User login
Recent cocaine use quadrupled stroke risk
SAN DIEGO – Cocaine use within the past 24 hours was reported by 2.4% of 1,101 patients aged 15-49 years who developed ischemic stroke, compared with 0.4% of 1,154 age-matched controls without stroke.
The recent cocaine use was associated with a fourfold increase in the odds of ischemic stroke after adjustment for the effects of age, current smoking status, sex, and ethnicity. The difference in cocaine use between groups and the increased risk with cocaine were statistically significant, Yu-Ching Cheng, Ph.D., reported.
Females seemed to be at particular risk from recent cocaine use, with an adjusted odds ratio for ischemic stroke of 11 in those who had used cocaine within the previous 24 hours, compared with controls. Males had an odds ratio of 2 for ischemic stroke after recent cocaine use, compared with controls, she reported in a poster presentation at the International Stroke Conference.
"With few exceptions, we believe every young stroke patient should be screened for drug abuse at the time of hospital admission, especially in the case where there’s no clear etiology" for the stroke, she said in an interview at the poster. Knowing about acute cocaine use probably wouldn’t change management of the stroke but might help optimize recovery if the patient is encouraged to seek drug abuse counseling. That also may help prevent secondary strokes from continued cocaine use, she added.
Other factors increased the risk of stroke in the case-control study, but none did as much as cocaine use within the past 24 hours. Diabetes was significantly more common in the stroke patients (17%), compared with controls (5%), and was associated with approximately 3.5-fold higher odds for stroke after adjustment for confounding factors. Stroke patients were significantly more likely than controls to have hypertension (42% vs. 18%, respectively), which was associated with a threefold greater likelihood of stroke. Significantly more stroke patients were current smokers (45% vs. 29%, respectively), which doubled the likelihood of stroke, reported Dr. Cheng of the University of Maryland, Baltimore.
The Stroke Prevention in Young Adults Study was a population-based, case-control study of people in the Baltimore-Washington area aged 15-49 years who either had a first ischemic stroke between 1992 and 2008 or were age-matched controls without stroke who were contacted by random-digit dialing. Investigators collected data through a standardized interview.
The stroke risk from recent cocaine use did not differ by race, with an adjusted odds ratio of nearly 5 for both blacks and whites after cocaine use in the previous 24 hours, she reported at the meeting, sponsored by the American Heart Association.
Ever having used cocaine or having used cocaine in the last 1-30 days was not significantly associated with increased risk for stroke.
Males comprised 53% of the stroke patients and 46% of controls.
Approximately 1.9 million U.S. residents have used cocaine in the past month, previous data have suggested, with adults aged 18-25 years most likely to use cocaine, she said. Cocaine is known to constrict blood vessels; increase heart rate, body temperature, and blood pressure; and decrease oxygen supply to the brain.
Dr. Cheng reported having no relevant financial disclosures. The study was funded by the National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, and the Centers for Disease Control and Prevention.
On Twitter @sherryboschert
SAN DIEGO – Cocaine use within the past 24 hours was reported by 2.4% of 1,101 patients aged 15-49 years who developed ischemic stroke, compared with 0.4% of 1,154 age-matched controls without stroke.
The recent cocaine use was associated with a fourfold increase in the odds of ischemic stroke after adjustment for the effects of age, current smoking status, sex, and ethnicity. The difference in cocaine use between groups and the increased risk with cocaine were statistically significant, Yu-Ching Cheng, Ph.D., reported.
Females seemed to be at particular risk from recent cocaine use, with an adjusted odds ratio for ischemic stroke of 11 in those who had used cocaine within the previous 24 hours, compared with controls. Males had an odds ratio of 2 for ischemic stroke after recent cocaine use, compared with controls, she reported in a poster presentation at the International Stroke Conference.
"With few exceptions, we believe every young stroke patient should be screened for drug abuse at the time of hospital admission, especially in the case where there’s no clear etiology" for the stroke, she said in an interview at the poster. Knowing about acute cocaine use probably wouldn’t change management of the stroke but might help optimize recovery if the patient is encouraged to seek drug abuse counseling. That also may help prevent secondary strokes from continued cocaine use, she added.
Other factors increased the risk of stroke in the case-control study, but none did as much as cocaine use within the past 24 hours. Diabetes was significantly more common in the stroke patients (17%), compared with controls (5%), and was associated with approximately 3.5-fold higher odds for stroke after adjustment for confounding factors. Stroke patients were significantly more likely than controls to have hypertension (42% vs. 18%, respectively), which was associated with a threefold greater likelihood of stroke. Significantly more stroke patients were current smokers (45% vs. 29%, respectively), which doubled the likelihood of stroke, reported Dr. Cheng of the University of Maryland, Baltimore.
The Stroke Prevention in Young Adults Study was a population-based, case-control study of people in the Baltimore-Washington area aged 15-49 years who either had a first ischemic stroke between 1992 and 2008 or were age-matched controls without stroke who were contacted by random-digit dialing. Investigators collected data through a standardized interview.
The stroke risk from recent cocaine use did not differ by race, with an adjusted odds ratio of nearly 5 for both blacks and whites after cocaine use in the previous 24 hours, she reported at the meeting, sponsored by the American Heart Association.
Ever having used cocaine or having used cocaine in the last 1-30 days was not significantly associated with increased risk for stroke.
Males comprised 53% of the stroke patients and 46% of controls.
Approximately 1.9 million U.S. residents have used cocaine in the past month, previous data have suggested, with adults aged 18-25 years most likely to use cocaine, she said. Cocaine is known to constrict blood vessels; increase heart rate, body temperature, and blood pressure; and decrease oxygen supply to the brain.
Dr. Cheng reported having no relevant financial disclosures. The study was funded by the National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, and the Centers for Disease Control and Prevention.
On Twitter @sherryboschert
SAN DIEGO – Cocaine use within the past 24 hours was reported by 2.4% of 1,101 patients aged 15-49 years who developed ischemic stroke, compared with 0.4% of 1,154 age-matched controls without stroke.
The recent cocaine use was associated with a fourfold increase in the odds of ischemic stroke after adjustment for the effects of age, current smoking status, sex, and ethnicity. The difference in cocaine use between groups and the increased risk with cocaine were statistically significant, Yu-Ching Cheng, Ph.D., reported.
Females seemed to be at particular risk from recent cocaine use, with an adjusted odds ratio for ischemic stroke of 11 in those who had used cocaine within the previous 24 hours, compared with controls. Males had an odds ratio of 2 for ischemic stroke after recent cocaine use, compared with controls, she reported in a poster presentation at the International Stroke Conference.
"With few exceptions, we believe every young stroke patient should be screened for drug abuse at the time of hospital admission, especially in the case where there’s no clear etiology" for the stroke, she said in an interview at the poster. Knowing about acute cocaine use probably wouldn’t change management of the stroke but might help optimize recovery if the patient is encouraged to seek drug abuse counseling. That also may help prevent secondary strokes from continued cocaine use, she added.
Other factors increased the risk of stroke in the case-control study, but none did as much as cocaine use within the past 24 hours. Diabetes was significantly more common in the stroke patients (17%), compared with controls (5%), and was associated with approximately 3.5-fold higher odds for stroke after adjustment for confounding factors. Stroke patients were significantly more likely than controls to have hypertension (42% vs. 18%, respectively), which was associated with a threefold greater likelihood of stroke. Significantly more stroke patients were current smokers (45% vs. 29%, respectively), which doubled the likelihood of stroke, reported Dr. Cheng of the University of Maryland, Baltimore.
The Stroke Prevention in Young Adults Study was a population-based, case-control study of people in the Baltimore-Washington area aged 15-49 years who either had a first ischemic stroke between 1992 and 2008 or were age-matched controls without stroke who were contacted by random-digit dialing. Investigators collected data through a standardized interview.
The stroke risk from recent cocaine use did not differ by race, with an adjusted odds ratio of nearly 5 for both blacks and whites after cocaine use in the previous 24 hours, she reported at the meeting, sponsored by the American Heart Association.
Ever having used cocaine or having used cocaine in the last 1-30 days was not significantly associated with increased risk for stroke.
Males comprised 53% of the stroke patients and 46% of controls.
Approximately 1.9 million U.S. residents have used cocaine in the past month, previous data have suggested, with adults aged 18-25 years most likely to use cocaine, she said. Cocaine is known to constrict blood vessels; increase heart rate, body temperature, and blood pressure; and decrease oxygen supply to the brain.
Dr. Cheng reported having no relevant financial disclosures. The study was funded by the National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, and the Centers for Disease Control and Prevention.
On Twitter @sherryboschert
AT THE INTERNATIONAL STROKE CONFERENCE
Major finding: Cocaine use in the past 24 hours was reported by 2.4% of young adults with ischemic stroke and 0.4% of age-matched controls without stroke, and was associated with a fourfold increased odds of stroke.
Data source: A case-control comparison of data on 1,101 ischemic stroke patients aged 15-49 years and 1,154 controls reached by random-digit dialing for phone questionnaires.
Disclosures: Dr. Cheng reported having no relevant financial disclosures. The study was funded by the National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, and the Centers for Disease Control and Prevention.
Half of stroke survivors returned to driving
SAN DIEGO – Only 6% of 162 stroke survivors underwent a formal driving evaluation, but 51% returned to driving within a year, a survey found.
Among the 83 who returned to driving, 9 (11%) did so despite reporting that their stroke greatly affected their ability to "engage in valued life activities," Dr. Shelly D. Ozark reported in a poster presentation at the International Stroke Conference.
Twenty-six (31%) of the 83 drivers said their stroke had some effect on their ability to engage in valued life activities, and 48 drivers (58%) said the stroke did not affect that part of their lives.
Formal driving evaluations were completed by 2 of the 9 stroke survivors who returned to driving despite great effects from the stroke, 3 of 26 drivers who reported some effects, and 4 of 48 drivers who reported no effects of the stroke on their abilities to engage in valued life activities, reported Dr. Ozark of the Medical University of South Carolina, Charleston.
Forty-nine (59%) of the 83 drivers returned to driving less than a month after their stroke, 21 (25%) resumed driving 1-3 months after their stroke, and 13 (16%) resumed driving more than 3 months post stroke, the investigators reported at the meeting, sponsored by the American Heart Association.
Thirty-eight (46%) of the 83 drivers said they had self-imposed limits on their driving. This included 17 (35%) of the 48 drivers who reported no effects of the stroke on valued life activities. It is unclear whether a formal driving evaluation would have supported their decision to limit driving, Dr. Ozark noted. Self-imposed driving limits also were reported by 16 of 26 drivers who experienced some effects of the stroke and 5 of 9 drivers who reported great effects of the stroke on their ability to engage in valued life activities.
The study was a secondary analysis of a subset of data from the longitudinal surveys of the Stroke Education and Prevention - South Carolina Project.
Dr. Ozark reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN DIEGO – Only 6% of 162 stroke survivors underwent a formal driving evaluation, but 51% returned to driving within a year, a survey found.
Among the 83 who returned to driving, 9 (11%) did so despite reporting that their stroke greatly affected their ability to "engage in valued life activities," Dr. Shelly D. Ozark reported in a poster presentation at the International Stroke Conference.
Twenty-six (31%) of the 83 drivers said their stroke had some effect on their ability to engage in valued life activities, and 48 drivers (58%) said the stroke did not affect that part of their lives.
Formal driving evaluations were completed by 2 of the 9 stroke survivors who returned to driving despite great effects from the stroke, 3 of 26 drivers who reported some effects, and 4 of 48 drivers who reported no effects of the stroke on their abilities to engage in valued life activities, reported Dr. Ozark of the Medical University of South Carolina, Charleston.
Forty-nine (59%) of the 83 drivers returned to driving less than a month after their stroke, 21 (25%) resumed driving 1-3 months after their stroke, and 13 (16%) resumed driving more than 3 months post stroke, the investigators reported at the meeting, sponsored by the American Heart Association.
Thirty-eight (46%) of the 83 drivers said they had self-imposed limits on their driving. This included 17 (35%) of the 48 drivers who reported no effects of the stroke on valued life activities. It is unclear whether a formal driving evaluation would have supported their decision to limit driving, Dr. Ozark noted. Self-imposed driving limits also were reported by 16 of 26 drivers who experienced some effects of the stroke and 5 of 9 drivers who reported great effects of the stroke on their ability to engage in valued life activities.
The study was a secondary analysis of a subset of data from the longitudinal surveys of the Stroke Education and Prevention - South Carolina Project.
Dr. Ozark reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN DIEGO – Only 6% of 162 stroke survivors underwent a formal driving evaluation, but 51% returned to driving within a year, a survey found.
Among the 83 who returned to driving, 9 (11%) did so despite reporting that their stroke greatly affected their ability to "engage in valued life activities," Dr. Shelly D. Ozark reported in a poster presentation at the International Stroke Conference.
Twenty-six (31%) of the 83 drivers said their stroke had some effect on their ability to engage in valued life activities, and 48 drivers (58%) said the stroke did not affect that part of their lives.
Formal driving evaluations were completed by 2 of the 9 stroke survivors who returned to driving despite great effects from the stroke, 3 of 26 drivers who reported some effects, and 4 of 48 drivers who reported no effects of the stroke on their abilities to engage in valued life activities, reported Dr. Ozark of the Medical University of South Carolina, Charleston.
Forty-nine (59%) of the 83 drivers returned to driving less than a month after their stroke, 21 (25%) resumed driving 1-3 months after their stroke, and 13 (16%) resumed driving more than 3 months post stroke, the investigators reported at the meeting, sponsored by the American Heart Association.
Thirty-eight (46%) of the 83 drivers said they had self-imposed limits on their driving. This included 17 (35%) of the 48 drivers who reported no effects of the stroke on valued life activities. It is unclear whether a formal driving evaluation would have supported their decision to limit driving, Dr. Ozark noted. Self-imposed driving limits also were reported by 16 of 26 drivers who experienced some effects of the stroke and 5 of 9 drivers who reported great effects of the stroke on their ability to engage in valued life activities.
The study was a secondary analysis of a subset of data from the longitudinal surveys of the Stroke Education and Prevention - South Carolina Project.
Dr. Ozark reported having no relevant financial disclosures.
On Twitter @sherryboschert
AT THE INTERNATIONAL STROKE CONFERENCE
Major finding: Six percent of stroke survivors underwent formal driving evaluations, but 51% of survivors returned to driving within 1 year.
Data source: A secondary analysis of survey responses from 162 stroke survivors, a subset of the Stroke Prevention and Education–South Carolina Project data
Disclosures: Dr. Ozark reported having no relevant financial disclosures.
Foods can trigger EoE after allergy is outgrown
SAN DIEGO – Seventeen of 425 children who had eosinophilic esophagitis caused by a specific food developed the condition after outgrowing the allergy to that food, a retrospective study found.
People who outgrow a food allergy may be at risk of developing eosinophilic esophagitis (EoE) to the same food, Dr. Jonathan Spergel said during a press briefing at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He and his associates studied data on 1,025 children with EoE seen at the Children’s Hospital of Philadelphia in 2000-2012 to assess the prevalence of food allergy. In 425 children (42%), a specific food was identified as the EoE culprit – reintroducing the food to the diet caused esophageal changes on biopsy or biopsy changes normalized when the food was removed from the diet.
Eighty-four children had a history of IgE-mediated food allergy. Milk, egg, wheat, and soy were the most common food triggers of EoE in the 425 children in the study and in a subset of 17 who had outgrown IgE-mediated allergy to the specific food, reported Dr. Spergel, chief of the allergy section at the Children’s Hospital of Philadelphia. Sixteen of the 17 patients had atopic disease. The most common foods causing IgE-mediated allergy were peanuts, tree nuts, eggs, and milk.
The development of EoE coincided with reintroducing the food triggers. The time between outgrowing an allergy and reintroducing the food, triggering EoE, averaged 2 years but ranged from 6 months to 5 years.
Notably, two of the children who outgrew their food allergy had a normal biopsy of the esophagus when they had the food allergy, he said.
The findings support other recent studies suggesting that the pathophysiologies of EoE and IgE-mediated food allergy are distinct from each other, and that both can occur in the same individual to the same food, Dr. Spergel said. The mechanism by which EoE develops is poorly understood.
"I think these kids probably always had EoE to the food, but they weren’t eating it" because of the allergy, he said. "From 1% to 15% on oral immunotherapy get EoE, depending on which group you look at. I don’t think we caused" EoE by giving oral immunotherapy, he added. "We uncovered it."
Although it is rare for children who outgrow a food allergy to later develop EoE to that food, it’s worth keeping in mind if a child starts vomiting often or complains of stomachaches months or years later, Dr. Spergel said. Keeping the possibility in mind may help clinicians rule out other etiologies and detect EoE faster. "You have to take it seriously and get it checked out," he said.
Of the 84 patients with IgE-mediated food allergy, the 17 who outgrew the allergy and then developed EoE to the same food were significantly older (12 years, on average), compared with 67 patients who developed EoE from a different food from the one that caused their allergy.
The lead author on the study was Dr. Solrun Melkorka Meggadottir, a fellow at the Children’s Hospital of Philadelphia. The findings have been submitted to the Journal of Allergy and Clinical Immunology.
Dr. Spergel and Dr. Meggadottir reported having no disclosures.
sboschert@frontlinemedcom.com On Twitter @sherryboschert
Eosinophilic esophagitis (EoE) is a chronic condition that affects children and adults across the world. Children present with feeding problems, abdominal pain, and reflux-like symptoms, whereas adults complain of dysphagia and food impactions. No Food and Drug Administration–approved treatments are available leaving practitioners with off-label use of swallowed steroids administered from multi-dose inhalers or dietary restrictions.
 |
|
The elimination of protein antigens has been accomplished through the use of elemental formulas, empiric elimination of the most common allergens, or targeted elimination based on skin or blood testing. A literature review revealed that these approaches are effective in inducing histologic remission in 90.8%, 72.1%, and 45.5% of patients, respectively.
This report by Meggadottir and Spergel provides at least two important findings for patients with EoE. Their data contribute to a growing body of literature and clinical observations indicating that mechanistic pathways other than IgE-mediated food reactions may contribute to the pathogenesis of EoE. Some recent reports and clinical experiences suggest the children with EoE may tolerate some allergenic foods that are cooked in a different way or children may outgrow their allergies. Results from this study suggest that this may not be the case. Clinical implications of their findings suggest that children and adults may need to continue to restrict common foods from their diets; the long-term impact of this on quality of life is not certain.
Dr. Glenn T. Furuta, professor of pediatrics, University of Colorado School of Medicine, Director of the Gastrointestinal Eosinophilic Diseases Program, Children’s Hospital Colorado, Denver. He is the co-founder of EnteroTrack.
Eosinophilic esophagitis (EoE) is a chronic condition that affects children and adults across the world. Children present with feeding problems, abdominal pain, and reflux-like symptoms, whereas adults complain of dysphagia and food impactions. No Food and Drug Administration–approved treatments are available leaving practitioners with off-label use of swallowed steroids administered from multi-dose inhalers or dietary restrictions.
 |
|
The elimination of protein antigens has been accomplished through the use of elemental formulas, empiric elimination of the most common allergens, or targeted elimination based on skin or blood testing. A literature review revealed that these approaches are effective in inducing histologic remission in 90.8%, 72.1%, and 45.5% of patients, respectively.
This report by Meggadottir and Spergel provides at least two important findings for patients with EoE. Their data contribute to a growing body of literature and clinical observations indicating that mechanistic pathways other than IgE-mediated food reactions may contribute to the pathogenesis of EoE. Some recent reports and clinical experiences suggest the children with EoE may tolerate some allergenic foods that are cooked in a different way or children may outgrow their allergies. Results from this study suggest that this may not be the case. Clinical implications of their findings suggest that children and adults may need to continue to restrict common foods from their diets; the long-term impact of this on quality of life is not certain.
Dr. Glenn T. Furuta, professor of pediatrics, University of Colorado School of Medicine, Director of the Gastrointestinal Eosinophilic Diseases Program, Children’s Hospital Colorado, Denver. He is the co-founder of EnteroTrack.
Eosinophilic esophagitis (EoE) is a chronic condition that affects children and adults across the world. Children present with feeding problems, abdominal pain, and reflux-like symptoms, whereas adults complain of dysphagia and food impactions. No Food and Drug Administration–approved treatments are available leaving practitioners with off-label use of swallowed steroids administered from multi-dose inhalers or dietary restrictions.
 |
|
The elimination of protein antigens has been accomplished through the use of elemental formulas, empiric elimination of the most common allergens, or targeted elimination based on skin or blood testing. A literature review revealed that these approaches are effective in inducing histologic remission in 90.8%, 72.1%, and 45.5% of patients, respectively.
This report by Meggadottir and Spergel provides at least two important findings for patients with EoE. Their data contribute to a growing body of literature and clinical observations indicating that mechanistic pathways other than IgE-mediated food reactions may contribute to the pathogenesis of EoE. Some recent reports and clinical experiences suggest the children with EoE may tolerate some allergenic foods that are cooked in a different way or children may outgrow their allergies. Results from this study suggest that this may not be the case. Clinical implications of their findings suggest that children and adults may need to continue to restrict common foods from their diets; the long-term impact of this on quality of life is not certain.
Dr. Glenn T. Furuta, professor of pediatrics, University of Colorado School of Medicine, Director of the Gastrointestinal Eosinophilic Diseases Program, Children’s Hospital Colorado, Denver. He is the co-founder of EnteroTrack.
SAN DIEGO – Seventeen of 425 children who had eosinophilic esophagitis caused by a specific food developed the condition after outgrowing the allergy to that food, a retrospective study found.
People who outgrow a food allergy may be at risk of developing eosinophilic esophagitis (EoE) to the same food, Dr. Jonathan Spergel said during a press briefing at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He and his associates studied data on 1,025 children with EoE seen at the Children’s Hospital of Philadelphia in 2000-2012 to assess the prevalence of food allergy. In 425 children (42%), a specific food was identified as the EoE culprit – reintroducing the food to the diet caused esophageal changes on biopsy or biopsy changes normalized when the food was removed from the diet.
Eighty-four children had a history of IgE-mediated food allergy. Milk, egg, wheat, and soy were the most common food triggers of EoE in the 425 children in the study and in a subset of 17 who had outgrown IgE-mediated allergy to the specific food, reported Dr. Spergel, chief of the allergy section at the Children’s Hospital of Philadelphia. Sixteen of the 17 patients had atopic disease. The most common foods causing IgE-mediated allergy were peanuts, tree nuts, eggs, and milk.
The development of EoE coincided with reintroducing the food triggers. The time between outgrowing an allergy and reintroducing the food, triggering EoE, averaged 2 years but ranged from 6 months to 5 years.
Notably, two of the children who outgrew their food allergy had a normal biopsy of the esophagus when they had the food allergy, he said.
The findings support other recent studies suggesting that the pathophysiologies of EoE and IgE-mediated food allergy are distinct from each other, and that both can occur in the same individual to the same food, Dr. Spergel said. The mechanism by which EoE develops is poorly understood.
"I think these kids probably always had EoE to the food, but they weren’t eating it" because of the allergy, he said. "From 1% to 15% on oral immunotherapy get EoE, depending on which group you look at. I don’t think we caused" EoE by giving oral immunotherapy, he added. "We uncovered it."
Although it is rare for children who outgrow a food allergy to later develop EoE to that food, it’s worth keeping in mind if a child starts vomiting often or complains of stomachaches months or years later, Dr. Spergel said. Keeping the possibility in mind may help clinicians rule out other etiologies and detect EoE faster. "You have to take it seriously and get it checked out," he said.
Of the 84 patients with IgE-mediated food allergy, the 17 who outgrew the allergy and then developed EoE to the same food were significantly older (12 years, on average), compared with 67 patients who developed EoE from a different food from the one that caused their allergy.
The lead author on the study was Dr. Solrun Melkorka Meggadottir, a fellow at the Children’s Hospital of Philadelphia. The findings have been submitted to the Journal of Allergy and Clinical Immunology.
Dr. Spergel and Dr. Meggadottir reported having no disclosures.
sboschert@frontlinemedcom.com On Twitter @sherryboschert
SAN DIEGO – Seventeen of 425 children who had eosinophilic esophagitis caused by a specific food developed the condition after outgrowing the allergy to that food, a retrospective study found.
People who outgrow a food allergy may be at risk of developing eosinophilic esophagitis (EoE) to the same food, Dr. Jonathan Spergel said during a press briefing at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He and his associates studied data on 1,025 children with EoE seen at the Children’s Hospital of Philadelphia in 2000-2012 to assess the prevalence of food allergy. In 425 children (42%), a specific food was identified as the EoE culprit – reintroducing the food to the diet caused esophageal changes on biopsy or biopsy changes normalized when the food was removed from the diet.
Eighty-four children had a history of IgE-mediated food allergy. Milk, egg, wheat, and soy were the most common food triggers of EoE in the 425 children in the study and in a subset of 17 who had outgrown IgE-mediated allergy to the specific food, reported Dr. Spergel, chief of the allergy section at the Children’s Hospital of Philadelphia. Sixteen of the 17 patients had atopic disease. The most common foods causing IgE-mediated allergy were peanuts, tree nuts, eggs, and milk.
The development of EoE coincided with reintroducing the food triggers. The time between outgrowing an allergy and reintroducing the food, triggering EoE, averaged 2 years but ranged from 6 months to 5 years.
Notably, two of the children who outgrew their food allergy had a normal biopsy of the esophagus when they had the food allergy, he said.
The findings support other recent studies suggesting that the pathophysiologies of EoE and IgE-mediated food allergy are distinct from each other, and that both can occur in the same individual to the same food, Dr. Spergel said. The mechanism by which EoE develops is poorly understood.
"I think these kids probably always had EoE to the food, but they weren’t eating it" because of the allergy, he said. "From 1% to 15% on oral immunotherapy get EoE, depending on which group you look at. I don’t think we caused" EoE by giving oral immunotherapy, he added. "We uncovered it."
Although it is rare for children who outgrow a food allergy to later develop EoE to that food, it’s worth keeping in mind if a child starts vomiting often or complains of stomachaches months or years later, Dr. Spergel said. Keeping the possibility in mind may help clinicians rule out other etiologies and detect EoE faster. "You have to take it seriously and get it checked out," he said.
Of the 84 patients with IgE-mediated food allergy, the 17 who outgrew the allergy and then developed EoE to the same food were significantly older (12 years, on average), compared with 67 patients who developed EoE from a different food from the one that caused their allergy.
The lead author on the study was Dr. Solrun Melkorka Meggadottir, a fellow at the Children’s Hospital of Philadelphia. The findings have been submitted to the Journal of Allergy and Clinical Immunology.
Dr. Spergel and Dr. Meggadottir reported having no disclosures.
sboschert@frontlinemedcom.com On Twitter @sherryboschert
AT 2014 AAAAI ANNUAL MEETING
Major finding: Seventeen of 425 children with eosinophilic esophagitis caused by a specific food redeveloped the condition after outgrowing an allergy to the same food.
Data source: A retrospective study of data on 1,025 children seen at one institution for eosinophilic esophagitis.
Disclosures: Dr. Spergel and Dr. Meggadottir reported having no relevant financial disclosures.
Biomarker unreliability scratches trial of customized chemotherapy
Customized adjuvant chemotherapy for non–small-cell lung cancer proved feasible in a phase II trial in 150 patients, but the unreliability of some biomarker tests led to cancellation of a planned phase III study of the efficacy of individualizing treatment based on biomarker status.
The prospective phase II trial at 29 French centers enrolled patients who had completely resected nonsquamous cell, stage II or IIIA (non-N2) tumors and had no prior chemotherapy. Within 2 months of surgery, 80% of all patients had complete biomarker status results and were able to start adjuvant treatment, showing that timely customized adjuvant therapy is feasible, Dr. Marie Wislez and her associates reported in the Journal of Clinical Oncology.
The study randomized 74 patients to a control group receiving conventional treatment with four courses of cisplatin (75 mg/m2) plus pemetrexed (500 mg/m2) every 21 days plus vitamin B12 supplements and corticosteroids. Among 76 patients randomized to the customized treatment group, 7 patients with activated epidermal growth factor receptor (mutations were treated with oral erlotinib 150 mg/day for 1 year. In the customized group, 53 patients who were negative for excision repair cross-complementation group 1 (ERCC1) were to receive the same cisplatin/pemetrexed regimen as the control group, and 16 ERCC1-positive patients were simply followed, the investigators reported (J. Clin. Oncol. 2014 March 17 [doi:10.1200/JCO.2013.53.1525]).
Patients were to be followed every 6 months for the first 5 years after surgery. The adjuvant therapies generally were well tolerated.
The investigators discovered unexpectedly, however, that assays for ERCC1 expression were unreliable, reported Dr. Wislez of Tenon Hospital, Paris, and her associates. ERCC1-positive patients made up 25% of patients in the study, compared with 44% of patients with non–small-cell lung cancer in the previous IALT (International Adjuvant Lung Cancer) trial (N. Engl. J. Med. 2006;355:983-91).
When Dr. Wislez and her associates restained biosamples from the IALT trial using the 8F1 antibody test commercially available in 2011, they found important discrepancies between the IALT’s results from 2006 and those obtained in 2001.The unreliability of the ERCC1 immunohistochemical readouts led them to cancel the planned phase III TASTE (Tailored Postsurgical Therapy in Early-Stage NSCLC) trial until ERCC1 testing methodology can be improved.
The current phase II trial also found that the epidermal growth factor receptor mutation rate (6.7%) was lower than expected based on previously published data, whether analyzed using probe-specific polymerase chain reaction or by direct sequencing methodology.
Some of Dr. Wislez’s associates in the study reported financial associations with Roche, Eli Lilly, Pfizer, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Pfizer, and/or Amgen.
On Twitter @sherryboschert
Customized adjuvant chemotherapy for non–small-cell lung cancer proved feasible in a phase II trial in 150 patients, but the unreliability of some biomarker tests led to cancellation of a planned phase III study of the efficacy of individualizing treatment based on biomarker status.
The prospective phase II trial at 29 French centers enrolled patients who had completely resected nonsquamous cell, stage II or IIIA (non-N2) tumors and had no prior chemotherapy. Within 2 months of surgery, 80% of all patients had complete biomarker status results and were able to start adjuvant treatment, showing that timely customized adjuvant therapy is feasible, Dr. Marie Wislez and her associates reported in the Journal of Clinical Oncology.
The study randomized 74 patients to a control group receiving conventional treatment with four courses of cisplatin (75 mg/m2) plus pemetrexed (500 mg/m2) every 21 days plus vitamin B12 supplements and corticosteroids. Among 76 patients randomized to the customized treatment group, 7 patients with activated epidermal growth factor receptor (mutations were treated with oral erlotinib 150 mg/day for 1 year. In the customized group, 53 patients who were negative for excision repair cross-complementation group 1 (ERCC1) were to receive the same cisplatin/pemetrexed regimen as the control group, and 16 ERCC1-positive patients were simply followed, the investigators reported (J. Clin. Oncol. 2014 March 17 [doi:10.1200/JCO.2013.53.1525]).
Patients were to be followed every 6 months for the first 5 years after surgery. The adjuvant therapies generally were well tolerated.
The investigators discovered unexpectedly, however, that assays for ERCC1 expression were unreliable, reported Dr. Wislez of Tenon Hospital, Paris, and her associates. ERCC1-positive patients made up 25% of patients in the study, compared with 44% of patients with non–small-cell lung cancer in the previous IALT (International Adjuvant Lung Cancer) trial (N. Engl. J. Med. 2006;355:983-91).
When Dr. Wislez and her associates restained biosamples from the IALT trial using the 8F1 antibody test commercially available in 2011, they found important discrepancies between the IALT’s results from 2006 and those obtained in 2001.The unreliability of the ERCC1 immunohistochemical readouts led them to cancel the planned phase III TASTE (Tailored Postsurgical Therapy in Early-Stage NSCLC) trial until ERCC1 testing methodology can be improved.
The current phase II trial also found that the epidermal growth factor receptor mutation rate (6.7%) was lower than expected based on previously published data, whether analyzed using probe-specific polymerase chain reaction or by direct sequencing methodology.
Some of Dr. Wislez’s associates in the study reported financial associations with Roche, Eli Lilly, Pfizer, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Pfizer, and/or Amgen.
On Twitter @sherryboschert
Customized adjuvant chemotherapy for non–small-cell lung cancer proved feasible in a phase II trial in 150 patients, but the unreliability of some biomarker tests led to cancellation of a planned phase III study of the efficacy of individualizing treatment based on biomarker status.
The prospective phase II trial at 29 French centers enrolled patients who had completely resected nonsquamous cell, stage II or IIIA (non-N2) tumors and had no prior chemotherapy. Within 2 months of surgery, 80% of all patients had complete biomarker status results and were able to start adjuvant treatment, showing that timely customized adjuvant therapy is feasible, Dr. Marie Wislez and her associates reported in the Journal of Clinical Oncology.
The study randomized 74 patients to a control group receiving conventional treatment with four courses of cisplatin (75 mg/m2) plus pemetrexed (500 mg/m2) every 21 days plus vitamin B12 supplements and corticosteroids. Among 76 patients randomized to the customized treatment group, 7 patients with activated epidermal growth factor receptor (mutations were treated with oral erlotinib 150 mg/day for 1 year. In the customized group, 53 patients who were negative for excision repair cross-complementation group 1 (ERCC1) were to receive the same cisplatin/pemetrexed regimen as the control group, and 16 ERCC1-positive patients were simply followed, the investigators reported (J. Clin. Oncol. 2014 March 17 [doi:10.1200/JCO.2013.53.1525]).
Patients were to be followed every 6 months for the first 5 years after surgery. The adjuvant therapies generally were well tolerated.
The investigators discovered unexpectedly, however, that assays for ERCC1 expression were unreliable, reported Dr. Wislez of Tenon Hospital, Paris, and her associates. ERCC1-positive patients made up 25% of patients in the study, compared with 44% of patients with non–small-cell lung cancer in the previous IALT (International Adjuvant Lung Cancer) trial (N. Engl. J. Med. 2006;355:983-91).
When Dr. Wislez and her associates restained biosamples from the IALT trial using the 8F1 antibody test commercially available in 2011, they found important discrepancies between the IALT’s results from 2006 and those obtained in 2001.The unreliability of the ERCC1 immunohistochemical readouts led them to cancel the planned phase III TASTE (Tailored Postsurgical Therapy in Early-Stage NSCLC) trial until ERCC1 testing methodology can be improved.
The current phase II trial also found that the epidermal growth factor receptor mutation rate (6.7%) was lower than expected based on previously published data, whether analyzed using probe-specific polymerase chain reaction or by direct sequencing methodology.
Some of Dr. Wislez’s associates in the study reported financial associations with Roche, Eli Lilly, Pfizer, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Pfizer, and/or Amgen.
On Twitter @sherryboschert
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Major finding: Eighty percent of patients had biomarker status results and could start adjuvant chemotherapy within 2 months of surgery, but test results for the biomarker ERCC1 appeared to be unreliable.
Data source: Prospective phase II study of 150 chemotherapy-naive patients with nonsquamous cell, stage II or IIIa non–small-cell lung cancer.
Disclosures: Some of Dr. Wislez’s associates in the study reported financial associations with Roche, Eli Lilly, Pfizer, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Pfizer, and/or Amgen.
Amrubicin combination fails in phase III trial
The combination of amrubicin plus cisplatin proved inferior to irinotecan plus cisplatin for the treatment of extensive-disease small-cell lung cancer in a phase III trial of 284 patients.
Patients in the amrubicin/cisplatin group survived a median of 15 months, significantly shorter than the median overall survival of 17.7 months in the irinotecan/cisplatin group.
The hazard ratio for the amrubicin/cisplatin group compared with the irinotecan/cisplatin group was 1.43, exceeding the predetermined noninferiority margin of 1.31, which triggered early publication of results in Journal of Clinical Oncology, Dr. Miyako Satouchi and associates reported (2014 March 17 [doi:10.1200/JCO.2013.53.5153]).
Enrollment ended in December 2010, and updated interim analyses included results as of May 2012.
Amrubicin is a synthetic anthracycline derivative that had shown some promise in phase I/II clinical trials. The investigators had hoped it might be as effective as irinotecan and cause less grade 3-4 diarrhea. Compared with the irinotecan/cisplatin group, patients in the amrubicin/cisplatin group in the current study were less likely to develop grade 3-4 diarrhea (1% vs. 8%) but were more likely to develop grade 4 neutropenia (79% vs. 22%) or grade 3-4 febrile neutropenia (32% vs. 11%), reported Dr. Satouchi of Hyogo Cancer Center, Akashi, Japan, and associates.
Initially, patients in the amrubicin/cisplatin group underwent four cycles of IV amrubicin 40 mg/m2 on days 1, 2, and 3 and IV cisplatin 60 mg/m2 on day 1 every 3 weeks. In an effort to reduce severe hematologic toxicities, the investigators revised the protocol during the study to reduce the initial dose of amrubicin to 35 mg/m2. Patients in the irinotecan/cisplatin group underwent four cycles of IV irinotecan 60 mg/m2 on days 1, 8, and 15 and IV cisplatin 60 mg/m2 on day 1.
Among secondary outcomes, in the amrubicin/cisplatin group, the response rate was 78%, and the median progression-free survival was 5.1 months, compared with a response rate of 72% and a median progression-free survival of 5.6 months on irinotecan/cisplatin.
An analysis of treatment after the study found that two-thirds of patients from the irinotecan/cisplatin group subsequently received amrubicin as a single agent, which may have contributed to the group’s better overall survival, the investigators said. It may be that amrubicin is less synergistic than irinotecan with cisplatin and thus is inferior as a first-line combination, but amrubicin may still be a valuable second- or third-line therapy for extensive-disease small-cell lung cancer, the investigators said.
Dr. Satouchi reported receiving honoraria from Nippon Kayaku, and some of the other investigators in the study reported financial relationships with Nippon Kayaku, Daiichi Sankyo, and/or Bristol-Myers Squibb.
On Twitter @sherryboschert
The combination of amrubicin plus cisplatin proved inferior to irinotecan plus cisplatin for the treatment of extensive-disease small-cell lung cancer in a phase III trial of 284 patients.
Patients in the amrubicin/cisplatin group survived a median of 15 months, significantly shorter than the median overall survival of 17.7 months in the irinotecan/cisplatin group.
The hazard ratio for the amrubicin/cisplatin group compared with the irinotecan/cisplatin group was 1.43, exceeding the predetermined noninferiority margin of 1.31, which triggered early publication of results in Journal of Clinical Oncology, Dr. Miyako Satouchi and associates reported (2014 March 17 [doi:10.1200/JCO.2013.53.5153]).
Enrollment ended in December 2010, and updated interim analyses included results as of May 2012.
Amrubicin is a synthetic anthracycline derivative that had shown some promise in phase I/II clinical trials. The investigators had hoped it might be as effective as irinotecan and cause less grade 3-4 diarrhea. Compared with the irinotecan/cisplatin group, patients in the amrubicin/cisplatin group in the current study were less likely to develop grade 3-4 diarrhea (1% vs. 8%) but were more likely to develop grade 4 neutropenia (79% vs. 22%) or grade 3-4 febrile neutropenia (32% vs. 11%), reported Dr. Satouchi of Hyogo Cancer Center, Akashi, Japan, and associates.
Initially, patients in the amrubicin/cisplatin group underwent four cycles of IV amrubicin 40 mg/m2 on days 1, 2, and 3 and IV cisplatin 60 mg/m2 on day 1 every 3 weeks. In an effort to reduce severe hematologic toxicities, the investigators revised the protocol during the study to reduce the initial dose of amrubicin to 35 mg/m2. Patients in the irinotecan/cisplatin group underwent four cycles of IV irinotecan 60 mg/m2 on days 1, 8, and 15 and IV cisplatin 60 mg/m2 on day 1.
Among secondary outcomes, in the amrubicin/cisplatin group, the response rate was 78%, and the median progression-free survival was 5.1 months, compared with a response rate of 72% and a median progression-free survival of 5.6 months on irinotecan/cisplatin.
An analysis of treatment after the study found that two-thirds of patients from the irinotecan/cisplatin group subsequently received amrubicin as a single agent, which may have contributed to the group’s better overall survival, the investigators said. It may be that amrubicin is less synergistic than irinotecan with cisplatin and thus is inferior as a first-line combination, but amrubicin may still be a valuable second- or third-line therapy for extensive-disease small-cell lung cancer, the investigators said.
Dr. Satouchi reported receiving honoraria from Nippon Kayaku, and some of the other investigators in the study reported financial relationships with Nippon Kayaku, Daiichi Sankyo, and/or Bristol-Myers Squibb.
On Twitter @sherryboschert
The combination of amrubicin plus cisplatin proved inferior to irinotecan plus cisplatin for the treatment of extensive-disease small-cell lung cancer in a phase III trial of 284 patients.
Patients in the amrubicin/cisplatin group survived a median of 15 months, significantly shorter than the median overall survival of 17.7 months in the irinotecan/cisplatin group.
The hazard ratio for the amrubicin/cisplatin group compared with the irinotecan/cisplatin group was 1.43, exceeding the predetermined noninferiority margin of 1.31, which triggered early publication of results in Journal of Clinical Oncology, Dr. Miyako Satouchi and associates reported (2014 March 17 [doi:10.1200/JCO.2013.53.5153]).
Enrollment ended in December 2010, and updated interim analyses included results as of May 2012.
Amrubicin is a synthetic anthracycline derivative that had shown some promise in phase I/II clinical trials. The investigators had hoped it might be as effective as irinotecan and cause less grade 3-4 diarrhea. Compared with the irinotecan/cisplatin group, patients in the amrubicin/cisplatin group in the current study were less likely to develop grade 3-4 diarrhea (1% vs. 8%) but were more likely to develop grade 4 neutropenia (79% vs. 22%) or grade 3-4 febrile neutropenia (32% vs. 11%), reported Dr. Satouchi of Hyogo Cancer Center, Akashi, Japan, and associates.
Initially, patients in the amrubicin/cisplatin group underwent four cycles of IV amrubicin 40 mg/m2 on days 1, 2, and 3 and IV cisplatin 60 mg/m2 on day 1 every 3 weeks. In an effort to reduce severe hematologic toxicities, the investigators revised the protocol during the study to reduce the initial dose of amrubicin to 35 mg/m2. Patients in the irinotecan/cisplatin group underwent four cycles of IV irinotecan 60 mg/m2 on days 1, 8, and 15 and IV cisplatin 60 mg/m2 on day 1.
Among secondary outcomes, in the amrubicin/cisplatin group, the response rate was 78%, and the median progression-free survival was 5.1 months, compared with a response rate of 72% and a median progression-free survival of 5.6 months on irinotecan/cisplatin.
An analysis of treatment after the study found that two-thirds of patients from the irinotecan/cisplatin group subsequently received amrubicin as a single agent, which may have contributed to the group’s better overall survival, the investigators said. It may be that amrubicin is less synergistic than irinotecan with cisplatin and thus is inferior as a first-line combination, but amrubicin may still be a valuable second- or third-line therapy for extensive-disease small-cell lung cancer, the investigators said.
Dr. Satouchi reported receiving honoraria from Nippon Kayaku, and some of the other investigators in the study reported financial relationships with Nippon Kayaku, Daiichi Sankyo, and/or Bristol-Myers Squibb.
On Twitter @sherryboschert
FROM JOURNAL OF CLINICAL ONCOLOGY
Major finding: Median overall survival was 15 months using amrubicin/cisplatin, significantly worse than 17.7 months using irinotecan/cisplatin.
Data source: A multicenter, randomized phase III noninferiority trial in 284 patients with extensive-disease small-cell lung cancer.
Disclosures: Dr. Satouchi reported receiving honoraria from Nippon Kayaku, and some of the other investigators in the study reported financial relationships with Nippon Kayaku, Daiichi Sankyo, and/or Bristol-Myers Squibb.
Palliative care shortens ICU, hospital stays, review shows
SAN FRANCISCO – Palliative care in the intensive care unit reduces the length of stay in the ICU and the hospital without changing mortality rates or family satisfaction, according to a review of the literature.
Although measurements of family satisfaction overall didn’t change much from palliative care of a loved one in the ICU, some measures of components of satisfaction increased with palliative care, such as improved communication with the physician, better consensus around the goals of care, and decreased anxiety and depression in family members, reported Dr. Rebecca A. Aslakson of Johns Hopkins University, Baltimore, and her colleagues.
Dr. Aslakson presented the findings at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Dr. Aslakson and her associates were unable to perform a formal meta-analysis of the 37 published trials of palliative care in the ICU because of the heterogeneity of the studies, which looked at more than 40 different outcomes.
Instead, their systematic review grouped results under four outcomes that commonly were measured, and assessed those either by the number of studies or by the number of patients studied.
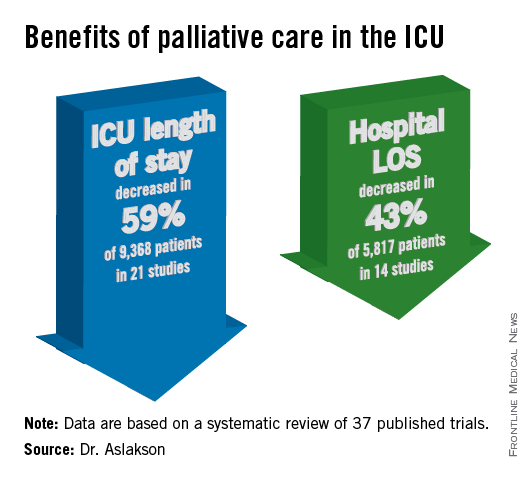
ICU length of stay decreased with palliative care in 13 of 21 studies (62%) that used this outcome and in 59% of 9,368 patients in those studies.
Hospital length of stay decreased with palliative care in 8 of 14 studies (57%) and in 43% of 5,817 patients. Family satisfaction did not decrease in any studies or families and increased in only 1 of 14 studies (7%) and in 2% of families of 4,927 patients, Dr. Aslakson and her colleagues reported (J. Palliat. Med. 2014;17:219-35).
Mortality rates did not change with palliative care in 14 of 16 studies (88%) that assessed mortality and in 57% of 5,969 patients in those studies. Mortality increased in one small study (6%) and decreased in one larger study (6%).
"Talking about big-picture issues and goals of care doesn’t lead to people dying," Dr. Aslakson said.
"No harm came in any of these studies." Some separate studies of palliative care outside of ICUs reported that this increases hope, "because people feel that they have more control over their choices and what’s happening to their loved ones," she added.
Integrative vs. consultative model
Dr. Aslakson and her associates also reviewed studies based on whether the interventions used integrative or consultative models of palliative care.
Generally, consultative models bring outsiders into the ICU to help provide palliative care, and integrative models train the ICU team to be the palliative care providers. In reality, the two models may overlap. For this review, the investigators applied mutually exclusive definitions to 36 of the studies.
In 18 studies of integrative interventions, members of the ICU team were the only caregivers in face-to-face interactions with the patient and families. In 18 studies of consultative interventions, palliative care providers included others besides the ICU team.
In the studies of integrative palliative care, ICU length of stay decreased with palliative care in four of nine studies (44%) that measured this outcome and in 52% of 6,963 patients in those studies, she reported. Hospital length of stay decreased in two of five studies (40%) and in 24% of 3,812 patients. Family satisfaction changed in none of 15 studies, and mortality decreased in 1 of 5 studies (20%) and in 34% of 3,807 patients.
In the studies of consultative care, ICU length of stay decreased with palliative care in 9 of 12 studies (75%) that measured this outcome and in 79% of 2,405 patients in those studies. Hospital length of stay decreased in six of nine studies (67%) and in 79% of 2,005 patients. Family satisfaction increased in one of four studies (25%) and in 21% of 429 patients. Mortality increased in 1 of 11 studies (9%) and in 5% of 2,162 patients.
One model isn’t necessarily better than the other, Dr. Aslakson said. Integrative palliative care may work best in a closed ICU with perhaps four or five intensivists in a relatively small unit. An integrative approach can be much more difficult in open or semiopen ICUs that have "40 different doctors floating around," she said. "We tried that in my unit, and it didn’t work that well."
Different ICUs need palliative care models that fit them. "Look at your unit, the way it works, and who the providers are, then look at the literature and see what matches that and what might work for your unit," she said.
Outcomes of better communication
A previous, separate review of the medical literature identified 21 controlled trials of 16 interventions to improve communication in ICUs between families and care providers. Overall, the interventions improved emotional outcomes for families and reduced ICU length of stay and treatment intensity (Chest 2011;139:543-54), she noted.
Yet another prior review of the literature reported that interventions to promote family meetings, use empathetic communication skills, and employ palliative care consultations improved family satisfaction and reduced ICU length of stay and the adverse effects of family bereavement (Curr. Opin. Crit. Care 2009;15:569-77).
Dr. Aslakson reported having no financial disclosures.
Dr. Jennifer Cox, FCCP, comments: Dr. Aslakson and colleagues’ systematic review adds to the body of literature that demonstrates no mortality increase when palliative care measures are initiated in the ICU. Shorter lengths of stay both in the ICU and hospital were other positive outcomes noted without a significant change in patient or family satisfaction.
These findings were independent of whether an integrative or consultative approach to palliative care was undertaken. This should encourage physicians to examine their practice setting and determine which approach meets the needs of their ICU and begin to utilize palliative care earlier and more aggressively without fear of increasing mortality.
sboschert@frontlinemedcom.com
On Twitter @sherryboschert
SAN FRANCISCO – Palliative care in the intensive care unit reduces the length of stay in the ICU and the hospital without changing mortality rates or family satisfaction, according to a review of the literature.
Although measurements of family satisfaction overall didn’t change much from palliative care of a loved one in the ICU, some measures of components of satisfaction increased with palliative care, such as improved communication with the physician, better consensus around the goals of care, and decreased anxiety and depression in family members, reported Dr. Rebecca A. Aslakson of Johns Hopkins University, Baltimore, and her colleagues.
Dr. Aslakson presented the findings at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.

Dr. Aslakson and her associates were unable to perform a formal meta-analysis of the 37 published trials of palliative care in the ICU because of the heterogeneity of the studies, which looked at more than 40 different outcomes.
Instead, their systematic review grouped results under four outcomes that commonly were measured, and assessed those either by the number of studies or by the number of patients studied.
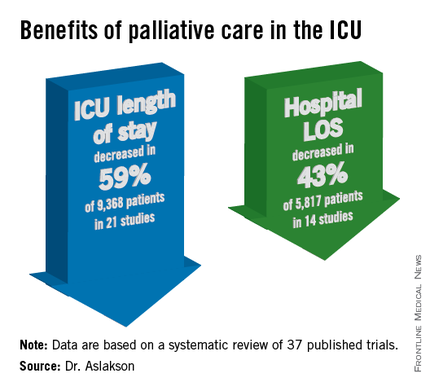
ICU length of stay decreased with palliative care in 13 of 21 studies (62%) that used this outcome and in 59% of 9,368 patients in those studies.
Hospital length of stay decreased with palliative care in 8 of 14 studies (57%) and in 43% of 5,817 patients. Family satisfaction did not decrease in any studies or families and increased in only 1 of 14 studies (7%) and in 2% of families of 4,927 patients, Dr. Aslakson and her colleagues reported (J. Palliat. Med. 2014;17:219-35).
Mortality rates did not change with palliative care in 14 of 16 studies (88%) that assessed mortality and in 57% of 5,969 patients in those studies. Mortality increased in one small study (6%) and decreased in one larger study (6%).
"Talking about big-picture issues and goals of care doesn’t lead to people dying," Dr. Aslakson said.
"No harm came in any of these studies." Some separate studies of palliative care outside of ICUs reported that this increases hope, "because people feel that they have more control over their choices and what’s happening to their loved ones," she added.
Integrative vs. consultative model
Dr. Aslakson and her associates also reviewed studies based on whether the interventions used integrative or consultative models of palliative care.
Generally, consultative models bring outsiders into the ICU to help provide palliative care, and integrative models train the ICU team to be the palliative care providers. In reality, the two models may overlap. For this review, the investigators applied mutually exclusive definitions to 36 of the studies.
In 18 studies of integrative interventions, members of the ICU team were the only caregivers in face-to-face interactions with the patient and families. In 18 studies of consultative interventions, palliative care providers included others besides the ICU team.
In the studies of integrative palliative care, ICU length of stay decreased with palliative care in four of nine studies (44%) that measured this outcome and in 52% of 6,963 patients in those studies, she reported. Hospital length of stay decreased in two of five studies (40%) and in 24% of 3,812 patients. Family satisfaction changed in none of 15 studies, and mortality decreased in 1 of 5 studies (20%) and in 34% of 3,807 patients.
In the studies of consultative care, ICU length of stay decreased with palliative care in 9 of 12 studies (75%) that measured this outcome and in 79% of 2,405 patients in those studies. Hospital length of stay decreased in six of nine studies (67%) and in 79% of 2,005 patients. Family satisfaction increased in one of four studies (25%) and in 21% of 429 patients. Mortality increased in 1 of 11 studies (9%) and in 5% of 2,162 patients.
One model isn’t necessarily better than the other, Dr. Aslakson said. Integrative palliative care may work best in a closed ICU with perhaps four or five intensivists in a relatively small unit. An integrative approach can be much more difficult in open or semiopen ICUs that have "40 different doctors floating around," she said. "We tried that in my unit, and it didn’t work that well."
Different ICUs need palliative care models that fit them. "Look at your unit, the way it works, and who the providers are, then look at the literature and see what matches that and what might work for your unit," she said.
Outcomes of better communication
A previous, separate review of the medical literature identified 21 controlled trials of 16 interventions to improve communication in ICUs between families and care providers. Overall, the interventions improved emotional outcomes for families and reduced ICU length of stay and treatment intensity (Chest 2011;139:543-54), she noted.
Yet another prior review of the literature reported that interventions to promote family meetings, use empathetic communication skills, and employ palliative care consultations improved family satisfaction and reduced ICU length of stay and the adverse effects of family bereavement (Curr. Opin. Crit. Care 2009;15:569-77).
Dr. Aslakson reported having no financial disclosures.
Dr. Jennifer Cox, FCCP, comments: Dr. Aslakson and colleagues’ systematic review adds to the body of literature that demonstrates no mortality increase when palliative care measures are initiated in the ICU. Shorter lengths of stay both in the ICU and hospital were other positive outcomes noted without a significant change in patient or family satisfaction.
These findings were independent of whether an integrative or consultative approach to palliative care was undertaken. This should encourage physicians to examine their practice setting and determine which approach meets the needs of their ICU and begin to utilize palliative care earlier and more aggressively without fear of increasing mortality.
sboschert@frontlinemedcom.com
On Twitter @sherryboschert
SAN FRANCISCO – Palliative care in the intensive care unit reduces the length of stay in the ICU and the hospital without changing mortality rates or family satisfaction, according to a review of the literature.
Although measurements of family satisfaction overall didn’t change much from palliative care of a loved one in the ICU, some measures of components of satisfaction increased with palliative care, such as improved communication with the physician, better consensus around the goals of care, and decreased anxiety and depression in family members, reported Dr. Rebecca A. Aslakson of Johns Hopkins University, Baltimore, and her colleagues.
Dr. Aslakson presented the findings at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.

Dr. Aslakson and her associates were unable to perform a formal meta-analysis of the 37 published trials of palliative care in the ICU because of the heterogeneity of the studies, which looked at more than 40 different outcomes.
Instead, their systematic review grouped results under four outcomes that commonly were measured, and assessed those either by the number of studies or by the number of patients studied.
ICU length of stay decreased with palliative care in 13 of 21 studies (62%) that used this outcome and in 59% of 9,368 patients in those studies.
Hospital length of stay decreased with palliative care in 8 of 14 studies (57%) and in 43% of 5,817 patients. Family satisfaction did not decrease in any studies or families and increased in only 1 of 14 studies (7%) and in 2% of families of 4,927 patients, Dr. Aslakson and her colleagues reported (J. Palliat. Med. 2014;17:219-35).
Mortality rates did not change with palliative care in 14 of 16 studies (88%) that assessed mortality and in 57% of 5,969 patients in those studies. Mortality increased in one small study (6%) and decreased in one larger study (6%).
"Talking about big-picture issues and goals of care doesn’t lead to people dying," Dr. Aslakson said.
"No harm came in any of these studies." Some separate studies of palliative care outside of ICUs reported that this increases hope, "because people feel that they have more control over their choices and what’s happening to their loved ones," she added.
Integrative vs. consultative model
Dr. Aslakson and her associates also reviewed studies based on whether the interventions used integrative or consultative models of palliative care.
Generally, consultative models bring outsiders into the ICU to help provide palliative care, and integrative models train the ICU team to be the palliative care providers. In reality, the two models may overlap. For this review, the investigators applied mutually exclusive definitions to 36 of the studies.
In 18 studies of integrative interventions, members of the ICU team were the only caregivers in face-to-face interactions with the patient and families. In 18 studies of consultative interventions, palliative care providers included others besides the ICU team.
In the studies of integrative palliative care, ICU length of stay decreased with palliative care in four of nine studies (44%) that measured this outcome and in 52% of 6,963 patients in those studies, she reported. Hospital length of stay decreased in two of five studies (40%) and in 24% of 3,812 patients. Family satisfaction changed in none of 15 studies, and mortality decreased in 1 of 5 studies (20%) and in 34% of 3,807 patients.
In the studies of consultative care, ICU length of stay decreased with palliative care in 9 of 12 studies (75%) that measured this outcome and in 79% of 2,405 patients in those studies. Hospital length of stay decreased in six of nine studies (67%) and in 79% of 2,005 patients. Family satisfaction increased in one of four studies (25%) and in 21% of 429 patients. Mortality increased in 1 of 11 studies (9%) and in 5% of 2,162 patients.
One model isn’t necessarily better than the other, Dr. Aslakson said. Integrative palliative care may work best in a closed ICU with perhaps four or five intensivists in a relatively small unit. An integrative approach can be much more difficult in open or semiopen ICUs that have "40 different doctors floating around," she said. "We tried that in my unit, and it didn’t work that well."
Different ICUs need palliative care models that fit them. "Look at your unit, the way it works, and who the providers are, then look at the literature and see what matches that and what might work for your unit," she said.
Outcomes of better communication
A previous, separate review of the medical literature identified 21 controlled trials of 16 interventions to improve communication in ICUs between families and care providers. Overall, the interventions improved emotional outcomes for families and reduced ICU length of stay and treatment intensity (Chest 2011;139:543-54), she noted.
Yet another prior review of the literature reported that interventions to promote family meetings, use empathetic communication skills, and employ palliative care consultations improved family satisfaction and reduced ICU length of stay and the adverse effects of family bereavement (Curr. Opin. Crit. Care 2009;15:569-77).
Dr. Aslakson reported having no financial disclosures.
Dr. Jennifer Cox, FCCP, comments: Dr. Aslakson and colleagues’ systematic review adds to the body of literature that demonstrates no mortality increase when palliative care measures are initiated in the ICU. Shorter lengths of stay both in the ICU and hospital were other positive outcomes noted without a significant change in patient or family satisfaction.
These findings were independent of whether an integrative or consultative approach to palliative care was undertaken. This should encourage physicians to examine their practice setting and determine which approach meets the needs of their ICU and begin to utilize palliative care earlier and more aggressively without fear of increasing mortality.
sboschert@frontlinemedcom.com
On Twitter @sherryboschert
Relatively few in ICUs get end-of-life dialogue training
SAN FRANCISCO – Despite training recommendations, half of physicians and less than a third of nurses surveyed in adult intensive care units at 56 California hospitals reported receiving formal training in talking with patients and families about the end of life.
A 2008 consensus statement by the American College of Critical Care Medicine included a recommendation for end-of-life communication skills training for clinicians to improve the care of patients dying in ICUs ((Crit. Care Med. 2008;36:953-63).
Dr. Matthew H.R. Anstey and his associates approached 149 California hospitals to gauge the extent of implementation of this recommendation. At 56 hospitals, doctors and nurses who work in adult ICUs voluntarily completed an anonymous web-based survey. Eighty-four percent of the 1,363 respondents were nurses, he reported in a poster presentation at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Overall, 32% of the respondents said they had received formal training in communication skills. A significantly higher percentage of doctors had undergone training (50%) compared with nurses (29%), said Dr. Anstey, who is currently a lecturer in anesthesia at Harvard Medical School, Boston.
Sixty-six percent of all respondents agreed that "nurses are present during the communication of end-of-life information to the family" at their institution. Nurses were significantly more likely to agree with this statement (69%) than were doctors (52%).
Both doctors and nurses were very supportive of the idea of formal communication training for ICU providers. When asked about possible strategies to reduce inappropriate care for ICU patients, 91% of respondents said communication training would have a positive effect, Dr. Anstey reported.
This could be accomplished by requiring ICU physicians to complete a communication training module for ongoing credentialing, he said in an e-mail interview. Either individual hospitals could require this as part of credentialing for privileges to work in the ICU, or state medical boards could require it, similar to the California Medical Board’s requirement that physicians obtain some continuing medical education in pain management, he suggested.
The characteristics of participating hospitals were similar to those of nonparticipating hospitals in the sizes of the hospitals and ICUs, their regional location in California, and the proportions of hospitals that are teaching facilities.
The 93 nonparticipating hospitals were significantly more likely to be for-profit hospitals (59%) compared with participating hospitals (7%), and significantly less likely to be part of a hospital system containing more than three hospitals (54%) compared with participating hospitals (75%).
Dr. Anstey reported having no financial disclosures. His research was in conjunction with a Commonwealth Fund Harkness Fellowship in Health Care Policy and Practice for which he was placed at Kaiser Permanente in California.
Dr. Paul A. Selecky, FCCP, comments: Physicians are notorious about not doing a good job of communicating with patients in general, and when you focus on a vital subject as end-of-life care, it is of even greater importance. The findings in this study are not surprising. The unanswered question is how to fix it.
On Twitter @sherryboschert
SAN FRANCISCO – Despite training recommendations, half of physicians and less than a third of nurses surveyed in adult intensive care units at 56 California hospitals reported receiving formal training in talking with patients and families about the end of life.
A 2008 consensus statement by the American College of Critical Care Medicine included a recommendation for end-of-life communication skills training for clinicians to improve the care of patients dying in ICUs ((Crit. Care Med. 2008;36:953-63).
Dr. Matthew H.R. Anstey and his associates approached 149 California hospitals to gauge the extent of implementation of this recommendation. At 56 hospitals, doctors and nurses who work in adult ICUs voluntarily completed an anonymous web-based survey. Eighty-four percent of the 1,363 respondents were nurses, he reported in a poster presentation at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Overall, 32% of the respondents said they had received formal training in communication skills. A significantly higher percentage of doctors had undergone training (50%) compared with nurses (29%), said Dr. Anstey, who is currently a lecturer in anesthesia at Harvard Medical School, Boston.
Sixty-six percent of all respondents agreed that "nurses are present during the communication of end-of-life information to the family" at their institution. Nurses were significantly more likely to agree with this statement (69%) than were doctors (52%).
Both doctors and nurses were very supportive of the idea of formal communication training for ICU providers. When asked about possible strategies to reduce inappropriate care for ICU patients, 91% of respondents said communication training would have a positive effect, Dr. Anstey reported.
This could be accomplished by requiring ICU physicians to complete a communication training module for ongoing credentialing, he said in an e-mail interview. Either individual hospitals could require this as part of credentialing for privileges to work in the ICU, or state medical boards could require it, similar to the California Medical Board’s requirement that physicians obtain some continuing medical education in pain management, he suggested.
The characteristics of participating hospitals were similar to those of nonparticipating hospitals in the sizes of the hospitals and ICUs, their regional location in California, and the proportions of hospitals that are teaching facilities.
The 93 nonparticipating hospitals were significantly more likely to be for-profit hospitals (59%) compared with participating hospitals (7%), and significantly less likely to be part of a hospital system containing more than three hospitals (54%) compared with participating hospitals (75%).
Dr. Anstey reported having no financial disclosures. His research was in conjunction with a Commonwealth Fund Harkness Fellowship in Health Care Policy and Practice for which he was placed at Kaiser Permanente in California.
Dr. Paul A. Selecky, FCCP, comments: Physicians are notorious about not doing a good job of communicating with patients in general, and when you focus on a vital subject as end-of-life care, it is of even greater importance. The findings in this study are not surprising. The unanswered question is how to fix it.
On Twitter @sherryboschert
SAN FRANCISCO – Despite training recommendations, half of physicians and less than a third of nurses surveyed in adult intensive care units at 56 California hospitals reported receiving formal training in talking with patients and families about the end of life.
A 2008 consensus statement by the American College of Critical Care Medicine included a recommendation for end-of-life communication skills training for clinicians to improve the care of patients dying in ICUs ((Crit. Care Med. 2008;36:953-63).
Dr. Matthew H.R. Anstey and his associates approached 149 California hospitals to gauge the extent of implementation of this recommendation. At 56 hospitals, doctors and nurses who work in adult ICUs voluntarily completed an anonymous web-based survey. Eighty-four percent of the 1,363 respondents were nurses, he reported in a poster presentation at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Overall, 32% of the respondents said they had received formal training in communication skills. A significantly higher percentage of doctors had undergone training (50%) compared with nurses (29%), said Dr. Anstey, who is currently a lecturer in anesthesia at Harvard Medical School, Boston.
Sixty-six percent of all respondents agreed that "nurses are present during the communication of end-of-life information to the family" at their institution. Nurses were significantly more likely to agree with this statement (69%) than were doctors (52%).
Both doctors and nurses were very supportive of the idea of formal communication training for ICU providers. When asked about possible strategies to reduce inappropriate care for ICU patients, 91% of respondents said communication training would have a positive effect, Dr. Anstey reported.
This could be accomplished by requiring ICU physicians to complete a communication training module for ongoing credentialing, he said in an e-mail interview. Either individual hospitals could require this as part of credentialing for privileges to work in the ICU, or state medical boards could require it, similar to the California Medical Board’s requirement that physicians obtain some continuing medical education in pain management, he suggested.
The characteristics of participating hospitals were similar to those of nonparticipating hospitals in the sizes of the hospitals and ICUs, their regional location in California, and the proportions of hospitals that are teaching facilities.
The 93 nonparticipating hospitals were significantly more likely to be for-profit hospitals (59%) compared with participating hospitals (7%), and significantly less likely to be part of a hospital system containing more than three hospitals (54%) compared with participating hospitals (75%).
Dr. Anstey reported having no financial disclosures. His research was in conjunction with a Commonwealth Fund Harkness Fellowship in Health Care Policy and Practice for which he was placed at Kaiser Permanente in California.
Dr. Paul A. Selecky, FCCP, comments: Physicians are notorious about not doing a good job of communicating with patients in general, and when you focus on a vital subject as end-of-life care, it is of even greater importance. The findings in this study are not surprising. The unanswered question is how to fix it.
On Twitter @sherryboschert
Major finding: Half of doctors and 29% of nurses in ICUs said they had received formal training in end-of-life communications.
Data source: A voluntary web-based survey of 1,363 doctors and nurses working in adult ICUs in 56 California hospitals.
Disclosures: Dr. Anstey reported having no financial disclosures. His research was in conjunction with a Commonwealth Fund Harkness Fellowship in Health Care Policy and Practice, for which he was placed at Kaiser Permanente in California.
Two definitions of Gulf War illness recommended
An Institute of Medicine committee could not reach consensus on a single definition of Gulf War illness and recommended in a new report that clinicians and researchers at least narrow it down to two definitions.
That’s the preferred term – Gulf War illness – for a slew of health problems that occur at a higher rate in the approximately 697,000 U.S. veterans who served in the 1990-1991 Gulf War than in other veterans, the committee said. Gulf War illness is widely used and is more accurate than other terms used over the years for the same problems, including Gulf War syndrome, chronic multisystem illness, unexplained illness, medically unexplained symptoms, and medically unexplained physical symptoms, the report stated.
The Institute of Medicine (IOM) drafted a committee of 16 experts at the request of the Department of Veterans Affairs to try to develop a case definition for what's now called Gulf War illness in order to help standardize diagnosis, inclusion in research studies, and treatments. The committee's 120-page report, "Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined," is available on the IOM website.
Unable to find validation for one definition because of methodologic limitations in the studies, the committee recommended using either a broader definition from the Centers for Disease Control and Prevention (CDC) or a more restrictive one from studies of Kansas veterans, depending on the situation. Between the two of them, the definitions capture the most common symptoms of Gulf War illness and provide a framework for more focused research and treatment, the report said.
The committee found 2,033 articles in the literature focused on Gulf War illness symptoms and closely reviewed 718 of them. It found no objective diagnostic criteria but saw cumulative evidence that Gulf War illness is real. Fatigue, pain, and neurocognitive, gastrointestinal, respiratory, and/or dermatologic symptoms were reported with higher frequency in Gulf War veterans than in veterans of the same era who had not been deployed or were deployed elsewhere.
To identify the illness in a Gulf War veteran, the CDC definition requires one or more symptoms in at least two of the following categories: fatigue, pain, or mood and cognition. The Kansas definition requires symptoms in at least three of the following domains: fatigue or sleep, pain, neurologic or cognitive or mood symptoms, gastrointestinal symptoms, respiratory symptoms, and skin symptoms.
The CDC definition identified the illness in 29%-60% of Gulf War veterans, depending on the population studied. The Kansas definition identified the illness in 34% of Kansas veterans of the Gulf War.
The reason the committee couldn’t compile a single case definition is that the studies in the literature do not report on key features of the illness including onset, duration, severity, frequency of symptoms, and exclusionary criteria. The Department of Veterans Affairs should boost efforts to collect these kinds of data, conduct earlier in-depth assessments after deployment when unexpected complaints occur, and track troops’ exposures to vaccinations, drugs, and environmental factors, the report recommends.
The name Gulf War illness won out over Gulf War syndrome because "syndrome" indicates a new group of signs and symptoms, while the types and patterns of symptoms in this illness commonly are seen with other illnesses. The committee rejected "chronic multisymptom illness" because the phrase is not specific to Gulf War veterans.
The committee included experts in clinical medicine, toxicology, psychiatry, neurology, gastroenterology, epidemiology, sociology, psychometrics, biostatistics, occupational medicine, and basic science.
The report does not include potential disclosures of conflicts of interest.
On Twitter @sherryboschert
There has long been debate about whether Gulf War syndrome or Gulf War illness existed. If you remember the time of the Gulf War and shortly afterwards, there were a lot of questions about whether this was "all in their heads." Nobody ever found the sources of the illness. This report says to me that it’s final – that this august institution says, yes, this illness does exist. Whether you use the wider or narrower definition, this is to be taken seriously. That’s very important for physicians to know.
In terms of the two definitions – one narrower to be used for research and one broader to be used clinically – I think this gives people a little bit of latitude, depending on where they fit. The take-home piece is that, since there has been such a smorgasbord of opinions, thoughts, and descriptions, this should help the field by solidifying it further. We do still have two working definitions and we still don’t know a lot such as onset, duration, severity, frequency, and exclusionary criteria. Certainly, more needs to be done, but I think this is a very important step.
 |
|
Finally, I think it’s also important that they said "Gulf War illness" rather than "chronic multisymptom illness." Again, I think that lends credibility to the claims of the veterans who have been there. This committee is willing to say, yes, there is something about having been deployed to the first Gulf War at a particular time that helps us understand your medical history. That’s not as far as we’d like to go, but it’s certainly some steps in the right direction.
That was a very short war a long time ago. Now, we’ve had 12 years of a very long war with multiple deployments, yet we haven’t really seen Gulf War illness or its equivalent in this generation of Iraq and Afghanistan veterans. I have expected that at some point we’re going to see these psychosomatic, vague symptoms coming out of this current war. I definitely think there’s time. Traumatic brain injury seems like the cardinal disorder from these wars, but I think we’re going to see some psychosomatic reactions over time.
Dr. Elspeth Cameron Ritchie is an expert on military health issues who retired as a colonel after 28 years in the U.S. Army. These are excerpts of an interview in which she spoke as an individual, not in her current roles as chief clinical officer of the Department of Behavioral Health for the District of Columbia and professor of psychiatry at the Uniformed Services University of the Health Sciences, Bethesda, Md., and at Georgetown University, Washington. She reported having no financial disclosures.
There has long been debate about whether Gulf War syndrome or Gulf War illness existed. If you remember the time of the Gulf War and shortly afterwards, there were a lot of questions about whether this was "all in their heads." Nobody ever found the sources of the illness. This report says to me that it’s final – that this august institution says, yes, this illness does exist. Whether you use the wider or narrower definition, this is to be taken seriously. That’s very important for physicians to know.
In terms of the two definitions – one narrower to be used for research and one broader to be used clinically – I think this gives people a little bit of latitude, depending on where they fit. The take-home piece is that, since there has been such a smorgasbord of opinions, thoughts, and descriptions, this should help the field by solidifying it further. We do still have two working definitions and we still don’t know a lot such as onset, duration, severity, frequency, and exclusionary criteria. Certainly, more needs to be done, but I think this is a very important step.
 |
|
Finally, I think it’s also important that they said "Gulf War illness" rather than "chronic multisymptom illness." Again, I think that lends credibility to the claims of the veterans who have been there. This committee is willing to say, yes, there is something about having been deployed to the first Gulf War at a particular time that helps us understand your medical history. That’s not as far as we’d like to go, but it’s certainly some steps in the right direction.
That was a very short war a long time ago. Now, we’ve had 12 years of a very long war with multiple deployments, yet we haven’t really seen Gulf War illness or its equivalent in this generation of Iraq and Afghanistan veterans. I have expected that at some point we’re going to see these psychosomatic, vague symptoms coming out of this current war. I definitely think there’s time. Traumatic brain injury seems like the cardinal disorder from these wars, but I think we’re going to see some psychosomatic reactions over time.
Dr. Elspeth Cameron Ritchie is an expert on military health issues who retired as a colonel after 28 years in the U.S. Army. These are excerpts of an interview in which she spoke as an individual, not in her current roles as chief clinical officer of the Department of Behavioral Health for the District of Columbia and professor of psychiatry at the Uniformed Services University of the Health Sciences, Bethesda, Md., and at Georgetown University, Washington. She reported having no financial disclosures.
There has long been debate about whether Gulf War syndrome or Gulf War illness existed. If you remember the time of the Gulf War and shortly afterwards, there were a lot of questions about whether this was "all in their heads." Nobody ever found the sources of the illness. This report says to me that it’s final – that this august institution says, yes, this illness does exist. Whether you use the wider or narrower definition, this is to be taken seriously. That’s very important for physicians to know.
In terms of the two definitions – one narrower to be used for research and one broader to be used clinically – I think this gives people a little bit of latitude, depending on where they fit. The take-home piece is that, since there has been such a smorgasbord of opinions, thoughts, and descriptions, this should help the field by solidifying it further. We do still have two working definitions and we still don’t know a lot such as onset, duration, severity, frequency, and exclusionary criteria. Certainly, more needs to be done, but I think this is a very important step.
 |
|
Finally, I think it’s also important that they said "Gulf War illness" rather than "chronic multisymptom illness." Again, I think that lends credibility to the claims of the veterans who have been there. This committee is willing to say, yes, there is something about having been deployed to the first Gulf War at a particular time that helps us understand your medical history. That’s not as far as we’d like to go, but it’s certainly some steps in the right direction.
That was a very short war a long time ago. Now, we’ve had 12 years of a very long war with multiple deployments, yet we haven’t really seen Gulf War illness or its equivalent in this generation of Iraq and Afghanistan veterans. I have expected that at some point we’re going to see these psychosomatic, vague symptoms coming out of this current war. I definitely think there’s time. Traumatic brain injury seems like the cardinal disorder from these wars, but I think we’re going to see some psychosomatic reactions over time.
Dr. Elspeth Cameron Ritchie is an expert on military health issues who retired as a colonel after 28 years in the U.S. Army. These are excerpts of an interview in which she spoke as an individual, not in her current roles as chief clinical officer of the Department of Behavioral Health for the District of Columbia and professor of psychiatry at the Uniformed Services University of the Health Sciences, Bethesda, Md., and at Georgetown University, Washington. She reported having no financial disclosures.
An Institute of Medicine committee could not reach consensus on a single definition of Gulf War illness and recommended in a new report that clinicians and researchers at least narrow it down to two definitions.
That’s the preferred term – Gulf War illness – for a slew of health problems that occur at a higher rate in the approximately 697,000 U.S. veterans who served in the 1990-1991 Gulf War than in other veterans, the committee said. Gulf War illness is widely used and is more accurate than other terms used over the years for the same problems, including Gulf War syndrome, chronic multisystem illness, unexplained illness, medically unexplained symptoms, and medically unexplained physical symptoms, the report stated.
The Institute of Medicine (IOM) drafted a committee of 16 experts at the request of the Department of Veterans Affairs to try to develop a case definition for what's now called Gulf War illness in order to help standardize diagnosis, inclusion in research studies, and treatments. The committee's 120-page report, "Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined," is available on the IOM website.
Unable to find validation for one definition because of methodologic limitations in the studies, the committee recommended using either a broader definition from the Centers for Disease Control and Prevention (CDC) or a more restrictive one from studies of Kansas veterans, depending on the situation. Between the two of them, the definitions capture the most common symptoms of Gulf War illness and provide a framework for more focused research and treatment, the report said.
The committee found 2,033 articles in the literature focused on Gulf War illness symptoms and closely reviewed 718 of them. It found no objective diagnostic criteria but saw cumulative evidence that Gulf War illness is real. Fatigue, pain, and neurocognitive, gastrointestinal, respiratory, and/or dermatologic symptoms were reported with higher frequency in Gulf War veterans than in veterans of the same era who had not been deployed or were deployed elsewhere.
To identify the illness in a Gulf War veteran, the CDC definition requires one or more symptoms in at least two of the following categories: fatigue, pain, or mood and cognition. The Kansas definition requires symptoms in at least three of the following domains: fatigue or sleep, pain, neurologic or cognitive or mood symptoms, gastrointestinal symptoms, respiratory symptoms, and skin symptoms.
The CDC definition identified the illness in 29%-60% of Gulf War veterans, depending on the population studied. The Kansas definition identified the illness in 34% of Kansas veterans of the Gulf War.
The reason the committee couldn’t compile a single case definition is that the studies in the literature do not report on key features of the illness including onset, duration, severity, frequency of symptoms, and exclusionary criteria. The Department of Veterans Affairs should boost efforts to collect these kinds of data, conduct earlier in-depth assessments after deployment when unexpected complaints occur, and track troops’ exposures to vaccinations, drugs, and environmental factors, the report recommends.
The name Gulf War illness won out over Gulf War syndrome because "syndrome" indicates a new group of signs and symptoms, while the types and patterns of symptoms in this illness commonly are seen with other illnesses. The committee rejected "chronic multisymptom illness" because the phrase is not specific to Gulf War veterans.
The committee included experts in clinical medicine, toxicology, psychiatry, neurology, gastroenterology, epidemiology, sociology, psychometrics, biostatistics, occupational medicine, and basic science.
The report does not include potential disclosures of conflicts of interest.
On Twitter @sherryboschert
An Institute of Medicine committee could not reach consensus on a single definition of Gulf War illness and recommended in a new report that clinicians and researchers at least narrow it down to two definitions.
That’s the preferred term – Gulf War illness – for a slew of health problems that occur at a higher rate in the approximately 697,000 U.S. veterans who served in the 1990-1991 Gulf War than in other veterans, the committee said. Gulf War illness is widely used and is more accurate than other terms used over the years for the same problems, including Gulf War syndrome, chronic multisystem illness, unexplained illness, medically unexplained symptoms, and medically unexplained physical symptoms, the report stated.
The Institute of Medicine (IOM) drafted a committee of 16 experts at the request of the Department of Veterans Affairs to try to develop a case definition for what's now called Gulf War illness in order to help standardize diagnosis, inclusion in research studies, and treatments. The committee's 120-page report, "Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined," is available on the IOM website.
Unable to find validation for one definition because of methodologic limitations in the studies, the committee recommended using either a broader definition from the Centers for Disease Control and Prevention (CDC) or a more restrictive one from studies of Kansas veterans, depending on the situation. Between the two of them, the definitions capture the most common symptoms of Gulf War illness and provide a framework for more focused research and treatment, the report said.
The committee found 2,033 articles in the literature focused on Gulf War illness symptoms and closely reviewed 718 of them. It found no objective diagnostic criteria but saw cumulative evidence that Gulf War illness is real. Fatigue, pain, and neurocognitive, gastrointestinal, respiratory, and/or dermatologic symptoms were reported with higher frequency in Gulf War veterans than in veterans of the same era who had not been deployed or were deployed elsewhere.
To identify the illness in a Gulf War veteran, the CDC definition requires one or more symptoms in at least two of the following categories: fatigue, pain, or mood and cognition. The Kansas definition requires symptoms in at least three of the following domains: fatigue or sleep, pain, neurologic or cognitive or mood symptoms, gastrointestinal symptoms, respiratory symptoms, and skin symptoms.
The CDC definition identified the illness in 29%-60% of Gulf War veterans, depending on the population studied. The Kansas definition identified the illness in 34% of Kansas veterans of the Gulf War.
The reason the committee couldn’t compile a single case definition is that the studies in the literature do not report on key features of the illness including onset, duration, severity, frequency of symptoms, and exclusionary criteria. The Department of Veterans Affairs should boost efforts to collect these kinds of data, conduct earlier in-depth assessments after deployment when unexpected complaints occur, and track troops’ exposures to vaccinations, drugs, and environmental factors, the report recommends.
The name Gulf War illness won out over Gulf War syndrome because "syndrome" indicates a new group of signs and symptoms, while the types and patterns of symptoms in this illness commonly are seen with other illnesses. The committee rejected "chronic multisymptom illness" because the phrase is not specific to Gulf War veterans.
The committee included experts in clinical medicine, toxicology, psychiatry, neurology, gastroenterology, epidemiology, sociology, psychometrics, biostatistics, occupational medicine, and basic science.
The report does not include potential disclosures of conflicts of interest.
On Twitter @sherryboschert
VIDEO: Eosinophilic esophagitis may follow outgrown food allergy
SAN DIEGO (FRONTLINE MEDICAL NEWS) – People who outgrow a food allergy may be at risk of developing eosinophilic esophagitis (EoE) to the same food, Dr. Jonathan Spergel said in a press briefing at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
In a video interview, Dr. Spergel outlined the signs and symptoms that could point to EoE in a child who has outgrown a food allergy.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN DIEGO (FRONTLINE MEDICAL NEWS) – People who outgrow a food allergy may be at risk of developing eosinophilic esophagitis (EoE) to the same food, Dr. Jonathan Spergel said in a press briefing at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
In a video interview, Dr. Spergel outlined the signs and symptoms that could point to EoE in a child who has outgrown a food allergy.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN DIEGO (FRONTLINE MEDICAL NEWS) – People who outgrow a food allergy may be at risk of developing eosinophilic esophagitis (EoE) to the same food, Dr. Jonathan Spergel said in a press briefing at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
In a video interview, Dr. Spergel outlined the signs and symptoms that could point to EoE in a child who has outgrown a food allergy.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
EXPERT ANALYSIS FROM 2014 AAAAI ANNUAL MEETING
List of allergy care ‘don’ts’ expanded
SAN DIEGO – Allergists added to their list of "don’ts" in the Choosing Wisely campaign by releasing a second set of five things that physicians should stop doing, bringing their number of recommendations to 10.
The American Academy of Allergy, Asthma, and Immunology was part of the first wave of groups in 2012 to compile lists of tests and procedures that are overused in diagnosis and treatment, as part of the ABIM Foundation’s Choosing Wisely campaign. Academy leaders released the new list at the annual meeting.
The second round of recommendations includes admonitions related to antihistamine use, food IgE testing, radiocontrast media, vaccinations, and penicillin allergy, with supporting statements and references to the evidence behind them. The five new recommendations are:
• Don’t rely on antihistamines as first-line treatment in severe allergic reactions. Data show that antihistamines are overused as first-line treatment for anaphylaxis, and they have been associated with delay of proper treatment and death. The first-line treatment of anaphylaxis is epinephrine, the statement said.
• Don’t perform food IgE testing without a history consistent with potential IgE-mediated food allergy. From 50% to 90% of patients with presumed food allergies have food intolerance or symptoms produced by non-food-related causes, not IgE-mediated reactions. Ordering panels of food tests in those patients wastes resources and leads to misdiagnoses and unnecessary food avoidance.
• Don’t routinely order low- or iso-osmolar radiocontrast media or pretreat with corticosteroids and antihistamines for patients with a history of seafood allergy who require radiocontrast media. Some patients do react to contrast media, but the reactions aren’t related to seafood allergy, data in the medical literature show. Don’t deny imaging to patients with seafood allergies or pretreat them with antihistamines or corticosteroids, and don’t tell patients who have had an anaphylactic reaction to contrast media that they are allergic to seafood. Patients with a history of anaphylaxis to contrast media are at risk for another reaction if exposed again, and the risk of serious anaphylaxis from radiographic contrast media is increased in patients with asthma or cardiovascular disease or who are taking beta-blockers.
• Don’t routinely avoid influenza vaccination in egg-allergic patients. These patients can be given egg-free influenza vaccine or egg-based influenza vaccine if they are observed for 30 minutes afterward. Give the vaccine and observe the patient in an allergist/immunologist’s office if the patient has a history of a reaction stronger than hives from eating eggs.
• Don’t overuse non–beta-lactam antibiotics in patients with a history of penicillin allergy without an appropriate evaluation. Although 10% of people say they are allergic to penicillin, data show that only 1% truly have a penicillin allergy. That suggests that 99% of the population can take these antibiotics safely and can avoid the higher cost and greater complications associated with alternative antibiotics.
The statement emphasizes that these are not hard and fast rules, but rather words of advice that should spark conversations between patients and physicians if management choices diverge from these recommendations.
The academy’s list is preceded by the five items it released earlier in the Choosing Wisely campaign: Do not perform unproven diagnostic tests such as IgG testing or an indiscriminate battery of IgE tests in the evaluation of allergy. Do not order sinus CT or indiscriminately prescribe antibiotics for uncomplicated acute rhinosinusitis. Do not routinely do diagnostic testing in patients with chronic urticaria, or recommend replacement immunoglobulin therapy for recurrent infections unless there’s demonstration of impaired antibody responses to vaccines. And lastly, don’t diagnose or manage asthma without spirometry.
Lists created by medical groups in the Choosing Wisely campaign can be found here.
On twitter @sherryboschert
SAN DIEGO – Allergists added to their list of "don’ts" in the Choosing Wisely campaign by releasing a second set of five things that physicians should stop doing, bringing their number of recommendations to 10.
The American Academy of Allergy, Asthma, and Immunology was part of the first wave of groups in 2012 to compile lists of tests and procedures that are overused in diagnosis and treatment, as part of the ABIM Foundation’s Choosing Wisely campaign. Academy leaders released the new list at the annual meeting.
The second round of recommendations includes admonitions related to antihistamine use, food IgE testing, radiocontrast media, vaccinations, and penicillin allergy, with supporting statements and references to the evidence behind them. The five new recommendations are:
• Don’t rely on antihistamines as first-line treatment in severe allergic reactions. Data show that antihistamines are overused as first-line treatment for anaphylaxis, and they have been associated with delay of proper treatment and death. The first-line treatment of anaphylaxis is epinephrine, the statement said.
• Don’t perform food IgE testing without a history consistent with potential IgE-mediated food allergy. From 50% to 90% of patients with presumed food allergies have food intolerance or symptoms produced by non-food-related causes, not IgE-mediated reactions. Ordering panels of food tests in those patients wastes resources and leads to misdiagnoses and unnecessary food avoidance.
• Don’t routinely order low- or iso-osmolar radiocontrast media or pretreat with corticosteroids and antihistamines for patients with a history of seafood allergy who require radiocontrast media. Some patients do react to contrast media, but the reactions aren’t related to seafood allergy, data in the medical literature show. Don’t deny imaging to patients with seafood allergies or pretreat them with antihistamines or corticosteroids, and don’t tell patients who have had an anaphylactic reaction to contrast media that they are allergic to seafood. Patients with a history of anaphylaxis to contrast media are at risk for another reaction if exposed again, and the risk of serious anaphylaxis from radiographic contrast media is increased in patients with asthma or cardiovascular disease or who are taking beta-blockers.
• Don’t routinely avoid influenza vaccination in egg-allergic patients. These patients can be given egg-free influenza vaccine or egg-based influenza vaccine if they are observed for 30 minutes afterward. Give the vaccine and observe the patient in an allergist/immunologist’s office if the patient has a history of a reaction stronger than hives from eating eggs.
• Don’t overuse non–beta-lactam antibiotics in patients with a history of penicillin allergy without an appropriate evaluation. Although 10% of people say they are allergic to penicillin, data show that only 1% truly have a penicillin allergy. That suggests that 99% of the population can take these antibiotics safely and can avoid the higher cost and greater complications associated with alternative antibiotics.
The statement emphasizes that these are not hard and fast rules, but rather words of advice that should spark conversations between patients and physicians if management choices diverge from these recommendations.
The academy’s list is preceded by the five items it released earlier in the Choosing Wisely campaign: Do not perform unproven diagnostic tests such as IgG testing or an indiscriminate battery of IgE tests in the evaluation of allergy. Do not order sinus CT or indiscriminately prescribe antibiotics for uncomplicated acute rhinosinusitis. Do not routinely do diagnostic testing in patients with chronic urticaria, or recommend replacement immunoglobulin therapy for recurrent infections unless there’s demonstration of impaired antibody responses to vaccines. And lastly, don’t diagnose or manage asthma without spirometry.
Lists created by medical groups in the Choosing Wisely campaign can be found here.
On twitter @sherryboschert
SAN DIEGO – Allergists added to their list of "don’ts" in the Choosing Wisely campaign by releasing a second set of five things that physicians should stop doing, bringing their number of recommendations to 10.
The American Academy of Allergy, Asthma, and Immunology was part of the first wave of groups in 2012 to compile lists of tests and procedures that are overused in diagnosis and treatment, as part of the ABIM Foundation’s Choosing Wisely campaign. Academy leaders released the new list at the annual meeting.
The second round of recommendations includes admonitions related to antihistamine use, food IgE testing, radiocontrast media, vaccinations, and penicillin allergy, with supporting statements and references to the evidence behind them. The five new recommendations are:
• Don’t rely on antihistamines as first-line treatment in severe allergic reactions. Data show that antihistamines are overused as first-line treatment for anaphylaxis, and they have been associated with delay of proper treatment and death. The first-line treatment of anaphylaxis is epinephrine, the statement said.
• Don’t perform food IgE testing without a history consistent with potential IgE-mediated food allergy. From 50% to 90% of patients with presumed food allergies have food intolerance or symptoms produced by non-food-related causes, not IgE-mediated reactions. Ordering panels of food tests in those patients wastes resources and leads to misdiagnoses and unnecessary food avoidance.
• Don’t routinely order low- or iso-osmolar radiocontrast media or pretreat with corticosteroids and antihistamines for patients with a history of seafood allergy who require radiocontrast media. Some patients do react to contrast media, but the reactions aren’t related to seafood allergy, data in the medical literature show. Don’t deny imaging to patients with seafood allergies or pretreat them with antihistamines or corticosteroids, and don’t tell patients who have had an anaphylactic reaction to contrast media that they are allergic to seafood. Patients with a history of anaphylaxis to contrast media are at risk for another reaction if exposed again, and the risk of serious anaphylaxis from radiographic contrast media is increased in patients with asthma or cardiovascular disease or who are taking beta-blockers.
• Don’t routinely avoid influenza vaccination in egg-allergic patients. These patients can be given egg-free influenza vaccine or egg-based influenza vaccine if they are observed for 30 minutes afterward. Give the vaccine and observe the patient in an allergist/immunologist’s office if the patient has a history of a reaction stronger than hives from eating eggs.
• Don’t overuse non–beta-lactam antibiotics in patients with a history of penicillin allergy without an appropriate evaluation. Although 10% of people say they are allergic to penicillin, data show that only 1% truly have a penicillin allergy. That suggests that 99% of the population can take these antibiotics safely and can avoid the higher cost and greater complications associated with alternative antibiotics.
The statement emphasizes that these are not hard and fast rules, but rather words of advice that should spark conversations between patients and physicians if management choices diverge from these recommendations.
The academy’s list is preceded by the five items it released earlier in the Choosing Wisely campaign: Do not perform unproven diagnostic tests such as IgG testing or an indiscriminate battery of IgE tests in the evaluation of allergy. Do not order sinus CT or indiscriminately prescribe antibiotics for uncomplicated acute rhinosinusitis. Do not routinely do diagnostic testing in patients with chronic urticaria, or recommend replacement immunoglobulin therapy for recurrent infections unless there’s demonstration of impaired antibody responses to vaccines. And lastly, don’t diagnose or manage asthma without spirometry.
Lists created by medical groups in the Choosing Wisely campaign can be found here.
On twitter @sherryboschert
AT 2014 AAAAI ANNUAL MEETING