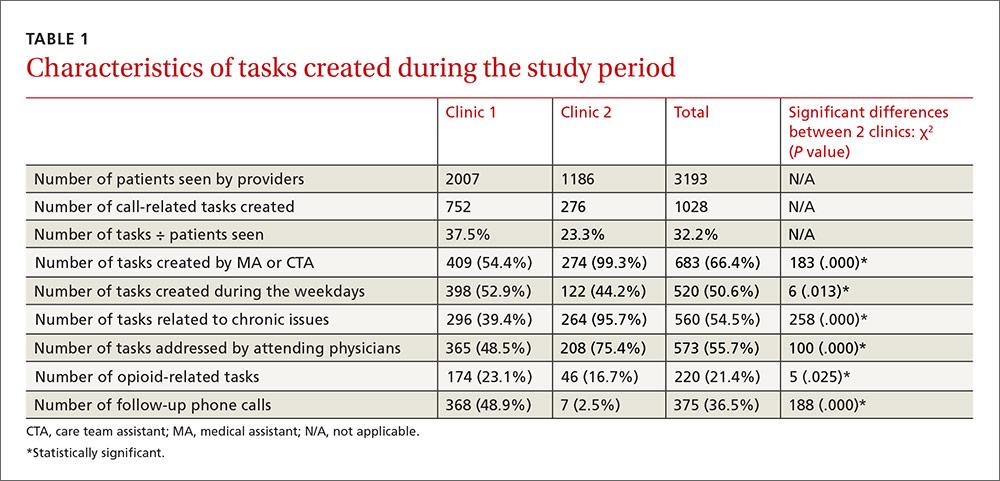
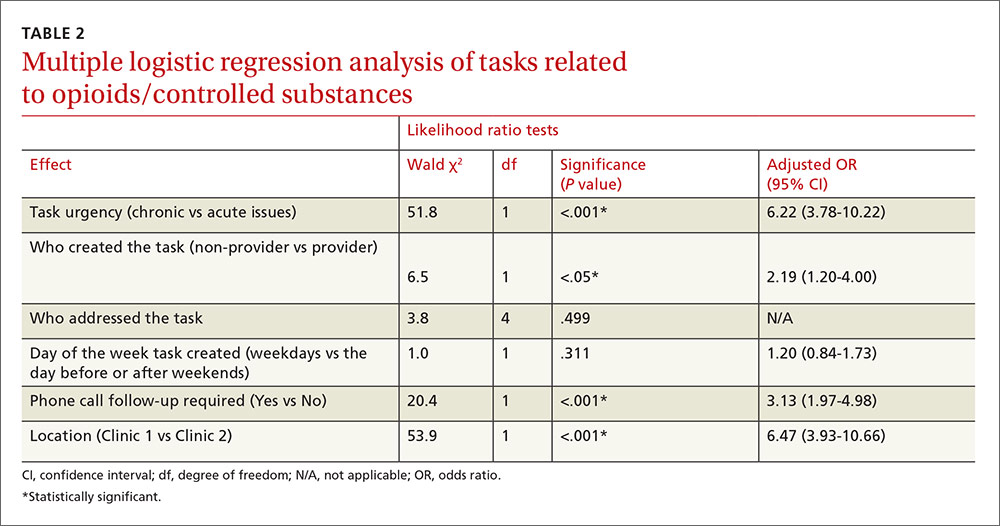
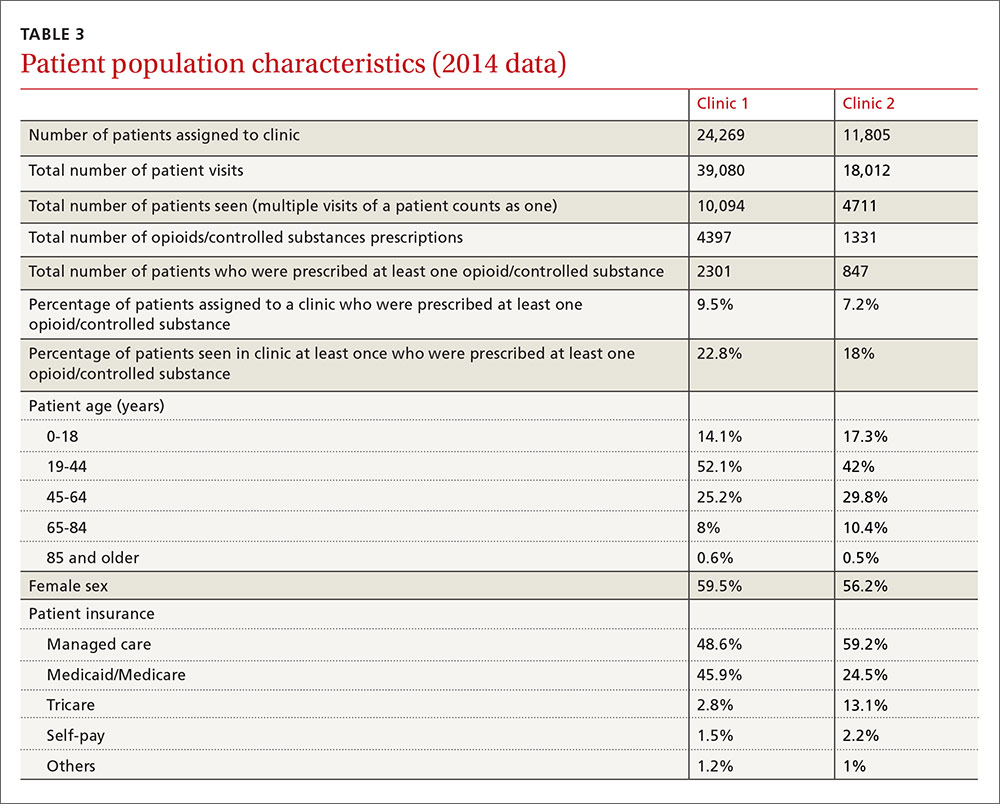
User login
Painful facial abscess
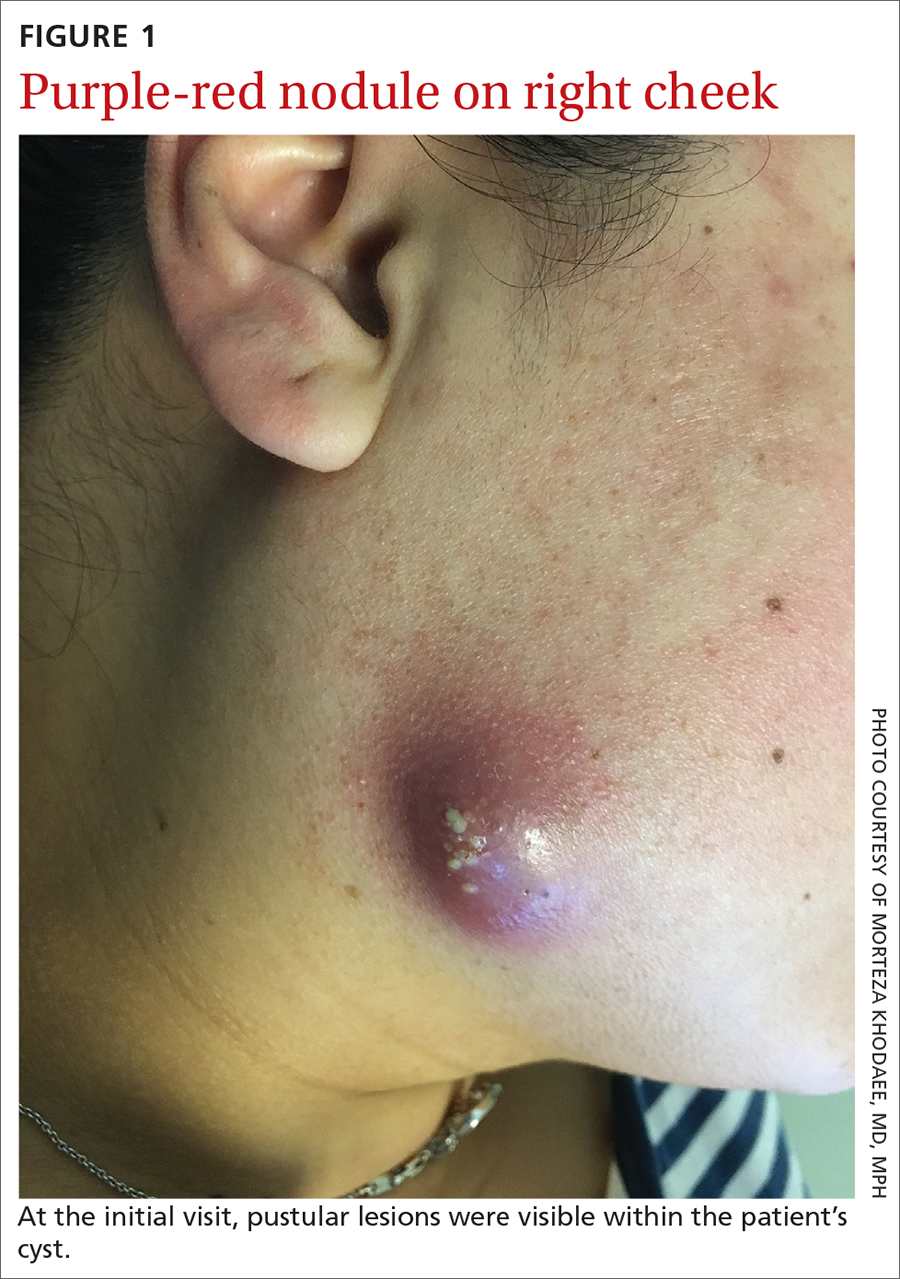
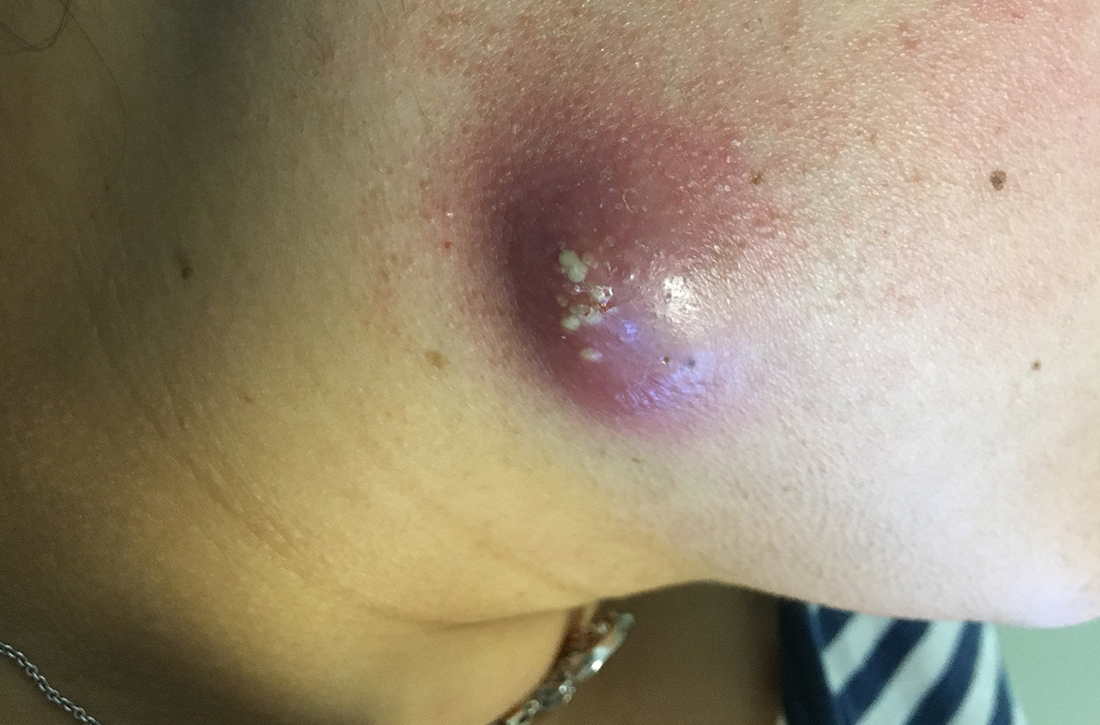
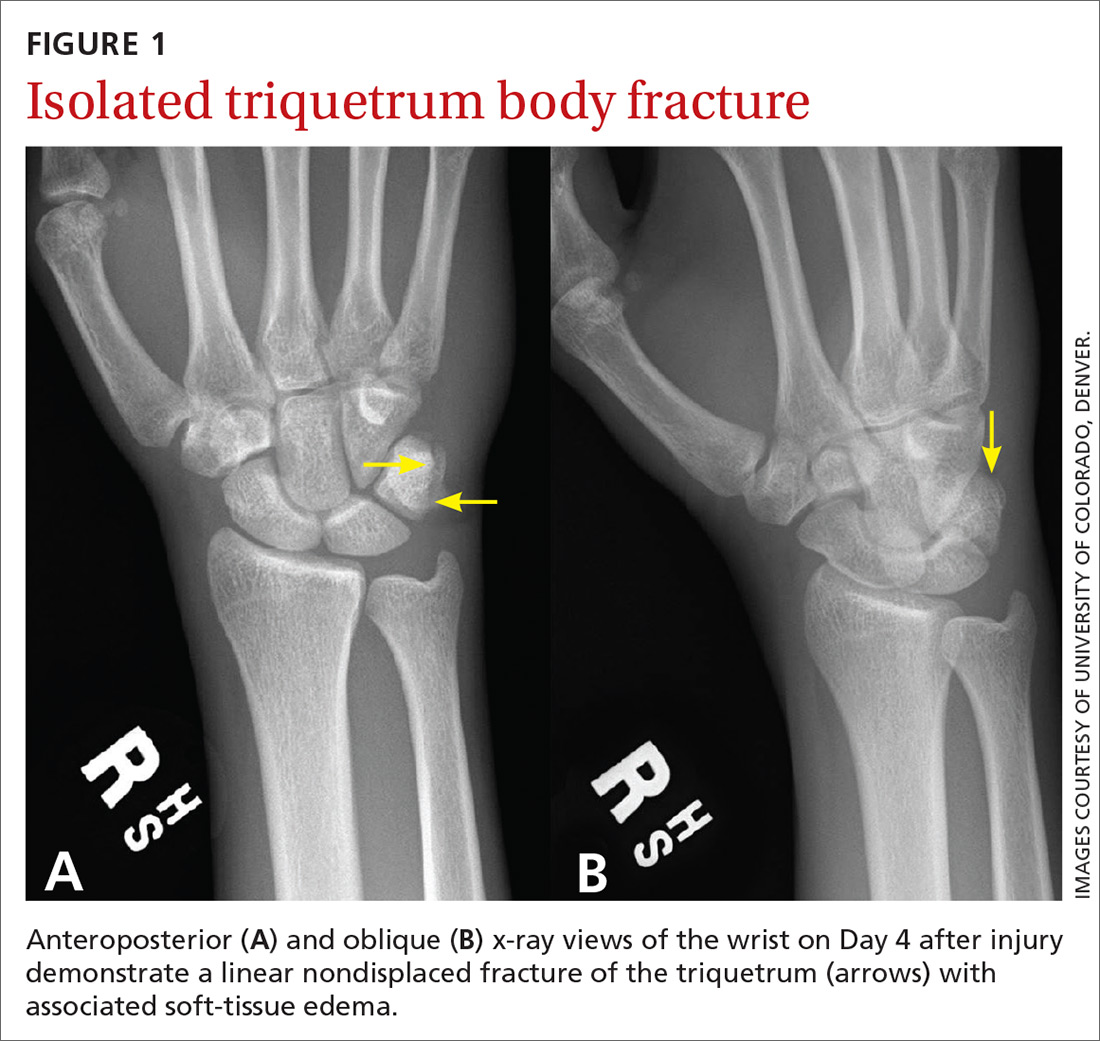
A 35-year-old woman presented to our clinic with a purple-red cyst on her right cheek that had been present for about 4 years but had worsened over the prior 2 weeks (FIGURE 1). She said she was experiencing excruciating pain and that the cyst had purulent drainage. She denied any history of diabetes, dental problems, recent trauma, or an inciting event.
On physical examination, there was no cervical lymphadenopathy, and her vital signs were normal. An incision and drainage procedure was performed. About 2 mL of purulent fluid was extracted and sent for aerobic and anaerobic cultures.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cervicofacial actinomycosis
Direct Gram stain showed gram-positive cocci, so the patient was started on a 7-day course of cephalexin 500 mg tid. Five days later, the anaerobic culture grew Actinomyces neuii, revealing the diagnosis as cervicofacial actinomycosis; the patient stopped taking cephalexin. The patient was then switched to a 3-month course of amoxicillin 875 mg bid.
Actinomyces are natural inhabitants of the human oropharynx and gastrointestinal and genitourinary tracts.1-4 They are filamentous, gram-positive rods with characteristic sulfur granules (although these are not always present).1-4 It is believed that actinomycosis is endogenously acquired from deep tissue either through dental trauma, penetrating wounds, or compound fractures.2,4
The most common presentations of actinomycosis include cervicofacial (sometimes referred to as “lumpy jaw syndrome”), followed by abdominopelvic and thoracic/pulmonary, manifestations.2-4 Primary cutaneous actinomycosis is rare.5-9 Actinomycosis infection often manifests with indolent constitutional symptoms such as fatigue and anorexia.1 Most cases occur in men ages 20 to 60 years, although cases in women are increasingly being reported.2-4
Risk factors include poor dental hygiene or dental procedures, alcoholism, intrauterine device use, immunosuppression, appendicitis, and diverticulitis.2-4 The exact cause of this patient’s actinomycosis was unknown, as she did not have any known risk factors.
Furunculosis and sporotrichosis are part of the differential
Actinomycosis is often called a “great mimicker” due to its ability to masquerade as infection, malignancy, or fungus.1 The differential diagnosis for this patient’s presentation included bacterial soft-tissue infection (eg, furunculosis), infected epidermoid cyst, cutaneous tuberculosis, sporotrichosis, deep fungal infection, and nocardiosis.
Continue to: Furunculosis was initially suspected
Furunculosis was initially suspected, but the original wound culture demonstrated actinomycoses instead of traditional gram-positive bacteria.
A clinical diagnosis
The diagnosis of actinomycosis is usually made clinically, but definitive confirmation requires culture, which can be challenging with a slow-growing facultative or strict anaerobe that may take up to 14 days to appear.2-4 A Gram stain can aid in the diagnosis, but overall, there is a high false-negative rate in identifying actinomycosis.1,3,4
Treatment time can be lengthy, but prognosis is favorable
Unfortunately, there are no randomized controlled studies for treatment of actinomycosis. The majority of evidence for treatment comes from in vitro and clinical case studies.2-4,10 In general, prognosis of actinomycosis is favorable with low mortality, but chronic infection without complete resolution of symptoms can occur.1-4,7,8,10
First-line therapy for actinomycosis is a beta-lactam antibiotic, typically penicillin G or amoxicillin.2-4,10 High doses of prolonged intravenous (IV) and oral antibiotic therapy (2 to 12 months) based on location and complexity are standard.3,11 However, if there is minimal bone involvement and the patient shows rapid improvement, treatment could be shortened to a 4 to 6–week oral regimen.1,11 Surgical intervention can also shorten the required length of antibiotic duration.1,10
Cutaneous actinomycosis Tx. Amoxicillin/clavulanic acid has been shown to be an effective treatment for cutaneous actinomycosis, especially if polymicrobial infection is suspected.5,6 Individualized regimens for cutaneous actinomycosis—based on severity, location, and treatment response—are acceptable with close monitoring.1,2,11
Continue to: A lengthy recovery for our patient
A lengthy recovery for our patient
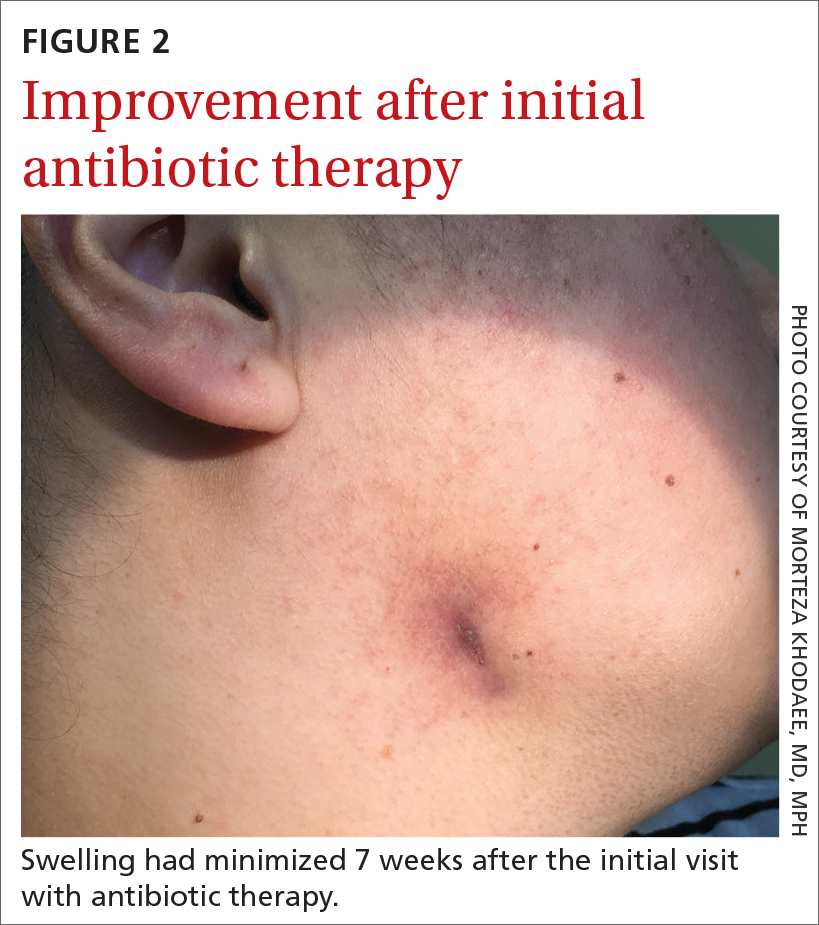
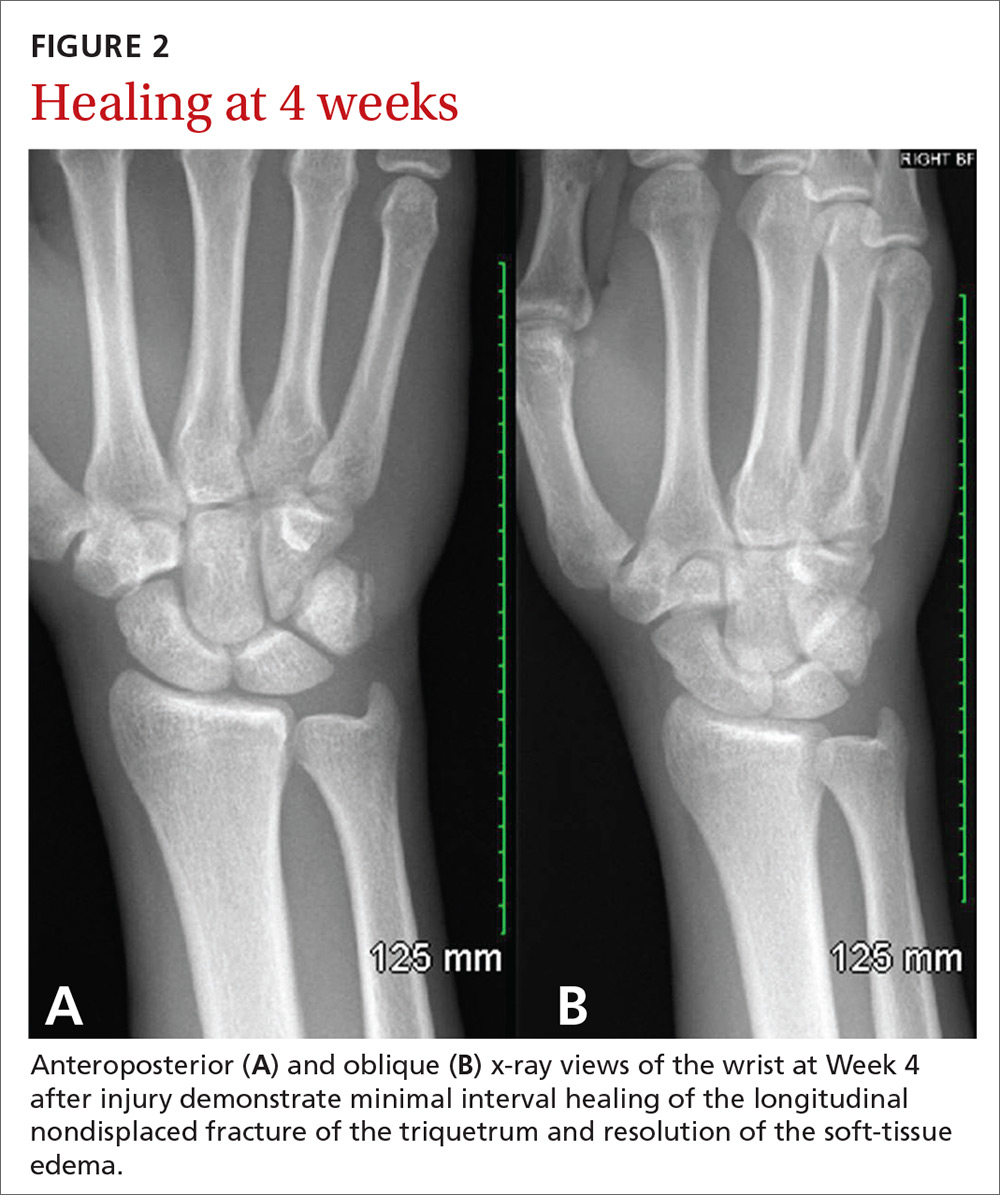
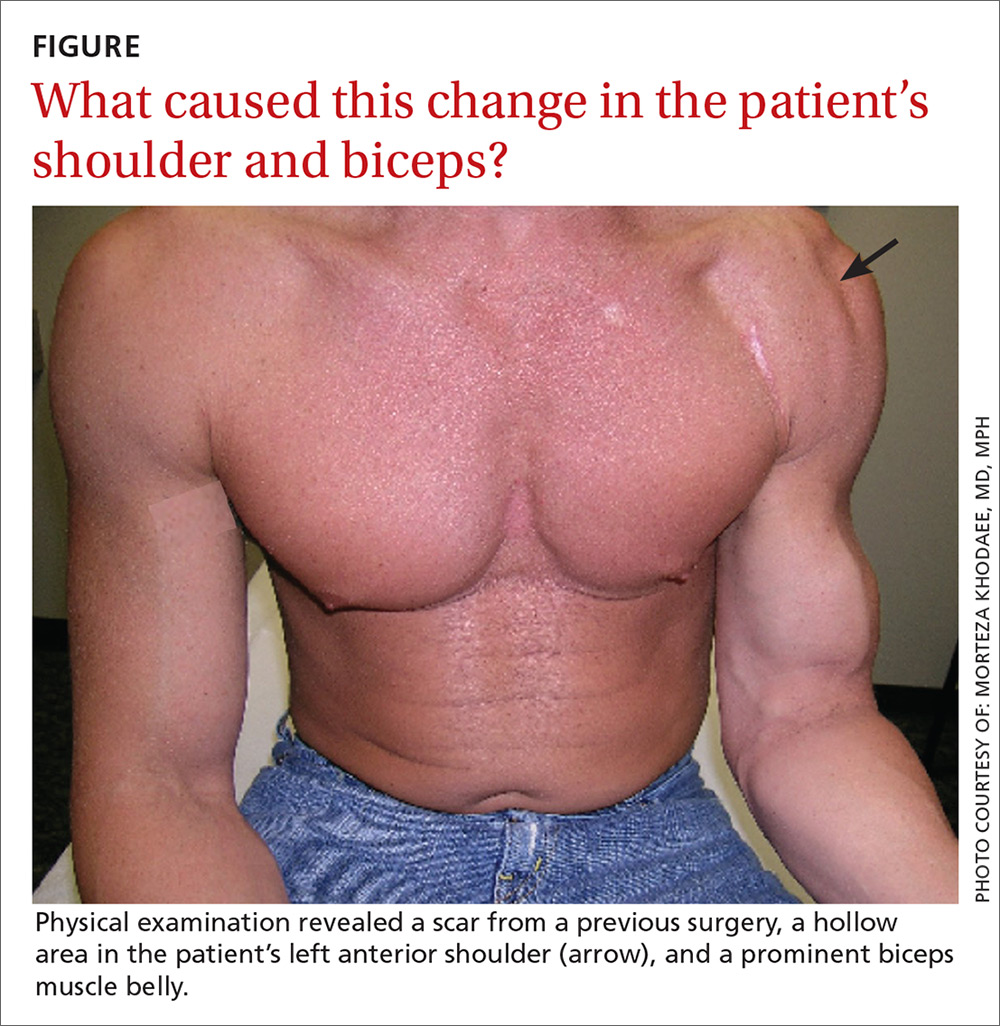
Seven weeks after the initial visit, the patient reported that she had taken only 20 days’ worth of the recommended 3-month course of amoxicillin. Fortunately, the lesion appeared to be healing well with no apparent fluid collection (FIGURE 2).
The patient was then prescribed, and completed, a 3-month course of amoxicillin/clavulanic acid
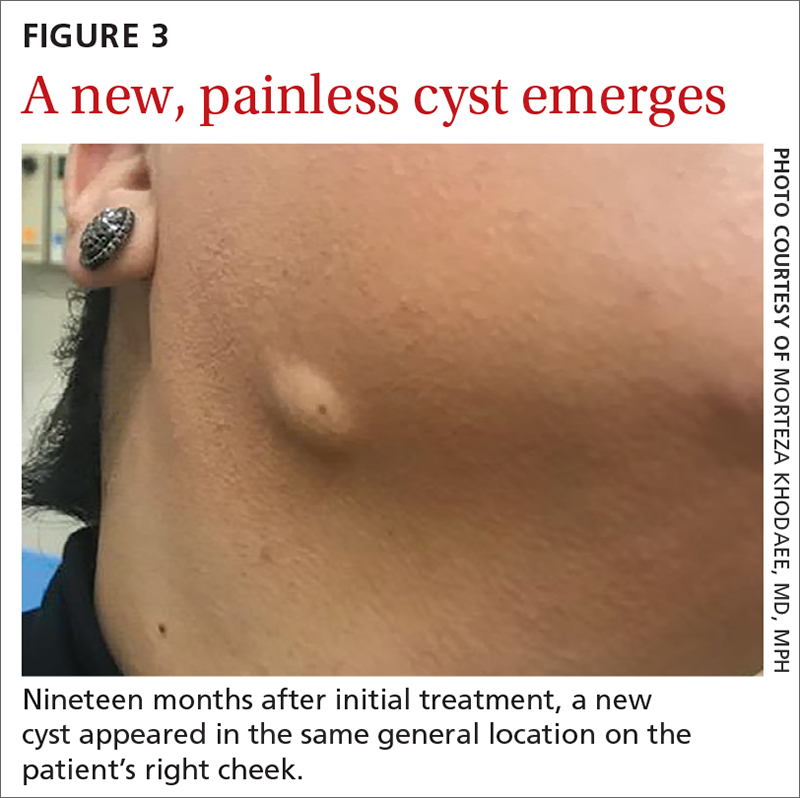
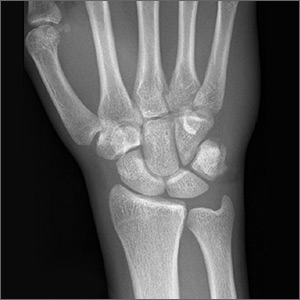
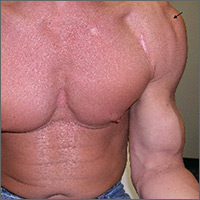
Nineteen months after initial treatment, the lesion reappeared as a painless cyst in a similar location (FIGURE 3). Plastic Surgery incised and drained the lesion and Infectious Diseases continued her on 3 months of amoxicillin/clavulanic acid 875 mg/125 mg bid, which she did complete.
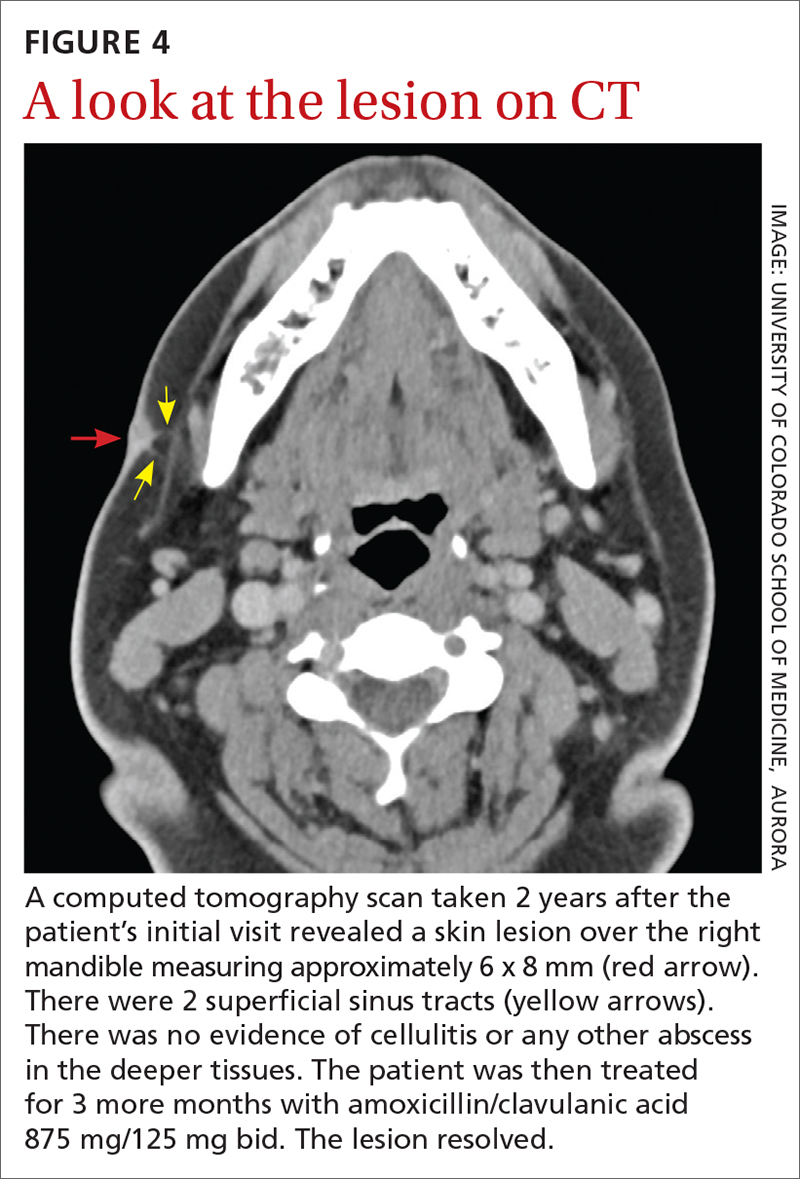
Due to the continued presence of the lesion, a computed tomography scan of the face was ordered 2 years after the initial visit and demonstrated a superficial skin lesion with no mandibular involvement (FIGURE 4). She was then treated with 3 more months of amoxicillin/clavulanic acid 875 mg/125 mg bid, with the possibility of deep debridement if not improved. However, debridement was unnecessary as the cyst did not recur.
We believe that the course of this patient’s treatment was protracted because she never took oral antibiotics for more than 3 months at a time, and thus, her infection never completely resolved. In retrospect, we would have treated her more aggressively from the outset.
1. Najmi AH, Najmi IH, Tawhari MMH, et al. Cutaneous actinomycosis and long-term management through using oral and topical antibiotics: a case report. Clin Pract. 2018;8:1102. doi: 10.4081/ cp.2018.1102
2. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. StatPearls Publishing; 2021.
3. Valour F, Sénécha A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-97. doi: 10.2147/IDR.S39601
4. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099
5. Akhtar M, Zade MP, Shahane PL, et al. Scalp actinomycosis presenting as soft tissue tumour: a case report with literature review. Int J Surg Case Rep. 2015;16:99-101. doi: 10.1016/ j.ijscr.2015.09.030
6. Bose M, Ghosh R, Mukherjee K, et al. Primary cutaneous actinomycosis:a case report. J Clin Diagn Res. 2014;8:YD03-5. doi: 10.7860/JCDR/2014/8286.4591
7. Cataño JC, Gómez Villegas SI. Images in clinical medicine. Cutaneous actinomycosis. N Engl J Med. 2016;374:1773. doi: 10.1056/ NEJMicm1511213
8. Mehta V, Balachandran C. Primary cutaneous actinomycosis on the chest wall. Dermatol Online J. 2008;14:13.
9. Piggott SA, Khodaee M. A bump in the groin: cutaneous actinomycosis. J Family Community Med. 2017;24:203. doi: 10.4103/jfcm.JFCM_79_17
10. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J Dermatolog Treat. 2017;28:59-64. doi: 10.1080/09546634.2016.1178373
11. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;;7:183-197. doi: 10.2147/IDR.S39601
A 35-year-old woman presented to our clinic with a purple-red cyst on her right cheek that had been present for about 4 years but had worsened over the prior 2 weeks (FIGURE 1). She said she was experiencing excruciating pain and that the cyst had purulent drainage. She denied any history of diabetes, dental problems, recent trauma, or an inciting event.
On physical examination, there was no cervical lymphadenopathy, and her vital signs were normal. An incision and drainage procedure was performed. About 2 mL of purulent fluid was extracted and sent for aerobic and anaerobic cultures.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cervicofacial actinomycosis
Direct Gram stain showed gram-positive cocci, so the patient was started on a 7-day course of cephalexin 500 mg tid. Five days later, the anaerobic culture grew Actinomyces neuii, revealing the diagnosis as cervicofacial actinomycosis; the patient stopped taking cephalexin. The patient was then switched to a 3-month course of amoxicillin 875 mg bid.
Actinomyces are natural inhabitants of the human oropharynx and gastrointestinal and genitourinary tracts.1-4 They are filamentous, gram-positive rods with characteristic sulfur granules (although these are not always present).1-4 It is believed that actinomycosis is endogenously acquired from deep tissue either through dental trauma, penetrating wounds, or compound fractures.2,4
The most common presentations of actinomycosis include cervicofacial (sometimes referred to as “lumpy jaw syndrome”), followed by abdominopelvic and thoracic/pulmonary, manifestations.2-4 Primary cutaneous actinomycosis is rare.5-9 Actinomycosis infection often manifests with indolent constitutional symptoms such as fatigue and anorexia.1 Most cases occur in men ages 20 to 60 years, although cases in women are increasingly being reported.2-4
Risk factors include poor dental hygiene or dental procedures, alcoholism, intrauterine device use, immunosuppression, appendicitis, and diverticulitis.2-4 The exact cause of this patient’s actinomycosis was unknown, as she did not have any known risk factors.
Furunculosis and sporotrichosis are part of the differential
Actinomycosis is often called a “great mimicker” due to its ability to masquerade as infection, malignancy, or fungus.1 The differential diagnosis for this patient’s presentation included bacterial soft-tissue infection (eg, furunculosis), infected epidermoid cyst, cutaneous tuberculosis, sporotrichosis, deep fungal infection, and nocardiosis.
Continue to: Furunculosis was initially suspected
Furunculosis was initially suspected, but the original wound culture demonstrated actinomycoses instead of traditional gram-positive bacteria.
A clinical diagnosis
The diagnosis of actinomycosis is usually made clinically, but definitive confirmation requires culture, which can be challenging with a slow-growing facultative or strict anaerobe that may take up to 14 days to appear.2-4 A Gram stain can aid in the diagnosis, but overall, there is a high false-negative rate in identifying actinomycosis.1,3,4
Treatment time can be lengthy, but prognosis is favorable
Unfortunately, there are no randomized controlled studies for treatment of actinomycosis. The majority of evidence for treatment comes from in vitro and clinical case studies.2-4,10 In general, prognosis of actinomycosis is favorable with low mortality, but chronic infection without complete resolution of symptoms can occur.1-4,7,8,10
First-line therapy for actinomycosis is a beta-lactam antibiotic, typically penicillin G or amoxicillin.2-4,10 High doses of prolonged intravenous (IV) and oral antibiotic therapy (2 to 12 months) based on location and complexity are standard.3,11 However, if there is minimal bone involvement and the patient shows rapid improvement, treatment could be shortened to a 4 to 6–week oral regimen.1,11 Surgical intervention can also shorten the required length of antibiotic duration.1,10
Cutaneous actinomycosis Tx. Amoxicillin/clavulanic acid has been shown to be an effective treatment for cutaneous actinomycosis, especially if polymicrobial infection is suspected.5,6 Individualized regimens for cutaneous actinomycosis—based on severity, location, and treatment response—are acceptable with close monitoring.1,2,11
Continue to: A lengthy recovery for our patient
A lengthy recovery for our patient
Seven weeks after the initial visit, the patient reported that she had taken only 20 days’ worth of the recommended 3-month course of amoxicillin. Fortunately, the lesion appeared to be healing well with no apparent fluid collection (FIGURE 2).
The patient was then prescribed, and completed, a 3-month course of amoxicillin/clavulanic acid
Nineteen months after initial treatment, the lesion reappeared as a painless cyst in a similar location (FIGURE 3). Plastic Surgery incised and drained the lesion and Infectious Diseases continued her on 3 months of amoxicillin/clavulanic acid 875 mg/125 mg bid, which she did complete.
Due to the continued presence of the lesion, a computed tomography scan of the face was ordered 2 years after the initial visit and demonstrated a superficial skin lesion with no mandibular involvement (FIGURE 4). She was then treated with 3 more months of amoxicillin/clavulanic acid 875 mg/125 mg bid, with the possibility of deep debridement if not improved. However, debridement was unnecessary as the cyst did not recur.

We believe that the course of this patient’s treatment was protracted because she never took oral antibiotics for more than 3 months at a time, and thus, her infection never completely resolved. In retrospect, we would have treated her more aggressively from the outset.
A 35-year-old woman presented to our clinic with a purple-red cyst on her right cheek that had been present for about 4 years but had worsened over the prior 2 weeks (FIGURE 1). She said she was experiencing excruciating pain and that the cyst had purulent drainage. She denied any history of diabetes, dental problems, recent trauma, or an inciting event.
On physical examination, there was no cervical lymphadenopathy, and her vital signs were normal. An incision and drainage procedure was performed. About 2 mL of purulent fluid was extracted and sent for aerobic and anaerobic cultures.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cervicofacial actinomycosis
Direct Gram stain showed gram-positive cocci, so the patient was started on a 7-day course of cephalexin 500 mg tid. Five days later, the anaerobic culture grew Actinomyces neuii, revealing the diagnosis as cervicofacial actinomycosis; the patient stopped taking cephalexin. The patient was then switched to a 3-month course of amoxicillin 875 mg bid.
Actinomyces are natural inhabitants of the human oropharynx and gastrointestinal and genitourinary tracts.1-4 They are filamentous, gram-positive rods with characteristic sulfur granules (although these are not always present).1-4 It is believed that actinomycosis is endogenously acquired from deep tissue either through dental trauma, penetrating wounds, or compound fractures.2,4
The most common presentations of actinomycosis include cervicofacial (sometimes referred to as “lumpy jaw syndrome”), followed by abdominopelvic and thoracic/pulmonary, manifestations.2-4 Primary cutaneous actinomycosis is rare.5-9 Actinomycosis infection often manifests with indolent constitutional symptoms such as fatigue and anorexia.1 Most cases occur in men ages 20 to 60 years, although cases in women are increasingly being reported.2-4
Risk factors include poor dental hygiene or dental procedures, alcoholism, intrauterine device use, immunosuppression, appendicitis, and diverticulitis.2-4 The exact cause of this patient’s actinomycosis was unknown, as she did not have any known risk factors.
Furunculosis and sporotrichosis are part of the differential
Actinomycosis is often called a “great mimicker” due to its ability to masquerade as infection, malignancy, or fungus.1 The differential diagnosis for this patient’s presentation included bacterial soft-tissue infection (eg, furunculosis), infected epidermoid cyst, cutaneous tuberculosis, sporotrichosis, deep fungal infection, and nocardiosis.
Continue to: Furunculosis was initially suspected
Furunculosis was initially suspected, but the original wound culture demonstrated actinomycoses instead of traditional gram-positive bacteria.
A clinical diagnosis
The diagnosis of actinomycosis is usually made clinically, but definitive confirmation requires culture, which can be challenging with a slow-growing facultative or strict anaerobe that may take up to 14 days to appear.2-4 A Gram stain can aid in the diagnosis, but overall, there is a high false-negative rate in identifying actinomycosis.1,3,4
Treatment time can be lengthy, but prognosis is favorable
Unfortunately, there are no randomized controlled studies for treatment of actinomycosis. The majority of evidence for treatment comes from in vitro and clinical case studies.2-4,10 In general, prognosis of actinomycosis is favorable with low mortality, but chronic infection without complete resolution of symptoms can occur.1-4,7,8,10
First-line therapy for actinomycosis is a beta-lactam antibiotic, typically penicillin G or amoxicillin.2-4,10 High doses of prolonged intravenous (IV) and oral antibiotic therapy (2 to 12 months) based on location and complexity are standard.3,11 However, if there is minimal bone involvement and the patient shows rapid improvement, treatment could be shortened to a 4 to 6–week oral regimen.1,11 Surgical intervention can also shorten the required length of antibiotic duration.1,10
Cutaneous actinomycosis Tx. Amoxicillin/clavulanic acid has been shown to be an effective treatment for cutaneous actinomycosis, especially if polymicrobial infection is suspected.5,6 Individualized regimens for cutaneous actinomycosis—based on severity, location, and treatment response—are acceptable with close monitoring.1,2,11
Continue to: A lengthy recovery for our patient
A lengthy recovery for our patient
Seven weeks after the initial visit, the patient reported that she had taken only 20 days’ worth of the recommended 3-month course of amoxicillin. Fortunately, the lesion appeared to be healing well with no apparent fluid collection (FIGURE 2).
The patient was then prescribed, and completed, a 3-month course of amoxicillin/clavulanic acid
Nineteen months after initial treatment, the lesion reappeared as a painless cyst in a similar location (FIGURE 3). Plastic Surgery incised and drained the lesion and Infectious Diseases continued her on 3 months of amoxicillin/clavulanic acid 875 mg/125 mg bid, which she did complete.
Due to the continued presence of the lesion, a computed tomography scan of the face was ordered 2 years after the initial visit and demonstrated a superficial skin lesion with no mandibular involvement (FIGURE 4). She was then treated with 3 more months of amoxicillin/clavulanic acid 875 mg/125 mg bid, with the possibility of deep debridement if not improved. However, debridement was unnecessary as the cyst did not recur.

We believe that the course of this patient’s treatment was protracted because she never took oral antibiotics for more than 3 months at a time, and thus, her infection never completely resolved. In retrospect, we would have treated her more aggressively from the outset.
1. Najmi AH, Najmi IH, Tawhari MMH, et al. Cutaneous actinomycosis and long-term management through using oral and topical antibiotics: a case report. Clin Pract. 2018;8:1102. doi: 10.4081/ cp.2018.1102
2. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. StatPearls Publishing; 2021.
3. Valour F, Sénécha A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-97. doi: 10.2147/IDR.S39601
4. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099
5. Akhtar M, Zade MP, Shahane PL, et al. Scalp actinomycosis presenting as soft tissue tumour: a case report with literature review. Int J Surg Case Rep. 2015;16:99-101. doi: 10.1016/ j.ijscr.2015.09.030
6. Bose M, Ghosh R, Mukherjee K, et al. Primary cutaneous actinomycosis:a case report. J Clin Diagn Res. 2014;8:YD03-5. doi: 10.7860/JCDR/2014/8286.4591
7. Cataño JC, Gómez Villegas SI. Images in clinical medicine. Cutaneous actinomycosis. N Engl J Med. 2016;374:1773. doi: 10.1056/ NEJMicm1511213
8. Mehta V, Balachandran C. Primary cutaneous actinomycosis on the chest wall. Dermatol Online J. 2008;14:13.
9. Piggott SA, Khodaee M. A bump in the groin: cutaneous actinomycosis. J Family Community Med. 2017;24:203. doi: 10.4103/jfcm.JFCM_79_17
10. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J Dermatolog Treat. 2017;28:59-64. doi: 10.1080/09546634.2016.1178373
11. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;;7:183-197. doi: 10.2147/IDR.S39601
1. Najmi AH, Najmi IH, Tawhari MMH, et al. Cutaneous actinomycosis and long-term management through using oral and topical antibiotics: a case report. Clin Pract. 2018;8:1102. doi: 10.4081/ cp.2018.1102
2. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. StatPearls Publishing; 2021.
3. Valour F, Sénécha A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-97. doi: 10.2147/IDR.S39601
4. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099
5. Akhtar M, Zade MP, Shahane PL, et al. Scalp actinomycosis presenting as soft tissue tumour: a case report with literature review. Int J Surg Case Rep. 2015;16:99-101. doi: 10.1016/ j.ijscr.2015.09.030
6. Bose M, Ghosh R, Mukherjee K, et al. Primary cutaneous actinomycosis:a case report. J Clin Diagn Res. 2014;8:YD03-5. doi: 10.7860/JCDR/2014/8286.4591
7. Cataño JC, Gómez Villegas SI. Images in clinical medicine. Cutaneous actinomycosis. N Engl J Med. 2016;374:1773. doi: 10.1056/ NEJMicm1511213
8. Mehta V, Balachandran C. Primary cutaneous actinomycosis on the chest wall. Dermatol Online J. 2008;14:13.
9. Piggott SA, Khodaee M. A bump in the groin: cutaneous actinomycosis. J Family Community Med. 2017;24:203. doi: 10.4103/jfcm.JFCM_79_17
10. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J Dermatolog Treat. 2017;28:59-64. doi: 10.1080/09546634.2016.1178373
11. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;;7:183-197. doi: 10.2147/IDR.S39601
37-year-old man • cough • increasing shortness of breath • pleuritic chest pain • Dx?
THE CASE
A 37-year-old man with a history of asthma, schizoaffective disorder, and tobacco use (36 packs per year) presented to the clinic after 5 days of worsening cough, reproducible left-sided chest pain, and increasing shortness of breath. He also experienced chills, fatigue, nausea, and vomiting but was afebrile. The patient had not travelled recently nor had direct contact with anyone sick. He also denied intravenous (IV) drug use, alcohol use, and bloody sputum. Recently, he had intentionally lost weight, as recommended by his psychiatrist.
Medication review revealed that he was taking many central-acting agents for schizoaffective disorder, including alprazolam, aripiprazole, desvenlafaxine, and quetiapine. Due to his intermittent asthma since childhood, he used an albuterol inhaler as needed, which currently offered only minimal relief. He denied any history of hospitalization or intubation for asthma.
During the clinic visit, his blood pressure was 90/60 mm Hg and his heart rate was normal. His pulse oximetry was 92% on room air. On physical examination, he had normal-appearing dentition. Auscultation revealed bilateral expiratory wheezes with decreased breath sounds at the left lower lobe.
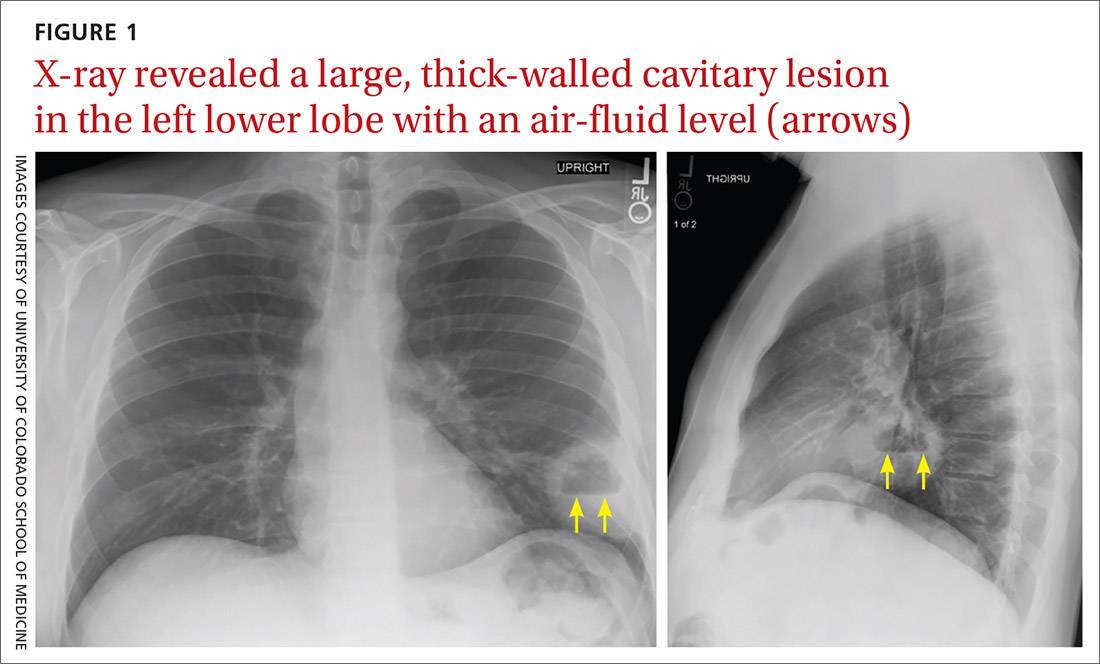
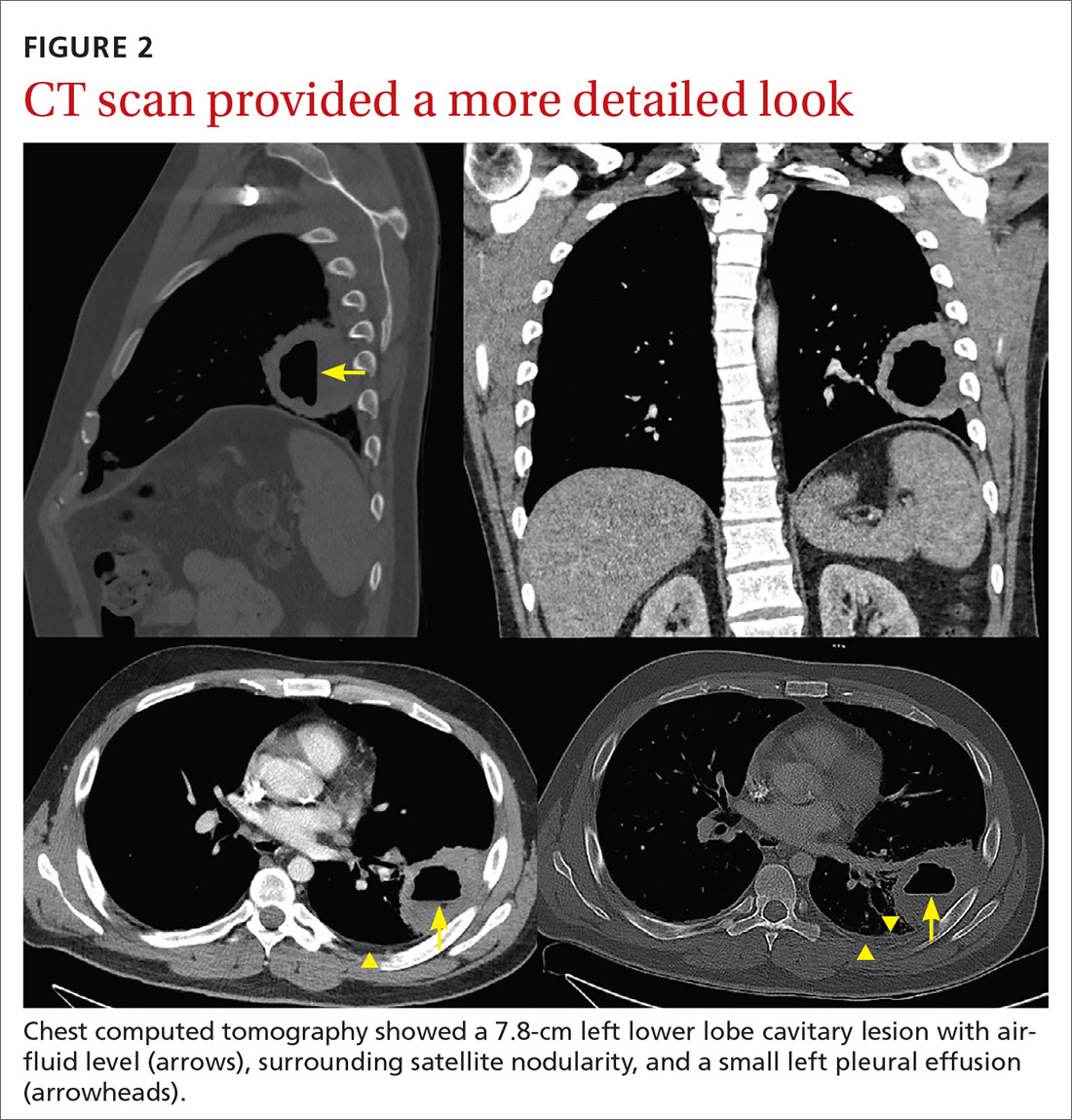
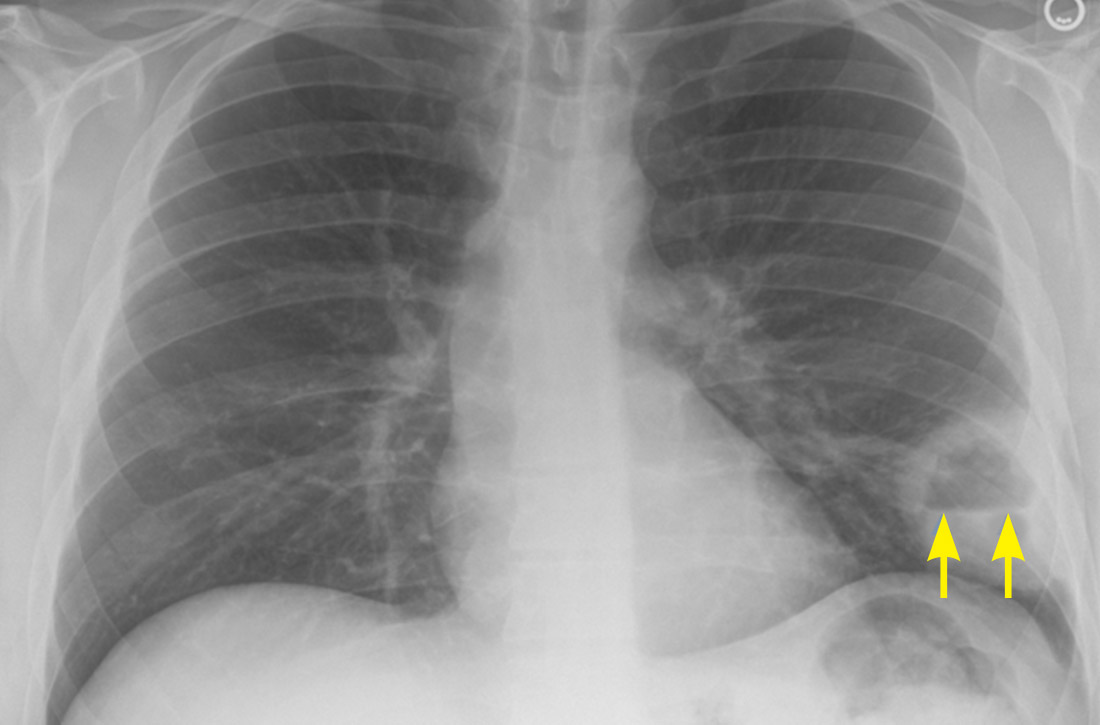
A plain chest radiograph (CXR) performed in the clinic (FIGURE 1) showed a large, thick-walled cavitary lesion with an air-fluid level in the left lower lobe. The patient was directly admitted to the Family Medicine Inpatient Service. Computed tomography (CT) of the chest with contrast was ordered to rule out empyema or malignancy. The chest CT confirmed the previous findings while also revealing a surrounding satellite nodularity in the left lower lobe (FIGURE 2). QuantiFERON-TB Gold and HIV tests were both negative.
THE DIAGNOSIS
The patient was given a diagnosis of a lung abscess based on symptoms and imaging. An extensive smoking history, as well as multiple sedating medications, increased his likelihood of aspiration.
DISCUSSION
Lung abscess is the probable diagnosis in a patient with indolent infectious symptoms (cough, fever, night sweats) developing over days to weeks and a CXR finding of pulmonary opacity, often with an air-fluid level.1-4 A lung abscess is a circumscribed collection of pus in the lung parenchyma that develops as a result of microbial infection.4
Primary vs secondary abscess. Lung abscesses can be divided into 2 groups: primary and secondary abscesses. Primary abscesses (60%) occur without any other medical condition or in patients prone to aspiration.5 Secondary abscesses occur in the setting of a comorbid medical condition, such as lung disease, heart disease, bronchogenic neoplasm, or immunocompromised status.5
Continue to: With a primary lung abscess...
With a primary lung abscess, oropharyngeal contents are aspirated (generally while the patient is unconscious) and contain mixed flora.2 The aspirate typically migrates to the posterior segments of the upper lobes and to the superior segments of the lower lobes. These abscesses are usually singular and have an air-fluid level.1,2
Secondary lung abscesses occur in bronchial obstruction (by tumor, foreign body, or enlarged lymph nodes), with coexisting lung diseases (bronchiectasis, cystic fibrosis, infected pulmonary infarcts, lung contusion) or by direct spread (broncho-esophageal fistula, subphrenic abscess).6 Secondary abscesses are associated with a poorer prognosis, dependent on the patient’s general condition and underlying disease.7
What to rule out
The differential diagnosis of cavitary lung lesion includes tuberculosis, necrotizing pneumonia, bronchial carcinoma, pulmonary embolism, vasculitis (eg, Churg-Strauss syndrome), and localized pleural empyema.1,4 A CT scan is helpful to differentiate between a parenchymal lesion and pleural collection, which may not be as clear on CXR.1,4
Tuberculosis manifests with fatigue, weight loss, and night sweats; a chest CT will reveal a cavitating lesion (usually upper lobe) with a characteristic “rim sign” that includes caseous necrosis surrounded by a peripheral enhancing rim.8
Necrotizing pneumonia manifests as acute, fulminant infection. The most common causative organisms on sputum culture are Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas species. Plain radiography will reveal multiple cavities and often associated pleural effusion and empyema.9
Continue to: Excavating bronchogenic carcinomas
Excavating bronchogenic carcinomas differ from a lung abscess in that a patient with the latter is typically, but not always, febrile and has purulent sputum. On imaging, a bronchogenic carcinoma has a thicker and more irregular wall than a lung abscess.10
Treatment
When antibiotics first became available, penicillin was used to treat lung abscess.11 Then IV clindamycin became the drug of choice after 2 trials demonstrated its superiority to IV penicillin.12,13 More recently, clindamycin alone has fallen out of favor due to growing anaerobic resistance.14
Current therapy includes beta-lactam with beta-lactamase inhibitors.14 Lung abscesses are typically polymicrobial and thus carry different degrees of antibiotic resistance.15,16 If culture data are available, targeted therapy is preferred, especially for secondary abscesses.7 Antibiotic therapy is usually continued until a CXR reveals a small lesion or is clear, which may require several months of outpatient oral antibiotic therapy.4
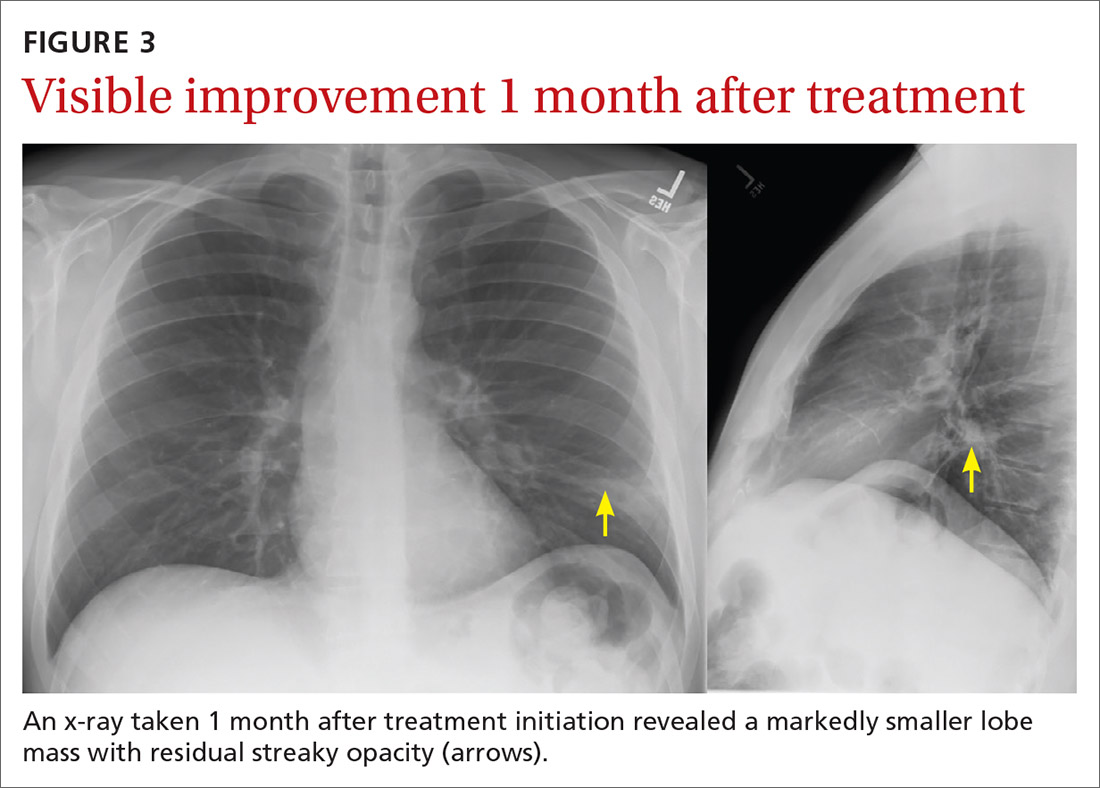
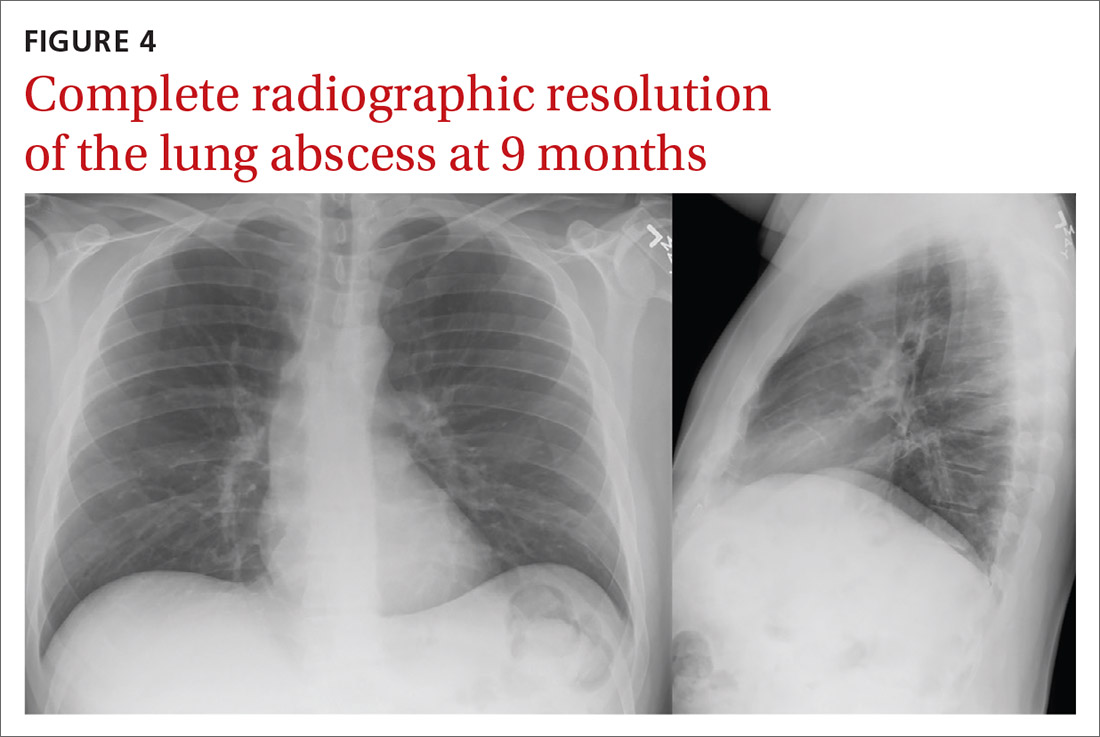
Our patient was treated with IV clindamycin for 3 days in the hospital. Clindamycin was chosen due to his penicillin allergy and started empirically without any culture data. He was transitioned to oral clindamycin and completed a total 3-week course as his CXR continued to show improvement (FIGURE 3). He did not undergo bronchoscopy. A follow-up CXR showed resolution of lung abscess at 9 months. (FIGURE 4).
THE TAKEAWAY
All patients with lung abscesses should have sputum culture with gram stain done—ideally prior to starting antibiotics.3,4 Bronchoscopy should be considered for patients with atypical presentations or those who fail standard therapy, but may be used in other cases, as well.3
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; morteza.khodaee@cuanschutz.edu
1. Hassan M, Asciak R, Rizk R, et al. Lung abscess or empyema? Taking a closer look. Thorax. 2018;73:887-889. https://doi. org/10.1136/thoraxjnl-2018-211604
2. Moreira J da SM, Camargo J de JP, Felicetti JC, et al. Lung abscess: analysis of 252 consecutive cases diagnosed between 1968 and 2004. J Bras Pneumol. 2006;32:136-43. https://doi.org/10.1590/ s1806-37132006000200009
3. Schiza S, Siafakas NM. Clinical presentation and management of empyema, lung abscess and pleural effusion. Curr Opin Pulm Med. 2006;12:205-211. https://doi.org/10.1097/01. mcp.0000219270.73180.8b
4. Yazbeck MF, Dahdel M, Kalra A, et al. Lung abscess: update on microbiology and management. Am J Ther. 2014;21:217-221. https://doi.org/10.1097/MJT.0b013e3182383c9b
5. Nicolini A, Cilloniz C, Senarega R, et al. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276-285. https://doi. org/10.5603/PiAP.2014.0033
6. Puligandla PS, Laberge J-M. Respiratory infections: pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42-52. https://doi.org/10.1053/j.sempedsurg.2007.10.007
7. Marra A, Hillejan L, Ukena D. [Management of Lung Abscess]. Zentralbl Chir. 2015;140 (suppl 1):S47-S53. https://doi. org/10.1055/s-0035-1557883
THE CASE
A 37-year-old man with a history of asthma, schizoaffective disorder, and tobacco use (36 packs per year) presented to the clinic after 5 days of worsening cough, reproducible left-sided chest pain, and increasing shortness of breath. He also experienced chills, fatigue, nausea, and vomiting but was afebrile. The patient had not travelled recently nor had direct contact with anyone sick. He also denied intravenous (IV) drug use, alcohol use, and bloody sputum. Recently, he had intentionally lost weight, as recommended by his psychiatrist.
Medication review revealed that he was taking many central-acting agents for schizoaffective disorder, including alprazolam, aripiprazole, desvenlafaxine, and quetiapine. Due to his intermittent asthma since childhood, he used an albuterol inhaler as needed, which currently offered only minimal relief. He denied any history of hospitalization or intubation for asthma.
During the clinic visit, his blood pressure was 90/60 mm Hg and his heart rate was normal. His pulse oximetry was 92% on room air. On physical examination, he had normal-appearing dentition. Auscultation revealed bilateral expiratory wheezes with decreased breath sounds at the left lower lobe.
A plain chest radiograph (CXR) performed in the clinic (FIGURE 1) showed a large, thick-walled cavitary lesion with an air-fluid level in the left lower lobe. The patient was directly admitted to the Family Medicine Inpatient Service. Computed tomography (CT) of the chest with contrast was ordered to rule out empyema or malignancy. The chest CT confirmed the previous findings while also revealing a surrounding satellite nodularity in the left lower lobe (FIGURE 2). QuantiFERON-TB Gold and HIV tests were both negative.
THE DIAGNOSIS
The patient was given a diagnosis of a lung abscess based on symptoms and imaging. An extensive smoking history, as well as multiple sedating medications, increased his likelihood of aspiration.
DISCUSSION
Lung abscess is the probable diagnosis in a patient with indolent infectious symptoms (cough, fever, night sweats) developing over days to weeks and a CXR finding of pulmonary opacity, often with an air-fluid level.1-4 A lung abscess is a circumscribed collection of pus in the lung parenchyma that develops as a result of microbial infection.4
Primary vs secondary abscess. Lung abscesses can be divided into 2 groups: primary and secondary abscesses. Primary abscesses (60%) occur without any other medical condition or in patients prone to aspiration.5 Secondary abscesses occur in the setting of a comorbid medical condition, such as lung disease, heart disease, bronchogenic neoplasm, or immunocompromised status.5
Continue to: With a primary lung abscess...
With a primary lung abscess, oropharyngeal contents are aspirated (generally while the patient is unconscious) and contain mixed flora.2 The aspirate typically migrates to the posterior segments of the upper lobes and to the superior segments of the lower lobes. These abscesses are usually singular and have an air-fluid level.1,2
Secondary lung abscesses occur in bronchial obstruction (by tumor, foreign body, or enlarged lymph nodes), with coexisting lung diseases (bronchiectasis, cystic fibrosis, infected pulmonary infarcts, lung contusion) or by direct spread (broncho-esophageal fistula, subphrenic abscess).6 Secondary abscesses are associated with a poorer prognosis, dependent on the patient’s general condition and underlying disease.7
What to rule out
The differential diagnosis of cavitary lung lesion includes tuberculosis, necrotizing pneumonia, bronchial carcinoma, pulmonary embolism, vasculitis (eg, Churg-Strauss syndrome), and localized pleural empyema.1,4 A CT scan is helpful to differentiate between a parenchymal lesion and pleural collection, which may not be as clear on CXR.1,4
Tuberculosis manifests with fatigue, weight loss, and night sweats; a chest CT will reveal a cavitating lesion (usually upper lobe) with a characteristic “rim sign” that includes caseous necrosis surrounded by a peripheral enhancing rim.8
Necrotizing pneumonia manifests as acute, fulminant infection. The most common causative organisms on sputum culture are Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas species. Plain radiography will reveal multiple cavities and often associated pleural effusion and empyema.9
Continue to: Excavating bronchogenic carcinomas
Excavating bronchogenic carcinomas differ from a lung abscess in that a patient with the latter is typically, but not always, febrile and has purulent sputum. On imaging, a bronchogenic carcinoma has a thicker and more irregular wall than a lung abscess.10
Treatment
When antibiotics first became available, penicillin was used to treat lung abscess.11 Then IV clindamycin became the drug of choice after 2 trials demonstrated its superiority to IV penicillin.12,13 More recently, clindamycin alone has fallen out of favor due to growing anaerobic resistance.14
Current therapy includes beta-lactam with beta-lactamase inhibitors.14 Lung abscesses are typically polymicrobial and thus carry different degrees of antibiotic resistance.15,16 If culture data are available, targeted therapy is preferred, especially for secondary abscesses.7 Antibiotic therapy is usually continued until a CXR reveals a small lesion or is clear, which may require several months of outpatient oral antibiotic therapy.4
Our patient was treated with IV clindamycin for 3 days in the hospital. Clindamycin was chosen due to his penicillin allergy and started empirically without any culture data. He was transitioned to oral clindamycin and completed a total 3-week course as his CXR continued to show improvement (FIGURE 3). He did not undergo bronchoscopy. A follow-up CXR showed resolution of lung abscess at 9 months. (FIGURE 4).
THE TAKEAWAY
All patients with lung abscesses should have sputum culture with gram stain done—ideally prior to starting antibiotics.3,4 Bronchoscopy should be considered for patients with atypical presentations or those who fail standard therapy, but may be used in other cases, as well.3
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; morteza.khodaee@cuanschutz.edu
THE CASE
A 37-year-old man with a history of asthma, schizoaffective disorder, and tobacco use (36 packs per year) presented to the clinic after 5 days of worsening cough, reproducible left-sided chest pain, and increasing shortness of breath. He also experienced chills, fatigue, nausea, and vomiting but was afebrile. The patient had not travelled recently nor had direct contact with anyone sick. He also denied intravenous (IV) drug use, alcohol use, and bloody sputum. Recently, he had intentionally lost weight, as recommended by his psychiatrist.
Medication review revealed that he was taking many central-acting agents for schizoaffective disorder, including alprazolam, aripiprazole, desvenlafaxine, and quetiapine. Due to his intermittent asthma since childhood, he used an albuterol inhaler as needed, which currently offered only minimal relief. He denied any history of hospitalization or intubation for asthma.
During the clinic visit, his blood pressure was 90/60 mm Hg and his heart rate was normal. His pulse oximetry was 92% on room air. On physical examination, he had normal-appearing dentition. Auscultation revealed bilateral expiratory wheezes with decreased breath sounds at the left lower lobe.
A plain chest radiograph (CXR) performed in the clinic (FIGURE 1) showed a large, thick-walled cavitary lesion with an air-fluid level in the left lower lobe. The patient was directly admitted to the Family Medicine Inpatient Service. Computed tomography (CT) of the chest with contrast was ordered to rule out empyema or malignancy. The chest CT confirmed the previous findings while also revealing a surrounding satellite nodularity in the left lower lobe (FIGURE 2). QuantiFERON-TB Gold and HIV tests were both negative.
THE DIAGNOSIS
The patient was given a diagnosis of a lung abscess based on symptoms and imaging. An extensive smoking history, as well as multiple sedating medications, increased his likelihood of aspiration.
DISCUSSION
Lung abscess is the probable diagnosis in a patient with indolent infectious symptoms (cough, fever, night sweats) developing over days to weeks and a CXR finding of pulmonary opacity, often with an air-fluid level.1-4 A lung abscess is a circumscribed collection of pus in the lung parenchyma that develops as a result of microbial infection.4
Primary vs secondary abscess. Lung abscesses can be divided into 2 groups: primary and secondary abscesses. Primary abscesses (60%) occur without any other medical condition or in patients prone to aspiration.5 Secondary abscesses occur in the setting of a comorbid medical condition, such as lung disease, heart disease, bronchogenic neoplasm, or immunocompromised status.5
Continue to: With a primary lung abscess...
With a primary lung abscess, oropharyngeal contents are aspirated (generally while the patient is unconscious) and contain mixed flora.2 The aspirate typically migrates to the posterior segments of the upper lobes and to the superior segments of the lower lobes. These abscesses are usually singular and have an air-fluid level.1,2
Secondary lung abscesses occur in bronchial obstruction (by tumor, foreign body, or enlarged lymph nodes), with coexisting lung diseases (bronchiectasis, cystic fibrosis, infected pulmonary infarcts, lung contusion) or by direct spread (broncho-esophageal fistula, subphrenic abscess).6 Secondary abscesses are associated with a poorer prognosis, dependent on the patient’s general condition and underlying disease.7
What to rule out
The differential diagnosis of cavitary lung lesion includes tuberculosis, necrotizing pneumonia, bronchial carcinoma, pulmonary embolism, vasculitis (eg, Churg-Strauss syndrome), and localized pleural empyema.1,4 A CT scan is helpful to differentiate between a parenchymal lesion and pleural collection, which may not be as clear on CXR.1,4
Tuberculosis manifests with fatigue, weight loss, and night sweats; a chest CT will reveal a cavitating lesion (usually upper lobe) with a characteristic “rim sign” that includes caseous necrosis surrounded by a peripheral enhancing rim.8
Necrotizing pneumonia manifests as acute, fulminant infection. The most common causative organisms on sputum culture are Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas species. Plain radiography will reveal multiple cavities and often associated pleural effusion and empyema.9
Continue to: Excavating bronchogenic carcinomas
Excavating bronchogenic carcinomas differ from a lung abscess in that a patient with the latter is typically, but not always, febrile and has purulent sputum. On imaging, a bronchogenic carcinoma has a thicker and more irregular wall than a lung abscess.10
Treatment
When antibiotics first became available, penicillin was used to treat lung abscess.11 Then IV clindamycin became the drug of choice after 2 trials demonstrated its superiority to IV penicillin.12,13 More recently, clindamycin alone has fallen out of favor due to growing anaerobic resistance.14
Current therapy includes beta-lactam with beta-lactamase inhibitors.14 Lung abscesses are typically polymicrobial and thus carry different degrees of antibiotic resistance.15,16 If culture data are available, targeted therapy is preferred, especially for secondary abscesses.7 Antibiotic therapy is usually continued until a CXR reveals a small lesion or is clear, which may require several months of outpatient oral antibiotic therapy.4
Our patient was treated with IV clindamycin for 3 days in the hospital. Clindamycin was chosen due to his penicillin allergy and started empirically without any culture data. He was transitioned to oral clindamycin and completed a total 3-week course as his CXR continued to show improvement (FIGURE 3). He did not undergo bronchoscopy. A follow-up CXR showed resolution of lung abscess at 9 months. (FIGURE 4).
THE TAKEAWAY
All patients with lung abscesses should have sputum culture with gram stain done—ideally prior to starting antibiotics.3,4 Bronchoscopy should be considered for patients with atypical presentations or those who fail standard therapy, but may be used in other cases, as well.3
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; morteza.khodaee@cuanschutz.edu
1. Hassan M, Asciak R, Rizk R, et al. Lung abscess or empyema? Taking a closer look. Thorax. 2018;73:887-889. https://doi. org/10.1136/thoraxjnl-2018-211604
2. Moreira J da SM, Camargo J de JP, Felicetti JC, et al. Lung abscess: analysis of 252 consecutive cases diagnosed between 1968 and 2004. J Bras Pneumol. 2006;32:136-43. https://doi.org/10.1590/ s1806-37132006000200009
3. Schiza S, Siafakas NM. Clinical presentation and management of empyema, lung abscess and pleural effusion. Curr Opin Pulm Med. 2006;12:205-211. https://doi.org/10.1097/01. mcp.0000219270.73180.8b
4. Yazbeck MF, Dahdel M, Kalra A, et al. Lung abscess: update on microbiology and management. Am J Ther. 2014;21:217-221. https://doi.org/10.1097/MJT.0b013e3182383c9b
5. Nicolini A, Cilloniz C, Senarega R, et al. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276-285. https://doi. org/10.5603/PiAP.2014.0033
6. Puligandla PS, Laberge J-M. Respiratory infections: pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42-52. https://doi.org/10.1053/j.sempedsurg.2007.10.007
7. Marra A, Hillejan L, Ukena D. [Management of Lung Abscess]. Zentralbl Chir. 2015;140 (suppl 1):S47-S53. https://doi. org/10.1055/s-0035-1557883
1. Hassan M, Asciak R, Rizk R, et al. Lung abscess or empyema? Taking a closer look. Thorax. 2018;73:887-889. https://doi. org/10.1136/thoraxjnl-2018-211604
2. Moreira J da SM, Camargo J de JP, Felicetti JC, et al. Lung abscess: analysis of 252 consecutive cases diagnosed between 1968 and 2004. J Bras Pneumol. 2006;32:136-43. https://doi.org/10.1590/ s1806-37132006000200009
3. Schiza S, Siafakas NM. Clinical presentation and management of empyema, lung abscess and pleural effusion. Curr Opin Pulm Med. 2006;12:205-211. https://doi.org/10.1097/01. mcp.0000219270.73180.8b
4. Yazbeck MF, Dahdel M, Kalra A, et al. Lung abscess: update on microbiology and management. Am J Ther. 2014;21:217-221. https://doi.org/10.1097/MJT.0b013e3182383c9b
5. Nicolini A, Cilloniz C, Senarega R, et al. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276-285. https://doi. org/10.5603/PiAP.2014.0033
6. Puligandla PS, Laberge J-M. Respiratory infections: pneumonia, lung abscess, and empyema. Semin Pediatr Surg. 2008;17:42-52. https://doi.org/10.1053/j.sempedsurg.2007.10.007
7. Marra A, Hillejan L, Ukena D. [Management of Lung Abscess]. Zentralbl Chir. 2015;140 (suppl 1):S47-S53. https://doi. org/10.1055/s-0035-1557883
Painful, swollen elbow
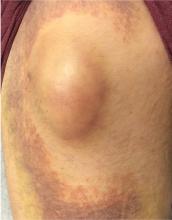
A 32-year-old woman presented to our clinic with left elbow swelling and pain of 6 days’ duration. She’d had a posterior interosseous nerve (PIN) injection (hydrodissection) at another facility 12 days earlier for refractory intersection syndrome.
During nerve hydrodissection, fluid is injected into the area surrounding the nerve in an effort to displace the muscles, tendons, and fascia and thus reduce friction on the nerve. This treatment, often completed with ultrasound guidance, is utilized by patients who want to obtain pain relief without undergoing surgery for nerve entrapment syndromes.
In this case, a combination of 1 mL (40 mg) of methylprednisolone acetate, 1 mL of lidocaine 2%, and 3 mL of normal saline was injected into the supinator muscle belly (proximal dorsal aspect of the forearm) under ultrasound guidance. Six days later, the patient began to experience elbow pain, redness, and swelling. The symptoms progressed within several hours and became so notable that she sought care at an urgent care facility the next morning. At this facility, she was told she had an infection and was prescribed oral levofloxacin 500 mg/d.
The patient presented to our clinic after 4 days of oral levofloxacin with no improvement of symptoms. She denied chills or fever and described her pain as moderate and radiating to her fingers. There was no history of trauma. The patient reported riding her bike more frequently, which had caused the original forearm pain that warranted the PIN injection. There were no other recent changes to activity. Her medical, social, and surgical histories were otherwise unremarkable.
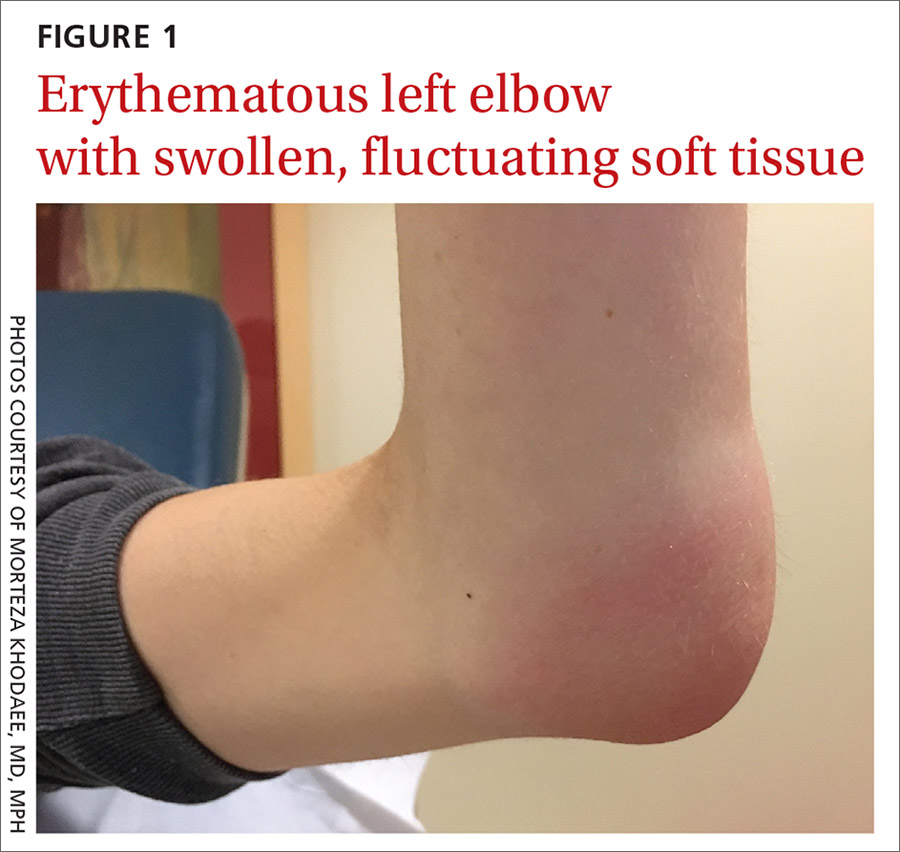
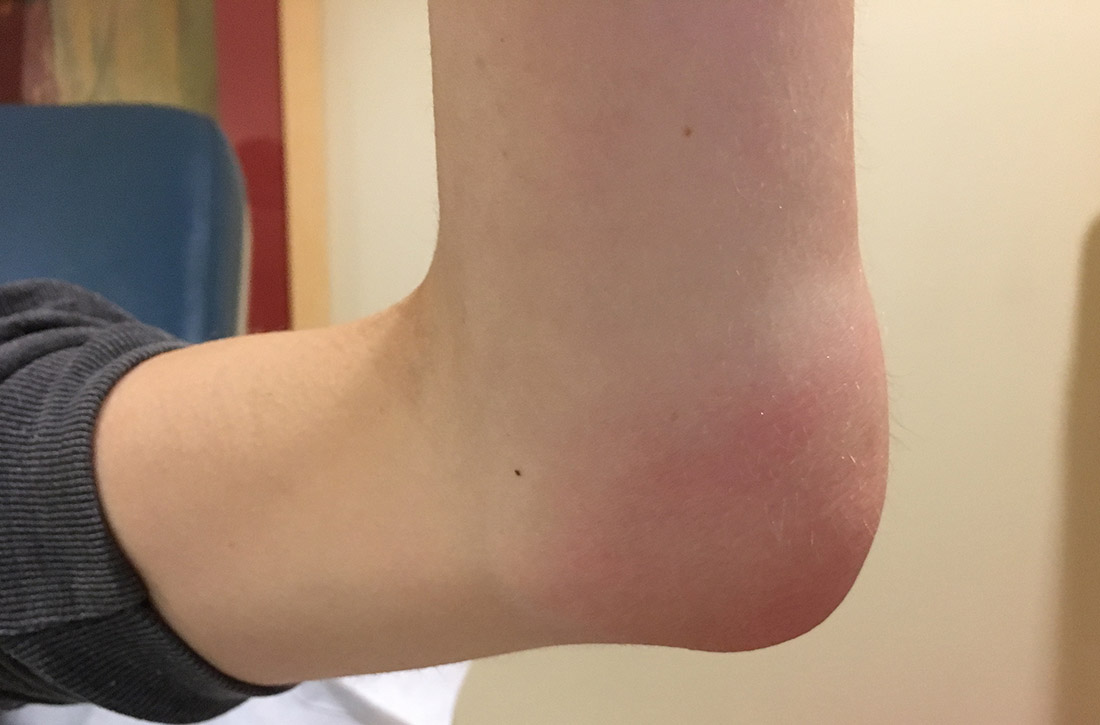
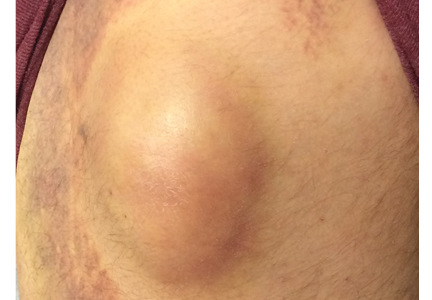
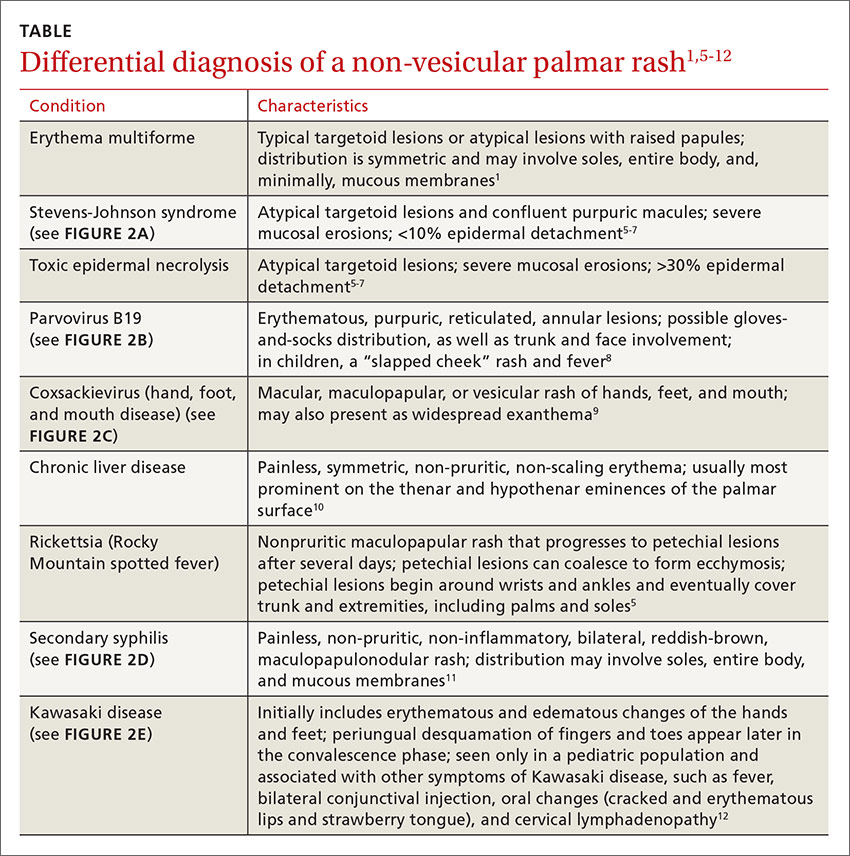
Her vital signs were normal. Physical exam revealed an erythematous and warm left elbow (FIGURE 1). Her left elbow range of motion (extension and flexion) was mildly decreased due to the pain and swelling.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Iatrogenic septic olecranon bursitis
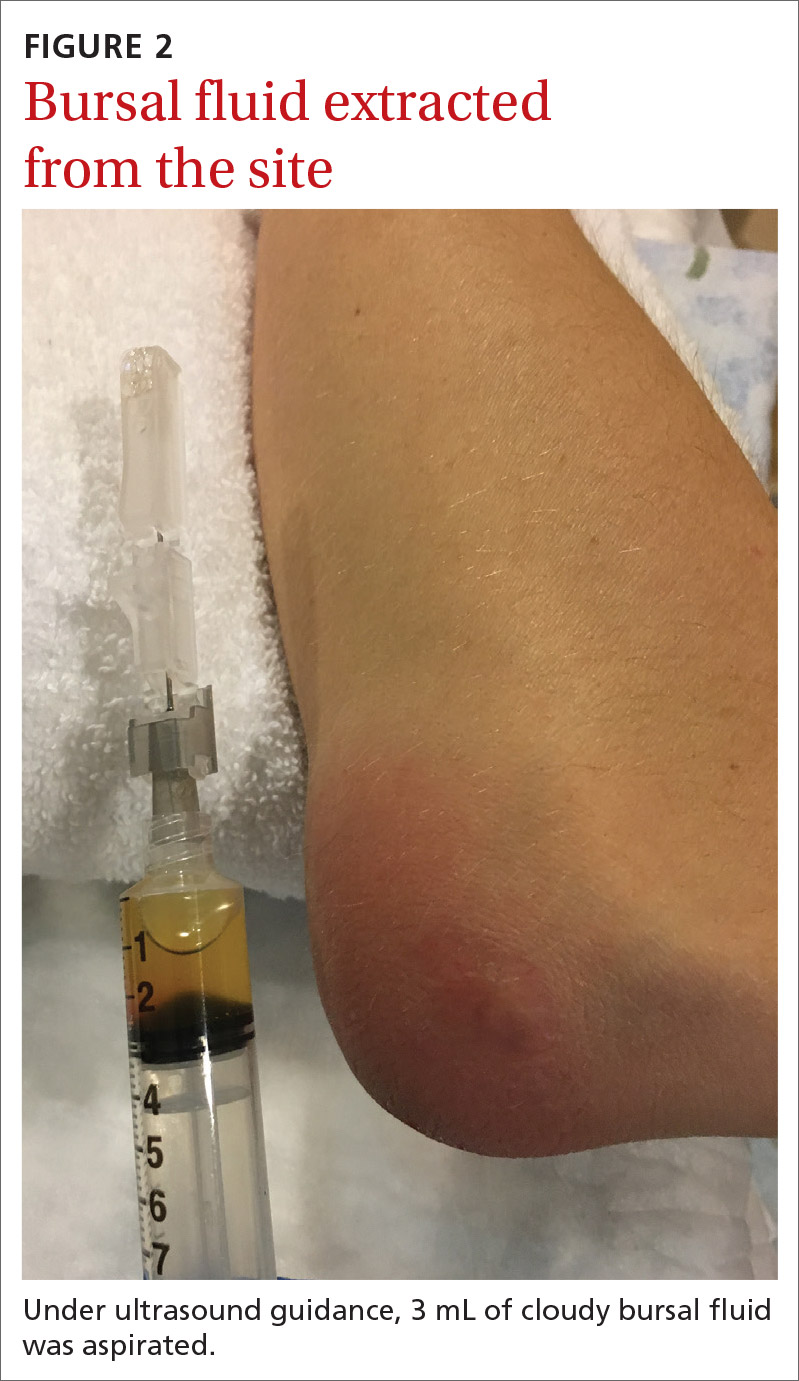
Aspiration of the patient’s olecranon bursa produced 3 mL of cloudy fluid (FIGURE 2). The patient’s painful, swollen, erythematous, warm elbow, cloudy aspirate, and history of preceding PIN hydrodissection were consistent with a diagnosis of septic olecranon bursitis.
Septic bursitis usually is caused by bacteria.1,2 Bursal infection can result from the spread of infection from nearby tissues or direct inoculation from skin trauma. It can also be iatrogenic and occur among healthy individuals.2,3 Injection anywhere close to the bursa can inoculate enough bacteria to progress to cellulitis first and then septic bursitis. Inflammatory conditions such as gout and rheumatoid arthritis also can cause acute and/or chronic superficial bursitis.1,2,4
Differentiating between septic and nonseptic bursitis can be challenging on history and physical exam alone, but specific signs and symptoms should warrant concern for infection.1,2,4,5 Fever is present in up to 75% of septic cases5; however, lack of fever does not rule out septic bursitis. Pain, erythema, warmth, and an overlying skin lesion also can indicate infection.4 Diagnostic imaging modalities may help distinguish different types of olecranon bursitis, but in most cases, they are not necessary.2
Other joint disorders factor into the differential
The differential diagnosis is broad and includes a variety of joint disorders in addition to septic (and nonseptic) bursitis.2,3
Septic arthritis is a deeper infection that involves the elbow joint and is considered an orthopedic emergency due to potential joint destruction.
Continue to: A simple joint effusion
A simple joint effusion also arises from the elbow joint, but this diagnosis becomes less likely when the joint aspirate appears cloudy. A simple joint effusion would not produce bacteria on gram stain and culture.
Crystalline inflammatory arthritis (gout, pseudogout) is due to intra-articular precipitation of crystals (uric acid crystals in gout, calcium pyrophosphate crystals in pseudogout).
Hematomas would produce gross blood or clot on joint aspiration.
Cellulitis is an infection of the superficial soft tissue (only) and thus, aspiration is not likely to yield fluid.
Diagnosis can be made with culture of fluid
Confirmation of septic olecranon bursitis is best attained by bursal needle aspiration and culture. Aspiration also can evaluate for other causes of elbow swelling. (If septic olecranon bursitis is suspected clinically, empiric antibiotics should be started while awaiting culture results.6) White blood cell counts from the aspirate also may be utilized but have a lower sensitivity and specificity for diagnosis.7
Continue to: In addition to aiding in diagnosis
In addition to aiding in diagnosis, bursal aspiration for a patient with septic bursitis can improve symptoms and reduce bacterial load.1-3,8 The use of a compressive bandage after aspiration may help reduce re-accumulation of the bursal fluid.1-3,8Staphylococcus aureus is responsible for the majority of septic olecranon bursitis cases.9-11
Tailoring the antibiotic regimen
There is wide variation in the treatment of septic olecranon bursitis due to the lack of strong evidence-based guidelines.1,2,8,11-13 When septic bursitis is strongly suspected (or confirmed) the patient should be started on an antibiotic regimen that covers S aureus.1,2 Once culture results and sensitivities return, the antibiotic regimen can be tailored appropriately.
In cases of mild-to-moderate septic olecranon bursitis in an immunocompetent host, the patient can be started on oral antibiotics and monitored closely as an outpatient.1-3,8 Patients with septic olecranon bursitis who meet the criteria for systemic inflammatory response syndrome or who are immunocompromised should be hospitalized and started on intravenous antibiotics.1-3 Recommended duration of antibiotic therapy varies but is usually about 10 to 14 days.1-3,8 In rare cases, surgical intervention with bursectomy may be necessary.1,2,14
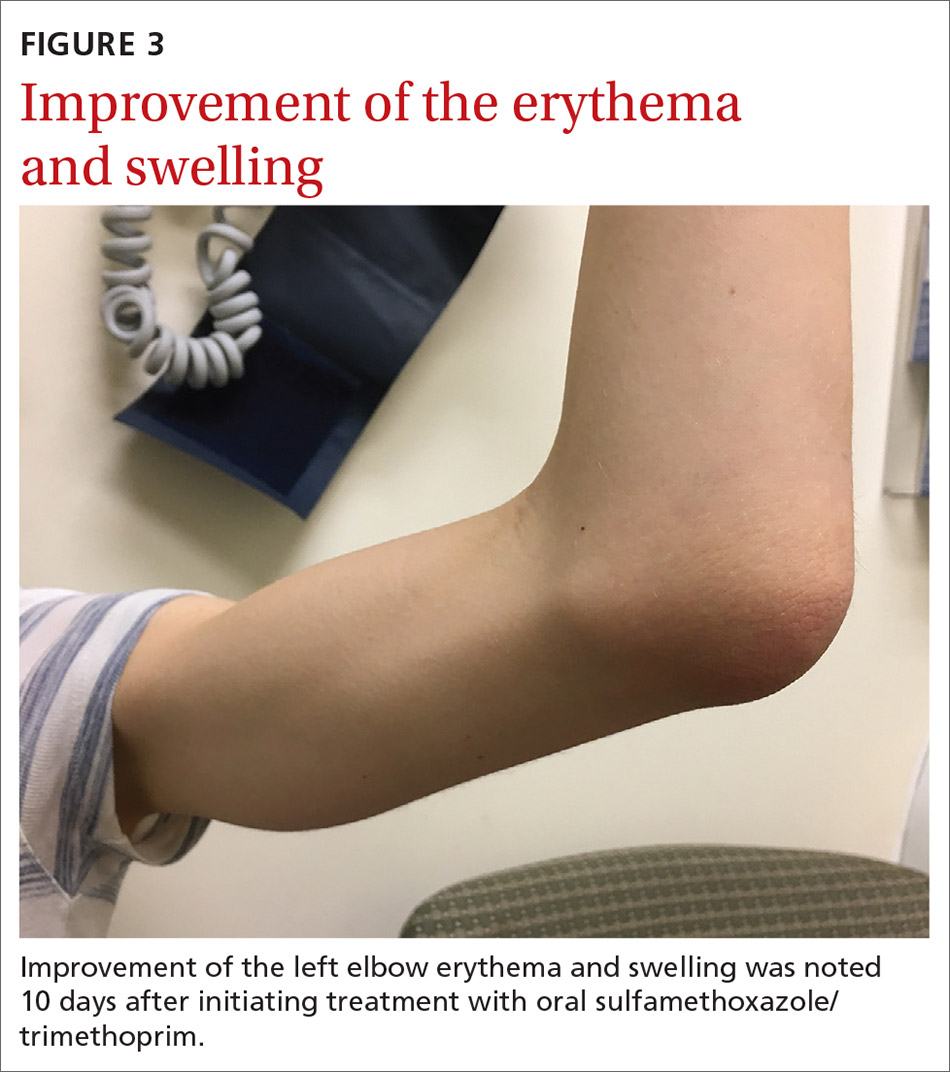
Our patient was given a dose of ceftriaxone 250 mg intramuscularly and was started on oral sulfamethoxazole/trimethoprim 800 mg/160 mg twice daily after aspiration of the bursa. Culture of the bursal fluid grew oxacillin-sensitive S aureus which was sensitive to a variety of antibiotics including levofloxacin and sulfamethoxazole/trimethoprim. Her symptoms gradually improved (FIGURE 3) and resolved after a 14-day course of oral sulfamethoxazole/trimethoprim.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine & Orthopedics, AFW Clinic, 3055 Roslyn St, Denver, CO 80238; morteza. khodaee@cuanschutz.edu
1. Baumbach SF, Lobo CM, Badyine I, et al. Prepatellar and olecranon bursitis: literature review and development of a treatment algorithm. Arch Orthop Trauma Surg. 2014;134:359-370.
2. Khodaee M. Common superficial bursitis. Am Fam Physician. 2017;95:224-231.
3. Harris-Spinks C, Nabhan D, Khodaee M. Noniatrogenic septic olecranon bursitis: report of two cases and review of the literature. Curr Sports Med Rep. 2016;15:33-37.
4. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25:158-167.
5. Blackwell JR, Hay BA, Bolt AM, et al. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6:182-190.
6. Del Buono A, Franceschi F, Palumbo A, et al. Diagnosis and management of olecranon bursitis. Surgeon. 2012;10:297-300.
7. Stell IM, Gransden WR. Simple tests for septic bursitis: comparative study. BMJ. 1998;316:1877.
8. Abzug JM, Chen NC, Jacoby SM. Septic olecranon bursitis. J Hand Surg Am. 2012;37:1252-1253.
9. Cea-Pereiro JC, Garcia-Meijide J, Mera-Varela A, et al. A comparison between septic bursitis caused by Staphylococcus aureus and those caused by other organisms. Clin Rheumatol. 2001;20:10-14.
10. Morrey BE. Bursitis. In: Morrey BE, Sanchez-Sotelo J, eds. The Elbow and its Disorders. 4th ed. Philadelphia, PA: Saunders Elsevier 2009:1164-1173.
11. Wingert NC, DeMaio M, Shenenberger DW. Septic olecranon bursitis, contact dermatitis, and pneumonitis in a gas turbine engine mechanic. J Shoulder Elbow Surg. 2012;21:E16-E20.
12. Baumbach SF, Michel M, Wyen H, et al. Current treatment concepts for olecranon and prepatellar bursitis in Austria. Z Orthop Unfall. 2013;151:149-155.
13. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134:1517-1536.
14. Ogilvie-Harris DJ, Gilbart M. Endoscopic bursal resection: the olecranon bursa and prepatellar bursa. Arthroscopy. 2000;16:249-253.
A 32-year-old woman presented to our clinic with left elbow swelling and pain of 6 days’ duration. She’d had a posterior interosseous nerve (PIN) injection (hydrodissection) at another facility 12 days earlier for refractory intersection syndrome.
During nerve hydrodissection, fluid is injected into the area surrounding the nerve in an effort to displace the muscles, tendons, and fascia and thus reduce friction on the nerve. This treatment, often completed with ultrasound guidance, is utilized by patients who want to obtain pain relief without undergoing surgery for nerve entrapment syndromes.
In this case, a combination of 1 mL (40 mg) of methylprednisolone acetate, 1 mL of lidocaine 2%, and 3 mL of normal saline was injected into the supinator muscle belly (proximal dorsal aspect of the forearm) under ultrasound guidance. Six days later, the patient began to experience elbow pain, redness, and swelling. The symptoms progressed within several hours and became so notable that she sought care at an urgent care facility the next morning. At this facility, she was told she had an infection and was prescribed oral levofloxacin 500 mg/d.
The patient presented to our clinic after 4 days of oral levofloxacin with no improvement of symptoms. She denied chills or fever and described her pain as moderate and radiating to her fingers. There was no history of trauma. The patient reported riding her bike more frequently, which had caused the original forearm pain that warranted the PIN injection. There were no other recent changes to activity. Her medical, social, and surgical histories were otherwise unremarkable.
Her vital signs were normal. Physical exam revealed an erythematous and warm left elbow (FIGURE 1). Her left elbow range of motion (extension and flexion) was mildly decreased due to the pain and swelling.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Iatrogenic septic olecranon bursitis
Aspiration of the patient’s olecranon bursa produced 3 mL of cloudy fluid (FIGURE 2). The patient’s painful, swollen, erythematous, warm elbow, cloudy aspirate, and history of preceding PIN hydrodissection were consistent with a diagnosis of septic olecranon bursitis.
Septic bursitis usually is caused by bacteria.1,2 Bursal infection can result from the spread of infection from nearby tissues or direct inoculation from skin trauma. It can also be iatrogenic and occur among healthy individuals.2,3 Injection anywhere close to the bursa can inoculate enough bacteria to progress to cellulitis first and then septic bursitis. Inflammatory conditions such as gout and rheumatoid arthritis also can cause acute and/or chronic superficial bursitis.1,2,4
Differentiating between septic and nonseptic bursitis can be challenging on history and physical exam alone, but specific signs and symptoms should warrant concern for infection.1,2,4,5 Fever is present in up to 75% of septic cases5; however, lack of fever does not rule out septic bursitis. Pain, erythema, warmth, and an overlying skin lesion also can indicate infection.4 Diagnostic imaging modalities may help distinguish different types of olecranon bursitis, but in most cases, they are not necessary.2
Other joint disorders factor into the differential
The differential diagnosis is broad and includes a variety of joint disorders in addition to septic (and nonseptic) bursitis.2,3
Septic arthritis is a deeper infection that involves the elbow joint and is considered an orthopedic emergency due to potential joint destruction.
Continue to: A simple joint effusion
A simple joint effusion also arises from the elbow joint, but this diagnosis becomes less likely when the joint aspirate appears cloudy. A simple joint effusion would not produce bacteria on gram stain and culture.
Crystalline inflammatory arthritis (gout, pseudogout) is due to intra-articular precipitation of crystals (uric acid crystals in gout, calcium pyrophosphate crystals in pseudogout).
Hematomas would produce gross blood or clot on joint aspiration.
Cellulitis is an infection of the superficial soft tissue (only) and thus, aspiration is not likely to yield fluid.
Diagnosis can be made with culture of fluid
Confirmation of septic olecranon bursitis is best attained by bursal needle aspiration and culture. Aspiration also can evaluate for other causes of elbow swelling. (If septic olecranon bursitis is suspected clinically, empiric antibiotics should be started while awaiting culture results.6) White blood cell counts from the aspirate also may be utilized but have a lower sensitivity and specificity for diagnosis.7
Continue to: In addition to aiding in diagnosis
In addition to aiding in diagnosis, bursal aspiration for a patient with septic bursitis can improve symptoms and reduce bacterial load.1-3,8 The use of a compressive bandage after aspiration may help reduce re-accumulation of the bursal fluid.1-3,8Staphylococcus aureus is responsible for the majority of septic olecranon bursitis cases.9-11
Tailoring the antibiotic regimen
There is wide variation in the treatment of septic olecranon bursitis due to the lack of strong evidence-based guidelines.1,2,8,11-13 When septic bursitis is strongly suspected (or confirmed) the patient should be started on an antibiotic regimen that covers S aureus.1,2 Once culture results and sensitivities return, the antibiotic regimen can be tailored appropriately.
In cases of mild-to-moderate septic olecranon bursitis in an immunocompetent host, the patient can be started on oral antibiotics and monitored closely as an outpatient.1-3,8 Patients with septic olecranon bursitis who meet the criteria for systemic inflammatory response syndrome or who are immunocompromised should be hospitalized and started on intravenous antibiotics.1-3 Recommended duration of antibiotic therapy varies but is usually about 10 to 14 days.1-3,8 In rare cases, surgical intervention with bursectomy may be necessary.1,2,14
Our patient was given a dose of ceftriaxone 250 mg intramuscularly and was started on oral sulfamethoxazole/trimethoprim 800 mg/160 mg twice daily after aspiration of the bursa. Culture of the bursal fluid grew oxacillin-sensitive S aureus which was sensitive to a variety of antibiotics including levofloxacin and sulfamethoxazole/trimethoprim. Her symptoms gradually improved (FIGURE 3) and resolved after a 14-day course of oral sulfamethoxazole/trimethoprim.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine & Orthopedics, AFW Clinic, 3055 Roslyn St, Denver, CO 80238; morteza. khodaee@cuanschutz.edu
A 32-year-old woman presented to our clinic with left elbow swelling and pain of 6 days’ duration. She’d had a posterior interosseous nerve (PIN) injection (hydrodissection) at another facility 12 days earlier for refractory intersection syndrome.
During nerve hydrodissection, fluid is injected into the area surrounding the nerve in an effort to displace the muscles, tendons, and fascia and thus reduce friction on the nerve. This treatment, often completed with ultrasound guidance, is utilized by patients who want to obtain pain relief without undergoing surgery for nerve entrapment syndromes.
In this case, a combination of 1 mL (40 mg) of methylprednisolone acetate, 1 mL of lidocaine 2%, and 3 mL of normal saline was injected into the supinator muscle belly (proximal dorsal aspect of the forearm) under ultrasound guidance. Six days later, the patient began to experience elbow pain, redness, and swelling. The symptoms progressed within several hours and became so notable that she sought care at an urgent care facility the next morning. At this facility, she was told she had an infection and was prescribed oral levofloxacin 500 mg/d.
The patient presented to our clinic after 4 days of oral levofloxacin with no improvement of symptoms. She denied chills or fever and described her pain as moderate and radiating to her fingers. There was no history of trauma. The patient reported riding her bike more frequently, which had caused the original forearm pain that warranted the PIN injection. There were no other recent changes to activity. Her medical, social, and surgical histories were otherwise unremarkable.
Her vital signs were normal. Physical exam revealed an erythematous and warm left elbow (FIGURE 1). Her left elbow range of motion (extension and flexion) was mildly decreased due to the pain and swelling.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Iatrogenic septic olecranon bursitis
Aspiration of the patient’s olecranon bursa produced 3 mL of cloudy fluid (FIGURE 2). The patient’s painful, swollen, erythematous, warm elbow, cloudy aspirate, and history of preceding PIN hydrodissection were consistent with a diagnosis of septic olecranon bursitis.
Septic bursitis usually is caused by bacteria.1,2 Bursal infection can result from the spread of infection from nearby tissues or direct inoculation from skin trauma. It can also be iatrogenic and occur among healthy individuals.2,3 Injection anywhere close to the bursa can inoculate enough bacteria to progress to cellulitis first and then septic bursitis. Inflammatory conditions such as gout and rheumatoid arthritis also can cause acute and/or chronic superficial bursitis.1,2,4
Differentiating between septic and nonseptic bursitis can be challenging on history and physical exam alone, but specific signs and symptoms should warrant concern for infection.1,2,4,5 Fever is present in up to 75% of septic cases5; however, lack of fever does not rule out septic bursitis. Pain, erythema, warmth, and an overlying skin lesion also can indicate infection.4 Diagnostic imaging modalities may help distinguish different types of olecranon bursitis, but in most cases, they are not necessary.2
Other joint disorders factor into the differential
The differential diagnosis is broad and includes a variety of joint disorders in addition to septic (and nonseptic) bursitis.2,3
Septic arthritis is a deeper infection that involves the elbow joint and is considered an orthopedic emergency due to potential joint destruction.
Continue to: A simple joint effusion
A simple joint effusion also arises from the elbow joint, but this diagnosis becomes less likely when the joint aspirate appears cloudy. A simple joint effusion would not produce bacteria on gram stain and culture.
Crystalline inflammatory arthritis (gout, pseudogout) is due to intra-articular precipitation of crystals (uric acid crystals in gout, calcium pyrophosphate crystals in pseudogout).
Hematomas would produce gross blood or clot on joint aspiration.
Cellulitis is an infection of the superficial soft tissue (only) and thus, aspiration is not likely to yield fluid.
Diagnosis can be made with culture of fluid
Confirmation of septic olecranon bursitis is best attained by bursal needle aspiration and culture. Aspiration also can evaluate for other causes of elbow swelling. (If septic olecranon bursitis is suspected clinically, empiric antibiotics should be started while awaiting culture results.6) White blood cell counts from the aspirate also may be utilized but have a lower sensitivity and specificity for diagnosis.7
Continue to: In addition to aiding in diagnosis
In addition to aiding in diagnosis, bursal aspiration for a patient with septic bursitis can improve symptoms and reduce bacterial load.1-3,8 The use of a compressive bandage after aspiration may help reduce re-accumulation of the bursal fluid.1-3,8Staphylococcus aureus is responsible for the majority of septic olecranon bursitis cases.9-11
Tailoring the antibiotic regimen
There is wide variation in the treatment of septic olecranon bursitis due to the lack of strong evidence-based guidelines.1,2,8,11-13 When septic bursitis is strongly suspected (or confirmed) the patient should be started on an antibiotic regimen that covers S aureus.1,2 Once culture results and sensitivities return, the antibiotic regimen can be tailored appropriately.
In cases of mild-to-moderate septic olecranon bursitis in an immunocompetent host, the patient can be started on oral antibiotics and monitored closely as an outpatient.1-3,8 Patients with septic olecranon bursitis who meet the criteria for systemic inflammatory response syndrome or who are immunocompromised should be hospitalized and started on intravenous antibiotics.1-3 Recommended duration of antibiotic therapy varies but is usually about 10 to 14 days.1-3,8 In rare cases, surgical intervention with bursectomy may be necessary.1,2,14
Our patient was given a dose of ceftriaxone 250 mg intramuscularly and was started on oral sulfamethoxazole/trimethoprim 800 mg/160 mg twice daily after aspiration of the bursa. Culture of the bursal fluid grew oxacillin-sensitive S aureus which was sensitive to a variety of antibiotics including levofloxacin and sulfamethoxazole/trimethoprim. Her symptoms gradually improved (FIGURE 3) and resolved after a 14-day course of oral sulfamethoxazole/trimethoprim.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine & Orthopedics, AFW Clinic, 3055 Roslyn St, Denver, CO 80238; morteza. khodaee@cuanschutz.edu
1. Baumbach SF, Lobo CM, Badyine I, et al. Prepatellar and olecranon bursitis: literature review and development of a treatment algorithm. Arch Orthop Trauma Surg. 2014;134:359-370.
2. Khodaee M. Common superficial bursitis. Am Fam Physician. 2017;95:224-231.
3. Harris-Spinks C, Nabhan D, Khodaee M. Noniatrogenic septic olecranon bursitis: report of two cases and review of the literature. Curr Sports Med Rep. 2016;15:33-37.
4. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25:158-167.
5. Blackwell JR, Hay BA, Bolt AM, et al. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6:182-190.
6. Del Buono A, Franceschi F, Palumbo A, et al. Diagnosis and management of olecranon bursitis. Surgeon. 2012;10:297-300.
7. Stell IM, Gransden WR. Simple tests for septic bursitis: comparative study. BMJ. 1998;316:1877.
8. Abzug JM, Chen NC, Jacoby SM. Septic olecranon bursitis. J Hand Surg Am. 2012;37:1252-1253.
9. Cea-Pereiro JC, Garcia-Meijide J, Mera-Varela A, et al. A comparison between septic bursitis caused by Staphylococcus aureus and those caused by other organisms. Clin Rheumatol. 2001;20:10-14.
10. Morrey BE. Bursitis. In: Morrey BE, Sanchez-Sotelo J, eds. The Elbow and its Disorders. 4th ed. Philadelphia, PA: Saunders Elsevier 2009:1164-1173.
11. Wingert NC, DeMaio M, Shenenberger DW. Septic olecranon bursitis, contact dermatitis, and pneumonitis in a gas turbine engine mechanic. J Shoulder Elbow Surg. 2012;21:E16-E20.
12. Baumbach SF, Michel M, Wyen H, et al. Current treatment concepts for olecranon and prepatellar bursitis in Austria. Z Orthop Unfall. 2013;151:149-155.
13. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134:1517-1536.
14. Ogilvie-Harris DJ, Gilbart M. Endoscopic bursal resection: the olecranon bursa and prepatellar bursa. Arthroscopy. 2000;16:249-253.
1. Baumbach SF, Lobo CM, Badyine I, et al. Prepatellar and olecranon bursitis: literature review and development of a treatment algorithm. Arch Orthop Trauma Surg. 2014;134:359-370.
2. Khodaee M. Common superficial bursitis. Am Fam Physician. 2017;95:224-231.
3. Harris-Spinks C, Nabhan D, Khodaee M. Noniatrogenic septic olecranon bursitis: report of two cases and review of the literature. Curr Sports Med Rep. 2016;15:33-37.
4. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25:158-167.
5. Blackwell JR, Hay BA, Bolt AM, et al. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6:182-190.
6. Del Buono A, Franceschi F, Palumbo A, et al. Diagnosis and management of olecranon bursitis. Surgeon. 2012;10:297-300.
7. Stell IM, Gransden WR. Simple tests for septic bursitis: comparative study. BMJ. 1998;316:1877.
8. Abzug JM, Chen NC, Jacoby SM. Septic olecranon bursitis. J Hand Surg Am. 2012;37:1252-1253.
9. Cea-Pereiro JC, Garcia-Meijide J, Mera-Varela A, et al. A comparison between septic bursitis caused by Staphylococcus aureus and those caused by other organisms. Clin Rheumatol. 2001;20:10-14.
10. Morrey BE. Bursitis. In: Morrey BE, Sanchez-Sotelo J, eds. The Elbow and its Disorders. 4th ed. Philadelphia, PA: Saunders Elsevier 2009:1164-1173.
11. Wingert NC, DeMaio M, Shenenberger DW. Septic olecranon bursitis, contact dermatitis, and pneumonitis in a gas turbine engine mechanic. J Shoulder Elbow Surg. 2012;21:E16-E20.
12. Baumbach SF, Michel M, Wyen H, et al. Current treatment concepts for olecranon and prepatellar bursitis in Austria. Z Orthop Unfall. 2013;151:149-155.
13. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134:1517-1536.
14. Ogilvie-Harris DJ, Gilbart M. Endoscopic bursal resection: the olecranon bursa and prepatellar bursa. Arthroscopy. 2000;16:249-253.
Acute bilateral hand edema and vesiculation
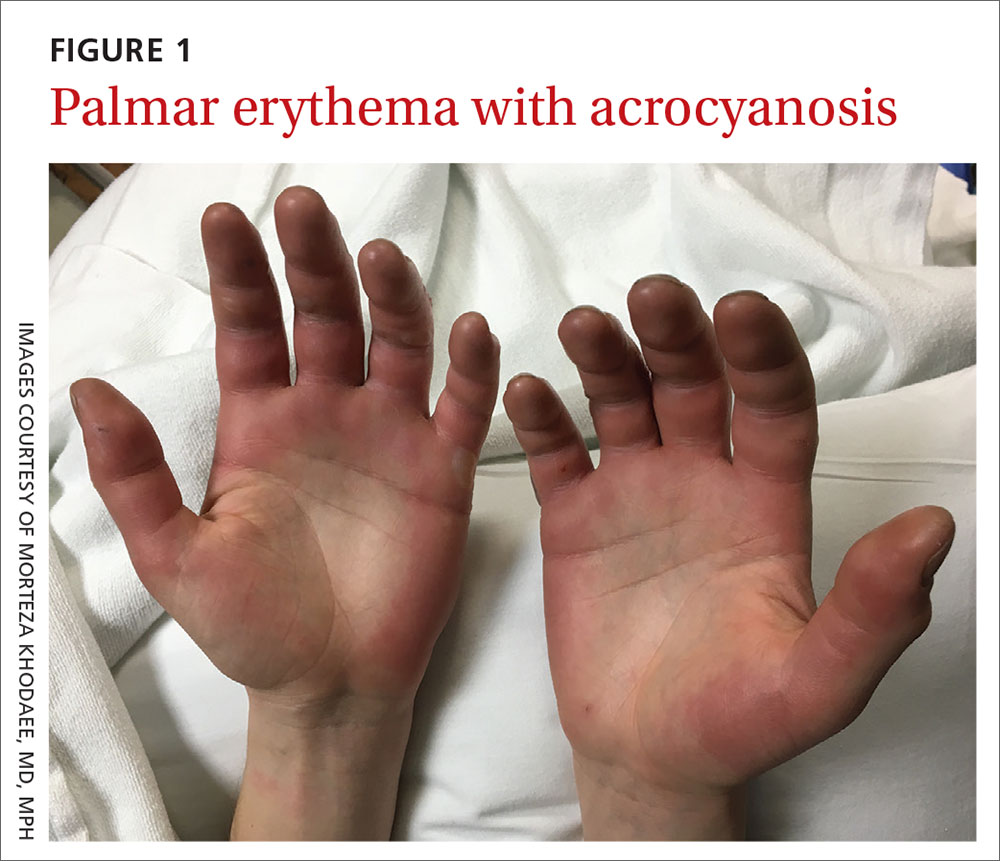
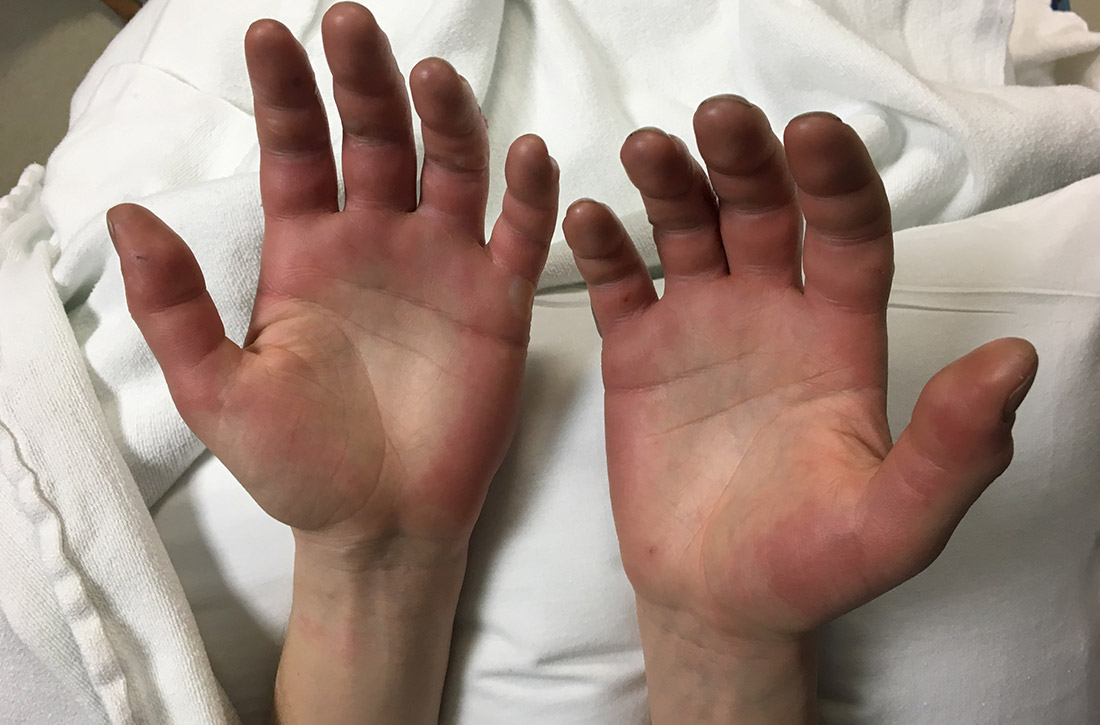
A 27-year-old man presented to the urgent care clinic with acute bilateral hand swelling, blisters, numbness, and pain. History taking revealed that these symptoms developed after he was locked outside of his apartment for 45 minutes in –22°C (–8°F) weather following a night of heavy drinking.
On physical examination, the patient had a temperature of 36.2°C (97.2°F) and a heart rate of 116 beats/min. He had
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
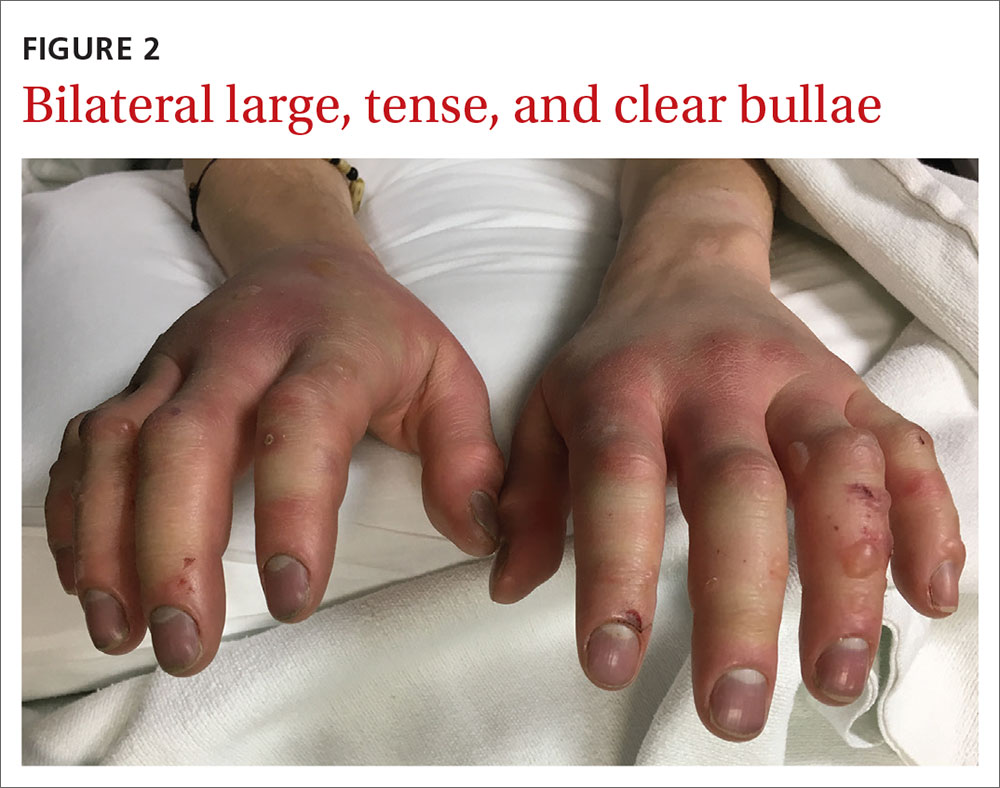
Diagnosis: Second-degree frostbite
Frostbite is the result of tissue freezing, which generally occurs after prolonged exposure to freezing temperatures (typically –4°C or below).1,2 The majority (~90%) of frostbite injuries occur in the hands and feet; however, frostbite has also been observed in the face, perineum, buttocks, and male genitalia.3
Frostbite is a clinical diagnosis based on a history of sustained exposure to freezing temperatures, paresthesia of affected areas, and typical skin changes. Evidence is lacking regarding the epidemiology of frostbite within the general population.2
Pathophysiology. Intra- and extracellular ice crystal formation causes fluid and electrolyte disturbances, cell dehydration, lipid denaturation, and subsequent cell death.1 After thawing, progressive tissue ischemia can occur as a result of endothelial damage and dysfunction, intravascular sludging, increased inflammatory markers, an influx of free radicals, and microvascular thrombosis.1
Classification. Traditionally, frostbite has been classified according to a 4-tiered system based on tissue appearance after rewarming.2 First-degree frostbite is characterized by white plaques with surrounding erythema; second degree by edema and clear or cloudy vesicles; third degree by hemorrhagic bullae; and fourth degree by cold and hard tissue that eventually progresses to gangrene.2
A simpler scheme designates frostbite as either superficial (corresponding to first- or second-degree frostbite) or deep (corresponding to third- or fourth-degree frostbite) with presumed muscle and bone involvement.2
Continue to: Risk factors
Risk factors. Frostbite is often associated with risk factors such as alcohol or drug intoxication, vehicular failure or trauma, immobilizing trauma, psychiatric illness, homelessness, Raynaud phenomenon, peripheral vascular disease, diabetes, inadequate clothing, previous cold-weather injury, outdoor winter recreation, and the use of certain medications (eg, beta-blockers).1-3 Apart from environmental exposure, frostbite can also occur by direct contact with freezing materials, such as ice packs or industrial refrigerants.3
Differential includes nonfreezing injuries
Frostnip, pernio, and trench foot are other cold-weather injuries distinguished by the absence of tissue freezing.4 Raynaud phenomenon is a condition that is triggered by either cold temperatures or emotional stress.5
Frostnip is characterized by pallor and paresthesia of exposed areas. It may precede frostbite, but it quickly resolves after rewarming.2
Pernio occurs when skin is exposed to damp, cold, nonfreezing environments.6 It results in edematous and inflammatory skin lesions that may be painful, pruritic, violaceous, or erythematous.6 These lesions are typically found over the fingers, toes, nose, ears, buttocks, or thighs.4,6 Pernio may be classified as either primary or secondary disease.5 Primary pernio is considered idiopathic.6 Secondary pernio is thought to be either drug induced or due to underlying autoimmune diseases, such as hepatitis or cryopathy.6
Trench foot develops under similar conditions to pernio but requires exposure to a wet environment for at least 10 to 14 hours.7 It is characterized by foot pain, paresthesia, pruritus, edema, erythema, cyanosis, blisters, and even gangrene if left untreated.7
Continue to: Raynaud phenomenon
Raynaud phenomenon results from transient, acral vasocontraction and manifests as well-demarcated pallor, cyanosis, and then erythema as the affected body part reperfuses.5 Similar to pernio, it can be categorized as either primary or secondary.5 Primary phenomenon is idiopathic. Secondary phenomenon is thought to be a result of autoimmune disease, use of certain medications, occupational vibratory exposure, obstructive vascular disease, or infection
In the absence of a history of exposure to subfreezing temperatures, frostbite can be excluded from the differential diagnosis.
Treatment entails rewarming
The aim of frostbite treatment is to save injured cells and minimize tissue loss.1 This is accomplished through rapid rewarming and—in severe cases—reperfusion techniques.
Tissue should be rewarmed in a 37°C to 39°C water bath with povidone iodine or chlorhexidine added for antiseptic effect.1 All efforts should be made to avoid refreezing or trauma, as this could worsen the initial injury.2 Oral or intravenous hydration may be offered to optimize fluid status.1 Supplemental oxygen may be administered to maintain saturations above 90%.1 Nonsteroidal anti-inflammatory drugs are helpful for analgesia and anti-inflammatory effect, and opioids can be used for breakthrough pain.1 It is recommended that blisters be drained in a sterile fashion and that all affected tissue be covered with topical aloe vera and a loose dressing.1,2,4
Treatment of severe frostbite. Angiography should be performed on all patients with third- or fourth-degree frostbite.3 If imaging shows evidence of vascular occlusion, tissue plasminogen activator (tPA) and heparin can be initiated within 24 hours to reduce the risk for amputation.8-10
Continue to: Iloprost is another...
Iloprost is another proposed treatment for severe frostbite. It is a prostacyclin analog that may lower the amputation rate in patients with at least third-degree frostbite.11 Unlike tPA, iloprost may be given to trauma patients, and it can be used more than 24 hours after injury.2
In cases of fourth-degree frostbite that is not successfully reperfused, amputation is delayed until dry gangrene develops. This often takes weeks to months.12
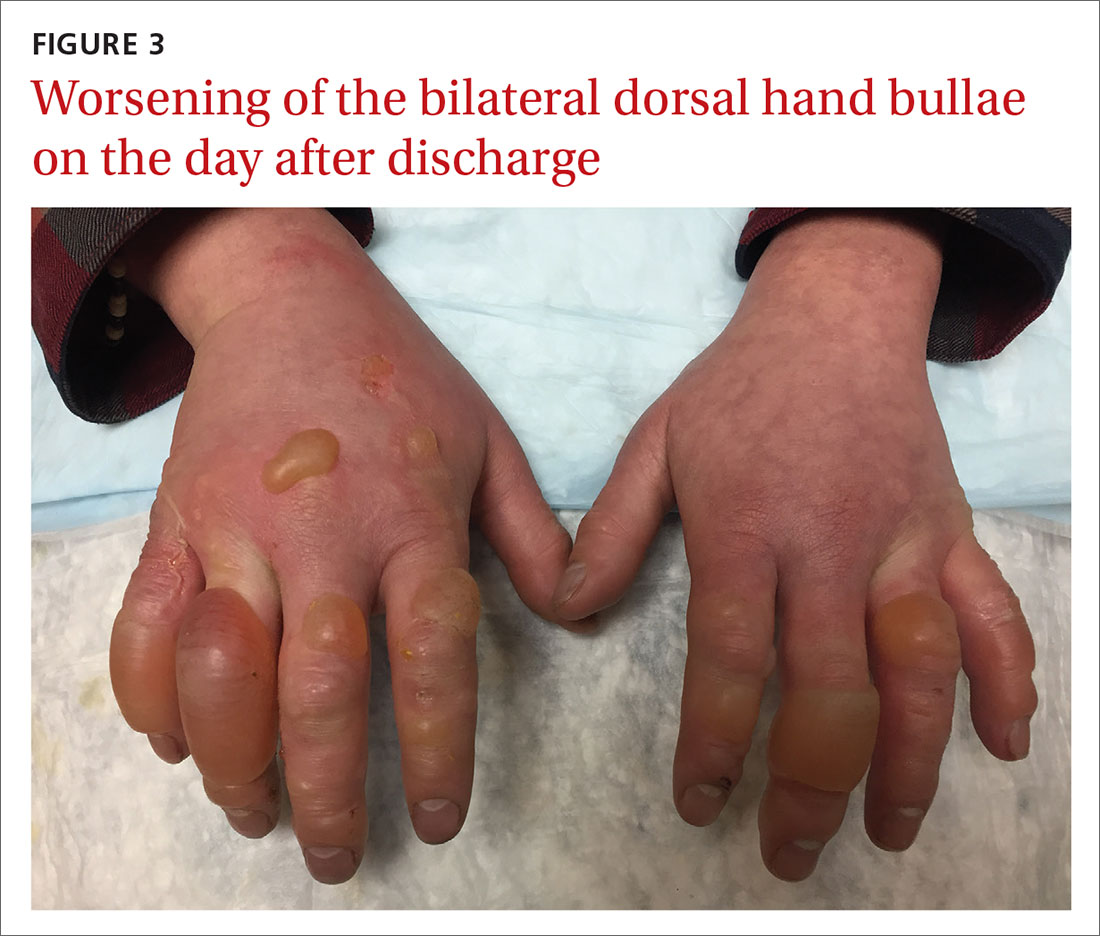
Our patient underwent rewarming and was orally rehydrated. He was discharged home with ibuprofen, oxycodone-acetaminophen, topical aloe vera, and loose dressings. His bullae enlarged the next day (FIGURE 3). One week later, his blisters were debrided and dressed with silver sulfadiazine at his plastic surgery follow-up. He experienced sensory deficits for a few months, but eventually made a full recovery after 6 months with no remaining sequelae.
ACKNOWLEDGEMENT
The authors thank Lisa Kim, MD, for her clinical care of this patient.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; morteza.khodaee@ cuanschutz.edu
1. Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
2. Heil K, Thomas R, Robertson G, et al. Freezing and non-freezing cold weather injuries: a systematic review. Br Med Bull. 2016;117:79-93.
3. Millet JD, Brown RK, Levi B, et al. Frostbite: spectrum of imaging findings and guidelines for management. Radiographics. 2016;36:2154-2169.
4. Long WB 3rd, Edlich RF, Winters KL, et al. Cold injuries. J Long Term Eff Med Implants. 2005;15:67-78.
5. Baker JS, Miranpuri S. Perniosis: a case report with literature review. J Am Podiatr Med Assoc. 2016;106:138-140.
6. Bush JS, Watson S. Trench foot. Updated February 3, 2020. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK482364/. Accessed April 22, 2020.
7. Musa R, Qurie A. Raynaud disease (Raynaud phenomenon, Raynaud syndrome). Updated February 14, 2019. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK499833/. Accessed April 22, 2020.
8. Bruen KJ, Ballard JR, Morris SE, et al. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142:546-551; discussion 551-553.
9. Gonzaga T, Jenabzadeh K, Anderson CP, et al. Use of intra-arterial thrombolytic therapy for acute treatment of frostbite in 62 patients with review of thrombolytic therapy in frostbite. J Burn Care Res. 2016;37:e323-e334.
10. Twomey JA, Peltier GL, Zera RT. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma. 2005;59:1350-1354; discussion 1354-1355.
11. Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med. 2011;364:189-190.
12. McIntosh SE, Opacic M, Freer L, et al; Wilderness Medical Society. Wilderness Medical Society practice guidelines for the prevention and treatment of frostbite: 2014 update. Wilderness Environ Med. 2014;25(4 suppl):S43-S54.
A 27-year-old man presented to the urgent care clinic with acute bilateral hand swelling, blisters, numbness, and pain. History taking revealed that these symptoms developed after he was locked outside of his apartment for 45 minutes in –22°C (–8°F) weather following a night of heavy drinking.
On physical examination, the patient had a temperature of 36.2°C (97.2°F) and a heart rate of 116 beats/min. He had
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Second-degree frostbite
Frostbite is the result of tissue freezing, which generally occurs after prolonged exposure to freezing temperatures (typically –4°C or below).1,2 The majority (~90%) of frostbite injuries occur in the hands and feet; however, frostbite has also been observed in the face, perineum, buttocks, and male genitalia.3
Frostbite is a clinical diagnosis based on a history of sustained exposure to freezing temperatures, paresthesia of affected areas, and typical skin changes. Evidence is lacking regarding the epidemiology of frostbite within the general population.2
Pathophysiology. Intra- and extracellular ice crystal formation causes fluid and electrolyte disturbances, cell dehydration, lipid denaturation, and subsequent cell death.1 After thawing, progressive tissue ischemia can occur as a result of endothelial damage and dysfunction, intravascular sludging, increased inflammatory markers, an influx of free radicals, and microvascular thrombosis.1
Classification. Traditionally, frostbite has been classified according to a 4-tiered system based on tissue appearance after rewarming.2 First-degree frostbite is characterized by white plaques with surrounding erythema; second degree by edema and clear or cloudy vesicles; third degree by hemorrhagic bullae; and fourth degree by cold and hard tissue that eventually progresses to gangrene.2
A simpler scheme designates frostbite as either superficial (corresponding to first- or second-degree frostbite) or deep (corresponding to third- or fourth-degree frostbite) with presumed muscle and bone involvement.2
Continue to: Risk factors
Risk factors. Frostbite is often associated with risk factors such as alcohol or drug intoxication, vehicular failure or trauma, immobilizing trauma, psychiatric illness, homelessness, Raynaud phenomenon, peripheral vascular disease, diabetes, inadequate clothing, previous cold-weather injury, outdoor winter recreation, and the use of certain medications (eg, beta-blockers).1-3 Apart from environmental exposure, frostbite can also occur by direct contact with freezing materials, such as ice packs or industrial refrigerants.3
Differential includes nonfreezing injuries
Frostnip, pernio, and trench foot are other cold-weather injuries distinguished by the absence of tissue freezing.4 Raynaud phenomenon is a condition that is triggered by either cold temperatures or emotional stress.5
Frostnip is characterized by pallor and paresthesia of exposed areas. It may precede frostbite, but it quickly resolves after rewarming.2
Pernio occurs when skin is exposed to damp, cold, nonfreezing environments.6 It results in edematous and inflammatory skin lesions that may be painful, pruritic, violaceous, or erythematous.6 These lesions are typically found over the fingers, toes, nose, ears, buttocks, or thighs.4,6 Pernio may be classified as either primary or secondary disease.5 Primary pernio is considered idiopathic.6 Secondary pernio is thought to be either drug induced or due to underlying autoimmune diseases, such as hepatitis or cryopathy.6
Trench foot develops under similar conditions to pernio but requires exposure to a wet environment for at least 10 to 14 hours.7 It is characterized by foot pain, paresthesia, pruritus, edema, erythema, cyanosis, blisters, and even gangrene if left untreated.7
Continue to: Raynaud phenomenon
Raynaud phenomenon results from transient, acral vasocontraction and manifests as well-demarcated pallor, cyanosis, and then erythema as the affected body part reperfuses.5 Similar to pernio, it can be categorized as either primary or secondary.5 Primary phenomenon is idiopathic. Secondary phenomenon is thought to be a result of autoimmune disease, use of certain medications, occupational vibratory exposure, obstructive vascular disease, or infection
In the absence of a history of exposure to subfreezing temperatures, frostbite can be excluded from the differential diagnosis.
Treatment entails rewarming
The aim of frostbite treatment is to save injured cells and minimize tissue loss.1 This is accomplished through rapid rewarming and—in severe cases—reperfusion techniques.
Tissue should be rewarmed in a 37°C to 39°C water bath with povidone iodine or chlorhexidine added for antiseptic effect.1 All efforts should be made to avoid refreezing or trauma, as this could worsen the initial injury.2 Oral or intravenous hydration may be offered to optimize fluid status.1 Supplemental oxygen may be administered to maintain saturations above 90%.1 Nonsteroidal anti-inflammatory drugs are helpful for analgesia and anti-inflammatory effect, and opioids can be used for breakthrough pain.1 It is recommended that blisters be drained in a sterile fashion and that all affected tissue be covered with topical aloe vera and a loose dressing.1,2,4
Treatment of severe frostbite. Angiography should be performed on all patients with third- or fourth-degree frostbite.3 If imaging shows evidence of vascular occlusion, tissue plasminogen activator (tPA) and heparin can be initiated within 24 hours to reduce the risk for amputation.8-10
Continue to: Iloprost is another...
Iloprost is another proposed treatment for severe frostbite. It is a prostacyclin analog that may lower the amputation rate in patients with at least third-degree frostbite.11 Unlike tPA, iloprost may be given to trauma patients, and it can be used more than 24 hours after injury.2
In cases of fourth-degree frostbite that is not successfully reperfused, amputation is delayed until dry gangrene develops. This often takes weeks to months.12
Our patient underwent rewarming and was orally rehydrated. He was discharged home with ibuprofen, oxycodone-acetaminophen, topical aloe vera, and loose dressings. His bullae enlarged the next day (FIGURE 3). One week later, his blisters were debrided and dressed with silver sulfadiazine at his plastic surgery follow-up. He experienced sensory deficits for a few months, but eventually made a full recovery after 6 months with no remaining sequelae.
ACKNOWLEDGEMENT
The authors thank Lisa Kim, MD, for her clinical care of this patient.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; morteza.khodaee@ cuanschutz.edu
A 27-year-old man presented to the urgent care clinic with acute bilateral hand swelling, blisters, numbness, and pain. History taking revealed that these symptoms developed after he was locked outside of his apartment for 45 minutes in –22°C (–8°F) weather following a night of heavy drinking.
On physical examination, the patient had a temperature of 36.2°C (97.2°F) and a heart rate of 116 beats/min. He had
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Second-degree frostbite
Frostbite is the result of tissue freezing, which generally occurs after prolonged exposure to freezing temperatures (typically –4°C or below).1,2 The majority (~90%) of frostbite injuries occur in the hands and feet; however, frostbite has also been observed in the face, perineum, buttocks, and male genitalia.3
Frostbite is a clinical diagnosis based on a history of sustained exposure to freezing temperatures, paresthesia of affected areas, and typical skin changes. Evidence is lacking regarding the epidemiology of frostbite within the general population.2
Pathophysiology. Intra- and extracellular ice crystal formation causes fluid and electrolyte disturbances, cell dehydration, lipid denaturation, and subsequent cell death.1 After thawing, progressive tissue ischemia can occur as a result of endothelial damage and dysfunction, intravascular sludging, increased inflammatory markers, an influx of free radicals, and microvascular thrombosis.1
Classification. Traditionally, frostbite has been classified according to a 4-tiered system based on tissue appearance after rewarming.2 First-degree frostbite is characterized by white plaques with surrounding erythema; second degree by edema and clear or cloudy vesicles; third degree by hemorrhagic bullae; and fourth degree by cold and hard tissue that eventually progresses to gangrene.2
A simpler scheme designates frostbite as either superficial (corresponding to first- or second-degree frostbite) or deep (corresponding to third- or fourth-degree frostbite) with presumed muscle and bone involvement.2
Continue to: Risk factors
Risk factors. Frostbite is often associated with risk factors such as alcohol or drug intoxication, vehicular failure or trauma, immobilizing trauma, psychiatric illness, homelessness, Raynaud phenomenon, peripheral vascular disease, diabetes, inadequate clothing, previous cold-weather injury, outdoor winter recreation, and the use of certain medications (eg, beta-blockers).1-3 Apart from environmental exposure, frostbite can also occur by direct contact with freezing materials, such as ice packs or industrial refrigerants.3
Differential includes nonfreezing injuries
Frostnip, pernio, and trench foot are other cold-weather injuries distinguished by the absence of tissue freezing.4 Raynaud phenomenon is a condition that is triggered by either cold temperatures or emotional stress.5
Frostnip is characterized by pallor and paresthesia of exposed areas. It may precede frostbite, but it quickly resolves after rewarming.2
Pernio occurs when skin is exposed to damp, cold, nonfreezing environments.6 It results in edematous and inflammatory skin lesions that may be painful, pruritic, violaceous, or erythematous.6 These lesions are typically found over the fingers, toes, nose, ears, buttocks, or thighs.4,6 Pernio may be classified as either primary or secondary disease.5 Primary pernio is considered idiopathic.6 Secondary pernio is thought to be either drug induced or due to underlying autoimmune diseases, such as hepatitis or cryopathy.6
Trench foot develops under similar conditions to pernio but requires exposure to a wet environment for at least 10 to 14 hours.7 It is characterized by foot pain, paresthesia, pruritus, edema, erythema, cyanosis, blisters, and even gangrene if left untreated.7
Continue to: Raynaud phenomenon
Raynaud phenomenon results from transient, acral vasocontraction and manifests as well-demarcated pallor, cyanosis, and then erythema as the affected body part reperfuses.5 Similar to pernio, it can be categorized as either primary or secondary.5 Primary phenomenon is idiopathic. Secondary phenomenon is thought to be a result of autoimmune disease, use of certain medications, occupational vibratory exposure, obstructive vascular disease, or infection
In the absence of a history of exposure to subfreezing temperatures, frostbite can be excluded from the differential diagnosis.
Treatment entails rewarming
The aim of frostbite treatment is to save injured cells and minimize tissue loss.1 This is accomplished through rapid rewarming and—in severe cases—reperfusion techniques.
Tissue should be rewarmed in a 37°C to 39°C water bath with povidone iodine or chlorhexidine added for antiseptic effect.1 All efforts should be made to avoid refreezing or trauma, as this could worsen the initial injury.2 Oral or intravenous hydration may be offered to optimize fluid status.1 Supplemental oxygen may be administered to maintain saturations above 90%.1 Nonsteroidal anti-inflammatory drugs are helpful for analgesia and anti-inflammatory effect, and opioids can be used for breakthrough pain.1 It is recommended that blisters be drained in a sterile fashion and that all affected tissue be covered with topical aloe vera and a loose dressing.1,2,4
Treatment of severe frostbite. Angiography should be performed on all patients with third- or fourth-degree frostbite.3 If imaging shows evidence of vascular occlusion, tissue plasminogen activator (tPA) and heparin can be initiated within 24 hours to reduce the risk for amputation.8-10
Continue to: Iloprost is another...
Iloprost is another proposed treatment for severe frostbite. It is a prostacyclin analog that may lower the amputation rate in patients with at least third-degree frostbite.11 Unlike tPA, iloprost may be given to trauma patients, and it can be used more than 24 hours after injury.2
In cases of fourth-degree frostbite that is not successfully reperfused, amputation is delayed until dry gangrene develops. This often takes weeks to months.12
Our patient underwent rewarming and was orally rehydrated. He was discharged home with ibuprofen, oxycodone-acetaminophen, topical aloe vera, and loose dressings. His bullae enlarged the next day (FIGURE 3). One week later, his blisters were debrided and dressed with silver sulfadiazine at his plastic surgery follow-up. He experienced sensory deficits for a few months, but eventually made a full recovery after 6 months with no remaining sequelae.
ACKNOWLEDGEMENT
The authors thank Lisa Kim, MD, for her clinical care of this patient.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; morteza.khodaee@ cuanschutz.edu
1. Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
2. Heil K, Thomas R, Robertson G, et al. Freezing and non-freezing cold weather injuries: a systematic review. Br Med Bull. 2016;117:79-93.
3. Millet JD, Brown RK, Levi B, et al. Frostbite: spectrum of imaging findings and guidelines for management. Radiographics. 2016;36:2154-2169.
4. Long WB 3rd, Edlich RF, Winters KL, et al. Cold injuries. J Long Term Eff Med Implants. 2005;15:67-78.
5. Baker JS, Miranpuri S. Perniosis: a case report with literature review. J Am Podiatr Med Assoc. 2016;106:138-140.
6. Bush JS, Watson S. Trench foot. Updated February 3, 2020. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK482364/. Accessed April 22, 2020.
7. Musa R, Qurie A. Raynaud disease (Raynaud phenomenon, Raynaud syndrome). Updated February 14, 2019. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK499833/. Accessed April 22, 2020.
8. Bruen KJ, Ballard JR, Morris SE, et al. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142:546-551; discussion 551-553.
9. Gonzaga T, Jenabzadeh K, Anderson CP, et al. Use of intra-arterial thrombolytic therapy for acute treatment of frostbite in 62 patients with review of thrombolytic therapy in frostbite. J Burn Care Res. 2016;37:e323-e334.
10. Twomey JA, Peltier GL, Zera RT. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma. 2005;59:1350-1354; discussion 1354-1355.
11. Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med. 2011;364:189-190.
12. McIntosh SE, Opacic M, Freer L, et al; Wilderness Medical Society. Wilderness Medical Society practice guidelines for the prevention and treatment of frostbite: 2014 update. Wilderness Environ Med. 2014;25(4 suppl):S43-S54.
1. Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
2. Heil K, Thomas R, Robertson G, et al. Freezing and non-freezing cold weather injuries: a systematic review. Br Med Bull. 2016;117:79-93.
3. Millet JD, Brown RK, Levi B, et al. Frostbite: spectrum of imaging findings and guidelines for management. Radiographics. 2016;36:2154-2169.
4. Long WB 3rd, Edlich RF, Winters KL, et al. Cold injuries. J Long Term Eff Med Implants. 2005;15:67-78.
5. Baker JS, Miranpuri S. Perniosis: a case report with literature review. J Am Podiatr Med Assoc. 2016;106:138-140.
6. Bush JS, Watson S. Trench foot. Updated February 3, 2020. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK482364/. Accessed April 22, 2020.
7. Musa R, Qurie A. Raynaud disease (Raynaud phenomenon, Raynaud syndrome). Updated February 14, 2019. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK499833/. Accessed April 22, 2020.
8. Bruen KJ, Ballard JR, Morris SE, et al. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142:546-551; discussion 551-553.
9. Gonzaga T, Jenabzadeh K, Anderson CP, et al. Use of intra-arterial thrombolytic therapy for acute treatment of frostbite in 62 patients with review of thrombolytic therapy in frostbite. J Burn Care Res. 2016;37:e323-e334.
10. Twomey JA, Peltier GL, Zera RT. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma. 2005;59:1350-1354; discussion 1354-1355.
11. Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med. 2011;364:189-190.
12. McIntosh SE, Opacic M, Freer L, et al; Wilderness Medical Society. Wilderness Medical Society practice guidelines for the prevention and treatment of frostbite: 2014 update. Wilderness Environ Med. 2014;25(4 suppl):S43-S54.
20-year-old male college basketball prospect • wrist pain after falling on wrist • normal ROM • pain with active/passive wrist extension • Dx?
THE CASE
A 20-year-old man presented to our family medicine clinic with right wrist pain 4 days after falling on his wrist and hand while playing basketball. He denied any other previous injury or trauma. The pain was unchanged since the injury occurred.
Examination demonstrated mild edema over the palmar and ulnar aspect of the patient’s right wrist with no apparent ecchymosis. He had normal range of motion of his right wrist and hand. However, he experienced pain with active and passive wrist extension and ulnar deviation. There was significant tenderness in the palmar and ulnar aspects of his right wrist just distal to the ulnar styloid process.
THE DIAGNOSIS
Standard plain x-rays of the right wrist revealed an isolated fracture of the body of the triquetrum (FIGURE 1). Since the patient refused to have a cast placed, his wrist was immobilized with a wrist brace. By Day 16 post injury, the pain and edema had improved significantly. After talking with the patient about the potential risks and benefits of continuing to play basketball—and despite our recommendation that he not play—he decided to continue playing since he was a college basketball prospect.
At 4 weeks post injury, x-rays demonstrated mild interval healing (FIGURE 2). At the 8-week visit, the patient had only very mild pain and tenderness, and x-ray images showed improvement (FIGURE 3). Within a few months, his symptoms resolved completely. No further imaging was performed.
DISCUSSION
In general, carpal fractures are uncommon.1 The triquetrum is the second most commonly injured carpal bone, involved in up to 18% of all carpal fractures.2,3 Triquetrum fractures most commonly occur as isolated injuries and are typically classified in 2 general categories: avulsion fractures (dorsal cortex or volar cortex) and fractures of the triquetrum body.4-8 Isolated avulsion fractures of the triquetral dorsal cortex are relatively common, occurring in about 95% of triquetrum injuries.4-9 Isolated fractures of the triquetrum body are less common, occurring in about 4% of triquetrum injuries, and can go unnoticed on conventional x-rays.4-9
Basketball presents a unique risk for hand or wrist fracture due to its high-impact nature, hard playing surfaces, and frequent use of the hands for dribbling, shooting, rebounding, and passing the ball.
In a retrospective study of sports-related fractures conducted at the Royal Infirmary of Edinburgh, basketball had the highest incidence of carpal injuries compared with other sports, including football, rugby, skiing, snowboarding, and ice-skating.4 Similarly, a retrospective study conducted at the University of California, Los Angeles, found that of all Division 1 collegiate athletes at the school, basketball players had the highest incidence of primary fractures, and the most common fracture location was the hand.10
Continue to: An injury that's easy to miss
An injury that’s easy to miss
Because the incidence of hand and wrist injuries is high among basketball players, it is imperative that triquetrum body fractures are not missed or misdiagnosed as more common hand and wrist injuries, such as triquetral dorsal avulsion fractures.
Our patient, who had an isolated triquetrum body fracture, presented with focal tenderness on the palmar and ulnar aspects of his wrist and pain with ulnar deviation. Since triquetral body fractures often have a clinical presentation quite similar to that of triquetral dorsal avulsion fractures, patients presenting with symptoms of wrist tenderness and pain should be treated with a high degree of clinical suspicion.
With our patient, anteroposterior and lateral x-rays were sufficient to demonstrate an isolated triquetrum body fracture; however, triquetral fractures can be missed in up to 20% of x-rays.4 Both magnetic resonance imaging and computerized tomography are useful in diagnosing occult triquetrum fractures and should be used to confirm clinical suspicion when traditional x-rays are inconclusive.11,12
Management varies
Management of isolated triquetrum body fractures varies depending on the fracture pattern and the status of bone consolidation. Triquetral body fractures typically heal well; it’s very rare that there is a nonunion. As our patient’s fracture was nondisplaced and stable, brace immobilization for 4 weeks was sufficient to facilitate healing and restore long-term hand and wrist functionality. This course of treatment is consistent with other cases of nondisplaced triquetrum body fractures reported in the literature.13
Long-term outcomes. The literature is sparse regarding the long-term functional outcome of nonsurgical treatment for nondisplaced triquetrum body fractures. Multiple carpal fractures, displaced triquetrum body fractures, and persistent pain for multiple months after nonsurgical management all indicate the need for referral to orthopedic surgery. In instances of fracture displacement or nonunion, management tends to be surgical, with open reduction and internal fixation (ORIF) used in multiple cases of nonunion for isolated triquetrum body fractures.3,14 Any diagnostic imaging that reveals displacement, malunion, or nonunion of the fracture is an indication for referral to an orthopedic surgeon.
Continue to: Return to play
Return to play. There is no evidence-based return-to-play recommendation for patients with a triquetrum fracture. However, our patient continued to play basketball through the early stages of injury management because he was a collegiate prospect. While medical, social, and economic factors should be considered when discussing treatment options with athletes, injuries should be managed so that there is no long-term loss of function or risk of injury exacerbation. When discussing early return from injury with athletes who have outside pressure to return to play, it’s important to make them aware of the associated long- and short-term risks.15
THE TAKEAWAY
Management of an isolated triquetrum body fracture is typically straightforward; however, if the fracture is displaced, refer the patient to an orthopedic surgeon as ORIF may be required. For this reason, it’s important to be able to promptly identify isolated triquetrum body fractures and to avoid confusing them with triquetrum dorsal avulsion fractures.
Depending on the sport played and the severity of the injury, athletes with conservatively managed nondisplaced triquetral body fractures may be candidates for early return to play. Nonetheless, athletes should understand both the short- and the long-term risks of playing with an injury, and they should never be advised to continue playing with an injury if it jeopardizes their well-being or the long-term functionality of the affected body part.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; Morteza.Khodaee@cuanschutz.edu
1. Suh N, Ek ET, Wolfe SW. Carpal fractures. J Hand Surg Am. 2014;39:785-791.
2. Hey HW, Chong AK, Murphy D. Prevalence of carpal fracture in Singapore. J Hand Surg Am. 2011;36:278-283.
3. Al Rashid M, Rasoli S, Khan WS. Non-union of isolated displaced triquetral body fracture—a case report. Ortop Traumatol Rehabil. 2012;14:71-74.
4. Becce F, Theumann N, Bollmann C, et al. Dorsal fractures of the triquetrum: MRI findings with an emphasis on dorsal carpal ligament injuries. AJR Am J Roentgenol. 2013;200:608-617.
5. Court-Brown CM, Wood AM, Aitken S. The epidemiology of acute sports-related fractures in adults. Injury. 2008;39:1365-1372.
6. Urch EY, Lee SK. Carpal fractures other than scaphoid. Clin Sports Med. 2015;34:51-67.
7. deWeber K. Triquetrum fractures. UpToDate. 2016. www.uptodate.com/contents/triquetrum-fractures. Accessed September 3, 2019.
8. Höcker K, Menschik A. Chip fractures of the triquetrum. Mechanism, classification and results. J Hand Surg Br. 1994;19:584-588.
9. Jarraya M, Hayashi D, Roemer FW, et al. Radiographically occult and subtle fractures: a pictorial review. Radiol Res Pract. 2013;2013:370169.
10. Hame SL, LaFemina JM, McAllister DR, et al. Fractures in the collegiate athlete. Am J Sports Med. 2004;32:446-451.
11. Hindman BW, Kulik WJ, Lee G, et al. Occult fractures of the carpals and metacarpals: demonstration by CT. AJR Am J Roentgenol. 1989;153:529-532.
12. Pierre-Jerome C, Moncayo V, Albastaki U, et al. Multiple occult wrist bone injuries and joint effusions: prevalence and distribution on MRI. Emerg Radiol. 2010;17:179-184.
13. Yildirim C, Akmaz I, Keklikçi K, et al. An unusual combined fracture pattern of the triquetrum. J Hand Surg Eur Vol. 2008;33:385-386.
14. Rasoli S, Ricks M, Packer G. Isolated displaced non-union of a triquetral body fracture: a case report. J Med Case Rep. 2012;6:54.
15. Strickland JW. Considerations for the treatment of the injured athlete. Clin Sports Med. 1998;17:397-400.
THE CASE
A 20-year-old man presented to our family medicine clinic with right wrist pain 4 days after falling on his wrist and hand while playing basketball. He denied any other previous injury or trauma. The pain was unchanged since the injury occurred.
Examination demonstrated mild edema over the palmar and ulnar aspect of the patient’s right wrist with no apparent ecchymosis. He had normal range of motion of his right wrist and hand. However, he experienced pain with active and passive wrist extension and ulnar deviation. There was significant tenderness in the palmar and ulnar aspects of his right wrist just distal to the ulnar styloid process.
THE DIAGNOSIS
Standard plain x-rays of the right wrist revealed an isolated fracture of the body of the triquetrum (FIGURE 1). Since the patient refused to have a cast placed, his wrist was immobilized with a wrist brace. By Day 16 post injury, the pain and edema had improved significantly. After talking with the patient about the potential risks and benefits of continuing to play basketball—and despite our recommendation that he not play—he decided to continue playing since he was a college basketball prospect.
At 4 weeks post injury, x-rays demonstrated mild interval healing (FIGURE 2). At the 8-week visit, the patient had only very mild pain and tenderness, and x-ray images showed improvement (FIGURE 3). Within a few months, his symptoms resolved completely. No further imaging was performed.
DISCUSSION
In general, carpal fractures are uncommon.1 The triquetrum is the second most commonly injured carpal bone, involved in up to 18% of all carpal fractures.2,3 Triquetrum fractures most commonly occur as isolated injuries and are typically classified in 2 general categories: avulsion fractures (dorsal cortex or volar cortex) and fractures of the triquetrum body.4-8 Isolated avulsion fractures of the triquetral dorsal cortex are relatively common, occurring in about 95% of triquetrum injuries.4-9 Isolated fractures of the triquetrum body are less common, occurring in about 4% of triquetrum injuries, and can go unnoticed on conventional x-rays.4-9
Basketball presents a unique risk for hand or wrist fracture due to its high-impact nature, hard playing surfaces, and frequent use of the hands for dribbling, shooting, rebounding, and passing the ball.
In a retrospective study of sports-related fractures conducted at the Royal Infirmary of Edinburgh, basketball had the highest incidence of carpal injuries compared with other sports, including football, rugby, skiing, snowboarding, and ice-skating.4 Similarly, a retrospective study conducted at the University of California, Los Angeles, found that of all Division 1 collegiate athletes at the school, basketball players had the highest incidence of primary fractures, and the most common fracture location was the hand.10
Continue to: An injury that's easy to miss
An injury that’s easy to miss
Because the incidence of hand and wrist injuries is high among basketball players, it is imperative that triquetrum body fractures are not missed or misdiagnosed as more common hand and wrist injuries, such as triquetral dorsal avulsion fractures.
Our patient, who had an isolated triquetrum body fracture, presented with focal tenderness on the palmar and ulnar aspects of his wrist and pain with ulnar deviation. Since triquetral body fractures often have a clinical presentation quite similar to that of triquetral dorsal avulsion fractures, patients presenting with symptoms of wrist tenderness and pain should be treated with a high degree of clinical suspicion.
With our patient, anteroposterior and lateral x-rays were sufficient to demonstrate an isolated triquetrum body fracture; however, triquetral fractures can be missed in up to 20% of x-rays.4 Both magnetic resonance imaging and computerized tomography are useful in diagnosing occult triquetrum fractures and should be used to confirm clinical suspicion when traditional x-rays are inconclusive.11,12
Management varies
Management of isolated triquetrum body fractures varies depending on the fracture pattern and the status of bone consolidation. Triquetral body fractures typically heal well; it’s very rare that there is a nonunion. As our patient’s fracture was nondisplaced and stable, brace immobilization for 4 weeks was sufficient to facilitate healing and restore long-term hand and wrist functionality. This course of treatment is consistent with other cases of nondisplaced triquetrum body fractures reported in the literature.13
Long-term outcomes. The literature is sparse regarding the long-term functional outcome of nonsurgical treatment for nondisplaced triquetrum body fractures. Multiple carpal fractures, displaced triquetrum body fractures, and persistent pain for multiple months after nonsurgical management all indicate the need for referral to orthopedic surgery. In instances of fracture displacement or nonunion, management tends to be surgical, with open reduction and internal fixation (ORIF) used in multiple cases of nonunion for isolated triquetrum body fractures.3,14 Any diagnostic imaging that reveals displacement, malunion, or nonunion of the fracture is an indication for referral to an orthopedic surgeon.
Continue to: Return to play
Return to play. There is no evidence-based return-to-play recommendation for patients with a triquetrum fracture. However, our patient continued to play basketball through the early stages of injury management because he was a collegiate prospect. While medical, social, and economic factors should be considered when discussing treatment options with athletes, injuries should be managed so that there is no long-term loss of function or risk of injury exacerbation. When discussing early return from injury with athletes who have outside pressure to return to play, it’s important to make them aware of the associated long- and short-term risks.15
THE TAKEAWAY
Management of an isolated triquetrum body fracture is typically straightforward; however, if the fracture is displaced, refer the patient to an orthopedic surgeon as ORIF may be required. For this reason, it’s important to be able to promptly identify isolated triquetrum body fractures and to avoid confusing them with triquetrum dorsal avulsion fractures.
Depending on the sport played and the severity of the injury, athletes with conservatively managed nondisplaced triquetral body fractures may be candidates for early return to play. Nonetheless, athletes should understand both the short- and the long-term risks of playing with an injury, and they should never be advised to continue playing with an injury if it jeopardizes their well-being or the long-term functionality of the affected body part.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; Morteza.Khodaee@cuanschutz.edu
THE CASE
A 20-year-old man presented to our family medicine clinic with right wrist pain 4 days after falling on his wrist and hand while playing basketball. He denied any other previous injury or trauma. The pain was unchanged since the injury occurred.
Examination demonstrated mild edema over the palmar and ulnar aspect of the patient’s right wrist with no apparent ecchymosis. He had normal range of motion of his right wrist and hand. However, he experienced pain with active and passive wrist extension and ulnar deviation. There was significant tenderness in the palmar and ulnar aspects of his right wrist just distal to the ulnar styloid process.
THE DIAGNOSIS
Standard plain x-rays of the right wrist revealed an isolated fracture of the body of the triquetrum (FIGURE 1). Since the patient refused to have a cast placed, his wrist was immobilized with a wrist brace. By Day 16 post injury, the pain and edema had improved significantly. After talking with the patient about the potential risks and benefits of continuing to play basketball—and despite our recommendation that he not play—he decided to continue playing since he was a college basketball prospect.
At 4 weeks post injury, x-rays demonstrated mild interval healing (FIGURE 2). At the 8-week visit, the patient had only very mild pain and tenderness, and x-ray images showed improvement (FIGURE 3). Within a few months, his symptoms resolved completely. No further imaging was performed.
DISCUSSION
In general, carpal fractures are uncommon.1 The triquetrum is the second most commonly injured carpal bone, involved in up to 18% of all carpal fractures.2,3 Triquetrum fractures most commonly occur as isolated injuries and are typically classified in 2 general categories: avulsion fractures (dorsal cortex or volar cortex) and fractures of the triquetrum body.4-8 Isolated avulsion fractures of the triquetral dorsal cortex are relatively common, occurring in about 95% of triquetrum injuries.4-9 Isolated fractures of the triquetrum body are less common, occurring in about 4% of triquetrum injuries, and can go unnoticed on conventional x-rays.4-9
Basketball presents a unique risk for hand or wrist fracture due to its high-impact nature, hard playing surfaces, and frequent use of the hands for dribbling, shooting, rebounding, and passing the ball.
In a retrospective study of sports-related fractures conducted at the Royal Infirmary of Edinburgh, basketball had the highest incidence of carpal injuries compared with other sports, including football, rugby, skiing, snowboarding, and ice-skating.4 Similarly, a retrospective study conducted at the University of California, Los Angeles, found that of all Division 1 collegiate athletes at the school, basketball players had the highest incidence of primary fractures, and the most common fracture location was the hand.10
Continue to: An injury that's easy to miss
An injury that’s easy to miss
Because the incidence of hand and wrist injuries is high among basketball players, it is imperative that triquetrum body fractures are not missed or misdiagnosed as more common hand and wrist injuries, such as triquetral dorsal avulsion fractures.
Our patient, who had an isolated triquetrum body fracture, presented with focal tenderness on the palmar and ulnar aspects of his wrist and pain with ulnar deviation. Since triquetral body fractures often have a clinical presentation quite similar to that of triquetral dorsal avulsion fractures, patients presenting with symptoms of wrist tenderness and pain should be treated with a high degree of clinical suspicion.
With our patient, anteroposterior and lateral x-rays were sufficient to demonstrate an isolated triquetrum body fracture; however, triquetral fractures can be missed in up to 20% of x-rays.4 Both magnetic resonance imaging and computerized tomography are useful in diagnosing occult triquetrum fractures and should be used to confirm clinical suspicion when traditional x-rays are inconclusive.11,12
Management varies
Management of isolated triquetrum body fractures varies depending on the fracture pattern and the status of bone consolidation. Triquetral body fractures typically heal well; it’s very rare that there is a nonunion. As our patient’s fracture was nondisplaced and stable, brace immobilization for 4 weeks was sufficient to facilitate healing and restore long-term hand and wrist functionality. This course of treatment is consistent with other cases of nondisplaced triquetrum body fractures reported in the literature.13
Long-term outcomes. The literature is sparse regarding the long-term functional outcome of nonsurgical treatment for nondisplaced triquetrum body fractures. Multiple carpal fractures, displaced triquetrum body fractures, and persistent pain for multiple months after nonsurgical management all indicate the need for referral to orthopedic surgery. In instances of fracture displacement or nonunion, management tends to be surgical, with open reduction and internal fixation (ORIF) used in multiple cases of nonunion for isolated triquetrum body fractures.3,14 Any diagnostic imaging that reveals displacement, malunion, or nonunion of the fracture is an indication for referral to an orthopedic surgeon.
Continue to: Return to play
Return to play. There is no evidence-based return-to-play recommendation for patients with a triquetrum fracture. However, our patient continued to play basketball through the early stages of injury management because he was a collegiate prospect. While medical, social, and economic factors should be considered when discussing treatment options with athletes, injuries should be managed so that there is no long-term loss of function or risk of injury exacerbation. When discussing early return from injury with athletes who have outside pressure to return to play, it’s important to make them aware of the associated long- and short-term risks.15
THE TAKEAWAY
Management of an isolated triquetrum body fracture is typically straightforward; however, if the fracture is displaced, refer the patient to an orthopedic surgeon as ORIF may be required. For this reason, it’s important to be able to promptly identify isolated triquetrum body fractures and to avoid confusing them with triquetrum dorsal avulsion fractures.
Depending on the sport played and the severity of the injury, athletes with conservatively managed nondisplaced triquetral body fractures may be candidates for early return to play. Nonetheless, athletes should understand both the short- and the long-term risks of playing with an injury, and they should never be advised to continue playing with an injury if it jeopardizes their well-being or the long-term functionality of the affected body part.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; Morteza.Khodaee@cuanschutz.edu
1. Suh N, Ek ET, Wolfe SW. Carpal fractures. J Hand Surg Am. 2014;39:785-791.
2. Hey HW, Chong AK, Murphy D. Prevalence of carpal fracture in Singapore. J Hand Surg Am. 2011;36:278-283.
3. Al Rashid M, Rasoli S, Khan WS. Non-union of isolated displaced triquetral body fracture—a case report. Ortop Traumatol Rehabil. 2012;14:71-74.
4. Becce F, Theumann N, Bollmann C, et al. Dorsal fractures of the triquetrum: MRI findings with an emphasis on dorsal carpal ligament injuries. AJR Am J Roentgenol. 2013;200:608-617.
5. Court-Brown CM, Wood AM, Aitken S. The epidemiology of acute sports-related fractures in adults. Injury. 2008;39:1365-1372.
6. Urch EY, Lee SK. Carpal fractures other than scaphoid. Clin Sports Med. 2015;34:51-67.
7. deWeber K. Triquetrum fractures. UpToDate. 2016. www.uptodate.com/contents/triquetrum-fractures. Accessed September 3, 2019.
8. Höcker K, Menschik A. Chip fractures of the triquetrum. Mechanism, classification and results. J Hand Surg Br. 1994;19:584-588.
9. Jarraya M, Hayashi D, Roemer FW, et al. Radiographically occult and subtle fractures: a pictorial review. Radiol Res Pract. 2013;2013:370169.
10. Hame SL, LaFemina JM, McAllister DR, et al. Fractures in the collegiate athlete. Am J Sports Med. 2004;32:446-451.
11. Hindman BW, Kulik WJ, Lee G, et al. Occult fractures of the carpals and metacarpals: demonstration by CT. AJR Am J Roentgenol. 1989;153:529-532.
12. Pierre-Jerome C, Moncayo V, Albastaki U, et al. Multiple occult wrist bone injuries and joint effusions: prevalence and distribution on MRI. Emerg Radiol. 2010;17:179-184.
13. Yildirim C, Akmaz I, Keklikçi K, et al. An unusual combined fracture pattern of the triquetrum. J Hand Surg Eur Vol. 2008;33:385-386.
14. Rasoli S, Ricks M, Packer G. Isolated displaced non-union of a triquetral body fracture: a case report. J Med Case Rep. 2012;6:54.
15. Strickland JW. Considerations for the treatment of the injured athlete. Clin Sports Med. 1998;17:397-400.
1. Suh N, Ek ET, Wolfe SW. Carpal fractures. J Hand Surg Am. 2014;39:785-791.
2. Hey HW, Chong AK, Murphy D. Prevalence of carpal fracture in Singapore. J Hand Surg Am. 2011;36:278-283.
3. Al Rashid M, Rasoli S, Khan WS. Non-union of isolated displaced triquetral body fracture—a case report. Ortop Traumatol Rehabil. 2012;14:71-74.
4. Becce F, Theumann N, Bollmann C, et al. Dorsal fractures of the triquetrum: MRI findings with an emphasis on dorsal carpal ligament injuries. AJR Am J Roentgenol. 2013;200:608-617.
5. Court-Brown CM, Wood AM, Aitken S. The epidemiology of acute sports-related fractures in adults. Injury. 2008;39:1365-1372.
6. Urch EY, Lee SK. Carpal fractures other than scaphoid. Clin Sports Med. 2015;34:51-67.
7. deWeber K. Triquetrum fractures. UpToDate. 2016. www.uptodate.com/contents/triquetrum-fractures. Accessed September 3, 2019.
8. Höcker K, Menschik A. Chip fractures of the triquetrum. Mechanism, classification and results. J Hand Surg Br. 1994;19:584-588.
9. Jarraya M, Hayashi D, Roemer FW, et al. Radiographically occult and subtle fractures: a pictorial review. Radiol Res Pract. 2013;2013:370169.
10. Hame SL, LaFemina JM, McAllister DR, et al. Fractures in the collegiate athlete. Am J Sports Med. 2004;32:446-451.
11. Hindman BW, Kulik WJ, Lee G, et al. Occult fractures of the carpals and metacarpals: demonstration by CT. AJR Am J Roentgenol. 1989;153:529-532.
12. Pierre-Jerome C, Moncayo V, Albastaki U, et al. Multiple occult wrist bone injuries and joint effusions: prevalence and distribution on MRI. Emerg Radiol. 2010;17:179-184.
13. Yildirim C, Akmaz I, Keklikçi K, et al. An unusual combined fracture pattern of the triquetrum. J Hand Surg Eur Vol. 2008;33:385-386.
14. Rasoli S, Ricks M, Packer G. Isolated displaced non-union of a triquetral body fracture: a case report. J Med Case Rep. 2012;6:54.
15. Strickland JW. Considerations for the treatment of the injured athlete. Clin Sports Med. 1998;17:397-400.
Weakness with left elbow flexion • left anterior shoulder pain • Dx?
THE CASE
A 41-year-old, right-hand dominant man sought care at our facility one day after trying to pull his boat out of the water. He’d tried to lift the boat with his hands while his forearms were fully supinated and his elbows were flexed to about 90°. He then felt a sharp burning sensation in his left anterior shoulder and was unable to lift the boat. The patient denied feeling a popping sensation at the time of the injury. He had mild pain at night, but was able to sleep. He said that he had mild diminished strength with elbow flexion, but denied having any numbness, tingling, or discoloration of his skin.
The patient said he did weightlifting and strength training of his upper and lower extremities 4 times/week. He was in good general health, was not taking any medications or supplements, and denied smoking or using illicit drugs. His surgical history was significant for a Bankart repair 8 years ago.
On physical examination, the patient had a scar from the previous surgery, a hollow area in his left anterior shoulder, and a prominent biceps muscle belly (FIGURE). His shoulder range of motion was normal. Left shoulder Neer, Hawkins-Kennedy, drop-arm, cross-arm, empty can, and apprehension tests were negative. A left Speed’s test (resisted elbow flexion when elbow is flexed 20° to 30° with the forearm in supination and the arm in about 60° of flexion) was positive for mild anterior shoulder pain. So, too, was a Yergason’s test (resisted forearm supination and elbow flexion when forearm is pronated and elbow is flexed to 90°). The patient’s elbow flexion strength was 4 out of 5, and his supination strength was 5 out of 5. Neurovascular and sensory examinations of his upper extremities, including radial and ulnar pulses, were normal.
THE DIAGNOSIS
A diagnostic musculoskeletal ultrasound revealed an empty tendon sheath of the long head of the biceps in the bicipital groove and a retracted echogenic stump with associated hematoma at the proximal musculotendinous junction. Based on the patient’s history, physical examination, and ultrasound, a diagnosis of an acute rupture of the left long head of the biceps brachii tendon was made.
DISCUSSION
Diagnosis of acute rupture is often made clinically based on a visually apparent defect proximally and a bulbous mass distally (“Popeye deformity”).1 Ultrasound and magnetic resonance imaging (MRI) may aid in the diagnosis by demonstrating an absence of the long head in the bicipital groove or at its insertion.
The biceps brachii tendon functions in flexion and supination of the forearm. The long head of the biceps also plays a stabilizing role in the glenohumeral joint during elbow flexion and supination.2 Injury to the biceps most often occurs in middle-aged men following a traumatic sudden eccentric bicipital contraction event, during which most patients describe a snapping or popping sensation.3,4
Rupture of the proximal biceps tendon represents about 90% of all biceps ruptures, which almost exclusively involve the long head of the biceps.3,5,6 Risk factors for tendon rupture include obesity, smoking, steroid injection in or around the tendon, and previous tendinopathy.7-10
Functional limitations. It is generally thought that functional limitations following a proximal biceps rupture are relatively minimal, due to the work of other flexors and supinators, including the brachialis and brachioradialis. However, because strength and endurance of the muscle can decrease by about 25%, physical laborers and high-demand athletes may notice a degree of residual weakness with supination and elbow flexion.11,12
Surgery is suitable for some, but not all
Surgical repair is recommended for acute ruptures in patients with high physical demands and for whom a slight loss of flexion and supination strength would not be well tolerated.13 Tenotomy and tenodesis are the main techniques used to surgically repair a rupture of the long head of the biceps brachii tendon. Although there is no consensus on which technique is superior, it seems that there is less cosmetic deformity and better post-surgery biomechanical strength with tenodesis compared with tenotomy.14 However, tenodesis is associated with a higher likelihood of bicipital pain,14 and recent case reports have suggested it is associated with an increased risk of humeral fracture.15 Therefore, each patient should be treated on an individual case basis, taking into account age, activity level, and physical demand.14
For most patients, treatment remains conservative with typically excellent outcomes. Nonoperative management includes gentle range-of-motion exercises for the prevention of contractures of the elbow and shoulder. Such exercises can be started almost immediately after injury. In one study, nonoperative management was recommended for patients with sedentary work, injury in the non-dominant arm, and acceptable cosmetic deformity. Researchers noted that patients who opt for a nonsurgical treatment generally do well with a home exercise program and rarely have stiffness.1
If the patient is a young athlete, if cosmetic deformity is unacceptable, or if the injury is in the dominant arm of a laborer, then the patient may want to consider tenodesis.1 Tangari et al found that in high-demand athletes, biceps tenodesis resulted in excellent functional and cosmetic results with no clinically significant decrease in strength after an average follow-up of 7.6 years.13 In a case s
Our patient elected to proceed with a tenodesis procedure. Two months after the surgery, he had fully recovered.
THE TAKEAWAY
Rupture of the biceps brachii tendon is relatively uncommon. In the vast majority of cases, it happens in the long head of the dominant arm of middle-aged men. Diagnosis is mainly clinical; however, ultrasound and MRI can confirm the diagnosis when there is doubt. Nonoperative management is appropriate for the majority of patients. Young athletes, patients who are concerned with cosmetic appearance, and labor workers with injury to their dominant arm should be referred to an orthopedic surgeon for possible surgery.
1. Geaney LE, Mazzocca AD. Biceps brachii tendon ruptures: a review of diagnosis and treatment of proximal and distal biceps tendon ruptures. Phys Sportsmed. 2010;38:117-125.
2. Payne LZ, Deng XH, Craig EV, et al. The combined dynamic and static contributions to subacromial impingement. A biomechanical analysis. Am J Sports Med. 1997;25:801-808.
3. Jayamoorthy T, Field JR, Costi JJ, et al. Biceps tenodesis: a biomechanical study of fixation methods. J Shoulder Elbow Surg. 2004;13:160-164.
4. Mazzocca AD, Spang JT, Arciero RA. Distal biceps rupture. Orthop Clin North Am. 2008;39:237-249, vii.
5. Carter AN, Erickson SM. Proximal biceps tendon rupture: primarily an injury of middle age. Phys Sportsmed. 1999;27:95-101.
6. Elser F, Braun S, Dewing CB, et al. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27:581-592.
7. Kelly MP, Perkinson SG, Ablove RH, et al. Distal biceps tendon ruptures: an epidemiological analysis using a large population database. Am J Sports Med. 2015;43:2012-2017.
8. Schneider A, Bennett JM, O’Connor DP, et al. Bilateral ruptures of the distal biceps brachii tendon. J Shoulder Elbow Surg. 2009;18:804-807.
9. Sethi N, Wright R, Yamaguchi K. Disorders of the long head of the biceps tendon. J Shoulder Elbow Surg. 1999;8:644-654.
10. The Physician and Sportsmedicine. Complete rupture of large tendons. Risk factors, signs, and definitive treatment. Available at: https://orthony.com/directory/uploads/flik_complete-rupture-of-large-tendons.pdf. Accessed December 8, 2017.
11. Pearl ML, Bessos K, Wong K. Strength deficits related to distal biceps tendon rupture and repair. A case report. Am J Sports Med. 1998;26:295-296.
12. Deutch SR, Gelineck J, Johannsen HV, et al. Permanent disabilities in the displaced muscle from rupture of the long head tendon of the biceps. Scand J Med Sci Sports. 2005;15:159-162.
13. Tangari M, Carbone S, Gallo M, et al. Long head of the biceps tendon rupture in professional wrestlers: treatment with a mini-open tenodesis. J Shoulder Elbow Surg. 2011;20:409-413.
14. Hsu AR, Ghodadra NS, Provencher MT, et al. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20:326-332.
15. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20:e7-e11.
THE CASE
A 41-year-old, right-hand dominant man sought care at our facility one day after trying to pull his boat out of the water. He’d tried to lift the boat with his hands while his forearms were fully supinated and his elbows were flexed to about 90°. He then felt a sharp burning sensation in his left anterior shoulder and was unable to lift the boat. The patient denied feeling a popping sensation at the time of the injury. He had mild pain at night, but was able to sleep. He said that he had mild diminished strength with elbow flexion, but denied having any numbness, tingling, or discoloration of his skin.
The patient said he did weightlifting and strength training of his upper and lower extremities 4 times/week. He was in good general health, was not taking any medications or supplements, and denied smoking or using illicit drugs. His surgical history was significant for a Bankart repair 8 years ago.
On physical examination, the patient had a scar from the previous surgery, a hollow area in his left anterior shoulder, and a prominent biceps muscle belly (FIGURE). His shoulder range of motion was normal. Left shoulder Neer, Hawkins-Kennedy, drop-arm, cross-arm, empty can, and apprehension tests were negative. A left Speed’s test (resisted elbow flexion when elbow is flexed 20° to 30° with the forearm in supination and the arm in about 60° of flexion) was positive for mild anterior shoulder pain. So, too, was a Yergason’s test (resisted forearm supination and elbow flexion when forearm is pronated and elbow is flexed to 90°). The patient’s elbow flexion strength was 4 out of 5, and his supination strength was 5 out of 5. Neurovascular and sensory examinations of his upper extremities, including radial and ulnar pulses, were normal.
THE DIAGNOSIS
A diagnostic musculoskeletal ultrasound revealed an empty tendon sheath of the long head of the biceps in the bicipital groove and a retracted echogenic stump with associated hematoma at the proximal musculotendinous junction. Based on the patient’s history, physical examination, and ultrasound, a diagnosis of an acute rupture of the left long head of the biceps brachii tendon was made.
DISCUSSION
Diagnosis of acute rupture is often made clinically based on a visually apparent defect proximally and a bulbous mass distally (“Popeye deformity”).1 Ultrasound and magnetic resonance imaging (MRI) may aid in the diagnosis by demonstrating an absence of the long head in the bicipital groove or at its insertion.
The biceps brachii tendon functions in flexion and supination of the forearm. The long head of the biceps also plays a stabilizing role in the glenohumeral joint during elbow flexion and supination.2 Injury to the biceps most often occurs in middle-aged men following a traumatic sudden eccentric bicipital contraction event, during which most patients describe a snapping or popping sensation.3,4
Rupture of the proximal biceps tendon represents about 90% of all biceps ruptures, which almost exclusively involve the long head of the biceps.3,5,6 Risk factors for tendon rupture include obesity, smoking, steroid injection in or around the tendon, and previous tendinopathy.7-10
Functional limitations. It is generally thought that functional limitations following a proximal biceps rupture are relatively minimal, due to the work of other flexors and supinators, including the brachialis and brachioradialis. However, because strength and endurance of the muscle can decrease by about 25%, physical laborers and high-demand athletes may notice a degree of residual weakness with supination and elbow flexion.11,12
Surgery is suitable for some, but not all
Surgical repair is recommended for acute ruptures in patients with high physical demands and for whom a slight loss of flexion and supination strength would not be well tolerated.13 Tenotomy and tenodesis are the main techniques used to surgically repair a rupture of the long head of the biceps brachii tendon. Although there is no consensus on which technique is superior, it seems that there is less cosmetic deformity and better post-surgery biomechanical strength with tenodesis compared with tenotomy.14 However, tenodesis is associated with a higher likelihood of bicipital pain,14 and recent case reports have suggested it is associated with an increased risk of humeral fracture.15 Therefore, each patient should be treated on an individual case basis, taking into account age, activity level, and physical demand.14
For most patients, treatment remains conservative with typically excellent outcomes. Nonoperative management includes gentle range-of-motion exercises for the prevention of contractures of the elbow and shoulder. Such exercises can be started almost immediately after injury. In one study, nonoperative management was recommended for patients with sedentary work, injury in the non-dominant arm, and acceptable cosmetic deformity. Researchers noted that patients who opt for a nonsurgical treatment generally do well with a home exercise program and rarely have stiffness.1
If the patient is a young athlete, if cosmetic deformity is unacceptable, or if the injury is in the dominant arm of a laborer, then the patient may want to consider tenodesis.1 Tangari et al found that in high-demand athletes, biceps tenodesis resulted in excellent functional and cosmetic results with no clinically significant decrease in strength after an average follow-up of 7.6 years.13 In a case s
Our patient elected to proceed with a tenodesis procedure. Two months after the surgery, he had fully recovered.
THE TAKEAWAY
Rupture of the biceps brachii tendon is relatively uncommon. In the vast majority of cases, it happens in the long head of the dominant arm of middle-aged men. Diagnosis is mainly clinical; however, ultrasound and MRI can confirm the diagnosis when there is doubt. Nonoperative management is appropriate for the majority of patients. Young athletes, patients who are concerned with cosmetic appearance, and labor workers with injury to their dominant arm should be referred to an orthopedic surgeon for possible surgery.
THE CASE
A 41-year-old, right-hand dominant man sought care at our facility one day after trying to pull his boat out of the water. He’d tried to lift the boat with his hands while his forearms were fully supinated and his elbows were flexed to about 90°. He then felt a sharp burning sensation in his left anterior shoulder and was unable to lift the boat. The patient denied feeling a popping sensation at the time of the injury. He had mild pain at night, but was able to sleep. He said that he had mild diminished strength with elbow flexion, but denied having any numbness, tingling, or discoloration of his skin.
The patient said he did weightlifting and strength training of his upper and lower extremities 4 times/week. He was in good general health, was not taking any medications or supplements, and denied smoking or using illicit drugs. His surgical history was significant for a Bankart repair 8 years ago.
On physical examination, the patient had a scar from the previous surgery, a hollow area in his left anterior shoulder, and a prominent biceps muscle belly (FIGURE). His shoulder range of motion was normal. Left shoulder Neer, Hawkins-Kennedy, drop-arm, cross-arm, empty can, and apprehension tests were negative. A left Speed’s test (resisted elbow flexion when elbow is flexed 20° to 30° with the forearm in supination and the arm in about 60° of flexion) was positive for mild anterior shoulder pain. So, too, was a Yergason’s test (resisted forearm supination and elbow flexion when forearm is pronated and elbow is flexed to 90°). The patient’s elbow flexion strength was 4 out of 5, and his supination strength was 5 out of 5. Neurovascular and sensory examinations of his upper extremities, including radial and ulnar pulses, were normal.
THE DIAGNOSIS
A diagnostic musculoskeletal ultrasound revealed an empty tendon sheath of the long head of the biceps in the bicipital groove and a retracted echogenic stump with associated hematoma at the proximal musculotendinous junction. Based on the patient’s history, physical examination, and ultrasound, a diagnosis of an acute rupture of the left long head of the biceps brachii tendon was made.
DISCUSSION
Diagnosis of acute rupture is often made clinically based on a visually apparent defect proximally and a bulbous mass distally (“Popeye deformity”).1 Ultrasound and magnetic resonance imaging (MRI) may aid in the diagnosis by demonstrating an absence of the long head in the bicipital groove or at its insertion.
The biceps brachii tendon functions in flexion and supination of the forearm. The long head of the biceps also plays a stabilizing role in the glenohumeral joint during elbow flexion and supination.2 Injury to the biceps most often occurs in middle-aged men following a traumatic sudden eccentric bicipital contraction event, during which most patients describe a snapping or popping sensation.3,4
Rupture of the proximal biceps tendon represents about 90% of all biceps ruptures, which almost exclusively involve the long head of the biceps.3,5,6 Risk factors for tendon rupture include obesity, smoking, steroid injection in or around the tendon, and previous tendinopathy.7-10
Functional limitations. It is generally thought that functional limitations following a proximal biceps rupture are relatively minimal, due to the work of other flexors and supinators, including the brachialis and brachioradialis. However, because strength and endurance of the muscle can decrease by about 25%, physical laborers and high-demand athletes may notice a degree of residual weakness with supination and elbow flexion.11,12
Surgery is suitable for some, but not all
Surgical repair is recommended for acute ruptures in patients with high physical demands and for whom a slight loss of flexion and supination strength would not be well tolerated.13 Tenotomy and tenodesis are the main techniques used to surgically repair a rupture of the long head of the biceps brachii tendon. Although there is no consensus on which technique is superior, it seems that there is less cosmetic deformity and better post-surgery biomechanical strength with tenodesis compared with tenotomy.14 However, tenodesis is associated with a higher likelihood of bicipital pain,14 and recent case reports have suggested it is associated with an increased risk of humeral fracture.15 Therefore, each patient should be treated on an individual case basis, taking into account age, activity level, and physical demand.14
For most patients, treatment remains conservative with typically excellent outcomes. Nonoperative management includes gentle range-of-motion exercises for the prevention of contractures of the elbow and shoulder. Such exercises can be started almost immediately after injury. In one study, nonoperative management was recommended for patients with sedentary work, injury in the non-dominant arm, and acceptable cosmetic deformity. Researchers noted that patients who opt for a nonsurgical treatment generally do well with a home exercise program and rarely have stiffness.1
If the patient is a young athlete, if cosmetic deformity is unacceptable, or if the injury is in the dominant arm of a laborer, then the patient may want to consider tenodesis.1 Tangari et al found that in high-demand athletes, biceps tenodesis resulted in excellent functional and cosmetic results with no clinically significant decrease in strength after an average follow-up of 7.6 years.13 In a case s
Our patient elected to proceed with a tenodesis procedure. Two months after the surgery, he had fully recovered.
THE TAKEAWAY
Rupture of the biceps brachii tendon is relatively uncommon. In the vast majority of cases, it happens in the long head of the dominant arm of middle-aged men. Diagnosis is mainly clinical; however, ultrasound and MRI can confirm the diagnosis when there is doubt. Nonoperative management is appropriate for the majority of patients. Young athletes, patients who are concerned with cosmetic appearance, and labor workers with injury to their dominant arm should be referred to an orthopedic surgeon for possible surgery.
1. Geaney LE, Mazzocca AD. Biceps brachii tendon ruptures: a review of diagnosis and treatment of proximal and distal biceps tendon ruptures. Phys Sportsmed. 2010;38:117-125.
2. Payne LZ, Deng XH, Craig EV, et al. The combined dynamic and static contributions to subacromial impingement. A biomechanical analysis. Am J Sports Med. 1997;25:801-808.
3. Jayamoorthy T, Field JR, Costi JJ, et al. Biceps tenodesis: a biomechanical study of fixation methods. J Shoulder Elbow Surg. 2004;13:160-164.
4. Mazzocca AD, Spang JT, Arciero RA. Distal biceps rupture. Orthop Clin North Am. 2008;39:237-249, vii.
5. Carter AN, Erickson SM. Proximal biceps tendon rupture: primarily an injury of middle age. Phys Sportsmed. 1999;27:95-101.
6. Elser F, Braun S, Dewing CB, et al. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27:581-592.
7. Kelly MP, Perkinson SG, Ablove RH, et al. Distal biceps tendon ruptures: an epidemiological analysis using a large population database. Am J Sports Med. 2015;43:2012-2017.
8. Schneider A, Bennett JM, O’Connor DP, et al. Bilateral ruptures of the distal biceps brachii tendon. J Shoulder Elbow Surg. 2009;18:804-807.
9. Sethi N, Wright R, Yamaguchi K. Disorders of the long head of the biceps tendon. J Shoulder Elbow Surg. 1999;8:644-654.
10. The Physician and Sportsmedicine. Complete rupture of large tendons. Risk factors, signs, and definitive treatment. Available at: https://orthony.com/directory/uploads/flik_complete-rupture-of-large-tendons.pdf. Accessed December 8, 2017.
11. Pearl ML, Bessos K, Wong K. Strength deficits related to distal biceps tendon rupture and repair. A case report. Am J Sports Med. 1998;26:295-296.
12. Deutch SR, Gelineck J, Johannsen HV, et al. Permanent disabilities in the displaced muscle from rupture of the long head tendon of the biceps. Scand J Med Sci Sports. 2005;15:159-162.
13. Tangari M, Carbone S, Gallo M, et al. Long head of the biceps tendon rupture in professional wrestlers: treatment with a mini-open tenodesis. J Shoulder Elbow Surg. 2011;20:409-413.
14. Hsu AR, Ghodadra NS, Provencher MT, et al. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20:326-332.
15. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20:e7-e11.
1. Geaney LE, Mazzocca AD. Biceps brachii tendon ruptures: a review of diagnosis and treatment of proximal and distal biceps tendon ruptures. Phys Sportsmed. 2010;38:117-125.
2. Payne LZ, Deng XH, Craig EV, et al. The combined dynamic and static contributions to subacromial impingement. A biomechanical analysis. Am J Sports Med. 1997;25:801-808.
3. Jayamoorthy T, Field JR, Costi JJ, et al. Biceps tenodesis: a biomechanical study of fixation methods. J Shoulder Elbow Surg. 2004;13:160-164.
4. Mazzocca AD, Spang JT, Arciero RA. Distal biceps rupture. Orthop Clin North Am. 2008;39:237-249, vii.
5. Carter AN, Erickson SM. Proximal biceps tendon rupture: primarily an injury of middle age. Phys Sportsmed. 1999;27:95-101.
6. Elser F, Braun S, Dewing CB, et al. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27:581-592.
7. Kelly MP, Perkinson SG, Ablove RH, et al. Distal biceps tendon ruptures: an epidemiological analysis using a large population database. Am J Sports Med. 2015;43:2012-2017.
8. Schneider A, Bennett JM, O’Connor DP, et al. Bilateral ruptures of the distal biceps brachii tendon. J Shoulder Elbow Surg. 2009;18:804-807.
9. Sethi N, Wright R, Yamaguchi K. Disorders of the long head of the biceps tendon. J Shoulder Elbow Surg. 1999;8:644-654.
10. The Physician and Sportsmedicine. Complete rupture of large tendons. Risk factors, signs, and definitive treatment. Available at: https://orthony.com/directory/uploads/flik_complete-rupture-of-large-tendons.pdf. Accessed December 8, 2017.
11. Pearl ML, Bessos K, Wong K. Strength deficits related to distal biceps tendon rupture and repair. A case report. Am J Sports Med. 1998;26:295-296.
12. Deutch SR, Gelineck J, Johannsen HV, et al. Permanent disabilities in the displaced muscle from rupture of the long head tendon of the biceps. Scand J Med Sci Sports. 2005;15:159-162.
13. Tangari M, Carbone S, Gallo M, et al. Long head of the biceps tendon rupture in professional wrestlers: treatment with a mini-open tenodesis. J Shoulder Elbow Surg. 2011;20:409-413.
14. Hsu AR, Ghodadra NS, Provencher MT, et al. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20:326-332.
15. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20:e7-e11.
A lump on the hip
A 42-year-old man presented with a lump on the side of his left hip, which had developed after he fell on his hip while playing basketball about 2 weeks earlier. He was able to continue playing and finished the game. After the game he noticed a lump, which rapidly increased in size. Significant bruising developed afterwards, and the area was mildly painful. The lump did not interfere with his daily activities, but it was annoying.
THE DIFFERENTIAL DIAGNOSIS
Morel-Lavallée lesion is an uncommon condition resulting from shearing trauma and collection of fluid in the space between deep fatty tissue and superficial fascia.6 It is usually the result of severe trauma, as in a motor vehicle accident, but it can also result from sports-related trauma, as in our patient.6–8 Lateral hip, gluteal, and sacral regions are the most common locations for Morel-Lavallée lesions and are often associated with an underlying fracture.6,9
Morel-Lavallée lesions usually develop hours or days after trauma, although they may develop weeks or even months later.2 Symptoms include bulging, pain, and loss of cutaneous sensation over the affected area. Although ultrasonography can be used, magnetic resonance imaging (MRI) is the gold standard for diagnosis and staging.6,10 If there is concern for fracture, plain radiography should be performed.
Mellado and Bencardino classified Morel-Lavallée lesions into 6 types based on their morphology, presence or absence of a capsule, signal behavior on MRI, and enhancement pattern.10 The exact rate of infection in patients with Morel-Lavallée lesions is unknown; however, the risk of infection seems to be highest after surgical intervention or aspiration.5,6
Another potential complication is fluid reaccumulation, which most often occurs with large lesions (> 50 mL) and lesions with a fibrous capsule or pseudocapsule.5 Large lesions can compromise adjacent neurovascular structures, particularly in the extremities.5 Potential consequences include dermal necrosis, compartment syndrome, and tissue necrosis.5
MANAGEMENT APPROACH
Aspiration of a fluid-filled mass is useful in both diagnosis and management of Morel-Lavallée lesions. Treatment includes watchful waiting; compression and pressure wraps; injection of a sclerosing agent (eg, doxycyline, alcohol); needle aspiration; percutaneous drainage with debridement, irrigation, and suction; and incision and evacuation.6
The approach to treatment depends on the stage of the lesion and whether an underlying fracture is present. Depending on the amount of blood and lymphatic products and the acuity of the collected fluid (hours to days post-trauma), aspiration with a large-bore needle (eg, 14 to 22 gauge) may or may not be successful.7 In general, traumatic serosanguinous fluid collections are less painful and resolve faster than well-formed coagulated hematomas.
Patients who have a large lesion, significant pain, or decreased range of motion should be referred to an orthopedic surgeon.
Our patient was managed conservatively, and his symptoms completely resolved in 2 months.
- Ahmad Z, Tibrewal S, Waters G, Nolan J. Solitary amyloidoma related to THA. Orthopedics 2013; 36:e971–e973.
- Harris-Spinks C, Nabhan D, Khodaee M. Noniatrogenic septic olecranon bursitis: report of two cases and review of the literature. Curr Sports Med Rep 2016; 15:33–37.
- Price MD, Busconi BD, McMillan S. Proximal femur fractures. In: Miller MD, Sanders TG, eds. Presentation, Imaging and Treatment of Common Musculoskeletal Conditions. Philadelphia, PA: Saunders; 2011:365–376.
- Stanton MC, Maloney MD, Dehaven KE, Giordano BD. Acute traumatic tear of gluteus medius and minimus tendons in a patient without antecedant peritrochanteric hip pain. Geriatr Orthop Surg Rehabil 2012; 3:84–88.
- Khodaee M, Deu RS, Mathern S, Bravman JT. Morel-Lavallée lesion in sports. Curr Sports Med Rep 2016; 15:417–422.
- Bonilla-Yoon I, Masih S, Patel DB, et al. The Morel-Lavallée lesion: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol 2014; 21:35–43.
- Khodaee M, Deu RS. Ankle Morel-Lavallée lesion in a recreational racquetball player. J Sports Med Phys Fitness 2016. Epub ahead of print.
- Shmerling A, Bravman JT, Khodaee M. Morel-Lavallée lesion of the knee in a recreational frisbee player. Case Rep Orthop 2016; 2016:8723489.
- Miller J, Daggett J, Ambay R, Payne WG. Morel-Lavallée lesion. Eplasty 2014; 14:ic12.
- Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am 2005; 13:775–782.
A 42-year-old man presented with a lump on the side of his left hip, which had developed after he fell on his hip while playing basketball about 2 weeks earlier. He was able to continue playing and finished the game. After the game he noticed a lump, which rapidly increased in size. Significant bruising developed afterwards, and the area was mildly painful. The lump did not interfere with his daily activities, but it was annoying.
THE DIFFERENTIAL DIAGNOSIS
Morel-Lavallée lesion is an uncommon condition resulting from shearing trauma and collection of fluid in the space between deep fatty tissue and superficial fascia.6 It is usually the result of severe trauma, as in a motor vehicle accident, but it can also result from sports-related trauma, as in our patient.6–8 Lateral hip, gluteal, and sacral regions are the most common locations for Morel-Lavallée lesions and are often associated with an underlying fracture.6,9
Morel-Lavallée lesions usually develop hours or days after trauma, although they may develop weeks or even months later.2 Symptoms include bulging, pain, and loss of cutaneous sensation over the affected area. Although ultrasonography can be used, magnetic resonance imaging (MRI) is the gold standard for diagnosis and staging.6,10 If there is concern for fracture, plain radiography should be performed.
Mellado and Bencardino classified Morel-Lavallée lesions into 6 types based on their morphology, presence or absence of a capsule, signal behavior on MRI, and enhancement pattern.10 The exact rate of infection in patients with Morel-Lavallée lesions is unknown; however, the risk of infection seems to be highest after surgical intervention or aspiration.5,6
Another potential complication is fluid reaccumulation, which most often occurs with large lesions (> 50 mL) and lesions with a fibrous capsule or pseudocapsule.5 Large lesions can compromise adjacent neurovascular structures, particularly in the extremities.5 Potential consequences include dermal necrosis, compartment syndrome, and tissue necrosis.5
MANAGEMENT APPROACH
Aspiration of a fluid-filled mass is useful in both diagnosis and management of Morel-Lavallée lesions. Treatment includes watchful waiting; compression and pressure wraps; injection of a sclerosing agent (eg, doxycyline, alcohol); needle aspiration; percutaneous drainage with debridement, irrigation, and suction; and incision and evacuation.6
The approach to treatment depends on the stage of the lesion and whether an underlying fracture is present. Depending on the amount of blood and lymphatic products and the acuity of the collected fluid (hours to days post-trauma), aspiration with a large-bore needle (eg, 14 to 22 gauge) may or may not be successful.7 In general, traumatic serosanguinous fluid collections are less painful and resolve faster than well-formed coagulated hematomas.
Patients who have a large lesion, significant pain, or decreased range of motion should be referred to an orthopedic surgeon.
Our patient was managed conservatively, and his symptoms completely resolved in 2 months.
A 42-year-old man presented with a lump on the side of his left hip, which had developed after he fell on his hip while playing basketball about 2 weeks earlier. He was able to continue playing and finished the game. After the game he noticed a lump, which rapidly increased in size. Significant bruising developed afterwards, and the area was mildly painful. The lump did not interfere with his daily activities, but it was annoying.
THE DIFFERENTIAL DIAGNOSIS
Morel-Lavallée lesion is an uncommon condition resulting from shearing trauma and collection of fluid in the space between deep fatty tissue and superficial fascia.6 It is usually the result of severe trauma, as in a motor vehicle accident, but it can also result from sports-related trauma, as in our patient.6–8 Lateral hip, gluteal, and sacral regions are the most common locations for Morel-Lavallée lesions and are often associated with an underlying fracture.6,9
Morel-Lavallée lesions usually develop hours or days after trauma, although they may develop weeks or even months later.2 Symptoms include bulging, pain, and loss of cutaneous sensation over the affected area. Although ultrasonography can be used, magnetic resonance imaging (MRI) is the gold standard for diagnosis and staging.6,10 If there is concern for fracture, plain radiography should be performed.
Mellado and Bencardino classified Morel-Lavallée lesions into 6 types based on their morphology, presence or absence of a capsule, signal behavior on MRI, and enhancement pattern.10 The exact rate of infection in patients with Morel-Lavallée lesions is unknown; however, the risk of infection seems to be highest after surgical intervention or aspiration.5,6
Another potential complication is fluid reaccumulation, which most often occurs with large lesions (> 50 mL) and lesions with a fibrous capsule or pseudocapsule.5 Large lesions can compromise adjacent neurovascular structures, particularly in the extremities.5 Potential consequences include dermal necrosis, compartment syndrome, and tissue necrosis.5
MANAGEMENT APPROACH
Aspiration of a fluid-filled mass is useful in both diagnosis and management of Morel-Lavallée lesions. Treatment includes watchful waiting; compression and pressure wraps; injection of a sclerosing agent (eg, doxycyline, alcohol); needle aspiration; percutaneous drainage with debridement, irrigation, and suction; and incision and evacuation.6
The approach to treatment depends on the stage of the lesion and whether an underlying fracture is present. Depending on the amount of blood and lymphatic products and the acuity of the collected fluid (hours to days post-trauma), aspiration with a large-bore needle (eg, 14 to 22 gauge) may or may not be successful.7 In general, traumatic serosanguinous fluid collections are less painful and resolve faster than well-formed coagulated hematomas.
Patients who have a large lesion, significant pain, or decreased range of motion should be referred to an orthopedic surgeon.
Our patient was managed conservatively, and his symptoms completely resolved in 2 months.
- Ahmad Z, Tibrewal S, Waters G, Nolan J. Solitary amyloidoma related to THA. Orthopedics 2013; 36:e971–e973.
- Harris-Spinks C, Nabhan D, Khodaee M. Noniatrogenic septic olecranon bursitis: report of two cases and review of the literature. Curr Sports Med Rep 2016; 15:33–37.
- Price MD, Busconi BD, McMillan S. Proximal femur fractures. In: Miller MD, Sanders TG, eds. Presentation, Imaging and Treatment of Common Musculoskeletal Conditions. Philadelphia, PA: Saunders; 2011:365–376.
- Stanton MC, Maloney MD, Dehaven KE, Giordano BD. Acute traumatic tear of gluteus medius and minimus tendons in a patient without antecedant peritrochanteric hip pain. Geriatr Orthop Surg Rehabil 2012; 3:84–88.
- Khodaee M, Deu RS, Mathern S, Bravman JT. Morel-Lavallée lesion in sports. Curr Sports Med Rep 2016; 15:417–422.
- Bonilla-Yoon I, Masih S, Patel DB, et al. The Morel-Lavallée lesion: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol 2014; 21:35–43.
- Khodaee M, Deu RS. Ankle Morel-Lavallée lesion in a recreational racquetball player. J Sports Med Phys Fitness 2016. Epub ahead of print.
- Shmerling A, Bravman JT, Khodaee M. Morel-Lavallée lesion of the knee in a recreational frisbee player. Case Rep Orthop 2016; 2016:8723489.
- Miller J, Daggett J, Ambay R, Payne WG. Morel-Lavallée lesion. Eplasty 2014; 14:ic12.
- Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am 2005; 13:775–782.
- Ahmad Z, Tibrewal S, Waters G, Nolan J. Solitary amyloidoma related to THA. Orthopedics 2013; 36:e971–e973.
- Harris-Spinks C, Nabhan D, Khodaee M. Noniatrogenic septic olecranon bursitis: report of two cases and review of the literature. Curr Sports Med Rep 2016; 15:33–37.
- Price MD, Busconi BD, McMillan S. Proximal femur fractures. In: Miller MD, Sanders TG, eds. Presentation, Imaging and Treatment of Common Musculoskeletal Conditions. Philadelphia, PA: Saunders; 2011:365–376.
- Stanton MC, Maloney MD, Dehaven KE, Giordano BD. Acute traumatic tear of gluteus medius and minimus tendons in a patient without antecedant peritrochanteric hip pain. Geriatr Orthop Surg Rehabil 2012; 3:84–88.
- Khodaee M, Deu RS, Mathern S, Bravman JT. Morel-Lavallée lesion in sports. Curr Sports Med Rep 2016; 15:417–422.
- Bonilla-Yoon I, Masih S, Patel DB, et al. The Morel-Lavallée lesion: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol 2014; 21:35–43.
- Khodaee M, Deu RS. Ankle Morel-Lavallée lesion in a recreational racquetball player. J Sports Med Phys Fitness 2016. Epub ahead of print.
- Shmerling A, Bravman JT, Khodaee M. Morel-Lavallée lesion of the knee in a recreational frisbee player. Case Rep Orthop 2016; 2016:8723489.
- Miller J, Daggett J, Ambay R, Payne WG. Morel-Lavallée lesion. Eplasty 2014; 14:ic12.
- Mellado JM, Bencardino JT. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am 2005; 13:775–782.
A look at the burden of opioid management in primary care
ABSTRACT
Purpose Pain management with opioids in primary care is challenging. The objective of this study was to identify the number of opioid-related tasks in our clinics and determine whether opioid-related tasks occur more often in a residency setting.
Methods This was a retrospective observational review of an electronic health record (EHR) system to evaluate tasks related to the use of opioids and other controlled substances. Tasks are created in the EHR when patients call the clinic; the task-box system is a means of communication within the EHR. The study setting was 2 university-based family medicine clinics. Clinic 1 has faculty and resident providers in an urban area. Clinic 2 has only faculty providers in a suburban area. We reviewed all tasks recorded in November 2010.
Results A total of 3193 patients were seen at the clinics. In addition, 1028 call-related tasks were created, 220 of which (21.4%) were opioid-related. More than half of the tasks were about chronic (ongoing) patient issues. More than one‑third of the tasks required follow-up phone calls. Multiple logistic regression analysis showed more opioid-related tasks in the residency setting (Clinic 1) compared with the nonresidency setting (Clinic 2), (23.1% vs 16.7%; P<.001). However, multiple logistic regression analysis did not show any correlations between opioid-related tasks and who addressed the tasks or the day tasks were created.
Conclusions Primary care physicians prescribe significant amounts of opioids. Due to the nature of opioid use and abuse, a well-planned protocol customized to the practice or institution is required to streamline this process and decrease the number of unnecessary phone calls and follow-ups.
Pain management with opioids in primary care is challenging,1,2 and many physicians find it unsatisfying and burdensome.3 More than 60 million patient visits for chronic pain occur annually in the United States, consuming large amounts of time and resources.4 Contributing to the challenge is the need to ensure patient safety and satisfaction, as well as staff satisfaction with pain management.5-8 Opioid-related death is a major cause of iatrogenic mortality in the United States:9,10 From 1999 to 2006, fatal opioid-involved intoxications more than tripled from 4000 to 13,800.7
At issue for many providers, as well as patients and staff, is dissatisfaction with current systems in place for managing chronic non-cancer pain with opioids.2,3,8,11 In developing this study, we decided to focus on the systems aspect of care with 2 primary outcome measures in mind. Specifically, we sought to identify the tasks related to managing opioids and other controlled substances in 2 primary care clinics in a university-based family medicine program and to determine what proportion of all routine tasks in these 2 clinics could be attributed to opioid-related issues. With our secondary outcome measures, we sought to compare the number of opioid-related tasks in the residency setting with those in a nonresidency setting, and to identify factors that might be associated with an increase in the number of opioid-related tasks.
METHODS
Setting and design
We conducted a retrospective observational pilot study reviewing our electronic health record (EHR) system (Allscripts TouchWorks) at 2 of our outpatient family medicine clinics at the University of Colorado. When patients call the clinics, or when patient-care-related concerns need to be addressed, an electronic task message is created and sent to the appropriate task box for staff or provider response. The task box system is how staff and providers communicate within the EHR. Each provider has a personal task box, and there are other task boxes in the system (eg, triage, medication refill) for urgent and non-urgent patient care issues.
For example, when a patient calls to request a refill, a medical assistant (MA), care team assistant (CTA), or nurse will create a task for the medication refill box. If the task is urgent, it is marked with a red asterisk and a triage provider will address the task that same day. Non-urgent triage tasks will be addressed by the patient’s primary care provider within 2 to 3 days. Depending on the issue at hand, the task may or may not require phone calls to the patient, pharmacy, or insurance company.
Clinic 1, in urban Denver, has 13 physicians (many of them part-time clinical faculty), one nurse practitioner (NP), one physician assistant (PA), and 18 family medicine residents. Clinic 2, in a suburb of Denver, has 5 physicians (only one is part-time) and one nurse practitioner. Clinic 1 is divided into 3 pods, and each has the same number of attending physicians, residents, and MAs, and either a PA or NP.
We reviewed, one by one, all tasks created from November 1 to 30, 2010. One of the study’s investigators categorized each task according to the following descriptors: who created the task, who addressed the task, what day of the week the task was created, urgency of the task, whether the task required a follow-up phone call, and whether the task was related to opioid/controlled-substance issues. The task was categorized as acute if the issue was related to a condition that had been present for fewer than 3 weeks. Chronic tasks were created for conditions present for ≥3 weeks. At the time the study was completed, our EHR had no portal through which we could communicate with patients.
ANALYSIS
We conducted statistical analyses with the IBM SPSS, version 22.0 (SPSS, Inc, Chicago, Illinois). We used descriptive statistics to examine the frequency and percentage for all variables. We used a chi-squared (χ2) test to assess the differences between the 2 clinics, and used a binary multiple logistic regression model to determine possible factors related to opioid-related tasks. P values <.05 were considered statistically significant. The Colorado Multiple Institutional Review Board approved this study.
RESULTS
Clinics 1 and 2, respectively, saw 2007 and 1186 patients during the study period (TABLE 1). The additional 1028 tasks generated by phone calls were almost equally distributed among the 3 pods of Clinic 1 (290, 202, and 260) and Clinic 2 (276). For data analysis, we compared Clinic 1 with Clinic 2 and also compared the 3 pods of Clinic 1 individually with Clinic 2. Both approaches produced similar results.
Most tasks (54% for Clinic 1 and 99% for Clinic 2) were created by MAs and CTAs. At Clinic 1, tasks were also created by residents (17%), PA/NPs (8%), attending physicians (7%), and others/clinical nurses (14%). Tasks at Clinic 1 were addressed by attending physicians (49%), residents (25%), PA/NPs (25%), and others (1%). At Clinic 2, tasks were addressed by attending physicians (75%) and PA/NPs (25%). Approximately half of the tasks (51%) in both clinics were created during weekdays, compared with the day after weekends/holidays (28%), the day before weekends/holidays (17%), and during weekends/holidays (4%). Chronic patient issues, acute patient issues, and other issues accounted for 54%, 29%, and 17% of tasks, respectively. Follow-up phone calls to patients, pharmacies, or others occurred in 37% of tasks. Two hundred twenty tasks (21%) in the clinics combined were related to opioids and controlled substances.
Multiple logistic regression analysis of data from both clinics (TABLE 2) showed more opioid-related tasks in Clinic 1 compared with Clinic 2 (P<.001), and that these tasks were more often related to chronic issues than to acute issues (P<.001). Tasks created by MAs, CTAs, clinical nurses, and others were more likely to be opioid-related compared with the tasks created by attending physicians, residents, NPs, or a PA (25% vs 15%; P<.05). Compared with non-opioid-related tasks, opioid-related tasks required more follow-up phone calls (P<.001). Follow-up phone calls to pharmacies occurred more often with opioid-related tasks than with non-opioid tasks (11% vs 5%), while follow-up phone calls to patients occurred more often for non-opioid related tasks than opioid-related tasks (28% vs 18%). No correlations with task creation were found for who addressed the opioid-related task or the day the task was created.
DISCUSSION
This study demonstrated that our process of handling patient issues related to opioids accounts for a large proportion of all tasks. Dealing with tasks is time consuming, not only for attending physicians and residents but also for clinic nurses and staff. Almost a quarter of clinic tasks were opioid related. As has been shown in previous studies,5-8 chronic pain management with opioids is an unsatisfying task for staff and care providers at our clinics. We also found that tasks created by non-providers were more likely to be opioid-related than were tasks created by providers. This is most likely due to the fact that non-providers cannot write prescriptions and they have to ask providers for further reviews.
Khalid et al found that, compared with attending physicians, residents had more patients on chronic opioids who displayed concerning behaviors, including early refills and refills from multiple providers.13 The higher number of part-time providers at Clinic 1 in our study may have also caused insufficient continuity of care at that site. Nevertheless, this model of practice is used in many academic primary care institutions.4 Another possible reason for the difference could be a lack of resident training on current guidelines for managing opiates for chronic pain.3,13,14 Again, this was a pilot study and we drew no solid conclusion about the reasons for differences between these 2 clinics.
It is obvious, however, that we spend a significant amount of time and resources dealing with chronic pain management. Our institution created an opioid/controlled-substance patient registry about 3 years ago. The data for 2014 showed that 22.8% and 18% of patients seen at least once at Clinic 1 and Clinic 2, respectively, were prescribed opioids/controlled substances (TABLE 3).
Possible solutions to reduce tasks related to opioid management. For both small and large practices, one way to reduce the number of tasks related to opioid management and, therefore, the time allocated to completing those tasks, would be to have a clear protocol to follow.3,4,8,11,14,15 The protocol may include the creation of an opioid/controlled-substance registry and the development and implementation of clinical decision support programs.
We also recommend the dissemination of tools for clinical management at the point of care. These can include a controlled-substance risk assessment tool for aberrant behaviors, a controlled-substance informed consent form, a functional and quality-of-life assessment, electronic clinical-note templates in the EHR, urine drug screening, and routine use of existing state pharmacy prescription drug monitoring programs. Also essential would be the provision of routine educational programs for clinicians regarding chronic pain management based on existing evidence and guidelines. (See “Opioids for chronic pain: The CDC’s 12 recommendations.”) It has been demonstrated that an EHR opioid dashboard or an EHR-based protocol improved adherence to guidelines for prescribing opiates.16
This study has several limitations. First, this was a small pilot study completed over a short period of time, although we believe the findings are likely representative of the prescribing practices in the 2 clinics we evaluated. Second, it was a retrospective study, which was appropriate for evaluating our questions. Third, we were unable to account for other factors that could potentially confound the results, including, but not limited to, the amount of time allocated to each task, and the total number of patients at each clinic who were on opioids for management of chronic pain during the study period. However, due to our recent addition of an opioid/controlled-substance patient registry, we were able to add information for the year 2014 (TABLE 3). Multi-center large scale studies are required to evaluate this further.
ACKNOWLEDGEMENTS
We thank Dr. Corey Lyon for his editorial assistance.
CORRESPONDENCE
Morteza Khodaee, MD, AFW Family Medicine Clinic, 3055 Roslyn Street, Denver, CO 80238; morteza.khodaee@ucdenver.edu.
1. Smith BH, Torrance N. Management of chronic pain in primary care. Curr Opin Support Palliat Care. 2011;5:137-142.
2. Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA. 2012;307:1377-1378.
3. Leverence RR, Williams RL, Potter M, et al; PRIME Net Clinicians. Chronic non-cancer pain: a siren for primary care—a report from the PRImary Care MultiEthnic Network (PRIME Net). J Am Board Fam Med. 2011;24:551-561.
4. Watkins A, Wasmann S, Dodson L, et al. An evaluation of the care provided to patients prescribed controlled substances for chronic nonmalignant pain at an academic family medicine center. Fam Med. 2004;36:487-489.
5. Brown J, Setnik B, Lee K, et al. Assessment, stratification, and monitoring of the risk for prescription opioid misuse and abuse in the primary care setting. J Opioid Manag. 2011;7:467-483.
6. Duensing L, Eksterowicz N, Macario A, et al. Patient and physician perceptions of treatment of moderate-to-severe chronic pain with oral opioids. Curr Med Res Opin. 2010;26:1579-1585.
7. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12:S26-S35.
8. Wenghofer EF, Wilson L, Kahan M, et al. Survey of Ontario primary care physicians’ experiences with opioid prescribing. Can Fam Physician. 2011;57:324-332.
9. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
10. Hartrick CT, Gatchel RJ, Conroy S. Identification and management of pain medication abuse and misuse: current state and future directions. Expert Rev Neurother. 2012;12:601-610.
11. Wiedemer NL, Harden PS, Arndt IO, et al. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8:573-584.
12. Colburn JL, Jasinski DR, Rastegar DA. Long-term opioid therapy, aberrant behaviors, and substance misuse: comparison of patients treated by resident and attending physicians in a general medical clinic. J Opioid Manag. 2012;8:153-160.
13. Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med. 2015;16:480-487.
14. Canada RE, DiRocco D, Day S. A better approach to opioid prescribing in primary care. J Fam Pract. 2014;63:E1-E8.
15. Clark LG, Upshur CC. Family medicine physicians’ views of how to improve chronic pain management. J Am Board Fam Med. 2007;20:479-482.
16. Anderson D, Zlateva I, Khatri K, et al. Using health information technology to improve adherence to opioid prescribing guidelines in primary care. Clin J Pain. 2015;31:573-579.
ABSTRACT
Purpose Pain management with opioids in primary care is challenging. The objective of this study was to identify the number of opioid-related tasks in our clinics and determine whether opioid-related tasks occur more often in a residency setting.
Methods This was a retrospective observational review of an electronic health record (EHR) system to evaluate tasks related to the use of opioids and other controlled substances. Tasks are created in the EHR when patients call the clinic; the task-box system is a means of communication within the EHR. The study setting was 2 university-based family medicine clinics. Clinic 1 has faculty and resident providers in an urban area. Clinic 2 has only faculty providers in a suburban area. We reviewed all tasks recorded in November 2010.
Results A total of 3193 patients were seen at the clinics. In addition, 1028 call-related tasks were created, 220 of which (21.4%) were opioid-related. More than half of the tasks were about chronic (ongoing) patient issues. More than one‑third of the tasks required follow-up phone calls. Multiple logistic regression analysis showed more opioid-related tasks in the residency setting (Clinic 1) compared with the nonresidency setting (Clinic 2), (23.1% vs 16.7%; P<.001). However, multiple logistic regression analysis did not show any correlations between opioid-related tasks and who addressed the tasks or the day tasks were created.
Conclusions Primary care physicians prescribe significant amounts of opioids. Due to the nature of opioid use and abuse, a well-planned protocol customized to the practice or institution is required to streamline this process and decrease the number of unnecessary phone calls and follow-ups.
Pain management with opioids in primary care is challenging,1,2 and many physicians find it unsatisfying and burdensome.3 More than 60 million patient visits for chronic pain occur annually in the United States, consuming large amounts of time and resources.4 Contributing to the challenge is the need to ensure patient safety and satisfaction, as well as staff satisfaction with pain management.5-8 Opioid-related death is a major cause of iatrogenic mortality in the United States:9,10 From 1999 to 2006, fatal opioid-involved intoxications more than tripled from 4000 to 13,800.7
At issue for many providers, as well as patients and staff, is dissatisfaction with current systems in place for managing chronic non-cancer pain with opioids.2,3,8,11 In developing this study, we decided to focus on the systems aspect of care with 2 primary outcome measures in mind. Specifically, we sought to identify the tasks related to managing opioids and other controlled substances in 2 primary care clinics in a university-based family medicine program and to determine what proportion of all routine tasks in these 2 clinics could be attributed to opioid-related issues. With our secondary outcome measures, we sought to compare the number of opioid-related tasks in the residency setting with those in a nonresidency setting, and to identify factors that might be associated with an increase in the number of opioid-related tasks.
METHODS
Setting and design
We conducted a retrospective observational pilot study reviewing our electronic health record (EHR) system (Allscripts TouchWorks) at 2 of our outpatient family medicine clinics at the University of Colorado. When patients call the clinics, or when patient-care-related concerns need to be addressed, an electronic task message is created and sent to the appropriate task box for staff or provider response. The task box system is how staff and providers communicate within the EHR. Each provider has a personal task box, and there are other task boxes in the system (eg, triage, medication refill) for urgent and non-urgent patient care issues.
For example, when a patient calls to request a refill, a medical assistant (MA), care team assistant (CTA), or nurse will create a task for the medication refill box. If the task is urgent, it is marked with a red asterisk and a triage provider will address the task that same day. Non-urgent triage tasks will be addressed by the patient’s primary care provider within 2 to 3 days. Depending on the issue at hand, the task may or may not require phone calls to the patient, pharmacy, or insurance company.
Clinic 1, in urban Denver, has 13 physicians (many of them part-time clinical faculty), one nurse practitioner (NP), one physician assistant (PA), and 18 family medicine residents. Clinic 2, in a suburb of Denver, has 5 physicians (only one is part-time) and one nurse practitioner. Clinic 1 is divided into 3 pods, and each has the same number of attending physicians, residents, and MAs, and either a PA or NP.
We reviewed, one by one, all tasks created from November 1 to 30, 2010. One of the study’s investigators categorized each task according to the following descriptors: who created the task, who addressed the task, what day of the week the task was created, urgency of the task, whether the task required a follow-up phone call, and whether the task was related to opioid/controlled-substance issues. The task was categorized as acute if the issue was related to a condition that had been present for fewer than 3 weeks. Chronic tasks were created for conditions present for ≥3 weeks. At the time the study was completed, our EHR had no portal through which we could communicate with patients.
ANALYSIS
We conducted statistical analyses with the IBM SPSS, version 22.0 (SPSS, Inc, Chicago, Illinois). We used descriptive statistics to examine the frequency and percentage for all variables. We used a chi-squared (χ2) test to assess the differences between the 2 clinics, and used a binary multiple logistic regression model to determine possible factors related to opioid-related tasks. P values <.05 were considered statistically significant. The Colorado Multiple Institutional Review Board approved this study.
RESULTS
Clinics 1 and 2, respectively, saw 2007 and 1186 patients during the study period (TABLE 1). The additional 1028 tasks generated by phone calls were almost equally distributed among the 3 pods of Clinic 1 (290, 202, and 260) and Clinic 2 (276). For data analysis, we compared Clinic 1 with Clinic 2 and also compared the 3 pods of Clinic 1 individually with Clinic 2. Both approaches produced similar results.
Most tasks (54% for Clinic 1 and 99% for Clinic 2) were created by MAs and CTAs. At Clinic 1, tasks were also created by residents (17%), PA/NPs (8%), attending physicians (7%), and others/clinical nurses (14%). Tasks at Clinic 1 were addressed by attending physicians (49%), residents (25%), PA/NPs (25%), and others (1%). At Clinic 2, tasks were addressed by attending physicians (75%) and PA/NPs (25%). Approximately half of the tasks (51%) in both clinics were created during weekdays, compared with the day after weekends/holidays (28%), the day before weekends/holidays (17%), and during weekends/holidays (4%). Chronic patient issues, acute patient issues, and other issues accounted for 54%, 29%, and 17% of tasks, respectively. Follow-up phone calls to patients, pharmacies, or others occurred in 37% of tasks. Two hundred twenty tasks (21%) in the clinics combined were related to opioids and controlled substances.
Multiple logistic regression analysis of data from both clinics (TABLE 2) showed more opioid-related tasks in Clinic 1 compared with Clinic 2 (P<.001), and that these tasks were more often related to chronic issues than to acute issues (P<.001). Tasks created by MAs, CTAs, clinical nurses, and others were more likely to be opioid-related compared with the tasks created by attending physicians, residents, NPs, or a PA (25% vs 15%; P<.05). Compared with non-opioid-related tasks, opioid-related tasks required more follow-up phone calls (P<.001). Follow-up phone calls to pharmacies occurred more often with opioid-related tasks than with non-opioid tasks (11% vs 5%), while follow-up phone calls to patients occurred more often for non-opioid related tasks than opioid-related tasks (28% vs 18%). No correlations with task creation were found for who addressed the opioid-related task or the day the task was created.
DISCUSSION
This study demonstrated that our process of handling patient issues related to opioids accounts for a large proportion of all tasks. Dealing with tasks is time consuming, not only for attending physicians and residents but also for clinic nurses and staff. Almost a quarter of clinic tasks were opioid related. As has been shown in previous studies,5-8 chronic pain management with opioids is an unsatisfying task for staff and care providers at our clinics. We also found that tasks created by non-providers were more likely to be opioid-related than were tasks created by providers. This is most likely due to the fact that non-providers cannot write prescriptions and they have to ask providers for further reviews.
Khalid et al found that, compared with attending physicians, residents had more patients on chronic opioids who displayed concerning behaviors, including early refills and refills from multiple providers.13 The higher number of part-time providers at Clinic 1 in our study may have also caused insufficient continuity of care at that site. Nevertheless, this model of practice is used in many academic primary care institutions.4 Another possible reason for the difference could be a lack of resident training on current guidelines for managing opiates for chronic pain.3,13,14 Again, this was a pilot study and we drew no solid conclusion about the reasons for differences between these 2 clinics.
It is obvious, however, that we spend a significant amount of time and resources dealing with chronic pain management. Our institution created an opioid/controlled-substance patient registry about 3 years ago. The data for 2014 showed that 22.8% and 18% of patients seen at least once at Clinic 1 and Clinic 2, respectively, were prescribed opioids/controlled substances (TABLE 3).
Possible solutions to reduce tasks related to opioid management. For both small and large practices, one way to reduce the number of tasks related to opioid management and, therefore, the time allocated to completing those tasks, would be to have a clear protocol to follow.3,4,8,11,14,15 The protocol may include the creation of an opioid/controlled-substance registry and the development and implementation of clinical decision support programs.
We also recommend the dissemination of tools for clinical management at the point of care. These can include a controlled-substance risk assessment tool for aberrant behaviors, a controlled-substance informed consent form, a functional and quality-of-life assessment, electronic clinical-note templates in the EHR, urine drug screening, and routine use of existing state pharmacy prescription drug monitoring programs. Also essential would be the provision of routine educational programs for clinicians regarding chronic pain management based on existing evidence and guidelines. (See “Opioids for chronic pain: The CDC’s 12 recommendations.”) It has been demonstrated that an EHR opioid dashboard or an EHR-based protocol improved adherence to guidelines for prescribing opiates.16
This study has several limitations. First, this was a small pilot study completed over a short period of time, although we believe the findings are likely representative of the prescribing practices in the 2 clinics we evaluated. Second, it was a retrospective study, which was appropriate for evaluating our questions. Third, we were unable to account for other factors that could potentially confound the results, including, but not limited to, the amount of time allocated to each task, and the total number of patients at each clinic who were on opioids for management of chronic pain during the study period. However, due to our recent addition of an opioid/controlled-substance patient registry, we were able to add information for the year 2014 (TABLE 3). Multi-center large scale studies are required to evaluate this further.
ACKNOWLEDGEMENTS
We thank Dr. Corey Lyon for his editorial assistance.
CORRESPONDENCE
Morteza Khodaee, MD, AFW Family Medicine Clinic, 3055 Roslyn Street, Denver, CO 80238; morteza.khodaee@ucdenver.edu.
ABSTRACT
Purpose Pain management with opioids in primary care is challenging. The objective of this study was to identify the number of opioid-related tasks in our clinics and determine whether opioid-related tasks occur more often in a residency setting.
Methods This was a retrospective observational review of an electronic health record (EHR) system to evaluate tasks related to the use of opioids and other controlled substances. Tasks are created in the EHR when patients call the clinic; the task-box system is a means of communication within the EHR. The study setting was 2 university-based family medicine clinics. Clinic 1 has faculty and resident providers in an urban area. Clinic 2 has only faculty providers in a suburban area. We reviewed all tasks recorded in November 2010.
Results A total of 3193 patients were seen at the clinics. In addition, 1028 call-related tasks were created, 220 of which (21.4%) were opioid-related. More than half of the tasks were about chronic (ongoing) patient issues. More than one‑third of the tasks required follow-up phone calls. Multiple logistic regression analysis showed more opioid-related tasks in the residency setting (Clinic 1) compared with the nonresidency setting (Clinic 2), (23.1% vs 16.7%; P<.001). However, multiple logistic regression analysis did not show any correlations between opioid-related tasks and who addressed the tasks or the day tasks were created.
Conclusions Primary care physicians prescribe significant amounts of opioids. Due to the nature of opioid use and abuse, a well-planned protocol customized to the practice or institution is required to streamline this process and decrease the number of unnecessary phone calls and follow-ups.
Pain management with opioids in primary care is challenging,1,2 and many physicians find it unsatisfying and burdensome.3 More than 60 million patient visits for chronic pain occur annually in the United States, consuming large amounts of time and resources.4 Contributing to the challenge is the need to ensure patient safety and satisfaction, as well as staff satisfaction with pain management.5-8 Opioid-related death is a major cause of iatrogenic mortality in the United States:9,10 From 1999 to 2006, fatal opioid-involved intoxications more than tripled from 4000 to 13,800.7
At issue for many providers, as well as patients and staff, is dissatisfaction with current systems in place for managing chronic non-cancer pain with opioids.2,3,8,11 In developing this study, we decided to focus on the systems aspect of care with 2 primary outcome measures in mind. Specifically, we sought to identify the tasks related to managing opioids and other controlled substances in 2 primary care clinics in a university-based family medicine program and to determine what proportion of all routine tasks in these 2 clinics could be attributed to opioid-related issues. With our secondary outcome measures, we sought to compare the number of opioid-related tasks in the residency setting with those in a nonresidency setting, and to identify factors that might be associated with an increase in the number of opioid-related tasks.
METHODS
Setting and design
We conducted a retrospective observational pilot study reviewing our electronic health record (EHR) system (Allscripts TouchWorks) at 2 of our outpatient family medicine clinics at the University of Colorado. When patients call the clinics, or when patient-care-related concerns need to be addressed, an electronic task message is created and sent to the appropriate task box for staff or provider response. The task box system is how staff and providers communicate within the EHR. Each provider has a personal task box, and there are other task boxes in the system (eg, triage, medication refill) for urgent and non-urgent patient care issues.
For example, when a patient calls to request a refill, a medical assistant (MA), care team assistant (CTA), or nurse will create a task for the medication refill box. If the task is urgent, it is marked with a red asterisk and a triage provider will address the task that same day. Non-urgent triage tasks will be addressed by the patient’s primary care provider within 2 to 3 days. Depending on the issue at hand, the task may or may not require phone calls to the patient, pharmacy, or insurance company.
Clinic 1, in urban Denver, has 13 physicians (many of them part-time clinical faculty), one nurse practitioner (NP), one physician assistant (PA), and 18 family medicine residents. Clinic 2, in a suburb of Denver, has 5 physicians (only one is part-time) and one nurse practitioner. Clinic 1 is divided into 3 pods, and each has the same number of attending physicians, residents, and MAs, and either a PA or NP.
We reviewed, one by one, all tasks created from November 1 to 30, 2010. One of the study’s investigators categorized each task according to the following descriptors: who created the task, who addressed the task, what day of the week the task was created, urgency of the task, whether the task required a follow-up phone call, and whether the task was related to opioid/controlled-substance issues. The task was categorized as acute if the issue was related to a condition that had been present for fewer than 3 weeks. Chronic tasks were created for conditions present for ≥3 weeks. At the time the study was completed, our EHR had no portal through which we could communicate with patients.
ANALYSIS
We conducted statistical analyses with the IBM SPSS, version 22.0 (SPSS, Inc, Chicago, Illinois). We used descriptive statistics to examine the frequency and percentage for all variables. We used a chi-squared (χ2) test to assess the differences between the 2 clinics, and used a binary multiple logistic regression model to determine possible factors related to opioid-related tasks. P values <.05 were considered statistically significant. The Colorado Multiple Institutional Review Board approved this study.
RESULTS
Clinics 1 and 2, respectively, saw 2007 and 1186 patients during the study period (TABLE 1). The additional 1028 tasks generated by phone calls were almost equally distributed among the 3 pods of Clinic 1 (290, 202, and 260) and Clinic 2 (276). For data analysis, we compared Clinic 1 with Clinic 2 and also compared the 3 pods of Clinic 1 individually with Clinic 2. Both approaches produced similar results.
Most tasks (54% for Clinic 1 and 99% for Clinic 2) were created by MAs and CTAs. At Clinic 1, tasks were also created by residents (17%), PA/NPs (8%), attending physicians (7%), and others/clinical nurses (14%). Tasks at Clinic 1 were addressed by attending physicians (49%), residents (25%), PA/NPs (25%), and others (1%). At Clinic 2, tasks were addressed by attending physicians (75%) and PA/NPs (25%). Approximately half of the tasks (51%) in both clinics were created during weekdays, compared with the day after weekends/holidays (28%), the day before weekends/holidays (17%), and during weekends/holidays (4%). Chronic patient issues, acute patient issues, and other issues accounted for 54%, 29%, and 17% of tasks, respectively. Follow-up phone calls to patients, pharmacies, or others occurred in 37% of tasks. Two hundred twenty tasks (21%) in the clinics combined were related to opioids and controlled substances.
Multiple logistic regression analysis of data from both clinics (TABLE 2) showed more opioid-related tasks in Clinic 1 compared with Clinic 2 (P<.001), and that these tasks were more often related to chronic issues than to acute issues (P<.001). Tasks created by MAs, CTAs, clinical nurses, and others were more likely to be opioid-related compared with the tasks created by attending physicians, residents, NPs, or a PA (25% vs 15%; P<.05). Compared with non-opioid-related tasks, opioid-related tasks required more follow-up phone calls (P<.001). Follow-up phone calls to pharmacies occurred more often with opioid-related tasks than with non-opioid tasks (11% vs 5%), while follow-up phone calls to patients occurred more often for non-opioid related tasks than opioid-related tasks (28% vs 18%). No correlations with task creation were found for who addressed the opioid-related task or the day the task was created.
DISCUSSION
This study demonstrated that our process of handling patient issues related to opioids accounts for a large proportion of all tasks. Dealing with tasks is time consuming, not only for attending physicians and residents but also for clinic nurses and staff. Almost a quarter of clinic tasks were opioid related. As has been shown in previous studies,5-8 chronic pain management with opioids is an unsatisfying task for staff and care providers at our clinics. We also found that tasks created by non-providers were more likely to be opioid-related than were tasks created by providers. This is most likely due to the fact that non-providers cannot write prescriptions and they have to ask providers for further reviews.
Khalid et al found that, compared with attending physicians, residents had more patients on chronic opioids who displayed concerning behaviors, including early refills and refills from multiple providers.13 The higher number of part-time providers at Clinic 1 in our study may have also caused insufficient continuity of care at that site. Nevertheless, this model of practice is used in many academic primary care institutions.4 Another possible reason for the difference could be a lack of resident training on current guidelines for managing opiates for chronic pain.3,13,14 Again, this was a pilot study and we drew no solid conclusion about the reasons for differences between these 2 clinics.
It is obvious, however, that we spend a significant amount of time and resources dealing with chronic pain management. Our institution created an opioid/controlled-substance patient registry about 3 years ago. The data for 2014 showed that 22.8% and 18% of patients seen at least once at Clinic 1 and Clinic 2, respectively, were prescribed opioids/controlled substances (TABLE 3).
Possible solutions to reduce tasks related to opioid management. For both small and large practices, one way to reduce the number of tasks related to opioid management and, therefore, the time allocated to completing those tasks, would be to have a clear protocol to follow.3,4,8,11,14,15 The protocol may include the creation of an opioid/controlled-substance registry and the development and implementation of clinical decision support programs.
We also recommend the dissemination of tools for clinical management at the point of care. These can include a controlled-substance risk assessment tool for aberrant behaviors, a controlled-substance informed consent form, a functional and quality-of-life assessment, electronic clinical-note templates in the EHR, urine drug screening, and routine use of existing state pharmacy prescription drug monitoring programs. Also essential would be the provision of routine educational programs for clinicians regarding chronic pain management based on existing evidence and guidelines. (See “Opioids for chronic pain: The CDC’s 12 recommendations.”) It has been demonstrated that an EHR opioid dashboard or an EHR-based protocol improved adherence to guidelines for prescribing opiates.16
This study has several limitations. First, this was a small pilot study completed over a short period of time, although we believe the findings are likely representative of the prescribing practices in the 2 clinics we evaluated. Second, it was a retrospective study, which was appropriate for evaluating our questions. Third, we were unable to account for other factors that could potentially confound the results, including, but not limited to, the amount of time allocated to each task, and the total number of patients at each clinic who were on opioids for management of chronic pain during the study period. However, due to our recent addition of an opioid/controlled-substance patient registry, we were able to add information for the year 2014 (TABLE 3). Multi-center large scale studies are required to evaluate this further.
ACKNOWLEDGEMENTS
We thank Dr. Corey Lyon for his editorial assistance.
CORRESPONDENCE
Morteza Khodaee, MD, AFW Family Medicine Clinic, 3055 Roslyn Street, Denver, CO 80238; morteza.khodaee@ucdenver.edu.
1. Smith BH, Torrance N. Management of chronic pain in primary care. Curr Opin Support Palliat Care. 2011;5:137-142.
2. Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA. 2012;307:1377-1378.
3. Leverence RR, Williams RL, Potter M, et al; PRIME Net Clinicians. Chronic non-cancer pain: a siren for primary care—a report from the PRImary Care MultiEthnic Network (PRIME Net). J Am Board Fam Med. 2011;24:551-561.
4. Watkins A, Wasmann S, Dodson L, et al. An evaluation of the care provided to patients prescribed controlled substances for chronic nonmalignant pain at an academic family medicine center. Fam Med. 2004;36:487-489.
5. Brown J, Setnik B, Lee K, et al. Assessment, stratification, and monitoring of the risk for prescription opioid misuse and abuse in the primary care setting. J Opioid Manag. 2011;7:467-483.
6. Duensing L, Eksterowicz N, Macario A, et al. Patient and physician perceptions of treatment of moderate-to-severe chronic pain with oral opioids. Curr Med Res Opin. 2010;26:1579-1585.
7. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12:S26-S35.
8. Wenghofer EF, Wilson L, Kahan M, et al. Survey of Ontario primary care physicians’ experiences with opioid prescribing. Can Fam Physician. 2011;57:324-332.
9. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
10. Hartrick CT, Gatchel RJ, Conroy S. Identification and management of pain medication abuse and misuse: current state and future directions. Expert Rev Neurother. 2012;12:601-610.
11. Wiedemer NL, Harden PS, Arndt IO, et al. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8:573-584.
12. Colburn JL, Jasinski DR, Rastegar DA. Long-term opioid therapy, aberrant behaviors, and substance misuse: comparison of patients treated by resident and attending physicians in a general medical clinic. J Opioid Manag. 2012;8:153-160.
13. Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med. 2015;16:480-487.
14. Canada RE, DiRocco D, Day S. A better approach to opioid prescribing in primary care. J Fam Pract. 2014;63:E1-E8.
15. Clark LG, Upshur CC. Family medicine physicians’ views of how to improve chronic pain management. J Am Board Fam Med. 2007;20:479-482.
16. Anderson D, Zlateva I, Khatri K, et al. Using health information technology to improve adherence to opioid prescribing guidelines in primary care. Clin J Pain. 2015;31:573-579.
1. Smith BH, Torrance N. Management of chronic pain in primary care. Curr Opin Support Palliat Care. 2011;5:137-142.
2. Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA. 2012;307:1377-1378.
3. Leverence RR, Williams RL, Potter M, et al; PRIME Net Clinicians. Chronic non-cancer pain: a siren for primary care—a report from the PRImary Care MultiEthnic Network (PRIME Net). J Am Board Fam Med. 2011;24:551-561.
4. Watkins A, Wasmann S, Dodson L, et al. An evaluation of the care provided to patients prescribed controlled substances for chronic nonmalignant pain at an academic family medicine center. Fam Med. 2004;36:487-489.
5. Brown J, Setnik B, Lee K, et al. Assessment, stratification, and monitoring of the risk for prescription opioid misuse and abuse in the primary care setting. J Opioid Manag. 2011;7:467-483.
6. Duensing L, Eksterowicz N, Macario A, et al. Patient and physician perceptions of treatment of moderate-to-severe chronic pain with oral opioids. Curr Med Res Opin. 2010;26:1579-1585.
7. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12:S26-S35.
8. Wenghofer EF, Wilson L, Kahan M, et al. Survey of Ontario primary care physicians’ experiences with opioid prescribing. Can Fam Physician. 2011;57:324-332.
9. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
10. Hartrick CT, Gatchel RJ, Conroy S. Identification and management of pain medication abuse and misuse: current state and future directions. Expert Rev Neurother. 2012;12:601-610.
11. Wiedemer NL, Harden PS, Arndt IO, et al. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8:573-584.
12. Colburn JL, Jasinski DR, Rastegar DA. Long-term opioid therapy, aberrant behaviors, and substance misuse: comparison of patients treated by resident and attending physicians in a general medical clinic. J Opioid Manag. 2012;8:153-160.
13. Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med. 2015;16:480-487.
14. Canada RE, DiRocco D, Day S. A better approach to opioid prescribing in primary care. J Fam Pract. 2014;63:E1-E8.
15. Clark LG, Upshur CC. Family medicine physicians’ views of how to improve chronic pain management. J Am Board Fam Med. 2007;20:479-482.
16. Anderson D, Zlateva I, Khatri K, et al. Using health information technology to improve adherence to opioid prescribing guidelines in primary care. Clin J Pain. 2015;31:573-579.
Mildly pruritic palmar rash
A 62-year-old man presented to the emergency department (ED) with a swollen, red, and painful right lower leg. He’d had bilateral lower leg swelling for 2 months, but the left leg became increasingly painful and red over the past 3 days. The patient also had a 3-day history of a diffuse rash that began on his right upper arm and spread to his left arm, both palms, both legs, and his back. It was mildly pruritic, but not painful.
The patient indicated that he had recently sought care from his primary care physician for lower respiratory symptoms. He had just completed a 5-day course of azithromycin and prednisone (50 mg/d for 5 days) the day before his ED visit.
A lower extremity venous ultrasound revealed that the patient had a deep vein thrombosis (DVT). Computed tomography (CT) imaging of the chest with contrast revealed pulmonary emboli. He was treated with enoxaparin and warfarin. We diagnosed the rash based on the patient’s history and the appearance of the rash, which was comprised of blanching and erythematous macules with central clearing (FIGURE 1). (There were no blisters or mucosal involvement.)
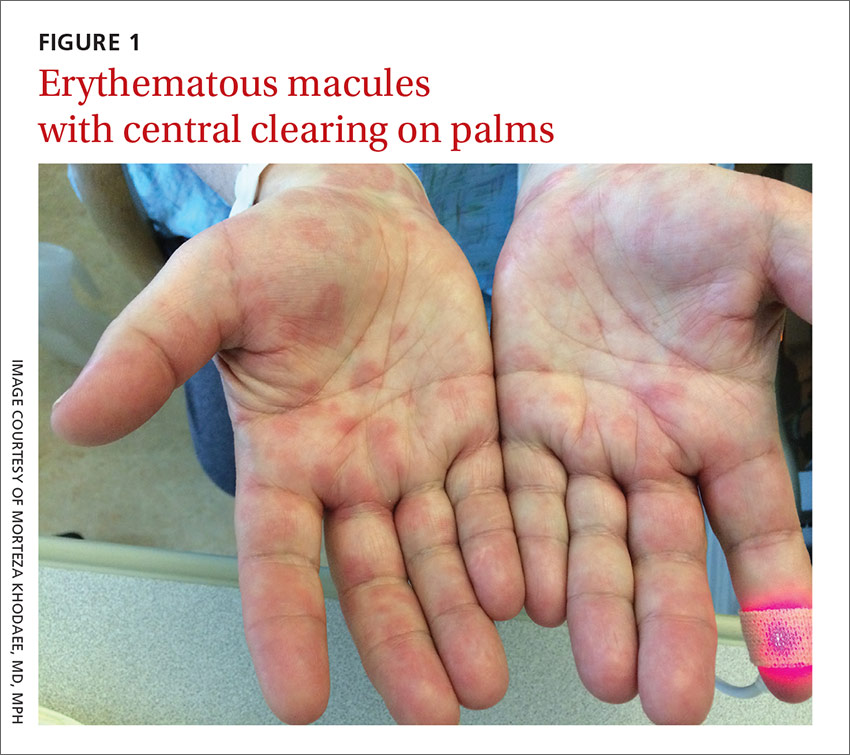
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythema multiforme
The clinical exam was consistent with the diagnosis of erythema multiforme (EM). A diagnosis of EM can usually be made based on the clinical exam alone.1 Typical targetoid lesions have a round shape and 3 concentric zones: A central dusky area of epidermal necrosis that may involve bullae, a paler pink or edematous zone, and a peripheral erythematous ring.2 Atypical lesions, such as raised papules, may also be seen.2
The skin lesions of EM usually appear symmetrically on the distal extremities and spread in a centripetal manner.1 Palms, soles, and mucosa can be involved.1 EM with mucosal involvement is called “erythema multiforme major,” and EM without mucosal disease (as in our patient’s case) is called “erythema multiforme minor.”2
EM is an acute, immune-mediated eruption thought to be caused by a cell-mediated hypersensitivity to certain infections or drugs.2 Ninety percent of cases are associated with an infection; herpes simplex virus (HSV) is the most common infectious agent.3 Mycoplasma pneumoniae is another culprit, especially in children. Medications are inciting factors about 10% of the time; nonsteroidal anti-inflammatory drugs, sulfonamides, antiepileptics, and antibiotics have been linked to EM eruptions.3
Interestingly, while azithromycin—the medication our patient had taken most recently—can cause EM, it has been mainly linked to cases of Stevens-Johnson syndrome (SJS).4 So, while we suspect that azithromycin was the trigger in our patient’s case, we can’t be sure. It’s also possible that Mycoplasma pneumoniae was the trigger for our patient’s EM. However, Mycoplasma pneumoniae is more common in adolescents.
Differential includes life-threatening conditions like SJS
The differential diagnosis for a non-vesicular palmar rash is discussed in the TABLE.1,5-12 There is a wide spectrum of possible etiologies—from infectious and rheumatologic disorders to chronic liver disease. Histologic testing may be useful in differentiating EM from other diseases, but in most cases, it is not required to make a diagnosis.1 Laboratory testing may reveal leukocytosis, an elevated erythrocyte sedimentation rate, and elevated liver function test results, but these are nonspecific.1
It’s important to differentiate EM from life-threatening conditions like SJS and toxic epidermal necrolysis (TEN).5 EM is characterized by typical and atypical targetoid lesions with minimal mucosal involvement.6,7 SJS is characterized by flat atypical targetoid lesions, confluent purpuric macules, severe mucosal erosions, and <10% epidermal detachment.6,7 TEN is characterized by severe mucosal erosions and >30% epidermal detachment.6,7
Lesions resolve on their own, but topical steroids can provide relief
EM is a self-limiting disease; lesions resolve within about 2 weeks.3 Management begins by treating any suspected infection or discontinuing any suspected drugs.1 In patients with co-existing or recurrent HSV infection, early treatment with an oral antiviral (such as acyclovir) may lessen the number and duration of lesions.1,6 In addition, oral antihistamines and topical steroids may be used to provide symptomatic relief.1,6 Use of oral corticosteroids can be considered in severe mucosal disease, although such use is considered controversial due to a lack of evidence.1,6
Our patient remained hospitalized for 4 days. As noted earlier, his DVT and pulmonary embolism were treated with enoxaparin and the patient was sent home with a prescription for warfarin. Regarding the EM, his rash and itching
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, Colorado 80238; morteza.khodaee@ucdenver.edu.
1. Lamoreux MR, Sternbach MR, Hsu WT. Erythema multiforme. Am Fam Physician. 2006;74:1883-1888.
2. Patel NN, Patel DN. Erythema multiforme syndrome. Am J Med. 2009;122:623-625.
3. Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
4. Nambudiri VE. More than skin deep—the costs of antibiotic overuse: a teachable moment. JAMA Intern Med. 2014;174:1724-1725.
5. Usatine RP, Sandy N. Dermatologic emergencies. Am Fam Physician. 2010;82:773-780.
6. Al-Johani KA, Fedele S, Porter SR. Erythema multiforme andrelated disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:642-654.
7. Assier H, Bastuji-Garin S, Revuz J, et al. Erythema multiforme with mucous membrane involvement and Stevens-Johnson syndrome are clinically different disorders with distinct causes. Arch Dermatol. 1995;131:539-543.
8. Mage V, Lipsker D, Barbarot S, et al. Different patterns of skin manifestations associated with parvovirus B19 primary infection in adults. J Am Acad Dermatol. 2014;71:62-69.
9. Hubiche T, Schuffenecker I, Boralevi F, et al; Clinical Research Group of the French Society of Pediatric Dermatology Groupe de Recherche Clinique de la Société Française de Dermatologie Pédiatrique. Dermatological spectrum of hand, foot and mouth disease from classical to generalized exanthema. Pediatr Infect Dis J. 2014;33:e92-e98.
10. Serrao R, Zirwas M, English JC. Palmar erythema. Am J Clin Dermatol. 2007;8:347-356.
11. Meffert JJ. Photo quiz. A palmar rash. Am Fam Physician. 1999;59:1259-1260.
12. Saguil A, Fargo M, Grogan S. Diagnosis and management of Kawasaki disease. Am Fam Physician. 2015;91:365-371.
A 62-year-old man presented to the emergency department (ED) with a swollen, red, and painful right lower leg. He’d had bilateral lower leg swelling for 2 months, but the left leg became increasingly painful and red over the past 3 days. The patient also had a 3-day history of a diffuse rash that began on his right upper arm and spread to his left arm, both palms, both legs, and his back. It was mildly pruritic, but not painful.
The patient indicated that he had recently sought care from his primary care physician for lower respiratory symptoms. He had just completed a 5-day course of azithromycin and prednisone (50 mg/d for 5 days) the day before his ED visit.
A lower extremity venous ultrasound revealed that the patient had a deep vein thrombosis (DVT). Computed tomography (CT) imaging of the chest with contrast revealed pulmonary emboli. He was treated with enoxaparin and warfarin. We diagnosed the rash based on the patient’s history and the appearance of the rash, which was comprised of blanching and erythematous macules with central clearing (FIGURE 1). (There were no blisters or mucosal involvement.)

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythema multiforme
The clinical exam was consistent with the diagnosis of erythema multiforme (EM). A diagnosis of EM can usually be made based on the clinical exam alone.1 Typical targetoid lesions have a round shape and 3 concentric zones: A central dusky area of epidermal necrosis that may involve bullae, a paler pink or edematous zone, and a peripheral erythematous ring.2 Atypical lesions, such as raised papules, may also be seen.2
The skin lesions of EM usually appear symmetrically on the distal extremities and spread in a centripetal manner.1 Palms, soles, and mucosa can be involved.1 EM with mucosal involvement is called “erythema multiforme major,” and EM without mucosal disease (as in our patient’s case) is called “erythema multiforme minor.”2
EM is an acute, immune-mediated eruption thought to be caused by a cell-mediated hypersensitivity to certain infections or drugs.2 Ninety percent of cases are associated with an infection; herpes simplex virus (HSV) is the most common infectious agent.3 Mycoplasma pneumoniae is another culprit, especially in children. Medications are inciting factors about 10% of the time; nonsteroidal anti-inflammatory drugs, sulfonamides, antiepileptics, and antibiotics have been linked to EM eruptions.3
Interestingly, while azithromycin—the medication our patient had taken most recently—can cause EM, it has been mainly linked to cases of Stevens-Johnson syndrome (SJS).4 So, while we suspect that azithromycin was the trigger in our patient’s case, we can’t be sure. It’s also possible that Mycoplasma pneumoniae was the trigger for our patient’s EM. However, Mycoplasma pneumoniae is more common in adolescents.
Differential includes life-threatening conditions like SJS
The differential diagnosis for a non-vesicular palmar rash is discussed in the TABLE.1,5-12 There is a wide spectrum of possible etiologies—from infectious and rheumatologic disorders to chronic liver disease. Histologic testing may be useful in differentiating EM from other diseases, but in most cases, it is not required to make a diagnosis.1 Laboratory testing may reveal leukocytosis, an elevated erythrocyte sedimentation rate, and elevated liver function test results, but these are nonspecific.1
It’s important to differentiate EM from life-threatening conditions like SJS and toxic epidermal necrolysis (TEN).5 EM is characterized by typical and atypical targetoid lesions with minimal mucosal involvement.6,7 SJS is characterized by flat atypical targetoid lesions, confluent purpuric macules, severe mucosal erosions, and <10% epidermal detachment.6,7 TEN is characterized by severe mucosal erosions and >30% epidermal detachment.6,7

Lesions resolve on their own, but topical steroids can provide relief
EM is a self-limiting disease; lesions resolve within about 2 weeks.3 Management begins by treating any suspected infection or discontinuing any suspected drugs.1 In patients with co-existing or recurrent HSV infection, early treatment with an oral antiviral (such as acyclovir) may lessen the number and duration of lesions.1,6 In addition, oral antihistamines and topical steroids may be used to provide symptomatic relief.1,6 Use of oral corticosteroids can be considered in severe mucosal disease, although such use is considered controversial due to a lack of evidence.1,6
Our patient remained hospitalized for 4 days. As noted earlier, his DVT and pulmonary embolism were treated with enoxaparin and the patient was sent home with a prescription for warfarin. Regarding the EM, his rash and itching
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, Colorado 80238; morteza.khodaee@ucdenver.edu.
A 62-year-old man presented to the emergency department (ED) with a swollen, red, and painful right lower leg. He’d had bilateral lower leg swelling for 2 months, but the left leg became increasingly painful and red over the past 3 days. The patient also had a 3-day history of a diffuse rash that began on his right upper arm and spread to his left arm, both palms, both legs, and his back. It was mildly pruritic, but not painful.
The patient indicated that he had recently sought care from his primary care physician for lower respiratory symptoms. He had just completed a 5-day course of azithromycin and prednisone (50 mg/d for 5 days) the day before his ED visit.
A lower extremity venous ultrasound revealed that the patient had a deep vein thrombosis (DVT). Computed tomography (CT) imaging of the chest with contrast revealed pulmonary emboli. He was treated with enoxaparin and warfarin. We diagnosed the rash based on the patient’s history and the appearance of the rash, which was comprised of blanching and erythematous macules with central clearing (FIGURE 1). (There were no blisters or mucosal involvement.)

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythema multiforme
The clinical exam was consistent with the diagnosis of erythema multiforme (EM). A diagnosis of EM can usually be made based on the clinical exam alone.1 Typical targetoid lesions have a round shape and 3 concentric zones: A central dusky area of epidermal necrosis that may involve bullae, a paler pink or edematous zone, and a peripheral erythematous ring.2 Atypical lesions, such as raised papules, may also be seen.2
The skin lesions of EM usually appear symmetrically on the distal extremities and spread in a centripetal manner.1 Palms, soles, and mucosa can be involved.1 EM with mucosal involvement is called “erythema multiforme major,” and EM without mucosal disease (as in our patient’s case) is called “erythema multiforme minor.”2
EM is an acute, immune-mediated eruption thought to be caused by a cell-mediated hypersensitivity to certain infections or drugs.2 Ninety percent of cases are associated with an infection; herpes simplex virus (HSV) is the most common infectious agent.3 Mycoplasma pneumoniae is another culprit, especially in children. Medications are inciting factors about 10% of the time; nonsteroidal anti-inflammatory drugs, sulfonamides, antiepileptics, and antibiotics have been linked to EM eruptions.3
Interestingly, while azithromycin—the medication our patient had taken most recently—can cause EM, it has been mainly linked to cases of Stevens-Johnson syndrome (SJS).4 So, while we suspect that azithromycin was the trigger in our patient’s case, we can’t be sure. It’s also possible that Mycoplasma pneumoniae was the trigger for our patient’s EM. However, Mycoplasma pneumoniae is more common in adolescents.
Differential includes life-threatening conditions like SJS
The differential diagnosis for a non-vesicular palmar rash is discussed in the TABLE.1,5-12 There is a wide spectrum of possible etiologies—from infectious and rheumatologic disorders to chronic liver disease. Histologic testing may be useful in differentiating EM from other diseases, but in most cases, it is not required to make a diagnosis.1 Laboratory testing may reveal leukocytosis, an elevated erythrocyte sedimentation rate, and elevated liver function test results, but these are nonspecific.1
It’s important to differentiate EM from life-threatening conditions like SJS and toxic epidermal necrolysis (TEN).5 EM is characterized by typical and atypical targetoid lesions with minimal mucosal involvement.6,7 SJS is characterized by flat atypical targetoid lesions, confluent purpuric macules, severe mucosal erosions, and <10% epidermal detachment.6,7 TEN is characterized by severe mucosal erosions and >30% epidermal detachment.6,7

Lesions resolve on their own, but topical steroids can provide relief
EM is a self-limiting disease; lesions resolve within about 2 weeks.3 Management begins by treating any suspected infection or discontinuing any suspected drugs.1 In patients with co-existing or recurrent HSV infection, early treatment with an oral antiviral (such as acyclovir) may lessen the number and duration of lesions.1,6 In addition, oral antihistamines and topical steroids may be used to provide symptomatic relief.1,6 Use of oral corticosteroids can be considered in severe mucosal disease, although such use is considered controversial due to a lack of evidence.1,6
Our patient remained hospitalized for 4 days. As noted earlier, his DVT and pulmonary embolism were treated with enoxaparin and the patient was sent home with a prescription for warfarin. Regarding the EM, his rash and itching
CORRESPONDENCE
Morteza Khodaee, MD, MPH, AFW Clinic, 3055 Roslyn Street, Denver, Colorado 80238; morteza.khodaee@ucdenver.edu.
1. Lamoreux MR, Sternbach MR, Hsu WT. Erythema multiforme. Am Fam Physician. 2006;74:1883-1888.
2. Patel NN, Patel DN. Erythema multiforme syndrome. Am J Med. 2009;122:623-625.
3. Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
4. Nambudiri VE. More than skin deep—the costs of antibiotic overuse: a teachable moment. JAMA Intern Med. 2014;174:1724-1725.
5. Usatine RP, Sandy N. Dermatologic emergencies. Am Fam Physician. 2010;82:773-780.
6. Al-Johani KA, Fedele S, Porter SR. Erythema multiforme andrelated disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:642-654.
7. Assier H, Bastuji-Garin S, Revuz J, et al. Erythema multiforme with mucous membrane involvement and Stevens-Johnson syndrome are clinically different disorders with distinct causes. Arch Dermatol. 1995;131:539-543.
8. Mage V, Lipsker D, Barbarot S, et al. Different patterns of skin manifestations associated with parvovirus B19 primary infection in adults. J Am Acad Dermatol. 2014;71:62-69.
9. Hubiche T, Schuffenecker I, Boralevi F, et al; Clinical Research Group of the French Society of Pediatric Dermatology Groupe de Recherche Clinique de la Société Française de Dermatologie Pédiatrique. Dermatological spectrum of hand, foot and mouth disease from classical to generalized exanthema. Pediatr Infect Dis J. 2014;33:e92-e98.
10. Serrao R, Zirwas M, English JC. Palmar erythema. Am J Clin Dermatol. 2007;8:347-356.
11. Meffert JJ. Photo quiz. A palmar rash. Am Fam Physician. 1999;59:1259-1260.
12. Saguil A, Fargo M, Grogan S. Diagnosis and management of Kawasaki disease. Am Fam Physician. 2015;91:365-371.
1. Lamoreux MR, Sternbach MR, Hsu WT. Erythema multiforme. Am Fam Physician. 2006;74:1883-1888.
2. Patel NN, Patel DN. Erythema multiforme syndrome. Am J Med. 2009;122:623-625.
3. Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
4. Nambudiri VE. More than skin deep—the costs of antibiotic overuse: a teachable moment. JAMA Intern Med. 2014;174:1724-1725.
5. Usatine RP, Sandy N. Dermatologic emergencies. Am Fam Physician. 2010;82:773-780.
6. Al-Johani KA, Fedele S, Porter SR. Erythema multiforme andrelated disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:642-654.
7. Assier H, Bastuji-Garin S, Revuz J, et al. Erythema multiforme with mucous membrane involvement and Stevens-Johnson syndrome are clinically different disorders with distinct causes. Arch Dermatol. 1995;131:539-543.
8. Mage V, Lipsker D, Barbarot S, et al. Different patterns of skin manifestations associated with parvovirus B19 primary infection in adults. J Am Acad Dermatol. 2014;71:62-69.
9. Hubiche T, Schuffenecker I, Boralevi F, et al; Clinical Research Group of the French Society of Pediatric Dermatology Groupe de Recherche Clinique de la Société Française de Dermatologie Pédiatrique. Dermatological spectrum of hand, foot and mouth disease from classical to generalized exanthema. Pediatr Infect Dis J. 2014;33:e92-e98.
10. Serrao R, Zirwas M, English JC. Palmar erythema. Am J Clin Dermatol. 2007;8:347-356.
11. Meffert JJ. Photo quiz. A palmar rash. Am Fam Physician. 1999;59:1259-1260.
12. Saguil A, Fargo M, Grogan S. Diagnosis and management of Kawasaki disease. Am Fam Physician. 2015;91:365-371.
Knee pain • no popping • no previous trauma • Dx?
THE CASE
A 36-year-old man sought care at our family medicine clinic for knee pain that he’d had for the past year. He denied any previous injury or trauma to the knee. The pain affected the posterolateral left knee and was aggravated by squatting and deep flexion. Daily activities did not bother him, but skiing, golfing, mountain biking, and lifting weights worsened the pain. His pain had gradually become more severe and frequent. He denied any mechanical symptoms such as catching, popping, or locking.
Examination of his left knee demonstrated range of motion from 0 to 120 degrees; further flexion caused significant pain. McMurray and Thessaly tests were positive for posterolateral pain, particularly with knee flexion >120 degrees. Physical examination was otherwise unremarkable. Standard x-rays of the left knee were normal. Our patient completed a month of physical therapy, but his symptoms did not improve.
THE DIAGNOSIS
After the patient completed physical therapy, magnetic resonance imaging (MRI) was performed. The MRI did not reveal any left knee effusion, and the menisci, collateral ligaments, and cartilage surfaces were normal. And, while the cruciate ligaments were intact, a large pericruciate ganglion cyst was noted (FIGURES 1 AND 2).
DISCUSSION
Ganglion cysts are dense, encapsulated structures filled with clear viscous fluid that often arise adjacent to tendon sheaths or joint capsules, most commonly over the dorsum of the hand.1 Intra-articular ganglia involving the cruciate ligaments of the knee are relatively uncommon.2 The estimated prevalence of cruciate ligament ganglion cysts at arthroscopy is 0.2% to 1.9%; similar rates have been demonstrated with MRI.3-6 There are more reported cases of these cysts involving the anterior cruciate ligament (ACL) compared to those affecting the posterior cruciate ligament (PCL).2,6
Classification of these cysts is based on relative location with respect to the ligaments. Type 1 cysts originate anterior to the ACL; type 2, between the ACL and PCL; and type 3, posterior to the PCL.6,7 Cruciate ligament ganglion cysts are more common in men, are typically discovered between age 20 and 40, and are usually incidental findings.8
The pathogenesis of ganglion cyst formation is unknown.1,6,7 The most widely accepted theory is that ganglion cysts result from mucinous degeneration of connective tissue in areas of repetitive stress.1,6,7 Other theories suggest hyaluronic acid production secondary to mesenchymal stem cell proliferation within the ligaments, synovial tissue herniation, or congenital translocation of synovial tissue as possible etiologies.2,6,7
Concurrent pathologies such as meniscal tears or chondral lesions may also be present; however, there is some disagreement as to what role, if any, antecedent trauma has in the pathogenesis of cyst formation.1,6 Several investigators have suggested that prior knee trauma is a likely risk factor.2,8,9
In most patients, cruciate ligament ganglion cysts are asymptomatic.7 The most common presenting symptom is nonspecific pain that is exacerbated by activity, such as stair climbing, squatting, or other activities that require extreme flexion or extension of the knee.6,9 Other possible symptoms include limited range of motion (extension block with ACL involvement, limited flexion with PCL lesions), a catching or locking sensation, instability, or joint line tenderness.5,6 A palpable mass on physical exam is not usually present.6 Some investigators suggest that larger lesions and those closer to the femoral ligamentous attachments are more likely to cause symptoms.5
Cruciate ligament ganglion cysts can be an easily overlooked source of a patient’s symptoms because they often mimic more common pathologies.2 The differential diagnosis of cruciate ligament ganglion cysts and posterior knee pain includes any other intra-articular cysts (eg, meniscal cysts), posterior meniscal tear, popliteus tendinopathy, or neoplasms (eg, hemangioma and synovial sarcoma).2,6
MRI is the best method of diagnosis
Because the symptoms of cruciate ligament ganglion cysts are variable and nonspecific, the diagnosis is rarely made on clinical grounds alone.1 The best method of evaluating suspected intra-articular pathologies such as cruciate ligament ganglion cysts is MRI.5,10
Cruciate ligament ganglion cysts typically follow fluid signal on all sequences, with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.1,2,5,6 A pericruciate location with a multilocular appearance is usually sufficient evidence to make a diagnosis. However, solid or semi-solid pathologies (such as synovial cell sarcoma, synovial hemangioma, or synovial chondromatosis) can have similar signal intensity.
If necessary, intravenous contrast can be helpful; a lack of central contrast enhancement can differentiate ganglion cysts from other solid, enhancing, or partially enhancing lesions. Other diagnostic modalities, such as ultrasound, computed tomography (CT), and diagnostic arthroscopy, are less practical and have a wide range of sensitivity and specificity.5,6,10
Arthroscopic excision is the treatment of choice
Asymptomatic cruciate ligament ganglion cysts are usually managed with clinical follow-up. For patients with symptomatic cysts, ultrasound- or CT-guided percutaneous cyst aspiration may temporarily improve symptoms, but recurrence rates have not been well studied.2,6,9,10 Additionally, accessibility to cysts in this location via these approaches is limited. Arthroscopic excision of the cyst is the treatment of choice for symptomatic cases.1,2,5,6,10
Our patient underwent arthroscopic cyst resection, which resulted in complete resolution of his symptoms. In 3 months, he returned to his regular physical activities with no pain or discomfort. One year later, he remained asymptomatic.
THE TAKEAWAY
Cruciate ligament ganglion cysts are a rare cause of posterior knee pain. An MRI is the best diagnostic modality to evaluate and confirm the diagnosis, as well as rule out other pathologies. The treatment of choice for symptomatic cases is arthroscopic excision of the cyst.
1. Mao Y, Dong Q, Wang Y. Ganglion cysts of the cruciate ligaments: a series of 31 cases and review of the literature. BMC Musculoskelet Disord. 2012;13:137.
2. Krudwig WK, Schulte KK, Heinemann C. Intra-articular ganglion cysts of the knee joint: a report of 85 cases and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2004;12:123-129.
3. Bergin D, Morrison WB, Carrino JA, et al. Anterior cruciate ligament ganglia and mucoid degeneration: coexistence and clinical correlation. AJR Am J Roentgenol. 2004;182:1283-1287.
4. Bui-Mansfield LT, Youngberg RA. Intraarticular ganglia of the knee: prevalence, presentation, etiology, and management. AJR Am J Roentgenol. 1997;168:123-127.
5. Lunhao B, Yu S, Jiashi W. Diagnosis and treatment of ganglion cysts of the cruciate ligaments. Arch Orthop Trauma Surg. 2011;131:1053-1057.
6. Stein D, Cantlon M, Mackay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
7. Zantop T, Rusch A, Hassenpflug J, et al. Intra-articular ganglion cysts of the cruciate ligaments: case report and review of the literature. Arch Orthop Trauma Surg. 2003;123:195-198.
8. Tsai TY, Yang YS, Tseng FJ, et al. Arthroscopic excision of ganglion cysts of the posterior cruciate ligaments using posterior trans-septal portal. Arthroscopy. 2012;28:95-99.
9. Huang GS, Lee CH, Chan WP, et al. Ganglion cysts of the cruciate ligaments. Acta Radiol. 2002;43:419-424.
10. Tyrrell PN, Cassar-Pullicino VN, McCall IW. Intra-articular ganglion cysts of the cruciate ligaments. Eur Radiol. 2000;10:1233-1238.
THE CASE
A 36-year-old man sought care at our family medicine clinic for knee pain that he’d had for the past year. He denied any previous injury or trauma to the knee. The pain affected the posterolateral left knee and was aggravated by squatting and deep flexion. Daily activities did not bother him, but skiing, golfing, mountain biking, and lifting weights worsened the pain. His pain had gradually become more severe and frequent. He denied any mechanical symptoms such as catching, popping, or locking.
Examination of his left knee demonstrated range of motion from 0 to 120 degrees; further flexion caused significant pain. McMurray and Thessaly tests were positive for posterolateral pain, particularly with knee flexion >120 degrees. Physical examination was otherwise unremarkable. Standard x-rays of the left knee were normal. Our patient completed a month of physical therapy, but his symptoms did not improve.
THE DIAGNOSIS
After the patient completed physical therapy, magnetic resonance imaging (MRI) was performed. The MRI did not reveal any left knee effusion, and the menisci, collateral ligaments, and cartilage surfaces were normal. And, while the cruciate ligaments were intact, a large pericruciate ganglion cyst was noted (FIGURES 1 AND 2).
DISCUSSION
Ganglion cysts are dense, encapsulated structures filled with clear viscous fluid that often arise adjacent to tendon sheaths or joint capsules, most commonly over the dorsum of the hand.1 Intra-articular ganglia involving the cruciate ligaments of the knee are relatively uncommon.2 The estimated prevalence of cruciate ligament ganglion cysts at arthroscopy is 0.2% to 1.9%; similar rates have been demonstrated with MRI.3-6 There are more reported cases of these cysts involving the anterior cruciate ligament (ACL) compared to those affecting the posterior cruciate ligament (PCL).2,6
Classification of these cysts is based on relative location with respect to the ligaments. Type 1 cysts originate anterior to the ACL; type 2, between the ACL and PCL; and type 3, posterior to the PCL.6,7 Cruciate ligament ganglion cysts are more common in men, are typically discovered between age 20 and 40, and are usually incidental findings.8
The pathogenesis of ganglion cyst formation is unknown.1,6,7 The most widely accepted theory is that ganglion cysts result from mucinous degeneration of connective tissue in areas of repetitive stress.1,6,7 Other theories suggest hyaluronic acid production secondary to mesenchymal stem cell proliferation within the ligaments, synovial tissue herniation, or congenital translocation of synovial tissue as possible etiologies.2,6,7
Concurrent pathologies such as meniscal tears or chondral lesions may also be present; however, there is some disagreement as to what role, if any, antecedent trauma has in the pathogenesis of cyst formation.1,6 Several investigators have suggested that prior knee trauma is a likely risk factor.2,8,9
In most patients, cruciate ligament ganglion cysts are asymptomatic.7 The most common presenting symptom is nonspecific pain that is exacerbated by activity, such as stair climbing, squatting, or other activities that require extreme flexion or extension of the knee.6,9 Other possible symptoms include limited range of motion (extension block with ACL involvement, limited flexion with PCL lesions), a catching or locking sensation, instability, or joint line tenderness.5,6 A palpable mass on physical exam is not usually present.6 Some investigators suggest that larger lesions and those closer to the femoral ligamentous attachments are more likely to cause symptoms.5
Cruciate ligament ganglion cysts can be an easily overlooked source of a patient’s symptoms because they often mimic more common pathologies.2 The differential diagnosis of cruciate ligament ganglion cysts and posterior knee pain includes any other intra-articular cysts (eg, meniscal cysts), posterior meniscal tear, popliteus tendinopathy, or neoplasms (eg, hemangioma and synovial sarcoma).2,6
MRI is the best method of diagnosis
Because the symptoms of cruciate ligament ganglion cysts are variable and nonspecific, the diagnosis is rarely made on clinical grounds alone.1 The best method of evaluating suspected intra-articular pathologies such as cruciate ligament ganglion cysts is MRI.5,10
Cruciate ligament ganglion cysts typically follow fluid signal on all sequences, with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.1,2,5,6 A pericruciate location with a multilocular appearance is usually sufficient evidence to make a diagnosis. However, solid or semi-solid pathologies (such as synovial cell sarcoma, synovial hemangioma, or synovial chondromatosis) can have similar signal intensity.
If necessary, intravenous contrast can be helpful; a lack of central contrast enhancement can differentiate ganglion cysts from other solid, enhancing, or partially enhancing lesions. Other diagnostic modalities, such as ultrasound, computed tomography (CT), and diagnostic arthroscopy, are less practical and have a wide range of sensitivity and specificity.5,6,10
Arthroscopic excision is the treatment of choice
Asymptomatic cruciate ligament ganglion cysts are usually managed with clinical follow-up. For patients with symptomatic cysts, ultrasound- or CT-guided percutaneous cyst aspiration may temporarily improve symptoms, but recurrence rates have not been well studied.2,6,9,10 Additionally, accessibility to cysts in this location via these approaches is limited. Arthroscopic excision of the cyst is the treatment of choice for symptomatic cases.1,2,5,6,10
Our patient underwent arthroscopic cyst resection, which resulted in complete resolution of his symptoms. In 3 months, he returned to his regular physical activities with no pain or discomfort. One year later, he remained asymptomatic.
THE TAKEAWAY
Cruciate ligament ganglion cysts are a rare cause of posterior knee pain. An MRI is the best diagnostic modality to evaluate and confirm the diagnosis, as well as rule out other pathologies. The treatment of choice for symptomatic cases is arthroscopic excision of the cyst.
THE CASE
A 36-year-old man sought care at our family medicine clinic for knee pain that he’d had for the past year. He denied any previous injury or trauma to the knee. The pain affected the posterolateral left knee and was aggravated by squatting and deep flexion. Daily activities did not bother him, but skiing, golfing, mountain biking, and lifting weights worsened the pain. His pain had gradually become more severe and frequent. He denied any mechanical symptoms such as catching, popping, or locking.
Examination of his left knee demonstrated range of motion from 0 to 120 degrees; further flexion caused significant pain. McMurray and Thessaly tests were positive for posterolateral pain, particularly with knee flexion >120 degrees. Physical examination was otherwise unremarkable. Standard x-rays of the left knee were normal. Our patient completed a month of physical therapy, but his symptoms did not improve.
THE DIAGNOSIS
After the patient completed physical therapy, magnetic resonance imaging (MRI) was performed. The MRI did not reveal any left knee effusion, and the menisci, collateral ligaments, and cartilage surfaces were normal. And, while the cruciate ligaments were intact, a large pericruciate ganglion cyst was noted (FIGURES 1 AND 2).
DISCUSSION
Ganglion cysts are dense, encapsulated structures filled with clear viscous fluid that often arise adjacent to tendon sheaths or joint capsules, most commonly over the dorsum of the hand.1 Intra-articular ganglia involving the cruciate ligaments of the knee are relatively uncommon.2 The estimated prevalence of cruciate ligament ganglion cysts at arthroscopy is 0.2% to 1.9%; similar rates have been demonstrated with MRI.3-6 There are more reported cases of these cysts involving the anterior cruciate ligament (ACL) compared to those affecting the posterior cruciate ligament (PCL).2,6
Classification of these cysts is based on relative location with respect to the ligaments. Type 1 cysts originate anterior to the ACL; type 2, between the ACL and PCL; and type 3, posterior to the PCL.6,7 Cruciate ligament ganglion cysts are more common in men, are typically discovered between age 20 and 40, and are usually incidental findings.8
The pathogenesis of ganglion cyst formation is unknown.1,6,7 The most widely accepted theory is that ganglion cysts result from mucinous degeneration of connective tissue in areas of repetitive stress.1,6,7 Other theories suggest hyaluronic acid production secondary to mesenchymal stem cell proliferation within the ligaments, synovial tissue herniation, or congenital translocation of synovial tissue as possible etiologies.2,6,7
Concurrent pathologies such as meniscal tears or chondral lesions may also be present; however, there is some disagreement as to what role, if any, antecedent trauma has in the pathogenesis of cyst formation.1,6 Several investigators have suggested that prior knee trauma is a likely risk factor.2,8,9
In most patients, cruciate ligament ganglion cysts are asymptomatic.7 The most common presenting symptom is nonspecific pain that is exacerbated by activity, such as stair climbing, squatting, or other activities that require extreme flexion or extension of the knee.6,9 Other possible symptoms include limited range of motion (extension block with ACL involvement, limited flexion with PCL lesions), a catching or locking sensation, instability, or joint line tenderness.5,6 A palpable mass on physical exam is not usually present.6 Some investigators suggest that larger lesions and those closer to the femoral ligamentous attachments are more likely to cause symptoms.5
Cruciate ligament ganglion cysts can be an easily overlooked source of a patient’s symptoms because they often mimic more common pathologies.2 The differential diagnosis of cruciate ligament ganglion cysts and posterior knee pain includes any other intra-articular cysts (eg, meniscal cysts), posterior meniscal tear, popliteus tendinopathy, or neoplasms (eg, hemangioma and synovial sarcoma).2,6
MRI is the best method of diagnosis
Because the symptoms of cruciate ligament ganglion cysts are variable and nonspecific, the diagnosis is rarely made on clinical grounds alone.1 The best method of evaluating suspected intra-articular pathologies such as cruciate ligament ganglion cysts is MRI.5,10
Cruciate ligament ganglion cysts typically follow fluid signal on all sequences, with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.1,2,5,6 A pericruciate location with a multilocular appearance is usually sufficient evidence to make a diagnosis. However, solid or semi-solid pathologies (such as synovial cell sarcoma, synovial hemangioma, or synovial chondromatosis) can have similar signal intensity.
If necessary, intravenous contrast can be helpful; a lack of central contrast enhancement can differentiate ganglion cysts from other solid, enhancing, or partially enhancing lesions. Other diagnostic modalities, such as ultrasound, computed tomography (CT), and diagnostic arthroscopy, are less practical and have a wide range of sensitivity and specificity.5,6,10
Arthroscopic excision is the treatment of choice
Asymptomatic cruciate ligament ganglion cysts are usually managed with clinical follow-up. For patients with symptomatic cysts, ultrasound- or CT-guided percutaneous cyst aspiration may temporarily improve symptoms, but recurrence rates have not been well studied.2,6,9,10 Additionally, accessibility to cysts in this location via these approaches is limited. Arthroscopic excision of the cyst is the treatment of choice for symptomatic cases.1,2,5,6,10
Our patient underwent arthroscopic cyst resection, which resulted in complete resolution of his symptoms. In 3 months, he returned to his regular physical activities with no pain or discomfort. One year later, he remained asymptomatic.
THE TAKEAWAY
Cruciate ligament ganglion cysts are a rare cause of posterior knee pain. An MRI is the best diagnostic modality to evaluate and confirm the diagnosis, as well as rule out other pathologies. The treatment of choice for symptomatic cases is arthroscopic excision of the cyst.
1. Mao Y, Dong Q, Wang Y. Ganglion cysts of the cruciate ligaments: a series of 31 cases and review of the literature. BMC Musculoskelet Disord. 2012;13:137.
2. Krudwig WK, Schulte KK, Heinemann C. Intra-articular ganglion cysts of the knee joint: a report of 85 cases and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2004;12:123-129.
3. Bergin D, Morrison WB, Carrino JA, et al. Anterior cruciate ligament ganglia and mucoid degeneration: coexistence and clinical correlation. AJR Am J Roentgenol. 2004;182:1283-1287.
4. Bui-Mansfield LT, Youngberg RA. Intraarticular ganglia of the knee: prevalence, presentation, etiology, and management. AJR Am J Roentgenol. 1997;168:123-127.
5. Lunhao B, Yu S, Jiashi W. Diagnosis and treatment of ganglion cysts of the cruciate ligaments. Arch Orthop Trauma Surg. 2011;131:1053-1057.
6. Stein D, Cantlon M, Mackay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
7. Zantop T, Rusch A, Hassenpflug J, et al. Intra-articular ganglion cysts of the cruciate ligaments: case report and review of the literature. Arch Orthop Trauma Surg. 2003;123:195-198.
8. Tsai TY, Yang YS, Tseng FJ, et al. Arthroscopic excision of ganglion cysts of the posterior cruciate ligaments using posterior trans-septal portal. Arthroscopy. 2012;28:95-99.
9. Huang GS, Lee CH, Chan WP, et al. Ganglion cysts of the cruciate ligaments. Acta Radiol. 2002;43:419-424.
10. Tyrrell PN, Cassar-Pullicino VN, McCall IW. Intra-articular ganglion cysts of the cruciate ligaments. Eur Radiol. 2000;10:1233-1238.
1. Mao Y, Dong Q, Wang Y. Ganglion cysts of the cruciate ligaments: a series of 31 cases and review of the literature. BMC Musculoskelet Disord. 2012;13:137.
2. Krudwig WK, Schulte KK, Heinemann C. Intra-articular ganglion cysts of the knee joint: a report of 85 cases and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2004;12:123-129.
3. Bergin D, Morrison WB, Carrino JA, et al. Anterior cruciate ligament ganglia and mucoid degeneration: coexistence and clinical correlation. AJR Am J Roentgenol. 2004;182:1283-1287.
4. Bui-Mansfield LT, Youngberg RA. Intraarticular ganglia of the knee: prevalence, presentation, etiology, and management. AJR Am J Roentgenol. 1997;168:123-127.
5. Lunhao B, Yu S, Jiashi W. Diagnosis and treatment of ganglion cysts of the cruciate ligaments. Arch Orthop Trauma Surg. 2011;131:1053-1057.
6. Stein D, Cantlon M, Mackay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
7. Zantop T, Rusch A, Hassenpflug J, et al. Intra-articular ganglion cysts of the cruciate ligaments: case report and review of the literature. Arch Orthop Trauma Surg. 2003;123:195-198.
8. Tsai TY, Yang YS, Tseng FJ, et al. Arthroscopic excision of ganglion cysts of the posterior cruciate ligaments using posterior trans-septal portal. Arthroscopy. 2012;28:95-99.
9. Huang GS, Lee CH, Chan WP, et al. Ganglion cysts of the cruciate ligaments. Acta Radiol. 2002;43:419-424.
10. Tyrrell PN, Cassar-Pullicino VN, McCall IW. Intra-articular ganglion cysts of the cruciate ligaments. Eur Radiol. 2000;10:1233-1238.