User login
CV risk factors go undiagnosed, untreated in many psoriatic patients
A significant proportion of patients with psoriasis and psoriatic arthritis (PsA) are underdiagnosed and undertreated for cardiovascular risk factors (CVRF), according to Lihi Eder, MD, of the University of Toronto, and her associates.
In a cross-sectional analysis published in the Journal of Rheumatology, researchers examined 2,254 patients (58.9% with PsA, 41.1% with psoriasis only) from eight centers in Canada, the United States, and Israel. They found that 1,017 of the patients had hypertension (PsA: 48.5%, psoriasis: 40.2%), including 233 who were not previously diagnosed with hypertension and were not taking any blood pressure–lowering medications (PsA: 19.9%, psoriasis: 39.1%). Many patients had low adherence to hypertension treatment recommendations: A total of 602 (PsA: 55.9%, psoriasis: 64.8%) were untreated or undertreated. Undertreatment of hypertension occurred in 60.9% patients with cardiovascular disease or diabetes mellitus.
“In this large international study, we found significant gaps in screening and treating CVRF in patients with psoriasis and PsA,” the researchers concluded. “Although questions exist regarding the optimal treatment targets for CVRF in psoriatic patients, adherence by physicians to, at a minimum, the general treatment recommendations for primary CV prevention is warranted.”
SOURCE: Eder L et al. J Rheumatol. 2018 Feb 1. doi: 10.3899/jrheum.170379.
A significant proportion of patients with psoriasis and psoriatic arthritis (PsA) are underdiagnosed and undertreated for cardiovascular risk factors (CVRF), according to Lihi Eder, MD, of the University of Toronto, and her associates.
In a cross-sectional analysis published in the Journal of Rheumatology, researchers examined 2,254 patients (58.9% with PsA, 41.1% with psoriasis only) from eight centers in Canada, the United States, and Israel. They found that 1,017 of the patients had hypertension (PsA: 48.5%, psoriasis: 40.2%), including 233 who were not previously diagnosed with hypertension and were not taking any blood pressure–lowering medications (PsA: 19.9%, psoriasis: 39.1%). Many patients had low adherence to hypertension treatment recommendations: A total of 602 (PsA: 55.9%, psoriasis: 64.8%) were untreated or undertreated. Undertreatment of hypertension occurred in 60.9% patients with cardiovascular disease or diabetes mellitus.
“In this large international study, we found significant gaps in screening and treating CVRF in patients with psoriasis and PsA,” the researchers concluded. “Although questions exist regarding the optimal treatment targets for CVRF in psoriatic patients, adherence by physicians to, at a minimum, the general treatment recommendations for primary CV prevention is warranted.”
SOURCE: Eder L et al. J Rheumatol. 2018 Feb 1. doi: 10.3899/jrheum.170379.
A significant proportion of patients with psoriasis and psoriatic arthritis (PsA) are underdiagnosed and undertreated for cardiovascular risk factors (CVRF), according to Lihi Eder, MD, of the University of Toronto, and her associates.
In a cross-sectional analysis published in the Journal of Rheumatology, researchers examined 2,254 patients (58.9% with PsA, 41.1% with psoriasis only) from eight centers in Canada, the United States, and Israel. They found that 1,017 of the patients had hypertension (PsA: 48.5%, psoriasis: 40.2%), including 233 who were not previously diagnosed with hypertension and were not taking any blood pressure–lowering medications (PsA: 19.9%, psoriasis: 39.1%). Many patients had low adherence to hypertension treatment recommendations: A total of 602 (PsA: 55.9%, psoriasis: 64.8%) were untreated or undertreated. Undertreatment of hypertension occurred in 60.9% patients with cardiovascular disease or diabetes mellitus.
“In this large international study, we found significant gaps in screening and treating CVRF in patients with psoriasis and PsA,” the researchers concluded. “Although questions exist regarding the optimal treatment targets for CVRF in psoriatic patients, adherence by physicians to, at a minimum, the general treatment recommendations for primary CV prevention is warranted.”
SOURCE: Eder L et al. J Rheumatol. 2018 Feb 1. doi: 10.3899/jrheum.170379.
FROM JOURNAL OF RHEUMATOLOGY
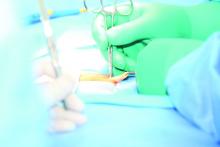
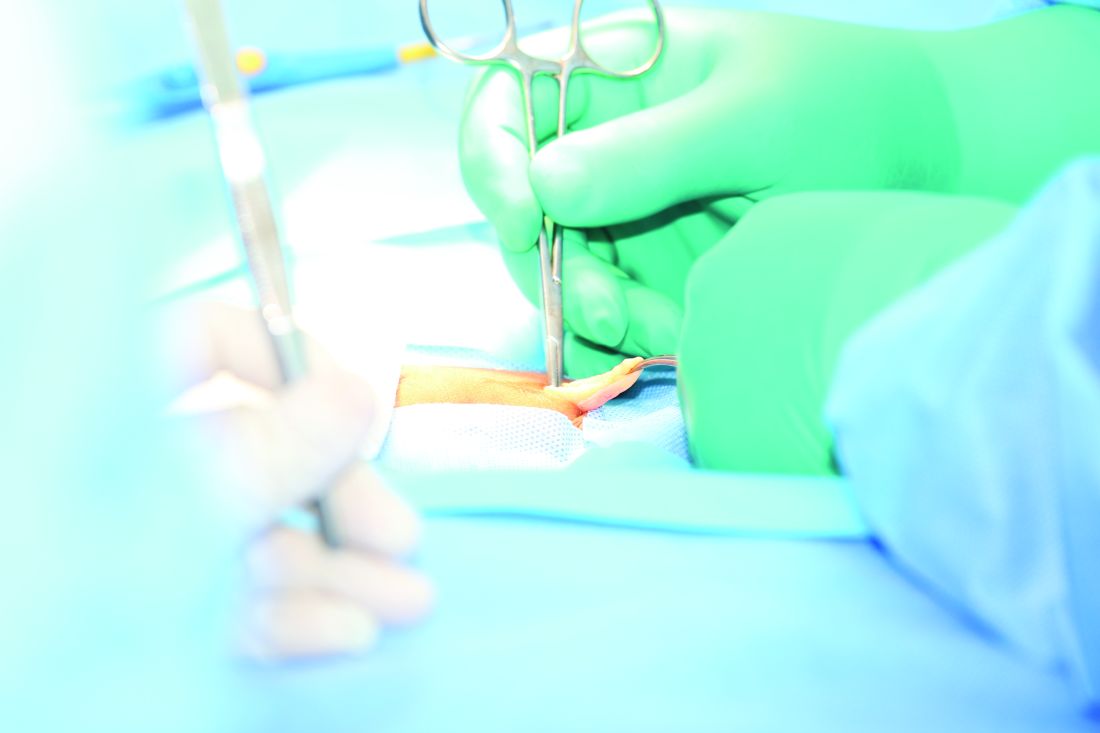
Hernia repair patients had less pain with lightweight mesh
and less discomfort at 1 year in a group of patients who had lightweight mesh, compared with patients who had heavyweight mesh, in a multicenter, randomized clinical trial.
Martin Rutegård, MD, of Umeå (Sweden) University, and his research associates reported that 3%-10% of all hernia surgeries “result in severe or moderately severe pain for more than a year after hernia surgery, which may have a significant impact on social activities, sex life, and quality of life. ... Interest in the use of lightweight meshes in groin hernia repair has increased in recent years, as it is assumed that this type of mesh may cause less discomfort and chronic pain.”
Patients were followed for 1-3 years and given questionnaires to report their outcomes. Patients were all male and were close in weight (mean body mass index, 25.2 kg/m2 in the lightweight-mesh group and 25.3 in the heavyweight-mesh group), age (59 and 58, respectively), and American Society of Anaesthesiologists classification of their hernia defect.
Of a total of 363 patients, 185 patients were randomized to the lightweight-mesh group and 178 patients to the heavyweight group. Investigators found that there were significant differences concerning awareness of a groin lump and groin discomfort, favoring the lightweight group 1 year after surgery. A total of 6% of the lightweight group reported the groin lump awareness at 1 year, vs. 18% of the heavyweight group. Groin discomfort was reported by 18% of the lightweight group and 28% of the heavyweight group.
After 1 year, that difference subsided. In terms of discomfort, the investigators found no statistically significant or clinically relevant differences between types of mesh, with 263/288 patients (91.3%) reporting improvement after 12 months, 19/288 patients (6.6%) experiencing no change, and 6/288 patients (2.1%) having worsened.
Additionally, there was no statistically significant difference in quality of life as measured by the EuroQol five dimensions (EQ-5D) between the different mesh groups. It was noted that all the patients had a statistically significantly better quality of life postoperatively from day 11 and onward, compared with before surgery. In addition, the investigators did not detect a significant difference between the mesh groups in their reported sexual life after surgery at 4 and 12 months subsequent to the operation.
The recurrence rate at the follow-up visit and clinical examination was 2.4% and equal between both groups.
The study was limited by possible bias of the expertise-based design and also some missing data, especially with regard to sexual life after surgery.
The study was funded by the Västerbotten County Council, VISARE NORR Fund, and Northern Country Councils Regional Federation. The investigators reported no conflicts of interest.
SOURCE: M. Rutegård et al. Hernia 2018 Jan 20. doi: 10.1007/s10029-018-1734-z.
and less discomfort at 1 year in a group of patients who had lightweight mesh, compared with patients who had heavyweight mesh, in a multicenter, randomized clinical trial.
Martin Rutegård, MD, of Umeå (Sweden) University, and his research associates reported that 3%-10% of all hernia surgeries “result in severe or moderately severe pain for more than a year after hernia surgery, which may have a significant impact on social activities, sex life, and quality of life. ... Interest in the use of lightweight meshes in groin hernia repair has increased in recent years, as it is assumed that this type of mesh may cause less discomfort and chronic pain.”
Patients were followed for 1-3 years and given questionnaires to report their outcomes. Patients were all male and were close in weight (mean body mass index, 25.2 kg/m2 in the lightweight-mesh group and 25.3 in the heavyweight-mesh group), age (59 and 58, respectively), and American Society of Anaesthesiologists classification of their hernia defect.
Of a total of 363 patients, 185 patients were randomized to the lightweight-mesh group and 178 patients to the heavyweight group. Investigators found that there were significant differences concerning awareness of a groin lump and groin discomfort, favoring the lightweight group 1 year after surgery. A total of 6% of the lightweight group reported the groin lump awareness at 1 year, vs. 18% of the heavyweight group. Groin discomfort was reported by 18% of the lightweight group and 28% of the heavyweight group.
After 1 year, that difference subsided. In terms of discomfort, the investigators found no statistically significant or clinically relevant differences between types of mesh, with 263/288 patients (91.3%) reporting improvement after 12 months, 19/288 patients (6.6%) experiencing no change, and 6/288 patients (2.1%) having worsened.
Additionally, there was no statistically significant difference in quality of life as measured by the EuroQol five dimensions (EQ-5D) between the different mesh groups. It was noted that all the patients had a statistically significantly better quality of life postoperatively from day 11 and onward, compared with before surgery. In addition, the investigators did not detect a significant difference between the mesh groups in their reported sexual life after surgery at 4 and 12 months subsequent to the operation.
The recurrence rate at the follow-up visit and clinical examination was 2.4% and equal between both groups.
The study was limited by possible bias of the expertise-based design and also some missing data, especially with regard to sexual life after surgery.
The study was funded by the Västerbotten County Council, VISARE NORR Fund, and Northern Country Councils Regional Federation. The investigators reported no conflicts of interest.
SOURCE: M. Rutegård et al. Hernia 2018 Jan 20. doi: 10.1007/s10029-018-1734-z.
and less discomfort at 1 year in a group of patients who had lightweight mesh, compared with patients who had heavyweight mesh, in a multicenter, randomized clinical trial.
Martin Rutegård, MD, of Umeå (Sweden) University, and his research associates reported that 3%-10% of all hernia surgeries “result in severe or moderately severe pain for more than a year after hernia surgery, which may have a significant impact on social activities, sex life, and quality of life. ... Interest in the use of lightweight meshes in groin hernia repair has increased in recent years, as it is assumed that this type of mesh may cause less discomfort and chronic pain.”
Patients were followed for 1-3 years and given questionnaires to report their outcomes. Patients were all male and were close in weight (mean body mass index, 25.2 kg/m2 in the lightweight-mesh group and 25.3 in the heavyweight-mesh group), age (59 and 58, respectively), and American Society of Anaesthesiologists classification of their hernia defect.
Of a total of 363 patients, 185 patients were randomized to the lightweight-mesh group and 178 patients to the heavyweight group. Investigators found that there were significant differences concerning awareness of a groin lump and groin discomfort, favoring the lightweight group 1 year after surgery. A total of 6% of the lightweight group reported the groin lump awareness at 1 year, vs. 18% of the heavyweight group. Groin discomfort was reported by 18% of the lightweight group and 28% of the heavyweight group.
After 1 year, that difference subsided. In terms of discomfort, the investigators found no statistically significant or clinically relevant differences between types of mesh, with 263/288 patients (91.3%) reporting improvement after 12 months, 19/288 patients (6.6%) experiencing no change, and 6/288 patients (2.1%) having worsened.
Additionally, there was no statistically significant difference in quality of life as measured by the EuroQol five dimensions (EQ-5D) between the different mesh groups. It was noted that all the patients had a statistically significantly better quality of life postoperatively from day 11 and onward, compared with before surgery. In addition, the investigators did not detect a significant difference between the mesh groups in their reported sexual life after surgery at 4 and 12 months subsequent to the operation.
The recurrence rate at the follow-up visit and clinical examination was 2.4% and equal between both groups.
The study was limited by possible bias of the expertise-based design and also some missing data, especially with regard to sexual life after surgery.
The study was funded by the Västerbotten County Council, VISARE NORR Fund, and Northern Country Councils Regional Federation. The investigators reported no conflicts of interest.
SOURCE: M. Rutegård et al. Hernia 2018 Jan 20. doi: 10.1007/s10029-018-1734-z.
FROM HERNIA
Key clinical point: The weight of mesh used for inguinal hernia repair did not impact outcomes.
Major finding: A total of 6% of the lightweight group reported groin lump awareness at 1 year, vs. 18% of the heavyweight group.
Study details: A randomized, multicenter study of 363 patients.
Disclosures: The study was funded by the Västerbotten County Council, VISARE NORR Fund, and Northern Country Councils Regional Federation. The investigators reported no conflicts of interest.
Source: Rutegård M et al. Hernia. 2018 Jan 20. doi: 10.1007/s10029-018-1734-z.
FDA approves implantable therapy for PAH
to treat adult patients with New York Heart Association (NYHA) Class I, II and III pulmonary arterial hypertension.
This infusion system is implanted into a patient for intravenous delivery of treprostinil (Remodulin) and is designed to help supply blood to the lungs and keep a patient’s blood pressure within a healthy range. The system comprises three parts: the pump, the programmer, and the catheter.
The implant should not be used for patients with NYHA Class IV heart failure, a known or suspected infection, bacteremia, or sepsis requiring antibiotics; vasculature that is inadequate for an 8 French introducer or catheter advancement without stylet guidance; implanted leads or catheters (active or abandoned) in the superior vena cava that cannot be removed prior to or at system implant; a body size not sufficient to accept the pump; or skin or soft tissue that would heal poorly or increase susceptibility to infections. Patients who are unable to tolerate a sudden cessation of treprostinil therapy also would not be able to receive the implantable device.
Read the full approval on the FDA’s website.
to treat adult patients with New York Heart Association (NYHA) Class I, II and III pulmonary arterial hypertension.
This infusion system is implanted into a patient for intravenous delivery of treprostinil (Remodulin) and is designed to help supply blood to the lungs and keep a patient’s blood pressure within a healthy range. The system comprises three parts: the pump, the programmer, and the catheter.
The implant should not be used for patients with NYHA Class IV heart failure, a known or suspected infection, bacteremia, or sepsis requiring antibiotics; vasculature that is inadequate for an 8 French introducer or catheter advancement without stylet guidance; implanted leads or catheters (active or abandoned) in the superior vena cava that cannot be removed prior to or at system implant; a body size not sufficient to accept the pump; or skin or soft tissue that would heal poorly or increase susceptibility to infections. Patients who are unable to tolerate a sudden cessation of treprostinil therapy also would not be able to receive the implantable device.
Read the full approval on the FDA’s website.
to treat adult patients with New York Heart Association (NYHA) Class I, II and III pulmonary arterial hypertension.
This infusion system is implanted into a patient for intravenous delivery of treprostinil (Remodulin) and is designed to help supply blood to the lungs and keep a patient’s blood pressure within a healthy range. The system comprises three parts: the pump, the programmer, and the catheter.
The implant should not be used for patients with NYHA Class IV heart failure, a known or suspected infection, bacteremia, or sepsis requiring antibiotics; vasculature that is inadequate for an 8 French introducer or catheter advancement without stylet guidance; implanted leads or catheters (active or abandoned) in the superior vena cava that cannot be removed prior to or at system implant; a body size not sufficient to accept the pump; or skin or soft tissue that would heal poorly or increase susceptibility to infections. Patients who are unable to tolerate a sudden cessation of treprostinil therapy also would not be able to receive the implantable device.
Read the full approval on the FDA’s website.
FDA issues safety alert for loperamide
The Food and Drug Administration announced Jan. 30 that is has issued a MedWatch safety alert on the use of the over-the-counter (OTC) antidiarrhea drug, loperamide.
Currently, the FDA is working with manufacturers to use blister packs or other single-dose packaging and to limit the number of doses in a package.
The alert comes after receiving continuous reports of serious heart problems and deaths with the use of much higher than recommended doses of loperamide, mainly among people who are intentionally misusing or abusing the product, regardless of the addition of a warning to the medicine label and a previous communication. The FDA states that loperamide is a safe drug when used as directed.
Loperamide is approved to help control symptoms of diarrhea. The maximum recommended daily dose for adults is 8 mg per day for OTC use and 16 mg per day for prescription use. It acts on opioid receptors in the gut to slow the movement in the intestines and decrease the number of bowel movements.
It is noted that much higher than recommended doses of loperamide, either intentionally or unintentionally, can result in serious cardiac adverse events, including QT interval prolongation, torsade de pointes or other ventricular arrhythmias, syncope, and cardiac arrest. Health care professionals and patients can report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
In 2016, the FDA issued a Drug Safety Communication and added warnings about serious heart problems to the drug label of prescription loperamide and to the Drug Facts label of OTC loperamide products. The FDA is working to evaluate this safety issue and will update the public when more information is available.
Read the full safety alert here.
The Food and Drug Administration announced Jan. 30 that is has issued a MedWatch safety alert on the use of the over-the-counter (OTC) antidiarrhea drug, loperamide.
Currently, the FDA is working with manufacturers to use blister packs or other single-dose packaging and to limit the number of doses in a package.
The alert comes after receiving continuous reports of serious heart problems and deaths with the use of much higher than recommended doses of loperamide, mainly among people who are intentionally misusing or abusing the product, regardless of the addition of a warning to the medicine label and a previous communication. The FDA states that loperamide is a safe drug when used as directed.
Loperamide is approved to help control symptoms of diarrhea. The maximum recommended daily dose for adults is 8 mg per day for OTC use and 16 mg per day for prescription use. It acts on opioid receptors in the gut to slow the movement in the intestines and decrease the number of bowel movements.
It is noted that much higher than recommended doses of loperamide, either intentionally or unintentionally, can result in serious cardiac adverse events, including QT interval prolongation, torsade de pointes or other ventricular arrhythmias, syncope, and cardiac arrest. Health care professionals and patients can report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
In 2016, the FDA issued a Drug Safety Communication and added warnings about serious heart problems to the drug label of prescription loperamide and to the Drug Facts label of OTC loperamide products. The FDA is working to evaluate this safety issue and will update the public when more information is available.
Read the full safety alert here.
The Food and Drug Administration announced Jan. 30 that is has issued a MedWatch safety alert on the use of the over-the-counter (OTC) antidiarrhea drug, loperamide.
Currently, the FDA is working with manufacturers to use blister packs or other single-dose packaging and to limit the number of doses in a package.
The alert comes after receiving continuous reports of serious heart problems and deaths with the use of much higher than recommended doses of loperamide, mainly among people who are intentionally misusing or abusing the product, regardless of the addition of a warning to the medicine label and a previous communication. The FDA states that loperamide is a safe drug when used as directed.
Loperamide is approved to help control symptoms of diarrhea. The maximum recommended daily dose for adults is 8 mg per day for OTC use and 16 mg per day for prescription use. It acts on opioid receptors in the gut to slow the movement in the intestines and decrease the number of bowel movements.
It is noted that much higher than recommended doses of loperamide, either intentionally or unintentionally, can result in serious cardiac adverse events, including QT interval prolongation, torsade de pointes or other ventricular arrhythmias, syncope, and cardiac arrest. Health care professionals and patients can report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
In 2016, the FDA issued a Drug Safety Communication and added warnings about serious heart problems to the drug label of prescription loperamide and to the Drug Facts label of OTC loperamide products. The FDA is working to evaluate this safety issue and will update the public when more information is available.
Read the full safety alert here.
FDA approves lutetium Lu 177 dotatate for GEP-NETs
The Food and Drug Administration has approved the first radiopharmaceutical, lutetium Lu 177 dotatate (Lutathera), for the treatment of somatostatin receptor positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs), including foregut, midgut, and hindgut neuroendocrine tumors in adults.
Approval is based on two studies, including the phase 3, NETTER-1, that compared lutetium Lu 177 dotatate plus octreotide to octreotide alone, and a subset of patients from an expanded access program in the Netherlands in patients with somatostatin receptor positive tumors, the FDA said in a statement.
In the expanded access study, complete or partial tumor shrinkage was reported in 16% of the patients in the subset of 360 patients with GEP-NETs.
Common side effects include lymphopenia, increased GGT, AST and/or ALT, vomiting, nausea, hyperglycemia and hypokalemia.
Serious side effects include myelosuppression, secondary myelodysplastic syndrome and leukemia, renal toxicity, hepatotoxicity, neuroendocrine hormonal crises, and infertility. Patients taking lutetium Lu 177 dotatate are exposed to radiation and exposure of other patients, medical personnel, and household members should be limited in accordance with radiation safety practices, the FDA said.
The Food and Drug Administration has approved the first radiopharmaceutical, lutetium Lu 177 dotatate (Lutathera), for the treatment of somatostatin receptor positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs), including foregut, midgut, and hindgut neuroendocrine tumors in adults.
Approval is based on two studies, including the phase 3, NETTER-1, that compared lutetium Lu 177 dotatate plus octreotide to octreotide alone, and a subset of patients from an expanded access program in the Netherlands in patients with somatostatin receptor positive tumors, the FDA said in a statement.
In the expanded access study, complete or partial tumor shrinkage was reported in 16% of the patients in the subset of 360 patients with GEP-NETs.
Common side effects include lymphopenia, increased GGT, AST and/or ALT, vomiting, nausea, hyperglycemia and hypokalemia.
Serious side effects include myelosuppression, secondary myelodysplastic syndrome and leukemia, renal toxicity, hepatotoxicity, neuroendocrine hormonal crises, and infertility. Patients taking lutetium Lu 177 dotatate are exposed to radiation and exposure of other patients, medical personnel, and household members should be limited in accordance with radiation safety practices, the FDA said.
The Food and Drug Administration has approved the first radiopharmaceutical, lutetium Lu 177 dotatate (Lutathera), for the treatment of somatostatin receptor positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs), including foregut, midgut, and hindgut neuroendocrine tumors in adults.
Approval is based on two studies, including the phase 3, NETTER-1, that compared lutetium Lu 177 dotatate plus octreotide to octreotide alone, and a subset of patients from an expanded access program in the Netherlands in patients with somatostatin receptor positive tumors, the FDA said in a statement.
In the expanded access study, complete or partial tumor shrinkage was reported in 16% of the patients in the subset of 360 patients with GEP-NETs.
Common side effects include lymphopenia, increased GGT, AST and/or ALT, vomiting, nausea, hyperglycemia and hypokalemia.
Serious side effects include myelosuppression, secondary myelodysplastic syndrome and leukemia, renal toxicity, hepatotoxicity, neuroendocrine hormonal crises, and infertility. Patients taking lutetium Lu 177 dotatate are exposed to radiation and exposure of other patients, medical personnel, and household members should be limited in accordance with radiation safety practices, the FDA said.
FDA grants ‘Breakthrough Therapy Designation’ for upadacitinib for atopic dermatitis
The Food and Drug Administration has granted “Breakthrough Therapy Designation” for the investigational, once-daily oral Janus kinase 1 (JAK1)-selective inhibitor upadacitinib (ABT-494) in adult patients with moderate to severe atopic dermatitis who are candidates for systemic therapy.
The Breakthrough Therapy Designation is based on positive phase 2b results announced in Sept. 2017. The study found that patients treated with upadacitinib achieved statistically significant improvements in the primary endpoint (greater mean percentage change from baseline in Eczema Area and Severity Index score) and in all skin- and itch-specific secondary endpoints across all doses (30 mg, 15mg, or 7.5 mg once-daily) at week 16, compared with placebo (P less than .05). Reduction in itch was observed within the first week and improvement in skin within the first 2 weeks (P less than .001 across all doses). Of patients receiving the 30 mg once-daily dose of upadacitinib, 50% had clear or almost clear skin, according to a press release. There were 42 patients in each of the three treatment groups and 41 patients in the placebo group in this randomized, double-blind, parallel-group study sponsored by AbbVie, which discovered and developed upadacitinib.
The phase 3 clinical program is expected to begin in the first half of 2018, according to AbbVie. Any additional information on the clinical trials for upadacitinib is available at clinicaltrials.gov.
SOURCE: Prnewswire.com.
The Food and Drug Administration has granted “Breakthrough Therapy Designation” for the investigational, once-daily oral Janus kinase 1 (JAK1)-selective inhibitor upadacitinib (ABT-494) in adult patients with moderate to severe atopic dermatitis who are candidates for systemic therapy.
The Breakthrough Therapy Designation is based on positive phase 2b results announced in Sept. 2017. The study found that patients treated with upadacitinib achieved statistically significant improvements in the primary endpoint (greater mean percentage change from baseline in Eczema Area and Severity Index score) and in all skin- and itch-specific secondary endpoints across all doses (30 mg, 15mg, or 7.5 mg once-daily) at week 16, compared with placebo (P less than .05). Reduction in itch was observed within the first week and improvement in skin within the first 2 weeks (P less than .001 across all doses). Of patients receiving the 30 mg once-daily dose of upadacitinib, 50% had clear or almost clear skin, according to a press release. There were 42 patients in each of the three treatment groups and 41 patients in the placebo group in this randomized, double-blind, parallel-group study sponsored by AbbVie, which discovered and developed upadacitinib.
The phase 3 clinical program is expected to begin in the first half of 2018, according to AbbVie. Any additional information on the clinical trials for upadacitinib is available at clinicaltrials.gov.
SOURCE: Prnewswire.com.
The Food and Drug Administration has granted “Breakthrough Therapy Designation” for the investigational, once-daily oral Janus kinase 1 (JAK1)-selective inhibitor upadacitinib (ABT-494) in adult patients with moderate to severe atopic dermatitis who are candidates for systemic therapy.
The Breakthrough Therapy Designation is based on positive phase 2b results announced in Sept. 2017. The study found that patients treated with upadacitinib achieved statistically significant improvements in the primary endpoint (greater mean percentage change from baseline in Eczema Area and Severity Index score) and in all skin- and itch-specific secondary endpoints across all doses (30 mg, 15mg, or 7.5 mg once-daily) at week 16, compared with placebo (P less than .05). Reduction in itch was observed within the first week and improvement in skin within the first 2 weeks (P less than .001 across all doses). Of patients receiving the 30 mg once-daily dose of upadacitinib, 50% had clear or almost clear skin, according to a press release. There were 42 patients in each of the three treatment groups and 41 patients in the placebo group in this randomized, double-blind, parallel-group study sponsored by AbbVie, which discovered and developed upadacitinib.
The phase 3 clinical program is expected to begin in the first half of 2018, according to AbbVie. Any additional information on the clinical trials for upadacitinib is available at clinicaltrials.gov.
SOURCE: Prnewswire.com.
FDA approves injection treatment for low-risk APL
The Food and Drug Administration announced the approval of arsenic trioxide injection (Trisenox) in combination with tretinoin for the treatment of adults with newly diagnosed, low-risk acute promyelocytic leukemia (APL) characterized by t(15;17) translocation or PML/RAR-alpha gene expression.
The expanded indication was granted by the FDA on Jan. 12 after priority review. It is based on published studies and a review of Teva’s global safety database for arsenic trioxide.
A recent randomized, phase 3 trial compared tretinoin plus arsenic trioxide with tretinoin plus chemotherapy as first-line treatment for APL (J Clin Oncol. 2017 Feb 20;35[6]:605-12). It found that 100% of 127 patients in the tretinoin plus arsenic trioxide arm achieved complete remission, compared with 97% of 136 patients in the tretinoin plus chemotherapy arm. After a median follow-up of 40.6 months, the event-free survival at 50 months for patients in the tretinoin/arsenic trioxide arm was 97.3% vs. 80% for tretinoin/chemotherapy (P = .001).
The arsenic trioxide injection carries a boxed warning for differentiation syndrome and cardiac conduction abnormalities.
The Food and Drug Administration announced the approval of arsenic trioxide injection (Trisenox) in combination with tretinoin for the treatment of adults with newly diagnosed, low-risk acute promyelocytic leukemia (APL) characterized by t(15;17) translocation or PML/RAR-alpha gene expression.
The expanded indication was granted by the FDA on Jan. 12 after priority review. It is based on published studies and a review of Teva’s global safety database for arsenic trioxide.
A recent randomized, phase 3 trial compared tretinoin plus arsenic trioxide with tretinoin plus chemotherapy as first-line treatment for APL (J Clin Oncol. 2017 Feb 20;35[6]:605-12). It found that 100% of 127 patients in the tretinoin plus arsenic trioxide arm achieved complete remission, compared with 97% of 136 patients in the tretinoin plus chemotherapy arm. After a median follow-up of 40.6 months, the event-free survival at 50 months for patients in the tretinoin/arsenic trioxide arm was 97.3% vs. 80% for tretinoin/chemotherapy (P = .001).
The arsenic trioxide injection carries a boxed warning for differentiation syndrome and cardiac conduction abnormalities.
The Food and Drug Administration announced the approval of arsenic trioxide injection (Trisenox) in combination with tretinoin for the treatment of adults with newly diagnosed, low-risk acute promyelocytic leukemia (APL) characterized by t(15;17) translocation or PML/RAR-alpha gene expression.
The expanded indication was granted by the FDA on Jan. 12 after priority review. It is based on published studies and a review of Teva’s global safety database for arsenic trioxide.
A recent randomized, phase 3 trial compared tretinoin plus arsenic trioxide with tretinoin plus chemotherapy as first-line treatment for APL (J Clin Oncol. 2017 Feb 20;35[6]:605-12). It found that 100% of 127 patients in the tretinoin plus arsenic trioxide arm achieved complete remission, compared with 97% of 136 patients in the tretinoin plus chemotherapy arm. After a median follow-up of 40.6 months, the event-free survival at 50 months for patients in the tretinoin/arsenic trioxide arm was 97.3% vs. 80% for tretinoin/chemotherapy (P = .001).
The arsenic trioxide injection carries a boxed warning for differentiation syndrome and cardiac conduction abnormalities.
FDA grants breakthrough therapy designation for severe aplastic anemia drug
The Food and Drug Administration has granted breakthrough therapy designation to eltrombopag (Promacta) for use in combination with standard immunosuppressive therapy as a first-line treatment for patients with severe aplastic anemia (SAA).
Eltrombopag already is approved as a second-line therapy in patients with refractory SAA and is approved for adults and children with refractory chronic immune thrombocytopenia (ITP).
“Promacta is a promising medicine that, if approved for first-line use in severe aplastic anemia, may redefine the standard of care for patients with this rare and serious bone marrow condition,” Samit Hirawat, MD, head of Novartis Oncology Global Drug Development, said in a statement.
The Food and Drug Administration has granted breakthrough therapy designation to eltrombopag (Promacta) for use in combination with standard immunosuppressive therapy as a first-line treatment for patients with severe aplastic anemia (SAA).
Eltrombopag already is approved as a second-line therapy in patients with refractory SAA and is approved for adults and children with refractory chronic immune thrombocytopenia (ITP).
“Promacta is a promising medicine that, if approved for first-line use in severe aplastic anemia, may redefine the standard of care for patients with this rare and serious bone marrow condition,” Samit Hirawat, MD, head of Novartis Oncology Global Drug Development, said in a statement.
The Food and Drug Administration has granted breakthrough therapy designation to eltrombopag (Promacta) for use in combination with standard immunosuppressive therapy as a first-line treatment for patients with severe aplastic anemia (SAA).
Eltrombopag already is approved as a second-line therapy in patients with refractory SAA and is approved for adults and children with refractory chronic immune thrombocytopenia (ITP).
“Promacta is a promising medicine that, if approved for first-line use in severe aplastic anemia, may redefine the standard of care for patients with this rare and serious bone marrow condition,” Samit Hirawat, MD, head of Novartis Oncology Global Drug Development, said in a statement.
Autologous stem-cell transplantation for scleroderma beats cyclophosphamide in long term
with improved event-free and overall survival over intravenous cyclophosphamide, according to Keith M. Sullivan, MD, and his associates.
A total of 75 patients with severe scleroderma (diffuse cutaneous systemic sclerosis) underwent randomization to open-label treatment with either transplantation or intravenous cyclophosphamide at an initial dose of 500 mg/m2 of body-surface area, followed by 11 monthly infusions of 750 mg/m2 with mesna prophylaxis.
Additionally in the per-protocol population, the rate of event-free survival at 54 months was 79% in the transplantation group and 50% in the cyclophosphamide group, while the Kaplan-Meier estimated rate of event-free survival at 72 months was 74% in the transplantation group and 47% in the cyclophosphamide group (P = .03), and the rate of overall survival was 86% and 51% (P = .02). Also, in the per-protocol population at 54 months, the percentage of participants who had initiated disease-modifying antirheumatic drugs was lower in the transplantation group than in the cyclophosphamide group (9% vs. 44%; P = .001).
The researchers noted that 21 deaths occurred over a period of 72 months, including 7 participants in the transplantation group, of whom 3 died without receiving a transplant. The percentage of participants who had serious adverse events in the 72-month period was lower in the cyclophosphamide group than in the transplantation group (51% vs. 74%).
“In conclusion, at 54 months after randomization, myeloablative CD34-positive selected autologous hematopoietic stem-cell transplantation resulted in significantly better clinical outcomes than 12 months of cyclophosphamide,” the researchers wrote. “Although there was greater hematopoietic toxicity and an unquantified risk of second cancers from exposure to total body irradiation, toxic effects should be weighed against the beneficial results of treatment and the seriousness of the underlying disease.”
with improved event-free and overall survival over intravenous cyclophosphamide, according to Keith M. Sullivan, MD, and his associates.
A total of 75 patients with severe scleroderma (diffuse cutaneous systemic sclerosis) underwent randomization to open-label treatment with either transplantation or intravenous cyclophosphamide at an initial dose of 500 mg/m2 of body-surface area, followed by 11 monthly infusions of 750 mg/m2 with mesna prophylaxis.
Additionally in the per-protocol population, the rate of event-free survival at 54 months was 79% in the transplantation group and 50% in the cyclophosphamide group, while the Kaplan-Meier estimated rate of event-free survival at 72 months was 74% in the transplantation group and 47% in the cyclophosphamide group (P = .03), and the rate of overall survival was 86% and 51% (P = .02). Also, in the per-protocol population at 54 months, the percentage of participants who had initiated disease-modifying antirheumatic drugs was lower in the transplantation group than in the cyclophosphamide group (9% vs. 44%; P = .001).
The researchers noted that 21 deaths occurred over a period of 72 months, including 7 participants in the transplantation group, of whom 3 died without receiving a transplant. The percentage of participants who had serious adverse events in the 72-month period was lower in the cyclophosphamide group than in the transplantation group (51% vs. 74%).
“In conclusion, at 54 months after randomization, myeloablative CD34-positive selected autologous hematopoietic stem-cell transplantation resulted in significantly better clinical outcomes than 12 months of cyclophosphamide,” the researchers wrote. “Although there was greater hematopoietic toxicity and an unquantified risk of second cancers from exposure to total body irradiation, toxic effects should be weighed against the beneficial results of treatment and the seriousness of the underlying disease.”
with improved event-free and overall survival over intravenous cyclophosphamide, according to Keith M. Sullivan, MD, and his associates.
A total of 75 patients with severe scleroderma (diffuse cutaneous systemic sclerosis) underwent randomization to open-label treatment with either transplantation or intravenous cyclophosphamide at an initial dose of 500 mg/m2 of body-surface area, followed by 11 monthly infusions of 750 mg/m2 with mesna prophylaxis.
Additionally in the per-protocol population, the rate of event-free survival at 54 months was 79% in the transplantation group and 50% in the cyclophosphamide group, while the Kaplan-Meier estimated rate of event-free survival at 72 months was 74% in the transplantation group and 47% in the cyclophosphamide group (P = .03), and the rate of overall survival was 86% and 51% (P = .02). Also, in the per-protocol population at 54 months, the percentage of participants who had initiated disease-modifying antirheumatic drugs was lower in the transplantation group than in the cyclophosphamide group (9% vs. 44%; P = .001).
The researchers noted that 21 deaths occurred over a period of 72 months, including 7 participants in the transplantation group, of whom 3 died without receiving a transplant. The percentage of participants who had serious adverse events in the 72-month period was lower in the cyclophosphamide group than in the transplantation group (51% vs. 74%).
“In conclusion, at 54 months after randomization, myeloablative CD34-positive selected autologous hematopoietic stem-cell transplantation resulted in significantly better clinical outcomes than 12 months of cyclophosphamide,” the researchers wrote. “Although there was greater hematopoietic toxicity and an unquantified risk of second cancers from exposure to total body irradiation, toxic effects should be weighed against the beneficial results of treatment and the seriousness of the underlying disease.”
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
FDA approves tofacitinib for psoriatic arthritis
(PsA) who have had an inadequate response or intolerance to methotrexate or other disease-modifying antirheumatic drugs (DMARDs), according to a Dec. 14 announcement from its manufacturer, Pfizer.
The approvals of tofacitinib (Xeljanz) at 5 mg twice daily and extended-release tofacitinib (Xeljanz XR) at 11 mg once daily are based on data from the phase 3 Oral Psoriatic Arthritis Trial (OPAL) clinical development program, which consisted of two pivotal studies, OPAL Broaden and OPAL Beyond.
In the OPAL Broaden study, 50% of patients who received tofacitinib 5 mg twice daily in combination with a nonbiologic DMARD achieved an ACR20 response at 3 months, compared with 33% of those treated with placebo (P equal to or less than .05). In the OPAL Beyond study, 50% of patients achieved an ACR20 response with tofacitinib 5 mg twice daily at 3 months, when compared with 24% of patients taking placebo (P equal to or less than .05).
Tofacitinib is the first and only Janus kinase inhibitor approved by the FDA to treat both moderate to severe rheumatoid arthritis and active PsA, Pfizer said in its announcement. It is noted that the recommended dose of tofacitinib is in combination with nonbiologic DMARDs, and use in combination with biologic DMARDs or with potent immunosuppressants such as azathioprine and cyclosporine is not recommended.
The safety of tofacitinib in these trials was consistent with the safety profile observed in rheumatoid arthritis patients. The most common adverse events that occurred in greater than 3% of patients on tofacitinib 5 mg twice daily were nasopharyngitis, upper respiratory tract infection, headache, and diarrhea.
(PsA) who have had an inadequate response or intolerance to methotrexate or other disease-modifying antirheumatic drugs (DMARDs), according to a Dec. 14 announcement from its manufacturer, Pfizer.
The approvals of tofacitinib (Xeljanz) at 5 mg twice daily and extended-release tofacitinib (Xeljanz XR) at 11 mg once daily are based on data from the phase 3 Oral Psoriatic Arthritis Trial (OPAL) clinical development program, which consisted of two pivotal studies, OPAL Broaden and OPAL Beyond.
In the OPAL Broaden study, 50% of patients who received tofacitinib 5 mg twice daily in combination with a nonbiologic DMARD achieved an ACR20 response at 3 months, compared with 33% of those treated with placebo (P equal to or less than .05). In the OPAL Beyond study, 50% of patients achieved an ACR20 response with tofacitinib 5 mg twice daily at 3 months, when compared with 24% of patients taking placebo (P equal to or less than .05).
Tofacitinib is the first and only Janus kinase inhibitor approved by the FDA to treat both moderate to severe rheumatoid arthritis and active PsA, Pfizer said in its announcement. It is noted that the recommended dose of tofacitinib is in combination with nonbiologic DMARDs, and use in combination with biologic DMARDs or with potent immunosuppressants such as azathioprine and cyclosporine is not recommended.
The safety of tofacitinib in these trials was consistent with the safety profile observed in rheumatoid arthritis patients. The most common adverse events that occurred in greater than 3% of patients on tofacitinib 5 mg twice daily were nasopharyngitis, upper respiratory tract infection, headache, and diarrhea.
(PsA) who have had an inadequate response or intolerance to methotrexate or other disease-modifying antirheumatic drugs (DMARDs), according to a Dec. 14 announcement from its manufacturer, Pfizer.
The approvals of tofacitinib (Xeljanz) at 5 mg twice daily and extended-release tofacitinib (Xeljanz XR) at 11 mg once daily are based on data from the phase 3 Oral Psoriatic Arthritis Trial (OPAL) clinical development program, which consisted of two pivotal studies, OPAL Broaden and OPAL Beyond.
In the OPAL Broaden study, 50% of patients who received tofacitinib 5 mg twice daily in combination with a nonbiologic DMARD achieved an ACR20 response at 3 months, compared with 33% of those treated with placebo (P equal to or less than .05). In the OPAL Beyond study, 50% of patients achieved an ACR20 response with tofacitinib 5 mg twice daily at 3 months, when compared with 24% of patients taking placebo (P equal to or less than .05).
Tofacitinib is the first and only Janus kinase inhibitor approved by the FDA to treat both moderate to severe rheumatoid arthritis and active PsA, Pfizer said in its announcement. It is noted that the recommended dose of tofacitinib is in combination with nonbiologic DMARDs, and use in combination with biologic DMARDs or with potent immunosuppressants such as azathioprine and cyclosporine is not recommended.
The safety of tofacitinib in these trials was consistent with the safety profile observed in rheumatoid arthritis patients. The most common adverse events that occurred in greater than 3% of patients on tofacitinib 5 mg twice daily were nasopharyngitis, upper respiratory tract infection, headache, and diarrhea.