User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Patients With Stable Lupus May Be Safely Weaned Off MMF
Patients with quiescent systemic lupus erythematosus (SLE) who are on maintenance therapy with mycophenolate mofetil (MMF) may be able to be safely weaned off the drug with the understanding that disease flare may occur and may require restarting immunosuppressive therapy.
That’s the conclusion of investigators in a multicenter randomized trial conducted at 19 US centers and published in The Lancet Rheumatology. They found that among 100 patients with stable SLE who were on MMF for at least 2 years for renal indications or at least 1 year for nonrenal indications, MMF withdrawal was not significantly inferior to MMF maintenance in terms of clinically significant disease reactivation within at least 1 year.
“Our findings suggest that mycophenolate mofetil could be safely withdrawn in patients with stable SLE. However, larger studies with a longer follow-up are still needed,” wrote Eliza F. Chakravarty, MD, MS, from the University of Oklahoma College of Medicine in Oklahoma City, and colleagues.
“Our study was only for 60 weeks, so we don’t have long-term data on what happens when patients taper off, but my recommendation — and I think the data support this — is that even if you do have a history of lupus nephritis, if you had stable disease or very little to no activity for a year or 2, then I think it’s worth stopping the medication and following for any signs of disease flare,” Dr. Chakravarty said in an interview with this news organization.
She added that “in clinical practice, we would follow patients regularly no matter what they’re on, even if they’re in remission, looking for clinical signs or laboratory evidence of flare, and then if they look like they might be having flare, treat them accordingly.”
Toxicities a Concern
Although MMF is effective for inducing prolonged disease quiescence, it is a known teratogen and has significant toxicities, and it’s desirable to wean patients off the drug if it can be done safely, Dr. Chakravarty said.
The optimal duration of maintenance therapy with MMF is not known, however, which prompted the researchers to conduct the open-label study.
Patients aged 18-70 years who met the American College of Rheumatology (ACR) 1997 SLE criteria and had a clinical SLE Disease Activity Index (SLEDAI) score ≤ 4 at screening and who also had been on stable or tapering MMF therapy for 2 or more years for renal indications or 1 or more year for nonrenal indications were eligible. All patients were on a background regimen of hydroxychloroquine.
Patients were randomly assigned on an equal basis to either withdrawal with a 12-week taper or to continued maintenance at their baseline dose, ranging from 1 to 3 g/day for 60 weeks.
The investigators used an adaptive random-allocation strategy to ensure that the groups were balanced for study site, renal vs nonrenal disease, and baseline MMF dose (≥ 2 g/day vs < 2 g/day).
A total of 100 patients with an average age of 42 years were included in a modified intention-to-treat analysis: 49 were randomly assigned to maintenance and 51 to withdrawal.
Overall, 84% of patients were women, 40% were White, and 41% were Black. Most patients, 76%, had a history of lupus nephritis.
Significant disease reactivation, the primary endpoints, was defined as the need to increase prednisone to ≥ 15 mg/day for 4 weeks, the need for two or more short steroid bursts, or the need to resume MMF or start patients on another immunosuppressive therapy.
By week 60, 18% of patients in the withdrawal group had clinically significant disease reactivation compared with 10% of patients in the maintenance group.
“Although the differences were not significant, this study used an estimation-based design to determine estimated increases in clinically significant disease reactivation risk with 75%, 85%, and 95% confidence limits to assist clinicians and patients in making informed treatment decisions. We found a 6%-8% increase with upper 85% confidence limits of 11%-19% in clinically significant disease reactivation and flare risk following mycophenolate mofetil withdrawal,” the investigators wrote.
Rates of adverse events were similar between the groups, occurring in 90% of patients in the maintenance arm and 88% of those in the withdrawal arm. Infections occurred more frequently among patients in the maintenance group, at 64% vs 46%.
Encouraging Data
In an accompanying editorial, Noémie Jourde-Chiche, MD, PhD, from Aix-Marseille University in Marseille, France, and Laurent Chiche, MD, from Hopital Europeen de Marseille, wrote that the study data “were clearly encouraging.” They noted that the results show that it’s feasible to wean select patients off immunosuppressive therapy and keep SLE in check and that the quantified risk assessment strategy will allow shared decision-making for each patient.
“Overall, the prospect of a time-limited (versus lifelong) treatment may favor compliance, as observed in other disease fields, which might consolidate remission and reduce the risk of subsequent relapse, using sequentially treat-to-target and think-to-untreat strategies for a win-wean era in SLE,” they wrote.
“We’ve been awaiting the results of this trial for quite a while, and so it is nice to see it out,” commented Karen H. Costenbader, MD, MPH, professor of medicine at Harvard Medical School, and chair of the division of rheumatology and director of the Lupus Program at Brigham and Women’s Hospital in Boston, Massachusetts.
“It does provide some data to address a question that comes up in discussions with patients all the time: A person with lupus has been doing really well, in what we call low disease activity state or remission, but on mycophenolate, possibly for several years,” she said in a reply to a request for objective commentary.
“The question is how and when to taper and can MMF be safely discontinued,” she said. “Personally, I always review the severity of the underlying disease and indication for the MMF in the first place. Really active SLE with rapidly progressing kidney or other organ damage has to be treated with tremendous respect and no one wants to go back there. I also think about how long it has been, which other medications are still being taken (hydroxychloroquine, belimumab [Benlysta], etc.) and whether the labs and symptoms have really returned to completely normal. Then I have discussions about all this with my patient and we often try a long, slow, gingerly taper with a lot of interim monitoring.”
The study was funded by the National Institute of Allergy and Infectious Diseases and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Chakravarty and Dr. Costenbader report no relevant financial relationships. Dr. Jourde-Chiche declares personal consulting fees from Otsuka and AstraZeneca, personal speaking fees from GlaxoSmithKline and Otsuka, and personal payment for expert testimony from Otsuka. Dr. Chiche declares research grants paid to his institution from AstraZeneca and GlaxoSmithKline, personal consulting fees from Novartis and AstraZeneca, and personal speaking fees from GlaxoSmithKline and Novartis.
A version of this article appeared on Medscape.com .
Patients with quiescent systemic lupus erythematosus (SLE) who are on maintenance therapy with mycophenolate mofetil (MMF) may be able to be safely weaned off the drug with the understanding that disease flare may occur and may require restarting immunosuppressive therapy.
That’s the conclusion of investigators in a multicenter randomized trial conducted at 19 US centers and published in The Lancet Rheumatology. They found that among 100 patients with stable SLE who were on MMF for at least 2 years for renal indications or at least 1 year for nonrenal indications, MMF withdrawal was not significantly inferior to MMF maintenance in terms of clinically significant disease reactivation within at least 1 year.
“Our findings suggest that mycophenolate mofetil could be safely withdrawn in patients with stable SLE. However, larger studies with a longer follow-up are still needed,” wrote Eliza F. Chakravarty, MD, MS, from the University of Oklahoma College of Medicine in Oklahoma City, and colleagues.
“Our study was only for 60 weeks, so we don’t have long-term data on what happens when patients taper off, but my recommendation — and I think the data support this — is that even if you do have a history of lupus nephritis, if you had stable disease or very little to no activity for a year or 2, then I think it’s worth stopping the medication and following for any signs of disease flare,” Dr. Chakravarty said in an interview with this news organization.
She added that “in clinical practice, we would follow patients regularly no matter what they’re on, even if they’re in remission, looking for clinical signs or laboratory evidence of flare, and then if they look like they might be having flare, treat them accordingly.”
Toxicities a Concern
Although MMF is effective for inducing prolonged disease quiescence, it is a known teratogen and has significant toxicities, and it’s desirable to wean patients off the drug if it can be done safely, Dr. Chakravarty said.
The optimal duration of maintenance therapy with MMF is not known, however, which prompted the researchers to conduct the open-label study.
Patients aged 18-70 years who met the American College of Rheumatology (ACR) 1997 SLE criteria and had a clinical SLE Disease Activity Index (SLEDAI) score ≤ 4 at screening and who also had been on stable or tapering MMF therapy for 2 or more years for renal indications or 1 or more year for nonrenal indications were eligible. All patients were on a background regimen of hydroxychloroquine.
Patients were randomly assigned on an equal basis to either withdrawal with a 12-week taper or to continued maintenance at their baseline dose, ranging from 1 to 3 g/day for 60 weeks.
The investigators used an adaptive random-allocation strategy to ensure that the groups were balanced for study site, renal vs nonrenal disease, and baseline MMF dose (≥ 2 g/day vs < 2 g/day).
A total of 100 patients with an average age of 42 years were included in a modified intention-to-treat analysis: 49 were randomly assigned to maintenance and 51 to withdrawal.
Overall, 84% of patients were women, 40% were White, and 41% were Black. Most patients, 76%, had a history of lupus nephritis.
Significant disease reactivation, the primary endpoints, was defined as the need to increase prednisone to ≥ 15 mg/day for 4 weeks, the need for two or more short steroid bursts, or the need to resume MMF or start patients on another immunosuppressive therapy.
By week 60, 18% of patients in the withdrawal group had clinically significant disease reactivation compared with 10% of patients in the maintenance group.
“Although the differences were not significant, this study used an estimation-based design to determine estimated increases in clinically significant disease reactivation risk with 75%, 85%, and 95% confidence limits to assist clinicians and patients in making informed treatment decisions. We found a 6%-8% increase with upper 85% confidence limits of 11%-19% in clinically significant disease reactivation and flare risk following mycophenolate mofetil withdrawal,” the investigators wrote.
Rates of adverse events were similar between the groups, occurring in 90% of patients in the maintenance arm and 88% of those in the withdrawal arm. Infections occurred more frequently among patients in the maintenance group, at 64% vs 46%.
Encouraging Data
In an accompanying editorial, Noémie Jourde-Chiche, MD, PhD, from Aix-Marseille University in Marseille, France, and Laurent Chiche, MD, from Hopital Europeen de Marseille, wrote that the study data “were clearly encouraging.” They noted that the results show that it’s feasible to wean select patients off immunosuppressive therapy and keep SLE in check and that the quantified risk assessment strategy will allow shared decision-making for each patient.
“Overall, the prospect of a time-limited (versus lifelong) treatment may favor compliance, as observed in other disease fields, which might consolidate remission and reduce the risk of subsequent relapse, using sequentially treat-to-target and think-to-untreat strategies for a win-wean era in SLE,” they wrote.
“We’ve been awaiting the results of this trial for quite a while, and so it is nice to see it out,” commented Karen H. Costenbader, MD, MPH, professor of medicine at Harvard Medical School, and chair of the division of rheumatology and director of the Lupus Program at Brigham and Women’s Hospital in Boston, Massachusetts.
“It does provide some data to address a question that comes up in discussions with patients all the time: A person with lupus has been doing really well, in what we call low disease activity state or remission, but on mycophenolate, possibly for several years,” she said in a reply to a request for objective commentary.
“The question is how and when to taper and can MMF be safely discontinued,” she said. “Personally, I always review the severity of the underlying disease and indication for the MMF in the first place. Really active SLE with rapidly progressing kidney or other organ damage has to be treated with tremendous respect and no one wants to go back there. I also think about how long it has been, which other medications are still being taken (hydroxychloroquine, belimumab [Benlysta], etc.) and whether the labs and symptoms have really returned to completely normal. Then I have discussions about all this with my patient and we often try a long, slow, gingerly taper with a lot of interim monitoring.”
The study was funded by the National Institute of Allergy and Infectious Diseases and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Chakravarty and Dr. Costenbader report no relevant financial relationships. Dr. Jourde-Chiche declares personal consulting fees from Otsuka and AstraZeneca, personal speaking fees from GlaxoSmithKline and Otsuka, and personal payment for expert testimony from Otsuka. Dr. Chiche declares research grants paid to his institution from AstraZeneca and GlaxoSmithKline, personal consulting fees from Novartis and AstraZeneca, and personal speaking fees from GlaxoSmithKline and Novartis.
A version of this article appeared on Medscape.com .
Patients with quiescent systemic lupus erythematosus (SLE) who are on maintenance therapy with mycophenolate mofetil (MMF) may be able to be safely weaned off the drug with the understanding that disease flare may occur and may require restarting immunosuppressive therapy.
That’s the conclusion of investigators in a multicenter randomized trial conducted at 19 US centers and published in The Lancet Rheumatology. They found that among 100 patients with stable SLE who were on MMF for at least 2 years for renal indications or at least 1 year for nonrenal indications, MMF withdrawal was not significantly inferior to MMF maintenance in terms of clinically significant disease reactivation within at least 1 year.
“Our findings suggest that mycophenolate mofetil could be safely withdrawn in patients with stable SLE. However, larger studies with a longer follow-up are still needed,” wrote Eliza F. Chakravarty, MD, MS, from the University of Oklahoma College of Medicine in Oklahoma City, and colleagues.
“Our study was only for 60 weeks, so we don’t have long-term data on what happens when patients taper off, but my recommendation — and I think the data support this — is that even if you do have a history of lupus nephritis, if you had stable disease or very little to no activity for a year or 2, then I think it’s worth stopping the medication and following for any signs of disease flare,” Dr. Chakravarty said in an interview with this news organization.
She added that “in clinical practice, we would follow patients regularly no matter what they’re on, even if they’re in remission, looking for clinical signs or laboratory evidence of flare, and then if they look like they might be having flare, treat them accordingly.”
Toxicities a Concern
Although MMF is effective for inducing prolonged disease quiescence, it is a known teratogen and has significant toxicities, and it’s desirable to wean patients off the drug if it can be done safely, Dr. Chakravarty said.
The optimal duration of maintenance therapy with MMF is not known, however, which prompted the researchers to conduct the open-label study.
Patients aged 18-70 years who met the American College of Rheumatology (ACR) 1997 SLE criteria and had a clinical SLE Disease Activity Index (SLEDAI) score ≤ 4 at screening and who also had been on stable or tapering MMF therapy for 2 or more years for renal indications or 1 or more year for nonrenal indications were eligible. All patients were on a background regimen of hydroxychloroquine.
Patients were randomly assigned on an equal basis to either withdrawal with a 12-week taper or to continued maintenance at their baseline dose, ranging from 1 to 3 g/day for 60 weeks.
The investigators used an adaptive random-allocation strategy to ensure that the groups were balanced for study site, renal vs nonrenal disease, and baseline MMF dose (≥ 2 g/day vs < 2 g/day).
A total of 100 patients with an average age of 42 years were included in a modified intention-to-treat analysis: 49 were randomly assigned to maintenance and 51 to withdrawal.
Overall, 84% of patients were women, 40% were White, and 41% were Black. Most patients, 76%, had a history of lupus nephritis.
Significant disease reactivation, the primary endpoints, was defined as the need to increase prednisone to ≥ 15 mg/day for 4 weeks, the need for two or more short steroid bursts, or the need to resume MMF or start patients on another immunosuppressive therapy.
By week 60, 18% of patients in the withdrawal group had clinically significant disease reactivation compared with 10% of patients in the maintenance group.
“Although the differences were not significant, this study used an estimation-based design to determine estimated increases in clinically significant disease reactivation risk with 75%, 85%, and 95% confidence limits to assist clinicians and patients in making informed treatment decisions. We found a 6%-8% increase with upper 85% confidence limits of 11%-19% in clinically significant disease reactivation and flare risk following mycophenolate mofetil withdrawal,” the investigators wrote.
Rates of adverse events were similar between the groups, occurring in 90% of patients in the maintenance arm and 88% of those in the withdrawal arm. Infections occurred more frequently among patients in the maintenance group, at 64% vs 46%.
Encouraging Data
In an accompanying editorial, Noémie Jourde-Chiche, MD, PhD, from Aix-Marseille University in Marseille, France, and Laurent Chiche, MD, from Hopital Europeen de Marseille, wrote that the study data “were clearly encouraging.” They noted that the results show that it’s feasible to wean select patients off immunosuppressive therapy and keep SLE in check and that the quantified risk assessment strategy will allow shared decision-making for each patient.
“Overall, the prospect of a time-limited (versus lifelong) treatment may favor compliance, as observed in other disease fields, which might consolidate remission and reduce the risk of subsequent relapse, using sequentially treat-to-target and think-to-untreat strategies for a win-wean era in SLE,” they wrote.
“We’ve been awaiting the results of this trial for quite a while, and so it is nice to see it out,” commented Karen H. Costenbader, MD, MPH, professor of medicine at Harvard Medical School, and chair of the division of rheumatology and director of the Lupus Program at Brigham and Women’s Hospital in Boston, Massachusetts.
“It does provide some data to address a question that comes up in discussions with patients all the time: A person with lupus has been doing really well, in what we call low disease activity state or remission, but on mycophenolate, possibly for several years,” she said in a reply to a request for objective commentary.
“The question is how and when to taper and can MMF be safely discontinued,” she said. “Personally, I always review the severity of the underlying disease and indication for the MMF in the first place. Really active SLE with rapidly progressing kidney or other organ damage has to be treated with tremendous respect and no one wants to go back there. I also think about how long it has been, which other medications are still being taken (hydroxychloroquine, belimumab [Benlysta], etc.) and whether the labs and symptoms have really returned to completely normal. Then I have discussions about all this with my patient and we often try a long, slow, gingerly taper with a lot of interim monitoring.”
The study was funded by the National Institute of Allergy and Infectious Diseases and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Chakravarty and Dr. Costenbader report no relevant financial relationships. Dr. Jourde-Chiche declares personal consulting fees from Otsuka and AstraZeneca, personal speaking fees from GlaxoSmithKline and Otsuka, and personal payment for expert testimony from Otsuka. Dr. Chiche declares research grants paid to his institution from AstraZeneca and GlaxoSmithKline, personal consulting fees from Novartis and AstraZeneca, and personal speaking fees from GlaxoSmithKline and Novartis.
A version of this article appeared on Medscape.com .
FROM THE LANCET RHEUMATOLOGY
Oral Cancer: New System May Improve Prognostic Accuracy
The TNM staging system is used by most facilities for cancer reporting, as defined by the National Cancer Institute. This system combines the size and extent of the primary tumor (T), the number of neighboring lymph nodes with cancer and subcategories (N), and whether or not metastasis has occurred (M).
In a new study published in the journal Cancer, the researchers created a novel classification system to better account for extranodal extension (ENE). The study population included 1460 adults with OSCC (696 with no lymph node involvement and 764 with positive lymph nodes), who underwent surgical resections at four centers.
“Our findings build on the growing evidence base that historical factors do not improve staging performance and that their omission results in improved N‐classification [i.e., the nodal status or lymph node involvement in cancer staging] performance,” John R. de Almeida, MD, of the University of Toronto, and colleagues, wrote in their new paper.
For patients with OSCC, this system, known as the 8th edition of American Joint Committee on Cancer/International Union Against Cancer TNM N‐classification (TNM‐8‐N), has several limitations, the researchers explained. These limitations include redundancy in the rare N3a category (i.e., having single or multiple lymph nodes greater than 6 cm or 3-7 lymph nodes without ENE) and the impact of ENE as a new prognostic feature, they said.
“Recent studies have shown that major ENE is associated with a significantly worse outcome than minor ENE, suggesting that these two subgroups should be considered as separate entities,” the authors wrote.
Study Methods and Results
The researchers created N-classifications based on adjusted hazard ratios and statistical analysis (recursive partitioning) with a focus on lymph node (LN) size and number and the extent of ENE. They compared their classifications of OSCC cases to those of the TNM-8-N’s classifications of the same cases.
Using the new classification system, lymph node number and size and the extent of ENE were associated with overall survival. The adjusted hazard ratios for LN counts of 1 vs. zero and greater than 1 vs. 0 were 1.92 and 3.21, respectively. The adjusted hazard ratios (aHRs) for LN size of greater than 3 cm vs. 3 cm or less, and for major vs. minor ENE were 1.88 and 1.40, respectively.
The use of an aHR improved cancer staging compared to the TNM-8-N by eliminating the N2c and 6-cm threshold, stratifying the extent of ENE, and stratifying N2b by 3-cm threshold, the researchers wrote.
The researchers compared their new system to the TNM-8 and also two other classification systems and their own recursive partitioning analysis (another statistical model).
The aHR-based system ranked first out of five in terms of correctly staging cancer, while the TNM-8 was fifth in the discovery cohort and fifth in the validation cohorts.
Outcome predictions (percentage variance explained) were 19.81 with the aHR vs. 18.95 in theTNM-8 in the discovery cohort, and similarly were 11.72 vs. 10.13, respectively, in the validation cohort.
“Overall, 25 patients staged as IVa in TNM‐8 were upstaged to IVb in the aHR proposal, and one patient staged as IVb was downstaged to IVa. Otherwise, overall stage between TNM‐8 and aHR remained the same,” the authors wrote.
“Our proposed N-classification based on aHR challenges previous tenets such as the importance of the 6-cm threshold and the importance of contralateral nodes,” the researchers wrote in their discussion.
The results from the new classification system were limited by the relatively small sample sizes and may not generalize to nonsquamous oral cancers, the researchers noted.
Further validation is needed before this system may be routinely applied in practice, but the results support evidence in favor of eliminating historical factors from staging, they said.
Experts Tout Advantages of Proposed Classification System
Cancer staging must be as accurate as possible and reviewed frequently, Shawn Li, MD, an otolaryngologist at University Hospitals, Cleveland, said in an interview. “This study aims to optimize nodal staging in oral cavity cancer. The current staging system doesn’t reflect updated data, and may not be specific enough to oral cavity cancers.”
This study notes the importance of stratifying extranodal extension (ENE) by micro (less than 2 mm) and macro (greater than 2 mm),” he said. It also points out that metastatic disease greater than 6 cm without ENE is infrequent enough not to require its own subcategory, he said.
Finally, in the new classification, proposed in this paper, “N2c was removed, because, statistically, it doesn’t seem to be a worse prognosis in cancers of the oral cavity,” he said.
“The data [described in this new paper] suggests that certain traditional criteria used in nodal staging for oral cavity cancer, such as [involving] very large lymph nodes greater than 6 cm in size and contralateral nodal involvement, may be less important than criteria that have not as of yet been incorporated into head and neck staging,” Wesley Talcott, MD, said in an interview. “The current study provides evidence that in oral cavity cancer, the prognostic accuracy of staging may improve by dropping these older criteria and incorporating degree of extranodal extension.”
This evidence is apparent in the ranking of the new aHR classification as first of the five strategies compared in the study, said Dr. Talcott, who was not involved in the study.
Highlighting the importance of microscopic vs. macroscopic extension may lead to doctors improving their identification of patients at highest risk for recurrence and refining treatment strategies, suggested Dr. Talcott, MD, a radiation oncologist at Northwell Health, New York, NY. However, a larger dataset is needed to validate the diagnostic accuracy of the authors’ proposed staging system, he said.
The TNM‐8‐N was updated in 2017, Dr. Li noted. “Since this system is widely referenced, it will likely need to be updated again before the changes in this study are widely adopted,” he said.
The study was supported by the National Institutes of Health and the National Cancer Institute. The researchers, Dr. Li, and Dr. Talcott had no financial conflicts to disclose.
The TNM staging system is used by most facilities for cancer reporting, as defined by the National Cancer Institute. This system combines the size and extent of the primary tumor (T), the number of neighboring lymph nodes with cancer and subcategories (N), and whether or not metastasis has occurred (M).
In a new study published in the journal Cancer, the researchers created a novel classification system to better account for extranodal extension (ENE). The study population included 1460 adults with OSCC (696 with no lymph node involvement and 764 with positive lymph nodes), who underwent surgical resections at four centers.
“Our findings build on the growing evidence base that historical factors do not improve staging performance and that their omission results in improved N‐classification [i.e., the nodal status or lymph node involvement in cancer staging] performance,” John R. de Almeida, MD, of the University of Toronto, and colleagues, wrote in their new paper.
For patients with OSCC, this system, known as the 8th edition of American Joint Committee on Cancer/International Union Against Cancer TNM N‐classification (TNM‐8‐N), has several limitations, the researchers explained. These limitations include redundancy in the rare N3a category (i.e., having single or multiple lymph nodes greater than 6 cm or 3-7 lymph nodes without ENE) and the impact of ENE as a new prognostic feature, they said.
“Recent studies have shown that major ENE is associated with a significantly worse outcome than minor ENE, suggesting that these two subgroups should be considered as separate entities,” the authors wrote.
Study Methods and Results
The researchers created N-classifications based on adjusted hazard ratios and statistical analysis (recursive partitioning) with a focus on lymph node (LN) size and number and the extent of ENE. They compared their classifications of OSCC cases to those of the TNM-8-N’s classifications of the same cases.
Using the new classification system, lymph node number and size and the extent of ENE were associated with overall survival. The adjusted hazard ratios for LN counts of 1 vs. zero and greater than 1 vs. 0 were 1.92 and 3.21, respectively. The adjusted hazard ratios (aHRs) for LN size of greater than 3 cm vs. 3 cm or less, and for major vs. minor ENE were 1.88 and 1.40, respectively.
The use of an aHR improved cancer staging compared to the TNM-8-N by eliminating the N2c and 6-cm threshold, stratifying the extent of ENE, and stratifying N2b by 3-cm threshold, the researchers wrote.
The researchers compared their new system to the TNM-8 and also two other classification systems and their own recursive partitioning analysis (another statistical model).
The aHR-based system ranked first out of five in terms of correctly staging cancer, while the TNM-8 was fifth in the discovery cohort and fifth in the validation cohorts.
Outcome predictions (percentage variance explained) were 19.81 with the aHR vs. 18.95 in theTNM-8 in the discovery cohort, and similarly were 11.72 vs. 10.13, respectively, in the validation cohort.
“Overall, 25 patients staged as IVa in TNM‐8 were upstaged to IVb in the aHR proposal, and one patient staged as IVb was downstaged to IVa. Otherwise, overall stage between TNM‐8 and aHR remained the same,” the authors wrote.
“Our proposed N-classification based on aHR challenges previous tenets such as the importance of the 6-cm threshold and the importance of contralateral nodes,” the researchers wrote in their discussion.
The results from the new classification system were limited by the relatively small sample sizes and may not generalize to nonsquamous oral cancers, the researchers noted.
Further validation is needed before this system may be routinely applied in practice, but the results support evidence in favor of eliminating historical factors from staging, they said.
Experts Tout Advantages of Proposed Classification System
Cancer staging must be as accurate as possible and reviewed frequently, Shawn Li, MD, an otolaryngologist at University Hospitals, Cleveland, said in an interview. “This study aims to optimize nodal staging in oral cavity cancer. The current staging system doesn’t reflect updated data, and may not be specific enough to oral cavity cancers.”
This study notes the importance of stratifying extranodal extension (ENE) by micro (less than 2 mm) and macro (greater than 2 mm),” he said. It also points out that metastatic disease greater than 6 cm without ENE is infrequent enough not to require its own subcategory, he said.
Finally, in the new classification, proposed in this paper, “N2c was removed, because, statistically, it doesn’t seem to be a worse prognosis in cancers of the oral cavity,” he said.
“The data [described in this new paper] suggests that certain traditional criteria used in nodal staging for oral cavity cancer, such as [involving] very large lymph nodes greater than 6 cm in size and contralateral nodal involvement, may be less important than criteria that have not as of yet been incorporated into head and neck staging,” Wesley Talcott, MD, said in an interview. “The current study provides evidence that in oral cavity cancer, the prognostic accuracy of staging may improve by dropping these older criteria and incorporating degree of extranodal extension.”
This evidence is apparent in the ranking of the new aHR classification as first of the five strategies compared in the study, said Dr. Talcott, who was not involved in the study.
Highlighting the importance of microscopic vs. macroscopic extension may lead to doctors improving their identification of patients at highest risk for recurrence and refining treatment strategies, suggested Dr. Talcott, MD, a radiation oncologist at Northwell Health, New York, NY. However, a larger dataset is needed to validate the diagnostic accuracy of the authors’ proposed staging system, he said.
The TNM‐8‐N was updated in 2017, Dr. Li noted. “Since this system is widely referenced, it will likely need to be updated again before the changes in this study are widely adopted,” he said.
The study was supported by the National Institutes of Health and the National Cancer Institute. The researchers, Dr. Li, and Dr. Talcott had no financial conflicts to disclose.
The TNM staging system is used by most facilities for cancer reporting, as defined by the National Cancer Institute. This system combines the size and extent of the primary tumor (T), the number of neighboring lymph nodes with cancer and subcategories (N), and whether or not metastasis has occurred (M).
In a new study published in the journal Cancer, the researchers created a novel classification system to better account for extranodal extension (ENE). The study population included 1460 adults with OSCC (696 with no lymph node involvement and 764 with positive lymph nodes), who underwent surgical resections at four centers.
“Our findings build on the growing evidence base that historical factors do not improve staging performance and that their omission results in improved N‐classification [i.e., the nodal status or lymph node involvement in cancer staging] performance,” John R. de Almeida, MD, of the University of Toronto, and colleagues, wrote in their new paper.
For patients with OSCC, this system, known as the 8th edition of American Joint Committee on Cancer/International Union Against Cancer TNM N‐classification (TNM‐8‐N), has several limitations, the researchers explained. These limitations include redundancy in the rare N3a category (i.e., having single or multiple lymph nodes greater than 6 cm or 3-7 lymph nodes without ENE) and the impact of ENE as a new prognostic feature, they said.
“Recent studies have shown that major ENE is associated with a significantly worse outcome than minor ENE, suggesting that these two subgroups should be considered as separate entities,” the authors wrote.
Study Methods and Results
The researchers created N-classifications based on adjusted hazard ratios and statistical analysis (recursive partitioning) with a focus on lymph node (LN) size and number and the extent of ENE. They compared their classifications of OSCC cases to those of the TNM-8-N’s classifications of the same cases.
Using the new classification system, lymph node number and size and the extent of ENE were associated with overall survival. The adjusted hazard ratios for LN counts of 1 vs. zero and greater than 1 vs. 0 were 1.92 and 3.21, respectively. The adjusted hazard ratios (aHRs) for LN size of greater than 3 cm vs. 3 cm or less, and for major vs. minor ENE were 1.88 and 1.40, respectively.
The use of an aHR improved cancer staging compared to the TNM-8-N by eliminating the N2c and 6-cm threshold, stratifying the extent of ENE, and stratifying N2b by 3-cm threshold, the researchers wrote.
The researchers compared their new system to the TNM-8 and also two other classification systems and their own recursive partitioning analysis (another statistical model).
The aHR-based system ranked first out of five in terms of correctly staging cancer, while the TNM-8 was fifth in the discovery cohort and fifth in the validation cohorts.
Outcome predictions (percentage variance explained) were 19.81 with the aHR vs. 18.95 in theTNM-8 in the discovery cohort, and similarly were 11.72 vs. 10.13, respectively, in the validation cohort.
“Overall, 25 patients staged as IVa in TNM‐8 were upstaged to IVb in the aHR proposal, and one patient staged as IVb was downstaged to IVa. Otherwise, overall stage between TNM‐8 and aHR remained the same,” the authors wrote.
“Our proposed N-classification based on aHR challenges previous tenets such as the importance of the 6-cm threshold and the importance of contralateral nodes,” the researchers wrote in their discussion.
The results from the new classification system were limited by the relatively small sample sizes and may not generalize to nonsquamous oral cancers, the researchers noted.
Further validation is needed before this system may be routinely applied in practice, but the results support evidence in favor of eliminating historical factors from staging, they said.
Experts Tout Advantages of Proposed Classification System
Cancer staging must be as accurate as possible and reviewed frequently, Shawn Li, MD, an otolaryngologist at University Hospitals, Cleveland, said in an interview. “This study aims to optimize nodal staging in oral cavity cancer. The current staging system doesn’t reflect updated data, and may not be specific enough to oral cavity cancers.”
This study notes the importance of stratifying extranodal extension (ENE) by micro (less than 2 mm) and macro (greater than 2 mm),” he said. It also points out that metastatic disease greater than 6 cm without ENE is infrequent enough not to require its own subcategory, he said.
Finally, in the new classification, proposed in this paper, “N2c was removed, because, statistically, it doesn’t seem to be a worse prognosis in cancers of the oral cavity,” he said.
“The data [described in this new paper] suggests that certain traditional criteria used in nodal staging for oral cavity cancer, such as [involving] very large lymph nodes greater than 6 cm in size and contralateral nodal involvement, may be less important than criteria that have not as of yet been incorporated into head and neck staging,” Wesley Talcott, MD, said in an interview. “The current study provides evidence that in oral cavity cancer, the prognostic accuracy of staging may improve by dropping these older criteria and incorporating degree of extranodal extension.”
This evidence is apparent in the ranking of the new aHR classification as first of the five strategies compared in the study, said Dr. Talcott, who was not involved in the study.
Highlighting the importance of microscopic vs. macroscopic extension may lead to doctors improving their identification of patients at highest risk for recurrence and refining treatment strategies, suggested Dr. Talcott, MD, a radiation oncologist at Northwell Health, New York, NY. However, a larger dataset is needed to validate the diagnostic accuracy of the authors’ proposed staging system, he said.
The TNM‐8‐N was updated in 2017, Dr. Li noted. “Since this system is widely referenced, it will likely need to be updated again before the changes in this study are widely adopted,” he said.
The study was supported by the National Institutes of Health and the National Cancer Institute. The researchers, Dr. Li, and Dr. Talcott had no financial conflicts to disclose.
FROM CANCER
Healthcare Workers Face Increased Risks During the Pandemic
Healthcare workers have been at an increased risk for SARS-CoV-2 infection and mental distress such as anxiety and depression during the pandemic, according to new research.
The risk for infection was higher among healthcare workers in the first two waves of the pandemic and again during the fifth wave.
“Previous publications, including ours, suggested that the main problem was in the early weeks and months of the pandemic, but this paper shows that it continued until the later stages,” senior author Nicola Cherry, MD, an occupational epidemiologist at the University of Alberta in Edmonton, Canada, told this news organization.
The findings were published in the Canadian Journal of Public Health.
Wave Upon Wave
In the current study, the investigators sought to compare the risk for SARS-CoV-2 infection and mental distress among healthcare workers and among community referents (CRs). They examined the following waves of the COVID-19 pandemic:
- Wave 1: From March to June 2020 (4 months).
- Wave 2: From July 2020 to February 2021 (8 months).
- Wave 3: From March to June 2021 (4 months).
- Wave 4: From July to October 2021 (4 months).
- Wave 5 (Omicron): From November 2021 to March 2022 (5 months).
Healthcare workers in Alberta were asked at recruitment for consent to match their individual records to the Alberta Administrative Health Database. As the pandemic progressed, participants were also asked for consent to be linked to COVID-19 immunization records maintained by the provinces, as well as for the results of all polymerase chain reaction (PCR) testing for the SARS-CoV-2 virus.
The investigators matched 2959 healthcare workers to 14,546 CRs according to their age, sex, geographic location in Alberta, and number of physician claims from April 1, 2019, to March 31, 2020.
Incident SARS-CoV-2 infection was examined using PCR testing and the first date of a physician consultation at which the code for SARS-CoV-2 infection had been recorded. Mental health disorders were identified from physician records. They included anxiety disorders, stress and adjustment reactions, and depressive disorders.
Most (79.5%) of the healthcare workers were registered nurses, followed by physicians (16.1%), healthcare aides (2.4%), and licensed practical nurses (2.0%). Most participants (87.5%) were female. The median age at recruitment was 44 years.
Healthcare workers were at a greater risk for COVID-19 overall, with the first SARS-CoV-2 infection defined from either PCR tests (odds ratio [OR], 1.96) or from physician records (OR, 1.33). They were also at an increased risk for anxiety (adjusted OR, 1.25; P < .001), stress/adjustment reaction (adjusted OR, 1.52; P < .001), and depressive condition (adjusted OR, 1.39; P < .001). Moreover, the excess risks for stress/adjustment reactions and depressive conditions increased with successive waves during the pandemic, peaking in the fourth wave and continuing in the fifth wave.
“Although the increase was less in the middle of the phases of the pandemic, it came back with a vengeance during the last phase, which was the Omicron phase,” said Dr. Cherry.
“Employers of healthcare workers can’t assume that everything is now under control, that they know what they’re doing, and that there is no risk. We are now having some increases in COVID. It’s going to go on. The pandemic is not over in that sense, and infection control continues to be major,” she added.
The finding that mental health worsened among healthcare workers was not surprising, Dr. Cherry said. Even before the pandemic, studies had shown that healthcare workers were at a greater risk for depression than the population overall.
“There is a lot of need for care in mental health support of healthcare workers, whether during a pandemic or not,” said Dr. Cherry.
Nurses Are Suffering
Commenting on the research for this news organization, Farinaz Havaei, PhD, RN, assistant professor of nursing at the University of British Columbia in Vancouver, Canada, said, “This is a very important and timely study that draws on objective clinical and administrative data, as opposed to healthcare workers’ subjective reports.” Dr. Havaei did not participate in the research.
Overall, the findings are consistent with previous research that drew upon healthcare workers’ reports. They speak to the chronic and cumulative impact of COVID-19 and its associated stressors on the mental health and well-being of healthcare workers, said Dr. Havaei.
“The likelihood of stress/adjustment reaction and depression showed a relatively steady increase with increasing COVID-19 waves. This increase can likely be explained by healthcare workers’ depleting emotional reserves for coping with chronic workplace stressors such as concerns about exposure to COVID-19, inadequate staffing, and work overload,” she said. Witnessing the suffering and trauma of patients and their families likely added to this risk.
Dr. Havaei also pointed out that most of the study participants were nurses. The findings are consistent with prepandemic research that showed that the suboptimal conditions that nurses increasingly faced resulted in high levels of exhaustion and burnout.
“While I agree with the authors’ call for more mental health support for healthcare workers, I think prevention efforts that address the root cause of the problem should be prioritized,” she said.
From Heroes to Zeros
The same phenomena have been observed in the United States, said John Q. Young, MD, MPP, PhD, professor and chair of psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. In various studies, Dr. Young and his colleagues have reported a strong association between exposure to the stressors of the pandemic and subsequent development of depression, anxiety, and posttraumatic stress disorder (PTSD) among healthcare workers.
“The findings from Alberta are remarkably consistent. In the beginning of the pandemic, there was a lot of acknowledgment of the work healthcare workers were doing. The fire department clapping as you leave work at night, being called heroes, even though a lot of healthcare workers feel uncomfortable with the hero language because they don’t feel like heroes. Yes, they’re afraid, but they are going to do what they need to do and help,” he said.
But as the pandemic continued, public sentiment changed, Dr. Young said. “They’ve gone from heroes to zeros. Now we are seeing the accumulated, chronic effects over months and years, and these are significant. Our healthcare workforce is vulnerable now. The reserves are low. There are serious shortages in nursing, with more retirements and more people leaving the field,” he said.
As part of a campaign to help healthcare workers cope, psychiatrists at Northwell Health have started a program called Stress First Aid at their Center for Traumatic Stress Response Resilience, where they train nurses, physicians, and other healthcare staff to use basic tools to recognize and respond to stress and distress in themselves and in their colleagues, said Dr. Young.
“For those healthcare workers who find that they are struggling and need more support, there is resilience coaching, which is one-on-one support. For those who need more clinical attention, there is a clinical program where our healthcare workers can meet with a psychologist, psychiatrist, or a therapist, to work through depression, PTSD, and anxiety. We didn’t have this before the pandemic, but it is now a big focus for our workforce,” he said. “We are trying to build resilience. The trauma is real.”
The study was supported by the College of Physicians and Surgeons of Alberta, the Canadian Institutes of Health Research, and the Canadian Immunology Task Force. Dr. Cherry and Dr. Havaei reported no relevant financial relationships. Dr. Young reported that he is senior vice president of behavioral health at Northwell.
A version of this article appeared on Medscape.com.
Healthcare workers have been at an increased risk for SARS-CoV-2 infection and mental distress such as anxiety and depression during the pandemic, according to new research.
The risk for infection was higher among healthcare workers in the first two waves of the pandemic and again during the fifth wave.
“Previous publications, including ours, suggested that the main problem was in the early weeks and months of the pandemic, but this paper shows that it continued until the later stages,” senior author Nicola Cherry, MD, an occupational epidemiologist at the University of Alberta in Edmonton, Canada, told this news organization.
The findings were published in the Canadian Journal of Public Health.
Wave Upon Wave
In the current study, the investigators sought to compare the risk for SARS-CoV-2 infection and mental distress among healthcare workers and among community referents (CRs). They examined the following waves of the COVID-19 pandemic:
- Wave 1: From March to June 2020 (4 months).
- Wave 2: From July 2020 to February 2021 (8 months).
- Wave 3: From March to June 2021 (4 months).
- Wave 4: From July to October 2021 (4 months).
- Wave 5 (Omicron): From November 2021 to March 2022 (5 months).
Healthcare workers in Alberta were asked at recruitment for consent to match their individual records to the Alberta Administrative Health Database. As the pandemic progressed, participants were also asked for consent to be linked to COVID-19 immunization records maintained by the provinces, as well as for the results of all polymerase chain reaction (PCR) testing for the SARS-CoV-2 virus.
The investigators matched 2959 healthcare workers to 14,546 CRs according to their age, sex, geographic location in Alberta, and number of physician claims from April 1, 2019, to March 31, 2020.
Incident SARS-CoV-2 infection was examined using PCR testing and the first date of a physician consultation at which the code for SARS-CoV-2 infection had been recorded. Mental health disorders were identified from physician records. They included anxiety disorders, stress and adjustment reactions, and depressive disorders.
Most (79.5%) of the healthcare workers were registered nurses, followed by physicians (16.1%), healthcare aides (2.4%), and licensed practical nurses (2.0%). Most participants (87.5%) were female. The median age at recruitment was 44 years.
Healthcare workers were at a greater risk for COVID-19 overall, with the first SARS-CoV-2 infection defined from either PCR tests (odds ratio [OR], 1.96) or from physician records (OR, 1.33). They were also at an increased risk for anxiety (adjusted OR, 1.25; P < .001), stress/adjustment reaction (adjusted OR, 1.52; P < .001), and depressive condition (adjusted OR, 1.39; P < .001). Moreover, the excess risks for stress/adjustment reactions and depressive conditions increased with successive waves during the pandemic, peaking in the fourth wave and continuing in the fifth wave.
“Although the increase was less in the middle of the phases of the pandemic, it came back with a vengeance during the last phase, which was the Omicron phase,” said Dr. Cherry.
“Employers of healthcare workers can’t assume that everything is now under control, that they know what they’re doing, and that there is no risk. We are now having some increases in COVID. It’s going to go on. The pandemic is not over in that sense, and infection control continues to be major,” she added.
The finding that mental health worsened among healthcare workers was not surprising, Dr. Cherry said. Even before the pandemic, studies had shown that healthcare workers were at a greater risk for depression than the population overall.
“There is a lot of need for care in mental health support of healthcare workers, whether during a pandemic or not,” said Dr. Cherry.
Nurses Are Suffering
Commenting on the research for this news organization, Farinaz Havaei, PhD, RN, assistant professor of nursing at the University of British Columbia in Vancouver, Canada, said, “This is a very important and timely study that draws on objective clinical and administrative data, as opposed to healthcare workers’ subjective reports.” Dr. Havaei did not participate in the research.
Overall, the findings are consistent with previous research that drew upon healthcare workers’ reports. They speak to the chronic and cumulative impact of COVID-19 and its associated stressors on the mental health and well-being of healthcare workers, said Dr. Havaei.
“The likelihood of stress/adjustment reaction and depression showed a relatively steady increase with increasing COVID-19 waves. This increase can likely be explained by healthcare workers’ depleting emotional reserves for coping with chronic workplace stressors such as concerns about exposure to COVID-19, inadequate staffing, and work overload,” she said. Witnessing the suffering and trauma of patients and their families likely added to this risk.
Dr. Havaei also pointed out that most of the study participants were nurses. The findings are consistent with prepandemic research that showed that the suboptimal conditions that nurses increasingly faced resulted in high levels of exhaustion and burnout.
“While I agree with the authors’ call for more mental health support for healthcare workers, I think prevention efforts that address the root cause of the problem should be prioritized,” she said.
From Heroes to Zeros
The same phenomena have been observed in the United States, said John Q. Young, MD, MPP, PhD, professor and chair of psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. In various studies, Dr. Young and his colleagues have reported a strong association between exposure to the stressors of the pandemic and subsequent development of depression, anxiety, and posttraumatic stress disorder (PTSD) among healthcare workers.
“The findings from Alberta are remarkably consistent. In the beginning of the pandemic, there was a lot of acknowledgment of the work healthcare workers were doing. The fire department clapping as you leave work at night, being called heroes, even though a lot of healthcare workers feel uncomfortable with the hero language because they don’t feel like heroes. Yes, they’re afraid, but they are going to do what they need to do and help,” he said.
But as the pandemic continued, public sentiment changed, Dr. Young said. “They’ve gone from heroes to zeros. Now we are seeing the accumulated, chronic effects over months and years, and these are significant. Our healthcare workforce is vulnerable now. The reserves are low. There are serious shortages in nursing, with more retirements and more people leaving the field,” he said.
As part of a campaign to help healthcare workers cope, psychiatrists at Northwell Health have started a program called Stress First Aid at their Center for Traumatic Stress Response Resilience, where they train nurses, physicians, and other healthcare staff to use basic tools to recognize and respond to stress and distress in themselves and in their colleagues, said Dr. Young.
“For those healthcare workers who find that they are struggling and need more support, there is resilience coaching, which is one-on-one support. For those who need more clinical attention, there is a clinical program where our healthcare workers can meet with a psychologist, psychiatrist, or a therapist, to work through depression, PTSD, and anxiety. We didn’t have this before the pandemic, but it is now a big focus for our workforce,” he said. “We are trying to build resilience. The trauma is real.”
The study was supported by the College of Physicians and Surgeons of Alberta, the Canadian Institutes of Health Research, and the Canadian Immunology Task Force. Dr. Cherry and Dr. Havaei reported no relevant financial relationships. Dr. Young reported that he is senior vice president of behavioral health at Northwell.
A version of this article appeared on Medscape.com.
Healthcare workers have been at an increased risk for SARS-CoV-2 infection and mental distress such as anxiety and depression during the pandemic, according to new research.
The risk for infection was higher among healthcare workers in the first two waves of the pandemic and again during the fifth wave.
“Previous publications, including ours, suggested that the main problem was in the early weeks and months of the pandemic, but this paper shows that it continued until the later stages,” senior author Nicola Cherry, MD, an occupational epidemiologist at the University of Alberta in Edmonton, Canada, told this news organization.
The findings were published in the Canadian Journal of Public Health.
Wave Upon Wave
In the current study, the investigators sought to compare the risk for SARS-CoV-2 infection and mental distress among healthcare workers and among community referents (CRs). They examined the following waves of the COVID-19 pandemic:
- Wave 1: From March to June 2020 (4 months).
- Wave 2: From July 2020 to February 2021 (8 months).
- Wave 3: From March to June 2021 (4 months).
- Wave 4: From July to October 2021 (4 months).
- Wave 5 (Omicron): From November 2021 to March 2022 (5 months).
Healthcare workers in Alberta were asked at recruitment for consent to match their individual records to the Alberta Administrative Health Database. As the pandemic progressed, participants were also asked for consent to be linked to COVID-19 immunization records maintained by the provinces, as well as for the results of all polymerase chain reaction (PCR) testing for the SARS-CoV-2 virus.
The investigators matched 2959 healthcare workers to 14,546 CRs according to their age, sex, geographic location in Alberta, and number of physician claims from April 1, 2019, to March 31, 2020.
Incident SARS-CoV-2 infection was examined using PCR testing and the first date of a physician consultation at which the code for SARS-CoV-2 infection had been recorded. Mental health disorders were identified from physician records. They included anxiety disorders, stress and adjustment reactions, and depressive disorders.
Most (79.5%) of the healthcare workers were registered nurses, followed by physicians (16.1%), healthcare aides (2.4%), and licensed practical nurses (2.0%). Most participants (87.5%) were female. The median age at recruitment was 44 years.
Healthcare workers were at a greater risk for COVID-19 overall, with the first SARS-CoV-2 infection defined from either PCR tests (odds ratio [OR], 1.96) or from physician records (OR, 1.33). They were also at an increased risk for anxiety (adjusted OR, 1.25; P < .001), stress/adjustment reaction (adjusted OR, 1.52; P < .001), and depressive condition (adjusted OR, 1.39; P < .001). Moreover, the excess risks for stress/adjustment reactions and depressive conditions increased with successive waves during the pandemic, peaking in the fourth wave and continuing in the fifth wave.
“Although the increase was less in the middle of the phases of the pandemic, it came back with a vengeance during the last phase, which was the Omicron phase,” said Dr. Cherry.
“Employers of healthcare workers can’t assume that everything is now under control, that they know what they’re doing, and that there is no risk. We are now having some increases in COVID. It’s going to go on. The pandemic is not over in that sense, and infection control continues to be major,” she added.
The finding that mental health worsened among healthcare workers was not surprising, Dr. Cherry said. Even before the pandemic, studies had shown that healthcare workers were at a greater risk for depression than the population overall.
“There is a lot of need for care in mental health support of healthcare workers, whether during a pandemic or not,” said Dr. Cherry.
Nurses Are Suffering
Commenting on the research for this news organization, Farinaz Havaei, PhD, RN, assistant professor of nursing at the University of British Columbia in Vancouver, Canada, said, “This is a very important and timely study that draws on objective clinical and administrative data, as opposed to healthcare workers’ subjective reports.” Dr. Havaei did not participate in the research.
Overall, the findings are consistent with previous research that drew upon healthcare workers’ reports. They speak to the chronic and cumulative impact of COVID-19 and its associated stressors on the mental health and well-being of healthcare workers, said Dr. Havaei.
“The likelihood of stress/adjustment reaction and depression showed a relatively steady increase with increasing COVID-19 waves. This increase can likely be explained by healthcare workers’ depleting emotional reserves for coping with chronic workplace stressors such as concerns about exposure to COVID-19, inadequate staffing, and work overload,” she said. Witnessing the suffering and trauma of patients and their families likely added to this risk.
Dr. Havaei also pointed out that most of the study participants were nurses. The findings are consistent with prepandemic research that showed that the suboptimal conditions that nurses increasingly faced resulted in high levels of exhaustion and burnout.
“While I agree with the authors’ call for more mental health support for healthcare workers, I think prevention efforts that address the root cause of the problem should be prioritized,” she said.
From Heroes to Zeros
The same phenomena have been observed in the United States, said John Q. Young, MD, MPP, PhD, professor and chair of psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. In various studies, Dr. Young and his colleagues have reported a strong association between exposure to the stressors of the pandemic and subsequent development of depression, anxiety, and posttraumatic stress disorder (PTSD) among healthcare workers.
“The findings from Alberta are remarkably consistent. In the beginning of the pandemic, there was a lot of acknowledgment of the work healthcare workers were doing. The fire department clapping as you leave work at night, being called heroes, even though a lot of healthcare workers feel uncomfortable with the hero language because they don’t feel like heroes. Yes, they’re afraid, but they are going to do what they need to do and help,” he said.
But as the pandemic continued, public sentiment changed, Dr. Young said. “They’ve gone from heroes to zeros. Now we are seeing the accumulated, chronic effects over months and years, and these are significant. Our healthcare workforce is vulnerable now. The reserves are low. There are serious shortages in nursing, with more retirements and more people leaving the field,” he said.
As part of a campaign to help healthcare workers cope, psychiatrists at Northwell Health have started a program called Stress First Aid at their Center for Traumatic Stress Response Resilience, where they train nurses, physicians, and other healthcare staff to use basic tools to recognize and respond to stress and distress in themselves and in their colleagues, said Dr. Young.
“For those healthcare workers who find that they are struggling and need more support, there is resilience coaching, which is one-on-one support. For those who need more clinical attention, there is a clinical program where our healthcare workers can meet with a psychologist, psychiatrist, or a therapist, to work through depression, PTSD, and anxiety. We didn’t have this before the pandemic, but it is now a big focus for our workforce,” he said. “We are trying to build resilience. The trauma is real.”
The study was supported by the College of Physicians and Surgeons of Alberta, the Canadian Institutes of Health Research, and the Canadian Immunology Task Force. Dr. Cherry and Dr. Havaei reported no relevant financial relationships. Dr. Young reported that he is senior vice president of behavioral health at Northwell.
A version of this article appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF PUBLIC HEALTH
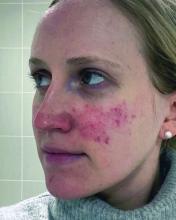
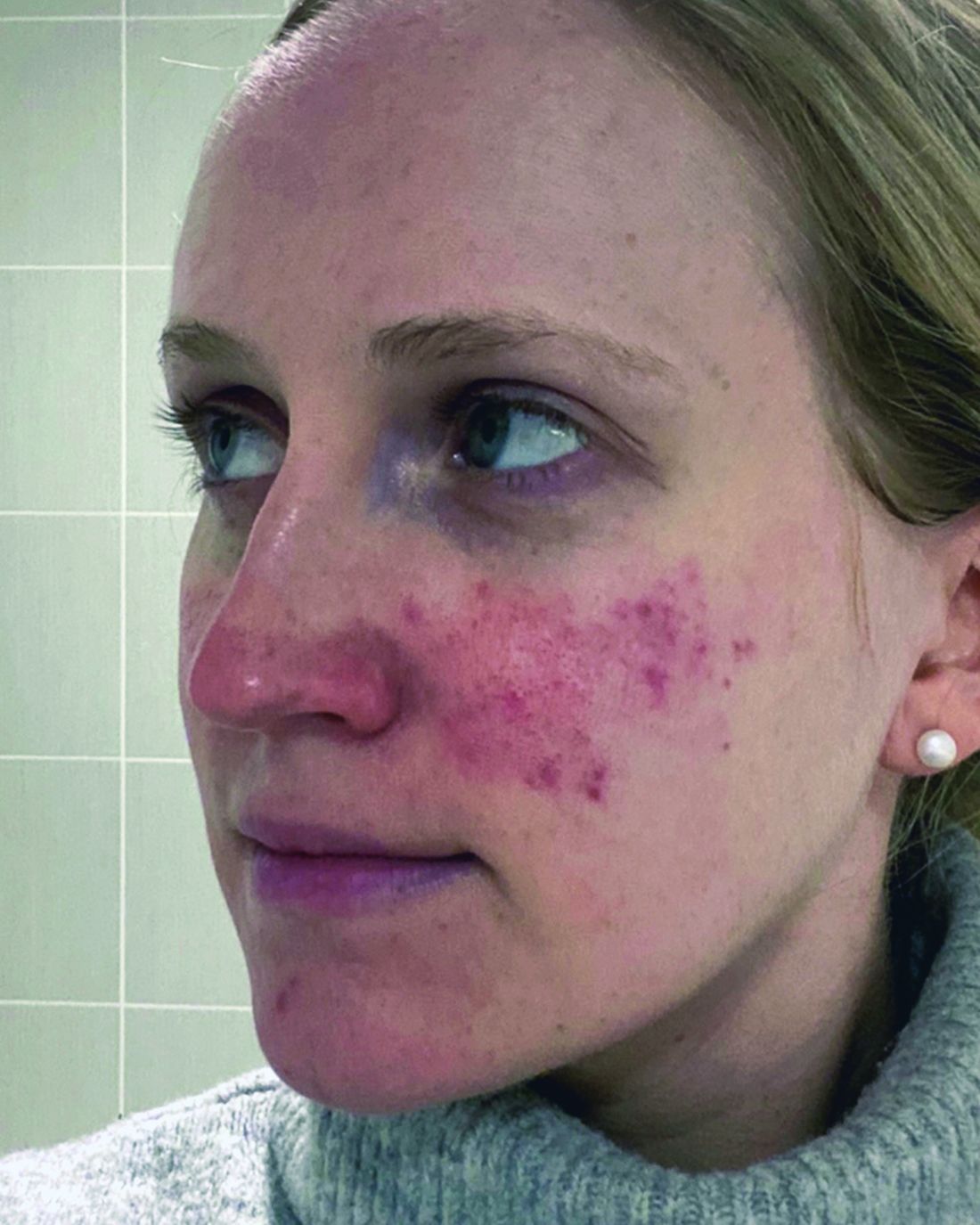
Despite An AI Assist, Imaging Study Shows Disparities in Diagnosing Different Skin Tones
When clinicians in a large-scale study viewed a series of digital images that showed skin diseases across skin tones and were asked to make a diagnosis, the accuracy was 38% among dermatologists and 19% among primary care physicians (PCPs). But when decision support from a deep learning system (DLS) was introduced, diagnostic accuracy increased by 33% among dermatologists and 69% among PCPs, results from a multicenter study showed.
However, the researchers found that across all images, diseases in dark skin (Fitzpatrick skin types 5 and 6) were diagnosed less accurately than diseases in light skin (Fitzpatrick skin types 1-4).
“,” researchers led by Matthew Groh, PhD, of Northwestern University’s Kellogg School of Management, wrote in their study, published online in Nature Medicine.
For the study, 389 board-certified dermatologists and 450 PCPs in 39 countries were presented with 364 images to view spanning 46 skin diseases and asked to submit up to four differential diagnoses. Nearly 80% of the images were of 8 diseases: atopic dermatitis, cutaneous T-cell lymphoma (CTCL), dermatomyositis, lichen planus, Lyme disease, pityriasis rosea, pityriasis rubra pilaris, and secondary syphilis.
Dermatologists and PCPs achieved a diagnostic accuracy of 38% and 19%, respectively, but both groups of clinicians were 4 percentage points less accurate for diagnosis of images of dark skin as compared with light skin. With assistance from DLS decision support, diagnostic accuracy increased by 33% among dermatologists and 69% among primary care physicians. Among dermatologists, DLS support generally increased diagnostic accuracy evenly across skin tones. However, among PCPs, DLS support increased their diagnostic accuracy more in light skin tones than in dark ones.
In the survey component of the study, when the participants were asked, “Do you feel you received sufficient training for diagnosing skin diseases in patients with skin of color (non-white patients)?” 67% of all PCPs and 33% of all dermatologists responded no. “Furthermore, we have found differences in how often BCDs [board-certified dermatologists] and PCPs refer patients with light and dark skin for biopsy,” the authors wrote. “Specifically, for CTCL (a life-threatening disease), we found that both BCDs and PCPs report that they would refer patients for biopsy significantly more often in light skin than dark skin. Moreover, for the common skin diseases atopic dermatitis and pityriasis rosea, we found that BCDs report they would refer patients for biopsy more often in dark skin than light skin, which creates an unnecessary overburden on patients with dark skin.”
In a press release about the study, Dr. Groh emphasized that he and other scientists who investigate human-computer interaction “have to find a way to incorporate underrepresented demographics in our research. That way we will be ready to accurately implement these models in the real world and build AI systems that serve as tools that are designed to avoid the kind of systematic errors we know humans and machines are prone to. Then you can update curricula, you can change norms in different fields and hopefully everyone gets better.”
Ronald Moy, MD, a dermatologist who practices in Beverly Hills, Calif., who was asked to comment on the work, said that the study contributes insights into physician-AI interaction and highlights the need for further training on diagnosing skin diseases in people with darker skin tones. “The strengths of this study include its large sample size of dermatologists and primary care physicians, use of quality-controlled images across skin tones, and thorough evaluation of diagnostic accuracy with and without AI assistance,” said Dr. Moy, who is a past president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, and the American Board of Facial Cosmetic Surgery.
“The study is limited to diagnosis and skin tone estimation based purely on a single image, which does not fully represent a clinical evaluation,” he added. However, “it does provide important benchmark data on diagnostic accuracy disparities across skin tones, but also demonstrates that while AI assistance can improve overall diagnostic accuracy, it may exacerbate disparities for non-specialists.”
Funding for the study was provided by MIT Media Lab consortium members and the Harold Horowitz Student Research Fund. One of the study authors, P. Murali Doraiswamy, MBBS, disclosed that he has received grants, advisory fees, and/or stock from several biotechnology companies outside the scope of this work and that he is a co-inventor on several patents through Duke University. The remaining authors reported having no disclosures. Dr. Moy reported having no disclosures.
When clinicians in a large-scale study viewed a series of digital images that showed skin diseases across skin tones and were asked to make a diagnosis, the accuracy was 38% among dermatologists and 19% among primary care physicians (PCPs). But when decision support from a deep learning system (DLS) was introduced, diagnostic accuracy increased by 33% among dermatologists and 69% among PCPs, results from a multicenter study showed.
However, the researchers found that across all images, diseases in dark skin (Fitzpatrick skin types 5 and 6) were diagnosed less accurately than diseases in light skin (Fitzpatrick skin types 1-4).
“,” researchers led by Matthew Groh, PhD, of Northwestern University’s Kellogg School of Management, wrote in their study, published online in Nature Medicine.
For the study, 389 board-certified dermatologists and 450 PCPs in 39 countries were presented with 364 images to view spanning 46 skin diseases and asked to submit up to four differential diagnoses. Nearly 80% of the images were of 8 diseases: atopic dermatitis, cutaneous T-cell lymphoma (CTCL), dermatomyositis, lichen planus, Lyme disease, pityriasis rosea, pityriasis rubra pilaris, and secondary syphilis.
Dermatologists and PCPs achieved a diagnostic accuracy of 38% and 19%, respectively, but both groups of clinicians were 4 percentage points less accurate for diagnosis of images of dark skin as compared with light skin. With assistance from DLS decision support, diagnostic accuracy increased by 33% among dermatologists and 69% among primary care physicians. Among dermatologists, DLS support generally increased diagnostic accuracy evenly across skin tones. However, among PCPs, DLS support increased their diagnostic accuracy more in light skin tones than in dark ones.
In the survey component of the study, when the participants were asked, “Do you feel you received sufficient training for diagnosing skin diseases in patients with skin of color (non-white patients)?” 67% of all PCPs and 33% of all dermatologists responded no. “Furthermore, we have found differences in how often BCDs [board-certified dermatologists] and PCPs refer patients with light and dark skin for biopsy,” the authors wrote. “Specifically, for CTCL (a life-threatening disease), we found that both BCDs and PCPs report that they would refer patients for biopsy significantly more often in light skin than dark skin. Moreover, for the common skin diseases atopic dermatitis and pityriasis rosea, we found that BCDs report they would refer patients for biopsy more often in dark skin than light skin, which creates an unnecessary overburden on patients with dark skin.”
In a press release about the study, Dr. Groh emphasized that he and other scientists who investigate human-computer interaction “have to find a way to incorporate underrepresented demographics in our research. That way we will be ready to accurately implement these models in the real world and build AI systems that serve as tools that are designed to avoid the kind of systematic errors we know humans and machines are prone to. Then you can update curricula, you can change norms in different fields and hopefully everyone gets better.”
Ronald Moy, MD, a dermatologist who practices in Beverly Hills, Calif., who was asked to comment on the work, said that the study contributes insights into physician-AI interaction and highlights the need for further training on diagnosing skin diseases in people with darker skin tones. “The strengths of this study include its large sample size of dermatologists and primary care physicians, use of quality-controlled images across skin tones, and thorough evaluation of diagnostic accuracy with and without AI assistance,” said Dr. Moy, who is a past president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, and the American Board of Facial Cosmetic Surgery.
“The study is limited to diagnosis and skin tone estimation based purely on a single image, which does not fully represent a clinical evaluation,” he added. However, “it does provide important benchmark data on diagnostic accuracy disparities across skin tones, but also demonstrates that while AI assistance can improve overall diagnostic accuracy, it may exacerbate disparities for non-specialists.”
Funding for the study was provided by MIT Media Lab consortium members and the Harold Horowitz Student Research Fund. One of the study authors, P. Murali Doraiswamy, MBBS, disclosed that he has received grants, advisory fees, and/or stock from several biotechnology companies outside the scope of this work and that he is a co-inventor on several patents through Duke University. The remaining authors reported having no disclosures. Dr. Moy reported having no disclosures.
When clinicians in a large-scale study viewed a series of digital images that showed skin diseases across skin tones and were asked to make a diagnosis, the accuracy was 38% among dermatologists and 19% among primary care physicians (PCPs). But when decision support from a deep learning system (DLS) was introduced, diagnostic accuracy increased by 33% among dermatologists and 69% among PCPs, results from a multicenter study showed.
However, the researchers found that across all images, diseases in dark skin (Fitzpatrick skin types 5 and 6) were diagnosed less accurately than diseases in light skin (Fitzpatrick skin types 1-4).
“,” researchers led by Matthew Groh, PhD, of Northwestern University’s Kellogg School of Management, wrote in their study, published online in Nature Medicine.
For the study, 389 board-certified dermatologists and 450 PCPs in 39 countries were presented with 364 images to view spanning 46 skin diseases and asked to submit up to four differential diagnoses. Nearly 80% of the images were of 8 diseases: atopic dermatitis, cutaneous T-cell lymphoma (CTCL), dermatomyositis, lichen planus, Lyme disease, pityriasis rosea, pityriasis rubra pilaris, and secondary syphilis.
Dermatologists and PCPs achieved a diagnostic accuracy of 38% and 19%, respectively, but both groups of clinicians were 4 percentage points less accurate for diagnosis of images of dark skin as compared with light skin. With assistance from DLS decision support, diagnostic accuracy increased by 33% among dermatologists and 69% among primary care physicians. Among dermatologists, DLS support generally increased diagnostic accuracy evenly across skin tones. However, among PCPs, DLS support increased their diagnostic accuracy more in light skin tones than in dark ones.
In the survey component of the study, when the participants were asked, “Do you feel you received sufficient training for diagnosing skin diseases in patients with skin of color (non-white patients)?” 67% of all PCPs and 33% of all dermatologists responded no. “Furthermore, we have found differences in how often BCDs [board-certified dermatologists] and PCPs refer patients with light and dark skin for biopsy,” the authors wrote. “Specifically, for CTCL (a life-threatening disease), we found that both BCDs and PCPs report that they would refer patients for biopsy significantly more often in light skin than dark skin. Moreover, for the common skin diseases atopic dermatitis and pityriasis rosea, we found that BCDs report they would refer patients for biopsy more often in dark skin than light skin, which creates an unnecessary overburden on patients with dark skin.”
In a press release about the study, Dr. Groh emphasized that he and other scientists who investigate human-computer interaction “have to find a way to incorporate underrepresented demographics in our research. That way we will be ready to accurately implement these models in the real world and build AI systems that serve as tools that are designed to avoid the kind of systematic errors we know humans and machines are prone to. Then you can update curricula, you can change norms in different fields and hopefully everyone gets better.”
Ronald Moy, MD, a dermatologist who practices in Beverly Hills, Calif., who was asked to comment on the work, said that the study contributes insights into physician-AI interaction and highlights the need for further training on diagnosing skin diseases in people with darker skin tones. “The strengths of this study include its large sample size of dermatologists and primary care physicians, use of quality-controlled images across skin tones, and thorough evaluation of diagnostic accuracy with and without AI assistance,” said Dr. Moy, who is a past president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, and the American Board of Facial Cosmetic Surgery.
“The study is limited to diagnosis and skin tone estimation based purely on a single image, which does not fully represent a clinical evaluation,” he added. However, “it does provide important benchmark data on diagnostic accuracy disparities across skin tones, but also demonstrates that while AI assistance can improve overall diagnostic accuracy, it may exacerbate disparities for non-specialists.”
Funding for the study was provided by MIT Media Lab consortium members and the Harold Horowitz Student Research Fund. One of the study authors, P. Murali Doraiswamy, MBBS, disclosed that he has received grants, advisory fees, and/or stock from several biotechnology companies outside the scope of this work and that he is a co-inventor on several patents through Duke University. The remaining authors reported having no disclosures. Dr. Moy reported having no disclosures.
FROM NATURE MEDICINE
Comorbidities and Disease Type Weigh Heavily in Pregnancy Outcomes of Immune-Mediated Inflammatory Diseases
Comorbidities may play a large role in driving poor pregnancy outcomes in pregnant people with certain immune-mediated inflammatory diseases (IMIDs).
In a new study of 12 individual IMIDs, people with rheumatoid arthritis (RA) or inflammatory bowel disease (IBD) did not have signficantly increased risk for preterm birth (PTB) or low birth weight (LBW), compared with people who did not have an IMID, after adjusting for additional chronic conditions and other confounding factors.
The study was published online on February 1 in eClinicalMedicine.
While many studies have explored the relationships between pregnancy outcomes and IMIDs, “the impact of comorbidities on the relation between IMIDs and pregnancy course is insufficiently examined,” the authors wrote. These previous studies also tended to have a small sample size.
Pregnancy Outcome Risks Varied Between IMIDs
To remedy this, researchers used electronic health record data from Providence St Joseph Health — a multistate integrated healthcare system — to identify more than 365,000 pregnant people with live births between January 1, 2013, and December 31, 2022. The cohort included more than 5700 people with at least one of 12 IMIDs: Psoriasis, IBD, RA, spondyloarthritis (SpA), multiple sclerosis, systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), antiphospholipid syndrome (APS), Sjögren syndrome (SjS), vasculitis, sarcoidosis, and systemic sclerosis. The study included only live births with a gestational age of 20 weeks or greater.
Researchers compared maternal-fetal health outcomes between the two groups, controlling for comorbidities including diabetes, cardiovascular disease, chronic kidney disease, obesity, and depression. They also accounted for confounding variables including race, age, smoking status, and socioeconomic status.
In total, 83% of people in the IMID group had no immunomodulatory medication prescriptions during their pregnancy. Of the 17% taking medication, 48%-70% continued taking their medication until delivery. Most patients were White, comprising 62.9% of the non-IMID group and 73.1% of the IMID group.
After adjusting for comorbidities, patients with any of the 12 IMIDs had a 10%-20% higher risk for PTB, LBW, small for gestation age (SGA), and cesarean section than did comparators.
But these risks varied between IMIDs. Patients with RA and IBD did not have an increased risk for PTB or LBW. However, when researchers did not control for comorbidities, pregnancy risks were higher and showed statistical significance in these two groups.
“This suggests that for RA and IBD, comorbidities may be a more important factor for adverse outcomes than the underlying autoimmune disease,” senior author Jennifer Hadlock, MD, an associate professor and director of medical data science at the Institute for Systems Biology in Seattle, Washington, said in a video accompanying a press release.
Overall, the analysis found that women with IMIDs were approximately two to three times more likely to have chronic comorbidities than the control group.
Like previous studies, there was a strong association between SLE and APS and poor pregnancy outcomes, even after controlling for confounding factors. Patients with SpA had a 50% increased risk for PTB, while those with SLE and APS had more than a twofold higher risk. Patients with SLE were 90% more likely than comparators to deliver babies with an SGA condition, while RA patients had a 30% higher risk. SLE was the only condition with an increased risk for LBW (relative risk, 3.5). IBD, RA, PsA, SpA, SLE, APS, and SjS were all associated with a higher likelihood of delivery via cesarean section.
“The findings of this study reveal that the associations between IMIDs and adverse pregnancy outcomes are influenced by the specific type of IMIDs and the presence of comorbidities,” the authors wrote.
A Large Study, But How Representative Is It?
Asked to comment on the study, Catherine Sims, MD, a rheumatologist at Duke University Medical Center in Durham, North Carolina, noted that the analysis was much larger than many reproductive rheumatology studies, and “their statistics were phenomenal.”
She agreed that “not all autoimmune diseases are created equal when it comes to pregnancy-associated risks.” However, she added that this study’s patient population may not be totally representative of pregnant people with IMIDs or autoimmune diseases.
“We’re making generalizations about autoimmune diseases based on this demographic of White women who are not taking immunosuppression,” she said.
“We know that race and ethnicity play a huge role in pregnancy outcomes, and Black women have higher maternal and fetal morbidity and mortality, which is likely related to systemic racism and biases in the medical system,” she added. “While the study did control for sociodemographic factors, the population studied is not diverse.”
Only 17% of people with IMID in the cohort were on immunosuppressive medication, which could suggest low disease activity in the study population, Dr. Sims said. If the population generally had well-controlled disease, that could have positioned them for better pregnancy outcomes.
The authors noted that their analysis did not have information on IMID disease activity or severity — one of the limitations of the study.
However, the authors argued that the observed low prescription rate during the study may have increased poor pregnancy outcomes.
“Although this reflects real-world care in the population studied, results from this study may show higher risk than might be achieved with recommended care guidelines,” they wrote.
Ultimately, the authors argued that these findings show how co-occurring health conditions can affect pregnancy outcomes in autoimmune diseases, particularly for RA and IBD.
“There is a need to take comorbidities into consideration for guidelines for patients with inflammatory bowel disease and rheumatoid arthritis and when designing future research to investigate maternal health in patients with IMIDs,” they wrote.
The study was funded by the National Institutes of Health. Dr. Sims declared no relevant financial relationships. Dr. Hadlock has received research funding (paid to the institute) from Pfizer, Novartis, Janssen, Bristol-Myers Squibb, and Gilead.
A version of this article first appeared on Medscape.com.
Comorbidities may play a large role in driving poor pregnancy outcomes in pregnant people with certain immune-mediated inflammatory diseases (IMIDs).
In a new study of 12 individual IMIDs, people with rheumatoid arthritis (RA) or inflammatory bowel disease (IBD) did not have signficantly increased risk for preterm birth (PTB) or low birth weight (LBW), compared with people who did not have an IMID, after adjusting for additional chronic conditions and other confounding factors.
The study was published online on February 1 in eClinicalMedicine.
While many studies have explored the relationships between pregnancy outcomes and IMIDs, “the impact of comorbidities on the relation between IMIDs and pregnancy course is insufficiently examined,” the authors wrote. These previous studies also tended to have a small sample size.
Pregnancy Outcome Risks Varied Between IMIDs
To remedy this, researchers used electronic health record data from Providence St Joseph Health — a multistate integrated healthcare system — to identify more than 365,000 pregnant people with live births between January 1, 2013, and December 31, 2022. The cohort included more than 5700 people with at least one of 12 IMIDs: Psoriasis, IBD, RA, spondyloarthritis (SpA), multiple sclerosis, systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), antiphospholipid syndrome (APS), Sjögren syndrome (SjS), vasculitis, sarcoidosis, and systemic sclerosis. The study included only live births with a gestational age of 20 weeks or greater.
Researchers compared maternal-fetal health outcomes between the two groups, controlling for comorbidities including diabetes, cardiovascular disease, chronic kidney disease, obesity, and depression. They also accounted for confounding variables including race, age, smoking status, and socioeconomic status.
In total, 83% of people in the IMID group had no immunomodulatory medication prescriptions during their pregnancy. Of the 17% taking medication, 48%-70% continued taking their medication until delivery. Most patients were White, comprising 62.9% of the non-IMID group and 73.1% of the IMID group.
After adjusting for comorbidities, patients with any of the 12 IMIDs had a 10%-20% higher risk for PTB, LBW, small for gestation age (SGA), and cesarean section than did comparators.
But these risks varied between IMIDs. Patients with RA and IBD did not have an increased risk for PTB or LBW. However, when researchers did not control for comorbidities, pregnancy risks were higher and showed statistical significance in these two groups.
“This suggests that for RA and IBD, comorbidities may be a more important factor for adverse outcomes than the underlying autoimmune disease,” senior author Jennifer Hadlock, MD, an associate professor and director of medical data science at the Institute for Systems Biology in Seattle, Washington, said in a video accompanying a press release.
Overall, the analysis found that women with IMIDs were approximately two to three times more likely to have chronic comorbidities than the control group.
Like previous studies, there was a strong association between SLE and APS and poor pregnancy outcomes, even after controlling for confounding factors. Patients with SpA had a 50% increased risk for PTB, while those with SLE and APS had more than a twofold higher risk. Patients with SLE were 90% more likely than comparators to deliver babies with an SGA condition, while RA patients had a 30% higher risk. SLE was the only condition with an increased risk for LBW (relative risk, 3.5). IBD, RA, PsA, SpA, SLE, APS, and SjS were all associated with a higher likelihood of delivery via cesarean section.
“The findings of this study reveal that the associations between IMIDs and adverse pregnancy outcomes are influenced by the specific type of IMIDs and the presence of comorbidities,” the authors wrote.
A Large Study, But How Representative Is It?
Asked to comment on the study, Catherine Sims, MD, a rheumatologist at Duke University Medical Center in Durham, North Carolina, noted that the analysis was much larger than many reproductive rheumatology studies, and “their statistics were phenomenal.”
She agreed that “not all autoimmune diseases are created equal when it comes to pregnancy-associated risks.” However, she added that this study’s patient population may not be totally representative of pregnant people with IMIDs or autoimmune diseases.
“We’re making generalizations about autoimmune diseases based on this demographic of White women who are not taking immunosuppression,” she said.
“We know that race and ethnicity play a huge role in pregnancy outcomes, and Black women have higher maternal and fetal morbidity and mortality, which is likely related to systemic racism and biases in the medical system,” she added. “While the study did control for sociodemographic factors, the population studied is not diverse.”
Only 17% of people with IMID in the cohort were on immunosuppressive medication, which could suggest low disease activity in the study population, Dr. Sims said. If the population generally had well-controlled disease, that could have positioned them for better pregnancy outcomes.
The authors noted that their analysis did not have information on IMID disease activity or severity — one of the limitations of the study.
However, the authors argued that the observed low prescription rate during the study may have increased poor pregnancy outcomes.
“Although this reflects real-world care in the population studied, results from this study may show higher risk than might be achieved with recommended care guidelines,” they wrote.
Ultimately, the authors argued that these findings show how co-occurring health conditions can affect pregnancy outcomes in autoimmune diseases, particularly for RA and IBD.
“There is a need to take comorbidities into consideration for guidelines for patients with inflammatory bowel disease and rheumatoid arthritis and when designing future research to investigate maternal health in patients with IMIDs,” they wrote.
The study was funded by the National Institutes of Health. Dr. Sims declared no relevant financial relationships. Dr. Hadlock has received research funding (paid to the institute) from Pfizer, Novartis, Janssen, Bristol-Myers Squibb, and Gilead.
A version of this article first appeared on Medscape.com.
Comorbidities may play a large role in driving poor pregnancy outcomes in pregnant people with certain immune-mediated inflammatory diseases (IMIDs).
In a new study of 12 individual IMIDs, people with rheumatoid arthritis (RA) or inflammatory bowel disease (IBD) did not have signficantly increased risk for preterm birth (PTB) or low birth weight (LBW), compared with people who did not have an IMID, after adjusting for additional chronic conditions and other confounding factors.
The study was published online on February 1 in eClinicalMedicine.
While many studies have explored the relationships between pregnancy outcomes and IMIDs, “the impact of comorbidities on the relation between IMIDs and pregnancy course is insufficiently examined,” the authors wrote. These previous studies also tended to have a small sample size.
Pregnancy Outcome Risks Varied Between IMIDs
To remedy this, researchers used electronic health record data from Providence St Joseph Health — a multistate integrated healthcare system — to identify more than 365,000 pregnant people with live births between January 1, 2013, and December 31, 2022. The cohort included more than 5700 people with at least one of 12 IMIDs: Psoriasis, IBD, RA, spondyloarthritis (SpA), multiple sclerosis, systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), antiphospholipid syndrome (APS), Sjögren syndrome (SjS), vasculitis, sarcoidosis, and systemic sclerosis. The study included only live births with a gestational age of 20 weeks or greater.
Researchers compared maternal-fetal health outcomes between the two groups, controlling for comorbidities including diabetes, cardiovascular disease, chronic kidney disease, obesity, and depression. They also accounted for confounding variables including race, age, smoking status, and socioeconomic status.
In total, 83% of people in the IMID group had no immunomodulatory medication prescriptions during their pregnancy. Of the 17% taking medication, 48%-70% continued taking their medication until delivery. Most patients were White, comprising 62.9% of the non-IMID group and 73.1% of the IMID group.
After adjusting for comorbidities, patients with any of the 12 IMIDs had a 10%-20% higher risk for PTB, LBW, small for gestation age (SGA), and cesarean section than did comparators.
But these risks varied between IMIDs. Patients with RA and IBD did not have an increased risk for PTB or LBW. However, when researchers did not control for comorbidities, pregnancy risks were higher and showed statistical significance in these two groups.
“This suggests that for RA and IBD, comorbidities may be a more important factor for adverse outcomes than the underlying autoimmune disease,” senior author Jennifer Hadlock, MD, an associate professor and director of medical data science at the Institute for Systems Biology in Seattle, Washington, said in a video accompanying a press release.
Overall, the analysis found that women with IMIDs were approximately two to three times more likely to have chronic comorbidities than the control group.
Like previous studies, there was a strong association between SLE and APS and poor pregnancy outcomes, even after controlling for confounding factors. Patients with SpA had a 50% increased risk for PTB, while those with SLE and APS had more than a twofold higher risk. Patients with SLE were 90% more likely than comparators to deliver babies with an SGA condition, while RA patients had a 30% higher risk. SLE was the only condition with an increased risk for LBW (relative risk, 3.5). IBD, RA, PsA, SpA, SLE, APS, and SjS were all associated with a higher likelihood of delivery via cesarean section.
“The findings of this study reveal that the associations between IMIDs and adverse pregnancy outcomes are influenced by the specific type of IMIDs and the presence of comorbidities,” the authors wrote.
A Large Study, But How Representative Is It?
Asked to comment on the study, Catherine Sims, MD, a rheumatologist at Duke University Medical Center in Durham, North Carolina, noted that the analysis was much larger than many reproductive rheumatology studies, and “their statistics were phenomenal.”
She agreed that “not all autoimmune diseases are created equal when it comes to pregnancy-associated risks.” However, she added that this study’s patient population may not be totally representative of pregnant people with IMIDs or autoimmune diseases.
“We’re making generalizations about autoimmune diseases based on this demographic of White women who are not taking immunosuppression,” she said.
“We know that race and ethnicity play a huge role in pregnancy outcomes, and Black women have higher maternal and fetal morbidity and mortality, which is likely related to systemic racism and biases in the medical system,” she added. “While the study did control for sociodemographic factors, the population studied is not diverse.”
Only 17% of people with IMID in the cohort were on immunosuppressive medication, which could suggest low disease activity in the study population, Dr. Sims said. If the population generally had well-controlled disease, that could have positioned them for better pregnancy outcomes.
The authors noted that their analysis did not have information on IMID disease activity or severity — one of the limitations of the study.
However, the authors argued that the observed low prescription rate during the study may have increased poor pregnancy outcomes.
“Although this reflects real-world care in the population studied, results from this study may show higher risk than might be achieved with recommended care guidelines,” they wrote.
Ultimately, the authors argued that these findings show how co-occurring health conditions can affect pregnancy outcomes in autoimmune diseases, particularly for RA and IBD.
“There is a need to take comorbidities into consideration for guidelines for patients with inflammatory bowel disease and rheumatoid arthritis and when designing future research to investigate maternal health in patients with IMIDs,” they wrote.
The study was funded by the National Institutes of Health. Dr. Sims declared no relevant financial relationships. Dr. Hadlock has received research funding (paid to the institute) from Pfizer, Novartis, Janssen, Bristol-Myers Squibb, and Gilead.
A version of this article first appeared on Medscape.com.
FROM ECLINICALMEDICINE
While Rare, Periocular Melanoma May Be Slightly Increasing
SAN DIEGO — Every year, about 4000 patients in the United States are diagnosed with periocular melanoma, according to Geva Mannor, MD, MPH.
“Though rare, the incidence is thought to be slightly increasing, while the onset tends to occur in patients over age 40 years,” Dr. Mannor, an oculofacial plastic surgeon in the division of ophthalmology at Scripps Clinic, San Diego, said at the annual Cutaneous Malignancy Update. “It may be more common in males and welding is a risk factor. Pain and vision loss are late symptoms, and there are amelanotic variants. Gene expression profiling and other genetic testing can predict metastasis, especially expression of BRCA1-associated protein 1 (BAP1).”
. Extrapolating from the best available data, Dr. Mannor said that the annual incidence of choroidal melanoma in the United States is less than 2500, the annual incidence of eyelid melanoma is less than 750, and the annual incidence of conjunctival melanoma is less than 250 — much lower than for cutaneous melanomas. Put another way, the ratio between cutaneous and choroid melanoma is 80:1, the ratio between cutaneous and eyelid melanoma is 266:1, and the ratio between cutaneous and conjunctival melanoma is 800:1.
According to an article published in 2021 on the topic, risk factors for periocular melanoma include light eye color (blue/gray; relative risk, 1.75), fair skin (RR, 1.80), and inability to tan (RR, 1.64), but not blonde hair. A review of 210 patients with melanoma of the eyelid from 11 studies showed that 57% were located on the lower lid, 13% were on the upper lid (“I think because the brow protects sun exposure to the upper lid,” Dr. Mannor said), 12% were on the brow, 10% were on the lateral canthus, and 2% were on the medial canthus. In addition, 35% of the eyelid melanomas were superficial spreading cases, 31% were lentigo maligna, and 19% were nodular. The mean Breslow depth was 1.36 mm and the mortality rate was 4.9%.
Dr. Mannor said that cheek and brow melanomas can extend to the inside of the eyelid and conjunctiva. “Therefore, you want to examine the inside of upper and lower eyelids,” he said at the meeting, which was hosted by the Scripps Cancer Center. “Margin-control excision of lid melanoma is the standard treatment.”
On a related note, he said that glaucoma eye drops that contain prostaglandin F2alpha induce cutaneous and iris pigmentation with varying rates depending on the specific type of prostaglandin. “There have been case reports of these eye drops causing periocular pigmentation that masquerades as suspicious, melanoma-like skin cancer, often necessitating a skin biopsy,” he said.
Dr. Mannor reported having no disclosures.
SAN DIEGO — Every year, about 4000 patients in the United States are diagnosed with periocular melanoma, according to Geva Mannor, MD, MPH.
“Though rare, the incidence is thought to be slightly increasing, while the onset tends to occur in patients over age 40 years,” Dr. Mannor, an oculofacial plastic surgeon in the division of ophthalmology at Scripps Clinic, San Diego, said at the annual Cutaneous Malignancy Update. “It may be more common in males and welding is a risk factor. Pain and vision loss are late symptoms, and there are amelanotic variants. Gene expression profiling and other genetic testing can predict metastasis, especially expression of BRCA1-associated protein 1 (BAP1).”
. Extrapolating from the best available data, Dr. Mannor said that the annual incidence of choroidal melanoma in the United States is less than 2500, the annual incidence of eyelid melanoma is less than 750, and the annual incidence of conjunctival melanoma is less than 250 — much lower than for cutaneous melanomas. Put another way, the ratio between cutaneous and choroid melanoma is 80:1, the ratio between cutaneous and eyelid melanoma is 266:1, and the ratio between cutaneous and conjunctival melanoma is 800:1.
According to an article published in 2021 on the topic, risk factors for periocular melanoma include light eye color (blue/gray; relative risk, 1.75), fair skin (RR, 1.80), and inability to tan (RR, 1.64), but not blonde hair. A review of 210 patients with melanoma of the eyelid from 11 studies showed that 57% were located on the lower lid, 13% were on the upper lid (“I think because the brow protects sun exposure to the upper lid,” Dr. Mannor said), 12% were on the brow, 10% were on the lateral canthus, and 2% were on the medial canthus. In addition, 35% of the eyelid melanomas were superficial spreading cases, 31% were lentigo maligna, and 19% were nodular. The mean Breslow depth was 1.36 mm and the mortality rate was 4.9%.
Dr. Mannor said that cheek and brow melanomas can extend to the inside of the eyelid and conjunctiva. “Therefore, you want to examine the inside of upper and lower eyelids,” he said at the meeting, which was hosted by the Scripps Cancer Center. “Margin-control excision of lid melanoma is the standard treatment.”
On a related note, he said that glaucoma eye drops that contain prostaglandin F2alpha induce cutaneous and iris pigmentation with varying rates depending on the specific type of prostaglandin. “There have been case reports of these eye drops causing periocular pigmentation that masquerades as suspicious, melanoma-like skin cancer, often necessitating a skin biopsy,” he said.
Dr. Mannor reported having no disclosures.
SAN DIEGO — Every year, about 4000 patients in the United States are diagnosed with periocular melanoma, according to Geva Mannor, MD, MPH.
“Though rare, the incidence is thought to be slightly increasing, while the onset tends to occur in patients over age 40 years,” Dr. Mannor, an oculofacial plastic surgeon in the division of ophthalmology at Scripps Clinic, San Diego, said at the annual Cutaneous Malignancy Update. “It may be more common in males and welding is a risk factor. Pain and vision loss are late symptoms, and there are amelanotic variants. Gene expression profiling and other genetic testing can predict metastasis, especially expression of BRCA1-associated protein 1 (BAP1).”
. Extrapolating from the best available data, Dr. Mannor said that the annual incidence of choroidal melanoma in the United States is less than 2500, the annual incidence of eyelid melanoma is less than 750, and the annual incidence of conjunctival melanoma is less than 250 — much lower than for cutaneous melanomas. Put another way, the ratio between cutaneous and choroid melanoma is 80:1, the ratio between cutaneous and eyelid melanoma is 266:1, and the ratio between cutaneous and conjunctival melanoma is 800:1.
According to an article published in 2021 on the topic, risk factors for periocular melanoma include light eye color (blue/gray; relative risk, 1.75), fair skin (RR, 1.80), and inability to tan (RR, 1.64), but not blonde hair. A review of 210 patients with melanoma of the eyelid from 11 studies showed that 57% were located on the lower lid, 13% were on the upper lid (“I think because the brow protects sun exposure to the upper lid,” Dr. Mannor said), 12% were on the brow, 10% were on the lateral canthus, and 2% were on the medial canthus. In addition, 35% of the eyelid melanomas were superficial spreading cases, 31% were lentigo maligna, and 19% were nodular. The mean Breslow depth was 1.36 mm and the mortality rate was 4.9%.
Dr. Mannor said that cheek and brow melanomas can extend to the inside of the eyelid and conjunctiva. “Therefore, you want to examine the inside of upper and lower eyelids,” he said at the meeting, which was hosted by the Scripps Cancer Center. “Margin-control excision of lid melanoma is the standard treatment.”
On a related note, he said that glaucoma eye drops that contain prostaglandin F2alpha induce cutaneous and iris pigmentation with varying rates depending on the specific type of prostaglandin. “There have been case reports of these eye drops causing periocular pigmentation that masquerades as suspicious, melanoma-like skin cancer, often necessitating a skin biopsy,” he said.
Dr. Mannor reported having no disclosures.
FROM MELANOMA 2024
A Neurotoxin, an Antidepressant, and More Emerging Options for Treating Rosacea
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
FROM ODAC 2024
Expert Shares Tips for Diagnosing, Managing Spitz Nevi
SAN DIEGO —
“For a pigmented Spitz nevus, we were taught to look for a starburst pattern, a central area of homogeneous pigment, and peripheral symmetrical streaks or pseudopods,” Dr. Piggott, an adult and pediatric dermatologist at Scripps Clinic, San Diego, said at the annual Cutaneous Malignancy Update. “For Spitz nevi without pigment, we were taught to look for symmetric dotted vessels.”
However, results from a retrospective study published in 2015 gave her pause in relying on dermoscopy alone for assessing Spitz nevi. Researchers from Italy, Japan, and Brazil studied excision specimens of 384 Spitzoid-looking lesions in patients 12 years and older. On histology, 86.7% were diagnosed as benign Spitz nevi and 13.3% were diagnosed as melanoma.
When the researchers looked at the dermoscopic images, many cases of atypical Spitz nevi were indistinguishable from the benign Spitz nevi. Now, Dr. Piggott said, “I respect the dermoscopy criteria, but I don’t rely solely on it.”
If a child presents with Spitzoid-looking lesion, biopsy is generally preferred to observation. “The traditional belief was that punch biopsy was preferable, followed by shave biopsy,” she said. “This is always on a case-by-case basis.”
However, results from a retrospective study of the records of 123 cases of biopsy-proven Spitz nevi with incomplete removal on biopsy suggests that the method of biopsy matters. The researchers found that the presence of residual lesion in the re-excision specimen was significantly higher when the initial biopsy was done by punch biopsy (90.9%) when compared with shave biopsy (48.9%) and formal excision (62.5%; P < .05).
“This suggests that shave may better than punch for the initial biopsy, but the study was limited by its retrospective design,” Dr. Piggott said at the meeting, which was hosted by Scripps Cancer Center. “Even today it remains controversial whether you should do a shave or punch biopsy.”
Parameters for diagnosing Spitzoid tumors that pathologists look for under the microscope are asymmetry, Clark’s level IV/V, lack of maturation, solid growth, nuclear pleomorphism, high nuclear-cytoplasmic ratio, and mitoses that are atypical, deep, or that exceed 6 mm2 in size.
In terms of treatment recommendations for children with biopsy-proven Spitz nevi, Dr. Piggott said that there is no consensus among pediatric dermatologists. If the biopsy comes back as a benign Spitz nevus, the most reasonable approach is observation, “especially if there is no clinical residue — no pigment on exam, no papule left over in the scar,” she said. “You also want to educate the family about the rare potential for transformation down the line. Monitor for recurrence and consider re-excision if recurrence occurs.”
If the initial Spitz nevus biopsy reveals any degree of atypia, excision is preferred. “In young children, you have to weigh the risks of anesthesia for removal,” she said. “If you’re unable to excise the lesion, close observation is recommended at 6 months or 1 year.”
Treatment for borderline atypical Spitz tumor is excision, she continued, but no outcomes data exist that document a survival benefit with sentinel lymph node (SLN) biopsy. “The decision on whether to do a SLN biopsy is usually made on a case-by-case basis,” Dr. Piggott said. “Nodal metastases from atypical Spitzoid tumors are not uncommon, but death from widespread disease is rare. If the SLN biopsy is positive, complete lymphadenectomy is associated with increased risk of morbidity and no evidence of increased survival. If lymph node disease is found, we in pediatric dermatology would consider referral to a pediatric oncologist for consideration of systemic therapy such as interferon or a newer immunotherapy.”
Dr. Piggott reported having no relevant disclosures.
SAN DIEGO —
“For a pigmented Spitz nevus, we were taught to look for a starburst pattern, a central area of homogeneous pigment, and peripheral symmetrical streaks or pseudopods,” Dr. Piggott, an adult and pediatric dermatologist at Scripps Clinic, San Diego, said at the annual Cutaneous Malignancy Update. “For Spitz nevi without pigment, we were taught to look for symmetric dotted vessels.”
However, results from a retrospective study published in 2015 gave her pause in relying on dermoscopy alone for assessing Spitz nevi. Researchers from Italy, Japan, and Brazil studied excision specimens of 384 Spitzoid-looking lesions in patients 12 years and older. On histology, 86.7% were diagnosed as benign Spitz nevi and 13.3% were diagnosed as melanoma.
When the researchers looked at the dermoscopic images, many cases of atypical Spitz nevi were indistinguishable from the benign Spitz nevi. Now, Dr. Piggott said, “I respect the dermoscopy criteria, but I don’t rely solely on it.”
If a child presents with Spitzoid-looking lesion, biopsy is generally preferred to observation. “The traditional belief was that punch biopsy was preferable, followed by shave biopsy,” she said. “This is always on a case-by-case basis.”
However, results from a retrospective study of the records of 123 cases of biopsy-proven Spitz nevi with incomplete removal on biopsy suggests that the method of biopsy matters. The researchers found that the presence of residual lesion in the re-excision specimen was significantly higher when the initial biopsy was done by punch biopsy (90.9%) when compared with shave biopsy (48.9%) and formal excision (62.5%; P < .05).
“This suggests that shave may better than punch for the initial biopsy, but the study was limited by its retrospective design,” Dr. Piggott said at the meeting, which was hosted by Scripps Cancer Center. “Even today it remains controversial whether you should do a shave or punch biopsy.”
Parameters for diagnosing Spitzoid tumors that pathologists look for under the microscope are asymmetry, Clark’s level IV/V, lack of maturation, solid growth, nuclear pleomorphism, high nuclear-cytoplasmic ratio, and mitoses that are atypical, deep, or that exceed 6 mm2 in size.
In terms of treatment recommendations for children with biopsy-proven Spitz nevi, Dr. Piggott said that there is no consensus among pediatric dermatologists. If the biopsy comes back as a benign Spitz nevus, the most reasonable approach is observation, “especially if there is no clinical residue — no pigment on exam, no papule left over in the scar,” she said. “You also want to educate the family about the rare potential for transformation down the line. Monitor for recurrence and consider re-excision if recurrence occurs.”
If the initial Spitz nevus biopsy reveals any degree of atypia, excision is preferred. “In young children, you have to weigh the risks of anesthesia for removal,” she said. “If you’re unable to excise the lesion, close observation is recommended at 6 months or 1 year.”
Treatment for borderline atypical Spitz tumor is excision, she continued, but no outcomes data exist that document a survival benefit with sentinel lymph node (SLN) biopsy. “The decision on whether to do a SLN biopsy is usually made on a case-by-case basis,” Dr. Piggott said. “Nodal metastases from atypical Spitzoid tumors are not uncommon, but death from widespread disease is rare. If the SLN biopsy is positive, complete lymphadenectomy is associated with increased risk of morbidity and no evidence of increased survival. If lymph node disease is found, we in pediatric dermatology would consider referral to a pediatric oncologist for consideration of systemic therapy such as interferon or a newer immunotherapy.”
Dr. Piggott reported having no relevant disclosures.
SAN DIEGO —
“For a pigmented Spitz nevus, we were taught to look for a starburst pattern, a central area of homogeneous pigment, and peripheral symmetrical streaks or pseudopods,” Dr. Piggott, an adult and pediatric dermatologist at Scripps Clinic, San Diego, said at the annual Cutaneous Malignancy Update. “For Spitz nevi without pigment, we were taught to look for symmetric dotted vessels.”
However, results from a retrospective study published in 2015 gave her pause in relying on dermoscopy alone for assessing Spitz nevi. Researchers from Italy, Japan, and Brazil studied excision specimens of 384 Spitzoid-looking lesions in patients 12 years and older. On histology, 86.7% were diagnosed as benign Spitz nevi and 13.3% were diagnosed as melanoma.
When the researchers looked at the dermoscopic images, many cases of atypical Spitz nevi were indistinguishable from the benign Spitz nevi. Now, Dr. Piggott said, “I respect the dermoscopy criteria, but I don’t rely solely on it.”
If a child presents with Spitzoid-looking lesion, biopsy is generally preferred to observation. “The traditional belief was that punch biopsy was preferable, followed by shave biopsy,” she said. “This is always on a case-by-case basis.”
However, results from a retrospective study of the records of 123 cases of biopsy-proven Spitz nevi with incomplete removal on biopsy suggests that the method of biopsy matters. The researchers found that the presence of residual lesion in the re-excision specimen was significantly higher when the initial biopsy was done by punch biopsy (90.9%) when compared with shave biopsy (48.9%) and formal excision (62.5%; P < .05).
“This suggests that shave may better than punch for the initial biopsy, but the study was limited by its retrospective design,” Dr. Piggott said at the meeting, which was hosted by Scripps Cancer Center. “Even today it remains controversial whether you should do a shave or punch biopsy.”
Parameters for diagnosing Spitzoid tumors that pathologists look for under the microscope are asymmetry, Clark’s level IV/V, lack of maturation, solid growth, nuclear pleomorphism, high nuclear-cytoplasmic ratio, and mitoses that are atypical, deep, or that exceed 6 mm2 in size.
In terms of treatment recommendations for children with biopsy-proven Spitz nevi, Dr. Piggott said that there is no consensus among pediatric dermatologists. If the biopsy comes back as a benign Spitz nevus, the most reasonable approach is observation, “especially if there is no clinical residue — no pigment on exam, no papule left over in the scar,” she said. “You also want to educate the family about the rare potential for transformation down the line. Monitor for recurrence and consider re-excision if recurrence occurs.”
If the initial Spitz nevus biopsy reveals any degree of atypia, excision is preferred. “In young children, you have to weigh the risks of anesthesia for removal,” she said. “If you’re unable to excise the lesion, close observation is recommended at 6 months or 1 year.”
Treatment for borderline atypical Spitz tumor is excision, she continued, but no outcomes data exist that document a survival benefit with sentinel lymph node (SLN) biopsy. “The decision on whether to do a SLN biopsy is usually made on a case-by-case basis,” Dr. Piggott said. “Nodal metastases from atypical Spitzoid tumors are not uncommon, but death from widespread disease is rare. If the SLN biopsy is positive, complete lymphadenectomy is associated with increased risk of morbidity and no evidence of increased survival. If lymph node disease is found, we in pediatric dermatology would consider referral to a pediatric oncologist for consideration of systemic therapy such as interferon or a newer immunotherapy.”
Dr. Piggott reported having no relevant disclosures.
FROM MELANOMA 2024
New Findings on Vitamin D, Omega-3 Supplements for Preventing Autoimmune Diseases
Two years after the end of a randomized trial that showed a benefit of daily vitamin D and omega-3 fatty acid (n-3 FA) supplementation for reducing risk for autoimmune diseases, the salubrious effects of daily vitamin D appear to have waned after the supplement was discontinued, while the protection from n-3 lived on for at least 2 additional years.
As previously reported, the randomized VITAL, which was designed primarily to study the effects of vitamin D and n-3 supplementation on incident cancer and cardiovascular disease, also showed that 5 years of vitamin D supplementation was associated with a 22% reduction in risk for confirmed autoimmune diseases, and 5 years of n-3 FA supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases.
Now, investigators Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston, Massachusetts, and colleagues reported that among 21,592 participants in VITAL who agreed to be followed for an additional 2 years after discontinuation, the protection against autoimmune diseases from daily vitamin D (cholecalciferol; 2000 IU/d) was no longer statistically significant, but the benefits of daily marine n-3 FAs (1 g/d as a fish-oil capsule containing 460 mg of eicosapentaenoic acid and 380 mg of docosahexaenoic acid) remained significant.
“VITAL observational extension results suggest that vitamin D supplementation should be given on a continuous basis for long-term prevention of [autoimmune diseases]. The beneficial effects of n-3 fatty acids, however, may be prolonged for at least 2 years after discontinuation,” they wrote in an article published in Arthritis & Rheumatology.
Dr. Costenbader told this news organization that the results of the observational extension study suggest that the benefits of vitamin D “wear off more quickly, and it should be continued for a longer period of time or indefinitely, rather than only for 5 years.”
In addition to the disparity in the duration of the protective effect, the investigators also saw differences in the effects across different autoimmune diseases.
“The protective effect of vitamin D seemed strongest for psoriasis, while for omega-3 fatty acids, the protective effects were strongest for rheumatoid arthritis and inflammatory bowel disease,” she said.
Mixed Effects
In an interview with this news organization, Janet Funk, MD, MS, vice chair of research in the Department of Medicine and professor in the School of Nutritional Science and Wellness at the University of Arizona, Tucson, Arizona, who was not involved in the study, saidthat the results suggest that while each supplement may offer protection against autoimmune diseases, the effects are inconsistent and may not apply to all patients.
“I think the VITAL extension results suggest that either supplement (or both together) may have benefits in reducing risk of autoimmune diseases, including possible persistent effects posttreatment, but that these effects are nuanced (ie, only in normal weight post-vitamin D treatment) and possibly not uniform across all autoimmune diseases (including possible adverse effects for some — eg, inverse association between prior omega-3 and psoriasis and tendency for increased autoimmune thyroid disease for vitamin D), although the study was not powered sufficiently to draw disease-specific conclusions,” she said.
In an editorial accompanying the study, rheumatologist Joel M. Kremer, MD, of Albany Medical College and the Corrona Research Foundation in Delray Beach, Florida, wrote that “[T]he studies by Dr. Costenbader, et al. have shed new light on the possibility that dietary supplements of n-3 FA [fatty acid] may prevent the onset of [autoimmune disease]. The sustained benefits they describe for as long as 2 years after the supplements are discontinued are consistent with the chronicity of FA species in cellular plasma membranes where they serve as substrates for a diverse array of salient metabolic and inflammatory pathways.”
VITAL Then
To test whether vitamin D or marine-derived long-chain n-3 FA supplementation could protect against autoimmune disease over time, Dr. Costenbader and colleagues piggybacked an ancillary study onto the VITAL trial, which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older and 13,085 women aged 55 and older. The study had a 2 × 2 factorial design, with patients randomly assigned to vitamin D 2000 IU/d or placebo and then further randomized to either 1 g/d n-3 FAs or placebo in both the vitamin D and placebo primary randomization arms.
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with a hazard ratio (HR) of 0.68 (P = .02) for incident autoimmune disease, n-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03). However, when probable incident autoimmune disease cases were included, the effect of n-3 became significant, with an HR of 0.82.
VITAL Now
In the current analysis, Dr. Costenbader and colleagues reported observational data on 21,592 VITAL participants, a sample representing 83.5% of those who were initially randomized, and 87.9% of those who were alive and could be contacted at the end of the study.
As in the initial trial, the investigators used annual questionnaires to assess incident autoimmune diseases during the randomized follow-up. Participants were asked about new-onset, doctor-diagnosed rheumatoid arthritis, polymyalgia rheumatica, psoriasis, autoimmune thyroid disease, and inflammatory bowel disease. Participants could also write in any other new autoimmune disease diagnoses.
There were 236 new cases of confirmed autoimmune disease that occurred since the initial publication of the trial results, as well as 65 probable cases identified during the median 5.3 years of the randomized portion, and 42 probable cases diagnosed during the 2-year observational phase.
The investigators found that after the 2-year observation period, 255 participants initially randomized to receive vitamin D had a newly developed confirmed autoimmune disease, compared with 259 of those initially randomized to a vitamin D placebo. This translated into a nonsignificant HR of 0.98.
Adding probable autoimmune cases to the confirmed cases made little difference, resulting in a nonsignificant adjusted HR of 0.95.
In contrast, there were 234 confirmed autoimmune disease cases among patients initially assigned to n-3, compared with 280 among patients randomized to the n-3 placebo, translating into a statistically significant HR of 0.83 for new-onset autoimmune disease with n-3.
Dr. Costenbader and colleagues acknowledged that the study was limited by the use of doses intended to prevent cancer or cardiovascular disease and that higher doses intended for high-risk or nutritionally deficient populations might reveal larger effects of supplementation. In addition, they noted the difficulty of identifying the timing and onset of incident disease, and that the small number of cases that occurred during the 2-year observational period precluded detailed analyses of individual autoimmune diseases.
The study was funded by grants from the National Institutes of Health. Dr. Costenbader, Dr. Funk, and Dr. Kremer reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Two years after the end of a randomized trial that showed a benefit of daily vitamin D and omega-3 fatty acid (n-3 FA) supplementation for reducing risk for autoimmune diseases, the salubrious effects of daily vitamin D appear to have waned after the supplement was discontinued, while the protection from n-3 lived on for at least 2 additional years.
As previously reported, the randomized VITAL, which was designed primarily to study the effects of vitamin D and n-3 supplementation on incident cancer and cardiovascular disease, also showed that 5 years of vitamin D supplementation was associated with a 22% reduction in risk for confirmed autoimmune diseases, and 5 years of n-3 FA supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases.
Now, investigators Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston, Massachusetts, and colleagues reported that among 21,592 participants in VITAL who agreed to be followed for an additional 2 years after discontinuation, the protection against autoimmune diseases from daily vitamin D (cholecalciferol; 2000 IU/d) was no longer statistically significant, but the benefits of daily marine n-3 FAs (1 g/d as a fish-oil capsule containing 460 mg of eicosapentaenoic acid and 380 mg of docosahexaenoic acid) remained significant.
“VITAL observational extension results suggest that vitamin D supplementation should be given on a continuous basis for long-term prevention of [autoimmune diseases]. The beneficial effects of n-3 fatty acids, however, may be prolonged for at least 2 years after discontinuation,” they wrote in an article published in Arthritis & Rheumatology.
Dr. Costenbader told this news organization that the results of the observational extension study suggest that the benefits of vitamin D “wear off more quickly, and it should be continued for a longer period of time or indefinitely, rather than only for 5 years.”
In addition to the disparity in the duration of the protective effect, the investigators also saw differences in the effects across different autoimmune diseases.
“The protective effect of vitamin D seemed strongest for psoriasis, while for omega-3 fatty acids, the protective effects were strongest for rheumatoid arthritis and inflammatory bowel disease,” she said.
Mixed Effects
In an interview with this news organization, Janet Funk, MD, MS, vice chair of research in the Department of Medicine and professor in the School of Nutritional Science and Wellness at the University of Arizona, Tucson, Arizona, who was not involved in the study, saidthat the results suggest that while each supplement may offer protection against autoimmune diseases, the effects are inconsistent and may not apply to all patients.
“I think the VITAL extension results suggest that either supplement (or both together) may have benefits in reducing risk of autoimmune diseases, including possible persistent effects posttreatment, but that these effects are nuanced (ie, only in normal weight post-vitamin D treatment) and possibly not uniform across all autoimmune diseases (including possible adverse effects for some — eg, inverse association between prior omega-3 and psoriasis and tendency for increased autoimmune thyroid disease for vitamin D), although the study was not powered sufficiently to draw disease-specific conclusions,” she said.
In an editorial accompanying the study, rheumatologist Joel M. Kremer, MD, of Albany Medical College and the Corrona Research Foundation in Delray Beach, Florida, wrote that “[T]he studies by Dr. Costenbader, et al. have shed new light on the possibility that dietary supplements of n-3 FA [fatty acid] may prevent the onset of [autoimmune disease]. The sustained benefits they describe for as long as 2 years after the supplements are discontinued are consistent with the chronicity of FA species in cellular plasma membranes where they serve as substrates for a diverse array of salient metabolic and inflammatory pathways.”
VITAL Then
To test whether vitamin D or marine-derived long-chain n-3 FA supplementation could protect against autoimmune disease over time, Dr. Costenbader and colleagues piggybacked an ancillary study onto the VITAL trial, which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older and 13,085 women aged 55 and older. The study had a 2 × 2 factorial design, with patients randomly assigned to vitamin D 2000 IU/d or placebo and then further randomized to either 1 g/d n-3 FAs or placebo in both the vitamin D and placebo primary randomization arms.
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with a hazard ratio (HR) of 0.68 (P = .02) for incident autoimmune disease, n-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03). However, when probable incident autoimmune disease cases were included, the effect of n-3 became significant, with an HR of 0.82.
VITAL Now
In the current analysis, Dr. Costenbader and colleagues reported observational data on 21,592 VITAL participants, a sample representing 83.5% of those who were initially randomized, and 87.9% of those who were alive and could be contacted at the end of the study.
As in the initial trial, the investigators used annual questionnaires to assess incident autoimmune diseases during the randomized follow-up. Participants were asked about new-onset, doctor-diagnosed rheumatoid arthritis, polymyalgia rheumatica, psoriasis, autoimmune thyroid disease, and inflammatory bowel disease. Participants could also write in any other new autoimmune disease diagnoses.
There were 236 new cases of confirmed autoimmune disease that occurred since the initial publication of the trial results, as well as 65 probable cases identified during the median 5.3 years of the randomized portion, and 42 probable cases diagnosed during the 2-year observational phase.
The investigators found that after the 2-year observation period, 255 participants initially randomized to receive vitamin D had a newly developed confirmed autoimmune disease, compared with 259 of those initially randomized to a vitamin D placebo. This translated into a nonsignificant HR of 0.98.
Adding probable autoimmune cases to the confirmed cases made little difference, resulting in a nonsignificant adjusted HR of 0.95.
In contrast, there were 234 confirmed autoimmune disease cases among patients initially assigned to n-3, compared with 280 among patients randomized to the n-3 placebo, translating into a statistically significant HR of 0.83 for new-onset autoimmune disease with n-3.
Dr. Costenbader and colleagues acknowledged that the study was limited by the use of doses intended to prevent cancer or cardiovascular disease and that higher doses intended for high-risk or nutritionally deficient populations might reveal larger effects of supplementation. In addition, they noted the difficulty of identifying the timing and onset of incident disease, and that the small number of cases that occurred during the 2-year observational period precluded detailed analyses of individual autoimmune diseases.
The study was funded by grants from the National Institutes of Health. Dr. Costenbader, Dr. Funk, and Dr. Kremer reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Two years after the end of a randomized trial that showed a benefit of daily vitamin D and omega-3 fatty acid (n-3 FA) supplementation for reducing risk for autoimmune diseases, the salubrious effects of daily vitamin D appear to have waned after the supplement was discontinued, while the protection from n-3 lived on for at least 2 additional years.
As previously reported, the randomized VITAL, which was designed primarily to study the effects of vitamin D and n-3 supplementation on incident cancer and cardiovascular disease, also showed that 5 years of vitamin D supplementation was associated with a 22% reduction in risk for confirmed autoimmune diseases, and 5 years of n-3 FA supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases.
Now, investigators Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston, Massachusetts, and colleagues reported that among 21,592 participants in VITAL who agreed to be followed for an additional 2 years after discontinuation, the protection against autoimmune diseases from daily vitamin D (cholecalciferol; 2000 IU/d) was no longer statistically significant, but the benefits of daily marine n-3 FAs (1 g/d as a fish-oil capsule containing 460 mg of eicosapentaenoic acid and 380 mg of docosahexaenoic acid) remained significant.
“VITAL observational extension results suggest that vitamin D supplementation should be given on a continuous basis for long-term prevention of [autoimmune diseases]. The beneficial effects of n-3 fatty acids, however, may be prolonged for at least 2 years after discontinuation,” they wrote in an article published in Arthritis & Rheumatology.
Dr. Costenbader told this news organization that the results of the observational extension study suggest that the benefits of vitamin D “wear off more quickly, and it should be continued for a longer period of time or indefinitely, rather than only for 5 years.”
In addition to the disparity in the duration of the protective effect, the investigators also saw differences in the effects across different autoimmune diseases.
“The protective effect of vitamin D seemed strongest for psoriasis, while for omega-3 fatty acids, the protective effects were strongest for rheumatoid arthritis and inflammatory bowel disease,” she said.
Mixed Effects
In an interview with this news organization, Janet Funk, MD, MS, vice chair of research in the Department of Medicine and professor in the School of Nutritional Science and Wellness at the University of Arizona, Tucson, Arizona, who was not involved in the study, saidthat the results suggest that while each supplement may offer protection against autoimmune diseases, the effects are inconsistent and may not apply to all patients.
“I think the VITAL extension results suggest that either supplement (or both together) may have benefits in reducing risk of autoimmune diseases, including possible persistent effects posttreatment, but that these effects are nuanced (ie, only in normal weight post-vitamin D treatment) and possibly not uniform across all autoimmune diseases (including possible adverse effects for some — eg, inverse association between prior omega-3 and psoriasis and tendency for increased autoimmune thyroid disease for vitamin D), although the study was not powered sufficiently to draw disease-specific conclusions,” she said.
In an editorial accompanying the study, rheumatologist Joel M. Kremer, MD, of Albany Medical College and the Corrona Research Foundation in Delray Beach, Florida, wrote that “[T]he studies by Dr. Costenbader, et al. have shed new light on the possibility that dietary supplements of n-3 FA [fatty acid] may prevent the onset of [autoimmune disease]. The sustained benefits they describe for as long as 2 years after the supplements are discontinued are consistent with the chronicity of FA species in cellular plasma membranes where they serve as substrates for a diverse array of salient metabolic and inflammatory pathways.”
VITAL Then
To test whether vitamin D or marine-derived long-chain n-3 FA supplementation could protect against autoimmune disease over time, Dr. Costenbader and colleagues piggybacked an ancillary study onto the VITAL trial, which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older and 13,085 women aged 55 and older. The study had a 2 × 2 factorial design, with patients randomly assigned to vitamin D 2000 IU/d or placebo and then further randomized to either 1 g/d n-3 FAs or placebo in both the vitamin D and placebo primary randomization arms.
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with a hazard ratio (HR) of 0.68 (P = .02) for incident autoimmune disease, n-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03). However, when probable incident autoimmune disease cases were included, the effect of n-3 became significant, with an HR of 0.82.
VITAL Now
In the current analysis, Dr. Costenbader and colleagues reported observational data on 21,592 VITAL participants, a sample representing 83.5% of those who were initially randomized, and 87.9% of those who were alive and could be contacted at the end of the study.
As in the initial trial, the investigators used annual questionnaires to assess incident autoimmune diseases during the randomized follow-up. Participants were asked about new-onset, doctor-diagnosed rheumatoid arthritis, polymyalgia rheumatica, psoriasis, autoimmune thyroid disease, and inflammatory bowel disease. Participants could also write in any other new autoimmune disease diagnoses.
There were 236 new cases of confirmed autoimmune disease that occurred since the initial publication of the trial results, as well as 65 probable cases identified during the median 5.3 years of the randomized portion, and 42 probable cases diagnosed during the 2-year observational phase.
The investigators found that after the 2-year observation period, 255 participants initially randomized to receive vitamin D had a newly developed confirmed autoimmune disease, compared with 259 of those initially randomized to a vitamin D placebo. This translated into a nonsignificant HR of 0.98.
Adding probable autoimmune cases to the confirmed cases made little difference, resulting in a nonsignificant adjusted HR of 0.95.
In contrast, there were 234 confirmed autoimmune disease cases among patients initially assigned to n-3, compared with 280 among patients randomized to the n-3 placebo, translating into a statistically significant HR of 0.83 for new-onset autoimmune disease with n-3.
Dr. Costenbader and colleagues acknowledged that the study was limited by the use of doses intended to prevent cancer or cardiovascular disease and that higher doses intended for high-risk or nutritionally deficient populations might reveal larger effects of supplementation. In addition, they noted the difficulty of identifying the timing and onset of incident disease, and that the small number of cases that occurred during the 2-year observational period precluded detailed analyses of individual autoimmune diseases.
The study was funded by grants from the National Institutes of Health. Dr. Costenbader, Dr. Funk, and Dr. Kremer reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
How to Avoid the $400,000 Med School Debt Mistakes I Made
It’s not always great to be tops among your peers.
, according to Medscape Medical News’ 2023 Residents Salary and Debt Report.
I’m smack in that upper percentile. I amassed nearly a half million dollars in student debt and currently stand at roughly $400,000. Yay me.
As a naive twentysomething making a major life decision, I never thought my loans would amount to this inconceivable figure, the proverbial “mortgage without a roof” you hear student debt experts talk about.
This isn’t a story about how the student loan industry needs to be reformed or how education has become increasingly expensive or regrets about going to medical school.
It’s also not a story about how you should be handling basics like consolidating and refinancing and paying extra toward your principal.
It’s about my experience as a physician 13 years after signing that first promissory note. In short: I completely miscalculated the impact loans would have on my life.
I bought money to go to school. I can’t undo that. But over the past decade, I have learned a lot, particularly how those with their own mountain of debt — or who will inevitably wind up with one — can manage things better than I have.
Mistake #1: Loan Forgiveness Is More Complicated Than it Seems
My parents and I were aware of the Public Service Loan Forgiveness (PSLF) program which began in 2007 shortly before I started exploring medical school options. I wanted to help people, so working in the nonprofit sector sounded like a no-brainer. Making 120 payments while practicing at a qualifying institution didn’t sound hard.
Newsflash: Not all healthcare organizations are 501(c)3 programs that qualify as nonprofit for the PSLF program. You can’t just snap your fingers and land at one. I graduated from fellowship just as the COVID-19 pandemic began, which meant I was launching my medical career in the midst of hiring freezes and an overnight disappearance of job opportunities.
I had to take a 2-year hiatus from the nonprofit sector and found a part-time position with a local private practice group. It still stings. Had I been working for a qualified employer, I could have benefited from the student loan payment pause and been closer to applying for loan forgiveness.
Avoid it: Be brutally honest with yourself about what kind of medicine you want to practice — especially within the opportunities you have on hand. Private practice is very different from working for the nonprofit sector. I didn›t know that. When weighing career choices, immediately ask, “How will this impact how I pay my loans?” You may not like the answer, but you›ll always know where you stand financially.
Mistake #2: I Forgot to Factor in Life Goals
To be fair, some things were out of my control: Not getting into a state school with cheaper tuition rates, graduating at the start of a once-in-a-lifetime global pandemic. I wasn’t prepared for a changing job landscape. But there were also “expected” life events like getting married, developing a geographical preference, and having a child. I didn’t consider those either.
How about the “expected” goal of buying a home? For years I didn’t feel financially comfortable enough to take on a mortgage. For so long, my attitude has been don’t take on any more debt. (A special shout-out to my 6.8% interest rate which has contributed over a third of my total loan amount.)
This even affected how my husband and I would talk about what a future home might look like. There’s always a giant unwelcome guest casting a shadow over my thoughts.
Avoid it: Don’t compartmentalize your personal and professional lives. Your student loans will hang over both, and you need to be honest with yourself about what “upward mobility” really means to you while in debt. There’s a reason people say “live like a resident” until your loans are paid off. My husband and I finally worked our numbers to where we bought our first home this past year — a moment years in the making. I still drive around in my beloved Honda CR-V like it’s a Mercedes G-Wagon.
Mistake #3: I Didn’t Ask Questions
I regret not talking to a practicing physician about their experience with student loans. I didn’t know any. There weren’t any physicians in my extended family or my community network. I was a first-generation Pakistani American kid trying to figure it out.
It’s difficult because even today, many physicians aren’t comfortable discussing their financial circumstances. The lack of financial transparency and even financial literacy is astounding among young medical professionals. We live in a medical culture where no one talks about the money. I was too diffident and nervous to even try.
Avoid it: Don’t be afraid to have uncomfortable conversations about money. Don’t allow yourself to make even one passive decision. It’s your life.
If you can’t find someone in medicine to talk to about their financial journey, there are plenty of credible resources. Medscape Medical News has a Physician Business Academy with hot topics like personal finance. The White Coat Investor is literally bookmarked on all my electronic devices. KevinMD.com has a ton of resources and articles answering common financial questions about retirement, savings, and house buying. And Travis Hornsby with www.studentloanplanner.com has wonderful advice on all kinds of different loans.
There are no stupid questions. Just ask. You might be surprised by what people are willing to share.
Mistake #4: Playing it Casual With My Lenders
If $400,000 in debt doesn’t sound bad enough, imagine lots more. It turns out my loan carrier had me at a much higher loan balance because they’d inadvertently duplicated one of my loans in the total. I didn’t know that until I transferred my loans to another handler and it came to light.
Imagine my relief at having a lower total. Imagine my anger at myself for not checking sooner.
Avoid it: Do a thorough self-audit on all your loans more than once a year. Pretend they’re a patient with odd symptoms you can’t pin down and you have the luxury of doing every diagnostic test available. It’s not fun studying your own debt, but it’s the only way to really know how much you have.
Mistake #5: Not Leaving Room to Change My Mind
I underestimated how I would evolve and how my goals would change after having the letters “MD” after my name. I never dreamed that a nonprofit salary might not be enough.
A lot of us assume that the bedside is where we will find professional satisfaction. But you might be surprised. In a climate where we’re constantly being pushed to do more in a broken healthcare system, a landscape where misinformation and technology are forcing medicine to change, there might be little joy in working clinically full time. Then what do you do?
Because I elected to go the PSLF route, I’m tied to this decision. And while it still makes the most economic sense for me personally, it now limits my professional exploration and freedom.
Avoid it: Consider how much time you really want to spend in clinical medicine. Be mindful that you have to work at least 0.8 full time equivalent to qualify for the PSLF program. It’s very hard to predict the future, let alone your future, but just know you›ll have moments where you ask, “Do I really want to stay on this career track?” Will you be able to pivot? Can you live with it if the answer is no?
Looking Ahead
Let me be clear about one thing. Despite all the negativity I feel toward my student loans — guilt about the burden I brought to my marriage and my adult life, disappointment about the cost of becoming a successful physician, and frustration that this has turned out to be the most influential factor shaping my professional and personal choices — the one thing I don’t feel is shame.
I worked hard to get to this point in my life. I am proud of being a physician.
My student loan burden will follow me to the grave. But progress is also possible. I have friends that have paid their loans down by hustling, working hard, and dropping every penny toward them.
I also have friends that have had their loans forgiven. There are options. Everyone’s experience looks a little different. But don’t be naive: Student loans will color every financial decision you make.
I’m finding solace now in recently moving and finding work at a nonprofit institution. I’m back at it; 77 payments made, and 43 to go.
Well, technically I’ve made 93 payments. I’m still waiting for my loan servicer to get around to updating my account.
You really have to stay on top of those folks.
A version of this article appeared on Medscape.com.
It’s not always great to be tops among your peers.
, according to Medscape Medical News’ 2023 Residents Salary and Debt Report.
I’m smack in that upper percentile. I amassed nearly a half million dollars in student debt and currently stand at roughly $400,000. Yay me.
As a naive twentysomething making a major life decision, I never thought my loans would amount to this inconceivable figure, the proverbial “mortgage without a roof” you hear student debt experts talk about.
This isn’t a story about how the student loan industry needs to be reformed or how education has become increasingly expensive or regrets about going to medical school.
It’s also not a story about how you should be handling basics like consolidating and refinancing and paying extra toward your principal.
It’s about my experience as a physician 13 years after signing that first promissory note. In short: I completely miscalculated the impact loans would have on my life.
I bought money to go to school. I can’t undo that. But over the past decade, I have learned a lot, particularly how those with their own mountain of debt — or who will inevitably wind up with one — can manage things better than I have.
Mistake #1: Loan Forgiveness Is More Complicated Than it Seems
My parents and I were aware of the Public Service Loan Forgiveness (PSLF) program which began in 2007 shortly before I started exploring medical school options. I wanted to help people, so working in the nonprofit sector sounded like a no-brainer. Making 120 payments while practicing at a qualifying institution didn’t sound hard.
Newsflash: Not all healthcare organizations are 501(c)3 programs that qualify as nonprofit for the PSLF program. You can’t just snap your fingers and land at one. I graduated from fellowship just as the COVID-19 pandemic began, which meant I was launching my medical career in the midst of hiring freezes and an overnight disappearance of job opportunities.
I had to take a 2-year hiatus from the nonprofit sector and found a part-time position with a local private practice group. It still stings. Had I been working for a qualified employer, I could have benefited from the student loan payment pause and been closer to applying for loan forgiveness.
Avoid it: Be brutally honest with yourself about what kind of medicine you want to practice — especially within the opportunities you have on hand. Private practice is very different from working for the nonprofit sector. I didn›t know that. When weighing career choices, immediately ask, “How will this impact how I pay my loans?” You may not like the answer, but you›ll always know where you stand financially.
Mistake #2: I Forgot to Factor in Life Goals
To be fair, some things were out of my control: Not getting into a state school with cheaper tuition rates, graduating at the start of a once-in-a-lifetime global pandemic. I wasn’t prepared for a changing job landscape. But there were also “expected” life events like getting married, developing a geographical preference, and having a child. I didn’t consider those either.
How about the “expected” goal of buying a home? For years I didn’t feel financially comfortable enough to take on a mortgage. For so long, my attitude has been don’t take on any more debt. (A special shout-out to my 6.8% interest rate which has contributed over a third of my total loan amount.)
This even affected how my husband and I would talk about what a future home might look like. There’s always a giant unwelcome guest casting a shadow over my thoughts.
Avoid it: Don’t compartmentalize your personal and professional lives. Your student loans will hang over both, and you need to be honest with yourself about what “upward mobility” really means to you while in debt. There’s a reason people say “live like a resident” until your loans are paid off. My husband and I finally worked our numbers to where we bought our first home this past year — a moment years in the making. I still drive around in my beloved Honda CR-V like it’s a Mercedes G-Wagon.
Mistake #3: I Didn’t Ask Questions
I regret not talking to a practicing physician about their experience with student loans. I didn’t know any. There weren’t any physicians in my extended family or my community network. I was a first-generation Pakistani American kid trying to figure it out.
It’s difficult because even today, many physicians aren’t comfortable discussing their financial circumstances. The lack of financial transparency and even financial literacy is astounding among young medical professionals. We live in a medical culture where no one talks about the money. I was too diffident and nervous to even try.
Avoid it: Don’t be afraid to have uncomfortable conversations about money. Don’t allow yourself to make even one passive decision. It’s your life.
If you can’t find someone in medicine to talk to about their financial journey, there are plenty of credible resources. Medscape Medical News has a Physician Business Academy with hot topics like personal finance. The White Coat Investor is literally bookmarked on all my electronic devices. KevinMD.com has a ton of resources and articles answering common financial questions about retirement, savings, and house buying. And Travis Hornsby with www.studentloanplanner.com has wonderful advice on all kinds of different loans.
There are no stupid questions. Just ask. You might be surprised by what people are willing to share.
Mistake #4: Playing it Casual With My Lenders
If $400,000 in debt doesn’t sound bad enough, imagine lots more. It turns out my loan carrier had me at a much higher loan balance because they’d inadvertently duplicated one of my loans in the total. I didn’t know that until I transferred my loans to another handler and it came to light.
Imagine my relief at having a lower total. Imagine my anger at myself for not checking sooner.
Avoid it: Do a thorough self-audit on all your loans more than once a year. Pretend they’re a patient with odd symptoms you can’t pin down and you have the luxury of doing every diagnostic test available. It’s not fun studying your own debt, but it’s the only way to really know how much you have.
Mistake #5: Not Leaving Room to Change My Mind
I underestimated how I would evolve and how my goals would change after having the letters “MD” after my name. I never dreamed that a nonprofit salary might not be enough.
A lot of us assume that the bedside is where we will find professional satisfaction. But you might be surprised. In a climate where we’re constantly being pushed to do more in a broken healthcare system, a landscape where misinformation and technology are forcing medicine to change, there might be little joy in working clinically full time. Then what do you do?
Because I elected to go the PSLF route, I’m tied to this decision. And while it still makes the most economic sense for me personally, it now limits my professional exploration and freedom.
Avoid it: Consider how much time you really want to spend in clinical medicine. Be mindful that you have to work at least 0.8 full time equivalent to qualify for the PSLF program. It’s very hard to predict the future, let alone your future, but just know you›ll have moments where you ask, “Do I really want to stay on this career track?” Will you be able to pivot? Can you live with it if the answer is no?
Looking Ahead
Let me be clear about one thing. Despite all the negativity I feel toward my student loans — guilt about the burden I brought to my marriage and my adult life, disappointment about the cost of becoming a successful physician, and frustration that this has turned out to be the most influential factor shaping my professional and personal choices — the one thing I don’t feel is shame.
I worked hard to get to this point in my life. I am proud of being a physician.
My student loan burden will follow me to the grave. But progress is also possible. I have friends that have paid their loans down by hustling, working hard, and dropping every penny toward them.
I also have friends that have had their loans forgiven. There are options. Everyone’s experience looks a little different. But don’t be naive: Student loans will color every financial decision you make.
I’m finding solace now in recently moving and finding work at a nonprofit institution. I’m back at it; 77 payments made, and 43 to go.
Well, technically I’ve made 93 payments. I’m still waiting for my loan servicer to get around to updating my account.
You really have to stay on top of those folks.
A version of this article appeared on Medscape.com.
It’s not always great to be tops among your peers.
, according to Medscape Medical News’ 2023 Residents Salary and Debt Report.
I’m smack in that upper percentile. I amassed nearly a half million dollars in student debt and currently stand at roughly $400,000. Yay me.
As a naive twentysomething making a major life decision, I never thought my loans would amount to this inconceivable figure, the proverbial “mortgage without a roof” you hear student debt experts talk about.
This isn’t a story about how the student loan industry needs to be reformed or how education has become increasingly expensive or regrets about going to medical school.
It’s also not a story about how you should be handling basics like consolidating and refinancing and paying extra toward your principal.
It’s about my experience as a physician 13 years after signing that first promissory note. In short: I completely miscalculated the impact loans would have on my life.
I bought money to go to school. I can’t undo that. But over the past decade, I have learned a lot, particularly how those with their own mountain of debt — or who will inevitably wind up with one — can manage things better than I have.
Mistake #1: Loan Forgiveness Is More Complicated Than it Seems
My parents and I were aware of the Public Service Loan Forgiveness (PSLF) program which began in 2007 shortly before I started exploring medical school options. I wanted to help people, so working in the nonprofit sector sounded like a no-brainer. Making 120 payments while practicing at a qualifying institution didn’t sound hard.
Newsflash: Not all healthcare organizations are 501(c)3 programs that qualify as nonprofit for the PSLF program. You can’t just snap your fingers and land at one. I graduated from fellowship just as the COVID-19 pandemic began, which meant I was launching my medical career in the midst of hiring freezes and an overnight disappearance of job opportunities.
I had to take a 2-year hiatus from the nonprofit sector and found a part-time position with a local private practice group. It still stings. Had I been working for a qualified employer, I could have benefited from the student loan payment pause and been closer to applying for loan forgiveness.
Avoid it: Be brutally honest with yourself about what kind of medicine you want to practice — especially within the opportunities you have on hand. Private practice is very different from working for the nonprofit sector. I didn›t know that. When weighing career choices, immediately ask, “How will this impact how I pay my loans?” You may not like the answer, but you›ll always know where you stand financially.
Mistake #2: I Forgot to Factor in Life Goals
To be fair, some things were out of my control: Not getting into a state school with cheaper tuition rates, graduating at the start of a once-in-a-lifetime global pandemic. I wasn’t prepared for a changing job landscape. But there were also “expected” life events like getting married, developing a geographical preference, and having a child. I didn’t consider those either.
How about the “expected” goal of buying a home? For years I didn’t feel financially comfortable enough to take on a mortgage. For so long, my attitude has been don’t take on any more debt. (A special shout-out to my 6.8% interest rate which has contributed over a third of my total loan amount.)
This even affected how my husband and I would talk about what a future home might look like. There’s always a giant unwelcome guest casting a shadow over my thoughts.
Avoid it: Don’t compartmentalize your personal and professional lives. Your student loans will hang over both, and you need to be honest with yourself about what “upward mobility” really means to you while in debt. There’s a reason people say “live like a resident” until your loans are paid off. My husband and I finally worked our numbers to where we bought our first home this past year — a moment years in the making. I still drive around in my beloved Honda CR-V like it’s a Mercedes G-Wagon.
Mistake #3: I Didn’t Ask Questions
I regret not talking to a practicing physician about their experience with student loans. I didn’t know any. There weren’t any physicians in my extended family or my community network. I was a first-generation Pakistani American kid trying to figure it out.
It’s difficult because even today, many physicians aren’t comfortable discussing their financial circumstances. The lack of financial transparency and even financial literacy is astounding among young medical professionals. We live in a medical culture where no one talks about the money. I was too diffident and nervous to even try.
Avoid it: Don’t be afraid to have uncomfortable conversations about money. Don’t allow yourself to make even one passive decision. It’s your life.
If you can’t find someone in medicine to talk to about their financial journey, there are plenty of credible resources. Medscape Medical News has a Physician Business Academy with hot topics like personal finance. The White Coat Investor is literally bookmarked on all my electronic devices. KevinMD.com has a ton of resources and articles answering common financial questions about retirement, savings, and house buying. And Travis Hornsby with www.studentloanplanner.com has wonderful advice on all kinds of different loans.
There are no stupid questions. Just ask. You might be surprised by what people are willing to share.
Mistake #4: Playing it Casual With My Lenders
If $400,000 in debt doesn’t sound bad enough, imagine lots more. It turns out my loan carrier had me at a much higher loan balance because they’d inadvertently duplicated one of my loans in the total. I didn’t know that until I transferred my loans to another handler and it came to light.
Imagine my relief at having a lower total. Imagine my anger at myself for not checking sooner.
Avoid it: Do a thorough self-audit on all your loans more than once a year. Pretend they’re a patient with odd symptoms you can’t pin down and you have the luxury of doing every diagnostic test available. It’s not fun studying your own debt, but it’s the only way to really know how much you have.
Mistake #5: Not Leaving Room to Change My Mind
I underestimated how I would evolve and how my goals would change after having the letters “MD” after my name. I never dreamed that a nonprofit salary might not be enough.
A lot of us assume that the bedside is where we will find professional satisfaction. But you might be surprised. In a climate where we’re constantly being pushed to do more in a broken healthcare system, a landscape where misinformation and technology are forcing medicine to change, there might be little joy in working clinically full time. Then what do you do?
Because I elected to go the PSLF route, I’m tied to this decision. And while it still makes the most economic sense for me personally, it now limits my professional exploration and freedom.
Avoid it: Consider how much time you really want to spend in clinical medicine. Be mindful that you have to work at least 0.8 full time equivalent to qualify for the PSLF program. It’s very hard to predict the future, let alone your future, but just know you›ll have moments where you ask, “Do I really want to stay on this career track?” Will you be able to pivot? Can you live with it if the answer is no?
Looking Ahead
Let me be clear about one thing. Despite all the negativity I feel toward my student loans — guilt about the burden I brought to my marriage and my adult life, disappointment about the cost of becoming a successful physician, and frustration that this has turned out to be the most influential factor shaping my professional and personal choices — the one thing I don’t feel is shame.
I worked hard to get to this point in my life. I am proud of being a physician.
My student loan burden will follow me to the grave. But progress is also possible. I have friends that have paid their loans down by hustling, working hard, and dropping every penny toward them.
I also have friends that have had their loans forgiven. There are options. Everyone’s experience looks a little different. But don’t be naive: Student loans will color every financial decision you make.
I’m finding solace now in recently moving and finding work at a nonprofit institution. I’m back at it; 77 payments made, and 43 to go.
Well, technically I’ve made 93 payments. I’m still waiting for my loan servicer to get around to updating my account.
You really have to stay on top of those folks.
A version of this article appeared on Medscape.com.