User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
PCOS associated with shorter lifespan
CHICAGO –
In the study, involving nearly 10,000 women with PCOS and matched controls from Finland, women with PCOS died on average a year earlier than their age-matched counterparts, primarily from diseases of the circulatory system, cancer, and diabetes.
PCOS is the most common endocrine disorder of reproductive-age women, of whom about 50%-70% also have obesity.
“I think we need to acknowledge that this is a health burden and not just a reproductive problem. In many cases we deal with the reproductive problem, and then these women are left alone. … So I think the message is we need to look beyond the reproductive outcomes, which are … really good. We can manage that,” said Terhi T. Piltonen, MD, PhD, during a press briefing held June 15 at the annual meeting of the Endocrine Society.
“I think the difficult part is [managing] the lifelong health for these women and supporting them to achieve the best health they can get. We need a multidisciplinary effort and to put more resources into the research,” added Dr. Piltonen, professor in the departments of ob.gyn. and reproductive endocrinology at the University of Oulu, Finland.
Indeed, Punith Kempegowda, MD, PhD, of the University of Birmingham (England) observed: “In our medical schools in the U.K., over 5 years, students get 45 minutes [of education] on PCOS, and they’re expected to learn about it.”
And over the last 20 years, funding for research into the condition has totaled less than a half percent of overall medical funding. “And we’re talking about 10% of all women. …We need to acknowledge it and educate people more. We need more published studies to understand more about it,” he noted.
Asked to comment, Greg Dodell, MD, owner and president of Central Park Endocrinology, New York, said: “PCOS is about a lot more than fertility, and that may not be the goal or on the mind of a woman at the time they start having symptoms of PCOS or get the diagnosis.”
“PCOS is largely a metabolic condition rooted in insulin resistance, and therefore, the potential clinical outcomes, including mortality, are important to recognize.”
Dr. Dodell, who has a special interest in PCOS, advised that, for women with the condition, “focus on reducing insulin resistance with health-promoting behaviors and medications as needed. Data demonstrate that improving fitness, irrespective of a change in weight, can improve metabolic markers.” And, he advised that these women be routinely screened for mental health issues.
He also noted, “PCOS occurs across the size spectrum, but those patients in larger bodies may face weight stigma which has negative health consequences. These patients may avoid going to doctors for routine health screenings, so it is an important issue to continue to address.”
Women with PCOS lose a year of life
The new data come from 9,839 women with PCOS and 70,705 age- and region-matched controls from the Finnish Care Register for Health Care. The group with PCOS had been diagnosed at a mean age of 27 years.
The mean follow-up time was 13.1 years in both groups, during which 1,003 controls and 177 women with PCOS died. The mean age at death was 51.4 years for the PCOS group versus 52.6 years for the control women, a significant difference (P < .001).
Causes of death that were significantly higher among the women with PCOS versus controls after adjustments were cancer (hazard ratio, 1.39), and diseases of the circulatory system (1.68).
In more specific subcategories, after adjustment for education, the women with PCOS had increased mortality from nonischemic diseases, such as hypertensive heart disease, pulmonary embolism, etc. (HR, 2.06), and diabetes (HR, 2.85).
One study limitation was the inability to adjust for body mass index, Dr. Piltonen noted.
Dr. Piltonen, Dr. Kempegowda, and Dr. Dodell have no disclosures.
CHICAGO –
In the study, involving nearly 10,000 women with PCOS and matched controls from Finland, women with PCOS died on average a year earlier than their age-matched counterparts, primarily from diseases of the circulatory system, cancer, and diabetes.
PCOS is the most common endocrine disorder of reproductive-age women, of whom about 50%-70% also have obesity.
“I think we need to acknowledge that this is a health burden and not just a reproductive problem. In many cases we deal with the reproductive problem, and then these women are left alone. … So I think the message is we need to look beyond the reproductive outcomes, which are … really good. We can manage that,” said Terhi T. Piltonen, MD, PhD, during a press briefing held June 15 at the annual meeting of the Endocrine Society.
“I think the difficult part is [managing] the lifelong health for these women and supporting them to achieve the best health they can get. We need a multidisciplinary effort and to put more resources into the research,” added Dr. Piltonen, professor in the departments of ob.gyn. and reproductive endocrinology at the University of Oulu, Finland.
Indeed, Punith Kempegowda, MD, PhD, of the University of Birmingham (England) observed: “In our medical schools in the U.K., over 5 years, students get 45 minutes [of education] on PCOS, and they’re expected to learn about it.”
And over the last 20 years, funding for research into the condition has totaled less than a half percent of overall medical funding. “And we’re talking about 10% of all women. …We need to acknowledge it and educate people more. We need more published studies to understand more about it,” he noted.
Asked to comment, Greg Dodell, MD, owner and president of Central Park Endocrinology, New York, said: “PCOS is about a lot more than fertility, and that may not be the goal or on the mind of a woman at the time they start having symptoms of PCOS or get the diagnosis.”
“PCOS is largely a metabolic condition rooted in insulin resistance, and therefore, the potential clinical outcomes, including mortality, are important to recognize.”
Dr. Dodell, who has a special interest in PCOS, advised that, for women with the condition, “focus on reducing insulin resistance with health-promoting behaviors and medications as needed. Data demonstrate that improving fitness, irrespective of a change in weight, can improve metabolic markers.” And, he advised that these women be routinely screened for mental health issues.
He also noted, “PCOS occurs across the size spectrum, but those patients in larger bodies may face weight stigma which has negative health consequences. These patients may avoid going to doctors for routine health screenings, so it is an important issue to continue to address.”
Women with PCOS lose a year of life
The new data come from 9,839 women with PCOS and 70,705 age- and region-matched controls from the Finnish Care Register for Health Care. The group with PCOS had been diagnosed at a mean age of 27 years.
The mean follow-up time was 13.1 years in both groups, during which 1,003 controls and 177 women with PCOS died. The mean age at death was 51.4 years for the PCOS group versus 52.6 years for the control women, a significant difference (P < .001).
Causes of death that were significantly higher among the women with PCOS versus controls after adjustments were cancer (hazard ratio, 1.39), and diseases of the circulatory system (1.68).
In more specific subcategories, after adjustment for education, the women with PCOS had increased mortality from nonischemic diseases, such as hypertensive heart disease, pulmonary embolism, etc. (HR, 2.06), and diabetes (HR, 2.85).
One study limitation was the inability to adjust for body mass index, Dr. Piltonen noted.
Dr. Piltonen, Dr. Kempegowda, and Dr. Dodell have no disclosures.
CHICAGO –
In the study, involving nearly 10,000 women with PCOS and matched controls from Finland, women with PCOS died on average a year earlier than their age-matched counterparts, primarily from diseases of the circulatory system, cancer, and diabetes.
PCOS is the most common endocrine disorder of reproductive-age women, of whom about 50%-70% also have obesity.
“I think we need to acknowledge that this is a health burden and not just a reproductive problem. In many cases we deal with the reproductive problem, and then these women are left alone. … So I think the message is we need to look beyond the reproductive outcomes, which are … really good. We can manage that,” said Terhi T. Piltonen, MD, PhD, during a press briefing held June 15 at the annual meeting of the Endocrine Society.
“I think the difficult part is [managing] the lifelong health for these women and supporting them to achieve the best health they can get. We need a multidisciplinary effort and to put more resources into the research,” added Dr. Piltonen, professor in the departments of ob.gyn. and reproductive endocrinology at the University of Oulu, Finland.
Indeed, Punith Kempegowda, MD, PhD, of the University of Birmingham (England) observed: “In our medical schools in the U.K., over 5 years, students get 45 minutes [of education] on PCOS, and they’re expected to learn about it.”
And over the last 20 years, funding for research into the condition has totaled less than a half percent of overall medical funding. “And we’re talking about 10% of all women. …We need to acknowledge it and educate people more. We need more published studies to understand more about it,” he noted.
Asked to comment, Greg Dodell, MD, owner and president of Central Park Endocrinology, New York, said: “PCOS is about a lot more than fertility, and that may not be the goal or on the mind of a woman at the time they start having symptoms of PCOS or get the diagnosis.”
“PCOS is largely a metabolic condition rooted in insulin resistance, and therefore, the potential clinical outcomes, including mortality, are important to recognize.”
Dr. Dodell, who has a special interest in PCOS, advised that, for women with the condition, “focus on reducing insulin resistance with health-promoting behaviors and medications as needed. Data demonstrate that improving fitness, irrespective of a change in weight, can improve metabolic markers.” And, he advised that these women be routinely screened for mental health issues.
He also noted, “PCOS occurs across the size spectrum, but those patients in larger bodies may face weight stigma which has negative health consequences. These patients may avoid going to doctors for routine health screenings, so it is an important issue to continue to address.”
Women with PCOS lose a year of life
The new data come from 9,839 women with PCOS and 70,705 age- and region-matched controls from the Finnish Care Register for Health Care. The group with PCOS had been diagnosed at a mean age of 27 years.
The mean follow-up time was 13.1 years in both groups, during which 1,003 controls and 177 women with PCOS died. The mean age at death was 51.4 years for the PCOS group versus 52.6 years for the control women, a significant difference (P < .001).
Causes of death that were significantly higher among the women with PCOS versus controls after adjustments were cancer (hazard ratio, 1.39), and diseases of the circulatory system (1.68).
In more specific subcategories, after adjustment for education, the women with PCOS had increased mortality from nonischemic diseases, such as hypertensive heart disease, pulmonary embolism, etc. (HR, 2.06), and diabetes (HR, 2.85).
One study limitation was the inability to adjust for body mass index, Dr. Piltonen noted.
Dr. Piltonen, Dr. Kempegowda, and Dr. Dodell have no disclosures.
AT ENDO 2023
International rights group calls out United States for allowing hospitals to push millions into debt
Human Rights Watch, the nonprofit that for decades has called attention to the victims of war, famine, and political repression around the world, is taking aim at U.S. hospitals for pushing millions of American patients into debt.
In a new report, the group calls for stronger government action to protect Americans from aggressive billing and debt collection by nonprofit hospitals, which Human Rights Watch said are systematically undermining patients’ human rights.
“Given the high prevalence of hospital-related medical debt in the U.S., this system is clearly not working,” concludes the report, which draws extensively on an ongoing investigation of medical debt by KFF Health News and NPR.
The report continues: “The U.S. model of subsidizing privately operated hospitals with tax exemptions in the hope that they will increase the accessibility of hospital care for un- and underinsured patients allows for abusive medical billing and debt collection practices and undermines human rights, including the right to health.”
Nationwide, about 100 million people – or 41% of adults – have some form of health care debt, a KFF survey conducted for the KFF Health News–NPR project found. And while patient debt is being driven by a range of medical and dental bills, polls and studies suggest hospitals are a major contributor.
About a third of U.S. adults with health care debt owed money for hospitalization, KFF’s polling found. Close to half of those owed at least $5,000. About a quarter owed $10,000 or more.
The scale of this crisis – which is unparalleled among wealthy nations – compelled Human Rights Watch to release the new report, said researcher Matt McConnell, its author. “Historically, Human Rights Watch has been an organization that has focused on international human rights issues,” he said. “But on medical debt, the U.S. is a real outlier. What you see is a system that privileges a few but creates large barriers to people accessing basic health rights.”
Hospital industry officials defend their work, citing hospitals’ broader work to help the communities they serve. “As a field, hospitals provide more benefit to their communities than any other sector in health care,” Melinda Hatton, general counsel at the American Hospital Association, wrote in a response to the Human Right Watch report.
Federal law requires private, tax-exempt hospitals – which make up more than half the nation’s medical centers – to provide care at no cost or at a discount to low-income patients. But reporting by KFF Health News and others has found that many hospitals make this aid difficult for patients to get.
At the same time, thousands of medical centers – including many tax-exempt ones – engage in aggressive debt collection tactics to pursue patients, including garnishing patients’ wages, placing liens on their homes, or selling their debt to third-party debt collectors.
Overall, KFF Health News found that most of the nation’s approximately 5,100 hospitals serving the general public have policies to use legal action or other aggressive tactics against patients. And one in five will deny nonemergency care to people with outstanding debt.
“Medical debt is drowning many low-income and working families while hospitals continue to benefit from nonprofit tax status as they pursue families for medical debt,” said Marceline White, executive director of Economic Action Maryland. The advocacy group has helped enact tighter rules to ensure Maryland hospitals make financial assistance more easily accessible and to restrict hospitals from some aggressive debt collection tactics, such as placing liens on patients’ homes.
Similar efforts are underway in other states, including Colorado, New Mexico, New York, Oregon, and Washington. But many patient and consumer advocates say stronger federal action is needed to expand patient protections.
The Human Rights Watch report – titled “In Sheep’s Clothing: United States’ Poorly Regulated Nonprofit Hospitals Undermine Health Care Access” – lists more than a dozen recommendations. These include:
- Congress should pass legislation to ensure that hospitals provide at least the same amount of charity care as they receive in public subsidies.
- The IRS should set uniform national standards on patients’ eligibility for financial assistance at nonprofit hospitals. Currently, hospitals are free to set their own standards, resulting in widespread variation, which can confuse patients.
- The Consumer Financial Protection Bureau, a federal watchdog agency, should crack down on debt collectors that do not ensure that patients have been screened for financial assistance before being pursued.
- The federal Centers for Medicare & Medicaid Services, which administers the two mammoth public insurance programs, should penalize hospitals that do not provide adequate financial assistance to patients.
“Nonprofit hospitals are contributing to medical debt and engaging in abusive billing and debt collection practices,” Mr. McConnell said. “The reason this keeps happening is the absence of clear guidelines and the federal government’s inadequate enforcement of existing regulations.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Human Rights Watch, the nonprofit that for decades has called attention to the victims of war, famine, and political repression around the world, is taking aim at U.S. hospitals for pushing millions of American patients into debt.
In a new report, the group calls for stronger government action to protect Americans from aggressive billing and debt collection by nonprofit hospitals, which Human Rights Watch said are systematically undermining patients’ human rights.
“Given the high prevalence of hospital-related medical debt in the U.S., this system is clearly not working,” concludes the report, which draws extensively on an ongoing investigation of medical debt by KFF Health News and NPR.
The report continues: “The U.S. model of subsidizing privately operated hospitals with tax exemptions in the hope that they will increase the accessibility of hospital care for un- and underinsured patients allows for abusive medical billing and debt collection practices and undermines human rights, including the right to health.”
Nationwide, about 100 million people – or 41% of adults – have some form of health care debt, a KFF survey conducted for the KFF Health News–NPR project found. And while patient debt is being driven by a range of medical and dental bills, polls and studies suggest hospitals are a major contributor.
About a third of U.S. adults with health care debt owed money for hospitalization, KFF’s polling found. Close to half of those owed at least $5,000. About a quarter owed $10,000 or more.
The scale of this crisis – which is unparalleled among wealthy nations – compelled Human Rights Watch to release the new report, said researcher Matt McConnell, its author. “Historically, Human Rights Watch has been an organization that has focused on international human rights issues,” he said. “But on medical debt, the U.S. is a real outlier. What you see is a system that privileges a few but creates large barriers to people accessing basic health rights.”
Hospital industry officials defend their work, citing hospitals’ broader work to help the communities they serve. “As a field, hospitals provide more benefit to their communities than any other sector in health care,” Melinda Hatton, general counsel at the American Hospital Association, wrote in a response to the Human Right Watch report.
Federal law requires private, tax-exempt hospitals – which make up more than half the nation’s medical centers – to provide care at no cost or at a discount to low-income patients. But reporting by KFF Health News and others has found that many hospitals make this aid difficult for patients to get.
At the same time, thousands of medical centers – including many tax-exempt ones – engage in aggressive debt collection tactics to pursue patients, including garnishing patients’ wages, placing liens on their homes, or selling their debt to third-party debt collectors.
Overall, KFF Health News found that most of the nation’s approximately 5,100 hospitals serving the general public have policies to use legal action or other aggressive tactics against patients. And one in five will deny nonemergency care to people with outstanding debt.
“Medical debt is drowning many low-income and working families while hospitals continue to benefit from nonprofit tax status as they pursue families for medical debt,” said Marceline White, executive director of Economic Action Maryland. The advocacy group has helped enact tighter rules to ensure Maryland hospitals make financial assistance more easily accessible and to restrict hospitals from some aggressive debt collection tactics, such as placing liens on patients’ homes.
Similar efforts are underway in other states, including Colorado, New Mexico, New York, Oregon, and Washington. But many patient and consumer advocates say stronger federal action is needed to expand patient protections.
The Human Rights Watch report – titled “In Sheep’s Clothing: United States’ Poorly Regulated Nonprofit Hospitals Undermine Health Care Access” – lists more than a dozen recommendations. These include:
- Congress should pass legislation to ensure that hospitals provide at least the same amount of charity care as they receive in public subsidies.
- The IRS should set uniform national standards on patients’ eligibility for financial assistance at nonprofit hospitals. Currently, hospitals are free to set their own standards, resulting in widespread variation, which can confuse patients.
- The Consumer Financial Protection Bureau, a federal watchdog agency, should crack down on debt collectors that do not ensure that patients have been screened for financial assistance before being pursued.
- The federal Centers for Medicare & Medicaid Services, which administers the two mammoth public insurance programs, should penalize hospitals that do not provide adequate financial assistance to patients.
“Nonprofit hospitals are contributing to medical debt and engaging in abusive billing and debt collection practices,” Mr. McConnell said. “The reason this keeps happening is the absence of clear guidelines and the federal government’s inadequate enforcement of existing regulations.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Human Rights Watch, the nonprofit that for decades has called attention to the victims of war, famine, and political repression around the world, is taking aim at U.S. hospitals for pushing millions of American patients into debt.
In a new report, the group calls for stronger government action to protect Americans from aggressive billing and debt collection by nonprofit hospitals, which Human Rights Watch said are systematically undermining patients’ human rights.
“Given the high prevalence of hospital-related medical debt in the U.S., this system is clearly not working,” concludes the report, which draws extensively on an ongoing investigation of medical debt by KFF Health News and NPR.
The report continues: “The U.S. model of subsidizing privately operated hospitals with tax exemptions in the hope that they will increase the accessibility of hospital care for un- and underinsured patients allows for abusive medical billing and debt collection practices and undermines human rights, including the right to health.”
Nationwide, about 100 million people – or 41% of adults – have some form of health care debt, a KFF survey conducted for the KFF Health News–NPR project found. And while patient debt is being driven by a range of medical and dental bills, polls and studies suggest hospitals are a major contributor.
About a third of U.S. adults with health care debt owed money for hospitalization, KFF’s polling found. Close to half of those owed at least $5,000. About a quarter owed $10,000 or more.
The scale of this crisis – which is unparalleled among wealthy nations – compelled Human Rights Watch to release the new report, said researcher Matt McConnell, its author. “Historically, Human Rights Watch has been an organization that has focused on international human rights issues,” he said. “But on medical debt, the U.S. is a real outlier. What you see is a system that privileges a few but creates large barriers to people accessing basic health rights.”
Hospital industry officials defend their work, citing hospitals’ broader work to help the communities they serve. “As a field, hospitals provide more benefit to their communities than any other sector in health care,” Melinda Hatton, general counsel at the American Hospital Association, wrote in a response to the Human Right Watch report.
Federal law requires private, tax-exempt hospitals – which make up more than half the nation’s medical centers – to provide care at no cost or at a discount to low-income patients. But reporting by KFF Health News and others has found that many hospitals make this aid difficult for patients to get.
At the same time, thousands of medical centers – including many tax-exempt ones – engage in aggressive debt collection tactics to pursue patients, including garnishing patients’ wages, placing liens on their homes, or selling their debt to third-party debt collectors.
Overall, KFF Health News found that most of the nation’s approximately 5,100 hospitals serving the general public have policies to use legal action or other aggressive tactics against patients. And one in five will deny nonemergency care to people with outstanding debt.
“Medical debt is drowning many low-income and working families while hospitals continue to benefit from nonprofit tax status as they pursue families for medical debt,” said Marceline White, executive director of Economic Action Maryland. The advocacy group has helped enact tighter rules to ensure Maryland hospitals make financial assistance more easily accessible and to restrict hospitals from some aggressive debt collection tactics, such as placing liens on patients’ homes.
Similar efforts are underway in other states, including Colorado, New Mexico, New York, Oregon, and Washington. But many patient and consumer advocates say stronger federal action is needed to expand patient protections.
The Human Rights Watch report – titled “In Sheep’s Clothing: United States’ Poorly Regulated Nonprofit Hospitals Undermine Health Care Access” – lists more than a dozen recommendations. These include:
- Congress should pass legislation to ensure that hospitals provide at least the same amount of charity care as they receive in public subsidies.
- The IRS should set uniform national standards on patients’ eligibility for financial assistance at nonprofit hospitals. Currently, hospitals are free to set their own standards, resulting in widespread variation, which can confuse patients.
- The Consumer Financial Protection Bureau, a federal watchdog agency, should crack down on debt collectors that do not ensure that patients have been screened for financial assistance before being pursued.
- The federal Centers for Medicare & Medicaid Services, which administers the two mammoth public insurance programs, should penalize hospitals that do not provide adequate financial assistance to patients.
“Nonprofit hospitals are contributing to medical debt and engaging in abusive billing and debt collection practices,” Mr. McConnell said. “The reason this keeps happening is the absence of clear guidelines and the federal government’s inadequate enforcement of existing regulations.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Rehabilitation improves walk test results for post–pulmonary embolism patients with persistent dyspnea
In patients with persistent dyspnea following a pulmonary embolism, rehabilitation should be considered as a treatment option, according to findings from a randomized, controlled trial comparing usual care to a twice-weekly, 8-week physical exercise program.
The prevalence of persistent dyspnea, functional limitations, and reduced quality of life (QoL) after pulmonary embolism (PE) ranges from 30% to 50% in published studies. While the underlying mechanisms remain unclear and are likely multifactorial, Øyvind Jervan, MD, and colleagues reported, research suggests that deconditioning and psychological factors contribute substantially to post-PE impairment. Optimal management remains unknown.
The investigators randomized adult patients 1:1 from two hospitals (Osfold Hospital and Akershus University Hospital) with PE identified via computed tomography pulmonary angiography 6-72 months prior to study inclusion to either a supervised outpatient exercise program or usual care. The once- or twice-weekly home-based program was tailored to each participant and included a 90-minute educational session on the cardiopulmonary system, diagnosis and treatment of PE and its possible long-term effects, the benefits of exercise and physical activity, and the management of breathlessness. Also during the intervention period, participants were given a simple home-based exercise program to be performed once or twice weekly. Differences between groups in the Incremental Shuttle Walk Test (ISWT), a standardized walking test that assesses exercise capacity, was the primary endpoint. Secondary endpoints included an endurance walk test (ESWT) and measures of symptoms and QoL.
Among 211 participants (median age 57 years; 56% men), the median time from diagnosis to inclusion was 10.3 months. Median baseline walking distance on the ISWT was 695 m with 21% achieving the 1,020-m maximum distance. At follow-up, a between-group difference of 53.0 m favored the rehabilitation group (89 evaluable subjects; 87 in usual care) (P = .0035). While subgroup analysis revealed a greater difference for those with shorter time from diagnosis (6-12 months vs. 12.1-72 months), the between-group differences were nonsignificant. Also, no ISWT differences between the intervention and control group were found for those with higher pulmonary embolism severity and dyspnea scores. The walk endurance test revealed no between-group differences.
Scores at follow-up on the Pulmonary Embolism-QoL questionnaire favored the rehabilitation group (mean difference –4%; P = .041), but there were no differences in generic QoL, dyspnea scores, or the ESWT.
“The present study adds to the growing evidence of the benefits of rehabilitation after PE,” the researchers stated. Although several recent studies have shown rehabilitation after PE results that were promising, the authors pointed out that most of these studies have been small or have lacked a control group, with great variations between them with respect to time, mode, and duration of intervention. In addition, the current study is the largest one addressing the effect of rehabilitation after PE to demonstrate in subjects with persistent dyspnea a positive effect on exercise capacity and QoL.
The researchers also commented that the small detected mean difference of 53 m in walking distance was lower than has been considered a worthwhile improvement by some, and its clinical relevance can be debated. Other studies, however, have used mean group differences of 40-62 m as clinically meaningful. The authors underscored also that the ISWT data were subject to a considerable ceiling effect which may underestimate the effect size.
Addressing study limitations, the researchers added that: “The rehabilitation program in the present study consisted mainly of exercise training. It is unknown whether the addition of occupational therapy, psychology, or dietary therapy would provide additional benefits for the participants. Most participants had mild symptoms, which may have limited the potential benefits of our rehabilitation program.”
The project was funded by Østfold Hospital Trust. Dr. Jervan reported no relevant conflicts of interest.
In patients with persistent dyspnea following a pulmonary embolism, rehabilitation should be considered as a treatment option, according to findings from a randomized, controlled trial comparing usual care to a twice-weekly, 8-week physical exercise program.
The prevalence of persistent dyspnea, functional limitations, and reduced quality of life (QoL) after pulmonary embolism (PE) ranges from 30% to 50% in published studies. While the underlying mechanisms remain unclear and are likely multifactorial, Øyvind Jervan, MD, and colleagues reported, research suggests that deconditioning and psychological factors contribute substantially to post-PE impairment. Optimal management remains unknown.
The investigators randomized adult patients 1:1 from two hospitals (Osfold Hospital and Akershus University Hospital) with PE identified via computed tomography pulmonary angiography 6-72 months prior to study inclusion to either a supervised outpatient exercise program or usual care. The once- or twice-weekly home-based program was tailored to each participant and included a 90-minute educational session on the cardiopulmonary system, diagnosis and treatment of PE and its possible long-term effects, the benefits of exercise and physical activity, and the management of breathlessness. Also during the intervention period, participants were given a simple home-based exercise program to be performed once or twice weekly. Differences between groups in the Incremental Shuttle Walk Test (ISWT), a standardized walking test that assesses exercise capacity, was the primary endpoint. Secondary endpoints included an endurance walk test (ESWT) and measures of symptoms and QoL.
Among 211 participants (median age 57 years; 56% men), the median time from diagnosis to inclusion was 10.3 months. Median baseline walking distance on the ISWT was 695 m with 21% achieving the 1,020-m maximum distance. At follow-up, a between-group difference of 53.0 m favored the rehabilitation group (89 evaluable subjects; 87 in usual care) (P = .0035). While subgroup analysis revealed a greater difference for those with shorter time from diagnosis (6-12 months vs. 12.1-72 months), the between-group differences were nonsignificant. Also, no ISWT differences between the intervention and control group were found for those with higher pulmonary embolism severity and dyspnea scores. The walk endurance test revealed no between-group differences.
Scores at follow-up on the Pulmonary Embolism-QoL questionnaire favored the rehabilitation group (mean difference –4%; P = .041), but there were no differences in generic QoL, dyspnea scores, or the ESWT.
“The present study adds to the growing evidence of the benefits of rehabilitation after PE,” the researchers stated. Although several recent studies have shown rehabilitation after PE results that were promising, the authors pointed out that most of these studies have been small or have lacked a control group, with great variations between them with respect to time, mode, and duration of intervention. In addition, the current study is the largest one addressing the effect of rehabilitation after PE to demonstrate in subjects with persistent dyspnea a positive effect on exercise capacity and QoL.
The researchers also commented that the small detected mean difference of 53 m in walking distance was lower than has been considered a worthwhile improvement by some, and its clinical relevance can be debated. Other studies, however, have used mean group differences of 40-62 m as clinically meaningful. The authors underscored also that the ISWT data were subject to a considerable ceiling effect which may underestimate the effect size.
Addressing study limitations, the researchers added that: “The rehabilitation program in the present study consisted mainly of exercise training. It is unknown whether the addition of occupational therapy, psychology, or dietary therapy would provide additional benefits for the participants. Most participants had mild symptoms, which may have limited the potential benefits of our rehabilitation program.”
The project was funded by Østfold Hospital Trust. Dr. Jervan reported no relevant conflicts of interest.
In patients with persistent dyspnea following a pulmonary embolism, rehabilitation should be considered as a treatment option, according to findings from a randomized, controlled trial comparing usual care to a twice-weekly, 8-week physical exercise program.
The prevalence of persistent dyspnea, functional limitations, and reduced quality of life (QoL) after pulmonary embolism (PE) ranges from 30% to 50% in published studies. While the underlying mechanisms remain unclear and are likely multifactorial, Øyvind Jervan, MD, and colleagues reported, research suggests that deconditioning and psychological factors contribute substantially to post-PE impairment. Optimal management remains unknown.
The investigators randomized adult patients 1:1 from two hospitals (Osfold Hospital and Akershus University Hospital) with PE identified via computed tomography pulmonary angiography 6-72 months prior to study inclusion to either a supervised outpatient exercise program or usual care. The once- or twice-weekly home-based program was tailored to each participant and included a 90-minute educational session on the cardiopulmonary system, diagnosis and treatment of PE and its possible long-term effects, the benefits of exercise and physical activity, and the management of breathlessness. Also during the intervention period, participants were given a simple home-based exercise program to be performed once or twice weekly. Differences between groups in the Incremental Shuttle Walk Test (ISWT), a standardized walking test that assesses exercise capacity, was the primary endpoint. Secondary endpoints included an endurance walk test (ESWT) and measures of symptoms and QoL.
Among 211 participants (median age 57 years; 56% men), the median time from diagnosis to inclusion was 10.3 months. Median baseline walking distance on the ISWT was 695 m with 21% achieving the 1,020-m maximum distance. At follow-up, a between-group difference of 53.0 m favored the rehabilitation group (89 evaluable subjects; 87 in usual care) (P = .0035). While subgroup analysis revealed a greater difference for those with shorter time from diagnosis (6-12 months vs. 12.1-72 months), the between-group differences were nonsignificant. Also, no ISWT differences between the intervention and control group were found for those with higher pulmonary embolism severity and dyspnea scores. The walk endurance test revealed no between-group differences.
Scores at follow-up on the Pulmonary Embolism-QoL questionnaire favored the rehabilitation group (mean difference –4%; P = .041), but there were no differences in generic QoL, dyspnea scores, or the ESWT.
“The present study adds to the growing evidence of the benefits of rehabilitation after PE,” the researchers stated. Although several recent studies have shown rehabilitation after PE results that were promising, the authors pointed out that most of these studies have been small or have lacked a control group, with great variations between them with respect to time, mode, and duration of intervention. In addition, the current study is the largest one addressing the effect of rehabilitation after PE to demonstrate in subjects with persistent dyspnea a positive effect on exercise capacity and QoL.
The researchers also commented that the small detected mean difference of 53 m in walking distance was lower than has been considered a worthwhile improvement by some, and its clinical relevance can be debated. Other studies, however, have used mean group differences of 40-62 m as clinically meaningful. The authors underscored also that the ISWT data were subject to a considerable ceiling effect which may underestimate the effect size.
Addressing study limitations, the researchers added that: “The rehabilitation program in the present study consisted mainly of exercise training. It is unknown whether the addition of occupational therapy, psychology, or dietary therapy would provide additional benefits for the participants. Most participants had mild symptoms, which may have limited the potential benefits of our rehabilitation program.”
The project was funded by Østfold Hospital Trust. Dr. Jervan reported no relevant conflicts of interest.
FROM THE JOURNAL CHEST
Patients with post-COVID cognitive symptoms may have gliosis
In a case-control study of 40 patients who were treated at a tertiary care psychiatric hospital in Canada, the level of translocator protein total distribution volume (TSPO VT), a marker of gliosis, was 9.23 mL/cm3 among patients with COVID-DC and 7.72 mL/cm3 among control persons. Differences were particularly notable in the ventral striatum and dorsal putamen.
“Most theories assume there is inflammation in the brain [with] long COVID,” but that assumption had not been studied, author Jeffrey H. Meyer, MD, PhD, Canada Research Chair in Neurochemistry of Major Depressive Disorder at the University of Toronto, said in an interview. “Such information is pivotal to developing treatments.”
The study was published online in JAMA Psychiatry.
Quantifiable marker
The investigators sought to determine whether levels of TSPO VT, which are quantifiable with PET, are elevated in the dorsal putamen, ventral striatum, prefrontal cortex, anterior cingulate cortex, and hippocampus of patients with COVID-DC, compared with patients without this syndrome. These brain regions were chosen, according to the authors, “because injury in these regions, which can cause gliosis, also induces symptoms of COVID-DC.”
The study was conducted from April 2021 through June 30, 2022. The investigators compared levels of TSPO VT in the selected brain regions of 20 participants with COVID-DC (mean age, 32.7 years; 60% women) with that of 20 control persons (mean age, 33.3 years; 55% women). TSPO VT was measured with fluorine F18–labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide PET.
The difference in TSPO VT was most noticeable in the ventral striatum (mean difference, 1.97 mL/cm3) and dorsal putamen (mean difference, 1.70 mL/cm3). The study authors suggest that gliosis in these areas may explain some of the persistent symptoms reported in structured clinical interviews and assessed on neuropsychological and psychological testing.
For patients with COVID-DC, motor speed on the finger-tapping test was negatively associated with dorsal putamen TSPO VT (r, −0.53). The 10 participants with COVID-DC whose speed was lowest had higher mean dorsal putamen TSPO VT levels than those of control persons by 2.3 mL/cm3.
The investigators could not assess a possible association between the ventral striatum TSPO VT and anhedonia because all participants had these symptoms. No significant correlations were found between depression and TSPO VT in the prefrontal cortex or anterior cingulate cortex.
The authors acknowledged that the study was cross-sectional, and so the duration of persistently elevated TSPO VT is not yet known. In addition, elevation in TSPO VT is not completely specific to glial cells, and although correlations with finger-tapping test performance reflect associations between brain changes and symptoms, they do not prove cause and effect.
“Presently, clinicians can use treatments for symptoms in other illnesses that are [also] common with long COVID. We need better than this,” said Dr. Meyer. “Clients with long COVID should be able to state their symptoms, and the practitioner should have an evidence-based matching treatment to recommend.”
Research is ongoing. “We are acquiring more information regarding different types of inflammation in the brain, whether there is ongoing injury, and whether treatments that influence inflammation are helpful,” said Dr. Meyer.
Jigsaw puzzle
“While this is an important piece in the jigsaw puzzle of neuroinflammation in chronic neurological disease, it is important to keep in mind that we still lack understanding of the complex picture for several reasons,” Alexander Gerhard, MD, honorary senior lecturer in neuroscience at the University of Manchester, England, wrote in an accompanying editorial.
Among these reasons is that the PET technique used in the study is noisy and not restricted to glial cells, he wrote. TSPO expression is only one part of the brain’s neuroinflammatory response, but PET techniques “do not currently allow us to distinguish between different states of microglial activation.” In addition, “a much more detailed understanding of microglial activation at different time points” is needed before neuroinflammatory changes can be targeted therapeutically, Dr. Gerhard wrote.
In a comment, Vilma Gabbay, MD, professor of psychiatry and neuroscience and director of biomarkers and dimensional psychiatry in the Psychiatry Research Institute at Montefiore Einstein, Albert Einstein College of Medicine, New York, said that “this is an important initial step to better understand the neuropsychiatric consequences of COVID even in only a mild and moderate viral illness.” TSPO imaging through PET scanning has been used as an index for neuroinflammation and gliosis. Researchers have used it to study neurodegenerative diseases, but as the authors noted, the ligand is not specific for gliosis.
“Follow-up large cohort studies including other measures of neuroimaging modalities assessing circuitry and neurochemistry are needed,” she said. “Similarly, studying the blood-brain barrier will also allow us to better understand how the immune reaction in the blood transitions to the brain.”
This field of research is evolving, and clinical trials are ongoing, Dr. Gabbay added. Meanwhile, clinicians should monitor for, assess, and treat neuropsychiatric symptoms and “follow the literature for new research and management recommendations.”
The study was primarily funded by a Canadian Institutes of Health Research Project grant to the authors, with some funding from the Canadian Institute for Military and Veteran Health Research. Dr. Meyer received support from their Canada Research Chair awards and received grants and support from several pharmaceutical companies outside of the submitted work. Dr. Gerhard and Dr. Gabbay disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a case-control study of 40 patients who were treated at a tertiary care psychiatric hospital in Canada, the level of translocator protein total distribution volume (TSPO VT), a marker of gliosis, was 9.23 mL/cm3 among patients with COVID-DC and 7.72 mL/cm3 among control persons. Differences were particularly notable in the ventral striatum and dorsal putamen.
“Most theories assume there is inflammation in the brain [with] long COVID,” but that assumption had not been studied, author Jeffrey H. Meyer, MD, PhD, Canada Research Chair in Neurochemistry of Major Depressive Disorder at the University of Toronto, said in an interview. “Such information is pivotal to developing treatments.”
The study was published online in JAMA Psychiatry.
Quantifiable marker
The investigators sought to determine whether levels of TSPO VT, which are quantifiable with PET, are elevated in the dorsal putamen, ventral striatum, prefrontal cortex, anterior cingulate cortex, and hippocampus of patients with COVID-DC, compared with patients without this syndrome. These brain regions were chosen, according to the authors, “because injury in these regions, which can cause gliosis, also induces symptoms of COVID-DC.”
The study was conducted from April 2021 through June 30, 2022. The investigators compared levels of TSPO VT in the selected brain regions of 20 participants with COVID-DC (mean age, 32.7 years; 60% women) with that of 20 control persons (mean age, 33.3 years; 55% women). TSPO VT was measured with fluorine F18–labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide PET.
The difference in TSPO VT was most noticeable in the ventral striatum (mean difference, 1.97 mL/cm3) and dorsal putamen (mean difference, 1.70 mL/cm3). The study authors suggest that gliosis in these areas may explain some of the persistent symptoms reported in structured clinical interviews and assessed on neuropsychological and psychological testing.
For patients with COVID-DC, motor speed on the finger-tapping test was negatively associated with dorsal putamen TSPO VT (r, −0.53). The 10 participants with COVID-DC whose speed was lowest had higher mean dorsal putamen TSPO VT levels than those of control persons by 2.3 mL/cm3.
The investigators could not assess a possible association between the ventral striatum TSPO VT and anhedonia because all participants had these symptoms. No significant correlations were found between depression and TSPO VT in the prefrontal cortex or anterior cingulate cortex.
The authors acknowledged that the study was cross-sectional, and so the duration of persistently elevated TSPO VT is not yet known. In addition, elevation in TSPO VT is not completely specific to glial cells, and although correlations with finger-tapping test performance reflect associations between brain changes and symptoms, they do not prove cause and effect.
“Presently, clinicians can use treatments for symptoms in other illnesses that are [also] common with long COVID. We need better than this,” said Dr. Meyer. “Clients with long COVID should be able to state their symptoms, and the practitioner should have an evidence-based matching treatment to recommend.”
Research is ongoing. “We are acquiring more information regarding different types of inflammation in the brain, whether there is ongoing injury, and whether treatments that influence inflammation are helpful,” said Dr. Meyer.
Jigsaw puzzle
“While this is an important piece in the jigsaw puzzle of neuroinflammation in chronic neurological disease, it is important to keep in mind that we still lack understanding of the complex picture for several reasons,” Alexander Gerhard, MD, honorary senior lecturer in neuroscience at the University of Manchester, England, wrote in an accompanying editorial.
Among these reasons is that the PET technique used in the study is noisy and not restricted to glial cells, he wrote. TSPO expression is only one part of the brain’s neuroinflammatory response, but PET techniques “do not currently allow us to distinguish between different states of microglial activation.” In addition, “a much more detailed understanding of microglial activation at different time points” is needed before neuroinflammatory changes can be targeted therapeutically, Dr. Gerhard wrote.
In a comment, Vilma Gabbay, MD, professor of psychiatry and neuroscience and director of biomarkers and dimensional psychiatry in the Psychiatry Research Institute at Montefiore Einstein, Albert Einstein College of Medicine, New York, said that “this is an important initial step to better understand the neuropsychiatric consequences of COVID even in only a mild and moderate viral illness.” TSPO imaging through PET scanning has been used as an index for neuroinflammation and gliosis. Researchers have used it to study neurodegenerative diseases, but as the authors noted, the ligand is not specific for gliosis.
“Follow-up large cohort studies including other measures of neuroimaging modalities assessing circuitry and neurochemistry are needed,” she said. “Similarly, studying the blood-brain barrier will also allow us to better understand how the immune reaction in the blood transitions to the brain.”
This field of research is evolving, and clinical trials are ongoing, Dr. Gabbay added. Meanwhile, clinicians should monitor for, assess, and treat neuropsychiatric symptoms and “follow the literature for new research and management recommendations.”
The study was primarily funded by a Canadian Institutes of Health Research Project grant to the authors, with some funding from the Canadian Institute for Military and Veteran Health Research. Dr. Meyer received support from their Canada Research Chair awards and received grants and support from several pharmaceutical companies outside of the submitted work. Dr. Gerhard and Dr. Gabbay disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a case-control study of 40 patients who were treated at a tertiary care psychiatric hospital in Canada, the level of translocator protein total distribution volume (TSPO VT), a marker of gliosis, was 9.23 mL/cm3 among patients with COVID-DC and 7.72 mL/cm3 among control persons. Differences were particularly notable in the ventral striatum and dorsal putamen.
“Most theories assume there is inflammation in the brain [with] long COVID,” but that assumption had not been studied, author Jeffrey H. Meyer, MD, PhD, Canada Research Chair in Neurochemistry of Major Depressive Disorder at the University of Toronto, said in an interview. “Such information is pivotal to developing treatments.”
The study was published online in JAMA Psychiatry.
Quantifiable marker
The investigators sought to determine whether levels of TSPO VT, which are quantifiable with PET, are elevated in the dorsal putamen, ventral striatum, prefrontal cortex, anterior cingulate cortex, and hippocampus of patients with COVID-DC, compared with patients without this syndrome. These brain regions were chosen, according to the authors, “because injury in these regions, which can cause gliosis, also induces symptoms of COVID-DC.”
The study was conducted from April 2021 through June 30, 2022. The investigators compared levels of TSPO VT in the selected brain regions of 20 participants with COVID-DC (mean age, 32.7 years; 60% women) with that of 20 control persons (mean age, 33.3 years; 55% women). TSPO VT was measured with fluorine F18–labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide PET.
The difference in TSPO VT was most noticeable in the ventral striatum (mean difference, 1.97 mL/cm3) and dorsal putamen (mean difference, 1.70 mL/cm3). The study authors suggest that gliosis in these areas may explain some of the persistent symptoms reported in structured clinical interviews and assessed on neuropsychological and psychological testing.
For patients with COVID-DC, motor speed on the finger-tapping test was negatively associated with dorsal putamen TSPO VT (r, −0.53). The 10 participants with COVID-DC whose speed was lowest had higher mean dorsal putamen TSPO VT levels than those of control persons by 2.3 mL/cm3.
The investigators could not assess a possible association between the ventral striatum TSPO VT and anhedonia because all participants had these symptoms. No significant correlations were found between depression and TSPO VT in the prefrontal cortex or anterior cingulate cortex.
The authors acknowledged that the study was cross-sectional, and so the duration of persistently elevated TSPO VT is not yet known. In addition, elevation in TSPO VT is not completely specific to glial cells, and although correlations with finger-tapping test performance reflect associations between brain changes and symptoms, they do not prove cause and effect.
“Presently, clinicians can use treatments for symptoms in other illnesses that are [also] common with long COVID. We need better than this,” said Dr. Meyer. “Clients with long COVID should be able to state their symptoms, and the practitioner should have an evidence-based matching treatment to recommend.”
Research is ongoing. “We are acquiring more information regarding different types of inflammation in the brain, whether there is ongoing injury, and whether treatments that influence inflammation are helpful,” said Dr. Meyer.
Jigsaw puzzle
“While this is an important piece in the jigsaw puzzle of neuroinflammation in chronic neurological disease, it is important to keep in mind that we still lack understanding of the complex picture for several reasons,” Alexander Gerhard, MD, honorary senior lecturer in neuroscience at the University of Manchester, England, wrote in an accompanying editorial.
Among these reasons is that the PET technique used in the study is noisy and not restricted to glial cells, he wrote. TSPO expression is only one part of the brain’s neuroinflammatory response, but PET techniques “do not currently allow us to distinguish between different states of microglial activation.” In addition, “a much more detailed understanding of microglial activation at different time points” is needed before neuroinflammatory changes can be targeted therapeutically, Dr. Gerhard wrote.
In a comment, Vilma Gabbay, MD, professor of psychiatry and neuroscience and director of biomarkers and dimensional psychiatry in the Psychiatry Research Institute at Montefiore Einstein, Albert Einstein College of Medicine, New York, said that “this is an important initial step to better understand the neuropsychiatric consequences of COVID even in only a mild and moderate viral illness.” TSPO imaging through PET scanning has been used as an index for neuroinflammation and gliosis. Researchers have used it to study neurodegenerative diseases, but as the authors noted, the ligand is not specific for gliosis.
“Follow-up large cohort studies including other measures of neuroimaging modalities assessing circuitry and neurochemistry are needed,” she said. “Similarly, studying the blood-brain barrier will also allow us to better understand how the immune reaction in the blood transitions to the brain.”
This field of research is evolving, and clinical trials are ongoing, Dr. Gabbay added. Meanwhile, clinicians should monitor for, assess, and treat neuropsychiatric symptoms and “follow the literature for new research and management recommendations.”
The study was primarily funded by a Canadian Institutes of Health Research Project grant to the authors, with some funding from the Canadian Institute for Military and Veteran Health Research. Dr. Meyer received support from their Canada Research Chair awards and received grants and support from several pharmaceutical companies outside of the submitted work. Dr. Gerhard and Dr. Gabbay disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
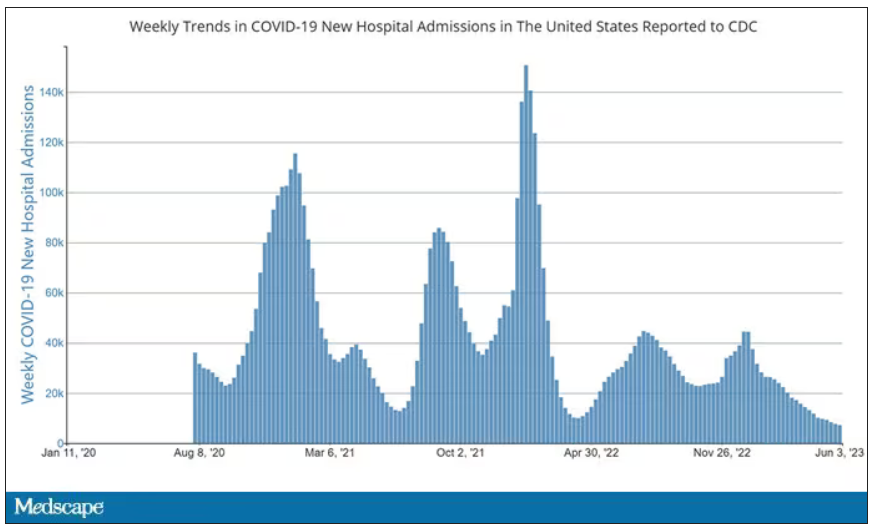
Latest data: COVID vaccine safety, protection, and breakthrough infections in inflammatory, autoimmune diseases
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
AT EULAR 2023
Millions who had COVID-19 still don’t have sense of smell, taste
Almost 36 million people were diagnosed in 2021, and 60% of them reported accompanying losses in smell or taste, according to the study by Mass Eye and Ear, which is affiliated with Harvard Medical School, Boston. The study was published in The Laryngoscope.
Most people fully regained the senses, but about 24% didn’t get smell back completely, and more than 3% had no recovery, the researchers reported. The numbers were similar among those who lost the sense of taste, they added.
“Many people never fully recovered,” Neil Bhattacharyya, MD, professor of otolaryngology and one of the study’s authors, told Fortune, estimating that up to 6 million people still have lingering symptoms. “If you lost your sense of smell, did you get it back? There’s about a one in four chance you didn’t. That’s terrible.”
Researchers looked at the records of 30,000 adults who had COVID-19 in 2021. They reported that patients who suffered more severe cases were less likely to regain some or all their senses.
Some patients said they lost appetite because they couldn’t smell food. There’s concern, too, about losing the ability to smell gas and smoke, spoiled food, and dirty diapers.
People with symptoms should see their doctors, Dr. Bhattacharyya said. The symptoms might be caused by something other than lingering COVID-19 effects and might be treatable.
A version of this article first appeared on WebMD.com.
Almost 36 million people were diagnosed in 2021, and 60% of them reported accompanying losses in smell or taste, according to the study by Mass Eye and Ear, which is affiliated with Harvard Medical School, Boston. The study was published in The Laryngoscope.
Most people fully regained the senses, but about 24% didn’t get smell back completely, and more than 3% had no recovery, the researchers reported. The numbers were similar among those who lost the sense of taste, they added.
“Many people never fully recovered,” Neil Bhattacharyya, MD, professor of otolaryngology and one of the study’s authors, told Fortune, estimating that up to 6 million people still have lingering symptoms. “If you lost your sense of smell, did you get it back? There’s about a one in four chance you didn’t. That’s terrible.”
Researchers looked at the records of 30,000 adults who had COVID-19 in 2021. They reported that patients who suffered more severe cases were less likely to regain some or all their senses.
Some patients said they lost appetite because they couldn’t smell food. There’s concern, too, about losing the ability to smell gas and smoke, spoiled food, and dirty diapers.
People with symptoms should see their doctors, Dr. Bhattacharyya said. The symptoms might be caused by something other than lingering COVID-19 effects and might be treatable.
A version of this article first appeared on WebMD.com.
Almost 36 million people were diagnosed in 2021, and 60% of them reported accompanying losses in smell or taste, according to the study by Mass Eye and Ear, which is affiliated with Harvard Medical School, Boston. The study was published in The Laryngoscope.
Most people fully regained the senses, but about 24% didn’t get smell back completely, and more than 3% had no recovery, the researchers reported. The numbers were similar among those who lost the sense of taste, they added.
“Many people never fully recovered,” Neil Bhattacharyya, MD, professor of otolaryngology and one of the study’s authors, told Fortune, estimating that up to 6 million people still have lingering symptoms. “If you lost your sense of smell, did you get it back? There’s about a one in four chance you didn’t. That’s terrible.”
Researchers looked at the records of 30,000 adults who had COVID-19 in 2021. They reported that patients who suffered more severe cases were less likely to regain some or all their senses.
Some patients said they lost appetite because they couldn’t smell food. There’s concern, too, about losing the ability to smell gas and smoke, spoiled food, and dirty diapers.
People with symptoms should see their doctors, Dr. Bhattacharyya said. The symptoms might be caused by something other than lingering COVID-19 effects and might be treatable.
A version of this article first appeared on WebMD.com.
FROM THE LARYNGOSCOPE
Alcohol may curb stress signaling in brain to protect heart
The study shows that light to moderate drinking was associated with lower major adverse cardiovascular events (MACE), and this was partly mediated by decreased stress signaling in the brain.
In addition, the benefit of light to moderate drinking with respect to MACE was most pronounced among people with a history of anxiety, a condition known to be associated with higher stress signaling in the brain.
However, the apparent CVD benefits of light to moderate drinking were counterbalanced by an increased risk of cancer.
“There is no safe level of alcohol consumption,” senior author and cardiologist Ahmed Tawakol, MD, codirector of the Cardiovascular Imaging Research Center at Massachusetts General Hospital, Boston, said in an interview.
“We see cancer risk even at the level that we see some protection from heart disease. And higher amounts of alcohol clearly increase heart disease risk,” Dr. Tawakol said.
The study was published online in the Journal of the American College of Cardiology.
Clear mechanistic link
Chronic stress is associated with MACE via stress-related neural network activity (SNA). Light to moderate alcohol consumption has been linked to lower MACE risk, but the mechanisms behind this connection remain unclear.
“We know that when the neural centers of stress are activated, they trigger downstream changes that result in heart disease. And we’ve long appreciated that alcohol in the short term reduces stress, so we hypothesized that maybe alcohol impacts those stress systems chronically and that might explain its cardiovascular effects,” Dr. Tawakol explained.
The study included roughly 53,000 adults (mean age, 60 years; 60% women) from the Mass General Brigham Biobank. The researchers first evaluated the relationship between light to moderate alcohol consumption and MACE after adjusting for a range of genetic, clinical, lifestyle, and socioeconomic factors.
During mean follow-up of 3.4 years, 1,914 individuals experienced MACE. Light to moderate alcohol consumption (compared to none/minimal) was associated with lower MACE risk (hazard ratio [HR], 0.786; 95% confidence interval [CI], 0.717-0.862; P < .0001) after adjustment for cardiovascular risk factors.
The researchers then studied a subset of 713 individuals who had undergone previous PET/CT brain imaging (primarily for cancer surveillance) to determine the effect of light to moderate alcohol consumption on resting SNA.
They found that light to moderate alcohol consumption correlated with decreased SNA (standardized beta, –0.192; 95% CI, –0.338 to 0.046; P = .01). Lower SNA partially mediated the beneficial effect of light to moderate alcohol intake on MACE risk (odds ratio [OR], –0.040; 95% CI, –0.097 to –0.003; P < .05).
Light to moderate alcohol consumption was associated with larger decreases in MACE risk among individuals with a history of anxiety (HR, 0.60; 95% CI, 0.50-0.72, vs. HR, 1.78; 95% CI, 0.73-0.80; P = .003).
The coauthors of an editorial say the discovery of a “new possible mechanism of action” for why light to moderate alcohol consumption might protect the heart “deserves closer attention in future investigations.”
However, Giovanni de Gaetano, MD, PhD, department of epidemiology and prevention, IRCCS NEUROMED, Pozzilli, Italy, one of the authors, emphasized that individuals who consume alcohol should not “exceed the recommended daily dose limits suggested in many countries and that no abstainer should start to drink, even in moderation, solely for the purpose of improving his/her health outcomes.”
Dr. Tawakol and colleagues said that, given alcohol’s adverse health effects, such as heightened cancer risk, new interventions that have positive effects on the neurobiology of stress but without the harmful effects of alcohol are needed.
To that end, they are studying the effect of exercise, stress-reduction interventions such as meditation, and pharmacologic therapies on stress-associated neural networks, and how they might induce CV benefits.
Dr. Tawakol said in an interview that one “additional important message is that anxiety and other related conditions like depression have really substantial health consequences, including increased MACE. Safer interventions that reduce anxiety may yet prove to reduce the risk of heart disease very nicely.”
The study was supported by the National Institutes of Health. Dr. Tawakol and Dr. de Gaetano have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The study shows that light to moderate drinking was associated with lower major adverse cardiovascular events (MACE), and this was partly mediated by decreased stress signaling in the brain.
In addition, the benefit of light to moderate drinking with respect to MACE was most pronounced among people with a history of anxiety, a condition known to be associated with higher stress signaling in the brain.
However, the apparent CVD benefits of light to moderate drinking were counterbalanced by an increased risk of cancer.
“There is no safe level of alcohol consumption,” senior author and cardiologist Ahmed Tawakol, MD, codirector of the Cardiovascular Imaging Research Center at Massachusetts General Hospital, Boston, said in an interview.
“We see cancer risk even at the level that we see some protection from heart disease. And higher amounts of alcohol clearly increase heart disease risk,” Dr. Tawakol said.
The study was published online in the Journal of the American College of Cardiology.
Clear mechanistic link
Chronic stress is associated with MACE via stress-related neural network activity (SNA). Light to moderate alcohol consumption has been linked to lower MACE risk, but the mechanisms behind this connection remain unclear.
“We know that when the neural centers of stress are activated, they trigger downstream changes that result in heart disease. And we’ve long appreciated that alcohol in the short term reduces stress, so we hypothesized that maybe alcohol impacts those stress systems chronically and that might explain its cardiovascular effects,” Dr. Tawakol explained.
The study included roughly 53,000 adults (mean age, 60 years; 60% women) from the Mass General Brigham Biobank. The researchers first evaluated the relationship between light to moderate alcohol consumption and MACE after adjusting for a range of genetic, clinical, lifestyle, and socioeconomic factors.
During mean follow-up of 3.4 years, 1,914 individuals experienced MACE. Light to moderate alcohol consumption (compared to none/minimal) was associated with lower MACE risk (hazard ratio [HR], 0.786; 95% confidence interval [CI], 0.717-0.862; P < .0001) after adjustment for cardiovascular risk factors.
The researchers then studied a subset of 713 individuals who had undergone previous PET/CT brain imaging (primarily for cancer surveillance) to determine the effect of light to moderate alcohol consumption on resting SNA.
They found that light to moderate alcohol consumption correlated with decreased SNA (standardized beta, –0.192; 95% CI, –0.338 to 0.046; P = .01). Lower SNA partially mediated the beneficial effect of light to moderate alcohol intake on MACE risk (odds ratio [OR], –0.040; 95% CI, –0.097 to –0.003; P < .05).
Light to moderate alcohol consumption was associated with larger decreases in MACE risk among individuals with a history of anxiety (HR, 0.60; 95% CI, 0.50-0.72, vs. HR, 1.78; 95% CI, 0.73-0.80; P = .003).
The coauthors of an editorial say the discovery of a “new possible mechanism of action” for why light to moderate alcohol consumption might protect the heart “deserves closer attention in future investigations.”
However, Giovanni de Gaetano, MD, PhD, department of epidemiology and prevention, IRCCS NEUROMED, Pozzilli, Italy, one of the authors, emphasized that individuals who consume alcohol should not “exceed the recommended daily dose limits suggested in many countries and that no abstainer should start to drink, even in moderation, solely for the purpose of improving his/her health outcomes.”
Dr. Tawakol and colleagues said that, given alcohol’s adverse health effects, such as heightened cancer risk, new interventions that have positive effects on the neurobiology of stress but without the harmful effects of alcohol are needed.
To that end, they are studying the effect of exercise, stress-reduction interventions such as meditation, and pharmacologic therapies on stress-associated neural networks, and how they might induce CV benefits.
Dr. Tawakol said in an interview that one “additional important message is that anxiety and other related conditions like depression have really substantial health consequences, including increased MACE. Safer interventions that reduce anxiety may yet prove to reduce the risk of heart disease very nicely.”
The study was supported by the National Institutes of Health. Dr. Tawakol and Dr. de Gaetano have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The study shows that light to moderate drinking was associated with lower major adverse cardiovascular events (MACE), and this was partly mediated by decreased stress signaling in the brain.
In addition, the benefit of light to moderate drinking with respect to MACE was most pronounced among people with a history of anxiety, a condition known to be associated with higher stress signaling in the brain.
However, the apparent CVD benefits of light to moderate drinking were counterbalanced by an increased risk of cancer.
“There is no safe level of alcohol consumption,” senior author and cardiologist Ahmed Tawakol, MD, codirector of the Cardiovascular Imaging Research Center at Massachusetts General Hospital, Boston, said in an interview.
“We see cancer risk even at the level that we see some protection from heart disease. And higher amounts of alcohol clearly increase heart disease risk,” Dr. Tawakol said.
The study was published online in the Journal of the American College of Cardiology.
Clear mechanistic link
Chronic stress is associated with MACE via stress-related neural network activity (SNA). Light to moderate alcohol consumption has been linked to lower MACE risk, but the mechanisms behind this connection remain unclear.
“We know that when the neural centers of stress are activated, they trigger downstream changes that result in heart disease. And we’ve long appreciated that alcohol in the short term reduces stress, so we hypothesized that maybe alcohol impacts those stress systems chronically and that might explain its cardiovascular effects,” Dr. Tawakol explained.
The study included roughly 53,000 adults (mean age, 60 years; 60% women) from the Mass General Brigham Biobank. The researchers first evaluated the relationship between light to moderate alcohol consumption and MACE after adjusting for a range of genetic, clinical, lifestyle, and socioeconomic factors.
During mean follow-up of 3.4 years, 1,914 individuals experienced MACE. Light to moderate alcohol consumption (compared to none/minimal) was associated with lower MACE risk (hazard ratio [HR], 0.786; 95% confidence interval [CI], 0.717-0.862; P < .0001) after adjustment for cardiovascular risk factors.
The researchers then studied a subset of 713 individuals who had undergone previous PET/CT brain imaging (primarily for cancer surveillance) to determine the effect of light to moderate alcohol consumption on resting SNA.
They found that light to moderate alcohol consumption correlated with decreased SNA (standardized beta, –0.192; 95% CI, –0.338 to 0.046; P = .01). Lower SNA partially mediated the beneficial effect of light to moderate alcohol intake on MACE risk (odds ratio [OR], –0.040; 95% CI, –0.097 to –0.003; P < .05).
Light to moderate alcohol consumption was associated with larger decreases in MACE risk among individuals with a history of anxiety (HR, 0.60; 95% CI, 0.50-0.72, vs. HR, 1.78; 95% CI, 0.73-0.80; P = .003).
The coauthors of an editorial say the discovery of a “new possible mechanism of action” for why light to moderate alcohol consumption might protect the heart “deserves closer attention in future investigations.”
However, Giovanni de Gaetano, MD, PhD, department of epidemiology and prevention, IRCCS NEUROMED, Pozzilli, Italy, one of the authors, emphasized that individuals who consume alcohol should not “exceed the recommended daily dose limits suggested in many countries and that no abstainer should start to drink, even in moderation, solely for the purpose of improving his/her health outcomes.”
Dr. Tawakol and colleagues said that, given alcohol’s adverse health effects, such as heightened cancer risk, new interventions that have positive effects on the neurobiology of stress but without the harmful effects of alcohol are needed.
To that end, they are studying the effect of exercise, stress-reduction interventions such as meditation, and pharmacologic therapies on stress-associated neural networks, and how they might induce CV benefits.
Dr. Tawakol said in an interview that one “additional important message is that anxiety and other related conditions like depression have really substantial health consequences, including increased MACE. Safer interventions that reduce anxiety may yet prove to reduce the risk of heart disease very nicely.”
The study was supported by the National Institutes of Health. Dr. Tawakol and Dr. de Gaetano have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
The cardiopulmonary effects of mask wearing
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
There was a time when I would have had to explain to you what an N95 mask is, how it is designed to filter out 95% of fine particles, defined as stuff in the air less than 2.5 microns in size.
But of course, you know that now. The N95 had its moment – a moment that seemed to be passing as the concentration of airborne coronavirus particles decreased.
But, as the poet said, all that is less than 2.5 microns in size is not coronavirus. Wildfire smoke is also chock full of fine particulate matter. And so, N95s are having something of a comeback.
That’s why an article that took a deep look at what happens to our cardiovascular system when we wear N95 masks caught my eye.
Mask wearing has been the subject of intense debate around the country. While the vast majority of evidence, as well as the personal experience of thousands of doctors, suggests that wearing a mask has no significant physiologic effects, it’s not hard to find those who suggest that mask wearing depletes oxygen levels, or leads to infection, or has other bizarre effects.
In a world of conflicting opinions, a controlled study is a wonderful thing, and that’s what appeared in JAMA Network Open.
This isn’t a huge study, but it’s big enough to make some important conclusions. Thirty individuals, all young and healthy, half female, were enrolled. Each participant spent 3 days in a metabolic chamber; this is essentially a giant, airtight room where all the inputs (oxygen levels and so on) and outputs (carbon dioxide levels and so on) can be precisely measured.
After a day of getting used to the environment, the participants spent a day either wearing an N95 mask or not for 16 waking hours. On the next day, they switched. Every other variable was controlled, from the calories in their diet to the temperature of the room itself.
They engaged in light exercise twice during the day – riding a stationary bike – and a host of physiologic parameters were measured. The question being, would the wearing of the mask for 16 hours straight change anything?
And the answer is yes, some things changed, but not by much.
Here’s a graph of the heart rate over time. You can see some separation, with higher heart rates during the mask-wearing day, particularly around 11 a.m. – when light exercise was scheduled.
Zooming in on the exercise period makes the difference more clear. The heart rate was about eight beats/min higher while masked and engaging in exercise. Systolic blood pressure was about 6 mm Hg higher. Oxygen saturation was lower by 0.7%.
So yes, exercising while wearing an N95 mask might be different from exercising without an N95 mask. But nothing here looks dangerous to me. The 0.7% decrease in oxygen saturation is smaller than the typical measurement error of a pulse oximeter. The authors write that venous pH decreased during the masked day, which is of more interest to me as a nephrologist, but they don’t show that data even in the supplement. I suspect it didn’t decrease much.
They also showed that respiratory rate during exercise decreased in the masked condition. That doesn’t really make sense when you think about it in the context of the other findings, which are all suggestive of increased metabolic rate and sympathetic drive. Does that call the whole procedure into question? No, but it’s worth noting.
These were young, healthy people. You could certainly argue that those with more vulnerable cardiopulmonary status might have had different effects from mask wearing, but without a specific study in those people, it’s just conjecture. Clearly, this study lets us conclude that mask wearing at rest has less of an effect than mask wearing during exercise.
But remember that, in reality, we are wearing masks for a reason. One could imagine a study where this metabolic chamber was filled with wildfire smoke at a concentration similar to what we saw in New York. In that situation, we might find that wearing an N95 is quite helpful. The thing is, studying masks in isolation is useful because you can control so many variables. But masks aren’t used in isolation. In fact, that’s sort of their defining characteristic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
There was a time when I would have had to explain to you what an N95 mask is, how it is designed to filter out 95% of fine particles, defined as stuff in the air less than 2.5 microns in size.
But of course, you know that now. The N95 had its moment – a moment that seemed to be passing as the concentration of airborne coronavirus particles decreased.
But, as the poet said, all that is less than 2.5 microns in size is not coronavirus. Wildfire smoke is also chock full of fine particulate matter. And so, N95s are having something of a comeback.
That’s why an article that took a deep look at what happens to our cardiovascular system when we wear N95 masks caught my eye.
Mask wearing has been the subject of intense debate around the country. While the vast majority of evidence, as well as the personal experience of thousands of doctors, suggests that wearing a mask has no significant physiologic effects, it’s not hard to find those who suggest that mask wearing depletes oxygen levels, or leads to infection, or has other bizarre effects.
In a world of conflicting opinions, a controlled study is a wonderful thing, and that’s what appeared in JAMA Network Open.
This isn’t a huge study, but it’s big enough to make some important conclusions. Thirty individuals, all young and healthy, half female, were enrolled. Each participant spent 3 days in a metabolic chamber; this is essentially a giant, airtight room where all the inputs (oxygen levels and so on) and outputs (carbon dioxide levels and so on) can be precisely measured.
After a day of getting used to the environment, the participants spent a day either wearing an N95 mask or not for 16 waking hours. On the next day, they switched. Every other variable was controlled, from the calories in their diet to the temperature of the room itself.
They engaged in light exercise twice during the day – riding a stationary bike – and a host of physiologic parameters were measured. The question being, would the wearing of the mask for 16 hours straight change anything?
And the answer is yes, some things changed, but not by much.
Here’s a graph of the heart rate over time. You can see some separation, with higher heart rates during the mask-wearing day, particularly around 11 a.m. – when light exercise was scheduled.
Zooming in on the exercise period makes the difference more clear. The heart rate was about eight beats/min higher while masked and engaging in exercise. Systolic blood pressure was about 6 mm Hg higher. Oxygen saturation was lower by 0.7%.
So yes, exercising while wearing an N95 mask might be different from exercising without an N95 mask. But nothing here looks dangerous to me. The 0.7% decrease in oxygen saturation is smaller than the typical measurement error of a pulse oximeter. The authors write that venous pH decreased during the masked day, which is of more interest to me as a nephrologist, but they don’t show that data even in the supplement. I suspect it didn’t decrease much.
They also showed that respiratory rate during exercise decreased in the masked condition. That doesn’t really make sense when you think about it in the context of the other findings, which are all suggestive of increased metabolic rate and sympathetic drive. Does that call the whole procedure into question? No, but it’s worth noting.
These were young, healthy people. You could certainly argue that those with more vulnerable cardiopulmonary status might have had different effects from mask wearing, but without a specific study in those people, it’s just conjecture. Clearly, this study lets us conclude that mask wearing at rest has less of an effect than mask wearing during exercise.
But remember that, in reality, we are wearing masks for a reason. One could imagine a study where this metabolic chamber was filled with wildfire smoke at a concentration similar to what we saw in New York. In that situation, we might find that wearing an N95 is quite helpful. The thing is, studying masks in isolation is useful because you can control so many variables. But masks aren’t used in isolation. In fact, that’s sort of their defining characteristic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
There was a time when I would have had to explain to you what an N95 mask is, how it is designed to filter out 95% of fine particles, defined as stuff in the air less than 2.5 microns in size.
But of course, you know that now. The N95 had its moment – a moment that seemed to be passing as the concentration of airborne coronavirus particles decreased.
But, as the poet said, all that is less than 2.5 microns in size is not coronavirus. Wildfire smoke is also chock full of fine particulate matter. And so, N95s are having something of a comeback.
That’s why an article that took a deep look at what happens to our cardiovascular system when we wear N95 masks caught my eye.
Mask wearing has been the subject of intense debate around the country. While the vast majority of evidence, as well as the personal experience of thousands of doctors, suggests that wearing a mask has no significant physiologic effects, it’s not hard to find those who suggest that mask wearing depletes oxygen levels, or leads to infection, or has other bizarre effects.
In a world of conflicting opinions, a controlled study is a wonderful thing, and that’s what appeared in JAMA Network Open.
This isn’t a huge study, but it’s big enough to make some important conclusions. Thirty individuals, all young and healthy, half female, were enrolled. Each participant spent 3 days in a metabolic chamber; this is essentially a giant, airtight room where all the inputs (oxygen levels and so on) and outputs (carbon dioxide levels and so on) can be precisely measured.
After a day of getting used to the environment, the participants spent a day either wearing an N95 mask or not for 16 waking hours. On the next day, they switched. Every other variable was controlled, from the calories in their diet to the temperature of the room itself.
They engaged in light exercise twice during the day – riding a stationary bike – and a host of physiologic parameters were measured. The question being, would the wearing of the mask for 16 hours straight change anything?
And the answer is yes, some things changed, but not by much.
Here’s a graph of the heart rate over time. You can see some separation, with higher heart rates during the mask-wearing day, particularly around 11 a.m. – when light exercise was scheduled.
Zooming in on the exercise period makes the difference more clear. The heart rate was about eight beats/min higher while masked and engaging in exercise. Systolic blood pressure was about 6 mm Hg higher. Oxygen saturation was lower by 0.7%.
So yes, exercising while wearing an N95 mask might be different from exercising without an N95 mask. But nothing here looks dangerous to me. The 0.7% decrease in oxygen saturation is smaller than the typical measurement error of a pulse oximeter. The authors write that venous pH decreased during the masked day, which is of more interest to me as a nephrologist, but they don’t show that data even in the supplement. I suspect it didn’t decrease much.
They also showed that respiratory rate during exercise decreased in the masked condition. That doesn’t really make sense when you think about it in the context of the other findings, which are all suggestive of increased metabolic rate and sympathetic drive. Does that call the whole procedure into question? No, but it’s worth noting.
These were young, healthy people. You could certainly argue that those with more vulnerable cardiopulmonary status might have had different effects from mask wearing, but without a specific study in those people, it’s just conjecture. Clearly, this study lets us conclude that mask wearing at rest has less of an effect than mask wearing during exercise.
But remember that, in reality, we are wearing masks for a reason. One could imagine a study where this metabolic chamber was filled with wildfire smoke at a concentration similar to what we saw in New York. In that situation, we might find that wearing an N95 is quite helpful. The thing is, studying masks in isolation is useful because you can control so many variables. But masks aren’t used in isolation. In fact, that’s sort of their defining characteristic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Insurers poised to crack down on off-label Ozempic prescriptions
The warning letters, first reported by The Washington Post, include threats such as the possibility of reporting “suspected inappropriate or fraudulent activity ... to the state licensure board, federal and/or state law enforcement.”
It’s the latest chapter in the story of the popular, highly effective, and very expensive drug intended for diabetes that results in quick weight loss. Off-label prescribing means a medicine has been prescribed for a reason other than the uses approved by the Food and Drug Administration. The practice is common and legal (the U.S. Agency for Healthcare Research and Quality says one in five prescriptions in the U.S. are off label).
But insurance companies are pushing back because many do not cover weight loss medications, while they do cover diabetes treatments. The insurance company letters suggest that prescribers are failing to document in a person’s medical record that the person actually has diabetes.
Ozempic, which is FDA approved for treatment of diabetes, is similar to the drug Wegovy, which is approved to be used for weight loss. Ozempic typically costs more than $900 per month. Both Wegovy and Ozempic contain semaglutide, which mimics a hormone that helps the brain regulate appetite and food intake. Clinical studies show that after taking semaglutide for more than 5 years, people lose on average 17% of their body weight. But once they stop taking it, most people regain much of the weight.
Demand for both Ozempic and Wegovy has been surging, leading to shortages and tactics to acquire the drugs outside of the United States, as well as warnings from public health officials about the dangers of knockoff versions of the drugs. The Centers for Disease Control and Prevention says 42% of people in the United States are obese.
“Obesity is a complex disease involving an excessive amount of body fat,” the Mayo Clinic explained. “Obesity isn’t just a cosmetic concern. It’s a medical problem that increases the risk of other diseases and health problems, such as heart disease, diabetes, high blood pressure, and certain cancers.”
A version of this article first appeared on WebMD.com.
The warning letters, first reported by The Washington Post, include threats such as the possibility of reporting “suspected inappropriate or fraudulent activity ... to the state licensure board, federal and/or state law enforcement.”
It’s the latest chapter in the story of the popular, highly effective, and very expensive drug intended for diabetes that results in quick weight loss. Off-label prescribing means a medicine has been prescribed for a reason other than the uses approved by the Food and Drug Administration. The practice is common and legal (the U.S. Agency for Healthcare Research and Quality says one in five prescriptions in the U.S. are off label).
But insurance companies are pushing back because many do not cover weight loss medications, while they do cover diabetes treatments. The insurance company letters suggest that prescribers are failing to document in a person’s medical record that the person actually has diabetes.
Ozempic, which is FDA approved for treatment of diabetes, is similar to the drug Wegovy, which is approved to be used for weight loss. Ozempic typically costs more than $900 per month. Both Wegovy and Ozempic contain semaglutide, which mimics a hormone that helps the brain regulate appetite and food intake. Clinical studies show that after taking semaglutide for more than 5 years, people lose on average 17% of their body weight. But once they stop taking it, most people regain much of the weight.
Demand for both Ozempic and Wegovy has been surging, leading to shortages and tactics to acquire the drugs outside of the United States, as well as warnings from public health officials about the dangers of knockoff versions of the drugs. The Centers for Disease Control and Prevention says 42% of people in the United States are obese.
“Obesity is a complex disease involving an excessive amount of body fat,” the Mayo Clinic explained. “Obesity isn’t just a cosmetic concern. It’s a medical problem that increases the risk of other diseases and health problems, such as heart disease, diabetes, high blood pressure, and certain cancers.”
A version of this article first appeared on WebMD.com.
The warning letters, first reported by The Washington Post, include threats such as the possibility of reporting “suspected inappropriate or fraudulent activity ... to the state licensure board, federal and/or state law enforcement.”
It’s the latest chapter in the story of the popular, highly effective, and very expensive drug intended for diabetes that results in quick weight loss. Off-label prescribing means a medicine has been prescribed for a reason other than the uses approved by the Food and Drug Administration. The practice is common and legal (the U.S. Agency for Healthcare Research and Quality says one in five prescriptions in the U.S. are off label).
But insurance companies are pushing back because many do not cover weight loss medications, while they do cover diabetes treatments. The insurance company letters suggest that prescribers are failing to document in a person’s medical record that the person actually has diabetes.
Ozempic, which is FDA approved for treatment of diabetes, is similar to the drug Wegovy, which is approved to be used for weight loss. Ozempic typically costs more than $900 per month. Both Wegovy and Ozempic contain semaglutide, which mimics a hormone that helps the brain regulate appetite and food intake. Clinical studies show that after taking semaglutide for more than 5 years, people lose on average 17% of their body weight. But once they stop taking it, most people regain much of the weight.
Demand for both Ozempic and Wegovy has been surging, leading to shortages and tactics to acquire the drugs outside of the United States, as well as warnings from public health officials about the dangers of knockoff versions of the drugs. The Centers for Disease Control and Prevention says 42% of people in the United States are obese.
“Obesity is a complex disease involving an excessive amount of body fat,” the Mayo Clinic explained. “Obesity isn’t just a cosmetic concern. It’s a medical problem that increases the risk of other diseases and health problems, such as heart disease, diabetes, high blood pressure, and certain cancers.”
A version of this article first appeared on WebMD.com.
Popular weight loss drugs can carry some unpleasant side effects
Johnna Mendenall had never been “the skinny friend,” she said, but the demands of motherhood – along with a sedentary desk job – made weight management even more difficult. Worried that family type 2 diabetes would catch up with her, she decided to start Wegovy shots for weight loss.
She was nervous about potential side effects. It took 5 days of staring at the Wegovy pen before she worked up the nerve for her first .25-milligram shot. And sure enough, the side effects came on strong.
“The nausea kicked in,” she said. “When I increased my dose to 1 milligram, I spent the entire night from 10 p.m. to 5 a.m. vomiting. I almost quit that day.”
While gastrointestinal (GI) symptoms seem to be the most common, a laundry list of others has been discussed in the news, on TikTok, and across online forums. Those include “Ozempic face,” or the gaunt look some get after taking the medication, along with hair loss, anxiety, depression, and debilitating fatigue.
Ms. Mendenall’s primary side effects have been vomiting, fatigue, and severe constipation, but she has also seen some positive changes: The “food noise,” or the urge to eat when she isn’t hungry, is gone. Since her first dose 12 weeks ago, she has gone from 236 pounds to 215.
Warning label
Wegovy’s active ingredient, semaglutide, mimics the role of a natural hormone called glucagonlike peptide–1 (GLP-1), which helps you feel well fed. Semaglutide is used at a lower dose under the brand name Ozempic, which is approved for type 2 diabetes and used off-label for weight loss.
Both Ozempic and Wegovy come with a warning label for potential side effects, the most common ones being nausea, diarrhea, stomach pain, and vomiting.
With the surging popularity of semaglutide, more people are getting prescriptions through telemedicine companies, forgoing more in-depth consultations, leading to more side effects, said Caroline Apovian, MD, professor of medicine at Harvard Medical School and codirector of the Center for Weight Management and Wellness at Brigham and Women’s Hospital, Boston.
Specialists say starting with low doses and gradually increasing over time helps avoid side effects, but insurance companies often require a faster timeline to continue covering the medication, Dr. Apovian said.
“Insurance companies are practicing medicine for us by demanding the patient go up in dosage [too quickly],” she explained.
Ms. Mendenall’s insurance has paid for her Wegovy shots, but without that coverage, she said it would cost her $1,200 per month.
There are similar medications on the market, such as liraglutide, sold under the name Saxenda. But it is a daily, rather than a weekly, shot and also comes with side effects and has been shown to be less effective. In one clinical trial, the people being studied saw their average body weight over 68 weeks drop by 15.8% with semaglutide, and by 6.4% with liraglutide.
Tirzepatide, branded Mounjaro – a type 2 diabetes drug made by Eli Lilly that may soon gain Food and Drug Administration approval for weight loss – could have fewer side effects. In clinical trials, 44% of those taking semaglutide had nausea and 31% reported diarrhea, compared with 33% and 23% of those taking tirzepatide, although no trial has directly compared the two agents.
Loss of bowel control
For now, Wegovy and Saxenda are the only GLP-1 agonist shots authorized for weight loss, and their maker, Danish drug company Novo Nordisk, is facing its second shortage of Wegovy amid growing demand.
Personal stories online about semaglutide range from overwhelmingly positive – just what some need to win a lifelong battle with obesity – to harsh scenarios with potentially long-term health consequences, and everything in between.
One private community on Reddit is dedicated to a particularly unpleasant side effect: loss of bowel control while sleeping. Others have reported uncontrollable vomiting.
Kimberly Carew of Clearwater, Fla., started on .5 milligrams of Ozempic last year after her rheumatologist and endocrinologist suggested it to treat her type 2 diabetes. She was told it came with the bonus of weight loss, which she was hoping would help with her joint and back pain.
But after she increased the dose to 1 milligram, her GI symptoms, which started out mild, became unbearable. She couldn’t keep food down, and when she vomited, the food would often come up whole, she said.
“One night I ate ramen before bed. And the next morning, it came out just as it went down,” said Ms. Carew, 42, a registered mental health counseling intern. “I was getting severe heartburn and could not take a couple bites of food without getting nauseous.”
She also had “sulfur burps,” a side effect discussed by some Ozempic users, causing her to taste rotten egg sometimes.
She was diagnosed with gastroparesis. Some types of gastroparesis can be resolved by discontinuing GLP-1 medications, as referenced in two case reports in the Journal of Investigative Medicine.
Gut hormone
GI symptoms are most common with semaglutide because the hormone it imitates, GLP-1, is secreted by cells in the stomach, small intestines, and pancreas, said Anne Peters, MD, director of the USC Clinical Diabetes Programs.
“This is the deal: The side effects are real because it’s a gut hormone. It’s increasing the level of something your body already has,” she said.
But, like Dr. Apovian, Dr. Peters said those side effects can likely be avoided if shots are started at the lowest doses and gradually adjusted up.
While the average starting dose is .25 milligrams, Dr. Peters said she often starts her patients on about an eighth of that – just “a whiff of a dose.”
“It’ll take them months to get up to the starting dose, but what’s the rush?”
Dr. Peters said she also avoids giving diabetes patients the maximum dose, which is 2 milligrams per week for Ozempic (and 2.4 milligrams for Wegovy for weight loss).
When asked about the drugs’ side effects, Novo Nordisk responded that “GLP-1 receptor agonists are a well-established class of medicines, which have demonstrated long-term safety in clinical trials. The most common adverse reactions, as with all GLP-1 [agonists], are gastrointestinal related.”
Is it the drug or the weight loss?
Still, non-gastrointestinal side effects such as hair loss, mood changes, and sunken facial features are reported among semaglutide users across the Internet. While these cases are often anecdotal, they can be very heartfelt.
Celina Horvath Myers, also known as CelinaSpookyBoo, a Canadian YouTuber who took Ozempic for type 2 diabetes, said she began having intense panic attacks and depression after starting the medication.
“Who I have been these last couple weeks, has probably been the scariest time of my life,” she said on her YouTube channel.
While severe depression and anxiety are not established side effects of the medication, some people get anhedonia, said W. Scott Butsch, MD, MSc, director of obesity medicine in the Bariatric and Metabolic Institute at Cleveland Clinic. But that could be a natural consequence of lower appetite, he said, given that food gives most people pleasure in the moment.
Many other reported changes come from the weight loss itself, not the medication, said Dr. Butsch.
“These are drugs that change the body’s weight regulatory system,” he said. “When someone loses weight, you get the shrinking of the fat cells, as well as the atrophy of the muscles. This rapid weight loss may give the appearance of one’s face changing.”
For some people, like Ms. Mendenall, the side effects are worth it. For others, like Ms. Carew, they’re intolerable.
Ms. Carew said she stopped the medication after about 7 months, and gradually worked up to eating solid foods again.
“It’s the American way, we’ve all got to be thin and beautiful,” she said. “But I feel like it’s very unsafe because we just don’t know how seriously our bodies will react to these things in the long term. People see it as a quick fix, but it comes with risks.”
A version of this article first appeared on WebMD.com.
Johnna Mendenall had never been “the skinny friend,” she said, but the demands of motherhood – along with a sedentary desk job – made weight management even more difficult. Worried that family type 2 diabetes would catch up with her, she decided to start Wegovy shots for weight loss.
She was nervous about potential side effects. It took 5 days of staring at the Wegovy pen before she worked up the nerve for her first .25-milligram shot. And sure enough, the side effects came on strong.
“The nausea kicked in,” she said. “When I increased my dose to 1 milligram, I spent the entire night from 10 p.m. to 5 a.m. vomiting. I almost quit that day.”
While gastrointestinal (GI) symptoms seem to be the most common, a laundry list of others has been discussed in the news, on TikTok, and across online forums. Those include “Ozempic face,” or the gaunt look some get after taking the medication, along with hair loss, anxiety, depression, and debilitating fatigue.
Ms. Mendenall’s primary side effects have been vomiting, fatigue, and severe constipation, but she has also seen some positive changes: The “food noise,” or the urge to eat when she isn’t hungry, is gone. Since her first dose 12 weeks ago, she has gone from 236 pounds to 215.
Warning label
Wegovy’s active ingredient, semaglutide, mimics the role of a natural hormone called glucagonlike peptide–1 (GLP-1), which helps you feel well fed. Semaglutide is used at a lower dose under the brand name Ozempic, which is approved for type 2 diabetes and used off-label for weight loss.
Both Ozempic and Wegovy come with a warning label for potential side effects, the most common ones being nausea, diarrhea, stomach pain, and vomiting.
With the surging popularity of semaglutide, more people are getting prescriptions through telemedicine companies, forgoing more in-depth consultations, leading to more side effects, said Caroline Apovian, MD, professor of medicine at Harvard Medical School and codirector of the Center for Weight Management and Wellness at Brigham and Women’s Hospital, Boston.
Specialists say starting with low doses and gradually increasing over time helps avoid side effects, but insurance companies often require a faster timeline to continue covering the medication, Dr. Apovian said.
“Insurance companies are practicing medicine for us by demanding the patient go up in dosage [too quickly],” she explained.
Ms. Mendenall’s insurance has paid for her Wegovy shots, but without that coverage, she said it would cost her $1,200 per month.
There are similar medications on the market, such as liraglutide, sold under the name Saxenda. But it is a daily, rather than a weekly, shot and also comes with side effects and has been shown to be less effective. In one clinical trial, the people being studied saw their average body weight over 68 weeks drop by 15.8% with semaglutide, and by 6.4% with liraglutide.
Tirzepatide, branded Mounjaro – a type 2 diabetes drug made by Eli Lilly that may soon gain Food and Drug Administration approval for weight loss – could have fewer side effects. In clinical trials, 44% of those taking semaglutide had nausea and 31% reported diarrhea, compared with 33% and 23% of those taking tirzepatide, although no trial has directly compared the two agents.
Loss of bowel control
For now, Wegovy and Saxenda are the only GLP-1 agonist shots authorized for weight loss, and their maker, Danish drug company Novo Nordisk, is facing its second shortage of Wegovy amid growing demand.
Personal stories online about semaglutide range from overwhelmingly positive – just what some need to win a lifelong battle with obesity – to harsh scenarios with potentially long-term health consequences, and everything in between.
One private community on Reddit is dedicated to a particularly unpleasant side effect: loss of bowel control while sleeping. Others have reported uncontrollable vomiting.
Kimberly Carew of Clearwater, Fla., started on .5 milligrams of Ozempic last year after her rheumatologist and endocrinologist suggested it to treat her type 2 diabetes. She was told it came with the bonus of weight loss, which she was hoping would help with her joint and back pain.
But after she increased the dose to 1 milligram, her GI symptoms, which started out mild, became unbearable. She couldn’t keep food down, and when she vomited, the food would often come up whole, she said.
“One night I ate ramen before bed. And the next morning, it came out just as it went down,” said Ms. Carew, 42, a registered mental health counseling intern. “I was getting severe heartburn and could not take a couple bites of food without getting nauseous.”
She also had “sulfur burps,” a side effect discussed by some Ozempic users, causing her to taste rotten egg sometimes.
She was diagnosed with gastroparesis. Some types of gastroparesis can be resolved by discontinuing GLP-1 medications, as referenced in two case reports in the Journal of Investigative Medicine.
Gut hormone
GI symptoms are most common with semaglutide because the hormone it imitates, GLP-1, is secreted by cells in the stomach, small intestines, and pancreas, said Anne Peters, MD, director of the USC Clinical Diabetes Programs.
“This is the deal: The side effects are real because it’s a gut hormone. It’s increasing the level of something your body already has,” she said.
But, like Dr. Apovian, Dr. Peters said those side effects can likely be avoided if shots are started at the lowest doses and gradually adjusted up.
While the average starting dose is .25 milligrams, Dr. Peters said she often starts her patients on about an eighth of that – just “a whiff of a dose.”
“It’ll take them months to get up to the starting dose, but what’s the rush?”
Dr. Peters said she also avoids giving diabetes patients the maximum dose, which is 2 milligrams per week for Ozempic (and 2.4 milligrams for Wegovy for weight loss).
When asked about the drugs’ side effects, Novo Nordisk responded that “GLP-1 receptor agonists are a well-established class of medicines, which have demonstrated long-term safety in clinical trials. The most common adverse reactions, as with all GLP-1 [agonists], are gastrointestinal related.”
Is it the drug or the weight loss?
Still, non-gastrointestinal side effects such as hair loss, mood changes, and sunken facial features are reported among semaglutide users across the Internet. While these cases are often anecdotal, they can be very heartfelt.
Celina Horvath Myers, also known as CelinaSpookyBoo, a Canadian YouTuber who took Ozempic for type 2 diabetes, said she began having intense panic attacks and depression after starting the medication.
“Who I have been these last couple weeks, has probably been the scariest time of my life,” she said on her YouTube channel.
While severe depression and anxiety are not established side effects of the medication, some people get anhedonia, said W. Scott Butsch, MD, MSc, director of obesity medicine in the Bariatric and Metabolic Institute at Cleveland Clinic. But that could be a natural consequence of lower appetite, he said, given that food gives most people pleasure in the moment.
Many other reported changes come from the weight loss itself, not the medication, said Dr. Butsch.
“These are drugs that change the body’s weight regulatory system,” he said. “When someone loses weight, you get the shrinking of the fat cells, as well as the atrophy of the muscles. This rapid weight loss may give the appearance of one’s face changing.”
For some people, like Ms. Mendenall, the side effects are worth it. For others, like Ms. Carew, they’re intolerable.
Ms. Carew said she stopped the medication after about 7 months, and gradually worked up to eating solid foods again.
“It’s the American way, we’ve all got to be thin and beautiful,” she said. “But I feel like it’s very unsafe because we just don’t know how seriously our bodies will react to these things in the long term. People see it as a quick fix, but it comes with risks.”
A version of this article first appeared on WebMD.com.
Johnna Mendenall had never been “the skinny friend,” she said, but the demands of motherhood – along with a sedentary desk job – made weight management even more difficult. Worried that family type 2 diabetes would catch up with her, she decided to start Wegovy shots for weight loss.
She was nervous about potential side effects. It took 5 days of staring at the Wegovy pen before she worked up the nerve for her first .25-milligram shot. And sure enough, the side effects came on strong.
“The nausea kicked in,” she said. “When I increased my dose to 1 milligram, I spent the entire night from 10 p.m. to 5 a.m. vomiting. I almost quit that day.”
While gastrointestinal (GI) symptoms seem to be the most common, a laundry list of others has been discussed in the news, on TikTok, and across online forums. Those include “Ozempic face,” or the gaunt look some get after taking the medication, along with hair loss, anxiety, depression, and debilitating fatigue.
Ms. Mendenall’s primary side effects have been vomiting, fatigue, and severe constipation, but she has also seen some positive changes: The “food noise,” or the urge to eat when she isn’t hungry, is gone. Since her first dose 12 weeks ago, she has gone from 236 pounds to 215.
Warning label
Wegovy’s active ingredient, semaglutide, mimics the role of a natural hormone called glucagonlike peptide–1 (GLP-1), which helps you feel well fed. Semaglutide is used at a lower dose under the brand name Ozempic, which is approved for type 2 diabetes and used off-label for weight loss.
Both Ozempic and Wegovy come with a warning label for potential side effects, the most common ones being nausea, diarrhea, stomach pain, and vomiting.
With the surging popularity of semaglutide, more people are getting prescriptions through telemedicine companies, forgoing more in-depth consultations, leading to more side effects, said Caroline Apovian, MD, professor of medicine at Harvard Medical School and codirector of the Center for Weight Management and Wellness at Brigham and Women’s Hospital, Boston.
Specialists say starting with low doses and gradually increasing over time helps avoid side effects, but insurance companies often require a faster timeline to continue covering the medication, Dr. Apovian said.
“Insurance companies are practicing medicine for us by demanding the patient go up in dosage [too quickly],” she explained.
Ms. Mendenall’s insurance has paid for her Wegovy shots, but without that coverage, she said it would cost her $1,200 per month.
There are similar medications on the market, such as liraglutide, sold under the name Saxenda. But it is a daily, rather than a weekly, shot and also comes with side effects and has been shown to be less effective. In one clinical trial, the people being studied saw their average body weight over 68 weeks drop by 15.8% with semaglutide, and by 6.4% with liraglutide.
Tirzepatide, branded Mounjaro – a type 2 diabetes drug made by Eli Lilly that may soon gain Food and Drug Administration approval for weight loss – could have fewer side effects. In clinical trials, 44% of those taking semaglutide had nausea and 31% reported diarrhea, compared with 33% and 23% of those taking tirzepatide, although no trial has directly compared the two agents.
Loss of bowel control
For now, Wegovy and Saxenda are the only GLP-1 agonist shots authorized for weight loss, and their maker, Danish drug company Novo Nordisk, is facing its second shortage of Wegovy amid growing demand.
Personal stories online about semaglutide range from overwhelmingly positive – just what some need to win a lifelong battle with obesity – to harsh scenarios with potentially long-term health consequences, and everything in between.
One private community on Reddit is dedicated to a particularly unpleasant side effect: loss of bowel control while sleeping. Others have reported uncontrollable vomiting.
Kimberly Carew of Clearwater, Fla., started on .5 milligrams of Ozempic last year after her rheumatologist and endocrinologist suggested it to treat her type 2 diabetes. She was told it came with the bonus of weight loss, which she was hoping would help with her joint and back pain.
But after she increased the dose to 1 milligram, her GI symptoms, which started out mild, became unbearable. She couldn’t keep food down, and when she vomited, the food would often come up whole, she said.
“One night I ate ramen before bed. And the next morning, it came out just as it went down,” said Ms. Carew, 42, a registered mental health counseling intern. “I was getting severe heartburn and could not take a couple bites of food without getting nauseous.”
She also had “sulfur burps,” a side effect discussed by some Ozempic users, causing her to taste rotten egg sometimes.
She was diagnosed with gastroparesis. Some types of gastroparesis can be resolved by discontinuing GLP-1 medications, as referenced in two case reports in the Journal of Investigative Medicine.
Gut hormone
GI symptoms are most common with semaglutide because the hormone it imitates, GLP-1, is secreted by cells in the stomach, small intestines, and pancreas, said Anne Peters, MD, director of the USC Clinical Diabetes Programs.
“This is the deal: The side effects are real because it’s a gut hormone. It’s increasing the level of something your body already has,” she said.
But, like Dr. Apovian, Dr. Peters said those side effects can likely be avoided if shots are started at the lowest doses and gradually adjusted up.
While the average starting dose is .25 milligrams, Dr. Peters said she often starts her patients on about an eighth of that – just “a whiff of a dose.”
“It’ll take them months to get up to the starting dose, but what’s the rush?”
Dr. Peters said she also avoids giving diabetes patients the maximum dose, which is 2 milligrams per week for Ozempic (and 2.4 milligrams for Wegovy for weight loss).
When asked about the drugs’ side effects, Novo Nordisk responded that “GLP-1 receptor agonists are a well-established class of medicines, which have demonstrated long-term safety in clinical trials. The most common adverse reactions, as with all GLP-1 [agonists], are gastrointestinal related.”
Is it the drug or the weight loss?
Still, non-gastrointestinal side effects such as hair loss, mood changes, and sunken facial features are reported among semaglutide users across the Internet. While these cases are often anecdotal, they can be very heartfelt.
Celina Horvath Myers, also known as CelinaSpookyBoo, a Canadian YouTuber who took Ozempic for type 2 diabetes, said she began having intense panic attacks and depression after starting the medication.
“Who I have been these last couple weeks, has probably been the scariest time of my life,” she said on her YouTube channel.
While severe depression and anxiety are not established side effects of the medication, some people get anhedonia, said W. Scott Butsch, MD, MSc, director of obesity medicine in the Bariatric and Metabolic Institute at Cleveland Clinic. But that could be a natural consequence of lower appetite, he said, given that food gives most people pleasure in the moment.
Many other reported changes come from the weight loss itself, not the medication, said Dr. Butsch.
“These are drugs that change the body’s weight regulatory system,” he said. “When someone loses weight, you get the shrinking of the fat cells, as well as the atrophy of the muscles. This rapid weight loss may give the appearance of one’s face changing.”
For some people, like Ms. Mendenall, the side effects are worth it. For others, like Ms. Carew, they’re intolerable.
Ms. Carew said she stopped the medication after about 7 months, and gradually worked up to eating solid foods again.
“It’s the American way, we’ve all got to be thin and beautiful,” she said. “But I feel like it’s very unsafe because we just don’t know how seriously our bodies will react to these things in the long term. People see it as a quick fix, but it comes with risks.”
A version of this article first appeared on WebMD.com.