User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Roflumilast foam gets nod as new option for seborrheic dermatitis
The in a press release.
The 0.3% foam, marketed as Zoryve, applied once-daily, is indicated for patients aged 9 years and older with seborrheic dermatitis, and can be used anywhere on the body, including areas with hair, with no limits on duration of use, according to the company, Arcutis. A 0.3% cream formulation of roflumilast was previously approved by the FDA for the topical treatment of plaque psoriasis in patients aged 6 years and older.
Approval was based on data from the phase 3 STRATUM trial and an accompanying phase 2 study known as Trial 203. These studies included a total of 683 adults and youth aged 9 years and older with seborrheic dermatitis. Participants were randomized to roflumilast or a placebo.
At 8 weeks, 79.5 % of patients on roflumilast met the primary efficacy endpoint of Investigator Global Assessment (IGA) scores of 0 or 1 (clear or almost clear) compared with 58.0% of patients on placebo (P < .001); the results were similar in the phase 2 Trial 203 (73.1% vs. 40.8%, respectively; P < .001). Overall, more than 50% of the patients on roflumilast achieved a clear score.
Patients in the roflumilast group also showed significant improvement in all secondary endpoints, including itching, scaling, and erythema, according to the company.
In the STRATUM study, 62.8% of roflumilast-treated patients and 40.6% of placebo patients achieved a 4-point or more reduction in itch based on the Worst Itch Numerical Rating Score (P =.0001), and 28% of roflumilast-treated patients reported significant itch improvement within the first 48 hours of use, compared with 13% of placebo patients (P = .0024).
Over a treatment period of up to 1 year, no treatment-related severe adverse events were reported in the phase 2 and 3 studies. The incidence of treatment emergent adverse events was similar between the treatment and placebo groups, and the most common adverse events (occurring in 1% of more of patients) across both studies were nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
Roflumilast foam is scheduled to be available by the end of January 2024, according to the company. The product is for topical use only, and contraindicated for individuals with severe liver impairment.
The in a press release.
The 0.3% foam, marketed as Zoryve, applied once-daily, is indicated for patients aged 9 years and older with seborrheic dermatitis, and can be used anywhere on the body, including areas with hair, with no limits on duration of use, according to the company, Arcutis. A 0.3% cream formulation of roflumilast was previously approved by the FDA for the topical treatment of plaque psoriasis in patients aged 6 years and older.
Approval was based on data from the phase 3 STRATUM trial and an accompanying phase 2 study known as Trial 203. These studies included a total of 683 adults and youth aged 9 years and older with seborrheic dermatitis. Participants were randomized to roflumilast or a placebo.
At 8 weeks, 79.5 % of patients on roflumilast met the primary efficacy endpoint of Investigator Global Assessment (IGA) scores of 0 or 1 (clear or almost clear) compared with 58.0% of patients on placebo (P < .001); the results were similar in the phase 2 Trial 203 (73.1% vs. 40.8%, respectively; P < .001). Overall, more than 50% of the patients on roflumilast achieved a clear score.
Patients in the roflumilast group also showed significant improvement in all secondary endpoints, including itching, scaling, and erythema, according to the company.
In the STRATUM study, 62.8% of roflumilast-treated patients and 40.6% of placebo patients achieved a 4-point or more reduction in itch based on the Worst Itch Numerical Rating Score (P =.0001), and 28% of roflumilast-treated patients reported significant itch improvement within the first 48 hours of use, compared with 13% of placebo patients (P = .0024).
Over a treatment period of up to 1 year, no treatment-related severe adverse events were reported in the phase 2 and 3 studies. The incidence of treatment emergent adverse events was similar between the treatment and placebo groups, and the most common adverse events (occurring in 1% of more of patients) across both studies were nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
Roflumilast foam is scheduled to be available by the end of January 2024, according to the company. The product is for topical use only, and contraindicated for individuals with severe liver impairment.
The in a press release.
The 0.3% foam, marketed as Zoryve, applied once-daily, is indicated for patients aged 9 years and older with seborrheic dermatitis, and can be used anywhere on the body, including areas with hair, with no limits on duration of use, according to the company, Arcutis. A 0.3% cream formulation of roflumilast was previously approved by the FDA for the topical treatment of plaque psoriasis in patients aged 6 years and older.
Approval was based on data from the phase 3 STRATUM trial and an accompanying phase 2 study known as Trial 203. These studies included a total of 683 adults and youth aged 9 years and older with seborrheic dermatitis. Participants were randomized to roflumilast or a placebo.
At 8 weeks, 79.5 % of patients on roflumilast met the primary efficacy endpoint of Investigator Global Assessment (IGA) scores of 0 or 1 (clear or almost clear) compared with 58.0% of patients on placebo (P < .001); the results were similar in the phase 2 Trial 203 (73.1% vs. 40.8%, respectively; P < .001). Overall, more than 50% of the patients on roflumilast achieved a clear score.
Patients in the roflumilast group also showed significant improvement in all secondary endpoints, including itching, scaling, and erythema, according to the company.
In the STRATUM study, 62.8% of roflumilast-treated patients and 40.6% of placebo patients achieved a 4-point or more reduction in itch based on the Worst Itch Numerical Rating Score (P =.0001), and 28% of roflumilast-treated patients reported significant itch improvement within the first 48 hours of use, compared with 13% of placebo patients (P = .0024).
Over a treatment period of up to 1 year, no treatment-related severe adverse events were reported in the phase 2 and 3 studies. The incidence of treatment emergent adverse events was similar between the treatment and placebo groups, and the most common adverse events (occurring in 1% of more of patients) across both studies were nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
Roflumilast foam is scheduled to be available by the end of January 2024, according to the company. The product is for topical use only, and contraindicated for individuals with severe liver impairment.
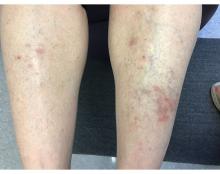
A 55-year-old female presented a with few years' history of pruritic plaques on her shins and wrists
. Lesions may have a covering of scale. HLP commonly affects middle aged men and women. Lesions are most commonly located bilaterally on the shins and ankles and can be painful or pruritic. The differential diagnosis for the condition includes lichen simplex chronicus, connective tissue disease, and other skin disorders that cause hyperkeratosis. This wide differential makes histopathological analysis a useful tool in confirming the diagnosis of HLP.
A definitive diagnosis can be made via skin biopsy. Histopathology reveals hyperkeratosis, acanthosis, and a band-like lymphocytic infiltrate in the dermis. An eosinophilic infiltrate may be present. Other common features include saw tooth rete ridges and Civatte bodies, which are apoptotic keratinocytes. The lymphocytic infiltrate may indicate an autoimmune etiology in which the body’s immune system erroneously attacks itself. However, the exact cause is not known and genetic and environmental factors may play a role.
The treatment of HLP includes symptomatic management and control of inflammation. Topical steroids can be prescribed to manage the inflammation and associated pruritus, and emollient creams and moisturizers are helpful in controlling the dryness. Oral steroids, immunosuppressant medications, or retinoids may be necessary in more severe cases. In addition, psoralen plus ultraviolet A (PUVA) light therapy has been found to be beneficial in some cases. Squamous cell carcinoma may arise in lesions.
This case and photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Donna Bilu Martin, MD; Premier Dermatology, MD, Aventura, Florida. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Arnold DL, Krishnamurthy K. Lichen Planus. [Updated 2023 Jun 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526126/
Jaime TJ et al. An Bras Dermatol. 2011 Jul-Aug;86(4 Suppl 1):S96-9.
Mirchandani S et al. Med Pharm Rep. 2020 Apr;93(2):210-2. .
Whittington CP et al. Arch Pathol Lab Med. 2023 Jun 19. doi: 10.5858/arpa.2022-0515-RA.
. Lesions may have a covering of scale. HLP commonly affects middle aged men and women. Lesions are most commonly located bilaterally on the shins and ankles and can be painful or pruritic. The differential diagnosis for the condition includes lichen simplex chronicus, connective tissue disease, and other skin disorders that cause hyperkeratosis. This wide differential makes histopathological analysis a useful tool in confirming the diagnosis of HLP.
A definitive diagnosis can be made via skin biopsy. Histopathology reveals hyperkeratosis, acanthosis, and a band-like lymphocytic infiltrate in the dermis. An eosinophilic infiltrate may be present. Other common features include saw tooth rete ridges and Civatte bodies, which are apoptotic keratinocytes. The lymphocytic infiltrate may indicate an autoimmune etiology in which the body’s immune system erroneously attacks itself. However, the exact cause is not known and genetic and environmental factors may play a role.
The treatment of HLP includes symptomatic management and control of inflammation. Topical steroids can be prescribed to manage the inflammation and associated pruritus, and emollient creams and moisturizers are helpful in controlling the dryness. Oral steroids, immunosuppressant medications, or retinoids may be necessary in more severe cases. In addition, psoralen plus ultraviolet A (PUVA) light therapy has been found to be beneficial in some cases. Squamous cell carcinoma may arise in lesions.
This case and photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Donna Bilu Martin, MD; Premier Dermatology, MD, Aventura, Florida. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Arnold DL, Krishnamurthy K. Lichen Planus. [Updated 2023 Jun 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526126/
Jaime TJ et al. An Bras Dermatol. 2011 Jul-Aug;86(4 Suppl 1):S96-9.
Mirchandani S et al. Med Pharm Rep. 2020 Apr;93(2):210-2. .
Whittington CP et al. Arch Pathol Lab Med. 2023 Jun 19. doi: 10.5858/arpa.2022-0515-RA.
. Lesions may have a covering of scale. HLP commonly affects middle aged men and women. Lesions are most commonly located bilaterally on the shins and ankles and can be painful or pruritic. The differential diagnosis for the condition includes lichen simplex chronicus, connective tissue disease, and other skin disorders that cause hyperkeratosis. This wide differential makes histopathological analysis a useful tool in confirming the diagnosis of HLP.
A definitive diagnosis can be made via skin biopsy. Histopathology reveals hyperkeratosis, acanthosis, and a band-like lymphocytic infiltrate in the dermis. An eosinophilic infiltrate may be present. Other common features include saw tooth rete ridges and Civatte bodies, which are apoptotic keratinocytes. The lymphocytic infiltrate may indicate an autoimmune etiology in which the body’s immune system erroneously attacks itself. However, the exact cause is not known and genetic and environmental factors may play a role.
The treatment of HLP includes symptomatic management and control of inflammation. Topical steroids can be prescribed to manage the inflammation and associated pruritus, and emollient creams and moisturizers are helpful in controlling the dryness. Oral steroids, immunosuppressant medications, or retinoids may be necessary in more severe cases. In addition, psoralen plus ultraviolet A (PUVA) light therapy has been found to be beneficial in some cases. Squamous cell carcinoma may arise in lesions.
This case and photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Donna Bilu Martin, MD; Premier Dermatology, MD, Aventura, Florida. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Arnold DL, Krishnamurthy K. Lichen Planus. [Updated 2023 Jun 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526126/
Jaime TJ et al. An Bras Dermatol. 2011 Jul-Aug;86(4 Suppl 1):S96-9.
Mirchandani S et al. Med Pharm Rep. 2020 Apr;93(2):210-2. .
Whittington CP et al. Arch Pathol Lab Med. 2023 Jun 19. doi: 10.5858/arpa.2022-0515-RA.
How does lebrikizumab perform across different racial and ethnic subgroups?
.
The finding comes from an analysis of the 16-week induction periods of the phase 3 ADvocate1 and ADvocate2 trials, which Raj Chovatiya, MD, PhD, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. The efficacy of lebrikizumab monotherapy to treat moderate-to-severe AD has been established in phase 3 studies, “but disease characteristic and efficacy outcomes may vary among racial and ethnic subgroups,” said Dr. Chovatiya, assistant professor in the department of dermatology at Northwestern University, Chicago. “The goal of the current study is to report the week 16 efficacy of lebrikizumab-treated patients in racial and ethnic subgroups from ADvocate1 and ADvocate2.”
Key eligibility criteria for both trials included adults or adolescents with a diagnosis of AD as defined by the American Academy of Dermatology Consensus Criteria, for at least 1 year prior to screening. They had moderate-to-severe AD, were candidates for systemic therapy, and were dupilumab- and tralokinumab-naive. Outcomes of interest were the Investigator’s Global Assessment 0 or 1, with at least a 2-point improvement; and the proportions of patients who achieved Eczema Area and Severity Index (EASI75) and EASI90 responses, and an improvement of 4 points or more on the Pruritus Numeric Rating Scale (NRS).
For statistical analysis, the researchers pooled data from Advocate1 and Advocate2 and applied imputation methodology to the 16-week induction period. Subsequent data from patients who received topical or systemic rescue medication or discontinued treatment due to lack of efficacy were imputed as nonresponders. Subsequent data from patients who discontinued treatment for other reasons were set to missing, and the researchers handled missing data with multiple imputation. They used logistic regression to test the interaction between the treatment and subgroup and the Cochran-Mantel-Haenszel method to evaluate the treatment effect within each subgroup after adjusting for stratification factors.
Dr. Chovatiya reported findings from the 851 study participants in the combined studies. Of these, 542 were White, 192 were Asian, 84 were Black, and 33 were from other racial subgroups. By ethnic subgroup, 748 were not Hispanic or Latino, 91 were Hispanic or Latino, and ethnicity was unknown or not reported for 12 subjects. At baseline, the mean body mass index was slightly higher among Blacks (30.4 kg/m2) and Hispanics (29.4 kg/m2) compared with other racial and ethnic groups, “which reflects general epidemiologic data among these groups in the United States,” Dr. Chovatiya said. “You can also see a difference in the balance of IGA scores — they were a little bit more severe in the Black or African American and Hispanic groups as well.” The researchers also observed differences in the baseline EASI score across some of these groups, particularly in the Asian individuals, who had higher EASI scores. Prior use of systemic therapy was lower in the Black and “other” subgroups, compared with other racial subgroups.
At week 16, key efficacy endpoints were generally similar between the different racial subgroups. Specifically, 25.1% of Asians in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 4.1% of those in the placebo group (P < .001), while 33.2% of Blacks in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 13.2% of those in the placebo group (no P value was established because this subgroup represented less than 10% of the entire study population). In addition, 43.3% of Whites in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 14.1% of those in the placebo group (P < .001).
In other findings, 45.5% of Asians in the lebrikizumab treatment group achieved an EASI75, compared with 8.5% of those in the placebo group (P < .001), while 51.7% of Blacks in the lebrikizumab treatment group achieved an EASI75, compared with 18.8% of those in the placebo group. Among whites in the lebrikizumab treatment group, 59.7% of achieved an EASI75, compared with 20.4% of those in the placebo group (P < .001).
Dr. Chovatiya said that 26.5% of Asians in the lebrikizumab treatment group achieved an EASI90, compared with 4.3% of those in the placebo group (P < .001), while 26.9% of Blacks in the lebrikizumab treatment group achieved an EASI90, compared with 13.2% of those in the placebo group. In addition, 38.3% of Whites in the lebrikizumab treatment group achieved an EASI90, compared with 10.9% of those in the placebo group (P < .001).
Finally, 36.4% of Asians in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 5.7% of those in the placebo group (P <. 001), while 41.7% of Blacks in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 17.4% of those in the placebo group. In addition, 45.9% of Whites in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 14.8% of those in the placebo group (P < .001). Statistical analyses of efficacy endpoints conducted by ethnic group yielded similar results.
Dr. Chovatiya acknowledged certain limitations of the study, including the fact that differences in baseline demographics and disease characteristics limit direct comparison across racial and ethnic subgroups. “Due to the relatively small sample size of some racial and ethnic subgroups and the post hoc nature of this analysis, additional studies are needed to verify these results,” he concluded. But for now, he said, the data available indicate that “lebrikizumab is effective across racial and ethnic subgroups for the treatment of moderate-to-severe AD after 16 weeks of monotherapy treatment.”
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly and Company. Dr. Chovatiya disclosed that he is speaker for and/or a consult and advisory board member to many pharmaceutical companies, including Eli Lilly.
.
The finding comes from an analysis of the 16-week induction periods of the phase 3 ADvocate1 and ADvocate2 trials, which Raj Chovatiya, MD, PhD, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. The efficacy of lebrikizumab monotherapy to treat moderate-to-severe AD has been established in phase 3 studies, “but disease characteristic and efficacy outcomes may vary among racial and ethnic subgroups,” said Dr. Chovatiya, assistant professor in the department of dermatology at Northwestern University, Chicago. “The goal of the current study is to report the week 16 efficacy of lebrikizumab-treated patients in racial and ethnic subgroups from ADvocate1 and ADvocate2.”
Key eligibility criteria for both trials included adults or adolescents with a diagnosis of AD as defined by the American Academy of Dermatology Consensus Criteria, for at least 1 year prior to screening. They had moderate-to-severe AD, were candidates for systemic therapy, and were dupilumab- and tralokinumab-naive. Outcomes of interest were the Investigator’s Global Assessment 0 or 1, with at least a 2-point improvement; and the proportions of patients who achieved Eczema Area and Severity Index (EASI75) and EASI90 responses, and an improvement of 4 points or more on the Pruritus Numeric Rating Scale (NRS).
For statistical analysis, the researchers pooled data from Advocate1 and Advocate2 and applied imputation methodology to the 16-week induction period. Subsequent data from patients who received topical or systemic rescue medication or discontinued treatment due to lack of efficacy were imputed as nonresponders. Subsequent data from patients who discontinued treatment for other reasons were set to missing, and the researchers handled missing data with multiple imputation. They used logistic regression to test the interaction between the treatment and subgroup and the Cochran-Mantel-Haenszel method to evaluate the treatment effect within each subgroup after adjusting for stratification factors.
Dr. Chovatiya reported findings from the 851 study participants in the combined studies. Of these, 542 were White, 192 were Asian, 84 were Black, and 33 were from other racial subgroups. By ethnic subgroup, 748 were not Hispanic or Latino, 91 were Hispanic or Latino, and ethnicity was unknown or not reported for 12 subjects. At baseline, the mean body mass index was slightly higher among Blacks (30.4 kg/m2) and Hispanics (29.4 kg/m2) compared with other racial and ethnic groups, “which reflects general epidemiologic data among these groups in the United States,” Dr. Chovatiya said. “You can also see a difference in the balance of IGA scores — they were a little bit more severe in the Black or African American and Hispanic groups as well.” The researchers also observed differences in the baseline EASI score across some of these groups, particularly in the Asian individuals, who had higher EASI scores. Prior use of systemic therapy was lower in the Black and “other” subgroups, compared with other racial subgroups.
At week 16, key efficacy endpoints were generally similar between the different racial subgroups. Specifically, 25.1% of Asians in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 4.1% of those in the placebo group (P < .001), while 33.2% of Blacks in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 13.2% of those in the placebo group (no P value was established because this subgroup represented less than 10% of the entire study population). In addition, 43.3% of Whites in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 14.1% of those in the placebo group (P < .001).
In other findings, 45.5% of Asians in the lebrikizumab treatment group achieved an EASI75, compared with 8.5% of those in the placebo group (P < .001), while 51.7% of Blacks in the lebrikizumab treatment group achieved an EASI75, compared with 18.8% of those in the placebo group. Among whites in the lebrikizumab treatment group, 59.7% of achieved an EASI75, compared with 20.4% of those in the placebo group (P < .001).
Dr. Chovatiya said that 26.5% of Asians in the lebrikizumab treatment group achieved an EASI90, compared with 4.3% of those in the placebo group (P < .001), while 26.9% of Blacks in the lebrikizumab treatment group achieved an EASI90, compared with 13.2% of those in the placebo group. In addition, 38.3% of Whites in the lebrikizumab treatment group achieved an EASI90, compared with 10.9% of those in the placebo group (P < .001).
Finally, 36.4% of Asians in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 5.7% of those in the placebo group (P <. 001), while 41.7% of Blacks in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 17.4% of those in the placebo group. In addition, 45.9% of Whites in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 14.8% of those in the placebo group (P < .001). Statistical analyses of efficacy endpoints conducted by ethnic group yielded similar results.
Dr. Chovatiya acknowledged certain limitations of the study, including the fact that differences in baseline demographics and disease characteristics limit direct comparison across racial and ethnic subgroups. “Due to the relatively small sample size of some racial and ethnic subgroups and the post hoc nature of this analysis, additional studies are needed to verify these results,” he concluded. But for now, he said, the data available indicate that “lebrikizumab is effective across racial and ethnic subgroups for the treatment of moderate-to-severe AD after 16 weeks of monotherapy treatment.”
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly and Company. Dr. Chovatiya disclosed that he is speaker for and/or a consult and advisory board member to many pharmaceutical companies, including Eli Lilly.
.
The finding comes from an analysis of the 16-week induction periods of the phase 3 ADvocate1 and ADvocate2 trials, which Raj Chovatiya, MD, PhD, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. The efficacy of lebrikizumab monotherapy to treat moderate-to-severe AD has been established in phase 3 studies, “but disease characteristic and efficacy outcomes may vary among racial and ethnic subgroups,” said Dr. Chovatiya, assistant professor in the department of dermatology at Northwestern University, Chicago. “The goal of the current study is to report the week 16 efficacy of lebrikizumab-treated patients in racial and ethnic subgroups from ADvocate1 and ADvocate2.”
Key eligibility criteria for both trials included adults or adolescents with a diagnosis of AD as defined by the American Academy of Dermatology Consensus Criteria, for at least 1 year prior to screening. They had moderate-to-severe AD, were candidates for systemic therapy, and were dupilumab- and tralokinumab-naive. Outcomes of interest were the Investigator’s Global Assessment 0 or 1, with at least a 2-point improvement; and the proportions of patients who achieved Eczema Area and Severity Index (EASI75) and EASI90 responses, and an improvement of 4 points or more on the Pruritus Numeric Rating Scale (NRS).
For statistical analysis, the researchers pooled data from Advocate1 and Advocate2 and applied imputation methodology to the 16-week induction period. Subsequent data from patients who received topical or systemic rescue medication or discontinued treatment due to lack of efficacy were imputed as nonresponders. Subsequent data from patients who discontinued treatment for other reasons were set to missing, and the researchers handled missing data with multiple imputation. They used logistic regression to test the interaction between the treatment and subgroup and the Cochran-Mantel-Haenszel method to evaluate the treatment effect within each subgroup after adjusting for stratification factors.
Dr. Chovatiya reported findings from the 851 study participants in the combined studies. Of these, 542 were White, 192 were Asian, 84 were Black, and 33 were from other racial subgroups. By ethnic subgroup, 748 were not Hispanic or Latino, 91 were Hispanic or Latino, and ethnicity was unknown or not reported for 12 subjects. At baseline, the mean body mass index was slightly higher among Blacks (30.4 kg/m2) and Hispanics (29.4 kg/m2) compared with other racial and ethnic groups, “which reflects general epidemiologic data among these groups in the United States,” Dr. Chovatiya said. “You can also see a difference in the balance of IGA scores — they were a little bit more severe in the Black or African American and Hispanic groups as well.” The researchers also observed differences in the baseline EASI score across some of these groups, particularly in the Asian individuals, who had higher EASI scores. Prior use of systemic therapy was lower in the Black and “other” subgroups, compared with other racial subgroups.
At week 16, key efficacy endpoints were generally similar between the different racial subgroups. Specifically, 25.1% of Asians in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 4.1% of those in the placebo group (P < .001), while 33.2% of Blacks in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 13.2% of those in the placebo group (no P value was established because this subgroup represented less than 10% of the entire study population). In addition, 43.3% of Whites in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 14.1% of those in the placebo group (P < .001).
In other findings, 45.5% of Asians in the lebrikizumab treatment group achieved an EASI75, compared with 8.5% of those in the placebo group (P < .001), while 51.7% of Blacks in the lebrikizumab treatment group achieved an EASI75, compared with 18.8% of those in the placebo group. Among whites in the lebrikizumab treatment group, 59.7% of achieved an EASI75, compared with 20.4% of those in the placebo group (P < .001).
Dr. Chovatiya said that 26.5% of Asians in the lebrikizumab treatment group achieved an EASI90, compared with 4.3% of those in the placebo group (P < .001), while 26.9% of Blacks in the lebrikizumab treatment group achieved an EASI90, compared with 13.2% of those in the placebo group. In addition, 38.3% of Whites in the lebrikizumab treatment group achieved an EASI90, compared with 10.9% of those in the placebo group (P < .001).
Finally, 36.4% of Asians in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 5.7% of those in the placebo group (P <. 001), while 41.7% of Blacks in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 17.4% of those in the placebo group. In addition, 45.9% of Whites in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 14.8% of those in the placebo group (P < .001). Statistical analyses of efficacy endpoints conducted by ethnic group yielded similar results.
Dr. Chovatiya acknowledged certain limitations of the study, including the fact that differences in baseline demographics and disease characteristics limit direct comparison across racial and ethnic subgroups. “Due to the relatively small sample size of some racial and ethnic subgroups and the post hoc nature of this analysis, additional studies are needed to verify these results,” he concluded. But for now, he said, the data available indicate that “lebrikizumab is effective across racial and ethnic subgroups for the treatment of moderate-to-severe AD after 16 weeks of monotherapy treatment.”
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly and Company. Dr. Chovatiya disclosed that he is speaker for and/or a consult and advisory board member to many pharmaceutical companies, including Eli Lilly.
FROM RAD 2023
10% of US physicians work for or under UnitedHealth. Is that a problem?
as more payers and private equity firms pursue medical practice acquisitions.
The company added 20,000 physicians in the last year alone, including a previously physician-owned multispecialty group practice of 400 doctors in New York. They join the growing web of doctors — about 90,000 of the 950,000 active US physicians — working for the UnitedHealth Group subsidiary, Optum Health, providing primary, specialty, urgent, and surgical care. Amar Desai, MD, chief executive officer of Optum Health, shared the updated workforce numbers during the health care conglomerate’s annual investor conference.
Health care mergers and consolidations have become more common as physician groups struggle to stay afloat amid dwindling payer reimbursements. Although private equity and health systems often acquire practices, payers like UHC are increasingly doing so as part of their model to advance value-based care.
Yashaswini Singh, PhD, health care economist and assistant professor of health services, policy, and practice at Brown University, says such moves mirror the broader trend in corporate consolidation of physician practices. She said in an interview that the integrated models could possibly enhance care coordination and improve outcomes, but the impact of payer-led consolidation has not been extensively studied.
Meanwhile, evidence considering private equity ownership is just emerging. In a 2022 study published in JAMA Health Forum, with Dr. Singh as lead author, findings showed that private equity involvement increased healthcare spending through higher prices and utilization.
Consolidation can also raise anti-trust concerns. “If payers incentivize referral patterns of their employed physicians to favor other physicians employed by the payer, it can reduce competition by restricting consumer choice,” said Dr. Singh.
A potential merger between Cigna and Humana that could happen by the end of the year will likely face intense scrutiny as it would create a company that rivals the size of UnitedHealth Group or CVS Health. If it goes through, the duo could streamline its insurance offerings and leverage each other’s care delivery platforms, clinics, and provider workforce.
The Biden Administration has sought to strengthen anti-trust statutes to prevent industry monopolies and consumer harm, and the US Department of Justice and Federal Trade Commission have proposed new merger guidelines that have yet to be finalized.
According to Dr. Singh, some of Optum’s medical practice purchases may bypass anti-trust statutes since most prospective mergers and acquisitions are reviewed only if they exceed a specific value ($101 million for 2023). Limited transparency in ownership structures further complicates matters. Plus, Dr. Singh said instances where physicians are hired instead of acquired through mergers would not be subject to current anti-trust laws.
The ‘corporatization’ of health care is not good for patients or physicians, said Robert McNamara, MD, chief medical officer of the American Academy of Emergency Medicine Physician Group and cofounder of Take Medicine Back, a physician group advocating to remove corporate interests from health care.
“If you ask a physician what causes them the most moral conflict, they’ll tell you it’s the insurance companies denying something they want to do for their patients,” he said. “To have the doctors now working for the insurance industry conflicts with a physician’s duty to put the patient first.”
Dr. McNamara, chair of emergency medicine at Temple University’s Katz School of Medicine, said in an interview that more than half the states in the United States have laws or court rulings that support protecting physician autonomy from corporate interests. Still, he hopes a federal prohibition on private equity’s involvement in healthcare can soon gain traction. In November, Take Medicine Back raised a resolution at the American Medical Association’s interim House of Delegates meeting, which he said was subsequently referred to a committee.
Emergency medicine was among the first specialties to succumb to private equity firms, but Dr. McNamara said that all types of health care providers and entities — from cardiology and urology to addiction treatment centers and nursing homes — are being swallowed up by larger organizations, including payers.
UHC was named in a class action suit recently for allegedly shirking doctors’ orders and relying on a flawed algorithm to determine the length of skilled nursing facility stays for Medicare Advantage policyholders.
At the investor meeting, Dr. Desai reiterated Optum’s desire to continue expanding care delivery options, especially in its pharmacy and behavioral health business lines, and focus on adopting value-based care. He credited the rapid growth to developing strong relationships with providers and standardizing technology and clinical systems.
A version of this article appeared on Medscape.com.
as more payers and private equity firms pursue medical practice acquisitions.
The company added 20,000 physicians in the last year alone, including a previously physician-owned multispecialty group practice of 400 doctors in New York. They join the growing web of doctors — about 90,000 of the 950,000 active US physicians — working for the UnitedHealth Group subsidiary, Optum Health, providing primary, specialty, urgent, and surgical care. Amar Desai, MD, chief executive officer of Optum Health, shared the updated workforce numbers during the health care conglomerate’s annual investor conference.
Health care mergers and consolidations have become more common as physician groups struggle to stay afloat amid dwindling payer reimbursements. Although private equity and health systems often acquire practices, payers like UHC are increasingly doing so as part of their model to advance value-based care.
Yashaswini Singh, PhD, health care economist and assistant professor of health services, policy, and practice at Brown University, says such moves mirror the broader trend in corporate consolidation of physician practices. She said in an interview that the integrated models could possibly enhance care coordination and improve outcomes, but the impact of payer-led consolidation has not been extensively studied.
Meanwhile, evidence considering private equity ownership is just emerging. In a 2022 study published in JAMA Health Forum, with Dr. Singh as lead author, findings showed that private equity involvement increased healthcare spending through higher prices and utilization.
Consolidation can also raise anti-trust concerns. “If payers incentivize referral patterns of their employed physicians to favor other physicians employed by the payer, it can reduce competition by restricting consumer choice,” said Dr. Singh.
A potential merger between Cigna and Humana that could happen by the end of the year will likely face intense scrutiny as it would create a company that rivals the size of UnitedHealth Group or CVS Health. If it goes through, the duo could streamline its insurance offerings and leverage each other’s care delivery platforms, clinics, and provider workforce.
The Biden Administration has sought to strengthen anti-trust statutes to prevent industry monopolies and consumer harm, and the US Department of Justice and Federal Trade Commission have proposed new merger guidelines that have yet to be finalized.
According to Dr. Singh, some of Optum’s medical practice purchases may bypass anti-trust statutes since most prospective mergers and acquisitions are reviewed only if they exceed a specific value ($101 million for 2023). Limited transparency in ownership structures further complicates matters. Plus, Dr. Singh said instances where physicians are hired instead of acquired through mergers would not be subject to current anti-trust laws.
The ‘corporatization’ of health care is not good for patients or physicians, said Robert McNamara, MD, chief medical officer of the American Academy of Emergency Medicine Physician Group and cofounder of Take Medicine Back, a physician group advocating to remove corporate interests from health care.
“If you ask a physician what causes them the most moral conflict, they’ll tell you it’s the insurance companies denying something they want to do for their patients,” he said. “To have the doctors now working for the insurance industry conflicts with a physician’s duty to put the patient first.”
Dr. McNamara, chair of emergency medicine at Temple University’s Katz School of Medicine, said in an interview that more than half the states in the United States have laws or court rulings that support protecting physician autonomy from corporate interests. Still, he hopes a federal prohibition on private equity’s involvement in healthcare can soon gain traction. In November, Take Medicine Back raised a resolution at the American Medical Association’s interim House of Delegates meeting, which he said was subsequently referred to a committee.
Emergency medicine was among the first specialties to succumb to private equity firms, but Dr. McNamara said that all types of health care providers and entities — from cardiology and urology to addiction treatment centers and nursing homes — are being swallowed up by larger organizations, including payers.
UHC was named in a class action suit recently for allegedly shirking doctors’ orders and relying on a flawed algorithm to determine the length of skilled nursing facility stays for Medicare Advantage policyholders.
At the investor meeting, Dr. Desai reiterated Optum’s desire to continue expanding care delivery options, especially in its pharmacy and behavioral health business lines, and focus on adopting value-based care. He credited the rapid growth to developing strong relationships with providers and standardizing technology and clinical systems.
A version of this article appeared on Medscape.com.
as more payers and private equity firms pursue medical practice acquisitions.
The company added 20,000 physicians in the last year alone, including a previously physician-owned multispecialty group practice of 400 doctors in New York. They join the growing web of doctors — about 90,000 of the 950,000 active US physicians — working for the UnitedHealth Group subsidiary, Optum Health, providing primary, specialty, urgent, and surgical care. Amar Desai, MD, chief executive officer of Optum Health, shared the updated workforce numbers during the health care conglomerate’s annual investor conference.
Health care mergers and consolidations have become more common as physician groups struggle to stay afloat amid dwindling payer reimbursements. Although private equity and health systems often acquire practices, payers like UHC are increasingly doing so as part of their model to advance value-based care.
Yashaswini Singh, PhD, health care economist and assistant professor of health services, policy, and practice at Brown University, says such moves mirror the broader trend in corporate consolidation of physician practices. She said in an interview that the integrated models could possibly enhance care coordination and improve outcomes, but the impact of payer-led consolidation has not been extensively studied.
Meanwhile, evidence considering private equity ownership is just emerging. In a 2022 study published in JAMA Health Forum, with Dr. Singh as lead author, findings showed that private equity involvement increased healthcare spending through higher prices and utilization.
Consolidation can also raise anti-trust concerns. “If payers incentivize referral patterns of their employed physicians to favor other physicians employed by the payer, it can reduce competition by restricting consumer choice,” said Dr. Singh.
A potential merger between Cigna and Humana that could happen by the end of the year will likely face intense scrutiny as it would create a company that rivals the size of UnitedHealth Group or CVS Health. If it goes through, the duo could streamline its insurance offerings and leverage each other’s care delivery platforms, clinics, and provider workforce.
The Biden Administration has sought to strengthen anti-trust statutes to prevent industry monopolies and consumer harm, and the US Department of Justice and Federal Trade Commission have proposed new merger guidelines that have yet to be finalized.
According to Dr. Singh, some of Optum’s medical practice purchases may bypass anti-trust statutes since most prospective mergers and acquisitions are reviewed only if they exceed a specific value ($101 million for 2023). Limited transparency in ownership structures further complicates matters. Plus, Dr. Singh said instances where physicians are hired instead of acquired through mergers would not be subject to current anti-trust laws.
The ‘corporatization’ of health care is not good for patients or physicians, said Robert McNamara, MD, chief medical officer of the American Academy of Emergency Medicine Physician Group and cofounder of Take Medicine Back, a physician group advocating to remove corporate interests from health care.
“If you ask a physician what causes them the most moral conflict, they’ll tell you it’s the insurance companies denying something they want to do for their patients,” he said. “To have the doctors now working for the insurance industry conflicts with a physician’s duty to put the patient first.”
Dr. McNamara, chair of emergency medicine at Temple University’s Katz School of Medicine, said in an interview that more than half the states in the United States have laws or court rulings that support protecting physician autonomy from corporate interests. Still, he hopes a federal prohibition on private equity’s involvement in healthcare can soon gain traction. In November, Take Medicine Back raised a resolution at the American Medical Association’s interim House of Delegates meeting, which he said was subsequently referred to a committee.
Emergency medicine was among the first specialties to succumb to private equity firms, but Dr. McNamara said that all types of health care providers and entities — from cardiology and urology to addiction treatment centers and nursing homes — are being swallowed up by larger organizations, including payers.
UHC was named in a class action suit recently for allegedly shirking doctors’ orders and relying on a flawed algorithm to determine the length of skilled nursing facility stays for Medicare Advantage policyholders.
At the investor meeting, Dr. Desai reiterated Optum’s desire to continue expanding care delivery options, especially in its pharmacy and behavioral health business lines, and focus on adopting value-based care. He credited the rapid growth to developing strong relationships with providers and standardizing technology and clinical systems.
A version of this article appeared on Medscape.com.
Pilot study educates barbers about pseudofolliculitis barbae
A .
The results were published in a research letter in JAMA Dermatology. “Educating barbers on dermatologic conditions that disproportionately affect Black males and establishing referral services between barbers and dermatologists could serve as plausible interventions,” the authors wrote.
PFB — or “razor bumps” in layman’s terms — is a chronic, inflammatory follicular disorder, which can occur in any racial group, but primarily affects Black men, noted the corresponding author of the study, Xavier Rice, MD, a dermatology resident at Washington University in Saint Louis, Missouri. PFB manifests as bumps and pustules or nodules along the beard line and are painful, he said in an interview. “They tend to leave scars once they resolve,” and impair the ability to shave, he noted.
In some communities, Black men may see their barbers more often than primary care doctors or dermatologists, “so if you equip the barbers with the knowledge to recognize the disease, make recommendations on how to prevent and to treat, and also form some allyship with barbers and dermatologists, then we can get referrals for people, especially the ones with severe disease,” he said. A lot of the barbers in the study said that “they didn’t receive much education on how to properly address it [PFB] and they had a lot of miseducation about what actually caused it,” added Dr. Rice, who was a medical student at the University of Texas Medical Branch, Galveston, when the study was conducted.
Study involved 40 barbers
For the study, Dr. Rice and his coauthors surveyed 40 barbers in the Houston, Texas, area; 39 were Black and one was Hispanic; 75% were men and 25% were women. Most (90%) said that at least 60% of their clients were Black. Between January and April 2022, the barbers received questionnaires before and after participating in a session that involved a review of a comprehensive educational brochure with information on the recognition, cause, prevention, and treatment of PFB, which they then kept for reference and to provide to clients as needed. “Common myths and nuanced home remedies from barber experience were also addressed,” the authors wrote.
No more than 2 weeks after the information session, each barber completed a posttest questionnaire.
Based on their responses to pretest questions, 39 of the 40 barbers understood that Black men were the group most impacted by PFB and that a person with severe PFB should see a physician. In the pretest survey, 12 barbers (30%) correctly recognized a photo of PFB, which increased to 39 (97.5%) in the posttest survey. In the pretest survey, two barbers (5%) identified laser hair removal as the most effective treatment for PFB, compared with 37 (92.5%) in the posttest survey.
Overall, the mean percentage of correct scores out of 20 questions was 54.8% in the pretest survey, increasing to 91% in the posttest survey (P <.001).
Limitations of the studies included heterogeneity in the survey response options that potentially could have introduced bias, the authors wrote. Another was that since there is a lack of evidence for ideal treatment strategies for PFB, there may have been some uncertainty among the correct answers for the survey that might have contributed to variability in responses. “Further research and implementation of these interventions are needed in efforts to improve health outcomes,” they added.
“Barbers can serve as allies in referral services,” Dr. Rice said in the interview. “They can be the first line for a number of diseases that are related to hair.”
Part of his role as a dermatologist, he added, includes going into a community with “boots on the ground” and talking to people who will see these patients “because access to care, presentation to big hospital systems can be challenging.”
Dr. Rice and the other study authors had no not report any financial disclosures.
A .
The results were published in a research letter in JAMA Dermatology. “Educating barbers on dermatologic conditions that disproportionately affect Black males and establishing referral services between barbers and dermatologists could serve as plausible interventions,” the authors wrote.
PFB — or “razor bumps” in layman’s terms — is a chronic, inflammatory follicular disorder, which can occur in any racial group, but primarily affects Black men, noted the corresponding author of the study, Xavier Rice, MD, a dermatology resident at Washington University in Saint Louis, Missouri. PFB manifests as bumps and pustules or nodules along the beard line and are painful, he said in an interview. “They tend to leave scars once they resolve,” and impair the ability to shave, he noted.
In some communities, Black men may see their barbers more often than primary care doctors or dermatologists, “so if you equip the barbers with the knowledge to recognize the disease, make recommendations on how to prevent and to treat, and also form some allyship with barbers and dermatologists, then we can get referrals for people, especially the ones with severe disease,” he said. A lot of the barbers in the study said that “they didn’t receive much education on how to properly address it [PFB] and they had a lot of miseducation about what actually caused it,” added Dr. Rice, who was a medical student at the University of Texas Medical Branch, Galveston, when the study was conducted.
Study involved 40 barbers
For the study, Dr. Rice and his coauthors surveyed 40 barbers in the Houston, Texas, area; 39 were Black and one was Hispanic; 75% were men and 25% were women. Most (90%) said that at least 60% of their clients were Black. Between January and April 2022, the barbers received questionnaires before and after participating in a session that involved a review of a comprehensive educational brochure with information on the recognition, cause, prevention, and treatment of PFB, which they then kept for reference and to provide to clients as needed. “Common myths and nuanced home remedies from barber experience were also addressed,” the authors wrote.
No more than 2 weeks after the information session, each barber completed a posttest questionnaire.
Based on their responses to pretest questions, 39 of the 40 barbers understood that Black men were the group most impacted by PFB and that a person with severe PFB should see a physician. In the pretest survey, 12 barbers (30%) correctly recognized a photo of PFB, which increased to 39 (97.5%) in the posttest survey. In the pretest survey, two barbers (5%) identified laser hair removal as the most effective treatment for PFB, compared with 37 (92.5%) in the posttest survey.
Overall, the mean percentage of correct scores out of 20 questions was 54.8% in the pretest survey, increasing to 91% in the posttest survey (P <.001).
Limitations of the studies included heterogeneity in the survey response options that potentially could have introduced bias, the authors wrote. Another was that since there is a lack of evidence for ideal treatment strategies for PFB, there may have been some uncertainty among the correct answers for the survey that might have contributed to variability in responses. “Further research and implementation of these interventions are needed in efforts to improve health outcomes,” they added.
“Barbers can serve as allies in referral services,” Dr. Rice said in the interview. “They can be the first line for a number of diseases that are related to hair.”
Part of his role as a dermatologist, he added, includes going into a community with “boots on the ground” and talking to people who will see these patients “because access to care, presentation to big hospital systems can be challenging.”
Dr. Rice and the other study authors had no not report any financial disclosures.
A .
The results were published in a research letter in JAMA Dermatology. “Educating barbers on dermatologic conditions that disproportionately affect Black males and establishing referral services between barbers and dermatologists could serve as plausible interventions,” the authors wrote.
PFB — or “razor bumps” in layman’s terms — is a chronic, inflammatory follicular disorder, which can occur in any racial group, but primarily affects Black men, noted the corresponding author of the study, Xavier Rice, MD, a dermatology resident at Washington University in Saint Louis, Missouri. PFB manifests as bumps and pustules or nodules along the beard line and are painful, he said in an interview. “They tend to leave scars once they resolve,” and impair the ability to shave, he noted.
In some communities, Black men may see their barbers more often than primary care doctors or dermatologists, “so if you equip the barbers with the knowledge to recognize the disease, make recommendations on how to prevent and to treat, and also form some allyship with barbers and dermatologists, then we can get referrals for people, especially the ones with severe disease,” he said. A lot of the barbers in the study said that “they didn’t receive much education on how to properly address it [PFB] and they had a lot of miseducation about what actually caused it,” added Dr. Rice, who was a medical student at the University of Texas Medical Branch, Galveston, when the study was conducted.
Study involved 40 barbers
For the study, Dr. Rice and his coauthors surveyed 40 barbers in the Houston, Texas, area; 39 were Black and one was Hispanic; 75% were men and 25% were women. Most (90%) said that at least 60% of their clients were Black. Between January and April 2022, the barbers received questionnaires before and after participating in a session that involved a review of a comprehensive educational brochure with information on the recognition, cause, prevention, and treatment of PFB, which they then kept for reference and to provide to clients as needed. “Common myths and nuanced home remedies from barber experience were also addressed,” the authors wrote.
No more than 2 weeks after the information session, each barber completed a posttest questionnaire.
Based on their responses to pretest questions, 39 of the 40 barbers understood that Black men were the group most impacted by PFB and that a person with severe PFB should see a physician. In the pretest survey, 12 barbers (30%) correctly recognized a photo of PFB, which increased to 39 (97.5%) in the posttest survey. In the pretest survey, two barbers (5%) identified laser hair removal as the most effective treatment for PFB, compared with 37 (92.5%) in the posttest survey.
Overall, the mean percentage of correct scores out of 20 questions was 54.8% in the pretest survey, increasing to 91% in the posttest survey (P <.001).
Limitations of the studies included heterogeneity in the survey response options that potentially could have introduced bias, the authors wrote. Another was that since there is a lack of evidence for ideal treatment strategies for PFB, there may have been some uncertainty among the correct answers for the survey that might have contributed to variability in responses. “Further research and implementation of these interventions are needed in efforts to improve health outcomes,” they added.
“Barbers can serve as allies in referral services,” Dr. Rice said in the interview. “They can be the first line for a number of diseases that are related to hair.”
Part of his role as a dermatologist, he added, includes going into a community with “boots on the ground” and talking to people who will see these patients “because access to care, presentation to big hospital systems can be challenging.”
Dr. Rice and the other study authors had no not report any financial disclosures.
FROM JAMA DERMATOLOGY
GVHD raises vitiligo risk in transplant recipients
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
FROM JAMA DERMATOLOGY
Adequate disease control elusive for many patients on systemic AD therapies, study finds
,” the study’s lead author, Jonathan I. Silverberg, MD, PhD, MPH, reported.
The findings come from an analysis of real-world outcomes from the TARGET-DERM AD registry, which Dr. Silverberg, professor and director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. He characterized the findings as “patients just getting stuck with a therapy and not advancing when they need to.”
TARGET-DERM AD is a longitudinal observational study of people with AD at 39 academic centers in the United States and Canada. Dr. Silverberg and his coinvestigators evaluated the proportion of patients who were experiencing an inadequate response after receiving systemic therapy and continuing on the same treatment for 3-12 months. “These are patients who are receiving their first or advanced systemic therapy, and the question is, how long did they stay on it, even if they’re not doing so well?” Dr. Silverberg said.
The researchers identified and compared the proportions of patients not achieving moderate or optimal clinician-reported outcome targets on patients with AD treated with their first systemic therapy. Advanced systemic therapy (AST) included abrocitinib, dupilumab, tralokinumab, or upadacitinib, while conventional systemic therapy (CST) included methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and systemic corticosteroids.
Patients in TARGET-DERM AD were treated and maintained on their first systemic therapy (either advanced or conventional) for up to 12 months. They had a validated Investigator’s Global Assessment of AD (vIGA-AD) score of 3 or 4 less than 45 days prior to initiation of systemic therapy or up to 14 days afterward. They also had at least one vIGA-AD assessment 3-12 months after initiating treatment. Outcome measures included IGA (defined as a score of 2 or less, with an optional target of 0 or 1), BSA (defined as a 50% BSA improvement, with an optimal target BSA of 2% or less), and the Worst-Itch Numeric Rating Scale (defined as at least a 4-point reduction, with an optimal target of 1 or less).
The analysis included 445 patients with a mean age of 31 years at enrollment. More than half (62%) were female and 45% were non-Hispanic Whites. Most patients (92%) were on an AST, mainly dupilumab, with smaller proportions treated with either tralokinumab, upadacitinib, or abrocitinib. Fewer than 10% of patients in the registry were being treated with CSTs.
At 6 months, 37% and approximately 67% of the AST-treated patients had inadequate responses in terms of skin clearance and itch outcomes, respectively. At 12 months, these figures were about 30% and 66%, respectively. CST-treated patients showed a similar trend. For patients starting an AST on or after Sept. 21, 2021, when three additional AST options were available (tralokinumab, upadacitinib, and abrocitinib), the proportion of patients demonstrating an adequate response over 12 months was generally similar to the overall cohort of those on ASTs.
“These findings suggest a need for alternative therapies and management strategies in AD treatment,” concluded Dr. Silverberg, who chaired the RAD symposium.
Dr. Silverberg reported being a consultant and/or an adviser for many pharmaceutical companies, and has received grant or research support from Galderma and Pfizer. The TARGET-DERM study is sponsored by Target PharmaSolutions.
,” the study’s lead author, Jonathan I. Silverberg, MD, PhD, MPH, reported.
The findings come from an analysis of real-world outcomes from the TARGET-DERM AD registry, which Dr. Silverberg, professor and director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. He characterized the findings as “patients just getting stuck with a therapy and not advancing when they need to.”
TARGET-DERM AD is a longitudinal observational study of people with AD at 39 academic centers in the United States and Canada. Dr. Silverberg and his coinvestigators evaluated the proportion of patients who were experiencing an inadequate response after receiving systemic therapy and continuing on the same treatment for 3-12 months. “These are patients who are receiving their first or advanced systemic therapy, and the question is, how long did they stay on it, even if they’re not doing so well?” Dr. Silverberg said.
The researchers identified and compared the proportions of patients not achieving moderate or optimal clinician-reported outcome targets on patients with AD treated with their first systemic therapy. Advanced systemic therapy (AST) included abrocitinib, dupilumab, tralokinumab, or upadacitinib, while conventional systemic therapy (CST) included methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and systemic corticosteroids.
Patients in TARGET-DERM AD were treated and maintained on their first systemic therapy (either advanced or conventional) for up to 12 months. They had a validated Investigator’s Global Assessment of AD (vIGA-AD) score of 3 or 4 less than 45 days prior to initiation of systemic therapy or up to 14 days afterward. They also had at least one vIGA-AD assessment 3-12 months after initiating treatment. Outcome measures included IGA (defined as a score of 2 or less, with an optional target of 0 or 1), BSA (defined as a 50% BSA improvement, with an optimal target BSA of 2% or less), and the Worst-Itch Numeric Rating Scale (defined as at least a 4-point reduction, with an optimal target of 1 or less).
The analysis included 445 patients with a mean age of 31 years at enrollment. More than half (62%) were female and 45% were non-Hispanic Whites. Most patients (92%) were on an AST, mainly dupilumab, with smaller proportions treated with either tralokinumab, upadacitinib, or abrocitinib. Fewer than 10% of patients in the registry were being treated with CSTs.
At 6 months, 37% and approximately 67% of the AST-treated patients had inadequate responses in terms of skin clearance and itch outcomes, respectively. At 12 months, these figures were about 30% and 66%, respectively. CST-treated patients showed a similar trend. For patients starting an AST on or after Sept. 21, 2021, when three additional AST options were available (tralokinumab, upadacitinib, and abrocitinib), the proportion of patients demonstrating an adequate response over 12 months was generally similar to the overall cohort of those on ASTs.
“These findings suggest a need for alternative therapies and management strategies in AD treatment,” concluded Dr. Silverberg, who chaired the RAD symposium.
Dr. Silverberg reported being a consultant and/or an adviser for many pharmaceutical companies, and has received grant or research support from Galderma and Pfizer. The TARGET-DERM study is sponsored by Target PharmaSolutions.
,” the study’s lead author, Jonathan I. Silverberg, MD, PhD, MPH, reported.
The findings come from an analysis of real-world outcomes from the TARGET-DERM AD registry, which Dr. Silverberg, professor and director of clinical research and contact dermatitis in the department of dermatology at George Washington University, Washington, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. He characterized the findings as “patients just getting stuck with a therapy and not advancing when they need to.”
TARGET-DERM AD is a longitudinal observational study of people with AD at 39 academic centers in the United States and Canada. Dr. Silverberg and his coinvestigators evaluated the proportion of patients who were experiencing an inadequate response after receiving systemic therapy and continuing on the same treatment for 3-12 months. “These are patients who are receiving their first or advanced systemic therapy, and the question is, how long did they stay on it, even if they’re not doing so well?” Dr. Silverberg said.
The researchers identified and compared the proportions of patients not achieving moderate or optimal clinician-reported outcome targets on patients with AD treated with their first systemic therapy. Advanced systemic therapy (AST) included abrocitinib, dupilumab, tralokinumab, or upadacitinib, while conventional systemic therapy (CST) included methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and systemic corticosteroids.
Patients in TARGET-DERM AD were treated and maintained on their first systemic therapy (either advanced or conventional) for up to 12 months. They had a validated Investigator’s Global Assessment of AD (vIGA-AD) score of 3 or 4 less than 45 days prior to initiation of systemic therapy or up to 14 days afterward. They also had at least one vIGA-AD assessment 3-12 months after initiating treatment. Outcome measures included IGA (defined as a score of 2 or less, with an optional target of 0 or 1), BSA (defined as a 50% BSA improvement, with an optimal target BSA of 2% or less), and the Worst-Itch Numeric Rating Scale (defined as at least a 4-point reduction, with an optimal target of 1 or less).
The analysis included 445 patients with a mean age of 31 years at enrollment. More than half (62%) were female and 45% were non-Hispanic Whites. Most patients (92%) were on an AST, mainly dupilumab, with smaller proportions treated with either tralokinumab, upadacitinib, or abrocitinib. Fewer than 10% of patients in the registry were being treated with CSTs.
At 6 months, 37% and approximately 67% of the AST-treated patients had inadequate responses in terms of skin clearance and itch outcomes, respectively. At 12 months, these figures were about 30% and 66%, respectively. CST-treated patients showed a similar trend. For patients starting an AST on or after Sept. 21, 2021, when three additional AST options were available (tralokinumab, upadacitinib, and abrocitinib), the proportion of patients demonstrating an adequate response over 12 months was generally similar to the overall cohort of those on ASTs.
“These findings suggest a need for alternative therapies and management strategies in AD treatment,” concluded Dr. Silverberg, who chaired the RAD symposium.
Dr. Silverberg reported being a consultant and/or an adviser for many pharmaceutical companies, and has received grant or research support from Galderma and Pfizer. The TARGET-DERM study is sponsored by Target PharmaSolutions.
FROM RAD 2023
Supercharge your medical practice with ChatGPT: Here’s why you should upgrade
Artificial intelligence (AI) has already demonstrated its potential in various areas of healthcare, from early disease detection and drug discovery to genomics and personalized care. OpenAI’s ChatGPT, a large language model, is one AI tool that has been transforming practices across the globe, including mine.
Let me walk you through it.
ChatGPT is essentially an AI-fueled assistant, capable of interpreting and generating human-like text in response to user inputs. Imagine a well-informed and competent trainee working with you, ready to tackle tasks from handling patient inquiries to summarizing intricate medical literature.
Currently, ChatGPT works on the “freemium” pricing model; there is a free version built upon GPT-3.5 as well as a subscription “ChatGPT Plus” version based on GPT-4 which offers additional features such as the use of third-party plug-ins.
Now, you may ask, “Isn’t the free version enough?” The free version is indeed impressive, but upgrading to the paid version for $20 per month unlocks the full potential of this tool, particularly if we add plug-ins.
Here are some of the best ways to incorporate ChatGPT Plus into your practice.
Time saver and efficiency multiplier. The paid version of ChatGPT is an extraordinary time-saving tool. It can help you sort through vast amounts of medical literature in a fraction of the time it would normally take. Imagine having to sift through hundreds of articles to find the latest research relevant to a patient’s case. With the paid version of ChatGPT, you can simply ask it to provide summaries of the most recent and relevant studies, all in seconds.
Did you forget about that PowerPoint you need to make but know the potential papers you would use? No problem. ChatGPT can create slides in a few minutes. It becomes your on-demand research assistant.
Of course, you need to provide the source you find most relevant to you. Using plug-ins such as ScholarAI and Link Reader are great.
Improved patient communication. Explaining complex medical terminology and procedures to patients can sometimes be a challenge. ChatGPT can generate simplified and personalized explanations for your patients, fostering their understanding and involvement in their care process.
Epic is currently collaborating with Nuance Communications, Microsoft’s speech recognition subsidiary, to use generative AI tools for medical note-taking in the electronic health record. However, you do not need to wait for it; it just takes a prompt in ChatGPT and then copying/pasting the results into the chart.
Smoother administrative management. The premium version of ChatGPT can automate administrative tasks such as creating letters of medical necessity, clearance to other physicians for services, or even communications to staff on specific topics. This frees you to focus more on your core work: providing patient care.
Precision medicine aid. ChatGPT can be a powerful ally in the field of precision medicine. Its capabilities for analyzing large datasets and unearthing valuable insights can help deliver more personalized and potentially effective treatment plans. For example, one can prompt ChatGPT to query the reported frequency of certain genomic variants and their implications; with the upgraded version and plug-ins, the results will have fewer hallucinations — inaccurate results — and key data references.
Unlimited accessibility. Uninterrupted access is a compelling reason to upgrade. While the free version may have usage limitations, the premium version provides unrestricted, round-the-clock access. Be it a late-night research quest or an early-morning patient query, your AI assistant will always be available.
Strengthened privacy and security. The premium version of ChatGPT includes heightened privacy and security measures. Just make sure to follow HIPAA and not include identifiers when making queries.
Embracing AI tools like ChatGPT in your practice can help you stay at the cutting edge of medical care, saving you time, enhancing patient communication, and supporting you in providing personalized care.
While the free version can serve as a good starting point (there are apps for both iOS and Android), upgrading to the paid version opens up a world of possibilities that can truly supercharge your practice.
I would love to hear your comments on this column or on future topics. Contact me at Arturo.AI.MedTech@gmail.com.
Arturo Loaiza-Bonilla, MD, MSEd, is the cofounder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr. Loaiza-Bonilla is Assistant Professor of Medicine, Drexel University School of Medicine, Philadelphia, Pennsylvania, and serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has financial relationships with Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, Guardant, Amgen, Eisai, Natera, Merck, and Bristol Myers Squibb.
A version of this article appeared on Medscape.com.
Artificial intelligence (AI) has already demonstrated its potential in various areas of healthcare, from early disease detection and drug discovery to genomics and personalized care. OpenAI’s ChatGPT, a large language model, is one AI tool that has been transforming practices across the globe, including mine.
Let me walk you through it.
ChatGPT is essentially an AI-fueled assistant, capable of interpreting and generating human-like text in response to user inputs. Imagine a well-informed and competent trainee working with you, ready to tackle tasks from handling patient inquiries to summarizing intricate medical literature.
Currently, ChatGPT works on the “freemium” pricing model; there is a free version built upon GPT-3.5 as well as a subscription “ChatGPT Plus” version based on GPT-4 which offers additional features such as the use of third-party plug-ins.
Now, you may ask, “Isn’t the free version enough?” The free version is indeed impressive, but upgrading to the paid version for $20 per month unlocks the full potential of this tool, particularly if we add plug-ins.
Here are some of the best ways to incorporate ChatGPT Plus into your practice.
Time saver and efficiency multiplier. The paid version of ChatGPT is an extraordinary time-saving tool. It can help you sort through vast amounts of medical literature in a fraction of the time it would normally take. Imagine having to sift through hundreds of articles to find the latest research relevant to a patient’s case. With the paid version of ChatGPT, you can simply ask it to provide summaries of the most recent and relevant studies, all in seconds.
Did you forget about that PowerPoint you need to make but know the potential papers you would use? No problem. ChatGPT can create slides in a few minutes. It becomes your on-demand research assistant.
Of course, you need to provide the source you find most relevant to you. Using plug-ins such as ScholarAI and Link Reader are great.
Improved patient communication. Explaining complex medical terminology and procedures to patients can sometimes be a challenge. ChatGPT can generate simplified and personalized explanations for your patients, fostering their understanding and involvement in their care process.
Epic is currently collaborating with Nuance Communications, Microsoft’s speech recognition subsidiary, to use generative AI tools for medical note-taking in the electronic health record. However, you do not need to wait for it; it just takes a prompt in ChatGPT and then copying/pasting the results into the chart.
Smoother administrative management. The premium version of ChatGPT can automate administrative tasks such as creating letters of medical necessity, clearance to other physicians for services, or even communications to staff on specific topics. This frees you to focus more on your core work: providing patient care.
Precision medicine aid. ChatGPT can be a powerful ally in the field of precision medicine. Its capabilities for analyzing large datasets and unearthing valuable insights can help deliver more personalized and potentially effective treatment plans. For example, one can prompt ChatGPT to query the reported frequency of certain genomic variants and their implications; with the upgraded version and plug-ins, the results will have fewer hallucinations — inaccurate results — and key data references.
Unlimited accessibility. Uninterrupted access is a compelling reason to upgrade. While the free version may have usage limitations, the premium version provides unrestricted, round-the-clock access. Be it a late-night research quest or an early-morning patient query, your AI assistant will always be available.
Strengthened privacy and security. The premium version of ChatGPT includes heightened privacy and security measures. Just make sure to follow HIPAA and not include identifiers when making queries.
Embracing AI tools like ChatGPT in your practice can help you stay at the cutting edge of medical care, saving you time, enhancing patient communication, and supporting you in providing personalized care.
While the free version can serve as a good starting point (there are apps for both iOS and Android), upgrading to the paid version opens up a world of possibilities that can truly supercharge your practice.
I would love to hear your comments on this column or on future topics. Contact me at Arturo.AI.MedTech@gmail.com.
Arturo Loaiza-Bonilla, MD, MSEd, is the cofounder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr. Loaiza-Bonilla is Assistant Professor of Medicine, Drexel University School of Medicine, Philadelphia, Pennsylvania, and serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has financial relationships with Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, Guardant, Amgen, Eisai, Natera, Merck, and Bristol Myers Squibb.
A version of this article appeared on Medscape.com.
Artificial intelligence (AI) has already demonstrated its potential in various areas of healthcare, from early disease detection and drug discovery to genomics and personalized care. OpenAI’s ChatGPT, a large language model, is one AI tool that has been transforming practices across the globe, including mine.
Let me walk you through it.
ChatGPT is essentially an AI-fueled assistant, capable of interpreting and generating human-like text in response to user inputs. Imagine a well-informed and competent trainee working with you, ready to tackle tasks from handling patient inquiries to summarizing intricate medical literature.
Currently, ChatGPT works on the “freemium” pricing model; there is a free version built upon GPT-3.5 as well as a subscription “ChatGPT Plus” version based on GPT-4 which offers additional features such as the use of third-party plug-ins.
Now, you may ask, “Isn’t the free version enough?” The free version is indeed impressive, but upgrading to the paid version for $20 per month unlocks the full potential of this tool, particularly if we add plug-ins.
Here are some of the best ways to incorporate ChatGPT Plus into your practice.
Time saver and efficiency multiplier. The paid version of ChatGPT is an extraordinary time-saving tool. It can help you sort through vast amounts of medical literature in a fraction of the time it would normally take. Imagine having to sift through hundreds of articles to find the latest research relevant to a patient’s case. With the paid version of ChatGPT, you can simply ask it to provide summaries of the most recent and relevant studies, all in seconds.
Did you forget about that PowerPoint you need to make but know the potential papers you would use? No problem. ChatGPT can create slides in a few minutes. It becomes your on-demand research assistant.
Of course, you need to provide the source you find most relevant to you. Using plug-ins such as ScholarAI and Link Reader are great.
Improved patient communication. Explaining complex medical terminology and procedures to patients can sometimes be a challenge. ChatGPT can generate simplified and personalized explanations for your patients, fostering their understanding and involvement in their care process.
Epic is currently collaborating with Nuance Communications, Microsoft’s speech recognition subsidiary, to use generative AI tools for medical note-taking in the electronic health record. However, you do not need to wait for it; it just takes a prompt in ChatGPT and then copying/pasting the results into the chart.
Smoother administrative management. The premium version of ChatGPT can automate administrative tasks such as creating letters of medical necessity, clearance to other physicians for services, or even communications to staff on specific topics. This frees you to focus more on your core work: providing patient care.
Precision medicine aid. ChatGPT can be a powerful ally in the field of precision medicine. Its capabilities for analyzing large datasets and unearthing valuable insights can help deliver more personalized and potentially effective treatment plans. For example, one can prompt ChatGPT to query the reported frequency of certain genomic variants and their implications; with the upgraded version and plug-ins, the results will have fewer hallucinations — inaccurate results — and key data references.
Unlimited accessibility. Uninterrupted access is a compelling reason to upgrade. While the free version may have usage limitations, the premium version provides unrestricted, round-the-clock access. Be it a late-night research quest or an early-morning patient query, your AI assistant will always be available.
Strengthened privacy and security. The premium version of ChatGPT includes heightened privacy and security measures. Just make sure to follow HIPAA and not include identifiers when making queries.
Embracing AI tools like ChatGPT in your practice can help you stay at the cutting edge of medical care, saving you time, enhancing patient communication, and supporting you in providing personalized care.
While the free version can serve as a good starting point (there are apps for both iOS and Android), upgrading to the paid version opens up a world of possibilities that can truly supercharge your practice.
I would love to hear your comments on this column or on future topics. Contact me at Arturo.AI.MedTech@gmail.com.
Arturo Loaiza-Bonilla, MD, MSEd, is the cofounder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr. Loaiza-Bonilla is Assistant Professor of Medicine, Drexel University School of Medicine, Philadelphia, Pennsylvania, and serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has financial relationships with Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, Guardant, Amgen, Eisai, Natera, Merck, and Bristol Myers Squibb.
A version of this article appeared on Medscape.com.
Electronic Health Records — Recent Survey Results
I have been writing about electronic health records since the mid-1990s. While the basic concept has always been sound, I have always been (and continue to be) a critic of its implementation, which I have compared to the work of the Underpants Gnomes from the television show South Park.
You may recall that Phase One of the Gnomes’ grand scheme was to collect underpants, and Phase Three was to reap enormous profits. Unfortunately, they never quite figured out Phase Two.
EHR’s problems have run a similar course, ever since George W. Bush introduced the EHR Incentive Program (later renamed the Promoting Interoperability Program) in 2000. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” That was the ultimate goal — Phase Three, if you will — but nearly a quarter-century later, we are still struggling with Phase Two.
According to the results of a recent survey by this news organization, progress has been made, but issues with usability, reliability, and patient privacy remain.
surveys, respectively. But 56% of them continue to worry about harmful effects from incorrect or misdirected information as a result of inputs from multiple sources, and the rapid turnover of staff that is doing the inputting. Many doctors worry about the potential for incorrect medications and “rule out” diagnoses getting embedded in some patients’ records and undermining future care.
The lack of information sharing among different EHR systems has been the technology’s greatest unmet promise, according to the survey. A lack of interoperability was cited as the most common reason for switching EHR systems. Other reasons included difficulties in clinical documentation and extracting data for quality reporting, as well as the inability to merge inpatient and outpatient records.
A clear majority (72%) felt EHR systems are getting easier to use. The recent decrease in government mandates has freed vendors to work on improving ease of documentation and information retrieval. The incorporation of virtual assistants and other artificial intelligence–based features (as I discussed in two recent columns) have also contributed to improved overall usability. Some newer applications even allow users to build workarounds to compensate for inherent deficiencies in the system.
Physicians tended to be most praiseworthy of functions related to electronic prescribing and retrieval of individual patient data. They felt that much more improvement was needed in helpful prompt features, internal messaging, and communications from patients.
The survey found that 38% of physicians “always” or “often” copy and paste information in patient charts, with another 37% doing so “occasionally.” Noting some of the problems inherent in copy and paste, such as note bloat, internal inconsistencies, error propagation, and documentation in the wrong patient chart, the survey authors suggest that EHR developers could help by shifting away from timelines that appear as one long note. They could also add functionality to allow new information to be displayed as updates on a digital chart.
Improvement is also needed in the way the EHR affects patient interactions, according to the survey results. Physicians are still often forced to click to a different screen to find lab results, another for current medications, and still another for past notes, all while trying to communicate with the patient. Such issues are likely to decrease in the next few years as doctors gain the ability to give voice commands to AI-based system add-ons to obtain this information.
Security concerns seem to be decreasing. In this year’s survey, nearly half of all physicians voiced no EHR privacy problems or concerns, even though a recent review of medical literature concluded that security risks remain meaningful. Those who did have privacy concerns were mostly worried about hackers and other unauthorized access to patient information.
The survey found that around 40% of EHR systems are not using patient portals to post lab results, diagnoses and procedure notes, or prescriptions. However, other physicians complained that their systems were too prompt in posting results, so that patients often received them before the doctor did. This is certainly another area where improvement at both extremes is necessary.
Other areas in which physicians saw a need for improvement were in system reliability, user training, and ongoing customer service. And among the dwindling ranks of physicians with no EHR experience, the most common reasons given for refusing to invest in an EHR system were affordability and interference with the doctor-patient relationship.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
I have been writing about electronic health records since the mid-1990s. While the basic concept has always been sound, I have always been (and continue to be) a critic of its implementation, which I have compared to the work of the Underpants Gnomes from the television show South Park.
You may recall that Phase One of the Gnomes’ grand scheme was to collect underpants, and Phase Three was to reap enormous profits. Unfortunately, they never quite figured out Phase Two.
EHR’s problems have run a similar course, ever since George W. Bush introduced the EHR Incentive Program (later renamed the Promoting Interoperability Program) in 2000. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” That was the ultimate goal — Phase Three, if you will — but nearly a quarter-century later, we are still struggling with Phase Two.
According to the results of a recent survey by this news organization, progress has been made, but issues with usability, reliability, and patient privacy remain.
surveys, respectively. But 56% of them continue to worry about harmful effects from incorrect or misdirected information as a result of inputs from multiple sources, and the rapid turnover of staff that is doing the inputting. Many doctors worry about the potential for incorrect medications and “rule out” diagnoses getting embedded in some patients’ records and undermining future care.
The lack of information sharing among different EHR systems has been the technology’s greatest unmet promise, according to the survey. A lack of interoperability was cited as the most common reason for switching EHR systems. Other reasons included difficulties in clinical documentation and extracting data for quality reporting, as well as the inability to merge inpatient and outpatient records.
A clear majority (72%) felt EHR systems are getting easier to use. The recent decrease in government mandates has freed vendors to work on improving ease of documentation and information retrieval. The incorporation of virtual assistants and other artificial intelligence–based features (as I discussed in two recent columns) have also contributed to improved overall usability. Some newer applications even allow users to build workarounds to compensate for inherent deficiencies in the system.
Physicians tended to be most praiseworthy of functions related to electronic prescribing and retrieval of individual patient data. They felt that much more improvement was needed in helpful prompt features, internal messaging, and communications from patients.
The survey found that 38% of physicians “always” or “often” copy and paste information in patient charts, with another 37% doing so “occasionally.” Noting some of the problems inherent in copy and paste, such as note bloat, internal inconsistencies, error propagation, and documentation in the wrong patient chart, the survey authors suggest that EHR developers could help by shifting away from timelines that appear as one long note. They could also add functionality to allow new information to be displayed as updates on a digital chart.
Improvement is also needed in the way the EHR affects patient interactions, according to the survey results. Physicians are still often forced to click to a different screen to find lab results, another for current medications, and still another for past notes, all while trying to communicate with the patient. Such issues are likely to decrease in the next few years as doctors gain the ability to give voice commands to AI-based system add-ons to obtain this information.
Security concerns seem to be decreasing. In this year’s survey, nearly half of all physicians voiced no EHR privacy problems or concerns, even though a recent review of medical literature concluded that security risks remain meaningful. Those who did have privacy concerns were mostly worried about hackers and other unauthorized access to patient information.
The survey found that around 40% of EHR systems are not using patient portals to post lab results, diagnoses and procedure notes, or prescriptions. However, other physicians complained that their systems were too prompt in posting results, so that patients often received them before the doctor did. This is certainly another area where improvement at both extremes is necessary.
Other areas in which physicians saw a need for improvement were in system reliability, user training, and ongoing customer service. And among the dwindling ranks of physicians with no EHR experience, the most common reasons given for refusing to invest in an EHR system were affordability and interference with the doctor-patient relationship.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
I have been writing about electronic health records since the mid-1990s. While the basic concept has always been sound, I have always been (and continue to be) a critic of its implementation, which I have compared to the work of the Underpants Gnomes from the television show South Park.
You may recall that Phase One of the Gnomes’ grand scheme was to collect underpants, and Phase Three was to reap enormous profits. Unfortunately, they never quite figured out Phase Two.
EHR’s problems have run a similar course, ever since George W. Bush introduced the EHR Incentive Program (later renamed the Promoting Interoperability Program) in 2000. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” That was the ultimate goal — Phase Three, if you will — but nearly a quarter-century later, we are still struggling with Phase Two.
According to the results of a recent survey by this news organization, progress has been made, but issues with usability, reliability, and patient privacy remain.
surveys, respectively. But 56% of them continue to worry about harmful effects from incorrect or misdirected information as a result of inputs from multiple sources, and the rapid turnover of staff that is doing the inputting. Many doctors worry about the potential for incorrect medications and “rule out” diagnoses getting embedded in some patients’ records and undermining future care.
The lack of information sharing among different EHR systems has been the technology’s greatest unmet promise, according to the survey. A lack of interoperability was cited as the most common reason for switching EHR systems. Other reasons included difficulties in clinical documentation and extracting data for quality reporting, as well as the inability to merge inpatient and outpatient records.
A clear majority (72%) felt EHR systems are getting easier to use. The recent decrease in government mandates has freed vendors to work on improving ease of documentation and information retrieval. The incorporation of virtual assistants and other artificial intelligence–based features (as I discussed in two recent columns) have also contributed to improved overall usability. Some newer applications even allow users to build workarounds to compensate for inherent deficiencies in the system.
Physicians tended to be most praiseworthy of functions related to electronic prescribing and retrieval of individual patient data. They felt that much more improvement was needed in helpful prompt features, internal messaging, and communications from patients.
The survey found that 38% of physicians “always” or “often” copy and paste information in patient charts, with another 37% doing so “occasionally.” Noting some of the problems inherent in copy and paste, such as note bloat, internal inconsistencies, error propagation, and documentation in the wrong patient chart, the survey authors suggest that EHR developers could help by shifting away from timelines that appear as one long note. They could also add functionality to allow new information to be displayed as updates on a digital chart.
Improvement is also needed in the way the EHR affects patient interactions, according to the survey results. Physicians are still often forced to click to a different screen to find lab results, another for current medications, and still another for past notes, all while trying to communicate with the patient. Such issues are likely to decrease in the next few years as doctors gain the ability to give voice commands to AI-based system add-ons to obtain this information.
Security concerns seem to be decreasing. In this year’s survey, nearly half of all physicians voiced no EHR privacy problems or concerns, even though a recent review of medical literature concluded that security risks remain meaningful. Those who did have privacy concerns were mostly worried about hackers and other unauthorized access to patient information.
The survey found that around 40% of EHR systems are not using patient portals to post lab results, diagnoses and procedure notes, or prescriptions. However, other physicians complained that their systems were too prompt in posting results, so that patients often received them before the doctor did. This is certainly another area where improvement at both extremes is necessary.
Other areas in which physicians saw a need for improvement were in system reliability, user training, and ongoing customer service. And among the dwindling ranks of physicians with no EHR experience, the most common reasons given for refusing to invest in an EHR system were affordability and interference with the doctor-patient relationship.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Acne stigma persists across social and professional settings
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
from more than 1300 individuals.

Self-stigma among people with acne has been examined in previous studies; however, “little is known about the prevalence and magnitude of stigmatizing attitudes of the general public toward individuals with acne,” wrote Ali Shields of Drexel University, Philadelphia, Pennsylvania, and her coauthors.
In the study, recently published in JAMA Dermatology, they reviewed survey data from 1357 adults aged 18 years and older who were identified through an online national research registry (ResearchMatch). The mean age of the participants was 42.4 years range). 67.7% were female.
Participants were randomly shown 1 of 12 standardized portraits of individuals that varied in skin tone, sex, and acne severity. They responded to questions about stigmatizing attitudes with respect to the portrait, including stereotype endorsement and desire for social distance.
With regard to social distance, survey participants were significantly less comfortable being friends with people with severe acne, compared with those who did not have acne (adjusted coefficient [aC], -0.28, P = .003). Compared with people without acne, participants also reported significantly less comfort in hiring someone with severe acne (aC, -0.33; P < .001), having physical contact (aC, -0.26; P = .006), dating (aC, -0.44; P = .004), and posting photos with that person on social media (aC, -0.50; P < .001).
With regard to common acne stereotypes, survey participants also rated individuals with severe acne as significantly more likely than those without acne to have poor hygiene and to be unattractive, unintelligent, unlikeable, immature, and untrustworthy (aCs, -1.04, -0.89, -0.42, -0.36, -0.52, and -0.40, respectively; P < .001 for all).
In a linear regression analysis, the researchers found no evidence of association modification by sex of the portraits presented, but found evidence that “the effect size of association of acne with stereotype endorsement was greater for individuals with dark skin.”
The findings were limited by several factors including the potential differences in degree of severity between images after the addition of acne because the baseline images were not exact controls for each other: Therefore comparisons between image sets based on skin tone or sex should be interpreted cautiously, the researchers noted. Other limitations included the homogeneous population of survey respondents and the inability to account for all aspects of stigma, they said.
However, the results illustrate the persistent stigma associated with acne and “highlight the need to identify approaches to reduce stigmatizing attitudes in the community and for adequate access to care, which might prevent negative downstream effects related to these stigmatizing attitudes,” the authors concluded.
The study was supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases to corresponding author John S. Barbieri, MD. Coauthor Arash Mostaghimi, MD, disclosed personal fees from hims & hers, AbbVie, Sun Pharmaceutical Industries, Pfizer, Digital Diagnostics, Lilly, Equillium, ASLAN Pharmaceuticals, Boehringer Ingelheim, Fig.1 Beauty, Acom Healthcare, and Olaplex outside the current study. Dr. Barbieri disclosed personal fees from Dexcel Pharma for consulting outside the current study.
FROM JAMA DERMATOLOGY