User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Kombucha benefits type 2 diabetes, study suggests
TOPLINE:
The sample size was too small for statistical significance.
METHODOLOGY:
- Prospective, randomized, double-blinded, crossover study at a single-center urban hospital system.
- A total of 12 participants with type 2 diabetes were randomly assigned to consume 240 mL of either a kombucha product or placebo daily with dinner for 4 weeks.
- After an 8-week washout, they were switched to the other product for another 4 weeks.
- Fasting blood glucose levels were self-determined at baseline and at 1 and 4 weeks, and questionnaires were used to assess secondary health outcomes.
- Questionnaire data were analyzed for all 12 participants, but only 7 who completed the study were included in the analysis of fasting blood glucose.
TAKEAWAY:
- Kombucha significantly lowered average fasting blood glucose levels at week 4, compared with baseline (164 vs. 116 mg/dL; P = .035), while the placebo was not associated with statistically significant change (162 vs. 141 mg/dL; P = .078).
- Among just the five participants with baseline fasting glucose > 130 mg/dL, kombucha consumption was associated with a mean fasting blood glucose decrease of 74.3 mg/dL, significantly greater than the 15.9 mg/dL drop with placebo (P = .017).
- On cultural enumeration, the kombucha contained mostly lactic acid bacteria, acetic acid bacteria, and yeast, with molds present.
IN PRACTICE:
“Kombucha is a growing part of the beverage market in the United States and the world, driven, in part, by the wide range of suggested health benefits. However, nearly all of these benefits are based on in vitro or animal studies, and human clinical trials are needed to validate biological outcomes.”
SOURCE:
The study was conducted by Chagai Mendelson, of MedStar Georgetown University Hospital, Washington, and colleagues. It was published in Frontiers in Nutrition.
LIMITATIONS:
- The number of participants was small, and attrition was high.
- Glucose levels were self-reported.
- Only one kombucha was studied.
DISCLOSURES:
One author is a cofounder of Synbiotic Health and another has a financial interest in the company. The other authors have no disclosures. Kombucha and placebo drinks were donated by Craft Kombucha, but the company did not have access to the data, and no authors have financial ties with that company.
A version of this article first appeared on Medscape.com.
TOPLINE:
The sample size was too small for statistical significance.
METHODOLOGY:
- Prospective, randomized, double-blinded, crossover study at a single-center urban hospital system.
- A total of 12 participants with type 2 diabetes were randomly assigned to consume 240 mL of either a kombucha product or placebo daily with dinner for 4 weeks.
- After an 8-week washout, they were switched to the other product for another 4 weeks.
- Fasting blood glucose levels were self-determined at baseline and at 1 and 4 weeks, and questionnaires were used to assess secondary health outcomes.
- Questionnaire data were analyzed for all 12 participants, but only 7 who completed the study were included in the analysis of fasting blood glucose.
TAKEAWAY:
- Kombucha significantly lowered average fasting blood glucose levels at week 4, compared with baseline (164 vs. 116 mg/dL; P = .035), while the placebo was not associated with statistically significant change (162 vs. 141 mg/dL; P = .078).
- Among just the five participants with baseline fasting glucose > 130 mg/dL, kombucha consumption was associated with a mean fasting blood glucose decrease of 74.3 mg/dL, significantly greater than the 15.9 mg/dL drop with placebo (P = .017).
- On cultural enumeration, the kombucha contained mostly lactic acid bacteria, acetic acid bacteria, and yeast, with molds present.
IN PRACTICE:
“Kombucha is a growing part of the beverage market in the United States and the world, driven, in part, by the wide range of suggested health benefits. However, nearly all of these benefits are based on in vitro or animal studies, and human clinical trials are needed to validate biological outcomes.”
SOURCE:
The study was conducted by Chagai Mendelson, of MedStar Georgetown University Hospital, Washington, and colleagues. It was published in Frontiers in Nutrition.
LIMITATIONS:
- The number of participants was small, and attrition was high.
- Glucose levels were self-reported.
- Only one kombucha was studied.
DISCLOSURES:
One author is a cofounder of Synbiotic Health and another has a financial interest in the company. The other authors have no disclosures. Kombucha and placebo drinks were donated by Craft Kombucha, but the company did not have access to the data, and no authors have financial ties with that company.
A version of this article first appeared on Medscape.com.
TOPLINE:
The sample size was too small for statistical significance.
METHODOLOGY:
- Prospective, randomized, double-blinded, crossover study at a single-center urban hospital system.
- A total of 12 participants with type 2 diabetes were randomly assigned to consume 240 mL of either a kombucha product or placebo daily with dinner for 4 weeks.
- After an 8-week washout, they were switched to the other product for another 4 weeks.
- Fasting blood glucose levels were self-determined at baseline and at 1 and 4 weeks, and questionnaires were used to assess secondary health outcomes.
- Questionnaire data were analyzed for all 12 participants, but only 7 who completed the study were included in the analysis of fasting blood glucose.
TAKEAWAY:
- Kombucha significantly lowered average fasting blood glucose levels at week 4, compared with baseline (164 vs. 116 mg/dL; P = .035), while the placebo was not associated with statistically significant change (162 vs. 141 mg/dL; P = .078).
- Among just the five participants with baseline fasting glucose > 130 mg/dL, kombucha consumption was associated with a mean fasting blood glucose decrease of 74.3 mg/dL, significantly greater than the 15.9 mg/dL drop with placebo (P = .017).
- On cultural enumeration, the kombucha contained mostly lactic acid bacteria, acetic acid bacteria, and yeast, with molds present.
IN PRACTICE:
“Kombucha is a growing part of the beverage market in the United States and the world, driven, in part, by the wide range of suggested health benefits. However, nearly all of these benefits are based on in vitro or animal studies, and human clinical trials are needed to validate biological outcomes.”
SOURCE:
The study was conducted by Chagai Mendelson, of MedStar Georgetown University Hospital, Washington, and colleagues. It was published in Frontiers in Nutrition.
LIMITATIONS:
- The number of participants was small, and attrition was high.
- Glucose levels were self-reported.
- Only one kombucha was studied.
DISCLOSURES:
One author is a cofounder of Synbiotic Health and another has a financial interest in the company. The other authors have no disclosures. Kombucha and placebo drinks were donated by Craft Kombucha, but the company did not have access to the data, and no authors have financial ties with that company.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN NUTRITION
The four questions you should ask about sexual health
This transcript has been edited for clarity.
When I went to med school, we were taught to take a sexual history. Do you smoke? Do you drink? Do you do drugs? Do you have sex? Men, women, or both? And that was it. We’re telling patients that sex is a vice, something that is dangerous and that you should feel bad about. But sex is how we’re all here and how we even continue as a species. We must get comfortable as doctors talking to our patients about sexual medicine.
What if we move away from sex being in the vice category – the part of the social history that’s the bad stuff you shouldn’t be doing? Maybe we should bring it into the review of systems.
As a very basic first step, I like to ask patients four things. As a sexual medicine doctor, I deal with these four things: libido, arousal, orgasm, and pain.
Why are these important? These are the things our patients really care about; 2.3 of every 1,000 people got divorced in 2021.
Libido. Women who have distressing low sexual desire have sex on average two and a half times per month. We call this mercy sex or duty sex. I don’t know what the half time per month looks like, but people genuinely care about desire and their doctors don’t really know that.
We have a biopsychosocial toolbox to help our patients. Let me give you an example: Antidepressants can have sexual side effects. Could there be medications in our toolbox that can help our patients? Of course there can, and there are. What about education or talk therapy? We should be asking our patients what they care about and why they care about it so we can help them achieve their quality-of-life goals.
Arousal. What about arousal? Did you know that erections are a marker of cardiovascular disease in men? We know this to be true for men, and I’m certain the research would be no different for women. We know that there are many biological causes for decrease in arousal, including sleep apnea, diabetes, hypertension, and smoking. I can convince a lot of men to quit smoking because I tell them it’s bad for their penis. We have to understand what our patients care about and then advise them on why we think we can help improve these issues.
Orgasm. How about orgasm? Have you ever been asked whether you can orgasm? Have you ever been asked whether you have questions about orgasm? About 15%-20% of women report having an orgasm disorder, and we rarely talk about this in an exam room. I’ve certainly never been asked, and everybody knows what I do for a living. Not to mention all the men that I and my colleagues see who have really distressing premature ejaculation or delayed orgasm. This is pathophysiology at its finest and most complex. It is so interesting, and we have so much to learn and understand about orgasm in general.
Pain. Finally, ask about pain. It seems obvious that we should be asking our patients about their pain, which includes pelvic pain, but oftentimes we avoid talking about private parts. Pain affects not just our patients, but also their partners and their families, when our patients can’t sit without discomfort, if they can’t go and perform the daily activities that bring them joy and belonging. We have to really work with our toolbox in a biopsychosocial manner to help our patients. I often use the incredible rehabilitation specialists called pelvic floor physical therapists.
Remember, we’re talking about libido, arousal, orgasm, and pain. Sex is important to us as a species. It’s important to our patients. Ask nonjudgmental and open-ended questions. You actually may be the only doctor to ever do so.
Dr. Rubin is an assistant clinical professor, department of urology, Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I went to med school, we were taught to take a sexual history. Do you smoke? Do you drink? Do you do drugs? Do you have sex? Men, women, or both? And that was it. We’re telling patients that sex is a vice, something that is dangerous and that you should feel bad about. But sex is how we’re all here and how we even continue as a species. We must get comfortable as doctors talking to our patients about sexual medicine.
What if we move away from sex being in the vice category – the part of the social history that’s the bad stuff you shouldn’t be doing? Maybe we should bring it into the review of systems.
As a very basic first step, I like to ask patients four things. As a sexual medicine doctor, I deal with these four things: libido, arousal, orgasm, and pain.
Why are these important? These are the things our patients really care about; 2.3 of every 1,000 people got divorced in 2021.
Libido. Women who have distressing low sexual desire have sex on average two and a half times per month. We call this mercy sex or duty sex. I don’t know what the half time per month looks like, but people genuinely care about desire and their doctors don’t really know that.
We have a biopsychosocial toolbox to help our patients. Let me give you an example: Antidepressants can have sexual side effects. Could there be medications in our toolbox that can help our patients? Of course there can, and there are. What about education or talk therapy? We should be asking our patients what they care about and why they care about it so we can help them achieve their quality-of-life goals.
Arousal. What about arousal? Did you know that erections are a marker of cardiovascular disease in men? We know this to be true for men, and I’m certain the research would be no different for women. We know that there are many biological causes for decrease in arousal, including sleep apnea, diabetes, hypertension, and smoking. I can convince a lot of men to quit smoking because I tell them it’s bad for their penis. We have to understand what our patients care about and then advise them on why we think we can help improve these issues.
Orgasm. How about orgasm? Have you ever been asked whether you can orgasm? Have you ever been asked whether you have questions about orgasm? About 15%-20% of women report having an orgasm disorder, and we rarely talk about this in an exam room. I’ve certainly never been asked, and everybody knows what I do for a living. Not to mention all the men that I and my colleagues see who have really distressing premature ejaculation or delayed orgasm. This is pathophysiology at its finest and most complex. It is so interesting, and we have so much to learn and understand about orgasm in general.
Pain. Finally, ask about pain. It seems obvious that we should be asking our patients about their pain, which includes pelvic pain, but oftentimes we avoid talking about private parts. Pain affects not just our patients, but also their partners and their families, when our patients can’t sit without discomfort, if they can’t go and perform the daily activities that bring them joy and belonging. We have to really work with our toolbox in a biopsychosocial manner to help our patients. I often use the incredible rehabilitation specialists called pelvic floor physical therapists.
Remember, we’re talking about libido, arousal, orgasm, and pain. Sex is important to us as a species. It’s important to our patients. Ask nonjudgmental and open-ended questions. You actually may be the only doctor to ever do so.
Dr. Rubin is an assistant clinical professor, department of urology, Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When I went to med school, we were taught to take a sexual history. Do you smoke? Do you drink? Do you do drugs? Do you have sex? Men, women, or both? And that was it. We’re telling patients that sex is a vice, something that is dangerous and that you should feel bad about. But sex is how we’re all here and how we even continue as a species. We must get comfortable as doctors talking to our patients about sexual medicine.
What if we move away from sex being in the vice category – the part of the social history that’s the bad stuff you shouldn’t be doing? Maybe we should bring it into the review of systems.
As a very basic first step, I like to ask patients four things. As a sexual medicine doctor, I deal with these four things: libido, arousal, orgasm, and pain.
Why are these important? These are the things our patients really care about; 2.3 of every 1,000 people got divorced in 2021.
Libido. Women who have distressing low sexual desire have sex on average two and a half times per month. We call this mercy sex or duty sex. I don’t know what the half time per month looks like, but people genuinely care about desire and their doctors don’t really know that.
We have a biopsychosocial toolbox to help our patients. Let me give you an example: Antidepressants can have sexual side effects. Could there be medications in our toolbox that can help our patients? Of course there can, and there are. What about education or talk therapy? We should be asking our patients what they care about and why they care about it so we can help them achieve their quality-of-life goals.
Arousal. What about arousal? Did you know that erections are a marker of cardiovascular disease in men? We know this to be true for men, and I’m certain the research would be no different for women. We know that there are many biological causes for decrease in arousal, including sleep apnea, diabetes, hypertension, and smoking. I can convince a lot of men to quit smoking because I tell them it’s bad for their penis. We have to understand what our patients care about and then advise them on why we think we can help improve these issues.
Orgasm. How about orgasm? Have you ever been asked whether you can orgasm? Have you ever been asked whether you have questions about orgasm? About 15%-20% of women report having an orgasm disorder, and we rarely talk about this in an exam room. I’ve certainly never been asked, and everybody knows what I do for a living. Not to mention all the men that I and my colleagues see who have really distressing premature ejaculation or delayed orgasm. This is pathophysiology at its finest and most complex. It is so interesting, and we have so much to learn and understand about orgasm in general.
Pain. Finally, ask about pain. It seems obvious that we should be asking our patients about their pain, which includes pelvic pain, but oftentimes we avoid talking about private parts. Pain affects not just our patients, but also their partners and their families, when our patients can’t sit without discomfort, if they can’t go and perform the daily activities that bring them joy and belonging. We have to really work with our toolbox in a biopsychosocial manner to help our patients. I often use the incredible rehabilitation specialists called pelvic floor physical therapists.
Remember, we’re talking about libido, arousal, orgasm, and pain. Sex is important to us as a species. It’s important to our patients. Ask nonjudgmental and open-ended questions. You actually may be the only doctor to ever do so.
Dr. Rubin is an assistant clinical professor, department of urology, Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
New and emerging options for treating recurrent C. difficile
This transcript has been edited for clarity.
Clostridioides difficile is a toxin-based infection that takes up residence in the colon due to disturbed normal bowel flora, usually after antibiotics.
Recurrent C. difficile can happen in up to a quarter of patients who receive oral vancomycin as a treatment for their infection. It can also occur with treatment with the newer agent, fidaxomicin, although possibly in fewer patients. In general, relapses are indeed common.
When I trained at Johns Hopkins under John Bartlett, he took the approach that after the second – and always after the third – relapse, an extended course of oral therapy with vancomycin could help get patients out of trouble. He used the so-called extended pulse method, where patients would take the drug for approximately 4-6 weeks and gradually reduce the dose.
This approach can also be done with fidaxomicin. However, I’m not sure it works much better than vancomycin, and there are often hurdles to using fidaxomicin because of insurers not approving it because of the expense.
What other therapies are there?
There is bezlotoxumab, which is a human monoclonal antibody targeting C. difficile toxin B. I’ve used it a few times. It is given as a one-time infusion, and there are challenges regarding cost, the logistics of setting up the infusion, and insurance approval.
Fecal microbiota transplant
In recent years, fecal microbiota transplants (FMT) have received a lot of attention as a different avenue of treatment that could lower the potential for relapses, with success rates usually around 80%-90%. However, in the past few years, there have been some serious safety signals because of possible transmission of dangerous pathogens, often with drug resistance, with FMT.
I’m therefore pleased to say that newer fecal microbiota products are coming in fast and furious. I thought I’d spend a few minutes speaking about these.
OpenBiome, an organization dedicated to microbiome research, offers an investigational product from screened donors that has not received Food and Drug Administration approval. It’s been around for some time. It can be used in either upper or lower GI applications, and the organization cites about an 84% success rate using this product.
There are also two new FDA-approved products I think are worth knowing about. They’ve just been approved recently and we’re a little uncertain of where they’re going to end up in the treatment landscape.
The first is from Ferring, and it goes by fecal microbiota, live-jslm (Rebyota). This is a product from qualified and screened donors, the main component of which is Bacteroides, which is given as a single dose by enema.
The company did a phase 3 trial with a Bayesian primary analysis, which I think convinced the FDA to approve this product. The success rate in people with multiple relapses was 70.6%, compared with 57.5% with placebo. The estimated treatment effect was 13.1%. Of those who did respond, over 90% were kept free of relapse over a 6-month period.
The other product, also FDA approved, is from Seres. It was previously called SER-109, and is now called fecal microbiota spores, live-brpk (Vowst). Unlike the previous product, this is orally administered, with patients taking four capsules daily for 3 days. Again, these donor-derived firmicutes have been appropriately screened and are free of potential pathogens.
The phase 3 randomized clinical trial results were published in the New England Journal of Medicine. They showed that 12% of those taking this product had a relapse, compared with 40% of those taking placebo, which is about the range we tend to see in people who have had multiple relapses. The safety profile was similar to placebo.
So, how will people use these treatments?
I think the FDA imprimatur will be attractive to people, but the products, I believe, will be priced fairly expensively, in the under $10,000 range. The first (Rebyota) is a rectal infusion; it is a one-and-done treatment but creates logistical issues. Interestingly, it could be a billable procedure for infectious disease clinicians. The ease of oral administration for Vowst, no doubt, will be very appealing. Both of these are given after completing a course of treatment with vancomycin or fidaxomicin so as not to interfere with the microbiome product.
I’ll also briefly mention a paper published in JAMA on yet another microbiome product, called VE303. This product was based on eight commensal strains of Clostridia and was given orally in a phase 2 trial. Interestingly, this worked about the same as the oral product that is already FDA approved. The study showed a recurrence rate of 13.8% in the high-dose group, compared with 45.5% in the placebo group.
I think this is exciting. And, of course, there is the expense.
But anything that can be done to help improve these patients is welcome, as once they get into the multiple-relapse phase, it is challenging to turn around. These commercialized products will hopefully become a bit more mainstream. Certainly, we’ll see how these will be utilized in the coming months and over the next few years.
Dr. Auwaerter is Clinical Director, Division of Infectious Diseases, Johns Hopkins University, Baltimore. He reported conflicts of interest with Gilead, Shionogi, and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Clostridioides difficile is a toxin-based infection that takes up residence in the colon due to disturbed normal bowel flora, usually after antibiotics.
Recurrent C. difficile can happen in up to a quarter of patients who receive oral vancomycin as a treatment for their infection. It can also occur with treatment with the newer agent, fidaxomicin, although possibly in fewer patients. In general, relapses are indeed common.
When I trained at Johns Hopkins under John Bartlett, he took the approach that after the second – and always after the third – relapse, an extended course of oral therapy with vancomycin could help get patients out of trouble. He used the so-called extended pulse method, where patients would take the drug for approximately 4-6 weeks and gradually reduce the dose.
This approach can also be done with fidaxomicin. However, I’m not sure it works much better than vancomycin, and there are often hurdles to using fidaxomicin because of insurers not approving it because of the expense.
What other therapies are there?
There is bezlotoxumab, which is a human monoclonal antibody targeting C. difficile toxin B. I’ve used it a few times. It is given as a one-time infusion, and there are challenges regarding cost, the logistics of setting up the infusion, and insurance approval.
Fecal microbiota transplant
In recent years, fecal microbiota transplants (FMT) have received a lot of attention as a different avenue of treatment that could lower the potential for relapses, with success rates usually around 80%-90%. However, in the past few years, there have been some serious safety signals because of possible transmission of dangerous pathogens, often with drug resistance, with FMT.
I’m therefore pleased to say that newer fecal microbiota products are coming in fast and furious. I thought I’d spend a few minutes speaking about these.
OpenBiome, an organization dedicated to microbiome research, offers an investigational product from screened donors that has not received Food and Drug Administration approval. It’s been around for some time. It can be used in either upper or lower GI applications, and the organization cites about an 84% success rate using this product.
There are also two new FDA-approved products I think are worth knowing about. They’ve just been approved recently and we’re a little uncertain of where they’re going to end up in the treatment landscape.
The first is from Ferring, and it goes by fecal microbiota, live-jslm (Rebyota). This is a product from qualified and screened donors, the main component of which is Bacteroides, which is given as a single dose by enema.
The company did a phase 3 trial with a Bayesian primary analysis, which I think convinced the FDA to approve this product. The success rate in people with multiple relapses was 70.6%, compared with 57.5% with placebo. The estimated treatment effect was 13.1%. Of those who did respond, over 90% were kept free of relapse over a 6-month period.
The other product, also FDA approved, is from Seres. It was previously called SER-109, and is now called fecal microbiota spores, live-brpk (Vowst). Unlike the previous product, this is orally administered, with patients taking four capsules daily for 3 days. Again, these donor-derived firmicutes have been appropriately screened and are free of potential pathogens.
The phase 3 randomized clinical trial results were published in the New England Journal of Medicine. They showed that 12% of those taking this product had a relapse, compared with 40% of those taking placebo, which is about the range we tend to see in people who have had multiple relapses. The safety profile was similar to placebo.
So, how will people use these treatments?
I think the FDA imprimatur will be attractive to people, but the products, I believe, will be priced fairly expensively, in the under $10,000 range. The first (Rebyota) is a rectal infusion; it is a one-and-done treatment but creates logistical issues. Interestingly, it could be a billable procedure for infectious disease clinicians. The ease of oral administration for Vowst, no doubt, will be very appealing. Both of these are given after completing a course of treatment with vancomycin or fidaxomicin so as not to interfere with the microbiome product.
I’ll also briefly mention a paper published in JAMA on yet another microbiome product, called VE303. This product was based on eight commensal strains of Clostridia and was given orally in a phase 2 trial. Interestingly, this worked about the same as the oral product that is already FDA approved. The study showed a recurrence rate of 13.8% in the high-dose group, compared with 45.5% in the placebo group.
I think this is exciting. And, of course, there is the expense.
But anything that can be done to help improve these patients is welcome, as once they get into the multiple-relapse phase, it is challenging to turn around. These commercialized products will hopefully become a bit more mainstream. Certainly, we’ll see how these will be utilized in the coming months and over the next few years.
Dr. Auwaerter is Clinical Director, Division of Infectious Diseases, Johns Hopkins University, Baltimore. He reported conflicts of interest with Gilead, Shionogi, and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Clostridioides difficile is a toxin-based infection that takes up residence in the colon due to disturbed normal bowel flora, usually after antibiotics.
Recurrent C. difficile can happen in up to a quarter of patients who receive oral vancomycin as a treatment for their infection. It can also occur with treatment with the newer agent, fidaxomicin, although possibly in fewer patients. In general, relapses are indeed common.
When I trained at Johns Hopkins under John Bartlett, he took the approach that after the second – and always after the third – relapse, an extended course of oral therapy with vancomycin could help get patients out of trouble. He used the so-called extended pulse method, where patients would take the drug for approximately 4-6 weeks and gradually reduce the dose.
This approach can also be done with fidaxomicin. However, I’m not sure it works much better than vancomycin, and there are often hurdles to using fidaxomicin because of insurers not approving it because of the expense.
What other therapies are there?
There is bezlotoxumab, which is a human monoclonal antibody targeting C. difficile toxin B. I’ve used it a few times. It is given as a one-time infusion, and there are challenges regarding cost, the logistics of setting up the infusion, and insurance approval.
Fecal microbiota transplant
In recent years, fecal microbiota transplants (FMT) have received a lot of attention as a different avenue of treatment that could lower the potential for relapses, with success rates usually around 80%-90%. However, in the past few years, there have been some serious safety signals because of possible transmission of dangerous pathogens, often with drug resistance, with FMT.
I’m therefore pleased to say that newer fecal microbiota products are coming in fast and furious. I thought I’d spend a few minutes speaking about these.
OpenBiome, an organization dedicated to microbiome research, offers an investigational product from screened donors that has not received Food and Drug Administration approval. It’s been around for some time. It can be used in either upper or lower GI applications, and the organization cites about an 84% success rate using this product.
There are also two new FDA-approved products I think are worth knowing about. They’ve just been approved recently and we’re a little uncertain of where they’re going to end up in the treatment landscape.
The first is from Ferring, and it goes by fecal microbiota, live-jslm (Rebyota). This is a product from qualified and screened donors, the main component of which is Bacteroides, which is given as a single dose by enema.
The company did a phase 3 trial with a Bayesian primary analysis, which I think convinced the FDA to approve this product. The success rate in people with multiple relapses was 70.6%, compared with 57.5% with placebo. The estimated treatment effect was 13.1%. Of those who did respond, over 90% were kept free of relapse over a 6-month period.
The other product, also FDA approved, is from Seres. It was previously called SER-109, and is now called fecal microbiota spores, live-brpk (Vowst). Unlike the previous product, this is orally administered, with patients taking four capsules daily for 3 days. Again, these donor-derived firmicutes have been appropriately screened and are free of potential pathogens.
The phase 3 randomized clinical trial results were published in the New England Journal of Medicine. They showed that 12% of those taking this product had a relapse, compared with 40% of those taking placebo, which is about the range we tend to see in people who have had multiple relapses. The safety profile was similar to placebo.
So, how will people use these treatments?
I think the FDA imprimatur will be attractive to people, but the products, I believe, will be priced fairly expensively, in the under $10,000 range. The first (Rebyota) is a rectal infusion; it is a one-and-done treatment but creates logistical issues. Interestingly, it could be a billable procedure for infectious disease clinicians. The ease of oral administration for Vowst, no doubt, will be very appealing. Both of these are given after completing a course of treatment with vancomycin or fidaxomicin so as not to interfere with the microbiome product.
I’ll also briefly mention a paper published in JAMA on yet another microbiome product, called VE303. This product was based on eight commensal strains of Clostridia and was given orally in a phase 2 trial. Interestingly, this worked about the same as the oral product that is already FDA approved. The study showed a recurrence rate of 13.8% in the high-dose group, compared with 45.5% in the placebo group.
I think this is exciting. And, of course, there is the expense.
But anything that can be done to help improve these patients is welcome, as once they get into the multiple-relapse phase, it is challenging to turn around. These commercialized products will hopefully become a bit more mainstream. Certainly, we’ll see how these will be utilized in the coming months and over the next few years.
Dr. Auwaerter is Clinical Director, Division of Infectious Diseases, Johns Hopkins University, Baltimore. He reported conflicts of interest with Gilead, Shionogi, and Medscape.
A version of this article first appeared on Medscape.com.
FDA clears AI-assisted colonoscopy device
, according to the Israeli-based manufacturer of the same name.
The device helps identify lesions in real time and is associated with a significant increase in the adenoma detection rate (ADR), according to the press release.
The device was cleared under the FDA’s 510(k) process, and follows the European CE Mark and Israel AMAR approval, which were received in mid-2021. It will be available in the United States in the coming weeks.
In a study performed in 2022 with 29 endoscopy experts and more than 950 patients, the device was validated as “one of the best-performing AI solutions in the category, increasing ADR by 26% relatively (7% in absolute values), which translated into a 21% decrease in colorectal cancer occurrence and a 35% decrease in patient mortality,” according to the press release.
In this multicenter, randomized, controlled trial conducted at 10 hospitals in Europe, the United States, and Israel, and presented at United European Gastroenterology Week 2022, the authors noted that “apart from diminutive lesions, [MAGENTIQ-COLO] increased the detection of 6- to 9-mm adenomas, suggesting that this novel [computer-aided polyp detection] system is also able to detect more clinically relevant lesions.”
The device “takes the video out of the colonoscopy device, breaks it into frames, and analyzes them in real time with its AI engine to detect polyps in them,” Dror Zur, founder and CEO of MAGENTIQ-EYE, explained in an interview. “If a polyp is detected, then MAGENTIQ-COLO signs it with a bounding box on the video’s overlay and sends it as a video with an overlay to the display monitor so the doctor can look at it and find more polyps.”
As previously reported by this news organization, research has shown that conventional colonoscopies miss about a quarter of adenomas. Many AI systems have recently come on the market, promising to improve detection by overcoming human error in detecting polyps.
Colonoscopy has become standard in most developed countries, with 15-20 million procedures performed every year in the United States alone; however, high missed rates and undetected adenomas during the procedures mean that even patients who get regular, recommended screenings are still at risk of developing colon cancer, notes the press release.
“A missed polyp can lead to interval cancer, which accounts for approximately 8%-10% of all CRC in the U.S., translated to over 13,500 cancer cases that could be prevented every year with better detection,” the press release also states.
According to the National Institutes of Health, colorectal cancer is the third leading cause of cancer-related death in the United States.
A version of this article first appeared on Medscape.com.
, according to the Israeli-based manufacturer of the same name.
The device helps identify lesions in real time and is associated with a significant increase in the adenoma detection rate (ADR), according to the press release.
The device was cleared under the FDA’s 510(k) process, and follows the European CE Mark and Israel AMAR approval, which were received in mid-2021. It will be available in the United States in the coming weeks.
In a study performed in 2022 with 29 endoscopy experts and more than 950 patients, the device was validated as “one of the best-performing AI solutions in the category, increasing ADR by 26% relatively (7% in absolute values), which translated into a 21% decrease in colorectal cancer occurrence and a 35% decrease in patient mortality,” according to the press release.
In this multicenter, randomized, controlled trial conducted at 10 hospitals in Europe, the United States, and Israel, and presented at United European Gastroenterology Week 2022, the authors noted that “apart from diminutive lesions, [MAGENTIQ-COLO] increased the detection of 6- to 9-mm adenomas, suggesting that this novel [computer-aided polyp detection] system is also able to detect more clinically relevant lesions.”
The device “takes the video out of the colonoscopy device, breaks it into frames, and analyzes them in real time with its AI engine to detect polyps in them,” Dror Zur, founder and CEO of MAGENTIQ-EYE, explained in an interview. “If a polyp is detected, then MAGENTIQ-COLO signs it with a bounding box on the video’s overlay and sends it as a video with an overlay to the display monitor so the doctor can look at it and find more polyps.”
As previously reported by this news organization, research has shown that conventional colonoscopies miss about a quarter of adenomas. Many AI systems have recently come on the market, promising to improve detection by overcoming human error in detecting polyps.
Colonoscopy has become standard in most developed countries, with 15-20 million procedures performed every year in the United States alone; however, high missed rates and undetected adenomas during the procedures mean that even patients who get regular, recommended screenings are still at risk of developing colon cancer, notes the press release.
“A missed polyp can lead to interval cancer, which accounts for approximately 8%-10% of all CRC in the U.S., translated to over 13,500 cancer cases that could be prevented every year with better detection,” the press release also states.
According to the National Institutes of Health, colorectal cancer is the third leading cause of cancer-related death in the United States.
A version of this article first appeared on Medscape.com.
, according to the Israeli-based manufacturer of the same name.
The device helps identify lesions in real time and is associated with a significant increase in the adenoma detection rate (ADR), according to the press release.
The device was cleared under the FDA’s 510(k) process, and follows the European CE Mark and Israel AMAR approval, which were received in mid-2021. It will be available in the United States in the coming weeks.
In a study performed in 2022 with 29 endoscopy experts and more than 950 patients, the device was validated as “one of the best-performing AI solutions in the category, increasing ADR by 26% relatively (7% in absolute values), which translated into a 21% decrease in colorectal cancer occurrence and a 35% decrease in patient mortality,” according to the press release.
In this multicenter, randomized, controlled trial conducted at 10 hospitals in Europe, the United States, and Israel, and presented at United European Gastroenterology Week 2022, the authors noted that “apart from diminutive lesions, [MAGENTIQ-COLO] increased the detection of 6- to 9-mm adenomas, suggesting that this novel [computer-aided polyp detection] system is also able to detect more clinically relevant lesions.”
The device “takes the video out of the colonoscopy device, breaks it into frames, and analyzes them in real time with its AI engine to detect polyps in them,” Dror Zur, founder and CEO of MAGENTIQ-EYE, explained in an interview. “If a polyp is detected, then MAGENTIQ-COLO signs it with a bounding box on the video’s overlay and sends it as a video with an overlay to the display monitor so the doctor can look at it and find more polyps.”
As previously reported by this news organization, research has shown that conventional colonoscopies miss about a quarter of adenomas. Many AI systems have recently come on the market, promising to improve detection by overcoming human error in detecting polyps.
Colonoscopy has become standard in most developed countries, with 15-20 million procedures performed every year in the United States alone; however, high missed rates and undetected adenomas during the procedures mean that even patients who get regular, recommended screenings are still at risk of developing colon cancer, notes the press release.
“A missed polyp can lead to interval cancer, which accounts for approximately 8%-10% of all CRC in the U.S., translated to over 13,500 cancer cases that could be prevented every year with better detection,” the press release also states.
According to the National Institutes of Health, colorectal cancer is the third leading cause of cancer-related death in the United States.
A version of this article first appeared on Medscape.com.
Injecting long-acting antiretrovirals into clinic care
At the Whitman-Walker Health Center, Washington, community health workers see about 3,200 antiretroviral users a year. With long-acting injections now available, the clinic opted to integrate the new medications into its peer staff program.
“Our peer workers are very competent,” said Rupa Patel, MD, MPH, medical liason of the pre-exposure prophylaxis for HIV prevention program at Washington University at St. Louis.* “They do phlebotomy, they give you your meds. They’re your main doctor until you really need to see the doctor.”
In the peer staff program, workers are trained in a 4-month medical residency–style program that shows them how to test for HIV, inject long-acting formulations of new drugs, and conduct follow-up visits.
Presenting the new approach at the International AIDS Society Conference on HIV Science, Dr. Patel reported that 139 people have received long-acting injections at the clinic since the program launched with a total of 314 injections administered.
The training program includes lectures, mock injection, and client care sessions, observation and supervised administration, a written exam, and case review sessions.
Retention for the second injection was 95%, with 91% of injections given within the 14-day window. For the third injection, retention was 91%, with 63% given within the window.
The program reports a high level of client satisfaction with the peer-administered injections, which are also given in a room decorated with a beach theme and music to help calm people who might be nervous of receiving shots.
“Our retention is going to be the highest compared to other clinics because your peer, your friend, is reminding you and comforting you and telling you: ‘Don’t worry, I’m on the injection too,’ ” Dr. Patel said.
Andrew Grulich, MD, PhD, head of the HIV epidemiology and prevention program at the Kirby Institute, Sydney, pointed out there is tension between wanting to use long-acting injectables for people who are struggling with taking oral therapies daily and the need to ensure that they come back for their injections on time.
“I think it’s a potential way forward – we’re learning as we’re going with these new forms of therapy,” he said in an interview. “It is absolutely critical that people turn up on time for those injections, and if they don’t, resistance can be an issue.”
Presenting new data from another project at the HIV Clinic at San Francisco General Hospital, Monica Gandhi, MD, MPH, told the conference: “There are multiple reasons why it’s hard to take oral antiretrovirals every day.”
At the HIV Clinic in San Francisco General, people without homes, those with mental illness, and those using stimulants receive care.
The clinical trials for long-acting injectable antiretrovirals included only people who were virologically suppressed, which is also the Food and Drug Administration criteria for use. However, this clinic offered long-acting injections to patients with viremia because it was too difficult for them to take a daily pill.
In a comment, Dr. Gandhi, director of the University of California, San Francisco’s Center for AIDS Research, said: “We don’t call people hard to reach, we call them hardly reached because it’s not their fault.” There are just all of these issues that have made it harder for them to take medication consistently.
Dr. Gandhi reported that, of the 133 people being treated with long-acting injectable cabotegravir and rilpivirine at the clinic through this program, 57 had viremia at baseline.
However, only two of these patients experienced virologic failure while on the injectable antiretroviral program. The overall virologic failure rate was 1.5%, which was equivalent to that seen in clinical trials in virologically suppressed individuals.
The results presented at the conference and were also published in Annals of Internal Medicine.
The clinic found that 73% of people attended their injection appointments on time, and those who did not were followed up with telephone calls to ensure they received their injection within the 14-day window.
Dr. Gandhi said people were highly motivated to turn up for their injection appointments. “They are virologically suppressed, so it feels so amazing. They’re self-motivated for the first time to want to get an injection.”
A version of this article first appeared on Medscape.com.
*Correction, 8/4/23: An earlier version of this article misstated Dr. Patel's university affiliation.
At the Whitman-Walker Health Center, Washington, community health workers see about 3,200 antiretroviral users a year. With long-acting injections now available, the clinic opted to integrate the new medications into its peer staff program.
“Our peer workers are very competent,” said Rupa Patel, MD, MPH, medical liason of the pre-exposure prophylaxis for HIV prevention program at Washington University at St. Louis.* “They do phlebotomy, they give you your meds. They’re your main doctor until you really need to see the doctor.”
In the peer staff program, workers are trained in a 4-month medical residency–style program that shows them how to test for HIV, inject long-acting formulations of new drugs, and conduct follow-up visits.
Presenting the new approach at the International AIDS Society Conference on HIV Science, Dr. Patel reported that 139 people have received long-acting injections at the clinic since the program launched with a total of 314 injections administered.
The training program includes lectures, mock injection, and client care sessions, observation and supervised administration, a written exam, and case review sessions.
Retention for the second injection was 95%, with 91% of injections given within the 14-day window. For the third injection, retention was 91%, with 63% given within the window.
The program reports a high level of client satisfaction with the peer-administered injections, which are also given in a room decorated with a beach theme and music to help calm people who might be nervous of receiving shots.
“Our retention is going to be the highest compared to other clinics because your peer, your friend, is reminding you and comforting you and telling you: ‘Don’t worry, I’m on the injection too,’ ” Dr. Patel said.
Andrew Grulich, MD, PhD, head of the HIV epidemiology and prevention program at the Kirby Institute, Sydney, pointed out there is tension between wanting to use long-acting injectables for people who are struggling with taking oral therapies daily and the need to ensure that they come back for their injections on time.
“I think it’s a potential way forward – we’re learning as we’re going with these new forms of therapy,” he said in an interview. “It is absolutely critical that people turn up on time for those injections, and if they don’t, resistance can be an issue.”
Presenting new data from another project at the HIV Clinic at San Francisco General Hospital, Monica Gandhi, MD, MPH, told the conference: “There are multiple reasons why it’s hard to take oral antiretrovirals every day.”
At the HIV Clinic in San Francisco General, people without homes, those with mental illness, and those using stimulants receive care.
The clinical trials for long-acting injectable antiretrovirals included only people who were virologically suppressed, which is also the Food and Drug Administration criteria for use. However, this clinic offered long-acting injections to patients with viremia because it was too difficult for them to take a daily pill.
In a comment, Dr. Gandhi, director of the University of California, San Francisco’s Center for AIDS Research, said: “We don’t call people hard to reach, we call them hardly reached because it’s not their fault.” There are just all of these issues that have made it harder for them to take medication consistently.
Dr. Gandhi reported that, of the 133 people being treated with long-acting injectable cabotegravir and rilpivirine at the clinic through this program, 57 had viremia at baseline.
However, only two of these patients experienced virologic failure while on the injectable antiretroviral program. The overall virologic failure rate was 1.5%, which was equivalent to that seen in clinical trials in virologically suppressed individuals.
The results presented at the conference and were also published in Annals of Internal Medicine.
The clinic found that 73% of people attended their injection appointments on time, and those who did not were followed up with telephone calls to ensure they received their injection within the 14-day window.
Dr. Gandhi said people were highly motivated to turn up for their injection appointments. “They are virologically suppressed, so it feels so amazing. They’re self-motivated for the first time to want to get an injection.”
A version of this article first appeared on Medscape.com.
*Correction, 8/4/23: An earlier version of this article misstated Dr. Patel's university affiliation.
At the Whitman-Walker Health Center, Washington, community health workers see about 3,200 antiretroviral users a year. With long-acting injections now available, the clinic opted to integrate the new medications into its peer staff program.
“Our peer workers are very competent,” said Rupa Patel, MD, MPH, medical liason of the pre-exposure prophylaxis for HIV prevention program at Washington University at St. Louis.* “They do phlebotomy, they give you your meds. They’re your main doctor until you really need to see the doctor.”
In the peer staff program, workers are trained in a 4-month medical residency–style program that shows them how to test for HIV, inject long-acting formulations of new drugs, and conduct follow-up visits.
Presenting the new approach at the International AIDS Society Conference on HIV Science, Dr. Patel reported that 139 people have received long-acting injections at the clinic since the program launched with a total of 314 injections administered.
The training program includes lectures, mock injection, and client care sessions, observation and supervised administration, a written exam, and case review sessions.
Retention for the second injection was 95%, with 91% of injections given within the 14-day window. For the third injection, retention was 91%, with 63% given within the window.
The program reports a high level of client satisfaction with the peer-administered injections, which are also given in a room decorated with a beach theme and music to help calm people who might be nervous of receiving shots.
“Our retention is going to be the highest compared to other clinics because your peer, your friend, is reminding you and comforting you and telling you: ‘Don’t worry, I’m on the injection too,’ ” Dr. Patel said.
Andrew Grulich, MD, PhD, head of the HIV epidemiology and prevention program at the Kirby Institute, Sydney, pointed out there is tension between wanting to use long-acting injectables for people who are struggling with taking oral therapies daily and the need to ensure that they come back for their injections on time.
“I think it’s a potential way forward – we’re learning as we’re going with these new forms of therapy,” he said in an interview. “It is absolutely critical that people turn up on time for those injections, and if they don’t, resistance can be an issue.”
Presenting new data from another project at the HIV Clinic at San Francisco General Hospital, Monica Gandhi, MD, MPH, told the conference: “There are multiple reasons why it’s hard to take oral antiretrovirals every day.”
At the HIV Clinic in San Francisco General, people without homes, those with mental illness, and those using stimulants receive care.
The clinical trials for long-acting injectable antiretrovirals included only people who were virologically suppressed, which is also the Food and Drug Administration criteria for use. However, this clinic offered long-acting injections to patients with viremia because it was too difficult for them to take a daily pill.
In a comment, Dr. Gandhi, director of the University of California, San Francisco’s Center for AIDS Research, said: “We don’t call people hard to reach, we call them hardly reached because it’s not their fault.” There are just all of these issues that have made it harder for them to take medication consistently.
Dr. Gandhi reported that, of the 133 people being treated with long-acting injectable cabotegravir and rilpivirine at the clinic through this program, 57 had viremia at baseline.
However, only two of these patients experienced virologic failure while on the injectable antiretroviral program. The overall virologic failure rate was 1.5%, which was equivalent to that seen in clinical trials in virologically suppressed individuals.
The results presented at the conference and were also published in Annals of Internal Medicine.
The clinic found that 73% of people attended their injection appointments on time, and those who did not were followed up with telephone calls to ensure they received their injection within the 14-day window.
Dr. Gandhi said people were highly motivated to turn up for their injection appointments. “They are virologically suppressed, so it feels so amazing. They’re self-motivated for the first time to want to get an injection.”
A version of this article first appeared on Medscape.com.
*Correction, 8/4/23: An earlier version of this article misstated Dr. Patel's university affiliation.
FROM IAS 2023
UNAIDS targets: Progress reported, but ‘HIV is far from over’
BRISBANE, AUSTRALIA – The year was 1987 and the Grim Reaper (a personification of death), holding a large scythe, rolled a 10-pin bowling ball through a dark, foggy place. In the advertisement on television, the cloaked skeleton aimed the bowling ball at the other end of a lane where a group of people stood in place of pins.
Who would fall next?
In the 1980s, cases of HIV were rising in the community and people in Australia and elsewhere were dying of AIDS. The Australian government opted to use mainstream media to deliver a blunt message through advertising to raise awareness about the health risk and how to manage HIV in the community.
But the campaign also contributed to stigma for those living with the disease and especially those in the gay community who felt ostracized by rising public concern.
In the inner city of Sydney, a few thousand people died of AIDS, Andrew Grulich, MD, PhD, from the Kirby Institute at the University of New South Wales, Sydney, and involved in tracking cases, said in an interview. “Sydney was devastated by AIDS, it was truly devastated.”
HIV and AIDS quickly became an even more severe problem for several countries around Australia in Thailand, Papua New Guinea, and beyond. After HIV was first reported in Thailand in 1984, the region had the highest prevalence of HIV in Southeast Asia. Through the 1990s in Papua New Guinea, HIV prevalence rose steeply as well.
By 2010, the Joint United Nations Programme on HIV/AIDS (UNAIDS) set a target of a 90% reduction in HIV incidence, a 90% reduction in AIDS deaths by 2030, and 95% of people living with HIV and AIDS being aware of their status, on treatment, and having an undetectable viral load.
Since then, significant progress has been made globally with 86% of people knowing their HIV status. However, new infections persist at a rate that has not dropped as fast as possible.
New infections
According to the latest UNAIDS report, regions of North America and western and central Europe showed a 23% decline in new infections from 2010 to 2022, below the target 90% reduction.
Some regions of the United States have seen significant declines in new HIV infections. San Francisco has a 67% drop in new diagnoses. And now, along with the District of Columbia, the four states with the highest HIV rates are New York, Maryland, Georgia, and Florida.
Several countries in eastern and southern Africa are close to achieving their target HIV reduction of 90%.
Mitchell Warren, executive director of AVAC for global health advocacy, access, and equity, said that many of the low- and middle-income countries that are on track to achieve targets are able to do so because of support from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund to Fight AIDS, Tuberculosis, and Malaria.
“That foreign development assistance is transforming the AIDS response in a number of African countries, and yet at home, in various states and municipalities, not only are we not reaching that effort, we don’t even use those targets,” Mr. Warren pointed out.
“We might see municipalities that are performing well, but at a national level it’s frankly a disgrace by comparison, because we know what’s possible,” Mr. Warren said.
Lowering cases
Today, in the inner city of Sydney, new HIV diagnoses have plummeted by 88%, which puts the area on track to achieve the 90% UNAIDS target ahead of schedule.
Dr. Grulich and his team at the Kirby Institute are tracking new diagnoses by postal code and reported their encouraging findings here this week at the International AIDS Society Conference on HIV Science.
“This 88% decline is happening in an area where, in the ’80s and ’90s, a few thousand people died of AIDS,” Dr. Grulich told this news organization. “It feels close to miraculous.”
Dr. Grulich attributes some of the success to long-term government leadership that for the most part has been apolitical. HIV has been perceived by the public as an important health issue to be addressed. “We’ve never had a political contest over it,” he added. “We have politicians who are committed to evidence-based policy.”
In inner city Sydney, HIV prevention campaigns are a visible part of community life, Dr. Grulich explained. At public events, it is discussed; at bus stops, posters are on display; and passing trains have messages plastered to the side of them.
That community effort has consistently received government funding for years – albeit linked to key performance indicators – but it has enabled a high level of communication among government, community, clinicians, and researchers.
Another advantage is Australia’s universal health coverage, said Sharon Lewin, PhD, president of the International AIDS Society and director of the Peter Doherty Institute for Infection and Immunity at the University of Melbourne. “One very clear difference for Australia is a health system that provides free medication and free prevention,” she said. “You can’t underestimate the impact that has on public health.”
Globally, significant progress has been made toward the UN’s 95-95-95 targets, with 86% of people with HIV now knowing their status, 88% of those being on treatment, and 93% of those having an undetectable viral load, “for a total of 75% of all people living with HIV worldwide with undetectable viral load,” Dr. Grulich pointed out.
But Dr. Lewin cautioned that now is not the time to take our eye off the ball, especially with respect to the 39 million or so people living with HIV globally, all of whom need lifelong treatment and care to manage their disease. “We also need to be aware that if we relax the investment, and people stop their treatment, transmission occurs again,” Dr. Lewin warned. “Despite the great news of potentially getting close to eliminating HIV transmission in Australia, HIV is far from over.”
A version of this article first appeared on Medscape.com.
BRISBANE, AUSTRALIA – The year was 1987 and the Grim Reaper (a personification of death), holding a large scythe, rolled a 10-pin bowling ball through a dark, foggy place. In the advertisement on television, the cloaked skeleton aimed the bowling ball at the other end of a lane where a group of people stood in place of pins.
Who would fall next?
In the 1980s, cases of HIV were rising in the community and people in Australia and elsewhere were dying of AIDS. The Australian government opted to use mainstream media to deliver a blunt message through advertising to raise awareness about the health risk and how to manage HIV in the community.
But the campaign also contributed to stigma for those living with the disease and especially those in the gay community who felt ostracized by rising public concern.
In the inner city of Sydney, a few thousand people died of AIDS, Andrew Grulich, MD, PhD, from the Kirby Institute at the University of New South Wales, Sydney, and involved in tracking cases, said in an interview. “Sydney was devastated by AIDS, it was truly devastated.”
HIV and AIDS quickly became an even more severe problem for several countries around Australia in Thailand, Papua New Guinea, and beyond. After HIV was first reported in Thailand in 1984, the region had the highest prevalence of HIV in Southeast Asia. Through the 1990s in Papua New Guinea, HIV prevalence rose steeply as well.
By 2010, the Joint United Nations Programme on HIV/AIDS (UNAIDS) set a target of a 90% reduction in HIV incidence, a 90% reduction in AIDS deaths by 2030, and 95% of people living with HIV and AIDS being aware of their status, on treatment, and having an undetectable viral load.
Since then, significant progress has been made globally with 86% of people knowing their HIV status. However, new infections persist at a rate that has not dropped as fast as possible.
New infections
According to the latest UNAIDS report, regions of North America and western and central Europe showed a 23% decline in new infections from 2010 to 2022, below the target 90% reduction.
Some regions of the United States have seen significant declines in new HIV infections. San Francisco has a 67% drop in new diagnoses. And now, along with the District of Columbia, the four states with the highest HIV rates are New York, Maryland, Georgia, and Florida.
Several countries in eastern and southern Africa are close to achieving their target HIV reduction of 90%.
Mitchell Warren, executive director of AVAC for global health advocacy, access, and equity, said that many of the low- and middle-income countries that are on track to achieve targets are able to do so because of support from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund to Fight AIDS, Tuberculosis, and Malaria.
“That foreign development assistance is transforming the AIDS response in a number of African countries, and yet at home, in various states and municipalities, not only are we not reaching that effort, we don’t even use those targets,” Mr. Warren pointed out.
“We might see municipalities that are performing well, but at a national level it’s frankly a disgrace by comparison, because we know what’s possible,” Mr. Warren said.
Lowering cases
Today, in the inner city of Sydney, new HIV diagnoses have plummeted by 88%, which puts the area on track to achieve the 90% UNAIDS target ahead of schedule.
Dr. Grulich and his team at the Kirby Institute are tracking new diagnoses by postal code and reported their encouraging findings here this week at the International AIDS Society Conference on HIV Science.
“This 88% decline is happening in an area where, in the ’80s and ’90s, a few thousand people died of AIDS,” Dr. Grulich told this news organization. “It feels close to miraculous.”
Dr. Grulich attributes some of the success to long-term government leadership that for the most part has been apolitical. HIV has been perceived by the public as an important health issue to be addressed. “We’ve never had a political contest over it,” he added. “We have politicians who are committed to evidence-based policy.”
In inner city Sydney, HIV prevention campaigns are a visible part of community life, Dr. Grulich explained. At public events, it is discussed; at bus stops, posters are on display; and passing trains have messages plastered to the side of them.
That community effort has consistently received government funding for years – albeit linked to key performance indicators – but it has enabled a high level of communication among government, community, clinicians, and researchers.
Another advantage is Australia’s universal health coverage, said Sharon Lewin, PhD, president of the International AIDS Society and director of the Peter Doherty Institute for Infection and Immunity at the University of Melbourne. “One very clear difference for Australia is a health system that provides free medication and free prevention,” she said. “You can’t underestimate the impact that has on public health.”
Globally, significant progress has been made toward the UN’s 95-95-95 targets, with 86% of people with HIV now knowing their status, 88% of those being on treatment, and 93% of those having an undetectable viral load, “for a total of 75% of all people living with HIV worldwide with undetectable viral load,” Dr. Grulich pointed out.
But Dr. Lewin cautioned that now is not the time to take our eye off the ball, especially with respect to the 39 million or so people living with HIV globally, all of whom need lifelong treatment and care to manage their disease. “We also need to be aware that if we relax the investment, and people stop their treatment, transmission occurs again,” Dr. Lewin warned. “Despite the great news of potentially getting close to eliminating HIV transmission in Australia, HIV is far from over.”
A version of this article first appeared on Medscape.com.
BRISBANE, AUSTRALIA – The year was 1987 and the Grim Reaper (a personification of death), holding a large scythe, rolled a 10-pin bowling ball through a dark, foggy place. In the advertisement on television, the cloaked skeleton aimed the bowling ball at the other end of a lane where a group of people stood in place of pins.
Who would fall next?
In the 1980s, cases of HIV were rising in the community and people in Australia and elsewhere were dying of AIDS. The Australian government opted to use mainstream media to deliver a blunt message through advertising to raise awareness about the health risk and how to manage HIV in the community.
But the campaign also contributed to stigma for those living with the disease and especially those in the gay community who felt ostracized by rising public concern.
In the inner city of Sydney, a few thousand people died of AIDS, Andrew Grulich, MD, PhD, from the Kirby Institute at the University of New South Wales, Sydney, and involved in tracking cases, said in an interview. “Sydney was devastated by AIDS, it was truly devastated.”
HIV and AIDS quickly became an even more severe problem for several countries around Australia in Thailand, Papua New Guinea, and beyond. After HIV was first reported in Thailand in 1984, the region had the highest prevalence of HIV in Southeast Asia. Through the 1990s in Papua New Guinea, HIV prevalence rose steeply as well.
By 2010, the Joint United Nations Programme on HIV/AIDS (UNAIDS) set a target of a 90% reduction in HIV incidence, a 90% reduction in AIDS deaths by 2030, and 95% of people living with HIV and AIDS being aware of their status, on treatment, and having an undetectable viral load.
Since then, significant progress has been made globally with 86% of people knowing their HIV status. However, new infections persist at a rate that has not dropped as fast as possible.
New infections
According to the latest UNAIDS report, regions of North America and western and central Europe showed a 23% decline in new infections from 2010 to 2022, below the target 90% reduction.
Some regions of the United States have seen significant declines in new HIV infections. San Francisco has a 67% drop in new diagnoses. And now, along with the District of Columbia, the four states with the highest HIV rates are New York, Maryland, Georgia, and Florida.
Several countries in eastern and southern Africa are close to achieving their target HIV reduction of 90%.
Mitchell Warren, executive director of AVAC for global health advocacy, access, and equity, said that many of the low- and middle-income countries that are on track to achieve targets are able to do so because of support from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund to Fight AIDS, Tuberculosis, and Malaria.
“That foreign development assistance is transforming the AIDS response in a number of African countries, and yet at home, in various states and municipalities, not only are we not reaching that effort, we don’t even use those targets,” Mr. Warren pointed out.
“We might see municipalities that are performing well, but at a national level it’s frankly a disgrace by comparison, because we know what’s possible,” Mr. Warren said.
Lowering cases
Today, in the inner city of Sydney, new HIV diagnoses have plummeted by 88%, which puts the area on track to achieve the 90% UNAIDS target ahead of schedule.
Dr. Grulich and his team at the Kirby Institute are tracking new diagnoses by postal code and reported their encouraging findings here this week at the International AIDS Society Conference on HIV Science.
“This 88% decline is happening in an area where, in the ’80s and ’90s, a few thousand people died of AIDS,” Dr. Grulich told this news organization. “It feels close to miraculous.”
Dr. Grulich attributes some of the success to long-term government leadership that for the most part has been apolitical. HIV has been perceived by the public as an important health issue to be addressed. “We’ve never had a political contest over it,” he added. “We have politicians who are committed to evidence-based policy.”
In inner city Sydney, HIV prevention campaigns are a visible part of community life, Dr. Grulich explained. At public events, it is discussed; at bus stops, posters are on display; and passing trains have messages plastered to the side of them.
That community effort has consistently received government funding for years – albeit linked to key performance indicators – but it has enabled a high level of communication among government, community, clinicians, and researchers.
Another advantage is Australia’s universal health coverage, said Sharon Lewin, PhD, president of the International AIDS Society and director of the Peter Doherty Institute for Infection and Immunity at the University of Melbourne. “One very clear difference for Australia is a health system that provides free medication and free prevention,” she said. “You can’t underestimate the impact that has on public health.”
Globally, significant progress has been made toward the UN’s 95-95-95 targets, with 86% of people with HIV now knowing their status, 88% of those being on treatment, and 93% of those having an undetectable viral load, “for a total of 75% of all people living with HIV worldwide with undetectable viral load,” Dr. Grulich pointed out.
But Dr. Lewin cautioned that now is not the time to take our eye off the ball, especially with respect to the 39 million or so people living with HIV globally, all of whom need lifelong treatment and care to manage their disease. “We also need to be aware that if we relax the investment, and people stop their treatment, transmission occurs again,” Dr. Lewin warned. “Despite the great news of potentially getting close to eliminating HIV transmission in Australia, HIV is far from over.”
A version of this article first appeared on Medscape.com.
Multiple trials of long COVID treatments advancing, more on the way
Additional clinical trials to test at least seven more treatments are expected to launch in the coming months, officials said.
The trials are part of the NIH’s research effort known as the Researching COVID to Enhance Recovery (RECOVER) Initiative. In December 2020, Congress approved $1.15 billion for the NIH to research and test treatments for long COVID. The new clinical trials are in phase 2 and will test safety and effectiveness.
“The condition affects nearly all body systems and presents with more than 200 symptoms,” said Walter J. Koroshetz, MD, director of the NIH National Institute of Neurological Disorders and Stroke and colead of the RECOVER Initiative. How many people have long COVID is uncertain, he told attendees at the briefing. “The answer kind of depends on how you define the problem and also what variant caused it. The incidence was higher in Delta.” Some estimates suggest that 5%-10% of those infected develop long COVID. “I don’t think we have solid numbers, as it’s a moving target,” Dr. Koroshetz said.
Patients with long COVID have grown increasingly frustrated at the lack of effective treatments. Some doctors have turned to off-label use of some drugs to treat them.
The four trials include the following:
- RECOVER-VITAL will focus on a treatment for viral persistence, which can occur if the virus lingers and causes the immune system to not work properly. One treatment will test a longer dose regimen of the antiviral Paxlovid (nirmatrelvir and ritonavir), which is currently used to treat mild to moderate COVID to halt progression to severe COVID.
- RECOVER-NEURO will target treatments for symptoms such as brain fog, memory problems, and attention challenges. Among the potential treatments are a program called BrainHQ, which provides Web-based training, and PASC-Cognitive Recovery (post-acute sequelae of COVID), a Web-based program developed by Mount Sinai Health System in New York. Also being tested is a direct current stimulation program to improve brain activity.
- RECOVER-SLEEP will evaluate treatments for sleep problems, which can include daytime sleepiness and other problems. According to Dr. Koroshetz, melatonin, light therapy, and an educational coaching system are among the treatments that will be studied.
- RECOVER-AUTONOMIC will evaluate treatments to address symptoms linked with problems of the autonomic nervous system. The first trial will target postural orthostatic tachycardia syndrome (POTS), which can include irregular heartbeat, fatigue, and dizziness. A treatment for immune disease and a drug currently used to treat chronic heart failure will be tested.
Timelines
The first trial, on viral persistence, has launched, said Kanecia Zimmerman, MD, a principal investigator at the Duke Clinical Research Institute, the clinical trials data coordinating center for the trials. “We are actively working to launch the second on cognitive dysfunction.” The sleep and autonomic trials will launch in the coming months, she said. Also planned is a trial to study exercise intolerance, which is reported by many with long COVID.
Information on how to join long COVID trials is available here.
A version of this article first appeared on Medscape.com.
Additional clinical trials to test at least seven more treatments are expected to launch in the coming months, officials said.
The trials are part of the NIH’s research effort known as the Researching COVID to Enhance Recovery (RECOVER) Initiative. In December 2020, Congress approved $1.15 billion for the NIH to research and test treatments for long COVID. The new clinical trials are in phase 2 and will test safety and effectiveness.
“The condition affects nearly all body systems and presents with more than 200 symptoms,” said Walter J. Koroshetz, MD, director of the NIH National Institute of Neurological Disorders and Stroke and colead of the RECOVER Initiative. How many people have long COVID is uncertain, he told attendees at the briefing. “The answer kind of depends on how you define the problem and also what variant caused it. The incidence was higher in Delta.” Some estimates suggest that 5%-10% of those infected develop long COVID. “I don’t think we have solid numbers, as it’s a moving target,” Dr. Koroshetz said.
Patients with long COVID have grown increasingly frustrated at the lack of effective treatments. Some doctors have turned to off-label use of some drugs to treat them.
The four trials include the following:
- RECOVER-VITAL will focus on a treatment for viral persistence, which can occur if the virus lingers and causes the immune system to not work properly. One treatment will test a longer dose regimen of the antiviral Paxlovid (nirmatrelvir and ritonavir), which is currently used to treat mild to moderate COVID to halt progression to severe COVID.
- RECOVER-NEURO will target treatments for symptoms such as brain fog, memory problems, and attention challenges. Among the potential treatments are a program called BrainHQ, which provides Web-based training, and PASC-Cognitive Recovery (post-acute sequelae of COVID), a Web-based program developed by Mount Sinai Health System in New York. Also being tested is a direct current stimulation program to improve brain activity.
- RECOVER-SLEEP will evaluate treatments for sleep problems, which can include daytime sleepiness and other problems. According to Dr. Koroshetz, melatonin, light therapy, and an educational coaching system are among the treatments that will be studied.
- RECOVER-AUTONOMIC will evaluate treatments to address symptoms linked with problems of the autonomic nervous system. The first trial will target postural orthostatic tachycardia syndrome (POTS), which can include irregular heartbeat, fatigue, and dizziness. A treatment for immune disease and a drug currently used to treat chronic heart failure will be tested.
Timelines
The first trial, on viral persistence, has launched, said Kanecia Zimmerman, MD, a principal investigator at the Duke Clinical Research Institute, the clinical trials data coordinating center for the trials. “We are actively working to launch the second on cognitive dysfunction.” The sleep and autonomic trials will launch in the coming months, she said. Also planned is a trial to study exercise intolerance, which is reported by many with long COVID.
Information on how to join long COVID trials is available here.
A version of this article first appeared on Medscape.com.
Additional clinical trials to test at least seven more treatments are expected to launch in the coming months, officials said.
The trials are part of the NIH’s research effort known as the Researching COVID to Enhance Recovery (RECOVER) Initiative. In December 2020, Congress approved $1.15 billion for the NIH to research and test treatments for long COVID. The new clinical trials are in phase 2 and will test safety and effectiveness.
“The condition affects nearly all body systems and presents with more than 200 symptoms,” said Walter J. Koroshetz, MD, director of the NIH National Institute of Neurological Disorders and Stroke and colead of the RECOVER Initiative. How many people have long COVID is uncertain, he told attendees at the briefing. “The answer kind of depends on how you define the problem and also what variant caused it. The incidence was higher in Delta.” Some estimates suggest that 5%-10% of those infected develop long COVID. “I don’t think we have solid numbers, as it’s a moving target,” Dr. Koroshetz said.
Patients with long COVID have grown increasingly frustrated at the lack of effective treatments. Some doctors have turned to off-label use of some drugs to treat them.
The four trials include the following:
- RECOVER-VITAL will focus on a treatment for viral persistence, which can occur if the virus lingers and causes the immune system to not work properly. One treatment will test a longer dose regimen of the antiviral Paxlovid (nirmatrelvir and ritonavir), which is currently used to treat mild to moderate COVID to halt progression to severe COVID.
- RECOVER-NEURO will target treatments for symptoms such as brain fog, memory problems, and attention challenges. Among the potential treatments are a program called BrainHQ, which provides Web-based training, and PASC-Cognitive Recovery (post-acute sequelae of COVID), a Web-based program developed by Mount Sinai Health System in New York. Also being tested is a direct current stimulation program to improve brain activity.
- RECOVER-SLEEP will evaluate treatments for sleep problems, which can include daytime sleepiness and other problems. According to Dr. Koroshetz, melatonin, light therapy, and an educational coaching system are among the treatments that will be studied.
- RECOVER-AUTONOMIC will evaluate treatments to address symptoms linked with problems of the autonomic nervous system. The first trial will target postural orthostatic tachycardia syndrome (POTS), which can include irregular heartbeat, fatigue, and dizziness. A treatment for immune disease and a drug currently used to treat chronic heart failure will be tested.
Timelines
The first trial, on viral persistence, has launched, said Kanecia Zimmerman, MD, a principal investigator at the Duke Clinical Research Institute, the clinical trials data coordinating center for the trials. “We are actively working to launch the second on cognitive dysfunction.” The sleep and autonomic trials will launch in the coming months, she said. Also planned is a trial to study exercise intolerance, which is reported by many with long COVID.
Information on how to join long COVID trials is available here.
A version of this article first appeared on Medscape.com.
Foot rash during self-treatment
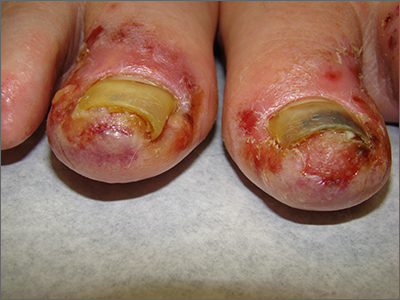
The patient’s toenail thickening appeared consistent with possible onychomycosis—but in addition, there was a marked inflammatory and vesicular eruption consistent with an allergic contact dermatitis.
TTO, also known as melaleuca oil, is a popular product used to treat many disorders including alopecia, seborrheic dermatitis, and onychomycosis.1 Unfortunately, it is a complex compound, and the rate of positive reactions to patch testing ranges from 0.1% to 3.5%.2
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis results from an irritating or relatively caustic substance causing direct damage and inflammation to the skin. In allergic contact dermatitis, as occurred here, there is sensitization to a substance that causes a type IV delayed cell-mediated immune response. Although radioallergosorbent blood testing will usually show immunoglobulin E antibodies to the inciting substance, patch testing is more specific and will show a reaction to the imputed substance on direct skin application. This usually is performed as a panel of antigens tested at the same time.
The mainstay of treatment is to identify, stop use of, and then avoid the sensitizing substance. Topical steroids (triamcinolone 0.1% ointment or clobetasol 0.05% ointment twice daily) are helpful in most cases. If the condition is severe or does not respond to initial therapy, systemic steroids (prednisone 40 mg/d for 5 days for most cases or a 2- to 3-week taper for Rhus dermatitis [eg, poison ivy]) are often effective.3
This patient was instructed to stop using TTO and counseled to avoid it in the future. She was told that her nails might fall off due to the inflammation, which might cure her onychomycosis, and that it takes 12 to 18 months to grow new toenails. She was advised to return for evaluation if the new nails developed any abnormalities or if her onychomycosis recurred. Oral terbinafine 250 mg/d for 90 days is usually a safe and effective therapy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790. doi: 10.1111/j.1365-4632.2012.05654.x
2. de Groot AC, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143. doi: 10.1111/cod.12591
3. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.

The patient’s toenail thickening appeared consistent with possible onychomycosis—but in addition, there was a marked inflammatory and vesicular eruption consistent with an allergic contact dermatitis.
TTO, also known as melaleuca oil, is a popular product used to treat many disorders including alopecia, seborrheic dermatitis, and onychomycosis.1 Unfortunately, it is a complex compound, and the rate of positive reactions to patch testing ranges from 0.1% to 3.5%.2
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis results from an irritating or relatively caustic substance causing direct damage and inflammation to the skin. In allergic contact dermatitis, as occurred here, there is sensitization to a substance that causes a type IV delayed cell-mediated immune response. Although radioallergosorbent blood testing will usually show immunoglobulin E antibodies to the inciting substance, patch testing is more specific and will show a reaction to the imputed substance on direct skin application. This usually is performed as a panel of antigens tested at the same time.
The mainstay of treatment is to identify, stop use of, and then avoid the sensitizing substance. Topical steroids (triamcinolone 0.1% ointment or clobetasol 0.05% ointment twice daily) are helpful in most cases. If the condition is severe or does not respond to initial therapy, systemic steroids (prednisone 40 mg/d for 5 days for most cases or a 2- to 3-week taper for Rhus dermatitis [eg, poison ivy]) are often effective.3
This patient was instructed to stop using TTO and counseled to avoid it in the future. She was told that her nails might fall off due to the inflammation, which might cure her onychomycosis, and that it takes 12 to 18 months to grow new toenails. She was advised to return for evaluation if the new nails developed any abnormalities or if her onychomycosis recurred. Oral terbinafine 250 mg/d for 90 days is usually a safe and effective therapy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The patient’s toenail thickening appeared consistent with possible onychomycosis—but in addition, there was a marked inflammatory and vesicular eruption consistent with an allergic contact dermatitis.
TTO, also known as melaleuca oil, is a popular product used to treat many disorders including alopecia, seborrheic dermatitis, and onychomycosis.1 Unfortunately, it is a complex compound, and the rate of positive reactions to patch testing ranges from 0.1% to 3.5%.2
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis results from an irritating or relatively caustic substance causing direct damage and inflammation to the skin. In allergic contact dermatitis, as occurred here, there is sensitization to a substance that causes a type IV delayed cell-mediated immune response. Although radioallergosorbent blood testing will usually show immunoglobulin E antibodies to the inciting substance, patch testing is more specific and will show a reaction to the imputed substance on direct skin application. This usually is performed as a panel of antigens tested at the same time.
The mainstay of treatment is to identify, stop use of, and then avoid the sensitizing substance. Topical steroids (triamcinolone 0.1% ointment or clobetasol 0.05% ointment twice daily) are helpful in most cases. If the condition is severe or does not respond to initial therapy, systemic steroids (prednisone 40 mg/d for 5 days for most cases or a 2- to 3-week taper for Rhus dermatitis [eg, poison ivy]) are often effective.3
This patient was instructed to stop using TTO and counseled to avoid it in the future. She was told that her nails might fall off due to the inflammation, which might cure her onychomycosis, and that it takes 12 to 18 months to grow new toenails. She was advised to return for evaluation if the new nails developed any abnormalities or if her onychomycosis recurred. Oral terbinafine 250 mg/d for 90 days is usually a safe and effective therapy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790. doi: 10.1111/j.1365-4632.2012.05654.x
2. de Groot AC, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143. doi: 10.1111/cod.12591
3. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
1. Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790. doi: 10.1111/j.1365-4632.2012.05654.x
2. de Groot AC, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143. doi: 10.1111/cod.12591
3. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
Hospital guards snoop through patient records, cost hospital $240K
Yakima Valley Memorial Hospital agreed to the voluntary settlement after an investigation into the actions of 23 emergency department security guards who allegedly used their login credentials to access the patient medical records of 419 patients.
The information accessed included names, dates of birth, medical record numbers, addresses, certain notes related to treatment, and insurance information, according to a release by the U.S .Department of Health & Human Services’ Office for Civil Rights (OCR). A breach notification report alerted OCR to the snooping.
As part of the agreement, OCR will monitor Yakima Valley Memorial Hospital for 2 years and the hospital must conduct a thorough risk analysis as well as develop a risk management plan to address and mitigate identified security risks and vulnerabilities. The settlement is not considered an admission of guilt by the hospital.
Is such snooping common?
The incident highlights the frequent practice of employees snooping through medical records and the steep consequences that can result for providers, said Paul Redding, vice president of partner engagement and cybersecurity at Compliancy Group, a company that offers guided HIPAA compliance software for healthcare providers and vendors.
“I think the problem is absolutely growing,” he said. “What’s crazy about this case is it’s actually a really small HIPAA violation. Less than 500 people were affected, and the hospital still must pay a quarter-of-a-million-dollar settlement. If you take the average HIPAA violation, which is in the thousands and thousands of [patients], this amount would be magnified many times over.”
In general, employees snoop through records out of curiosity or to find out information about people they know – or want to learn about, said J. David Sims, a cybersecurity expert and CEO of Security First IT, a company that provides cybersecurity solutions and IT support to health care businesses.
Mr. Sims says he has heard of cases where health professionals snooped through records to find information about the new love interests of ex-partners or to learn about people on dating websites whom they’re interested in dating.
“Most of the time, it’s people being nosy,” he said. “In a lot of cases, it’s curiosity about famous people. You see it a lot in areas where you have football players who come in with injuries or you have an actor or actress who come in for something.”
“Data breaches caused by current and former workforce members impermissibly accessing patient records are a recurring issue across the health care industry. Health care organizations must ensure that workforce members can only access the patient information needed to do their jobs,” OCR director Melanie Fontes Rainer said in a June statement. “HIPAA-covered entities must have robust policies and procedures in place to ensure patient health information is protected from identify theft and fraud.”
Yakima Valley Memorial Hospital did not return a message seeking comment.
According to OCR’s latest report to Congress, complaints about HIPAA violations increased by 39% between 2017 and 2021. Breaches affecting fewer than 500 individuals rose by 5% during the same time period, and breaches impacting 500 or more individuals increased by 58%.
Common reasons employees snoop
The OCR announcement does not specify why the 23 security guards were accessing the medical records, but the incident raises questions about why the security guards had access to protected health information (PHI) in the first place, Mr. Redding said.
“I have yet to have anyone explain to me why the security guards would have access to PHI at all, at any level,” he said. “Was it by design or was it by error?”
In 2019 for instance, dozens of employees at Northwestern Memorial Hospital in Chicago were fired for accessing the health records of former Empire actor Jussie Smollett. In another high-profile case, nearly a dozen emergency medical service employees were caught snooping through 911 records connected to the treatment and, later, death of Joan Rivers.
“Sadly, there is a lack of education around what compliance really means inside the medical industry as a whole,” Mr. Redding said. “There is a lack of employee training and a lack of emphasis on accountability for employees.”
Privacy breaches fuel lawsuits
Health professionals caught snooping through records are frequently terminated and employers can face a range of ramifications, including civil and criminal penalties.
A growing trend is class action lawsuits associated with privacy violations, Mr. Redding adds.
Because patients are unable to sue in civil court for HIPAA breaches, they frequently sue for “breach of an implied contract,” he explained. In such cases, patients allege that the privacy documents they signed with health care providers established an implied contract, and their records being exposed constituted a contract breach.
“Class action lawsuits are starting to become extremely common,” Mr. Redding said. “It’s happening in many cases, even sometimes before Health & Human Services issue a fine, that [providers] are being wrapped into a class action lawsuit.”
Mayo Clinic, for example, was recently slapped with a class action suit after a former employee inappropriately accessed the records of 1,600 patients. Mayo settled the suit in January 2023, the terms of which were not publicly disclosed.
Multiple patients also filed a class action suit against San Diego–based Scripps Health after its data were hit with a cyberattack and subsequent breach that impacted close to 2 million people. Scripps reached a $3.5 million settlement with the plaintiffs in 2023.
Some practices and employers may also face state penalties for data privacy breaches, depending on their jurisdiction. In July, Connecticut became the fifth state to enact a comprehensive data privacy law. The measure, which creates a robust framework for protecting health-related records and other data, includes civil penalties of up to $5,000 for violations. Other states, including California, Virginia, Utah, and Colorado, also have state data privacy laws on the books.
How can practices stop snooping?
A first step to preventing snooping is conducting a thorough risk assessment, said David Harlow, a health care attorney and chief compliance and privacy officer for Insulet Corporation, a medical device company. The analysis should address who has access to what data and whether they really need such access, he said.
“Then it’s putting in place the proper controls to ensure access is limited and use is limited to the appropriate individuals and circumstances,” Mr. Harlow said.
Regulators don’t expect a giant academic medical center and a small private physician practice to take an identical HIPAA compliance approach, he stressed. The ideal approach will vary by entity. Providers just need to address the standards in a way that makes sense for their operation, he said.
Training is also a critical component, adds Mr. Sims.
“Having training is key,” he said. “Oftentimes, an employee might think, ‘Well, if I can click on this data and it comes up, obviously, I can look at it.’ They need to understand what information they are and are not allowed to access.”
Keep in mind that settings or controls might change when larger transitions take place, such as moving to a new electronic health record system, Mr. Sims said. It’s essential to reevaluate controls when changes in the practice take place to ensure that everything is functioning correctly.
Mr. Sims also suggests that practices create a type of “If you see something, say something,” policy that encourages fellow physicians and employees to report anything that looks suspicious within electronic logs. If an employee, for instance, is suddenly looking at many more records than usual or at odd times of the day or night, this should raise red flags.
“It’s great to stop it early so that it doesn’t become a bigger issue for the practice to deal with, but also, from a legal standpoint, you want to have a defensible argument that you were doing all you could to stop this as quickly as possible,” he said. “It puts you in a better position to defend yourself.”
The snooping security guards case holds an important lesson for all health providers, Mr. Harlow said.
“This is a message to all of us, that you need to have done the assessment up front,” he said. You need to have the right controls in place up front. This is not a situation where somebody managed to hack into a system for some devious means. This is someone who was given keys. Why were they given the keys?”
A version of this article first appeared on Medscape.com.
Yakima Valley Memorial Hospital agreed to the voluntary settlement after an investigation into the actions of 23 emergency department security guards who allegedly used their login credentials to access the patient medical records of 419 patients.
The information accessed included names, dates of birth, medical record numbers, addresses, certain notes related to treatment, and insurance information, according to a release by the U.S .Department of Health & Human Services’ Office for Civil Rights (OCR). A breach notification report alerted OCR to the snooping.
As part of the agreement, OCR will monitor Yakima Valley Memorial Hospital for 2 years and the hospital must conduct a thorough risk analysis as well as develop a risk management plan to address and mitigate identified security risks and vulnerabilities. The settlement is not considered an admission of guilt by the hospital.
Is such snooping common?
The incident highlights the frequent practice of employees snooping through medical records and the steep consequences that can result for providers, said Paul Redding, vice president of partner engagement and cybersecurity at Compliancy Group, a company that offers guided HIPAA compliance software for healthcare providers and vendors.
“I think the problem is absolutely growing,” he said. “What’s crazy about this case is it’s actually a really small HIPAA violation. Less than 500 people were affected, and the hospital still must pay a quarter-of-a-million-dollar settlement. If you take the average HIPAA violation, which is in the thousands and thousands of [patients], this amount would be magnified many times over.”
In general, employees snoop through records out of curiosity or to find out information about people they know – or want to learn about, said J. David Sims, a cybersecurity expert and CEO of Security First IT, a company that provides cybersecurity solutions and IT support to health care businesses.
Mr. Sims says he has heard of cases where health professionals snooped through records to find information about the new love interests of ex-partners or to learn about people on dating websites whom they’re interested in dating.
“Most of the time, it’s people being nosy,” he said. “In a lot of cases, it’s curiosity about famous people. You see it a lot in areas where you have football players who come in with injuries or you have an actor or actress who come in for something.”
“Data breaches caused by current and former workforce members impermissibly accessing patient records are a recurring issue across the health care industry. Health care organizations must ensure that workforce members can only access the patient information needed to do their jobs,” OCR director Melanie Fontes Rainer said in a June statement. “HIPAA-covered entities must have robust policies and procedures in place to ensure patient health information is protected from identify theft and fraud.”
Yakima Valley Memorial Hospital did not return a message seeking comment.
According to OCR’s latest report to Congress, complaints about HIPAA violations increased by 39% between 2017 and 2021. Breaches affecting fewer than 500 individuals rose by 5% during the same time period, and breaches impacting 500 or more individuals increased by 58%.
Common reasons employees snoop
The OCR announcement does not specify why the 23 security guards were accessing the medical records, but the incident raises questions about why the security guards had access to protected health information (PHI) in the first place, Mr. Redding said.
“I have yet to have anyone explain to me why the security guards would have access to PHI at all, at any level,” he said. “Was it by design or was it by error?”
In 2019 for instance, dozens of employees at Northwestern Memorial Hospital in Chicago were fired for accessing the health records of former Empire actor Jussie Smollett. In another high-profile case, nearly a dozen emergency medical service employees were caught snooping through 911 records connected to the treatment and, later, death of Joan Rivers.
“Sadly, there is a lack of education around what compliance really means inside the medical industry as a whole,” Mr. Redding said. “There is a lack of employee training and a lack of emphasis on accountability for employees.”
Privacy breaches fuel lawsuits
Health professionals caught snooping through records are frequently terminated and employers can face a range of ramifications, including civil and criminal penalties.
A growing trend is class action lawsuits associated with privacy violations, Mr. Redding adds.
Because patients are unable to sue in civil court for HIPAA breaches, they frequently sue for “breach of an implied contract,” he explained. In such cases, patients allege that the privacy documents they signed with health care providers established an implied contract, and their records being exposed constituted a contract breach.
“Class action lawsuits are starting to become extremely common,” Mr. Redding said. “It’s happening in many cases, even sometimes before Health & Human Services issue a fine, that [providers] are being wrapped into a class action lawsuit.”
Mayo Clinic, for example, was recently slapped with a class action suit after a former employee inappropriately accessed the records of 1,600 patients. Mayo settled the suit in January 2023, the terms of which were not publicly disclosed.
Multiple patients also filed a class action suit against San Diego–based Scripps Health after its data were hit with a cyberattack and subsequent breach that impacted close to 2 million people. Scripps reached a $3.5 million settlement with the plaintiffs in 2023.
Some practices and employers may also face state penalties for data privacy breaches, depending on their jurisdiction. In July, Connecticut became the fifth state to enact a comprehensive data privacy law. The measure, which creates a robust framework for protecting health-related records and other data, includes civil penalties of up to $5,000 for violations. Other states, including California, Virginia, Utah, and Colorado, also have state data privacy laws on the books.
How can practices stop snooping?
A first step to preventing snooping is conducting a thorough risk assessment, said David Harlow, a health care attorney and chief compliance and privacy officer for Insulet Corporation, a medical device company. The analysis should address who has access to what data and whether they really need such access, he said.
“Then it’s putting in place the proper controls to ensure access is limited and use is limited to the appropriate individuals and circumstances,” Mr. Harlow said.
Regulators don’t expect a giant academic medical center and a small private physician practice to take an identical HIPAA compliance approach, he stressed. The ideal approach will vary by entity. Providers just need to address the standards in a way that makes sense for their operation, he said.
Training is also a critical component, adds Mr. Sims.
“Having training is key,” he said. “Oftentimes, an employee might think, ‘Well, if I can click on this data and it comes up, obviously, I can look at it.’ They need to understand what information they are and are not allowed to access.”
Keep in mind that settings or controls might change when larger transitions take place, such as moving to a new electronic health record system, Mr. Sims said. It’s essential to reevaluate controls when changes in the practice take place to ensure that everything is functioning correctly.
Mr. Sims also suggests that practices create a type of “If you see something, say something,” policy that encourages fellow physicians and employees to report anything that looks suspicious within electronic logs. If an employee, for instance, is suddenly looking at many more records than usual or at odd times of the day or night, this should raise red flags.
“It’s great to stop it early so that it doesn’t become a bigger issue for the practice to deal with, but also, from a legal standpoint, you want to have a defensible argument that you were doing all you could to stop this as quickly as possible,” he said. “It puts you in a better position to defend yourself.”
The snooping security guards case holds an important lesson for all health providers, Mr. Harlow said.
“This is a message to all of us, that you need to have done the assessment up front,” he said. You need to have the right controls in place up front. This is not a situation where somebody managed to hack into a system for some devious means. This is someone who was given keys. Why were they given the keys?”
A version of this article first appeared on Medscape.com.
Yakima Valley Memorial Hospital agreed to the voluntary settlement after an investigation into the actions of 23 emergency department security guards who allegedly used their login credentials to access the patient medical records of 419 patients.
The information accessed included names, dates of birth, medical record numbers, addresses, certain notes related to treatment, and insurance information, according to a release by the U.S .Department of Health & Human Services’ Office for Civil Rights (OCR). A breach notification report alerted OCR to the snooping.
As part of the agreement, OCR will monitor Yakima Valley Memorial Hospital for 2 years and the hospital must conduct a thorough risk analysis as well as develop a risk management plan to address and mitigate identified security risks and vulnerabilities. The settlement is not considered an admission of guilt by the hospital.
Is such snooping common?
The incident highlights the frequent practice of employees snooping through medical records and the steep consequences that can result for providers, said Paul Redding, vice president of partner engagement and cybersecurity at Compliancy Group, a company that offers guided HIPAA compliance software for healthcare providers and vendors.
“I think the problem is absolutely growing,” he said. “What’s crazy about this case is it’s actually a really small HIPAA violation. Less than 500 people were affected, and the hospital still must pay a quarter-of-a-million-dollar settlement. If you take the average HIPAA violation, which is in the thousands and thousands of [patients], this amount would be magnified many times over.”
In general, employees snoop through records out of curiosity or to find out information about people they know – or want to learn about, said J. David Sims, a cybersecurity expert and CEO of Security First IT, a company that provides cybersecurity solutions and IT support to health care businesses.
Mr. Sims says he has heard of cases where health professionals snooped through records to find information about the new love interests of ex-partners or to learn about people on dating websites whom they’re interested in dating.
“Most of the time, it’s people being nosy,” he said. “In a lot of cases, it’s curiosity about famous people. You see it a lot in areas where you have football players who come in with injuries or you have an actor or actress who come in for something.”
“Data breaches caused by current and former workforce members impermissibly accessing patient records are a recurring issue across the health care industry. Health care organizations must ensure that workforce members can only access the patient information needed to do their jobs,” OCR director Melanie Fontes Rainer said in a June statement. “HIPAA-covered entities must have robust policies and procedures in place to ensure patient health information is protected from identify theft and fraud.”
Yakima Valley Memorial Hospital did not return a message seeking comment.
According to OCR’s latest report to Congress, complaints about HIPAA violations increased by 39% between 2017 and 2021. Breaches affecting fewer than 500 individuals rose by 5% during the same time period, and breaches impacting 500 or more individuals increased by 58%.
Common reasons employees snoop
The OCR announcement does not specify why the 23 security guards were accessing the medical records, but the incident raises questions about why the security guards had access to protected health information (PHI) in the first place, Mr. Redding said.
“I have yet to have anyone explain to me why the security guards would have access to PHI at all, at any level,” he said. “Was it by design or was it by error?”
In 2019 for instance, dozens of employees at Northwestern Memorial Hospital in Chicago were fired for accessing the health records of former Empire actor Jussie Smollett. In another high-profile case, nearly a dozen emergency medical service employees were caught snooping through 911 records connected to the treatment and, later, death of Joan Rivers.
“Sadly, there is a lack of education around what compliance really means inside the medical industry as a whole,” Mr. Redding said. “There is a lack of employee training and a lack of emphasis on accountability for employees.”
Privacy breaches fuel lawsuits
Health professionals caught snooping through records are frequently terminated and employers can face a range of ramifications, including civil and criminal penalties.
A growing trend is class action lawsuits associated with privacy violations, Mr. Redding adds.
Because patients are unable to sue in civil court for HIPAA breaches, they frequently sue for “breach of an implied contract,” he explained. In such cases, patients allege that the privacy documents they signed with health care providers established an implied contract, and their records being exposed constituted a contract breach.
“Class action lawsuits are starting to become extremely common,” Mr. Redding said. “It’s happening in many cases, even sometimes before Health & Human Services issue a fine, that [providers] are being wrapped into a class action lawsuit.”
Mayo Clinic, for example, was recently slapped with a class action suit after a former employee inappropriately accessed the records of 1,600 patients. Mayo settled the suit in January 2023, the terms of which were not publicly disclosed.
Multiple patients also filed a class action suit against San Diego–based Scripps Health after its data were hit with a cyberattack and subsequent breach that impacted close to 2 million people. Scripps reached a $3.5 million settlement with the plaintiffs in 2023.
Some practices and employers may also face state penalties for data privacy breaches, depending on their jurisdiction. In July, Connecticut became the fifth state to enact a comprehensive data privacy law. The measure, which creates a robust framework for protecting health-related records and other data, includes civil penalties of up to $5,000 for violations. Other states, including California, Virginia, Utah, and Colorado, also have state data privacy laws on the books.
How can practices stop snooping?
A first step to preventing snooping is conducting a thorough risk assessment, said David Harlow, a health care attorney and chief compliance and privacy officer for Insulet Corporation, a medical device company. The analysis should address who has access to what data and whether they really need such access, he said.
“Then it’s putting in place the proper controls to ensure access is limited and use is limited to the appropriate individuals and circumstances,” Mr. Harlow said.
Regulators don’t expect a giant academic medical center and a small private physician practice to take an identical HIPAA compliance approach, he stressed. The ideal approach will vary by entity. Providers just need to address the standards in a way that makes sense for their operation, he said.
Training is also a critical component, adds Mr. Sims.
“Having training is key,” he said. “Oftentimes, an employee might think, ‘Well, if I can click on this data and it comes up, obviously, I can look at it.’ They need to understand what information they are and are not allowed to access.”
Keep in mind that settings or controls might change when larger transitions take place, such as moving to a new electronic health record system, Mr. Sims said. It’s essential to reevaluate controls when changes in the practice take place to ensure that everything is functioning correctly.
Mr. Sims also suggests that practices create a type of “If you see something, say something,” policy that encourages fellow physicians and employees to report anything that looks suspicious within electronic logs. If an employee, for instance, is suddenly looking at many more records than usual or at odd times of the day or night, this should raise red flags.
“It’s great to stop it early so that it doesn’t become a bigger issue for the practice to deal with, but also, from a legal standpoint, you want to have a defensible argument that you were doing all you could to stop this as quickly as possible,” he said. “It puts you in a better position to defend yourself.”
The snooping security guards case holds an important lesson for all health providers, Mr. Harlow said.
“This is a message to all of us, that you need to have done the assessment up front,” he said. You need to have the right controls in place up front. This is not a situation where somebody managed to hack into a system for some devious means. This is someone who was given keys. Why were they given the keys?”
A version of this article first appeared on Medscape.com.
Women increasingly dying of alcohol-related causes
The most dramatic rise occurred in the last 3 years covered by the study, published in JAMA Network Open.
“From 2018 to 2020, there was an increase of 14.7% per year” in alcohol-related deaths in women, said study researcher Ibraheem M. Karaye, MD, DrPH, assistant professor of population health, and director of the health science program at Hofstra University in Hempstead, N.Y. While alcohol-related deaths in men also rose greatly during that same 3-year period, the increase was less than in women, at 12.5% per year.
Researchers have known for several years that the sex gap related to alcohol use and complications is narrowing. Women are drinking more, engaging in more high-risk drinking, and increasingly developing alcohol use disorder, Dr. Karaye said. “However, we know very little about the trends in alcohol-related deaths.”
Using a Centers for Disease Control and Prevention database that spanned the years 1999 to 2020, Dr. Karaye and his coresearchers analyzed files that identified underlying causes of death. During those years, more than 605,000 alcohol-attributed deaths were identified. Overall, men were still nearly three times more likely to die from alcohol-related issues than were women. However, the rate of alcohol-related deaths in women increased steadily and, in the latest years studied, more greatly than in men.
“We found there were three different segments of trends in women,” Dr. Karaye said. The rates increased slowly, then steadily picked up speed. For instance:
- 1999-2007: “We found that mortality rates from alcohol were increasing by 1% per year” in women, he said.
- 2007-2018: “The rate increased 4.3% per year. That was a big one, but not as phenomenal as the most recent, the most concerning,” he said.
- 2018 to 2020: The rate increased 14.7% per year in women, compared with 12.5% per year for men.
The findings stayed strong, Dr. Karaye said, even when the researchers excluded data from the year 2020, the first pandemic year.
Explaining the increase
“Our study is descriptive; it tells us the ‘what’ but not the ‘why,’” Dr. Karaye said. “However, we can speculate based on what’s known and previous research.” Women are drinking at higher rates than before and tend to develop more alcohol-related complications than men do.
Women have lower concentrations of the enzyme called alcohol dehydrogenase, which helps breaks down and metabolize alcohol. “We know that in women the concentration of fat to water is higher, so that also leads to a possibly higher concentration of alcohol,” Dr. Karaye said.
The study findings point to the need for more research to focus on causes for the rise in women, Dr. Karaye said. Studies on the use of medication for alcohol use disorder need to represent women more equitably, he said.
Other findings on women, alcohol
Other recent research has found that the proportion of suicides that involved alcohol has also increased for women of all age groups, but not men. In research published in 2022, researchers analyzed more than 115,000 deaths by suicide from 2003 to 2018 and found the proportion of those deaths involving alcohol at a level above the legal limit increased annually for women in all age groups, but not for men.
A review by Mayo Clinic researchers found that women are increasingly affected by liver disease linked to alcohol and develop more severe disease at lower levels of drinking than do men. Among other factors, the researchers said that an increase in obesity, which can worsen the liver-damaging effects of alcohol, is a contributor.
Expert perspectives
Overall, recent research is showing that, “not only are women drinking more but potentially are developing more problems later on as a result of the alcohol,” said Mark S. Kaplan, DrPH, professor emeritus of social welfare at the University of California, Los Angeles. He conducted the study finding growing alcohol use involvement in women’s death by suicide.
“I think this new study is strong,” he said. In future research, “we should focus on some of the issues that may have to do with social circumstances.”
In particular, he said, research should examine the increase in alcohol-involved death found in the new study among American Indian or Alaska Native women. While the overall annual increase was 14.7% for the years 2018-2020, the rate among American Indian or Alaska Native women was 22.8% annually.
While the new study and others find the gap between the sexes is narrowing for alcohol-related complications, “unfortunately, alcohol use disorder and alcohol-related deaths are increasing in both men and women,” said Camille A. Kezer, MD, a gastroenterology and hepatology fellow at Mayo Clinic, who led the review on sex differences in alcohol-linked liver disease.
However, she said, “we know that there are risks of alcohol that are unique to women for a variety of reasons, including differences in metabolism and the impact of hormones, as well as the increasing prevalence of obesity and bariatric surgery in women.”
Bariatric surgery has been linked with an increase in alcohol consumption and disorder in some studies.
Dr. Kezer’s advice to women: “Limit alcohol intake to one drink per day or less. If you are concerned about your alcohol intake, you should seek help.”
Health care providers are committed to helping their patients recognize and treat alcohol-related disorders, she said.
A version of this article first appeared on WebMD.com.
The most dramatic rise occurred in the last 3 years covered by the study, published in JAMA Network Open.
“From 2018 to 2020, there was an increase of 14.7% per year” in alcohol-related deaths in women, said study researcher Ibraheem M. Karaye, MD, DrPH, assistant professor of population health, and director of the health science program at Hofstra University in Hempstead, N.Y. While alcohol-related deaths in men also rose greatly during that same 3-year period, the increase was less than in women, at 12.5% per year.
Researchers have known for several years that the sex gap related to alcohol use and complications is narrowing. Women are drinking more, engaging in more high-risk drinking, and increasingly developing alcohol use disorder, Dr. Karaye said. “However, we know very little about the trends in alcohol-related deaths.”
Using a Centers for Disease Control and Prevention database that spanned the years 1999 to 2020, Dr. Karaye and his coresearchers analyzed files that identified underlying causes of death. During those years, more than 605,000 alcohol-attributed deaths were identified. Overall, men were still nearly three times more likely to die from alcohol-related issues than were women. However, the rate of alcohol-related deaths in women increased steadily and, in the latest years studied, more greatly than in men.
“We found there were three different segments of trends in women,” Dr. Karaye said. The rates increased slowly, then steadily picked up speed. For instance:
- 1999-2007: “We found that mortality rates from alcohol were increasing by 1% per year” in women, he said.
- 2007-2018: “The rate increased 4.3% per year. That was a big one, but not as phenomenal as the most recent, the most concerning,” he said.
- 2018 to 2020: The rate increased 14.7% per year in women, compared with 12.5% per year for men.
The findings stayed strong, Dr. Karaye said, even when the researchers excluded data from the year 2020, the first pandemic year.
Explaining the increase
“Our study is descriptive; it tells us the ‘what’ but not the ‘why,’” Dr. Karaye said. “However, we can speculate based on what’s known and previous research.” Women are drinking at higher rates than before and tend to develop more alcohol-related complications than men do.
Women have lower concentrations of the enzyme called alcohol dehydrogenase, which helps breaks down and metabolize alcohol. “We know that in women the concentration of fat to water is higher, so that also leads to a possibly higher concentration of alcohol,” Dr. Karaye said.
The study findings point to the need for more research to focus on causes for the rise in women, Dr. Karaye said. Studies on the use of medication for alcohol use disorder need to represent women more equitably, he said.
Other findings on women, alcohol
Other recent research has found that the proportion of suicides that involved alcohol has also increased for women of all age groups, but not men. In research published in 2022, researchers analyzed more than 115,000 deaths by suicide from 2003 to 2018 and found the proportion of those deaths involving alcohol at a level above the legal limit increased annually for women in all age groups, but not for men.
A review by Mayo Clinic researchers found that women are increasingly affected by liver disease linked to alcohol and develop more severe disease at lower levels of drinking than do men. Among other factors, the researchers said that an increase in obesity, which can worsen the liver-damaging effects of alcohol, is a contributor.
Expert perspectives
Overall, recent research is showing that, “not only are women drinking more but potentially are developing more problems later on as a result of the alcohol,” said Mark S. Kaplan, DrPH, professor emeritus of social welfare at the University of California, Los Angeles. He conducted the study finding growing alcohol use involvement in women’s death by suicide.
“I think this new study is strong,” he said. In future research, “we should focus on some of the issues that may have to do with social circumstances.”
In particular, he said, research should examine the increase in alcohol-involved death found in the new study among American Indian or Alaska Native women. While the overall annual increase was 14.7% for the years 2018-2020, the rate among American Indian or Alaska Native women was 22.8% annually.
While the new study and others find the gap between the sexes is narrowing for alcohol-related complications, “unfortunately, alcohol use disorder and alcohol-related deaths are increasing in both men and women,” said Camille A. Kezer, MD, a gastroenterology and hepatology fellow at Mayo Clinic, who led the review on sex differences in alcohol-linked liver disease.
However, she said, “we know that there are risks of alcohol that are unique to women for a variety of reasons, including differences in metabolism and the impact of hormones, as well as the increasing prevalence of obesity and bariatric surgery in women.”
Bariatric surgery has been linked with an increase in alcohol consumption and disorder in some studies.
Dr. Kezer’s advice to women: “Limit alcohol intake to one drink per day or less. If you are concerned about your alcohol intake, you should seek help.”
Health care providers are committed to helping their patients recognize and treat alcohol-related disorders, she said.
A version of this article first appeared on WebMD.com.
The most dramatic rise occurred in the last 3 years covered by the study, published in JAMA Network Open.
“From 2018 to 2020, there was an increase of 14.7% per year” in alcohol-related deaths in women, said study researcher Ibraheem M. Karaye, MD, DrPH, assistant professor of population health, and director of the health science program at Hofstra University in Hempstead, N.Y. While alcohol-related deaths in men also rose greatly during that same 3-year period, the increase was less than in women, at 12.5% per year.
Researchers have known for several years that the sex gap related to alcohol use and complications is narrowing. Women are drinking more, engaging in more high-risk drinking, and increasingly developing alcohol use disorder, Dr. Karaye said. “However, we know very little about the trends in alcohol-related deaths.”
Using a Centers for Disease Control and Prevention database that spanned the years 1999 to 2020, Dr. Karaye and his coresearchers analyzed files that identified underlying causes of death. During those years, more than 605,000 alcohol-attributed deaths were identified. Overall, men were still nearly three times more likely to die from alcohol-related issues than were women. However, the rate of alcohol-related deaths in women increased steadily and, in the latest years studied, more greatly than in men.
“We found there were three different segments of trends in women,” Dr. Karaye said. The rates increased slowly, then steadily picked up speed. For instance:
- 1999-2007: “We found that mortality rates from alcohol were increasing by 1% per year” in women, he said.
- 2007-2018: “The rate increased 4.3% per year. That was a big one, but not as phenomenal as the most recent, the most concerning,” he said.
- 2018 to 2020: The rate increased 14.7% per year in women, compared with 12.5% per year for men.
The findings stayed strong, Dr. Karaye said, even when the researchers excluded data from the year 2020, the first pandemic year.
Explaining the increase
“Our study is descriptive; it tells us the ‘what’ but not the ‘why,’” Dr. Karaye said. “However, we can speculate based on what’s known and previous research.” Women are drinking at higher rates than before and tend to develop more alcohol-related complications than men do.
Women have lower concentrations of the enzyme called alcohol dehydrogenase, which helps breaks down and metabolize alcohol. “We know that in women the concentration of fat to water is higher, so that also leads to a possibly higher concentration of alcohol,” Dr. Karaye said.
The study findings point to the need for more research to focus on causes for the rise in women, Dr. Karaye said. Studies on the use of medication for alcohol use disorder need to represent women more equitably, he said.
Other findings on women, alcohol
Other recent research has found that the proportion of suicides that involved alcohol has also increased for women of all age groups, but not men. In research published in 2022, researchers analyzed more than 115,000 deaths by suicide from 2003 to 2018 and found the proportion of those deaths involving alcohol at a level above the legal limit increased annually for women in all age groups, but not for men.
A review by Mayo Clinic researchers found that women are increasingly affected by liver disease linked to alcohol and develop more severe disease at lower levels of drinking than do men. Among other factors, the researchers said that an increase in obesity, which can worsen the liver-damaging effects of alcohol, is a contributor.
Expert perspectives
Overall, recent research is showing that, “not only are women drinking more but potentially are developing more problems later on as a result of the alcohol,” said Mark S. Kaplan, DrPH, professor emeritus of social welfare at the University of California, Los Angeles. He conducted the study finding growing alcohol use involvement in women’s death by suicide.
“I think this new study is strong,” he said. In future research, “we should focus on some of the issues that may have to do with social circumstances.”
In particular, he said, research should examine the increase in alcohol-involved death found in the new study among American Indian or Alaska Native women. While the overall annual increase was 14.7% for the years 2018-2020, the rate among American Indian or Alaska Native women was 22.8% annually.
While the new study and others find the gap between the sexes is narrowing for alcohol-related complications, “unfortunately, alcohol use disorder and alcohol-related deaths are increasing in both men and women,” said Camille A. Kezer, MD, a gastroenterology and hepatology fellow at Mayo Clinic, who led the review on sex differences in alcohol-linked liver disease.
However, she said, “we know that there are risks of alcohol that are unique to women for a variety of reasons, including differences in metabolism and the impact of hormones, as well as the increasing prevalence of obesity and bariatric surgery in women.”
Bariatric surgery has been linked with an increase in alcohol consumption and disorder in some studies.
Dr. Kezer’s advice to women: “Limit alcohol intake to one drink per day or less. If you are concerned about your alcohol intake, you should seek help.”
Health care providers are committed to helping their patients recognize and treat alcohol-related disorders, she said.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN