User login
Inpatient Management of Hidradenitis Suppurativa: A Delphi Consensus Study
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that affects approximately 0.1% of the US population.1,2 Severe disease or HS flares can lead patients to seek care through the emergency department (ED), with some requiring inpatient admission. 3 Inpatient hospitalization of patients with HS has increased over the last 2 decades, and patients with HS utilize emergency and inpatient care more frequently than those with other dermatologic conditions.4,5 Minority patients and those of lower socioeconomic status are more likely to present to the ED for HS management due to limited access to care and other existing comorbid conditions. 4 In a 2022 study of the Nationwide Readmissions Database, the authors looked at hospital readmission rates of patients with HS compared with those with heart failure—both patient populations with chronic debilitating conditions. Results indicated that the hospital readmission rates for patients with HS surpassed those of patients with heart failure for that year, highlighting the need for improved inpatient management of HS.6
Patients with HS present to the ED with severe pain, fever, wound care, or the need for surgical intervention. The ED and inpatient hospital setting are locations in which physicians may not be as familiar with the diagnosis or treatment of HS, specifically flares or severe disease. 7 The inpatient care setting provides access to certain resources that can be challenging to obtain in the outpatient clinical setting, such as social workers and pain specialists, but also can prove challenging in obtaining other resources for HS management, such as advanced medical therapies. Given the increase in hospital- based care for HS and lack of widespread inpatient access to dermatology and HS experts, consensus recommendations for management of HS in the acute hospital setting would be beneficial. In our study, we sought to generate a collection of expert consensus statements providers can refer to when managing patients with HS in the inpatient setting.
Methods
The study team at the Wake Forest University School of Medicine (Winston-Salem, North Carolina)(M.N., R.P., L.C.S.) developed an initial set of consensus statements based on current published HS treatment guidelines,8,9 publications on management of inpatient HS,3 published supportive care guidelines for Stevens-Johnson syndrome, 10 and personal clinical experience in managing inpatient HS, which resulted in 50 statements organized into the following categories: overall care, wound care, genital care, pain management, infection control, medical management, surgical management, nutrition, and transitional care guidelines. This study was approved by the Wake Forest University institutional review board (IRB00084257).
Participant Recruitment—Dermatologists were identified for participation in the study based on membership in the Society of Dermatology Hospitalists and the Hidradenitis Suppurativa Foundation or authorship of publications relevant to HS or inpatient dermatology. Dermatologists from larger academic institutions with HS specialty clinics and inpatient dermatology services also were identified. Participants were invited via email and could suggest other experts for inclusion. A total of 31 dermatologists were invited to participate in the study, with 26 agreeing to participate. All participating dermatologists were practicing in the United States.
Delphi Study—In the first round of the Delphi study, the participants were sent an online survey via REDCap in which they were asked to rank the appropriateness of each of the proposed 50 guideline statements on a scale of 1 (very inappropriate) to 9 (very appropriate). Participants also were able to provide commentary and feedback on each of the statements. Survey results were analyzed using the RAND/ UCLA Appropriateness Method.11 For each statement, the median rating for appropriateness, interpercentile range (IPR), IPR adjusted for symmetry, and disagreement index (DI) were calculated (DI=IPR/IPR adjusted for symmetry). The 30th and 70th percentiles were used in the DI calculation as the upper and lower limits, respectively. A median rating for appropriateness of 1.0 to 3.9 was considered “inappropriate,” 4.0 to 6.9 was considered “uncertain appropriateness,” and 7.0 to 9.0 was “appropriate.” A DI value greater than or equal to 1 indicated a lack of consensus regarding the appropriateness of the statement. Following each round, participants received a copy of their responses along with the group median rank of each statement. Statements that did not reach consensus in the first Delphi round were revised based on feedback received by the participants, and a second survey with 14 statements was sent via REDCap 2 weeks later. The RAND/UCLA Appropriateness Method also was applied to this second Delphi round. After the second survey, participants received a copy of anonymized comments regarding the consensus statements and were allowed to provide additional final commentary to be included in the discussion of these recommendations.
Results
Twenty-six dermatologists completed the first-round survey, and 24 participants completed the second-round survey. All participants self-identified as having expertise in either HS (n=22 [85%]) or inpatient dermatology (n=17 [65%]), and 13 (50%) participants self-identified as experts in both HS and inpatient dermatology. All participants, except 1, were affiliated with an academic health system with inpatient dermatology services. The average length of time in practice as a dermatologist was 10 years (median, 9 years [range, 3–27 years]).
Of the 50 initial proposed consensus statements, 26 (52%) achieved consensus after the first round; 21 statements revealed DI calculations that did not achieve consensus. Two statements achieved consensus but received median ratings for appropriateness, indicating uncertain appropriateness; because of this, 1 statement was removed and 1 was revised based on participant feedback, resulting in 13 revised statements (eTable 1). Controversial topics in the consensus process included obtaining wound cultures and meaningful culture data interpretation, use of specific biologic medications in the inpatient setting, and use of intravenous ertapenem. Participant responses to these topics are discussed in detail below. Of these secondround statements, all achieved consensus. The final set of consensus statements can be found in eTable 2.
Comment
Our Delphi consensus study combined the expertise of both dermatologists who care for patients with HS and those with inpatient dermatology experience to produce a set of recommendations for the management of HS in the hospital care setting. A strength of this study is inclusion of many national leaders in both HS and inpatient dermatology, with some participants having developed the previously published HS treatment guidelines and others having participated in inpatient dermatology Delphi studies.8-10 The expertise is further strengthened by the geographically diverse institutional representation within the United States.
The final consensus recommendations included 40 statements covering a range of patient care issues, including use of appropriate inpatient subspecialists (care team), supportive care measures (wound care, pain control, genital care), disease-oriented treatment (medical management, surgical management), inpatient complications (infection control, nutrition), and successful transition back to outpatient management (transitional care). These recommendations are meant to serve as a resource for providers to consider when taking care of inpatient HS flares, recognizing that the complexity and individual circumstances of each patient are unique.
Delphi Consensus Recommendations Compared to Prior Guidelines—Several recommendations in the current study align with the previously published North American clinical management guidelines for HS.8,9 Our recommendations agree with prior guidelines on the importance of disease staging and pain assessment using validated assessment tools as well as screening for HS comorbidities. There also is agreement in the potential benefit of involving pain specialists in the development of a comprehensive pain management plan. The inpatient care setting provides a unique opportunity to engage multiple specialists and collaborate on patient care in a timely manner. Our recommendations regarding surgical care also align with established guidelines in recommending incision and drainage as an acute bedside procedure best utilized for symptom relief in inflamed abscesses and relegating most other surgical management to the outpatient setting. Wound care recommendations also are similar, with our expert participants agreeing on individualizing dressing choices based on wound characteristics. A benefit of inpatient wound care is access to skilled nursing for dressing changes and potentially improved access to more sophisticated dressing materials. Our recommendations differ from the prior guidelines in our focus on severe HS, HS flares, and HS complications, which constitute the majority of inpatient disease management. We provide additional guidance on management of secondary infections, perianal fistulous disease, and importantly transitional care to optimize discharge planning.
Differing Opinions in Our Analysis—Despite the success of our Delphi consensus process, there were some differing opinions regarding certain aspects of inpatient HS management, which is to be expected given the lack of strong evidence-based research to support some of the recommended practices. There were differing opinions on the utility of wound culture data, with some participants feeling culture data could help with antibiotic susceptibility and resistance patterns, while others felt wound cultures represent bacterial colonization or biofilm formation.
Initial consensus statements in the first Delphi round were created for individual biologic medications but did not achieve consensus, and feedback on the use of biologics in the inpatient environment was mixed, largely due to logistic and insurance issues. Many participants felt biologic medication cost, difficulty obtaining inpatient reimbursement, health care resource utilization, and availability of biologics in different hospital systems prevented recommending the use of specific biologics during hospitalization. The one exception was in the case of a hospitalized patient who was already receiving infliximab for HS: there was consensus on ensuring the patient dosing was maximized, if appropriate, to 10 mg/kg.12 Ertapenem use also was controversial, with some participants using it as a bridge therapy to either outpatient biologic use or surgery, while others felt it was onerous and difficult to establish reliable access to secure intravenous administration and regular dosing once the patient left the inpatient setting.13 Others said they have experienced objections from infectious disease colleagues on the use of intravenous antibiotics, citing antibiotic stewardship concerns.
Patient Care in the Inpatient Setting—Prior literature suggests patients admitted as inpatients for HS tend to be of lower socioeconomic status and are admitted to larger urban teaching hospitals.14,15 Patients with lower socioeconomic status have increased difficulty accessing health care resources; therefore, inpatient admission serves as an opportunity to provide a holistic HS assessment and coordinate resources for chronic outpatient management.
Study Limitations—This Delphi consensus study has some limitations. The existing literature on inpatient management of HS is limited, challenging our ability to assess the extent to which these published recommendations are already being implemented. Additionally, the study included HS and inpatient dermatology experts from the United States, which means the recommendations may not be generalizable to other countries. Most participants practiced dermatology at large tertiary care academic medical centers, which may limit the ability to implement recommendations in all US inpatient care settings such as small community-based hospitals; however, many of the supportive care guidelines such as pain control, wound care, nutritional support, and social work should be achievable in most inpatient care settings.
Conclusion
Given the increase in inpatient and ED health care utilization for HS, there is an urgent need for expert consensus recommendations on inpatient management of this unique patient population, which requires complex multidisciplinary care. Our recommendations are a resource for providers to utilize and potentially improve the standard of care we provide these patients.
Acknowledgment—We thank the Wake Forest University Clinical and Translational Science Institute (Winston- Salem, North Carolina) for providing statistical help.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Ingram JR. The epidemiology of hidradenitis suppurativa. Br J Dermatol. 2020;183:990-998. doi:10.1111/bjd.19435
- Charrow A, Savage KT, Flood K, et al. Hidradenitis suppurativa for the dermatologic hospitalist. Cutis. 2019;104:276-280.
- Anzaldi L, Perkins JA, Byrd AS, et al. Characterizing inpatient hospitalizations for hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2020;82:510-513. doi:10.1016/j.jaad.2019.09.019
- Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73:609-614. doi:10.1016/j.jaad.2015.06.053
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192. doi:10.1016/j.jaad.2021.06.894
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150:937-944. doi:10.1001/jamadermatol.2014.691
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j .jaad.2019.02.067
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016/j .jaad.2020.02.066
- Fitch K, Bernstein SJ, Burnand B, et al. The RAND/UCLA Appropriateness Method: User’s Manual. Rand; 2001.
- Oskardmay AN, Miles JA, Sayed CJ. Determining the optimal dose of infliximab for treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;81:702-708. doi:10.1016/j.jaad.2019.05.022
- Join-Lambert O, Coignard-Biehler H, Jais JP, et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother. 2016;71:513-520. doi:10.1093/jac/dkv361
- Khanna R, Whang KA, Huang AH, et al. Inpatient burden of hidradenitis suppurativa in the United States: analysis of the 2016 National Inpatient Sample. J Dermatolog Treat. 2022;33:1150-1152. doi:10.1080/09 546634.2020.1773380
- Patel A, Patel A, Solanki D, et al. Hidradenitis suppurativa in the United States: insights from the national inpatient sample (2008-2017) on contemporary trends in demographics, hospitalization rates, chronic comorbid conditions, and mortality. Cureus. 2022;14:E24755. doi:10.7759/cureus.24755
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that affects approximately 0.1% of the US population.1,2 Severe disease or HS flares can lead patients to seek care through the emergency department (ED), with some requiring inpatient admission. 3 Inpatient hospitalization of patients with HS has increased over the last 2 decades, and patients with HS utilize emergency and inpatient care more frequently than those with other dermatologic conditions.4,5 Minority patients and those of lower socioeconomic status are more likely to present to the ED for HS management due to limited access to care and other existing comorbid conditions. 4 In a 2022 study of the Nationwide Readmissions Database, the authors looked at hospital readmission rates of patients with HS compared with those with heart failure—both patient populations with chronic debilitating conditions. Results indicated that the hospital readmission rates for patients with HS surpassed those of patients with heart failure for that year, highlighting the need for improved inpatient management of HS.6
Patients with HS present to the ED with severe pain, fever, wound care, or the need for surgical intervention. The ED and inpatient hospital setting are locations in which physicians may not be as familiar with the diagnosis or treatment of HS, specifically flares or severe disease. 7 The inpatient care setting provides access to certain resources that can be challenging to obtain in the outpatient clinical setting, such as social workers and pain specialists, but also can prove challenging in obtaining other resources for HS management, such as advanced medical therapies. Given the increase in hospital- based care for HS and lack of widespread inpatient access to dermatology and HS experts, consensus recommendations for management of HS in the acute hospital setting would be beneficial. In our study, we sought to generate a collection of expert consensus statements providers can refer to when managing patients with HS in the inpatient setting.
Methods
The study team at the Wake Forest University School of Medicine (Winston-Salem, North Carolina)(M.N., R.P., L.C.S.) developed an initial set of consensus statements based on current published HS treatment guidelines,8,9 publications on management of inpatient HS,3 published supportive care guidelines for Stevens-Johnson syndrome, 10 and personal clinical experience in managing inpatient HS, which resulted in 50 statements organized into the following categories: overall care, wound care, genital care, pain management, infection control, medical management, surgical management, nutrition, and transitional care guidelines. This study was approved by the Wake Forest University institutional review board (IRB00084257).
Participant Recruitment—Dermatologists were identified for participation in the study based on membership in the Society of Dermatology Hospitalists and the Hidradenitis Suppurativa Foundation or authorship of publications relevant to HS or inpatient dermatology. Dermatologists from larger academic institutions with HS specialty clinics and inpatient dermatology services also were identified. Participants were invited via email and could suggest other experts for inclusion. A total of 31 dermatologists were invited to participate in the study, with 26 agreeing to participate. All participating dermatologists were practicing in the United States.
Delphi Study—In the first round of the Delphi study, the participants were sent an online survey via REDCap in which they were asked to rank the appropriateness of each of the proposed 50 guideline statements on a scale of 1 (very inappropriate) to 9 (very appropriate). Participants also were able to provide commentary and feedback on each of the statements. Survey results were analyzed using the RAND/ UCLA Appropriateness Method.11 For each statement, the median rating for appropriateness, interpercentile range (IPR), IPR adjusted for symmetry, and disagreement index (DI) were calculated (DI=IPR/IPR adjusted for symmetry). The 30th and 70th percentiles were used in the DI calculation as the upper and lower limits, respectively. A median rating for appropriateness of 1.0 to 3.9 was considered “inappropriate,” 4.0 to 6.9 was considered “uncertain appropriateness,” and 7.0 to 9.0 was “appropriate.” A DI value greater than or equal to 1 indicated a lack of consensus regarding the appropriateness of the statement. Following each round, participants received a copy of their responses along with the group median rank of each statement. Statements that did not reach consensus in the first Delphi round were revised based on feedback received by the participants, and a second survey with 14 statements was sent via REDCap 2 weeks later. The RAND/UCLA Appropriateness Method also was applied to this second Delphi round. After the second survey, participants received a copy of anonymized comments regarding the consensus statements and were allowed to provide additional final commentary to be included in the discussion of these recommendations.
Results
Twenty-six dermatologists completed the first-round survey, and 24 participants completed the second-round survey. All participants self-identified as having expertise in either HS (n=22 [85%]) or inpatient dermatology (n=17 [65%]), and 13 (50%) participants self-identified as experts in both HS and inpatient dermatology. All participants, except 1, were affiliated with an academic health system with inpatient dermatology services. The average length of time in practice as a dermatologist was 10 years (median, 9 years [range, 3–27 years]).
Of the 50 initial proposed consensus statements, 26 (52%) achieved consensus after the first round; 21 statements revealed DI calculations that did not achieve consensus. Two statements achieved consensus but received median ratings for appropriateness, indicating uncertain appropriateness; because of this, 1 statement was removed and 1 was revised based on participant feedback, resulting in 13 revised statements (eTable 1). Controversial topics in the consensus process included obtaining wound cultures and meaningful culture data interpretation, use of specific biologic medications in the inpatient setting, and use of intravenous ertapenem. Participant responses to these topics are discussed in detail below. Of these secondround statements, all achieved consensus. The final set of consensus statements can be found in eTable 2.
Comment
Our Delphi consensus study combined the expertise of both dermatologists who care for patients with HS and those with inpatient dermatology experience to produce a set of recommendations for the management of HS in the hospital care setting. A strength of this study is inclusion of many national leaders in both HS and inpatient dermatology, with some participants having developed the previously published HS treatment guidelines and others having participated in inpatient dermatology Delphi studies.8-10 The expertise is further strengthened by the geographically diverse institutional representation within the United States.
The final consensus recommendations included 40 statements covering a range of patient care issues, including use of appropriate inpatient subspecialists (care team), supportive care measures (wound care, pain control, genital care), disease-oriented treatment (medical management, surgical management), inpatient complications (infection control, nutrition), and successful transition back to outpatient management (transitional care). These recommendations are meant to serve as a resource for providers to consider when taking care of inpatient HS flares, recognizing that the complexity and individual circumstances of each patient are unique.
Delphi Consensus Recommendations Compared to Prior Guidelines—Several recommendations in the current study align with the previously published North American clinical management guidelines for HS.8,9 Our recommendations agree with prior guidelines on the importance of disease staging and pain assessment using validated assessment tools as well as screening for HS comorbidities. There also is agreement in the potential benefit of involving pain specialists in the development of a comprehensive pain management plan. The inpatient care setting provides a unique opportunity to engage multiple specialists and collaborate on patient care in a timely manner. Our recommendations regarding surgical care also align with established guidelines in recommending incision and drainage as an acute bedside procedure best utilized for symptom relief in inflamed abscesses and relegating most other surgical management to the outpatient setting. Wound care recommendations also are similar, with our expert participants agreeing on individualizing dressing choices based on wound characteristics. A benefit of inpatient wound care is access to skilled nursing for dressing changes and potentially improved access to more sophisticated dressing materials. Our recommendations differ from the prior guidelines in our focus on severe HS, HS flares, and HS complications, which constitute the majority of inpatient disease management. We provide additional guidance on management of secondary infections, perianal fistulous disease, and importantly transitional care to optimize discharge planning.
Differing Opinions in Our Analysis—Despite the success of our Delphi consensus process, there were some differing opinions regarding certain aspects of inpatient HS management, which is to be expected given the lack of strong evidence-based research to support some of the recommended practices. There were differing opinions on the utility of wound culture data, with some participants feeling culture data could help with antibiotic susceptibility and resistance patterns, while others felt wound cultures represent bacterial colonization or biofilm formation.
Initial consensus statements in the first Delphi round were created for individual biologic medications but did not achieve consensus, and feedback on the use of biologics in the inpatient environment was mixed, largely due to logistic and insurance issues. Many participants felt biologic medication cost, difficulty obtaining inpatient reimbursement, health care resource utilization, and availability of biologics in different hospital systems prevented recommending the use of specific biologics during hospitalization. The one exception was in the case of a hospitalized patient who was already receiving infliximab for HS: there was consensus on ensuring the patient dosing was maximized, if appropriate, to 10 mg/kg.12 Ertapenem use also was controversial, with some participants using it as a bridge therapy to either outpatient biologic use or surgery, while others felt it was onerous and difficult to establish reliable access to secure intravenous administration and regular dosing once the patient left the inpatient setting.13 Others said they have experienced objections from infectious disease colleagues on the use of intravenous antibiotics, citing antibiotic stewardship concerns.
Patient Care in the Inpatient Setting—Prior literature suggests patients admitted as inpatients for HS tend to be of lower socioeconomic status and are admitted to larger urban teaching hospitals.14,15 Patients with lower socioeconomic status have increased difficulty accessing health care resources; therefore, inpatient admission serves as an opportunity to provide a holistic HS assessment and coordinate resources for chronic outpatient management.
Study Limitations—This Delphi consensus study has some limitations. The existing literature on inpatient management of HS is limited, challenging our ability to assess the extent to which these published recommendations are already being implemented. Additionally, the study included HS and inpatient dermatology experts from the United States, which means the recommendations may not be generalizable to other countries. Most participants practiced dermatology at large tertiary care academic medical centers, which may limit the ability to implement recommendations in all US inpatient care settings such as small community-based hospitals; however, many of the supportive care guidelines such as pain control, wound care, nutritional support, and social work should be achievable in most inpatient care settings.
Conclusion
Given the increase in inpatient and ED health care utilization for HS, there is an urgent need for expert consensus recommendations on inpatient management of this unique patient population, which requires complex multidisciplinary care. Our recommendations are a resource for providers to utilize and potentially improve the standard of care we provide these patients.
Acknowledgment—We thank the Wake Forest University Clinical and Translational Science Institute (Winston- Salem, North Carolina) for providing statistical help.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that affects approximately 0.1% of the US population.1,2 Severe disease or HS flares can lead patients to seek care through the emergency department (ED), with some requiring inpatient admission. 3 Inpatient hospitalization of patients with HS has increased over the last 2 decades, and patients with HS utilize emergency and inpatient care more frequently than those with other dermatologic conditions.4,5 Minority patients and those of lower socioeconomic status are more likely to present to the ED for HS management due to limited access to care and other existing comorbid conditions. 4 In a 2022 study of the Nationwide Readmissions Database, the authors looked at hospital readmission rates of patients with HS compared with those with heart failure—both patient populations with chronic debilitating conditions. Results indicated that the hospital readmission rates for patients with HS surpassed those of patients with heart failure for that year, highlighting the need for improved inpatient management of HS.6
Patients with HS present to the ED with severe pain, fever, wound care, or the need for surgical intervention. The ED and inpatient hospital setting are locations in which physicians may not be as familiar with the diagnosis or treatment of HS, specifically flares or severe disease. 7 The inpatient care setting provides access to certain resources that can be challenging to obtain in the outpatient clinical setting, such as social workers and pain specialists, but also can prove challenging in obtaining other resources for HS management, such as advanced medical therapies. Given the increase in hospital- based care for HS and lack of widespread inpatient access to dermatology and HS experts, consensus recommendations for management of HS in the acute hospital setting would be beneficial. In our study, we sought to generate a collection of expert consensus statements providers can refer to when managing patients with HS in the inpatient setting.
Methods
The study team at the Wake Forest University School of Medicine (Winston-Salem, North Carolina)(M.N., R.P., L.C.S.) developed an initial set of consensus statements based on current published HS treatment guidelines,8,9 publications on management of inpatient HS,3 published supportive care guidelines for Stevens-Johnson syndrome, 10 and personal clinical experience in managing inpatient HS, which resulted in 50 statements organized into the following categories: overall care, wound care, genital care, pain management, infection control, medical management, surgical management, nutrition, and transitional care guidelines. This study was approved by the Wake Forest University institutional review board (IRB00084257).
Participant Recruitment—Dermatologists were identified for participation in the study based on membership in the Society of Dermatology Hospitalists and the Hidradenitis Suppurativa Foundation or authorship of publications relevant to HS or inpatient dermatology. Dermatologists from larger academic institutions with HS specialty clinics and inpatient dermatology services also were identified. Participants were invited via email and could suggest other experts for inclusion. A total of 31 dermatologists were invited to participate in the study, with 26 agreeing to participate. All participating dermatologists were practicing in the United States.
Delphi Study—In the first round of the Delphi study, the participants were sent an online survey via REDCap in which they were asked to rank the appropriateness of each of the proposed 50 guideline statements on a scale of 1 (very inappropriate) to 9 (very appropriate). Participants also were able to provide commentary and feedback on each of the statements. Survey results were analyzed using the RAND/ UCLA Appropriateness Method.11 For each statement, the median rating for appropriateness, interpercentile range (IPR), IPR adjusted for symmetry, and disagreement index (DI) were calculated (DI=IPR/IPR adjusted for symmetry). The 30th and 70th percentiles were used in the DI calculation as the upper and lower limits, respectively. A median rating for appropriateness of 1.0 to 3.9 was considered “inappropriate,” 4.0 to 6.9 was considered “uncertain appropriateness,” and 7.0 to 9.0 was “appropriate.” A DI value greater than or equal to 1 indicated a lack of consensus regarding the appropriateness of the statement. Following each round, participants received a copy of their responses along with the group median rank of each statement. Statements that did not reach consensus in the first Delphi round were revised based on feedback received by the participants, and a second survey with 14 statements was sent via REDCap 2 weeks later. The RAND/UCLA Appropriateness Method also was applied to this second Delphi round. After the second survey, participants received a copy of anonymized comments regarding the consensus statements and were allowed to provide additional final commentary to be included in the discussion of these recommendations.
Results
Twenty-six dermatologists completed the first-round survey, and 24 participants completed the second-round survey. All participants self-identified as having expertise in either HS (n=22 [85%]) or inpatient dermatology (n=17 [65%]), and 13 (50%) participants self-identified as experts in both HS and inpatient dermatology. All participants, except 1, were affiliated with an academic health system with inpatient dermatology services. The average length of time in practice as a dermatologist was 10 years (median, 9 years [range, 3–27 years]).
Of the 50 initial proposed consensus statements, 26 (52%) achieved consensus after the first round; 21 statements revealed DI calculations that did not achieve consensus. Two statements achieved consensus but received median ratings for appropriateness, indicating uncertain appropriateness; because of this, 1 statement was removed and 1 was revised based on participant feedback, resulting in 13 revised statements (eTable 1). Controversial topics in the consensus process included obtaining wound cultures and meaningful culture data interpretation, use of specific biologic medications in the inpatient setting, and use of intravenous ertapenem. Participant responses to these topics are discussed in detail below. Of these secondround statements, all achieved consensus. The final set of consensus statements can be found in eTable 2.
Comment
Our Delphi consensus study combined the expertise of both dermatologists who care for patients with HS and those with inpatient dermatology experience to produce a set of recommendations for the management of HS in the hospital care setting. A strength of this study is inclusion of many national leaders in both HS and inpatient dermatology, with some participants having developed the previously published HS treatment guidelines and others having participated in inpatient dermatology Delphi studies.8-10 The expertise is further strengthened by the geographically diverse institutional representation within the United States.
The final consensus recommendations included 40 statements covering a range of patient care issues, including use of appropriate inpatient subspecialists (care team), supportive care measures (wound care, pain control, genital care), disease-oriented treatment (medical management, surgical management), inpatient complications (infection control, nutrition), and successful transition back to outpatient management (transitional care). These recommendations are meant to serve as a resource for providers to consider when taking care of inpatient HS flares, recognizing that the complexity and individual circumstances of each patient are unique.
Delphi Consensus Recommendations Compared to Prior Guidelines—Several recommendations in the current study align with the previously published North American clinical management guidelines for HS.8,9 Our recommendations agree with prior guidelines on the importance of disease staging and pain assessment using validated assessment tools as well as screening for HS comorbidities. There also is agreement in the potential benefit of involving pain specialists in the development of a comprehensive pain management plan. The inpatient care setting provides a unique opportunity to engage multiple specialists and collaborate on patient care in a timely manner. Our recommendations regarding surgical care also align with established guidelines in recommending incision and drainage as an acute bedside procedure best utilized for symptom relief in inflamed abscesses and relegating most other surgical management to the outpatient setting. Wound care recommendations also are similar, with our expert participants agreeing on individualizing dressing choices based on wound characteristics. A benefit of inpatient wound care is access to skilled nursing for dressing changes and potentially improved access to more sophisticated dressing materials. Our recommendations differ from the prior guidelines in our focus on severe HS, HS flares, and HS complications, which constitute the majority of inpatient disease management. We provide additional guidance on management of secondary infections, perianal fistulous disease, and importantly transitional care to optimize discharge planning.
Differing Opinions in Our Analysis—Despite the success of our Delphi consensus process, there were some differing opinions regarding certain aspects of inpatient HS management, which is to be expected given the lack of strong evidence-based research to support some of the recommended practices. There were differing opinions on the utility of wound culture data, with some participants feeling culture data could help with antibiotic susceptibility and resistance patterns, while others felt wound cultures represent bacterial colonization or biofilm formation.
Initial consensus statements in the first Delphi round were created for individual biologic medications but did not achieve consensus, and feedback on the use of biologics in the inpatient environment was mixed, largely due to logistic and insurance issues. Many participants felt biologic medication cost, difficulty obtaining inpatient reimbursement, health care resource utilization, and availability of biologics in different hospital systems prevented recommending the use of specific biologics during hospitalization. The one exception was in the case of a hospitalized patient who was already receiving infliximab for HS: there was consensus on ensuring the patient dosing was maximized, if appropriate, to 10 mg/kg.12 Ertapenem use also was controversial, with some participants using it as a bridge therapy to either outpatient biologic use or surgery, while others felt it was onerous and difficult to establish reliable access to secure intravenous administration and regular dosing once the patient left the inpatient setting.13 Others said they have experienced objections from infectious disease colleagues on the use of intravenous antibiotics, citing antibiotic stewardship concerns.
Patient Care in the Inpatient Setting—Prior literature suggests patients admitted as inpatients for HS tend to be of lower socioeconomic status and are admitted to larger urban teaching hospitals.14,15 Patients with lower socioeconomic status have increased difficulty accessing health care resources; therefore, inpatient admission serves as an opportunity to provide a holistic HS assessment and coordinate resources for chronic outpatient management.
Study Limitations—This Delphi consensus study has some limitations. The existing literature on inpatient management of HS is limited, challenging our ability to assess the extent to which these published recommendations are already being implemented. Additionally, the study included HS and inpatient dermatology experts from the United States, which means the recommendations may not be generalizable to other countries. Most participants practiced dermatology at large tertiary care academic medical centers, which may limit the ability to implement recommendations in all US inpatient care settings such as small community-based hospitals; however, many of the supportive care guidelines such as pain control, wound care, nutritional support, and social work should be achievable in most inpatient care settings.
Conclusion
Given the increase in inpatient and ED health care utilization for HS, there is an urgent need for expert consensus recommendations on inpatient management of this unique patient population, which requires complex multidisciplinary care. Our recommendations are a resource for providers to utilize and potentially improve the standard of care we provide these patients.
Acknowledgment—We thank the Wake Forest University Clinical and Translational Science Institute (Winston- Salem, North Carolina) for providing statistical help.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Ingram JR. The epidemiology of hidradenitis suppurativa. Br J Dermatol. 2020;183:990-998. doi:10.1111/bjd.19435
- Charrow A, Savage KT, Flood K, et al. Hidradenitis suppurativa for the dermatologic hospitalist. Cutis. 2019;104:276-280.
- Anzaldi L, Perkins JA, Byrd AS, et al. Characterizing inpatient hospitalizations for hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2020;82:510-513. doi:10.1016/j.jaad.2019.09.019
- Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73:609-614. doi:10.1016/j.jaad.2015.06.053
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192. doi:10.1016/j.jaad.2021.06.894
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150:937-944. doi:10.1001/jamadermatol.2014.691
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j .jaad.2019.02.067
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016/j .jaad.2020.02.066
- Fitch K, Bernstein SJ, Burnand B, et al. The RAND/UCLA Appropriateness Method: User’s Manual. Rand; 2001.
- Oskardmay AN, Miles JA, Sayed CJ. Determining the optimal dose of infliximab for treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;81:702-708. doi:10.1016/j.jaad.2019.05.022
- Join-Lambert O, Coignard-Biehler H, Jais JP, et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother. 2016;71:513-520. doi:10.1093/jac/dkv361
- Khanna R, Whang KA, Huang AH, et al. Inpatient burden of hidradenitis suppurativa in the United States: analysis of the 2016 National Inpatient Sample. J Dermatolog Treat. 2022;33:1150-1152. doi:10.1080/09 546634.2020.1773380
- Patel A, Patel A, Solanki D, et al. Hidradenitis suppurativa in the United States: insights from the national inpatient sample (2008-2017) on contemporary trends in demographics, hospitalization rates, chronic comorbid conditions, and mortality. Cureus. 2022;14:E24755. doi:10.7759/cureus.24755
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Ingram JR. The epidemiology of hidradenitis suppurativa. Br J Dermatol. 2020;183:990-998. doi:10.1111/bjd.19435
- Charrow A, Savage KT, Flood K, et al. Hidradenitis suppurativa for the dermatologic hospitalist. Cutis. 2019;104:276-280.
- Anzaldi L, Perkins JA, Byrd AS, et al. Characterizing inpatient hospitalizations for hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2020;82:510-513. doi:10.1016/j.jaad.2019.09.019
- Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73:609-614. doi:10.1016/j.jaad.2015.06.053
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192. doi:10.1016/j.jaad.2021.06.894
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150:937-944. doi:10.1001/jamadermatol.2014.691
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j .jaad.2019.02.067
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016/j .jaad.2020.02.066
- Fitch K, Bernstein SJ, Burnand B, et al. The RAND/UCLA Appropriateness Method: User’s Manual. Rand; 2001.
- Oskardmay AN, Miles JA, Sayed CJ. Determining the optimal dose of infliximab for treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;81:702-708. doi:10.1016/j.jaad.2019.05.022
- Join-Lambert O, Coignard-Biehler H, Jais JP, et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother. 2016;71:513-520. doi:10.1093/jac/dkv361
- Khanna R, Whang KA, Huang AH, et al. Inpatient burden of hidradenitis suppurativa in the United States: analysis of the 2016 National Inpatient Sample. J Dermatolog Treat. 2022;33:1150-1152. doi:10.1080/09 546634.2020.1773380
- Patel A, Patel A, Solanki D, et al. Hidradenitis suppurativa in the United States: insights from the national inpatient sample (2008-2017) on contemporary trends in demographics, hospitalization rates, chronic comorbid conditions, and mortality. Cureus. 2022;14:E24755. doi:10.7759/cureus.24755
Practice Points
- Given the increase in hospital-based care for hidradenitis suppurativa (HS) and the lack of widespread inpatient access to dermatology and HS experts, consensus recommendations for management of HS in the acute hospital setting would be beneficial.
- Our Delphi study yielded 40 statements that reached consensus covering a range of patient care issues (eg, appropriate inpatient subspecialists [care team]), supportive care measures (wound care, pain control, genital care), disease-oriented treatment (medical management, surgical management), inpatient complications (infection control, nutrition), and successful transition to outpatient management (transitional care).
- These recommendations serve as an important resource for providers caring for inpatients with HS and represent a successful collaboration between inpatient dermatology and HS experts.
Persistent ‘postherpetic neuralgia’ and well-demarcated plaque
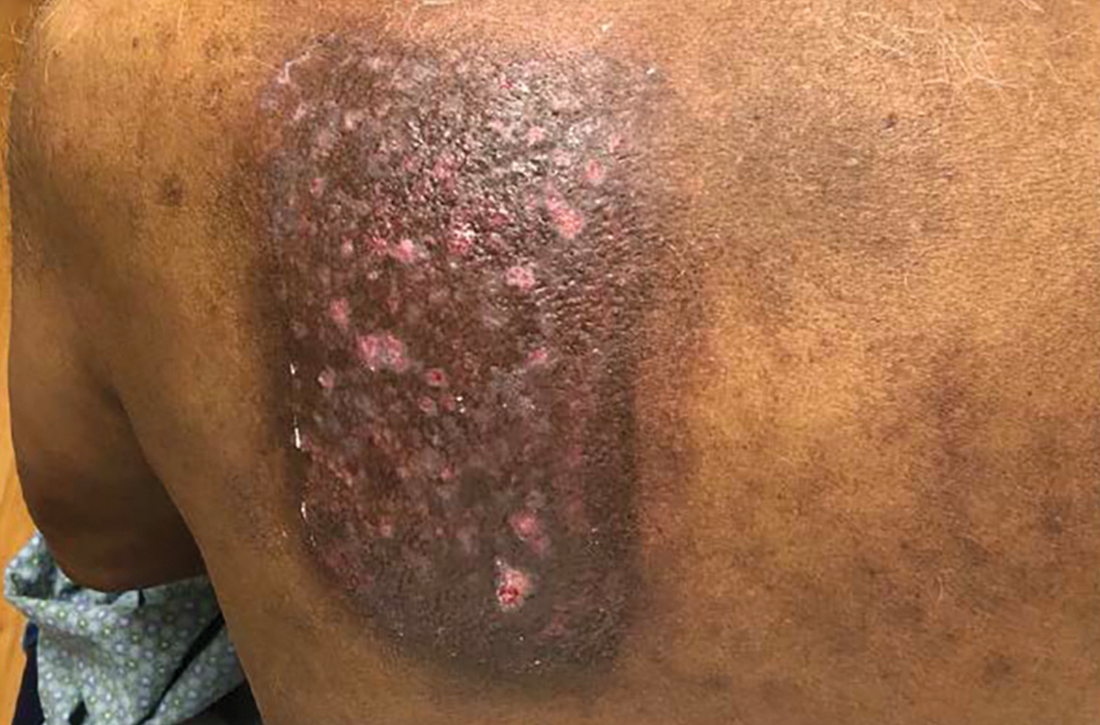
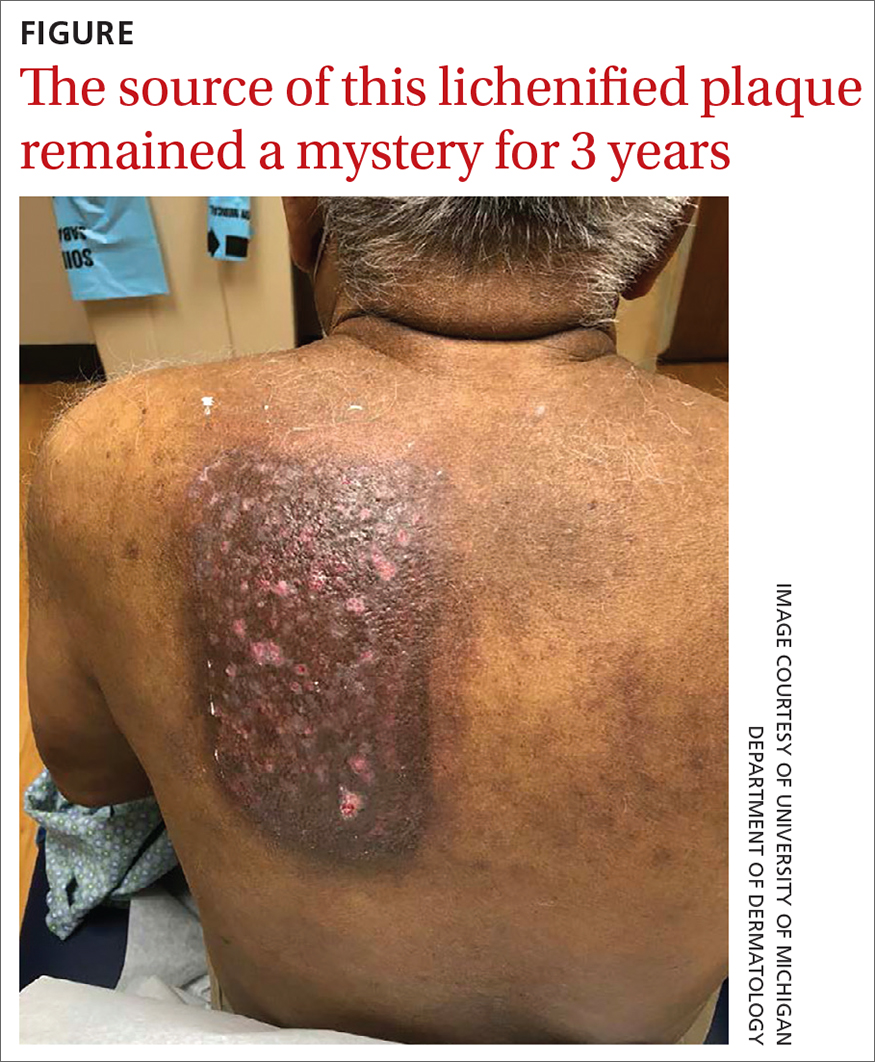
A 75-YEAR-OLD MAN presented to the dermatology clinic for evaluation of localized, persistent burning pain and discomfort attributed to shingles and postherpetic neuralgia. He had received a diagnosis of shingles on his left upper back about 3 years prior to this presentation.
In the ensuing years, the patient had been evaluated and treated by his primary care physician, a pain management team, and a neurologist. These clinicians treated the symptoms as postherpetic neuralgia, with no consensus explanation for the skin findings. The patient reported that his symptoms were unresponsive to trials of gabapentin 800 mg tid, duloxetine 60 mg PO qd, and acetaminophen 1 to 3 g/d PO. He also had undergone several rounds of acupuncture, thoracic and cervical spine steroid injections, and epidurals, without resolution of symptoms. The patient believed the only treatment that helped was a lidocaine 4% patch, which he had used nearly every day for the previous 3 years.
Physical exam by the dermatologist revealed a lidocaine patch applied to the patient’s left upper back. Upon its removal, skin examination showed a well-demarcated, erythematous, hyperpigmented, lichenified plaque with excoriations and erosions where the patch had been (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Contact dermatitis
The patient’s history and skin exam provided enough information to diagnose contact dermatitis. The pruritus, burning, and pain the patient had experienced were due to continuous application of the lidocaine patch to the area rather than postherpetic neuralgia.
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis is an inflammatory reaction caused directly by a substance, while allergic contact dermatitis is a delayed hypersensitivity reaction to specific allergens.1 While data to elucidate the incidence and prevalence of allergic contact dermatitis are unknown, common causes include latex, dyes, oils, resins, and compounds in textiles, rubber, cosmetics, and other products used in daily life.1
Allergic contact dermatitis due to lidocaine is becoming more prevalent with increased use and availability of over-the-counter products.2 A retrospective chart review of 1819 patch-tested patients from the University of British Columbia Contact Dermatitis Clinic showed a significant proportion of patients (2.4%) were found to have
The differential varies by area affected
The differential diagnosis for contact dermatitis varies by area affected and the distribution of rash. Atopic dermatitis, lichen planus, and psoriasis are a few dermatologic conditions to consider in the differential diagnosis. They can look similar to contact dermatitis, but the patient’s history can help to discern the most likely diagnosis.1
Atopic dermatitis is a complex dysfunction of the skin barrier and immune factors that often begins in childhood and persists in some patients throughout their lifetime. Atopic dermatitis is associated with other forms of atopy including asthma, allergic rhinitis, and food and contact allergies. Atopic dermatitis in the absence of contact allergies may manifest with chronic, diffuse, scaly patches with poorly defined borders. The patches appear in a symmetrical distribution and favor the flexural surfaces, such as the antecubital fossa, wrists, and neck.
Continue to: Lichen planus
Lichen planus most often manifests in the fourth through sixth decade of life as flat-topped itchy pink-to-purple polygonal papules to plaques. Lesions range from 2 to 10 mm and favor the volar wrists, shins, and lower back, although they may be widespread. Oral lesions manifesting as ulcers or white lacy patches in the buccal mucosa are common and may be a clue to the diagnosis. Unlike more generalized contact dermatitis, lichen planus lesions are discrete.
Psoriasis manifests as well-demarcated scaly plaques distributed symmetrically over extensor surfaces. The plaques commonly are found on the elbows, knees, and scalp. When psoriasis manifests in a very limited form (as just a single plaque or limited number of plaques), it can be hard to confidently exclude other etiologies. In these circumstances, look for psoriasis signs in more unique locations (eg, pitting in the nails or plaques on the scalp or in the gluteal cleft). Adding those findings to an otherwise solitary plaque significantly adds to diagnostic certainty.
Diagnosis entails getting the shape of things
Diagnosis is based on history of exposure to irritating or allergic substances, as well as a clinical exam. Skin examination of contact dermatitis can vary based on how long it has been present: Acute manifestations include erythema, oozing, scale, vesicles, and bullae, while chronic contact dermatitis tends to demonstrate lichenification and scale.1
Distinctive findings. The most distinctive physical exam findings in patients with contact dermatitis are often shape and distribution of the rash, which reflect points of contact with the offending agent. This clue helped to elucidate the diagnosis in our patient: his rash was perfectly demarcated within the precise area where the patch was applied daily.
Irritant vs allergic. Patch testing can be performed to differentiate irritant vs allergic contact dermatitis.1 Irritant contact dermatitis usually is apparent when removing a patch and will resolve over a day, whereas allergic contact dermatitis forms over time and the skin rash is most prominent several days after the patch has been removed.1
Continue to: Treatment
Treatment: First, stop the offense
Treatment of both variants of contact dermatitis includes avoidance of the causative substance and symptomatic treatment with topical steroids, antihistamines, and possibly oral steroids depending on the severity.1
For our patient, a viral swab was taken and submitted for varicella zoster virus polymerase chain reaction testing to rule out persistent herpes zoster infection; the result was negative. The patient was counseled to discontinue use of the lidocaine patch.
Given the severity and protracted duration of the patient’s symptoms, he also was started on high-potency topical steroids (clobetasol 0.05% ointment to be applied twice daily under occlusion for 2 months), a 4-week prednisone taper (60 mg × 1 week, 40 mg × 1 week, 20 mg × 1 week, 10 mg × 1 week, then stop), and hydroxyzine (25 mg nightly as needed for pruritus). The patient’s rash and symptoms improved dramatically within the first few doses of prednisone and completely cleared by Week 4 of the prednisone taper. At his follow-up appointment 1 month after completing the prednisone taper, he stated that the pain on his back had resolved
1. Li Y, Li L. Contact dermatitis: classifications and management. Clin Rev Allergy Immunol. 2021;61:245-281. doi: 10.1007/s12016-021-08875-0
2. Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271. doi: 10.1097/DER.0000000000000216
3. To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372. doi: 10.1097/DSS.0000000000000190
A 75-YEAR-OLD MAN presented to the dermatology clinic for evaluation of localized, persistent burning pain and discomfort attributed to shingles and postherpetic neuralgia. He had received a diagnosis of shingles on his left upper back about 3 years prior to this presentation.
In the ensuing years, the patient had been evaluated and treated by his primary care physician, a pain management team, and a neurologist. These clinicians treated the symptoms as postherpetic neuralgia, with no consensus explanation for the skin findings. The patient reported that his symptoms were unresponsive to trials of gabapentin 800 mg tid, duloxetine 60 mg PO qd, and acetaminophen 1 to 3 g/d PO. He also had undergone several rounds of acupuncture, thoracic and cervical spine steroid injections, and epidurals, without resolution of symptoms. The patient believed the only treatment that helped was a lidocaine 4% patch, which he had used nearly every day for the previous 3 years.
Physical exam by the dermatologist revealed a lidocaine patch applied to the patient’s left upper back. Upon its removal, skin examination showed a well-demarcated, erythematous, hyperpigmented, lichenified plaque with excoriations and erosions where the patch had been (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Contact dermatitis
The patient’s history and skin exam provided enough information to diagnose contact dermatitis. The pruritus, burning, and pain the patient had experienced were due to continuous application of the lidocaine patch to the area rather than postherpetic neuralgia.
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis is an inflammatory reaction caused directly by a substance, while allergic contact dermatitis is a delayed hypersensitivity reaction to specific allergens.1 While data to elucidate the incidence and prevalence of allergic contact dermatitis are unknown, common causes include latex, dyes, oils, resins, and compounds in textiles, rubber, cosmetics, and other products used in daily life.1
Allergic contact dermatitis due to lidocaine is becoming more prevalent with increased use and availability of over-the-counter products.2 A retrospective chart review of 1819 patch-tested patients from the University of British Columbia Contact Dermatitis Clinic showed a significant proportion of patients (2.4%) were found to have
The differential varies by area affected
The differential diagnosis for contact dermatitis varies by area affected and the distribution of rash. Atopic dermatitis, lichen planus, and psoriasis are a few dermatologic conditions to consider in the differential diagnosis. They can look similar to contact dermatitis, but the patient’s history can help to discern the most likely diagnosis.1
Atopic dermatitis is a complex dysfunction of the skin barrier and immune factors that often begins in childhood and persists in some patients throughout their lifetime. Atopic dermatitis is associated with other forms of atopy including asthma, allergic rhinitis, and food and contact allergies. Atopic dermatitis in the absence of contact allergies may manifest with chronic, diffuse, scaly patches with poorly defined borders. The patches appear in a symmetrical distribution and favor the flexural surfaces, such as the antecubital fossa, wrists, and neck.
Continue to: Lichen planus
Lichen planus most often manifests in the fourth through sixth decade of life as flat-topped itchy pink-to-purple polygonal papules to plaques. Lesions range from 2 to 10 mm and favor the volar wrists, shins, and lower back, although they may be widespread. Oral lesions manifesting as ulcers or white lacy patches in the buccal mucosa are common and may be a clue to the diagnosis. Unlike more generalized contact dermatitis, lichen planus lesions are discrete.
Psoriasis manifests as well-demarcated scaly plaques distributed symmetrically over extensor surfaces. The plaques commonly are found on the elbows, knees, and scalp. When psoriasis manifests in a very limited form (as just a single plaque or limited number of plaques), it can be hard to confidently exclude other etiologies. In these circumstances, look for psoriasis signs in more unique locations (eg, pitting in the nails or plaques on the scalp or in the gluteal cleft). Adding those findings to an otherwise solitary plaque significantly adds to diagnostic certainty.
Diagnosis entails getting the shape of things
Diagnosis is based on history of exposure to irritating or allergic substances, as well as a clinical exam. Skin examination of contact dermatitis can vary based on how long it has been present: Acute manifestations include erythema, oozing, scale, vesicles, and bullae, while chronic contact dermatitis tends to demonstrate lichenification and scale.1
Distinctive findings. The most distinctive physical exam findings in patients with contact dermatitis are often shape and distribution of the rash, which reflect points of contact with the offending agent. This clue helped to elucidate the diagnosis in our patient: his rash was perfectly demarcated within the precise area where the patch was applied daily.
Irritant vs allergic. Patch testing can be performed to differentiate irritant vs allergic contact dermatitis.1 Irritant contact dermatitis usually is apparent when removing a patch and will resolve over a day, whereas allergic contact dermatitis forms over time and the skin rash is most prominent several days after the patch has been removed.1
Continue to: Treatment
Treatment: First, stop the offense
Treatment of both variants of contact dermatitis includes avoidance of the causative substance and symptomatic treatment with topical steroids, antihistamines, and possibly oral steroids depending on the severity.1
For our patient, a viral swab was taken and submitted for varicella zoster virus polymerase chain reaction testing to rule out persistent herpes zoster infection; the result was negative. The patient was counseled to discontinue use of the lidocaine patch.
Given the severity and protracted duration of the patient’s symptoms, he also was started on high-potency topical steroids (clobetasol 0.05% ointment to be applied twice daily under occlusion for 2 months), a 4-week prednisone taper (60 mg × 1 week, 40 mg × 1 week, 20 mg × 1 week, 10 mg × 1 week, then stop), and hydroxyzine (25 mg nightly as needed for pruritus). The patient’s rash and symptoms improved dramatically within the first few doses of prednisone and completely cleared by Week 4 of the prednisone taper. At his follow-up appointment 1 month after completing the prednisone taper, he stated that the pain on his back had resolved
A 75-YEAR-OLD MAN presented to the dermatology clinic for evaluation of localized, persistent burning pain and discomfort attributed to shingles and postherpetic neuralgia. He had received a diagnosis of shingles on his left upper back about 3 years prior to this presentation.
In the ensuing years, the patient had been evaluated and treated by his primary care physician, a pain management team, and a neurologist. These clinicians treated the symptoms as postherpetic neuralgia, with no consensus explanation for the skin findings. The patient reported that his symptoms were unresponsive to trials of gabapentin 800 mg tid, duloxetine 60 mg PO qd, and acetaminophen 1 to 3 g/d PO. He also had undergone several rounds of acupuncture, thoracic and cervical spine steroid injections, and epidurals, without resolution of symptoms. The patient believed the only treatment that helped was a lidocaine 4% patch, which he had used nearly every day for the previous 3 years.
Physical exam by the dermatologist revealed a lidocaine patch applied to the patient’s left upper back. Upon its removal, skin examination showed a well-demarcated, erythematous, hyperpigmented, lichenified plaque with excoriations and erosions where the patch had been (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Contact dermatitis
The patient’s history and skin exam provided enough information to diagnose contact dermatitis. The pruritus, burning, and pain the patient had experienced were due to continuous application of the lidocaine patch to the area rather than postherpetic neuralgia.
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis is an inflammatory reaction caused directly by a substance, while allergic contact dermatitis is a delayed hypersensitivity reaction to specific allergens.1 While data to elucidate the incidence and prevalence of allergic contact dermatitis are unknown, common causes include latex, dyes, oils, resins, and compounds in textiles, rubber, cosmetics, and other products used in daily life.1
Allergic contact dermatitis due to lidocaine is becoming more prevalent with increased use and availability of over-the-counter products.2 A retrospective chart review of 1819 patch-tested patients from the University of British Columbia Contact Dermatitis Clinic showed a significant proportion of patients (2.4%) were found to have
The differential varies by area affected
The differential diagnosis for contact dermatitis varies by area affected and the distribution of rash. Atopic dermatitis, lichen planus, and psoriasis are a few dermatologic conditions to consider in the differential diagnosis. They can look similar to contact dermatitis, but the patient’s history can help to discern the most likely diagnosis.1
Atopic dermatitis is a complex dysfunction of the skin barrier and immune factors that often begins in childhood and persists in some patients throughout their lifetime. Atopic dermatitis is associated with other forms of atopy including asthma, allergic rhinitis, and food and contact allergies. Atopic dermatitis in the absence of contact allergies may manifest with chronic, diffuse, scaly patches with poorly defined borders. The patches appear in a symmetrical distribution and favor the flexural surfaces, such as the antecubital fossa, wrists, and neck.
Continue to: Lichen planus
Lichen planus most often manifests in the fourth through sixth decade of life as flat-topped itchy pink-to-purple polygonal papules to plaques. Lesions range from 2 to 10 mm and favor the volar wrists, shins, and lower back, although they may be widespread. Oral lesions manifesting as ulcers or white lacy patches in the buccal mucosa are common and may be a clue to the diagnosis. Unlike more generalized contact dermatitis, lichen planus lesions are discrete.
Psoriasis manifests as well-demarcated scaly plaques distributed symmetrically over extensor surfaces. The plaques commonly are found on the elbows, knees, and scalp. When psoriasis manifests in a very limited form (as just a single plaque or limited number of plaques), it can be hard to confidently exclude other etiologies. In these circumstances, look for psoriasis signs in more unique locations (eg, pitting in the nails or plaques on the scalp or in the gluteal cleft). Adding those findings to an otherwise solitary plaque significantly adds to diagnostic certainty.
Diagnosis entails getting the shape of things
Diagnosis is based on history of exposure to irritating or allergic substances, as well as a clinical exam. Skin examination of contact dermatitis can vary based on how long it has been present: Acute manifestations include erythema, oozing, scale, vesicles, and bullae, while chronic contact dermatitis tends to demonstrate lichenification and scale.1
Distinctive findings. The most distinctive physical exam findings in patients with contact dermatitis are often shape and distribution of the rash, which reflect points of contact with the offending agent. This clue helped to elucidate the diagnosis in our patient: his rash was perfectly demarcated within the precise area where the patch was applied daily.
Irritant vs allergic. Patch testing can be performed to differentiate irritant vs allergic contact dermatitis.1 Irritant contact dermatitis usually is apparent when removing a patch and will resolve over a day, whereas allergic contact dermatitis forms over time and the skin rash is most prominent several days after the patch has been removed.1
Continue to: Treatment
Treatment: First, stop the offense
Treatment of both variants of contact dermatitis includes avoidance of the causative substance and symptomatic treatment with topical steroids, antihistamines, and possibly oral steroids depending on the severity.1
For our patient, a viral swab was taken and submitted for varicella zoster virus polymerase chain reaction testing to rule out persistent herpes zoster infection; the result was negative. The patient was counseled to discontinue use of the lidocaine patch.
Given the severity and protracted duration of the patient’s symptoms, he also was started on high-potency topical steroids (clobetasol 0.05% ointment to be applied twice daily under occlusion for 2 months), a 4-week prednisone taper (60 mg × 1 week, 40 mg × 1 week, 20 mg × 1 week, 10 mg × 1 week, then stop), and hydroxyzine (25 mg nightly as needed for pruritus). The patient’s rash and symptoms improved dramatically within the first few doses of prednisone and completely cleared by Week 4 of the prednisone taper. At his follow-up appointment 1 month after completing the prednisone taper, he stated that the pain on his back had resolved
1. Li Y, Li L. Contact dermatitis: classifications and management. Clin Rev Allergy Immunol. 2021;61:245-281. doi: 10.1007/s12016-021-08875-0
2. Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271. doi: 10.1097/DER.0000000000000216
3. To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372. doi: 10.1097/DSS.0000000000000190
1. Li Y, Li L. Contact dermatitis: classifications and management. Clin Rev Allergy Immunol. 2021;61:245-281. doi: 10.1007/s12016-021-08875-0
2. Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271. doi: 10.1097/DER.0000000000000216
3. To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372. doi: 10.1097/DSS.0000000000000190