User login
Psychosocial Impact of Psoriasis: A Review for Dermatology Residents
The psychosocial impact of psoriasis is a critical component of disease burden. Psoriatic patients have high rates of depression and anxiety, problems at work, and difficulties with interpersonal relationships and intimacy.1 A National Psoriasis Foundation (NPF) survey from 2003 to 2011 reported that psoriasis affects overall emotional well-being in 88% of patients and enjoyment of life in 82% of patients.2
The reasons for psychosocial burden stem from public misconceptions and disease stigma. A survey of 1005 individuals (age range, 16–64 years) about their perceptions of psoriasis revealed that 16.5% believed that psoriasis is contagious and 6.8% believed that psoriasis is related to personal hygiene.3 Fifty percent practiced discriminatory behavior toward psoriatic patients, including reluctance to shake hands (28.8%) and engage in sexual relations/intercourse (44.1%). Sixty-five percent of psoriatic patients felt their appearance is unsightly, and 73% felt self-conscious about having psoriasis.2
The psychosocial burden exists despite medical treatment of the disease. In a cross-sectional study of 1184 psoriatic patients, 70.2% had impaired quality of life (QOL) as measured by the dermatology life quality index (DLQI), even after receiving a 4-week treatment for psoriasis.4 Medical treatment of psoriasis is not enough; providers need to assess overall QOL and provide treatment and resources for these patients in addition to symptomatic management.
There have been many studies on the psychosocial burden of psoriasis, but few have focused on a dermatology resident’s role in addressing this issue. This article will review psychosocial domains—psychiatric comorbidities and social functioning including occupational functioning, interpersonal relationships, and sexual functioning— and discuss a dermatology resident’s role in assessing and addressing each of these areas.
Methods
A PubMed search of articles indexed for MEDLINE was conducted using the following terms: psoriasis, depression, anxiety, work productivity, sexual functioning, and interpersonal relationships. Selected articles covered prevalence, assessment, and management of each psychosocial domain.
Results
Psychiatric Comorbidities
Prevalence
A high prevalence of psychiatric comorbidities exists in psoriatic patients. In a study of 469,097 patients with psoriasis, depression was the third most prevalent comorbidity (17.91%), following hyperlipidemia (45.64%) and hypertension (42.19%).5 In a 10-year longitudinal, population-based, prospective cohort study, antidepressant prescriptions were twice as frequent in psoriatic patients (17.8%) compared to control (7.9%)(P<.001).6 In a meta-analysis of 98 studies investigating psoriatic patients and psychiatric comorbidities, patients with psoriasis were 1.5 times more likely to experience depression (odds ratio [OR]: 1.57; 95% CI, 1.40-1.76) and use antidepressants (OR: 4.24; 95% CI, 1.53-11.76) compared to control.7 Patients with psoriasis were more likely to attempt suicide (OR: 1.32; 95% CI, 1.14-1.54) and complete suicide (OR: 1.20; 95% CI, 1.04-1.39) compared to people without psoriasis.8 A 1-year cross-sectional study of 90 psoriatic patients reported 78.7% were diagnosed with depression and 76.7% were diagnosed with anxiety. Seventy-two percent reported both anxiety and depression, correlating with worse QOL (χ2=26.7; P<.05).9
Assessment
Psychiatric comorbidities are assessed using clinical judgment and formal screening questionnaires in research studies. Signs of depression in patients with psoriasis can manifest as poor treatment adherence and recurrent flares of psoriasis.10,11 Psoriatic patients with psychiatric comorbidities were less likely to be adherent to treatment (risk ratio: 0.35; P<.003).10 The patient health questionnaire (PHQ) 9 and generalized anxiety disorder scale (GAD) 7 are validated and reliable questionnaires. The first 2 questions in PHQ-9 and GAD-7 screen for depression and anxiety, respectively.12-14 These 2-question screens are practical in a fast-paced dermatology outpatient setting. Systematic questionnaires specifically targeting mood disorders may be more beneficial than the widely used DLQI, which may not adequately capture mood disorders. Over the course of 10 months, 607 patients with psoriasis were asked to fill out the PHQ-9, GAD-7, and DLQI. Thirty-eight percent of patients with major depressive disorder had a DLQI score lower than 10, while 46% of patients with generalized anxiety disorder had a DLQI score lower than 10.15 Other questionnaires, including the hospital anxiety and depression scale and Beck depression inventory, are valid instruments with high sensitivity but are commonly used for research purposes and may not be clinically feasible.16
Management
Dermatologists should refer patients with depression and/or anxiety to psychiatry. Interventions include pharmacologic and nonpharmacologic management. First-line therapy for depression and anxiety is a combination of selective serotonin reuptake inhibitors and cognitive behavioral therapy.17 In addition, providers can direct patients to online resources such as the NPF website, where patients with psoriasis can access information about the signs and symptoms of mood disorders and contact the patient navigation center for further help.18
Social Functioning
Occupational Prevalence
The NPF found that 92% of patients with psoriasis or psoriatic arthritis (PsA) surveyed between 2003 and 2011 cited their psoriasis as reason for unemployment.2 In a survey of 43 patients asked about social and occupational functioning using the social and occupational assessment scale, 62.5% of psoriatic patients reported distress at work and 51.1% reported decreased efficiency at work.19 A national online survey that was conducted in France and issued to patients with and without psoriasis assessed overall QOL and work productivity using the work productivity and activity impairment questionnaire for psoriasis (WPAI-PSO). Of 714 patients with psoriasis and PsA, the latter had a 57.6% decrease in work productivity over 7 days compared to 27.9% in controls (P<.05).20 Occupational impairment leads to lost wages and hinders advancement, further exacerbating the psychosocial burden of psoriasis.21
Occupational Assessment
Formal assessment of occupational function can be done with the WPAI-PSO, a 6-question valid instrument.22 Providers may look for risk factors associated with greater loss in work productivity to help identify and offer support for patients. Patients with increased severity of itching, pain, and scaling experienced a greater decrease in work productivity.21,23 Patients with PsA warrant early detection and treatment because they experience greater physical restraints that can interfere with work activities. Of the 459 psoriatic patients without a prior diagnosis of PsA who filled out the PsA screening and evaluation questionnaire, 144 (31.4%) received a score of 44 or higher and were referred to rheumatology for further evaluation with the classification criteria for PsA. Nine percent of patients failed to be screened and remained undiagnosed with PsA.24 In a study using the health assessment questionnaire to assess 400 patients with PsA, those with worse physical function due to joint pain and stiffness were less likely to remain employed (OR: 0.56; P=.02).25
Occupational Management
Identifying and coordinating symptoms of PsA between dermatology and rheumatology is beneficial for patients who experience debilitating symptoms. There are a variety of treatments available for PsA. According to the European League Against Rheumatism 2015 guidelines developed from expert opinion and systematic reviews for PsA management, there are 4 phases of treatment, with reassessment every 3 to 6 months for effectiveness of therapy.26,27 Phase I involves initiating nonsteroidal anti-inflammatory drugs with or without glucocorticoid injections. Phase II involves synthetic disease-modifying drugs, including methotrexate, leflunomide, sulfasalazine, or cyclosporine. Phase III involves adding a second synthetic disease-modifying drug or starting a biologic, such as an anti–tumor necrosis factor, IL-12/IL-23, or IL-17 inhibitor. Phase IV involves switching to a different drug in either aforementioned class.26,27 Treatment with biologics improves work productivity as assessed by WPAI-PSO for psoriasis and PsA.28-30 Encouraging patients to speak up in the workplace and request small accommodations such as timely breaks or ergonomic chairs can help patients feel more comfortable and supported in the work environment.18 Patients who felt supported at work were more likely to remain employed.25
Interpersonal Relationships Prevalence
Misinformation about psoriasis, fear of rejection, and feelings of isolation may contribute to interpersonal conflict. Patients have feelings of shame and self-consciousness that hinder them from engaging in social activities and seeking out relationships.31 Twenty-nine percent of patients feel that psoriasis has interfered with establishing relationships because of negative self-esteem associated with the disease,32 and 26.3% have experienced people avoiding physical contact.33 Family and spouses of patients with psoriasis may be secondarily affected due to economic and emotional distress. Ninety-eight percent of family members of psoriatic patients experienced emotional distress and 54% experienced the burden of care.34 In a survey of 63 relatives and partners of patients with psoriasis, 57% experienced psychological distress, including anxiety and worry over a psoriatic patient’s future.35
Interpersonal Relationships Assessment
Current available tools, including the DLQI and short form health survey, measure overall QOL, including social functioning, but may not be practical in a clinic setting. Although no quick-screening test to assess for this domain exists, providers are encouraged to ask patients about disease impact on interpersonal relationships. The family DLQI questionnaire, adapted from the DLQI, may help physicians and social workers evaluate the burden on a patient’s family members.34
Interpersonal Relationships Management
It may be difficult for providers to address problems with interpersonal relationships without accessible tools. Patients may not be accompanied by family or friends during appointments, and it is difficult to screen for these issues during visits. Providers may offer resources such as the NPF website, which provides information about support groups. It also provides tips on dating and connecting to others in the community who share similar experiences.18 Encouraging patients to seek family or couples therapy also may be beneficial. Increased social support can lead to better QOL and fewer depressive symptoms.36
Sexual Functioning Prevalence
Psoriasis affects both physical and psychological components of sexual function. Among 3485 patients with skin conditions who were surveyed about sexual function, 34% of psoriatic patients reported that psoriasis interfered with sexual functioning at least to a certain degree.37 Sexual impairment was strongly associated with depression, anxiety, and suicidal ideation; 24% of depressed patients and 20% of anxious patients experienced sexual problems a lot or very much, based on the DLQI.37 Depending on the questionnaire used, the prevalence of sexual dysfunction due to psoriasis ranged from 35.5% to 71.3%.38 In an observational cohort study of 158 participants (n=79 psoriasis patients and n=79 controls), 34.2% of patients with psoriasis experienced erectile dysfunction compared to 17.7% of controls.39 Forty-two percent of psoriatic patients with genital involvement reported dyspareunia, 32% reported worsening of genital psoriasis after intercourse, and 43% reported decreased frequency of intercourse.40
Sexual Functioning Assessment
The Skindex-29, DLQI, and psoriasis disability index are available QOL tools that include one question evaluating difficulties with sexual function. The
Sexual Functioning Management
Better disease control leads to improved sexual function, as patients experience fewer feelings of shame, anxiety, and depression, as well as improvement of physical symptoms that can interfere with sexual functioning.38,43,44 Reducing friction, warmth, and moisture, as well as avoiding tight clothing, can help those with genital psoriasis. Patients are advised to reapply topical medications after sexual intercourse. Patients also can apply makeup to disguise psoriasis and help reduce feelings of self-consciousness that can impede sexual intimacy.18
Comment
The psychosocial burden of psoriasis penetrates many facets of patient lives. Psoriasis can invoke feelings of shame and embarrassment that are worsened by the public’s misconceptions about psoriasis, resulting in serious mental health issues that can cause even greater disability. Depression and anxiety are prevalent in patients with psoriasis. The characteristic symptoms of pain and pruritus along with psychiatric comorbidities can have an underestimated impact on daily activities, including employment, interpersonal relationships, and sexual function. Such dysfunctions have serious implications toward wages, professional advancement, social support, and overall QOL.
Dermatology providers play an important role in screening for these problems through validated questionnaires and identifying risks. Simple screening questions such as the PHQ-9 can be beneficial and feasible during dermatology visits. Screening for PsA can help patients avoid problems at work. Sexual dysfunction is a sensitive topic; however, providers can use a 1-question screen from valid questionnaires and inquire about the location of lesions as opportunities to address this issue.
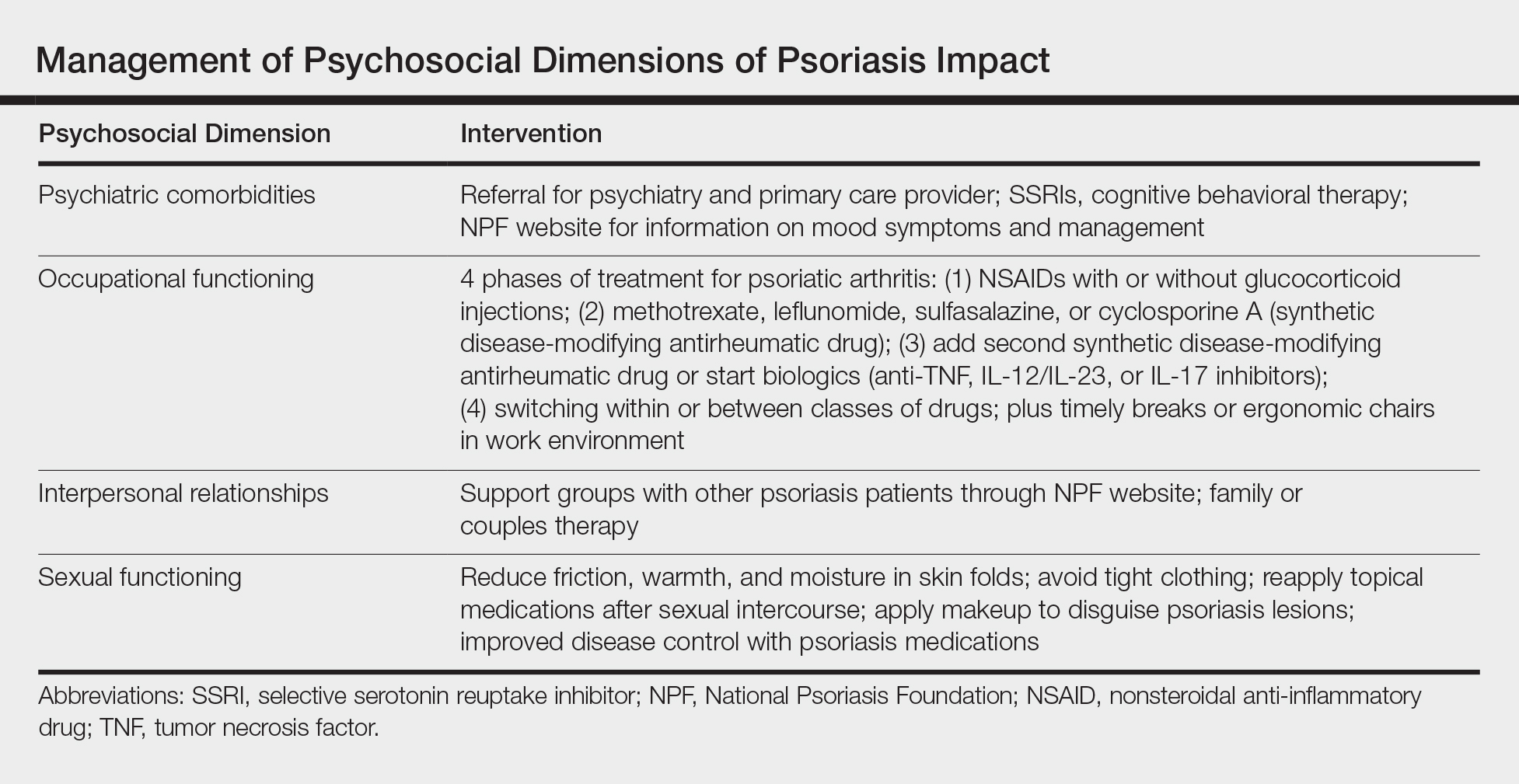
Interventions lead to better disease control, which concurrently improves overall QOL. These interventions depend on both patient adherence and a physician’s commitment to finding an optimal treatment regimen for each individual. Medical management; coordinating care; developing treatment plans with psychiatry, rheumatology, and primary care providers; and psychological counseling and services may be necessary and beneficial (Table). Offering accessible resources such as the NPF website helps patients access information outside the clinic when it is not feasible to address all these concerns in a single visit. Psoriasis requires more than just medical management; it requires dermatology providers to use a multidisciplinary approach to address the psychosocial aspects of the disease.
Conclusion
The psychosocial burden of psoriasis is immense. Stigma, public misconception, mental health concerns, and occupational and interpersonal difficulty are the basis of disease burden. Providers play a vital role in assessing the effect psoriasis has on different areas of patients’ lives and providing appropriate interventions and resources to reduce disease burden.
- Kimball AB, Jacobson C, Weiss S, et al. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6:383-392.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
- Wolf P, Weger W, Legat F, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges. 2018;16:981-990.
- Shah K, Mellars L, Changolkar A, et al. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.e4.
- Dowlatshahi EA, Wakkee M, Herings RM, et al. Increased antidepressant drug exposure in psoriasis patients: a longitudinal population-based cohort study. Acta Derm Venereol. 2013;93:544-550.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425.e2-440.e2.
- Lakshmy S, Balasundaram S, Sarkar S, et al. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J Psychol Med. 2015;37:434-440.
- Renzi C, Picardi A, Abeni D, et al. Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol. 2002;138:337-342.
- Kulkarni AS, Balkrishnan R, Camacho FT, et al. Medication and health care service utilization related to depressive symptoms in older adults with psoriasis. J Drugs Dermatol. 2004;3:661-666.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284-1292.
- Lamb RC, Matcham F, Turner MA, et al. Screening for anxiety and depression in people with psoriasis: a cross-sectional study in a tertiary referral setting. Br J Dermatol. 2017;176:1028-1034.
- Law M, Naughton MT, Dhar A, et al. Validation of two depression screening instruments in a sleep disorders clinic. J Clin Sleep Med. 2014;10:683-688.
- Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70:1219-1229.
- National Psoriasis Foundation. Living with psoriatic arthritis. https://www.psoriasis.org/life-with-psoriatic-arthritis. Accessed September 23, 2018.
- Gaikwad R, Deshpande S, Raje S, et al. Evaluation of functional impairment in psoriasis. Indian J Dermatol Venereol Leprol. 2006;72:37-40.
- Claudepierre P, Lahfa M, Levy P, et al. The impact of psoriasis on professional life: PsoPRO, a French national survey [published online April 6, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14986.
- Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41:514-521.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353-365.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21.
- Spelman L, Su JC, Fernandez-Penas P, et al. Frequency of undiagnosed psoriatic arthritis among psoriasis patients in Australian dermatology practice. J Eur Acad Dermatol Venereol. 2015;29:2184-2191.
- Tillett W, Shaddick G, Askari A, et al. Factors influencing work disability in psoriatic arthritis: first results from a large UK multicentre study. Rheumatology (Oxford). 2015;54:157-162.
- Raychaudhuri SP, Wilken R, Sukhov AC, et al. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21-37.
- Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the manegement of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499-510.
- Beroukhim K, Danesh M, Nguyen C, et al. A prospective, interventional assessment of the impact of ustekinumab treatment on psoriasis-related work productivity and activity impairment. J Dermatol Treat. 2016;27:552-555.
- Armstrong AW, Lynde CW, McBride SR, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol. 2016;152:661-669.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;66:e67-76.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20.
- Reich A, Welz-Kubiak K, Rams Ł. Apprehension of the disease by patients suffering from psoriasis. Postepy Dermatol Alergol. 2014;31:289-293.
- Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experience in dermatologic disorders. Cutis. 1998;61:339-342.
- Basra MK, Finlay AY. The family impact of skin diseases: the Greater Patient concept. Br J Dermatol. 2007;156:929-937.
- Eghlileb AM, Davies EE, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156:1245-1250.
- Janowski K, Steuden S, Pietrzak A, et al. Social support and adaptation to the disease in men and women with psoriasis. Arch Dermatol Res. 2012;304:421-432.
- Sampogna F, Abeni D, Gieler U, et al. Impairment of sexual life in 3,485 dermatological outpatients from a multicentre study in 13 European countries. Acta Derm Venereol. 2017;97:478-482.
- Sampogna F, Gisondi P, Tabolli S, et al. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214:144-150.
- Molina-Leyva A, Molina-Leyva I, Almodovar-Real A, et al. Prevalence and associated factors of erectile dysfunction in patients with moderate to severe psoriasis and healthy population: a comparative study considering physical and psychological factors. Arch Sex Behav. 2016;45:2047-2055.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom. 2001;70:221-225.
- Molina-Leyva A, Almodovar-Real A, Ruiz-Carrascosa JC, et al. Distribution pattern of psoriasis affects sexual function in moderate to severe psoriasis: a prospective case series study. J Sex Med. 2014;11:2882-2889.
- Guenther L, Han C, Szapary P, et al. Impact of ustekinumab on health-related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. J Eur Acad Dermatol Venereol. 2011;25:851-857.
- Guenther L, Warren RB, Cather JC, et al. Impact of ixekizumab treatment on skin-related personal relationship difficulties in moderate-to-severe psoriasis patients: 12-week results from two Phase 3 trials. J Eur Acad Dermatol Venereol. 2017;31:1867-1875.
The psychosocial impact of psoriasis is a critical component of disease burden. Psoriatic patients have high rates of depression and anxiety, problems at work, and difficulties with interpersonal relationships and intimacy.1 A National Psoriasis Foundation (NPF) survey from 2003 to 2011 reported that psoriasis affects overall emotional well-being in 88% of patients and enjoyment of life in 82% of patients.2
The reasons for psychosocial burden stem from public misconceptions and disease stigma. A survey of 1005 individuals (age range, 16–64 years) about their perceptions of psoriasis revealed that 16.5% believed that psoriasis is contagious and 6.8% believed that psoriasis is related to personal hygiene.3 Fifty percent practiced discriminatory behavior toward psoriatic patients, including reluctance to shake hands (28.8%) and engage in sexual relations/intercourse (44.1%). Sixty-five percent of psoriatic patients felt their appearance is unsightly, and 73% felt self-conscious about having psoriasis.2
The psychosocial burden exists despite medical treatment of the disease. In a cross-sectional study of 1184 psoriatic patients, 70.2% had impaired quality of life (QOL) as measured by the dermatology life quality index (DLQI), even after receiving a 4-week treatment for psoriasis.4 Medical treatment of psoriasis is not enough; providers need to assess overall QOL and provide treatment and resources for these patients in addition to symptomatic management.
There have been many studies on the psychosocial burden of psoriasis, but few have focused on a dermatology resident’s role in addressing this issue. This article will review psychosocial domains—psychiatric comorbidities and social functioning including occupational functioning, interpersonal relationships, and sexual functioning— and discuss a dermatology resident’s role in assessing and addressing each of these areas.
Methods
A PubMed search of articles indexed for MEDLINE was conducted using the following terms: psoriasis, depression, anxiety, work productivity, sexual functioning, and interpersonal relationships. Selected articles covered prevalence, assessment, and management of each psychosocial domain.
Results
Psychiatric Comorbidities
Prevalence
A high prevalence of psychiatric comorbidities exists in psoriatic patients. In a study of 469,097 patients with psoriasis, depression was the third most prevalent comorbidity (17.91%), following hyperlipidemia (45.64%) and hypertension (42.19%).5 In a 10-year longitudinal, population-based, prospective cohort study, antidepressant prescriptions were twice as frequent in psoriatic patients (17.8%) compared to control (7.9%)(P<.001).6 In a meta-analysis of 98 studies investigating psoriatic patients and psychiatric comorbidities, patients with psoriasis were 1.5 times more likely to experience depression (odds ratio [OR]: 1.57; 95% CI, 1.40-1.76) and use antidepressants (OR: 4.24; 95% CI, 1.53-11.76) compared to control.7 Patients with psoriasis were more likely to attempt suicide (OR: 1.32; 95% CI, 1.14-1.54) and complete suicide (OR: 1.20; 95% CI, 1.04-1.39) compared to people without psoriasis.8 A 1-year cross-sectional study of 90 psoriatic patients reported 78.7% were diagnosed with depression and 76.7% were diagnosed with anxiety. Seventy-two percent reported both anxiety and depression, correlating with worse QOL (χ2=26.7; P<.05).9
Assessment
Psychiatric comorbidities are assessed using clinical judgment and formal screening questionnaires in research studies. Signs of depression in patients with psoriasis can manifest as poor treatment adherence and recurrent flares of psoriasis.10,11 Psoriatic patients with psychiatric comorbidities were less likely to be adherent to treatment (risk ratio: 0.35; P<.003).10 The patient health questionnaire (PHQ) 9 and generalized anxiety disorder scale (GAD) 7 are validated and reliable questionnaires. The first 2 questions in PHQ-9 and GAD-7 screen for depression and anxiety, respectively.12-14 These 2-question screens are practical in a fast-paced dermatology outpatient setting. Systematic questionnaires specifically targeting mood disorders may be more beneficial than the widely used DLQI, which may not adequately capture mood disorders. Over the course of 10 months, 607 patients with psoriasis were asked to fill out the PHQ-9, GAD-7, and DLQI. Thirty-eight percent of patients with major depressive disorder had a DLQI score lower than 10, while 46% of patients with generalized anxiety disorder had a DLQI score lower than 10.15 Other questionnaires, including the hospital anxiety and depression scale and Beck depression inventory, are valid instruments with high sensitivity but are commonly used for research purposes and may not be clinically feasible.16
Management
Dermatologists should refer patients with depression and/or anxiety to psychiatry. Interventions include pharmacologic and nonpharmacologic management. First-line therapy for depression and anxiety is a combination of selective serotonin reuptake inhibitors and cognitive behavioral therapy.17 In addition, providers can direct patients to online resources such as the NPF website, where patients with psoriasis can access information about the signs and symptoms of mood disorders and contact the patient navigation center for further help.18
Social Functioning
Occupational Prevalence
The NPF found that 92% of patients with psoriasis or psoriatic arthritis (PsA) surveyed between 2003 and 2011 cited their psoriasis as reason for unemployment.2 In a survey of 43 patients asked about social and occupational functioning using the social and occupational assessment scale, 62.5% of psoriatic patients reported distress at work and 51.1% reported decreased efficiency at work.19 A national online survey that was conducted in France and issued to patients with and without psoriasis assessed overall QOL and work productivity using the work productivity and activity impairment questionnaire for psoriasis (WPAI-PSO). Of 714 patients with psoriasis and PsA, the latter had a 57.6% decrease in work productivity over 7 days compared to 27.9% in controls (P<.05).20 Occupational impairment leads to lost wages and hinders advancement, further exacerbating the psychosocial burden of psoriasis.21
Occupational Assessment
Formal assessment of occupational function can be done with the WPAI-PSO, a 6-question valid instrument.22 Providers may look for risk factors associated with greater loss in work productivity to help identify and offer support for patients. Patients with increased severity of itching, pain, and scaling experienced a greater decrease in work productivity.21,23 Patients with PsA warrant early detection and treatment because they experience greater physical restraints that can interfere with work activities. Of the 459 psoriatic patients without a prior diagnosis of PsA who filled out the PsA screening and evaluation questionnaire, 144 (31.4%) received a score of 44 or higher and were referred to rheumatology for further evaluation with the classification criteria for PsA. Nine percent of patients failed to be screened and remained undiagnosed with PsA.24 In a study using the health assessment questionnaire to assess 400 patients with PsA, those with worse physical function due to joint pain and stiffness were less likely to remain employed (OR: 0.56; P=.02).25
Occupational Management
Identifying and coordinating symptoms of PsA between dermatology and rheumatology is beneficial for patients who experience debilitating symptoms. There are a variety of treatments available for PsA. According to the European League Against Rheumatism 2015 guidelines developed from expert opinion and systematic reviews for PsA management, there are 4 phases of treatment, with reassessment every 3 to 6 months for effectiveness of therapy.26,27 Phase I involves initiating nonsteroidal anti-inflammatory drugs with or without glucocorticoid injections. Phase II involves synthetic disease-modifying drugs, including methotrexate, leflunomide, sulfasalazine, or cyclosporine. Phase III involves adding a second synthetic disease-modifying drug or starting a biologic, such as an anti–tumor necrosis factor, IL-12/IL-23, or IL-17 inhibitor. Phase IV involves switching to a different drug in either aforementioned class.26,27 Treatment with biologics improves work productivity as assessed by WPAI-PSO for psoriasis and PsA.28-30 Encouraging patients to speak up in the workplace and request small accommodations such as timely breaks or ergonomic chairs can help patients feel more comfortable and supported in the work environment.18 Patients who felt supported at work were more likely to remain employed.25
Interpersonal Relationships Prevalence
Misinformation about psoriasis, fear of rejection, and feelings of isolation may contribute to interpersonal conflict. Patients have feelings of shame and self-consciousness that hinder them from engaging in social activities and seeking out relationships.31 Twenty-nine percent of patients feel that psoriasis has interfered with establishing relationships because of negative self-esteem associated with the disease,32 and 26.3% have experienced people avoiding physical contact.33 Family and spouses of patients with psoriasis may be secondarily affected due to economic and emotional distress. Ninety-eight percent of family members of psoriatic patients experienced emotional distress and 54% experienced the burden of care.34 In a survey of 63 relatives and partners of patients with psoriasis, 57% experienced psychological distress, including anxiety and worry over a psoriatic patient’s future.35
Interpersonal Relationships Assessment
Current available tools, including the DLQI and short form health survey, measure overall QOL, including social functioning, but may not be practical in a clinic setting. Although no quick-screening test to assess for this domain exists, providers are encouraged to ask patients about disease impact on interpersonal relationships. The family DLQI questionnaire, adapted from the DLQI, may help physicians and social workers evaluate the burden on a patient’s family members.34
Interpersonal Relationships Management
It may be difficult for providers to address problems with interpersonal relationships without accessible tools. Patients may not be accompanied by family or friends during appointments, and it is difficult to screen for these issues during visits. Providers may offer resources such as the NPF website, which provides information about support groups. It also provides tips on dating and connecting to others in the community who share similar experiences.18 Encouraging patients to seek family or couples therapy also may be beneficial. Increased social support can lead to better QOL and fewer depressive symptoms.36
Sexual Functioning Prevalence
Psoriasis affects both physical and psychological components of sexual function. Among 3485 patients with skin conditions who were surveyed about sexual function, 34% of psoriatic patients reported that psoriasis interfered with sexual functioning at least to a certain degree.37 Sexual impairment was strongly associated with depression, anxiety, and suicidal ideation; 24% of depressed patients and 20% of anxious patients experienced sexual problems a lot or very much, based on the DLQI.37 Depending on the questionnaire used, the prevalence of sexual dysfunction due to psoriasis ranged from 35.5% to 71.3%.38 In an observational cohort study of 158 participants (n=79 psoriasis patients and n=79 controls), 34.2% of patients with psoriasis experienced erectile dysfunction compared to 17.7% of controls.39 Forty-two percent of psoriatic patients with genital involvement reported dyspareunia, 32% reported worsening of genital psoriasis after intercourse, and 43% reported decreased frequency of intercourse.40
Sexual Functioning Assessment
The Skindex-29, DLQI, and psoriasis disability index are available QOL tools that include one question evaluating difficulties with sexual function. The
Sexual Functioning Management
Better disease control leads to improved sexual function, as patients experience fewer feelings of shame, anxiety, and depression, as well as improvement of physical symptoms that can interfere with sexual functioning.38,43,44 Reducing friction, warmth, and moisture, as well as avoiding tight clothing, can help those with genital psoriasis. Patients are advised to reapply topical medications after sexual intercourse. Patients also can apply makeup to disguise psoriasis and help reduce feelings of self-consciousness that can impede sexual intimacy.18
Comment
The psychosocial burden of psoriasis penetrates many facets of patient lives. Psoriasis can invoke feelings of shame and embarrassment that are worsened by the public’s misconceptions about psoriasis, resulting in serious mental health issues that can cause even greater disability. Depression and anxiety are prevalent in patients with psoriasis. The characteristic symptoms of pain and pruritus along with psychiatric comorbidities can have an underestimated impact on daily activities, including employment, interpersonal relationships, and sexual function. Such dysfunctions have serious implications toward wages, professional advancement, social support, and overall QOL.
Dermatology providers play an important role in screening for these problems through validated questionnaires and identifying risks. Simple screening questions such as the PHQ-9 can be beneficial and feasible during dermatology visits. Screening for PsA can help patients avoid problems at work. Sexual dysfunction is a sensitive topic; however, providers can use a 1-question screen from valid questionnaires and inquire about the location of lesions as opportunities to address this issue.
Interventions lead to better disease control, which concurrently improves overall QOL. These interventions depend on both patient adherence and a physician’s commitment to finding an optimal treatment regimen for each individual. Medical management; coordinating care; developing treatment plans with psychiatry, rheumatology, and primary care providers; and psychological counseling and services may be necessary and beneficial (Table). Offering accessible resources such as the NPF website helps patients access information outside the clinic when it is not feasible to address all these concerns in a single visit. Psoriasis requires more than just medical management; it requires dermatology providers to use a multidisciplinary approach to address the psychosocial aspects of the disease.
Conclusion
The psychosocial burden of psoriasis is immense. Stigma, public misconception, mental health concerns, and occupational and interpersonal difficulty are the basis of disease burden. Providers play a vital role in assessing the effect psoriasis has on different areas of patients’ lives and providing appropriate interventions and resources to reduce disease burden.
The psychosocial impact of psoriasis is a critical component of disease burden. Psoriatic patients have high rates of depression and anxiety, problems at work, and difficulties with interpersonal relationships and intimacy.1 A National Psoriasis Foundation (NPF) survey from 2003 to 2011 reported that psoriasis affects overall emotional well-being in 88% of patients and enjoyment of life in 82% of patients.2
The reasons for psychosocial burden stem from public misconceptions and disease stigma. A survey of 1005 individuals (age range, 16–64 years) about their perceptions of psoriasis revealed that 16.5% believed that psoriasis is contagious and 6.8% believed that psoriasis is related to personal hygiene.3 Fifty percent practiced discriminatory behavior toward psoriatic patients, including reluctance to shake hands (28.8%) and engage in sexual relations/intercourse (44.1%). Sixty-five percent of psoriatic patients felt their appearance is unsightly, and 73% felt self-conscious about having psoriasis.2
The psychosocial burden exists despite medical treatment of the disease. In a cross-sectional study of 1184 psoriatic patients, 70.2% had impaired quality of life (QOL) as measured by the dermatology life quality index (DLQI), even after receiving a 4-week treatment for psoriasis.4 Medical treatment of psoriasis is not enough; providers need to assess overall QOL and provide treatment and resources for these patients in addition to symptomatic management.
There have been many studies on the psychosocial burden of psoriasis, but few have focused on a dermatology resident’s role in addressing this issue. This article will review psychosocial domains—psychiatric comorbidities and social functioning including occupational functioning, interpersonal relationships, and sexual functioning— and discuss a dermatology resident’s role in assessing and addressing each of these areas.
Methods
A PubMed search of articles indexed for MEDLINE was conducted using the following terms: psoriasis, depression, anxiety, work productivity, sexual functioning, and interpersonal relationships. Selected articles covered prevalence, assessment, and management of each psychosocial domain.
Results
Psychiatric Comorbidities
Prevalence
A high prevalence of psychiatric comorbidities exists in psoriatic patients. In a study of 469,097 patients with psoriasis, depression was the third most prevalent comorbidity (17.91%), following hyperlipidemia (45.64%) and hypertension (42.19%).5 In a 10-year longitudinal, population-based, prospective cohort study, antidepressant prescriptions were twice as frequent in psoriatic patients (17.8%) compared to control (7.9%)(P<.001).6 In a meta-analysis of 98 studies investigating psoriatic patients and psychiatric comorbidities, patients with psoriasis were 1.5 times more likely to experience depression (odds ratio [OR]: 1.57; 95% CI, 1.40-1.76) and use antidepressants (OR: 4.24; 95% CI, 1.53-11.76) compared to control.7 Patients with psoriasis were more likely to attempt suicide (OR: 1.32; 95% CI, 1.14-1.54) and complete suicide (OR: 1.20; 95% CI, 1.04-1.39) compared to people without psoriasis.8 A 1-year cross-sectional study of 90 psoriatic patients reported 78.7% were diagnosed with depression and 76.7% were diagnosed with anxiety. Seventy-two percent reported both anxiety and depression, correlating with worse QOL (χ2=26.7; P<.05).9
Assessment
Psychiatric comorbidities are assessed using clinical judgment and formal screening questionnaires in research studies. Signs of depression in patients with psoriasis can manifest as poor treatment adherence and recurrent flares of psoriasis.10,11 Psoriatic patients with psychiatric comorbidities were less likely to be adherent to treatment (risk ratio: 0.35; P<.003).10 The patient health questionnaire (PHQ) 9 and generalized anxiety disorder scale (GAD) 7 are validated and reliable questionnaires. The first 2 questions in PHQ-9 and GAD-7 screen for depression and anxiety, respectively.12-14 These 2-question screens are practical in a fast-paced dermatology outpatient setting. Systematic questionnaires specifically targeting mood disorders may be more beneficial than the widely used DLQI, which may not adequately capture mood disorders. Over the course of 10 months, 607 patients with psoriasis were asked to fill out the PHQ-9, GAD-7, and DLQI. Thirty-eight percent of patients with major depressive disorder had a DLQI score lower than 10, while 46% of patients with generalized anxiety disorder had a DLQI score lower than 10.15 Other questionnaires, including the hospital anxiety and depression scale and Beck depression inventory, are valid instruments with high sensitivity but are commonly used for research purposes and may not be clinically feasible.16
Management
Dermatologists should refer patients with depression and/or anxiety to psychiatry. Interventions include pharmacologic and nonpharmacologic management. First-line therapy for depression and anxiety is a combination of selective serotonin reuptake inhibitors and cognitive behavioral therapy.17 In addition, providers can direct patients to online resources such as the NPF website, where patients with psoriasis can access information about the signs and symptoms of mood disorders and contact the patient navigation center for further help.18
Social Functioning
Occupational Prevalence
The NPF found that 92% of patients with psoriasis or psoriatic arthritis (PsA) surveyed between 2003 and 2011 cited their psoriasis as reason for unemployment.2 In a survey of 43 patients asked about social and occupational functioning using the social and occupational assessment scale, 62.5% of psoriatic patients reported distress at work and 51.1% reported decreased efficiency at work.19 A national online survey that was conducted in France and issued to patients with and without psoriasis assessed overall QOL and work productivity using the work productivity and activity impairment questionnaire for psoriasis (WPAI-PSO). Of 714 patients with psoriasis and PsA, the latter had a 57.6% decrease in work productivity over 7 days compared to 27.9% in controls (P<.05).20 Occupational impairment leads to lost wages and hinders advancement, further exacerbating the psychosocial burden of psoriasis.21
Occupational Assessment
Formal assessment of occupational function can be done with the WPAI-PSO, a 6-question valid instrument.22 Providers may look for risk factors associated with greater loss in work productivity to help identify and offer support for patients. Patients with increased severity of itching, pain, and scaling experienced a greater decrease in work productivity.21,23 Patients with PsA warrant early detection and treatment because they experience greater physical restraints that can interfere with work activities. Of the 459 psoriatic patients without a prior diagnosis of PsA who filled out the PsA screening and evaluation questionnaire, 144 (31.4%) received a score of 44 or higher and were referred to rheumatology for further evaluation with the classification criteria for PsA. Nine percent of patients failed to be screened and remained undiagnosed with PsA.24 In a study using the health assessment questionnaire to assess 400 patients with PsA, those with worse physical function due to joint pain and stiffness were less likely to remain employed (OR: 0.56; P=.02).25
Occupational Management
Identifying and coordinating symptoms of PsA between dermatology and rheumatology is beneficial for patients who experience debilitating symptoms. There are a variety of treatments available for PsA. According to the European League Against Rheumatism 2015 guidelines developed from expert opinion and systematic reviews for PsA management, there are 4 phases of treatment, with reassessment every 3 to 6 months for effectiveness of therapy.26,27 Phase I involves initiating nonsteroidal anti-inflammatory drugs with or without glucocorticoid injections. Phase II involves synthetic disease-modifying drugs, including methotrexate, leflunomide, sulfasalazine, or cyclosporine. Phase III involves adding a second synthetic disease-modifying drug or starting a biologic, such as an anti–tumor necrosis factor, IL-12/IL-23, or IL-17 inhibitor. Phase IV involves switching to a different drug in either aforementioned class.26,27 Treatment with biologics improves work productivity as assessed by WPAI-PSO for psoriasis and PsA.28-30 Encouraging patients to speak up in the workplace and request small accommodations such as timely breaks or ergonomic chairs can help patients feel more comfortable and supported in the work environment.18 Patients who felt supported at work were more likely to remain employed.25
Interpersonal Relationships Prevalence
Misinformation about psoriasis, fear of rejection, and feelings of isolation may contribute to interpersonal conflict. Patients have feelings of shame and self-consciousness that hinder them from engaging in social activities and seeking out relationships.31 Twenty-nine percent of patients feel that psoriasis has interfered with establishing relationships because of negative self-esteem associated with the disease,32 and 26.3% have experienced people avoiding physical contact.33 Family and spouses of patients with psoriasis may be secondarily affected due to economic and emotional distress. Ninety-eight percent of family members of psoriatic patients experienced emotional distress and 54% experienced the burden of care.34 In a survey of 63 relatives and partners of patients with psoriasis, 57% experienced psychological distress, including anxiety and worry over a psoriatic patient’s future.35
Interpersonal Relationships Assessment
Current available tools, including the DLQI and short form health survey, measure overall QOL, including social functioning, but may not be practical in a clinic setting. Although no quick-screening test to assess for this domain exists, providers are encouraged to ask patients about disease impact on interpersonal relationships. The family DLQI questionnaire, adapted from the DLQI, may help physicians and social workers evaluate the burden on a patient’s family members.34
Interpersonal Relationships Management
It may be difficult for providers to address problems with interpersonal relationships without accessible tools. Patients may not be accompanied by family or friends during appointments, and it is difficult to screen for these issues during visits. Providers may offer resources such as the NPF website, which provides information about support groups. It also provides tips on dating and connecting to others in the community who share similar experiences.18 Encouraging patients to seek family or couples therapy also may be beneficial. Increased social support can lead to better QOL and fewer depressive symptoms.36
Sexual Functioning Prevalence
Psoriasis affects both physical and psychological components of sexual function. Among 3485 patients with skin conditions who were surveyed about sexual function, 34% of psoriatic patients reported that psoriasis interfered with sexual functioning at least to a certain degree.37 Sexual impairment was strongly associated with depression, anxiety, and suicidal ideation; 24% of depressed patients and 20% of anxious patients experienced sexual problems a lot or very much, based on the DLQI.37 Depending on the questionnaire used, the prevalence of sexual dysfunction due to psoriasis ranged from 35.5% to 71.3%.38 In an observational cohort study of 158 participants (n=79 psoriasis patients and n=79 controls), 34.2% of patients with psoriasis experienced erectile dysfunction compared to 17.7% of controls.39 Forty-two percent of psoriatic patients with genital involvement reported dyspareunia, 32% reported worsening of genital psoriasis after intercourse, and 43% reported decreased frequency of intercourse.40
Sexual Functioning Assessment
The Skindex-29, DLQI, and psoriasis disability index are available QOL tools that include one question evaluating difficulties with sexual function. The
Sexual Functioning Management
Better disease control leads to improved sexual function, as patients experience fewer feelings of shame, anxiety, and depression, as well as improvement of physical symptoms that can interfere with sexual functioning.38,43,44 Reducing friction, warmth, and moisture, as well as avoiding tight clothing, can help those with genital psoriasis. Patients are advised to reapply topical medications after sexual intercourse. Patients also can apply makeup to disguise psoriasis and help reduce feelings of self-consciousness that can impede sexual intimacy.18
Comment
The psychosocial burden of psoriasis penetrates many facets of patient lives. Psoriasis can invoke feelings of shame and embarrassment that are worsened by the public’s misconceptions about psoriasis, resulting in serious mental health issues that can cause even greater disability. Depression and anxiety are prevalent in patients with psoriasis. The characteristic symptoms of pain and pruritus along with psychiatric comorbidities can have an underestimated impact on daily activities, including employment, interpersonal relationships, and sexual function. Such dysfunctions have serious implications toward wages, professional advancement, social support, and overall QOL.
Dermatology providers play an important role in screening for these problems through validated questionnaires and identifying risks. Simple screening questions such as the PHQ-9 can be beneficial and feasible during dermatology visits. Screening for PsA can help patients avoid problems at work. Sexual dysfunction is a sensitive topic; however, providers can use a 1-question screen from valid questionnaires and inquire about the location of lesions as opportunities to address this issue.
Interventions lead to better disease control, which concurrently improves overall QOL. These interventions depend on both patient adherence and a physician’s commitment to finding an optimal treatment regimen for each individual. Medical management; coordinating care; developing treatment plans with psychiatry, rheumatology, and primary care providers; and psychological counseling and services may be necessary and beneficial (Table). Offering accessible resources such as the NPF website helps patients access information outside the clinic when it is not feasible to address all these concerns in a single visit. Psoriasis requires more than just medical management; it requires dermatology providers to use a multidisciplinary approach to address the psychosocial aspects of the disease.
Conclusion
The psychosocial burden of psoriasis is immense. Stigma, public misconception, mental health concerns, and occupational and interpersonal difficulty are the basis of disease burden. Providers play a vital role in assessing the effect psoriasis has on different areas of patients’ lives and providing appropriate interventions and resources to reduce disease burden.
- Kimball AB, Jacobson C, Weiss S, et al. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6:383-392.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
- Wolf P, Weger W, Legat F, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges. 2018;16:981-990.
- Shah K, Mellars L, Changolkar A, et al. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.e4.
- Dowlatshahi EA, Wakkee M, Herings RM, et al. Increased antidepressant drug exposure in psoriasis patients: a longitudinal population-based cohort study. Acta Derm Venereol. 2013;93:544-550.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425.e2-440.e2.
- Lakshmy S, Balasundaram S, Sarkar S, et al. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J Psychol Med. 2015;37:434-440.
- Renzi C, Picardi A, Abeni D, et al. Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol. 2002;138:337-342.
- Kulkarni AS, Balkrishnan R, Camacho FT, et al. Medication and health care service utilization related to depressive symptoms in older adults with psoriasis. J Drugs Dermatol. 2004;3:661-666.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284-1292.
- Lamb RC, Matcham F, Turner MA, et al. Screening for anxiety and depression in people with psoriasis: a cross-sectional study in a tertiary referral setting. Br J Dermatol. 2017;176:1028-1034.
- Law M, Naughton MT, Dhar A, et al. Validation of two depression screening instruments in a sleep disorders clinic. J Clin Sleep Med. 2014;10:683-688.
- Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70:1219-1229.
- National Psoriasis Foundation. Living with psoriatic arthritis. https://www.psoriasis.org/life-with-psoriatic-arthritis. Accessed September 23, 2018.
- Gaikwad R, Deshpande S, Raje S, et al. Evaluation of functional impairment in psoriasis. Indian J Dermatol Venereol Leprol. 2006;72:37-40.
- Claudepierre P, Lahfa M, Levy P, et al. The impact of psoriasis on professional life: PsoPRO, a French national survey [published online April 6, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14986.
- Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41:514-521.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353-365.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21.
- Spelman L, Su JC, Fernandez-Penas P, et al. Frequency of undiagnosed psoriatic arthritis among psoriasis patients in Australian dermatology practice. J Eur Acad Dermatol Venereol. 2015;29:2184-2191.
- Tillett W, Shaddick G, Askari A, et al. Factors influencing work disability in psoriatic arthritis: first results from a large UK multicentre study. Rheumatology (Oxford). 2015;54:157-162.
- Raychaudhuri SP, Wilken R, Sukhov AC, et al. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21-37.
- Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the manegement of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499-510.
- Beroukhim K, Danesh M, Nguyen C, et al. A prospective, interventional assessment of the impact of ustekinumab treatment on psoriasis-related work productivity and activity impairment. J Dermatol Treat. 2016;27:552-555.
- Armstrong AW, Lynde CW, McBride SR, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol. 2016;152:661-669.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;66:e67-76.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20.
- Reich A, Welz-Kubiak K, Rams Ł. Apprehension of the disease by patients suffering from psoriasis. Postepy Dermatol Alergol. 2014;31:289-293.
- Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experience in dermatologic disorders. Cutis. 1998;61:339-342.
- Basra MK, Finlay AY. The family impact of skin diseases: the Greater Patient concept. Br J Dermatol. 2007;156:929-937.
- Eghlileb AM, Davies EE, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156:1245-1250.
- Janowski K, Steuden S, Pietrzak A, et al. Social support and adaptation to the disease in men and women with psoriasis. Arch Dermatol Res. 2012;304:421-432.
- Sampogna F, Abeni D, Gieler U, et al. Impairment of sexual life in 3,485 dermatological outpatients from a multicentre study in 13 European countries. Acta Derm Venereol. 2017;97:478-482.
- Sampogna F, Gisondi P, Tabolli S, et al. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214:144-150.
- Molina-Leyva A, Molina-Leyva I, Almodovar-Real A, et al. Prevalence and associated factors of erectile dysfunction in patients with moderate to severe psoriasis and healthy population: a comparative study considering physical and psychological factors. Arch Sex Behav. 2016;45:2047-2055.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom. 2001;70:221-225.
- Molina-Leyva A, Almodovar-Real A, Ruiz-Carrascosa JC, et al. Distribution pattern of psoriasis affects sexual function in moderate to severe psoriasis: a prospective case series study. J Sex Med. 2014;11:2882-2889.
- Guenther L, Han C, Szapary P, et al. Impact of ustekinumab on health-related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. J Eur Acad Dermatol Venereol. 2011;25:851-857.
- Guenther L, Warren RB, Cather JC, et al. Impact of ixekizumab treatment on skin-related personal relationship difficulties in moderate-to-severe psoriasis patients: 12-week results from two Phase 3 trials. J Eur Acad Dermatol Venereol. 2017;31:1867-1875.
- Kimball AB, Jacobson C, Weiss S, et al. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6:383-392.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
- Wolf P, Weger W, Legat F, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges. 2018;16:981-990.
- Shah K, Mellars L, Changolkar A, et al. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.e4.
- Dowlatshahi EA, Wakkee M, Herings RM, et al. Increased antidepressant drug exposure in psoriasis patients: a longitudinal population-based cohort study. Acta Derm Venereol. 2013;93:544-550.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425.e2-440.e2.
- Lakshmy S, Balasundaram S, Sarkar S, et al. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J Psychol Med. 2015;37:434-440.
- Renzi C, Picardi A, Abeni D, et al. Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol. 2002;138:337-342.
- Kulkarni AS, Balkrishnan R, Camacho FT, et al. Medication and health care service utilization related to depressive symptoms in older adults with psoriasis. J Drugs Dermatol. 2004;3:661-666.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284-1292.
- Lamb RC, Matcham F, Turner MA, et al. Screening for anxiety and depression in people with psoriasis: a cross-sectional study in a tertiary referral setting. Br J Dermatol. 2017;176:1028-1034.
- Law M, Naughton MT, Dhar A, et al. Validation of two depression screening instruments in a sleep disorders clinic. J Clin Sleep Med. 2014;10:683-688.
- Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70:1219-1229.
- National Psoriasis Foundation. Living with psoriatic arthritis. https://www.psoriasis.org/life-with-psoriatic-arthritis. Accessed September 23, 2018.
- Gaikwad R, Deshpande S, Raje S, et al. Evaluation of functional impairment in psoriasis. Indian J Dermatol Venereol Leprol. 2006;72:37-40.
- Claudepierre P, Lahfa M, Levy P, et al. The impact of psoriasis on professional life: PsoPRO, a French national survey [published online April 6, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14986.
- Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41:514-521.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353-365.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21.
- Spelman L, Su JC, Fernandez-Penas P, et al. Frequency of undiagnosed psoriatic arthritis among psoriasis patients in Australian dermatology practice. J Eur Acad Dermatol Venereol. 2015;29:2184-2191.
- Tillett W, Shaddick G, Askari A, et al. Factors influencing work disability in psoriatic arthritis: first results from a large UK multicentre study. Rheumatology (Oxford). 2015;54:157-162.
- Raychaudhuri SP, Wilken R, Sukhov AC, et al. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21-37.
- Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the manegement of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499-510.
- Beroukhim K, Danesh M, Nguyen C, et al. A prospective, interventional assessment of the impact of ustekinumab treatment on psoriasis-related work productivity and activity impairment. J Dermatol Treat. 2016;27:552-555.
- Armstrong AW, Lynde CW, McBride SR, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol. 2016;152:661-669.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;66:e67-76.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20.
- Reich A, Welz-Kubiak K, Rams Ł. Apprehension of the disease by patients suffering from psoriasis. Postepy Dermatol Alergol. 2014;31:289-293.
- Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experience in dermatologic disorders. Cutis. 1998;61:339-342.
- Basra MK, Finlay AY. The family impact of skin diseases: the Greater Patient concept. Br J Dermatol. 2007;156:929-937.
- Eghlileb AM, Davies EE, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156:1245-1250.
- Janowski K, Steuden S, Pietrzak A, et al. Social support and adaptation to the disease in men and women with psoriasis. Arch Dermatol Res. 2012;304:421-432.
- Sampogna F, Abeni D, Gieler U, et al. Impairment of sexual life in 3,485 dermatological outpatients from a multicentre study in 13 European countries. Acta Derm Venereol. 2017;97:478-482.
- Sampogna F, Gisondi P, Tabolli S, et al. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214:144-150.
- Molina-Leyva A, Molina-Leyva I, Almodovar-Real A, et al. Prevalence and associated factors of erectile dysfunction in patients with moderate to severe psoriasis and healthy population: a comparative study considering physical and psychological factors. Arch Sex Behav. 2016;45:2047-2055.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom. 2001;70:221-225.
- Molina-Leyva A, Almodovar-Real A, Ruiz-Carrascosa JC, et al. Distribution pattern of psoriasis affects sexual function in moderate to severe psoriasis: a prospective case series study. J Sex Med. 2014;11:2882-2889.
- Guenther L, Han C, Szapary P, et al. Impact of ustekinumab on health-related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. J Eur Acad Dermatol Venereol. 2011;25:851-857.
- Guenther L, Warren RB, Cather JC, et al. Impact of ixekizumab treatment on skin-related personal relationship difficulties in moderate-to-severe psoriasis patients: 12-week results from two Phase 3 trials. J Eur Acad Dermatol Venereol. 2017;31:1867-1875.
Practice Points
- The psychosocial impact of psoriasis is an important component of the disease burden leading to reduced quality of life.
- Assessment of psychosocial dysfunction can be done through short questionnaires, asking patients directly about these issues and anticipating these problems in patients who are most vulnerable.
- Management of psychosocial impact ranges from pharmacological interventions to helpful resources such as the National Psoriasis Foundation website.
Topical Corticosteroid Tachyphylaxis: Why Don’t Patients Adhere to Treatment?
Topical Corticosteroids for Treatment-Resistant Atopic Dermatitis
Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
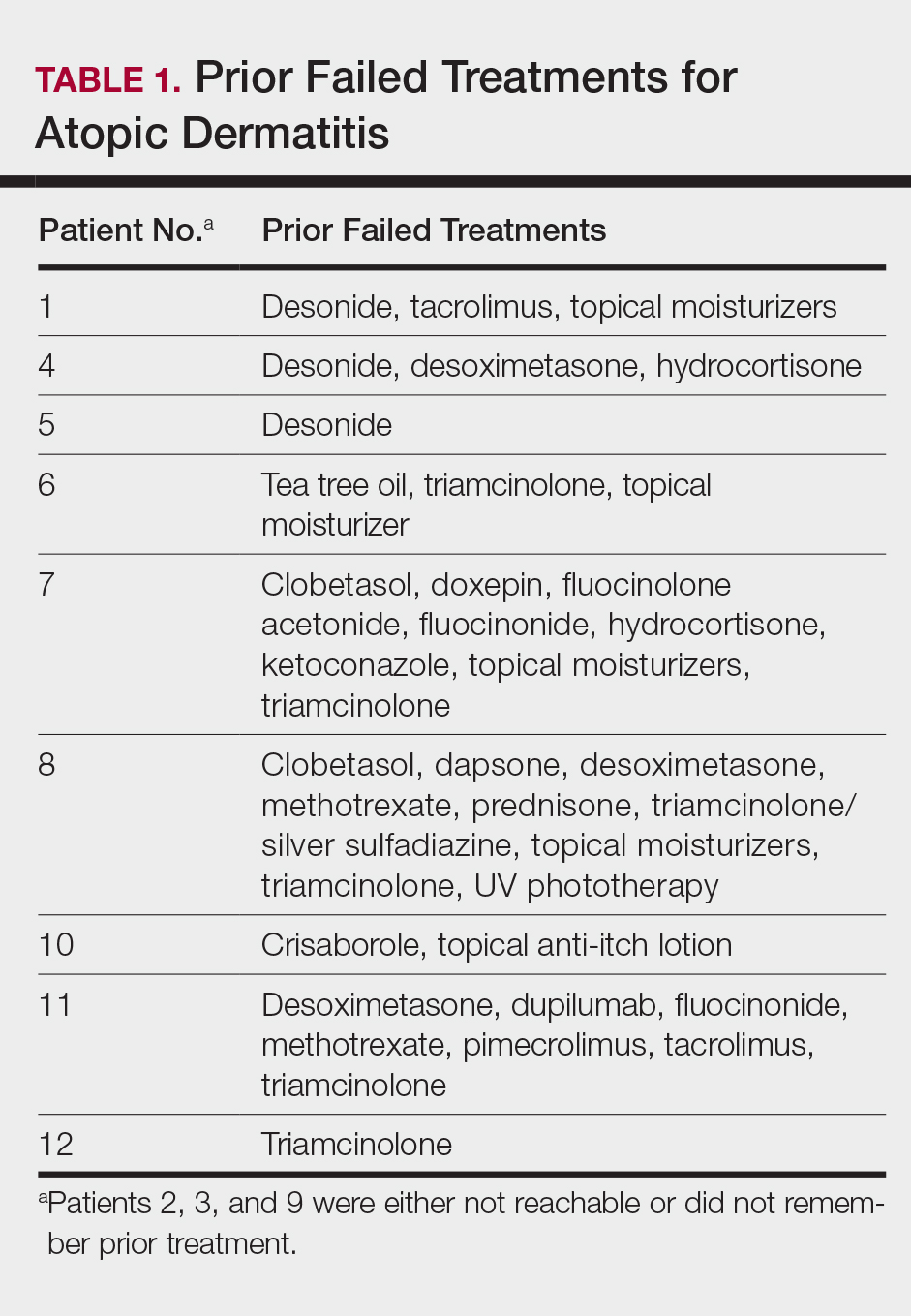
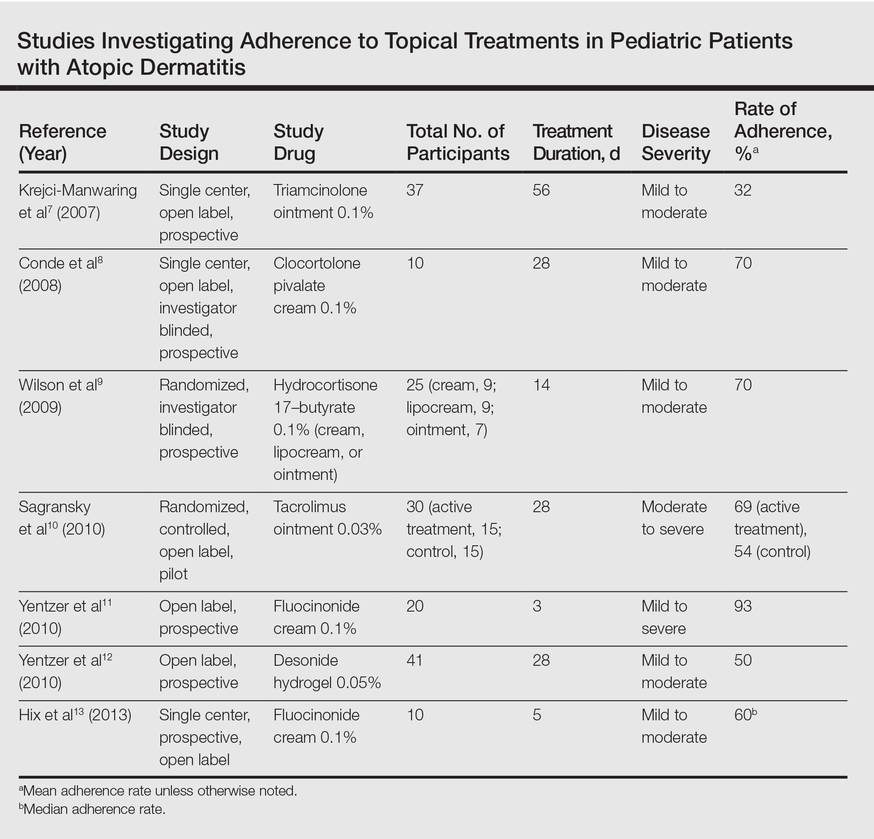
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.
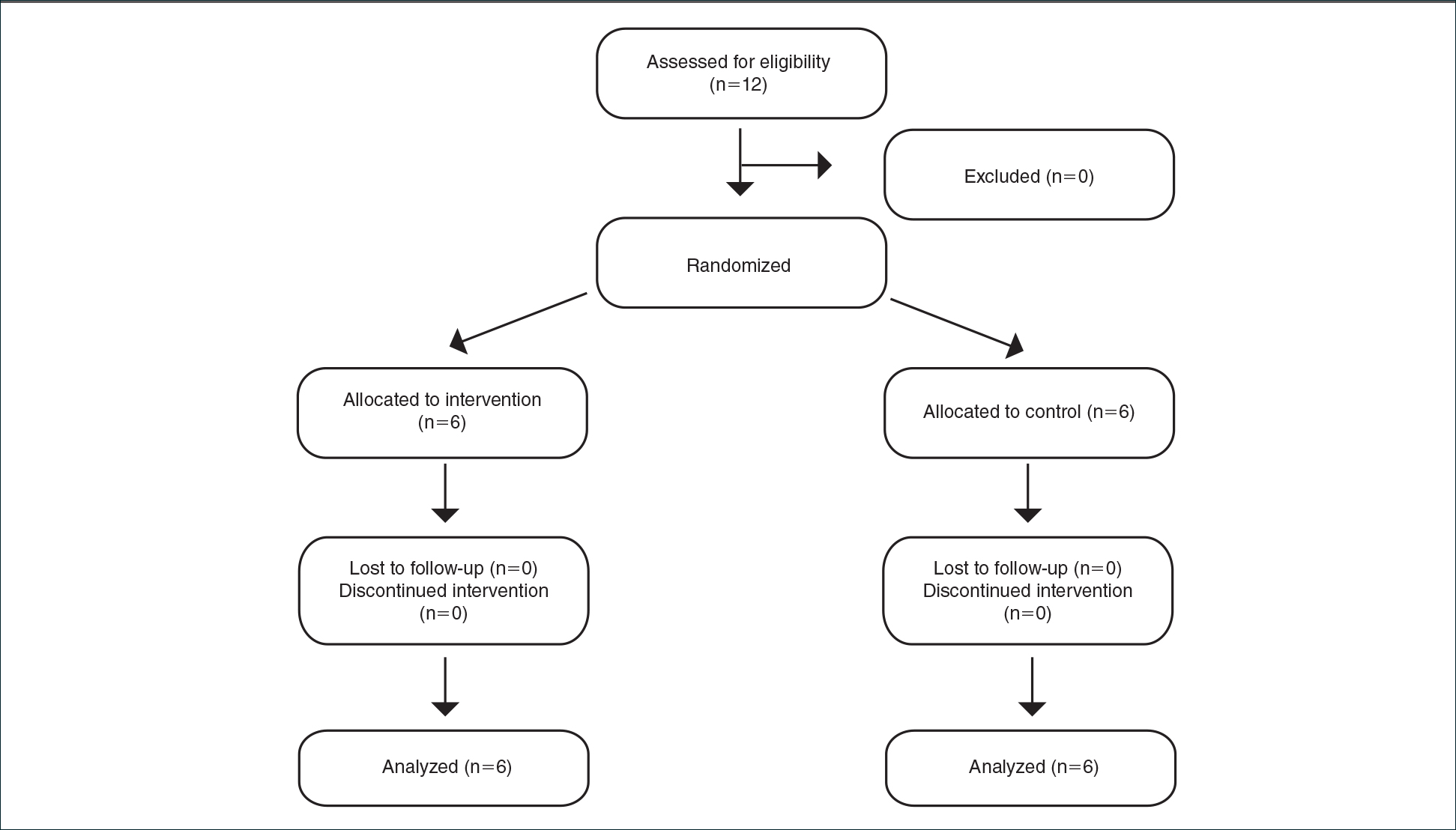
Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.
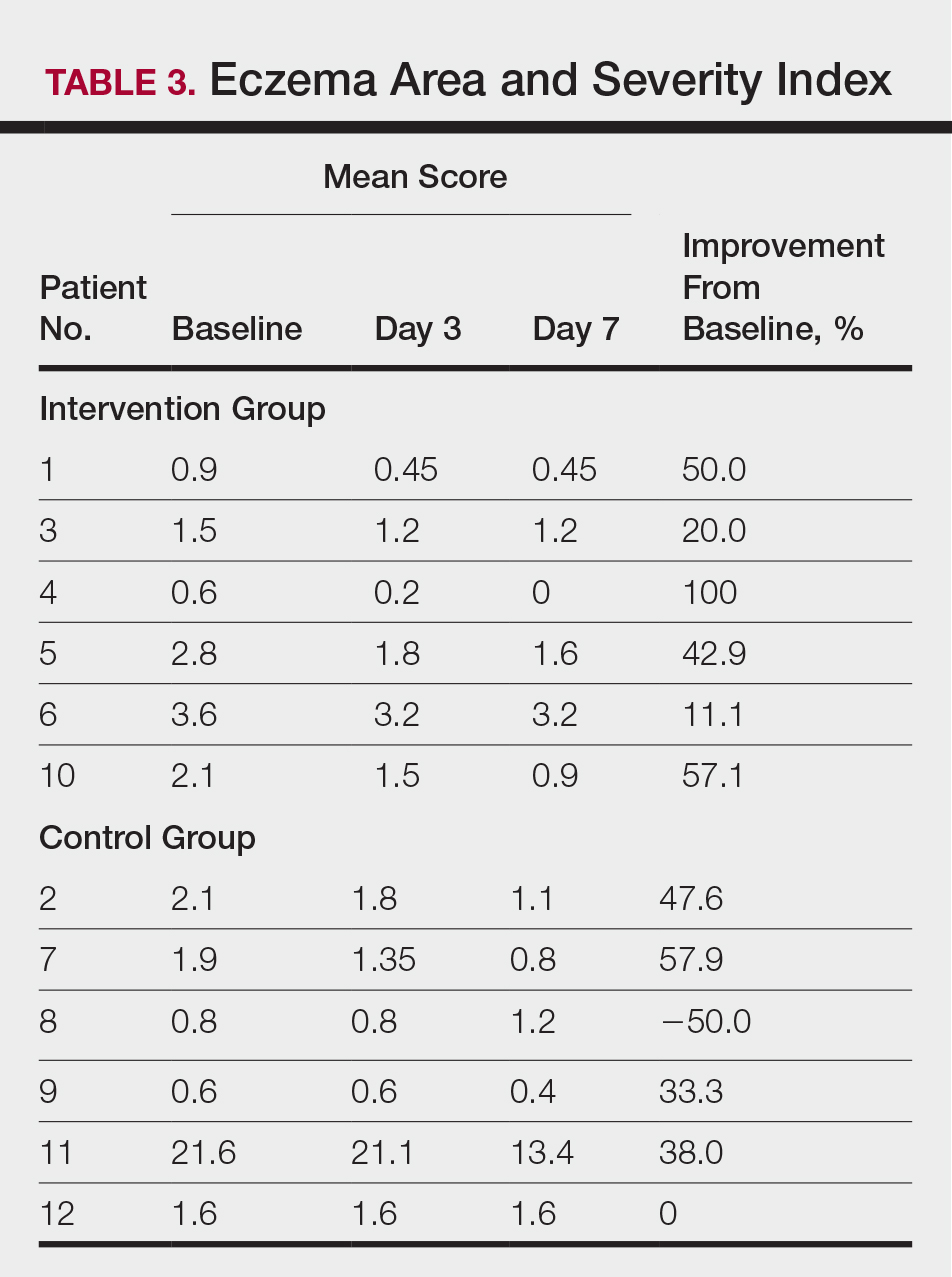
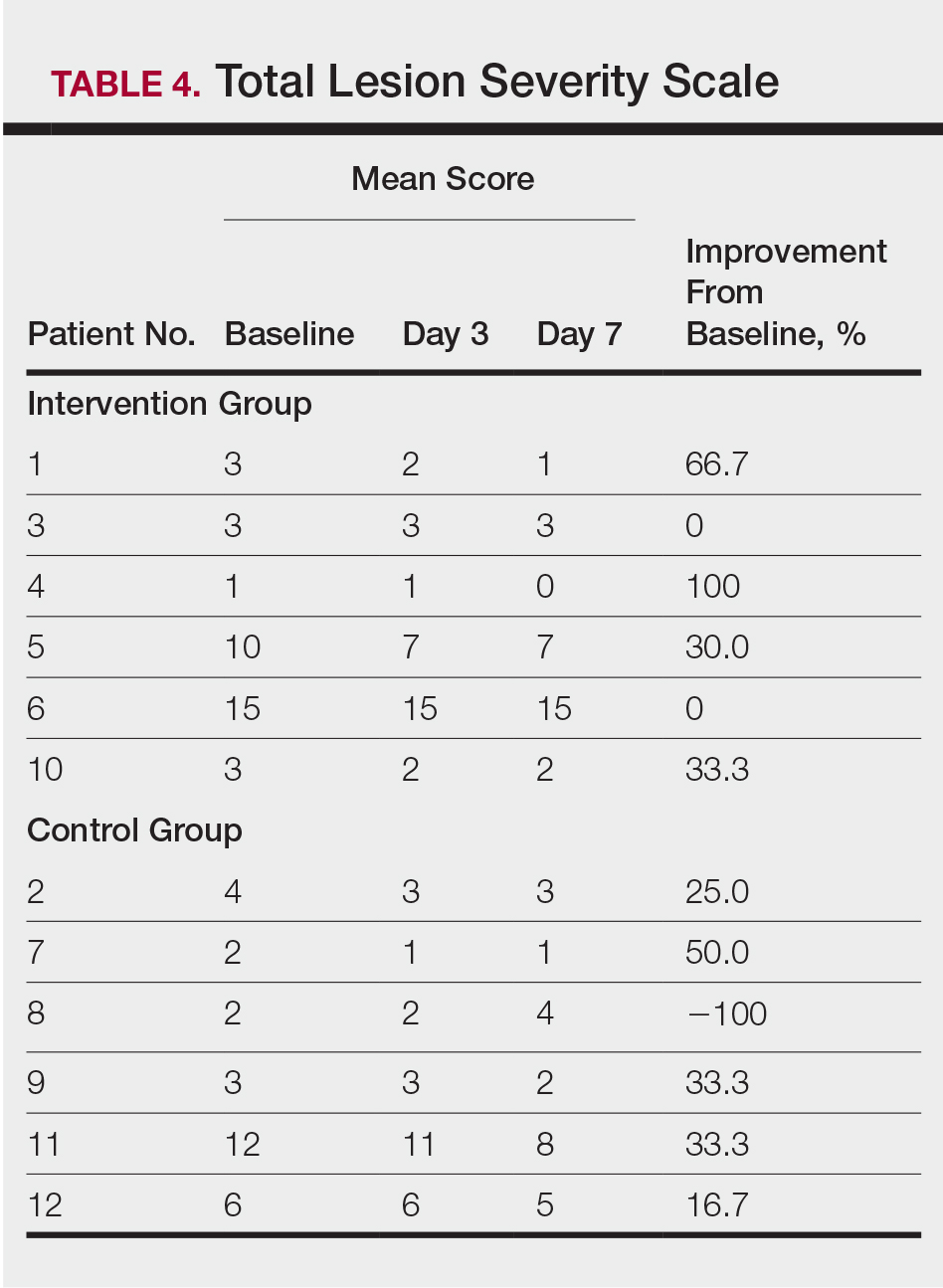
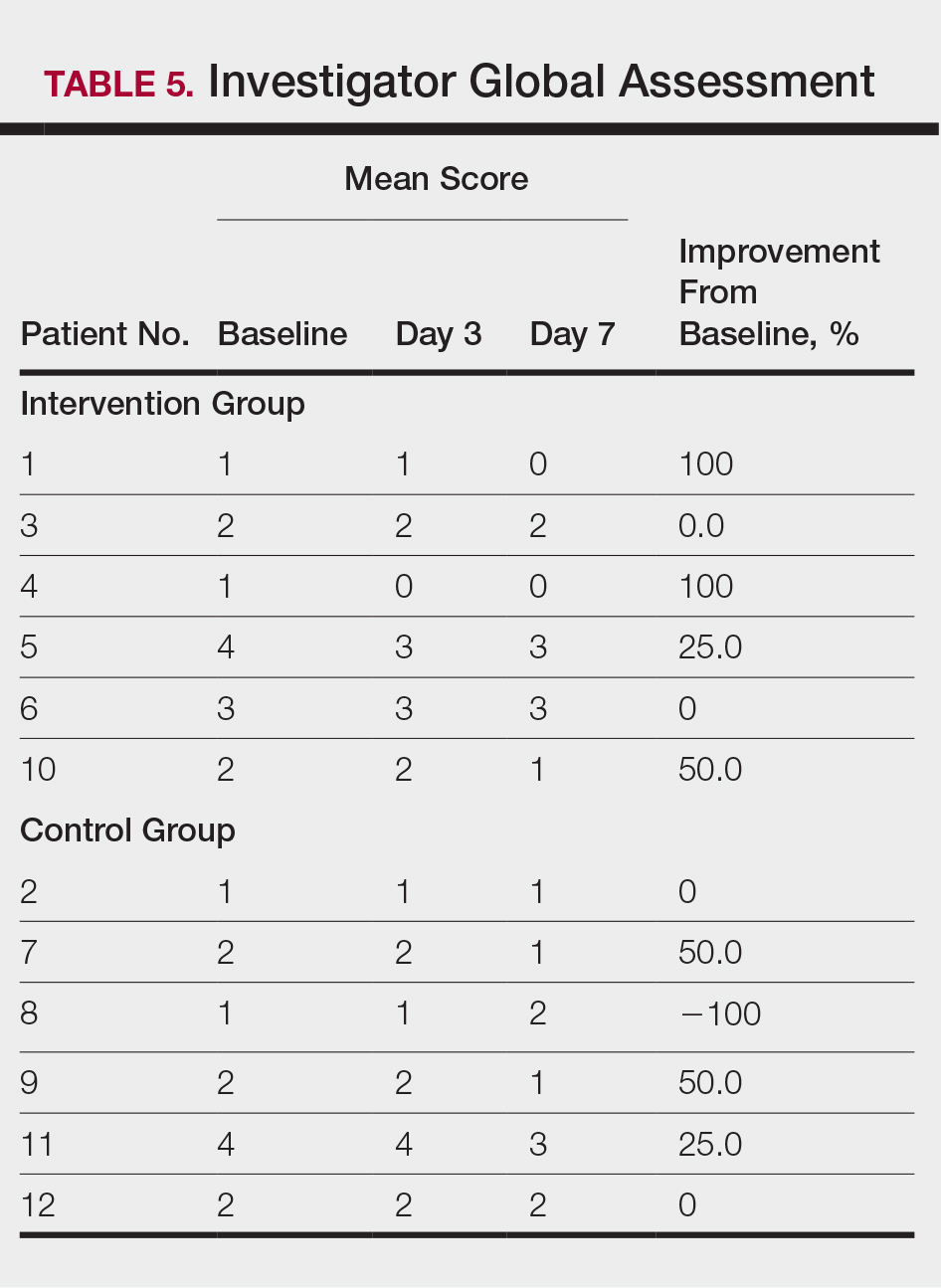
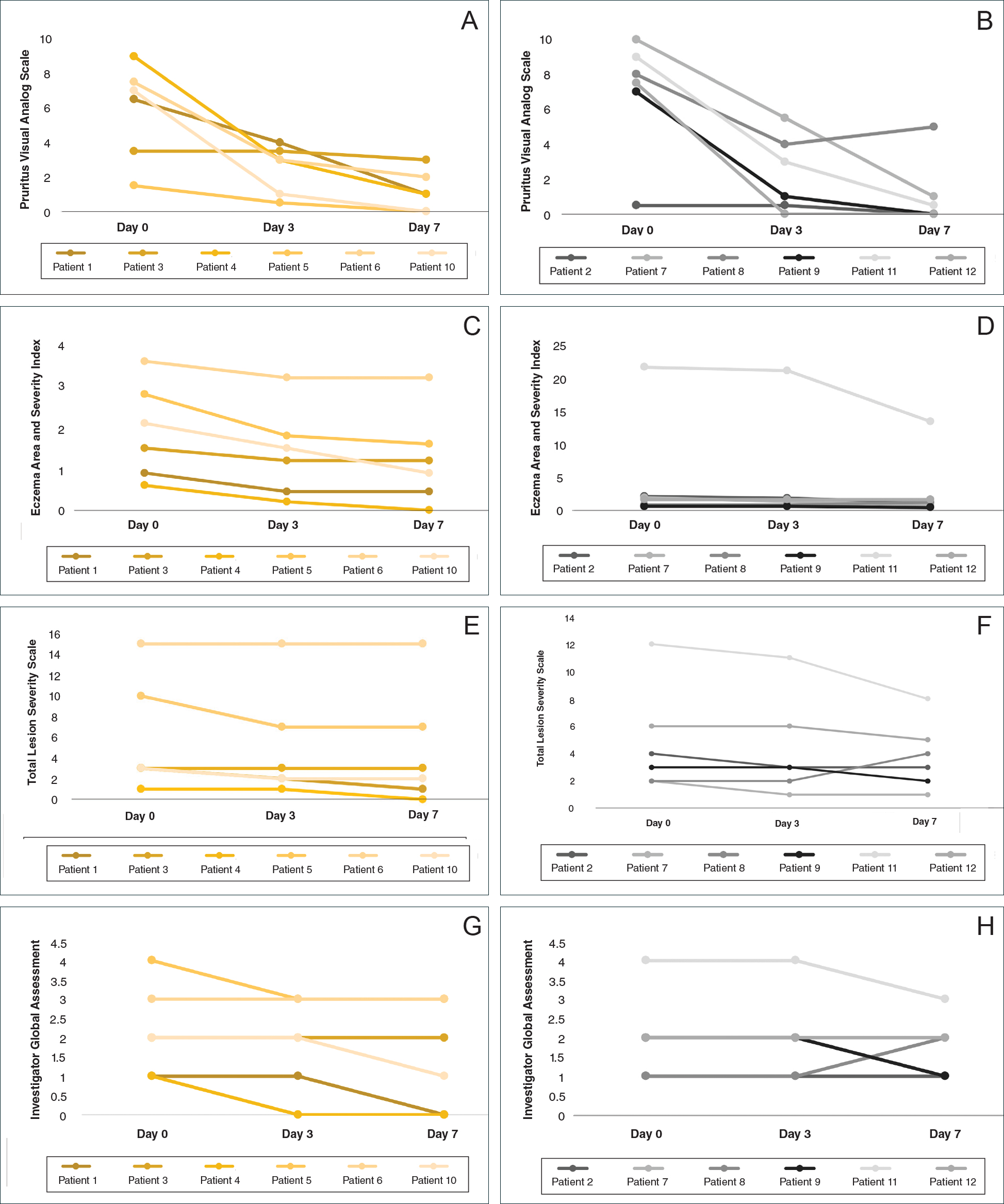
All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
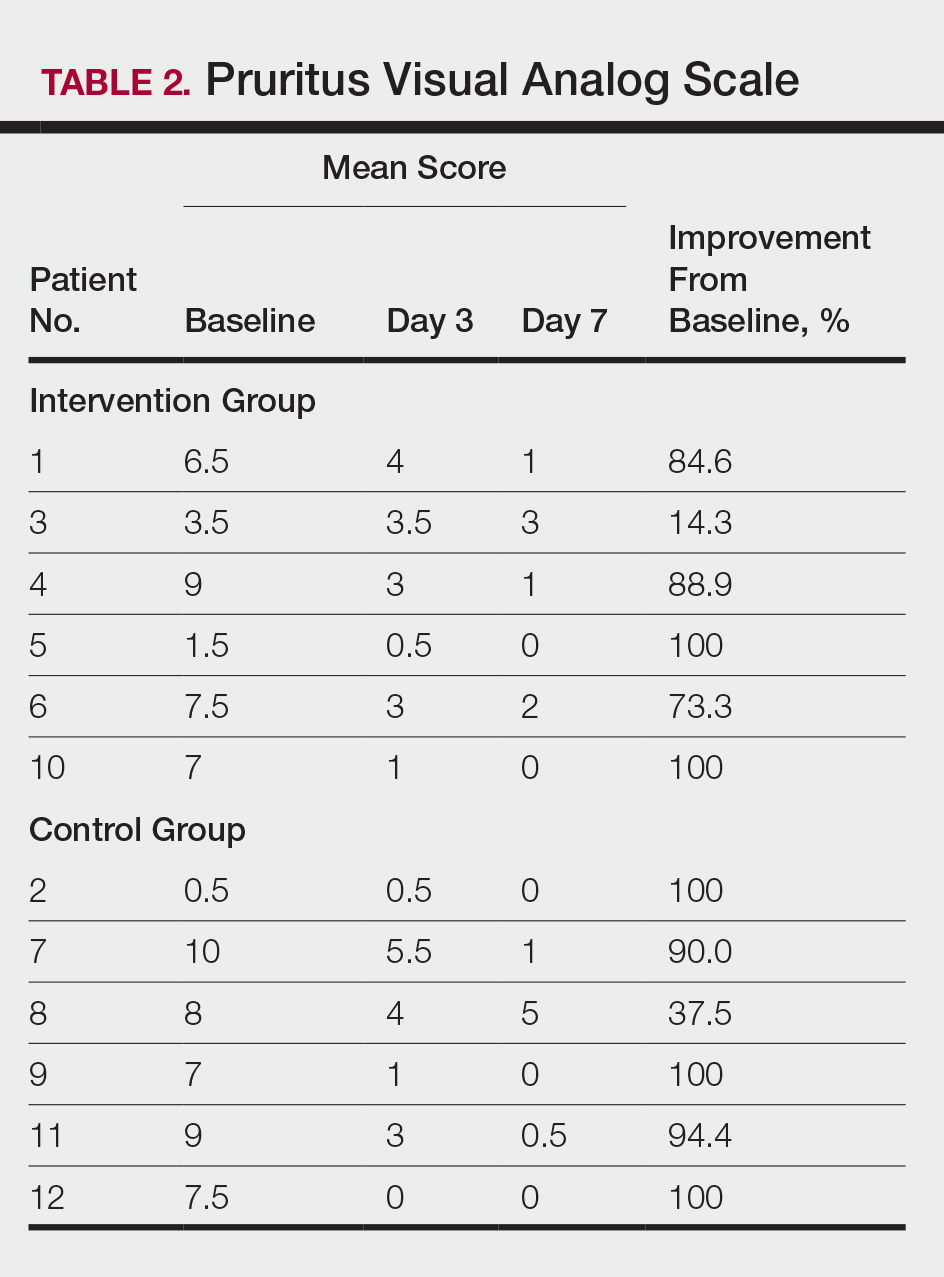
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.
Comment
Although topical corticosteroids are the mainstay for treatment of AD, many patients report treatment resistance after a period of a few doses or longer.6-9 There is strong evidence demonstrating rapid corticosteroid receptor downregulation in tissues after corticosteroid therapy, which is the accepted mechanism for tachyphylaxis, but the timing of this effect does not match up with clinical experiences. The physiologic significance of corticosteroid agonist-induced receptor downregulation is unknown and may not have any considerable effect on corticosteroid efficacy.3 A systematic review by Taheri et al3 on the development of resistance to topical corticosteroids proposed 2 theories for the underlying pathogenesis of tachyphylaxis: (1) long-term patient nonadherence, and (2) the initial maximal response during the first few weeks of therapy eventually plateaus. Because corticosteroids may plateau after a certain number of doses, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis.10 The treatment “resistance” reported by the patients in our study may have been due to this plateau effect or to poor adherence.
Our finding that nearly all patients had rapid improvement of AD with the topical corticosteroid is not definitive proof but supports the notion that tachyphylaxis is largely mediated by poor adherence to treatment. Patients rapidly improved over the short study period. The short duration of treatment and multiple visits over the study period were designed to help ensure patient adherence. Rapid improvement in AD when topical corticosteroids are used should be expected, as AD patients have rapid improvement with application of topical corticosteroids in inpatient settings.11,12
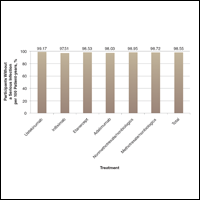
Poor adherence to topical medication is common. In a Danish study, 99 of 322 patients (31%) did not redeem their AD prescriptions.13 In a single-center, 5-day, prospective study evaluating the use of fluocinonide cream 0.1% for treatment of children and adults with AD, the median percentage of prescribed doses taken was 40%, according to objective electronic monitors, even though patients reported 100% adherence in their medication diaries.Better adherence was seen on day 1 of treatment in which 66.6% (6/9) of patients adhered to their treatment strategy versus day 5 in which only 11.1% (1/9) of patients used their medication.1
Topical corticosteroids are safe and efficacious if used appropriately; however, patients commonly express fear and anxiety about using them. Topical corticosteroid phobia may stem from a misconception that these products carry the same adverse effects as their oral and systemic counterparts, which may be perpetuated by the media.1 Of 200 dermatology patients surveyed, 72.5% expressed concern about using topical corticosteroids on themselves or their children’s skin, and 24% of these patients stated they were noncompliant with their medication because of these worries. Almost 50% of patients requested prescriptions for corticosteroid-sparing medications such as tacrolimus.1 Patient education is important to help ensure treatment adherence. Other factors that can affect treatment adherence include forgetfulness; the chronic nature of AD; the need for ongoing application of topical treatments; prohibitive costs of some topical agents; and complexities in coordinating school, work, and family plans with the treatment regimen.2
We attempted to ensure good treatment adherence in our study by calling the patients in the intervention group twice daily. The mean improvement in EASI, TLSS, and IGA scores was higher in the intervention group versus the control group, which suggests that patient reminders have at least some benefit. Because AD treatment resistance appears more closely tied to nonadherence rather than loss of medication efficacy, it seems prudent to focus on interventions that would improve treatment adherence; however, such interventions generally are not well tested. Recommended interventions have included educating patients about the side effects of topical corticosteroids, avoiding use of medical jargon, and taking patient vehicle preference into account when prescribing treatments.8 Patients should be scheduled for a return visit within 1 to 2 weeks, as early return visits can augment treatment adherence.14 At the return visit, there can be a more detailed discussion of long-term management and side effects.8
Limitations of our study included a small sample size and brief treatment duration. Even though the patients had previously reported treatment failure with topical corticosteroids, all demonstrated improvement in only 1 week with a potent topical corticosteroid. The treatment resistance that initially was reported likely was due to poor adherence, but it is possible for AD patients to be resistant to treatment with topical corticosteroids due to allergic contact dermatitis. Patients could theoretically be allergic to components of the vehicle used in topical corticosteroids, which could aggravate their dermatitis; however, this effect seems unlikely in our patient population, as all the patients in our study showed improvement following treatment. Another study limitation was that adherence was not measured. The frequent follow-up visits were designed to encourage treatment adherence, but adherence was not specifically assessed. Although patients were encouraged to only use the desoximetasone spray during the study, it is not known whether patients used other products.
Conclusion
Some AD patients exhibit apparent decreased efficacy of topical corticosteroids over time, but this tachyphylaxis phenomenon is more likely due to poor treatment adherence than to loss of corticosteroid responsiveness. In our study, AD patients who reported treatment failure with topical corticosteroids improved rapidly with topical corticosteroids under conditions designed to promote good adherence to treatment. The majority of patients improved in all 4 parameters used for evaluating disease severity, with 100% of patients reporting improvement in pruritus. Intervention to improve treatment adherence may lead to better health outcomes. When AD appears resistant to topical corticosteroids, addressing adherence issues may be critical.
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18:323-332.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Miller JJ, Roling D, Margolis D, et al. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546-549.
- Smith SD, Harris V, Lee A, et al. General practitioners knowledge about use of topical corticosteroids in paediatric atopic dermatitis in Australia. Aust Fam Physician. 2017;46:335-340.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656-661.
- Reitamo S, Remitz A. Topical agents for atopic dermatitis. In: Bieber T, ed. Advances in the Management of Atopic Dermatitis. London, United Kingdom: Future Medicine Ltd; 2013:62-72.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Fukaya M. Cortisol homeostasis in the epidermis is influenced by topical corticosteroids in patients with atopic dermatitis. Indian J Dermatol. 2017;62:440.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- van der Schaft J, Keijzer WW, Sanders KJ, et al. Is there an additional value of inpatient treatment for patients with atopic dermatitis? Acta Derm Venereol. 2016;96:797-801.
- Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2011;67:100-106.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.
Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.
All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.

Comment
Although topical corticosteroids are the mainstay for treatment of AD, many patients report treatment resistance after a period of a few doses or longer.6-9 There is strong evidence demonstrating rapid corticosteroid receptor downregulation in tissues after corticosteroid therapy, which is the accepted mechanism for tachyphylaxis, but the timing of this effect does not match up with clinical experiences. The physiologic significance of corticosteroid agonist-induced receptor downregulation is unknown and may not have any considerable effect on corticosteroid efficacy.3 A systematic review by Taheri et al3 on the development of resistance to topical corticosteroids proposed 2 theories for the underlying pathogenesis of tachyphylaxis: (1) long-term patient nonadherence, and (2) the initial maximal response during the first few weeks of therapy eventually plateaus. Because corticosteroids may plateau after a certain number of doses, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis.10 The treatment “resistance” reported by the patients in our study may have been due to this plateau effect or to poor adherence.
Our finding that nearly all patients had rapid improvement of AD with the topical corticosteroid is not definitive proof but supports the notion that tachyphylaxis is largely mediated by poor adherence to treatment. Patients rapidly improved over the short study period. The short duration of treatment and multiple visits over the study period were designed to help ensure patient adherence. Rapid improvement in AD when topical corticosteroids are used should be expected, as AD patients have rapid improvement with application of topical corticosteroids in inpatient settings.11,12
Poor adherence to topical medication is common. In a Danish study, 99 of 322 patients (31%) did not redeem their AD prescriptions.13 In a single-center, 5-day, prospective study evaluating the use of fluocinonide cream 0.1% for treatment of children and adults with AD, the median percentage of prescribed doses taken was 40%, according to objective electronic monitors, even though patients reported 100% adherence in their medication diaries.Better adherence was seen on day 1 of treatment in which 66.6% (6/9) of patients adhered to their treatment strategy versus day 5 in which only 11.1% (1/9) of patients used their medication.1
Topical corticosteroids are safe and efficacious if used appropriately; however, patients commonly express fear and anxiety about using them. Topical corticosteroid phobia may stem from a misconception that these products carry the same adverse effects as their oral and systemic counterparts, which may be perpetuated by the media.1 Of 200 dermatology patients surveyed, 72.5% expressed concern about using topical corticosteroids on themselves or their children’s skin, and 24% of these patients stated they were noncompliant with their medication because of these worries. Almost 50% of patients requested prescriptions for corticosteroid-sparing medications such as tacrolimus.1 Patient education is important to help ensure treatment adherence. Other factors that can affect treatment adherence include forgetfulness; the chronic nature of AD; the need for ongoing application of topical treatments; prohibitive costs of some topical agents; and complexities in coordinating school, work, and family plans with the treatment regimen.2
We attempted to ensure good treatment adherence in our study by calling the patients in the intervention group twice daily. The mean improvement in EASI, TLSS, and IGA scores was higher in the intervention group versus the control group, which suggests that patient reminders have at least some benefit. Because AD treatment resistance appears more closely tied to nonadherence rather than loss of medication efficacy, it seems prudent to focus on interventions that would improve treatment adherence; however, such interventions generally are not well tested. Recommended interventions have included educating patients about the side effects of topical corticosteroids, avoiding use of medical jargon, and taking patient vehicle preference into account when prescribing treatments.8 Patients should be scheduled for a return visit within 1 to 2 weeks, as early return visits can augment treatment adherence.14 At the return visit, there can be a more detailed discussion of long-term management and side effects.8
Limitations of our study included a small sample size and brief treatment duration. Even though the patients had previously reported treatment failure with topical corticosteroids, all demonstrated improvement in only 1 week with a potent topical corticosteroid. The treatment resistance that initially was reported likely was due to poor adherence, but it is possible for AD patients to be resistant to treatment with topical corticosteroids due to allergic contact dermatitis. Patients could theoretically be allergic to components of the vehicle used in topical corticosteroids, which could aggravate their dermatitis; however, this effect seems unlikely in our patient population, as all the patients in our study showed improvement following treatment. Another study limitation was that adherence was not measured. The frequent follow-up visits were designed to encourage treatment adherence, but adherence was not specifically assessed. Although patients were encouraged to only use the desoximetasone spray during the study, it is not known whether patients used other products.
Conclusion
Some AD patients exhibit apparent decreased efficacy of topical corticosteroids over time, but this tachyphylaxis phenomenon is more likely due to poor treatment adherence than to loss of corticosteroid responsiveness. In our study, AD patients who reported treatment failure with topical corticosteroids improved rapidly with topical corticosteroids under conditions designed to promote good adherence to treatment. The majority of patients improved in all 4 parameters used for evaluating disease severity, with 100% of patients reporting improvement in pruritus. Intervention to improve treatment adherence may lead to better health outcomes. When AD appears resistant to topical corticosteroids, addressing adherence issues may be critical.
Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.
Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.
All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.

Comment
Although topical corticosteroids are the mainstay for treatment of AD, many patients report treatment resistance after a period of a few doses or longer.6-9 There is strong evidence demonstrating rapid corticosteroid receptor downregulation in tissues after corticosteroid therapy, which is the accepted mechanism for tachyphylaxis, but the timing of this effect does not match up with clinical experiences. The physiologic significance of corticosteroid agonist-induced receptor downregulation is unknown and may not have any considerable effect on corticosteroid efficacy.3 A systematic review by Taheri et al3 on the development of resistance to topical corticosteroids proposed 2 theories for the underlying pathogenesis of tachyphylaxis: (1) long-term patient nonadherence, and (2) the initial maximal response during the first few weeks of therapy eventually plateaus. Because corticosteroids may plateau after a certain number of doses, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis.10 The treatment “resistance” reported by the patients in our study may have been due to this plateau effect or to poor adherence.
Our finding that nearly all patients had rapid improvement of AD with the topical corticosteroid is not definitive proof but supports the notion that tachyphylaxis is largely mediated by poor adherence to treatment. Patients rapidly improved over the short study period. The short duration of treatment and multiple visits over the study period were designed to help ensure patient adherence. Rapid improvement in AD when topical corticosteroids are used should be expected, as AD patients have rapid improvement with application of topical corticosteroids in inpatient settings.11,12
Poor adherence to topical medication is common. In a Danish study, 99 of 322 patients (31%) did not redeem their AD prescriptions.13 In a single-center, 5-day, prospective study evaluating the use of fluocinonide cream 0.1% for treatment of children and adults with AD, the median percentage of prescribed doses taken was 40%, according to objective electronic monitors, even though patients reported 100% adherence in their medication diaries.Better adherence was seen on day 1 of treatment in which 66.6% (6/9) of patients adhered to their treatment strategy versus day 5 in which only 11.1% (1/9) of patients used their medication.1
Topical corticosteroids are safe and efficacious if used appropriately; however, patients commonly express fear and anxiety about using them. Topical corticosteroid phobia may stem from a misconception that these products carry the same adverse effects as their oral and systemic counterparts, which may be perpetuated by the media.1 Of 200 dermatology patients surveyed, 72.5% expressed concern about using topical corticosteroids on themselves or their children’s skin, and 24% of these patients stated they were noncompliant with their medication because of these worries. Almost 50% of patients requested prescriptions for corticosteroid-sparing medications such as tacrolimus.1 Patient education is important to help ensure treatment adherence. Other factors that can affect treatment adherence include forgetfulness; the chronic nature of AD; the need for ongoing application of topical treatments; prohibitive costs of some topical agents; and complexities in coordinating school, work, and family plans with the treatment regimen.2
We attempted to ensure good treatment adherence in our study by calling the patients in the intervention group twice daily. The mean improvement in EASI, TLSS, and IGA scores was higher in the intervention group versus the control group, which suggests that patient reminders have at least some benefit. Because AD treatment resistance appears more closely tied to nonadherence rather than loss of medication efficacy, it seems prudent to focus on interventions that would improve treatment adherence; however, such interventions generally are not well tested. Recommended interventions have included educating patients about the side effects of topical corticosteroids, avoiding use of medical jargon, and taking patient vehicle preference into account when prescribing treatments.8 Patients should be scheduled for a return visit within 1 to 2 weeks, as early return visits can augment treatment adherence.14 At the return visit, there can be a more detailed discussion of long-term management and side effects.8
Limitations of our study included a small sample size and brief treatment duration. Even though the patients had previously reported treatment failure with topical corticosteroids, all demonstrated improvement in only 1 week with a potent topical corticosteroid. The treatment resistance that initially was reported likely was due to poor adherence, but it is possible for AD patients to be resistant to treatment with topical corticosteroids due to allergic contact dermatitis. Patients could theoretically be allergic to components of the vehicle used in topical corticosteroids, which could aggravate their dermatitis; however, this effect seems unlikely in our patient population, as all the patients in our study showed improvement following treatment. Another study limitation was that adherence was not measured. The frequent follow-up visits were designed to encourage treatment adherence, but adherence was not specifically assessed. Although patients were encouraged to only use the desoximetasone spray during the study, it is not known whether patients used other products.
Conclusion
Some AD patients exhibit apparent decreased efficacy of topical corticosteroids over time, but this tachyphylaxis phenomenon is more likely due to poor treatment adherence than to loss of corticosteroid responsiveness. In our study, AD patients who reported treatment failure with topical corticosteroids improved rapidly with topical corticosteroids under conditions designed to promote good adherence to treatment. The majority of patients improved in all 4 parameters used for evaluating disease severity, with 100% of patients reporting improvement in pruritus. Intervention to improve treatment adherence may lead to better health outcomes. When AD appears resistant to topical corticosteroids, addressing adherence issues may be critical.
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18:323-332.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Miller JJ, Roling D, Margolis D, et al. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546-549.
- Smith SD, Harris V, Lee A, et al. General practitioners knowledge about use of topical corticosteroids in paediatric atopic dermatitis in Australia. Aust Fam Physician. 2017;46:335-340.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656-661.
- Reitamo S, Remitz A. Topical agents for atopic dermatitis. In: Bieber T, ed. Advances in the Management of Atopic Dermatitis. London, United Kingdom: Future Medicine Ltd; 2013:62-72.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Fukaya M. Cortisol homeostasis in the epidermis is influenced by topical corticosteroids in patients with atopic dermatitis. Indian J Dermatol. 2017;62:440.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- van der Schaft J, Keijzer WW, Sanders KJ, et al. Is there an additional value of inpatient treatment for patients with atopic dermatitis? Acta Derm Venereol. 2016;96:797-801.
- Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2011;67:100-106.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18:323-332.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Miller JJ, Roling D, Margolis D, et al. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546-549.
- Smith SD, Harris V, Lee A, et al. General practitioners knowledge about use of topical corticosteroids in paediatric atopic dermatitis in Australia. Aust Fam Physician. 2017;46:335-340.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656-661.
- Reitamo S, Remitz A. Topical agents for atopic dermatitis. In: Bieber T, ed. Advances in the Management of Atopic Dermatitis. London, United Kingdom: Future Medicine Ltd; 2013:62-72.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Fukaya M. Cortisol homeostasis in the epidermis is influenced by topical corticosteroids in patients with atopic dermatitis. Indian J Dermatol. 2017;62:440.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- van der Schaft J, Keijzer WW, Sanders KJ, et al. Is there an additional value of inpatient treatment for patients with atopic dermatitis? Acta Derm Venereol. 2016;96:797-801.
- Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2011;67:100-106.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
Practice Points
- Mid-potency corticosteroids are the first-line treatment of atopic dermatitis (AD).
- Atopic dermatitis may fail to respond to topical corticosteroids initially or lose response over time, a phenomenon known as tachyphylaxis.
- Nonadherence to medication is the most likely cause of treatment resistance in patients with AD.
Screening for Depression in Rosacea Patients
Rosacea is a chronic skin condition that can be classified into 4 subtypes: erythematotelangiectatic, papulopustular, phymatous, and ocular. Erythematotelangiectatic rosacea is characterized by redness of the face and excessive blushing. Papulopustular rosacea is a more severe form of disease that is characterized by papules and pustules of the central face. If left untreated, these subtypes may progress to phymatous rosacea, which is characterized by skin thickening, fibrosis, and cosmetic disfigurement. Ocular rosacea is characterized by redness and irritation of the eyes.1 Rosacea patients often are burdened with embarrassment, social anxiety, and psychiatric comorbidities.
The Patient Health Questionnaire 9 (PHQ-9) is a validated and reliable self-administered tool for diagnosis of depression and designation of depression severity. This instrument could prove useful in screening for depression in rosacea patients given the high incidence of psychiatric comorbidities in this patient population.2 The PHQ-9 consists of 9 questions that assess for criteria used to define depressive disorders in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).3 The questionnaire is brief, easy to administer, and has 88% specificity and sensitivity.4
Other studies have evaluated the relationship between rosacea and psychiatric illness, but the PHQ-9 was not used as a screening tool.7,8 Rosacea patients are at increased risk for having psychiatrist-diagnosed depression.5 In one assessment, a positive correlation between rosacea and psychiatric illness was noted using the Dermatology Life Quality Index, the rejection scale of the Questionnaire on Experience with Skin Complaints, and the German version of the Hospital Anxiety and Depression Scale.6 Interpretation of Rosacea Quality of Life and Dermatology Life Quality Index scores indicated that rosacea has a negative impact on quality of life.7
The purpose of this study was to examine the relationship between self-assessed rosacea severity scores and level of depression using the validated rosacea self-assessment tool and the PHQ-9 questionnaire, respectively.
Methods
Study Population
Study participants were adult patients from the Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) dermatology clinic from January 2011 to December 2014 who had received a diagnosis of rosacea (International Classification of Diseases, Ninth Revision [ICD-9] code 695.3) from a Wake Forest dermatologist. Institutional review board approval was obtained prior to initiation of the study. Data collection occurred from October 2014 through February 2015. A total of 478 patients met criteria for participation in the study and were identified from the Wake Forest Baptist Hospital Transitional Data Warehouse and the electronic medical record. Because rosacea typically is not diagnosed in children and the data measures are not validated in children, this demographic group was excluded from participation.
Of 478 eligible patients who were invited to participate via mail or telephone, 46 completed the rosacea self-assessment tool and PHQ-9 survey in person. A total of 432 patients were mailed a presurvey recruitment letter notifying them that they would be receiving a survey in the mail unless they contacted the study team to decline participation. An email address and telephone number for the study team was provided. Twenty patients declined to participate in the study; surveys were then mailed to the remaining 412 patients. Sixteen of the mailed surveys were returned by the post office due to an incorrect address.
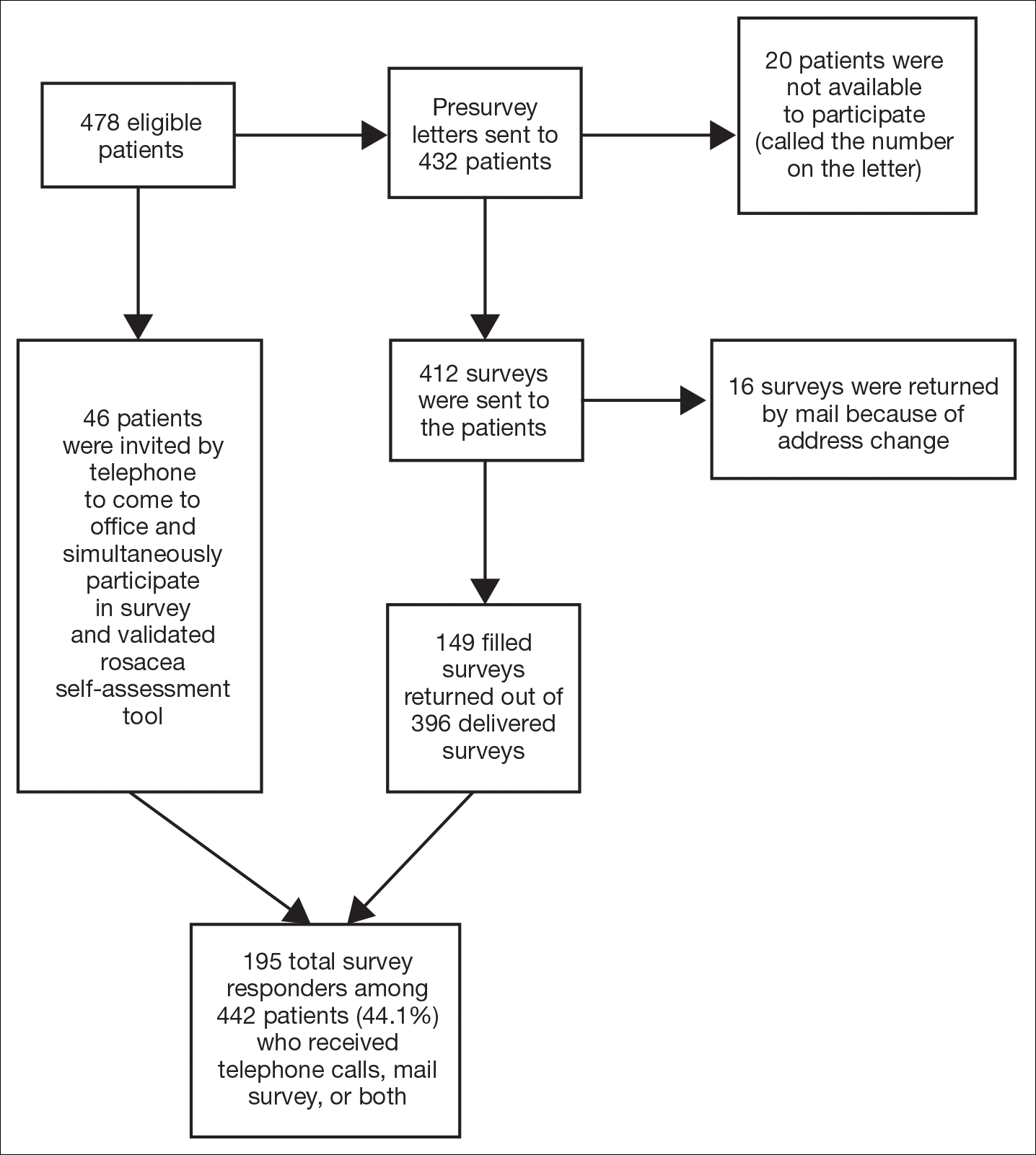
Self-assessments
Patients selected images to self-identify the severity of their rosacea symptoms, including erythema, papulopustular lesions, ocular symptoms, and nasal involvement by looking at photographs on the self-assessment tool, which showed various rosacea severity levels. Scores ranged from 2 (least severe) to 8 (most severe). The PHQ-9 survey was completed by participants to assess mental health and mood.
Statistical Analysis
Results were reported using descriptive statistics. Regression analysis was performed to identify independent outcome predictors. To study the relationship between age and demographic variables, the population was divided into 2 groups: patients aged 60 years and older and patients younger than 60 years. Correlation of variables with duration of disease also was studied by creating 2 groups: patients with a disease duration of 11 years or longer and patients with a disease duration of less than 11 years. Comparisons were completed between groups using χ2 tests for proportions and t tests or analysis of variance for continuous variables. Analysis of variance was applied among all patients classified according to the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe).
Results
There is a direct relationship between rosacea severity and depression when comparing across the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe)(P=.006; F=5.18; N=183)
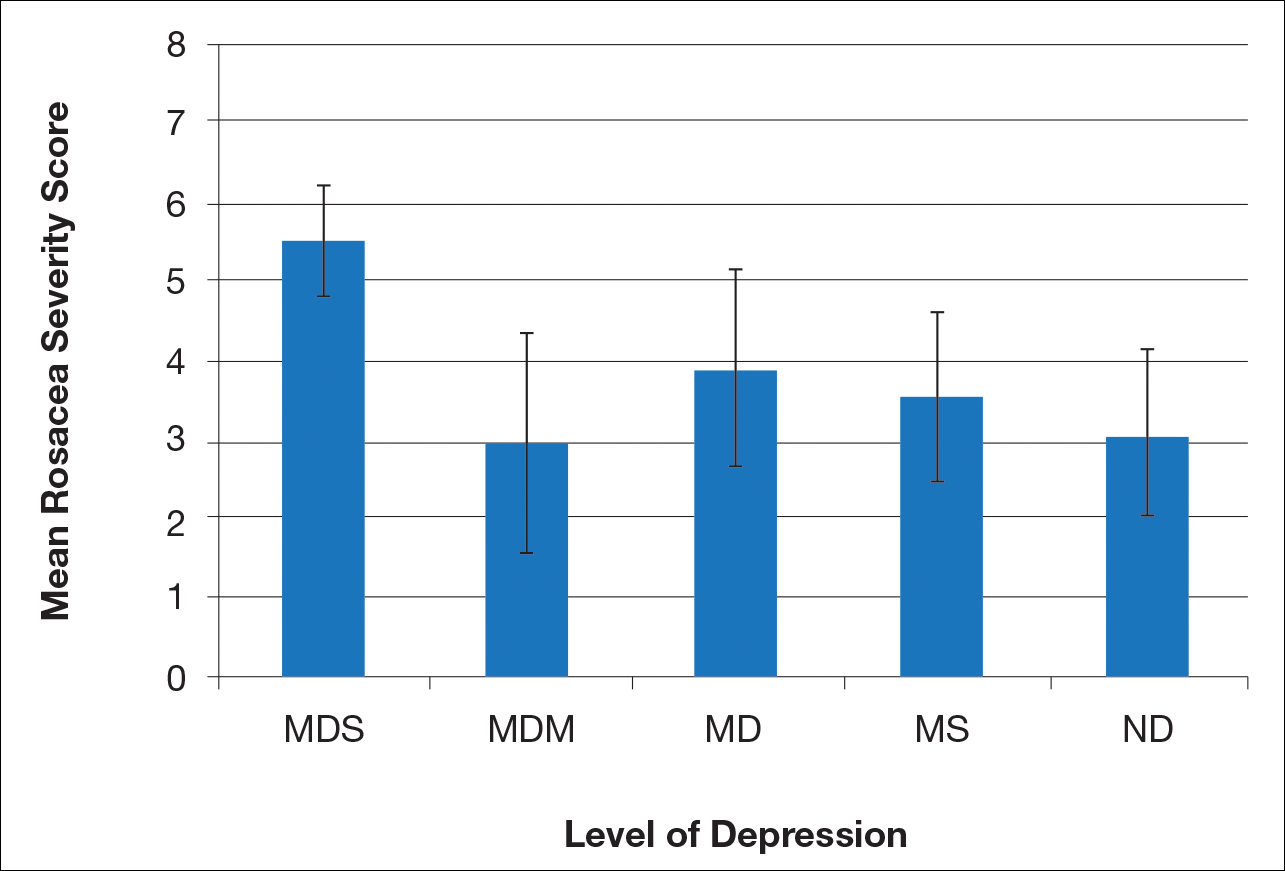
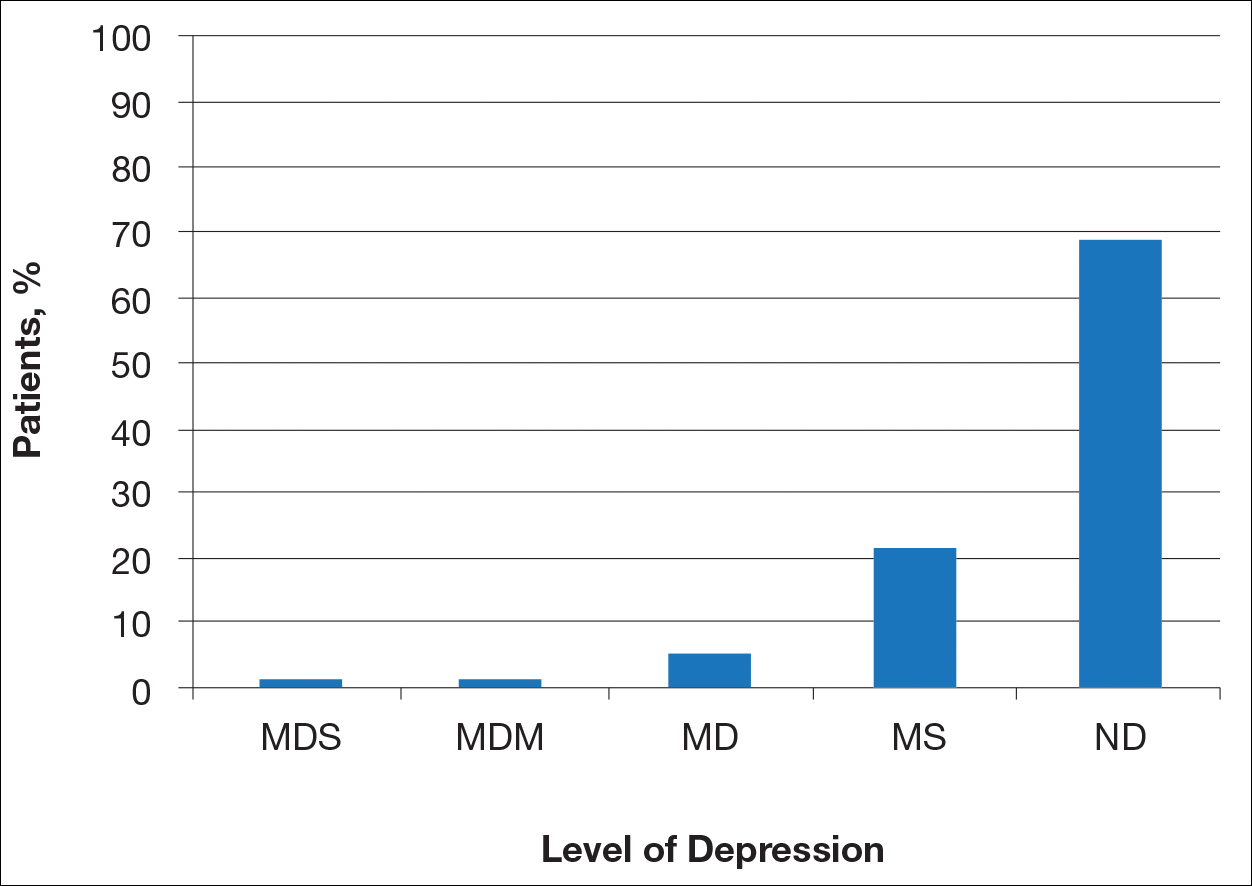
Most patients reported they were nondepressed (68.9%). As measured by the PHQ-9, 31.1% of patients experienced some level of depression: 21.9% reported minimal depression symptoms, 7.1% reported minor depression, 1.1% reported major depression (moderate), and 1.1% reported major depression (severe)(Table).
Comment
There is a direct relationship between rosacea severity and level of depression. In our study, nearly one-third of patients reported some degree of depression. The reason for this correlation may be due to disease stigmatization and decreased quality of life due to the somatic symptoms of rosacea. Our study reinforced the results of other studies evaluating the psychosocial impact of rosacea.8,9 Depression is associated with poor treatment adherence and poor outcomes in rosacea patients; therefore, depression may serve as an important outcome measure.10 The psychosocial impact of rosacea can be severe, but with disease improvement, there often is an improvement in the patient’s psychosocial status.7
There are several limitations to our study. The study population consisted of patients at a university dermatology clinic who may not be representative of patients in the general population; however, our hospital system does not require referral and provides care to a large percentage of the surrounding community.
Clinical implementation of the validated rosacea self-assessment tool and PHQ-9 may have several benefits. Patient-assessed rosacea severity and psychosocial impact obtained via use of these tools would provide physicians with information to fine-tune rosacea treatment regimens. Patients with the greatest social impact may require a more aggressive treatment approach. Early detection of depression in the rosacea population is important in informing treatment strategy and improving outcomes. Physicians should pay close attention to signs of depression in rosacea patients and determine if psychiatric treatment or referral for psychiatric evaluation is indicated. The correlation between rosacea and depression underscores the importance of treating this highly impactful disease; however, the low number of responders from the major depression (moderate) subgroup prevented us from making any strong conclusion about this specific subgroup.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychol Ann. 2002;32:509-515.
- America Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Gupta MA, Gupta AK, Chen SJ, et al. Comorbidity of rosacea and depression: an analysis of the National Ambulatory Medical Care Survey and National Hospital Ambulatory Care Survey—outpatient department data collected by the US National Center for Health Statistics from 1995 to 2002. Br J Dermatol. 2005;153:1176-1181.
- Böhm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Halioua B, Cribier B, Frey M, et al. Feelings of stigmatization in patients with rosacea [published online June 21, 2016]. J Eur Acad Dermatol Venereol. 2016;31:163-168.
- Bewley A, Fowler J, Schöfer H, et al. Erythema of rosacea impairs health-related quality of life: results of a meta-analysis [published online March 16, 2016]. Dermatol Ther (Heidelb). 2016;6:237-247.
- Korman AM, Hill D, Alikhan A, et al. Impact and management of depression in psoriasis patients [published online January 4, 2016]. Expert Opin Pharmacother. 2016;17:147-152.
Rosacea is a chronic skin condition that can be classified into 4 subtypes: erythematotelangiectatic, papulopustular, phymatous, and ocular. Erythematotelangiectatic rosacea is characterized by redness of the face and excessive blushing. Papulopustular rosacea is a more severe form of disease that is characterized by papules and pustules of the central face. If left untreated, these subtypes may progress to phymatous rosacea, which is characterized by skin thickening, fibrosis, and cosmetic disfigurement. Ocular rosacea is characterized by redness and irritation of the eyes.1 Rosacea patients often are burdened with embarrassment, social anxiety, and psychiatric comorbidities.
The Patient Health Questionnaire 9 (PHQ-9) is a validated and reliable self-administered tool for diagnosis of depression and designation of depression severity. This instrument could prove useful in screening for depression in rosacea patients given the high incidence of psychiatric comorbidities in this patient population.2 The PHQ-9 consists of 9 questions that assess for criteria used to define depressive disorders in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).3 The questionnaire is brief, easy to administer, and has 88% specificity and sensitivity.4
Other studies have evaluated the relationship between rosacea and psychiatric illness, but the PHQ-9 was not used as a screening tool.7,8 Rosacea patients are at increased risk for having psychiatrist-diagnosed depression.5 In one assessment, a positive correlation between rosacea and psychiatric illness was noted using the Dermatology Life Quality Index, the rejection scale of the Questionnaire on Experience with Skin Complaints, and the German version of the Hospital Anxiety and Depression Scale.6 Interpretation of Rosacea Quality of Life and Dermatology Life Quality Index scores indicated that rosacea has a negative impact on quality of life.7
The purpose of this study was to examine the relationship between self-assessed rosacea severity scores and level of depression using the validated rosacea self-assessment tool and the PHQ-9 questionnaire, respectively.
Methods
Study Population
Study participants were adult patients from the Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) dermatology clinic from January 2011 to December 2014 who had received a diagnosis of rosacea (International Classification of Diseases, Ninth Revision [ICD-9] code 695.3) from a Wake Forest dermatologist. Institutional review board approval was obtained prior to initiation of the study. Data collection occurred from October 2014 through February 2015. A total of 478 patients met criteria for participation in the study and were identified from the Wake Forest Baptist Hospital Transitional Data Warehouse and the electronic medical record. Because rosacea typically is not diagnosed in children and the data measures are not validated in children, this demographic group was excluded from participation.
Of 478 eligible patients who were invited to participate via mail or telephone, 46 completed the rosacea self-assessment tool and PHQ-9 survey in person. A total of 432 patients were mailed a presurvey recruitment letter notifying them that they would be receiving a survey in the mail unless they contacted the study team to decline participation. An email address and telephone number for the study team was provided. Twenty patients declined to participate in the study; surveys were then mailed to the remaining 412 patients. Sixteen of the mailed surveys were returned by the post office due to an incorrect address.

Self-assessments
Patients selected images to self-identify the severity of their rosacea symptoms, including erythema, papulopustular lesions, ocular symptoms, and nasal involvement by looking at photographs on the self-assessment tool, which showed various rosacea severity levels. Scores ranged from 2 (least severe) to 8 (most severe). The PHQ-9 survey was completed by participants to assess mental health and mood.
Statistical Analysis
Results were reported using descriptive statistics. Regression analysis was performed to identify independent outcome predictors. To study the relationship between age and demographic variables, the population was divided into 2 groups: patients aged 60 years and older and patients younger than 60 years. Correlation of variables with duration of disease also was studied by creating 2 groups: patients with a disease duration of 11 years or longer and patients with a disease duration of less than 11 years. Comparisons were completed between groups using χ2 tests for proportions and t tests or analysis of variance for continuous variables. Analysis of variance was applied among all patients classified according to the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe).
Results
There is a direct relationship between rosacea severity and depression when comparing across the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe)(P=.006; F=5.18; N=183)


Most patients reported they were nondepressed (68.9%). As measured by the PHQ-9, 31.1% of patients experienced some level of depression: 21.9% reported minimal depression symptoms, 7.1% reported minor depression, 1.1% reported major depression (moderate), and 1.1% reported major depression (severe)(Table).
Comment
There is a direct relationship between rosacea severity and level of depression. In our study, nearly one-third of patients reported some degree of depression. The reason for this correlation may be due to disease stigmatization and decreased quality of life due to the somatic symptoms of rosacea. Our study reinforced the results of other studies evaluating the psychosocial impact of rosacea.8,9 Depression is associated with poor treatment adherence and poor outcomes in rosacea patients; therefore, depression may serve as an important outcome measure.10 The psychosocial impact of rosacea can be severe, but with disease improvement, there often is an improvement in the patient’s psychosocial status.7
There are several limitations to our study. The study population consisted of patients at a university dermatology clinic who may not be representative of patients in the general population; however, our hospital system does not require referral and provides care to a large percentage of the surrounding community.
Clinical implementation of the validated rosacea self-assessment tool and PHQ-9 may have several benefits. Patient-assessed rosacea severity and psychosocial impact obtained via use of these tools would provide physicians with information to fine-tune rosacea treatment regimens. Patients with the greatest social impact may require a more aggressive treatment approach. Early detection of depression in the rosacea population is important in informing treatment strategy and improving outcomes. Physicians should pay close attention to signs of depression in rosacea patients and determine if psychiatric treatment or referral for psychiatric evaluation is indicated. The correlation between rosacea and depression underscores the importance of treating this highly impactful disease; however, the low number of responders from the major depression (moderate) subgroup prevented us from making any strong conclusion about this specific subgroup.
Rosacea is a chronic skin condition that can be classified into 4 subtypes: erythematotelangiectatic, papulopustular, phymatous, and ocular. Erythematotelangiectatic rosacea is characterized by redness of the face and excessive blushing. Papulopustular rosacea is a more severe form of disease that is characterized by papules and pustules of the central face. If left untreated, these subtypes may progress to phymatous rosacea, which is characterized by skin thickening, fibrosis, and cosmetic disfigurement. Ocular rosacea is characterized by redness and irritation of the eyes.1 Rosacea patients often are burdened with embarrassment, social anxiety, and psychiatric comorbidities.
The Patient Health Questionnaire 9 (PHQ-9) is a validated and reliable self-administered tool for diagnosis of depression and designation of depression severity. This instrument could prove useful in screening for depression in rosacea patients given the high incidence of psychiatric comorbidities in this patient population.2 The PHQ-9 consists of 9 questions that assess for criteria used to define depressive disorders in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).3 The questionnaire is brief, easy to administer, and has 88% specificity and sensitivity.4
Other studies have evaluated the relationship between rosacea and psychiatric illness, but the PHQ-9 was not used as a screening tool.7,8 Rosacea patients are at increased risk for having psychiatrist-diagnosed depression.5 In one assessment, a positive correlation between rosacea and psychiatric illness was noted using the Dermatology Life Quality Index, the rejection scale of the Questionnaire on Experience with Skin Complaints, and the German version of the Hospital Anxiety and Depression Scale.6 Interpretation of Rosacea Quality of Life and Dermatology Life Quality Index scores indicated that rosacea has a negative impact on quality of life.7
The purpose of this study was to examine the relationship between self-assessed rosacea severity scores and level of depression using the validated rosacea self-assessment tool and the PHQ-9 questionnaire, respectively.
Methods
Study Population
Study participants were adult patients from the Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) dermatology clinic from January 2011 to December 2014 who had received a diagnosis of rosacea (International Classification of Diseases, Ninth Revision [ICD-9] code 695.3) from a Wake Forest dermatologist. Institutional review board approval was obtained prior to initiation of the study. Data collection occurred from October 2014 through February 2015. A total of 478 patients met criteria for participation in the study and were identified from the Wake Forest Baptist Hospital Transitional Data Warehouse and the electronic medical record. Because rosacea typically is not diagnosed in children and the data measures are not validated in children, this demographic group was excluded from participation.
Of 478 eligible patients who were invited to participate via mail or telephone, 46 completed the rosacea self-assessment tool and PHQ-9 survey in person. A total of 432 patients were mailed a presurvey recruitment letter notifying them that they would be receiving a survey in the mail unless they contacted the study team to decline participation. An email address and telephone number for the study team was provided. Twenty patients declined to participate in the study; surveys were then mailed to the remaining 412 patients. Sixteen of the mailed surveys were returned by the post office due to an incorrect address.

Self-assessments
Patients selected images to self-identify the severity of their rosacea symptoms, including erythema, papulopustular lesions, ocular symptoms, and nasal involvement by looking at photographs on the self-assessment tool, which showed various rosacea severity levels. Scores ranged from 2 (least severe) to 8 (most severe). The PHQ-9 survey was completed by participants to assess mental health and mood.
Statistical Analysis
Results were reported using descriptive statistics. Regression analysis was performed to identify independent outcome predictors. To study the relationship between age and demographic variables, the population was divided into 2 groups: patients aged 60 years and older and patients younger than 60 years. Correlation of variables with duration of disease also was studied by creating 2 groups: patients with a disease duration of 11 years or longer and patients with a disease duration of less than 11 years. Comparisons were completed between groups using χ2 tests for proportions and t tests or analysis of variance for continuous variables. Analysis of variance was applied among all patients classified according to the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe).
Results
There is a direct relationship between rosacea severity and depression when comparing across the following levels of depression: nondepressed, minimal depression symptoms, minor depression, major depression (moderate), and major depression (severe)(P=.006; F=5.18; N=183)


Most patients reported they were nondepressed (68.9%). As measured by the PHQ-9, 31.1% of patients experienced some level of depression: 21.9% reported minimal depression symptoms, 7.1% reported minor depression, 1.1% reported major depression (moderate), and 1.1% reported major depression (severe)(Table).
Comment
There is a direct relationship between rosacea severity and level of depression. In our study, nearly one-third of patients reported some degree of depression. The reason for this correlation may be due to disease stigmatization and decreased quality of life due to the somatic symptoms of rosacea. Our study reinforced the results of other studies evaluating the psychosocial impact of rosacea.8,9 Depression is associated with poor treatment adherence and poor outcomes in rosacea patients; therefore, depression may serve as an important outcome measure.10 The psychosocial impact of rosacea can be severe, but with disease improvement, there often is an improvement in the patient’s psychosocial status.7
There are several limitations to our study. The study population consisted of patients at a university dermatology clinic who may not be representative of patients in the general population; however, our hospital system does not require referral and provides care to a large percentage of the surrounding community.
Clinical implementation of the validated rosacea self-assessment tool and PHQ-9 may have several benefits. Patient-assessed rosacea severity and psychosocial impact obtained via use of these tools would provide physicians with information to fine-tune rosacea treatment regimens. Patients with the greatest social impact may require a more aggressive treatment approach. Early detection of depression in the rosacea population is important in informing treatment strategy and improving outcomes. Physicians should pay close attention to signs of depression in rosacea patients and determine if psychiatric treatment or referral for psychiatric evaluation is indicated. The correlation between rosacea and depression underscores the importance of treating this highly impactful disease; however, the low number of responders from the major depression (moderate) subgroup prevented us from making any strong conclusion about this specific subgroup.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychol Ann. 2002;32:509-515.
- America Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Gupta MA, Gupta AK, Chen SJ, et al. Comorbidity of rosacea and depression: an analysis of the National Ambulatory Medical Care Survey and National Hospital Ambulatory Care Survey—outpatient department data collected by the US National Center for Health Statistics from 1995 to 2002. Br J Dermatol. 2005;153:1176-1181.
- Böhm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Halioua B, Cribier B, Frey M, et al. Feelings of stigmatization in patients with rosacea [published online June 21, 2016]. J Eur Acad Dermatol Venereol. 2016;31:163-168.
- Bewley A, Fowler J, Schöfer H, et al. Erythema of rosacea impairs health-related quality of life: results of a meta-analysis [published online March 16, 2016]. Dermatol Ther (Heidelb). 2016;6:237-247.
- Korman AM, Hill D, Alikhan A, et al. Impact and management of depression in psoriasis patients [published online January 4, 2016]. Expert Opin Pharmacother. 2016;17:147-152.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychol Ann. 2002;32:509-515.
- America Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Gupta MA, Gupta AK, Chen SJ, et al. Comorbidity of rosacea and depression: an analysis of the National Ambulatory Medical Care Survey and National Hospital Ambulatory Care Survey—outpatient department data collected by the US National Center for Health Statistics from 1995 to 2002. Br J Dermatol. 2005;153:1176-1181.
- Böhm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Halioua B, Cribier B, Frey M, et al. Feelings of stigmatization in patients with rosacea [published online June 21, 2016]. J Eur Acad Dermatol Venereol. 2016;31:163-168.
- Bewley A, Fowler J, Schöfer H, et al. Erythema of rosacea impairs health-related quality of life: results of a meta-analysis [published online March 16, 2016]. Dermatol Ther (Heidelb). 2016;6:237-247.
- Korman AM, Hill D, Alikhan A, et al. Impact and management of depression in psoriasis patients [published online January 4, 2016]. Expert Opin Pharmacother. 2016;17:147-152.
Practice Points
- Rosacea patients often are burdened with embarrassment, social anxiety, and psychiatric comorbidities.
- There is a direct relationship between rosacea severity and degree of depression.
- Physicians should pay close attention to signs of depression in rosacea patients and determine if psychiatric treatment or referral for psychiatric evaluation is indicated.
Cost of Diagnosing Psoriasis and Rosacea for Dermatologists Versus Primary Care Physicians
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i
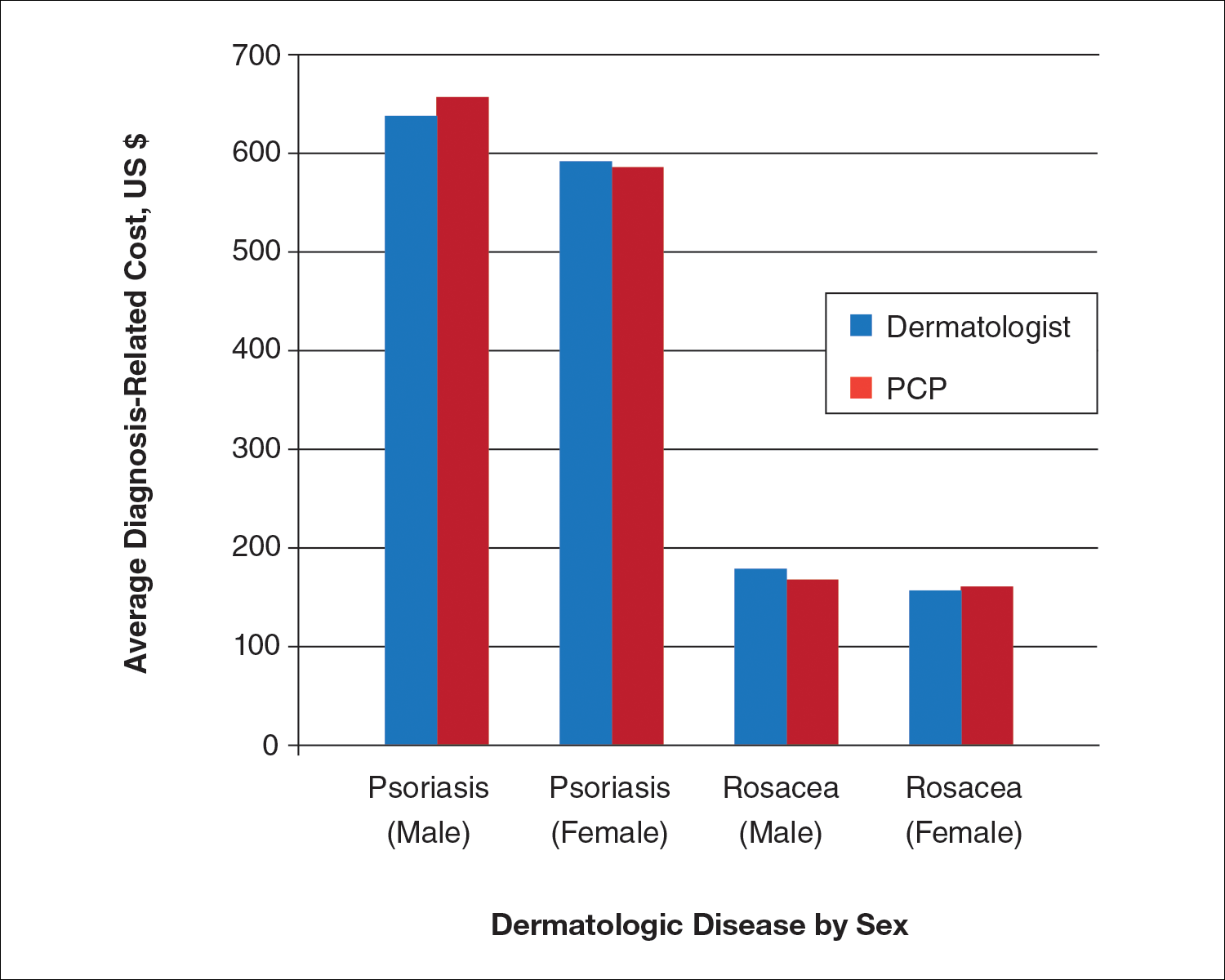
Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i

Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i

Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
Practice Points
- Growing health care costs are causing accountable care organizations (ACOs) to reconsider how to best manage skin disease.
- There is little difference in average diagnosis-related cost between primary care physicians and dermatologists in diagnosing psoriasis or rosacea.
- With diagnosis costs essentially equal and increased dermatologist diagnostic accuracy, ACOs may encourage skin disease to be managed by dermatologists.
Presenting Treatment Safety Data: Subjective Interpretations of Objective Information
The Nuremberg Code in 1947,1 the Declaration of Helsinki in 1964,2 and the Belmont Report in 19793 were cornerstones in the establishment of ethical principles in the medical field. These documents specifically highlight the concept of informed consent, which maintains that to practice ethical medicine, physicians must fully inform patients of all therapeutic benefits and especially risks as well as treatment alternatives before they consent to therapeutic intervention. Educating patients about risks of treatment is obligatory. Risk communication involves a mutual exchange of information between physicians and patients; the physician presents risk information in an understandable manner that adequately conveys pertinent data that is critical for the patient to make an informed therapeutic decision.4
An inherent problem with risk education is that patients may be terrified about risks associated with treatment. Some patients will refuse needed treatment because of fear.5 When patients have concerns about the safety profile of a treatment regimen and potential adverse effects, they may be less compliant with treatment.6 The intelligent noncompliance phenomenon occurs when a patient knowingly makes the choice to not adhere to treatment, and concern regarding treatment risks relative to benefits is a common reason underlying this phenomenon.7,8
Behavioral economists have studied how individuals weigh risks. Kahneman and Tversky’s9 prospect theory asserts that individuals tend to overweigh unlikely risks and underweigh more certain risks, which they call the certainty effect; it is the basis of the human tendency to avoid risks in situations of likely gain and to pursue risks in situations of likely loss. The tendency to overweigh rare risks is even more pronounced for affect-rich events such as serious side effects.10 The way data are presented can affect how patients interpret the information. Context and framing of data affect patients’ perceptions.11 We describe several ways to present safety data using graphical presentation of psoriasis treatment safety data as an example and explain how each one can affect patients’ perception of treatment risks.
Approaches to Presenting Safety Data
There are numerous ways to present safety data to patients, including verbal, numeric, and visual strategies.12 Many methods of presentation are a combination of these strategies. Graphs are visual strategies to further categorize and present numeric data, and physicians may choose to incorporate these aids when presenting safety information to patients. Graphical presentations give the patient a mental picture of the data. Numerous types of graphs can be constructed. Kalb et al13 determined the effect of psoriasis treatment on the risk of serious infection from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). We used the results from this study to demonstrate multiple ways of presenting safety data (Figures 1–3).
A graphical presentation with a truncated y-axis is a common approach (Figure 1). Graphs with truncated axes are sometimes used to conserve space or to accentuate certain differences in the graph that would otherwise be less obvious without the zoomed in y-axis.14 These graphs present quantitatively accurate information that can be visually misleading at the same time. Truncated axes accentuate differences, creating mental impressions that are not reflective of the magnitude of the numeric differences. Alternatively, a graph with a full y-axis includes both the maximum and minimum data values on the y-axis (Figure 2). The y-axis also extends maximally to the total number of patients or patient-years studied. This type of graph presents all of the numeric data without distortion.
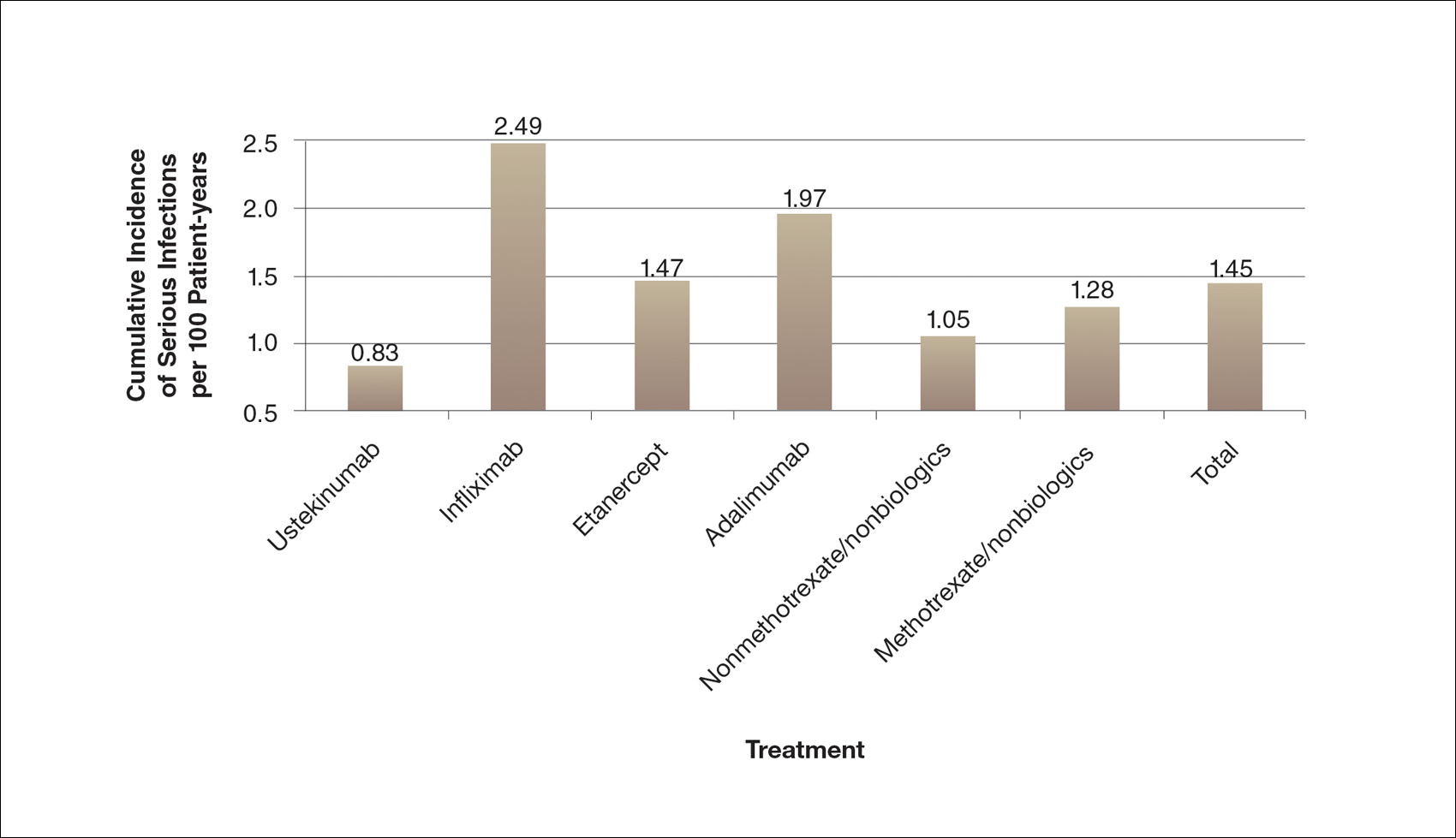
A graph also can present the percentage of patients or patient-years that do not have an adverse effect (Figure 3). This inverse presentation of the data does not emphasize rare cases of patients who have had adverse effects; instead, it emphasizes the large percentage of patients who did not have adverse effects and presents a far more reassuring perspective, even though mathematically the information is identical.
Focus on the Patients Who Do Not Have Adverse Effects of Treatments
Fear of adverse effects is one of the most commonly reported causes of poor treatment adherence.15 New therapies for psoriasis are highly effective and safe, but as with all treatments, they also are associated with some risks. Patients may latch onto those risks too tightly or perhaps, in other circumstances, not tightly enough. The method used by a physician to present safety data to a patient may determine the patient’s perception about treatments.
When trying to give patients an accurate impression of treatment risks, it may be helpful to avoid approaches that focus on presenting the (few) cases of severe adverse drug effects since patients (and physicians) are likely to overweigh the unlikely risk of having an adverse effect if presented with this information. It may be more reassuring to focus on presenting information about the chance of not having an adverse drug effect, assuming the physician’s goal is to be reassuring.
Poor communication with patients when presenting safety data can foster exaggerated fears of an unlikely consequence to the point that patients can be left undertreated and sustaining disease symptoms.16 Physicians may strive to do no harm to their patients, but without careful presentation of safety data in the process of helping the patient make an informed decision, it is possible to do mental harm to patients in the form of fear or even, in the case of nonadherence or treatment refusal, physical harm in the form of continued disease symptoms.
One limitation of this review is that we only used graphical presentation of data as an example. Similar concerns apply to numerical data presentation. Telling a patient the risk of a severe adverse reaction is doubled by a certain treatment may be terrifying, though if the baseline risk is rare, doubling the baseline risk may represent only a minimal increase in the absolute risk. Telling a patient the risk is only 1 in 1000 may still be alarming because many patients tend to focus on the 1, but telling a patient that 999 of 1000 patients do not have a problem can be much more reassuring.
The physician’s goal—to help patients make informed decisions about their treatment—calls for him/her to assimilate safety data into useful information that the patient can use to make an informed decision.17 Overly comforting or alarming, confusing, and inaccurate information can misguide the patient, violating the ethical principle of nonmaleficence. Although there is an obligation to educate patients about risks, there may not be a purely objective way to do it. When physicians present objective data to patients, whether in numerical or graphical form, there will be an unavoidable subjective interpretation of the data. The form of presentation will have a critical effect on patients’ subjective perceptions. Physicians can present objective data in such a way as to be reassuring or frightening.
Conclusion
Despite physicians’ best-intentioned efforts, it may be impossible to avoid presenting safety data in a way that will be subjectively interpreted by patients. Physicians have a choice in how they present data to patients; their best judgment should be used in how they present data to inform patients, guide them, and offer them the best treatment outcomes.
Acknowledgment
We thank Scott Jaros, BA (Winston-Salem, North Carolina), for his assistance in the revision of the manuscript.
- Freyhofer HH. The Nuremberg Medical Trial: The Holocaust and the Origin of the Nuremberg Medical Code. New York, NY: Peter Lang Publishing; 2004.
- Carlson R, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713.
- Office for Human Research Protections. The Belmont Report. Rockville, MD: US Department of Health and Human Services; 1979.
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827-830.
- Hayden C, Neame R, Tarrant C. Patients’ adherence-related beliefs about methotrexate: a qualitative study of the role of written patient information. BMJ Open. 2015;5:e006918.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Weintraub M. Intelligent noncompliance with special emphasis on the elderly. Contemp Pharm Pract. 1981;4:8-11.
- Horne R. Representations of medication and treatment: advances in theory and measurement. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. London, England: Routledge, Taylor & Francis Group; 1997:155-188.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263-291.
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185-190.
- Kessler JB, Zhang CY. Behavioural economics and health. In: Detels R, Gulliford M, Abdool Karim Q, et al, eds. Oxford Textbook of Global Public Health. 6th ed. Oxford, UK: Oxford University Press; 2015:775-789.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations [published online September 14, 2007]. Med Decis Making. 2007;27:696-713.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Rensberger B. Slanting the slopes of graphs. The Washington Post. May 10, 1995. http://www.washingtonpost.com/archive/1995/05/10/slanting-the-slope-of-graphs/08a34412-60a2-4719-86e5-d7433938c166/. Accessed September 21, 2016.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26(5, pt 1):607-611.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745-748.
The Nuremberg Code in 1947,1 the Declaration of Helsinki in 1964,2 and the Belmont Report in 19793 were cornerstones in the establishment of ethical principles in the medical field. These documents specifically highlight the concept of informed consent, which maintains that to practice ethical medicine, physicians must fully inform patients of all therapeutic benefits and especially risks as well as treatment alternatives before they consent to therapeutic intervention. Educating patients about risks of treatment is obligatory. Risk communication involves a mutual exchange of information between physicians and patients; the physician presents risk information in an understandable manner that adequately conveys pertinent data that is critical for the patient to make an informed therapeutic decision.4
An inherent problem with risk education is that patients may be terrified about risks associated with treatment. Some patients will refuse needed treatment because of fear.5 When patients have concerns about the safety profile of a treatment regimen and potential adverse effects, they may be less compliant with treatment.6 The intelligent noncompliance phenomenon occurs when a patient knowingly makes the choice to not adhere to treatment, and concern regarding treatment risks relative to benefits is a common reason underlying this phenomenon.7,8
Behavioral economists have studied how individuals weigh risks. Kahneman and Tversky’s9 prospect theory asserts that individuals tend to overweigh unlikely risks and underweigh more certain risks, which they call the certainty effect; it is the basis of the human tendency to avoid risks in situations of likely gain and to pursue risks in situations of likely loss. The tendency to overweigh rare risks is even more pronounced for affect-rich events such as serious side effects.10 The way data are presented can affect how patients interpret the information. Context and framing of data affect patients’ perceptions.11 We describe several ways to present safety data using graphical presentation of psoriasis treatment safety data as an example and explain how each one can affect patients’ perception of treatment risks.
Approaches to Presenting Safety Data
There are numerous ways to present safety data to patients, including verbal, numeric, and visual strategies.12 Many methods of presentation are a combination of these strategies. Graphs are visual strategies to further categorize and present numeric data, and physicians may choose to incorporate these aids when presenting safety information to patients. Graphical presentations give the patient a mental picture of the data. Numerous types of graphs can be constructed. Kalb et al13 determined the effect of psoriasis treatment on the risk of serious infection from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). We used the results from this study to demonstrate multiple ways of presenting safety data (Figures 1–3).
A graphical presentation with a truncated y-axis is a common approach (Figure 1). Graphs with truncated axes are sometimes used to conserve space or to accentuate certain differences in the graph that would otherwise be less obvious without the zoomed in y-axis.14 These graphs present quantitatively accurate information that can be visually misleading at the same time. Truncated axes accentuate differences, creating mental impressions that are not reflective of the magnitude of the numeric differences. Alternatively, a graph with a full y-axis includes both the maximum and minimum data values on the y-axis (Figure 2). The y-axis also extends maximally to the total number of patients or patient-years studied. This type of graph presents all of the numeric data without distortion.


A graph also can present the percentage of patients or patient-years that do not have an adverse effect (Figure 3). This inverse presentation of the data does not emphasize rare cases of patients who have had adverse effects; instead, it emphasizes the large percentage of patients who did not have adverse effects and presents a far more reassuring perspective, even though mathematically the information is identical.

Focus on the Patients Who Do Not Have Adverse Effects of Treatments
Fear of adverse effects is one of the most commonly reported causes of poor treatment adherence.15 New therapies for psoriasis are highly effective and safe, but as with all treatments, they also are associated with some risks. Patients may latch onto those risks too tightly or perhaps, in other circumstances, not tightly enough. The method used by a physician to present safety data to a patient may determine the patient’s perception about treatments.
When trying to give patients an accurate impression of treatment risks, it may be helpful to avoid approaches that focus on presenting the (few) cases of severe adverse drug effects since patients (and physicians) are likely to overweigh the unlikely risk of having an adverse effect if presented with this information. It may be more reassuring to focus on presenting information about the chance of not having an adverse drug effect, assuming the physician’s goal is to be reassuring.
Poor communication with patients when presenting safety data can foster exaggerated fears of an unlikely consequence to the point that patients can be left undertreated and sustaining disease symptoms.16 Physicians may strive to do no harm to their patients, but without careful presentation of safety data in the process of helping the patient make an informed decision, it is possible to do mental harm to patients in the form of fear or even, in the case of nonadherence or treatment refusal, physical harm in the form of continued disease symptoms.
One limitation of this review is that we only used graphical presentation of data as an example. Similar concerns apply to numerical data presentation. Telling a patient the risk of a severe adverse reaction is doubled by a certain treatment may be terrifying, though if the baseline risk is rare, doubling the baseline risk may represent only a minimal increase in the absolute risk. Telling a patient the risk is only 1 in 1000 may still be alarming because many patients tend to focus on the 1, but telling a patient that 999 of 1000 patients do not have a problem can be much more reassuring.
The physician’s goal—to help patients make informed decisions about their treatment—calls for him/her to assimilate safety data into useful information that the patient can use to make an informed decision.17 Overly comforting or alarming, confusing, and inaccurate information can misguide the patient, violating the ethical principle of nonmaleficence. Although there is an obligation to educate patients about risks, there may not be a purely objective way to do it. When physicians present objective data to patients, whether in numerical or graphical form, there will be an unavoidable subjective interpretation of the data. The form of presentation will have a critical effect on patients’ subjective perceptions. Physicians can present objective data in such a way as to be reassuring or frightening.
Conclusion
Despite physicians’ best-intentioned efforts, it may be impossible to avoid presenting safety data in a way that will be subjectively interpreted by patients. Physicians have a choice in how they present data to patients; their best judgment should be used in how they present data to inform patients, guide them, and offer them the best treatment outcomes.
Acknowledgment
We thank Scott Jaros, BA (Winston-Salem, North Carolina), for his assistance in the revision of the manuscript.
The Nuremberg Code in 1947,1 the Declaration of Helsinki in 1964,2 and the Belmont Report in 19793 were cornerstones in the establishment of ethical principles in the medical field. These documents specifically highlight the concept of informed consent, which maintains that to practice ethical medicine, physicians must fully inform patients of all therapeutic benefits and especially risks as well as treatment alternatives before they consent to therapeutic intervention. Educating patients about risks of treatment is obligatory. Risk communication involves a mutual exchange of information between physicians and patients; the physician presents risk information in an understandable manner that adequately conveys pertinent data that is critical for the patient to make an informed therapeutic decision.4
An inherent problem with risk education is that patients may be terrified about risks associated with treatment. Some patients will refuse needed treatment because of fear.5 When patients have concerns about the safety profile of a treatment regimen and potential adverse effects, they may be less compliant with treatment.6 The intelligent noncompliance phenomenon occurs when a patient knowingly makes the choice to not adhere to treatment, and concern regarding treatment risks relative to benefits is a common reason underlying this phenomenon.7,8
Behavioral economists have studied how individuals weigh risks. Kahneman and Tversky’s9 prospect theory asserts that individuals tend to overweigh unlikely risks and underweigh more certain risks, which they call the certainty effect; it is the basis of the human tendency to avoid risks in situations of likely gain and to pursue risks in situations of likely loss. The tendency to overweigh rare risks is even more pronounced for affect-rich events such as serious side effects.10 The way data are presented can affect how patients interpret the information. Context and framing of data affect patients’ perceptions.11 We describe several ways to present safety data using graphical presentation of psoriasis treatment safety data as an example and explain how each one can affect patients’ perception of treatment risks.
Approaches to Presenting Safety Data
There are numerous ways to present safety data to patients, including verbal, numeric, and visual strategies.12 Many methods of presentation are a combination of these strategies. Graphs are visual strategies to further categorize and present numeric data, and physicians may choose to incorporate these aids when presenting safety information to patients. Graphical presentations give the patient a mental picture of the data. Numerous types of graphs can be constructed. Kalb et al13 determined the effect of psoriasis treatment on the risk of serious infection from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). We used the results from this study to demonstrate multiple ways of presenting safety data (Figures 1–3).
A graphical presentation with a truncated y-axis is a common approach (Figure 1). Graphs with truncated axes are sometimes used to conserve space or to accentuate certain differences in the graph that would otherwise be less obvious without the zoomed in y-axis.14 These graphs present quantitatively accurate information that can be visually misleading at the same time. Truncated axes accentuate differences, creating mental impressions that are not reflective of the magnitude of the numeric differences. Alternatively, a graph with a full y-axis includes both the maximum and minimum data values on the y-axis (Figure 2). The y-axis also extends maximally to the total number of patients or patient-years studied. This type of graph presents all of the numeric data without distortion.


A graph also can present the percentage of patients or patient-years that do not have an adverse effect (Figure 3). This inverse presentation of the data does not emphasize rare cases of patients who have had adverse effects; instead, it emphasizes the large percentage of patients who did not have adverse effects and presents a far more reassuring perspective, even though mathematically the information is identical.

Focus on the Patients Who Do Not Have Adverse Effects of Treatments
Fear of adverse effects is one of the most commonly reported causes of poor treatment adherence.15 New therapies for psoriasis are highly effective and safe, but as with all treatments, they also are associated with some risks. Patients may latch onto those risks too tightly or perhaps, in other circumstances, not tightly enough. The method used by a physician to present safety data to a patient may determine the patient’s perception about treatments.
When trying to give patients an accurate impression of treatment risks, it may be helpful to avoid approaches that focus on presenting the (few) cases of severe adverse drug effects since patients (and physicians) are likely to overweigh the unlikely risk of having an adverse effect if presented with this information. It may be more reassuring to focus on presenting information about the chance of not having an adverse drug effect, assuming the physician’s goal is to be reassuring.
Poor communication with patients when presenting safety data can foster exaggerated fears of an unlikely consequence to the point that patients can be left undertreated and sustaining disease symptoms.16 Physicians may strive to do no harm to their patients, but without careful presentation of safety data in the process of helping the patient make an informed decision, it is possible to do mental harm to patients in the form of fear or even, in the case of nonadherence or treatment refusal, physical harm in the form of continued disease symptoms.
One limitation of this review is that we only used graphical presentation of data as an example. Similar concerns apply to numerical data presentation. Telling a patient the risk of a severe adverse reaction is doubled by a certain treatment may be terrifying, though if the baseline risk is rare, doubling the baseline risk may represent only a minimal increase in the absolute risk. Telling a patient the risk is only 1 in 1000 may still be alarming because many patients tend to focus on the 1, but telling a patient that 999 of 1000 patients do not have a problem can be much more reassuring.
The physician’s goal—to help patients make informed decisions about their treatment—calls for him/her to assimilate safety data into useful information that the patient can use to make an informed decision.17 Overly comforting or alarming, confusing, and inaccurate information can misguide the patient, violating the ethical principle of nonmaleficence. Although there is an obligation to educate patients about risks, there may not be a purely objective way to do it. When physicians present objective data to patients, whether in numerical or graphical form, there will be an unavoidable subjective interpretation of the data. The form of presentation will have a critical effect on patients’ subjective perceptions. Physicians can present objective data in such a way as to be reassuring or frightening.
Conclusion
Despite physicians’ best-intentioned efforts, it may be impossible to avoid presenting safety data in a way that will be subjectively interpreted by patients. Physicians have a choice in how they present data to patients; their best judgment should be used in how they present data to inform patients, guide them, and offer them the best treatment outcomes.
Acknowledgment
We thank Scott Jaros, BA (Winston-Salem, North Carolina), for his assistance in the revision of the manuscript.
- Freyhofer HH. The Nuremberg Medical Trial: The Holocaust and the Origin of the Nuremberg Medical Code. New York, NY: Peter Lang Publishing; 2004.
- Carlson R, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713.
- Office for Human Research Protections. The Belmont Report. Rockville, MD: US Department of Health and Human Services; 1979.
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827-830.
- Hayden C, Neame R, Tarrant C. Patients’ adherence-related beliefs about methotrexate: a qualitative study of the role of written patient information. BMJ Open. 2015;5:e006918.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Weintraub M. Intelligent noncompliance with special emphasis on the elderly. Contemp Pharm Pract. 1981;4:8-11.
- Horne R. Representations of medication and treatment: advances in theory and measurement. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. London, England: Routledge, Taylor & Francis Group; 1997:155-188.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263-291.
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185-190.
- Kessler JB, Zhang CY. Behavioural economics and health. In: Detels R, Gulliford M, Abdool Karim Q, et al, eds. Oxford Textbook of Global Public Health. 6th ed. Oxford, UK: Oxford University Press; 2015:775-789.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations [published online September 14, 2007]. Med Decis Making. 2007;27:696-713.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Rensberger B. Slanting the slopes of graphs. The Washington Post. May 10, 1995. http://www.washingtonpost.com/archive/1995/05/10/slanting-the-slope-of-graphs/08a34412-60a2-4719-86e5-d7433938c166/. Accessed September 21, 2016.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26(5, pt 1):607-611.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745-748.
- Freyhofer HH. The Nuremberg Medical Trial: The Holocaust and the Origin of the Nuremberg Medical Code. New York, NY: Peter Lang Publishing; 2004.
- Carlson R, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713.
- Office for Human Research Protections. The Belmont Report. Rockville, MD: US Department of Health and Human Services; 1979.
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827-830.
- Hayden C, Neame R, Tarrant C. Patients’ adherence-related beliefs about methotrexate: a qualitative study of the role of written patient information. BMJ Open. 2015;5:e006918.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Weintraub M. Intelligent noncompliance with special emphasis on the elderly. Contemp Pharm Pract. 1981;4:8-11.
- Horne R. Representations of medication and treatment: advances in theory and measurement. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. London, England: Routledge, Taylor & Francis Group; 1997:155-188.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263-291.
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185-190.
- Kessler JB, Zhang CY. Behavioural economics and health. In: Detels R, Gulliford M, Abdool Karim Q, et al, eds. Oxford Textbook of Global Public Health. 6th ed. Oxford, UK: Oxford University Press; 2015:775-789.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations [published online September 14, 2007]. Med Decis Making. 2007;27:696-713.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Rensberger B. Slanting the slopes of graphs. The Washington Post. May 10, 1995. http://www.washingtonpost.com/archive/1995/05/10/slanting-the-slope-of-graphs/08a34412-60a2-4719-86e5-d7433938c166/. Accessed September 21, 2016.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26(5, pt 1):607-611.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745-748.
Practice Points
- Physicians can guide patients’ perceptions of drug safety by the way safety data are presented.
- For patients who are concerned about rare treatment risks, presenting data on the patients who have not experienced adverse effects can be reassuring.
A Boxed Warning for Inadequate Psoriasis Treatment
The US Food and Drug Administration uses the term boxed warning to highlight potentially dangerous situations associated with prescription drugs. A boxed warning is used when “[T]here is an adverse reaction so serious in proportion to the potential benefit from the drug (e.g., a fatal, life-threatening or permanently disabling adverse reaction) that it is essential that it be considered in assessing the risks and benefits of using the drug.”1 However, drugs are not the only potential cause of severe adverse outcomes in patients with psoriasis. Untreated psoriasis also is a well-established cause of serious morbidity and mortality. What are the risks of inadequate psoriasis treatment?
Psoriasis is associated with an increased risk for cardiovascular disease.2-4 Patients with psoriasis also have a higher prevalence of classic cardiovascular risk factors including smoking, diabetes mellitus, hypertension, obesity, and hyperlipidemia.5,6 Psoriasis is a T-cell mediated disease process driven by IL-23 and TH17 helper cell–derived proinflammatory cytokines, sharing certain genetic aspects with metabolic syndrome.6 Cytokine actions on insulin signaling, lipid metabolism, and adipogenesis may underlie the increased prevalence of metabolic syndrome and cardiovascular risk factors in patients with psoriasis. In addition to treating the cutaneous manifestations of psoriasis, reducing inflammation in these patients reduces C-reactive protein and lipid peroxidation and increases high-density lipoprotein levels.6 Tumor necrosis factor α blockers decrease the risk for cardiovascular disease in patients with psoriasis.7,8 Lower than expected rates of cardiovascular disease also have been reported in a large cohort of psoriasis patients (ie, PSOLAR [Psoriasis Longitudinal Assessment and Registry] registry) being treated with either ustekinumab or tumor necrosis factor α blockers.9
Psoriatic arthritis is a chronic inflammatory disease in which active inflammation results in progressive joint destruction.10 Tumor necrosis factor α inhibitors suppress disease progression, preserve function, and delay destruction of the joints. Ustekinumab also helps control psoriatic arthritis and inhibits radiographic progression of joint disease.11
Importantly, untreated moderate to severe psoriasis is associated with several comorbidities that may lead to early death such as heart attacks and strokes.12 Furthermore, patients not taking biologic medications may have higher death rates than patients taking biologic medications.9 Psoriasis also is associated with tremendous suffering and negative psychosocial effects. The mental and physical impact of the disease is comparable to other major medical conditions (eg, cancer, arthritis, hypertension, heart disease, diabetes, depression).13 Patients also may experience physical discomfort from pain and itching.14 Children with psoriasis may experience bullying, which is associated with an increased number of depressive episodes, thereby increasing their risk for developing psychiatric conditions such as depression and anxiety as adults.15 The stigma associated with psoriasis may affect patients’ ability to build relationships. Patients with psoriasis experience higher divorce rates than patients with other chronic medical conditions, and direct involvement of genital regions may negatively impact patients’ sex lives. Patients have noted that the stigma of psoriasis also is associated with the inability to obtain employment.15 Almost one-third of patients with psoriasis who are either not working or are retired base their work status on their skin condition.16 Furthermore, psoriasis may contribute to economic burden for patients due to indirect costs associated with work absenteeism.17
Adequate treatment of psoriasis improves patients’ physical and psychological health as well as their ability to function in the workplace. However, despite the benefits of treatment, 30% of patients with severe psoriasis and 53% of patients with moderate psoriasis receive no treatment or only topical medications instead of systemic therapies.16 The potential adverse events of inadequate psoriasis treatment far outweigh any potential benefits of withholding treatment. Perhaps a boxed warning should be issued for inadequate treatment of psoriasis patients.
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for industry: warning and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products—content and format. US Food and Drug Administration website. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm075096.pdf. Published October 6, 2011. Accessed August 10, 2016.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Rose S, Sheth NH, Baker JF, et al. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis. 2013;3:273-278.
- Shlyankevich J, Mehta NN, Krueger JG, et al. Accumulating evidence for the association and shared pathogenic mechanisms between psoriasis and cardiovascular-related comorbidities. Am J Med. 2014;127:1148-1153.
- Lee MK, Kim HS, Cho EB, et al. A study of awareness and screening behavior of cardiovascular risk factors in patients with psoriasis and dermatologists. Ann Dermatol. 2015;27:59-65.
- Voiculescu VM, Lupu M, Papagheorghe L, et al. Psoriasis and metabolic syndrome—scientific evidence and therapeutic implications. J Med Life. 2014;7:468-471.
- Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
- Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular comorbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448.
- Chimenti MS, Graceffa D, Perricone R. Anti-TNFα discontinuation in rheumatoid and psoriatic arthritis: is it possible after disease remission [published online Apr 21, 2011]? Autoimmun Rev. 2011;10:636-640.
- Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73:1000-1006.
- Pietrzak A, Bartosinska J, Blaszczyk R, et al. Increased serum level of N-terminal Pro-B-type natriuretic peptide as a possible biomarker of cardiovascular risk in psoriatic patients. J Eur Acad Dermatol Venereol. 2015;29:1010-1014.
- Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3, pt 1):401-407.
- Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
- Garshick MK, Kimball AB. Psoriasis and the life cycle of persistent life effects. Dermatol Clin. 2015;33:25-39.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. August 17, 2014;20. pii:13030/qt48r4w8h2.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
The US Food and Drug Administration uses the term boxed warning to highlight potentially dangerous situations associated with prescription drugs. A boxed warning is used when “[T]here is an adverse reaction so serious in proportion to the potential benefit from the drug (e.g., a fatal, life-threatening or permanently disabling adverse reaction) that it is essential that it be considered in assessing the risks and benefits of using the drug.”1 However, drugs are not the only potential cause of severe adverse outcomes in patients with psoriasis. Untreated psoriasis also is a well-established cause of serious morbidity and mortality. What are the risks of inadequate psoriasis treatment?
Psoriasis is associated with an increased risk for cardiovascular disease.2-4 Patients with psoriasis also have a higher prevalence of classic cardiovascular risk factors including smoking, diabetes mellitus, hypertension, obesity, and hyperlipidemia.5,6 Psoriasis is a T-cell mediated disease process driven by IL-23 and TH17 helper cell–derived proinflammatory cytokines, sharing certain genetic aspects with metabolic syndrome.6 Cytokine actions on insulin signaling, lipid metabolism, and adipogenesis may underlie the increased prevalence of metabolic syndrome and cardiovascular risk factors in patients with psoriasis. In addition to treating the cutaneous manifestations of psoriasis, reducing inflammation in these patients reduces C-reactive protein and lipid peroxidation and increases high-density lipoprotein levels.6 Tumor necrosis factor α blockers decrease the risk for cardiovascular disease in patients with psoriasis.7,8 Lower than expected rates of cardiovascular disease also have been reported in a large cohort of psoriasis patients (ie, PSOLAR [Psoriasis Longitudinal Assessment and Registry] registry) being treated with either ustekinumab or tumor necrosis factor α blockers.9
Psoriatic arthritis is a chronic inflammatory disease in which active inflammation results in progressive joint destruction.10 Tumor necrosis factor α inhibitors suppress disease progression, preserve function, and delay destruction of the joints. Ustekinumab also helps control psoriatic arthritis and inhibits radiographic progression of joint disease.11
Importantly, untreated moderate to severe psoriasis is associated with several comorbidities that may lead to early death such as heart attacks and strokes.12 Furthermore, patients not taking biologic medications may have higher death rates than patients taking biologic medications.9 Psoriasis also is associated with tremendous suffering and negative psychosocial effects. The mental and physical impact of the disease is comparable to other major medical conditions (eg, cancer, arthritis, hypertension, heart disease, diabetes, depression).13 Patients also may experience physical discomfort from pain and itching.14 Children with psoriasis may experience bullying, which is associated with an increased number of depressive episodes, thereby increasing their risk for developing psychiatric conditions such as depression and anxiety as adults.15 The stigma associated with psoriasis may affect patients’ ability to build relationships. Patients with psoriasis experience higher divorce rates than patients with other chronic medical conditions, and direct involvement of genital regions may negatively impact patients’ sex lives. Patients have noted that the stigma of psoriasis also is associated with the inability to obtain employment.15 Almost one-third of patients with psoriasis who are either not working or are retired base their work status on their skin condition.16 Furthermore, psoriasis may contribute to economic burden for patients due to indirect costs associated with work absenteeism.17
Adequate treatment of psoriasis improves patients’ physical and psychological health as well as their ability to function in the workplace. However, despite the benefits of treatment, 30% of patients with severe psoriasis and 53% of patients with moderate psoriasis receive no treatment or only topical medications instead of systemic therapies.16 The potential adverse events of inadequate psoriasis treatment far outweigh any potential benefits of withholding treatment. Perhaps a boxed warning should be issued for inadequate treatment of psoriasis patients.
The US Food and Drug Administration uses the term boxed warning to highlight potentially dangerous situations associated with prescription drugs. A boxed warning is used when “[T]here is an adverse reaction so serious in proportion to the potential benefit from the drug (e.g., a fatal, life-threatening or permanently disabling adverse reaction) that it is essential that it be considered in assessing the risks and benefits of using the drug.”1 However, drugs are not the only potential cause of severe adverse outcomes in patients with psoriasis. Untreated psoriasis also is a well-established cause of serious morbidity and mortality. What are the risks of inadequate psoriasis treatment?
Psoriasis is associated with an increased risk for cardiovascular disease.2-4 Patients with psoriasis also have a higher prevalence of classic cardiovascular risk factors including smoking, diabetes mellitus, hypertension, obesity, and hyperlipidemia.5,6 Psoriasis is a T-cell mediated disease process driven by IL-23 and TH17 helper cell–derived proinflammatory cytokines, sharing certain genetic aspects with metabolic syndrome.6 Cytokine actions on insulin signaling, lipid metabolism, and adipogenesis may underlie the increased prevalence of metabolic syndrome and cardiovascular risk factors in patients with psoriasis. In addition to treating the cutaneous manifestations of psoriasis, reducing inflammation in these patients reduces C-reactive protein and lipid peroxidation and increases high-density lipoprotein levels.6 Tumor necrosis factor α blockers decrease the risk for cardiovascular disease in patients with psoriasis.7,8 Lower than expected rates of cardiovascular disease also have been reported in a large cohort of psoriasis patients (ie, PSOLAR [Psoriasis Longitudinal Assessment and Registry] registry) being treated with either ustekinumab or tumor necrosis factor α blockers.9
Psoriatic arthritis is a chronic inflammatory disease in which active inflammation results in progressive joint destruction.10 Tumor necrosis factor α inhibitors suppress disease progression, preserve function, and delay destruction of the joints. Ustekinumab also helps control psoriatic arthritis and inhibits radiographic progression of joint disease.11
Importantly, untreated moderate to severe psoriasis is associated with several comorbidities that may lead to early death such as heart attacks and strokes.12 Furthermore, patients not taking biologic medications may have higher death rates than patients taking biologic medications.9 Psoriasis also is associated with tremendous suffering and negative psychosocial effects. The mental and physical impact of the disease is comparable to other major medical conditions (eg, cancer, arthritis, hypertension, heart disease, diabetes, depression).13 Patients also may experience physical discomfort from pain and itching.14 Children with psoriasis may experience bullying, which is associated with an increased number of depressive episodes, thereby increasing their risk for developing psychiatric conditions such as depression and anxiety as adults.15 The stigma associated with psoriasis may affect patients’ ability to build relationships. Patients with psoriasis experience higher divorce rates than patients with other chronic medical conditions, and direct involvement of genital regions may negatively impact patients’ sex lives. Patients have noted that the stigma of psoriasis also is associated with the inability to obtain employment.15 Almost one-third of patients with psoriasis who are either not working or are retired base their work status on their skin condition.16 Furthermore, psoriasis may contribute to economic burden for patients due to indirect costs associated with work absenteeism.17
Adequate treatment of psoriasis improves patients’ physical and psychological health as well as their ability to function in the workplace. However, despite the benefits of treatment, 30% of patients with severe psoriasis and 53% of patients with moderate psoriasis receive no treatment or only topical medications instead of systemic therapies.16 The potential adverse events of inadequate psoriasis treatment far outweigh any potential benefits of withholding treatment. Perhaps a boxed warning should be issued for inadequate treatment of psoriasis patients.
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for industry: warning and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products—content and format. US Food and Drug Administration website. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm075096.pdf. Published October 6, 2011. Accessed August 10, 2016.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Rose S, Sheth NH, Baker JF, et al. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis. 2013;3:273-278.
- Shlyankevich J, Mehta NN, Krueger JG, et al. Accumulating evidence for the association and shared pathogenic mechanisms between psoriasis and cardiovascular-related comorbidities. Am J Med. 2014;127:1148-1153.
- Lee MK, Kim HS, Cho EB, et al. A study of awareness and screening behavior of cardiovascular risk factors in patients with psoriasis and dermatologists. Ann Dermatol. 2015;27:59-65.
- Voiculescu VM, Lupu M, Papagheorghe L, et al. Psoriasis and metabolic syndrome—scientific evidence and therapeutic implications. J Med Life. 2014;7:468-471.
- Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
- Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular comorbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448.
- Chimenti MS, Graceffa D, Perricone R. Anti-TNFα discontinuation in rheumatoid and psoriatic arthritis: is it possible after disease remission [published online Apr 21, 2011]? Autoimmun Rev. 2011;10:636-640.
- Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73:1000-1006.
- Pietrzak A, Bartosinska J, Blaszczyk R, et al. Increased serum level of N-terminal Pro-B-type natriuretic peptide as a possible biomarker of cardiovascular risk in psoriatic patients. J Eur Acad Dermatol Venereol. 2015;29:1010-1014.
- Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3, pt 1):401-407.
- Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
- Garshick MK, Kimball AB. Psoriasis and the life cycle of persistent life effects. Dermatol Clin. 2015;33:25-39.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. August 17, 2014;20. pii:13030/qt48r4w8h2.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for industry: warning and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products—content and format. US Food and Drug Administration website. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm075096.pdf. Published October 6, 2011. Accessed August 10, 2016.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Rose S, Sheth NH, Baker JF, et al. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis. 2013;3:273-278.
- Shlyankevich J, Mehta NN, Krueger JG, et al. Accumulating evidence for the association and shared pathogenic mechanisms between psoriasis and cardiovascular-related comorbidities. Am J Med. 2014;127:1148-1153.
- Lee MK, Kim HS, Cho EB, et al. A study of awareness and screening behavior of cardiovascular risk factors in patients with psoriasis and dermatologists. Ann Dermatol. 2015;27:59-65.
- Voiculescu VM, Lupu M, Papagheorghe L, et al. Psoriasis and metabolic syndrome—scientific evidence and therapeutic implications. J Med Life. 2014;7:468-471.
- Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
- Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular comorbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448.
- Chimenti MS, Graceffa D, Perricone R. Anti-TNFα discontinuation in rheumatoid and psoriatic arthritis: is it possible after disease remission [published online Apr 21, 2011]? Autoimmun Rev. 2011;10:636-640.
- Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73:1000-1006.
- Pietrzak A, Bartosinska J, Blaszczyk R, et al. Increased serum level of N-terminal Pro-B-type natriuretic peptide as a possible biomarker of cardiovascular risk in psoriatic patients. J Eur Acad Dermatol Venereol. 2015;29:1010-1014.
- Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3, pt 1):401-407.
- Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
- Garshick MK, Kimball AB. Psoriasis and the life cycle of persistent life effects. Dermatol Clin. 2015;33:25-39.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. August 17, 2014;20. pii:13030/qt48r4w8h2.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
A Review of Patient Adherence to Topical Therapies for Treatment of Atopic Dermatitis
Atopic dermatitis (AD) is a chronic inflammatory skin disease that typically begins in early childhood (Figure). It is one of the most commonly diagnosed dermatologic conditions, affecting up to 25% of children and 2% to 3% of adults in the United States.1,2 The mainstays of treatment for AD are topical emollients and topical medications, of which corticosteroids are most commonly prescribed.3 Although treatments for AD generally are straightforward and efficacious when used correctly, poor adherence to treatment often prevents patients from achieving disease control.4 Patient adherence to therapy is a familiar challenge in dermatology, especially for diseases like AD that require long-term treatment with topical medications.4,5 In some instances, poor adherence may be misconstrued as poor response to treatment, which may lead to escalation to more powerful and potentially dangerous systemic medications.6 Ensuring good adherence to treatment leads to better outcomes and disease control, averts unnecessary treatment, prevents disease complications, improves quality of life, and decreases treatment cost.4,5 This article provides a review of the literature on patient adherence to topical therapies for AD as well as a discussion of methods to improve patient adherence to treatment in the clinical setting.
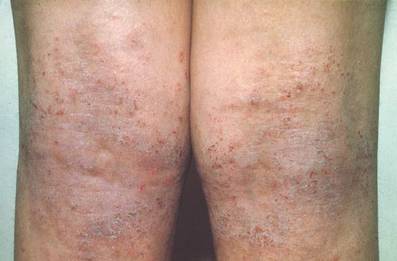
Methods
A PubMed search of articles indexed for MEDLINE from January 2005 to May 2015 was conducted to identify studies that focused on treatment adherence in AD using the search terms atopic dermatitis and medication adherence and atopic dermatitis and patient compliance After excluding duplicate results and those that were not in the English language, a final list of clinical trials that investigated patient adherence/compliance to topical medications for the treatment of AD was extracted for evaluation.
Results
Our review of the literature yielded 7 quantitative studies that evaluated adherence to topical medications in AD using electronic monitoring and/or self-reporting (Table).7-13 Participant demographics, disease severity, drug and vehicle used, duration of treatment, and number of follow-up visits varied. All studies used medication event monitoring system caps on medication jars to objectively track patient adherence by recording the date and time when the cap was removed. To assess disease response, the studies used such measures as the Investigator Global Assessment scale, Eczema Area and Severity Index score, or other visual analog scales.
In all of the studies, treatment proved effective and disease severity declined from baseline regardless of the rate of adherence, with benefit continuing after treatment had ended.7-13 Some results suggested that better adherence increased treatment efficacy and reduced disease severity.8,9 However, one 10-day trial found no difference in severity and efficacy among participants who applied the medication at least once daily, missed applications some days, or applied the medication more than twice daily.13
Study participants typically overestimated their adherence to treatment compared to actual adherence rates, with most reporting near 100% adherence.7-9,11,12 Average measured adherence rates ranged from 32% to 93% (Table). Adherence rates typically were highest at the beginning of the study and decreased as the study continued.7-13 The study with the best average adherence rate of 93% had the shortest treatment period of 3 days,11 and the study with the lowest average adherence rate of 32% had the longest treatment period of 8 weeks.7 The study with the lowest adherence rate was the only study wherein participants were blinded to their enrollment in the study, which would most closely mimic adherence rates in clinical practice.7 The participants in the other studies were not aware that their adherence was being monitored, but their behavior may have been influenced since they were aware of their enrollment in the study.
Many variables affect treatment adherence in patients with AD. Average adherence rates were significantly higher (P=.03) in participants with greater disease severity.7 There is conflicting evidence regarding the role of medication vehicle in treatment adherence. While Wilson et al9 did not find any difference in adherence based on medication vehicle, Yentzer et al12 found vehicle characteristics and medication side effects were among patients’ top-ranked concerns about using topical medications. Sagransky et al10 compared treatment adherence between 2 groups of AD patients: one control group received a standard-of-care 4-week follow-up, and an active group received an additional 1-week follow-up. The mean adherence rate of the treatment group was 69% compared with 54% in the control group.10
Comment
Poor adherence to treatment is a pervasive problem in patients with AD. Our review of the literature confirmed that patients generally are not accurate historians of their medication usage, often reporting near-perfect treatment adherence even when actual adherence is poor. Rates of adherence from clinical trials are likely higher than those seen in clinical practice due in part to study incentives and differences between how patients in a study are treated compared to those in a physician’s clinic; for example, research study participants often have additional follow-up visits compared to those being treated in the clinical population and by virtue of being enrolled in a study are aware that their behavior is being monitored, which can increase treatment adherence.7
The dogma suggesting that tachyphylaxis can occur with long-term use of topical corticosteroids is not supported by clinical trials.14 Furthermore, in our review of the literature patient adherence was highest in the shortest study11 and lowest in the longest study.7 Given that AD patients cannot benefit from a treatment if they do not use it, the supposed decrease in efficacy of topical corticosteroids over time may be because patients fail to use them consistently.
Our review of the literature was limited by the small body of research that exists on treatment adherence in AD patients, especially relating to topical medications, and did not reveal any studies evaluating systemic medications in AD. Of the studies we examined, sample sizes were small and treatment and follow-up periods were short. Our review only covered adherence to prescribed topical medications in AD, chiefly corticosteroids; thus, we did not evaluate adherence to other therapies (eg, emollients) in this patient population.
The existing research also is limited by the relative paucity of data showing a correlation between improved adherence to topical treatment and improved disease outcomes, which may be due to the methodological limitations of the study designs that have been used; for instance, studies may use objective monitors to describe daily adherence to treatment, but disease severity typically is measured over longer periods of time, usually every few weeks or months. Short-term data may not be an accurate demonstration of how participants’ actual treatment adherence impacts disease outcome, as the data does not account for more complex adherence factors; for example, participants who achieve good disease control using topical corticosteroids for an 8-week study period may actually demonstrate poor treatment adherence overall, as topical corticosteroids have good short-term efficacy and the patient may have stopped using the product after the first few weeks of the treatment period. In contrast, poorly adherent patients may never use the medication well enough to achieve improvement and may continue low-level use throughout the study period. Therefore, studies that measure disease severity at more regular intervals are required to show the true effect of treatment adherence on disease outcomes.
Since AD mainly affects children, family issues can pose special challenges to attaining good treatment adherence.15,16 The physician–patient (or parent) relationship and the family’s perception of the patient’s disease severity are strong predictors of adherence to topical treatment.16 Potential barriers to adherence in the pediatric population are caregivers with negative beliefs about treatment, the time-consuming nature of applying topical therapies, or a child who is uncooperative.15,17 In the treatment of infants, critical factors are caregiver availability and beliefs and fears about medications and their side effects, while in the teenage population, the desire to “fit in” and oppositional behavior can lead to poor adherence to treatment.17 Regardless of age, other barriers to treatment adherence are forgetfulness, belief that the drug is not working, and the messiness of treatment.17
Educational tools (eg, action plans, instructions about how to apply topical medications correctly) may be underutilized in patients with AD. If consistently implemented, these tools could have a positive impact on adherence to medication in patients with AD. For example, written action plans pioneered in the asthma community have shown to improve quality of life and reduce disease severity and may offer the same benefits for AD patients due to the similarities of the diseases.18 Since AD patients and their caregivers often are not well versed in how to apply topical medications correctly, efforts to educate patients could potentially increase adherence to treatment. In one study, AD patients began to use medications more effectively after applying a fluorescent cream to reveal affected areas they had missed, and clinicians were able to provide additional instruction based on the findings.19
Adherence to topical treatments among AD patients is a multifactorial issue. Regimens often are complex and inconvenient due to the need for multiple medications, the topical nature of the products, and the need for frequent application. To optimize prescription treatments, patients also must be diligent with preventive measures such as application of topical emollients and use of bathing techniques (eg, bleach baths). A way to overcome treatment complexity and increase adherence may be to provide a written action plan and involve the patient and caregiver in the plan’s development. If a drug formulation is not aesthetically acceptable to the patient (eg, the greasiness of an ointment), allowing the patient to choose the medication vehicle may increase satisfaction and use.12 Fear of steroid side effects also is common among patients and caregivers and could be overcome with education about the product.20
Conclusion
Treatment adherence can have a dramatic effect on diseases outcomes and can be particularly challenging in AD due to the use of topical medications with complex treatment regimens. Additionally, a large majority of patients with AD are children, from infants to teenagers, adding another layer of treatment challenges. Further research is needed to more definitively develop effective methods for enhancing treatment adherence in this patient population. Although enormous amounts of money are being spent to develop improved treatments for AD, we may be able to achieve far more benefit at a much lower cost by figuring out how to get patients to adhere to the treatments that are already available.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Landis ET, Davis SA, Taheri A, et al. Top dermatologic diagnoses by age. Dermatol Online J. 2014;20:22368.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Lee IA, Maibach HI. Pharmionics in dermatology: a review of topical medication adherence. Am J Clin Dermatol. 2006;7:231-236.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Conde JF, Kaur M, Fleischer AB Jr, et al. Adherence to clocortolone pivalate cream 0.1% in a pediatric population with atopic dermatitis. Cutis. 2008;81:435-441.
- Wilson R, Camacho F, Clark AR, et al. Adherence to topical hydrocortisone 17-butyrate 0.1% in different vehicles in adults with atopic dermatitis. J Am Acad Dermatol. 2009;60:166-168.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
- Yentzer BA, Ade RA, Fountain JM, et al. Improvement in treatment adherence with a 3-day course of fluocinonide cream 0.1% for atopic dermatitis. Cutis. 2010;86:208-213.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Hix E, Gustafson CJ, O’Neill JL, et al. Adherence to a five day treatment course of topical fluocinonide 0.1% cream in atopic dermatitis. Dermatol Online J. 2013;19:20029.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Santer M, Burgess H, Yardley L, et al. Managing childhood eczema: qualitative study exploring carers’ experiences of barriers and facilitators to treatment adherence. J Adv Nurs. 2013;69:2493-2501.
- Ohya Y, Williams H, Steptoe A, et al. Psychosocial factors and adherence to treatment advice in childhood atopic dermatitis. J Invest Dermatol. 2001;117:852-857.
- Ou HT, Feldman SR, Balkrishnan R. Understanding and improving treatment adherence in pediatric patients. Semin Cutan Med Surg. 2010;29:137-140.
- Chisolm SS, Taylor SL, Balkrishnan R, et al. Written action plans: potential for improving outcomes in children with atopic dermatitis. J Am Acad Dermatol. 2008;59:677-683.
- Ulff E, Maroti M, Serup J. Fluorescent cream used as an educational intervention to improve the effectiveness of self-application by patients with atopic dermatitis. J Dermatolog Treat. 2013;24:268-271.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
Atopic dermatitis (AD) is a chronic inflammatory skin disease that typically begins in early childhood (Figure). It is one of the most commonly diagnosed dermatologic conditions, affecting up to 25% of children and 2% to 3% of adults in the United States.1,2 The mainstays of treatment for AD are topical emollients and topical medications, of which corticosteroids are most commonly prescribed.3 Although treatments for AD generally are straightforward and efficacious when used correctly, poor adherence to treatment often prevents patients from achieving disease control.4 Patient adherence to therapy is a familiar challenge in dermatology, especially for diseases like AD that require long-term treatment with topical medications.4,5 In some instances, poor adherence may be misconstrued as poor response to treatment, which may lead to escalation to more powerful and potentially dangerous systemic medications.6 Ensuring good adherence to treatment leads to better outcomes and disease control, averts unnecessary treatment, prevents disease complications, improves quality of life, and decreases treatment cost.4,5 This article provides a review of the literature on patient adherence to topical therapies for AD as well as a discussion of methods to improve patient adherence to treatment in the clinical setting.

Methods
A PubMed search of articles indexed for MEDLINE from January 2005 to May 2015 was conducted to identify studies that focused on treatment adherence in AD using the search terms atopic dermatitis and medication adherence and atopic dermatitis and patient compliance After excluding duplicate results and those that were not in the English language, a final list of clinical trials that investigated patient adherence/compliance to topical medications for the treatment of AD was extracted for evaluation.
Results
Our review of the literature yielded 7 quantitative studies that evaluated adherence to topical medications in AD using electronic monitoring and/or self-reporting (Table).7-13 Participant demographics, disease severity, drug and vehicle used, duration of treatment, and number of follow-up visits varied. All studies used medication event monitoring system caps on medication jars to objectively track patient adherence by recording the date and time when the cap was removed. To assess disease response, the studies used such measures as the Investigator Global Assessment scale, Eczema Area and Severity Index score, or other visual analog scales.
In all of the studies, treatment proved effective and disease severity declined from baseline regardless of the rate of adherence, with benefit continuing after treatment had ended.7-13 Some results suggested that better adherence increased treatment efficacy and reduced disease severity.8,9 However, one 10-day trial found no difference in severity and efficacy among participants who applied the medication at least once daily, missed applications some days, or applied the medication more than twice daily.13
Study participants typically overestimated their adherence to treatment compared to actual adherence rates, with most reporting near 100% adherence.7-9,11,12 Average measured adherence rates ranged from 32% to 93% (Table). Adherence rates typically were highest at the beginning of the study and decreased as the study continued.7-13 The study with the best average adherence rate of 93% had the shortest treatment period of 3 days,11 and the study with the lowest average adherence rate of 32% had the longest treatment period of 8 weeks.7 The study with the lowest adherence rate was the only study wherein participants were blinded to their enrollment in the study, which would most closely mimic adherence rates in clinical practice.7 The participants in the other studies were not aware that their adherence was being monitored, but their behavior may have been influenced since they were aware of their enrollment in the study.
Many variables affect treatment adherence in patients with AD. Average adherence rates were significantly higher (P=.03) in participants with greater disease severity.7 There is conflicting evidence regarding the role of medication vehicle in treatment adherence. While Wilson et al9 did not find any difference in adherence based on medication vehicle, Yentzer et al12 found vehicle characteristics and medication side effects were among patients’ top-ranked concerns about using topical medications. Sagransky et al10 compared treatment adherence between 2 groups of AD patients: one control group received a standard-of-care 4-week follow-up, and an active group received an additional 1-week follow-up. The mean adherence rate of the treatment group was 69% compared with 54% in the control group.10
Comment
Poor adherence to treatment is a pervasive problem in patients with AD. Our review of the literature confirmed that patients generally are not accurate historians of their medication usage, often reporting near-perfect treatment adherence even when actual adherence is poor. Rates of adherence from clinical trials are likely higher than those seen in clinical practice due in part to study incentives and differences between how patients in a study are treated compared to those in a physician’s clinic; for example, research study participants often have additional follow-up visits compared to those being treated in the clinical population and by virtue of being enrolled in a study are aware that their behavior is being monitored, which can increase treatment adherence.7
The dogma suggesting that tachyphylaxis can occur with long-term use of topical corticosteroids is not supported by clinical trials.14 Furthermore, in our review of the literature patient adherence was highest in the shortest study11 and lowest in the longest study.7 Given that AD patients cannot benefit from a treatment if they do not use it, the supposed decrease in efficacy of topical corticosteroids over time may be because patients fail to use them consistently.
Our review of the literature was limited by the small body of research that exists on treatment adherence in AD patients, especially relating to topical medications, and did not reveal any studies evaluating systemic medications in AD. Of the studies we examined, sample sizes were small and treatment and follow-up periods were short. Our review only covered adherence to prescribed topical medications in AD, chiefly corticosteroids; thus, we did not evaluate adherence to other therapies (eg, emollients) in this patient population.
The existing research also is limited by the relative paucity of data showing a correlation between improved adherence to topical treatment and improved disease outcomes, which may be due to the methodological limitations of the study designs that have been used; for instance, studies may use objective monitors to describe daily adherence to treatment, but disease severity typically is measured over longer periods of time, usually every few weeks or months. Short-term data may not be an accurate demonstration of how participants’ actual treatment adherence impacts disease outcome, as the data does not account for more complex adherence factors; for example, participants who achieve good disease control using topical corticosteroids for an 8-week study period may actually demonstrate poor treatment adherence overall, as topical corticosteroids have good short-term efficacy and the patient may have stopped using the product after the first few weeks of the treatment period. In contrast, poorly adherent patients may never use the medication well enough to achieve improvement and may continue low-level use throughout the study period. Therefore, studies that measure disease severity at more regular intervals are required to show the true effect of treatment adherence on disease outcomes.
Since AD mainly affects children, family issues can pose special challenges to attaining good treatment adherence.15,16 The physician–patient (or parent) relationship and the family’s perception of the patient’s disease severity are strong predictors of adherence to topical treatment.16 Potential barriers to adherence in the pediatric population are caregivers with negative beliefs about treatment, the time-consuming nature of applying topical therapies, or a child who is uncooperative.15,17 In the treatment of infants, critical factors are caregiver availability and beliefs and fears about medications and their side effects, while in the teenage population, the desire to “fit in” and oppositional behavior can lead to poor adherence to treatment.17 Regardless of age, other barriers to treatment adherence are forgetfulness, belief that the drug is not working, and the messiness of treatment.17
Educational tools (eg, action plans, instructions about how to apply topical medications correctly) may be underutilized in patients with AD. If consistently implemented, these tools could have a positive impact on adherence to medication in patients with AD. For example, written action plans pioneered in the asthma community have shown to improve quality of life and reduce disease severity and may offer the same benefits for AD patients due to the similarities of the diseases.18 Since AD patients and their caregivers often are not well versed in how to apply topical medications correctly, efforts to educate patients could potentially increase adherence to treatment. In one study, AD patients began to use medications more effectively after applying a fluorescent cream to reveal affected areas they had missed, and clinicians were able to provide additional instruction based on the findings.19
Adherence to topical treatments among AD patients is a multifactorial issue. Regimens often are complex and inconvenient due to the need for multiple medications, the topical nature of the products, and the need for frequent application. To optimize prescription treatments, patients also must be diligent with preventive measures such as application of topical emollients and use of bathing techniques (eg, bleach baths). A way to overcome treatment complexity and increase adherence may be to provide a written action plan and involve the patient and caregiver in the plan’s development. If a drug formulation is not aesthetically acceptable to the patient (eg, the greasiness of an ointment), allowing the patient to choose the medication vehicle may increase satisfaction and use.12 Fear of steroid side effects also is common among patients and caregivers and could be overcome with education about the product.20
Conclusion
Treatment adherence can have a dramatic effect on diseases outcomes and can be particularly challenging in AD due to the use of topical medications with complex treatment regimens. Additionally, a large majority of patients with AD are children, from infants to teenagers, adding another layer of treatment challenges. Further research is needed to more definitively develop effective methods for enhancing treatment adherence in this patient population. Although enormous amounts of money are being spent to develop improved treatments for AD, we may be able to achieve far more benefit at a much lower cost by figuring out how to get patients to adhere to the treatments that are already available.
Atopic dermatitis (AD) is a chronic inflammatory skin disease that typically begins in early childhood (Figure). It is one of the most commonly diagnosed dermatologic conditions, affecting up to 25% of children and 2% to 3% of adults in the United States.1,2 The mainstays of treatment for AD are topical emollients and topical medications, of which corticosteroids are most commonly prescribed.3 Although treatments for AD generally are straightforward and efficacious when used correctly, poor adherence to treatment often prevents patients from achieving disease control.4 Patient adherence to therapy is a familiar challenge in dermatology, especially for diseases like AD that require long-term treatment with topical medications.4,5 In some instances, poor adherence may be misconstrued as poor response to treatment, which may lead to escalation to more powerful and potentially dangerous systemic medications.6 Ensuring good adherence to treatment leads to better outcomes and disease control, averts unnecessary treatment, prevents disease complications, improves quality of life, and decreases treatment cost.4,5 This article provides a review of the literature on patient adherence to topical therapies for AD as well as a discussion of methods to improve patient adherence to treatment in the clinical setting.

Methods
A PubMed search of articles indexed for MEDLINE from January 2005 to May 2015 was conducted to identify studies that focused on treatment adherence in AD using the search terms atopic dermatitis and medication adherence and atopic dermatitis and patient compliance After excluding duplicate results and those that were not in the English language, a final list of clinical trials that investigated patient adherence/compliance to topical medications for the treatment of AD was extracted for evaluation.
Results
Our review of the literature yielded 7 quantitative studies that evaluated adherence to topical medications in AD using electronic monitoring and/or self-reporting (Table).7-13 Participant demographics, disease severity, drug and vehicle used, duration of treatment, and number of follow-up visits varied. All studies used medication event monitoring system caps on medication jars to objectively track patient adherence by recording the date and time when the cap was removed. To assess disease response, the studies used such measures as the Investigator Global Assessment scale, Eczema Area and Severity Index score, or other visual analog scales.
In all of the studies, treatment proved effective and disease severity declined from baseline regardless of the rate of adherence, with benefit continuing after treatment had ended.7-13 Some results suggested that better adherence increased treatment efficacy and reduced disease severity.8,9 However, one 10-day trial found no difference in severity and efficacy among participants who applied the medication at least once daily, missed applications some days, or applied the medication more than twice daily.13
Study participants typically overestimated their adherence to treatment compared to actual adherence rates, with most reporting near 100% adherence.7-9,11,12 Average measured adherence rates ranged from 32% to 93% (Table). Adherence rates typically were highest at the beginning of the study and decreased as the study continued.7-13 The study with the best average adherence rate of 93% had the shortest treatment period of 3 days,11 and the study with the lowest average adherence rate of 32% had the longest treatment period of 8 weeks.7 The study with the lowest adherence rate was the only study wherein participants were blinded to their enrollment in the study, which would most closely mimic adherence rates in clinical practice.7 The participants in the other studies were not aware that their adherence was being monitored, but their behavior may have been influenced since they were aware of their enrollment in the study.
Many variables affect treatment adherence in patients with AD. Average adherence rates were significantly higher (P=.03) in participants with greater disease severity.7 There is conflicting evidence regarding the role of medication vehicle in treatment adherence. While Wilson et al9 did not find any difference in adherence based on medication vehicle, Yentzer et al12 found vehicle characteristics and medication side effects were among patients’ top-ranked concerns about using topical medications. Sagransky et al10 compared treatment adherence between 2 groups of AD patients: one control group received a standard-of-care 4-week follow-up, and an active group received an additional 1-week follow-up. The mean adherence rate of the treatment group was 69% compared with 54% in the control group.10
Comment
Poor adherence to treatment is a pervasive problem in patients with AD. Our review of the literature confirmed that patients generally are not accurate historians of their medication usage, often reporting near-perfect treatment adherence even when actual adherence is poor. Rates of adherence from clinical trials are likely higher than those seen in clinical practice due in part to study incentives and differences between how patients in a study are treated compared to those in a physician’s clinic; for example, research study participants often have additional follow-up visits compared to those being treated in the clinical population and by virtue of being enrolled in a study are aware that their behavior is being monitored, which can increase treatment adherence.7
The dogma suggesting that tachyphylaxis can occur with long-term use of topical corticosteroids is not supported by clinical trials.14 Furthermore, in our review of the literature patient adherence was highest in the shortest study11 and lowest in the longest study.7 Given that AD patients cannot benefit from a treatment if they do not use it, the supposed decrease in efficacy of topical corticosteroids over time may be because patients fail to use them consistently.
Our review of the literature was limited by the small body of research that exists on treatment adherence in AD patients, especially relating to topical medications, and did not reveal any studies evaluating systemic medications in AD. Of the studies we examined, sample sizes were small and treatment and follow-up periods were short. Our review only covered adherence to prescribed topical medications in AD, chiefly corticosteroids; thus, we did not evaluate adherence to other therapies (eg, emollients) in this patient population.
The existing research also is limited by the relative paucity of data showing a correlation between improved adherence to topical treatment and improved disease outcomes, which may be due to the methodological limitations of the study designs that have been used; for instance, studies may use objective monitors to describe daily adherence to treatment, but disease severity typically is measured over longer periods of time, usually every few weeks or months. Short-term data may not be an accurate demonstration of how participants’ actual treatment adherence impacts disease outcome, as the data does not account for more complex adherence factors; for example, participants who achieve good disease control using topical corticosteroids for an 8-week study period may actually demonstrate poor treatment adherence overall, as topical corticosteroids have good short-term efficacy and the patient may have stopped using the product after the first few weeks of the treatment period. In contrast, poorly adherent patients may never use the medication well enough to achieve improvement and may continue low-level use throughout the study period. Therefore, studies that measure disease severity at more regular intervals are required to show the true effect of treatment adherence on disease outcomes.
Since AD mainly affects children, family issues can pose special challenges to attaining good treatment adherence.15,16 The physician–patient (or parent) relationship and the family’s perception of the patient’s disease severity are strong predictors of adherence to topical treatment.16 Potential barriers to adherence in the pediatric population are caregivers with negative beliefs about treatment, the time-consuming nature of applying topical therapies, or a child who is uncooperative.15,17 In the treatment of infants, critical factors are caregiver availability and beliefs and fears about medications and their side effects, while in the teenage population, the desire to “fit in” and oppositional behavior can lead to poor adherence to treatment.17 Regardless of age, other barriers to treatment adherence are forgetfulness, belief that the drug is not working, and the messiness of treatment.17
Educational tools (eg, action plans, instructions about how to apply topical medications correctly) may be underutilized in patients with AD. If consistently implemented, these tools could have a positive impact on adherence to medication in patients with AD. For example, written action plans pioneered in the asthma community have shown to improve quality of life and reduce disease severity and may offer the same benefits for AD patients due to the similarities of the diseases.18 Since AD patients and their caregivers often are not well versed in how to apply topical medications correctly, efforts to educate patients could potentially increase adherence to treatment. In one study, AD patients began to use medications more effectively after applying a fluorescent cream to reveal affected areas they had missed, and clinicians were able to provide additional instruction based on the findings.19
Adherence to topical treatments among AD patients is a multifactorial issue. Regimens often are complex and inconvenient due to the need for multiple medications, the topical nature of the products, and the need for frequent application. To optimize prescription treatments, patients also must be diligent with preventive measures such as application of topical emollients and use of bathing techniques (eg, bleach baths). A way to overcome treatment complexity and increase adherence may be to provide a written action plan and involve the patient and caregiver in the plan’s development. If a drug formulation is not aesthetically acceptable to the patient (eg, the greasiness of an ointment), allowing the patient to choose the medication vehicle may increase satisfaction and use.12 Fear of steroid side effects also is common among patients and caregivers and could be overcome with education about the product.20
Conclusion
Treatment adherence can have a dramatic effect on diseases outcomes and can be particularly challenging in AD due to the use of topical medications with complex treatment regimens. Additionally, a large majority of patients with AD are children, from infants to teenagers, adding another layer of treatment challenges. Further research is needed to more definitively develop effective methods for enhancing treatment adherence in this patient population. Although enormous amounts of money are being spent to develop improved treatments for AD, we may be able to achieve far more benefit at a much lower cost by figuring out how to get patients to adhere to the treatments that are already available.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Landis ET, Davis SA, Taheri A, et al. Top dermatologic diagnoses by age. Dermatol Online J. 2014;20:22368.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Lee IA, Maibach HI. Pharmionics in dermatology: a review of topical medication adherence. Am J Clin Dermatol. 2006;7:231-236.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Conde JF, Kaur M, Fleischer AB Jr, et al. Adherence to clocortolone pivalate cream 0.1% in a pediatric population with atopic dermatitis. Cutis. 2008;81:435-441.
- Wilson R, Camacho F, Clark AR, et al. Adherence to topical hydrocortisone 17-butyrate 0.1% in different vehicles in adults with atopic dermatitis. J Am Acad Dermatol. 2009;60:166-168.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
- Yentzer BA, Ade RA, Fountain JM, et al. Improvement in treatment adherence with a 3-day course of fluocinonide cream 0.1% for atopic dermatitis. Cutis. 2010;86:208-213.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Hix E, Gustafson CJ, O’Neill JL, et al. Adherence to a five day treatment course of topical fluocinonide 0.1% cream in atopic dermatitis. Dermatol Online J. 2013;19:20029.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Santer M, Burgess H, Yardley L, et al. Managing childhood eczema: qualitative study exploring carers’ experiences of barriers and facilitators to treatment adherence. J Adv Nurs. 2013;69:2493-2501.
- Ohya Y, Williams H, Steptoe A, et al. Psychosocial factors and adherence to treatment advice in childhood atopic dermatitis. J Invest Dermatol. 2001;117:852-857.
- Ou HT, Feldman SR, Balkrishnan R. Understanding and improving treatment adherence in pediatric patients. Semin Cutan Med Surg. 2010;29:137-140.
- Chisolm SS, Taylor SL, Balkrishnan R, et al. Written action plans: potential for improving outcomes in children with atopic dermatitis. J Am Acad Dermatol. 2008;59:677-683.
- Ulff E, Maroti M, Serup J. Fluorescent cream used as an educational intervention to improve the effectiveness of self-application by patients with atopic dermatitis. J Dermatolog Treat. 2013;24:268-271.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Landis ET, Davis SA, Taheri A, et al. Top dermatologic diagnoses by age. Dermatol Online J. 2014;20:22368.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Lee IA, Maibach HI. Pharmionics in dermatology: a review of topical medication adherence. Am J Clin Dermatol. 2006;7:231-236.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Conde JF, Kaur M, Fleischer AB Jr, et al. Adherence to clocortolone pivalate cream 0.1% in a pediatric population with atopic dermatitis. Cutis. 2008;81:435-441.
- Wilson R, Camacho F, Clark AR, et al. Adherence to topical hydrocortisone 17-butyrate 0.1% in different vehicles in adults with atopic dermatitis. J Am Acad Dermatol. 2009;60:166-168.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
- Yentzer BA, Ade RA, Fountain JM, et al. Improvement in treatment adherence with a 3-day course of fluocinonide cream 0.1% for atopic dermatitis. Cutis. 2010;86:208-213.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Hix E, Gustafson CJ, O’Neill JL, et al. Adherence to a five day treatment course of topical fluocinonide 0.1% cream in atopic dermatitis. Dermatol Online J. 2013;19:20029.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Santer M, Burgess H, Yardley L, et al. Managing childhood eczema: qualitative study exploring carers’ experiences of barriers and facilitators to treatment adherence. J Adv Nurs. 2013;69:2493-2501.
- Ohya Y, Williams H, Steptoe A, et al. Psychosocial factors and adherence to treatment advice in childhood atopic dermatitis. J Invest Dermatol. 2001;117:852-857.
- Ou HT, Feldman SR, Balkrishnan R. Understanding and improving treatment adherence in pediatric patients. Semin Cutan Med Surg. 2010;29:137-140.
- Chisolm SS, Taylor SL, Balkrishnan R, et al. Written action plans: potential for improving outcomes in children with atopic dermatitis. J Am Acad Dermatol. 2008;59:677-683.
- Ulff E, Maroti M, Serup J. Fluorescent cream used as an educational intervention to improve the effectiveness of self-application by patients with atopic dermatitis. J Dermatolog Treat. 2013;24:268-271.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
Practice Points
- When used correctly, topical treatments for atopic dermatitis (AD) generally are straightforward and efficacious, but poor adherence to treatment can prevent patients from achieving disease control.
- Patients tend to overestimate their adherence to topical treatment regimens for AD compared to actual adherence rates.
- Improved treatment adherence in this patient population may be achieved by allowing patients to choose their preferred topical vehicle and providing patient education about how to apply medications effectively; for pediatric patients, AD action plans also may be useful.
Maintaining Adherence to Psoriasis Treatments
Adherence to psoriasis therapies, especially topical therapy, is remarkably poor. Dr. Steven Feldman discusses the patient-physician relationship and ways that physicians can help patients so they're not fearful of taking their medications. He discusses follow-up with patients and special considerations for patients with scalp psoriasis.
The psoriasis audiocast series is created in collaboration with Cutis® and the National Psoriasis Foundation®.
Adherence to psoriasis therapies, especially topical therapy, is remarkably poor. Dr. Steven Feldman discusses the patient-physician relationship and ways that physicians can help patients so they're not fearful of taking their medications. He discusses follow-up with patients and special considerations for patients with scalp psoriasis.
The psoriasis audiocast series is created in collaboration with Cutis® and the National Psoriasis Foundation®.
Adherence to psoriasis therapies, especially topical therapy, is remarkably poor. Dr. Steven Feldman discusses the patient-physician relationship and ways that physicians can help patients so they're not fearful of taking their medications. He discusses follow-up with patients and special considerations for patients with scalp psoriasis.
The psoriasis audiocast series is created in collaboration with Cutis® and the National Psoriasis Foundation®.
Most Common Dermatologic Conditions Encountered by Dermatologists and Nondermatologists
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).
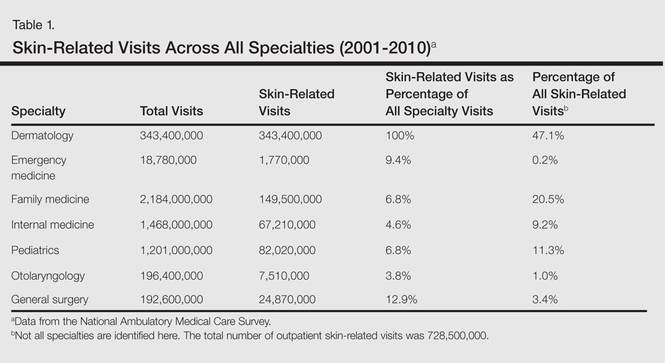
Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).
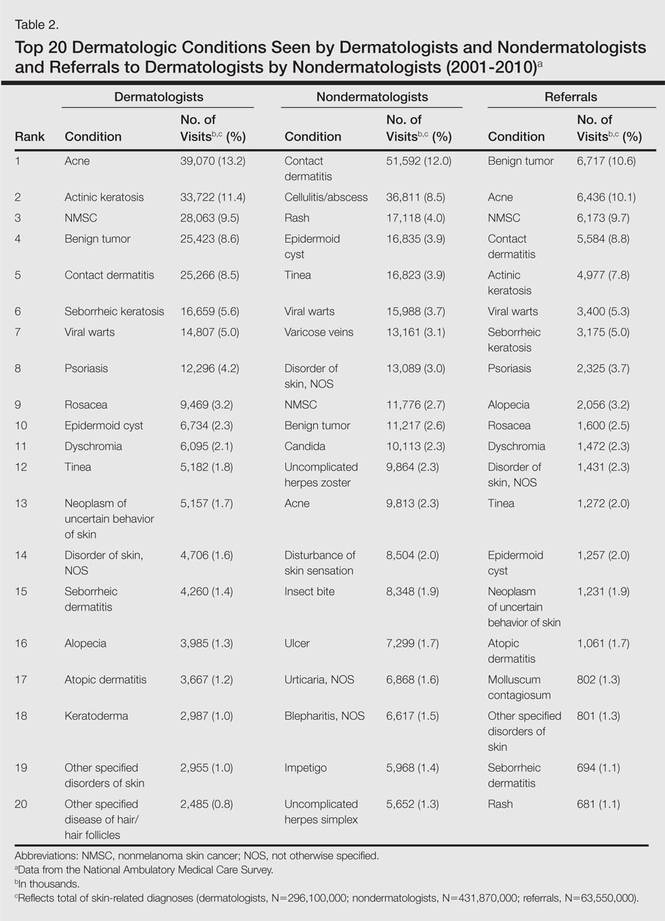
The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.
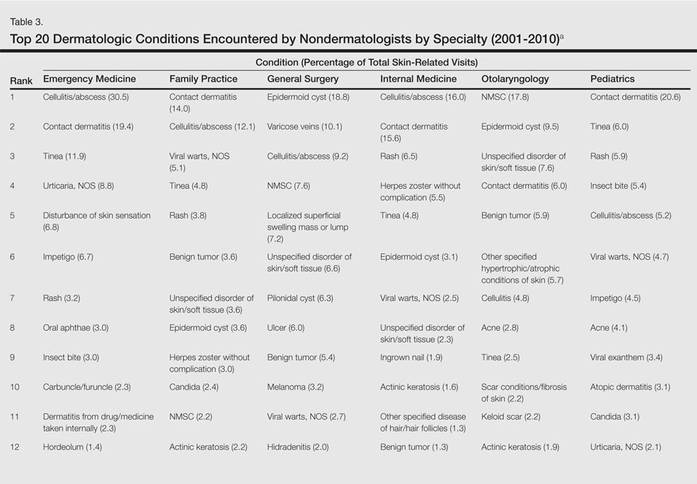
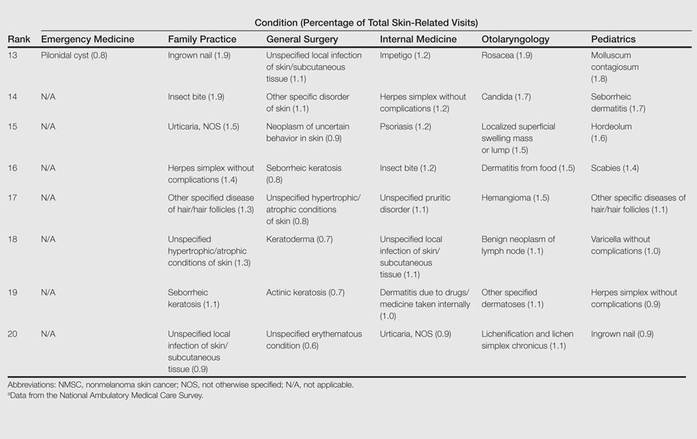
Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).

Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).

The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.


Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).

Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).

The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.


Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
Practice Points
- Approximately half of skin-related visits are to nondermatologists, such as family medicine physicians, pediatricians, and internists.
- Skin conditions that most frequently present to nondermatologists are different from those seen by dermatologists.
- Education efforts in nondermatology specialties should be targeted toward the common skin diseases that present to these specialties to maximize the yield of medical education and improve diagnostic accuracy and patient outcomes.