User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
AHA backs screening for cognitive impairment after stroke
Screening for cognitive impairment should be part of multidisciplinary care for stroke survivors, the American Heart Association says in a new scientific statement.
“Cognitive impairment after stroke is very common, is associated with other post-stroke outcomes, and often has significant impact on the quality of life,” Nada El Husseini, MD, MHSc, chair of the scientific statement writing group, told this news organization.
“It is important to screen stroke survivors for cognitive impairment as well as for associated comorbidities such as mood and sleep disorders,” said Dr. El Husseini, associate professor of neurology at Duke University Medical Center in Durham, N.C.
The scientific statement was published online in Stroke. It’s the first to specifically focus on the cognitive impairment resulting from an overt stroke (ischemic or hemorrhagic).
‘Actionable’ considerations for care
The writing group performed a “scoping” review of the literature on the prevalence, diagnosis, and management of poststroke cognitive impairment (PSCI) to provide a framework for “actionable considerations” for clinical practice as well as to highlight gaps needing additional studies, Dr. El Husseini explained.
PSCI, ranging from mild to severe, occurs in up to 60% of stroke survivors in the first year after stroke; yet, it is often underreported and underdiagnosed, the writing group notes.
Up to 20% of stroke survivors who experience mild cognitive impairment fully recover cognitive function, and cognitive recovery is most likely within the first 6 months after a stroke.
However, improvement in cognitive impairment without return to prestroke levels is more frequent than is complete recovery. As many as one in three stroke survivors may develop dementia within 5 years of stroke.
The writing group also notes that PSCI is often associated with other conditions, including physical disability, sleep disorders, behavioral and personality changes, depression, and other neuropsychological changes – each of which may contribute to lower quality of life.
Currently, there is no “gold standard” for cognitive screening following stroke, but several brief cognitive screening tests, including the Mini–Mental State Examination and the Montreal Cognitive Assessment, are widely used to identify cognitive impairment after stroke.
The statement also highlights the importance of assessing cognitive changes over time after stroke. Stroke survivors who experience unexplained difficulties with cognitive-related activities of daily living, following care instructions, or providing a reliable health history may be candidates for additional cognitive screening.
Manage risk factors to prevent repeat stroke
“Anticipatory guidance regarding home and driving safety and, return to work (if applicable) along with interdisciplinary collaboration among different medical and ancillary specialists in the diagnosis and management of cognitive impairment is key for the holistic care of stroke survivors,” Dr. El Husseini told this news organization.
The multidisciplinary poststroke health care team could include neurologists, occupational therapists, speech therapists, nurses, neuropsychologists, gerontologists, and primary care providers.
“Because recurrent stroke is strongly associated with the development of cognitive impairment and dementia, prevention of recurrent strokes should be sought to decrease that risk,” Dr. El Husseini said. This includes addressing stroke risk factors, including high blood pressure, high cholesterol, type 2 diabetes, and atrial fibrillation.
The writing group says research is needed in the future to determine how cognitive impairment develops after stroke and the impact of nonbrain factors, including infection, frailty, and social factors.
Further research is also needed to determine best practices for cognitive screening after stroke, including the development and use of screening instruments that consider demographic, cultural, and linguistic factors in determining “normal” function.
“Perhaps the most pressing need, however, is the development of effective and culturally relevant treatments for poststroke cognitive impairment,” Dr. El Husseini said in a news release.
“We hope to see big enough clinical trials that assess various techniques, medications, and lifestyle changes in diverse groups of patients that may help improve cognitive function,” she added.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Stroke Council, the Council on Cardiovascular Radiology and Intervention, the Council on Hypertension, and the Council on Lifestyle and Cardiometabolic Health.
Screening for cognitive impairment should be part of multidisciplinary care for stroke survivors, the American Heart Association says in a new scientific statement.
“Cognitive impairment after stroke is very common, is associated with other post-stroke outcomes, and often has significant impact on the quality of life,” Nada El Husseini, MD, MHSc, chair of the scientific statement writing group, told this news organization.
“It is important to screen stroke survivors for cognitive impairment as well as for associated comorbidities such as mood and sleep disorders,” said Dr. El Husseini, associate professor of neurology at Duke University Medical Center in Durham, N.C.
The scientific statement was published online in Stroke. It’s the first to specifically focus on the cognitive impairment resulting from an overt stroke (ischemic or hemorrhagic).
‘Actionable’ considerations for care
The writing group performed a “scoping” review of the literature on the prevalence, diagnosis, and management of poststroke cognitive impairment (PSCI) to provide a framework for “actionable considerations” for clinical practice as well as to highlight gaps needing additional studies, Dr. El Husseini explained.
PSCI, ranging from mild to severe, occurs in up to 60% of stroke survivors in the first year after stroke; yet, it is often underreported and underdiagnosed, the writing group notes.
Up to 20% of stroke survivors who experience mild cognitive impairment fully recover cognitive function, and cognitive recovery is most likely within the first 6 months after a stroke.
However, improvement in cognitive impairment without return to prestroke levels is more frequent than is complete recovery. As many as one in three stroke survivors may develop dementia within 5 years of stroke.
The writing group also notes that PSCI is often associated with other conditions, including physical disability, sleep disorders, behavioral and personality changes, depression, and other neuropsychological changes – each of which may contribute to lower quality of life.
Currently, there is no “gold standard” for cognitive screening following stroke, but several brief cognitive screening tests, including the Mini–Mental State Examination and the Montreal Cognitive Assessment, are widely used to identify cognitive impairment after stroke.
The statement also highlights the importance of assessing cognitive changes over time after stroke. Stroke survivors who experience unexplained difficulties with cognitive-related activities of daily living, following care instructions, or providing a reliable health history may be candidates for additional cognitive screening.
Manage risk factors to prevent repeat stroke
“Anticipatory guidance regarding home and driving safety and, return to work (if applicable) along with interdisciplinary collaboration among different medical and ancillary specialists in the diagnosis and management of cognitive impairment is key for the holistic care of stroke survivors,” Dr. El Husseini told this news organization.
The multidisciplinary poststroke health care team could include neurologists, occupational therapists, speech therapists, nurses, neuropsychologists, gerontologists, and primary care providers.
“Because recurrent stroke is strongly associated with the development of cognitive impairment and dementia, prevention of recurrent strokes should be sought to decrease that risk,” Dr. El Husseini said. This includes addressing stroke risk factors, including high blood pressure, high cholesterol, type 2 diabetes, and atrial fibrillation.
The writing group says research is needed in the future to determine how cognitive impairment develops after stroke and the impact of nonbrain factors, including infection, frailty, and social factors.
Further research is also needed to determine best practices for cognitive screening after stroke, including the development and use of screening instruments that consider demographic, cultural, and linguistic factors in determining “normal” function.
“Perhaps the most pressing need, however, is the development of effective and culturally relevant treatments for poststroke cognitive impairment,” Dr. El Husseini said in a news release.
“We hope to see big enough clinical trials that assess various techniques, medications, and lifestyle changes in diverse groups of patients that may help improve cognitive function,” she added.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Stroke Council, the Council on Cardiovascular Radiology and Intervention, the Council on Hypertension, and the Council on Lifestyle and Cardiometabolic Health.
Screening for cognitive impairment should be part of multidisciplinary care for stroke survivors, the American Heart Association says in a new scientific statement.
“Cognitive impairment after stroke is very common, is associated with other post-stroke outcomes, and often has significant impact on the quality of life,” Nada El Husseini, MD, MHSc, chair of the scientific statement writing group, told this news organization.
“It is important to screen stroke survivors for cognitive impairment as well as for associated comorbidities such as mood and sleep disorders,” said Dr. El Husseini, associate professor of neurology at Duke University Medical Center in Durham, N.C.
The scientific statement was published online in Stroke. It’s the first to specifically focus on the cognitive impairment resulting from an overt stroke (ischemic or hemorrhagic).
‘Actionable’ considerations for care
The writing group performed a “scoping” review of the literature on the prevalence, diagnosis, and management of poststroke cognitive impairment (PSCI) to provide a framework for “actionable considerations” for clinical practice as well as to highlight gaps needing additional studies, Dr. El Husseini explained.
PSCI, ranging from mild to severe, occurs in up to 60% of stroke survivors in the first year after stroke; yet, it is often underreported and underdiagnosed, the writing group notes.
Up to 20% of stroke survivors who experience mild cognitive impairment fully recover cognitive function, and cognitive recovery is most likely within the first 6 months after a stroke.
However, improvement in cognitive impairment without return to prestroke levels is more frequent than is complete recovery. As many as one in three stroke survivors may develop dementia within 5 years of stroke.
The writing group also notes that PSCI is often associated with other conditions, including physical disability, sleep disorders, behavioral and personality changes, depression, and other neuropsychological changes – each of which may contribute to lower quality of life.
Currently, there is no “gold standard” for cognitive screening following stroke, but several brief cognitive screening tests, including the Mini–Mental State Examination and the Montreal Cognitive Assessment, are widely used to identify cognitive impairment after stroke.
The statement also highlights the importance of assessing cognitive changes over time after stroke. Stroke survivors who experience unexplained difficulties with cognitive-related activities of daily living, following care instructions, or providing a reliable health history may be candidates for additional cognitive screening.
Manage risk factors to prevent repeat stroke
“Anticipatory guidance regarding home and driving safety and, return to work (if applicable) along with interdisciplinary collaboration among different medical and ancillary specialists in the diagnosis and management of cognitive impairment is key for the holistic care of stroke survivors,” Dr. El Husseini told this news organization.
The multidisciplinary poststroke health care team could include neurologists, occupational therapists, speech therapists, nurses, neuropsychologists, gerontologists, and primary care providers.
“Because recurrent stroke is strongly associated with the development of cognitive impairment and dementia, prevention of recurrent strokes should be sought to decrease that risk,” Dr. El Husseini said. This includes addressing stroke risk factors, including high blood pressure, high cholesterol, type 2 diabetes, and atrial fibrillation.
The writing group says research is needed in the future to determine how cognitive impairment develops after stroke and the impact of nonbrain factors, including infection, frailty, and social factors.
Further research is also needed to determine best practices for cognitive screening after stroke, including the development and use of screening instruments that consider demographic, cultural, and linguistic factors in determining “normal” function.
“Perhaps the most pressing need, however, is the development of effective and culturally relevant treatments for poststroke cognitive impairment,” Dr. El Husseini said in a news release.
“We hope to see big enough clinical trials that assess various techniques, medications, and lifestyle changes in diverse groups of patients that may help improve cognitive function,” she added.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Stroke Council, the Council on Cardiovascular Radiology and Intervention, the Council on Hypertension, and the Council on Lifestyle and Cardiometabolic Health.
Step count–heart rate link confirmed in children
, according to a study presented at the Pediatric Academic Societies annual meeting.
The new findings provide a new means for pediatricians to measure physical fitness, the researchers said.
“It really changes the way we evaluate kids’ fitness and gives us a new method of judging physical fitness other than body mass index,” said Susan Gasparino, MD, an instructor in pediatrics at the University of Rochester (N.Y.) Medical Center, who led the study.
Using data from the 2005 to 2006 National Health and Nutrition Examination Survey, Dr. Gasparino and her colleagues examined the association between resting heart rate (RHR) and step count among 899 children and 1,640 adolescents aged 6-19 years.
In the adolescent group, the mean RHR was 74.9 among those who walked more than 10,000 steps per day (n = 414) and 79.3 for those whose step counts fell below that cutoff (n = 1,226) (P < .001). For each additional 1,000 steps per day, RHR decreased by an average of 0.7 beats per minute in this group (P < .001).
In the younger age group, mean RHR was 85.3 among children who took more than 10,000 steps per day (n = 447) and 86.3 among those who did not reach that threshold (n = 452) (P = .29). For each additional 1,000 steps per day, RHR decreased by an average of 0.3 bpm in this group (P = .02)
Dr. Gasparino said next steps in research could include controlling for confounders, such as baseline anxiety and medications that could blunt the heart rate.
Broader implications
If similar results bear out in future studies, monitoring RHR could be incorporated into fitness programs for children and adolescents. Doing so could obviate “the need for intensive treadmill assessments using VO2max, time-consuming and emotionally fraught school-based physical fitness tests, and the fear and potential shame of the scale,” the researchers said.
Dr. Gasparino said measuring RHR during a 3-minute step test could help organizations and governments determine whether fitness programs are improving cardiovascular and overall health and could help them direct “funding and resources to the programs that are effective.” Such a test could also be incorporated into pediatrician wellness checks, she noted.
“It’s an exciting development, and [RHR measurement] holds a lot of promise as a clinical tool that can be applicable in a lot of settings,” said Nicholas M. Edwards, MD, MPH, a sports medicine pediatrician and an associate professor of orthopedics at the University of Minnesota in Minneapolis.
Dr. Edwards said that, because measurement of fitness in clinical settings is difficult, finding ways to “assess fitness in the office with the equipment already at hand would be a superb development.”
If use of RHR to measure fitness “is validated in a clinical setting,” Dr. Edwards said, “I think adoption would be a natural next step.”
Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, according to a study presented at the Pediatric Academic Societies annual meeting.
The new findings provide a new means for pediatricians to measure physical fitness, the researchers said.
“It really changes the way we evaluate kids’ fitness and gives us a new method of judging physical fitness other than body mass index,” said Susan Gasparino, MD, an instructor in pediatrics at the University of Rochester (N.Y.) Medical Center, who led the study.
Using data from the 2005 to 2006 National Health and Nutrition Examination Survey, Dr. Gasparino and her colleagues examined the association between resting heart rate (RHR) and step count among 899 children and 1,640 adolescents aged 6-19 years.
In the adolescent group, the mean RHR was 74.9 among those who walked more than 10,000 steps per day (n = 414) and 79.3 for those whose step counts fell below that cutoff (n = 1,226) (P < .001). For each additional 1,000 steps per day, RHR decreased by an average of 0.7 beats per minute in this group (P < .001).
In the younger age group, mean RHR was 85.3 among children who took more than 10,000 steps per day (n = 447) and 86.3 among those who did not reach that threshold (n = 452) (P = .29). For each additional 1,000 steps per day, RHR decreased by an average of 0.3 bpm in this group (P = .02)
Dr. Gasparino said next steps in research could include controlling for confounders, such as baseline anxiety and medications that could blunt the heart rate.
Broader implications
If similar results bear out in future studies, monitoring RHR could be incorporated into fitness programs for children and adolescents. Doing so could obviate “the need for intensive treadmill assessments using VO2max, time-consuming and emotionally fraught school-based physical fitness tests, and the fear and potential shame of the scale,” the researchers said.
Dr. Gasparino said measuring RHR during a 3-minute step test could help organizations and governments determine whether fitness programs are improving cardiovascular and overall health and could help them direct “funding and resources to the programs that are effective.” Such a test could also be incorporated into pediatrician wellness checks, she noted.
“It’s an exciting development, and [RHR measurement] holds a lot of promise as a clinical tool that can be applicable in a lot of settings,” said Nicholas M. Edwards, MD, MPH, a sports medicine pediatrician and an associate professor of orthopedics at the University of Minnesota in Minneapolis.
Dr. Edwards said that, because measurement of fitness in clinical settings is difficult, finding ways to “assess fitness in the office with the equipment already at hand would be a superb development.”
If use of RHR to measure fitness “is validated in a clinical setting,” Dr. Edwards said, “I think adoption would be a natural next step.”
Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, according to a study presented at the Pediatric Academic Societies annual meeting.
The new findings provide a new means for pediatricians to measure physical fitness, the researchers said.
“It really changes the way we evaluate kids’ fitness and gives us a new method of judging physical fitness other than body mass index,” said Susan Gasparino, MD, an instructor in pediatrics at the University of Rochester (N.Y.) Medical Center, who led the study.
Using data from the 2005 to 2006 National Health and Nutrition Examination Survey, Dr. Gasparino and her colleagues examined the association between resting heart rate (RHR) and step count among 899 children and 1,640 adolescents aged 6-19 years.
In the adolescent group, the mean RHR was 74.9 among those who walked more than 10,000 steps per day (n = 414) and 79.3 for those whose step counts fell below that cutoff (n = 1,226) (P < .001). For each additional 1,000 steps per day, RHR decreased by an average of 0.7 beats per minute in this group (P < .001).
In the younger age group, mean RHR was 85.3 among children who took more than 10,000 steps per day (n = 447) and 86.3 among those who did not reach that threshold (n = 452) (P = .29). For each additional 1,000 steps per day, RHR decreased by an average of 0.3 bpm in this group (P = .02)
Dr. Gasparino said next steps in research could include controlling for confounders, such as baseline anxiety and medications that could blunt the heart rate.
Broader implications
If similar results bear out in future studies, monitoring RHR could be incorporated into fitness programs for children and adolescents. Doing so could obviate “the need for intensive treadmill assessments using VO2max, time-consuming and emotionally fraught school-based physical fitness tests, and the fear and potential shame of the scale,” the researchers said.
Dr. Gasparino said measuring RHR during a 3-minute step test could help organizations and governments determine whether fitness programs are improving cardiovascular and overall health and could help them direct “funding and resources to the programs that are effective.” Such a test could also be incorporated into pediatrician wellness checks, she noted.
“It’s an exciting development, and [RHR measurement] holds a lot of promise as a clinical tool that can be applicable in a lot of settings,” said Nicholas M. Edwards, MD, MPH, a sports medicine pediatrician and an associate professor of orthopedics at the University of Minnesota in Minneapolis.
Dr. Edwards said that, because measurement of fitness in clinical settings is difficult, finding ways to “assess fitness in the office with the equipment already at hand would be a superb development.”
If use of RHR to measure fitness “is validated in a clinical setting,” Dr. Edwards said, “I think adoption would be a natural next step.”
Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM PAS 2023
Long-COVID rate may be higher with rheumatic diseases
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
AT BSR 2023
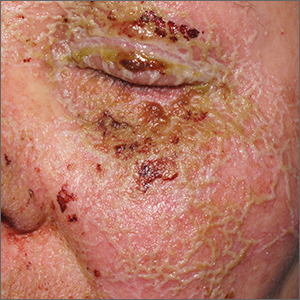
Pustules on face
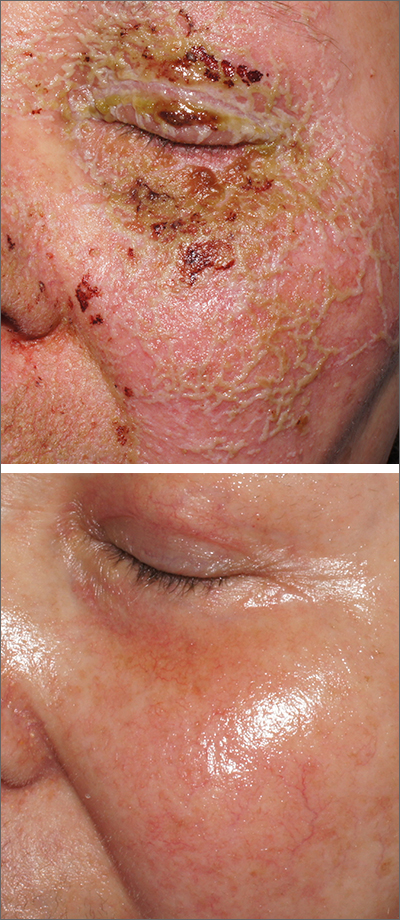
A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bouché O, Ben Abdelghani M, Labourey JL, et al. Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol. 2019;25:4007-4018. doi: 10.3748/wjg.v25.i29.4007

A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bouché O, Ben Abdelghani M, Labourey JL, et al. Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol. 2019;25:4007-4018. doi: 10.3748/wjg.v25.i29.4007
1. Bouché O, Ben Abdelghani M, Labourey JL, et al. Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol. 2019;25:4007-4018. doi: 10.3748/wjg.v25.i29.4007
Lose weight, gain huge debt: N.Y. provider has sued more than 300 patients who had bariatric surgery
Seven months after Lahavah Wallace’s weight-loss operation, a New York bariatric surgery practice sued her, accusing her of “intentionally” failing to pay nearly $18,000 of her bill.
Long Island Minimally Invasive Surgery, which does business as the New York Bariatric Group, went on to accuse Ms. Wallace of “embezzlement,” alleging she kept insurance payments that should have been turned over to the practice.
Ms. Wallace denies the allegations, which the bariatric practice has leveled against patients in hundreds of debt-collection lawsuits filed over the past 4 years, court records in New York state show.
In about 60 cases, the lawsuits demanded $100,000 or more from patients. Some patients were found liable for tens of thousands of dollars in interest charges or wound up shackled with debt that could take a decade or more to shake. Others are facing the likely prospect of six-figure financial penalties, court records show.
Backed by a major private equity firm, the bariatric practice spends millions each year on advertisements featuring patients who have dropped 100 pounds or more after bariatric procedures, sometimes having had a portion of their stomachs removed. The ads have run on TV, online, and on New York City subway posters.
The online ads, often showcasing the slogan “Stop obesity for life,” appealed to Ms. Wallace, who lives in Brooklyn and works as a legal assistant for the state of New York. She said she turned over checks from her insurer to the bariatric group and was stunned when the medical practice hauled her into court citing an “out-of-network payment agreement” she had signed before her surgery.
“I really didn’t know what I was signing,” Ms. Wallace told KFF Health News. “I didn’t pay enough attention.”
Shawn Garber, MD, a bariatric surgeon who founded the practice in 2000 on Long Island and serves as its CEO, said that “prior to rendering services” his office staff advises patients of the costs and their responsibility to pay the bill.
The bariatric group has cited these out-of-network payment agreements in at least 300 lawsuits filed against patients from January 2019 to 2022 demanding nearly $19 million to cover medical bills, interest charges, and attorney’s fees, a KFF Health News review of New York state court records found.
Danny De Voe, a partner at Sahn Ward Braff Koblenz law firm in Uniondale, N.Y., who filed many of those suits, declined to comment, citing attorney-client privilege.
In most cases, the medical practice had agreed to accept an insurance company’s out-of-network rate as full payment for its services – with caveats, according to court filings.
In the agreements they signed, patients promised to pay any coinsurance, meeting any deductible, and pass on to the medical practice any reimbursement checks they received from their health plans within 7 days.
KFF Health News found – while legal fees and other costs can layer on thousands more.
Elisabeth Benjamin, a lawyer with the Community Service Society of New York, said conflicts can arise when insurers send checks to pay for out-of-network medical services to patients rather than reimbursing a medical provider directly.
“We would prefer to see regulators step in and stop that practice,” she said, adding it “causes tension between providers and patients.”
That’s certainly true for Ms. Wallace. The surgery practice sued her in August 2022demanding $17,981 in fees it said remained unpaid after her January 2022 laparoscopic sleeve gastrectomy, an operation in which much of the stomach is removed to assist weight loss.
The lawsuit also tacked on a demand for $5,993 in attorney’s fees, court records show.
The suit alleges Ms. Wallace signed the contract even though she “had no intention” of paying her bills. The complaint goes on to accuse her of “committing embezzlement” by “willfully, intentionally, deliberately and maliciously” depositing checks from her health plan into her personal account.
The suit doesn’t include details to substantiate these claims, and Ms. Wallace said in her court response they are not true. Ms. Wallace said she turned over checks for the charges.
“They billed the insurance for everything they possibly could,” Ms. Wallace said.
In September, Ms. Wallace filed for bankruptcy, hoping to discharge the bariatric care debt along with about $4,700 in unrelated credit card charges.
The medical practice fired back in November by filing an “adversary complaint” in her Brooklyn bankruptcy court proceeding that argues her medical debt should not be forgiven because Ms. Wallace committed fraud.
The adversary complaint, which is pending in the bankruptcy case, accuses Ms. Wallace of “fraudulently” inducing the surgery center to perform “elective medical procedures” without requiring payment up front.
Both the harsh wording and claims of wrongdoing have infuriated Ms. Wallace and her attorney, Jacob Silver, of Brooklyn.
Mr. Silver wants the medical practice to turn over records of the payments received from Ms. Wallace. “There is no fraud here,” he said. “This is frivolous. We are taking a no-settlement position.”
Gaining debt
Few patients sued by the bariatric practice mount a defense in court and those who do fight often lose, court records show.
The medical practice won default judgments totaling nearly $6 million in about 90 of the 300 cases in the sample reviewed by KFF Health News. Default judgments are entered when the defendant fails to respond.
Many cases either are pending, or it is not clear from court filings how they were resolved.
Some patients tried to argue that the fees were too high or that they didn’t understand going in how much they could owe. One woman, trying to push back against a demand for more than $100,000, said in a legal filing that she “was given numerous papers to sign without anyone of the staff members explaining to me what it actually meant.” Another patient, who was sued for more than $40,000, wrote: “I don’t have the means to pay this bill.”
Among the cases described in court records:
- A Westchester County, N.Y., woman was sued for $102,556 and settled for $72,000 in May 2021. She agreed to pay $7,500 upon signing the settlement and $500 a month from September 2021 to May 2032.
- A Peekskill, N.Y., woman in a December 2019 judgment was held liable for $384,092, which included $94,047 in interest.
- A Newburgh, N.Y., man was sued in 2021 for $252,309 in medical bills, 12% interest, and $84,103 in attorneys’ fees. The case is pending.
Robert Cohen, a longtime attorney for the bariatric practice, testified in a November 2021 hearing that the lawyers take “a contingency fee of one-third of our recovery” in these cases. In that case, Mr. Cohen had requested $13,578 based on his contingency fee arrangement. He testified that he spent 7.3 hours on the case and that his customary billing rate was $475 per hour, which came to $3,467.50. The judge awarded the lower amount, according to a transcript of the hearing.
Teresa LaMasters, MD, president of the American Society for Metabolic and Bariatric Surgery, said suing patients for large sums “is not a common practice” among bariatric surgeons.
“This is not what the vast majority in the field would espouse,” she said.
But Dr. Garber, the NYBG’s chief executive, suggested patients deserve blame.
“These lawsuits stem from these patients stealing the insurance money rather than forwarding it onto NYBG as they are morally and contractually obligated to do,” Dr. Garber wrote in an email to KFF Health News.
Dr. Garber added: “The issue is not with what we bill, but rather with the fact that the insurance companies refuse to send payment directly to us.”
‘A kooky system’
Defense attorneys argue that many patients don’t fully comprehend the perils of failing to pay on time – for whatever reason.
In a few cases, patients admitted pocketing checks they were obligated to turn over to the medical practice. But for the most part, court records don’t specify how many such checks were issued and for what amounts – or whether the patient improperly cashed them.
“It’s a kooky system,” said Paul Brite, an attorney who has faced off against the bariatric practice in court.
“You sign these documents that could cost you tons of money. It shouldn’t be that way,” he said. “This can ruin their financial life.”
New York lawmakers have acted to limit the damage from medical debt, including “surprise bills.”
In November, Democratic Gov. Kathy Hochul signed legislation that prohibits health care providers from slapping liens on a primary residence or garnishing wages.
But contracts with onerous repayment terms represent an “evolving area of law” and an alarming “new twist” on concerns over medical debt, said Ms. Benjamin, the community service society lawyer.
She said contract “accelerator clauses” that trigger severe penalties if patients miss payments should not be permitted for medical debt.
“If you default, the full amount is due,” she said. “This is really a bummer.”
‘Fair market value’
The debt collection lawsuits argue that weight-loss patients had agreed to pay “fair market value” for services – and the doctors are only trying to secure money they are due.
But some prices far exceed typical insurance payments for obesity treatments across the country, according to a medical billing data registry. Surgeons performed about 200,000 bariatric operations in 2020, according to the bariatric surgery society.
Ms. Wallace, the Brooklyn legal assistant, was billed $60,500 for her lap sleeve gastrectomy, though how much her insurance actually paid remains to be hashed out in court.
Michael Arrigo, a California medical billing expert at No World Borders, called the prices “outrageous” and “unreasonable and, in fact, likely unconscionable.”
“I disagree that these are fair market charges,” he said.
Dr. LaMasters called the gastrectomy price billed to Ms. Wallace “really expensive” and “a severe outlier.” While charges vary by region, she quoted a typical price of around $22,000.
Dr. Garber said NYBG “bills at usual and customary rates” determined by Fair Health, a New York City-based repository of insurance claims data. Fair Health “sets these rates based upon the acceptable price for our geographic location,” he said.
But Rachel Kent, Fair Health’s senior director of marketing, told KFF Health News that the group “does not set rates, nor determine or take any position on what constitutes ‘usual and customary rates.’ ” Instead, it reports the prices providers are charging in a given area.
Overall, Fair Health data shows huge price variations even in adjacent ZIP codes in the metro area. In Long Island’s Roslyn Heights neighborhood, where NYBG is based, Fair Health lists the out-of-network price charged by providers in the area as $60,500, the figure Ms. Wallace was billed.
But in several other New York City–area ZIP codes the price charged for the gastrectomy procedure hovers around $20,000, according to the data bank. The price in Manhattan is $17,500, for instance, according to Fair Health.
Nationwide, the average cost in 2021 for bariatric surgery done in a hospital was $32,868, according to a KFF analysis of health insurance claims.
Private equity arrives
Dr. Garber said in a court affidavit in May 2022 that he founded the bariatric practice “with a singular focus: providing safe, effective care to patients suffering from obesity and its resulting complications.”
Under his leadership, the practice has “developed into New York’s elite institution for obesity treatment,” Dr. Garber said. He said the group’s surgeons are “highly sought after to train other bariatric surgeons throughout the country and are active in the development of new, cutting-edge bariatric surgery techniques.”
In 2017, Dr. Garber and partners agreed on a business plan to help spur growth and “attract private equity investment,” according to the affidavit.
They formed a separate company to handle the bariatric practice’s business side. Known as management services organizations, such companies provide a way for private equity investors to circumvent laws in some states that prohibit nonphysicians from owning a stake in a medical practice.
In August 2019, the private equity firm Sentinel Capital Partners bought 65% of the MSO for $156.5 million, according to Dr. Garber’s affidavit. The management company is now known as New You Bariatric Group. The private equity firm did not respond to requests for comment.
Dr. Garber, in a September 2021 American Society for Metabolic and Bariatric Surgery webinar viewable online, said the weight-loss practice spends $6 million a year on media and marketing directly to patients – and is on a roll. Nationally, bariatric surgery is growing 6% annually, he said. NYBG boasts two dozen offices in the tri-state area of New York, New Jersey, and Connecticut and is poised to expand into more states.
“Since private equity, we’ve been growing at 30%-40% year over year,” Dr. Garber said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Seven months after Lahavah Wallace’s weight-loss operation, a New York bariatric surgery practice sued her, accusing her of “intentionally” failing to pay nearly $18,000 of her bill.
Long Island Minimally Invasive Surgery, which does business as the New York Bariatric Group, went on to accuse Ms. Wallace of “embezzlement,” alleging she kept insurance payments that should have been turned over to the practice.
Ms. Wallace denies the allegations, which the bariatric practice has leveled against patients in hundreds of debt-collection lawsuits filed over the past 4 years, court records in New York state show.
In about 60 cases, the lawsuits demanded $100,000 or more from patients. Some patients were found liable for tens of thousands of dollars in interest charges or wound up shackled with debt that could take a decade or more to shake. Others are facing the likely prospect of six-figure financial penalties, court records show.
Backed by a major private equity firm, the bariatric practice spends millions each year on advertisements featuring patients who have dropped 100 pounds or more after bariatric procedures, sometimes having had a portion of their stomachs removed. The ads have run on TV, online, and on New York City subway posters.
The online ads, often showcasing the slogan “Stop obesity for life,” appealed to Ms. Wallace, who lives in Brooklyn and works as a legal assistant for the state of New York. She said she turned over checks from her insurer to the bariatric group and was stunned when the medical practice hauled her into court citing an “out-of-network payment agreement” she had signed before her surgery.
“I really didn’t know what I was signing,” Ms. Wallace told KFF Health News. “I didn’t pay enough attention.”
Shawn Garber, MD, a bariatric surgeon who founded the practice in 2000 on Long Island and serves as its CEO, said that “prior to rendering services” his office staff advises patients of the costs and their responsibility to pay the bill.
The bariatric group has cited these out-of-network payment agreements in at least 300 lawsuits filed against patients from January 2019 to 2022 demanding nearly $19 million to cover medical bills, interest charges, and attorney’s fees, a KFF Health News review of New York state court records found.
Danny De Voe, a partner at Sahn Ward Braff Koblenz law firm in Uniondale, N.Y., who filed many of those suits, declined to comment, citing attorney-client privilege.
In most cases, the medical practice had agreed to accept an insurance company’s out-of-network rate as full payment for its services – with caveats, according to court filings.
In the agreements they signed, patients promised to pay any coinsurance, meeting any deductible, and pass on to the medical practice any reimbursement checks they received from their health plans within 7 days.
KFF Health News found – while legal fees and other costs can layer on thousands more.
Elisabeth Benjamin, a lawyer with the Community Service Society of New York, said conflicts can arise when insurers send checks to pay for out-of-network medical services to patients rather than reimbursing a medical provider directly.
“We would prefer to see regulators step in and stop that practice,” she said, adding it “causes tension between providers and patients.”
That’s certainly true for Ms. Wallace. The surgery practice sued her in August 2022demanding $17,981 in fees it said remained unpaid after her January 2022 laparoscopic sleeve gastrectomy, an operation in which much of the stomach is removed to assist weight loss.
The lawsuit also tacked on a demand for $5,993 in attorney’s fees, court records show.
The suit alleges Ms. Wallace signed the contract even though she “had no intention” of paying her bills. The complaint goes on to accuse her of “committing embezzlement” by “willfully, intentionally, deliberately and maliciously” depositing checks from her health plan into her personal account.
The suit doesn’t include details to substantiate these claims, and Ms. Wallace said in her court response they are not true. Ms. Wallace said she turned over checks for the charges.
“They billed the insurance for everything they possibly could,” Ms. Wallace said.
In September, Ms. Wallace filed for bankruptcy, hoping to discharge the bariatric care debt along with about $4,700 in unrelated credit card charges.
The medical practice fired back in November by filing an “adversary complaint” in her Brooklyn bankruptcy court proceeding that argues her medical debt should not be forgiven because Ms. Wallace committed fraud.
The adversary complaint, which is pending in the bankruptcy case, accuses Ms. Wallace of “fraudulently” inducing the surgery center to perform “elective medical procedures” without requiring payment up front.
Both the harsh wording and claims of wrongdoing have infuriated Ms. Wallace and her attorney, Jacob Silver, of Brooklyn.
Mr. Silver wants the medical practice to turn over records of the payments received from Ms. Wallace. “There is no fraud here,” he said. “This is frivolous. We are taking a no-settlement position.”
Gaining debt
Few patients sued by the bariatric practice mount a defense in court and those who do fight often lose, court records show.
The medical practice won default judgments totaling nearly $6 million in about 90 of the 300 cases in the sample reviewed by KFF Health News. Default judgments are entered when the defendant fails to respond.
Many cases either are pending, or it is not clear from court filings how they were resolved.
Some patients tried to argue that the fees were too high or that they didn’t understand going in how much they could owe. One woman, trying to push back against a demand for more than $100,000, said in a legal filing that she “was given numerous papers to sign without anyone of the staff members explaining to me what it actually meant.” Another patient, who was sued for more than $40,000, wrote: “I don’t have the means to pay this bill.”
Among the cases described in court records:
- A Westchester County, N.Y., woman was sued for $102,556 and settled for $72,000 in May 2021. She agreed to pay $7,500 upon signing the settlement and $500 a month from September 2021 to May 2032.
- A Peekskill, N.Y., woman in a December 2019 judgment was held liable for $384,092, which included $94,047 in interest.
- A Newburgh, N.Y., man was sued in 2021 for $252,309 in medical bills, 12% interest, and $84,103 in attorneys’ fees. The case is pending.
Robert Cohen, a longtime attorney for the bariatric practice, testified in a November 2021 hearing that the lawyers take “a contingency fee of one-third of our recovery” in these cases. In that case, Mr. Cohen had requested $13,578 based on his contingency fee arrangement. He testified that he spent 7.3 hours on the case and that his customary billing rate was $475 per hour, which came to $3,467.50. The judge awarded the lower amount, according to a transcript of the hearing.
Teresa LaMasters, MD, president of the American Society for Metabolic and Bariatric Surgery, said suing patients for large sums “is not a common practice” among bariatric surgeons.
“This is not what the vast majority in the field would espouse,” she said.
But Dr. Garber, the NYBG’s chief executive, suggested patients deserve blame.
“These lawsuits stem from these patients stealing the insurance money rather than forwarding it onto NYBG as they are morally and contractually obligated to do,” Dr. Garber wrote in an email to KFF Health News.
Dr. Garber added: “The issue is not with what we bill, but rather with the fact that the insurance companies refuse to send payment directly to us.”
‘A kooky system’
Defense attorneys argue that many patients don’t fully comprehend the perils of failing to pay on time – for whatever reason.
In a few cases, patients admitted pocketing checks they were obligated to turn over to the medical practice. But for the most part, court records don’t specify how many such checks were issued and for what amounts – or whether the patient improperly cashed them.
“It’s a kooky system,” said Paul Brite, an attorney who has faced off against the bariatric practice in court.
“You sign these documents that could cost you tons of money. It shouldn’t be that way,” he said. “This can ruin their financial life.”
New York lawmakers have acted to limit the damage from medical debt, including “surprise bills.”
In November, Democratic Gov. Kathy Hochul signed legislation that prohibits health care providers from slapping liens on a primary residence or garnishing wages.
But contracts with onerous repayment terms represent an “evolving area of law” and an alarming “new twist” on concerns over medical debt, said Ms. Benjamin, the community service society lawyer.
She said contract “accelerator clauses” that trigger severe penalties if patients miss payments should not be permitted for medical debt.
“If you default, the full amount is due,” she said. “This is really a bummer.”
‘Fair market value’
The debt collection lawsuits argue that weight-loss patients had agreed to pay “fair market value” for services – and the doctors are only trying to secure money they are due.
But some prices far exceed typical insurance payments for obesity treatments across the country, according to a medical billing data registry. Surgeons performed about 200,000 bariatric operations in 2020, according to the bariatric surgery society.
Ms. Wallace, the Brooklyn legal assistant, was billed $60,500 for her lap sleeve gastrectomy, though how much her insurance actually paid remains to be hashed out in court.
Michael Arrigo, a California medical billing expert at No World Borders, called the prices “outrageous” and “unreasonable and, in fact, likely unconscionable.”
“I disagree that these are fair market charges,” he said.
Dr. LaMasters called the gastrectomy price billed to Ms. Wallace “really expensive” and “a severe outlier.” While charges vary by region, she quoted a typical price of around $22,000.
Dr. Garber said NYBG “bills at usual and customary rates” determined by Fair Health, a New York City-based repository of insurance claims data. Fair Health “sets these rates based upon the acceptable price for our geographic location,” he said.
But Rachel Kent, Fair Health’s senior director of marketing, told KFF Health News that the group “does not set rates, nor determine or take any position on what constitutes ‘usual and customary rates.’ ” Instead, it reports the prices providers are charging in a given area.
Overall, Fair Health data shows huge price variations even in adjacent ZIP codes in the metro area. In Long Island’s Roslyn Heights neighborhood, where NYBG is based, Fair Health lists the out-of-network price charged by providers in the area as $60,500, the figure Ms. Wallace was billed.
But in several other New York City–area ZIP codes the price charged for the gastrectomy procedure hovers around $20,000, according to the data bank. The price in Manhattan is $17,500, for instance, according to Fair Health.
Nationwide, the average cost in 2021 for bariatric surgery done in a hospital was $32,868, according to a KFF analysis of health insurance claims.
Private equity arrives
Dr. Garber said in a court affidavit in May 2022 that he founded the bariatric practice “with a singular focus: providing safe, effective care to patients suffering from obesity and its resulting complications.”
Under his leadership, the practice has “developed into New York’s elite institution for obesity treatment,” Dr. Garber said. He said the group’s surgeons are “highly sought after to train other bariatric surgeons throughout the country and are active in the development of new, cutting-edge bariatric surgery techniques.”
In 2017, Dr. Garber and partners agreed on a business plan to help spur growth and “attract private equity investment,” according to the affidavit.
They formed a separate company to handle the bariatric practice’s business side. Known as management services organizations, such companies provide a way for private equity investors to circumvent laws in some states that prohibit nonphysicians from owning a stake in a medical practice.
In August 2019, the private equity firm Sentinel Capital Partners bought 65% of the MSO for $156.5 million, according to Dr. Garber’s affidavit. The management company is now known as New You Bariatric Group. The private equity firm did not respond to requests for comment.
Dr. Garber, in a September 2021 American Society for Metabolic and Bariatric Surgery webinar viewable online, said the weight-loss practice spends $6 million a year on media and marketing directly to patients – and is on a roll. Nationally, bariatric surgery is growing 6% annually, he said. NYBG boasts two dozen offices in the tri-state area of New York, New Jersey, and Connecticut and is poised to expand into more states.
“Since private equity, we’ve been growing at 30%-40% year over year,” Dr. Garber said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Seven months after Lahavah Wallace’s weight-loss operation, a New York bariatric surgery practice sued her, accusing her of “intentionally” failing to pay nearly $18,000 of her bill.
Long Island Minimally Invasive Surgery, which does business as the New York Bariatric Group, went on to accuse Ms. Wallace of “embezzlement,” alleging she kept insurance payments that should have been turned over to the practice.
Ms. Wallace denies the allegations, which the bariatric practice has leveled against patients in hundreds of debt-collection lawsuits filed over the past 4 years, court records in New York state show.
In about 60 cases, the lawsuits demanded $100,000 or more from patients. Some patients were found liable for tens of thousands of dollars in interest charges or wound up shackled with debt that could take a decade or more to shake. Others are facing the likely prospect of six-figure financial penalties, court records show.
Backed by a major private equity firm, the bariatric practice spends millions each year on advertisements featuring patients who have dropped 100 pounds or more after bariatric procedures, sometimes having had a portion of their stomachs removed. The ads have run on TV, online, and on New York City subway posters.
The online ads, often showcasing the slogan “Stop obesity for life,” appealed to Ms. Wallace, who lives in Brooklyn and works as a legal assistant for the state of New York. She said she turned over checks from her insurer to the bariatric group and was stunned when the medical practice hauled her into court citing an “out-of-network payment agreement” she had signed before her surgery.
“I really didn’t know what I was signing,” Ms. Wallace told KFF Health News. “I didn’t pay enough attention.”
Shawn Garber, MD, a bariatric surgeon who founded the practice in 2000 on Long Island and serves as its CEO, said that “prior to rendering services” his office staff advises patients of the costs and their responsibility to pay the bill.
The bariatric group has cited these out-of-network payment agreements in at least 300 lawsuits filed against patients from January 2019 to 2022 demanding nearly $19 million to cover medical bills, interest charges, and attorney’s fees, a KFF Health News review of New York state court records found.
Danny De Voe, a partner at Sahn Ward Braff Koblenz law firm in Uniondale, N.Y., who filed many of those suits, declined to comment, citing attorney-client privilege.
In most cases, the medical practice had agreed to accept an insurance company’s out-of-network rate as full payment for its services – with caveats, according to court filings.
In the agreements they signed, patients promised to pay any coinsurance, meeting any deductible, and pass on to the medical practice any reimbursement checks they received from their health plans within 7 days.
KFF Health News found – while legal fees and other costs can layer on thousands more.
Elisabeth Benjamin, a lawyer with the Community Service Society of New York, said conflicts can arise when insurers send checks to pay for out-of-network medical services to patients rather than reimbursing a medical provider directly.
“We would prefer to see regulators step in and stop that practice,” she said, adding it “causes tension between providers and patients.”
That’s certainly true for Ms. Wallace. The surgery practice sued her in August 2022demanding $17,981 in fees it said remained unpaid after her January 2022 laparoscopic sleeve gastrectomy, an operation in which much of the stomach is removed to assist weight loss.
The lawsuit also tacked on a demand for $5,993 in attorney’s fees, court records show.
The suit alleges Ms. Wallace signed the contract even though she “had no intention” of paying her bills. The complaint goes on to accuse her of “committing embezzlement” by “willfully, intentionally, deliberately and maliciously” depositing checks from her health plan into her personal account.
The suit doesn’t include details to substantiate these claims, and Ms. Wallace said in her court response they are not true. Ms. Wallace said she turned over checks for the charges.
“They billed the insurance for everything they possibly could,” Ms. Wallace said.
In September, Ms. Wallace filed for bankruptcy, hoping to discharge the bariatric care debt along with about $4,700 in unrelated credit card charges.
The medical practice fired back in November by filing an “adversary complaint” in her Brooklyn bankruptcy court proceeding that argues her medical debt should not be forgiven because Ms. Wallace committed fraud.
The adversary complaint, which is pending in the bankruptcy case, accuses Ms. Wallace of “fraudulently” inducing the surgery center to perform “elective medical procedures” without requiring payment up front.
Both the harsh wording and claims of wrongdoing have infuriated Ms. Wallace and her attorney, Jacob Silver, of Brooklyn.
Mr. Silver wants the medical practice to turn over records of the payments received from Ms. Wallace. “There is no fraud here,” he said. “This is frivolous. We are taking a no-settlement position.”
Gaining debt
Few patients sued by the bariatric practice mount a defense in court and those who do fight often lose, court records show.
The medical practice won default judgments totaling nearly $6 million in about 90 of the 300 cases in the sample reviewed by KFF Health News. Default judgments are entered when the defendant fails to respond.
Many cases either are pending, or it is not clear from court filings how they were resolved.
Some patients tried to argue that the fees were too high or that they didn’t understand going in how much they could owe. One woman, trying to push back against a demand for more than $100,000, said in a legal filing that she “was given numerous papers to sign without anyone of the staff members explaining to me what it actually meant.” Another patient, who was sued for more than $40,000, wrote: “I don’t have the means to pay this bill.”
Among the cases described in court records:
- A Westchester County, N.Y., woman was sued for $102,556 and settled for $72,000 in May 2021. She agreed to pay $7,500 upon signing the settlement and $500 a month from September 2021 to May 2032.
- A Peekskill, N.Y., woman in a December 2019 judgment was held liable for $384,092, which included $94,047 in interest.
- A Newburgh, N.Y., man was sued in 2021 for $252,309 in medical bills, 12% interest, and $84,103 in attorneys’ fees. The case is pending.
Robert Cohen, a longtime attorney for the bariatric practice, testified in a November 2021 hearing that the lawyers take “a contingency fee of one-third of our recovery” in these cases. In that case, Mr. Cohen had requested $13,578 based on his contingency fee arrangement. He testified that he spent 7.3 hours on the case and that his customary billing rate was $475 per hour, which came to $3,467.50. The judge awarded the lower amount, according to a transcript of the hearing.
Teresa LaMasters, MD, president of the American Society for Metabolic and Bariatric Surgery, said suing patients for large sums “is not a common practice” among bariatric surgeons.
“This is not what the vast majority in the field would espouse,” she said.
But Dr. Garber, the NYBG’s chief executive, suggested patients deserve blame.
“These lawsuits stem from these patients stealing the insurance money rather than forwarding it onto NYBG as they are morally and contractually obligated to do,” Dr. Garber wrote in an email to KFF Health News.
Dr. Garber added: “The issue is not with what we bill, but rather with the fact that the insurance companies refuse to send payment directly to us.”
‘A kooky system’
Defense attorneys argue that many patients don’t fully comprehend the perils of failing to pay on time – for whatever reason.
In a few cases, patients admitted pocketing checks they were obligated to turn over to the medical practice. But for the most part, court records don’t specify how many such checks were issued and for what amounts – or whether the patient improperly cashed them.
“It’s a kooky system,” said Paul Brite, an attorney who has faced off against the bariatric practice in court.
“You sign these documents that could cost you tons of money. It shouldn’t be that way,” he said. “This can ruin their financial life.”
New York lawmakers have acted to limit the damage from medical debt, including “surprise bills.”
In November, Democratic Gov. Kathy Hochul signed legislation that prohibits health care providers from slapping liens on a primary residence or garnishing wages.
But contracts with onerous repayment terms represent an “evolving area of law” and an alarming “new twist” on concerns over medical debt, said Ms. Benjamin, the community service society lawyer.
She said contract “accelerator clauses” that trigger severe penalties if patients miss payments should not be permitted for medical debt.
“If you default, the full amount is due,” she said. “This is really a bummer.”
‘Fair market value’
The debt collection lawsuits argue that weight-loss patients had agreed to pay “fair market value” for services – and the doctors are only trying to secure money they are due.
But some prices far exceed typical insurance payments for obesity treatments across the country, according to a medical billing data registry. Surgeons performed about 200,000 bariatric operations in 2020, according to the bariatric surgery society.
Ms. Wallace, the Brooklyn legal assistant, was billed $60,500 for her lap sleeve gastrectomy, though how much her insurance actually paid remains to be hashed out in court.
Michael Arrigo, a California medical billing expert at No World Borders, called the prices “outrageous” and “unreasonable and, in fact, likely unconscionable.”
“I disagree that these are fair market charges,” he said.
Dr. LaMasters called the gastrectomy price billed to Ms. Wallace “really expensive” and “a severe outlier.” While charges vary by region, she quoted a typical price of around $22,000.
Dr. Garber said NYBG “bills at usual and customary rates” determined by Fair Health, a New York City-based repository of insurance claims data. Fair Health “sets these rates based upon the acceptable price for our geographic location,” he said.
But Rachel Kent, Fair Health’s senior director of marketing, told KFF Health News that the group “does not set rates, nor determine or take any position on what constitutes ‘usual and customary rates.’ ” Instead, it reports the prices providers are charging in a given area.
Overall, Fair Health data shows huge price variations even in adjacent ZIP codes in the metro area. In Long Island’s Roslyn Heights neighborhood, where NYBG is based, Fair Health lists the out-of-network price charged by providers in the area as $60,500, the figure Ms. Wallace was billed.
But in several other New York City–area ZIP codes the price charged for the gastrectomy procedure hovers around $20,000, according to the data bank. The price in Manhattan is $17,500, for instance, according to Fair Health.
Nationwide, the average cost in 2021 for bariatric surgery done in a hospital was $32,868, according to a KFF analysis of health insurance claims.
Private equity arrives
Dr. Garber said in a court affidavit in May 2022 that he founded the bariatric practice “with a singular focus: providing safe, effective care to patients suffering from obesity and its resulting complications.”
Under his leadership, the practice has “developed into New York’s elite institution for obesity treatment,” Dr. Garber said. He said the group’s surgeons are “highly sought after to train other bariatric surgeons throughout the country and are active in the development of new, cutting-edge bariatric surgery techniques.”
In 2017, Dr. Garber and partners agreed on a business plan to help spur growth and “attract private equity investment,” according to the affidavit.
They formed a separate company to handle the bariatric practice’s business side. Known as management services organizations, such companies provide a way for private equity investors to circumvent laws in some states that prohibit nonphysicians from owning a stake in a medical practice.
In August 2019, the private equity firm Sentinel Capital Partners bought 65% of the MSO for $156.5 million, according to Dr. Garber’s affidavit. The management company is now known as New You Bariatric Group. The private equity firm did not respond to requests for comment.
Dr. Garber, in a September 2021 American Society for Metabolic and Bariatric Surgery webinar viewable online, said the weight-loss practice spends $6 million a year on media and marketing directly to patients – and is on a roll. Nationally, bariatric surgery is growing 6% annually, he said. NYBG boasts two dozen offices in the tri-state area of New York, New Jersey, and Connecticut and is poised to expand into more states.
“Since private equity, we’ve been growing at 30%-40% year over year,” Dr. Garber said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Researchers seek to understand post-COVID autoimmune disease risk
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Gut microbiome may guide personalized heart failure therapy
Understanding more about the gut microbiome and how it may affect the development and treatment of heart failure could lead to a more personalized approach to managing the condition, a new review article suggests.
the authors note. “Interactions among the gut microbiome, diet, and medications offer potentially innovative modalities for management of patients with heart failure,” they add.
The review was published online in the Journal of the American College of Cardiology.
“Over the past years we have gathered more understanding about how important the gut microbiome is in relation to how our bodies function overall and even though the cardiovascular system and the heart itself may appear to be quite distant from the gut, we know the gut microbiome affects the cardiovascular system and the physiology of heart failure,” lead author Petra Mamic, MD, Stanford (Calif.) University, told this news organization.
“We’ve also learnt that the microbiome is very personalized. It seems to be affected by a lot of intrinsic and as well as extrinsic factors. For cardiovascular diseases in particular, we always knew that diet and lifestyle were part of the environmental risk, and we now believe that the gut microbiome may be one of the factors that mediates that risk,” she said.
“Studies on the gut microbiome are difficult to do and we are right at the beginning of this type of research. But we have learned that the microbiome is altered or dysregulated in many diseases including many cardiovascular diseases, and many of the changes in the microbiome we see in different cardiovascular diseases seem to overlap,” she added.
Dr. Mamic explained that patients with heart failure have a microbiome that appears different and dysregulated, compared with the microbiome in healthy individuals.
“The difficulty is teasing out whether the microbiome changes are causing heart failure or if they are a consequence of the heart failure and all the medications and comorbidities associated with heart failure,” she commented.
Animal studies have shown that many microbial products, small molecules made by the microbiome, seem to affect how the heart recovers from injury, for example after a myocardial infarction, and how much the heart scars and hypertrophies after an injury, Dr. Mamic reported. These microbiome-derived small molecules can also affect blood pressure, which is dysregulated in heart failure.
Other products of the microbiome can be pro-inflammatory or anti-inflammatory, which can again affect the cardiovascular physiology and the heart, she noted.
High-fiber diet may be beneficial
One area of particular interest at present involves the role of short-chain fatty acids, which are a byproduct of microbes in the gut that digest fiber.
“These short chain fatty acids seem to have positive effects on the host physiology. They are anti-inflammatory; they lower blood pressure; and they seem to protect the heart from scarring and hypertrophy after injury. In heart failure, the gut microbes that make these short-chain fatty acids are significantly depleted,” Dr. Mamic explained.
They are an obvious focus of interest because these short-chain fatty acids are produced when gut bacteria break down dietary fiber, raising the possibility of beneficial effects from eating a high-fiber diet.
Another product of the gut microbiome of interest is trimethylamine N-oxide, formed when gut bacteria break down nutrients such as L-carnitine and phosphatidyl choline, nutrients abundant in foods of animal origin, especially red meat. This metabolite has proatherogenic and prothrombotic effects, and negatively affected cardiac remodeling in a mouse heart failure model, the review notes.
However, though it is too early to make specific dietary recommendations based on these findings, Dr. Mamic points out that a high-fiber diet is thought to be beneficial.
“Nutritional research is very hard to do and the data is limited, but as best as we can summarize things, we know that plant-based diets such as the Mediterranean and DASH diets seem to prevent some of the risk factors for the development of heart failure and seem to slow the progression of heart failure,” she added.
One of the major recommendations in these diets is a high intake of fiber, including whole foods, vegetables, fruits, legumes, and nuts, and less intake of processed food and red meat. “In general, I think everyone should eat like that, but I specifically recommend a plant-based diet with a high amount of fiber to my heart failure patients,” Dr. Mamic said.
Large variation in microbiome composition
The review also explores the idea of personalization of diet or specific treatments dependent on an individual’s gut microbiome composition.
Dr. Mamic explains: “When we look at the microbiome composition between individuals, it is very different. There is very little overlap between individuals, even in people who are related. It seems to be more to do with the environment – people who are living together are more likely to have similarities in their microbiome. We are still trying to understand what drives these differences.”
It is thought that these differences may affect the response to a specific diet or medication. Dr. Mamic gives the example of fiber. “Not all bacteria can digest the same types of fiber, so not everyone responds in the same way to a high-fiber diet. That’s probably because of differences in their microbiome.”
Another example is the response to the heart failure drug digoxin, which is metabolized by one particular strain of bacteria in the gut. The toxicity or effectiveness of digoxin seems to be influenced by levels of this bacterial strain, and this again can be influenced by diet, Dr. Mamic says.
Manipulating the microbiome as a therapeutic strategy
Microbiome-targeting therapies may also become part of future treatment strategies for many conditions, including heart failure, the review authors say.
Probiotics (foods and dietary supplements that contain live microbes) interact with the gut microbiota to alter host physiology beneficially. Certain probiotics may specifically modulate processes dysregulated in heart failure, as was suggested in a rodent heart failure model in which supplementation with Lactobacillus-containing and Bifidobacterium-containing probiotics resulted in markedly improved cardiac function, the authors report.
However, a randomized trial (GutHeart) of probiotic yeast Saccharomyces boulardii in patients with heart failure found no improvement in cardiac function, compared with standard care.
Commenting on this, Dr. Mamic suggested that a more specific approach may be needed.
“Some of our preliminary data have shown people who have heart failure have severely depleted Bifidobacteria,” Dr. Mamic said. These bacteria are commercially available as a probiotic, and the researchers are planning a study to give patients with heart failure these specific probiotics. “We are trying to find practical ways forward and to be guided by the data. These people have very little Bifidobacteria, and we know that probiotics seem to be accepted best by the host where there is a specific need for them, so this seems like a sensible approach.”
Dr. Mamic does not recommend that heart failure patients take general probiotic products at present, but she tells her patients about the study she is doing. “Probiotics are quite different from each other. It is a very unregulated market. A general probiotic product may not contain the specific bacteria needed.”
Include microbiome data in biobanks
The review calls for more research on the subject and a more systematic approach to collecting data on the microbiome.
“At present for medical research, blood samples are collected, stored, and analyzed routinely. I think we should also be collecting stool samples in the same way to analyze the microbiome,” Dr. Mamic suggests.
“If we can combine that with data from blood tests on various metabolites/cytokines and look at how the microbiome changes over time or with medication, or with diet, and how the host responds including clinically relevant data, that would be really important. Given how quickly the field is growing I would think there would be biobanks including the microbiome in a few years’ time.”
“We need to gather this data. We would be looking for which bacteria are there, what their functionality is, how it changes over time, with diet or medication, and even whether we can use the microbiome data to predict who will respond to a specific drug.”
Dr. Mamic believes that in the future, analysis of the microbiome could be a routine part of deciding what people eat for good health and to characterize patients for personalized therapies.
“It is clear that the microbiome can influence health, and a dysregulated microbiome negatively affects the host, but there is lot of work to do. We need to learn a lot more about it, but we shouldn’t miss the opportunity to do this,” she concluded.
Dr. Mamic reported no disclosures.
A version of this article first appeared on Medscape.com.
Understanding more about the gut microbiome and how it may affect the development and treatment of heart failure could lead to a more personalized approach to managing the condition, a new review article suggests.
the authors note. “Interactions among the gut microbiome, diet, and medications offer potentially innovative modalities for management of patients with heart failure,” they add.
The review was published online in the Journal of the American College of Cardiology.
“Over the past years we have gathered more understanding about how important the gut microbiome is in relation to how our bodies function overall and even though the cardiovascular system and the heart itself may appear to be quite distant from the gut, we know the gut microbiome affects the cardiovascular system and the physiology of heart failure,” lead author Petra Mamic, MD, Stanford (Calif.) University, told this news organization.
“We’ve also learnt that the microbiome is very personalized. It seems to be affected by a lot of intrinsic and as well as extrinsic factors. For cardiovascular diseases in particular, we always knew that diet and lifestyle were part of the environmental risk, and we now believe that the gut microbiome may be one of the factors that mediates that risk,” she said.
“Studies on the gut microbiome are difficult to do and we are right at the beginning of this type of research. But we have learned that the microbiome is altered or dysregulated in many diseases including many cardiovascular diseases, and many of the changes in the microbiome we see in different cardiovascular diseases seem to overlap,” she added.
Dr. Mamic explained that patients with heart failure have a microbiome that appears different and dysregulated, compared with the microbiome in healthy individuals.
“The difficulty is teasing out whether the microbiome changes are causing heart failure or if they are a consequence of the heart failure and all the medications and comorbidities associated with heart failure,” she commented.
Animal studies have shown that many microbial products, small molecules made by the microbiome, seem to affect how the heart recovers from injury, for example after a myocardial infarction, and how much the heart scars and hypertrophies after an injury, Dr. Mamic reported. These microbiome-derived small molecules can also affect blood pressure, which is dysregulated in heart failure.
Other products of the microbiome can be pro-inflammatory or anti-inflammatory, which can again affect the cardiovascular physiology and the heart, she noted.
High-fiber diet may be beneficial
One area of particular interest at present involves the role of short-chain fatty acids, which are a byproduct of microbes in the gut that digest fiber.
“These short chain fatty acids seem to have positive effects on the host physiology. They are anti-inflammatory; they lower blood pressure; and they seem to protect the heart from scarring and hypertrophy after injury. In heart failure, the gut microbes that make these short-chain fatty acids are significantly depleted,” Dr. Mamic explained.
They are an obvious focus of interest because these short-chain fatty acids are produced when gut bacteria break down dietary fiber, raising the possibility of beneficial effects from eating a high-fiber diet.
Another product of the gut microbiome of interest is trimethylamine N-oxide, formed when gut bacteria break down nutrients such as L-carnitine and phosphatidyl choline, nutrients abundant in foods of animal origin, especially red meat. This metabolite has proatherogenic and prothrombotic effects, and negatively affected cardiac remodeling in a mouse heart failure model, the review notes.
However, though it is too early to make specific dietary recommendations based on these findings, Dr. Mamic points out that a high-fiber diet is thought to be beneficial.
“Nutritional research is very hard to do and the data is limited, but as best as we can summarize things, we know that plant-based diets such as the Mediterranean and DASH diets seem to prevent some of the risk factors for the development of heart failure and seem to slow the progression of heart failure,” she added.
One of the major recommendations in these diets is a high intake of fiber, including whole foods, vegetables, fruits, legumes, and nuts, and less intake of processed food and red meat. “In general, I think everyone should eat like that, but I specifically recommend a plant-based diet with a high amount of fiber to my heart failure patients,” Dr. Mamic said.
Large variation in microbiome composition
The review also explores the idea of personalization of diet or specific treatments dependent on an individual’s gut microbiome composition.
Dr. Mamic explains: “When we look at the microbiome composition between individuals, it is very different. There is very little overlap between individuals, even in people who are related. It seems to be more to do with the environment – people who are living together are more likely to have similarities in their microbiome. We are still trying to understand what drives these differences.”
It is thought that these differences may affect the response to a specific diet or medication. Dr. Mamic gives the example of fiber. “Not all bacteria can digest the same types of fiber, so not everyone responds in the same way to a high-fiber diet. That’s probably because of differences in their microbiome.”
Another example is the response to the heart failure drug digoxin, which is metabolized by one particular strain of bacteria in the gut. The toxicity or effectiveness of digoxin seems to be influenced by levels of this bacterial strain, and this again can be influenced by diet, Dr. Mamic says.
Manipulating the microbiome as a therapeutic strategy
Microbiome-targeting therapies may also become part of future treatment strategies for many conditions, including heart failure, the review authors say.
Probiotics (foods and dietary supplements that contain live microbes) interact with the gut microbiota to alter host physiology beneficially. Certain probiotics may specifically modulate processes dysregulated in heart failure, as was suggested in a rodent heart failure model in which supplementation with Lactobacillus-containing and Bifidobacterium-containing probiotics resulted in markedly improved cardiac function, the authors report.
However, a randomized trial (GutHeart) of probiotic yeast Saccharomyces boulardii in patients with heart failure found no improvement in cardiac function, compared with standard care.
Commenting on this, Dr. Mamic suggested that a more specific approach may be needed.
“Some of our preliminary data have shown people who have heart failure have severely depleted Bifidobacteria,” Dr. Mamic said. These bacteria are commercially available as a probiotic, and the researchers are planning a study to give patients with heart failure these specific probiotics. “We are trying to find practical ways forward and to be guided by the data. These people have very little Bifidobacteria, and we know that probiotics seem to be accepted best by the host where there is a specific need for them, so this seems like a sensible approach.”
Dr. Mamic does not recommend that heart failure patients take general probiotic products at present, but she tells her patients about the study she is doing. “Probiotics are quite different from each other. It is a very unregulated market. A general probiotic product may not contain the specific bacteria needed.”
Include microbiome data in biobanks
The review calls for more research on the subject and a more systematic approach to collecting data on the microbiome.
“At present for medical research, blood samples are collected, stored, and analyzed routinely. I think we should also be collecting stool samples in the same way to analyze the microbiome,” Dr. Mamic suggests.
“If we can combine that with data from blood tests on various metabolites/cytokines and look at how the microbiome changes over time or with medication, or with diet, and how the host responds including clinically relevant data, that would be really important. Given how quickly the field is growing I would think there would be biobanks including the microbiome in a few years’ time.”
“We need to gather this data. We would be looking for which bacteria are there, what their functionality is, how it changes over time, with diet or medication, and even whether we can use the microbiome data to predict who will respond to a specific drug.”
Dr. Mamic believes that in the future, analysis of the microbiome could be a routine part of deciding what people eat for good health and to characterize patients for personalized therapies.
“It is clear that the microbiome can influence health, and a dysregulated microbiome negatively affects the host, but there is lot of work to do. We need to learn a lot more about it, but we shouldn’t miss the opportunity to do this,” she concluded.
Dr. Mamic reported no disclosures.
A version of this article first appeared on Medscape.com.
Understanding more about the gut microbiome and how it may affect the development and treatment of heart failure could lead to a more personalized approach to managing the condition, a new review article suggests.
the authors note. “Interactions among the gut microbiome, diet, and medications offer potentially innovative modalities for management of patients with heart failure,” they add.
The review was published online in the Journal of the American College of Cardiology.
“Over the past years we have gathered more understanding about how important the gut microbiome is in relation to how our bodies function overall and even though the cardiovascular system and the heart itself may appear to be quite distant from the gut, we know the gut microbiome affects the cardiovascular system and the physiology of heart failure,” lead author Petra Mamic, MD, Stanford (Calif.) University, told this news organization.
“We’ve also learnt that the microbiome is very personalized. It seems to be affected by a lot of intrinsic and as well as extrinsic factors. For cardiovascular diseases in particular, we always knew that diet and lifestyle were part of the environmental risk, and we now believe that the gut microbiome may be one of the factors that mediates that risk,” she said.
“Studies on the gut microbiome are difficult to do and we are right at the beginning of this type of research. But we have learned that the microbiome is altered or dysregulated in many diseases including many cardiovascular diseases, and many of the changes in the microbiome we see in different cardiovascular diseases seem to overlap,” she added.
Dr. Mamic explained that patients with heart failure have a microbiome that appears different and dysregulated, compared with the microbiome in healthy individuals.
“The difficulty is teasing out whether the microbiome changes are causing heart failure or if they are a consequence of the heart failure and all the medications and comorbidities associated with heart failure,” she commented.
Animal studies have shown that many microbial products, small molecules made by the microbiome, seem to affect how the heart recovers from injury, for example after a myocardial infarction, and how much the heart scars and hypertrophies after an injury, Dr. Mamic reported. These microbiome-derived small molecules can also affect blood pressure, which is dysregulated in heart failure.
Other products of the microbiome can be pro-inflammatory or anti-inflammatory, which can again affect the cardiovascular physiology and the heart, she noted.
High-fiber diet may be beneficial
One area of particular interest at present involves the role of short-chain fatty acids, which are a byproduct of microbes in the gut that digest fiber.
“These short chain fatty acids seem to have positive effects on the host physiology. They are anti-inflammatory; they lower blood pressure; and they seem to protect the heart from scarring and hypertrophy after injury. In heart failure, the gut microbes that make these short-chain fatty acids are significantly depleted,” Dr. Mamic explained.
They are an obvious focus of interest because these short-chain fatty acids are produced when gut bacteria break down dietary fiber, raising the possibility of beneficial effects from eating a high-fiber diet.
Another product of the gut microbiome of interest is trimethylamine N-oxide, formed when gut bacteria break down nutrients such as L-carnitine and phosphatidyl choline, nutrients abundant in foods of animal origin, especially red meat. This metabolite has proatherogenic and prothrombotic effects, and negatively affected cardiac remodeling in a mouse heart failure model, the review notes.
However, though it is too early to make specific dietary recommendations based on these findings, Dr. Mamic points out that a high-fiber diet is thought to be beneficial.
“Nutritional research is very hard to do and the data is limited, but as best as we can summarize things, we know that plant-based diets such as the Mediterranean and DASH diets seem to prevent some of the risk factors for the development of heart failure and seem to slow the progression of heart failure,” she added.
One of the major recommendations in these diets is a high intake of fiber, including whole foods, vegetables, fruits, legumes, and nuts, and less intake of processed food and red meat. “In general, I think everyone should eat like that, but I specifically recommend a plant-based diet with a high amount of fiber to my heart failure patients,” Dr. Mamic said.
Large variation in microbiome composition
The review also explores the idea of personalization of diet or specific treatments dependent on an individual’s gut microbiome composition.
Dr. Mamic explains: “When we look at the microbiome composition between individuals, it is very different. There is very little overlap between individuals, even in people who are related. It seems to be more to do with the environment – people who are living together are more likely to have similarities in their microbiome. We are still trying to understand what drives these differences.”
It is thought that these differences may affect the response to a specific diet or medication. Dr. Mamic gives the example of fiber. “Not all bacteria can digest the same types of fiber, so not everyone responds in the same way to a high-fiber diet. That’s probably because of differences in their microbiome.”
Another example is the response to the heart failure drug digoxin, which is metabolized by one particular strain of bacteria in the gut. The toxicity or effectiveness of digoxin seems to be influenced by levels of this bacterial strain, and this again can be influenced by diet, Dr. Mamic says.
Manipulating the microbiome as a therapeutic strategy
Microbiome-targeting therapies may also become part of future treatment strategies for many conditions, including heart failure, the review authors say.
Probiotics (foods and dietary supplements that contain live microbes) interact with the gut microbiota to alter host physiology beneficially. Certain probiotics may specifically modulate processes dysregulated in heart failure, as was suggested in a rodent heart failure model in which supplementation with Lactobacillus-containing and Bifidobacterium-containing probiotics resulted in markedly improved cardiac function, the authors report.
However, a randomized trial (GutHeart) of probiotic yeast Saccharomyces boulardii in patients with heart failure found no improvement in cardiac function, compared with standard care.
Commenting on this, Dr. Mamic suggested that a more specific approach may be needed.
“Some of our preliminary data have shown people who have heart failure have severely depleted Bifidobacteria,” Dr. Mamic said. These bacteria are commercially available as a probiotic, and the researchers are planning a study to give patients with heart failure these specific probiotics. “We are trying to find practical ways forward and to be guided by the data. These people have very little Bifidobacteria, and we know that probiotics seem to be accepted best by the host where there is a specific need for them, so this seems like a sensible approach.”
Dr. Mamic does not recommend that heart failure patients take general probiotic products at present, but she tells her patients about the study she is doing. “Probiotics are quite different from each other. It is a very unregulated market. A general probiotic product may not contain the specific bacteria needed.”
Include microbiome data in biobanks
The review calls for more research on the subject and a more systematic approach to collecting data on the microbiome.
“At present for medical research, blood samples are collected, stored, and analyzed routinely. I think we should also be collecting stool samples in the same way to analyze the microbiome,” Dr. Mamic suggests.
“If we can combine that with data from blood tests on various metabolites/cytokines and look at how the microbiome changes over time or with medication, or with diet, and how the host responds including clinically relevant data, that would be really important. Given how quickly the field is growing I would think there would be biobanks including the microbiome in a few years’ time.”
“We need to gather this data. We would be looking for which bacteria are there, what their functionality is, how it changes over time, with diet or medication, and even whether we can use the microbiome data to predict who will respond to a specific drug.”
Dr. Mamic believes that in the future, analysis of the microbiome could be a routine part of deciding what people eat for good health and to characterize patients for personalized therapies.
“It is clear that the microbiome can influence health, and a dysregulated microbiome negatively affects the host, but there is lot of work to do. We need to learn a lot more about it, but we shouldn’t miss the opportunity to do this,” she concluded.
Dr. Mamic reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
New blood pressure thresholds: How do they affect the evaluation and treatment of hypertension?
In a major shift in the definition of hypertension, guidelines published in 2017 reclassified 130/80 mm Hg as high blood pressure, or stage 1 hypertension. Previous guidelines classified 130/80 mm Hg as elevated, and 140/90 mm Hg used to be the threshold for stage 1 hypertension.
“This shift in classification criteria may cause confusion among clinicians caring for patients with hypertension and has a significant impact on how we diagnose and manage hypertension in our practice,” said Shawna D. Nesbitt, MD, professor of internal medicine at the University of Texas Southwestern Medical Center and medical director at Parkland Hypertension Clinic in Dallas. Dr. Nesbitt is an expert in the diagnosis and treatment of hypertension, particularly complex and refractory cases.
Cardiovascular disease (CVD) is the leading cause of death in the United States, accounting for nearly one-quarter of all deaths in men and in women. Hypertension is a key factor contributing to CVD. The hypertension‐related CVD mortality is currently on the rise in many U.S. demographic groups, including younger individuals (35-64 years old), she said.
When asked about the potential causes of this trend, Dr. Nesbitt explained that the epidemics of obesity and overweight are critical contributors to the high prevalence of hypertension.
The new definition means a wider gap in the prevalence of hypertension between men and women, as well as between Black and White people in the United States. The U.S. rates of hypertension and hypertension‐related CVD mortality are much higher in Black than in White people in this country. Hypertension control rates are the lowest in Black, Hispanic, and Asian males, Dr. Nesbitt said.
Accurate measurement of blood pressure is crucial
The changes in classification criteria for hypertension have made accurate measurements of blood pressure important. A key challenge in the evaluation of hypertension in the clinic is the difference in the methods used to measure blood pressure between trials and real-world clinical practice.
“We can’t easily translate data collected in clinical trials into real-life scenarios, and this can have important implications in our expectations of treatment outcome,” Dr. Nesbitt cautioned.
Commenting on the best practices in blood pressure measurements in the office, Dr. Nesbitt said that patients need to be seated with their feet on the floor and their backs and arms supported. In addition, patients need to have at least 5 minutes of rest without talking.
“It is very important to help patients understand what triggers their blood pressure to be elevated and teach them how and when to measure their blood pressure at home using their own devices,” she added.
Another critical question is how to translate the new guidelines into changes in clinical care, she said.
Current treatment landscape of hypertension
Ensuring a healthy diet, weight, and sleep, participating in physical activity, avoiding nicotine, and managing blood pressure, cholesterol, and sugar levels are the new “Life’s Essential 8” strategies proposed by the American Heart Association (AHA) to reduce CVD risk.
“Sleep has recently been added to the AHA guidelines because it modulates many factors contributing to hypertension,” Dr. Nesbitt pointed out. She advised that clinicians should ask patients about their sleep and educate them on healthy sleeping habits.
Some of the evidence used to develop the new AHA guidelines is derived from the SPRINT trial, which showed that controlling blood pressure reduces the risk of major adverse cardiovascular events. “This is our ultimate goal for our patients with hypertension,” Dr. Nesbitt noted.
Regarding the best practice in hypertension management, Dr. Nesbitt explained that with the new blood pressure thresholds, more patients will be diagnosed with stage 1 hypertension and need the nonpharmacological therapy suggested by the AHA. But patients with stage 1 hypertension and with a high CVD risk (at least 10%) also should receive blood pressure-lowering medications, so an accurate assessment of the risk of clinical atherosclerotic cardiovascular disease (ASCVD) or the estimated 10-year CVD risk is crucial. “If we are not careful, we might miss some patients who need to be treated,” she said.
Calcium channel blockers, thiazide diuretics, and ACE inhibitors or angiotensin receptor blockers (ARBs) are the treatment of choice for patients with newly diagnosed hypertension. Although extensively used in the past, beta-blockers are no longer a first-line treatment for hypertension.
When asked why beta-blockers are no longer suitable for routine initial treatment of hypertension, Dr. Nesbitt said that they are effective in controlling palpitations but “other antihypertensive drugs have proven far better in controlling blood pressure.”
Hypertension is multifactorial and often occurs in combination with other conditions, including diabetes and chronic kidney disease. When developing a treatment plan for patients with hypertension, comorbidities need to be considered, because their management may also help control blood pressure, especially for conditions that may contribute to the development of hypertension.
Common conditions that contribute to and often coexist with hypertension include sleep apnea, obesity, anxiety, and depression. However, convincing people to seek mental health support can be very challenging, Dr. Nesbitt said.
She added that hypertension is a complex disease with a strong social component. Understanding its pathophysiology and social determinants is paramount for successfully managing hypertension at the individual level, as well as at the community level.
Identification and management of side effects is key
Dr. Nesbitt also discussed the importance of the identification and management of side effects associated with blood pressure-lowering drugs. She cautioned that, if not managed, side effects can lead to treatment nonadherence and pseudo‐resistance, both of which can jeopardize the successful management of hypertension.
When asked about her approach to managing side effects and convincing patients to continue taking their medications, Dr. Nesbitt noted that “setting realistic expectations and goals is key.”
In an interview after Dr. Nesbitt’s presentation, Jesica Naanous, MD, agreed that having an honest conversation with the patients is the best way to convince them to keep taking their medications. She also explains to patients that the complications of uncontrolled blood pressure are worse than the side effects of the drugs.
“As a last resort, I change a blood pressure-lowering agent to another,” added Dr. Naanous, an internist at the American British Cowdray (ABC) Medical Center in Mexico City. She explained that many antihypertensive drugs have different toxicity profiles, and simply changing to another agent can make treatment more tolerable for the patient.
Dr. Nesbitt reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing health care products used by or on patients.
In a major shift in the definition of hypertension, guidelines published in 2017 reclassified 130/80 mm Hg as high blood pressure, or stage 1 hypertension. Previous guidelines classified 130/80 mm Hg as elevated, and 140/90 mm Hg used to be the threshold for stage 1 hypertension.
“This shift in classification criteria may cause confusion among clinicians caring for patients with hypertension and has a significant impact on how we diagnose and manage hypertension in our practice,” said Shawna D. Nesbitt, MD, professor of internal medicine at the University of Texas Southwestern Medical Center and medical director at Parkland Hypertension Clinic in Dallas. Dr. Nesbitt is an expert in the diagnosis and treatment of hypertension, particularly complex and refractory cases.
Cardiovascular disease (CVD) is the leading cause of death in the United States, accounting for nearly one-quarter of all deaths in men and in women. Hypertension is a key factor contributing to CVD. The hypertension‐related CVD mortality is currently on the rise in many U.S. demographic groups, including younger individuals (35-64 years old), she said.
When asked about the potential causes of this trend, Dr. Nesbitt explained that the epidemics of obesity and overweight are critical contributors to the high prevalence of hypertension.
The new definition means a wider gap in the prevalence of hypertension between men and women, as well as between Black and White people in the United States. The U.S. rates of hypertension and hypertension‐related CVD mortality are much higher in Black than in White people in this country. Hypertension control rates are the lowest in Black, Hispanic, and Asian males, Dr. Nesbitt said.
Accurate measurement of blood pressure is crucial
The changes in classification criteria for hypertension have made accurate measurements of blood pressure important. A key challenge in the evaluation of hypertension in the clinic is the difference in the methods used to measure blood pressure between trials and real-world clinical practice.
“We can’t easily translate data collected in clinical trials into real-life scenarios, and this can have important implications in our expectations of treatment outcome,” Dr. Nesbitt cautioned.
Commenting on the best practices in blood pressure measurements in the office, Dr. Nesbitt said that patients need to be seated with their feet on the floor and their backs and arms supported. In addition, patients need to have at least 5 minutes of rest without talking.
“It is very important to help patients understand what triggers their blood pressure to be elevated and teach them how and when to measure their blood pressure at home using their own devices,” she added.
Another critical question is how to translate the new guidelines into changes in clinical care, she said.
Current treatment landscape of hypertension
Ensuring a healthy diet, weight, and sleep, participating in physical activity, avoiding nicotine, and managing blood pressure, cholesterol, and sugar levels are the new “Life’s Essential 8” strategies proposed by the American Heart Association (AHA) to reduce CVD risk.
“Sleep has recently been added to the AHA guidelines because it modulates many factors contributing to hypertension,” Dr. Nesbitt pointed out. She advised that clinicians should ask patients about their sleep and educate them on healthy sleeping habits.
Some of the evidence used to develop the new AHA guidelines is derived from the SPRINT trial, which showed that controlling blood pressure reduces the risk of major adverse cardiovascular events. “This is our ultimate goal for our patients with hypertension,” Dr. Nesbitt noted.
Regarding the best practice in hypertension management, Dr. Nesbitt explained that with the new blood pressure thresholds, more patients will be diagnosed with stage 1 hypertension and need the nonpharmacological therapy suggested by the AHA. But patients with stage 1 hypertension and with a high CVD risk (at least 10%) also should receive blood pressure-lowering medications, so an accurate assessment of the risk of clinical atherosclerotic cardiovascular disease (ASCVD) or the estimated 10-year CVD risk is crucial. “If we are not careful, we might miss some patients who need to be treated,” she said.
Calcium channel blockers, thiazide diuretics, and ACE inhibitors or angiotensin receptor blockers (ARBs) are the treatment of choice for patients with newly diagnosed hypertension. Although extensively used in the past, beta-blockers are no longer a first-line treatment for hypertension.
When asked why beta-blockers are no longer suitable for routine initial treatment of hypertension, Dr. Nesbitt said that they are effective in controlling palpitations but “other antihypertensive drugs have proven far better in controlling blood pressure.”
Hypertension is multifactorial and often occurs in combination with other conditions, including diabetes and chronic kidney disease. When developing a treatment plan for patients with hypertension, comorbidities need to be considered, because their management may also help control blood pressure, especially for conditions that may contribute to the development of hypertension.
Common conditions that contribute to and often coexist with hypertension include sleep apnea, obesity, anxiety, and depression. However, convincing people to seek mental health support can be very challenging, Dr. Nesbitt said.
She added that hypertension is a complex disease with a strong social component. Understanding its pathophysiology and social determinants is paramount for successfully managing hypertension at the individual level, as well as at the community level.
Identification and management of side effects is key
Dr. Nesbitt also discussed the importance of the identification and management of side effects associated with blood pressure-lowering drugs. She cautioned that, if not managed, side effects can lead to treatment nonadherence and pseudo‐resistance, both of which can jeopardize the successful management of hypertension.
When asked about her approach to managing side effects and convincing patients to continue taking their medications, Dr. Nesbitt noted that “setting realistic expectations and goals is key.”
In an interview after Dr. Nesbitt’s presentation, Jesica Naanous, MD, agreed that having an honest conversation with the patients is the best way to convince them to keep taking their medications. She also explains to patients that the complications of uncontrolled blood pressure are worse than the side effects of the drugs.
“As a last resort, I change a blood pressure-lowering agent to another,” added Dr. Naanous, an internist at the American British Cowdray (ABC) Medical Center in Mexico City. She explained that many antihypertensive drugs have different toxicity profiles, and simply changing to another agent can make treatment more tolerable for the patient.
Dr. Nesbitt reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing health care products used by or on patients.
In a major shift in the definition of hypertension, guidelines published in 2017 reclassified 130/80 mm Hg as high blood pressure, or stage 1 hypertension. Previous guidelines classified 130/80 mm Hg as elevated, and 140/90 mm Hg used to be the threshold for stage 1 hypertension.
“This shift in classification criteria may cause confusion among clinicians caring for patients with hypertension and has a significant impact on how we diagnose and manage hypertension in our practice,” said Shawna D. Nesbitt, MD, professor of internal medicine at the University of Texas Southwestern Medical Center and medical director at Parkland Hypertension Clinic in Dallas. Dr. Nesbitt is an expert in the diagnosis and treatment of hypertension, particularly complex and refractory cases.
Cardiovascular disease (CVD) is the leading cause of death in the United States, accounting for nearly one-quarter of all deaths in men and in women. Hypertension is a key factor contributing to CVD. The hypertension‐related CVD mortality is currently on the rise in many U.S. demographic groups, including younger individuals (35-64 years old), she said.
When asked about the potential causes of this trend, Dr. Nesbitt explained that the epidemics of obesity and overweight are critical contributors to the high prevalence of hypertension.
The new definition means a wider gap in the prevalence of hypertension between men and women, as well as between Black and White people in the United States. The U.S. rates of hypertension and hypertension‐related CVD mortality are much higher in Black than in White people in this country. Hypertension control rates are the lowest in Black, Hispanic, and Asian males, Dr. Nesbitt said.
Accurate measurement of blood pressure is crucial
The changes in classification criteria for hypertension have made accurate measurements of blood pressure important. A key challenge in the evaluation of hypertension in the clinic is the difference in the methods used to measure blood pressure between trials and real-world clinical practice.
“We can’t easily translate data collected in clinical trials into real-life scenarios, and this can have important implications in our expectations of treatment outcome,” Dr. Nesbitt cautioned.
Commenting on the best practices in blood pressure measurements in the office, Dr. Nesbitt said that patients need to be seated with their feet on the floor and their backs and arms supported. In addition, patients need to have at least 5 minutes of rest without talking.
“It is very important to help patients understand what triggers their blood pressure to be elevated and teach them how and when to measure their blood pressure at home using their own devices,” she added.
Another critical question is how to translate the new guidelines into changes in clinical care, she said.
Current treatment landscape of hypertension
Ensuring a healthy diet, weight, and sleep, participating in physical activity, avoiding nicotine, and managing blood pressure, cholesterol, and sugar levels are the new “Life’s Essential 8” strategies proposed by the American Heart Association (AHA) to reduce CVD risk.
“Sleep has recently been added to the AHA guidelines because it modulates many factors contributing to hypertension,” Dr. Nesbitt pointed out. She advised that clinicians should ask patients about their sleep and educate them on healthy sleeping habits.
Some of the evidence used to develop the new AHA guidelines is derived from the SPRINT trial, which showed that controlling blood pressure reduces the risk of major adverse cardiovascular events. “This is our ultimate goal for our patients with hypertension,” Dr. Nesbitt noted.
Regarding the best practice in hypertension management, Dr. Nesbitt explained that with the new blood pressure thresholds, more patients will be diagnosed with stage 1 hypertension and need the nonpharmacological therapy suggested by the AHA. But patients with stage 1 hypertension and with a high CVD risk (at least 10%) also should receive blood pressure-lowering medications, so an accurate assessment of the risk of clinical atherosclerotic cardiovascular disease (ASCVD) or the estimated 10-year CVD risk is crucial. “If we are not careful, we might miss some patients who need to be treated,” she said.
Calcium channel blockers, thiazide diuretics, and ACE inhibitors or angiotensin receptor blockers (ARBs) are the treatment of choice for patients with newly diagnosed hypertension. Although extensively used in the past, beta-blockers are no longer a first-line treatment for hypertension.
When asked why beta-blockers are no longer suitable for routine initial treatment of hypertension, Dr. Nesbitt said that they are effective in controlling palpitations but “other antihypertensive drugs have proven far better in controlling blood pressure.”
Hypertension is multifactorial and often occurs in combination with other conditions, including diabetes and chronic kidney disease. When developing a treatment plan for patients with hypertension, comorbidities need to be considered, because their management may also help control blood pressure, especially for conditions that may contribute to the development of hypertension.
Common conditions that contribute to and often coexist with hypertension include sleep apnea, obesity, anxiety, and depression. However, convincing people to seek mental health support can be very challenging, Dr. Nesbitt said.
She added that hypertension is a complex disease with a strong social component. Understanding its pathophysiology and social determinants is paramount for successfully managing hypertension at the individual level, as well as at the community level.
Identification and management of side effects is key
Dr. Nesbitt also discussed the importance of the identification and management of side effects associated with blood pressure-lowering drugs. She cautioned that, if not managed, side effects can lead to treatment nonadherence and pseudo‐resistance, both of which can jeopardize the successful management of hypertension.
When asked about her approach to managing side effects and convincing patients to continue taking their medications, Dr. Nesbitt noted that “setting realistic expectations and goals is key.”
In an interview after Dr. Nesbitt’s presentation, Jesica Naanous, MD, agreed that having an honest conversation with the patients is the best way to convince them to keep taking their medications. She also explains to patients that the complications of uncontrolled blood pressure are worse than the side effects of the drugs.
“As a last resort, I change a blood pressure-lowering agent to another,” added Dr. Naanous, an internist at the American British Cowdray (ABC) Medical Center in Mexico City. She explained that many antihypertensive drugs have different toxicity profiles, and simply changing to another agent can make treatment more tolerable for the patient.
Dr. Nesbitt reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing health care products used by or on patients.
AT INTERNAL MEDICINE 2023
Can an endoscopic procedure treat type 2 diabetes?
Called recellularization via electroporation therapy (ReCET), the technology, manufactured by Endogenex, uses a specialized catheter to deliver alternating electric pulses to the duodenum to induce cellular regeneration. This process is thought to improve insulin sensitivity, in part, by altering gut hormones and nutritional sensing, principal investigator Jacques Bergman, MD, PhD, said in a press briefing held in conjunction with the annual Digestive Disease Week® (DDW), where he will present the data on May 9.
In the first-in-human study of ReCET, 12 of 14 patients were able to come off insulin for up to a year following the procedure when combined with the use of the glucagonlike peptide–1 agonist semaglutide.
“This might be a game changer in the management of type 2 diabetes because a single outpatient endoscopic intervention was suggested to have a pretty long therapeutic effect, which is compliance-free, as opposed to drug therapy that relies on patients taking the drugs on a daily basis,” said Dr. Bergman, professor of gastrointestinal endoscopy at Amsterdam University Medical Center.
Moreover, he added, “this technique is disease-modifying, so it goes to the root cause of type 2 diabetes and tackles the insulin resistance, as opposed to drug therapy, which at best, is disease-controlling, and the effect is immediately gone if you stop the medication.”
ReCET is similar to another product, Fractyl’s Revita DMR, for which Dr. Bergman was involved in a randomized clinical trial. He said in an interview that the two technologies differ in that the Revita uses heat with submucosal lifting to avoid deeper heat penetration, whereas ReCET is nonthermal. He is also involved in a second randomized trial of the Revita.
Is semaglutide muddying the findings?
Asked to comment about the current study with ReCET, Ali Aminian, MD, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic, said that the treatment effect is certainly plausible.
“The observation that hyperglycemia rapidly and substantially improves after bariatric surgery has prompted innovators to search for novel endoscopic procedures targeting the GI tract to improve diabetes and metabolic disease. Over the years, we learned that in addition to its role in digestion and absorption, the GI tract is actually a large endocrine organ which contributes to development of diabetes and metabolic disease.”
However, Dr. Aminian said that, “while these preliminary findings on a very small number of patients with a very short follow-up time are interesting,” he faulted the study design for including semaglutide. “When patients are treated with a combination of therapies, it will be hard to understand the true effect of each therapy,” and particularly, “when we add a strong diabetes medication like semaglutide.”
Dr. Bergman said semaglutide was used to “boost the insulin-resistant effect of the endoscopic treatment,” and that a planned double-blind, randomized trial will “show how much semaglutide actually contributed to the effect.” The ultimate goal, he noted, is to eliminate the need for all medications.
Moreover, when people with type 2 diabetes add semaglutide to insulin treatment, only about 20% typically are able to quit taking the insulin, in contrast to the 86% seen in this study, lead author Celine Busch, MBBS, a PhD candidate in gastroenterology at Amsterdam University, said in a DDW statement.
Dr. Aminian said, “I’m looking forward to better quality data ... from studies with a stronger design to prove safety, efficacy, and durability of this endoscopic intervention in patients with diabetes.”
But, he also cautioned, “in the past few years, other endoscopic procedures targeting the duodenum were introduced with exciting initial findings based on a small series [with a] short-term follow-up time. However, their safety, efficacy, and durability were not proven in subsequent studies.”
All patients stopped insulin, most for a year
The single-arm, single-center study involved 14 patients with type 2 diabetes taking basal but not premeal insulin. All underwent the 1-hour outpatient ReCET procedure, which involved placing a catheter into the first part of the small bowel and delivering electrical pulses to the duodenum.
Patients adhered to a calorie-controlled liquid diet for 2 weeks, after which they were initiated on semaglutide. All 14 patients were able to come off insulin for 3 months while maintaining glycemic control, and 12 were able to come off insulin for 12 months. They also experienced a 50% reduction in liver fat.
Dr. Bergman said a randomized, double-blind study using a sham procedure for controls is expected to start in about 2 months. “But for now, we are very encouraged by the potential for controlling type 2 diabetes with a single endoscopic treatment.”
Dr. Bergman has reported serving on the advisory board for Endogenex. Dr. Aminian has reported receiving research support and honorarium from Medtronic and Ethicon.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
Called recellularization via electroporation therapy (ReCET), the technology, manufactured by Endogenex, uses a specialized catheter to deliver alternating electric pulses to the duodenum to induce cellular regeneration. This process is thought to improve insulin sensitivity, in part, by altering gut hormones and nutritional sensing, principal investigator Jacques Bergman, MD, PhD, said in a press briefing held in conjunction with the annual Digestive Disease Week® (DDW), where he will present the data on May 9.
In the first-in-human study of ReCET, 12 of 14 patients were able to come off insulin for up to a year following the procedure when combined with the use of the glucagonlike peptide–1 agonist semaglutide.
“This might be a game changer in the management of type 2 diabetes because a single outpatient endoscopic intervention was suggested to have a pretty long therapeutic effect, which is compliance-free, as opposed to drug therapy that relies on patients taking the drugs on a daily basis,” said Dr. Bergman, professor of gastrointestinal endoscopy at Amsterdam University Medical Center.
Moreover, he added, “this technique is disease-modifying, so it goes to the root cause of type 2 diabetes and tackles the insulin resistance, as opposed to drug therapy, which at best, is disease-controlling, and the effect is immediately gone if you stop the medication.”
ReCET is similar to another product, Fractyl’s Revita DMR, for which Dr. Bergman was involved in a randomized clinical trial. He said in an interview that the two technologies differ in that the Revita uses heat with submucosal lifting to avoid deeper heat penetration, whereas ReCET is nonthermal. He is also involved in a second randomized trial of the Revita.
Is semaglutide muddying the findings?
Asked to comment about the current study with ReCET, Ali Aminian, MD, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic, said that the treatment effect is certainly plausible.
“The observation that hyperglycemia rapidly and substantially improves after bariatric surgery has prompted innovators to search for novel endoscopic procedures targeting the GI tract to improve diabetes and metabolic disease. Over the years, we learned that in addition to its role in digestion and absorption, the GI tract is actually a large endocrine organ which contributes to development of diabetes and metabolic disease.”
However, Dr. Aminian said that, “while these preliminary findings on a very small number of patients with a very short follow-up time are interesting,” he faulted the study design for including semaglutide. “When patients are treated with a combination of therapies, it will be hard to understand the true effect of each therapy,” and particularly, “when we add a strong diabetes medication like semaglutide.”
Dr. Bergman said semaglutide was used to “boost the insulin-resistant effect of the endoscopic treatment,” and that a planned double-blind, randomized trial will “show how much semaglutide actually contributed to the effect.” The ultimate goal, he noted, is to eliminate the need for all medications.
Moreover, when people with type 2 diabetes add semaglutide to insulin treatment, only about 20% typically are able to quit taking the insulin, in contrast to the 86% seen in this study, lead author Celine Busch, MBBS, a PhD candidate in gastroenterology at Amsterdam University, said in a DDW statement.
Dr. Aminian said, “I’m looking forward to better quality data ... from studies with a stronger design to prove safety, efficacy, and durability of this endoscopic intervention in patients with diabetes.”
But, he also cautioned, “in the past few years, other endoscopic procedures targeting the duodenum were introduced with exciting initial findings based on a small series [with a] short-term follow-up time. However, their safety, efficacy, and durability were not proven in subsequent studies.”
All patients stopped insulin, most for a year
The single-arm, single-center study involved 14 patients with type 2 diabetes taking basal but not premeal insulin. All underwent the 1-hour outpatient ReCET procedure, which involved placing a catheter into the first part of the small bowel and delivering electrical pulses to the duodenum.
Patients adhered to a calorie-controlled liquid diet for 2 weeks, after which they were initiated on semaglutide. All 14 patients were able to come off insulin for 3 months while maintaining glycemic control, and 12 were able to come off insulin for 12 months. They also experienced a 50% reduction in liver fat.
Dr. Bergman said a randomized, double-blind study using a sham procedure for controls is expected to start in about 2 months. “But for now, we are very encouraged by the potential for controlling type 2 diabetes with a single endoscopic treatment.”
Dr. Bergman has reported serving on the advisory board for Endogenex. Dr. Aminian has reported receiving research support and honorarium from Medtronic and Ethicon.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
Called recellularization via electroporation therapy (ReCET), the technology, manufactured by Endogenex, uses a specialized catheter to deliver alternating electric pulses to the duodenum to induce cellular regeneration. This process is thought to improve insulin sensitivity, in part, by altering gut hormones and nutritional sensing, principal investigator Jacques Bergman, MD, PhD, said in a press briefing held in conjunction with the annual Digestive Disease Week® (DDW), where he will present the data on May 9.
In the first-in-human study of ReCET, 12 of 14 patients were able to come off insulin for up to a year following the procedure when combined with the use of the glucagonlike peptide–1 agonist semaglutide.
“This might be a game changer in the management of type 2 diabetes because a single outpatient endoscopic intervention was suggested to have a pretty long therapeutic effect, which is compliance-free, as opposed to drug therapy that relies on patients taking the drugs on a daily basis,” said Dr. Bergman, professor of gastrointestinal endoscopy at Amsterdam University Medical Center.
Moreover, he added, “this technique is disease-modifying, so it goes to the root cause of type 2 diabetes and tackles the insulin resistance, as opposed to drug therapy, which at best, is disease-controlling, and the effect is immediately gone if you stop the medication.”
ReCET is similar to another product, Fractyl’s Revita DMR, for which Dr. Bergman was involved in a randomized clinical trial. He said in an interview that the two technologies differ in that the Revita uses heat with submucosal lifting to avoid deeper heat penetration, whereas ReCET is nonthermal. He is also involved in a second randomized trial of the Revita.
Is semaglutide muddying the findings?
Asked to comment about the current study with ReCET, Ali Aminian, MD, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic, said that the treatment effect is certainly plausible.
“The observation that hyperglycemia rapidly and substantially improves after bariatric surgery has prompted innovators to search for novel endoscopic procedures targeting the GI tract to improve diabetes and metabolic disease. Over the years, we learned that in addition to its role in digestion and absorption, the GI tract is actually a large endocrine organ which contributes to development of diabetes and metabolic disease.”
However, Dr. Aminian said that, “while these preliminary findings on a very small number of patients with a very short follow-up time are interesting,” he faulted the study design for including semaglutide. “When patients are treated with a combination of therapies, it will be hard to understand the true effect of each therapy,” and particularly, “when we add a strong diabetes medication like semaglutide.”
Dr. Bergman said semaglutide was used to “boost the insulin-resistant effect of the endoscopic treatment,” and that a planned double-blind, randomized trial will “show how much semaglutide actually contributed to the effect.” The ultimate goal, he noted, is to eliminate the need for all medications.
Moreover, when people with type 2 diabetes add semaglutide to insulin treatment, only about 20% typically are able to quit taking the insulin, in contrast to the 86% seen in this study, lead author Celine Busch, MBBS, a PhD candidate in gastroenterology at Amsterdam University, said in a DDW statement.
Dr. Aminian said, “I’m looking forward to better quality data ... from studies with a stronger design to prove safety, efficacy, and durability of this endoscopic intervention in patients with diabetes.”
But, he also cautioned, “in the past few years, other endoscopic procedures targeting the duodenum were introduced with exciting initial findings based on a small series [with a] short-term follow-up time. However, their safety, efficacy, and durability were not proven in subsequent studies.”
All patients stopped insulin, most for a year
The single-arm, single-center study involved 14 patients with type 2 diabetes taking basal but not premeal insulin. All underwent the 1-hour outpatient ReCET procedure, which involved placing a catheter into the first part of the small bowel and delivering electrical pulses to the duodenum.
Patients adhered to a calorie-controlled liquid diet for 2 weeks, after which they were initiated on semaglutide. All 14 patients were able to come off insulin for 3 months while maintaining glycemic control, and 12 were able to come off insulin for 12 months. They also experienced a 50% reduction in liver fat.
Dr. Bergman said a randomized, double-blind study using a sham procedure for controls is expected to start in about 2 months. “But for now, we are very encouraged by the potential for controlling type 2 diabetes with a single endoscopic treatment.”
Dr. Bergman has reported serving on the advisory board for Endogenex. Dr. Aminian has reported receiving research support and honorarium from Medtronic and Ethicon.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
FROM DDW 2023
Long-COVID patients respond differently to COVID vaccines
A new study shows that people with long COVID respond differently to COVID vaccines and that the condition may be caused by a dysfunction of the immune system – possibly explaining why some people experience symptoms for months while others recover and resume normal lives.
The study compared people who already had long COVID with people who had recovered from the virus. Both groups had not yet been vaccinated prior to the study. When researchers analyzed blood samples after people received an initial vaccine dose, they found that people with long COVID and people who had already recovered from the virus had similar immune responses at first. But
The long-COVID group also showed an extra immune response that tried to fight the virus in a secondary way that researchers didn’t expect. Both groups showed an initial increase in their blood of antibodies that primarily target what’s known as the “spike” protein of the coronavirus, which allows the virus to invade healthy cells. But the long-COVID group also showed a prolonged increased immune response that tried to fight the part of the virus related to how it replicates.
“Theoretically, the production of these antibodies could mean that people are more protected from infection,” said researcher Catherine Le, MD, in a statement. “We also need to investigate if the elevated immune response corresponds with severity or number of long–COVID-19 symptoms.”
Dr. Le is codirector of the COVID-19 Recovery Program at Cedars-Sinai Medical Center in Los Angeles, where the study was conducted.
Study participants agreed in September 2020 to participate in long-term COVID research at Cedars-Sinai. The new analysis was published earlier this year in BMC Infectious Diseases and included 245 people who had long COVID and 86 health care workers who had recovered from COVID but did not have long-term symptoms.
For the study, long COVID was defined as having symptoms that lasted more than 12 weeks. Common long-COVID symptoms are fatigue, shortness of breath, and brain dysfunction such as confusion and forgetfulness.
The authors said it’s unclear why the two groups had different immune responses and also noted that their study was limited by a small sample size. Their research of blood samples is ongoing, with the goals of identifying a way to diagnose long COVID with a laboratory test and of better understanding what causes the condition.
A version of this article first appeared on WebMD.com.
A new study shows that people with long COVID respond differently to COVID vaccines and that the condition may be caused by a dysfunction of the immune system – possibly explaining why some people experience symptoms for months while others recover and resume normal lives.
The study compared people who already had long COVID with people who had recovered from the virus. Both groups had not yet been vaccinated prior to the study. When researchers analyzed blood samples after people received an initial vaccine dose, they found that people with long COVID and people who had already recovered from the virus had similar immune responses at first. But
The long-COVID group also showed an extra immune response that tried to fight the virus in a secondary way that researchers didn’t expect. Both groups showed an initial increase in their blood of antibodies that primarily target what’s known as the “spike” protein of the coronavirus, which allows the virus to invade healthy cells. But the long-COVID group also showed a prolonged increased immune response that tried to fight the part of the virus related to how it replicates.
“Theoretically, the production of these antibodies could mean that people are more protected from infection,” said researcher Catherine Le, MD, in a statement. “We also need to investigate if the elevated immune response corresponds with severity or number of long–COVID-19 symptoms.”
Dr. Le is codirector of the COVID-19 Recovery Program at Cedars-Sinai Medical Center in Los Angeles, where the study was conducted.
Study participants agreed in September 2020 to participate in long-term COVID research at Cedars-Sinai. The new analysis was published earlier this year in BMC Infectious Diseases and included 245 people who had long COVID and 86 health care workers who had recovered from COVID but did not have long-term symptoms.
For the study, long COVID was defined as having symptoms that lasted more than 12 weeks. Common long-COVID symptoms are fatigue, shortness of breath, and brain dysfunction such as confusion and forgetfulness.
The authors said it’s unclear why the two groups had different immune responses and also noted that their study was limited by a small sample size. Their research of blood samples is ongoing, with the goals of identifying a way to diagnose long COVID with a laboratory test and of better understanding what causes the condition.
A version of this article first appeared on WebMD.com.
A new study shows that people with long COVID respond differently to COVID vaccines and that the condition may be caused by a dysfunction of the immune system – possibly explaining why some people experience symptoms for months while others recover and resume normal lives.
The study compared people who already had long COVID with people who had recovered from the virus. Both groups had not yet been vaccinated prior to the study. When researchers analyzed blood samples after people received an initial vaccine dose, they found that people with long COVID and people who had already recovered from the virus had similar immune responses at first. But
The long-COVID group also showed an extra immune response that tried to fight the virus in a secondary way that researchers didn’t expect. Both groups showed an initial increase in their blood of antibodies that primarily target what’s known as the “spike” protein of the coronavirus, which allows the virus to invade healthy cells. But the long-COVID group also showed a prolonged increased immune response that tried to fight the part of the virus related to how it replicates.
“Theoretically, the production of these antibodies could mean that people are more protected from infection,” said researcher Catherine Le, MD, in a statement. “We also need to investigate if the elevated immune response corresponds with severity or number of long–COVID-19 symptoms.”
Dr. Le is codirector of the COVID-19 Recovery Program at Cedars-Sinai Medical Center in Los Angeles, where the study was conducted.
Study participants agreed in September 2020 to participate in long-term COVID research at Cedars-Sinai. The new analysis was published earlier this year in BMC Infectious Diseases and included 245 people who had long COVID and 86 health care workers who had recovered from COVID but did not have long-term symptoms.
For the study, long COVID was defined as having symptoms that lasted more than 12 weeks. Common long-COVID symptoms are fatigue, shortness of breath, and brain dysfunction such as confusion and forgetfulness.
The authors said it’s unclear why the two groups had different immune responses and also noted that their study was limited by a small sample size. Their research of blood samples is ongoing, with the goals of identifying a way to diagnose long COVID with a laboratory test and of better understanding what causes the condition.
A version of this article first appeared on WebMD.com.