, referrals to radiology delay performance of LPs, and delayed diagnosis of acute bacterial meningitis, the most common yet serious condition for which LPs are performed, is associated with increased morbidity and mortality.4-8 Furthermore, although initiating empiric antibiotic treatment for suspected acute bacterial meningitis is recommended in some cases, doing so routinely can cause false-negative cerebrospinal fluid (CSF) culture results, complicating decisions about de-escalation and duration of antibiotics that could have been safely avoided by promptly performing an LP.9
Delaying the performance of LP has been associated with increased mortality.10 Demonstration of proficiency in performance of lumbar puncture is considered a core competency for hospitalists,11 and with the increasing availability of point-of-care ultrasound, hospitalists can use ultrasound to guide performance of LPs at the bedside.12 However, 30% of patients requiring LP in emergency departments have difficult-to-palpate lumbar spine landmarks,13 and lumbar puncture performed based on palpation of landmarks alone has been reported to fail or be traumatic in 28% of patients.14 Use of ultrasound guidance for lumbar puncture has been shown in randomized controlled trials to improve procedural success rates, while reducing the time to successful LP, needle passes, patient pain scores, and risk of a traumatic LP.15-17
The purpose of this position statement is to review the literature and present consensus-based recommendations on the performance of ultrasound-guided LP in adult patients. This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP. Similar to previously published Society of Hospital Medicine (SHM) position statements,12,18,19 this document presents recommendations with supporting evidence for the clinical outcomes, techniques, and training for using ultrasound guidance for LP. A manuscript describing the technique of ultrasound guidance for LPs has been previously published by some of the authors of this position statement.20
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the six working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. Updated searches were conducted in November 2016, January 2018, and October 2018. The search strings are included in Appendix 3. All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide LP were selected. In addition, the following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. Moreover, studies focusing on the use of ultrasound guidance for spinal nerve root injections, regional anesthesia, and assessment of lumbar spine anatomy alone were excluded. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided LP were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into the draft recommendations.
The Research and Development (RAND) Appropriateness Method that required panel judgment and consensus was used.21 The 27 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (REDCap™) in February 2018 and April 2018 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale. The three zones of the 9-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” A strong recommendation required 80% of the votes within one integer of the median, following the RAND rules. Disagreement was defined as >30% of panelists voting outside of the zone of the median.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording of each recommendation (Table 2). The revised consensus-based recommendations underwent internal and external reviews by POCUS experts from different subspecialties. The final review of this position statement was performed by members of the SHM POCUS Task Force, SHM Education Committee, and SHM Executive Committee. The SHM Executive Committee endorsed this position statement in June 2018 before submission to the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 4,389 references were pooled from four different sources: a search by a certified medical librarian in December 2015 (3,212 citations) that was updated in November 2016 (380 citations), January 2018 (282 citations), and October 2018 (274 citations); working group members’ personal bibliographies and searches (31 citations); and a search focusing on ultrasound-guided LP training (210 citations). A total of 232 full-text articles were reviewed, and the final selection included 77 articles that were abstracted into a data table and incorporated into the draft recommendations. Details of the literature search strategy are presented in Appendix 3.
RECOMMENDATIONS
Four domains (clinical outcomes, technique, training, and knowledge gaps) with 16 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation were drafted citing supporting evidence. After two rounds of panel voting, five recommendations did not achieve agreement based on the RAND rules, one recommendation was combined with another recommendation during peer review, and 10 statements received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Nine statements were approved as strong recommendations, and one was approved as a conditional recommendation. Therefore, the final recommendation count was 10. The strength of the recommendation and degree of consensus for each recommendation are summarized in Table 1.
Terminology
LP is a procedure in which a spinal needle is introduced into the subarachnoid space for the purpose of collecting CSF for diagnostic evaluation and/or therapeutic relief.
Throughout this document, the phrases “ultrasound-guided” and “ultrasound guidance” refer to the use of ultrasound to mark a needle insertion site immediately before performing the procedure. This is also known as static ultrasound guidance. Real-time or dynamic ultrasound guidance refers to direct visualization of the needle tip as it traverses through the skin and soft tissues to reach the ligamentum flavum. Any reference to real-time ultrasound guidance is explicitly stated.
Clinical outcomes
1) When ultrasound equipment is available, along with providers who are appropriately trained to use it, we recommend that ultrasound guidance should be used for site selection of LPs to reduce the number of needle insertion attempts and needle redirections and increase the overall procedure success rates, especially in patients who are obese or have difficult-to-palpate landmarks.
Rationale. LPs have historically been performed by selecting a needle insertion site based on palpation of anatomical landmarks. However, an estimated 30% of patients requiring LP in emergency departments have lumbar spine landmarks that are difficult to palpate, most commonly due to obesity.13 Furthermore, lumbar puncture performed based on palpation of landmarks alone has been reported to fail in 28% of patients.14
Ultrasound can be used at the bedside to elucidate the lumbar spine anatomy to guide performance of LP or epidural catheterization. Since the early 2000s, randomized studies comparing the use of ultrasound guidance (ultrasound-guided) versus anatomical landmarks (landmark-guided) to map the lumbar spine for epidural catheterization have emerged. It is important to recognize that the exact same ultrasound technique is used for site marking of LP, epidural catheterization, and spinal anesthesia—the key difference is how deep the needle tip is inserted. Therefore, data from these three ultrasound-guided procedures are often pooled. Currently, at least 33 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP, epidural catheterization, or spinal anesthesia have been published.22-49 We present three meta-analyses below that pooled data primarily from randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP or spinal anesthesia.
In 2013, Shaikh et al. published the first meta-analysis with 14 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP (n = 5) or epidural catheterization (n = 9). The pooled data showed that use of ultrasound guidance decreased the proportion of failed procedures (risk ratio 0.21, 95% CI 0.10-0.43) with an absolute risk reduction of 6.3% (95% CI 4.1%-8.4%) and a number needed to treat of 16 (95% CI 12-25) to prevent one failed procedure. In addition, the use of ultrasound reduced the mean number of attempts by 0.44 (95% CI 0.24-0.64) and reduced the mean number of needle redirections by 1.00 (95% CI 0.75-1.24). The reduction in risk of a failed procedure was similar for LPs (risk ratio 0.19 [95% CI 0.07-0.56]) and epidural catheterizations (risk ratio 0.23 [95% CI 0.09-0.60]).16
A similar meta-analysis published by Perlas et al. in 2016 included a total of 31 studies, both randomized controlled and cohort studies, evaluating the use of ultrasound guidance for LP, spinal anesthesia, and epidural catheterization.50 The goal of this systematic review and meta-analysis was to establish clinical practice recommendations. The authors concluded (1) the data consistently suggest that ultrasound is more accurate than palpation for lumbar interspace identification, (2) ultrasound allows accurate measurement of the needle insertion depth to reach the epidural space with a mean difference of <3 mm compared with the actual needle insertion depth, and (3) ultrasound increases the efficacy of lumbar epidural or spinal anesthesia by decreasing the mean number of needle passes for success by 0.75 (95% CI 0.44-1.07) and reducing the risk of a failed procedure (risk ratio 0.51 [95% CI 0.32-0.80]), both in patients with normal surface anatomy and in those with technically difficult surface anatomy due to obesity, scoliosis, or previous spine surgery.
Compared to the two earlier meta-analyses that included studies of both LP and spinal anesthesia procedures, the meta-analysis conducted by Gottlieb et al. in 2018 pooled data from 12 randomized controlled studies of ultrasound guidance for LPs only. For the primary outcome, pooled data from both adult and pediatric studies demonstrated higher procedural success rates with ultrasound-guided vs landmark-guided LPs (90% vs 81%) with an odds ratio of 2.1 (95% CI 0.66-7.44) in favor of ultrasound; however, there were no statistically significant differences when the adult and pediatric subgroups were analyzed separately, probably due to underpowering. For the secondary outcomes, data from the adult subgroup showed that use of ultrasound guidance was associated with fewer traumatic LPs (OR 0.28, 95% CI 0.14-0.59), shorter time to procedural success (adjusted mean difference –3.03 minutes, 95% CI –3.54 to –2.52), fewer number of needle passes (adjusted mean difference –0.81 passes, 95% CI –1.57 to –0.05), and lower patient pain scores (adjusted mean difference –2.53, 95% CI –3.89 to –1.17).
At least 12 randomized controlled studies have been published comparing the use of ultrasound guidance vs landmarks for the performance of LP or spinal anesthesia in adult patients, which were not included in the abovementioned meta-analyses. These individual studies demonstrated similar benefits of using ultrasound guidance: reduced needle insertion attempts, reduced needle redirections, and increased overall procedural success rates.17,31,37,40,41,43-49
It is important to recognize that four randomized controlled studies did not demonstrate any benefits of ultrasound guidance on the number of attempts or procedural success rates,23,33,41,51 and three of these studies were included in the abovementioned meta-analyses.23,33,51 Limitations of these negative studies include potential selection bias, inadequate sample sizes, and varying levels of operator skills in procedures, ultrasound guidance, or both. One study included emergency medicine residents as operators with varying degrees of ultrasound skills, and more importantly, patient enrollment occurred by convenience sampling, which may have introduced selection bias. Furthermore, most of the patients were not obese (median BMI of 27 kg/m2), and it is unclear why 10 years lapsed from data collection until publication.33 Another study with three experienced anesthesiologists as operators performing spinal anesthesia enrolled only patients who were not obese (mean BMI of 29 kg/m2) and had easily palpable bony landmarks—two patient characteristics associated with the least benefit of using ultrasound guidance in other studies.23 Another negative study had one experienced anesthesiologist marking obstetric patients with ultrasound, but junior residents performing the actual procedure in the absence of the anesthesiologist who had marked the patient.41
In general, the greatest benefit of using ultrasound guidance for LP has been demonstrated in obese patients.24,32,34,35,52,53 Benefits have been shown in specific obese patient populations, including obstetric,31,54,55 orthopedic,24,56,57 and emergency department patients.30
By increasing the procedural success rates with the use of ultrasound at the bedside, fewer patients may be referred to interventional radiology for fluoroscopic-guided LP, decreasing the patient exposure to ionizing radiation. A randomized study (n = 112) that compared site marking with ultrasound guidance versus fluoroscopic guidance for epidural steroid injections found the two techniques to be equivalent with respect to mean procedure time, number of needle insertion attempts, or needle passes.58 Another randomized study found that the performance time of ultrasound guidance was two minutes shorter (P < .05) than fluoroscopic guidance.59
Techniques
2) We recommend that ultrasound should be used to more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients.
Rationale. Traditionally, an imaginary line connecting the iliac crests (intercristal line, Tuffier’s line, or Jacoby’s line) was considered to identify the L4 vertebra or the L4-L5 interspinous space in the midline; however, studies have revealed this traditional landmark to be much less accurate than previously thought. In general, palpating the iliac crests to mark the intercristal line identifies an interspinous space that is one space cephalad (ie, the L2-L3 interspinous space) but can range from L1-L2 to L4-L5.46,60-64 If an LP is inadvertently performed in the L1-L2 interspinous space, the risk of spinal cord injury is higher than that when performed in a more distal interspinous space.
A study by Margarido et al. with 45 patients with a mean BMI of 30 kg/m2 found that the intercristal line was located above the L4-L5 interspinous space in 100% of patients. More importantly, the intercristal line was above L2-L3 in 36% of patients and above L1-L2 in 4% of patients. It is important to note that patients with scoliosis or previous spine surgery were excluded from this study, and all examinations were performed by two experienced anesthesiologists with patients in a sitting position—all factors that would favor accurate palpation and marking of the iliac crests.60
In a study of nonobese patients (mean BMI 28 kg/m2) undergoing spinal anesthesia, Duniec et al. compared the lumbar level identified by palpation versus ultrasound and found discordance between the two techniques in 36% of patients; 18% were one space too cephalad, 16% were one space too caudal, and 2% were off by two interspinous spaces.61 Another study found discordance in 64% of patients (mean BMI 28 kg/m2) when comparing the interspinous level where spinal anesthesia had been performed by palpation versus a post-procedural ultrasound examination. This study revealed that the interspinous space was more cephalad in 50% of patients with 6% of punctures performed in the L1-L2 interspace.62 A similar study compared the accuracy of palpation vs ultrasound to identify the L3-L4 interspinous space in obese (mean BMI 34 kg/m2) versus nonobese (mean BMI 27 kg/m2) patients. This study found marking a space above L3-L4 in 51% of obese and 40% of nonobese patients and marking of the L1-L2 interspace in 7% of obese and 4% of nonobese patients.64
A study comparing palpation vs ultrasound found that 68% of obese patients with a BMI of >30 kg/m2 had difficult-to-palpate lumbar spine landmarks, but with the use of ultrasound, landmarks were identified in 76% of all patients, including obese and nonobese, with difficult-to-palpate landmarks.65
3) We suggest using ultrasound for selecting and marking a needle insertion site just before performing LPs in either a lateral decubitus or sitting position. The patient should remain in the same position after marking the needle insertion site.
Rationale. Ultrasound mapping of the lumbar spine can be performed in either a lateral decubitus or sitting position. Selecting and marking a needle insertion site should be performed at the bedside just before performing the procedure. The patient must remain in the same position in the interim between marking and inserting the needle, as a slight change in position can alter the needle trajectory, lowering the LP success rate. Although performing LPs in a lateral decubitus position has the advantage of accurately measuring the opening pressure, misalignment of the shoulder and pelvic girdles and bowing of the bed in a lateral decubitus position may lower LP success rates.
One randomized study comparing ultrasound-guided spinal anesthesia in a lateral decubitus versus sitting position found no difference in the number of needle insertion attempts or measurement of the skin-dura distance; however, the needle insertion depth was 0.73 cm greater in a lateral decubitus vs sitting position (P = .002).66 Procedural success rates of LP with ultrasound guidance have not been directly compared in a sitting versus lateral decubitus position, although the overall procedural success rates were higher in one study that allowed the operator to choose either sitting or lateral decubitus position when ultrasound was used.32
4) We recommend that a low-frequency transducer, preferably a curvilinear array transducer, should be used to evaluate the lumbar spine and mark a needle insertion site in most patients. A high-frequency linear array transducer may be used in nonobese patients.
Rationale. Low-frequency transducers emit sound waves that penetrate deep tissues, allowing visualization of bones and ligaments of the lumbar spine. A high-frequency linear transducer offers better resolution but shallower penetration to approximately 6-9 cm, limiting its use for site marking in overweight and obese patients. In obese patients, the ligamentum flavum is often deeper than 6 cm, which requires a low-frequency transducer to be visualized.
Most of the randomized controlled studies demonstrating benefits of using ultrasound guidance compared with landmark guidance for performance of LP, epidural anesthesia, or spinal anesthesia have used a low-frequency, curvilinear transducer.22,24,26-28,31,34-36,39,43-45,67 Two randomized controlled trials used a high-frequency linear transducer for site marking of lumbar procedures.30,32,37 Using a high-frequency linear transducer has been described in real-time, ultrasound-guided LPs, the advantage being better needle visualization with a linear transducer.29 Detection of blood vessels by color flow Doppler may be another advantage of using a high-frequency linear transducer, although a study by Grau et al. showed that use of color flow Doppler with a low-frequency curvilinear transducer permitted visualization of interspinous vessels as small as 0.5 mm in size.68
5) We recommend that ultrasound should be used to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad, sequentially identifying the lumbar spine interspaces.Rationale. Although no studies have directly compared different ultrasound scanning protocols to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad to sequentially identify the lumbar interspinous spaces is the most commonly described technique in studies demonstrating improved clinical outcomes with the use of ultrasound.24,31,34,37,39,40,45,56,57,67 Because the sacrum can be easily recognized, identifying it first is most beneficial in patients with few or no palpable landmarks.
All five lumbar spinous processes and interspinous spaces can be mapped from the sacrum using either a midline or a paramedian approach, and the widest interspinous space can be selected. In a midline approach, either a transverse or a longitudinal view is obtained. The transducer is centered on the sacrum and slid cephalad from L5 to L1 to identify each spinous process and interspinous space. In a paramedian approach, longitudinal paramedian views are obtained from the L5–sacrum interspace to the L1–L2 interspace, and each interspinous space is identified as the transducer is slid cephalad. Both these approaches are effective for mapping the lumbar spine. Whether the entire lumbar spine is mapped, and whether a midline or a paramedian approach is utilized, will depend on the operator’s preference.
6) We recommend that ultrasound should be used in a transverse plane to mark the midline of the lumbar spine and a longitudinal plane to mark the interspinous spaces. The intersection of these two lines marks the needle insertion site.
Rationale. The most common technique described in comparative studies of ultrasound vs landmarks includes visualization of the lumbar spine in two planes, a transverse plane to identify the midline and a longitudinal plane to identify the interspinous spaces. The majority of randomized controlled studies that demonstrated a reduction in the number of needle insertion attempts and an increase in the procedural success rates have used this technique (see Clinical Outcomes).22,24,28,32,35-37,43,44 Marking the midline and interspinous space(s) for LP may be performed in any order, starting with either the transverse or longitudinal plane first.
The midline of the spine is marked by placing the transducer in a transverse plane over the lumbar spine, centering over the spinous processes that have a distinct hyperechoic tip and a prominent acoustic shadow deep to the bone, and drawing a line perpendicular to the center of the transducer delineating the midline. The midline should be marked over a minimum of two or three spinous processes.
To identify the interspinous spaces, the transducer is aligned longitudinally over the midline. The transducer is slid along the midline to identify the widest interspinous space. Once the transducer is centered over the widest interspinous space, a line perpendicular to the center of the transducer is drawn to mark the interspinous space. The intersection of the lines marking the spinal midline and the selected interspinous space identifies the needle entry point.
To visualize the ligamentum flavum from a paramedian view, the transducer is oriented longitudinally over the midline, slid approximately 1 cm laterally, and tilted approximately 15 degrees aiming the ultrasound beam toward the midline. The skin–ligamentum flavum distance is most reliably measured from a paramedian view. Alternatively, in some patients, the ligamentum flavum may be visualized in the midline and the depth can be measured.
7) We recommend that ultrasound should be used during a preprocedural evaluation to measure the distance from the skin surface to the ligamentum flavum from a longitudinal paramedian view to estimate the needle insertion depth and ensure that a spinal needle of adequate length is used.
Rationale. The distance from the skin to the ligamentum flavum can be measured using ultrasound during preprocedural planning. Knowing the depth to the ligamentum flavum preprocedurally allows the operator to procure a spinal needle of adequate length, anticipate the insertion depth before CSF can be obtained, determine the depth to which a local anesthetic will need to be injected, and decide whether the anticipated difficulty of the procedure warrants referral to or consultation with another specialist.
The skin–ligamentum flavum distance can be measured from a transverse midline view or a longitudinal paramedian view. A longitudinal paramedian view provides an unobstructed view of the ligamentum flavum due to less shadowing from bony structures compared with a midline view. Several studies have demonstrated a strong correlation between the skin–ligamentum flavum distance measured by ultrasound and the actual needle insertion depth in both midline and paramedian views.28,34,36,53,54,57,69,70
A meta-analysis that included 13 comparative studies evaluating the correlation between ultrasound-measured depth and actual needle insertion depth to reach the epidural or intrathecal space consistently demonstrated a strong correlation between the measured and actual depth.50 A few studies have reported near-perfect Pearson correlation coefficients of 0.98.55,71,72 The pooled correlation was 0.91 (95% CI 0.87-0.94). All studies measured the depth from the skin to the ventral side of the ligamentum flavum or the intrathecal space from either a longitudinal paramedian view (n = 4) or a transverse midline view (n = 9). Eight of the more recent studies evaluated the accuracy of the ultrasound measurements and found the depth measurements by ultrasound to be accurate within 1-13 mm of the actual needle insertion depth, with seven of the eight studies reporting a mean difference of ≤3 mm.50
Measurement of the distance between the skin and the ligamentum flavum generally underestimates the needle insertion depth. One study reported that measurement of the skin–ligamentum flavum distance underestimates the needle insertion depth by 7.6 mm to obtain CSF, whereas measurement of the skin–posterior longitudinal ligament distance overestimates the needle insertion depth by 2.5 mm.57 A well-accepted contributor to underestimation of the depth measurements using ultrasound is compression of the skin and soft tissues by the transducer, and therefore, pressure on the skin must be released before freezing an image and measuring the depth to the subarachnoid space.
Training
8) We recommend that novices should undergo simulation-based training, where available, before attempting ultrasound-guided LPs on actual patients.
Rationale. Similar to training for other bedside procedures, dedicated training sessions, including didactics, supervised practice on patients, and simulation-based practice, should be considered when teaching novices to perform ultrasound-guided LP. Simulation-based training facilitates acquisition of knowledge and skills to perform invasive bedside procedures, including LP.73 Simulation-based training has been commonly incorporated into procedure training for trainees using an immersive experience, such as a “boot camp,”74-77 or a standardized curriculum,78,79 and has demonstrated improvements in post-course procedural knowledge, technical skills, and operator confidence. Two of these studies included training in the use of ultrasound guidance for LP. These studies showed that simulation-based practice improved skill acquisition and confidence.80,81 Simulation using novel computer software may improve skill acquisition in the use of ultrasound guidance for LP.82
9) We recommend that training in ultrasound-guided LPs should be adapted based on prior ultrasound experience, as learning curves will vary.Rationale. The learning curve to achieve competency in the use of ultrasound guidance for LP has not been well studied. The rate of attaining competency in identifying lumbar spine structures using ultrasound will vary by provider based on prior skills in ultrasound-guided procedures.83 Thus, providers with prior ultrasound experience may require less training than those without such experience to achieve competency. However, extensive experience in performing landmark-guided LPs does not necessarily translate into rapid acquisition of skills to perform the procedure with ultrasound guidance. A study of practicing anesthesiologists with no prior ultrasound experience demonstrated that 20 supervised trials of ultrasound-guided spinal anesthesia were insufficient to achieve competency.84 Although minimums may be a necessary step to gain competence, using them as a sole means to define competence does not account for variable learning curves.12 Based on a national survey of 21 hospitalist procedure experts, the mean current vs suggested minimums for initial and ongoing hospital privileging for LPs were 1.8 vs 6.9 and 2.2 vs 4.6 annually in one report.85
A fundamental question that needs to be answered is how to define competency in the use of ultrasound guidance for LP, including the specific skills and knowledge that must be mastered. At a minimum, providers must be able to identify lumbar spinous processes and distinguish them from the sacrum, identify the lumbar interspinous spaces and their corresponding levels, and estimate the depth from the skin to the ligamentum flavum from the midline and paramedian planes. Novice operators may benefit from practicing lumbar spine mapping of nonobese patients using a high-frequency linear transducer that generates high-resolution images and facilitates recognition of lumbar spine structures.
10) We recommend that novice providers should be supervised when performing ultrasound-guided LPs before performing the procedure independently on patients.
Rationale: Demonstration of competency in the use of ultrasound to identify lumbar spine anatomy should be achieved before routinely performing the procedure independently on patients.18 All providers will require a variable period of supervised practice to demonstrate the appropriate technique, followed by a period of unsupervised practice before competency is achieved. Supervised practice with guidance and feedback has been shown to significantly improve providers’ ability to delineate lumbar spine anatomy.86
KNOWLEDGE GAPS
The process of producing these guidelines revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for LP.
First, it is unclear whether the use of ultrasound guidance for LP reduces postprocedural back pain and whether it improves patient satisfaction. Several studies have evaluated postprocedural back pain28,30,32,33,52 and patient satisfaction28,29,33,51 with the use of ultrasound guidance, but these studies have found inconsistent results. Some of these results were probably due to insufficient statistical power or confounding variables. Furthermore, benefits have been demonstrated in certain subgroups, such as overweight patients or those with anatomical abnormalities, as was found in two studies.52,87 Use of ultrasound guidance for spinal anesthesia has been shown to reduce postprocedural headache28 and improve patient satisfaction51, although similar benefit has not been demonstrated in patients undergoing LP.
Second, the effect of using ultrasound guidance on the frequency of traumatic LPs is an area of uncertainty. A “traumatic tap” is defined as an inadvertent puncture of an epidural vein during passage of the spinal needle through the dura. It remains difficult to discern in these studies whether red blood cells detected in the CSF resulted from puncture of an epidural vein or from needle trauma of the skin and soft tissues. Despite this uncertainty, at least seven randomized controlled studies have assessed the effect of ultrasound guidance on traumatic LPs. The meta-analysis by Shaikh et al. included five randomized controlled studies that assessed the effect of ultrasound guidance on the reporting of traumatic taps. The study found a reduced risk of traumatic taps (risk ratio 0.27 [95% CI 0.11-0.67]), an absolute risk reduction of 5.9% (95% CI 2.3%-9.5%), and a number needed to treat of 17 (95% CI 11-44) to prevent one traumatic tap.16 Similarly, the meta-analysis by Gottlieb et al. showed a lower risk of traumatic taps among adults undergoing LP with ultrasound guidance in five randomized controlled studies with an odds ratio of 0.28 (95% CI 0.14-0.59). The meta-analysis by Gottlieb et al. included two adult studies that were not included by Shaikh et al.
Third, several important questions about the technique of ultrasound-guided LP remain unanswered. In addition to the static technique, a dynamic technique with real-time needle tracking has been described to perform ultrasound-guided LP, epidural catheterization, and spinal anesthesia. A pilot study by Grau et al. found that ultrasound used either statically or dynamically had fewer insertion attempts and needle redirections than use of landmarks alone.29 Three other pilot studies showed successful spinal anesthesia in almost all patients88-90 and one large study demonstrated successful spinal anesthesia with real-time ultrasound guidance in 97 of 100 patients with a median of three needle passes.91 Furthermore, a few industry-sponsored studies with small numbers of patients have described the use of novel needle tracking systems that facilitate needle visualization during real-time ultrasound-guided LP.92,93 However, to our knowledge, no comparative studies of static versus dynamic guidance using novel needle tracking systems in human subjects have been published, and any potential role for these novel needle tracking systems has not yet been defined.
Finally, the effects of using ultrasound guidance on clinical decision-making, timeliness, and cost-effectiveness of LP have not yet been explored but could have important clinical practice implications.
CONCLUSION
Randomized controlled trials have demonstrated that using ultrasound guidance for LPs can reduce the number of needle insertion attempts and needle redirections and increase the overall procedural success rates. Ultrasound can more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients, although the greatest benefit of using ultrasound guidance for LPs has been shown in obese patients.
Ultrasound permits assessment of the interspinous space width and measurement of the ligamentum flavum depth to select an optimal needle insertion site and adequate length spinal needle. Although the use of real-time ultrasound guidance has been described, the use of static ultrasound guidance for LP site marking remains the standard technique.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators from Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Paul Mayo, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclosures
The authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1).
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Approximately 400,000 lumbar punctures (LPs) are performed in the United States annually for either diagnostic workup or therapeutic relief.1 Lumbar punctures are increasingly being performed in the United States, with an estimated 97,000 LPs performed on Medicare fee-for-service beneficiaries in 2011 alone, which is an increase of approximately 4,000 LPs in the same population from 1991.2 Approximately 273,612 LPs were performed on hospitalized patients in the United States in 2010,1 and the inpatient hospital setting is the most common site for LPs.2,3
Many LPs are referred to radiologists who have access to imaging guidance to aid with needle insertion.2 However, referrals to radiology delay performance of LPs, and delayed diagnosis of acute bacterial meningitis, the most common yet serious condition for which LPs are performed, is associated with increased morbidity and mortality.4-8 Furthermore, although initiating empiric antibiotic treatment for suspected acute bacterial meningitis is recommended in some cases, doing so routinely can cause false-negative cerebrospinal fluid (CSF) culture results, complicating decisions about de-escalation and duration of antibiotics that could have been safely avoided by promptly performing an LP.9
Delaying the performance of LP has been associated with increased mortality.10 Demonstration of proficiency in performance of lumbar puncture is considered a core competency for hospitalists,11 and with the increasing availability of point-of-care ultrasound, hospitalists can use ultrasound to guide performance of LPs at the bedside.12 However, 30% of patients requiring LP in emergency departments have difficult-to-palpate lumbar spine landmarks,13 and lumbar puncture performed based on palpation of landmarks alone has been reported to fail or be traumatic in 28% of patients.14 Use of ultrasound guidance for lumbar puncture has been shown in randomized controlled trials to improve procedural success rates, while reducing the time to successful LP, needle passes, patient pain scores, and risk of a traumatic LP.15-17
The purpose of this position statement is to review the literature and present consensus-based recommendations on the performance of ultrasound-guided LP in adult patients. This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP. Similar to previously published Society of Hospital Medicine (SHM) position statements,12,18,19 this document presents recommendations with supporting evidence for the clinical outcomes, techniques, and training for using ultrasound guidance for LP. A manuscript describing the technique of ultrasound guidance for LPs has been previously published by some of the authors of this position statement.20
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the six working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. Updated searches were conducted in November 2016, January 2018, and October 2018. The search strings are included in Appendix 3. All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide LP were selected. In addition, the following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. Moreover, studies focusing on the use of ultrasound guidance for spinal nerve root injections, regional anesthesia, and assessment of lumbar spine anatomy alone were excluded. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided LP were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into the draft recommendations.
The Research and Development (RAND) Appropriateness Method that required panel judgment and consensus was used.21 The 27 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (REDCap™) in February 2018 and April 2018 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale. The three zones of the 9-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” A strong recommendation required 80% of the votes within one integer of the median, following the RAND rules. Disagreement was defined as >30% of panelists voting outside of the zone of the median.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording of each recommendation (Table 2). The revised consensus-based recommendations underwent internal and external reviews by POCUS experts from different subspecialties. The final review of this position statement was performed by members of the SHM POCUS Task Force, SHM Education Committee, and SHM Executive Committee. The SHM Executive Committee endorsed this position statement in June 2018 before submission to the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 4,389 references were pooled from four different sources: a search by a certified medical librarian in December 2015 (3,212 citations) that was updated in November 2016 (380 citations), January 2018 (282 citations), and October 2018 (274 citations); working group members’ personal bibliographies and searches (31 citations); and a search focusing on ultrasound-guided LP training (210 citations). A total of 232 full-text articles were reviewed, and the final selection included 77 articles that were abstracted into a data table and incorporated into the draft recommendations. Details of the literature search strategy are presented in Appendix 3.
RECOMMENDATIONS
Four domains (clinical outcomes, technique, training, and knowledge gaps) with 16 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation were drafted citing supporting evidence. After two rounds of panel voting, five recommendations did not achieve agreement based on the RAND rules, one recommendation was combined with another recommendation during peer review, and 10 statements received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Nine statements were approved as strong recommendations, and one was approved as a conditional recommendation. Therefore, the final recommendation count was 10. The strength of the recommendation and degree of consensus for each recommendation are summarized in Table 1.
Terminology
LP is a procedure in which a spinal needle is introduced into the subarachnoid space for the purpose of collecting CSF for diagnostic evaluation and/or therapeutic relief.
Throughout this document, the phrases “ultrasound-guided” and “ultrasound guidance” refer to the use of ultrasound to mark a needle insertion site immediately before performing the procedure. This is also known as static ultrasound guidance. Real-time or dynamic ultrasound guidance refers to direct visualization of the needle tip as it traverses through the skin and soft tissues to reach the ligamentum flavum. Any reference to real-time ultrasound guidance is explicitly stated.
Clinical outcomes
1) When ultrasound equipment is available, along with providers who are appropriately trained to use it, we recommend that ultrasound guidance should be used for site selection of LPs to reduce the number of needle insertion attempts and needle redirections and increase the overall procedure success rates, especially in patients who are obese or have difficult-to-palpate landmarks.
Rationale. LPs have historically been performed by selecting a needle insertion site based on palpation of anatomical landmarks. However, an estimated 30% of patients requiring LP in emergency departments have lumbar spine landmarks that are difficult to palpate, most commonly due to obesity.13 Furthermore, lumbar puncture performed based on palpation of landmarks alone has been reported to fail in 28% of patients.14
Ultrasound can be used at the bedside to elucidate the lumbar spine anatomy to guide performance of LP or epidural catheterization. Since the early 2000s, randomized studies comparing the use of ultrasound guidance (ultrasound-guided) versus anatomical landmarks (landmark-guided) to map the lumbar spine for epidural catheterization have emerged. It is important to recognize that the exact same ultrasound technique is used for site marking of LP, epidural catheterization, and spinal anesthesia—the key difference is how deep the needle tip is inserted. Therefore, data from these three ultrasound-guided procedures are often pooled. Currently, at least 33 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP, epidural catheterization, or spinal anesthesia have been published.22-49 We present three meta-analyses below that pooled data primarily from randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP or spinal anesthesia.
In 2013, Shaikh et al. published the first meta-analysis with 14 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP (n = 5) or epidural catheterization (n = 9). The pooled data showed that use of ultrasound guidance decreased the proportion of failed procedures (risk ratio 0.21, 95% CI 0.10-0.43) with an absolute risk reduction of 6.3% (95% CI 4.1%-8.4%) and a number needed to treat of 16 (95% CI 12-25) to prevent one failed procedure. In addition, the use of ultrasound reduced the mean number of attempts by 0.44 (95% CI 0.24-0.64) and reduced the mean number of needle redirections by 1.00 (95% CI 0.75-1.24). The reduction in risk of a failed procedure was similar for LPs (risk ratio 0.19 [95% CI 0.07-0.56]) and epidural catheterizations (risk ratio 0.23 [95% CI 0.09-0.60]).16
A similar meta-analysis published by Perlas et al. in 2016 included a total of 31 studies, both randomized controlled and cohort studies, evaluating the use of ultrasound guidance for LP, spinal anesthesia, and epidural catheterization.50 The goal of this systematic review and meta-analysis was to establish clinical practice recommendations. The authors concluded (1) the data consistently suggest that ultrasound is more accurate than palpation for lumbar interspace identification, (2) ultrasound allows accurate measurement of the needle insertion depth to reach the epidural space with a mean difference of <3 mm compared with the actual needle insertion depth, and (3) ultrasound increases the efficacy of lumbar epidural or spinal anesthesia by decreasing the mean number of needle passes for success by 0.75 (95% CI 0.44-1.07) and reducing the risk of a failed procedure (risk ratio 0.51 [95% CI 0.32-0.80]), both in patients with normal surface anatomy and in those with technically difficult surface anatomy due to obesity, scoliosis, or previous spine surgery.
Compared to the two earlier meta-analyses that included studies of both LP and spinal anesthesia procedures, the meta-analysis conducted by Gottlieb et al. in 2018 pooled data from 12 randomized controlled studies of ultrasound guidance for LPs only. For the primary outcome, pooled data from both adult and pediatric studies demonstrated higher procedural success rates with ultrasound-guided vs landmark-guided LPs (90% vs 81%) with an odds ratio of 2.1 (95% CI 0.66-7.44) in favor of ultrasound; however, there were no statistically significant differences when the adult and pediatric subgroups were analyzed separately, probably due to underpowering. For the secondary outcomes, data from the adult subgroup showed that use of ultrasound guidance was associated with fewer traumatic LPs (OR 0.28, 95% CI 0.14-0.59), shorter time to procedural success (adjusted mean difference –3.03 minutes, 95% CI –3.54 to –2.52), fewer number of needle passes (adjusted mean difference –0.81 passes, 95% CI –1.57 to –0.05), and lower patient pain scores (adjusted mean difference –2.53, 95% CI –3.89 to –1.17).
At least 12 randomized controlled studies have been published comparing the use of ultrasound guidance vs landmarks for the performance of LP or spinal anesthesia in adult patients, which were not included in the abovementioned meta-analyses. These individual studies demonstrated similar benefits of using ultrasound guidance: reduced needle insertion attempts, reduced needle redirections, and increased overall procedural success rates.17,31,37,40,41,43-49
It is important to recognize that four randomized controlled studies did not demonstrate any benefits of ultrasound guidance on the number of attempts or procedural success rates,23,33,41,51 and three of these studies were included in the abovementioned meta-analyses.23,33,51 Limitations of these negative studies include potential selection bias, inadequate sample sizes, and varying levels of operator skills in procedures, ultrasound guidance, or both. One study included emergency medicine residents as operators with varying degrees of ultrasound skills, and more importantly, patient enrollment occurred by convenience sampling, which may have introduced selection bias. Furthermore, most of the patients were not obese (median BMI of 27 kg/m2), and it is unclear why 10 years lapsed from data collection until publication.33 Another study with three experienced anesthesiologists as operators performing spinal anesthesia enrolled only patients who were not obese (mean BMI of 29 kg/m2) and had easily palpable bony landmarks—two patient characteristics associated with the least benefit of using ultrasound guidance in other studies.23 Another negative study had one experienced anesthesiologist marking obstetric patients with ultrasound, but junior residents performing the actual procedure in the absence of the anesthesiologist who had marked the patient.41
In general, the greatest benefit of using ultrasound guidance for LP has been demonstrated in obese patients.24,32,34,35,52,53 Benefits have been shown in specific obese patient populations, including obstetric,31,54,55 orthopedic,24,56,57 and emergency department patients.30
By increasing the procedural success rates with the use of ultrasound at the bedside, fewer patients may be referred to interventional radiology for fluoroscopic-guided LP, decreasing the patient exposure to ionizing radiation. A randomized study (n = 112) that compared site marking with ultrasound guidance versus fluoroscopic guidance for epidural steroid injections found the two techniques to be equivalent with respect to mean procedure time, number of needle insertion attempts, or needle passes.58 Another randomized study found that the performance time of ultrasound guidance was two minutes shorter (P < .05) than fluoroscopic guidance.59
Techniques
2) We recommend that ultrasound should be used to more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients.
Rationale. Traditionally, an imaginary line connecting the iliac crests (intercristal line, Tuffier’s line, or Jacoby’s line) was considered to identify the L4 vertebra or the L4-L5 interspinous space in the midline; however, studies have revealed this traditional landmark to be much less accurate than previously thought. In general, palpating the iliac crests to mark the intercristal line identifies an interspinous space that is one space cephalad (ie, the L2-L3 interspinous space) but can range from L1-L2 to L4-L5.46,60-64 If an LP is inadvertently performed in the L1-L2 interspinous space, the risk of spinal cord injury is higher than that when performed in a more distal interspinous space.
A study by Margarido et al. with 45 patients with a mean BMI of 30 kg/m2 found that the intercristal line was located above the L4-L5 interspinous space in 100% of patients. More importantly, the intercristal line was above L2-L3 in 36% of patients and above L1-L2 in 4% of patients. It is important to note that patients with scoliosis or previous spine surgery were excluded from this study, and all examinations were performed by two experienced anesthesiologists with patients in a sitting position—all factors that would favor accurate palpation and marking of the iliac crests.60
In a study of nonobese patients (mean BMI 28 kg/m2) undergoing spinal anesthesia, Duniec et al. compared the lumbar level identified by palpation versus ultrasound and found discordance between the two techniques in 36% of patients; 18% were one space too cephalad, 16% were one space too caudal, and 2% were off by two interspinous spaces.61 Another study found discordance in 64% of patients (mean BMI 28 kg/m2) when comparing the interspinous level where spinal anesthesia had been performed by palpation versus a post-procedural ultrasound examination. This study revealed that the interspinous space was more cephalad in 50% of patients with 6% of punctures performed in the L1-L2 interspace.62 A similar study compared the accuracy of palpation vs ultrasound to identify the L3-L4 interspinous space in obese (mean BMI 34 kg/m2) versus nonobese (mean BMI 27 kg/m2) patients. This study found marking a space above L3-L4 in 51% of obese and 40% of nonobese patients and marking of the L1-L2 interspace in 7% of obese and 4% of nonobese patients.64
A study comparing palpation vs ultrasound found that 68% of obese patients with a BMI of >30 kg/m2 had difficult-to-palpate lumbar spine landmarks, but with the use of ultrasound, landmarks were identified in 76% of all patients, including obese and nonobese, with difficult-to-palpate landmarks.65
3) We suggest using ultrasound for selecting and marking a needle insertion site just before performing LPs in either a lateral decubitus or sitting position. The patient should remain in the same position after marking the needle insertion site.
Rationale. Ultrasound mapping of the lumbar spine can be performed in either a lateral decubitus or sitting position. Selecting and marking a needle insertion site should be performed at the bedside just before performing the procedure. The patient must remain in the same position in the interim between marking and inserting the needle, as a slight change in position can alter the needle trajectory, lowering the LP success rate. Although performing LPs in a lateral decubitus position has the advantage of accurately measuring the opening pressure, misalignment of the shoulder and pelvic girdles and bowing of the bed in a lateral decubitus position may lower LP success rates.
One randomized study comparing ultrasound-guided spinal anesthesia in a lateral decubitus versus sitting position found no difference in the number of needle insertion attempts or measurement of the skin-dura distance; however, the needle insertion depth was 0.73 cm greater in a lateral decubitus vs sitting position (P = .002).66 Procedural success rates of LP with ultrasound guidance have not been directly compared in a sitting versus lateral decubitus position, although the overall procedural success rates were higher in one study that allowed the operator to choose either sitting or lateral decubitus position when ultrasound was used.32
4) We recommend that a low-frequency transducer, preferably a curvilinear array transducer, should be used to evaluate the lumbar spine and mark a needle insertion site in most patients. A high-frequency linear array transducer may be used in nonobese patients.
Rationale. Low-frequency transducers emit sound waves that penetrate deep tissues, allowing visualization of bones and ligaments of the lumbar spine. A high-frequency linear transducer offers better resolution but shallower penetration to approximately 6-9 cm, limiting its use for site marking in overweight and obese patients. In obese patients, the ligamentum flavum is often deeper than 6 cm, which requires a low-frequency transducer to be visualized.
Most of the randomized controlled studies demonstrating benefits of using ultrasound guidance compared with landmark guidance for performance of LP, epidural anesthesia, or spinal anesthesia have used a low-frequency, curvilinear transducer.22,24,26-28,31,34-36,39,43-45,67 Two randomized controlled trials used a high-frequency linear transducer for site marking of lumbar procedures.30,32,37 Using a high-frequency linear transducer has been described in real-time, ultrasound-guided LPs, the advantage being better needle visualization with a linear transducer.29 Detection of blood vessels by color flow Doppler may be another advantage of using a high-frequency linear transducer, although a study by Grau et al. showed that use of color flow Doppler with a low-frequency curvilinear transducer permitted visualization of interspinous vessels as small as 0.5 mm in size.68
5) We recommend that ultrasound should be used to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad, sequentially identifying the lumbar spine interspaces.Rationale. Although no studies have directly compared different ultrasound scanning protocols to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad to sequentially identify the lumbar interspinous spaces is the most commonly described technique in studies demonstrating improved clinical outcomes with the use of ultrasound.24,31,34,37,39,40,45,56,57,67 Because the sacrum can be easily recognized, identifying it first is most beneficial in patients with few or no palpable landmarks.
All five lumbar spinous processes and interspinous spaces can be mapped from the sacrum using either a midline or a paramedian approach, and the widest interspinous space can be selected. In a midline approach, either a transverse or a longitudinal view is obtained. The transducer is centered on the sacrum and slid cephalad from L5 to L1 to identify each spinous process and interspinous space. In a paramedian approach, longitudinal paramedian views are obtained from the L5–sacrum interspace to the L1–L2 interspace, and each interspinous space is identified as the transducer is slid cephalad. Both these approaches are effective for mapping the lumbar spine. Whether the entire lumbar spine is mapped, and whether a midline or a paramedian approach is utilized, will depend on the operator’s preference.
6) We recommend that ultrasound should be used in a transverse plane to mark the midline of the lumbar spine and a longitudinal plane to mark the interspinous spaces. The intersection of these two lines marks the needle insertion site.
Rationale. The most common technique described in comparative studies of ultrasound vs landmarks includes visualization of the lumbar spine in two planes, a transverse plane to identify the midline and a longitudinal plane to identify the interspinous spaces. The majority of randomized controlled studies that demonstrated a reduction in the number of needle insertion attempts and an increase in the procedural success rates have used this technique (see Clinical Outcomes).22,24,28,32,35-37,43,44 Marking the midline and interspinous space(s) for LP may be performed in any order, starting with either the transverse or longitudinal plane first.
The midline of the spine is marked by placing the transducer in a transverse plane over the lumbar spine, centering over the spinous processes that have a distinct hyperechoic tip and a prominent acoustic shadow deep to the bone, and drawing a line perpendicular to the center of the transducer delineating the midline. The midline should be marked over a minimum of two or three spinous processes.
To identify the interspinous spaces, the transducer is aligned longitudinally over the midline. The transducer is slid along the midline to identify the widest interspinous space. Once the transducer is centered over the widest interspinous space, a line perpendicular to the center of the transducer is drawn to mark the interspinous space. The intersection of the lines marking the spinal midline and the selected interspinous space identifies the needle entry point.
To visualize the ligamentum flavum from a paramedian view, the transducer is oriented longitudinally over the midline, slid approximately 1 cm laterally, and tilted approximately 15 degrees aiming the ultrasound beam toward the midline. The skin–ligamentum flavum distance is most reliably measured from a paramedian view. Alternatively, in some patients, the ligamentum flavum may be visualized in the midline and the depth can be measured.
7) We recommend that ultrasound should be used during a preprocedural evaluation to measure the distance from the skin surface to the ligamentum flavum from a longitudinal paramedian view to estimate the needle insertion depth and ensure that a spinal needle of adequate length is used.
Rationale. The distance from the skin to the ligamentum flavum can be measured using ultrasound during preprocedural planning. Knowing the depth to the ligamentum flavum preprocedurally allows the operator to procure a spinal needle of adequate length, anticipate the insertion depth before CSF can be obtained, determine the depth to which a local anesthetic will need to be injected, and decide whether the anticipated difficulty of the procedure warrants referral to or consultation with another specialist.
The skin–ligamentum flavum distance can be measured from a transverse midline view or a longitudinal paramedian view. A longitudinal paramedian view provides an unobstructed view of the ligamentum flavum due to less shadowing from bony structures compared with a midline view. Several studies have demonstrated a strong correlation between the skin–ligamentum flavum distance measured by ultrasound and the actual needle insertion depth in both midline and paramedian views.28,34,36,53,54,57,69,70
A meta-analysis that included 13 comparative studies evaluating the correlation between ultrasound-measured depth and actual needle insertion depth to reach the epidural or intrathecal space consistently demonstrated a strong correlation between the measured and actual depth.50 A few studies have reported near-perfect Pearson correlation coefficients of 0.98.55,71,72 The pooled correlation was 0.91 (95% CI 0.87-0.94). All studies measured the depth from the skin to the ventral side of the ligamentum flavum or the intrathecal space from either a longitudinal paramedian view (n = 4) or a transverse midline view (n = 9). Eight of the more recent studies evaluated the accuracy of the ultrasound measurements and found the depth measurements by ultrasound to be accurate within 1-13 mm of the actual needle insertion depth, with seven of the eight studies reporting a mean difference of ≤3 mm.50
Measurement of the distance between the skin and the ligamentum flavum generally underestimates the needle insertion depth. One study reported that measurement of the skin–ligamentum flavum distance underestimates the needle insertion depth by 7.6 mm to obtain CSF, whereas measurement of the skin–posterior longitudinal ligament distance overestimates the needle insertion depth by 2.5 mm.57 A well-accepted contributor to underestimation of the depth measurements using ultrasound is compression of the skin and soft tissues by the transducer, and therefore, pressure on the skin must be released before freezing an image and measuring the depth to the subarachnoid space.
Training
8) We recommend that novices should undergo simulation-based training, where available, before attempting ultrasound-guided LPs on actual patients.
Rationale. Similar to training for other bedside procedures, dedicated training sessions, including didactics, supervised practice on patients, and simulation-based practice, should be considered when teaching novices to perform ultrasound-guided LP. Simulation-based training facilitates acquisition of knowledge and skills to perform invasive bedside procedures, including LP.73 Simulation-based training has been commonly incorporated into procedure training for trainees using an immersive experience, such as a “boot camp,”74-77 or a standardized curriculum,78,79 and has demonstrated improvements in post-course procedural knowledge, technical skills, and operator confidence. Two of these studies included training in the use of ultrasound guidance for LP. These studies showed that simulation-based practice improved skill acquisition and confidence.80,81 Simulation using novel computer software may improve skill acquisition in the use of ultrasound guidance for LP.82
9) We recommend that training in ultrasound-guided LPs should be adapted based on prior ultrasound experience, as learning curves will vary.Rationale. The learning curve to achieve competency in the use of ultrasound guidance for LP has not been well studied. The rate of attaining competency in identifying lumbar spine structures using ultrasound will vary by provider based on prior skills in ultrasound-guided procedures.83 Thus, providers with prior ultrasound experience may require less training than those without such experience to achieve competency. However, extensive experience in performing landmark-guided LPs does not necessarily translate into rapid acquisition of skills to perform the procedure with ultrasound guidance. A study of practicing anesthesiologists with no prior ultrasound experience demonstrated that 20 supervised trials of ultrasound-guided spinal anesthesia were insufficient to achieve competency.84 Although minimums may be a necessary step to gain competence, using them as a sole means to define competence does not account for variable learning curves.12 Based on a national survey of 21 hospitalist procedure experts, the mean current vs suggested minimums for initial and ongoing hospital privileging for LPs were 1.8 vs 6.9 and 2.2 vs 4.6 annually in one report.85
A fundamental question that needs to be answered is how to define competency in the use of ultrasound guidance for LP, including the specific skills and knowledge that must be mastered. At a minimum, providers must be able to identify lumbar spinous processes and distinguish them from the sacrum, identify the lumbar interspinous spaces and their corresponding levels, and estimate the depth from the skin to the ligamentum flavum from the midline and paramedian planes. Novice operators may benefit from practicing lumbar spine mapping of nonobese patients using a high-frequency linear transducer that generates high-resolution images and facilitates recognition of lumbar spine structures.
10) We recommend that novice providers should be supervised when performing ultrasound-guided LPs before performing the procedure independently on patients.
Rationale: Demonstration of competency in the use of ultrasound to identify lumbar spine anatomy should be achieved before routinely performing the procedure independently on patients.18 All providers will require a variable period of supervised practice to demonstrate the appropriate technique, followed by a period of unsupervised practice before competency is achieved. Supervised practice with guidance and feedback has been shown to significantly improve providers’ ability to delineate lumbar spine anatomy.86
KNOWLEDGE GAPS
The process of producing these guidelines revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for LP.
First, it is unclear whether the use of ultrasound guidance for LP reduces postprocedural back pain and whether it improves patient satisfaction. Several studies have evaluated postprocedural back pain28,30,32,33,52 and patient satisfaction28,29,33,51 with the use of ultrasound guidance, but these studies have found inconsistent results. Some of these results were probably due to insufficient statistical power or confounding variables. Furthermore, benefits have been demonstrated in certain subgroups, such as overweight patients or those with anatomical abnormalities, as was found in two studies.52,87 Use of ultrasound guidance for spinal anesthesia has been shown to reduce postprocedural headache28 and improve patient satisfaction51, although similar benefit has not been demonstrated in patients undergoing LP.
Second, the effect of using ultrasound guidance on the frequency of traumatic LPs is an area of uncertainty. A “traumatic tap” is defined as an inadvertent puncture of an epidural vein during passage of the spinal needle through the dura. It remains difficult to discern in these studies whether red blood cells detected in the CSF resulted from puncture of an epidural vein or from needle trauma of the skin and soft tissues. Despite this uncertainty, at least seven randomized controlled studies have assessed the effect of ultrasound guidance on traumatic LPs. The meta-analysis by Shaikh et al. included five randomized controlled studies that assessed the effect of ultrasound guidance on the reporting of traumatic taps. The study found a reduced risk of traumatic taps (risk ratio 0.27 [95% CI 0.11-0.67]), an absolute risk reduction of 5.9% (95% CI 2.3%-9.5%), and a number needed to treat of 17 (95% CI 11-44) to prevent one traumatic tap.16 Similarly, the meta-analysis by Gottlieb et al. showed a lower risk of traumatic taps among adults undergoing LP with ultrasound guidance in five randomized controlled studies with an odds ratio of 0.28 (95% CI 0.14-0.59). The meta-analysis by Gottlieb et al. included two adult studies that were not included by Shaikh et al.
Third, several important questions about the technique of ultrasound-guided LP remain unanswered. In addition to the static technique, a dynamic technique with real-time needle tracking has been described to perform ultrasound-guided LP, epidural catheterization, and spinal anesthesia. A pilot study by Grau et al. found that ultrasound used either statically or dynamically had fewer insertion attempts and needle redirections than use of landmarks alone.29 Three other pilot studies showed successful spinal anesthesia in almost all patients88-90 and one large study demonstrated successful spinal anesthesia with real-time ultrasound guidance in 97 of 100 patients with a median of three needle passes.91 Furthermore, a few industry-sponsored studies with small numbers of patients have described the use of novel needle tracking systems that facilitate needle visualization during real-time ultrasound-guided LP.92,93 However, to our knowledge, no comparative studies of static versus dynamic guidance using novel needle tracking systems in human subjects have been published, and any potential role for these novel needle tracking systems has not yet been defined.
Finally, the effects of using ultrasound guidance on clinical decision-making, timeliness, and cost-effectiveness of LP have not yet been explored but could have important clinical practice implications.
CONCLUSION
Randomized controlled trials have demonstrated that using ultrasound guidance for LPs can reduce the number of needle insertion attempts and needle redirections and increase the overall procedural success rates. Ultrasound can more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients, although the greatest benefit of using ultrasound guidance for LPs has been shown in obese patients.
Ultrasound permits assessment of the interspinous space width and measurement of the ligamentum flavum depth to select an optimal needle insertion site and adequate length spinal needle. Although the use of real-time ultrasound guidance has been described, the use of static ultrasound guidance for LP site marking remains the standard technique.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators from Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Paul Mayo, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclosures
The authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1).
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023.
2. Kroll H, Duszak R, Jr, Nsiah E, et al. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. AJR Am J Roentgenol. 2015;204(1):15-19. https://doi.org/10.2214/AJR.14.12622.
3. Vickers A, Donnelly JP, Moore JX, Wang HE. 263EMF epidemiology of lumbar punctures in hospitalized patients in United States. Ann Emerg Med. 2017;70(4):S104. https://doi.org/10.1016/j.annemergmed.2017.07.241.
4. Køster-Rasmussen R, Korshin A, Meyer CN. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008;57(6):449-454. https://doi.org/10.1016/j.jinf.2008.09.033.
5. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129(11):862-869. https://doi.org/10.7326/0003-4819-129-11_Part_1-199812010-00004.
6. Lepur D, Barsić B. Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection. 2007;35(4):225-231. https://doi.org/10.1007/s15010-007-6202-0.
7. Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98(4):291-298. https://doi.org/10.1093/qjmed/hci047.
8. Auburtin M, Wolff M, Charpentier J, et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit Care Med. 2006;34(11):2758-2765. https://doi.org/10.1097/01.CCM.0000239434.26669.65.
9. Michael B, Menezes BF, Cunniffe J, et al. Effect of delayed lumbar punctures on the diagnosis of acute bacterial meningitis in adults. Emerg Med J. 2010;27(6):433-438. https://doi.org/10.1136/emj.2009.075598.
10. Glimåker M, Johansson B, Grindborg Ö, et al. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis. 2015;60(8):1162-1169. https://doi.org/10.1093/cid/civ011.
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 Revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715.
12. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079.
13. Shah KH, McGillicuddy D, Spear J, Edlow JA. Predicting difficult and traumatic lumbar punctures. Am J Emerg Med. 2007;25(6):608-611. https://doi.org/10.1016/j.ajem.2006.11.025.
14. Williams P, Tait G, Wijeratne T. Success rate of elective lumbar puncture at a major Melbourne neurology unit. Surg Neurol Int. 2018;9:12. https://doi.org/10.4103/sni.sni_426_17.
15. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2018;26(1). https://doi.org/10.1111/acem.13558.
16. Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ. 2013;346:f1720. https://doi.org/10.1136/bmj.f1720.
17. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
18. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
19. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
20. Soni NJ, Franco-Sadud R, Schnobrich D, et al. Ultrasound guidance for lumbar puncture. Neurol Clin Pract. 2016;6(4):358-368. https://doi.org/10.1212/CPJ.0000000000000265.
21. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The Rand/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand Corp; 2001.
22. Abdelhamid SA, Mansour MA. Ultrasound-guided intrathecal anesthesia: does scanning help? Egypt J Anaesth. 2013;29(4):389-394. https://doi.org/10.1016/j.egja.2013.06.003.
23. Ansari T, Yousef A, El Gamassy A, Fayez M. Ultrasound-guided spinal anaesthesia in obstetrics: is there an advantage over the landmark technique in patients with easily palpable spines? Int J Obstet Anesth. 2014;23(3):213-216. https://doi.org/10.1016/j.ijoa.2014.03.001.
24. Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115(1):94-101. https://doi.org/10.1097/ALN.0b013e31821a8ad4.
25. Cho YC, Koo DH, Oh SK, et al. Comparison of ultrasound-assisted lumbar puncture with lumbar puncture using palpation of landmarks in aged patients in an emergency center. J Korean Soc Emerg Med. 2009;20(3):304.
26. Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45(6):766-771. https://doi.org/10.1034/j.1399-6576.2001.045006766.x.
27. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Ultrasound imaging facilitates localization of the epidural space during combined spinal and epidural anesthesia. Reg Anesth Pain Med. 2001;26(1):64-67. https://doi.org/10.1053/rapm.2001.19633.
28. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth. 2002;14(3):169-175. https://doi.org/10.1016/S0952-8180(01)00378-6.
29. Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2004;21(1):25-31. https://doi.org/10.1017/S026502150400105X.
30. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
31. Nassar M, Abdelazim IA. Pre-puncture ultrasound guided epidural insertion before vaginal delivery. J Clin Monit Comput. 2015;29(5):573-577. https://doi.org/10.1007/s10877-014-9634-y.
32. Nomura JT, Leech SJ, Shenbagamurthi S, et al. A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med. 2007;26(10):1341-1348. https://doi.org/10.7863/jum.2007.26.10.1341.
33. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
34. Sahin T, Balaban O, Sahin L, Solak M, Toker K. A randomized controlled trial of preinsertion ultrasound guidance for spinal anaesthesia in pregnancy: outcomes among obese and lean parturients: ultrasound for spinal anesthesia in pregnancy. J Anesth. 2014;28(3):413-419. https://doi.org/10.1007/s00540-013-1726-1.
35. Wang Q, Yin C, Wang TL. Ultrasound facilitates identification of combined spinal-epidural puncture in obese parturients. Chin Med J (Engl). 2012;125(21):3840-3843. PubMed
36. Vallejo MC, Phelps AL, Singh S, Orebaugh SL, Sah N. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19(4):373-378. https://doi.org/10.1016/j.ijoa.2010.04.002.
37. Darrieutort-Laffite C, Bart G, Planche L, et al. Usefulness of a pre-procedure ultrasound scanning of the lumbar spine before epidural injection in patients with a presumed difficult puncture: a randomized controlled trial. Joint Bone Spine. 2015;82(5):356-361. https://doi.org/10.1016/j.jbspin.2015.02.001.
38. Vosko MR, Brunner C, Schreiber S. Lumbar puncture with ultrasound study (lupus study)-international prospective randomized multicentre trial. Int J Stroke. 2017;12(1):22. https://doi.org/10.1055/s-0037-1606991.
39. Urfalioğlu A, Bilal B, Öksüz G, et al. Comparison of the landmark and ultrasound methods in cesarean sections performed under spinal anesthesia on obese pregnants. J Matern Fetal Neonatal Med. 2017;30(9):1051-1056. https://doi.org/10.1080/14767058.2016.1199677.
40. Tawfik MM, Atallah MM, Elkharboutly WS, Allakkany NS, Abdelkhalek M. Does preprocedural ultrasound increase the first-pass success rate of epidural catheterization before cesarean delivery? A randomized controlled trial. Anesth Analg. 2017;124(3):851-856. https://doi.org/10.1213/ANE.0000000000001325.
41. Turkstra TP, Marmai KL, Armstrong KP, Kumar K, Singh SI. Preprocedural ultrasound assessment does not improve trainee performance of spinal anesthesia for obstetrical patients: a randomized controlled trial. J Clin Anesth. 2017;37:21-24. https://doi.org/10.1016/j.jclinane.2016.10.034.
42. Chong SE, Mohd Nikman A, Saedah A, et al. Real-time ultrasound-guided paramedian spinal anaesthesia: evaluation of the efficacy and the success rate of single needle pass. Br J Anaesth. 2017;118(5):799-801. https://doi.org/10.1093/bja/aex108.
43. Creaney M, Mullane D, Casby C, Tan T. Ultrasound to identify the lumbar space in women with impalpable bony landmarks presenting for elective caesarean delivery under spinal anaesthesia: a randomised trial. Int J Obstet Anesth. 2016;28:12-16. https://doi.org/10.1016/j.ijoa.2016.07.007.
44. Ekinci M, Alici HA, Ahiskalioglu A, et al. The use of ultrasound in planned cesarean delivery under spinal anesthesia for patients having nonprominent anatomic landmarks. J Clin Anesth. 2017;37:82-85. https://doi.org/10.1016/j.jclinane.2016.10.014.
45. Perna P, Gioia A, Ragazzi R, Volta CA, Innamorato M. Can pre-procedure neuroaxial ultrasound improve the identification of the potential epidural space when compared with anatomical landmarks? A prospective randomized study. Minerva Anestesiol. 2017;83(1):41-49. https://doi.org/10.23736/S0375-9393.16.11399-9.
46. Chin A, Crooke B, Heywood L, et al. A randomised controlled trial comparing needle movements during combined spinal-epidural anaesthesia with and without ultrasound assistance. Anaesthesia. 2018;73(4):466-473. https://doi.org/10.1111/anae.14206.
47. Dhanger S, Vinayagam S, Vaidhyanathan B, Rajesh IJ, Tripathy DK. Comparison of landmark versus pre-procedural ultrasonography-assisted midline approach for identification of subarachnoid space in elective caesarean section: a randomised controlled trial. Indian J Anaesth. 2018;62(4):280-284. https://doi.org/10.4103/ija.IJA_488_17.
48. Evans DP, Tozer J, Joyce M, Vitto MJ. Comparison of ultrasound-guided and landmark-based lumbar punctures in inexperienced resident physicians. J Ultrasound Med. 2019;38(3):613-620. https://doi.org/10.1002/jum.14728.
49. Srinivasan KK, Leo AM, Iohom G, Loughnane F, Lee PJ. Pre-procedure ultrasound-guided paramedian spinal anaesthesia at L5-S1: is this better than landmark-guided midline approach? A randomised controlled trial. Indian J Anaesth. 2018;62(1):53-60. https://doi.org/10.4103/ija.IJA_448_17.
50. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
51. Lim YC, Choo CY, Tan KT. A randomised controlled trial of ultrasound-assisted spinal anaesthesia. Anaesth Intensive Care. 2014;42(2):191-198. https://doi.org/10.1177/0310057X1404200205.
52. Honarbakhsh S, Osman C, Teo JTH, Gabriel C. Ultrasound-guided lumbar puncture as a diagnostic aid to reduce number of attempts and complication rates. Ultrasound. 2013;21(4):170-175. https://doi.org/10.1177/1742271X13504332.
53. Sahota JS, Carvalho JC, Balki M, Fanning N, Arzola C. Ultrasound estimates for midline epidural punctures in the obese parturient: paramedian sagittal oblique is comparable to transverse median plane. Anesth Analg. 2013;116(4):829-835. https://doi.org/10.1213/ANE.0b013e31827f55f0.
54. Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108(6):1876-1881. https://doi.org/10.1213/ane.0b013e3181a323f6.
55. Wallace DH, Currie JM, Gilstrap LC, Santos R. Indirect sonographic guidance for epidural anesthesia in obese pregnant patients. Reg Anesth. 1992;17(4):233-236. PubMed
56. Srinivasan KK, Iohom G, Loughnane F, Lee PJ. Conventional landmark-guided midline versus preprocedure ultrasound-guided paramedian techniques in spinal anesthesia. Anesth Analg. 2015;21(4):1089-1096. https://doi.org/10.1213/ANE.0000000000000911.
57. Chin KJ, Perlas A, Singh M, et al. An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can J Anaesth. 2009;56(9):643-650. https://doi.org/10.1007/s12630-009-9132-8.
58. Evansa I, Logina I, Vanags I, Borgeat A. Ultrasound versus fluoroscopic-guided epidural steroid injections in patients with degenerative spinal diseases: a randomised study. Eur J Anaesthesiol. 2015;32(4):262-268. https://doi.org/10.1097/EJA.0000000000000103.
59. Park Y, Lee JH, Park KD, et al. Ultrasound-guided vs fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: a prospective, randomized, single-blind clinical study. Am J Phys Med Rehabil. 2013;92(7):575-586. https://doi.org/10.1097/PHM.0b013e318292356b.
60. Margarido CB, Mikhael R, Arzola C, Balki M, Carvalho JC. The intercristal line determined by palpation is not a reliable anatomical landmark for neuraxial anesthesia. Can J Anaesth. 2011;58(3):262-266. https://doi.org/10.1007/s12630-010-9432-z.
61. Duniec L, Nowakowski P, Kosson D, Łazowski T. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther. 2013;45(1):1-6. https://doi.org/10.5603/AIT.2013.0001.
62. Schlotterbeck H, Schaeffer R, Dow WA, et al. Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia. Br J Anaesth. 2008;100(2):230-234. https://doi.org/10.1093/bja/aem371.
63. Whitty R, Moore M, Macarthur A. Identification of the lumbar interspinous spaces: palpation versus ultrasound. Anesth Analg. 2008;106(2):538-540, table of contents. https://doi.org/10.1213/ane.0b013e31816069d9.
64. Locks Gde F, Almeida MC, Pereira AA. Use of the ultrasound to determine the level of lumbar puncture in pregnant women. Rev Bras Anestesiol. 2010;60(1):13-19. https://doi.org/10.1016/S0034-7094(10)70002-7.
65. Stiffler KA, Jwayyed S, Wilber ST, Robinson A. The use of ultrasound to identify pertinent landmarks for lumbar puncture. Am J Emerg Med. 2007;25(3):331-334. https://doi.org/10.1016/j.ajem.2006.07.010.
66. Gulay U, Meltem T, Nadir SS, Aysin A. Ultrasound-guided evaluation of the lumbar subarachnoid space in lateral and sitting positions in pregnant patients to receive elective cesarean operation. Pak J Med Sci. 2015;31(1):76-81. https://doi.org/10.12669/pjms.311.5647.
67. Kawaguchi R, Yamauchi M, Sugino S, Yamakage M. Ultrasound-aided ipsilateral-dominant epidural block for total hip arthroplasty: a randomised controlled single-blind study. Eur J Anaesthesiol. 2011;28(2):137-140. https://doi.org/10.1097/EJA.0b013e3283423457.
68. Grau T, Leipold RW, Horter J, Martin E, Motsch J. Colour Doppler imaging of the interspinous and epidural space. Eur J Anaesthesiol. 2001;18(11):706-712. https://doi.org/10.1097/00003643-200111000-00002.
69. Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 2007;104(5):1188-92, tables of contents. https://doi.org/10.1213/01.ane.0000250912.66057.41.
70. Chauhan AK, Bhatia R, Agrawal S. Lumbar epidural depth using transverse ultrasound scan and its correlation with loss of resistance technique: a prospective observational study in Indian population. Saudi J Anaesth. 2018;12(2):279-282. https://doi.org/10.4103/sja.SJA_679_17.
71. Gnaho A, Nguyen V, Villevielle T, et al. Assessing the depth of the subarachnoid space by ultrasound. Rev Bras Anestesiol. 2012;62(4):520-530. https://doi.org/10.1016/S0034-7094(12)70150-2.
72. Cork RC, Kryc JJ, Vaughan RW. Ultrasonic localization of the lumbar epidural space. Anesthesiology. 1980;52(6):513-516. https://doi.org/10.1097/00000542-198006000-00013.
73. Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. https://doi.org/10.1212/WNL.0b013e31825dd39d.
74. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. https://doi.org/10.3109/0142159X.2010.509412.
75. Wayne DB, Cohen ER, Singer BD, et al. Progress toward improving medical school graduates’ skills via a “boot camp” curriculum. Simul Healthc. 2014;9(1):33-39. https://doi.org/10.1097/SIH.0000000000000001.
76. Cohen ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern boot camp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a.
77. Martin R, Gannon D, Riggle J, et al. A comprehensive workshop using simulation to train internal medicine residents in bedside procedures performed by internists. Chest. 2012;142(4):545A. https://doi.org/10.1378/chest.1390093.
78. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
79. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1.
80. Restrepo CG, Baker MD, Pruitt CM, Gullett JP, Pigott DC. Ability of pediatric emergency medicine physicians to identify anatomic landmarks with the assistance of ultrasound prior to lumbar puncture in a simulated obese model. Pediatr Emerg Care. 2015;31(1):15-19. https://doi.org/10.1097/PEC.0000000000000330.
81. VanderWielen BA, Harris R, Galgon RE, VanderWielen LM, Schroeder KM. Teaching sonoanatomy to anesthesia faculty and residents: utility of hands-on gel phantom and instructional video training models. J Clin Anesth. 2015;27(3):188-194. https://doi.org/10.1016/j.jclinane.2014.07.007.
82. Keri Z, Sydor D, Ungi T, et al. Computerized training system for ultrasound-guided lumbar puncture on abnormal spine models: a randomized controlled trial. Can J Anaesth. 2015;62(7):777-784. https://doi.org/10.1007/s12630-015-0367-2.
83. Deacon AJ, Melhuishi NS, Terblanche NC. CUSUM method for construction of trainee spinal ultrasound learning curves following standardised teaching. Anaesth Intensive Care. 2014;42(4):480-486. https://doi.org/10.1177/0310057X1404200409.
84. Margarido CB, Arzola C, Balki M, Carvalho JC. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anaesth. 2010;57(2):120-126. https://doi.org/10.1007/s12630-009-9219-2.
85. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
86. Terblanche NC, Arzola C, Wills KE, et al. Standardised training program in spinal ultrasound for epidural insertion: protocol driven versus non-protocol driven teaching approach. Anaesth Intensive Care. 2014;42(4):460-466. https://doi.org/10.1177/0310057X1404200406.
87. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
88. Karmakar MK, Li X, Ho AM, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth. 2009;102(6):845-854. https://doi.org/10.1093/bja/aep079.
89. Tran D, Kamani AA, Al-Attas E, et al. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anaesth. 2010;57(4):313-321. https://doi.org/10.1007/s12630-009-9252-1.
90. Liu Y, Qian W, Ke XJ, Mei W. Real-time ultrasound-guided spinal anesthesia using a new paramedian transverse approach. Curr Med Sci. 2018;38(5):910-913. https://doi.org/10.1007/s11596-018-1961-7.
91. Conroy PH, Luyet C, McCartney CJ, McHardy PG. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol Res Pract. 2013;2013:525818. https://doi.org/10.1155/2013/525818.
92. Brinkmann S, Tang R, Sawka A, Vaghadia H. Single-operator real-time ultrasound-guided spinal injection using SonixGPS™: a case series. Can J Anaesth. 2013;60(9):896-901. https://doi.org/10.1007/s12630-013-9984-9.
93. Niazi AU, Chin KJ, Jin R, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS ultrasound guidance system: a feasibility study. Acta Anaesthesiol Scand. 2014;58(7):875-881. https://doi.org/10.1111/aas.12353.
1. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023.
2. Kroll H, Duszak R, Jr, Nsiah E, et al. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. AJR Am J Roentgenol. 2015;204(1):15-19. https://doi.org/10.2214/AJR.14.12622.
3. Vickers A, Donnelly JP, Moore JX, Wang HE. 263EMF epidemiology of lumbar punctures in hospitalized patients in United States. Ann Emerg Med. 2017;70(4):S104. https://doi.org/10.1016/j.annemergmed.2017.07.241.
4. Køster-Rasmussen R, Korshin A, Meyer CN. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008;57(6):449-454. https://doi.org/10.1016/j.jinf.2008.09.033.
5. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129(11):862-869. https://doi.org/10.7326/0003-4819-129-11_Part_1-199812010-00004.
6. Lepur D, Barsić B. Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection. 2007;35(4):225-231. https://doi.org/10.1007/s15010-007-6202-0.
7. Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98(4):291-298. https://doi.org/10.1093/qjmed/hci047.
8. Auburtin M, Wolff M, Charpentier J, et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit Care Med. 2006;34(11):2758-2765. https://doi.org/10.1097/01.CCM.0000239434.26669.65.
9. Michael B, Menezes BF, Cunniffe J, et al. Effect of delayed lumbar punctures on the diagnosis of acute bacterial meningitis in adults. Emerg Med J. 2010;27(6):433-438. https://doi.org/10.1136/emj.2009.075598.
10. Glimåker M, Johansson B, Grindborg Ö, et al. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis. 2015;60(8):1162-1169. https://doi.org/10.1093/cid/civ011.
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 Revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715.
12. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079.
13. Shah KH, McGillicuddy D, Spear J, Edlow JA. Predicting difficult and traumatic lumbar punctures. Am J Emerg Med. 2007;25(6):608-611. https://doi.org/10.1016/j.ajem.2006.11.025.
14. Williams P, Tait G, Wijeratne T. Success rate of elective lumbar puncture at a major Melbourne neurology unit. Surg Neurol Int. 2018;9:12. https://doi.org/10.4103/sni.sni_426_17.
15. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2018;26(1). https://doi.org/10.1111/acem.13558.
16. Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ. 2013;346:f1720. https://doi.org/10.1136/bmj.f1720.
17. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
18. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
19. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
20. Soni NJ, Franco-Sadud R, Schnobrich D, et al. Ultrasound guidance for lumbar puncture. Neurol Clin Pract. 2016;6(4):358-368. https://doi.org/10.1212/CPJ.0000000000000265.
21. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The Rand/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand Corp; 2001.
22. Abdelhamid SA, Mansour MA. Ultrasound-guided intrathecal anesthesia: does scanning help? Egypt J Anaesth. 2013;29(4):389-394. https://doi.org/10.1016/j.egja.2013.06.003.
23. Ansari T, Yousef A, El Gamassy A, Fayez M. Ultrasound-guided spinal anaesthesia in obstetrics: is there an advantage over the landmark technique in patients with easily palpable spines? Int J Obstet Anesth. 2014;23(3):213-216. https://doi.org/10.1016/j.ijoa.2014.03.001.
24. Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115(1):94-101. https://doi.org/10.1097/ALN.0b013e31821a8ad4.
25. Cho YC, Koo DH, Oh SK, et al. Comparison of ultrasound-assisted lumbar puncture with lumbar puncture using palpation of landmarks in aged patients in an emergency center. J Korean Soc Emerg Med. 2009;20(3):304.
26. Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45(6):766-771. https://doi.org/10.1034/j.1399-6576.2001.045006766.x.
27. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Ultrasound imaging facilitates localization of the epidural space during combined spinal and epidural anesthesia. Reg Anesth Pain Med. 2001;26(1):64-67. https://doi.org/10.1053/rapm.2001.19633.
28. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth. 2002;14(3):169-175. https://doi.org/10.1016/S0952-8180(01)00378-6.
29. Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2004;21(1):25-31. https://doi.org/10.1017/S026502150400105X.
30. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
31. Nassar M, Abdelazim IA. Pre-puncture ultrasound guided epidural insertion before vaginal delivery. J Clin Monit Comput. 2015;29(5):573-577. https://doi.org/10.1007/s10877-014-9634-y.
32. Nomura JT, Leech SJ, Shenbagamurthi S, et al. A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med. 2007;26(10):1341-1348. https://doi.org/10.7863/jum.2007.26.10.1341.
33. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
34. Sahin T, Balaban O, Sahin L, Solak M, Toker K. A randomized controlled trial of preinsertion ultrasound guidance for spinal anaesthesia in pregnancy: outcomes among obese and lean parturients: ultrasound for spinal anesthesia in pregnancy. J Anesth. 2014;28(3):413-419. https://doi.org/10.1007/s00540-013-1726-1.
35. Wang Q, Yin C, Wang TL. Ultrasound facilitates identification of combined spinal-epidural puncture in obese parturients. Chin Med J (Engl). 2012;125(21):3840-3843. PubMed
36. Vallejo MC, Phelps AL, Singh S, Orebaugh SL, Sah N. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19(4):373-378. https://doi.org/10.1016/j.ijoa.2010.04.002.
37. Darrieutort-Laffite C, Bart G, Planche L, et al. Usefulness of a pre-procedure ultrasound scanning of the lumbar spine before epidural injection in patients with a presumed difficult puncture: a randomized controlled trial. Joint Bone Spine. 2015;82(5):356-361. https://doi.org/10.1016/j.jbspin.2015.02.001.
38. Vosko MR, Brunner C, Schreiber S. Lumbar puncture with ultrasound study (lupus study)-international prospective randomized multicentre trial. Int J Stroke. 2017;12(1):22. https://doi.org/10.1055/s-0037-1606991.
39. Urfalioğlu A, Bilal B, Öksüz G, et al. Comparison of the landmark and ultrasound methods in cesarean sections performed under spinal anesthesia on obese pregnants. J Matern Fetal Neonatal Med. 2017;30(9):1051-1056. https://doi.org/10.1080/14767058.2016.1199677.
40. Tawfik MM, Atallah MM, Elkharboutly WS, Allakkany NS, Abdelkhalek M. Does preprocedural ultrasound increase the first-pass success rate of epidural catheterization before cesarean delivery? A randomized controlled trial. Anesth Analg. 2017;124(3):851-856. https://doi.org/10.1213/ANE.0000000000001325.
41. Turkstra TP, Marmai KL, Armstrong KP, Kumar K, Singh SI. Preprocedural ultrasound assessment does not improve trainee performance of spinal anesthesia for obstetrical patients: a randomized controlled trial. J Clin Anesth. 2017;37:21-24. https://doi.org/10.1016/j.jclinane.2016.10.034.
42. Chong SE, Mohd Nikman A, Saedah A, et al. Real-time ultrasound-guided paramedian spinal anaesthesia: evaluation of the efficacy and the success rate of single needle pass. Br J Anaesth. 2017;118(5):799-801. https://doi.org/10.1093/bja/aex108.
43. Creaney M, Mullane D, Casby C, Tan T. Ultrasound to identify the lumbar space in women with impalpable bony landmarks presenting for elective caesarean delivery under spinal anaesthesia: a randomised trial. Int J Obstet Anesth. 2016;28:12-16. https://doi.org/10.1016/j.ijoa.2016.07.007.
44. Ekinci M, Alici HA, Ahiskalioglu A, et al. The use of ultrasound in planned cesarean delivery under spinal anesthesia for patients having nonprominent anatomic landmarks. J Clin Anesth. 2017;37:82-85. https://doi.org/10.1016/j.jclinane.2016.10.014.
45. Perna P, Gioia A, Ragazzi R, Volta CA, Innamorato M. Can pre-procedure neuroaxial ultrasound improve the identification of the potential epidural space when compared with anatomical landmarks? A prospective randomized study. Minerva Anestesiol. 2017;83(1):41-49. https://doi.org/10.23736/S0375-9393.16.11399-9.
46. Chin A, Crooke B, Heywood L, et al. A randomised controlled trial comparing needle movements during combined spinal-epidural anaesthesia with and without ultrasound assistance. Anaesthesia. 2018;73(4):466-473. https://doi.org/10.1111/anae.14206.
47. Dhanger S, Vinayagam S, Vaidhyanathan B, Rajesh IJ, Tripathy DK. Comparison of landmark versus pre-procedural ultrasonography-assisted midline approach for identification of subarachnoid space in elective caesarean section: a randomised controlled trial. Indian J Anaesth. 2018;62(4):280-284. https://doi.org/10.4103/ija.IJA_488_17.
48. Evans DP, Tozer J, Joyce M, Vitto MJ. Comparison of ultrasound-guided and landmark-based lumbar punctures in inexperienced resident physicians. J Ultrasound Med. 2019;38(3):613-620. https://doi.org/10.1002/jum.14728.
49. Srinivasan KK, Leo AM, Iohom G, Loughnane F, Lee PJ. Pre-procedure ultrasound-guided paramedian spinal anaesthesia at L5-S1: is this better than landmark-guided midline approach? A randomised controlled trial. Indian J Anaesth. 2018;62(1):53-60. https://doi.org/10.4103/ija.IJA_448_17.
50. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
51. Lim YC, Choo CY, Tan KT. A randomised controlled trial of ultrasound-assisted spinal anaesthesia. Anaesth Intensive Care. 2014;42(2):191-198. https://doi.org/10.1177/0310057X1404200205.
52. Honarbakhsh S, Osman C, Teo JTH, Gabriel C. Ultrasound-guided lumbar puncture as a diagnostic aid to reduce number of attempts and complication rates. Ultrasound. 2013;21(4):170-175. https://doi.org/10.1177/1742271X13504332.
53. Sahota JS, Carvalho JC, Balki M, Fanning N, Arzola C. Ultrasound estimates for midline epidural punctures in the obese parturient: paramedian sagittal oblique is comparable to transverse median plane. Anesth Analg. 2013;116(4):829-835. https://doi.org/10.1213/ANE.0b013e31827f55f0.
54. Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108(6):1876-1881. https://doi.org/10.1213/ane.0b013e3181a323f6.
55. Wallace DH, Currie JM, Gilstrap LC, Santos R. Indirect sonographic guidance for epidural anesthesia in obese pregnant patients. Reg Anesth. 1992;17(4):233-236. PubMed
56. Srinivasan KK, Iohom G, Loughnane F, Lee PJ. Conventional landmark-guided midline versus preprocedure ultrasound-guided paramedian techniques in spinal anesthesia. Anesth Analg. 2015;21(4):1089-1096. https://doi.org/10.1213/ANE.0000000000000911.
57. Chin KJ, Perlas A, Singh M, et al. An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can J Anaesth. 2009;56(9):643-650. https://doi.org/10.1007/s12630-009-9132-8.
58. Evansa I, Logina I, Vanags I, Borgeat A. Ultrasound versus fluoroscopic-guided epidural steroid injections in patients with degenerative spinal diseases: a randomised study. Eur J Anaesthesiol. 2015;32(4):262-268. https://doi.org/10.1097/EJA.0000000000000103.
59. Park Y, Lee JH, Park KD, et al. Ultrasound-guided vs fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: a prospective, randomized, single-blind clinical study. Am J Phys Med Rehabil. 2013;92(7):575-586. https://doi.org/10.1097/PHM.0b013e318292356b.
60. Margarido CB, Mikhael R, Arzola C, Balki M, Carvalho JC. The intercristal line determined by palpation is not a reliable anatomical landmark for neuraxial anesthesia. Can J Anaesth. 2011;58(3):262-266. https://doi.org/10.1007/s12630-010-9432-z.
61. Duniec L, Nowakowski P, Kosson D, Łazowski T. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther. 2013;45(1):1-6. https://doi.org/10.5603/AIT.2013.0001.
62. Schlotterbeck H, Schaeffer R, Dow WA, et al. Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia. Br J Anaesth. 2008;100(2):230-234. https://doi.org/10.1093/bja/aem371.
63. Whitty R, Moore M, Macarthur A. Identification of the lumbar interspinous spaces: palpation versus ultrasound. Anesth Analg. 2008;106(2):538-540, table of contents. https://doi.org/10.1213/ane.0b013e31816069d9.
64. Locks Gde F, Almeida MC, Pereira AA. Use of the ultrasound to determine the level of lumbar puncture in pregnant women. Rev Bras Anestesiol. 2010;60(1):13-19. https://doi.org/10.1016/S0034-7094(10)70002-7.
65. Stiffler KA, Jwayyed S, Wilber ST, Robinson A. The use of ultrasound to identify pertinent landmarks for lumbar puncture. Am J Emerg Med. 2007;25(3):331-334. https://doi.org/10.1016/j.ajem.2006.07.010.
66. Gulay U, Meltem T, Nadir SS, Aysin A. Ultrasound-guided evaluation of the lumbar subarachnoid space in lateral and sitting positions in pregnant patients to receive elective cesarean operation. Pak J Med Sci. 2015;31(1):76-81. https://doi.org/10.12669/pjms.311.5647.
67. Kawaguchi R, Yamauchi M, Sugino S, Yamakage M. Ultrasound-aided ipsilateral-dominant epidural block for total hip arthroplasty: a randomised controlled single-blind study. Eur J Anaesthesiol. 2011;28(2):137-140. https://doi.org/10.1097/EJA.0b013e3283423457.
68. Grau T, Leipold RW, Horter J, Martin E, Motsch J. Colour Doppler imaging of the interspinous and epidural space. Eur J Anaesthesiol. 2001;18(11):706-712. https://doi.org/10.1097/00003643-200111000-00002.
69. Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 2007;104(5):1188-92, tables of contents. https://doi.org/10.1213/01.ane.0000250912.66057.41.
70. Chauhan AK, Bhatia R, Agrawal S. Lumbar epidural depth using transverse ultrasound scan and its correlation with loss of resistance technique: a prospective observational study in Indian population. Saudi J Anaesth. 2018;12(2):279-282. https://doi.org/10.4103/sja.SJA_679_17.
71. Gnaho A, Nguyen V, Villevielle T, et al. Assessing the depth of the subarachnoid space by ultrasound. Rev Bras Anestesiol. 2012;62(4):520-530. https://doi.org/10.1016/S0034-7094(12)70150-2.
72. Cork RC, Kryc JJ, Vaughan RW. Ultrasonic localization of the lumbar epidural space. Anesthesiology. 1980;52(6):513-516. https://doi.org/10.1097/00000542-198006000-00013.
73. Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. https://doi.org/10.1212/WNL.0b013e31825dd39d.
74. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. https://doi.org/10.3109/0142159X.2010.509412.
75. Wayne DB, Cohen ER, Singer BD, et al. Progress toward improving medical school graduates’ skills via a “boot camp” curriculum. Simul Healthc. 2014;9(1):33-39. https://doi.org/10.1097/SIH.0000000000000001.
76. Cohen ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern boot camp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a.
77. Martin R, Gannon D, Riggle J, et al. A comprehensive workshop using simulation to train internal medicine residents in bedside procedures performed by internists. Chest. 2012;142(4):545A. https://doi.org/10.1378/chest.1390093.
78. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
79. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1.
80. Restrepo CG, Baker MD, Pruitt CM, Gullett JP, Pigott DC. Ability of pediatric emergency medicine physicians to identify anatomic landmarks with the assistance of ultrasound prior to lumbar puncture in a simulated obese model. Pediatr Emerg Care. 2015;31(1):15-19. https://doi.org/10.1097/PEC.0000000000000330.
81. VanderWielen BA, Harris R, Galgon RE, VanderWielen LM, Schroeder KM. Teaching sonoanatomy to anesthesia faculty and residents: utility of hands-on gel phantom and instructional video training models. J Clin Anesth. 2015;27(3):188-194. https://doi.org/10.1016/j.jclinane.2014.07.007.
82. Keri Z, Sydor D, Ungi T, et al. Computerized training system for ultrasound-guided lumbar puncture on abnormal spine models: a randomized controlled trial. Can J Anaesth. 2015;62(7):777-784. https://doi.org/10.1007/s12630-015-0367-2.
83. Deacon AJ, Melhuishi NS, Terblanche NC. CUSUM method for construction of trainee spinal ultrasound learning curves following standardised teaching. Anaesth Intensive Care. 2014;42(4):480-486. https://doi.org/10.1177/0310057X1404200409.
84. Margarido CB, Arzola C, Balki M, Carvalho JC. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anaesth. 2010;57(2):120-126. https://doi.org/10.1007/s12630-009-9219-2.
85. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
86. Terblanche NC, Arzola C, Wills KE, et al. Standardised training program in spinal ultrasound for epidural insertion: protocol driven versus non-protocol driven teaching approach. Anaesth Intensive Care. 2014;42(4):460-466. https://doi.org/10.1177/0310057X1404200406.
87. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
88. Karmakar MK, Li X, Ho AM, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth. 2009;102(6):845-854. https://doi.org/10.1093/bja/aep079.
89. Tran D, Kamani AA, Al-Attas E, et al. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anaesth. 2010;57(4):313-321. https://doi.org/10.1007/s12630-009-9252-1.
90. Liu Y, Qian W, Ke XJ, Mei W. Real-time ultrasound-guided spinal anesthesia using a new paramedian transverse approach. Curr Med Sci. 2018;38(5):910-913. https://doi.org/10.1007/s11596-018-1961-7.
91. Conroy PH, Luyet C, McCartney CJ, McHardy PG. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol Res Pract. 2013;2013:525818. https://doi.org/10.1155/2013/525818.
92. Brinkmann S, Tang R, Sawka A, Vaghadia H. Single-operator real-time ultrasound-guided spinal injection using SonixGPS™: a case series. Can J Anaesth. 2013;60(9):896-901. https://doi.org/10.1007/s12630-013-9984-9.
93. Niazi AU, Chin KJ, Jin R, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS ultrasound guidance system: a feasibility study. Acta Anaesthesiol Scand. 2014;58(7):875-881. https://doi.org/10.1111/aas.12353.
© 2019 Society of Hospital Medicine
IVC filter placement increases mortality in some VTE patients
Clinical question: How does inferior vena cava (IVC) filter placement affect 30-day mortality in patients with venous thromboembolism (VTE) with increased risk of bleeding when anticoagulation is not feasible?
Background: Standard treatment for VTE, including deep venous thrombosis (DVT) and pulmonary embolism (PE), is anticoagulation. However, for patients with active bleeding or increased risk of bleeding, anticoagulation may be contraindicated. In these circumstances, placing an IVC filter is recommended by major professional societies; however, the mortality benefit of IVC filter placement is uncertain.
Study design: A retrospective cohort study.
Setting: State Inpatient and Emergency Department Databases from California, Florida, and New York hospitals from 2005 to 2012.
Synopsis: The authors compared the 30-day mortality rates in 45,771 hospitalized adult patients with inpatient diagnosis codes of PE and/or DVT, as well as a contraindication to anticoagulation, who underwent IVC filter placement with 80,259 similar patients who did not undergo IVC filter placement. Baseline characteristics and coexisting conditions were similar in the two populations. The authors found that patients with IVC filter placement had an increased risk of 30-day mortality, compared with patients without an IVC filter placed (HR, 1.18; 95% CI, 1.13-1.22; P less than .001).
This study used observational data derived from reimbursement codes, which lacked unmeasured confounders (for example, severity of VTE and fragility score), so randomized, controlled trials are required to confirm the results. Nevertheless, this study should prompt physicians to carefully consider decisions to place an IVC filter in the setting of a contraindication to anticoagulation.
Bottom line: IVC filter placement in patients with VTE and contraindication for anticoagulation was associated with an increased 30-day mortality. Randomized, controlled trials are required to confirm the observed results.
Citation: Turner TE et al. Association of inferior vena cava filter placement for venous thromboembolic disease and a contraindication to anticoagulation with 30-day mortality. JAMA Network Open. 2018;1(3):e180452.
Dr. Kobaidze is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Clinical question: How does inferior vena cava (IVC) filter placement affect 30-day mortality in patients with venous thromboembolism (VTE) with increased risk of bleeding when anticoagulation is not feasible?
Background: Standard treatment for VTE, including deep venous thrombosis (DVT) and pulmonary embolism (PE), is anticoagulation. However, for patients with active bleeding or increased risk of bleeding, anticoagulation may be contraindicated. In these circumstances, placing an IVC filter is recommended by major professional societies; however, the mortality benefit of IVC filter placement is uncertain.
Study design: A retrospective cohort study.
Setting: State Inpatient and Emergency Department Databases from California, Florida, and New York hospitals from 2005 to 2012.
Synopsis: The authors compared the 30-day mortality rates in 45,771 hospitalized adult patients with inpatient diagnosis codes of PE and/or DVT, as well as a contraindication to anticoagulation, who underwent IVC filter placement with 80,259 similar patients who did not undergo IVC filter placement. Baseline characteristics and coexisting conditions were similar in the two populations. The authors found that patients with IVC filter placement had an increased risk of 30-day mortality, compared with patients without an IVC filter placed (HR, 1.18; 95% CI, 1.13-1.22; P less than .001).
This study used observational data derived from reimbursement codes, which lacked unmeasured confounders (for example, severity of VTE and fragility score), so randomized, controlled trials are required to confirm the results. Nevertheless, this study should prompt physicians to carefully consider decisions to place an IVC filter in the setting of a contraindication to anticoagulation.
Bottom line: IVC filter placement in patients with VTE and contraindication for anticoagulation was associated with an increased 30-day mortality. Randomized, controlled trials are required to confirm the observed results.
Citation: Turner TE et al. Association of inferior vena cava filter placement for venous thromboembolic disease and a contraindication to anticoagulation with 30-day mortality. JAMA Network Open. 2018;1(3):e180452.
Dr. Kobaidze is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Clinical question: How does inferior vena cava (IVC) filter placement affect 30-day mortality in patients with venous thromboembolism (VTE) with increased risk of bleeding when anticoagulation is not feasible?
Background: Standard treatment for VTE, including deep venous thrombosis (DVT) and pulmonary embolism (PE), is anticoagulation. However, for patients with active bleeding or increased risk of bleeding, anticoagulation may be contraindicated. In these circumstances, placing an IVC filter is recommended by major professional societies; however, the mortality benefit of IVC filter placement is uncertain.
Study design: A retrospective cohort study.
Setting: State Inpatient and Emergency Department Databases from California, Florida, and New York hospitals from 2005 to 2012.
Synopsis: The authors compared the 30-day mortality rates in 45,771 hospitalized adult patients with inpatient diagnosis codes of PE and/or DVT, as well as a contraindication to anticoagulation, who underwent IVC filter placement with 80,259 similar patients who did not undergo IVC filter placement. Baseline characteristics and coexisting conditions were similar in the two populations. The authors found that patients with IVC filter placement had an increased risk of 30-day mortality, compared with patients without an IVC filter placed (HR, 1.18; 95% CI, 1.13-1.22; P less than .001).
This study used observational data derived from reimbursement codes, which lacked unmeasured confounders (for example, severity of VTE and fragility score), so randomized, controlled trials are required to confirm the results. Nevertheless, this study should prompt physicians to carefully consider decisions to place an IVC filter in the setting of a contraindication to anticoagulation.
Bottom line: IVC filter placement in patients with VTE and contraindication for anticoagulation was associated with an increased 30-day mortality. Randomized, controlled trials are required to confirm the observed results.
Citation: Turner TE et al. Association of inferior vena cava filter placement for venous thromboembolic disease and a contraindication to anticoagulation with 30-day mortality. JAMA Network Open. 2018;1(3):e180452.
Dr. Kobaidze is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Conference highlights growing HAI concerns
The Fifth Decennial International Conference on Healthcare-Associated Infections 2010, held in March in Atlanta, featured experts from several different fields discussing the significant prevalence of healthcare-associated infections (HAIs) and strategies that may be implemented to reduce their occurrence.
HAIs precipitated by the use of such devices as central venous catheters (CVCs), mechanical ventilators, and indwelling urinary catheters received special emphasis as important sources of patient morbidity and mortality.
Naomi O’Grady of the National Institutes of Health (NIH) summarized the current available knowledge regarding the prevention of central-line-associated bloodstream infections (CLABSIs). Strategies targeting appropriate line maintenance include:
- Chlorhexidine sponge dressings at the CVC insertion site in patients with short-term catheters;
- Cleanse catheter hubs and connectors with alcoholic-chlorhexidine (rather than alcohol alone) after each use; and
- Consider daily bathing of patients with chlorhexidine soap.
Speakers stressed that novel technologies, such as antimicrobial lock solutions and antiseptic- or antibiotic-impregnated catheters, should be considered when CLABSI rates remain high. Mark Shelly, MD, of Rochester, N.Y., emphasized awareness that CLABSIs occur frequently outside the ICU. “If you are only looking for CLABSI in the ICU, then you are missing more than half of the story,” Dr. Shelly said. Researchers from the National Health Safety Network (NHSN) provided more information about the substantial numbers of CLABSIs that occur on general medical wards.
Carolyn Gould, MD, MS, of the Centers for Disease Control and Prevention (CDC) confirmed that catheter-associated urinary tract infections (CAUTIs) are the most common type of HAI. CAUTIs occur at a frequency of >560,000 infections per year and cost as much as $500 million per year, she explained. Strategies to prevent CAUTIs include inserting urinary catheters only for appropriate indications and leaving them in place for the shortest possible duration.
In recent years, concern has grown about the prevalence of healthcare-associated Clostridium difficile infection (HA-CDI), which can lead to uncomplicated diarrhea, sepsis, or even death. Several speakers described strategies that reduce HA-CDI development, including the identification and removal of environmental sources of C. diff, accommodating CDI patients in a private room with contact precautions, and minimizing both the frequency and duration of antimicrobial therapy.
Uncertainty about the most reliable tests to confirm CDI was a topic of focus. Enzyme immunoassay (EIA) testing, cell cytotoxin assays, and polymerase chain reaction (PCR) testing are readily available in most U.S. hospitals; however, PCR testing might prove to be the most advantageous since it is rapid, sensitive, and specific.
Neil Fishman, MD, of the University of Pennsylvania School of Medicine in Philadelphia was one of several speakers to address the important role of antimicrobial stewardship program (ASP) development. According to Dr. Fishman, ASP goals should be to “ensure the proper use of antimicrobials” and to “promote cost-effectiveness.” By taking actions that promote the appropriate use of antimicrobials, the following positive consequences can be anticipated:
- Improved clinical outcomes;
- Reduced risk of adverse drug effects; and
- A reduction in, or stabilization of, the rate of antimicrobial resistance.
Multidrug-resistant (MDR) gram-negative Bacillus is a major challenge for hospitals worldwide. The CDC offers two guidelines for the optimal management and isolation of MDR organisms (MDRO): HICPAC 2006 (a management guideline) and HICPAC 2007 (MDRO isolation precaution guidelines). Consistent utilization of these guidelines is crucial to control the spread of MDRO.
The CDC’s Alexander Killen, MD, discussed the increasing proportion of MDR Acinetobacter and Enterobacteriaceae. Emerging issues among these organisms include the development of highly resistant strains, the incidence of which is increasing in nonacute-care settings.
The CDC’s Karen Anderson reported laboratory data on carbapenem-resistant Enterobacteriaceae (CRE) in a long-term-care facility. Her team demonstrated that CRE colonization can persist for up to six months. She speculated that the transfer of resistance between different species occurs, as does patient-to-patient transmission.
The CDC recommends the use of surveillance cultures as part of enhanced precautions. Surveillance is to continue until no new cases are detected.
Karen Clarke, MD, MS, MPH
Ketino Kobaidze, MD, PhD
Mohamad Moussa, MD
Sheri Tejedor, MD
Emory University
School of Medicine, Atlanta
The Fifth Decennial International Conference on Healthcare-Associated Infections 2010, held in March in Atlanta, featured experts from several different fields discussing the significant prevalence of healthcare-associated infections (HAIs) and strategies that may be implemented to reduce their occurrence.
HAIs precipitated by the use of such devices as central venous catheters (CVCs), mechanical ventilators, and indwelling urinary catheters received special emphasis as important sources of patient morbidity and mortality.
Naomi O’Grady of the National Institutes of Health (NIH) summarized the current available knowledge regarding the prevention of central-line-associated bloodstream infections (CLABSIs). Strategies targeting appropriate line maintenance include:
- Chlorhexidine sponge dressings at the CVC insertion site in patients with short-term catheters;
- Cleanse catheter hubs and connectors with alcoholic-chlorhexidine (rather than alcohol alone) after each use; and
- Consider daily bathing of patients with chlorhexidine soap.
Speakers stressed that novel technologies, such as antimicrobial lock solutions and antiseptic- or antibiotic-impregnated catheters, should be considered when CLABSI rates remain high. Mark Shelly, MD, of Rochester, N.Y., emphasized awareness that CLABSIs occur frequently outside the ICU. “If you are only looking for CLABSI in the ICU, then you are missing more than half of the story,” Dr. Shelly said. Researchers from the National Health Safety Network (NHSN) provided more information about the substantial numbers of CLABSIs that occur on general medical wards.
Carolyn Gould, MD, MS, of the Centers for Disease Control and Prevention (CDC) confirmed that catheter-associated urinary tract infections (CAUTIs) are the most common type of HAI. CAUTIs occur at a frequency of >560,000 infections per year and cost as much as $500 million per year, she explained. Strategies to prevent CAUTIs include inserting urinary catheters only for appropriate indications and leaving them in place for the shortest possible duration.
In recent years, concern has grown about the prevalence of healthcare-associated Clostridium difficile infection (HA-CDI), which can lead to uncomplicated diarrhea, sepsis, or even death. Several speakers described strategies that reduce HA-CDI development, including the identification and removal of environmental sources of C. diff, accommodating CDI patients in a private room with contact precautions, and minimizing both the frequency and duration of antimicrobial therapy.
Uncertainty about the most reliable tests to confirm CDI was a topic of focus. Enzyme immunoassay (EIA) testing, cell cytotoxin assays, and polymerase chain reaction (PCR) testing are readily available in most U.S. hospitals; however, PCR testing might prove to be the most advantageous since it is rapid, sensitive, and specific.
Neil Fishman, MD, of the University of Pennsylvania School of Medicine in Philadelphia was one of several speakers to address the important role of antimicrobial stewardship program (ASP) development. According to Dr. Fishman, ASP goals should be to “ensure the proper use of antimicrobials” and to “promote cost-effectiveness.” By taking actions that promote the appropriate use of antimicrobials, the following positive consequences can be anticipated:
- Improved clinical outcomes;
- Reduced risk of adverse drug effects; and
- A reduction in, or stabilization of, the rate of antimicrobial resistance.
Multidrug-resistant (MDR) gram-negative Bacillus is a major challenge for hospitals worldwide. The CDC offers two guidelines for the optimal management and isolation of MDR organisms (MDRO): HICPAC 2006 (a management guideline) and HICPAC 2007 (MDRO isolation precaution guidelines). Consistent utilization of these guidelines is crucial to control the spread of MDRO.
The CDC’s Alexander Killen, MD, discussed the increasing proportion of MDR Acinetobacter and Enterobacteriaceae. Emerging issues among these organisms include the development of highly resistant strains, the incidence of which is increasing in nonacute-care settings.
The CDC’s Karen Anderson reported laboratory data on carbapenem-resistant Enterobacteriaceae (CRE) in a long-term-care facility. Her team demonstrated that CRE colonization can persist for up to six months. She speculated that the transfer of resistance between different species occurs, as does patient-to-patient transmission.
The CDC recommends the use of surveillance cultures as part of enhanced precautions. Surveillance is to continue until no new cases are detected.
Karen Clarke, MD, MS, MPH
Ketino Kobaidze, MD, PhD
Mohamad Moussa, MD
Sheri Tejedor, MD
Emory University
School of Medicine, Atlanta
The Fifth Decennial International Conference on Healthcare-Associated Infections 2010, held in March in Atlanta, featured experts from several different fields discussing the significant prevalence of healthcare-associated infections (HAIs) and strategies that may be implemented to reduce their occurrence.
HAIs precipitated by the use of such devices as central venous catheters (CVCs), mechanical ventilators, and indwelling urinary catheters received special emphasis as important sources of patient morbidity and mortality.
Naomi O’Grady of the National Institutes of Health (NIH) summarized the current available knowledge regarding the prevention of central-line-associated bloodstream infections (CLABSIs). Strategies targeting appropriate line maintenance include:
- Chlorhexidine sponge dressings at the CVC insertion site in patients with short-term catheters;
- Cleanse catheter hubs and connectors with alcoholic-chlorhexidine (rather than alcohol alone) after each use; and
- Consider daily bathing of patients with chlorhexidine soap.
Speakers stressed that novel technologies, such as antimicrobial lock solutions and antiseptic- or antibiotic-impregnated catheters, should be considered when CLABSI rates remain high. Mark Shelly, MD, of Rochester, N.Y., emphasized awareness that CLABSIs occur frequently outside the ICU. “If you are only looking for CLABSI in the ICU, then you are missing more than half of the story,” Dr. Shelly said. Researchers from the National Health Safety Network (NHSN) provided more information about the substantial numbers of CLABSIs that occur on general medical wards.
Carolyn Gould, MD, MS, of the Centers for Disease Control and Prevention (CDC) confirmed that catheter-associated urinary tract infections (CAUTIs) are the most common type of HAI. CAUTIs occur at a frequency of >560,000 infections per year and cost as much as $500 million per year, she explained. Strategies to prevent CAUTIs include inserting urinary catheters only for appropriate indications and leaving them in place for the shortest possible duration.
In recent years, concern has grown about the prevalence of healthcare-associated Clostridium difficile infection (HA-CDI), which can lead to uncomplicated diarrhea, sepsis, or even death. Several speakers described strategies that reduce HA-CDI development, including the identification and removal of environmental sources of C. diff, accommodating CDI patients in a private room with contact precautions, and minimizing both the frequency and duration of antimicrobial therapy.
Uncertainty about the most reliable tests to confirm CDI was a topic of focus. Enzyme immunoassay (EIA) testing, cell cytotoxin assays, and polymerase chain reaction (PCR) testing are readily available in most U.S. hospitals; however, PCR testing might prove to be the most advantageous since it is rapid, sensitive, and specific.
Neil Fishman, MD, of the University of Pennsylvania School of Medicine in Philadelphia was one of several speakers to address the important role of antimicrobial stewardship program (ASP) development. According to Dr. Fishman, ASP goals should be to “ensure the proper use of antimicrobials” and to “promote cost-effectiveness.” By taking actions that promote the appropriate use of antimicrobials, the following positive consequences can be anticipated:
- Improved clinical outcomes;
- Reduced risk of adverse drug effects; and
- A reduction in, or stabilization of, the rate of antimicrobial resistance.
Multidrug-resistant (MDR) gram-negative Bacillus is a major challenge for hospitals worldwide. The CDC offers two guidelines for the optimal management and isolation of MDR organisms (MDRO): HICPAC 2006 (a management guideline) and HICPAC 2007 (MDRO isolation precaution guidelines). Consistent utilization of these guidelines is crucial to control the spread of MDRO.
The CDC’s Alexander Killen, MD, discussed the increasing proportion of MDR Acinetobacter and Enterobacteriaceae. Emerging issues among these organisms include the development of highly resistant strains, the incidence of which is increasing in nonacute-care settings.
The CDC’s Karen Anderson reported laboratory data on carbapenem-resistant Enterobacteriaceae (CRE) in a long-term-care facility. Her team demonstrated that CRE colonization can persist for up to six months. She speculated that the transfer of resistance between different species occurs, as does patient-to-patient transmission.
The CDC recommends the use of surveillance cultures as part of enhanced precautions. Surveillance is to continue until no new cases are detected.
Karen Clarke, MD, MS, MPH
Ketino Kobaidze, MD, PhD
Mohamad Moussa, MD
Sheri Tejedor, MD
Emory University
School of Medicine, Atlanta