User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
These adverse events linked to improved cancer prognosis
TOPLINE:
.
METHODOLOGY:
- Emerging evidence suggests that the presence of cutaneous immune-related adverse events may be linked with favorable outcomes among patients with cancer who receive ICIs.
- Researchers conducted a systematic review and meta-analysis that included 23 studies and a total of 22,749 patients with cancer who received ICI treatment; studies compared outcomes among patients with and those without cutaneous immune-related adverse events.
- The major outcomes evaluated in the analysis were overall survival and progression-free survival (PFS); subgroup analyses assessed cutaneous immune-related adverse event type, cancer type, and other factors.
TAKEAWAY:
- The occurrence of cutaneous immune-related adverse events was associated with improved PFS (hazard ratio, 0.52; P < .001) and overall survival (HR, 0.61; P < .001).
- In the subgroup analysis, patients with eczematous (HR, 0.69), lichenoid or lichen planus–like skin lesions (HR, 0.51), pruritus without rash (HR, 0.70), psoriasis (HR, 0.63), or vitiligo (HR, 0.30) demonstrated a significant overall survival advantage. Vitiligo was the only adverse event associated with a PFS advantage (HR, 0.28).
- Among patients with melanoma, analyses revealed a significant association between the incidence of cutaneous immune-related adverse events and improved overall survival (HR, 0.51) and PFS (HR, 0.45). The authors highlighted similar findings among patients with non–small cell lung cancer (HR, 0.50 for overall survival and 0.61 for PFS).
IN PRACTICE:
“These data suggest that [cutaneous immune-related adverse events] may have useful prognostic value in ICI treatment,” the authors concluded.
SOURCE:
The analysis, led by Fei Wang, MD, Zhong Da Hospital, School of Medicine, Southeast University, Nanjing, China, was published online in JAMA Dermatology.
LIMITATIONS:
Most of the data came from retrospective studies, and there were limited data on specific patient subgroups. The Egger tests, used to assess potential publication bias in meta-analyses, revealed publication bias.
DISCLOSURES:
No disclosures were reported. The study was supported by a grant from the Postgraduate Research and Practice Innovation Program of Jiangsu Province.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Emerging evidence suggests that the presence of cutaneous immune-related adverse events may be linked with favorable outcomes among patients with cancer who receive ICIs.
- Researchers conducted a systematic review and meta-analysis that included 23 studies and a total of 22,749 patients with cancer who received ICI treatment; studies compared outcomes among patients with and those without cutaneous immune-related adverse events.
- The major outcomes evaluated in the analysis were overall survival and progression-free survival (PFS); subgroup analyses assessed cutaneous immune-related adverse event type, cancer type, and other factors.
TAKEAWAY:
- The occurrence of cutaneous immune-related adverse events was associated with improved PFS (hazard ratio, 0.52; P < .001) and overall survival (HR, 0.61; P < .001).
- In the subgroup analysis, patients with eczematous (HR, 0.69), lichenoid or lichen planus–like skin lesions (HR, 0.51), pruritus without rash (HR, 0.70), psoriasis (HR, 0.63), or vitiligo (HR, 0.30) demonstrated a significant overall survival advantage. Vitiligo was the only adverse event associated with a PFS advantage (HR, 0.28).
- Among patients with melanoma, analyses revealed a significant association between the incidence of cutaneous immune-related adverse events and improved overall survival (HR, 0.51) and PFS (HR, 0.45). The authors highlighted similar findings among patients with non–small cell lung cancer (HR, 0.50 for overall survival and 0.61 for PFS).
IN PRACTICE:
“These data suggest that [cutaneous immune-related adverse events] may have useful prognostic value in ICI treatment,” the authors concluded.
SOURCE:
The analysis, led by Fei Wang, MD, Zhong Da Hospital, School of Medicine, Southeast University, Nanjing, China, was published online in JAMA Dermatology.
LIMITATIONS:
Most of the data came from retrospective studies, and there were limited data on specific patient subgroups. The Egger tests, used to assess potential publication bias in meta-analyses, revealed publication bias.
DISCLOSURES:
No disclosures were reported. The study was supported by a grant from the Postgraduate Research and Practice Innovation Program of Jiangsu Province.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Emerging evidence suggests that the presence of cutaneous immune-related adverse events may be linked with favorable outcomes among patients with cancer who receive ICIs.
- Researchers conducted a systematic review and meta-analysis that included 23 studies and a total of 22,749 patients with cancer who received ICI treatment; studies compared outcomes among patients with and those without cutaneous immune-related adverse events.
- The major outcomes evaluated in the analysis were overall survival and progression-free survival (PFS); subgroup analyses assessed cutaneous immune-related adverse event type, cancer type, and other factors.
TAKEAWAY:
- The occurrence of cutaneous immune-related adverse events was associated with improved PFS (hazard ratio, 0.52; P < .001) and overall survival (HR, 0.61; P < .001).
- In the subgroup analysis, patients with eczematous (HR, 0.69), lichenoid or lichen planus–like skin lesions (HR, 0.51), pruritus without rash (HR, 0.70), psoriasis (HR, 0.63), or vitiligo (HR, 0.30) demonstrated a significant overall survival advantage. Vitiligo was the only adverse event associated with a PFS advantage (HR, 0.28).
- Among patients with melanoma, analyses revealed a significant association between the incidence of cutaneous immune-related adverse events and improved overall survival (HR, 0.51) and PFS (HR, 0.45). The authors highlighted similar findings among patients with non–small cell lung cancer (HR, 0.50 for overall survival and 0.61 for PFS).
IN PRACTICE:
“These data suggest that [cutaneous immune-related adverse events] may have useful prognostic value in ICI treatment,” the authors concluded.
SOURCE:
The analysis, led by Fei Wang, MD, Zhong Da Hospital, School of Medicine, Southeast University, Nanjing, China, was published online in JAMA Dermatology.
LIMITATIONS:
Most of the data came from retrospective studies, and there were limited data on specific patient subgroups. The Egger tests, used to assess potential publication bias in meta-analyses, revealed publication bias.
DISCLOSURES:
No disclosures were reported. The study was supported by a grant from the Postgraduate Research and Practice Innovation Program of Jiangsu Province.
A version of this article first appeared on Medscape.com.
Does the number of primary melanomas affect survival?
TOPLINE:
.
METHODOLOGY:
- The difference in outcomes between people with multiple primary melanomas (MPMs) and a single primary melanoma (SPM) has not been established.
- To compare 10-year melanoma-specific mortality and overall mortality between people with MPMs and SPM, researchers drew from the Melanoma Patterns of Care study, a population-based observational analysis of residents in the state of New South Wales, Australia, who had a melanoma reported to the state cancer registry over 12 months in 2006-2007, and were followed up until 2018, for a median of almost 12 years.
- The researchers performed logistic regression analyses to assess 10-year melanoma-specific mortality differences between the two groups.
TAKEAWAY:
- Of 3,404 people included in the analysis, 2,830 had an SPM and 574 developed MPMs during follow-up.
- On multivariable regression adjusted for pathologic characteristics of the thickest lesion in the MPM group, no significant differences were seen in 10-year melanoma-specific mortality between the two groups (odds ratio, 0.85; 95% confidence interval, 0.58-1.24; P = .40).
- Sensitivity analyses adjusted for parameters of the first primary melanoma among patients with MPMs revealed similar findings (OR, 1.34; 95% CI, 0.92-1.96; P = .12).
- On multivariable analysis using data from the thickest lesion, factors independently associated with melanoma-specific mortality were male sex, disadvantaged socioeconomic status (based on location of residence), and Breslow thickness.
- Factors independently associated with 10-year overall mortality were like those seen in other studies and included sex, Breslow thickness, ulceration status, and socioeconomic disadvantage.
IN PRACTICE:
“The results of our study suggest that the number of primary melanomas is not an independent risk factor for mortality,” the researchers concluded. “In addition, the detection of melanoma at an early stage (with a thin Breslow thickness) rather than an intrinsic biologic factor remains the biggest influence on melanoma mortality after diagnosis of one or more melanomas.”
SOURCE:
Corresponding author Serigne N. Lo, PhD, of the Melanoma Institute Australia, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
No adjustments for treatment modality were made in the study, and at baseline survey, effective systemic treatments for melanoma were not available.
DISCLOSURES:
This study was supported by the Australian National Health and Medical Research Council, Cancer Institute New South Wales, and the New South Wale State Government via a grant to the New South Wales Melanoma Network. Additional support was provided by Melanoma Institute Australia and the New South Wales Melanoma Network.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- The difference in outcomes between people with multiple primary melanomas (MPMs) and a single primary melanoma (SPM) has not been established.
- To compare 10-year melanoma-specific mortality and overall mortality between people with MPMs and SPM, researchers drew from the Melanoma Patterns of Care study, a population-based observational analysis of residents in the state of New South Wales, Australia, who had a melanoma reported to the state cancer registry over 12 months in 2006-2007, and were followed up until 2018, for a median of almost 12 years.
- The researchers performed logistic regression analyses to assess 10-year melanoma-specific mortality differences between the two groups.
TAKEAWAY:
- Of 3,404 people included in the analysis, 2,830 had an SPM and 574 developed MPMs during follow-up.
- On multivariable regression adjusted for pathologic characteristics of the thickest lesion in the MPM group, no significant differences were seen in 10-year melanoma-specific mortality between the two groups (odds ratio, 0.85; 95% confidence interval, 0.58-1.24; P = .40).
- Sensitivity analyses adjusted for parameters of the first primary melanoma among patients with MPMs revealed similar findings (OR, 1.34; 95% CI, 0.92-1.96; P = .12).
- On multivariable analysis using data from the thickest lesion, factors independently associated with melanoma-specific mortality were male sex, disadvantaged socioeconomic status (based on location of residence), and Breslow thickness.
- Factors independently associated with 10-year overall mortality were like those seen in other studies and included sex, Breslow thickness, ulceration status, and socioeconomic disadvantage.
IN PRACTICE:
“The results of our study suggest that the number of primary melanomas is not an independent risk factor for mortality,” the researchers concluded. “In addition, the detection of melanoma at an early stage (with a thin Breslow thickness) rather than an intrinsic biologic factor remains the biggest influence on melanoma mortality after diagnosis of one or more melanomas.”
SOURCE:
Corresponding author Serigne N. Lo, PhD, of the Melanoma Institute Australia, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
No adjustments for treatment modality were made in the study, and at baseline survey, effective systemic treatments for melanoma were not available.
DISCLOSURES:
This study was supported by the Australian National Health and Medical Research Council, Cancer Institute New South Wales, and the New South Wale State Government via a grant to the New South Wales Melanoma Network. Additional support was provided by Melanoma Institute Australia and the New South Wales Melanoma Network.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- The difference in outcomes between people with multiple primary melanomas (MPMs) and a single primary melanoma (SPM) has not been established.
- To compare 10-year melanoma-specific mortality and overall mortality between people with MPMs and SPM, researchers drew from the Melanoma Patterns of Care study, a population-based observational analysis of residents in the state of New South Wales, Australia, who had a melanoma reported to the state cancer registry over 12 months in 2006-2007, and were followed up until 2018, for a median of almost 12 years.
- The researchers performed logistic regression analyses to assess 10-year melanoma-specific mortality differences between the two groups.
TAKEAWAY:
- Of 3,404 people included in the analysis, 2,830 had an SPM and 574 developed MPMs during follow-up.
- On multivariable regression adjusted for pathologic characteristics of the thickest lesion in the MPM group, no significant differences were seen in 10-year melanoma-specific mortality between the two groups (odds ratio, 0.85; 95% confidence interval, 0.58-1.24; P = .40).
- Sensitivity analyses adjusted for parameters of the first primary melanoma among patients with MPMs revealed similar findings (OR, 1.34; 95% CI, 0.92-1.96; P = .12).
- On multivariable analysis using data from the thickest lesion, factors independently associated with melanoma-specific mortality were male sex, disadvantaged socioeconomic status (based on location of residence), and Breslow thickness.
- Factors independently associated with 10-year overall mortality were like those seen in other studies and included sex, Breslow thickness, ulceration status, and socioeconomic disadvantage.
IN PRACTICE:
“The results of our study suggest that the number of primary melanomas is not an independent risk factor for mortality,” the researchers concluded. “In addition, the detection of melanoma at an early stage (with a thin Breslow thickness) rather than an intrinsic biologic factor remains the biggest influence on melanoma mortality after diagnosis of one or more melanomas.”
SOURCE:
Corresponding author Serigne N. Lo, PhD, of the Melanoma Institute Australia, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
No adjustments for treatment modality were made in the study, and at baseline survey, effective systemic treatments for melanoma were not available.
DISCLOSURES:
This study was supported by the Australian National Health and Medical Research Council, Cancer Institute New South Wales, and the New South Wale State Government via a grant to the New South Wales Melanoma Network. Additional support was provided by Melanoma Institute Australia and the New South Wales Melanoma Network.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
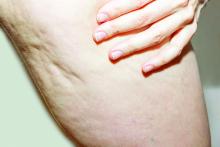
A glimpse at devices designed to tackle cellulite
SAN DIEGO –
A noninvasive treatment, rapid acoustic pulse (RAP) technology (RESONIC), was cleared by the Food and Drug Administration in 2021 for short-term improvement in the appearance of cellulite. The device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or shear the fibrotic septa; release of the septa results in the smoothing of skin dimples, Arisa E. Ortiz, MD, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. RAP, however, was “taken off the market temporarily to refine the design.”
According to Dr. Ortiz, RAP’s repetition rate and short rise time provides microscopic mechanical disruption to the targeted cellular level structures and vacuoles, while high peak pressure and the fast repetition rate exploit the viscoelastic nature of tissue. Compressed pulses from electronic filtering and the reflector shape eliminate cavitation, heat, and pain. Researchers have postulated that the procedure stimulates collagenesis and angiogenesis.
“There’s no heat to this; it just uses sound,” Dr. Ortiz explained. “It’s also time dependent. The longer you do the treatment, the more disruption of the septa you see. The procedure takes 20-30 minutes. Unlike other treatments, it’s not just for discreet dimples. You can treat entire areas like the buttock or posterior leg,” said Dr. Ortiz, who did not use the RAP device in her practice.
Another device, targeted verifiable subcision (TVS, marketed as Avéli), is FDA cleared for temporary reduction in the appearance of cellulite in the buttock and thigh areas of adult women. “But studies show lasting results, at least through a year,” Dr. Ortiz said. The device features a light-guided probe and a hook. The light enables clinicians to navigate under the skin, while the hook releases a tiny blade that severs the septa. “Once you find the septa, then you activate the blade and release the septa. You go right to left because the direction of the blade is on the left of the probe. Then you go back to verify that you got everything that was creating that dimple.”
Previous devices, she said, would “blindly shear the area, so you would find the dimple and blindly cut, so there was no way to verify that you got the target dimple. The results were sometimes mediocre because you didn’t really know if you effectively treated the area.”
She emphasized that TVS is only useful for discreet dimples. “Many patients who come in asking for cellulite treatment have a lot of laxity and rippled texture,” said Dr. Ortiz, who is also president-elect of the American Society for Laser Medicine and Surgery. “This is not going to be appropriate for those cases. Setting expectations is important. If patients have laxity and discreet dimples and it’s just the dimples that bother them, that’s fine. They just need to understand the difference,” she said, noting that this is “safe for all skin types.”
Tumescent anesthesia is used to control pain during the procedure. The most common adverse events are bruising and soreness. Results from a pivotal trial showed that clinically significant improvements in the primary endpoint, Cellulite Severity Scale scores, were sustained 1 year after treatment. “Hemosiderin staining can occur, but it eventually dissipates on its own,” Dr. Ortiz added. “You can use laser to speed up healing but sometimes that can make it worse, so you want to be careful with that. Most of the time I have patients wait it out; it does go away on its own.”
She noted that the development of RAP and TVS have helped clinicians better understand the makeup of septa. “We used to think of septa as singular bands that are vertically oriented in cellulite, but what we’ve realized is that it’s more like a network of septa,” she said.
Another noninvasive technology, synchronous parallel ultrasound beam technology from Sofwave (marketed as SUPERB), was FDA cleared in December 2022 for the short-term improvement in the appearance of cellulite. The device has seven parallel beam transducers that increase tissue temperatures of the treatment area to 60-70° C, inducing collagen remodeling and collagen denaturation, she said.
In the pivotal study of 68 women, two blinded reviewers reported an 89% improvement rate for both cellulite and skin laxity, after two treatments 2-4 weeks apart, according to data she presented at the meeting. The mean pain score during treatment was 4.55 on a scale of 1-10. No safety issues were observed and immediate responses were limited to erythema and edema.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies, including receipt of speaker fees and honoraria from Sofwave. She is also cochair of MOAS.
SAN DIEGO –
A noninvasive treatment, rapid acoustic pulse (RAP) technology (RESONIC), was cleared by the Food and Drug Administration in 2021 for short-term improvement in the appearance of cellulite. The device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or shear the fibrotic septa; release of the septa results in the smoothing of skin dimples, Arisa E. Ortiz, MD, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. RAP, however, was “taken off the market temporarily to refine the design.”
According to Dr. Ortiz, RAP’s repetition rate and short rise time provides microscopic mechanical disruption to the targeted cellular level structures and vacuoles, while high peak pressure and the fast repetition rate exploit the viscoelastic nature of tissue. Compressed pulses from electronic filtering and the reflector shape eliminate cavitation, heat, and pain. Researchers have postulated that the procedure stimulates collagenesis and angiogenesis.
“There’s no heat to this; it just uses sound,” Dr. Ortiz explained. “It’s also time dependent. The longer you do the treatment, the more disruption of the septa you see. The procedure takes 20-30 minutes. Unlike other treatments, it’s not just for discreet dimples. You can treat entire areas like the buttock or posterior leg,” said Dr. Ortiz, who did not use the RAP device in her practice.
Another device, targeted verifiable subcision (TVS, marketed as Avéli), is FDA cleared for temporary reduction in the appearance of cellulite in the buttock and thigh areas of adult women. “But studies show lasting results, at least through a year,” Dr. Ortiz said. The device features a light-guided probe and a hook. The light enables clinicians to navigate under the skin, while the hook releases a tiny blade that severs the septa. “Once you find the septa, then you activate the blade and release the septa. You go right to left because the direction of the blade is on the left of the probe. Then you go back to verify that you got everything that was creating that dimple.”
Previous devices, she said, would “blindly shear the area, so you would find the dimple and blindly cut, so there was no way to verify that you got the target dimple. The results were sometimes mediocre because you didn’t really know if you effectively treated the area.”
She emphasized that TVS is only useful for discreet dimples. “Many patients who come in asking for cellulite treatment have a lot of laxity and rippled texture,” said Dr. Ortiz, who is also president-elect of the American Society for Laser Medicine and Surgery. “This is not going to be appropriate for those cases. Setting expectations is important. If patients have laxity and discreet dimples and it’s just the dimples that bother them, that’s fine. They just need to understand the difference,” she said, noting that this is “safe for all skin types.”
Tumescent anesthesia is used to control pain during the procedure. The most common adverse events are bruising and soreness. Results from a pivotal trial showed that clinically significant improvements in the primary endpoint, Cellulite Severity Scale scores, were sustained 1 year after treatment. “Hemosiderin staining can occur, but it eventually dissipates on its own,” Dr. Ortiz added. “You can use laser to speed up healing but sometimes that can make it worse, so you want to be careful with that. Most of the time I have patients wait it out; it does go away on its own.”
She noted that the development of RAP and TVS have helped clinicians better understand the makeup of septa. “We used to think of septa as singular bands that are vertically oriented in cellulite, but what we’ve realized is that it’s more like a network of septa,” she said.
Another noninvasive technology, synchronous parallel ultrasound beam technology from Sofwave (marketed as SUPERB), was FDA cleared in December 2022 for the short-term improvement in the appearance of cellulite. The device has seven parallel beam transducers that increase tissue temperatures of the treatment area to 60-70° C, inducing collagen remodeling and collagen denaturation, she said.
In the pivotal study of 68 women, two blinded reviewers reported an 89% improvement rate for both cellulite and skin laxity, after two treatments 2-4 weeks apart, according to data she presented at the meeting. The mean pain score during treatment was 4.55 on a scale of 1-10. No safety issues were observed and immediate responses were limited to erythema and edema.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies, including receipt of speaker fees and honoraria from Sofwave. She is also cochair of MOAS.
SAN DIEGO –
A noninvasive treatment, rapid acoustic pulse (RAP) technology (RESONIC), was cleared by the Food and Drug Administration in 2021 for short-term improvement in the appearance of cellulite. The device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or shear the fibrotic septa; release of the septa results in the smoothing of skin dimples, Arisa E. Ortiz, MD, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. RAP, however, was “taken off the market temporarily to refine the design.”
According to Dr. Ortiz, RAP’s repetition rate and short rise time provides microscopic mechanical disruption to the targeted cellular level structures and vacuoles, while high peak pressure and the fast repetition rate exploit the viscoelastic nature of tissue. Compressed pulses from electronic filtering and the reflector shape eliminate cavitation, heat, and pain. Researchers have postulated that the procedure stimulates collagenesis and angiogenesis.
“There’s no heat to this; it just uses sound,” Dr. Ortiz explained. “It’s also time dependent. The longer you do the treatment, the more disruption of the septa you see. The procedure takes 20-30 minutes. Unlike other treatments, it’s not just for discreet dimples. You can treat entire areas like the buttock or posterior leg,” said Dr. Ortiz, who did not use the RAP device in her practice.
Another device, targeted verifiable subcision (TVS, marketed as Avéli), is FDA cleared for temporary reduction in the appearance of cellulite in the buttock and thigh areas of adult women. “But studies show lasting results, at least through a year,” Dr. Ortiz said. The device features a light-guided probe and a hook. The light enables clinicians to navigate under the skin, while the hook releases a tiny blade that severs the septa. “Once you find the septa, then you activate the blade and release the septa. You go right to left because the direction of the blade is on the left of the probe. Then you go back to verify that you got everything that was creating that dimple.”
Previous devices, she said, would “blindly shear the area, so you would find the dimple and blindly cut, so there was no way to verify that you got the target dimple. The results were sometimes mediocre because you didn’t really know if you effectively treated the area.”
She emphasized that TVS is only useful for discreet dimples. “Many patients who come in asking for cellulite treatment have a lot of laxity and rippled texture,” said Dr. Ortiz, who is also president-elect of the American Society for Laser Medicine and Surgery. “This is not going to be appropriate for those cases. Setting expectations is important. If patients have laxity and discreet dimples and it’s just the dimples that bother them, that’s fine. They just need to understand the difference,” she said, noting that this is “safe for all skin types.”
Tumescent anesthesia is used to control pain during the procedure. The most common adverse events are bruising and soreness. Results from a pivotal trial showed that clinically significant improvements in the primary endpoint, Cellulite Severity Scale scores, were sustained 1 year after treatment. “Hemosiderin staining can occur, but it eventually dissipates on its own,” Dr. Ortiz added. “You can use laser to speed up healing but sometimes that can make it worse, so you want to be careful with that. Most of the time I have patients wait it out; it does go away on its own.”
She noted that the development of RAP and TVS have helped clinicians better understand the makeup of septa. “We used to think of septa as singular bands that are vertically oriented in cellulite, but what we’ve realized is that it’s more like a network of septa,” she said.
Another noninvasive technology, synchronous parallel ultrasound beam technology from Sofwave (marketed as SUPERB), was FDA cleared in December 2022 for the short-term improvement in the appearance of cellulite. The device has seven parallel beam transducers that increase tissue temperatures of the treatment area to 60-70° C, inducing collagen remodeling and collagen denaturation, she said.
In the pivotal study of 68 women, two blinded reviewers reported an 89% improvement rate for both cellulite and skin laxity, after two treatments 2-4 weeks apart, according to data she presented at the meeting. The mean pain score during treatment was 4.55 on a scale of 1-10. No safety issues were observed and immediate responses were limited to erythema and edema.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies, including receipt of speaker fees and honoraria from Sofwave. She is also cochair of MOAS.
AT MOAS 2023
Hidradenitis suppurativa experts reach consensus on treatment outcome measures
TOPLINE:
Hidradenitis suppurativa (HS) experts collaborated to reach consensus on a core set of outcome measures, with the intent of improving the management of HS in clinical practice.
METHODOLOGY:
- Participants in the study were 55 HS experts from the HiSTORIC group (dermatologists, internists, surgeons, and nurses) and 24 patient research partners.
- The group identified clinician- and patient-reported HS outcome measures in the literature, then participated in an online item reduction survey, followed by an electronic Delphi survey to reach consensus on which measures should be used in clinical practice. Consensus was defined as at least 67% of participants agreeing/strongly agreeing or disagreeing/strongly disagreeing about the use of a measure in clinical practice.
- The initial literature search yielded 11 HS studies with clinician-reported outcome measures and 12 with patient-reported outcomes; of these, eight and five, respectively, were included in the final reduction survey.
TAKEAWAY:
- The group reached consensus on two HS outcome measures for use in clinical practice: the HS Investigator Global Assessment (HS-IGA) score, a clinician-reported outcome measure selected by the HS experts, and the HS Quality of Life (HiSQOL) score, a patient-reported outcome measure selected by the patient research partners.
- The HS-IGA score uses a number between 0 and 5 based on the sum of abscesses, inflammatory and noninflammatory nodules, and tunnels in regions of the upper or lower body.
- The HiSQOL, a disease-specific quality-of-life measure for adults with HS, is designed to capture unique features of HS, including symptoms (such as pain, itch, odor, and drainage) and psychosocial outcomes and activities that may be affected by the disease.
IN PRACTICE:
“The intent of these recommendations is to provide an objective framework with both clinician and patient input that can facilitate bidirectional discussion, trust building, and decision making on the current treatment strategy and the need to adjust or escalate treatment in an appropriate time frame,” the authors wrote.
SOURCE:
The study was published online in JAMA Dermatology. The lead author was Nicole Mastacouris, MS, and the corresponding author was Amit Garg, MD, both of Northwell Health, New Hyde Park, N.Y.
LIMITATIONS:
The consensus results may have been affected by variations in HS management by region. Neither measure has been studied in clinical practice, and practice variability may limit their implementation.
DISCLOSURES:
The study was supported by grants from UCB and AbbVie. Ms. Mastacouris had no financial disclosures. Dr. Garg disclosed grant support from AbbVie and UCB during the conduct of the study, as well as personal fees from AbbVie, UCB, Aclaris Therapeutics, Anaptys Bio, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, Insmed, Janssen, Novartis, Pfizer, Sonoma Biotherapeutics, Union Therapeutics, Ventyx Biosciences, and Viela Biosciences during the conduct of the study; Dr. Garg also holds patents for HS-IGA and HiSQOL. Many other coauthors disclosed relationships with multiple companies, including AbbVie and UCB, and some also disclosed patents, including patents for HiSQOL and HS Area and Severity Index.
A version of this article first appeared on Medscape.com.
TOPLINE:
Hidradenitis suppurativa (HS) experts collaborated to reach consensus on a core set of outcome measures, with the intent of improving the management of HS in clinical practice.
METHODOLOGY:
- Participants in the study were 55 HS experts from the HiSTORIC group (dermatologists, internists, surgeons, and nurses) and 24 patient research partners.
- The group identified clinician- and patient-reported HS outcome measures in the literature, then participated in an online item reduction survey, followed by an electronic Delphi survey to reach consensus on which measures should be used in clinical practice. Consensus was defined as at least 67% of participants agreeing/strongly agreeing or disagreeing/strongly disagreeing about the use of a measure in clinical practice.
- The initial literature search yielded 11 HS studies with clinician-reported outcome measures and 12 with patient-reported outcomes; of these, eight and five, respectively, were included in the final reduction survey.
TAKEAWAY:
- The group reached consensus on two HS outcome measures for use in clinical practice: the HS Investigator Global Assessment (HS-IGA) score, a clinician-reported outcome measure selected by the HS experts, and the HS Quality of Life (HiSQOL) score, a patient-reported outcome measure selected by the patient research partners.
- The HS-IGA score uses a number between 0 and 5 based on the sum of abscesses, inflammatory and noninflammatory nodules, and tunnels in regions of the upper or lower body.
- The HiSQOL, a disease-specific quality-of-life measure for adults with HS, is designed to capture unique features of HS, including symptoms (such as pain, itch, odor, and drainage) and psychosocial outcomes and activities that may be affected by the disease.
IN PRACTICE:
“The intent of these recommendations is to provide an objective framework with both clinician and patient input that can facilitate bidirectional discussion, trust building, and decision making on the current treatment strategy and the need to adjust or escalate treatment in an appropriate time frame,” the authors wrote.
SOURCE:
The study was published online in JAMA Dermatology. The lead author was Nicole Mastacouris, MS, and the corresponding author was Amit Garg, MD, both of Northwell Health, New Hyde Park, N.Y.
LIMITATIONS:
The consensus results may have been affected by variations in HS management by region. Neither measure has been studied in clinical practice, and practice variability may limit their implementation.
DISCLOSURES:
The study was supported by grants from UCB and AbbVie. Ms. Mastacouris had no financial disclosures. Dr. Garg disclosed grant support from AbbVie and UCB during the conduct of the study, as well as personal fees from AbbVie, UCB, Aclaris Therapeutics, Anaptys Bio, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, Insmed, Janssen, Novartis, Pfizer, Sonoma Biotherapeutics, Union Therapeutics, Ventyx Biosciences, and Viela Biosciences during the conduct of the study; Dr. Garg also holds patents for HS-IGA and HiSQOL. Many other coauthors disclosed relationships with multiple companies, including AbbVie and UCB, and some also disclosed patents, including patents for HiSQOL and HS Area and Severity Index.
A version of this article first appeared on Medscape.com.
TOPLINE:
Hidradenitis suppurativa (HS) experts collaborated to reach consensus on a core set of outcome measures, with the intent of improving the management of HS in clinical practice.
METHODOLOGY:
- Participants in the study were 55 HS experts from the HiSTORIC group (dermatologists, internists, surgeons, and nurses) and 24 patient research partners.
- The group identified clinician- and patient-reported HS outcome measures in the literature, then participated in an online item reduction survey, followed by an electronic Delphi survey to reach consensus on which measures should be used in clinical practice. Consensus was defined as at least 67% of participants agreeing/strongly agreeing or disagreeing/strongly disagreeing about the use of a measure in clinical practice.
- The initial literature search yielded 11 HS studies with clinician-reported outcome measures and 12 with patient-reported outcomes; of these, eight and five, respectively, were included in the final reduction survey.
TAKEAWAY:
- The group reached consensus on two HS outcome measures for use in clinical practice: the HS Investigator Global Assessment (HS-IGA) score, a clinician-reported outcome measure selected by the HS experts, and the HS Quality of Life (HiSQOL) score, a patient-reported outcome measure selected by the patient research partners.
- The HS-IGA score uses a number between 0 and 5 based on the sum of abscesses, inflammatory and noninflammatory nodules, and tunnels in regions of the upper or lower body.
- The HiSQOL, a disease-specific quality-of-life measure for adults with HS, is designed to capture unique features of HS, including symptoms (such as pain, itch, odor, and drainage) and psychosocial outcomes and activities that may be affected by the disease.
IN PRACTICE:
“The intent of these recommendations is to provide an objective framework with both clinician and patient input that can facilitate bidirectional discussion, trust building, and decision making on the current treatment strategy and the need to adjust or escalate treatment in an appropriate time frame,” the authors wrote.
SOURCE:
The study was published online in JAMA Dermatology. The lead author was Nicole Mastacouris, MS, and the corresponding author was Amit Garg, MD, both of Northwell Health, New Hyde Park, N.Y.
LIMITATIONS:
The consensus results may have been affected by variations in HS management by region. Neither measure has been studied in clinical practice, and practice variability may limit their implementation.
DISCLOSURES:
The study was supported by grants from UCB and AbbVie. Ms. Mastacouris had no financial disclosures. Dr. Garg disclosed grant support from AbbVie and UCB during the conduct of the study, as well as personal fees from AbbVie, UCB, Aclaris Therapeutics, Anaptys Bio, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, Insmed, Janssen, Novartis, Pfizer, Sonoma Biotherapeutics, Union Therapeutics, Ventyx Biosciences, and Viela Biosciences during the conduct of the study; Dr. Garg also holds patents for HS-IGA and HiSQOL. Many other coauthors disclosed relationships with multiple companies, including AbbVie and UCB, and some also disclosed patents, including patents for HiSQOL and HS Area and Severity Index.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Hidradenitis suppurativa experts reach consensus on treatment outcome measures
TOPLINE:
METHODOLOGY:
- Participants in the study were 55 HS experts from the HiSTORIC group (dermatologists, internists, surgeons, and nurses) and 24 patient research partners.
- The group identified clinician- and patient-reported HS outcome measures in the literature, then participated in an online item reduction survey, followed by an electronic Delphi survey to reach consensus on which measures should be used in clinical practice. Consensus was defined as at least 67% of participants agreeing/strongly agreeing or disagreeing/strongly disagreeing about the use of a measure in clinical practice.
- The initial literature search yielded 11 HS studies with clinician-reported outcome measures and 12 with patient-reported outcomes; of these, eight and five, respectively, were included in the final reduction survey.
TAKEAWAY:
- The group reached consensus on two HS outcome measures for use in clinical practice: the HS Investigator Global Assessment (HS-IGA) score, a clinician-reported outcome measure selected by the HS experts, and the HS Quality of Life (HiSQOL) score, a patient-reported outcome measure selected by the patient research partners.
- The HS-IGA score uses a number between 0 and 5 based on the sum of abscesses, inflammatory and noninflammatory nodules, and tunnels in regions of the upper or lower body.
- The HiSQOL, a disease-specific quality-of-life measure for adults with HS, is designed to capture unique features of HS, including symptoms (such as pain, itch, odor, and drainage) and psychosocial outcomes and activities that may be affected by the disease.
IN PRACTICE:
“The intent of these recommendations is to provide an objective framework with both clinician and patient input that can facilitate bidirectional discussion, trust building, and decision making on the current treatment strategy and the need to adjust or escalate treatment in an appropriate time frame,” the authors wrote.
SOURCE:
The study was published online in JAMA Dermatology. The lead author was Nicole Mastacouris, MS, and the corresponding author was Amit Garg, MD, both of Northwell Health, New Hyde Park, N.Y.
LIMITATIONS:
The consensus results may have been affected by variations in HS management by region. Neither measure has been studied in clinical practice, and practice variability may limit their implementation.
DISCLOSURES:
The study was supported by grants from UCB and AbbVie. Ms. Mastacouris had no financial disclosures. Dr. Garg disclosed grant support from AbbVie and UCB during the conduct of the study, as well as personal fees from AbbVie, UCB, Aclaris Therapeutics, Anaptys Bio, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, Insmed, Janssen, Novartis, Pfizer, Sonoma Biotherapeutics, Union Therapeutics, Ventyx Biosciences, and Viela Biosciences during the conduct of the study; Dr. Garg also holds patents for HS-IGA and HiSQOL. Many other coauthors disclosed relationships with multiple companies, including AbbVie and UCB, and some also disclosed patents, including patents for HiSQOL and HS Area and Severity Index.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Participants in the study were 55 HS experts from the HiSTORIC group (dermatologists, internists, surgeons, and nurses) and 24 patient research partners.
- The group identified clinician- and patient-reported HS outcome measures in the literature, then participated in an online item reduction survey, followed by an electronic Delphi survey to reach consensus on which measures should be used in clinical practice. Consensus was defined as at least 67% of participants agreeing/strongly agreeing or disagreeing/strongly disagreeing about the use of a measure in clinical practice.
- The initial literature search yielded 11 HS studies with clinician-reported outcome measures and 12 with patient-reported outcomes; of these, eight and five, respectively, were included in the final reduction survey.
TAKEAWAY:
- The group reached consensus on two HS outcome measures for use in clinical practice: the HS Investigator Global Assessment (HS-IGA) score, a clinician-reported outcome measure selected by the HS experts, and the HS Quality of Life (HiSQOL) score, a patient-reported outcome measure selected by the patient research partners.
- The HS-IGA score uses a number between 0 and 5 based on the sum of abscesses, inflammatory and noninflammatory nodules, and tunnels in regions of the upper or lower body.
- The HiSQOL, a disease-specific quality-of-life measure for adults with HS, is designed to capture unique features of HS, including symptoms (such as pain, itch, odor, and drainage) and psychosocial outcomes and activities that may be affected by the disease.
IN PRACTICE:
“The intent of these recommendations is to provide an objective framework with both clinician and patient input that can facilitate bidirectional discussion, trust building, and decision making on the current treatment strategy and the need to adjust or escalate treatment in an appropriate time frame,” the authors wrote.
SOURCE:
The study was published online in JAMA Dermatology. The lead author was Nicole Mastacouris, MS, and the corresponding author was Amit Garg, MD, both of Northwell Health, New Hyde Park, N.Y.
LIMITATIONS:
The consensus results may have been affected by variations in HS management by region. Neither measure has been studied in clinical practice, and practice variability may limit their implementation.
DISCLOSURES:
The study was supported by grants from UCB and AbbVie. Ms. Mastacouris had no financial disclosures. Dr. Garg disclosed grant support from AbbVie and UCB during the conduct of the study, as well as personal fees from AbbVie, UCB, Aclaris Therapeutics, Anaptys Bio, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, Insmed, Janssen, Novartis, Pfizer, Sonoma Biotherapeutics, Union Therapeutics, Ventyx Biosciences, and Viela Biosciences during the conduct of the study; Dr. Garg also holds patents for HS-IGA and HiSQOL. Many other coauthors disclosed relationships with multiple companies, including AbbVie and UCB, and some also disclosed patents, including patents for HiSQOL and HS Area and Severity Index.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Participants in the study were 55 HS experts from the HiSTORIC group (dermatologists, internists, surgeons, and nurses) and 24 patient research partners.
- The group identified clinician- and patient-reported HS outcome measures in the literature, then participated in an online item reduction survey, followed by an electronic Delphi survey to reach consensus on which measures should be used in clinical practice. Consensus was defined as at least 67% of participants agreeing/strongly agreeing or disagreeing/strongly disagreeing about the use of a measure in clinical practice.
- The initial literature search yielded 11 HS studies with clinician-reported outcome measures and 12 with patient-reported outcomes; of these, eight and five, respectively, were included in the final reduction survey.
TAKEAWAY:
- The group reached consensus on two HS outcome measures for use in clinical practice: the HS Investigator Global Assessment (HS-IGA) score, a clinician-reported outcome measure selected by the HS experts, and the HS Quality of Life (HiSQOL) score, a patient-reported outcome measure selected by the patient research partners.
- The HS-IGA score uses a number between 0 and 5 based on the sum of abscesses, inflammatory and noninflammatory nodules, and tunnels in regions of the upper or lower body.
- The HiSQOL, a disease-specific quality-of-life measure for adults with HS, is designed to capture unique features of HS, including symptoms (such as pain, itch, odor, and drainage) and psychosocial outcomes and activities that may be affected by the disease.
IN PRACTICE:
“The intent of these recommendations is to provide an objective framework with both clinician and patient input that can facilitate bidirectional discussion, trust building, and decision making on the current treatment strategy and the need to adjust or escalate treatment in an appropriate time frame,” the authors wrote.
SOURCE:
The study was published online in JAMA Dermatology. The lead author was Nicole Mastacouris, MS, and the corresponding author was Amit Garg, MD, both of Northwell Health, New Hyde Park, N.Y.
LIMITATIONS:
The consensus results may have been affected by variations in HS management by region. Neither measure has been studied in clinical practice, and practice variability may limit their implementation.
DISCLOSURES:
The study was supported by grants from UCB and AbbVie. Ms. Mastacouris had no financial disclosures. Dr. Garg disclosed grant support from AbbVie and UCB during the conduct of the study, as well as personal fees from AbbVie, UCB, Aclaris Therapeutics, Anaptys Bio, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, Insmed, Janssen, Novartis, Pfizer, Sonoma Biotherapeutics, Union Therapeutics, Ventyx Biosciences, and Viela Biosciences during the conduct of the study; Dr. Garg also holds patents for HS-IGA and HiSQOL. Many other coauthors disclosed relationships with multiple companies, including AbbVie and UCB, and some also disclosed patents, including patents for HiSQOL and HS Area and Severity Index.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Hidradenitis suppurativa experts reach consensus on treatment outcome measures
TOPLINE:
METHODOLOGY:
- Participants in the study were 55 HS experts from the HiSTORIC group (dermatologists, internists, surgeons, and nurses) and 24 patient research partners.
- The group identified clinician- and patient-reported HS outcome measures in the literature, then participated in an online item reduction survey, followed by an electronic Delphi survey to reach consensus on which measures should be used in clinical practice. Consensus was defined as at least 67% of participants agreeing/strongly agreeing or disagreeing/strongly disagreeing about the use of a measure in clinical practice.
- The initial literature search yielded 11 HS studies with clinician-reported outcome measures and 12 with patient-reported outcomes; of these, eight and five, respectively, were included in the final reduction survey.
TAKEAWAY:
- The group reached consensus on two HS outcome measures for use in clinical practice: the HS Investigator Global Assessment (HS-IGA) score, a clinician-reported outcome measure selected by the HS experts, and the HS Quality of Life (HiSQOL) score, a patient-reported outcome measure selected by the patient research partners.
- The HS-IGA score uses a number between 0 and 5 based on the sum of abscesses, inflammatory and noninflammatory nodules, and tunnels in regions of the upper or lower body.
- The HiSQOL, a disease-specific quality-of-life measure for adults with HS, is designed to capture unique features of HS, including symptoms (such as pain, itch, odor, and drainage) and psychosocial outcomes and activities that may be affected by the disease.
IN PRACTICE:
“The intent of these recommendations is to provide an objective framework with both clinician and patient input that can facilitate bidirectional discussion, trust building, and decision making on the current treatment strategy and the need to adjust or escalate treatment in an appropriate time frame,” the authors wrote.
SOURCE:
The study was published online in JAMA Dermatology. The lead author was Nicole Mastacouris, MS, and the corresponding author was Amit Garg, MD, both of Northwell Health, New Hyde Park, N.Y.
LIMITATIONS:
The consensus results may have been affected by variations in HS management by region. Neither measure has been studied in clinical practice, and practice variability may limit their implementation.
DISCLOSURES:
The study was supported by grants from UCB and AbbVie. Ms. Mastacouris had no financial disclosures. Dr. Garg disclosed grant support from AbbVie and UCB during the conduct of the study, as well as personal fees from AbbVie, UCB, Aclaris Therapeutics, Anaptys Bio, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, Insmed, Janssen, Novartis, Pfizer, Sonoma Biotherapeutics, Union Therapeutics, Ventyx Biosciences, and Viela Biosciences during the conduct of the study; Dr. Garg also holds patents for HS-IGA and HiSQOL. Many other coauthors disclosed relationships with multiple companies, including AbbVie and UCB, and some also disclosed patents, including patents for HiSQOL and HS Area and Severity Index.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Participants in the study were 55 HS experts from the HiSTORIC group (dermatologists, internists, surgeons, and nurses) and 24 patient research partners.
- The group identified clinician- and patient-reported HS outcome measures in the literature, then participated in an online item reduction survey, followed by an electronic Delphi survey to reach consensus on which measures should be used in clinical practice. Consensus was defined as at least 67% of participants agreeing/strongly agreeing or disagreeing/strongly disagreeing about the use of a measure in clinical practice.
- The initial literature search yielded 11 HS studies with clinician-reported outcome measures and 12 with patient-reported outcomes; of these, eight and five, respectively, were included in the final reduction survey.
TAKEAWAY:
- The group reached consensus on two HS outcome measures for use in clinical practice: the HS Investigator Global Assessment (HS-IGA) score, a clinician-reported outcome measure selected by the HS experts, and the HS Quality of Life (HiSQOL) score, a patient-reported outcome measure selected by the patient research partners.
- The HS-IGA score uses a number between 0 and 5 based on the sum of abscesses, inflammatory and noninflammatory nodules, and tunnels in regions of the upper or lower body.
- The HiSQOL, a disease-specific quality-of-life measure for adults with HS, is designed to capture unique features of HS, including symptoms (such as pain, itch, odor, and drainage) and psychosocial outcomes and activities that may be affected by the disease.
IN PRACTICE:
“The intent of these recommendations is to provide an objective framework with both clinician and patient input that can facilitate bidirectional discussion, trust building, and decision making on the current treatment strategy and the need to adjust or escalate treatment in an appropriate time frame,” the authors wrote.
SOURCE:
The study was published online in JAMA Dermatology. The lead author was Nicole Mastacouris, MS, and the corresponding author was Amit Garg, MD, both of Northwell Health, New Hyde Park, N.Y.
LIMITATIONS:
The consensus results may have been affected by variations in HS management by region. Neither measure has been studied in clinical practice, and practice variability may limit their implementation.
DISCLOSURES:
The study was supported by grants from UCB and AbbVie. Ms. Mastacouris had no financial disclosures. Dr. Garg disclosed grant support from AbbVie and UCB during the conduct of the study, as well as personal fees from AbbVie, UCB, Aclaris Therapeutics, Anaptys Bio, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, Insmed, Janssen, Novartis, Pfizer, Sonoma Biotherapeutics, Union Therapeutics, Ventyx Biosciences, and Viela Biosciences during the conduct of the study; Dr. Garg also holds patents for HS-IGA and HiSQOL. Many other coauthors disclosed relationships with multiple companies, including AbbVie and UCB, and some also disclosed patents, including patents for HiSQOL and HS Area and Severity Index.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Participants in the study were 55 HS experts from the HiSTORIC group (dermatologists, internists, surgeons, and nurses) and 24 patient research partners.
- The group identified clinician- and patient-reported HS outcome measures in the literature, then participated in an online item reduction survey, followed by an electronic Delphi survey to reach consensus on which measures should be used in clinical practice. Consensus was defined as at least 67% of participants agreeing/strongly agreeing or disagreeing/strongly disagreeing about the use of a measure in clinical practice.
- The initial literature search yielded 11 HS studies with clinician-reported outcome measures and 12 with patient-reported outcomes; of these, eight and five, respectively, were included in the final reduction survey.
TAKEAWAY:
- The group reached consensus on two HS outcome measures for use in clinical practice: the HS Investigator Global Assessment (HS-IGA) score, a clinician-reported outcome measure selected by the HS experts, and the HS Quality of Life (HiSQOL) score, a patient-reported outcome measure selected by the patient research partners.
- The HS-IGA score uses a number between 0 and 5 based on the sum of abscesses, inflammatory and noninflammatory nodules, and tunnels in regions of the upper or lower body.
- The HiSQOL, a disease-specific quality-of-life measure for adults with HS, is designed to capture unique features of HS, including symptoms (such as pain, itch, odor, and drainage) and psychosocial outcomes and activities that may be affected by the disease.
IN PRACTICE:
“The intent of these recommendations is to provide an objective framework with both clinician and patient input that can facilitate bidirectional discussion, trust building, and decision making on the current treatment strategy and the need to adjust or escalate treatment in an appropriate time frame,” the authors wrote.
SOURCE:
The study was published online in JAMA Dermatology. The lead author was Nicole Mastacouris, MS, and the corresponding author was Amit Garg, MD, both of Northwell Health, New Hyde Park, N.Y.
LIMITATIONS:
The consensus results may have been affected by variations in HS management by region. Neither measure has been studied in clinical practice, and practice variability may limit their implementation.
DISCLOSURES:
The study was supported by grants from UCB and AbbVie. Ms. Mastacouris had no financial disclosures. Dr. Garg disclosed grant support from AbbVie and UCB during the conduct of the study, as well as personal fees from AbbVie, UCB, Aclaris Therapeutics, Anaptys Bio, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, Insmed, Janssen, Novartis, Pfizer, Sonoma Biotherapeutics, Union Therapeutics, Ventyx Biosciences, and Viela Biosciences during the conduct of the study; Dr. Garg also holds patents for HS-IGA and HiSQOL. Many other coauthors disclosed relationships with multiple companies, including AbbVie and UCB, and some also disclosed patents, including patents for HiSQOL and HS Area and Severity Index.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
ACP addresses ethical issues for ‘grateful patients’ physician fundraising
Patients sometimes want to give back to their physician or hospital. In recent years, the practice of soliciting donations from these patients has grown into structured fundraising initiatives at some health care organizations. Some employers mandate clinicians solicit donations, while other doctors participate voluntarily.
“In recent decades, more physician practices have become part of large health systems: these arrangements can offer benefits to care but can also lead to interference in the patient-physician relationship and challenges to the physician’s ethical responsibilities to patients,” said Omar T. Atiq, MD, president of the American College of Physicians.
Grateful patient fundraising (GPF) is largely based on models of charitable giving outside of health care and is relatively new to the industry. Simply defined, it is the solicitation of donations by doctors from current and former patients. Funds may be used for operating costs, clinical research, equipment upgrades, or facility improvements.
In a newly published position paper, the ACP, which represents roughly 161,000 physicians, is clear that clinicians should not try to convert their patients into donors.
“Physicians who directly solicit funds from their own patients do risk interfering with the physician-patient relationship, which is supposed to be based on the patient’s best interests, not the physicians’ interests,” said Stacey A. Tovino, JD, PhD, director of health care law programs at the University of Oklahoma, Norman.
Once involved in fundraising, patients may also develop an unrealistic expectation of what kind of care they should receive, according to the ACP.
Another pitfall clinicians may fall into is the HIPAA Privacy Rule. In 2013, HIPAA was expanded to allow hospital fundraisers to access privileged health information, including demographic, health insurance, treating clinician, and data on outcomes. Dr. Atiq said that, since then, electronic health records have been used as tools to aide fundraising efforts. For instance, some health care organizations have embedded a feature inside EHRs to allow physicians to flag development officers when a patient or family member might be a potential donor.
Patients may be unaware that hospital fundraising departments have access to their electronic health records, or that they have the right to opt out of fundraising solicitations.
“Physicians should not use or reveal patient information for fundraising,” Dr. Atiq said. “Even acknowledging that a person is under one’s care can make it possible for protected health information to be revealed.”
Data-mining EHRs may be legal, Ms. Tovino said, but it hugs a fine ethical line.
“A patient may not expect that their information will be used for these purposes and may not know how to opt out of having their information used in these ways,” Ms. Tovino said.
A clinician’s employment contract, whether it be a full-time position or for specific admitting privileges, may make it hard for them to push back against expectations to ask patients for money or screen for donors. Metrics or expectations to approach potential donors create ethical snares for clinicians – and it pits them between their patient and place of employment.
“GPF does raise ethical concerns, including those surrounding confidentiality and privacy, and whether physicians are being remunerated or evaluated based on their participation,” Ms. Tovino said.
Asked how doctors can avoid being involved in GPF, Dr. Atiq referred to the ACP ethics manual, which separates clinicians from fundraising.
“Redirecting the patient to discuss donations with institutional administrators provides the appropriate venue and firewall,” he said.
An author of the ACP paper reported a paid position on the board of the Government Employees Health Association.
A version of this article first appeared on Medscape.com.
Patients sometimes want to give back to their physician or hospital. In recent years, the practice of soliciting donations from these patients has grown into structured fundraising initiatives at some health care organizations. Some employers mandate clinicians solicit donations, while other doctors participate voluntarily.
“In recent decades, more physician practices have become part of large health systems: these arrangements can offer benefits to care but can also lead to interference in the patient-physician relationship and challenges to the physician’s ethical responsibilities to patients,” said Omar T. Atiq, MD, president of the American College of Physicians.
Grateful patient fundraising (GPF) is largely based on models of charitable giving outside of health care and is relatively new to the industry. Simply defined, it is the solicitation of donations by doctors from current and former patients. Funds may be used for operating costs, clinical research, equipment upgrades, or facility improvements.
In a newly published position paper, the ACP, which represents roughly 161,000 physicians, is clear that clinicians should not try to convert their patients into donors.
“Physicians who directly solicit funds from their own patients do risk interfering with the physician-patient relationship, which is supposed to be based on the patient’s best interests, not the physicians’ interests,” said Stacey A. Tovino, JD, PhD, director of health care law programs at the University of Oklahoma, Norman.
Once involved in fundraising, patients may also develop an unrealistic expectation of what kind of care they should receive, according to the ACP.
Another pitfall clinicians may fall into is the HIPAA Privacy Rule. In 2013, HIPAA was expanded to allow hospital fundraisers to access privileged health information, including demographic, health insurance, treating clinician, and data on outcomes. Dr. Atiq said that, since then, electronic health records have been used as tools to aide fundraising efforts. For instance, some health care organizations have embedded a feature inside EHRs to allow physicians to flag development officers when a patient or family member might be a potential donor.
Patients may be unaware that hospital fundraising departments have access to their electronic health records, or that they have the right to opt out of fundraising solicitations.
“Physicians should not use or reveal patient information for fundraising,” Dr. Atiq said. “Even acknowledging that a person is under one’s care can make it possible for protected health information to be revealed.”
Data-mining EHRs may be legal, Ms. Tovino said, but it hugs a fine ethical line.
“A patient may not expect that their information will be used for these purposes and may not know how to opt out of having their information used in these ways,” Ms. Tovino said.
A clinician’s employment contract, whether it be a full-time position or for specific admitting privileges, may make it hard for them to push back against expectations to ask patients for money or screen for donors. Metrics or expectations to approach potential donors create ethical snares for clinicians – and it pits them between their patient and place of employment.
“GPF does raise ethical concerns, including those surrounding confidentiality and privacy, and whether physicians are being remunerated or evaluated based on their participation,” Ms. Tovino said.
Asked how doctors can avoid being involved in GPF, Dr. Atiq referred to the ACP ethics manual, which separates clinicians from fundraising.
“Redirecting the patient to discuss donations with institutional administrators provides the appropriate venue and firewall,” he said.
An author of the ACP paper reported a paid position on the board of the Government Employees Health Association.
A version of this article first appeared on Medscape.com.
Patients sometimes want to give back to their physician or hospital. In recent years, the practice of soliciting donations from these patients has grown into structured fundraising initiatives at some health care organizations. Some employers mandate clinicians solicit donations, while other doctors participate voluntarily.
“In recent decades, more physician practices have become part of large health systems: these arrangements can offer benefits to care but can also lead to interference in the patient-physician relationship and challenges to the physician’s ethical responsibilities to patients,” said Omar T. Atiq, MD, president of the American College of Physicians.
Grateful patient fundraising (GPF) is largely based on models of charitable giving outside of health care and is relatively new to the industry. Simply defined, it is the solicitation of donations by doctors from current and former patients. Funds may be used for operating costs, clinical research, equipment upgrades, or facility improvements.
In a newly published position paper, the ACP, which represents roughly 161,000 physicians, is clear that clinicians should not try to convert their patients into donors.
“Physicians who directly solicit funds from their own patients do risk interfering with the physician-patient relationship, which is supposed to be based on the patient’s best interests, not the physicians’ interests,” said Stacey A. Tovino, JD, PhD, director of health care law programs at the University of Oklahoma, Norman.
Once involved in fundraising, patients may also develop an unrealistic expectation of what kind of care they should receive, according to the ACP.
Another pitfall clinicians may fall into is the HIPAA Privacy Rule. In 2013, HIPAA was expanded to allow hospital fundraisers to access privileged health information, including demographic, health insurance, treating clinician, and data on outcomes. Dr. Atiq said that, since then, electronic health records have been used as tools to aide fundraising efforts. For instance, some health care organizations have embedded a feature inside EHRs to allow physicians to flag development officers when a patient or family member might be a potential donor.
Patients may be unaware that hospital fundraising departments have access to their electronic health records, or that they have the right to opt out of fundraising solicitations.
“Physicians should not use or reveal patient information for fundraising,” Dr. Atiq said. “Even acknowledging that a person is under one’s care can make it possible for protected health information to be revealed.”
Data-mining EHRs may be legal, Ms. Tovino said, but it hugs a fine ethical line.
“A patient may not expect that their information will be used for these purposes and may not know how to opt out of having their information used in these ways,” Ms. Tovino said.
A clinician’s employment contract, whether it be a full-time position or for specific admitting privileges, may make it hard for them to push back against expectations to ask patients for money or screen for donors. Metrics or expectations to approach potential donors create ethical snares for clinicians – and it pits them between their patient and place of employment.
“GPF does raise ethical concerns, including those surrounding confidentiality and privacy, and whether physicians are being remunerated or evaluated based on their participation,” Ms. Tovino said.
Asked how doctors can avoid being involved in GPF, Dr. Atiq referred to the ACP ethics manual, which separates clinicians from fundraising.
“Redirecting the patient to discuss donations with institutional administrators provides the appropriate venue and firewall,” he said.
An author of the ACP paper reported a paid position on the board of the Government Employees Health Association.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Study spotlights paucity of black dermatologists in academia
TOPLINE:
METHODOLOGY:
- To assess the prevalence of Black dermatologists in academic dermatology programs, researchers obtained an inventory of all 142 U.S.-accredited dermatology residency programs from the Accreditation Council for Graduate Medical Education.
- The researchers drew from institutional websites, the Black Derm Directory (an online repository of Black dermatologists), and other sources to identify full- and part-time Black faculty.
- Variables of interest for each Black dermatologist included gender, institution, department title, academic and nonacademic leadership roles, publication number, National Institutes of Health grant funding, degrees, subspecialties, medical school attended, place of residency, and fellowship training.
- The researchers used Pearson’s chi-squared testing to calculate associations.
TAKEAWAY:
- Of the 86 Black faculty identified, 81.4% were female; most (42.4%) were in the southern United States, followed by the Midwest (23.5%); and 83% held full-time positions.
- Slightly more than one-quarter (26.7%) of the Black faculty attended a top 10 medical school, 16.3% graduated from a historically Black college and university medical school, and 43.5% of those with 25 or more research publications had attended a top 10 medical school.
- Only three dermatology department chairs were Black, and all were female. In addition, more than half of Black faculty (59.2%) were assistant professors, 37.7% held leadership positions at their institutions, and 32.6% held outside leadership roles in dermatology (such as leadership titles at professional dermatology organizations or editorial positions at a journal).
IN PRACTICE:
“Greater efforts are needed to recruit Black dermatology graduates into academic faculty positions,” and “faculty development programs offered by academic institutions and dermatologic associations ... should continue to be expanded,” the authors conclude.
SOURCE:
Corresponding author Nada Elbuluk, MD, MSc, director of the skin of color and pigmentary disorders program and the diversity and inclusion program in the department of dermatology at the University of Southern California, Los Angeles, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The process for identifying Black faculty and insufficient or outdated information on department websites were limitations.
DISCLOSURES:
Dr. Elbuluk disclosed that she has served as a consultant for Avita, Scientis, Incyte, VisualDx, La Roche Posay, Beiersdorf, and Unilever. She has served on advisory boards for Allergan, Eli Lilly, Galderma, Incyte, Pfizer, Janssen, La Roche Posay, L’Oreal, McGraw Hill, and Dior. She has been a speaker for La Roche Posay, Scientis, Medscape, Beiersdorf, and Dior, and has served as investigator for Avita. Another author is an investigator and speaker for Castle Biosciences.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- To assess the prevalence of Black dermatologists in academic dermatology programs, researchers obtained an inventory of all 142 U.S.-accredited dermatology residency programs from the Accreditation Council for Graduate Medical Education.
- The researchers drew from institutional websites, the Black Derm Directory (an online repository of Black dermatologists), and other sources to identify full- and part-time Black faculty.
- Variables of interest for each Black dermatologist included gender, institution, department title, academic and nonacademic leadership roles, publication number, National Institutes of Health grant funding, degrees, subspecialties, medical school attended, place of residency, and fellowship training.
- The researchers used Pearson’s chi-squared testing to calculate associations.
TAKEAWAY:
- Of the 86 Black faculty identified, 81.4% were female; most (42.4%) were in the southern United States, followed by the Midwest (23.5%); and 83% held full-time positions.
- Slightly more than one-quarter (26.7%) of the Black faculty attended a top 10 medical school, 16.3% graduated from a historically Black college and university medical school, and 43.5% of those with 25 or more research publications had attended a top 10 medical school.
- Only three dermatology department chairs were Black, and all were female. In addition, more than half of Black faculty (59.2%) were assistant professors, 37.7% held leadership positions at their institutions, and 32.6% held outside leadership roles in dermatology (such as leadership titles at professional dermatology organizations or editorial positions at a journal).
IN PRACTICE:
“Greater efforts are needed to recruit Black dermatology graduates into academic faculty positions,” and “faculty development programs offered by academic institutions and dermatologic associations ... should continue to be expanded,” the authors conclude.
SOURCE:
Corresponding author Nada Elbuluk, MD, MSc, director of the skin of color and pigmentary disorders program and the diversity and inclusion program in the department of dermatology at the University of Southern California, Los Angeles, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The process for identifying Black faculty and insufficient or outdated information on department websites were limitations.
DISCLOSURES:
Dr. Elbuluk disclosed that she has served as a consultant for Avita, Scientis, Incyte, VisualDx, La Roche Posay, Beiersdorf, and Unilever. She has served on advisory boards for Allergan, Eli Lilly, Galderma, Incyte, Pfizer, Janssen, La Roche Posay, L’Oreal, McGraw Hill, and Dior. She has been a speaker for La Roche Posay, Scientis, Medscape, Beiersdorf, and Dior, and has served as investigator for Avita. Another author is an investigator and speaker for Castle Biosciences.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- To assess the prevalence of Black dermatologists in academic dermatology programs, researchers obtained an inventory of all 142 U.S.-accredited dermatology residency programs from the Accreditation Council for Graduate Medical Education.
- The researchers drew from institutional websites, the Black Derm Directory (an online repository of Black dermatologists), and other sources to identify full- and part-time Black faculty.
- Variables of interest for each Black dermatologist included gender, institution, department title, academic and nonacademic leadership roles, publication number, National Institutes of Health grant funding, degrees, subspecialties, medical school attended, place of residency, and fellowship training.
- The researchers used Pearson’s chi-squared testing to calculate associations.
TAKEAWAY:
- Of the 86 Black faculty identified, 81.4% were female; most (42.4%) were in the southern United States, followed by the Midwest (23.5%); and 83% held full-time positions.
- Slightly more than one-quarter (26.7%) of the Black faculty attended a top 10 medical school, 16.3% graduated from a historically Black college and university medical school, and 43.5% of those with 25 or more research publications had attended a top 10 medical school.
- Only three dermatology department chairs were Black, and all were female. In addition, more than half of Black faculty (59.2%) were assistant professors, 37.7% held leadership positions at their institutions, and 32.6% held outside leadership roles in dermatology (such as leadership titles at professional dermatology organizations or editorial positions at a journal).
IN PRACTICE:
“Greater efforts are needed to recruit Black dermatology graduates into academic faculty positions,” and “faculty development programs offered by academic institutions and dermatologic associations ... should continue to be expanded,” the authors conclude.
SOURCE:
Corresponding author Nada Elbuluk, MD, MSc, director of the skin of color and pigmentary disorders program and the diversity and inclusion program in the department of dermatology at the University of Southern California, Los Angeles, led the research. The study was published in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The process for identifying Black faculty and insufficient or outdated information on department websites were limitations.
DISCLOSURES:
Dr. Elbuluk disclosed that she has served as a consultant for Avita, Scientis, Incyte, VisualDx, La Roche Posay, Beiersdorf, and Unilever. She has served on advisory boards for Allergan, Eli Lilly, Galderma, Incyte, Pfizer, Janssen, La Roche Posay, L’Oreal, McGraw Hill, and Dior. She has been a speaker for La Roche Posay, Scientis, Medscape, Beiersdorf, and Dior, and has served as investigator for Avita. Another author is an investigator and speaker for Castle Biosciences.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Most adults with alopecia areata untreated 1 year after diagnosis
TOPLINE:
using data from more than 45,000 individuals.
METHODOLOGY:
- The study population included 45,483 adults aged 18 years and older with new diagnoses of AA between Oct. 15, 2015, and Feb. 28, 2020. Data were from a large U.S. health care database that included medical and pharmacy claims.
- The mean age of the participants was 43.8 years, and 65.7% were female.
- The researchers measured variables that might relate to AA and its treatment patterns within 1 year of starting the study and during the first year of the study, with data collected at 1, 42, 84, and 365 days after study entry.
TAKEAWAYS:
- During the first year after diagnosis, 66.4% of patients received at least one treatment for AA at one or more time points.
- At 1 year, 71.8% of patients were not receiving any active treatment for AA.
- Among those who received treatment, intralesional injections were the most often prescribed therapy (41.8% of patients), followed by topical corticosteroids (40.9%), intramuscular corticosteroids (38.1%), and oral corticosteroids (20.6%).
- Patients diagnosed with either alopecia totalis or alopecia universalis were significantly less likely to receive intralesional steroids and significantly more likely to receive topical corticosteroids than those without these diagnoses (11.1% vs. 44.1% and 25.4% vs. 42.1, respectively).
IN PRACTICE:
The results highlight the need to determine why so many alopecia patients with AA were no longer on treatment after 1 year, although treatment trends may change with the emergence of new therapies, such as JAK inhibitors and others, according to the authors.
SOURCE:
The lead author of the study was Hemin Lee, MD, MPH, Brigham and Women’s Hospital, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The use of insurance claims data did not allow analysis of over-the-counter medications and treatments, and the lack of a single ICD-10 code for defining AA could have resulted in misclassification of outcomes.
DISCLOSURES:
The study received no outside funding, and Dr. Lee had no disclosures. One author had disclosures that included receiving personal fees from Pfizer and Concert outside of the submitted study and participating in alopecia-related trials with Lilly, Concert, Aclaris, and Incyte. Another author’s disclosures included receiving personal fees from companies that included Pfizer, Concert, Lilly, and AbbVie. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
using data from more than 45,000 individuals.
METHODOLOGY:
- The study population included 45,483 adults aged 18 years and older with new diagnoses of AA between Oct. 15, 2015, and Feb. 28, 2020. Data were from a large U.S. health care database that included medical and pharmacy claims.
- The mean age of the participants was 43.8 years, and 65.7% were female.
- The researchers measured variables that might relate to AA and its treatment patterns within 1 year of starting the study and during the first year of the study, with data collected at 1, 42, 84, and 365 days after study entry.
TAKEAWAYS:
- During the first year after diagnosis, 66.4% of patients received at least one treatment for AA at one or more time points.
- At 1 year, 71.8% of patients were not receiving any active treatment for AA.
- Among those who received treatment, intralesional injections were the most often prescribed therapy (41.8% of patients), followed by topical corticosteroids (40.9%), intramuscular corticosteroids (38.1%), and oral corticosteroids (20.6%).
- Patients diagnosed with either alopecia totalis or alopecia universalis were significantly less likely to receive intralesional steroids and significantly more likely to receive topical corticosteroids than those without these diagnoses (11.1% vs. 44.1% and 25.4% vs. 42.1, respectively).
IN PRACTICE:
The results highlight the need to determine why so many alopecia patients with AA were no longer on treatment after 1 year, although treatment trends may change with the emergence of new therapies, such as JAK inhibitors and others, according to the authors.
SOURCE:
The lead author of the study was Hemin Lee, MD, MPH, Brigham and Women’s Hospital, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The use of insurance claims data did not allow analysis of over-the-counter medications and treatments, and the lack of a single ICD-10 code for defining AA could have resulted in misclassification of outcomes.
DISCLOSURES:
The study received no outside funding, and Dr. Lee had no disclosures. One author had disclosures that included receiving personal fees from Pfizer and Concert outside of the submitted study and participating in alopecia-related trials with Lilly, Concert, Aclaris, and Incyte. Another author’s disclosures included receiving personal fees from companies that included Pfizer, Concert, Lilly, and AbbVie. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
using data from more than 45,000 individuals.
METHODOLOGY:
- The study population included 45,483 adults aged 18 years and older with new diagnoses of AA between Oct. 15, 2015, and Feb. 28, 2020. Data were from a large U.S. health care database that included medical and pharmacy claims.
- The mean age of the participants was 43.8 years, and 65.7% were female.
- The researchers measured variables that might relate to AA and its treatment patterns within 1 year of starting the study and during the first year of the study, with data collected at 1, 42, 84, and 365 days after study entry.
TAKEAWAYS:
- During the first year after diagnosis, 66.4% of patients received at least one treatment for AA at one or more time points.
- At 1 year, 71.8% of patients were not receiving any active treatment for AA.
- Among those who received treatment, intralesional injections were the most often prescribed therapy (41.8% of patients), followed by topical corticosteroids (40.9%), intramuscular corticosteroids (38.1%), and oral corticosteroids (20.6%).
- Patients diagnosed with either alopecia totalis or alopecia universalis were significantly less likely to receive intralesional steroids and significantly more likely to receive topical corticosteroids than those without these diagnoses (11.1% vs. 44.1% and 25.4% vs. 42.1, respectively).
IN PRACTICE:
The results highlight the need to determine why so many alopecia patients with AA were no longer on treatment after 1 year, although treatment trends may change with the emergence of new therapies, such as JAK inhibitors and others, according to the authors.
SOURCE:
The lead author of the study was Hemin Lee, MD, MPH, Brigham and Women’s Hospital, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The use of insurance claims data did not allow analysis of over-the-counter medications and treatments, and the lack of a single ICD-10 code for defining AA could have resulted in misclassification of outcomes.
DISCLOSURES:
The study received no outside funding, and Dr. Lee had no disclosures. One author had disclosures that included receiving personal fees from Pfizer and Concert outside of the submitted study and participating in alopecia-related trials with Lilly, Concert, Aclaris, and Incyte. Another author’s disclosures included receiving personal fees from companies that included Pfizer, Concert, Lilly, and AbbVie. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Overburdened: Health care workers more likely to die by suicide
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
If you run into a health care provider these days and ask, “How are you doing?” you’re likely to get a response like this one: “You know, hanging in there.” You smile and move on. But it may be time to go a step further. If you ask that next question – “No, really, how are you doing?” Well, you might need to carve out some time.
It’s been a rough few years for those of us in the health care professions. Our lives, dominated by COVID-related concerns at home, were equally dominated by COVID concerns at work. On the job, there were fewer and fewer of us around as exploitation and COVID-related stressors led doctors, nurses, and others to leave the profession entirely or take early retirement. Even now, I’m not sure we’ve recovered. Staffing in the hospitals is still a huge problem, and the persistence of impersonal meetings via teleconference – which not only prevent any sort of human connection but, audaciously, run from one into another without a break – robs us of even the subtle joy of walking from one hallway to another for 5 minutes of reflection before sitting down to view the next hastily cobbled together PowerPoint.
I’m speaking in generalities, of course.
I’m talking about how bad things are now because, in truth, they’ve never been great. And that may be why health care workers – people with jobs focused on serving others – are nevertheless at substantially increased risk for suicide.
Analyses through the years have shown that physicians tend to have higher rates of death from suicide than the general population. There are reasons for this that may not entirely be because of work-related stress. Doctors’ suicide attempts are more often lethal – we know what is likely to work, after all.
And, according to this paper in JAMA, it is those people who may be suffering most of all.
The study is a nationally representative sample based on the 2008 American Community Survey. Records were linked to the National Death Index through 2019.
Survey respondents were classified into five categories of health care worker, as you can see here. And 1,666,000 non–health care workers served as the control group.
Let’s take a look at the numbers.
I’m showing you age- and sex-standardized rates of death from suicide, starting with non–health care workers. In this study, physicians have similar rates of death from suicide to the general population. Nurses have higher rates, but health care support workers – nurses’ aides, home health aides – have rates nearly twice that of the general population.
Only social and behavioral health workers had rates lower than those in the general population, perhaps because they know how to access life-saving resources.
Of course, these groups differ in a lot of ways – education and income, for example. But even after adjustment for these factors as well as for sex, race, and marital status, the results persist. The only group with even a trend toward lower suicide rates are social and behavioral health workers.
There has been much hand-wringing about rates of physician suicide in the past. It is still a very real problem. But this paper finally highlights that there is a lot more to the health care profession than physicians. It’s time we acknowledge and support the people in our profession who seem to be suffering more than any of us: the aides, the techs, the support staff – the overworked and underpaid who have to deal with all the stresses that physicians like me face and then some.
There’s more to suicide risk than just your job; I know that. Family matters. Relationships matter. Medical and psychiatric illnesses matter. But to ignore this problem when it is right here, in our own house so to speak, can’t continue.
Might I suggest we start by asking someone in our profession – whether doctor, nurse, aide, or tech – how they are doing. How they are really doing. And when we are done listening, we use what we hear to advocate for real change.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
If you run into a health care provider these days and ask, “How are you doing?” you’re likely to get a response like this one: “You know, hanging in there.” You smile and move on. But it may be time to go a step further. If you ask that next question – “No, really, how are you doing?” Well, you might need to carve out some time.
It’s been a rough few years for those of us in the health care professions. Our lives, dominated by COVID-related concerns at home, were equally dominated by COVID concerns at work. On the job, there were fewer and fewer of us around as exploitation and COVID-related stressors led doctors, nurses, and others to leave the profession entirely or take early retirement. Even now, I’m not sure we’ve recovered. Staffing in the hospitals is still a huge problem, and the persistence of impersonal meetings via teleconference – which not only prevent any sort of human connection but, audaciously, run from one into another without a break – robs us of even the subtle joy of walking from one hallway to another for 5 minutes of reflection before sitting down to view the next hastily cobbled together PowerPoint.
I’m speaking in generalities, of course.
I’m talking about how bad things are now because, in truth, they’ve never been great. And that may be why health care workers – people with jobs focused on serving others – are nevertheless at substantially increased risk for suicide.
Analyses through the years have shown that physicians tend to have higher rates of death from suicide than the general population. There are reasons for this that may not entirely be because of work-related stress. Doctors’ suicide attempts are more often lethal – we know what is likely to work, after all.
And, according to this paper in JAMA, it is those people who may be suffering most of all.
The study is a nationally representative sample based on the 2008 American Community Survey. Records were linked to the National Death Index through 2019.
Survey respondents were classified into five categories of health care worker, as you can see here. And 1,666,000 non–health care workers served as the control group.
Let’s take a look at the numbers.
I’m showing you age- and sex-standardized rates of death from suicide, starting with non–health care workers. In this study, physicians have similar rates of death from suicide to the general population. Nurses have higher rates, but health care support workers – nurses’ aides, home health aides – have rates nearly twice that of the general population.
Only social and behavioral health workers had rates lower than those in the general population, perhaps because they know how to access life-saving resources.
Of course, these groups differ in a lot of ways – education and income, for example. But even after adjustment for these factors as well as for sex, race, and marital status, the results persist. The only group with even a trend toward lower suicide rates are social and behavioral health workers.
There has been much hand-wringing about rates of physician suicide in the past. It is still a very real problem. But this paper finally highlights that there is a lot more to the health care profession than physicians. It’s time we acknowledge and support the people in our profession who seem to be suffering more than any of us: the aides, the techs, the support staff – the overworked and underpaid who have to deal with all the stresses that physicians like me face and then some.
There’s more to suicide risk than just your job; I know that. Family matters. Relationships matter. Medical and psychiatric illnesses matter. But to ignore this problem when it is right here, in our own house so to speak, can’t continue.
Might I suggest we start by asking someone in our profession – whether doctor, nurse, aide, or tech – how they are doing. How they are really doing. And when we are done listening, we use what we hear to advocate for real change.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
If you run into a health care provider these days and ask, “How are you doing?” you’re likely to get a response like this one: “You know, hanging in there.” You smile and move on. But it may be time to go a step further. If you ask that next question – “No, really, how are you doing?” Well, you might need to carve out some time.
It’s been a rough few years for those of us in the health care professions. Our lives, dominated by COVID-related concerns at home, were equally dominated by COVID concerns at work. On the job, there were fewer and fewer of us around as exploitation and COVID-related stressors led doctors, nurses, and others to leave the profession entirely or take early retirement. Even now, I’m not sure we’ve recovered. Staffing in the hospitals is still a huge problem, and the persistence of impersonal meetings via teleconference – which not only prevent any sort of human connection but, audaciously, run from one into another without a break – robs us of even the subtle joy of walking from one hallway to another for 5 minutes of reflection before sitting down to view the next hastily cobbled together PowerPoint.
I’m speaking in generalities, of course.
I’m talking about how bad things are now because, in truth, they’ve never been great. And that may be why health care workers – people with jobs focused on serving others – are nevertheless at substantially increased risk for suicide.
Analyses through the years have shown that physicians tend to have higher rates of death from suicide than the general population. There are reasons for this that may not entirely be because of work-related stress. Doctors’ suicide attempts are more often lethal – we know what is likely to work, after all.
And, according to this paper in JAMA, it is those people who may be suffering most of all.
The study is a nationally representative sample based on the 2008 American Community Survey. Records were linked to the National Death Index through 2019.
Survey respondents were classified into five categories of health care worker, as you can see here. And 1,666,000 non–health care workers served as the control group.
Let’s take a look at the numbers.
I’m showing you age- and sex-standardized rates of death from suicide, starting with non–health care workers. In this study, physicians have similar rates of death from suicide to the general population. Nurses have higher rates, but health care support workers – nurses’ aides, home health aides – have rates nearly twice that of the general population.
Only social and behavioral health workers had rates lower than those in the general population, perhaps because they know how to access life-saving resources.
Of course, these groups differ in a lot of ways – education and income, for example. But even after adjustment for these factors as well as for sex, race, and marital status, the results persist. The only group with even a trend toward lower suicide rates are social and behavioral health workers.
There has been much hand-wringing about rates of physician suicide in the past. It is still a very real problem. But this paper finally highlights that there is a lot more to the health care profession than physicians. It’s time we acknowledge and support the people in our profession who seem to be suffering more than any of us: the aides, the techs, the support staff – the overworked and underpaid who have to deal with all the stresses that physicians like me face and then some.
There’s more to suicide risk than just your job; I know that. Family matters. Relationships matter. Medical and psychiatric illnesses matter. But to ignore this problem when it is right here, in our own house so to speak, can’t continue.
Might I suggest we start by asking someone in our profession – whether doctor, nurse, aide, or tech – how they are doing. How they are really doing. And when we are done listening, we use what we hear to advocate for real change.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.