User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
How the Change Healthcare Cyberattack Affects Oncology Care
Change Healthcare, a subsidiary of UnitedHealth, took its systems offline after a cyberattack by BlackCat/ALPHV ransomware group.
The American Hospital Association said that this massive interruption is the “most significant cyberattack on the US healthcare system in American history.”
What Is the Change Healthcare Attack?
On February 21, Change Healthcare experienced an outside cybersecurity threat. When it became aware of the issue, the company disconnected its systems to prevent any further issues. Change Healthcare said that it has a “high level” of confidence that the cyberattack did not affect Optum, UnitedHealthcare, and UnitedHealth Group systems, stating it was an isolated attack on Change Healthcare. However, Change Healthcare has not said whether patient information has been compromised.
Who Is Behind the Attack?
In a statement, Change Healthcare announced that BlackCat/ALPHV identified itself to the company, claiming responsibility for the cybercrime. According to the US Department of Justice, BlackCat/ALPHV is the second most prolific ransomware-as-a-service entity in the world, with over 1000 victims of cybercrimes across the globe.
This news organization reached out to the Cybersecurity and Infrastructure Security Agency (CISA), a component of the US Department of Homeland Security, for comment on whether CISA or other agencies had taken any previous action to stop the group after other attacks.
“CISA is working with our partners and Change Healthcare to support remediation, assist impacted organizations, and share timely information to reduce the likelihood of similar intrusions,” Eric Goldstein, executive assistant director for cybersecurity, responded in a statement.
How Has the Attack Affected Oncology Practices?
Change Healthcare is a technology company that provides services to hospitals and clinics across the country, including pharmacy claims transactions, clinician claims processing, patient access and financial clearance, clinician payments, and prior authorizations.
The Community Oncology Alliance (COA) said that the cyberattack has caused a massive disruption in claims processing. COA also said that practices have reported the disruption of benefits verification for patients, prior authorizations, and financial assistance from the attack.
“It’s impacting pretty much every facet of the practice and practice management,” said Nicolas Ferreyros, managing director of policy, advocacy, and communications at COA. “Right now, practices are making do, they’re working around these challenges.”
However, Ferreyros cautioned, continuing to manage these challenges “is absolutely, 100% unsustainable” for oncology practices.
“Very soon you’re going to find practices that are having to make tough decisions about what to do, how are they going to make payroll, are they going to take financial risks on filling prescriptions and treating patients?” he added.
What Are Current Workarounds for Clinicians?
Change Healthcare recommends that clinicians use manual methods such as calling the payer’s provider service line to check patients’ claim status and complete eligibility verification and prior authorizations.
The Department of Health & Human Services has issued guidance to Medicare Advantage organizations and Part D sponsors asking them to “remove or relax prior authorization, other utilization management, and timely filing requirements” while systems are offline. The department is also asking Medicare Advantage to offer advance funding to clinicians who have been affected the most.
How Common Are Attacks Like These?
In 2023, a record-setting 725 healthcare security breaches were reported to the Department of Health & Human Services Office for Civil Rights, according to a report from The HIPAA Journal. The number of breachers has increased yearly. Last year, an average of 370,000 healthcare records were breached every day.
A version of this article first appeared on Medscape.com.
Change Healthcare, a subsidiary of UnitedHealth, took its systems offline after a cyberattack by BlackCat/ALPHV ransomware group.
The American Hospital Association said that this massive interruption is the “most significant cyberattack on the US healthcare system in American history.”
What Is the Change Healthcare Attack?
On February 21, Change Healthcare experienced an outside cybersecurity threat. When it became aware of the issue, the company disconnected its systems to prevent any further issues. Change Healthcare said that it has a “high level” of confidence that the cyberattack did not affect Optum, UnitedHealthcare, and UnitedHealth Group systems, stating it was an isolated attack on Change Healthcare. However, Change Healthcare has not said whether patient information has been compromised.
Who Is Behind the Attack?
In a statement, Change Healthcare announced that BlackCat/ALPHV identified itself to the company, claiming responsibility for the cybercrime. According to the US Department of Justice, BlackCat/ALPHV is the second most prolific ransomware-as-a-service entity in the world, with over 1000 victims of cybercrimes across the globe.
This news organization reached out to the Cybersecurity and Infrastructure Security Agency (CISA), a component of the US Department of Homeland Security, for comment on whether CISA or other agencies had taken any previous action to stop the group after other attacks.
“CISA is working with our partners and Change Healthcare to support remediation, assist impacted organizations, and share timely information to reduce the likelihood of similar intrusions,” Eric Goldstein, executive assistant director for cybersecurity, responded in a statement.
How Has the Attack Affected Oncology Practices?
Change Healthcare is a technology company that provides services to hospitals and clinics across the country, including pharmacy claims transactions, clinician claims processing, patient access and financial clearance, clinician payments, and prior authorizations.
The Community Oncology Alliance (COA) said that the cyberattack has caused a massive disruption in claims processing. COA also said that practices have reported the disruption of benefits verification for patients, prior authorizations, and financial assistance from the attack.
“It’s impacting pretty much every facet of the practice and practice management,” said Nicolas Ferreyros, managing director of policy, advocacy, and communications at COA. “Right now, practices are making do, they’re working around these challenges.”
However, Ferreyros cautioned, continuing to manage these challenges “is absolutely, 100% unsustainable” for oncology practices.
“Very soon you’re going to find practices that are having to make tough decisions about what to do, how are they going to make payroll, are they going to take financial risks on filling prescriptions and treating patients?” he added.
What Are Current Workarounds for Clinicians?
Change Healthcare recommends that clinicians use manual methods such as calling the payer’s provider service line to check patients’ claim status and complete eligibility verification and prior authorizations.
The Department of Health & Human Services has issued guidance to Medicare Advantage organizations and Part D sponsors asking them to “remove or relax prior authorization, other utilization management, and timely filing requirements” while systems are offline. The department is also asking Medicare Advantage to offer advance funding to clinicians who have been affected the most.
How Common Are Attacks Like These?
In 2023, a record-setting 725 healthcare security breaches were reported to the Department of Health & Human Services Office for Civil Rights, according to a report from The HIPAA Journal. The number of breachers has increased yearly. Last year, an average of 370,000 healthcare records were breached every day.
A version of this article first appeared on Medscape.com.
Change Healthcare, a subsidiary of UnitedHealth, took its systems offline after a cyberattack by BlackCat/ALPHV ransomware group.
The American Hospital Association said that this massive interruption is the “most significant cyberattack on the US healthcare system in American history.”
What Is the Change Healthcare Attack?
On February 21, Change Healthcare experienced an outside cybersecurity threat. When it became aware of the issue, the company disconnected its systems to prevent any further issues. Change Healthcare said that it has a “high level” of confidence that the cyberattack did not affect Optum, UnitedHealthcare, and UnitedHealth Group systems, stating it was an isolated attack on Change Healthcare. However, Change Healthcare has not said whether patient information has been compromised.
Who Is Behind the Attack?
In a statement, Change Healthcare announced that BlackCat/ALPHV identified itself to the company, claiming responsibility for the cybercrime. According to the US Department of Justice, BlackCat/ALPHV is the second most prolific ransomware-as-a-service entity in the world, with over 1000 victims of cybercrimes across the globe.
This news organization reached out to the Cybersecurity and Infrastructure Security Agency (CISA), a component of the US Department of Homeland Security, for comment on whether CISA or other agencies had taken any previous action to stop the group after other attacks.
“CISA is working with our partners and Change Healthcare to support remediation, assist impacted organizations, and share timely information to reduce the likelihood of similar intrusions,” Eric Goldstein, executive assistant director for cybersecurity, responded in a statement.
How Has the Attack Affected Oncology Practices?
Change Healthcare is a technology company that provides services to hospitals and clinics across the country, including pharmacy claims transactions, clinician claims processing, patient access and financial clearance, clinician payments, and prior authorizations.
The Community Oncology Alliance (COA) said that the cyberattack has caused a massive disruption in claims processing. COA also said that practices have reported the disruption of benefits verification for patients, prior authorizations, and financial assistance from the attack.
“It’s impacting pretty much every facet of the practice and practice management,” said Nicolas Ferreyros, managing director of policy, advocacy, and communications at COA. “Right now, practices are making do, they’re working around these challenges.”
However, Ferreyros cautioned, continuing to manage these challenges “is absolutely, 100% unsustainable” for oncology practices.
“Very soon you’re going to find practices that are having to make tough decisions about what to do, how are they going to make payroll, are they going to take financial risks on filling prescriptions and treating patients?” he added.
What Are Current Workarounds for Clinicians?
Change Healthcare recommends that clinicians use manual methods such as calling the payer’s provider service line to check patients’ claim status and complete eligibility verification and prior authorizations.
The Department of Health & Human Services has issued guidance to Medicare Advantage organizations and Part D sponsors asking them to “remove or relax prior authorization, other utilization management, and timely filing requirements” while systems are offline. The department is also asking Medicare Advantage to offer advance funding to clinicians who have been affected the most.
How Common Are Attacks Like These?
In 2023, a record-setting 725 healthcare security breaches were reported to the Department of Health & Human Services Office for Civil Rights, according to a report from The HIPAA Journal. The number of breachers has increased yearly. Last year, an average of 370,000 healthcare records were breached every day.
A version of this article first appeared on Medscape.com.
Study Finds No Increased Cancer Risk With Spironolactone
TOPLINE:
than that of unexposed women.
METHODOLOGY:
- Spironolactone, used off-label for several skin conditions in women, carries a warning about an increased tumor risk associated with high doses in rat models, and its antiandrogen properties have prompted hypotheses about a possible increased risk for breast or gynecologic cancers.
- The researchers reviewed data on 420 women with a history of spironolactone use for acne, hair loss, and hirsutism and 3272 women with no spironolactone use at the authors› institution. Their mean age ranged from 42 to 63 years; the majority were White, and 38% were non-White.
- Median spironolactone doses ranged from 25 mg to 225 mg; chart reviews included 5-year follow-up data from the first spironolactone exposure to allow time for tumor development.
TAKEAWAY:
- A total of 37 of the 420 women exposed to spironolactone developed any tumors, as did 546 of the 3272 with no spironolactone exposure.
- After the researchers controlled for age and race, women exposed to spironolactone were no more likely to develop a malignant tumor than a benign tumor, compared with unexposed women (odds ratio [OR], 0.48, P = .2).
- The risk for breast or uterine cancer was not significantly different in the spironolactone and non-spironolactone groups (OR, 0.95, P > .9).
IN PRACTICE:
“Women taking spironolactone for acne, hair loss, and hirsutism and who are at low risk of breast or gynecologic cancers may be counseled to have regular gynecology follow-up, but no more frequently than the general population,” but more studies are needed to evaluate risk over longer periods of time, the researchers wrote.
SOURCE:
The lead author of the study was Rachel C. Hill, BS, a student at Weill Cornell Medical College, New York City, and Shari R. Lipner, MD, PhD, of the department of dermatology at Weill Cornell Medical College, was the corresponding author. The study was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The findings were limited by the retrospective design, as well as the small number of spironolactone patients analyzed, the short follow-up period, the lack of information about spironolactone courses, and the inability to control for family history of malignancy.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences and a grant from the Clinical and Translational Science Center at Weill Cornell Medical College awarded to Ms. Hill. None of the authors had relevant disclosures; Dr. Lipner disclosed serving as a consultant for Ortho-Dermatologics, Eli Lilly, Moberg Pharmaceuticals, and BelleTorus Corporation.
A version of this article appeared on Medscape.com.
TOPLINE:
than that of unexposed women.
METHODOLOGY:
- Spironolactone, used off-label for several skin conditions in women, carries a warning about an increased tumor risk associated with high doses in rat models, and its antiandrogen properties have prompted hypotheses about a possible increased risk for breast or gynecologic cancers.
- The researchers reviewed data on 420 women with a history of spironolactone use for acne, hair loss, and hirsutism and 3272 women with no spironolactone use at the authors› institution. Their mean age ranged from 42 to 63 years; the majority were White, and 38% were non-White.
- Median spironolactone doses ranged from 25 mg to 225 mg; chart reviews included 5-year follow-up data from the first spironolactone exposure to allow time for tumor development.
TAKEAWAY:
- A total of 37 of the 420 women exposed to spironolactone developed any tumors, as did 546 of the 3272 with no spironolactone exposure.
- After the researchers controlled for age and race, women exposed to spironolactone were no more likely to develop a malignant tumor than a benign tumor, compared with unexposed women (odds ratio [OR], 0.48, P = .2).
- The risk for breast or uterine cancer was not significantly different in the spironolactone and non-spironolactone groups (OR, 0.95, P > .9).
IN PRACTICE:
“Women taking spironolactone for acne, hair loss, and hirsutism and who are at low risk of breast or gynecologic cancers may be counseled to have regular gynecology follow-up, but no more frequently than the general population,” but more studies are needed to evaluate risk over longer periods of time, the researchers wrote.
SOURCE:
The lead author of the study was Rachel C. Hill, BS, a student at Weill Cornell Medical College, New York City, and Shari R. Lipner, MD, PhD, of the department of dermatology at Weill Cornell Medical College, was the corresponding author. The study was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The findings were limited by the retrospective design, as well as the small number of spironolactone patients analyzed, the short follow-up period, the lack of information about spironolactone courses, and the inability to control for family history of malignancy.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences and a grant from the Clinical and Translational Science Center at Weill Cornell Medical College awarded to Ms. Hill. None of the authors had relevant disclosures; Dr. Lipner disclosed serving as a consultant for Ortho-Dermatologics, Eli Lilly, Moberg Pharmaceuticals, and BelleTorus Corporation.
A version of this article appeared on Medscape.com.
TOPLINE:
than that of unexposed women.
METHODOLOGY:
- Spironolactone, used off-label for several skin conditions in women, carries a warning about an increased tumor risk associated with high doses in rat models, and its antiandrogen properties have prompted hypotheses about a possible increased risk for breast or gynecologic cancers.
- The researchers reviewed data on 420 women with a history of spironolactone use for acne, hair loss, and hirsutism and 3272 women with no spironolactone use at the authors› institution. Their mean age ranged from 42 to 63 years; the majority were White, and 38% were non-White.
- Median spironolactone doses ranged from 25 mg to 225 mg; chart reviews included 5-year follow-up data from the first spironolactone exposure to allow time for tumor development.
TAKEAWAY:
- A total of 37 of the 420 women exposed to spironolactone developed any tumors, as did 546 of the 3272 with no spironolactone exposure.
- After the researchers controlled for age and race, women exposed to spironolactone were no more likely to develop a malignant tumor than a benign tumor, compared with unexposed women (odds ratio [OR], 0.48, P = .2).
- The risk for breast or uterine cancer was not significantly different in the spironolactone and non-spironolactone groups (OR, 0.95, P > .9).
IN PRACTICE:
“Women taking spironolactone for acne, hair loss, and hirsutism and who are at low risk of breast or gynecologic cancers may be counseled to have regular gynecology follow-up, but no more frequently than the general population,” but more studies are needed to evaluate risk over longer periods of time, the researchers wrote.
SOURCE:
The lead author of the study was Rachel C. Hill, BS, a student at Weill Cornell Medical College, New York City, and Shari R. Lipner, MD, PhD, of the department of dermatology at Weill Cornell Medical College, was the corresponding author. The study was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The findings were limited by the retrospective design, as well as the small number of spironolactone patients analyzed, the short follow-up period, the lack of information about spironolactone courses, and the inability to control for family history of malignancy.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences and a grant from the Clinical and Translational Science Center at Weill Cornell Medical College awarded to Ms. Hill. None of the authors had relevant disclosures; Dr. Lipner disclosed serving as a consultant for Ortho-Dermatologics, Eli Lilly, Moberg Pharmaceuticals, and BelleTorus Corporation.
A version of this article appeared on Medscape.com.
High-Fiber Gut Microbe Makeover Aids Weight Loss
TOPLINE:
A fiber supplement also found in beans and other foods may lead to weight loss and improved insulin sensitivity in people with excess body weight, partly due to changes in the gut microbiota.
METHODOLOGY:
- In animal studies, resistant starch (RS), a kind of dietary fiber, has shown a potential to reduce body fat along with other metabolic benefits, but human dietary studies of RS have been inconsistent, especially with a high-fat diet.
- Researchers conducted a crossover, randomized trial to study the effect of RS as a dietary supplement on 37 individuals with overweight or obesity (average age, 33.43 years; 15 women; body mass index > 24 or higher waist circumference).
- Participants were fed a similar background diet and either 40 g of RS (high-amylose maize) or an energy-matched placebo starch daily for 8 weeks and then switched between the two in a separate 8-week period.
- The primary outcome was body weight, and the secondary outcomes were visceral and subcutaneous fat mass, waist circumference, lipid profiles, insulin sensitivity, metabolome, and gut microbiome.
- RS’s impact on gut microbiota composition and function was assessed with metagenomics and metabolomics, and RS-modified gut microbiota’s effect on host body fat and glucose was confirmed by transferring from select average participants to mice.
TAKEAWAY:
- Participants showed a mean weight loss of 2.8 kg after consuming RS for 8 weeks (P < .001), but there was no significant change in body weight in those on placebo starch.
- RS improved insulin sensitivity in people to a greater extent than placebo starch (P = .025) and showed a greater reduction in fat mass, waist circumference, and other obesity-related outcomes.
- The abundance in the gut of the microbe Bifidobacterium adolescentis increased significantly following RS intervention, an increase that exhibited a strong correlation with decreased BMI, suggesting a role of RS in reducing obesity.
- The levels of pro-inflammatory cytokines, such as serum tumor necrosis factor-alpha and interleukin-1 beta, were significantly lower in participants who consumed RS than in those who had placebo starch.
IN PRACTICE:
“Our study provided an effective dietary recommendation using RS as a supplement (40 g/d with a balanced background diet containing 25%-30% fat), which may help to achieve significant weight loss,” the authors wrote.
SOURCE:
This study was led and corresponded by Huating Li, Shanghai Clinical Center for Diabetes, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China, and University of Hong Kong, Pok Fu Lam, and published online in Nature Metabolism.
LIMITATIONS:
This study was limited by the small sample size and stringent inclusion criteria for participants. The use of database-driven and taxane-based methodology might have led to difficult-to-classify sequences being discarded and strain-level functional diversity being overlooked. The authors also acknowledged the need to validate the findings of this study in larger and more diverse cohorts.
DISCLOSURES:
This work was supported by the National Key Research and Development Program of China, Shanghai Municipal Key Clinical Specialty, National Natural Science Foundation of China, and other sources. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
A fiber supplement also found in beans and other foods may lead to weight loss and improved insulin sensitivity in people with excess body weight, partly due to changes in the gut microbiota.
METHODOLOGY:
- In animal studies, resistant starch (RS), a kind of dietary fiber, has shown a potential to reduce body fat along with other metabolic benefits, but human dietary studies of RS have been inconsistent, especially with a high-fat diet.
- Researchers conducted a crossover, randomized trial to study the effect of RS as a dietary supplement on 37 individuals with overweight or obesity (average age, 33.43 years; 15 women; body mass index > 24 or higher waist circumference).
- Participants were fed a similar background diet and either 40 g of RS (high-amylose maize) or an energy-matched placebo starch daily for 8 weeks and then switched between the two in a separate 8-week period.
- The primary outcome was body weight, and the secondary outcomes were visceral and subcutaneous fat mass, waist circumference, lipid profiles, insulin sensitivity, metabolome, and gut microbiome.
- RS’s impact on gut microbiota composition and function was assessed with metagenomics and metabolomics, and RS-modified gut microbiota’s effect on host body fat and glucose was confirmed by transferring from select average participants to mice.
TAKEAWAY:
- Participants showed a mean weight loss of 2.8 kg after consuming RS for 8 weeks (P < .001), but there was no significant change in body weight in those on placebo starch.
- RS improved insulin sensitivity in people to a greater extent than placebo starch (P = .025) and showed a greater reduction in fat mass, waist circumference, and other obesity-related outcomes.
- The abundance in the gut of the microbe Bifidobacterium adolescentis increased significantly following RS intervention, an increase that exhibited a strong correlation with decreased BMI, suggesting a role of RS in reducing obesity.
- The levels of pro-inflammatory cytokines, such as serum tumor necrosis factor-alpha and interleukin-1 beta, were significantly lower in participants who consumed RS than in those who had placebo starch.
IN PRACTICE:
“Our study provided an effective dietary recommendation using RS as a supplement (40 g/d with a balanced background diet containing 25%-30% fat), which may help to achieve significant weight loss,” the authors wrote.
SOURCE:
This study was led and corresponded by Huating Li, Shanghai Clinical Center for Diabetes, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China, and University of Hong Kong, Pok Fu Lam, and published online in Nature Metabolism.
LIMITATIONS:
This study was limited by the small sample size and stringent inclusion criteria for participants. The use of database-driven and taxane-based methodology might have led to difficult-to-classify sequences being discarded and strain-level functional diversity being overlooked. The authors also acknowledged the need to validate the findings of this study in larger and more diverse cohorts.
DISCLOSURES:
This work was supported by the National Key Research and Development Program of China, Shanghai Municipal Key Clinical Specialty, National Natural Science Foundation of China, and other sources. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
A fiber supplement also found in beans and other foods may lead to weight loss and improved insulin sensitivity in people with excess body weight, partly due to changes in the gut microbiota.
METHODOLOGY:
- In animal studies, resistant starch (RS), a kind of dietary fiber, has shown a potential to reduce body fat along with other metabolic benefits, but human dietary studies of RS have been inconsistent, especially with a high-fat diet.
- Researchers conducted a crossover, randomized trial to study the effect of RS as a dietary supplement on 37 individuals with overweight or obesity (average age, 33.43 years; 15 women; body mass index > 24 or higher waist circumference).
- Participants were fed a similar background diet and either 40 g of RS (high-amylose maize) or an energy-matched placebo starch daily for 8 weeks and then switched between the two in a separate 8-week period.
- The primary outcome was body weight, and the secondary outcomes were visceral and subcutaneous fat mass, waist circumference, lipid profiles, insulin sensitivity, metabolome, and gut microbiome.
- RS’s impact on gut microbiota composition and function was assessed with metagenomics and metabolomics, and RS-modified gut microbiota’s effect on host body fat and glucose was confirmed by transferring from select average participants to mice.
TAKEAWAY:
- Participants showed a mean weight loss of 2.8 kg after consuming RS for 8 weeks (P < .001), but there was no significant change in body weight in those on placebo starch.
- RS improved insulin sensitivity in people to a greater extent than placebo starch (P = .025) and showed a greater reduction in fat mass, waist circumference, and other obesity-related outcomes.
- The abundance in the gut of the microbe Bifidobacterium adolescentis increased significantly following RS intervention, an increase that exhibited a strong correlation with decreased BMI, suggesting a role of RS in reducing obesity.
- The levels of pro-inflammatory cytokines, such as serum tumor necrosis factor-alpha and interleukin-1 beta, were significantly lower in participants who consumed RS than in those who had placebo starch.
IN PRACTICE:
“Our study provided an effective dietary recommendation using RS as a supplement (40 g/d with a balanced background diet containing 25%-30% fat), which may help to achieve significant weight loss,” the authors wrote.
SOURCE:
This study was led and corresponded by Huating Li, Shanghai Clinical Center for Diabetes, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China, and University of Hong Kong, Pok Fu Lam, and published online in Nature Metabolism.
LIMITATIONS:
This study was limited by the small sample size and stringent inclusion criteria for participants. The use of database-driven and taxane-based methodology might have led to difficult-to-classify sequences being discarded and strain-level functional diversity being overlooked. The authors also acknowledged the need to validate the findings of this study in larger and more diverse cohorts.
DISCLOSURES:
This work was supported by the National Key Research and Development Program of China, Shanghai Municipal Key Clinical Specialty, National Natural Science Foundation of China, and other sources. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
No Increase in Autoimmune Risk Seen With GLP-1 Receptor Agonists and SGLT2 Inhibitors
TOPLINE:
In patients with type 2 diabetes, there was no difference in risk of developing autoimmune disease if prescribed glucagon-like peptide 1 receptor agonists (GLP-1-RAs), sodium-glucose cotransporter-2 (SGLT2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors.
METHODOLOGY:
- The effect of GLP-1-RAs and SGLT2 inhibitors on autoimmune rheumatic disease (ARD) is understudied, though previous case reports and one study have hinted at increased risk.
- Researchers used administrative health data from 2014 to 2021 to identify 34,400 patients prescribed GLP-1-RAs and 83,500 patients prescribed SGLT2 inhibitors.
- They compared patients prescribed GLP-1-RAs or SGLT2 inhibitors with 68,400 patients prescribed DPP-4 inhibitors, which previous studies suggest do not increase ARD risk.
- Primary outcome was ARD incidence, defined by diagnostic codes.
TAKEAWAY:
- There were no significant differences in incident ARDs between the three groups.
- Mean follow-up time was 0.88-1.53 years.
- The hazard ratio (HR) for developing ARDs with GLP-1-RAs exposure was 0.93 (95% CI, 0.66-1.30) compared with DPP-4 inhibitors.
- The HR for developing ARDs with SGLT2 inhibitor exposure was 0.97 (95% CI, 0.76-1.24).
IN PRACTICE:
“Extended longitudinal data are needed to assess risk and benefit with longer-term exposure,” the authors wrote.
SOURCE:
First author Derin Karacabeyli, MD, of the University of British Columbia, Vancouver, Canada, presented the study in abstract form at the Canadian Rheumatology Association (CRA) 2024 Annual Meeting in Winnipeg on February 29.
LIMITATIONS:
The study was observational, which could have some residual or unmeasured confounding of data. The researchers relied on diagnostic codes and the average follow-up time was short.
DISCLOSURES:
The study was funded by the Canadian Institutes of Health Research. The authors had no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
In patients with type 2 diabetes, there was no difference in risk of developing autoimmune disease if prescribed glucagon-like peptide 1 receptor agonists (GLP-1-RAs), sodium-glucose cotransporter-2 (SGLT2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors.
METHODOLOGY:
- The effect of GLP-1-RAs and SGLT2 inhibitors on autoimmune rheumatic disease (ARD) is understudied, though previous case reports and one study have hinted at increased risk.
- Researchers used administrative health data from 2014 to 2021 to identify 34,400 patients prescribed GLP-1-RAs and 83,500 patients prescribed SGLT2 inhibitors.
- They compared patients prescribed GLP-1-RAs or SGLT2 inhibitors with 68,400 patients prescribed DPP-4 inhibitors, which previous studies suggest do not increase ARD risk.
- Primary outcome was ARD incidence, defined by diagnostic codes.
TAKEAWAY:
- There were no significant differences in incident ARDs between the three groups.
- Mean follow-up time was 0.88-1.53 years.
- The hazard ratio (HR) for developing ARDs with GLP-1-RAs exposure was 0.93 (95% CI, 0.66-1.30) compared with DPP-4 inhibitors.
- The HR for developing ARDs with SGLT2 inhibitor exposure was 0.97 (95% CI, 0.76-1.24).
IN PRACTICE:
“Extended longitudinal data are needed to assess risk and benefit with longer-term exposure,” the authors wrote.
SOURCE:
First author Derin Karacabeyli, MD, of the University of British Columbia, Vancouver, Canada, presented the study in abstract form at the Canadian Rheumatology Association (CRA) 2024 Annual Meeting in Winnipeg on February 29.
LIMITATIONS:
The study was observational, which could have some residual or unmeasured confounding of data. The researchers relied on diagnostic codes and the average follow-up time was short.
DISCLOSURES:
The study was funded by the Canadian Institutes of Health Research. The authors had no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
In patients with type 2 diabetes, there was no difference in risk of developing autoimmune disease if prescribed glucagon-like peptide 1 receptor agonists (GLP-1-RAs), sodium-glucose cotransporter-2 (SGLT2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors.
METHODOLOGY:
- The effect of GLP-1-RAs and SGLT2 inhibitors on autoimmune rheumatic disease (ARD) is understudied, though previous case reports and one study have hinted at increased risk.
- Researchers used administrative health data from 2014 to 2021 to identify 34,400 patients prescribed GLP-1-RAs and 83,500 patients prescribed SGLT2 inhibitors.
- They compared patients prescribed GLP-1-RAs or SGLT2 inhibitors with 68,400 patients prescribed DPP-4 inhibitors, which previous studies suggest do not increase ARD risk.
- Primary outcome was ARD incidence, defined by diagnostic codes.
TAKEAWAY:
- There were no significant differences in incident ARDs between the three groups.
- Mean follow-up time was 0.88-1.53 years.
- The hazard ratio (HR) for developing ARDs with GLP-1-RAs exposure was 0.93 (95% CI, 0.66-1.30) compared with DPP-4 inhibitors.
- The HR for developing ARDs with SGLT2 inhibitor exposure was 0.97 (95% CI, 0.76-1.24).
IN PRACTICE:
“Extended longitudinal data are needed to assess risk and benefit with longer-term exposure,” the authors wrote.
SOURCE:
First author Derin Karacabeyli, MD, of the University of British Columbia, Vancouver, Canada, presented the study in abstract form at the Canadian Rheumatology Association (CRA) 2024 Annual Meeting in Winnipeg on February 29.
LIMITATIONS:
The study was observational, which could have some residual or unmeasured confounding of data. The researchers relied on diagnostic codes and the average follow-up time was short.
DISCLOSURES:
The study was funded by the Canadian Institutes of Health Research. The authors had no disclosures.
A version of this article appeared on Medscape.com.
First Denosumab Biosimilar Approved in Two Different Formulations
The US Food and Drug Administration (FDA) has approved the first biosimilar to denosumab, denosumab-bddz (Wyost/Jubbonti).
The biosimilar was also granted interchangeability status, which allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). Sandoz announced the approval on March 5, 2024. The lower dosage of denosumab-bddz, marketed as Jubbonti, was also approved by Health Canada in February.
The FDA approval “is based on robust clinical studies and accompanied by labeling with safety warnings,” according to the press release. Like the reference products Prolia and Xgeva, denosumab-bddz is approved for two indications at separate doses.
Wyost (120-mg/1.7-mL injection) is approved to:
- Prevent skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors
- Treat adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity
- Treat hypercalcemia of cancer that is refractory to bisphosphonate therapy
Jubbonti (60-mg/1-mL injection) is approved to:
- Treat postmenopausal women with osteoporosis who are at high risk for fracture
- Increase bone mass in men with osteoporosis who are at high risk for fracture
- Treat glucocorticoid-induced osteoporosis in men and women who are at high risk for fracture
- Increase bone mass in men who are at high risk for fracture who are receiving androgen deprivation therapy for nonmetastatic prostate cancer
- Increase bone mass in women who are at high risk for fracture who are receiving adjuvant aromatase inhibitor therapy for breast cancer.
Both doses are contraindicated for hypocalcemia and known clinically significant hypersensitivity to denosumab products. Exposure to denosumab products during pregnancy can cause fetal harm, so women of reproductive potential should be advised to use effective contraception during therapy and for at least 5 months after the last dose of denosumab-bddz.
Sandoz did not provide information on US launch details, citing “ongoing patent litigation around these products.”
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved the first biosimilar to denosumab, denosumab-bddz (Wyost/Jubbonti).
The biosimilar was also granted interchangeability status, which allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). Sandoz announced the approval on March 5, 2024. The lower dosage of denosumab-bddz, marketed as Jubbonti, was also approved by Health Canada in February.
The FDA approval “is based on robust clinical studies and accompanied by labeling with safety warnings,” according to the press release. Like the reference products Prolia and Xgeva, denosumab-bddz is approved for two indications at separate doses.
Wyost (120-mg/1.7-mL injection) is approved to:
- Prevent skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors
- Treat adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity
- Treat hypercalcemia of cancer that is refractory to bisphosphonate therapy
Jubbonti (60-mg/1-mL injection) is approved to:
- Treat postmenopausal women with osteoporosis who are at high risk for fracture
- Increase bone mass in men with osteoporosis who are at high risk for fracture
- Treat glucocorticoid-induced osteoporosis in men and women who are at high risk for fracture
- Increase bone mass in men who are at high risk for fracture who are receiving androgen deprivation therapy for nonmetastatic prostate cancer
- Increase bone mass in women who are at high risk for fracture who are receiving adjuvant aromatase inhibitor therapy for breast cancer.
Both doses are contraindicated for hypocalcemia and known clinically significant hypersensitivity to denosumab products. Exposure to denosumab products during pregnancy can cause fetal harm, so women of reproductive potential should be advised to use effective contraception during therapy and for at least 5 months after the last dose of denosumab-bddz.
Sandoz did not provide information on US launch details, citing “ongoing patent litigation around these products.”
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved the first biosimilar to denosumab, denosumab-bddz (Wyost/Jubbonti).
The biosimilar was also granted interchangeability status, which allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). Sandoz announced the approval on March 5, 2024. The lower dosage of denosumab-bddz, marketed as Jubbonti, was also approved by Health Canada in February.
The FDA approval “is based on robust clinical studies and accompanied by labeling with safety warnings,” according to the press release. Like the reference products Prolia and Xgeva, denosumab-bddz is approved for two indications at separate doses.
Wyost (120-mg/1.7-mL injection) is approved to:
- Prevent skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors
- Treat adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity
- Treat hypercalcemia of cancer that is refractory to bisphosphonate therapy
Jubbonti (60-mg/1-mL injection) is approved to:
- Treat postmenopausal women with osteoporosis who are at high risk for fracture
- Increase bone mass in men with osteoporosis who are at high risk for fracture
- Treat glucocorticoid-induced osteoporosis in men and women who are at high risk for fracture
- Increase bone mass in men who are at high risk for fracture who are receiving androgen deprivation therapy for nonmetastatic prostate cancer
- Increase bone mass in women who are at high risk for fracture who are receiving adjuvant aromatase inhibitor therapy for breast cancer.
Both doses are contraindicated for hypocalcemia and known clinically significant hypersensitivity to denosumab products. Exposure to denosumab products during pregnancy can cause fetal harm, so women of reproductive potential should be advised to use effective contraception during therapy and for at least 5 months after the last dose of denosumab-bddz.
Sandoz did not provide information on US launch details, citing “ongoing patent litigation around these products.”
A version of this article appeared on Medscape.com.
COVID-19 Is a Very Weird Virus
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.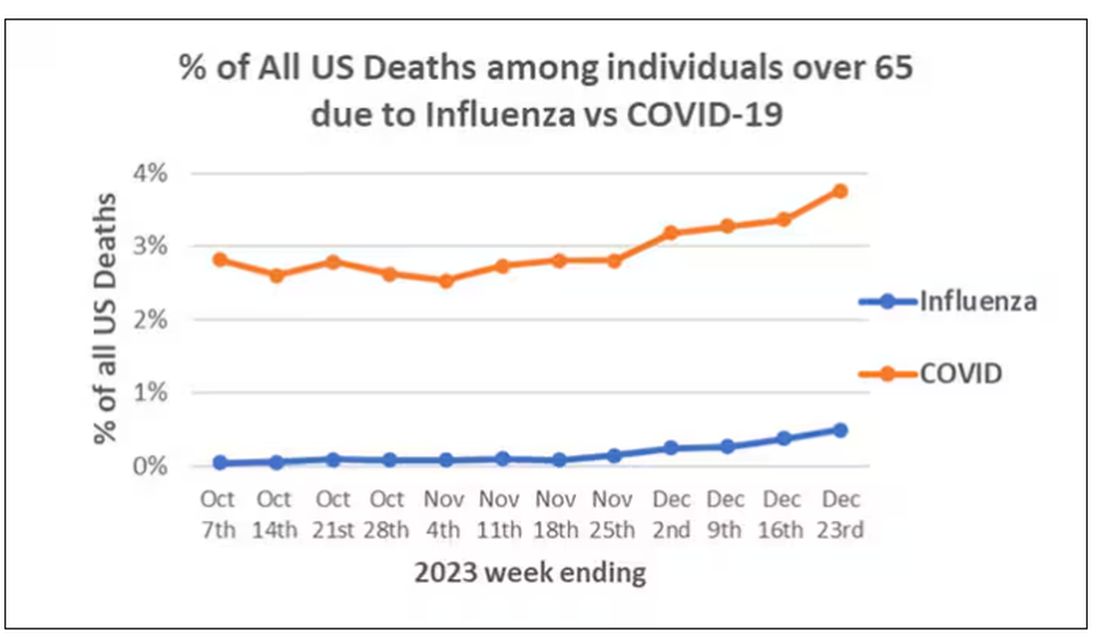
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)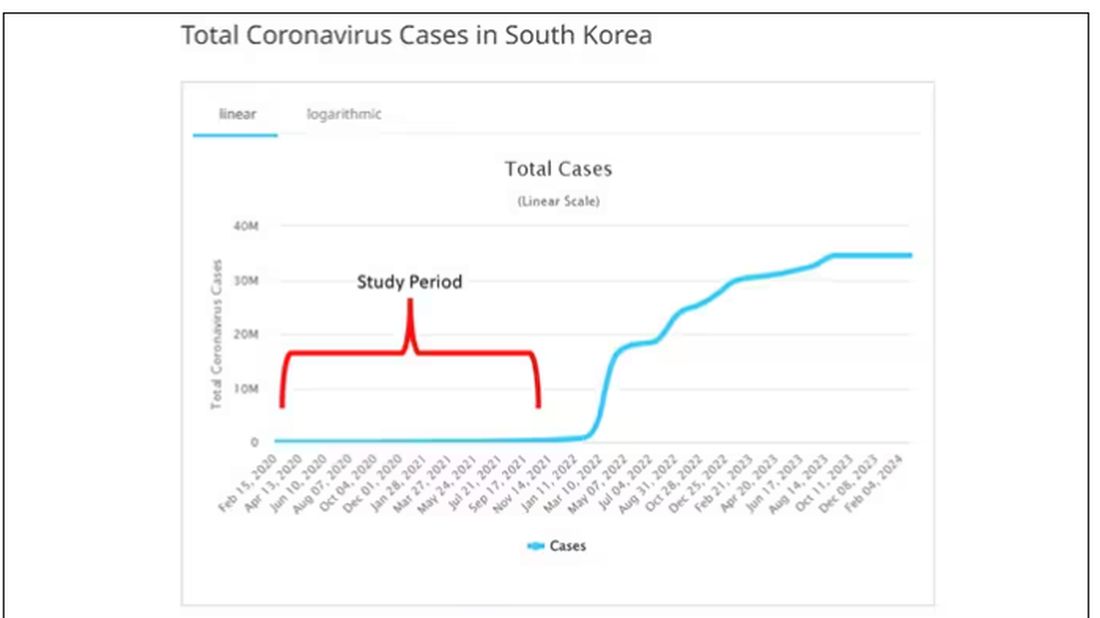
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.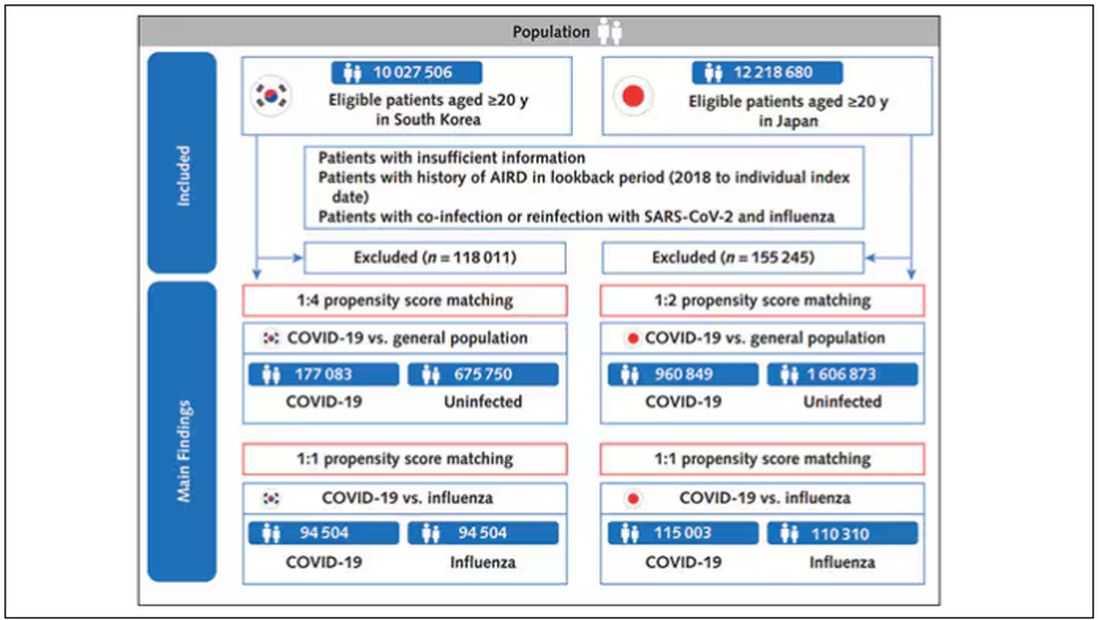
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).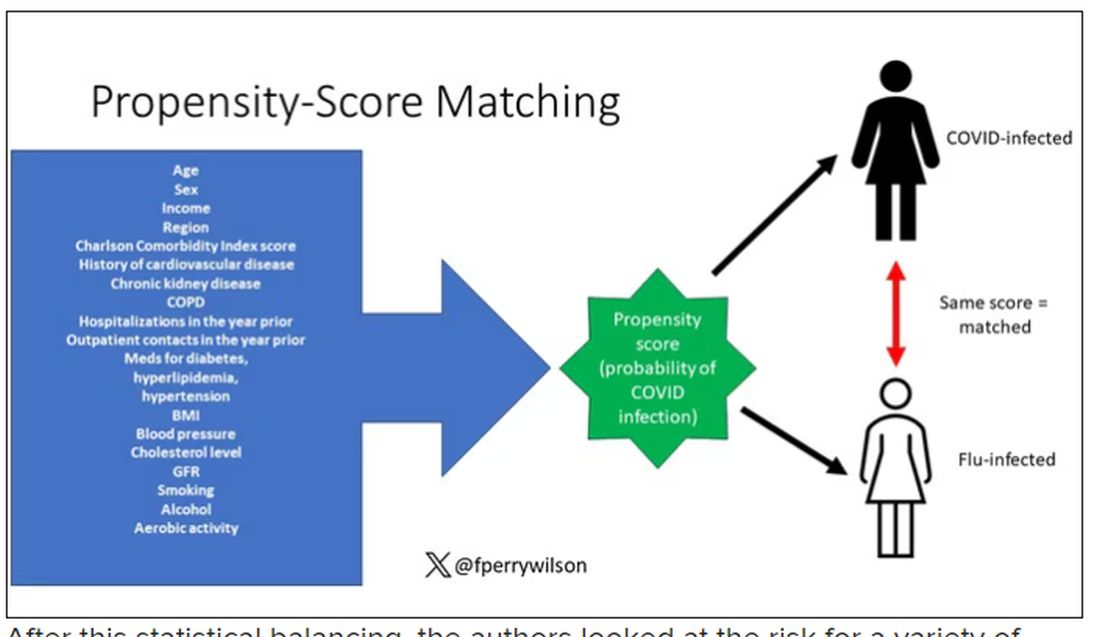
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.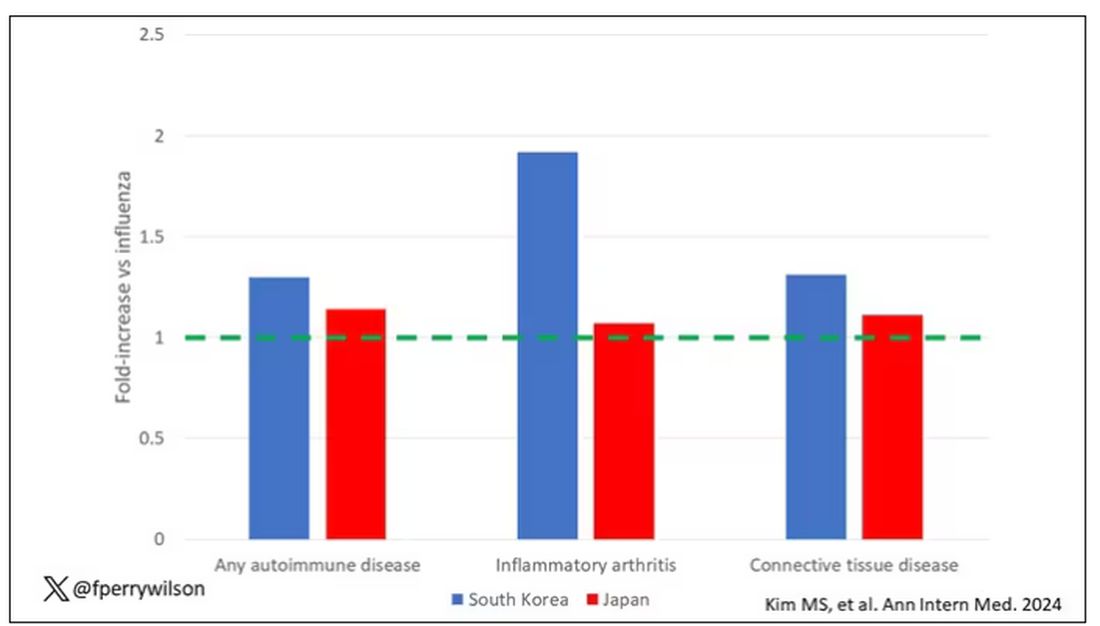
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.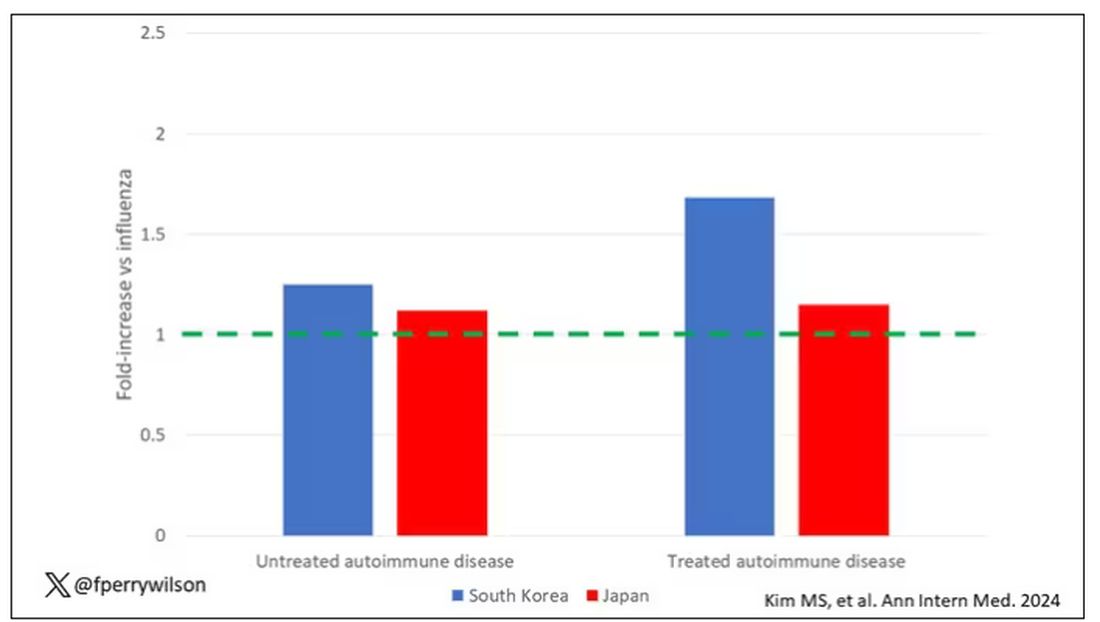
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.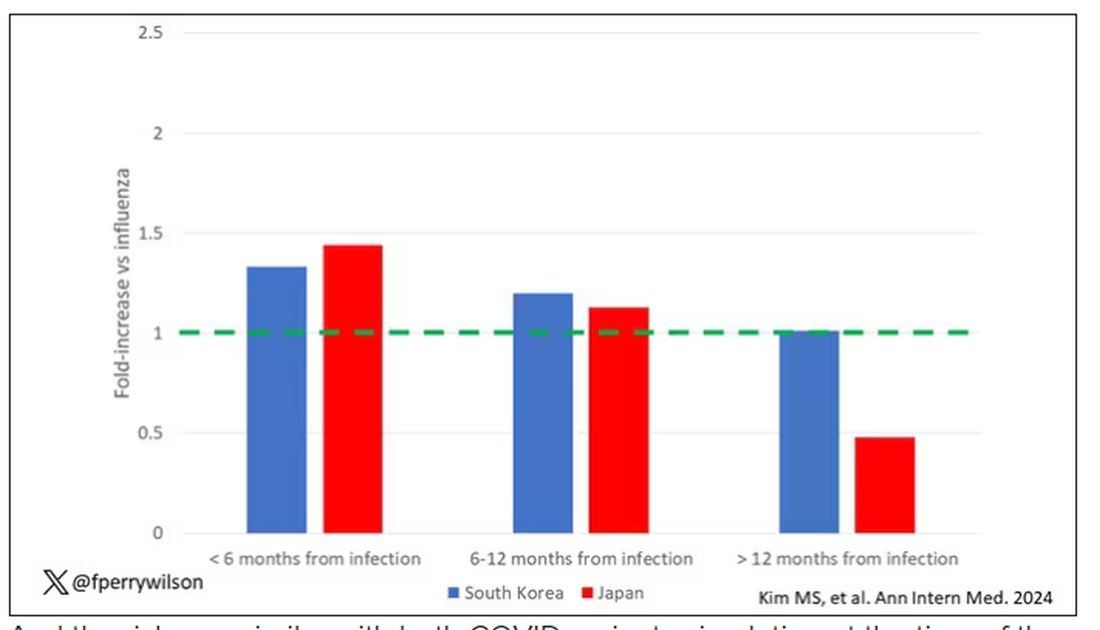
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?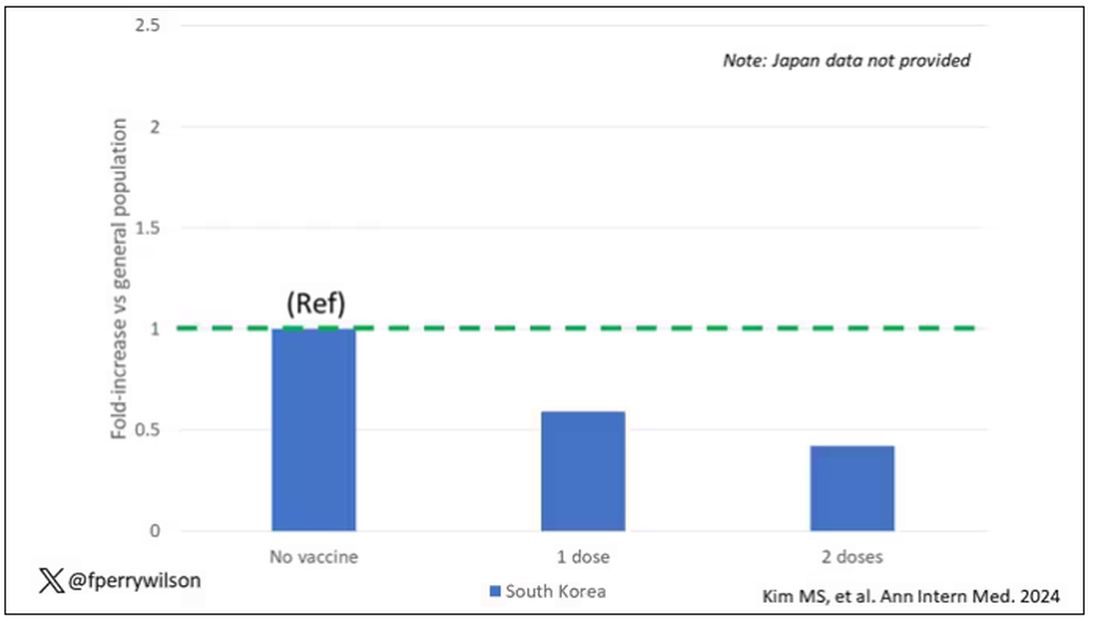
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Artificially Sweetened Drinks Linked to Increased AF Risk
TOPLINE:
(AF) in a new observational study.
METHODOLOGY:
- The population-based cohort study looked at the associations of sugar-sweetened beverages, artificial sweetened beverages, and pure fruit juice consumption with the risk for incident AF and evaluated whether genetic susceptibility modifies these associations.
- The authors analyzed data from the UK Biobank on 201,856 participants who were free of baseline AF, had genetic data available, and completed a 24-hour diet questionnaire. The diagnosis of AF was obtained by linkage from primary care, hospital inpatient, and death register records.
- The results were adjusted for a wide range of potential confounders including age, sex, ethnicity, education level, socioeconomic status, smoking, alcohol consumption, physical activity level, sleep duration, body mass index, blood pressure, kidney function, sleep apnea, coronary heart disease, diabetes, and the use of lipid-lowering or antihypertensive medication.
TAKEAWAY:
- During a median follow-up of 9.9 years, 9362 incident AF cases were documented.
- Compared with nonconsumers, individuals who consumed more than 2 L per week of artificially sweetened beverages had a 20% increased risk of developing AF (hazard ratio [HR], 1.20; 95% CI, 1.10-1.31).
- Those who drank more than 2 L per week of sugar-sweetened beverages had a 10% increased risk for AF (HR, 1.10; 95% CI, 1.01-1.20).
- Consumption of 1 L or less per week of pure fruit juice was associated with an 8% lower risk of developing AF (HR, 0.92; 95% CI, 0.87-0.97).
- The associations persisted after adjustment for genetic susceptibility for AF.
IN PRACTICE:
The study authors concluded that this study does not demonstrate that consumption of sugar-sweetened or artificially sweetened beverages alters AF risk but rather that the consumption of these drinks may predict AF risk beyond traditional risk factors. They added that intervention studies and basic research are warranted to confirm whether the observed associations are causal. Commenting on the study, Duane Mellor, MD, registered dietitian at Aston University, Birmingham, England, said it is unclear if the observations in this study are a chance finding as there is a lack of a clear biological link. Naveed Sattar, MD, professor of metabolic medicine at the University of Glasgow, Glasgow, Scotland, added that although the authors tried to adjust for many factors, there is a strong chance that other behavioral aspects linked to beverage choice could be more relevant as a cause of AF rather than the drinks themselves. Tom Sanders, MD, professor emeritus of nutrition and dietetics, King’s College London, London, England, pointed out that as this is the first study that has reported such an effect with artificially sweetened drinks, the finding needs replication before any conclusions can be drawn. “It remains good dietary advice to recommend the consumption of low-calorie artificially sweetened drink in place of sugar-sweetened drinks and alcohol,” he added.
SOURCE:
The study, led by Ying Sun, MD, Shanghai Jiao Tong University School of Medicine, Shanghai, China, was published online in Circulation: Arrhythmia and Electrophysiology.
LIMITATIONS:
The consumption of beverages was self-reported and based on only five separate single-day food intake recalls which were taken over the first 3 years of the study, which was extrapolated to estimate weekly intake. The researchers could not tell whether the sugar-sweetened and artificially sweetened drinks were caffeinated and could not rule out residual confounding by other unmeasured or unknown factors.
DISCLOSURES:
This study was supported by the National Natural Science Foundation of China, Shanghai Municipal Health Commission, Shanghai Municipal Human Resources and Social Security Bureau, Clinical Research Plan of Shanghai Hospital Development Center, Postdoctoral Scientific Research Foundation of Shanghai Ninth People’s Hospital, and Shanghai Jiao Tong University School of Medicine.
A version of this article appeared on Medscape.com.
TOPLINE:
(AF) in a new observational study.
METHODOLOGY:
- The population-based cohort study looked at the associations of sugar-sweetened beverages, artificial sweetened beverages, and pure fruit juice consumption with the risk for incident AF and evaluated whether genetic susceptibility modifies these associations.
- The authors analyzed data from the UK Biobank on 201,856 participants who were free of baseline AF, had genetic data available, and completed a 24-hour diet questionnaire. The diagnosis of AF was obtained by linkage from primary care, hospital inpatient, and death register records.
- The results were adjusted for a wide range of potential confounders including age, sex, ethnicity, education level, socioeconomic status, smoking, alcohol consumption, physical activity level, sleep duration, body mass index, blood pressure, kidney function, sleep apnea, coronary heart disease, diabetes, and the use of lipid-lowering or antihypertensive medication.
TAKEAWAY:
- During a median follow-up of 9.9 years, 9362 incident AF cases were documented.
- Compared with nonconsumers, individuals who consumed more than 2 L per week of artificially sweetened beverages had a 20% increased risk of developing AF (hazard ratio [HR], 1.20; 95% CI, 1.10-1.31).
- Those who drank more than 2 L per week of sugar-sweetened beverages had a 10% increased risk for AF (HR, 1.10; 95% CI, 1.01-1.20).
- Consumption of 1 L or less per week of pure fruit juice was associated with an 8% lower risk of developing AF (HR, 0.92; 95% CI, 0.87-0.97).
- The associations persisted after adjustment for genetic susceptibility for AF.
IN PRACTICE:
The study authors concluded that this study does not demonstrate that consumption of sugar-sweetened or artificially sweetened beverages alters AF risk but rather that the consumption of these drinks may predict AF risk beyond traditional risk factors. They added that intervention studies and basic research are warranted to confirm whether the observed associations are causal. Commenting on the study, Duane Mellor, MD, registered dietitian at Aston University, Birmingham, England, said it is unclear if the observations in this study are a chance finding as there is a lack of a clear biological link. Naveed Sattar, MD, professor of metabolic medicine at the University of Glasgow, Glasgow, Scotland, added that although the authors tried to adjust for many factors, there is a strong chance that other behavioral aspects linked to beverage choice could be more relevant as a cause of AF rather than the drinks themselves. Tom Sanders, MD, professor emeritus of nutrition and dietetics, King’s College London, London, England, pointed out that as this is the first study that has reported such an effect with artificially sweetened drinks, the finding needs replication before any conclusions can be drawn. “It remains good dietary advice to recommend the consumption of low-calorie artificially sweetened drink in place of sugar-sweetened drinks and alcohol,” he added.
SOURCE:
The study, led by Ying Sun, MD, Shanghai Jiao Tong University School of Medicine, Shanghai, China, was published online in Circulation: Arrhythmia and Electrophysiology.
LIMITATIONS:
The consumption of beverages was self-reported and based on only five separate single-day food intake recalls which were taken over the first 3 years of the study, which was extrapolated to estimate weekly intake. The researchers could not tell whether the sugar-sweetened and artificially sweetened drinks were caffeinated and could not rule out residual confounding by other unmeasured or unknown factors.
DISCLOSURES:
This study was supported by the National Natural Science Foundation of China, Shanghai Municipal Health Commission, Shanghai Municipal Human Resources and Social Security Bureau, Clinical Research Plan of Shanghai Hospital Development Center, Postdoctoral Scientific Research Foundation of Shanghai Ninth People’s Hospital, and Shanghai Jiao Tong University School of Medicine.
A version of this article appeared on Medscape.com.
TOPLINE:
(AF) in a new observational study.
METHODOLOGY:
- The population-based cohort study looked at the associations of sugar-sweetened beverages, artificial sweetened beverages, and pure fruit juice consumption with the risk for incident AF and evaluated whether genetic susceptibility modifies these associations.
- The authors analyzed data from the UK Biobank on 201,856 participants who were free of baseline AF, had genetic data available, and completed a 24-hour diet questionnaire. The diagnosis of AF was obtained by linkage from primary care, hospital inpatient, and death register records.
- The results were adjusted for a wide range of potential confounders including age, sex, ethnicity, education level, socioeconomic status, smoking, alcohol consumption, physical activity level, sleep duration, body mass index, blood pressure, kidney function, sleep apnea, coronary heart disease, diabetes, and the use of lipid-lowering or antihypertensive medication.
TAKEAWAY:
- During a median follow-up of 9.9 years, 9362 incident AF cases were documented.
- Compared with nonconsumers, individuals who consumed more than 2 L per week of artificially sweetened beverages had a 20% increased risk of developing AF (hazard ratio [HR], 1.20; 95% CI, 1.10-1.31).
- Those who drank more than 2 L per week of sugar-sweetened beverages had a 10% increased risk for AF (HR, 1.10; 95% CI, 1.01-1.20).
- Consumption of 1 L or less per week of pure fruit juice was associated with an 8% lower risk of developing AF (HR, 0.92; 95% CI, 0.87-0.97).
- The associations persisted after adjustment for genetic susceptibility for AF.
IN PRACTICE:
The study authors concluded that this study does not demonstrate that consumption of sugar-sweetened or artificially sweetened beverages alters AF risk but rather that the consumption of these drinks may predict AF risk beyond traditional risk factors. They added that intervention studies and basic research are warranted to confirm whether the observed associations are causal. Commenting on the study, Duane Mellor, MD, registered dietitian at Aston University, Birmingham, England, said it is unclear if the observations in this study are a chance finding as there is a lack of a clear biological link. Naveed Sattar, MD, professor of metabolic medicine at the University of Glasgow, Glasgow, Scotland, added that although the authors tried to adjust for many factors, there is a strong chance that other behavioral aspects linked to beverage choice could be more relevant as a cause of AF rather than the drinks themselves. Tom Sanders, MD, professor emeritus of nutrition and dietetics, King’s College London, London, England, pointed out that as this is the first study that has reported such an effect with artificially sweetened drinks, the finding needs replication before any conclusions can be drawn. “It remains good dietary advice to recommend the consumption of low-calorie artificially sweetened drink in place of sugar-sweetened drinks and alcohol,” he added.
SOURCE:
The study, led by Ying Sun, MD, Shanghai Jiao Tong University School of Medicine, Shanghai, China, was published online in Circulation: Arrhythmia and Electrophysiology.
LIMITATIONS:
The consumption of beverages was self-reported and based on only five separate single-day food intake recalls which were taken over the first 3 years of the study, which was extrapolated to estimate weekly intake. The researchers could not tell whether the sugar-sweetened and artificially sweetened drinks were caffeinated and could not rule out residual confounding by other unmeasured or unknown factors.
DISCLOSURES:
This study was supported by the National Natural Science Foundation of China, Shanghai Municipal Health Commission, Shanghai Municipal Human Resources and Social Security Bureau, Clinical Research Plan of Shanghai Hospital Development Center, Postdoctoral Scientific Research Foundation of Shanghai Ninth People’s Hospital, and Shanghai Jiao Tong University School of Medicine.
A version of this article appeared on Medscape.com.
Another Neurotoxin for Frown Lines Enters the Market
The Food and Drug Administration (FDA) has approved letibotulinumtoxinA-wlbg, an injectable neurotoxin long used in South Korea for the treatment of moderate to severe glabellar (frown) lines in adults. Developed by Hugel, the product is being marketed under the brand name Letybo.
The FDA’s approval was based on positive results from three phase 3 trials of letibotulinumtoxinA-wlbg that enrolled more than 1000 individuals in the United States and Europe. According to information in the package insert, the most common adverse reaction reported in the trials was headache, which occurred in 2% of trial participants. Other adverse events reported by fewer than 1% of trial participants included brow ptosis, eyelid ptosis, and blepharospasm, while the most frequently reported injection site reactions included administrative site swelling, facial pain, folliculitis, and periorbital hematoma.
According to a press release from the company, letibotulinumtoxinA-wlbg has been the leading neurotoxin brand in South Korea for 7 consecutive years, and the product has been sold in more than 50 different countries. Hugel plans to launch Letybo for US-based aesthetic clinicians in the latter half of 2024.
A version of this article appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved letibotulinumtoxinA-wlbg, an injectable neurotoxin long used in South Korea for the treatment of moderate to severe glabellar (frown) lines in adults. Developed by Hugel, the product is being marketed under the brand name Letybo.
The FDA’s approval was based on positive results from three phase 3 trials of letibotulinumtoxinA-wlbg that enrolled more than 1000 individuals in the United States and Europe. According to information in the package insert, the most common adverse reaction reported in the trials was headache, which occurred in 2% of trial participants. Other adverse events reported by fewer than 1% of trial participants included brow ptosis, eyelid ptosis, and blepharospasm, while the most frequently reported injection site reactions included administrative site swelling, facial pain, folliculitis, and periorbital hematoma.
According to a press release from the company, letibotulinumtoxinA-wlbg has been the leading neurotoxin brand in South Korea for 7 consecutive years, and the product has been sold in more than 50 different countries. Hugel plans to launch Letybo for US-based aesthetic clinicians in the latter half of 2024.
A version of this article appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved letibotulinumtoxinA-wlbg, an injectable neurotoxin long used in South Korea for the treatment of moderate to severe glabellar (frown) lines in adults. Developed by Hugel, the product is being marketed under the brand name Letybo.
The FDA’s approval was based on positive results from three phase 3 trials of letibotulinumtoxinA-wlbg that enrolled more than 1000 individuals in the United States and Europe. According to information in the package insert, the most common adverse reaction reported in the trials was headache, which occurred in 2% of trial participants. Other adverse events reported by fewer than 1% of trial participants included brow ptosis, eyelid ptosis, and blepharospasm, while the most frequently reported injection site reactions included administrative site swelling, facial pain, folliculitis, and periorbital hematoma.
According to a press release from the company, letibotulinumtoxinA-wlbg has been the leading neurotoxin brand in South Korea for 7 consecutive years, and the product has been sold in more than 50 different countries. Hugel plans to launch Letybo for US-based aesthetic clinicians in the latter half of 2024.
A version of this article appeared on Medscape.com.
Effect of Metformin Across Renal Function States in Diabetes
TOPLINE:
Metformin cuts the risk for diabetic nephropathy (DN) and major kidney and cardiovascular events in patients with newly diagnosed type 2 diabetes (T2D) across various renal function states.
METHODOLOGY:
Metformin is a first-line treatment in US and South Korean T2D management guidelines, except for patients with advanced chronic kidney disease (CKD) (stage, ≥ 4; estimated glomerular filtration rate [eGFR], < 30).
The study used data from the databases of three tertiary hospitals in South Korea to assess the effect of metformin on long-term renal and cardiovascular outcomes across various renal function states in patients with newly diagnosed T2D.
Four groups of treatment-control comparative cohorts were identified at each hospital: Patients who had not yet developed DN at T2D diagnosis (mean age in treatment and control cohorts, 61-65 years) and those with reduced renal function (CKD stages 3A, 3B, and 4).
Patients who continuously received metformin after T2D diagnosis and beyond the observation period were 1:1 propensity score matched with controls who were prescribed oral hypoglycemic agents other than metformin.
Primary outcomes were net major adverse cardiovascular events including strokes (MACEs) or in-hospital death and a composite of major adverse kidney events (MAKEs) or in-hospital death.
TAKEAWAY:
Among patients without DN at T2D diagnosis, the continuous use of metformin vs other oral hypoglycemic agents was associated with a lower risk for:
Overt DN (incidence rate ratio [IRR], 0.82; 95% CI, 0.71-0.95),
MACEs (IRR, 0.76; 95% CI, 0.64-0.92), and
MAKEs (IRR, 0.45; 95% CI, 0.33-0.62).
Compared with non-metformin or discontinued metformin use, the continuous use of metformin was associated with a lower risk for MACE across CKD stages 3A (IRR, 0.70; 95% CI, 0.57-0.87), 3B (IRR, 0.83; 95% CI, 0.74-0.93), and 4 (IRR, 0.71; 95% CI, 0.60-0.85).
Similarly, the risk for MAKE was lower among continuous metformin users than in nonusers or discontinuous metformin users across CKD stage 3A (IRR, 0.39; 95% CI, 0.35-0.43), 3B (IRR, 0.44; 95% CI, 0.40-0.48), and 4 (IRR, 0.45; 95% CI, 0.39-0.51).
IN PRACTICE:
“The significance of the current study is highlighted by its integration of real-world clinical data, which encompasses patients diagnosed with CDK4 [eGRF, 15-29 mL/min/1.73 m2], a group currently considered contraindicated,” the authors wrote.
SOURCE:
The study, led by Yongjin Yi, MD, PhD, Department of Internal Medicine, Dankook University College of Medicine, Cheonan-si, Republic of Korea, was published in Scientific Reports.
LIMITATIONS:
There may be a possibility of selection bias because of the retrospective and observational nature of this study. Despite achieving a 1:1 propensity score matching to address the confounding factors, some variables, such as serum albumin and A1c levels, remained unbalanced after matching. The paper did not include observation length or patient numbers, but in response to an email query from Medscape, Yi notes that in one hospital, the mean duration of observation for the control and treatment groups was about 6.5 years, and the total number in the treatment groups across data from three hospitals was 11,675, with the same number of matched controls.
DISCLOSURES:
This study was supported by a Young Investigator Research Grant from the Korean Society of Nephrology, a grant from the Seoul National University Bundang Hospital Research Fund, and the Bio&Medical Technology Development Program of the National Research Foundation funded by the Korean government. The authors disclosed no competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
Metformin cuts the risk for diabetic nephropathy (DN) and major kidney and cardiovascular events in patients with newly diagnosed type 2 diabetes (T2D) across various renal function states.
METHODOLOGY:
Metformin is a first-line treatment in US and South Korean T2D management guidelines, except for patients with advanced chronic kidney disease (CKD) (stage, ≥ 4; estimated glomerular filtration rate [eGFR], < 30).
The study used data from the databases of three tertiary hospitals in South Korea to assess the effect of metformin on long-term renal and cardiovascular outcomes across various renal function states in patients with newly diagnosed T2D.
Four groups of treatment-control comparative cohorts were identified at each hospital: Patients who had not yet developed DN at T2D diagnosis (mean age in treatment and control cohorts, 61-65 years) and those with reduced renal function (CKD stages 3A, 3B, and 4).
Patients who continuously received metformin after T2D diagnosis and beyond the observation period were 1:1 propensity score matched with controls who were prescribed oral hypoglycemic agents other than metformin.
Primary outcomes were net major adverse cardiovascular events including strokes (MACEs) or in-hospital death and a composite of major adverse kidney events (MAKEs) or in-hospital death.
TAKEAWAY:
Among patients without DN at T2D diagnosis, the continuous use of metformin vs other oral hypoglycemic agents was associated with a lower risk for:
Overt DN (incidence rate ratio [IRR], 0.82; 95% CI, 0.71-0.95),
MACEs (IRR, 0.76; 95% CI, 0.64-0.92), and
MAKEs (IRR, 0.45; 95% CI, 0.33-0.62).
Compared with non-metformin or discontinued metformin use, the continuous use of metformin was associated with a lower risk for MACE across CKD stages 3A (IRR, 0.70; 95% CI, 0.57-0.87), 3B (IRR, 0.83; 95% CI, 0.74-0.93), and 4 (IRR, 0.71; 95% CI, 0.60-0.85).
Similarly, the risk for MAKE was lower among continuous metformin users than in nonusers or discontinuous metformin users across CKD stage 3A (IRR, 0.39; 95% CI, 0.35-0.43), 3B (IRR, 0.44; 95% CI, 0.40-0.48), and 4 (IRR, 0.45; 95% CI, 0.39-0.51).
IN PRACTICE:
“The significance of the current study is highlighted by its integration of real-world clinical data, which encompasses patients diagnosed with CDK4 [eGRF, 15-29 mL/min/1.73 m2], a group currently considered contraindicated,” the authors wrote.
SOURCE:
The study, led by Yongjin Yi, MD, PhD, Department of Internal Medicine, Dankook University College of Medicine, Cheonan-si, Republic of Korea, was published in Scientific Reports.
LIMITATIONS:
There may be a possibility of selection bias because of the retrospective and observational nature of this study. Despite achieving a 1:1 propensity score matching to address the confounding factors, some variables, such as serum albumin and A1c levels, remained unbalanced after matching. The paper did not include observation length or patient numbers, but in response to an email query from Medscape, Yi notes that in one hospital, the mean duration of observation for the control and treatment groups was about 6.5 years, and the total number in the treatment groups across data from three hospitals was 11,675, with the same number of matched controls.
DISCLOSURES:
This study was supported by a Young Investigator Research Grant from the Korean Society of Nephrology, a grant from the Seoul National University Bundang Hospital Research Fund, and the Bio&Medical Technology Development Program of the National Research Foundation funded by the Korean government. The authors disclosed no competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
Metformin cuts the risk for diabetic nephropathy (DN) and major kidney and cardiovascular events in patients with newly diagnosed type 2 diabetes (T2D) across various renal function states.
METHODOLOGY:
Metformin is a first-line treatment in US and South Korean T2D management guidelines, except for patients with advanced chronic kidney disease (CKD) (stage, ≥ 4; estimated glomerular filtration rate [eGFR], < 30).
The study used data from the databases of three tertiary hospitals in South Korea to assess the effect of metformin on long-term renal and cardiovascular outcomes across various renal function states in patients with newly diagnosed T2D.
Four groups of treatment-control comparative cohorts were identified at each hospital: Patients who had not yet developed DN at T2D diagnosis (mean age in treatment and control cohorts, 61-65 years) and those with reduced renal function (CKD stages 3A, 3B, and 4).
Patients who continuously received metformin after T2D diagnosis and beyond the observation period were 1:1 propensity score matched with controls who were prescribed oral hypoglycemic agents other than metformin.
Primary outcomes were net major adverse cardiovascular events including strokes (MACEs) or in-hospital death and a composite of major adverse kidney events (MAKEs) or in-hospital death.
TAKEAWAY:
Among patients without DN at T2D diagnosis, the continuous use of metformin vs other oral hypoglycemic agents was associated with a lower risk for:
Overt DN (incidence rate ratio [IRR], 0.82; 95% CI, 0.71-0.95),
MACEs (IRR, 0.76; 95% CI, 0.64-0.92), and
MAKEs (IRR, 0.45; 95% CI, 0.33-0.62).
Compared with non-metformin or discontinued metformin use, the continuous use of metformin was associated with a lower risk for MACE across CKD stages 3A (IRR, 0.70; 95% CI, 0.57-0.87), 3B (IRR, 0.83; 95% CI, 0.74-0.93), and 4 (IRR, 0.71; 95% CI, 0.60-0.85).
Similarly, the risk for MAKE was lower among continuous metformin users than in nonusers or discontinuous metformin users across CKD stage 3A (IRR, 0.39; 95% CI, 0.35-0.43), 3B (IRR, 0.44; 95% CI, 0.40-0.48), and 4 (IRR, 0.45; 95% CI, 0.39-0.51).
IN PRACTICE:
“The significance of the current study is highlighted by its integration of real-world clinical data, which encompasses patients diagnosed with CDK4 [eGRF, 15-29 mL/min/1.73 m2], a group currently considered contraindicated,” the authors wrote.
SOURCE:
The study, led by Yongjin Yi, MD, PhD, Department of Internal Medicine, Dankook University College of Medicine, Cheonan-si, Republic of Korea, was published in Scientific Reports.
LIMITATIONS:
There may be a possibility of selection bias because of the retrospective and observational nature of this study. Despite achieving a 1:1 propensity score matching to address the confounding factors, some variables, such as serum albumin and A1c levels, remained unbalanced after matching. The paper did not include observation length or patient numbers, but in response to an email query from Medscape, Yi notes that in one hospital, the mean duration of observation for the control and treatment groups was about 6.5 years, and the total number in the treatment groups across data from three hospitals was 11,675, with the same number of matched controls.
DISCLOSURES:
This study was supported by a Young Investigator Research Grant from the Korean Society of Nephrology, a grant from the Seoul National University Bundang Hospital Research Fund, and the Bio&Medical Technology Development Program of the National Research Foundation funded by the Korean government. The authors disclosed no competing interests.
A version of this article appeared on Medscape.com.
Increased Risk of New Rheumatic Disease Follows COVID-19 Infection
The risk of developing a new autoimmune inflammatory rheumatic disease (AIRD) is greater following a COVID-19 infection than after an influenza infection or in the general population, according to a study published March 5 in Annals of Internal Medicine. More severe COVID-19 infections were linked to a greater risk of incident rheumatic disease, but vaccination appeared protective against development of a new AIRD.
“Importantly, this study shows the value of vaccination to prevent severe disease and these types of sequelae,” Anne Davidson, MBBS, a professor in the Institute of Molecular Medicine at The Feinstein Institutes for Medical Research in Manhasset, New York, who was not involved in the study, said in an interview.
Previous research had already identified the likelihood of an association between SARS-CoV-2 infection and subsequent development of a new AIRD. This new study, however, includes much larger cohorts from two different countries and relies on more robust methodology than previous studies, experts said.
“Unique steps were taken by the study authors to make sure that what they were looking at in terms of signal was most likely true,” Alfred Kim, MD, PhD, assistant professor of medicine in rheumatology at Washington University in St. Louis, who was not involved in the study, said in an interview. Dr. Davidson agreed, noting that these authors “were a bit more rigorous with ascertainment of the autoimmune diagnosis, using two codes and also checking that appropriate medications were administered.”
More Robust and Rigorous Research
Past cohort studies finding an increased risk of rheumatic disease after COVID-19 “based their findings solely on comparisons between infected and uninfected groups, which could be influenced by ascertainment bias due to disparities in care, differences in health-seeking tendencies, and inherent risks among the groups,” Min Seo Kim, MD, of the Broad Institute of MIT and Harvard, Cambridge, Massachusetts, and his colleagues reported. Their study, however, required at least two claims with codes for rheumatic disease and compared patients with COVID-19 to those with flu “to adjust for the potentially heightened detection of AIRD in SARS-CoV-2–infected persons owing to their interactions with the health care system.”
Dr. Alfred Kim said the fact that they used at least two claims codes “gives a little more credence that the patients were actually experiencing some sort of autoimmune inflammatory condition as opposed to a very transient issue post COVID that just went away on its own.”
He acknowledged that the previous research was reasonably strong, “especially in light of the fact that there has been so much work done on a molecular level demonstrating that COVID-19 is associated with a substantial increase in autoantibodies in a significant proportion of patients, so this always opened up the possibility that this could associate with some sort of autoimmune disease downstream.”
While the study is well done with a large population, “it still has limitations that might overestimate the effect,” Kevin W. Byram, MD, associate professor of medicine in rheumatology and immunology at Vanderbilt University Medical Center in Nashville, Tennessee, who was not involved in the study, said in an interview. “We certainly have seen individual cases of new rheumatic disease where COVID-19 infection is likely the trigger,” but the phenomenon is not new, he added.
“Many autoimmune diseases are spurred by a loss of tolerance that might be induced by a pathogen of some sort,” Dr. Byram said. “The study is right to point out different forms of bias that might be at play. One in particular that is important to consider in a study like this is the lack of case-level adjudication regarding the diagnosis of rheumatic disease” since the study relied on available ICD-10 codes and medication prescriptions.
The researchers used national claims data to compare risk of incident AIRD in 10,027,506 South Korean and 12,218,680 Japanese adults, aged 20 and older, at 1 month, 6 months, and 12 months after COVID-19 infection, influenza infection, or a matched index date for uninfected control participants. Only patients with at least two claims for AIRD were considered to have a new diagnosis.
Patients who had COVID-19 between January 2020 and December 2021, confirmed by PCR or antigen testing, were matched 1:1 with patients who had test-confirmed influenza during that time and 1:4 with uninfected control participants, whose index date was set to the infection date of their matched COVID-19 patient.
The propensity score matching was based on age, sex, household income, urban versus rural residence, and various clinical characteristics and history: body mass index; blood pressure; fasting blood glucose; glomerular filtration rate; smoking status; alcohol consumption; weekly aerobic physical activity; comorbidity index; hospitalizations and outpatient visits in the previous year; past use of diabetes, hyperlipidemia, or hypertension medication; and history of cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, or respiratory infectious disease.
Patients with a history of AIRD or with coinfection or reinfection of COVID-19 and influenza were excluded, as were patients diagnosed with rheumatic disease within a month of COVID-19 infection.
Risk Varied With Disease Severity and Vaccination Status
Among the Korean patients, 3.9% had a COVID-19 infection and 0.98% had an influenza infection. After matching, the comparison populations included 94,504 patients with COVID-19 versus 94,504 patients with flu, and 177,083 patients with COVID-19 versus 675,750 uninfected controls.
The risk of developing an AIRD at least 1 month after infection in South Korean patients with COVID-19 was 25% higher than in uninfected control participants (adjusted hazard ratio [aHR], 1.25; 95% CI, 1.18–1.31; P < .05) and 30% higher than in influenza patients (aHR, 1.3; 95% CI, 1.02–1.59; P < .05). Specifically, risk in South Korean patients with COVID-19 was significantly increased for connective tissue disease and both treated and untreated AIRD but not for inflammatory arthritis.
Among the Japanese patients, 8.2% had COVID-19 and 0.99% had flu, resulting in matched populations of 115,003 with COVID-19 versus 110,310 with flu, and 960,849 with COVID-19 versus 1,606,873 uninfected patients. The effect size was larger in Japanese patients, with a 79% increased risk for AIRD in patients with COVID-19, compared with the general population (aHR, 1.79; 95% CI, 1.77–1.82; P < .05) and a 14% increased risk, compared with patients with influenza infection (aHR, 1.14; 95% CI, 1.10–1.17; P < .05). In Japanese patients, risk was increased across all four categories, including a doubled risk for inflammatory arthritis (aHR, 2.02; 95% CI, 1.96–2.07; P < .05), compared with the general population.
The researchers had data only from the South Korean cohort to calculate risk based on vaccination status, SARS-CoV-2 variant (wild type versus Delta), and COVID-19 severity. Researchers determined a COVID-19 infection to be moderate-to-severe based on billing codes for ICU admission or requiring oxygen therapy, extracorporeal membrane oxygenation, renal replacement, or CPR.
Infection with both the original strain and the Delta variant were linked to similar increased risks for AIRD, but moderate to severe COVID-19 infections had greater risk of subsequent AIRD (aHR, 1.42; P < .05) than mild infections (aHR, 1.22; P < .05). Vaccination was linked to a lower risk of AIRD within the COVID-19 patient population: One dose was linked to a 41% reduced risk (HR, 0.59; P < .05) and two doses were linked to a 58% reduced risk (HR, 0.42; P < .05), regardless of the vaccine type, compared with unvaccinated patients with COVID-19. The apparent protective effect of vaccination was true only for patients with mild COVID-19, not those with moderate to severe infection.
“One has to wonder whether or not these people were at much higher risk of developing autoimmune disease that just got exposed because they got COVID, so that a fraction of these would have gotten an autoimmune disease downstream,” Dr. Alfred Kim said. Regardless, one clinical implication of the findings is the reduced risk in vaccinated patients, regardless of the vaccine type, given the fact that “mRNA vaccination in particular has not been associated with any autoantibody development,” he said.
Though the correlations in the study cannot translate to causation, several mechanisms might be at play in a viral infection contributing to autoimmune risk, Dr. Davidson said. Given that viral nucleic acids also recognize self-nucleic acids, “a large load of viral nucleic acid may break tolerance,” or “viral proteins could also mimic self-proteins,” she said. “In addition, tolerance may be broken by a highly inflammatory environment associated with the release of cytokines and other inflammatory mediators.”
The association between new-onset autoimmune disease and severe COVID-19 infection suggests multiple mechanisms may be involved in excess immune stimulation, Dr. Davidson said. But she added that it’s unclear how these findings, involving the original strain and Delta variant of SARS-CoV-2, might relate to currently circulating variants.
The research was funded by the National Research Foundation of Korea, the Korea Health Industry Development Institute, and the Ministry of Food and Drug Safety of the Republic of Korea. The authors reported no relevant financial relationships with industry. Dr. Alfred Kim has sponsored research agreements with AstraZeneca, Bristol-Myers Squibb, and Novartis; receives royalties from a patent with Kypha Inc.; and has done consulting or speaking for Amgen, ANI Pharmaceuticals, Aurinia Pharmaceuticals, Exagen Diagnostics, GlaxoSmithKline, Kypha, Miltenyi Biotech, Pfizer, Rheumatology & Arthritis Learning Network, Synthekine, Techtonic Therapeutics, and UpToDate. Dr. Byram reported consulting for TenSixteen Bio. Dr. Davidson had no disclosures.
The risk of developing a new autoimmune inflammatory rheumatic disease (AIRD) is greater following a COVID-19 infection than after an influenza infection or in the general population, according to a study published March 5 in Annals of Internal Medicine. More severe COVID-19 infections were linked to a greater risk of incident rheumatic disease, but vaccination appeared protective against development of a new AIRD.
“Importantly, this study shows the value of vaccination to prevent severe disease and these types of sequelae,” Anne Davidson, MBBS, a professor in the Institute of Molecular Medicine at The Feinstein Institutes for Medical Research in Manhasset, New York, who was not involved in the study, said in an interview.
Previous research had already identified the likelihood of an association between SARS-CoV-2 infection and subsequent development of a new AIRD. This new study, however, includes much larger cohorts from two different countries and relies on more robust methodology than previous studies, experts said.
“Unique steps were taken by the study authors to make sure that what they were looking at in terms of signal was most likely true,” Alfred Kim, MD, PhD, assistant professor of medicine in rheumatology at Washington University in St. Louis, who was not involved in the study, said in an interview. Dr. Davidson agreed, noting that these authors “were a bit more rigorous with ascertainment of the autoimmune diagnosis, using two codes and also checking that appropriate medications were administered.”
More Robust and Rigorous Research
Past cohort studies finding an increased risk of rheumatic disease after COVID-19 “based their findings solely on comparisons between infected and uninfected groups, which could be influenced by ascertainment bias due to disparities in care, differences in health-seeking tendencies, and inherent risks among the groups,” Min Seo Kim, MD, of the Broad Institute of MIT and Harvard, Cambridge, Massachusetts, and his colleagues reported. Their study, however, required at least two claims with codes for rheumatic disease and compared patients with COVID-19 to those with flu “to adjust for the potentially heightened detection of AIRD in SARS-CoV-2–infected persons owing to their interactions with the health care system.”
Dr. Alfred Kim said the fact that they used at least two claims codes “gives a little more credence that the patients were actually experiencing some sort of autoimmune inflammatory condition as opposed to a very transient issue post COVID that just went away on its own.”
He acknowledged that the previous research was reasonably strong, “especially in light of the fact that there has been so much work done on a molecular level demonstrating that COVID-19 is associated with a substantial increase in autoantibodies in a significant proportion of patients, so this always opened up the possibility that this could associate with some sort of autoimmune disease downstream.”
While the study is well done with a large population, “it still has limitations that might overestimate the effect,” Kevin W. Byram, MD, associate professor of medicine in rheumatology and immunology at Vanderbilt University Medical Center in Nashville, Tennessee, who was not involved in the study, said in an interview. “We certainly have seen individual cases of new rheumatic disease where COVID-19 infection is likely the trigger,” but the phenomenon is not new, he added.
“Many autoimmune diseases are spurred by a loss of tolerance that might be induced by a pathogen of some sort,” Dr. Byram said. “The study is right to point out different forms of bias that might be at play. One in particular that is important to consider in a study like this is the lack of case-level adjudication regarding the diagnosis of rheumatic disease” since the study relied on available ICD-10 codes and medication prescriptions.
The researchers used national claims data to compare risk of incident AIRD in 10,027,506 South Korean and 12,218,680 Japanese adults, aged 20 and older, at 1 month, 6 months, and 12 months after COVID-19 infection, influenza infection, or a matched index date for uninfected control participants. Only patients with at least two claims for AIRD were considered to have a new diagnosis.
Patients who had COVID-19 between January 2020 and December 2021, confirmed by PCR or antigen testing, were matched 1:1 with patients who had test-confirmed influenza during that time and 1:4 with uninfected control participants, whose index date was set to the infection date of their matched COVID-19 patient.
The propensity score matching was based on age, sex, household income, urban versus rural residence, and various clinical characteristics and history: body mass index; blood pressure; fasting blood glucose; glomerular filtration rate; smoking status; alcohol consumption; weekly aerobic physical activity; comorbidity index; hospitalizations and outpatient visits in the previous year; past use of diabetes, hyperlipidemia, or hypertension medication; and history of cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, or respiratory infectious disease.
Patients with a history of AIRD or with coinfection or reinfection of COVID-19 and influenza were excluded, as were patients diagnosed with rheumatic disease within a month of COVID-19 infection.
Risk Varied With Disease Severity and Vaccination Status
Among the Korean patients, 3.9% had a COVID-19 infection and 0.98% had an influenza infection. After matching, the comparison populations included 94,504 patients with COVID-19 versus 94,504 patients with flu, and 177,083 patients with COVID-19 versus 675,750 uninfected controls.
The risk of developing an AIRD at least 1 month after infection in South Korean patients with COVID-19 was 25% higher than in uninfected control participants (adjusted hazard ratio [aHR], 1.25; 95% CI, 1.18–1.31; P < .05) and 30% higher than in influenza patients (aHR, 1.3; 95% CI, 1.02–1.59; P < .05). Specifically, risk in South Korean patients with COVID-19 was significantly increased for connective tissue disease and both treated and untreated AIRD but not for inflammatory arthritis.
Among the Japanese patients, 8.2% had COVID-19 and 0.99% had flu, resulting in matched populations of 115,003 with COVID-19 versus 110,310 with flu, and 960,849 with COVID-19 versus 1,606,873 uninfected patients. The effect size was larger in Japanese patients, with a 79% increased risk for AIRD in patients with COVID-19, compared with the general population (aHR, 1.79; 95% CI, 1.77–1.82; P < .05) and a 14% increased risk, compared with patients with influenza infection (aHR, 1.14; 95% CI, 1.10–1.17; P < .05). In Japanese patients, risk was increased across all four categories, including a doubled risk for inflammatory arthritis (aHR, 2.02; 95% CI, 1.96–2.07; P < .05), compared with the general population.
The researchers had data only from the South Korean cohort to calculate risk based on vaccination status, SARS-CoV-2 variant (wild type versus Delta), and COVID-19 severity. Researchers determined a COVID-19 infection to be moderate-to-severe based on billing codes for ICU admission or requiring oxygen therapy, extracorporeal membrane oxygenation, renal replacement, or CPR.
Infection with both the original strain and the Delta variant were linked to similar increased risks for AIRD, but moderate to severe COVID-19 infections had greater risk of subsequent AIRD (aHR, 1.42; P < .05) than mild infections (aHR, 1.22; P < .05). Vaccination was linked to a lower risk of AIRD within the COVID-19 patient population: One dose was linked to a 41% reduced risk (HR, 0.59; P < .05) and two doses were linked to a 58% reduced risk (HR, 0.42; P < .05), regardless of the vaccine type, compared with unvaccinated patients with COVID-19. The apparent protective effect of vaccination was true only for patients with mild COVID-19, not those with moderate to severe infection.
“One has to wonder whether or not these people were at much higher risk of developing autoimmune disease that just got exposed because they got COVID, so that a fraction of these would have gotten an autoimmune disease downstream,” Dr. Alfred Kim said. Regardless, one clinical implication of the findings is the reduced risk in vaccinated patients, regardless of the vaccine type, given the fact that “mRNA vaccination in particular has not been associated with any autoantibody development,” he said.
Though the correlations in the study cannot translate to causation, several mechanisms might be at play in a viral infection contributing to autoimmune risk, Dr. Davidson said. Given that viral nucleic acids also recognize self-nucleic acids, “a large load of viral nucleic acid may break tolerance,” or “viral proteins could also mimic self-proteins,” she said. “In addition, tolerance may be broken by a highly inflammatory environment associated with the release of cytokines and other inflammatory mediators.”
The association between new-onset autoimmune disease and severe COVID-19 infection suggests multiple mechanisms may be involved in excess immune stimulation, Dr. Davidson said. But she added that it’s unclear how these findings, involving the original strain and Delta variant of SARS-CoV-2, might relate to currently circulating variants.
The research was funded by the National Research Foundation of Korea, the Korea Health Industry Development Institute, and the Ministry of Food and Drug Safety of the Republic of Korea. The authors reported no relevant financial relationships with industry. Dr. Alfred Kim has sponsored research agreements with AstraZeneca, Bristol-Myers Squibb, and Novartis; receives royalties from a patent with Kypha Inc.; and has done consulting or speaking for Amgen, ANI Pharmaceuticals, Aurinia Pharmaceuticals, Exagen Diagnostics, GlaxoSmithKline, Kypha, Miltenyi Biotech, Pfizer, Rheumatology & Arthritis Learning Network, Synthekine, Techtonic Therapeutics, and UpToDate. Dr. Byram reported consulting for TenSixteen Bio. Dr. Davidson had no disclosures.
The risk of developing a new autoimmune inflammatory rheumatic disease (AIRD) is greater following a COVID-19 infection than after an influenza infection or in the general population, according to a study published March 5 in Annals of Internal Medicine. More severe COVID-19 infections were linked to a greater risk of incident rheumatic disease, but vaccination appeared protective against development of a new AIRD.
“Importantly, this study shows the value of vaccination to prevent severe disease and these types of sequelae,” Anne Davidson, MBBS, a professor in the Institute of Molecular Medicine at The Feinstein Institutes for Medical Research in Manhasset, New York, who was not involved in the study, said in an interview.
Previous research had already identified the likelihood of an association between SARS-CoV-2 infection and subsequent development of a new AIRD. This new study, however, includes much larger cohorts from two different countries and relies on more robust methodology than previous studies, experts said.
“Unique steps were taken by the study authors to make sure that what they were looking at in terms of signal was most likely true,” Alfred Kim, MD, PhD, assistant professor of medicine in rheumatology at Washington University in St. Louis, who was not involved in the study, said in an interview. Dr. Davidson agreed, noting that these authors “were a bit more rigorous with ascertainment of the autoimmune diagnosis, using two codes and also checking that appropriate medications were administered.”
More Robust and Rigorous Research
Past cohort studies finding an increased risk of rheumatic disease after COVID-19 “based their findings solely on comparisons between infected and uninfected groups, which could be influenced by ascertainment bias due to disparities in care, differences in health-seeking tendencies, and inherent risks among the groups,” Min Seo Kim, MD, of the Broad Institute of MIT and Harvard, Cambridge, Massachusetts, and his colleagues reported. Their study, however, required at least two claims with codes for rheumatic disease and compared patients with COVID-19 to those with flu “to adjust for the potentially heightened detection of AIRD in SARS-CoV-2–infected persons owing to their interactions with the health care system.”
Dr. Alfred Kim said the fact that they used at least two claims codes “gives a little more credence that the patients were actually experiencing some sort of autoimmune inflammatory condition as opposed to a very transient issue post COVID that just went away on its own.”
He acknowledged that the previous research was reasonably strong, “especially in light of the fact that there has been so much work done on a molecular level demonstrating that COVID-19 is associated with a substantial increase in autoantibodies in a significant proportion of patients, so this always opened up the possibility that this could associate with some sort of autoimmune disease downstream.”
While the study is well done with a large population, “it still has limitations that might overestimate the effect,” Kevin W. Byram, MD, associate professor of medicine in rheumatology and immunology at Vanderbilt University Medical Center in Nashville, Tennessee, who was not involved in the study, said in an interview. “We certainly have seen individual cases of new rheumatic disease where COVID-19 infection is likely the trigger,” but the phenomenon is not new, he added.
“Many autoimmune diseases are spurred by a loss of tolerance that might be induced by a pathogen of some sort,” Dr. Byram said. “The study is right to point out different forms of bias that might be at play. One in particular that is important to consider in a study like this is the lack of case-level adjudication regarding the diagnosis of rheumatic disease” since the study relied on available ICD-10 codes and medication prescriptions.
The researchers used national claims data to compare risk of incident AIRD in 10,027,506 South Korean and 12,218,680 Japanese adults, aged 20 and older, at 1 month, 6 months, and 12 months after COVID-19 infection, influenza infection, or a matched index date for uninfected control participants. Only patients with at least two claims for AIRD were considered to have a new diagnosis.
Patients who had COVID-19 between January 2020 and December 2021, confirmed by PCR or antigen testing, were matched 1:1 with patients who had test-confirmed influenza during that time and 1:4 with uninfected control participants, whose index date was set to the infection date of their matched COVID-19 patient.
The propensity score matching was based on age, sex, household income, urban versus rural residence, and various clinical characteristics and history: body mass index; blood pressure; fasting blood glucose; glomerular filtration rate; smoking status; alcohol consumption; weekly aerobic physical activity; comorbidity index; hospitalizations and outpatient visits in the previous year; past use of diabetes, hyperlipidemia, or hypertension medication; and history of cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, or respiratory infectious disease.
Patients with a history of AIRD or with coinfection or reinfection of COVID-19 and influenza were excluded, as were patients diagnosed with rheumatic disease within a month of COVID-19 infection.
Risk Varied With Disease Severity and Vaccination Status
Among the Korean patients, 3.9% had a COVID-19 infection and 0.98% had an influenza infection. After matching, the comparison populations included 94,504 patients with COVID-19 versus 94,504 patients with flu, and 177,083 patients with COVID-19 versus 675,750 uninfected controls.
The risk of developing an AIRD at least 1 month after infection in South Korean patients with COVID-19 was 25% higher than in uninfected control participants (adjusted hazard ratio [aHR], 1.25; 95% CI, 1.18–1.31; P < .05) and 30% higher than in influenza patients (aHR, 1.3; 95% CI, 1.02–1.59; P < .05). Specifically, risk in South Korean patients with COVID-19 was significantly increased for connective tissue disease and both treated and untreated AIRD but not for inflammatory arthritis.
Among the Japanese patients, 8.2% had COVID-19 and 0.99% had flu, resulting in matched populations of 115,003 with COVID-19 versus 110,310 with flu, and 960,849 with COVID-19 versus 1,606,873 uninfected patients. The effect size was larger in Japanese patients, with a 79% increased risk for AIRD in patients with COVID-19, compared with the general population (aHR, 1.79; 95% CI, 1.77–1.82; P < .05) and a 14% increased risk, compared with patients with influenza infection (aHR, 1.14; 95% CI, 1.10–1.17; P < .05). In Japanese patients, risk was increased across all four categories, including a doubled risk for inflammatory arthritis (aHR, 2.02; 95% CI, 1.96–2.07; P < .05), compared with the general population.
The researchers had data only from the South Korean cohort to calculate risk based on vaccination status, SARS-CoV-2 variant (wild type versus Delta), and COVID-19 severity. Researchers determined a COVID-19 infection to be moderate-to-severe based on billing codes for ICU admission or requiring oxygen therapy, extracorporeal membrane oxygenation, renal replacement, or CPR.
Infection with both the original strain and the Delta variant were linked to similar increased risks for AIRD, but moderate to severe COVID-19 infections had greater risk of subsequent AIRD (aHR, 1.42; P < .05) than mild infections (aHR, 1.22; P < .05). Vaccination was linked to a lower risk of AIRD within the COVID-19 patient population: One dose was linked to a 41% reduced risk (HR, 0.59; P < .05) and two doses were linked to a 58% reduced risk (HR, 0.42; P < .05), regardless of the vaccine type, compared with unvaccinated patients with COVID-19. The apparent protective effect of vaccination was true only for patients with mild COVID-19, not those with moderate to severe infection.
“One has to wonder whether or not these people were at much higher risk of developing autoimmune disease that just got exposed because they got COVID, so that a fraction of these would have gotten an autoimmune disease downstream,” Dr. Alfred Kim said. Regardless, one clinical implication of the findings is the reduced risk in vaccinated patients, regardless of the vaccine type, given the fact that “mRNA vaccination in particular has not been associated with any autoantibody development,” he said.
Though the correlations in the study cannot translate to causation, several mechanisms might be at play in a viral infection contributing to autoimmune risk, Dr. Davidson said. Given that viral nucleic acids also recognize self-nucleic acids, “a large load of viral nucleic acid may break tolerance,” or “viral proteins could also mimic self-proteins,” she said. “In addition, tolerance may be broken by a highly inflammatory environment associated with the release of cytokines and other inflammatory mediators.”
The association between new-onset autoimmune disease and severe COVID-19 infection suggests multiple mechanisms may be involved in excess immune stimulation, Dr. Davidson said. But she added that it’s unclear how these findings, involving the original strain and Delta variant of SARS-CoV-2, might relate to currently circulating variants.
The research was funded by the National Research Foundation of Korea, the Korea Health Industry Development Institute, and the Ministry of Food and Drug Safety of the Republic of Korea. The authors reported no relevant financial relationships with industry. Dr. Alfred Kim has sponsored research agreements with AstraZeneca, Bristol-Myers Squibb, and Novartis; receives royalties from a patent with Kypha Inc.; and has done consulting or speaking for Amgen, ANI Pharmaceuticals, Aurinia Pharmaceuticals, Exagen Diagnostics, GlaxoSmithKline, Kypha, Miltenyi Biotech, Pfizer, Rheumatology & Arthritis Learning Network, Synthekine, Techtonic Therapeutics, and UpToDate. Dr. Byram reported consulting for TenSixteen Bio. Dr. Davidson had no disclosures.
FROM ANNALS OF INTERNAL MEDICINE



