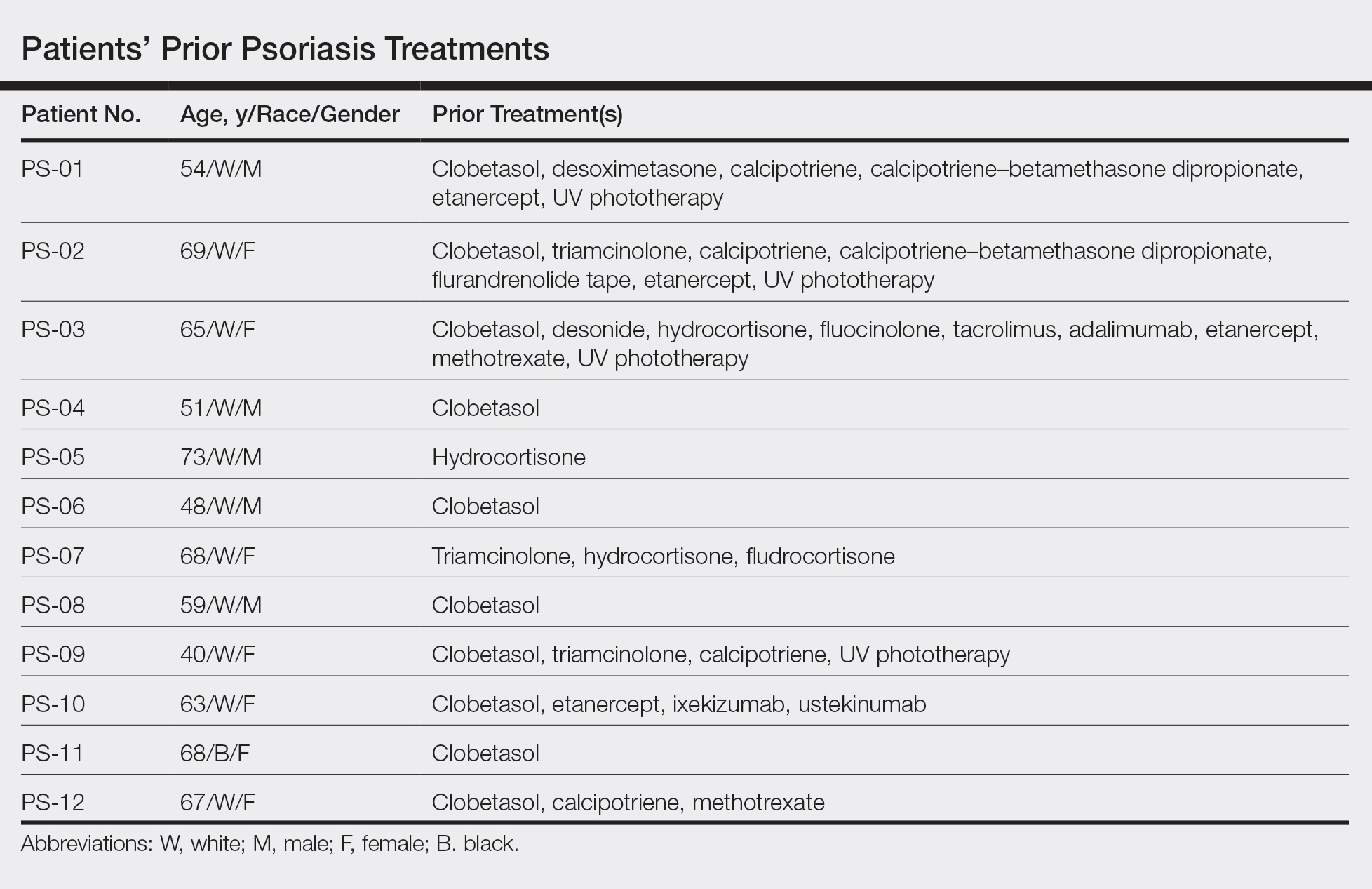
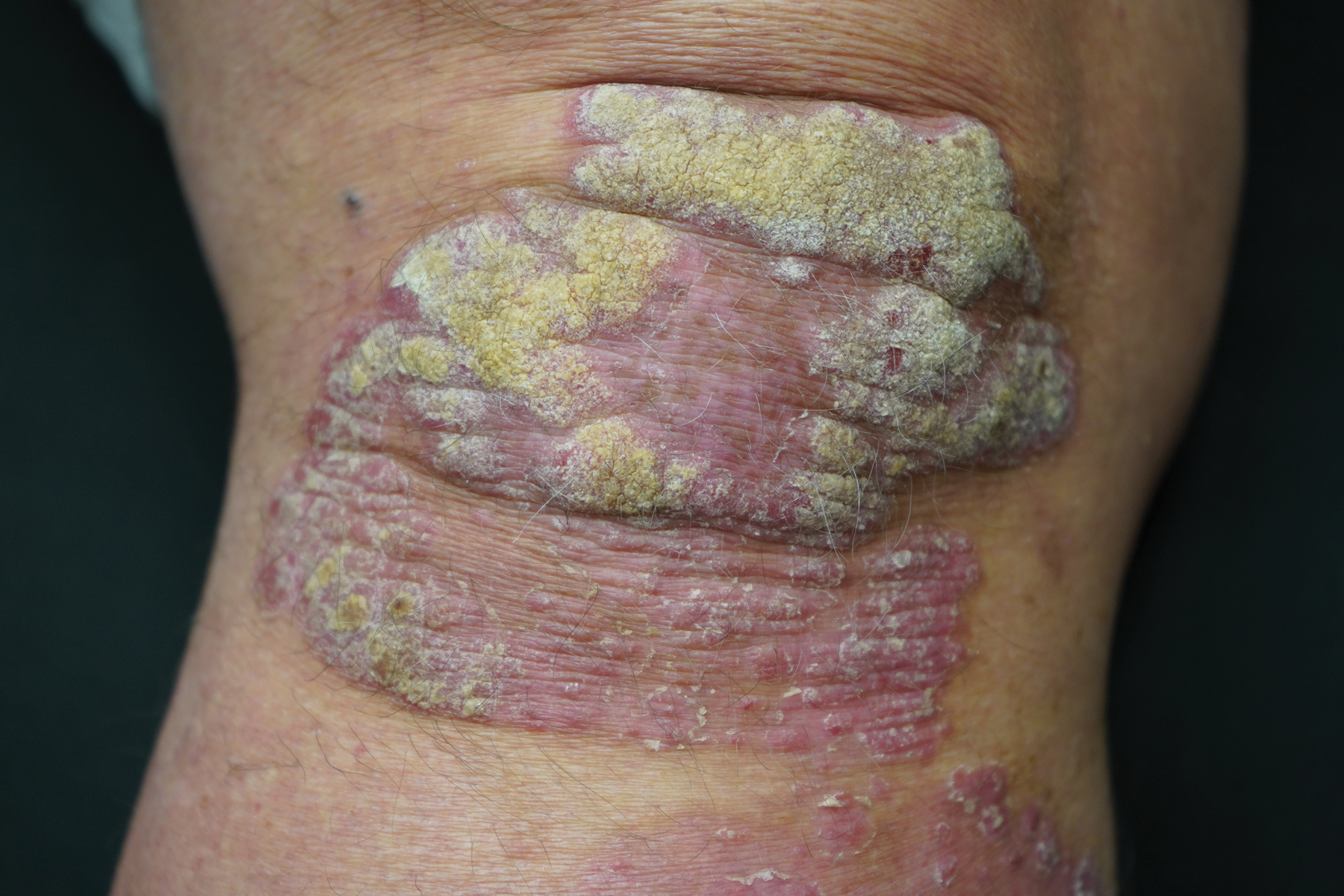
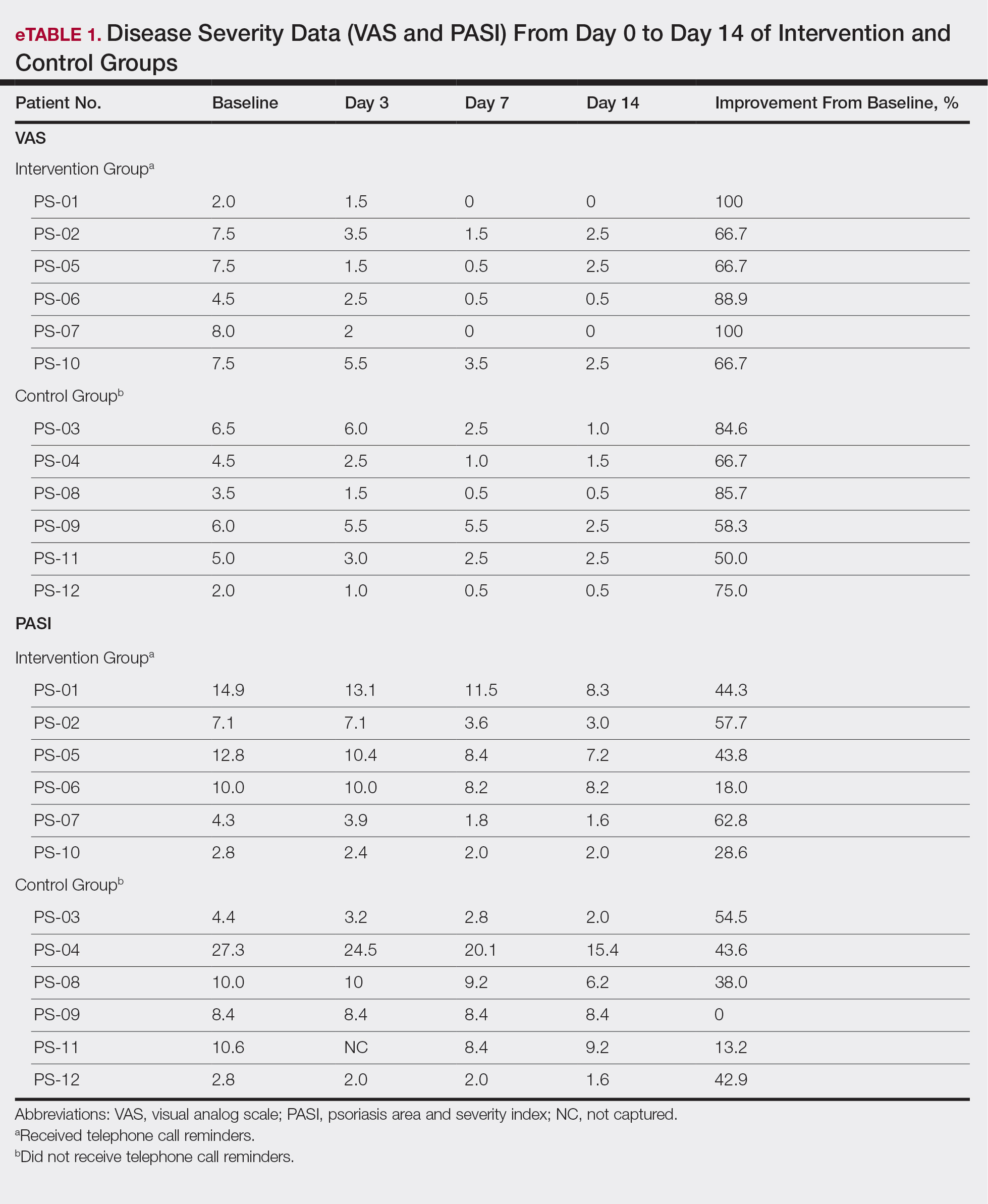
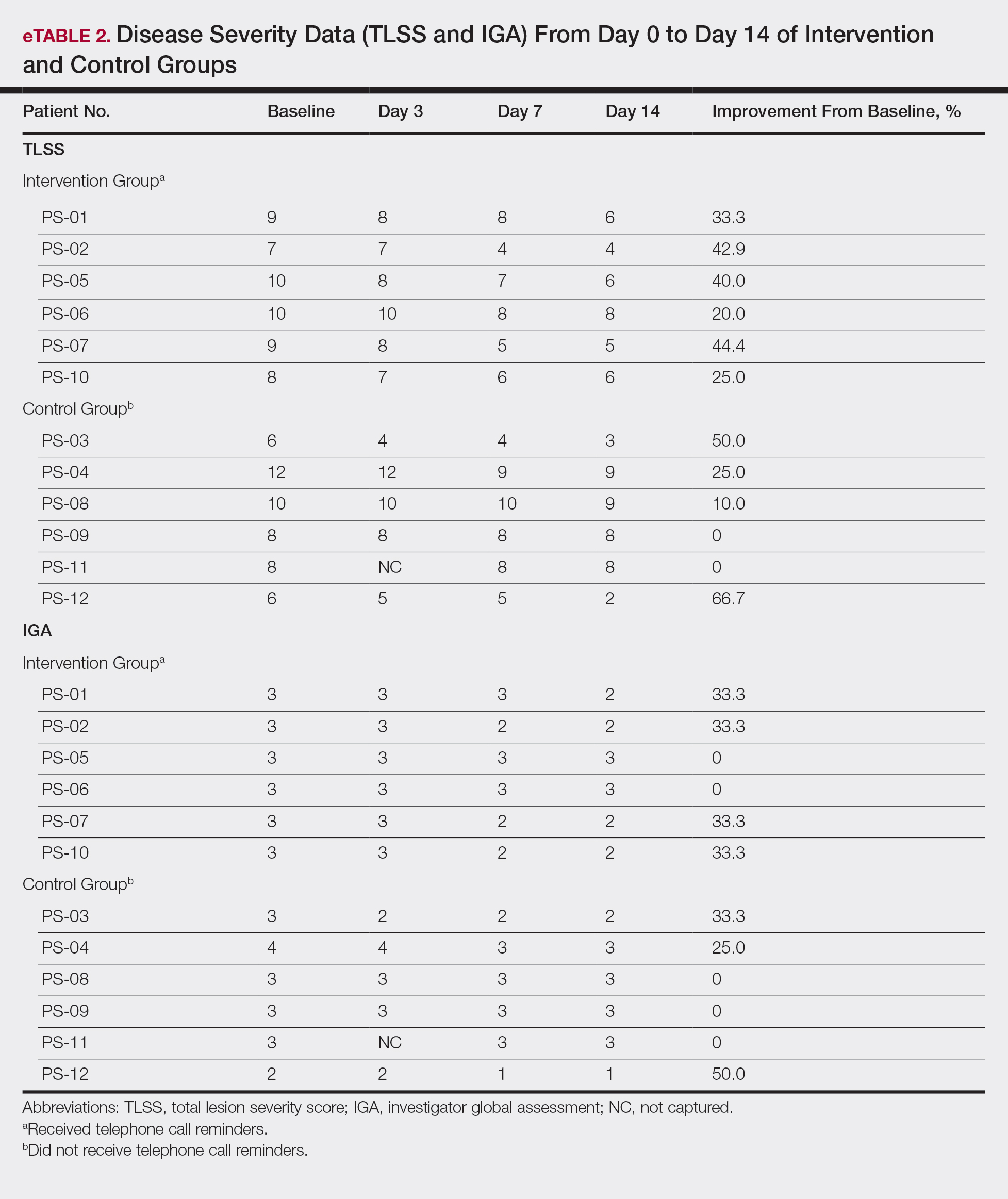
User login
Factors Influencing Patient Preferences for Phototherapy: A Survey Study
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
Practice Points
- Patients have different priorities when selecting phototherapy, including safety, costs, effectiveness, insurance issues, and convenience.
- By offering and educating patients on all forms of phototherapy, dermatologists may help guide patients to their optimal treatment plan according to patient priorities.
Views and Beliefs of Vitiligo Patients in Online Discussion Forums: A Qualitative Study
Vitiligo is a chronic dermatologic condition that negatively affects quality of life (QOL), with substantial burden on the psychosocial well-being of patients.1 There is no cure, and current treatment modalities are aimed at controlling the chronic relapsing condition.1-3 Despite topical and cosmetic treatments for stabilization and repigmentation, vitiligo remains unpredictable.3
All genders, races, ethnicities, and socioeconomic classes are equally affected.4 The underlying etiology of vitiligo remains unknown to a great extent and is more poorly understood by the general public compared with other skin diseases (eg, acne).5 Patients with vitiligo experience social withdrawal, decreased sense of self-esteem, anxiety, depression, and suicidal ideation.5,6 Stigmatization has the greatest impact on QOL, with strong correlations between avoidance behaviors and lesion concealment.6-8 Although the condition is especially disfiguring for darker skin types, lighter skin types also are substantially affected, with similar overall self-reported stress.6,7
Individuals with chronic illnesses such as vitiligo turn to online communities for health information and social support, commiserating with others who have the same condition.9,10 Online forums are platforms for asynchronous peer-to-peer exchange of disease-related information for better management of long-term disease.11 Moreover, of all available internet resources, online forum posts are the most commonly accessed source of information (91%) for patients following visits with their doctors.12
Qualitative research involving chronic skin conditions and the information exchanged in online forums has been conducted for patients with acne, psoriasis, and atopic dermatitis, but not for patients with vitiligo.13-16 Although online questionnaires have been administered to patients with vitiligo, the content within online forums is not well characterized.2,17
The purpose of this qualitative study was to evaluate the online content exchanged by individuals with vitiligo to better understand the general attitudes and help-seeking behaviors in online forums.
Methods
Study Design—This qualitative study sought to investigate health beliefs and messages about vitiligo posted by users in US-based online discussion forums. An interpretive research paradigm was utilized so that all content collected in online forums were the views expressed by individuals.18-20 An integrated approach was used in the development of the coding manual, with pre-established major themes and subthemes as a guiding framework.16,21,22 We adhered to an inductive grounded method by means of de novo line-by-line coding, such that we had flexibility for new subthemes to emerge throughout the duration of the entire coding process.23
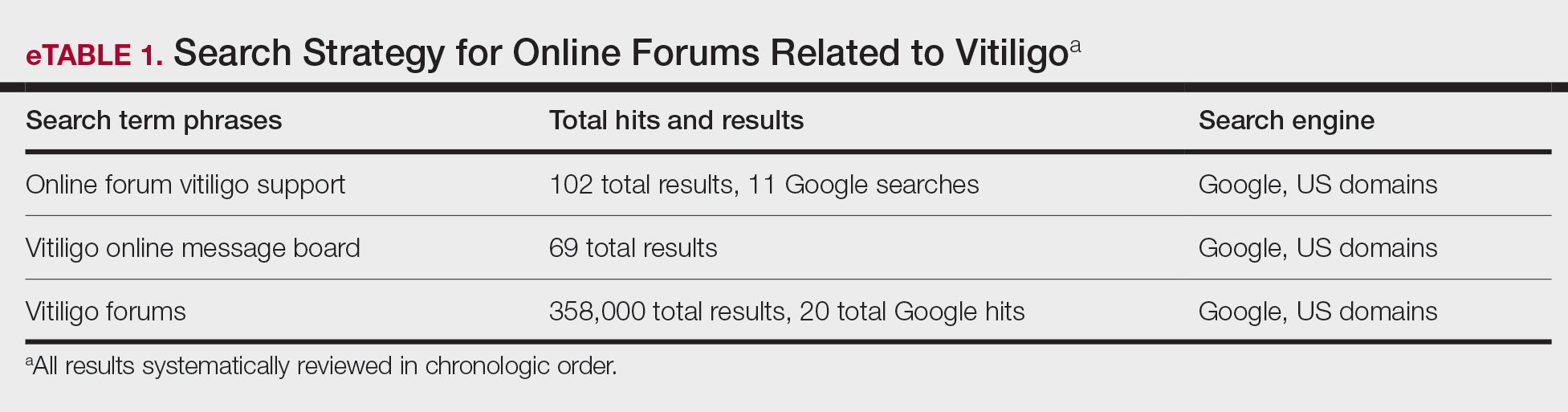
Individual posts and subsequent replies embedded within public online forums were used as the collected data source. Google was utilized as the primary search engine to identify forums pertaining to vitiligo, as 80% of US adults with chronic disease report that their inquiries for health information start with Google, Bing, or Yahoo.24 The institutional review board at the Wake Forest School of Medicine (Winston-Salem, North Carolina) granted approval of the study (IRB00063073). Online forums were considered “property” of the public domain and were accessible to all, eliminating the need for written informed consent.24-26
Search Criteria—We conducted our forum search in February 2020 with a systematic approach using predetermined phrases—online forum vitiligo support, vitiligo online message board, and vitiligo forums—which yielded more than 358,171 total results (eTable 1). Threads were identified in chronological order (from newest to oldest) based on how they appeared during each internet search, and all Google results for the respective search phrases were reviewed. Dates of selected threads ranged from 2005 to 2020. Only sites with US domains were included. Posts that either included views and understandings of vitiligo or belonged to a thread that contained a vitiligo discussion were deemed relevant for inclusion. Forums were excluded if registration or means of payment was required to view posts, if there were fewer than 2 user replies to a thread, if threads contained patient photographs, or if no posts had been made in the last 2 years (rendering the thread inactive). No social media platforms, such as Facebook, or formal online platforms, such as MyVitiligoTeam, were included in the search. A no-fee-for-access was chosen for this study, as the majority of those with a chronic condition who encounter a required paywall find the information elsewhere.25
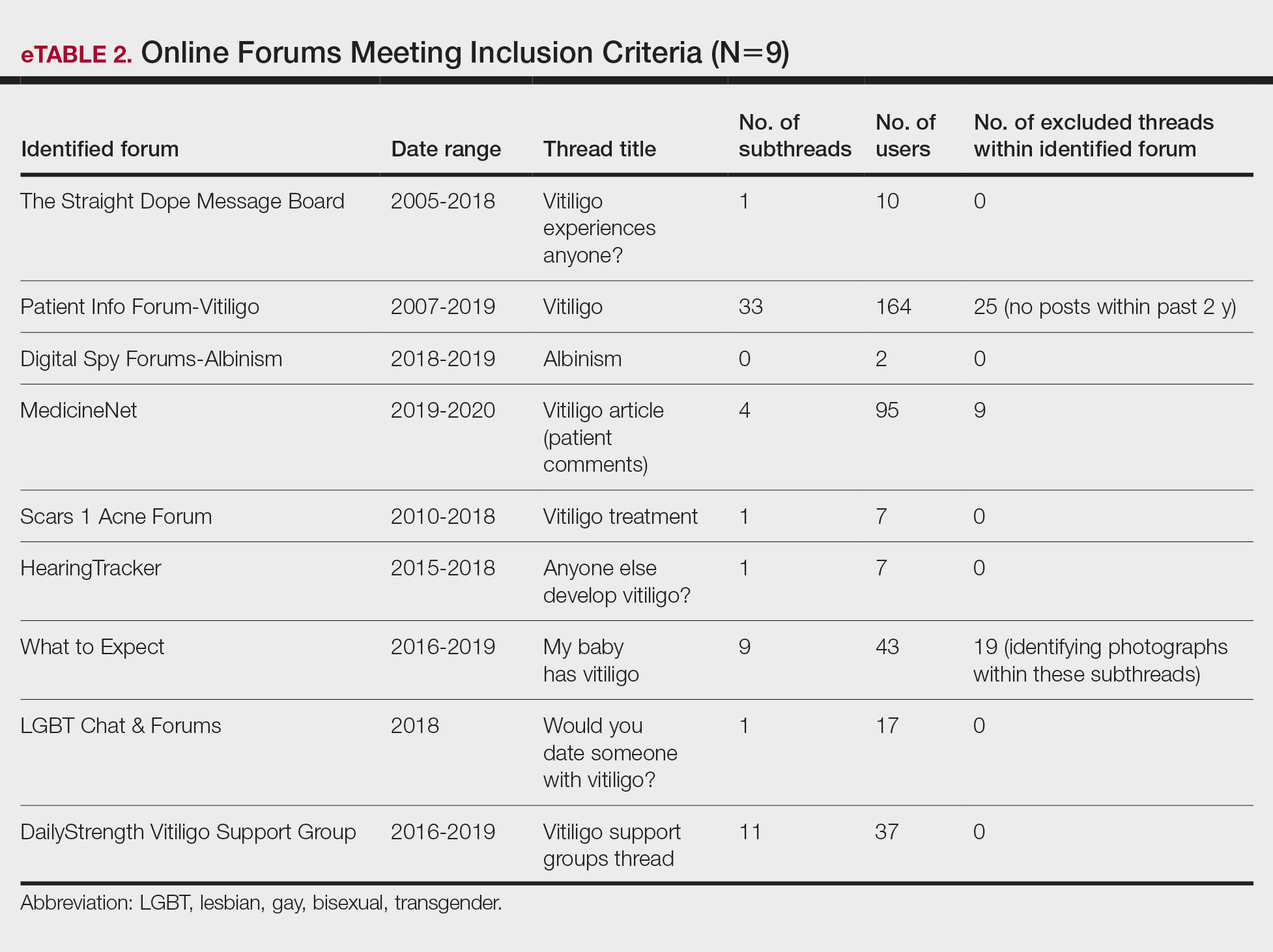
Data Analysis—A total of 39 online forums were deemed relevant to the topic of vitiligo; 9 of them met inclusion criteria (eTable 2). The messages within the forums were copied verbatim into a password-encrypted text document, and usernames in the threads were de-identified, ensuring user confidentiality.
An inductive thematic analysis was utilized to explore the views and beliefs of online forum users discussing vitiligo. One author (M.B.G.) read the extracted message threads, developed an initial codebook, and established a finalized version with the agreement of another author (A.M.B.)(eTable 3). The forums were independently coded (M.B.G. and A.M.B.) in a line-by-line manner according to the codebook. Discrepancies were documented and resolved. Data saturation was adequately achieved, such that no new themes emerged during the iterative coding process. NVivo was used for qualitative analysis.
Results
Nine forums met inclusion criteria, comprising 105 pages of text. There were 61 total discussion threads, with 382 anonymous contributing users. Most users initiated a thread by posting either a question, an advice statement, or a request for help. The psychosocial impact of the disease permeated multiple domains,including personal relationships and daily life. Several threads discussed treatment, including effective camouflage and makeup, as well as peer validation of physician-prescribed treatments, along with threads dedicated to “cures” or homeopathy regimens. In several instances, commercial product endorsement, testimonials, and marketing links were reposted by the same user multiple times.
Inductive thematic analysis highlighted diverse themes and subthemes related to the beliefs and perspectives of users with vitiligo or with relatives or friends with vitiligo: psychosocial impact, disease management and camouflage/concealment, alternative medicine/homeopathy/cures, interactions with the public and health care providers, and skin tone and race. Quotes from individuals were included to demonstrate themes and subthemes.
Psychosocial Impact: QOL, Sources of Support, and Coping—There was a broad range of comments on how patients cope with and view their vitiligo. Some individuals felt vitiligo made them special, and others were at peace with and accepted their condition. In contrast, others reported the disease had devastated them and interfered with relationships. Individuals shared their stories of grief and hardships through childhood and adulthood and their concerns, especially on affected visible areas or the potential for disease progression. Users were vocal about how vitiligo affected their daily routines and lives, sharing how they felt uncomfortable outside the home, no longer engaged in swimming or exposing their legs, and preferred to stay inside instead. Some users adopted a “tough love” approach to coping, sharing how they have learned to either embrace their vitiligo or “live with it.” Some examples include:
“My best advice is go with the flow, vitiligo is not the worst thing that can happen.”
“I hate my life with vitiligo yet really I feel so selfish that there is much worse suffering in the world than a few white patches.”
Other advice was very practical:
“I hope it isn’t vanity that is tearing you apart because that is only skin deep. Make a fashion statement with hats.”
Some users acknowledged and adopted the mantra that vitiligo is not a somatic condition or “physical ailment,” while others emphasized its pervasive psychological burden:
“I still deal with this psychologically . . . You must keep a positive attitude and frame of mind . . . Vitiligo will not kill you, but you do need to stay strong and keep your head up emotionally.”
“I am just really thankful that I have a disease that will not kill me or that has [not] affected me physically at all. I consider myself lucky.”
Disease Management: Treatment, Vitiligo Course, Advice-Seeking, Camouflage—The range of information discussed for treatment was highly variable. There were many accounts in which users advised others to seek professional help, namely that of a dermatologist, for a formal assessment. Many expressed frustrations with treatments and their ineffectiveness, to which the majority of users said to consult with a professional and to remain patient and hopeful/optimistic:
“The best thing to do would be to take an appointment with a dermatologist and have the discoloration checked out. That’s the only way to know whether it is vitiligo or not.”
“My way of dealing with it is to gain control by camouflage.”
“The calming effect of being in control of my vitiligo, whether with concealers, self-tan or anything else, has stopped my feelings of despair.”
Beliefs on Alternative Medicine: Homeopathy and Alternative Regimens—Although some threads started with a post asking for the best treatments, others initiated a discussion by posting “best herbal treatments for cure” or “how to cure my vitiligo,” emphasizing the beliefs and wishes for a cure for vitiligo. Alternative therapies that users endorsed included apple cider vinegar, toothpaste, vitamins, and Ayurvedic treatment, among others. Dietary plans were popular, with users claiming success with dietary alterations in stopping and preventing lesion progression. For example, individuals felt that avoidance of sugar, meat, dairy, and citrus fruits or drinks and consumption of only filtered water were crucial to preventing further lesion spread and resulted in their “cure”:
“Don’t eat chocolate, wine (made of grapes), coffee, or tea if you don’t want to have vitiligo or let it get worse. Take Vitamin B, biotin, and nuts for Vitamin E.”
Other dangerous messages pitted treatments by health professionals against beliefs in homeopathy:
“I feel that vitiligo treatment is all in your diet and vitamins. All that medicine and UV lights is a no-no . . .w ith every medicine there is a side effect. The doctors could be healing your vitiligo and severely damaging you inside and out, and you won’t know until years later.”
There was a minor presence of users advising against homeopathy and the associated misinformation and inaccurate claims on curing vitiligo, though this group was small in comparison to the number of users posting outlandish claims on cure:
“There is no cure . . . It’s where your immune system attacks your skin cells causing loss of pigmentation. The skin that has lost the pigmentation can’t be reversed.”
Interactions With the Public and Health Care Providers—Those with vitiligo encounter unique situations in public and in their daily lives. Many of the accounts shared anecdotal stories on how patients have handled the stigma and discrimination faced:
“I have had to face discrimination at school, public places, college, functions, and every new person I have met has asked me this: ‘how did this happen?’”
Those with vitiligo even stated how they wished others would deal with their condition out in public, hoping that others would directly ask what the lesions were instead of the more hurtful staring. There were many stories in which users said others feel vitiligo was contagious or “dirty” and stressed that the condition is not infectious:
“I refer to myself as ‘camo-man’ and reassure people I come into contact with that it is not contagious.”
“Once I was eating at a restaurant . . . and a little girl said to her mom, ‘Look, Mom, that lady doesn’t wash her arms, look how dirty they are.’ That just broke my heart.”
Skin Tone and Implications—The belief that vitiligo lesions are less dramatic or less anxiety provoking for individuals with lighter skin was noted by users themselves and by health care providers in certain cases. Skin tone and its impact on QOL was confusing and contentious. Some users with fair skin stated their vitiligo was “less of an annoyance” or “less obvious” compared with individuals with darker complexions. Conversely, other accounts of self-reported White users vehemently stressed the anxieties felt by depigmented lesions, despite being “already white at baseline.”
“Was told by my dermatologist (upon diagnosis) that ‘You’re lucky you’re not African American—it shows up on them much worse. You’re so fair, it doesn’t really matter.’ ”
“You didn’t say what race you are. I could imagine it has a bigger impact if you are anything other than White.”
Comment
Patients Looking for Cures—The general attitude within the forums was uplifting and encouraging, with users detailing how they respond to others in public and sharing their personal perspectives. We found a mix of information regarding disease management and treatment of vitiligo. Overall, there was uncertainty about treatments, with individuals expressing concern that their treatments were ineffective or had failed or that better alternatives would be more suitable for their condition. We found many anecdotal endorsements of homeopathic remedies for vitiligo, with users boasting that their disease had not only been cured but had never returned. Some users completely denounced these statements, while other threads seemed to revolve completely around “cure” discussions with no dissenting voices. The number of discussions related to homeopathy was concerning. Furthermore, there often were no moderators within threads to remove cure-related content, whether commercially endorsed or anecdotal. It is plausible that supplements and vitamins recommended by some physicians may be incorrectly interpreted as a “cure” in online discussions. Our findings are consistent with prior reports that forums are a platform to express dissatisfaction with treatment and the need for additional treatment options.15,22
Concern Expressed by Health Care Providers—Prior qualitative research has described how patients with chronic dermatologic conditions believe that health care providers minimize patients’ psychological distress.27,28 We found several accounts in which an individual had explicitly stated their provider had “belittled” the extent and impact of vitiligo when comparing skin phototypes. This suggests either that physicians underestimate the impact of vitiligo on their patients or that physicians are not expressing enough empathic concern about the impact the condition has on those affected.
Cosmetic Aspects of Vitiligo—Few clinical trials have investigated QOL and cosmetic acceptability of treatments as outcome measures.29 We found several instances in which users with vitiligo had reported being dismissed as having a “cosmetic disease,” consistent with other work demonstrating the negative impact on such dismissals.22 Moreover, concealment and camouflage techniques frequently were discussed, demonstrating the relevance of cosmetic management as an important research topic.
Trustworthy Sources of Health Information—Patients still view physicians as trustworthy and a key source of health care information and advice.30-32 Patients with vitiligo who have been directed to reliable information sources often express gratitude22 and want health professionals to remain an important source in their health information-seeking.31 Given the range in information discussed online, it may be valuable to invite patients to share what information they have encountered online.
Our study highlights the conflicting health information and advice shared by users in online forums, complicating an already psychologically burdensome condition. Guiding patients to credible, moderated sites and resources that are accurate, understandable, and easy to access may help dispel the conflicting messages and stories discussed in the online community.
Study Strengths and Limitations—Limitations included reporting bias and reliance on self-reported information on the diagnosis and extent of individuals’ vitiligo. Excluding social media websites and platforms from the data collection is a limitation to comprehensively assessing the topic of internet users with vitiligo. Many social media platforms direct patients and their family members to support groups and therefore may have excluded these particular individuals. Social media platforms were excluded from our research owing to the prerequisite of creating user accounts or registering as an online member. Our inclusion criteria were specific to forums that did not require registering or creating an account and were therefore freely accessible to all internet viewers. There is an inherent lack of context present in online forums, preventing data collection on individuals’ demographics and socioeconomic backgrounds. However, anonymity may have allowed individuals to express their thoughts more freely.
An integrated approach, along with our sampling method of online forums not requiring registration, allows for greater transferability and understanding of the health needs of the general public with vitiligo.
Conclusion
Individuals with vitiligo continue to seek peer psychosocial support for the physical and emotional management of their disease. Counseling those with vitiligo about cosmetic concealment options, homeopathy, and treatment scams remains paramount. Directing patients to evidence-based resources, along with providing structured sources of support, may help to improve the psychosocial burden and QOL experienced by patients with vitiligo. Connecting patients with local and national support groups moderated by physicians, such as the Global Vitiligo Foundation (https://globalvitiligofoundation.org/), may provide benefit to patients with vitiligo.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883-885.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo—part 2—classification, histopathology and treatment. An Bras Dermatol. 2014;89:784-790.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383-392.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Grimes PE, Billips M. Childhood vitiligo: clinical spectrum and therapeutic approaches. In: Hann SK, Nordlund JJ, eds. Vitiligo: A Monograph on the Basic and Clinical Science. Blackwell Science; 2000.
- Sawant NS, Vanjari NA, Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019:6879412.
- Liu Y, Kornfield R, Shaw BR, et al. When support is needed: social support solicitation and provision in an online alcohol use disorder forum. Digit Health. 2017;3:2055207617704274.
- Health 2.0. The Economist. 2007;384:14.
- Fox S. Peer-to-peer health care. Pew Research Center. February 28, 2011. Accessed December 14, 2021. https://www.pewinternet.org/wp-content/uploads/sites/9/media/Files/Reports/2011/Pew_P2PHealthcare_2011.pdf
- Li N, Orrange S, Kravitz RL, et al. Reasons for and predictors of patients’ online health information seeking following a medical appointment. Fam Pract. 2014;31:550-556.
- Idriss SZ, Kvedar JC, Watson AJ. The role of online support communities: benefits of expanded social networks to patients with psoriasis. Arch Dermatol. 2009;145:46-51.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol 2017;176:1500-1507.
- Santer M, Chandler D, Lown M, et al. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol. 2017;177:751-757.
- Santer M, Burgess H, Yardley L, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62:e261-e267.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736-739.
- Guba EG, Lincoln YS. Competing paradigms in qualitative research. In: Denzin NK, Lincoln YS, eds. Handbook of Qualitative Research. Sage Publications, Inc; 1994:105-117.
- Lincoln YS. Emerging criteria for quality in qualitative and interpretive research. Qualitative Inquiry. 2016;1:275-289.
- O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol. 2017;176:1500-1507.
- Teasdale E, Muller I, Sani AA, et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open. 2018;8:e018652.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772.
- Hewson C, Buchanan T, Brown I, et al. Ethics Guidelines for Internet-mediated Research. The British Psychological Society; 2017.
- Coulson NS. Sharing, supporting and sobriety: a qualitative analysis of messages posted to alcohol-related online discussion forums in the United Kingdom. J Subst Use. 2014;19:176-180.
- Attard A, Coulson NS. A thematic analysis of patient communication in Parkinson’s disease online support group discussion forums. Comput Hum Behav. 2012;28:500-506.
- Nelson PA, Chew-Graham CA, Griffiths CE, et al. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol. 2013;168:354-361.
- Gore C, Johnson RJ, Caress AL, et al. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy. 2005;60:938-943.
- Eleftheriadou V, Thomas KS, Whitton ME, et al. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804-814.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
- Sillence E, Briggs P, Harris PR, et al. How do patients evaluate and make use of online health information? Soc Sci Med. 2007;64:1853-1862.
- Hay MC, Cadigan RJ, Khanna D, et al. Prepared patients: internet information seeking by new rheumatology patients. Arthritis Rheum. 2008;59:575-582.
Vitiligo is a chronic dermatologic condition that negatively affects quality of life (QOL), with substantial burden on the psychosocial well-being of patients.1 There is no cure, and current treatment modalities are aimed at controlling the chronic relapsing condition.1-3 Despite topical and cosmetic treatments for stabilization and repigmentation, vitiligo remains unpredictable.3
All genders, races, ethnicities, and socioeconomic classes are equally affected.4 The underlying etiology of vitiligo remains unknown to a great extent and is more poorly understood by the general public compared with other skin diseases (eg, acne).5 Patients with vitiligo experience social withdrawal, decreased sense of self-esteem, anxiety, depression, and suicidal ideation.5,6 Stigmatization has the greatest impact on QOL, with strong correlations between avoidance behaviors and lesion concealment.6-8 Although the condition is especially disfiguring for darker skin types, lighter skin types also are substantially affected, with similar overall self-reported stress.6,7
Individuals with chronic illnesses such as vitiligo turn to online communities for health information and social support, commiserating with others who have the same condition.9,10 Online forums are platforms for asynchronous peer-to-peer exchange of disease-related information for better management of long-term disease.11 Moreover, of all available internet resources, online forum posts are the most commonly accessed source of information (91%) for patients following visits with their doctors.12
Qualitative research involving chronic skin conditions and the information exchanged in online forums has been conducted for patients with acne, psoriasis, and atopic dermatitis, but not for patients with vitiligo.13-16 Although online questionnaires have been administered to patients with vitiligo, the content within online forums is not well characterized.2,17
The purpose of this qualitative study was to evaluate the online content exchanged by individuals with vitiligo to better understand the general attitudes and help-seeking behaviors in online forums.
Methods
Study Design—This qualitative study sought to investigate health beliefs and messages about vitiligo posted by users in US-based online discussion forums. An interpretive research paradigm was utilized so that all content collected in online forums were the views expressed by individuals.18-20 An integrated approach was used in the development of the coding manual, with pre-established major themes and subthemes as a guiding framework.16,21,22 We adhered to an inductive grounded method by means of de novo line-by-line coding, such that we had flexibility for new subthemes to emerge throughout the duration of the entire coding process.23
Individual posts and subsequent replies embedded within public online forums were used as the collected data source. Google was utilized as the primary search engine to identify forums pertaining to vitiligo, as 80% of US adults with chronic disease report that their inquiries for health information start with Google, Bing, or Yahoo.24 The institutional review board at the Wake Forest School of Medicine (Winston-Salem, North Carolina) granted approval of the study (IRB00063073). Online forums were considered “property” of the public domain and were accessible to all, eliminating the need for written informed consent.24-26
Search Criteria—We conducted our forum search in February 2020 with a systematic approach using predetermined phrases—online forum vitiligo support, vitiligo online message board, and vitiligo forums—which yielded more than 358,171 total results (eTable 1). Threads were identified in chronological order (from newest to oldest) based on how they appeared during each internet search, and all Google results for the respective search phrases were reviewed. Dates of selected threads ranged from 2005 to 2020. Only sites with US domains were included. Posts that either included views and understandings of vitiligo or belonged to a thread that contained a vitiligo discussion were deemed relevant for inclusion. Forums were excluded if registration or means of payment was required to view posts, if there were fewer than 2 user replies to a thread, if threads contained patient photographs, or if no posts had been made in the last 2 years (rendering the thread inactive). No social media platforms, such as Facebook, or formal online platforms, such as MyVitiligoTeam, were included in the search. A no-fee-for-access was chosen for this study, as the majority of those with a chronic condition who encounter a required paywall find the information elsewhere.25
Data Analysis—A total of 39 online forums were deemed relevant to the topic of vitiligo; 9 of them met inclusion criteria (eTable 2). The messages within the forums were copied verbatim into a password-encrypted text document, and usernames in the threads were de-identified, ensuring user confidentiality.
An inductive thematic analysis was utilized to explore the views and beliefs of online forum users discussing vitiligo. One author (M.B.G.) read the extracted message threads, developed an initial codebook, and established a finalized version with the agreement of another author (A.M.B.)(eTable 3). The forums were independently coded (M.B.G. and A.M.B.) in a line-by-line manner according to the codebook. Discrepancies were documented and resolved. Data saturation was adequately achieved, such that no new themes emerged during the iterative coding process. NVivo was used for qualitative analysis.
Results
Nine forums met inclusion criteria, comprising 105 pages of text. There were 61 total discussion threads, with 382 anonymous contributing users. Most users initiated a thread by posting either a question, an advice statement, or a request for help. The psychosocial impact of the disease permeated multiple domains,including personal relationships and daily life. Several threads discussed treatment, including effective camouflage and makeup, as well as peer validation of physician-prescribed treatments, along with threads dedicated to “cures” or homeopathy regimens. In several instances, commercial product endorsement, testimonials, and marketing links were reposted by the same user multiple times.
Inductive thematic analysis highlighted diverse themes and subthemes related to the beliefs and perspectives of users with vitiligo or with relatives or friends with vitiligo: psychosocial impact, disease management and camouflage/concealment, alternative medicine/homeopathy/cures, interactions with the public and health care providers, and skin tone and race. Quotes from individuals were included to demonstrate themes and subthemes.
Psychosocial Impact: QOL, Sources of Support, and Coping—There was a broad range of comments on how patients cope with and view their vitiligo. Some individuals felt vitiligo made them special, and others were at peace with and accepted their condition. In contrast, others reported the disease had devastated them and interfered with relationships. Individuals shared their stories of grief and hardships through childhood and adulthood and their concerns, especially on affected visible areas or the potential for disease progression. Users were vocal about how vitiligo affected their daily routines and lives, sharing how they felt uncomfortable outside the home, no longer engaged in swimming or exposing their legs, and preferred to stay inside instead. Some users adopted a “tough love” approach to coping, sharing how they have learned to either embrace their vitiligo or “live with it.” Some examples include:
“My best advice is go with the flow, vitiligo is not the worst thing that can happen.”
“I hate my life with vitiligo yet really I feel so selfish that there is much worse suffering in the world than a few white patches.”
Other advice was very practical:
“I hope it isn’t vanity that is tearing you apart because that is only skin deep. Make a fashion statement with hats.”
Some users acknowledged and adopted the mantra that vitiligo is not a somatic condition or “physical ailment,” while others emphasized its pervasive psychological burden:
“I still deal with this psychologically . . . You must keep a positive attitude and frame of mind . . . Vitiligo will not kill you, but you do need to stay strong and keep your head up emotionally.”
“I am just really thankful that I have a disease that will not kill me or that has [not] affected me physically at all. I consider myself lucky.”
Disease Management: Treatment, Vitiligo Course, Advice-Seeking, Camouflage—The range of information discussed for treatment was highly variable. There were many accounts in which users advised others to seek professional help, namely that of a dermatologist, for a formal assessment. Many expressed frustrations with treatments and their ineffectiveness, to which the majority of users said to consult with a professional and to remain patient and hopeful/optimistic:
“The best thing to do would be to take an appointment with a dermatologist and have the discoloration checked out. That’s the only way to know whether it is vitiligo or not.”
“My way of dealing with it is to gain control by camouflage.”
“The calming effect of being in control of my vitiligo, whether with concealers, self-tan or anything else, has stopped my feelings of despair.”
Beliefs on Alternative Medicine: Homeopathy and Alternative Regimens—Although some threads started with a post asking for the best treatments, others initiated a discussion by posting “best herbal treatments for cure” or “how to cure my vitiligo,” emphasizing the beliefs and wishes for a cure for vitiligo. Alternative therapies that users endorsed included apple cider vinegar, toothpaste, vitamins, and Ayurvedic treatment, among others. Dietary plans were popular, with users claiming success with dietary alterations in stopping and preventing lesion progression. For example, individuals felt that avoidance of sugar, meat, dairy, and citrus fruits or drinks and consumption of only filtered water were crucial to preventing further lesion spread and resulted in their “cure”:
“Don’t eat chocolate, wine (made of grapes), coffee, or tea if you don’t want to have vitiligo or let it get worse. Take Vitamin B, biotin, and nuts for Vitamin E.”
Other dangerous messages pitted treatments by health professionals against beliefs in homeopathy:
“I feel that vitiligo treatment is all in your diet and vitamins. All that medicine and UV lights is a no-no . . .w ith every medicine there is a side effect. The doctors could be healing your vitiligo and severely damaging you inside and out, and you won’t know until years later.”
There was a minor presence of users advising against homeopathy and the associated misinformation and inaccurate claims on curing vitiligo, though this group was small in comparison to the number of users posting outlandish claims on cure:
“There is no cure . . . It’s where your immune system attacks your skin cells causing loss of pigmentation. The skin that has lost the pigmentation can’t be reversed.”
Interactions With the Public and Health Care Providers—Those with vitiligo encounter unique situations in public and in their daily lives. Many of the accounts shared anecdotal stories on how patients have handled the stigma and discrimination faced:
“I have had to face discrimination at school, public places, college, functions, and every new person I have met has asked me this: ‘how did this happen?’”
Those with vitiligo even stated how they wished others would deal with their condition out in public, hoping that others would directly ask what the lesions were instead of the more hurtful staring. There were many stories in which users said others feel vitiligo was contagious or “dirty” and stressed that the condition is not infectious:
“I refer to myself as ‘camo-man’ and reassure people I come into contact with that it is not contagious.”
“Once I was eating at a restaurant . . . and a little girl said to her mom, ‘Look, Mom, that lady doesn’t wash her arms, look how dirty they are.’ That just broke my heart.”
Skin Tone and Implications—The belief that vitiligo lesions are less dramatic or less anxiety provoking for individuals with lighter skin was noted by users themselves and by health care providers in certain cases. Skin tone and its impact on QOL was confusing and contentious. Some users with fair skin stated their vitiligo was “less of an annoyance” or “less obvious” compared with individuals with darker complexions. Conversely, other accounts of self-reported White users vehemently stressed the anxieties felt by depigmented lesions, despite being “already white at baseline.”
“Was told by my dermatologist (upon diagnosis) that ‘You’re lucky you’re not African American—it shows up on them much worse. You’re so fair, it doesn’t really matter.’ ”
“You didn’t say what race you are. I could imagine it has a bigger impact if you are anything other than White.”
Comment
Patients Looking for Cures—The general attitude within the forums was uplifting and encouraging, with users detailing how they respond to others in public and sharing their personal perspectives. We found a mix of information regarding disease management and treatment of vitiligo. Overall, there was uncertainty about treatments, with individuals expressing concern that their treatments were ineffective or had failed or that better alternatives would be more suitable for their condition. We found many anecdotal endorsements of homeopathic remedies for vitiligo, with users boasting that their disease had not only been cured but had never returned. Some users completely denounced these statements, while other threads seemed to revolve completely around “cure” discussions with no dissenting voices. The number of discussions related to homeopathy was concerning. Furthermore, there often were no moderators within threads to remove cure-related content, whether commercially endorsed or anecdotal. It is plausible that supplements and vitamins recommended by some physicians may be incorrectly interpreted as a “cure” in online discussions. Our findings are consistent with prior reports that forums are a platform to express dissatisfaction with treatment and the need for additional treatment options.15,22
Concern Expressed by Health Care Providers—Prior qualitative research has described how patients with chronic dermatologic conditions believe that health care providers minimize patients’ psychological distress.27,28 We found several accounts in which an individual had explicitly stated their provider had “belittled” the extent and impact of vitiligo when comparing skin phototypes. This suggests either that physicians underestimate the impact of vitiligo on their patients or that physicians are not expressing enough empathic concern about the impact the condition has on those affected.
Cosmetic Aspects of Vitiligo—Few clinical trials have investigated QOL and cosmetic acceptability of treatments as outcome measures.29 We found several instances in which users with vitiligo had reported being dismissed as having a “cosmetic disease,” consistent with other work demonstrating the negative impact on such dismissals.22 Moreover, concealment and camouflage techniques frequently were discussed, demonstrating the relevance of cosmetic management as an important research topic.
Trustworthy Sources of Health Information—Patients still view physicians as trustworthy and a key source of health care information and advice.30-32 Patients with vitiligo who have been directed to reliable information sources often express gratitude22 and want health professionals to remain an important source in their health information-seeking.31 Given the range in information discussed online, it may be valuable to invite patients to share what information they have encountered online.
Our study highlights the conflicting health information and advice shared by users in online forums, complicating an already psychologically burdensome condition. Guiding patients to credible, moderated sites and resources that are accurate, understandable, and easy to access may help dispel the conflicting messages and stories discussed in the online community.
Study Strengths and Limitations—Limitations included reporting bias and reliance on self-reported information on the diagnosis and extent of individuals’ vitiligo. Excluding social media websites and platforms from the data collection is a limitation to comprehensively assessing the topic of internet users with vitiligo. Many social media platforms direct patients and their family members to support groups and therefore may have excluded these particular individuals. Social media platforms were excluded from our research owing to the prerequisite of creating user accounts or registering as an online member. Our inclusion criteria were specific to forums that did not require registering or creating an account and were therefore freely accessible to all internet viewers. There is an inherent lack of context present in online forums, preventing data collection on individuals’ demographics and socioeconomic backgrounds. However, anonymity may have allowed individuals to express their thoughts more freely.
An integrated approach, along with our sampling method of online forums not requiring registration, allows for greater transferability and understanding of the health needs of the general public with vitiligo.
Conclusion
Individuals with vitiligo continue to seek peer psychosocial support for the physical and emotional management of their disease. Counseling those with vitiligo about cosmetic concealment options, homeopathy, and treatment scams remains paramount. Directing patients to evidence-based resources, along with providing structured sources of support, may help to improve the psychosocial burden and QOL experienced by patients with vitiligo. Connecting patients with local and national support groups moderated by physicians, such as the Global Vitiligo Foundation (https://globalvitiligofoundation.org/), may provide benefit to patients with vitiligo.
Vitiligo is a chronic dermatologic condition that negatively affects quality of life (QOL), with substantial burden on the psychosocial well-being of patients.1 There is no cure, and current treatment modalities are aimed at controlling the chronic relapsing condition.1-3 Despite topical and cosmetic treatments for stabilization and repigmentation, vitiligo remains unpredictable.3
All genders, races, ethnicities, and socioeconomic classes are equally affected.4 The underlying etiology of vitiligo remains unknown to a great extent and is more poorly understood by the general public compared with other skin diseases (eg, acne).5 Patients with vitiligo experience social withdrawal, decreased sense of self-esteem, anxiety, depression, and suicidal ideation.5,6 Stigmatization has the greatest impact on QOL, with strong correlations between avoidance behaviors and lesion concealment.6-8 Although the condition is especially disfiguring for darker skin types, lighter skin types also are substantially affected, with similar overall self-reported stress.6,7
Individuals with chronic illnesses such as vitiligo turn to online communities for health information and social support, commiserating with others who have the same condition.9,10 Online forums are platforms for asynchronous peer-to-peer exchange of disease-related information for better management of long-term disease.11 Moreover, of all available internet resources, online forum posts are the most commonly accessed source of information (91%) for patients following visits with their doctors.12
Qualitative research involving chronic skin conditions and the information exchanged in online forums has been conducted for patients with acne, psoriasis, and atopic dermatitis, but not for patients with vitiligo.13-16 Although online questionnaires have been administered to patients with vitiligo, the content within online forums is not well characterized.2,17
The purpose of this qualitative study was to evaluate the online content exchanged by individuals with vitiligo to better understand the general attitudes and help-seeking behaviors in online forums.
Methods
Study Design—This qualitative study sought to investigate health beliefs and messages about vitiligo posted by users in US-based online discussion forums. An interpretive research paradigm was utilized so that all content collected in online forums were the views expressed by individuals.18-20 An integrated approach was used in the development of the coding manual, with pre-established major themes and subthemes as a guiding framework.16,21,22 We adhered to an inductive grounded method by means of de novo line-by-line coding, such that we had flexibility for new subthemes to emerge throughout the duration of the entire coding process.23
Individual posts and subsequent replies embedded within public online forums were used as the collected data source. Google was utilized as the primary search engine to identify forums pertaining to vitiligo, as 80% of US adults with chronic disease report that their inquiries for health information start with Google, Bing, or Yahoo.24 The institutional review board at the Wake Forest School of Medicine (Winston-Salem, North Carolina) granted approval of the study (IRB00063073). Online forums were considered “property” of the public domain and were accessible to all, eliminating the need for written informed consent.24-26
Search Criteria—We conducted our forum search in February 2020 with a systematic approach using predetermined phrases—online forum vitiligo support, vitiligo online message board, and vitiligo forums—which yielded more than 358,171 total results (eTable 1). Threads were identified in chronological order (from newest to oldest) based on how they appeared during each internet search, and all Google results for the respective search phrases were reviewed. Dates of selected threads ranged from 2005 to 2020. Only sites with US domains were included. Posts that either included views and understandings of vitiligo or belonged to a thread that contained a vitiligo discussion were deemed relevant for inclusion. Forums were excluded if registration or means of payment was required to view posts, if there were fewer than 2 user replies to a thread, if threads contained patient photographs, or if no posts had been made in the last 2 years (rendering the thread inactive). No social media platforms, such as Facebook, or formal online platforms, such as MyVitiligoTeam, were included in the search. A no-fee-for-access was chosen for this study, as the majority of those with a chronic condition who encounter a required paywall find the information elsewhere.25
Data Analysis—A total of 39 online forums were deemed relevant to the topic of vitiligo; 9 of them met inclusion criteria (eTable 2). The messages within the forums were copied verbatim into a password-encrypted text document, and usernames in the threads were de-identified, ensuring user confidentiality.
An inductive thematic analysis was utilized to explore the views and beliefs of online forum users discussing vitiligo. One author (M.B.G.) read the extracted message threads, developed an initial codebook, and established a finalized version with the agreement of another author (A.M.B.)(eTable 3). The forums were independently coded (M.B.G. and A.M.B.) in a line-by-line manner according to the codebook. Discrepancies were documented and resolved. Data saturation was adequately achieved, such that no new themes emerged during the iterative coding process. NVivo was used for qualitative analysis.
Results
Nine forums met inclusion criteria, comprising 105 pages of text. There were 61 total discussion threads, with 382 anonymous contributing users. Most users initiated a thread by posting either a question, an advice statement, or a request for help. The psychosocial impact of the disease permeated multiple domains,including personal relationships and daily life. Several threads discussed treatment, including effective camouflage and makeup, as well as peer validation of physician-prescribed treatments, along with threads dedicated to “cures” or homeopathy regimens. In several instances, commercial product endorsement, testimonials, and marketing links were reposted by the same user multiple times.
Inductive thematic analysis highlighted diverse themes and subthemes related to the beliefs and perspectives of users with vitiligo or with relatives or friends with vitiligo: psychosocial impact, disease management and camouflage/concealment, alternative medicine/homeopathy/cures, interactions with the public and health care providers, and skin tone and race. Quotes from individuals were included to demonstrate themes and subthemes.
Psychosocial Impact: QOL, Sources of Support, and Coping—There was a broad range of comments on how patients cope with and view their vitiligo. Some individuals felt vitiligo made them special, and others were at peace with and accepted their condition. In contrast, others reported the disease had devastated them and interfered with relationships. Individuals shared their stories of grief and hardships through childhood and adulthood and their concerns, especially on affected visible areas or the potential for disease progression. Users were vocal about how vitiligo affected their daily routines and lives, sharing how they felt uncomfortable outside the home, no longer engaged in swimming or exposing their legs, and preferred to stay inside instead. Some users adopted a “tough love” approach to coping, sharing how they have learned to either embrace their vitiligo or “live with it.” Some examples include:
“My best advice is go with the flow, vitiligo is not the worst thing that can happen.”
“I hate my life with vitiligo yet really I feel so selfish that there is much worse suffering in the world than a few white patches.”
Other advice was very practical:
“I hope it isn’t vanity that is tearing you apart because that is only skin deep. Make a fashion statement with hats.”
Some users acknowledged and adopted the mantra that vitiligo is not a somatic condition or “physical ailment,” while others emphasized its pervasive psychological burden:
“I still deal with this psychologically . . . You must keep a positive attitude and frame of mind . . . Vitiligo will not kill you, but you do need to stay strong and keep your head up emotionally.”
“I am just really thankful that I have a disease that will not kill me or that has [not] affected me physically at all. I consider myself lucky.”
Disease Management: Treatment, Vitiligo Course, Advice-Seeking, Camouflage—The range of information discussed for treatment was highly variable. There were many accounts in which users advised others to seek professional help, namely that of a dermatologist, for a formal assessment. Many expressed frustrations with treatments and their ineffectiveness, to which the majority of users said to consult with a professional and to remain patient and hopeful/optimistic:
“The best thing to do would be to take an appointment with a dermatologist and have the discoloration checked out. That’s the only way to know whether it is vitiligo or not.”
“My way of dealing with it is to gain control by camouflage.”
“The calming effect of being in control of my vitiligo, whether with concealers, self-tan or anything else, has stopped my feelings of despair.”
Beliefs on Alternative Medicine: Homeopathy and Alternative Regimens—Although some threads started with a post asking for the best treatments, others initiated a discussion by posting “best herbal treatments for cure” or “how to cure my vitiligo,” emphasizing the beliefs and wishes for a cure for vitiligo. Alternative therapies that users endorsed included apple cider vinegar, toothpaste, vitamins, and Ayurvedic treatment, among others. Dietary plans were popular, with users claiming success with dietary alterations in stopping and preventing lesion progression. For example, individuals felt that avoidance of sugar, meat, dairy, and citrus fruits or drinks and consumption of only filtered water were crucial to preventing further lesion spread and resulted in their “cure”:
“Don’t eat chocolate, wine (made of grapes), coffee, or tea if you don’t want to have vitiligo or let it get worse. Take Vitamin B, biotin, and nuts for Vitamin E.”
Other dangerous messages pitted treatments by health professionals against beliefs in homeopathy:
“I feel that vitiligo treatment is all in your diet and vitamins. All that medicine and UV lights is a no-no . . .w ith every medicine there is a side effect. The doctors could be healing your vitiligo and severely damaging you inside and out, and you won’t know until years later.”
There was a minor presence of users advising against homeopathy and the associated misinformation and inaccurate claims on curing vitiligo, though this group was small in comparison to the number of users posting outlandish claims on cure:
“There is no cure . . . It’s where your immune system attacks your skin cells causing loss of pigmentation. The skin that has lost the pigmentation can’t be reversed.”
Interactions With the Public and Health Care Providers—Those with vitiligo encounter unique situations in public and in their daily lives. Many of the accounts shared anecdotal stories on how patients have handled the stigma and discrimination faced:
“I have had to face discrimination at school, public places, college, functions, and every new person I have met has asked me this: ‘how did this happen?’”
Those with vitiligo even stated how they wished others would deal with their condition out in public, hoping that others would directly ask what the lesions were instead of the more hurtful staring. There were many stories in which users said others feel vitiligo was contagious or “dirty” and stressed that the condition is not infectious:
“I refer to myself as ‘camo-man’ and reassure people I come into contact with that it is not contagious.”
“Once I was eating at a restaurant . . . and a little girl said to her mom, ‘Look, Mom, that lady doesn’t wash her arms, look how dirty they are.’ That just broke my heart.”
Skin Tone and Implications—The belief that vitiligo lesions are less dramatic or less anxiety provoking for individuals with lighter skin was noted by users themselves and by health care providers in certain cases. Skin tone and its impact on QOL was confusing and contentious. Some users with fair skin stated their vitiligo was “less of an annoyance” or “less obvious” compared with individuals with darker complexions. Conversely, other accounts of self-reported White users vehemently stressed the anxieties felt by depigmented lesions, despite being “already white at baseline.”
“Was told by my dermatologist (upon diagnosis) that ‘You’re lucky you’re not African American—it shows up on them much worse. You’re so fair, it doesn’t really matter.’ ”
“You didn’t say what race you are. I could imagine it has a bigger impact if you are anything other than White.”
Comment
Patients Looking for Cures—The general attitude within the forums was uplifting and encouraging, with users detailing how they respond to others in public and sharing their personal perspectives. We found a mix of information regarding disease management and treatment of vitiligo. Overall, there was uncertainty about treatments, with individuals expressing concern that their treatments were ineffective or had failed or that better alternatives would be more suitable for their condition. We found many anecdotal endorsements of homeopathic remedies for vitiligo, with users boasting that their disease had not only been cured but had never returned. Some users completely denounced these statements, while other threads seemed to revolve completely around “cure” discussions with no dissenting voices. The number of discussions related to homeopathy was concerning. Furthermore, there often were no moderators within threads to remove cure-related content, whether commercially endorsed or anecdotal. It is plausible that supplements and vitamins recommended by some physicians may be incorrectly interpreted as a “cure” in online discussions. Our findings are consistent with prior reports that forums are a platform to express dissatisfaction with treatment and the need for additional treatment options.15,22
Concern Expressed by Health Care Providers—Prior qualitative research has described how patients with chronic dermatologic conditions believe that health care providers minimize patients’ psychological distress.27,28 We found several accounts in which an individual had explicitly stated their provider had “belittled” the extent and impact of vitiligo when comparing skin phototypes. This suggests either that physicians underestimate the impact of vitiligo on their patients or that physicians are not expressing enough empathic concern about the impact the condition has on those affected.
Cosmetic Aspects of Vitiligo—Few clinical trials have investigated QOL and cosmetic acceptability of treatments as outcome measures.29 We found several instances in which users with vitiligo had reported being dismissed as having a “cosmetic disease,” consistent with other work demonstrating the negative impact on such dismissals.22 Moreover, concealment and camouflage techniques frequently were discussed, demonstrating the relevance of cosmetic management as an important research topic.
Trustworthy Sources of Health Information—Patients still view physicians as trustworthy and a key source of health care information and advice.30-32 Patients with vitiligo who have been directed to reliable information sources often express gratitude22 and want health professionals to remain an important source in their health information-seeking.31 Given the range in information discussed online, it may be valuable to invite patients to share what information they have encountered online.
Our study highlights the conflicting health information and advice shared by users in online forums, complicating an already psychologically burdensome condition. Guiding patients to credible, moderated sites and resources that are accurate, understandable, and easy to access may help dispel the conflicting messages and stories discussed in the online community.
Study Strengths and Limitations—Limitations included reporting bias and reliance on self-reported information on the diagnosis and extent of individuals’ vitiligo. Excluding social media websites and platforms from the data collection is a limitation to comprehensively assessing the topic of internet users with vitiligo. Many social media platforms direct patients and their family members to support groups and therefore may have excluded these particular individuals. Social media platforms were excluded from our research owing to the prerequisite of creating user accounts or registering as an online member. Our inclusion criteria were specific to forums that did not require registering or creating an account and were therefore freely accessible to all internet viewers. There is an inherent lack of context present in online forums, preventing data collection on individuals’ demographics and socioeconomic backgrounds. However, anonymity may have allowed individuals to express their thoughts more freely.
An integrated approach, along with our sampling method of online forums not requiring registration, allows for greater transferability and understanding of the health needs of the general public with vitiligo.
Conclusion
Individuals with vitiligo continue to seek peer psychosocial support for the physical and emotional management of their disease. Counseling those with vitiligo about cosmetic concealment options, homeopathy, and treatment scams remains paramount. Directing patients to evidence-based resources, along with providing structured sources of support, may help to improve the psychosocial burden and QOL experienced by patients with vitiligo. Connecting patients with local and national support groups moderated by physicians, such as the Global Vitiligo Foundation (https://globalvitiligofoundation.org/), may provide benefit to patients with vitiligo.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883-885.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo—part 2—classification, histopathology and treatment. An Bras Dermatol. 2014;89:784-790.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383-392.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Grimes PE, Billips M. Childhood vitiligo: clinical spectrum and therapeutic approaches. In: Hann SK, Nordlund JJ, eds. Vitiligo: A Monograph on the Basic and Clinical Science. Blackwell Science; 2000.
- Sawant NS, Vanjari NA, Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019:6879412.
- Liu Y, Kornfield R, Shaw BR, et al. When support is needed: social support solicitation and provision in an online alcohol use disorder forum. Digit Health. 2017;3:2055207617704274.
- Health 2.0. The Economist. 2007;384:14.
- Fox S. Peer-to-peer health care. Pew Research Center. February 28, 2011. Accessed December 14, 2021. https://www.pewinternet.org/wp-content/uploads/sites/9/media/Files/Reports/2011/Pew_P2PHealthcare_2011.pdf
- Li N, Orrange S, Kravitz RL, et al. Reasons for and predictors of patients’ online health information seeking following a medical appointment. Fam Pract. 2014;31:550-556.
- Idriss SZ, Kvedar JC, Watson AJ. The role of online support communities: benefits of expanded social networks to patients with psoriasis. Arch Dermatol. 2009;145:46-51.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol 2017;176:1500-1507.
- Santer M, Chandler D, Lown M, et al. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol. 2017;177:751-757.
- Santer M, Burgess H, Yardley L, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62:e261-e267.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736-739.
- Guba EG, Lincoln YS. Competing paradigms in qualitative research. In: Denzin NK, Lincoln YS, eds. Handbook of Qualitative Research. Sage Publications, Inc; 1994:105-117.
- Lincoln YS. Emerging criteria for quality in qualitative and interpretive research. Qualitative Inquiry. 2016;1:275-289.
- O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol. 2017;176:1500-1507.
- Teasdale E, Muller I, Sani AA, et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open. 2018;8:e018652.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772.
- Hewson C, Buchanan T, Brown I, et al. Ethics Guidelines for Internet-mediated Research. The British Psychological Society; 2017.
- Coulson NS. Sharing, supporting and sobriety: a qualitative analysis of messages posted to alcohol-related online discussion forums in the United Kingdom. J Subst Use. 2014;19:176-180.
- Attard A, Coulson NS. A thematic analysis of patient communication in Parkinson’s disease online support group discussion forums. Comput Hum Behav. 2012;28:500-506.
- Nelson PA, Chew-Graham CA, Griffiths CE, et al. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol. 2013;168:354-361.
- Gore C, Johnson RJ, Caress AL, et al. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy. 2005;60:938-943.
- Eleftheriadou V, Thomas KS, Whitton ME, et al. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804-814.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
- Sillence E, Briggs P, Harris PR, et al. How do patients evaluate and make use of online health information? Soc Sci Med. 2007;64:1853-1862.
- Hay MC, Cadigan RJ, Khanna D, et al. Prepared patients: internet information seeking by new rheumatology patients. Arthritis Rheum. 2008;59:575-582.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883-885.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo—part 2—classification, histopathology and treatment. An Bras Dermatol. 2014;89:784-790.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383-392.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Grimes PE, Billips M. Childhood vitiligo: clinical spectrum and therapeutic approaches. In: Hann SK, Nordlund JJ, eds. Vitiligo: A Monograph on the Basic and Clinical Science. Blackwell Science; 2000.
- Sawant NS, Vanjari NA, Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019:6879412.
- Liu Y, Kornfield R, Shaw BR, et al. When support is needed: social support solicitation and provision in an online alcohol use disorder forum. Digit Health. 2017;3:2055207617704274.
- Health 2.0. The Economist. 2007;384:14.
- Fox S. Peer-to-peer health care. Pew Research Center. February 28, 2011. Accessed December 14, 2021. https://www.pewinternet.org/wp-content/uploads/sites/9/media/Files/Reports/2011/Pew_P2PHealthcare_2011.pdf
- Li N, Orrange S, Kravitz RL, et al. Reasons for and predictors of patients’ online health information seeking following a medical appointment. Fam Pract. 2014;31:550-556.
- Idriss SZ, Kvedar JC, Watson AJ. The role of online support communities: benefits of expanded social networks to patients with psoriasis. Arch Dermatol. 2009;145:46-51.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol 2017;176:1500-1507.
- Santer M, Chandler D, Lown M, et al. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol. 2017;177:751-757.
- Santer M, Burgess H, Yardley L, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62:e261-e267.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736-739.
- Guba EG, Lincoln YS. Competing paradigms in qualitative research. In: Denzin NK, Lincoln YS, eds. Handbook of Qualitative Research. Sage Publications, Inc; 1994:105-117.
- Lincoln YS. Emerging criteria for quality in qualitative and interpretive research. Qualitative Inquiry. 2016;1:275-289.
- O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol. 2017;176:1500-1507.
- Teasdale E, Muller I, Sani AA, et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open. 2018;8:e018652.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772.
- Hewson C, Buchanan T, Brown I, et al. Ethics Guidelines for Internet-mediated Research. The British Psychological Society; 2017.
- Coulson NS. Sharing, supporting and sobriety: a qualitative analysis of messages posted to alcohol-related online discussion forums in the United Kingdom. J Subst Use. 2014;19:176-180.
- Attard A, Coulson NS. A thematic analysis of patient communication in Parkinson’s disease online support group discussion forums. Comput Hum Behav. 2012;28:500-506.
- Nelson PA, Chew-Graham CA, Griffiths CE, et al. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol. 2013;168:354-361.
- Gore C, Johnson RJ, Caress AL, et al. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy. 2005;60:938-943.
- Eleftheriadou V, Thomas KS, Whitton ME, et al. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804-814.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
- Sillence E, Briggs P, Harris PR, et al. How do patients evaluate and make use of online health information? Soc Sci Med. 2007;64:1853-1862.
- Hay MC, Cadigan RJ, Khanna D, et al. Prepared patients: internet information seeking by new rheumatology patients. Arthritis Rheum. 2008;59:575-582.
Practice Points
- Online forums provide invaluable insight on vitiligo disease management, psychosocial impact, and burden on quality of life. Patient care can be improved by inquiring where patients seek information and whether online forums are utilized.
- Commonly discussed topics in online forums were cosmetic concealment of vitiligo lesions and homeopathy or “cure” discussions. Health care providers can engage in honest conversations about evidence-based medical treatments for vitiligo. The interest in cosmetic management highlights a relevant research area in this field.
- Health care providers can better serve patients with vitiligo by providing online resources that are reputable and can help guide patients to credible internet sources such as the Global Vitiligo Foundation.
Anecdote Increases Patient Willingness to Take a Biologic Medication for Psoriasis
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
Practice Points
- Patients often are apprehensive to start biologic medications for their psoriasis.
- Clinical trial evidence of a biologic medication’s efficacy and safety as well as anecdotes of patient experiences appear to be important factors for patients when considering taking a medication.
- The use of an anecdote—alone or in combination with clinical trial evidence—to help patients overcome fears of starting a biologic medication for their psoriasis may be an effective way to improve patients’ willingness to take treatment.
Psoriasis Highlights From AADVMX 2021
Key studies on psoriasis presented at the American Academy of Dermatology Virtual Meeting Experience (AAD VMX) 2021included data on new topical treatments and biological therapies.
Dr Steven Feldman, of Wake Forest School of Medicine, reviews trial data demonstrating the efficacy of a topical formulation of roflumilast, a phosphodiesterase type 4 (PDE-4) inhibitor previously used in oral systemic form to treat psoriasis.
He also discusses a meta-analysis of the efficacy of biologics favoring newer treatments, such as drugs targeting IL-17 and IL-23.
Dr Feldman reviews the results of two pivotal phase 3 trials presented at the meeting. The POETYK study examined deucravacitinib, a TYK2 inhibitor. In a head-to-head comparison, deucravacitinib was found to be more effective and better tolerated than apremilast in treating psoriasis. BE RADIANT, another head-to-head study, compared the IL-17 blockers bimekizumab and secukinumab. The year-long study favored bimekizumab, though it was associated with a higher risk for candidiasis.
Finally, Dr Feldman discusses the significance of a study showing that psoriasis patients have an approximately 20% higher risk for COVID-19 infection compared with a control group.
--
Steven R. Feldman, MD, PhD, Professor, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Steven R. Feldman, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AbbVie; Alvotech; Advance Medical; Almirall; Arena; Bristol-Myers Squibb; Caremark; Amgen; Celgene; Galderma Laboratories; Gerson Lehrman Group; Guidepoint Global; Helsinn; Janssen; Kikaku; Leo Pharma; Eli Lilly and Company; Merck; Mylan; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sienna; Sun Pharma; Suncare Research; Xenoport
Serve(d) as a speaker for: AbbVie; Amgen; Celgene; Janssen; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sun Pharma
Receive(d) grant support from: AbbVie; Amgen; Celgene; Galderma Laboratories; Janssen; Eli Lilly and Company; Novartis; Pfizer; Regeneron; Sanofi
Receive(d) royalties from: Informa; UpToDate; Xlibris
Holds stock in: Causa Technologies; Medical Quality Enhancement Corporation
Serves as founder and chief technology officer for: Causa Technologies
Key studies on psoriasis presented at the American Academy of Dermatology Virtual Meeting Experience (AAD VMX) 2021included data on new topical treatments and biological therapies.
Dr Steven Feldman, of Wake Forest School of Medicine, reviews trial data demonstrating the efficacy of a topical formulation of roflumilast, a phosphodiesterase type 4 (PDE-4) inhibitor previously used in oral systemic form to treat psoriasis.
He also discusses a meta-analysis of the efficacy of biologics favoring newer treatments, such as drugs targeting IL-17 and IL-23.
Dr Feldman reviews the results of two pivotal phase 3 trials presented at the meeting. The POETYK study examined deucravacitinib, a TYK2 inhibitor. In a head-to-head comparison, deucravacitinib was found to be more effective and better tolerated than apremilast in treating psoriasis. BE RADIANT, another head-to-head study, compared the IL-17 blockers bimekizumab and secukinumab. The year-long study favored bimekizumab, though it was associated with a higher risk for candidiasis.
Finally, Dr Feldman discusses the significance of a study showing that psoriasis patients have an approximately 20% higher risk for COVID-19 infection compared with a control group.
--
Steven R. Feldman, MD, PhD, Professor, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Steven R. Feldman, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AbbVie; Alvotech; Advance Medical; Almirall; Arena; Bristol-Myers Squibb; Caremark; Amgen; Celgene; Galderma Laboratories; Gerson Lehrman Group; Guidepoint Global; Helsinn; Janssen; Kikaku; Leo Pharma; Eli Lilly and Company; Merck; Mylan; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sienna; Sun Pharma; Suncare Research; Xenoport
Serve(d) as a speaker for: AbbVie; Amgen; Celgene; Janssen; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sun Pharma
Receive(d) grant support from: AbbVie; Amgen; Celgene; Galderma Laboratories; Janssen; Eli Lilly and Company; Novartis; Pfizer; Regeneron; Sanofi
Receive(d) royalties from: Informa; UpToDate; Xlibris
Holds stock in: Causa Technologies; Medical Quality Enhancement Corporation
Serves as founder and chief technology officer for: Causa Technologies
Key studies on psoriasis presented at the American Academy of Dermatology Virtual Meeting Experience (AAD VMX) 2021included data on new topical treatments and biological therapies.
Dr Steven Feldman, of Wake Forest School of Medicine, reviews trial data demonstrating the efficacy of a topical formulation of roflumilast, a phosphodiesterase type 4 (PDE-4) inhibitor previously used in oral systemic form to treat psoriasis.
He also discusses a meta-analysis of the efficacy of biologics favoring newer treatments, such as drugs targeting IL-17 and IL-23.
Dr Feldman reviews the results of two pivotal phase 3 trials presented at the meeting. The POETYK study examined deucravacitinib, a TYK2 inhibitor. In a head-to-head comparison, deucravacitinib was found to be more effective and better tolerated than apremilast in treating psoriasis. BE RADIANT, another head-to-head study, compared the IL-17 blockers bimekizumab and secukinumab. The year-long study favored bimekizumab, though it was associated with a higher risk for candidiasis.
Finally, Dr Feldman discusses the significance of a study showing that psoriasis patients have an approximately 20% higher risk for COVID-19 infection compared with a control group.
--
Steven R. Feldman, MD, PhD, Professor, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Steven R. Feldman, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AbbVie; Alvotech; Advance Medical; Almirall; Arena; Bristol-Myers Squibb; Caremark; Amgen; Celgene; Galderma Laboratories; Gerson Lehrman Group; Guidepoint Global; Helsinn; Janssen; Kikaku; Leo Pharma; Eli Lilly and Company; Merck; Mylan; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sienna; Sun Pharma; Suncare Research; Xenoport
Serve(d) as a speaker for: AbbVie; Amgen; Celgene; Janssen; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sun Pharma
Receive(d) grant support from: AbbVie; Amgen; Celgene; Galderma Laboratories; Janssen; Eli Lilly and Company; Novartis; Pfizer; Regeneron; Sanofi
Receive(d) royalties from: Informa; UpToDate; Xlibris
Holds stock in: Causa Technologies; Medical Quality Enhancement Corporation
Serves as founder and chief technology officer for: Causa Technologies

Gaps in Treatment Guidelines for Atopic Dermatitis
Treatment options for atopic dermatitis have evolved significantly in the past several years, but the current guidelines have yet to catch up.
Drs Steven Feldman and Lindsay Strowd, from Wake Forest School of Medicine, discuss gaps in the American Academy of Dermatology treatment guidelines for atopic dermatitis.
The current guidelines have not been updated to include medications approved for atopic dermatitis, including crisaborole, a steroid-sparing ointment used to treat mild to moderate disease in patients 3 months of age and older.
Another drug that has been approved since the 2014 guidelines is the biologic dupilumab, which is a monoclonal antibody that acts on the IL-4 receptor. The agent inhibits the binding of IL-4 receptors to the principal cytokines responsible for mediating the disease. Dupilumab is administered by injection and is approved for patients 6 years and older with moderate to severe disease.
The doctors also discuss therapies for atopic dermatitis currently in development, including topical and oral JAK inhibitors. They agree that the long-term benefit of topical JAK inhibitors may be limited, but that oral JAK inhibitors have the potential to be more effective than dupilumab and more acceptable to patients who do not like injections.
--
Steven R. Feldman, MD, PhD, Professor, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Steven R. Feldman, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AbbVie; Alvotech; Advance Medical; Almirall; Arena; Bristol-Myers Squibb; Caremark; Amgen; Celgene; Galderma Laboratories; Gerson Lehrman Group; Guidepoint Global; Helsinn; Janssen; Kikaku; Leo Pharma; Eli Lilly and Company; Merck; Mylan; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sienna; Sun Pharma; Suncare Research; Xenoport
Serve(d) as a speaker for: AbbVie; Amgen; Celgene; Janssen; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sun Pharma
Receive(d) grant support from: AbbVie; Amgen; Celgene; Galderma Laboratories; Janssen; Eli Lilly and Company; Novartis; Pfizer; Regeneron; Sanofi
Receive(d) royalties from: Informa; UpToDate; Xlibris
Holds stock in: Causa Technologies; Medical Quality Enhancement Corporation
Serves as founder and chief technology officer for: Causa Technologies
Lindsay C. Strowd, MD, Associate Professor, Vice Chair, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Lindsay C. Strowd, MD, has disclosed no relevant financial relationships.
Treatment options for atopic dermatitis have evolved significantly in the past several years, but the current guidelines have yet to catch up.
Drs Steven Feldman and Lindsay Strowd, from Wake Forest School of Medicine, discuss gaps in the American Academy of Dermatology treatment guidelines for atopic dermatitis.
The current guidelines have not been updated to include medications approved for atopic dermatitis, including crisaborole, a steroid-sparing ointment used to treat mild to moderate disease in patients 3 months of age and older.
Another drug that has been approved since the 2014 guidelines is the biologic dupilumab, which is a monoclonal antibody that acts on the IL-4 receptor. The agent inhibits the binding of IL-4 receptors to the principal cytokines responsible for mediating the disease. Dupilumab is administered by injection and is approved for patients 6 years and older with moderate to severe disease.
The doctors also discuss therapies for atopic dermatitis currently in development, including topical and oral JAK inhibitors. They agree that the long-term benefit of topical JAK inhibitors may be limited, but that oral JAK inhibitors have the potential to be more effective than dupilumab and more acceptable to patients who do not like injections.
--
Steven R. Feldman, MD, PhD, Professor, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Steven R. Feldman, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AbbVie; Alvotech; Advance Medical; Almirall; Arena; Bristol-Myers Squibb; Caremark; Amgen; Celgene; Galderma Laboratories; Gerson Lehrman Group; Guidepoint Global; Helsinn; Janssen; Kikaku; Leo Pharma; Eli Lilly and Company; Merck; Mylan; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sienna; Sun Pharma; Suncare Research; Xenoport
Serve(d) as a speaker for: AbbVie; Amgen; Celgene; Janssen; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sun Pharma
Receive(d) grant support from: AbbVie; Amgen; Celgene; Galderma Laboratories; Janssen; Eli Lilly and Company; Novartis; Pfizer; Regeneron; Sanofi
Receive(d) royalties from: Informa; UpToDate; Xlibris
Holds stock in: Causa Technologies; Medical Quality Enhancement Corporation
Serves as founder and chief technology officer for: Causa Technologies
Lindsay C. Strowd, MD, Associate Professor, Vice Chair, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Lindsay C. Strowd, MD, has disclosed no relevant financial relationships.
Treatment options for atopic dermatitis have evolved significantly in the past several years, but the current guidelines have yet to catch up.
Drs Steven Feldman and Lindsay Strowd, from Wake Forest School of Medicine, discuss gaps in the American Academy of Dermatology treatment guidelines for atopic dermatitis.
The current guidelines have not been updated to include medications approved for atopic dermatitis, including crisaborole, a steroid-sparing ointment used to treat mild to moderate disease in patients 3 months of age and older.
Another drug that has been approved since the 2014 guidelines is the biologic dupilumab, which is a monoclonal antibody that acts on the IL-4 receptor. The agent inhibits the binding of IL-4 receptors to the principal cytokines responsible for mediating the disease. Dupilumab is administered by injection and is approved for patients 6 years and older with moderate to severe disease.
The doctors also discuss therapies for atopic dermatitis currently in development, including topical and oral JAK inhibitors. They agree that the long-term benefit of topical JAK inhibitors may be limited, but that oral JAK inhibitors have the potential to be more effective than dupilumab and more acceptable to patients who do not like injections.
--
Steven R. Feldman, MD, PhD, Professor, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Steven R. Feldman, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AbbVie; Alvotech; Advance Medical; Almirall; Arena; Bristol-Myers Squibb; Caremark; Amgen; Celgene; Galderma Laboratories; Gerson Lehrman Group; Guidepoint Global; Helsinn; Janssen; Kikaku; Leo Pharma; Eli Lilly and Company; Merck; Mylan; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sienna; Sun Pharma; Suncare Research; Xenoport
Serve(d) as a speaker for: AbbVie; Amgen; Celgene; Janssen; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sun Pharma
Receive(d) grant support from: AbbVie; Amgen; Celgene; Galderma Laboratories; Janssen; Eli Lilly and Company; Novartis; Pfizer; Regeneron; Sanofi
Receive(d) royalties from: Informa; UpToDate; Xlibris
Holds stock in: Causa Technologies; Medical Quality Enhancement Corporation
Serves as founder and chief technology officer for: Causa Technologies
Lindsay C. Strowd, MD, Associate Professor, Vice Chair, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Lindsay C. Strowd, MD, has disclosed no relevant financial relationships.

Clinical Case-Viewing Sessions in Dermatology: The Patient Perspective
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.
The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.
The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.
The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
Practice Points
- Patient willingness to attend dermatology clinical case-viewing (CCV) sessions is relatively unchanged before and after the session.
- Participants generally consider CCV sessions to be a positive experience.
Adherence to Topical Treatment Can Improve Treatment-Resistant Moderate Psoriasis
High-potency topical corticosteroids are first-line treatments for psoriasis, but many patients report that they are ineffective or lose effectiveness over time.1-5 The mechanism underlying the lack or loss of activity is not well characterized but may be due to poor adherence to treatment. Adherence to topical treatment is poor in the short run and even worse in the long run.6,7 We evaluated 12 patients with psoriasis resistant to topical corticosteroids to determine if they would respond to topical corticosteroids under conditions designed to promote adherence to treatment.
Methods
This open-label, randomized, single-center clinical study recruited 12 patients with plaque psoriasis that previously failed treatment with topical corticosteroids and other therapies (Table). We stratified disease by body surface area: mild (<3%), moderate (3%–10%), and severe (>10%). Inclusion criteria included adult patients with plaque psoriasis amenable to topical corticosteroid therapy, ability to comply with requirements of the study, and a history of failed topical corticosteroid treatment (Figure). Patients were excluded if they were pregnant, breastfeeding, had conditions that would affect adherence or potentially bias results (eg, dementia, Alzheimer disease), had a history of allergy or sensitivity to corticosteroids, and had a history of drug hypersensitivity.
All patients received desoximetasone spray 0.25% twice daily for 14 days. At the baseline visit, 6 patients were randomly selected to also receive a twice-daily reminder telephone call. Study visits occurred frequently—at baseline and on days 3, 7, and 14—to further assure good adherence to the treatment regimen.
During visits, disease severity was scored using the visual analog scale for pruritus, psoriasis area and severity index (PASI), total lesion severity score (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
The study was designed to assess the number of topical treatment–resistant patients who would improve with topical treatment but was not designed or powered to test if the telephone call reminders increased adherence.
Results
All patients completed the study; 10 of 12 patients (83.3%) had previously used topical clobetasol and it failed (Table). At the 2-week end-of-study visit, most patients improved on all measures. Patients who received telephone call reminders improved more than patients who did not. All 12 patients (100%) reported relief of itching; 11 of 12 (91.7%) had an improved PASI; 10 of 12 (83.3%) had an improved TLSS; and 7 of 12 (58.3%) had an improved IGA (eTables 1 and 2).
The percentage reduction in pruritus ranged from 66.7% to 100% and 50.0% to 85.7% with and without telephone call reminders, respectively. Improvement in PASI ranged from 18.0% to 62.8% and 0% to 54.5% with and without telephone call reminders, respectively. Improvement in TLSS and IGA was of lower magnitude but showed a similar pattern, with numerically greater improvement in the telephone call reminders group compared to the group that was not called (eTable 2). No patients showed a worse score for pruritus on the visual analog scale, PASI, TLSS, or IGA.
Discussion
Topical corticosteroids are highly effective for psoriasis in clinical trials, with clearance in 2 to 4 weeks in 60% to 80% of patients, a rapidity of response not matched by even the most potent biologic treatments.8,9 However, topical corticosteroids are not always effective in clinical practice. There may be primary inefficacy (they do not work at first) or secondary inefficacy (a previously effective treatment loses efficacy over time).10 Poor adherence can explain both phenomena. Primary adherence occurs when patients fill their prescription; secondary adherence occurs when patients follow the medication recommendations.11 Primary nonadherence is common in patients with psoriasis; in one study, 50% of psoriasis prescriptions were not filled.12 Secondary adherence also is poor and declines over time; electronic monitoring revealed adherence to topical treatments in psoriasis patients decreased from 85% initially to 51% at the end of 8 weeks.7 Given the high efficacy of topical corticosteroids in clinical trials and the poor adherence to topical treatment in patients with psoriasis, we anticipated that psoriasis that is resistant to topical corticosteroids would improve rapidly under conditions designed to promote adherence.
As expected, disease improved in almost every patient in this small cohort when they were given a potent topical corticosteroid, even though they previously reported that their psoriasis was resistant to potent topical corticosteroids. Although this study enrolled only a small cohort, it appears that the majority of patients with limited psoriasis that was reported to be resistant to topical treatment can see a response to topical treatment under conditions designed to encourage good adherence.
We believe that the good outcomes seen in our study were a result of good adherence. Although the desoximetasone spray 0.25% used in this study is a superpotent topical corticosteroid,8 the response to treatment was unlikely due to changing corticosteroid potency because 10 of 12 patients had tried another superpotent topical corticosteroid (clobetasol) and it failed. We chose a spray product for this study rather than an ointment to promote adherence; however, this choice limited the ability to assess adherence directly, as adherence-monitoring devices for spray delivery systems are not readily available.
Our study was limited by the small sample size and brief duration of treatment. However, the effect size is so large (ie, the topical treatment was so effective) that only a small sample size and brief treatment duration were needed to show that a high percentage of patients with psoriasis that had previously failed treatment with topical corticosteroids can in fact respond to this treatment.
We used telephone calls as reminders in 50% of patients to further encourage adherence. The study was not designed or powered to assess the effect of the telephone call reminders, but patients receiving those calls appeared to have slightly greater reduction in disease severity. Nonetheless, twice-daily telephone call reminders are unlikely to be a wanted or practical intervention; other approaches to encourage adherence are needed.
Frequent follow-up visits were incorporated in our study design to maximize adherence. Although it might not be feasible for clinical practices to schedule follow-up visits as often as in our study, other approaches such as virtual visits and electronic interaction might provide a practical alternative. Multifaceted approaches to increasing adherence include encouraging patients to participate in the treatment plan, prescribing therapy consistent with a patient’s preferred vehicle, and extensive patient education.13 If patients do not respond as expected, poor adherence can be considered. Other potential causes of poor outcomes include error in diagnosis; resistance to the prescribed treatment; concomitant infection; irritant exposure; and, in the case of biologics, antidrug antibody formation.14,15
- Feldman SR, Fleischer AB Jr, Cooper JZ. New topical treatments change the pattern of treatment of psoriasis: dermatologists remain the primary providers of this care. Int J Dermatol. 2000;39:41-44.
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis. 2007;80(suppl 5):12-19.
- Saleem MD, Negus D, Feldman SR. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: a randomized clinical trial. J Dermatolog Treat. 2018;29:32-35.
- Mraz S, Leonardi C, Colón LE, et al. Different treatment outcomes with different formulations of clobetasol propionate 0.05% for the treatment of plaque psoriasis. J Dermatolog Treat. 2008;19:354-359.
- Chiricozzi A, Pimpinelli N, Ricceri F, et al. Treatment of psoriasis with topical agents: recommendations from a Tuscany Consensus. Dermatol Ther. 2017;30:e12549.
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015;14:835-840.
- Beutner K, Chakrabarty A, Lemke S, et al. An intra-individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. J Drugs Dermatol. 2006;5:357-360.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- Blais L, Kettani FZ, Forget A, et al. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2016;73:91-97.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Zschocke I, Mrowietz U, Karakasili E, et al. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4-9.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Varada S, Tintle SJ, Gottlieb AB. Apremilast for the treatment of psoriatic arthritis. Expert Rev Clin Pharmacol. 2014;7:239-250.
High-potency topical corticosteroids are first-line treatments for psoriasis, but many patients report that they are ineffective or lose effectiveness over time.1-5 The mechanism underlying the lack or loss of activity is not well characterized but may be due to poor adherence to treatment. Adherence to topical treatment is poor in the short run and even worse in the long run.6,7 We evaluated 12 patients with psoriasis resistant to topical corticosteroids to determine if they would respond to topical corticosteroids under conditions designed to promote adherence to treatment.
Methods
This open-label, randomized, single-center clinical study recruited 12 patients with plaque psoriasis that previously failed treatment with topical corticosteroids and other therapies (Table). We stratified disease by body surface area: mild (<3%), moderate (3%–10%), and severe (>10%). Inclusion criteria included adult patients with plaque psoriasis amenable to topical corticosteroid therapy, ability to comply with requirements of the study, and a history of failed topical corticosteroid treatment (Figure). Patients were excluded if they were pregnant, breastfeeding, had conditions that would affect adherence or potentially bias results (eg, dementia, Alzheimer disease), had a history of allergy or sensitivity to corticosteroids, and had a history of drug hypersensitivity.
All patients received desoximetasone spray 0.25% twice daily for 14 days. At the baseline visit, 6 patients were randomly selected to also receive a twice-daily reminder telephone call. Study visits occurred frequently—at baseline and on days 3, 7, and 14—to further assure good adherence to the treatment regimen.
During visits, disease severity was scored using the visual analog scale for pruritus, psoriasis area and severity index (PASI), total lesion severity score (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
The study was designed to assess the number of topical treatment–resistant patients who would improve with topical treatment but was not designed or powered to test if the telephone call reminders increased adherence.
Results
All patients completed the study; 10 of 12 patients (83.3%) had previously used topical clobetasol and it failed (Table). At the 2-week end-of-study visit, most patients improved on all measures. Patients who received telephone call reminders improved more than patients who did not. All 12 patients (100%) reported relief of itching; 11 of 12 (91.7%) had an improved PASI; 10 of 12 (83.3%) had an improved TLSS; and 7 of 12 (58.3%) had an improved IGA (eTables 1 and 2).
The percentage reduction in pruritus ranged from 66.7% to 100% and 50.0% to 85.7% with and without telephone call reminders, respectively. Improvement in PASI ranged from 18.0% to 62.8% and 0% to 54.5% with and without telephone call reminders, respectively. Improvement in TLSS and IGA was of lower magnitude but showed a similar pattern, with numerically greater improvement in the telephone call reminders group compared to the group that was not called (eTable 2). No patients showed a worse score for pruritus on the visual analog scale, PASI, TLSS, or IGA.
Discussion
Topical corticosteroids are highly effective for psoriasis in clinical trials, with clearance in 2 to 4 weeks in 60% to 80% of patients, a rapidity of response not matched by even the most potent biologic treatments.8,9 However, topical corticosteroids are not always effective in clinical practice. There may be primary inefficacy (they do not work at first) or secondary inefficacy (a previously effective treatment loses efficacy over time).10 Poor adherence can explain both phenomena. Primary adherence occurs when patients fill their prescription; secondary adherence occurs when patients follow the medication recommendations.11 Primary nonadherence is common in patients with psoriasis; in one study, 50% of psoriasis prescriptions were not filled.12 Secondary adherence also is poor and declines over time; electronic monitoring revealed adherence to topical treatments in psoriasis patients decreased from 85% initially to 51% at the end of 8 weeks.7 Given the high efficacy of topical corticosteroids in clinical trials and the poor adherence to topical treatment in patients with psoriasis, we anticipated that psoriasis that is resistant to topical corticosteroids would improve rapidly under conditions designed to promote adherence.
As expected, disease improved in almost every patient in this small cohort when they were given a potent topical corticosteroid, even though they previously reported that their psoriasis was resistant to potent topical corticosteroids. Although this study enrolled only a small cohort, it appears that the majority of patients with limited psoriasis that was reported to be resistant to topical treatment can see a response to topical treatment under conditions designed to encourage good adherence.
We believe that the good outcomes seen in our study were a result of good adherence. Although the desoximetasone spray 0.25% used in this study is a superpotent topical corticosteroid,8 the response to treatment was unlikely due to changing corticosteroid potency because 10 of 12 patients had tried another superpotent topical corticosteroid (clobetasol) and it failed. We chose a spray product for this study rather than an ointment to promote adherence; however, this choice limited the ability to assess adherence directly, as adherence-monitoring devices for spray delivery systems are not readily available.
Our study was limited by the small sample size and brief duration of treatment. However, the effect size is so large (ie, the topical treatment was so effective) that only a small sample size and brief treatment duration were needed to show that a high percentage of patients with psoriasis that had previously failed treatment with topical corticosteroids can in fact respond to this treatment.
We used telephone calls as reminders in 50% of patients to further encourage adherence. The study was not designed or powered to assess the effect of the telephone call reminders, but patients receiving those calls appeared to have slightly greater reduction in disease severity. Nonetheless, twice-daily telephone call reminders are unlikely to be a wanted or practical intervention; other approaches to encourage adherence are needed.
Frequent follow-up visits were incorporated in our study design to maximize adherence. Although it might not be feasible for clinical practices to schedule follow-up visits as often as in our study, other approaches such as virtual visits and electronic interaction might provide a practical alternative. Multifaceted approaches to increasing adherence include encouraging patients to participate in the treatment plan, prescribing therapy consistent with a patient’s preferred vehicle, and extensive patient education.13 If patients do not respond as expected, poor adherence can be considered. Other potential causes of poor outcomes include error in diagnosis; resistance to the prescribed treatment; concomitant infection; irritant exposure; and, in the case of biologics, antidrug antibody formation.14,15
High-potency topical corticosteroids are first-line treatments for psoriasis, but many patients report that they are ineffective or lose effectiveness over time.1-5 The mechanism underlying the lack or loss of activity is not well characterized but may be due to poor adherence to treatment. Adherence to topical treatment is poor in the short run and even worse in the long run.6,7 We evaluated 12 patients with psoriasis resistant to topical corticosteroids to determine if they would respond to topical corticosteroids under conditions designed to promote adherence to treatment.
Methods
This open-label, randomized, single-center clinical study recruited 12 patients with plaque psoriasis that previously failed treatment with topical corticosteroids and other therapies (Table). We stratified disease by body surface area: mild (<3%), moderate (3%–10%), and severe (>10%). Inclusion criteria included adult patients with plaque psoriasis amenable to topical corticosteroid therapy, ability to comply with requirements of the study, and a history of failed topical corticosteroid treatment (Figure). Patients were excluded if they were pregnant, breastfeeding, had conditions that would affect adherence or potentially bias results (eg, dementia, Alzheimer disease), had a history of allergy or sensitivity to corticosteroids, and had a history of drug hypersensitivity.
All patients received desoximetasone spray 0.25% twice daily for 14 days. At the baseline visit, 6 patients were randomly selected to also receive a twice-daily reminder telephone call. Study visits occurred frequently—at baseline and on days 3, 7, and 14—to further assure good adherence to the treatment regimen.
During visits, disease severity was scored using the visual analog scale for pruritus, psoriasis area and severity index (PASI), total lesion severity score (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
The study was designed to assess the number of topical treatment–resistant patients who would improve with topical treatment but was not designed or powered to test if the telephone call reminders increased adherence.
Results
All patients completed the study; 10 of 12 patients (83.3%) had previously used topical clobetasol and it failed (Table). At the 2-week end-of-study visit, most patients improved on all measures. Patients who received telephone call reminders improved more than patients who did not. All 12 patients (100%) reported relief of itching; 11 of 12 (91.7%) had an improved PASI; 10 of 12 (83.3%) had an improved TLSS; and 7 of 12 (58.3%) had an improved IGA (eTables 1 and 2).
The percentage reduction in pruritus ranged from 66.7% to 100% and 50.0% to 85.7% with and without telephone call reminders, respectively. Improvement in PASI ranged from 18.0% to 62.8% and 0% to 54.5% with and without telephone call reminders, respectively. Improvement in TLSS and IGA was of lower magnitude but showed a similar pattern, with numerically greater improvement in the telephone call reminders group compared to the group that was not called (eTable 2). No patients showed a worse score for pruritus on the visual analog scale, PASI, TLSS, or IGA.
Discussion
Topical corticosteroids are highly effective for psoriasis in clinical trials, with clearance in 2 to 4 weeks in 60% to 80% of patients, a rapidity of response not matched by even the most potent biologic treatments.8,9 However, topical corticosteroids are not always effective in clinical practice. There may be primary inefficacy (they do not work at first) or secondary inefficacy (a previously effective treatment loses efficacy over time).10 Poor adherence can explain both phenomena. Primary adherence occurs when patients fill their prescription; secondary adherence occurs when patients follow the medication recommendations.11 Primary nonadherence is common in patients with psoriasis; in one study, 50% of psoriasis prescriptions were not filled.12 Secondary adherence also is poor and declines over time; electronic monitoring revealed adherence to topical treatments in psoriasis patients decreased from 85% initially to 51% at the end of 8 weeks.7 Given the high efficacy of topical corticosteroids in clinical trials and the poor adherence to topical treatment in patients with psoriasis, we anticipated that psoriasis that is resistant to topical corticosteroids would improve rapidly under conditions designed to promote adherence.
As expected, disease improved in almost every patient in this small cohort when they were given a potent topical corticosteroid, even though they previously reported that their psoriasis was resistant to potent topical corticosteroids. Although this study enrolled only a small cohort, it appears that the majority of patients with limited psoriasis that was reported to be resistant to topical treatment can see a response to topical treatment under conditions designed to encourage good adherence.
We believe that the good outcomes seen in our study were a result of good adherence. Although the desoximetasone spray 0.25% used in this study is a superpotent topical corticosteroid,8 the response to treatment was unlikely due to changing corticosteroid potency because 10 of 12 patients had tried another superpotent topical corticosteroid (clobetasol) and it failed. We chose a spray product for this study rather than an ointment to promote adherence; however, this choice limited the ability to assess adherence directly, as adherence-monitoring devices for spray delivery systems are not readily available.
Our study was limited by the small sample size and brief duration of treatment. However, the effect size is so large (ie, the topical treatment was so effective) that only a small sample size and brief treatment duration were needed to show that a high percentage of patients with psoriasis that had previously failed treatment with topical corticosteroids can in fact respond to this treatment.
We used telephone calls as reminders in 50% of patients to further encourage adherence. The study was not designed or powered to assess the effect of the telephone call reminders, but patients receiving those calls appeared to have slightly greater reduction in disease severity. Nonetheless, twice-daily telephone call reminders are unlikely to be a wanted or practical intervention; other approaches to encourage adherence are needed.
Frequent follow-up visits were incorporated in our study design to maximize adherence. Although it might not be feasible for clinical practices to schedule follow-up visits as often as in our study, other approaches such as virtual visits and electronic interaction might provide a practical alternative. Multifaceted approaches to increasing adherence include encouraging patients to participate in the treatment plan, prescribing therapy consistent with a patient’s preferred vehicle, and extensive patient education.13 If patients do not respond as expected, poor adherence can be considered. Other potential causes of poor outcomes include error in diagnosis; resistance to the prescribed treatment; concomitant infection; irritant exposure; and, in the case of biologics, antidrug antibody formation.14,15
- Feldman SR, Fleischer AB Jr, Cooper JZ. New topical treatments change the pattern of treatment of psoriasis: dermatologists remain the primary providers of this care. Int J Dermatol. 2000;39:41-44.
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis. 2007;80(suppl 5):12-19.
- Saleem MD, Negus D, Feldman SR. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: a randomized clinical trial. J Dermatolog Treat. 2018;29:32-35.
- Mraz S, Leonardi C, Colón LE, et al. Different treatment outcomes with different formulations of clobetasol propionate 0.05% for the treatment of plaque psoriasis. J Dermatolog Treat. 2008;19:354-359.
- Chiricozzi A, Pimpinelli N, Ricceri F, et al. Treatment of psoriasis with topical agents: recommendations from a Tuscany Consensus. Dermatol Ther. 2017;30:e12549.
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015;14:835-840.
- Beutner K, Chakrabarty A, Lemke S, et al. An intra-individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. J Drugs Dermatol. 2006;5:357-360.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- Blais L, Kettani FZ, Forget A, et al. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2016;73:91-97.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Zschocke I, Mrowietz U, Karakasili E, et al. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4-9.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Varada S, Tintle SJ, Gottlieb AB. Apremilast for the treatment of psoriatic arthritis. Expert Rev Clin Pharmacol. 2014;7:239-250.
- Feldman SR, Fleischer AB Jr, Cooper JZ. New topical treatments change the pattern of treatment of psoriasis: dermatologists remain the primary providers of this care. Int J Dermatol. 2000;39:41-44.
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis. 2007;80(suppl 5):12-19.
- Saleem MD, Negus D, Feldman SR. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: a randomized clinical trial. J Dermatolog Treat. 2018;29:32-35.
- Mraz S, Leonardi C, Colón LE, et al. Different treatment outcomes with different formulations of clobetasol propionate 0.05% for the treatment of plaque psoriasis. J Dermatolog Treat. 2008;19:354-359.
- Chiricozzi A, Pimpinelli N, Ricceri F, et al. Treatment of psoriasis with topical agents: recommendations from a Tuscany Consensus. Dermatol Ther. 2017;30:e12549.
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015;14:835-840.
- Beutner K, Chakrabarty A, Lemke S, et al. An intra-individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. J Drugs Dermatol. 2006;5:357-360.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- Blais L, Kettani FZ, Forget A, et al. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2016;73:91-97.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Zschocke I, Mrowietz U, Karakasili E, et al. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4-9.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Varada S, Tintle SJ, Gottlieb AB. Apremilast for the treatment of psoriatic arthritis. Expert Rev Clin Pharmacol. 2014;7:239-250.
Practice Points
- Most patients with psoriasis are good candidates for topical treatment.
- Topical treatment of psoriasis often is ineffective.
- Topical treatment of psoriasis can be rapidly effective, even in patients who reported disease that was resistant to topical treatment.
How Do Drug Shortages Affect Dermatologists?
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
Automobile Injury: A Common Familiar Risk for Presenting and Comparing Risks in Dermatology
Numerous highly efficacious treatment modalities exist in dermatology, yet patients may be highly wary of their possible adverse events, even when those risks are rare.1,2 Such fears can lead to poor medication adherence and treatment refusal. A key determinant in successful patient-provider care is to effectively communicate risk. The communication of risk is hampered by the lack of any common currency for comparing risks. The development of a standardized unit of risk could help facilitate risk comparisons, allowing physicians and patients to put risk levels into better perspective.
One easily relatable event is the risk of injury in an automobile crash. Driving, whether to the dermatology clinic for a monitoring visit or to the supermarket for weekly groceries, is associated with risk of injury and death. The risk of automobile-related injury warranting a visit to the emergency department could provide a comparator that physicians can use to give patients a more objective sense of treatment risks or to introduce the justification of a monitoring visit. The objective of this study was to develop a standard risk unit based on the lifetime risk (LTR) of automobile injury and to compare this unit of risk to various risks of dermatologic treatments.
Methods
Literature Review
We first identified common risks in dermatology that would be illustrative and then identified keywords. PubMed searches for articles indexed for MEDLINE from November 1996 to February 2017 were performed combining the following terms: (relative risk, odds ratio, lifetime risk) and (isotretinoin, IBD; melanoma, SCC, transplantation; indoor tanning, BCC, SCC; transplant and SCC; biologics and tuberculosis; hydroxychloroquine retinal toxicity; psoriasis and psoriatic arthritis). An additional search was performed in June 2018 including the term blindness and injectable fillers. Our search combined these terms in numerous ways. Results were focused on meta-analyses and observational studies.
The references of relevant studies were included. Articles not focused on meta-analyses but rather on observational studies were individually analyzed for quality and bias using the 9-point Newcastle-Ottawa Scale, with a score of 7 or more as a cutoff for inclusion.
Determination of Risk Comparators
Data from the 2016 National Safety Council’s Injury Facts report were searched for nonmedical-related risk comparators, such as the risk of death by dog attack, by lightning, and by fire or smoke.3 Data from the 2015 US Department of Transportation Traffic Safety Facts were searched for relatable risk comparators, such as the LTR of automobile death and injury.4
Definitions
Automobile injury was defined as an injury warranting a visit to the emergency department.5 Automobile was defined as a road vehicle with 4 wheels and powered by an internal combustion engine or electric motor.6 This definition excluded light trucks, large trucks, and motorcycles.
LTR Calculation
Lifetime risk was used as the comparative measure. Lifetime risk is a type of absolute risk that depicts the probability that a specific disease or event will occur in an individual’s lifespan. The LTRof developing a disease or adverse event due to a dermatologic therapy or interventionwas denoted as LTRadverse event and calculated by the following equation7,8:

In this equation, LTRgeneral population is the LTR of developing the disease or adverse event without being subject to the therapy or intervention, and RRintervention is the relative risk (RR) from previously published RR data (relating to the development of the disease in question or an adverse event of the intervention). The use of equation (1) holds true only when the absolute risk of developing the disease or adverse event (LTRgeneral population) is low.7 Although the calculation of an LTR using a constant lifetime RR may require major approximations, studies evaluating the variation of RR over time are sparse.7,9 The Newcastle-Ottawa Scale was used to control such variance; only high-quality, nonrandomized studies were included. Although the use of residual LTR would be preferable, as LTR depends on age, such epidemiological data do not exist for complex diseases.
When not available, the LTRgeneral population was calculated from the rate of disease (cases per 100,000 individuals per year) multiplied by the average lifespan of an American (78.8 years)10:
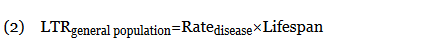
When an odds ratio (OR) was presented, its conversion to RR followed11:
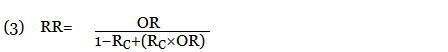
In this equation, RC is the absolute risk in the unexposed group. If the prevalence of the disease was considered low, the rare disease assumption was implemented as the following11,12:

The use of this approximation overestimates the LTR of an event. From a patient perspective, this approach is conservative. If prior LTR values were available, such as the LTR of automobile injury, automobile death, or other intervention, they were used without the need for calculation.
Unit Comparator
The LTRs of all adverse events were normalized to a unit comparator, using the LTR of an automobile injury as reference point, denoted as 1 risk unit (RU):

This equation allows for quick comparison of the magnitude of LTRs between events. Events with an RU less than 1 are less likely to occur than the risk of automobile injury; events with an RU greater than 1 are more likely than the risk of automobile injury. All RR, LTR, and unit comparators were presented as a single pooled estimate of their respective upper-limit CIs. The use of the upper-limit CI conservatively overestimates the LTR of an event.
Results
Ten dermatologic interventions were identified as illustrative, to be presented alongside the risk of automobile injury and death. The LTR of automobile injury was 32%, defined as 1.0 RU. The LTR of automobile death was 0.89% (1/36 RU).
Two events had LTRs roughly similar to automobile injury: development of a subsequent basal cell carcinoma within 3 years (1.4 RU) and development of a squamous cell carcinoma (SCC) secondary to indoor tanning (1.6 RU). Development of SCC following organ transplantation (34 RU) was considerably more likely than automobile injury. All other identified events had lower RUs than automobile injury (Table). Three events with small RUs included tuberculosis development with a tumor necrosis factor α inhibitor (1/32 RU), Crohn disease development with isotretinoin (1/41 RU), and blindness following facial hyaluronic acid injection (1/80 RU). The LTR of death by dog attack (1/42,436 RU) and death by lightning strike (1/36,542 RU) also had small RUs.
The unit comparators from the Table were adapted into graphic form to depict risk relative to the risk of automobile injury (Figure).
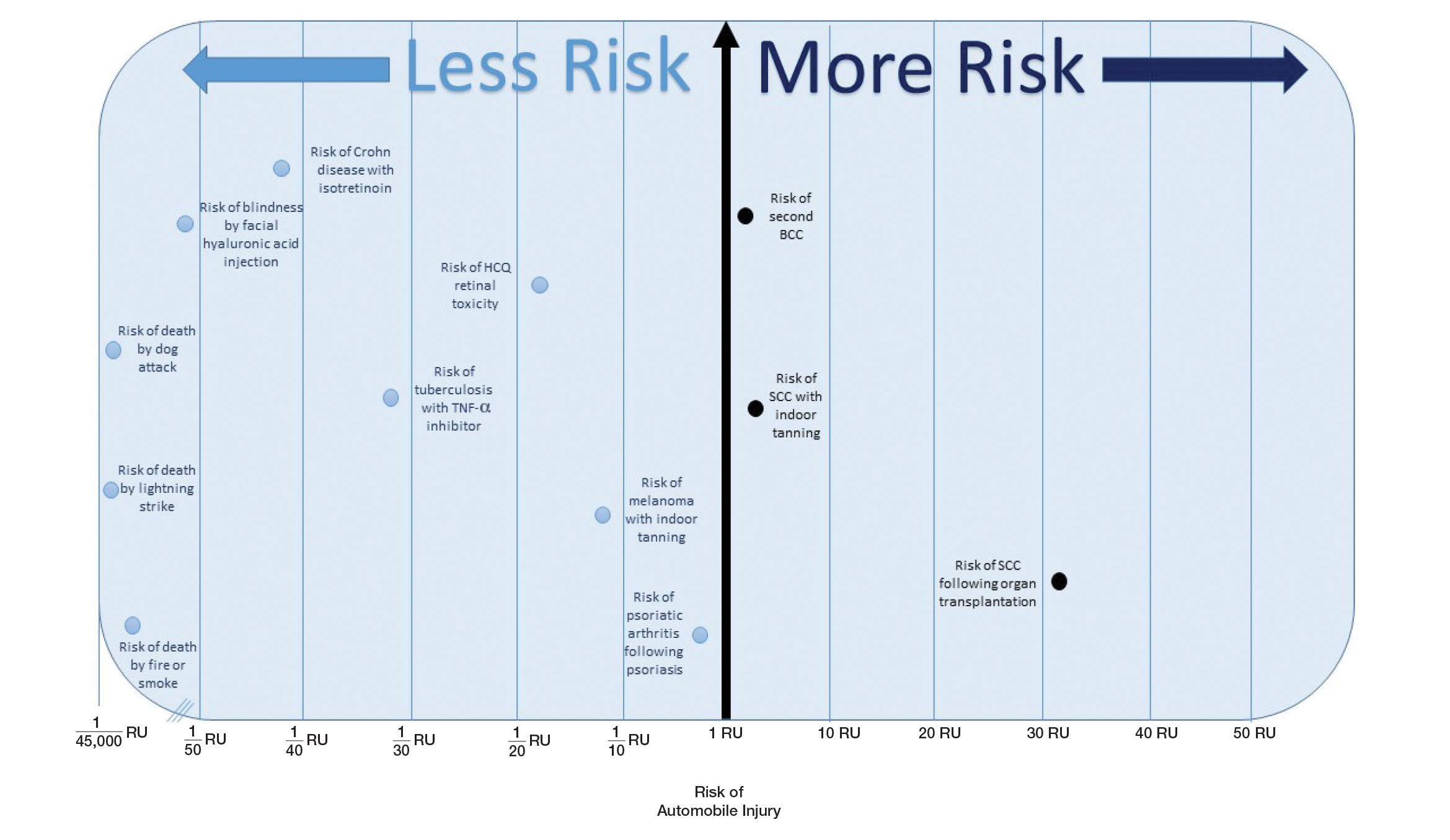
Comment
Numerous interventions in dermatology offer much less risk of an adverse event than the LTR of automobile injury. However, this concept of risk includes only the likelihood of development of an event, not the severity of the measured event, as our numerical and visual tool objectively captures the related risks using an RU comparator. Such use of a standardized RU demonstrates the essence of risk; “zero risk” does not exist, and each intervention or treatment, albeit how small, must be justified in concordance with other types of risk, such as the automobile.
The development of adverse events secondary to dermatologic intervention or therapy, for which monitoring visits are utilized, were used as important comparators to the risk of automobile injury. The continuous practice of monitoring visits may increase patient’s fears regarding possible adverse events secondary to therapy. Hydroxychloroquine retinal toxicity (1/16 RU) and psoriatic arthritis development following severe psoriasis (1/3.9 RU) were less likely to occur than automobile injury. The development of abnormal blood counts or blood tests secondary to therapy or intervention could not be formatted into an RU. The use of equation (1) for the calculation of LTRadverse eventholds true only when the absolute risk of developing the adverse event in the general population—in this case, abnormal blood counts or blood tests—is low.7
Although the unit comparator allows for the comparison of different dermatologic risk, a limitation of the RU model and its visual tool are a dependence on RR, a value that changes following publication of new studies. A solution was the use of a single pooled estimate to represent the upper-limit CIs of LTR. This practice overestimates risk. As with RR, new automobile injury rates are published annually.10 In the last 5 years, the LTR of automobile injury has stayed relatively constant: between 32% and 33%.4 Although the RU calculations and Figure included a wide variety of interventions in dermatology, select clinical situations were not included. It is beyond the scope of this article to systematically review all risk in dermatology but rather introduce the concept of the RU founded on automobile-associated risks. With the introduction of a methodical framework, the reader is invited to calculate RUs pertinent to their clinical interests.
Any intervention or treatment in dermatology is accompanied by risk. The use of a unit comparator using an easily relatable event—the LTR of automobile injury—allows the patient to easily compare risk and internally justify the practice of monitoring visits. Inclusion of a visual tool, such as the Figure, might alleviate many irrational fears that accompany some of the highly effective treatments and interventions used in dermatology and thus lead to better patient outcomes.
Acknowledgment
We thank Taranjeet Singh, MS (Dunn, North Carolina), for her comments on an earlier version of the manuscript.
- Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making. 2003;23:511-517.
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014;15:165-180.
- National Safety Council. Odds of dying. Injury Facts website. http://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/. Accessed November 4, 2018.
- National Center for Statistics and Analysis (NCSA) motor vehicle traffic crash data resource page. National Highway Traffic Safety Administration website. https://crashstats.nhtsa.dot.gov/#/. Accessed November 4, 2018.
- CDC report shows motor vehicle crash injuries are frequent and costly. Centers for Disease Control and Prevention website. http://www.cdc.gov/media/releases/2014/p1007-crash-injuries.html. Published October 7, 2014. Accessed November 4, 2018.
- Automobile. Business Dictionary website. http://www.businessdictionary.com/definition/automobile.html. Accessed November 4, 2018.
- Dupont WD, Plummer WD Jr. Understanding the relationship between relative and absolute risk. Cancer. 1996;77:2193-2199.
- Kaminska E, Patel I, Dabade TS, et al. Comparing the lifetime risks of TNF-alpha inhibitor use to common benchmarks of risk. J Dermatolog Treat. 2011;24:101-106.
- Dupont WD. Converting relative risks to absolute risks: a graphical approach. Stat Med. 1989;8:641-651.
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1-122.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration website. http://handbook.cochrane.org. Updated March 2011. Accessed November 15, 2018.
- Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142:761-764.
- Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207-214.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424-1429.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
- Lee SY, Jamal MM, Nguyen ET, et al. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:210-216.
- Injury Facts, 2017. Itasca, IL: National Safety Council; 2017.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-1394.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-1460.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847-857.e1-18.
- Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta Derm Venereol. 2015;95:923-927.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- National Highway Traffic Safety Administration (NHTSA). Traffic Safety Facts 2015. Washington, DC: US Department of Transportation; 2015.
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:E5909.
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-519.
Numerous highly efficacious treatment modalities exist in dermatology, yet patients may be highly wary of their possible adverse events, even when those risks are rare.1,2 Such fears can lead to poor medication adherence and treatment refusal. A key determinant in successful patient-provider care is to effectively communicate risk. The communication of risk is hampered by the lack of any common currency for comparing risks. The development of a standardized unit of risk could help facilitate risk comparisons, allowing physicians and patients to put risk levels into better perspective.
One easily relatable event is the risk of injury in an automobile crash. Driving, whether to the dermatology clinic for a monitoring visit or to the supermarket for weekly groceries, is associated with risk of injury and death. The risk of automobile-related injury warranting a visit to the emergency department could provide a comparator that physicians can use to give patients a more objective sense of treatment risks or to introduce the justification of a monitoring visit. The objective of this study was to develop a standard risk unit based on the lifetime risk (LTR) of automobile injury and to compare this unit of risk to various risks of dermatologic treatments.
Methods
Literature Review
We first identified common risks in dermatology that would be illustrative and then identified keywords. PubMed searches for articles indexed for MEDLINE from November 1996 to February 2017 were performed combining the following terms: (relative risk, odds ratio, lifetime risk) and (isotretinoin, IBD; melanoma, SCC, transplantation; indoor tanning, BCC, SCC; transplant and SCC; biologics and tuberculosis; hydroxychloroquine retinal toxicity; psoriasis and psoriatic arthritis). An additional search was performed in June 2018 including the term blindness and injectable fillers. Our search combined these terms in numerous ways. Results were focused on meta-analyses and observational studies.
The references of relevant studies were included. Articles not focused on meta-analyses but rather on observational studies were individually analyzed for quality and bias using the 9-point Newcastle-Ottawa Scale, with a score of 7 or more as a cutoff for inclusion.
Determination of Risk Comparators
Data from the 2016 National Safety Council’s Injury Facts report were searched for nonmedical-related risk comparators, such as the risk of death by dog attack, by lightning, and by fire or smoke.3 Data from the 2015 US Department of Transportation Traffic Safety Facts were searched for relatable risk comparators, such as the LTR of automobile death and injury.4
Definitions
Automobile injury was defined as an injury warranting a visit to the emergency department.5 Automobile was defined as a road vehicle with 4 wheels and powered by an internal combustion engine or electric motor.6 This definition excluded light trucks, large trucks, and motorcycles.
LTR Calculation
Lifetime risk was used as the comparative measure. Lifetime risk is a type of absolute risk that depicts the probability that a specific disease or event will occur in an individual’s lifespan. The LTRof developing a disease or adverse event due to a dermatologic therapy or interventionwas denoted as LTRadverse event and calculated by the following equation7,8:

In this equation, LTRgeneral population is the LTR of developing the disease or adverse event without being subject to the therapy or intervention, and RRintervention is the relative risk (RR) from previously published RR data (relating to the development of the disease in question or an adverse event of the intervention). The use of equation (1) holds true only when the absolute risk of developing the disease or adverse event (LTRgeneral population) is low.7 Although the calculation of an LTR using a constant lifetime RR may require major approximations, studies evaluating the variation of RR over time are sparse.7,9 The Newcastle-Ottawa Scale was used to control such variance; only high-quality, nonrandomized studies were included. Although the use of residual LTR would be preferable, as LTR depends on age, such epidemiological data do not exist for complex diseases.
When not available, the LTRgeneral population was calculated from the rate of disease (cases per 100,000 individuals per year) multiplied by the average lifespan of an American (78.8 years)10:
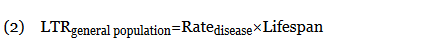
When an odds ratio (OR) was presented, its conversion to RR followed11:
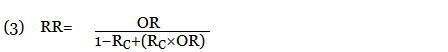
In this equation, RC is the absolute risk in the unexposed group. If the prevalence of the disease was considered low, the rare disease assumption was implemented as the following11,12:

The use of this approximation overestimates the LTR of an event. From a patient perspective, this approach is conservative. If prior LTR values were available, such as the LTR of automobile injury, automobile death, or other intervention, they were used without the need for calculation.
Unit Comparator
The LTRs of all adverse events were normalized to a unit comparator, using the LTR of an automobile injury as reference point, denoted as 1 risk unit (RU):

This equation allows for quick comparison of the magnitude of LTRs between events. Events with an RU less than 1 are less likely to occur than the risk of automobile injury; events with an RU greater than 1 are more likely than the risk of automobile injury. All RR, LTR, and unit comparators were presented as a single pooled estimate of their respective upper-limit CIs. The use of the upper-limit CI conservatively overestimates the LTR of an event.
Results
Ten dermatologic interventions were identified as illustrative, to be presented alongside the risk of automobile injury and death. The LTR of automobile injury was 32%, defined as 1.0 RU. The LTR of automobile death was 0.89% (1/36 RU).
Two events had LTRs roughly similar to automobile injury: development of a subsequent basal cell carcinoma within 3 years (1.4 RU) and development of a squamous cell carcinoma (SCC) secondary to indoor tanning (1.6 RU). Development of SCC following organ transplantation (34 RU) was considerably more likely than automobile injury. All other identified events had lower RUs than automobile injury (Table). Three events with small RUs included tuberculosis development with a tumor necrosis factor α inhibitor (1/32 RU), Crohn disease development with isotretinoin (1/41 RU), and blindness following facial hyaluronic acid injection (1/80 RU). The LTR of death by dog attack (1/42,436 RU) and death by lightning strike (1/36,542 RU) also had small RUs.
The unit comparators from the Table were adapted into graphic form to depict risk relative to the risk of automobile injury (Figure).

Comment
Numerous interventions in dermatology offer much less risk of an adverse event than the LTR of automobile injury. However, this concept of risk includes only the likelihood of development of an event, not the severity of the measured event, as our numerical and visual tool objectively captures the related risks using an RU comparator. Such use of a standardized RU demonstrates the essence of risk; “zero risk” does not exist, and each intervention or treatment, albeit how small, must be justified in concordance with other types of risk, such as the automobile.
The development of adverse events secondary to dermatologic intervention or therapy, for which monitoring visits are utilized, were used as important comparators to the risk of automobile injury. The continuous practice of monitoring visits may increase patient’s fears regarding possible adverse events secondary to therapy. Hydroxychloroquine retinal toxicity (1/16 RU) and psoriatic arthritis development following severe psoriasis (1/3.9 RU) were less likely to occur than automobile injury. The development of abnormal blood counts or blood tests secondary to therapy or intervention could not be formatted into an RU. The use of equation (1) for the calculation of LTRadverse eventholds true only when the absolute risk of developing the adverse event in the general population—in this case, abnormal blood counts or blood tests—is low.7
Although the unit comparator allows for the comparison of different dermatologic risk, a limitation of the RU model and its visual tool are a dependence on RR, a value that changes following publication of new studies. A solution was the use of a single pooled estimate to represent the upper-limit CIs of LTR. This practice overestimates risk. As with RR, new automobile injury rates are published annually.10 In the last 5 years, the LTR of automobile injury has stayed relatively constant: between 32% and 33%.4 Although the RU calculations and Figure included a wide variety of interventions in dermatology, select clinical situations were not included. It is beyond the scope of this article to systematically review all risk in dermatology but rather introduce the concept of the RU founded on automobile-associated risks. With the introduction of a methodical framework, the reader is invited to calculate RUs pertinent to their clinical interests.
Any intervention or treatment in dermatology is accompanied by risk. The use of a unit comparator using an easily relatable event—the LTR of automobile injury—allows the patient to easily compare risk and internally justify the practice of monitoring visits. Inclusion of a visual tool, such as the Figure, might alleviate many irrational fears that accompany some of the highly effective treatments and interventions used in dermatology and thus lead to better patient outcomes.
Acknowledgment
We thank Taranjeet Singh, MS (Dunn, North Carolina), for her comments on an earlier version of the manuscript.
Numerous highly efficacious treatment modalities exist in dermatology, yet patients may be highly wary of their possible adverse events, even when those risks are rare.1,2 Such fears can lead to poor medication adherence and treatment refusal. A key determinant in successful patient-provider care is to effectively communicate risk. The communication of risk is hampered by the lack of any common currency for comparing risks. The development of a standardized unit of risk could help facilitate risk comparisons, allowing physicians and patients to put risk levels into better perspective.
One easily relatable event is the risk of injury in an automobile crash. Driving, whether to the dermatology clinic for a monitoring visit or to the supermarket for weekly groceries, is associated with risk of injury and death. The risk of automobile-related injury warranting a visit to the emergency department could provide a comparator that physicians can use to give patients a more objective sense of treatment risks or to introduce the justification of a monitoring visit. The objective of this study was to develop a standard risk unit based on the lifetime risk (LTR) of automobile injury and to compare this unit of risk to various risks of dermatologic treatments.
Methods
Literature Review
We first identified common risks in dermatology that would be illustrative and then identified keywords. PubMed searches for articles indexed for MEDLINE from November 1996 to February 2017 were performed combining the following terms: (relative risk, odds ratio, lifetime risk) and (isotretinoin, IBD; melanoma, SCC, transplantation; indoor tanning, BCC, SCC; transplant and SCC; biologics and tuberculosis; hydroxychloroquine retinal toxicity; psoriasis and psoriatic arthritis). An additional search was performed in June 2018 including the term blindness and injectable fillers. Our search combined these terms in numerous ways. Results were focused on meta-analyses and observational studies.
The references of relevant studies were included. Articles not focused on meta-analyses but rather on observational studies were individually analyzed for quality and bias using the 9-point Newcastle-Ottawa Scale, with a score of 7 or more as a cutoff for inclusion.
Determination of Risk Comparators
Data from the 2016 National Safety Council’s Injury Facts report were searched for nonmedical-related risk comparators, such as the risk of death by dog attack, by lightning, and by fire or smoke.3 Data from the 2015 US Department of Transportation Traffic Safety Facts were searched for relatable risk comparators, such as the LTR of automobile death and injury.4
Definitions
Automobile injury was defined as an injury warranting a visit to the emergency department.5 Automobile was defined as a road vehicle with 4 wheels and powered by an internal combustion engine or electric motor.6 This definition excluded light trucks, large trucks, and motorcycles.
LTR Calculation
Lifetime risk was used as the comparative measure. Lifetime risk is a type of absolute risk that depicts the probability that a specific disease or event will occur in an individual’s lifespan. The LTRof developing a disease or adverse event due to a dermatologic therapy or interventionwas denoted as LTRadverse event and calculated by the following equation7,8:

In this equation, LTRgeneral population is the LTR of developing the disease or adverse event without being subject to the therapy or intervention, and RRintervention is the relative risk (RR) from previously published RR data (relating to the development of the disease in question or an adverse event of the intervention). The use of equation (1) holds true only when the absolute risk of developing the disease or adverse event (LTRgeneral population) is low.7 Although the calculation of an LTR using a constant lifetime RR may require major approximations, studies evaluating the variation of RR over time are sparse.7,9 The Newcastle-Ottawa Scale was used to control such variance; only high-quality, nonrandomized studies were included. Although the use of residual LTR would be preferable, as LTR depends on age, such epidemiological data do not exist for complex diseases.
When not available, the LTRgeneral population was calculated from the rate of disease (cases per 100,000 individuals per year) multiplied by the average lifespan of an American (78.8 years)10:
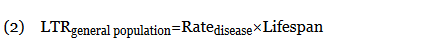
When an odds ratio (OR) was presented, its conversion to RR followed11:
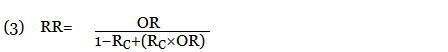
In this equation, RC is the absolute risk in the unexposed group. If the prevalence of the disease was considered low, the rare disease assumption was implemented as the following11,12:

The use of this approximation overestimates the LTR of an event. From a patient perspective, this approach is conservative. If prior LTR values were available, such as the LTR of automobile injury, automobile death, or other intervention, they were used without the need for calculation.
Unit Comparator
The LTRs of all adverse events were normalized to a unit comparator, using the LTR of an automobile injury as reference point, denoted as 1 risk unit (RU):

This equation allows for quick comparison of the magnitude of LTRs between events. Events with an RU less than 1 are less likely to occur than the risk of automobile injury; events with an RU greater than 1 are more likely than the risk of automobile injury. All RR, LTR, and unit comparators were presented as a single pooled estimate of their respective upper-limit CIs. The use of the upper-limit CI conservatively overestimates the LTR of an event.
Results
Ten dermatologic interventions were identified as illustrative, to be presented alongside the risk of automobile injury and death. The LTR of automobile injury was 32%, defined as 1.0 RU. The LTR of automobile death was 0.89% (1/36 RU).
Two events had LTRs roughly similar to automobile injury: development of a subsequent basal cell carcinoma within 3 years (1.4 RU) and development of a squamous cell carcinoma (SCC) secondary to indoor tanning (1.6 RU). Development of SCC following organ transplantation (34 RU) was considerably more likely than automobile injury. All other identified events had lower RUs than automobile injury (Table). Three events with small RUs included tuberculosis development with a tumor necrosis factor α inhibitor (1/32 RU), Crohn disease development with isotretinoin (1/41 RU), and blindness following facial hyaluronic acid injection (1/80 RU). The LTR of death by dog attack (1/42,436 RU) and death by lightning strike (1/36,542 RU) also had small RUs.
The unit comparators from the Table were adapted into graphic form to depict risk relative to the risk of automobile injury (Figure).

Comment
Numerous interventions in dermatology offer much less risk of an adverse event than the LTR of automobile injury. However, this concept of risk includes only the likelihood of development of an event, not the severity of the measured event, as our numerical and visual tool objectively captures the related risks using an RU comparator. Such use of a standardized RU demonstrates the essence of risk; “zero risk” does not exist, and each intervention or treatment, albeit how small, must be justified in concordance with other types of risk, such as the automobile.
The development of adverse events secondary to dermatologic intervention or therapy, for which monitoring visits are utilized, were used as important comparators to the risk of automobile injury. The continuous practice of monitoring visits may increase patient’s fears regarding possible adverse events secondary to therapy. Hydroxychloroquine retinal toxicity (1/16 RU) and psoriatic arthritis development following severe psoriasis (1/3.9 RU) were less likely to occur than automobile injury. The development of abnormal blood counts or blood tests secondary to therapy or intervention could not be formatted into an RU. The use of equation (1) for the calculation of LTRadverse eventholds true only when the absolute risk of developing the adverse event in the general population—in this case, abnormal blood counts or blood tests—is low.7
Although the unit comparator allows for the comparison of different dermatologic risk, a limitation of the RU model and its visual tool are a dependence on RR, a value that changes following publication of new studies. A solution was the use of a single pooled estimate to represent the upper-limit CIs of LTR. This practice overestimates risk. As with RR, new automobile injury rates are published annually.10 In the last 5 years, the LTR of automobile injury has stayed relatively constant: between 32% and 33%.4 Although the RU calculations and Figure included a wide variety of interventions in dermatology, select clinical situations were not included. It is beyond the scope of this article to systematically review all risk in dermatology but rather introduce the concept of the RU founded on automobile-associated risks. With the introduction of a methodical framework, the reader is invited to calculate RUs pertinent to their clinical interests.
Any intervention or treatment in dermatology is accompanied by risk. The use of a unit comparator using an easily relatable event—the LTR of automobile injury—allows the patient to easily compare risk and internally justify the practice of monitoring visits. Inclusion of a visual tool, such as the Figure, might alleviate many irrational fears that accompany some of the highly effective treatments and interventions used in dermatology and thus lead to better patient outcomes.
Acknowledgment
We thank Taranjeet Singh, MS (Dunn, North Carolina), for her comments on an earlier version of the manuscript.
- Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making. 2003;23:511-517.
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014;15:165-180.
- National Safety Council. Odds of dying. Injury Facts website. http://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/. Accessed November 4, 2018.
- National Center for Statistics and Analysis (NCSA) motor vehicle traffic crash data resource page. National Highway Traffic Safety Administration website. https://crashstats.nhtsa.dot.gov/#/. Accessed November 4, 2018.
- CDC report shows motor vehicle crash injuries are frequent and costly. Centers for Disease Control and Prevention website. http://www.cdc.gov/media/releases/2014/p1007-crash-injuries.html. Published October 7, 2014. Accessed November 4, 2018.
- Automobile. Business Dictionary website. http://www.businessdictionary.com/definition/automobile.html. Accessed November 4, 2018.
- Dupont WD, Plummer WD Jr. Understanding the relationship between relative and absolute risk. Cancer. 1996;77:2193-2199.
- Kaminska E, Patel I, Dabade TS, et al. Comparing the lifetime risks of TNF-alpha inhibitor use to common benchmarks of risk. J Dermatolog Treat. 2011;24:101-106.
- Dupont WD. Converting relative risks to absolute risks: a graphical approach. Stat Med. 1989;8:641-651.
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1-122.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration website. http://handbook.cochrane.org. Updated March 2011. Accessed November 15, 2018.
- Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142:761-764.
- Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207-214.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424-1429.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
- Lee SY, Jamal MM, Nguyen ET, et al. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:210-216.
- Injury Facts, 2017. Itasca, IL: National Safety Council; 2017.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-1394.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-1460.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847-857.e1-18.
- Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta Derm Venereol. 2015;95:923-927.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- National Highway Traffic Safety Administration (NHTSA). Traffic Safety Facts 2015. Washington, DC: US Department of Transportation; 2015.
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:E5909.
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-519.
- Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making. 2003;23:511-517.
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014;15:165-180.
- National Safety Council. Odds of dying. Injury Facts website. http://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/. Accessed November 4, 2018.
- National Center for Statistics and Analysis (NCSA) motor vehicle traffic crash data resource page. National Highway Traffic Safety Administration website. https://crashstats.nhtsa.dot.gov/#/. Accessed November 4, 2018.
- CDC report shows motor vehicle crash injuries are frequent and costly. Centers for Disease Control and Prevention website. http://www.cdc.gov/media/releases/2014/p1007-crash-injuries.html. Published October 7, 2014. Accessed November 4, 2018.
- Automobile. Business Dictionary website. http://www.businessdictionary.com/definition/automobile.html. Accessed November 4, 2018.
- Dupont WD, Plummer WD Jr. Understanding the relationship between relative and absolute risk. Cancer. 1996;77:2193-2199.
- Kaminska E, Patel I, Dabade TS, et al. Comparing the lifetime risks of TNF-alpha inhibitor use to common benchmarks of risk. J Dermatolog Treat. 2011;24:101-106.
- Dupont WD. Converting relative risks to absolute risks: a graphical approach. Stat Med. 1989;8:641-651.
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1-122.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration website. http://handbook.cochrane.org. Updated March 2011. Accessed November 15, 2018.
- Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142:761-764.
- Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207-214.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424-1429.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
- Lee SY, Jamal MM, Nguyen ET, et al. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:210-216.
- Injury Facts, 2017. Itasca, IL: National Safety Council; 2017.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-1394.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-1460.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847-857.e1-18.
- Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta Derm Venereol. 2015;95:923-927.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- National Highway Traffic Safety Administration (NHTSA). Traffic Safety Facts 2015. Washington, DC: US Department of Transportation; 2015.
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:E5909.
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-519.
Practice Points
- Using common identifiable risks may help patients put the risk of certain dermatologic interventions into perspective.
- Numerous interventions in dermatology offer much less risk of an adverse event than the lifetime risk of automobile injury.
Strategies to Reduce Youth Indoor Tanning Injuries
Perusal of any lifestyle magazine reveals photographs of movie stars with sun-kissed skin. One can imagine their carefree lives afford ample time outdoors, a vast departure from the pasty masses trapped in their office cubicles. Our cultural norms dictate that a glowing look is a sign of health and attractiveness. Light-skinned individuals must receive regular exposure to sunlight to maintain their bronzed color. Over the last century, the indoor tanning industry has expanded to fill the niche created by the ceaseless pursuit of the ideal complexion.
Indoor tanning use causes up to 170,000 cases of skin cancer per year worldwide.1 Accumulating sunburns early in life is a leading risk factor for melanoma, the deadliest form of skin cancer. Campaigns to spread awareness about the link between UV radiation and skin cancer are ubiquitous. The US Food and Drug Administration (FDA) recommends against the use of tanning beds by minors, and several states have passed laws restricting their access. However, adolescents continue to engage. White female high school students remain frequenters of this practice, with more than 15% reporting current use of indoor tanning facilities.2 It seems targeted outreach and media campaigns are unsuccessful in influencing their behavior, and new approaches are needed.
Tanning-Related Injuries
Concentrated exposure to UV radiation during indoor tanning sessions carries the potential for immediate harm. Public health campaigns have focused on long-term skin cancer risk while overlooking thousands of injuries that occur annually at tanning salons across the country. The US Consumer Product Safety Commission first noted injuries associated with the largely unregulated tanning industry in 1974.3 In response, the FDA limited radiation levels, required indoor tanning devices to have timers and manual off switches, and mandated the use of protective eyewear. These changes sparked industry backlash due to the cost of compliance. The Indoor Tanning Association (no longer in operation) hired a lobbying firm in 2009 that successfully fought to resist further regulation.3
More than 3000 indoor tanning–related injuries are treated in emergency departments annually.4 White women aged 18 to 24 years who visit tanning salons are most likely to sustain injuries. In one study, severe skin burns accounted for 80% of emergency department visits, while the rest were due to fainting, eye injuries, and infections from unsanitary equipment.Timer malfunctions may play a role in tanning bed injuries, as several injured patients have reported falling asleep while tanning.4 Only 5% of tanning salons in North Carolina complied with FDA-recommended exposure schedules in 2003, suggesting that neglect or deliberate override of safety features also may contribute to injury.5
Challenges in Changing Tanning Behaviors
Use of indoor tanning facilities by adolescents is boosted by their misperceptions of peer engagement. Many teenagers overestimate the number of their peers who tan, which influences their own behavior.6 This phenomenon illustrates the importance of perceived social norms in this demographic group. Motivating adolescents to take actions that violate these norms poses a considerable challenge.
To teenagers, the perceived immediate benefits of indoor tanning far outweigh perceived costs. The immediate benefit of indoor tanning is having attractive skin, which may improve social standing and perceived self-worth. When adolescents weigh costs and benefits at different points in time, the present value of future events is discounted when compared to current events. For example, an immediate loss of $1000 is more impactful than losing $1000 ten years down the road. Adolescents are motivated to succeed in the short-term and may heavily discount future adverse effects such as the risk for developing cancer or premature aging of the skin. Therefore, getting a tan may be the “rational” decision even if there is an increased risk of future skin cancer.7
The addiction theory of tanning seeks to explain why individuals continue to tan despite knowledge of the associated risks. Exposure to UV radiation releases endorphins, producing a natural narcotic effect.8 The relaxing feeling sunbathers experience may lead to a phenomenon similar to addictions to opioids, alcohol, tobacco, and sugar. Behavior change is a process that unfolds over time. The 5 stages are precontemplation, contemplation, preparation, action, and maintenance.9 Education falls on deaf ears when the recipients are not ready to consider change. Individuals who are already thinking about cutting back on tanning fall into the category of contemplators and are the most open to educational techniques.9
Potential Solutions
Despite the dire long-term consequences of melanoma, warning adolescents of the increased cancer risk from tanning is an ineffective dissuasion strategy.10 Solutions that aim to limit tanning behaviors in this population should instead center on decreasing the present utility of a tan. Emphasis on the risk of immediate injury may be one effective route. The costs of potential damage to current appearance, vision, and overall health are not readily discounted by adolescents. Teens who devote time and money to the pursuit of a golden glow place high value on attractiveness. Such individuals respond best to loss-framed messages that focus on the impact of UV exposure on appearance, not just their health.11 However, appearance-motivated individuals may feel threatened by interventions that aim to reduce their decision freedom and display high reactance, leading them to reassert their freedom by resisting antitanning messages.12 Another strategy is altering media messaging to support a wider swathe of skin tones, reducing the social benefits of a tan. To swing the needle on our cultural norms, this intervention will require an enduring effort with backing from media outlets and celebrities.
Taxes on tanning salons and devices provide a basic economic disincentive to adolescents who typically have limited funds. State cigarette tax increases successfully reduced youth consumption of tobacco in the 1990s.13 A provision of the Patient Protection and Affordable Care Act levied a 10% excise tax on tanning salons with promising early results.14 Further taxation may generate revenue for educational campaigns on the injury risks of tanning. Continued safety improvements that limit user exposure to UV radiation and enforcement of FDA regulations also will decrease injury rates. Minimizing the UV output of tanning beds and designing protective equipment for tanners are 2 potential objectives. Improvement of over-the-counter sunless tanning agents also will provide alternatives to catching rays for adolescents who wish to attain a bronzed complexion.
Final Thoughts
Health care providers must assess a patient’s readiness for change and tailor interventions accordingly. Regardless of the method, new approaches to combat adolescent tanning injuries may reduce health care costs and minimize serious public health concerns for the next generation.
- Firger J. Indoor tanning injuries send thousands to the ER each year. CBS News. December 16, 2014. https://www.cbsnews.com/news/skin-cancer-burns-indoor-tanning-salon-injuries/. Accessed November 7, 2018.
- Guy GP, Berkowitz Z, Everett Jones S, et al. Prevalence of indoor tanning and association with sunburn among youth in the United States. JAMA Dermatol. 2017;153:387-390.
- Pulley MK. Government tan lines: examining the reach and effectiveness of federal and state efforts to protect consumers from the dangers of indoor tanning. Pepperdine Law Review. 2009;36:1163-1181.
- Guy GP Jr, Watson M, Haileyesus T, et al. Indoor tanning–related injuries treated in a national sample of US hospital emergency departments. JAMA Intern Med. 2015;175:309-311.
- Hornung RL, Magee KH, Lee WJ, et al. Tanning facility use: are we exceeding Food and Drug Administration limits? J Am Acad Dermatol. 2003;49:655-661.
- Hoerster KD, Mayer JA, Woodruff SI, et al. The influence of parents and peers on adolescent indoor tanning behavior: findings from a multi-city sample. J Am Acad Dermatol. 2007;57:990-997.
- Feldman SR, Dempsey JR, Grummer S, et al. Implications of a utility model for ultraviolet exposure behavior. J Am Acad Dermatol. 2001;45:718-722.
- Okhovat J, Feldman SR. Tanning: an addiction? The Melanoma Letter. 2013 Winter;31:5-7. https://www.skincancer.org/Media/Default/File/File/SCF_ML_31-3.pdf. Accessed November 11, 2017.
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. applications to addictive behaviors. Am Psychol. 1992;47:1102-1114.
- Baker MK. Preventing Skin Cancer in Adolescent Girls Through Intervention With Their Mothers [dissertation]. Johnson City, TN: East Tennessee State University; 2013.
- Thomas K, Hevey D, Pertl M, et al. Appearance matters: the frame and focus of health messages influences beliefs about skin cancer. Br J Health Psychol. 2011;16(pt 2):418-429.
- Jones JL, Leary MR. Effects of appearance-based admonitions against sun exposure on tanning intentions in young adults. Health Psychol. 1994;13:86-90.
- Carpenter C, Cook PJ. Cigarette taxes and youth smoking: new evidence from national, state, and local youth risk behavior surveys. J Health Econ. 2008;27:287-99.
- Ryan E. The ‘tanning tax’ is a public health success story. Health Affairs website. https://www.healthaffairs.org/do/10.1377/hblog20170815.061547/full/. Published August 15, 2017. Accessed November 7, 2018.
Perusal of any lifestyle magazine reveals photographs of movie stars with sun-kissed skin. One can imagine their carefree lives afford ample time outdoors, a vast departure from the pasty masses trapped in their office cubicles. Our cultural norms dictate that a glowing look is a sign of health and attractiveness. Light-skinned individuals must receive regular exposure to sunlight to maintain their bronzed color. Over the last century, the indoor tanning industry has expanded to fill the niche created by the ceaseless pursuit of the ideal complexion.
Indoor tanning use causes up to 170,000 cases of skin cancer per year worldwide.1 Accumulating sunburns early in life is a leading risk factor for melanoma, the deadliest form of skin cancer. Campaigns to spread awareness about the link between UV radiation and skin cancer are ubiquitous. The US Food and Drug Administration (FDA) recommends against the use of tanning beds by minors, and several states have passed laws restricting their access. However, adolescents continue to engage. White female high school students remain frequenters of this practice, with more than 15% reporting current use of indoor tanning facilities.2 It seems targeted outreach and media campaigns are unsuccessful in influencing their behavior, and new approaches are needed.
Tanning-Related Injuries
Concentrated exposure to UV radiation during indoor tanning sessions carries the potential for immediate harm. Public health campaigns have focused on long-term skin cancer risk while overlooking thousands of injuries that occur annually at tanning salons across the country. The US Consumer Product Safety Commission first noted injuries associated with the largely unregulated tanning industry in 1974.3 In response, the FDA limited radiation levels, required indoor tanning devices to have timers and manual off switches, and mandated the use of protective eyewear. These changes sparked industry backlash due to the cost of compliance. The Indoor Tanning Association (no longer in operation) hired a lobbying firm in 2009 that successfully fought to resist further regulation.3
More than 3000 indoor tanning–related injuries are treated in emergency departments annually.4 White women aged 18 to 24 years who visit tanning salons are most likely to sustain injuries. In one study, severe skin burns accounted for 80% of emergency department visits, while the rest were due to fainting, eye injuries, and infections from unsanitary equipment.Timer malfunctions may play a role in tanning bed injuries, as several injured patients have reported falling asleep while tanning.4 Only 5% of tanning salons in North Carolina complied with FDA-recommended exposure schedules in 2003, suggesting that neglect or deliberate override of safety features also may contribute to injury.5
Challenges in Changing Tanning Behaviors
Use of indoor tanning facilities by adolescents is boosted by their misperceptions of peer engagement. Many teenagers overestimate the number of their peers who tan, which influences their own behavior.6 This phenomenon illustrates the importance of perceived social norms in this demographic group. Motivating adolescents to take actions that violate these norms poses a considerable challenge.
To teenagers, the perceived immediate benefits of indoor tanning far outweigh perceived costs. The immediate benefit of indoor tanning is having attractive skin, which may improve social standing and perceived self-worth. When adolescents weigh costs and benefits at different points in time, the present value of future events is discounted when compared to current events. For example, an immediate loss of $1000 is more impactful than losing $1000 ten years down the road. Adolescents are motivated to succeed in the short-term and may heavily discount future adverse effects such as the risk for developing cancer or premature aging of the skin. Therefore, getting a tan may be the “rational” decision even if there is an increased risk of future skin cancer.7
The addiction theory of tanning seeks to explain why individuals continue to tan despite knowledge of the associated risks. Exposure to UV radiation releases endorphins, producing a natural narcotic effect.8 The relaxing feeling sunbathers experience may lead to a phenomenon similar to addictions to opioids, alcohol, tobacco, and sugar. Behavior change is a process that unfolds over time. The 5 stages are precontemplation, contemplation, preparation, action, and maintenance.9 Education falls on deaf ears when the recipients are not ready to consider change. Individuals who are already thinking about cutting back on tanning fall into the category of contemplators and are the most open to educational techniques.9
Potential Solutions
Despite the dire long-term consequences of melanoma, warning adolescents of the increased cancer risk from tanning is an ineffective dissuasion strategy.10 Solutions that aim to limit tanning behaviors in this population should instead center on decreasing the present utility of a tan. Emphasis on the risk of immediate injury may be one effective route. The costs of potential damage to current appearance, vision, and overall health are not readily discounted by adolescents. Teens who devote time and money to the pursuit of a golden glow place high value on attractiveness. Such individuals respond best to loss-framed messages that focus on the impact of UV exposure on appearance, not just their health.11 However, appearance-motivated individuals may feel threatened by interventions that aim to reduce their decision freedom and display high reactance, leading them to reassert their freedom by resisting antitanning messages.12 Another strategy is altering media messaging to support a wider swathe of skin tones, reducing the social benefits of a tan. To swing the needle on our cultural norms, this intervention will require an enduring effort with backing from media outlets and celebrities.
Taxes on tanning salons and devices provide a basic economic disincentive to adolescents who typically have limited funds. State cigarette tax increases successfully reduced youth consumption of tobacco in the 1990s.13 A provision of the Patient Protection and Affordable Care Act levied a 10% excise tax on tanning salons with promising early results.14 Further taxation may generate revenue for educational campaigns on the injury risks of tanning. Continued safety improvements that limit user exposure to UV radiation and enforcement of FDA regulations also will decrease injury rates. Minimizing the UV output of tanning beds and designing protective equipment for tanners are 2 potential objectives. Improvement of over-the-counter sunless tanning agents also will provide alternatives to catching rays for adolescents who wish to attain a bronzed complexion.
Final Thoughts
Health care providers must assess a patient’s readiness for change and tailor interventions accordingly. Regardless of the method, new approaches to combat adolescent tanning injuries may reduce health care costs and minimize serious public health concerns for the next generation.
Perusal of any lifestyle magazine reveals photographs of movie stars with sun-kissed skin. One can imagine their carefree lives afford ample time outdoors, a vast departure from the pasty masses trapped in their office cubicles. Our cultural norms dictate that a glowing look is a sign of health and attractiveness. Light-skinned individuals must receive regular exposure to sunlight to maintain their bronzed color. Over the last century, the indoor tanning industry has expanded to fill the niche created by the ceaseless pursuit of the ideal complexion.
Indoor tanning use causes up to 170,000 cases of skin cancer per year worldwide.1 Accumulating sunburns early in life is a leading risk factor for melanoma, the deadliest form of skin cancer. Campaigns to spread awareness about the link between UV radiation and skin cancer are ubiquitous. The US Food and Drug Administration (FDA) recommends against the use of tanning beds by minors, and several states have passed laws restricting their access. However, adolescents continue to engage. White female high school students remain frequenters of this practice, with more than 15% reporting current use of indoor tanning facilities.2 It seems targeted outreach and media campaigns are unsuccessful in influencing their behavior, and new approaches are needed.
Tanning-Related Injuries
Concentrated exposure to UV radiation during indoor tanning sessions carries the potential for immediate harm. Public health campaigns have focused on long-term skin cancer risk while overlooking thousands of injuries that occur annually at tanning salons across the country. The US Consumer Product Safety Commission first noted injuries associated with the largely unregulated tanning industry in 1974.3 In response, the FDA limited radiation levels, required indoor tanning devices to have timers and manual off switches, and mandated the use of protective eyewear. These changes sparked industry backlash due to the cost of compliance. The Indoor Tanning Association (no longer in operation) hired a lobbying firm in 2009 that successfully fought to resist further regulation.3
More than 3000 indoor tanning–related injuries are treated in emergency departments annually.4 White women aged 18 to 24 years who visit tanning salons are most likely to sustain injuries. In one study, severe skin burns accounted for 80% of emergency department visits, while the rest were due to fainting, eye injuries, and infections from unsanitary equipment.Timer malfunctions may play a role in tanning bed injuries, as several injured patients have reported falling asleep while tanning.4 Only 5% of tanning salons in North Carolina complied with FDA-recommended exposure schedules in 2003, suggesting that neglect or deliberate override of safety features also may contribute to injury.5
Challenges in Changing Tanning Behaviors
Use of indoor tanning facilities by adolescents is boosted by their misperceptions of peer engagement. Many teenagers overestimate the number of their peers who tan, which influences their own behavior.6 This phenomenon illustrates the importance of perceived social norms in this demographic group. Motivating adolescents to take actions that violate these norms poses a considerable challenge.
To teenagers, the perceived immediate benefits of indoor tanning far outweigh perceived costs. The immediate benefit of indoor tanning is having attractive skin, which may improve social standing and perceived self-worth. When adolescents weigh costs and benefits at different points in time, the present value of future events is discounted when compared to current events. For example, an immediate loss of $1000 is more impactful than losing $1000 ten years down the road. Adolescents are motivated to succeed in the short-term and may heavily discount future adverse effects such as the risk for developing cancer or premature aging of the skin. Therefore, getting a tan may be the “rational” decision even if there is an increased risk of future skin cancer.7
The addiction theory of tanning seeks to explain why individuals continue to tan despite knowledge of the associated risks. Exposure to UV radiation releases endorphins, producing a natural narcotic effect.8 The relaxing feeling sunbathers experience may lead to a phenomenon similar to addictions to opioids, alcohol, tobacco, and sugar. Behavior change is a process that unfolds over time. The 5 stages are precontemplation, contemplation, preparation, action, and maintenance.9 Education falls on deaf ears when the recipients are not ready to consider change. Individuals who are already thinking about cutting back on tanning fall into the category of contemplators and are the most open to educational techniques.9
Potential Solutions
Despite the dire long-term consequences of melanoma, warning adolescents of the increased cancer risk from tanning is an ineffective dissuasion strategy.10 Solutions that aim to limit tanning behaviors in this population should instead center on decreasing the present utility of a tan. Emphasis on the risk of immediate injury may be one effective route. The costs of potential damage to current appearance, vision, and overall health are not readily discounted by adolescents. Teens who devote time and money to the pursuit of a golden glow place high value on attractiveness. Such individuals respond best to loss-framed messages that focus on the impact of UV exposure on appearance, not just their health.11 However, appearance-motivated individuals may feel threatened by interventions that aim to reduce their decision freedom and display high reactance, leading them to reassert their freedom by resisting antitanning messages.12 Another strategy is altering media messaging to support a wider swathe of skin tones, reducing the social benefits of a tan. To swing the needle on our cultural norms, this intervention will require an enduring effort with backing from media outlets and celebrities.
Taxes on tanning salons and devices provide a basic economic disincentive to adolescents who typically have limited funds. State cigarette tax increases successfully reduced youth consumption of tobacco in the 1990s.13 A provision of the Patient Protection and Affordable Care Act levied a 10% excise tax on tanning salons with promising early results.14 Further taxation may generate revenue for educational campaigns on the injury risks of tanning. Continued safety improvements that limit user exposure to UV radiation and enforcement of FDA regulations also will decrease injury rates. Minimizing the UV output of tanning beds and designing protective equipment for tanners are 2 potential objectives. Improvement of over-the-counter sunless tanning agents also will provide alternatives to catching rays for adolescents who wish to attain a bronzed complexion.
Final Thoughts
Health care providers must assess a patient’s readiness for change and tailor interventions accordingly. Regardless of the method, new approaches to combat adolescent tanning injuries may reduce health care costs and minimize serious public health concerns for the next generation.
- Firger J. Indoor tanning injuries send thousands to the ER each year. CBS News. December 16, 2014. https://www.cbsnews.com/news/skin-cancer-burns-indoor-tanning-salon-injuries/. Accessed November 7, 2018.
- Guy GP, Berkowitz Z, Everett Jones S, et al. Prevalence of indoor tanning and association with sunburn among youth in the United States. JAMA Dermatol. 2017;153:387-390.
- Pulley MK. Government tan lines: examining the reach and effectiveness of federal and state efforts to protect consumers from the dangers of indoor tanning. Pepperdine Law Review. 2009;36:1163-1181.
- Guy GP Jr, Watson M, Haileyesus T, et al. Indoor tanning–related injuries treated in a national sample of US hospital emergency departments. JAMA Intern Med. 2015;175:309-311.
- Hornung RL, Magee KH, Lee WJ, et al. Tanning facility use: are we exceeding Food and Drug Administration limits? J Am Acad Dermatol. 2003;49:655-661.
- Hoerster KD, Mayer JA, Woodruff SI, et al. The influence of parents and peers on adolescent indoor tanning behavior: findings from a multi-city sample. J Am Acad Dermatol. 2007;57:990-997.
- Feldman SR, Dempsey JR, Grummer S, et al. Implications of a utility model for ultraviolet exposure behavior. J Am Acad Dermatol. 2001;45:718-722.
- Okhovat J, Feldman SR. Tanning: an addiction? The Melanoma Letter. 2013 Winter;31:5-7. https://www.skincancer.org/Media/Default/File/File/SCF_ML_31-3.pdf. Accessed November 11, 2017.
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. applications to addictive behaviors. Am Psychol. 1992;47:1102-1114.
- Baker MK. Preventing Skin Cancer in Adolescent Girls Through Intervention With Their Mothers [dissertation]. Johnson City, TN: East Tennessee State University; 2013.
- Thomas K, Hevey D, Pertl M, et al. Appearance matters: the frame and focus of health messages influences beliefs about skin cancer. Br J Health Psychol. 2011;16(pt 2):418-429.
- Jones JL, Leary MR. Effects of appearance-based admonitions against sun exposure on tanning intentions in young adults. Health Psychol. 1994;13:86-90.
- Carpenter C, Cook PJ. Cigarette taxes and youth smoking: new evidence from national, state, and local youth risk behavior surveys. J Health Econ. 2008;27:287-99.
- Ryan E. The ‘tanning tax’ is a public health success story. Health Affairs website. https://www.healthaffairs.org/do/10.1377/hblog20170815.061547/full/. Published August 15, 2017. Accessed November 7, 2018.
- Firger J. Indoor tanning injuries send thousands to the ER each year. CBS News. December 16, 2014. https://www.cbsnews.com/news/skin-cancer-burns-indoor-tanning-salon-injuries/. Accessed November 7, 2018.
- Guy GP, Berkowitz Z, Everett Jones S, et al. Prevalence of indoor tanning and association with sunburn among youth in the United States. JAMA Dermatol. 2017;153:387-390.
- Pulley MK. Government tan lines: examining the reach and effectiveness of federal and state efforts to protect consumers from the dangers of indoor tanning. Pepperdine Law Review. 2009;36:1163-1181.
- Guy GP Jr, Watson M, Haileyesus T, et al. Indoor tanning–related injuries treated in a national sample of US hospital emergency departments. JAMA Intern Med. 2015;175:309-311.
- Hornung RL, Magee KH, Lee WJ, et al. Tanning facility use: are we exceeding Food and Drug Administration limits? J Am Acad Dermatol. 2003;49:655-661.
- Hoerster KD, Mayer JA, Woodruff SI, et al. The influence of parents and peers on adolescent indoor tanning behavior: findings from a multi-city sample. J Am Acad Dermatol. 2007;57:990-997.
- Feldman SR, Dempsey JR, Grummer S, et al. Implications of a utility model for ultraviolet exposure behavior. J Am Acad Dermatol. 2001;45:718-722.
- Okhovat J, Feldman SR. Tanning: an addiction? The Melanoma Letter. 2013 Winter;31:5-7. https://www.skincancer.org/Media/Default/File/File/SCF_ML_31-3.pdf. Accessed November 11, 2017.
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. applications to addictive behaviors. Am Psychol. 1992;47:1102-1114.
- Baker MK. Preventing Skin Cancer in Adolescent Girls Through Intervention With Their Mothers [dissertation]. Johnson City, TN: East Tennessee State University; 2013.
- Thomas K, Hevey D, Pertl M, et al. Appearance matters: the frame and focus of health messages influences beliefs about skin cancer. Br J Health Psychol. 2011;16(pt 2):418-429.
- Jones JL, Leary MR. Effects of appearance-based admonitions against sun exposure on tanning intentions in young adults. Health Psychol. 1994;13:86-90.
- Carpenter C, Cook PJ. Cigarette taxes and youth smoking: new evidence from national, state, and local youth risk behavior surveys. J Health Econ. 2008;27:287-99.
- Ryan E. The ‘tanning tax’ is a public health success story. Health Affairs website. https://www.healthaffairs.org/do/10.1377/hblog20170815.061547/full/. Published August 15, 2017. Accessed November 7, 2018.