User login
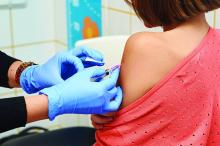
CDC now offering CME course on HPV vaccination
The Centers for Disease Control and Prevention is now offering a CME course to educate clinicians about the importance of human papillomavirus (HPV) vaccination in protecting adolescents from certain types of cancer and to provide them with the skills and resources to make effective HPV vaccine recommendations.
The course is a Web-on-demand video that will teach clinicians how to be successful in making HPV vaccination recommendations, how to communicate HPV vaccination information to parents and patients, and how to properly answer parents’ questions. Currently, the CDC recommends the HPV vaccine for adolescents at 11- to 12-years-of-age. The CDC hopes this will reduce missed opportunities to protect patients against HPV.
Speakers in the video include Alix Casler, MD, of the Orlando Family Physician Association; Linda Fu, MD, MS, of Children’s National Health System in Washington; Todd Wolynn, MD, president and CEO of Kids Plus Pediatrics, Pittsburgh; and Wendy Sue Swanson, MD, MBE, a pediatrician who is chief of digital innovation at Seattle Children’s Hospital.
The course was initiated in Jan. 16, 2018, and will continue until Jan. 16, 2020. Anyone who provides immunization to patients can participate.
The course is called “Routinely Recommending Cancer Prevention: HPV Vaccination at 11 and 12 as a Standard of Care.” Read more about the course on and download it from the CDC’s website.
The Centers for Disease Control and Prevention is now offering a CME course to educate clinicians about the importance of human papillomavirus (HPV) vaccination in protecting adolescents from certain types of cancer and to provide them with the skills and resources to make effective HPV vaccine recommendations.
The course is a Web-on-demand video that will teach clinicians how to be successful in making HPV vaccination recommendations, how to communicate HPV vaccination information to parents and patients, and how to properly answer parents’ questions. Currently, the CDC recommends the HPV vaccine for adolescents at 11- to 12-years-of-age. The CDC hopes this will reduce missed opportunities to protect patients against HPV.
Speakers in the video include Alix Casler, MD, of the Orlando Family Physician Association; Linda Fu, MD, MS, of Children’s National Health System in Washington; Todd Wolynn, MD, president and CEO of Kids Plus Pediatrics, Pittsburgh; and Wendy Sue Swanson, MD, MBE, a pediatrician who is chief of digital innovation at Seattle Children’s Hospital.
The course was initiated in Jan. 16, 2018, and will continue until Jan. 16, 2020. Anyone who provides immunization to patients can participate.
The course is called “Routinely Recommending Cancer Prevention: HPV Vaccination at 11 and 12 as a Standard of Care.” Read more about the course on and download it from the CDC’s website.
The Centers for Disease Control and Prevention is now offering a CME course to educate clinicians about the importance of human papillomavirus (HPV) vaccination in protecting adolescents from certain types of cancer and to provide them with the skills and resources to make effective HPV vaccine recommendations.
The course is a Web-on-demand video that will teach clinicians how to be successful in making HPV vaccination recommendations, how to communicate HPV vaccination information to parents and patients, and how to properly answer parents’ questions. Currently, the CDC recommends the HPV vaccine for adolescents at 11- to 12-years-of-age. The CDC hopes this will reduce missed opportunities to protect patients against HPV.
Speakers in the video include Alix Casler, MD, of the Orlando Family Physician Association; Linda Fu, MD, MS, of Children’s National Health System in Washington; Todd Wolynn, MD, president and CEO of Kids Plus Pediatrics, Pittsburgh; and Wendy Sue Swanson, MD, MBE, a pediatrician who is chief of digital innovation at Seattle Children’s Hospital.
The course was initiated in Jan. 16, 2018, and will continue until Jan. 16, 2020. Anyone who provides immunization to patients can participate.
The course is called “Routinely Recommending Cancer Prevention: HPV Vaccination at 11 and 12 as a Standard of Care.” Read more about the course on and download it from the CDC’s website.
CDC releases website for ME/CFS information
The Centers for Disease Control and Prevention has released a website for health care providers about myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
The website is designed to help clinicians by offering information about how they can better assess and help their patients manage the illness. The website includes information about the presentation and clinical course of ME/CFS, diagnosis, clinical care, understanding historical case definitions and criteria, and free continuing education.
The website’s resource section includes the National Academy of Medicine report published in 2015, as well as primers and clinical practice guidelines.
Currently, it is estimated that 836,000 to 2.5 million Americans have ME/CFS. Roughly 90% of people who have ME/CFS have not been diagnosed.
The Centers for Disease Control and Prevention has released a website for health care providers about myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
The website is designed to help clinicians by offering information about how they can better assess and help their patients manage the illness. The website includes information about the presentation and clinical course of ME/CFS, diagnosis, clinical care, understanding historical case definitions and criteria, and free continuing education.
The website’s resource section includes the National Academy of Medicine report published in 2015, as well as primers and clinical practice guidelines.
Currently, it is estimated that 836,000 to 2.5 million Americans have ME/CFS. Roughly 90% of people who have ME/CFS have not been diagnosed.
The Centers for Disease Control and Prevention has released a website for health care providers about myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
The website is designed to help clinicians by offering information about how they can better assess and help their patients manage the illness. The website includes information about the presentation and clinical course of ME/CFS, diagnosis, clinical care, understanding historical case definitions and criteria, and free continuing education.
The website’s resource section includes the National Academy of Medicine report published in 2015, as well as primers and clinical practice guidelines.
Currently, it is estimated that 836,000 to 2.5 million Americans have ME/CFS. Roughly 90% of people who have ME/CFS have not been diagnosed.
FDA expands Xeljanz approval to certain adults with ulcerative colitis
In two 8-week placebo-controlled trials, 10 mg of Xeljanz given twice daily induced remissions in 17%-18% of patients. In a placebo-controlled trial among the patients who responded by week 8, Xeljanz, at a 5-mg or 10-mg dose given twice daily, was effective in inducing remission by week 52 in 34% and 41% of patients, respectively. Additionally, 35% and 47% of those patients sustained corticosteroid-free remissions when treated with 5-mg and 10-mg doses, respectively.
“New treatments are needed for patients with moderately to severely active ulcerative colitis,” said Julie Beitz, MD, director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research in a press release. “Today’s approval provides an alternative therapy for a debilitating disease with limited treatment options.”
Xeljanz is the first oral medication approved for chronic use in moderately to severely active UC. The FDA states that other FDA-approved treatments for the chronic treatment of moderately to severely active ulcerative colitis must be administered through an intravenous infusion or subcutaneous injection.
Xeljanz, made by Pfizer Labs, was previously approved in 2012 for rheumatoid arthritis and in 2017 for psoriatic arthritis.
Find the full press release on the FDA’s website.
In two 8-week placebo-controlled trials, 10 mg of Xeljanz given twice daily induced remissions in 17%-18% of patients. In a placebo-controlled trial among the patients who responded by week 8, Xeljanz, at a 5-mg or 10-mg dose given twice daily, was effective in inducing remission by week 52 in 34% and 41% of patients, respectively. Additionally, 35% and 47% of those patients sustained corticosteroid-free remissions when treated with 5-mg and 10-mg doses, respectively.
“New treatments are needed for patients with moderately to severely active ulcerative colitis,” said Julie Beitz, MD, director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research in a press release. “Today’s approval provides an alternative therapy for a debilitating disease with limited treatment options.”
Xeljanz is the first oral medication approved for chronic use in moderately to severely active UC. The FDA states that other FDA-approved treatments for the chronic treatment of moderately to severely active ulcerative colitis must be administered through an intravenous infusion or subcutaneous injection.
Xeljanz, made by Pfizer Labs, was previously approved in 2012 for rheumatoid arthritis and in 2017 for psoriatic arthritis.
Find the full press release on the FDA’s website.
In two 8-week placebo-controlled trials, 10 mg of Xeljanz given twice daily induced remissions in 17%-18% of patients. In a placebo-controlled trial among the patients who responded by week 8, Xeljanz, at a 5-mg or 10-mg dose given twice daily, was effective in inducing remission by week 52 in 34% and 41% of patients, respectively. Additionally, 35% and 47% of those patients sustained corticosteroid-free remissions when treated with 5-mg and 10-mg doses, respectively.
“New treatments are needed for patients with moderately to severely active ulcerative colitis,” said Julie Beitz, MD, director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research in a press release. “Today’s approval provides an alternative therapy for a debilitating disease with limited treatment options.”
Xeljanz is the first oral medication approved for chronic use in moderately to severely active UC. The FDA states that other FDA-approved treatments for the chronic treatment of moderately to severely active ulcerative colitis must be administered through an intravenous infusion or subcutaneous injection.
Xeljanz, made by Pfizer Labs, was previously approved in 2012 for rheumatoid arthritis and in 2017 for psoriatic arthritis.
Find the full press release on the FDA’s website.
Suicidal ideation, maladaptive beliefs appear linked in some psychotic disorders
Maladaptive beliefs and face emotion processing appear associated with suicidal ideation and behavior in psychosis, results of a retrospective, cross-sectional study suggest.
“The present findings suggest that maladaptive beliefs are associated with a tendency to misperceive neutral stimuli as threatening, and are associated with suicidal ideation and behavior,” wrote Jennifer Villa of San Diego State University and her associates.
In the study, published in the Journal of Psychiatric Research, 101 outpatients aged 18 and older with psychotic disorders were assessed via the Interpersonal Needs Questionnaire–15 (INQ-15) and the Penn Emotion Recognition Task (ER-40). The participants also were assessed via several other measures, including the Modified Scale for Suicidal Ideation (MSSI), reported Ms. Villa. The INQ-15, a self-report measure, assesses interpersonal beliefs that underlie the desire for suicide. The MSSI, an 18-item instrument, measures the presence of ideation in the previous 48 hours.
Ms. Villa and her associates found that , compared with those without any past history of attempts or current ideation. In addition, MSSI total scores were correlated with INQ total scores (P = .002). When comparing disorders, patients with bipolar disorder with MSSI current ideation had higher INQ total scores than did patients without ideation (P = .010) vs. no MSSI current ideation.
They cited several limitations. One is that the findings might not be generalizable, because causal relationships between maladaptive beliefs, emotion recognition, or the risk of suicidal ideation or behavior cannot be inferred. Also, because most of the patients in the sample were middle-aged adults, the findings might not apply to patients who are experiencing first-episode psychosis. Nevertheless, they said, “the results are of clinical interest demonstrating the growing importance of social cognition to the cumulative evaluation of suicide risk in psychosis and identification of potential targets for suicide prevention.”
Ms. Villa reported no conflicts of interest. Two of the authors reported relationships with several pharmaceutical companies.
SOURCE: Villa J et al. J Psychiatr Res. 2018 Ma;100:107-12.
Maladaptive beliefs and face emotion processing appear associated with suicidal ideation and behavior in psychosis, results of a retrospective, cross-sectional study suggest.
“The present findings suggest that maladaptive beliefs are associated with a tendency to misperceive neutral stimuli as threatening, and are associated with suicidal ideation and behavior,” wrote Jennifer Villa of San Diego State University and her associates.
In the study, published in the Journal of Psychiatric Research, 101 outpatients aged 18 and older with psychotic disorders were assessed via the Interpersonal Needs Questionnaire–15 (INQ-15) and the Penn Emotion Recognition Task (ER-40). The participants also were assessed via several other measures, including the Modified Scale for Suicidal Ideation (MSSI), reported Ms. Villa. The INQ-15, a self-report measure, assesses interpersonal beliefs that underlie the desire for suicide. The MSSI, an 18-item instrument, measures the presence of ideation in the previous 48 hours.
Ms. Villa and her associates found that , compared with those without any past history of attempts or current ideation. In addition, MSSI total scores were correlated with INQ total scores (P = .002). When comparing disorders, patients with bipolar disorder with MSSI current ideation had higher INQ total scores than did patients without ideation (P = .010) vs. no MSSI current ideation.
They cited several limitations. One is that the findings might not be generalizable, because causal relationships between maladaptive beliefs, emotion recognition, or the risk of suicidal ideation or behavior cannot be inferred. Also, because most of the patients in the sample were middle-aged adults, the findings might not apply to patients who are experiencing first-episode psychosis. Nevertheless, they said, “the results are of clinical interest demonstrating the growing importance of social cognition to the cumulative evaluation of suicide risk in psychosis and identification of potential targets for suicide prevention.”
Ms. Villa reported no conflicts of interest. Two of the authors reported relationships with several pharmaceutical companies.
SOURCE: Villa J et al. J Psychiatr Res. 2018 Ma;100:107-12.
Maladaptive beliefs and face emotion processing appear associated with suicidal ideation and behavior in psychosis, results of a retrospective, cross-sectional study suggest.
“The present findings suggest that maladaptive beliefs are associated with a tendency to misperceive neutral stimuli as threatening, and are associated with suicidal ideation and behavior,” wrote Jennifer Villa of San Diego State University and her associates.
In the study, published in the Journal of Psychiatric Research, 101 outpatients aged 18 and older with psychotic disorders were assessed via the Interpersonal Needs Questionnaire–15 (INQ-15) and the Penn Emotion Recognition Task (ER-40). The participants also were assessed via several other measures, including the Modified Scale for Suicidal Ideation (MSSI), reported Ms. Villa. The INQ-15, a self-report measure, assesses interpersonal beliefs that underlie the desire for suicide. The MSSI, an 18-item instrument, measures the presence of ideation in the previous 48 hours.
Ms. Villa and her associates found that , compared with those without any past history of attempts or current ideation. In addition, MSSI total scores were correlated with INQ total scores (P = .002). When comparing disorders, patients with bipolar disorder with MSSI current ideation had higher INQ total scores than did patients without ideation (P = .010) vs. no MSSI current ideation.
They cited several limitations. One is that the findings might not be generalizable, because causal relationships between maladaptive beliefs, emotion recognition, or the risk of suicidal ideation or behavior cannot be inferred. Also, because most of the patients in the sample were middle-aged adults, the findings might not apply to patients who are experiencing first-episode psychosis. Nevertheless, they said, “the results are of clinical interest demonstrating the growing importance of social cognition to the cumulative evaluation of suicide risk in psychosis and identification of potential targets for suicide prevention.”
Ms. Villa reported no conflicts of interest. Two of the authors reported relationships with several pharmaceutical companies.
SOURCE: Villa J et al. J Psychiatr Res. 2018 Ma;100:107-12.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Behavioral sleep intervention linked to sleep improvement in infants
(BSI) in a real-world setting, Sarah M. Honaker, PhD, of Indiana University, Indianapolis, and her associates, reported in the Journal of Pediatrics.
In a study of 652 parents who participated, parents started BSI when their infants were as young as less than 1 month of age and as late as 18 months of age. Most parents started BSI at 3-5 months.
Crying generally was greatest the first night, occurring in 45% of cases when all BSI approaches were considered. It lasted a mean 43 minutes, which dropped significantly after 1 week to a mean 9 minutes (P less than .001). Crying was considered most intense (on a 1-5 scale) on the initial night of BSI, a mean 4.42, and this “was equally true for all of the BSI approaches,” Dr. Honaker and her colleagues wrote.
In most cases, the parents’ first attempt at BSI worked (83%). Success varied by BSI approach, with the highest first attempt success rate in the unmodified extinction group (90%), followed by parental presence without support (83%), modified extinction (81%), and parental presence with support (65%). Eventually, 27% of parents were successful with a different approach than the one with which they started. Most commonly, they changed from modified extinction to unmodified extinction (66% of those who changed approaches).
“The majority of parents report successfully implementing BSI at a variety of ages across infancy, primarily using extinction-based approaches,” the researchers concluded. “Few significant differences were found between approaches, suggesting that health providers should offer parents options for BSI implementation.”
SOURCE: Honaker SM et al., J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.04.009.
(BSI) in a real-world setting, Sarah M. Honaker, PhD, of Indiana University, Indianapolis, and her associates, reported in the Journal of Pediatrics.
In a study of 652 parents who participated, parents started BSI when their infants were as young as less than 1 month of age and as late as 18 months of age. Most parents started BSI at 3-5 months.
Crying generally was greatest the first night, occurring in 45% of cases when all BSI approaches were considered. It lasted a mean 43 minutes, which dropped significantly after 1 week to a mean 9 minutes (P less than .001). Crying was considered most intense (on a 1-5 scale) on the initial night of BSI, a mean 4.42, and this “was equally true for all of the BSI approaches,” Dr. Honaker and her colleagues wrote.
In most cases, the parents’ first attempt at BSI worked (83%). Success varied by BSI approach, with the highest first attempt success rate in the unmodified extinction group (90%), followed by parental presence without support (83%), modified extinction (81%), and parental presence with support (65%). Eventually, 27% of parents were successful with a different approach than the one with which they started. Most commonly, they changed from modified extinction to unmodified extinction (66% of those who changed approaches).
“The majority of parents report successfully implementing BSI at a variety of ages across infancy, primarily using extinction-based approaches,” the researchers concluded. “Few significant differences were found between approaches, suggesting that health providers should offer parents options for BSI implementation.”
SOURCE: Honaker SM et al., J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.04.009.
(BSI) in a real-world setting, Sarah M. Honaker, PhD, of Indiana University, Indianapolis, and her associates, reported in the Journal of Pediatrics.
In a study of 652 parents who participated, parents started BSI when their infants were as young as less than 1 month of age and as late as 18 months of age. Most parents started BSI at 3-5 months.
Crying generally was greatest the first night, occurring in 45% of cases when all BSI approaches were considered. It lasted a mean 43 minutes, which dropped significantly after 1 week to a mean 9 minutes (P less than .001). Crying was considered most intense (on a 1-5 scale) on the initial night of BSI, a mean 4.42, and this “was equally true for all of the BSI approaches,” Dr. Honaker and her colleagues wrote.
In most cases, the parents’ first attempt at BSI worked (83%). Success varied by BSI approach, with the highest first attempt success rate in the unmodified extinction group (90%), followed by parental presence without support (83%), modified extinction (81%), and parental presence with support (65%). Eventually, 27% of parents were successful with a different approach than the one with which they started. Most commonly, they changed from modified extinction to unmodified extinction (66% of those who changed approaches).
“The majority of parents report successfully implementing BSI at a variety of ages across infancy, primarily using extinction-based approaches,” the researchers concluded. “Few significant differences were found between approaches, suggesting that health providers should offer parents options for BSI implementation.”
SOURCE: Honaker SM et al., J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.04.009.
FROM THE JOURNAL OF PEDIATRICS
FDA approves marketing of device to control GI bleeding
The Food and Drug Administration announced May 7 that it has permitted marketing of .
Hemospray is an aerosolized spray device that delivers a mineral blend to the bleeding site in the GI tract and is applied during endoscopic procedures and can cover large ulcers or tumors.
The FDA evaluated data from clinical studies consisting of 228 patients with upper and lower GI bleeding, supplemented with evidence from medical literature, including an additional 522 patients. The studies found that Hemospray stopped GI bleeding in 95% of patients within 5 minutes of device usage. Results also found that bleeding recurred, usually within 72 hours, and up to 30 days following device usage, in 20% of patients. Bowel perforation was observed as a serious side effect in approximately 1% of patients.
“The device provides an additional, nonsurgical option for treating upper and lower GI bleeding in certain patients, and may help reduce the risk of death from a GI bleed for many patients,” said Binita Ashar, MD, director, division of surgical devices, in the FDA’s Center for Devices and Radiological Health in a press release.
Hemospray is not intended for patients who have a gastrointestinal fistula or are at high risk for GI perforation. The device is not intended for use in patients with variceal bleeding. The FDA permitted the marketing of the Hemospray device to Wilson-Cook Medical.*
Read the full press release here.
Correction, 5/10/18: An earlier version of this article incorrectly described the patient population that should not be treated with Hemospray.
The Food and Drug Administration announced May 7 that it has permitted marketing of .
Hemospray is an aerosolized spray device that delivers a mineral blend to the bleeding site in the GI tract and is applied during endoscopic procedures and can cover large ulcers or tumors.
The FDA evaluated data from clinical studies consisting of 228 patients with upper and lower GI bleeding, supplemented with evidence from medical literature, including an additional 522 patients. The studies found that Hemospray stopped GI bleeding in 95% of patients within 5 minutes of device usage. Results also found that bleeding recurred, usually within 72 hours, and up to 30 days following device usage, in 20% of patients. Bowel perforation was observed as a serious side effect in approximately 1% of patients.
“The device provides an additional, nonsurgical option for treating upper and lower GI bleeding in certain patients, and may help reduce the risk of death from a GI bleed for many patients,” said Binita Ashar, MD, director, division of surgical devices, in the FDA’s Center for Devices and Radiological Health in a press release.
Hemospray is not intended for patients who have a gastrointestinal fistula or are at high risk for GI perforation. The device is not intended for use in patients with variceal bleeding. The FDA permitted the marketing of the Hemospray device to Wilson-Cook Medical.*
Read the full press release here.
Correction, 5/10/18: An earlier version of this article incorrectly described the patient population that should not be treated with Hemospray.
The Food and Drug Administration announced May 7 that it has permitted marketing of .
Hemospray is an aerosolized spray device that delivers a mineral blend to the bleeding site in the GI tract and is applied during endoscopic procedures and can cover large ulcers or tumors.
The FDA evaluated data from clinical studies consisting of 228 patients with upper and lower GI bleeding, supplemented with evidence from medical literature, including an additional 522 patients. The studies found that Hemospray stopped GI bleeding in 95% of patients within 5 minutes of device usage. Results also found that bleeding recurred, usually within 72 hours, and up to 30 days following device usage, in 20% of patients. Bowel perforation was observed as a serious side effect in approximately 1% of patients.
“The device provides an additional, nonsurgical option for treating upper and lower GI bleeding in certain patients, and may help reduce the risk of death from a GI bleed for many patients,” said Binita Ashar, MD, director, division of surgical devices, in the FDA’s Center for Devices and Radiological Health in a press release.
Hemospray is not intended for patients who have a gastrointestinal fistula or are at high risk for GI perforation. The device is not intended for use in patients with variceal bleeding. The FDA permitted the marketing of the Hemospray device to Wilson-Cook Medical.*
Read the full press release here.
Correction, 5/10/18: An earlier version of this article incorrectly described the patient population that should not be treated with Hemospray.
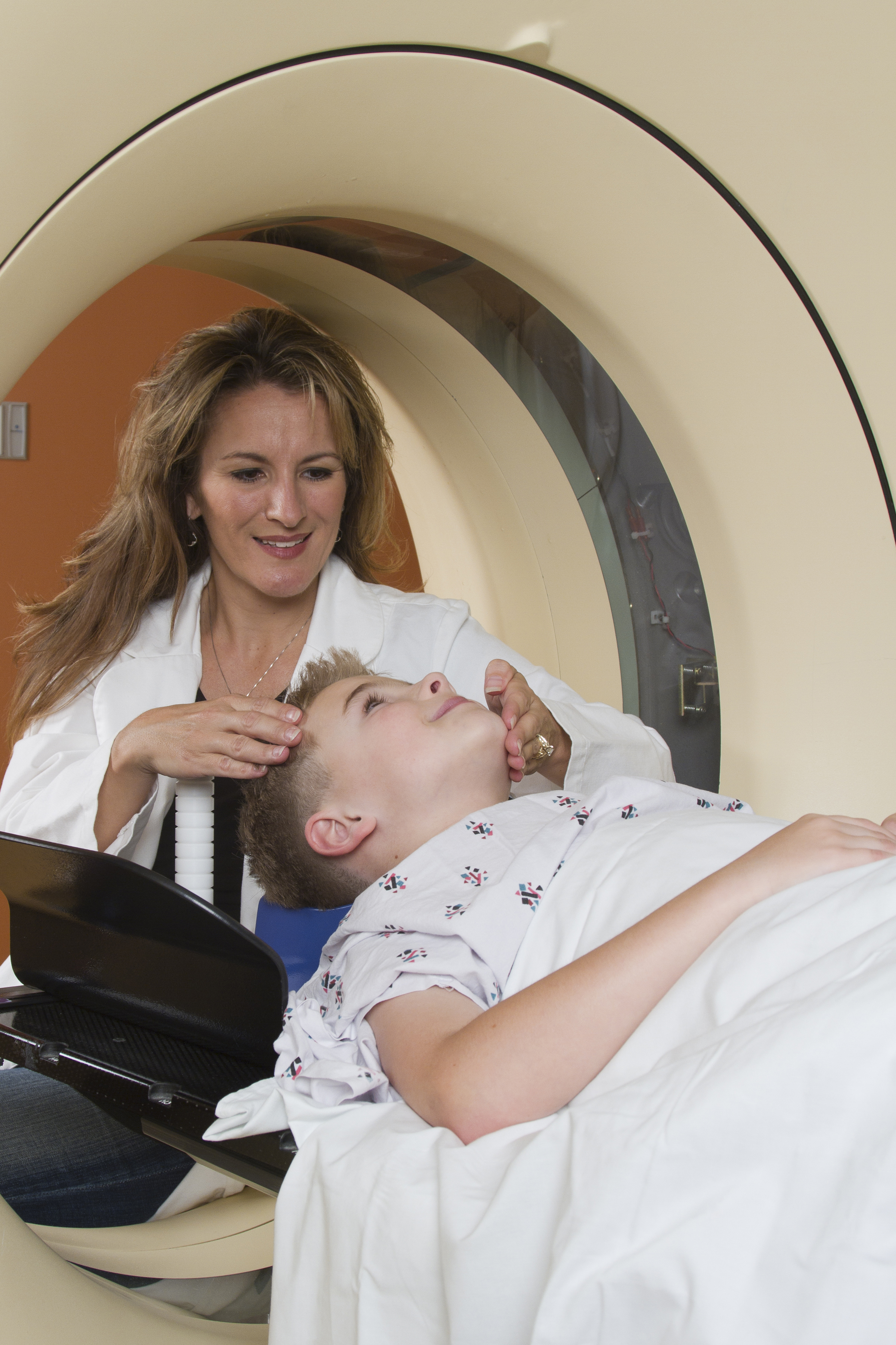
S100B biomarker could reduce CT scans in children with mTBI
according to Charlotte Oris, PharmD, of the University Hospital of Clermont-Ferrand, France, and her associates.
In a meta-analysis of eight prospective cohort studies including a total of 601 children published in Pediatrics, researchers looked at the association between S100B serum levels and CT findings in 373 patients. The median serum concentrations of S100B were 0.47 mcg/L for patients with intracerebral lesions and 0.21 mcg/L for those without lesions (P less than .001).
Additionally, researchers collected data from 358 individuals included in two studies for the origin of mTBI. The median concentrations of S100B were 0.39 mcg/L for road accidents, 0.29 mcg/L for domestic accidents, and 0.18 mcg/L for sport-related accidents. The difference was statistically significant between the road accidents group and the domestic accidents group (P less than .001) and the difference between the road accidents group and the sport-related accidents group (P less than .001). It is noted that S100B specificity could be higher after a sport-related trauma.
“S100B protein serum levels, in combination with the PECARN [Pediatric Emergency Care Applied Research Network] algorithm, could reduce the need for CT scans by one-third. In our additional analysis, based on 373 children, the importance of taking a blood sample 3 hours or less after trauma was underscored,” the researchers said.
“S100B represents a promising biomarker with 100% sensitivity. The limited specificity of S100B could be reevaluated for future research by using a combination of different brain biomarkers,” Dr. Oris and her colleagues concluded.
SOURCE: Oris C et al. Pediatrics. 2018. doi: 10.1542/peds.2018-0037.
according to Charlotte Oris, PharmD, of the University Hospital of Clermont-Ferrand, France, and her associates.
In a meta-analysis of eight prospective cohort studies including a total of 601 children published in Pediatrics, researchers looked at the association between S100B serum levels and CT findings in 373 patients. The median serum concentrations of S100B were 0.47 mcg/L for patients with intracerebral lesions and 0.21 mcg/L for those without lesions (P less than .001).
Additionally, researchers collected data from 358 individuals included in two studies for the origin of mTBI. The median concentrations of S100B were 0.39 mcg/L for road accidents, 0.29 mcg/L for domestic accidents, and 0.18 mcg/L for sport-related accidents. The difference was statistically significant between the road accidents group and the domestic accidents group (P less than .001) and the difference between the road accidents group and the sport-related accidents group (P less than .001). It is noted that S100B specificity could be higher after a sport-related trauma.
“S100B protein serum levels, in combination with the PECARN [Pediatric Emergency Care Applied Research Network] algorithm, could reduce the need for CT scans by one-third. In our additional analysis, based on 373 children, the importance of taking a blood sample 3 hours or less after trauma was underscored,” the researchers said.
“S100B represents a promising biomarker with 100% sensitivity. The limited specificity of S100B could be reevaluated for future research by using a combination of different brain biomarkers,” Dr. Oris and her colleagues concluded.
SOURCE: Oris C et al. Pediatrics. 2018. doi: 10.1542/peds.2018-0037.
according to Charlotte Oris, PharmD, of the University Hospital of Clermont-Ferrand, France, and her associates.
In a meta-analysis of eight prospective cohort studies including a total of 601 children published in Pediatrics, researchers looked at the association between S100B serum levels and CT findings in 373 patients. The median serum concentrations of S100B were 0.47 mcg/L for patients with intracerebral lesions and 0.21 mcg/L for those without lesions (P less than .001).
Additionally, researchers collected data from 358 individuals included in two studies for the origin of mTBI. The median concentrations of S100B were 0.39 mcg/L for road accidents, 0.29 mcg/L for domestic accidents, and 0.18 mcg/L for sport-related accidents. The difference was statistically significant between the road accidents group and the domestic accidents group (P less than .001) and the difference between the road accidents group and the sport-related accidents group (P less than .001). It is noted that S100B specificity could be higher after a sport-related trauma.
“S100B protein serum levels, in combination with the PECARN [Pediatric Emergency Care Applied Research Network] algorithm, could reduce the need for CT scans by one-third. In our additional analysis, based on 373 children, the importance of taking a blood sample 3 hours or less after trauma was underscored,” the researchers said.
“S100B represents a promising biomarker with 100% sensitivity. The limited specificity of S100B could be reevaluated for future research by using a combination of different brain biomarkers,” Dr. Oris and her colleagues concluded.
SOURCE: Oris C et al. Pediatrics. 2018. doi: 10.1542/peds.2018-0037.
IL-22 blocker investigated in phase 2a atopic dermatitis study
Fezakinumab, an interleukin-22 monoclonal antibody, “resulted in consistent improvements in clinical and molecular disease scores as compared with placebo” in a phase 2a study of adults with moderate to severe atopic dermatitis (AD), according to Emma Guttman-Yassky, MD, of Icahn School of Medicine at Mount Sinai, New York, and her associates.
In the double-blind, placebo-controlled trial, 60 patients were randomized to intravenous fezakinumab every 2 weeks for 10 weeks (40 patients) or placebo (20). Beginning at week 4, those who received fezakinumab “showed a consistently stronger and more significant mean SCORAD decline from baseline” compared with those on placebo. This became statistically significant at weeks 6-10 (P less than .05). “Differences between drug and placebo extended beyond the last dose” at week 10, they noted.*
The primary endpoint, the change in the SCORAD score from baseline at 12 weeks, was not statistically significant, however.
In addition, progressive reductions were seen during weeks 14-20, with a significant difference between the drug and placebo arms (P = .049) observed at week 20.
The mean decline in body surface area was “consistently stronger” among those on the biologic, “and was significantly different from the placebo group starting from week 8 until the end of study,” which included the 12th week (P = .009), the researchers noted.
In addition, among those on fezakinumab, mean improvements in Investigator Global Assessment scores compared with baseline were stronger and appeared earlier and were significantly different compared with those on placebo at week 16 (P less than .001).
There were two serious adverse events among those in the treatment group: facial cellulitis after a dental procedure and a pregnancy with elective termination, which were considered “most likely unrelated” to treatment. In the fezakinumab group, four patients had upper respiratory tract infections, the most common adverse event.
“This is the first clinical trial investigating IL-22 blockade in patients with AD, and the first to suggest a pathogenic role of IL-22 in any human disease,” the authors concluded.
SOURCE: Guttman-Yassky et al. J Am Acad Dermatol. 78(5);872-81.
Correction, 4/27/17: An earlier version of this article misstated the statistical significance of the primary endpoint.
Fezakinumab, an interleukin-22 monoclonal antibody, “resulted in consistent improvements in clinical and molecular disease scores as compared with placebo” in a phase 2a study of adults with moderate to severe atopic dermatitis (AD), according to Emma Guttman-Yassky, MD, of Icahn School of Medicine at Mount Sinai, New York, and her associates.
In the double-blind, placebo-controlled trial, 60 patients were randomized to intravenous fezakinumab every 2 weeks for 10 weeks (40 patients) or placebo (20). Beginning at week 4, those who received fezakinumab “showed a consistently stronger and more significant mean SCORAD decline from baseline” compared with those on placebo. This became statistically significant at weeks 6-10 (P less than .05). “Differences between drug and placebo extended beyond the last dose” at week 10, they noted.*
The primary endpoint, the change in the SCORAD score from baseline at 12 weeks, was not statistically significant, however.
In addition, progressive reductions were seen during weeks 14-20, with a significant difference between the drug and placebo arms (P = .049) observed at week 20.
The mean decline in body surface area was “consistently stronger” among those on the biologic, “and was significantly different from the placebo group starting from week 8 until the end of study,” which included the 12th week (P = .009), the researchers noted.
In addition, among those on fezakinumab, mean improvements in Investigator Global Assessment scores compared with baseline were stronger and appeared earlier and were significantly different compared with those on placebo at week 16 (P less than .001).
There were two serious adverse events among those in the treatment group: facial cellulitis after a dental procedure and a pregnancy with elective termination, which were considered “most likely unrelated” to treatment. In the fezakinumab group, four patients had upper respiratory tract infections, the most common adverse event.
“This is the first clinical trial investigating IL-22 blockade in patients with AD, and the first to suggest a pathogenic role of IL-22 in any human disease,” the authors concluded.
SOURCE: Guttman-Yassky et al. J Am Acad Dermatol. 78(5);872-81.
Correction, 4/27/17: An earlier version of this article misstated the statistical significance of the primary endpoint.
Fezakinumab, an interleukin-22 monoclonal antibody, “resulted in consistent improvements in clinical and molecular disease scores as compared with placebo” in a phase 2a study of adults with moderate to severe atopic dermatitis (AD), according to Emma Guttman-Yassky, MD, of Icahn School of Medicine at Mount Sinai, New York, and her associates.
In the double-blind, placebo-controlled trial, 60 patients were randomized to intravenous fezakinumab every 2 weeks for 10 weeks (40 patients) or placebo (20). Beginning at week 4, those who received fezakinumab “showed a consistently stronger and more significant mean SCORAD decline from baseline” compared with those on placebo. This became statistically significant at weeks 6-10 (P less than .05). “Differences between drug and placebo extended beyond the last dose” at week 10, they noted.*
The primary endpoint, the change in the SCORAD score from baseline at 12 weeks, was not statistically significant, however.
In addition, progressive reductions were seen during weeks 14-20, with a significant difference between the drug and placebo arms (P = .049) observed at week 20.
The mean decline in body surface area was “consistently stronger” among those on the biologic, “and was significantly different from the placebo group starting from week 8 until the end of study,” which included the 12th week (P = .009), the researchers noted.
In addition, among those on fezakinumab, mean improvements in Investigator Global Assessment scores compared with baseline were stronger and appeared earlier and were significantly different compared with those on placebo at week 16 (P less than .001).
There were two serious adverse events among those in the treatment group: facial cellulitis after a dental procedure and a pregnancy with elective termination, which were considered “most likely unrelated” to treatment. In the fezakinumab group, four patients had upper respiratory tract infections, the most common adverse event.
“This is the first clinical trial investigating IL-22 blockade in patients with AD, and the first to suggest a pathogenic role of IL-22 in any human disease,” the authors concluded.
SOURCE: Guttman-Yassky et al. J Am Acad Dermatol. 78(5);872-81.
Correction, 4/27/17: An earlier version of this article misstated the statistical significance of the primary endpoint.
FROM THE JOURNAL OF the AMERICAN ACADEMY OF DERMATOLOGY
E-cigarette use in teens increases risk of cannabis use
, reported Hongying Dai, PhD, of Children’s Mercy Kansas City (Mo.), and her associates.
In an analysis of data from the Population Assessment of Tobacco and Health (PATH) survey, 11,996 participants aged 12-17 years completed both the wave 1 and wave 2 surveys. Researchers found one in four adolescents (26.6%) who used e-cigarettes at wave 1 reported marijuana use at wave 2, compared with 7.7% of adolescents who never used e-cigarettes at wave 1 (P less than .05). E-cigarette users at wave 1 were more likely to report marijuana use in the past 12 months at wave 2 (adjusted odds ratio = 1.9). In addition, 2.8% of participants who had never used marijuana at wave 1 reported heavy use of marijuana at wave 2.
“Our study revealed that e-cigarette use was associated with an increased risk of subsequent marijuana use among youth, with a stronger temporal association among younger adolescents,” the researchers concluded. “With these findings, we suggest that the widespread use of e-cigarettes among youth may have implications for the uptake of other drugs of abuse beyond nicotine and tobacco products.”
SOURCE: Dai H et al. Pediatrics. 2018;141(5):e20173787.
, reported Hongying Dai, PhD, of Children’s Mercy Kansas City (Mo.), and her associates.
In an analysis of data from the Population Assessment of Tobacco and Health (PATH) survey, 11,996 participants aged 12-17 years completed both the wave 1 and wave 2 surveys. Researchers found one in four adolescents (26.6%) who used e-cigarettes at wave 1 reported marijuana use at wave 2, compared with 7.7% of adolescents who never used e-cigarettes at wave 1 (P less than .05). E-cigarette users at wave 1 were more likely to report marijuana use in the past 12 months at wave 2 (adjusted odds ratio = 1.9). In addition, 2.8% of participants who had never used marijuana at wave 1 reported heavy use of marijuana at wave 2.
“Our study revealed that e-cigarette use was associated with an increased risk of subsequent marijuana use among youth, with a stronger temporal association among younger adolescents,” the researchers concluded. “With these findings, we suggest that the widespread use of e-cigarettes among youth may have implications for the uptake of other drugs of abuse beyond nicotine and tobacco products.”
SOURCE: Dai H et al. Pediatrics. 2018;141(5):e20173787.
, reported Hongying Dai, PhD, of Children’s Mercy Kansas City (Mo.), and her associates.
In an analysis of data from the Population Assessment of Tobacco and Health (PATH) survey, 11,996 participants aged 12-17 years completed both the wave 1 and wave 2 surveys. Researchers found one in four adolescents (26.6%) who used e-cigarettes at wave 1 reported marijuana use at wave 2, compared with 7.7% of adolescents who never used e-cigarettes at wave 1 (P less than .05). E-cigarette users at wave 1 were more likely to report marijuana use in the past 12 months at wave 2 (adjusted odds ratio = 1.9). In addition, 2.8% of participants who had never used marijuana at wave 1 reported heavy use of marijuana at wave 2.
“Our study revealed that e-cigarette use was associated with an increased risk of subsequent marijuana use among youth, with a stronger temporal association among younger adolescents,” the researchers concluded. “With these findings, we suggest that the widespread use of e-cigarettes among youth may have implications for the uptake of other drugs of abuse beyond nicotine and tobacco products.”
SOURCE: Dai H et al. Pediatrics. 2018;141(5):e20173787.
FROM PEDIATRICS
Peanut exposure in at-risk infants does not reduce eczema, other allergic diseases
and does not hasten the resolution of eczema, according to George du Toit, MB, and his associates.
In a study published in The Journal of Allergy and Clinical Immunology, they reported on the results of a 12-month extension of the Learning Early About Peanut Allergy (LEAP) study, which found that early introduction of peanuts into the diets of infants at high risk of peanut allergy markedly reduced their risk of developing peanut allergy at age 5, compared with those who did not consume peanuts. As infants, the majority had severe eczema, which, with egg allergy, was used to identify those at high risk.
In the extension study, the rates of eczema decreased across study time points to 72 months of age in both groups, to 39% in the peanut avoidance group and 37% in the peanut consumption group. Eczema severity decreased across study time points, and there were no significant differences in severity between the two groups at any time point.
There were also no differences between the two groups in the rates of asthma, seasonal rhinoconjunctivitis, and perennial rhinoconjunctivitis at 30, 60, and 72 months of age.
“The underlying immune mechanisms associated with tolerance to peanut do not alter the natural history of allergic disease,” the researchers concluded. “Different prevention strategies or strategies that include multiple dietary interventions need to be tested to assess whether the reduction in peanut allergy observed in the LEAP consumption group can be extended to other common food allergens and allergic diseases.”
SOURCE: Du Toit G et al. J Allergy Clin Immunol. 2018 Apr;141[4]1343-53.
and does not hasten the resolution of eczema, according to George du Toit, MB, and his associates.
In a study published in The Journal of Allergy and Clinical Immunology, they reported on the results of a 12-month extension of the Learning Early About Peanut Allergy (LEAP) study, which found that early introduction of peanuts into the diets of infants at high risk of peanut allergy markedly reduced their risk of developing peanut allergy at age 5, compared with those who did not consume peanuts. As infants, the majority had severe eczema, which, with egg allergy, was used to identify those at high risk.
In the extension study, the rates of eczema decreased across study time points to 72 months of age in both groups, to 39% in the peanut avoidance group and 37% in the peanut consumption group. Eczema severity decreased across study time points, and there were no significant differences in severity between the two groups at any time point.
There were also no differences between the two groups in the rates of asthma, seasonal rhinoconjunctivitis, and perennial rhinoconjunctivitis at 30, 60, and 72 months of age.
“The underlying immune mechanisms associated with tolerance to peanut do not alter the natural history of allergic disease,” the researchers concluded. “Different prevention strategies or strategies that include multiple dietary interventions need to be tested to assess whether the reduction in peanut allergy observed in the LEAP consumption group can be extended to other common food allergens and allergic diseases.”
SOURCE: Du Toit G et al. J Allergy Clin Immunol. 2018 Apr;141[4]1343-53.
and does not hasten the resolution of eczema, according to George du Toit, MB, and his associates.
In a study published in The Journal of Allergy and Clinical Immunology, they reported on the results of a 12-month extension of the Learning Early About Peanut Allergy (LEAP) study, which found that early introduction of peanuts into the diets of infants at high risk of peanut allergy markedly reduced their risk of developing peanut allergy at age 5, compared with those who did not consume peanuts. As infants, the majority had severe eczema, which, with egg allergy, was used to identify those at high risk.
In the extension study, the rates of eczema decreased across study time points to 72 months of age in both groups, to 39% in the peanut avoidance group and 37% in the peanut consumption group. Eczema severity decreased across study time points, and there were no significant differences in severity between the two groups at any time point.
There were also no differences between the two groups in the rates of asthma, seasonal rhinoconjunctivitis, and perennial rhinoconjunctivitis at 30, 60, and 72 months of age.
“The underlying immune mechanisms associated with tolerance to peanut do not alter the natural history of allergic disease,” the researchers concluded. “Different prevention strategies or strategies that include multiple dietary interventions need to be tested to assess whether the reduction in peanut allergy observed in the LEAP consumption group can be extended to other common food allergens and allergic diseases.”
SOURCE: Du Toit G et al. J Allergy Clin Immunol. 2018 Apr;141[4]1343-53.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY