User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
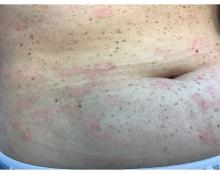
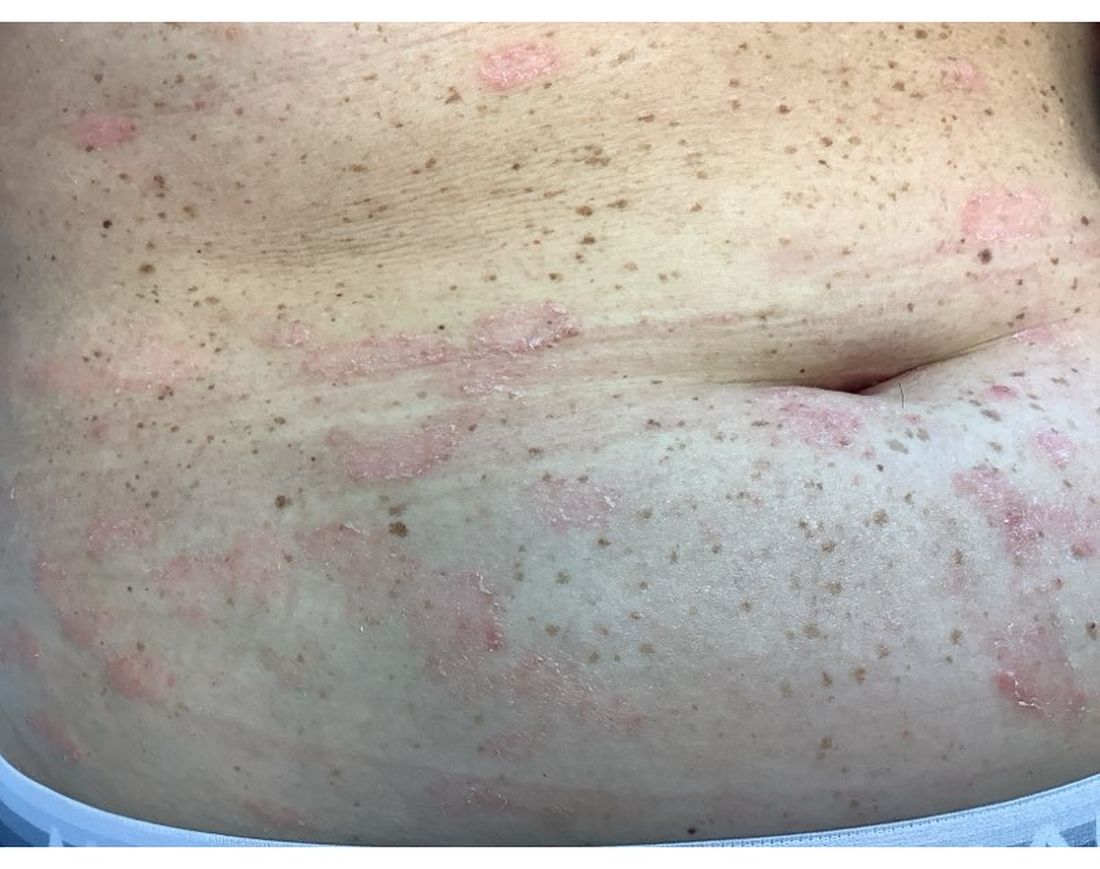
A 30-Year-Old White Female Presented With a 4-Month History of Scaly, Erythematous Patches and Plaques on Her Trunk and Extremities
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Mild Hidradenitis Suppurativa: Positive Results Reported for Topical Therapy
SAN DIEGO — cream, in a phase 2 trial.
“HS is a chronic, recurring inflammatory skin disease that is associated with painful inflammatory modules and abscesses,” said presenting author Martina J. Porter, MD, a dermatologist at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, Massachusetts. Dr. Porter presented the data during a late-breaking session at the annual meeting of the American Academy of Dermatology.
“Over time, these patients may progress to having tunnels, ulcerations, malodorous discharge, and permanent scarring,” she said. “Currently, there are no approved therapies for milder HS, and the standard treatments that we apply in clinical practice are often inadequate.”
Ruxolitinib is a selective Janus kinase (JAK) 1/JAK2 inhibitor that has demonstrated efficacy in other inflammatory and autoimmune skin diseases. Ruxolitinib cream, 1.5%, is approved for treating mild to moderate atopic dermatitis and nonsegmental vitiligo in patients ages 12 years and older.
The phase 2 double-blind, vehicle-controlled trial evaluated the efficacy and safety of ruxolitinib cream for mild HS. Researchers assigned 69 adults with Hurley stage I or II HS to receive 1.5% ruxolitinib cream or vehicle cream twice daily for 16 weeks. The primary endpoint was the change from baseline in AN count at week 16. To be eligible, patients had to have an AN count between 3 and 10.
“This is much more mild than what we have seen in any systemic therapy trials,” Dr. Porter said. “And, if patients had 3 lesions, they all needed to be in one anatomic area, but if they had 4-10 lesions, they had to have two anatomic areas involved. Also, no patients with active draining tunnels were allowed in the study.”
Of the 69 patients, 34 received ruxolitinib cream and 35 received vehicle. About 51% of patients in the vehicle arm were Black and 34% were White, while about 32% of patients in the ruxolitinib arm were Black and 56% were White.
The mean age of patients overall was 29 years, and about half the patients in both study arms had Hurley stage I disease, while the other half had Hurley stage II disease. Their average AN count ranged between 5.3 and 5.6 — mostly inflammatory nodules and few abscesses. Patients were not allowed to receive any type of intervention or rescue therapy during the study.
Dr. Porter reported that the least square mean change in AN count from baseline to week 16 was -2.42 in the vehicle arm vs -3.61 in the ruxolitinib cream arm (P <.05). The proportion of patients who achieved a 50% decrease in AN count was 79.2% in the ruxolitinib cream arm, compared with 56.5% of patients in the vehicle arm, respectively. More patients in the ruxolitinib cream arm achieved a 75% decrease in AN count (54.2% vs 25%), a 90% decrease in AN count (20.8 vs 12.5%), and a 100% decrease in AN count (20.8% vs 12.5%).
In other findings, 79.2% of patients in the ruxolitinib cream arm achieved a Hidradenitis Suppurativa Clinical Response score from baseline through week 16, compared with 50% of those in the vehicle group. The International Hidradenitis Suppurativa Severity Score System results favored the ruxolitinib cream arm (-4.46 vs -2.66 in the vehicle arm). Skin Pain and Itch numeric rating scale scores were moderate at baseline and improved similarly in both groups during the study.
Ruxolitinib cream was generally well tolerated over 16 weeks. No serious treatment-emergent adverse events were reported. The most common adverse event reported in the ruxolitinib cream group was COVID-19 and nasopharyngitis (two cases each) and one case of an application site reaction.
“Twice-daily 1.5% ruxolitinib cream was effective in patients with milder HS,” Dr. Porter concluded. “Modifications to our traditionally accepted clinical endpoints may be needed in studies of patients with milder HS.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the results, characterized the study as exciting for several reasons.
“First, with the global push in recent years to increase HS awareness, I am already seeing more patients earlier in their disease course with milder disease, and there is currently a gap in approved therapies for this patient population,” she told this news organization.
“Second, patients are very interested in topical therapies for HS and are thrilled whenever they learn that topical options are under investigation. This study had small patient numbers, but it was encouraging to see the positive results for ruxolitinib cream and that the treatment appeared well-tolerated.”
The trial was sponsored by the Incyte Corporation. Dr. Porter disclosed that she has received consulting fees from AbbVie, Alumis, Eli Lilly, Incyte, Janssen, Novartis, Pfizer, Prometheus Laboratories, Sanofi, Sonoma Biotherapeutics, Trifecta Clinical, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article appeared on Medscape.com .
SAN DIEGO — cream, in a phase 2 trial.
“HS is a chronic, recurring inflammatory skin disease that is associated with painful inflammatory modules and abscesses,” said presenting author Martina J. Porter, MD, a dermatologist at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, Massachusetts. Dr. Porter presented the data during a late-breaking session at the annual meeting of the American Academy of Dermatology.
“Over time, these patients may progress to having tunnels, ulcerations, malodorous discharge, and permanent scarring,” she said. “Currently, there are no approved therapies for milder HS, and the standard treatments that we apply in clinical practice are often inadequate.”
Ruxolitinib is a selective Janus kinase (JAK) 1/JAK2 inhibitor that has demonstrated efficacy in other inflammatory and autoimmune skin diseases. Ruxolitinib cream, 1.5%, is approved for treating mild to moderate atopic dermatitis and nonsegmental vitiligo in patients ages 12 years and older.
The phase 2 double-blind, vehicle-controlled trial evaluated the efficacy and safety of ruxolitinib cream for mild HS. Researchers assigned 69 adults with Hurley stage I or II HS to receive 1.5% ruxolitinib cream or vehicle cream twice daily for 16 weeks. The primary endpoint was the change from baseline in AN count at week 16. To be eligible, patients had to have an AN count between 3 and 10.
“This is much more mild than what we have seen in any systemic therapy trials,” Dr. Porter said. “And, if patients had 3 lesions, they all needed to be in one anatomic area, but if they had 4-10 lesions, they had to have two anatomic areas involved. Also, no patients with active draining tunnels were allowed in the study.”
Of the 69 patients, 34 received ruxolitinib cream and 35 received vehicle. About 51% of patients in the vehicle arm were Black and 34% were White, while about 32% of patients in the ruxolitinib arm were Black and 56% were White.
The mean age of patients overall was 29 years, and about half the patients in both study arms had Hurley stage I disease, while the other half had Hurley stage II disease. Their average AN count ranged between 5.3 and 5.6 — mostly inflammatory nodules and few abscesses. Patients were not allowed to receive any type of intervention or rescue therapy during the study.
Dr. Porter reported that the least square mean change in AN count from baseline to week 16 was -2.42 in the vehicle arm vs -3.61 in the ruxolitinib cream arm (P <.05). The proportion of patients who achieved a 50% decrease in AN count was 79.2% in the ruxolitinib cream arm, compared with 56.5% of patients in the vehicle arm, respectively. More patients in the ruxolitinib cream arm achieved a 75% decrease in AN count (54.2% vs 25%), a 90% decrease in AN count (20.8 vs 12.5%), and a 100% decrease in AN count (20.8% vs 12.5%).
In other findings, 79.2% of patients in the ruxolitinib cream arm achieved a Hidradenitis Suppurativa Clinical Response score from baseline through week 16, compared with 50% of those in the vehicle group. The International Hidradenitis Suppurativa Severity Score System results favored the ruxolitinib cream arm (-4.46 vs -2.66 in the vehicle arm). Skin Pain and Itch numeric rating scale scores were moderate at baseline and improved similarly in both groups during the study.
Ruxolitinib cream was generally well tolerated over 16 weeks. No serious treatment-emergent adverse events were reported. The most common adverse event reported in the ruxolitinib cream group was COVID-19 and nasopharyngitis (two cases each) and one case of an application site reaction.
“Twice-daily 1.5% ruxolitinib cream was effective in patients with milder HS,” Dr. Porter concluded. “Modifications to our traditionally accepted clinical endpoints may be needed in studies of patients with milder HS.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the results, characterized the study as exciting for several reasons.
“First, with the global push in recent years to increase HS awareness, I am already seeing more patients earlier in their disease course with milder disease, and there is currently a gap in approved therapies for this patient population,” she told this news organization.
“Second, patients are very interested in topical therapies for HS and are thrilled whenever they learn that topical options are under investigation. This study had small patient numbers, but it was encouraging to see the positive results for ruxolitinib cream and that the treatment appeared well-tolerated.”
The trial was sponsored by the Incyte Corporation. Dr. Porter disclosed that she has received consulting fees from AbbVie, Alumis, Eli Lilly, Incyte, Janssen, Novartis, Pfizer, Prometheus Laboratories, Sanofi, Sonoma Biotherapeutics, Trifecta Clinical, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article appeared on Medscape.com .
SAN DIEGO — cream, in a phase 2 trial.
“HS is a chronic, recurring inflammatory skin disease that is associated with painful inflammatory modules and abscesses,” said presenting author Martina J. Porter, MD, a dermatologist at Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, Massachusetts. Dr. Porter presented the data during a late-breaking session at the annual meeting of the American Academy of Dermatology.
“Over time, these patients may progress to having tunnels, ulcerations, malodorous discharge, and permanent scarring,” she said. “Currently, there are no approved therapies for milder HS, and the standard treatments that we apply in clinical practice are often inadequate.”
Ruxolitinib is a selective Janus kinase (JAK) 1/JAK2 inhibitor that has demonstrated efficacy in other inflammatory and autoimmune skin diseases. Ruxolitinib cream, 1.5%, is approved for treating mild to moderate atopic dermatitis and nonsegmental vitiligo in patients ages 12 years and older.
The phase 2 double-blind, vehicle-controlled trial evaluated the efficacy and safety of ruxolitinib cream for mild HS. Researchers assigned 69 adults with Hurley stage I or II HS to receive 1.5% ruxolitinib cream or vehicle cream twice daily for 16 weeks. The primary endpoint was the change from baseline in AN count at week 16. To be eligible, patients had to have an AN count between 3 and 10.
“This is much more mild than what we have seen in any systemic therapy trials,” Dr. Porter said. “And, if patients had 3 lesions, they all needed to be in one anatomic area, but if they had 4-10 lesions, they had to have two anatomic areas involved. Also, no patients with active draining tunnels were allowed in the study.”
Of the 69 patients, 34 received ruxolitinib cream and 35 received vehicle. About 51% of patients in the vehicle arm were Black and 34% were White, while about 32% of patients in the ruxolitinib arm were Black and 56% were White.
The mean age of patients overall was 29 years, and about half the patients in both study arms had Hurley stage I disease, while the other half had Hurley stage II disease. Their average AN count ranged between 5.3 and 5.6 — mostly inflammatory nodules and few abscesses. Patients were not allowed to receive any type of intervention or rescue therapy during the study.
Dr. Porter reported that the least square mean change in AN count from baseline to week 16 was -2.42 in the vehicle arm vs -3.61 in the ruxolitinib cream arm (P <.05). The proportion of patients who achieved a 50% decrease in AN count was 79.2% in the ruxolitinib cream arm, compared with 56.5% of patients in the vehicle arm, respectively. More patients in the ruxolitinib cream arm achieved a 75% decrease in AN count (54.2% vs 25%), a 90% decrease in AN count (20.8 vs 12.5%), and a 100% decrease in AN count (20.8% vs 12.5%).
In other findings, 79.2% of patients in the ruxolitinib cream arm achieved a Hidradenitis Suppurativa Clinical Response score from baseline through week 16, compared with 50% of those in the vehicle group. The International Hidradenitis Suppurativa Severity Score System results favored the ruxolitinib cream arm (-4.46 vs -2.66 in the vehicle arm). Skin Pain and Itch numeric rating scale scores were moderate at baseline and improved similarly in both groups during the study.
Ruxolitinib cream was generally well tolerated over 16 weeks. No serious treatment-emergent adverse events were reported. The most common adverse event reported in the ruxolitinib cream group was COVID-19 and nasopharyngitis (two cases each) and one case of an application site reaction.
“Twice-daily 1.5% ruxolitinib cream was effective in patients with milder HS,” Dr. Porter concluded. “Modifications to our traditionally accepted clinical endpoints may be needed in studies of patients with milder HS.”
Jennifer L. Hsiao, MD, a dermatologist who directs the HS clinic at the University of Southern California, Los Angeles, who was asked to comment on the results, characterized the study as exciting for several reasons.
“First, with the global push in recent years to increase HS awareness, I am already seeing more patients earlier in their disease course with milder disease, and there is currently a gap in approved therapies for this patient population,” she told this news organization.
“Second, patients are very interested in topical therapies for HS and are thrilled whenever they learn that topical options are under investigation. This study had small patient numbers, but it was encouraging to see the positive results for ruxolitinib cream and that the treatment appeared well-tolerated.”
The trial was sponsored by the Incyte Corporation. Dr. Porter disclosed that she has received consulting fees from AbbVie, Alumis, Eli Lilly, Incyte, Janssen, Novartis, Pfizer, Prometheus Laboratories, Sanofi, Sonoma Biotherapeutics, Trifecta Clinical, and UCB. Dr. Hsiao disclosed that she is a member of the board of directors for the HS Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, and UCB; as a speaker for AbbVie, Novartis, and UCB; and as an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article appeared on Medscape.com .
FROM AAD 2024
Study Identifies Several Factors That Influence Longterm Antibiotic Prescribing for Acne
to follow them, according to the authors of a recently published study.
“This study explored why dermatologists still prescribe a good number of long-term antibiotics for people with acne,” the study’s senior author Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview. “And we found a lot of reasons.” The study was published online in JAMA Dermatology.
Using online surveys and semi-structured video interviews of 30 dermatologists, infectious disease physicians with expertise in antimicrobial stewardship, dermatology residents, and nonphysician clinicians, the investigators assessed respondents’ knowledge and attitudes regarding long-term antibiotics in acne. Salient themes impacting long-term antibiotic prescriptions included the following:
- A perceived dearth of evidence to justify changes in practice.
- Difficulties with iPLEDGE, the Risk Evaluation and Mitigation Strategy (REMS) for managing the teratogenic risks associated with isotretinoin, and with discussing oral contraceptives.
- “Navigating” discussions with about tapering-off of antibiotics.
- Challenging patient demands.
- A lack of effective tools for monitoring progress in antibiotic stewardship.
“It’s surprising there are so many barriers that make it difficult for dermatologists to stick with the guidelines even if they want to,” said Dr. Yeung, a coauthor of the recently released updated American Academy of Dermatology (AAD) acne management guidelines.
A dermatologist who wants to stop systemic antibiotics within 3 months may not know how to do so, he explained, or high demand for appointments may prevent timely follow-ups.
A major reason why dermatologists struggle to limit long-term antibiotic use is that there are very few substitutes that are perceived to work as well, said David J. Margolis, MD, PhD, who was not involved with the study and was asked to comment on the results. He is professor of epidemiology and dermatology at the University of Pennsylvania, Philadelphia.
“Part of the reason antibiotics are being used to treat acne is that they’re effective, and effective for severe disease,” he said. The alternatives, which are mostly topicals, said Dr. Margolis, do not work as well for moderate to severe disease or, with isotretinoin, involve time-consuming hurdles. Dr. Margolis said that he often hears such concerns from individual dermatologists. “But it’s helpful to see these in a well-organized, well-reported qualitative study.”
Infectious disease specialists surveyed considered limiting long-term antibiotic use as extremely important, while several dermatologists “argued that other specialties ‘underestimate the impact acne has on people’s lives,’ ” the authors wrote. Other respondents prioritized making the right choice for the patient at hand.
Although guidelines were never meant to be black and white, Dr. Yeung said, it is crucial to target the goal of tapering off after about 3-4 months — a cutoff with which guidelines from groups including the AAD, the Japanese Dermatological Association in guidelines from 2016, and 2017, respectively, and others concur.
He added, “Some folks believe that if the oral antibiotic is working, why stop? We need to develop evidence to show that reducing oral antibiotic use is important to our patients, not just to a theoretical problem of antibiotic resistance in society.” For example, in a study published in The Lancet in 2004, patients who used strictly topical regimens achieved efficacy similar to that of those who used only oral antibiotics.
In addition, some clinicians worried that limiting antibiotics could reduce patient satisfaction, spurring switches to other providers. However, he and the other authors of the JAMA Dermatology study noted that in a survey of patients with acne published in the Journal of Clinical and Aesthetic Dermatology in 2019, 76.9% said they would be “very or extremely likely” to use effective antibiotic-free treatments if offered.
Because most respondents were highly aware of the importance of antibiotic stewardship, Dr. Yeung said, additional passive education is not necessarily the answer. “It will take a concerted effort by our national societies to come up with resources and solutions for individual dermatologists to overcome some of these larger barriers.” Such solutions could range from training in communication and shared decision-making to implementing systems that provide individualized feedback to support antibiotic stewardship.
Many ongoing studies are examining antibiotic stewardship, Dr. Margolis said in the interview. However, he added, dermatologists’ idea of long-term use is 3 months, versus 1 month or less in other specialties. “Moreover, dermatology patients tend to be much healthier individuals and are rarely hospitalized, so there may be some issues comparing the ongoing studies to individuals with acne.” Future research will need to account for such differences, he said.
The study was funded by an American Acne & Rosacea Society Clinical Research Award. Dr. Yeung is associate editor of JAMA Dermatology. Dr. Margolis has received a National Institutes of Health grant to study doxycycline versus spironolactone in acne.
to follow them, according to the authors of a recently published study.
“This study explored why dermatologists still prescribe a good number of long-term antibiotics for people with acne,” the study’s senior author Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview. “And we found a lot of reasons.” The study was published online in JAMA Dermatology.
Using online surveys and semi-structured video interviews of 30 dermatologists, infectious disease physicians with expertise in antimicrobial stewardship, dermatology residents, and nonphysician clinicians, the investigators assessed respondents’ knowledge and attitudes regarding long-term antibiotics in acne. Salient themes impacting long-term antibiotic prescriptions included the following:
- A perceived dearth of evidence to justify changes in practice.
- Difficulties with iPLEDGE, the Risk Evaluation and Mitigation Strategy (REMS) for managing the teratogenic risks associated with isotretinoin, and with discussing oral contraceptives.
- “Navigating” discussions with about tapering-off of antibiotics.
- Challenging patient demands.
- A lack of effective tools for monitoring progress in antibiotic stewardship.
“It’s surprising there are so many barriers that make it difficult for dermatologists to stick with the guidelines even if they want to,” said Dr. Yeung, a coauthor of the recently released updated American Academy of Dermatology (AAD) acne management guidelines.
A dermatologist who wants to stop systemic antibiotics within 3 months may not know how to do so, he explained, or high demand for appointments may prevent timely follow-ups.
A major reason why dermatologists struggle to limit long-term antibiotic use is that there are very few substitutes that are perceived to work as well, said David J. Margolis, MD, PhD, who was not involved with the study and was asked to comment on the results. He is professor of epidemiology and dermatology at the University of Pennsylvania, Philadelphia.
“Part of the reason antibiotics are being used to treat acne is that they’re effective, and effective for severe disease,” he said. The alternatives, which are mostly topicals, said Dr. Margolis, do not work as well for moderate to severe disease or, with isotretinoin, involve time-consuming hurdles. Dr. Margolis said that he often hears such concerns from individual dermatologists. “But it’s helpful to see these in a well-organized, well-reported qualitative study.”
Infectious disease specialists surveyed considered limiting long-term antibiotic use as extremely important, while several dermatologists “argued that other specialties ‘underestimate the impact acne has on people’s lives,’ ” the authors wrote. Other respondents prioritized making the right choice for the patient at hand.
Although guidelines were never meant to be black and white, Dr. Yeung said, it is crucial to target the goal of tapering off after about 3-4 months — a cutoff with which guidelines from groups including the AAD, the Japanese Dermatological Association in guidelines from 2016, and 2017, respectively, and others concur.
He added, “Some folks believe that if the oral antibiotic is working, why stop? We need to develop evidence to show that reducing oral antibiotic use is important to our patients, not just to a theoretical problem of antibiotic resistance in society.” For example, in a study published in The Lancet in 2004, patients who used strictly topical regimens achieved efficacy similar to that of those who used only oral antibiotics.
In addition, some clinicians worried that limiting antibiotics could reduce patient satisfaction, spurring switches to other providers. However, he and the other authors of the JAMA Dermatology study noted that in a survey of patients with acne published in the Journal of Clinical and Aesthetic Dermatology in 2019, 76.9% said they would be “very or extremely likely” to use effective antibiotic-free treatments if offered.
Because most respondents were highly aware of the importance of antibiotic stewardship, Dr. Yeung said, additional passive education is not necessarily the answer. “It will take a concerted effort by our national societies to come up with resources and solutions for individual dermatologists to overcome some of these larger barriers.” Such solutions could range from training in communication and shared decision-making to implementing systems that provide individualized feedback to support antibiotic stewardship.
Many ongoing studies are examining antibiotic stewardship, Dr. Margolis said in the interview. However, he added, dermatologists’ idea of long-term use is 3 months, versus 1 month or less in other specialties. “Moreover, dermatology patients tend to be much healthier individuals and are rarely hospitalized, so there may be some issues comparing the ongoing studies to individuals with acne.” Future research will need to account for such differences, he said.
The study was funded by an American Acne & Rosacea Society Clinical Research Award. Dr. Yeung is associate editor of JAMA Dermatology. Dr. Margolis has received a National Institutes of Health grant to study doxycycline versus spironolactone in acne.
to follow them, according to the authors of a recently published study.
“This study explored why dermatologists still prescribe a good number of long-term antibiotics for people with acne,” the study’s senior author Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview. “And we found a lot of reasons.” The study was published online in JAMA Dermatology.
Using online surveys and semi-structured video interviews of 30 dermatologists, infectious disease physicians with expertise in antimicrobial stewardship, dermatology residents, and nonphysician clinicians, the investigators assessed respondents’ knowledge and attitudes regarding long-term antibiotics in acne. Salient themes impacting long-term antibiotic prescriptions included the following:
- A perceived dearth of evidence to justify changes in practice.
- Difficulties with iPLEDGE, the Risk Evaluation and Mitigation Strategy (REMS) for managing the teratogenic risks associated with isotretinoin, and with discussing oral contraceptives.
- “Navigating” discussions with about tapering-off of antibiotics.
- Challenging patient demands.
- A lack of effective tools for monitoring progress in antibiotic stewardship.
“It’s surprising there are so many barriers that make it difficult for dermatologists to stick with the guidelines even if they want to,” said Dr. Yeung, a coauthor of the recently released updated American Academy of Dermatology (AAD) acne management guidelines.
A dermatologist who wants to stop systemic antibiotics within 3 months may not know how to do so, he explained, or high demand for appointments may prevent timely follow-ups.
A major reason why dermatologists struggle to limit long-term antibiotic use is that there are very few substitutes that are perceived to work as well, said David J. Margolis, MD, PhD, who was not involved with the study and was asked to comment on the results. He is professor of epidemiology and dermatology at the University of Pennsylvania, Philadelphia.
“Part of the reason antibiotics are being used to treat acne is that they’re effective, and effective for severe disease,” he said. The alternatives, which are mostly topicals, said Dr. Margolis, do not work as well for moderate to severe disease or, with isotretinoin, involve time-consuming hurdles. Dr. Margolis said that he often hears such concerns from individual dermatologists. “But it’s helpful to see these in a well-organized, well-reported qualitative study.”
Infectious disease specialists surveyed considered limiting long-term antibiotic use as extremely important, while several dermatologists “argued that other specialties ‘underestimate the impact acne has on people’s lives,’ ” the authors wrote. Other respondents prioritized making the right choice for the patient at hand.
Although guidelines were never meant to be black and white, Dr. Yeung said, it is crucial to target the goal of tapering off after about 3-4 months — a cutoff with which guidelines from groups including the AAD, the Japanese Dermatological Association in guidelines from 2016, and 2017, respectively, and others concur.
He added, “Some folks believe that if the oral antibiotic is working, why stop? We need to develop evidence to show that reducing oral antibiotic use is important to our patients, not just to a theoretical problem of antibiotic resistance in society.” For example, in a study published in The Lancet in 2004, patients who used strictly topical regimens achieved efficacy similar to that of those who used only oral antibiotics.
In addition, some clinicians worried that limiting antibiotics could reduce patient satisfaction, spurring switches to other providers. However, he and the other authors of the JAMA Dermatology study noted that in a survey of patients with acne published in the Journal of Clinical and Aesthetic Dermatology in 2019, 76.9% said they would be “very or extremely likely” to use effective antibiotic-free treatments if offered.
Because most respondents were highly aware of the importance of antibiotic stewardship, Dr. Yeung said, additional passive education is not necessarily the answer. “It will take a concerted effort by our national societies to come up with resources and solutions for individual dermatologists to overcome some of these larger barriers.” Such solutions could range from training in communication and shared decision-making to implementing systems that provide individualized feedback to support antibiotic stewardship.
Many ongoing studies are examining antibiotic stewardship, Dr. Margolis said in the interview. However, he added, dermatologists’ idea of long-term use is 3 months, versus 1 month or less in other specialties. “Moreover, dermatology patients tend to be much healthier individuals and are rarely hospitalized, so there may be some issues comparing the ongoing studies to individuals with acne.” Future research will need to account for such differences, he said.
The study was funded by an American Acne & Rosacea Society Clinical Research Award. Dr. Yeung is associate editor of JAMA Dermatology. Dr. Margolis has received a National Institutes of Health grant to study doxycycline versus spironolactone in acne.
FROM JAMA DERMATOLOGY
Androgenetic Alopecia: Study Finds Efficacy of Topical and Oral Minoxidil Similar
Oral minoxidil, 5 mg once a day, “did not demonstrate superiority” over topical minoxidil, 5%, applied twice a day, after 24 weeks, reported Mariana Alvares Penha, MD, of the department of dermatology at São Paulo State University, in Botucatu, Brazil, and coauthors. Their randomized, controlled, double-blind study was published online in JAMA Dermatology.
Topical minoxidil is approved by the US Food and Drug Administration (FDA) for androgenetic alopecia (AGA), but there has been increasing interest worldwide in the use of low-dose oral minoxidil, a vasodilator approved as an antihypertensive, as an alternative treatment.
The trial “is important information that’s never been elucidated before,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview. The data, he added, can be used to reassure patients who do not want to take the oral form of the drug that a topical is just as effective.
“This study does let us counsel patients better and really give them the evidence,” said Shari Lipner, MD, PhD, associate professor of clinical dermatology at Weill Cornell Medicine, New York, who was also asked to comment on the results.
Both Dr. Lipner and Dr. Friedman said the study was well-designed.
The investigators enrolled 90 men aged 18-55; 68 completed the trial. Most had mild to moderate AGA. Men were excluded if they had received treatment for alopecia in the previous 6 months, a history of hair transplant, cardiopathy, nephropathy, dermatoses involving the scalp, any clinical conditions causing hair loss, or hypersensitivity to minoxidil.
They were randomized to receive either 5 mg of oral minoxidil a day, plus a placebo solution to apply to the scalp, or topical minoxidil solution (5%) applied twice a day plus placebo capsules. They were told to take a capsule at bedtime and to apply 1 mL of the solution to dry hair in the morning and at night.
The final analysis included 35 men in the topical group and 33 in the oral group (mean age, 36.6 years). Seven people in the topical group and 11 in the oral group were not able to attend the final appointment at 24 weeks. Three additional patients in the topical group dropped out for insomnia, hair shedding, and scalp eczema, while one dropped out of the oral group because of headache.
At 24 weeks, the percentage increase in terminal hair density in the oral minoxidil group was 27% higher (P = .005) in the vertex and 13% higher (P = .15) in the frontal scalp, compared with the topical-treated group.
Total hair density increased by 2% in the oral group compared with topical treatment in the vertex and decreased by 0.2% in the frontal area compared with topical treatment. None of these differences were statistically significant.
Three dermatologists blinded to the treatments, who analyzed photographs, determined that 60% of the men in the oral group and 48% in the topical group had clinical improvement in the frontal area, which was not statistically significant. More orally-treated patients had improvement in the vertex area: 70% compared with 46% of those on topical treatment (P = .04).
Hypertrichosis, Headache
Of the original 90 patients in the trial, more men taking oral minoxidil had hypertrichosis: 49% compared with 25% in the topical formulation group. Headache was also more common among those on oral minoxidil: six cases (14%) vs. one case (2%) among those on topical minoxidil. There was no difference in mean arterial blood pressure or resting heart rate between the two groups. Transient hair loss was more common with topical treatment, but it was not significant.
Dr. Friedman said that the study results would not change how he practices, but that it would give him data to use to inform patients who do not want to take oral minoxidil. He generally prescribes the oral form, unless patients do not want to take it or there is a medical contraindication, which he said is rare.
“I personally think oral is superior to topical,” mainly “because the patient’s actually using it,” said Dr. Friedman. “They’re more likely to take a pill a day versus apply something topically twice a day,” he added.
Both Dr. Lipner and Dr. Friedman said that they doubted that individuals could — or would want to — follow the twice-daily topical regimen used in the trial.
“In real life, not in the clinical trial scenario, it may be very hard for patients to comply with putting on the topical minoxidil twice a day or even once a day,” Dr. Lipner said.
However, she continues to prescribe more topical minoxidil than oral, because she believes “there’s less potential for side effects.” For patients who can adhere to the topical regimen, the study shows that they will get results, said Dr. Lipner.
Dr. Friedman, however, said that for patients who are looking at a lifetime of medication, “an oral will always win out on a topical to the scalp from an adherence perspective.”
The study was supported by the Brazilian Dermatology Society Support Fund. Dr. Penha reported receiving grants from the fund; no other disclosures were reported. Dr. Friedman and Dr. Lipner reported no conflicts related to minoxidil.
Oral minoxidil, 5 mg once a day, “did not demonstrate superiority” over topical minoxidil, 5%, applied twice a day, after 24 weeks, reported Mariana Alvares Penha, MD, of the department of dermatology at São Paulo State University, in Botucatu, Brazil, and coauthors. Their randomized, controlled, double-blind study was published online in JAMA Dermatology.
Topical minoxidil is approved by the US Food and Drug Administration (FDA) for androgenetic alopecia (AGA), but there has been increasing interest worldwide in the use of low-dose oral minoxidil, a vasodilator approved as an antihypertensive, as an alternative treatment.
The trial “is important information that’s never been elucidated before,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview. The data, he added, can be used to reassure patients who do not want to take the oral form of the drug that a topical is just as effective.
“This study does let us counsel patients better and really give them the evidence,” said Shari Lipner, MD, PhD, associate professor of clinical dermatology at Weill Cornell Medicine, New York, who was also asked to comment on the results.
Both Dr. Lipner and Dr. Friedman said the study was well-designed.
The investigators enrolled 90 men aged 18-55; 68 completed the trial. Most had mild to moderate AGA. Men were excluded if they had received treatment for alopecia in the previous 6 months, a history of hair transplant, cardiopathy, nephropathy, dermatoses involving the scalp, any clinical conditions causing hair loss, or hypersensitivity to minoxidil.
They were randomized to receive either 5 mg of oral minoxidil a day, plus a placebo solution to apply to the scalp, or topical minoxidil solution (5%) applied twice a day plus placebo capsules. They were told to take a capsule at bedtime and to apply 1 mL of the solution to dry hair in the morning and at night.
The final analysis included 35 men in the topical group and 33 in the oral group (mean age, 36.6 years). Seven people in the topical group and 11 in the oral group were not able to attend the final appointment at 24 weeks. Three additional patients in the topical group dropped out for insomnia, hair shedding, and scalp eczema, while one dropped out of the oral group because of headache.
At 24 weeks, the percentage increase in terminal hair density in the oral minoxidil group was 27% higher (P = .005) in the vertex and 13% higher (P = .15) in the frontal scalp, compared with the topical-treated group.
Total hair density increased by 2% in the oral group compared with topical treatment in the vertex and decreased by 0.2% in the frontal area compared with topical treatment. None of these differences were statistically significant.
Three dermatologists blinded to the treatments, who analyzed photographs, determined that 60% of the men in the oral group and 48% in the topical group had clinical improvement in the frontal area, which was not statistically significant. More orally-treated patients had improvement in the vertex area: 70% compared with 46% of those on topical treatment (P = .04).
Hypertrichosis, Headache
Of the original 90 patients in the trial, more men taking oral minoxidil had hypertrichosis: 49% compared with 25% in the topical formulation group. Headache was also more common among those on oral minoxidil: six cases (14%) vs. one case (2%) among those on topical minoxidil. There was no difference in mean arterial blood pressure or resting heart rate between the two groups. Transient hair loss was more common with topical treatment, but it was not significant.
Dr. Friedman said that the study results would not change how he practices, but that it would give him data to use to inform patients who do not want to take oral minoxidil. He generally prescribes the oral form, unless patients do not want to take it or there is a medical contraindication, which he said is rare.
“I personally think oral is superior to topical,” mainly “because the patient’s actually using it,” said Dr. Friedman. “They’re more likely to take a pill a day versus apply something topically twice a day,” he added.
Both Dr. Lipner and Dr. Friedman said that they doubted that individuals could — or would want to — follow the twice-daily topical regimen used in the trial.
“In real life, not in the clinical trial scenario, it may be very hard for patients to comply with putting on the topical minoxidil twice a day or even once a day,” Dr. Lipner said.
However, she continues to prescribe more topical minoxidil than oral, because she believes “there’s less potential for side effects.” For patients who can adhere to the topical regimen, the study shows that they will get results, said Dr. Lipner.
Dr. Friedman, however, said that for patients who are looking at a lifetime of medication, “an oral will always win out on a topical to the scalp from an adherence perspective.”
The study was supported by the Brazilian Dermatology Society Support Fund. Dr. Penha reported receiving grants from the fund; no other disclosures were reported. Dr. Friedman and Dr. Lipner reported no conflicts related to minoxidil.
Oral minoxidil, 5 mg once a day, “did not demonstrate superiority” over topical minoxidil, 5%, applied twice a day, after 24 weeks, reported Mariana Alvares Penha, MD, of the department of dermatology at São Paulo State University, in Botucatu, Brazil, and coauthors. Their randomized, controlled, double-blind study was published online in JAMA Dermatology.
Topical minoxidil is approved by the US Food and Drug Administration (FDA) for androgenetic alopecia (AGA), but there has been increasing interest worldwide in the use of low-dose oral minoxidil, a vasodilator approved as an antihypertensive, as an alternative treatment.
The trial “is important information that’s never been elucidated before,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview. The data, he added, can be used to reassure patients who do not want to take the oral form of the drug that a topical is just as effective.
“This study does let us counsel patients better and really give them the evidence,” said Shari Lipner, MD, PhD, associate professor of clinical dermatology at Weill Cornell Medicine, New York, who was also asked to comment on the results.
Both Dr. Lipner and Dr. Friedman said the study was well-designed.
The investigators enrolled 90 men aged 18-55; 68 completed the trial. Most had mild to moderate AGA. Men were excluded if they had received treatment for alopecia in the previous 6 months, a history of hair transplant, cardiopathy, nephropathy, dermatoses involving the scalp, any clinical conditions causing hair loss, or hypersensitivity to minoxidil.
They were randomized to receive either 5 mg of oral minoxidil a day, plus a placebo solution to apply to the scalp, or topical minoxidil solution (5%) applied twice a day plus placebo capsules. They were told to take a capsule at bedtime and to apply 1 mL of the solution to dry hair in the morning and at night.
The final analysis included 35 men in the topical group and 33 in the oral group (mean age, 36.6 years). Seven people in the topical group and 11 in the oral group were not able to attend the final appointment at 24 weeks. Three additional patients in the topical group dropped out for insomnia, hair shedding, and scalp eczema, while one dropped out of the oral group because of headache.
At 24 weeks, the percentage increase in terminal hair density in the oral minoxidil group was 27% higher (P = .005) in the vertex and 13% higher (P = .15) in the frontal scalp, compared with the topical-treated group.
Total hair density increased by 2% in the oral group compared with topical treatment in the vertex and decreased by 0.2% in the frontal area compared with topical treatment. None of these differences were statistically significant.
Three dermatologists blinded to the treatments, who analyzed photographs, determined that 60% of the men in the oral group and 48% in the topical group had clinical improvement in the frontal area, which was not statistically significant. More orally-treated patients had improvement in the vertex area: 70% compared with 46% of those on topical treatment (P = .04).
Hypertrichosis, Headache
Of the original 90 patients in the trial, more men taking oral minoxidil had hypertrichosis: 49% compared with 25% in the topical formulation group. Headache was also more common among those on oral minoxidil: six cases (14%) vs. one case (2%) among those on topical minoxidil. There was no difference in mean arterial blood pressure or resting heart rate between the two groups. Transient hair loss was more common with topical treatment, but it was not significant.
Dr. Friedman said that the study results would not change how he practices, but that it would give him data to use to inform patients who do not want to take oral minoxidil. He generally prescribes the oral form, unless patients do not want to take it or there is a medical contraindication, which he said is rare.
“I personally think oral is superior to topical,” mainly “because the patient’s actually using it,” said Dr. Friedman. “They’re more likely to take a pill a day versus apply something topically twice a day,” he added.
Both Dr. Lipner and Dr. Friedman said that they doubted that individuals could — or would want to — follow the twice-daily topical regimen used in the trial.
“In real life, not in the clinical trial scenario, it may be very hard for patients to comply with putting on the topical minoxidil twice a day or even once a day,” Dr. Lipner said.
However, she continues to prescribe more topical minoxidil than oral, because she believes “there’s less potential for side effects.” For patients who can adhere to the topical regimen, the study shows that they will get results, said Dr. Lipner.
Dr. Friedman, however, said that for patients who are looking at a lifetime of medication, “an oral will always win out on a topical to the scalp from an adherence perspective.”
The study was supported by the Brazilian Dermatology Society Support Fund. Dr. Penha reported receiving grants from the fund; no other disclosures were reported. Dr. Friedman and Dr. Lipner reported no conflicts related to minoxidil.
FROM JAMA DERMATOLOGY
Bimekizumab Under FDA Review for Hidradenitis Suppurativa
On April 4, 2024, the US .
The agency also accepted a second sBLA for a bimekizumab-bkzx 2-mL device.
The developments were announced in a press release from UCB, the manufacturer of bimekizumab-bkzx (Bimzelx), which was first approved in the United States in October 2023 for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
According to the press release, acceptance of the sBLA was based on results from two phase 3 studies known as BE HEARD I and BE HEARD II, which found that bimekizumab-bkzx showed clinically meaningful improvements compared with placebo at week 16 and were sustained to week 48. If approved, this would be the first HS approval for bimekizumab-bkzx worldwide. In the European Union, it is approved for treating adults with psoriatic arthritis and axial spondyloarthritis, in addition to moderate to severe psoriasis.
According to the company, approval of the 2-mL injection device would mean that patients would have an alternative one-injection regimen option; currently, one dose for psoriasis is administered as two 1-mL injections. Full US prescribing information for bimekizumab-bkzx can be found here.
A version of this article first appeared on Medscape.com.
On April 4, 2024, the US .
The agency also accepted a second sBLA for a bimekizumab-bkzx 2-mL device.
The developments were announced in a press release from UCB, the manufacturer of bimekizumab-bkzx (Bimzelx), which was first approved in the United States in October 2023 for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
According to the press release, acceptance of the sBLA was based on results from two phase 3 studies known as BE HEARD I and BE HEARD II, which found that bimekizumab-bkzx showed clinically meaningful improvements compared with placebo at week 16 and were sustained to week 48. If approved, this would be the first HS approval for bimekizumab-bkzx worldwide. In the European Union, it is approved for treating adults with psoriatic arthritis and axial spondyloarthritis, in addition to moderate to severe psoriasis.
According to the company, approval of the 2-mL injection device would mean that patients would have an alternative one-injection regimen option; currently, one dose for psoriasis is administered as two 1-mL injections. Full US prescribing information for bimekizumab-bkzx can be found here.
A version of this article first appeared on Medscape.com.
On April 4, 2024, the US .
The agency also accepted a second sBLA for a bimekizumab-bkzx 2-mL device.
The developments were announced in a press release from UCB, the manufacturer of bimekizumab-bkzx (Bimzelx), which was first approved in the United States in October 2023 for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
According to the press release, acceptance of the sBLA was based on results from two phase 3 studies known as BE HEARD I and BE HEARD II, which found that bimekizumab-bkzx showed clinically meaningful improvements compared with placebo at week 16 and were sustained to week 48. If approved, this would be the first HS approval for bimekizumab-bkzx worldwide. In the European Union, it is approved for treating adults with psoriatic arthritis and axial spondyloarthritis, in addition to moderate to severe psoriasis.
According to the company, approval of the 2-mL injection device would mean that patients would have an alternative one-injection regimen option; currently, one dose for psoriasis is administered as two 1-mL injections. Full US prescribing information for bimekizumab-bkzx can be found here.
A version of this article first appeared on Medscape.com.
Childhood Atopic Dermatitis Linked to IBD Risk
TOPLINE:
, but atopic manifestations are generally not associated with IBD.
METHODOLOGY:
- Studies examining the link between atopy and IBD have yielded inconsistent results. Many of these studies included adults, introducing recall bias, or relied on physician diagnoses that might have overlooked mild cases.
- Researchers analyzed prospectively collected data on 83,311 children from two cohort studies, ABIS (1997-1999) and MoBa (1999-2008), who were followed up from birth until 2021 or a diagnosis of IBD.
- Information on parents was collected prospectively via questionnaires on any atopy their children might have developed by the age of 3 years. Atopy included conditions such as AD, asthma, food allergy, or allergic rhinitis.
TAKEAWAY:
- A total of 301 participants were diagnosed with IBD over 1,174,756 person-years of follow-up. By the age of 3 years, 31,671 children (38%) were reported to have any atopic manifestation.
- Children with AD at the age of 3 years demonstrated a significantly higher risk for IBD (pooled adjusted hazard ratio [aHR], 1.46), Crohn’s disease (pooled aHR, 1.53), and ulcerative colitis (pooled aHR, 1.78).
- Any atopic manifestation by the age of 3 years was not associated with a subsequent risk for IBD, Crohn’s disease, or ulcerative colitis, nor were analyses focused on early-life food-related allergy, asthma, and allergic rhinitis.
IN PRACTICE:
According to the authors, these findings suggested potential shared underlying causes between AD and IBD, which could help identify individuals at risk, and “a deeper understanding could significantly benefit the development of novel treatment approaches capable of effectively addressing both conditions, consequently enhancing patient outcomes.”
SOURCE:
This study, led by Tereza Lerchova, MD, PhD, Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, was published online in The Journal of Pediatrics.
LIMITATIONS:
The findings of this study were mostly related to childhood-onset IBD instead of IBD in adult life. Lower participation in the MoBa study could limit generalizability to a broader population. In addition, there might have been lower participation from families without atopic manifestations.
DISCLOSURES:
The study was funded by the Swedish Society for Medical Research, Swedish Research Council, and ALF and supported by grants from the Swedish Child Diabetes Foundation, Swedish Council for Working Life and Social Research, Swedish Research Council, Medical Research Council of Southeast Sweden, JDRF Wallenberg Foundation, Linkoping University, and Joanna Cocozza Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, but atopic manifestations are generally not associated with IBD.
METHODOLOGY:
- Studies examining the link between atopy and IBD have yielded inconsistent results. Many of these studies included adults, introducing recall bias, or relied on physician diagnoses that might have overlooked mild cases.
- Researchers analyzed prospectively collected data on 83,311 children from two cohort studies, ABIS (1997-1999) and MoBa (1999-2008), who were followed up from birth until 2021 or a diagnosis of IBD.
- Information on parents was collected prospectively via questionnaires on any atopy their children might have developed by the age of 3 years. Atopy included conditions such as AD, asthma, food allergy, or allergic rhinitis.
TAKEAWAY:
- A total of 301 participants were diagnosed with IBD over 1,174,756 person-years of follow-up. By the age of 3 years, 31,671 children (38%) were reported to have any atopic manifestation.
- Children with AD at the age of 3 years demonstrated a significantly higher risk for IBD (pooled adjusted hazard ratio [aHR], 1.46), Crohn’s disease (pooled aHR, 1.53), and ulcerative colitis (pooled aHR, 1.78).
- Any atopic manifestation by the age of 3 years was not associated with a subsequent risk for IBD, Crohn’s disease, or ulcerative colitis, nor were analyses focused on early-life food-related allergy, asthma, and allergic rhinitis.
IN PRACTICE:
According to the authors, these findings suggested potential shared underlying causes between AD and IBD, which could help identify individuals at risk, and “a deeper understanding could significantly benefit the development of novel treatment approaches capable of effectively addressing both conditions, consequently enhancing patient outcomes.”
SOURCE:
This study, led by Tereza Lerchova, MD, PhD, Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, was published online in The Journal of Pediatrics.
LIMITATIONS:
The findings of this study were mostly related to childhood-onset IBD instead of IBD in adult life. Lower participation in the MoBa study could limit generalizability to a broader population. In addition, there might have been lower participation from families without atopic manifestations.
DISCLOSURES:
The study was funded by the Swedish Society for Medical Research, Swedish Research Council, and ALF and supported by grants from the Swedish Child Diabetes Foundation, Swedish Council for Working Life and Social Research, Swedish Research Council, Medical Research Council of Southeast Sweden, JDRF Wallenberg Foundation, Linkoping University, and Joanna Cocozza Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, but atopic manifestations are generally not associated with IBD.
METHODOLOGY:
- Studies examining the link between atopy and IBD have yielded inconsistent results. Many of these studies included adults, introducing recall bias, or relied on physician diagnoses that might have overlooked mild cases.
- Researchers analyzed prospectively collected data on 83,311 children from two cohort studies, ABIS (1997-1999) and MoBa (1999-2008), who were followed up from birth until 2021 or a diagnosis of IBD.
- Information on parents was collected prospectively via questionnaires on any atopy their children might have developed by the age of 3 years. Atopy included conditions such as AD, asthma, food allergy, or allergic rhinitis.
TAKEAWAY:
- A total of 301 participants were diagnosed with IBD over 1,174,756 person-years of follow-up. By the age of 3 years, 31,671 children (38%) were reported to have any atopic manifestation.
- Children with AD at the age of 3 years demonstrated a significantly higher risk for IBD (pooled adjusted hazard ratio [aHR], 1.46), Crohn’s disease (pooled aHR, 1.53), and ulcerative colitis (pooled aHR, 1.78).
- Any atopic manifestation by the age of 3 years was not associated with a subsequent risk for IBD, Crohn’s disease, or ulcerative colitis, nor were analyses focused on early-life food-related allergy, asthma, and allergic rhinitis.
IN PRACTICE:
According to the authors, these findings suggested potential shared underlying causes between AD and IBD, which could help identify individuals at risk, and “a deeper understanding could significantly benefit the development of novel treatment approaches capable of effectively addressing both conditions, consequently enhancing patient outcomes.”
SOURCE:
This study, led by Tereza Lerchova, MD, PhD, Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, was published online in The Journal of Pediatrics.
LIMITATIONS:
The findings of this study were mostly related to childhood-onset IBD instead of IBD in adult life. Lower participation in the MoBa study could limit generalizability to a broader population. In addition, there might have been lower participation from families without atopic manifestations.
DISCLOSURES:
The study was funded by the Swedish Society for Medical Research, Swedish Research Council, and ALF and supported by grants from the Swedish Child Diabetes Foundation, Swedish Council for Working Life and Social Research, Swedish Research Council, Medical Research Council of Southeast Sweden, JDRF Wallenberg Foundation, Linkoping University, and Joanna Cocozza Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Repeat MCED Testing May ID Early-Stage and Unscreened Cancers
This was the conclusion of recent data presented by Ora Karp Gordon, MD, MS, during a session at the American Association for Cancer Research annual meeting.
The MCED test, known as Galleri, was made clinically available in the United States in April 2021. Developed by GRAIL LLC, the test analyzes cell-free DNA in the blood using targeted methylation analysis and machine learning to detect the presence of a cancer signal and determine its organ of origin or cancer signal origin. The initial screening of over 53,000 individuals with the Galleri test detected a cancer signal in 1.1% of participants.
The new real-world analysis examines the outcomes of repeat MCED testing in 5,794 individuals.
The study looked at individuals who initially received a ‘no cancer signal detected’ result and then underwent a second Galleri test. Over 80% of participants received their follow-up test 10-18 months after the first, with a median interval between blood draws of 12.9 months.
“The repeat tests detect those cancer cases that have reached the detection threshold since their last MCED test, which should be less than one year of incidence,” Dr. Gordon, professor at Saint John’s Cancer Institute, Santa Monica, California, said in an interview. “We are just now starting to see results from patients who get their second and even third round of screening.”
“Galleri is recommended to be used annually in addition to USPSTF [US Preventive Services Task Force]–recommended cancer screening tests, like mammography and colonoscopy,” she said.
This recommendation is based on a modeling study suggesting that annual screening would improve stage shift, diagnostic yield, and potentially mortality when compared to biennial screening, although biennial screening was still favorable compared with no screening, she explained.
Early Real-World Evidence of Repeat Testing
Among the cohort of 5,794 individuals who received repeat testing, 26 received a positive cancer signal on their second test, yielding a cancer signal detection rate of 0.45% (95% CI: 0.31%-0.66%). The cancer signal detection rate was slightly higher in men. The rate was 0.50% (95% CI: 0.32%-0.81%; 17 of 3367) in men versus 0.37% (95% CI: 0.2%-0.7%; 9 of 2427) in women.
During her presentation, Dr. Gordon highlighted that the repeat testing signal detection rate was lower than the initial 0.95% rate (95% CI: 0.87-1.0; 510 of 53,744) seen in the previous larger cohort of patients who were retested at 1 year.
She acknowledged that the lower cancer signal detection rate of repeat testing may indicate some degree of ‘early adopter’ bias, where those who return for a second test are systematically different from the general screening population. This could suggest that broader population-level screening may yield different results, she continued.
Shift Toward Unscreened Cancers
The top cancer types identified in the second round of testing were lymphoid, head and neck, bladder/urothelial, colorectal, and anal cancers. Clinicians were able to confirm clinical outcomes in 12 of 26 cases, in which cancer signals were detected. Of those 12 cases, 8 individuals received a cancer diagnosis and 4 did not have cancer. The remaining 14 of 26 cases in which cancer signals were detected are still under investigation.
“We found a shift away from USPSTF screen-detected cancers, like breast, lung, and prostate, and relative increase in unscreened urinary, head and neck, and lymphoid cancers, with 75% of cancers being those without any screening guidelines,” Dr. Gordon said in an interview.
She added that patients who choose to retest may have different cancer rates for several reasons, including bias toward a population that is health conscious and adhered to all recommended cancer screening.
“So the shift toward unscreened cancers is not unexpected and highlights the value of Galleri,” she said, but also acknowledged that “continued monitoring is needed to see if this translates in a persistent finding over time and tests.”
Shift Toward Early-Stage Cancers
Staging information was available for five cases, and Dr. Gordon highlighted in her talk that four of these confirmed cancers were stage I, including cancers of the anus, head and neck, bladder, and lymphoma. The fifth confirmed cancer with staging information was stage IV ovarian cancer.
“It is still early, and the numbers are very small, but the detection of early-stage cancers with second annual testing is very encouraging as these are the cases where MCED testing could have the greatest impact in improving outcomes through earlier treatment,” Dr. Gordon told this publication.
During an interview after the talk, Kenneth L. Kehl, MD, MPH, echoed that data must be confirmed in larger cohorts.
“The shift toward earlier stage cancers that are less detectable by standard screening methods is an interesting result, but we need to be cautious since the numbers were relatively small, and we do not have data on cancers that were diagnosed among patients whose second MCED test was also negative,” said Dr. Kehl, a medical oncologist at Dana-Farber Cancer Institute, Boston.
MCED Results Could Help Direct Diagnostic Workup
The test’s ability to predict the organ of origin was highly accurate, correctly identifying the cancer type in all eight confirmed cases. Among the eight cases with a confirmed cancer diagnosis, the accuracy of the first prediction was 100%, and diagnoses included invasive cancers across multiple tissues and organs, including anus, colon, head and neck, urothelial tract, ovary, and the lymphatic system.
“The fact that the site of origin for 100% of confirmed cancers was accurately predicted with GRAIL’s CSO by Galleri test confirms the promise that this can guide workup when a cancer signal is detected,” Dr. Gordon noted in the interview.
Looking Ahead
Dr. Kehl, who was not involved in the MCED study, noted in an interview that “further data on test characteristics beyond positive predictive value, including the sensitivity, specificity, and negative predictive value, as well as demonstration of clinical benefit — ideally in a randomized trial — will likely be required for MCED testing to become a standard public health recommendation.”
He added that challenges associated with implementing annual screening with MCED tests include the risks of both false positives and false negatives as testing becomes more widely available.
“False positives cause anxiety and lead to additional testing that may carry its own risks, and we need to understand if potentially false negative tests will be associated with less uptake of established screening strategies,” Dr. Kehl said in an interview. However, he noted that serial testing could lead to more frequent diagnoses of early-stage cancers that may be less detectable by standard methods.
Dr. Gordon reported financial relationships with GRAIL LLC and Genetic Technologies Corporation. Dr. Kehl reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing healthcare products used by or on patients.
This was the conclusion of recent data presented by Ora Karp Gordon, MD, MS, during a session at the American Association for Cancer Research annual meeting.
The MCED test, known as Galleri, was made clinically available in the United States in April 2021. Developed by GRAIL LLC, the test analyzes cell-free DNA in the blood using targeted methylation analysis and machine learning to detect the presence of a cancer signal and determine its organ of origin or cancer signal origin. The initial screening of over 53,000 individuals with the Galleri test detected a cancer signal in 1.1% of participants.
The new real-world analysis examines the outcomes of repeat MCED testing in 5,794 individuals.
The study looked at individuals who initially received a ‘no cancer signal detected’ result and then underwent a second Galleri test. Over 80% of participants received their follow-up test 10-18 months after the first, with a median interval between blood draws of 12.9 months.
“The repeat tests detect those cancer cases that have reached the detection threshold since their last MCED test, which should be less than one year of incidence,” Dr. Gordon, professor at Saint John’s Cancer Institute, Santa Monica, California, said in an interview. “We are just now starting to see results from patients who get their second and even third round of screening.”
“Galleri is recommended to be used annually in addition to USPSTF [US Preventive Services Task Force]–recommended cancer screening tests, like mammography and colonoscopy,” she said.
This recommendation is based on a modeling study suggesting that annual screening would improve stage shift, diagnostic yield, and potentially mortality when compared to biennial screening, although biennial screening was still favorable compared with no screening, she explained.
Early Real-World Evidence of Repeat Testing
Among the cohort of 5,794 individuals who received repeat testing, 26 received a positive cancer signal on their second test, yielding a cancer signal detection rate of 0.45% (95% CI: 0.31%-0.66%). The cancer signal detection rate was slightly higher in men. The rate was 0.50% (95% CI: 0.32%-0.81%; 17 of 3367) in men versus 0.37% (95% CI: 0.2%-0.7%; 9 of 2427) in women.
During her presentation, Dr. Gordon highlighted that the repeat testing signal detection rate was lower than the initial 0.95% rate (95% CI: 0.87-1.0; 510 of 53,744) seen in the previous larger cohort of patients who were retested at 1 year.
She acknowledged that the lower cancer signal detection rate of repeat testing may indicate some degree of ‘early adopter’ bias, where those who return for a second test are systematically different from the general screening population. This could suggest that broader population-level screening may yield different results, she continued.
Shift Toward Unscreened Cancers
The top cancer types identified in the second round of testing were lymphoid, head and neck, bladder/urothelial, colorectal, and anal cancers. Clinicians were able to confirm clinical outcomes in 12 of 26 cases, in which cancer signals were detected. Of those 12 cases, 8 individuals received a cancer diagnosis and 4 did not have cancer. The remaining 14 of 26 cases in which cancer signals were detected are still under investigation.
“We found a shift away from USPSTF screen-detected cancers, like breast, lung, and prostate, and relative increase in unscreened urinary, head and neck, and lymphoid cancers, with 75% of cancers being those without any screening guidelines,” Dr. Gordon said in an interview.
She added that patients who choose to retest may have different cancer rates for several reasons, including bias toward a population that is health conscious and adhered to all recommended cancer screening.
“So the shift toward unscreened cancers is not unexpected and highlights the value of Galleri,” she said, but also acknowledged that “continued monitoring is needed to see if this translates in a persistent finding over time and tests.”
Shift Toward Early-Stage Cancers
Staging information was available for five cases, and Dr. Gordon highlighted in her talk that four of these confirmed cancers were stage I, including cancers of the anus, head and neck, bladder, and lymphoma. The fifth confirmed cancer with staging information was stage IV ovarian cancer.
“It is still early, and the numbers are very small, but the detection of early-stage cancers with second annual testing is very encouraging as these are the cases where MCED testing could have the greatest impact in improving outcomes through earlier treatment,” Dr. Gordon told this publication.
During an interview after the talk, Kenneth L. Kehl, MD, MPH, echoed that data must be confirmed in larger cohorts.
“The shift toward earlier stage cancers that are less detectable by standard screening methods is an interesting result, but we need to be cautious since the numbers were relatively small, and we do not have data on cancers that were diagnosed among patients whose second MCED test was also negative,” said Dr. Kehl, a medical oncologist at Dana-Farber Cancer Institute, Boston.
MCED Results Could Help Direct Diagnostic Workup
The test’s ability to predict the organ of origin was highly accurate, correctly identifying the cancer type in all eight confirmed cases. Among the eight cases with a confirmed cancer diagnosis, the accuracy of the first prediction was 100%, and diagnoses included invasive cancers across multiple tissues and organs, including anus, colon, head and neck, urothelial tract, ovary, and the lymphatic system.
“The fact that the site of origin for 100% of confirmed cancers was accurately predicted with GRAIL’s CSO by Galleri test confirms the promise that this can guide workup when a cancer signal is detected,” Dr. Gordon noted in the interview.
Looking Ahead
Dr. Kehl, who was not involved in the MCED study, noted in an interview that “further data on test characteristics beyond positive predictive value, including the sensitivity, specificity, and negative predictive value, as well as demonstration of clinical benefit — ideally in a randomized trial — will likely be required for MCED testing to become a standard public health recommendation.”
He added that challenges associated with implementing annual screening with MCED tests include the risks of both false positives and false negatives as testing becomes more widely available.
“False positives cause anxiety and lead to additional testing that may carry its own risks, and we need to understand if potentially false negative tests will be associated with less uptake of established screening strategies,” Dr. Kehl said in an interview. However, he noted that serial testing could lead to more frequent diagnoses of early-stage cancers that may be less detectable by standard methods.
Dr. Gordon reported financial relationships with GRAIL LLC and Genetic Technologies Corporation. Dr. Kehl reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing healthcare products used by or on patients.
This was the conclusion of recent data presented by Ora Karp Gordon, MD, MS, during a session at the American Association for Cancer Research annual meeting.
The MCED test, known as Galleri, was made clinically available in the United States in April 2021. Developed by GRAIL LLC, the test analyzes cell-free DNA in the blood using targeted methylation analysis and machine learning to detect the presence of a cancer signal and determine its organ of origin or cancer signal origin. The initial screening of over 53,000 individuals with the Galleri test detected a cancer signal in 1.1% of participants.
The new real-world analysis examines the outcomes of repeat MCED testing in 5,794 individuals.
The study looked at individuals who initially received a ‘no cancer signal detected’ result and then underwent a second Galleri test. Over 80% of participants received their follow-up test 10-18 months after the first, with a median interval between blood draws of 12.9 months.
“The repeat tests detect those cancer cases that have reached the detection threshold since their last MCED test, which should be less than one year of incidence,” Dr. Gordon, professor at Saint John’s Cancer Institute, Santa Monica, California, said in an interview. “We are just now starting to see results from patients who get their second and even third round of screening.”
“Galleri is recommended to be used annually in addition to USPSTF [US Preventive Services Task Force]–recommended cancer screening tests, like mammography and colonoscopy,” she said.
This recommendation is based on a modeling study suggesting that annual screening would improve stage shift, diagnostic yield, and potentially mortality when compared to biennial screening, although biennial screening was still favorable compared with no screening, she explained.
Early Real-World Evidence of Repeat Testing
Among the cohort of 5,794 individuals who received repeat testing, 26 received a positive cancer signal on their second test, yielding a cancer signal detection rate of 0.45% (95% CI: 0.31%-0.66%). The cancer signal detection rate was slightly higher in men. The rate was 0.50% (95% CI: 0.32%-0.81%; 17 of 3367) in men versus 0.37% (95% CI: 0.2%-0.7%; 9 of 2427) in women.
During her presentation, Dr. Gordon highlighted that the repeat testing signal detection rate was lower than the initial 0.95% rate (95% CI: 0.87-1.0; 510 of 53,744) seen in the previous larger cohort of patients who were retested at 1 year.
She acknowledged that the lower cancer signal detection rate of repeat testing may indicate some degree of ‘early adopter’ bias, where those who return for a second test are systematically different from the general screening population. This could suggest that broader population-level screening may yield different results, she continued.
Shift Toward Unscreened Cancers
The top cancer types identified in the second round of testing were lymphoid, head and neck, bladder/urothelial, colorectal, and anal cancers. Clinicians were able to confirm clinical outcomes in 12 of 26 cases, in which cancer signals were detected. Of those 12 cases, 8 individuals received a cancer diagnosis and 4 did not have cancer. The remaining 14 of 26 cases in which cancer signals were detected are still under investigation.
“We found a shift away from USPSTF screen-detected cancers, like breast, lung, and prostate, and relative increase in unscreened urinary, head and neck, and lymphoid cancers, with 75% of cancers being those without any screening guidelines,” Dr. Gordon said in an interview.
She added that patients who choose to retest may have different cancer rates for several reasons, including bias toward a population that is health conscious and adhered to all recommended cancer screening.
“So the shift toward unscreened cancers is not unexpected and highlights the value of Galleri,” she said, but also acknowledged that “continued monitoring is needed to see if this translates in a persistent finding over time and tests.”
Shift Toward Early-Stage Cancers
Staging information was available for five cases, and Dr. Gordon highlighted in her talk that four of these confirmed cancers were stage I, including cancers of the anus, head and neck, bladder, and lymphoma. The fifth confirmed cancer with staging information was stage IV ovarian cancer.
“It is still early, and the numbers are very small, but the detection of early-stage cancers with second annual testing is very encouraging as these are the cases where MCED testing could have the greatest impact in improving outcomes through earlier treatment,” Dr. Gordon told this publication.
During an interview after the talk, Kenneth L. Kehl, MD, MPH, echoed that data must be confirmed in larger cohorts.
“The shift toward earlier stage cancers that are less detectable by standard screening methods is an interesting result, but we need to be cautious since the numbers were relatively small, and we do not have data on cancers that were diagnosed among patients whose second MCED test was also negative,” said Dr. Kehl, a medical oncologist at Dana-Farber Cancer Institute, Boston.
MCED Results Could Help Direct Diagnostic Workup
The test’s ability to predict the organ of origin was highly accurate, correctly identifying the cancer type in all eight confirmed cases. Among the eight cases with a confirmed cancer diagnosis, the accuracy of the first prediction was 100%, and diagnoses included invasive cancers across multiple tissues and organs, including anus, colon, head and neck, urothelial tract, ovary, and the lymphatic system.
“The fact that the site of origin for 100% of confirmed cancers was accurately predicted with GRAIL’s CSO by Galleri test confirms the promise that this can guide workup when a cancer signal is detected,” Dr. Gordon noted in the interview.
Looking Ahead
Dr. Kehl, who was not involved in the MCED study, noted in an interview that “further data on test characteristics beyond positive predictive value, including the sensitivity, specificity, and negative predictive value, as well as demonstration of clinical benefit — ideally in a randomized trial — will likely be required for MCED testing to become a standard public health recommendation.”
He added that challenges associated with implementing annual screening with MCED tests include the risks of both false positives and false negatives as testing becomes more widely available.
“False positives cause anxiety and lead to additional testing that may carry its own risks, and we need to understand if potentially false negative tests will be associated with less uptake of established screening strategies,” Dr. Kehl said in an interview. However, he noted that serial testing could lead to more frequent diagnoses of early-stage cancers that may be less detectable by standard methods.
Dr. Gordon reported financial relationships with GRAIL LLC and Genetic Technologies Corporation. Dr. Kehl reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing healthcare products used by or on patients.
FROM AACR 2024
Medicine or Politics? Doctors Defend Their Social Activism
It should come as no surprise that when physicians speak out on social and political issues, there is sometimes a backlash. This can range from the typical trolling that occurs online to rarer cases of professional penalties. Two doctors were fired by NYU Langone Health late last year after they posted social media messages about the Israel-Hamas war. Still, many physicians are not only willing to stand up for what they believe in, but they see it as an essential part of their profession.
"We're now at a place where doctors need to engage in public advocacy as an urgent part of our job," wrote Rob Davidson, MD, an emergency department physician, at the onslaught of the COVID-19 pandemic. In an Op-Ed piece for The Guardian, Dr. Davidson noted how the virus forced many physicians into becoming "activist doctors," calling for adequate personal protective equipment and correcting misinformation. "What we want above all is for the administration to listen to doctors, nurses, and frontline health workers - and stop playing politics," he wrote.
'It's Not About Being Political'
The intersection of medicine and politics is hardly new. Doctors frequently testify before Congress, sharing their expertise on issues concerning public health. This, however, isn't the same as "playing politics."
"I'm not taking political stances," said Megan Ranney, MD, Dean of the Yale School of Public Health. "Rather, I'm using science to inform best practices, and I'm vocal around the area where I have expertise where we could do collectively better."
Dr. Ranney's work to end firearm injury and death garnered particular attention when she co-authored an open letter to the National Rifle Association (NRA) in 2018. She wrote the letter in response to a tweet by the organization, admonishing physicians to "stay in their lane" when it comes to gun control.
Dr. Ranney's letter discussed gun violence as a public health crisis and urged the NRA to "be part of the solution" by joining the collective effort to reduce firearm injury and death through research, education, and advocacy. "We are not anti-gun," she stated. "We are anti-bullet hole," adding that "almost half of doctors own guns."
The NRA disagreed. When Dr. Ranney testified before Congress during a hearing on gun violence in 2023, NRA spokesperson Billy McLaughlin condemned her testimony as an effort to "dismantle the Second Amendment," calling Dr. Ranney "a known gun control extremist."
"If you actually read what I write, or if you actually listen to what I say, I'm not saying things on behalf of one political party or another," said Dr. Ranney. "It's not about being political. It's about recognizing our role in describing what's happening and making it clear for the world to see. Showing where, based off of data, there may be a better path to improve health and wellbeing."
In spite of the backlash, Dr. Ranney has no regrets about being an activist. "In the current media landscape, folks love to slap labels on people that may or may not be accurate. To me, what matters isn't where I land with a particular politician or political party, but how the work that I do improves health for populations."
When the Need to Act Outweighs the Fear
Laura Andreson, DO, an ob.gyn, took activism a step further when she joined a group of women in Tennessee to file a suit against the state, the attorney general, and the state board of medical examiners. The issue was the Tennessee's abortion ban, which the suit claimed prevented women from getting "necessary and potentially life-saving medical care."
Dr. Andreson, who says she was "not at all" politically active in the past, began to realize how the abortion ban could drastically affect her profession and her patients. "I don't know what flipped in me, but I just felt like I could do this," she said.
Like Dr. Ranney, Dr. Andreson has been as visible as she has been vocal, giving press conferences and interviews, but she acknowledges she has some fears about safety. In fact, after filing the lawsuit, the Center for Reproductive Rights recommended that she go to a website, DeleteMe, that removes personal data from the internet, making it more difficult for people to find her information. "But my need to do this and my desire to do this is stronger than my fears," she added.
Dr. Andreson, who is part of a small practice, did check with both her coworkers and the hospital administration before moving forward with the lawsuit. She was relieved to find that she had the support of her practice and that there wasn't anything in the hospital bylaws to prevent her from filing the lawsuit. "But the people in the bigger institutions who probably have an even better expert base than I do, they are handcuffed," she said.
It has been, in Dr. Andreson's words, "a little uncomfortable" being on the board of the Tennessee Medical Association when the Tennessee Board of Medical Examiners is part of the lawsuit. "We're all members of the same group," she said. "But I'm not suing them as individuals; I'm suing them as an entity that is under our government."
Dr. Andreson said most people have been supportive of her activist work, though she admitted to feeling frustrated when she encounters apathy from fellow ob.gyns. She got little response when she circulated information explaining the abortion laws and trying to get others involved. But she still sees education as being a key part of making change happen.
"I think advocacy, as someone who is considered a responsible, trustworthy person by your community, is important, because you can sway some people just by educating them," she said.
Fighting Inequities in Medicine and Beyond
Christina Chen, MD, says she felt very supported by her medical community at the Mayo Clinic in Rochester, Minnesota, when she and 16 other Asian American physicians posted a video on Instagram in 2020 highlighting increased violence and harassment of Asian Americans during COVID-19. It soon went viral, and the Mayo Clinic distributed it across their social media channels. The only negative repercussions Mayo faced were a few posts on social media saying that politics should not be brought into the healthcare space. Dr. Chen disagrees.
"Social issues and political decisions have direct impact on the health of our communities," Dr. Chen said. "We know that we still have a long way to go to solve health inequities, which is a public health problem, and we all play a huge role in voicing our concerns."
Activism, however, seems to be more complicated when it involves physicians being critical of inequities within the medical field. Nephrologist, Vanessa Grubbs, MD, MPH, founded the nonprofit Black Doc Village in 2022 to raise awareness about the wrongful dismissal of Black residents and expand the Black physician workforce.
Dr. Grubbs said that the medical community has not been supportive of her activism. "The reason why I'm no longer in academia is in part because they got very upset with me tweeting about how some trainees are biased in their treatment of attendings," she said. "Senior White men attendings are often treated very differently than junior women of color faculty."
Dr. Grubbs also expressed her views in 2020 essay in the New England Journal of Medicine where she criticized academic medical institutions for ignoring systemic racism, paying lip service to diversity, equity, and inclusion, and staying "deafeningly silent" when issues of racism are raised.
Today, Black Doc Village is focused on conducting research that can be used to change policy. And Dr. Grubbs now has the full support of her colleagues at West Oakland Health, in Oakland, California, which aspires to advance the Bay Area Black community's health and dignity. "So, no one here has a problem with me speaking out," she added.
The emphasis on data-driven activism as opposed to "playing politics," is a recurring theme for many physicians who publicly engage with social issues.
"It's not partisan," Dr. Ranney said. "Rather, it's a commitment to translating science into actionable steps that can be used regardless of what political party you are in. My job is not to be on one side or the other, but to advance human health." These doctors challenge their critics to explain how such a goal is outside their purview.
A version of this article first appeared on Medscape.com.
It should come as no surprise that when physicians speak out on social and political issues, there is sometimes a backlash. This can range from the typical trolling that occurs online to rarer cases of professional penalties. Two doctors were fired by NYU Langone Health late last year after they posted social media messages about the Israel-Hamas war. Still, many physicians are not only willing to stand up for what they believe in, but they see it as an essential part of their profession.
"We're now at a place where doctors need to engage in public advocacy as an urgent part of our job," wrote Rob Davidson, MD, an emergency department physician, at the onslaught of the COVID-19 pandemic. In an Op-Ed piece for The Guardian, Dr. Davidson noted how the virus forced many physicians into becoming "activist doctors," calling for adequate personal protective equipment and correcting misinformation. "What we want above all is for the administration to listen to doctors, nurses, and frontline health workers - and stop playing politics," he wrote.
'It's Not About Being Political'
The intersection of medicine and politics is hardly new. Doctors frequently testify before Congress, sharing their expertise on issues concerning public health. This, however, isn't the same as "playing politics."
"I'm not taking political stances," said Megan Ranney, MD, Dean of the Yale School of Public Health. "Rather, I'm using science to inform best practices, and I'm vocal around the area where I have expertise where we could do collectively better."
Dr. Ranney's work to end firearm injury and death garnered particular attention when she co-authored an open letter to the National Rifle Association (NRA) in 2018. She wrote the letter in response to a tweet by the organization, admonishing physicians to "stay in their lane" when it comes to gun control.
Dr. Ranney's letter discussed gun violence as a public health crisis and urged the NRA to "be part of the solution" by joining the collective effort to reduce firearm injury and death through research, education, and advocacy. "We are not anti-gun," she stated. "We are anti-bullet hole," adding that "almost half of doctors own guns."
The NRA disagreed. When Dr. Ranney testified before Congress during a hearing on gun violence in 2023, NRA spokesperson Billy McLaughlin condemned her testimony as an effort to "dismantle the Second Amendment," calling Dr. Ranney "a known gun control extremist."
"If you actually read what I write, or if you actually listen to what I say, I'm not saying things on behalf of one political party or another," said Dr. Ranney. "It's not about being political. It's about recognizing our role in describing what's happening and making it clear for the world to see. Showing where, based off of data, there may be a better path to improve health and wellbeing."
In spite of the backlash, Dr. Ranney has no regrets about being an activist. "In the current media landscape, folks love to slap labels on people that may or may not be accurate. To me, what matters isn't where I land with a particular politician or political party, but how the work that I do improves health for populations."
When the Need to Act Outweighs the Fear
Laura Andreson, DO, an ob.gyn, took activism a step further when she joined a group of women in Tennessee to file a suit against the state, the attorney general, and the state board of medical examiners. The issue was the Tennessee's abortion ban, which the suit claimed prevented women from getting "necessary and potentially life-saving medical care."
Dr. Andreson, who says she was "not at all" politically active in the past, began to realize how the abortion ban could drastically affect her profession and her patients. "I don't know what flipped in me, but I just felt like I could do this," she said.
Like Dr. Ranney, Dr. Andreson has been as visible as she has been vocal, giving press conferences and interviews, but she acknowledges she has some fears about safety. In fact, after filing the lawsuit, the Center for Reproductive Rights recommended that she go to a website, DeleteMe, that removes personal data from the internet, making it more difficult for people to find her information. "But my need to do this and my desire to do this is stronger than my fears," she added.
Dr. Andreson, who is part of a small practice, did check with both her coworkers and the hospital administration before moving forward with the lawsuit. She was relieved to find that she had the support of her practice and that there wasn't anything in the hospital bylaws to prevent her from filing the lawsuit. "But the people in the bigger institutions who probably have an even better expert base than I do, they are handcuffed," she said.
It has been, in Dr. Andreson's words, "a little uncomfortable" being on the board of the Tennessee Medical Association when the Tennessee Board of Medical Examiners is part of the lawsuit. "We're all members of the same group," she said. "But I'm not suing them as individuals; I'm suing them as an entity that is under our government."
Dr. Andreson said most people have been supportive of her activist work, though she admitted to feeling frustrated when she encounters apathy from fellow ob.gyns. She got little response when she circulated information explaining the abortion laws and trying to get others involved. But she still sees education as being a key part of making change happen.
"I think advocacy, as someone who is considered a responsible, trustworthy person by your community, is important, because you can sway some people just by educating them," she said.
Fighting Inequities in Medicine and Beyond
Christina Chen, MD, says she felt very supported by her medical community at the Mayo Clinic in Rochester, Minnesota, when she and 16 other Asian American physicians posted a video on Instagram in 2020 highlighting increased violence and harassment of Asian Americans during COVID-19. It soon went viral, and the Mayo Clinic distributed it across their social media channels. The only negative repercussions Mayo faced were a few posts on social media saying that politics should not be brought into the healthcare space. Dr. Chen disagrees.
"Social issues and political decisions have direct impact on the health of our communities," Dr. Chen said. "We know that we still have a long way to go to solve health inequities, which is a public health problem, and we all play a huge role in voicing our concerns."
Activism, however, seems to be more complicated when it involves physicians being critical of inequities within the medical field. Nephrologist, Vanessa Grubbs, MD, MPH, founded the nonprofit Black Doc Village in 2022 to raise awareness about the wrongful dismissal of Black residents and expand the Black physician workforce.
Dr. Grubbs said that the medical community has not been supportive of her activism. "The reason why I'm no longer in academia is in part because they got very upset with me tweeting about how some trainees are biased in their treatment of attendings," she said. "Senior White men attendings are often treated very differently than junior women of color faculty."
Dr. Grubbs also expressed her views in 2020 essay in the New England Journal of Medicine where she criticized academic medical institutions for ignoring systemic racism, paying lip service to diversity, equity, and inclusion, and staying "deafeningly silent" when issues of racism are raised.
Today, Black Doc Village is focused on conducting research that can be used to change policy. And Dr. Grubbs now has the full support of her colleagues at West Oakland Health, in Oakland, California, which aspires to advance the Bay Area Black community's health and dignity. "So, no one here has a problem with me speaking out," she added.
The emphasis on data-driven activism as opposed to "playing politics," is a recurring theme for many physicians who publicly engage with social issues.
"It's not partisan," Dr. Ranney said. "Rather, it's a commitment to translating science into actionable steps that can be used regardless of what political party you are in. My job is not to be on one side or the other, but to advance human health." These doctors challenge their critics to explain how such a goal is outside their purview.
A version of this article first appeared on Medscape.com.
It should come as no surprise that when physicians speak out on social and political issues, there is sometimes a backlash. This can range from the typical trolling that occurs online to rarer cases of professional penalties. Two doctors were fired by NYU Langone Health late last year after they posted social media messages about the Israel-Hamas war. Still, many physicians are not only willing to stand up for what they believe in, but they see it as an essential part of their profession.
"We're now at a place where doctors need to engage in public advocacy as an urgent part of our job," wrote Rob Davidson, MD, an emergency department physician, at the onslaught of the COVID-19 pandemic. In an Op-Ed piece for The Guardian, Dr. Davidson noted how the virus forced many physicians into becoming "activist doctors," calling for adequate personal protective equipment and correcting misinformation. "What we want above all is for the administration to listen to doctors, nurses, and frontline health workers - and stop playing politics," he wrote.
'It's Not About Being Political'
The intersection of medicine and politics is hardly new. Doctors frequently testify before Congress, sharing their expertise on issues concerning public health. This, however, isn't the same as "playing politics."
"I'm not taking political stances," said Megan Ranney, MD, Dean of the Yale School of Public Health. "Rather, I'm using science to inform best practices, and I'm vocal around the area where I have expertise where we could do collectively better."
Dr. Ranney's work to end firearm injury and death garnered particular attention when she co-authored an open letter to the National Rifle Association (NRA) in 2018. She wrote the letter in response to a tweet by the organization, admonishing physicians to "stay in their lane" when it comes to gun control.
Dr. Ranney's letter discussed gun violence as a public health crisis and urged the NRA to "be part of the solution" by joining the collective effort to reduce firearm injury and death through research, education, and advocacy. "We are not anti-gun," she stated. "We are anti-bullet hole," adding that "almost half of doctors own guns."
The NRA disagreed. When Dr. Ranney testified before Congress during a hearing on gun violence in 2023, NRA spokesperson Billy McLaughlin condemned her testimony as an effort to "dismantle the Second Amendment," calling Dr. Ranney "a known gun control extremist."
"If you actually read what I write, or if you actually listen to what I say, I'm not saying things on behalf of one political party or another," said Dr. Ranney. "It's not about being political. It's about recognizing our role in describing what's happening and making it clear for the world to see. Showing where, based off of data, there may be a better path to improve health and wellbeing."
In spite of the backlash, Dr. Ranney has no regrets about being an activist. "In the current media landscape, folks love to slap labels on people that may or may not be accurate. To me, what matters isn't where I land with a particular politician or political party, but how the work that I do improves health for populations."
When the Need to Act Outweighs the Fear
Laura Andreson, DO, an ob.gyn, took activism a step further when she joined a group of women in Tennessee to file a suit against the state, the attorney general, and the state board of medical examiners. The issue was the Tennessee's abortion ban, which the suit claimed prevented women from getting "necessary and potentially life-saving medical care."
Dr. Andreson, who says she was "not at all" politically active in the past, began to realize how the abortion ban could drastically affect her profession and her patients. "I don't know what flipped in me, but I just felt like I could do this," she said.
Like Dr. Ranney, Dr. Andreson has been as visible as she has been vocal, giving press conferences and interviews, but she acknowledges she has some fears about safety. In fact, after filing the lawsuit, the Center for Reproductive Rights recommended that she go to a website, DeleteMe, that removes personal data from the internet, making it more difficult for people to find her information. "But my need to do this and my desire to do this is stronger than my fears," she added.
Dr. Andreson, who is part of a small practice, did check with both her coworkers and the hospital administration before moving forward with the lawsuit. She was relieved to find that she had the support of her practice and that there wasn't anything in the hospital bylaws to prevent her from filing the lawsuit. "But the people in the bigger institutions who probably have an even better expert base than I do, they are handcuffed," she said.
It has been, in Dr. Andreson's words, "a little uncomfortable" being on the board of the Tennessee Medical Association when the Tennessee Board of Medical Examiners is part of the lawsuit. "We're all members of the same group," she said. "But I'm not suing them as individuals; I'm suing them as an entity that is under our government."
Dr. Andreson said most people have been supportive of her activist work, though she admitted to feeling frustrated when she encounters apathy from fellow ob.gyns. She got little response when she circulated information explaining the abortion laws and trying to get others involved. But she still sees education as being a key part of making change happen.
"I think advocacy, as someone who is considered a responsible, trustworthy person by your community, is important, because you can sway some people just by educating them," she said.
Fighting Inequities in Medicine and Beyond
Christina Chen, MD, says she felt very supported by her medical community at the Mayo Clinic in Rochester, Minnesota, when she and 16 other Asian American physicians posted a video on Instagram in 2020 highlighting increased violence and harassment of Asian Americans during COVID-19. It soon went viral, and the Mayo Clinic distributed it across their social media channels. The only negative repercussions Mayo faced were a few posts on social media saying that politics should not be brought into the healthcare space. Dr. Chen disagrees.
"Social issues and political decisions have direct impact on the health of our communities," Dr. Chen said. "We know that we still have a long way to go to solve health inequities, which is a public health problem, and we all play a huge role in voicing our concerns."
Activism, however, seems to be more complicated when it involves physicians being critical of inequities within the medical field. Nephrologist, Vanessa Grubbs, MD, MPH, founded the nonprofit Black Doc Village in 2022 to raise awareness about the wrongful dismissal of Black residents and expand the Black physician workforce.
Dr. Grubbs said that the medical community has not been supportive of her activism. "The reason why I'm no longer in academia is in part because they got very upset with me tweeting about how some trainees are biased in their treatment of attendings," she said. "Senior White men attendings are often treated very differently than junior women of color faculty."
Dr. Grubbs also expressed her views in 2020 essay in the New England Journal of Medicine where she criticized academic medical institutions for ignoring systemic racism, paying lip service to diversity, equity, and inclusion, and staying "deafeningly silent" when issues of racism are raised.
Today, Black Doc Village is focused on conducting research that can be used to change policy. And Dr. Grubbs now has the full support of her colleagues at West Oakland Health, in Oakland, California, which aspires to advance the Bay Area Black community's health and dignity. "So, no one here has a problem with me speaking out," she added.
The emphasis on data-driven activism as opposed to "playing politics," is a recurring theme for many physicians who publicly engage with social issues.
"It's not partisan," Dr. Ranney said. "Rather, it's a commitment to translating science into actionable steps that can be used regardless of what political party you are in. My job is not to be on one side or the other, but to advance human health." These doctors challenge their critics to explain how such a goal is outside their purview.
A version of this article first appeared on Medscape.com.
New Tool Helps Clinicians Detect Zoom Dysmorphia in Virtual Settings
SAN DIEGO — , according to George Kroumpouzos, MD, PhD, who, with colleagues, recently proposed a screening tool to help identify patients with zoom dysmorphia.
The term, coined in 2020 by dermatologist Shadi Kourosh, MD, MPH, and colleagues at Harvard Medical School, Boston, refers to an altered or skewed negative perception of one’s body image that results from spending extended amounts of time on video calls. Speaking at the annual meeting of the American Academy of Dermatology, Dr. Kroumpouzos, clinical associate professor of dermatology at Brown University, Providence Rhode Island, explained that most people believe that zoom dysmorphia falls within the spectrum of body dysmorphic disorder (BDD). He described zoom dysmorphia as “a facial dysmorphia triggered or aggravated by frequent virtual meetings. Frequent use of videoconferencing platforms is linked to a distorted perception of facial images, which leads to dysmorphic concerns.”
Individuals with zoom dysmorphia tend to scrutinize their facial features and fixate on what they think needs to improve, he continued. They experience anxiety about attending video conferences with the camera on and feel pressured to appear perfect before virtual meetings. “They find facial flaws during virtual meetings, and they believe others notice their perceived flaws,” he said. “This all has drastic effects on body dissatisfaction and self-esteem, which leads to a desire to seek cosmetic procedures. It interferes with an individual’s life and can trigger or aggravate body dysmorphic disorder.”
While several tools have been validated in cosmetic settings to screen for BDD, such as the 9-item Body Dysmorphic Disorder Questionnaire–Dermatology questionnaire, the 7-item Body Dysmorphic Disorder Questionnaire–Aesthetic Surgery questionnaire, the Cosmetic Procedure Screening Questionnaire, and the Body Dysmorphic Disorder Symptom Scale, no formal screening tools exist to identify zoom dysmorphia. To complicate matters, “identifying dysmorphic concerns in virtual settings can be challenging,” Dr. Kroumpouzos added. “This makes the recognition of zoom dysmorphia during telehealth visits even more difficult.”
Individuals who may have zoom dysmorphia may fear being misunderstood, judged, or ridiculed because of a perceived flaw in appearance, he said, making establishing rapport and eye contact difficult. “There’s a reticence and silence due to the individual’s avoidant characteristics,” he said. “Patients may become easily distracted or disengaged during telehealth visits in case of technical issues. Psychiatric comorbidities can mask symptoms related to dysmorphic concerns.”
To bridge this gap, Dr. Kroumpouzos and colleagues have proposed a screening tool, a questionnaire related to features of zoom dysmorphia, to facilitate recognition of zoom dysmorphia in virtual settings.
The first component consists of open-ended questions such as “Are you comfortable with being interviewed in a virtual appointment?” and “How do you feel about your appearance during virtual meetings?” Such questions “aim to start the dialogue, to facilitate the discussion with a patient who may be shy or avoidant,” Dr. Kroumpouzos explained.
The second component of the tool consists of questions more specific to screening for zoom dysmorphia, starting with “Are you concerned about facial flaws?” If the patient answers no, they don’t qualify for any others, he said. “But, if they answer yes to that question and yes to at least one more [question], they may have zoom dysmorphia.”
Other questions include, “Do you think that your face is not friendly to the camera?” “Do you hesitate to open the camera?” “Have you tried to hide or camouflage your flaw with your hands, hair, makeup, or clothing?” “Have you sought advice from others to improve your appearance or image?” “Do you often use the filter features of the video conferencing platform?” “Did you consider buying a new camera or equipment that helps improve your image?”
If the clinician deems the patient a candidate for the diagnosis of zoom dysmorphia, the tool recommends asking a BDD-focused question: “In the past month, have you been very concerned that there is something wrong with your physical appearance or the way one or more parts of your body look?” If the patient answers yes, “that individual should be invited to fill out a questionnaire specifically for BDD or come to the office for further evaluation,” Dr. Kroumpouzos said.
In his view, the brevity of the proposed screening tool makes it easy to incorporate into clinical practice, and the “yes or no” questions are practical. “It is crucial to elicit the presence of zoom dysmorphia in its early stage,” he said. “Zoom dysmorphia may trigger an increase in BDD, [so] it is essential to identify the presence of BDD in zoom dysmorphia sufferers and treat it appropriately.”
Dr. Kroumpouzos reported having no relevant financial disclosures.
SAN DIEGO — , according to George Kroumpouzos, MD, PhD, who, with colleagues, recently proposed a screening tool to help identify patients with zoom dysmorphia.
The term, coined in 2020 by dermatologist Shadi Kourosh, MD, MPH, and colleagues at Harvard Medical School, Boston, refers to an altered or skewed negative perception of one’s body image that results from spending extended amounts of time on video calls. Speaking at the annual meeting of the American Academy of Dermatology, Dr. Kroumpouzos, clinical associate professor of dermatology at Brown University, Providence Rhode Island, explained that most people believe that zoom dysmorphia falls within the spectrum of body dysmorphic disorder (BDD). He described zoom dysmorphia as “a facial dysmorphia triggered or aggravated by frequent virtual meetings. Frequent use of videoconferencing platforms is linked to a distorted perception of facial images, which leads to dysmorphic concerns.”
Individuals with zoom dysmorphia tend to scrutinize their facial features and fixate on what they think needs to improve, he continued. They experience anxiety about attending video conferences with the camera on and feel pressured to appear perfect before virtual meetings. “They find facial flaws during virtual meetings, and they believe others notice their perceived flaws,” he said. “This all has drastic effects on body dissatisfaction and self-esteem, which leads to a desire to seek cosmetic procedures. It interferes with an individual’s life and can trigger or aggravate body dysmorphic disorder.”
While several tools have been validated in cosmetic settings to screen for BDD, such as the 9-item Body Dysmorphic Disorder Questionnaire–Dermatology questionnaire, the 7-item Body Dysmorphic Disorder Questionnaire–Aesthetic Surgery questionnaire, the Cosmetic Procedure Screening Questionnaire, and the Body Dysmorphic Disorder Symptom Scale, no formal screening tools exist to identify zoom dysmorphia. To complicate matters, “identifying dysmorphic concerns in virtual settings can be challenging,” Dr. Kroumpouzos added. “This makes the recognition of zoom dysmorphia during telehealth visits even more difficult.”
Individuals who may have zoom dysmorphia may fear being misunderstood, judged, or ridiculed because of a perceived flaw in appearance, he said, making establishing rapport and eye contact difficult. “There’s a reticence and silence due to the individual’s avoidant characteristics,” he said. “Patients may become easily distracted or disengaged during telehealth visits in case of technical issues. Psychiatric comorbidities can mask symptoms related to dysmorphic concerns.”
To bridge this gap, Dr. Kroumpouzos and colleagues have proposed a screening tool, a questionnaire related to features of zoom dysmorphia, to facilitate recognition of zoom dysmorphia in virtual settings.
The first component consists of open-ended questions such as “Are you comfortable with being interviewed in a virtual appointment?” and “How do you feel about your appearance during virtual meetings?” Such questions “aim to start the dialogue, to facilitate the discussion with a patient who may be shy or avoidant,” Dr. Kroumpouzos explained.
The second component of the tool consists of questions more specific to screening for zoom dysmorphia, starting with “Are you concerned about facial flaws?” If the patient answers no, they don’t qualify for any others, he said. “But, if they answer yes to that question and yes to at least one more [question], they may have zoom dysmorphia.”
Other questions include, “Do you think that your face is not friendly to the camera?” “Do you hesitate to open the camera?” “Have you tried to hide or camouflage your flaw with your hands, hair, makeup, or clothing?” “Have you sought advice from others to improve your appearance or image?” “Do you often use the filter features of the video conferencing platform?” “Did you consider buying a new camera or equipment that helps improve your image?”
If the clinician deems the patient a candidate for the diagnosis of zoom dysmorphia, the tool recommends asking a BDD-focused question: “In the past month, have you been very concerned that there is something wrong with your physical appearance or the way one or more parts of your body look?” If the patient answers yes, “that individual should be invited to fill out a questionnaire specifically for BDD or come to the office for further evaluation,” Dr. Kroumpouzos said.
In his view, the brevity of the proposed screening tool makes it easy to incorporate into clinical practice, and the “yes or no” questions are practical. “It is crucial to elicit the presence of zoom dysmorphia in its early stage,” he said. “Zoom dysmorphia may trigger an increase in BDD, [so] it is essential to identify the presence of BDD in zoom dysmorphia sufferers and treat it appropriately.”
Dr. Kroumpouzos reported having no relevant financial disclosures.
SAN DIEGO — , according to George Kroumpouzos, MD, PhD, who, with colleagues, recently proposed a screening tool to help identify patients with zoom dysmorphia.
The term, coined in 2020 by dermatologist Shadi Kourosh, MD, MPH, and colleagues at Harvard Medical School, Boston, refers to an altered or skewed negative perception of one’s body image that results from spending extended amounts of time on video calls. Speaking at the annual meeting of the American Academy of Dermatology, Dr. Kroumpouzos, clinical associate professor of dermatology at Brown University, Providence Rhode Island, explained that most people believe that zoom dysmorphia falls within the spectrum of body dysmorphic disorder (BDD). He described zoom dysmorphia as “a facial dysmorphia triggered or aggravated by frequent virtual meetings. Frequent use of videoconferencing platforms is linked to a distorted perception of facial images, which leads to dysmorphic concerns.”
Individuals with zoom dysmorphia tend to scrutinize their facial features and fixate on what they think needs to improve, he continued. They experience anxiety about attending video conferences with the camera on and feel pressured to appear perfect before virtual meetings. “They find facial flaws during virtual meetings, and they believe others notice their perceived flaws,” he said. “This all has drastic effects on body dissatisfaction and self-esteem, which leads to a desire to seek cosmetic procedures. It interferes with an individual’s life and can trigger or aggravate body dysmorphic disorder.”
While several tools have been validated in cosmetic settings to screen for BDD, such as the 9-item Body Dysmorphic Disorder Questionnaire–Dermatology questionnaire, the 7-item Body Dysmorphic Disorder Questionnaire–Aesthetic Surgery questionnaire, the Cosmetic Procedure Screening Questionnaire, and the Body Dysmorphic Disorder Symptom Scale, no formal screening tools exist to identify zoom dysmorphia. To complicate matters, “identifying dysmorphic concerns in virtual settings can be challenging,” Dr. Kroumpouzos added. “This makes the recognition of zoom dysmorphia during telehealth visits even more difficult.”
Individuals who may have zoom dysmorphia may fear being misunderstood, judged, or ridiculed because of a perceived flaw in appearance, he said, making establishing rapport and eye contact difficult. “There’s a reticence and silence due to the individual’s avoidant characteristics,” he said. “Patients may become easily distracted or disengaged during telehealth visits in case of technical issues. Psychiatric comorbidities can mask symptoms related to dysmorphic concerns.”
To bridge this gap, Dr. Kroumpouzos and colleagues have proposed a screening tool, a questionnaire related to features of zoom dysmorphia, to facilitate recognition of zoom dysmorphia in virtual settings.
The first component consists of open-ended questions such as “Are you comfortable with being interviewed in a virtual appointment?” and “How do you feel about your appearance during virtual meetings?” Such questions “aim to start the dialogue, to facilitate the discussion with a patient who may be shy or avoidant,” Dr. Kroumpouzos explained.
The second component of the tool consists of questions more specific to screening for zoom dysmorphia, starting with “Are you concerned about facial flaws?” If the patient answers no, they don’t qualify for any others, he said. “But, if they answer yes to that question and yes to at least one more [question], they may have zoom dysmorphia.”
Other questions include, “Do you think that your face is not friendly to the camera?” “Do you hesitate to open the camera?” “Have you tried to hide or camouflage your flaw with your hands, hair, makeup, or clothing?” “Have you sought advice from others to improve your appearance or image?” “Do you often use the filter features of the video conferencing platform?” “Did you consider buying a new camera or equipment that helps improve your image?”
If the clinician deems the patient a candidate for the diagnosis of zoom dysmorphia, the tool recommends asking a BDD-focused question: “In the past month, have you been very concerned that there is something wrong with your physical appearance or the way one or more parts of your body look?” If the patient answers yes, “that individual should be invited to fill out a questionnaire specifically for BDD or come to the office for further evaluation,” Dr. Kroumpouzos said.
In his view, the brevity of the proposed screening tool makes it easy to incorporate into clinical practice, and the “yes or no” questions are practical. “It is crucial to elicit the presence of zoom dysmorphia in its early stage,” he said. “Zoom dysmorphia may trigger an increase in BDD, [so] it is essential to identify the presence of BDD in zoom dysmorphia sufferers and treat it appropriately.”
Dr. Kroumpouzos reported having no relevant financial disclosures.
FROM AAD 2024
Less Than 50% of Accelerated Approvals Show Clinical Benefit
despite being on the US market for more than 5 years, according to a new study.
Under the program, drugs are approved for marketing if they show benefit in surrogate markers thought to indicate efficacy. Progression-free survival, tumor response, and duration of response are the most used surrogate markers for accelerated approvals of cancer drugs. These are based largely on imaging studies that show either a stop in growth in the case of progression-free survival or tumor shrinkage in the case of tumor response.
Following accelerated approvals, companies are then supposed to show actual clinical benefit in confirmatory trials.
The problem with relying on surrogate markers for drug approvals is that they don’t always correlate with longer survival or improved quality of life, said Edward Cliff, MBBS, who presented the findings at the American Association for Cancer Research 2024 annual meeting (abstract 918). The study was also published in JAMA to coincide with the meeting presentation.
In some cancers, these markers work well, but in others they don’t, said Dr. Cliff, a hematology trainee at Brigham and Women’s Hospital, Boston, when the work was conducted, and now a hematology fellow at the Peter MacCallum Cancer Centre in Melbourne, Australia.
To determine whether cancer drugs granted accelerated approval ultimately show an overall survival or quality of life benefit, researchers reviewed 46 cancer drugs granted accelerated approvals between 2013 and 2017. Twenty (43%) were granted full approval after demonstrating survival or quality-of-life benefits.
Nine, however, were converted to full approvals on the basis of surrogate markers. These include a full approval for pembrolizumab in previously treated recurrent or refractory head and neck squamous cell carcinoma and a full approval for nivolumab for refractory locally advanced or metastatic urothelial carcinoma, both based on tumor response rate and duration of response.
Of the remaining 17 drugs evaluated in the trial, 10 have been withdrawn and seven do not yet have confirmatory trial results.
The reliance on surrogate markers means that these drugs are used for treatment, covered by insurance, and added to guidelines — all without solid evidence of real-world clinical benefit, said Dr. Cliff.
However, the goal should not be to do away with the accelerated approval process, because it sometimes does deliver powerful agents to patients quickly. Instead, Dr. Cliff told this news organization, the system needs to be improved so that “we keep the speed while getting certainty around clinical benefits” with robust and timely confirmatory trials.
In the meantime, “clinicians should communicate with patients about any residual uncertainty of clinical benefit when they offer novel therapies,” Dr. Cliff explained. “It’s important for them to have the information.”
There has been some progress on the issue. In December 2022, the US Congress passed the Food and Drug Administration Omnibus Reform Act. Among other things, the Act requires companies to have confirmation trials underway as a condition for accelerated approval, and to provide regular reports on their progress. The Act also expedites the withdrawal process for drugs that don’t show a benefit.
The Act has been put to the test twice recently. In February, FDA used the expedited process to remove the multiple myeloma drug melphalan flufenamide from the market. Melphalan flufenamide hadn’t been sold in the US for quite some time, so the process wasn’t contentious.
In March, Regeneron announced that accelerated approval for the follicular and diffuse B cell lymphoma drug odronextamab has been delayed pending enrollment in a confirmatory trial.
“There have been some promising steps,” Dr. Cliff said, but much work needs to be done.
Study moderator Shivaani Kummar, MD, agreed, noting that “the data is showing that the confirmatory trials aren’t happening at the pace which they should.”
But the solution is not to curtail approvals; it’s to make sure that accelerated approval commitments are met, said Dr. Kummar.
Still, “as a practicing oncologist, I welcome the accelerated pathway,” Dr. Kummar, a medical oncologist/hematologist at Oregon Health & Science University, Portland, told this news organization. “I want the availability to my patients.”
Having drugs approved on the basis of surrogate markers doesn’t necessarily mean patients are getting ineffective therapies, Dr. Kummar noted. For instance, if an agent just shrinks the tumor, it can sometimes still be “a huge clinical benefit because it can take the symptoms away.”
As for prescribing drugs based on accelerated approvals, she said she tells her patients that trials have been promising, but we don’t know what the long-term effects are. She and her patient then make a decision together.
The study was funded by Arnold Ventures. Dr. Kummar reported support from several companies, including Bayer, Gilead, and others. Dr. Cliff had no disclosures.
A version of this article appeared on Medscape.com.
despite being on the US market for more than 5 years, according to a new study.
Under the program, drugs are approved for marketing if they show benefit in surrogate markers thought to indicate efficacy. Progression-free survival, tumor response, and duration of response are the most used surrogate markers for accelerated approvals of cancer drugs. These are based largely on imaging studies that show either a stop in growth in the case of progression-free survival or tumor shrinkage in the case of tumor response.
Following accelerated approvals, companies are then supposed to show actual clinical benefit in confirmatory trials.
The problem with relying on surrogate markers for drug approvals is that they don’t always correlate with longer survival or improved quality of life, said Edward Cliff, MBBS, who presented the findings at the American Association for Cancer Research 2024 annual meeting (abstract 918). The study was also published in JAMA to coincide with the meeting presentation.
In some cancers, these markers work well, but in others they don’t, said Dr. Cliff, a hematology trainee at Brigham and Women’s Hospital, Boston, when the work was conducted, and now a hematology fellow at the Peter MacCallum Cancer Centre in Melbourne, Australia.
To determine whether cancer drugs granted accelerated approval ultimately show an overall survival or quality of life benefit, researchers reviewed 46 cancer drugs granted accelerated approvals between 2013 and 2017. Twenty (43%) were granted full approval after demonstrating survival or quality-of-life benefits.
Nine, however, were converted to full approvals on the basis of surrogate markers. These include a full approval for pembrolizumab in previously treated recurrent or refractory head and neck squamous cell carcinoma and a full approval for nivolumab for refractory locally advanced or metastatic urothelial carcinoma, both based on tumor response rate and duration of response.
Of the remaining 17 drugs evaluated in the trial, 10 have been withdrawn and seven do not yet have confirmatory trial results.
The reliance on surrogate markers means that these drugs are used for treatment, covered by insurance, and added to guidelines — all without solid evidence of real-world clinical benefit, said Dr. Cliff.
However, the goal should not be to do away with the accelerated approval process, because it sometimes does deliver powerful agents to patients quickly. Instead, Dr. Cliff told this news organization, the system needs to be improved so that “we keep the speed while getting certainty around clinical benefits” with robust and timely confirmatory trials.
In the meantime, “clinicians should communicate with patients about any residual uncertainty of clinical benefit when they offer novel therapies,” Dr. Cliff explained. “It’s important for them to have the information.”
There has been some progress on the issue. In December 2022, the US Congress passed the Food and Drug Administration Omnibus Reform Act. Among other things, the Act requires companies to have confirmation trials underway as a condition for accelerated approval, and to provide regular reports on their progress. The Act also expedites the withdrawal process for drugs that don’t show a benefit.
The Act has been put to the test twice recently. In February, FDA used the expedited process to remove the multiple myeloma drug melphalan flufenamide from the market. Melphalan flufenamide hadn’t been sold in the US for quite some time, so the process wasn’t contentious.
In March, Regeneron announced that accelerated approval for the follicular and diffuse B cell lymphoma drug odronextamab has been delayed pending enrollment in a confirmatory trial.
“There have been some promising steps,” Dr. Cliff said, but much work needs to be done.
Study moderator Shivaani Kummar, MD, agreed, noting that “the data is showing that the confirmatory trials aren’t happening at the pace which they should.”
But the solution is not to curtail approvals; it’s to make sure that accelerated approval commitments are met, said Dr. Kummar.
Still, “as a practicing oncologist, I welcome the accelerated pathway,” Dr. Kummar, a medical oncologist/hematologist at Oregon Health & Science University, Portland, told this news organization. “I want the availability to my patients.”
Having drugs approved on the basis of surrogate markers doesn’t necessarily mean patients are getting ineffective therapies, Dr. Kummar noted. For instance, if an agent just shrinks the tumor, it can sometimes still be “a huge clinical benefit because it can take the symptoms away.”
As for prescribing drugs based on accelerated approvals, she said she tells her patients that trials have been promising, but we don’t know what the long-term effects are. She and her patient then make a decision together.
The study was funded by Arnold Ventures. Dr. Kummar reported support from several companies, including Bayer, Gilead, and others. Dr. Cliff had no disclosures.
A version of this article appeared on Medscape.com.
despite being on the US market for more than 5 years, according to a new study.
Under the program, drugs are approved for marketing if they show benefit in surrogate markers thought to indicate efficacy. Progression-free survival, tumor response, and duration of response are the most used surrogate markers for accelerated approvals of cancer drugs. These are based largely on imaging studies that show either a stop in growth in the case of progression-free survival or tumor shrinkage in the case of tumor response.
Following accelerated approvals, companies are then supposed to show actual clinical benefit in confirmatory trials.
The problem with relying on surrogate markers for drug approvals is that they don’t always correlate with longer survival or improved quality of life, said Edward Cliff, MBBS, who presented the findings at the American Association for Cancer Research 2024 annual meeting (abstract 918). The study was also published in JAMA to coincide with the meeting presentation.
In some cancers, these markers work well, but in others they don’t, said Dr. Cliff, a hematology trainee at Brigham and Women’s Hospital, Boston, when the work was conducted, and now a hematology fellow at the Peter MacCallum Cancer Centre in Melbourne, Australia.
To determine whether cancer drugs granted accelerated approval ultimately show an overall survival or quality of life benefit, researchers reviewed 46 cancer drugs granted accelerated approvals between 2013 and 2017. Twenty (43%) were granted full approval after demonstrating survival or quality-of-life benefits.
Nine, however, were converted to full approvals on the basis of surrogate markers. These include a full approval for pembrolizumab in previously treated recurrent or refractory head and neck squamous cell carcinoma and a full approval for nivolumab for refractory locally advanced or metastatic urothelial carcinoma, both based on tumor response rate and duration of response.
Of the remaining 17 drugs evaluated in the trial, 10 have been withdrawn and seven do not yet have confirmatory trial results.
The reliance on surrogate markers means that these drugs are used for treatment, covered by insurance, and added to guidelines — all without solid evidence of real-world clinical benefit, said Dr. Cliff.
However, the goal should not be to do away with the accelerated approval process, because it sometimes does deliver powerful agents to patients quickly. Instead, Dr. Cliff told this news organization, the system needs to be improved so that “we keep the speed while getting certainty around clinical benefits” with robust and timely confirmatory trials.
In the meantime, “clinicians should communicate with patients about any residual uncertainty of clinical benefit when they offer novel therapies,” Dr. Cliff explained. “It’s important for them to have the information.”
There has been some progress on the issue. In December 2022, the US Congress passed the Food and Drug Administration Omnibus Reform Act. Among other things, the Act requires companies to have confirmation trials underway as a condition for accelerated approval, and to provide regular reports on their progress. The Act also expedites the withdrawal process for drugs that don’t show a benefit.
The Act has been put to the test twice recently. In February, FDA used the expedited process to remove the multiple myeloma drug melphalan flufenamide from the market. Melphalan flufenamide hadn’t been sold in the US for quite some time, so the process wasn’t contentious.
In March, Regeneron announced that accelerated approval for the follicular and diffuse B cell lymphoma drug odronextamab has been delayed pending enrollment in a confirmatory trial.
“There have been some promising steps,” Dr. Cliff said, but much work needs to be done.
Study moderator Shivaani Kummar, MD, agreed, noting that “the data is showing that the confirmatory trials aren’t happening at the pace which they should.”
But the solution is not to curtail approvals; it’s to make sure that accelerated approval commitments are met, said Dr. Kummar.
Still, “as a practicing oncologist, I welcome the accelerated pathway,” Dr. Kummar, a medical oncologist/hematologist at Oregon Health & Science University, Portland, told this news organization. “I want the availability to my patients.”
Having drugs approved on the basis of surrogate markers doesn’t necessarily mean patients are getting ineffective therapies, Dr. Kummar noted. For instance, if an agent just shrinks the tumor, it can sometimes still be “a huge clinical benefit because it can take the symptoms away.”
As for prescribing drugs based on accelerated approvals, she said she tells her patients that trials have been promising, but we don’t know what the long-term effects are. She and her patient then make a decision together.
The study was funded by Arnold Ventures. Dr. Kummar reported support from several companies, including Bayer, Gilead, and others. Dr. Cliff had no disclosures.
A version of this article appeared on Medscape.com.