User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Lawsuit alleges undisclosed stomach risks from Ozempic, Mounjaro
The two drugs, which are Food and Drug Administration approved to treat type 2 diabetes, have become well known for their weight loss properties. Ozempic is made by Danish drug maker Novo Nordisk, and Mounjaro is made by Indiana-based Eli Lilly and Co.
In the lawsuit, Jaclyn Bjorklund, 44, of Louisiana, asserts that she was “severely injured” after using Ozempic and Mounjaro and that the pharmaceutical companies failed to disclose the drugs’ risk of causing vomiting and diarrhea due to inflammation of the stomach lining, as well as the risk of gastroparesis.
The prescribing labels for Mounjaro and Ozempic state that each “delays gastric emptying” and warn of the risk of severe gastrointestinal adverse reactions. The prescribing labels for both drugs state that the most common side effects include vomiting, diarrhea, and stomach pain. The Ozempic label does not mention gastroparesis, and the Mounjaro label states that the drug has not been studied in people with the condition and is therefore not recommended for people who have it.
Ms. Bjorklund has not been diagnosed with gastroparesis, but her symptoms are “indicative of” the condition, her lawyer, Paul Pennock, told NBC News.
Ms. Bjorklund used Ozempic for more than 1 year, and in July 2023 switched to Mounjaro, the lawsuit states. The document, posted on her law firm’s website, details that using the drugs resulted in “severe vomiting, stomach pain, gastrointestinal burning, being hospitalized for stomach issues on several occasions including visits to the emergency room, [and] teeth falling out due to excessive vomiting, requiring additional medications to alleviate her excessive vomiting, and throwing up whole food hours after eating.”
Novo Nordisk spokesperson Natalia Salomao told NBC News that patient safety is “of utmost importance to Novo Nordisk,” and she also noted that gastroparesis is a known risk for people with diabetes. The Food and Drug Administration declined to comment on the case, and Eli Lilly did not immediately respond to a request for comment, NBC News reported.
A version of this article first appeared on WebMD.com.
The two drugs, which are Food and Drug Administration approved to treat type 2 diabetes, have become well known for their weight loss properties. Ozempic is made by Danish drug maker Novo Nordisk, and Mounjaro is made by Indiana-based Eli Lilly and Co.
In the lawsuit, Jaclyn Bjorklund, 44, of Louisiana, asserts that she was “severely injured” after using Ozempic and Mounjaro and that the pharmaceutical companies failed to disclose the drugs’ risk of causing vomiting and diarrhea due to inflammation of the stomach lining, as well as the risk of gastroparesis.
The prescribing labels for Mounjaro and Ozempic state that each “delays gastric emptying” and warn of the risk of severe gastrointestinal adverse reactions. The prescribing labels for both drugs state that the most common side effects include vomiting, diarrhea, and stomach pain. The Ozempic label does not mention gastroparesis, and the Mounjaro label states that the drug has not been studied in people with the condition and is therefore not recommended for people who have it.
Ms. Bjorklund has not been diagnosed with gastroparesis, but her symptoms are “indicative of” the condition, her lawyer, Paul Pennock, told NBC News.
Ms. Bjorklund used Ozempic for more than 1 year, and in July 2023 switched to Mounjaro, the lawsuit states. The document, posted on her law firm’s website, details that using the drugs resulted in “severe vomiting, stomach pain, gastrointestinal burning, being hospitalized for stomach issues on several occasions including visits to the emergency room, [and] teeth falling out due to excessive vomiting, requiring additional medications to alleviate her excessive vomiting, and throwing up whole food hours after eating.”
Novo Nordisk spokesperson Natalia Salomao told NBC News that patient safety is “of utmost importance to Novo Nordisk,” and she also noted that gastroparesis is a known risk for people with diabetes. The Food and Drug Administration declined to comment on the case, and Eli Lilly did not immediately respond to a request for comment, NBC News reported.
A version of this article first appeared on WebMD.com.
The two drugs, which are Food and Drug Administration approved to treat type 2 diabetes, have become well known for their weight loss properties. Ozempic is made by Danish drug maker Novo Nordisk, and Mounjaro is made by Indiana-based Eli Lilly and Co.
In the lawsuit, Jaclyn Bjorklund, 44, of Louisiana, asserts that she was “severely injured” after using Ozempic and Mounjaro and that the pharmaceutical companies failed to disclose the drugs’ risk of causing vomiting and diarrhea due to inflammation of the stomach lining, as well as the risk of gastroparesis.
The prescribing labels for Mounjaro and Ozempic state that each “delays gastric emptying” and warn of the risk of severe gastrointestinal adverse reactions. The prescribing labels for both drugs state that the most common side effects include vomiting, diarrhea, and stomach pain. The Ozempic label does not mention gastroparesis, and the Mounjaro label states that the drug has not been studied in people with the condition and is therefore not recommended for people who have it.
Ms. Bjorklund has not been diagnosed with gastroparesis, but her symptoms are “indicative of” the condition, her lawyer, Paul Pennock, told NBC News.
Ms. Bjorklund used Ozempic for more than 1 year, and in July 2023 switched to Mounjaro, the lawsuit states. The document, posted on her law firm’s website, details that using the drugs resulted in “severe vomiting, stomach pain, gastrointestinal burning, being hospitalized for stomach issues on several occasions including visits to the emergency room, [and] teeth falling out due to excessive vomiting, requiring additional medications to alleviate her excessive vomiting, and throwing up whole food hours after eating.”
Novo Nordisk spokesperson Natalia Salomao told NBC News that patient safety is “of utmost importance to Novo Nordisk,” and she also noted that gastroparesis is a known risk for people with diabetes. The Food and Drug Administration declined to comment on the case, and Eli Lilly did not immediately respond to a request for comment, NBC News reported.
A version of this article first appeared on WebMD.com.
Higher occurrence of kidney stones with more added sugar
Consuming a higher percentage of calories from added sugars is linked with a higher prevalence of kidney stones, new research suggests.
Though added sugars have been linked with multiple poor health outcomes, their link with kidney stones has been unclear.
Added sugars are sugars or caloric sweeteners added to foods or drinks during processing or preparation to add flavor or shelf life. They do not include natural sugars such as lactose in milk and fructose in fruits.
Researchers, led by Shan Yin, a urologist at Affiliated Hospital of North Sichuan Medical College, in Nanchong, China, compared the added-sugar intake by quartiles in the U.S. National Health and Nutrition Examination Survey 2007-2018.
A total of 28,303 adults were included in this study, with an average age of 48. Women who consumed less than 600 or more than 3,500 kcal or men who consumed less than 800 or more than 4,200 kcal were excluded.
Researchers adjusted for factors including age, race, education, income, physical activity, and marital, employment, and smoking status.
Compared with the first quartile of percentage added-sugar calorie intake, the population in the fourth quartile, with the highest added sugar intake, had a higher prevalence of kidney stones (odds ratio, 1.39; 95% confidence interval, 1.17-1.65).
Compared with the group with fewer than 5% of calories from added sugar, the group that consumed at least 25% of calories from added sugar had nearly twice the prevalence of kidney stones (OR, 1.88; 95% CI, 1.52-2.32).
Findings were published online in Frontiers in Nutrition.
“By identifying this association, policymakers and health professionals can emphasize the need for public health initiatives to reduce added sugar consumption and promote healthy dietary habits,” the authors write.
Added sugar in the U.S. diet
Sugar-sweetened beverages such as soft drinks and energy and sports drinks account for 34.4% of added sugars in the American diet. Previous studies have shown the relationship between consuming sugar-sweetened beverages and a higher risk of obesity, diabetes, and cardiovascular disease, diseases that often co-occur with kidney stones.
Researchers note that even though most added sugars in the United States come from sugar-sweetened beverages, it’s unclear whether the association between added sugars and kidney stones is caused by the beverages or other sources. For instance, fructose intake has been found to be independently associated with kidney stones.
How much is too much?
The recommended upper limit on added sugar is controversial and varies widely by health organization. The American Heart Association says daily average intake from added sugars should be no more than 150 kcal for adult males (about 9 teaspoons) and no more than 100 kcal for women (about 6 teaspoons). The Institute of Medicine allows up to 25% of calories to be consumed from added sugars. The 2020 Dietary Guidelines for Americans and World Health Organization set 10% of calories as the recommended upper limit.
Further investigating what causes kidney stones is critical as kidney stones are common worldwide, affecting about 1 in 10 people in the United States alone, and occurrence is increasing. Kidney stones have a high recurrence rate – about half of people who get them have a second episode within 10 years, the authors note.
The researchers acknowledge that because participants self-reported food intake, there is the potential for recall bias. Additionally, because of the cross-sectional design, the researchers were not able to determine whether sugar intake or kidney stone occurrence came first.
This work was supported by the Doctoral Fund Project of North Sichuan Medical College. The authors declare no relevant financial relationships.
Consuming a higher percentage of calories from added sugars is linked with a higher prevalence of kidney stones, new research suggests.
Though added sugars have been linked with multiple poor health outcomes, their link with kidney stones has been unclear.
Added sugars are sugars or caloric sweeteners added to foods or drinks during processing or preparation to add flavor or shelf life. They do not include natural sugars such as lactose in milk and fructose in fruits.
Researchers, led by Shan Yin, a urologist at Affiliated Hospital of North Sichuan Medical College, in Nanchong, China, compared the added-sugar intake by quartiles in the U.S. National Health and Nutrition Examination Survey 2007-2018.
A total of 28,303 adults were included in this study, with an average age of 48. Women who consumed less than 600 or more than 3,500 kcal or men who consumed less than 800 or more than 4,200 kcal were excluded.
Researchers adjusted for factors including age, race, education, income, physical activity, and marital, employment, and smoking status.
Compared with the first quartile of percentage added-sugar calorie intake, the population in the fourth quartile, with the highest added sugar intake, had a higher prevalence of kidney stones (odds ratio, 1.39; 95% confidence interval, 1.17-1.65).
Compared with the group with fewer than 5% of calories from added sugar, the group that consumed at least 25% of calories from added sugar had nearly twice the prevalence of kidney stones (OR, 1.88; 95% CI, 1.52-2.32).
Findings were published online in Frontiers in Nutrition.
“By identifying this association, policymakers and health professionals can emphasize the need for public health initiatives to reduce added sugar consumption and promote healthy dietary habits,” the authors write.
Added sugar in the U.S. diet
Sugar-sweetened beverages such as soft drinks and energy and sports drinks account for 34.4% of added sugars in the American diet. Previous studies have shown the relationship between consuming sugar-sweetened beverages and a higher risk of obesity, diabetes, and cardiovascular disease, diseases that often co-occur with kidney stones.
Researchers note that even though most added sugars in the United States come from sugar-sweetened beverages, it’s unclear whether the association between added sugars and kidney stones is caused by the beverages or other sources. For instance, fructose intake has been found to be independently associated with kidney stones.
How much is too much?
The recommended upper limit on added sugar is controversial and varies widely by health organization. The American Heart Association says daily average intake from added sugars should be no more than 150 kcal for adult males (about 9 teaspoons) and no more than 100 kcal for women (about 6 teaspoons). The Institute of Medicine allows up to 25% of calories to be consumed from added sugars. The 2020 Dietary Guidelines for Americans and World Health Organization set 10% of calories as the recommended upper limit.
Further investigating what causes kidney stones is critical as kidney stones are common worldwide, affecting about 1 in 10 people in the United States alone, and occurrence is increasing. Kidney stones have a high recurrence rate – about half of people who get them have a second episode within 10 years, the authors note.
The researchers acknowledge that because participants self-reported food intake, there is the potential for recall bias. Additionally, because of the cross-sectional design, the researchers were not able to determine whether sugar intake or kidney stone occurrence came first.
This work was supported by the Doctoral Fund Project of North Sichuan Medical College. The authors declare no relevant financial relationships.
Consuming a higher percentage of calories from added sugars is linked with a higher prevalence of kidney stones, new research suggests.
Though added sugars have been linked with multiple poor health outcomes, their link with kidney stones has been unclear.
Added sugars are sugars or caloric sweeteners added to foods or drinks during processing or preparation to add flavor or shelf life. They do not include natural sugars such as lactose in milk and fructose in fruits.
Researchers, led by Shan Yin, a urologist at Affiliated Hospital of North Sichuan Medical College, in Nanchong, China, compared the added-sugar intake by quartiles in the U.S. National Health and Nutrition Examination Survey 2007-2018.
A total of 28,303 adults were included in this study, with an average age of 48. Women who consumed less than 600 or more than 3,500 kcal or men who consumed less than 800 or more than 4,200 kcal were excluded.
Researchers adjusted for factors including age, race, education, income, physical activity, and marital, employment, and smoking status.
Compared with the first quartile of percentage added-sugar calorie intake, the population in the fourth quartile, with the highest added sugar intake, had a higher prevalence of kidney stones (odds ratio, 1.39; 95% confidence interval, 1.17-1.65).
Compared with the group with fewer than 5% of calories from added sugar, the group that consumed at least 25% of calories from added sugar had nearly twice the prevalence of kidney stones (OR, 1.88; 95% CI, 1.52-2.32).
Findings were published online in Frontiers in Nutrition.
“By identifying this association, policymakers and health professionals can emphasize the need for public health initiatives to reduce added sugar consumption and promote healthy dietary habits,” the authors write.
Added sugar in the U.S. diet
Sugar-sweetened beverages such as soft drinks and energy and sports drinks account for 34.4% of added sugars in the American diet. Previous studies have shown the relationship between consuming sugar-sweetened beverages and a higher risk of obesity, diabetes, and cardiovascular disease, diseases that often co-occur with kidney stones.
Researchers note that even though most added sugars in the United States come from sugar-sweetened beverages, it’s unclear whether the association between added sugars and kidney stones is caused by the beverages or other sources. For instance, fructose intake has been found to be independently associated with kidney stones.
How much is too much?
The recommended upper limit on added sugar is controversial and varies widely by health organization. The American Heart Association says daily average intake from added sugars should be no more than 150 kcal for adult males (about 9 teaspoons) and no more than 100 kcal for women (about 6 teaspoons). The Institute of Medicine allows up to 25% of calories to be consumed from added sugars. The 2020 Dietary Guidelines for Americans and World Health Organization set 10% of calories as the recommended upper limit.
Further investigating what causes kidney stones is critical as kidney stones are common worldwide, affecting about 1 in 10 people in the United States alone, and occurrence is increasing. Kidney stones have a high recurrence rate – about half of people who get them have a second episode within 10 years, the authors note.
The researchers acknowledge that because participants self-reported food intake, there is the potential for recall bias. Additionally, because of the cross-sectional design, the researchers were not able to determine whether sugar intake or kidney stone occurrence came first.
This work was supported by the Doctoral Fund Project of North Sichuan Medical College. The authors declare no relevant financial relationships.
FROM FRONTIERS IN NUTRITION
Using Ozempic for ‘minor’ weight loss: Fair or foul?
Ashley Raibick is familiar with the weight loss yo-yo. She’s bounced through the big names: Weight Watchers, Jenny Craig, and so on. She drops 10 pounds and then slides off the plan only to see her weight pop back up.
But a day at her local med spa – where she gets facials, Botox, and fillers – changed all that for the 28-year-old hairstylist who just wanted to lose 18 pounds.
During one of her visits, she noticed that the spa’s owner was thinner. When Ms. Raibick asked her how she did it, the owner explained that she was on semaglutide and talked Ms. Raibick through the process. Ms. Raibick was convinced. That same day, she got a prescription from a doctor at the spa and got her first shot.
“Are people going to think I’m crazy for doing this?” she recalls thinking.
At 5 foot 4, her starting weight before the drug was 158, which would put her in the overweight, but not obese, category based on body mass index (BMI). And she really just wanted to get down to 140 and stop there.
Ozempic is part of an ever-growing group of GLP-1 receptor agonists that contain a peptide called semaglutide as its main ingredient. Although first meant to treat type 2 diabetes, the reputation of Ozempic and its siblings picked up when already-thin celebrities were suspected of using the injectable drugs to become even slimmer.
The FDA approved Ozempic’s cousin, Wegovy, for “weight management” in patients with obesity a few years ago, whereas Ozempic is currently approved only for diabetes treatment. Curious patients who don’t fit the criteria can – and do – get off-label prescriptions if they can afford to pay out of pocket, often to the tune of more than $1,400 a month.
For many – mainly those who have been on the drug for a couple of months and have lost weight as a result – taking Ozempic has not only helped them shed stubborn weight, but has also freed them from the constant internal chatter around eating, commonly called “food noise.” But experts do not all agree that semaglutide is the right path for those who aren’t technically obese – especially in the long term.
After her first 9 weeks on semaglutide, Ms. Raibick had already lost 18 pounds. That’s when she decided to post about it on TikTok, and her videos on GLP-1s were viewed hundreds of thousands of times.
For the time being, there is no data on how many semaglutide takers are using the drug for diabetes and/or obesity, and how many are using it off-label for weight loss alone. But the company that makes Ozempic, Novo Nordisk, has reported sharp increases in sales and projects more profits down the road.
Ms. Raibick knows of others like her, who sought out the drug for more minor weight loss but aren’t as candid about their journeys. Some feel a stigma about having to resort to a weight-loss drug intended to treat obesity, rather than achieving their goals with diet and lifestyle change alone.
Another reason for the secrecy is the guilt some who take Ozempic feel about using their financial privilege to get a drug that had serious shortages, which made it harder for some patients who need the drug for diabetes or obesity treatment to get their doses.
That’s what Diana Thiara, MD, the medical director of the University of California, San Francisco’s weight management program, has been seeing on the ground.
“It’s one of the most depressing things I’ve experienced as a physician,” she said. In her practice, she has seen patients who have finally been able to access GLP-1s and have started to lose weight, only for them to regain the weight in the time it takes to find another prescription under their insurance coverage.
“It’s just horrible, there are patients spending all day calling dozens of pharmacies. I’ve never had a situation like this in my career,” said Dr. Thiara.
Ann, 48, a mom who works from home full-time, has been taking Ozempic since the end of January. (Ann is not her real name; she asked that we use a pseudonym in order to feel comfortable speaking publicly about her use of Ozempic). Like Ms. Raibick, she has been paying out of pocket for her shots. At first, she was going to have to pay $1,400 a month, but she found a pharmacy in Canada that offers the medication for $350. It’s sourced globally, she said, so sometimes her Ozempic boxes will be in Czech or another foreign language.
Unlike a lot of women, Ann never had any qualms with her weight or the way her body looked. She was never big on exercise, but it wasn’t until the pandemic that she started to gain weight. She noticed the changes in her body once places started opening back up, and her clothes didn’t fit anymore.
She tried moving more and eating healthier. She tried former Real Housewives of Beverly Hills cast member Teddi Mellencamp’s controversial weight-loss program, infamous for its incredibly restrictive dietary plan and excessive cardio recommendations. Nothing worked until another mom at her daughter’s school mentioned that she was on Ozempic.
Ann also started to get hot flashes and missed periods. The doctor who prescribed her Ozempic confirmed that she was perimenopausal and that, for women in this stage of life, losing weight can be harder than ever.
Ann, who is 5 foot 7, started out at 176 pounds (considered overweight) and now weighs in at 151, which is considered a normal weight by BMI measurements. She’s still on Ozempic but continues to struggle with the shame around the idea she’s potentially taking the drug away from someone else who might desperately need it. And she doesn’t know how long she’ll have to stay on Ozempic to maintain her weight loss.
Ann has reason for concern. A 2022 study found that most people regain the weight they lost within a year of stopping Ozempic.
Once Ms. Raibick hit her initial goal weight, she felt that she could keep going and lose a little more. It wasn’t until she got into the 120-pound range that she decided it was time to wean off the dose of semaglutide she had been taking.
“I got to the point where my mom was like, ‘All right, you’re a little too thin.’ But I’m just so happy where I’m at. I’m not mentally stressed out about fitting into clothes or getting into a bathing suit,” said Ms. Raibick, who has now lost around 30 pounds in total since she started the shots.
At one point, she stopped taking the drug altogether, and all of the hunger cravings and food noise semaglutide had suppressed came back to the surface. She didn’t gain any weight that month, she said, but the internal chatter around food was enough to make her start back on a lower dose, geared toward weight maintenance.
There’s also the issue of side effects. Ms. Raibick says she never had the overwhelming nausea and digestive problems that so many on the drug – including Ann – have reported. But Dr. Thiara said that even beyond these more common side effects, there are a number of other concerns – like the long-lasting effects on thyroid and reproductive health, especially for women – that we still don’t know enough about. And just recently, CNN reported that some Ozempic users have developed stomach paralysis due to the drug’s ability to slow down the passage of food through the digestive tract.
For Ms. Raibick, the out-of-pocket cost for the drug is around $600 a month. It’s an expense she’s willing to keep paying for, even just for the peace of mind the drug provides. She doesn’t have any plans to stop her semaglutide shots soon.
“There is nothing stopping me from – a year from now, when I’ve put a little weight back on – looking back at photos from this time and thinking I was way too skinny.”
Dan Azagury, MD, a bariatric surgeon and associate professor of surgery at Stanford (Calif.) University, tries GLP-1s for patients with obesity before considering bariatric surgery. For his patient population, it’s possible that drugs like Ozempic will be part of their lifelong treatment plans.
“We’re not doing it for the cosmetic part of it, we’re doing it for health,” he said. “What I tell my patients is, if you’re planning to start on this medication, you should be OK with the idea of staying on it forever.”
For doctors like Dr. Thiara who specialize in weight management, using Ozempic long-term for patients in a healthy weight range is the wrong approach.
“It’s not about the way people look, it’s about health. If you’re a normal weight or even in an overweight category, but not showing signs of risk of having elevated cardiometabolic disease ... You don’t need to be taking medications for weight loss,” she said. “This idea of using medications for aesthetic reasons is really more related to societal ills around how we value fitness above anything else. That’s not the goal, and it’s not safe.”
A version of this article first appeared on WebMD.com.
Ashley Raibick is familiar with the weight loss yo-yo. She’s bounced through the big names: Weight Watchers, Jenny Craig, and so on. She drops 10 pounds and then slides off the plan only to see her weight pop back up.
But a day at her local med spa – where she gets facials, Botox, and fillers – changed all that for the 28-year-old hairstylist who just wanted to lose 18 pounds.
During one of her visits, she noticed that the spa’s owner was thinner. When Ms. Raibick asked her how she did it, the owner explained that she was on semaglutide and talked Ms. Raibick through the process. Ms. Raibick was convinced. That same day, she got a prescription from a doctor at the spa and got her first shot.
“Are people going to think I’m crazy for doing this?” she recalls thinking.
At 5 foot 4, her starting weight before the drug was 158, which would put her in the overweight, but not obese, category based on body mass index (BMI). And she really just wanted to get down to 140 and stop there.
Ozempic is part of an ever-growing group of GLP-1 receptor agonists that contain a peptide called semaglutide as its main ingredient. Although first meant to treat type 2 diabetes, the reputation of Ozempic and its siblings picked up when already-thin celebrities were suspected of using the injectable drugs to become even slimmer.
The FDA approved Ozempic’s cousin, Wegovy, for “weight management” in patients with obesity a few years ago, whereas Ozempic is currently approved only for diabetes treatment. Curious patients who don’t fit the criteria can – and do – get off-label prescriptions if they can afford to pay out of pocket, often to the tune of more than $1,400 a month.
For many – mainly those who have been on the drug for a couple of months and have lost weight as a result – taking Ozempic has not only helped them shed stubborn weight, but has also freed them from the constant internal chatter around eating, commonly called “food noise.” But experts do not all agree that semaglutide is the right path for those who aren’t technically obese – especially in the long term.
After her first 9 weeks on semaglutide, Ms. Raibick had already lost 18 pounds. That’s when she decided to post about it on TikTok, and her videos on GLP-1s were viewed hundreds of thousands of times.
For the time being, there is no data on how many semaglutide takers are using the drug for diabetes and/or obesity, and how many are using it off-label for weight loss alone. But the company that makes Ozempic, Novo Nordisk, has reported sharp increases in sales and projects more profits down the road.
Ms. Raibick knows of others like her, who sought out the drug for more minor weight loss but aren’t as candid about their journeys. Some feel a stigma about having to resort to a weight-loss drug intended to treat obesity, rather than achieving their goals with diet and lifestyle change alone.
Another reason for the secrecy is the guilt some who take Ozempic feel about using their financial privilege to get a drug that had serious shortages, which made it harder for some patients who need the drug for diabetes or obesity treatment to get their doses.
That’s what Diana Thiara, MD, the medical director of the University of California, San Francisco’s weight management program, has been seeing on the ground.
“It’s one of the most depressing things I’ve experienced as a physician,” she said. In her practice, she has seen patients who have finally been able to access GLP-1s and have started to lose weight, only for them to regain the weight in the time it takes to find another prescription under their insurance coverage.
“It’s just horrible, there are patients spending all day calling dozens of pharmacies. I’ve never had a situation like this in my career,” said Dr. Thiara.
Ann, 48, a mom who works from home full-time, has been taking Ozempic since the end of January. (Ann is not her real name; she asked that we use a pseudonym in order to feel comfortable speaking publicly about her use of Ozempic). Like Ms. Raibick, she has been paying out of pocket for her shots. At first, she was going to have to pay $1,400 a month, but she found a pharmacy in Canada that offers the medication for $350. It’s sourced globally, she said, so sometimes her Ozempic boxes will be in Czech or another foreign language.
Unlike a lot of women, Ann never had any qualms with her weight or the way her body looked. She was never big on exercise, but it wasn’t until the pandemic that she started to gain weight. She noticed the changes in her body once places started opening back up, and her clothes didn’t fit anymore.
She tried moving more and eating healthier. She tried former Real Housewives of Beverly Hills cast member Teddi Mellencamp’s controversial weight-loss program, infamous for its incredibly restrictive dietary plan and excessive cardio recommendations. Nothing worked until another mom at her daughter’s school mentioned that she was on Ozempic.
Ann also started to get hot flashes and missed periods. The doctor who prescribed her Ozempic confirmed that she was perimenopausal and that, for women in this stage of life, losing weight can be harder than ever.
Ann, who is 5 foot 7, started out at 176 pounds (considered overweight) and now weighs in at 151, which is considered a normal weight by BMI measurements. She’s still on Ozempic but continues to struggle with the shame around the idea she’s potentially taking the drug away from someone else who might desperately need it. And she doesn’t know how long she’ll have to stay on Ozempic to maintain her weight loss.
Ann has reason for concern. A 2022 study found that most people regain the weight they lost within a year of stopping Ozempic.
Once Ms. Raibick hit her initial goal weight, she felt that she could keep going and lose a little more. It wasn’t until she got into the 120-pound range that she decided it was time to wean off the dose of semaglutide she had been taking.
“I got to the point where my mom was like, ‘All right, you’re a little too thin.’ But I’m just so happy where I’m at. I’m not mentally stressed out about fitting into clothes or getting into a bathing suit,” said Ms. Raibick, who has now lost around 30 pounds in total since she started the shots.
At one point, she stopped taking the drug altogether, and all of the hunger cravings and food noise semaglutide had suppressed came back to the surface. She didn’t gain any weight that month, she said, but the internal chatter around food was enough to make her start back on a lower dose, geared toward weight maintenance.
There’s also the issue of side effects. Ms. Raibick says she never had the overwhelming nausea and digestive problems that so many on the drug – including Ann – have reported. But Dr. Thiara said that even beyond these more common side effects, there are a number of other concerns – like the long-lasting effects on thyroid and reproductive health, especially for women – that we still don’t know enough about. And just recently, CNN reported that some Ozempic users have developed stomach paralysis due to the drug’s ability to slow down the passage of food through the digestive tract.
For Ms. Raibick, the out-of-pocket cost for the drug is around $600 a month. It’s an expense she’s willing to keep paying for, even just for the peace of mind the drug provides. She doesn’t have any plans to stop her semaglutide shots soon.
“There is nothing stopping me from – a year from now, when I’ve put a little weight back on – looking back at photos from this time and thinking I was way too skinny.”
Dan Azagury, MD, a bariatric surgeon and associate professor of surgery at Stanford (Calif.) University, tries GLP-1s for patients with obesity before considering bariatric surgery. For his patient population, it’s possible that drugs like Ozempic will be part of their lifelong treatment plans.
“We’re not doing it for the cosmetic part of it, we’re doing it for health,” he said. “What I tell my patients is, if you’re planning to start on this medication, you should be OK with the idea of staying on it forever.”
For doctors like Dr. Thiara who specialize in weight management, using Ozempic long-term for patients in a healthy weight range is the wrong approach.
“It’s not about the way people look, it’s about health. If you’re a normal weight or even in an overweight category, but not showing signs of risk of having elevated cardiometabolic disease ... You don’t need to be taking medications for weight loss,” she said. “This idea of using medications for aesthetic reasons is really more related to societal ills around how we value fitness above anything else. That’s not the goal, and it’s not safe.”
A version of this article first appeared on WebMD.com.
Ashley Raibick is familiar with the weight loss yo-yo. She’s bounced through the big names: Weight Watchers, Jenny Craig, and so on. She drops 10 pounds and then slides off the plan only to see her weight pop back up.
But a day at her local med spa – where she gets facials, Botox, and fillers – changed all that for the 28-year-old hairstylist who just wanted to lose 18 pounds.
During one of her visits, she noticed that the spa’s owner was thinner. When Ms. Raibick asked her how she did it, the owner explained that she was on semaglutide and talked Ms. Raibick through the process. Ms. Raibick was convinced. That same day, she got a prescription from a doctor at the spa and got her first shot.
“Are people going to think I’m crazy for doing this?” she recalls thinking.
At 5 foot 4, her starting weight before the drug was 158, which would put her in the overweight, but not obese, category based on body mass index (BMI). And she really just wanted to get down to 140 and stop there.
Ozempic is part of an ever-growing group of GLP-1 receptor agonists that contain a peptide called semaglutide as its main ingredient. Although first meant to treat type 2 diabetes, the reputation of Ozempic and its siblings picked up when already-thin celebrities were suspected of using the injectable drugs to become even slimmer.
The FDA approved Ozempic’s cousin, Wegovy, for “weight management” in patients with obesity a few years ago, whereas Ozempic is currently approved only for diabetes treatment. Curious patients who don’t fit the criteria can – and do – get off-label prescriptions if they can afford to pay out of pocket, often to the tune of more than $1,400 a month.
For many – mainly those who have been on the drug for a couple of months and have lost weight as a result – taking Ozempic has not only helped them shed stubborn weight, but has also freed them from the constant internal chatter around eating, commonly called “food noise.” But experts do not all agree that semaglutide is the right path for those who aren’t technically obese – especially in the long term.
After her first 9 weeks on semaglutide, Ms. Raibick had already lost 18 pounds. That’s when she decided to post about it on TikTok, and her videos on GLP-1s were viewed hundreds of thousands of times.
For the time being, there is no data on how many semaglutide takers are using the drug for diabetes and/or obesity, and how many are using it off-label for weight loss alone. But the company that makes Ozempic, Novo Nordisk, has reported sharp increases in sales and projects more profits down the road.
Ms. Raibick knows of others like her, who sought out the drug for more minor weight loss but aren’t as candid about their journeys. Some feel a stigma about having to resort to a weight-loss drug intended to treat obesity, rather than achieving their goals with diet and lifestyle change alone.
Another reason for the secrecy is the guilt some who take Ozempic feel about using their financial privilege to get a drug that had serious shortages, which made it harder for some patients who need the drug for diabetes or obesity treatment to get their doses.
That’s what Diana Thiara, MD, the medical director of the University of California, San Francisco’s weight management program, has been seeing on the ground.
“It’s one of the most depressing things I’ve experienced as a physician,” she said. In her practice, she has seen patients who have finally been able to access GLP-1s and have started to lose weight, only for them to regain the weight in the time it takes to find another prescription under their insurance coverage.
“It’s just horrible, there are patients spending all day calling dozens of pharmacies. I’ve never had a situation like this in my career,” said Dr. Thiara.
Ann, 48, a mom who works from home full-time, has been taking Ozempic since the end of January. (Ann is not her real name; she asked that we use a pseudonym in order to feel comfortable speaking publicly about her use of Ozempic). Like Ms. Raibick, she has been paying out of pocket for her shots. At first, she was going to have to pay $1,400 a month, but she found a pharmacy in Canada that offers the medication for $350. It’s sourced globally, she said, so sometimes her Ozempic boxes will be in Czech or another foreign language.
Unlike a lot of women, Ann never had any qualms with her weight or the way her body looked. She was never big on exercise, but it wasn’t until the pandemic that she started to gain weight. She noticed the changes in her body once places started opening back up, and her clothes didn’t fit anymore.
She tried moving more and eating healthier. She tried former Real Housewives of Beverly Hills cast member Teddi Mellencamp’s controversial weight-loss program, infamous for its incredibly restrictive dietary plan and excessive cardio recommendations. Nothing worked until another mom at her daughter’s school mentioned that she was on Ozempic.
Ann also started to get hot flashes and missed periods. The doctor who prescribed her Ozempic confirmed that she was perimenopausal and that, for women in this stage of life, losing weight can be harder than ever.
Ann, who is 5 foot 7, started out at 176 pounds (considered overweight) and now weighs in at 151, which is considered a normal weight by BMI measurements. She’s still on Ozempic but continues to struggle with the shame around the idea she’s potentially taking the drug away from someone else who might desperately need it. And she doesn’t know how long she’ll have to stay on Ozempic to maintain her weight loss.
Ann has reason for concern. A 2022 study found that most people regain the weight they lost within a year of stopping Ozempic.
Once Ms. Raibick hit her initial goal weight, she felt that she could keep going and lose a little more. It wasn’t until she got into the 120-pound range that she decided it was time to wean off the dose of semaglutide she had been taking.
“I got to the point where my mom was like, ‘All right, you’re a little too thin.’ But I’m just so happy where I’m at. I’m not mentally stressed out about fitting into clothes or getting into a bathing suit,” said Ms. Raibick, who has now lost around 30 pounds in total since she started the shots.
At one point, she stopped taking the drug altogether, and all of the hunger cravings and food noise semaglutide had suppressed came back to the surface. She didn’t gain any weight that month, she said, but the internal chatter around food was enough to make her start back on a lower dose, geared toward weight maintenance.
There’s also the issue of side effects. Ms. Raibick says she never had the overwhelming nausea and digestive problems that so many on the drug – including Ann – have reported. But Dr. Thiara said that even beyond these more common side effects, there are a number of other concerns – like the long-lasting effects on thyroid and reproductive health, especially for women – that we still don’t know enough about. And just recently, CNN reported that some Ozempic users have developed stomach paralysis due to the drug’s ability to slow down the passage of food through the digestive tract.
For Ms. Raibick, the out-of-pocket cost for the drug is around $600 a month. It’s an expense she’s willing to keep paying for, even just for the peace of mind the drug provides. She doesn’t have any plans to stop her semaglutide shots soon.
“There is nothing stopping me from – a year from now, when I’ve put a little weight back on – looking back at photos from this time and thinking I was way too skinny.”
Dan Azagury, MD, a bariatric surgeon and associate professor of surgery at Stanford (Calif.) University, tries GLP-1s for patients with obesity before considering bariatric surgery. For his patient population, it’s possible that drugs like Ozempic will be part of their lifelong treatment plans.
“We’re not doing it for the cosmetic part of it, we’re doing it for health,” he said. “What I tell my patients is, if you’re planning to start on this medication, you should be OK with the idea of staying on it forever.”
For doctors like Dr. Thiara who specialize in weight management, using Ozempic long-term for patients in a healthy weight range is the wrong approach.
“It’s not about the way people look, it’s about health. If you’re a normal weight or even in an overweight category, but not showing signs of risk of having elevated cardiometabolic disease ... You don’t need to be taking medications for weight loss,” she said. “This idea of using medications for aesthetic reasons is really more related to societal ills around how we value fitness above anything else. That’s not the goal, and it’s not safe.”
A version of this article first appeared on WebMD.com.
How soybean oil could lead to gut inflammation
A popular ingredient in the American diet has been linked to ulcerative colitis. The ingredient is soybean oil, which is very common in processed foods. In fact, U.S. per capita consumption of soybean oil increased more than 1,000-fold during the 20th century.
In a study from the University of California, Riverside, and UC Davis, published in Gut Microbes, mice fed a diet high in soybean oil were more at risk of developing colitis.
The likely culprit? Linoleic acid, an omega-6 fatty acid that composes up to 60% of soybean oil.
Small amounts of linoleic acid help maintain the body’s water balance. But Americans derive as much as 10% of their daily energy from linoleic acid, when they need only 1%-2%, the researchers say.
The findings build on earlier research linking a high-linoleic acid diet with inflammatory bowel disease, or IBD, in humans. (Previous research in mice has also linked high consumption of the oil with obesity and diabetes in the rodents.)
For the new study, the researchers wanted to drill down into how linoleic acid affects the gut.
How linoleic acid may promote inflammation
In mice, the soybean oil diet upset the ratio of omega-3 to omega-6 fatty acids in the gut. This led to a decrease in endocannabinoids, lipid-based molecules that help block inflammation.
Enzymes that metabolize fatty acids are “shared between two pathways,” said study coauthor Frances Sladek, PhD, professor of cell biology at UC Riverside. “If you swamp the system with linoleic acid, you’ll have less enzymes available to metabolize omega-3s into good endocannabinoids.”
The endocannabinoid system has been linked to “visceral pain” in the gut, said Punyanganie de Silva, MD, MPH, an assistant professor at Brigham & Women’s Hospital, Boston, who was not involved in the study. But the relationship between the endocannabinoid system and inflammation has yet to be fully explored.
“This is one of the first papers that has looked at the association between linoleic acid and the endocannabinoid system,” Dr. de Silva said.
Changes in the gut microbiome
The gut microbiome of the mice also showed increased amounts of adherent invasive E. coli, a type of bacteria that grows by using linoleic acid as a carbon source. A “very close relative” of this bacteria has been linked to IBD in humans, Dr. Sladek said.
Using a method known as metabolomics, the researchers studied 3,000 metabolites in the intestinal cells of both the mice and the bacteria. Endocannabinoids decreased in both.
“We were actually quite surprised. I didn’t realize that bacteria made endocannabinoids,” Dr. Sladek said.
Helpful bacteria, such as the probiotic lactobacillus species, died off. The mice also had increased levels of oxylipins, which are correlated with obesity in mice and colitis in humans.
A high–linoleic acid diet could mean a leaky gut
Linoleic acid binds to a protein known as HNF-4 alpha. Disrupting the expression of this protein can weaken the intestinal barrier, letting toxins flow into the body – more commonly known as leaky gut. Mice on the soybean oil diet had decreased levels of the protein and more porous intestinal barriers, raising the risk for inflammation and colitis. “The HNF-4 alpha protein is conserved from mouse to human, so whatever’s happening to it in the context of the mouse gut, there’s a very high chance that a similar effect could be seen in humans as well,” said study coauthor Poonamjot Deol, PhD, an assistant professional researcher at UC Riverside.
Still, Dr. de Silva urges “some caution when interpreting these results,” given that “this is still experimental and needs to be reproduced in clinical studies as humans have a far more varied microbiome and more variable environmental exposures than these very controlled mouse model studies.”
Dr. de Silva says cooking with olive oil can “help increase omega-3 to omega-6 ratios” and advises eating a varied diet that includes omega-3 fats, such as flaxseed and walnuts, and minimal amounts of processed foods and saturated fats.
Looking ahead, endocannabinoids are being explored as “a potential therapy for treating IBD symptoms,” said Dr. Deol. She hopes to delve further into how linoleic acid affects the endocannabinoid system.
A version of this article first appeared on WebMD.com.
A popular ingredient in the American diet has been linked to ulcerative colitis. The ingredient is soybean oil, which is very common in processed foods. In fact, U.S. per capita consumption of soybean oil increased more than 1,000-fold during the 20th century.
In a study from the University of California, Riverside, and UC Davis, published in Gut Microbes, mice fed a diet high in soybean oil were more at risk of developing colitis.
The likely culprit? Linoleic acid, an omega-6 fatty acid that composes up to 60% of soybean oil.
Small amounts of linoleic acid help maintain the body’s water balance. But Americans derive as much as 10% of their daily energy from linoleic acid, when they need only 1%-2%, the researchers say.
The findings build on earlier research linking a high-linoleic acid diet with inflammatory bowel disease, or IBD, in humans. (Previous research in mice has also linked high consumption of the oil with obesity and diabetes in the rodents.)
For the new study, the researchers wanted to drill down into how linoleic acid affects the gut.
How linoleic acid may promote inflammation
In mice, the soybean oil diet upset the ratio of omega-3 to omega-6 fatty acids in the gut. This led to a decrease in endocannabinoids, lipid-based molecules that help block inflammation.
Enzymes that metabolize fatty acids are “shared between two pathways,” said study coauthor Frances Sladek, PhD, professor of cell biology at UC Riverside. “If you swamp the system with linoleic acid, you’ll have less enzymes available to metabolize omega-3s into good endocannabinoids.”
The endocannabinoid system has been linked to “visceral pain” in the gut, said Punyanganie de Silva, MD, MPH, an assistant professor at Brigham & Women’s Hospital, Boston, who was not involved in the study. But the relationship between the endocannabinoid system and inflammation has yet to be fully explored.
“This is one of the first papers that has looked at the association between linoleic acid and the endocannabinoid system,” Dr. de Silva said.
Changes in the gut microbiome
The gut microbiome of the mice also showed increased amounts of adherent invasive E. coli, a type of bacteria that grows by using linoleic acid as a carbon source. A “very close relative” of this bacteria has been linked to IBD in humans, Dr. Sladek said.
Using a method known as metabolomics, the researchers studied 3,000 metabolites in the intestinal cells of both the mice and the bacteria. Endocannabinoids decreased in both.
“We were actually quite surprised. I didn’t realize that bacteria made endocannabinoids,” Dr. Sladek said.
Helpful bacteria, such as the probiotic lactobacillus species, died off. The mice also had increased levels of oxylipins, which are correlated with obesity in mice and colitis in humans.
A high–linoleic acid diet could mean a leaky gut
Linoleic acid binds to a protein known as HNF-4 alpha. Disrupting the expression of this protein can weaken the intestinal barrier, letting toxins flow into the body – more commonly known as leaky gut. Mice on the soybean oil diet had decreased levels of the protein and more porous intestinal barriers, raising the risk for inflammation and colitis. “The HNF-4 alpha protein is conserved from mouse to human, so whatever’s happening to it in the context of the mouse gut, there’s a very high chance that a similar effect could be seen in humans as well,” said study coauthor Poonamjot Deol, PhD, an assistant professional researcher at UC Riverside.
Still, Dr. de Silva urges “some caution when interpreting these results,” given that “this is still experimental and needs to be reproduced in clinical studies as humans have a far more varied microbiome and more variable environmental exposures than these very controlled mouse model studies.”
Dr. de Silva says cooking with olive oil can “help increase omega-3 to omega-6 ratios” and advises eating a varied diet that includes omega-3 fats, such as flaxseed and walnuts, and minimal amounts of processed foods and saturated fats.
Looking ahead, endocannabinoids are being explored as “a potential therapy for treating IBD symptoms,” said Dr. Deol. She hopes to delve further into how linoleic acid affects the endocannabinoid system.
A version of this article first appeared on WebMD.com.
A popular ingredient in the American diet has been linked to ulcerative colitis. The ingredient is soybean oil, which is very common in processed foods. In fact, U.S. per capita consumption of soybean oil increased more than 1,000-fold during the 20th century.
In a study from the University of California, Riverside, and UC Davis, published in Gut Microbes, mice fed a diet high in soybean oil were more at risk of developing colitis.
The likely culprit? Linoleic acid, an omega-6 fatty acid that composes up to 60% of soybean oil.
Small amounts of linoleic acid help maintain the body’s water balance. But Americans derive as much as 10% of their daily energy from linoleic acid, when they need only 1%-2%, the researchers say.
The findings build on earlier research linking a high-linoleic acid diet with inflammatory bowel disease, or IBD, in humans. (Previous research in mice has also linked high consumption of the oil with obesity and diabetes in the rodents.)
For the new study, the researchers wanted to drill down into how linoleic acid affects the gut.
How linoleic acid may promote inflammation
In mice, the soybean oil diet upset the ratio of omega-3 to omega-6 fatty acids in the gut. This led to a decrease in endocannabinoids, lipid-based molecules that help block inflammation.
Enzymes that metabolize fatty acids are “shared between two pathways,” said study coauthor Frances Sladek, PhD, professor of cell biology at UC Riverside. “If you swamp the system with linoleic acid, you’ll have less enzymes available to metabolize omega-3s into good endocannabinoids.”
The endocannabinoid system has been linked to “visceral pain” in the gut, said Punyanganie de Silva, MD, MPH, an assistant professor at Brigham & Women’s Hospital, Boston, who was not involved in the study. But the relationship between the endocannabinoid system and inflammation has yet to be fully explored.
“This is one of the first papers that has looked at the association between linoleic acid and the endocannabinoid system,” Dr. de Silva said.
Changes in the gut microbiome
The gut microbiome of the mice also showed increased amounts of adherent invasive E. coli, a type of bacteria that grows by using linoleic acid as a carbon source. A “very close relative” of this bacteria has been linked to IBD in humans, Dr. Sladek said.
Using a method known as metabolomics, the researchers studied 3,000 metabolites in the intestinal cells of both the mice and the bacteria. Endocannabinoids decreased in both.
“We were actually quite surprised. I didn’t realize that bacteria made endocannabinoids,” Dr. Sladek said.
Helpful bacteria, such as the probiotic lactobacillus species, died off. The mice also had increased levels of oxylipins, which are correlated with obesity in mice and colitis in humans.
A high–linoleic acid diet could mean a leaky gut
Linoleic acid binds to a protein known as HNF-4 alpha. Disrupting the expression of this protein can weaken the intestinal barrier, letting toxins flow into the body – more commonly known as leaky gut. Mice on the soybean oil diet had decreased levels of the protein and more porous intestinal barriers, raising the risk for inflammation and colitis. “The HNF-4 alpha protein is conserved from mouse to human, so whatever’s happening to it in the context of the mouse gut, there’s a very high chance that a similar effect could be seen in humans as well,” said study coauthor Poonamjot Deol, PhD, an assistant professional researcher at UC Riverside.
Still, Dr. de Silva urges “some caution when interpreting these results,” given that “this is still experimental and needs to be reproduced in clinical studies as humans have a far more varied microbiome and more variable environmental exposures than these very controlled mouse model studies.”
Dr. de Silva says cooking with olive oil can “help increase omega-3 to omega-6 ratios” and advises eating a varied diet that includes omega-3 fats, such as flaxseed and walnuts, and minimal amounts of processed foods and saturated fats.
Looking ahead, endocannabinoids are being explored as “a potential therapy for treating IBD symptoms,” said Dr. Deol. She hopes to delve further into how linoleic acid affects the endocannabinoid system.
A version of this article first appeared on WebMD.com.
FROM GUT MICROBES
Rosacea look-alike
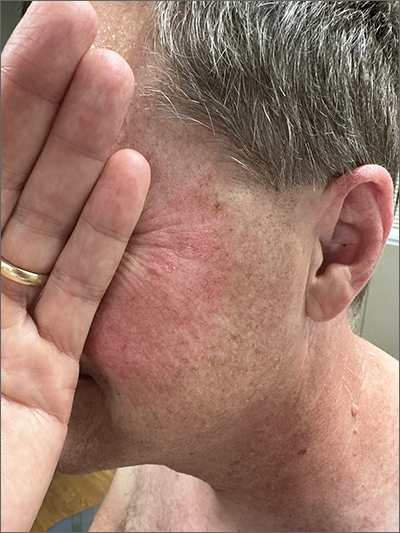
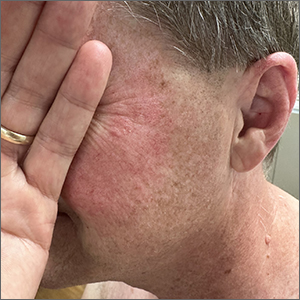
Although it’s easy to jump to the conclusion that facial erythema is rosacea, there are multiple other conditions that can lead to reddening of the face. In this case, excessive sun exposure had resulted in a diffuse actinic change of the malar and lateral aspects of this patient’s face. The palpably rough lesions were actinic keratoses.
Actinic keratoses are caused by exposure to ultraviolet radiation. These lesions are premalignant and common. Areas of the body at greatest risk include those not typically covered by clothing (eg, face, hands, arms, ears, forehead, and top of the scalp—especially in individuals with hair loss). There is a range of estimates regarding the percentage of actinic keratoses that will progress to squamous cell carcinoma in situ, and then invasive squamous cell carcinoma. One study determined that 10% of actinic keratoses progress to squamous cell carcinoma over the course of 2 years.1
In patients with broad areas of multiple clinically palpable lesions with rough sandpapery texture or visible white scale, there are likely preclinical lesions in the same areas. With so many lesions, field therapy of the entire region is often performed instead of treating the lesions 1 at a time.
There are multiple topical agents for field therapy, including 5-fluorouracil, diclofenac gel, and imiquimod gel.2 Since significant erythema and inflammation usually follow application of the topical agent, clinicians may want to have patients treat in segments to make the process more tolerable.
5-fluorouracil has a complete clearance rate (CCR) of 75% to 90% and is usually applied twice daily for 2 weeks, although there are multiple different protocols. Diclofenac has a CCR of 58% over a 60- to 90-day course, and imiquimod has a CCR of 54% after a 120-day course. Photodynamic therapy (PDT) has the advantage of a single treatment but a CCR of 38%. PDT may be advantageous for a patient who has difficulty applying topical medication over a period of weeks.
Niacinamide has been shown to help with skin repair and reduce the risk of additional nonmelanoma skin cancers (NMSC) by 23% and additional actinic keratoses by about 15% in individuals with a history of actinic keratoses or NMSC.3 In contrast to niacin, niacinamide does not cause flushing. Niacinamide is used long term; if discontinued, it no longer confers benefit in helping the skin repair itself.
The patient in this case was prescribed topical 5% fluorouracil cream to be applied twice daily to the malar regions bilaterally for 2 weeks and, if not inflamed by 2 weeks, to extend the treatment until there is robust inflammation (but not to exceed 3 weeks). He was scheduled to follow up in 3 months for reexamination. He was also advised to start taking niacinamide 500 mg twice daily to reduce his risk of additional precancerous and cancerous skin lesions and counseled on the importance of sunscreen, hats, and sun-protective clothing.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33:1099-1101. doi: 10.1111/j.1524-4725.2007.33224.x
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
- Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.

Although it’s easy to jump to the conclusion that facial erythema is rosacea, there are multiple other conditions that can lead to reddening of the face. In this case, excessive sun exposure had resulted in a diffuse actinic change of the malar and lateral aspects of this patient’s face. The palpably rough lesions were actinic keratoses.
Actinic keratoses are caused by exposure to ultraviolet radiation. These lesions are premalignant and common. Areas of the body at greatest risk include those not typically covered by clothing (eg, face, hands, arms, ears, forehead, and top of the scalp—especially in individuals with hair loss). There is a range of estimates regarding the percentage of actinic keratoses that will progress to squamous cell carcinoma in situ, and then invasive squamous cell carcinoma. One study determined that 10% of actinic keratoses progress to squamous cell carcinoma over the course of 2 years.1
In patients with broad areas of multiple clinically palpable lesions with rough sandpapery texture or visible white scale, there are likely preclinical lesions in the same areas. With so many lesions, field therapy of the entire region is often performed instead of treating the lesions 1 at a time.
There are multiple topical agents for field therapy, including 5-fluorouracil, diclofenac gel, and imiquimod gel.2 Since significant erythema and inflammation usually follow application of the topical agent, clinicians may want to have patients treat in segments to make the process more tolerable.
5-fluorouracil has a complete clearance rate (CCR) of 75% to 90% and is usually applied twice daily for 2 weeks, although there are multiple different protocols. Diclofenac has a CCR of 58% over a 60- to 90-day course, and imiquimod has a CCR of 54% after a 120-day course. Photodynamic therapy (PDT) has the advantage of a single treatment but a CCR of 38%. PDT may be advantageous for a patient who has difficulty applying topical medication over a period of weeks.
Niacinamide has been shown to help with skin repair and reduce the risk of additional nonmelanoma skin cancers (NMSC) by 23% and additional actinic keratoses by about 15% in individuals with a history of actinic keratoses or NMSC.3 In contrast to niacin, niacinamide does not cause flushing. Niacinamide is used long term; if discontinued, it no longer confers benefit in helping the skin repair itself.
The patient in this case was prescribed topical 5% fluorouracil cream to be applied twice daily to the malar regions bilaterally for 2 weeks and, if not inflamed by 2 weeks, to extend the treatment until there is robust inflammation (but not to exceed 3 weeks). He was scheduled to follow up in 3 months for reexamination. He was also advised to start taking niacinamide 500 mg twice daily to reduce his risk of additional precancerous and cancerous skin lesions and counseled on the importance of sunscreen, hats, and sun-protective clothing.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Although it’s easy to jump to the conclusion that facial erythema is rosacea, there are multiple other conditions that can lead to reddening of the face. In this case, excessive sun exposure had resulted in a diffuse actinic change of the malar and lateral aspects of this patient’s face. The palpably rough lesions were actinic keratoses.
Actinic keratoses are caused by exposure to ultraviolet radiation. These lesions are premalignant and common. Areas of the body at greatest risk include those not typically covered by clothing (eg, face, hands, arms, ears, forehead, and top of the scalp—especially in individuals with hair loss). There is a range of estimates regarding the percentage of actinic keratoses that will progress to squamous cell carcinoma in situ, and then invasive squamous cell carcinoma. One study determined that 10% of actinic keratoses progress to squamous cell carcinoma over the course of 2 years.1
In patients with broad areas of multiple clinically palpable lesions with rough sandpapery texture or visible white scale, there are likely preclinical lesions in the same areas. With so many lesions, field therapy of the entire region is often performed instead of treating the lesions 1 at a time.
There are multiple topical agents for field therapy, including 5-fluorouracil, diclofenac gel, and imiquimod gel.2 Since significant erythema and inflammation usually follow application of the topical agent, clinicians may want to have patients treat in segments to make the process more tolerable.
5-fluorouracil has a complete clearance rate (CCR) of 75% to 90% and is usually applied twice daily for 2 weeks, although there are multiple different protocols. Diclofenac has a CCR of 58% over a 60- to 90-day course, and imiquimod has a CCR of 54% after a 120-day course. Photodynamic therapy (PDT) has the advantage of a single treatment but a CCR of 38%. PDT may be advantageous for a patient who has difficulty applying topical medication over a period of weeks.
Niacinamide has been shown to help with skin repair and reduce the risk of additional nonmelanoma skin cancers (NMSC) by 23% and additional actinic keratoses by about 15% in individuals with a history of actinic keratoses or NMSC.3 In contrast to niacin, niacinamide does not cause flushing. Niacinamide is used long term; if discontinued, it no longer confers benefit in helping the skin repair itself.
The patient in this case was prescribed topical 5% fluorouracil cream to be applied twice daily to the malar regions bilaterally for 2 weeks and, if not inflamed by 2 weeks, to extend the treatment until there is robust inflammation (but not to exceed 3 weeks). He was scheduled to follow up in 3 months for reexamination. He was also advised to start taking niacinamide 500 mg twice daily to reduce his risk of additional precancerous and cancerous skin lesions and counseled on the importance of sunscreen, hats, and sun-protective clothing.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33:1099-1101. doi: 10.1111/j.1524-4725.2007.33224.x
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
- Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33:1099-1101. doi: 10.1111/j.1524-4725.2007.33224.x
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
- Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.
Skin reactions common at insulin pump infusion sites
new research suggests.
Insulin pump use is increasingly common, but many patients experience infusion-site failure that in some cases leads to discontinuation. In a novel investigation, researchers at the University of Washington, Seattle, used biopsies and noninvasive imaging to compare insulin pump sites with control sites in 30 patients. Several differences were found at pump sites in comparison with control sites, including fibrosis, inflammation, eosinophils, and increased vessel density.
“These findings support allergic sensitization as a potentially common reaction at [insulin pump] sites. The leading candidates causing this include insulin preservatives, plastic materials, and adhesive glue used in device manufacturing,” wrote Andrea Kalus, MD, of the university’s dermatology division, and colleagues. The findings were published recently in Diabetes Care.
The inflammatory response, they wrote, “may result in tissue changes responsible for the infusion-site failures seen frequently in clinical practice.”
Such infusion site problems represent an “Achilles heel” of these otherwise highly beneficial devices, lead author Irl Hirsch, MD, professor of medicine in the division of metabolism, endocrinology, and nutrition, said in a statement. “It doesn’t really matter how good the technology is. We still don’t understand what is happening with the infusion sites, much less to [be able to] fix it.”
Significant differences between pump and nonpump sites
In the cross-sectional study, Dr. Kalus and colleagues used noninvasive optical coherence tomography (OCT) immediately prior to performing punch biopsies at three sites: the site currently in active use, the “recovery site” used 3-5 days prior to the procedures, and control sites never used for pump infusion. Punch biopsies were also performed at those sites.
The mean age of the patients was 48.3 years, the mean diabetes duration was 30.4 years, and the mean duration of pump use was 15.8 years. Nearly all patients (93.3%) reported itchiness at the site, and 76.7% reported skin redness.
Of the 25 patients for whom OCT imaging was successful, statistical analysis showed significant differences in vascular area density and the optical attenuation coefficient, a surrogate for skin inflammation, between the pump and control sites and between recovery sites and current pump sites. The greater vessel density is likely a result of injury and repair related to catheter insertion, the authors said.
In the biopsy samples, both current and recovery sites showed increased fibrosis, fibrin, inflammation, fat necrosis, vascularity, and eosinophils, compared with the control sites, but no significant differences were found between current and recovery sites.
Eosinophils: ‘The most surprising histologic finding’
Eosinophils were found in 73% of skin biopsy specimens from current sites and in 75% of specimens from recovery sites, compared with none from the control sites (for both, P < .01). In all study participants, eosinophils were found in at least one current and/or recovery infusion site deep in the dermis near the interface with fat. The number of eosinophils ranged from 0 to 31 per high-power field, with a median of 4.
The number of eosinophils didn’t vary by type of insulin or brand of pump, but higher counts were seen in those who had used pumps for less than 10 years, compared with more than 20 years (P = .02).
The prevalence and degree of eosinophils were “the most surprising histologic finding,” the authors wrote, adding that “eosinophils are not typically present as a component of resident inflammatory cells in the skin.”
While eosinophils may be present in normal wound healing, “the absolute number and density of eosinophil in these samples support a delayed-type hypersensitivity response, which is typically observed between 2 and 7 days after exposure to an allergen. ... Eosinophils are often correlated with symptoms of itchiness and likely explain the high percentage of participants who reported itchiness in this study,” Dr. Kalus and colleagues wrote.
Correlation found between inflammation and glycemic control
All participants used the Dexcom G6 continuous glucose monitor as part of their usual care. Inflammation scores were positively correlated with insulin dose (P = .009) and were negatively correlated with time in range (P = .01).
No other OCT or biopsy findings differed by duration of pump use, previous use of animal insulin, or type of insulin.
The reason for these findings is unclear, Dr. Hirsch said. “How much was the catheter or the insulin causing the irritation around the sites? How much was it from the preservatives, or is this because of the insulin pump itself? All these questions need to be answered in future studies. ... The real goal of all of this is to minimize skin damage and improve the experience for our patients.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust. Dr. Hirsch reported grants and contracts from Insulet, Medtronic, and Dexcom outside the submitted work; consulting fees from Abbott Diabetes Care, Lifescan, and Hagar outside the submitted work; and honoraria for lectures, presentations, participation on speaker’s bureaus, manuscript writing, or educational events as section editor for UpToDate outside the submitted work. Dr. Kalus has no disclosures.
A version of this article first appeared on Medscape.com.
new research suggests.
Insulin pump use is increasingly common, but many patients experience infusion-site failure that in some cases leads to discontinuation. In a novel investigation, researchers at the University of Washington, Seattle, used biopsies and noninvasive imaging to compare insulin pump sites with control sites in 30 patients. Several differences were found at pump sites in comparison with control sites, including fibrosis, inflammation, eosinophils, and increased vessel density.
“These findings support allergic sensitization as a potentially common reaction at [insulin pump] sites. The leading candidates causing this include insulin preservatives, plastic materials, and adhesive glue used in device manufacturing,” wrote Andrea Kalus, MD, of the university’s dermatology division, and colleagues. The findings were published recently in Diabetes Care.
The inflammatory response, they wrote, “may result in tissue changes responsible for the infusion-site failures seen frequently in clinical practice.”
Such infusion site problems represent an “Achilles heel” of these otherwise highly beneficial devices, lead author Irl Hirsch, MD, professor of medicine in the division of metabolism, endocrinology, and nutrition, said in a statement. “It doesn’t really matter how good the technology is. We still don’t understand what is happening with the infusion sites, much less to [be able to] fix it.”
Significant differences between pump and nonpump sites
In the cross-sectional study, Dr. Kalus and colleagues used noninvasive optical coherence tomography (OCT) immediately prior to performing punch biopsies at three sites: the site currently in active use, the “recovery site” used 3-5 days prior to the procedures, and control sites never used for pump infusion. Punch biopsies were also performed at those sites.
The mean age of the patients was 48.3 years, the mean diabetes duration was 30.4 years, and the mean duration of pump use was 15.8 years. Nearly all patients (93.3%) reported itchiness at the site, and 76.7% reported skin redness.
Of the 25 patients for whom OCT imaging was successful, statistical analysis showed significant differences in vascular area density and the optical attenuation coefficient, a surrogate for skin inflammation, between the pump and control sites and between recovery sites and current pump sites. The greater vessel density is likely a result of injury and repair related to catheter insertion, the authors said.
In the biopsy samples, both current and recovery sites showed increased fibrosis, fibrin, inflammation, fat necrosis, vascularity, and eosinophils, compared with the control sites, but no significant differences were found between current and recovery sites.
Eosinophils: ‘The most surprising histologic finding’
Eosinophils were found in 73% of skin biopsy specimens from current sites and in 75% of specimens from recovery sites, compared with none from the control sites (for both, P < .01). In all study participants, eosinophils were found in at least one current and/or recovery infusion site deep in the dermis near the interface with fat. The number of eosinophils ranged from 0 to 31 per high-power field, with a median of 4.
The number of eosinophils didn’t vary by type of insulin or brand of pump, but higher counts were seen in those who had used pumps for less than 10 years, compared with more than 20 years (P = .02).
The prevalence and degree of eosinophils were “the most surprising histologic finding,” the authors wrote, adding that “eosinophils are not typically present as a component of resident inflammatory cells in the skin.”
While eosinophils may be present in normal wound healing, “the absolute number and density of eosinophil in these samples support a delayed-type hypersensitivity response, which is typically observed between 2 and 7 days after exposure to an allergen. ... Eosinophils are often correlated with symptoms of itchiness and likely explain the high percentage of participants who reported itchiness in this study,” Dr. Kalus and colleagues wrote.
Correlation found between inflammation and glycemic control
All participants used the Dexcom G6 continuous glucose monitor as part of their usual care. Inflammation scores were positively correlated with insulin dose (P = .009) and were negatively correlated with time in range (P = .01).
No other OCT or biopsy findings differed by duration of pump use, previous use of animal insulin, or type of insulin.
The reason for these findings is unclear, Dr. Hirsch said. “How much was the catheter or the insulin causing the irritation around the sites? How much was it from the preservatives, or is this because of the insulin pump itself? All these questions need to be answered in future studies. ... The real goal of all of this is to minimize skin damage and improve the experience for our patients.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust. Dr. Hirsch reported grants and contracts from Insulet, Medtronic, and Dexcom outside the submitted work; consulting fees from Abbott Diabetes Care, Lifescan, and Hagar outside the submitted work; and honoraria for lectures, presentations, participation on speaker’s bureaus, manuscript writing, or educational events as section editor for UpToDate outside the submitted work. Dr. Kalus has no disclosures.
A version of this article first appeared on Medscape.com.
new research suggests.
Insulin pump use is increasingly common, but many patients experience infusion-site failure that in some cases leads to discontinuation. In a novel investigation, researchers at the University of Washington, Seattle, used biopsies and noninvasive imaging to compare insulin pump sites with control sites in 30 patients. Several differences were found at pump sites in comparison with control sites, including fibrosis, inflammation, eosinophils, and increased vessel density.
“These findings support allergic sensitization as a potentially common reaction at [insulin pump] sites. The leading candidates causing this include insulin preservatives, plastic materials, and adhesive glue used in device manufacturing,” wrote Andrea Kalus, MD, of the university’s dermatology division, and colleagues. The findings were published recently in Diabetes Care.
The inflammatory response, they wrote, “may result in tissue changes responsible for the infusion-site failures seen frequently in clinical practice.”
Such infusion site problems represent an “Achilles heel” of these otherwise highly beneficial devices, lead author Irl Hirsch, MD, professor of medicine in the division of metabolism, endocrinology, and nutrition, said in a statement. “It doesn’t really matter how good the technology is. We still don’t understand what is happening with the infusion sites, much less to [be able to] fix it.”
Significant differences between pump and nonpump sites
In the cross-sectional study, Dr. Kalus and colleagues used noninvasive optical coherence tomography (OCT) immediately prior to performing punch biopsies at three sites: the site currently in active use, the “recovery site” used 3-5 days prior to the procedures, and control sites never used for pump infusion. Punch biopsies were also performed at those sites.
The mean age of the patients was 48.3 years, the mean diabetes duration was 30.4 years, and the mean duration of pump use was 15.8 years. Nearly all patients (93.3%) reported itchiness at the site, and 76.7% reported skin redness.
Of the 25 patients for whom OCT imaging was successful, statistical analysis showed significant differences in vascular area density and the optical attenuation coefficient, a surrogate for skin inflammation, between the pump and control sites and between recovery sites and current pump sites. The greater vessel density is likely a result of injury and repair related to catheter insertion, the authors said.
In the biopsy samples, both current and recovery sites showed increased fibrosis, fibrin, inflammation, fat necrosis, vascularity, and eosinophils, compared with the control sites, but no significant differences were found between current and recovery sites.
Eosinophils: ‘The most surprising histologic finding’
Eosinophils were found in 73% of skin biopsy specimens from current sites and in 75% of specimens from recovery sites, compared with none from the control sites (for both, P < .01). In all study participants, eosinophils were found in at least one current and/or recovery infusion site deep in the dermis near the interface with fat. The number of eosinophils ranged from 0 to 31 per high-power field, with a median of 4.
The number of eosinophils didn’t vary by type of insulin or brand of pump, but higher counts were seen in those who had used pumps for less than 10 years, compared with more than 20 years (P = .02).
The prevalence and degree of eosinophils were “the most surprising histologic finding,” the authors wrote, adding that “eosinophils are not typically present as a component of resident inflammatory cells in the skin.”
While eosinophils may be present in normal wound healing, “the absolute number and density of eosinophil in these samples support a delayed-type hypersensitivity response, which is typically observed between 2 and 7 days after exposure to an allergen. ... Eosinophils are often correlated with symptoms of itchiness and likely explain the high percentage of participants who reported itchiness in this study,” Dr. Kalus and colleagues wrote.
Correlation found between inflammation and glycemic control
All participants used the Dexcom G6 continuous glucose monitor as part of their usual care. Inflammation scores were positively correlated with insulin dose (P = .009) and were negatively correlated with time in range (P = .01).
No other OCT or biopsy findings differed by duration of pump use, previous use of animal insulin, or type of insulin.
The reason for these findings is unclear, Dr. Hirsch said. “How much was the catheter or the insulin causing the irritation around the sites? How much was it from the preservatives, or is this because of the insulin pump itself? All these questions need to be answered in future studies. ... The real goal of all of this is to minimize skin damage and improve the experience for our patients.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust. Dr. Hirsch reported grants and contracts from Insulet, Medtronic, and Dexcom outside the submitted work; consulting fees from Abbott Diabetes Care, Lifescan, and Hagar outside the submitted work; and honoraria for lectures, presentations, participation on speaker’s bureaus, manuscript writing, or educational events as section editor for UpToDate outside the submitted work. Dr. Kalus has no disclosures.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Nonalcohol substance use disorder tied to bariatric surgery
Nonalcohol substance use disorder (SUD) was 2.5 times more common in people who had gastric bypass surgery, compared with a control group who received usual obesity care, a new prospective study has found.
The findings suggest that the risk for nonalcohol SUD should be carefully explained to patients getting a gastric bypass and that the risk should be considered in care before and after the surgery, said the study authors and editorialists.
Though alcohol use disorder is a well-known side effect for some bariatric procedures, little is known about the link between the procedures and other substance abuse, wrote the study authors, led by Per-Arne Svensson, PhD, with the department of molecular and clinical medicine, Institute of Medicine, at the University of Gothenburg (Sweden).
The study was published online in Obesity.
The researchers analyzed data from the SOS study. It was originally designed to compare bariatric surgery with usual obesity care, with overall mortality as the primary outcome. The protocol also called for reporting negative effects of included treatments.
The study was conducted throughout Sweden at 25 public surgical departments and 480 primary health centers. Participants were between ages 37 and 60 years and had a body mass index of at least 34 kg/m2 for men and 38 for women.
After people with previous nonalcoholic SUD were excluded, the study population included 1,990 patients who had undergone bariatric surgery between September 1987 and January 2001, as well as 2,030 matched controls who received usual obesity care. The three types of bariatric surgery were gastric bypass (264 patients), vertical banded gastroplasty (1,353), and gastric banding (373), as chosen by the surgeons.
The follow-up was nearly 24 years.
Link found only with gastric bypass
The researchers identified participants who had nonalcoholic SUDs using the ICD from the Swedish National Patient Register covering hospital treatment (hospital stays or hospital-based outpatient care) but not primary care.
Only gastric bypass was associated with an increased incidence of nonalcoholic SUD (adjusted hazard ratio, 2.54; 95% confidence interval, 1.14-5.65), compared with controls during the follow-up period.
Among those who had gastric bypass surgery, three developed opioid-related disorders; three had sedative-, hypnotic-, or anxiolytic-related disorders; and three had other psychoactive substance–related disorders, the study authors wrote.
The researchers found no statistical difference in the incidence of nonalcoholic SUD when the groups who had undergone different surgical procedures were compared with each other.
“It is important to acknowledge that the number of affected patients was relatively low, in the single digits,” Jihad Kudsi, MD, a bariatric surgeon and chairman of surgery at Duly Health and Care, Oak Brook, Ill., said in a press release.
The findings “highlight the critical role of bariatric behavioral health clinicians in the comprehensive evaluation and care of patients both before and after weight loss surgery,” added Dr. Kudsi, who was not associated with the research.
Bariatric surgery candidates should be warned, monitored
The data indicate that patients who are candidates for bariatric surgery should be “carefully warned” about risks for nonalcoholic SUD and be monitored after the procedure, wrote James E. Mitchell, MD, a psychiatrist with the department of psychiatry and behavioral science, University of North Dakota, Fargo, and Devika Umashanker, MD, with Obesity Medicine, Hartford (Conn.) Health Care, in an accompanying editorial.
They acknowledged, however, that monitoring can be difficult given the typical low rate of follow-up of these patients.
Though the reasons for the rise in nonalcoholic SUD are not clear, Dr. Mitchell and Dr. Umashanker said biologic and psychosocial issues may be contributors to the increase.
The persistence of medical comorbidities and a lack of noted improvement in quality of life or physical mobility after the surgery has been addressed in a paper on suicide risk after bariatric surgery, the study authors also noted.
Dr. Svensson said in an interview that a mechanism for alcohol abuse after gastric bypass surgery is more evident, as measured by “increased blood alcohol levels after the surgery for a given amount of alcohol.” However, for other addictive substances, the mechanism is not obvious and needs further study.
The editorialists reminded clinicians that measuring phosphatidylethanol can be very useful in identifying and quantifying recent alcohol intake, suggesting that all clinicians, not just those in bariatric surgery clinics, should be aware of the connection between the procedures and subsequent alcohol abuse and monitor those patients carefully.
Both the study authors and the editorialists pointed out that the SOS cohort was recruited when vertical banded gastroplasty and banding were commonly used, and both methods are now rarely, if ever, used. Gastric sleeve procedures are now the most common approach, and those patients were not included in the study.
“However, gastric bypass surgery patients were included, albeit in a minority of the sample,” Dr. Mitchell and Dr. Umashanker wrote. In addition, the sample size of patients with SUD was too small to determine the drugs that were being abused.
Dr. Svensson said in an interview the main limitation is that SUD events were identified in the Swedish National Patient Register, which misses nonhospitalized patients.
“This register is very complete for hospitals, but it does not include SUD events detected in the primary health care setting,” he said. “Hence, the absolute number of events is probably a clear underestimation. However, it is unlikely that this limitation would affect the study groups (control group vs. groups with different surgical procedures) in different ways and hence the conclusions from this study are most likely valid.”
The study authors and the editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonalcohol substance use disorder (SUD) was 2.5 times more common in people who had gastric bypass surgery, compared with a control group who received usual obesity care, a new prospective study has found.
The findings suggest that the risk for nonalcohol SUD should be carefully explained to patients getting a gastric bypass and that the risk should be considered in care before and after the surgery, said the study authors and editorialists.
Though alcohol use disorder is a well-known side effect for some bariatric procedures, little is known about the link between the procedures and other substance abuse, wrote the study authors, led by Per-Arne Svensson, PhD, with the department of molecular and clinical medicine, Institute of Medicine, at the University of Gothenburg (Sweden).
The study was published online in Obesity.
The researchers analyzed data from the SOS study. It was originally designed to compare bariatric surgery with usual obesity care, with overall mortality as the primary outcome. The protocol also called for reporting negative effects of included treatments.
The study was conducted throughout Sweden at 25 public surgical departments and 480 primary health centers. Participants were between ages 37 and 60 years and had a body mass index of at least 34 kg/m2 for men and 38 for women.
After people with previous nonalcoholic SUD were excluded, the study population included 1,990 patients who had undergone bariatric surgery between September 1987 and January 2001, as well as 2,030 matched controls who received usual obesity care. The three types of bariatric surgery were gastric bypass (264 patients), vertical banded gastroplasty (1,353), and gastric banding (373), as chosen by the surgeons.
The follow-up was nearly 24 years.
Link found only with gastric bypass
The researchers identified participants who had nonalcoholic SUDs using the ICD from the Swedish National Patient Register covering hospital treatment (hospital stays or hospital-based outpatient care) but not primary care.
Only gastric bypass was associated with an increased incidence of nonalcoholic SUD (adjusted hazard ratio, 2.54; 95% confidence interval, 1.14-5.65), compared with controls during the follow-up period.
Among those who had gastric bypass surgery, three developed opioid-related disorders; three had sedative-, hypnotic-, or anxiolytic-related disorders; and three had other psychoactive substance–related disorders, the study authors wrote.
The researchers found no statistical difference in the incidence of nonalcoholic SUD when the groups who had undergone different surgical procedures were compared with each other.
“It is important to acknowledge that the number of affected patients was relatively low, in the single digits,” Jihad Kudsi, MD, a bariatric surgeon and chairman of surgery at Duly Health and Care, Oak Brook, Ill., said in a press release.
The findings “highlight the critical role of bariatric behavioral health clinicians in the comprehensive evaluation and care of patients both before and after weight loss surgery,” added Dr. Kudsi, who was not associated with the research.
Bariatric surgery candidates should be warned, monitored
The data indicate that patients who are candidates for bariatric surgery should be “carefully warned” about risks for nonalcoholic SUD and be monitored after the procedure, wrote James E. Mitchell, MD, a psychiatrist with the department of psychiatry and behavioral science, University of North Dakota, Fargo, and Devika Umashanker, MD, with Obesity Medicine, Hartford (Conn.) Health Care, in an accompanying editorial.
They acknowledged, however, that monitoring can be difficult given the typical low rate of follow-up of these patients.
Though the reasons for the rise in nonalcoholic SUD are not clear, Dr. Mitchell and Dr. Umashanker said biologic and psychosocial issues may be contributors to the increase.
The persistence of medical comorbidities and a lack of noted improvement in quality of life or physical mobility after the surgery has been addressed in a paper on suicide risk after bariatric surgery, the study authors also noted.
Dr. Svensson said in an interview that a mechanism for alcohol abuse after gastric bypass surgery is more evident, as measured by “increased blood alcohol levels after the surgery for a given amount of alcohol.” However, for other addictive substances, the mechanism is not obvious and needs further study.
The editorialists reminded clinicians that measuring phosphatidylethanol can be very useful in identifying and quantifying recent alcohol intake, suggesting that all clinicians, not just those in bariatric surgery clinics, should be aware of the connection between the procedures and subsequent alcohol abuse and monitor those patients carefully.
Both the study authors and the editorialists pointed out that the SOS cohort was recruited when vertical banded gastroplasty and banding were commonly used, and both methods are now rarely, if ever, used. Gastric sleeve procedures are now the most common approach, and those patients were not included in the study.
“However, gastric bypass surgery patients were included, albeit in a minority of the sample,” Dr. Mitchell and Dr. Umashanker wrote. In addition, the sample size of patients with SUD was too small to determine the drugs that were being abused.
Dr. Svensson said in an interview the main limitation is that SUD events were identified in the Swedish National Patient Register, which misses nonhospitalized patients.
“This register is very complete for hospitals, but it does not include SUD events detected in the primary health care setting,” he said. “Hence, the absolute number of events is probably a clear underestimation. However, it is unlikely that this limitation would affect the study groups (control group vs. groups with different surgical procedures) in different ways and hence the conclusions from this study are most likely valid.”
The study authors and the editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonalcohol substance use disorder (SUD) was 2.5 times more common in people who had gastric bypass surgery, compared with a control group who received usual obesity care, a new prospective study has found.
The findings suggest that the risk for nonalcohol SUD should be carefully explained to patients getting a gastric bypass and that the risk should be considered in care before and after the surgery, said the study authors and editorialists.
Though alcohol use disorder is a well-known side effect for some bariatric procedures, little is known about the link between the procedures and other substance abuse, wrote the study authors, led by Per-Arne Svensson, PhD, with the department of molecular and clinical medicine, Institute of Medicine, at the University of Gothenburg (Sweden).
The study was published online in Obesity.
The researchers analyzed data from the SOS study. It was originally designed to compare bariatric surgery with usual obesity care, with overall mortality as the primary outcome. The protocol also called for reporting negative effects of included treatments.
The study was conducted throughout Sweden at 25 public surgical departments and 480 primary health centers. Participants were between ages 37 and 60 years and had a body mass index of at least 34 kg/m2 for men and 38 for women.
After people with previous nonalcoholic SUD were excluded, the study population included 1,990 patients who had undergone bariatric surgery between September 1987 and January 2001, as well as 2,030 matched controls who received usual obesity care. The three types of bariatric surgery were gastric bypass (264 patients), vertical banded gastroplasty (1,353), and gastric banding (373), as chosen by the surgeons.
The follow-up was nearly 24 years.
Link found only with gastric bypass
The researchers identified participants who had nonalcoholic SUDs using the ICD from the Swedish National Patient Register covering hospital treatment (hospital stays or hospital-based outpatient care) but not primary care.
Only gastric bypass was associated with an increased incidence of nonalcoholic SUD (adjusted hazard ratio, 2.54; 95% confidence interval, 1.14-5.65), compared with controls during the follow-up period.
Among those who had gastric bypass surgery, three developed opioid-related disorders; three had sedative-, hypnotic-, or anxiolytic-related disorders; and three had other psychoactive substance–related disorders, the study authors wrote.
The researchers found no statistical difference in the incidence of nonalcoholic SUD when the groups who had undergone different surgical procedures were compared with each other.
“It is important to acknowledge that the number of affected patients was relatively low, in the single digits,” Jihad Kudsi, MD, a bariatric surgeon and chairman of surgery at Duly Health and Care, Oak Brook, Ill., said in a press release.
The findings “highlight the critical role of bariatric behavioral health clinicians in the comprehensive evaluation and care of patients both before and after weight loss surgery,” added Dr. Kudsi, who was not associated with the research.
Bariatric surgery candidates should be warned, monitored
The data indicate that patients who are candidates for bariatric surgery should be “carefully warned” about risks for nonalcoholic SUD and be monitored after the procedure, wrote James E. Mitchell, MD, a psychiatrist with the department of psychiatry and behavioral science, University of North Dakota, Fargo, and Devika Umashanker, MD, with Obesity Medicine, Hartford (Conn.) Health Care, in an accompanying editorial.
They acknowledged, however, that monitoring can be difficult given the typical low rate of follow-up of these patients.
Though the reasons for the rise in nonalcoholic SUD are not clear, Dr. Mitchell and Dr. Umashanker said biologic and psychosocial issues may be contributors to the increase.
The persistence of medical comorbidities and a lack of noted improvement in quality of life or physical mobility after the surgery has been addressed in a paper on suicide risk after bariatric surgery, the study authors also noted.
Dr. Svensson said in an interview that a mechanism for alcohol abuse after gastric bypass surgery is more evident, as measured by “increased blood alcohol levels after the surgery for a given amount of alcohol.” However, for other addictive substances, the mechanism is not obvious and needs further study.
The editorialists reminded clinicians that measuring phosphatidylethanol can be very useful in identifying and quantifying recent alcohol intake, suggesting that all clinicians, not just those in bariatric surgery clinics, should be aware of the connection between the procedures and subsequent alcohol abuse and monitor those patients carefully.
Both the study authors and the editorialists pointed out that the SOS cohort was recruited when vertical banded gastroplasty and banding were commonly used, and both methods are now rarely, if ever, used. Gastric sleeve procedures are now the most common approach, and those patients were not included in the study.
“However, gastric bypass surgery patients were included, albeit in a minority of the sample,” Dr. Mitchell and Dr. Umashanker wrote. In addition, the sample size of patients with SUD was too small to determine the drugs that were being abused.
Dr. Svensson said in an interview the main limitation is that SUD events were identified in the Swedish National Patient Register, which misses nonhospitalized patients.
“This register is very complete for hospitals, but it does not include SUD events detected in the primary health care setting,” he said. “Hence, the absolute number of events is probably a clear underestimation. However, it is unlikely that this limitation would affect the study groups (control group vs. groups with different surgical procedures) in different ways and hence the conclusions from this study are most likely valid.”
The study authors and the editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM OBESITY
What factors cause multiple biologic failure in psoriasis?
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Even one drink a day tied to increased BP in healthy adults
“A vexing question has been whether usual intake of small amounts of alcohol is associated with a higher level of BP. We identified a continuous, more or less linear association, with no evidence of a threshold for the association,” study coauthor Paul Whelton, MD, of Tulane University School of Public Health and Tropical Medicine, New Orleans, said in an interview.
For systolic BP (SBP), “the most important BP risk indicator for CVD [cardiovascular disease], the association was robust, being present in both men and women and in both North America as well as Asia,” Dr. Whelton noted.
Based on the results, “the lower the better, and no consumption even better, as we did not find any indication that human health may benefit from consumption of very small amounts of alcohol,” senior author Marco Vinceti, MD, PhD, of University of Modena and Reggio Emilia University in Italy, told this news organization.
“Clearly, alcohol is not the only or necessarily the main determinant of high blood pressure, and the effects of small intakes of alcohol emerging from our pooled analysis were certainly not biologically as relevant and meaningful as those induced by high intakes,” Dr. Vinceti added.
The study was published online in Hypertension.
The researchers analyzed data from seven large, observational studies conducted in the United States, Korea, and Japan involving 19,548 adults (65% men).
Participants ranged in age from 20 years to the early 70s at baseline and were followed for a median of 5.3 years (range, 4-12 years). None of the participants had previously been diagnosed with hypertension or other CVD, diabetes, liver disease, alcoholism, or binge drinking.
Compared with nondrinkers, SBP was 1.25 mm Hg higher in adults who consumed an average of 12 grams of alcohol per day, rising to 4.90 mm Hg in adults consuming an average of 48 grams of alcohol per day.
For reference, in the United States, 12 ounces of regular beer, 5 ounces of wine, or a 1.5-ounce shot of distilled spirits contains about 14 grams of alcohol.
Diastolic BP (DBP) was 1.14 mm Hg higher in adults who consumed an average of 12 grams of alcohol per day, rising to 3.10 mm Hg in those who consumed an average of 48 grams of alcohol per day.
Subgroup analyses by gender showed an almost linear association between baseline alcohol intake and SBP changes in men and women and for DBP in men, while in women, there was an inverted U-shaped association.
No safe level
“From a BP perspective, it’s best to avoid alcohol intake. This is what the WHO [World Health Organization] recommends,” Dr. Whelton said.
“If someone is already drinking alcohol and does not want to stop doing so, minimizing alcohol consumption is desirable; many guidelines recommend not starting to drink alcohol but in those already drinking alcohol, consumption of two or less standard drinks per day for men and one or less standard drinks of alcohol per day for women,” Dr. Whelton noted.
Commenting on the study for this article, Alberto Ascherio, MD, of Harvard T. H. Chan School of Public Health, Boston, said it’s been known for more than 30 years that alcohol intake is associated with increased systolic and diastolic BP. The added value of this new study is a “refinement of the estimate of the dose response.”
Dr. Ascherio noted that “moderate alcohol consumption is associated with a modest increase in risk of cancer, but, in spite of the adverse association with BP, with a potentially beneficial effect on cardiovascular disease.” However, “the causality of the latter association has been questioned, but there is no consensus on this.”
Also weighing in, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York City, said this new study represents “yet another piece of evidence suggesting that there simply is no ‘healthy’ amount of alcohol use in humans.
“Even small amounts of alcohol intake can have negative health effects, as demonstrated in this study,” Dr. Brennan said. “There is still a widely held belief among people that drinking in moderation is good for you. It is becoming more and more clear that this is simply not the case. As health authorities grapple with drinking ‘recommendations,’ additional datasets like these will be helpful.”
The study had no specific funding. Dr. Whelton, Dr. Vinceti, Dr. Ascherio, and Dr. Brennan have no relevant disclosures.
A version of this article first appeared on Medscape.com.
“A vexing question has been whether usual intake of small amounts of alcohol is associated with a higher level of BP. We identified a continuous, more or less linear association, with no evidence of a threshold for the association,” study coauthor Paul Whelton, MD, of Tulane University School of Public Health and Tropical Medicine, New Orleans, said in an interview.
For systolic BP (SBP), “the most important BP risk indicator for CVD [cardiovascular disease], the association was robust, being present in both men and women and in both North America as well as Asia,” Dr. Whelton noted.
Based on the results, “the lower the better, and no consumption even better, as we did not find any indication that human health may benefit from consumption of very small amounts of alcohol,” senior author Marco Vinceti, MD, PhD, of University of Modena and Reggio Emilia University in Italy, told this news organization.
“Clearly, alcohol is not the only or necessarily the main determinant of high blood pressure, and the effects of small intakes of alcohol emerging from our pooled analysis were certainly not biologically as relevant and meaningful as those induced by high intakes,” Dr. Vinceti added.
The study was published online in Hypertension.
The researchers analyzed data from seven large, observational studies conducted in the United States, Korea, and Japan involving 19,548 adults (65% men).
Participants ranged in age from 20 years to the early 70s at baseline and were followed for a median of 5.3 years (range, 4-12 years). None of the participants had previously been diagnosed with hypertension or other CVD, diabetes, liver disease, alcoholism, or binge drinking.
Compared with nondrinkers, SBP was 1.25 mm Hg higher in adults who consumed an average of 12 grams of alcohol per day, rising to 4.90 mm Hg in adults consuming an average of 48 grams of alcohol per day.
For reference, in the United States, 12 ounces of regular beer, 5 ounces of wine, or a 1.5-ounce shot of distilled spirits contains about 14 grams of alcohol.
Diastolic BP (DBP) was 1.14 mm Hg higher in adults who consumed an average of 12 grams of alcohol per day, rising to 3.10 mm Hg in those who consumed an average of 48 grams of alcohol per day.
Subgroup analyses by gender showed an almost linear association between baseline alcohol intake and SBP changes in men and women and for DBP in men, while in women, there was an inverted U-shaped association.
No safe level
“From a BP perspective, it’s best to avoid alcohol intake. This is what the WHO [World Health Organization] recommends,” Dr. Whelton said.
“If someone is already drinking alcohol and does not want to stop doing so, minimizing alcohol consumption is desirable; many guidelines recommend not starting to drink alcohol but in those already drinking alcohol, consumption of two or less standard drinks per day for men and one or less standard drinks of alcohol per day for women,” Dr. Whelton noted.
Commenting on the study for this article, Alberto Ascherio, MD, of Harvard T. H. Chan School of Public Health, Boston, said it’s been known for more than 30 years that alcohol intake is associated with increased systolic and diastolic BP. The added value of this new study is a “refinement of the estimate of the dose response.”
Dr. Ascherio noted that “moderate alcohol consumption is associated with a modest increase in risk of cancer, but, in spite of the adverse association with BP, with a potentially beneficial effect on cardiovascular disease.” However, “the causality of the latter association has been questioned, but there is no consensus on this.”
Also weighing in, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York City, said this new study represents “yet another piece of evidence suggesting that there simply is no ‘healthy’ amount of alcohol use in humans.
“Even small amounts of alcohol intake can have negative health effects, as demonstrated in this study,” Dr. Brennan said. “There is still a widely held belief among people that drinking in moderation is good for you. It is becoming more and more clear that this is simply not the case. As health authorities grapple with drinking ‘recommendations,’ additional datasets like these will be helpful.”
The study had no specific funding. Dr. Whelton, Dr. Vinceti, Dr. Ascherio, and Dr. Brennan have no relevant disclosures.
A version of this article first appeared on Medscape.com.
“A vexing question has been whether usual intake of small amounts of alcohol is associated with a higher level of BP. We identified a continuous, more or less linear association, with no evidence of a threshold for the association,” study coauthor Paul Whelton, MD, of Tulane University School of Public Health and Tropical Medicine, New Orleans, said in an interview.
For systolic BP (SBP), “the most important BP risk indicator for CVD [cardiovascular disease], the association was robust, being present in both men and women and in both North America as well as Asia,” Dr. Whelton noted.
Based on the results, “the lower the better, and no consumption even better, as we did not find any indication that human health may benefit from consumption of very small amounts of alcohol,” senior author Marco Vinceti, MD, PhD, of University of Modena and Reggio Emilia University in Italy, told this news organization.
“Clearly, alcohol is not the only or necessarily the main determinant of high blood pressure, and the effects of small intakes of alcohol emerging from our pooled analysis were certainly not biologically as relevant and meaningful as those induced by high intakes,” Dr. Vinceti added.
The study was published online in Hypertension.
The researchers analyzed data from seven large, observational studies conducted in the United States, Korea, and Japan involving 19,548 adults (65% men).
Participants ranged in age from 20 years to the early 70s at baseline and were followed for a median of 5.3 years (range, 4-12 years). None of the participants had previously been diagnosed with hypertension or other CVD, diabetes, liver disease, alcoholism, or binge drinking.
Compared with nondrinkers, SBP was 1.25 mm Hg higher in adults who consumed an average of 12 grams of alcohol per day, rising to 4.90 mm Hg in adults consuming an average of 48 grams of alcohol per day.
For reference, in the United States, 12 ounces of regular beer, 5 ounces of wine, or a 1.5-ounce shot of distilled spirits contains about 14 grams of alcohol.
Diastolic BP (DBP) was 1.14 mm Hg higher in adults who consumed an average of 12 grams of alcohol per day, rising to 3.10 mm Hg in those who consumed an average of 48 grams of alcohol per day.
Subgroup analyses by gender showed an almost linear association between baseline alcohol intake and SBP changes in men and women and for DBP in men, while in women, there was an inverted U-shaped association.
No safe level
“From a BP perspective, it’s best to avoid alcohol intake. This is what the WHO [World Health Organization] recommends,” Dr. Whelton said.
“If someone is already drinking alcohol and does not want to stop doing so, minimizing alcohol consumption is desirable; many guidelines recommend not starting to drink alcohol but in those already drinking alcohol, consumption of two or less standard drinks per day for men and one or less standard drinks of alcohol per day for women,” Dr. Whelton noted.
Commenting on the study for this article, Alberto Ascherio, MD, of Harvard T. H. Chan School of Public Health, Boston, said it’s been known for more than 30 years that alcohol intake is associated with increased systolic and diastolic BP. The added value of this new study is a “refinement of the estimate of the dose response.”
Dr. Ascherio noted that “moderate alcohol consumption is associated with a modest increase in risk of cancer, but, in spite of the adverse association with BP, with a potentially beneficial effect on cardiovascular disease.” However, “the causality of the latter association has been questioned, but there is no consensus on this.”
Also weighing in, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York City, said this new study represents “yet another piece of evidence suggesting that there simply is no ‘healthy’ amount of alcohol use in humans.
“Even small amounts of alcohol intake can have negative health effects, as demonstrated in this study,” Dr. Brennan said. “There is still a widely held belief among people that drinking in moderation is good for you. It is becoming more and more clear that this is simply not the case. As health authorities grapple with drinking ‘recommendations,’ additional datasets like these will be helpful.”
The study had no specific funding. Dr. Whelton, Dr. Vinceti, Dr. Ascherio, and Dr. Brennan have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION
Heat waves plus air pollution tied to doubling of fatal MI
, a study from China suggests.

The researchers estimate that up to 3% of all deaths due to MI could be attributed to the combination of extreme temperatures and high levels of ambient fine particulate matter (PM2.5).
“Our findings provide evidence that reducing exposure to both extreme temperatures and fine particulate pollution may be useful to prevent premature deaths from heart attack,” senior author Yuewei Liu, MD, PhD, with Sun Yat-sen University in Guangzhou, China, said in a statement.
There is “long-standing evidence” of the harmful cardiovascular effects of air pollution, Jonathan Newman, MD, MPH, cardiologist at NYU Langone Heart in New York, who wasn’t involved in the study, said in an interview.
The added value of this study was finding an interaction between extreme hot temperatures and air pollution, “which is worrisome with global warming,” said Dr. Newman, assistant professor, department of medicine, the Leon H. Charney Division of Cardiology at NYU Langone Health.
The study was published online in Circulation.
Intensity and duration matter
The researchers analyzed data on 202,678 adults (mean age, 77.6 years; 52% male) who suffered fatal MI between 2015 and 2020 in Jiangsu province, a region with four distinct seasons and a wide range of temperatures and ambient PM2.5.
They evaluated the association of exposure to extreme temperature events, including both hot and cold spells, and PM2.5 with MI mortality, and their interactive effects.
Among the key findings:
- The risk of fatal MI was 18% higher during 2-day heat waves with heat indexes at or above the 90th percentile (ranging from 82.6° to 97.9° F) and 74% higher during 4-day heat waves with heat indexes at or above the 97.5th percentile (ranging from 94.8° to 109.4° F), compared with control days.
- The risk of fatal MI was 4% higher during 2-day cold snaps with temperatures at or below the 10th percentile (ranging from 33.3° to 40.5° F) and 12% higher during 3-day cold snaps with temperatures at or below the 2.5th percentile (ranging from 27.0° to 37.2° F).
- The risk of fatal MI was twice as high during 4-day heat waves that had PM2.5 above 37.5 mcg/m3. Days with high levels of PM2.5 during cold snaps did not have an equivalent increase in the risk of fatal MI.
- Up to 2.8% of MI deaths during the 5-year study period may be attributable to the combination of extreme temperature exposure and PM2.5 at levels exceeding World Health Organization air quality guidelines (37.5 mcg/m3).
- The risk of fatal MI was generally higher among women than men during heat waves and was higher among adults 80 years old and older than in younger adults during heat waves, cold snaps, or days with high levels of PM2.5.
The finding that adults over age 80 are particularly susceptible to the effects of heat and air pollution and the interaction of the two is “notable and particularly relevant given the aging of the population,” Dr. Newman told this news organization.
Mitigating both extreme temperature events and PM2.5 exposures “may bring health cobenefits in preventing premature deaths from MI,” the researchers write.
“To improve public health, it is important to take fine particulate pollution into consideration when providing extreme temperature warnings to the public,” Dr. Liu adds in the statement.
In an earlier study, Dr. Liu and colleagues showed that exposure to both large and small particulate matter, as well as nitrogen dioxide, was significantly associated with increased odds of death from MI.
This study was funded by China’s Ministry of Science and Technology. The authors and Dr. Newman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a study from China suggests.

The researchers estimate that up to 3% of all deaths due to MI could be attributed to the combination of extreme temperatures and high levels of ambient fine particulate matter (PM2.5).
“Our findings provide evidence that reducing exposure to both extreme temperatures and fine particulate pollution may be useful to prevent premature deaths from heart attack,” senior author Yuewei Liu, MD, PhD, with Sun Yat-sen University in Guangzhou, China, said in a statement.
There is “long-standing evidence” of the harmful cardiovascular effects of air pollution, Jonathan Newman, MD, MPH, cardiologist at NYU Langone Heart in New York, who wasn’t involved in the study, said in an interview.
The added value of this study was finding an interaction between extreme hot temperatures and air pollution, “which is worrisome with global warming,” said Dr. Newman, assistant professor, department of medicine, the Leon H. Charney Division of Cardiology at NYU Langone Health.
The study was published online in Circulation.
Intensity and duration matter
The researchers analyzed data on 202,678 adults (mean age, 77.6 years; 52% male) who suffered fatal MI between 2015 and 2020 in Jiangsu province, a region with four distinct seasons and a wide range of temperatures and ambient PM2.5.
They evaluated the association of exposure to extreme temperature events, including both hot and cold spells, and PM2.5 with MI mortality, and their interactive effects.
Among the key findings:
- The risk of fatal MI was 18% higher during 2-day heat waves with heat indexes at or above the 90th percentile (ranging from 82.6° to 97.9° F) and 74% higher during 4-day heat waves with heat indexes at or above the 97.5th percentile (ranging from 94.8° to 109.4° F), compared with control days.
- The risk of fatal MI was 4% higher during 2-day cold snaps with temperatures at or below the 10th percentile (ranging from 33.3° to 40.5° F) and 12% higher during 3-day cold snaps with temperatures at or below the 2.5th percentile (ranging from 27.0° to 37.2° F).
- The risk of fatal MI was twice as high during 4-day heat waves that had PM2.5 above 37.5 mcg/m3. Days with high levels of PM2.5 during cold snaps did not have an equivalent increase in the risk of fatal MI.
- Up to 2.8% of MI deaths during the 5-year study period may be attributable to the combination of extreme temperature exposure and PM2.5 at levels exceeding World Health Organization air quality guidelines (37.5 mcg/m3).
- The risk of fatal MI was generally higher among women than men during heat waves and was higher among adults 80 years old and older than in younger adults during heat waves, cold snaps, or days with high levels of PM2.5.
The finding that adults over age 80 are particularly susceptible to the effects of heat and air pollution and the interaction of the two is “notable and particularly relevant given the aging of the population,” Dr. Newman told this news organization.
Mitigating both extreme temperature events and PM2.5 exposures “may bring health cobenefits in preventing premature deaths from MI,” the researchers write.
“To improve public health, it is important to take fine particulate pollution into consideration when providing extreme temperature warnings to the public,” Dr. Liu adds in the statement.
In an earlier study, Dr. Liu and colleagues showed that exposure to both large and small particulate matter, as well as nitrogen dioxide, was significantly associated with increased odds of death from MI.
This study was funded by China’s Ministry of Science and Technology. The authors and Dr. Newman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a study from China suggests.

The researchers estimate that up to 3% of all deaths due to MI could be attributed to the combination of extreme temperatures and high levels of ambient fine particulate matter (PM2.5).
“Our findings provide evidence that reducing exposure to both extreme temperatures and fine particulate pollution may be useful to prevent premature deaths from heart attack,” senior author Yuewei Liu, MD, PhD, with Sun Yat-sen University in Guangzhou, China, said in a statement.
There is “long-standing evidence” of the harmful cardiovascular effects of air pollution, Jonathan Newman, MD, MPH, cardiologist at NYU Langone Heart in New York, who wasn’t involved in the study, said in an interview.
The added value of this study was finding an interaction between extreme hot temperatures and air pollution, “which is worrisome with global warming,” said Dr. Newman, assistant professor, department of medicine, the Leon H. Charney Division of Cardiology at NYU Langone Health.
The study was published online in Circulation.
Intensity and duration matter
The researchers analyzed data on 202,678 adults (mean age, 77.6 years; 52% male) who suffered fatal MI between 2015 and 2020 in Jiangsu province, a region with four distinct seasons and a wide range of temperatures and ambient PM2.5.
They evaluated the association of exposure to extreme temperature events, including both hot and cold spells, and PM2.5 with MI mortality, and their interactive effects.
Among the key findings:
- The risk of fatal MI was 18% higher during 2-day heat waves with heat indexes at or above the 90th percentile (ranging from 82.6° to 97.9° F) and 74% higher during 4-day heat waves with heat indexes at or above the 97.5th percentile (ranging from 94.8° to 109.4° F), compared with control days.
- The risk of fatal MI was 4% higher during 2-day cold snaps with temperatures at or below the 10th percentile (ranging from 33.3° to 40.5° F) and 12% higher during 3-day cold snaps with temperatures at or below the 2.5th percentile (ranging from 27.0° to 37.2° F).
- The risk of fatal MI was twice as high during 4-day heat waves that had PM2.5 above 37.5 mcg/m3. Days with high levels of PM2.5 during cold snaps did not have an equivalent increase in the risk of fatal MI.
- Up to 2.8% of MI deaths during the 5-year study period may be attributable to the combination of extreme temperature exposure and PM2.5 at levels exceeding World Health Organization air quality guidelines (37.5 mcg/m3).
- The risk of fatal MI was generally higher among women than men during heat waves and was higher among adults 80 years old and older than in younger adults during heat waves, cold snaps, or days with high levels of PM2.5.
The finding that adults over age 80 are particularly susceptible to the effects of heat and air pollution and the interaction of the two is “notable and particularly relevant given the aging of the population,” Dr. Newman told this news organization.
Mitigating both extreme temperature events and PM2.5 exposures “may bring health cobenefits in preventing premature deaths from MI,” the researchers write.
“To improve public health, it is important to take fine particulate pollution into consideration when providing extreme temperature warnings to the public,” Dr. Liu adds in the statement.
In an earlier study, Dr. Liu and colleagues showed that exposure to both large and small particulate matter, as well as nitrogen dioxide, was significantly associated with increased odds of death from MI.
This study was funded by China’s Ministry of Science and Technology. The authors and Dr. Newman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION