User login
Epidemiology of Organ System Dysfunction
The International Consensus Conference (ICC) for sepsis defines severe sepsis as an infection leading to acute organ dysfunction.[1, 2] Severe sepsis afflicts over 1 million patients each year in Medicare alone, and is substantially more common among older Americans than acute myocardial infarction.[3, 4, 5] Recently, the Agency for Healthcare Research and Quality identified severe sepsis as the single most expensive cause of hospitalization in the United States.[6] The incidence of severe sepsis continues to rise.[4, 5]
Severe sepsis is often mischaracterized as a diagnosis cared for primarily in the intensive care unit (ICU). Yet, studies indicate that only 32% to 50% of patients with severe sepsis require ICU care, leaving the majority on the general care wards.[7, 8] These studies also reveal mortality rates of 26% to 30% among patients with severe sepsis who are not admitted to an ICU compared to 11% to 33% in the ICU.[7, 8]
Although a number of epidemiologic and interventional studies have focused on severe sepsis in the ICU,[3, 9, 10] much less is known about patients cared for on the general medicine wards. Without this information, clinicians cannot make informed choices about important management decisions such as targeted diagnostic testing, empirical antimicrobials, and other therapies. To this end, we sought to further characterize the infectious etiologies and resultant organ system dysfunctions in the subset of patients with severe sepsis admitted to non‐ICU medical services at a tertiary academic medical center.
METHODS
Population/Setting
All hospitalizations of adult patients (18 years old) who were initially admitted to non‐ICU medical services at the University of Michigan Hospital during 2009 through 2010 were included. The University of Michigan Hospital has 610 general medical‐surgical beds, including telemetry beds, with closed ICUs comprised of 179 beds staffed by intensivists. Patients transferred from other hospitals and those admitted to non‐medical services were excluded.
Data Abstraction and Definitions
All International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes for hospitalizations were screened using a previously published and validated algorithm for severe sepsis.[11] Following this screening, 3 randomly selected round‐numbered batches of hospitalizations were sampled with subsequent application of the exclusion criteria. Medical records including physicians' notes, consultants' notes, nurses' notes, physical therapy notes, discharge coordinators' notes, emergency room flow sheets, as well as ward flow sheets were reviewed in detail by 3 practicing hospitalists using a structured instrument closely aligned with the ICC definition of severe sepsis.[2] We also sampled a smaller number of patients whose ICD‐9‐CM diagnoses screened negative for severe sepsis. Sample size was selected as part of a project with multiple objectives, and reflected a pragmatic balance between the anticipated precision of the results and the resources available to conduct chart review.[11] All discrepancies were reconciled among the 3 reviewers.
Reviewers first assessed whether infection was present, then evaluated for evidence of each organ system dysfunction, and finally determined the extent to which those organ dysfunctions were a response to the infection. Infection was defined either as a patient with a microbiologic culture growing a pathologic organism in a normally sterile site or documentation of a suspected infection with other confirmatory evidence (radiological, physical exam finding) with resultant systemic inflammatory response and administration of antimicrobials. Community‐acquired and healthcare‐associated infections were not differentiated. Microbiologic data, confirmatory tests, and site of infection were abstracted in detail.
Organ dysfunction was defined as per the 2001 ICC criteria,[2] and was assessed for neurological, pulmonary, cardiovascular, renal, gastrointestinal, hematological, and hepatic system involvement in all patients. A summary of these clinical definitions is included in Table 1. Data on important comorbidities were also abstracted. Immunosuppression was defined as having any of the following: solid organ transplant, bone marrow/stem cell transplant, human immunodeficiency virus/acquired immunodeficiency syndrome, neutropenia (absolute neutrophil count <1000), hematologic malignancy, solid organ malignancy with chemotherapy within the past 12 months, or pharmacologic immunosuppression (prednisone >20 mg daily for >4 weeks, calcineurin inhibitor, methotrexate, tumor necrosis factor inhibitors, azathioprine, sulfasalazine, hydroxychloroquine). Last, each chart was evaluated for the presence of explicit documentation with the presence of the words or phrases: sepsis, septic shock, or severe sepsis, indicating that the clinical service recognized and fully documented that a patient had severe sepsis.
| Organ System | Parameters to Indicate Dysfunction |
|---|---|
| |
| Cardiovascular | Systolic BP <90, elevated lactate, MAP <70, requiring pressors >2 hours, decrease in systolic BP of >40 |
| Renal | Creatinine increase >0.5 mg/dL, oliguria |
| Neurological | Acute mental status changes |
| Pulmonary | Intubation, BiPAP, supplemental oxygen >6 LPM or 40% face mask, PaO2/FiO2 <300 |
| Hematologic | INR >1.5 or PTT >60 not on anticoagulation, platelets <100 or 50% of baseline |
| Ileus | Decreased bowel motility requiring a change in diet |
| Hepatic | Bilirubin >4 mg/dL and >1.5 baseline |
Data Analysis
Methods for assessment of reviewer concordance have been previously described and were summarized using the kappa statistic.[11] Initial data extraction was performed in SAS 9.1 (SAS Institute, Cary, NC) and all analyses were conducted in Stata 12 (StataCorp LP, College Station, TX). Binomial 95% confidence intervals (CIs) are presented. This project was approved by the University of Michigan Institutional Review Board.
RESULTS
Of 23,288 hospitalizations examined from 2009 through 2010, the ICD‐9based automated screen for severe sepsis was positive for 3,146 (14 %). A random sample of 111 medical records, of which 92 had screened positive for severe sepsis and 19 had screened negative, was reviewed in detail. After review by the hospitalists, 64 of these 111 hospitalizations were judged to have severe sepsis, 61 of the 92 screened positive cases (66%), and 3 of the 19 screened negative cases (16%). The 3 reviewers had a kappa of 0.70, indicating good agreement.
Characteristics of the 64 patients with severe sepsis are shown in Table 2. The mean age was 63 years old (standard deviation [SD]=17.7), and 41% were male. The mean length of stay was 13.7 days (SD=20.8). Thirty‐nine percent (95% CI, 27%‐52%) of patients (25/64) were immunosuppressed. Of patients initially admitted to the general medical ward, 25% (16/64; 95% CI, 15%‐37%) ultimately required ICU care during their admission. The overall in‐hospital mortality rate was 13% (8/64; 95% CI, 6%‐23%). Immunosuppressed patients had a mortality rate of 20% and nonimmunosuppressed patients had a mortality rate of 8%. Only 47% (30/64; 95% CI, 34%‐60%) of the medical records had explicit clinician documentation of severe sepsis.
| Age, mean (SD), y | 63 (18) |
|---|---|
| |
| Male sex, no. (%) | 26 (41) |
| Preexisting conditions, no. (%) | |
| History of diabetes | 20 (31) |
| End stage renal disease on chronic dialysis | 2 (3) |
| Chronic obstructive pulmonary disease on oxygen | 3 (5) |
| History of cancer | 15 (23) |
| Liver cirrhosis | 5 (8) |
| Immunosuppression | 25 (39) |
| Median length of stay (days) | 7.5 |
| Mean length of stay (SD) | 13.7 (20.8) |
The most common site of infection was found to be the genitourinary system, occurring in 41% (26/64; 95% CI, 29%‐54%) of patients (Table 3). Pulmonary and intra‐abdominal sites were also common, accounting for 14% (95% CI, 6.6%‐25%) and 13% (95% CI, 5.6%‐23%) of sites, respectively. An infecting organism was identified by culture in 66% (42/64; 95% CI, 53%‐77%) of case patients with specific pathogens listed in Table 4. Among patients with positive culture results, the majority grew Gram‐negative organisms (57%; 95% CI, 41%‐72%). Non‐Clostridium difficile Gram‐positive organisms were also prominent and identified in 48% (95% CI, 32%‐64%) of positive cultures. Candida was less common (12%, 95% CI, 4.0%‐26%). Fourteen cases (22%, 95% CI, 10%‐30%) had 2 or more concomitant infectious pathogens.
| Site | No. (%) |
|---|---|
| |
| Genitourinary | 26 (41) |
| Pulmonary | 9 (14) |
| Intra‐abdominal (not intraluminal) | 8 (13) |
| Bloodstream/cardiac | 5 (8) |
| Skin and soft tissue | 4 (6) |
| GI lumen | 4 (6) |
| Joint | 2 (3) |
| Multiple sites | 4 (6) |
| Unknown | 2 (2) |
| Absolute Frequency, Total Positive Culture Results, N=64, No. (%)*?>a | Patients With Cultures Growing at Least One of the Pathogens, N=42, No. (%)*?>a | |
|---|---|---|
| ||
| Gram‐negative pathogens | 30 (47) | 24 (57) |
| Escherichia coli | 12 (19) | 12 (29) |
| Escherichia coli (multidrug resistant) | 2 (3) | 2 (5) |
| Klebsiella | 6 (9) | 5 (12) |
| Pseudomonas aeruginosa | 6 (9) | 4 (10) |
| Pseudomonas aeruginosa (multidrug resistant) | 2 (3) | 2 (5) |
| Otherb | 6 (9) | 6 (14) |
| Gram‐positive pathogens | 29 (45) | 25 (59) |
| Enterococcus | 14 (22) | 13 (31) |
| Vancomycin‐resistant Enterococcus species | 5 (8) | 4 (10) |
| Staphylococcus aureus | 7 (11) | 7 (17) |
| Methicillin‐resistant Staphylococcus aureus | 3 (5) | 3 (7) |
| Streptococcus pneumoniae | 2 (3) | 2 (5) |
| Coagulase‐negative staphylococci | 1 (2) | 1 (2) |
| Clostridium difficile | 5 (8) | 5 (12) |
| Fungi | ||
| Candida species | 5 (8) | 5 (12) |
| Mycobacterium avium | 1 (2) | 1 (2) |
| Two organisms | 9 (21) | |
| Three or more organisms | 5 (12) | |
All 64 patients had at least 1 organ dysfunction, as required by the ICC definition of severe sepsis. Organ dysfunction in 2 or more organ systems occurred in 77% (95% CI, 64%‐86%) of the cases (49/64). The incidence for each organ system dysfunction is presented in Table 5, as well as its relationship to both mortality and ICU admission. The most common organ system dysfunctions were found to be cardiovascular (hypotension) and renal dysfunction occurring in 66% and 64% of the cases, respectively. In this non‐ICU population, pulmonary dysfunction occurred in 30% of cases, but was frequently associated with transfer to the ICU, as 63% of the patients with pulmonary failure required ICU care. Patients with more organ systems affected were more likely to be transferred to the ICU and to die.
| No. (%) | ICU Transfer, No. (%) | Mortality, No. (%) | |
|---|---|---|---|
| |||
| Number of failed organs, N = 64 | |||
| 1 | 15 (23%) | 0 (0%) | 0 (0%) |
| 2 | 25 (39%) | 2 (8%) | 0 (0%) |
| 3 | 7 (11%) | 2 (29%) | 1 (14%) |
| 4 | 10 (16%) | 6 (60%) | 3 (30%) |
| >4 | 7 (11%) | 6 (86%) | 4 (57%) |
| Types of organ system dysfunction, all patients, N = 64*?>a | |||
| Cardiovascular | 42 (66%) | 16 (38%)b | 8 (19%)c |
| Renal | 41 (64%) | 10 (24%)b | 5 (12%)c |
| Central nervous system | 35 (54%) | 14 (40%)b | 7 (18%)c |
| Pulmonary | 19 (30%) | 12 (63%)b | 8 (42%)c |
| Hematologic | 15 (23%) | 6 (40%)b | 6 (40%)c |
| GI (ileus) | 8 (13%) | 5 (63%)b | 1 (13%)c |
| Hepatic | 5 (8%) | 4 (80%)b | 2 (40%)c |
DISCUSSION
Severe sepsis was common among patients admitted to the general medical ward in this tertiary care center. Our patient cohort differed in important ways from previously described typical cases of severe sepsis among ICU populations. Severe sepsis on the general medical wards was more commonly associated with Gram‐negative pathogens in the setting of genitourinary tract infections. This is in contrast to Gram‐positive organisms and respiratory tract infections, which are more common in the ICU.[3, 10] Renal and cardiac dysfunction were commonly observed organ failures, whereas in the ICU, severe sepsis has been reported to more likely involve respiratory failure. These results suggest that hospitalists seeking to provide evidence‐based care to prevent postsepsis morbidity and mortality for their non‐ICU patients need to heighten their index of suspicion when caring for an infected patient and appreciate that many severe sepsis patients may not fit neatly into traditional sepsis treatment algorithms.
Studies characterizing severe sepsis in the ICU setting indicate a predominance of pulmonary infections and respiratory failure with occurrence rates of 74% to 95% and 54% to 61%, respectively.[3, 12, 13] Given that either shock or pulmonary dysfunction is often required for admission to many ICUs, it is perhaps not surprising that these rates are dramatically different on the general medicine ward, with a relative scarcity of pulmonary infections (14%) and respiratory dysfunction (30%). Instead, genitourinary infections were noted in 41% (95% CI, 29%‐54%) of the cases, in contrast to the rates of genitourinary infections in ICU patients with severe sepsis, which have rates of 5.4% to 9.1%.[3, 10] Likely as a result of this, a Gram‐negative predominance is noted in the associated microbiology. Furthermore, our study indicates that C difficile and vancomycin‐resistant Enterococcus (VRE) species appear to represent an emerging cause of severe sepsis on the general medicine wards, as they have not been noted to be causative micro‐organisms in previous studies of sepsis. This is concordant with other studies showing increases in incidence and severity of disease for C difficile as well as VRE.[14, 15]
Previous epidemiologic studies of severe sepsis originating outside the ICU are lacking, but some work has been done. One study on the epidemiology of sepsis both with and without organ dysfunction aggregated all hospitalized patients and included those both admitted to the general medicine wards and directly to the ICU.[7] Similar to our study, this study also found a predominance of Gram‐negative causative organisms, as well as comparable in‐hospital mortality rates (12.8% vs 13%). Additionally, genitourinary infections were noted in 20% of the patients, notably higher than rates reported to have been found in patients with severe sepsis in the ICU, but not the magnitude found in our study, perhaps as a result of the combined ICU‐ward population studied. A similar high prevalence of genitourinary infections was also noted in a recent administrative data‐based study of emergency medical services‐transported patients with severe sepsis, half of whom required intensive care during their hospitalization.[16]
Our study is unique in that it focuses on severe sepsis in patients, commonly cared for by hospitalists, who were admitted to the general medical ward, and uses patient level data to elucidate more characteristics of the defining organ dysfunction. Furthermore, our results suggest that severe sepsis was poorly documented in this setting, indicating a potential impact on billing, coding, case mix index, and hospital mortality statistics that rely on very specific wording, as well as a possible need for increased awareness among hospitalists. Without this awareness, an opportunity may be missed for improved patient care via specific sepsis‐targeted measures,[13, 17, 18] including more aggressive resuscitative measures[19] or intensive physical and occupational therapy interventions aimed at impacting the cognitive and functional debilities[20] that result from severe sepsis. Highlighting this growing need to better assist clinicians assess the severity of septic patients and recognize these complex cases on the general medicine wards, 1 recent study evaluated the fitness of several clinical disease‐severity scoring systems for patients with sepsis in general internal medicine departments.[21] Perhaps with the help of tools such as these, which are being piloted in some hospitals, the care of this growing population can be enhanced.
Our study has a number of limitations that should be kept in mind. First, this is a single center study performed at an academic tertiary care center with a relatively high incidence of immunosuppression, which may influence the spectrum of infecting organisms. Our center also has a relatively large, closed‐model ICU, which often operates at near capacity, potentially affecting the severity of our non‐ICU population. Second, although we screened a large number of patients, as necessitated by our intensive and detailed review of clinical information, our sample size with hospitalist‐validated severe sepsis is relatively small. With this small sample size, less prevalent infections, patient characteristics, and organ dysfunctions may by chance have been under or over‐represented, and one could expect some variance in the occurrence rates of organ system dysfunction and infection rates by sampling error alone. Further larger scale studies are warranted to confirm these data and their generalizability. Third, the data necessary to calculate sequential organ failure assessment or multiple organ dysfunction score were not collected. This may limit the ability to directly compare the organ dysfunction noted in this study with others. Additionally, given the ICC definitions of organ dysfunction, some of the organ dysfunction noted, particularly for neurological dysfunction, was reliant on subjective clinical findings documented in the record. Finally, we relied on the lack of specific terminology to indicate a lack of documentation of sepsis, which does not necessarily indicate a lack of recognition or undertreatment of this condition. However, these limitations are offset by the strengths of this study, including the patient‐level medical record validation of severe sepsis by trained hospitalist physicians, high kappa statistic, and strict application of guideline‐based definitions.
This work has important implications for both clinicians and for future research on severe sepsis. The results suggest that severe sepsis may be quite common outside the ICU, and that patients presenting with this condition who are admitted to general medical wards are not routinely characterized by the profound hypoxemia and refractory shock of iconic cases. Certainly, further study looking at larger numbers of cases is needed to better understand the specifics and nuances of this important topic as well as to further evaluate clinicians' ability to recognize and treat such patients in this setting. Furthermore, future research on the treatment of severe sepsis, including both antimicrobials and disease‐modifying agents (eg, anti‐inflammatories) must continue to include and even focus on this large population of non‐ICU patients with severe sepsis, as the risk/benefit ratios of such potential treatments may vary with severity of illness.
In conclusion, severe sepsis was commonly found in patients admitted on the general medicine wards. The epidemiology of the infections and resultant organ dysfunction appears to differ from that found in the ICU. More studies are needed to provide a deeper understanding of this disease process, as this will enable clinicians to better recognize and treat patients thus afflicted, no matter the setting.
Acknowledgments
The authors thank Laetitia Shapiro, AM, for her programming assistance.
Disclosures: This work was supported in part by the US National Institutes of HealthK08, HL091249 (TJI) and the University of Michigan SpecialistHospitalist Allied Research Program (SHARP). This work was also supported in part by VA Ann Arbor Healthcare System, Geriatric Research Education and Clinical Center (GRECC).
- , , , et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- , , , et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
- , , , , , . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , , . Population burden of long‐term survivorship after severe sepsis in older americans. J Am Geriatr Soc. 2012;60(6):1070–1077.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. October 2011. In: Healthcare Cost and Utilization Project Statistical Briefs. Rockville, MD: Agency for Health Care Policy and Research; 2006. Available from: http://www.ncbi.nlm.nih.gov/books/NBK65391. Accessed June 2, 2012.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35(5):1284–1289.
- , , , , . Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33(1):71–80.
- , , , et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis‐related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307(22):2390–2399.
- , , , , . Incidence and impact of organ dysfunctions associated with sepsis. Chest. 2005;127(3):942–951.
- , , , et al. Identifying patients with severe sepsis using administrative claims: patient‐level validation of the Angus Implementation of the International Consensus Conference definition of severe sepsis [published online ahead of print September 18, 2012]. Medical Care. doi: 10.1097/MLR.0b013e318268ac86.
- , , , . Current epidemiology of septic shock: the CUB‐Rea Network. Am J Respir Crit Care Med. 2003;168(2):165–172.
- . Management of sepsis. N Engl J Med. 2006;355(16):1699–1713.
- , , . Current status of Clostridium difficile infection ipidemiology. Clin Infect Dis. 2012;55(suppl 2):S65–S70.
- , . Vancomycin‐resistant enterococci. Semin Respir Infect. 2000;15(4):314–326.
- , , , , , . Severe sepsis in prehospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med. 2012;186(12):1264–1271.
- , . Novel Therapies for Septic Shock Over the Past 4 Decades. JAMA. 2011;306(2):194–199.
- , , , et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med. 2010;38(4):1036–1043.
- , . Diagnosis and treatment of severe sepsis. Crit Care. 2007;11(suppl 5):S2.
- , , , . Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794.
- , , , , . Assessment of disease‐severity scoring systems for patients with sepsis in general internal medicine departments. Crit Care. 2011;15:R95.
The International Consensus Conference (ICC) for sepsis defines severe sepsis as an infection leading to acute organ dysfunction.[1, 2] Severe sepsis afflicts over 1 million patients each year in Medicare alone, and is substantially more common among older Americans than acute myocardial infarction.[3, 4, 5] Recently, the Agency for Healthcare Research and Quality identified severe sepsis as the single most expensive cause of hospitalization in the United States.[6] The incidence of severe sepsis continues to rise.[4, 5]
Severe sepsis is often mischaracterized as a diagnosis cared for primarily in the intensive care unit (ICU). Yet, studies indicate that only 32% to 50% of patients with severe sepsis require ICU care, leaving the majority on the general care wards.[7, 8] These studies also reveal mortality rates of 26% to 30% among patients with severe sepsis who are not admitted to an ICU compared to 11% to 33% in the ICU.[7, 8]
Although a number of epidemiologic and interventional studies have focused on severe sepsis in the ICU,[3, 9, 10] much less is known about patients cared for on the general medicine wards. Without this information, clinicians cannot make informed choices about important management decisions such as targeted diagnostic testing, empirical antimicrobials, and other therapies. To this end, we sought to further characterize the infectious etiologies and resultant organ system dysfunctions in the subset of patients with severe sepsis admitted to non‐ICU medical services at a tertiary academic medical center.
METHODS
Population/Setting
All hospitalizations of adult patients (18 years old) who were initially admitted to non‐ICU medical services at the University of Michigan Hospital during 2009 through 2010 were included. The University of Michigan Hospital has 610 general medical‐surgical beds, including telemetry beds, with closed ICUs comprised of 179 beds staffed by intensivists. Patients transferred from other hospitals and those admitted to non‐medical services were excluded.
Data Abstraction and Definitions
All International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes for hospitalizations were screened using a previously published and validated algorithm for severe sepsis.[11] Following this screening, 3 randomly selected round‐numbered batches of hospitalizations were sampled with subsequent application of the exclusion criteria. Medical records including physicians' notes, consultants' notes, nurses' notes, physical therapy notes, discharge coordinators' notes, emergency room flow sheets, as well as ward flow sheets were reviewed in detail by 3 practicing hospitalists using a structured instrument closely aligned with the ICC definition of severe sepsis.[2] We also sampled a smaller number of patients whose ICD‐9‐CM diagnoses screened negative for severe sepsis. Sample size was selected as part of a project with multiple objectives, and reflected a pragmatic balance between the anticipated precision of the results and the resources available to conduct chart review.[11] All discrepancies were reconciled among the 3 reviewers.
Reviewers first assessed whether infection was present, then evaluated for evidence of each organ system dysfunction, and finally determined the extent to which those organ dysfunctions were a response to the infection. Infection was defined either as a patient with a microbiologic culture growing a pathologic organism in a normally sterile site or documentation of a suspected infection with other confirmatory evidence (radiological, physical exam finding) with resultant systemic inflammatory response and administration of antimicrobials. Community‐acquired and healthcare‐associated infections were not differentiated. Microbiologic data, confirmatory tests, and site of infection were abstracted in detail.
Organ dysfunction was defined as per the 2001 ICC criteria,[2] and was assessed for neurological, pulmonary, cardiovascular, renal, gastrointestinal, hematological, and hepatic system involvement in all patients. A summary of these clinical definitions is included in Table 1. Data on important comorbidities were also abstracted. Immunosuppression was defined as having any of the following: solid organ transplant, bone marrow/stem cell transplant, human immunodeficiency virus/acquired immunodeficiency syndrome, neutropenia (absolute neutrophil count <1000), hematologic malignancy, solid organ malignancy with chemotherapy within the past 12 months, or pharmacologic immunosuppression (prednisone >20 mg daily for >4 weeks, calcineurin inhibitor, methotrexate, tumor necrosis factor inhibitors, azathioprine, sulfasalazine, hydroxychloroquine). Last, each chart was evaluated for the presence of explicit documentation with the presence of the words or phrases: sepsis, septic shock, or severe sepsis, indicating that the clinical service recognized and fully documented that a patient had severe sepsis.
| Organ System | Parameters to Indicate Dysfunction |
|---|---|
| |
| Cardiovascular | Systolic BP <90, elevated lactate, MAP <70, requiring pressors >2 hours, decrease in systolic BP of >40 |
| Renal | Creatinine increase >0.5 mg/dL, oliguria |
| Neurological | Acute mental status changes |
| Pulmonary | Intubation, BiPAP, supplemental oxygen >6 LPM or 40% face mask, PaO2/FiO2 <300 |
| Hematologic | INR >1.5 or PTT >60 not on anticoagulation, platelets <100 or 50% of baseline |
| Ileus | Decreased bowel motility requiring a change in diet |
| Hepatic | Bilirubin >4 mg/dL and >1.5 baseline |
Data Analysis
Methods for assessment of reviewer concordance have been previously described and were summarized using the kappa statistic.[11] Initial data extraction was performed in SAS 9.1 (SAS Institute, Cary, NC) and all analyses were conducted in Stata 12 (StataCorp LP, College Station, TX). Binomial 95% confidence intervals (CIs) are presented. This project was approved by the University of Michigan Institutional Review Board.
RESULTS
Of 23,288 hospitalizations examined from 2009 through 2010, the ICD‐9based automated screen for severe sepsis was positive for 3,146 (14 %). A random sample of 111 medical records, of which 92 had screened positive for severe sepsis and 19 had screened negative, was reviewed in detail. After review by the hospitalists, 64 of these 111 hospitalizations were judged to have severe sepsis, 61 of the 92 screened positive cases (66%), and 3 of the 19 screened negative cases (16%). The 3 reviewers had a kappa of 0.70, indicating good agreement.
Characteristics of the 64 patients with severe sepsis are shown in Table 2. The mean age was 63 years old (standard deviation [SD]=17.7), and 41% were male. The mean length of stay was 13.7 days (SD=20.8). Thirty‐nine percent (95% CI, 27%‐52%) of patients (25/64) were immunosuppressed. Of patients initially admitted to the general medical ward, 25% (16/64; 95% CI, 15%‐37%) ultimately required ICU care during their admission. The overall in‐hospital mortality rate was 13% (8/64; 95% CI, 6%‐23%). Immunosuppressed patients had a mortality rate of 20% and nonimmunosuppressed patients had a mortality rate of 8%. Only 47% (30/64; 95% CI, 34%‐60%) of the medical records had explicit clinician documentation of severe sepsis.
| Age, mean (SD), y | 63 (18) |
|---|---|
| |
| Male sex, no. (%) | 26 (41) |
| Preexisting conditions, no. (%) | |
| History of diabetes | 20 (31) |
| End stage renal disease on chronic dialysis | 2 (3) |
| Chronic obstructive pulmonary disease on oxygen | 3 (5) |
| History of cancer | 15 (23) |
| Liver cirrhosis | 5 (8) |
| Immunosuppression | 25 (39) |
| Median length of stay (days) | 7.5 |
| Mean length of stay (SD) | 13.7 (20.8) |
The most common site of infection was found to be the genitourinary system, occurring in 41% (26/64; 95% CI, 29%‐54%) of patients (Table 3). Pulmonary and intra‐abdominal sites were also common, accounting for 14% (95% CI, 6.6%‐25%) and 13% (95% CI, 5.6%‐23%) of sites, respectively. An infecting organism was identified by culture in 66% (42/64; 95% CI, 53%‐77%) of case patients with specific pathogens listed in Table 4. Among patients with positive culture results, the majority grew Gram‐negative organisms (57%; 95% CI, 41%‐72%). Non‐Clostridium difficile Gram‐positive organisms were also prominent and identified in 48% (95% CI, 32%‐64%) of positive cultures. Candida was less common (12%, 95% CI, 4.0%‐26%). Fourteen cases (22%, 95% CI, 10%‐30%) had 2 or more concomitant infectious pathogens.
| Site | No. (%) |
|---|---|
| |
| Genitourinary | 26 (41) |
| Pulmonary | 9 (14) |
| Intra‐abdominal (not intraluminal) | 8 (13) |
| Bloodstream/cardiac | 5 (8) |
| Skin and soft tissue | 4 (6) |
| GI lumen | 4 (6) |
| Joint | 2 (3) |
| Multiple sites | 4 (6) |
| Unknown | 2 (2) |
| Absolute Frequency, Total Positive Culture Results, N=64, No. (%)*?>a | Patients With Cultures Growing at Least One of the Pathogens, N=42, No. (%)*?>a | |
|---|---|---|
| ||
| Gram‐negative pathogens | 30 (47) | 24 (57) |
| Escherichia coli | 12 (19) | 12 (29) |
| Escherichia coli (multidrug resistant) | 2 (3) | 2 (5) |
| Klebsiella | 6 (9) | 5 (12) |
| Pseudomonas aeruginosa | 6 (9) | 4 (10) |
| Pseudomonas aeruginosa (multidrug resistant) | 2 (3) | 2 (5) |
| Otherb | 6 (9) | 6 (14) |
| Gram‐positive pathogens | 29 (45) | 25 (59) |
| Enterococcus | 14 (22) | 13 (31) |
| Vancomycin‐resistant Enterococcus species | 5 (8) | 4 (10) |
| Staphylococcus aureus | 7 (11) | 7 (17) |
| Methicillin‐resistant Staphylococcus aureus | 3 (5) | 3 (7) |
| Streptococcus pneumoniae | 2 (3) | 2 (5) |
| Coagulase‐negative staphylococci | 1 (2) | 1 (2) |
| Clostridium difficile | 5 (8) | 5 (12) |
| Fungi | ||
| Candida species | 5 (8) | 5 (12) |
| Mycobacterium avium | 1 (2) | 1 (2) |
| Two organisms | 9 (21) | |
| Three or more organisms | 5 (12) | |
All 64 patients had at least 1 organ dysfunction, as required by the ICC definition of severe sepsis. Organ dysfunction in 2 or more organ systems occurred in 77% (95% CI, 64%‐86%) of the cases (49/64). The incidence for each organ system dysfunction is presented in Table 5, as well as its relationship to both mortality and ICU admission. The most common organ system dysfunctions were found to be cardiovascular (hypotension) and renal dysfunction occurring in 66% and 64% of the cases, respectively. In this non‐ICU population, pulmonary dysfunction occurred in 30% of cases, but was frequently associated with transfer to the ICU, as 63% of the patients with pulmonary failure required ICU care. Patients with more organ systems affected were more likely to be transferred to the ICU and to die.
| No. (%) | ICU Transfer, No. (%) | Mortality, No. (%) | |
|---|---|---|---|
| |||
| Number of failed organs, N = 64 | |||
| 1 | 15 (23%) | 0 (0%) | 0 (0%) |
| 2 | 25 (39%) | 2 (8%) | 0 (0%) |
| 3 | 7 (11%) | 2 (29%) | 1 (14%) |
| 4 | 10 (16%) | 6 (60%) | 3 (30%) |
| >4 | 7 (11%) | 6 (86%) | 4 (57%) |
| Types of organ system dysfunction, all patients, N = 64*?>a | |||
| Cardiovascular | 42 (66%) | 16 (38%)b | 8 (19%)c |
| Renal | 41 (64%) | 10 (24%)b | 5 (12%)c |
| Central nervous system | 35 (54%) | 14 (40%)b | 7 (18%)c |
| Pulmonary | 19 (30%) | 12 (63%)b | 8 (42%)c |
| Hematologic | 15 (23%) | 6 (40%)b | 6 (40%)c |
| GI (ileus) | 8 (13%) | 5 (63%)b | 1 (13%)c |
| Hepatic | 5 (8%) | 4 (80%)b | 2 (40%)c |
DISCUSSION
Severe sepsis was common among patients admitted to the general medical ward in this tertiary care center. Our patient cohort differed in important ways from previously described typical cases of severe sepsis among ICU populations. Severe sepsis on the general medical wards was more commonly associated with Gram‐negative pathogens in the setting of genitourinary tract infections. This is in contrast to Gram‐positive organisms and respiratory tract infections, which are more common in the ICU.[3, 10] Renal and cardiac dysfunction were commonly observed organ failures, whereas in the ICU, severe sepsis has been reported to more likely involve respiratory failure. These results suggest that hospitalists seeking to provide evidence‐based care to prevent postsepsis morbidity and mortality for their non‐ICU patients need to heighten their index of suspicion when caring for an infected patient and appreciate that many severe sepsis patients may not fit neatly into traditional sepsis treatment algorithms.
Studies characterizing severe sepsis in the ICU setting indicate a predominance of pulmonary infections and respiratory failure with occurrence rates of 74% to 95% and 54% to 61%, respectively.[3, 12, 13] Given that either shock or pulmonary dysfunction is often required for admission to many ICUs, it is perhaps not surprising that these rates are dramatically different on the general medicine ward, with a relative scarcity of pulmonary infections (14%) and respiratory dysfunction (30%). Instead, genitourinary infections were noted in 41% (95% CI, 29%‐54%) of the cases, in contrast to the rates of genitourinary infections in ICU patients with severe sepsis, which have rates of 5.4% to 9.1%.[3, 10] Likely as a result of this, a Gram‐negative predominance is noted in the associated microbiology. Furthermore, our study indicates that C difficile and vancomycin‐resistant Enterococcus (VRE) species appear to represent an emerging cause of severe sepsis on the general medicine wards, as they have not been noted to be causative micro‐organisms in previous studies of sepsis. This is concordant with other studies showing increases in incidence and severity of disease for C difficile as well as VRE.[14, 15]
Previous epidemiologic studies of severe sepsis originating outside the ICU are lacking, but some work has been done. One study on the epidemiology of sepsis both with and without organ dysfunction aggregated all hospitalized patients and included those both admitted to the general medicine wards and directly to the ICU.[7] Similar to our study, this study also found a predominance of Gram‐negative causative organisms, as well as comparable in‐hospital mortality rates (12.8% vs 13%). Additionally, genitourinary infections were noted in 20% of the patients, notably higher than rates reported to have been found in patients with severe sepsis in the ICU, but not the magnitude found in our study, perhaps as a result of the combined ICU‐ward population studied. A similar high prevalence of genitourinary infections was also noted in a recent administrative data‐based study of emergency medical services‐transported patients with severe sepsis, half of whom required intensive care during their hospitalization.[16]
Our study is unique in that it focuses on severe sepsis in patients, commonly cared for by hospitalists, who were admitted to the general medical ward, and uses patient level data to elucidate more characteristics of the defining organ dysfunction. Furthermore, our results suggest that severe sepsis was poorly documented in this setting, indicating a potential impact on billing, coding, case mix index, and hospital mortality statistics that rely on very specific wording, as well as a possible need for increased awareness among hospitalists. Without this awareness, an opportunity may be missed for improved patient care via specific sepsis‐targeted measures,[13, 17, 18] including more aggressive resuscitative measures[19] or intensive physical and occupational therapy interventions aimed at impacting the cognitive and functional debilities[20] that result from severe sepsis. Highlighting this growing need to better assist clinicians assess the severity of septic patients and recognize these complex cases on the general medicine wards, 1 recent study evaluated the fitness of several clinical disease‐severity scoring systems for patients with sepsis in general internal medicine departments.[21] Perhaps with the help of tools such as these, which are being piloted in some hospitals, the care of this growing population can be enhanced.
Our study has a number of limitations that should be kept in mind. First, this is a single center study performed at an academic tertiary care center with a relatively high incidence of immunosuppression, which may influence the spectrum of infecting organisms. Our center also has a relatively large, closed‐model ICU, which often operates at near capacity, potentially affecting the severity of our non‐ICU population. Second, although we screened a large number of patients, as necessitated by our intensive and detailed review of clinical information, our sample size with hospitalist‐validated severe sepsis is relatively small. With this small sample size, less prevalent infections, patient characteristics, and organ dysfunctions may by chance have been under or over‐represented, and one could expect some variance in the occurrence rates of organ system dysfunction and infection rates by sampling error alone. Further larger scale studies are warranted to confirm these data and their generalizability. Third, the data necessary to calculate sequential organ failure assessment or multiple organ dysfunction score were not collected. This may limit the ability to directly compare the organ dysfunction noted in this study with others. Additionally, given the ICC definitions of organ dysfunction, some of the organ dysfunction noted, particularly for neurological dysfunction, was reliant on subjective clinical findings documented in the record. Finally, we relied on the lack of specific terminology to indicate a lack of documentation of sepsis, which does not necessarily indicate a lack of recognition or undertreatment of this condition. However, these limitations are offset by the strengths of this study, including the patient‐level medical record validation of severe sepsis by trained hospitalist physicians, high kappa statistic, and strict application of guideline‐based definitions.
This work has important implications for both clinicians and for future research on severe sepsis. The results suggest that severe sepsis may be quite common outside the ICU, and that patients presenting with this condition who are admitted to general medical wards are not routinely characterized by the profound hypoxemia and refractory shock of iconic cases. Certainly, further study looking at larger numbers of cases is needed to better understand the specifics and nuances of this important topic as well as to further evaluate clinicians' ability to recognize and treat such patients in this setting. Furthermore, future research on the treatment of severe sepsis, including both antimicrobials and disease‐modifying agents (eg, anti‐inflammatories) must continue to include and even focus on this large population of non‐ICU patients with severe sepsis, as the risk/benefit ratios of such potential treatments may vary with severity of illness.
In conclusion, severe sepsis was commonly found in patients admitted on the general medicine wards. The epidemiology of the infections and resultant organ dysfunction appears to differ from that found in the ICU. More studies are needed to provide a deeper understanding of this disease process, as this will enable clinicians to better recognize and treat patients thus afflicted, no matter the setting.
Acknowledgments
The authors thank Laetitia Shapiro, AM, for her programming assistance.
Disclosures: This work was supported in part by the US National Institutes of HealthK08, HL091249 (TJI) and the University of Michigan SpecialistHospitalist Allied Research Program (SHARP). This work was also supported in part by VA Ann Arbor Healthcare System, Geriatric Research Education and Clinical Center (GRECC).
The International Consensus Conference (ICC) for sepsis defines severe sepsis as an infection leading to acute organ dysfunction.[1, 2] Severe sepsis afflicts over 1 million patients each year in Medicare alone, and is substantially more common among older Americans than acute myocardial infarction.[3, 4, 5] Recently, the Agency for Healthcare Research and Quality identified severe sepsis as the single most expensive cause of hospitalization in the United States.[6] The incidence of severe sepsis continues to rise.[4, 5]
Severe sepsis is often mischaracterized as a diagnosis cared for primarily in the intensive care unit (ICU). Yet, studies indicate that only 32% to 50% of patients with severe sepsis require ICU care, leaving the majority on the general care wards.[7, 8] These studies also reveal mortality rates of 26% to 30% among patients with severe sepsis who are not admitted to an ICU compared to 11% to 33% in the ICU.[7, 8]
Although a number of epidemiologic and interventional studies have focused on severe sepsis in the ICU,[3, 9, 10] much less is known about patients cared for on the general medicine wards. Without this information, clinicians cannot make informed choices about important management decisions such as targeted diagnostic testing, empirical antimicrobials, and other therapies. To this end, we sought to further characterize the infectious etiologies and resultant organ system dysfunctions in the subset of patients with severe sepsis admitted to non‐ICU medical services at a tertiary academic medical center.
METHODS
Population/Setting
All hospitalizations of adult patients (18 years old) who were initially admitted to non‐ICU medical services at the University of Michigan Hospital during 2009 through 2010 were included. The University of Michigan Hospital has 610 general medical‐surgical beds, including telemetry beds, with closed ICUs comprised of 179 beds staffed by intensivists. Patients transferred from other hospitals and those admitted to non‐medical services were excluded.
Data Abstraction and Definitions
All International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes for hospitalizations were screened using a previously published and validated algorithm for severe sepsis.[11] Following this screening, 3 randomly selected round‐numbered batches of hospitalizations were sampled with subsequent application of the exclusion criteria. Medical records including physicians' notes, consultants' notes, nurses' notes, physical therapy notes, discharge coordinators' notes, emergency room flow sheets, as well as ward flow sheets were reviewed in detail by 3 practicing hospitalists using a structured instrument closely aligned with the ICC definition of severe sepsis.[2] We also sampled a smaller number of patients whose ICD‐9‐CM diagnoses screened negative for severe sepsis. Sample size was selected as part of a project with multiple objectives, and reflected a pragmatic balance between the anticipated precision of the results and the resources available to conduct chart review.[11] All discrepancies were reconciled among the 3 reviewers.
Reviewers first assessed whether infection was present, then evaluated for evidence of each organ system dysfunction, and finally determined the extent to which those organ dysfunctions were a response to the infection. Infection was defined either as a patient with a microbiologic culture growing a pathologic organism in a normally sterile site or documentation of a suspected infection with other confirmatory evidence (radiological, physical exam finding) with resultant systemic inflammatory response and administration of antimicrobials. Community‐acquired and healthcare‐associated infections were not differentiated. Microbiologic data, confirmatory tests, and site of infection were abstracted in detail.
Organ dysfunction was defined as per the 2001 ICC criteria,[2] and was assessed for neurological, pulmonary, cardiovascular, renal, gastrointestinal, hematological, and hepatic system involvement in all patients. A summary of these clinical definitions is included in Table 1. Data on important comorbidities were also abstracted. Immunosuppression was defined as having any of the following: solid organ transplant, bone marrow/stem cell transplant, human immunodeficiency virus/acquired immunodeficiency syndrome, neutropenia (absolute neutrophil count <1000), hematologic malignancy, solid organ malignancy with chemotherapy within the past 12 months, or pharmacologic immunosuppression (prednisone >20 mg daily for >4 weeks, calcineurin inhibitor, methotrexate, tumor necrosis factor inhibitors, azathioprine, sulfasalazine, hydroxychloroquine). Last, each chart was evaluated for the presence of explicit documentation with the presence of the words or phrases: sepsis, septic shock, or severe sepsis, indicating that the clinical service recognized and fully documented that a patient had severe sepsis.
| Organ System | Parameters to Indicate Dysfunction |
|---|---|
| |
| Cardiovascular | Systolic BP <90, elevated lactate, MAP <70, requiring pressors >2 hours, decrease in systolic BP of >40 |
| Renal | Creatinine increase >0.5 mg/dL, oliguria |
| Neurological | Acute mental status changes |
| Pulmonary | Intubation, BiPAP, supplemental oxygen >6 LPM or 40% face mask, PaO2/FiO2 <300 |
| Hematologic | INR >1.5 or PTT >60 not on anticoagulation, platelets <100 or 50% of baseline |
| Ileus | Decreased bowel motility requiring a change in diet |
| Hepatic | Bilirubin >4 mg/dL and >1.5 baseline |
Data Analysis
Methods for assessment of reviewer concordance have been previously described and were summarized using the kappa statistic.[11] Initial data extraction was performed in SAS 9.1 (SAS Institute, Cary, NC) and all analyses were conducted in Stata 12 (StataCorp LP, College Station, TX). Binomial 95% confidence intervals (CIs) are presented. This project was approved by the University of Michigan Institutional Review Board.
RESULTS
Of 23,288 hospitalizations examined from 2009 through 2010, the ICD‐9based automated screen for severe sepsis was positive for 3,146 (14 %). A random sample of 111 medical records, of which 92 had screened positive for severe sepsis and 19 had screened negative, was reviewed in detail. After review by the hospitalists, 64 of these 111 hospitalizations were judged to have severe sepsis, 61 of the 92 screened positive cases (66%), and 3 of the 19 screened negative cases (16%). The 3 reviewers had a kappa of 0.70, indicating good agreement.
Characteristics of the 64 patients with severe sepsis are shown in Table 2. The mean age was 63 years old (standard deviation [SD]=17.7), and 41% were male. The mean length of stay was 13.7 days (SD=20.8). Thirty‐nine percent (95% CI, 27%‐52%) of patients (25/64) were immunosuppressed. Of patients initially admitted to the general medical ward, 25% (16/64; 95% CI, 15%‐37%) ultimately required ICU care during their admission. The overall in‐hospital mortality rate was 13% (8/64; 95% CI, 6%‐23%). Immunosuppressed patients had a mortality rate of 20% and nonimmunosuppressed patients had a mortality rate of 8%. Only 47% (30/64; 95% CI, 34%‐60%) of the medical records had explicit clinician documentation of severe sepsis.
| Age, mean (SD), y | 63 (18) |
|---|---|
| |
| Male sex, no. (%) | 26 (41) |
| Preexisting conditions, no. (%) | |
| History of diabetes | 20 (31) |
| End stage renal disease on chronic dialysis | 2 (3) |
| Chronic obstructive pulmonary disease on oxygen | 3 (5) |
| History of cancer | 15 (23) |
| Liver cirrhosis | 5 (8) |
| Immunosuppression | 25 (39) |
| Median length of stay (days) | 7.5 |
| Mean length of stay (SD) | 13.7 (20.8) |
The most common site of infection was found to be the genitourinary system, occurring in 41% (26/64; 95% CI, 29%‐54%) of patients (Table 3). Pulmonary and intra‐abdominal sites were also common, accounting for 14% (95% CI, 6.6%‐25%) and 13% (95% CI, 5.6%‐23%) of sites, respectively. An infecting organism was identified by culture in 66% (42/64; 95% CI, 53%‐77%) of case patients with specific pathogens listed in Table 4. Among patients with positive culture results, the majority grew Gram‐negative organisms (57%; 95% CI, 41%‐72%). Non‐Clostridium difficile Gram‐positive organisms were also prominent and identified in 48% (95% CI, 32%‐64%) of positive cultures. Candida was less common (12%, 95% CI, 4.0%‐26%). Fourteen cases (22%, 95% CI, 10%‐30%) had 2 or more concomitant infectious pathogens.
| Site | No. (%) |
|---|---|
| |
| Genitourinary | 26 (41) |
| Pulmonary | 9 (14) |
| Intra‐abdominal (not intraluminal) | 8 (13) |
| Bloodstream/cardiac | 5 (8) |
| Skin and soft tissue | 4 (6) |
| GI lumen | 4 (6) |
| Joint | 2 (3) |
| Multiple sites | 4 (6) |
| Unknown | 2 (2) |
| Absolute Frequency, Total Positive Culture Results, N=64, No. (%)*?>a | Patients With Cultures Growing at Least One of the Pathogens, N=42, No. (%)*?>a | |
|---|---|---|
| ||
| Gram‐negative pathogens | 30 (47) | 24 (57) |
| Escherichia coli | 12 (19) | 12 (29) |
| Escherichia coli (multidrug resistant) | 2 (3) | 2 (5) |
| Klebsiella | 6 (9) | 5 (12) |
| Pseudomonas aeruginosa | 6 (9) | 4 (10) |
| Pseudomonas aeruginosa (multidrug resistant) | 2 (3) | 2 (5) |
| Otherb | 6 (9) | 6 (14) |
| Gram‐positive pathogens | 29 (45) | 25 (59) |
| Enterococcus | 14 (22) | 13 (31) |
| Vancomycin‐resistant Enterococcus species | 5 (8) | 4 (10) |
| Staphylococcus aureus | 7 (11) | 7 (17) |
| Methicillin‐resistant Staphylococcus aureus | 3 (5) | 3 (7) |
| Streptococcus pneumoniae | 2 (3) | 2 (5) |
| Coagulase‐negative staphylococci | 1 (2) | 1 (2) |
| Clostridium difficile | 5 (8) | 5 (12) |
| Fungi | ||
| Candida species | 5 (8) | 5 (12) |
| Mycobacterium avium | 1 (2) | 1 (2) |
| Two organisms | 9 (21) | |
| Three or more organisms | 5 (12) | |
All 64 patients had at least 1 organ dysfunction, as required by the ICC definition of severe sepsis. Organ dysfunction in 2 or more organ systems occurred in 77% (95% CI, 64%‐86%) of the cases (49/64). The incidence for each organ system dysfunction is presented in Table 5, as well as its relationship to both mortality and ICU admission. The most common organ system dysfunctions were found to be cardiovascular (hypotension) and renal dysfunction occurring in 66% and 64% of the cases, respectively. In this non‐ICU population, pulmonary dysfunction occurred in 30% of cases, but was frequently associated with transfer to the ICU, as 63% of the patients with pulmonary failure required ICU care. Patients with more organ systems affected were more likely to be transferred to the ICU and to die.
| No. (%) | ICU Transfer, No. (%) | Mortality, No. (%) | |
|---|---|---|---|
| |||
| Number of failed organs, N = 64 | |||
| 1 | 15 (23%) | 0 (0%) | 0 (0%) |
| 2 | 25 (39%) | 2 (8%) | 0 (0%) |
| 3 | 7 (11%) | 2 (29%) | 1 (14%) |
| 4 | 10 (16%) | 6 (60%) | 3 (30%) |
| >4 | 7 (11%) | 6 (86%) | 4 (57%) |
| Types of organ system dysfunction, all patients, N = 64*?>a | |||
| Cardiovascular | 42 (66%) | 16 (38%)b | 8 (19%)c |
| Renal | 41 (64%) | 10 (24%)b | 5 (12%)c |
| Central nervous system | 35 (54%) | 14 (40%)b | 7 (18%)c |
| Pulmonary | 19 (30%) | 12 (63%)b | 8 (42%)c |
| Hematologic | 15 (23%) | 6 (40%)b | 6 (40%)c |
| GI (ileus) | 8 (13%) | 5 (63%)b | 1 (13%)c |
| Hepatic | 5 (8%) | 4 (80%)b | 2 (40%)c |
DISCUSSION
Severe sepsis was common among patients admitted to the general medical ward in this tertiary care center. Our patient cohort differed in important ways from previously described typical cases of severe sepsis among ICU populations. Severe sepsis on the general medical wards was more commonly associated with Gram‐negative pathogens in the setting of genitourinary tract infections. This is in contrast to Gram‐positive organisms and respiratory tract infections, which are more common in the ICU.[3, 10] Renal and cardiac dysfunction were commonly observed organ failures, whereas in the ICU, severe sepsis has been reported to more likely involve respiratory failure. These results suggest that hospitalists seeking to provide evidence‐based care to prevent postsepsis morbidity and mortality for their non‐ICU patients need to heighten their index of suspicion when caring for an infected patient and appreciate that many severe sepsis patients may not fit neatly into traditional sepsis treatment algorithms.
Studies characterizing severe sepsis in the ICU setting indicate a predominance of pulmonary infections and respiratory failure with occurrence rates of 74% to 95% and 54% to 61%, respectively.[3, 12, 13] Given that either shock or pulmonary dysfunction is often required for admission to many ICUs, it is perhaps not surprising that these rates are dramatically different on the general medicine ward, with a relative scarcity of pulmonary infections (14%) and respiratory dysfunction (30%). Instead, genitourinary infections were noted in 41% (95% CI, 29%‐54%) of the cases, in contrast to the rates of genitourinary infections in ICU patients with severe sepsis, which have rates of 5.4% to 9.1%.[3, 10] Likely as a result of this, a Gram‐negative predominance is noted in the associated microbiology. Furthermore, our study indicates that C difficile and vancomycin‐resistant Enterococcus (VRE) species appear to represent an emerging cause of severe sepsis on the general medicine wards, as they have not been noted to be causative micro‐organisms in previous studies of sepsis. This is concordant with other studies showing increases in incidence and severity of disease for C difficile as well as VRE.[14, 15]
Previous epidemiologic studies of severe sepsis originating outside the ICU are lacking, but some work has been done. One study on the epidemiology of sepsis both with and without organ dysfunction aggregated all hospitalized patients and included those both admitted to the general medicine wards and directly to the ICU.[7] Similar to our study, this study also found a predominance of Gram‐negative causative organisms, as well as comparable in‐hospital mortality rates (12.8% vs 13%). Additionally, genitourinary infections were noted in 20% of the patients, notably higher than rates reported to have been found in patients with severe sepsis in the ICU, but not the magnitude found in our study, perhaps as a result of the combined ICU‐ward population studied. A similar high prevalence of genitourinary infections was also noted in a recent administrative data‐based study of emergency medical services‐transported patients with severe sepsis, half of whom required intensive care during their hospitalization.[16]
Our study is unique in that it focuses on severe sepsis in patients, commonly cared for by hospitalists, who were admitted to the general medical ward, and uses patient level data to elucidate more characteristics of the defining organ dysfunction. Furthermore, our results suggest that severe sepsis was poorly documented in this setting, indicating a potential impact on billing, coding, case mix index, and hospital mortality statistics that rely on very specific wording, as well as a possible need for increased awareness among hospitalists. Without this awareness, an opportunity may be missed for improved patient care via specific sepsis‐targeted measures,[13, 17, 18] including more aggressive resuscitative measures[19] or intensive physical and occupational therapy interventions aimed at impacting the cognitive and functional debilities[20] that result from severe sepsis. Highlighting this growing need to better assist clinicians assess the severity of septic patients and recognize these complex cases on the general medicine wards, 1 recent study evaluated the fitness of several clinical disease‐severity scoring systems for patients with sepsis in general internal medicine departments.[21] Perhaps with the help of tools such as these, which are being piloted in some hospitals, the care of this growing population can be enhanced.
Our study has a number of limitations that should be kept in mind. First, this is a single center study performed at an academic tertiary care center with a relatively high incidence of immunosuppression, which may influence the spectrum of infecting organisms. Our center also has a relatively large, closed‐model ICU, which often operates at near capacity, potentially affecting the severity of our non‐ICU population. Second, although we screened a large number of patients, as necessitated by our intensive and detailed review of clinical information, our sample size with hospitalist‐validated severe sepsis is relatively small. With this small sample size, less prevalent infections, patient characteristics, and organ dysfunctions may by chance have been under or over‐represented, and one could expect some variance in the occurrence rates of organ system dysfunction and infection rates by sampling error alone. Further larger scale studies are warranted to confirm these data and their generalizability. Third, the data necessary to calculate sequential organ failure assessment or multiple organ dysfunction score were not collected. This may limit the ability to directly compare the organ dysfunction noted in this study with others. Additionally, given the ICC definitions of organ dysfunction, some of the organ dysfunction noted, particularly for neurological dysfunction, was reliant on subjective clinical findings documented in the record. Finally, we relied on the lack of specific terminology to indicate a lack of documentation of sepsis, which does not necessarily indicate a lack of recognition or undertreatment of this condition. However, these limitations are offset by the strengths of this study, including the patient‐level medical record validation of severe sepsis by trained hospitalist physicians, high kappa statistic, and strict application of guideline‐based definitions.
This work has important implications for both clinicians and for future research on severe sepsis. The results suggest that severe sepsis may be quite common outside the ICU, and that patients presenting with this condition who are admitted to general medical wards are not routinely characterized by the profound hypoxemia and refractory shock of iconic cases. Certainly, further study looking at larger numbers of cases is needed to better understand the specifics and nuances of this important topic as well as to further evaluate clinicians' ability to recognize and treat such patients in this setting. Furthermore, future research on the treatment of severe sepsis, including both antimicrobials and disease‐modifying agents (eg, anti‐inflammatories) must continue to include and even focus on this large population of non‐ICU patients with severe sepsis, as the risk/benefit ratios of such potential treatments may vary with severity of illness.
In conclusion, severe sepsis was commonly found in patients admitted on the general medicine wards. The epidemiology of the infections and resultant organ dysfunction appears to differ from that found in the ICU. More studies are needed to provide a deeper understanding of this disease process, as this will enable clinicians to better recognize and treat patients thus afflicted, no matter the setting.
Acknowledgments
The authors thank Laetitia Shapiro, AM, for her programming assistance.
Disclosures: This work was supported in part by the US National Institutes of HealthK08, HL091249 (TJI) and the University of Michigan SpecialistHospitalist Allied Research Program (SHARP). This work was also supported in part by VA Ann Arbor Healthcare System, Geriatric Research Education and Clinical Center (GRECC).
- , , , et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- , , , et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
- , , , , , . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , , . Population burden of long‐term survivorship after severe sepsis in older americans. J Am Geriatr Soc. 2012;60(6):1070–1077.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. October 2011. In: Healthcare Cost and Utilization Project Statistical Briefs. Rockville, MD: Agency for Health Care Policy and Research; 2006. Available from: http://www.ncbi.nlm.nih.gov/books/NBK65391. Accessed June 2, 2012.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35(5):1284–1289.
- , , , , . Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33(1):71–80.
- , , , et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis‐related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307(22):2390–2399.
- , , , , . Incidence and impact of organ dysfunctions associated with sepsis. Chest. 2005;127(3):942–951.
- , , , et al. Identifying patients with severe sepsis using administrative claims: patient‐level validation of the Angus Implementation of the International Consensus Conference definition of severe sepsis [published online ahead of print September 18, 2012]. Medical Care. doi: 10.1097/MLR.0b013e318268ac86.
- , , , . Current epidemiology of septic shock: the CUB‐Rea Network. Am J Respir Crit Care Med. 2003;168(2):165–172.
- . Management of sepsis. N Engl J Med. 2006;355(16):1699–1713.
- , , . Current status of Clostridium difficile infection ipidemiology. Clin Infect Dis. 2012;55(suppl 2):S65–S70.
- , . Vancomycin‐resistant enterococci. Semin Respir Infect. 2000;15(4):314–326.
- , , , , , . Severe sepsis in prehospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med. 2012;186(12):1264–1271.
- , . Novel Therapies for Septic Shock Over the Past 4 Decades. JAMA. 2011;306(2):194–199.
- , , , et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med. 2010;38(4):1036–1043.
- , . Diagnosis and treatment of severe sepsis. Crit Care. 2007;11(suppl 5):S2.
- , , , . Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794.
- , , , , . Assessment of disease‐severity scoring systems for patients with sepsis in general internal medicine departments. Crit Care. 2011;15:R95.
- , , , et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.
- , , , et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
- , , , , , . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , , . Population burden of long‐term survivorship after severe sepsis in older americans. J Am Geriatr Soc. 2012;60(6):1070–1077.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. October 2011. In: Healthcare Cost and Utilization Project Statistical Briefs. Rockville, MD: Agency for Health Care Policy and Research; 2006. Available from: http://www.ncbi.nlm.nih.gov/books/NBK65391. Accessed June 2, 2012.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35(5):1284–1289.
- , , , , . Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33(1):71–80.
- , , , et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis‐related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307(22):2390–2399.
- , , , , . Incidence and impact of organ dysfunctions associated with sepsis. Chest. 2005;127(3):942–951.
- , , , et al. Identifying patients with severe sepsis using administrative claims: patient‐level validation of the Angus Implementation of the International Consensus Conference definition of severe sepsis [published online ahead of print September 18, 2012]. Medical Care. doi: 10.1097/MLR.0b013e318268ac86.
- , , , . Current epidemiology of septic shock: the CUB‐Rea Network. Am J Respir Crit Care Med. 2003;168(2):165–172.
- . Management of sepsis. N Engl J Med. 2006;355(16):1699–1713.
- , , . Current status of Clostridium difficile infection ipidemiology. Clin Infect Dis. 2012;55(suppl 2):S65–S70.
- , . Vancomycin‐resistant enterococci. Semin Respir Infect. 2000;15(4):314–326.
- , , , , , . Severe sepsis in prehospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med. 2012;186(12):1264–1271.
- , . Novel Therapies for Septic Shock Over the Past 4 Decades. JAMA. 2011;306(2):194–199.
- , , , et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med. 2010;38(4):1036–1043.
- , . Diagnosis and treatment of severe sepsis. Crit Care. 2007;11(suppl 5):S2.
- , , , . Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794.
- , , , , . Assessment of disease‐severity scoring systems for patients with sepsis in general internal medicine departments. Crit Care. 2011;15:R95.
Copyright © 2013 Society of Hospital Medicine
Hospitalist Time Usage and Cyclicality
Many academic medical centers (AMCs) employ hospitalists to provide care for patients on resident services as supervising attendings,1, 2 as well as on nonresident services.3 The number of hospitalists working on nonresident services at AMCs has grown exponentially, as the Accreditation Council for Graduate Medical Education (ACGME) implemented duty‐hour standards for residents.3 According to the latest Society of Hospital Medicine (SHM) estimates, the number of practicing hospitalists is projected to grow to 30,000 by 2010.4 As astonishing as this growth may sound, it is anticipated that more hospitalists will be needed to meet the demand for these physicians.5 Further, as financial realities require AMCs to be increasingly efficient without compromising patient care, and hospitalists provide a greater range of clinical services, it is important to better understand how hospitalists spend their time in the hospital. Understanding the daily work flow of hospitalists can identify how these physicians can be better supported. A previous report by O'Leary et al.6 highlighted how hospitalists spent their time during their usual day shifts at an AMC. It is important to validate their study to determine broadly applicable findings. We performed a time‐motion study where we followed the admitting hospitalists during the day and night shifts. We felt it was important to focus on hospitalists who are admitting patients, as this has potential patient safety and quality implications related to multitasking, triaging, and helping patients navigate through a complex admission process involving multiple clinical services. Our goal was to better understand how the flow of patients impacted these physicians, and determine how our hospitalists spent their time providing direct and indirect patient care‐related activities. In addition, we looked for predictable variations in activities throughout the day that might be associated with the timely care of patients.
Materials and Methods
Setting
The University of Michigan Health System (UMHS) is a tertiary care AMC, with more than 800 beds, and over 34,000 annual adult discharges. Internal Medicine services comprise a large proportion of those discharged, accounting for over 17,000 discharges per year; and is projected to grow at an annual rate of 4%. As service caps and work‐hour restrictions have limited the total number of patients that medical residents are able to care for, our hospitalist group has increased the number of physicians on the nonresident hospitalist service. At the time of the study, there were 23 hospitalists, equivalent to 18.25 full‐time equivalents (FTEs), staffing the service. The hospitalists provide in‐house patient care 24 hours a day and 7 days a week. Hospitalists also provide general medicine consult services, surgical comanagement and perioperative care, procedures, inpatient cardiopulmonary arrest response, rapid response team supervision, and observation care; and are also the primary inpatient physicians for many of the hospitalized interventional radiology and dermatology patients. These direct patient care activities account for 4500 annual discharges from the nonresident service.
Data Collection
Four university undergraduate business administration program students shadowed 11 hospitalists over a 3‐week period in 4‐hour to 12‐hour time blocks. The students followed the hospitalist on the shift that was taking admission calls, during day and night. A data collection tool was designed to track physicians' actions in 1‐minute increments, using categories similar to those used in a previously published time‐motion study of hospitalists' activities (Table 1).6 Physicians' activities each minute were assigned to a single category that most represented their action during that time period. At our AMC, 6 hospitalists work during the day shifts, and 2 on the night shifts. Our hospitalists may have patients in any of the 14 general care units in the hospital, as our hospitalists' services are not geographically based. The day hospitalists' shifts are scheduled from 7 AM to 7 PM. Two of the 6 hospitalists rotate through a 3‐day cycle as the admitting physician. Their duties include triaging and admitting patients until 2 PM, providing the day‐to‐day care for their patients until 7 PM, and occasionally cross‐covering for the other day‐shift hospitalists that have left for the day. The 4 other day‐shift hospitalists, not on their rotation as the admitting physician, may sign out and leave as early as 4 PM if their work for the day is done. At 2 PM, a separate swing‐shift hospitalist takes over the role of triaging and admitting until 7 PM. During the day shift, consults and perioperative management of patients are provided by a separate hospitalist on the consult service. At 7 PM, 2 nocturnists arrive for their 7 PM to 7 AM shift. The nocturnists, in addition to cross‐covering service patients, admit a maximum of 6 patients each, or until midnightwhichever comes first.
| Category | Code | Description |
|---|---|---|
| Direct patient care | DPIH | Initial history |
| DPDI | Discharge instructions | |
| DPFM | Family meetings | |
| DPRV | Revisit | |
| DPCC | Cross‐cover | |
| Indirect patient care | ||
| Documentation | IDGD | General documentation |
| IDDN | Daily notes | |
| IDDD | Discharge navigator | |
| Records/Results | IPMR | Review medical records |
| Communication | ICHH | Patient handoffs |
| ICFF | Face‐to‐face | |
| ICIP | Incoming page | |
| ICOP | Outgoing page | |
| ICIC | Incoming call | |
| ICOC | Outgoing call | |
| ICEE | E‐mail communications | |
| ICDP | Discharge planner | |
| Orders | IOWO | Writing orders |
| Professional development | PDRR | Reading articles, textbooks, references |
| Education | EEWR | Teaching during work rounds |
| Travel | TTTT | Travel |
| Personal | PPPP | Personal |
| Down time | DDDD | Downtime |
The students observed 11 different hospitalists, and followed these physicians during 9 weekday shifts, 5 weekday swing shifts, 10 weekday night shifts, and 4 weekend night shifts. The variance in the number of each type of shifts monitored was likely due to scheduling limitations of the students. In total, they collected data on 8,915 minutes of hospitalists' activities. The students monitored the hospitalists representing time periods from 7 AM to 2 AM. Analysis from 2 AM to 7 AM was excluded, because after 2 AM the hospitalists did not routinely evaluate new patients with the exception of emergent requests. New admissions after midnight are handled by a night float service staffed by residents.
Results
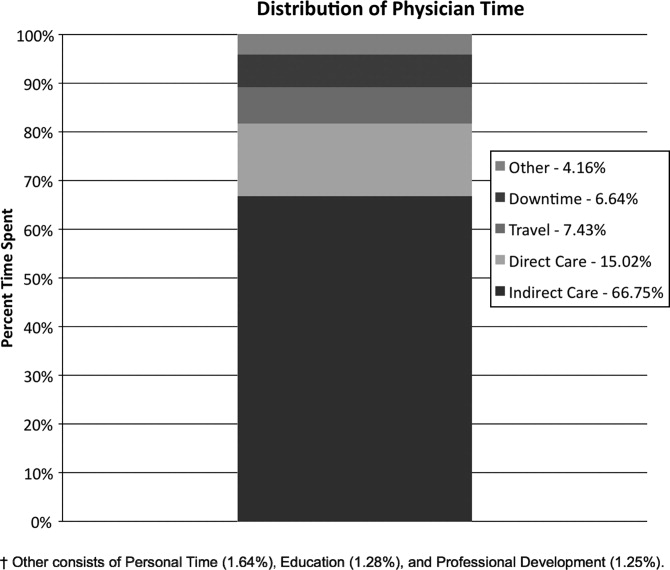
Overall, time spent on patient care activities comprised the bulk of hospitalists' shifts (82%) (Figure 1). Patient care activities were further categorized as direct patient caredefined as face‐to‐face patient or family time; and indirect patient caredefined as activities related to patient care, but without patient or family contact. Direct and indirect patient care accounted for 15% and 67% of the hospitalists' time, respectively. The other 18% of the hospitalists' time spent in the hospital were broadly categorized into: professional development, education, personal, downtime, and travel. Professional development included activities such as looking up information (eg, literature search); education included times that hospitalists spent with residents or medical students; personal time included only restroom and food breaks; and travel included time spent moving from 1 area to the next during their shift.
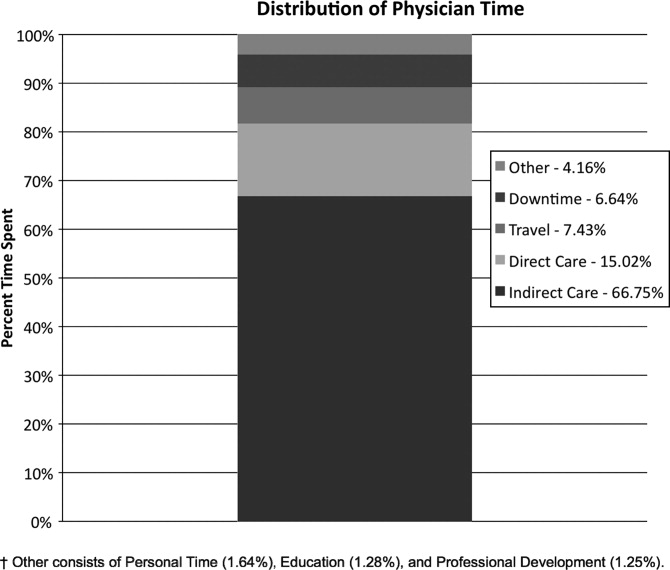
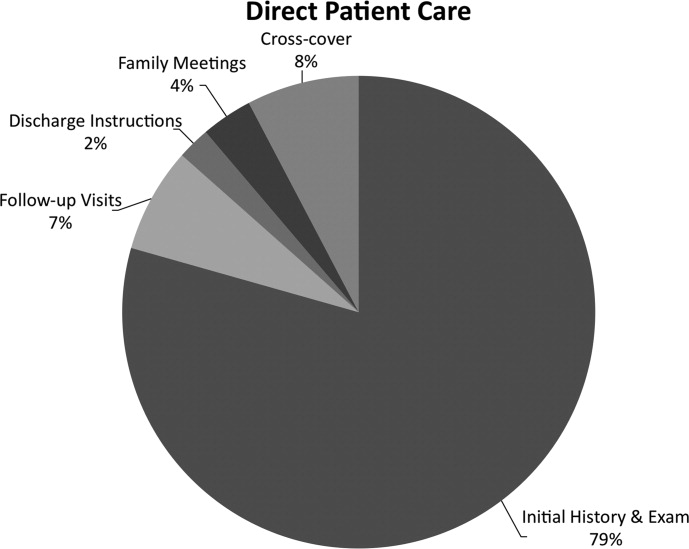
The majority of the hospitalists' direct patient care time was spent on evaluating new patients (79%). Significantly smaller amounts of time were spent on other direct care activities: cross‐covering other patients (8%), follow‐up visits (7%), family meetings (4%), and discharge instructions (2%) (Figure 2).
Indirect patient care activities included, 41% of time used to communicate with other healthcare providers, 26% on medical documentation, 20% reviewing medical records and results, and 13% of time writing orders (Figure 3). Communication accounted for a large proportion of a hospitalists' work, and included telephone conversations with Emergency Department (ED) or other admitting providers, handoffs, paging, face‐to‐face conversations with consultants and other support staff, and e‐mail.
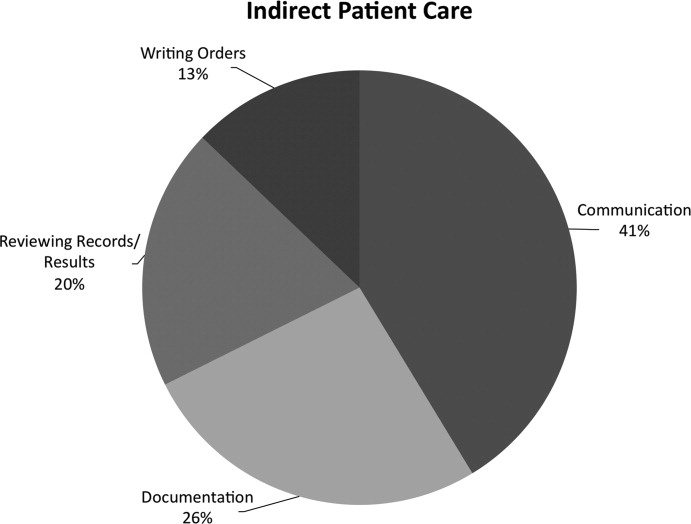
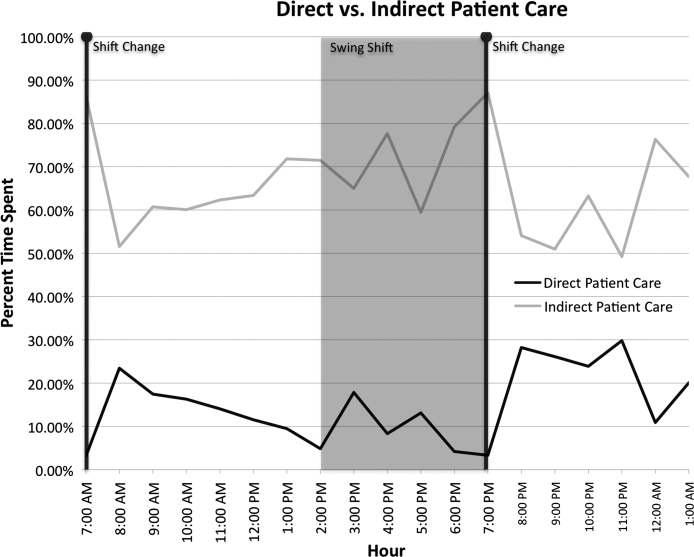
Figure 4 shows the hourly distribution of time spent on direct and indirect patient care by a hospitalist throughout the day. The day‐time hospitalists pick up their signout from the nocturnists at 7 AM to begin their shift. The swing hospitalists arrive at 2 PM during the weekdays, and their primary duty is to triage and admit patients until 7 PM. The nocturnists start their shift at 7 PM, at which time the daytime and swing‐shift hospitalists all sign out for the night.
Discussion
Hospitalists on the nonresident service at our AMC utilize about 15% of their time on face‐to‐face patient care activities, 67% on indirect patient care activities, and 7% of time on moving from 1 part of the hospital to another. Hospitalists are valuable members of the physician work force who address the increasing patient care demands in the face of increasing limitations on residency work‐hours, a growing aging population, and existing inefficiencies in AMCs. The only other work‐flow study of hospitalists of which we are aware provided a single institution's perspective on time utilization by hospitalists. Our study in a different AMC setting revealed strong consistency with the O'Leary et al.6 study in the fraction of time hospitalists spent on direct patient care (15% and 18%, respectively), indirect patient care (67% and 69%); and within indirect patient care the time spent on documentation (26% and 37% of total time) and communications (41% and 35%). While travel in the O'Leary et al.6 study took up only 3% of hospitalists' time, the conclusions in that paper clearly suggest that the authors consider it an area of concern. Our study found that travel accounted for over 7% of hospitalists' time, confirming that intuition. The significant travel time may in part reflect the effects of a non‐geographically‐located hospitalist service. From these 2 studies we can be more confident that in large, tertiary care AMCs the time hospitalists spend on indirect patient care dominates that for direct patient care (by a factor of 4 in these studies), that within indirect patient care documentation and communication are dominant activities, and that travel can take a significant amount of time when patients are dispersed throughout the facility.
Both studies demonstrated that communication accounted for a significant proportion of a hospitalist's time. In our study communication accounted for 28% of their total time in the hospital, and 41% of the indirect patient care portion (Figure 3). A closer look within our communication category revealed that phone calls and handoffs accounted for two‐thirds of all communication time observed. As the hospitalists who carry the admitting pager, they receive the pages to take admission calls, but also take calls from consultants who have recommendations, as well as from nursing and other hospital staff. Depending on the nature of the conversation, the phone calls can last several minutes. While ensuring the communication between health care providers is complete and thorough, there may be opportunities to develop novel approaches to the way hospitalists communicate with other care providers. For example, at the UMHS, alternative communication methods with nursing staff have been proposed such as utilizing a website or a handheld device to help hospitalists prioritize their communications back to the nursing staff7; while standardizing the intake information from the ED or other admitting providers may help reduce the total time spent on phone calls. We will need to further explore the potential benefits of these ideas in future work.
Our data also reveal an interesting cyclicality of daily activities for the hospitalists, as shown in Figure 4. We identified batching behaviors throughout the day, which cause delays in seeing patients and can be deleterious to smooth workflows in support services. Spikes in indirect patient care, followed closely by spikes in direct patient care, occur regularly at shift changes (7 AM, 2 PM, and 7 PM). Also, in the night shift, indirect patient care drops to its lowest levels (in % of time spent) throughout the day, and direct patient care reaches its highest levels. The day‐shift indirect care profile is counter‐cyclical with direct care, as the hospitalist shifts between direct care and indirect care depending on the time of the day. We discuss these phenomena in turn.
It is known that variability in any operation causes congestion and delay, as an unavoidable consequence of the physics of material and information flows.8 Indeed, an entire subindustry based on Lean manufacturing principles has evolved from the Toyota Production System based on the elimination of unnecessary variability in operations.9 Lean processes have been ongoing in manufacturing facilities for decades, and these efforts are just recently being embraced by the service sector in general, and health care specifically.10, 11 Batching is an extreme form of variability, where there is a lull in the amount of work being done and then a burst of work is done over a short period of time. This means that jobs pile up in the queue waiting for the next spike of activity. Our data indicate batching seems to be a common phenomenon for our hospitalists. The majority of the patients admitted to our hospitalist service are unscheduled admissions that arrive primarily through the ED. One potential result of the unscheduled admissions is that patients could be referred to our hospitalist service at a pace that is not well predictable on an hour‐to‐hour basis. This could lead to an unintended result of multiple patients admitted over a short period of time. This means that many patients wait for intake, delaying the onset of their care by the inpatient physician. Also, since an initial exam often results in orders for laboratory tests and studies, batching on the floor will translate into batching of orders going to nursing, pathology, radiology, and other hospital support services. This imposes the cost of variability on these other services in the hospital. From a systems perspective, efficiency will improve if these activities can be smoothed throughout the day. This may suggest opportunities to work with the ED, to help smooth the inflow of patients into the hospital system.
Within the hospital, all of the day‐shift hospitalists can be reached about the needs of their respective patients, however, the physician carrying the admission pager also fields calls for admissions, and acts as the default contact person for the hospitalist group. As this hospitalist receives information on new admissions, he/she is aware of patients ready for intake but cannot evaluate them at the rate they are being referred, so the queue builds. This continues into the swing shift, which also fields referrals faster than they can attend to them. The volatility in indirect care during the swing shift, 2 PM to 7 PM, reflects a significant amount of triaging and fielding general calls about hospitalist patients. These activities further reduce the swing shift's ability to clear the intake queue. The night shift finally gets to these patients and, eventually, clears the queue. There may be an opportunity to consider the use of multiple input pagers or other process changes that can smooth this flow and rationalize the recurring tasks of finding patients and the responsible physician.
Another concept in Lean thinking is that variability is costly when it represents a mismatch between demand for a service and the capacity to serve. With regards to admitted patients, when demand outpaces capacity, patients will wait. When capacity outpaces demand, there is excess capacity in the system. The ideal is to match demand and capacity at all times, so nobody waits and the system carries no costly excess capacity. As the intake providers for admitted patients, we can attack this problem from the capacity side. Here, 2 generic Lean tactics are to: (1) reallocate resources to a bottleneck that is holding up the entire system, and (2) relieve workers of time‐consuming but non‐value‐adding work so they have more capacity to devote to serving demand. In our study, carrying multiple input pagers is an example of tactic (1), and efficient communication technologies and practices that reduce indirect time is an example of (2). Systemwide improvements would require further investigation by working with the variability on the input side (eg, ED admissions).
Our study also found that a significant percent of the time observed was spent traveling (7.4%) from room to room between different floors in the hospital. Travel time, which is non‐value‐adding, is one of the major forms of waste Lean thinking.12 Our hospitalists can provide care to patients at any of the general medical‐surgical beds we have available at our health system. These beds are distributed across 14 units on 5 different floors, as well as in the ED if a bed is not available for an admitted patient. In hospitals routinely operating at high occupancy, such as our AMC, patients often get distributed throughout the facility for lack of beds on the appropriate service's ward. One cost for this is a potential mismatch between a patient's needs and floor nurses' training. Our study reveals another cost, and that is its contribution to the significant amount of time hospitalists spent on travel, which is largely driven by the need to see dispersed patients. Reducing this cost requires a systemic, rather than service‐specific, solution. Our AMC is adding observation‐status beds to relieve some of the pressure on licensed beds, and considering bed management (including parts of the admissions and discharge processes) changes designed to promote better collocation of patients with services. Further study on these and other collocation tactics is warranted.
The spike in indirect activities at 4 PM represents, in part, an early signout by 1 or more of the hospitalists who are not scheduled to hold the admission pager, and have completed their work for the day. This handoff will be replicated at 7 PM when the nocturnists arrive for their night shift. In addition to a significant indirect load on physicians, multiple handoffs have been associated with decreased quality of care.13 Again, it is worthwhile considering the feasibility of alternative shift schedules that can minimize handoffs.
Finally, our findings revealed that a low percentage of time was dedicated to providing discharge instructions (2.24% of direct patient care time, and 0.34% of total time). Because the task of discharging patients falls primarily on the day‐shift hospitalists, when combined with swing‐shift and night‐shift hospitalists' data, the low percentage measured on discharge instructions may have been diluted. Nonetheless, this may point to the need for further investigation on how hospitalists provide direct patient encounter time during this critical phase of transition out of the hospital.
Our study is not without limitations. The student observers shadowed a representative group of hospitalists, but they were not able to follow everyone in the group. More specifically, their observations were made on the hospitalist who was carrying the primary hospitalist service admitting pager. Although it was the intent of our study to focus on the hospitalists we felt would be the busiest, our results may not be generalizable to all hospitalists. Although our research supports the previous findings by O'Leary et al.,6 a second limitation to our study is that our analysis was done at a single hospitalist group in an AMC, and hence the results may not be generalizable to other hospitalist groups. Another limitation may be that we did not do an evaluation of the hours between 2 AM to 7 AM. This period of time is used to catch up on medical documentation and to be available for medical emergencies. As more hospitalist programs are employing the use of nocturnists, it may be informative to have this time period tracked for activities.
Conclusions
Our study supports the broad allocation of hospitalist time found in an earlier study at a different AMC,6 suggesting that these might be generally representative in other AMCs. We found that travel constitutes a significant claim in hospitalists' time, due in part to the inability to collocate hospitalist service patients. Remedies are not likely to be service‐specific, but will require systemwide analyses of admission and discharge processes. Communication takes a significant amount of hospitalist time, with pages and phone calls related to handoffs accounting for most of the total communication time. As hospitalists working at non‐AMC settings may experience different work flow issues, we would like to see time‐motion studies of hospitalists in other types of hospitals. Future studies should also seek to better understand the how hospitals at high occupancy may reduce batching and streamline both the discharge and admission process, determine the factors that account for the significant communication time and how these processes could be streamlined, and evaluate the potential benefits of geographical localization of hospitalists' patients.
Acknowledgements
The authors thank Tracey Jackson, Michael Paulsen, Deepak Srinivasin, and Ryan Werblow, who were students in the undergraduate business school program, for their invaluable contribution in shadowing hospitalists to collect the time study data.
- , , , .Where should hospitalists sit within the academic medical center?J Gen Intern Med.2008;23:1269–1272.
- , .Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- , , , , .Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3:247–255.
- Society of Hospital Medicine. Society of Hospital Medicine Releases Results of the 2007–2008 Survey on the State of the Hospital Medicine Movement.2008. Available at: http://www.hospitalmedicine.org/AM/Template.cfm? Section=Press_Releases3:398–402.
- , , .How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- , .MCOMM: Redefining Medical Communication in the 21st Century, University of Michigan Health System. In: Society of Hospital Medicine Annual Meeting, Best of Innovations Presentation; 2009; Chicago, IL;2009.
- , .Factory Physics: Foundations of Manufacturing Management.Boston:Irwin, McGraw‐Hill;1996.
- .The Toyota Way.1st ed.Madison, WI:McGraw‐Hill;2004.
- Going Lean in Health Care.White Paper.Boston, MA:Institute for Healthcare Improvement;2005 January and February, 2005. Available at: http://www.ihconline.org/toolkits/LeanInHealthcare/GoingLeaninHealth CareWhitePaper.pdf. Accessed September 2009.
- , , , .Lean health care: what can hospitals learn from a world‐class automaker?J Hosp Med.2006;1:191–199.
- , , , , .Managing Business Process Flows.Upper Saddle River, NJ:Prentice Hall;2006.
- , .The patient handoff: medicine's Formula One moment.Chest.2008;134:9–12.
Many academic medical centers (AMCs) employ hospitalists to provide care for patients on resident services as supervising attendings,1, 2 as well as on nonresident services.3 The number of hospitalists working on nonresident services at AMCs has grown exponentially, as the Accreditation Council for Graduate Medical Education (ACGME) implemented duty‐hour standards for residents.3 According to the latest Society of Hospital Medicine (SHM) estimates, the number of practicing hospitalists is projected to grow to 30,000 by 2010.4 As astonishing as this growth may sound, it is anticipated that more hospitalists will be needed to meet the demand for these physicians.5 Further, as financial realities require AMCs to be increasingly efficient without compromising patient care, and hospitalists provide a greater range of clinical services, it is important to better understand how hospitalists spend their time in the hospital. Understanding the daily work flow of hospitalists can identify how these physicians can be better supported. A previous report by O'Leary et al.6 highlighted how hospitalists spent their time during their usual day shifts at an AMC. It is important to validate their study to determine broadly applicable findings. We performed a time‐motion study where we followed the admitting hospitalists during the day and night shifts. We felt it was important to focus on hospitalists who are admitting patients, as this has potential patient safety and quality implications related to multitasking, triaging, and helping patients navigate through a complex admission process involving multiple clinical services. Our goal was to better understand how the flow of patients impacted these physicians, and determine how our hospitalists spent their time providing direct and indirect patient care‐related activities. In addition, we looked for predictable variations in activities throughout the day that might be associated with the timely care of patients.
Materials and Methods
Setting
The University of Michigan Health System (UMHS) is a tertiary care AMC, with more than 800 beds, and over 34,000 annual adult discharges. Internal Medicine services comprise a large proportion of those discharged, accounting for over 17,000 discharges per year; and is projected to grow at an annual rate of 4%. As service caps and work‐hour restrictions have limited the total number of patients that medical residents are able to care for, our hospitalist group has increased the number of physicians on the nonresident hospitalist service. At the time of the study, there were 23 hospitalists, equivalent to 18.25 full‐time equivalents (FTEs), staffing the service. The hospitalists provide in‐house patient care 24 hours a day and 7 days a week. Hospitalists also provide general medicine consult services, surgical comanagement and perioperative care, procedures, inpatient cardiopulmonary arrest response, rapid response team supervision, and observation care; and are also the primary inpatient physicians for many of the hospitalized interventional radiology and dermatology patients. These direct patient care activities account for 4500 annual discharges from the nonresident service.
Data Collection
Four university undergraduate business administration program students shadowed 11 hospitalists over a 3‐week period in 4‐hour to 12‐hour time blocks. The students followed the hospitalist on the shift that was taking admission calls, during day and night. A data collection tool was designed to track physicians' actions in 1‐minute increments, using categories similar to those used in a previously published time‐motion study of hospitalists' activities (Table 1).6 Physicians' activities each minute were assigned to a single category that most represented their action during that time period. At our AMC, 6 hospitalists work during the day shifts, and 2 on the night shifts. Our hospitalists may have patients in any of the 14 general care units in the hospital, as our hospitalists' services are not geographically based. The day hospitalists' shifts are scheduled from 7 AM to 7 PM. Two of the 6 hospitalists rotate through a 3‐day cycle as the admitting physician. Their duties include triaging and admitting patients until 2 PM, providing the day‐to‐day care for their patients until 7 PM, and occasionally cross‐covering for the other day‐shift hospitalists that have left for the day. The 4 other day‐shift hospitalists, not on their rotation as the admitting physician, may sign out and leave as early as 4 PM if their work for the day is done. At 2 PM, a separate swing‐shift hospitalist takes over the role of triaging and admitting until 7 PM. During the day shift, consults and perioperative management of patients are provided by a separate hospitalist on the consult service. At 7 PM, 2 nocturnists arrive for their 7 PM to 7 AM shift. The nocturnists, in addition to cross‐covering service patients, admit a maximum of 6 patients each, or until midnightwhichever comes first.
| Category | Code | Description |
|---|---|---|
| Direct patient care | DPIH | Initial history |
| DPDI | Discharge instructions | |
| DPFM | Family meetings | |
| DPRV | Revisit | |
| DPCC | Cross‐cover | |
| Indirect patient care | ||
| Documentation | IDGD | General documentation |
| IDDN | Daily notes | |
| IDDD | Discharge navigator | |
| Records/Results | IPMR | Review medical records |
| Communication | ICHH | Patient handoffs |
| ICFF | Face‐to‐face | |
| ICIP | Incoming page | |
| ICOP | Outgoing page | |
| ICIC | Incoming call | |
| ICOC | Outgoing call | |
| ICEE | E‐mail communications | |
| ICDP | Discharge planner | |
| Orders | IOWO | Writing orders |
| Professional development | PDRR | Reading articles, textbooks, references |
| Education | EEWR | Teaching during work rounds |
| Travel | TTTT | Travel |
| Personal | PPPP | Personal |
| Down time | DDDD | Downtime |
The students observed 11 different hospitalists, and followed these physicians during 9 weekday shifts, 5 weekday swing shifts, 10 weekday night shifts, and 4 weekend night shifts. The variance in the number of each type of shifts monitored was likely due to scheduling limitations of the students. In total, they collected data on 8,915 minutes of hospitalists' activities. The students monitored the hospitalists representing time periods from 7 AM to 2 AM. Analysis from 2 AM to 7 AM was excluded, because after 2 AM the hospitalists did not routinely evaluate new patients with the exception of emergent requests. New admissions after midnight are handled by a night float service staffed by residents.
Results
Overall, time spent on patient care activities comprised the bulk of hospitalists' shifts (82%) (Figure 1). Patient care activities were further categorized as direct patient caredefined as face‐to‐face patient or family time; and indirect patient caredefined as activities related to patient care, but without patient or family contact. Direct and indirect patient care accounted for 15% and 67% of the hospitalists' time, respectively. The other 18% of the hospitalists' time spent in the hospital were broadly categorized into: professional development, education, personal, downtime, and travel. Professional development included activities such as looking up information (eg, literature search); education included times that hospitalists spent with residents or medical students; personal time included only restroom and food breaks; and travel included time spent moving from 1 area to the next during their shift.

The majority of the hospitalists' direct patient care time was spent on evaluating new patients (79%). Significantly smaller amounts of time were spent on other direct care activities: cross‐covering other patients (8%), follow‐up visits (7%), family meetings (4%), and discharge instructions (2%) (Figure 2).

Indirect patient care activities included, 41% of time used to communicate with other healthcare providers, 26% on medical documentation, 20% reviewing medical records and results, and 13% of time writing orders (Figure 3). Communication accounted for a large proportion of a hospitalists' work, and included telephone conversations with Emergency Department (ED) or other admitting providers, handoffs, paging, face‐to‐face conversations with consultants and other support staff, and e‐mail.

Figure 4 shows the hourly distribution of time spent on direct and indirect patient care by a hospitalist throughout the day. The day‐time hospitalists pick up their signout from the nocturnists at 7 AM to begin their shift. The swing hospitalists arrive at 2 PM during the weekdays, and their primary duty is to triage and admit patients until 7 PM. The nocturnists start their shift at 7 PM, at which time the daytime and swing‐shift hospitalists all sign out for the night.

Discussion
Hospitalists on the nonresident service at our AMC utilize about 15% of their time on face‐to‐face patient care activities, 67% on indirect patient care activities, and 7% of time on moving from 1 part of the hospital to another. Hospitalists are valuable members of the physician work force who address the increasing patient care demands in the face of increasing limitations on residency work‐hours, a growing aging population, and existing inefficiencies in AMCs. The only other work‐flow study of hospitalists of which we are aware provided a single institution's perspective on time utilization by hospitalists. Our study in a different AMC setting revealed strong consistency with the O'Leary et al.6 study in the fraction of time hospitalists spent on direct patient care (15% and 18%, respectively), indirect patient care (67% and 69%); and within indirect patient care the time spent on documentation (26% and 37% of total time) and communications (41% and 35%). While travel in the O'Leary et al.6 study took up only 3% of hospitalists' time, the conclusions in that paper clearly suggest that the authors consider it an area of concern. Our study found that travel accounted for over 7% of hospitalists' time, confirming that intuition. The significant travel time may in part reflect the effects of a non‐geographically‐located hospitalist service. From these 2 studies we can be more confident that in large, tertiary care AMCs the time hospitalists spend on indirect patient care dominates that for direct patient care (by a factor of 4 in these studies), that within indirect patient care documentation and communication are dominant activities, and that travel can take a significant amount of time when patients are dispersed throughout the facility.
Both studies demonstrated that communication accounted for a significant proportion of a hospitalist's time. In our study communication accounted for 28% of their total time in the hospital, and 41% of the indirect patient care portion (Figure 3). A closer look within our communication category revealed that phone calls and handoffs accounted for two‐thirds of all communication time observed. As the hospitalists who carry the admitting pager, they receive the pages to take admission calls, but also take calls from consultants who have recommendations, as well as from nursing and other hospital staff. Depending on the nature of the conversation, the phone calls can last several minutes. While ensuring the communication between health care providers is complete and thorough, there may be opportunities to develop novel approaches to the way hospitalists communicate with other care providers. For example, at the UMHS, alternative communication methods with nursing staff have been proposed such as utilizing a website or a handheld device to help hospitalists prioritize their communications back to the nursing staff7; while standardizing the intake information from the ED or other admitting providers may help reduce the total time spent on phone calls. We will need to further explore the potential benefits of these ideas in future work.
Our data also reveal an interesting cyclicality of daily activities for the hospitalists, as shown in Figure 4. We identified batching behaviors throughout the day, which cause delays in seeing patients and can be deleterious to smooth workflows in support services. Spikes in indirect patient care, followed closely by spikes in direct patient care, occur regularly at shift changes (7 AM, 2 PM, and 7 PM). Also, in the night shift, indirect patient care drops to its lowest levels (in % of time spent) throughout the day, and direct patient care reaches its highest levels. The day‐shift indirect care profile is counter‐cyclical with direct care, as the hospitalist shifts between direct care and indirect care depending on the time of the day. We discuss these phenomena in turn.
It is known that variability in any operation causes congestion and delay, as an unavoidable consequence of the physics of material and information flows.8 Indeed, an entire subindustry based on Lean manufacturing principles has evolved from the Toyota Production System based on the elimination of unnecessary variability in operations.9 Lean processes have been ongoing in manufacturing facilities for decades, and these efforts are just recently being embraced by the service sector in general, and health care specifically.10, 11 Batching is an extreme form of variability, where there is a lull in the amount of work being done and then a burst of work is done over a short period of time. This means that jobs pile up in the queue waiting for the next spike of activity. Our data indicate batching seems to be a common phenomenon for our hospitalists. The majority of the patients admitted to our hospitalist service are unscheduled admissions that arrive primarily through the ED. One potential result of the unscheduled admissions is that patients could be referred to our hospitalist service at a pace that is not well predictable on an hour‐to‐hour basis. This could lead to an unintended result of multiple patients admitted over a short period of time. This means that many patients wait for intake, delaying the onset of their care by the inpatient physician. Also, since an initial exam often results in orders for laboratory tests and studies, batching on the floor will translate into batching of orders going to nursing, pathology, radiology, and other hospital support services. This imposes the cost of variability on these other services in the hospital. From a systems perspective, efficiency will improve if these activities can be smoothed throughout the day. This may suggest opportunities to work with the ED, to help smooth the inflow of patients into the hospital system.
Within the hospital, all of the day‐shift hospitalists can be reached about the needs of their respective patients, however, the physician carrying the admission pager also fields calls for admissions, and acts as the default contact person for the hospitalist group. As this hospitalist receives information on new admissions, he/she is aware of patients ready for intake but cannot evaluate them at the rate they are being referred, so the queue builds. This continues into the swing shift, which also fields referrals faster than they can attend to them. The volatility in indirect care during the swing shift, 2 PM to 7 PM, reflects a significant amount of triaging and fielding general calls about hospitalist patients. These activities further reduce the swing shift's ability to clear the intake queue. The night shift finally gets to these patients and, eventually, clears the queue. There may be an opportunity to consider the use of multiple input pagers or other process changes that can smooth this flow and rationalize the recurring tasks of finding patients and the responsible physician.
Another concept in Lean thinking is that variability is costly when it represents a mismatch between demand for a service and the capacity to serve. With regards to admitted patients, when demand outpaces capacity, patients will wait. When capacity outpaces demand, there is excess capacity in the system. The ideal is to match demand and capacity at all times, so nobody waits and the system carries no costly excess capacity. As the intake providers for admitted patients, we can attack this problem from the capacity side. Here, 2 generic Lean tactics are to: (1) reallocate resources to a bottleneck that is holding up the entire system, and (2) relieve workers of time‐consuming but non‐value‐adding work so they have more capacity to devote to serving demand. In our study, carrying multiple input pagers is an example of tactic (1), and efficient communication technologies and practices that reduce indirect time is an example of (2). Systemwide improvements would require further investigation by working with the variability on the input side (eg, ED admissions).
Our study also found that a significant percent of the time observed was spent traveling (7.4%) from room to room between different floors in the hospital. Travel time, which is non‐value‐adding, is one of the major forms of waste Lean thinking.12 Our hospitalists can provide care to patients at any of the general medical‐surgical beds we have available at our health system. These beds are distributed across 14 units on 5 different floors, as well as in the ED if a bed is not available for an admitted patient. In hospitals routinely operating at high occupancy, such as our AMC, patients often get distributed throughout the facility for lack of beds on the appropriate service's ward. One cost for this is a potential mismatch between a patient's needs and floor nurses' training. Our study reveals another cost, and that is its contribution to the significant amount of time hospitalists spent on travel, which is largely driven by the need to see dispersed patients. Reducing this cost requires a systemic, rather than service‐specific, solution. Our AMC is adding observation‐status beds to relieve some of the pressure on licensed beds, and considering bed management (including parts of the admissions and discharge processes) changes designed to promote better collocation of patients with services. Further study on these and other collocation tactics is warranted.
The spike in indirect activities at 4 PM represents, in part, an early signout by 1 or more of the hospitalists who are not scheduled to hold the admission pager, and have completed their work for the day. This handoff will be replicated at 7 PM when the nocturnists arrive for their night shift. In addition to a significant indirect load on physicians, multiple handoffs have been associated with decreased quality of care.13 Again, it is worthwhile considering the feasibility of alternative shift schedules that can minimize handoffs.
Finally, our findings revealed that a low percentage of time was dedicated to providing discharge instructions (2.24% of direct patient care time, and 0.34% of total time). Because the task of discharging patients falls primarily on the day‐shift hospitalists, when combined with swing‐shift and night‐shift hospitalists' data, the low percentage measured on discharge instructions may have been diluted. Nonetheless, this may point to the need for further investigation on how hospitalists provide direct patient encounter time during this critical phase of transition out of the hospital.
Our study is not without limitations. The student observers shadowed a representative group of hospitalists, but they were not able to follow everyone in the group. More specifically, their observations were made on the hospitalist who was carrying the primary hospitalist service admitting pager. Although it was the intent of our study to focus on the hospitalists we felt would be the busiest, our results may not be generalizable to all hospitalists. Although our research supports the previous findings by O'Leary et al.,6 a second limitation to our study is that our analysis was done at a single hospitalist group in an AMC, and hence the results may not be generalizable to other hospitalist groups. Another limitation may be that we did not do an evaluation of the hours between 2 AM to 7 AM. This period of time is used to catch up on medical documentation and to be available for medical emergencies. As more hospitalist programs are employing the use of nocturnists, it may be informative to have this time period tracked for activities.
Conclusions
Our study supports the broad allocation of hospitalist time found in an earlier study at a different AMC,6 suggesting that these might be generally representative in other AMCs. We found that travel constitutes a significant claim in hospitalists' time, due in part to the inability to collocate hospitalist service patients. Remedies are not likely to be service‐specific, but will require systemwide analyses of admission and discharge processes. Communication takes a significant amount of hospitalist time, with pages and phone calls related to handoffs accounting for most of the total communication time. As hospitalists working at non‐AMC settings may experience different work flow issues, we would like to see time‐motion studies of hospitalists in other types of hospitals. Future studies should also seek to better understand the how hospitals at high occupancy may reduce batching and streamline both the discharge and admission process, determine the factors that account for the significant communication time and how these processes could be streamlined, and evaluate the potential benefits of geographical localization of hospitalists' patients.
Acknowledgements
The authors thank Tracey Jackson, Michael Paulsen, Deepak Srinivasin, and Ryan Werblow, who were students in the undergraduate business school program, for their invaluable contribution in shadowing hospitalists to collect the time study data.
Many academic medical centers (AMCs) employ hospitalists to provide care for patients on resident services as supervising attendings,1, 2 as well as on nonresident services.3 The number of hospitalists working on nonresident services at AMCs has grown exponentially, as the Accreditation Council for Graduate Medical Education (ACGME) implemented duty‐hour standards for residents.3 According to the latest Society of Hospital Medicine (SHM) estimates, the number of practicing hospitalists is projected to grow to 30,000 by 2010.4 As astonishing as this growth may sound, it is anticipated that more hospitalists will be needed to meet the demand for these physicians.5 Further, as financial realities require AMCs to be increasingly efficient without compromising patient care, and hospitalists provide a greater range of clinical services, it is important to better understand how hospitalists spend their time in the hospital. Understanding the daily work flow of hospitalists can identify how these physicians can be better supported. A previous report by O'Leary et al.6 highlighted how hospitalists spent their time during their usual day shifts at an AMC. It is important to validate their study to determine broadly applicable findings. We performed a time‐motion study where we followed the admitting hospitalists during the day and night shifts. We felt it was important to focus on hospitalists who are admitting patients, as this has potential patient safety and quality implications related to multitasking, triaging, and helping patients navigate through a complex admission process involving multiple clinical services. Our goal was to better understand how the flow of patients impacted these physicians, and determine how our hospitalists spent their time providing direct and indirect patient care‐related activities. In addition, we looked for predictable variations in activities throughout the day that might be associated with the timely care of patients.
Materials and Methods
Setting
The University of Michigan Health System (UMHS) is a tertiary care AMC, with more than 800 beds, and over 34,000 annual adult discharges. Internal Medicine services comprise a large proportion of those discharged, accounting for over 17,000 discharges per year; and is projected to grow at an annual rate of 4%. As service caps and work‐hour restrictions have limited the total number of patients that medical residents are able to care for, our hospitalist group has increased the number of physicians on the nonresident hospitalist service. At the time of the study, there were 23 hospitalists, equivalent to 18.25 full‐time equivalents (FTEs), staffing the service. The hospitalists provide in‐house patient care 24 hours a day and 7 days a week. Hospitalists also provide general medicine consult services, surgical comanagement and perioperative care, procedures, inpatient cardiopulmonary arrest response, rapid response team supervision, and observation care; and are also the primary inpatient physicians for many of the hospitalized interventional radiology and dermatology patients. These direct patient care activities account for 4500 annual discharges from the nonresident service.
Data Collection
Four university undergraduate business administration program students shadowed 11 hospitalists over a 3‐week period in 4‐hour to 12‐hour time blocks. The students followed the hospitalist on the shift that was taking admission calls, during day and night. A data collection tool was designed to track physicians' actions in 1‐minute increments, using categories similar to those used in a previously published time‐motion study of hospitalists' activities (Table 1).6 Physicians' activities each minute were assigned to a single category that most represented their action during that time period. At our AMC, 6 hospitalists work during the day shifts, and 2 on the night shifts. Our hospitalists may have patients in any of the 14 general care units in the hospital, as our hospitalists' services are not geographically based. The day hospitalists' shifts are scheduled from 7 AM to 7 PM. Two of the 6 hospitalists rotate through a 3‐day cycle as the admitting physician. Their duties include triaging and admitting patients until 2 PM, providing the day‐to‐day care for their patients until 7 PM, and occasionally cross‐covering for the other day‐shift hospitalists that have left for the day. The 4 other day‐shift hospitalists, not on their rotation as the admitting physician, may sign out and leave as early as 4 PM if their work for the day is done. At 2 PM, a separate swing‐shift hospitalist takes over the role of triaging and admitting until 7 PM. During the day shift, consults and perioperative management of patients are provided by a separate hospitalist on the consult service. At 7 PM, 2 nocturnists arrive for their 7 PM to 7 AM shift. The nocturnists, in addition to cross‐covering service patients, admit a maximum of 6 patients each, or until midnightwhichever comes first.
| Category | Code | Description |
|---|---|---|
| Direct patient care | DPIH | Initial history |
| DPDI | Discharge instructions | |
| DPFM | Family meetings | |
| DPRV | Revisit | |
| DPCC | Cross‐cover | |
| Indirect patient care | ||
| Documentation | IDGD | General documentation |
| IDDN | Daily notes | |
| IDDD | Discharge navigator | |
| Records/Results | IPMR | Review medical records |
| Communication | ICHH | Patient handoffs |
| ICFF | Face‐to‐face | |
| ICIP | Incoming page | |
| ICOP | Outgoing page | |
| ICIC | Incoming call | |
| ICOC | Outgoing call | |
| ICEE | E‐mail communications | |
| ICDP | Discharge planner | |
| Orders | IOWO | Writing orders |
| Professional development | PDRR | Reading articles, textbooks, references |
| Education | EEWR | Teaching during work rounds |
| Travel | TTTT | Travel |
| Personal | PPPP | Personal |
| Down time | DDDD | Downtime |
The students observed 11 different hospitalists, and followed these physicians during 9 weekday shifts, 5 weekday swing shifts, 10 weekday night shifts, and 4 weekend night shifts. The variance in the number of each type of shifts monitored was likely due to scheduling limitations of the students. In total, they collected data on 8,915 minutes of hospitalists' activities. The students monitored the hospitalists representing time periods from 7 AM to 2 AM. Analysis from 2 AM to 7 AM was excluded, because after 2 AM the hospitalists did not routinely evaluate new patients with the exception of emergent requests. New admissions after midnight are handled by a night float service staffed by residents.
Results
Overall, time spent on patient care activities comprised the bulk of hospitalists' shifts (82%) (Figure 1). Patient care activities were further categorized as direct patient caredefined as face‐to‐face patient or family time; and indirect patient caredefined as activities related to patient care, but without patient or family contact. Direct and indirect patient care accounted for 15% and 67% of the hospitalists' time, respectively. The other 18% of the hospitalists' time spent in the hospital were broadly categorized into: professional development, education, personal, downtime, and travel. Professional development included activities such as looking up information (eg, literature search); education included times that hospitalists spent with residents or medical students; personal time included only restroom and food breaks; and travel included time spent moving from 1 area to the next during their shift.

The majority of the hospitalists' direct patient care time was spent on evaluating new patients (79%). Significantly smaller amounts of time were spent on other direct care activities: cross‐covering other patients (8%), follow‐up visits (7%), family meetings (4%), and discharge instructions (2%) (Figure 2).

Indirect patient care activities included, 41% of time used to communicate with other healthcare providers, 26% on medical documentation, 20% reviewing medical records and results, and 13% of time writing orders (Figure 3). Communication accounted for a large proportion of a hospitalists' work, and included telephone conversations with Emergency Department (ED) or other admitting providers, handoffs, paging, face‐to‐face conversations with consultants and other support staff, and e‐mail.

Figure 4 shows the hourly distribution of time spent on direct and indirect patient care by a hospitalist throughout the day. The day‐time hospitalists pick up their signout from the nocturnists at 7 AM to begin their shift. The swing hospitalists arrive at 2 PM during the weekdays, and their primary duty is to triage and admit patients until 7 PM. The nocturnists start their shift at 7 PM, at which time the daytime and swing‐shift hospitalists all sign out for the night.

Discussion
Hospitalists on the nonresident service at our AMC utilize about 15% of their time on face‐to‐face patient care activities, 67% on indirect patient care activities, and 7% of time on moving from 1 part of the hospital to another. Hospitalists are valuable members of the physician work force who address the increasing patient care demands in the face of increasing limitations on residency work‐hours, a growing aging population, and existing inefficiencies in AMCs. The only other work‐flow study of hospitalists of which we are aware provided a single institution's perspective on time utilization by hospitalists. Our study in a different AMC setting revealed strong consistency with the O'Leary et al.6 study in the fraction of time hospitalists spent on direct patient care (15% and 18%, respectively), indirect patient care (67% and 69%); and within indirect patient care the time spent on documentation (26% and 37% of total time) and communications (41% and 35%). While travel in the O'Leary et al.6 study took up only 3% of hospitalists' time, the conclusions in that paper clearly suggest that the authors consider it an area of concern. Our study found that travel accounted for over 7% of hospitalists' time, confirming that intuition. The significant travel time may in part reflect the effects of a non‐geographically‐located hospitalist service. From these 2 studies we can be more confident that in large, tertiary care AMCs the time hospitalists spend on indirect patient care dominates that for direct patient care (by a factor of 4 in these studies), that within indirect patient care documentation and communication are dominant activities, and that travel can take a significant amount of time when patients are dispersed throughout the facility.
Both studies demonstrated that communication accounted for a significant proportion of a hospitalist's time. In our study communication accounted for 28% of their total time in the hospital, and 41% of the indirect patient care portion (Figure 3). A closer look within our communication category revealed that phone calls and handoffs accounted for two‐thirds of all communication time observed. As the hospitalists who carry the admitting pager, they receive the pages to take admission calls, but also take calls from consultants who have recommendations, as well as from nursing and other hospital staff. Depending on the nature of the conversation, the phone calls can last several minutes. While ensuring the communication between health care providers is complete and thorough, there may be opportunities to develop novel approaches to the way hospitalists communicate with other care providers. For example, at the UMHS, alternative communication methods with nursing staff have been proposed such as utilizing a website or a handheld device to help hospitalists prioritize their communications back to the nursing staff7; while standardizing the intake information from the ED or other admitting providers may help reduce the total time spent on phone calls. We will need to further explore the potential benefits of these ideas in future work.
Our data also reveal an interesting cyclicality of daily activities for the hospitalists, as shown in Figure 4. We identified batching behaviors throughout the day, which cause delays in seeing patients and can be deleterious to smooth workflows in support services. Spikes in indirect patient care, followed closely by spikes in direct patient care, occur regularly at shift changes (7 AM, 2 PM, and 7 PM). Also, in the night shift, indirect patient care drops to its lowest levels (in % of time spent) throughout the day, and direct patient care reaches its highest levels. The day‐shift indirect care profile is counter‐cyclical with direct care, as the hospitalist shifts between direct care and indirect care depending on the time of the day. We discuss these phenomena in turn.
It is known that variability in any operation causes congestion and delay, as an unavoidable consequence of the physics of material and information flows.8 Indeed, an entire subindustry based on Lean manufacturing principles has evolved from the Toyota Production System based on the elimination of unnecessary variability in operations.9 Lean processes have been ongoing in manufacturing facilities for decades, and these efforts are just recently being embraced by the service sector in general, and health care specifically.10, 11 Batching is an extreme form of variability, where there is a lull in the amount of work being done and then a burst of work is done over a short period of time. This means that jobs pile up in the queue waiting for the next spike of activity. Our data indicate batching seems to be a common phenomenon for our hospitalists. The majority of the patients admitted to our hospitalist service are unscheduled admissions that arrive primarily through the ED. One potential result of the unscheduled admissions is that patients could be referred to our hospitalist service at a pace that is not well predictable on an hour‐to‐hour basis. This could lead to an unintended result of multiple patients admitted over a short period of time. This means that many patients wait for intake, delaying the onset of their care by the inpatient physician. Also, since an initial exam often results in orders for laboratory tests and studies, batching on the floor will translate into batching of orders going to nursing, pathology, radiology, and other hospital support services. This imposes the cost of variability on these other services in the hospital. From a systems perspective, efficiency will improve if these activities can be smoothed throughout the day. This may suggest opportunities to work with the ED, to help smooth the inflow of patients into the hospital system.
Within the hospital, all of the day‐shift hospitalists can be reached about the needs of their respective patients, however, the physician carrying the admission pager also fields calls for admissions, and acts as the default contact person for the hospitalist group. As this hospitalist receives information on new admissions, he/she is aware of patients ready for intake but cannot evaluate them at the rate they are being referred, so the queue builds. This continues into the swing shift, which also fields referrals faster than they can attend to them. The volatility in indirect care during the swing shift, 2 PM to 7 PM, reflects a significant amount of triaging and fielding general calls about hospitalist patients. These activities further reduce the swing shift's ability to clear the intake queue. The night shift finally gets to these patients and, eventually, clears the queue. There may be an opportunity to consider the use of multiple input pagers or other process changes that can smooth this flow and rationalize the recurring tasks of finding patients and the responsible physician.
Another concept in Lean thinking is that variability is costly when it represents a mismatch between demand for a service and the capacity to serve. With regards to admitted patients, when demand outpaces capacity, patients will wait. When capacity outpaces demand, there is excess capacity in the system. The ideal is to match demand and capacity at all times, so nobody waits and the system carries no costly excess capacity. As the intake providers for admitted patients, we can attack this problem from the capacity side. Here, 2 generic Lean tactics are to: (1) reallocate resources to a bottleneck that is holding up the entire system, and (2) relieve workers of time‐consuming but non‐value‐adding work so they have more capacity to devote to serving demand. In our study, carrying multiple input pagers is an example of tactic (1), and efficient communication technologies and practices that reduce indirect time is an example of (2). Systemwide improvements would require further investigation by working with the variability on the input side (eg, ED admissions).
Our study also found that a significant percent of the time observed was spent traveling (7.4%) from room to room between different floors in the hospital. Travel time, which is non‐value‐adding, is one of the major forms of waste Lean thinking.12 Our hospitalists can provide care to patients at any of the general medical‐surgical beds we have available at our health system. These beds are distributed across 14 units on 5 different floors, as well as in the ED if a bed is not available for an admitted patient. In hospitals routinely operating at high occupancy, such as our AMC, patients often get distributed throughout the facility for lack of beds on the appropriate service's ward. One cost for this is a potential mismatch between a patient's needs and floor nurses' training. Our study reveals another cost, and that is its contribution to the significant amount of time hospitalists spent on travel, which is largely driven by the need to see dispersed patients. Reducing this cost requires a systemic, rather than service‐specific, solution. Our AMC is adding observation‐status beds to relieve some of the pressure on licensed beds, and considering bed management (including parts of the admissions and discharge processes) changes designed to promote better collocation of patients with services. Further study on these and other collocation tactics is warranted.
The spike in indirect activities at 4 PM represents, in part, an early signout by 1 or more of the hospitalists who are not scheduled to hold the admission pager, and have completed their work for the day. This handoff will be replicated at 7 PM when the nocturnists arrive for their night shift. In addition to a significant indirect load on physicians, multiple handoffs have been associated with decreased quality of care.13 Again, it is worthwhile considering the feasibility of alternative shift schedules that can minimize handoffs.
Finally, our findings revealed that a low percentage of time was dedicated to providing discharge instructions (2.24% of direct patient care time, and 0.34% of total time). Because the task of discharging patients falls primarily on the day‐shift hospitalists, when combined with swing‐shift and night‐shift hospitalists' data, the low percentage measured on discharge instructions may have been diluted. Nonetheless, this may point to the need for further investigation on how hospitalists provide direct patient encounter time during this critical phase of transition out of the hospital.
Our study is not without limitations. The student observers shadowed a representative group of hospitalists, but they were not able to follow everyone in the group. More specifically, their observations were made on the hospitalist who was carrying the primary hospitalist service admitting pager. Although it was the intent of our study to focus on the hospitalists we felt would be the busiest, our results may not be generalizable to all hospitalists. Although our research supports the previous findings by O'Leary et al.,6 a second limitation to our study is that our analysis was done at a single hospitalist group in an AMC, and hence the results may not be generalizable to other hospitalist groups. Another limitation may be that we did not do an evaluation of the hours between 2 AM to 7 AM. This period of time is used to catch up on medical documentation and to be available for medical emergencies. As more hospitalist programs are employing the use of nocturnists, it may be informative to have this time period tracked for activities.
Conclusions
Our study supports the broad allocation of hospitalist time found in an earlier study at a different AMC,6 suggesting that these might be generally representative in other AMCs. We found that travel constitutes a significant claim in hospitalists' time, due in part to the inability to collocate hospitalist service patients. Remedies are not likely to be service‐specific, but will require systemwide analyses of admission and discharge processes. Communication takes a significant amount of hospitalist time, with pages and phone calls related to handoffs accounting for most of the total communication time. As hospitalists working at non‐AMC settings may experience different work flow issues, we would like to see time‐motion studies of hospitalists in other types of hospitals. Future studies should also seek to better understand the how hospitals at high occupancy may reduce batching and streamline both the discharge and admission process, determine the factors that account for the significant communication time and how these processes could be streamlined, and evaluate the potential benefits of geographical localization of hospitalists' patients.
Acknowledgements
The authors thank Tracey Jackson, Michael Paulsen, Deepak Srinivasin, and Ryan Werblow, who were students in the undergraduate business school program, for their invaluable contribution in shadowing hospitalists to collect the time study data.
- , , , .Where should hospitalists sit within the academic medical center?J Gen Intern Med.2008;23:1269–1272.
- , .Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- , , , , .Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3:247–255.
- Society of Hospital Medicine. Society of Hospital Medicine Releases Results of the 2007–2008 Survey on the State of the Hospital Medicine Movement.2008. Available at: http://www.hospitalmedicine.org/AM/Template.cfm? Section=Press_Releases3:398–402.
- , , .How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- , .MCOMM: Redefining Medical Communication in the 21st Century, University of Michigan Health System. In: Society of Hospital Medicine Annual Meeting, Best of Innovations Presentation; 2009; Chicago, IL;2009.
- , .Factory Physics: Foundations of Manufacturing Management.Boston:Irwin, McGraw‐Hill;1996.
- .The Toyota Way.1st ed.Madison, WI:McGraw‐Hill;2004.
- Going Lean in Health Care.White Paper.Boston, MA:Institute for Healthcare Improvement;2005 January and February, 2005. Available at: http://www.ihconline.org/toolkits/LeanInHealthcare/GoingLeaninHealth CareWhitePaper.pdf. Accessed September 2009.
- , , , .Lean health care: what can hospitals learn from a world‐class automaker?J Hosp Med.2006;1:191–199.
- , , , , .Managing Business Process Flows.Upper Saddle River, NJ:Prentice Hall;2006.
- , .The patient handoff: medicine's Formula One moment.Chest.2008;134:9–12.
- , , , .Where should hospitalists sit within the academic medical center?J Gen Intern Med.2008;23:1269–1272.
- , .Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- , , , , .Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3:247–255.
- Society of Hospital Medicine. Society of Hospital Medicine Releases Results of the 2007–2008 Survey on the State of the Hospital Medicine Movement.2008. Available at: http://www.hospitalmedicine.org/AM/Template.cfm? Section=Press_Releases3:398–402.
- , , .How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- , .MCOMM: Redefining Medical Communication in the 21st Century, University of Michigan Health System. In: Society of Hospital Medicine Annual Meeting, Best of Innovations Presentation; 2009; Chicago, IL;2009.
- , .Factory Physics: Foundations of Manufacturing Management.Boston:Irwin, McGraw‐Hill;1996.
- .The Toyota Way.1st ed.Madison, WI:McGraw‐Hill;2004.
- Going Lean in Health Care.White Paper.Boston, MA:Institute for Healthcare Improvement;2005 January and February, 2005. Available at: http://www.ihconline.org/toolkits/LeanInHealthcare/GoingLeaninHealth CareWhitePaper.pdf. Accessed September 2009.
- , , , .Lean health care: what can hospitals learn from a world‐class automaker?J Hosp Med.2006;1:191–199.
- , , , , .Managing Business Process Flows.Upper Saddle River, NJ:Prentice Hall;2006.
- , .The patient handoff: medicine's Formula One moment.Chest.2008;134:9–12.
Copyright © 2010 Society of Hospital Medicine
Evidence for Thromboembolism Prophylaxis
Deep venous thrombosis (DVT) and pulmonary embolism (PE), collectively referred to as venous thromboembolism (VTE), are common events in hospitalized patients and result in significant morbidity and mortality. Often silent and frequently unexpected, VTE is preventable. Accordingly, the American College of Chest Physicians recommends that pharmacologic prophylaxis be given to acutely ill medical patients admitted to the hospital with congestive heart failure or severe respiratory disease, or to patients who are confined to bed who have additional risk factors, such as cancer or previous VTE.1 Three recent meta‐analyses24 demonstrated significant reductions in VTE in general medicine patients with pharmacologic prophylaxis. Recently the National Quality Forum advocated that hospitals evaluate each patient upon admission and regularly thereafter, for the risk of developing DVT/VTE and utilize clinically appropriate methods to prevent DVT/VTE.5
Despite recommendations for prophylaxis, multiple studies demonstrate utilization in <50% of at‐risk general medical patients.68 Physicians' lack of awareness may partially explain this underutilization, but other likely factors include physicians' questions about the clinical importance of the outcome (eg, some studies have shown reductions primarily in asymptomatic distal DVT), doubt regarding the best form of prophylaxis (ie, unfractionated heparin [UFH] vs. low molecular weight heparin [LMWH]), uncertainty regarding optimal dosing regimens, and comparable uncertainty regarding which patients have sufficiently high risk for VTE to outweigh the risks of anticoagulation.
We undertook the current meta‐analysis to address questions about thromboembolism prevention in general medicine patients. Does pharmacologic prophylaxis prevent clinically relevant events? Is LMWH or UFH preferable in terms of either efficacy or safety?
MATERIALS AND METHODS
Search Strategy
We conducted an extensive search that included reviewing electronic databases (MEDLINE, EMBASE, and CINAHL) through June 2008, reviewing conference proceedings, and contacting drug manufacturers. The MEDLINE search combined the key words deep venous thrombosis, thromboembolism, AND pulmonary embolism with the terms primary prevention, prophylaxis, OR prevention. We limited the search results using the filter for randomized controlled trials in PubMed. Similar strategies (available on request) were used to search EMBASE, CINAHL, and the Cochrane Central Register of Controlled Trials. We also searched the Cochrane Database of Systematic Reviews to identify previous reviews on the same topic. We obtained translations of eligible, non‐English‐language articles.
The proceedings of annual meetings from the American Thoracic Society, the American Society of Hematology, and the Society for General Internal Medicine from 1994 to 2008 were hand‐searched for reports on DVT or PE prevention published in abstract form only. (Note: the American Society of Hematology was only available through 2007). We contacted the 3 main manufacturers of LMWHPfizer (dalteparin), Aventis (enoxaparin), Glaxo Smith Kline (nadoparin)and requested information on unpublished pharmaceutical sponsored trials. First authors from the trials included in this meta‐analysis were also contacted to determine if they knew of additional published or unpublished trials.
Inclusion and Exclusion Criteria
Studies were required to be prospective randomized controlled trials comparing UFH or LMWH to mechanical prophylaxis, placebo, or no intervention. We also included randomized head‐to‐head comparisons of UFH and LMWH. Eligible studies enrolled general medical patients. Trials including predominantly intensive care unit (ICU) patients; stroke, spinal cord, or acute myocardial infarction patients were excluded. We excluded trials focused on these populations because the risk for VTE may differ from that for general medical patients and because patients in these groups already commonly receive anticoagulants as a preventive measure or as active treatment (eg, for acute myocardial infarction [MI] care). Trials assessing thrombosis in patients with long‐term central venous access/catheters were also excluded. Articles focusing on long‐term rehabilitation patients were excluded.
Studies had to employ objective criteria for diagnosing VTE. For DVT these included duplex ultrasonography, venography, fibrinogen uptake scanning, impedance plethysmography, or autopsy as a primary or secondary outcome. Studies utilizing thermographic techniques were excluded.9 Eligible diagnostic modalities for PE consisted of pulmonary arteriogram, ventilation/perfusion scan, CT angiography, and autopsy.
After an initial review of article titles and abstracts, the full texts of all articles that potentially met our inclusion criteria were independently reviewed for eligibility by 2 authors (G.M.B., M.D.). In cases of disagreement, a third author (S.F.) independently reviewed the article and adjudicated decisions.
Quantitative Data Synthesis and Statistical Analysis
For all included articles, 2 reviewers independently abstracted data on key study features (including population size, trial design, modality of VTE diagnosis, and interventions delivered to treatment and control groups), results (including the rates of all DVT, proximal DVT, symptomatic DVT, PE, and death), as well as adverse events (such as bleeding and thrombocytopenia). We accepted the endpoint of DVT when assessed by duplex ultrasonography, venography, autopsy, or when diagnosed by fibrinogen uptake scanning or impedance plethysmography. For all endpoints we abstracted event rates as number of events based on intention to treat. Each study was assessed for quality using the Jadad scale.10 The Jadad scale is a validated tool for characterizing study quality that accounts for randomization, blinding, and description of withdrawals and dropouts in individual trials. The Jadad score ranges from 0 to 5 with higher numbers identifying trials of greater methodological rigor.
The trials were divided into 4 groups based on the prophylaxis agent used and the method of comparison (UFH vs. control, LMWH vs. control, LMWH vs. UFH, and LMWH/UFH combined vs. control). After combining trials for each group, we calculated a pooled relative risk (RR) and a 95% confidence interval (CI) based on both fixed and a random effects model using the DerSimonian and Laird method. Heterogeneity of the included studies was evaluated with a chi‐square statistic. The percentage of variation in the pooled RR attributable to heterogeneity was calculated and reported using the I‐squared statistic.11 Sensitivity analyses were performed and included repeating all analyses using high‐quality studies only (Jadad score 3 or higher). Publication bias was assessed using the methods developed by Egger et al.12 and Begg and Mazumdar.13 All analyses were performed using STATA SE version 9 (Stata Corp, College Station, TX).
RESULTS
Study Identification and Selection
The computerized literature search resulted in 5284 articles. Three additional citations were found by review of bibliographies. No additional trials were identified from reviews of abstracts from national meetings. Representatives from the 3 pharmaceutical companies reported no knowledge of additional published or unpublished data. Of the 5287 studies identified by the search, 14 studies met all eligibility criteria (Figure 1).
Study Characteristics
The 14 trials eligible for inclusion in the analysis consisted of 8 comparisons of UFH or LMWH vs. control (Table 1) and 6 head‐to‐head comparisons of UFH and LMWH (Table 2). The 14 studies included 8 multicenter trials and enrolled a total of 24,515 patients: 20,594 in the 8 trials that compared UFH or LMWH with placebo and 3921 in the 6 trials that compared LMWH with UFH. Two trials exclusively enrolled patients with either congestive heart failure or severe respiratory disease,14, 15 while 12 trials enrolled mixed populations. In 8 trials a period of immobility was necessary for study entry,14, 1621 while in 2 trials immobility was not required.22, 23 In the 4 remaining trials immobility was not explicitly discussed.15, 2426 One‐half of the trials required a length of stay greater than 3 days.1719, 2225
| Study (Year)Reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Belch et al. (1981)15 | 100 | 8 | Age 40‐80 years; CHF; chest infection | UFH TID | None | FUS | VQ | No | 1 |
| Dahan et al. (1986)23 | 270 | 10* | Age >65 years | Enoxaparin 60 mg | Placebo | FUS | Autopsy | Yes | 3 |
| Halkin et al. (1982)20 | 1358 | Not reported | Age >40 years; immobile | UFH BID | None | No | No | No | 1 |
| Mahe et al. (2005)16 | 2474 | 13.08 | Age >40 years; immobile | Nadroparin 7500 IU | Placebo | Autopsy | Autopsy | Yes | 5 |
| Gardlund (1996)21 | 11693 | 8.2 | Age >55 years; immobile | UFH BID | None | Autopsy | Autopsy | No | 2 |
| Samama et al. (1999)24 | 738 | 7 | Age >40 years; length of stay 6 days; CHF; respiratory failure or 1 additional risk factor | Enoxaparin 40 mg | Placebo | Venography | Composite | Yes | 4 |
| Leizorovicz et al. (2004)25 | 3681 | 12.6 | Age >40 years; length of stay 4 days; CHF; respiratory failure or 1 additional risk factor | Dalteparin 5000 IU | Placebo | DUS | Composite | Yes | 4 |
| Lederle et al. (2006)22 | 280 | 13.4 | Age >60 years; length of stay 3 days | Enoxaparin 40 mg | Placebo | DUS | Composite | Yes | 5 |
| Study (Year)reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug/Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Bergmann and Neuhart (1996)27 | 442 | 9.5 | Age >65 years; immobile | Enoxaparin 20 mg | UFH BID | FUS | Composite | Yes | 5 |
| Harenberg et al. (1990)19 | 166 | 10* | Age 40‐80 years; 1 week of bed rest | LMWH 1.500 aPTT units QD | UFH TID | IP | No | Yes | 3 |
| Kleber et al. (2003)14 | 665 | 9.8 | Age 18 years; severe CHF or respiratory disease; immobile | Enoxaparin 40 mg | UFH TID | Venography | Composite | No | 3 |
| Aquino et al. (1990)26 | 99 | Not reported | Age >70 years | Nadoparine 7500 IU | UFH BID | DUS | Composite | No | 1 |
| Harenberg et al. (1996)18 | 1590 | 10* | Age 50‐80 years; immobile + 1 additional risk factor | Nadoparine 36 mg | UFH TID | DUS | Composite | Yes | 4 |
| Lechler et al. (1996)17 | 959 | 7* | Age >18 years; immobile + 1 additional risk factor | Enoxaparin 40 mg | UFH TID | DUS | Composite | Yes | 3 |
While minimum age for study entry varied, the patient population predominantly ranged from 65 to 85 years of age. Many of the trials reported expected, not actual, treatment duration. The range of expected treatment was 7 to 21 days, with 10 days of treatment the most frequently mentioned. In the 8 trials1416, 21, 22, 24, 25, 27 that reported actual treatment duration, the range was 8 to 13.4 days. Most trials did not report number of VTE risk factors per patient, nor was there uniform acceptance of risk factors across trials.
UFH or LMWH vs. Control
DVT
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of all DVT (RR = 0.55; 95% CI: 0.36‐0.83) (Figure 2A). When stratified by methodological quality, 5 trials16, 2225 with Jadad scores of 3 or higher showed an RR reduction of 0.53 (95% CI: 0.38‐0.72) in reducing all DVT. All of the higher‐quality trials compared LMWH to placebo. Across 4 trials that reported data for symptomatic DVT there was a nonsignificant reduction in RR compared with placebo (RR = 0.73; 95% CI: 0.45‐1.16) (Figure 2B). Only 2 trials24, 25 (both LMWH trials) reported results for proximal DVT and demonstrated significant benefit of prophylaxis with a pooled RR of 0.46 (95% CI: 0.31‐0.69) (Figure 2C).
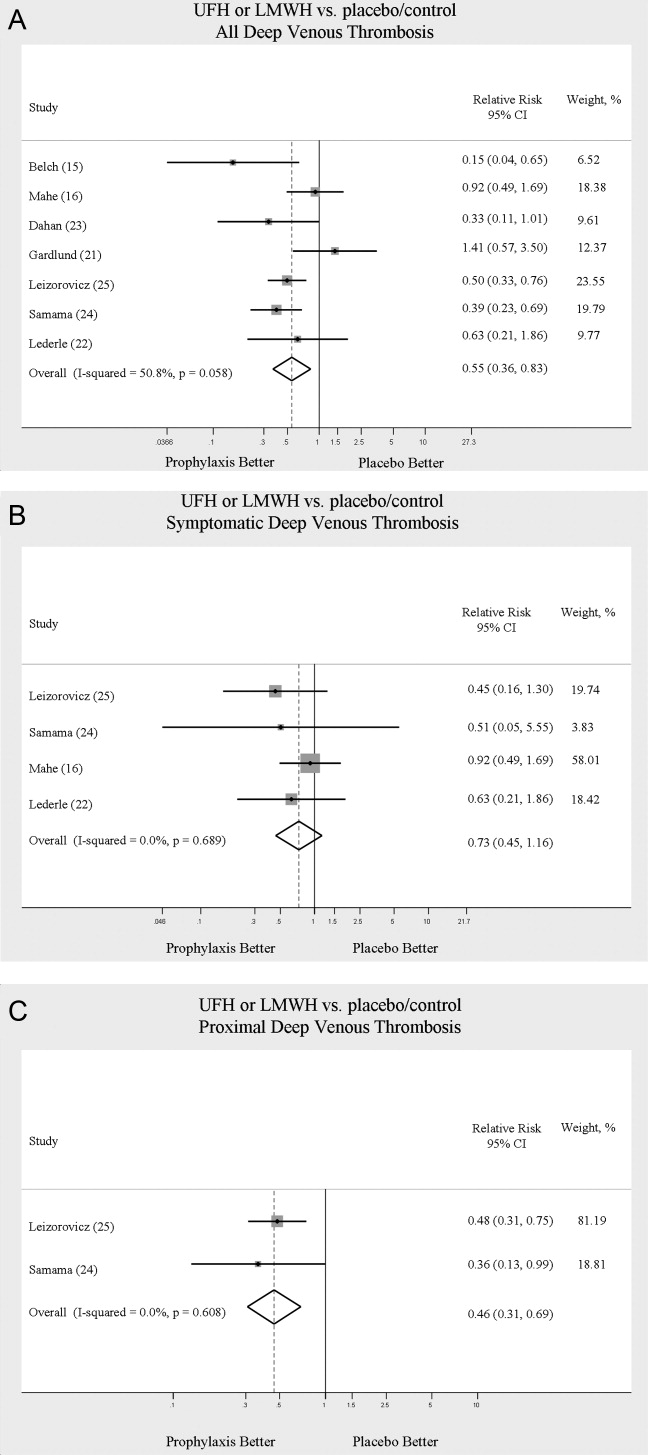
PE
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of PE (RR = 0.70; 95% CI: 0.53‐0.93) (Figure 3A). The 5 trials16, 2225 with Jadad scores of 3 or greater showed a similar relative risk reduction, but the result was no longer statistically significant (RR = 0.56; 95% CI: 0.31‐1.02). Two of the trials16, 21 relied solely on the results of autopsy to diagnose PE, which may have given rise to chance differences in detection due to generally low autopsy rates. Eliminating these 2 studies from the analysis resulted in loss of statistical significance for the reduction in risk for PE (RR = 0.48; 95% CI: 0.20‐1.15).
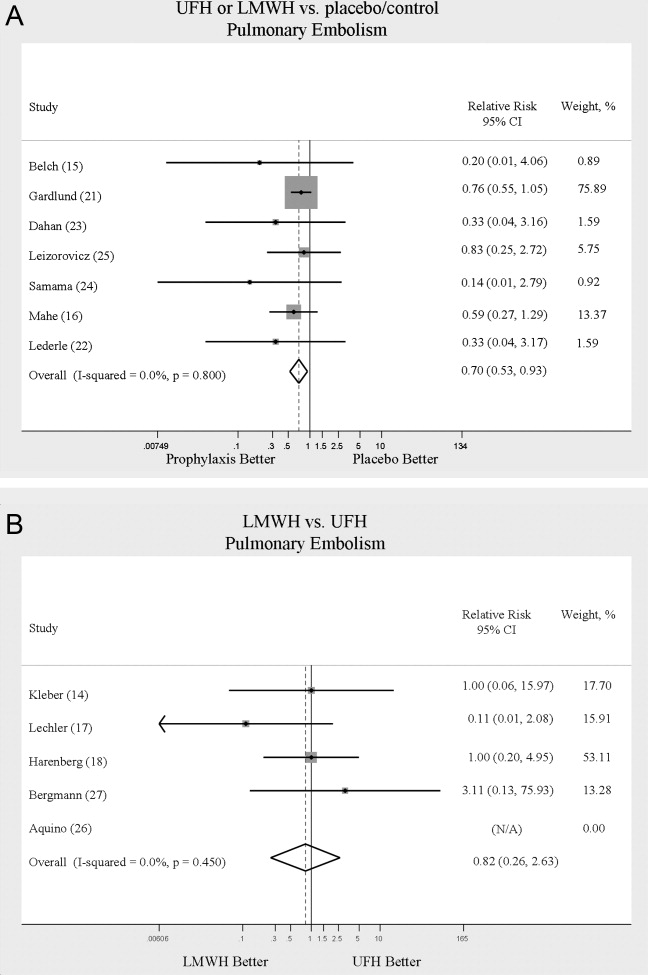
Death
Seven trials16, 2025 comparing either UFH or LMWH to control examined the impact of pharmacologic prophylaxis on death and found no significant difference between treated and untreated patients across all trials (RR = 0.92; 95% CI: 0.82‐1.03) and those limited to studies with Jadad scores of 3 or higher (RR = 0.97; 95% CI: 0.80‐1.17).
LMWH vs. UFH
DVT
In 6 trials14, 1719, 26, 27 comparing LMWH to UFH given either twice a day (BID) or 3 times a day (TID), there was no statistically significant difference in all DVT (RR = 0.90; 95% CI: 0.57‐1.43). (For all analyses RRs <1 favor LMWH, while RRs >1 favor UFH.) A total of 2 trials14, 18 reported results separately for proximal DVT with no statistically significant difference noted between UFH and LMWH (RR = 1.60; 95% CI: 0.53‐4.88). One small trial26 reported findings comparing UFH to LMWH for prevention of symptomatic DVT with no difference noted.
PE
Pooled data from the 5 trials14, 17, 18, 26, 27 comparing UFH to LMWH in the prevention of PE showed no statistically significant difference in rates of pulmonary embolism (RR = 0.82; 95% CI: 0.26‐2.63) (Figure 3B). In sensitivity analysis this result was not impacted by Jadad score.
Death
When UFH was compared to LMWH no statistically significant difference in the rate of death was found (RR = 0.96; 95% CI: 0.50‐1.85). Here again, no difference was noted when limited to studies with Jadad scores of 3 or higher.
Complications
We evaluated adverse events of heparin products used for prophylaxis and whether there were differences between UFH and LMWH. Reporting of complications was not uniform from study to study, making pooling more difficult. However, we were able to abstract data on any bleeding, major bleeding, and thrombocytopenia from several studies. In 5 studies15, 16, 2325 of either UFH or LMWH vs. control, a significantly increased risk of any bleeding (RR = 1.54; 95% CI: 1.15‐2.06) (Figure 4A) was found. When only major bleeding was evaluated, no statistically significant difference was noted (RR = 1.20; 95% CI: 0.55‐2.58) (Figure 4B). In 4 trials16, 22, 24, 25 the occurrence of thrombocytopenia was not significantly different when comparing UFH or LMWH to control (RR = 0.92; 95% CI: 0.46‐1.86).
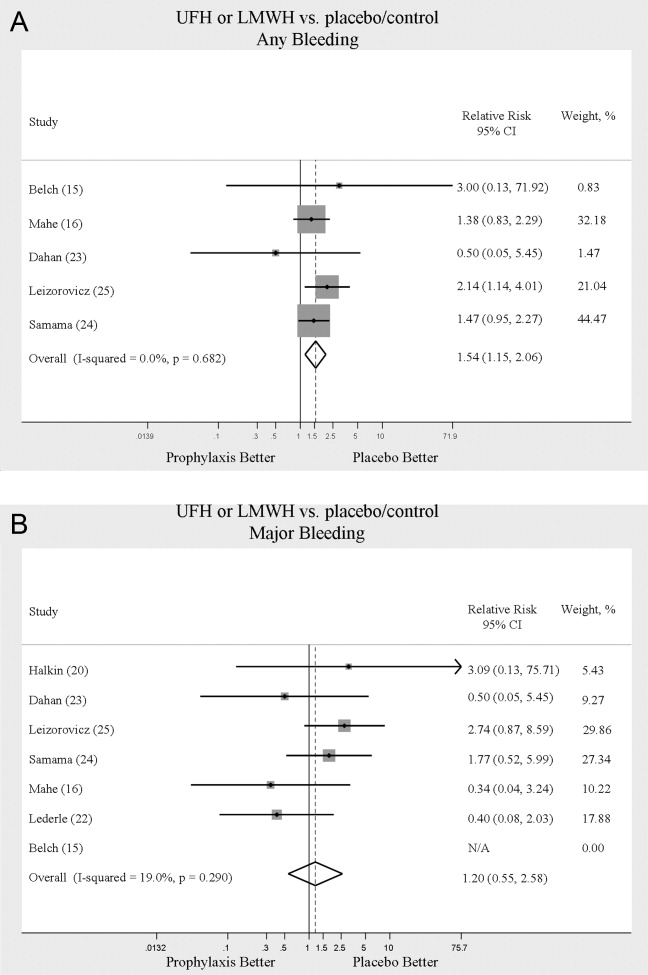
When LMWH was compared to UFH in 4 trials,14, 17, 18, 27 a nonsignificant trend toward a decrease in any bleeding was found in the LMWH group (RR = 0.72; 95% CI: 0.44‐1.16) (Figure 5A). A similar trend was seen favoring LMWH in rates of major bleeding (RR = 0.57; 95% CI: 0.25‐1.32) (Figure 5B). Neither trend was statistically significant. Three trials comparing LMWH to UFH reported on thrombocytopenia17, 18, 27 with no significant difference noted (RR = 0.52; 95% CI: 0.06‐4.18).
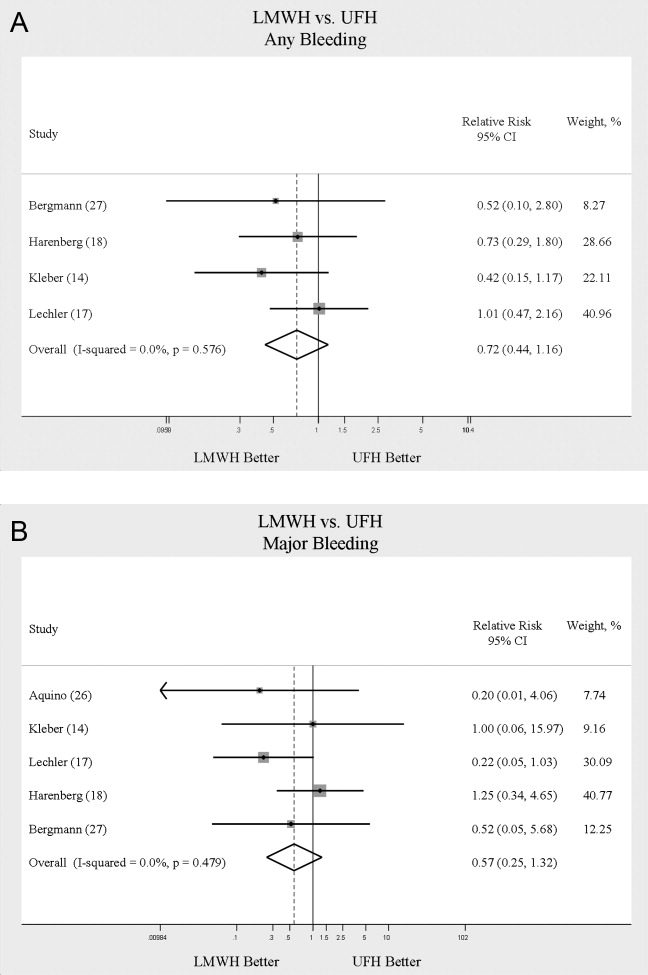
Heterogeneity and Publication Bias
No statistically significant heterogeneity was identified between trials for any outcomes. The highest I‐squared value was 54.5% (P = 0.14) for the endpoint of thrombocytopenia when UFH was compared to LMWH. In some cases, the nonsignificant results for tests of heterogeneity may have reflected small numbers of trials, but the values for I‐squared for all other endpoints were close to zero indicating that little nonrandom variation existed in the results across studies. All analyses were run using both random effects and fixed effects modeling. While we report results for random effects, no significant differences were observed using fixed effects.
We tested for publication bias using the methods developed by Egger et al.12 and Begg and Mazumdar.13 There was evidence of bias only for the outcome of PE when prophylaxis was compared to control, as the results for both tests were significant (Begg and Mazumdar:13 P = 0.035; Egger et al.:12 P = 0.010). For other outcomes tested, including all DVT (prophylaxis compared to control, and LMWH vs. UFH) as well as PE (LMWH vs. UFH), the P‐values were not significant.
DISCUSSION
When compared to control, LMWH or UFH decreased the risk of all DVT by 45% (RR = 0.55; 95% CI: 0.36‐0.83) and proximal DVT by 54% (RR = 0.46; 95% CI: 0.31‐0.69). PE was also decreased by 30% (RR = 0.70; 95% CI: 0.53‐0.93). Of note, when prophylaxis was compared with placebo all of the high‐quality studies showing a benefit were done using LMWH. The benefits of prophylaxis occurred at the cost of a 54% increased overall risk of bleeding (RR = 1.54; 95% CI 1.15‐2.06). However, the risk of major bleeding was not significantly increased. We did not find a mortality benefit to pharmacologic thromboembolism prophylaxis.
When comparing UFH to LMWH, we noted no difference in all DVT, symptomatic DVT, proximal DVT, PE, or death. While there was a trend toward less bleeding with LMWH, this was not statistically significant.
Taken in aggregate, our findings are in agreement with previous published meta‐analyses reporting net benefit for thromboembolism prophylaxis in medical patients.24, 22, 28, 29 Our meta‐analysis has several methodological strengths over the prior studies, including a comprehensive search of both the published and unpublished literature and assessment of the relationship between methodological quality of included trials and reported benefit. In contrast to previous reviews, our analysis highlights several limitations of the current evidence.
First, many of the studies are older, with predicted lengths of stay of greater than 1 week. The 8‐13‐day range of treatment duration we found in this study is longer than the average length of stay in today's hospitals. Second, there is variability in the diagnostic tests used to diagnose DVT, as well as variation in the definition of DVT among studies. Studies using fibrinogen uptake scanning reported rates of DVT as high as 26%15 while studies using venography reported DVT rates of almost 15% in the placebo arm.24 These rates are higher than most physicians' routine practice. One reason for this discrepancy is most studies did not distinguish below‐the‐knee DVT from more clinically relevant above‐the‐knee DVT. Systematic reviews of medical and surgical patients have found rates of proximal propagation from 0% to 29% in untreated patients.30, 31 Though controversial, below‐the‐knee DVT is believed less morbid than proximal DVT or symptomatic DVT. We addressed this by focusing specifically on clinically relevant endpoints of proximal and symptomatic DVT. When we restricted our analysis to proximal DVT we found a 54% RR reduction in 2 pooled trials of LMWH compared to placebo. In pooled analyses symptomatic DVT was not affected by prophylaxis. When compared head‐to‐head there were no differences between LMWH and UFH for proximal DVT or symptomatic DVT.
When considering PE, the utilization of autopsy as the sole diagnostic method in 2 large trials16, 21 is particularly problematic. In the trial by Garlund,21 the mortality rate was 5.4%, with an autopsy rate of 60.1%. Similarly, in the trial by Mahe et al.,16 the mortality rate was 10%, with an autopsy rate of 49%. Given the low absolute number of deaths and substantial proportion of decedents without autopsy, the potential for chance to produce an imbalance in detection of PE is high in these studies. When we excluded these 2 trials, we found that PE was no longer reduced to a statistically significant degree by prophylaxis. Loss of significance for PE in 2 sensitivity analyses (when excluding studies of lower quality, or using autopsy as a sole diagnostic study) is problematic and calls into question the true benefit of prophylaxis for prevention of PE.
Another limitation of the current literature centers on the variability of dosing used. We pooled trials of UFH whether given BID or TID. Given the small number of trials we did not do sensitivity analyses by dosage. A recent meta‐analysis3 found both doses are efficacious, while a recent review article32 suggested superiority of TID dosing. We believe the available literature does not clearly address this issue. Regarding comparisons of LMWH to UFH, dosing variability was also noted. The trial by Bergmann and Neuhart27 used enoxaparin 20 mg per day and found similar efficacy to UFH BID, while the Samama et al.24 trial found enoxaparin 20 mg per day no more efficacious than placebo. While the literature does not clearly define a best dose, we believe enoxaparin doses lower than 40 mg daily do not reflect the standard of care.
An additional limitation of the literature is publication bias. We assessed the possibility of publication bias by a variety of means. We did find statistical evidence of publication bias for the outcome of PE when prophylaxis was compared to control. Importantly, two meta‐analyses2, 4 on thromboembolism prophylaxis for general medicine patients suggested publication bias is present and our finding supports this conclusion. While no test for publication bias is foolproof, the best protection against publication bias, which we pursued in our study, consists of a thorough search for unpublished studies, including a search of conference proceedings, contact with experts in the field, and manufacturers of LMWH.
A final limitation of the current literature centers on risk assessment. All of the trials in this meta‐analysis included patients with an elevated level of risk. Unfortunately, risk was not clearly defined in many studies, and there was no minimum level of risk between trials. While immobility, age, and length of stay were reported for most studies, other risk factors such as personal history of thromboembolism and malignancy were not uniformly reported. Based on our analysis we are not confident our results can be extrapolated to all general medicine patients.
In conclusion, we found good evidence that pharmacologic prophylaxis significantly decreases the risk of all DVT and proximal DVT in at‐risk general medical patients. However, only LMWH was shown to prevent proximal DVT. We found inconclusive evidence that prophylaxis prevents PE. When compared directly we did not find clear superiority between UFH and LMWH, though several limitations of the current literature hamper decision‐making. Given the lower cost, it may seem justified to use UFH. However, there are other practical issues, such as the fact that LMWH is given once daily, and so potentially preferred by patients and more efficient for nurses. All of these results pertain to patients with elevated risk. While we did not find significant safety concerns with prophylaxis we do not know if these results can be extrapolated to lower‐risk patients. We believe that recommending widespread prophylaxis of all general medicine patients requires additional evidence about appropriate patient selection.
Acknowledgements
The authors thank Emmanuelle Williams, MD, for translating articles from French; Claudia Figueroa, MS, for translating articles from Spanish; Vikas Gulani, MD, for translating articles from German; and Rebecca Lee, MS, for translating articles from German, Dutch, and Italian. In addition, the authors thank Dr. Dilzer from Pfizer Global Pharmaceuticals, Kathleen E. Moigis from Aventis, and Carol McCullen from Glaxo Smith Kline for their search for unpublished pharmaceutical trials of low molecular weight heparins. Finally, the authors thank the Veterans Administration/University of Michigan Patient Safety Enhancement Program for research support.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(suppl):338S–400S.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- ,,,,.Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis.Chest.2007;131(2):507–516.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum. National Consensus Standards for the Prevention and Care of Venous Thromboembolism (including Deep Vein Thrombosis and Pulmonary Embolism). Available at: http://www.qualityforum.org/projects/completed/vte/index.asp. Accessed May2009.
- ,.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17(10):788–791.
- ,,,.[Effectiveness of low molecular weight heparin (Fragmin) in the prevention of thromboembolism in internal medicine patients. A randomized double‐blind study].Med Klin (Munich).1988;83(7):241–245, 278.
- ,,, et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials.1996;17(1):1–12.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21(11):1539–1558.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315(7109):629–634.
- ,.Operating characteristics of a rank correlation test for publication bias.Biometrics.1994;50(4):1088–1101.
- ,,,,,.Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease.Am Heart J.2003;145(4):614–621.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26(2):115–117.
- ,,,,.Lack of effect of a low‐molecular‐weight heparin (nadroparin) on mortality in bedridden medical in‐patients: a prospective randomised double‐blind study.Eur J Clin Pharmacol.2005;61(5‐6):347–351.
- ,,.The venous thrombotic risk in non‐surgical patients: epidemiological data and efficacy/safety profile of a low‐molecular‐weight heparin (enoxaparin). The Prime Study Group.Haemostasis.1996;26(suppl 2):49–56.
- ,,.Subcutaneous low‐molecular‐weight heparin versus standard heparin and the prevention of thromboembolism in medical inpatients. The Heparin Study in Internal Medicine Group.Haemostasis.1996;26(3):127–139.
- ,,, et al.Randomized controlled study of heparin and low molecular weight heparin for prevention of deep‐vein thrombosis in medical patients.Thromb Res.1990;59(3):639–650.
- ,,,.Reduction of mortality in general medical in‐patients by low‐dose heparin prophylaxis.Ann Intern Med.1982;96(5):561–565.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,, et al.The prophylaxis of medical patients for thromboembolism pilot study.Am J Med.2006;119(1):54–59.
- ,,, et al.Prevention of deep vein thrombosis in elderly medical in‐patients by a low molecular weight heparin: a randomized double‐blind trial.Haemostasis.1986;16(2):159–164.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- ,,.Prevention of thromboembolic accidents in elderly subjects with Fraxiparine. In: Bounameaux H, Samama MM, Ten Cate JW, eds.Fraxiaparine. 2nd International Symposium. Recent pharmacological and clinical data.New York:Schattauer;1990:51‐54.
- ,.A multicenter randomized double‐blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in‐patients bedridden for an acute medical illness. The Enoxaparin in Medicine Study Group.Thromb Haemost.1996;76(4):529–534.
- ,,, et al.Prevention of venous thromboembolism in internal medicine with unfractionated or low‐molecular‐weight heparins: a meta‐analysis of randomised clinical trials.Thromb Haemost.2000;83(1):14–19.
- ,,,,.Meta‐analysis of venous thromboembolism prophylaxis in medically Ill patients.Clin Ther.2007;29(11):2395–2405.
- ,,,,,.Clinical relevance of distal deep vein thrombosis. Review of literature data.Thromb Haemost.2006;95(1):56–64.
- .Natural history of venous thromboembolism.Circulation.2003;107(suppl 1):I22–I30.
- .Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients.N Engl J Med.2007;356(14):1438–1444.
Deep venous thrombosis (DVT) and pulmonary embolism (PE), collectively referred to as venous thromboembolism (VTE), are common events in hospitalized patients and result in significant morbidity and mortality. Often silent and frequently unexpected, VTE is preventable. Accordingly, the American College of Chest Physicians recommends that pharmacologic prophylaxis be given to acutely ill medical patients admitted to the hospital with congestive heart failure or severe respiratory disease, or to patients who are confined to bed who have additional risk factors, such as cancer or previous VTE.1 Three recent meta‐analyses24 demonstrated significant reductions in VTE in general medicine patients with pharmacologic prophylaxis. Recently the National Quality Forum advocated that hospitals evaluate each patient upon admission and regularly thereafter, for the risk of developing DVT/VTE and utilize clinically appropriate methods to prevent DVT/VTE.5
Despite recommendations for prophylaxis, multiple studies demonstrate utilization in <50% of at‐risk general medical patients.68 Physicians' lack of awareness may partially explain this underutilization, but other likely factors include physicians' questions about the clinical importance of the outcome (eg, some studies have shown reductions primarily in asymptomatic distal DVT), doubt regarding the best form of prophylaxis (ie, unfractionated heparin [UFH] vs. low molecular weight heparin [LMWH]), uncertainty regarding optimal dosing regimens, and comparable uncertainty regarding which patients have sufficiently high risk for VTE to outweigh the risks of anticoagulation.
We undertook the current meta‐analysis to address questions about thromboembolism prevention in general medicine patients. Does pharmacologic prophylaxis prevent clinically relevant events? Is LMWH or UFH preferable in terms of either efficacy or safety?
MATERIALS AND METHODS
Search Strategy
We conducted an extensive search that included reviewing electronic databases (MEDLINE, EMBASE, and CINAHL) through June 2008, reviewing conference proceedings, and contacting drug manufacturers. The MEDLINE search combined the key words deep venous thrombosis, thromboembolism, AND pulmonary embolism with the terms primary prevention, prophylaxis, OR prevention. We limited the search results using the filter for randomized controlled trials in PubMed. Similar strategies (available on request) were used to search EMBASE, CINAHL, and the Cochrane Central Register of Controlled Trials. We also searched the Cochrane Database of Systematic Reviews to identify previous reviews on the same topic. We obtained translations of eligible, non‐English‐language articles.
The proceedings of annual meetings from the American Thoracic Society, the American Society of Hematology, and the Society for General Internal Medicine from 1994 to 2008 were hand‐searched for reports on DVT or PE prevention published in abstract form only. (Note: the American Society of Hematology was only available through 2007). We contacted the 3 main manufacturers of LMWHPfizer (dalteparin), Aventis (enoxaparin), Glaxo Smith Kline (nadoparin)and requested information on unpublished pharmaceutical sponsored trials. First authors from the trials included in this meta‐analysis were also contacted to determine if they knew of additional published or unpublished trials.
Inclusion and Exclusion Criteria
Studies were required to be prospective randomized controlled trials comparing UFH or LMWH to mechanical prophylaxis, placebo, or no intervention. We also included randomized head‐to‐head comparisons of UFH and LMWH. Eligible studies enrolled general medical patients. Trials including predominantly intensive care unit (ICU) patients; stroke, spinal cord, or acute myocardial infarction patients were excluded. We excluded trials focused on these populations because the risk for VTE may differ from that for general medical patients and because patients in these groups already commonly receive anticoagulants as a preventive measure or as active treatment (eg, for acute myocardial infarction [MI] care). Trials assessing thrombosis in patients with long‐term central venous access/catheters were also excluded. Articles focusing on long‐term rehabilitation patients were excluded.
Studies had to employ objective criteria for diagnosing VTE. For DVT these included duplex ultrasonography, venography, fibrinogen uptake scanning, impedance plethysmography, or autopsy as a primary or secondary outcome. Studies utilizing thermographic techniques were excluded.9 Eligible diagnostic modalities for PE consisted of pulmonary arteriogram, ventilation/perfusion scan, CT angiography, and autopsy.
After an initial review of article titles and abstracts, the full texts of all articles that potentially met our inclusion criteria were independently reviewed for eligibility by 2 authors (G.M.B., M.D.). In cases of disagreement, a third author (S.F.) independently reviewed the article and adjudicated decisions.
Quantitative Data Synthesis and Statistical Analysis
For all included articles, 2 reviewers independently abstracted data on key study features (including population size, trial design, modality of VTE diagnosis, and interventions delivered to treatment and control groups), results (including the rates of all DVT, proximal DVT, symptomatic DVT, PE, and death), as well as adverse events (such as bleeding and thrombocytopenia). We accepted the endpoint of DVT when assessed by duplex ultrasonography, venography, autopsy, or when diagnosed by fibrinogen uptake scanning or impedance plethysmography. For all endpoints we abstracted event rates as number of events based on intention to treat. Each study was assessed for quality using the Jadad scale.10 The Jadad scale is a validated tool for characterizing study quality that accounts for randomization, blinding, and description of withdrawals and dropouts in individual trials. The Jadad score ranges from 0 to 5 with higher numbers identifying trials of greater methodological rigor.
The trials were divided into 4 groups based on the prophylaxis agent used and the method of comparison (UFH vs. control, LMWH vs. control, LMWH vs. UFH, and LMWH/UFH combined vs. control). After combining trials for each group, we calculated a pooled relative risk (RR) and a 95% confidence interval (CI) based on both fixed and a random effects model using the DerSimonian and Laird method. Heterogeneity of the included studies was evaluated with a chi‐square statistic. The percentage of variation in the pooled RR attributable to heterogeneity was calculated and reported using the I‐squared statistic.11 Sensitivity analyses were performed and included repeating all analyses using high‐quality studies only (Jadad score 3 or higher). Publication bias was assessed using the methods developed by Egger et al.12 and Begg and Mazumdar.13 All analyses were performed using STATA SE version 9 (Stata Corp, College Station, TX).
RESULTS
Study Identification and Selection
The computerized literature search resulted in 5284 articles. Three additional citations were found by review of bibliographies. No additional trials were identified from reviews of abstracts from national meetings. Representatives from the 3 pharmaceutical companies reported no knowledge of additional published or unpublished data. Of the 5287 studies identified by the search, 14 studies met all eligibility criteria (Figure 1).

Study Characteristics
The 14 trials eligible for inclusion in the analysis consisted of 8 comparisons of UFH or LMWH vs. control (Table 1) and 6 head‐to‐head comparisons of UFH and LMWH (Table 2). The 14 studies included 8 multicenter trials and enrolled a total of 24,515 patients: 20,594 in the 8 trials that compared UFH or LMWH with placebo and 3921 in the 6 trials that compared LMWH with UFH. Two trials exclusively enrolled patients with either congestive heart failure or severe respiratory disease,14, 15 while 12 trials enrolled mixed populations. In 8 trials a period of immobility was necessary for study entry,14, 1621 while in 2 trials immobility was not required.22, 23 In the 4 remaining trials immobility was not explicitly discussed.15, 2426 One‐half of the trials required a length of stay greater than 3 days.1719, 2225
| Study (Year)Reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Belch et al. (1981)15 | 100 | 8 | Age 40‐80 years; CHF; chest infection | UFH TID | None | FUS | VQ | No | 1 |
| Dahan et al. (1986)23 | 270 | 10* | Age >65 years | Enoxaparin 60 mg | Placebo | FUS | Autopsy | Yes | 3 |
| Halkin et al. (1982)20 | 1358 | Not reported | Age >40 years; immobile | UFH BID | None | No | No | No | 1 |
| Mahe et al. (2005)16 | 2474 | 13.08 | Age >40 years; immobile | Nadroparin 7500 IU | Placebo | Autopsy | Autopsy | Yes | 5 |
| Gardlund (1996)21 | 11693 | 8.2 | Age >55 years; immobile | UFH BID | None | Autopsy | Autopsy | No | 2 |
| Samama et al. (1999)24 | 738 | 7 | Age >40 years; length of stay 6 days; CHF; respiratory failure or 1 additional risk factor | Enoxaparin 40 mg | Placebo | Venography | Composite | Yes | 4 |
| Leizorovicz et al. (2004)25 | 3681 | 12.6 | Age >40 years; length of stay 4 days; CHF; respiratory failure or 1 additional risk factor | Dalteparin 5000 IU | Placebo | DUS | Composite | Yes | 4 |
| Lederle et al. (2006)22 | 280 | 13.4 | Age >60 years; length of stay 3 days | Enoxaparin 40 mg | Placebo | DUS | Composite | Yes | 5 |
| Study (Year)reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug/Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Bergmann and Neuhart (1996)27 | 442 | 9.5 | Age >65 years; immobile | Enoxaparin 20 mg | UFH BID | FUS | Composite | Yes | 5 |
| Harenberg et al. (1990)19 | 166 | 10* | Age 40‐80 years; 1 week of bed rest | LMWH 1.500 aPTT units QD | UFH TID | IP | No | Yes | 3 |
| Kleber et al. (2003)14 | 665 | 9.8 | Age 18 years; severe CHF or respiratory disease; immobile | Enoxaparin 40 mg | UFH TID | Venography | Composite | No | 3 |
| Aquino et al. (1990)26 | 99 | Not reported | Age >70 years | Nadoparine 7500 IU | UFH BID | DUS | Composite | No | 1 |
| Harenberg et al. (1996)18 | 1590 | 10* | Age 50‐80 years; immobile + 1 additional risk factor | Nadoparine 36 mg | UFH TID | DUS | Composite | Yes | 4 |
| Lechler et al. (1996)17 | 959 | 7* | Age >18 years; immobile + 1 additional risk factor | Enoxaparin 40 mg | UFH TID | DUS | Composite | Yes | 3 |
While minimum age for study entry varied, the patient population predominantly ranged from 65 to 85 years of age. Many of the trials reported expected, not actual, treatment duration. The range of expected treatment was 7 to 21 days, with 10 days of treatment the most frequently mentioned. In the 8 trials1416, 21, 22, 24, 25, 27 that reported actual treatment duration, the range was 8 to 13.4 days. Most trials did not report number of VTE risk factors per patient, nor was there uniform acceptance of risk factors across trials.
UFH or LMWH vs. Control
DVT
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of all DVT (RR = 0.55; 95% CI: 0.36‐0.83) (Figure 2A). When stratified by methodological quality, 5 trials16, 2225 with Jadad scores of 3 or higher showed an RR reduction of 0.53 (95% CI: 0.38‐0.72) in reducing all DVT. All of the higher‐quality trials compared LMWH to placebo. Across 4 trials that reported data for symptomatic DVT there was a nonsignificant reduction in RR compared with placebo (RR = 0.73; 95% CI: 0.45‐1.16) (Figure 2B). Only 2 trials24, 25 (both LMWH trials) reported results for proximal DVT and demonstrated significant benefit of prophylaxis with a pooled RR of 0.46 (95% CI: 0.31‐0.69) (Figure 2C).

PE
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of PE (RR = 0.70; 95% CI: 0.53‐0.93) (Figure 3A). The 5 trials16, 2225 with Jadad scores of 3 or greater showed a similar relative risk reduction, but the result was no longer statistically significant (RR = 0.56; 95% CI: 0.31‐1.02). Two of the trials16, 21 relied solely on the results of autopsy to diagnose PE, which may have given rise to chance differences in detection due to generally low autopsy rates. Eliminating these 2 studies from the analysis resulted in loss of statistical significance for the reduction in risk for PE (RR = 0.48; 95% CI: 0.20‐1.15).

Death
Seven trials16, 2025 comparing either UFH or LMWH to control examined the impact of pharmacologic prophylaxis on death and found no significant difference between treated and untreated patients across all trials (RR = 0.92; 95% CI: 0.82‐1.03) and those limited to studies with Jadad scores of 3 or higher (RR = 0.97; 95% CI: 0.80‐1.17).
LMWH vs. UFH
DVT
In 6 trials14, 1719, 26, 27 comparing LMWH to UFH given either twice a day (BID) or 3 times a day (TID), there was no statistically significant difference in all DVT (RR = 0.90; 95% CI: 0.57‐1.43). (For all analyses RRs <1 favor LMWH, while RRs >1 favor UFH.) A total of 2 trials14, 18 reported results separately for proximal DVT with no statistically significant difference noted between UFH and LMWH (RR = 1.60; 95% CI: 0.53‐4.88). One small trial26 reported findings comparing UFH to LMWH for prevention of symptomatic DVT with no difference noted.
PE
Pooled data from the 5 trials14, 17, 18, 26, 27 comparing UFH to LMWH in the prevention of PE showed no statistically significant difference in rates of pulmonary embolism (RR = 0.82; 95% CI: 0.26‐2.63) (Figure 3B). In sensitivity analysis this result was not impacted by Jadad score.
Death
When UFH was compared to LMWH no statistically significant difference in the rate of death was found (RR = 0.96; 95% CI: 0.50‐1.85). Here again, no difference was noted when limited to studies with Jadad scores of 3 or higher.
Complications
We evaluated adverse events of heparin products used for prophylaxis and whether there were differences between UFH and LMWH. Reporting of complications was not uniform from study to study, making pooling more difficult. However, we were able to abstract data on any bleeding, major bleeding, and thrombocytopenia from several studies. In 5 studies15, 16, 2325 of either UFH or LMWH vs. control, a significantly increased risk of any bleeding (RR = 1.54; 95% CI: 1.15‐2.06) (Figure 4A) was found. When only major bleeding was evaluated, no statistically significant difference was noted (RR = 1.20; 95% CI: 0.55‐2.58) (Figure 4B). In 4 trials16, 22, 24, 25 the occurrence of thrombocytopenia was not significantly different when comparing UFH or LMWH to control (RR = 0.92; 95% CI: 0.46‐1.86).

When LMWH was compared to UFH in 4 trials,14, 17, 18, 27 a nonsignificant trend toward a decrease in any bleeding was found in the LMWH group (RR = 0.72; 95% CI: 0.44‐1.16) (Figure 5A). A similar trend was seen favoring LMWH in rates of major bleeding (RR = 0.57; 95% CI: 0.25‐1.32) (Figure 5B). Neither trend was statistically significant. Three trials comparing LMWH to UFH reported on thrombocytopenia17, 18, 27 with no significant difference noted (RR = 0.52; 95% CI: 0.06‐4.18).

Heterogeneity and Publication Bias
No statistically significant heterogeneity was identified between trials for any outcomes. The highest I‐squared value was 54.5% (P = 0.14) for the endpoint of thrombocytopenia when UFH was compared to LMWH. In some cases, the nonsignificant results for tests of heterogeneity may have reflected small numbers of trials, but the values for I‐squared for all other endpoints were close to zero indicating that little nonrandom variation existed in the results across studies. All analyses were run using both random effects and fixed effects modeling. While we report results for random effects, no significant differences were observed using fixed effects.
We tested for publication bias using the methods developed by Egger et al.12 and Begg and Mazumdar.13 There was evidence of bias only for the outcome of PE when prophylaxis was compared to control, as the results for both tests were significant (Begg and Mazumdar:13 P = 0.035; Egger et al.:12 P = 0.010). For other outcomes tested, including all DVT (prophylaxis compared to control, and LMWH vs. UFH) as well as PE (LMWH vs. UFH), the P‐values were not significant.
DISCUSSION
When compared to control, LMWH or UFH decreased the risk of all DVT by 45% (RR = 0.55; 95% CI: 0.36‐0.83) and proximal DVT by 54% (RR = 0.46; 95% CI: 0.31‐0.69). PE was also decreased by 30% (RR = 0.70; 95% CI: 0.53‐0.93). Of note, when prophylaxis was compared with placebo all of the high‐quality studies showing a benefit were done using LMWH. The benefits of prophylaxis occurred at the cost of a 54% increased overall risk of bleeding (RR = 1.54; 95% CI 1.15‐2.06). However, the risk of major bleeding was not significantly increased. We did not find a mortality benefit to pharmacologic thromboembolism prophylaxis.
When comparing UFH to LMWH, we noted no difference in all DVT, symptomatic DVT, proximal DVT, PE, or death. While there was a trend toward less bleeding with LMWH, this was not statistically significant.
Taken in aggregate, our findings are in agreement with previous published meta‐analyses reporting net benefit for thromboembolism prophylaxis in medical patients.24, 22, 28, 29 Our meta‐analysis has several methodological strengths over the prior studies, including a comprehensive search of both the published and unpublished literature and assessment of the relationship between methodological quality of included trials and reported benefit. In contrast to previous reviews, our analysis highlights several limitations of the current evidence.
First, many of the studies are older, with predicted lengths of stay of greater than 1 week. The 8‐13‐day range of treatment duration we found in this study is longer than the average length of stay in today's hospitals. Second, there is variability in the diagnostic tests used to diagnose DVT, as well as variation in the definition of DVT among studies. Studies using fibrinogen uptake scanning reported rates of DVT as high as 26%15 while studies using venography reported DVT rates of almost 15% in the placebo arm.24 These rates are higher than most physicians' routine practice. One reason for this discrepancy is most studies did not distinguish below‐the‐knee DVT from more clinically relevant above‐the‐knee DVT. Systematic reviews of medical and surgical patients have found rates of proximal propagation from 0% to 29% in untreated patients.30, 31 Though controversial, below‐the‐knee DVT is believed less morbid than proximal DVT or symptomatic DVT. We addressed this by focusing specifically on clinically relevant endpoints of proximal and symptomatic DVT. When we restricted our analysis to proximal DVT we found a 54% RR reduction in 2 pooled trials of LMWH compared to placebo. In pooled analyses symptomatic DVT was not affected by prophylaxis. When compared head‐to‐head there were no differences between LMWH and UFH for proximal DVT or symptomatic DVT.
When considering PE, the utilization of autopsy as the sole diagnostic method in 2 large trials16, 21 is particularly problematic. In the trial by Garlund,21 the mortality rate was 5.4%, with an autopsy rate of 60.1%. Similarly, in the trial by Mahe et al.,16 the mortality rate was 10%, with an autopsy rate of 49%. Given the low absolute number of deaths and substantial proportion of decedents without autopsy, the potential for chance to produce an imbalance in detection of PE is high in these studies. When we excluded these 2 trials, we found that PE was no longer reduced to a statistically significant degree by prophylaxis. Loss of significance for PE in 2 sensitivity analyses (when excluding studies of lower quality, or using autopsy as a sole diagnostic study) is problematic and calls into question the true benefit of prophylaxis for prevention of PE.
Another limitation of the current literature centers on the variability of dosing used. We pooled trials of UFH whether given BID or TID. Given the small number of trials we did not do sensitivity analyses by dosage. A recent meta‐analysis3 found both doses are efficacious, while a recent review article32 suggested superiority of TID dosing. We believe the available literature does not clearly address this issue. Regarding comparisons of LMWH to UFH, dosing variability was also noted. The trial by Bergmann and Neuhart27 used enoxaparin 20 mg per day and found similar efficacy to UFH BID, while the Samama et al.24 trial found enoxaparin 20 mg per day no more efficacious than placebo. While the literature does not clearly define a best dose, we believe enoxaparin doses lower than 40 mg daily do not reflect the standard of care.
An additional limitation of the literature is publication bias. We assessed the possibility of publication bias by a variety of means. We did find statistical evidence of publication bias for the outcome of PE when prophylaxis was compared to control. Importantly, two meta‐analyses2, 4 on thromboembolism prophylaxis for general medicine patients suggested publication bias is present and our finding supports this conclusion. While no test for publication bias is foolproof, the best protection against publication bias, which we pursued in our study, consists of a thorough search for unpublished studies, including a search of conference proceedings, contact with experts in the field, and manufacturers of LMWH.
A final limitation of the current literature centers on risk assessment. All of the trials in this meta‐analysis included patients with an elevated level of risk. Unfortunately, risk was not clearly defined in many studies, and there was no minimum level of risk between trials. While immobility, age, and length of stay were reported for most studies, other risk factors such as personal history of thromboembolism and malignancy were not uniformly reported. Based on our analysis we are not confident our results can be extrapolated to all general medicine patients.
In conclusion, we found good evidence that pharmacologic prophylaxis significantly decreases the risk of all DVT and proximal DVT in at‐risk general medical patients. However, only LMWH was shown to prevent proximal DVT. We found inconclusive evidence that prophylaxis prevents PE. When compared directly we did not find clear superiority between UFH and LMWH, though several limitations of the current literature hamper decision‐making. Given the lower cost, it may seem justified to use UFH. However, there are other practical issues, such as the fact that LMWH is given once daily, and so potentially preferred by patients and more efficient for nurses. All of these results pertain to patients with elevated risk. While we did not find significant safety concerns with prophylaxis we do not know if these results can be extrapolated to lower‐risk patients. We believe that recommending widespread prophylaxis of all general medicine patients requires additional evidence about appropriate patient selection.
Acknowledgements
The authors thank Emmanuelle Williams, MD, for translating articles from French; Claudia Figueroa, MS, for translating articles from Spanish; Vikas Gulani, MD, for translating articles from German; and Rebecca Lee, MS, for translating articles from German, Dutch, and Italian. In addition, the authors thank Dr. Dilzer from Pfizer Global Pharmaceuticals, Kathleen E. Moigis from Aventis, and Carol McCullen from Glaxo Smith Kline for their search for unpublished pharmaceutical trials of low molecular weight heparins. Finally, the authors thank the Veterans Administration/University of Michigan Patient Safety Enhancement Program for research support.
Deep venous thrombosis (DVT) and pulmonary embolism (PE), collectively referred to as venous thromboembolism (VTE), are common events in hospitalized patients and result in significant morbidity and mortality. Often silent and frequently unexpected, VTE is preventable. Accordingly, the American College of Chest Physicians recommends that pharmacologic prophylaxis be given to acutely ill medical patients admitted to the hospital with congestive heart failure or severe respiratory disease, or to patients who are confined to bed who have additional risk factors, such as cancer or previous VTE.1 Three recent meta‐analyses24 demonstrated significant reductions in VTE in general medicine patients with pharmacologic prophylaxis. Recently the National Quality Forum advocated that hospitals evaluate each patient upon admission and regularly thereafter, for the risk of developing DVT/VTE and utilize clinically appropriate methods to prevent DVT/VTE.5
Despite recommendations for prophylaxis, multiple studies demonstrate utilization in <50% of at‐risk general medical patients.68 Physicians' lack of awareness may partially explain this underutilization, but other likely factors include physicians' questions about the clinical importance of the outcome (eg, some studies have shown reductions primarily in asymptomatic distal DVT), doubt regarding the best form of prophylaxis (ie, unfractionated heparin [UFH] vs. low molecular weight heparin [LMWH]), uncertainty regarding optimal dosing regimens, and comparable uncertainty regarding which patients have sufficiently high risk for VTE to outweigh the risks of anticoagulation.
We undertook the current meta‐analysis to address questions about thromboembolism prevention in general medicine patients. Does pharmacologic prophylaxis prevent clinically relevant events? Is LMWH or UFH preferable in terms of either efficacy or safety?
MATERIALS AND METHODS
Search Strategy
We conducted an extensive search that included reviewing electronic databases (MEDLINE, EMBASE, and CINAHL) through June 2008, reviewing conference proceedings, and contacting drug manufacturers. The MEDLINE search combined the key words deep venous thrombosis, thromboembolism, AND pulmonary embolism with the terms primary prevention, prophylaxis, OR prevention. We limited the search results using the filter for randomized controlled trials in PubMed. Similar strategies (available on request) were used to search EMBASE, CINAHL, and the Cochrane Central Register of Controlled Trials. We also searched the Cochrane Database of Systematic Reviews to identify previous reviews on the same topic. We obtained translations of eligible, non‐English‐language articles.
The proceedings of annual meetings from the American Thoracic Society, the American Society of Hematology, and the Society for General Internal Medicine from 1994 to 2008 were hand‐searched for reports on DVT or PE prevention published in abstract form only. (Note: the American Society of Hematology was only available through 2007). We contacted the 3 main manufacturers of LMWHPfizer (dalteparin), Aventis (enoxaparin), Glaxo Smith Kline (nadoparin)and requested information on unpublished pharmaceutical sponsored trials. First authors from the trials included in this meta‐analysis were also contacted to determine if they knew of additional published or unpublished trials.
Inclusion and Exclusion Criteria
Studies were required to be prospective randomized controlled trials comparing UFH or LMWH to mechanical prophylaxis, placebo, or no intervention. We also included randomized head‐to‐head comparisons of UFH and LMWH. Eligible studies enrolled general medical patients. Trials including predominantly intensive care unit (ICU) patients; stroke, spinal cord, or acute myocardial infarction patients were excluded. We excluded trials focused on these populations because the risk for VTE may differ from that for general medical patients and because patients in these groups already commonly receive anticoagulants as a preventive measure or as active treatment (eg, for acute myocardial infarction [MI] care). Trials assessing thrombosis in patients with long‐term central venous access/catheters were also excluded. Articles focusing on long‐term rehabilitation patients were excluded.
Studies had to employ objective criteria for diagnosing VTE. For DVT these included duplex ultrasonography, venography, fibrinogen uptake scanning, impedance plethysmography, or autopsy as a primary or secondary outcome. Studies utilizing thermographic techniques were excluded.9 Eligible diagnostic modalities for PE consisted of pulmonary arteriogram, ventilation/perfusion scan, CT angiography, and autopsy.
After an initial review of article titles and abstracts, the full texts of all articles that potentially met our inclusion criteria were independently reviewed for eligibility by 2 authors (G.M.B., M.D.). In cases of disagreement, a third author (S.F.) independently reviewed the article and adjudicated decisions.
Quantitative Data Synthesis and Statistical Analysis
For all included articles, 2 reviewers independently abstracted data on key study features (including population size, trial design, modality of VTE diagnosis, and interventions delivered to treatment and control groups), results (including the rates of all DVT, proximal DVT, symptomatic DVT, PE, and death), as well as adverse events (such as bleeding and thrombocytopenia). We accepted the endpoint of DVT when assessed by duplex ultrasonography, venography, autopsy, or when diagnosed by fibrinogen uptake scanning or impedance plethysmography. For all endpoints we abstracted event rates as number of events based on intention to treat. Each study was assessed for quality using the Jadad scale.10 The Jadad scale is a validated tool for characterizing study quality that accounts for randomization, blinding, and description of withdrawals and dropouts in individual trials. The Jadad score ranges from 0 to 5 with higher numbers identifying trials of greater methodological rigor.
The trials were divided into 4 groups based on the prophylaxis agent used and the method of comparison (UFH vs. control, LMWH vs. control, LMWH vs. UFH, and LMWH/UFH combined vs. control). After combining trials for each group, we calculated a pooled relative risk (RR) and a 95% confidence interval (CI) based on both fixed and a random effects model using the DerSimonian and Laird method. Heterogeneity of the included studies was evaluated with a chi‐square statistic. The percentage of variation in the pooled RR attributable to heterogeneity was calculated and reported using the I‐squared statistic.11 Sensitivity analyses were performed and included repeating all analyses using high‐quality studies only (Jadad score 3 or higher). Publication bias was assessed using the methods developed by Egger et al.12 and Begg and Mazumdar.13 All analyses were performed using STATA SE version 9 (Stata Corp, College Station, TX).
RESULTS
Study Identification and Selection
The computerized literature search resulted in 5284 articles. Three additional citations were found by review of bibliographies. No additional trials were identified from reviews of abstracts from national meetings. Representatives from the 3 pharmaceutical companies reported no knowledge of additional published or unpublished data. Of the 5287 studies identified by the search, 14 studies met all eligibility criteria (Figure 1).

Study Characteristics
The 14 trials eligible for inclusion in the analysis consisted of 8 comparisons of UFH or LMWH vs. control (Table 1) and 6 head‐to‐head comparisons of UFH and LMWH (Table 2). The 14 studies included 8 multicenter trials and enrolled a total of 24,515 patients: 20,594 in the 8 trials that compared UFH or LMWH with placebo and 3921 in the 6 trials that compared LMWH with UFH. Two trials exclusively enrolled patients with either congestive heart failure or severe respiratory disease,14, 15 while 12 trials enrolled mixed populations. In 8 trials a period of immobility was necessary for study entry,14, 1621 while in 2 trials immobility was not required.22, 23 In the 4 remaining trials immobility was not explicitly discussed.15, 2426 One‐half of the trials required a length of stay greater than 3 days.1719, 2225
| Study (Year)Reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Belch et al. (1981)15 | 100 | 8 | Age 40‐80 years; CHF; chest infection | UFH TID | None | FUS | VQ | No | 1 |
| Dahan et al. (1986)23 | 270 | 10* | Age >65 years | Enoxaparin 60 mg | Placebo | FUS | Autopsy | Yes | 3 |
| Halkin et al. (1982)20 | 1358 | Not reported | Age >40 years; immobile | UFH BID | None | No | No | No | 1 |
| Mahe et al. (2005)16 | 2474 | 13.08 | Age >40 years; immobile | Nadroparin 7500 IU | Placebo | Autopsy | Autopsy | Yes | 5 |
| Gardlund (1996)21 | 11693 | 8.2 | Age >55 years; immobile | UFH BID | None | Autopsy | Autopsy | No | 2 |
| Samama et al. (1999)24 | 738 | 7 | Age >40 years; length of stay 6 days; CHF; respiratory failure or 1 additional risk factor | Enoxaparin 40 mg | Placebo | Venography | Composite | Yes | 4 |
| Leizorovicz et al. (2004)25 | 3681 | 12.6 | Age >40 years; length of stay 4 days; CHF; respiratory failure or 1 additional risk factor | Dalteparin 5000 IU | Placebo | DUS | Composite | Yes | 4 |
| Lederle et al. (2006)22 | 280 | 13.4 | Age >60 years; length of stay 3 days | Enoxaparin 40 mg | Placebo | DUS | Composite | Yes | 5 |
| Study (Year)reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug/Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Bergmann and Neuhart (1996)27 | 442 | 9.5 | Age >65 years; immobile | Enoxaparin 20 mg | UFH BID | FUS | Composite | Yes | 5 |
| Harenberg et al. (1990)19 | 166 | 10* | Age 40‐80 years; 1 week of bed rest | LMWH 1.500 aPTT units QD | UFH TID | IP | No | Yes | 3 |
| Kleber et al. (2003)14 | 665 | 9.8 | Age 18 years; severe CHF or respiratory disease; immobile | Enoxaparin 40 mg | UFH TID | Venography | Composite | No | 3 |
| Aquino et al. (1990)26 | 99 | Not reported | Age >70 years | Nadoparine 7500 IU | UFH BID | DUS | Composite | No | 1 |
| Harenberg et al. (1996)18 | 1590 | 10* | Age 50‐80 years; immobile + 1 additional risk factor | Nadoparine 36 mg | UFH TID | DUS | Composite | Yes | 4 |
| Lechler et al. (1996)17 | 959 | 7* | Age >18 years; immobile + 1 additional risk factor | Enoxaparin 40 mg | UFH TID | DUS | Composite | Yes | 3 |
While minimum age for study entry varied, the patient population predominantly ranged from 65 to 85 years of age. Many of the trials reported expected, not actual, treatment duration. The range of expected treatment was 7 to 21 days, with 10 days of treatment the most frequently mentioned. In the 8 trials1416, 21, 22, 24, 25, 27 that reported actual treatment duration, the range was 8 to 13.4 days. Most trials did not report number of VTE risk factors per patient, nor was there uniform acceptance of risk factors across trials.
UFH or LMWH vs. Control
DVT
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of all DVT (RR = 0.55; 95% CI: 0.36‐0.83) (Figure 2A). When stratified by methodological quality, 5 trials16, 2225 with Jadad scores of 3 or higher showed an RR reduction of 0.53 (95% CI: 0.38‐0.72) in reducing all DVT. All of the higher‐quality trials compared LMWH to placebo. Across 4 trials that reported data for symptomatic DVT there was a nonsignificant reduction in RR compared with placebo (RR = 0.73; 95% CI: 0.45‐1.16) (Figure 2B). Only 2 trials24, 25 (both LMWH trials) reported results for proximal DVT and demonstrated significant benefit of prophylaxis with a pooled RR of 0.46 (95% CI: 0.31‐0.69) (Figure 2C).

PE
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of PE (RR = 0.70; 95% CI: 0.53‐0.93) (Figure 3A). The 5 trials16, 2225 with Jadad scores of 3 or greater showed a similar relative risk reduction, but the result was no longer statistically significant (RR = 0.56; 95% CI: 0.31‐1.02). Two of the trials16, 21 relied solely on the results of autopsy to diagnose PE, which may have given rise to chance differences in detection due to generally low autopsy rates. Eliminating these 2 studies from the analysis resulted in loss of statistical significance for the reduction in risk for PE (RR = 0.48; 95% CI: 0.20‐1.15).

Death
Seven trials16, 2025 comparing either UFH or LMWH to control examined the impact of pharmacologic prophylaxis on death and found no significant difference between treated and untreated patients across all trials (RR = 0.92; 95% CI: 0.82‐1.03) and those limited to studies with Jadad scores of 3 or higher (RR = 0.97; 95% CI: 0.80‐1.17).
LMWH vs. UFH
DVT
In 6 trials14, 1719, 26, 27 comparing LMWH to UFH given either twice a day (BID) or 3 times a day (TID), there was no statistically significant difference in all DVT (RR = 0.90; 95% CI: 0.57‐1.43). (For all analyses RRs <1 favor LMWH, while RRs >1 favor UFH.) A total of 2 trials14, 18 reported results separately for proximal DVT with no statistically significant difference noted between UFH and LMWH (RR = 1.60; 95% CI: 0.53‐4.88). One small trial26 reported findings comparing UFH to LMWH for prevention of symptomatic DVT with no difference noted.
PE
Pooled data from the 5 trials14, 17, 18, 26, 27 comparing UFH to LMWH in the prevention of PE showed no statistically significant difference in rates of pulmonary embolism (RR = 0.82; 95% CI: 0.26‐2.63) (Figure 3B). In sensitivity analysis this result was not impacted by Jadad score.
Death
When UFH was compared to LMWH no statistically significant difference in the rate of death was found (RR = 0.96; 95% CI: 0.50‐1.85). Here again, no difference was noted when limited to studies with Jadad scores of 3 or higher.
Complications
We evaluated adverse events of heparin products used for prophylaxis and whether there were differences between UFH and LMWH. Reporting of complications was not uniform from study to study, making pooling more difficult. However, we were able to abstract data on any bleeding, major bleeding, and thrombocytopenia from several studies. In 5 studies15, 16, 2325 of either UFH or LMWH vs. control, a significantly increased risk of any bleeding (RR = 1.54; 95% CI: 1.15‐2.06) (Figure 4A) was found. When only major bleeding was evaluated, no statistically significant difference was noted (RR = 1.20; 95% CI: 0.55‐2.58) (Figure 4B). In 4 trials16, 22, 24, 25 the occurrence of thrombocytopenia was not significantly different when comparing UFH or LMWH to control (RR = 0.92; 95% CI: 0.46‐1.86).

When LMWH was compared to UFH in 4 trials,14, 17, 18, 27 a nonsignificant trend toward a decrease in any bleeding was found in the LMWH group (RR = 0.72; 95% CI: 0.44‐1.16) (Figure 5A). A similar trend was seen favoring LMWH in rates of major bleeding (RR = 0.57; 95% CI: 0.25‐1.32) (Figure 5B). Neither trend was statistically significant. Three trials comparing LMWH to UFH reported on thrombocytopenia17, 18, 27 with no significant difference noted (RR = 0.52; 95% CI: 0.06‐4.18).

Heterogeneity and Publication Bias
No statistically significant heterogeneity was identified between trials for any outcomes. The highest I‐squared value was 54.5% (P = 0.14) for the endpoint of thrombocytopenia when UFH was compared to LMWH. In some cases, the nonsignificant results for tests of heterogeneity may have reflected small numbers of trials, but the values for I‐squared for all other endpoints were close to zero indicating that little nonrandom variation existed in the results across studies. All analyses were run using both random effects and fixed effects modeling. While we report results for random effects, no significant differences were observed using fixed effects.
We tested for publication bias using the methods developed by Egger et al.12 and Begg and Mazumdar.13 There was evidence of bias only for the outcome of PE when prophylaxis was compared to control, as the results for both tests were significant (Begg and Mazumdar:13 P = 0.035; Egger et al.:12 P = 0.010). For other outcomes tested, including all DVT (prophylaxis compared to control, and LMWH vs. UFH) as well as PE (LMWH vs. UFH), the P‐values were not significant.
DISCUSSION
When compared to control, LMWH or UFH decreased the risk of all DVT by 45% (RR = 0.55; 95% CI: 0.36‐0.83) and proximal DVT by 54% (RR = 0.46; 95% CI: 0.31‐0.69). PE was also decreased by 30% (RR = 0.70; 95% CI: 0.53‐0.93). Of note, when prophylaxis was compared with placebo all of the high‐quality studies showing a benefit were done using LMWH. The benefits of prophylaxis occurred at the cost of a 54% increased overall risk of bleeding (RR = 1.54; 95% CI 1.15‐2.06). However, the risk of major bleeding was not significantly increased. We did not find a mortality benefit to pharmacologic thromboembolism prophylaxis.
When comparing UFH to LMWH, we noted no difference in all DVT, symptomatic DVT, proximal DVT, PE, or death. While there was a trend toward less bleeding with LMWH, this was not statistically significant.
Taken in aggregate, our findings are in agreement with previous published meta‐analyses reporting net benefit for thromboembolism prophylaxis in medical patients.24, 22, 28, 29 Our meta‐analysis has several methodological strengths over the prior studies, including a comprehensive search of both the published and unpublished literature and assessment of the relationship between methodological quality of included trials and reported benefit. In contrast to previous reviews, our analysis highlights several limitations of the current evidence.
First, many of the studies are older, with predicted lengths of stay of greater than 1 week. The 8‐13‐day range of treatment duration we found in this study is longer than the average length of stay in today's hospitals. Second, there is variability in the diagnostic tests used to diagnose DVT, as well as variation in the definition of DVT among studies. Studies using fibrinogen uptake scanning reported rates of DVT as high as 26%15 while studies using venography reported DVT rates of almost 15% in the placebo arm.24 These rates are higher than most physicians' routine practice. One reason for this discrepancy is most studies did not distinguish below‐the‐knee DVT from more clinically relevant above‐the‐knee DVT. Systematic reviews of medical and surgical patients have found rates of proximal propagation from 0% to 29% in untreated patients.30, 31 Though controversial, below‐the‐knee DVT is believed less morbid than proximal DVT or symptomatic DVT. We addressed this by focusing specifically on clinically relevant endpoints of proximal and symptomatic DVT. When we restricted our analysis to proximal DVT we found a 54% RR reduction in 2 pooled trials of LMWH compared to placebo. In pooled analyses symptomatic DVT was not affected by prophylaxis. When compared head‐to‐head there were no differences between LMWH and UFH for proximal DVT or symptomatic DVT.
When considering PE, the utilization of autopsy as the sole diagnostic method in 2 large trials16, 21 is particularly problematic. In the trial by Garlund,21 the mortality rate was 5.4%, with an autopsy rate of 60.1%. Similarly, in the trial by Mahe et al.,16 the mortality rate was 10%, with an autopsy rate of 49%. Given the low absolute number of deaths and substantial proportion of decedents without autopsy, the potential for chance to produce an imbalance in detection of PE is high in these studies. When we excluded these 2 trials, we found that PE was no longer reduced to a statistically significant degree by prophylaxis. Loss of significance for PE in 2 sensitivity analyses (when excluding studies of lower quality, or using autopsy as a sole diagnostic study) is problematic and calls into question the true benefit of prophylaxis for prevention of PE.
Another limitation of the current literature centers on the variability of dosing used. We pooled trials of UFH whether given BID or TID. Given the small number of trials we did not do sensitivity analyses by dosage. A recent meta‐analysis3 found both doses are efficacious, while a recent review article32 suggested superiority of TID dosing. We believe the available literature does not clearly address this issue. Regarding comparisons of LMWH to UFH, dosing variability was also noted. The trial by Bergmann and Neuhart27 used enoxaparin 20 mg per day and found similar efficacy to UFH BID, while the Samama et al.24 trial found enoxaparin 20 mg per day no more efficacious than placebo. While the literature does not clearly define a best dose, we believe enoxaparin doses lower than 40 mg daily do not reflect the standard of care.
An additional limitation of the literature is publication bias. We assessed the possibility of publication bias by a variety of means. We did find statistical evidence of publication bias for the outcome of PE when prophylaxis was compared to control. Importantly, two meta‐analyses2, 4 on thromboembolism prophylaxis for general medicine patients suggested publication bias is present and our finding supports this conclusion. While no test for publication bias is foolproof, the best protection against publication bias, which we pursued in our study, consists of a thorough search for unpublished studies, including a search of conference proceedings, contact with experts in the field, and manufacturers of LMWH.
A final limitation of the current literature centers on risk assessment. All of the trials in this meta‐analysis included patients with an elevated level of risk. Unfortunately, risk was not clearly defined in many studies, and there was no minimum level of risk between trials. While immobility, age, and length of stay were reported for most studies, other risk factors such as personal history of thromboembolism and malignancy were not uniformly reported. Based on our analysis we are not confident our results can be extrapolated to all general medicine patients.
In conclusion, we found good evidence that pharmacologic prophylaxis significantly decreases the risk of all DVT and proximal DVT in at‐risk general medical patients. However, only LMWH was shown to prevent proximal DVT. We found inconclusive evidence that prophylaxis prevents PE. When compared directly we did not find clear superiority between UFH and LMWH, though several limitations of the current literature hamper decision‐making. Given the lower cost, it may seem justified to use UFH. However, there are other practical issues, such as the fact that LMWH is given once daily, and so potentially preferred by patients and more efficient for nurses. All of these results pertain to patients with elevated risk. While we did not find significant safety concerns with prophylaxis we do not know if these results can be extrapolated to lower‐risk patients. We believe that recommending widespread prophylaxis of all general medicine patients requires additional evidence about appropriate patient selection.
Acknowledgements
The authors thank Emmanuelle Williams, MD, for translating articles from French; Claudia Figueroa, MS, for translating articles from Spanish; Vikas Gulani, MD, for translating articles from German; and Rebecca Lee, MS, for translating articles from German, Dutch, and Italian. In addition, the authors thank Dr. Dilzer from Pfizer Global Pharmaceuticals, Kathleen E. Moigis from Aventis, and Carol McCullen from Glaxo Smith Kline for their search for unpublished pharmaceutical trials of low molecular weight heparins. Finally, the authors thank the Veterans Administration/University of Michigan Patient Safety Enhancement Program for research support.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(suppl):338S–400S.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- ,,,,.Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis.Chest.2007;131(2):507–516.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum. National Consensus Standards for the Prevention and Care of Venous Thromboembolism (including Deep Vein Thrombosis and Pulmonary Embolism). Available at: http://www.qualityforum.org/projects/completed/vte/index.asp. Accessed May2009.
- ,.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17(10):788–791.
- ,,,.[Effectiveness of low molecular weight heparin (Fragmin) in the prevention of thromboembolism in internal medicine patients. A randomized double‐blind study].Med Klin (Munich).1988;83(7):241–245, 278.
- ,,, et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials.1996;17(1):1–12.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21(11):1539–1558.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315(7109):629–634.
- ,.Operating characteristics of a rank correlation test for publication bias.Biometrics.1994;50(4):1088–1101.
- ,,,,,.Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease.Am Heart J.2003;145(4):614–621.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26(2):115–117.
- ,,,,.Lack of effect of a low‐molecular‐weight heparin (nadroparin) on mortality in bedridden medical in‐patients: a prospective randomised double‐blind study.Eur J Clin Pharmacol.2005;61(5‐6):347–351.
- ,,.The venous thrombotic risk in non‐surgical patients: epidemiological data and efficacy/safety profile of a low‐molecular‐weight heparin (enoxaparin). The Prime Study Group.Haemostasis.1996;26(suppl 2):49–56.
- ,,.Subcutaneous low‐molecular‐weight heparin versus standard heparin and the prevention of thromboembolism in medical inpatients. The Heparin Study in Internal Medicine Group.Haemostasis.1996;26(3):127–139.
- ,,, et al.Randomized controlled study of heparin and low molecular weight heparin for prevention of deep‐vein thrombosis in medical patients.Thromb Res.1990;59(3):639–650.
- ,,,.Reduction of mortality in general medical in‐patients by low‐dose heparin prophylaxis.Ann Intern Med.1982;96(5):561–565.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,, et al.The prophylaxis of medical patients for thromboembolism pilot study.Am J Med.2006;119(1):54–59.
- ,,, et al.Prevention of deep vein thrombosis in elderly medical in‐patients by a low molecular weight heparin: a randomized double‐blind trial.Haemostasis.1986;16(2):159–164.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- ,,.Prevention of thromboembolic accidents in elderly subjects with Fraxiparine. In: Bounameaux H, Samama MM, Ten Cate JW, eds.Fraxiaparine. 2nd International Symposium. Recent pharmacological and clinical data.New York:Schattauer;1990:51‐54.
- ,.A multicenter randomized double‐blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in‐patients bedridden for an acute medical illness. The Enoxaparin in Medicine Study Group.Thromb Haemost.1996;76(4):529–534.
- ,,, et al.Prevention of venous thromboembolism in internal medicine with unfractionated or low‐molecular‐weight heparins: a meta‐analysis of randomised clinical trials.Thromb Haemost.2000;83(1):14–19.
- ,,,,.Meta‐analysis of venous thromboembolism prophylaxis in medically Ill patients.Clin Ther.2007;29(11):2395–2405.
- ,,,,,.Clinical relevance of distal deep vein thrombosis. Review of literature data.Thromb Haemost.2006;95(1):56–64.
- .Natural history of venous thromboembolism.Circulation.2003;107(suppl 1):I22–I30.
- .Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients.N Engl J Med.2007;356(14):1438–1444.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(suppl):338S–400S.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- ,,,,.Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis.Chest.2007;131(2):507–516.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum. National Consensus Standards for the Prevention and Care of Venous Thromboembolism (including Deep Vein Thrombosis and Pulmonary Embolism). Available at: http://www.qualityforum.org/projects/completed/vte/index.asp. Accessed May2009.
- ,.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17(10):788–791.
- ,,,.[Effectiveness of low molecular weight heparin (Fragmin) in the prevention of thromboembolism in internal medicine patients. A randomized double‐blind study].Med Klin (Munich).1988;83(7):241–245, 278.
- ,,, et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials.1996;17(1):1–12.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21(11):1539–1558.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315(7109):629–634.
- ,.Operating characteristics of a rank correlation test for publication bias.Biometrics.1994;50(4):1088–1101.
- ,,,,,.Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease.Am Heart J.2003;145(4):614–621.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26(2):115–117.
- ,,,,.Lack of effect of a low‐molecular‐weight heparin (nadroparin) on mortality in bedridden medical in‐patients: a prospective randomised double‐blind study.Eur J Clin Pharmacol.2005;61(5‐6):347–351.
- ,,.The venous thrombotic risk in non‐surgical patients: epidemiological data and efficacy/safety profile of a low‐molecular‐weight heparin (enoxaparin). The Prime Study Group.Haemostasis.1996;26(suppl 2):49–56.
- ,,.Subcutaneous low‐molecular‐weight heparin versus standard heparin and the prevention of thromboembolism in medical inpatients. The Heparin Study in Internal Medicine Group.Haemostasis.1996;26(3):127–139.
- ,,, et al.Randomized controlled study of heparin and low molecular weight heparin for prevention of deep‐vein thrombosis in medical patients.Thromb Res.1990;59(3):639–650.
- ,,,.Reduction of mortality in general medical in‐patients by low‐dose heparin prophylaxis.Ann Intern Med.1982;96(5):561–565.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,, et al.The prophylaxis of medical patients for thromboembolism pilot study.Am J Med.2006;119(1):54–59.
- ,,, et al.Prevention of deep vein thrombosis in elderly medical in‐patients by a low molecular weight heparin: a randomized double‐blind trial.Haemostasis.1986;16(2):159–164.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- ,,.Prevention of thromboembolic accidents in elderly subjects with Fraxiparine. In: Bounameaux H, Samama MM, Ten Cate JW, eds.Fraxiaparine. 2nd International Symposium. Recent pharmacological and clinical data.New York:Schattauer;1990:51‐54.
- ,.A multicenter randomized double‐blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in‐patients bedridden for an acute medical illness. The Enoxaparin in Medicine Study Group.Thromb Haemost.1996;76(4):529–534.
- ,,, et al.Prevention of venous thromboembolism in internal medicine with unfractionated or low‐molecular‐weight heparins: a meta‐analysis of randomised clinical trials.Thromb Haemost.2000;83(1):14–19.
- ,,,,.Meta‐analysis of venous thromboembolism prophylaxis in medically Ill patients.Clin Ther.2007;29(11):2395–2405.
- ,,,,,.Clinical relevance of distal deep vein thrombosis. Review of literature data.Thromb Haemost.2006;95(1):56–64.
- .Natural history of venous thromboembolism.Circulation.2003;107(suppl 1):I22–I30.
- .Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients.N Engl J Med.2007;356(14):1438–1444.
Copyright © 2009 Society of Hospital Medicine
Academic Hospital Medicine
The past decade has seen hospital medicine grow from fewer than 1000 hospitalists nationwide to more than 20,000.1 In fact, survey data suggest that hospital medicine is the fastest growing field of internal medicine in the history of the US, and the growth of hospital medicine has produced a net increase in the number of generalists in the US.2
Although few direct estimates exist, academic hospital medicine (AHM) is also growing rapidly.1 Fueled by potential efficiency gains, a need for increased educational oversight of teaching services, and new residency work hour limitations, many academic medical centers and teaching hospitals have developed large hospital medicine programs. Internal medicine residency graduates interested in general medicine are finding hospital medicine an increasingly popular career choice. As a result, AHM groups have many recent residency graduates with an average age that is generally younger than 40.3
Over 85% of hospitalists are generalists and should find natural alliances with the nonhospitalist side of general internal medicine by collaborating in the course of clinical care, by teaching residents and students, or by designing quality improvement or research projects. In many academic centers, hospitalists are part of the division of general internal medicine, whereas in a few centers, hospitalists either have a separate division or lie outside the internal medicine department (employed by their hospitals).
Despite sharing a common training background and generalist mindset, many new academic hospitalists face different challenges than those faced by pure outpatient‐based academic generalists. First, at many centers, the financial arrangements between the AHM group and the hospital discourage hospitalists from traditional academic pursuits and draw them into clinical, operational, or administrative duties (such as responsibility for utilization review) that, although locally valuable, may not count as academic products in themselves or may take time away from more academic activities. Close alignment between hospitals and AHM may result in hospital administrators dictating hospitalists' practice in a way that further impedes academic viability. Reductions in resident training hours and an increasing need to provide 24‐hour coverage have facilitated growth in AHM into roles beyond those of the traditional academic generalist, such as medical comanagement of surgical patients and coverage of nonteaching services.4, 5 The youth of the field may exacerbate these problems. Most academic hospitalist groups have few senior leaders, whether they are clinical‐, education‐, or research‐focused. Young faculty need senior leaders as mentors to buffer them from relentless clinical demands that would compromise their hopes for academic success.
In order to better characterize these concerns and develop a shared work plan for future activities in support of AHM, the Society of Hospital Medicine (SHM) and the Society of General Internal Medicine (SGIM) convened an AHM consensus conference, a collaborative meeting developed and attended by representatives from SHM, SGIM, the Association of Chiefs of General Internal Medicine (ACGIM), the Association of Professors of Medicine, the Association of Program Directors in Internal Medicine, and the Association of Administrators in Internal Medicine. Using a structured consensus‐building format, we identified key barriers and challenges to AHM, then developed potential solutions.
Consensus Conference Format
Consensus Conference Steering Committee
The consensus conference was developed first by the sponsoring professional societies (SGIM, SHM, and ACGIM) being asked to nominate 2 people to be part of the consensus conference steering committee. The steering committee's main functions were to identify key tasks for the consensus conference, invite consensus conference attendees, ensure adequate representation from all participating organizations, synthesize the results of the consensus conference, and work with the individual professional societies so that results from the consensus conference were acted upon in a coordinated and effective manner.
Consensus Conference Prework
The consensus conference co‐chairs convened a series of conference calls in the spring of 2007, during which the steering committee developed a series of key areas to be explored during the conference. Topic areas were selected on the basis of the group's expertise and referred to past work by AHM taskforces convened by both SGIM/ACGIM and SHM.
The steering committee then invited stakeholders from each invited society so that each professional organization would provide at least 1 representative with expertise appropriate to 1 of the key domains identified:
-
Clinical and financial issues (within which topics such as optimal job descriptions and salary structures would be explored).
-
Teaching and education mission (within which topics such as mentorship for AHM junior faculty might be discussed).
-
Research and promotable activities (within which issues related to the development of promotable activities for AHM would be discussed).
Invitees to the consensus conference were assigned to one working group, given a general description of the potential areas within their domain, and instructed to consider a number of broad questions relevant to the topic area. These questions were as follows:
-
What are the key barriers to AHM in each key domain?
-
What viewpoints or barriers are the most pressing and/or actionable?
-
What solutions could be implemented or initiated in the next 1 to 3 years?
In order to facilitate discussion, consensus conference invitees were provided copies of findings from the SGIM/ACGIM and SHM Academic Hospitalist Task Forces, preliminary results from a survey of AHM leaders, and key literature related to the field.
Consensus Conference Format
The AHM consensus conference followed a modified Delphi consensus‐building format, in which the members of each working group developed findings relevant to their area, presented these viewpoints back to the overall group for feedback, and returned to their working group to refine their initial recommendations or move on to subsequent areas.
We used Day 1 of the meeting to confirm and refine lists of key barriers and opportunities to AHM. On Day 2, we developed actionable solutions and identified barriers with no ready solution but which were felt to be worth highlighting.
Each cycle of feedback (1 on Day 1 and 2 on Day 2) was used to identify additional barriers or opportunities prespecified by the steering committee, prioritize issues/opportunities, clarify uncertainties or point them out when they existed, and identify new areas requiring consensus. Between each cycle, workflow and interim results were summarized by the co‐chairs and a professional meeting coordinator to ensure that the group felt consensus had been achieved and to identify where additional work was required.
Writing Group/Peer Review
After the consensus conference adjourned, minutes were circulated to the group and approved, whereupon a summary of the meeting was reformatted into manuscript form. The manuscript was circulated to the steering committee, consensus conference attendees, and 2 selected peer reviewers as an additional check on the external validity of the study's results.
Consensus Findings 1: Current Challenges in AHM (Table 1)
Clinical and Financial Issues in AHM
The consensus group identified misalignment of the mission of hospitals (which often provide substantial financial support for hospital medicine programs) and the mission of departments of internal medicine (or divisions of general internal medicine) in which adult hospitalists reside as a fundamental barrier in AHM. Misalignment of missions produces challenges to the development of hospitalist groups in that their primary funder, the hospital, focuses on clinical care delivery, productivity, efficiency, and, in some cases, participation in patient safety and quality improvement efforts, whereas academic departments place considerable value on education, research, grants received, dissemination of scholarly work, and the national reputation of its faculty. Further exacerbating this tension is the fact that hospitalists do not always reside within traditional academic divisions (such as divisions of general medicine) and are therefore viewed by the hospital and their peers as hospital employees more than academic faculty.
As yet, few hospital medicine programs have successfully integrated academic and clinical needs. In many AHM programs, clinical demands have trumped academic pursuits and, as a result, produced jobs that have frequent turnover. This occurs most often when hospitalists are hired by academic medical centers primarily to staff nonresident services. Hospitalists who join these academic programs expecting ample opportunity to teach and pursue scholarly work often leave when they realize these jobs differ little from those in community settings (with the exception of less pay and, in most cases, a less efficient clinical delivery system). This turnover contributes to the perception of hospitalists as transient nonacademic faculty. The participants felt that we needed to define the ideal academic hospitalist job description.
| Clinical and Financial Issues | Teaching and Education Mission | Research and Promotable Activities | Cross‐Cutting Issues |
|---|---|---|---|
| |||
| Hospitalists' functions more often explicitly linked to hospital initiatives (clinical care, quality improvement, utilization, and throughput) | Distinguishing jobs that are predominantly clinical (C‐e) from those that are predominantly education‐focused (c‐E), which is important given the high clinical burdens | Lack of a pipeline producing hospitalist clinician investigators | Lack of leadership or negotiation skill training |
| Differing political, financial, and scientific priorities between hospitalists and administrators | Further exacerbation of C‐e/c‐E distinctions by the emergence of uncovered services | Few national funders focusing on inpatient general internal medicine | Little infrastructure for academic functions |
| Little guidance on the best models for each job type | Little recognition of quality improvement as a promotable/testable activity | Rapidly moving/growing field | |
| Decreasing interest in general internal medicine as a career path | |||
Teaching and Education Mission in AHM
Traditionally, faculty in academic medical centers have had prominent roles in resident teaching services, supervising medical residents, interns, and students. Hospitalists fill these roles at some institutions and in many cases have replaced senior faculty who are no longer able (because of competing demands from clinics or labs) or willing (because of an increased need for oversight and availability) to staff the teaching service. The teaching hospitalists start at these positions straight out of residency with little experience, training, or mentoring in how to succeed as a clinician educator. The creation of nonresident hospitalist services to address residency work hour requirements has removed many hospitalists from teaching opportunities as these services often have few if any teaching opportunities. The consensus group identified the lack of teaching opportunities and a lack of any formal preparation for those who do teach as the key challenges for new hospitalist clinician educators.
Research and Promotable Activities in AHM
Numerous challenges to promotion and success in hospital medicine research were identified. Most conference attendees felt that chairs of departments of medicine do not fully understand what the roles of academic hospitalists are, how they fit into the department's mission, or what is needed to better integrate hospitalists into the research and academic activities of the department. In addition, there are few hospital medicine fellowship programs, and those that have been created focus primarily on improving teaching skills or quality improvement rather than on research or the development of academic products. Aspiring academic hospitalists could pursue research fellowship training in existing programs (ie, the Robert Wood Johnson Foundation), but few graduates currently pursue these opportunities, and federally funded fellowships (eg, the National Research Service Awards and Health Resources and Services Administration T32 awards) explicitly exclude physicians who are not focused on primary care research. The group noted that a number of Veterans Administration fellowships (such as the Quality Scholars programs) may provide avenues for the training of hospital medicinefocused researchers, but they have been underused.
For researchers who focus on hospital medicine, federal funding sources are limited for both career development awards (K‐series) and later (R‐series) grants, particularly those funding the quality and safety research that hospitalists often pursue. Agencies of the National Institutes of Health currently do not provide many opportunities for hospital‐based general internal medicine research, and thus academic hospitalist research is undervalued by many promotion committees.
Cross‐Cutting Issues
Challenges in leadership and mentorship were identified as cross‐cutting. Many AHM programs are young, and so are their leaders. As a result, hospital medicine leaders often lack the experience and skills necessary to successfully negotiate for the support that is critical for the ideal program's success. As a young field, hospital medicine lacks faculty who have succeeded in careers as hospitalists, have been promoted in tenure tracks, and can mentor and guide young faculty through the complexities of academic medicine. Absent leadership and mentoring, few hospital medicine programs will succeed in traditional academic pursuits.
Consensus Findings 2: Overcoming Challenges to the Development of AHM (Table 2)
Summit attendees spent considerable time developing and refining solutions to the challenges described previously. Addressing the challenges resulted in a diverse group of proposed products that included educating key stakeholders, designing meetings, courses, or workshops, and gathering and disseminating data. There was considerable overlap among the solutions (Table 2).
| Solutions | Proposed Products | Challenge Domains Addressed* |
|---|---|---|
| ||
| 1. Educate stakeholders | Workshops at professional society meetings (SHM, SGIM, ACGIM, APM, and APDIM) | Addresses all domains |
| Publications highlighting issues | ||
| 2. Define the sustainable job | Data gathering and publication | Clinical/financial |
| 3. Quality improvement portfolio | Development and dissemination of criteria for the QI portfolio | Research/promotion |
| 4. Hospitalist training/mentoring | Academic hospitalist boot camp | Teaching/education |
| Research/promotion | ||
| Cross‐cutting | ||
| 5. Enhance research career pathways | Advocacy for enhanced training programs and funding sources | Research/promotion |
| 6. Improved relationships among general medicine societies | Society collaboration on product development | Addresses all domains |
Outreach to and Education of Stakeholders in Academic Medicine
The focus of the educational and outreach efforts suggested by the consensus group is to help leaders in academic medicine (not just AHM) and academic medical centers understand the challenges facing AHM. More importantly, efforts should reinforce the value of academic hospitalists to their hospital, department, and division. Efforts to engage these critical stakeholders to discuss and potentially address a number of the conference's proposed solutions are needed. Leaders include deans of medical schools, chairs of departments of medicine, division chiefs, and hospital administrative leadership.
Suggested outreach and educational activities included the publication of articles in key journals with the goal of increasing the visibility of AHM in professional societies as well as meetings and workshops focusing on teaching hospitalists and academic leaders methods to overcome challenges. Professional societies with a stake in AHM should better understand the challenges and position themselves to address these issues. The AHM task forces of SHM and SGIM can help give academic hospitalists a voice in having their needs addressed.
Publications
Articles have been commissioned in the following areas: descriptions of challenges and proposed solutions, best practices for nonresident hospitalist services, and metrics for the success of hospital medicine programs.
Meetings/Workshops
Meetings and workshops, sponsored by professional societies with a vested interest in AHM, were thought to be an effective way to address the needs of hospitalists, particularly those pursuing careers as clinician educators. Such workshops would provide skills in teaching and early career survival (eg, how to bill correctly) and in developing an educator's portfolio. Leadership training offerings, perhaps building on examples from SHM and ACGIM, were also thought to be valuable resources and venues that should be directed toward hospitalists, their chiefs, and relevant leaders.
Defining a Sustainable Job Description for Academic Hospitalists
The group strongly endorsed the need for transparent and readily available data aimed at developing sustainable academic hospitalist positions. For example, required information would include how academic jobs are constructed (in terms of months on service per year and the number of nights or weekends of coverage) and what successful programs and their hospitalists have found to be acceptable. Over the longer term, empiric comparisons based on key metrics are needed to not only help guide career development and retention but also facilitate negotiations for programmatic support.
The group pointed out that embedded in delineating an optimal academic hospitalist job description is the longstanding work of general medicine societies in supporting and fostering the development of clinician educators. In many ways, the pressures of academic physicians to be mostly clinician and less educator versus someone who focuses heavily on educational work is similar for hospitalists and outpatient generalists. Academic general internal medicine divisions hired many general internists in the early 1990s to expand the reach of academic medical centers and increase the outpatient base.6 Many university hospitals are now hiring hospitalists to provide the inpatient care for these patients, but residency work hour reductions have added a layer of complexity, creating the need for entirely new roles for academic generalists (such as surgical comanagement of medically complex patients).7, 8 Past experiences in refining and reinforcing education as a key function (
Development of a Quality‐Improvement Portfolio Akin to an Educator's Portfolio
Many hospitalists actively participate in administrative work related to quality improvement activities, and we should develop this additional pathway for promotable academic activities (eg, clinician administrator); however, such a pathway may not be recognized by all promotion committees. The group observed that many aspects of quality improvement are similar to those of education (eg, developing a curriculum, leading a team, evaluating a process, defining generalizability, and disseminating locally proven interventions) and as such would be amenable to the development of a quality improvement portfolio, which candidates could submit to promotion committees. Again, past work in developing the importance and value of the educator's portfolio would facilitate the development of a quality improvement portfolio, which would require endorsement from key stakeholders (eg, the Association of Professors of Medicine, SGIM, and SHM).9 Importantly, this work may also benefit many outpatient‐based generalists who are increasingly focusing their careers on quality and safety improvement.
Developing Mentoring and Training Opportunities for Newly Hired and Junior Hospitalists
We reached a strong consensus about the need to develop a retreat‐format training opportunity by which junior academic hospitalists would be able to gain training in tasks critical to early‐career success. These were envisioned as an initial 2‐ to 3‐day meeting followed by mentorship at a distance and continued collaboration within the class of attendees. Topics would include key functions in AHM, such as becoming an effective attending physician and teacher, leadership, quality improvement, the business of medicine, effective billing, and maintaining a curriculum vitae. A number of professional societies have developed leadership or mentoring retreats, and at the time of this article's preparation, both regional and national efforts were underway to develop these products.
Developing Training and Mentorship Pathways for Hospitalist Researchers
There are few funded hospitalist researchers in the midcareer phase and a small but growing number of academic hospitalists entering the field with a focus on research. Enhancing a pipeline of researchers is a critical need for the field, as cementing AHM as an equal member of the academic medical community will be predicated on the successful development of hospitalist investigators. To this end, academic hospitalist groups should be encouraged to partner with other established research units (particularly general internal medicine) to create mentoring relationships and increase collaborative activities. The emergence of the Clinical and Translational Science Awards consortium sites, with a focus on implementation and effectiveness research, may also provide local opportunities for hospitalists to partner in research important for early‐career grant submission. Furthermore, building the pipeline of academic hospitalist researchers will require a strong focus on identifying students and residents through outreach at individual sites as well as presentations at national meetings (eg, the American College of Physicians).
Two other issues were also thought to be important. First, professional societies should work to encourage funders of primary carefocused general medicine training programs (the National Research Service Awards and the Health Resources and Services Administration) to allow hospitalists to qualify for such critical research training. Second, continuing to advocate for increasing funding for implementation and effectiveness research, via either the Agency for Healthcare Research and Quality or individual agencies of the National Institutes of Health, will be key; the emergence of a medical effectiveness institute would also be a potential boon.
Improving Relationships Between the Professional Homes of Academic Generalists
Relationships between outpatient‐based general medicine and hospital medicine were rocky as the field of AHM first took shape, and some residua of initial tensions persist a decade later. These tensions persist in part because hospitalists remain underdeveloped members of the academic community, and this perhaps gives some license to aver that hospitalists are merely transient faculty in a stage between residency and fellowship hired to improve throughput.
Overcoming this perception will require more engagement between academic generalists of all types, not less. The consensus group felt strongly that there need not be a single professional home for academic hospitalists and that generalists should be willing and even encouraged to self‐identify as hospital‐ or clinic‐focused, much as they might be geriatrics‐focused, informatics‐focused, or women's healthfocused. In fact, in some academic centers, a few generalists have successfully integrated themselves into both clinic‐based and hospitalist roles. In this way, the emergence and growth of AHM should be viewed as a boon to the practice of general medicine, not a challenge.
Resources
Much of what is proposed to enhance AHM will require resources. Academic hospitals have a vested interest in supporting AHM as a way to reduce turnover in a group that is increasingly critical for hospital operations, not to mention key leadership roles. Negotiating for these resources should emphasize that hospitals benefit directly from the revenue and margin that comes from incremental hospital admissions, collect most of the federal graduate medical education dollars, and benefit from improved care processes that are a result of hospitalist quality improvement efforts.
Deans and Departments, a key audience for the conference findings, also have a clear stake in fostering a less transient, more professionally satisfied and academically successful work force, particularly when hospitalists are increasingly the key educators of medical residents. Moreover, schools have a vested interest in the academic accomplishments and national reputation of their hospitalists. The financial arrangements will be unique to each setting and institution, and it is clear that the sources to be tapped will vary from site to site, but these resources are clearly necessary for the field.
Conclusions
AHM is at a crossroads. Unparalleled growth has created a large cadre of hospitalists who are struggling to meet the clinical demands of practice and the requirements for academic promotion; this situation will likely lead to, at a minimum, worsening problems with faculty turnover, and even greater losses of talented and passionate clinicians from the field of academic General Internal Medicine.
The challenges are numerous but not insurmountable, and our process identified issues and potential solutions which address clinical, educational, and research aspects of academic hospitalists' lives. We acknowledge that our findings are most relevant to hospitalists at academic medical centers or large academically oriented community teaching hospitals rather than hospitalists at community hospitals whose work is predominantly clinical with smaller teaching roles. However, we feel the academic hospitalists we targeted are in greater need of assistance. We believe that the most important issues are unsustainable, nonacademic positions, poor job preparation and training, inadequate prioritization of academic roles, and insufficient leadership and mentoring within the field.
It is the hope of all the consensus conference attendees that efforts focusing on academic hospitalists in the short term are not viewed as effort diverted from general internal medicine; in fact, the group felt that while many of the products of the consensus conference were probably most needed by AHM in the short term, these same solutions would likely be useful to outpatient‐based generalists as well. Despite the concerns and challenges outlined, the consensus conference group was also very hopeful that, in the setting where resources and collaboration are appropriately marshaled, that AHM will flourish quickly. In doing so, academic hospitalists will become better role models for residents and students, attracting the next generation of generalists needed to provide care to an increasingly complex patient population, and further advance the mission of General Internal Medicine.
Acknowledgements
The authors thank Dr. Jeff Glasheen and Dr. Robert Wachter for their comments on an earlier version of this article. In addition, the authors thank the following conference participants: Dan Brotman, MD, Johns Hopkins University; Deborah M. DeMarco, MD, President of the Association of Program Directors in Internal Medicine; Jeff Glasheen, MD, University of Colorado; Rusty Holman, MD, President of the Society of Hospital Medicine; Martha A. Hooven, President of the Administrators of Internal Medicine; Peter Kaboli, MD, University of Iowa; David O Meltzer, MD, PhD, University of Chicago; Vikas Parekh, MD, University of Michigan; Russell Phillips, MD MPH, Harvard Medical School; Sanjay Saint, MD, MPH, University of Michigan; Barbara Schuster, MD, President of the Association of Professors of Medicine; Brad Sharpe, MD, University of California San Francisco; Jeff Wiese, MD, Tulane University; David Kushner, Facilitator of the Kushner Companies; Geri Barnes, Support Staff of the Society of Hospital Medicine; and Amy Woodward, Support Staff of the Society of General Internal Medicine.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,,,,.Trends in market demand for internal medicine 1999 to 2004: an analysis of physician job advertisements.J Gen Intern Med.2006;21:1079–1085.
- Society of Hospital Medicine. 2006 Hospital Medicine Survey. Available at: http://www.hospitalmedicine.org/content/navigationmenu/media/mediakit/media_kit.htm. Accessed January 2009.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- UCLA rewrites the script for academic networks.Med Netw Strategy Rep.1998;7:1–5.
- ,,,,,.Systematic review: effects of resident work hours on patient safety.Ann Intern Med.2004;141:851–857.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136:591–596.
- ,,,.Documentation systems for educators seeking academic promotion in U.S. medical schools.Acad Med.2004;79:783–790.
The past decade has seen hospital medicine grow from fewer than 1000 hospitalists nationwide to more than 20,000.1 In fact, survey data suggest that hospital medicine is the fastest growing field of internal medicine in the history of the US, and the growth of hospital medicine has produced a net increase in the number of generalists in the US.2
Although few direct estimates exist, academic hospital medicine (AHM) is also growing rapidly.1 Fueled by potential efficiency gains, a need for increased educational oversight of teaching services, and new residency work hour limitations, many academic medical centers and teaching hospitals have developed large hospital medicine programs. Internal medicine residency graduates interested in general medicine are finding hospital medicine an increasingly popular career choice. As a result, AHM groups have many recent residency graduates with an average age that is generally younger than 40.3
Over 85% of hospitalists are generalists and should find natural alliances with the nonhospitalist side of general internal medicine by collaborating in the course of clinical care, by teaching residents and students, or by designing quality improvement or research projects. In many academic centers, hospitalists are part of the division of general internal medicine, whereas in a few centers, hospitalists either have a separate division or lie outside the internal medicine department (employed by their hospitals).
Despite sharing a common training background and generalist mindset, many new academic hospitalists face different challenges than those faced by pure outpatient‐based academic generalists. First, at many centers, the financial arrangements between the AHM group and the hospital discourage hospitalists from traditional academic pursuits and draw them into clinical, operational, or administrative duties (such as responsibility for utilization review) that, although locally valuable, may not count as academic products in themselves or may take time away from more academic activities. Close alignment between hospitals and AHM may result in hospital administrators dictating hospitalists' practice in a way that further impedes academic viability. Reductions in resident training hours and an increasing need to provide 24‐hour coverage have facilitated growth in AHM into roles beyond those of the traditional academic generalist, such as medical comanagement of surgical patients and coverage of nonteaching services.4, 5 The youth of the field may exacerbate these problems. Most academic hospitalist groups have few senior leaders, whether they are clinical‐, education‐, or research‐focused. Young faculty need senior leaders as mentors to buffer them from relentless clinical demands that would compromise their hopes for academic success.
In order to better characterize these concerns and develop a shared work plan for future activities in support of AHM, the Society of Hospital Medicine (SHM) and the Society of General Internal Medicine (SGIM) convened an AHM consensus conference, a collaborative meeting developed and attended by representatives from SHM, SGIM, the Association of Chiefs of General Internal Medicine (ACGIM), the Association of Professors of Medicine, the Association of Program Directors in Internal Medicine, and the Association of Administrators in Internal Medicine. Using a structured consensus‐building format, we identified key barriers and challenges to AHM, then developed potential solutions.
Consensus Conference Format
Consensus Conference Steering Committee
The consensus conference was developed first by the sponsoring professional societies (SGIM, SHM, and ACGIM) being asked to nominate 2 people to be part of the consensus conference steering committee. The steering committee's main functions were to identify key tasks for the consensus conference, invite consensus conference attendees, ensure adequate representation from all participating organizations, synthesize the results of the consensus conference, and work with the individual professional societies so that results from the consensus conference were acted upon in a coordinated and effective manner.
Consensus Conference Prework
The consensus conference co‐chairs convened a series of conference calls in the spring of 2007, during which the steering committee developed a series of key areas to be explored during the conference. Topic areas were selected on the basis of the group's expertise and referred to past work by AHM taskforces convened by both SGIM/ACGIM and SHM.
The steering committee then invited stakeholders from each invited society so that each professional organization would provide at least 1 representative with expertise appropriate to 1 of the key domains identified:
-
Clinical and financial issues (within which topics such as optimal job descriptions and salary structures would be explored).
-
Teaching and education mission (within which topics such as mentorship for AHM junior faculty might be discussed).
-
Research and promotable activities (within which issues related to the development of promotable activities for AHM would be discussed).
Invitees to the consensus conference were assigned to one working group, given a general description of the potential areas within their domain, and instructed to consider a number of broad questions relevant to the topic area. These questions were as follows:
-
What are the key barriers to AHM in each key domain?
-
What viewpoints or barriers are the most pressing and/or actionable?
-
What solutions could be implemented or initiated in the next 1 to 3 years?
In order to facilitate discussion, consensus conference invitees were provided copies of findings from the SGIM/ACGIM and SHM Academic Hospitalist Task Forces, preliminary results from a survey of AHM leaders, and key literature related to the field.
Consensus Conference Format
The AHM consensus conference followed a modified Delphi consensus‐building format, in which the members of each working group developed findings relevant to their area, presented these viewpoints back to the overall group for feedback, and returned to their working group to refine their initial recommendations or move on to subsequent areas.
We used Day 1 of the meeting to confirm and refine lists of key barriers and opportunities to AHM. On Day 2, we developed actionable solutions and identified barriers with no ready solution but which were felt to be worth highlighting.
Each cycle of feedback (1 on Day 1 and 2 on Day 2) was used to identify additional barriers or opportunities prespecified by the steering committee, prioritize issues/opportunities, clarify uncertainties or point them out when they existed, and identify new areas requiring consensus. Between each cycle, workflow and interim results were summarized by the co‐chairs and a professional meeting coordinator to ensure that the group felt consensus had been achieved and to identify where additional work was required.
Writing Group/Peer Review
After the consensus conference adjourned, minutes were circulated to the group and approved, whereupon a summary of the meeting was reformatted into manuscript form. The manuscript was circulated to the steering committee, consensus conference attendees, and 2 selected peer reviewers as an additional check on the external validity of the study's results.
Consensus Findings 1: Current Challenges in AHM (Table 1)
Clinical and Financial Issues in AHM
The consensus group identified misalignment of the mission of hospitals (which often provide substantial financial support for hospital medicine programs) and the mission of departments of internal medicine (or divisions of general internal medicine) in which adult hospitalists reside as a fundamental barrier in AHM. Misalignment of missions produces challenges to the development of hospitalist groups in that their primary funder, the hospital, focuses on clinical care delivery, productivity, efficiency, and, in some cases, participation in patient safety and quality improvement efforts, whereas academic departments place considerable value on education, research, grants received, dissemination of scholarly work, and the national reputation of its faculty. Further exacerbating this tension is the fact that hospitalists do not always reside within traditional academic divisions (such as divisions of general medicine) and are therefore viewed by the hospital and their peers as hospital employees more than academic faculty.
As yet, few hospital medicine programs have successfully integrated academic and clinical needs. In many AHM programs, clinical demands have trumped academic pursuits and, as a result, produced jobs that have frequent turnover. This occurs most often when hospitalists are hired by academic medical centers primarily to staff nonresident services. Hospitalists who join these academic programs expecting ample opportunity to teach and pursue scholarly work often leave when they realize these jobs differ little from those in community settings (with the exception of less pay and, in most cases, a less efficient clinical delivery system). This turnover contributes to the perception of hospitalists as transient nonacademic faculty. The participants felt that we needed to define the ideal academic hospitalist job description.
| Clinical and Financial Issues | Teaching and Education Mission | Research and Promotable Activities | Cross‐Cutting Issues |
|---|---|---|---|
| |||
| Hospitalists' functions more often explicitly linked to hospital initiatives (clinical care, quality improvement, utilization, and throughput) | Distinguishing jobs that are predominantly clinical (C‐e) from those that are predominantly education‐focused (c‐E), which is important given the high clinical burdens | Lack of a pipeline producing hospitalist clinician investigators | Lack of leadership or negotiation skill training |
| Differing political, financial, and scientific priorities between hospitalists and administrators | Further exacerbation of C‐e/c‐E distinctions by the emergence of uncovered services | Few national funders focusing on inpatient general internal medicine | Little infrastructure for academic functions |
| Little guidance on the best models for each job type | Little recognition of quality improvement as a promotable/testable activity | Rapidly moving/growing field | |
| Decreasing interest in general internal medicine as a career path | |||
Teaching and Education Mission in AHM
Traditionally, faculty in academic medical centers have had prominent roles in resident teaching services, supervising medical residents, interns, and students. Hospitalists fill these roles at some institutions and in many cases have replaced senior faculty who are no longer able (because of competing demands from clinics or labs) or willing (because of an increased need for oversight and availability) to staff the teaching service. The teaching hospitalists start at these positions straight out of residency with little experience, training, or mentoring in how to succeed as a clinician educator. The creation of nonresident hospitalist services to address residency work hour requirements has removed many hospitalists from teaching opportunities as these services often have few if any teaching opportunities. The consensus group identified the lack of teaching opportunities and a lack of any formal preparation for those who do teach as the key challenges for new hospitalist clinician educators.
Research and Promotable Activities in AHM
Numerous challenges to promotion and success in hospital medicine research were identified. Most conference attendees felt that chairs of departments of medicine do not fully understand what the roles of academic hospitalists are, how they fit into the department's mission, or what is needed to better integrate hospitalists into the research and academic activities of the department. In addition, there are few hospital medicine fellowship programs, and those that have been created focus primarily on improving teaching skills or quality improvement rather than on research or the development of academic products. Aspiring academic hospitalists could pursue research fellowship training in existing programs (ie, the Robert Wood Johnson Foundation), but few graduates currently pursue these opportunities, and federally funded fellowships (eg, the National Research Service Awards and Health Resources and Services Administration T32 awards) explicitly exclude physicians who are not focused on primary care research. The group noted that a number of Veterans Administration fellowships (such as the Quality Scholars programs) may provide avenues for the training of hospital medicinefocused researchers, but they have been underused.
For researchers who focus on hospital medicine, federal funding sources are limited for both career development awards (K‐series) and later (R‐series) grants, particularly those funding the quality and safety research that hospitalists often pursue. Agencies of the National Institutes of Health currently do not provide many opportunities for hospital‐based general internal medicine research, and thus academic hospitalist research is undervalued by many promotion committees.
Cross‐Cutting Issues
Challenges in leadership and mentorship were identified as cross‐cutting. Many AHM programs are young, and so are their leaders. As a result, hospital medicine leaders often lack the experience and skills necessary to successfully negotiate for the support that is critical for the ideal program's success. As a young field, hospital medicine lacks faculty who have succeeded in careers as hospitalists, have been promoted in tenure tracks, and can mentor and guide young faculty through the complexities of academic medicine. Absent leadership and mentoring, few hospital medicine programs will succeed in traditional academic pursuits.
Consensus Findings 2: Overcoming Challenges to the Development of AHM (Table 2)
Summit attendees spent considerable time developing and refining solutions to the challenges described previously. Addressing the challenges resulted in a diverse group of proposed products that included educating key stakeholders, designing meetings, courses, or workshops, and gathering and disseminating data. There was considerable overlap among the solutions (Table 2).
| Solutions | Proposed Products | Challenge Domains Addressed* |
|---|---|---|
| ||
| 1. Educate stakeholders | Workshops at professional society meetings (SHM, SGIM, ACGIM, APM, and APDIM) | Addresses all domains |
| Publications highlighting issues | ||
| 2. Define the sustainable job | Data gathering and publication | Clinical/financial |
| 3. Quality improvement portfolio | Development and dissemination of criteria for the QI portfolio | Research/promotion |
| 4. Hospitalist training/mentoring | Academic hospitalist boot camp | Teaching/education |
| Research/promotion | ||
| Cross‐cutting | ||
| 5. Enhance research career pathways | Advocacy for enhanced training programs and funding sources | Research/promotion |
| 6. Improved relationships among general medicine societies | Society collaboration on product development | Addresses all domains |
Outreach to and Education of Stakeholders in Academic Medicine
The focus of the educational and outreach efforts suggested by the consensus group is to help leaders in academic medicine (not just AHM) and academic medical centers understand the challenges facing AHM. More importantly, efforts should reinforce the value of academic hospitalists to their hospital, department, and division. Efforts to engage these critical stakeholders to discuss and potentially address a number of the conference's proposed solutions are needed. Leaders include deans of medical schools, chairs of departments of medicine, division chiefs, and hospital administrative leadership.
Suggested outreach and educational activities included the publication of articles in key journals with the goal of increasing the visibility of AHM in professional societies as well as meetings and workshops focusing on teaching hospitalists and academic leaders methods to overcome challenges. Professional societies with a stake in AHM should better understand the challenges and position themselves to address these issues. The AHM task forces of SHM and SGIM can help give academic hospitalists a voice in having their needs addressed.
Publications
Articles have been commissioned in the following areas: descriptions of challenges and proposed solutions, best practices for nonresident hospitalist services, and metrics for the success of hospital medicine programs.
Meetings/Workshops
Meetings and workshops, sponsored by professional societies with a vested interest in AHM, were thought to be an effective way to address the needs of hospitalists, particularly those pursuing careers as clinician educators. Such workshops would provide skills in teaching and early career survival (eg, how to bill correctly) and in developing an educator's portfolio. Leadership training offerings, perhaps building on examples from SHM and ACGIM, were also thought to be valuable resources and venues that should be directed toward hospitalists, their chiefs, and relevant leaders.
Defining a Sustainable Job Description for Academic Hospitalists
The group strongly endorsed the need for transparent and readily available data aimed at developing sustainable academic hospitalist positions. For example, required information would include how academic jobs are constructed (in terms of months on service per year and the number of nights or weekends of coverage) and what successful programs and their hospitalists have found to be acceptable. Over the longer term, empiric comparisons based on key metrics are needed to not only help guide career development and retention but also facilitate negotiations for programmatic support.
The group pointed out that embedded in delineating an optimal academic hospitalist job description is the longstanding work of general medicine societies in supporting and fostering the development of clinician educators. In many ways, the pressures of academic physicians to be mostly clinician and less educator versus someone who focuses heavily on educational work is similar for hospitalists and outpatient generalists. Academic general internal medicine divisions hired many general internists in the early 1990s to expand the reach of academic medical centers and increase the outpatient base.6 Many university hospitals are now hiring hospitalists to provide the inpatient care for these patients, but residency work hour reductions have added a layer of complexity, creating the need for entirely new roles for academic generalists (such as surgical comanagement of medically complex patients).7, 8 Past experiences in refining and reinforcing education as a key function (
Development of a Quality‐Improvement Portfolio Akin to an Educator's Portfolio
Many hospitalists actively participate in administrative work related to quality improvement activities, and we should develop this additional pathway for promotable academic activities (eg, clinician administrator); however, such a pathway may not be recognized by all promotion committees. The group observed that many aspects of quality improvement are similar to those of education (eg, developing a curriculum, leading a team, evaluating a process, defining generalizability, and disseminating locally proven interventions) and as such would be amenable to the development of a quality improvement portfolio, which candidates could submit to promotion committees. Again, past work in developing the importance and value of the educator's portfolio would facilitate the development of a quality improvement portfolio, which would require endorsement from key stakeholders (eg, the Association of Professors of Medicine, SGIM, and SHM).9 Importantly, this work may also benefit many outpatient‐based generalists who are increasingly focusing their careers on quality and safety improvement.
Developing Mentoring and Training Opportunities for Newly Hired and Junior Hospitalists
We reached a strong consensus about the need to develop a retreat‐format training opportunity by which junior academic hospitalists would be able to gain training in tasks critical to early‐career success. These were envisioned as an initial 2‐ to 3‐day meeting followed by mentorship at a distance and continued collaboration within the class of attendees. Topics would include key functions in AHM, such as becoming an effective attending physician and teacher, leadership, quality improvement, the business of medicine, effective billing, and maintaining a curriculum vitae. A number of professional societies have developed leadership or mentoring retreats, and at the time of this article's preparation, both regional and national efforts were underway to develop these products.
Developing Training and Mentorship Pathways for Hospitalist Researchers
There are few funded hospitalist researchers in the midcareer phase and a small but growing number of academic hospitalists entering the field with a focus on research. Enhancing a pipeline of researchers is a critical need for the field, as cementing AHM as an equal member of the academic medical community will be predicated on the successful development of hospitalist investigators. To this end, academic hospitalist groups should be encouraged to partner with other established research units (particularly general internal medicine) to create mentoring relationships and increase collaborative activities. The emergence of the Clinical and Translational Science Awards consortium sites, with a focus on implementation and effectiveness research, may also provide local opportunities for hospitalists to partner in research important for early‐career grant submission. Furthermore, building the pipeline of academic hospitalist researchers will require a strong focus on identifying students and residents through outreach at individual sites as well as presentations at national meetings (eg, the American College of Physicians).
Two other issues were also thought to be important. First, professional societies should work to encourage funders of primary carefocused general medicine training programs (the National Research Service Awards and the Health Resources and Services Administration) to allow hospitalists to qualify for such critical research training. Second, continuing to advocate for increasing funding for implementation and effectiveness research, via either the Agency for Healthcare Research and Quality or individual agencies of the National Institutes of Health, will be key; the emergence of a medical effectiveness institute would also be a potential boon.
Improving Relationships Between the Professional Homes of Academic Generalists
Relationships between outpatient‐based general medicine and hospital medicine were rocky as the field of AHM first took shape, and some residua of initial tensions persist a decade later. These tensions persist in part because hospitalists remain underdeveloped members of the academic community, and this perhaps gives some license to aver that hospitalists are merely transient faculty in a stage between residency and fellowship hired to improve throughput.
Overcoming this perception will require more engagement between academic generalists of all types, not less. The consensus group felt strongly that there need not be a single professional home for academic hospitalists and that generalists should be willing and even encouraged to self‐identify as hospital‐ or clinic‐focused, much as they might be geriatrics‐focused, informatics‐focused, or women's healthfocused. In fact, in some academic centers, a few generalists have successfully integrated themselves into both clinic‐based and hospitalist roles. In this way, the emergence and growth of AHM should be viewed as a boon to the practice of general medicine, not a challenge.
Resources
Much of what is proposed to enhance AHM will require resources. Academic hospitals have a vested interest in supporting AHM as a way to reduce turnover in a group that is increasingly critical for hospital operations, not to mention key leadership roles. Negotiating for these resources should emphasize that hospitals benefit directly from the revenue and margin that comes from incremental hospital admissions, collect most of the federal graduate medical education dollars, and benefit from improved care processes that are a result of hospitalist quality improvement efforts.
Deans and Departments, a key audience for the conference findings, also have a clear stake in fostering a less transient, more professionally satisfied and academically successful work force, particularly when hospitalists are increasingly the key educators of medical residents. Moreover, schools have a vested interest in the academic accomplishments and national reputation of their hospitalists. The financial arrangements will be unique to each setting and institution, and it is clear that the sources to be tapped will vary from site to site, but these resources are clearly necessary for the field.
Conclusions
AHM is at a crossroads. Unparalleled growth has created a large cadre of hospitalists who are struggling to meet the clinical demands of practice and the requirements for academic promotion; this situation will likely lead to, at a minimum, worsening problems with faculty turnover, and even greater losses of talented and passionate clinicians from the field of academic General Internal Medicine.
The challenges are numerous but not insurmountable, and our process identified issues and potential solutions which address clinical, educational, and research aspects of academic hospitalists' lives. We acknowledge that our findings are most relevant to hospitalists at academic medical centers or large academically oriented community teaching hospitals rather than hospitalists at community hospitals whose work is predominantly clinical with smaller teaching roles. However, we feel the academic hospitalists we targeted are in greater need of assistance. We believe that the most important issues are unsustainable, nonacademic positions, poor job preparation and training, inadequate prioritization of academic roles, and insufficient leadership and mentoring within the field.
It is the hope of all the consensus conference attendees that efforts focusing on academic hospitalists in the short term are not viewed as effort diverted from general internal medicine; in fact, the group felt that while many of the products of the consensus conference were probably most needed by AHM in the short term, these same solutions would likely be useful to outpatient‐based generalists as well. Despite the concerns and challenges outlined, the consensus conference group was also very hopeful that, in the setting where resources and collaboration are appropriately marshaled, that AHM will flourish quickly. In doing so, academic hospitalists will become better role models for residents and students, attracting the next generation of generalists needed to provide care to an increasingly complex patient population, and further advance the mission of General Internal Medicine.
Acknowledgements
The authors thank Dr. Jeff Glasheen and Dr. Robert Wachter for their comments on an earlier version of this article. In addition, the authors thank the following conference participants: Dan Brotman, MD, Johns Hopkins University; Deborah M. DeMarco, MD, President of the Association of Program Directors in Internal Medicine; Jeff Glasheen, MD, University of Colorado; Rusty Holman, MD, President of the Society of Hospital Medicine; Martha A. Hooven, President of the Administrators of Internal Medicine; Peter Kaboli, MD, University of Iowa; David O Meltzer, MD, PhD, University of Chicago; Vikas Parekh, MD, University of Michigan; Russell Phillips, MD MPH, Harvard Medical School; Sanjay Saint, MD, MPH, University of Michigan; Barbara Schuster, MD, President of the Association of Professors of Medicine; Brad Sharpe, MD, University of California San Francisco; Jeff Wiese, MD, Tulane University; David Kushner, Facilitator of the Kushner Companies; Geri Barnes, Support Staff of the Society of Hospital Medicine; and Amy Woodward, Support Staff of the Society of General Internal Medicine.
The past decade has seen hospital medicine grow from fewer than 1000 hospitalists nationwide to more than 20,000.1 In fact, survey data suggest that hospital medicine is the fastest growing field of internal medicine in the history of the US, and the growth of hospital medicine has produced a net increase in the number of generalists in the US.2
Although few direct estimates exist, academic hospital medicine (AHM) is also growing rapidly.1 Fueled by potential efficiency gains, a need for increased educational oversight of teaching services, and new residency work hour limitations, many academic medical centers and teaching hospitals have developed large hospital medicine programs. Internal medicine residency graduates interested in general medicine are finding hospital medicine an increasingly popular career choice. As a result, AHM groups have many recent residency graduates with an average age that is generally younger than 40.3
Over 85% of hospitalists are generalists and should find natural alliances with the nonhospitalist side of general internal medicine by collaborating in the course of clinical care, by teaching residents and students, or by designing quality improvement or research projects. In many academic centers, hospitalists are part of the division of general internal medicine, whereas in a few centers, hospitalists either have a separate division or lie outside the internal medicine department (employed by their hospitals).
Despite sharing a common training background and generalist mindset, many new academic hospitalists face different challenges than those faced by pure outpatient‐based academic generalists. First, at many centers, the financial arrangements between the AHM group and the hospital discourage hospitalists from traditional academic pursuits and draw them into clinical, operational, or administrative duties (such as responsibility for utilization review) that, although locally valuable, may not count as academic products in themselves or may take time away from more academic activities. Close alignment between hospitals and AHM may result in hospital administrators dictating hospitalists' practice in a way that further impedes academic viability. Reductions in resident training hours and an increasing need to provide 24‐hour coverage have facilitated growth in AHM into roles beyond those of the traditional academic generalist, such as medical comanagement of surgical patients and coverage of nonteaching services.4, 5 The youth of the field may exacerbate these problems. Most academic hospitalist groups have few senior leaders, whether they are clinical‐, education‐, or research‐focused. Young faculty need senior leaders as mentors to buffer them from relentless clinical demands that would compromise their hopes for academic success.
In order to better characterize these concerns and develop a shared work plan for future activities in support of AHM, the Society of Hospital Medicine (SHM) and the Society of General Internal Medicine (SGIM) convened an AHM consensus conference, a collaborative meeting developed and attended by representatives from SHM, SGIM, the Association of Chiefs of General Internal Medicine (ACGIM), the Association of Professors of Medicine, the Association of Program Directors in Internal Medicine, and the Association of Administrators in Internal Medicine. Using a structured consensus‐building format, we identified key barriers and challenges to AHM, then developed potential solutions.
Consensus Conference Format
Consensus Conference Steering Committee
The consensus conference was developed first by the sponsoring professional societies (SGIM, SHM, and ACGIM) being asked to nominate 2 people to be part of the consensus conference steering committee. The steering committee's main functions were to identify key tasks for the consensus conference, invite consensus conference attendees, ensure adequate representation from all participating organizations, synthesize the results of the consensus conference, and work with the individual professional societies so that results from the consensus conference were acted upon in a coordinated and effective manner.
Consensus Conference Prework
The consensus conference co‐chairs convened a series of conference calls in the spring of 2007, during which the steering committee developed a series of key areas to be explored during the conference. Topic areas were selected on the basis of the group's expertise and referred to past work by AHM taskforces convened by both SGIM/ACGIM and SHM.
The steering committee then invited stakeholders from each invited society so that each professional organization would provide at least 1 representative with expertise appropriate to 1 of the key domains identified:
-
Clinical and financial issues (within which topics such as optimal job descriptions and salary structures would be explored).
-
Teaching and education mission (within which topics such as mentorship for AHM junior faculty might be discussed).
-
Research and promotable activities (within which issues related to the development of promotable activities for AHM would be discussed).
Invitees to the consensus conference were assigned to one working group, given a general description of the potential areas within their domain, and instructed to consider a number of broad questions relevant to the topic area. These questions were as follows:
-
What are the key barriers to AHM in each key domain?
-
What viewpoints or barriers are the most pressing and/or actionable?
-
What solutions could be implemented or initiated in the next 1 to 3 years?
In order to facilitate discussion, consensus conference invitees were provided copies of findings from the SGIM/ACGIM and SHM Academic Hospitalist Task Forces, preliminary results from a survey of AHM leaders, and key literature related to the field.
Consensus Conference Format
The AHM consensus conference followed a modified Delphi consensus‐building format, in which the members of each working group developed findings relevant to their area, presented these viewpoints back to the overall group for feedback, and returned to their working group to refine their initial recommendations or move on to subsequent areas.
We used Day 1 of the meeting to confirm and refine lists of key barriers and opportunities to AHM. On Day 2, we developed actionable solutions and identified barriers with no ready solution but which were felt to be worth highlighting.
Each cycle of feedback (1 on Day 1 and 2 on Day 2) was used to identify additional barriers or opportunities prespecified by the steering committee, prioritize issues/opportunities, clarify uncertainties or point them out when they existed, and identify new areas requiring consensus. Between each cycle, workflow and interim results were summarized by the co‐chairs and a professional meeting coordinator to ensure that the group felt consensus had been achieved and to identify where additional work was required.
Writing Group/Peer Review
After the consensus conference adjourned, minutes were circulated to the group and approved, whereupon a summary of the meeting was reformatted into manuscript form. The manuscript was circulated to the steering committee, consensus conference attendees, and 2 selected peer reviewers as an additional check on the external validity of the study's results.
Consensus Findings 1: Current Challenges in AHM (Table 1)
Clinical and Financial Issues in AHM
The consensus group identified misalignment of the mission of hospitals (which often provide substantial financial support for hospital medicine programs) and the mission of departments of internal medicine (or divisions of general internal medicine) in which adult hospitalists reside as a fundamental barrier in AHM. Misalignment of missions produces challenges to the development of hospitalist groups in that their primary funder, the hospital, focuses on clinical care delivery, productivity, efficiency, and, in some cases, participation in patient safety and quality improvement efforts, whereas academic departments place considerable value on education, research, grants received, dissemination of scholarly work, and the national reputation of its faculty. Further exacerbating this tension is the fact that hospitalists do not always reside within traditional academic divisions (such as divisions of general medicine) and are therefore viewed by the hospital and their peers as hospital employees more than academic faculty.
As yet, few hospital medicine programs have successfully integrated academic and clinical needs. In many AHM programs, clinical demands have trumped academic pursuits and, as a result, produced jobs that have frequent turnover. This occurs most often when hospitalists are hired by academic medical centers primarily to staff nonresident services. Hospitalists who join these academic programs expecting ample opportunity to teach and pursue scholarly work often leave when they realize these jobs differ little from those in community settings (with the exception of less pay and, in most cases, a less efficient clinical delivery system). This turnover contributes to the perception of hospitalists as transient nonacademic faculty. The participants felt that we needed to define the ideal academic hospitalist job description.
| Clinical and Financial Issues | Teaching and Education Mission | Research and Promotable Activities | Cross‐Cutting Issues |
|---|---|---|---|
| |||
| Hospitalists' functions more often explicitly linked to hospital initiatives (clinical care, quality improvement, utilization, and throughput) | Distinguishing jobs that are predominantly clinical (C‐e) from those that are predominantly education‐focused (c‐E), which is important given the high clinical burdens | Lack of a pipeline producing hospitalist clinician investigators | Lack of leadership or negotiation skill training |
| Differing political, financial, and scientific priorities between hospitalists and administrators | Further exacerbation of C‐e/c‐E distinctions by the emergence of uncovered services | Few national funders focusing on inpatient general internal medicine | Little infrastructure for academic functions |
| Little guidance on the best models for each job type | Little recognition of quality improvement as a promotable/testable activity | Rapidly moving/growing field | |
| Decreasing interest in general internal medicine as a career path | |||
Teaching and Education Mission in AHM
Traditionally, faculty in academic medical centers have had prominent roles in resident teaching services, supervising medical residents, interns, and students. Hospitalists fill these roles at some institutions and in many cases have replaced senior faculty who are no longer able (because of competing demands from clinics or labs) or willing (because of an increased need for oversight and availability) to staff the teaching service. The teaching hospitalists start at these positions straight out of residency with little experience, training, or mentoring in how to succeed as a clinician educator. The creation of nonresident hospitalist services to address residency work hour requirements has removed many hospitalists from teaching opportunities as these services often have few if any teaching opportunities. The consensus group identified the lack of teaching opportunities and a lack of any formal preparation for those who do teach as the key challenges for new hospitalist clinician educators.
Research and Promotable Activities in AHM
Numerous challenges to promotion and success in hospital medicine research were identified. Most conference attendees felt that chairs of departments of medicine do not fully understand what the roles of academic hospitalists are, how they fit into the department's mission, or what is needed to better integrate hospitalists into the research and academic activities of the department. In addition, there are few hospital medicine fellowship programs, and those that have been created focus primarily on improving teaching skills or quality improvement rather than on research or the development of academic products. Aspiring academic hospitalists could pursue research fellowship training in existing programs (ie, the Robert Wood Johnson Foundation), but few graduates currently pursue these opportunities, and federally funded fellowships (eg, the National Research Service Awards and Health Resources and Services Administration T32 awards) explicitly exclude physicians who are not focused on primary care research. The group noted that a number of Veterans Administration fellowships (such as the Quality Scholars programs) may provide avenues for the training of hospital medicinefocused researchers, but they have been underused.
For researchers who focus on hospital medicine, federal funding sources are limited for both career development awards (K‐series) and later (R‐series) grants, particularly those funding the quality and safety research that hospitalists often pursue. Agencies of the National Institutes of Health currently do not provide many opportunities for hospital‐based general internal medicine research, and thus academic hospitalist research is undervalued by many promotion committees.
Cross‐Cutting Issues
Challenges in leadership and mentorship were identified as cross‐cutting. Many AHM programs are young, and so are their leaders. As a result, hospital medicine leaders often lack the experience and skills necessary to successfully negotiate for the support that is critical for the ideal program's success. As a young field, hospital medicine lacks faculty who have succeeded in careers as hospitalists, have been promoted in tenure tracks, and can mentor and guide young faculty through the complexities of academic medicine. Absent leadership and mentoring, few hospital medicine programs will succeed in traditional academic pursuits.
Consensus Findings 2: Overcoming Challenges to the Development of AHM (Table 2)
Summit attendees spent considerable time developing and refining solutions to the challenges described previously. Addressing the challenges resulted in a diverse group of proposed products that included educating key stakeholders, designing meetings, courses, or workshops, and gathering and disseminating data. There was considerable overlap among the solutions (Table 2).
| Solutions | Proposed Products | Challenge Domains Addressed* |
|---|---|---|
| ||
| 1. Educate stakeholders | Workshops at professional society meetings (SHM, SGIM, ACGIM, APM, and APDIM) | Addresses all domains |
| Publications highlighting issues | ||
| 2. Define the sustainable job | Data gathering and publication | Clinical/financial |
| 3. Quality improvement portfolio | Development and dissemination of criteria for the QI portfolio | Research/promotion |
| 4. Hospitalist training/mentoring | Academic hospitalist boot camp | Teaching/education |
| Research/promotion | ||
| Cross‐cutting | ||
| 5. Enhance research career pathways | Advocacy for enhanced training programs and funding sources | Research/promotion |
| 6. Improved relationships among general medicine societies | Society collaboration on product development | Addresses all domains |
Outreach to and Education of Stakeholders in Academic Medicine
The focus of the educational and outreach efforts suggested by the consensus group is to help leaders in academic medicine (not just AHM) and academic medical centers understand the challenges facing AHM. More importantly, efforts should reinforce the value of academic hospitalists to their hospital, department, and division. Efforts to engage these critical stakeholders to discuss and potentially address a number of the conference's proposed solutions are needed. Leaders include deans of medical schools, chairs of departments of medicine, division chiefs, and hospital administrative leadership.
Suggested outreach and educational activities included the publication of articles in key journals with the goal of increasing the visibility of AHM in professional societies as well as meetings and workshops focusing on teaching hospitalists and academic leaders methods to overcome challenges. Professional societies with a stake in AHM should better understand the challenges and position themselves to address these issues. The AHM task forces of SHM and SGIM can help give academic hospitalists a voice in having their needs addressed.
Publications
Articles have been commissioned in the following areas: descriptions of challenges and proposed solutions, best practices for nonresident hospitalist services, and metrics for the success of hospital medicine programs.
Meetings/Workshops
Meetings and workshops, sponsored by professional societies with a vested interest in AHM, were thought to be an effective way to address the needs of hospitalists, particularly those pursuing careers as clinician educators. Such workshops would provide skills in teaching and early career survival (eg, how to bill correctly) and in developing an educator's portfolio. Leadership training offerings, perhaps building on examples from SHM and ACGIM, were also thought to be valuable resources and venues that should be directed toward hospitalists, their chiefs, and relevant leaders.
Defining a Sustainable Job Description for Academic Hospitalists
The group strongly endorsed the need for transparent and readily available data aimed at developing sustainable academic hospitalist positions. For example, required information would include how academic jobs are constructed (in terms of months on service per year and the number of nights or weekends of coverage) and what successful programs and their hospitalists have found to be acceptable. Over the longer term, empiric comparisons based on key metrics are needed to not only help guide career development and retention but also facilitate negotiations for programmatic support.
The group pointed out that embedded in delineating an optimal academic hospitalist job description is the longstanding work of general medicine societies in supporting and fostering the development of clinician educators. In many ways, the pressures of academic physicians to be mostly clinician and less educator versus someone who focuses heavily on educational work is similar for hospitalists and outpatient generalists. Academic general internal medicine divisions hired many general internists in the early 1990s to expand the reach of academic medical centers and increase the outpatient base.6 Many university hospitals are now hiring hospitalists to provide the inpatient care for these patients, but residency work hour reductions have added a layer of complexity, creating the need for entirely new roles for academic generalists (such as surgical comanagement of medically complex patients).7, 8 Past experiences in refining and reinforcing education as a key function (
Development of a Quality‐Improvement Portfolio Akin to an Educator's Portfolio
Many hospitalists actively participate in administrative work related to quality improvement activities, and we should develop this additional pathway for promotable academic activities (eg, clinician administrator); however, such a pathway may not be recognized by all promotion committees. The group observed that many aspects of quality improvement are similar to those of education (eg, developing a curriculum, leading a team, evaluating a process, defining generalizability, and disseminating locally proven interventions) and as such would be amenable to the development of a quality improvement portfolio, which candidates could submit to promotion committees. Again, past work in developing the importance and value of the educator's portfolio would facilitate the development of a quality improvement portfolio, which would require endorsement from key stakeholders (eg, the Association of Professors of Medicine, SGIM, and SHM).9 Importantly, this work may also benefit many outpatient‐based generalists who are increasingly focusing their careers on quality and safety improvement.
Developing Mentoring and Training Opportunities for Newly Hired and Junior Hospitalists
We reached a strong consensus about the need to develop a retreat‐format training opportunity by which junior academic hospitalists would be able to gain training in tasks critical to early‐career success. These were envisioned as an initial 2‐ to 3‐day meeting followed by mentorship at a distance and continued collaboration within the class of attendees. Topics would include key functions in AHM, such as becoming an effective attending physician and teacher, leadership, quality improvement, the business of medicine, effective billing, and maintaining a curriculum vitae. A number of professional societies have developed leadership or mentoring retreats, and at the time of this article's preparation, both regional and national efforts were underway to develop these products.
Developing Training and Mentorship Pathways for Hospitalist Researchers
There are few funded hospitalist researchers in the midcareer phase and a small but growing number of academic hospitalists entering the field with a focus on research. Enhancing a pipeline of researchers is a critical need for the field, as cementing AHM as an equal member of the academic medical community will be predicated on the successful development of hospitalist investigators. To this end, academic hospitalist groups should be encouraged to partner with other established research units (particularly general internal medicine) to create mentoring relationships and increase collaborative activities. The emergence of the Clinical and Translational Science Awards consortium sites, with a focus on implementation and effectiveness research, may also provide local opportunities for hospitalists to partner in research important for early‐career grant submission. Furthermore, building the pipeline of academic hospitalist researchers will require a strong focus on identifying students and residents through outreach at individual sites as well as presentations at national meetings (eg, the American College of Physicians).
Two other issues were also thought to be important. First, professional societies should work to encourage funders of primary carefocused general medicine training programs (the National Research Service Awards and the Health Resources and Services Administration) to allow hospitalists to qualify for such critical research training. Second, continuing to advocate for increasing funding for implementation and effectiveness research, via either the Agency for Healthcare Research and Quality or individual agencies of the National Institutes of Health, will be key; the emergence of a medical effectiveness institute would also be a potential boon.
Improving Relationships Between the Professional Homes of Academic Generalists
Relationships between outpatient‐based general medicine and hospital medicine were rocky as the field of AHM first took shape, and some residua of initial tensions persist a decade later. These tensions persist in part because hospitalists remain underdeveloped members of the academic community, and this perhaps gives some license to aver that hospitalists are merely transient faculty in a stage between residency and fellowship hired to improve throughput.
Overcoming this perception will require more engagement between academic generalists of all types, not less. The consensus group felt strongly that there need not be a single professional home for academic hospitalists and that generalists should be willing and even encouraged to self‐identify as hospital‐ or clinic‐focused, much as they might be geriatrics‐focused, informatics‐focused, or women's healthfocused. In fact, in some academic centers, a few generalists have successfully integrated themselves into both clinic‐based and hospitalist roles. In this way, the emergence and growth of AHM should be viewed as a boon to the practice of general medicine, not a challenge.
Resources
Much of what is proposed to enhance AHM will require resources. Academic hospitals have a vested interest in supporting AHM as a way to reduce turnover in a group that is increasingly critical for hospital operations, not to mention key leadership roles. Negotiating for these resources should emphasize that hospitals benefit directly from the revenue and margin that comes from incremental hospital admissions, collect most of the federal graduate medical education dollars, and benefit from improved care processes that are a result of hospitalist quality improvement efforts.
Deans and Departments, a key audience for the conference findings, also have a clear stake in fostering a less transient, more professionally satisfied and academically successful work force, particularly when hospitalists are increasingly the key educators of medical residents. Moreover, schools have a vested interest in the academic accomplishments and national reputation of their hospitalists. The financial arrangements will be unique to each setting and institution, and it is clear that the sources to be tapped will vary from site to site, but these resources are clearly necessary for the field.
Conclusions
AHM is at a crossroads. Unparalleled growth has created a large cadre of hospitalists who are struggling to meet the clinical demands of practice and the requirements for academic promotion; this situation will likely lead to, at a minimum, worsening problems with faculty turnover, and even greater losses of talented and passionate clinicians from the field of academic General Internal Medicine.
The challenges are numerous but not insurmountable, and our process identified issues and potential solutions which address clinical, educational, and research aspects of academic hospitalists' lives. We acknowledge that our findings are most relevant to hospitalists at academic medical centers or large academically oriented community teaching hospitals rather than hospitalists at community hospitals whose work is predominantly clinical with smaller teaching roles. However, we feel the academic hospitalists we targeted are in greater need of assistance. We believe that the most important issues are unsustainable, nonacademic positions, poor job preparation and training, inadequate prioritization of academic roles, and insufficient leadership and mentoring within the field.
It is the hope of all the consensus conference attendees that efforts focusing on academic hospitalists in the short term are not viewed as effort diverted from general internal medicine; in fact, the group felt that while many of the products of the consensus conference were probably most needed by AHM in the short term, these same solutions would likely be useful to outpatient‐based generalists as well. Despite the concerns and challenges outlined, the consensus conference group was also very hopeful that, in the setting where resources and collaboration are appropriately marshaled, that AHM will flourish quickly. In doing so, academic hospitalists will become better role models for residents and students, attracting the next generation of generalists needed to provide care to an increasingly complex patient population, and further advance the mission of General Internal Medicine.
Acknowledgements
The authors thank Dr. Jeff Glasheen and Dr. Robert Wachter for their comments on an earlier version of this article. In addition, the authors thank the following conference participants: Dan Brotman, MD, Johns Hopkins University; Deborah M. DeMarco, MD, President of the Association of Program Directors in Internal Medicine; Jeff Glasheen, MD, University of Colorado; Rusty Holman, MD, President of the Society of Hospital Medicine; Martha A. Hooven, President of the Administrators of Internal Medicine; Peter Kaboli, MD, University of Iowa; David O Meltzer, MD, PhD, University of Chicago; Vikas Parekh, MD, University of Michigan; Russell Phillips, MD MPH, Harvard Medical School; Sanjay Saint, MD, MPH, University of Michigan; Barbara Schuster, MD, President of the Association of Professors of Medicine; Brad Sharpe, MD, University of California San Francisco; Jeff Wiese, MD, Tulane University; David Kushner, Facilitator of the Kushner Companies; Geri Barnes, Support Staff of the Society of Hospital Medicine; and Amy Woodward, Support Staff of the Society of General Internal Medicine.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,,,,.Trends in market demand for internal medicine 1999 to 2004: an analysis of physician job advertisements.J Gen Intern Med.2006;21:1079–1085.
- Society of Hospital Medicine. 2006 Hospital Medicine Survey. Available at: http://www.hospitalmedicine.org/content/navigationmenu/media/mediakit/media_kit.htm. Accessed January 2009.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- UCLA rewrites the script for academic networks.Med Netw Strategy Rep.1998;7:1–5.
- ,,,,,.Systematic review: effects of resident work hours on patient safety.Ann Intern Med.2004;141:851–857.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136:591–596.
- ,,,.Documentation systems for educators seeking academic promotion in U.S. medical schools.Acad Med.2004;79:783–790.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,,,,.Trends in market demand for internal medicine 1999 to 2004: an analysis of physician job advertisements.J Gen Intern Med.2006;21:1079–1085.
- Society of Hospital Medicine. 2006 Hospital Medicine Survey. Available at: http://www.hospitalmedicine.org/content/navigationmenu/media/mediakit/media_kit.htm. Accessed January 2009.
- ,,,,,.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294:1088–1100.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- UCLA rewrites the script for academic networks.Med Netw Strategy Rep.1998;7:1–5.
- ,,,,,.Systematic review: effects of resident work hours on patient safety.Ann Intern Med.2004;141:851–857.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136:591–596.
- ,,,.Documentation systems for educators seeking academic promotion in U.S. medical schools.Acad Med.2004;79:783–790.
Jumpstarting Hospital Medicine Research
Dramatic changes in the organization, financing, and delivery of hospital care that began a decade ago continue to accelerate. One of the most important changes has been the emergence of hospitalists as providers of inpatient care.1 Hospitalists are physicians, usually general internists, whose clinical focus is the hospitalized patient. As patient illnesses have become more severe and complex, physicians have found it difficult to balance inpatient and outpatient care and have focused on one of the two.25 It is estimated that there are currently 15,000 practicing hospitalists nationally, and projections suggest that this number may exceed 30,000 by 2010, which is equal to the number of cardiologists currently practicing in the United States.6 A 2003 survey from the American Hospital Association showed that more than 30% of the nation's 4900 community hospitals have hospital medicine groups.7 Furthermore, more than 70% of the nation's largest hospitals (>500 beds) and 66% of major teaching hospitals use hospitalists.7
The transition to a hospitalist model generates multiple new research questions about the best approach to caring for the hospitalized patient. Additionally, hospitalists may spawn new areas of clinical research by tackling clinical issues that formerly lacked a large number of specialist investigators. Examples include implementation‐based studies,8, 9 inpatient safety practices,1012 quasi‐experimental studies focusing on common inpatient issues,13, 14 and the evaluation of new methods for reducing resource utilization within various inpatient care delivery structures.15, 16
Similarly, if future clinical trials are to be carried out in real‐world settings, by necessity these will require the participation of hospitalists. Clinical research performed by hospitalists and hospital medicine programs, however, remains underdeveloped. Although this has been attributed to several variables, including the youth of the field, a paucity of fellowship‐trained hospitalist researchers, and a lack of a hospitalist‐oriented national funding source, we also believe that additional barriers exist which could be overcome if hospitalists actively partnered with specialists to perform hospital‐based clinical and translational research.
Hospitalists lack clinical expertise in many clinical issues. In both academic and nonacademic settings, the diagnostic approach, individual treatment decisions, and follow‐up of complex patients occur with frequent consultation of specialists. Specialists often provide a deeper understanding of both the pathophysiologic concepts and scientific principles underlying important clinical questions and are more likely to have had fellowship training that included clinical research experience. Specialists also have more access to extramural funding for disease‐based investigation, and thus their involvement in hospital‐based clinical research would likely enhance funding opportunities, improve project feasibility, and increase dissemination of the results. A successful clinical research program will therefore be one that combines specialists and hospitalists working collaboratively to determine the best way to care for inpatients. With that in mind, we created the University of Michigan SpecialistHospitalist Allied Research Program (SHARP).
METHODS
Setting
The University of Michigan Medical Center includes a 900‐bed teaching hospital with more than 44,000 yearly inpatient discharges, and the Department of Internal Medicine manages nearly 15,000 annual discharges. The University of Michigan Hospital Medicine Program has grown dramatically over the past few years and now includes more than 30 hospitalists. These hospitalists will manage nearly 8000 admissions in the upcoming year, which represent more than half of all the patients admitted to the Department of Internal Medicine. Five years ago, these 8000 admissions would have been cared for by 3 to 4 times as many providers, most of whom would have been specialists. Currently, specialists consult regularly on patients cared for by hospitalists, and as a result, a few loosely formed research collaborations developed spontaneously but lacked resources or infrastructure to facilitate their completion. SHARP was intended to organize these clinical research pilot studies and jumpstart hospital‐based clinical and translational research.
The SHARP Intervention
Objectives
In 2006, hospitalists and specialists with an interest in expanding clinical and translational research aimed at caring for inpatients were brought together for the SHARP intervention. This intervention had several objectives:
To develop a clinical research infrastructure within the University of Michigan Hospital Medicine Program to facilitate patient participation.
To foster increased specialisthospitalist collaboration for addressing common inpatient problems.
To facilitate pilot projects and preliminary data collection that enhance the ability to obtain subsequent extramural funding for collaborative research projects.
To facilitate multicenter investigation led by the University of Michigan by allowing the SHARP investigators to use an existing hospitalist consortium to expand the scope of research projects.
Ultimately, to develop the ability to perform multicenter intervention‐based clinical trials.
Structure
The key to SHARP's infrastructure is its personnel and governance structure. At the head of SHARP is an academic hospitalist as principal investigator (PI) and an academic cardiologist with health services research training serving as coprincipal investigator (Co‐PI). Key personnel also include a hospitalist investigator, a masters‐level research associate, a PhD clinical epidemiologist, and the hospitalists and subspecialists who serve as investigators. Although the program leadership has research experience, many of the hospitalist and specialist investigators are junior faculty without extensive prior research experience. Thus, SHARP was specifically designed to build the capacity to enhance inpatient clinical and translational research and to remove barriers for new investigators developing their academic careers.
It is critical that oversight provides direction for the research program, assists with project identification and selection, and facilitates collaborations that tie diverse projects together. We believe that this is best accomplished by the creation of a steering committee chaired by both the PI and Co‐PI. The steering committee also includes key individuals such as the Vice Chair of the Department of Medicine and the Associate Dean for Clinical and Translational Research at the University of Michigan. The 2 cochairs are responsible for overseeing the program and reporting the progress of SHARP to the University of Michigan Department of Internal Medicine. They will help identify and produce viable research proposals that can be brought to the full committee. To help the program understand and overcome bureaucratic obstacles, we have also included a former high‐level administrator on the steering committee as a consultant. Given the initial scope of the program, the SHARP steering committee has had a small number of key individuals. As the program grows and increases its number of ongoing collaborative projects, we will likely need to expand committee membership.
SHARP leadership meets regularly to plan projects, discuss grant ideas, make hiring decisions, and troubleshoot problems in existing projects. The entire steering committee meets quarterly to help chart the overall course of the program. A more thorough description of the program and its structure can be found on the SHARP Web site (
SHARP Funding
SHARP could not exist without resources. The funding for the program comes from the Department of Internal Medicine and uses revenue from the hospital medicine program that flows to the department. To garner support for the program, SHARP leadership sought buy‐in from the Chair of Medicine, all the division chiefs, and key faculty active in clinical research. The fact that the program has the potential to benefit not just hospitalists but also other department faculty such as specialists facilitated departmental funding. The program is funded for 3 years with an 18‐month program review to gauge progress. Funding is used to build clinical research infrastructure and facilitate collection of pilot data. SHARP resources support a portion of the salaries of key personnel for the 3‐year duration of the project (research associate, 50%; PI, 10%; Co‐PI, 5%; and epidemiologist, 5%), after which time intramural funding ends. Every SHARP project is, therefore, expected to apply for extramural funding with the goal of full extramural programmatic support after 3 years.
SHARP Performance Metrics
Measuring the accomplishments of SHARP is clearly important. As the program is intended to jumpstart collaborative inpatient clinical research, the number of such projects is important to track. An additional goal is to support work that leads to extramural funding. As the program started from scratch, it is unrealistic to have completed peer‐reviewed manuscripts or successful extramural grants as the sole metrics by which the program is judged, especially early in its initiation. In a yearly report to the department chair, we will report on primary and secondary outcomes (see Table 1).
| Primary outcomes |
|---|
|
| 1. Number of ongoing research projects involving SHARP support and a brief description of the aims and status of each |
| 2. Number of extramural grants submitted in which SHARP is mentioned or involved |
| 3. Extramural grants received (total and direct dollars) |
| 4. Peer‐reviewed publications authored by SHARP investigators |
| Secondary outcomes |
| 1. Abstracts accepted for presentation at national or international scientific meetings |
| 2. Non‐peer‐reviewed publications related to SHARP |
| 3. Invited presentations by SHARP investigators |
| 4. People who have visited the University of Michigan in conjunction with SHARP work (eg, visiting professors) |
Initial SHARP Projects
SHARP has a formal process for evaluating potential projects. A steering committee ultimately decides how best to use SHARP‐related resources. Key components in this decision are related to the proposal's innovation, feasibility, and importance as well as the extent of specialisthospitalist collaboration. The 2 projects described next are our initial areas of focus and exemplify these concepts. One project partners hospitalists with infectious disease specialists, whereas the second pairs hospitalists with geriatricians and clinical pharmacists.
Reducing False Positive Blood Cultures
The blood culture is an important tool for the diagnosis and management of bloodstream infections. As a result, physicians have a low threshold for obtaining blood cultures. Unfortunately, up to half of all positive blood cultures are positive because of contamination. These false positive cultures lead to additional diagnostic testing, unnecessary antibiotics, and increased healthcare costs.17 A variety of antiseptic agents and techniques are used to prevent falsely positive cultures. However, a recent evidence‐based systematic review performed by University of Michigan investigators found no clear evidence to suggest which antiseptic agent should be routinely used. They concluded that a randomized controlled trial was urgently needed.18
SHARP and its infrastructure have begun a cluster‐randomized crossover trial at the university hospital. The trial compares the effects of a variety of skin antiseptic agents on peripheral blood culture contamination rates. The study population includes hospitalized patients undergoing venipuncture for peripheral blood cultures on 3 general medicine and surgery floors. The trial will include over 12,000 blood culture sets and will have 85% power to detect a 0.5% difference in effectiveness between antiseptic agents. Key outcomes will be rates of positive blood cultures (true positive versus false positive), quantity of additional diagnostic testing generated by positive cultures, resource use (including antibiotics), and associated costs. Clinical outcomes such as length of stay and inpatient mortality will also be measured as secondary outcomes.
Pharmacist‐Facilitated Hospital Discharge
Hospital discharge is a complex process in which patients must be transferred from the care of an inpatient team to that of an outpatient provider. During most hospitalizations, a patient will have new medications added, a chronic medication stopped, or a change in medication dosage. Studies have revealed that the most common adverse events that have an impact on patients after discharge are related to medications.1921 In our experience at the University of Michigan, patients frequently have medication‐related adverse events after discharge because they do not understand what medications they should be taking, what they are used for, how to manage side effects, or whom to call with problems. In addition, predictable medication‐related issues (such as the ability to pay for a medicine or expected serum electrolyte changes with newly added medications) are not universally anticipated. The frail elderly are especially vulnerable to medication‐related adverse events.
Building on the work of others in the field, we proposed studying the impact of an inpatient clinical pharmacist to address medication misadventures related to hospital discharge in our elderly population.22 The study uses an interrupted time series design (the pharmacist will alternate months at a nonresident hospitalist service and a resident general medicine service) to measure the impact of the clinical pharmacist. The pharmacist will focus on patients over the age of 65 meeting criteria that identify them to be at high risk for an adverse medication event after discharge. These factors include any new medication started in the hospital, medication noncompliance or an adverse medication event that led to the admission, or use of a high‐risk medication (eg, anticoagulants, narcotics, diuretics, diabetic agents, and immunosuppressives). The pharmacist and inpatient physicians will identify high‐risk patients who will receive predischarge medication counseling. This process will identify problem medications and needed follow‐up (eg, laboratory testing) and assess compliance issues. After discharge, patients will be contacted by the pharmacist both within 72 hours and at 30 days. Standardized questions will be asked of patients to troubleshoot medication issues, assess them for problems with medications or follow‐up, and identify patients who may need more urgent access to a healthcare provider to address medication‐related problems.
Key outcomes will include the pharmacist's actions at discharge (eg, dose changes made, medication class switches, and side‐effect monitoring implemented). In addition, we will track types of medication issues identified after discharge and interventions made. Important clinical outcomes will include return to the emergency department after discharge, 30‐day readmission rates, and healthcare‐related costs.
DISCUSSION AND NEXT STEPS
SHARP is a novel clinical research program partnering hospitalists with specialists. Its current focus targets single‐institution studies that generate pilot data leading to larger projects. The ultimate goal is to develop the ability to do larger multicenter investigator‐initiated projects. The SHARP program will also have the ability to perform observational studies to identify predictors and risk factors and the ability to carry out implementation studies that show how best to translate results from published articles to direct patient care.
A specialisthospitalist collaboration overcomes barriers that we feel may impede hospital medicine research at an academic medical center. For a similar program to succeed at other institutions, key components from our program will have to be replicated. First, senior, fellowship‐trained researchers are required to mentor junior investigators (who may or may not have additional fellowship training), help guide project selection, oversee grant and manuscript submissions, and troubleshoot problems that arise in the course of any clinical research project. In our institution, this comes from within our hospitalist program and from our specialist collaborators. In institutions lacking hospitalists with research experience, this guidance could come from within a division of general medicine, internal medicine specialty divisions, internal medicine department leadership, or even noninternal medicine departments (eg, emergency medicine, neurology, and surgery) that have traditionally been involved in clinical research programs.
A second key component that must be considered is funding. An initial investment is necessary to fund key personnel dedicated to getting projects started on the right track, collecting pilot data, and ensuring project completion and dissemination of the results. The positive margin generated by our hospitalist program facilitated the initial investment. In the absence of a positive margin, resources could come directly from the hospital, the medical school, the department of internal medicine, or perhaps a foundation. The case would need to be made that an initial short‐term investment would enhance the academic standing of the institution, enhance the careers of young investigators, and over time lead to a self‐sustaining program through investigator‐initiated grants and extramural funding. In addition to experienced leadership and funding, we created an oversight committee, but we feel that this is not a critical component. A potential concern with a program that partners with specialists might be that research topics become too disease‐specific or specialty‐oriented. We specifically created the oversight committee to protect against this possibility, and other institutions might need similar safeguards.
Our next step includes leveraging existing hospitalist collaboratives that reach beyond academic medical centers to expand further the reach of SHARP. Ultimately, any new therapy, clinical tool, diagnostic paradigm, or implementation strategy that is developed or evaluated bythe SHARP program would need to be tested in a real‐world setting to assess external validity. With support from the Blue Cross Blue Shield of Michigan Foundation, we have created a multihospital patient safety consortium, the Hospitalists as Emerging Leaders in Patient Safety Consortium, which includes academic, government, urban, rural, teaching, and nonteaching hospitals.23 Although the initial focus is patient safety, our goal for the consortium is to develop it into a multihospital clinical research program that could take pilot projects developed by SHARP and test them in real‐world settings. We believe that full‐scale multihospital studies based on SHARP pilot data will be very attractive to external funding agencies and will help SHARP become financially self‐sufficient after the initial 3‐year start‐up.
Hospital medicine research is desperately needed.24, 25 Unfortunately, the clinical research capabilities of most hospital medicine programs are quite underdeveloped. We believe that partnering hospitalists with specialists can facilitate collaborative research to identify the best way to care for inpatients. If successful, we believe that variations of this model can be replicated at other institutions and will be a critical factor in jumpstarting hospital medicine clinical research.
Acknowledgements
The authors thank Dr. Marc E. Lippman, Dr. Robert F. Todd, Dr. Larry McMahon, Dr. Timothy J. Laing, and Mr. Lindsay J. Graham, whose support made this program possible.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,,,.What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction?J Gen Intern Med.2003;18:725–729.
- ,,,,.Characteristics of general internists who practice only outpatient medicine: results from the physician worklife study.Semin Med Pract.2002;5:5–11.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,.The potential size of the hospitalist workforce in the United States.Am J Med.1999;106:441–445.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Translating infection prevention evidence into practice using quantitative and qualitative research.Am J Infect Control.2006;34:507–512.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,,,.Making health care safer: a critical analysis of patient safety practices.Evid Rep Technol Assess (Summ).2001;(43):i–x,1–668.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,, et al.Are antiseptic‐coated central venous catheters effective in a real‐world setting?Am J Infect Control.2006;34:388–393.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,.What effect does physician “profiling” have on inpatient physician satisfaction and hospital length of stay?BMC Health Serv Res.2006;6:45.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,,.Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination.Infect Control Hosp Epidemiol.2007;28:892–895.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,.Hospitalists as emerging leaders in patient safety: targeting a few to affect many.JPatient Saf.2005;1:78–82.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72 e1–7.
Dramatic changes in the organization, financing, and delivery of hospital care that began a decade ago continue to accelerate. One of the most important changes has been the emergence of hospitalists as providers of inpatient care.1 Hospitalists are physicians, usually general internists, whose clinical focus is the hospitalized patient. As patient illnesses have become more severe and complex, physicians have found it difficult to balance inpatient and outpatient care and have focused on one of the two.25 It is estimated that there are currently 15,000 practicing hospitalists nationally, and projections suggest that this number may exceed 30,000 by 2010, which is equal to the number of cardiologists currently practicing in the United States.6 A 2003 survey from the American Hospital Association showed that more than 30% of the nation's 4900 community hospitals have hospital medicine groups.7 Furthermore, more than 70% of the nation's largest hospitals (>500 beds) and 66% of major teaching hospitals use hospitalists.7
The transition to a hospitalist model generates multiple new research questions about the best approach to caring for the hospitalized patient. Additionally, hospitalists may spawn new areas of clinical research by tackling clinical issues that formerly lacked a large number of specialist investigators. Examples include implementation‐based studies,8, 9 inpatient safety practices,1012 quasi‐experimental studies focusing on common inpatient issues,13, 14 and the evaluation of new methods for reducing resource utilization within various inpatient care delivery structures.15, 16
Similarly, if future clinical trials are to be carried out in real‐world settings, by necessity these will require the participation of hospitalists. Clinical research performed by hospitalists and hospital medicine programs, however, remains underdeveloped. Although this has been attributed to several variables, including the youth of the field, a paucity of fellowship‐trained hospitalist researchers, and a lack of a hospitalist‐oriented national funding source, we also believe that additional barriers exist which could be overcome if hospitalists actively partnered with specialists to perform hospital‐based clinical and translational research.
Hospitalists lack clinical expertise in many clinical issues. In both academic and nonacademic settings, the diagnostic approach, individual treatment decisions, and follow‐up of complex patients occur with frequent consultation of specialists. Specialists often provide a deeper understanding of both the pathophysiologic concepts and scientific principles underlying important clinical questions and are more likely to have had fellowship training that included clinical research experience. Specialists also have more access to extramural funding for disease‐based investigation, and thus their involvement in hospital‐based clinical research would likely enhance funding opportunities, improve project feasibility, and increase dissemination of the results. A successful clinical research program will therefore be one that combines specialists and hospitalists working collaboratively to determine the best way to care for inpatients. With that in mind, we created the University of Michigan SpecialistHospitalist Allied Research Program (SHARP).
METHODS
Setting
The University of Michigan Medical Center includes a 900‐bed teaching hospital with more than 44,000 yearly inpatient discharges, and the Department of Internal Medicine manages nearly 15,000 annual discharges. The University of Michigan Hospital Medicine Program has grown dramatically over the past few years and now includes more than 30 hospitalists. These hospitalists will manage nearly 8000 admissions in the upcoming year, which represent more than half of all the patients admitted to the Department of Internal Medicine. Five years ago, these 8000 admissions would have been cared for by 3 to 4 times as many providers, most of whom would have been specialists. Currently, specialists consult regularly on patients cared for by hospitalists, and as a result, a few loosely formed research collaborations developed spontaneously but lacked resources or infrastructure to facilitate their completion. SHARP was intended to organize these clinical research pilot studies and jumpstart hospital‐based clinical and translational research.
The SHARP Intervention
Objectives
In 2006, hospitalists and specialists with an interest in expanding clinical and translational research aimed at caring for inpatients were brought together for the SHARP intervention. This intervention had several objectives:
To develop a clinical research infrastructure within the University of Michigan Hospital Medicine Program to facilitate patient participation.
To foster increased specialisthospitalist collaboration for addressing common inpatient problems.
To facilitate pilot projects and preliminary data collection that enhance the ability to obtain subsequent extramural funding for collaborative research projects.
To facilitate multicenter investigation led by the University of Michigan by allowing the SHARP investigators to use an existing hospitalist consortium to expand the scope of research projects.
Ultimately, to develop the ability to perform multicenter intervention‐based clinical trials.
Structure
The key to SHARP's infrastructure is its personnel and governance structure. At the head of SHARP is an academic hospitalist as principal investigator (PI) and an academic cardiologist with health services research training serving as coprincipal investigator (Co‐PI). Key personnel also include a hospitalist investigator, a masters‐level research associate, a PhD clinical epidemiologist, and the hospitalists and subspecialists who serve as investigators. Although the program leadership has research experience, many of the hospitalist and specialist investigators are junior faculty without extensive prior research experience. Thus, SHARP was specifically designed to build the capacity to enhance inpatient clinical and translational research and to remove barriers for new investigators developing their academic careers.
It is critical that oversight provides direction for the research program, assists with project identification and selection, and facilitates collaborations that tie diverse projects together. We believe that this is best accomplished by the creation of a steering committee chaired by both the PI and Co‐PI. The steering committee also includes key individuals such as the Vice Chair of the Department of Medicine and the Associate Dean for Clinical and Translational Research at the University of Michigan. The 2 cochairs are responsible for overseeing the program and reporting the progress of SHARP to the University of Michigan Department of Internal Medicine. They will help identify and produce viable research proposals that can be brought to the full committee. To help the program understand and overcome bureaucratic obstacles, we have also included a former high‐level administrator on the steering committee as a consultant. Given the initial scope of the program, the SHARP steering committee has had a small number of key individuals. As the program grows and increases its number of ongoing collaborative projects, we will likely need to expand committee membership.
SHARP leadership meets regularly to plan projects, discuss grant ideas, make hiring decisions, and troubleshoot problems in existing projects. The entire steering committee meets quarterly to help chart the overall course of the program. A more thorough description of the program and its structure can be found on the SHARP Web site (
SHARP Funding
SHARP could not exist without resources. The funding for the program comes from the Department of Internal Medicine and uses revenue from the hospital medicine program that flows to the department. To garner support for the program, SHARP leadership sought buy‐in from the Chair of Medicine, all the division chiefs, and key faculty active in clinical research. The fact that the program has the potential to benefit not just hospitalists but also other department faculty such as specialists facilitated departmental funding. The program is funded for 3 years with an 18‐month program review to gauge progress. Funding is used to build clinical research infrastructure and facilitate collection of pilot data. SHARP resources support a portion of the salaries of key personnel for the 3‐year duration of the project (research associate, 50%; PI, 10%; Co‐PI, 5%; and epidemiologist, 5%), after which time intramural funding ends. Every SHARP project is, therefore, expected to apply for extramural funding with the goal of full extramural programmatic support after 3 years.
SHARP Performance Metrics
Measuring the accomplishments of SHARP is clearly important. As the program is intended to jumpstart collaborative inpatient clinical research, the number of such projects is important to track. An additional goal is to support work that leads to extramural funding. As the program started from scratch, it is unrealistic to have completed peer‐reviewed manuscripts or successful extramural grants as the sole metrics by which the program is judged, especially early in its initiation. In a yearly report to the department chair, we will report on primary and secondary outcomes (see Table 1).
| Primary outcomes |
|---|
|
| 1. Number of ongoing research projects involving SHARP support and a brief description of the aims and status of each |
| 2. Number of extramural grants submitted in which SHARP is mentioned or involved |
| 3. Extramural grants received (total and direct dollars) |
| 4. Peer‐reviewed publications authored by SHARP investigators |
| Secondary outcomes |
| 1. Abstracts accepted for presentation at national or international scientific meetings |
| 2. Non‐peer‐reviewed publications related to SHARP |
| 3. Invited presentations by SHARP investigators |
| 4. People who have visited the University of Michigan in conjunction with SHARP work (eg, visiting professors) |
Initial SHARP Projects
SHARP has a formal process for evaluating potential projects. A steering committee ultimately decides how best to use SHARP‐related resources. Key components in this decision are related to the proposal's innovation, feasibility, and importance as well as the extent of specialisthospitalist collaboration. The 2 projects described next are our initial areas of focus and exemplify these concepts. One project partners hospitalists with infectious disease specialists, whereas the second pairs hospitalists with geriatricians and clinical pharmacists.
Reducing False Positive Blood Cultures
The blood culture is an important tool for the diagnosis and management of bloodstream infections. As a result, physicians have a low threshold for obtaining blood cultures. Unfortunately, up to half of all positive blood cultures are positive because of contamination. These false positive cultures lead to additional diagnostic testing, unnecessary antibiotics, and increased healthcare costs.17 A variety of antiseptic agents and techniques are used to prevent falsely positive cultures. However, a recent evidence‐based systematic review performed by University of Michigan investigators found no clear evidence to suggest which antiseptic agent should be routinely used. They concluded that a randomized controlled trial was urgently needed.18
SHARP and its infrastructure have begun a cluster‐randomized crossover trial at the university hospital. The trial compares the effects of a variety of skin antiseptic agents on peripheral blood culture contamination rates. The study population includes hospitalized patients undergoing venipuncture for peripheral blood cultures on 3 general medicine and surgery floors. The trial will include over 12,000 blood culture sets and will have 85% power to detect a 0.5% difference in effectiveness between antiseptic agents. Key outcomes will be rates of positive blood cultures (true positive versus false positive), quantity of additional diagnostic testing generated by positive cultures, resource use (including antibiotics), and associated costs. Clinical outcomes such as length of stay and inpatient mortality will also be measured as secondary outcomes.
Pharmacist‐Facilitated Hospital Discharge
Hospital discharge is a complex process in which patients must be transferred from the care of an inpatient team to that of an outpatient provider. During most hospitalizations, a patient will have new medications added, a chronic medication stopped, or a change in medication dosage. Studies have revealed that the most common adverse events that have an impact on patients after discharge are related to medications.1921 In our experience at the University of Michigan, patients frequently have medication‐related adverse events after discharge because they do not understand what medications they should be taking, what they are used for, how to manage side effects, or whom to call with problems. In addition, predictable medication‐related issues (such as the ability to pay for a medicine or expected serum electrolyte changes with newly added medications) are not universally anticipated. The frail elderly are especially vulnerable to medication‐related adverse events.
Building on the work of others in the field, we proposed studying the impact of an inpatient clinical pharmacist to address medication misadventures related to hospital discharge in our elderly population.22 The study uses an interrupted time series design (the pharmacist will alternate months at a nonresident hospitalist service and a resident general medicine service) to measure the impact of the clinical pharmacist. The pharmacist will focus on patients over the age of 65 meeting criteria that identify them to be at high risk for an adverse medication event after discharge. These factors include any new medication started in the hospital, medication noncompliance or an adverse medication event that led to the admission, or use of a high‐risk medication (eg, anticoagulants, narcotics, diuretics, diabetic agents, and immunosuppressives). The pharmacist and inpatient physicians will identify high‐risk patients who will receive predischarge medication counseling. This process will identify problem medications and needed follow‐up (eg, laboratory testing) and assess compliance issues. After discharge, patients will be contacted by the pharmacist both within 72 hours and at 30 days. Standardized questions will be asked of patients to troubleshoot medication issues, assess them for problems with medications or follow‐up, and identify patients who may need more urgent access to a healthcare provider to address medication‐related problems.
Key outcomes will include the pharmacist's actions at discharge (eg, dose changes made, medication class switches, and side‐effect monitoring implemented). In addition, we will track types of medication issues identified after discharge and interventions made. Important clinical outcomes will include return to the emergency department after discharge, 30‐day readmission rates, and healthcare‐related costs.
DISCUSSION AND NEXT STEPS
SHARP is a novel clinical research program partnering hospitalists with specialists. Its current focus targets single‐institution studies that generate pilot data leading to larger projects. The ultimate goal is to develop the ability to do larger multicenter investigator‐initiated projects. The SHARP program will also have the ability to perform observational studies to identify predictors and risk factors and the ability to carry out implementation studies that show how best to translate results from published articles to direct patient care.
A specialisthospitalist collaboration overcomes barriers that we feel may impede hospital medicine research at an academic medical center. For a similar program to succeed at other institutions, key components from our program will have to be replicated. First, senior, fellowship‐trained researchers are required to mentor junior investigators (who may or may not have additional fellowship training), help guide project selection, oversee grant and manuscript submissions, and troubleshoot problems that arise in the course of any clinical research project. In our institution, this comes from within our hospitalist program and from our specialist collaborators. In institutions lacking hospitalists with research experience, this guidance could come from within a division of general medicine, internal medicine specialty divisions, internal medicine department leadership, or even noninternal medicine departments (eg, emergency medicine, neurology, and surgery) that have traditionally been involved in clinical research programs.
A second key component that must be considered is funding. An initial investment is necessary to fund key personnel dedicated to getting projects started on the right track, collecting pilot data, and ensuring project completion and dissemination of the results. The positive margin generated by our hospitalist program facilitated the initial investment. In the absence of a positive margin, resources could come directly from the hospital, the medical school, the department of internal medicine, or perhaps a foundation. The case would need to be made that an initial short‐term investment would enhance the academic standing of the institution, enhance the careers of young investigators, and over time lead to a self‐sustaining program through investigator‐initiated grants and extramural funding. In addition to experienced leadership and funding, we created an oversight committee, but we feel that this is not a critical component. A potential concern with a program that partners with specialists might be that research topics become too disease‐specific or specialty‐oriented. We specifically created the oversight committee to protect against this possibility, and other institutions might need similar safeguards.
Our next step includes leveraging existing hospitalist collaboratives that reach beyond academic medical centers to expand further the reach of SHARP. Ultimately, any new therapy, clinical tool, diagnostic paradigm, or implementation strategy that is developed or evaluated bythe SHARP program would need to be tested in a real‐world setting to assess external validity. With support from the Blue Cross Blue Shield of Michigan Foundation, we have created a multihospital patient safety consortium, the Hospitalists as Emerging Leaders in Patient Safety Consortium, which includes academic, government, urban, rural, teaching, and nonteaching hospitals.23 Although the initial focus is patient safety, our goal for the consortium is to develop it into a multihospital clinical research program that could take pilot projects developed by SHARP and test them in real‐world settings. We believe that full‐scale multihospital studies based on SHARP pilot data will be very attractive to external funding agencies and will help SHARP become financially self‐sufficient after the initial 3‐year start‐up.
Hospital medicine research is desperately needed.24, 25 Unfortunately, the clinical research capabilities of most hospital medicine programs are quite underdeveloped. We believe that partnering hospitalists with specialists can facilitate collaborative research to identify the best way to care for inpatients. If successful, we believe that variations of this model can be replicated at other institutions and will be a critical factor in jumpstarting hospital medicine clinical research.
Acknowledgements
The authors thank Dr. Marc E. Lippman, Dr. Robert F. Todd, Dr. Larry McMahon, Dr. Timothy J. Laing, and Mr. Lindsay J. Graham, whose support made this program possible.
Dramatic changes in the organization, financing, and delivery of hospital care that began a decade ago continue to accelerate. One of the most important changes has been the emergence of hospitalists as providers of inpatient care.1 Hospitalists are physicians, usually general internists, whose clinical focus is the hospitalized patient. As patient illnesses have become more severe and complex, physicians have found it difficult to balance inpatient and outpatient care and have focused on one of the two.25 It is estimated that there are currently 15,000 practicing hospitalists nationally, and projections suggest that this number may exceed 30,000 by 2010, which is equal to the number of cardiologists currently practicing in the United States.6 A 2003 survey from the American Hospital Association showed that more than 30% of the nation's 4900 community hospitals have hospital medicine groups.7 Furthermore, more than 70% of the nation's largest hospitals (>500 beds) and 66% of major teaching hospitals use hospitalists.7
The transition to a hospitalist model generates multiple new research questions about the best approach to caring for the hospitalized patient. Additionally, hospitalists may spawn new areas of clinical research by tackling clinical issues that formerly lacked a large number of specialist investigators. Examples include implementation‐based studies,8, 9 inpatient safety practices,1012 quasi‐experimental studies focusing on common inpatient issues,13, 14 and the evaluation of new methods for reducing resource utilization within various inpatient care delivery structures.15, 16
Similarly, if future clinical trials are to be carried out in real‐world settings, by necessity these will require the participation of hospitalists. Clinical research performed by hospitalists and hospital medicine programs, however, remains underdeveloped. Although this has been attributed to several variables, including the youth of the field, a paucity of fellowship‐trained hospitalist researchers, and a lack of a hospitalist‐oriented national funding source, we also believe that additional barriers exist which could be overcome if hospitalists actively partnered with specialists to perform hospital‐based clinical and translational research.
Hospitalists lack clinical expertise in many clinical issues. In both academic and nonacademic settings, the diagnostic approach, individual treatment decisions, and follow‐up of complex patients occur with frequent consultation of specialists. Specialists often provide a deeper understanding of both the pathophysiologic concepts and scientific principles underlying important clinical questions and are more likely to have had fellowship training that included clinical research experience. Specialists also have more access to extramural funding for disease‐based investigation, and thus their involvement in hospital‐based clinical research would likely enhance funding opportunities, improve project feasibility, and increase dissemination of the results. A successful clinical research program will therefore be one that combines specialists and hospitalists working collaboratively to determine the best way to care for inpatients. With that in mind, we created the University of Michigan SpecialistHospitalist Allied Research Program (SHARP).
METHODS
Setting
The University of Michigan Medical Center includes a 900‐bed teaching hospital with more than 44,000 yearly inpatient discharges, and the Department of Internal Medicine manages nearly 15,000 annual discharges. The University of Michigan Hospital Medicine Program has grown dramatically over the past few years and now includes more than 30 hospitalists. These hospitalists will manage nearly 8000 admissions in the upcoming year, which represent more than half of all the patients admitted to the Department of Internal Medicine. Five years ago, these 8000 admissions would have been cared for by 3 to 4 times as many providers, most of whom would have been specialists. Currently, specialists consult regularly on patients cared for by hospitalists, and as a result, a few loosely formed research collaborations developed spontaneously but lacked resources or infrastructure to facilitate their completion. SHARP was intended to organize these clinical research pilot studies and jumpstart hospital‐based clinical and translational research.
The SHARP Intervention
Objectives
In 2006, hospitalists and specialists with an interest in expanding clinical and translational research aimed at caring for inpatients were brought together for the SHARP intervention. This intervention had several objectives:
To develop a clinical research infrastructure within the University of Michigan Hospital Medicine Program to facilitate patient participation.
To foster increased specialisthospitalist collaboration for addressing common inpatient problems.
To facilitate pilot projects and preliminary data collection that enhance the ability to obtain subsequent extramural funding for collaborative research projects.
To facilitate multicenter investigation led by the University of Michigan by allowing the SHARP investigators to use an existing hospitalist consortium to expand the scope of research projects.
Ultimately, to develop the ability to perform multicenter intervention‐based clinical trials.
Structure
The key to SHARP's infrastructure is its personnel and governance structure. At the head of SHARP is an academic hospitalist as principal investigator (PI) and an academic cardiologist with health services research training serving as coprincipal investigator (Co‐PI). Key personnel also include a hospitalist investigator, a masters‐level research associate, a PhD clinical epidemiologist, and the hospitalists and subspecialists who serve as investigators. Although the program leadership has research experience, many of the hospitalist and specialist investigators are junior faculty without extensive prior research experience. Thus, SHARP was specifically designed to build the capacity to enhance inpatient clinical and translational research and to remove barriers for new investigators developing their academic careers.
It is critical that oversight provides direction for the research program, assists with project identification and selection, and facilitates collaborations that tie diverse projects together. We believe that this is best accomplished by the creation of a steering committee chaired by both the PI and Co‐PI. The steering committee also includes key individuals such as the Vice Chair of the Department of Medicine and the Associate Dean for Clinical and Translational Research at the University of Michigan. The 2 cochairs are responsible for overseeing the program and reporting the progress of SHARP to the University of Michigan Department of Internal Medicine. They will help identify and produce viable research proposals that can be brought to the full committee. To help the program understand and overcome bureaucratic obstacles, we have also included a former high‐level administrator on the steering committee as a consultant. Given the initial scope of the program, the SHARP steering committee has had a small number of key individuals. As the program grows and increases its number of ongoing collaborative projects, we will likely need to expand committee membership.
SHARP leadership meets regularly to plan projects, discuss grant ideas, make hiring decisions, and troubleshoot problems in existing projects. The entire steering committee meets quarterly to help chart the overall course of the program. A more thorough description of the program and its structure can be found on the SHARP Web site (
SHARP Funding
SHARP could not exist without resources. The funding for the program comes from the Department of Internal Medicine and uses revenue from the hospital medicine program that flows to the department. To garner support for the program, SHARP leadership sought buy‐in from the Chair of Medicine, all the division chiefs, and key faculty active in clinical research. The fact that the program has the potential to benefit not just hospitalists but also other department faculty such as specialists facilitated departmental funding. The program is funded for 3 years with an 18‐month program review to gauge progress. Funding is used to build clinical research infrastructure and facilitate collection of pilot data. SHARP resources support a portion of the salaries of key personnel for the 3‐year duration of the project (research associate, 50%; PI, 10%; Co‐PI, 5%; and epidemiologist, 5%), after which time intramural funding ends. Every SHARP project is, therefore, expected to apply for extramural funding with the goal of full extramural programmatic support after 3 years.
SHARP Performance Metrics
Measuring the accomplishments of SHARP is clearly important. As the program is intended to jumpstart collaborative inpatient clinical research, the number of such projects is important to track. An additional goal is to support work that leads to extramural funding. As the program started from scratch, it is unrealistic to have completed peer‐reviewed manuscripts or successful extramural grants as the sole metrics by which the program is judged, especially early in its initiation. In a yearly report to the department chair, we will report on primary and secondary outcomes (see Table 1).
| Primary outcomes |
|---|
|
| 1. Number of ongoing research projects involving SHARP support and a brief description of the aims and status of each |
| 2. Number of extramural grants submitted in which SHARP is mentioned or involved |
| 3. Extramural grants received (total and direct dollars) |
| 4. Peer‐reviewed publications authored by SHARP investigators |
| Secondary outcomes |
| 1. Abstracts accepted for presentation at national or international scientific meetings |
| 2. Non‐peer‐reviewed publications related to SHARP |
| 3. Invited presentations by SHARP investigators |
| 4. People who have visited the University of Michigan in conjunction with SHARP work (eg, visiting professors) |
Initial SHARP Projects
SHARP has a formal process for evaluating potential projects. A steering committee ultimately decides how best to use SHARP‐related resources. Key components in this decision are related to the proposal's innovation, feasibility, and importance as well as the extent of specialisthospitalist collaboration. The 2 projects described next are our initial areas of focus and exemplify these concepts. One project partners hospitalists with infectious disease specialists, whereas the second pairs hospitalists with geriatricians and clinical pharmacists.
Reducing False Positive Blood Cultures
The blood culture is an important tool for the diagnosis and management of bloodstream infections. As a result, physicians have a low threshold for obtaining blood cultures. Unfortunately, up to half of all positive blood cultures are positive because of contamination. These false positive cultures lead to additional diagnostic testing, unnecessary antibiotics, and increased healthcare costs.17 A variety of antiseptic agents and techniques are used to prevent falsely positive cultures. However, a recent evidence‐based systematic review performed by University of Michigan investigators found no clear evidence to suggest which antiseptic agent should be routinely used. They concluded that a randomized controlled trial was urgently needed.18
SHARP and its infrastructure have begun a cluster‐randomized crossover trial at the university hospital. The trial compares the effects of a variety of skin antiseptic agents on peripheral blood culture contamination rates. The study population includes hospitalized patients undergoing venipuncture for peripheral blood cultures on 3 general medicine and surgery floors. The trial will include over 12,000 blood culture sets and will have 85% power to detect a 0.5% difference in effectiveness between antiseptic agents. Key outcomes will be rates of positive blood cultures (true positive versus false positive), quantity of additional diagnostic testing generated by positive cultures, resource use (including antibiotics), and associated costs. Clinical outcomes such as length of stay and inpatient mortality will also be measured as secondary outcomes.
Pharmacist‐Facilitated Hospital Discharge
Hospital discharge is a complex process in which patients must be transferred from the care of an inpatient team to that of an outpatient provider. During most hospitalizations, a patient will have new medications added, a chronic medication stopped, or a change in medication dosage. Studies have revealed that the most common adverse events that have an impact on patients after discharge are related to medications.1921 In our experience at the University of Michigan, patients frequently have medication‐related adverse events after discharge because they do not understand what medications they should be taking, what they are used for, how to manage side effects, or whom to call with problems. In addition, predictable medication‐related issues (such as the ability to pay for a medicine or expected serum electrolyte changes with newly added medications) are not universally anticipated. The frail elderly are especially vulnerable to medication‐related adverse events.
Building on the work of others in the field, we proposed studying the impact of an inpatient clinical pharmacist to address medication misadventures related to hospital discharge in our elderly population.22 The study uses an interrupted time series design (the pharmacist will alternate months at a nonresident hospitalist service and a resident general medicine service) to measure the impact of the clinical pharmacist. The pharmacist will focus on patients over the age of 65 meeting criteria that identify them to be at high risk for an adverse medication event after discharge. These factors include any new medication started in the hospital, medication noncompliance or an adverse medication event that led to the admission, or use of a high‐risk medication (eg, anticoagulants, narcotics, diuretics, diabetic agents, and immunosuppressives). The pharmacist and inpatient physicians will identify high‐risk patients who will receive predischarge medication counseling. This process will identify problem medications and needed follow‐up (eg, laboratory testing) and assess compliance issues. After discharge, patients will be contacted by the pharmacist both within 72 hours and at 30 days. Standardized questions will be asked of patients to troubleshoot medication issues, assess them for problems with medications or follow‐up, and identify patients who may need more urgent access to a healthcare provider to address medication‐related problems.
Key outcomes will include the pharmacist's actions at discharge (eg, dose changes made, medication class switches, and side‐effect monitoring implemented). In addition, we will track types of medication issues identified after discharge and interventions made. Important clinical outcomes will include return to the emergency department after discharge, 30‐day readmission rates, and healthcare‐related costs.
DISCUSSION AND NEXT STEPS
SHARP is a novel clinical research program partnering hospitalists with specialists. Its current focus targets single‐institution studies that generate pilot data leading to larger projects. The ultimate goal is to develop the ability to do larger multicenter investigator‐initiated projects. The SHARP program will also have the ability to perform observational studies to identify predictors and risk factors and the ability to carry out implementation studies that show how best to translate results from published articles to direct patient care.
A specialisthospitalist collaboration overcomes barriers that we feel may impede hospital medicine research at an academic medical center. For a similar program to succeed at other institutions, key components from our program will have to be replicated. First, senior, fellowship‐trained researchers are required to mentor junior investigators (who may or may not have additional fellowship training), help guide project selection, oversee grant and manuscript submissions, and troubleshoot problems that arise in the course of any clinical research project. In our institution, this comes from within our hospitalist program and from our specialist collaborators. In institutions lacking hospitalists with research experience, this guidance could come from within a division of general medicine, internal medicine specialty divisions, internal medicine department leadership, or even noninternal medicine departments (eg, emergency medicine, neurology, and surgery) that have traditionally been involved in clinical research programs.
A second key component that must be considered is funding. An initial investment is necessary to fund key personnel dedicated to getting projects started on the right track, collecting pilot data, and ensuring project completion and dissemination of the results. The positive margin generated by our hospitalist program facilitated the initial investment. In the absence of a positive margin, resources could come directly from the hospital, the medical school, the department of internal medicine, or perhaps a foundation. The case would need to be made that an initial short‐term investment would enhance the academic standing of the institution, enhance the careers of young investigators, and over time lead to a self‐sustaining program through investigator‐initiated grants and extramural funding. In addition to experienced leadership and funding, we created an oversight committee, but we feel that this is not a critical component. A potential concern with a program that partners with specialists might be that research topics become too disease‐specific or specialty‐oriented. We specifically created the oversight committee to protect against this possibility, and other institutions might need similar safeguards.
Our next step includes leveraging existing hospitalist collaboratives that reach beyond academic medical centers to expand further the reach of SHARP. Ultimately, any new therapy, clinical tool, diagnostic paradigm, or implementation strategy that is developed or evaluated bythe SHARP program would need to be tested in a real‐world setting to assess external validity. With support from the Blue Cross Blue Shield of Michigan Foundation, we have created a multihospital patient safety consortium, the Hospitalists as Emerging Leaders in Patient Safety Consortium, which includes academic, government, urban, rural, teaching, and nonteaching hospitals.23 Although the initial focus is patient safety, our goal for the consortium is to develop it into a multihospital clinical research program that could take pilot projects developed by SHARP and test them in real‐world settings. We believe that full‐scale multihospital studies based on SHARP pilot data will be very attractive to external funding agencies and will help SHARP become financially self‐sufficient after the initial 3‐year start‐up.
Hospital medicine research is desperately needed.24, 25 Unfortunately, the clinical research capabilities of most hospital medicine programs are quite underdeveloped. We believe that partnering hospitalists with specialists can facilitate collaborative research to identify the best way to care for inpatients. If successful, we believe that variations of this model can be replicated at other institutions and will be a critical factor in jumpstarting hospital medicine clinical research.
Acknowledgements
The authors thank Dr. Marc E. Lippman, Dr. Robert F. Todd, Dr. Larry McMahon, Dr. Timothy J. Laing, and Mr. Lindsay J. Graham, whose support made this program possible.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,,,.What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction?J Gen Intern Med.2003;18:725–729.
- ,,,,.Characteristics of general internists who practice only outpatient medicine: results from the physician worklife study.Semin Med Pract.2002;5:5–11.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,.The potential size of the hospitalist workforce in the United States.Am J Med.1999;106:441–445.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Translating infection prevention evidence into practice using quantitative and qualitative research.Am J Infect Control.2006;34:507–512.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,,,.Making health care safer: a critical analysis of patient safety practices.Evid Rep Technol Assess (Summ).2001;(43):i–x,1–668.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,, et al.Are antiseptic‐coated central venous catheters effective in a real‐world setting?Am J Infect Control.2006;34:388–393.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,.What effect does physician “profiling” have on inpatient physician satisfaction and hospital length of stay?BMC Health Serv Res.2006;6:45.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,,.Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination.Infect Control Hosp Epidemiol.2007;28:892–895.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,.Hospitalists as emerging leaders in patient safety: targeting a few to affect many.JPatient Saf.2005;1:78–82.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72 e1–7.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,,,.What effect does increasing inpatient time have on outpatient‐oriented internist satisfaction?J Gen Intern Med.2003;18:725–729.
- ,,,,.Characteristics of general internists who practice only outpatient medicine: results from the physician worklife study.Semin Med Pract.2002;5:5–11.
- ,.Hospitalists in teaching hospitals: opportunities but not without danger.J Gen Intern Med.2004;19:392–393.
- ,.Hospitalists: the new model of inpatient medical care in the United States.Eur J Intern Med.2003;14:65–70.
- ,,,,.The potential size of the hospitalist workforce in the United States.Am J Med.1999;106:441–445.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Translating infection prevention evidence into practice using quantitative and qualitative research.Am J Infect Control.2006;34:507–512.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,,,.Making health care safer: a critical analysis of patient safety practices.Evid Rep Technol Assess (Summ).2001;(43):i–x,1–668.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,,.Clinical pharmacists and inpatient medical care: a systematic review.Arch Intern Med.2006;166:955–964.
- ,,, et al.Are antiseptic‐coated central venous catheters effective in a real‐world setting?Am J Infect Control.2006;34:388–393.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,.What effect does physician “profiling” have on inpatient physician satisfaction and hospital length of stay?BMC Health Serv Res.2006;6:45.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,,,,.Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination.Infect Control Hosp Epidemiol.2007;28:892–895.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,.Hospitalists as emerging leaders in patient safety: targeting a few to affect many.JPatient Saf.2005;1:78–82.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119:72 e1–7.
Copyright © 2008 Society of Hospital Medicine
Community‐Acquired Pneumonia
Pneumonia may well be called the friend of the aged. Taken off by it in an acute, short, not often painful illness, the old man escapes those cold gradations of decay so distressing of himself and to his friends.
William Osler, MD, 1898
Community‐acquired pneumonia (CAP) is commonly defined as an infection of the pulmonary parenchyma that is associated with at least some symptoms and signs of acute infection, accompanied by the presence of an acute infiltrate on chest radiograph, in a patient not hospitalized or residing in a long‐term‐care facility for 14 days prior to the onset of symptoms.1 CAP continues to be a common and serious illness, causing substantial morbidity and mortality in the adult population. There are an estimated 56 million cases a year in the United States, with greater than 1 million hospitalizations. Community‐acquired pneumonia is one of the most common admitting diagnoses among adults, and with a 30‐day mortality between 10% and 14% for patients admitted to the hospital, it is the leading cause of infectious death in the United States.2 In elderly patients, hospitalization for CAP portends a poor long‐term prognosis. In a Medicare database, the 1‐year mortality for patients with CAP was nearly 40%, compared to 29% in patients with other diagnoses.3 Community‐acquired pneumonia is a model illness in hospital medicineit is a common disease that allows for evidence‐based and cost‐effective management. In addition, many national organizations have proposed multiple quality indicators for community‐acquired pneumonia, thus providing an opportunity for institutional quality improvement. This review article outlines the assessment and management of patients admitted to the hospital with community‐acquired pneumonia.
Etiology
Although many pathogens can cause community‐acquired pneumonia, the clinical syndromes and microbiology of CAP have traditionally been characterized as either typical or atypical. The typical organisms include Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, and the atypical organisms include Chlamydia spp., Mycoplasma pneumoniae, Legionella spp., and viruses. This historical distinction has recently come into question. It is now clear that the presenting symptoms, signs, and basic laboratory findings (including the chest radiograph) cannot be reliably used to predict the etiologic pathogen or to distinguish typical from atypical organisms.4 Rather, the specific causative agent of CAP depends more on the degree of patient illness. Table 1 shows what prospective studies with comprehensive diagnostic strategies determined to be the most common pathogens in patients hospitalized for CAP in ICU and non‐ICU settings.5 Streptococcus pneumoniae remains the most common cause of CAP in hospitalized patients and is the most common cause of fatal pneumonia, whereas Legionella spp. is a common cause of severe CAP, more often found in patients requiring admission to the intensive care unit. Gram‐negative bacilli can cause CAP in elderly patients and those recently treated with broad‐spectrum antibiotics or with underlying lung disease. Notably, though, despite improved diagnostic testing, only one quarter of all admitted patients with CAP have the etiologic agent defined, and therefore empiric therapy should be directed broadly at the most likely organisms.6
| Non‐ICU inpatients | ICU inpatients (severe) |
|---|---|
| S. pneumoniae | S. pneumoniae |
| M. pneumoniae | Legionella spp |
| C. pneumoniae | H. influenzae |
| H. influenzae | Gram‐negative bacilli |
| Legionella spp | S. aureus |
| Aspiration | |
| Respiratory viruses |
Clinical Presentation
Patients admitted to the hospital with CAP typically present with a brief history of respiratory complaints, including cough (greater than 90%), dyspnea (66%), sputum production (66%), and pleuritic chest pain (50%); see Table 2.7, 8 In 10%30% of patients, nonrespiratory complaints predominate, including headache, myalgias, fatigue, and gastrointestinal symptoms.6 Elderly patients, an increasing percentage of hospitalized patients, are less likely to present with typical CAP symptoms (such as cough) and more likely to have altered mental status as a presenting symptom.9
| Symptoms | Signs (exam) |
|---|---|
| Cough 90% | Fever 80% |
| Dyspnea 66% | Tachypnea 70% |
| Sputum 66% | Tachycardia 50% |
| Pleuritic chest pain 50% | Focal lung exam >90% |
On physical examination, patients with CAP usually have signs of fever (80%), tachypnea (70%), and tachycardia (50%); see Table 2. Most will have a focal lung exam (>90%) with findings ranging from crackles to bronchial breath sounds.10 No exam finding is specific for the diagnosis of pneumonia, but the absence of fever, tachycardia, and tachypnea significantly reduces the probability of CAP in patients with suspected pneumonia.10 Furthermore, similar to the clinical history, the physical examination of elderly patients with community‐acquired pneumonia is not specific or sensitive for the diagnosis of CAP. For example, up to 40% of elderly patients subsequently determined to have CAP may not have fever.11
Leukocytosis is common in patients with CAP; however, its absence does not rule out disease.12 A number of guidelines recommend laboratory evaluation of electrolytes, urea nitrogen, creatinine, liver enzymes, and bilirubin, although these are used primarily for prognostication and are not specifically useful in the diagnosis of CAP.
DIAGNOSIS
Differential Diagnosis
Given the nonspecific nature of the symptoms and signs associated with CAP, there is no single clinical feature or combination of clinical features that adequately rules in or out the diagnosis of CAP. Consequently, the differential diagnosis to be considered in patients with suspected CAP is broad. Noninfectious diseases can often present with similar clinical syndromes; these include congestive heart failure, exacerbation of chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism, and hypersensitivity pneumonitis. These diseases can often be distinguished with a thorough history and physical examination.
In addition, other upper‐ and lower‐airway infectious diseases can have similar nonspecific signs and symptoms. In particular, pneumonia must often be differentiated from acute bronchitis, which as a diagnosis accounts for up to 40% of patients evaluated for cough (versus 5% for pneumonia).10 Patients with acute bronchitis frequently do not present with high fevers or hypoxia and in general will not benefit from antibiotic therapy.13 Patients believed to have community‐acquired pneumonia might also be suffering from other pneumonia syndromes including aspiration pneumonia, postobstructive pneumonia, and pneumonia in immunocompromised patients (eg, those with HIV, on steroids, receiving chemotherapy). Determining the correct diagnosis can have implications for therapy and prognosis.
Diagnostic Studies
The diagnosis of community‐acquired pneumonia requires that a patient have both signs and symptoms consistent with pulmonary infection and evidence of a new radiographic infiltrate. Therefore, most guidelines recommend that all patients with a possible diagnosis of CAP be evaluated with chest radiography.1, 14, 15
The specific radiographic findings in community‐acquired pneumonia range from lobar consolidation to hazy focal infiltrate to diffuse bilateral interstitial opacities (see Figure 1). Although chest radiography has traditionally been considered the gold standard for the diagnosis of CAP, its exact performance characteristics are unknown, and it is clearly not 100% sensitive or 100% specific. The utility of the chest radiograph can be limited by patient body habitus, underlying lung disease, or dehydration. Computed tomography (CT) scanning, although not recommended for routine use, can identify pulmonary consolidation in up to 30% of patients with a normal or equivocal chest radiograph in whom pneumonia is suspected and can also identify complications of pneumonia including an empyema or pulmonary abscess.16
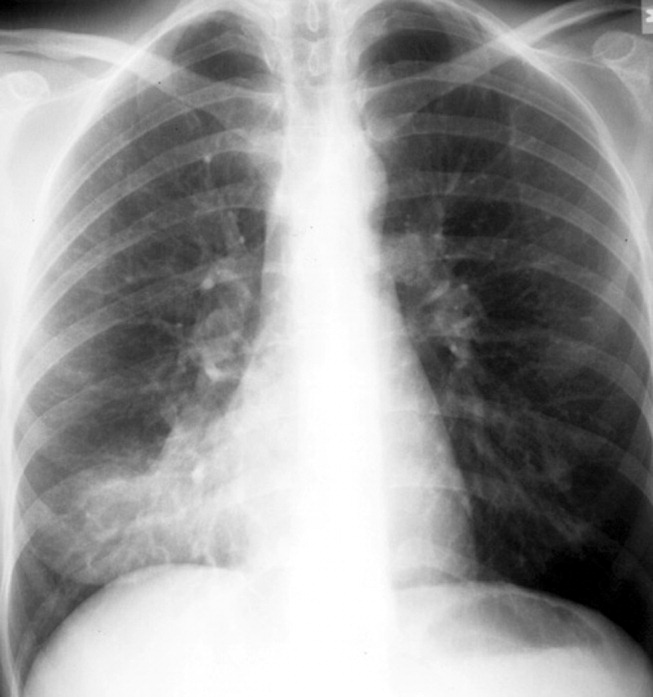
Limitations in the performance of the chest radiograph have resulted in an interest in the diagnostic performance of serologic markers of infection such as C‐reactive protein (CRP), procalcitonin, and soluble triggering receptor expressed on myeloid cells (s‐TREM).1719 Preliminary evidence suggests these inflammatory markers may ultimately prove useful in differentiating infectious from noninfectious pulmonary processes, but regular use of these new tests cannot currently be recommended.
Most expert guidelines state that 2 sets of blood cultures should be taken and analyzed prior to antibiotic administration in all patients admitted to the hospital with suspected community‐acquired pneumonia.1, 14, 15 Isolation of bacteria from blood cultures in CAP is a very specific way to identify a causative organism in order to subsequently narrow therapy and also identifies a high‐risk group of patients because bacteremia is associated with increased mortality. Obtaining blood cultures within 24 hours of admission has been associated with 10% lower odds of 30‐day mortality in patients with CAP,20 and as a result, drawing blood cultures prior to antibiotic administration is a national quality indicator for CAP.
There are, however, a number of problems with the routine acquisition of blood cultures in all patients admitted with CAP. Practically, the cultures can be difficult to obtain, can potentially delay the initiation of antibiotics, and are often contaminated, which has been shown to increase both cost and length of stay.21, 22 The yield is generally low: the true‐positive bacteremia rate for admitted patients with CAP ranges from 6% to 9%, and the culture results rarely change management or outcomes.23, 24 Given these limitations, many have argued that blood cultures should be obtained with a more targeted approach. A recent study used a Medicare database to create a decision‐support tool to help maximize the value of blood cultures in CAP.25 The predictors of a positive blood culture are shown in Table 3. Not obtaining cultures on patients who had received prior antibiotics or had no risk factors resulted in about 40% fewer overall cultures while identifying approximately 90% of bacteremias. In their guidelines, the British Thoracic Society (BTS) advocates a similar strategy, recommending blood cultures be omitted in nonsevere pneumonia and in patients without comorbidities.15, 26 Although recommendations vary for non‐severe CAP in hospitalized patients, all professional society guidelines agree that blood cultures should be obtained in critically ill patients, and if cultures are obtained, they should be drawn prior to antibiotics.1, 14, 15, 26
| Comorbidities |
| Liver disease |
| Vital signs |
| Systolic blood pressure < 90 mm Hg |
| Temperature < 35C or 40C |
| Pulse 125 beats/min |
| Laboratory and radiographic data |
| Blood urea nitrogen (BUN) 30 mg/dL |
| Sodium < 130 mmol/L |
| White blood cells < 5000/mm3 or > 20,000/mm3 |
| Prior use of antibiotics (negative predictor) |
Substantial controversy surrounds the utility of routine sputum gram stains and cultures for patients admitted to the hospital with CAP. The Infectious Disease Society of America (IDSA) and the British Thoracic Society (BTS) both recommend that all patients admitted to the hospital with community‐acquired pneumonia should have a gram stain and culture of expectorated sputum.1, 15, 26 Both organizations argue sputum collection is a simple and inexpensive procedure that can potentially identify pathogenic organisms and can affect both initial and long‐term antibiotic therapy. Most notably, they highlight gram stain specificity of greater than 80% for pneumococcal pneumonia. Conversely, the American Thoracic Society (ATS) argues that sputum gram stains and cultures generally have low sensitivity, specificity, and positive predictive value.14 Furthermore, they argue the utility of sputum testing is also limited practically; in one study 30% of patients could not produce an adequate sputum specimen and up to 30% had received prior antibiotic therapy, substantially reducing the yield.27 In another study, good‐quality sputum with a predominant morphotype could be obtained in only 14% of patients admitted with CAP.28 However, targeting sputum analysis to patients who have not received prior antibiotics and are able to produce an adequate sample improved the yield significantly.29 In addition, with increasing rates of antibiotic resistance among common community isolates (ie, S. Pneumoniae) and the increasing prevalence of infecting organisms not targeted by routine empiric therapy (methicillin‐resistant Staphylococcus Aureus [MRSA]), isolation of potential causative pathogens is increasingly important. We believe that severely ill patients with CAP (such as patients admitted to the ICU), as well as patients with identifiable risk factors for uncommon or drug‐resistant pathogens (eg, Pseudomonas aeruginosa, enteric gram‐negative rods, MRSA, etc.) should have sputum sent for gram stain and culture. Ideally, sputum obtained for gram stain and culture should be:
-
Prior to antibiotic therapy,
-
A deep‐cough, expectorated specimen,
-
A purulent specimen (>25 polymorphonucleacytes and less than 10 squamous cells per high‐powered field), and
-
Rapidly transported to the laboratory.
Subsequent gram stain and culture results should be interpreted in the specific clinical context and antibiotic choices targeted appropriately.
Alternative Diagnostic Tests
In recent years, there has been growth in additional diagnostic tests targeting specific organisms. The pneumococcal urinary antigen assay is a relatively sensitive (50%80%) and highly specific (90%) test for the detection of pneumococcal pneumonia, when compared with conventional diagnostic methods.27 The test is simple, convenient, rapid ( 15 min), and, with its high specificity, may allow for more focused antimicrobial therapy early in management. Current limitations include the possibility of false‐positive tests in patients colonized with S. pneumoniae or infected with other streptococcal species, as well as the inability to determine antibiotic sensitivity from positive tests. Updated IDSA and BTS guidelines state pneumococcal urinary antigen testing is an acceptable adjunct to other diagnostic tests, but blood and sputum analyses should still be performed.26, 27 For patients with suspected Legionella pneumonia (primarily critically ill and immunocompromised patients or in association with regional outbreaks), the urinary Legionella antigen assay is the test of choice, which detects 80%95% of community‐acquired cases of Legionnaires' disease with a specificity of 90%.27
During the winter months (typically from October to March), rapid antigen testing for influenza is generally recommended for patients with signs or symptoms consistent with influenza.27 The sensitivity of these tests is approximately 50%70%, so negative test results do not exclude the diagnosis, but results can be important epidemiologically and therapeutically (differentiating influenza A and B strains).27 Diagnostic tests targeting other common CAP pathogens, such as serologic tests for Mycoplasma pneumoniae or Chlamydia spp, should not be routinely performed. Testing for less common causative pathogens such as Mycobacterium tuberculosis should only be employed in the appropriate clinical setting.
ADMISSION DECISION
Once the diagnosis of CAP has been made, the initial site where treatment will occur, whether the hospital or the home, must be determined. The decision to hospitalize should be based on 3 factors: 1) evaluation of the safety of home treatment, 2) calculation of the Pneumonia Severity Index (PSI), and 3) clinical judgment of the physician.27 The PSI, or PORT (Pneumonia Outcomes Research Team) score, is a validated prediction rule that quantifies mortality and allows for risk stratification of patients with community‐acquired pneumonia.2 The PSI combines clinical history, physical examination, and laboratory data at the time of admission to divide patients into 5 risk classes and to estimate 30‐day mortality (Figure 2), which ranges from 0.1% of patients in risk class I to 27.0% in risk class V.2
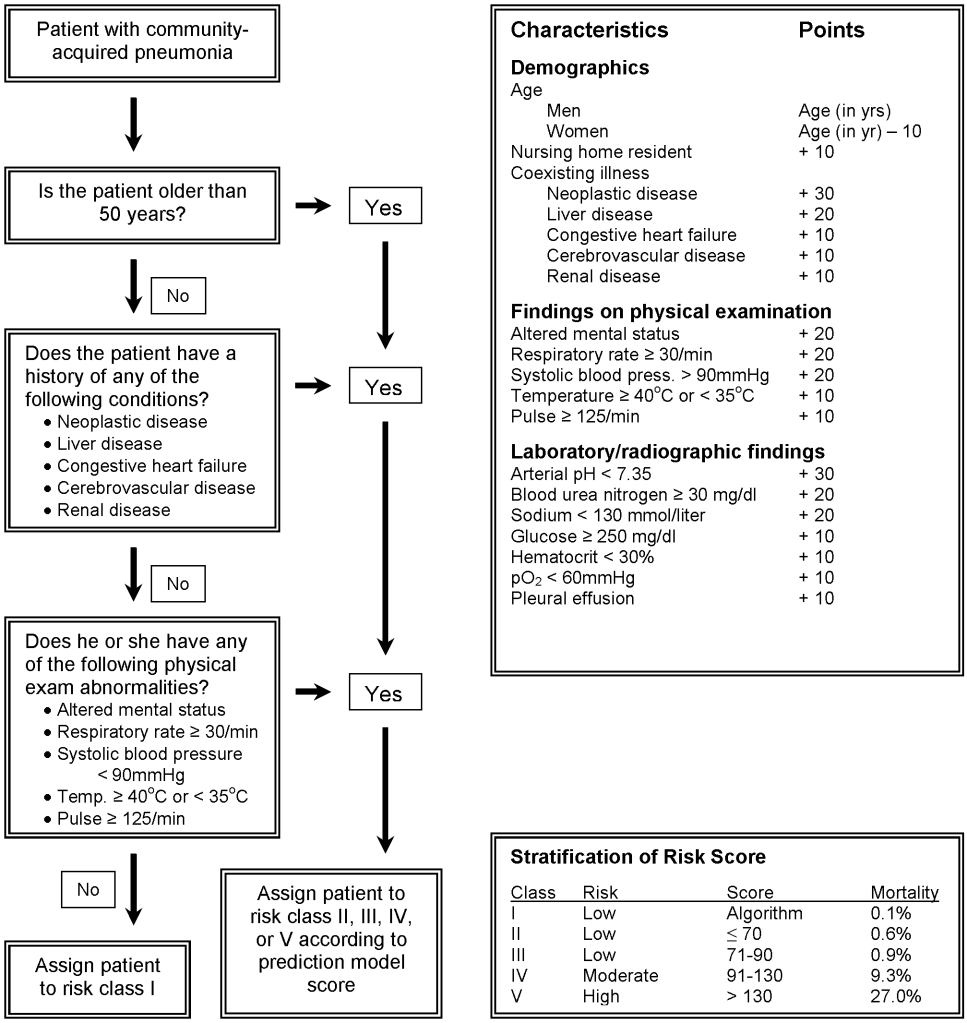
On the basis of the estimated prognosis and in the absence of concerns about home safety or comorbidities, patients in risk classes I, II, and III should be managed at home. Many prospective trials have shown that implementation of PSI significantly increases the number of low‐risk patients managed outside the hospital, with no differences in quality of life, complications, readmissions, or short‐term mortality.30, 31 Most recently, a trial randomizing patients in risk classes II and III to treatment in the hospital or at home found no significant differences in clinical outcomes but did find that patients were more satisfied with care at home.32 Because the number of patients with CAP being treated at home is increasing, the American College of Chest Physicians recently published a consensus statement on the management of community‐acquired pneumonia in the home.33 All national guidelines for the management of community‐acquired pneumonia recommend using the PSI to help determine the initial location of treatment, with the caveat that using the prediction rule should never supersede clinical judgment in the decision about whether to admit.1, 14, 15, 26, 27 A practical decision tree for the use of the PSI is shown in Figure 3.
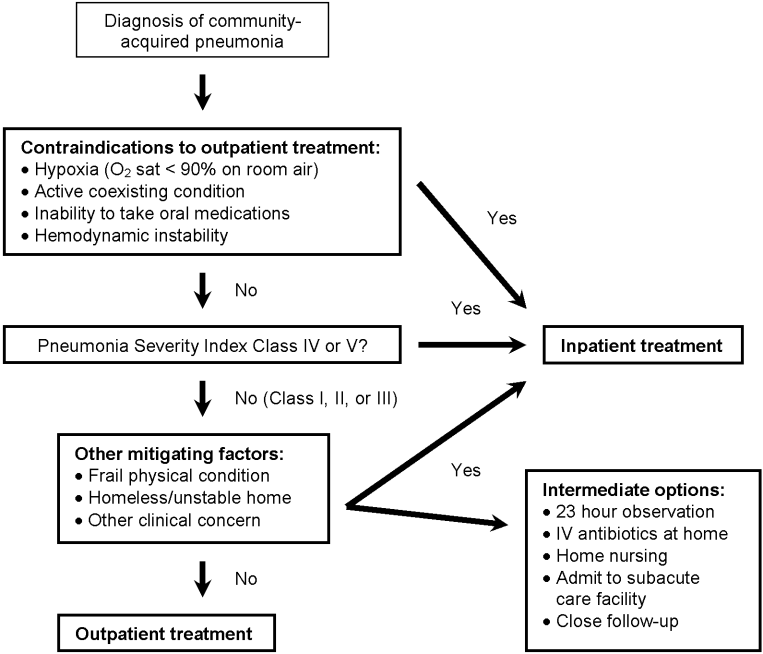
There are no reliable prediction rules for deciding on whether admission to the intensive care unit is necessary. Hemodynamic instability requiring resuscitation and monitoring or respiratory failure requiring ventilatory support are clear indications for ICU admission. Additional variables such as tachypnea (respiratory rate 30), altered mental status, multilobar disease, and azotemia are associated with severe CAP and should prompt consideration of ICU admission, especially when 2 or more variables coexist.14
TREATMENT
Initial Treatment
Once the admission decision is made and the initial diagnostic tests are completed (including blood and sputum cultures), patients with presumed community‐acquired pneumonia should receive necessary supportive care (O2, intravenous fluids, etc.) and prompt antimicrobial therapy. Antibiotics should be administered within 4 hours of arrival to patients with suspected CAP, as such prompt administration may be associated with shorter in‐hospital stays and decreased 30‐day mortality.34, 35 Regulatory organizations such as the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the Center for Medicare Services (CMS) have made delivery of antibiotics in less than 4 hours a hospital quality measure.
Despite diagnostic testing, the specific etiologic agent causing the pneumonia of a patient remains unknown in up to 75% of those admitted to the hospital.14 Most expert guidelines therefore recommend broad‐spectrum empiric therapy targeting both the typical and the atypical organisms that commonly cause CAP (Table 1).
Recommendations for empiric antibiotics are driven by 2 key factors: antibiotic resistance by S. pneumoniae and the results of studies of CAP treatment outcomes. Historically, patients with suspected community‐acquired pneumonia were treated with penicillin with generally good outcomes. Recently, the rate of S. pneumoniae isolates resistant to penicillin has risen dramatically in the United States, ranging between 20% and 30%, with high‐level resistance (MIC 4 mg/L) as high as 5.7%.36, 37 Concurrently, the rates of resistance of S. pneumoniae to many other antibiotics commonly used to treat CAP have also risen.37 Despite increasing resistance overall, most U.S. pneumococcal isolates have low resistance to third‐generation cephalosporins and fluoroquinolones with enhanced activity against S. pneumoniae.3638 In addition, despite increasing resistance by pneumococcal isolates to penicillin, several observational studies have shown that regardless of initial therapy, resistance to penicillin as well as third‐generation cephalosporins is not associated with higher mortality or worse outcomes when controlled for other risk factors for drug resistance.39, 40 An exception to that rule is pneumococcal isolates that are very highly resistant to PCN (MIC 4 mg/dL). At least one study has shown that patients with such isolates may be at higher risk for adverse outcomes and should probably not be treated with penicillins.1, 14, 15, 41 However, nationally, fewer than 6% of pneumococcal isolates have this level of resistance.37
The rationale for empiric broad‐spectrum coverage against both typical and atypical organisms has arisen from many retrospective and observational studies that have suggested that there is clinical benefit and improved outcomes with such regimens. One large retrospective study showed that in elderly patients with CAP, fluoroquinolone monotherapy was associated with lower 30‐day mortality when compared to monotherapy with a third‐generation cephalosporin.34 Adding an extended‐spectrum macrolide (eg, azithromycin) to an extended‐spectrum ‐lactam (eg, ceftriaxone) in the treatment of patients hospitalized with nonsevere CAP also appears to be associated with improved outcomes. Adding a macrolide has resulted in shorter lengths of stay (LOS), less treatment failure, and lower mortality.34, 4244 Similarly, according to unpublished observations, adding doxycycline to a ‐lactam as initial therapy was associated with a benefit of decreased mortality.45 The presumed etiology of the benefit has been the addition of specific coverage of atypical organisms, such as Mycoplasma pneumoniae and Chlamydia pneumoniae, which are common causes of CAP (Table 1). Others have proposed that the benefit of therapy with macrolides may be derived from the inherent anti‐inflammatory properties of macrolides.46 Because research has shown a benefit of dual versus monotherapy across a spectrum of antibiotics, others have proposed the benefit is simply a result of receiving double antibiotic coverage. In particular, 2 studies found a benefit of reduced mortality from combination therapy over monotherapy in bacteremic pneumococcal pneumonia.47, 48
Yet the accumulated evidence for adding coverage of atypical organisms has been only retrospective and observational. Because of this, the recommendation to routinely add antibiotics active against atypical organisms has been questioned by some. Two recent meta‐analyses and a systematic review examined all the available data on the need for atypical coverage in the treatment of patients with community‐acquired pneumonia.4951 Surprisingly, none showed a benefit in clinical efficacy or survival in patients treated with agents active against both atypical and typical organisms when compared to regimens with only typical coverage. In subset analyses, there was a benefit to providing empiric atypical coverage in patients subsequently shown to have Legionella spp. as a causative pathogen. However, this organism was uncommon in all 3 studies. Unfortunately, most studies included in the meta‐analyses compared fluoroquinolone or macrolide monotherapy with third‐generation cephalosporin monotherapy. There have been no high‐quality randomized, controlled trials of the treatment of hospitalized patients with CAP assessing combination therapy covering both typical and atypical organisms with monotherapy targeting typical organisms alone. High‐quality trials are warranted.
Despite the recent articles questioning the importance of atypical coverage, citing the substantial retrospective data and the general inability to identify causative organisms in most cases of CAP, adding a second agent with atypical coverage to a ‐lactam currently appears to be the most efficacious empiric treatment for CAP. Nearly all expert guidelines for the management of community‐acquired pneumonia recommend this empiric approach.1, 14, 27
Table 4 displays our recommendations for the treatment of community‐acquired pneumonia requiring hospitalization. Before implementation of these guidelines, hospitalists should consult with their infectious disease experts and consider local resistance patterns. In general, a typical adult patient with non‐severe CAP without additional risk factors should receive a parenteral extended‐spectrum ‐lactam plus either doxycycline or an advanced macrolide (see Table 4). Extended‐spectrum ‐lactams include cefotaxime, ceftriaxone, ampicillin‐sulbactam, and ertapenem. A respiratory fluoroquinolone as a single agent can be used for non‐ICU patients with CAP, but some agencies, including the Centers for Disease Control (CDC), discourage routine use of these agents in all patients secondary to concerns about cost and increasing gram‐negative rod fluoroquinolone resistance.52, 53
| Patient group | Empiric antibiotic therapy |
|---|---|
| |
| Inpatient, non‐ICU | ‐Lactama + either doxycycline or an advanced macrolideb |
| Severe ‐lactam allergyc | Respiratory fluoroquinoloned |
| Inpatient, ICU | |
| No risk for Pseudomonas | ‐Lactam + either an advanced macrolide or a respiratory fluoroquinolone |
| Severe ‐lactam allergy | Respiratory fluoroquinolone + clindamycin |
| Pseudomonas risk factorse | Antipseudomonal ‐lactamf + an antipseudomonal fluoroquinoloneg |
| Severe ‐lactam allergy | Aztreonam + a respiratory fluoroquinolone |
| MRSA risk factorsh | Add vancomycin to above regimens |
| From nursing home | Should be treated as nosocomial/health‐care‐associated pneumonia |
| Aspiration pneumonia | ‐Lactam or respiratory fluoroquinolone clindamycini |
Patients hospitalized with severe CAP who require ICU‐level care are at increased risk of Legionella spp. and drug‐resistant S. pneumoniae, which must be reflected in their initial antibiotic therapy.5 Patients with severe pneumonia should receive an intravenous extended‐spectrum ‐lactam plus either an intravenous macrolide or an intravenous respiratory fluoroquinolone.
All patients with severe CAP who are admitted to the intensive care unit should be routinely screened for risk factors for Pseudomonas aeruginosa. The known risk factors for pseudomonal infection are: bronchiectasis, immunosuppression including more than 10 mg/day of prednisone, malnutrition, and treatment with broad‐spectrum antibiotics in the last month.14 Those at risk for Pseudomonas aeruginosa or other resistant gram‐negative rod infection should be treated with an antipseudomonal ‐lactam plus an antipseudomonal fluoroquinolone. Many patients with severe CAP have risk factors for MRSA infection including recent prolonged hospitalization, recent use of broad‐spectrum antibiotics, and significant underlying lung disease, which should be considered in choosing initial antibiotic therapy.54 In addition, there have been reports of patients without underlying risk factors presenting with severe community‐acquired MRSA pneumonia. Many of these patients were younger and the MRSA pneumonia was associated with a necrotizing or cavitary disease requiring prolonged ICU stays.5558 In such cases or if an institution's rate of methicillin resistance in S. aureus community isolates is high (>15%20%), it may be appropriate to add initial empiric MRSA coverage for patients admitted to the ICU with CAP.55
Some patients will have unique risk factors and clinical presentations, which may require modification of these empiric recommendations. Several studies found 5%15% of cases of community‐acquired pneumonia to be aspiration pneumonia.57 Risk factors for aspiration events include, among others, dysphagia, history of stroke, altered level of consciousness, poor dentition, and tube feeding. Aspiration pneumonia traditionally was believed to be secondary to oral anaerobes, but recent research suggests gram‐positive cocci and gram‐negative rods are the predominant organisms.58 Antibiotic therapy in patients with clear aspiration pneumonia should be directed at these microbes with an extended‐spectrum ‐lactam (eg, ceftriaxone) or a respiratory fluoroquinolone (eg, levofloxacin or moxifloxacin). Anaerobic bacterial coverage can be added in patients with severe periodontal disease, alcoholism, concern for empyema, or evidence of aspiration with pulmonary abscess.58
Patients residing in long‐term care facilities are at high risk of contracting pneumonia. The microbiology of infections acquired in nursing facilities is similar to that in hospital‐acquired cases.59, 60 As a result, patients who develop pneumonia in institutional settings such as nursing homes should be treated with broad‐spectrum antibiotics, including coverage for MRSA.
Subsequent Treatment
Initial empiric antibiotic treatment should be modified based on the results of diagnostic testing. Although the specific etiologic agent is determined in only 25% of cases of CAP,35 when an organism is isolated, antibiotic coverage should be narrowed to cover that particular organism with an antibiotic with adequate lung penetration. Evidence suggests clinicians often do not adjust or narrow antibiotics based on sensitivity results, potentially breeding resistant organisms.61
Patients hospitalized with CAP usually improve quickly if they receive early, appropriate antibiotic therapy and supportive care. Excluding patients with severe CAP requiring intensive care unit admission, most patients resolve their tachycardia, tachypnea, and fever by day 2 or 3.62 Recent practice experience, evidence, and published guidelines14, 27 all indicate that patients can safely be transitioned to oral antibiotic therapy earlier in their hospital course. Table 5 outlines criteria that can be used to identify patients who have had an adequate response to parenteral therapy and can be considered for a switch to oral antibiotics. If these criteria are met, patients have less than a 1% chance of clinical deterioration necessitating admission to an ICU or transitional care unit.62 When an etiologic organism is not identified, oral therapy should reflect a spectrum of coverage to that of the initial intravenous therapy. In some cases, this may require use of more than one oral agent. We have had success, however, transitioning non‐ICU patients initially treated with intravenous ceftriaxone plus oral doxycycline, typically for 4872 hours, to oral doxycycline monotherapy at discharge.45
| Stable vital signs and clinical criteria for 24 hours |
| Temperature 37.8C (100F) |
| Heart rate 100 beats per minute |
| Respiratory rate 24 breaths per minute |
| Systolic blood pressure 90 mm Hg |
| Oxygen saturation (on room air) 90% |
| Ability to take oral medications |
There have been a limited number of high‐quality randomized trials examining the optimal duration of treatment for community‐acquired pneumonia. Most practice guidelines recommend 710 days for patients with CAP requiring hospitalization, with 14 days for documented Mycoplasma pneumoniae or Chlamydia pneumoniae. One recent randomized trial of patients with mild to severe CAP showed a short course of high‐dose levofloxacin (750 mg daily 5 days) was at least as effective as normal dosing (500 mg daily 10 days).63 Clinical experience with high‐dose levofloxacin is limited, but this regimen can be considered because it may reduce costs and exposure to antibiotics. When diagnosed, Legionella is usually treated for 1021 days, but 14 days is adequate with macrolides because of their long half‐life.27 Patients with more virulent pathogens like Staphylococcus aureus or Pseudomonas aeruginosa or other suppurative complications should be treated for at least 14 days.1, 14, 15, 27 In determining length of therapy, clinicians should use these durations of treatment as guides, and to individualize therapy, they should always consider patient age and frailty, comorbid conditions, severity of illness, and hospital course.
Failure to Respond
Although most patients hospitalized for CAP will improve rapidly and reach clinical stability in 23 days, some patients fail to respond. Some studies have estimated that failure to improve or clinical deterioration occurs in 5%10% of patients in the first 23 days.64 The common reasons for clinical decline or nonresponse to treatment, highlighted in Table 6, are:
-
Incorrect diagnosis: Illnesses such as congestive heart failure, pulmonary embolism, neoplasms, and hypersensitivity pneumonitis can mimick CAP.
-
Inadequate antibiotic selection: The etiologic agent may be resistant to empiric antibiotic selections. Examples would include methicillin‐resistant Staphylococcus aureus (MRSA) or multiresistant gram‐negative bacilli.
-
Unusual pathogen: CAP syndromes can be caused by myriad unusual organisms including Pneumocystis jirovecii, mycobacterium tuberculosis, endemic fungal infections (eg, coccidioidomycosis), and nocardiosis.
-
Complications of pneumonia: Specific complications of CAP include empyema, pulmonary abscess, extrapulmonary spread including meningitis or endocarditis, or other organ dysfunctions such as renal failure or myocardial infarction.
-
Inadequate host response: Despite appropriate antibiotic and supportive therapy, patients with CAP often fail to respond.
| Incorrect diagnosis of CAP. |
| Inadequate or inappropriate antibiotic selection for CAP. |
| Unusual pathogen causing CAP. |
| Pulmonary or extrapulmonary complication of CAP. |
| Inadequate or poor host response. |
Progressive pneumonia despite appropriate therapy and empyema were the most common causes of failure to respond in the first 72 hours in a recent study.64 Risk factors for early failure were older age (>65 years), Pneumonia Severity Index > 90, Legionella pneumonia, gram‐negative pneumonia, and initial antimicrobial therapy discordant with final culture and susceptibility results. The initial evaluation of the nonresponding patient should address these common causes and is likely to include additional imaging (CT), sampling of potential extrapulmonary infection (thoracentesis), and, in some cases, bronchoscopy.
DISCHARGE/FOLLOW‐UP PLANS
Patients hospitalized for community‐acquired pneumonia can be safely discharged when they have reached clinical stability, are able to tolerate oral medications, have no other active comorbid conditions, and have safe, close, appropriate outpatient follow‐up (see Table 7). Clinical pathways employing these discharge criteria have been found to be safe and effective in reducing the length of stay for CAP. Most important, patients should have met most if not all of the vital sign and clinical criteria noted in Table 5 in the criteria for switching to oral therapy. Patients with 2 or more abnormal vital signs (instabilities) within 24 hours prior to discharge are at high risk of readmission and mortality, but those with one or no abnormal vital signs generally have good outcomes.65 Absent other clinical factors or extenuating circumstances (persistent hypoxia, poor functional status, etc.), most patients with CAP should reach clinical stability by day 3 or 4, be considered for a switch to oral therapy, and, if stable, be discharged shortly thereafter.
| Patients should: |
|
Meet clinical criteria in Table V. |
| Be able to tolerate oral medications (no need to observe for 24 hours on oral therapy). |
| Have no evidence of active comorbid conditions (myocardial ischemia, pulmonary edema, etc.). |
| Have a normal mental status (or have returned to their baseline). |
| Have safe, appropriate outpatient follow‐up. |
When patients with CAP are discharged from the hospital, they should be counseled about the expected course of recovery. Most important, patients and families must be informed that many symptoms of CAP may persist well after hospitalization. In one study, up to 80% of patients reported persistent cough and fatigue 1 week after discharge, and up to 50% still had dyspnea and sputum production. In some, the cough can last for 46 weeks.8
All patients discharged after treatment of community‐acquired pneumonia should have follow‐up with their outpatient provider. The physician responsible for their inpatient care should communicate directly with the provider and outline the hospital course, the discharge medications, and the duration of antibiotic therapy. There is no specific time frame within which patients must be seen, but follow‐up should be dictated by patient age, comorbidities, clinical stability at discharge, and degree of illness. The American Thoracic Society guidelines do recommend patients with a substantial smoking history who are hospitalized with CAP have a follow‐up chest radiograph 46 weeks after discharge to establish a radiographic baseline and exclude the possibility of underlying malignancy.14 However, several studies have suggested that radiographic resolution may take 3 or more months in some patients, especially the elderly and those with multilobar disease.66
PREVENTION
Prevention of community‐acquired pneumonia and pneumonic syndromes has traditionally relied on vaccination with the polysaccharide pneumococcal pneumonia vaccine and the seasonal influenza vaccine. The vaccine for S. pneumoniae used in adults is composed of the 23 serotypes that cause 85%90% of the invasive pneumococcal infections in the United States. Although in randomized trials the vaccine has not consistently prevented community‐acquired pneumonia or death in elderly patients or those with comorbidities, it likely prevents invasive pneumococcal infection.67 National guidelines and the CDC recommend the pneumococcal vaccine be given to all patients older than 65 years and those with chronic medical conditions.1, 14, 15
The seasonal influenza vaccine has clearly been shown to decrease influenza‐related illness in elderly and high‐risk patient populations. As well, in a meta‐analysis and a large observational study of patients older than 65 years, vaccination against influenza prevented pneumonia, hospitalization, and death.68, 69 Vaccination of health care workers may also confer a benefit to elderly patients of reduced mortality. The CDC recommends the influenza vaccine for all patients more than 50 years old, those with comorbidities, those at high risk for influenza, and health care workers in both inpatient and outpatient settings.
Pneumococcal and influenza vaccination have traditionally been relegated to the outpatient setting. National guidelines and the CDC recommend vaccination of all eligible hospitalized patients. Vaccination is safe and effective with almost any medical illness, and both vaccines can be given simultaneously at discharge.69 Both JCAHO and CMS have defined administration of the pneumococcal and influenza vaccines to patients hospitalized with CAP as a quality measure. Using standing orders is the most effective means of ensuring vaccination.
Some evidence suggests that tobacco smokers are at increased risk of invasive pneumococcal disease or pneumonia.70 Patients hospitalized (for all illnesses, but for CAP in particular) should be counseled about smoking cessation and offered pharmacotherapy and outpatient follow‐up. And, finally, recent observational data suggests that use of acid suppressive therapy, including proton pump inhibitors and H‐2 receptor antagonists, may be associated with an increased risk of developing CAP.71 Patients using these agents who are admitted with CAP should have their indications for treatment reviewed, especially when the pneumonia has been recurrent and there is no clear indication for continued use of acid suppressive therapy, in which case they should be discontinued in the hospital.
CONCLUSIONS
Community‐acquired pneumonia remains a common cause for hospitalization of adult patients, with significant associated morbidity and mortality. Although there are multiple expert guidelines for the management of community‐acquired pneumonia, further research is urgently needed. Clinicians need improved diagnostic tests that enable an earlier and more accurate diagnosis of CAP. In addition, the etiologic agent causing CAP is rarely discovered; improved microbiologic studies might enable antibiotic therapy to be targeted to the organisms responsible. High‐quality randomized, controlled trials examining empiric antibiotic therapy in CAP are needed, especially related to the addition of agents covering atypical organisms. Last, the general management of patients hospitalized with CAP is marked by significant heterogeneity, and research and initiatives focusing on improving the quality and process of care of patients with CAP are needed.
- ,,,,,.Guidelines from the Infectious Disease Society of America. Practice guidelines for the management of community‐acquired pneumonia.Clin Infect Dis.2000;31:347–382.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,, et al.Pneumonia: still the old man's friend?Arch Intern Med.2003;163:317–323.
- ,,, et al.New and emerging etiologies for community‐acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases.Medicine.1990;69:307–316.
- .Community‐acquired pneumonia.Lancet.2003;362:1991–2001.
- ,,, et al.Processes and outcomes of care for patients with community‐acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team (PORT) cohort study.Arch Intern Med.1999;159:970–980.
- ,.Management of community‐acquired pneumonia.N Engl J Med.2002;347:2039–2045.
- ,,, et al.Measuring symptomatic and functional recovery in patients with community‐acquired pneumonia.J Gen Intern Med.1997;12:423–430.
- ,,, et al.Influence of age on symptoms at presentation with patients with community‐acquired pneumonia.Arch Intern Med.1997;157:1453–1459.
- ,,.Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination.JAMA.1997;278:1440–1445.
- ,,, et al.Community‐acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes.Medicine.2003;82:159–169.
- ,.Testing strategies in the initial management of patients with community‐acquired pneumonia.Ann Intern Med.2003;138:109–118.
- ,.Uncomplicated acute bronchitis.Ann Intern Med.2000;133:981–991.
- ,,, et al.American Thoracic Society: Guidelines for the management of community‐acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,, et al.British Thoracic Society guidelines for the management of community acquired pneumonia in adults.Thorax.2001;56:Suppl. 4,IV1–IV64.
- ,,,,.High‐resolution computed tomography for the diagnosis of community‐acquired pneumonia.Clin Infect Dis.1998;27:358–363.
- ,,, et al.Performance of a bedside C‐reactive protein test in the diagnosis of community‐acquired pneumonia in adults with acute cough.Am J Med.2004;116:529–535.
- ,,, et al.Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial.Lancet.2004;363:600–607.
- ,,, et al.Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia.N Engl J Med.2004:350:451–458.
- ,,, et al.Quality of care, process, and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- ,,,.Limited usefulness of initial blood cultures in community acquired pneumonia.Emerg Med J.2004;21:446–448.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,, et al.The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study.Chest.2003;123:1142–1150.
- ,,, et al.Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks.Chest.1995;108:932–936.
- ,,,.Predicting bacteremia in patients with community‐acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- British Thoracic Society. BTS Guidelines for the management of community acquired pneumonia in adults—2004 update. Available at: www.brit‐thoracic.org/guidelines.
- ,,,,,.Guidelines from the Infectious Disease Society of America. Update of guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433.
- ,,, et al.Assessment of the usefulness of sputum culture for diagnosis of community‐acquired pneumonia using the PORT predictive scoring system.Arch Intern Med.2004;164:1807–1811.
- ,,.Diagnostic value of microscopic examination of gram‐stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia.Clin Infect Dis.2004;39(2):165–169.
- ,,, et al.Safely increasing the proportion of patients with community‐acquired pneumonia treated as outpatients: an interventional trial.Arch Intern Med.1998;158:1350–1356.
- ,,, et al.A critical pathway for treatment of community‐acquired pneumonia.JAMA.2000;283:2654–2655.
- ,,, et al.Outpatient care compared with hospitalization for community‐acquired pneumonia. A randomized control trial in low‐risk patients.Ann Intern Med.2005;142:165–172.
- ,,.Management of community‐acquired pneumonia in the home.Chest.2005;127:1752–1763.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159:2562–2572.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,, et al.Increasing prevalence of multidrug‐resistant Streptococcus pneumoniae in the United States.N Engl J Med.2000;343:1917–1924.
- ,,, et al.Susceptibility patterns of Streptococcus pneumoniae isolates in North America (2002–2003): contemporary in vitro activities of amoxicillin/clavulanate and 15 other antimicrobial agents.Int J Antimicrob Agents.2005;25(4):282–289.
- ,,, et al.Antimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes?Clin Infect Dis.2005;41(2):139–148.
- ,,, et al.Pneumonia acquired in the community through drug‐resistant Streptococcus pneumoniae.Am J Respir Crit Care.1999;159:1835–1842.
- ,,, et al.Drug‐resistant pneumococcal pneumonia: clinical relevance and related factors.Clin Infect Dis.2004;38:787–798.
- ,,, et al.Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997.Am J Public Health.2000;90(2):223–9.
- ,,, et al.Lower mortality among patients with community‐acquired pneumonia treated with a macrolide plus a beta‐lactam agent versus a beta‐lactam alone.Eur J Clin Microbiol Infect Dis2005;24:190–195.
- ,,,.Impact of initial antibiotic choice on clinical outcomes in community‐acquired pneumonia: analysis of a hospital claims‐made database.Chest.2003;123:1503–1511.
- ,,,.Antimicrobial selection for hospitalized patients with presumed community‐acquired pneumonia: a survey of nonteaching US community hospitals.Ann Pharmacother2000;34:446–452.
- ,,,,.J Hosp Med.2006;1:7–12.
- .Anti‐inflammatory effects of macrolides—an underappreciated benefit in the treatment of community‐acquired respiratory tract infections and chronic inflammatory pulmonary conditions?J Antimicrob Chemother.2005;55:10–21.
- ,,, et al.Addition of a macrolide to a beta‐lactam based empirical antibiotic regimen is associated with lover in‐hospital mortality for patients with bacteremic pneumococcal pneumonia.Clin Infect Dis.2003;36:389–395.
- ,,, et al.Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia.Am J Respir Crit Care Med.2004;170:440–444.
- ,,,.Empiric antibiotic coverage of atypical pathogens for community‐acquired pneumonia in hospitalized adults.Cochrane Database Syst Rev.2005;2:CD004418.pub2.
- ,,.Effectiveness of β lactam antibiotics compared with antibiotics active against atypical pathogens in non‐severe community‐acquired pneumonia: meta‐analysis.Br Med J.2005;330:456.
- ,,,.Empirical atypical coverage for inpatients with community‐acquired pneumonia.Arch Intern Med.2005;165:1992–2000.
- ,,, et al.Antibiotic resistance among gram‐negative bacilli in US intensive care units: implications for fluoroquinolone use.JAMA.2003;289:885–888.
- ,,, et al.First‐generation fluoroquinolone use and subsequent emergence of multiple drug‐resistant bacteria in the intensive care unit.Crit Care Med.2005;33(2):283–289.
- ,.Etiology of community‐acquired pneumonia.Clin Chest Med.2005;26:47–55.
- .Community‐associated methicillin‐resistant Staphylococcus aureus: not only a cause of skin infections, also a new cause of pneumonia.Curr Opin Infect Dis.2005;18:123–124.
- ,,, et al.Severe community‐onset pneumonia in healthy adults caused by methicillin‐resistant Staphylococcus aureus carrying the Panton‐Valentine leukocidin genes.Clin Infect Dis.2005;40(1):100–107.
- ,,,.Fatal community‐associated methicillin‐resistant Staphylococcus aureus pneumonia in an immunocompetent young adult.Ann Emerg Med.2005;46:401–404.
- .Aspiration pneumonitis and aspiration pneumonia.N Engl J Med.2001;344:665–671.
- ,,, et al.Health care‐associated bloodstream infections in adults: a reason to change the accepted definition of community‐acquired infections.Ann Intern Med.2002;137:791–797.
- American Thoracic Society and theInfectious Diseases Society of America.Guidelines for the management of adults with hospital‐acquired, ventilator‐acquired, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,, et al.Blood culture and susceptibility results and allergy history do not influence fluoroquinolone use in the treatment of community‐acquired pneumonia.Pharmacotherapy.2005;25(1):59–66.
- ,,, et al.Time to clinical stability in patients hospitalized with community‐acquired pneumonia: implications for practice guidelines.JAMA.1998;279:1452–1457.
- ,,, et al.High‐dose, short‐course levofloxacin for community‐acquired pneumonia: a new treatment paradigm.Clin Infect Dis.2003;37:752–760.
- ,,, et al.Causes and factors associated with early failure in hospitalized patients with community‐acquired pneumonia.Arch Intern Med.2004;164:502–508.
- ,,, et al.Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia.Arch Intern Med.2002;162:1278–1284.
- ,,,.Radiographic resolution of community‐acquired bacterial pneumonia in the elderly.J Am Geriatr Soc.2004;52(2):224–229.
- ,,,.Vaccines for preventing pneumococcal infection in adults.Cochrane Database Syst Rev.2003;4:CD000422.
- ,,,,.The efficacy of influenza vaccine in elderly persons: a meta‐analysis and review of the literature.Ann Intern Med.1995;123:518–527.
- ,,, et al.Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly.N Engl J Med.2003;348:1322–1332.
- ,,,.Proportion of community‐acquired pneumonia cases attributable to tobacco smoking.Chest.1999;116:375–379.
- ,,, et al.Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs.JAMA.2004;292:1955–1960.
Pneumonia may well be called the friend of the aged. Taken off by it in an acute, short, not often painful illness, the old man escapes those cold gradations of decay so distressing of himself and to his friends.
William Osler, MD, 1898
Community‐acquired pneumonia (CAP) is commonly defined as an infection of the pulmonary parenchyma that is associated with at least some symptoms and signs of acute infection, accompanied by the presence of an acute infiltrate on chest radiograph, in a patient not hospitalized or residing in a long‐term‐care facility for 14 days prior to the onset of symptoms.1 CAP continues to be a common and serious illness, causing substantial morbidity and mortality in the adult population. There are an estimated 56 million cases a year in the United States, with greater than 1 million hospitalizations. Community‐acquired pneumonia is one of the most common admitting diagnoses among adults, and with a 30‐day mortality between 10% and 14% for patients admitted to the hospital, it is the leading cause of infectious death in the United States.2 In elderly patients, hospitalization for CAP portends a poor long‐term prognosis. In a Medicare database, the 1‐year mortality for patients with CAP was nearly 40%, compared to 29% in patients with other diagnoses.3 Community‐acquired pneumonia is a model illness in hospital medicineit is a common disease that allows for evidence‐based and cost‐effective management. In addition, many national organizations have proposed multiple quality indicators for community‐acquired pneumonia, thus providing an opportunity for institutional quality improvement. This review article outlines the assessment and management of patients admitted to the hospital with community‐acquired pneumonia.
Etiology
Although many pathogens can cause community‐acquired pneumonia, the clinical syndromes and microbiology of CAP have traditionally been characterized as either typical or atypical. The typical organisms include Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, and the atypical organisms include Chlamydia spp., Mycoplasma pneumoniae, Legionella spp., and viruses. This historical distinction has recently come into question. It is now clear that the presenting symptoms, signs, and basic laboratory findings (including the chest radiograph) cannot be reliably used to predict the etiologic pathogen or to distinguish typical from atypical organisms.4 Rather, the specific causative agent of CAP depends more on the degree of patient illness. Table 1 shows what prospective studies with comprehensive diagnostic strategies determined to be the most common pathogens in patients hospitalized for CAP in ICU and non‐ICU settings.5 Streptococcus pneumoniae remains the most common cause of CAP in hospitalized patients and is the most common cause of fatal pneumonia, whereas Legionella spp. is a common cause of severe CAP, more often found in patients requiring admission to the intensive care unit. Gram‐negative bacilli can cause CAP in elderly patients and those recently treated with broad‐spectrum antibiotics or with underlying lung disease. Notably, though, despite improved diagnostic testing, only one quarter of all admitted patients with CAP have the etiologic agent defined, and therefore empiric therapy should be directed broadly at the most likely organisms.6
| Non‐ICU inpatients | ICU inpatients (severe) |
|---|---|
| S. pneumoniae | S. pneumoniae |
| M. pneumoniae | Legionella spp |
| C. pneumoniae | H. influenzae |
| H. influenzae | Gram‐negative bacilli |
| Legionella spp | S. aureus |
| Aspiration | |
| Respiratory viruses |
Clinical Presentation
Patients admitted to the hospital with CAP typically present with a brief history of respiratory complaints, including cough (greater than 90%), dyspnea (66%), sputum production (66%), and pleuritic chest pain (50%); see Table 2.7, 8 In 10%30% of patients, nonrespiratory complaints predominate, including headache, myalgias, fatigue, and gastrointestinal symptoms.6 Elderly patients, an increasing percentage of hospitalized patients, are less likely to present with typical CAP symptoms (such as cough) and more likely to have altered mental status as a presenting symptom.9
| Symptoms | Signs (exam) |
|---|---|
| Cough 90% | Fever 80% |
| Dyspnea 66% | Tachypnea 70% |
| Sputum 66% | Tachycardia 50% |
| Pleuritic chest pain 50% | Focal lung exam >90% |
On physical examination, patients with CAP usually have signs of fever (80%), tachypnea (70%), and tachycardia (50%); see Table 2. Most will have a focal lung exam (>90%) with findings ranging from crackles to bronchial breath sounds.10 No exam finding is specific for the diagnosis of pneumonia, but the absence of fever, tachycardia, and tachypnea significantly reduces the probability of CAP in patients with suspected pneumonia.10 Furthermore, similar to the clinical history, the physical examination of elderly patients with community‐acquired pneumonia is not specific or sensitive for the diagnosis of CAP. For example, up to 40% of elderly patients subsequently determined to have CAP may not have fever.11
Leukocytosis is common in patients with CAP; however, its absence does not rule out disease.12 A number of guidelines recommend laboratory evaluation of electrolytes, urea nitrogen, creatinine, liver enzymes, and bilirubin, although these are used primarily for prognostication and are not specifically useful in the diagnosis of CAP.
DIAGNOSIS
Differential Diagnosis
Given the nonspecific nature of the symptoms and signs associated with CAP, there is no single clinical feature or combination of clinical features that adequately rules in or out the diagnosis of CAP. Consequently, the differential diagnosis to be considered in patients with suspected CAP is broad. Noninfectious diseases can often present with similar clinical syndromes; these include congestive heart failure, exacerbation of chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism, and hypersensitivity pneumonitis. These diseases can often be distinguished with a thorough history and physical examination.
In addition, other upper‐ and lower‐airway infectious diseases can have similar nonspecific signs and symptoms. In particular, pneumonia must often be differentiated from acute bronchitis, which as a diagnosis accounts for up to 40% of patients evaluated for cough (versus 5% for pneumonia).10 Patients with acute bronchitis frequently do not present with high fevers or hypoxia and in general will not benefit from antibiotic therapy.13 Patients believed to have community‐acquired pneumonia might also be suffering from other pneumonia syndromes including aspiration pneumonia, postobstructive pneumonia, and pneumonia in immunocompromised patients (eg, those with HIV, on steroids, receiving chemotherapy). Determining the correct diagnosis can have implications for therapy and prognosis.
Diagnostic Studies
The diagnosis of community‐acquired pneumonia requires that a patient have both signs and symptoms consistent with pulmonary infection and evidence of a new radiographic infiltrate. Therefore, most guidelines recommend that all patients with a possible diagnosis of CAP be evaluated with chest radiography.1, 14, 15
The specific radiographic findings in community‐acquired pneumonia range from lobar consolidation to hazy focal infiltrate to diffuse bilateral interstitial opacities (see Figure 1). Although chest radiography has traditionally been considered the gold standard for the diagnosis of CAP, its exact performance characteristics are unknown, and it is clearly not 100% sensitive or 100% specific. The utility of the chest radiograph can be limited by patient body habitus, underlying lung disease, or dehydration. Computed tomography (CT) scanning, although not recommended for routine use, can identify pulmonary consolidation in up to 30% of patients with a normal or equivocal chest radiograph in whom pneumonia is suspected and can also identify complications of pneumonia including an empyema or pulmonary abscess.16

Limitations in the performance of the chest radiograph have resulted in an interest in the diagnostic performance of serologic markers of infection such as C‐reactive protein (CRP), procalcitonin, and soluble triggering receptor expressed on myeloid cells (s‐TREM).1719 Preliminary evidence suggests these inflammatory markers may ultimately prove useful in differentiating infectious from noninfectious pulmonary processes, but regular use of these new tests cannot currently be recommended.
Most expert guidelines state that 2 sets of blood cultures should be taken and analyzed prior to antibiotic administration in all patients admitted to the hospital with suspected community‐acquired pneumonia.1, 14, 15 Isolation of bacteria from blood cultures in CAP is a very specific way to identify a causative organism in order to subsequently narrow therapy and also identifies a high‐risk group of patients because bacteremia is associated with increased mortality. Obtaining blood cultures within 24 hours of admission has been associated with 10% lower odds of 30‐day mortality in patients with CAP,20 and as a result, drawing blood cultures prior to antibiotic administration is a national quality indicator for CAP.
There are, however, a number of problems with the routine acquisition of blood cultures in all patients admitted with CAP. Practically, the cultures can be difficult to obtain, can potentially delay the initiation of antibiotics, and are often contaminated, which has been shown to increase both cost and length of stay.21, 22 The yield is generally low: the true‐positive bacteremia rate for admitted patients with CAP ranges from 6% to 9%, and the culture results rarely change management or outcomes.23, 24 Given these limitations, many have argued that blood cultures should be obtained with a more targeted approach. A recent study used a Medicare database to create a decision‐support tool to help maximize the value of blood cultures in CAP.25 The predictors of a positive blood culture are shown in Table 3. Not obtaining cultures on patients who had received prior antibiotics or had no risk factors resulted in about 40% fewer overall cultures while identifying approximately 90% of bacteremias. In their guidelines, the British Thoracic Society (BTS) advocates a similar strategy, recommending blood cultures be omitted in nonsevere pneumonia and in patients without comorbidities.15, 26 Although recommendations vary for non‐severe CAP in hospitalized patients, all professional society guidelines agree that blood cultures should be obtained in critically ill patients, and if cultures are obtained, they should be drawn prior to antibiotics.1, 14, 15, 26
| Comorbidities |
| Liver disease |
| Vital signs |
| Systolic blood pressure < 90 mm Hg |
| Temperature < 35C or 40C |
| Pulse 125 beats/min |
| Laboratory and radiographic data |
| Blood urea nitrogen (BUN) 30 mg/dL |
| Sodium < 130 mmol/L |
| White blood cells < 5000/mm3 or > 20,000/mm3 |
| Prior use of antibiotics (negative predictor) |
Substantial controversy surrounds the utility of routine sputum gram stains and cultures for patients admitted to the hospital with CAP. The Infectious Disease Society of America (IDSA) and the British Thoracic Society (BTS) both recommend that all patients admitted to the hospital with community‐acquired pneumonia should have a gram stain and culture of expectorated sputum.1, 15, 26 Both organizations argue sputum collection is a simple and inexpensive procedure that can potentially identify pathogenic organisms and can affect both initial and long‐term antibiotic therapy. Most notably, they highlight gram stain specificity of greater than 80% for pneumococcal pneumonia. Conversely, the American Thoracic Society (ATS) argues that sputum gram stains and cultures generally have low sensitivity, specificity, and positive predictive value.14 Furthermore, they argue the utility of sputum testing is also limited practically; in one study 30% of patients could not produce an adequate sputum specimen and up to 30% had received prior antibiotic therapy, substantially reducing the yield.27 In another study, good‐quality sputum with a predominant morphotype could be obtained in only 14% of patients admitted with CAP.28 However, targeting sputum analysis to patients who have not received prior antibiotics and are able to produce an adequate sample improved the yield significantly.29 In addition, with increasing rates of antibiotic resistance among common community isolates (ie, S. Pneumoniae) and the increasing prevalence of infecting organisms not targeted by routine empiric therapy (methicillin‐resistant Staphylococcus Aureus [MRSA]), isolation of potential causative pathogens is increasingly important. We believe that severely ill patients with CAP (such as patients admitted to the ICU), as well as patients with identifiable risk factors for uncommon or drug‐resistant pathogens (eg, Pseudomonas aeruginosa, enteric gram‐negative rods, MRSA, etc.) should have sputum sent for gram stain and culture. Ideally, sputum obtained for gram stain and culture should be:
-
Prior to antibiotic therapy,
-
A deep‐cough, expectorated specimen,
-
A purulent specimen (>25 polymorphonucleacytes and less than 10 squamous cells per high‐powered field), and
-
Rapidly transported to the laboratory.
Subsequent gram stain and culture results should be interpreted in the specific clinical context and antibiotic choices targeted appropriately.
Alternative Diagnostic Tests
In recent years, there has been growth in additional diagnostic tests targeting specific organisms. The pneumococcal urinary antigen assay is a relatively sensitive (50%80%) and highly specific (90%) test for the detection of pneumococcal pneumonia, when compared with conventional diagnostic methods.27 The test is simple, convenient, rapid ( 15 min), and, with its high specificity, may allow for more focused antimicrobial therapy early in management. Current limitations include the possibility of false‐positive tests in patients colonized with S. pneumoniae or infected with other streptococcal species, as well as the inability to determine antibiotic sensitivity from positive tests. Updated IDSA and BTS guidelines state pneumococcal urinary antigen testing is an acceptable adjunct to other diagnostic tests, but blood and sputum analyses should still be performed.26, 27 For patients with suspected Legionella pneumonia (primarily critically ill and immunocompromised patients or in association with regional outbreaks), the urinary Legionella antigen assay is the test of choice, which detects 80%95% of community‐acquired cases of Legionnaires' disease with a specificity of 90%.27
During the winter months (typically from October to March), rapid antigen testing for influenza is generally recommended for patients with signs or symptoms consistent with influenza.27 The sensitivity of these tests is approximately 50%70%, so negative test results do not exclude the diagnosis, but results can be important epidemiologically and therapeutically (differentiating influenza A and B strains).27 Diagnostic tests targeting other common CAP pathogens, such as serologic tests for Mycoplasma pneumoniae or Chlamydia spp, should not be routinely performed. Testing for less common causative pathogens such as Mycobacterium tuberculosis should only be employed in the appropriate clinical setting.
ADMISSION DECISION
Once the diagnosis of CAP has been made, the initial site where treatment will occur, whether the hospital or the home, must be determined. The decision to hospitalize should be based on 3 factors: 1) evaluation of the safety of home treatment, 2) calculation of the Pneumonia Severity Index (PSI), and 3) clinical judgment of the physician.27 The PSI, or PORT (Pneumonia Outcomes Research Team) score, is a validated prediction rule that quantifies mortality and allows for risk stratification of patients with community‐acquired pneumonia.2 The PSI combines clinical history, physical examination, and laboratory data at the time of admission to divide patients into 5 risk classes and to estimate 30‐day mortality (Figure 2), which ranges from 0.1% of patients in risk class I to 27.0% in risk class V.2

On the basis of the estimated prognosis and in the absence of concerns about home safety or comorbidities, patients in risk classes I, II, and III should be managed at home. Many prospective trials have shown that implementation of PSI significantly increases the number of low‐risk patients managed outside the hospital, with no differences in quality of life, complications, readmissions, or short‐term mortality.30, 31 Most recently, a trial randomizing patients in risk classes II and III to treatment in the hospital or at home found no significant differences in clinical outcomes but did find that patients were more satisfied with care at home.32 Because the number of patients with CAP being treated at home is increasing, the American College of Chest Physicians recently published a consensus statement on the management of community‐acquired pneumonia in the home.33 All national guidelines for the management of community‐acquired pneumonia recommend using the PSI to help determine the initial location of treatment, with the caveat that using the prediction rule should never supersede clinical judgment in the decision about whether to admit.1, 14, 15, 26, 27 A practical decision tree for the use of the PSI is shown in Figure 3.

There are no reliable prediction rules for deciding on whether admission to the intensive care unit is necessary. Hemodynamic instability requiring resuscitation and monitoring or respiratory failure requiring ventilatory support are clear indications for ICU admission. Additional variables such as tachypnea (respiratory rate 30), altered mental status, multilobar disease, and azotemia are associated with severe CAP and should prompt consideration of ICU admission, especially when 2 or more variables coexist.14
TREATMENT
Initial Treatment
Once the admission decision is made and the initial diagnostic tests are completed (including blood and sputum cultures), patients with presumed community‐acquired pneumonia should receive necessary supportive care (O2, intravenous fluids, etc.) and prompt antimicrobial therapy. Antibiotics should be administered within 4 hours of arrival to patients with suspected CAP, as such prompt administration may be associated with shorter in‐hospital stays and decreased 30‐day mortality.34, 35 Regulatory organizations such as the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the Center for Medicare Services (CMS) have made delivery of antibiotics in less than 4 hours a hospital quality measure.
Despite diagnostic testing, the specific etiologic agent causing the pneumonia of a patient remains unknown in up to 75% of those admitted to the hospital.14 Most expert guidelines therefore recommend broad‐spectrum empiric therapy targeting both the typical and the atypical organisms that commonly cause CAP (Table 1).
Recommendations for empiric antibiotics are driven by 2 key factors: antibiotic resistance by S. pneumoniae and the results of studies of CAP treatment outcomes. Historically, patients with suspected community‐acquired pneumonia were treated with penicillin with generally good outcomes. Recently, the rate of S. pneumoniae isolates resistant to penicillin has risen dramatically in the United States, ranging between 20% and 30%, with high‐level resistance (MIC 4 mg/L) as high as 5.7%.36, 37 Concurrently, the rates of resistance of S. pneumoniae to many other antibiotics commonly used to treat CAP have also risen.37 Despite increasing resistance overall, most U.S. pneumococcal isolates have low resistance to third‐generation cephalosporins and fluoroquinolones with enhanced activity against S. pneumoniae.3638 In addition, despite increasing resistance by pneumococcal isolates to penicillin, several observational studies have shown that regardless of initial therapy, resistance to penicillin as well as third‐generation cephalosporins is not associated with higher mortality or worse outcomes when controlled for other risk factors for drug resistance.39, 40 An exception to that rule is pneumococcal isolates that are very highly resistant to PCN (MIC 4 mg/dL). At least one study has shown that patients with such isolates may be at higher risk for adverse outcomes and should probably not be treated with penicillins.1, 14, 15, 41 However, nationally, fewer than 6% of pneumococcal isolates have this level of resistance.37
The rationale for empiric broad‐spectrum coverage against both typical and atypical organisms has arisen from many retrospective and observational studies that have suggested that there is clinical benefit and improved outcomes with such regimens. One large retrospective study showed that in elderly patients with CAP, fluoroquinolone monotherapy was associated with lower 30‐day mortality when compared to monotherapy with a third‐generation cephalosporin.34 Adding an extended‐spectrum macrolide (eg, azithromycin) to an extended‐spectrum ‐lactam (eg, ceftriaxone) in the treatment of patients hospitalized with nonsevere CAP also appears to be associated with improved outcomes. Adding a macrolide has resulted in shorter lengths of stay (LOS), less treatment failure, and lower mortality.34, 4244 Similarly, according to unpublished observations, adding doxycycline to a ‐lactam as initial therapy was associated with a benefit of decreased mortality.45 The presumed etiology of the benefit has been the addition of specific coverage of atypical organisms, such as Mycoplasma pneumoniae and Chlamydia pneumoniae, which are common causes of CAP (Table 1). Others have proposed that the benefit of therapy with macrolides may be derived from the inherent anti‐inflammatory properties of macrolides.46 Because research has shown a benefit of dual versus monotherapy across a spectrum of antibiotics, others have proposed the benefit is simply a result of receiving double antibiotic coverage. In particular, 2 studies found a benefit of reduced mortality from combination therapy over monotherapy in bacteremic pneumococcal pneumonia.47, 48
Yet the accumulated evidence for adding coverage of atypical organisms has been only retrospective and observational. Because of this, the recommendation to routinely add antibiotics active against atypical organisms has been questioned by some. Two recent meta‐analyses and a systematic review examined all the available data on the need for atypical coverage in the treatment of patients with community‐acquired pneumonia.4951 Surprisingly, none showed a benefit in clinical efficacy or survival in patients treated with agents active against both atypical and typical organisms when compared to regimens with only typical coverage. In subset analyses, there was a benefit to providing empiric atypical coverage in patients subsequently shown to have Legionella spp. as a causative pathogen. However, this organism was uncommon in all 3 studies. Unfortunately, most studies included in the meta‐analyses compared fluoroquinolone or macrolide monotherapy with third‐generation cephalosporin monotherapy. There have been no high‐quality randomized, controlled trials of the treatment of hospitalized patients with CAP assessing combination therapy covering both typical and atypical organisms with monotherapy targeting typical organisms alone. High‐quality trials are warranted.
Despite the recent articles questioning the importance of atypical coverage, citing the substantial retrospective data and the general inability to identify causative organisms in most cases of CAP, adding a second agent with atypical coverage to a ‐lactam currently appears to be the most efficacious empiric treatment for CAP. Nearly all expert guidelines for the management of community‐acquired pneumonia recommend this empiric approach.1, 14, 27
Table 4 displays our recommendations for the treatment of community‐acquired pneumonia requiring hospitalization. Before implementation of these guidelines, hospitalists should consult with their infectious disease experts and consider local resistance patterns. In general, a typical adult patient with non‐severe CAP without additional risk factors should receive a parenteral extended‐spectrum ‐lactam plus either doxycycline or an advanced macrolide (see Table 4). Extended‐spectrum ‐lactams include cefotaxime, ceftriaxone, ampicillin‐sulbactam, and ertapenem. A respiratory fluoroquinolone as a single agent can be used for non‐ICU patients with CAP, but some agencies, including the Centers for Disease Control (CDC), discourage routine use of these agents in all patients secondary to concerns about cost and increasing gram‐negative rod fluoroquinolone resistance.52, 53
| Patient group | Empiric antibiotic therapy |
|---|---|
| |
| Inpatient, non‐ICU | ‐Lactama + either doxycycline or an advanced macrolideb |
| Severe ‐lactam allergyc | Respiratory fluoroquinoloned |
| Inpatient, ICU | |
| No risk for Pseudomonas | ‐Lactam + either an advanced macrolide or a respiratory fluoroquinolone |
| Severe ‐lactam allergy | Respiratory fluoroquinolone + clindamycin |
| Pseudomonas risk factorse | Antipseudomonal ‐lactamf + an antipseudomonal fluoroquinoloneg |
| Severe ‐lactam allergy | Aztreonam + a respiratory fluoroquinolone |
| MRSA risk factorsh | Add vancomycin to above regimens |
| From nursing home | Should be treated as nosocomial/health‐care‐associated pneumonia |
| Aspiration pneumonia | ‐Lactam or respiratory fluoroquinolone clindamycini |
Patients hospitalized with severe CAP who require ICU‐level care are at increased risk of Legionella spp. and drug‐resistant S. pneumoniae, which must be reflected in their initial antibiotic therapy.5 Patients with severe pneumonia should receive an intravenous extended‐spectrum ‐lactam plus either an intravenous macrolide or an intravenous respiratory fluoroquinolone.
All patients with severe CAP who are admitted to the intensive care unit should be routinely screened for risk factors for Pseudomonas aeruginosa. The known risk factors for pseudomonal infection are: bronchiectasis, immunosuppression including more than 10 mg/day of prednisone, malnutrition, and treatment with broad‐spectrum antibiotics in the last month.14 Those at risk for Pseudomonas aeruginosa or other resistant gram‐negative rod infection should be treated with an antipseudomonal ‐lactam plus an antipseudomonal fluoroquinolone. Many patients with severe CAP have risk factors for MRSA infection including recent prolonged hospitalization, recent use of broad‐spectrum antibiotics, and significant underlying lung disease, which should be considered in choosing initial antibiotic therapy.54 In addition, there have been reports of patients without underlying risk factors presenting with severe community‐acquired MRSA pneumonia. Many of these patients were younger and the MRSA pneumonia was associated with a necrotizing or cavitary disease requiring prolonged ICU stays.5558 In such cases or if an institution's rate of methicillin resistance in S. aureus community isolates is high (>15%20%), it may be appropriate to add initial empiric MRSA coverage for patients admitted to the ICU with CAP.55
Some patients will have unique risk factors and clinical presentations, which may require modification of these empiric recommendations. Several studies found 5%15% of cases of community‐acquired pneumonia to be aspiration pneumonia.57 Risk factors for aspiration events include, among others, dysphagia, history of stroke, altered level of consciousness, poor dentition, and tube feeding. Aspiration pneumonia traditionally was believed to be secondary to oral anaerobes, but recent research suggests gram‐positive cocci and gram‐negative rods are the predominant organisms.58 Antibiotic therapy in patients with clear aspiration pneumonia should be directed at these microbes with an extended‐spectrum ‐lactam (eg, ceftriaxone) or a respiratory fluoroquinolone (eg, levofloxacin or moxifloxacin). Anaerobic bacterial coverage can be added in patients with severe periodontal disease, alcoholism, concern for empyema, or evidence of aspiration with pulmonary abscess.58
Patients residing in long‐term care facilities are at high risk of contracting pneumonia. The microbiology of infections acquired in nursing facilities is similar to that in hospital‐acquired cases.59, 60 As a result, patients who develop pneumonia in institutional settings such as nursing homes should be treated with broad‐spectrum antibiotics, including coverage for MRSA.
Subsequent Treatment
Initial empiric antibiotic treatment should be modified based on the results of diagnostic testing. Although the specific etiologic agent is determined in only 25% of cases of CAP,35 when an organism is isolated, antibiotic coverage should be narrowed to cover that particular organism with an antibiotic with adequate lung penetration. Evidence suggests clinicians often do not adjust or narrow antibiotics based on sensitivity results, potentially breeding resistant organisms.61
Patients hospitalized with CAP usually improve quickly if they receive early, appropriate antibiotic therapy and supportive care. Excluding patients with severe CAP requiring intensive care unit admission, most patients resolve their tachycardia, tachypnea, and fever by day 2 or 3.62 Recent practice experience, evidence, and published guidelines14, 27 all indicate that patients can safely be transitioned to oral antibiotic therapy earlier in their hospital course. Table 5 outlines criteria that can be used to identify patients who have had an adequate response to parenteral therapy and can be considered for a switch to oral antibiotics. If these criteria are met, patients have less than a 1% chance of clinical deterioration necessitating admission to an ICU or transitional care unit.62 When an etiologic organism is not identified, oral therapy should reflect a spectrum of coverage to that of the initial intravenous therapy. In some cases, this may require use of more than one oral agent. We have had success, however, transitioning non‐ICU patients initially treated with intravenous ceftriaxone plus oral doxycycline, typically for 4872 hours, to oral doxycycline monotherapy at discharge.45
| Stable vital signs and clinical criteria for 24 hours |
| Temperature 37.8C (100F) |
| Heart rate 100 beats per minute |
| Respiratory rate 24 breaths per minute |
| Systolic blood pressure 90 mm Hg |
| Oxygen saturation (on room air) 90% |
| Ability to take oral medications |
There have been a limited number of high‐quality randomized trials examining the optimal duration of treatment for community‐acquired pneumonia. Most practice guidelines recommend 710 days for patients with CAP requiring hospitalization, with 14 days for documented Mycoplasma pneumoniae or Chlamydia pneumoniae. One recent randomized trial of patients with mild to severe CAP showed a short course of high‐dose levofloxacin (750 mg daily 5 days) was at least as effective as normal dosing (500 mg daily 10 days).63 Clinical experience with high‐dose levofloxacin is limited, but this regimen can be considered because it may reduce costs and exposure to antibiotics. When diagnosed, Legionella is usually treated for 1021 days, but 14 days is adequate with macrolides because of their long half‐life.27 Patients with more virulent pathogens like Staphylococcus aureus or Pseudomonas aeruginosa or other suppurative complications should be treated for at least 14 days.1, 14, 15, 27 In determining length of therapy, clinicians should use these durations of treatment as guides, and to individualize therapy, they should always consider patient age and frailty, comorbid conditions, severity of illness, and hospital course.
Failure to Respond
Although most patients hospitalized for CAP will improve rapidly and reach clinical stability in 23 days, some patients fail to respond. Some studies have estimated that failure to improve or clinical deterioration occurs in 5%10% of patients in the first 23 days.64 The common reasons for clinical decline or nonresponse to treatment, highlighted in Table 6, are:
-
Incorrect diagnosis: Illnesses such as congestive heart failure, pulmonary embolism, neoplasms, and hypersensitivity pneumonitis can mimick CAP.
-
Inadequate antibiotic selection: The etiologic agent may be resistant to empiric antibiotic selections. Examples would include methicillin‐resistant Staphylococcus aureus (MRSA) or multiresistant gram‐negative bacilli.
-
Unusual pathogen: CAP syndromes can be caused by myriad unusual organisms including Pneumocystis jirovecii, mycobacterium tuberculosis, endemic fungal infections (eg, coccidioidomycosis), and nocardiosis.
-
Complications of pneumonia: Specific complications of CAP include empyema, pulmonary abscess, extrapulmonary spread including meningitis or endocarditis, or other organ dysfunctions such as renal failure or myocardial infarction.
-
Inadequate host response: Despite appropriate antibiotic and supportive therapy, patients with CAP often fail to respond.
| Incorrect diagnosis of CAP. |
| Inadequate or inappropriate antibiotic selection for CAP. |
| Unusual pathogen causing CAP. |
| Pulmonary or extrapulmonary complication of CAP. |
| Inadequate or poor host response. |
Progressive pneumonia despite appropriate therapy and empyema were the most common causes of failure to respond in the first 72 hours in a recent study.64 Risk factors for early failure were older age (>65 years), Pneumonia Severity Index > 90, Legionella pneumonia, gram‐negative pneumonia, and initial antimicrobial therapy discordant with final culture and susceptibility results. The initial evaluation of the nonresponding patient should address these common causes and is likely to include additional imaging (CT), sampling of potential extrapulmonary infection (thoracentesis), and, in some cases, bronchoscopy.
DISCHARGE/FOLLOW‐UP PLANS
Patients hospitalized for community‐acquired pneumonia can be safely discharged when they have reached clinical stability, are able to tolerate oral medications, have no other active comorbid conditions, and have safe, close, appropriate outpatient follow‐up (see Table 7). Clinical pathways employing these discharge criteria have been found to be safe and effective in reducing the length of stay for CAP. Most important, patients should have met most if not all of the vital sign and clinical criteria noted in Table 5 in the criteria for switching to oral therapy. Patients with 2 or more abnormal vital signs (instabilities) within 24 hours prior to discharge are at high risk of readmission and mortality, but those with one or no abnormal vital signs generally have good outcomes.65 Absent other clinical factors or extenuating circumstances (persistent hypoxia, poor functional status, etc.), most patients with CAP should reach clinical stability by day 3 or 4, be considered for a switch to oral therapy, and, if stable, be discharged shortly thereafter.
| Patients should: |
|
Meet clinical criteria in Table V. |
| Be able to tolerate oral medications (no need to observe for 24 hours on oral therapy). |
| Have no evidence of active comorbid conditions (myocardial ischemia, pulmonary edema, etc.). |
| Have a normal mental status (or have returned to their baseline). |
| Have safe, appropriate outpatient follow‐up. |
When patients with CAP are discharged from the hospital, they should be counseled about the expected course of recovery. Most important, patients and families must be informed that many symptoms of CAP may persist well after hospitalization. In one study, up to 80% of patients reported persistent cough and fatigue 1 week after discharge, and up to 50% still had dyspnea and sputum production. In some, the cough can last for 46 weeks.8
All patients discharged after treatment of community‐acquired pneumonia should have follow‐up with their outpatient provider. The physician responsible for their inpatient care should communicate directly with the provider and outline the hospital course, the discharge medications, and the duration of antibiotic therapy. There is no specific time frame within which patients must be seen, but follow‐up should be dictated by patient age, comorbidities, clinical stability at discharge, and degree of illness. The American Thoracic Society guidelines do recommend patients with a substantial smoking history who are hospitalized with CAP have a follow‐up chest radiograph 46 weeks after discharge to establish a radiographic baseline and exclude the possibility of underlying malignancy.14 However, several studies have suggested that radiographic resolution may take 3 or more months in some patients, especially the elderly and those with multilobar disease.66
PREVENTION
Prevention of community‐acquired pneumonia and pneumonic syndromes has traditionally relied on vaccination with the polysaccharide pneumococcal pneumonia vaccine and the seasonal influenza vaccine. The vaccine for S. pneumoniae used in adults is composed of the 23 serotypes that cause 85%90% of the invasive pneumococcal infections in the United States. Although in randomized trials the vaccine has not consistently prevented community‐acquired pneumonia or death in elderly patients or those with comorbidities, it likely prevents invasive pneumococcal infection.67 National guidelines and the CDC recommend the pneumococcal vaccine be given to all patients older than 65 years and those with chronic medical conditions.1, 14, 15
The seasonal influenza vaccine has clearly been shown to decrease influenza‐related illness in elderly and high‐risk patient populations. As well, in a meta‐analysis and a large observational study of patients older than 65 years, vaccination against influenza prevented pneumonia, hospitalization, and death.68, 69 Vaccination of health care workers may also confer a benefit to elderly patients of reduced mortality. The CDC recommends the influenza vaccine for all patients more than 50 years old, those with comorbidities, those at high risk for influenza, and health care workers in both inpatient and outpatient settings.
Pneumococcal and influenza vaccination have traditionally been relegated to the outpatient setting. National guidelines and the CDC recommend vaccination of all eligible hospitalized patients. Vaccination is safe and effective with almost any medical illness, and both vaccines can be given simultaneously at discharge.69 Both JCAHO and CMS have defined administration of the pneumococcal and influenza vaccines to patients hospitalized with CAP as a quality measure. Using standing orders is the most effective means of ensuring vaccination.
Some evidence suggests that tobacco smokers are at increased risk of invasive pneumococcal disease or pneumonia.70 Patients hospitalized (for all illnesses, but for CAP in particular) should be counseled about smoking cessation and offered pharmacotherapy and outpatient follow‐up. And, finally, recent observational data suggests that use of acid suppressive therapy, including proton pump inhibitors and H‐2 receptor antagonists, may be associated with an increased risk of developing CAP.71 Patients using these agents who are admitted with CAP should have their indications for treatment reviewed, especially when the pneumonia has been recurrent and there is no clear indication for continued use of acid suppressive therapy, in which case they should be discontinued in the hospital.
CONCLUSIONS
Community‐acquired pneumonia remains a common cause for hospitalization of adult patients, with significant associated morbidity and mortality. Although there are multiple expert guidelines for the management of community‐acquired pneumonia, further research is urgently needed. Clinicians need improved diagnostic tests that enable an earlier and more accurate diagnosis of CAP. In addition, the etiologic agent causing CAP is rarely discovered; improved microbiologic studies might enable antibiotic therapy to be targeted to the organisms responsible. High‐quality randomized, controlled trials examining empiric antibiotic therapy in CAP are needed, especially related to the addition of agents covering atypical organisms. Last, the general management of patients hospitalized with CAP is marked by significant heterogeneity, and research and initiatives focusing on improving the quality and process of care of patients with CAP are needed.
Pneumonia may well be called the friend of the aged. Taken off by it in an acute, short, not often painful illness, the old man escapes those cold gradations of decay so distressing of himself and to his friends.
William Osler, MD, 1898
Community‐acquired pneumonia (CAP) is commonly defined as an infection of the pulmonary parenchyma that is associated with at least some symptoms and signs of acute infection, accompanied by the presence of an acute infiltrate on chest radiograph, in a patient not hospitalized or residing in a long‐term‐care facility for 14 days prior to the onset of symptoms.1 CAP continues to be a common and serious illness, causing substantial morbidity and mortality in the adult population. There are an estimated 56 million cases a year in the United States, with greater than 1 million hospitalizations. Community‐acquired pneumonia is one of the most common admitting diagnoses among adults, and with a 30‐day mortality between 10% and 14% for patients admitted to the hospital, it is the leading cause of infectious death in the United States.2 In elderly patients, hospitalization for CAP portends a poor long‐term prognosis. In a Medicare database, the 1‐year mortality for patients with CAP was nearly 40%, compared to 29% in patients with other diagnoses.3 Community‐acquired pneumonia is a model illness in hospital medicineit is a common disease that allows for evidence‐based and cost‐effective management. In addition, many national organizations have proposed multiple quality indicators for community‐acquired pneumonia, thus providing an opportunity for institutional quality improvement. This review article outlines the assessment and management of patients admitted to the hospital with community‐acquired pneumonia.
Etiology
Although many pathogens can cause community‐acquired pneumonia, the clinical syndromes and microbiology of CAP have traditionally been characterized as either typical or atypical. The typical organisms include Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, and the atypical organisms include Chlamydia spp., Mycoplasma pneumoniae, Legionella spp., and viruses. This historical distinction has recently come into question. It is now clear that the presenting symptoms, signs, and basic laboratory findings (including the chest radiograph) cannot be reliably used to predict the etiologic pathogen or to distinguish typical from atypical organisms.4 Rather, the specific causative agent of CAP depends more on the degree of patient illness. Table 1 shows what prospective studies with comprehensive diagnostic strategies determined to be the most common pathogens in patients hospitalized for CAP in ICU and non‐ICU settings.5 Streptococcus pneumoniae remains the most common cause of CAP in hospitalized patients and is the most common cause of fatal pneumonia, whereas Legionella spp. is a common cause of severe CAP, more often found in patients requiring admission to the intensive care unit. Gram‐negative bacilli can cause CAP in elderly patients and those recently treated with broad‐spectrum antibiotics or with underlying lung disease. Notably, though, despite improved diagnostic testing, only one quarter of all admitted patients with CAP have the etiologic agent defined, and therefore empiric therapy should be directed broadly at the most likely organisms.6
| Non‐ICU inpatients | ICU inpatients (severe) |
|---|---|
| S. pneumoniae | S. pneumoniae |
| M. pneumoniae | Legionella spp |
| C. pneumoniae | H. influenzae |
| H. influenzae | Gram‐negative bacilli |
| Legionella spp | S. aureus |
| Aspiration | |
| Respiratory viruses |
Clinical Presentation
Patients admitted to the hospital with CAP typically present with a brief history of respiratory complaints, including cough (greater than 90%), dyspnea (66%), sputum production (66%), and pleuritic chest pain (50%); see Table 2.7, 8 In 10%30% of patients, nonrespiratory complaints predominate, including headache, myalgias, fatigue, and gastrointestinal symptoms.6 Elderly patients, an increasing percentage of hospitalized patients, are less likely to present with typical CAP symptoms (such as cough) and more likely to have altered mental status as a presenting symptom.9
| Symptoms | Signs (exam) |
|---|---|
| Cough 90% | Fever 80% |
| Dyspnea 66% | Tachypnea 70% |
| Sputum 66% | Tachycardia 50% |
| Pleuritic chest pain 50% | Focal lung exam >90% |
On physical examination, patients with CAP usually have signs of fever (80%), tachypnea (70%), and tachycardia (50%); see Table 2. Most will have a focal lung exam (>90%) with findings ranging from crackles to bronchial breath sounds.10 No exam finding is specific for the diagnosis of pneumonia, but the absence of fever, tachycardia, and tachypnea significantly reduces the probability of CAP in patients with suspected pneumonia.10 Furthermore, similar to the clinical history, the physical examination of elderly patients with community‐acquired pneumonia is not specific or sensitive for the diagnosis of CAP. For example, up to 40% of elderly patients subsequently determined to have CAP may not have fever.11
Leukocytosis is common in patients with CAP; however, its absence does not rule out disease.12 A number of guidelines recommend laboratory evaluation of electrolytes, urea nitrogen, creatinine, liver enzymes, and bilirubin, although these are used primarily for prognostication and are not specifically useful in the diagnosis of CAP.
DIAGNOSIS
Differential Diagnosis
Given the nonspecific nature of the symptoms and signs associated with CAP, there is no single clinical feature or combination of clinical features that adequately rules in or out the diagnosis of CAP. Consequently, the differential diagnosis to be considered in patients with suspected CAP is broad. Noninfectious diseases can often present with similar clinical syndromes; these include congestive heart failure, exacerbation of chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism, and hypersensitivity pneumonitis. These diseases can often be distinguished with a thorough history and physical examination.
In addition, other upper‐ and lower‐airway infectious diseases can have similar nonspecific signs and symptoms. In particular, pneumonia must often be differentiated from acute bronchitis, which as a diagnosis accounts for up to 40% of patients evaluated for cough (versus 5% for pneumonia).10 Patients with acute bronchitis frequently do not present with high fevers or hypoxia and in general will not benefit from antibiotic therapy.13 Patients believed to have community‐acquired pneumonia might also be suffering from other pneumonia syndromes including aspiration pneumonia, postobstructive pneumonia, and pneumonia in immunocompromised patients (eg, those with HIV, on steroids, receiving chemotherapy). Determining the correct diagnosis can have implications for therapy and prognosis.
Diagnostic Studies
The diagnosis of community‐acquired pneumonia requires that a patient have both signs and symptoms consistent with pulmonary infection and evidence of a new radiographic infiltrate. Therefore, most guidelines recommend that all patients with a possible diagnosis of CAP be evaluated with chest radiography.1, 14, 15
The specific radiographic findings in community‐acquired pneumonia range from lobar consolidation to hazy focal infiltrate to diffuse bilateral interstitial opacities (see Figure 1). Although chest radiography has traditionally been considered the gold standard for the diagnosis of CAP, its exact performance characteristics are unknown, and it is clearly not 100% sensitive or 100% specific. The utility of the chest radiograph can be limited by patient body habitus, underlying lung disease, or dehydration. Computed tomography (CT) scanning, although not recommended for routine use, can identify pulmonary consolidation in up to 30% of patients with a normal or equivocal chest radiograph in whom pneumonia is suspected and can also identify complications of pneumonia including an empyema or pulmonary abscess.16

Limitations in the performance of the chest radiograph have resulted in an interest in the diagnostic performance of serologic markers of infection such as C‐reactive protein (CRP), procalcitonin, and soluble triggering receptor expressed on myeloid cells (s‐TREM).1719 Preliminary evidence suggests these inflammatory markers may ultimately prove useful in differentiating infectious from noninfectious pulmonary processes, but regular use of these new tests cannot currently be recommended.
Most expert guidelines state that 2 sets of blood cultures should be taken and analyzed prior to antibiotic administration in all patients admitted to the hospital with suspected community‐acquired pneumonia.1, 14, 15 Isolation of bacteria from blood cultures in CAP is a very specific way to identify a causative organism in order to subsequently narrow therapy and also identifies a high‐risk group of patients because bacteremia is associated with increased mortality. Obtaining blood cultures within 24 hours of admission has been associated with 10% lower odds of 30‐day mortality in patients with CAP,20 and as a result, drawing blood cultures prior to antibiotic administration is a national quality indicator for CAP.
There are, however, a number of problems with the routine acquisition of blood cultures in all patients admitted with CAP. Practically, the cultures can be difficult to obtain, can potentially delay the initiation of antibiotics, and are often contaminated, which has been shown to increase both cost and length of stay.21, 22 The yield is generally low: the true‐positive bacteremia rate for admitted patients with CAP ranges from 6% to 9%, and the culture results rarely change management or outcomes.23, 24 Given these limitations, many have argued that blood cultures should be obtained with a more targeted approach. A recent study used a Medicare database to create a decision‐support tool to help maximize the value of blood cultures in CAP.25 The predictors of a positive blood culture are shown in Table 3. Not obtaining cultures on patients who had received prior antibiotics or had no risk factors resulted in about 40% fewer overall cultures while identifying approximately 90% of bacteremias. In their guidelines, the British Thoracic Society (BTS) advocates a similar strategy, recommending blood cultures be omitted in nonsevere pneumonia and in patients without comorbidities.15, 26 Although recommendations vary for non‐severe CAP in hospitalized patients, all professional society guidelines agree that blood cultures should be obtained in critically ill patients, and if cultures are obtained, they should be drawn prior to antibiotics.1, 14, 15, 26
| Comorbidities |
| Liver disease |
| Vital signs |
| Systolic blood pressure < 90 mm Hg |
| Temperature < 35C or 40C |
| Pulse 125 beats/min |
| Laboratory and radiographic data |
| Blood urea nitrogen (BUN) 30 mg/dL |
| Sodium < 130 mmol/L |
| White blood cells < 5000/mm3 or > 20,000/mm3 |
| Prior use of antibiotics (negative predictor) |
Substantial controversy surrounds the utility of routine sputum gram stains and cultures for patients admitted to the hospital with CAP. The Infectious Disease Society of America (IDSA) and the British Thoracic Society (BTS) both recommend that all patients admitted to the hospital with community‐acquired pneumonia should have a gram stain and culture of expectorated sputum.1, 15, 26 Both organizations argue sputum collection is a simple and inexpensive procedure that can potentially identify pathogenic organisms and can affect both initial and long‐term antibiotic therapy. Most notably, they highlight gram stain specificity of greater than 80% for pneumococcal pneumonia. Conversely, the American Thoracic Society (ATS) argues that sputum gram stains and cultures generally have low sensitivity, specificity, and positive predictive value.14 Furthermore, they argue the utility of sputum testing is also limited practically; in one study 30% of patients could not produce an adequate sputum specimen and up to 30% had received prior antibiotic therapy, substantially reducing the yield.27 In another study, good‐quality sputum with a predominant morphotype could be obtained in only 14% of patients admitted with CAP.28 However, targeting sputum analysis to patients who have not received prior antibiotics and are able to produce an adequate sample improved the yield significantly.29 In addition, with increasing rates of antibiotic resistance among common community isolates (ie, S. Pneumoniae) and the increasing prevalence of infecting organisms not targeted by routine empiric therapy (methicillin‐resistant Staphylococcus Aureus [MRSA]), isolation of potential causative pathogens is increasingly important. We believe that severely ill patients with CAP (such as patients admitted to the ICU), as well as patients with identifiable risk factors for uncommon or drug‐resistant pathogens (eg, Pseudomonas aeruginosa, enteric gram‐negative rods, MRSA, etc.) should have sputum sent for gram stain and culture. Ideally, sputum obtained for gram stain and culture should be:
-
Prior to antibiotic therapy,
-
A deep‐cough, expectorated specimen,
-
A purulent specimen (>25 polymorphonucleacytes and less than 10 squamous cells per high‐powered field), and
-
Rapidly transported to the laboratory.
Subsequent gram stain and culture results should be interpreted in the specific clinical context and antibiotic choices targeted appropriately.
Alternative Diagnostic Tests
In recent years, there has been growth in additional diagnostic tests targeting specific organisms. The pneumococcal urinary antigen assay is a relatively sensitive (50%80%) and highly specific (90%) test for the detection of pneumococcal pneumonia, when compared with conventional diagnostic methods.27 The test is simple, convenient, rapid ( 15 min), and, with its high specificity, may allow for more focused antimicrobial therapy early in management. Current limitations include the possibility of false‐positive tests in patients colonized with S. pneumoniae or infected with other streptococcal species, as well as the inability to determine antibiotic sensitivity from positive tests. Updated IDSA and BTS guidelines state pneumococcal urinary antigen testing is an acceptable adjunct to other diagnostic tests, but blood and sputum analyses should still be performed.26, 27 For patients with suspected Legionella pneumonia (primarily critically ill and immunocompromised patients or in association with regional outbreaks), the urinary Legionella antigen assay is the test of choice, which detects 80%95% of community‐acquired cases of Legionnaires' disease with a specificity of 90%.27
During the winter months (typically from October to March), rapid antigen testing for influenza is generally recommended for patients with signs or symptoms consistent with influenza.27 The sensitivity of these tests is approximately 50%70%, so negative test results do not exclude the diagnosis, but results can be important epidemiologically and therapeutically (differentiating influenza A and B strains).27 Diagnostic tests targeting other common CAP pathogens, such as serologic tests for Mycoplasma pneumoniae or Chlamydia spp, should not be routinely performed. Testing for less common causative pathogens such as Mycobacterium tuberculosis should only be employed in the appropriate clinical setting.
ADMISSION DECISION
Once the diagnosis of CAP has been made, the initial site where treatment will occur, whether the hospital or the home, must be determined. The decision to hospitalize should be based on 3 factors: 1) evaluation of the safety of home treatment, 2) calculation of the Pneumonia Severity Index (PSI), and 3) clinical judgment of the physician.27 The PSI, or PORT (Pneumonia Outcomes Research Team) score, is a validated prediction rule that quantifies mortality and allows for risk stratification of patients with community‐acquired pneumonia.2 The PSI combines clinical history, physical examination, and laboratory data at the time of admission to divide patients into 5 risk classes and to estimate 30‐day mortality (Figure 2), which ranges from 0.1% of patients in risk class I to 27.0% in risk class V.2

On the basis of the estimated prognosis and in the absence of concerns about home safety or comorbidities, patients in risk classes I, II, and III should be managed at home. Many prospective trials have shown that implementation of PSI significantly increases the number of low‐risk patients managed outside the hospital, with no differences in quality of life, complications, readmissions, or short‐term mortality.30, 31 Most recently, a trial randomizing patients in risk classes II and III to treatment in the hospital or at home found no significant differences in clinical outcomes but did find that patients were more satisfied with care at home.32 Because the number of patients with CAP being treated at home is increasing, the American College of Chest Physicians recently published a consensus statement on the management of community‐acquired pneumonia in the home.33 All national guidelines for the management of community‐acquired pneumonia recommend using the PSI to help determine the initial location of treatment, with the caveat that using the prediction rule should never supersede clinical judgment in the decision about whether to admit.1, 14, 15, 26, 27 A practical decision tree for the use of the PSI is shown in Figure 3.

There are no reliable prediction rules for deciding on whether admission to the intensive care unit is necessary. Hemodynamic instability requiring resuscitation and monitoring or respiratory failure requiring ventilatory support are clear indications for ICU admission. Additional variables such as tachypnea (respiratory rate 30), altered mental status, multilobar disease, and azotemia are associated with severe CAP and should prompt consideration of ICU admission, especially when 2 or more variables coexist.14
TREATMENT
Initial Treatment
Once the admission decision is made and the initial diagnostic tests are completed (including blood and sputum cultures), patients with presumed community‐acquired pneumonia should receive necessary supportive care (O2, intravenous fluids, etc.) and prompt antimicrobial therapy. Antibiotics should be administered within 4 hours of arrival to patients with suspected CAP, as such prompt administration may be associated with shorter in‐hospital stays and decreased 30‐day mortality.34, 35 Regulatory organizations such as the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the Center for Medicare Services (CMS) have made delivery of antibiotics in less than 4 hours a hospital quality measure.
Despite diagnostic testing, the specific etiologic agent causing the pneumonia of a patient remains unknown in up to 75% of those admitted to the hospital.14 Most expert guidelines therefore recommend broad‐spectrum empiric therapy targeting both the typical and the atypical organisms that commonly cause CAP (Table 1).
Recommendations for empiric antibiotics are driven by 2 key factors: antibiotic resistance by S. pneumoniae and the results of studies of CAP treatment outcomes. Historically, patients with suspected community‐acquired pneumonia were treated with penicillin with generally good outcomes. Recently, the rate of S. pneumoniae isolates resistant to penicillin has risen dramatically in the United States, ranging between 20% and 30%, with high‐level resistance (MIC 4 mg/L) as high as 5.7%.36, 37 Concurrently, the rates of resistance of S. pneumoniae to many other antibiotics commonly used to treat CAP have also risen.37 Despite increasing resistance overall, most U.S. pneumococcal isolates have low resistance to third‐generation cephalosporins and fluoroquinolones with enhanced activity against S. pneumoniae.3638 In addition, despite increasing resistance by pneumococcal isolates to penicillin, several observational studies have shown that regardless of initial therapy, resistance to penicillin as well as third‐generation cephalosporins is not associated with higher mortality or worse outcomes when controlled for other risk factors for drug resistance.39, 40 An exception to that rule is pneumococcal isolates that are very highly resistant to PCN (MIC 4 mg/dL). At least one study has shown that patients with such isolates may be at higher risk for adverse outcomes and should probably not be treated with penicillins.1, 14, 15, 41 However, nationally, fewer than 6% of pneumococcal isolates have this level of resistance.37
The rationale for empiric broad‐spectrum coverage against both typical and atypical organisms has arisen from many retrospective and observational studies that have suggested that there is clinical benefit and improved outcomes with such regimens. One large retrospective study showed that in elderly patients with CAP, fluoroquinolone monotherapy was associated with lower 30‐day mortality when compared to monotherapy with a third‐generation cephalosporin.34 Adding an extended‐spectrum macrolide (eg, azithromycin) to an extended‐spectrum ‐lactam (eg, ceftriaxone) in the treatment of patients hospitalized with nonsevere CAP also appears to be associated with improved outcomes. Adding a macrolide has resulted in shorter lengths of stay (LOS), less treatment failure, and lower mortality.34, 4244 Similarly, according to unpublished observations, adding doxycycline to a ‐lactam as initial therapy was associated with a benefit of decreased mortality.45 The presumed etiology of the benefit has been the addition of specific coverage of atypical organisms, such as Mycoplasma pneumoniae and Chlamydia pneumoniae, which are common causes of CAP (Table 1). Others have proposed that the benefit of therapy with macrolides may be derived from the inherent anti‐inflammatory properties of macrolides.46 Because research has shown a benefit of dual versus monotherapy across a spectrum of antibiotics, others have proposed the benefit is simply a result of receiving double antibiotic coverage. In particular, 2 studies found a benefit of reduced mortality from combination therapy over monotherapy in bacteremic pneumococcal pneumonia.47, 48
Yet the accumulated evidence for adding coverage of atypical organisms has been only retrospective and observational. Because of this, the recommendation to routinely add antibiotics active against atypical organisms has been questioned by some. Two recent meta‐analyses and a systematic review examined all the available data on the need for atypical coverage in the treatment of patients with community‐acquired pneumonia.4951 Surprisingly, none showed a benefit in clinical efficacy or survival in patients treated with agents active against both atypical and typical organisms when compared to regimens with only typical coverage. In subset analyses, there was a benefit to providing empiric atypical coverage in patients subsequently shown to have Legionella spp. as a causative pathogen. However, this organism was uncommon in all 3 studies. Unfortunately, most studies included in the meta‐analyses compared fluoroquinolone or macrolide monotherapy with third‐generation cephalosporin monotherapy. There have been no high‐quality randomized, controlled trials of the treatment of hospitalized patients with CAP assessing combination therapy covering both typical and atypical organisms with monotherapy targeting typical organisms alone. High‐quality trials are warranted.
Despite the recent articles questioning the importance of atypical coverage, citing the substantial retrospective data and the general inability to identify causative organisms in most cases of CAP, adding a second agent with atypical coverage to a ‐lactam currently appears to be the most efficacious empiric treatment for CAP. Nearly all expert guidelines for the management of community‐acquired pneumonia recommend this empiric approach.1, 14, 27
Table 4 displays our recommendations for the treatment of community‐acquired pneumonia requiring hospitalization. Before implementation of these guidelines, hospitalists should consult with their infectious disease experts and consider local resistance patterns. In general, a typical adult patient with non‐severe CAP without additional risk factors should receive a parenteral extended‐spectrum ‐lactam plus either doxycycline or an advanced macrolide (see Table 4). Extended‐spectrum ‐lactams include cefotaxime, ceftriaxone, ampicillin‐sulbactam, and ertapenem. A respiratory fluoroquinolone as a single agent can be used for non‐ICU patients with CAP, but some agencies, including the Centers for Disease Control (CDC), discourage routine use of these agents in all patients secondary to concerns about cost and increasing gram‐negative rod fluoroquinolone resistance.52, 53
| Patient group | Empiric antibiotic therapy |
|---|---|
| |
| Inpatient, non‐ICU | ‐Lactama + either doxycycline or an advanced macrolideb |
| Severe ‐lactam allergyc | Respiratory fluoroquinoloned |
| Inpatient, ICU | |
| No risk for Pseudomonas | ‐Lactam + either an advanced macrolide or a respiratory fluoroquinolone |
| Severe ‐lactam allergy | Respiratory fluoroquinolone + clindamycin |
| Pseudomonas risk factorse | Antipseudomonal ‐lactamf + an antipseudomonal fluoroquinoloneg |
| Severe ‐lactam allergy | Aztreonam + a respiratory fluoroquinolone |
| MRSA risk factorsh | Add vancomycin to above regimens |
| From nursing home | Should be treated as nosocomial/health‐care‐associated pneumonia |
| Aspiration pneumonia | ‐Lactam or respiratory fluoroquinolone clindamycini |
Patients hospitalized with severe CAP who require ICU‐level care are at increased risk of Legionella spp. and drug‐resistant S. pneumoniae, which must be reflected in their initial antibiotic therapy.5 Patients with severe pneumonia should receive an intravenous extended‐spectrum ‐lactam plus either an intravenous macrolide or an intravenous respiratory fluoroquinolone.
All patients with severe CAP who are admitted to the intensive care unit should be routinely screened for risk factors for Pseudomonas aeruginosa. The known risk factors for pseudomonal infection are: bronchiectasis, immunosuppression including more than 10 mg/day of prednisone, malnutrition, and treatment with broad‐spectrum antibiotics in the last month.14 Those at risk for Pseudomonas aeruginosa or other resistant gram‐negative rod infection should be treated with an antipseudomonal ‐lactam plus an antipseudomonal fluoroquinolone. Many patients with severe CAP have risk factors for MRSA infection including recent prolonged hospitalization, recent use of broad‐spectrum antibiotics, and significant underlying lung disease, which should be considered in choosing initial antibiotic therapy.54 In addition, there have been reports of patients without underlying risk factors presenting with severe community‐acquired MRSA pneumonia. Many of these patients were younger and the MRSA pneumonia was associated with a necrotizing or cavitary disease requiring prolonged ICU stays.5558 In such cases or if an institution's rate of methicillin resistance in S. aureus community isolates is high (>15%20%), it may be appropriate to add initial empiric MRSA coverage for patients admitted to the ICU with CAP.55
Some patients will have unique risk factors and clinical presentations, which may require modification of these empiric recommendations. Several studies found 5%15% of cases of community‐acquired pneumonia to be aspiration pneumonia.57 Risk factors for aspiration events include, among others, dysphagia, history of stroke, altered level of consciousness, poor dentition, and tube feeding. Aspiration pneumonia traditionally was believed to be secondary to oral anaerobes, but recent research suggests gram‐positive cocci and gram‐negative rods are the predominant organisms.58 Antibiotic therapy in patients with clear aspiration pneumonia should be directed at these microbes with an extended‐spectrum ‐lactam (eg, ceftriaxone) or a respiratory fluoroquinolone (eg, levofloxacin or moxifloxacin). Anaerobic bacterial coverage can be added in patients with severe periodontal disease, alcoholism, concern for empyema, or evidence of aspiration with pulmonary abscess.58
Patients residing in long‐term care facilities are at high risk of contracting pneumonia. The microbiology of infections acquired in nursing facilities is similar to that in hospital‐acquired cases.59, 60 As a result, patients who develop pneumonia in institutional settings such as nursing homes should be treated with broad‐spectrum antibiotics, including coverage for MRSA.
Subsequent Treatment
Initial empiric antibiotic treatment should be modified based on the results of diagnostic testing. Although the specific etiologic agent is determined in only 25% of cases of CAP,35 when an organism is isolated, antibiotic coverage should be narrowed to cover that particular organism with an antibiotic with adequate lung penetration. Evidence suggests clinicians often do not adjust or narrow antibiotics based on sensitivity results, potentially breeding resistant organisms.61
Patients hospitalized with CAP usually improve quickly if they receive early, appropriate antibiotic therapy and supportive care. Excluding patients with severe CAP requiring intensive care unit admission, most patients resolve their tachycardia, tachypnea, and fever by day 2 or 3.62 Recent practice experience, evidence, and published guidelines14, 27 all indicate that patients can safely be transitioned to oral antibiotic therapy earlier in their hospital course. Table 5 outlines criteria that can be used to identify patients who have had an adequate response to parenteral therapy and can be considered for a switch to oral antibiotics. If these criteria are met, patients have less than a 1% chance of clinical deterioration necessitating admission to an ICU or transitional care unit.62 When an etiologic organism is not identified, oral therapy should reflect a spectrum of coverage to that of the initial intravenous therapy. In some cases, this may require use of more than one oral agent. We have had success, however, transitioning non‐ICU patients initially treated with intravenous ceftriaxone plus oral doxycycline, typically for 4872 hours, to oral doxycycline monotherapy at discharge.45
| Stable vital signs and clinical criteria for 24 hours |
| Temperature 37.8C (100F) |
| Heart rate 100 beats per minute |
| Respiratory rate 24 breaths per minute |
| Systolic blood pressure 90 mm Hg |
| Oxygen saturation (on room air) 90% |
| Ability to take oral medications |
There have been a limited number of high‐quality randomized trials examining the optimal duration of treatment for community‐acquired pneumonia. Most practice guidelines recommend 710 days for patients with CAP requiring hospitalization, with 14 days for documented Mycoplasma pneumoniae or Chlamydia pneumoniae. One recent randomized trial of patients with mild to severe CAP showed a short course of high‐dose levofloxacin (750 mg daily 5 days) was at least as effective as normal dosing (500 mg daily 10 days).63 Clinical experience with high‐dose levofloxacin is limited, but this regimen can be considered because it may reduce costs and exposure to antibiotics. When diagnosed, Legionella is usually treated for 1021 days, but 14 days is adequate with macrolides because of their long half‐life.27 Patients with more virulent pathogens like Staphylococcus aureus or Pseudomonas aeruginosa or other suppurative complications should be treated for at least 14 days.1, 14, 15, 27 In determining length of therapy, clinicians should use these durations of treatment as guides, and to individualize therapy, they should always consider patient age and frailty, comorbid conditions, severity of illness, and hospital course.
Failure to Respond
Although most patients hospitalized for CAP will improve rapidly and reach clinical stability in 23 days, some patients fail to respond. Some studies have estimated that failure to improve or clinical deterioration occurs in 5%10% of patients in the first 23 days.64 The common reasons for clinical decline or nonresponse to treatment, highlighted in Table 6, are:
-
Incorrect diagnosis: Illnesses such as congestive heart failure, pulmonary embolism, neoplasms, and hypersensitivity pneumonitis can mimick CAP.
-
Inadequate antibiotic selection: The etiologic agent may be resistant to empiric antibiotic selections. Examples would include methicillin‐resistant Staphylococcus aureus (MRSA) or multiresistant gram‐negative bacilli.
-
Unusual pathogen: CAP syndromes can be caused by myriad unusual organisms including Pneumocystis jirovecii, mycobacterium tuberculosis, endemic fungal infections (eg, coccidioidomycosis), and nocardiosis.
-
Complications of pneumonia: Specific complications of CAP include empyema, pulmonary abscess, extrapulmonary spread including meningitis or endocarditis, or other organ dysfunctions such as renal failure or myocardial infarction.
-
Inadequate host response: Despite appropriate antibiotic and supportive therapy, patients with CAP often fail to respond.
| Incorrect diagnosis of CAP. |
| Inadequate or inappropriate antibiotic selection for CAP. |
| Unusual pathogen causing CAP. |
| Pulmonary or extrapulmonary complication of CAP. |
| Inadequate or poor host response. |
Progressive pneumonia despite appropriate therapy and empyema were the most common causes of failure to respond in the first 72 hours in a recent study.64 Risk factors for early failure were older age (>65 years), Pneumonia Severity Index > 90, Legionella pneumonia, gram‐negative pneumonia, and initial antimicrobial therapy discordant with final culture and susceptibility results. The initial evaluation of the nonresponding patient should address these common causes and is likely to include additional imaging (CT), sampling of potential extrapulmonary infection (thoracentesis), and, in some cases, bronchoscopy.
DISCHARGE/FOLLOW‐UP PLANS
Patients hospitalized for community‐acquired pneumonia can be safely discharged when they have reached clinical stability, are able to tolerate oral medications, have no other active comorbid conditions, and have safe, close, appropriate outpatient follow‐up (see Table 7). Clinical pathways employing these discharge criteria have been found to be safe and effective in reducing the length of stay for CAP. Most important, patients should have met most if not all of the vital sign and clinical criteria noted in Table 5 in the criteria for switching to oral therapy. Patients with 2 or more abnormal vital signs (instabilities) within 24 hours prior to discharge are at high risk of readmission and mortality, but those with one or no abnormal vital signs generally have good outcomes.65 Absent other clinical factors or extenuating circumstances (persistent hypoxia, poor functional status, etc.), most patients with CAP should reach clinical stability by day 3 or 4, be considered for a switch to oral therapy, and, if stable, be discharged shortly thereafter.
| Patients should: |
|
Meet clinical criteria in Table V. |
| Be able to tolerate oral medications (no need to observe for 24 hours on oral therapy). |
| Have no evidence of active comorbid conditions (myocardial ischemia, pulmonary edema, etc.). |
| Have a normal mental status (or have returned to their baseline). |
| Have safe, appropriate outpatient follow‐up. |
When patients with CAP are discharged from the hospital, they should be counseled about the expected course of recovery. Most important, patients and families must be informed that many symptoms of CAP may persist well after hospitalization. In one study, up to 80% of patients reported persistent cough and fatigue 1 week after discharge, and up to 50% still had dyspnea and sputum production. In some, the cough can last for 46 weeks.8
All patients discharged after treatment of community‐acquired pneumonia should have follow‐up with their outpatient provider. The physician responsible for their inpatient care should communicate directly with the provider and outline the hospital course, the discharge medications, and the duration of antibiotic therapy. There is no specific time frame within which patients must be seen, but follow‐up should be dictated by patient age, comorbidities, clinical stability at discharge, and degree of illness. The American Thoracic Society guidelines do recommend patients with a substantial smoking history who are hospitalized with CAP have a follow‐up chest radiograph 46 weeks after discharge to establish a radiographic baseline and exclude the possibility of underlying malignancy.14 However, several studies have suggested that radiographic resolution may take 3 or more months in some patients, especially the elderly and those with multilobar disease.66
PREVENTION
Prevention of community‐acquired pneumonia and pneumonic syndromes has traditionally relied on vaccination with the polysaccharide pneumococcal pneumonia vaccine and the seasonal influenza vaccine. The vaccine for S. pneumoniae used in adults is composed of the 23 serotypes that cause 85%90% of the invasive pneumococcal infections in the United States. Although in randomized trials the vaccine has not consistently prevented community‐acquired pneumonia or death in elderly patients or those with comorbidities, it likely prevents invasive pneumococcal infection.67 National guidelines and the CDC recommend the pneumococcal vaccine be given to all patients older than 65 years and those with chronic medical conditions.1, 14, 15
The seasonal influenza vaccine has clearly been shown to decrease influenza‐related illness in elderly and high‐risk patient populations. As well, in a meta‐analysis and a large observational study of patients older than 65 years, vaccination against influenza prevented pneumonia, hospitalization, and death.68, 69 Vaccination of health care workers may also confer a benefit to elderly patients of reduced mortality. The CDC recommends the influenza vaccine for all patients more than 50 years old, those with comorbidities, those at high risk for influenza, and health care workers in both inpatient and outpatient settings.
Pneumococcal and influenza vaccination have traditionally been relegated to the outpatient setting. National guidelines and the CDC recommend vaccination of all eligible hospitalized patients. Vaccination is safe and effective with almost any medical illness, and both vaccines can be given simultaneously at discharge.69 Both JCAHO and CMS have defined administration of the pneumococcal and influenza vaccines to patients hospitalized with CAP as a quality measure. Using standing orders is the most effective means of ensuring vaccination.
Some evidence suggests that tobacco smokers are at increased risk of invasive pneumococcal disease or pneumonia.70 Patients hospitalized (for all illnesses, but for CAP in particular) should be counseled about smoking cessation and offered pharmacotherapy and outpatient follow‐up. And, finally, recent observational data suggests that use of acid suppressive therapy, including proton pump inhibitors and H‐2 receptor antagonists, may be associated with an increased risk of developing CAP.71 Patients using these agents who are admitted with CAP should have their indications for treatment reviewed, especially when the pneumonia has been recurrent and there is no clear indication for continued use of acid suppressive therapy, in which case they should be discontinued in the hospital.
CONCLUSIONS
Community‐acquired pneumonia remains a common cause for hospitalization of adult patients, with significant associated morbidity and mortality. Although there are multiple expert guidelines for the management of community‐acquired pneumonia, further research is urgently needed. Clinicians need improved diagnostic tests that enable an earlier and more accurate diagnosis of CAP. In addition, the etiologic agent causing CAP is rarely discovered; improved microbiologic studies might enable antibiotic therapy to be targeted to the organisms responsible. High‐quality randomized, controlled trials examining empiric antibiotic therapy in CAP are needed, especially related to the addition of agents covering atypical organisms. Last, the general management of patients hospitalized with CAP is marked by significant heterogeneity, and research and initiatives focusing on improving the quality and process of care of patients with CAP are needed.
- ,,,,,.Guidelines from the Infectious Disease Society of America. Practice guidelines for the management of community‐acquired pneumonia.Clin Infect Dis.2000;31:347–382.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,, et al.Pneumonia: still the old man's friend?Arch Intern Med.2003;163:317–323.
- ,,, et al.New and emerging etiologies for community‐acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases.Medicine.1990;69:307–316.
- .Community‐acquired pneumonia.Lancet.2003;362:1991–2001.
- ,,, et al.Processes and outcomes of care for patients with community‐acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team (PORT) cohort study.Arch Intern Med.1999;159:970–980.
- ,.Management of community‐acquired pneumonia.N Engl J Med.2002;347:2039–2045.
- ,,, et al.Measuring symptomatic and functional recovery in patients with community‐acquired pneumonia.J Gen Intern Med.1997;12:423–430.
- ,,, et al.Influence of age on symptoms at presentation with patients with community‐acquired pneumonia.Arch Intern Med.1997;157:1453–1459.
- ,,.Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination.JAMA.1997;278:1440–1445.
- ,,, et al.Community‐acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes.Medicine.2003;82:159–169.
- ,.Testing strategies in the initial management of patients with community‐acquired pneumonia.Ann Intern Med.2003;138:109–118.
- ,.Uncomplicated acute bronchitis.Ann Intern Med.2000;133:981–991.
- ,,, et al.American Thoracic Society: Guidelines for the management of community‐acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,, et al.British Thoracic Society guidelines for the management of community acquired pneumonia in adults.Thorax.2001;56:Suppl. 4,IV1–IV64.
- ,,,,.High‐resolution computed tomography for the diagnosis of community‐acquired pneumonia.Clin Infect Dis.1998;27:358–363.
- ,,, et al.Performance of a bedside C‐reactive protein test in the diagnosis of community‐acquired pneumonia in adults with acute cough.Am J Med.2004;116:529–535.
- ,,, et al.Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial.Lancet.2004;363:600–607.
- ,,, et al.Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia.N Engl J Med.2004:350:451–458.
- ,,, et al.Quality of care, process, and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- ,,,.Limited usefulness of initial blood cultures in community acquired pneumonia.Emerg Med J.2004;21:446–448.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,, et al.The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study.Chest.2003;123:1142–1150.
- ,,, et al.Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks.Chest.1995;108:932–936.
- ,,,.Predicting bacteremia in patients with community‐acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- British Thoracic Society. BTS Guidelines for the management of community acquired pneumonia in adults—2004 update. Available at: www.brit‐thoracic.org/guidelines.
- ,,,,,.Guidelines from the Infectious Disease Society of America. Update of guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433.
- ,,, et al.Assessment of the usefulness of sputum culture for diagnosis of community‐acquired pneumonia using the PORT predictive scoring system.Arch Intern Med.2004;164:1807–1811.
- ,,.Diagnostic value of microscopic examination of gram‐stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia.Clin Infect Dis.2004;39(2):165–169.
- ,,, et al.Safely increasing the proportion of patients with community‐acquired pneumonia treated as outpatients: an interventional trial.Arch Intern Med.1998;158:1350–1356.
- ,,, et al.A critical pathway for treatment of community‐acquired pneumonia.JAMA.2000;283:2654–2655.
- ,,, et al.Outpatient care compared with hospitalization for community‐acquired pneumonia. A randomized control trial in low‐risk patients.Ann Intern Med.2005;142:165–172.
- ,,.Management of community‐acquired pneumonia in the home.Chest.2005;127:1752–1763.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159:2562–2572.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,, et al.Increasing prevalence of multidrug‐resistant Streptococcus pneumoniae in the United States.N Engl J Med.2000;343:1917–1924.
- ,,, et al.Susceptibility patterns of Streptococcus pneumoniae isolates in North America (2002–2003): contemporary in vitro activities of amoxicillin/clavulanate and 15 other antimicrobial agents.Int J Antimicrob Agents.2005;25(4):282–289.
- ,,, et al.Antimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes?Clin Infect Dis.2005;41(2):139–148.
- ,,, et al.Pneumonia acquired in the community through drug‐resistant Streptococcus pneumoniae.Am J Respir Crit Care.1999;159:1835–1842.
- ,,, et al.Drug‐resistant pneumococcal pneumonia: clinical relevance and related factors.Clin Infect Dis.2004;38:787–798.
- ,,, et al.Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997.Am J Public Health.2000;90(2):223–9.
- ,,, et al.Lower mortality among patients with community‐acquired pneumonia treated with a macrolide plus a beta‐lactam agent versus a beta‐lactam alone.Eur J Clin Microbiol Infect Dis2005;24:190–195.
- ,,,.Impact of initial antibiotic choice on clinical outcomes in community‐acquired pneumonia: analysis of a hospital claims‐made database.Chest.2003;123:1503–1511.
- ,,,.Antimicrobial selection for hospitalized patients with presumed community‐acquired pneumonia: a survey of nonteaching US community hospitals.Ann Pharmacother2000;34:446–452.
- ,,,,.J Hosp Med.2006;1:7–12.
- .Anti‐inflammatory effects of macrolides—an underappreciated benefit in the treatment of community‐acquired respiratory tract infections and chronic inflammatory pulmonary conditions?J Antimicrob Chemother.2005;55:10–21.
- ,,, et al.Addition of a macrolide to a beta‐lactam based empirical antibiotic regimen is associated with lover in‐hospital mortality for patients with bacteremic pneumococcal pneumonia.Clin Infect Dis.2003;36:389–395.
- ,,, et al.Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia.Am J Respir Crit Care Med.2004;170:440–444.
- ,,,.Empiric antibiotic coverage of atypical pathogens for community‐acquired pneumonia in hospitalized adults.Cochrane Database Syst Rev.2005;2:CD004418.pub2.
- ,,.Effectiveness of β lactam antibiotics compared with antibiotics active against atypical pathogens in non‐severe community‐acquired pneumonia: meta‐analysis.Br Med J.2005;330:456.
- ,,,.Empirical atypical coverage for inpatients with community‐acquired pneumonia.Arch Intern Med.2005;165:1992–2000.
- ,,, et al.Antibiotic resistance among gram‐negative bacilli in US intensive care units: implications for fluoroquinolone use.JAMA.2003;289:885–888.
- ,,, et al.First‐generation fluoroquinolone use and subsequent emergence of multiple drug‐resistant bacteria in the intensive care unit.Crit Care Med.2005;33(2):283–289.
- ,.Etiology of community‐acquired pneumonia.Clin Chest Med.2005;26:47–55.
- .Community‐associated methicillin‐resistant Staphylococcus aureus: not only a cause of skin infections, also a new cause of pneumonia.Curr Opin Infect Dis.2005;18:123–124.
- ,,, et al.Severe community‐onset pneumonia in healthy adults caused by methicillin‐resistant Staphylococcus aureus carrying the Panton‐Valentine leukocidin genes.Clin Infect Dis.2005;40(1):100–107.
- ,,,.Fatal community‐associated methicillin‐resistant Staphylococcus aureus pneumonia in an immunocompetent young adult.Ann Emerg Med.2005;46:401–404.
- .Aspiration pneumonitis and aspiration pneumonia.N Engl J Med.2001;344:665–671.
- ,,, et al.Health care‐associated bloodstream infections in adults: a reason to change the accepted definition of community‐acquired infections.Ann Intern Med.2002;137:791–797.
- American Thoracic Society and theInfectious Diseases Society of America.Guidelines for the management of adults with hospital‐acquired, ventilator‐acquired, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,, et al.Blood culture and susceptibility results and allergy history do not influence fluoroquinolone use in the treatment of community‐acquired pneumonia.Pharmacotherapy.2005;25(1):59–66.
- ,,, et al.Time to clinical stability in patients hospitalized with community‐acquired pneumonia: implications for practice guidelines.JAMA.1998;279:1452–1457.
- ,,, et al.High‐dose, short‐course levofloxacin for community‐acquired pneumonia: a new treatment paradigm.Clin Infect Dis.2003;37:752–760.
- ,,, et al.Causes and factors associated with early failure in hospitalized patients with community‐acquired pneumonia.Arch Intern Med.2004;164:502–508.
- ,,, et al.Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia.Arch Intern Med.2002;162:1278–1284.
- ,,,.Radiographic resolution of community‐acquired bacterial pneumonia in the elderly.J Am Geriatr Soc.2004;52(2):224–229.
- ,,,.Vaccines for preventing pneumococcal infection in adults.Cochrane Database Syst Rev.2003;4:CD000422.
- ,,,,.The efficacy of influenza vaccine in elderly persons: a meta‐analysis and review of the literature.Ann Intern Med.1995;123:518–527.
- ,,, et al.Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly.N Engl J Med.2003;348:1322–1332.
- ,,,.Proportion of community‐acquired pneumonia cases attributable to tobacco smoking.Chest.1999;116:375–379.
- ,,, et al.Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs.JAMA.2004;292:1955–1960.
- ,,,,,.Guidelines from the Infectious Disease Society of America. Practice guidelines for the management of community‐acquired pneumonia.Clin Infect Dis.2000;31:347–382.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,, et al.Pneumonia: still the old man's friend?Arch Intern Med.2003;163:317–323.
- ,,, et al.New and emerging etiologies for community‐acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases.Medicine.1990;69:307–316.
- .Community‐acquired pneumonia.Lancet.2003;362:1991–2001.
- ,,, et al.Processes and outcomes of care for patients with community‐acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team (PORT) cohort study.Arch Intern Med.1999;159:970–980.
- ,.Management of community‐acquired pneumonia.N Engl J Med.2002;347:2039–2045.
- ,,, et al.Measuring symptomatic and functional recovery in patients with community‐acquired pneumonia.J Gen Intern Med.1997;12:423–430.
- ,,, et al.Influence of age on symptoms at presentation with patients with community‐acquired pneumonia.Arch Intern Med.1997;157:1453–1459.
- ,,.Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination.JAMA.1997;278:1440–1445.
- ,,, et al.Community‐acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes.Medicine.2003;82:159–169.
- ,.Testing strategies in the initial management of patients with community‐acquired pneumonia.Ann Intern Med.2003;138:109–118.
- ,.Uncomplicated acute bronchitis.Ann Intern Med.2000;133:981–991.
- ,,, et al.American Thoracic Society: Guidelines for the management of community‐acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,, et al.British Thoracic Society guidelines for the management of community acquired pneumonia in adults.Thorax.2001;56:Suppl. 4,IV1–IV64.
- ,,,,.High‐resolution computed tomography for the diagnosis of community‐acquired pneumonia.Clin Infect Dis.1998;27:358–363.
- ,,, et al.Performance of a bedside C‐reactive protein test in the diagnosis of community‐acquired pneumonia in adults with acute cough.Am J Med.2004;116:529–535.
- ,,, et al.Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial.Lancet.2004;363:600–607.
- ,,, et al.Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia.N Engl J Med.2004:350:451–458.
- ,,, et al.Quality of care, process, and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- ,,,.Limited usefulness of initial blood cultures in community acquired pneumonia.Emerg Med J.2004;21:446–448.
- ,,.Contaminant blood cultures and resource utilization. The true consequences of false‐positive results.JAMA.1991;265:365–369.
- ,,, et al.The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study.Chest.2003;123:1142–1150.
- ,,, et al.Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks.Chest.1995;108:932–936.
- ,,,.Predicting bacteremia in patients with community‐acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- British Thoracic Society. BTS Guidelines for the management of community acquired pneumonia in adults—2004 update. Available at: www.brit‐thoracic.org/guidelines.
- ,,,,,.Guidelines from the Infectious Disease Society of America. Update of guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433.
- ,,, et al.Assessment of the usefulness of sputum culture for diagnosis of community‐acquired pneumonia using the PORT predictive scoring system.Arch Intern Med.2004;164:1807–1811.
- ,,.Diagnostic value of microscopic examination of gram‐stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia.Clin Infect Dis.2004;39(2):165–169.
- ,,, et al.Safely increasing the proportion of patients with community‐acquired pneumonia treated as outpatients: an interventional trial.Arch Intern Med.1998;158:1350–1356.
- ,,, et al.A critical pathway for treatment of community‐acquired pneumonia.JAMA.2000;283:2654–2655.
- ,,, et al.Outpatient care compared with hospitalization for community‐acquired pneumonia. A randomized control trial in low‐risk patients.Ann Intern Med.2005;142:165–172.
- ,,.Management of community‐acquired pneumonia in the home.Chest.2005;127:1752–1763.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159:2562–2572.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,, et al.Increasing prevalence of multidrug‐resistant Streptococcus pneumoniae in the United States.N Engl J Med.2000;343:1917–1924.
- ,,, et al.Susceptibility patterns of Streptococcus pneumoniae isolates in North America (2002–2003): contemporary in vitro activities of amoxicillin/clavulanate and 15 other antimicrobial agents.Int J Antimicrob Agents.2005;25(4):282–289.
- ,,, et al.Antimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes?Clin Infect Dis.2005;41(2):139–148.
- ,,, et al.Pneumonia acquired in the community through drug‐resistant Streptococcus pneumoniae.Am J Respir Crit Care.1999;159:1835–1842.
- ,,, et al.Drug‐resistant pneumococcal pneumonia: clinical relevance and related factors.Clin Infect Dis.2004;38:787–798.
- ,,, et al.Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997.Am J Public Health.2000;90(2):223–9.
- ,,, et al.Lower mortality among patients with community‐acquired pneumonia treated with a macrolide plus a beta‐lactam agent versus a beta‐lactam alone.Eur J Clin Microbiol Infect Dis2005;24:190–195.
- ,,,.Impact of initial antibiotic choice on clinical outcomes in community‐acquired pneumonia: analysis of a hospital claims‐made database.Chest.2003;123:1503–1511.
- ,,,.Antimicrobial selection for hospitalized patients with presumed community‐acquired pneumonia: a survey of nonteaching US community hospitals.Ann Pharmacother2000;34:446–452.
- ,,,,.J Hosp Med.2006;1:7–12.
- .Anti‐inflammatory effects of macrolides—an underappreciated benefit in the treatment of community‐acquired respiratory tract infections and chronic inflammatory pulmonary conditions?J Antimicrob Chemother.2005;55:10–21.
- ,,, et al.Addition of a macrolide to a beta‐lactam based empirical antibiotic regimen is associated with lover in‐hospital mortality for patients with bacteremic pneumococcal pneumonia.Clin Infect Dis.2003;36:389–395.
- ,,, et al.Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia.Am J Respir Crit Care Med.2004;170:440–444.
- ,,,.Empiric antibiotic coverage of atypical pathogens for community‐acquired pneumonia in hospitalized adults.Cochrane Database Syst Rev.2005;2:CD004418.pub2.
- ,,.Effectiveness of β lactam antibiotics compared with antibiotics active against atypical pathogens in non‐severe community‐acquired pneumonia: meta‐analysis.Br Med J.2005;330:456.
- ,,,.Empirical atypical coverage for inpatients with community‐acquired pneumonia.Arch Intern Med.2005;165:1992–2000.
- ,,, et al.Antibiotic resistance among gram‐negative bacilli in US intensive care units: implications for fluoroquinolone use.JAMA.2003;289:885–888.
- ,,, et al.First‐generation fluoroquinolone use and subsequent emergence of multiple drug‐resistant bacteria in the intensive care unit.Crit Care Med.2005;33(2):283–289.
- ,.Etiology of community‐acquired pneumonia.Clin Chest Med.2005;26:47–55.
- .Community‐associated methicillin‐resistant Staphylococcus aureus: not only a cause of skin infections, also a new cause of pneumonia.Curr Opin Infect Dis.2005;18:123–124.
- ,,, et al.Severe community‐onset pneumonia in healthy adults caused by methicillin‐resistant Staphylococcus aureus carrying the Panton‐Valentine leukocidin genes.Clin Infect Dis.2005;40(1):100–107.
- ,,,.Fatal community‐associated methicillin‐resistant Staphylococcus aureus pneumonia in an immunocompetent young adult.Ann Emerg Med.2005;46:401–404.
- .Aspiration pneumonitis and aspiration pneumonia.N Engl J Med.2001;344:665–671.
- ,,, et al.Health care‐associated bloodstream infections in adults: a reason to change the accepted definition of community‐acquired infections.Ann Intern Med.2002;137:791–797.
- American Thoracic Society and theInfectious Diseases Society of America.Guidelines for the management of adults with hospital‐acquired, ventilator‐acquired, and healthcare‐associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,, et al.Blood culture and susceptibility results and allergy history do not influence fluoroquinolone use in the treatment of community‐acquired pneumonia.Pharmacotherapy.2005;25(1):59–66.
- ,,, et al.Time to clinical stability in patients hospitalized with community‐acquired pneumonia: implications for practice guidelines.JAMA.1998;279:1452–1457.
- ,,, et al.High‐dose, short‐course levofloxacin for community‐acquired pneumonia: a new treatment paradigm.Clin Infect Dis.2003;37:752–760.
- ,,, et al.Causes and factors associated with early failure in hospitalized patients with community‐acquired pneumonia.Arch Intern Med.2004;164:502–508.
- ,,, et al.Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia.Arch Intern Med.2002;162:1278–1284.
- ,,,.Radiographic resolution of community‐acquired bacterial pneumonia in the elderly.J Am Geriatr Soc.2004;52(2):224–229.
- ,,,.Vaccines for preventing pneumococcal infection in adults.Cochrane Database Syst Rev.2003;4:CD000422.
- ,,,,.The efficacy of influenza vaccine in elderly persons: a meta‐analysis and review of the literature.Ann Intern Med.1995;123:518–527.
- ,,, et al.Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly.N Engl J Med.2003;348:1322–1332.
- ,,,.Proportion of community‐acquired pneumonia cases attributable to tobacco smoking.Chest.1999;116:375–379.
- ,,, et al.Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs.JAMA.2004;292:1955–1960.
Treatment of Community‐Acquired Pneumonia
In the United States, community‐acquired pneumonia (CAP) leads to nearly 1 million hospitalizations annually, with aggregate costs of hospitalization approaching $9 billion.1, 2 In an effort to improve the appropriate, cost‐effective care for patients with CAP, several professional societies have developed clinical practice guidelines and pathways for pneumonia.37 Although the guidelines address all aspects of care, they devote substantial attention to antibiotic recommendations. Most U.S. guidelines recommend treatment of hospitalized patients with an intravenous beta‐lactam combined with a macrolide, or a fluoroquinolone with activity against Streptococcus pneumoniae. Of the major U.S. CAP practice guidelines, only one6 recommends doxycycline as an alternative to a macrolide for inpatients.
Doxycycline is an attractive alternative to macrolides. Similar to macrolides, doxycycline is active against a wide variety of organisms including atypical bacteria (Chlamydia pneumoniae, Legionella pneumophilus, and Mycoplasma pneumoniae) and is well tolerated.810 In addition, it is inexpensive (cost of $1.00/day [awp] for 100 mg p.o. bid), and rates of tetracycline/doxycycline resistance among S. pneumoniae isolates have remained low, in contrast to the increasing rates of resistance to macrolides and fluoroquinolones.11, 12 The most recent guidelines from the Infectious Diseases Society of America cited limited published clinical data on the effectiveness of doxycycline in CAP as a barrier to increased use.7 Only one study of hospitalized patients has been published in the era of penicillin‐resistant pneumococcus, and this study included only 43 low‐risk patients treated with doxycycline.13 At the university hospital affiliated with the University of California, San Francisco, ceftriaxone plus doxycycline is generally recommended as initial empiric antibiotic therapy for patients hospitalized with CAP, but significant variability in prescribing exists, allowing for comparisons between patients treated with different initial empiric antibiotic regimens. We compared outcomes of hospitalized patients with CAP treated with ceftriaxone plus doxycycline to those of patients treated with alternative initial empiric therapy at an academic medical center.
METHODS
Study Population
A retrospective cohort study of all adults (age 18 years) discharged from the inpatient general medicine service of Moffitt‐Long Hospital at the University of California, San Francisco, was conducted from January 1999 through July 2001. Eligibility criteria included a principal discharge diagnosis of CAP and a chest radiograph demonstrating an infiltrate within 48 hours of admission. Exclusion criteria included infection with the human immunodeficiency virus, history of organ transplantation or use of immunosuppressive therapy (including prednisone > 15 mg/day), cystic fibrosis, postobstructive pneumonia, active tuberculosis, recent hospitalization (within 10 days), or admission for comfort care. The study protocol and procedures were reviewed and approved by the UCSF Committee for Human Research.
Data Collection
Medical record review by trained research assistants blinded to the research question was used to gather demographic data, comorbid illnesses, physical examination findings on initial presentation, and laboratory or radiographic results on initial presentation. The pneumonia severity index (PSI) score was calculated for each patient using the above data.14 In addition, data were collected on antibiotic allergies, antibiotics used within the 30 days prior to admission, results of sputum or blood cultures, and admission location (intensive care unit [ICU] versus medical floor).
Data from TSI (Transition Systems Inc., Boston, MA), the hospital administrative database, were used to identify the initial empiric antibiotic regimen. All antibiotics prescribed within the first 48 hours of hospitalization were considered initial empiric therapy with few exceptions. Initial empiric therapy was classified as 1) ceftriaxone plus doxycycline (including patients treated with these agents alone in the first 48 hours, as well as patients treated with both agents in the first 24 hours who were switched to alternative therapy [broader coverage] on the second day), or 2) other appropriate therapy (treatment consistent with current national guideline recommendations including at least a beta‐lactam plus a macrolide or a beta‐lactam plus a fluoroquinolone, or fluoroquinolone monotherapy). Patients receiving therapy inconsistent with current national guideline recommendations were excluded.
Outcomes
TSI data were used to identify length of stay, death during the index hospitalization, and return to the emergency department or readmission within 30 days of discharge. The National Death Index was used to identify all deaths that occurred after hospital discharge. The 30‐day mortality data included deaths occurring during the index hospitalization and in the 30 days after the index hospitalization discharge.
Statistical Analysis
For the purposes of this analysis we compared patients treated with ceftriaxone plus doxycycline to patients treated with other appropriate therapy. To examine demographic and clinical differences between the two groups, statistical tests of comparison were performed using chi‐square tests for the dichotomous variables and t tests for the numeric variables, all of which were normally distributed (after log transformation in the case of length of stay).
To adjust for clinical variables that might contribute to differences in outcomes between the two groups, we used backward stepwise logistic regression analysis to construct a propensity score15 for the likelihood of ceftriaxone plus doxycycline use. The propensity score reflected the conditional probability of exposure to ceftriaxone plus doxycycline and allowed for stratification and, subsequently, comparisons by quintiles of propensity score. Propensity scores often have distinct advantages over direct adjustment for a large number of confounding variables and allow direct comparisons between groups with a similar propensity for receiving ceftriaxone plus doxycycline.15 Unlike random assignment of treatment, however, the propensity score cannot balance unmeasured variables that may affect treatment assignment. Thus, the possibility of bias remains. The variables used to build the score included age, presence of comorbid illness, admission from a nursing home or long‐term care facility, antibiotic allergy, prior antibiotic use, PSI score, PSI risk class, diagnosis of aspiration, admission to the ICU, and positive blood cultures. The propensity score was then stratified and used as an adjustment variable in comparisons between groups for in‐hospital mortality, 30‐day mortality, and 30‐day readmission rates. As expected, length of stay was highly skewed and was therefore log‐transformed and compared between groups with adjustment for the propensity score.
To further address issues related to potential selection bias, a separate analysis was performed on a subset of the original cohort that excluded patients for whom ceftriaxone plus doxycycline would not generally be recommended as first‐line therapy. For this analysis, patients admitted from a nursing home or long‐term care facility, patients admitted to the ICU, and patients with a principal diagnosis of aspiration pneumonia were excluded. A propensity score was rederived for this subset, which was used to adjust for differences in outcomes. All statistical procedures were performed using STATA (Ver. 7.0, Stata Corporation, College Station TX).
RESULTS
Patient Characteristics
A total of 341 patients were eligible for analysis. Of this group, 216 were treated with ceftriaxone plus doxycycline and 125 received other appropriate therapy. Both groups of patients were similar in age. Patients treated with ceftriaxone plus doxycycline had a lower median PSI score and fewer comorbid illnesses than did patients treated with other appropriate therapy (Table 1). Blood cultures were positive in 30 (8.8%) of the 341 patients included in the analysis, with S. pneumoniae the most commonly isolated organism (n = 17, 5.0%). Of S. pneumoniae isolates, 4 (24%) were resistant to penicillin (MIC 1 g/mL), and 2 (12%) were resistant to tetracycline (MIC 8 g/mL).
| Ceftriaxone/doxycycline | Other appropriate therapy | |
|---|---|---|
| ||
| Patients (n) | 216 | 125 |
| Age (median) | 76 | 74 |
| PSI Score (median)a | 97 | 108 |
| PSI Risk Class (%)a | ||
| Class I | 9.3 | 5.6 |
| Class II | 11.1 | 8.8 |
| Class III | 21.8 | 13.6 |
| Class IV | 40.7 | 40.0 |
| Class V | 17.1 | 32.0 |
| Comorbid Illness (%)a | 36.1 | 47.2 |
| Nursing Home/LCF (%)a | 5.1 | 14.4 |
| Aspiration (%)a | 3.2 | 20.0 |
| Admission to ICU (%)a | 6.0 | 28.0 |
Common antibiotic choices in patients receiving other appropriate therapy included a beta‐lactam/beta‐lactamase inhibitor plus doxycycline or a macrolide (n = 36, 29%), fluoroquinolone monotherapy (n = 16, 13%), and a variety of other antibiotic combinations with activity against S. pneumoniae and atypical bacteria (n = 52, 42%).
Clinical Outcomes
Analyses of unadjusted outcomes showed that patients treated with ceftriaxone plus doxycycline had significantly lower inpatient (2% vs. 14%, P < .001) and 30‐day (6% vs. 20%, P < .001) mortality compared to patients treated with other regimens (Table 2). Multivariable logistic regression analysis identified three variables (diagnosis of congestive heart failure, admission to the ICU, and the presence of comorbid illness) associated with initial antibiotic selection, which were used to build a propensity score. After adjustment for the propensity score, use of ceftriaxone plus doxycycline remained significantly associated with lower inpatient mortality (OR = 0.26, 95% CI: 0.080.81) and 30‐day mortality (OR = 0.37, 95% CI: 0.170.81). Differences in length of stay and 30‐day readmission rates between the treatment groups were not significant (Table 2).
| Ceftriaxone + doxycycline (n = 216) | Other appropriate therapy (n = 125) | Adjusted odds ratio (95% confidence interval) | |
|---|---|---|---|
| |||
| Inpatient Mortality | 2.3% | 14.4% | 0.26 (0.080.81) |
| 30‐day mortality | 6.0% | 20.0% | 0.37 (0.170.81) |
| Length of stay (median days) | 3.0 | 4.0 | 0.09 (0.250.06)a |
| 30‐day readmission | 10.7% | 12.0% | 0.87 (0.421.81) |
Subset Analysis
To address issues related to selection bias, we performed an analysis of a subset of the patients after excluding those admitted from a nursing home, diagnosed with aspiration, or admitted to the ICU, for whom ceftriaxone plus doxycycline would not be considered recommended (or first‐line) therapy. The two resulting groups were similar, except there were fewer patients with comorbid illness in the ceftriaxone plus doxycycline group (34% vs. 50%, P = .015). The propensity score was rederived for this subset and used for adjustment. Unadjusted and adjusted outcomes are shown in Table 3. Use of ceftriaxone plus doxycycline in this subset also was associated with reduced odds of inpatient mortality (OR = 0.17, 95% CI: 0.040.77). The odds of 30‐day mortality also were reduced but not significantly, as the confidence interval included 1.0 (OR = 0.43, 95% CI: 0.141.31). There were no differences between groups in length of stay or in 30‐day readmission rate.
| Ceftriaxone + doxycycline (n = 188) | Other appropriate therapy (n = 70) | Adjusted odds ratio (95% CI) | |
|---|---|---|---|
| |||
| Age (median years) | 75 | 71 | |
| PSI score (mean) | 95 | 98 | |
| Comorbid illness (%)a | 33.5 | 50.0 | |
| Inpatient mortality | 1.6% | 7.1% | 0.17 (0.040.77) |
| 30‐day mortality | 4.8% | 8.6% | 0.43 (0.141.31) |
| LOS (median days) | 3 | 3 | 0.06 (0.240.12)b |
| 30‐day readmission | 11.9% | 10.0% | 1.31 (0.523.28) |
DISCUSSION
In our hospital setting, the use of ceftriaxone plus doxycycline as the initial empiric antibiotic therapy for patients hospitalized with community‐acquired pneumonia was associated with significantly lower inpatient and 30‐day mortality, even after adjusting for clinical differences between groups. We did not find a difference between regimens in hospital length of stay or 30‐day readmission rate. In case the multivariable model was insufficient to account for the clinical differences (i.e., selection bias) between groups, we also performed an analysis of a subgroup of less severely ill patients by excluding those admitted from nursing homes, those admitted to the intensive care unit, and those with aspiration pneumonia. In this subset, use of ceftriaxone plus doxycycline remained associated with lower inpatient mortality but not with lower 30‐day mortality. Although, as an observational study, the results of our findings could still be a result of residual confounding, we believe the results provide valuable information regarding doxycycline.
Combination therapy with a macrolide, but not doxycycline, is advocated by the practice guidelines of several major U.S. professional societies,3, 4, 7 apparently because of a lack of data on the effectiveness of combination therapy with doxycycline.7 Only one randomized, unblinded study, in 87 low‐risk patients hospitalized with CAP, that compared monotherapy with IV doxycycline versus physician‐determined therapy has been conducted.13 This study found no differences between treatment groups in clinical outcomes but did find that use of doxycycline was associated with shorter hospital stays and reduced costs. Our results, achieved in a real‐world setting in relatively ill hospitalized patients (58% were in PSI risk class IV or V), provide further support for the use of combination therapy with doxycycline.
Hospitalized patients treated with a beta‐lactam in combination with a macrolide are often discharged on macrolide monotherapy. In our population most patients treated with ceftriaxone plus doxycycline were discharged on doxycycline if they required continued therapy (data not shown). In the current era of resistance of Streptococcus pneumoniae to antibiotics, there is good reason to believe doxycycline may perform as well, if not better, than macrolides when hospitalized patients with CAP are discharged on oral monotherapy. Macrolide resistance rates among invasive pneumococcal isolates in the United States doubled from 10% to 20% during a period in which prescriptions for macrolides increased by 13%.12 In addition, a large surveillance study of more than 1500 isolates collected in 1999 and 2000 found that 26% of the isolates were resistant to macrolides, whereas only 16% were resistant to tetracycline.16 In vitro testing against S. Pneumoniae has also suggested that tetracycline resistance overestimates doxycycline resistance.17, 18 More recently, Streptococcus pneumoniae susceptibility data from the SENTRY Antimicrobial Surveillance program reaffirmed doxycycline's in vitro superiority over macrolides.17
Our study had several limitations. The study design adopted precluded determining whether favorable results with the use of ceftriaxone plus doxycycline resulted from an effect unique to this combination of antibiotics, the possible anti‐inflammatory properties of doxycycline alone,19, 20 or unmeasured confounders. For example, processes of care that affect clinical outcomes for patients hospitalized with CAP, such as the timing of antibiotic delivery, the timing of blood cultures, and stability assessment on discharge were not measured in this study. To affect outcomes, these processes of care would need to be differentially distributed between our comparison groups. However, because this study was performed in a single institution during a single interval, it is likely that the performance of these processes of care would be similar for all patients.
In conclusion, ceftriaxone plus doxycycline appears to be an effective, and possibly superior, therapy for patients hospitalized with CAP. Randomized controlled trials of doxycycline‐containing regimens versus other regimens are warranted.
- ,,, et al.Hospitalized pneumonia. Outcomes, treatment patterns, and costs in urban and rural areas.J Gen Intern Med.1996;11:415–421.
- ,,, et al.The cost of treating community‐acquired pneumonia.Clin Ther.1998;20:820–837.
- ,,, et al.Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious Diseases Society of America.Clin Infect Dis.2000;31:347–382.
- ,,, et al.Management of community‐acquired pneumonia in the era of pneumococcal resistance: a report from the Drug‐Resistant Streptococcus pneumoniae Therapeutic Working Group.Arch Intern Med.2000;160:1399–1408.
- ,,, et al.Canadian guidelines for the initial management of community‐acquired pneumonia: an evidence‐based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Canadian Community‐Acquired Pneumonia Working Group.Clin Infect Dis.2000;31:383–421.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,, et al.Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433
- ,,.Doxycycline.Ther Drug Monit1982;4:115–135
- ,.Chloramphenicol and tetracyclines.Med Clin North Am.1987;71:1155–1168
- ,.Tetracyclines.Med Clin North Am.1995;79:789–801
- ,,, et al.Antibiotic resistance among gram‐negative bacilli in US intensive care units: implications for fluoroquinolone use.JAMA.2003;289:885–888
- ,,, et al.Macrolide resistance among invasive Streptococcus pneumoniae isolates.JAMA.2001;286:1857–1862.
- ,,, et al.Doxycycline is a cost‐effective therapy for hospitalized patients with community‐acquired pneumonia.Arch Intern Med.1999;159:266–270.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- .Reducing bias in observational studies using subclassification on the propensity score.J Am Stat Assoc.1984;79:516–524
- ,,, et al.Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999—2000, including a comparison of resistance rates since 1994—1995.Antimicrob Agents Chemother.2001;45:1721–1729
- ,,.Doxycycline use for community‐acquired pneumonia: contemporary in vitro spectrum of activity against Streptococcus pneumoniae (1999–2002).Diagn Microbiol Infect Dis.2004;49:147–149
- ,,, et al.Doxycycline activity against Streptococcus pneumoniae.Chest.1995;108:1775–1776.
- ,,, et al.Inhibition of enzymatic activity of phospholipases A2 by minocycline and doxycycline.Biochem Pharmacol.1992;44:1165–1170.
- ,,, et al.Doxycycline reduces mortality to lethal endotoxemia by reducing nitric oxide synthesis via an interleukin‐10‐independent mechanism.J Infect Dis.1998;177:489–492.
In the United States, community‐acquired pneumonia (CAP) leads to nearly 1 million hospitalizations annually, with aggregate costs of hospitalization approaching $9 billion.1, 2 In an effort to improve the appropriate, cost‐effective care for patients with CAP, several professional societies have developed clinical practice guidelines and pathways for pneumonia.37 Although the guidelines address all aspects of care, they devote substantial attention to antibiotic recommendations. Most U.S. guidelines recommend treatment of hospitalized patients with an intravenous beta‐lactam combined with a macrolide, or a fluoroquinolone with activity against Streptococcus pneumoniae. Of the major U.S. CAP practice guidelines, only one6 recommends doxycycline as an alternative to a macrolide for inpatients.
Doxycycline is an attractive alternative to macrolides. Similar to macrolides, doxycycline is active against a wide variety of organisms including atypical bacteria (Chlamydia pneumoniae, Legionella pneumophilus, and Mycoplasma pneumoniae) and is well tolerated.810 In addition, it is inexpensive (cost of $1.00/day [awp] for 100 mg p.o. bid), and rates of tetracycline/doxycycline resistance among S. pneumoniae isolates have remained low, in contrast to the increasing rates of resistance to macrolides and fluoroquinolones.11, 12 The most recent guidelines from the Infectious Diseases Society of America cited limited published clinical data on the effectiveness of doxycycline in CAP as a barrier to increased use.7 Only one study of hospitalized patients has been published in the era of penicillin‐resistant pneumococcus, and this study included only 43 low‐risk patients treated with doxycycline.13 At the university hospital affiliated with the University of California, San Francisco, ceftriaxone plus doxycycline is generally recommended as initial empiric antibiotic therapy for patients hospitalized with CAP, but significant variability in prescribing exists, allowing for comparisons between patients treated with different initial empiric antibiotic regimens. We compared outcomes of hospitalized patients with CAP treated with ceftriaxone plus doxycycline to those of patients treated with alternative initial empiric therapy at an academic medical center.
METHODS
Study Population
A retrospective cohort study of all adults (age 18 years) discharged from the inpatient general medicine service of Moffitt‐Long Hospital at the University of California, San Francisco, was conducted from January 1999 through July 2001. Eligibility criteria included a principal discharge diagnosis of CAP and a chest radiograph demonstrating an infiltrate within 48 hours of admission. Exclusion criteria included infection with the human immunodeficiency virus, history of organ transplantation or use of immunosuppressive therapy (including prednisone > 15 mg/day), cystic fibrosis, postobstructive pneumonia, active tuberculosis, recent hospitalization (within 10 days), or admission for comfort care. The study protocol and procedures were reviewed and approved by the UCSF Committee for Human Research.
Data Collection
Medical record review by trained research assistants blinded to the research question was used to gather demographic data, comorbid illnesses, physical examination findings on initial presentation, and laboratory or radiographic results on initial presentation. The pneumonia severity index (PSI) score was calculated for each patient using the above data.14 In addition, data were collected on antibiotic allergies, antibiotics used within the 30 days prior to admission, results of sputum or blood cultures, and admission location (intensive care unit [ICU] versus medical floor).
Data from TSI (Transition Systems Inc., Boston, MA), the hospital administrative database, were used to identify the initial empiric antibiotic regimen. All antibiotics prescribed within the first 48 hours of hospitalization were considered initial empiric therapy with few exceptions. Initial empiric therapy was classified as 1) ceftriaxone plus doxycycline (including patients treated with these agents alone in the first 48 hours, as well as patients treated with both agents in the first 24 hours who were switched to alternative therapy [broader coverage] on the second day), or 2) other appropriate therapy (treatment consistent with current national guideline recommendations including at least a beta‐lactam plus a macrolide or a beta‐lactam plus a fluoroquinolone, or fluoroquinolone monotherapy). Patients receiving therapy inconsistent with current national guideline recommendations were excluded.
Outcomes
TSI data were used to identify length of stay, death during the index hospitalization, and return to the emergency department or readmission within 30 days of discharge. The National Death Index was used to identify all deaths that occurred after hospital discharge. The 30‐day mortality data included deaths occurring during the index hospitalization and in the 30 days after the index hospitalization discharge.
Statistical Analysis
For the purposes of this analysis we compared patients treated with ceftriaxone plus doxycycline to patients treated with other appropriate therapy. To examine demographic and clinical differences between the two groups, statistical tests of comparison were performed using chi‐square tests for the dichotomous variables and t tests for the numeric variables, all of which were normally distributed (after log transformation in the case of length of stay).
To adjust for clinical variables that might contribute to differences in outcomes between the two groups, we used backward stepwise logistic regression analysis to construct a propensity score15 for the likelihood of ceftriaxone plus doxycycline use. The propensity score reflected the conditional probability of exposure to ceftriaxone plus doxycycline and allowed for stratification and, subsequently, comparisons by quintiles of propensity score. Propensity scores often have distinct advantages over direct adjustment for a large number of confounding variables and allow direct comparisons between groups with a similar propensity for receiving ceftriaxone plus doxycycline.15 Unlike random assignment of treatment, however, the propensity score cannot balance unmeasured variables that may affect treatment assignment. Thus, the possibility of bias remains. The variables used to build the score included age, presence of comorbid illness, admission from a nursing home or long‐term care facility, antibiotic allergy, prior antibiotic use, PSI score, PSI risk class, diagnosis of aspiration, admission to the ICU, and positive blood cultures. The propensity score was then stratified and used as an adjustment variable in comparisons between groups for in‐hospital mortality, 30‐day mortality, and 30‐day readmission rates. As expected, length of stay was highly skewed and was therefore log‐transformed and compared between groups with adjustment for the propensity score.
To further address issues related to potential selection bias, a separate analysis was performed on a subset of the original cohort that excluded patients for whom ceftriaxone plus doxycycline would not generally be recommended as first‐line therapy. For this analysis, patients admitted from a nursing home or long‐term care facility, patients admitted to the ICU, and patients with a principal diagnosis of aspiration pneumonia were excluded. A propensity score was rederived for this subset, which was used to adjust for differences in outcomes. All statistical procedures were performed using STATA (Ver. 7.0, Stata Corporation, College Station TX).
RESULTS
Patient Characteristics
A total of 341 patients were eligible for analysis. Of this group, 216 were treated with ceftriaxone plus doxycycline and 125 received other appropriate therapy. Both groups of patients were similar in age. Patients treated with ceftriaxone plus doxycycline had a lower median PSI score and fewer comorbid illnesses than did patients treated with other appropriate therapy (Table 1). Blood cultures were positive in 30 (8.8%) of the 341 patients included in the analysis, with S. pneumoniae the most commonly isolated organism (n = 17, 5.0%). Of S. pneumoniae isolates, 4 (24%) were resistant to penicillin (MIC 1 g/mL), and 2 (12%) were resistant to tetracycline (MIC 8 g/mL).
| Ceftriaxone/doxycycline | Other appropriate therapy | |
|---|---|---|
| ||
| Patients (n) | 216 | 125 |
| Age (median) | 76 | 74 |
| PSI Score (median)a | 97 | 108 |
| PSI Risk Class (%)a | ||
| Class I | 9.3 | 5.6 |
| Class II | 11.1 | 8.8 |
| Class III | 21.8 | 13.6 |
| Class IV | 40.7 | 40.0 |
| Class V | 17.1 | 32.0 |
| Comorbid Illness (%)a | 36.1 | 47.2 |
| Nursing Home/LCF (%)a | 5.1 | 14.4 |
| Aspiration (%)a | 3.2 | 20.0 |
| Admission to ICU (%)a | 6.0 | 28.0 |
Common antibiotic choices in patients receiving other appropriate therapy included a beta‐lactam/beta‐lactamase inhibitor plus doxycycline or a macrolide (n = 36, 29%), fluoroquinolone monotherapy (n = 16, 13%), and a variety of other antibiotic combinations with activity against S. pneumoniae and atypical bacteria (n = 52, 42%).
Clinical Outcomes
Analyses of unadjusted outcomes showed that patients treated with ceftriaxone plus doxycycline had significantly lower inpatient (2% vs. 14%, P < .001) and 30‐day (6% vs. 20%, P < .001) mortality compared to patients treated with other regimens (Table 2). Multivariable logistic regression analysis identified three variables (diagnosis of congestive heart failure, admission to the ICU, and the presence of comorbid illness) associated with initial antibiotic selection, which were used to build a propensity score. After adjustment for the propensity score, use of ceftriaxone plus doxycycline remained significantly associated with lower inpatient mortality (OR = 0.26, 95% CI: 0.080.81) and 30‐day mortality (OR = 0.37, 95% CI: 0.170.81). Differences in length of stay and 30‐day readmission rates between the treatment groups were not significant (Table 2).
| Ceftriaxone + doxycycline (n = 216) | Other appropriate therapy (n = 125) | Adjusted odds ratio (95% confidence interval) | |
|---|---|---|---|
| |||
| Inpatient Mortality | 2.3% | 14.4% | 0.26 (0.080.81) |
| 30‐day mortality | 6.0% | 20.0% | 0.37 (0.170.81) |
| Length of stay (median days) | 3.0 | 4.0 | 0.09 (0.250.06)a |
| 30‐day readmission | 10.7% | 12.0% | 0.87 (0.421.81) |
Subset Analysis
To address issues related to selection bias, we performed an analysis of a subset of the patients after excluding those admitted from a nursing home, diagnosed with aspiration, or admitted to the ICU, for whom ceftriaxone plus doxycycline would not be considered recommended (or first‐line) therapy. The two resulting groups were similar, except there were fewer patients with comorbid illness in the ceftriaxone plus doxycycline group (34% vs. 50%, P = .015). The propensity score was rederived for this subset and used for adjustment. Unadjusted and adjusted outcomes are shown in Table 3. Use of ceftriaxone plus doxycycline in this subset also was associated with reduced odds of inpatient mortality (OR = 0.17, 95% CI: 0.040.77). The odds of 30‐day mortality also were reduced but not significantly, as the confidence interval included 1.0 (OR = 0.43, 95% CI: 0.141.31). There were no differences between groups in length of stay or in 30‐day readmission rate.
| Ceftriaxone + doxycycline (n = 188) | Other appropriate therapy (n = 70) | Adjusted odds ratio (95% CI) | |
|---|---|---|---|
| |||
| Age (median years) | 75 | 71 | |
| PSI score (mean) | 95 | 98 | |
| Comorbid illness (%)a | 33.5 | 50.0 | |
| Inpatient mortality | 1.6% | 7.1% | 0.17 (0.040.77) |
| 30‐day mortality | 4.8% | 8.6% | 0.43 (0.141.31) |
| LOS (median days) | 3 | 3 | 0.06 (0.240.12)b |
| 30‐day readmission | 11.9% | 10.0% | 1.31 (0.523.28) |
DISCUSSION
In our hospital setting, the use of ceftriaxone plus doxycycline as the initial empiric antibiotic therapy for patients hospitalized with community‐acquired pneumonia was associated with significantly lower inpatient and 30‐day mortality, even after adjusting for clinical differences between groups. We did not find a difference between regimens in hospital length of stay or 30‐day readmission rate. In case the multivariable model was insufficient to account for the clinical differences (i.e., selection bias) between groups, we also performed an analysis of a subgroup of less severely ill patients by excluding those admitted from nursing homes, those admitted to the intensive care unit, and those with aspiration pneumonia. In this subset, use of ceftriaxone plus doxycycline remained associated with lower inpatient mortality but not with lower 30‐day mortality. Although, as an observational study, the results of our findings could still be a result of residual confounding, we believe the results provide valuable information regarding doxycycline.
Combination therapy with a macrolide, but not doxycycline, is advocated by the practice guidelines of several major U.S. professional societies,3, 4, 7 apparently because of a lack of data on the effectiveness of combination therapy with doxycycline.7 Only one randomized, unblinded study, in 87 low‐risk patients hospitalized with CAP, that compared monotherapy with IV doxycycline versus physician‐determined therapy has been conducted.13 This study found no differences between treatment groups in clinical outcomes but did find that use of doxycycline was associated with shorter hospital stays and reduced costs. Our results, achieved in a real‐world setting in relatively ill hospitalized patients (58% were in PSI risk class IV or V), provide further support for the use of combination therapy with doxycycline.
Hospitalized patients treated with a beta‐lactam in combination with a macrolide are often discharged on macrolide monotherapy. In our population most patients treated with ceftriaxone plus doxycycline were discharged on doxycycline if they required continued therapy (data not shown). In the current era of resistance of Streptococcus pneumoniae to antibiotics, there is good reason to believe doxycycline may perform as well, if not better, than macrolides when hospitalized patients with CAP are discharged on oral monotherapy. Macrolide resistance rates among invasive pneumococcal isolates in the United States doubled from 10% to 20% during a period in which prescriptions for macrolides increased by 13%.12 In addition, a large surveillance study of more than 1500 isolates collected in 1999 and 2000 found that 26% of the isolates were resistant to macrolides, whereas only 16% were resistant to tetracycline.16 In vitro testing against S. Pneumoniae has also suggested that tetracycline resistance overestimates doxycycline resistance.17, 18 More recently, Streptococcus pneumoniae susceptibility data from the SENTRY Antimicrobial Surveillance program reaffirmed doxycycline's in vitro superiority over macrolides.17
Our study had several limitations. The study design adopted precluded determining whether favorable results with the use of ceftriaxone plus doxycycline resulted from an effect unique to this combination of antibiotics, the possible anti‐inflammatory properties of doxycycline alone,19, 20 or unmeasured confounders. For example, processes of care that affect clinical outcomes for patients hospitalized with CAP, such as the timing of antibiotic delivery, the timing of blood cultures, and stability assessment on discharge were not measured in this study. To affect outcomes, these processes of care would need to be differentially distributed between our comparison groups. However, because this study was performed in a single institution during a single interval, it is likely that the performance of these processes of care would be similar for all patients.
In conclusion, ceftriaxone plus doxycycline appears to be an effective, and possibly superior, therapy for patients hospitalized with CAP. Randomized controlled trials of doxycycline‐containing regimens versus other regimens are warranted.
In the United States, community‐acquired pneumonia (CAP) leads to nearly 1 million hospitalizations annually, with aggregate costs of hospitalization approaching $9 billion.1, 2 In an effort to improve the appropriate, cost‐effective care for patients with CAP, several professional societies have developed clinical practice guidelines and pathways for pneumonia.37 Although the guidelines address all aspects of care, they devote substantial attention to antibiotic recommendations. Most U.S. guidelines recommend treatment of hospitalized patients with an intravenous beta‐lactam combined with a macrolide, or a fluoroquinolone with activity against Streptococcus pneumoniae. Of the major U.S. CAP practice guidelines, only one6 recommends doxycycline as an alternative to a macrolide for inpatients.
Doxycycline is an attractive alternative to macrolides. Similar to macrolides, doxycycline is active against a wide variety of organisms including atypical bacteria (Chlamydia pneumoniae, Legionella pneumophilus, and Mycoplasma pneumoniae) and is well tolerated.810 In addition, it is inexpensive (cost of $1.00/day [awp] for 100 mg p.o. bid), and rates of tetracycline/doxycycline resistance among S. pneumoniae isolates have remained low, in contrast to the increasing rates of resistance to macrolides and fluoroquinolones.11, 12 The most recent guidelines from the Infectious Diseases Society of America cited limited published clinical data on the effectiveness of doxycycline in CAP as a barrier to increased use.7 Only one study of hospitalized patients has been published in the era of penicillin‐resistant pneumococcus, and this study included only 43 low‐risk patients treated with doxycycline.13 At the university hospital affiliated with the University of California, San Francisco, ceftriaxone plus doxycycline is generally recommended as initial empiric antibiotic therapy for patients hospitalized with CAP, but significant variability in prescribing exists, allowing for comparisons between patients treated with different initial empiric antibiotic regimens. We compared outcomes of hospitalized patients with CAP treated with ceftriaxone plus doxycycline to those of patients treated with alternative initial empiric therapy at an academic medical center.
METHODS
Study Population
A retrospective cohort study of all adults (age 18 years) discharged from the inpatient general medicine service of Moffitt‐Long Hospital at the University of California, San Francisco, was conducted from January 1999 through July 2001. Eligibility criteria included a principal discharge diagnosis of CAP and a chest radiograph demonstrating an infiltrate within 48 hours of admission. Exclusion criteria included infection with the human immunodeficiency virus, history of organ transplantation or use of immunosuppressive therapy (including prednisone > 15 mg/day), cystic fibrosis, postobstructive pneumonia, active tuberculosis, recent hospitalization (within 10 days), or admission for comfort care. The study protocol and procedures were reviewed and approved by the UCSF Committee for Human Research.
Data Collection
Medical record review by trained research assistants blinded to the research question was used to gather demographic data, comorbid illnesses, physical examination findings on initial presentation, and laboratory or radiographic results on initial presentation. The pneumonia severity index (PSI) score was calculated for each patient using the above data.14 In addition, data were collected on antibiotic allergies, antibiotics used within the 30 days prior to admission, results of sputum or blood cultures, and admission location (intensive care unit [ICU] versus medical floor).
Data from TSI (Transition Systems Inc., Boston, MA), the hospital administrative database, were used to identify the initial empiric antibiotic regimen. All antibiotics prescribed within the first 48 hours of hospitalization were considered initial empiric therapy with few exceptions. Initial empiric therapy was classified as 1) ceftriaxone plus doxycycline (including patients treated with these agents alone in the first 48 hours, as well as patients treated with both agents in the first 24 hours who were switched to alternative therapy [broader coverage] on the second day), or 2) other appropriate therapy (treatment consistent with current national guideline recommendations including at least a beta‐lactam plus a macrolide or a beta‐lactam plus a fluoroquinolone, or fluoroquinolone monotherapy). Patients receiving therapy inconsistent with current national guideline recommendations were excluded.
Outcomes
TSI data were used to identify length of stay, death during the index hospitalization, and return to the emergency department or readmission within 30 days of discharge. The National Death Index was used to identify all deaths that occurred after hospital discharge. The 30‐day mortality data included deaths occurring during the index hospitalization and in the 30 days after the index hospitalization discharge.
Statistical Analysis
For the purposes of this analysis we compared patients treated with ceftriaxone plus doxycycline to patients treated with other appropriate therapy. To examine demographic and clinical differences between the two groups, statistical tests of comparison were performed using chi‐square tests for the dichotomous variables and t tests for the numeric variables, all of which were normally distributed (after log transformation in the case of length of stay).
To adjust for clinical variables that might contribute to differences in outcomes between the two groups, we used backward stepwise logistic regression analysis to construct a propensity score15 for the likelihood of ceftriaxone plus doxycycline use. The propensity score reflected the conditional probability of exposure to ceftriaxone plus doxycycline and allowed for stratification and, subsequently, comparisons by quintiles of propensity score. Propensity scores often have distinct advantages over direct adjustment for a large number of confounding variables and allow direct comparisons between groups with a similar propensity for receiving ceftriaxone plus doxycycline.15 Unlike random assignment of treatment, however, the propensity score cannot balance unmeasured variables that may affect treatment assignment. Thus, the possibility of bias remains. The variables used to build the score included age, presence of comorbid illness, admission from a nursing home or long‐term care facility, antibiotic allergy, prior antibiotic use, PSI score, PSI risk class, diagnosis of aspiration, admission to the ICU, and positive blood cultures. The propensity score was then stratified and used as an adjustment variable in comparisons between groups for in‐hospital mortality, 30‐day mortality, and 30‐day readmission rates. As expected, length of stay was highly skewed and was therefore log‐transformed and compared between groups with adjustment for the propensity score.
To further address issues related to potential selection bias, a separate analysis was performed on a subset of the original cohort that excluded patients for whom ceftriaxone plus doxycycline would not generally be recommended as first‐line therapy. For this analysis, patients admitted from a nursing home or long‐term care facility, patients admitted to the ICU, and patients with a principal diagnosis of aspiration pneumonia were excluded. A propensity score was rederived for this subset, which was used to adjust for differences in outcomes. All statistical procedures were performed using STATA (Ver. 7.0, Stata Corporation, College Station TX).
RESULTS
Patient Characteristics
A total of 341 patients were eligible for analysis. Of this group, 216 were treated with ceftriaxone plus doxycycline and 125 received other appropriate therapy. Both groups of patients were similar in age. Patients treated with ceftriaxone plus doxycycline had a lower median PSI score and fewer comorbid illnesses than did patients treated with other appropriate therapy (Table 1). Blood cultures were positive in 30 (8.8%) of the 341 patients included in the analysis, with S. pneumoniae the most commonly isolated organism (n = 17, 5.0%). Of S. pneumoniae isolates, 4 (24%) were resistant to penicillin (MIC 1 g/mL), and 2 (12%) were resistant to tetracycline (MIC 8 g/mL).
| Ceftriaxone/doxycycline | Other appropriate therapy | |
|---|---|---|
| ||
| Patients (n) | 216 | 125 |
| Age (median) | 76 | 74 |
| PSI Score (median)a | 97 | 108 |
| PSI Risk Class (%)a | ||
| Class I | 9.3 | 5.6 |
| Class II | 11.1 | 8.8 |
| Class III | 21.8 | 13.6 |
| Class IV | 40.7 | 40.0 |
| Class V | 17.1 | 32.0 |
| Comorbid Illness (%)a | 36.1 | 47.2 |
| Nursing Home/LCF (%)a | 5.1 | 14.4 |
| Aspiration (%)a | 3.2 | 20.0 |
| Admission to ICU (%)a | 6.0 | 28.0 |
Common antibiotic choices in patients receiving other appropriate therapy included a beta‐lactam/beta‐lactamase inhibitor plus doxycycline or a macrolide (n = 36, 29%), fluoroquinolone monotherapy (n = 16, 13%), and a variety of other antibiotic combinations with activity against S. pneumoniae and atypical bacteria (n = 52, 42%).
Clinical Outcomes
Analyses of unadjusted outcomes showed that patients treated with ceftriaxone plus doxycycline had significantly lower inpatient (2% vs. 14%, P < .001) and 30‐day (6% vs. 20%, P < .001) mortality compared to patients treated with other regimens (Table 2). Multivariable logistic regression analysis identified three variables (diagnosis of congestive heart failure, admission to the ICU, and the presence of comorbid illness) associated with initial antibiotic selection, which were used to build a propensity score. After adjustment for the propensity score, use of ceftriaxone plus doxycycline remained significantly associated with lower inpatient mortality (OR = 0.26, 95% CI: 0.080.81) and 30‐day mortality (OR = 0.37, 95% CI: 0.170.81). Differences in length of stay and 30‐day readmission rates between the treatment groups were not significant (Table 2).
| Ceftriaxone + doxycycline (n = 216) | Other appropriate therapy (n = 125) | Adjusted odds ratio (95% confidence interval) | |
|---|---|---|---|
| |||
| Inpatient Mortality | 2.3% | 14.4% | 0.26 (0.080.81) |
| 30‐day mortality | 6.0% | 20.0% | 0.37 (0.170.81) |
| Length of stay (median days) | 3.0 | 4.0 | 0.09 (0.250.06)a |
| 30‐day readmission | 10.7% | 12.0% | 0.87 (0.421.81) |
Subset Analysis
To address issues related to selection bias, we performed an analysis of a subset of the patients after excluding those admitted from a nursing home, diagnosed with aspiration, or admitted to the ICU, for whom ceftriaxone plus doxycycline would not be considered recommended (or first‐line) therapy. The two resulting groups were similar, except there were fewer patients with comorbid illness in the ceftriaxone plus doxycycline group (34% vs. 50%, P = .015). The propensity score was rederived for this subset and used for adjustment. Unadjusted and adjusted outcomes are shown in Table 3. Use of ceftriaxone plus doxycycline in this subset also was associated with reduced odds of inpatient mortality (OR = 0.17, 95% CI: 0.040.77). The odds of 30‐day mortality also were reduced but not significantly, as the confidence interval included 1.0 (OR = 0.43, 95% CI: 0.141.31). There were no differences between groups in length of stay or in 30‐day readmission rate.
| Ceftriaxone + doxycycline (n = 188) | Other appropriate therapy (n = 70) | Adjusted odds ratio (95% CI) | |
|---|---|---|---|
| |||
| Age (median years) | 75 | 71 | |
| PSI score (mean) | 95 | 98 | |
| Comorbid illness (%)a | 33.5 | 50.0 | |
| Inpatient mortality | 1.6% | 7.1% | 0.17 (0.040.77) |
| 30‐day mortality | 4.8% | 8.6% | 0.43 (0.141.31) |
| LOS (median days) | 3 | 3 | 0.06 (0.240.12)b |
| 30‐day readmission | 11.9% | 10.0% | 1.31 (0.523.28) |
DISCUSSION
In our hospital setting, the use of ceftriaxone plus doxycycline as the initial empiric antibiotic therapy for patients hospitalized with community‐acquired pneumonia was associated with significantly lower inpatient and 30‐day mortality, even after adjusting for clinical differences between groups. We did not find a difference between regimens in hospital length of stay or 30‐day readmission rate. In case the multivariable model was insufficient to account for the clinical differences (i.e., selection bias) between groups, we also performed an analysis of a subgroup of less severely ill patients by excluding those admitted from nursing homes, those admitted to the intensive care unit, and those with aspiration pneumonia. In this subset, use of ceftriaxone plus doxycycline remained associated with lower inpatient mortality but not with lower 30‐day mortality. Although, as an observational study, the results of our findings could still be a result of residual confounding, we believe the results provide valuable information regarding doxycycline.
Combination therapy with a macrolide, but not doxycycline, is advocated by the practice guidelines of several major U.S. professional societies,3, 4, 7 apparently because of a lack of data on the effectiveness of combination therapy with doxycycline.7 Only one randomized, unblinded study, in 87 low‐risk patients hospitalized with CAP, that compared monotherapy with IV doxycycline versus physician‐determined therapy has been conducted.13 This study found no differences between treatment groups in clinical outcomes but did find that use of doxycycline was associated with shorter hospital stays and reduced costs. Our results, achieved in a real‐world setting in relatively ill hospitalized patients (58% were in PSI risk class IV or V), provide further support for the use of combination therapy with doxycycline.
Hospitalized patients treated with a beta‐lactam in combination with a macrolide are often discharged on macrolide monotherapy. In our population most patients treated with ceftriaxone plus doxycycline were discharged on doxycycline if they required continued therapy (data not shown). In the current era of resistance of Streptococcus pneumoniae to antibiotics, there is good reason to believe doxycycline may perform as well, if not better, than macrolides when hospitalized patients with CAP are discharged on oral monotherapy. Macrolide resistance rates among invasive pneumococcal isolates in the United States doubled from 10% to 20% during a period in which prescriptions for macrolides increased by 13%.12 In addition, a large surveillance study of more than 1500 isolates collected in 1999 and 2000 found that 26% of the isolates were resistant to macrolides, whereas only 16% were resistant to tetracycline.16 In vitro testing against S. Pneumoniae has also suggested that tetracycline resistance overestimates doxycycline resistance.17, 18 More recently, Streptococcus pneumoniae susceptibility data from the SENTRY Antimicrobial Surveillance program reaffirmed doxycycline's in vitro superiority over macrolides.17
Our study had several limitations. The study design adopted precluded determining whether favorable results with the use of ceftriaxone plus doxycycline resulted from an effect unique to this combination of antibiotics, the possible anti‐inflammatory properties of doxycycline alone,19, 20 or unmeasured confounders. For example, processes of care that affect clinical outcomes for patients hospitalized with CAP, such as the timing of antibiotic delivery, the timing of blood cultures, and stability assessment on discharge were not measured in this study. To affect outcomes, these processes of care would need to be differentially distributed between our comparison groups. However, because this study was performed in a single institution during a single interval, it is likely that the performance of these processes of care would be similar for all patients.
In conclusion, ceftriaxone plus doxycycline appears to be an effective, and possibly superior, therapy for patients hospitalized with CAP. Randomized controlled trials of doxycycline‐containing regimens versus other regimens are warranted.
- ,,, et al.Hospitalized pneumonia. Outcomes, treatment patterns, and costs in urban and rural areas.J Gen Intern Med.1996;11:415–421.
- ,,, et al.The cost of treating community‐acquired pneumonia.Clin Ther.1998;20:820–837.
- ,,, et al.Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious Diseases Society of America.Clin Infect Dis.2000;31:347–382.
- ,,, et al.Management of community‐acquired pneumonia in the era of pneumococcal resistance: a report from the Drug‐Resistant Streptococcus pneumoniae Therapeutic Working Group.Arch Intern Med.2000;160:1399–1408.
- ,,, et al.Canadian guidelines for the initial management of community‐acquired pneumonia: an evidence‐based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Canadian Community‐Acquired Pneumonia Working Group.Clin Infect Dis.2000;31:383–421.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,, et al.Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433
- ,,.Doxycycline.Ther Drug Monit1982;4:115–135
- ,.Chloramphenicol and tetracyclines.Med Clin North Am.1987;71:1155–1168
- ,.Tetracyclines.Med Clin North Am.1995;79:789–801
- ,,, et al.Antibiotic resistance among gram‐negative bacilli in US intensive care units: implications for fluoroquinolone use.JAMA.2003;289:885–888
- ,,, et al.Macrolide resistance among invasive Streptococcus pneumoniae isolates.JAMA.2001;286:1857–1862.
- ,,, et al.Doxycycline is a cost‐effective therapy for hospitalized patients with community‐acquired pneumonia.Arch Intern Med.1999;159:266–270.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- .Reducing bias in observational studies using subclassification on the propensity score.J Am Stat Assoc.1984;79:516–524
- ,,, et al.Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999—2000, including a comparison of resistance rates since 1994—1995.Antimicrob Agents Chemother.2001;45:1721–1729
- ,,.Doxycycline use for community‐acquired pneumonia: contemporary in vitro spectrum of activity against Streptococcus pneumoniae (1999–2002).Diagn Microbiol Infect Dis.2004;49:147–149
- ,,, et al.Doxycycline activity against Streptococcus pneumoniae.Chest.1995;108:1775–1776.
- ,,, et al.Inhibition of enzymatic activity of phospholipases A2 by minocycline and doxycycline.Biochem Pharmacol.1992;44:1165–1170.
- ,,, et al.Doxycycline reduces mortality to lethal endotoxemia by reducing nitric oxide synthesis via an interleukin‐10‐independent mechanism.J Infect Dis.1998;177:489–492.
- ,,, et al.Hospitalized pneumonia. Outcomes, treatment patterns, and costs in urban and rural areas.J Gen Intern Med.1996;11:415–421.
- ,,, et al.The cost of treating community‐acquired pneumonia.Clin Ther.1998;20:820–837.
- ,,, et al.Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious Diseases Society of America.Clin Infect Dis.2000;31:347–382.
- ,,, et al.Management of community‐acquired pneumonia in the era of pneumococcal resistance: a report from the Drug‐Resistant Streptococcus pneumoniae Therapeutic Working Group.Arch Intern Med.2000;160:1399–1408.
- ,,, et al.Canadian guidelines for the initial management of community‐acquired pneumonia: an evidence‐based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Canadian Community‐Acquired Pneumonia Working Group.Clin Infect Dis.2000;31:383–421.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,, et al.Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433
- ,,.Doxycycline.Ther Drug Monit1982;4:115–135
- ,.Chloramphenicol and tetracyclines.Med Clin North Am.1987;71:1155–1168
- ,.Tetracyclines.Med Clin North Am.1995;79:789–801
- ,,, et al.Antibiotic resistance among gram‐negative bacilli in US intensive care units: implications for fluoroquinolone use.JAMA.2003;289:885–888
- ,,, et al.Macrolide resistance among invasive Streptococcus pneumoniae isolates.JAMA.2001;286:1857–1862.
- ,,, et al.Doxycycline is a cost‐effective therapy for hospitalized patients with community‐acquired pneumonia.Arch Intern Med.1999;159:266–270.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- .Reducing bias in observational studies using subclassification on the propensity score.J Am Stat Assoc.1984;79:516–524
- ,,, et al.Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999—2000, including a comparison of resistance rates since 1994—1995.Antimicrob Agents Chemother.2001;45:1721–1729
- ,,.Doxycycline use for community‐acquired pneumonia: contemporary in vitro spectrum of activity against Streptococcus pneumoniae (1999–2002).Diagn Microbiol Infect Dis.2004;49:147–149
- ,,, et al.Doxycycline activity against Streptococcus pneumoniae.Chest.1995;108:1775–1776.
- ,,, et al.Inhibition of enzymatic activity of phospholipases A2 by minocycline and doxycycline.Biochem Pharmacol.1992;44:1165–1170.
- ,,, et al.Doxycycline reduces mortality to lethal endotoxemia by reducing nitric oxide synthesis via an interleukin‐10‐independent mechanism.J Infect Dis.1998;177:489–492.
Copyright © 2006 Society of Hospital Medicine