User login
Infective endocarditis: Refer for expert team care as soon as possible
In this issue of the Journal, Soud et al discuss the timing of referral of patients with infective endocarditis to surgery.1 When having this discussion, it is important to understand the nature of the disease and the role of surgery in its treatment.
Unless successfully treated and cured, infective endocarditis is fatal. It is associated with septic embolism (systemic with left-sided infective endocarditis and pulmonary with right-sided infective endocarditis), destruction of valve tissue, and invasion outside the aortic root or into the atrioventricular groove. Antimicrobials kill sensitive and exposed organisms but cannot reach those hiding in vegetations or biofilm, on foreign material, or in invaded extravascular tissue.
The objectives of surgery are to eliminate the source of embolism, debride and remove infected tissue and foreign material, expose and make residual organisms vulnerable to antimicrobials, and restore functional valves and cardiac integrity. Surgery to treat infective endocarditis is difficult and high-risk and requires an experienced surgeon. But final cure of the infection is still by antimicrobial treatment.
INFECTIVE ENDOCARDITIS NEEDS MULTIDISCIPLINARY CARE
Every aspect of infective endocarditis—diagnosis, medical management, management of complications, and surgery—is difficult. Recent guidelines2–6 therefore favor care by a multidisciplinary team that includes an infectious disease specialist, cardiologist, and cardiac surgeon from the very beginning, with access to any other needed discipline, often including neurology, neurosurgery, nephrology, and dependence specialists. Patients with infective endocarditis should be referred early to a center with access to a full endocarditis treatment team. The need for surgery and the optimal timing of it are team decisions. The American Association for Thoracic Surgery infective endocarditis guidelines are question-based and address most aspects that surgeons must consider before, during, and after operation.2
IF SURGERY IS INDICATED, IT IS BEST DONE SOONER
Once there is an indication to operate, the operation should be expedited. Delays mean continued risk of disease progression, invasion, heart block, and embolic events. Determining the timing of surgery is difficult in patients who have suffered an embolic stroke—nonhemorrhagic or hemorrhagic—or who have suffered brain bleeding; management of these issues has recently triggered expert opinion and review articles.7,8 The recommendation for early surgery is based on the conviction that once the patient has been stabilized (or has overwhelming mechanical hemodynamic problems requiring emergency surgery) and adequate antimicrobial coverage is on board, there are no additional benefits to delaying surgery.9 When the indication to operate is large mobile vegetations associated with a high risk of stroke, surgery before another event can make all the difference.
In the operating room, the first aspect addressed is adequate debridement. There is wide agreement that repair is preferable to replacement for the mitral and tricuspid valves, but there is no agreement that an allograft (although favored by our team) is the best replacement alternative for a destroyed aortic root. The key is that surgeons and their surgical teams must have the experience and tools that work for them.
Our recommendation is to refer all patients with infective endocarditis to a center with access to a full team of experienced experts able to address all aspects of the disease and its complications.
- Soud M, Pacha HM, Alraies MC. How soon should patients with infective endocarditis be referred for valve surgery? Cleve Clin J Med 2018; 85(5):362–364. doi:10.3949/ccjm.85a:17052
- Pettersson GB, Coselli JS, Pettersson GB, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease:executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Byrne JG, Rezai K, Sanchez JA, et al. Surgical management of endocarditis: the Society of Thoracic Surgeons clinical practice guideline. Ann Thorac Surg 2011; 91(6):2012–2019. doi:10.1016/j.athoracsur.2011.01.106
- Yanagawa B, Pettersson GB, Habib G, et al. Surgical management of infective endocarditis complicated by embolic stroke: practical recommendations for clinicians. Circulation 2016; 134(17):1280–1292. doi:10.1161/CIRCULATIONAHA.116.024156
- Cahill TJ , Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
In this issue of the Journal, Soud et al discuss the timing of referral of patients with infective endocarditis to surgery.1 When having this discussion, it is important to understand the nature of the disease and the role of surgery in its treatment.
Unless successfully treated and cured, infective endocarditis is fatal. It is associated with septic embolism (systemic with left-sided infective endocarditis and pulmonary with right-sided infective endocarditis), destruction of valve tissue, and invasion outside the aortic root or into the atrioventricular groove. Antimicrobials kill sensitive and exposed organisms but cannot reach those hiding in vegetations or biofilm, on foreign material, or in invaded extravascular tissue.
The objectives of surgery are to eliminate the source of embolism, debride and remove infected tissue and foreign material, expose and make residual organisms vulnerable to antimicrobials, and restore functional valves and cardiac integrity. Surgery to treat infective endocarditis is difficult and high-risk and requires an experienced surgeon. But final cure of the infection is still by antimicrobial treatment.
INFECTIVE ENDOCARDITIS NEEDS MULTIDISCIPLINARY CARE
Every aspect of infective endocarditis—diagnosis, medical management, management of complications, and surgery—is difficult. Recent guidelines2–6 therefore favor care by a multidisciplinary team that includes an infectious disease specialist, cardiologist, and cardiac surgeon from the very beginning, with access to any other needed discipline, often including neurology, neurosurgery, nephrology, and dependence specialists. Patients with infective endocarditis should be referred early to a center with access to a full endocarditis treatment team. The need for surgery and the optimal timing of it are team decisions. The American Association for Thoracic Surgery infective endocarditis guidelines are question-based and address most aspects that surgeons must consider before, during, and after operation.2
IF SURGERY IS INDICATED, IT IS BEST DONE SOONER
Once there is an indication to operate, the operation should be expedited. Delays mean continued risk of disease progression, invasion, heart block, and embolic events. Determining the timing of surgery is difficult in patients who have suffered an embolic stroke—nonhemorrhagic or hemorrhagic—or who have suffered brain bleeding; management of these issues has recently triggered expert opinion and review articles.7,8 The recommendation for early surgery is based on the conviction that once the patient has been stabilized (or has overwhelming mechanical hemodynamic problems requiring emergency surgery) and adequate antimicrobial coverage is on board, there are no additional benefits to delaying surgery.9 When the indication to operate is large mobile vegetations associated with a high risk of stroke, surgery before another event can make all the difference.
In the operating room, the first aspect addressed is adequate debridement. There is wide agreement that repair is preferable to replacement for the mitral and tricuspid valves, but there is no agreement that an allograft (although favored by our team) is the best replacement alternative for a destroyed aortic root. The key is that surgeons and their surgical teams must have the experience and tools that work for them.
Our recommendation is to refer all patients with infective endocarditis to a center with access to a full team of experienced experts able to address all aspects of the disease and its complications.
In this issue of the Journal, Soud et al discuss the timing of referral of patients with infective endocarditis to surgery.1 When having this discussion, it is important to understand the nature of the disease and the role of surgery in its treatment.
Unless successfully treated and cured, infective endocarditis is fatal. It is associated with septic embolism (systemic with left-sided infective endocarditis and pulmonary with right-sided infective endocarditis), destruction of valve tissue, and invasion outside the aortic root or into the atrioventricular groove. Antimicrobials kill sensitive and exposed organisms but cannot reach those hiding in vegetations or biofilm, on foreign material, or in invaded extravascular tissue.
The objectives of surgery are to eliminate the source of embolism, debride and remove infected tissue and foreign material, expose and make residual organisms vulnerable to antimicrobials, and restore functional valves and cardiac integrity. Surgery to treat infective endocarditis is difficult and high-risk and requires an experienced surgeon. But final cure of the infection is still by antimicrobial treatment.
INFECTIVE ENDOCARDITIS NEEDS MULTIDISCIPLINARY CARE
Every aspect of infective endocarditis—diagnosis, medical management, management of complications, and surgery—is difficult. Recent guidelines2–6 therefore favor care by a multidisciplinary team that includes an infectious disease specialist, cardiologist, and cardiac surgeon from the very beginning, with access to any other needed discipline, often including neurology, neurosurgery, nephrology, and dependence specialists. Patients with infective endocarditis should be referred early to a center with access to a full endocarditis treatment team. The need for surgery and the optimal timing of it are team decisions. The American Association for Thoracic Surgery infective endocarditis guidelines are question-based and address most aspects that surgeons must consider before, during, and after operation.2
IF SURGERY IS INDICATED, IT IS BEST DONE SOONER
Once there is an indication to operate, the operation should be expedited. Delays mean continued risk of disease progression, invasion, heart block, and embolic events. Determining the timing of surgery is difficult in patients who have suffered an embolic stroke—nonhemorrhagic or hemorrhagic—or who have suffered brain bleeding; management of these issues has recently triggered expert opinion and review articles.7,8 The recommendation for early surgery is based on the conviction that once the patient has been stabilized (or has overwhelming mechanical hemodynamic problems requiring emergency surgery) and adequate antimicrobial coverage is on board, there are no additional benefits to delaying surgery.9 When the indication to operate is large mobile vegetations associated with a high risk of stroke, surgery before another event can make all the difference.
In the operating room, the first aspect addressed is adequate debridement. There is wide agreement that repair is preferable to replacement for the mitral and tricuspid valves, but there is no agreement that an allograft (although favored by our team) is the best replacement alternative for a destroyed aortic root. The key is that surgeons and their surgical teams must have the experience and tools that work for them.
Our recommendation is to refer all patients with infective endocarditis to a center with access to a full team of experienced experts able to address all aspects of the disease and its complications.
- Soud M, Pacha HM, Alraies MC. How soon should patients with infective endocarditis be referred for valve surgery? Cleve Clin J Med 2018; 85(5):362–364. doi:10.3949/ccjm.85a:17052
- Pettersson GB, Coselli JS, Pettersson GB, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease:executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Byrne JG, Rezai K, Sanchez JA, et al. Surgical management of endocarditis: the Society of Thoracic Surgeons clinical practice guideline. Ann Thorac Surg 2011; 91(6):2012–2019. doi:10.1016/j.athoracsur.2011.01.106
- Yanagawa B, Pettersson GB, Habib G, et al. Surgical management of infective endocarditis complicated by embolic stroke: practical recommendations for clinicians. Circulation 2016; 134(17):1280–1292. doi:10.1161/CIRCULATIONAHA.116.024156
- Cahill TJ , Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Soud M, Pacha HM, Alraies MC. How soon should patients with infective endocarditis be referred for valve surgery? Cleve Clin J Med 2018; 85(5):362–364. doi:10.3949/ccjm.85a:17052
- Pettersson GB, Coselli JS, Pettersson GB, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease:executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Byrne JG, Rezai K, Sanchez JA, et al. Surgical management of endocarditis: the Society of Thoracic Surgeons clinical practice guideline. Ann Thorac Surg 2011; 91(6):2012–2019. doi:10.1016/j.athoracsur.2011.01.106
- Yanagawa B, Pettersson GB, Habib G, et al. Surgical management of infective endocarditis complicated by embolic stroke: practical recommendations for clinicians. Circulation 2016; 134(17):1280–1292. doi:10.1161/CIRCULATIONAHA.116.024156
- Cahill TJ , Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
Contribution of Predischarge ID Consult
With dramatically increasing costs of healthcare, it has become increasingly necessary for healthcare providers to demonstrate value in the delivery of care. Porter and Teisberg have strongly advocated that healthcare reform efforts should focus on improving value rather than limiting cost, with value being defined as quality per unit cost.1 However, it has been pointed out that value means different things to different people.2 The biggest challenge in defining value stems mainly from the difficulty in defining quality, because it, too, means vastly different things to different people. Modern medicine is increasingly characterized by multidisciplinary care. With limited or shrinking resources, it will become necessary for individual specialists to describe and articulate, in quantitative terms, their specific contributions to the overall outcome of individual patients.
Previous publications have provided broad descriptions of the value provided by infectious disease (ID) specialists in the domains of sepsis, infection control, outpatient antibiotic therapy, antimicrobial stewardship, and directive care and teaching.3, 4 Studies have also shown the value of ID physicians in specific disease conditions. ID consultation is associated with lower mortality5, 6 and lower relapse rates7 in hospitalized patients with Staphylococcus aureus bacteremia. In another study evaluating the impact of ID consultants, patients seen by ID consultants had longer lengths of hospital stay, longer intensive care unit lengths of stay, and higher antibiotic costs than matched controls not seen by ID consultants.8 It can be argued that a major limitation of the study was that controls were not matched for the ID diagnosis, nor for the causative microorganisms, but it is clear that ID physicians are challenged to demonstrate their contribution to the care of patients.
A unique activity of ID physicians is the management of community‐based parenteral anti‐infective therapy (CoPAT). At Baystate Medical Center, a policy of mandatory ID consultation was instituted for patients leaving hospital on parenteral antibiotics. A study was conducted on the impact of predischarge ID consultation for 44 patients who were not already being followed by the ID service. The study documented change from intravenous (IV) to oral formulation, change of antibiotic choice, and change of dose/duration of treatment in a substantial proportion of patients.9 These are significant changes, but ID consultation contributes more than the themes explored in the study.
The purpose of this study was to evaluate the contribution of ID consultation when consulted for CoPAT, an activity specific to ID practice, in a different institution, and using an expanded definition of medical contribution.
METHODS
The Cleveland Clinic's Department of Infectious Disease has 24 staff physicians and 11 inpatient ID consultative services. These include: 2 solid organ transplant services; a bone marrow transplant and oncologic service; 2 infective endocarditis/cardiac device infection services; an intensive care unit (ICU) service; a bone and joint infection service; a neuroinfection service; and 3 general ID consult services. Consultative services are provided 7 days a week. At the Cleveland Clinic, ID consultation is required prior to discharge on parenteral antibiotic therapy.10, 11 ID consultation for CoPAT usually occurs when the primary service deems the patient is close to being discharged from hospital. This circumstance allows for assessing the specific contribution of ID physicians beyond that of the primary service and other consulting services.
Case Ascertainment
The study was approved by the institutional review board. In February 2010, an electronic form for requesting ID consultations had been introduced into the computerized provider order entry (CPOE) system at the Cleveland Clinic. One of the required questions on the form was whether the consultation was regarding CoPAT, with options of Yes, No, or Not sure. These electronic ID consultation requests were screened to identify consultation requests for this study.
Inclusion and Exclusion Criteria
All adult ID consultations between February 11, 2010 and May 15, 2010 for which the CoPAT consult? field was marked Yes were included in the study. All other consultations, including not sure for CoPAT, were excluded.
Definitions
The first ID consultation during a hospitalization was considered an initial consultation. ID consultations for patients whom an ID service had previously seen during the same hospitalization were deemed reconsultations. Value provided was defined as contribution of the ID consultation team in the following domains: 1) optimization of antimicrobial therapy, 2) significant change in patient assessment, 3) additional medical care contribution. Specific contributions included in each domain are outlined in Table 1.
|
| Domain 1: Optimization of antibiotic therapy |
| Alteration of an antibiotic (change of antibiotic or route of administration) |
| Defining duration of therapy |
| Identification of psychosocial factors (eg, injection drug use) that influence treatment |
| Domain 2: Significant change in patient assessment |
| Diagnosis of an infectious process |
| Better appreciation of extent of disease |
| Refutation of a false infectious disease diagnosis |
| Recognition of a noninfectious process needing urgent attention |
| Identification of a positive culture as contaminant/colonization |
| Recognition of a need for additional testing (testing needed to arrive at a diagnosis or clarify a treatment plan before a patient could be safely discharged from hospital) |
| Recognition of need for surgery/emnvasive intervention |
| Refutation of antibiotic allergy by history or allergy testing |
| Domain 3: Additional medical care contribution |
| Administration of vaccines |
| Identification of an unrecognized medical problem that needed to be addressed after discharge from hospital |
| Provision of effective transition of care (ensuring that the same ID physician who saw the patient in hospital followed the patient after discharge from hospital) |
Data Collected
For each ID consultation episode, clinicians' notes were reviewed from the day of the ID consultation to the day the patient was discharged from hospital or the day the ID service signed off, whichever happened sooner. Results of recommended tests were followed up to determine if results led to a change in patient assessment. Data elements collected for each consultation episode included patient age, gender, race, date of hospitalization, date of discharge, date of ID consultation or reconsultation, primary service, and documentation of ID service contributions. Data were collected and entered in a Microsoft Access relational database. To minimize bias, the data collection was performed by physicians who had not participated in the care of the patient.
Analysis
The proportion of ID consultations in which the ID team contributed in the defined domains were enumerated, and described for the group overall and also separately for initial consultations and reconsultations.
RESULTS
In the time period studied, there were 1326 CPOE requests for ID consultation. The response to the question, CoPAT consult? was Yes for 304, No for 507, and Not sure for 515 requests. Of the 304 consultation requests marked Yes, 41 were excluded. Reasons for exclusion were: no ID consultation note (21), wrong service consulted (8), consultation request placed while the ID service was already following the patient (7), and duplicate consultation request (5). The remaining 263 consultation requests corresponded to 1 or more CoPAT consultation requests for 249 patients (across different hospitalizations). Of the 263 consultation requests, 172 were initial consultations, while the remaining 91 were reconsultations (patients not actively being followed by the ID service, but previously seen during the same hospitalization).
Consultation characteristics are outlined in Table 2. The most common group of infections for which CoPAT was sought was bone and joint infections, accounting for over 20% of the consultation requests. CoPAT consultations were requested a median of 4 days after hospitalization. Patients were discharged from hospital a median of 3 days after they were seen by the ID service. ID consultation did not delay discharge. The ID service usually saw the patient the same day, and followed the patient in hospital for a median of 1 day. There was no difference in hospital days after consult for patients who did not need antibiotics versus those who did.
| Characteristic | Initial Consultation [172] n (%)* | Reconsultation [91] n (%)* | Overall [263] n (%)* |
|---|---|---|---|
| |||
| Patient age in years, mean (SD) | 58 (14) | 62 (13) | 59 (14) |
| Male gender | 98 (60) | 91 (56) | 149 (57) |
| Caucasian race | 126 (73) | 74 (81) | 200 (76) |
| Services requesting consults (5 most common overall) | |||
| Medicine | 41 (17) | 14 (15) | 55 (21) |
| Orthopedics | 34 (14) | 0 (0) | 34 (13) |
| Hematology/Oncology | 16 (7) | 10 (11) | 26 (10) |
| Cardiology | 9 (4) | 15 (16) | 24 (9) |
| Gastroenterology | 14 (6) | 5 (5) | 19 (7) |
| Consult diagnosis (5 most common overall) | |||
| Bone and joint infection | 45 (26) | 9 (10) | 54 (21) |
| Skin or soft tissue infection or rash | 21 (12) | 8 (9) | 29 (11) |
| Endocarditis or cardiac device infection | 7 (4) | 15 (16) | 22 (8) |
| IV catheter or other endovascular infection | 9 (5) | 8 (9) | 17 (6) |
| Urinary tract infection | 12 (7) | 5 (5) | 17 (6) |
| Days from admission to ID consult, median (IQR) | 4 (1‐11) | 7 (2‐19) | 4 (1‐14) |
| Days to respond to consult request, median (IQR) | 0 (0‐1) | 0 (0‐0) | 0 (0‐0) |
| Days from ID consult to discharge, median (IQR) | 3 (2‐7) | 2 (1‐4.5) | 3 (1‐6) |
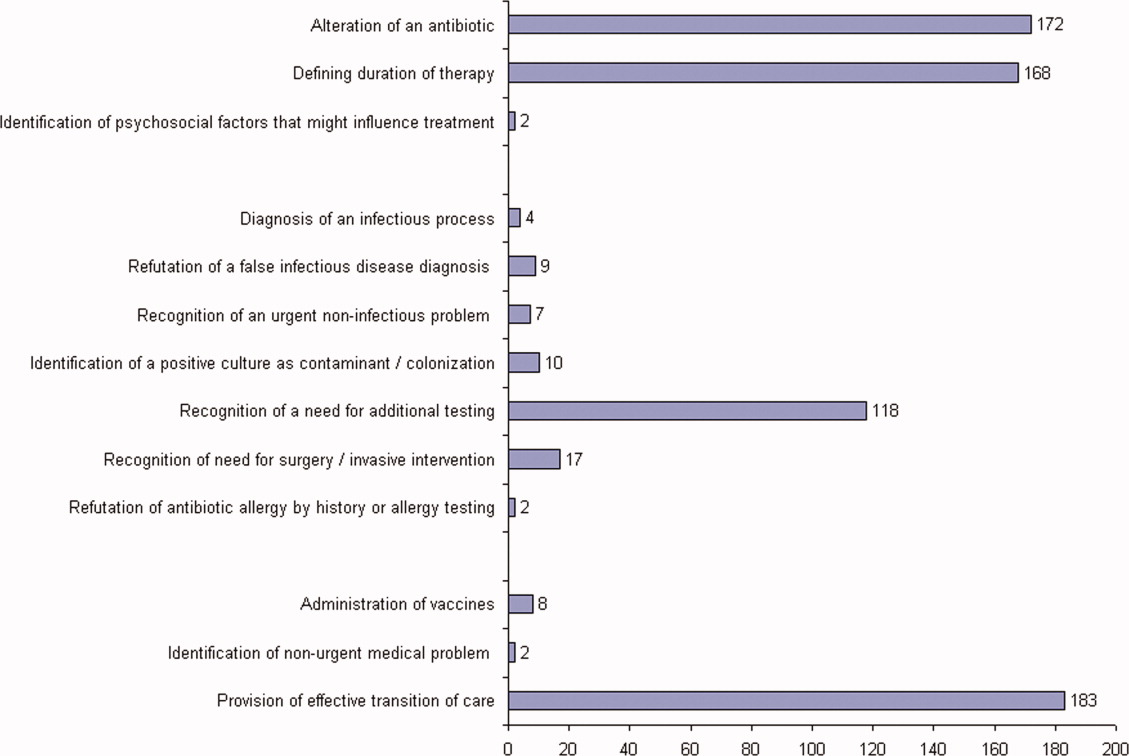
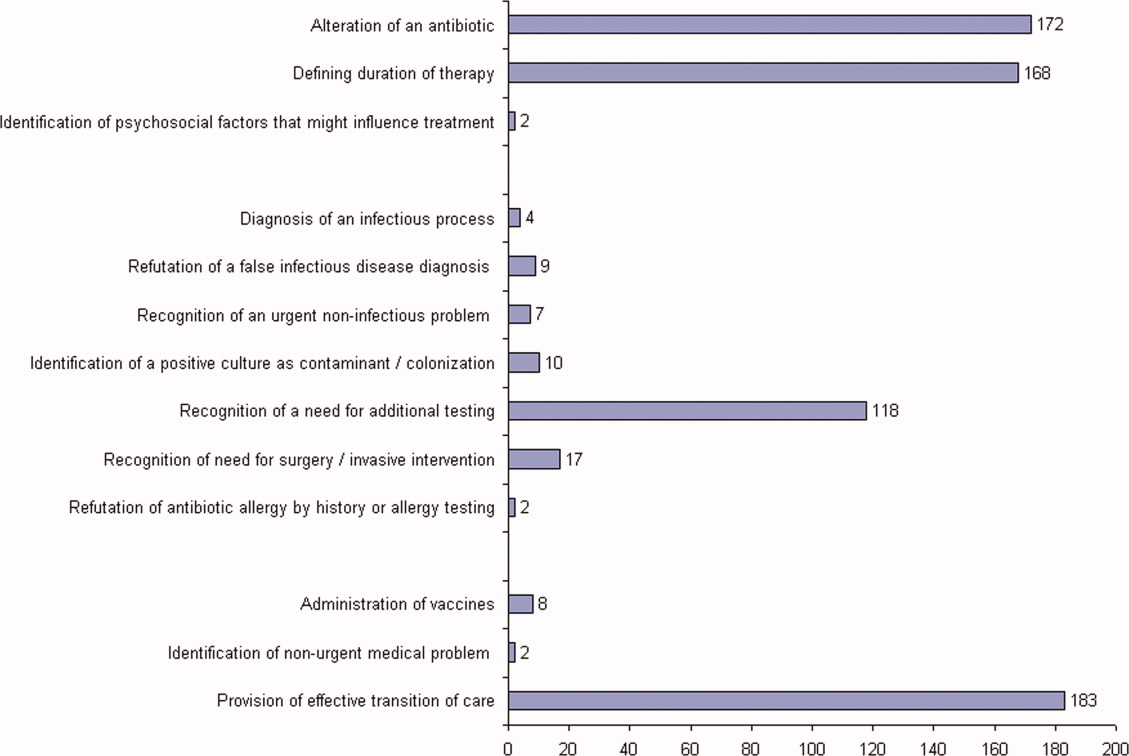
ID consultation provided value in at least 1 domain in 260 of the 263 consultations. This included optimization of antimicrobial treatment in 84%, significant alteration of patient assessment in 52%, and additional medical care contribution in 71% of consultations. Substantial contributions were made in all domains in both initial consultations and in reconsultations. Specific ID contributions within each of the domains are shown in Figure 1. There was wide overlap of contributions across the 3 domains for individual consultations (Figure 2), with contributions in all domains occurring in 34% of consultations. CoPAT was deemed not to be necessary in 27% of consultations. Among patients who did not require CoPAT, 60% received oral antibiotics and 40% were deemed not to need any antibiotics at hospital discharge. Among the patients discharged on CoPAT, a follow‐up appointment with a Cleveland Clinic ID physician familiar with the patient was set up 86% of the time; the rest either followed up with another physician or it was deemed that a scheduled follow‐up ID visit was not necessary.0

DISCUSSION
Physicians practicing in the specialty of infectious diseases face challenges and opportunities, as they adapt to changing demands within hospital practice in regard to reimbursement in an Accountable Care environment. Other challenges include emerging infections, antimicrobial resistance, need for antimicrobial stewardship, and increasing numbers of immunocompromised patients.12 From a health systems perspective, the overall value of care provided by the entire organization, and overall outcomes, are ultimately what matter. However, healthcare administrators need an appreciation of contributions of individual providers and specialties to fairly allocate resources and compensation for care provided. Articulating unique contributions is particularly challenging for individuals or services that provide purely cognitive input. Shrinking healthcare resources makes it critically important for cognitive specialists to be able to define their unique role in the care of patients with complex problems.
Our study found that a major contribution of ID consultation for CoPAT is that the process identifies a large number of patients who do not need CoPAT, thus effecting a powerful antimicrobial stewardship function. In our study, CoPAT was deemed unnecessary 27% of the time. The Infectious Diseases Society of America practice guidelines on outpatient parenteral antimicrobial therapy emphasize the importance of careful evaluation of patients considered for parenteral antibiotics outside the hospital setting.13 The focus on careful selection of appropriate patients for CoPAT has been a cornerstone of the Cleveland Clinic model of care. Nearly 30 years ago, we found that outpatient parenteral antibiotic therapy was unnecessary or not feasible in 40% of the patients referred for evaluation.10 If we adjust the numbers with the assumption that reimbursement issues present at that time are now less of an issue, the proportion of patients who were referred for CoPAT but not discharged on it was 29%, a figure remarkably similar to that found in the current study.
Another major contribution of ID consultation is the provision of effective transition of care from the inpatient to the outpatient setting. Frequent occurrence of postdischarge adverse events has been recognized as a problem in clinical practice.14 Primary care physicians are rarely involved in discussions about hospital discharge.15 A consensus conference including the American College of Physicians, Society of Hospital Medicine, and Society of General Internal Medicine, convened in July 2007 to address quality gaps in transitions of care between inpatient and outpatient settings. It identified 5 principles for effective care transitions: accountability, communication, timeliness, patient and family involvement, and respect for the hub of coordination of care.16 Recognizing gaps in care transition, hospitalists in a hospital‐based infusion program developed a model of care that successfully bridged the hospital‐to‐home care transition for patients who could return to hospital for daily antimicrobial infusions.17 In our system, ID physicians take ownership for directing parenteral antibiotic therapy for the episode of illness, specifying the physician, date, and time of follow‐up before the patient is discharged from hospital, thereby essentially satisfying the principles of effective care transitions identified. The purpose of the ID follow‐up is not to replace other follow‐up care for patients but to ensure safe transition of care while treating an episode of infection.
Attribution of identified contributions to the ID consultation could be done because our study was limited to CoPAT consultations. Such consultations typically occur when patients are deemed close to hospital discharge by the primary service. There should be little controversy about attribution of cognitive input in such consultations, because from the primary service's perspective, the patient is ready or almost ready to be discharged from hospital. It would be fair to state that most of the identified contributions in the study would not have occurred had it not been for the ID consultation.
We acknowledge that the study suffers from many limitations. The biggest limitation is that the contribution elements are defined by ID physicians and sought in the medical record by physicians from the same specialty. This arrangement certainly has potential for significant bias. To limit this bias, data collection was performed by physicians who had not participated in the care of the patient. In addition, we only could assess what was documented in the electronic health record. Our study found that alteration of antibiotic therapy was a substantial contribution, however, documentation of recommendation to change antibiotics in the medical record rarely specified exactly why the change was recommended. Reasons for antibiotic change recommendations included bug‐drug mismatch, minimum inhibitory concentration (MIC) considerations, pharmacokinetic considerations, adverse effects, convenience of dosing, drug interactions, and insurance coverage. However, it is not possible to quantify the specific contribution of each of these reasons, in a retrospective study, without making assumptions about why specific ID physicians made specific antibiotic change recommendations. There may have been more contributions that might not have been apparent on a retrospective chart review. The lack of a control group also lessens the impact of our findings. We could not have a control group, because no patient is discharged from the Cleveland Clinic on CoPAT without having been seen by an ID physician. Mandatory ID consultation for CoPAT has previously been shown to reduce costs,9 however, our study was not designed to evaluate cost.
The perceived value of ID consultation in our institution can be appreciated when one considers the longstanding institutional policy of requiring ID consultation for CoPAT.10, 11 The perpetuation of this tradition in the hospital is testament to the presumption that mandatory ID consultation is seen to be of value by the institution.
In summary, ID consultation in our institution contributes to the care of inpatients being considered for CoPAT by substantially reducing unnecessary parenteral antibiotic use, optimizing antibiotic therapy, recognizing need for additional testing before discharge from hospital, and by providing effective transition of care from the inpatient to the outpatient setting.
- ,.How physicians can change the future of health care.JAMA.2007;297:1103–1111.
- .Value of the infectious diseases specialist.Clin Infect Dis.1997;24:456.
- ,,, et al.The value of an infectious diseases specialist.Clin Infect Dis.2003;36:1013–1017.
- ,,,,,.The value of infectious diseases specialists: non‐patient care activities.Clin Infect Dis.2008;47:1051–1063.
- ,,,,.The value of infectious diseases consultation in Staphylococcus aureus bacteremia.Am J Med.2010;123:631–637.
- ,,,,.Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia.Medicine (Baltimore).2009;88:263–267.
- ,,, et al.Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients.Clin Infect Dis.1998;27:478–486.
- ,,.Infectious diseases consultation: impact on outcomes for hospitalized patients and results of a preliminary study.Clin Infect Dis.1997;24:468–470.
- ,,.Impact of mandatory inpatient infectious disease consultation on outpatient parenteral antibiotic therapy.Am J Med Sci.2005;330:60–64.
- ,.Home intravenous antibiotic therapy: a team approach.Ann Intern Med.1983;99:388–392.
- ,,.Transitioning antimicrobial stewardship beyond the hospital: the Cleveland Clinic's community‐based parenteral anti‐infective therapy (CoPAT) program.J Hosp Med.2011;6(suppl 1):S24–S30.
- ,,.Professional challenges and opportunities in clinical microbiology and infectious diseases in Europe.Lancet Infect Dis.2011;11:408–415.
- ,,, et al.Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines.Clin Infect Dis.2004;38:1651–1672.
- ,.Addressing postdischarge adverse events: a neglected area.Jt Comm J Qual Patient Saf.2008;34:85–97.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,, et al.Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4:364–370.
- .Hospitalist to home: outpatient parenteral antimicrobial therapy at an academic center.Clin Infect Dis.2010;51(suppl 2):S220–S223.
With dramatically increasing costs of healthcare, it has become increasingly necessary for healthcare providers to demonstrate value in the delivery of care. Porter and Teisberg have strongly advocated that healthcare reform efforts should focus on improving value rather than limiting cost, with value being defined as quality per unit cost.1 However, it has been pointed out that value means different things to different people.2 The biggest challenge in defining value stems mainly from the difficulty in defining quality, because it, too, means vastly different things to different people. Modern medicine is increasingly characterized by multidisciplinary care. With limited or shrinking resources, it will become necessary for individual specialists to describe and articulate, in quantitative terms, their specific contributions to the overall outcome of individual patients.
Previous publications have provided broad descriptions of the value provided by infectious disease (ID) specialists in the domains of sepsis, infection control, outpatient antibiotic therapy, antimicrobial stewardship, and directive care and teaching.3, 4 Studies have also shown the value of ID physicians in specific disease conditions. ID consultation is associated with lower mortality5, 6 and lower relapse rates7 in hospitalized patients with Staphylococcus aureus bacteremia. In another study evaluating the impact of ID consultants, patients seen by ID consultants had longer lengths of hospital stay, longer intensive care unit lengths of stay, and higher antibiotic costs than matched controls not seen by ID consultants.8 It can be argued that a major limitation of the study was that controls were not matched for the ID diagnosis, nor for the causative microorganisms, but it is clear that ID physicians are challenged to demonstrate their contribution to the care of patients.
A unique activity of ID physicians is the management of community‐based parenteral anti‐infective therapy (CoPAT). At Baystate Medical Center, a policy of mandatory ID consultation was instituted for patients leaving hospital on parenteral antibiotics. A study was conducted on the impact of predischarge ID consultation for 44 patients who were not already being followed by the ID service. The study documented change from intravenous (IV) to oral formulation, change of antibiotic choice, and change of dose/duration of treatment in a substantial proportion of patients.9 These are significant changes, but ID consultation contributes more than the themes explored in the study.
The purpose of this study was to evaluate the contribution of ID consultation when consulted for CoPAT, an activity specific to ID practice, in a different institution, and using an expanded definition of medical contribution.
METHODS
The Cleveland Clinic's Department of Infectious Disease has 24 staff physicians and 11 inpatient ID consultative services. These include: 2 solid organ transplant services; a bone marrow transplant and oncologic service; 2 infective endocarditis/cardiac device infection services; an intensive care unit (ICU) service; a bone and joint infection service; a neuroinfection service; and 3 general ID consult services. Consultative services are provided 7 days a week. At the Cleveland Clinic, ID consultation is required prior to discharge on parenteral antibiotic therapy.10, 11 ID consultation for CoPAT usually occurs when the primary service deems the patient is close to being discharged from hospital. This circumstance allows for assessing the specific contribution of ID physicians beyond that of the primary service and other consulting services.
Case Ascertainment
The study was approved by the institutional review board. In February 2010, an electronic form for requesting ID consultations had been introduced into the computerized provider order entry (CPOE) system at the Cleveland Clinic. One of the required questions on the form was whether the consultation was regarding CoPAT, with options of Yes, No, or Not sure. These electronic ID consultation requests were screened to identify consultation requests for this study.
Inclusion and Exclusion Criteria
All adult ID consultations between February 11, 2010 and May 15, 2010 for which the CoPAT consult? field was marked Yes were included in the study. All other consultations, including not sure for CoPAT, were excluded.
Definitions
The first ID consultation during a hospitalization was considered an initial consultation. ID consultations for patients whom an ID service had previously seen during the same hospitalization were deemed reconsultations. Value provided was defined as contribution of the ID consultation team in the following domains: 1) optimization of antimicrobial therapy, 2) significant change in patient assessment, 3) additional medical care contribution. Specific contributions included in each domain are outlined in Table 1.
|
| Domain 1: Optimization of antibiotic therapy |
| Alteration of an antibiotic (change of antibiotic or route of administration) |
| Defining duration of therapy |
| Identification of psychosocial factors (eg, injection drug use) that influence treatment |
| Domain 2: Significant change in patient assessment |
| Diagnosis of an infectious process |
| Better appreciation of extent of disease |
| Refutation of a false infectious disease diagnosis |
| Recognition of a noninfectious process needing urgent attention |
| Identification of a positive culture as contaminant/colonization |
| Recognition of a need for additional testing (testing needed to arrive at a diagnosis or clarify a treatment plan before a patient could be safely discharged from hospital) |
| Recognition of need for surgery/emnvasive intervention |
| Refutation of antibiotic allergy by history or allergy testing |
| Domain 3: Additional medical care contribution |
| Administration of vaccines |
| Identification of an unrecognized medical problem that needed to be addressed after discharge from hospital |
| Provision of effective transition of care (ensuring that the same ID physician who saw the patient in hospital followed the patient after discharge from hospital) |
Data Collected
For each ID consultation episode, clinicians' notes were reviewed from the day of the ID consultation to the day the patient was discharged from hospital or the day the ID service signed off, whichever happened sooner. Results of recommended tests were followed up to determine if results led to a change in patient assessment. Data elements collected for each consultation episode included patient age, gender, race, date of hospitalization, date of discharge, date of ID consultation or reconsultation, primary service, and documentation of ID service contributions. Data were collected and entered in a Microsoft Access relational database. To minimize bias, the data collection was performed by physicians who had not participated in the care of the patient.
Analysis
The proportion of ID consultations in which the ID team contributed in the defined domains were enumerated, and described for the group overall and also separately for initial consultations and reconsultations.
RESULTS
In the time period studied, there were 1326 CPOE requests for ID consultation. The response to the question, CoPAT consult? was Yes for 304, No for 507, and Not sure for 515 requests. Of the 304 consultation requests marked Yes, 41 were excluded. Reasons for exclusion were: no ID consultation note (21), wrong service consulted (8), consultation request placed while the ID service was already following the patient (7), and duplicate consultation request (5). The remaining 263 consultation requests corresponded to 1 or more CoPAT consultation requests for 249 patients (across different hospitalizations). Of the 263 consultation requests, 172 were initial consultations, while the remaining 91 were reconsultations (patients not actively being followed by the ID service, but previously seen during the same hospitalization).
Consultation characteristics are outlined in Table 2. The most common group of infections for which CoPAT was sought was bone and joint infections, accounting for over 20% of the consultation requests. CoPAT consultations were requested a median of 4 days after hospitalization. Patients were discharged from hospital a median of 3 days after they were seen by the ID service. ID consultation did not delay discharge. The ID service usually saw the patient the same day, and followed the patient in hospital for a median of 1 day. There was no difference in hospital days after consult for patients who did not need antibiotics versus those who did.
| Characteristic | Initial Consultation [172] n (%)* | Reconsultation [91] n (%)* | Overall [263] n (%)* |
|---|---|---|---|
| |||
| Patient age in years, mean (SD) | 58 (14) | 62 (13) | 59 (14) |
| Male gender | 98 (60) | 91 (56) | 149 (57) |
| Caucasian race | 126 (73) | 74 (81) | 200 (76) |
| Services requesting consults (5 most common overall) | |||
| Medicine | 41 (17) | 14 (15) | 55 (21) |
| Orthopedics | 34 (14) | 0 (0) | 34 (13) |
| Hematology/Oncology | 16 (7) | 10 (11) | 26 (10) |
| Cardiology | 9 (4) | 15 (16) | 24 (9) |
| Gastroenterology | 14 (6) | 5 (5) | 19 (7) |
| Consult diagnosis (5 most common overall) | |||
| Bone and joint infection | 45 (26) | 9 (10) | 54 (21) |
| Skin or soft tissue infection or rash | 21 (12) | 8 (9) | 29 (11) |
| Endocarditis or cardiac device infection | 7 (4) | 15 (16) | 22 (8) |
| IV catheter or other endovascular infection | 9 (5) | 8 (9) | 17 (6) |
| Urinary tract infection | 12 (7) | 5 (5) | 17 (6) |
| Days from admission to ID consult, median (IQR) | 4 (1‐11) | 7 (2‐19) | 4 (1‐14) |
| Days to respond to consult request, median (IQR) | 0 (0‐1) | 0 (0‐0) | 0 (0‐0) |
| Days from ID consult to discharge, median (IQR) | 3 (2‐7) | 2 (1‐4.5) | 3 (1‐6) |
ID consultation provided value in at least 1 domain in 260 of the 263 consultations. This included optimization of antimicrobial treatment in 84%, significant alteration of patient assessment in 52%, and additional medical care contribution in 71% of consultations. Substantial contributions were made in all domains in both initial consultations and in reconsultations. Specific ID contributions within each of the domains are shown in Figure 1. There was wide overlap of contributions across the 3 domains for individual consultations (Figure 2), with contributions in all domains occurring in 34% of consultations. CoPAT was deemed not to be necessary in 27% of consultations. Among patients who did not require CoPAT, 60% received oral antibiotics and 40% were deemed not to need any antibiotics at hospital discharge. Among the patients discharged on CoPAT, a follow‐up appointment with a Cleveland Clinic ID physician familiar with the patient was set up 86% of the time; the rest either followed up with another physician or it was deemed that a scheduled follow‐up ID visit was not necessary.0


DISCUSSION
Physicians practicing in the specialty of infectious diseases face challenges and opportunities, as they adapt to changing demands within hospital practice in regard to reimbursement in an Accountable Care environment. Other challenges include emerging infections, antimicrobial resistance, need for antimicrobial stewardship, and increasing numbers of immunocompromised patients.12 From a health systems perspective, the overall value of care provided by the entire organization, and overall outcomes, are ultimately what matter. However, healthcare administrators need an appreciation of contributions of individual providers and specialties to fairly allocate resources and compensation for care provided. Articulating unique contributions is particularly challenging for individuals or services that provide purely cognitive input. Shrinking healthcare resources makes it critically important for cognitive specialists to be able to define their unique role in the care of patients with complex problems.
Our study found that a major contribution of ID consultation for CoPAT is that the process identifies a large number of patients who do not need CoPAT, thus effecting a powerful antimicrobial stewardship function. In our study, CoPAT was deemed unnecessary 27% of the time. The Infectious Diseases Society of America practice guidelines on outpatient parenteral antimicrobial therapy emphasize the importance of careful evaluation of patients considered for parenteral antibiotics outside the hospital setting.13 The focus on careful selection of appropriate patients for CoPAT has been a cornerstone of the Cleveland Clinic model of care. Nearly 30 years ago, we found that outpatient parenteral antibiotic therapy was unnecessary or not feasible in 40% of the patients referred for evaluation.10 If we adjust the numbers with the assumption that reimbursement issues present at that time are now less of an issue, the proportion of patients who were referred for CoPAT but not discharged on it was 29%, a figure remarkably similar to that found in the current study.
Another major contribution of ID consultation is the provision of effective transition of care from the inpatient to the outpatient setting. Frequent occurrence of postdischarge adverse events has been recognized as a problem in clinical practice.14 Primary care physicians are rarely involved in discussions about hospital discharge.15 A consensus conference including the American College of Physicians, Society of Hospital Medicine, and Society of General Internal Medicine, convened in July 2007 to address quality gaps in transitions of care between inpatient and outpatient settings. It identified 5 principles for effective care transitions: accountability, communication, timeliness, patient and family involvement, and respect for the hub of coordination of care.16 Recognizing gaps in care transition, hospitalists in a hospital‐based infusion program developed a model of care that successfully bridged the hospital‐to‐home care transition for patients who could return to hospital for daily antimicrobial infusions.17 In our system, ID physicians take ownership for directing parenteral antibiotic therapy for the episode of illness, specifying the physician, date, and time of follow‐up before the patient is discharged from hospital, thereby essentially satisfying the principles of effective care transitions identified. The purpose of the ID follow‐up is not to replace other follow‐up care for patients but to ensure safe transition of care while treating an episode of infection.
Attribution of identified contributions to the ID consultation could be done because our study was limited to CoPAT consultations. Such consultations typically occur when patients are deemed close to hospital discharge by the primary service. There should be little controversy about attribution of cognitive input in such consultations, because from the primary service's perspective, the patient is ready or almost ready to be discharged from hospital. It would be fair to state that most of the identified contributions in the study would not have occurred had it not been for the ID consultation.
We acknowledge that the study suffers from many limitations. The biggest limitation is that the contribution elements are defined by ID physicians and sought in the medical record by physicians from the same specialty. This arrangement certainly has potential for significant bias. To limit this bias, data collection was performed by physicians who had not participated in the care of the patient. In addition, we only could assess what was documented in the electronic health record. Our study found that alteration of antibiotic therapy was a substantial contribution, however, documentation of recommendation to change antibiotics in the medical record rarely specified exactly why the change was recommended. Reasons for antibiotic change recommendations included bug‐drug mismatch, minimum inhibitory concentration (MIC) considerations, pharmacokinetic considerations, adverse effects, convenience of dosing, drug interactions, and insurance coverage. However, it is not possible to quantify the specific contribution of each of these reasons, in a retrospective study, without making assumptions about why specific ID physicians made specific antibiotic change recommendations. There may have been more contributions that might not have been apparent on a retrospective chart review. The lack of a control group also lessens the impact of our findings. We could not have a control group, because no patient is discharged from the Cleveland Clinic on CoPAT without having been seen by an ID physician. Mandatory ID consultation for CoPAT has previously been shown to reduce costs,9 however, our study was not designed to evaluate cost.
The perceived value of ID consultation in our institution can be appreciated when one considers the longstanding institutional policy of requiring ID consultation for CoPAT.10, 11 The perpetuation of this tradition in the hospital is testament to the presumption that mandatory ID consultation is seen to be of value by the institution.
In summary, ID consultation in our institution contributes to the care of inpatients being considered for CoPAT by substantially reducing unnecessary parenteral antibiotic use, optimizing antibiotic therapy, recognizing need for additional testing before discharge from hospital, and by providing effective transition of care from the inpatient to the outpatient setting.
With dramatically increasing costs of healthcare, it has become increasingly necessary for healthcare providers to demonstrate value in the delivery of care. Porter and Teisberg have strongly advocated that healthcare reform efforts should focus on improving value rather than limiting cost, with value being defined as quality per unit cost.1 However, it has been pointed out that value means different things to different people.2 The biggest challenge in defining value stems mainly from the difficulty in defining quality, because it, too, means vastly different things to different people. Modern medicine is increasingly characterized by multidisciplinary care. With limited or shrinking resources, it will become necessary for individual specialists to describe and articulate, in quantitative terms, their specific contributions to the overall outcome of individual patients.
Previous publications have provided broad descriptions of the value provided by infectious disease (ID) specialists in the domains of sepsis, infection control, outpatient antibiotic therapy, antimicrobial stewardship, and directive care and teaching.3, 4 Studies have also shown the value of ID physicians in specific disease conditions. ID consultation is associated with lower mortality5, 6 and lower relapse rates7 in hospitalized patients with Staphylococcus aureus bacteremia. In another study evaluating the impact of ID consultants, patients seen by ID consultants had longer lengths of hospital stay, longer intensive care unit lengths of stay, and higher antibiotic costs than matched controls not seen by ID consultants.8 It can be argued that a major limitation of the study was that controls were not matched for the ID diagnosis, nor for the causative microorganisms, but it is clear that ID physicians are challenged to demonstrate their contribution to the care of patients.
A unique activity of ID physicians is the management of community‐based parenteral anti‐infective therapy (CoPAT). At Baystate Medical Center, a policy of mandatory ID consultation was instituted for patients leaving hospital on parenteral antibiotics. A study was conducted on the impact of predischarge ID consultation for 44 patients who were not already being followed by the ID service. The study documented change from intravenous (IV) to oral formulation, change of antibiotic choice, and change of dose/duration of treatment in a substantial proportion of patients.9 These are significant changes, but ID consultation contributes more than the themes explored in the study.
The purpose of this study was to evaluate the contribution of ID consultation when consulted for CoPAT, an activity specific to ID practice, in a different institution, and using an expanded definition of medical contribution.
METHODS
The Cleveland Clinic's Department of Infectious Disease has 24 staff physicians and 11 inpatient ID consultative services. These include: 2 solid organ transplant services; a bone marrow transplant and oncologic service; 2 infective endocarditis/cardiac device infection services; an intensive care unit (ICU) service; a bone and joint infection service; a neuroinfection service; and 3 general ID consult services. Consultative services are provided 7 days a week. At the Cleveland Clinic, ID consultation is required prior to discharge on parenteral antibiotic therapy.10, 11 ID consultation for CoPAT usually occurs when the primary service deems the patient is close to being discharged from hospital. This circumstance allows for assessing the specific contribution of ID physicians beyond that of the primary service and other consulting services.
Case Ascertainment
The study was approved by the institutional review board. In February 2010, an electronic form for requesting ID consultations had been introduced into the computerized provider order entry (CPOE) system at the Cleveland Clinic. One of the required questions on the form was whether the consultation was regarding CoPAT, with options of Yes, No, or Not sure. These electronic ID consultation requests were screened to identify consultation requests for this study.
Inclusion and Exclusion Criteria
All adult ID consultations between February 11, 2010 and May 15, 2010 for which the CoPAT consult? field was marked Yes were included in the study. All other consultations, including not sure for CoPAT, were excluded.
Definitions
The first ID consultation during a hospitalization was considered an initial consultation. ID consultations for patients whom an ID service had previously seen during the same hospitalization were deemed reconsultations. Value provided was defined as contribution of the ID consultation team in the following domains: 1) optimization of antimicrobial therapy, 2) significant change in patient assessment, 3) additional medical care contribution. Specific contributions included in each domain are outlined in Table 1.
|
| Domain 1: Optimization of antibiotic therapy |
| Alteration of an antibiotic (change of antibiotic or route of administration) |
| Defining duration of therapy |
| Identification of psychosocial factors (eg, injection drug use) that influence treatment |
| Domain 2: Significant change in patient assessment |
| Diagnosis of an infectious process |
| Better appreciation of extent of disease |
| Refutation of a false infectious disease diagnosis |
| Recognition of a noninfectious process needing urgent attention |
| Identification of a positive culture as contaminant/colonization |
| Recognition of a need for additional testing (testing needed to arrive at a diagnosis or clarify a treatment plan before a patient could be safely discharged from hospital) |
| Recognition of need for surgery/emnvasive intervention |
| Refutation of antibiotic allergy by history or allergy testing |
| Domain 3: Additional medical care contribution |
| Administration of vaccines |
| Identification of an unrecognized medical problem that needed to be addressed after discharge from hospital |
| Provision of effective transition of care (ensuring that the same ID physician who saw the patient in hospital followed the patient after discharge from hospital) |
Data Collected
For each ID consultation episode, clinicians' notes were reviewed from the day of the ID consultation to the day the patient was discharged from hospital or the day the ID service signed off, whichever happened sooner. Results of recommended tests were followed up to determine if results led to a change in patient assessment. Data elements collected for each consultation episode included patient age, gender, race, date of hospitalization, date of discharge, date of ID consultation or reconsultation, primary service, and documentation of ID service contributions. Data were collected and entered in a Microsoft Access relational database. To minimize bias, the data collection was performed by physicians who had not participated in the care of the patient.
Analysis
The proportion of ID consultations in which the ID team contributed in the defined domains were enumerated, and described for the group overall and also separately for initial consultations and reconsultations.
RESULTS
In the time period studied, there were 1326 CPOE requests for ID consultation. The response to the question, CoPAT consult? was Yes for 304, No for 507, and Not sure for 515 requests. Of the 304 consultation requests marked Yes, 41 were excluded. Reasons for exclusion were: no ID consultation note (21), wrong service consulted (8), consultation request placed while the ID service was already following the patient (7), and duplicate consultation request (5). The remaining 263 consultation requests corresponded to 1 or more CoPAT consultation requests for 249 patients (across different hospitalizations). Of the 263 consultation requests, 172 were initial consultations, while the remaining 91 were reconsultations (patients not actively being followed by the ID service, but previously seen during the same hospitalization).
Consultation characteristics are outlined in Table 2. The most common group of infections for which CoPAT was sought was bone and joint infections, accounting for over 20% of the consultation requests. CoPAT consultations were requested a median of 4 days after hospitalization. Patients were discharged from hospital a median of 3 days after they were seen by the ID service. ID consultation did not delay discharge. The ID service usually saw the patient the same day, and followed the patient in hospital for a median of 1 day. There was no difference in hospital days after consult for patients who did not need antibiotics versus those who did.
| Characteristic | Initial Consultation [172] n (%)* | Reconsultation [91] n (%)* | Overall [263] n (%)* |
|---|---|---|---|
| |||
| Patient age in years, mean (SD) | 58 (14) | 62 (13) | 59 (14) |
| Male gender | 98 (60) | 91 (56) | 149 (57) |
| Caucasian race | 126 (73) | 74 (81) | 200 (76) |
| Services requesting consults (5 most common overall) | |||
| Medicine | 41 (17) | 14 (15) | 55 (21) |
| Orthopedics | 34 (14) | 0 (0) | 34 (13) |
| Hematology/Oncology | 16 (7) | 10 (11) | 26 (10) |
| Cardiology | 9 (4) | 15 (16) | 24 (9) |
| Gastroenterology | 14 (6) | 5 (5) | 19 (7) |
| Consult diagnosis (5 most common overall) | |||
| Bone and joint infection | 45 (26) | 9 (10) | 54 (21) |
| Skin or soft tissue infection or rash | 21 (12) | 8 (9) | 29 (11) |
| Endocarditis or cardiac device infection | 7 (4) | 15 (16) | 22 (8) |
| IV catheter or other endovascular infection | 9 (5) | 8 (9) | 17 (6) |
| Urinary tract infection | 12 (7) | 5 (5) | 17 (6) |
| Days from admission to ID consult, median (IQR) | 4 (1‐11) | 7 (2‐19) | 4 (1‐14) |
| Days to respond to consult request, median (IQR) | 0 (0‐1) | 0 (0‐0) | 0 (0‐0) |
| Days from ID consult to discharge, median (IQR) | 3 (2‐7) | 2 (1‐4.5) | 3 (1‐6) |
ID consultation provided value in at least 1 domain in 260 of the 263 consultations. This included optimization of antimicrobial treatment in 84%, significant alteration of patient assessment in 52%, and additional medical care contribution in 71% of consultations. Substantial contributions were made in all domains in both initial consultations and in reconsultations. Specific ID contributions within each of the domains are shown in Figure 1. There was wide overlap of contributions across the 3 domains for individual consultations (Figure 2), with contributions in all domains occurring in 34% of consultations. CoPAT was deemed not to be necessary in 27% of consultations. Among patients who did not require CoPAT, 60% received oral antibiotics and 40% were deemed not to need any antibiotics at hospital discharge. Among the patients discharged on CoPAT, a follow‐up appointment with a Cleveland Clinic ID physician familiar with the patient was set up 86% of the time; the rest either followed up with another physician or it was deemed that a scheduled follow‐up ID visit was not necessary.0


DISCUSSION
Physicians practicing in the specialty of infectious diseases face challenges and opportunities, as they adapt to changing demands within hospital practice in regard to reimbursement in an Accountable Care environment. Other challenges include emerging infections, antimicrobial resistance, need for antimicrobial stewardship, and increasing numbers of immunocompromised patients.12 From a health systems perspective, the overall value of care provided by the entire organization, and overall outcomes, are ultimately what matter. However, healthcare administrators need an appreciation of contributions of individual providers and specialties to fairly allocate resources and compensation for care provided. Articulating unique contributions is particularly challenging for individuals or services that provide purely cognitive input. Shrinking healthcare resources makes it critically important for cognitive specialists to be able to define their unique role in the care of patients with complex problems.
Our study found that a major contribution of ID consultation for CoPAT is that the process identifies a large number of patients who do not need CoPAT, thus effecting a powerful antimicrobial stewardship function. In our study, CoPAT was deemed unnecessary 27% of the time. The Infectious Diseases Society of America practice guidelines on outpatient parenteral antimicrobial therapy emphasize the importance of careful evaluation of patients considered for parenteral antibiotics outside the hospital setting.13 The focus on careful selection of appropriate patients for CoPAT has been a cornerstone of the Cleveland Clinic model of care. Nearly 30 years ago, we found that outpatient parenteral antibiotic therapy was unnecessary or not feasible in 40% of the patients referred for evaluation.10 If we adjust the numbers with the assumption that reimbursement issues present at that time are now less of an issue, the proportion of patients who were referred for CoPAT but not discharged on it was 29%, a figure remarkably similar to that found in the current study.
Another major contribution of ID consultation is the provision of effective transition of care from the inpatient to the outpatient setting. Frequent occurrence of postdischarge adverse events has been recognized as a problem in clinical practice.14 Primary care physicians are rarely involved in discussions about hospital discharge.15 A consensus conference including the American College of Physicians, Society of Hospital Medicine, and Society of General Internal Medicine, convened in July 2007 to address quality gaps in transitions of care between inpatient and outpatient settings. It identified 5 principles for effective care transitions: accountability, communication, timeliness, patient and family involvement, and respect for the hub of coordination of care.16 Recognizing gaps in care transition, hospitalists in a hospital‐based infusion program developed a model of care that successfully bridged the hospital‐to‐home care transition for patients who could return to hospital for daily antimicrobial infusions.17 In our system, ID physicians take ownership for directing parenteral antibiotic therapy for the episode of illness, specifying the physician, date, and time of follow‐up before the patient is discharged from hospital, thereby essentially satisfying the principles of effective care transitions identified. The purpose of the ID follow‐up is not to replace other follow‐up care for patients but to ensure safe transition of care while treating an episode of infection.
Attribution of identified contributions to the ID consultation could be done because our study was limited to CoPAT consultations. Such consultations typically occur when patients are deemed close to hospital discharge by the primary service. There should be little controversy about attribution of cognitive input in such consultations, because from the primary service's perspective, the patient is ready or almost ready to be discharged from hospital. It would be fair to state that most of the identified contributions in the study would not have occurred had it not been for the ID consultation.
We acknowledge that the study suffers from many limitations. The biggest limitation is that the contribution elements are defined by ID physicians and sought in the medical record by physicians from the same specialty. This arrangement certainly has potential for significant bias. To limit this bias, data collection was performed by physicians who had not participated in the care of the patient. In addition, we only could assess what was documented in the electronic health record. Our study found that alteration of antibiotic therapy was a substantial contribution, however, documentation of recommendation to change antibiotics in the medical record rarely specified exactly why the change was recommended. Reasons for antibiotic change recommendations included bug‐drug mismatch, minimum inhibitory concentration (MIC) considerations, pharmacokinetic considerations, adverse effects, convenience of dosing, drug interactions, and insurance coverage. However, it is not possible to quantify the specific contribution of each of these reasons, in a retrospective study, without making assumptions about why specific ID physicians made specific antibiotic change recommendations. There may have been more contributions that might not have been apparent on a retrospective chart review. The lack of a control group also lessens the impact of our findings. We could not have a control group, because no patient is discharged from the Cleveland Clinic on CoPAT without having been seen by an ID physician. Mandatory ID consultation for CoPAT has previously been shown to reduce costs,9 however, our study was not designed to evaluate cost.
The perceived value of ID consultation in our institution can be appreciated when one considers the longstanding institutional policy of requiring ID consultation for CoPAT.10, 11 The perpetuation of this tradition in the hospital is testament to the presumption that mandatory ID consultation is seen to be of value by the institution.
In summary, ID consultation in our institution contributes to the care of inpatients being considered for CoPAT by substantially reducing unnecessary parenteral antibiotic use, optimizing antibiotic therapy, recognizing need for additional testing before discharge from hospital, and by providing effective transition of care from the inpatient to the outpatient setting.
- ,.How physicians can change the future of health care.JAMA.2007;297:1103–1111.
- .Value of the infectious diseases specialist.Clin Infect Dis.1997;24:456.
- ,,, et al.The value of an infectious diseases specialist.Clin Infect Dis.2003;36:1013–1017.
- ,,,,,.The value of infectious diseases specialists: non‐patient care activities.Clin Infect Dis.2008;47:1051–1063.
- ,,,,.The value of infectious diseases consultation in Staphylococcus aureus bacteremia.Am J Med.2010;123:631–637.
- ,,,,.Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia.Medicine (Baltimore).2009;88:263–267.
- ,,, et al.Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients.Clin Infect Dis.1998;27:478–486.
- ,,.Infectious diseases consultation: impact on outcomes for hospitalized patients and results of a preliminary study.Clin Infect Dis.1997;24:468–470.
- ,,.Impact of mandatory inpatient infectious disease consultation on outpatient parenteral antibiotic therapy.Am J Med Sci.2005;330:60–64.
- ,.Home intravenous antibiotic therapy: a team approach.Ann Intern Med.1983;99:388–392.
- ,,.Transitioning antimicrobial stewardship beyond the hospital: the Cleveland Clinic's community‐based parenteral anti‐infective therapy (CoPAT) program.J Hosp Med.2011;6(suppl 1):S24–S30.
- ,,.Professional challenges and opportunities in clinical microbiology and infectious diseases in Europe.Lancet Infect Dis.2011;11:408–415.
- ,,, et al.Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines.Clin Infect Dis.2004;38:1651–1672.
- ,.Addressing postdischarge adverse events: a neglected area.Jt Comm J Qual Patient Saf.2008;34:85–97.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,, et al.Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4:364–370.
- .Hospitalist to home: outpatient parenteral antimicrobial therapy at an academic center.Clin Infect Dis.2010;51(suppl 2):S220–S223.
- ,.How physicians can change the future of health care.JAMA.2007;297:1103–1111.
- .Value of the infectious diseases specialist.Clin Infect Dis.1997;24:456.
- ,,, et al.The value of an infectious diseases specialist.Clin Infect Dis.2003;36:1013–1017.
- ,,,,,.The value of infectious diseases specialists: non‐patient care activities.Clin Infect Dis.2008;47:1051–1063.
- ,,,,.The value of infectious diseases consultation in Staphylococcus aureus bacteremia.Am J Med.2010;123:631–637.
- ,,,,.Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia.Medicine (Baltimore).2009;88:263–267.
- ,,, et al.Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients.Clin Infect Dis.1998;27:478–486.
- ,,.Infectious diseases consultation: impact on outcomes for hospitalized patients and results of a preliminary study.Clin Infect Dis.1997;24:468–470.
- ,,.Impact of mandatory inpatient infectious disease consultation on outpatient parenteral antibiotic therapy.Am J Med Sci.2005;330:60–64.
- ,.Home intravenous antibiotic therapy: a team approach.Ann Intern Med.1983;99:388–392.
- ,,.Transitioning antimicrobial stewardship beyond the hospital: the Cleveland Clinic's community‐based parenteral anti‐infective therapy (CoPAT) program.J Hosp Med.2011;6(suppl 1):S24–S30.
- ,,.Professional challenges and opportunities in clinical microbiology and infectious diseases in Europe.Lancet Infect Dis.2011;11:408–415.
- ,,, et al.Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines.Clin Infect Dis.2004;38:1651–1672.
- ,.Addressing postdischarge adverse events: a neglected area.Jt Comm J Qual Patient Saf.2008;34:85–97.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,, et al.Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4:364–370.
- .Hospitalist to home: outpatient parenteral antimicrobial therapy at an academic center.Clin Infect Dis.2010;51(suppl 2):S220–S223.
Community‐Based Parenteral Antimicrobial Therapy
. . . For the secret of the care of the patient is caring for the patient.Francis W. Peabody, October 21, 19251
Collaboration between members of a multidisciplinary team is a key component of an effective institutional antimicrobial stewardship program, which itself is a key component of optimizing the care of hospitalized patients being treated with antimicrobial agents for proven or suspected infectious diseases. However, patient care does not and should not end once the patient is discharged from the hospital. In fact, high‐quality, value‐based health care across the full range of a medical condition depends on planning for optimization of care within the hospital as well as transitions of care to the outpatient setting. This extended care plan includes collaboration with multiple members of the health care community, both inside and outside the institution. The current review examines 3 aspects of patient care across the full cycle of an infectious disease condition: (1) value‐based health care, (2) stewardship of antimicrobials, and (3) community‐based parenteral anti‐infective therapy (CoPAT) as a model for antimicrobial stewardship outside the institutional setting.
Value‐Based Health Care
Patients first want to know that the health care professionals treating them actually care about them as individuals, and only then are patients concerned about how much the medical team knows. Patient‐centered care is a critical component of value‐based health care, a term that was bandied about quite a bit during the recent and ongoing health care debate in the United States. But what exactly does it mean? First, value in health care is defined by health care outcomes as a function of or divided by the cost of delivery of care. As Dr. Michael Porter and Dr. Elizabeth Olmsted Teisberg delineated in their 2007 article in the Journal of the American Medical Association,2 as well as in the 2006 book Redefining Health Care,3 The purpose of the healthcare system is not to minimize costs but to deliver value to patients, that is, better health per dollar spent. As they discuss value, it is a patient‐centric measure, and is focused on individual patient (not just diagnosis‐related group) outcomes and the cost of care across the full cycle. In this way of looking at things, an episode of care goes beyond the treatment provided during the acute admission to also include the transition of care to the outpatient or posthospital setting.
The reforms proposed by Porter and Teisberg are best achieved when the participating health care institutions have developed an information technology platform able to integrate and fully measure care across the full cycle of a medical condition. Furthermore, there is strong evidence that patient value increases with physician and team experience and volume for a particular condition.2 High volumes tend to correlate with the development of better information technology, as well as the formation of dedicated teams with tailored facilities, and with a greater capacity for constructive feedback to improve patient outcomes. The more experience a physician and team have with the management of a particular medical condition, the greater is the opportunity to learn and refine practices to provide greater value to the patient.
The Institute of Medicine has recommended that all healthcare professionals should be educated to deliver patient‐centered care as members of an interdisciplinary team emphasizing evidence‐based practice, quality improvement, and informatics.4 As has been demonstrated for patients with congestive heart failure59 and other conditions,10, 11 outcomes improve when components of care are integrated (often by nurse‐directed teams), preparing for the transition of care from the hospital to the home.12 This concept is the basis for the community‐based parenteral anti‐infective therapy program (CoPAT) at the Cleveland Clinic as a model for antimicrobial stewardship for patients requiring parenteral antimicrobial therapy at the time of discharge from the inpatient setting.
Stewardship of Antimicrobials
The Merriam‐Webster dictionary alternatively defines a steward as: (1) an employee on a ship, airplane, bus, or train who manages the provisioning of food and attends to passengers or (2) one who actively manages affairs (manager).13 In the context of health care within an institution, one can think of clinicians as stewards or employees charged with managing patients and the drugs and other care they receive while they are attendants (passengers) at the institution. In the value‐based approach just discussed, where medical practice is organized around managing medical conditions for the entire care cycle, a medical steward would also be charged with managing or planning for patient care after discharge from the hospital or other institutional setting. Management or stewardship of antimicrobial agents is a key component of the care for patients with infectious diseases. In 2007, the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America (IDSA/SHEA) presented guidelines to aid institutions in the development of an effective hospital‐based antimicrobial stewardship program (a more detailed overview of antimicrobial stewardship is presented in the accompanying supplemental article by Dr. Ohl).14 The focus of the IDSA/SHEA guidelines was on development of programs within hospitals. Although the authors acknowledged that antimicrobial stewardship is also important in outpatient clinics and long‐term care facilities, transition of antimicrobial management after patient discharge from the hospital was not a focus of the 2007 guidelines.
A key objective of antimicrobial stewardship is to optimize antimicrobial drug selection and dosing to improve clinical outcomes while reducing drug toxicity and other potential untoward consequences of antimicrobial therapy, including selection of opportunistic organisms (eg, Clostridium difficile) or emergence of multidrug resistance in pathogens.14 A secondary objective is to reduce overall health care costs,14 which ideally would include inpatient as well as outpatient costs and those related to hospital readmission due to the initial infection or its outpatient treatment. Useful metrics for evaluation of an antimicrobial stewardship program include measures of pathogen/drug mismatch, antimicrobial costs, incidence of redundant therapy, compliance with antimicrobial drug restrictions (if applicable), days undergoing antimicrobial therapy, and number of cases of intravenous to oral conversion.14
Although the IDSA/SHEA guidelines for institutional antimicrobial stewardship programs suggest that an infectious diseases physician and clinical pharmacist with infectious diseases training should be core members of a multidisciplinary stewardship team,14 many community hospitals or smaller institutions do not have an infectious diseases physician or a readily available infectious diseases specialist for consultation. Hospitalists are often very effective advocates of appropriate use of antimicrobials and may play a leadership role on institutional antimicrobial stewardship teams. A recent study demonstrated that a hospitalist‐delivered academic detailing intervention (which included an individual appraisal of the provider's prescription pattern) significantly improved patterns of antibiotic prescribing for inpatients.15
Community‐Based Parenteral Anti‐Infective Therapy as a Systems‐Based Approach to Antimicrobial Stewardship
A systems‐based approach for antimicrobial stewardship, CoPAT has been in operation at the Cleveland Clinic, a 1200‐bed hospital in downtown Cleveland, Ohio, since November 1979. The experiences of the authors and their colleagues demonstrate it to be a value‐based proposition for the patient that uses an antimicrobial stewardship platform. Also known as outpatient parenteral antimicrobial therapy (OPAT), CoPAT refers to the practice of administering antimicrobial therapy in the home or other outpatient settings, first introduced by Rucker and Harrison in 1974 in the context of outpatient management of cystic fibrosis.16 In the United States, CoPAT is a common practice today, and the IDSA has created practice guidelines for it.17
In 1983, Rehm and Weinstein coauthored an article describing their experiences at the Cleveland Clinic, in which selected patients were trained for home‐based antimicrobial therapy.12 Figure 1 illustrates the astronomical growth that has occurred over the years at the Cleveland Clinic in the number of patients discharged from the acute care center undergoing CoPAT (Gordon, unpublished data). It is anticipated that this growth will continue and in large part reflects the complexity of patients being seen and the desire to reduce length of stay. Evaluating the quality of any medical care is difficult, but there are 3 general approaches to assessing or measuring the quality of medical care: assessing the structure of care, assessing processes of care, and assessing outcomes.18 The quality of the CoPAT program at the Cleveland Clinic can be examined in the context of these 3 areas of assessment.

Settings or the Structure of Care
In a 1966 publication on quality of medical care evaluations, Donabedian described assessment of the structure of care as one of the primary approaches to measuring the quality of care.18 By structure, Donabedian meant the settings in which medical care takes place, including the adequacy of facilities and equipment, qualifications or expertise of medical staff and their organization, the administrative structure of the institution or institutional program of interest, and other administrative and related processes supporting and directing the delivery of care. Although the structure of care has the advantage of being concrete and relatively easy to assess, to be most meaningful, it ultimately needs to be related to the processes and outcomes of care.
With respect to the CoPAT program at the Cleveland Clinic main hospital, infectious diseases consultation is required for every patient being considered for discharge with parenteral antibiotics, whether the patient is going home or to another facility, including the clinic's own skilled nursing facility (SNF). Arrangements are then made for the delivery of antibiotics at home or in SNFs or long‐term acute care (LTAC) centers. The Cleveland Clinic CoPAT program does not use an outpatient infusion center.
The Cleveland Clinic uses a mandatory infectious diseases consultation for CoPAT because there are a number of important issues that need to be addressed before the patient is discharged, and for our system this is best accomplished by an infectious diseases specialist.12 For example, is antimicrobial therapy actually required in the first place? If it is, what is the optimal type, route, and duration of therapy? Are there other medical issues that need to be addressed? Decisions also need to be made about optimal vascular access and antimicrobial selection and administration, as well as arrangements being made for monitoring clinical and laboratory aspects. It is important that there is a smooth transition of care and prescheduled follow‐up in the outpatient clinic. The identification and use of an infectious diseases clinician directing the process leads to accountability. Notably, mandatory infectious disease consultation for outpatient parenteral antibiotic therapy has been used at Baystate Medical Center with improvement in reducing costs.19
The Process of Care
Assessments of the process of care involve examination of the particulars of medical care delivery, or whether what is recognized or accepted as good medical care has been applied. As discussed by Donabedian, process of care deals with issues such as the appropriateness and completeness of information obtained through clinical history, physical examination, and diagnostic tests; justification of diagnosis and therapy; technical competence in the performance of diagnostic and therapeutic procedures; and coordination and continuity of care.18
The CoPAT initiation process at the Cleveland Clinic is illustrated in Figure 2. It is a bundled process. As already mentioned, an infectious diseases consultation and evaluation is scheduled for all patients considered for CoPAT, after which a CoPAT form is completed and a follow‐up appointment made before the patient is discharged. In addition, the vascular access team is consulted and an appropriate vascular access device is placed in the patient prior to discharge. Likewise, a case manager is enlisted to identify a health care agency or SNF for patient placement or to determine whether the patient will receive home treatment. Once the appropriate setting is identified, the case manager transmits a completed CoPAT form to the health care agency or SNF, while forwarding a copy to the CoPAT nurse coordinator in the infectious disease department.
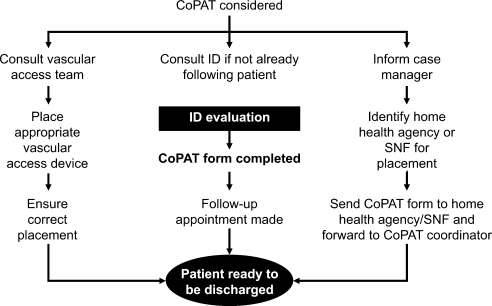
An electronic health record system is used at the Cleveland Clinic to provide real‐time information relevant for patient management. In 2007, a structured data form for CoPAT start‐of‐care was created within the Cleveland Clinic hospital electronic health record (EHR). This form contains a number of elements relevant for setting up patients for transition to CoPAT. In particular, the electronic CoPAT form contains information about the infection(s) and microorganism(s) being treated, intravenous antibiotic(s) prescribed (including treatment stop date), concurrent oral antibiotics, premedication recommendations (if appropriate), and recommended monitoring of laboratory tests. In addition, the form contains the telephone and fax numbers of the CoPAT coordinator and the name of the responsible physician, including a scheduled appointment for follow‐up (Fig. 3). The staff physician is responsible for completing the electronic CoPAT form or prescription. This CoPAT prescription then becomes part of the patient's electronic record and is transmissible and viewable by anyone with access to the EHR. This is important in terms of follow‐up and care accountability: an infectious disease staff clinician is identified as the contact person for clinical issues when a patient is on CoPAT.
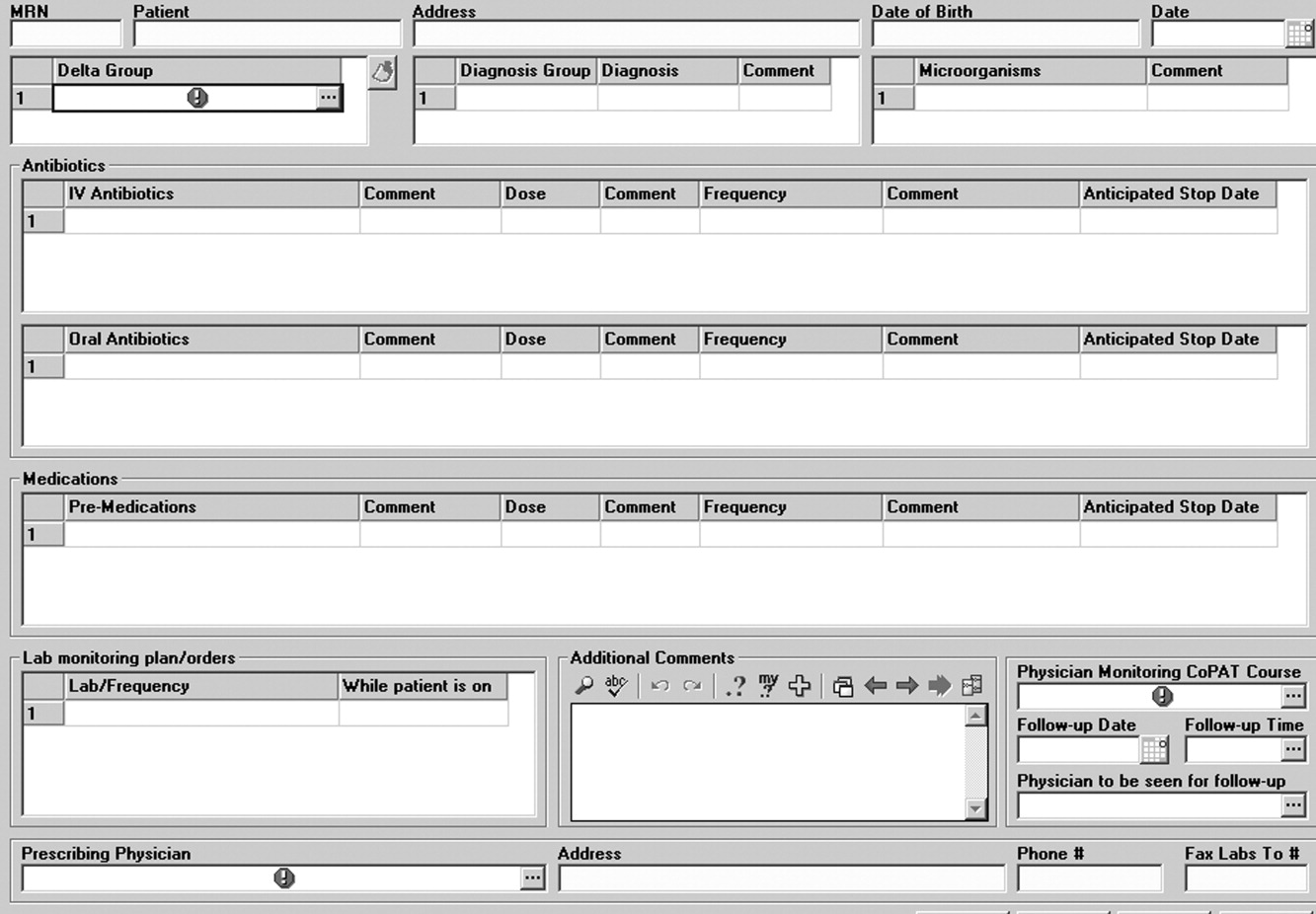
After the patient is discharged, the CoPAT coordinator in the infectious disease department becomes responsible, together with the clinic's outpatient pharmacy, for reviewing laboratory results and notifying clinicians of potential problems that need to be addressed. These issues can pertain to laboratory findings, vascular access, or new symptoms or signs observed by the home nurse or patient. All this information is communicated via electronic health record messaging and/or through direct calls to the physician, when needed.
The CoPAT program has been widely accepted by internal customers of the Cleveland Clinic, which include hospitalists. This is probably because there is autonomy and accountability with the infectious diseases staff, the program or team is available 7 days per week, and the EHR facilitates communication. In addition, the use of infectious disease‐specific subspecialty groups (eg, bone marrow and solid‐organ transplant, bone and joint, and infective endocarditis groups) increases clinical credibility, as well as value received by patients of the clinic. Furthermore, the electronic CoPAT script facilitates discharge planning. CoPATs constitute approximately 25% of all ID consultation requests at the Cleveland Clinic and help to justify the 20 clinical ID clinical FTEs.
Outcomes of Medical Care
Assessment of medical care outcomes is another frequently used approach for measuring the quality of medical care.18 Medical care outcomes that have been examined as measures of quality of care include survival, number of hospital readmissions, time between discharge and readmissions, length of initial hospital stay and subsequent readmissions, quality of life, and health care costs. As has often been said, If you cannot measure it, you cannot manage it. The CoPAT program using the EHR has facilitated retrieval of structured reports in a format that provides clinicians with real‐time data enabling assessment of outcomes. By examining this data, the CoPAT team is in a better position to contemplate potential interventions for improving outpatient care and the value patients receive.
A 36‐month review of Cleveland Clinic CoPAT patient demographics from July 2007 to June 2010 demonstrated 6287 patients (56% male) had been prescribed 9471 courses of CoPAT (Gordon, unpublished data). Seventy‐nine percent of the patients were white, 16% African American, and 5% of other races. Most patients received 1 antibiotic per CoPAT course (79.1%), whereas 18.2%, 2.5%, and 0.2% received 2, 3, and 4 antibiotics per CoPAT course, respectively. Figure 4 highlights CoPAT distribution by source for anatomic site of infection. Bone and joint infections were the most common diagnoses associated with CoPAT at the Cleveland Clinic, followed by abdominal, cardiovascular, primary disseminated disease (eg, catheter‐associated bloodstream infections), and skin and soft‐tissue infection.
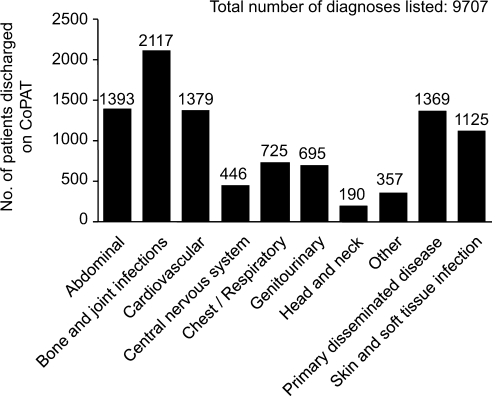
Figure 5 highlights the top‐10 pathogenic microorganisms in patients being discharged from the Cleveland Clinic with CoPAT, and the top‐10 antimicrobials prescribed for these patients. As can be seen, Staphylococcus aureus (methicillin susceptible and methicillin resistant) was the number one pathogen identified for patients undergoing CoPAT, followed by coagulase‐negative Staphylococcus and Enterococcus species. The most commonly identified gram‐negative bacteria among discharged patients was Pseudomonas aeruginosa. Only 2 of the top 10 pathogens were nonbacterial: Candida species and cytomegalovirus (CMV), the latter being the result of the high volume of transplantations performed at the clinic. With respect to the intravenous antimicrobials prescribed for patients undergoing CoPAT, the most commonly prescribed agent was vancomycin, followed by piperacillin/tazobactam. Of the 10 agents, only micafungin and ganciclovir were not antibacterial agents, indicating that the vast majority of patients discharged from the Cleveland Clinic with CoPAT had had bacterial, rather than fungal or viral, infections.
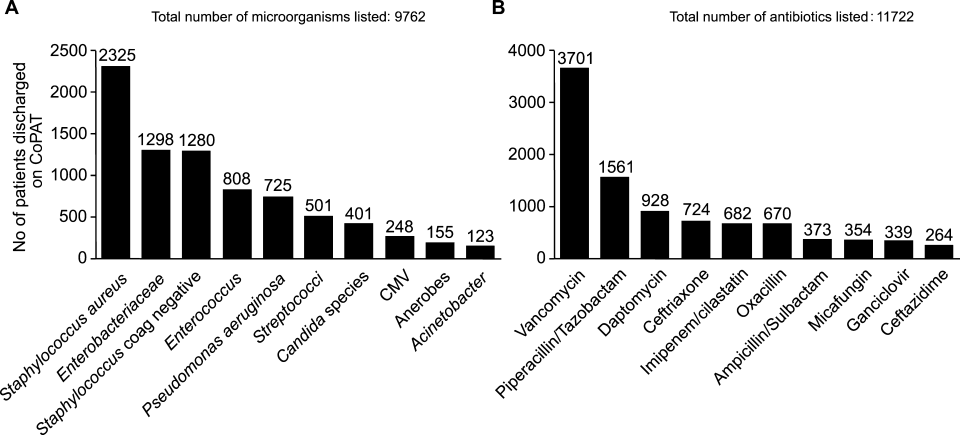
Of particular note, data collected from July 2007 through December 2008 demonstrated that more than 80% of patients discharged from the hospital with CoPAT did so with a prescheduled follow‐up visit. This patient‐centric measure is important because patients may not follow through with establishing appointments for follow‐up visits once discharge has already occurred. The Cleveland Clinic prides itself on making sure that a follow‐up appointment is actually made before the time of discharge for the vast majority of patients. The process also facilitates continuity of care with a specific infectious disease physician.
The various outcomes data collected by the Cleveland Clinic CoPAT Registry puts it in the position of being able to use the data to identify areas for improvement. Some of the projects made possible by the CoPAT Registry include analysis of: (1) outcomes of CoPAT in patients with bone and joint infections, (2) intensity of care in patients with cardiac and cardiac device infections while undergoing CoPAT, (3) C. difficile infections in patients undergoing CoPAT, and (4) emergency department (ED) visits or unanticipated readmissions in patients undergoing CoPAT. With respect to the last point, a 2009 article by Jencks and colleagues reported that 19.6% of the approximately 12 million Medicare beneficiaries who had been discharged from a hospital were rehospitalized within 30 days.20 Moreover, more than a third (34%) were rehospitalized within 90 days of discharge. It was estimated that no more than 10% of these readmissions were scheduled. More than 50% of patients with a medical condition who were rehospitalized within 30 days of discharge had not been billed for a physician visit between the time of discharge and hospitalization.20 This suggests that scheduling a follow‐up visit at the time of discharge might have reduced the need for many of these rehospitalizations. Unplanned rehospitalizations among the Medicare patients examined were not only relatively common but were also costly, resulting in an estimated $17.4 billion in additional Medicare costs.20 A New York Times editorial accompanying publication of the Jencks article noted that rehospitalizations and accompanying costs might be reduced by better discharge planning and closer cooperation between hospitals and physicians to ensure follow‐up care.21
At the Cleveland Clinic, data have recently been collected on the reasons for ED visits or hospital readmissions for patients receiving CoPAT at home through the Cleveland Clinic home care program. As illustrated in Figure 6, 24% of ED visits22 and 41% of hospital readmissions (Gordon, unpublished data) were for the infection being treated. Vascular access complications accounted for 23% of ED visits but only 2% of hospital readmissions. Nearly 50% of ED visits and 60% of hospital readmissions were for a reason unrelated to the infection being treated or CoPAT. It is hoped that closer examination of the data and perhaps additional analyses will suggest interventions to further reduce preventable readmissions or ED visits among patients discharged from the Cleveland Clinic on CoPAT.
Conclusions
Attention to antimicrobial stewardship and patient care should not end once the patient is discharged from the hospital or other institutional setting. Patients expect and should receive value‐based health care across the full cycle of their medical condition, and it is the responsibility of those caring for them to prepare for and provide such care during as well as after hospital discharge. The CoPAT program at the Cleveland Clinic provides a model for the extension of antimicrobial stewardship into the outpatient setting. The effectiveness of the program depends on a patient‐centric approach involving coordination and use of the expertise of multiple members of a team dedicated to patient value and facilitated by hospital‐based EHRs specialized for optimizing the transition of care into the outpatient setting for all patients scheduled to receive CoPAT. The quality of medical care provided by the Cleveland Clinic or other hospitals can be accessed through measurements of the structure, processes, and outcomes of care provided by the respective institutions. The data obtained can then be used to further refine care to optimize outcomes and provide high value for the patients treated at the institution. Achieving and then maintaining high‐quality medical care that provides value to patients is an ongoing process that should never be taken for granted.
- .The caring physician: the life of Dr. Francis W. Peabody [book review].N Engl J Med.1993;328:817–818.
- ,.How physicians can change the future of health care.JAMA.2007;297:1103–1111.
- ,.Redefining Health Care: Creating Value‐Based Competition on Results.Boston, MA:Harvard Business Press;2006.
- Greiner AC, Knebel E, eds.Health Professions Education: A Bridge to Quality. Committee on the Health Professions Education Summit.Washington, DC:National Academies Press;2003.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,,,,.Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial.J Am Geriatr Soc.2004;52:675–684.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta‐analysis.JAMA.2004;291:1358–1367.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- ,,,.Prolonged beneficial effects of a home‐based intervention on unplanned readmissions and mortality among patients with congestive heart failure.Arch Intern Med.1999;159:257–261.
- ,,,.A randomized, controlled trial of comprehensive geriatric assessment and multidisciplinary intervention after discharge of elderly from the emergency department–the DEED II study.J Am Geriatr Soc.2004;52:1417–1423.
- ,,,,.A randomized trial of comprehensive geriatric assessment and home intervention in the care of hospitalized patients.Age Ageing.1999;28:543–550.
- ,.Home intravenous antibiotic therapy: a team approach.Ann Intern Med.1983;99:388–392.
- Merriam‐Webster Dictionary Online. Definition of steward. Available at http://www.merriam‐webster.com/dictionary/steward. Accessed July 14,2010.
- ,,, et al.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44:159–177.
- ,,,.Improving antibiotic utilization among hospitalists: a pilot academic detailing project with a public health approach.J Hosp Med.2008;3:64–70.
- ,.Outpatient intravenous medications in the management of cystic fibrosis.Pediatrics.1974;54:358–360.
- ,,, et al.Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines.Clin Infect Dis.2004;38:1651–1672.
- .Evaluating the quality of medical care.Milbank Mem Fund Q.1966;44(Suppl):166–206.
- ,,.Impact of mandatory inpatient infectious disease consultation on outpatient parenteral antibiotic therapy.Am J Med Sci.2005;330:60–64.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360:1418–1428.
- The New York Times. Editorial: Back in the Hospital Again. April 15, 2009. Available at http://www.nytimes.com/2009/04/16/opinion/16thu2.html. Accessed July 16,2010.
- ,,,,,.Emergency department visits of patients on community‐based parenteral anti‐infective therapy at home. Presented at the 47th annual meeting of IDSA, Philadelphia, PA, October 29‐November 1, 2009. Poster 462.
. . . For the secret of the care of the patient is caring for the patient.Francis W. Peabody, October 21, 19251
Collaboration between members of a multidisciplinary team is a key component of an effective institutional antimicrobial stewardship program, which itself is a key component of optimizing the care of hospitalized patients being treated with antimicrobial agents for proven or suspected infectious diseases. However, patient care does not and should not end once the patient is discharged from the hospital. In fact, high‐quality, value‐based health care across the full range of a medical condition depends on planning for optimization of care within the hospital as well as transitions of care to the outpatient setting. This extended care plan includes collaboration with multiple members of the health care community, both inside and outside the institution. The current review examines 3 aspects of patient care across the full cycle of an infectious disease condition: (1) value‐based health care, (2) stewardship of antimicrobials, and (3) community‐based parenteral anti‐infective therapy (CoPAT) as a model for antimicrobial stewardship outside the institutional setting.
Value‐Based Health Care
Patients first want to know that the health care professionals treating them actually care about them as individuals, and only then are patients concerned about how much the medical team knows. Patient‐centered care is a critical component of value‐based health care, a term that was bandied about quite a bit during the recent and ongoing health care debate in the United States. But what exactly does it mean? First, value in health care is defined by health care outcomes as a function of or divided by the cost of delivery of care. As Dr. Michael Porter and Dr. Elizabeth Olmsted Teisberg delineated in their 2007 article in the Journal of the American Medical Association,2 as well as in the 2006 book Redefining Health Care,3 The purpose of the healthcare system is not to minimize costs but to deliver value to patients, that is, better health per dollar spent. As they discuss value, it is a patient‐centric measure, and is focused on individual patient (not just diagnosis‐related group) outcomes and the cost of care across the full cycle. In this way of looking at things, an episode of care goes beyond the treatment provided during the acute admission to also include the transition of care to the outpatient or posthospital setting.
The reforms proposed by Porter and Teisberg are best achieved when the participating health care institutions have developed an information technology platform able to integrate and fully measure care across the full cycle of a medical condition. Furthermore, there is strong evidence that patient value increases with physician and team experience and volume for a particular condition.2 High volumes tend to correlate with the development of better information technology, as well as the formation of dedicated teams with tailored facilities, and with a greater capacity for constructive feedback to improve patient outcomes. The more experience a physician and team have with the management of a particular medical condition, the greater is the opportunity to learn and refine practices to provide greater value to the patient.
The Institute of Medicine has recommended that all healthcare professionals should be educated to deliver patient‐centered care as members of an interdisciplinary team emphasizing evidence‐based practice, quality improvement, and informatics.4 As has been demonstrated for patients with congestive heart failure59 and other conditions,10, 11 outcomes improve when components of care are integrated (often by nurse‐directed teams), preparing for the transition of care from the hospital to the home.12 This concept is the basis for the community‐based parenteral anti‐infective therapy program (CoPAT) at the Cleveland Clinic as a model for antimicrobial stewardship for patients requiring parenteral antimicrobial therapy at the time of discharge from the inpatient setting.
Stewardship of Antimicrobials
The Merriam‐Webster dictionary alternatively defines a steward as: (1) an employee on a ship, airplane, bus, or train who manages the provisioning of food and attends to passengers or (2) one who actively manages affairs (manager).13 In the context of health care within an institution, one can think of clinicians as stewards or employees charged with managing patients and the drugs and other care they receive while they are attendants (passengers) at the institution. In the value‐based approach just discussed, where medical practice is organized around managing medical conditions for the entire care cycle, a medical steward would also be charged with managing or planning for patient care after discharge from the hospital or other institutional setting. Management or stewardship of antimicrobial agents is a key component of the care for patients with infectious diseases. In 2007, the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America (IDSA/SHEA) presented guidelines to aid institutions in the development of an effective hospital‐based antimicrobial stewardship program (a more detailed overview of antimicrobial stewardship is presented in the accompanying supplemental article by Dr. Ohl).14 The focus of the IDSA/SHEA guidelines was on development of programs within hospitals. Although the authors acknowledged that antimicrobial stewardship is also important in outpatient clinics and long‐term care facilities, transition of antimicrobial management after patient discharge from the hospital was not a focus of the 2007 guidelines.
A key objective of antimicrobial stewardship is to optimize antimicrobial drug selection and dosing to improve clinical outcomes while reducing drug toxicity and other potential untoward consequences of antimicrobial therapy, including selection of opportunistic organisms (eg, Clostridium difficile) or emergence of multidrug resistance in pathogens.14 A secondary objective is to reduce overall health care costs,14 which ideally would include inpatient as well as outpatient costs and those related to hospital readmission due to the initial infection or its outpatient treatment. Useful metrics for evaluation of an antimicrobial stewardship program include measures of pathogen/drug mismatch, antimicrobial costs, incidence of redundant therapy, compliance with antimicrobial drug restrictions (if applicable), days undergoing antimicrobial therapy, and number of cases of intravenous to oral conversion.14
Although the IDSA/SHEA guidelines for institutional antimicrobial stewardship programs suggest that an infectious diseases physician and clinical pharmacist with infectious diseases training should be core members of a multidisciplinary stewardship team,14 many community hospitals or smaller institutions do not have an infectious diseases physician or a readily available infectious diseases specialist for consultation. Hospitalists are often very effective advocates of appropriate use of antimicrobials and may play a leadership role on institutional antimicrobial stewardship teams. A recent study demonstrated that a hospitalist‐delivered academic detailing intervention (which included an individual appraisal of the provider's prescription pattern) significantly improved patterns of antibiotic prescribing for inpatients.15
Community‐Based Parenteral Anti‐Infective Therapy as a Systems‐Based Approach to Antimicrobial Stewardship
A systems‐based approach for antimicrobial stewardship, CoPAT has been in operation at the Cleveland Clinic, a 1200‐bed hospital in downtown Cleveland, Ohio, since November 1979. The experiences of the authors and their colleagues demonstrate it to be a value‐based proposition for the patient that uses an antimicrobial stewardship platform. Also known as outpatient parenteral antimicrobial therapy (OPAT), CoPAT refers to the practice of administering antimicrobial therapy in the home or other outpatient settings, first introduced by Rucker and Harrison in 1974 in the context of outpatient management of cystic fibrosis.16 In the United States, CoPAT is a common practice today, and the IDSA has created practice guidelines for it.17
In 1983, Rehm and Weinstein coauthored an article describing their experiences at the Cleveland Clinic, in which selected patients were trained for home‐based antimicrobial therapy.12 Figure 1 illustrates the astronomical growth that has occurred over the years at the Cleveland Clinic in the number of patients discharged from the acute care center undergoing CoPAT (Gordon, unpublished data). It is anticipated that this growth will continue and in large part reflects the complexity of patients being seen and the desire to reduce length of stay. Evaluating the quality of any medical care is difficult, but there are 3 general approaches to assessing or measuring the quality of medical care: assessing the structure of care, assessing processes of care, and assessing outcomes.18 The quality of the CoPAT program at the Cleveland Clinic can be examined in the context of these 3 areas of assessment.

Settings or the Structure of Care
In a 1966 publication on quality of medical care evaluations, Donabedian described assessment of the structure of care as one of the primary approaches to measuring the quality of care.18 By structure, Donabedian meant the settings in which medical care takes place, including the adequacy of facilities and equipment, qualifications or expertise of medical staff and their organization, the administrative structure of the institution or institutional program of interest, and other administrative and related processes supporting and directing the delivery of care. Although the structure of care has the advantage of being concrete and relatively easy to assess, to be most meaningful, it ultimately needs to be related to the processes and outcomes of care.
With respect to the CoPAT program at the Cleveland Clinic main hospital, infectious diseases consultation is required for every patient being considered for discharge with parenteral antibiotics, whether the patient is going home or to another facility, including the clinic's own skilled nursing facility (SNF). Arrangements are then made for the delivery of antibiotics at home or in SNFs or long‐term acute care (LTAC) centers. The Cleveland Clinic CoPAT program does not use an outpatient infusion center.
The Cleveland Clinic uses a mandatory infectious diseases consultation for CoPAT because there are a number of important issues that need to be addressed before the patient is discharged, and for our system this is best accomplished by an infectious diseases specialist.12 For example, is antimicrobial therapy actually required in the first place? If it is, what is the optimal type, route, and duration of therapy? Are there other medical issues that need to be addressed? Decisions also need to be made about optimal vascular access and antimicrobial selection and administration, as well as arrangements being made for monitoring clinical and laboratory aspects. It is important that there is a smooth transition of care and prescheduled follow‐up in the outpatient clinic. The identification and use of an infectious diseases clinician directing the process leads to accountability. Notably, mandatory infectious disease consultation for outpatient parenteral antibiotic therapy has been used at Baystate Medical Center with improvement in reducing costs.19
The Process of Care
Assessments of the process of care involve examination of the particulars of medical care delivery, or whether what is recognized or accepted as good medical care has been applied. As discussed by Donabedian, process of care deals with issues such as the appropriateness and completeness of information obtained through clinical history, physical examination, and diagnostic tests; justification of diagnosis and therapy; technical competence in the performance of diagnostic and therapeutic procedures; and coordination and continuity of care.18
The CoPAT initiation process at the Cleveland Clinic is illustrated in Figure 2. It is a bundled process. As already mentioned, an infectious diseases consultation and evaluation is scheduled for all patients considered for CoPAT, after which a CoPAT form is completed and a follow‐up appointment made before the patient is discharged. In addition, the vascular access team is consulted and an appropriate vascular access device is placed in the patient prior to discharge. Likewise, a case manager is enlisted to identify a health care agency or SNF for patient placement or to determine whether the patient will receive home treatment. Once the appropriate setting is identified, the case manager transmits a completed CoPAT form to the health care agency or SNF, while forwarding a copy to the CoPAT nurse coordinator in the infectious disease department.

An electronic health record system is used at the Cleveland Clinic to provide real‐time information relevant for patient management. In 2007, a structured data form for CoPAT start‐of‐care was created within the Cleveland Clinic hospital electronic health record (EHR). This form contains a number of elements relevant for setting up patients for transition to CoPAT. In particular, the electronic CoPAT form contains information about the infection(s) and microorganism(s) being treated, intravenous antibiotic(s) prescribed (including treatment stop date), concurrent oral antibiotics, premedication recommendations (if appropriate), and recommended monitoring of laboratory tests. In addition, the form contains the telephone and fax numbers of the CoPAT coordinator and the name of the responsible physician, including a scheduled appointment for follow‐up (Fig. 3). The staff physician is responsible for completing the electronic CoPAT form or prescription. This CoPAT prescription then becomes part of the patient's electronic record and is transmissible and viewable by anyone with access to the EHR. This is important in terms of follow‐up and care accountability: an infectious disease staff clinician is identified as the contact person for clinical issues when a patient is on CoPAT.

After the patient is discharged, the CoPAT coordinator in the infectious disease department becomes responsible, together with the clinic's outpatient pharmacy, for reviewing laboratory results and notifying clinicians of potential problems that need to be addressed. These issues can pertain to laboratory findings, vascular access, or new symptoms or signs observed by the home nurse or patient. All this information is communicated via electronic health record messaging and/or through direct calls to the physician, when needed.
The CoPAT program has been widely accepted by internal customers of the Cleveland Clinic, which include hospitalists. This is probably because there is autonomy and accountability with the infectious diseases staff, the program or team is available 7 days per week, and the EHR facilitates communication. In addition, the use of infectious disease‐specific subspecialty groups (eg, bone marrow and solid‐organ transplant, bone and joint, and infective endocarditis groups) increases clinical credibility, as well as value received by patients of the clinic. Furthermore, the electronic CoPAT script facilitates discharge planning. CoPATs constitute approximately 25% of all ID consultation requests at the Cleveland Clinic and help to justify the 20 clinical ID clinical FTEs.
Outcomes of Medical Care
Assessment of medical care outcomes is another frequently used approach for measuring the quality of medical care.18 Medical care outcomes that have been examined as measures of quality of care include survival, number of hospital readmissions, time between discharge and readmissions, length of initial hospital stay and subsequent readmissions, quality of life, and health care costs. As has often been said, If you cannot measure it, you cannot manage it. The CoPAT program using the EHR has facilitated retrieval of structured reports in a format that provides clinicians with real‐time data enabling assessment of outcomes. By examining this data, the CoPAT team is in a better position to contemplate potential interventions for improving outpatient care and the value patients receive.
A 36‐month review of Cleveland Clinic CoPAT patient demographics from July 2007 to June 2010 demonstrated 6287 patients (56% male) had been prescribed 9471 courses of CoPAT (Gordon, unpublished data). Seventy‐nine percent of the patients were white, 16% African American, and 5% of other races. Most patients received 1 antibiotic per CoPAT course (79.1%), whereas 18.2%, 2.5%, and 0.2% received 2, 3, and 4 antibiotics per CoPAT course, respectively. Figure 4 highlights CoPAT distribution by source for anatomic site of infection. Bone and joint infections were the most common diagnoses associated with CoPAT at the Cleveland Clinic, followed by abdominal, cardiovascular, primary disseminated disease (eg, catheter‐associated bloodstream infections), and skin and soft‐tissue infection.

Figure 5 highlights the top‐10 pathogenic microorganisms in patients being discharged from the Cleveland Clinic with CoPAT, and the top‐10 antimicrobials prescribed for these patients. As can be seen, Staphylococcus aureus (methicillin susceptible and methicillin resistant) was the number one pathogen identified for patients undergoing CoPAT, followed by coagulase‐negative Staphylococcus and Enterococcus species. The most commonly identified gram‐negative bacteria among discharged patients was Pseudomonas aeruginosa. Only 2 of the top 10 pathogens were nonbacterial: Candida species and cytomegalovirus (CMV), the latter being the result of the high volume of transplantations performed at the clinic. With respect to the intravenous antimicrobials prescribed for patients undergoing CoPAT, the most commonly prescribed agent was vancomycin, followed by piperacillin/tazobactam. Of the 10 agents, only micafungin and ganciclovir were not antibacterial agents, indicating that the vast majority of patients discharged from the Cleveland Clinic with CoPAT had had bacterial, rather than fungal or viral, infections.

Of particular note, data collected from July 2007 through December 2008 demonstrated that more than 80% of patients discharged from the hospital with CoPAT did so with a prescheduled follow‐up visit. This patient‐centric measure is important because patients may not follow through with establishing appointments for follow‐up visits once discharge has already occurred. The Cleveland Clinic prides itself on making sure that a follow‐up appointment is actually made before the time of discharge for the vast majority of patients. The process also facilitates continuity of care with a specific infectious disease physician.
The various outcomes data collected by the Cleveland Clinic CoPAT Registry puts it in the position of being able to use the data to identify areas for improvement. Some of the projects made possible by the CoPAT Registry include analysis of: (1) outcomes of CoPAT in patients with bone and joint infections, (2) intensity of care in patients with cardiac and cardiac device infections while undergoing CoPAT, (3) C. difficile infections in patients undergoing CoPAT, and (4) emergency department (ED) visits or unanticipated readmissions in patients undergoing CoPAT. With respect to the last point, a 2009 article by Jencks and colleagues reported that 19.6% of the approximately 12 million Medicare beneficiaries who had been discharged from a hospital were rehospitalized within 30 days.20 Moreover, more than a third (34%) were rehospitalized within 90 days of discharge. It was estimated that no more than 10% of these readmissions were scheduled. More than 50% of patients with a medical condition who were rehospitalized within 30 days of discharge had not been billed for a physician visit between the time of discharge and hospitalization.20 This suggests that scheduling a follow‐up visit at the time of discharge might have reduced the need for many of these rehospitalizations. Unplanned rehospitalizations among the Medicare patients examined were not only relatively common but were also costly, resulting in an estimated $17.4 billion in additional Medicare costs.20 A New York Times editorial accompanying publication of the Jencks article noted that rehospitalizations and accompanying costs might be reduced by better discharge planning and closer cooperation between hospitals and physicians to ensure follow‐up care.21
At the Cleveland Clinic, data have recently been collected on the reasons for ED visits or hospital readmissions for patients receiving CoPAT at home through the Cleveland Clinic home care program. As illustrated in Figure 6, 24% of ED visits22 and 41% of hospital readmissions (Gordon, unpublished data) were for the infection being treated. Vascular access complications accounted for 23% of ED visits but only 2% of hospital readmissions. Nearly 50% of ED visits and 60% of hospital readmissions were for a reason unrelated to the infection being treated or CoPAT. It is hoped that closer examination of the data and perhaps additional analyses will suggest interventions to further reduce preventable readmissions or ED visits among patients discharged from the Cleveland Clinic on CoPAT.

Conclusions
Attention to antimicrobial stewardship and patient care should not end once the patient is discharged from the hospital or other institutional setting. Patients expect and should receive value‐based health care across the full cycle of their medical condition, and it is the responsibility of those caring for them to prepare for and provide such care during as well as after hospital discharge. The CoPAT program at the Cleveland Clinic provides a model for the extension of antimicrobial stewardship into the outpatient setting. The effectiveness of the program depends on a patient‐centric approach involving coordination and use of the expertise of multiple members of a team dedicated to patient value and facilitated by hospital‐based EHRs specialized for optimizing the transition of care into the outpatient setting for all patients scheduled to receive CoPAT. The quality of medical care provided by the Cleveland Clinic or other hospitals can be accessed through measurements of the structure, processes, and outcomes of care provided by the respective institutions. The data obtained can then be used to further refine care to optimize outcomes and provide high value for the patients treated at the institution. Achieving and then maintaining high‐quality medical care that provides value to patients is an ongoing process that should never be taken for granted.
. . . For the secret of the care of the patient is caring for the patient.Francis W. Peabody, October 21, 19251
Collaboration between members of a multidisciplinary team is a key component of an effective institutional antimicrobial stewardship program, which itself is a key component of optimizing the care of hospitalized patients being treated with antimicrobial agents for proven or suspected infectious diseases. However, patient care does not and should not end once the patient is discharged from the hospital. In fact, high‐quality, value‐based health care across the full range of a medical condition depends on planning for optimization of care within the hospital as well as transitions of care to the outpatient setting. This extended care plan includes collaboration with multiple members of the health care community, both inside and outside the institution. The current review examines 3 aspects of patient care across the full cycle of an infectious disease condition: (1) value‐based health care, (2) stewardship of antimicrobials, and (3) community‐based parenteral anti‐infective therapy (CoPAT) as a model for antimicrobial stewardship outside the institutional setting.
Value‐Based Health Care
Patients first want to know that the health care professionals treating them actually care about them as individuals, and only then are patients concerned about how much the medical team knows. Patient‐centered care is a critical component of value‐based health care, a term that was bandied about quite a bit during the recent and ongoing health care debate in the United States. But what exactly does it mean? First, value in health care is defined by health care outcomes as a function of or divided by the cost of delivery of care. As Dr. Michael Porter and Dr. Elizabeth Olmsted Teisberg delineated in their 2007 article in the Journal of the American Medical Association,2 as well as in the 2006 book Redefining Health Care,3 The purpose of the healthcare system is not to minimize costs but to deliver value to patients, that is, better health per dollar spent. As they discuss value, it is a patient‐centric measure, and is focused on individual patient (not just diagnosis‐related group) outcomes and the cost of care across the full cycle. In this way of looking at things, an episode of care goes beyond the treatment provided during the acute admission to also include the transition of care to the outpatient or posthospital setting.
The reforms proposed by Porter and Teisberg are best achieved when the participating health care institutions have developed an information technology platform able to integrate and fully measure care across the full cycle of a medical condition. Furthermore, there is strong evidence that patient value increases with physician and team experience and volume for a particular condition.2 High volumes tend to correlate with the development of better information technology, as well as the formation of dedicated teams with tailored facilities, and with a greater capacity for constructive feedback to improve patient outcomes. The more experience a physician and team have with the management of a particular medical condition, the greater is the opportunity to learn and refine practices to provide greater value to the patient.
The Institute of Medicine has recommended that all healthcare professionals should be educated to deliver patient‐centered care as members of an interdisciplinary team emphasizing evidence‐based practice, quality improvement, and informatics.4 As has been demonstrated for patients with congestive heart failure59 and other conditions,10, 11 outcomes improve when components of care are integrated (often by nurse‐directed teams), preparing for the transition of care from the hospital to the home.12 This concept is the basis for the community‐based parenteral anti‐infective therapy program (CoPAT) at the Cleveland Clinic as a model for antimicrobial stewardship for patients requiring parenteral antimicrobial therapy at the time of discharge from the inpatient setting.
Stewardship of Antimicrobials
The Merriam‐Webster dictionary alternatively defines a steward as: (1) an employee on a ship, airplane, bus, or train who manages the provisioning of food and attends to passengers or (2) one who actively manages affairs (manager).13 In the context of health care within an institution, one can think of clinicians as stewards or employees charged with managing patients and the drugs and other care they receive while they are attendants (passengers) at the institution. In the value‐based approach just discussed, where medical practice is organized around managing medical conditions for the entire care cycle, a medical steward would also be charged with managing or planning for patient care after discharge from the hospital or other institutional setting. Management or stewardship of antimicrobial agents is a key component of the care for patients with infectious diseases. In 2007, the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America (IDSA/SHEA) presented guidelines to aid institutions in the development of an effective hospital‐based antimicrobial stewardship program (a more detailed overview of antimicrobial stewardship is presented in the accompanying supplemental article by Dr. Ohl).14 The focus of the IDSA/SHEA guidelines was on development of programs within hospitals. Although the authors acknowledged that antimicrobial stewardship is also important in outpatient clinics and long‐term care facilities, transition of antimicrobial management after patient discharge from the hospital was not a focus of the 2007 guidelines.
A key objective of antimicrobial stewardship is to optimize antimicrobial drug selection and dosing to improve clinical outcomes while reducing drug toxicity and other potential untoward consequences of antimicrobial therapy, including selection of opportunistic organisms (eg, Clostridium difficile) or emergence of multidrug resistance in pathogens.14 A secondary objective is to reduce overall health care costs,14 which ideally would include inpatient as well as outpatient costs and those related to hospital readmission due to the initial infection or its outpatient treatment. Useful metrics for evaluation of an antimicrobial stewardship program include measures of pathogen/drug mismatch, antimicrobial costs, incidence of redundant therapy, compliance with antimicrobial drug restrictions (if applicable), days undergoing antimicrobial therapy, and number of cases of intravenous to oral conversion.14
Although the IDSA/SHEA guidelines for institutional antimicrobial stewardship programs suggest that an infectious diseases physician and clinical pharmacist with infectious diseases training should be core members of a multidisciplinary stewardship team,14 many community hospitals or smaller institutions do not have an infectious diseases physician or a readily available infectious diseases specialist for consultation. Hospitalists are often very effective advocates of appropriate use of antimicrobials and may play a leadership role on institutional antimicrobial stewardship teams. A recent study demonstrated that a hospitalist‐delivered academic detailing intervention (which included an individual appraisal of the provider's prescription pattern) significantly improved patterns of antibiotic prescribing for inpatients.15
Community‐Based Parenteral Anti‐Infective Therapy as a Systems‐Based Approach to Antimicrobial Stewardship
A systems‐based approach for antimicrobial stewardship, CoPAT has been in operation at the Cleveland Clinic, a 1200‐bed hospital in downtown Cleveland, Ohio, since November 1979. The experiences of the authors and their colleagues demonstrate it to be a value‐based proposition for the patient that uses an antimicrobial stewardship platform. Also known as outpatient parenteral antimicrobial therapy (OPAT), CoPAT refers to the practice of administering antimicrobial therapy in the home or other outpatient settings, first introduced by Rucker and Harrison in 1974 in the context of outpatient management of cystic fibrosis.16 In the United States, CoPAT is a common practice today, and the IDSA has created practice guidelines for it.17
In 1983, Rehm and Weinstein coauthored an article describing their experiences at the Cleveland Clinic, in which selected patients were trained for home‐based antimicrobial therapy.12 Figure 1 illustrates the astronomical growth that has occurred over the years at the Cleveland Clinic in the number of patients discharged from the acute care center undergoing CoPAT (Gordon, unpublished data). It is anticipated that this growth will continue and in large part reflects the complexity of patients being seen and the desire to reduce length of stay. Evaluating the quality of any medical care is difficult, but there are 3 general approaches to assessing or measuring the quality of medical care: assessing the structure of care, assessing processes of care, and assessing outcomes.18 The quality of the CoPAT program at the Cleveland Clinic can be examined in the context of these 3 areas of assessment.

Settings or the Structure of Care
In a 1966 publication on quality of medical care evaluations, Donabedian described assessment of the structure of care as one of the primary approaches to measuring the quality of care.18 By structure, Donabedian meant the settings in which medical care takes place, including the adequacy of facilities and equipment, qualifications or expertise of medical staff and their organization, the administrative structure of the institution or institutional program of interest, and other administrative and related processes supporting and directing the delivery of care. Although the structure of care has the advantage of being concrete and relatively easy to assess, to be most meaningful, it ultimately needs to be related to the processes and outcomes of care.
With respect to the CoPAT program at the Cleveland Clinic main hospital, infectious diseases consultation is required for every patient being considered for discharge with parenteral antibiotics, whether the patient is going home or to another facility, including the clinic's own skilled nursing facility (SNF). Arrangements are then made for the delivery of antibiotics at home or in SNFs or long‐term acute care (LTAC) centers. The Cleveland Clinic CoPAT program does not use an outpatient infusion center.
The Cleveland Clinic uses a mandatory infectious diseases consultation for CoPAT because there are a number of important issues that need to be addressed before the patient is discharged, and for our system this is best accomplished by an infectious diseases specialist.12 For example, is antimicrobial therapy actually required in the first place? If it is, what is the optimal type, route, and duration of therapy? Are there other medical issues that need to be addressed? Decisions also need to be made about optimal vascular access and antimicrobial selection and administration, as well as arrangements being made for monitoring clinical and laboratory aspects. It is important that there is a smooth transition of care and prescheduled follow‐up in the outpatient clinic. The identification and use of an infectious diseases clinician directing the process leads to accountability. Notably, mandatory infectious disease consultation for outpatient parenteral antibiotic therapy has been used at Baystate Medical Center with improvement in reducing costs.19
The Process of Care
Assessments of the process of care involve examination of the particulars of medical care delivery, or whether what is recognized or accepted as good medical care has been applied. As discussed by Donabedian, process of care deals with issues such as the appropriateness and completeness of information obtained through clinical history, physical examination, and diagnostic tests; justification of diagnosis and therapy; technical competence in the performance of diagnostic and therapeutic procedures; and coordination and continuity of care.18
The CoPAT initiation process at the Cleveland Clinic is illustrated in Figure 2. It is a bundled process. As already mentioned, an infectious diseases consultation and evaluation is scheduled for all patients considered for CoPAT, after which a CoPAT form is completed and a follow‐up appointment made before the patient is discharged. In addition, the vascular access team is consulted and an appropriate vascular access device is placed in the patient prior to discharge. Likewise, a case manager is enlisted to identify a health care agency or SNF for patient placement or to determine whether the patient will receive home treatment. Once the appropriate setting is identified, the case manager transmits a completed CoPAT form to the health care agency or SNF, while forwarding a copy to the CoPAT nurse coordinator in the infectious disease department.

An electronic health record system is used at the Cleveland Clinic to provide real‐time information relevant for patient management. In 2007, a structured data form for CoPAT start‐of‐care was created within the Cleveland Clinic hospital electronic health record (EHR). This form contains a number of elements relevant for setting up patients for transition to CoPAT. In particular, the electronic CoPAT form contains information about the infection(s) and microorganism(s) being treated, intravenous antibiotic(s) prescribed (including treatment stop date), concurrent oral antibiotics, premedication recommendations (if appropriate), and recommended monitoring of laboratory tests. In addition, the form contains the telephone and fax numbers of the CoPAT coordinator and the name of the responsible physician, including a scheduled appointment for follow‐up (Fig. 3). The staff physician is responsible for completing the electronic CoPAT form or prescription. This CoPAT prescription then becomes part of the patient's electronic record and is transmissible and viewable by anyone with access to the EHR. This is important in terms of follow‐up and care accountability: an infectious disease staff clinician is identified as the contact person for clinical issues when a patient is on CoPAT.

After the patient is discharged, the CoPAT coordinator in the infectious disease department becomes responsible, together with the clinic's outpatient pharmacy, for reviewing laboratory results and notifying clinicians of potential problems that need to be addressed. These issues can pertain to laboratory findings, vascular access, or new symptoms or signs observed by the home nurse or patient. All this information is communicated via electronic health record messaging and/or through direct calls to the physician, when needed.
The CoPAT program has been widely accepted by internal customers of the Cleveland Clinic, which include hospitalists. This is probably because there is autonomy and accountability with the infectious diseases staff, the program or team is available 7 days per week, and the EHR facilitates communication. In addition, the use of infectious disease‐specific subspecialty groups (eg, bone marrow and solid‐organ transplant, bone and joint, and infective endocarditis groups) increases clinical credibility, as well as value received by patients of the clinic. Furthermore, the electronic CoPAT script facilitates discharge planning. CoPATs constitute approximately 25% of all ID consultation requests at the Cleveland Clinic and help to justify the 20 clinical ID clinical FTEs.
Outcomes of Medical Care
Assessment of medical care outcomes is another frequently used approach for measuring the quality of medical care.18 Medical care outcomes that have been examined as measures of quality of care include survival, number of hospital readmissions, time between discharge and readmissions, length of initial hospital stay and subsequent readmissions, quality of life, and health care costs. As has often been said, If you cannot measure it, you cannot manage it. The CoPAT program using the EHR has facilitated retrieval of structured reports in a format that provides clinicians with real‐time data enabling assessment of outcomes. By examining this data, the CoPAT team is in a better position to contemplate potential interventions for improving outpatient care and the value patients receive.
A 36‐month review of Cleveland Clinic CoPAT patient demographics from July 2007 to June 2010 demonstrated 6287 patients (56% male) had been prescribed 9471 courses of CoPAT (Gordon, unpublished data). Seventy‐nine percent of the patients were white, 16% African American, and 5% of other races. Most patients received 1 antibiotic per CoPAT course (79.1%), whereas 18.2%, 2.5%, and 0.2% received 2, 3, and 4 antibiotics per CoPAT course, respectively. Figure 4 highlights CoPAT distribution by source for anatomic site of infection. Bone and joint infections were the most common diagnoses associated with CoPAT at the Cleveland Clinic, followed by abdominal, cardiovascular, primary disseminated disease (eg, catheter‐associated bloodstream infections), and skin and soft‐tissue infection.

Figure 5 highlights the top‐10 pathogenic microorganisms in patients being discharged from the Cleveland Clinic with CoPAT, and the top‐10 antimicrobials prescribed for these patients. As can be seen, Staphylococcus aureus (methicillin susceptible and methicillin resistant) was the number one pathogen identified for patients undergoing CoPAT, followed by coagulase‐negative Staphylococcus and Enterococcus species. The most commonly identified gram‐negative bacteria among discharged patients was Pseudomonas aeruginosa. Only 2 of the top 10 pathogens were nonbacterial: Candida species and cytomegalovirus (CMV), the latter being the result of the high volume of transplantations performed at the clinic. With respect to the intravenous antimicrobials prescribed for patients undergoing CoPAT, the most commonly prescribed agent was vancomycin, followed by piperacillin/tazobactam. Of the 10 agents, only micafungin and ganciclovir were not antibacterial agents, indicating that the vast majority of patients discharged from the Cleveland Clinic with CoPAT had had bacterial, rather than fungal or viral, infections.

Of particular note, data collected from July 2007 through December 2008 demonstrated that more than 80% of patients discharged from the hospital with CoPAT did so with a prescheduled follow‐up visit. This patient‐centric measure is important because patients may not follow through with establishing appointments for follow‐up visits once discharge has already occurred. The Cleveland Clinic prides itself on making sure that a follow‐up appointment is actually made before the time of discharge for the vast majority of patients. The process also facilitates continuity of care with a specific infectious disease physician.
The various outcomes data collected by the Cleveland Clinic CoPAT Registry puts it in the position of being able to use the data to identify areas for improvement. Some of the projects made possible by the CoPAT Registry include analysis of: (1) outcomes of CoPAT in patients with bone and joint infections, (2) intensity of care in patients with cardiac and cardiac device infections while undergoing CoPAT, (3) C. difficile infections in patients undergoing CoPAT, and (4) emergency department (ED) visits or unanticipated readmissions in patients undergoing CoPAT. With respect to the last point, a 2009 article by Jencks and colleagues reported that 19.6% of the approximately 12 million Medicare beneficiaries who had been discharged from a hospital were rehospitalized within 30 days.20 Moreover, more than a third (34%) were rehospitalized within 90 days of discharge. It was estimated that no more than 10% of these readmissions were scheduled. More than 50% of patients with a medical condition who were rehospitalized within 30 days of discharge had not been billed for a physician visit between the time of discharge and hospitalization.20 This suggests that scheduling a follow‐up visit at the time of discharge might have reduced the need for many of these rehospitalizations. Unplanned rehospitalizations among the Medicare patients examined were not only relatively common but were also costly, resulting in an estimated $17.4 billion in additional Medicare costs.20 A New York Times editorial accompanying publication of the Jencks article noted that rehospitalizations and accompanying costs might be reduced by better discharge planning and closer cooperation between hospitals and physicians to ensure follow‐up care.21
At the Cleveland Clinic, data have recently been collected on the reasons for ED visits or hospital readmissions for patients receiving CoPAT at home through the Cleveland Clinic home care program. As illustrated in Figure 6, 24% of ED visits22 and 41% of hospital readmissions (Gordon, unpublished data) were for the infection being treated. Vascular access complications accounted for 23% of ED visits but only 2% of hospital readmissions. Nearly 50% of ED visits and 60% of hospital readmissions were for a reason unrelated to the infection being treated or CoPAT. It is hoped that closer examination of the data and perhaps additional analyses will suggest interventions to further reduce preventable readmissions or ED visits among patients discharged from the Cleveland Clinic on CoPAT.

Conclusions
Attention to antimicrobial stewardship and patient care should not end once the patient is discharged from the hospital or other institutional setting. Patients expect and should receive value‐based health care across the full cycle of their medical condition, and it is the responsibility of those caring for them to prepare for and provide such care during as well as after hospital discharge. The CoPAT program at the Cleveland Clinic provides a model for the extension of antimicrobial stewardship into the outpatient setting. The effectiveness of the program depends on a patient‐centric approach involving coordination and use of the expertise of multiple members of a team dedicated to patient value and facilitated by hospital‐based EHRs specialized for optimizing the transition of care into the outpatient setting for all patients scheduled to receive CoPAT. The quality of medical care provided by the Cleveland Clinic or other hospitals can be accessed through measurements of the structure, processes, and outcomes of care provided by the respective institutions. The data obtained can then be used to further refine care to optimize outcomes and provide high value for the patients treated at the institution. Achieving and then maintaining high‐quality medical care that provides value to patients is an ongoing process that should never be taken for granted.
- .The caring physician: the life of Dr. Francis W. Peabody [book review].N Engl J Med.1993;328:817–818.
- ,.How physicians can change the future of health care.JAMA.2007;297:1103–1111.
- ,.Redefining Health Care: Creating Value‐Based Competition on Results.Boston, MA:Harvard Business Press;2006.
- Greiner AC, Knebel E, eds.Health Professions Education: A Bridge to Quality. Committee on the Health Professions Education Summit.Washington, DC:National Academies Press;2003.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,,,,.Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial.J Am Geriatr Soc.2004;52:675–684.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta‐analysis.JAMA.2004;291:1358–1367.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- ,,,.Prolonged beneficial effects of a home‐based intervention on unplanned readmissions and mortality among patients with congestive heart failure.Arch Intern Med.1999;159:257–261.
- ,,,.A randomized, controlled trial of comprehensive geriatric assessment and multidisciplinary intervention after discharge of elderly from the emergency department–the DEED II study.J Am Geriatr Soc.2004;52:1417–1423.
- ,,,,.A randomized trial of comprehensive geriatric assessment and home intervention in the care of hospitalized patients.Age Ageing.1999;28:543–550.
- ,.Home intravenous antibiotic therapy: a team approach.Ann Intern Med.1983;99:388–392.
- Merriam‐Webster Dictionary Online. Definition of steward. Available at http://www.merriam‐webster.com/dictionary/steward. Accessed July 14,2010.
- ,,, et al.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44:159–177.
- ,,,.Improving antibiotic utilization among hospitalists: a pilot academic detailing project with a public health approach.J Hosp Med.2008;3:64–70.
- ,.Outpatient intravenous medications in the management of cystic fibrosis.Pediatrics.1974;54:358–360.
- ,,, et al.Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines.Clin Infect Dis.2004;38:1651–1672.
- .Evaluating the quality of medical care.Milbank Mem Fund Q.1966;44(Suppl):166–206.
- ,,.Impact of mandatory inpatient infectious disease consultation on outpatient parenteral antibiotic therapy.Am J Med Sci.2005;330:60–64.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360:1418–1428.
- The New York Times. Editorial: Back in the Hospital Again. April 15, 2009. Available at http://www.nytimes.com/2009/04/16/opinion/16thu2.html. Accessed July 16,2010.
- ,,,,,.Emergency department visits of patients on community‐based parenteral anti‐infective therapy at home. Presented at the 47th annual meeting of IDSA, Philadelphia, PA, October 29‐November 1, 2009. Poster 462.
- .The caring physician: the life of Dr. Francis W. Peabody [book review].N Engl J Med.1993;328:817–818.
- ,.How physicians can change the future of health care.JAMA.2007;297:1103–1111.
- ,.Redefining Health Care: Creating Value‐Based Competition on Results.Boston, MA:Harvard Business Press;2006.
- Greiner AC, Knebel E, eds.Health Professions Education: A Bridge to Quality. Committee on the Health Professions Education Summit.Washington, DC:National Academies Press;2003.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,,,,.Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial.J Am Geriatr Soc.2004;52:675–684.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta‐analysis.JAMA.2004;291:1358–1367.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- ,,,.Prolonged beneficial effects of a home‐based intervention on unplanned readmissions and mortality among patients with congestive heart failure.Arch Intern Med.1999;159:257–261.
- ,,,.A randomized, controlled trial of comprehensive geriatric assessment and multidisciplinary intervention after discharge of elderly from the emergency department–the DEED II study.J Am Geriatr Soc.2004;52:1417–1423.
- ,,,,.A randomized trial of comprehensive geriatric assessment and home intervention in the care of hospitalized patients.Age Ageing.1999;28:543–550.
- ,.Home intravenous antibiotic therapy: a team approach.Ann Intern Med.1983;99:388–392.
- Merriam‐Webster Dictionary Online. Definition of steward. Available at http://www.merriam‐webster.com/dictionary/steward. Accessed July 14,2010.
- ,,, et al.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44:159–177.
- ,,,.Improving antibiotic utilization among hospitalists: a pilot academic detailing project with a public health approach.J Hosp Med.2008;3:64–70.
- ,.Outpatient intravenous medications in the management of cystic fibrosis.Pediatrics.1974;54:358–360.
- ,,, et al.Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines.Clin Infect Dis.2004;38:1651–1672.
- .Evaluating the quality of medical care.Milbank Mem Fund Q.1966;44(Suppl):166–206.
- ,,.Impact of mandatory inpatient infectious disease consultation on outpatient parenteral antibiotic therapy.Am J Med Sci.2005;330:60–64.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360:1418–1428.
- The New York Times. Editorial: Back in the Hospital Again. April 15, 2009. Available at http://www.nytimes.com/2009/04/16/opinion/16thu2.html. Accessed July 16,2010.
- ,,,,,.Emergency department visits of patients on community‐based parenteral anti‐infective therapy at home. Presented at the 47th annual meeting of IDSA, Philadelphia, PA, October 29‐November 1, 2009. Poster 462.
Interferon-gamma-release assays: Better than tuberculin skin testing?
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.
CASE 1: A FOREIGN-BORN HEALTH CARE WORKER WITH A POSITIVE RESULT
A 30-year-old woman, an immigrant from the Philippines, is applying for a position as a registered nurse. On preemployment screening, her QFT-GIT test is positive: 8.1 IU/mL in the tuberculin antigen tube minus 0.6 IU/mL in the nil tube, for a tuberculin response of 7.5 IU/mL. Her medical record shows that previous tuberculin skin tests were positive. Her current screening examination and chest radiograph are normal. She received BCG vaccination as a child.
Comment. This case illustrates how the assays are useful in diagnosing latent tuberculosis in foreign-born health care workers. Whereas this patient’s previous positive skin tests may have been falsely positive because of her childhood BCG vaccination, BCG vaccination does not affect the results of interferon-gamma-release assays, and thus a positive QFT-GIT test is likely to indicate latent tuberculosis.
Case continued
We believe our patient has latent tuberculosis, and we recommend isoniazid therapy. However, she does not want to take isoniazid: she says she underwent a tuberculin skin test 2 days before the QFT-GIT test, and she thinks that may have affected her QFT-GIT test result.
Comment. Can tuberculin skin testing influence the results of interferon-gamma-release assays? The question is important, considering that the UK National Institute for Health and Clinical Excellence recommends a two-step procedure, with tuberculin skin testing first, then an interferon-gamma-release assay if the skin test is positive.10
Studies have found conflicting results.14 However, van Zyl-Smit et al14 obtained blood samples for QFT-GIT and T-SPOT.TB testing in 26 South Africans at 21, 14, and 7 days before tuberculin skin testing, and also on the day of the test and at 3, 7, 28, and 84 days after. They observed higher interferon-gamma responses after tuberculin skin testing, greater than the within-subject variability. This “boosting” effect was evident on day 7 but not on day 3, leading the investigators to conclude that interferon-gamma-release assays should ideally be performed no more than 3 days after a skin test.
The Canadian guidelines15 recommend an interferon-gamma-release assay on or before the day the skin test is read if both types of tests will be used. It is important to note that interferon-gamma-release assay testing does not boost subsequent test results,9 such as when used for serial or periodic testing.
For our patient in this case, isoniazid therapy is still recommended.
CASE 2: A MAN AT LOW RISK WITH A POSITIVE RESULT
A 26-year-old man applying for a position in health data services has a positive QFT-GIT test on preemployment health screening. He was born and raised in the United States, and has no known contacts with tuberculosis. He has never had a tuberculin skin test. A chest radiograph shows no evidence of tuberculosis, and he has no symptoms. His quantitative result (ie, the interferon-gamma level in his blood incubated with tuberculin antigens, minus the interferon-gamma level in his blood cultured without antigens) is 0.37 IU/mL.
Comment. QFT-GIT results are considered positive if the tuberculin response (tuberculin antigen tube minus nil tube) is 0.35 IU/mL or higher, and at least 25% higher than in the nil sample (Table 1), so this man’s result is just above the cutoff. T-cell responses can vary from time to time in the same person and from person to person, and this variation is reflected in the 15% variance accepted by the FDA.16 Given the applicant’s history, he is unlikely to have latent tuberculosis or to need isoniazid treatment.
This case shows the importance of having the actual quantitative interferon-gamma value when evaluating a patient with a positive interferon-gamma-release assay, particularly a patient at low risk of tuberculosis.
CASE 3: SEROCONVERSION
A 59-year-old woman, born and raised in the United States and working in the hospital environmental services department, has a positive QFT-GIT result on routine annual screening. Previous tuberculin skin tests were negative, and her first QFT-GIT test result on annual screening was negative. Her chest radiograph is negative, and she has no symptoms. One year ago her QFT-GIT value (tuberculin antigen tube minus nil tube) was 0.09 IU/mL; now it is 0.61 IU/mL. A tuberculin skin test is placed and is negative.
Comment. This case illustrates “QFT-GIT conversion,” ie, a positive test result in a person who previously had negative results.17 However, as with the man in case 2, 0.61 IU/mL can also be considered a weakly positive result. If the QFT-GIT result is weakly positive and the skin test is negative, results must be interpreted with caution. Nonspecific variations can occur with serial testing, and weakly positive responses may fluctuate over time.18
Veerapathran et al18 studied the shortterm reproducibility of the QFT-GIT test in 14 health care workers who underwent serial testing; discordance was mostly noted in those who had interferon-gamma values around the cutoff point. They suggested that a QFT-GIT conversion should be defined as a change from a negative to a positive result and at least a 30% increase in the baseline interferon-gamma response.17
Also, a small prospective series in a highrisk US immigrant population showed that the QFT-GIT test had inconsistent results in 13% of those tested, particularly in those with low positive responses (< 0.69 IU/mL).19
For clinicians, the question remains whether we need to use another cutoff to distinguish new infection from nonspecific variations, and whether the cutoff should vary depending on risk of infection.
CASE 4: AN INDETERMINATE RESULT IN A WOMAN AT LOW RISK
A 65-year-old woman, also from the United States, has an indeterminate QFT-GIT result on preemployment screening. She has no known contacts with tuberculosis.
Comment. An indeterminate result can mean either that the person is immunosuppressed (in which case her blood would show a low response to mitogen; Table 1), or that there could have been errors in the performance of the test, such as improper transport, handling, or storage of the blood specimen.6 Previously at our institution, 8% of the results in our health care workers were indeterminate, a finding that led to changes in specimen collection and laboratory analysis that significantly decreased the number of indeterminate results.12 We also found that using the newer QuantiFERON test, ie, the QFT-GIT, further decreased the indeterminate rate.12
A person with an indeterminate result should be tested again and be evaluated by a physician for underlying immunosuppression or to rule out active tuberculosis (eg, via chest radiography).
There are only limited data on the use of interferon-gamma-release assays in immunosuppressed people, such as patients with human immunodeficiency virus (HIV) infection. False-negative and indeterminate results are increasingly more common in HIV patients with declining CD4 counts.20 In immunocompromised patients at high risk of infection, use of both an assay and skin testing may be reasonable.16
CASE 5: SCREENING THE CONTACTS OF A MAN WITH ACTIVE TUBERCULOSIS
A 39-year-old male health care worker is diagnosed with active tuberculosis. The QFT-GIT test is then used to determine exposure in all possible contacts.
Comment. The CDC guidelines recommend using QuantiFERON tests in all circumstances in which the tuberculin skin test has been used, including contact investigation screening.9 The QFT-GIT test can be used to screen possible contacts of infected health care workers at baseline, and it is recommended that the test be repeated 8 to 10 weeks after the exposure.9 In our experience, contact investigation has been more efficient and easier to conduct with the use of the QFT-GIT than with the tuberculin skin test.21
THE FUTURE OF TUBERCULOSIS TESTING
Given the wide availability of interferon-gamma-release assays and laboratories that process them, more tuberculosis control programs will probably start using them rather than tuberculin skin testing. Successful implementation requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians. Further study is needed to evaluate the feasibility, utility, cost-effectiveness, and value of using these new tests.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007; 131:1898–1906.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000; 356:1099–1104.
- Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med 2007; 20:540–547.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006; 6:47.
- QuantiFERON®-TB GOLD (In-Tube Method) Package Insert. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023. Accessed August 11, 2010.
- T-SPOT.TB. www.oxfordimmunotec.com. Accessed August 11, 2010.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–184.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Recomm Rep 2010; 59:1–25.
- National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. CG33. http://www.evidence.nhs.uk/search.aspx?t=CG33. Accessed June 10, 2010.
- Sahni R, Miranda C, Yen-Lieberman B, et al. Does the implementation of an interferon-gamma release assay in lieu of a tuberculin skin test increase acceptance of preventive therapy for latent tuberculosis among healthcare workers? Infect Control Hosp Epidemiol 2009; 30:197–199.
- Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol 2009; 30:296–298.
- de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009; 169:179–187.
- van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58.
- Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008; 34:1–13.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. Curr Opin Infect Dis 2009; 22:174–182.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon RCDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141.
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008; 3:e1850.
- Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB Gold In-Tube assay. Clin Vaccine Immunol 2008; 15:425–432.
- Lalvani A, Pareek M. A 100-year update on diagnosis of tuberculosis infection. Br Med Bull 2010; 93:69–84.
- Miranda C, Schnellinger P, Scarpeli M, Tomford JW, Fraser TG, Gordon SM. Use of interferon gamma release assay (IGRA) for contact investigation in coworkers of a fast food worker with pulmonary tuberculosis (abstract). Presented at the Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Atlanta, GA, March 18–21, 2010.
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.
CASE 1: A FOREIGN-BORN HEALTH CARE WORKER WITH A POSITIVE RESULT
A 30-year-old woman, an immigrant from the Philippines, is applying for a position as a registered nurse. On preemployment screening, her QFT-GIT test is positive: 8.1 IU/mL in the tuberculin antigen tube minus 0.6 IU/mL in the nil tube, for a tuberculin response of 7.5 IU/mL. Her medical record shows that previous tuberculin skin tests were positive. Her current screening examination and chest radiograph are normal. She received BCG vaccination as a child.
Comment. This case illustrates how the assays are useful in diagnosing latent tuberculosis in foreign-born health care workers. Whereas this patient’s previous positive skin tests may have been falsely positive because of her childhood BCG vaccination, BCG vaccination does not affect the results of interferon-gamma-release assays, and thus a positive QFT-GIT test is likely to indicate latent tuberculosis.
Case continued
We believe our patient has latent tuberculosis, and we recommend isoniazid therapy. However, she does not want to take isoniazid: she says she underwent a tuberculin skin test 2 days before the QFT-GIT test, and she thinks that may have affected her QFT-GIT test result.
Comment. Can tuberculin skin testing influence the results of interferon-gamma-release assays? The question is important, considering that the UK National Institute for Health and Clinical Excellence recommends a two-step procedure, with tuberculin skin testing first, then an interferon-gamma-release assay if the skin test is positive.10
Studies have found conflicting results.14 However, van Zyl-Smit et al14 obtained blood samples for QFT-GIT and T-SPOT.TB testing in 26 South Africans at 21, 14, and 7 days before tuberculin skin testing, and also on the day of the test and at 3, 7, 28, and 84 days after. They observed higher interferon-gamma responses after tuberculin skin testing, greater than the within-subject variability. This “boosting” effect was evident on day 7 but not on day 3, leading the investigators to conclude that interferon-gamma-release assays should ideally be performed no more than 3 days after a skin test.
The Canadian guidelines15 recommend an interferon-gamma-release assay on or before the day the skin test is read if both types of tests will be used. It is important to note that interferon-gamma-release assay testing does not boost subsequent test results,9 such as when used for serial or periodic testing.
For our patient in this case, isoniazid therapy is still recommended.
CASE 2: A MAN AT LOW RISK WITH A POSITIVE RESULT
A 26-year-old man applying for a position in health data services has a positive QFT-GIT test on preemployment health screening. He was born and raised in the United States, and has no known contacts with tuberculosis. He has never had a tuberculin skin test. A chest radiograph shows no evidence of tuberculosis, and he has no symptoms. His quantitative result (ie, the interferon-gamma level in his blood incubated with tuberculin antigens, minus the interferon-gamma level in his blood cultured without antigens) is 0.37 IU/mL.
Comment. QFT-GIT results are considered positive if the tuberculin response (tuberculin antigen tube minus nil tube) is 0.35 IU/mL or higher, and at least 25% higher than in the nil sample (Table 1), so this man’s result is just above the cutoff. T-cell responses can vary from time to time in the same person and from person to person, and this variation is reflected in the 15% variance accepted by the FDA.16 Given the applicant’s history, he is unlikely to have latent tuberculosis or to need isoniazid treatment.
This case shows the importance of having the actual quantitative interferon-gamma value when evaluating a patient with a positive interferon-gamma-release assay, particularly a patient at low risk of tuberculosis.
CASE 3: SEROCONVERSION
A 59-year-old woman, born and raised in the United States and working in the hospital environmental services department, has a positive QFT-GIT result on routine annual screening. Previous tuberculin skin tests were negative, and her first QFT-GIT test result on annual screening was negative. Her chest radiograph is negative, and she has no symptoms. One year ago her QFT-GIT value (tuberculin antigen tube minus nil tube) was 0.09 IU/mL; now it is 0.61 IU/mL. A tuberculin skin test is placed and is negative.
Comment. This case illustrates “QFT-GIT conversion,” ie, a positive test result in a person who previously had negative results.17 However, as with the man in case 2, 0.61 IU/mL can also be considered a weakly positive result. If the QFT-GIT result is weakly positive and the skin test is negative, results must be interpreted with caution. Nonspecific variations can occur with serial testing, and weakly positive responses may fluctuate over time.18
Veerapathran et al18 studied the shortterm reproducibility of the QFT-GIT test in 14 health care workers who underwent serial testing; discordance was mostly noted in those who had interferon-gamma values around the cutoff point. They suggested that a QFT-GIT conversion should be defined as a change from a negative to a positive result and at least a 30% increase in the baseline interferon-gamma response.17
Also, a small prospective series in a highrisk US immigrant population showed that the QFT-GIT test had inconsistent results in 13% of those tested, particularly in those with low positive responses (< 0.69 IU/mL).19
For clinicians, the question remains whether we need to use another cutoff to distinguish new infection from nonspecific variations, and whether the cutoff should vary depending on risk of infection.
CASE 4: AN INDETERMINATE RESULT IN A WOMAN AT LOW RISK
A 65-year-old woman, also from the United States, has an indeterminate QFT-GIT result on preemployment screening. She has no known contacts with tuberculosis.
Comment. An indeterminate result can mean either that the person is immunosuppressed (in which case her blood would show a low response to mitogen; Table 1), or that there could have been errors in the performance of the test, such as improper transport, handling, or storage of the blood specimen.6 Previously at our institution, 8% of the results in our health care workers were indeterminate, a finding that led to changes in specimen collection and laboratory analysis that significantly decreased the number of indeterminate results.12 We also found that using the newer QuantiFERON test, ie, the QFT-GIT, further decreased the indeterminate rate.12
A person with an indeterminate result should be tested again and be evaluated by a physician for underlying immunosuppression or to rule out active tuberculosis (eg, via chest radiography).
There are only limited data on the use of interferon-gamma-release assays in immunosuppressed people, such as patients with human immunodeficiency virus (HIV) infection. False-negative and indeterminate results are increasingly more common in HIV patients with declining CD4 counts.20 In immunocompromised patients at high risk of infection, use of both an assay and skin testing may be reasonable.16
CASE 5: SCREENING THE CONTACTS OF A MAN WITH ACTIVE TUBERCULOSIS
A 39-year-old male health care worker is diagnosed with active tuberculosis. The QFT-GIT test is then used to determine exposure in all possible contacts.
Comment. The CDC guidelines recommend using QuantiFERON tests in all circumstances in which the tuberculin skin test has been used, including contact investigation screening.9 The QFT-GIT test can be used to screen possible contacts of infected health care workers at baseline, and it is recommended that the test be repeated 8 to 10 weeks after the exposure.9 In our experience, contact investigation has been more efficient and easier to conduct with the use of the QFT-GIT than with the tuberculin skin test.21
THE FUTURE OF TUBERCULOSIS TESTING
Given the wide availability of interferon-gamma-release assays and laboratories that process them, more tuberculosis control programs will probably start using them rather than tuberculin skin testing. Successful implementation requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians. Further study is needed to evaluate the feasibility, utility, cost-effectiveness, and value of using these new tests.
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.
CASE 1: A FOREIGN-BORN HEALTH CARE WORKER WITH A POSITIVE RESULT
A 30-year-old woman, an immigrant from the Philippines, is applying for a position as a registered nurse. On preemployment screening, her QFT-GIT test is positive: 8.1 IU/mL in the tuberculin antigen tube minus 0.6 IU/mL in the nil tube, for a tuberculin response of 7.5 IU/mL. Her medical record shows that previous tuberculin skin tests were positive. Her current screening examination and chest radiograph are normal. She received BCG vaccination as a child.
Comment. This case illustrates how the assays are useful in diagnosing latent tuberculosis in foreign-born health care workers. Whereas this patient’s previous positive skin tests may have been falsely positive because of her childhood BCG vaccination, BCG vaccination does not affect the results of interferon-gamma-release assays, and thus a positive QFT-GIT test is likely to indicate latent tuberculosis.
Case continued
We believe our patient has latent tuberculosis, and we recommend isoniazid therapy. However, she does not want to take isoniazid: she says she underwent a tuberculin skin test 2 days before the QFT-GIT test, and she thinks that may have affected her QFT-GIT test result.
Comment. Can tuberculin skin testing influence the results of interferon-gamma-release assays? The question is important, considering that the UK National Institute for Health and Clinical Excellence recommends a two-step procedure, with tuberculin skin testing first, then an interferon-gamma-release assay if the skin test is positive.10
Studies have found conflicting results.14 However, van Zyl-Smit et al14 obtained blood samples for QFT-GIT and T-SPOT.TB testing in 26 South Africans at 21, 14, and 7 days before tuberculin skin testing, and also on the day of the test and at 3, 7, 28, and 84 days after. They observed higher interferon-gamma responses after tuberculin skin testing, greater than the within-subject variability. This “boosting” effect was evident on day 7 but not on day 3, leading the investigators to conclude that interferon-gamma-release assays should ideally be performed no more than 3 days after a skin test.
The Canadian guidelines15 recommend an interferon-gamma-release assay on or before the day the skin test is read if both types of tests will be used. It is important to note that interferon-gamma-release assay testing does not boost subsequent test results,9 such as when used for serial or periodic testing.
For our patient in this case, isoniazid therapy is still recommended.
CASE 2: A MAN AT LOW RISK WITH A POSITIVE RESULT
A 26-year-old man applying for a position in health data services has a positive QFT-GIT test on preemployment health screening. He was born and raised in the United States, and has no known contacts with tuberculosis. He has never had a tuberculin skin test. A chest radiograph shows no evidence of tuberculosis, and he has no symptoms. His quantitative result (ie, the interferon-gamma level in his blood incubated with tuberculin antigens, minus the interferon-gamma level in his blood cultured without antigens) is 0.37 IU/mL.
Comment. QFT-GIT results are considered positive if the tuberculin response (tuberculin antigen tube minus nil tube) is 0.35 IU/mL or higher, and at least 25% higher than in the nil sample (Table 1), so this man’s result is just above the cutoff. T-cell responses can vary from time to time in the same person and from person to person, and this variation is reflected in the 15% variance accepted by the FDA.16 Given the applicant’s history, he is unlikely to have latent tuberculosis or to need isoniazid treatment.
This case shows the importance of having the actual quantitative interferon-gamma value when evaluating a patient with a positive interferon-gamma-release assay, particularly a patient at low risk of tuberculosis.
CASE 3: SEROCONVERSION
A 59-year-old woman, born and raised in the United States and working in the hospital environmental services department, has a positive QFT-GIT result on routine annual screening. Previous tuberculin skin tests were negative, and her first QFT-GIT test result on annual screening was negative. Her chest radiograph is negative, and she has no symptoms. One year ago her QFT-GIT value (tuberculin antigen tube minus nil tube) was 0.09 IU/mL; now it is 0.61 IU/mL. A tuberculin skin test is placed and is negative.
Comment. This case illustrates “QFT-GIT conversion,” ie, a positive test result in a person who previously had negative results.17 However, as with the man in case 2, 0.61 IU/mL can also be considered a weakly positive result. If the QFT-GIT result is weakly positive and the skin test is negative, results must be interpreted with caution. Nonspecific variations can occur with serial testing, and weakly positive responses may fluctuate over time.18
Veerapathran et al18 studied the shortterm reproducibility of the QFT-GIT test in 14 health care workers who underwent serial testing; discordance was mostly noted in those who had interferon-gamma values around the cutoff point. They suggested that a QFT-GIT conversion should be defined as a change from a negative to a positive result and at least a 30% increase in the baseline interferon-gamma response.17
Also, a small prospective series in a highrisk US immigrant population showed that the QFT-GIT test had inconsistent results in 13% of those tested, particularly in those with low positive responses (< 0.69 IU/mL).19
For clinicians, the question remains whether we need to use another cutoff to distinguish new infection from nonspecific variations, and whether the cutoff should vary depending on risk of infection.
CASE 4: AN INDETERMINATE RESULT IN A WOMAN AT LOW RISK
A 65-year-old woman, also from the United States, has an indeterminate QFT-GIT result on preemployment screening. She has no known contacts with tuberculosis.
Comment. An indeterminate result can mean either that the person is immunosuppressed (in which case her blood would show a low response to mitogen; Table 1), or that there could have been errors in the performance of the test, such as improper transport, handling, or storage of the blood specimen.6 Previously at our institution, 8% of the results in our health care workers were indeterminate, a finding that led to changes in specimen collection and laboratory analysis that significantly decreased the number of indeterminate results.12 We also found that using the newer QuantiFERON test, ie, the QFT-GIT, further decreased the indeterminate rate.12
A person with an indeterminate result should be tested again and be evaluated by a physician for underlying immunosuppression or to rule out active tuberculosis (eg, via chest radiography).
There are only limited data on the use of interferon-gamma-release assays in immunosuppressed people, such as patients with human immunodeficiency virus (HIV) infection. False-negative and indeterminate results are increasingly more common in HIV patients with declining CD4 counts.20 In immunocompromised patients at high risk of infection, use of both an assay and skin testing may be reasonable.16
CASE 5: SCREENING THE CONTACTS OF A MAN WITH ACTIVE TUBERCULOSIS
A 39-year-old male health care worker is diagnosed with active tuberculosis. The QFT-GIT test is then used to determine exposure in all possible contacts.
Comment. The CDC guidelines recommend using QuantiFERON tests in all circumstances in which the tuberculin skin test has been used, including contact investigation screening.9 The QFT-GIT test can be used to screen possible contacts of infected health care workers at baseline, and it is recommended that the test be repeated 8 to 10 weeks after the exposure.9 In our experience, contact investigation has been more efficient and easier to conduct with the use of the QFT-GIT than with the tuberculin skin test.21
THE FUTURE OF TUBERCULOSIS TESTING
Given the wide availability of interferon-gamma-release assays and laboratories that process them, more tuberculosis control programs will probably start using them rather than tuberculin skin testing. Successful implementation requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians. Further study is needed to evaluate the feasibility, utility, cost-effectiveness, and value of using these new tests.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007; 131:1898–1906.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000; 356:1099–1104.
- Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med 2007; 20:540–547.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006; 6:47.
- QuantiFERON®-TB GOLD (In-Tube Method) Package Insert. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023. Accessed August 11, 2010.
- T-SPOT.TB. www.oxfordimmunotec.com. Accessed August 11, 2010.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–184.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Recomm Rep 2010; 59:1–25.
- National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. CG33. http://www.evidence.nhs.uk/search.aspx?t=CG33. Accessed June 10, 2010.
- Sahni R, Miranda C, Yen-Lieberman B, et al. Does the implementation of an interferon-gamma release assay in lieu of a tuberculin skin test increase acceptance of preventive therapy for latent tuberculosis among healthcare workers? Infect Control Hosp Epidemiol 2009; 30:197–199.
- Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol 2009; 30:296–298.
- de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009; 169:179–187.
- van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58.
- Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008; 34:1–13.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. Curr Opin Infect Dis 2009; 22:174–182.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon RCDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141.
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008; 3:e1850.
- Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB Gold In-Tube assay. Clin Vaccine Immunol 2008; 15:425–432.
- Lalvani A, Pareek M. A 100-year update on diagnosis of tuberculosis infection. Br Med Bull 2010; 93:69–84.
- Miranda C, Schnellinger P, Scarpeli M, Tomford JW, Fraser TG, Gordon SM. Use of interferon gamma release assay (IGRA) for contact investigation in coworkers of a fast food worker with pulmonary tuberculosis (abstract). Presented at the Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Atlanta, GA, March 18–21, 2010.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007; 131:1898–1906.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000; 356:1099–1104.
- Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med 2007; 20:540–547.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006; 6:47.
- QuantiFERON®-TB GOLD (In-Tube Method) Package Insert. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023. Accessed August 11, 2010.
- T-SPOT.TB. www.oxfordimmunotec.com. Accessed August 11, 2010.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–184.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Recomm Rep 2010; 59:1–25.
- National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. CG33. http://www.evidence.nhs.uk/search.aspx?t=CG33. Accessed June 10, 2010.
- Sahni R, Miranda C, Yen-Lieberman B, et al. Does the implementation of an interferon-gamma release assay in lieu of a tuberculin skin test increase acceptance of preventive therapy for latent tuberculosis among healthcare workers? Infect Control Hosp Epidemiol 2009; 30:197–199.
- Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol 2009; 30:296–298.
- de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009; 169:179–187.
- van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58.
- Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008; 34:1–13.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. Curr Opin Infect Dis 2009; 22:174–182.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon RCDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141.
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008; 3:e1850.
- Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB Gold In-Tube assay. Clin Vaccine Immunol 2008; 15:425–432.
- Lalvani A, Pareek M. A 100-year update on diagnosis of tuberculosis infection. Br Med Bull 2010; 93:69–84.
- Miranda C, Schnellinger P, Scarpeli M, Tomford JW, Fraser TG, Gordon SM. Use of interferon gamma release assay (IGRA) for contact investigation in coworkers of a fast food worker with pulmonary tuberculosis (abstract). Presented at the Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Atlanta, GA, March 18–21, 2010.
KEY POINTS
- Prior vaccination with bacille Calmette-Guérin can cause the results of skin testing to be falsely positive, but it does not affect interferon-gamma-release assays.
- In 2005, the US Centers for Disease Control and Prevention recommended that interferon-gamma-release assays be used in all situations in which skin testing is currently used. Updated guidelines were published on June 25, 2010.
- Successful implementation of interferon-gamma-release assay testing requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians.
Update on 2009 pandemic influenza A (H1N1) virus
A 69-year-old ohio man with leukemia was treated in another state in late June. During the car trip back to Ohio, he developed a sore throat, fever, cough, and nasal congestion. He was admitted to Cleveland Clinic with a presumed diagnosis of neutropenic fever; his absolute neutrophil count was 0.4 × 109/L (reference range 1.8–7.7). His chest radiograph was normal. He was treated with empiric broad-spectrum antimicrobials. On his second day in the hospital, he was tested for influenza by a polymerase chain reaction (PCR) test, which was positive for influenza A. He was moved to a private room and started on oseltamivir (Tamiflu) and rimantadine (Flumadine). The patient’s previous roommate subsequently tested positive for influenza A, as did two health care workers working on the ward. All patients on the floor received prophylactic oseltamivir.
The patient’s condition worsened, and he subsequently went into respiratory distress with diffuse pulmonary infiltrates. He was transferred to the intensive care unit, where he was intubated. Influenza A was isolated from a bronchoscopic specimen. He subsequently recovered after a prolonged course and was discharged on hospital day 50. Testing by the Ohio Department of Health confirmed that this was the 2009 pandemic influenza A (H1N1) virus.
THE CHALLENGES WE FACE
We are now in the midst of an influenza pandemic of the 2009 influenza A (H1N1) virus, with pandemic defined as “worldwide sustained community transmission.” The circulation of seasonal and 2009 pandemic influenza A (H1N1) strains will make this flu season both interesting and challenging.
The approaches to vaccination, prophylaxis, and treatment will be more complex. As of this writing (mid-September 2009), it is clear that we will be giving two influenza vaccines this season: a trivalent vaccine for seasonal influenza, and a monovalent vaccine for pandemic H1N1. It appears the monovalent vaccine may require only one dose to provide protective immunity.1 Fortunately, the vast majority of cases of pandemic H1N1 are relatively mild and uncomplicated. Still, some people are at higher risk of complications, including young patients, pregnant women, and people with immune deficiency or concomitant health conditions that put them at higher risk of flu-associated complications. Thus, clinicians will need to be educated about whom to test, who needs prophylaxis, and who should not be treated.
As our case demonstrates, unsuspected cases of influenza in hospitalized patients or health care workers working with influenza pose the greatest threat for transmission of influenza within the hospital. Adults hospitalized with influenza tend to present late (more than 48 hours after the onset of symptoms) and tend to have prolonged illness.2 Ambulatory adults shed virus for 3 to 6 days; virus shedding is more prolonged for hospitalized patients. Antiviral agents started within 4 days of illness enhance viral clearance and are associated with a shorter stay.3 Therefore, we should have a low threshold for testing for influenza and for isolating all suspected cases.
This is also creating a paradigm shift for health care workers, who are notorious for working through an illness. If you are sick, stay home! This applies whether you have pandemic H1N1 or something else.
EPIDEMIOLOGY OF PANDEMIC 2009 INFLUENZA A (H1N1) VIRUS
The location of cases can now be found on Google Maps; the US Centers for Disease Control and Prevention (CDC) provides weekly influenza reports at www.cdc.gov/flu/weekly/fluactivity.htm.
Pandemic H1N1 appeared in the spring of 2009, and cases continued to mount all summer in the United States (when influenza is normally absent) and around the world. In Mexico in March and April 2009, 2,155 cases of pneumonia, 821 hospitalizations, and 100 deaths were reported.4
In contrast with seasonal influenza, children and younger adults were hit the hardest in Mexico. The age group 5 through 59 years accounted for 87% of the deaths (usually, they account for about 17%) and 71% of the cases of severe pneumonia (usually, they account for 32%). These observations may be explained in part by the possibility that people who were alive during the 1957 pandemic (which was an H1N1 strain) have some immunity to the new virus. However, the case-fatality rate was highest in people age 65 and older.4
As of July 2009, there were more than 43,000 confirmed cases of pandemic H1N1 in the United States, and actual cases probably exceed 1 million, with more than 400 deaths. An underlying risk factor was identified in more than half of the fatal cases.5 Ten percent of the women who died were pregnant.
Pandemic H1N1 has several distinctive epidemiologic features:
- The distribution of cases is similar across multiple geographic areas.
- The distribution of cases by age group is markedly different than that of seasonal influenza, with more cases in school children and fewer cases in older adults.
- Fewer cases have been reported in older adults, but this group has the highest case-fatality rate.
2009 PANDEMIC H1N1 IS A MONGREL
There are three types of influenza viruses, designated A, B, and C. Type A undergoes antigenic shift (rapid changes) and antigenic drift (gradual changes) from year to year, and so it is the type associated with pandemics. In contrast, type B undergoes antigenic drift only, and type C is relatively stable.
Influenza virus is subtyped on the basis of surface glycoproteins: 16 hemagglutinins and nine neuraminidases. The circulating subtypes change every year; the current circulating human subtypes are a seasonal subtype of H1N1 that is different than the pandemic H1N1 subtype, and H3N2.
The 2009 pandemic H1N1 is a new virus never seen before in North America.6 Genetically, it is a mongrel, coming from three recognized sources (pigs, birds, and humans) which were combined in pigs.7 It is similar to subtypes that circulated in the 1920s through the 1940s.
Most influenza in the Western world comes from Asia every fall, and its arrival is probably facilitated by air travel. The spread is usually unidirectional and is unlikely to contribute to long-term viral evolution.8 It appears that 2009 H1N1 virus is the predominant strain circulating in the current influenza season in the Southern Hemisphere. Virologic studies indicate that the H1N1 virus strain has remained antigenically stable since it appeared in April 2009. Thus, it appears likely that the strain selected by the United States for vaccine manufacturing will match the currently circulating seasonal and pandemic H1N1 strains.
VACCINATION IS THE FIRST LINE OF DEFENSE
In addition to the trivalent vaccine against seasonal influenza, a monovalent vaccine for pandemic H1N1 virus is being produced. The CDC has indicated that 45 million doses of pandemic influenza vaccine are expected in October 2009, with an average of 20 million doses each week thereafter. It is anticipated that half of these will be in multidose vials, that 20% will be in prefilled syringes for children over 5 years old and for pregnant women, and that 20% will be in the form of live-attenuated influenza vaccine (nasal spray). The inhaled vaccine should not be given to children under 2 years old, to children under 5 years old who have recurrent wheezing, or to anyone with severe asthma. Neither vaccine should be given to people allergic to hen eggs, from which the vaccine is produced.
An ample supply of the seasonal trivalent vaccine should be available. Once the CDC has more information about specific product availability of the pandemic H1N1 vaccine, that vaccine will be distributed. It can be given concurrently with seasonal influenza vaccine.
Several definitions should be kept in mind when discussing vaccination strategies. Supply is the number of vaccine doses available for distribution. Availability is the ability of a person recommended to be vaccinated to do so in a local venue. Prioritization is the recommendation to vaccination venues to selectively use vaccine for certain population groups first. Targeting is the recommendation that immunization programs encourage and promote vaccination for certain population groups.
The Advisory Committee on Immunization Practices and the CDC recommend both seasonal and H1N1 vaccinations for anyone 6 months of age or older who is at risk of becoming ill or of transmitting the viruses to others. Based on a review of epidemiologic data, the recommendation is for targeting the following five groups for H1N1 vaccination: children and young adults aged 6 months through 24 years; pregnant women; health care workers and emergency medical service workers; people ages 25 through 64 years who have certain health conditions (eg, diabetes, heart disease, lung disease); and people who live with or care for children younger than 6 months of age. This represents approximately 159 million people in the United States.
If the estimates for the vaccine supply are met, and if pandemic H1N1 vaccine requires only a single injection, there should be no need for prioritization of vaccine. If the supply of pandemic H1N1 vaccine is inadequate, then those groups who are targeted would also receive the first doses of the pandemic H1N1 vaccine. It should be used only with caution after consideration of potential benefits and risks in people who have had Guillain-Barré syndrome during the previous 6 weeks, in people with altered immunocompetence, or in people with medical conditions predisposing to influenza complications.
A mass vaccination campaign involving two separate flu vaccines can pose challenges in execution and messaging for public health officials and politicians. In 1976, an aggressive vaccination program turned into a disaster, as there was no pandemic and the vaccine was associated with adverse effects such as Guillain-Barré syndrome. The government and the medical profession need to prepare for a vaccine controversy and to communicate and continue to explain the plan to the public. As pointed out in a recent op-ed piece,9 we would hope that all expectant women in the fall flu season will get the flu vaccines. We also know that, normally, one in seven pregnancies would be expected to miscarry. The challenge for public health officials and physicians will be to explain to these patients that there may be an association rather than a causal relationship.
In health care workers, the average vaccination rate is only 37%. We should be doing much better. Cleveland Clinic previously increased the rate of vaccination among its employees via a program in which all workers must either be vaccinated or formally declare (on an internal Web site) that they decline to be vaccinated.10 This season, even more resources are being directed at decreasing the barriers to flu vaccinations for our health care workers with the support from hospital leadership.
INFECTION CONTROL IN THE HOSPITAL AND IN THE COMMUNITY
Influenza is very contagious and is spread in droplets via sneezing and coughing (within a 3-foot radius), or via unwashed hands—thus the infection-control campaigns urging you to cover your cough and wash your hands.
As noted, for patients being admitted or transferred to the hospital, we need to have a low threshold for testing for influenza and for isolating patients suspected of having influenza. For patients with suspected or proven seasonal influenza, transmission precautions are those recommended by the CDC for droplet precautions (www.cdc.gov/ncidod/dhqp/gl_isolation_droplet.html). A face mask is deemed adequate to protect transmission when coming within 3 feet of an infected person. CDC guidelines for pandemic H1N1 recommends airborne-transmission-based precautions for health care workers who are in close contact with patients with proven or possible H1N1 (www.cdc.gov/ncidod/dhqp/gl_isolation_airborne.html). This recommendation implies the use of fit-tested N95 respirators and negative air pressure rooms (if available).
The recent Institute of Medicine report, Respiratory Protection for Healthcare Workers in the Workplace Against Novel H1N1 Influenza A (www.iom.edu/CMS/3740/71769/72967/72970.aspx) endorses the current CDC guidelines and recommends following these guidelines until we have evidence that other forms of protection or guidelines are equally or more effective.
Personally, I am against this requirement because it creates a terrible administrative burden with no proven benefit. Requiring a respirator means requiring fit-testing, and this will negatively affect our ability to deliver patient care. Recent studies have shown that surgical masks may not be as effective11 but are probably sufficient. Lim et al12 reported that 79 (37%) of 212 workers who responded to a survey experienced headaches while wearing N95 masks. This remains a controversial issue.
Besides getting the flu shot, what can one do to avoid getting influenza or transmitting to others?
- Cover your cough (cough etiquette) and sneeze.
- Practice good hand hygiene.
- Avoid close contact with people who are sick.
- Do not go to school or work if sick.
A recent study of influenza in households suggested that having the person with flu and household contacts wear face masks and practice hand hygiene within the first 36 hours decreased transmission of flu within the household.13
The United States does have a national influenza pandemic plan that outlines specific roles in the event of a pandemic, and I urge you to peruse it at www.hhs.gov/pandemicflu/plan/.
RECOGNIZING AND DIAGNOSING INFLUENZA
The familiar signs and symptoms of influenza—fever, cough, muscle aches, and headache—are nonspecific. Call et al14 analyzed the diagnostic accuracy of symptoms and signs of influenza and found that fever and cough during an epidemic suggest but do not confirm influenza, and that sneezing in those over age 60 argues against influenza. They concluded that signs and symptoms can tell us whether a patient has an influenza-like illness, but do not confirm or exclude the diagnosis of influenza: “Clinicians need to consider whether influenza is circulating in their communities, and then either treat patients with influenza-like illness empirically or obtain a rapid influenza test.”14
The signs and symptoms of pandemic 2009 H1N1 are the same as for seasonal flu, except that about 25% of patients with pandemic flu develop gastrointestinal symptoms. It has not been more virulent than seasonal influenza to date.
Should you order a test for influenza?
Most people with influenza are neither tested nor treated. Before ordering a test for influenza, ask, “Does this patient actually have influenza?” Patients diagnosed with “influenza” may have a range of infectious and noninfectious causes, such as vasculitis, endocarditis, or any other condition that can cause a fever and cough.
If I truly suspect influenza, I would still only order a test if the results would change how I manage the patient—for example, a patient being admitted to the hospital where isolation would be required.
Pandemic H1N1 will be detected only as influenza A in our current PCR screen for human influenza. The test does not differentiate between seasonal strains of influenza A (which is resistant to oseltamivir) and pandemic H1N1 (which is susceptible to oseltamivir). This means if you intend to treat, you will have to address further complexity.
Testing for influenza
The clinician should be familiar with the types of tests available. Each test has advantages and disadvantages15:
Rapid antigen assay is a point-of-care test that can give results in 15 minutes but unfortunately is only 20% to 30% sensitive, so a negative result does not exclude the diagnosis. The positive predictive value is high, meaning a positive test means the patient does have the flu.
Direct fluorescent antibody testing takes about 2.5 hours to complete and requires special training for technicians. It has a sensitivity of 47%, a positive predictive value of 95%, and a negative predictive value of 92%.
PCR testing takes about 6 hours and has a sensitivity of 98%, a positive predictive value of 100%, and a negative predictive value of 98%. This is probably the best test, in view of its all-around performance, but it is not a point-of-care test.
Culture takes 2 to 3 days, has a sensitivity of 89%, a positive predictive value of 100%, and a negative predictive value of 88%.
These tests can determine that the patient has influenza A, but a confirmatory test is always required to confirm pandemic H1N1. This confirmatory testing can be done by the CDC, by state public health laboratories, and by commercial reference laboratories.
ANTIVIRAL TREATMENT
Since influenza test results do not specify whether the patient has seasonal or pandemic influenza, treatment decisions are a sticky wicket. Most patients with pandemic H1N1 do not need to be tested or treated.
Several drugs are approved for treating influenza and shorten the duration of symptoms by about 1 day. The earlier the treatment is started, the better: the time of antiviral initiation affects influenza viral load and the duration of viral shedding.3
The neuraminidase inhibitors oseltamivir and zanamivir (Relenza) block release of virus from the cell. Resistance to oseltamivir is emerging in seasonal influenza A, while most pandemic H1N1 strains are susceptible.
Oseltamivir resistance in pandemic H1N1
A total of 11 cases of oseltamivir-resistant pandemic H1N1 have been confirmed worldwide, including 3 in the United States (2 in immunosuppressed patients in Seattle, WA). Ten of the 11 cases occurred with oseltamivir exposure. All involved a histidine-to-tyrosine substitution at position 275 (H275Y) of the neuraminidase gene. Most were susceptible to zanamivir.
Supplies of oseltamivir and zanamivir are limited, so they should be used only in those who will benefit the most, ie, those at higher risk of influenza complications. These include children under 5 years old, adults age 65 and older, children and adolescents on long-term aspirin therapy, pregnant women, patients who have chronic conditions or who are immunosuppressed, and residents of long-term care facilities.
- Greenberg MA, Lai MH, Hartel GF. Response after one dose of a monovalent influenza A (H1N1) 2009 vaccine—preliminary report. N Engl J Med 2009;361doi:10.1056/NEJMoa0907413 [published online ahead of print].
- Ison M. Influenza in hospitalized adults: gaining insight into a significant problem. J Infect Dis 2009; 200:485–488.
- Lee N, Chan PKS, Hui DSC, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009; 200:492–500.
- Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674–679.
- Vaillant L, La Ruche G, Tarantola A, Barboza P; for the Epidemic Intelligence Team at InVS. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill 2009; 14(33):1–6. Available online at www.eurosurveillance.org/ViewArticle.aspx?ArticleID=19309.
- Zimmer SM, Burke DS. Historical perspective—emergence of influenza A (H1N1) viruses. N Engl J Med 2009; 361:279–285.
- Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197–201.
- Russell CA, Jones TC, Barr IG, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008; 320:340–346.
- Allen A. Prepare for a vaccine controversy. New York Times. 9/1/2009.
- Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37.
- Johnson DF, Druce JD, Birch C, Grayson ML. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis 2009; 49:275–277.
- Lim EC, Seet RC, Lee KH, Wilder-Smith EP, Chuah BY, Ong BK. Headaches and the N95 face-mask amongst healthcare providers. Acta Neurol Scand 2006; 113:199–202.
- Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a randomized trial. Ann Intern Med 2009; 151(6 Oct) [published online ahead of print].
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005; 293:987–997.
- Ginocchio CC, Zhang F, Manji R, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol 2009; 45:191–195.
- US Centers for Disease Control and Prevention. Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients—Seattle, Washington, 2009. MMWR 2009; 58:893–896.
A 69-year-old ohio man with leukemia was treated in another state in late June. During the car trip back to Ohio, he developed a sore throat, fever, cough, and nasal congestion. He was admitted to Cleveland Clinic with a presumed diagnosis of neutropenic fever; his absolute neutrophil count was 0.4 × 109/L (reference range 1.8–7.7). His chest radiograph was normal. He was treated with empiric broad-spectrum antimicrobials. On his second day in the hospital, he was tested for influenza by a polymerase chain reaction (PCR) test, which was positive for influenza A. He was moved to a private room and started on oseltamivir (Tamiflu) and rimantadine (Flumadine). The patient’s previous roommate subsequently tested positive for influenza A, as did two health care workers working on the ward. All patients on the floor received prophylactic oseltamivir.
The patient’s condition worsened, and he subsequently went into respiratory distress with diffuse pulmonary infiltrates. He was transferred to the intensive care unit, where he was intubated. Influenza A was isolated from a bronchoscopic specimen. He subsequently recovered after a prolonged course and was discharged on hospital day 50. Testing by the Ohio Department of Health confirmed that this was the 2009 pandemic influenza A (H1N1) virus.
THE CHALLENGES WE FACE
We are now in the midst of an influenza pandemic of the 2009 influenza A (H1N1) virus, with pandemic defined as “worldwide sustained community transmission.” The circulation of seasonal and 2009 pandemic influenza A (H1N1) strains will make this flu season both interesting and challenging.
The approaches to vaccination, prophylaxis, and treatment will be more complex. As of this writing (mid-September 2009), it is clear that we will be giving two influenza vaccines this season: a trivalent vaccine for seasonal influenza, and a monovalent vaccine for pandemic H1N1. It appears the monovalent vaccine may require only one dose to provide protective immunity.1 Fortunately, the vast majority of cases of pandemic H1N1 are relatively mild and uncomplicated. Still, some people are at higher risk of complications, including young patients, pregnant women, and people with immune deficiency or concomitant health conditions that put them at higher risk of flu-associated complications. Thus, clinicians will need to be educated about whom to test, who needs prophylaxis, and who should not be treated.
As our case demonstrates, unsuspected cases of influenza in hospitalized patients or health care workers working with influenza pose the greatest threat for transmission of influenza within the hospital. Adults hospitalized with influenza tend to present late (more than 48 hours after the onset of symptoms) and tend to have prolonged illness.2 Ambulatory adults shed virus for 3 to 6 days; virus shedding is more prolonged for hospitalized patients. Antiviral agents started within 4 days of illness enhance viral clearance and are associated with a shorter stay.3 Therefore, we should have a low threshold for testing for influenza and for isolating all suspected cases.
This is also creating a paradigm shift for health care workers, who are notorious for working through an illness. If you are sick, stay home! This applies whether you have pandemic H1N1 or something else.
EPIDEMIOLOGY OF PANDEMIC 2009 INFLUENZA A (H1N1) VIRUS
The location of cases can now be found on Google Maps; the US Centers for Disease Control and Prevention (CDC) provides weekly influenza reports at www.cdc.gov/flu/weekly/fluactivity.htm.
Pandemic H1N1 appeared in the spring of 2009, and cases continued to mount all summer in the United States (when influenza is normally absent) and around the world. In Mexico in March and April 2009, 2,155 cases of pneumonia, 821 hospitalizations, and 100 deaths were reported.4
In contrast with seasonal influenza, children and younger adults were hit the hardest in Mexico. The age group 5 through 59 years accounted for 87% of the deaths (usually, they account for about 17%) and 71% of the cases of severe pneumonia (usually, they account for 32%). These observations may be explained in part by the possibility that people who were alive during the 1957 pandemic (which was an H1N1 strain) have some immunity to the new virus. However, the case-fatality rate was highest in people age 65 and older.4
As of July 2009, there were more than 43,000 confirmed cases of pandemic H1N1 in the United States, and actual cases probably exceed 1 million, with more than 400 deaths. An underlying risk factor was identified in more than half of the fatal cases.5 Ten percent of the women who died were pregnant.
Pandemic H1N1 has several distinctive epidemiologic features:
- The distribution of cases is similar across multiple geographic areas.
- The distribution of cases by age group is markedly different than that of seasonal influenza, with more cases in school children and fewer cases in older adults.
- Fewer cases have been reported in older adults, but this group has the highest case-fatality rate.
2009 PANDEMIC H1N1 IS A MONGREL
There are three types of influenza viruses, designated A, B, and C. Type A undergoes antigenic shift (rapid changes) and antigenic drift (gradual changes) from year to year, and so it is the type associated with pandemics. In contrast, type B undergoes antigenic drift only, and type C is relatively stable.
Influenza virus is subtyped on the basis of surface glycoproteins: 16 hemagglutinins and nine neuraminidases. The circulating subtypes change every year; the current circulating human subtypes are a seasonal subtype of H1N1 that is different than the pandemic H1N1 subtype, and H3N2.
The 2009 pandemic H1N1 is a new virus never seen before in North America.6 Genetically, it is a mongrel, coming from three recognized sources (pigs, birds, and humans) which were combined in pigs.7 It is similar to subtypes that circulated in the 1920s through the 1940s.
Most influenza in the Western world comes from Asia every fall, and its arrival is probably facilitated by air travel. The spread is usually unidirectional and is unlikely to contribute to long-term viral evolution.8 It appears that 2009 H1N1 virus is the predominant strain circulating in the current influenza season in the Southern Hemisphere. Virologic studies indicate that the H1N1 virus strain has remained antigenically stable since it appeared in April 2009. Thus, it appears likely that the strain selected by the United States for vaccine manufacturing will match the currently circulating seasonal and pandemic H1N1 strains.
VACCINATION IS THE FIRST LINE OF DEFENSE
In addition to the trivalent vaccine against seasonal influenza, a monovalent vaccine for pandemic H1N1 virus is being produced. The CDC has indicated that 45 million doses of pandemic influenza vaccine are expected in October 2009, with an average of 20 million doses each week thereafter. It is anticipated that half of these will be in multidose vials, that 20% will be in prefilled syringes for children over 5 years old and for pregnant women, and that 20% will be in the form of live-attenuated influenza vaccine (nasal spray). The inhaled vaccine should not be given to children under 2 years old, to children under 5 years old who have recurrent wheezing, or to anyone with severe asthma. Neither vaccine should be given to people allergic to hen eggs, from which the vaccine is produced.
An ample supply of the seasonal trivalent vaccine should be available. Once the CDC has more information about specific product availability of the pandemic H1N1 vaccine, that vaccine will be distributed. It can be given concurrently with seasonal influenza vaccine.
Several definitions should be kept in mind when discussing vaccination strategies. Supply is the number of vaccine doses available for distribution. Availability is the ability of a person recommended to be vaccinated to do so in a local venue. Prioritization is the recommendation to vaccination venues to selectively use vaccine for certain population groups first. Targeting is the recommendation that immunization programs encourage and promote vaccination for certain population groups.
The Advisory Committee on Immunization Practices and the CDC recommend both seasonal and H1N1 vaccinations for anyone 6 months of age or older who is at risk of becoming ill or of transmitting the viruses to others. Based on a review of epidemiologic data, the recommendation is for targeting the following five groups for H1N1 vaccination: children and young adults aged 6 months through 24 years; pregnant women; health care workers and emergency medical service workers; people ages 25 through 64 years who have certain health conditions (eg, diabetes, heart disease, lung disease); and people who live with or care for children younger than 6 months of age. This represents approximately 159 million people in the United States.
If the estimates for the vaccine supply are met, and if pandemic H1N1 vaccine requires only a single injection, there should be no need for prioritization of vaccine. If the supply of pandemic H1N1 vaccine is inadequate, then those groups who are targeted would also receive the first doses of the pandemic H1N1 vaccine. It should be used only with caution after consideration of potential benefits and risks in people who have had Guillain-Barré syndrome during the previous 6 weeks, in people with altered immunocompetence, or in people with medical conditions predisposing to influenza complications.
A mass vaccination campaign involving two separate flu vaccines can pose challenges in execution and messaging for public health officials and politicians. In 1976, an aggressive vaccination program turned into a disaster, as there was no pandemic and the vaccine was associated with adverse effects such as Guillain-Barré syndrome. The government and the medical profession need to prepare for a vaccine controversy and to communicate and continue to explain the plan to the public. As pointed out in a recent op-ed piece,9 we would hope that all expectant women in the fall flu season will get the flu vaccines. We also know that, normally, one in seven pregnancies would be expected to miscarry. The challenge for public health officials and physicians will be to explain to these patients that there may be an association rather than a causal relationship.
In health care workers, the average vaccination rate is only 37%. We should be doing much better. Cleveland Clinic previously increased the rate of vaccination among its employees via a program in which all workers must either be vaccinated or formally declare (on an internal Web site) that they decline to be vaccinated.10 This season, even more resources are being directed at decreasing the barriers to flu vaccinations for our health care workers with the support from hospital leadership.
INFECTION CONTROL IN THE HOSPITAL AND IN THE COMMUNITY
Influenza is very contagious and is spread in droplets via sneezing and coughing (within a 3-foot radius), or via unwashed hands—thus the infection-control campaigns urging you to cover your cough and wash your hands.
As noted, for patients being admitted or transferred to the hospital, we need to have a low threshold for testing for influenza and for isolating patients suspected of having influenza. For patients with suspected or proven seasonal influenza, transmission precautions are those recommended by the CDC for droplet precautions (www.cdc.gov/ncidod/dhqp/gl_isolation_droplet.html). A face mask is deemed adequate to protect transmission when coming within 3 feet of an infected person. CDC guidelines for pandemic H1N1 recommends airborne-transmission-based precautions for health care workers who are in close contact with patients with proven or possible H1N1 (www.cdc.gov/ncidod/dhqp/gl_isolation_airborne.html). This recommendation implies the use of fit-tested N95 respirators and negative air pressure rooms (if available).
The recent Institute of Medicine report, Respiratory Protection for Healthcare Workers in the Workplace Against Novel H1N1 Influenza A (www.iom.edu/CMS/3740/71769/72967/72970.aspx) endorses the current CDC guidelines and recommends following these guidelines until we have evidence that other forms of protection or guidelines are equally or more effective.
Personally, I am against this requirement because it creates a terrible administrative burden with no proven benefit. Requiring a respirator means requiring fit-testing, and this will negatively affect our ability to deliver patient care. Recent studies have shown that surgical masks may not be as effective11 but are probably sufficient. Lim et al12 reported that 79 (37%) of 212 workers who responded to a survey experienced headaches while wearing N95 masks. This remains a controversial issue.
Besides getting the flu shot, what can one do to avoid getting influenza or transmitting to others?
- Cover your cough (cough etiquette) and sneeze.
- Practice good hand hygiene.
- Avoid close contact with people who are sick.
- Do not go to school or work if sick.
A recent study of influenza in households suggested that having the person with flu and household contacts wear face masks and practice hand hygiene within the first 36 hours decreased transmission of flu within the household.13
The United States does have a national influenza pandemic plan that outlines specific roles in the event of a pandemic, and I urge you to peruse it at www.hhs.gov/pandemicflu/plan/.
RECOGNIZING AND DIAGNOSING INFLUENZA
The familiar signs and symptoms of influenza—fever, cough, muscle aches, and headache—are nonspecific. Call et al14 analyzed the diagnostic accuracy of symptoms and signs of influenza and found that fever and cough during an epidemic suggest but do not confirm influenza, and that sneezing in those over age 60 argues against influenza. They concluded that signs and symptoms can tell us whether a patient has an influenza-like illness, but do not confirm or exclude the diagnosis of influenza: “Clinicians need to consider whether influenza is circulating in their communities, and then either treat patients with influenza-like illness empirically or obtain a rapid influenza test.”14
The signs and symptoms of pandemic 2009 H1N1 are the same as for seasonal flu, except that about 25% of patients with pandemic flu develop gastrointestinal symptoms. It has not been more virulent than seasonal influenza to date.
Should you order a test for influenza?
Most people with influenza are neither tested nor treated. Before ordering a test for influenza, ask, “Does this patient actually have influenza?” Patients diagnosed with “influenza” may have a range of infectious and noninfectious causes, such as vasculitis, endocarditis, or any other condition that can cause a fever and cough.
If I truly suspect influenza, I would still only order a test if the results would change how I manage the patient—for example, a patient being admitted to the hospital where isolation would be required.
Pandemic H1N1 will be detected only as influenza A in our current PCR screen for human influenza. The test does not differentiate between seasonal strains of influenza A (which is resistant to oseltamivir) and pandemic H1N1 (which is susceptible to oseltamivir). This means if you intend to treat, you will have to address further complexity.
Testing for influenza
The clinician should be familiar with the types of tests available. Each test has advantages and disadvantages15:
Rapid antigen assay is a point-of-care test that can give results in 15 minutes but unfortunately is only 20% to 30% sensitive, so a negative result does not exclude the diagnosis. The positive predictive value is high, meaning a positive test means the patient does have the flu.
Direct fluorescent antibody testing takes about 2.5 hours to complete and requires special training for technicians. It has a sensitivity of 47%, a positive predictive value of 95%, and a negative predictive value of 92%.
PCR testing takes about 6 hours and has a sensitivity of 98%, a positive predictive value of 100%, and a negative predictive value of 98%. This is probably the best test, in view of its all-around performance, but it is not a point-of-care test.
Culture takes 2 to 3 days, has a sensitivity of 89%, a positive predictive value of 100%, and a negative predictive value of 88%.
These tests can determine that the patient has influenza A, but a confirmatory test is always required to confirm pandemic H1N1. This confirmatory testing can be done by the CDC, by state public health laboratories, and by commercial reference laboratories.
ANTIVIRAL TREATMENT
Since influenza test results do not specify whether the patient has seasonal or pandemic influenza, treatment decisions are a sticky wicket. Most patients with pandemic H1N1 do not need to be tested or treated.
Several drugs are approved for treating influenza and shorten the duration of symptoms by about 1 day. The earlier the treatment is started, the better: the time of antiviral initiation affects influenza viral load and the duration of viral shedding.3
The neuraminidase inhibitors oseltamivir and zanamivir (Relenza) block release of virus from the cell. Resistance to oseltamivir is emerging in seasonal influenza A, while most pandemic H1N1 strains are susceptible.
Oseltamivir resistance in pandemic H1N1
A total of 11 cases of oseltamivir-resistant pandemic H1N1 have been confirmed worldwide, including 3 in the United States (2 in immunosuppressed patients in Seattle, WA). Ten of the 11 cases occurred with oseltamivir exposure. All involved a histidine-to-tyrosine substitution at position 275 (H275Y) of the neuraminidase gene. Most were susceptible to zanamivir.
Supplies of oseltamivir and zanamivir are limited, so they should be used only in those who will benefit the most, ie, those at higher risk of influenza complications. These include children under 5 years old, adults age 65 and older, children and adolescents on long-term aspirin therapy, pregnant women, patients who have chronic conditions or who are immunosuppressed, and residents of long-term care facilities.
A 69-year-old ohio man with leukemia was treated in another state in late June. During the car trip back to Ohio, he developed a sore throat, fever, cough, and nasal congestion. He was admitted to Cleveland Clinic with a presumed diagnosis of neutropenic fever; his absolute neutrophil count was 0.4 × 109/L (reference range 1.8–7.7). His chest radiograph was normal. He was treated with empiric broad-spectrum antimicrobials. On his second day in the hospital, he was tested for influenza by a polymerase chain reaction (PCR) test, which was positive for influenza A. He was moved to a private room and started on oseltamivir (Tamiflu) and rimantadine (Flumadine). The patient’s previous roommate subsequently tested positive for influenza A, as did two health care workers working on the ward. All patients on the floor received prophylactic oseltamivir.
The patient’s condition worsened, and he subsequently went into respiratory distress with diffuse pulmonary infiltrates. He was transferred to the intensive care unit, where he was intubated. Influenza A was isolated from a bronchoscopic specimen. He subsequently recovered after a prolonged course and was discharged on hospital day 50. Testing by the Ohio Department of Health confirmed that this was the 2009 pandemic influenza A (H1N1) virus.
THE CHALLENGES WE FACE
We are now in the midst of an influenza pandemic of the 2009 influenza A (H1N1) virus, with pandemic defined as “worldwide sustained community transmission.” The circulation of seasonal and 2009 pandemic influenza A (H1N1) strains will make this flu season both interesting and challenging.
The approaches to vaccination, prophylaxis, and treatment will be more complex. As of this writing (mid-September 2009), it is clear that we will be giving two influenza vaccines this season: a trivalent vaccine for seasonal influenza, and a monovalent vaccine for pandemic H1N1. It appears the monovalent vaccine may require only one dose to provide protective immunity.1 Fortunately, the vast majority of cases of pandemic H1N1 are relatively mild and uncomplicated. Still, some people are at higher risk of complications, including young patients, pregnant women, and people with immune deficiency or concomitant health conditions that put them at higher risk of flu-associated complications. Thus, clinicians will need to be educated about whom to test, who needs prophylaxis, and who should not be treated.
As our case demonstrates, unsuspected cases of influenza in hospitalized patients or health care workers working with influenza pose the greatest threat for transmission of influenza within the hospital. Adults hospitalized with influenza tend to present late (more than 48 hours after the onset of symptoms) and tend to have prolonged illness.2 Ambulatory adults shed virus for 3 to 6 days; virus shedding is more prolonged for hospitalized patients. Antiviral agents started within 4 days of illness enhance viral clearance and are associated with a shorter stay.3 Therefore, we should have a low threshold for testing for influenza and for isolating all suspected cases.
This is also creating a paradigm shift for health care workers, who are notorious for working through an illness. If you are sick, stay home! This applies whether you have pandemic H1N1 or something else.
EPIDEMIOLOGY OF PANDEMIC 2009 INFLUENZA A (H1N1) VIRUS
The location of cases can now be found on Google Maps; the US Centers for Disease Control and Prevention (CDC) provides weekly influenza reports at www.cdc.gov/flu/weekly/fluactivity.htm.
Pandemic H1N1 appeared in the spring of 2009, and cases continued to mount all summer in the United States (when influenza is normally absent) and around the world. In Mexico in March and April 2009, 2,155 cases of pneumonia, 821 hospitalizations, and 100 deaths were reported.4
In contrast with seasonal influenza, children and younger adults were hit the hardest in Mexico. The age group 5 through 59 years accounted for 87% of the deaths (usually, they account for about 17%) and 71% of the cases of severe pneumonia (usually, they account for 32%). These observations may be explained in part by the possibility that people who were alive during the 1957 pandemic (which was an H1N1 strain) have some immunity to the new virus. However, the case-fatality rate was highest in people age 65 and older.4
As of July 2009, there were more than 43,000 confirmed cases of pandemic H1N1 in the United States, and actual cases probably exceed 1 million, with more than 400 deaths. An underlying risk factor was identified in more than half of the fatal cases.5 Ten percent of the women who died were pregnant.
Pandemic H1N1 has several distinctive epidemiologic features:
- The distribution of cases is similar across multiple geographic areas.
- The distribution of cases by age group is markedly different than that of seasonal influenza, with more cases in school children and fewer cases in older adults.
- Fewer cases have been reported in older adults, but this group has the highest case-fatality rate.
2009 PANDEMIC H1N1 IS A MONGREL
There are three types of influenza viruses, designated A, B, and C. Type A undergoes antigenic shift (rapid changes) and antigenic drift (gradual changes) from year to year, and so it is the type associated with pandemics. In contrast, type B undergoes antigenic drift only, and type C is relatively stable.
Influenza virus is subtyped on the basis of surface glycoproteins: 16 hemagglutinins and nine neuraminidases. The circulating subtypes change every year; the current circulating human subtypes are a seasonal subtype of H1N1 that is different than the pandemic H1N1 subtype, and H3N2.
The 2009 pandemic H1N1 is a new virus never seen before in North America.6 Genetically, it is a mongrel, coming from three recognized sources (pigs, birds, and humans) which were combined in pigs.7 It is similar to subtypes that circulated in the 1920s through the 1940s.
Most influenza in the Western world comes from Asia every fall, and its arrival is probably facilitated by air travel. The spread is usually unidirectional and is unlikely to contribute to long-term viral evolution.8 It appears that 2009 H1N1 virus is the predominant strain circulating in the current influenza season in the Southern Hemisphere. Virologic studies indicate that the H1N1 virus strain has remained antigenically stable since it appeared in April 2009. Thus, it appears likely that the strain selected by the United States for vaccine manufacturing will match the currently circulating seasonal and pandemic H1N1 strains.
VACCINATION IS THE FIRST LINE OF DEFENSE
In addition to the trivalent vaccine against seasonal influenza, a monovalent vaccine for pandemic H1N1 virus is being produced. The CDC has indicated that 45 million doses of pandemic influenza vaccine are expected in October 2009, with an average of 20 million doses each week thereafter. It is anticipated that half of these will be in multidose vials, that 20% will be in prefilled syringes for children over 5 years old and for pregnant women, and that 20% will be in the form of live-attenuated influenza vaccine (nasal spray). The inhaled vaccine should not be given to children under 2 years old, to children under 5 years old who have recurrent wheezing, or to anyone with severe asthma. Neither vaccine should be given to people allergic to hen eggs, from which the vaccine is produced.
An ample supply of the seasonal trivalent vaccine should be available. Once the CDC has more information about specific product availability of the pandemic H1N1 vaccine, that vaccine will be distributed. It can be given concurrently with seasonal influenza vaccine.
Several definitions should be kept in mind when discussing vaccination strategies. Supply is the number of vaccine doses available for distribution. Availability is the ability of a person recommended to be vaccinated to do so in a local venue. Prioritization is the recommendation to vaccination venues to selectively use vaccine for certain population groups first. Targeting is the recommendation that immunization programs encourage and promote vaccination for certain population groups.
The Advisory Committee on Immunization Practices and the CDC recommend both seasonal and H1N1 vaccinations for anyone 6 months of age or older who is at risk of becoming ill or of transmitting the viruses to others. Based on a review of epidemiologic data, the recommendation is for targeting the following five groups for H1N1 vaccination: children and young adults aged 6 months through 24 years; pregnant women; health care workers and emergency medical service workers; people ages 25 through 64 years who have certain health conditions (eg, diabetes, heart disease, lung disease); and people who live with or care for children younger than 6 months of age. This represents approximately 159 million people in the United States.
If the estimates for the vaccine supply are met, and if pandemic H1N1 vaccine requires only a single injection, there should be no need for prioritization of vaccine. If the supply of pandemic H1N1 vaccine is inadequate, then those groups who are targeted would also receive the first doses of the pandemic H1N1 vaccine. It should be used only with caution after consideration of potential benefits and risks in people who have had Guillain-Barré syndrome during the previous 6 weeks, in people with altered immunocompetence, or in people with medical conditions predisposing to influenza complications.
A mass vaccination campaign involving two separate flu vaccines can pose challenges in execution and messaging for public health officials and politicians. In 1976, an aggressive vaccination program turned into a disaster, as there was no pandemic and the vaccine was associated with adverse effects such as Guillain-Barré syndrome. The government and the medical profession need to prepare for a vaccine controversy and to communicate and continue to explain the plan to the public. As pointed out in a recent op-ed piece,9 we would hope that all expectant women in the fall flu season will get the flu vaccines. We also know that, normally, one in seven pregnancies would be expected to miscarry. The challenge for public health officials and physicians will be to explain to these patients that there may be an association rather than a causal relationship.
In health care workers, the average vaccination rate is only 37%. We should be doing much better. Cleveland Clinic previously increased the rate of vaccination among its employees via a program in which all workers must either be vaccinated or formally declare (on an internal Web site) that they decline to be vaccinated.10 This season, even more resources are being directed at decreasing the barriers to flu vaccinations for our health care workers with the support from hospital leadership.
INFECTION CONTROL IN THE HOSPITAL AND IN THE COMMUNITY
Influenza is very contagious and is spread in droplets via sneezing and coughing (within a 3-foot radius), or via unwashed hands—thus the infection-control campaigns urging you to cover your cough and wash your hands.
As noted, for patients being admitted or transferred to the hospital, we need to have a low threshold for testing for influenza and for isolating patients suspected of having influenza. For patients with suspected or proven seasonal influenza, transmission precautions are those recommended by the CDC for droplet precautions (www.cdc.gov/ncidod/dhqp/gl_isolation_droplet.html). A face mask is deemed adequate to protect transmission when coming within 3 feet of an infected person. CDC guidelines for pandemic H1N1 recommends airborne-transmission-based precautions for health care workers who are in close contact with patients with proven or possible H1N1 (www.cdc.gov/ncidod/dhqp/gl_isolation_airborne.html). This recommendation implies the use of fit-tested N95 respirators and negative air pressure rooms (if available).
The recent Institute of Medicine report, Respiratory Protection for Healthcare Workers in the Workplace Against Novel H1N1 Influenza A (www.iom.edu/CMS/3740/71769/72967/72970.aspx) endorses the current CDC guidelines and recommends following these guidelines until we have evidence that other forms of protection or guidelines are equally or more effective.
Personally, I am against this requirement because it creates a terrible administrative burden with no proven benefit. Requiring a respirator means requiring fit-testing, and this will negatively affect our ability to deliver patient care. Recent studies have shown that surgical masks may not be as effective11 but are probably sufficient. Lim et al12 reported that 79 (37%) of 212 workers who responded to a survey experienced headaches while wearing N95 masks. This remains a controversial issue.
Besides getting the flu shot, what can one do to avoid getting influenza or transmitting to others?
- Cover your cough (cough etiquette) and sneeze.
- Practice good hand hygiene.
- Avoid close contact with people who are sick.
- Do not go to school or work if sick.
A recent study of influenza in households suggested that having the person with flu and household contacts wear face masks and practice hand hygiene within the first 36 hours decreased transmission of flu within the household.13
The United States does have a national influenza pandemic plan that outlines specific roles in the event of a pandemic, and I urge you to peruse it at www.hhs.gov/pandemicflu/plan/.
RECOGNIZING AND DIAGNOSING INFLUENZA
The familiar signs and symptoms of influenza—fever, cough, muscle aches, and headache—are nonspecific. Call et al14 analyzed the diagnostic accuracy of symptoms and signs of influenza and found that fever and cough during an epidemic suggest but do not confirm influenza, and that sneezing in those over age 60 argues against influenza. They concluded that signs and symptoms can tell us whether a patient has an influenza-like illness, but do not confirm or exclude the diagnosis of influenza: “Clinicians need to consider whether influenza is circulating in their communities, and then either treat patients with influenza-like illness empirically or obtain a rapid influenza test.”14
The signs and symptoms of pandemic 2009 H1N1 are the same as for seasonal flu, except that about 25% of patients with pandemic flu develop gastrointestinal symptoms. It has not been more virulent than seasonal influenza to date.
Should you order a test for influenza?
Most people with influenza are neither tested nor treated. Before ordering a test for influenza, ask, “Does this patient actually have influenza?” Patients diagnosed with “influenza” may have a range of infectious and noninfectious causes, such as vasculitis, endocarditis, or any other condition that can cause a fever and cough.
If I truly suspect influenza, I would still only order a test if the results would change how I manage the patient—for example, a patient being admitted to the hospital where isolation would be required.
Pandemic H1N1 will be detected only as influenza A in our current PCR screen for human influenza. The test does not differentiate between seasonal strains of influenza A (which is resistant to oseltamivir) and pandemic H1N1 (which is susceptible to oseltamivir). This means if you intend to treat, you will have to address further complexity.
Testing for influenza
The clinician should be familiar with the types of tests available. Each test has advantages and disadvantages15:
Rapid antigen assay is a point-of-care test that can give results in 15 minutes but unfortunately is only 20% to 30% sensitive, so a negative result does not exclude the diagnosis. The positive predictive value is high, meaning a positive test means the patient does have the flu.
Direct fluorescent antibody testing takes about 2.5 hours to complete and requires special training for technicians. It has a sensitivity of 47%, a positive predictive value of 95%, and a negative predictive value of 92%.
PCR testing takes about 6 hours and has a sensitivity of 98%, a positive predictive value of 100%, and a negative predictive value of 98%. This is probably the best test, in view of its all-around performance, but it is not a point-of-care test.
Culture takes 2 to 3 days, has a sensitivity of 89%, a positive predictive value of 100%, and a negative predictive value of 88%.
These tests can determine that the patient has influenza A, but a confirmatory test is always required to confirm pandemic H1N1. This confirmatory testing can be done by the CDC, by state public health laboratories, and by commercial reference laboratories.
ANTIVIRAL TREATMENT
Since influenza test results do not specify whether the patient has seasonal or pandemic influenza, treatment decisions are a sticky wicket. Most patients with pandemic H1N1 do not need to be tested or treated.
Several drugs are approved for treating influenza and shorten the duration of symptoms by about 1 day. The earlier the treatment is started, the better: the time of antiviral initiation affects influenza viral load and the duration of viral shedding.3
The neuraminidase inhibitors oseltamivir and zanamivir (Relenza) block release of virus from the cell. Resistance to oseltamivir is emerging in seasonal influenza A, while most pandemic H1N1 strains are susceptible.
Oseltamivir resistance in pandemic H1N1
A total of 11 cases of oseltamivir-resistant pandemic H1N1 have been confirmed worldwide, including 3 in the United States (2 in immunosuppressed patients in Seattle, WA). Ten of the 11 cases occurred with oseltamivir exposure. All involved a histidine-to-tyrosine substitution at position 275 (H275Y) of the neuraminidase gene. Most were susceptible to zanamivir.
Supplies of oseltamivir and zanamivir are limited, so they should be used only in those who will benefit the most, ie, those at higher risk of influenza complications. These include children under 5 years old, adults age 65 and older, children and adolescents on long-term aspirin therapy, pregnant women, patients who have chronic conditions or who are immunosuppressed, and residents of long-term care facilities.
- Greenberg MA, Lai MH, Hartel GF. Response after one dose of a monovalent influenza A (H1N1) 2009 vaccine—preliminary report. N Engl J Med 2009;361doi:10.1056/NEJMoa0907413 [published online ahead of print].
- Ison M. Influenza in hospitalized adults: gaining insight into a significant problem. J Infect Dis 2009; 200:485–488.
- Lee N, Chan PKS, Hui DSC, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009; 200:492–500.
- Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674–679.
- Vaillant L, La Ruche G, Tarantola A, Barboza P; for the Epidemic Intelligence Team at InVS. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill 2009; 14(33):1–6. Available online at www.eurosurveillance.org/ViewArticle.aspx?ArticleID=19309.
- Zimmer SM, Burke DS. Historical perspective—emergence of influenza A (H1N1) viruses. N Engl J Med 2009; 361:279–285.
- Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197–201.
- Russell CA, Jones TC, Barr IG, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008; 320:340–346.
- Allen A. Prepare for a vaccine controversy. New York Times. 9/1/2009.
- Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37.
- Johnson DF, Druce JD, Birch C, Grayson ML. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis 2009; 49:275–277.
- Lim EC, Seet RC, Lee KH, Wilder-Smith EP, Chuah BY, Ong BK. Headaches and the N95 face-mask amongst healthcare providers. Acta Neurol Scand 2006; 113:199–202.
- Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a randomized trial. Ann Intern Med 2009; 151(6 Oct) [published online ahead of print].
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005; 293:987–997.
- Ginocchio CC, Zhang F, Manji R, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol 2009; 45:191–195.
- US Centers for Disease Control and Prevention. Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients—Seattle, Washington, 2009. MMWR 2009; 58:893–896.
- Greenberg MA, Lai MH, Hartel GF. Response after one dose of a monovalent influenza A (H1N1) 2009 vaccine—preliminary report. N Engl J Med 2009;361doi:10.1056/NEJMoa0907413 [published online ahead of print].
- Ison M. Influenza in hospitalized adults: gaining insight into a significant problem. J Infect Dis 2009; 200:485–488.
- Lee N, Chan PKS, Hui DSC, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009; 200:492–500.
- Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674–679.
- Vaillant L, La Ruche G, Tarantola A, Barboza P; for the Epidemic Intelligence Team at InVS. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill 2009; 14(33):1–6. Available online at www.eurosurveillance.org/ViewArticle.aspx?ArticleID=19309.
- Zimmer SM, Burke DS. Historical perspective—emergence of influenza A (H1N1) viruses. N Engl J Med 2009; 361:279–285.
- Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197–201.
- Russell CA, Jones TC, Barr IG, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008; 320:340–346.
- Allen A. Prepare for a vaccine controversy. New York Times. 9/1/2009.
- Bertin M, Scarpelli M, Proctor AW, et al. Novel use of the intranet to document health care personnel participation in a mandatory influenza vaccination reporting program. Am J Infect Control 2007; 35:33–37.
- Johnson DF, Druce JD, Birch C, Grayson ML. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis 2009; 49:275–277.
- Lim EC, Seet RC, Lee KH, Wilder-Smith EP, Chuah BY, Ong BK. Headaches and the N95 face-mask amongst healthcare providers. Acta Neurol Scand 2006; 113:199–202.
- Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a randomized trial. Ann Intern Med 2009; 151(6 Oct) [published online ahead of print].
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005; 293:987–997.
- Ginocchio CC, Zhang F, Manji R, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol 2009; 45:191–195.
- US Centers for Disease Control and Prevention. Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients—Seattle, Washington, 2009. MMWR 2009; 58:893–896.
KEY POINTS
- Vaccination this season will require two vaccines: a trivalent vaccine for seasonal influenza and a monovalent vaccine for 2009 pandemic influenza A (H1N1).
- Recent studies indicate that the monovalent vaccine for 2009 pandemic influenza A (H1N1) may require only one injection.
- To date, 2009 pandemic influenza A (H1N1) virus has not been exceptionally virulent and differs from conventional influenza in that it seems to disproportionately affect children and young adults. Pregnant women are at a higher risk of complications.
- Most people with 2009 pandemic influenza A (H1N1) do not need to be tested, treated, or seen by a clinician.
- Antiviral drugs should be reserved only for those at high risk of influenza complications.
Health care worker, vaccinate thyself: Toward better compliance with influenza vaccination
Pandemic Influenza and the Hospitalist
Background
Influenza viruses are among the most common respiratory viral infections in humans. There are two major types of human influenza viruses, A and B, with influenza A strains responsible for seasonal or pandemic influenza. Influenza illness is characterized by fever, lower respiratory and often upper respiratory symptoms, myalgia, and malaise and occurs seasonally in temperate climates between late fall and early spring. The average flu season in the United States is marked by 30,000‐40,000 deaths, primarily in elderly patients with significant comorbidity and in the very young. Many of these deaths are caused by secondary bacterial pneumonias. Long interpandemic periods, including the current one of almost 40 years, involve minor mutations of the predominant influenza strain from year to year. Typically, adequate time exists to predict the prevailing strain with reasonable accuracy and to tailor a vaccine accordingly. Periodically an influenza pandemic involving a novel influenza strain emerges, attended by greater‐than‐expected morbidity and mortality.
All influenza viruses are subtyped on the basis of two surface glycoproteins. One of these, hemagglutinin (H), is responsible for viral cell entry; whereas the other, neuraminidase (N), facilitates release of the virus from infected cells, thus allowing perpetuation and amplification of infection. Antigenic drift is the ongoing process of genetic mutations that lead to new strains demonstrating variable change in antigenicity and is the basis for the annual updating of vaccine strains. Antigenic shift is the emergence of a novel influenza A subtype among humans, usually as the result of a recombination event. This radical change is necessary but not sufficient to initiate pandemic influenza, with efficient transmission from person to person also a critical feature. Pandemic influenza strains arise in 1 of 2 fashions. Genetic reassortment may occur when a mammalian host (human or porcine) is infected with both an avian and a human influenza virus, with subsequent dramatic movement into human populations, the source of the 1957 and 1968 pandemics. Alternatively, a novel virus may, after sufficient mutation, move directly from the avian population to humans, as appears to have occurred in 1918.
The 1918‐19 Pandemic
Abruptly in 1918, an influenza pandemic of seemingly unprecedented severity swept the world. Although disagreement remains regarding the source of the outbreak (China, the front lines of World War I, and even the United States have all been suggested), within 6‐9 months essentially the entire globe had been affected. Unlike more typical influenza seasons, the virus preferentially infected previously healthy young individuals, with those aged 15‐40 bearing the brunt of the illness. US military training installations, overcrowded with troops staging for service on the European front, played a particularly ill‐fated role in the pandemic as it swept through the United States.
Estimates of the pandemic's worldwide impact on mortality are sketchy at best, but many authorities believe that at least 50 million deaths resulted, with some suggesting a figure as high as 100 million. In the United States the virus was responsible for an estimated 700,000 deaths, with an untold burden of morbidity. Economic and social disruption was the norm in many areas, with widespread closure of businesses and schools and suspension of public gatherings of any kind. Many communities were simply overwhelmed by the sheer numbers of dying individuals. In Philadelphia, steam shovels were used to dig mass graves for influenza victims.1 The pandemic's effect on the health care system was likewise profound. Most hospitals counted their own physicians and nurses among those who died during the pandemic, and many of the health care workers who succumbed were infected in the course of caring for influenza patients. Overall, an estimated 2%‐3% of those infected with the virus died, a far higher percentage than is seen during interpandemic seasons. Strikingly, the vast majority of deaths do not appear to have resulted from secondary bacterial pneumonias, but rather to have been directly virally mediated through ARDS, a necrotizing viral pneumonia, or both.
The mystery of the 1918 pandemic has recently been partially unlocked, with the successful sequencing of the entire RNA genome of strains recovered from pathology tissue of two soldiers, as well as from lung tissue of a victim frozen in Alaskan permafrost since 1918.2, 3 The data suggest that the 1918 virus was derived from an avian source. Notably, some of the same changes in the polymerase proteins have been found in the highly pathogenic H5N1 viruses.
Avian Influenza Viruses
Influenza viruses that primarily infect birds are characterized as avian influenza viruses. These are always type A and are classified as either of low or high pathogenicity on the basis of the severity of the illness they cause in birds. The currently circulating H5N1 avian viruses are highly pathogenic.
Avian influenza viruses do not usually infect humans; however, several instances of human infections have been reported since 1997. The 1997 Hong Kong outbreak of avian (H5N1) influenza in 18 humans resulted in 6 deaths and was a seminal event that provided evidence that avian influenza viruses can infect people. It also provided the epidemiologic link between avian influenza infection in poultry with disease in humans and was proclaimed as a pandemic warning. These sentinel human infections led to the culling of the entire Hong Kong poultry population, with no subsequent human infection reported at that time. In 2003, more than 80 cases of avian influenza A (H7N7) illness occurred in the Netherlands among persons who handled infected poultry. Sustained human‐to‐human transmission did not occur in this or other outbreaks of avian influenza to date.
Since 2003, sporadic human cases of H5N1 have occurred, most recently reported from Turkey and Iraq. Human cases have also occurred in Vietnam, China, Cambodia, Thailand, and Indonesia, with a total of 173 reported cases and a case fatality rate exceeding 50% as of this writing.4 This mortality rate may be artificially inflated, as less severe cases have certainly gone unreported. All countries reporting human avian influenza diseases since 2003 have had concurrent epizoonotics in birds (both poultry and migratory birds).
Human cases of H5N1 influenza illness have been characterized by high fever and symptoms in the lower respiratory tract, as would be expected. Less predictable has been the presence of watery diarrhea in many patients and of abdominal and pleuritic pain and bleeding from the nose and gums in some. Sputum production has been variably present, and hemoptysis has been seen in some individuals. Most patients have had clinical and radiological evidence of pneumonia at the time they sought medical care, and progression to ARDS and multiorgan failure has been common. The majority of patients to date have required the initiation of mechanical ventilation early in their hospital course. Laboratory studies have typically shown lymphopenia, thrombocytopenia, and, in many cases, modestly elevated transaminase levels.5 Notably, the currently predominant strain of H5N1 (Z strain) is resistant to the M2 ion channel inhibitors amantadine and rimantadine but is susceptible to the newer class of neuraminidase inhibitors, zanamivir (Relenza) and oseltamivir (Tamiflu). Neuraminidase inhibitors and corticosteroids have been used to treat patients, although their efficacy in this setting is unclear. To date, virtually all cases appear to have been transmitted directly from poultry, although person‐to‐person transmission appears likely to have occurred in at least one family in Thailand.6 A recent study of the 14 clusters of avian influenza among humans emphasized the lack of sustained person‐to‐person transmission of H5N1 to date.7
Three factors are necessary for the emergence of a pandemic influenza strain: the ability to infect humans, a novel genetic makeup, and the ability for sustained transmission between people. A virus that in addition proves highly virulent, as did the 1918‐19 H1N1 strain, essentially creates the perfect storm. H5N1 influenza has currently fulfilled 2 of these 3 criteria. The virus is highly pathogenic, although how much of this fitness would be sacrificed with mutation to a more transmissible strain is uncertain. As many have observed, whether there will be another influenza pandemic does not seem in doubt; rather, it is when such a pandemic will occur and whether the pandemic will be caused by H5N1 or another influenza virus, that are the questions.
Potential Effects of the Next Pandemic
The global and national effects of an influenza pandemic will vary in direct proportion to the virulence of the circulating viral strain, but if such a virus is highly virulent, significant and perhaps severe economic and social disruption are likely.
The global economic impact has been estimated to be $800 billion with anticipated quarantines and interruption in global trade. On a national level, it has been estimated that in the United States a pandemic virus whose severity is comparable to that of the 1968 Hong Kong influenza pandemic would lead to approximately 200,000 deaths and 700,000 hospitalizations, of which roughly 100,000 would require treatment in intensive care unit settings. A more virulent strain, similar to that of the 1918‐19 pandemic, might easily result in 1 million deaths; with the number of patients hospitalized approaching 10 million, well over 1 million of which would require ICU‐level care. As an estimated 75% of the 105,000 ventilators in this country are in use at any given time under normal circumstances, the potential for demand to greatly outstrip supply is evident.8 Depending on the severity of a pandemic, suspension or curtailment of international trade and travel could be reasonably likely. Although the World Health Organization has recommended against closing borders or quarantining countries even in the throes of a pandemic, the prospect of this occurring does not seem implausible. In a worst‐case scenario, even the type of national and international chaos envisioned in the Dark Winter smallpox planning exercise might occur.9
Fortress America Versus Containment Strategies
Although the pandemic influenza plan calls for stockpiling antiviral drugs and increasing vaccine production capabilities, the most effective plan for pandemic preparedness may involve a surveillance and containment strategy. No country has enough medicines or vaccines to control a widespread outbreak of pandemic avian influenza. The best solution to prevention of a pandemic is stopping any virus from spreading in the first place. Increased surveillance for avian influenza among poultry and migratory birds in key Asian countries, along with provision of funds to compensate farmers for culling of potentially infected flocks, would align incentives for early detection and eradication. Containing an initial outbreak wherever it occurs is the best defense against a pandemic. Notably, China is thought to be a potential hot zone for emergence of pandemic avian influenza. China is not only the most populous nation in the world but has one quarter of the world's chickens, two thirds of the world's domesticated ducks, and 90% of the world's domesticated geese.
The challenges of biosecurity (protecting humans against animal‐borne diseases such as bird flu) in developing countries include the reality that populations living in close proximity to poultry are also the most illiterate and impoverished, with the most limited access to health care. The recent introduction of H5N1 into Europe has heightened surveillance efforts in the United States. The introduction of H5N1 into the United States may occur through movement of migratory birds and/or importation of exotic birds. The surveillance system has been expanded to include sampling for the influenza virus not only in poultry but also in bodies of water, as the virus is shed in bird feces.
Pandemic Planning
In the setting of a severe pandemic, hospitals will face an enormous burden of patients, with a huge influx of individuals requiring both intensive care unit as well as regular nursing floor care. At the local height of such a pandemic, the ability to successfully discharge every patient whose condition will permit this to the community or elsewhere will be critical, and almost certainly hospitals will need to expand to accept more patients than they are normally configured to hold. Hospitals staffs, particularly nurses and physicians, will be required to handle very large patient censuses. Among medical staffs, emergency physicians, hospitalists, critical care specialists, and infectious disease specialists will certainly be called on to play leading roles, much as they were during and in the aftermath of Hurricane Katrina recently. Despite all of the above, the ability of existing hospitals to accommodate all gravely ill patients may be outstripped, and auxiliary hospitals in schools and other public edifices may need to be established. Hospitalists are likely to be called on to play a major role in such temporary hospitals. The frustration and anguish of not being able to provide a standard level of care to patients (for example, being forced to triage which patients are most deserving of mechanical ventilation) should not be underestimated.
Although characterized by a relatively limited number of patients, the 2003 severe acute respiratory syndrome (SARS) outbreak in Toronto, Ontario, Canada, presented some of the same challenges that will be encountered in a virulent influenza pandemic. These include the need to quickly and drastically modify the usual emergency department and inpatient procedures, as hospitals initially serve to amplify the epidemic, as well as the additional stressor of health care workers becoming ill as a result of work‐related exposure. That fewer than 400 cases of SARS pushed the medical system of one of North America's largest cities nearly to its breaking point is both sobering and instructive.10, 11 Interested readers are directed to an excellent summary of lessons learned from the SARS outbreak, most of which are widely applicable to preparations for future infectious epidemics.12
Infection Control
Although the CDC and other Web sites currently recommend airborne isolation (respiratory personal protection) for avian influenza in humans, there is not strong epidemiologic evidence of transmission other than via droplets (the transmission mode of human influenza). The emergence of a limited number of cases of avian influenza in the United States would allow employment of airborne isolation measures; but in the event of a larger outbreak, the use of surgical masks and the practice of good hand hygiene would be sufficient by health care workers caring for persons with suspected or proven disease.
The CDC recently released proposed changes to help prevent disease outbreaks from contacts of those exposed to ill persons on airplanes. Proposed guidelines would require airlines to maintain computerized lists of passengers taken at point of departure in order to facilitate tracking of contacts and implementation of quarantine if necessary. These measures are part of pandemic planning and result from problems in tracking passengers on planes with SARS cases. By executive order, imposition of quarantine is limited to 9 diseases: cholera, diphtheria, smallpox, yellow fever, viral hemorrhagic fevers (eg, Ebola), plague, infectious tuberculosis, SARS and influenza caused by new strains with pandemic potential.
What Can Be Done?
Although valuable time has elapsed to prepare for the possibility of an H5N1 influenza pandemic, the US and global communities are presently taking the threat seriously and are engaging in a variety of activities to prepare for such an eventuality. Although currently available influenza vaccines do not provide any appreciable protection against H5N1, significant work is under way to develop an effective vaccine; with Chiron and sanofi pasteur preparing vaccine trials in association with the National Institute of Allergy and Infectious Diseases. Current influenza vaccine production is hampered by use of obsolete egg‐based manufacturing processes requiring 6 months, along with a limited capacity to manufacture adequate vaccine supplies even in many usual influenza seasons. The herculean task of providing hundreds of millions of doses of vaccine as soon as possible after the emergence of a pandemic strain, as daunting as it is, is further complicated by the fact that a successful H5N1 vaccine would not necessarily be effective against a strain that mutated sufficiently to move efficiently from person to person. Nonetheless, even partially solving these problems will pay dividends, whether or not H5N1 proves to be responsible for the next pandemic.
Given these difficulties with vaccine development and production, the backbone of any successful early response to a pandemic in the near future will be development of an adequate stockpile of antiviral medication, accompanied by a successful plan to distribute the drug when and where disease erupts. Despite uncertainties regarding their effectiveness as well as questions regarding optimal dose and duration in the setting of avian influenza, the neuraminidase inhibitors are the current drugs of choice. Of the 2 currently available agents, oseltamivir is the preferred drug for pandemic use, given its oral administration,. Unfortunately, the ability to manufacture the drug in sufficient quantities to stockpile has thus far proved problematic. Roche, the manufacturer of Tamiflu, has recently opened a new manufacturing plant and has stated that it can increase its current production of 55 million doses per year to 300 million doses by 2007. We do not recommend a role for personal stockpiling of neuraminidase inhibitors. Concerns include a shortage of the drug for seasonal influenza, absence of a pandemic at present, ignorance regarding the efficacy and optimal dose for H5N1, inappropriate use by individuals, and inequitable distribution. Recent case reports of oseltamivir resistance emerging during prophylaxis13 and treatment14 are of potential concern but do not alter current recommendations.
What can be done locally and specifically, and what can hospitalists do to prepare? First, although we are not sure that Dr Michael Osterholm's goal that planning for a pandemic must be on the agenda of every public health agency, school board, manufacturing plant, investment firm, mortuary, state legislature, and food distributor8 is entirely realistic, every hospital clearly needs to include pandemic influenza as a significant part of its disaster preparedness plan. Such planning will have broad overlap with planning for other potential disasters, including bioterrorist attacks, SARS outbreaks, and others. Hospitals must develop a plan for surge capacity, and such a plan should include not only coordination with other local hospitals, but also planning with local communities to identify sites where temporary flu hospitals can be established. Within hospital medicine groups, emergency staffing plans should be established before pandemic influenza (or another disaster) strikes. Such staffing plans need to include the ability to care for a much higher than normal number of patients for an extended period. Conceivably, a large number of patients will need to be manually ventilated for prolonged periods, which of course will tax the resources of any institution. Prompt discharge of all patients stable enough to leave the hospital will be critical, and given the investment of most hospital medicine groups in hospital throughput issues under normal conditions, much of the responsibility for helping to create beds during a crisis will inevitably fall on the shoulders of hospitalists.
Experiences during and shortly after Hurricane Katrina served to underscore that issues such as physical and mental fatigue, concern for the safety of family members, lack of supplies, communication difficulties, and absenteeism all add additional layers of complexity to the task of providing hospital care under extraordinary conditions such as during a natural disaster. These lessons can and should be extended to a major epidemic. This disaster also showed the importance of military involvement in the response to disasters that exceed local and state capabilities. The primary objective of the federal government in responding to disaster is to maintain security and essential services while preventing chaos. A pandemic of virulent influenza will raise the stakes still further, as physicians and nurses become casualties themselves. Despite these challenges, we are confident that the vast majority of hospitalists and other health care workers will rise to the occasion, and just as during the peri‐Katrina period, stories of selflessness and heroism will be de rigueur. Appropriate advance planning on all levels will serve to reduce the morbidity and mortality associated with the next pandemic and will help to ensure that health care workers do not sacrifice needlessly.0
|
1. World Health Organization (WHO) Website: |
|
2. Centers for Disease Control and Prevention (CDC): |
|
3. U.S. Government Avian Influenza Website: |
|
4. U.S. Department of Health and Human Services Pandemic Influenza Plan: |
|
5. Infectious Diseases Society of America (IDSA) Website: |
- .The Great Influenza.New York, NY:Viking Penguin,2004.
- ,,,,,.Characterization of the 1918 influenza virus polymerase genes.Nature.2005;437:889–893.
- ,,, et al.Characterization of the reconstructed 1918 Spanish influenza pandemic virus.Science.2005;310:77–80.
- WHO Epidemic and Pandemic Alert and Response. Confirmed cases of avian influenza A (H5N1). Available at http://www.who.int/csr/disease/avian_influenza/country/en/index.html. Accessed on February 28,2006.
- Writing Committee of the WHO Consultation on Human Influenza A/H5.Avian influenza A (H5N1) infection in humans.N Engl J Med.2005;353:1374–1385.
- ,,, et al.Probable person‐to‐person transmission of avian influenza A (H5N1).N Engl J Med.2005;352:333–40.
- ,,, et al.Family clustering of avian influenza A (H5N1).EID.2005;11:1799–1801.
- .Preparing for the next pandemic.N Engl J Med.2005;352:1839–1842.
- Center for Biosecurity. Dark Winter overview. Available at http://www.upmc‐biosecurity.org/pages/events/dark_winter/dark_winter.html. Accessed November 28,2005.
- ,,, et al.SARS outbreak in the Greater Toronto Area: the emergency department experience.CMAJ.2004;171:1342–1344.
- ,.Severe acute respiratory syndrome and critical care medicine: The Toronto experience.Crit Care Med.2005;33(suppl):S53–S60.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291:2483–2487.
- ,,, et al.Avian flu: Isolation of drug‐resistant H5N1 virus.Nature.2005;438:754.
- ,,, et al.Oseltamivir resistance during treatment of influenza A (H5N1) infection.N Engl J Med.2005;353:2667–2672.
Background
Influenza viruses are among the most common respiratory viral infections in humans. There are two major types of human influenza viruses, A and B, with influenza A strains responsible for seasonal or pandemic influenza. Influenza illness is characterized by fever, lower respiratory and often upper respiratory symptoms, myalgia, and malaise and occurs seasonally in temperate climates between late fall and early spring. The average flu season in the United States is marked by 30,000‐40,000 deaths, primarily in elderly patients with significant comorbidity and in the very young. Many of these deaths are caused by secondary bacterial pneumonias. Long interpandemic periods, including the current one of almost 40 years, involve minor mutations of the predominant influenza strain from year to year. Typically, adequate time exists to predict the prevailing strain with reasonable accuracy and to tailor a vaccine accordingly. Periodically an influenza pandemic involving a novel influenza strain emerges, attended by greater‐than‐expected morbidity and mortality.
All influenza viruses are subtyped on the basis of two surface glycoproteins. One of these, hemagglutinin (H), is responsible for viral cell entry; whereas the other, neuraminidase (N), facilitates release of the virus from infected cells, thus allowing perpetuation and amplification of infection. Antigenic drift is the ongoing process of genetic mutations that lead to new strains demonstrating variable change in antigenicity and is the basis for the annual updating of vaccine strains. Antigenic shift is the emergence of a novel influenza A subtype among humans, usually as the result of a recombination event. This radical change is necessary but not sufficient to initiate pandemic influenza, with efficient transmission from person to person also a critical feature. Pandemic influenza strains arise in 1 of 2 fashions. Genetic reassortment may occur when a mammalian host (human or porcine) is infected with both an avian and a human influenza virus, with subsequent dramatic movement into human populations, the source of the 1957 and 1968 pandemics. Alternatively, a novel virus may, after sufficient mutation, move directly from the avian population to humans, as appears to have occurred in 1918.
The 1918‐19 Pandemic
Abruptly in 1918, an influenza pandemic of seemingly unprecedented severity swept the world. Although disagreement remains regarding the source of the outbreak (China, the front lines of World War I, and even the United States have all been suggested), within 6‐9 months essentially the entire globe had been affected. Unlike more typical influenza seasons, the virus preferentially infected previously healthy young individuals, with those aged 15‐40 bearing the brunt of the illness. US military training installations, overcrowded with troops staging for service on the European front, played a particularly ill‐fated role in the pandemic as it swept through the United States.
Estimates of the pandemic's worldwide impact on mortality are sketchy at best, but many authorities believe that at least 50 million deaths resulted, with some suggesting a figure as high as 100 million. In the United States the virus was responsible for an estimated 700,000 deaths, with an untold burden of morbidity. Economic and social disruption was the norm in many areas, with widespread closure of businesses and schools and suspension of public gatherings of any kind. Many communities were simply overwhelmed by the sheer numbers of dying individuals. In Philadelphia, steam shovels were used to dig mass graves for influenza victims.1 The pandemic's effect on the health care system was likewise profound. Most hospitals counted their own physicians and nurses among those who died during the pandemic, and many of the health care workers who succumbed were infected in the course of caring for influenza patients. Overall, an estimated 2%‐3% of those infected with the virus died, a far higher percentage than is seen during interpandemic seasons. Strikingly, the vast majority of deaths do not appear to have resulted from secondary bacterial pneumonias, but rather to have been directly virally mediated through ARDS, a necrotizing viral pneumonia, or both.
The mystery of the 1918 pandemic has recently been partially unlocked, with the successful sequencing of the entire RNA genome of strains recovered from pathology tissue of two soldiers, as well as from lung tissue of a victim frozen in Alaskan permafrost since 1918.2, 3 The data suggest that the 1918 virus was derived from an avian source. Notably, some of the same changes in the polymerase proteins have been found in the highly pathogenic H5N1 viruses.
Avian Influenza Viruses
Influenza viruses that primarily infect birds are characterized as avian influenza viruses. These are always type A and are classified as either of low or high pathogenicity on the basis of the severity of the illness they cause in birds. The currently circulating H5N1 avian viruses are highly pathogenic.
Avian influenza viruses do not usually infect humans; however, several instances of human infections have been reported since 1997. The 1997 Hong Kong outbreak of avian (H5N1) influenza in 18 humans resulted in 6 deaths and was a seminal event that provided evidence that avian influenza viruses can infect people. It also provided the epidemiologic link between avian influenza infection in poultry with disease in humans and was proclaimed as a pandemic warning. These sentinel human infections led to the culling of the entire Hong Kong poultry population, with no subsequent human infection reported at that time. In 2003, more than 80 cases of avian influenza A (H7N7) illness occurred in the Netherlands among persons who handled infected poultry. Sustained human‐to‐human transmission did not occur in this or other outbreaks of avian influenza to date.
Since 2003, sporadic human cases of H5N1 have occurred, most recently reported from Turkey and Iraq. Human cases have also occurred in Vietnam, China, Cambodia, Thailand, and Indonesia, with a total of 173 reported cases and a case fatality rate exceeding 50% as of this writing.4 This mortality rate may be artificially inflated, as less severe cases have certainly gone unreported. All countries reporting human avian influenza diseases since 2003 have had concurrent epizoonotics in birds (both poultry and migratory birds).
Human cases of H5N1 influenza illness have been characterized by high fever and symptoms in the lower respiratory tract, as would be expected. Less predictable has been the presence of watery diarrhea in many patients and of abdominal and pleuritic pain and bleeding from the nose and gums in some. Sputum production has been variably present, and hemoptysis has been seen in some individuals. Most patients have had clinical and radiological evidence of pneumonia at the time they sought medical care, and progression to ARDS and multiorgan failure has been common. The majority of patients to date have required the initiation of mechanical ventilation early in their hospital course. Laboratory studies have typically shown lymphopenia, thrombocytopenia, and, in many cases, modestly elevated transaminase levels.5 Notably, the currently predominant strain of H5N1 (Z strain) is resistant to the M2 ion channel inhibitors amantadine and rimantadine but is susceptible to the newer class of neuraminidase inhibitors, zanamivir (Relenza) and oseltamivir (Tamiflu). Neuraminidase inhibitors and corticosteroids have been used to treat patients, although their efficacy in this setting is unclear. To date, virtually all cases appear to have been transmitted directly from poultry, although person‐to‐person transmission appears likely to have occurred in at least one family in Thailand.6 A recent study of the 14 clusters of avian influenza among humans emphasized the lack of sustained person‐to‐person transmission of H5N1 to date.7
Three factors are necessary for the emergence of a pandemic influenza strain: the ability to infect humans, a novel genetic makeup, and the ability for sustained transmission between people. A virus that in addition proves highly virulent, as did the 1918‐19 H1N1 strain, essentially creates the perfect storm. H5N1 influenza has currently fulfilled 2 of these 3 criteria. The virus is highly pathogenic, although how much of this fitness would be sacrificed with mutation to a more transmissible strain is uncertain. As many have observed, whether there will be another influenza pandemic does not seem in doubt; rather, it is when such a pandemic will occur and whether the pandemic will be caused by H5N1 or another influenza virus, that are the questions.
Potential Effects of the Next Pandemic
The global and national effects of an influenza pandemic will vary in direct proportion to the virulence of the circulating viral strain, but if such a virus is highly virulent, significant and perhaps severe economic and social disruption are likely.
The global economic impact has been estimated to be $800 billion with anticipated quarantines and interruption in global trade. On a national level, it has been estimated that in the United States a pandemic virus whose severity is comparable to that of the 1968 Hong Kong influenza pandemic would lead to approximately 200,000 deaths and 700,000 hospitalizations, of which roughly 100,000 would require treatment in intensive care unit settings. A more virulent strain, similar to that of the 1918‐19 pandemic, might easily result in 1 million deaths; with the number of patients hospitalized approaching 10 million, well over 1 million of which would require ICU‐level care. As an estimated 75% of the 105,000 ventilators in this country are in use at any given time under normal circumstances, the potential for demand to greatly outstrip supply is evident.8 Depending on the severity of a pandemic, suspension or curtailment of international trade and travel could be reasonably likely. Although the World Health Organization has recommended against closing borders or quarantining countries even in the throes of a pandemic, the prospect of this occurring does not seem implausible. In a worst‐case scenario, even the type of national and international chaos envisioned in the Dark Winter smallpox planning exercise might occur.9
Fortress America Versus Containment Strategies
Although the pandemic influenza plan calls for stockpiling antiviral drugs and increasing vaccine production capabilities, the most effective plan for pandemic preparedness may involve a surveillance and containment strategy. No country has enough medicines or vaccines to control a widespread outbreak of pandemic avian influenza. The best solution to prevention of a pandemic is stopping any virus from spreading in the first place. Increased surveillance for avian influenza among poultry and migratory birds in key Asian countries, along with provision of funds to compensate farmers for culling of potentially infected flocks, would align incentives for early detection and eradication. Containing an initial outbreak wherever it occurs is the best defense against a pandemic. Notably, China is thought to be a potential hot zone for emergence of pandemic avian influenza. China is not only the most populous nation in the world but has one quarter of the world's chickens, two thirds of the world's domesticated ducks, and 90% of the world's domesticated geese.
The challenges of biosecurity (protecting humans against animal‐borne diseases such as bird flu) in developing countries include the reality that populations living in close proximity to poultry are also the most illiterate and impoverished, with the most limited access to health care. The recent introduction of H5N1 into Europe has heightened surveillance efforts in the United States. The introduction of H5N1 into the United States may occur through movement of migratory birds and/or importation of exotic birds. The surveillance system has been expanded to include sampling for the influenza virus not only in poultry but also in bodies of water, as the virus is shed in bird feces.
Pandemic Planning
In the setting of a severe pandemic, hospitals will face an enormous burden of patients, with a huge influx of individuals requiring both intensive care unit as well as regular nursing floor care. At the local height of such a pandemic, the ability to successfully discharge every patient whose condition will permit this to the community or elsewhere will be critical, and almost certainly hospitals will need to expand to accept more patients than they are normally configured to hold. Hospitals staffs, particularly nurses and physicians, will be required to handle very large patient censuses. Among medical staffs, emergency physicians, hospitalists, critical care specialists, and infectious disease specialists will certainly be called on to play leading roles, much as they were during and in the aftermath of Hurricane Katrina recently. Despite all of the above, the ability of existing hospitals to accommodate all gravely ill patients may be outstripped, and auxiliary hospitals in schools and other public edifices may need to be established. Hospitalists are likely to be called on to play a major role in such temporary hospitals. The frustration and anguish of not being able to provide a standard level of care to patients (for example, being forced to triage which patients are most deserving of mechanical ventilation) should not be underestimated.
Although characterized by a relatively limited number of patients, the 2003 severe acute respiratory syndrome (SARS) outbreak in Toronto, Ontario, Canada, presented some of the same challenges that will be encountered in a virulent influenza pandemic. These include the need to quickly and drastically modify the usual emergency department and inpatient procedures, as hospitals initially serve to amplify the epidemic, as well as the additional stressor of health care workers becoming ill as a result of work‐related exposure. That fewer than 400 cases of SARS pushed the medical system of one of North America's largest cities nearly to its breaking point is both sobering and instructive.10, 11 Interested readers are directed to an excellent summary of lessons learned from the SARS outbreak, most of which are widely applicable to preparations for future infectious epidemics.12
Infection Control
Although the CDC and other Web sites currently recommend airborne isolation (respiratory personal protection) for avian influenza in humans, there is not strong epidemiologic evidence of transmission other than via droplets (the transmission mode of human influenza). The emergence of a limited number of cases of avian influenza in the United States would allow employment of airborne isolation measures; but in the event of a larger outbreak, the use of surgical masks and the practice of good hand hygiene would be sufficient by health care workers caring for persons with suspected or proven disease.
The CDC recently released proposed changes to help prevent disease outbreaks from contacts of those exposed to ill persons on airplanes. Proposed guidelines would require airlines to maintain computerized lists of passengers taken at point of departure in order to facilitate tracking of contacts and implementation of quarantine if necessary. These measures are part of pandemic planning and result from problems in tracking passengers on planes with SARS cases. By executive order, imposition of quarantine is limited to 9 diseases: cholera, diphtheria, smallpox, yellow fever, viral hemorrhagic fevers (eg, Ebola), plague, infectious tuberculosis, SARS and influenza caused by new strains with pandemic potential.
What Can Be Done?
Although valuable time has elapsed to prepare for the possibility of an H5N1 influenza pandemic, the US and global communities are presently taking the threat seriously and are engaging in a variety of activities to prepare for such an eventuality. Although currently available influenza vaccines do not provide any appreciable protection against H5N1, significant work is under way to develop an effective vaccine; with Chiron and sanofi pasteur preparing vaccine trials in association with the National Institute of Allergy and Infectious Diseases. Current influenza vaccine production is hampered by use of obsolete egg‐based manufacturing processes requiring 6 months, along with a limited capacity to manufacture adequate vaccine supplies even in many usual influenza seasons. The herculean task of providing hundreds of millions of doses of vaccine as soon as possible after the emergence of a pandemic strain, as daunting as it is, is further complicated by the fact that a successful H5N1 vaccine would not necessarily be effective against a strain that mutated sufficiently to move efficiently from person to person. Nonetheless, even partially solving these problems will pay dividends, whether or not H5N1 proves to be responsible for the next pandemic.
Given these difficulties with vaccine development and production, the backbone of any successful early response to a pandemic in the near future will be development of an adequate stockpile of antiviral medication, accompanied by a successful plan to distribute the drug when and where disease erupts. Despite uncertainties regarding their effectiveness as well as questions regarding optimal dose and duration in the setting of avian influenza, the neuraminidase inhibitors are the current drugs of choice. Of the 2 currently available agents, oseltamivir is the preferred drug for pandemic use, given its oral administration,. Unfortunately, the ability to manufacture the drug in sufficient quantities to stockpile has thus far proved problematic. Roche, the manufacturer of Tamiflu, has recently opened a new manufacturing plant and has stated that it can increase its current production of 55 million doses per year to 300 million doses by 2007. We do not recommend a role for personal stockpiling of neuraminidase inhibitors. Concerns include a shortage of the drug for seasonal influenza, absence of a pandemic at present, ignorance regarding the efficacy and optimal dose for H5N1, inappropriate use by individuals, and inequitable distribution. Recent case reports of oseltamivir resistance emerging during prophylaxis13 and treatment14 are of potential concern but do not alter current recommendations.
What can be done locally and specifically, and what can hospitalists do to prepare? First, although we are not sure that Dr Michael Osterholm's goal that planning for a pandemic must be on the agenda of every public health agency, school board, manufacturing plant, investment firm, mortuary, state legislature, and food distributor8 is entirely realistic, every hospital clearly needs to include pandemic influenza as a significant part of its disaster preparedness plan. Such planning will have broad overlap with planning for other potential disasters, including bioterrorist attacks, SARS outbreaks, and others. Hospitals must develop a plan for surge capacity, and such a plan should include not only coordination with other local hospitals, but also planning with local communities to identify sites where temporary flu hospitals can be established. Within hospital medicine groups, emergency staffing plans should be established before pandemic influenza (or another disaster) strikes. Such staffing plans need to include the ability to care for a much higher than normal number of patients for an extended period. Conceivably, a large number of patients will need to be manually ventilated for prolonged periods, which of course will tax the resources of any institution. Prompt discharge of all patients stable enough to leave the hospital will be critical, and given the investment of most hospital medicine groups in hospital throughput issues under normal conditions, much of the responsibility for helping to create beds during a crisis will inevitably fall on the shoulders of hospitalists.
Experiences during and shortly after Hurricane Katrina served to underscore that issues such as physical and mental fatigue, concern for the safety of family members, lack of supplies, communication difficulties, and absenteeism all add additional layers of complexity to the task of providing hospital care under extraordinary conditions such as during a natural disaster. These lessons can and should be extended to a major epidemic. This disaster also showed the importance of military involvement in the response to disasters that exceed local and state capabilities. The primary objective of the federal government in responding to disaster is to maintain security and essential services while preventing chaos. A pandemic of virulent influenza will raise the stakes still further, as physicians and nurses become casualties themselves. Despite these challenges, we are confident that the vast majority of hospitalists and other health care workers will rise to the occasion, and just as during the peri‐Katrina period, stories of selflessness and heroism will be de rigueur. Appropriate advance planning on all levels will serve to reduce the morbidity and mortality associated with the next pandemic and will help to ensure that health care workers do not sacrifice needlessly.0
|
1. World Health Organization (WHO) Website: |
|
2. Centers for Disease Control and Prevention (CDC): |
|
3. U.S. Government Avian Influenza Website: |
|
4. U.S. Department of Health and Human Services Pandemic Influenza Plan: |
|
5. Infectious Diseases Society of America (IDSA) Website: |
Background
Influenza viruses are among the most common respiratory viral infections in humans. There are two major types of human influenza viruses, A and B, with influenza A strains responsible for seasonal or pandemic influenza. Influenza illness is characterized by fever, lower respiratory and often upper respiratory symptoms, myalgia, and malaise and occurs seasonally in temperate climates between late fall and early spring. The average flu season in the United States is marked by 30,000‐40,000 deaths, primarily in elderly patients with significant comorbidity and in the very young. Many of these deaths are caused by secondary bacterial pneumonias. Long interpandemic periods, including the current one of almost 40 years, involve minor mutations of the predominant influenza strain from year to year. Typically, adequate time exists to predict the prevailing strain with reasonable accuracy and to tailor a vaccine accordingly. Periodically an influenza pandemic involving a novel influenza strain emerges, attended by greater‐than‐expected morbidity and mortality.
All influenza viruses are subtyped on the basis of two surface glycoproteins. One of these, hemagglutinin (H), is responsible for viral cell entry; whereas the other, neuraminidase (N), facilitates release of the virus from infected cells, thus allowing perpetuation and amplification of infection. Antigenic drift is the ongoing process of genetic mutations that lead to new strains demonstrating variable change in antigenicity and is the basis for the annual updating of vaccine strains. Antigenic shift is the emergence of a novel influenza A subtype among humans, usually as the result of a recombination event. This radical change is necessary but not sufficient to initiate pandemic influenza, with efficient transmission from person to person also a critical feature. Pandemic influenza strains arise in 1 of 2 fashions. Genetic reassortment may occur when a mammalian host (human or porcine) is infected with both an avian and a human influenza virus, with subsequent dramatic movement into human populations, the source of the 1957 and 1968 pandemics. Alternatively, a novel virus may, after sufficient mutation, move directly from the avian population to humans, as appears to have occurred in 1918.
The 1918‐19 Pandemic
Abruptly in 1918, an influenza pandemic of seemingly unprecedented severity swept the world. Although disagreement remains regarding the source of the outbreak (China, the front lines of World War I, and even the United States have all been suggested), within 6‐9 months essentially the entire globe had been affected. Unlike more typical influenza seasons, the virus preferentially infected previously healthy young individuals, with those aged 15‐40 bearing the brunt of the illness. US military training installations, overcrowded with troops staging for service on the European front, played a particularly ill‐fated role in the pandemic as it swept through the United States.
Estimates of the pandemic's worldwide impact on mortality are sketchy at best, but many authorities believe that at least 50 million deaths resulted, with some suggesting a figure as high as 100 million. In the United States the virus was responsible for an estimated 700,000 deaths, with an untold burden of morbidity. Economic and social disruption was the norm in many areas, with widespread closure of businesses and schools and suspension of public gatherings of any kind. Many communities were simply overwhelmed by the sheer numbers of dying individuals. In Philadelphia, steam shovels were used to dig mass graves for influenza victims.1 The pandemic's effect on the health care system was likewise profound. Most hospitals counted their own physicians and nurses among those who died during the pandemic, and many of the health care workers who succumbed were infected in the course of caring for influenza patients. Overall, an estimated 2%‐3% of those infected with the virus died, a far higher percentage than is seen during interpandemic seasons. Strikingly, the vast majority of deaths do not appear to have resulted from secondary bacterial pneumonias, but rather to have been directly virally mediated through ARDS, a necrotizing viral pneumonia, or both.
The mystery of the 1918 pandemic has recently been partially unlocked, with the successful sequencing of the entire RNA genome of strains recovered from pathology tissue of two soldiers, as well as from lung tissue of a victim frozen in Alaskan permafrost since 1918.2, 3 The data suggest that the 1918 virus was derived from an avian source. Notably, some of the same changes in the polymerase proteins have been found in the highly pathogenic H5N1 viruses.
Avian Influenza Viruses
Influenza viruses that primarily infect birds are characterized as avian influenza viruses. These are always type A and are classified as either of low or high pathogenicity on the basis of the severity of the illness they cause in birds. The currently circulating H5N1 avian viruses are highly pathogenic.
Avian influenza viruses do not usually infect humans; however, several instances of human infections have been reported since 1997. The 1997 Hong Kong outbreak of avian (H5N1) influenza in 18 humans resulted in 6 deaths and was a seminal event that provided evidence that avian influenza viruses can infect people. It also provided the epidemiologic link between avian influenza infection in poultry with disease in humans and was proclaimed as a pandemic warning. These sentinel human infections led to the culling of the entire Hong Kong poultry population, with no subsequent human infection reported at that time. In 2003, more than 80 cases of avian influenza A (H7N7) illness occurred in the Netherlands among persons who handled infected poultry. Sustained human‐to‐human transmission did not occur in this or other outbreaks of avian influenza to date.
Since 2003, sporadic human cases of H5N1 have occurred, most recently reported from Turkey and Iraq. Human cases have also occurred in Vietnam, China, Cambodia, Thailand, and Indonesia, with a total of 173 reported cases and a case fatality rate exceeding 50% as of this writing.4 This mortality rate may be artificially inflated, as less severe cases have certainly gone unreported. All countries reporting human avian influenza diseases since 2003 have had concurrent epizoonotics in birds (both poultry and migratory birds).
Human cases of H5N1 influenza illness have been characterized by high fever and symptoms in the lower respiratory tract, as would be expected. Less predictable has been the presence of watery diarrhea in many patients and of abdominal and pleuritic pain and bleeding from the nose and gums in some. Sputum production has been variably present, and hemoptysis has been seen in some individuals. Most patients have had clinical and radiological evidence of pneumonia at the time they sought medical care, and progression to ARDS and multiorgan failure has been common. The majority of patients to date have required the initiation of mechanical ventilation early in their hospital course. Laboratory studies have typically shown lymphopenia, thrombocytopenia, and, in many cases, modestly elevated transaminase levels.5 Notably, the currently predominant strain of H5N1 (Z strain) is resistant to the M2 ion channel inhibitors amantadine and rimantadine but is susceptible to the newer class of neuraminidase inhibitors, zanamivir (Relenza) and oseltamivir (Tamiflu). Neuraminidase inhibitors and corticosteroids have been used to treat patients, although their efficacy in this setting is unclear. To date, virtually all cases appear to have been transmitted directly from poultry, although person‐to‐person transmission appears likely to have occurred in at least one family in Thailand.6 A recent study of the 14 clusters of avian influenza among humans emphasized the lack of sustained person‐to‐person transmission of H5N1 to date.7
Three factors are necessary for the emergence of a pandemic influenza strain: the ability to infect humans, a novel genetic makeup, and the ability for sustained transmission between people. A virus that in addition proves highly virulent, as did the 1918‐19 H1N1 strain, essentially creates the perfect storm. H5N1 influenza has currently fulfilled 2 of these 3 criteria. The virus is highly pathogenic, although how much of this fitness would be sacrificed with mutation to a more transmissible strain is uncertain. As many have observed, whether there will be another influenza pandemic does not seem in doubt; rather, it is when such a pandemic will occur and whether the pandemic will be caused by H5N1 or another influenza virus, that are the questions.
Potential Effects of the Next Pandemic
The global and national effects of an influenza pandemic will vary in direct proportion to the virulence of the circulating viral strain, but if such a virus is highly virulent, significant and perhaps severe economic and social disruption are likely.
The global economic impact has been estimated to be $800 billion with anticipated quarantines and interruption in global trade. On a national level, it has been estimated that in the United States a pandemic virus whose severity is comparable to that of the 1968 Hong Kong influenza pandemic would lead to approximately 200,000 deaths and 700,000 hospitalizations, of which roughly 100,000 would require treatment in intensive care unit settings. A more virulent strain, similar to that of the 1918‐19 pandemic, might easily result in 1 million deaths; with the number of patients hospitalized approaching 10 million, well over 1 million of which would require ICU‐level care. As an estimated 75% of the 105,000 ventilators in this country are in use at any given time under normal circumstances, the potential for demand to greatly outstrip supply is evident.8 Depending on the severity of a pandemic, suspension or curtailment of international trade and travel could be reasonably likely. Although the World Health Organization has recommended against closing borders or quarantining countries even in the throes of a pandemic, the prospect of this occurring does not seem implausible. In a worst‐case scenario, even the type of national and international chaos envisioned in the Dark Winter smallpox planning exercise might occur.9
Fortress America Versus Containment Strategies
Although the pandemic influenza plan calls for stockpiling antiviral drugs and increasing vaccine production capabilities, the most effective plan for pandemic preparedness may involve a surveillance and containment strategy. No country has enough medicines or vaccines to control a widespread outbreak of pandemic avian influenza. The best solution to prevention of a pandemic is stopping any virus from spreading in the first place. Increased surveillance for avian influenza among poultry and migratory birds in key Asian countries, along with provision of funds to compensate farmers for culling of potentially infected flocks, would align incentives for early detection and eradication. Containing an initial outbreak wherever it occurs is the best defense against a pandemic. Notably, China is thought to be a potential hot zone for emergence of pandemic avian influenza. China is not only the most populous nation in the world but has one quarter of the world's chickens, two thirds of the world's domesticated ducks, and 90% of the world's domesticated geese.
The challenges of biosecurity (protecting humans against animal‐borne diseases such as bird flu) in developing countries include the reality that populations living in close proximity to poultry are also the most illiterate and impoverished, with the most limited access to health care. The recent introduction of H5N1 into Europe has heightened surveillance efforts in the United States. The introduction of H5N1 into the United States may occur through movement of migratory birds and/or importation of exotic birds. The surveillance system has been expanded to include sampling for the influenza virus not only in poultry but also in bodies of water, as the virus is shed in bird feces.
Pandemic Planning
In the setting of a severe pandemic, hospitals will face an enormous burden of patients, with a huge influx of individuals requiring both intensive care unit as well as regular nursing floor care. At the local height of such a pandemic, the ability to successfully discharge every patient whose condition will permit this to the community or elsewhere will be critical, and almost certainly hospitals will need to expand to accept more patients than they are normally configured to hold. Hospitals staffs, particularly nurses and physicians, will be required to handle very large patient censuses. Among medical staffs, emergency physicians, hospitalists, critical care specialists, and infectious disease specialists will certainly be called on to play leading roles, much as they were during and in the aftermath of Hurricane Katrina recently. Despite all of the above, the ability of existing hospitals to accommodate all gravely ill patients may be outstripped, and auxiliary hospitals in schools and other public edifices may need to be established. Hospitalists are likely to be called on to play a major role in such temporary hospitals. The frustration and anguish of not being able to provide a standard level of care to patients (for example, being forced to triage which patients are most deserving of mechanical ventilation) should not be underestimated.
Although characterized by a relatively limited number of patients, the 2003 severe acute respiratory syndrome (SARS) outbreak in Toronto, Ontario, Canada, presented some of the same challenges that will be encountered in a virulent influenza pandemic. These include the need to quickly and drastically modify the usual emergency department and inpatient procedures, as hospitals initially serve to amplify the epidemic, as well as the additional stressor of health care workers becoming ill as a result of work‐related exposure. That fewer than 400 cases of SARS pushed the medical system of one of North America's largest cities nearly to its breaking point is both sobering and instructive.10, 11 Interested readers are directed to an excellent summary of lessons learned from the SARS outbreak, most of which are widely applicable to preparations for future infectious epidemics.12
Infection Control
Although the CDC and other Web sites currently recommend airborne isolation (respiratory personal protection) for avian influenza in humans, there is not strong epidemiologic evidence of transmission other than via droplets (the transmission mode of human influenza). The emergence of a limited number of cases of avian influenza in the United States would allow employment of airborne isolation measures; but in the event of a larger outbreak, the use of surgical masks and the practice of good hand hygiene would be sufficient by health care workers caring for persons with suspected or proven disease.
The CDC recently released proposed changes to help prevent disease outbreaks from contacts of those exposed to ill persons on airplanes. Proposed guidelines would require airlines to maintain computerized lists of passengers taken at point of departure in order to facilitate tracking of contacts and implementation of quarantine if necessary. These measures are part of pandemic planning and result from problems in tracking passengers on planes with SARS cases. By executive order, imposition of quarantine is limited to 9 diseases: cholera, diphtheria, smallpox, yellow fever, viral hemorrhagic fevers (eg, Ebola), plague, infectious tuberculosis, SARS and influenza caused by new strains with pandemic potential.
What Can Be Done?
Although valuable time has elapsed to prepare for the possibility of an H5N1 influenza pandemic, the US and global communities are presently taking the threat seriously and are engaging in a variety of activities to prepare for such an eventuality. Although currently available influenza vaccines do not provide any appreciable protection against H5N1, significant work is under way to develop an effective vaccine; with Chiron and sanofi pasteur preparing vaccine trials in association with the National Institute of Allergy and Infectious Diseases. Current influenza vaccine production is hampered by use of obsolete egg‐based manufacturing processes requiring 6 months, along with a limited capacity to manufacture adequate vaccine supplies even in many usual influenza seasons. The herculean task of providing hundreds of millions of doses of vaccine as soon as possible after the emergence of a pandemic strain, as daunting as it is, is further complicated by the fact that a successful H5N1 vaccine would not necessarily be effective against a strain that mutated sufficiently to move efficiently from person to person. Nonetheless, even partially solving these problems will pay dividends, whether or not H5N1 proves to be responsible for the next pandemic.
Given these difficulties with vaccine development and production, the backbone of any successful early response to a pandemic in the near future will be development of an adequate stockpile of antiviral medication, accompanied by a successful plan to distribute the drug when and where disease erupts. Despite uncertainties regarding their effectiveness as well as questions regarding optimal dose and duration in the setting of avian influenza, the neuraminidase inhibitors are the current drugs of choice. Of the 2 currently available agents, oseltamivir is the preferred drug for pandemic use, given its oral administration,. Unfortunately, the ability to manufacture the drug in sufficient quantities to stockpile has thus far proved problematic. Roche, the manufacturer of Tamiflu, has recently opened a new manufacturing plant and has stated that it can increase its current production of 55 million doses per year to 300 million doses by 2007. We do not recommend a role for personal stockpiling of neuraminidase inhibitors. Concerns include a shortage of the drug for seasonal influenza, absence of a pandemic at present, ignorance regarding the efficacy and optimal dose for H5N1, inappropriate use by individuals, and inequitable distribution. Recent case reports of oseltamivir resistance emerging during prophylaxis13 and treatment14 are of potential concern but do not alter current recommendations.
What can be done locally and specifically, and what can hospitalists do to prepare? First, although we are not sure that Dr Michael Osterholm's goal that planning for a pandemic must be on the agenda of every public health agency, school board, manufacturing plant, investment firm, mortuary, state legislature, and food distributor8 is entirely realistic, every hospital clearly needs to include pandemic influenza as a significant part of its disaster preparedness plan. Such planning will have broad overlap with planning for other potential disasters, including bioterrorist attacks, SARS outbreaks, and others. Hospitals must develop a plan for surge capacity, and such a plan should include not only coordination with other local hospitals, but also planning with local communities to identify sites where temporary flu hospitals can be established. Within hospital medicine groups, emergency staffing plans should be established before pandemic influenza (or another disaster) strikes. Such staffing plans need to include the ability to care for a much higher than normal number of patients for an extended period. Conceivably, a large number of patients will need to be manually ventilated for prolonged periods, which of course will tax the resources of any institution. Prompt discharge of all patients stable enough to leave the hospital will be critical, and given the investment of most hospital medicine groups in hospital throughput issues under normal conditions, much of the responsibility for helping to create beds during a crisis will inevitably fall on the shoulders of hospitalists.
Experiences during and shortly after Hurricane Katrina served to underscore that issues such as physical and mental fatigue, concern for the safety of family members, lack of supplies, communication difficulties, and absenteeism all add additional layers of complexity to the task of providing hospital care under extraordinary conditions such as during a natural disaster. These lessons can and should be extended to a major epidemic. This disaster also showed the importance of military involvement in the response to disasters that exceed local and state capabilities. The primary objective of the federal government in responding to disaster is to maintain security and essential services while preventing chaos. A pandemic of virulent influenza will raise the stakes still further, as physicians and nurses become casualties themselves. Despite these challenges, we are confident that the vast majority of hospitalists and other health care workers will rise to the occasion, and just as during the peri‐Katrina period, stories of selflessness and heroism will be de rigueur. Appropriate advance planning on all levels will serve to reduce the morbidity and mortality associated with the next pandemic and will help to ensure that health care workers do not sacrifice needlessly.0
|
1. World Health Organization (WHO) Website: |
|
2. Centers for Disease Control and Prevention (CDC): |
|
3. U.S. Government Avian Influenza Website: |
|
4. U.S. Department of Health and Human Services Pandemic Influenza Plan: |
|
5. Infectious Diseases Society of America (IDSA) Website: |
- .The Great Influenza.New York, NY:Viking Penguin,2004.
- ,,,,,.Characterization of the 1918 influenza virus polymerase genes.Nature.2005;437:889–893.
- ,,, et al.Characterization of the reconstructed 1918 Spanish influenza pandemic virus.Science.2005;310:77–80.
- WHO Epidemic and Pandemic Alert and Response. Confirmed cases of avian influenza A (H5N1). Available at http://www.who.int/csr/disease/avian_influenza/country/en/index.html. Accessed on February 28,2006.
- Writing Committee of the WHO Consultation on Human Influenza A/H5.Avian influenza A (H5N1) infection in humans.N Engl J Med.2005;353:1374–1385.
- ,,, et al.Probable person‐to‐person transmission of avian influenza A (H5N1).N Engl J Med.2005;352:333–40.
- ,,, et al.Family clustering of avian influenza A (H5N1).EID.2005;11:1799–1801.
- .Preparing for the next pandemic.N Engl J Med.2005;352:1839–1842.
- Center for Biosecurity. Dark Winter overview. Available at http://www.upmc‐biosecurity.org/pages/events/dark_winter/dark_winter.html. Accessed November 28,2005.
- ,,, et al.SARS outbreak in the Greater Toronto Area: the emergency department experience.CMAJ.2004;171:1342–1344.
- ,.Severe acute respiratory syndrome and critical care medicine: The Toronto experience.Crit Care Med.2005;33(suppl):S53–S60.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291:2483–2487.
- ,,, et al.Avian flu: Isolation of drug‐resistant H5N1 virus.Nature.2005;438:754.
- ,,, et al.Oseltamivir resistance during treatment of influenza A (H5N1) infection.N Engl J Med.2005;353:2667–2672.
- .The Great Influenza.New York, NY:Viking Penguin,2004.
- ,,,,,.Characterization of the 1918 influenza virus polymerase genes.Nature.2005;437:889–893.
- ,,, et al.Characterization of the reconstructed 1918 Spanish influenza pandemic virus.Science.2005;310:77–80.
- WHO Epidemic and Pandemic Alert and Response. Confirmed cases of avian influenza A (H5N1). Available at http://www.who.int/csr/disease/avian_influenza/country/en/index.html. Accessed on February 28,2006.
- Writing Committee of the WHO Consultation on Human Influenza A/H5.Avian influenza A (H5N1) infection in humans.N Engl J Med.2005;353:1374–1385.
- ,,, et al.Probable person‐to‐person transmission of avian influenza A (H5N1).N Engl J Med.2005;352:333–40.
- ,,, et al.Family clustering of avian influenza A (H5N1).EID.2005;11:1799–1801.
- .Preparing for the next pandemic.N Engl J Med.2005;352:1839–1842.
- Center for Biosecurity. Dark Winter overview. Available at http://www.upmc‐biosecurity.org/pages/events/dark_winter/dark_winter.html. Accessed November 28,2005.
- ,,, et al.SARS outbreak in the Greater Toronto Area: the emergency department experience.CMAJ.2004;171:1342–1344.
- ,.Severe acute respiratory syndrome and critical care medicine: The Toronto experience.Crit Care Med.2005;33(suppl):S53–S60.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291:2483–2487.
- ,,, et al.Avian flu: Isolation of drug‐resistant H5N1 virus.Nature.2005;438:754.
- ,,, et al.Oseltamivir resistance during treatment of influenza A (H5N1) infection.N Engl J Med.2005;353:2667–2672.