User login
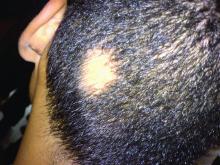
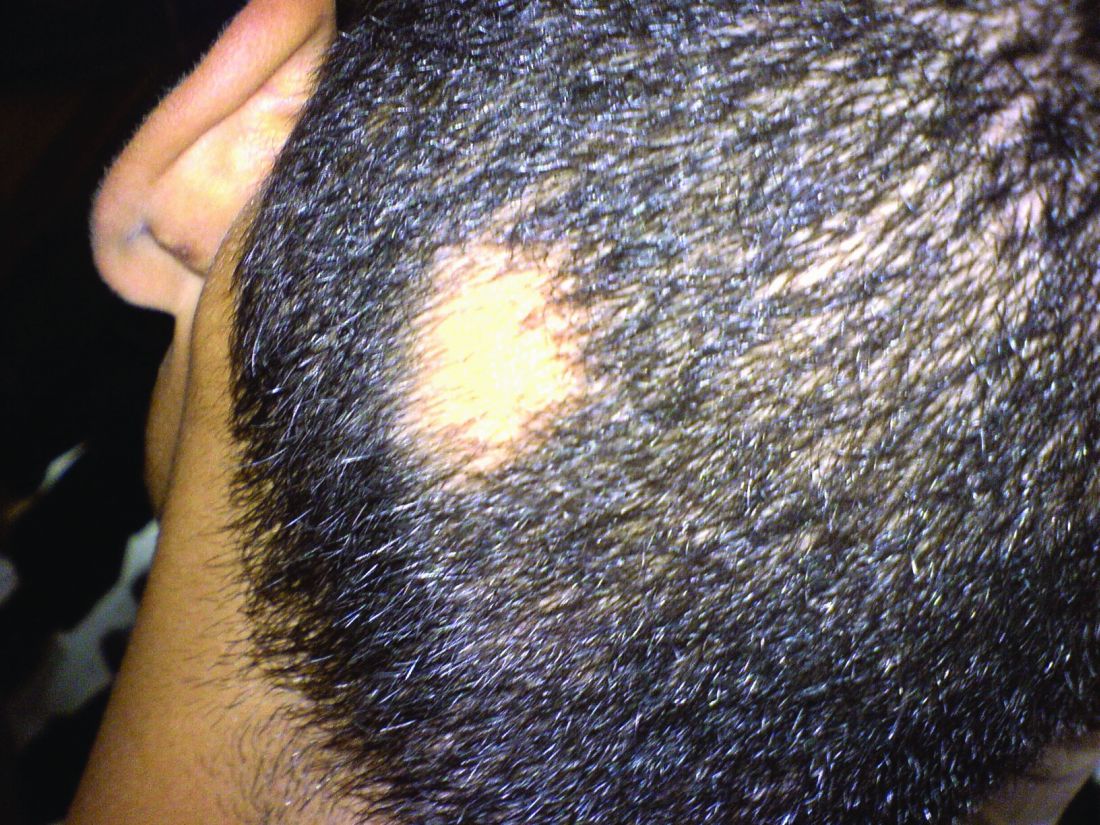
Consider hydroxychloroquine in treating pediatric alopecia areata
according to Duri Yun, MD, of the University of Chicago Medicine, and associates.
In a retrospective review published in Pediatric Dermatology, nine children aged 6-16 years with AA and diverse ethnicities were treated with hydroxychloroquine between July 1, 2013, and July 1, 2015; all had failed multiple previous treatment modalities. In patient 1, hydroxychloroquine therapy was initiated, fine hair regrowth occurred after 5 months of therapy and was maintained, with dosage tapered to 200 mg once daily after 1 year. After 2 years of therapy, hair had nearly completely regrown. Similar results occurred in patient 2, who had nearly complete hair loss within 2 weeks of initiating hydroxychloroquine. Steady regrowth continued to near-complete regrowth after 1 year of treatment, when dosage was tapered to 200 mg once daily.
Four patients (44%) had no evidence of regrowth after 4-6 months of hydroxychloroquine therapy so they discontinued therapy. The most common adverse events while taking hydroxychloroquine were abdominal pain in two patients (22%) and headache in two patients (22%).
“In the context of children with severe AA failing multiple first-line therapies, our findings suggest that there may be a subgroup that benefits from therapy with hydroxychloroquine,” the researchers concluded. “Determining which factors might predict response to various therapies will come from combined efforts to conduct well-controlled clinical trials of treatments for AA.”
SOURCE: Yun D et al., Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13451.
according to Duri Yun, MD, of the University of Chicago Medicine, and associates.
In a retrospective review published in Pediatric Dermatology, nine children aged 6-16 years with AA and diverse ethnicities were treated with hydroxychloroquine between July 1, 2013, and July 1, 2015; all had failed multiple previous treatment modalities. In patient 1, hydroxychloroquine therapy was initiated, fine hair regrowth occurred after 5 months of therapy and was maintained, with dosage tapered to 200 mg once daily after 1 year. After 2 years of therapy, hair had nearly completely regrown. Similar results occurred in patient 2, who had nearly complete hair loss within 2 weeks of initiating hydroxychloroquine. Steady regrowth continued to near-complete regrowth after 1 year of treatment, when dosage was tapered to 200 mg once daily.
Four patients (44%) had no evidence of regrowth after 4-6 months of hydroxychloroquine therapy so they discontinued therapy. The most common adverse events while taking hydroxychloroquine were abdominal pain in two patients (22%) and headache in two patients (22%).
“In the context of children with severe AA failing multiple first-line therapies, our findings suggest that there may be a subgroup that benefits from therapy with hydroxychloroquine,” the researchers concluded. “Determining which factors might predict response to various therapies will come from combined efforts to conduct well-controlled clinical trials of treatments for AA.”
SOURCE: Yun D et al., Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13451.
according to Duri Yun, MD, of the University of Chicago Medicine, and associates.
In a retrospective review published in Pediatric Dermatology, nine children aged 6-16 years with AA and diverse ethnicities were treated with hydroxychloroquine between July 1, 2013, and July 1, 2015; all had failed multiple previous treatment modalities. In patient 1, hydroxychloroquine therapy was initiated, fine hair regrowth occurred after 5 months of therapy and was maintained, with dosage tapered to 200 mg once daily after 1 year. After 2 years of therapy, hair had nearly completely regrown. Similar results occurred in patient 2, who had nearly complete hair loss within 2 weeks of initiating hydroxychloroquine. Steady regrowth continued to near-complete regrowth after 1 year of treatment, when dosage was tapered to 200 mg once daily.
Four patients (44%) had no evidence of regrowth after 4-6 months of hydroxychloroquine therapy so they discontinued therapy. The most common adverse events while taking hydroxychloroquine were abdominal pain in two patients (22%) and headache in two patients (22%).
“In the context of children with severe AA failing multiple first-line therapies, our findings suggest that there may be a subgroup that benefits from therapy with hydroxychloroquine,” the researchers concluded. “Determining which factors might predict response to various therapies will come from combined efforts to conduct well-controlled clinical trials of treatments for AA.”
SOURCE: Yun D et al., Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13451.
FROM PEDIATRIC DERMATOLOGY
ciTBI uncommon in minor head injuries with isolated vomiting
said Meredith L. Borland, MD, of the Princess Margaret Hospital for Children, Perth, Australia, and her associates.
Likewise, traumatic brain injury evident on computed tomography (TBI-CT) is rare in such cases.
In a study published in Pediatrics, 19,920 eligible children younger than 18 years were enrolled in the Australasian Paediatric Head Injury Rule Study (APHIRST); 3,389 had a history of any vomiting, and 1,006 had isolated vomiting without any other clinical decision rules predictors. Results found 76 of the 172 (44%) children with a ciTBI and 123 of the 285 (43%) children with TBI-CT had any history of vomiting. When the Children’s Head Injury Algorithm for the Prediction of Important Clinical Events (CHALICE) rule predictors for those with isolated vomiting – both fewer than three times (n = 662 of 1,006; 66%) and also three or more times (n = 344 of 1,006; 34%) – was applied, there was only one child with ciTBI, and there were only two children with a TBI-CT.
Within the subsample comprising 457 children younger than 2 years old with isolated vomiting out of the overall 1,006 (45%), there were none with ciTBI or TBI-CT. In the 549 (55%) children 2 years old and older with isolated vomiting, one (0.3%) had ciTBI, and two (0.6%) had TBI-CT.
In multivariate regression, signs of skull fracture, altered mental status, headache, and acting abnormally were significantly associated with ciTBI. Signs of a skull fracture, nonaccidental injury concern, headache, and acting abnormally were significantly associated with TBI-CT.
“TBI-CT is uncommon, and ciTBI is uncommon in children with minor blunt head injury when vomiting is their only sign or symptom,” Dr. Borland and her associates concluded. “In children with isolated vomiting, strategies such as observation should be considered before conducting an immediate CT scan.”
Read the full study in Pediatrics.
said Meredith L. Borland, MD, of the Princess Margaret Hospital for Children, Perth, Australia, and her associates.
Likewise, traumatic brain injury evident on computed tomography (TBI-CT) is rare in such cases.
In a study published in Pediatrics, 19,920 eligible children younger than 18 years were enrolled in the Australasian Paediatric Head Injury Rule Study (APHIRST); 3,389 had a history of any vomiting, and 1,006 had isolated vomiting without any other clinical decision rules predictors. Results found 76 of the 172 (44%) children with a ciTBI and 123 of the 285 (43%) children with TBI-CT had any history of vomiting. When the Children’s Head Injury Algorithm for the Prediction of Important Clinical Events (CHALICE) rule predictors for those with isolated vomiting – both fewer than three times (n = 662 of 1,006; 66%) and also three or more times (n = 344 of 1,006; 34%) – was applied, there was only one child with ciTBI, and there were only two children with a TBI-CT.
Within the subsample comprising 457 children younger than 2 years old with isolated vomiting out of the overall 1,006 (45%), there were none with ciTBI or TBI-CT. In the 549 (55%) children 2 years old and older with isolated vomiting, one (0.3%) had ciTBI, and two (0.6%) had TBI-CT.
In multivariate regression, signs of skull fracture, altered mental status, headache, and acting abnormally were significantly associated with ciTBI. Signs of a skull fracture, nonaccidental injury concern, headache, and acting abnormally were significantly associated with TBI-CT.
“TBI-CT is uncommon, and ciTBI is uncommon in children with minor blunt head injury when vomiting is their only sign or symptom,” Dr. Borland and her associates concluded. “In children with isolated vomiting, strategies such as observation should be considered before conducting an immediate CT scan.”
Read the full study in Pediatrics.
said Meredith L. Borland, MD, of the Princess Margaret Hospital for Children, Perth, Australia, and her associates.
Likewise, traumatic brain injury evident on computed tomography (TBI-CT) is rare in such cases.
In a study published in Pediatrics, 19,920 eligible children younger than 18 years were enrolled in the Australasian Paediatric Head Injury Rule Study (APHIRST); 3,389 had a history of any vomiting, and 1,006 had isolated vomiting without any other clinical decision rules predictors. Results found 76 of the 172 (44%) children with a ciTBI and 123 of the 285 (43%) children with TBI-CT had any history of vomiting. When the Children’s Head Injury Algorithm for the Prediction of Important Clinical Events (CHALICE) rule predictors for those with isolated vomiting – both fewer than three times (n = 662 of 1,006; 66%) and also three or more times (n = 344 of 1,006; 34%) – was applied, there was only one child with ciTBI, and there were only two children with a TBI-CT.
Within the subsample comprising 457 children younger than 2 years old with isolated vomiting out of the overall 1,006 (45%), there were none with ciTBI or TBI-CT. In the 549 (55%) children 2 years old and older with isolated vomiting, one (0.3%) had ciTBI, and two (0.6%) had TBI-CT.
In multivariate regression, signs of skull fracture, altered mental status, headache, and acting abnormally were significantly associated with ciTBI. Signs of a skull fracture, nonaccidental injury concern, headache, and acting abnormally were significantly associated with TBI-CT.
“TBI-CT is uncommon, and ciTBI is uncommon in children with minor blunt head injury when vomiting is their only sign or symptom,” Dr. Borland and her associates concluded. “In children with isolated vomiting, strategies such as observation should be considered before conducting an immediate CT scan.”
Read the full study in Pediatrics.
FROM PEDIATRICS
FDA approves certolizumab label update for pregnancy, breastfeeding
The manufacturer of certolizumab pegol, UCB, announced March 22 that the Food and Drug Administration approved a label update to the biologic that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.
In the CRIB study, certolizumab levels were below the lower limit of quantification (defined as 0.032 mcg/mL) in 13 out of 15 infant blood samples at birth and in all samples at weeks 4 and 8. No anticertolizumab antibodies were detected in mothers, umbilical cords, or infants.
In the CRADLE study, 56% of 137 breast milk samples from 17 mothers had no measurable certolizumab, and the remaining samples showed minimal levels of the biologic. No serious adverse reactions were noted in the 17 infants in the study.
“It is well recognized that women with chronic inflammatory disease face uncertainty during motherhood given the lack of information on treatment during pregnancy and breastfeeding. Many women with chronic inflammatory disease discontinue their biologic treatment during pregnancy, often when they need disease control the most,” said CRADLE lead study author Megan E. B. Clowse, MD, of Duke University, Durham, N.C., in a press release issued by UCB. “These data for Cimzia provide important information to empower women and healthcare providers making decisions about treatment during pregnancy and breastfeeding.”
UCB said that limited data from an ongoing pregnancy registry regarding the use of certolizumab in pregnant women are not sufficient to inform a risk of major birth defects or other adverse pregnancy outcomes.
The manufacturer of certolizumab pegol, UCB, announced March 22 that the Food and Drug Administration approved a label update to the biologic that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.
In the CRIB study, certolizumab levels were below the lower limit of quantification (defined as 0.032 mcg/mL) in 13 out of 15 infant blood samples at birth and in all samples at weeks 4 and 8. No anticertolizumab antibodies were detected in mothers, umbilical cords, or infants.
In the CRADLE study, 56% of 137 breast milk samples from 17 mothers had no measurable certolizumab, and the remaining samples showed minimal levels of the biologic. No serious adverse reactions were noted in the 17 infants in the study.
“It is well recognized that women with chronic inflammatory disease face uncertainty during motherhood given the lack of information on treatment during pregnancy and breastfeeding. Many women with chronic inflammatory disease discontinue their biologic treatment during pregnancy, often when they need disease control the most,” said CRADLE lead study author Megan E. B. Clowse, MD, of Duke University, Durham, N.C., in a press release issued by UCB. “These data for Cimzia provide important information to empower women and healthcare providers making decisions about treatment during pregnancy and breastfeeding.”
UCB said that limited data from an ongoing pregnancy registry regarding the use of certolizumab in pregnant women are not sufficient to inform a risk of major birth defects or other adverse pregnancy outcomes.
The manufacturer of certolizumab pegol, UCB, announced March 22 that the Food and Drug Administration approved a label update to the biologic that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.
In the CRIB study, certolizumab levels were below the lower limit of quantification (defined as 0.032 mcg/mL) in 13 out of 15 infant blood samples at birth and in all samples at weeks 4 and 8. No anticertolizumab antibodies were detected in mothers, umbilical cords, or infants.
In the CRADLE study, 56% of 137 breast milk samples from 17 mothers had no measurable certolizumab, and the remaining samples showed minimal levels of the biologic. No serious adverse reactions were noted in the 17 infants in the study.
“It is well recognized that women with chronic inflammatory disease face uncertainty during motherhood given the lack of information on treatment during pregnancy and breastfeeding. Many women with chronic inflammatory disease discontinue their biologic treatment during pregnancy, often when they need disease control the most,” said CRADLE lead study author Megan E. B. Clowse, MD, of Duke University, Durham, N.C., in a press release issued by UCB. “These data for Cimzia provide important information to empower women and healthcare providers making decisions about treatment during pregnancy and breastfeeding.”
UCB said that limited data from an ongoing pregnancy registry regarding the use of certolizumab in pregnant women are not sufficient to inform a risk of major birth defects or other adverse pregnancy outcomes.
Early family intervention tied to reduced AUDs in Mexican American youth
A family-focused middle school intervention can help reduce alcohol abuse and alcohol use disorders (AUD) in Mexican American adolescents who are at heightened risk for problem drinking, according to Nancy A. Gonzales, PhD, and her associates.
The report was published March 21 in JAMA Psychiatry.
The investigators examined 5-year follow-up results of a randomized controlled trial, Bridges/Puentes, a 9-week, evidence-based intervention aimed at helping urban, low-income Mexican American teens succeed at school. Among other features, the Bridges/Puentes intervention promoted cultural strengths that had been identified in previous interventions aimed at Latino youth.
“This blend of evidence-based practices and good recruitment rates, retention, and fidelity provided a strong foundation for testing the sustained results of middle school prevention for Latinos,” wrote Dr. Gonzales of the department of psychology and the REACH Institute at Arizona State University, Tempe, and her associates.
The study’s focus on Mexican American youth, which makes it difficult to generalize the results to other populations, was a limitation, the authors noted. Nevertheless, the intervention “is a viable method to not only reduce substance use initiation in the short-term ... but also to reduce later rates of AUDs and alcohol misuse among Mexican American adolescents at heightened risk for problem drinking,” they wrote.
The study was funded by the National Institute of Mental Health. No conflicts of interest were reported.
SOURCE: Gonzales NA et al. doi: 10.1001/jamapsychiatry.2018.0058.
A family-focused middle school intervention can help reduce alcohol abuse and alcohol use disorders (AUD) in Mexican American adolescents who are at heightened risk for problem drinking, according to Nancy A. Gonzales, PhD, and her associates.
The report was published March 21 in JAMA Psychiatry.
The investigators examined 5-year follow-up results of a randomized controlled trial, Bridges/Puentes, a 9-week, evidence-based intervention aimed at helping urban, low-income Mexican American teens succeed at school. Among other features, the Bridges/Puentes intervention promoted cultural strengths that had been identified in previous interventions aimed at Latino youth.
“This blend of evidence-based practices and good recruitment rates, retention, and fidelity provided a strong foundation for testing the sustained results of middle school prevention for Latinos,” wrote Dr. Gonzales of the department of psychology and the REACH Institute at Arizona State University, Tempe, and her associates.
The study’s focus on Mexican American youth, which makes it difficult to generalize the results to other populations, was a limitation, the authors noted. Nevertheless, the intervention “is a viable method to not only reduce substance use initiation in the short-term ... but also to reduce later rates of AUDs and alcohol misuse among Mexican American adolescents at heightened risk for problem drinking,” they wrote.
The study was funded by the National Institute of Mental Health. No conflicts of interest were reported.
SOURCE: Gonzales NA et al. doi: 10.1001/jamapsychiatry.2018.0058.
A family-focused middle school intervention can help reduce alcohol abuse and alcohol use disorders (AUD) in Mexican American adolescents who are at heightened risk for problem drinking, according to Nancy A. Gonzales, PhD, and her associates.
The report was published March 21 in JAMA Psychiatry.
The investigators examined 5-year follow-up results of a randomized controlled trial, Bridges/Puentes, a 9-week, evidence-based intervention aimed at helping urban, low-income Mexican American teens succeed at school. Among other features, the Bridges/Puentes intervention promoted cultural strengths that had been identified in previous interventions aimed at Latino youth.
“This blend of evidence-based practices and good recruitment rates, retention, and fidelity provided a strong foundation for testing the sustained results of middle school prevention for Latinos,” wrote Dr. Gonzales of the department of psychology and the REACH Institute at Arizona State University, Tempe, and her associates.
The study’s focus on Mexican American youth, which makes it difficult to generalize the results to other populations, was a limitation, the authors noted. Nevertheless, the intervention “is a viable method to not only reduce substance use initiation in the short-term ... but also to reduce later rates of AUDs and alcohol misuse among Mexican American adolescents at heightened risk for problem drinking,” they wrote.
The study was funded by the National Institute of Mental Health. No conflicts of interest were reported.
SOURCE: Gonzales NA et al. doi: 10.1001/jamapsychiatry.2018.0058.
FROM JAMA PSYCHIATRY
FDA issues warning to all duodenoscope manufacturers
The Food and Drug Administration on March 9 issued warning letters to all three duodenoscope manufacturers for failing to comply with the requirements of federal law under which they were ordered to conduct postmarket surveillance studies to assess the effectiveness of reprocessing the devices.
The warning is part of an ongoing effort to prevent patient infections associated with the transmission of bacteria from contaminated duodenoscopes. The three manufacturers – Olympus, Fujifilm, and Pentax – are required to conduct studies to sample and culture reprocessed duodenoscopes that are in clinical use to learn more about issues that contribute to contamination, and to study human factors to determine how hospital staff who have had training are following the reprocessing instructions. In 2015, the FDA ordered the companies to conduct a postmarket surveillance study to determine whether health care facilities were able to properly clean and disinfect the devices.
Currently, the Olympus manufacturer has failed to start data collection, while both Pentax and Fujifilm have failed to provide sufficient data required for their respective studies to sample and culture reprocessed duodenoscopes that are in clinical use. In addition, Olympus and Pentax have not complied with requirements to assess how well staff members have followed the reprocessing instructions after the human factors studies and Fujifilm has been meeting its requirements for its human factors study only.
“The FDA has taken important steps to improve the reprocessing of duodenoscopes, and we’ve seen a reduction in reports of patient infections, but we need the required postmarket studies to determine whether these measures are being properly implemented in real-world clinical settings and whether we need to take additional action to further improve the safety of these devices,” said Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health in a press release. “We expect these device manufacturers to meet their study obligations to ensure patient safety.”
The companies have until March 24 to submit a plan that outlines how study milestones will be achieved. If the companies fail to respond to the warning letter, the FDA states that they may take additional action, such as seizure, injunction, and civil monetary penalties.
Read the full press release on the FDA’s website.
The Food and Drug Administration on March 9 issued warning letters to all three duodenoscope manufacturers for failing to comply with the requirements of federal law under which they were ordered to conduct postmarket surveillance studies to assess the effectiveness of reprocessing the devices.
The warning is part of an ongoing effort to prevent patient infections associated with the transmission of bacteria from contaminated duodenoscopes. The three manufacturers – Olympus, Fujifilm, and Pentax – are required to conduct studies to sample and culture reprocessed duodenoscopes that are in clinical use to learn more about issues that contribute to contamination, and to study human factors to determine how hospital staff who have had training are following the reprocessing instructions. In 2015, the FDA ordered the companies to conduct a postmarket surveillance study to determine whether health care facilities were able to properly clean and disinfect the devices.
Currently, the Olympus manufacturer has failed to start data collection, while both Pentax and Fujifilm have failed to provide sufficient data required for their respective studies to sample and culture reprocessed duodenoscopes that are in clinical use. In addition, Olympus and Pentax have not complied with requirements to assess how well staff members have followed the reprocessing instructions after the human factors studies and Fujifilm has been meeting its requirements for its human factors study only.
“The FDA has taken important steps to improve the reprocessing of duodenoscopes, and we’ve seen a reduction in reports of patient infections, but we need the required postmarket studies to determine whether these measures are being properly implemented in real-world clinical settings and whether we need to take additional action to further improve the safety of these devices,” said Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health in a press release. “We expect these device manufacturers to meet their study obligations to ensure patient safety.”
The companies have until March 24 to submit a plan that outlines how study milestones will be achieved. If the companies fail to respond to the warning letter, the FDA states that they may take additional action, such as seizure, injunction, and civil monetary penalties.
Read the full press release on the FDA’s website.
The Food and Drug Administration on March 9 issued warning letters to all three duodenoscope manufacturers for failing to comply with the requirements of federal law under which they were ordered to conduct postmarket surveillance studies to assess the effectiveness of reprocessing the devices.
The warning is part of an ongoing effort to prevent patient infections associated with the transmission of bacteria from contaminated duodenoscopes. The three manufacturers – Olympus, Fujifilm, and Pentax – are required to conduct studies to sample and culture reprocessed duodenoscopes that are in clinical use to learn more about issues that contribute to contamination, and to study human factors to determine how hospital staff who have had training are following the reprocessing instructions. In 2015, the FDA ordered the companies to conduct a postmarket surveillance study to determine whether health care facilities were able to properly clean and disinfect the devices.
Currently, the Olympus manufacturer has failed to start data collection, while both Pentax and Fujifilm have failed to provide sufficient data required for their respective studies to sample and culture reprocessed duodenoscopes that are in clinical use. In addition, Olympus and Pentax have not complied with requirements to assess how well staff members have followed the reprocessing instructions after the human factors studies and Fujifilm has been meeting its requirements for its human factors study only.
“The FDA has taken important steps to improve the reprocessing of duodenoscopes, and we’ve seen a reduction in reports of patient infections, but we need the required postmarket studies to determine whether these measures are being properly implemented in real-world clinical settings and whether we need to take additional action to further improve the safety of these devices,” said Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health in a press release. “We expect these device manufacturers to meet their study obligations to ensure patient safety.”
The companies have until March 24 to submit a plan that outlines how study milestones will be achieved. If the companies fail to respond to the warning letter, the FDA states that they may take additional action, such as seizure, injunction, and civil monetary penalties.
Read the full press release on the FDA’s website.
Functional capacity, life skills critical for patients with schizophrenia
Improving the ability of people with schizophrenia to perform tasks that are important to daily functioning is a key component for any therapeutic intervention, a cross-sectional study of 740 patients shows.
“Our study confirms that [the four domains of] social cognition, neurocognition, resilience, and real-life functioning represent robust and independent constructs,” wrote Silvana Galderisi, MD, and her associates. The report was published in JAMA Psychiatry.
Dr. Galderisi and her associates recruited community-dwelling patients with schizophrenia as defined in the DSM-IV over an 18-month period. The patients were stabilized on antipsychotics and were seen in the outpatient units of 26 psychiatric clinics and/or mental health departments in Italy.
Several measures were administered, including the Brief Negative Symptom Scale (BNSS), the Calgary Depression Scale for Schizophrenia (CDSS), the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB), and the Positive and Negative Syndrome Scale (PANSS).
Using network analysis, the researchers analyzed the results found by the measures and the relationships between the four domains. “The study clearly shows that functional capacity and everyday life skills play a key role,” Dr. Galderisi and her associates wrote. “Functional capacity ... links neurocognition and social cognition with real-life functioning nodes, in particular with everyday life skills, such as household activities, handling of personal finances, and use of the telephone or public transportation.”
Dr. Galderisi and her associates said their findings show that patients with schizophrenia need treatment that goes beyond antipsychotics. “Therefore, with schizophrenia,” they wrote.
Read the full study in JAMA Psychiatry. 2018 Feb 14. doi: 10.1001/jamapsychiatry.2017.4607.
Improving the ability of people with schizophrenia to perform tasks that are important to daily functioning is a key component for any therapeutic intervention, a cross-sectional study of 740 patients shows.
“Our study confirms that [the four domains of] social cognition, neurocognition, resilience, and real-life functioning represent robust and independent constructs,” wrote Silvana Galderisi, MD, and her associates. The report was published in JAMA Psychiatry.
Dr. Galderisi and her associates recruited community-dwelling patients with schizophrenia as defined in the DSM-IV over an 18-month period. The patients were stabilized on antipsychotics and were seen in the outpatient units of 26 psychiatric clinics and/or mental health departments in Italy.
Several measures were administered, including the Brief Negative Symptom Scale (BNSS), the Calgary Depression Scale for Schizophrenia (CDSS), the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB), and the Positive and Negative Syndrome Scale (PANSS).
Using network analysis, the researchers analyzed the results found by the measures and the relationships between the four domains. “The study clearly shows that functional capacity and everyday life skills play a key role,” Dr. Galderisi and her associates wrote. “Functional capacity ... links neurocognition and social cognition with real-life functioning nodes, in particular with everyday life skills, such as household activities, handling of personal finances, and use of the telephone or public transportation.”
Dr. Galderisi and her associates said their findings show that patients with schizophrenia need treatment that goes beyond antipsychotics. “Therefore, with schizophrenia,” they wrote.
Read the full study in JAMA Psychiatry. 2018 Feb 14. doi: 10.1001/jamapsychiatry.2017.4607.
Improving the ability of people with schizophrenia to perform tasks that are important to daily functioning is a key component for any therapeutic intervention, a cross-sectional study of 740 patients shows.
“Our study confirms that [the four domains of] social cognition, neurocognition, resilience, and real-life functioning represent robust and independent constructs,” wrote Silvana Galderisi, MD, and her associates. The report was published in JAMA Psychiatry.
Dr. Galderisi and her associates recruited community-dwelling patients with schizophrenia as defined in the DSM-IV over an 18-month period. The patients were stabilized on antipsychotics and were seen in the outpatient units of 26 psychiatric clinics and/or mental health departments in Italy.
Several measures were administered, including the Brief Negative Symptom Scale (BNSS), the Calgary Depression Scale for Schizophrenia (CDSS), the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB), and the Positive and Negative Syndrome Scale (PANSS).
Using network analysis, the researchers analyzed the results found by the measures and the relationships between the four domains. “The study clearly shows that functional capacity and everyday life skills play a key role,” Dr. Galderisi and her associates wrote. “Functional capacity ... links neurocognition and social cognition with real-life functioning nodes, in particular with everyday life skills, such as household activities, handling of personal finances, and use of the telephone or public transportation.”
Dr. Galderisi and her associates said their findings show that patients with schizophrenia need treatment that goes beyond antipsychotics. “Therefore, with schizophrenia,” they wrote.
Read the full study in JAMA Psychiatry. 2018 Feb 14. doi: 10.1001/jamapsychiatry.2017.4607.
FROM JAMA PSYCHIATRY
Experts cite five orthopedic tests that physicians and patients should question
The American Academy of Pediatrics–Section on Orthopaedics and the Pediatric Orthopaedic Society of North America (POSNA) provide the following list:
- Do not order a screening hip ultrasound to rule out developmental hip dysplasia or developmental hip dislocation if the baby has no risk factors and has a clinically stable hip examination.
- Do not order radiographs or advise bracing or surgery for a child less than 8 years of age with simple in-toeing gait.
- Do not order custom orthotics or shoe inserts for a child with minimally symptomatic or asymptomatic flat feet.
- Do not order advanced imaging studies (MRI or CT) for most musculoskeletal conditions in a child until all appropriate clinical, laboratory, and plain radiographic examinations have been completed.
- Do not order follow-up x-rays for buckle (or torus) fractures if they are no longer tender or painful.
This list was developed based on data collected from 2014-2015 from the POSNA Evidence Based Committee and Advocacy Committee. Approximately 20 members of the two committees participated in the process. They submitted five items each from their practices and experience of tests or procedures that they found were commonly overutilized. The items were placed in order of number of times listed by each surgeon; a total of 30 items were submitted. The two committees then agreed on a final list of five procedures, based on frequency of responses and importance of the condition. After the list was reviewed and feedback provided, the POSNA board of directors voted on a final list. The AAP Executive Committee then provided final approval.
The committee noted that the list is provided for informational purposes and is not intended as a substitute for consultation with a physician.
Read the full list with more details here.
The American Academy of Pediatrics–Section on Orthopaedics and the Pediatric Orthopaedic Society of North America (POSNA) provide the following list:
- Do not order a screening hip ultrasound to rule out developmental hip dysplasia or developmental hip dislocation if the baby has no risk factors and has a clinically stable hip examination.
- Do not order radiographs or advise bracing or surgery for a child less than 8 years of age with simple in-toeing gait.
- Do not order custom orthotics or shoe inserts for a child with minimally symptomatic or asymptomatic flat feet.
- Do not order advanced imaging studies (MRI or CT) for most musculoskeletal conditions in a child until all appropriate clinical, laboratory, and plain radiographic examinations have been completed.
- Do not order follow-up x-rays for buckle (or torus) fractures if they are no longer tender or painful.
This list was developed based on data collected from 2014-2015 from the POSNA Evidence Based Committee and Advocacy Committee. Approximately 20 members of the two committees participated in the process. They submitted five items each from their practices and experience of tests or procedures that they found were commonly overutilized. The items were placed in order of number of times listed by each surgeon; a total of 30 items were submitted. The two committees then agreed on a final list of five procedures, based on frequency of responses and importance of the condition. After the list was reviewed and feedback provided, the POSNA board of directors voted on a final list. The AAP Executive Committee then provided final approval.
The committee noted that the list is provided for informational purposes and is not intended as a substitute for consultation with a physician.
Read the full list with more details here.
The American Academy of Pediatrics–Section on Orthopaedics and the Pediatric Orthopaedic Society of North America (POSNA) provide the following list:
- Do not order a screening hip ultrasound to rule out developmental hip dysplasia or developmental hip dislocation if the baby has no risk factors and has a clinically stable hip examination.
- Do not order radiographs or advise bracing or surgery for a child less than 8 years of age with simple in-toeing gait.
- Do not order custom orthotics or shoe inserts for a child with minimally symptomatic or asymptomatic flat feet.
- Do not order advanced imaging studies (MRI or CT) for most musculoskeletal conditions in a child until all appropriate clinical, laboratory, and plain radiographic examinations have been completed.
- Do not order follow-up x-rays for buckle (or torus) fractures if they are no longer tender or painful.
This list was developed based on data collected from 2014-2015 from the POSNA Evidence Based Committee and Advocacy Committee. Approximately 20 members of the two committees participated in the process. They submitted five items each from their practices and experience of tests or procedures that they found were commonly overutilized. The items were placed in order of number of times listed by each surgeon; a total of 30 items were submitted. The two committees then agreed on a final list of five procedures, based on frequency of responses and importance of the condition. After the list was reviewed and feedback provided, the POSNA board of directors voted on a final list. The AAP Executive Committee then provided final approval.
The committee noted that the list is provided for informational purposes and is not intended as a substitute for consultation with a physician.
Read the full list with more details here.
Sleep disturbance not linked to age or IQ in early ASD
Sleep disturbance is not associated with age and IQ in young children with autism spectrum disorder (ASD) and disruptive behaviors, according to Cynthia R. Johnson, PhD, and her associates.
They assessed 177 children aged 3-7 who were participating in the Research Units on Behavioral Intervention study, a 24-week trial. All of the children had a diagnosis of autism spectrum disorder, based on DSM-IV criteria. The diagnoses were corroborated by the Autism Diagnostic Observation Schedule and the Autism Diagnostic Interview–Revised.
The children were randomized into a parent training group or a parent education group. After getting parents to complete several forms, including the Children’s Sleep Habits Questionnaire, Dr. Johnson and her associates found no age differences between children who fell into the category of “good sleepers” (n = 52), and those characterized as “poor sleepers” (n = 46) (P = .57). In both sleep groups, more than 70% of the children had an IQ of 70 or above, and no significant difference was found in good sleepers, compared with poor sleepers, in IQ variable (P = .87) (Sleep Med. 2018. 44:61-6).
In addition, the researchers found that poor sleepers had significantly higher scores on the Aberrant Behavior Checklist subscales of irritability, hyperactivity, stereotypic behavior, and social withdrawal/lethargy, compared with good sleepers. All subscales of the parenting stress index and the PSI total score also were significantly higher in the poor sleepers group, compared with children in the good sleepers group, reported Dr. Johnson of the University of Florida, Gainesville, and her associates.
Additional studies are needed within a “comprehensive biopsychosocial model” to advance the understanding of why some children with autism experience disrupted sleep patterns and others do not. “, regardless of age and cognitive level,” Dr. Johnson and her associates concluded. “With improved sleep, better outcomes for children with ASD could be expected.”
Read the full study in Sleep Medicine
Sleep disturbance is not associated with age and IQ in young children with autism spectrum disorder (ASD) and disruptive behaviors, according to Cynthia R. Johnson, PhD, and her associates.
They assessed 177 children aged 3-7 who were participating in the Research Units on Behavioral Intervention study, a 24-week trial. All of the children had a diagnosis of autism spectrum disorder, based on DSM-IV criteria. The diagnoses were corroborated by the Autism Diagnostic Observation Schedule and the Autism Diagnostic Interview–Revised.
The children were randomized into a parent training group or a parent education group. After getting parents to complete several forms, including the Children’s Sleep Habits Questionnaire, Dr. Johnson and her associates found no age differences between children who fell into the category of “good sleepers” (n = 52), and those characterized as “poor sleepers” (n = 46) (P = .57). In both sleep groups, more than 70% of the children had an IQ of 70 or above, and no significant difference was found in good sleepers, compared with poor sleepers, in IQ variable (P = .87) (Sleep Med. 2018. 44:61-6).
In addition, the researchers found that poor sleepers had significantly higher scores on the Aberrant Behavior Checklist subscales of irritability, hyperactivity, stereotypic behavior, and social withdrawal/lethargy, compared with good sleepers. All subscales of the parenting stress index and the PSI total score also were significantly higher in the poor sleepers group, compared with children in the good sleepers group, reported Dr. Johnson of the University of Florida, Gainesville, and her associates.
Additional studies are needed within a “comprehensive biopsychosocial model” to advance the understanding of why some children with autism experience disrupted sleep patterns and others do not. “, regardless of age and cognitive level,” Dr. Johnson and her associates concluded. “With improved sleep, better outcomes for children with ASD could be expected.”
Read the full study in Sleep Medicine
Sleep disturbance is not associated with age and IQ in young children with autism spectrum disorder (ASD) and disruptive behaviors, according to Cynthia R. Johnson, PhD, and her associates.
They assessed 177 children aged 3-7 who were participating in the Research Units on Behavioral Intervention study, a 24-week trial. All of the children had a diagnosis of autism spectrum disorder, based on DSM-IV criteria. The diagnoses were corroborated by the Autism Diagnostic Observation Schedule and the Autism Diagnostic Interview–Revised.
The children were randomized into a parent training group or a parent education group. After getting parents to complete several forms, including the Children’s Sleep Habits Questionnaire, Dr. Johnson and her associates found no age differences between children who fell into the category of “good sleepers” (n = 52), and those characterized as “poor sleepers” (n = 46) (P = .57). In both sleep groups, more than 70% of the children had an IQ of 70 or above, and no significant difference was found in good sleepers, compared with poor sleepers, in IQ variable (P = .87) (Sleep Med. 2018. 44:61-6).
In addition, the researchers found that poor sleepers had significantly higher scores on the Aberrant Behavior Checklist subscales of irritability, hyperactivity, stereotypic behavior, and social withdrawal/lethargy, compared with good sleepers. All subscales of the parenting stress index and the PSI total score also were significantly higher in the poor sleepers group, compared with children in the good sleepers group, reported Dr. Johnson of the University of Florida, Gainesville, and her associates.
Additional studies are needed within a “comprehensive biopsychosocial model” to advance the understanding of why some children with autism experience disrupted sleep patterns and others do not. “, regardless of age and cognitive level,” Dr. Johnson and her associates concluded. “With improved sleep, better outcomes for children with ASD could be expected.”
Read the full study in Sleep Medicine
FROM SLEEP MEDICINE
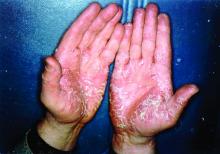
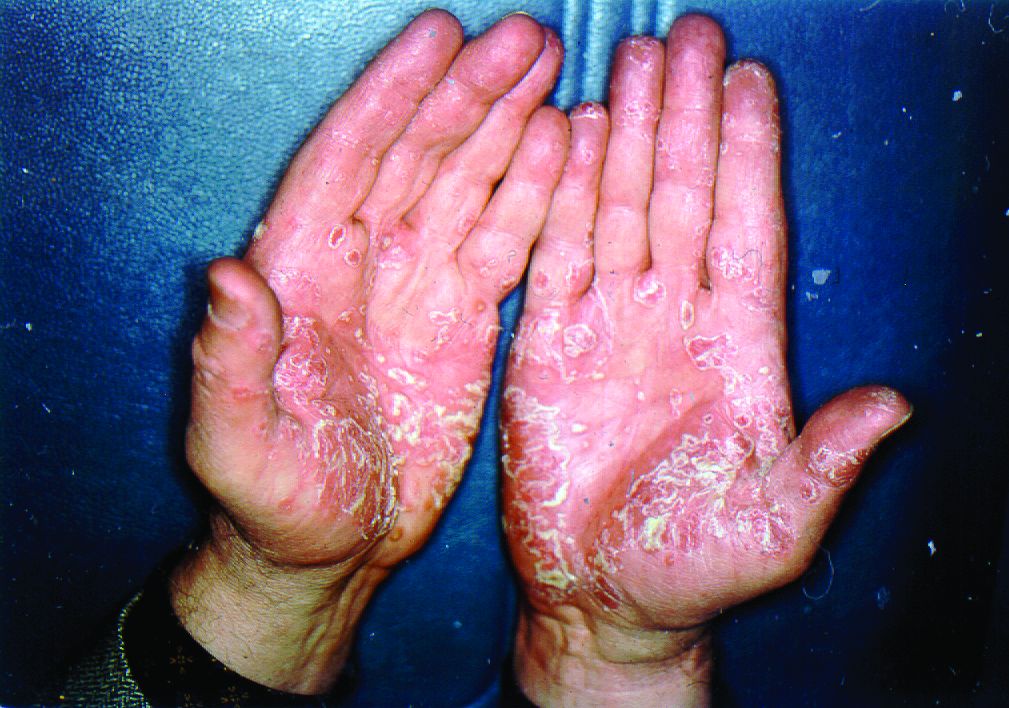
Biologics have best chance of achieving PASI 90 in psoriasis
The biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab pegol, and ustekinumab provide the best chances for achieving Psoriasis Area and Severity Index (PASI) 90 when compared with placebo in patients with moderate to severe psoriasis, according to Emilie Sbidian, MD, and her associates.
Dr. Sbidian of Henri Mondor Hospital, Créteil, France, and her colleagues conducted a network meta-analysis of 109 randomized, controlled trials that collectively had a total of 39,882 participants. The results showed that all of the interventions appeared superior to placebo in terms of reaching PASI 90.
The investigators also found that there was no significant difference between the three anti–IL-17 agents (brodalumab, ixekizumab, and secukinumab) and the two anti–IL-23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. However, all of the anti–IL-17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti–IL-23) were significantly more effective than three anti–TNF-alpha agents (infliximab, adalimumab, and etanercept).
Additionally, in the network meta-analysis, anti–IL-17 drugs were the best for achieving PASI 90, compared with placebo (RR, 30.81), followed by anti–IL-12/23 (RR, 23.16), anti–IL-23 (RR, 16.53), and anti–TNF-alpha (RR, 11.58). At the individual drug level, results showed ixekizumab was the best treatment for attaining PASI 90 when compared with placebo (RR, 32.45), followed by secukinumab (RR, 26.55), brodalumab (RR, 25.45), guselkumab (RR, 21.03), certolizumab pegol (RR, 24.58), and ustekinumab (RR, 19.91). The investigators found that “there was no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects.”
“Our main results do not reflect the way patients are managed in ‘real life,’ ” the researchers concluded. “Currently, biological treatments have been positioned as third-line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents).”
SOURCE: Sbidian E et al. Cochrane Database Syst Rev. 2017 Dec 22. doi: 10.1002/14651858.CD011535.pub2.
The biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab pegol, and ustekinumab provide the best chances for achieving Psoriasis Area and Severity Index (PASI) 90 when compared with placebo in patients with moderate to severe psoriasis, according to Emilie Sbidian, MD, and her associates.
Dr. Sbidian of Henri Mondor Hospital, Créteil, France, and her colleagues conducted a network meta-analysis of 109 randomized, controlled trials that collectively had a total of 39,882 participants. The results showed that all of the interventions appeared superior to placebo in terms of reaching PASI 90.
The investigators also found that there was no significant difference between the three anti–IL-17 agents (brodalumab, ixekizumab, and secukinumab) and the two anti–IL-23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. However, all of the anti–IL-17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti–IL-23) were significantly more effective than three anti–TNF-alpha agents (infliximab, adalimumab, and etanercept).
Additionally, in the network meta-analysis, anti–IL-17 drugs were the best for achieving PASI 90, compared with placebo (RR, 30.81), followed by anti–IL-12/23 (RR, 23.16), anti–IL-23 (RR, 16.53), and anti–TNF-alpha (RR, 11.58). At the individual drug level, results showed ixekizumab was the best treatment for attaining PASI 90 when compared with placebo (RR, 32.45), followed by secukinumab (RR, 26.55), brodalumab (RR, 25.45), guselkumab (RR, 21.03), certolizumab pegol (RR, 24.58), and ustekinumab (RR, 19.91). The investigators found that “there was no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects.”
“Our main results do not reflect the way patients are managed in ‘real life,’ ” the researchers concluded. “Currently, biological treatments have been positioned as third-line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents).”
SOURCE: Sbidian E et al. Cochrane Database Syst Rev. 2017 Dec 22. doi: 10.1002/14651858.CD011535.pub2.
The biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab pegol, and ustekinumab provide the best chances for achieving Psoriasis Area and Severity Index (PASI) 90 when compared with placebo in patients with moderate to severe psoriasis, according to Emilie Sbidian, MD, and her associates.
Dr. Sbidian of Henri Mondor Hospital, Créteil, France, and her colleagues conducted a network meta-analysis of 109 randomized, controlled trials that collectively had a total of 39,882 participants. The results showed that all of the interventions appeared superior to placebo in terms of reaching PASI 90.
The investigators also found that there was no significant difference between the three anti–IL-17 agents (brodalumab, ixekizumab, and secukinumab) and the two anti–IL-23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. However, all of the anti–IL-17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti–IL-23) were significantly more effective than three anti–TNF-alpha agents (infliximab, adalimumab, and etanercept).
Additionally, in the network meta-analysis, anti–IL-17 drugs were the best for achieving PASI 90, compared with placebo (RR, 30.81), followed by anti–IL-12/23 (RR, 23.16), anti–IL-23 (RR, 16.53), and anti–TNF-alpha (RR, 11.58). At the individual drug level, results showed ixekizumab was the best treatment for attaining PASI 90 when compared with placebo (RR, 32.45), followed by secukinumab (RR, 26.55), brodalumab (RR, 25.45), guselkumab (RR, 21.03), certolizumab pegol (RR, 24.58), and ustekinumab (RR, 19.91). The investigators found that “there was no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects.”
“Our main results do not reflect the way patients are managed in ‘real life,’ ” the researchers concluded. “Currently, biological treatments have been positioned as third-line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents).”
SOURCE: Sbidian E et al. Cochrane Database Syst Rev. 2017 Dec 22. doi: 10.1002/14651858.CD011535.pub2.
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS
More time with digital media impacts childhood well-being
In school-aged children, according to Stephanie Ruest, MD, of Hasbro Children’s Hospital, Providence, R.I., and her associates.
In a study surveying parents from the 2011-2012 National Survey of Children’s Health, behavior of 64,464 children aged 6-17 years was examined. Results found that 31% of children were reported to have a combined daily digital media exposure (DME) of less than 2 hours/day, 36% had 2-4 hours, 17% had 4-6 hours, and 16% had at least 6 hours/day of DME. Among the children with less than 2 hours of DME, 38% had access to media devices in their bedroom, compared with 73% in the greater than 6-hour exposure group.
Additionally, children who had 2-4 hours of DME per weekday not related to schoolwork had 22% lower odds of always/usually finishing their homework, compared with children who had less than 2 hours. Children with 4-6 hours/day had 46% lower odds and those with greater than 6 hours/day had 57% lower odds of demonstrating this marker, compared with children with less than 2 hours (P less than .001). “There was a similar decrease in odds seen with each of the remaining four flourishing markers (test for trend P less than .001 for each marker),” Dr. Ruest and her associates said.
“DME, when measured in combined daily hours, is inversely associated with behaviors and attitudes that have been identified as markers of childhood flourishing in a dose-dependent manner,” the researchers concluded. “Future studies should continue to work towards elucidating the complex relationship between total DME time in conjunction with the content of the media and how the interplay affects childhood flourishing.”
SOURCE: Ruest S et al. J Pediatr. 2018 Feb 1. doi: 10.1016/j.jpeds.2017.12.016.
In school-aged children, according to Stephanie Ruest, MD, of Hasbro Children’s Hospital, Providence, R.I., and her associates.
In a study surveying parents from the 2011-2012 National Survey of Children’s Health, behavior of 64,464 children aged 6-17 years was examined. Results found that 31% of children were reported to have a combined daily digital media exposure (DME) of less than 2 hours/day, 36% had 2-4 hours, 17% had 4-6 hours, and 16% had at least 6 hours/day of DME. Among the children with less than 2 hours of DME, 38% had access to media devices in their bedroom, compared with 73% in the greater than 6-hour exposure group.
Additionally, children who had 2-4 hours of DME per weekday not related to schoolwork had 22% lower odds of always/usually finishing their homework, compared with children who had less than 2 hours. Children with 4-6 hours/day had 46% lower odds and those with greater than 6 hours/day had 57% lower odds of demonstrating this marker, compared with children with less than 2 hours (P less than .001). “There was a similar decrease in odds seen with each of the remaining four flourishing markers (test for trend P less than .001 for each marker),” Dr. Ruest and her associates said.
“DME, when measured in combined daily hours, is inversely associated with behaviors and attitudes that have been identified as markers of childhood flourishing in a dose-dependent manner,” the researchers concluded. “Future studies should continue to work towards elucidating the complex relationship between total DME time in conjunction with the content of the media and how the interplay affects childhood flourishing.”
SOURCE: Ruest S et al. J Pediatr. 2018 Feb 1. doi: 10.1016/j.jpeds.2017.12.016.
In school-aged children, according to Stephanie Ruest, MD, of Hasbro Children’s Hospital, Providence, R.I., and her associates.
In a study surveying parents from the 2011-2012 National Survey of Children’s Health, behavior of 64,464 children aged 6-17 years was examined. Results found that 31% of children were reported to have a combined daily digital media exposure (DME) of less than 2 hours/day, 36% had 2-4 hours, 17% had 4-6 hours, and 16% had at least 6 hours/day of DME. Among the children with less than 2 hours of DME, 38% had access to media devices in their bedroom, compared with 73% in the greater than 6-hour exposure group.
Additionally, children who had 2-4 hours of DME per weekday not related to schoolwork had 22% lower odds of always/usually finishing their homework, compared with children who had less than 2 hours. Children with 4-6 hours/day had 46% lower odds and those with greater than 6 hours/day had 57% lower odds of demonstrating this marker, compared with children with less than 2 hours (P less than .001). “There was a similar decrease in odds seen with each of the remaining four flourishing markers (test for trend P less than .001 for each marker),” Dr. Ruest and her associates said.
“DME, when measured in combined daily hours, is inversely associated with behaviors and attitudes that have been identified as markers of childhood flourishing in a dose-dependent manner,” the researchers concluded. “Future studies should continue to work towards elucidating the complex relationship between total DME time in conjunction with the content of the media and how the interplay affects childhood flourishing.”
SOURCE: Ruest S et al. J Pediatr. 2018 Feb 1. doi: 10.1016/j.jpeds.2017.12.016.
FROM THE JOURNAL OF PEDIATRICS