User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
‘Real-World’ Registry Study of Upadacitinib Supports Clinical Trial Data
, results from an analysis of registry data showed.
“We know from clinical trials that upadacitinib is effective, but we have very little real-world experience on its effectiveness,” presenting study author Melinda Gooderham, MD, medical director of the Skin Center for Dermatology in Peterborough, Ontario, Canada, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference.
For the analysis, she and her coauthors evaluated the real-world clinical and patient-reported outcomes of 335 adults enrolled in the CorEvitas AD Registry from July 21, 2020, through Aug. 7, 2023. They included patients who were on upadacitinib for at least 4 weeks and persisted on the drug until the time of evaluation.
The CorEvitas AD Registry is a prospective, non-interventional registry of adults diagnosed with AD by a dermatologist or qualified dermatology practitioner. Outcomes measures included the proportion of patients who achieved skin clearance as defined by a Validated Investigators Global Assessment Scale for Atopic Dermatitis (vIGA) score of 0 or 1, an Eczema Area and Severity Index (EASI) score of 3 or less, a Peak Pruritus Numeric Rating Scale (PP-NRS) score of 0 or 1, Patient-Oriented Eczema Measure (POEM) scores of 0-2, Dermatology Life Quality Index (DLQI) scores of 0 or 1, or an Atopic Dermatitis Control Tool (ADCT) score of <7.
The researchers evaluated cross-sectional data from three different cohorts: data from the last registry visit (the overall cohort), data from a visit within 1 month to less than 5 months of upadacitinib initiation (the 1-5 months cohort), and data from a visit within 5-9 months following upadacitinib initiation (the 5-9 months cohort). They also conducted subgroup analyses of patients with prior use of biologics for AD (bio-experience) and those with no such history (bio-naive). Safety events were not assessed in this analysis.
The mean age of the 335 patients was 45.6 years, 51.6% were female, 64.2% were White, 64.2% were based in the United States and the rest were based in Canada. Most patients (70.8%) were treated with the 15-mg dose of upadacitinib. The median duration of treatment was 6.9 months. Slightly more than one-quarter of patients (28.1%) reported concomitant use of topical corticosteroids for AD, while 45.4% reported prior use of dupilumab and 6% reported prior use of tralokinumab.
Dr. Gooderham reported that 57.5% of patients in the total cohort total cohort achieved clear or almost clear skin (a vIGA-AD score of 0 or 1), with slight differences between the bio-naive (60.6%) and bio-experienced (54.1%) subgroups.
The other outcomes were similarly close between the 176 bio-naive and 159 bio-experienced patients. Specifically, 74.8% in the total cohort, 79.4% in the bio-naive subgroup, and 69.6% in the bio-experienced subgroup achieved an EASI score of 3 or less. In the measure of the worst itch in the past 24 hours, 45.3%, 47.7%, and 42.8% respectively achieved a PP-NRS of 0 or 1. In the patient-reported disease burden, 36.4%, 41%, and 31.4% achieved a POEM score of 0-2. In the quality of life measure, 39.8%, 42.8%, and 36.5% achieved a DLQI score of 0 or 1. In the measure of disease control, 69.3%, 70.5%, and 67.9% achieved an ADCT score of <7. In a combination of skin clearance and itch control, 40.9%, 43.2%, and 38.4% of the total cohort, bio-naive, and bio-experienced respectively achieved both an EASI score of 3 or less and a PP-NRS of 0 or 1.
The study outcomes were similar between the 1-5 months cohort and the 5-9 months cohort, but there was a trend toward more clearance the longer patients were on therapy.
“The findings suggest that low levels of disease severity are observed in patients on upadacitinib in a real-world setting,” Dr. Gooderham concluded. “This confirms what we see in the clinical trials.”
She disclosed that she is a consultant to, a speaker for, and/or a member of the advisory board for many pharmaceutical companies, including AbbVie.
, results from an analysis of registry data showed.
“We know from clinical trials that upadacitinib is effective, but we have very little real-world experience on its effectiveness,” presenting study author Melinda Gooderham, MD, medical director of the Skin Center for Dermatology in Peterborough, Ontario, Canada, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference.
For the analysis, she and her coauthors evaluated the real-world clinical and patient-reported outcomes of 335 adults enrolled in the CorEvitas AD Registry from July 21, 2020, through Aug. 7, 2023. They included patients who were on upadacitinib for at least 4 weeks and persisted on the drug until the time of evaluation.
The CorEvitas AD Registry is a prospective, non-interventional registry of adults diagnosed with AD by a dermatologist or qualified dermatology practitioner. Outcomes measures included the proportion of patients who achieved skin clearance as defined by a Validated Investigators Global Assessment Scale for Atopic Dermatitis (vIGA) score of 0 or 1, an Eczema Area and Severity Index (EASI) score of 3 or less, a Peak Pruritus Numeric Rating Scale (PP-NRS) score of 0 or 1, Patient-Oriented Eczema Measure (POEM) scores of 0-2, Dermatology Life Quality Index (DLQI) scores of 0 or 1, or an Atopic Dermatitis Control Tool (ADCT) score of <7.
The researchers evaluated cross-sectional data from three different cohorts: data from the last registry visit (the overall cohort), data from a visit within 1 month to less than 5 months of upadacitinib initiation (the 1-5 months cohort), and data from a visit within 5-9 months following upadacitinib initiation (the 5-9 months cohort). They also conducted subgroup analyses of patients with prior use of biologics for AD (bio-experience) and those with no such history (bio-naive). Safety events were not assessed in this analysis.
The mean age of the 335 patients was 45.6 years, 51.6% were female, 64.2% were White, 64.2% were based in the United States and the rest were based in Canada. Most patients (70.8%) were treated with the 15-mg dose of upadacitinib. The median duration of treatment was 6.9 months. Slightly more than one-quarter of patients (28.1%) reported concomitant use of topical corticosteroids for AD, while 45.4% reported prior use of dupilumab and 6% reported prior use of tralokinumab.
Dr. Gooderham reported that 57.5% of patients in the total cohort total cohort achieved clear or almost clear skin (a vIGA-AD score of 0 or 1), with slight differences between the bio-naive (60.6%) and bio-experienced (54.1%) subgroups.
The other outcomes were similarly close between the 176 bio-naive and 159 bio-experienced patients. Specifically, 74.8% in the total cohort, 79.4% in the bio-naive subgroup, and 69.6% in the bio-experienced subgroup achieved an EASI score of 3 or less. In the measure of the worst itch in the past 24 hours, 45.3%, 47.7%, and 42.8% respectively achieved a PP-NRS of 0 or 1. In the patient-reported disease burden, 36.4%, 41%, and 31.4% achieved a POEM score of 0-2. In the quality of life measure, 39.8%, 42.8%, and 36.5% achieved a DLQI score of 0 or 1. In the measure of disease control, 69.3%, 70.5%, and 67.9% achieved an ADCT score of <7. In a combination of skin clearance and itch control, 40.9%, 43.2%, and 38.4% of the total cohort, bio-naive, and bio-experienced respectively achieved both an EASI score of 3 or less and a PP-NRS of 0 or 1.
The study outcomes were similar between the 1-5 months cohort and the 5-9 months cohort, but there was a trend toward more clearance the longer patients were on therapy.
“The findings suggest that low levels of disease severity are observed in patients on upadacitinib in a real-world setting,” Dr. Gooderham concluded. “This confirms what we see in the clinical trials.”
She disclosed that she is a consultant to, a speaker for, and/or a member of the advisory board for many pharmaceutical companies, including AbbVie.
, results from an analysis of registry data showed.
“We know from clinical trials that upadacitinib is effective, but we have very little real-world experience on its effectiveness,” presenting study author Melinda Gooderham, MD, medical director of the Skin Center for Dermatology in Peterborough, Ontario, Canada, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference.
For the analysis, she and her coauthors evaluated the real-world clinical and patient-reported outcomes of 335 adults enrolled in the CorEvitas AD Registry from July 21, 2020, through Aug. 7, 2023. They included patients who were on upadacitinib for at least 4 weeks and persisted on the drug until the time of evaluation.
The CorEvitas AD Registry is a prospective, non-interventional registry of adults diagnosed with AD by a dermatologist or qualified dermatology practitioner. Outcomes measures included the proportion of patients who achieved skin clearance as defined by a Validated Investigators Global Assessment Scale for Atopic Dermatitis (vIGA) score of 0 or 1, an Eczema Area and Severity Index (EASI) score of 3 or less, a Peak Pruritus Numeric Rating Scale (PP-NRS) score of 0 or 1, Patient-Oriented Eczema Measure (POEM) scores of 0-2, Dermatology Life Quality Index (DLQI) scores of 0 or 1, or an Atopic Dermatitis Control Tool (ADCT) score of <7.
The researchers evaluated cross-sectional data from three different cohorts: data from the last registry visit (the overall cohort), data from a visit within 1 month to less than 5 months of upadacitinib initiation (the 1-5 months cohort), and data from a visit within 5-9 months following upadacitinib initiation (the 5-9 months cohort). They also conducted subgroup analyses of patients with prior use of biologics for AD (bio-experience) and those with no such history (bio-naive). Safety events were not assessed in this analysis.
The mean age of the 335 patients was 45.6 years, 51.6% were female, 64.2% were White, 64.2% were based in the United States and the rest were based in Canada. Most patients (70.8%) were treated with the 15-mg dose of upadacitinib. The median duration of treatment was 6.9 months. Slightly more than one-quarter of patients (28.1%) reported concomitant use of topical corticosteroids for AD, while 45.4% reported prior use of dupilumab and 6% reported prior use of tralokinumab.
Dr. Gooderham reported that 57.5% of patients in the total cohort total cohort achieved clear or almost clear skin (a vIGA-AD score of 0 or 1), with slight differences between the bio-naive (60.6%) and bio-experienced (54.1%) subgroups.
The other outcomes were similarly close between the 176 bio-naive and 159 bio-experienced patients. Specifically, 74.8% in the total cohort, 79.4% in the bio-naive subgroup, and 69.6% in the bio-experienced subgroup achieved an EASI score of 3 or less. In the measure of the worst itch in the past 24 hours, 45.3%, 47.7%, and 42.8% respectively achieved a PP-NRS of 0 or 1. In the patient-reported disease burden, 36.4%, 41%, and 31.4% achieved a POEM score of 0-2. In the quality of life measure, 39.8%, 42.8%, and 36.5% achieved a DLQI score of 0 or 1. In the measure of disease control, 69.3%, 70.5%, and 67.9% achieved an ADCT score of <7. In a combination of skin clearance and itch control, 40.9%, 43.2%, and 38.4% of the total cohort, bio-naive, and bio-experienced respectively achieved both an EASI score of 3 or less and a PP-NRS of 0 or 1.
The study outcomes were similar between the 1-5 months cohort and the 5-9 months cohort, but there was a trend toward more clearance the longer patients were on therapy.
“The findings suggest that low levels of disease severity are observed in patients on upadacitinib in a real-world setting,” Dr. Gooderham concluded. “This confirms what we see in the clinical trials.”
She disclosed that she is a consultant to, a speaker for, and/or a member of the advisory board for many pharmaceutical companies, including AbbVie.
FROM RAD 2023
What Makes Patients Vulnerable to Delusions of Parasitosis?
, reported researchers in a small retrospective case-control study.
Delusions of parasitosis (DOP) affects mostly middle-aged women and has associations with renal failure and some medications, wrote corresponding author Colleen Reisz, MD, a dermatologist with the department of internal medicine at the University of Missouri–Kansas City School of Medicine, and her coauthors. The study was published online December 15, 2023, in the Journal of the American Academy of Dermatology.
“We hypothesize that vulnerability to DOP emerges when multiple factors combine, such as age, sex, medications, and changes in [drug] clearance capacity,” Dr. Reisz and her coauthors wrote. “Changes in health care, such as the dramatic increase in stimulant prescriptions and alternatives to opioids in pain management, may be contributing to off target drug effects on the brain.”
To test their hypothesis, the researchers conducted a case-control study of biometric and pharmaceutical data from 34 patients with DOP which they compared to an age-matched control group of 53 women presenting with a dermatitis above the clavicle from a general dermatology practice between 2012 and 2020. They de-identified the data and performed statistical analysis on variables that included biometric data and intake of pharmaceuticals and nutraceuticals. Polypharmacy was defined as five or more drugs.
Of the 34 patients with DOP, 27 were women with a mean age of 58 years and 7 were men with a mean age of 60 years. Dr. Reisz and her colleagues observed statistical significance between cases and controls in terms of polypharmacy (P = .011), attention-deficit/hyperactivity disorder medications (P < .001), selective serotonin reuptake inhibitors (P = .005), opioids (P = .003), and gabapentin (P = .003).
In other findings, half of DOP cases presented with samples of perceived parasitic material, and four associated the perceived infestation with a single emotion-laden event. This prompted the researchers “to consider that DOP may share mechanisms with fear conditioning and extinction,” they wrote. “Fear conditioning refers to the process of memory acquisition and extinction. This process is essential for survival and has been studied in posttraumatic stress disorder.”
They acknowledged certain limitations of the study, including its retrospective single-center design and the lack of control for factors such as socioeconomic background and level of education.
“Patients with DOP should undergo detailed drug histories and examination of clearance profiles, especially renal function,” the researchers concluded.
Evan A. Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, said that delusional infestation is one of the most difficult medical conditions to treat and study.
“Though the numbers of cases in this research letter are small, they are instructive in demonstrating a high burden of polypharmacy including psychostimulants, opioids, and SSRIs in such patients,” he told this news organization. “Dermatologists should be performing detailed drug histories, obtaining comprehensive lab work, and considering the effects of medications — both illicit and prescribed — on clinical presentations. While in many cases, delusional patients refuse to consent to psychopharmacologic medications (or treatment in general), the elimination or decrease in dose of certain problematic medications may be helpful in and of themselves.”
The researchers reported having no financial disclosures. Dr. Rieder disclosed that he is a consultant for AbbVie, L’Oréal, Pierre Fabre, Procter & Gamble, and Unilever.
, reported researchers in a small retrospective case-control study.
Delusions of parasitosis (DOP) affects mostly middle-aged women and has associations with renal failure and some medications, wrote corresponding author Colleen Reisz, MD, a dermatologist with the department of internal medicine at the University of Missouri–Kansas City School of Medicine, and her coauthors. The study was published online December 15, 2023, in the Journal of the American Academy of Dermatology.
“We hypothesize that vulnerability to DOP emerges when multiple factors combine, such as age, sex, medications, and changes in [drug] clearance capacity,” Dr. Reisz and her coauthors wrote. “Changes in health care, such as the dramatic increase in stimulant prescriptions and alternatives to opioids in pain management, may be contributing to off target drug effects on the brain.”
To test their hypothesis, the researchers conducted a case-control study of biometric and pharmaceutical data from 34 patients with DOP which they compared to an age-matched control group of 53 women presenting with a dermatitis above the clavicle from a general dermatology practice between 2012 and 2020. They de-identified the data and performed statistical analysis on variables that included biometric data and intake of pharmaceuticals and nutraceuticals. Polypharmacy was defined as five or more drugs.
Of the 34 patients with DOP, 27 were women with a mean age of 58 years and 7 were men with a mean age of 60 years. Dr. Reisz and her colleagues observed statistical significance between cases and controls in terms of polypharmacy (P = .011), attention-deficit/hyperactivity disorder medications (P < .001), selective serotonin reuptake inhibitors (P = .005), opioids (P = .003), and gabapentin (P = .003).
In other findings, half of DOP cases presented with samples of perceived parasitic material, and four associated the perceived infestation with a single emotion-laden event. This prompted the researchers “to consider that DOP may share mechanisms with fear conditioning and extinction,” they wrote. “Fear conditioning refers to the process of memory acquisition and extinction. This process is essential for survival and has been studied in posttraumatic stress disorder.”
They acknowledged certain limitations of the study, including its retrospective single-center design and the lack of control for factors such as socioeconomic background and level of education.
“Patients with DOP should undergo detailed drug histories and examination of clearance profiles, especially renal function,” the researchers concluded.
Evan A. Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, said that delusional infestation is one of the most difficult medical conditions to treat and study.
“Though the numbers of cases in this research letter are small, they are instructive in demonstrating a high burden of polypharmacy including psychostimulants, opioids, and SSRIs in such patients,” he told this news organization. “Dermatologists should be performing detailed drug histories, obtaining comprehensive lab work, and considering the effects of medications — both illicit and prescribed — on clinical presentations. While in many cases, delusional patients refuse to consent to psychopharmacologic medications (or treatment in general), the elimination or decrease in dose of certain problematic medications may be helpful in and of themselves.”
The researchers reported having no financial disclosures. Dr. Rieder disclosed that he is a consultant for AbbVie, L’Oréal, Pierre Fabre, Procter & Gamble, and Unilever.
, reported researchers in a small retrospective case-control study.
Delusions of parasitosis (DOP) affects mostly middle-aged women and has associations with renal failure and some medications, wrote corresponding author Colleen Reisz, MD, a dermatologist with the department of internal medicine at the University of Missouri–Kansas City School of Medicine, and her coauthors. The study was published online December 15, 2023, in the Journal of the American Academy of Dermatology.
“We hypothesize that vulnerability to DOP emerges when multiple factors combine, such as age, sex, medications, and changes in [drug] clearance capacity,” Dr. Reisz and her coauthors wrote. “Changes in health care, such as the dramatic increase in stimulant prescriptions and alternatives to opioids in pain management, may be contributing to off target drug effects on the brain.”
To test their hypothesis, the researchers conducted a case-control study of biometric and pharmaceutical data from 34 patients with DOP which they compared to an age-matched control group of 53 women presenting with a dermatitis above the clavicle from a general dermatology practice between 2012 and 2020. They de-identified the data and performed statistical analysis on variables that included biometric data and intake of pharmaceuticals and nutraceuticals. Polypharmacy was defined as five or more drugs.
Of the 34 patients with DOP, 27 were women with a mean age of 58 years and 7 were men with a mean age of 60 years. Dr. Reisz and her colleagues observed statistical significance between cases and controls in terms of polypharmacy (P = .011), attention-deficit/hyperactivity disorder medications (P < .001), selective serotonin reuptake inhibitors (P = .005), opioids (P = .003), and gabapentin (P = .003).
In other findings, half of DOP cases presented with samples of perceived parasitic material, and four associated the perceived infestation with a single emotion-laden event. This prompted the researchers “to consider that DOP may share mechanisms with fear conditioning and extinction,” they wrote. “Fear conditioning refers to the process of memory acquisition and extinction. This process is essential for survival and has been studied in posttraumatic stress disorder.”
They acknowledged certain limitations of the study, including its retrospective single-center design and the lack of control for factors such as socioeconomic background and level of education.
“Patients with DOP should undergo detailed drug histories and examination of clearance profiles, especially renal function,” the researchers concluded.
Evan A. Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, said that delusional infestation is one of the most difficult medical conditions to treat and study.
“Though the numbers of cases in this research letter are small, they are instructive in demonstrating a high burden of polypharmacy including psychostimulants, opioids, and SSRIs in such patients,” he told this news organization. “Dermatologists should be performing detailed drug histories, obtaining comprehensive lab work, and considering the effects of medications — both illicit and prescribed — on clinical presentations. While in many cases, delusional patients refuse to consent to psychopharmacologic medications (or treatment in general), the elimination or decrease in dose of certain problematic medications may be helpful in and of themselves.”
The researchers reported having no financial disclosures. Dr. Rieder disclosed that he is a consultant for AbbVie, L’Oréal, Pierre Fabre, Procter & Gamble, and Unilever.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
FDA Approves Topical Gel For Wounds Associated With JEB and DEB
The FDA has approved a topical gel containing birch triterpenes for the treatment of partial thickness wounds in patients 6 months and older with junctional epidermolysis bullosa (JEB) and dystrophic epidermolysis bullosa (DEB).
The gel is marketed under the name Filsuvez. It is the first approved treatment for wounds associated with JEB and the second for patients with DEB, following the approval of Vyjuvek (Krystal Biotech), a topical gene therapy gel, in May 2023.
First developed by Amryt Pharma and intended for home use, Filsuvez is now marketed by Chiesi Global Rare Diseases, which acquired Amryt in January 2023. The gel is applied topically to the wound at each dressing change.
The approval of Filsuvez is based on results from the Efficacy and Safety Study of Oleogel-S10 in Epidermolysis Bullosa (EASE), a randomized, placebo-controlled study of 223 people, the largest-ever phase 3 clinical trial for the treatment of EB, according to the Chiesi news release. The gel was well tolerated and met the primary endpoint with statistical significance, with 41.3% of patients achieving first complete target wound closure within 45 days (compared with 28.9% on placebo).
“I am so excited to say that this is another hurdle cleared and milestone achieved for the EB Community,” Brett Kopelan, executive director at debra of America said in a blog post. “We are now on the road to being able to treat EB more effectively, and to make the worst disease you’ve never heard of chronic, but livable, by making use of multiple therapeutic options in conjunction with each other.”
The FDA has approved a topical gel containing birch triterpenes for the treatment of partial thickness wounds in patients 6 months and older with junctional epidermolysis bullosa (JEB) and dystrophic epidermolysis bullosa (DEB).
The gel is marketed under the name Filsuvez. It is the first approved treatment for wounds associated with JEB and the second for patients with DEB, following the approval of Vyjuvek (Krystal Biotech), a topical gene therapy gel, in May 2023.
First developed by Amryt Pharma and intended for home use, Filsuvez is now marketed by Chiesi Global Rare Diseases, which acquired Amryt in January 2023. The gel is applied topically to the wound at each dressing change.
The approval of Filsuvez is based on results from the Efficacy and Safety Study of Oleogel-S10 in Epidermolysis Bullosa (EASE), a randomized, placebo-controlled study of 223 people, the largest-ever phase 3 clinical trial for the treatment of EB, according to the Chiesi news release. The gel was well tolerated and met the primary endpoint with statistical significance, with 41.3% of patients achieving first complete target wound closure within 45 days (compared with 28.9% on placebo).
“I am so excited to say that this is another hurdle cleared and milestone achieved for the EB Community,” Brett Kopelan, executive director at debra of America said in a blog post. “We are now on the road to being able to treat EB more effectively, and to make the worst disease you’ve never heard of chronic, but livable, by making use of multiple therapeutic options in conjunction with each other.”
The FDA has approved a topical gel containing birch triterpenes for the treatment of partial thickness wounds in patients 6 months and older with junctional epidermolysis bullosa (JEB) and dystrophic epidermolysis bullosa (DEB).
The gel is marketed under the name Filsuvez. It is the first approved treatment for wounds associated with JEB and the second for patients with DEB, following the approval of Vyjuvek (Krystal Biotech), a topical gene therapy gel, in May 2023.
First developed by Amryt Pharma and intended for home use, Filsuvez is now marketed by Chiesi Global Rare Diseases, which acquired Amryt in January 2023. The gel is applied topically to the wound at each dressing change.
The approval of Filsuvez is based on results from the Efficacy and Safety Study of Oleogel-S10 in Epidermolysis Bullosa (EASE), a randomized, placebo-controlled study of 223 people, the largest-ever phase 3 clinical trial for the treatment of EB, according to the Chiesi news release. The gel was well tolerated and met the primary endpoint with statistical significance, with 41.3% of patients achieving first complete target wound closure within 45 days (compared with 28.9% on placebo).
“I am so excited to say that this is another hurdle cleared and milestone achieved for the EB Community,” Brett Kopelan, executive director at debra of America said in a blog post. “We are now on the road to being able to treat EB more effectively, and to make the worst disease you’ve never heard of chronic, but livable, by making use of multiple therapeutic options in conjunction with each other.”
Is It Time to Air Grievances?
‘Twas the night before Festivus and all through the house, everyone was griping.
In case you’ve only been watching Friends reruns lately, Festivus is a holiday that originated 25 years ago in the last season of Seinfeld. George’s father created it as an alternative to Christmas hype. In addition to an aluminum pole, the holiday features the annual airing of grievances, when one is encouraged to voice complaints. Aluminum poles haven’t replaced Christmas trees, but the spirit of Festivus is still with us in the widespread airing of grievances in 2023.
Complaining isn’t just a post-pandemic problem. Hector spends quite a bit of time complaining about Paris in the Iliad. That was a few pandemics ago. And repining is ubiquitous in literature — as human as walking on two limbs it seems. Ostensibly, we complain to effect change: Something is wrong and we expect it to be different. But that’s not the whole story. No one believes the weather will improve or the Patriots will play better because we complain about them. So why do we bother?
Even if nothing changes on the outside, it does seem to alter our internal state, serving a healthy psychological function. Putting to words what is aggravating can have the same benefit of deep breathing. We describe it as “getting something off our chest” because that’s what it feels like. We feel unburdened just by saying it out loud. Think about the last time you complained: Cranky staff, prior auths, Medicare, disrespectful patients, many of your colleagues will nod in agreement, validating your feelings and making you feel less isolated.
There are also maladaptive reasons for whining. It’s obviously an elementary way to get attention or to remove responsibility. It can also be a political weapon (office politics included). It’s such a potent way to connect that it’s used to build alliances and clout. “Washington is doing a great job,” said no candidate ever. No, if you want to get people on your side, find something irritating and complain to everyone how annoying it is. This solidifies “us” versus “them,” which can harm organizations and families alike.
Yet, eliminating all complaints is neither feasible, nor probably advisable. You could try to make your office a complaint-free zone, but the likely result would be to push any griping to the remote corners where you can no longer hear them. These criticisms might have uncovered missed opportunities, identify problems, and even improve cohesion if done in a safe and transparent setting. If they are left unaddressed or if the underlying culture isn’t sound, then they can propagate and lead to factions that harm productivity.
Griping is as much part of the holiday season as jingle bells and jelly donuts. I don’t believe complaining is up now because people were grumpier in 2023. Rather I think people just craved connection more than ever. So join in: Traffic after the time change, Tesla service, (super) late patients, prior auths, perioral dermatitis, post-COVID telogen effluvium.
I feel better.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X (formerly Twitter). Write to him at dermnews@mdedge.com.
‘Twas the night before Festivus and all through the house, everyone was griping.
In case you’ve only been watching Friends reruns lately, Festivus is a holiday that originated 25 years ago in the last season of Seinfeld. George’s father created it as an alternative to Christmas hype. In addition to an aluminum pole, the holiday features the annual airing of grievances, when one is encouraged to voice complaints. Aluminum poles haven’t replaced Christmas trees, but the spirit of Festivus is still with us in the widespread airing of grievances in 2023.
Complaining isn’t just a post-pandemic problem. Hector spends quite a bit of time complaining about Paris in the Iliad. That was a few pandemics ago. And repining is ubiquitous in literature — as human as walking on two limbs it seems. Ostensibly, we complain to effect change: Something is wrong and we expect it to be different. But that’s not the whole story. No one believes the weather will improve or the Patriots will play better because we complain about them. So why do we bother?
Even if nothing changes on the outside, it does seem to alter our internal state, serving a healthy psychological function. Putting to words what is aggravating can have the same benefit of deep breathing. We describe it as “getting something off our chest” because that’s what it feels like. We feel unburdened just by saying it out loud. Think about the last time you complained: Cranky staff, prior auths, Medicare, disrespectful patients, many of your colleagues will nod in agreement, validating your feelings and making you feel less isolated.
There are also maladaptive reasons for whining. It’s obviously an elementary way to get attention or to remove responsibility. It can also be a political weapon (office politics included). It’s such a potent way to connect that it’s used to build alliances and clout. “Washington is doing a great job,” said no candidate ever. No, if you want to get people on your side, find something irritating and complain to everyone how annoying it is. This solidifies “us” versus “them,” which can harm organizations and families alike.
Yet, eliminating all complaints is neither feasible, nor probably advisable. You could try to make your office a complaint-free zone, but the likely result would be to push any griping to the remote corners where you can no longer hear them. These criticisms might have uncovered missed opportunities, identify problems, and even improve cohesion if done in a safe and transparent setting. If they are left unaddressed or if the underlying culture isn’t sound, then they can propagate and lead to factions that harm productivity.
Griping is as much part of the holiday season as jingle bells and jelly donuts. I don’t believe complaining is up now because people were grumpier in 2023. Rather I think people just craved connection more than ever. So join in: Traffic after the time change, Tesla service, (super) late patients, prior auths, perioral dermatitis, post-COVID telogen effluvium.
I feel better.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X (formerly Twitter). Write to him at dermnews@mdedge.com.
‘Twas the night before Festivus and all through the house, everyone was griping.
In case you’ve only been watching Friends reruns lately, Festivus is a holiday that originated 25 years ago in the last season of Seinfeld. George’s father created it as an alternative to Christmas hype. In addition to an aluminum pole, the holiday features the annual airing of grievances, when one is encouraged to voice complaints. Aluminum poles haven’t replaced Christmas trees, but the spirit of Festivus is still with us in the widespread airing of grievances in 2023.
Complaining isn’t just a post-pandemic problem. Hector spends quite a bit of time complaining about Paris in the Iliad. That was a few pandemics ago. And repining is ubiquitous in literature — as human as walking on two limbs it seems. Ostensibly, we complain to effect change: Something is wrong and we expect it to be different. But that’s not the whole story. No one believes the weather will improve or the Patriots will play better because we complain about them. So why do we bother?
Even if nothing changes on the outside, it does seem to alter our internal state, serving a healthy psychological function. Putting to words what is aggravating can have the same benefit of deep breathing. We describe it as “getting something off our chest” because that’s what it feels like. We feel unburdened just by saying it out loud. Think about the last time you complained: Cranky staff, prior auths, Medicare, disrespectful patients, many of your colleagues will nod in agreement, validating your feelings and making you feel less isolated.
There are also maladaptive reasons for whining. It’s obviously an elementary way to get attention or to remove responsibility. It can also be a political weapon (office politics included). It’s such a potent way to connect that it’s used to build alliances and clout. “Washington is doing a great job,” said no candidate ever. No, if you want to get people on your side, find something irritating and complain to everyone how annoying it is. This solidifies “us” versus “them,” which can harm organizations and families alike.
Yet, eliminating all complaints is neither feasible, nor probably advisable. You could try to make your office a complaint-free zone, but the likely result would be to push any griping to the remote corners where you can no longer hear them. These criticisms might have uncovered missed opportunities, identify problems, and even improve cohesion if done in a safe and transparent setting. If they are left unaddressed or if the underlying culture isn’t sound, then they can propagate and lead to factions that harm productivity.
Griping is as much part of the holiday season as jingle bells and jelly donuts. I don’t believe complaining is up now because people were grumpier in 2023. Rather I think people just craved connection more than ever. So join in: Traffic after the time change, Tesla service, (super) late patients, prior auths, perioral dermatitis, post-COVID telogen effluvium.
I feel better.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X (formerly Twitter). Write to him at dermnews@mdedge.com.
Paradoxical Eczema Risk Low With Biologic Psoriasis Treatments
examined in a large observational analysis.
Using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) database, Ali Al-Janabi, MA, from the University of Manchester (England) and associates found that 273 (1%) of approximately 25,000 drug exposures in 13,699 biologic-treated patients with psoriasis were associated with paradoxical eczema.
The incidence of paradoxical eczema was found to vary by class. The highest rate was seen for IL-17 inhibitors, at 1.22 per 100,000 person-years, and the lowest rate was seen with IL-23 inhibitors, at 0.56 per 100,000 person-years. The respective incidence rates for tumor necrosis factor (TNF) inhibitors and IL-12/IL-23 inhibitors were a respective 0.94 and 0.80 per 100,000 person-years.
“Compared with TNF inhibitors, IL-23 inhibitor exposure was associated with significantly lower risk of paradoxical eczema,” the BADBIR Study Group reported in JAMA Dermatology. Indeed, patients treated with IL-23 inhibitors were 61% less likely than were those taking TNF-inhibitors to experience a paradoxical eczema event.
“These findings remained when restricting the analysis to first-line biologic exposures and were specific to this eczema phenotype” the group said.
Cautious Interpretation
As the corresponding author for the work, Mr. Al-Janabi observed in an email that the research needs to be replicated, and the findings need to be interpreted with caution.
“As well as usual clinical variables influencing biologic selection, clinicians could consider IL-23 inhibitors in patients with previous atopic dermatitis, hay fever, or paradoxical eczema episodes, as this class was associated with the lowest risk of paradoxical eczema,” he suggested.
A prior history of atopic dermatitis (AD) and hay fever appears to be particularly relevant, as both substantially upped the chances that paradoxical eczema would occur, with hazard ratios of 12.40 and 3.78, respectively. Increasing age also increased the risk, albeit slightly (hazard ratio [HR], 1.02 per year), and there was an apparent lower risk (HR, 0.60) comparing men and women.
The BADBIR Study Group authors believe that, to the best of their knowledge, this is the first study to compare paradoxical eczema risk by biologic class. “Based on clinical experience and prevalence of eczematous reactions reported in some IL-17 inhibitor clinical trials, we suspected an association between IL-17 inhibitor exposure and paradoxical eczema,” they wrote.
“While the incidence of paradoxical eczema was numerically highest among IL-17 inhibitor exposures, it was not significantly different from the incidence among TNF inhibitor exposures.” The low overall incidence of paradoxical eczema “may be reassuring for patients and clinicians,” they added, “but it is possible that the incidence was underestimated due to underreporting or exclusion of adverse events with insufficient detail.”
Details of the Analysis, Other Findings
To explore the risk of paradoxical eczema by biologic class and identify possible risk factors, the BADBIR Study Group performed a prospective cohort study using data held within the BADBIR database between September 2007 and December 2022.
Adults over the age of 18 year or older with plaque psoriasis and who had been treated with at least one of the following biologics were eligible for inclusion: the TNF inhibitors adalimumab, certolizumab pegol, etanercept, and infliximab; the IL-17 inhibitors bimekizumab, brodalumab, ixekizumab, and secukinumab; the IL-12/23 inhibitor ustekinumab; and the IL-23 inhibitors guselkumab, risankizumab, and tildrakizumab.
Patient records and adverse event data were reviewed to determine the incidence of paradoxical eczema events, using terms such as eczema, eczematized, eczematous, atopy, atopic, and dermatitis.
Of 24,952 drug exposures analyzed, the majority (11,819) were for TNF inhibitors, followed by IL-17 inhibitors (4,776), IL-12/23 inhibitors (6,423), and finally, IL-23 inhibitors (1,934).
Mr. Al-Janabi and coauthors reported that the median time to onset of paradoxical eczema events was 294 days — approximately 9.8 months. The earliest that these events were recorded was at 120 days (4 months), and the latest at 699 days (almost 2 years).
The face and neck were the most common sites affected (26% of exposures), with other sites including the limbs (23%), the trunk (13%), and hands or feet (12%). Itching (18%), redness (7%), and dryness (4%) were the most commonly reported symptoms.
The researchers noted that 21 patients had skin biopsies taken and “all showed spongiosis or a feature of eczema, with 1 having overlapping features of psoriasis.”
In the majority (92 %) of cases, patients experienced only one eczema event. Of the 20 patients who had more than one event, just over one-fifth of repeat events occurred after receiving the same biologic as for the index event. A quarter of events occurred after a different biologic of the same class had been used, and just over half of events occurred after a different class of biologic had been given.
Strengths and Limitations
The “large sample size and inclusion of multiple lines of exposure per participant” are strengths of the study, said the researchers. “We included data for all currently available biologics, originating from more than 160 dermatology centers in the UK and Ireland.”
They added, however, that the “main limitation is the small numbers of observations within certain subgroups, such as specific biologic exposures or participants in ethnic minority groups, restricting generalizability of our findings and the interpretation of some subgroup analyses.”
Moreover, the small number of paradoxical eczema events seen may have resulted in imprecise effect estimates, they observe, noting that the number of exposures to IL-23 inhibitors was low compared with other classes.
“Future studies with more exposures and paradoxical eczema events would enable a more robust analysis of individual drugs and patient subgroups,” the authors concluded.
The study was funded by the Medical Research Council. BADBIR is coordinated by The University of Manchester, and funded by the British Association of Dermatologists (BAD). The BAD receives income from AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Samsung Bioepis, Sandoz Hexal AG, and UCB Pharma for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester, which coordinates BADBIR. Mr. Al-Janabi reported receiving grants from the Medical Research Council during the conduct of the study; nonfinancial support from UCB, Almirall, and Janssen; and personal fees from UCB outside the submitted work.
examined in a large observational analysis.
Using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) database, Ali Al-Janabi, MA, from the University of Manchester (England) and associates found that 273 (1%) of approximately 25,000 drug exposures in 13,699 biologic-treated patients with psoriasis were associated with paradoxical eczema.
The incidence of paradoxical eczema was found to vary by class. The highest rate was seen for IL-17 inhibitors, at 1.22 per 100,000 person-years, and the lowest rate was seen with IL-23 inhibitors, at 0.56 per 100,000 person-years. The respective incidence rates for tumor necrosis factor (TNF) inhibitors and IL-12/IL-23 inhibitors were a respective 0.94 and 0.80 per 100,000 person-years.
“Compared with TNF inhibitors, IL-23 inhibitor exposure was associated with significantly lower risk of paradoxical eczema,” the BADBIR Study Group reported in JAMA Dermatology. Indeed, patients treated with IL-23 inhibitors were 61% less likely than were those taking TNF-inhibitors to experience a paradoxical eczema event.
“These findings remained when restricting the analysis to first-line biologic exposures and were specific to this eczema phenotype” the group said.
Cautious Interpretation
As the corresponding author for the work, Mr. Al-Janabi observed in an email that the research needs to be replicated, and the findings need to be interpreted with caution.
“As well as usual clinical variables influencing biologic selection, clinicians could consider IL-23 inhibitors in patients with previous atopic dermatitis, hay fever, or paradoxical eczema episodes, as this class was associated with the lowest risk of paradoxical eczema,” he suggested.
A prior history of atopic dermatitis (AD) and hay fever appears to be particularly relevant, as both substantially upped the chances that paradoxical eczema would occur, with hazard ratios of 12.40 and 3.78, respectively. Increasing age also increased the risk, albeit slightly (hazard ratio [HR], 1.02 per year), and there was an apparent lower risk (HR, 0.60) comparing men and women.
The BADBIR Study Group authors believe that, to the best of their knowledge, this is the first study to compare paradoxical eczema risk by biologic class. “Based on clinical experience and prevalence of eczematous reactions reported in some IL-17 inhibitor clinical trials, we suspected an association between IL-17 inhibitor exposure and paradoxical eczema,” they wrote.
“While the incidence of paradoxical eczema was numerically highest among IL-17 inhibitor exposures, it was not significantly different from the incidence among TNF inhibitor exposures.” The low overall incidence of paradoxical eczema “may be reassuring for patients and clinicians,” they added, “but it is possible that the incidence was underestimated due to underreporting or exclusion of adverse events with insufficient detail.”
Details of the Analysis, Other Findings
To explore the risk of paradoxical eczema by biologic class and identify possible risk factors, the BADBIR Study Group performed a prospective cohort study using data held within the BADBIR database between September 2007 and December 2022.
Adults over the age of 18 year or older with plaque psoriasis and who had been treated with at least one of the following biologics were eligible for inclusion: the TNF inhibitors adalimumab, certolizumab pegol, etanercept, and infliximab; the IL-17 inhibitors bimekizumab, brodalumab, ixekizumab, and secukinumab; the IL-12/23 inhibitor ustekinumab; and the IL-23 inhibitors guselkumab, risankizumab, and tildrakizumab.
Patient records and adverse event data were reviewed to determine the incidence of paradoxical eczema events, using terms such as eczema, eczematized, eczematous, atopy, atopic, and dermatitis.
Of 24,952 drug exposures analyzed, the majority (11,819) were for TNF inhibitors, followed by IL-17 inhibitors (4,776), IL-12/23 inhibitors (6,423), and finally, IL-23 inhibitors (1,934).
Mr. Al-Janabi and coauthors reported that the median time to onset of paradoxical eczema events was 294 days — approximately 9.8 months. The earliest that these events were recorded was at 120 days (4 months), and the latest at 699 days (almost 2 years).
The face and neck were the most common sites affected (26% of exposures), with other sites including the limbs (23%), the trunk (13%), and hands or feet (12%). Itching (18%), redness (7%), and dryness (4%) were the most commonly reported symptoms.
The researchers noted that 21 patients had skin biopsies taken and “all showed spongiosis or a feature of eczema, with 1 having overlapping features of psoriasis.”
In the majority (92 %) of cases, patients experienced only one eczema event. Of the 20 patients who had more than one event, just over one-fifth of repeat events occurred after receiving the same biologic as for the index event. A quarter of events occurred after a different biologic of the same class had been used, and just over half of events occurred after a different class of biologic had been given.
Strengths and Limitations
The “large sample size and inclusion of multiple lines of exposure per participant” are strengths of the study, said the researchers. “We included data for all currently available biologics, originating from more than 160 dermatology centers in the UK and Ireland.”
They added, however, that the “main limitation is the small numbers of observations within certain subgroups, such as specific biologic exposures or participants in ethnic minority groups, restricting generalizability of our findings and the interpretation of some subgroup analyses.”
Moreover, the small number of paradoxical eczema events seen may have resulted in imprecise effect estimates, they observe, noting that the number of exposures to IL-23 inhibitors was low compared with other classes.
“Future studies with more exposures and paradoxical eczema events would enable a more robust analysis of individual drugs and patient subgroups,” the authors concluded.
The study was funded by the Medical Research Council. BADBIR is coordinated by The University of Manchester, and funded by the British Association of Dermatologists (BAD). The BAD receives income from AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Samsung Bioepis, Sandoz Hexal AG, and UCB Pharma for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester, which coordinates BADBIR. Mr. Al-Janabi reported receiving grants from the Medical Research Council during the conduct of the study; nonfinancial support from UCB, Almirall, and Janssen; and personal fees from UCB outside the submitted work.
examined in a large observational analysis.
Using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) database, Ali Al-Janabi, MA, from the University of Manchester (England) and associates found that 273 (1%) of approximately 25,000 drug exposures in 13,699 biologic-treated patients with psoriasis were associated with paradoxical eczema.
The incidence of paradoxical eczema was found to vary by class. The highest rate was seen for IL-17 inhibitors, at 1.22 per 100,000 person-years, and the lowest rate was seen with IL-23 inhibitors, at 0.56 per 100,000 person-years. The respective incidence rates for tumor necrosis factor (TNF) inhibitors and IL-12/IL-23 inhibitors were a respective 0.94 and 0.80 per 100,000 person-years.
“Compared with TNF inhibitors, IL-23 inhibitor exposure was associated with significantly lower risk of paradoxical eczema,” the BADBIR Study Group reported in JAMA Dermatology. Indeed, patients treated with IL-23 inhibitors were 61% less likely than were those taking TNF-inhibitors to experience a paradoxical eczema event.
“These findings remained when restricting the analysis to first-line biologic exposures and were specific to this eczema phenotype” the group said.
Cautious Interpretation
As the corresponding author for the work, Mr. Al-Janabi observed in an email that the research needs to be replicated, and the findings need to be interpreted with caution.
“As well as usual clinical variables influencing biologic selection, clinicians could consider IL-23 inhibitors in patients with previous atopic dermatitis, hay fever, or paradoxical eczema episodes, as this class was associated with the lowest risk of paradoxical eczema,” he suggested.
A prior history of atopic dermatitis (AD) and hay fever appears to be particularly relevant, as both substantially upped the chances that paradoxical eczema would occur, with hazard ratios of 12.40 and 3.78, respectively. Increasing age also increased the risk, albeit slightly (hazard ratio [HR], 1.02 per year), and there was an apparent lower risk (HR, 0.60) comparing men and women.
The BADBIR Study Group authors believe that, to the best of their knowledge, this is the first study to compare paradoxical eczema risk by biologic class. “Based on clinical experience and prevalence of eczematous reactions reported in some IL-17 inhibitor clinical trials, we suspected an association between IL-17 inhibitor exposure and paradoxical eczema,” they wrote.
“While the incidence of paradoxical eczema was numerically highest among IL-17 inhibitor exposures, it was not significantly different from the incidence among TNF inhibitor exposures.” The low overall incidence of paradoxical eczema “may be reassuring for patients and clinicians,” they added, “but it is possible that the incidence was underestimated due to underreporting or exclusion of adverse events with insufficient detail.”
Details of the Analysis, Other Findings
To explore the risk of paradoxical eczema by biologic class and identify possible risk factors, the BADBIR Study Group performed a prospective cohort study using data held within the BADBIR database between September 2007 and December 2022.
Adults over the age of 18 year or older with plaque psoriasis and who had been treated with at least one of the following biologics were eligible for inclusion: the TNF inhibitors adalimumab, certolizumab pegol, etanercept, and infliximab; the IL-17 inhibitors bimekizumab, brodalumab, ixekizumab, and secukinumab; the IL-12/23 inhibitor ustekinumab; and the IL-23 inhibitors guselkumab, risankizumab, and tildrakizumab.
Patient records and adverse event data were reviewed to determine the incidence of paradoxical eczema events, using terms such as eczema, eczematized, eczematous, atopy, atopic, and dermatitis.
Of 24,952 drug exposures analyzed, the majority (11,819) were for TNF inhibitors, followed by IL-17 inhibitors (4,776), IL-12/23 inhibitors (6,423), and finally, IL-23 inhibitors (1,934).
Mr. Al-Janabi and coauthors reported that the median time to onset of paradoxical eczema events was 294 days — approximately 9.8 months. The earliest that these events were recorded was at 120 days (4 months), and the latest at 699 days (almost 2 years).
The face and neck were the most common sites affected (26% of exposures), with other sites including the limbs (23%), the trunk (13%), and hands or feet (12%). Itching (18%), redness (7%), and dryness (4%) were the most commonly reported symptoms.
The researchers noted that 21 patients had skin biopsies taken and “all showed spongiosis or a feature of eczema, with 1 having overlapping features of psoriasis.”
In the majority (92 %) of cases, patients experienced only one eczema event. Of the 20 patients who had more than one event, just over one-fifth of repeat events occurred after receiving the same biologic as for the index event. A quarter of events occurred after a different biologic of the same class had been used, and just over half of events occurred after a different class of biologic had been given.
Strengths and Limitations
The “large sample size and inclusion of multiple lines of exposure per participant” are strengths of the study, said the researchers. “We included data for all currently available biologics, originating from more than 160 dermatology centers in the UK and Ireland.”
They added, however, that the “main limitation is the small numbers of observations within certain subgroups, such as specific biologic exposures or participants in ethnic minority groups, restricting generalizability of our findings and the interpretation of some subgroup analyses.”
Moreover, the small number of paradoxical eczema events seen may have resulted in imprecise effect estimates, they observe, noting that the number of exposures to IL-23 inhibitors was low compared with other classes.
“Future studies with more exposures and paradoxical eczema events would enable a more robust analysis of individual drugs and patient subgroups,” the authors concluded.
The study was funded by the Medical Research Council. BADBIR is coordinated by The University of Manchester, and funded by the British Association of Dermatologists (BAD). The BAD receives income from AbbVie, Almirall, Amgen, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Samsung Bioepis, Sandoz Hexal AG, and UCB Pharma for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester, which coordinates BADBIR. Mr. Al-Janabi reported receiving grants from the Medical Research Council during the conduct of the study; nonfinancial support from UCB, Almirall, and Janssen; and personal fees from UCB outside the submitted work.
FROM JAMA DERMATOLOGY
Study evaluates aesthetic concerns among Hispanic, Latinx women
CHICAGO — , according to the results of a study that involved a survey of almost 4000 women.
To date, the aesthetic needs of Hispanic/Latinx patients, the second largest ethnic group in the United States, have been poorly understood. “Most [aesthetic] marketing materials are gauged toward Caucasian patients,” Sabrina Fabi, MD, a dermatologist and dermatologic cosmetic surgeon in San Diego, California, said at the annual meeting of the American Society for Dermatologic Surgery (ASDS), where she presented the study results.
In addition, Dr. Fabi noted that current studies of facial and body aesthetics are limited in terms of representation. “When we look at studies, they are more Fitzpatrick type IIs and IIIs,” she said. Addressing this gap, she and her colleagues conducted the large multicenter study to learn more about cosmetic concerns unique to Hispanic/Latinx women, across different ethnic groups, and how they may differ by age.
In the study, an online survey was administered to aesthetically-inclined adults across different demographic groups in the United States. Specifically, respondents were surveyed regarding 41 facial and 31 body characteristics, identifying those they found bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3974 women surveyed, 748 self-identified as Hispanic/Latinx and female. Most participants (86%) were born in the United States and were interested in aesthetic treatments (93%). The majority of patients identified as Generation X (42-57 years, 40.0%), followed by older Millennials (31-41 years, 33.0%), Generation Z/young Millennials (under 30 years, 16.7%), and Baby Boomers and older (over 57 years, 10.3%). Participants most commonly reported Fitzpatrick skin types III (24%) and IV (56%), and BMIs of 18.5 kg/m2 to <25 kg/m2 (42%) and 25 to <30 kg/m2 (27%).
Among Hispanic/Latinx women, the top facial concerns were related to submental fat (36%) and under-eye hollowing (35%). This is in contrast to White counterparts, who tended to find wrinkles more bothersome, according to Dr. Fabi. Among Hispanic/Latinx women, the top body concerns were related to stubborn fat involving the stomach (50%), sides (44%), and bra or the back area (40%).
Despite the shared concern of stubborn body fat across age groups, facial concerns shifted from skin quality (50%) and under-eye issues (43%) in the younger generations to upper facial lines (52%) and jowls/sagging skin (57%) in the older generations.
Dr. Fabi stated that approximately 30% of the population she sees is Hispanic/Latinx, and the results of this study substantiate what she sees in her practice. “This magnifies the things we need to be talking to them more about specifically.” The findings from this survey may aid in the customization of treatment plans to better serve this population, she said.
The study was sponsored by Allergan Aesthetics, which participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication. Dr. Fabi and three other authors are speakers, consultants, and investigators for Allergan. Other authors are on the advisory board, or are employees of Abbvie, Allergan’s parent company, and may own stock.
CHICAGO — , according to the results of a study that involved a survey of almost 4000 women.
To date, the aesthetic needs of Hispanic/Latinx patients, the second largest ethnic group in the United States, have been poorly understood. “Most [aesthetic] marketing materials are gauged toward Caucasian patients,” Sabrina Fabi, MD, a dermatologist and dermatologic cosmetic surgeon in San Diego, California, said at the annual meeting of the American Society for Dermatologic Surgery (ASDS), where she presented the study results.
In addition, Dr. Fabi noted that current studies of facial and body aesthetics are limited in terms of representation. “When we look at studies, they are more Fitzpatrick type IIs and IIIs,” she said. Addressing this gap, she and her colleagues conducted the large multicenter study to learn more about cosmetic concerns unique to Hispanic/Latinx women, across different ethnic groups, and how they may differ by age.
In the study, an online survey was administered to aesthetically-inclined adults across different demographic groups in the United States. Specifically, respondents were surveyed regarding 41 facial and 31 body characteristics, identifying those they found bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3974 women surveyed, 748 self-identified as Hispanic/Latinx and female. Most participants (86%) were born in the United States and were interested in aesthetic treatments (93%). The majority of patients identified as Generation X (42-57 years, 40.0%), followed by older Millennials (31-41 years, 33.0%), Generation Z/young Millennials (under 30 years, 16.7%), and Baby Boomers and older (over 57 years, 10.3%). Participants most commonly reported Fitzpatrick skin types III (24%) and IV (56%), and BMIs of 18.5 kg/m2 to <25 kg/m2 (42%) and 25 to <30 kg/m2 (27%).
Among Hispanic/Latinx women, the top facial concerns were related to submental fat (36%) and under-eye hollowing (35%). This is in contrast to White counterparts, who tended to find wrinkles more bothersome, according to Dr. Fabi. Among Hispanic/Latinx women, the top body concerns were related to stubborn fat involving the stomach (50%), sides (44%), and bra or the back area (40%).
Despite the shared concern of stubborn body fat across age groups, facial concerns shifted from skin quality (50%) and under-eye issues (43%) in the younger generations to upper facial lines (52%) and jowls/sagging skin (57%) in the older generations.
Dr. Fabi stated that approximately 30% of the population she sees is Hispanic/Latinx, and the results of this study substantiate what she sees in her practice. “This magnifies the things we need to be talking to them more about specifically.” The findings from this survey may aid in the customization of treatment plans to better serve this population, she said.
The study was sponsored by Allergan Aesthetics, which participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication. Dr. Fabi and three other authors are speakers, consultants, and investigators for Allergan. Other authors are on the advisory board, or are employees of Abbvie, Allergan’s parent company, and may own stock.
CHICAGO — , according to the results of a study that involved a survey of almost 4000 women.
To date, the aesthetic needs of Hispanic/Latinx patients, the second largest ethnic group in the United States, have been poorly understood. “Most [aesthetic] marketing materials are gauged toward Caucasian patients,” Sabrina Fabi, MD, a dermatologist and dermatologic cosmetic surgeon in San Diego, California, said at the annual meeting of the American Society for Dermatologic Surgery (ASDS), where she presented the study results.
In addition, Dr. Fabi noted that current studies of facial and body aesthetics are limited in terms of representation. “When we look at studies, they are more Fitzpatrick type IIs and IIIs,” she said. Addressing this gap, she and her colleagues conducted the large multicenter study to learn more about cosmetic concerns unique to Hispanic/Latinx women, across different ethnic groups, and how they may differ by age.
In the study, an online survey was administered to aesthetically-inclined adults across different demographic groups in the United States. Specifically, respondents were surveyed regarding 41 facial and 31 body characteristics, identifying those they found bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3974 women surveyed, 748 self-identified as Hispanic/Latinx and female. Most participants (86%) were born in the United States and were interested in aesthetic treatments (93%). The majority of patients identified as Generation X (42-57 years, 40.0%), followed by older Millennials (31-41 years, 33.0%), Generation Z/young Millennials (under 30 years, 16.7%), and Baby Boomers and older (over 57 years, 10.3%). Participants most commonly reported Fitzpatrick skin types III (24%) and IV (56%), and BMIs of 18.5 kg/m2 to <25 kg/m2 (42%) and 25 to <30 kg/m2 (27%).
Among Hispanic/Latinx women, the top facial concerns were related to submental fat (36%) and under-eye hollowing (35%). This is in contrast to White counterparts, who tended to find wrinkles more bothersome, according to Dr. Fabi. Among Hispanic/Latinx women, the top body concerns were related to stubborn fat involving the stomach (50%), sides (44%), and bra or the back area (40%).
Despite the shared concern of stubborn body fat across age groups, facial concerns shifted from skin quality (50%) and under-eye issues (43%) in the younger generations to upper facial lines (52%) and jowls/sagging skin (57%) in the older generations.
Dr. Fabi stated that approximately 30% of the population she sees is Hispanic/Latinx, and the results of this study substantiate what she sees in her practice. “This magnifies the things we need to be talking to them more about specifically.” The findings from this survey may aid in the customization of treatment plans to better serve this population, she said.
The study was sponsored by Allergan Aesthetics, which participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication. Dr. Fabi and three other authors are speakers, consultants, and investigators for Allergan. Other authors are on the advisory board, or are employees of Abbvie, Allergan’s parent company, and may own stock.
AT ASDS 2023
Sodium deoxycholate and triamcinolone: A good mix?
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at dermnews@mdedge.com. She was an investigator in clinical trials of Kybella.
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at dermnews@mdedge.com. She was an investigator in clinical trials of Kybella.
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at dermnews@mdedge.com. She was an investigator in clinical trials of Kybella.
How to Reduce Cardiovascular Morbidity and Mortality in Psoriasis and PsA
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.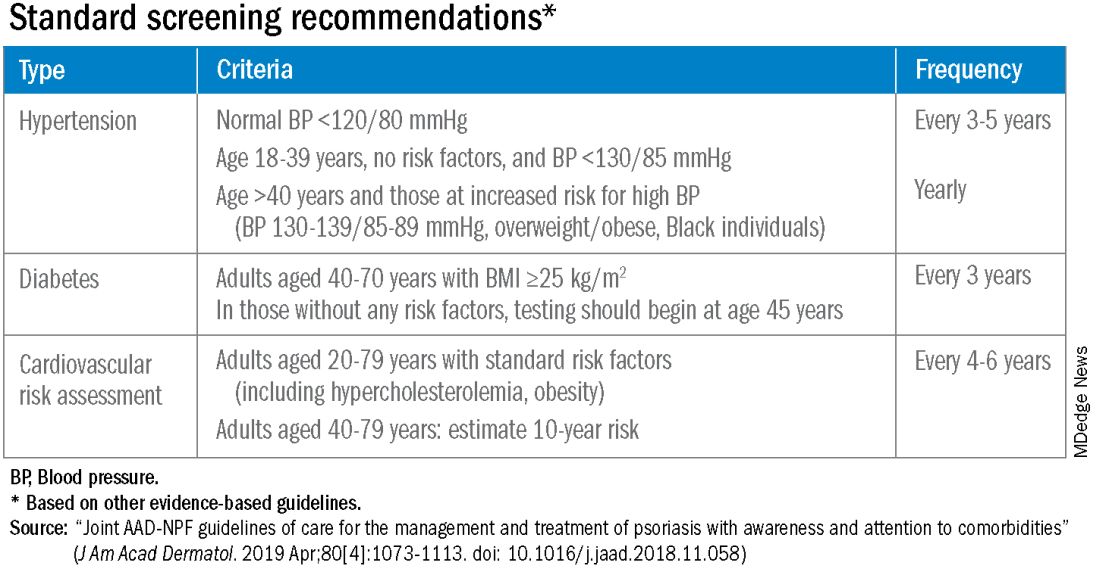
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Cluster of Eye Syphilis Cases Prompts CDC Concern
, according to a report by the Centers for Disease Control and Prevention.
With the incidence of syphilis infection in women increasing in the United States, experts are asking clinicians to be on the lookout for unusual ocular presentations.
“This is the first time such a cluster has been reported in the US,” the International Society for Infectious Diseases posted on ProMED.
Five women in Southwest Michigan who had a common male sex partner developed syphilis infections in their eyes. No new cases have been found related to these five cases after the women and the man received medical care.
If left untreated, the bacterium, Treponema pallidum, can infect the eyes, the ears, and the central nervous system.
The women, identified as non-Hispanic White, were aged 40-60 years and were not infected with HIV. They were diagnosed with early-stage syphilis and all were hospitalized and treated with intravenous penicillin. Routes of sexual exposure among the women included anal (40%), oral (40%), and vaginal (100%), the report states.
The common male sex partner they all met online was found to have early latent syphilis but never developed ocular syphilis.
It is not the eyes that are being exposed. Rather, it is an ocular presentation brought about by a systemic infection carried through the bloodstream after sexual exposure, explains William Nettleton, MD, MPH, medical director of the Kalamazoo and Calhoun public health departments in Michigan and lead author of the report.
“If we screen, identify, and treat syphilis promptly, we can prevent systemic manifestations,” he says.
Clinicians should be aware that the ocular manifestations can come at different stages of syphilis. “For patients you think may have ocular syphilis,” Dr. Nettleton says, “an immediate ophthalmologic evaluation is indicated.”
Symptoms Differed
The five women presented with a variety of symptoms.
Multiple attempts to contact the male partner by telephone and text were made by Michigan Department of Health and Human Services, but he did not respond. Local public health physicians reviewed the man’s electronic health record and discovered that he had sought care at a hospital emergency department in January 2022 for ulcerative penile and anal lesions.
He reported having multiple female sex partners during the previous 12 months but declined to disclose their identities; he reported no male or transgender sexual contact, according to the CDC report. Eventually he agreed to an evaluation, was found to have early latent syphilis, and was treated with penicillin.
Cases of syphilis have been soaring in the United States in recent years, reaching a 70-year high.
A version of this article appeared on Medscape.com.
, according to a report by the Centers for Disease Control and Prevention.
With the incidence of syphilis infection in women increasing in the United States, experts are asking clinicians to be on the lookout for unusual ocular presentations.
“This is the first time such a cluster has been reported in the US,” the International Society for Infectious Diseases posted on ProMED.
Five women in Southwest Michigan who had a common male sex partner developed syphilis infections in their eyes. No new cases have been found related to these five cases after the women and the man received medical care.
If left untreated, the bacterium, Treponema pallidum, can infect the eyes, the ears, and the central nervous system.
The women, identified as non-Hispanic White, were aged 40-60 years and were not infected with HIV. They were diagnosed with early-stage syphilis and all were hospitalized and treated with intravenous penicillin. Routes of sexual exposure among the women included anal (40%), oral (40%), and vaginal (100%), the report states.
The common male sex partner they all met online was found to have early latent syphilis but never developed ocular syphilis.
It is not the eyes that are being exposed. Rather, it is an ocular presentation brought about by a systemic infection carried through the bloodstream after sexual exposure, explains William Nettleton, MD, MPH, medical director of the Kalamazoo and Calhoun public health departments in Michigan and lead author of the report.
“If we screen, identify, and treat syphilis promptly, we can prevent systemic manifestations,” he says.
Clinicians should be aware that the ocular manifestations can come at different stages of syphilis. “For patients you think may have ocular syphilis,” Dr. Nettleton says, “an immediate ophthalmologic evaluation is indicated.”
Symptoms Differed
The five women presented with a variety of symptoms.
Multiple attempts to contact the male partner by telephone and text were made by Michigan Department of Health and Human Services, but he did not respond. Local public health physicians reviewed the man’s electronic health record and discovered that he had sought care at a hospital emergency department in January 2022 for ulcerative penile and anal lesions.
He reported having multiple female sex partners during the previous 12 months but declined to disclose their identities; he reported no male or transgender sexual contact, according to the CDC report. Eventually he agreed to an evaluation, was found to have early latent syphilis, and was treated with penicillin.
Cases of syphilis have been soaring in the United States in recent years, reaching a 70-year high.
A version of this article appeared on Medscape.com.
, according to a report by the Centers for Disease Control and Prevention.
With the incidence of syphilis infection in women increasing in the United States, experts are asking clinicians to be on the lookout for unusual ocular presentations.
“This is the first time such a cluster has been reported in the US,” the International Society for Infectious Diseases posted on ProMED.
Five women in Southwest Michigan who had a common male sex partner developed syphilis infections in their eyes. No new cases have been found related to these five cases after the women and the man received medical care.
If left untreated, the bacterium, Treponema pallidum, can infect the eyes, the ears, and the central nervous system.
The women, identified as non-Hispanic White, were aged 40-60 years and were not infected with HIV. They were diagnosed with early-stage syphilis and all were hospitalized and treated with intravenous penicillin. Routes of sexual exposure among the women included anal (40%), oral (40%), and vaginal (100%), the report states.
The common male sex partner they all met online was found to have early latent syphilis but never developed ocular syphilis.
It is not the eyes that are being exposed. Rather, it is an ocular presentation brought about by a systemic infection carried through the bloodstream after sexual exposure, explains William Nettleton, MD, MPH, medical director of the Kalamazoo and Calhoun public health departments in Michigan and lead author of the report.
“If we screen, identify, and treat syphilis promptly, we can prevent systemic manifestations,” he says.
Clinicians should be aware that the ocular manifestations can come at different stages of syphilis. “For patients you think may have ocular syphilis,” Dr. Nettleton says, “an immediate ophthalmologic evaluation is indicated.”
Symptoms Differed
The five women presented with a variety of symptoms.
Multiple attempts to contact the male partner by telephone and text were made by Michigan Department of Health and Human Services, but he did not respond. Local public health physicians reviewed the man’s electronic health record and discovered that he had sought care at a hospital emergency department in January 2022 for ulcerative penile and anal lesions.
He reported having multiple female sex partners during the previous 12 months but declined to disclose their identities; he reported no male or transgender sexual contact, according to the CDC report. Eventually he agreed to an evaluation, was found to have early latent syphilis, and was treated with penicillin.
Cases of syphilis have been soaring in the United States in recent years, reaching a 70-year high.
A version of this article appeared on Medscape.com.
FROM MMWR
Debate grows over facility fees as lawmakers urge greater transparency
Can the US healthcare system learn something about how to operate from car dealerships? Lawrence Kosinski, MD, MBA, a governing board member of American Gastroenterological Association (AGA), believes so.
There’s growing concern in the United States about the lack of clarity surrounding facility fees, which are intended to cover costs of maintaining medical facilities. Dr. Kosinski thinks that Congress should look into the transparency mandate it created for car prices as a model for how to address this.
A 1958 federal law set the stage for the consumer-friendly breakdown of costs and relevant performance data that anyone who has bought a new vehicle in the United States would recognize.
“You look at that and you know exactly what you are paying for,” Dr. Kosinski told this news organization. “In healthcare, we need something like that.”
Novel solutions like Dr. Kosinski’s will be increasingly necessary, as lawmakers on the state and federal level have begun to set their sights on tackling this issue.
The Biden administration in July expressed concern about an increased use of facility fees for healthcare provided at doctors’ offices, saying these additional costs often surprise consumers. House Energy and Commerce Chairwoman Cathy McMorris Rodgers (R-WA) also raised this issue several times this year, including at a May meeting about pending legislation on price transparency for health services, where she mentioned the case of a man who underwent eye surgery in Maine.
“His bill included three separate facility fees totaling $7800 and professional fees totaling $6200,” Ms. Rodgers said. “Why are three facility fees necessary for 1 hour of surgery in one O.R.?”
AGA’s Dr. Kosinski said facility fees cover the additional costs hospitals and clinics face in providing even routine treatments for some patients. For example, colonoscopy for a patient with a body mass index of 50 would pose special challenges for the anesthesiologist.
These factors need to be considered in setting policies on facility fees, he said. But there is no reason hospitals and other sites of medical care can’t make the information about facility fees easy for patients to find and understand, Dr. Kosinski said.
“I’m struggling to see a reason why we can’t be more transparent,” he said.
Big Battles Ahead
There are two connected battles ahead regarding facility fees: Efforts to restrict these additional charges for many medical services and fights over the need for greater transparency in general about health costs.
Senate Health, Education, Labor and Pensions Chairman Bernie Sanders (I-VT) is seeking to broadly restrict facility fees through his pending Primary Care and Health Workforce Act (S. 2840). The measure would block hospitals from charging health plans facility fees for many evaluation, management, and telehealth services.
The American Hospital Association (AHA) opposes it. They argue that the current payment approach rightly accounts for the added costs incurred when hospitals treat patients who are more likely to be ill or have chronic conditions than those seen in independent practices.
AHA said hospitals also need to maintain standby capacity for natural and man-made disasters, public health emergencies, and unexpected traumatic events. In September, AHA launched a television ad campaign to oppose any drive toward site-neutral policies. AHA says reducing the extra payments could cause more hospitals to shut their doors.
But there’s persistent interest in site-neutral payment, the term describing when the same reimbursement is given for care regardless of setting. This would lower pay for hospitals.
Among those pressing for change is an umbrella group of medical organizations known as the Alliance for Site Neutral Payment Reform. Its members include the American Academy of Family Physicians, American Academy of Orthopaedic Surgeons, American College of Physicians, Community Oncology Alliance, and Digestive Health Physicians Association.
And on November 9, Sen. Maggie Hassan (D-NH) argued for eventually including a site-neutral Medicare provision to a major healthcare package that the Senate Finance Committee is putting together.
Sen. Hassan is seeking to end what she called the “the practice of charging patients unfair hospital facility fees for care provided in the off-campus outpatient setting, like at a regular doctor’s office.”
Senate Finance Chairman Ron Wyden (D-OR) and the ranking Republican on the committee, Sen. Mike Crapo (R-ID), told Sen. Hassan they intended to work with her to see if this issue could be addressed in the pending legislative package.
A 2015 budget deal marked the last time Congress took a major step to address the higher cost of services provided in hospital-owned facilities.
Lawmakers then were scrambling to find cuts to offset spending in what became the 2015 Bipartisan Budget Act. This law established site-neutral payments under Medicare for services received at off-campus outpatient departments but exempted hospitals that already ran these kinds of operations or had advanced plans to create them.
Lawmakers are well aware of the potential savings from site-neutral policies and could look in time again to use them as part of a future budget deal.
In fact, in June, Sen. Hassan and Sens. Mike Braun (R-IN) and John Kennedy (R-LA) introduced a bill meant to basically end the exemption given in the 2015 deal to existing hospital outpatient departments, which has allowed higher Medicare payments. In a press release, Braun estimated that their proposed site-neutral change could save taxpayers $40 billion over a decade.
As Debate Continues, States Are Moving Ahead With Changes
Consumer activists have won a few battles this year at the state level about facility fees.
In July, Maine Gov. Janet Mills, a Democrat, signed a law that requires medical organizations to report facility fees to the state, which will share them publicly. Facility fees can pop up after a patient has received an insurance company estimate of the out-of-pocket costs for care.
“Patients receive bills bloated by healthcare providers that overcharge for services and insurance companies that deny claims without explanation,” the Portland Press Herald reported in a 2022 story. “And with little clout to fight back or even negotiate, feeling helpless, they often give up and pay, worn down by a system that is as time-consuming as it is obtuse.”
In May, Colorado enacted a law that will require patient notification about facility fees at many hospitals in the state.
In June, Connecticut expanded its law regarding facility fees and prohibited them for certain routine outpatient healthcare services. A statement from Gov. Ned Lamont’s office said the original intent of these facility fees was to ensure hospitals could maintain the around-the-clock care needed for inpatient and emergency care.
“However, these fees have been increasingly applied to services such as diagnostic testing and other routine services,” the statement said.
But there have been setbacks as well for those seeking to curb facilities.
The Texas Hospital Association (THA) in May said its advocacy defeated a pair of state bills, House bill 1692 and Senate bill 1275, that sought to limit facility fees for outpatient services.
In rallying opposition to these bills, THA said the loss of facility fees would threaten care for patients. Facility fees help cover costs “beyond the doctor’s bill,” such as “lab technicians, interpreters, medical records, security personnel, janitorial staff, and others,” THA said.
More Patients Shopping?
It’s unclear when — or if — Congress and other states will take major steps to reduce additional payments to hospitals for outpatient care.
But the increased use of high deductibles in health plans is driving more consumers to try to understand all of the costs of medical procedures ahead of time and, thus, drawing attention to facility fees, said Charlie Byrge, the chief operating officer of MDsave.
The average annual deductible levels for an individual increased by 3.0% to $2004 from 2020 to 2021 and for a family plan by 3.9% to $3868, according to a federal report. Some people have higher deductibles, exceeding $5000, Mr. Byrge said.
“That’s creating an opportunity for firms that can connect physicians directly with patients who will pay part or all of the costs of a treatment out of pocket,” he told this news organization.
Doctors and hospitals work with MDsave to charge preset prices for certain services, such as colonoscopies and mammograms. Consumers then can shop online to see if they can save. For example, in Nashville, Tennessee, where MDsave is based, the cost of a colonoscopy through MDsave is $2334, about half of the $4714 national average, according to the firm’s website.
This model for pricing routine medical care is akin to those used for other products and services, where companies decide ahead of time what to charge, he said.
“You don’t buy an airline ticket from Southwest or United or Delta and then there’s a bill after the fact because the price of gas went up a little bit on your flight,” Mr. Byrge said.
This will drive more competition among hospitals and clinics, in places where there are several sites of care in a region, Mr. Byrge said. But there are advantages for physicians and hospitals from the MDsave approach, he said.
“They know they’re getting paid upfront. They’re not going through the delays and headaches of the insurance reimbursement process. There are no denials. It’s just an upfront payment, and I think that’s what we’re starting to see the market really moving toward,” he said.
A version of this article appeared on Medscape.com.
Can the US healthcare system learn something about how to operate from car dealerships? Lawrence Kosinski, MD, MBA, a governing board member of American Gastroenterological Association (AGA), believes so.
There’s growing concern in the United States about the lack of clarity surrounding facility fees, which are intended to cover costs of maintaining medical facilities. Dr. Kosinski thinks that Congress should look into the transparency mandate it created for car prices as a model for how to address this.
A 1958 federal law set the stage for the consumer-friendly breakdown of costs and relevant performance data that anyone who has bought a new vehicle in the United States would recognize.
“You look at that and you know exactly what you are paying for,” Dr. Kosinski told this news organization. “In healthcare, we need something like that.”
Novel solutions like Dr. Kosinski’s will be increasingly necessary, as lawmakers on the state and federal level have begun to set their sights on tackling this issue.
The Biden administration in July expressed concern about an increased use of facility fees for healthcare provided at doctors’ offices, saying these additional costs often surprise consumers. House Energy and Commerce Chairwoman Cathy McMorris Rodgers (R-WA) also raised this issue several times this year, including at a May meeting about pending legislation on price transparency for health services, where she mentioned the case of a man who underwent eye surgery in Maine.
“His bill included three separate facility fees totaling $7800 and professional fees totaling $6200,” Ms. Rodgers said. “Why are three facility fees necessary for 1 hour of surgery in one O.R.?”
AGA’s Dr. Kosinski said facility fees cover the additional costs hospitals and clinics face in providing even routine treatments for some patients. For example, colonoscopy for a patient with a body mass index of 50 would pose special challenges for the anesthesiologist.
These factors need to be considered in setting policies on facility fees, he said. But there is no reason hospitals and other sites of medical care can’t make the information about facility fees easy for patients to find and understand, Dr. Kosinski said.
“I’m struggling to see a reason why we can’t be more transparent,” he said.
Big Battles Ahead
There are two connected battles ahead regarding facility fees: Efforts to restrict these additional charges for many medical services and fights over the need for greater transparency in general about health costs.
Senate Health, Education, Labor and Pensions Chairman Bernie Sanders (I-VT) is seeking to broadly restrict facility fees through his pending Primary Care and Health Workforce Act (S. 2840). The measure would block hospitals from charging health plans facility fees for many evaluation, management, and telehealth services.
The American Hospital Association (AHA) opposes it. They argue that the current payment approach rightly accounts for the added costs incurred when hospitals treat patients who are more likely to be ill or have chronic conditions than those seen in independent practices.
AHA said hospitals also need to maintain standby capacity for natural and man-made disasters, public health emergencies, and unexpected traumatic events. In September, AHA launched a television ad campaign to oppose any drive toward site-neutral policies. AHA says reducing the extra payments could cause more hospitals to shut their doors.
But there’s persistent interest in site-neutral payment, the term describing when the same reimbursement is given for care regardless of setting. This would lower pay for hospitals.
Among those pressing for change is an umbrella group of medical organizations known as the Alliance for Site Neutral Payment Reform. Its members include the American Academy of Family Physicians, American Academy of Orthopaedic Surgeons, American College of Physicians, Community Oncology Alliance, and Digestive Health Physicians Association.
And on November 9, Sen. Maggie Hassan (D-NH) argued for eventually including a site-neutral Medicare provision to a major healthcare package that the Senate Finance Committee is putting together.
Sen. Hassan is seeking to end what she called the “the practice of charging patients unfair hospital facility fees for care provided in the off-campus outpatient setting, like at a regular doctor’s office.”
Senate Finance Chairman Ron Wyden (D-OR) and the ranking Republican on the committee, Sen. Mike Crapo (R-ID), told Sen. Hassan they intended to work with her to see if this issue could be addressed in the pending legislative package.
A 2015 budget deal marked the last time Congress took a major step to address the higher cost of services provided in hospital-owned facilities.
Lawmakers then were scrambling to find cuts to offset spending in what became the 2015 Bipartisan Budget Act. This law established site-neutral payments under Medicare for services received at off-campus outpatient departments but exempted hospitals that already ran these kinds of operations or had advanced plans to create them.
Lawmakers are well aware of the potential savings from site-neutral policies and could look in time again to use them as part of a future budget deal.
In fact, in June, Sen. Hassan and Sens. Mike Braun (R-IN) and John Kennedy (R-LA) introduced a bill meant to basically end the exemption given in the 2015 deal to existing hospital outpatient departments, which has allowed higher Medicare payments. In a press release, Braun estimated that their proposed site-neutral change could save taxpayers $40 billion over a decade.
As Debate Continues, States Are Moving Ahead With Changes
Consumer activists have won a few battles this year at the state level about facility fees.
In July, Maine Gov. Janet Mills, a Democrat, signed a law that requires medical organizations to report facility fees to the state, which will share them publicly. Facility fees can pop up after a patient has received an insurance company estimate of the out-of-pocket costs for care.
“Patients receive bills bloated by healthcare providers that overcharge for services and insurance companies that deny claims without explanation,” the Portland Press Herald reported in a 2022 story. “And with little clout to fight back or even negotiate, feeling helpless, they often give up and pay, worn down by a system that is as time-consuming as it is obtuse.”
In May, Colorado enacted a law that will require patient notification about facility fees at many hospitals in the state.
In June, Connecticut expanded its law regarding facility fees and prohibited them for certain routine outpatient healthcare services. A statement from Gov. Ned Lamont’s office said the original intent of these facility fees was to ensure hospitals could maintain the around-the-clock care needed for inpatient and emergency care.
“However, these fees have been increasingly applied to services such as diagnostic testing and other routine services,” the statement said.
But there have been setbacks as well for those seeking to curb facilities.
The Texas Hospital Association (THA) in May said its advocacy defeated a pair of state bills, House bill 1692 and Senate bill 1275, that sought to limit facility fees for outpatient services.
In rallying opposition to these bills, THA said the loss of facility fees would threaten care for patients. Facility fees help cover costs “beyond the doctor’s bill,” such as “lab technicians, interpreters, medical records, security personnel, janitorial staff, and others,” THA said.
More Patients Shopping?
It’s unclear when — or if — Congress and other states will take major steps to reduce additional payments to hospitals for outpatient care.
But the increased use of high deductibles in health plans is driving more consumers to try to understand all of the costs of medical procedures ahead of time and, thus, drawing attention to facility fees, said Charlie Byrge, the chief operating officer of MDsave.
The average annual deductible levels for an individual increased by 3.0% to $2004 from 2020 to 2021 and for a family plan by 3.9% to $3868, according to a federal report. Some people have higher deductibles, exceeding $5000, Mr. Byrge said.
“That’s creating an opportunity for firms that can connect physicians directly with patients who will pay part or all of the costs of a treatment out of pocket,” he told this news organization.
Doctors and hospitals work with MDsave to charge preset prices for certain services, such as colonoscopies and mammograms. Consumers then can shop online to see if they can save. For example, in Nashville, Tennessee, where MDsave is based, the cost of a colonoscopy through MDsave is $2334, about half of the $4714 national average, according to the firm’s website.
This model for pricing routine medical care is akin to those used for other products and services, where companies decide ahead of time what to charge, he said.
“You don’t buy an airline ticket from Southwest or United or Delta and then there’s a bill after the fact because the price of gas went up a little bit on your flight,” Mr. Byrge said.
This will drive more competition among hospitals and clinics, in places where there are several sites of care in a region, Mr. Byrge said. But there are advantages for physicians and hospitals from the MDsave approach, he said.
“They know they’re getting paid upfront. They’re not going through the delays and headaches of the insurance reimbursement process. There are no denials. It’s just an upfront payment, and I think that’s what we’re starting to see the market really moving toward,” he said.
A version of this article appeared on Medscape.com.
Can the US healthcare system learn something about how to operate from car dealerships? Lawrence Kosinski, MD, MBA, a governing board member of American Gastroenterological Association (AGA), believes so.
There’s growing concern in the United States about the lack of clarity surrounding facility fees, which are intended to cover costs of maintaining medical facilities. Dr. Kosinski thinks that Congress should look into the transparency mandate it created for car prices as a model for how to address this.
A 1958 federal law set the stage for the consumer-friendly breakdown of costs and relevant performance data that anyone who has bought a new vehicle in the United States would recognize.
“You look at that and you know exactly what you are paying for,” Dr. Kosinski told this news organization. “In healthcare, we need something like that.”
Novel solutions like Dr. Kosinski’s will be increasingly necessary, as lawmakers on the state and federal level have begun to set their sights on tackling this issue.
The Biden administration in July expressed concern about an increased use of facility fees for healthcare provided at doctors’ offices, saying these additional costs often surprise consumers. House Energy and Commerce Chairwoman Cathy McMorris Rodgers (R-WA) also raised this issue several times this year, including at a May meeting about pending legislation on price transparency for health services, where she mentioned the case of a man who underwent eye surgery in Maine.
“His bill included three separate facility fees totaling $7800 and professional fees totaling $6200,” Ms. Rodgers said. “Why are three facility fees necessary for 1 hour of surgery in one O.R.?”
AGA’s Dr. Kosinski said facility fees cover the additional costs hospitals and clinics face in providing even routine treatments for some patients. For example, colonoscopy for a patient with a body mass index of 50 would pose special challenges for the anesthesiologist.
These factors need to be considered in setting policies on facility fees, he said. But there is no reason hospitals and other sites of medical care can’t make the information about facility fees easy for patients to find and understand, Dr. Kosinski said.
“I’m struggling to see a reason why we can’t be more transparent,” he said.
Big Battles Ahead
There are two connected battles ahead regarding facility fees: Efforts to restrict these additional charges for many medical services and fights over the need for greater transparency in general about health costs.
Senate Health, Education, Labor and Pensions Chairman Bernie Sanders (I-VT) is seeking to broadly restrict facility fees through his pending Primary Care and Health Workforce Act (S. 2840). The measure would block hospitals from charging health plans facility fees for many evaluation, management, and telehealth services.
The American Hospital Association (AHA) opposes it. They argue that the current payment approach rightly accounts for the added costs incurred when hospitals treat patients who are more likely to be ill or have chronic conditions than those seen in independent practices.
AHA said hospitals also need to maintain standby capacity for natural and man-made disasters, public health emergencies, and unexpected traumatic events. In September, AHA launched a television ad campaign to oppose any drive toward site-neutral policies. AHA says reducing the extra payments could cause more hospitals to shut their doors.
But there’s persistent interest in site-neutral payment, the term describing when the same reimbursement is given for care regardless of setting. This would lower pay for hospitals.
Among those pressing for change is an umbrella group of medical organizations known as the Alliance for Site Neutral Payment Reform. Its members include the American Academy of Family Physicians, American Academy of Orthopaedic Surgeons, American College of Physicians, Community Oncology Alliance, and Digestive Health Physicians Association.
And on November 9, Sen. Maggie Hassan (D-NH) argued for eventually including a site-neutral Medicare provision to a major healthcare package that the Senate Finance Committee is putting together.
Sen. Hassan is seeking to end what she called the “the practice of charging patients unfair hospital facility fees for care provided in the off-campus outpatient setting, like at a regular doctor’s office.”
Senate Finance Chairman Ron Wyden (D-OR) and the ranking Republican on the committee, Sen. Mike Crapo (R-ID), told Sen. Hassan they intended to work with her to see if this issue could be addressed in the pending legislative package.
A 2015 budget deal marked the last time Congress took a major step to address the higher cost of services provided in hospital-owned facilities.
Lawmakers then were scrambling to find cuts to offset spending in what became the 2015 Bipartisan Budget Act. This law established site-neutral payments under Medicare for services received at off-campus outpatient departments but exempted hospitals that already ran these kinds of operations or had advanced plans to create them.
Lawmakers are well aware of the potential savings from site-neutral policies and could look in time again to use them as part of a future budget deal.
In fact, in June, Sen. Hassan and Sens. Mike Braun (R-IN) and John Kennedy (R-LA) introduced a bill meant to basically end the exemption given in the 2015 deal to existing hospital outpatient departments, which has allowed higher Medicare payments. In a press release, Braun estimated that their proposed site-neutral change could save taxpayers $40 billion over a decade.
As Debate Continues, States Are Moving Ahead With Changes
Consumer activists have won a few battles this year at the state level about facility fees.
In July, Maine Gov. Janet Mills, a Democrat, signed a law that requires medical organizations to report facility fees to the state, which will share them publicly. Facility fees can pop up after a patient has received an insurance company estimate of the out-of-pocket costs for care.
“Patients receive bills bloated by healthcare providers that overcharge for services and insurance companies that deny claims without explanation,” the Portland Press Herald reported in a 2022 story. “And with little clout to fight back or even negotiate, feeling helpless, they often give up and pay, worn down by a system that is as time-consuming as it is obtuse.”
In May, Colorado enacted a law that will require patient notification about facility fees at many hospitals in the state.
In June, Connecticut expanded its law regarding facility fees and prohibited them for certain routine outpatient healthcare services. A statement from Gov. Ned Lamont’s office said the original intent of these facility fees was to ensure hospitals could maintain the around-the-clock care needed for inpatient and emergency care.
“However, these fees have been increasingly applied to services such as diagnostic testing and other routine services,” the statement said.
But there have been setbacks as well for those seeking to curb facilities.
The Texas Hospital Association (THA) in May said its advocacy defeated a pair of state bills, House bill 1692 and Senate bill 1275, that sought to limit facility fees for outpatient services.
In rallying opposition to these bills, THA said the loss of facility fees would threaten care for patients. Facility fees help cover costs “beyond the doctor’s bill,” such as “lab technicians, interpreters, medical records, security personnel, janitorial staff, and others,” THA said.
More Patients Shopping?
It’s unclear when — or if — Congress and other states will take major steps to reduce additional payments to hospitals for outpatient care.
But the increased use of high deductibles in health plans is driving more consumers to try to understand all of the costs of medical procedures ahead of time and, thus, drawing attention to facility fees, said Charlie Byrge, the chief operating officer of MDsave.
The average annual deductible levels for an individual increased by 3.0% to $2004 from 2020 to 2021 and for a family plan by 3.9% to $3868, according to a federal report. Some people have higher deductibles, exceeding $5000, Mr. Byrge said.
“That’s creating an opportunity for firms that can connect physicians directly with patients who will pay part or all of the costs of a treatment out of pocket,” he told this news organization.
Doctors and hospitals work with MDsave to charge preset prices for certain services, such as colonoscopies and mammograms. Consumers then can shop online to see if they can save. For example, in Nashville, Tennessee, where MDsave is based, the cost of a colonoscopy through MDsave is $2334, about half of the $4714 national average, according to the firm’s website.
This model for pricing routine medical care is akin to those used for other products and services, where companies decide ahead of time what to charge, he said.
“You don’t buy an airline ticket from Southwest or United or Delta and then there’s a bill after the fact because the price of gas went up a little bit on your flight,” Mr. Byrge said.
This will drive more competition among hospitals and clinics, in places where there are several sites of care in a region, Mr. Byrge said. But there are advantages for physicians and hospitals from the MDsave approach, he said.
“They know they’re getting paid upfront. They’re not going through the delays and headaches of the insurance reimbursement process. There are no denials. It’s just an upfront payment, and I think that’s what we’re starting to see the market really moving toward,” he said.
A version of this article appeared on Medscape.com.












