Risk Assessment Tools
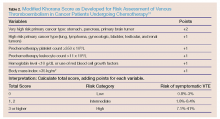
The modified Korana score is a well-validated risk-assessment model incorporating clinical parameters and laboratory biomarkers to stratify cancer patients according to their propensity to develop VTE, and represents a practical approach for clinical practice.31 The multivariate model identified the five most predictive variables: cancer site, pretreatment platelet count and leukocyte count, hemoglobin level, and body mass index (Table 2). For example, in the case presented, considering this patient’s age, primary site of the cancer, and his laboratory results, he was at a high-risk (up to 40%) for developing VTE.
Diagnosis
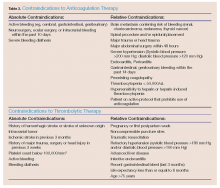
In patients with a high clinical suspicion of VTE and without contraindications to anticoagulation (Table 3), early initiation of anticoagulants should be considered while awaiting results from laboratory and imaging studies.5 The diagnostic accuracy for VTE improves with the following tests and scores: complete blood count, platelet count, D-dimer, Wells criteria, Modified Khorana Score, duplex ultrasonography, computed tomography angiography (CTA); and ventilation perfusion (V/Q) scan.
Complete Blood Count and Platelet Count. As previously discussed, a prechemotherapy platelet count ≥350 x 109/L, hemoglobin level <10g/dL, or prechemotherapy leukocyte count >11 x 109/L are good predictive biomarkers to consider VTE in a cancer patient.
D-Dimer. In view of the lack of specificity of D-dimer testing (most cancer patients have elevated D-dimer levels) and the high prevalence of VTE in cancer patients, D-dimer testing is less useful in this setting. The number of false positive D-dimer assays was 3-fold higher in cancer patients compared with noncancer patients.33 Hence, D-dimer testing is not recommended for the diagnosis of VTE in cancer patients.
Wells Criteria. Clinical prediction models such as the Wells criteria for VTE have proven useful in predicting the diagnosis of VTE: 62.5% of the 40 patients who were categorized as high-risk had positive studies; 16.1% of the 492 patients who were classified as moderate-risk and 10.2% of the 219 patients who were classified as low-risk had positive results.34 However, since cancer patients comprised a minority of the subjects in this study, it is unclear whether Wells criteria is as predictive for VTE in this patient population.
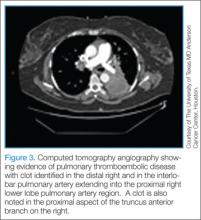
Duplex Venous Ultrasonography. The most recommended and preferred choice of investigation for initial diagnosis of DVT is duplex venous ultrasonography. This test is noninvasive, inexpensive, may be performed at bedside, and does not require IV contrast. It has excellent sensitivity and specificity for proximal vein DVT, but only 50% sensitivity for calf vein DVT. Duplex ultrasonography can analyze venous compressibility (which is considered to be more definitive) and Doppler imaging of venous blood flow.35 Limitations with Doppler ultrasonography include its inability to access more central veins (eg, large pelvic and iliac veins, proximal subclavian veins, inferior and superior venae cavae), and may not be used in bandaged or casted areas. Its effectiveness is operator dependent.Computed Tomography/Computed Tomography Angiography. Computed tomography angiography is the preferred imaging technique for the initial diagnosis of PE in most patients.5 Along with accurate imaging of mediastinal and parenchymal structures and visualization of emboli in the pulmonary vasculature, CTA is also useful to detect signs of right heart enlargement, which can be used in assessing the patient’s risk for adverse clinical outcomes.36 (See Figures 2 and 3 for examples of CT and CTA, demonstrating venous thrombosis and pulmonary thromboembolism.)
Computed Tomography Venography. The Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) II investigators recommend stratification of all patients with suspected PE according to an objective probability assessment. A negative D-dimer rapid enzyme-linked immunosorbent assay with a low or moderate probability clinical assessment can safely exclude PE. If PE is not excluded, CTA/CT venography (CTV) is recommended by most PIOPED II investigators, though CTA alone is an option. Since there is currently a lack of evidence to support CTV and since this study was comprised only a noncancer patient population, the authors do not recommend its use in the cancer patients.37Ventilation Perfusion Scan. As it is associated with less fetal radiation exposure than CTA, the V/Q scan is useful for assessing pregnant patients and those with renal insufficiency or untreatable contrast allergies in whom IV contrast is not feasible. A normal V/Q scan essentially rules out PE.38
Chest X-Ray and Electrocardiogram. Both a chest X-ray and electrocardiogram (ECG) facilitate the diagnosis of comorbidities and conditions with clinically similar presentations and can also assist in evaluating existing cardiac conditions. Findings on chest X-ray suspicious for PE include pleural effusion, localized infiltrate, Hamptons hump, and Westermark sign. In ECG, the most common finding in PE is nonspecific ST-T wave changes; a new right heart strain or an S1Q3T3 pattern is suspicious for PE.
Transthoracic Echocardiogram. More than 80% of patients with a documented PE will have some type of right heart abnormality, most commonly direct visualization of the thrombus, right ventricular dilatation, right ventricular hypokinesia with apical sparing, tricuspid regurgitation, abnormal interventricular septal motion, and pulmonary artery dilatation.39