User login
Research and Reviews for the Practicing Oncologist
Prescriber adherence to antiemetic guidelines with the new agent trifluridine-tipiracil
Cancer drugs are becoming available at an unprecedented rate. In 2015 alone, the US Food and Drug Administration (FDA) approved 18 new agents.1 Although many of those agents have adverse event profiles that are more favorable than those seen with conventional chemotherapy, nausea and vomiting still occur. In fact, nausea and vomiting continue to be ranked as among the most common and distressing of cancer symptoms.2,3 In a 2004 study, Grunberg and colleagues reported that as many as 75% of health care providers misjudge the risk for chemotherapy-induced nausea and vomiting (CINV), even when prescribing cancer drugs that have been available for years,4 thus amplifying concerns that such risk assessment might be even worse when new cancer agents are prescribed for the first time.
In this study, we hypothesized that patients prescribed a new cancer drug, trifluridine-tipiracil, would be at risk for CINV because of poor guideline adherence on the part of health care providers. The correct matching of antiemetics to chemotherapy is important. Inadequate antiemetic prophylaxis predisposes to nausea and vomiting with dehydration and metabolic and electrolyte derangements – complications that can occur in up to one-third of patients who receive moderately or highly emetogenic chemotherapy and who have been reported to achieve poor symptom control.4 Over-prophylaxis also has drawbacks. For example, antiemetics are expensive and, at times, they can induce their own adverse events, such as lethargy, dyskinesia, constipation, headaches, hiccups, fatigue, and even cardiac arrhythmias.5 The best approach is to appropriately match the antiemetic to the chemotherapy. Indeed, adherence to evidence-based guidelines has yielded success in symptom control, but the guidelines work on the assumption that the emetogenic potential of new chemotherapy agents has been accurately determined and then disseminated to and acted upon by health care providers.6,7 To our knowledge, no previous studies have tested that assumption, as we do in the present study.
Trifluridine-tipiracil was selected as the focus of this project and as illustrative of other newly approved chemotherapy agents for two reasons. First, it became available for routine prescribing in pretreated patients with metastatic colorectal cancer in the United States in September 2015.1 That timing allowed us to analyze much of the early prescribing period, both during the 9 months before approval, when the drug was available on a compassionate-use basis at our institution, and the 3 months after approval. Second, trifluridine-tipiracil has classifiably low emetogenic potential, and mismatching of antiemetics tends to occur more often with low emetogenic chemotherapy.9 Trifluridine-tipiracil and placebo patients manifest rates of nausea at 48% and 24%, respectively, and rates of vomiting at 28% and 14%, respectively.8
Hence, the goal of this study was to explore whether a guideline-based prophylactic antiemetic regimen was appropriately matched to the new chemotherapy agent, trifluridine-tipiracil, to report whether such symptoms of nausea and vomiting are kept at bay, and to identify a potentially vulnerable interval – immediately after drug approval – when cancer patients may be at risk for CINV because of poor adherence to antiemetic guideline prescribing practices by health care providers.
Methods
Overview
The Mayo Clinic Institutional Review Board approved this study. We obtained the identifying information of all patients treated with trifluridine-tipiracil at our institution from the Mayo Clinic Specialty Pharmacy, which uses an electronic prescribing system that contributed to the comprehensiveness of the data set. Patients included those who had participated in a colorectal cancer compassionate-use program before the September 2015 approval of the drug and those who received the drug shortly after its approval. In essence, this retrospective, single-institution study included every patient who received trifluridine-tipiracil for metastatic colorectal cancer in 2015 (January through December); this approach enabled us to systematically report on early first-cycle prescribing practices 9 months before and 3 months after the drug’s approval in September of 2015.
Determination of guideline adherence
This project relied on the National Comprehensive Cancer Network (NCCN) Guidelines (v1.2015, behind paywall) because they had been updated in 2015 (and hence coincided with this project’s study dates) to incorporate recommendations specific to oral chemotherapy and because they seemed concordant with other guidelines.10,11
Antiemetic prophylaxis for a specific patient was deemed guideline adherent if a version of the recommended NCCN antiemetic regimen had been prescribed during the first cycle of chemotherapy. This regimen consisted of metoclopramide, prochlorperazine, haloperidol, or a 5-hydroxytryptamine receptor antagonist. In contrast, if a patient had been prescribed a more aggressive or less aggressive regimen, such prescribing practices were deemed non–guideline adherent/aggressive (received more prophylaxis than called for) or non–guideline adherent/less aggressive (including no antiemetics), respectively. Again, medical record prescribing determined adherence.
Data reporting
The primary goal of this study was to report the percentage of patients who had been prescribed a first-cycle antiemetic prophylaxis regimen concordant with NCCN guidelines. Secondary goals included reporting the incidence of nausea and vomiting, the use of rescue antiemetics other than those prescribed up front, the need for an unplanned medical encounter to address nausea and vomiting, and change in antiemetic prescribing before the second chemotherapy cycle. Confidence intervals were calculated with JMP Pro 10.0.0. This study was too limited in sample size to assess sex-based differences in outcomes.
Results
Demographics
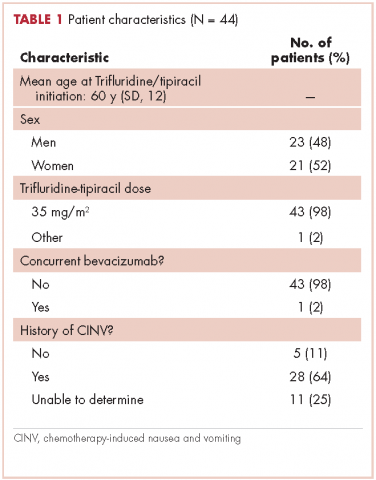
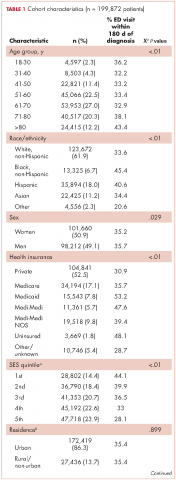
This report focuses on 44 patients who received first-cycle trifluridine-tipiracil during the first calendar year of the drug’s FDA approval. All patients had metastatic colorectal cancer and had previous exposures to other chemotherapy agents (Table 1). Of note, 28 patients (64%) had experienced CINV before starting trifluridine-tipiracil and all these patients had been heavily pretreated with multiple lines of chemotherapy.
Guideline adherence
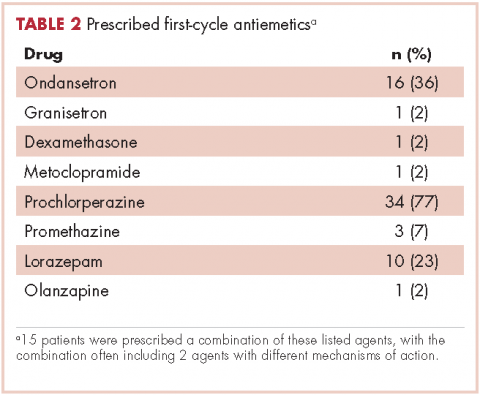
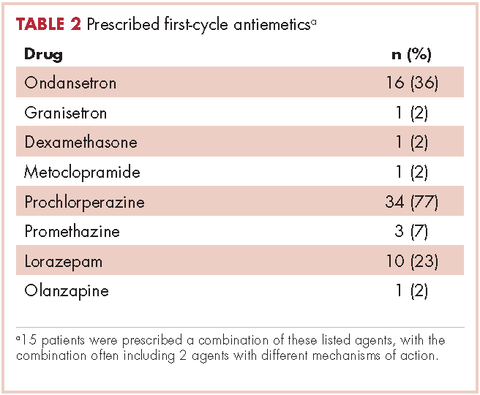
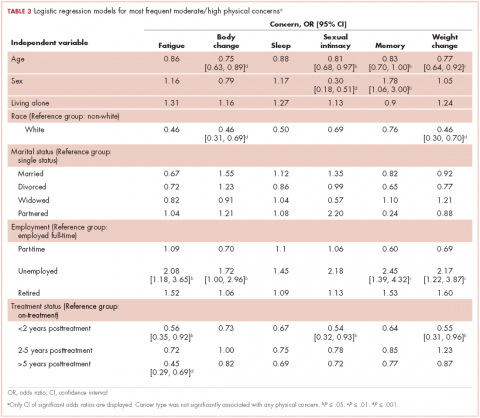
Patients were most commonly prescribed prochlorperazine and ondansetron prophylaxis for CINV before the first chemotherapy cycle of trifluridine-tipiracil (Table 2): 15 patients were prescribed combination antiemetic therapy, typically two of the most commonly prescribed single agents with different mechanisms of action. Twenty-five patients (57%; 95% confidence interval (CI): 42%, 70%) were prescribed antiemetics in a manner consistent with guidelines; 15 (34%; 95% CI: 22%, 49%) were prescribed antiemetics in a non–guideline-adherent/more aggressive manner (received more prophylaxis than called for); and 4 (9%; 95% CI: 4%, 21%) were prescribed them in a non–guideline-adherent/less aggressive manner.
Clinical outcomes based on guideline adherence
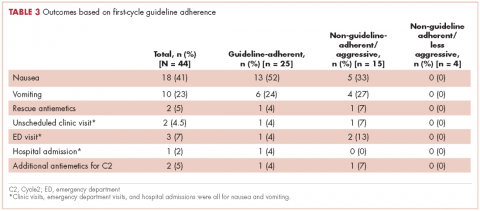
In guideline-adherent patients, first-cycle nausea and vomiting occurred in 13 patients (52%) and 6 patients (24%), respectively, with 1 patient requiring an unscheduled clinic visit and another an emergency department visit and hospital admission – all for nausea and vomiting (Table 3). In non–guideline-adherent/more aggressive patients, those symptoms occurred in 5 patients (33%, nausea) and 4 patients (27%, vomiting), with 1 patient requiring a clinic visit and emergency department visit and another an emergency department visit – again, all for nausea and vomiting. In non–guideline-adherent/less aggressive patients, no nausea or vomiting was reported.
Discussion
This study examined adherence to antiemetic guidelines in the setting of a soon-to-be-approved or newly approved antineoplastic agent. As hypothesized, a substantial proportion of patients (43% in this study) were prescribed antiemetics in a nonadherent manner with respect to guidelines, thus identifying the period shortly before and after FDA approval as a particularly vulnerable interval with respect to antiemetic guideline adherence. It is possible that our institution’s practice of testing novel chemotherapy agents for the treatment of colorectal cancer prompted a heightened awareness of potential adverse events, leading to greater guideline adherence than might have occurred in other settings and resulting in judicious straying from guideline adherence only when appropriate.12-14 Thus, these high rates of poor adherence may in fact represent an underestimate of what one might see in other clinical practices; and, similarly, these rates of symptom control might also be more favorable than those one might see in other clinical practices. To our knowledge, antiemetic prescribing practices with newer chemotherapy agents have not been explored before now, and our data underscore a clear need to do so – particularly during this limited interval when health care providers begin to prescribe new chemotherapy agents for the first time.
It is worth noting that despite the high rates of guideline nonadherence, rates of nausea and vomiting seemed to be comparable in patients prescribed antiemetics in a guideline-adherent manner and those prescribed antiemetics in a non–guideline-adherent/aggressive manner.A small number of patients in both the guideline-adherent and non–guideline-adherent/aggressive groups required rescue medications, unscheduled medical visits for nausea and vomiting, and additional antiemetics during the second cycle of chemotherapy. Of note,none of those interventions occurred in patients who were prescribed antiemetics in a non–guideline-adherent/less aggressive manner. These findings might reflect the fact that the patients had proven themselves to be at risk for nausea and vomiting with previous chemotherapy. Before they became candidates for trifluridine-tipiracil, patients had been heavily pretreated with other chemotherapy agents, most had experienced CINV, and many were therefore highly predisposed to nausea and vomiting. These observations underscore the fact that guidelines – even those that are well accepted and widely used – should be implemented in concert with good clinical judgment.10,11 This study has shortcomings, most notably its small sample size. However, had we extended our study beyond 3 months of the FDA approval to include more patients, our findings would have reflected more experienced prescribing practices and we thereby would have deviated from our primary goal of assessing antiemetic prescribing practices with only recently-approved and available chemotherapy agents. In this context, this limited sample size aptly serves a primary role of capturing outcomes within a fleeting but critical interval of new drug availability.In summary, this study found a notable rate of poor guideline adherence when prescribing antiemetics for trifluridine-tipiracil, a new chemotherapy agent of low emetogenic potential. Although the resultant rates of nausea and vomiting suggest that good clinical judgment might have influenced whether or not guidelines were adhered to, these findings nonetheless underscore the need to assess adherence to antiemetic guidelines when new chemotherapy drugs become available and potentially to put in place institutional infrastructure rapidly to promote improved adherence. Such an assessment should be deliberate, formalized, and prompt within individual oncology clinics and cancer centers after a new cancer drug becomes available. In conjunction with clinical judgment, such measures might lead to improved symptom control.
Acknowledgment
This paper is based on a poster that was presented at the 2016 Palliative Care in Oncology Symposium, on September 10, 2016: Adherence to antiemetic guidelines with a newly approved chemotherapy agent, trifluridine-tipiracil (TAS-102): a single-institution study. Daniel Childs and Aminah Jatoi, Mayo Clinic, Rochester, MN. http://meetinglibrary.asco.org/record/136444/abstract. J Clin Oncol. 2016;34(suppl 26S):abstract 221.
1. CenterWatch. FDA website. FDA approved drugs for oncology: drugs approved for 2015. https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Last updated April 2017. Accessed June 4, 2016.
2. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-1367.
3. Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3. Support Care Cancer. 2016;24:2661-2667.
4. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
5. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf. 2016; 15:343-356.
6. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract. 2014;10:68-74.
7. Mertens WC, Higby DJ, Brown D, et al. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol. 2003;21:1373-1378.
8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
9. Schwartzberg L, Morrow G, Balu S, et al. Chemotherapy-induced nausea and vomiting and antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists in patients with cancer treated with low emetogenic chemotherapy in a hospital outpatient setting in the United States. Curr Med Res Opin. 2011;27:1613-1622.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Antiemesis, Version1,2015 [behind paywall]. https://www.nccn.org. Last update not known. Accessed June 4, 2016.
11. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:v232-v243.
12. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 study. Lancet. 2013;381:303-312.
13. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393.
14. Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353.
Cancer drugs are becoming available at an unprecedented rate. In 2015 alone, the US Food and Drug Administration (FDA) approved 18 new agents.1 Although many of those agents have adverse event profiles that are more favorable than those seen with conventional chemotherapy, nausea and vomiting still occur. In fact, nausea and vomiting continue to be ranked as among the most common and distressing of cancer symptoms.2,3 In a 2004 study, Grunberg and colleagues reported that as many as 75% of health care providers misjudge the risk for chemotherapy-induced nausea and vomiting (CINV), even when prescribing cancer drugs that have been available for years,4 thus amplifying concerns that such risk assessment might be even worse when new cancer agents are prescribed for the first time.
In this study, we hypothesized that patients prescribed a new cancer drug, trifluridine-tipiracil, would be at risk for CINV because of poor guideline adherence on the part of health care providers. The correct matching of antiemetics to chemotherapy is important. Inadequate antiemetic prophylaxis predisposes to nausea and vomiting with dehydration and metabolic and electrolyte derangements – complications that can occur in up to one-third of patients who receive moderately or highly emetogenic chemotherapy and who have been reported to achieve poor symptom control.4 Over-prophylaxis also has drawbacks. For example, antiemetics are expensive and, at times, they can induce their own adverse events, such as lethargy, dyskinesia, constipation, headaches, hiccups, fatigue, and even cardiac arrhythmias.5 The best approach is to appropriately match the antiemetic to the chemotherapy. Indeed, adherence to evidence-based guidelines has yielded success in symptom control, but the guidelines work on the assumption that the emetogenic potential of new chemotherapy agents has been accurately determined and then disseminated to and acted upon by health care providers.6,7 To our knowledge, no previous studies have tested that assumption, as we do in the present study.
Trifluridine-tipiracil was selected as the focus of this project and as illustrative of other newly approved chemotherapy agents for two reasons. First, it became available for routine prescribing in pretreated patients with metastatic colorectal cancer in the United States in September 2015.1 That timing allowed us to analyze much of the early prescribing period, both during the 9 months before approval, when the drug was available on a compassionate-use basis at our institution, and the 3 months after approval. Second, trifluridine-tipiracil has classifiably low emetogenic potential, and mismatching of antiemetics tends to occur more often with low emetogenic chemotherapy.9 Trifluridine-tipiracil and placebo patients manifest rates of nausea at 48% and 24%, respectively, and rates of vomiting at 28% and 14%, respectively.8
Hence, the goal of this study was to explore whether a guideline-based prophylactic antiemetic regimen was appropriately matched to the new chemotherapy agent, trifluridine-tipiracil, to report whether such symptoms of nausea and vomiting are kept at bay, and to identify a potentially vulnerable interval – immediately after drug approval – when cancer patients may be at risk for CINV because of poor adherence to antiemetic guideline prescribing practices by health care providers.
Methods
Overview
The Mayo Clinic Institutional Review Board approved this study. We obtained the identifying information of all patients treated with trifluridine-tipiracil at our institution from the Mayo Clinic Specialty Pharmacy, which uses an electronic prescribing system that contributed to the comprehensiveness of the data set. Patients included those who had participated in a colorectal cancer compassionate-use program before the September 2015 approval of the drug and those who received the drug shortly after its approval. In essence, this retrospective, single-institution study included every patient who received trifluridine-tipiracil for metastatic colorectal cancer in 2015 (January through December); this approach enabled us to systematically report on early first-cycle prescribing practices 9 months before and 3 months after the drug’s approval in September of 2015.
Determination of guideline adherence
This project relied on the National Comprehensive Cancer Network (NCCN) Guidelines (v1.2015, behind paywall) because they had been updated in 2015 (and hence coincided with this project’s study dates) to incorporate recommendations specific to oral chemotherapy and because they seemed concordant with other guidelines.10,11
Antiemetic prophylaxis for a specific patient was deemed guideline adherent if a version of the recommended NCCN antiemetic regimen had been prescribed during the first cycle of chemotherapy. This regimen consisted of metoclopramide, prochlorperazine, haloperidol, or a 5-hydroxytryptamine receptor antagonist. In contrast, if a patient had been prescribed a more aggressive or less aggressive regimen, such prescribing practices were deemed non–guideline adherent/aggressive (received more prophylaxis than called for) or non–guideline adherent/less aggressive (including no antiemetics), respectively. Again, medical record prescribing determined adherence.
Data reporting
The primary goal of this study was to report the percentage of patients who had been prescribed a first-cycle antiemetic prophylaxis regimen concordant with NCCN guidelines. Secondary goals included reporting the incidence of nausea and vomiting, the use of rescue antiemetics other than those prescribed up front, the need for an unplanned medical encounter to address nausea and vomiting, and change in antiemetic prescribing before the second chemotherapy cycle. Confidence intervals were calculated with JMP Pro 10.0.0. This study was too limited in sample size to assess sex-based differences in outcomes.
Results
Demographics
This report focuses on 44 patients who received first-cycle trifluridine-tipiracil during the first calendar year of the drug’s FDA approval. All patients had metastatic colorectal cancer and had previous exposures to other chemotherapy agents (Table 1). Of note, 28 patients (64%) had experienced CINV before starting trifluridine-tipiracil and all these patients had been heavily pretreated with multiple lines of chemotherapy.
Guideline adherence
Patients were most commonly prescribed prochlorperazine and ondansetron prophylaxis for CINV before the first chemotherapy cycle of trifluridine-tipiracil (Table 2): 15 patients were prescribed combination antiemetic therapy, typically two of the most commonly prescribed single agents with different mechanisms of action. Twenty-five patients (57%; 95% confidence interval (CI): 42%, 70%) were prescribed antiemetics in a manner consistent with guidelines; 15 (34%; 95% CI: 22%, 49%) were prescribed antiemetics in a non–guideline-adherent/more aggressive manner (received more prophylaxis than called for); and 4 (9%; 95% CI: 4%, 21%) were prescribed them in a non–guideline-adherent/less aggressive manner.
Clinical outcomes based on guideline adherence
In guideline-adherent patients, first-cycle nausea and vomiting occurred in 13 patients (52%) and 6 patients (24%), respectively, with 1 patient requiring an unscheduled clinic visit and another an emergency department visit and hospital admission – all for nausea and vomiting (Table 3). In non–guideline-adherent/more aggressive patients, those symptoms occurred in 5 patients (33%, nausea) and 4 patients (27%, vomiting), with 1 patient requiring a clinic visit and emergency department visit and another an emergency department visit – again, all for nausea and vomiting. In non–guideline-adherent/less aggressive patients, no nausea or vomiting was reported.
Discussion
This study examined adherence to antiemetic guidelines in the setting of a soon-to-be-approved or newly approved antineoplastic agent. As hypothesized, a substantial proportion of patients (43% in this study) were prescribed antiemetics in a nonadherent manner with respect to guidelines, thus identifying the period shortly before and after FDA approval as a particularly vulnerable interval with respect to antiemetic guideline adherence. It is possible that our institution’s practice of testing novel chemotherapy agents for the treatment of colorectal cancer prompted a heightened awareness of potential adverse events, leading to greater guideline adherence than might have occurred in other settings and resulting in judicious straying from guideline adherence only when appropriate.12-14 Thus, these high rates of poor adherence may in fact represent an underestimate of what one might see in other clinical practices; and, similarly, these rates of symptom control might also be more favorable than those one might see in other clinical practices. To our knowledge, antiemetic prescribing practices with newer chemotherapy agents have not been explored before now, and our data underscore a clear need to do so – particularly during this limited interval when health care providers begin to prescribe new chemotherapy agents for the first time.
It is worth noting that despite the high rates of guideline nonadherence, rates of nausea and vomiting seemed to be comparable in patients prescribed antiemetics in a guideline-adherent manner and those prescribed antiemetics in a non–guideline-adherent/aggressive manner.A small number of patients in both the guideline-adherent and non–guideline-adherent/aggressive groups required rescue medications, unscheduled medical visits for nausea and vomiting, and additional antiemetics during the second cycle of chemotherapy. Of note,none of those interventions occurred in patients who were prescribed antiemetics in a non–guideline-adherent/less aggressive manner. These findings might reflect the fact that the patients had proven themselves to be at risk for nausea and vomiting with previous chemotherapy. Before they became candidates for trifluridine-tipiracil, patients had been heavily pretreated with other chemotherapy agents, most had experienced CINV, and many were therefore highly predisposed to nausea and vomiting. These observations underscore the fact that guidelines – even those that are well accepted and widely used – should be implemented in concert with good clinical judgment.10,11 This study has shortcomings, most notably its small sample size. However, had we extended our study beyond 3 months of the FDA approval to include more patients, our findings would have reflected more experienced prescribing practices and we thereby would have deviated from our primary goal of assessing antiemetic prescribing practices with only recently-approved and available chemotherapy agents. In this context, this limited sample size aptly serves a primary role of capturing outcomes within a fleeting but critical interval of new drug availability.In summary, this study found a notable rate of poor guideline adherence when prescribing antiemetics for trifluridine-tipiracil, a new chemotherapy agent of low emetogenic potential. Although the resultant rates of nausea and vomiting suggest that good clinical judgment might have influenced whether or not guidelines were adhered to, these findings nonetheless underscore the need to assess adherence to antiemetic guidelines when new chemotherapy drugs become available and potentially to put in place institutional infrastructure rapidly to promote improved adherence. Such an assessment should be deliberate, formalized, and prompt within individual oncology clinics and cancer centers after a new cancer drug becomes available. In conjunction with clinical judgment, such measures might lead to improved symptom control.
Acknowledgment
This paper is based on a poster that was presented at the 2016 Palliative Care in Oncology Symposium, on September 10, 2016: Adherence to antiemetic guidelines with a newly approved chemotherapy agent, trifluridine-tipiracil (TAS-102): a single-institution study. Daniel Childs and Aminah Jatoi, Mayo Clinic, Rochester, MN. http://meetinglibrary.asco.org/record/136444/abstract. J Clin Oncol. 2016;34(suppl 26S):abstract 221.
Cancer drugs are becoming available at an unprecedented rate. In 2015 alone, the US Food and Drug Administration (FDA) approved 18 new agents.1 Although many of those agents have adverse event profiles that are more favorable than those seen with conventional chemotherapy, nausea and vomiting still occur. In fact, nausea and vomiting continue to be ranked as among the most common and distressing of cancer symptoms.2,3 In a 2004 study, Grunberg and colleagues reported that as many as 75% of health care providers misjudge the risk for chemotherapy-induced nausea and vomiting (CINV), even when prescribing cancer drugs that have been available for years,4 thus amplifying concerns that such risk assessment might be even worse when new cancer agents are prescribed for the first time.
In this study, we hypothesized that patients prescribed a new cancer drug, trifluridine-tipiracil, would be at risk for CINV because of poor guideline adherence on the part of health care providers. The correct matching of antiemetics to chemotherapy is important. Inadequate antiemetic prophylaxis predisposes to nausea and vomiting with dehydration and metabolic and electrolyte derangements – complications that can occur in up to one-third of patients who receive moderately or highly emetogenic chemotherapy and who have been reported to achieve poor symptom control.4 Over-prophylaxis also has drawbacks. For example, antiemetics are expensive and, at times, they can induce their own adverse events, such as lethargy, dyskinesia, constipation, headaches, hiccups, fatigue, and even cardiac arrhythmias.5 The best approach is to appropriately match the antiemetic to the chemotherapy. Indeed, adherence to evidence-based guidelines has yielded success in symptom control, but the guidelines work on the assumption that the emetogenic potential of new chemotherapy agents has been accurately determined and then disseminated to and acted upon by health care providers.6,7 To our knowledge, no previous studies have tested that assumption, as we do in the present study.
Trifluridine-tipiracil was selected as the focus of this project and as illustrative of other newly approved chemotherapy agents for two reasons. First, it became available for routine prescribing in pretreated patients with metastatic colorectal cancer in the United States in September 2015.1 That timing allowed us to analyze much of the early prescribing period, both during the 9 months before approval, when the drug was available on a compassionate-use basis at our institution, and the 3 months after approval. Second, trifluridine-tipiracil has classifiably low emetogenic potential, and mismatching of antiemetics tends to occur more often with low emetogenic chemotherapy.9 Trifluridine-tipiracil and placebo patients manifest rates of nausea at 48% and 24%, respectively, and rates of vomiting at 28% and 14%, respectively.8
Hence, the goal of this study was to explore whether a guideline-based prophylactic antiemetic regimen was appropriately matched to the new chemotherapy agent, trifluridine-tipiracil, to report whether such symptoms of nausea and vomiting are kept at bay, and to identify a potentially vulnerable interval – immediately after drug approval – when cancer patients may be at risk for CINV because of poor adherence to antiemetic guideline prescribing practices by health care providers.
Methods
Overview
The Mayo Clinic Institutional Review Board approved this study. We obtained the identifying information of all patients treated with trifluridine-tipiracil at our institution from the Mayo Clinic Specialty Pharmacy, which uses an electronic prescribing system that contributed to the comprehensiveness of the data set. Patients included those who had participated in a colorectal cancer compassionate-use program before the September 2015 approval of the drug and those who received the drug shortly after its approval. In essence, this retrospective, single-institution study included every patient who received trifluridine-tipiracil for metastatic colorectal cancer in 2015 (January through December); this approach enabled us to systematically report on early first-cycle prescribing practices 9 months before and 3 months after the drug’s approval in September of 2015.
Determination of guideline adherence
This project relied on the National Comprehensive Cancer Network (NCCN) Guidelines (v1.2015, behind paywall) because they had been updated in 2015 (and hence coincided with this project’s study dates) to incorporate recommendations specific to oral chemotherapy and because they seemed concordant with other guidelines.10,11
Antiemetic prophylaxis for a specific patient was deemed guideline adherent if a version of the recommended NCCN antiemetic regimen had been prescribed during the first cycle of chemotherapy. This regimen consisted of metoclopramide, prochlorperazine, haloperidol, or a 5-hydroxytryptamine receptor antagonist. In contrast, if a patient had been prescribed a more aggressive or less aggressive regimen, such prescribing practices were deemed non–guideline adherent/aggressive (received more prophylaxis than called for) or non–guideline adherent/less aggressive (including no antiemetics), respectively. Again, medical record prescribing determined adherence.
Data reporting
The primary goal of this study was to report the percentage of patients who had been prescribed a first-cycle antiemetic prophylaxis regimen concordant with NCCN guidelines. Secondary goals included reporting the incidence of nausea and vomiting, the use of rescue antiemetics other than those prescribed up front, the need for an unplanned medical encounter to address nausea and vomiting, and change in antiemetic prescribing before the second chemotherapy cycle. Confidence intervals were calculated with JMP Pro 10.0.0. This study was too limited in sample size to assess sex-based differences in outcomes.
Results
Demographics
This report focuses on 44 patients who received first-cycle trifluridine-tipiracil during the first calendar year of the drug’s FDA approval. All patients had metastatic colorectal cancer and had previous exposures to other chemotherapy agents (Table 1). Of note, 28 patients (64%) had experienced CINV before starting trifluridine-tipiracil and all these patients had been heavily pretreated with multiple lines of chemotherapy.
Guideline adherence
Patients were most commonly prescribed prochlorperazine and ondansetron prophylaxis for CINV before the first chemotherapy cycle of trifluridine-tipiracil (Table 2): 15 patients were prescribed combination antiemetic therapy, typically two of the most commonly prescribed single agents with different mechanisms of action. Twenty-five patients (57%; 95% confidence interval (CI): 42%, 70%) were prescribed antiemetics in a manner consistent with guidelines; 15 (34%; 95% CI: 22%, 49%) were prescribed antiemetics in a non–guideline-adherent/more aggressive manner (received more prophylaxis than called for); and 4 (9%; 95% CI: 4%, 21%) were prescribed them in a non–guideline-adherent/less aggressive manner.
Clinical outcomes based on guideline adherence
In guideline-adherent patients, first-cycle nausea and vomiting occurred in 13 patients (52%) and 6 patients (24%), respectively, with 1 patient requiring an unscheduled clinic visit and another an emergency department visit and hospital admission – all for nausea and vomiting (Table 3). In non–guideline-adherent/more aggressive patients, those symptoms occurred in 5 patients (33%, nausea) and 4 patients (27%, vomiting), with 1 patient requiring a clinic visit and emergency department visit and another an emergency department visit – again, all for nausea and vomiting. In non–guideline-adherent/less aggressive patients, no nausea or vomiting was reported.
Discussion
This study examined adherence to antiemetic guidelines in the setting of a soon-to-be-approved or newly approved antineoplastic agent. As hypothesized, a substantial proportion of patients (43% in this study) were prescribed antiemetics in a nonadherent manner with respect to guidelines, thus identifying the period shortly before and after FDA approval as a particularly vulnerable interval with respect to antiemetic guideline adherence. It is possible that our institution’s practice of testing novel chemotherapy agents for the treatment of colorectal cancer prompted a heightened awareness of potential adverse events, leading to greater guideline adherence than might have occurred in other settings and resulting in judicious straying from guideline adherence only when appropriate.12-14 Thus, these high rates of poor adherence may in fact represent an underestimate of what one might see in other clinical practices; and, similarly, these rates of symptom control might also be more favorable than those one might see in other clinical practices. To our knowledge, antiemetic prescribing practices with newer chemotherapy agents have not been explored before now, and our data underscore a clear need to do so – particularly during this limited interval when health care providers begin to prescribe new chemotherapy agents for the first time.
It is worth noting that despite the high rates of guideline nonadherence, rates of nausea and vomiting seemed to be comparable in patients prescribed antiemetics in a guideline-adherent manner and those prescribed antiemetics in a non–guideline-adherent/aggressive manner.A small number of patients in both the guideline-adherent and non–guideline-adherent/aggressive groups required rescue medications, unscheduled medical visits for nausea and vomiting, and additional antiemetics during the second cycle of chemotherapy. Of note,none of those interventions occurred in patients who were prescribed antiemetics in a non–guideline-adherent/less aggressive manner. These findings might reflect the fact that the patients had proven themselves to be at risk for nausea and vomiting with previous chemotherapy. Before they became candidates for trifluridine-tipiracil, patients had been heavily pretreated with other chemotherapy agents, most had experienced CINV, and many were therefore highly predisposed to nausea and vomiting. These observations underscore the fact that guidelines – even those that are well accepted and widely used – should be implemented in concert with good clinical judgment.10,11 This study has shortcomings, most notably its small sample size. However, had we extended our study beyond 3 months of the FDA approval to include more patients, our findings would have reflected more experienced prescribing practices and we thereby would have deviated from our primary goal of assessing antiemetic prescribing practices with only recently-approved and available chemotherapy agents. In this context, this limited sample size aptly serves a primary role of capturing outcomes within a fleeting but critical interval of new drug availability.In summary, this study found a notable rate of poor guideline adherence when prescribing antiemetics for trifluridine-tipiracil, a new chemotherapy agent of low emetogenic potential. Although the resultant rates of nausea and vomiting suggest that good clinical judgment might have influenced whether or not guidelines were adhered to, these findings nonetheless underscore the need to assess adherence to antiemetic guidelines when new chemotherapy drugs become available and potentially to put in place institutional infrastructure rapidly to promote improved adherence. Such an assessment should be deliberate, formalized, and prompt within individual oncology clinics and cancer centers after a new cancer drug becomes available. In conjunction with clinical judgment, such measures might lead to improved symptom control.
Acknowledgment
This paper is based on a poster that was presented at the 2016 Palliative Care in Oncology Symposium, on September 10, 2016: Adherence to antiemetic guidelines with a newly approved chemotherapy agent, trifluridine-tipiracil (TAS-102): a single-institution study. Daniel Childs and Aminah Jatoi, Mayo Clinic, Rochester, MN. http://meetinglibrary.asco.org/record/136444/abstract. J Clin Oncol. 2016;34(suppl 26S):abstract 221.
1. CenterWatch. FDA website. FDA approved drugs for oncology: drugs approved for 2015. https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Last updated April 2017. Accessed June 4, 2016.
2. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-1367.
3. Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3. Support Care Cancer. 2016;24:2661-2667.
4. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
5. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf. 2016; 15:343-356.
6. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract. 2014;10:68-74.
7. Mertens WC, Higby DJ, Brown D, et al. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol. 2003;21:1373-1378.
8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
9. Schwartzberg L, Morrow G, Balu S, et al. Chemotherapy-induced nausea and vomiting and antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists in patients with cancer treated with low emetogenic chemotherapy in a hospital outpatient setting in the United States. Curr Med Res Opin. 2011;27:1613-1622.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Antiemesis, Version1,2015 [behind paywall]. https://www.nccn.org. Last update not known. Accessed June 4, 2016.
11. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:v232-v243.
12. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 study. Lancet. 2013;381:303-312.
13. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393.
14. Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353.
1. CenterWatch. FDA website. FDA approved drugs for oncology: drugs approved for 2015. https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Last updated April 2017. Accessed June 4, 2016.
2. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-1367.
3. Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3. Support Care Cancer. 2016;24:2661-2667.
4. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
5. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf. 2016; 15:343-356.
6. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract. 2014;10:68-74.
7. Mertens WC, Higby DJ, Brown D, et al. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol. 2003;21:1373-1378.
8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
9. Schwartzberg L, Morrow G, Balu S, et al. Chemotherapy-induced nausea and vomiting and antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists in patients with cancer treated with low emetogenic chemotherapy in a hospital outpatient setting in the United States. Curr Med Res Opin. 2011;27:1613-1622.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Antiemesis, Version1,2015 [behind paywall]. https://www.nccn.org. Last update not known. Accessed June 4, 2016.
11. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:v232-v243.
12. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 study. Lancet. 2013;381:303-312.
13. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393.
14. Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353.
Physician attitudes and prevalence of molecular testing in lung cancer
Lung cancer is the leading cause of cancer death in the United States. It is estimated that there will be 222,500 new cases of lung cancer and 155,870 deaths from lung cancer in 2017. Non–small-cell lung carcinoma (NSCLC) accounts for 80%-85% of lung cancers, with adenocarcinoma being the most common histologic subtype. Other less common subtypes include squamous-cell carcinoma, large-cell carcinoma, and NSCLC that cannot be further classified.1 Nearly 70% of patients present with locally advanced or metastatic disease at the time of diagnosis and are not candidates for surgical resection.2 For that group of patients, the mainstay of treatment is platinum-based chemotherapy with or without radiation therapy. Patients who are chemotherapy naive often experience a modest response, however; durable remission is short lived, and the 5-year survival rate remains staggeringly low.3 Improved understanding of the molecular pathways that drive malignancy in NSCLC has led to the development of drugs that target specific molecular pathways.4 By definition, these driver mutations facilitate oncogenesis by conferring a selective advantage during clonal evolution.5 Moreover, agents targeting these pathways are extremely active and induce durable responses in many patients.6,7,8
Predictive biomarkers in NSCLC include anaplastic lymphoma kinase (ALK) fusion oncogene and sensitizing epidermal growth factor receptor (EGFR) mutations. Mutations in the EGFR tyrosine kinase are observed in about 15%-20% of NSCLC adenocarcinomas in the United States and upward of 60% in Asian populations. They are also found more frequently in nonsmokers and women.6 The two most prevalent mutations in the EGFR tyrosine kinase domain are in-frame deletions of exon 19 and L858R substitution in exon 21, representing about 45% and 40% of mutations, respectively.9 Both mutations result in activation of the tyrosine kinase domain, and both are associated with sensitivity to the small-molecule tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, and afatinib.10 Other drug-sensitive mutations include point mutations at exon 21 (L861Q) and exon 18 (G719X).11 Targeted therapy produces durable responses in the majority of patients.12,13,14 Unfortunately, most patients develop acquired resistance to these therapies, which leads to disease progression.4,15-17
ALK gene rearrangements, although less prevalent, are another important molecular target in NSCLC and are seen in 2%-7% of cases in the United States.7 As with EGFR mutations, these mutations are more prevalent in nonsmokers, and they are found more commonly in younger patients and in men.8
Identification of driver mutations early in the course of disease and acquired resistance mutations later are crucial for the optimal management of advanced NSCLC. DNA analysis using polymerase chain reaction (PCR) and next-generation sequencing is the preferred method for testing for EGFR mutations, and ALK rearrangements are generally tested either by flourescence in situ hybridization (FISH) or immunohistochemistry.18,19 Newer blood-based assays have shown great promise, and clinicians may soon have the ability to monitor subtle genetic changes, identify resistance patterns, and change therapy when acquired resistance occurs.20
The American College of Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology have proposed guidelines for molecular testing in lung cancer. It is recommended that all advanced squamous and nonsquamous cell lung cancers with an adenocarcinoma component should be tested for EGFR and ALK mutations independent of age, sex, ethnicity, or smoking history. In the setting of smaller lung cancer specimens (eg, from biopsies, cytology) where an adenocarcinoma component cannot be completely excluded, EGFR and ALK testing may be performed in cases showing squamous or small cell histology but clinical criteria (eg, young age, lack of smoking history) may be useful in selecting a subset of these samples for testing. Samples obtained through surgical resection, open biopsy, endoscopy, transthoracic needle biopsy, fine-needle aspiration, and thoracentesis are all considered suitable for testing, but large biopsy samples are generally preferred over small biopsy samples, cell-blocks, and cytology samples.21 Despite this recommendation, not all patients who are eligible for mutation analysis are tested. At our institution, preliminary observations suggested that the percentage of patients being tested and the prevalence of driver mutations were significantly lower compared with published data. The purpose of this study was to evaluate physician attitudes about molecular testing, and to determine the rate of testing, the effect of biopsy sample size on rate of testing, and the prevalence of driver mutations at our institution.
Methods
In this retrospective clinical study, we identified 206 cases of advanced nsNSCLC from the tumor registry (February 2011-February 2013). Registry data was obtained from three hospitals within our health network – two academic tertiary care centers, and one community-based hospital. The other hospitals in the network were excluded because their EHR systems were not integrated with the rest of the hospitals and/or there was a lack of registry data. The testing rates for driver mutations, prevalence of driver mutations, and the tissue procurement techniques were obtained from individual chart review. Surgical specimens, core biopsy samples, and large volume thoracentesis specimens were categorized as large biopsy samples, and samples obtained by fine-needle aspiration, bronchial washing, and bronchial brushing were considered small biopsy samples. We used a chi-square analysis to compare mutation testing rates between the large and small biopsy sample groups. The prevalence of driver mutations was determined, excluding unknown or inadequate samples.
EGFR analysis had been conducted at Integrated Oncology, using formalin-fixed, paraffin-embedded tissue. Genomic DNA was isolated, and EGFR mutation analysis was performed using SNaPShot multiplex PCR, primer extension assay for exons 18-21; samples with >4mm2 and ≥50% tumor content were preferred. Macrodissection was used to enrich for tumor cells when samples had lower tumor cellularity and content. ALK rearrangements were tested in the hospital using the Vysis ALK Break Apart FISH probe kit (Abott Molecular Inc, Des Plaines, IL).
We conducted a web-based, 20-question survey about molecular profiling among 110 practitioners to gauge their knowledge and opinions about molecular testing. The practitioners included medical oncologists, thoracic surgeons, pulmonologists, and interventional radiologists. Each received an initial e-mail informing them of the study, inviting them to complete survey, and providing a link to it, and two reminder e-mails at biweekly intervals to maximize survey participation and responses. The questions were aimed at understanding the challenges surrounding molecular testing within our network. Apart from the questions gathering demographic information about the respondents, the questions were intended to highlight the disparities between guideline recommendations and physician practices; to gauge the perceived importance of molecular evaluation; to identify individual, subspecialty, and hospital-based challenges; and to assess physician attitudes toward alternatives to traditional tissue-based testing (Table 1, p. e150). Nineteen of the questions were structured as single or best answer, whereas Question 9, which was aimed at identifying system-based challenges, allowed for multiple answer selections.
Results
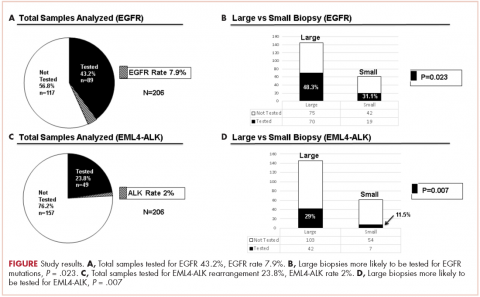
There were a total of 206 cases of advanced stage IIIb or IV nsNSCLC identified at three hospitals during 2011-2013. Of those 206 cases, 161 (78.2%) were recorded at the two large academic medical centers, and 45 (21.9%) were recorded at the smaller community-based hospital. Of the total, there were 145 (70.4%) large biopsy specimens and 61 (29.6%) small biopsy specimens. We found that 89 of the 206 cases (43.2 %) had been tested for EGFR mutations, and 49 (23.8%) had been tested for ALK rearrangements (Figure, A and C). In all, 70 (48.3%) large-sample biopsies and 19 (31.1%) small-sample biopsies were submitted for EGFR analysis (Figure, B), and 42 (29%) large-sample biopsies and 7 (11.5%) small-sample biopsies were tested for ALK rearrangements (Figure, D). Large-sample biopsies were more likely to be analyzed for EGFR mutations and ALK rearrangements, with the results reaching statistical significance (P = .023 and P = .007, respectively). Across all samples, a total of 7 EGFR mutations and 1 ALK rearrangement were identified, yielding a prevalence of 7.9% and 2% respectively (Figure, A and C).
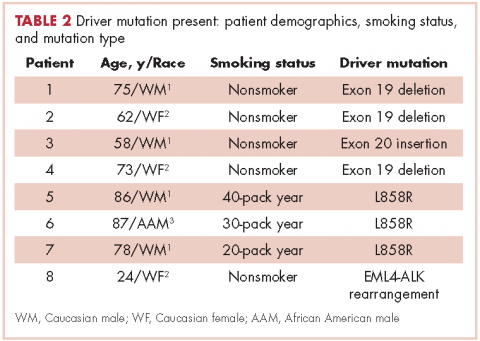
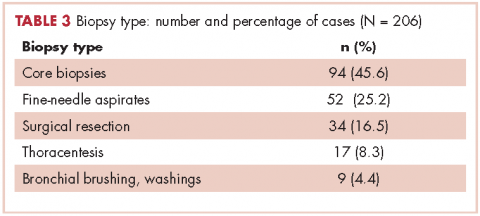
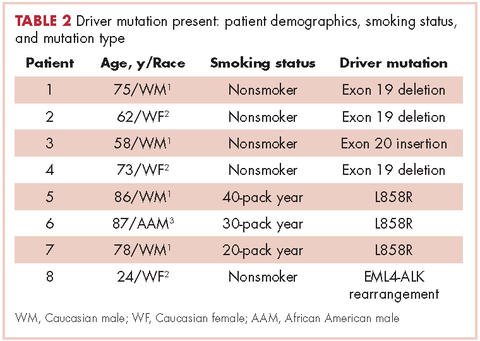
Table 2 shows the demographics, smoking status and type of driver mutation identified. Core biopsies were obtained in 45.6% of the cases and fine-needle aspiration biopsies were obtained in 25.2% of the cases with surgical resections, with thoracentesis and bronchial washings comprising the rest of the biopsies (Table 3).
The average age at diagnosis of the patients in the cases that were analyzed was 69.3 years. Most of the patients (83.9%) identified as white, 3.8% were African American, and 12.6% were in the Unknown category. Of the total number of patients, 11 were identified as never-smokers (5.3%), 50 (24.3%) had a 1-15 pack-year smoking history, 104 (50.5%) had a 16-45 pack-year smoking history, and 41 (19.9%) had a >45 pack-year smoking history.
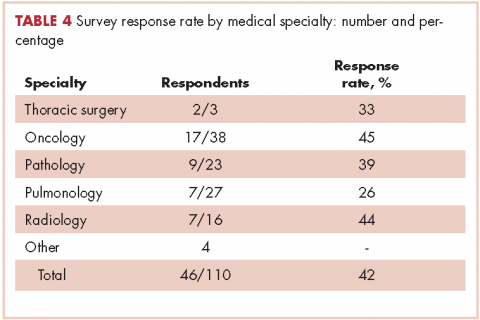
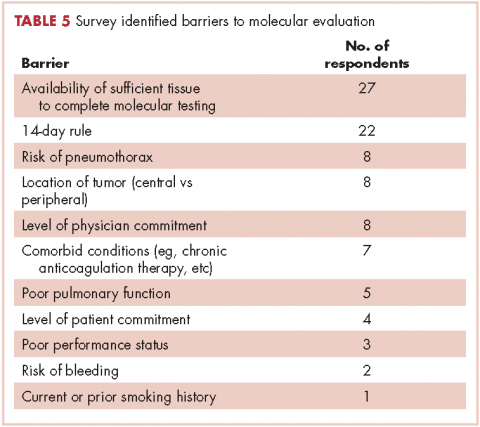
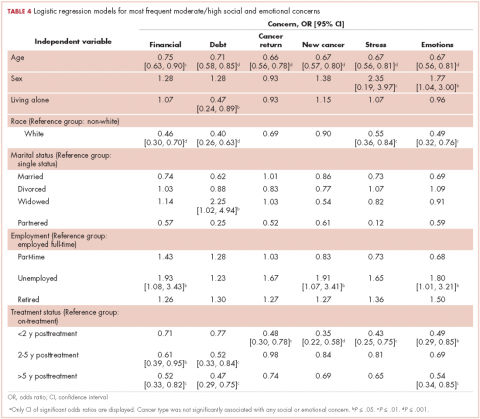
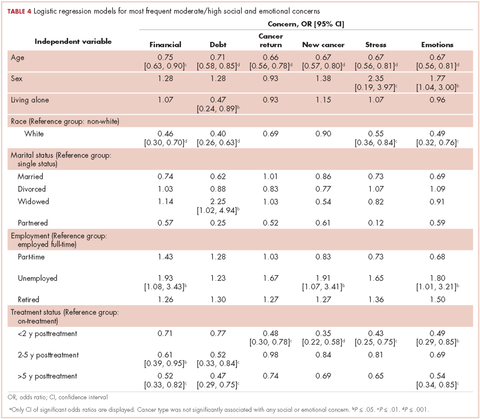
In regard to the survey, 46 of the 110 physicians asked to participate in the survey responded, representing a response rate of 41.8% (range across medical specialties, 26%-45%, Table 4). Of those respondents, 38 (82.6%) indicated they believed molecular evaluation was a very important aspect of NSCLC care, with the remainder indicating it was somewhat important. 91.4% of the respondents who routinely ordered molecular testing agreed that stage IIIb or IV nsNSCLC should undergo molecular evaluation.
The top barriers to molecular evaluation identified through this survey were the availability of sufficient tissue to complete molecular testing and the Center for Medicare and Medicaid Services’s (CMS’s) 14-day rule that requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing (Table 5).
Discussion
The treatment of advanced nsNSCLC has evolved significantly over the past decade. Molecular profiling is now an essential part of initial evaluation, and larger-sample biopsies are needed to ensure accurate evaluation and appropriate treatment. The detection of EGFR and EML4-ALK driver mutations are associated with increased response to tyrosine kinase inhibitors and are associated with improvement in progression-free survival, patient quality of life, and even overall survival in some studies.12,22,23,24 Early identification of these driver mutations is crucial, however, preliminary observation in our network suggested that a large percentage of patients with advanced nsNSCLC in were not being appropriately evaluated for those mutations. To evaluate our molecular profiling rates, we conducted a retrospective study and reviewed 3 years of registry data at 3 hospitals within our health system. Two of the hospitals included in our analysis were large tertiary academic centers, and one was a community hospital. Our findings confirmed that a large percentage of our patients who are eligible for molecular evaluation are not tested: 56.7% of cases were not tested for EGFR mutations, and 76.2% of cases were not tested for ALK rearrangements.
In a similar study, the Association for Community Cancer Centers conducted a project aimed at understanding the landscape and current challenges for molecular profiling in NSCLC. Eight institutions participated in the study, and baseline testing rates were analyzed. The findings demonstrated that high-volume institutions (treating >100 lung cancer patients a year tested 62% and 60% of advanced lung cancer patients for EGFR and EML4-ALK, respectively, and low-volume institutions (treating <100 lung cancer patients a year tested 52% and 47% for EGFR and EML4-ALK, respectively.25,26 In a recent international physician self-reported survey, Spicer and colleagues found that EGFR testing was requested before first-line therapy in patients with stage IIIB or IV disease in 81% of cases, and mutation results were available before start of therapy in 77% of the cases.27 Those percentages are relatively low, given that current guidelines recommend that molecular testing should be done for all patients with stage IIIB or IV nsNSCLC. This highlights the need for objective performance feedback so oncologists can make the necessary practice changes so that molecular testing is done before the start of therapy to ensure high-quality cancer care that will translate into better, cost-effective outcomes and improved patient quality of life.
Our study findings showed that the prevalence of EGFR and ALK mutations is substantially lower among the patients we treat in our network compared with other published data on prevalence. The reason for those low rates is not clear, but it is likely multifactorial. First, Western Pennsylvania, the region our network serves, has a large proportion of older adults – 17.3% of the population is older than 65 years (national average, 14.5%) and advanced age might have contributed to the lower EGFR and ALK rates measured in our study.28 Second, the smoking rate in Pennsylvania is higher than the national average, 20%-24% compared with 18%, respectively.29 Third, the air quality in Western Pennsylvania has historically been very poor as a result of the large steel and coal mining industries. Even though the air quality has improved in recent decades, the American Lung Association’s 2017 State of the Air report ranked Pittsburgh and surrounding areas in Western Pennsylvania among the top 25 most air polluted areas in the United States.30 It is not certain whether air pollution and air quality have any impact on driver mutation rates, but the correlation with smoking, ethnicity, and geographic distribution highlight the need for further epidemiologic studies.
Biopsy sufficiency – getting an adequate amount of sample tissue during biopsy – is a known challenge to molecular profiling, and we found that biopsy sample size had an impact on the testing rates in a large percentage of our cases. To fully understand the impact of biopsy sufficiency, we conducted a subset analysis and compared the testing rates between our large and small biopsy samples. Our analysis showed that larger-sample biopsies were more likely to be tested for mutations than were smaller-sample biopsies (EGFR: P = .023; ALK: P = .007).
Those results suggest that larger-sample biopsies should be encouraged, but procedural risks, tumor location, and patient age and wishes need to be considered before tissue acquisition.21 Furthermore, clinicians who are responsible for tissue procurement need to be properly educated on the tissue sample requirements and the impact these results have on treatment decisions.31 Our institution, like many others, has adopted rapid onsite evaluation (ROSE) of biopsy samples, whereby a trained cytopathologist reviews sample adequacy at the time of tissue procurement. Although there is scant data directly comparing molecular testing success rates with and without the ROSE protocol, a meta-analysis conducted by Schmidt and colleagues concluded that ROSE improved the adequacy rate of fine-needle aspiration cytology by 12%.32,33 Given that molecular profiling depends on both the absolute and relative amount of tumor cells present in the sample, the ROSE protocol likely enhances the procedural success rate and reduces the need for repeat and subsequent biopsies.
It is interesting to note that our data also demonstrated that we are obtaining large-sample biopsies in most of our patients (about 70%). However, we are still failing to test more than half of our cases for driver mutations (Figure, A and C). This strongly suggests there are additional factors beyond tissue adequacy that are contributing to our high failure rate. It is essential to understand the dynamics and system practices that influence testing rates if we are to improve the care and outcomes of our cancer patients. To better understand those barriers, we surveyed 110 practitioners (including medical oncologists, pulmonologists, thoracic surgeons, and interventional radiologists) about the molecular profiling process and their responses highlighted several important areas that deserve special attention (Tables 1, 4, 5).
In our institution, testing initiation is primarily the responsibility of the treating medical oncologist. This presents a challenge because there is often a significant delay between tissue acquisition, histologic confirmation, and oncologic review. Many institutions have adopted pathology-driven reflex testing to help overcome such delays. Automatic testing after pathologic confirmation streamlines the process, increases testing rates, and eliminates unnecessary delay between the time of diagnosis and the time of test ordering.34 It also allows for the molecular and histologic diagnosis to be integrated into a single pathology report before therapy is initiated.
Another barrier to timely testing according to the respondents, was the CMS’s 14-day rule. The 14-day rule requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing and was frequently identified as a cause for significant delay in testing and having an impact on first-line treatment decisions.35,36
Often clinicians will choose to defer testing until this time has elapsed to reduce the financial burden placed on the hospital but by that time, they might well have initiated treatment without knowing if the patient has a mutation. This is a significant challenge identified by many of our oncologists, and is a limitation to our analysis above as it is unclear what percentage of patients received follow up testing once care was established at an outside facility and once the 14-day time period had elapsed.
The data from our institution suggests there is discordance between physician attitudes and molecular testing practices. However, there are several limitations in our study. First, most of the survey respondents agreed that molecular testing is an important aspect of treating advanced lung cancer patients, but the retrospective nature of the study made it difficult to identify why testing was deferred or never conducted. Second, the absence of a centralized reporting system for molecular testing results at our institution, may have resulted in an overestimation of our testing failure rate in cases where results were not integrated our electronic medical record.
Third, the low survey response rate only allowed us to make generalizations regarding the conclusions, although it does provide a framework for future process improvements.
We believe the poor testing rates observed in our study are not isolated to our institution and reflect a significant challenge within the broader oncology community.27 A system of best practices is essential for capturing this subset of patients who are never tested. There is agreement among oncologists that improving our current testing rates will require a multidisciplinary approach, a refined process for molecular evaluation, a push toward reflex testing, and standardization of biopsy techniques and tissue handling procedures. In our institution, we have initiated a Lean Six Sigma and PDSA (plan, do, study, act) initiative to improve our current molecular testing process. In addition, because obtaining larger-sample biopsies or additional biopsies is often not feasible for many of our advanced cancer patients, we have started using whole blood circulating tumor cells (CTC) and plasma ctDNA (cell-free circulating DNA) for molecular testing. Recent studies have shown high concordance (89%) between tissue biopsies and blood-based mutation testing, which will likely have a positive impact on the cancer care of our patients and help to capture a subset of patients who are not candidates for traditional biopsies.37
Conclusions
Despite current guidelines for testing driver mutations in advanced nsNSCLC, a large segment of our patients are not being tested for those genetic aberrations. There are several barriers that continue to thwart the recommendation, including failure to integrate driver mutation testing into routine pathology practice (ie, reflex testing), insufficient tissue obtained from biopsy, and difficulty in obtaining tissue because of tumor location or risk of complications from the biopsy procedure. More important, these trends are not isolated to our institution and reflect a significant challenge within the oncology community. Our data show that for the purpose of driver mutation testing, larger-sample biopsies, such as surgical/core biopsies, are better than small-sample biopsies, such as needle aspiration. We have also demonstrated that the prevalence of driver mutations is lower in Western Pennsylvania, which is served by our network, than elsewhere in the United States.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
3. Kim TE, Murren JR. Therapy for stage IIIB and stage IV non-small cell lung cancer. Clin Chest Med. 2002;23(1):209-224.
4. Black RC, Khurshid H. NSCLC: An update of driver mutations, their role in pathogenesis and clinical significance. R I Med J (2013). 2015;98(10):25-28.
5. Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306-313.
6. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334.
7. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866-2874.
8. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246.
9. Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(suppl 1):S24-31.
10. Langer CJ. Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: is afatinib better or simply newer? J Clin Oncol. 2013;31(27):3303-3306.
11. Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12(24):7232-7241.
12. Shi Y, Siu-Kie JA, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154-162.
13. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957.
14. Khozin S, Blumenthal GM, Jiang X, et al. US Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19(7):774-779.
15. Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220-229.
16. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73.
17. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240-2247.
18. Ellison G, Zhu G, Moulis A, Dearden S, et al. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66(2):79-89.
19. Alì G, Proietti A, Pelliccioni S, et al. ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med. 2014;138(11):1449-1158.
20. Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472-484.
21. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
22. Kwak EL, Bany YJ, Cambridge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693-1703.
23. Shaw A, Yeap BY, Kenudson MM, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247-4253.
24. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388.
25. Association of Community Cancer Centers. Molecular Testing in the Community Setting. In: Molecular testing: resources and tools for the multidisciplinary team. http://accc-cancer.org/resources/molecularTesting-Overview.asp. Accessed November 15, 2015.
26. Association of Community Cancer Centers. Molecular testing: ACCC peer-to-peer webinars. The tissue issue: sampling and testing with Gail Probst, RN, MS, AOCN. https://www.youtube.com/watch?v=lapmni938Mc&feature=youtu.be. Published September 14, 2015. Accessed November 2015.
27. Spicer J S, Tischer B, Peters M. EGFR mutation testing and oncologist treatment choice in advanced NSCLC: global trends and differences. Ann Oncol. 2015;26(suppl 1):i60.
28. West L, Cole S, Goodkind D. US Census Bureau, 65+ in the United States: 2010, U.S. Government Printing Office, Washington, DC, 2014
29. Centers for Disease Control and Prevention. State tobacco activities tracking and evaluation system. Current cigarette use among adults (Behavior Risk Factor Surveillance System) 2015. https://www.cdc.gov/statesystem/cigaretteuseadult.html. Last updated September 16, 2016. Accessed May 26, 2017.
30. The American Lung Association. State of the Air 2017. http://www.lung.org/assets/documents/healthy-air/state-of-the-air/state-of-the-air-2017.pdf. Published 2017. Accessed May 26, 2017.
31. Gaga M, Powell CA, Schraufnagel DE, Schönfeld N, et al. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. 2013;188(4):503-507.
32. Ferguson PE, Sales CM, Hodges DC, et al. Effects of a multidisciplinary approach to improve volume of diagnostic material in CT-guided lung biopsies. PLoS One. 2015 Oct 19;10(10).
33. Schmidt RL, Witt BL, Lopez-Calderon LE, et al. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol. 2013;139(3):300-309.
34. Cengiz Inal, Yilmaz E, Chenget H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: Experience from a community-based academic center. J Clin Oncol. 2014;32(suppl):5s. [abstract 8098].
35. Grzegorz K, Leighl, M. Challenges in NSCLC molecular testing barriers to implementation. Oncology Exchange. 2012;11(4):8-10.
36. Lynch JA, Khoury MJ, Ann Borzecket A, et al. Utilization of epidermal growth factor receptor (EGFR) testing in the United States: a case study of T3 translational research. Genet Med. 2013;15(8):630-638.
37. Reck M. Investigating the utility of circulating-free tumour-derived DNA (ctDNA) in plasma for the detection of epidermal growth factor receptor (EGFR) mutation status in European and Japanese patients (pts) with advanced non-small-cell lung cancer (NSCLC): ASSESS study. Presented at the European Lung Cancer Conference (ELCC) Annual Meeting, Geneva; 15-18 April 2015.
Lung cancer is the leading cause of cancer death in the United States. It is estimated that there will be 222,500 new cases of lung cancer and 155,870 deaths from lung cancer in 2017. Non–small-cell lung carcinoma (NSCLC) accounts for 80%-85% of lung cancers, with adenocarcinoma being the most common histologic subtype. Other less common subtypes include squamous-cell carcinoma, large-cell carcinoma, and NSCLC that cannot be further classified.1 Nearly 70% of patients present with locally advanced or metastatic disease at the time of diagnosis and are not candidates for surgical resection.2 For that group of patients, the mainstay of treatment is platinum-based chemotherapy with or without radiation therapy. Patients who are chemotherapy naive often experience a modest response, however; durable remission is short lived, and the 5-year survival rate remains staggeringly low.3 Improved understanding of the molecular pathways that drive malignancy in NSCLC has led to the development of drugs that target specific molecular pathways.4 By definition, these driver mutations facilitate oncogenesis by conferring a selective advantage during clonal evolution.5 Moreover, agents targeting these pathways are extremely active and induce durable responses in many patients.6,7,8
Predictive biomarkers in NSCLC include anaplastic lymphoma kinase (ALK) fusion oncogene and sensitizing epidermal growth factor receptor (EGFR) mutations. Mutations in the EGFR tyrosine kinase are observed in about 15%-20% of NSCLC adenocarcinomas in the United States and upward of 60% in Asian populations. They are also found more frequently in nonsmokers and women.6 The two most prevalent mutations in the EGFR tyrosine kinase domain are in-frame deletions of exon 19 and L858R substitution in exon 21, representing about 45% and 40% of mutations, respectively.9 Both mutations result in activation of the tyrosine kinase domain, and both are associated with sensitivity to the small-molecule tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, and afatinib.10 Other drug-sensitive mutations include point mutations at exon 21 (L861Q) and exon 18 (G719X).11 Targeted therapy produces durable responses in the majority of patients.12,13,14 Unfortunately, most patients develop acquired resistance to these therapies, which leads to disease progression.4,15-17
ALK gene rearrangements, although less prevalent, are another important molecular target in NSCLC and are seen in 2%-7% of cases in the United States.7 As with EGFR mutations, these mutations are more prevalent in nonsmokers, and they are found more commonly in younger patients and in men.8
Identification of driver mutations early in the course of disease and acquired resistance mutations later are crucial for the optimal management of advanced NSCLC. DNA analysis using polymerase chain reaction (PCR) and next-generation sequencing is the preferred method for testing for EGFR mutations, and ALK rearrangements are generally tested either by flourescence in situ hybridization (FISH) or immunohistochemistry.18,19 Newer blood-based assays have shown great promise, and clinicians may soon have the ability to monitor subtle genetic changes, identify resistance patterns, and change therapy when acquired resistance occurs.20
The American College of Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology have proposed guidelines for molecular testing in lung cancer. It is recommended that all advanced squamous and nonsquamous cell lung cancers with an adenocarcinoma component should be tested for EGFR and ALK mutations independent of age, sex, ethnicity, or smoking history. In the setting of smaller lung cancer specimens (eg, from biopsies, cytology) where an adenocarcinoma component cannot be completely excluded, EGFR and ALK testing may be performed in cases showing squamous or small cell histology but clinical criteria (eg, young age, lack of smoking history) may be useful in selecting a subset of these samples for testing. Samples obtained through surgical resection, open biopsy, endoscopy, transthoracic needle biopsy, fine-needle aspiration, and thoracentesis are all considered suitable for testing, but large biopsy samples are generally preferred over small biopsy samples, cell-blocks, and cytology samples.21 Despite this recommendation, not all patients who are eligible for mutation analysis are tested. At our institution, preliminary observations suggested that the percentage of patients being tested and the prevalence of driver mutations were significantly lower compared with published data. The purpose of this study was to evaluate physician attitudes about molecular testing, and to determine the rate of testing, the effect of biopsy sample size on rate of testing, and the prevalence of driver mutations at our institution.
Methods
In this retrospective clinical study, we identified 206 cases of advanced nsNSCLC from the tumor registry (February 2011-February 2013). Registry data was obtained from three hospitals within our health network – two academic tertiary care centers, and one community-based hospital. The other hospitals in the network were excluded because their EHR systems were not integrated with the rest of the hospitals and/or there was a lack of registry data. The testing rates for driver mutations, prevalence of driver mutations, and the tissue procurement techniques were obtained from individual chart review. Surgical specimens, core biopsy samples, and large volume thoracentesis specimens were categorized as large biopsy samples, and samples obtained by fine-needle aspiration, bronchial washing, and bronchial brushing were considered small biopsy samples. We used a chi-square analysis to compare mutation testing rates between the large and small biopsy sample groups. The prevalence of driver mutations was determined, excluding unknown or inadequate samples.
EGFR analysis had been conducted at Integrated Oncology, using formalin-fixed, paraffin-embedded tissue. Genomic DNA was isolated, and EGFR mutation analysis was performed using SNaPShot multiplex PCR, primer extension assay for exons 18-21; samples with >4mm2 and ≥50% tumor content were preferred. Macrodissection was used to enrich for tumor cells when samples had lower tumor cellularity and content. ALK rearrangements were tested in the hospital using the Vysis ALK Break Apart FISH probe kit (Abott Molecular Inc, Des Plaines, IL).
We conducted a web-based, 20-question survey about molecular profiling among 110 practitioners to gauge their knowledge and opinions about molecular testing. The practitioners included medical oncologists, thoracic surgeons, pulmonologists, and interventional radiologists. Each received an initial e-mail informing them of the study, inviting them to complete survey, and providing a link to it, and two reminder e-mails at biweekly intervals to maximize survey participation and responses. The questions were aimed at understanding the challenges surrounding molecular testing within our network. Apart from the questions gathering demographic information about the respondents, the questions were intended to highlight the disparities between guideline recommendations and physician practices; to gauge the perceived importance of molecular evaluation; to identify individual, subspecialty, and hospital-based challenges; and to assess physician attitudes toward alternatives to traditional tissue-based testing (Table 1, p. e150). Nineteen of the questions were structured as single or best answer, whereas Question 9, which was aimed at identifying system-based challenges, allowed for multiple answer selections.
Results
There were a total of 206 cases of advanced stage IIIb or IV nsNSCLC identified at three hospitals during 2011-2013. Of those 206 cases, 161 (78.2%) were recorded at the two large academic medical centers, and 45 (21.9%) were recorded at the smaller community-based hospital. Of the total, there were 145 (70.4%) large biopsy specimens and 61 (29.6%) small biopsy specimens. We found that 89 of the 206 cases (43.2 %) had been tested for EGFR mutations, and 49 (23.8%) had been tested for ALK rearrangements (Figure, A and C). In all, 70 (48.3%) large-sample biopsies and 19 (31.1%) small-sample biopsies were submitted for EGFR analysis (Figure, B), and 42 (29%) large-sample biopsies and 7 (11.5%) small-sample biopsies were tested for ALK rearrangements (Figure, D). Large-sample biopsies were more likely to be analyzed for EGFR mutations and ALK rearrangements, with the results reaching statistical significance (P = .023 and P = .007, respectively). Across all samples, a total of 7 EGFR mutations and 1 ALK rearrangement were identified, yielding a prevalence of 7.9% and 2% respectively (Figure, A and C).
Table 2 shows the demographics, smoking status and type of driver mutation identified. Core biopsies were obtained in 45.6% of the cases and fine-needle aspiration biopsies were obtained in 25.2% of the cases with surgical resections, with thoracentesis and bronchial washings comprising the rest of the biopsies (Table 3).
The average age at diagnosis of the patients in the cases that were analyzed was 69.3 years. Most of the patients (83.9%) identified as white, 3.8% were African American, and 12.6% were in the Unknown category. Of the total number of patients, 11 were identified as never-smokers (5.3%), 50 (24.3%) had a 1-15 pack-year smoking history, 104 (50.5%) had a 16-45 pack-year smoking history, and 41 (19.9%) had a >45 pack-year smoking history.
In regard to the survey, 46 of the 110 physicians asked to participate in the survey responded, representing a response rate of 41.8% (range across medical specialties, 26%-45%, Table 4). Of those respondents, 38 (82.6%) indicated they believed molecular evaluation was a very important aspect of NSCLC care, with the remainder indicating it was somewhat important. 91.4% of the respondents who routinely ordered molecular testing agreed that stage IIIb or IV nsNSCLC should undergo molecular evaluation.
The top barriers to molecular evaluation identified through this survey were the availability of sufficient tissue to complete molecular testing and the Center for Medicare and Medicaid Services’s (CMS’s) 14-day rule that requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing (Table 5).
Discussion
The treatment of advanced nsNSCLC has evolved significantly over the past decade. Molecular profiling is now an essential part of initial evaluation, and larger-sample biopsies are needed to ensure accurate evaluation and appropriate treatment. The detection of EGFR and EML4-ALK driver mutations are associated with increased response to tyrosine kinase inhibitors and are associated with improvement in progression-free survival, patient quality of life, and even overall survival in some studies.12,22,23,24 Early identification of these driver mutations is crucial, however, preliminary observation in our network suggested that a large percentage of patients with advanced nsNSCLC in were not being appropriately evaluated for those mutations. To evaluate our molecular profiling rates, we conducted a retrospective study and reviewed 3 years of registry data at 3 hospitals within our health system. Two of the hospitals included in our analysis were large tertiary academic centers, and one was a community hospital. Our findings confirmed that a large percentage of our patients who are eligible for molecular evaluation are not tested: 56.7% of cases were not tested for EGFR mutations, and 76.2% of cases were not tested for ALK rearrangements.
In a similar study, the Association for Community Cancer Centers conducted a project aimed at understanding the landscape and current challenges for molecular profiling in NSCLC. Eight institutions participated in the study, and baseline testing rates were analyzed. The findings demonstrated that high-volume institutions (treating >100 lung cancer patients a year tested 62% and 60% of advanced lung cancer patients for EGFR and EML4-ALK, respectively, and low-volume institutions (treating <100 lung cancer patients a year tested 52% and 47% for EGFR and EML4-ALK, respectively.25,26 In a recent international physician self-reported survey, Spicer and colleagues found that EGFR testing was requested before first-line therapy in patients with stage IIIB or IV disease in 81% of cases, and mutation results were available before start of therapy in 77% of the cases.27 Those percentages are relatively low, given that current guidelines recommend that molecular testing should be done for all patients with stage IIIB or IV nsNSCLC. This highlights the need for objective performance feedback so oncologists can make the necessary practice changes so that molecular testing is done before the start of therapy to ensure high-quality cancer care that will translate into better, cost-effective outcomes and improved patient quality of life.
Our study findings showed that the prevalence of EGFR and ALK mutations is substantially lower among the patients we treat in our network compared with other published data on prevalence. The reason for those low rates is not clear, but it is likely multifactorial. First, Western Pennsylvania, the region our network serves, has a large proportion of older adults – 17.3% of the population is older than 65 years (national average, 14.5%) and advanced age might have contributed to the lower EGFR and ALK rates measured in our study.28 Second, the smoking rate in Pennsylvania is higher than the national average, 20%-24% compared with 18%, respectively.29 Third, the air quality in Western Pennsylvania has historically been very poor as a result of the large steel and coal mining industries. Even though the air quality has improved in recent decades, the American Lung Association’s 2017 State of the Air report ranked Pittsburgh and surrounding areas in Western Pennsylvania among the top 25 most air polluted areas in the United States.30 It is not certain whether air pollution and air quality have any impact on driver mutation rates, but the correlation with smoking, ethnicity, and geographic distribution highlight the need for further epidemiologic studies.
Biopsy sufficiency – getting an adequate amount of sample tissue during biopsy – is a known challenge to molecular profiling, and we found that biopsy sample size had an impact on the testing rates in a large percentage of our cases. To fully understand the impact of biopsy sufficiency, we conducted a subset analysis and compared the testing rates between our large and small biopsy samples. Our analysis showed that larger-sample biopsies were more likely to be tested for mutations than were smaller-sample biopsies (EGFR: P = .023; ALK: P = .007).
Those results suggest that larger-sample biopsies should be encouraged, but procedural risks, tumor location, and patient age and wishes need to be considered before tissue acquisition.21 Furthermore, clinicians who are responsible for tissue procurement need to be properly educated on the tissue sample requirements and the impact these results have on treatment decisions.31 Our institution, like many others, has adopted rapid onsite evaluation (ROSE) of biopsy samples, whereby a trained cytopathologist reviews sample adequacy at the time of tissue procurement. Although there is scant data directly comparing molecular testing success rates with and without the ROSE protocol, a meta-analysis conducted by Schmidt and colleagues concluded that ROSE improved the adequacy rate of fine-needle aspiration cytology by 12%.32,33 Given that molecular profiling depends on both the absolute and relative amount of tumor cells present in the sample, the ROSE protocol likely enhances the procedural success rate and reduces the need for repeat and subsequent biopsies.
It is interesting to note that our data also demonstrated that we are obtaining large-sample biopsies in most of our patients (about 70%). However, we are still failing to test more than half of our cases for driver mutations (Figure, A and C). This strongly suggests there are additional factors beyond tissue adequacy that are contributing to our high failure rate. It is essential to understand the dynamics and system practices that influence testing rates if we are to improve the care and outcomes of our cancer patients. To better understand those barriers, we surveyed 110 practitioners (including medical oncologists, pulmonologists, thoracic surgeons, and interventional radiologists) about the molecular profiling process and their responses highlighted several important areas that deserve special attention (Tables 1, 4, 5).
In our institution, testing initiation is primarily the responsibility of the treating medical oncologist. This presents a challenge because there is often a significant delay between tissue acquisition, histologic confirmation, and oncologic review. Many institutions have adopted pathology-driven reflex testing to help overcome such delays. Automatic testing after pathologic confirmation streamlines the process, increases testing rates, and eliminates unnecessary delay between the time of diagnosis and the time of test ordering.34 It also allows for the molecular and histologic diagnosis to be integrated into a single pathology report before therapy is initiated.
Another barrier to timely testing according to the respondents, was the CMS’s 14-day rule. The 14-day rule requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing and was frequently identified as a cause for significant delay in testing and having an impact on first-line treatment decisions.35,36
Often clinicians will choose to defer testing until this time has elapsed to reduce the financial burden placed on the hospital but by that time, they might well have initiated treatment without knowing if the patient has a mutation. This is a significant challenge identified by many of our oncologists, and is a limitation to our analysis above as it is unclear what percentage of patients received follow up testing once care was established at an outside facility and once the 14-day time period had elapsed.
The data from our institution suggests there is discordance between physician attitudes and molecular testing practices. However, there are several limitations in our study. First, most of the survey respondents agreed that molecular testing is an important aspect of treating advanced lung cancer patients, but the retrospective nature of the study made it difficult to identify why testing was deferred or never conducted. Second, the absence of a centralized reporting system for molecular testing results at our institution, may have resulted in an overestimation of our testing failure rate in cases where results were not integrated our electronic medical record.
Third, the low survey response rate only allowed us to make generalizations regarding the conclusions, although it does provide a framework for future process improvements.
We believe the poor testing rates observed in our study are not isolated to our institution and reflect a significant challenge within the broader oncology community.27 A system of best practices is essential for capturing this subset of patients who are never tested. There is agreement among oncologists that improving our current testing rates will require a multidisciplinary approach, a refined process for molecular evaluation, a push toward reflex testing, and standardization of biopsy techniques and tissue handling procedures. In our institution, we have initiated a Lean Six Sigma and PDSA (plan, do, study, act) initiative to improve our current molecular testing process. In addition, because obtaining larger-sample biopsies or additional biopsies is often not feasible for many of our advanced cancer patients, we have started using whole blood circulating tumor cells (CTC) and plasma ctDNA (cell-free circulating DNA) for molecular testing. Recent studies have shown high concordance (89%) between tissue biopsies and blood-based mutation testing, which will likely have a positive impact on the cancer care of our patients and help to capture a subset of patients who are not candidates for traditional biopsies.37
Conclusions
Despite current guidelines for testing driver mutations in advanced nsNSCLC, a large segment of our patients are not being tested for those genetic aberrations. There are several barriers that continue to thwart the recommendation, including failure to integrate driver mutation testing into routine pathology practice (ie, reflex testing), insufficient tissue obtained from biopsy, and difficulty in obtaining tissue because of tumor location or risk of complications from the biopsy procedure. More important, these trends are not isolated to our institution and reflect a significant challenge within the oncology community. Our data show that for the purpose of driver mutation testing, larger-sample biopsies, such as surgical/core biopsies, are better than small-sample biopsies, such as needle aspiration. We have also demonstrated that the prevalence of driver mutations is lower in Western Pennsylvania, which is served by our network, than elsewhere in the United States.
Lung cancer is the leading cause of cancer death in the United States. It is estimated that there will be 222,500 new cases of lung cancer and 155,870 deaths from lung cancer in 2017. Non–small-cell lung carcinoma (NSCLC) accounts for 80%-85% of lung cancers, with adenocarcinoma being the most common histologic subtype. Other less common subtypes include squamous-cell carcinoma, large-cell carcinoma, and NSCLC that cannot be further classified.1 Nearly 70% of patients present with locally advanced or metastatic disease at the time of diagnosis and are not candidates for surgical resection.2 For that group of patients, the mainstay of treatment is platinum-based chemotherapy with or without radiation therapy. Patients who are chemotherapy naive often experience a modest response, however; durable remission is short lived, and the 5-year survival rate remains staggeringly low.3 Improved understanding of the molecular pathways that drive malignancy in NSCLC has led to the development of drugs that target specific molecular pathways.4 By definition, these driver mutations facilitate oncogenesis by conferring a selective advantage during clonal evolution.5 Moreover, agents targeting these pathways are extremely active and induce durable responses in many patients.6,7,8
Predictive biomarkers in NSCLC include anaplastic lymphoma kinase (ALK) fusion oncogene and sensitizing epidermal growth factor receptor (EGFR) mutations. Mutations in the EGFR tyrosine kinase are observed in about 15%-20% of NSCLC adenocarcinomas in the United States and upward of 60% in Asian populations. They are also found more frequently in nonsmokers and women.6 The two most prevalent mutations in the EGFR tyrosine kinase domain are in-frame deletions of exon 19 and L858R substitution in exon 21, representing about 45% and 40% of mutations, respectively.9 Both mutations result in activation of the tyrosine kinase domain, and both are associated with sensitivity to the small-molecule tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, and afatinib.10 Other drug-sensitive mutations include point mutations at exon 21 (L861Q) and exon 18 (G719X).11 Targeted therapy produces durable responses in the majority of patients.12,13,14 Unfortunately, most patients develop acquired resistance to these therapies, which leads to disease progression.4,15-17
ALK gene rearrangements, although less prevalent, are another important molecular target in NSCLC and are seen in 2%-7% of cases in the United States.7 As with EGFR mutations, these mutations are more prevalent in nonsmokers, and they are found more commonly in younger patients and in men.8
Identification of driver mutations early in the course of disease and acquired resistance mutations later are crucial for the optimal management of advanced NSCLC. DNA analysis using polymerase chain reaction (PCR) and next-generation sequencing is the preferred method for testing for EGFR mutations, and ALK rearrangements are generally tested either by flourescence in situ hybridization (FISH) or immunohistochemistry.18,19 Newer blood-based assays have shown great promise, and clinicians may soon have the ability to monitor subtle genetic changes, identify resistance patterns, and change therapy when acquired resistance occurs.20
The American College of Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology have proposed guidelines for molecular testing in lung cancer. It is recommended that all advanced squamous and nonsquamous cell lung cancers with an adenocarcinoma component should be tested for EGFR and ALK mutations independent of age, sex, ethnicity, or smoking history. In the setting of smaller lung cancer specimens (eg, from biopsies, cytology) where an adenocarcinoma component cannot be completely excluded, EGFR and ALK testing may be performed in cases showing squamous or small cell histology but clinical criteria (eg, young age, lack of smoking history) may be useful in selecting a subset of these samples for testing. Samples obtained through surgical resection, open biopsy, endoscopy, transthoracic needle biopsy, fine-needle aspiration, and thoracentesis are all considered suitable for testing, but large biopsy samples are generally preferred over small biopsy samples, cell-blocks, and cytology samples.21 Despite this recommendation, not all patients who are eligible for mutation analysis are tested. At our institution, preliminary observations suggested that the percentage of patients being tested and the prevalence of driver mutations were significantly lower compared with published data. The purpose of this study was to evaluate physician attitudes about molecular testing, and to determine the rate of testing, the effect of biopsy sample size on rate of testing, and the prevalence of driver mutations at our institution.
Methods
In this retrospective clinical study, we identified 206 cases of advanced nsNSCLC from the tumor registry (February 2011-February 2013). Registry data was obtained from three hospitals within our health network – two academic tertiary care centers, and one community-based hospital. The other hospitals in the network were excluded because their EHR systems were not integrated with the rest of the hospitals and/or there was a lack of registry data. The testing rates for driver mutations, prevalence of driver mutations, and the tissue procurement techniques were obtained from individual chart review. Surgical specimens, core biopsy samples, and large volume thoracentesis specimens were categorized as large biopsy samples, and samples obtained by fine-needle aspiration, bronchial washing, and bronchial brushing were considered small biopsy samples. We used a chi-square analysis to compare mutation testing rates between the large and small biopsy sample groups. The prevalence of driver mutations was determined, excluding unknown or inadequate samples.
EGFR analysis had been conducted at Integrated Oncology, using formalin-fixed, paraffin-embedded tissue. Genomic DNA was isolated, and EGFR mutation analysis was performed using SNaPShot multiplex PCR, primer extension assay for exons 18-21; samples with >4mm2 and ≥50% tumor content were preferred. Macrodissection was used to enrich for tumor cells when samples had lower tumor cellularity and content. ALK rearrangements were tested in the hospital using the Vysis ALK Break Apart FISH probe kit (Abott Molecular Inc, Des Plaines, IL).
We conducted a web-based, 20-question survey about molecular profiling among 110 practitioners to gauge their knowledge and opinions about molecular testing. The practitioners included medical oncologists, thoracic surgeons, pulmonologists, and interventional radiologists. Each received an initial e-mail informing them of the study, inviting them to complete survey, and providing a link to it, and two reminder e-mails at biweekly intervals to maximize survey participation and responses. The questions were aimed at understanding the challenges surrounding molecular testing within our network. Apart from the questions gathering demographic information about the respondents, the questions were intended to highlight the disparities between guideline recommendations and physician practices; to gauge the perceived importance of molecular evaluation; to identify individual, subspecialty, and hospital-based challenges; and to assess physician attitudes toward alternatives to traditional tissue-based testing (Table 1, p. e150). Nineteen of the questions were structured as single or best answer, whereas Question 9, which was aimed at identifying system-based challenges, allowed for multiple answer selections.
Results
There were a total of 206 cases of advanced stage IIIb or IV nsNSCLC identified at three hospitals during 2011-2013. Of those 206 cases, 161 (78.2%) were recorded at the two large academic medical centers, and 45 (21.9%) were recorded at the smaller community-based hospital. Of the total, there were 145 (70.4%) large biopsy specimens and 61 (29.6%) small biopsy specimens. We found that 89 of the 206 cases (43.2 %) had been tested for EGFR mutations, and 49 (23.8%) had been tested for ALK rearrangements (Figure, A and C). In all, 70 (48.3%) large-sample biopsies and 19 (31.1%) small-sample biopsies were submitted for EGFR analysis (Figure, B), and 42 (29%) large-sample biopsies and 7 (11.5%) small-sample biopsies were tested for ALK rearrangements (Figure, D). Large-sample biopsies were more likely to be analyzed for EGFR mutations and ALK rearrangements, with the results reaching statistical significance (P = .023 and P = .007, respectively). Across all samples, a total of 7 EGFR mutations and 1 ALK rearrangement were identified, yielding a prevalence of 7.9% and 2% respectively (Figure, A and C).
Table 2 shows the demographics, smoking status and type of driver mutation identified. Core biopsies were obtained in 45.6% of the cases and fine-needle aspiration biopsies were obtained in 25.2% of the cases with surgical resections, with thoracentesis and bronchial washings comprising the rest of the biopsies (Table 3).
The average age at diagnosis of the patients in the cases that were analyzed was 69.3 years. Most of the patients (83.9%) identified as white, 3.8% were African American, and 12.6% were in the Unknown category. Of the total number of patients, 11 were identified as never-smokers (5.3%), 50 (24.3%) had a 1-15 pack-year smoking history, 104 (50.5%) had a 16-45 pack-year smoking history, and 41 (19.9%) had a >45 pack-year smoking history.
In regard to the survey, 46 of the 110 physicians asked to participate in the survey responded, representing a response rate of 41.8% (range across medical specialties, 26%-45%, Table 4). Of those respondents, 38 (82.6%) indicated they believed molecular evaluation was a very important aspect of NSCLC care, with the remainder indicating it was somewhat important. 91.4% of the respondents who routinely ordered molecular testing agreed that stage IIIb or IV nsNSCLC should undergo molecular evaluation.
The top barriers to molecular evaluation identified through this survey were the availability of sufficient tissue to complete molecular testing and the Center for Medicare and Medicaid Services’s (CMS’s) 14-day rule that requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing (Table 5).
Discussion
The treatment of advanced nsNSCLC has evolved significantly over the past decade. Molecular profiling is now an essential part of initial evaluation, and larger-sample biopsies are needed to ensure accurate evaluation and appropriate treatment. The detection of EGFR and EML4-ALK driver mutations are associated with increased response to tyrosine kinase inhibitors and are associated with improvement in progression-free survival, patient quality of life, and even overall survival in some studies.12,22,23,24 Early identification of these driver mutations is crucial, however, preliminary observation in our network suggested that a large percentage of patients with advanced nsNSCLC in were not being appropriately evaluated for those mutations. To evaluate our molecular profiling rates, we conducted a retrospective study and reviewed 3 years of registry data at 3 hospitals within our health system. Two of the hospitals included in our analysis were large tertiary academic centers, and one was a community hospital. Our findings confirmed that a large percentage of our patients who are eligible for molecular evaluation are not tested: 56.7% of cases were not tested for EGFR mutations, and 76.2% of cases were not tested for ALK rearrangements.
In a similar study, the Association for Community Cancer Centers conducted a project aimed at understanding the landscape and current challenges for molecular profiling in NSCLC. Eight institutions participated in the study, and baseline testing rates were analyzed. The findings demonstrated that high-volume institutions (treating >100 lung cancer patients a year tested 62% and 60% of advanced lung cancer patients for EGFR and EML4-ALK, respectively, and low-volume institutions (treating <100 lung cancer patients a year tested 52% and 47% for EGFR and EML4-ALK, respectively.25,26 In a recent international physician self-reported survey, Spicer and colleagues found that EGFR testing was requested before first-line therapy in patients with stage IIIB or IV disease in 81% of cases, and mutation results were available before start of therapy in 77% of the cases.27 Those percentages are relatively low, given that current guidelines recommend that molecular testing should be done for all patients with stage IIIB or IV nsNSCLC. This highlights the need for objective performance feedback so oncologists can make the necessary practice changes so that molecular testing is done before the start of therapy to ensure high-quality cancer care that will translate into better, cost-effective outcomes and improved patient quality of life.
Our study findings showed that the prevalence of EGFR and ALK mutations is substantially lower among the patients we treat in our network compared with other published data on prevalence. The reason for those low rates is not clear, but it is likely multifactorial. First, Western Pennsylvania, the region our network serves, has a large proportion of older adults – 17.3% of the population is older than 65 years (national average, 14.5%) and advanced age might have contributed to the lower EGFR and ALK rates measured in our study.28 Second, the smoking rate in Pennsylvania is higher than the national average, 20%-24% compared with 18%, respectively.29 Third, the air quality in Western Pennsylvania has historically been very poor as a result of the large steel and coal mining industries. Even though the air quality has improved in recent decades, the American Lung Association’s 2017 State of the Air report ranked Pittsburgh and surrounding areas in Western Pennsylvania among the top 25 most air polluted areas in the United States.30 It is not certain whether air pollution and air quality have any impact on driver mutation rates, but the correlation with smoking, ethnicity, and geographic distribution highlight the need for further epidemiologic studies.
Biopsy sufficiency – getting an adequate amount of sample tissue during biopsy – is a known challenge to molecular profiling, and we found that biopsy sample size had an impact on the testing rates in a large percentage of our cases. To fully understand the impact of biopsy sufficiency, we conducted a subset analysis and compared the testing rates between our large and small biopsy samples. Our analysis showed that larger-sample biopsies were more likely to be tested for mutations than were smaller-sample biopsies (EGFR: P = .023; ALK: P = .007).
Those results suggest that larger-sample biopsies should be encouraged, but procedural risks, tumor location, and patient age and wishes need to be considered before tissue acquisition.21 Furthermore, clinicians who are responsible for tissue procurement need to be properly educated on the tissue sample requirements and the impact these results have on treatment decisions.31 Our institution, like many others, has adopted rapid onsite evaluation (ROSE) of biopsy samples, whereby a trained cytopathologist reviews sample adequacy at the time of tissue procurement. Although there is scant data directly comparing molecular testing success rates with and without the ROSE protocol, a meta-analysis conducted by Schmidt and colleagues concluded that ROSE improved the adequacy rate of fine-needle aspiration cytology by 12%.32,33 Given that molecular profiling depends on both the absolute and relative amount of tumor cells present in the sample, the ROSE protocol likely enhances the procedural success rate and reduces the need for repeat and subsequent biopsies.
It is interesting to note that our data also demonstrated that we are obtaining large-sample biopsies in most of our patients (about 70%). However, we are still failing to test more than half of our cases for driver mutations (Figure, A and C). This strongly suggests there are additional factors beyond tissue adequacy that are contributing to our high failure rate. It is essential to understand the dynamics and system practices that influence testing rates if we are to improve the care and outcomes of our cancer patients. To better understand those barriers, we surveyed 110 practitioners (including medical oncologists, pulmonologists, thoracic surgeons, and interventional radiologists) about the molecular profiling process and their responses highlighted several important areas that deserve special attention (Tables 1, 4, 5).
In our institution, testing initiation is primarily the responsibility of the treating medical oncologist. This presents a challenge because there is often a significant delay between tissue acquisition, histologic confirmation, and oncologic review. Many institutions have adopted pathology-driven reflex testing to help overcome such delays. Automatic testing after pathologic confirmation streamlines the process, increases testing rates, and eliminates unnecessary delay between the time of diagnosis and the time of test ordering.34 It also allows for the molecular and histologic diagnosis to be integrated into a single pathology report before therapy is initiated.
Another barrier to timely testing according to the respondents, was the CMS’s 14-day rule. The 14-day rule requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing and was frequently identified as a cause for significant delay in testing and having an impact on first-line treatment decisions.35,36
Often clinicians will choose to defer testing until this time has elapsed to reduce the financial burden placed on the hospital but by that time, they might well have initiated treatment without knowing if the patient has a mutation. This is a significant challenge identified by many of our oncologists, and is a limitation to our analysis above as it is unclear what percentage of patients received follow up testing once care was established at an outside facility and once the 14-day time period had elapsed.
The data from our institution suggests there is discordance between physician attitudes and molecular testing practices. However, there are several limitations in our study. First, most of the survey respondents agreed that molecular testing is an important aspect of treating advanced lung cancer patients, but the retrospective nature of the study made it difficult to identify why testing was deferred or never conducted. Second, the absence of a centralized reporting system for molecular testing results at our institution, may have resulted in an overestimation of our testing failure rate in cases where results were not integrated our electronic medical record.
Third, the low survey response rate only allowed us to make generalizations regarding the conclusions, although it does provide a framework for future process improvements.
We believe the poor testing rates observed in our study are not isolated to our institution and reflect a significant challenge within the broader oncology community.27 A system of best practices is essential for capturing this subset of patients who are never tested. There is agreement among oncologists that improving our current testing rates will require a multidisciplinary approach, a refined process for molecular evaluation, a push toward reflex testing, and standardization of biopsy techniques and tissue handling procedures. In our institution, we have initiated a Lean Six Sigma and PDSA (plan, do, study, act) initiative to improve our current molecular testing process. In addition, because obtaining larger-sample biopsies or additional biopsies is often not feasible for many of our advanced cancer patients, we have started using whole blood circulating tumor cells (CTC) and plasma ctDNA (cell-free circulating DNA) for molecular testing. Recent studies have shown high concordance (89%) between tissue biopsies and blood-based mutation testing, which will likely have a positive impact on the cancer care of our patients and help to capture a subset of patients who are not candidates for traditional biopsies.37
Conclusions
Despite current guidelines for testing driver mutations in advanced nsNSCLC, a large segment of our patients are not being tested for those genetic aberrations. There are several barriers that continue to thwart the recommendation, including failure to integrate driver mutation testing into routine pathology practice (ie, reflex testing), insufficient tissue obtained from biopsy, and difficulty in obtaining tissue because of tumor location or risk of complications from the biopsy procedure. More important, these trends are not isolated to our institution and reflect a significant challenge within the oncology community. Our data show that for the purpose of driver mutation testing, larger-sample biopsies, such as surgical/core biopsies, are better than small-sample biopsies, such as needle aspiration. We have also demonstrated that the prevalence of driver mutations is lower in Western Pennsylvania, which is served by our network, than elsewhere in the United States.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
3. Kim TE, Murren JR. Therapy for stage IIIB and stage IV non-small cell lung cancer. Clin Chest Med. 2002;23(1):209-224.
4. Black RC, Khurshid H. NSCLC: An update of driver mutations, their role in pathogenesis and clinical significance. R I Med J (2013). 2015;98(10):25-28.
5. Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306-313.
6. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334.
7. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866-2874.
8. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246.
9. Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(suppl 1):S24-31.
10. Langer CJ. Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: is afatinib better or simply newer? J Clin Oncol. 2013;31(27):3303-3306.
11. Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12(24):7232-7241.
12. Shi Y, Siu-Kie JA, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154-162.
13. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957.
14. Khozin S, Blumenthal GM, Jiang X, et al. US Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19(7):774-779.
15. Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220-229.
16. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73.
17. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240-2247.
18. Ellison G, Zhu G, Moulis A, Dearden S, et al. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66(2):79-89.
19. Alì G, Proietti A, Pelliccioni S, et al. ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med. 2014;138(11):1449-1158.
20. Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472-484.
21. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
22. Kwak EL, Bany YJ, Cambridge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693-1703.
23. Shaw A, Yeap BY, Kenudson MM, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247-4253.
24. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388.
25. Association of Community Cancer Centers. Molecular Testing in the Community Setting. In: Molecular testing: resources and tools for the multidisciplinary team. http://accc-cancer.org/resources/molecularTesting-Overview.asp. Accessed November 15, 2015.
26. Association of Community Cancer Centers. Molecular testing: ACCC peer-to-peer webinars. The tissue issue: sampling and testing with Gail Probst, RN, MS, AOCN. https://www.youtube.com/watch?v=lapmni938Mc&feature=youtu.be. Published September 14, 2015. Accessed November 2015.
27. Spicer J S, Tischer B, Peters M. EGFR mutation testing and oncologist treatment choice in advanced NSCLC: global trends and differences. Ann Oncol. 2015;26(suppl 1):i60.
28. West L, Cole S, Goodkind D. US Census Bureau, 65+ in the United States: 2010, U.S. Government Printing Office, Washington, DC, 2014
29. Centers for Disease Control and Prevention. State tobacco activities tracking and evaluation system. Current cigarette use among adults (Behavior Risk Factor Surveillance System) 2015. https://www.cdc.gov/statesystem/cigaretteuseadult.html. Last updated September 16, 2016. Accessed May 26, 2017.
30. The American Lung Association. State of the Air 2017. http://www.lung.org/assets/documents/healthy-air/state-of-the-air/state-of-the-air-2017.pdf. Published 2017. Accessed May 26, 2017.
31. Gaga M, Powell CA, Schraufnagel DE, Schönfeld N, et al. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. 2013;188(4):503-507.
32. Ferguson PE, Sales CM, Hodges DC, et al. Effects of a multidisciplinary approach to improve volume of diagnostic material in CT-guided lung biopsies. PLoS One. 2015 Oct 19;10(10).
33. Schmidt RL, Witt BL, Lopez-Calderon LE, et al. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol. 2013;139(3):300-309.
34. Cengiz Inal, Yilmaz E, Chenget H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: Experience from a community-based academic center. J Clin Oncol. 2014;32(suppl):5s. [abstract 8098].
35. Grzegorz K, Leighl, M. Challenges in NSCLC molecular testing barriers to implementation. Oncology Exchange. 2012;11(4):8-10.
36. Lynch JA, Khoury MJ, Ann Borzecket A, et al. Utilization of epidermal growth factor receptor (EGFR) testing in the United States: a case study of T3 translational research. Genet Med. 2013;15(8):630-638.
37. Reck M. Investigating the utility of circulating-free tumour-derived DNA (ctDNA) in plasma for the detection of epidermal growth factor receptor (EGFR) mutation status in European and Japanese patients (pts) with advanced non-small-cell lung cancer (NSCLC): ASSESS study. Presented at the European Lung Cancer Conference (ELCC) Annual Meeting, Geneva; 15-18 April 2015.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
3. Kim TE, Murren JR. Therapy for stage IIIB and stage IV non-small cell lung cancer. Clin Chest Med. 2002;23(1):209-224.
4. Black RC, Khurshid H. NSCLC: An update of driver mutations, their role in pathogenesis and clinical significance. R I Med J (2013). 2015;98(10):25-28.
5. Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306-313.
6. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334.
7. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866-2874.
8. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246.
9. Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(suppl 1):S24-31.
10. Langer CJ. Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: is afatinib better or simply newer? J Clin Oncol. 2013;31(27):3303-3306.
11. Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12(24):7232-7241.
12. Shi Y, Siu-Kie JA, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154-162.
13. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957.
14. Khozin S, Blumenthal GM, Jiang X, et al. US Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19(7):774-779.
15. Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220-229.
16. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73.
17. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240-2247.
18. Ellison G, Zhu G, Moulis A, Dearden S, et al. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66(2):79-89.
19. Alì G, Proietti A, Pelliccioni S, et al. ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med. 2014;138(11):1449-1158.
20. Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472-484.
21. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
22. Kwak EL, Bany YJ, Cambridge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693-1703.
23. Shaw A, Yeap BY, Kenudson MM, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247-4253.
24. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388.
25. Association of Community Cancer Centers. Molecular Testing in the Community Setting. In: Molecular testing: resources and tools for the multidisciplinary team. http://accc-cancer.org/resources/molecularTesting-Overview.asp. Accessed November 15, 2015.
26. Association of Community Cancer Centers. Molecular testing: ACCC peer-to-peer webinars. The tissue issue: sampling and testing with Gail Probst, RN, MS, AOCN. https://www.youtube.com/watch?v=lapmni938Mc&feature=youtu.be. Published September 14, 2015. Accessed November 2015.
27. Spicer J S, Tischer B, Peters M. EGFR mutation testing and oncologist treatment choice in advanced NSCLC: global trends and differences. Ann Oncol. 2015;26(suppl 1):i60.
28. West L, Cole S, Goodkind D. US Census Bureau, 65+ in the United States: 2010, U.S. Government Printing Office, Washington, DC, 2014
29. Centers for Disease Control and Prevention. State tobacco activities tracking and evaluation system. Current cigarette use among adults (Behavior Risk Factor Surveillance System) 2015. https://www.cdc.gov/statesystem/cigaretteuseadult.html. Last updated September 16, 2016. Accessed May 26, 2017.
30. The American Lung Association. State of the Air 2017. http://www.lung.org/assets/documents/healthy-air/state-of-the-air/state-of-the-air-2017.pdf. Published 2017. Accessed May 26, 2017.
31. Gaga M, Powell CA, Schraufnagel DE, Schönfeld N, et al. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. 2013;188(4):503-507.
32. Ferguson PE, Sales CM, Hodges DC, et al. Effects of a multidisciplinary approach to improve volume of diagnostic material in CT-guided lung biopsies. PLoS One. 2015 Oct 19;10(10).
33. Schmidt RL, Witt BL, Lopez-Calderon LE, et al. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol. 2013;139(3):300-309.
34. Cengiz Inal, Yilmaz E, Chenget H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: Experience from a community-based academic center. J Clin Oncol. 2014;32(suppl):5s. [abstract 8098].
35. Grzegorz K, Leighl, M. Challenges in NSCLC molecular testing barriers to implementation. Oncology Exchange. 2012;11(4):8-10.
36. Lynch JA, Khoury MJ, Ann Borzecket A, et al. Utilization of epidermal growth factor receptor (EGFR) testing in the United States: a case study of T3 translational research. Genet Med. 2013;15(8):630-638.
37. Reck M. Investigating the utility of circulating-free tumour-derived DNA (ctDNA) in plasma for the detection of epidermal growth factor receptor (EGFR) mutation status in European and Japanese patients (pts) with advanced non-small-cell lung cancer (NSCLC): ASSESS study. Presented at the European Lung Cancer Conference (ELCC) Annual Meeting, Geneva; 15-18 April 2015.
Comprehensive assessment of cancer survivors’ concerns to inform program development
Complex cancer treatments, limited personnel resources, and a growing number of cancer survivors are challenging cancer health care professionals’ abilities to provide comprehensive care. Cancer survivors have a range of needs that extend over the cancer care trajectory and that represent physical, psychological, social, and spiritual domains. Numerous studies have explored supportive care needs and recent systematic reviews have highlighted the supportive care needs related to cancer1 and to specific cancer types, including prostate cancer,2 breast cancer,3 gynecologic cancer,4 hematological cancer,5 and lung cancer.6 However, reviews are limited in that they do not always assess needs across the cancer trajectory or identify demographic or clinical variables that are associated with needs. These data are needed to focus survivorship program development in cancer centers in order to target populations most likely at risk for unmet needs, identify what salient concerns to address, and to appropriately schedule supportive care programs.
The importance of assessing the patient’s subjective view of his/her needs or concerns is well acknowledged as being fundamental to patient-centered care.7 Clinicians routinely assess needs in practice using a variety of screening tools. However, there needs to be a broader assessment of concerns and needs in a population of survivors with mixed cancer diagnoses, along with their appraisal of how well their needs were addressed by their health care team, to provide an overall identification of gaps in supportive care. The primary purpose of the present study was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs; ascertain survivors’ perceived importance of those needs and the extent to which our institution, the University Hospitals Seidman Cancer Center, was attentive to those needs; and to identify who might be at risk for having greater concerns. The overall goal was to use the data to inform survivorship and supportive care program development.
Methods
Design, sample and setting
We used a cross-sectional design. Surveys were mailed once to a convenience sample of 2,750 adult patients who had been seen in follow-up during the previous 2 years (2010-2011) at all clinical sites of University Hospitals Seidman Cancer Center, a Midwestern National Cancer Institute-designated Comprehensive Cancer Center. Patients who had a noncancer diagnosis were excluded. The distribution list was screened for deceased individuals and those patients who had multiple visits during the time period. The project was reviewed and approved as nonresearch by the Case Western Reserve University Cancer Institutional Review Board.
Survey
An interdisciplinary team of clinicians, administrators, and researchers adapted the Mayo Clinic Cancer Center’s Cancer Survivors Survey of Needs8 to create a comprehensive survey for the cancer center. Input regarding the scope of the survey was sought from the Patient and Family Advisory Council of the cancer center. The survey, which was formatted for scanning purposes, consisted of 33 questions that were compiled into 4 sections. Sections 1 and 2 focused on demographic and treatment-related information, including use of community and hospital support services and preferences for follow-up care. In section 3, a quality-of-life framework was used to assess physical, social, emotional, and spiritual needs. Respondents were asked to rate their current level of concern for 19 physical effects, 10 social effects, 10 emotional effects, and 5 spiritual effects on a scale ranging from 0 (no concern) to 5 (extreme concern). In section 4, respondents were asked to indicate the importance of the cancer team addressing their physical, social, emotional, and spiritual needs. This was followed by their rating of the cancer team’s attention to their needs as Poor, Fair, Good, Excellent, or They did not ask about my needs. Respondents were asked about preferences for learning about physical, social, emotional, and spiritual effects. In addition to the 33 questions, there were 6 open-ended questions in which respondents were encouraged to share additional information about their needs, sources of support, and other concerns.
Procedures
Eligible respondents were mailed a cover letter explaining the survey from both the director and president of the cancer center, a survey, and a postage-paid return envelope. The option to respond to the survey by a telephone call to the director of the Office of Cancer Survivorship was offered in the cover letter.
Data analysis
Returned surveys were scanned into a Teleform database, verified, and exported into an SPSS data file. Data quality was checked by running frequency analyses and summarizing variables. Time-since-treatment responses were collapsed into 4 categories: on treatment, up to 2 years posttreatment, 2-5 years posttreatment, and more than 5 years posttreatment. Descriptive statistics were used to summarize demographic and medical characteristics of the respondents and to calculate the mean score for each concern for the total sample and then for each category of time since treatment. Because of the large number of respondents with breast cancer, the respondents were stratified into two groups, one of breast cancer the other of nonbreast cancer respondents. Then, the Mann-Whitney test was performed for each concern to examine differences between respondents with and without breast cancer.
To identify the most prevalent concerns, ratings for concerns were recoded into no concern (rated as 0), low concern (1 or 2), and moderate/high concern (3, 4, or 5). Since our interest was in the moderate and high concerns, the responses were dichotomized into moderate/high concerns and all other levels. Logistic regression models were then used to identify associations between a set of survivor characteristics or covariates (age, sex, living status, marital status, employment status, cancer type, and time since treatment) with the 12 most highly rated moderate/high concerns. All the analyses were performed using statistical software SPSS 20 and Stata 13.0
Results
Respondents
A total of 1,005 surveys were returned for a 37% response rate. Forty-two patients responded by telephone. The mean age of respondents was 64.9 years (range, 22-98; SD, 12.8). The typical respondent was female, white, and married (Table 1). Twenty-four percent of the respondents (n = 240) reported living alone. Although about 47% of respondents (n = 473) reported a breast cancer diagnosis, more than 17 cancers were identified, and 14% of respondents (n = 145) listed multiple diagnoses. About a third of respondents were receiving treatment when they completed the survey.
Just under half of the respondents (n = 498) reported using community resources for support and information about cancer, and 29.5% (n = 296) sought information on the internet during their cancer experience. The most commonly used community resources were The Gathering Place, a local organization offering free supportive programs and services to individuals with cancer and their families (n = 167), and the American Cancer Society (n = 138). Of the 496 respondents who reported accessing hospital resources, most (n = 322) said they used information that their health care team recommended. Other supportive options were used to a lesser degree: support groups (n = 92), chemotherapy and radiation therapy classes (n = 129), and supportive/educational programs offered by the cancer center (n = 27). Most of the respondents (n = 822, 88.6%) preferred to have their follow-up care remain with their cancer care team 1 year after treatments are completed. Almost two-thirds of respondents (n = 601, 64%) cited being seen at the cancer center for follow-up care as the most important factor in considering follow-up care.
Concerns In determining whether the large proportion of respondents with breast cancer skewed the study results, it was determined that median scores differed significantly in only four concerns. Compared with respondents without breast cancer, respondents with breast cancer were more likely to have significantly lower scores for concerns related to fatigue (P <.001) and sexual issues/intimacy (P = .001). Respondents with breast cancer were more likely to have significantly higher scores than respondents without breast cancer for concerns related to genetic counseling (P = .001) and fear of developing a new cancer (P = .010).
Fears of the cancer returning and developing a new cancer were the two most prevalent concerns, identified by 51% (n = 486) and 47.5% (n = 459), respectively (Table 2). Physical concerns, rated as moderate/high concerns by at least 25% of the sample, were fatigue (n = 336, 34.8%), changes in [the] body after cancer (n = 323, 33.7%), trouble sleeping (n = 302, 31.0%), sexual issues/intimacy (n = 263, 28.0%), memory and concentration (n = 261, 26.7%), and weight changes (n = 248, 25.5%). The most prevalent moderate/high social concerns were related to finances (n = 265, 27.5%) and debt from medical bills (n = 232, 25.1%). Managing stress (n = 279, 29.2%) and difficult emotions (n = 244, 25.1%) were prevalent moderate/high emotional concerns. Spiritual concerns were less often rated as moderate/high concerns. Having a breast cancer diagnosis was not significantly related to the number of reported moderate to high concerns (P = 1.00).
Variables associated with the 12 most frequent moderate/high concerns are shown in Tables 3 and 4. Age was associated with the most moderate/high concerns. With every decade of age, the odds of having the following moderate/high concerns decreased: bodily changes after cancer (odds ratio [OR], 0.75), sexual intimacy (OR, 0.81), memory and concentration (OR, 0.83), weight changes (OR, 0.77), financial (OR, 0.75), debt (OR, 0.71), cancer returning (OR, 0.66), developing a new cancer (OR, 0.67), managing stress (OR, 0.67), and managing difficult emotions (OR, 0.67).
Female sex was associated with lower odds of having a concern about sexual intimacy (OR, 0.30) and increased odds of having concerns related to memory and concentration (OR, 1.78), managing stress (OR, 2.35), and managing difficult emotions (OR, 1.77). Race was another demographic characteristic statistically associated with numerous moderate/high concerns. Survivors who identified white, were more likely than other people of other races to have fewer moderate/high concerns regarding bodily changes after cancer (OR, 0.46), weight change (OR, 0.46), finances (OR, 0.46), debt (OR, 0.40), managing stress (OR, 0.55), and managing difficult emotions (OR, 0.49). The odds of having a moderate/high concern regarding debt was 2.25 times higher given widowed marital status compared with those survivors who were single. Unemployment status, when compared with full-time employment, was significantly associated with increased odds of having moderate/high concerns related to fatigue (OR, 2.08), bodily changes after cancer (OR, 1.72), memory and concentration (OR, 2.45), weight changes (OR, 2.17), finances (OR, 1.93), developing a new cancer (OR, 1.91), and managing difficult emotions (OR, 1.80).
As expected, respondents who had completed treatment were less likely to have many of the moderate/high concerns as those still undergoing treatment. Survivors who were up to 2 years posttreatment were significantly more likely than those survivors receiving treatment to have fewer moderate/high concerns regarding fatigue (OR, 0.56), sexual intimacy (OR, 0.54), weight change (OR, 0.55), fears of the cancer returning (OR, 0.48), developing a new cancer (OR, 0.35), managing stress (OR, 0.43), and managing difficult emotions (OR, 0.49).
However, those improved odds were not sustained over the cancer trajectory. Compared with survivors who were receiving treatment, survivors who were between 2-5 years posttreatment did not have significantly reduced odds for moderate/high concerns related to fatigue, sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress, and managing difficult emotions. They did have significantly reduced odds for having concerns only related to finances (OR, 0.61) and debt (OR, 0.52).
Long-term survivors, who were beyond 5 years posttreatment, had significantly reduced odds for having moderate/high concerns related to fatigue (OR, 0.45), finances (OR, 0.52), debt (OR, 0.47), and managing difficult emotions (OR, 0.54), compared with survivors receiving treatment. Moderate/high concerns related to sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress did not have improved odds for these long-term survivors.
Attention to needs
The health care teams were rated highly for their attention to the patients’ physical needs. Most respondents (n = 845, 92.4%) viewed the health care team’s attention their physical needs as important and 763 (77.6%) survivors rated the team’s attention to these needs as excellent. The importance of addressing emotional needs was affirmed by 723 (78.5%) respondents, and although 454 (46.8%) viewed the team’s attention to these needs as excellent, 119 (12.3%) reported that the health care team did not ask about emotional needs. In addition, 566 respondents (60%) viewed having the health care team address their social needs as important, and most (n = 715, 74.2%) rated the team’s attention to social needs as good or excellent. Yet, 162 (16.8%) respondents reported that team did not ask about their social needs. The health care team’s addressing of spiritual needs was viewed as important by 346 (37.5%) respondents and ratings for how well the team attended to spiritual needs were: 148 (15.6%) poor or fair, 204 (21.5%) good, and 150 (15.8%) excellent. However, 448 (47.2%) respondents reported that the health care team did not ask about their spiritual needs.
Discussion
The primary purpose of this project was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs and to assess the perceived importance of these needs and the extent to which the cancer center staff were attentive to those needs. The overall goal of this assessment was to inform the development of survivorship and supportive care programs by highlighting common concerns, demographic and medical factors associated with specific concerns, and timing of moderate/high level concerns along the cancer trajectory. There were 3 main findings.
First, the results support the need for enhancing supportive care services to meet emotional concerns of survivors beyond the treatment phase. Similar to other studies,8,9 emotional concerns ranked higher than all other concerns in this study with about 50% of the sample rating “fear the cancer will return” and “fear of developing a new cancer” as moderate/high concern. Although the odds of not having these emotional concerns improved up to 2 years posttreatment, these concerns are likely to resurface, as odds for survivors beyond 2 years were not significantly different from those receiving treatment. A recent systematic review reported that fear of cancer recurrence is experienced by about 73% of cancer survivors, with 49% reporting a moderate to high degree.10 It can have a chronic, stable trajectory for some survivors and is strongly associated with higher levels of anxiety, distress, and depression, and less global, emotional/mental, physical, role, social, and cognitive quality of life.10 In this sample, managing stress and difficult emotions were also rated as moderate/high concerns by at least 25% of the sample.
Second, the findings identified patients at risk for cancer-related concerns throughout the cancer trajectory. As demonstrated in other studies, younger age was associated with greater odds of having multiple greater moderate/high concerns.11-13 Unemployment was the second most common demographic factor associated with multiple moderate/high concerns related to physical symptoms, finances and emotions. Similarly, identifying as black, Asian, American Indian/Alaskan Native, or other was also associated with greater odds of having numerous physical, financial, and emotional concerns. Women had greater concerns related to memory, sexual intimacy, coping with difficult emotions, and stress.
Third, the results helped to identify gaps in supportive care at our cancer center. Although spiritual concerns were not prevalent as being moderate/high, they were still viewed by about a third of survivors as being an important area for the health care team to address. Yet, consistent with other need assessments, spiritual concerns in this study were least often addressed by staff.1 Assessment of spiritual care needs, screening for spiritual distress, and providing spiritual care are essential components of a clinician-patient relationship that supports healing.14 The importance of attending to spiritual care needs was underscored by a recent systematic review that found a positive association between overall spiritual well-being and quality of life in patients with cancer, with the meaning/peace factor consistently and positively associated with physical and mental health.15 Another identified gap was the health care team’s lack of attention to the patient’s social needs, which included concerns related to finances and debt from medical bills. In all, 46% of the respondents reported having financial concerns, with the odds of having moderate/high financial concerns being greatest during treatment to 2 years posttreatment. Attention to the financial burden of cancer patients is critical because the magnitude of cancer-related financial concerns is a significant, strong predictor of quality of life and adverse psychological issues such as depression, anxiety, and distress.16,17
There were several program implications based on the results. A periodic audit of the concerns of survivors and their views on how well their needs were being met was a relatively low cost endeavor. Although the findings were consistent with the literature, the results, when shared with administrators and clinicians, were instrumental in effecting change because they represented the concerns of survivors at the cancer center. Another program directive, based on the results, was to extend the routine screening of patients’ needs during treatment to posttreatment survivorship. Patients who are young, unemployed, do not identify as white, and female warrant more thorough assessment of needs and concerns along the cancer trajectory. Integral to these screenings is the need for patient-centered communication, with discussion of how cancer is affecting the different domains of quality of life within the context of the patient’s life. Lastly, the results clearly indicated the need for additional training of health care providers on how to assess and address spiritual well-being in cancer survivors.
There were limitations to this study, including use of a nonvalidated survey and cross-sectional approach that limited our ability to explore how concerns might change over the trajectory. Also, it was not possible to clarify medical information of the respondents, such as cancer stage. Although the response rate of this study was not high, we are confident in the results because of the large sample size and the finding that the large proportion of respondents with breast cancer was not influential. Despite these limitations, this needs assessment of cancer survivors over the trajectory of care provided insight into the scope of their concerns, identified vulnerable groups of survivors, and highlighted gaps in addressing those concerns. A quality- of-life framework for assessing needs assured a comprehensive focus and generated practice changes to strengthen holistic, comprehensive oncology care.
1. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
2. Paterson C, Robertson A, Smith A, Nabi G. Identifying the unmet supportive care needs of men living with and beyond prostate cancer: A systematic review. Eur J Oncol Nurs. 2015;19:405-418.
3. Fiszer C, Dolbeault S, Sultan S, Bredart A. Prevalence, intensity, and predictors of the supportive care needs of women diagnosed with breast cancer: A systematic review. Psychooncology. 2014;23:361-374.
4. Maguire R, Kotronoulas G, Simpson M, Paterson C. A systematic review of the supportive care needs of women living with and beyond cervical cancer. Gynecol Oncol. 2015;136:478-490.
5. Hall A, Lynagh M, Bryant J, Sanson-Fisher R. Supportive care needs of hematological cancer survivors: A critical review of the literature. Crit Rev Oncol Hematol. 2013;88:102-116.
6. Maguire R, Papadopoulou C, Kotronoulas G, Simpson MF, McPhelim J, Irvine L. A systematic review of supportive care needs of people living with lung cancer. Eur J Oncol Nurs. 2013;17:449-464.
7. Adler NE, Page EK. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press; Institute of Medicine, 2008.
8. Ness S, Kokal J, Fee-Schroeder K, Novotny P, Satele D, Barton D. Concerns across the survivorship trajectory: results from a survey of cancer survivors. Oncol Nurs Forum. 2013;40:35-42.
9. Swash B, Hulbert-Williams N, Bramwell R. Unmet psychosocial needs in haematological cancer: A systematic review. Support Care Cancer. 2014;22:1131-1141.
10. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J Cancer Surviv. 2013;7:300-322.
11. Choi KH, Park JH, Park JH, Park JS. Psychosocial needs of cancer patients and related factors: A multi-center, cross-sectional study in Korea. Psychooncology. 2013;22:1073-1080.
12. Pauwels EE, Charlier C, De Bourdeaudhuij I, Lechner L, Van Hoof E. Care needs after primary breast cancer treatment. Survivors’ associated sociodemographic and medical characteristics. Psychooncology. 2013;22:125-132.
13. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
14. Puchalski CM, Blatt B, Kogan M, Butler A. Spirituality and health: The development of a field. Academic Medicine. 2014;89:10-16.
15. Bai M, Lazenby M. A systematic review of associations between spiritual well-being and quality of life at the scale and factor levels in studies among patients with cancer. J Palliat Med. 2015;18:286-298.
16. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332-338.
17. Sharp L, Carsin AE, Timmons A. Associations between cancer-related financial stress and strain and psychological wellbeing among individuals living with cancer. Psychooncology. 2013;22:745-755.
Complex cancer treatments, limited personnel resources, and a growing number of cancer survivors are challenging cancer health care professionals’ abilities to provide comprehensive care. Cancer survivors have a range of needs that extend over the cancer care trajectory and that represent physical, psychological, social, and spiritual domains. Numerous studies have explored supportive care needs and recent systematic reviews have highlighted the supportive care needs related to cancer1 and to specific cancer types, including prostate cancer,2 breast cancer,3 gynecologic cancer,4 hematological cancer,5 and lung cancer.6 However, reviews are limited in that they do not always assess needs across the cancer trajectory or identify demographic or clinical variables that are associated with needs. These data are needed to focus survivorship program development in cancer centers in order to target populations most likely at risk for unmet needs, identify what salient concerns to address, and to appropriately schedule supportive care programs.
The importance of assessing the patient’s subjective view of his/her needs or concerns is well acknowledged as being fundamental to patient-centered care.7 Clinicians routinely assess needs in practice using a variety of screening tools. However, there needs to be a broader assessment of concerns and needs in a population of survivors with mixed cancer diagnoses, along with their appraisal of how well their needs were addressed by their health care team, to provide an overall identification of gaps in supportive care. The primary purpose of the present study was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs; ascertain survivors’ perceived importance of those needs and the extent to which our institution, the University Hospitals Seidman Cancer Center, was attentive to those needs; and to identify who might be at risk for having greater concerns. The overall goal was to use the data to inform survivorship and supportive care program development.
Methods
Design, sample and setting
We used a cross-sectional design. Surveys were mailed once to a convenience sample of 2,750 adult patients who had been seen in follow-up during the previous 2 years (2010-2011) at all clinical sites of University Hospitals Seidman Cancer Center, a Midwestern National Cancer Institute-designated Comprehensive Cancer Center. Patients who had a noncancer diagnosis were excluded. The distribution list was screened for deceased individuals and those patients who had multiple visits during the time period. The project was reviewed and approved as nonresearch by the Case Western Reserve University Cancer Institutional Review Board.
Survey
An interdisciplinary team of clinicians, administrators, and researchers adapted the Mayo Clinic Cancer Center’s Cancer Survivors Survey of Needs8 to create a comprehensive survey for the cancer center. Input regarding the scope of the survey was sought from the Patient and Family Advisory Council of the cancer center. The survey, which was formatted for scanning purposes, consisted of 33 questions that were compiled into 4 sections. Sections 1 and 2 focused on demographic and treatment-related information, including use of community and hospital support services and preferences for follow-up care. In section 3, a quality-of-life framework was used to assess physical, social, emotional, and spiritual needs. Respondents were asked to rate their current level of concern for 19 physical effects, 10 social effects, 10 emotional effects, and 5 spiritual effects on a scale ranging from 0 (no concern) to 5 (extreme concern). In section 4, respondents were asked to indicate the importance of the cancer team addressing their physical, social, emotional, and spiritual needs. This was followed by their rating of the cancer team’s attention to their needs as Poor, Fair, Good, Excellent, or They did not ask about my needs. Respondents were asked about preferences for learning about physical, social, emotional, and spiritual effects. In addition to the 33 questions, there were 6 open-ended questions in which respondents were encouraged to share additional information about their needs, sources of support, and other concerns.
Procedures
Eligible respondents were mailed a cover letter explaining the survey from both the director and president of the cancer center, a survey, and a postage-paid return envelope. The option to respond to the survey by a telephone call to the director of the Office of Cancer Survivorship was offered in the cover letter.
Data analysis
Returned surveys were scanned into a Teleform database, verified, and exported into an SPSS data file. Data quality was checked by running frequency analyses and summarizing variables. Time-since-treatment responses were collapsed into 4 categories: on treatment, up to 2 years posttreatment, 2-5 years posttreatment, and more than 5 years posttreatment. Descriptive statistics were used to summarize demographic and medical characteristics of the respondents and to calculate the mean score for each concern for the total sample and then for each category of time since treatment. Because of the large number of respondents with breast cancer, the respondents were stratified into two groups, one of breast cancer the other of nonbreast cancer respondents. Then, the Mann-Whitney test was performed for each concern to examine differences between respondents with and without breast cancer.
To identify the most prevalent concerns, ratings for concerns were recoded into no concern (rated as 0), low concern (1 or 2), and moderate/high concern (3, 4, or 5). Since our interest was in the moderate and high concerns, the responses were dichotomized into moderate/high concerns and all other levels. Logistic regression models were then used to identify associations between a set of survivor characteristics or covariates (age, sex, living status, marital status, employment status, cancer type, and time since treatment) with the 12 most highly rated moderate/high concerns. All the analyses were performed using statistical software SPSS 20 and Stata 13.0
Results
Respondents
A total of 1,005 surveys were returned for a 37% response rate. Forty-two patients responded by telephone. The mean age of respondents was 64.9 years (range, 22-98; SD, 12.8). The typical respondent was female, white, and married (Table 1). Twenty-four percent of the respondents (n = 240) reported living alone. Although about 47% of respondents (n = 473) reported a breast cancer diagnosis, more than 17 cancers were identified, and 14% of respondents (n = 145) listed multiple diagnoses. About a third of respondents were receiving treatment when they completed the survey.
Just under half of the respondents (n = 498) reported using community resources for support and information about cancer, and 29.5% (n = 296) sought information on the internet during their cancer experience. The most commonly used community resources were The Gathering Place, a local organization offering free supportive programs and services to individuals with cancer and their families (n = 167), and the American Cancer Society (n = 138). Of the 496 respondents who reported accessing hospital resources, most (n = 322) said they used information that their health care team recommended. Other supportive options were used to a lesser degree: support groups (n = 92), chemotherapy and radiation therapy classes (n = 129), and supportive/educational programs offered by the cancer center (n = 27). Most of the respondents (n = 822, 88.6%) preferred to have their follow-up care remain with their cancer care team 1 year after treatments are completed. Almost two-thirds of respondents (n = 601, 64%) cited being seen at the cancer center for follow-up care as the most important factor in considering follow-up care.
Concerns In determining whether the large proportion of respondents with breast cancer skewed the study results, it was determined that median scores differed significantly in only four concerns. Compared with respondents without breast cancer, respondents with breast cancer were more likely to have significantly lower scores for concerns related to fatigue (P <.001) and sexual issues/intimacy (P = .001). Respondents with breast cancer were more likely to have significantly higher scores than respondents without breast cancer for concerns related to genetic counseling (P = .001) and fear of developing a new cancer (P = .010).
Fears of the cancer returning and developing a new cancer were the two most prevalent concerns, identified by 51% (n = 486) and 47.5% (n = 459), respectively (Table 2). Physical concerns, rated as moderate/high concerns by at least 25% of the sample, were fatigue (n = 336, 34.8%), changes in [the] body after cancer (n = 323, 33.7%), trouble sleeping (n = 302, 31.0%), sexual issues/intimacy (n = 263, 28.0%), memory and concentration (n = 261, 26.7%), and weight changes (n = 248, 25.5%). The most prevalent moderate/high social concerns were related to finances (n = 265, 27.5%) and debt from medical bills (n = 232, 25.1%). Managing stress (n = 279, 29.2%) and difficult emotions (n = 244, 25.1%) were prevalent moderate/high emotional concerns. Spiritual concerns were less often rated as moderate/high concerns. Having a breast cancer diagnosis was not significantly related to the number of reported moderate to high concerns (P = 1.00).
Variables associated with the 12 most frequent moderate/high concerns are shown in Tables 3 and 4. Age was associated with the most moderate/high concerns. With every decade of age, the odds of having the following moderate/high concerns decreased: bodily changes after cancer (odds ratio [OR], 0.75), sexual intimacy (OR, 0.81), memory and concentration (OR, 0.83), weight changes (OR, 0.77), financial (OR, 0.75), debt (OR, 0.71), cancer returning (OR, 0.66), developing a new cancer (OR, 0.67), managing stress (OR, 0.67), and managing difficult emotions (OR, 0.67).
Female sex was associated with lower odds of having a concern about sexual intimacy (OR, 0.30) and increased odds of having concerns related to memory and concentration (OR, 1.78), managing stress (OR, 2.35), and managing difficult emotions (OR, 1.77). Race was another demographic characteristic statistically associated with numerous moderate/high concerns. Survivors who identified white, were more likely than other people of other races to have fewer moderate/high concerns regarding bodily changes after cancer (OR, 0.46), weight change (OR, 0.46), finances (OR, 0.46), debt (OR, 0.40), managing stress (OR, 0.55), and managing difficult emotions (OR, 0.49). The odds of having a moderate/high concern regarding debt was 2.25 times higher given widowed marital status compared with those survivors who were single. Unemployment status, when compared with full-time employment, was significantly associated with increased odds of having moderate/high concerns related to fatigue (OR, 2.08), bodily changes after cancer (OR, 1.72), memory and concentration (OR, 2.45), weight changes (OR, 2.17), finances (OR, 1.93), developing a new cancer (OR, 1.91), and managing difficult emotions (OR, 1.80).
As expected, respondents who had completed treatment were less likely to have many of the moderate/high concerns as those still undergoing treatment. Survivors who were up to 2 years posttreatment were significantly more likely than those survivors receiving treatment to have fewer moderate/high concerns regarding fatigue (OR, 0.56), sexual intimacy (OR, 0.54), weight change (OR, 0.55), fears of the cancer returning (OR, 0.48), developing a new cancer (OR, 0.35), managing stress (OR, 0.43), and managing difficult emotions (OR, 0.49).
However, those improved odds were not sustained over the cancer trajectory. Compared with survivors who were receiving treatment, survivors who were between 2-5 years posttreatment did not have significantly reduced odds for moderate/high concerns related to fatigue, sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress, and managing difficult emotions. They did have significantly reduced odds for having concerns only related to finances (OR, 0.61) and debt (OR, 0.52).
Long-term survivors, who were beyond 5 years posttreatment, had significantly reduced odds for having moderate/high concerns related to fatigue (OR, 0.45), finances (OR, 0.52), debt (OR, 0.47), and managing difficult emotions (OR, 0.54), compared with survivors receiving treatment. Moderate/high concerns related to sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress did not have improved odds for these long-term survivors.
Attention to needs
The health care teams were rated highly for their attention to the patients’ physical needs. Most respondents (n = 845, 92.4%) viewed the health care team’s attention their physical needs as important and 763 (77.6%) survivors rated the team’s attention to these needs as excellent. The importance of addressing emotional needs was affirmed by 723 (78.5%) respondents, and although 454 (46.8%) viewed the team’s attention to these needs as excellent, 119 (12.3%) reported that the health care team did not ask about emotional needs. In addition, 566 respondents (60%) viewed having the health care team address their social needs as important, and most (n = 715, 74.2%) rated the team’s attention to social needs as good or excellent. Yet, 162 (16.8%) respondents reported that team did not ask about their social needs. The health care team’s addressing of spiritual needs was viewed as important by 346 (37.5%) respondents and ratings for how well the team attended to spiritual needs were: 148 (15.6%) poor or fair, 204 (21.5%) good, and 150 (15.8%) excellent. However, 448 (47.2%) respondents reported that the health care team did not ask about their spiritual needs.
Discussion
The primary purpose of this project was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs and to assess the perceived importance of these needs and the extent to which the cancer center staff were attentive to those needs. The overall goal of this assessment was to inform the development of survivorship and supportive care programs by highlighting common concerns, demographic and medical factors associated with specific concerns, and timing of moderate/high level concerns along the cancer trajectory. There were 3 main findings.
First, the results support the need for enhancing supportive care services to meet emotional concerns of survivors beyond the treatment phase. Similar to other studies,8,9 emotional concerns ranked higher than all other concerns in this study with about 50% of the sample rating “fear the cancer will return” and “fear of developing a new cancer” as moderate/high concern. Although the odds of not having these emotional concerns improved up to 2 years posttreatment, these concerns are likely to resurface, as odds for survivors beyond 2 years were not significantly different from those receiving treatment. A recent systematic review reported that fear of cancer recurrence is experienced by about 73% of cancer survivors, with 49% reporting a moderate to high degree.10 It can have a chronic, stable trajectory for some survivors and is strongly associated with higher levels of anxiety, distress, and depression, and less global, emotional/mental, physical, role, social, and cognitive quality of life.10 In this sample, managing stress and difficult emotions were also rated as moderate/high concerns by at least 25% of the sample.
Second, the findings identified patients at risk for cancer-related concerns throughout the cancer trajectory. As demonstrated in other studies, younger age was associated with greater odds of having multiple greater moderate/high concerns.11-13 Unemployment was the second most common demographic factor associated with multiple moderate/high concerns related to physical symptoms, finances and emotions. Similarly, identifying as black, Asian, American Indian/Alaskan Native, or other was also associated with greater odds of having numerous physical, financial, and emotional concerns. Women had greater concerns related to memory, sexual intimacy, coping with difficult emotions, and stress.
Third, the results helped to identify gaps in supportive care at our cancer center. Although spiritual concerns were not prevalent as being moderate/high, they were still viewed by about a third of survivors as being an important area for the health care team to address. Yet, consistent with other need assessments, spiritual concerns in this study were least often addressed by staff.1 Assessment of spiritual care needs, screening for spiritual distress, and providing spiritual care are essential components of a clinician-patient relationship that supports healing.14 The importance of attending to spiritual care needs was underscored by a recent systematic review that found a positive association between overall spiritual well-being and quality of life in patients with cancer, with the meaning/peace factor consistently and positively associated with physical and mental health.15 Another identified gap was the health care team’s lack of attention to the patient’s social needs, which included concerns related to finances and debt from medical bills. In all, 46% of the respondents reported having financial concerns, with the odds of having moderate/high financial concerns being greatest during treatment to 2 years posttreatment. Attention to the financial burden of cancer patients is critical because the magnitude of cancer-related financial concerns is a significant, strong predictor of quality of life and adverse psychological issues such as depression, anxiety, and distress.16,17
There were several program implications based on the results. A periodic audit of the concerns of survivors and their views on how well their needs were being met was a relatively low cost endeavor. Although the findings were consistent with the literature, the results, when shared with administrators and clinicians, were instrumental in effecting change because they represented the concerns of survivors at the cancer center. Another program directive, based on the results, was to extend the routine screening of patients’ needs during treatment to posttreatment survivorship. Patients who are young, unemployed, do not identify as white, and female warrant more thorough assessment of needs and concerns along the cancer trajectory. Integral to these screenings is the need for patient-centered communication, with discussion of how cancer is affecting the different domains of quality of life within the context of the patient’s life. Lastly, the results clearly indicated the need for additional training of health care providers on how to assess and address spiritual well-being in cancer survivors.
There were limitations to this study, including use of a nonvalidated survey and cross-sectional approach that limited our ability to explore how concerns might change over the trajectory. Also, it was not possible to clarify medical information of the respondents, such as cancer stage. Although the response rate of this study was not high, we are confident in the results because of the large sample size and the finding that the large proportion of respondents with breast cancer was not influential. Despite these limitations, this needs assessment of cancer survivors over the trajectory of care provided insight into the scope of their concerns, identified vulnerable groups of survivors, and highlighted gaps in addressing those concerns. A quality- of-life framework for assessing needs assured a comprehensive focus and generated practice changes to strengthen holistic, comprehensive oncology care.
Complex cancer treatments, limited personnel resources, and a growing number of cancer survivors are challenging cancer health care professionals’ abilities to provide comprehensive care. Cancer survivors have a range of needs that extend over the cancer care trajectory and that represent physical, psychological, social, and spiritual domains. Numerous studies have explored supportive care needs and recent systematic reviews have highlighted the supportive care needs related to cancer1 and to specific cancer types, including prostate cancer,2 breast cancer,3 gynecologic cancer,4 hematological cancer,5 and lung cancer.6 However, reviews are limited in that they do not always assess needs across the cancer trajectory or identify demographic or clinical variables that are associated with needs. These data are needed to focus survivorship program development in cancer centers in order to target populations most likely at risk for unmet needs, identify what salient concerns to address, and to appropriately schedule supportive care programs.
The importance of assessing the patient’s subjective view of his/her needs or concerns is well acknowledged as being fundamental to patient-centered care.7 Clinicians routinely assess needs in practice using a variety of screening tools. However, there needs to be a broader assessment of concerns and needs in a population of survivors with mixed cancer diagnoses, along with their appraisal of how well their needs were addressed by their health care team, to provide an overall identification of gaps in supportive care. The primary purpose of the present study was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs; ascertain survivors’ perceived importance of those needs and the extent to which our institution, the University Hospitals Seidman Cancer Center, was attentive to those needs; and to identify who might be at risk for having greater concerns. The overall goal was to use the data to inform survivorship and supportive care program development.
Methods
Design, sample and setting
We used a cross-sectional design. Surveys were mailed once to a convenience sample of 2,750 adult patients who had been seen in follow-up during the previous 2 years (2010-2011) at all clinical sites of University Hospitals Seidman Cancer Center, a Midwestern National Cancer Institute-designated Comprehensive Cancer Center. Patients who had a noncancer diagnosis were excluded. The distribution list was screened for deceased individuals and those patients who had multiple visits during the time period. The project was reviewed and approved as nonresearch by the Case Western Reserve University Cancer Institutional Review Board.
Survey
An interdisciplinary team of clinicians, administrators, and researchers adapted the Mayo Clinic Cancer Center’s Cancer Survivors Survey of Needs8 to create a comprehensive survey for the cancer center. Input regarding the scope of the survey was sought from the Patient and Family Advisory Council of the cancer center. The survey, which was formatted for scanning purposes, consisted of 33 questions that were compiled into 4 sections. Sections 1 and 2 focused on demographic and treatment-related information, including use of community and hospital support services and preferences for follow-up care. In section 3, a quality-of-life framework was used to assess physical, social, emotional, and spiritual needs. Respondents were asked to rate their current level of concern for 19 physical effects, 10 social effects, 10 emotional effects, and 5 spiritual effects on a scale ranging from 0 (no concern) to 5 (extreme concern). In section 4, respondents were asked to indicate the importance of the cancer team addressing their physical, social, emotional, and spiritual needs. This was followed by their rating of the cancer team’s attention to their needs as Poor, Fair, Good, Excellent, or They did not ask about my needs. Respondents were asked about preferences for learning about physical, social, emotional, and spiritual effects. In addition to the 33 questions, there were 6 open-ended questions in which respondents were encouraged to share additional information about their needs, sources of support, and other concerns.
Procedures
Eligible respondents were mailed a cover letter explaining the survey from both the director and president of the cancer center, a survey, and a postage-paid return envelope. The option to respond to the survey by a telephone call to the director of the Office of Cancer Survivorship was offered in the cover letter.
Data analysis
Returned surveys were scanned into a Teleform database, verified, and exported into an SPSS data file. Data quality was checked by running frequency analyses and summarizing variables. Time-since-treatment responses were collapsed into 4 categories: on treatment, up to 2 years posttreatment, 2-5 years posttreatment, and more than 5 years posttreatment. Descriptive statistics were used to summarize demographic and medical characteristics of the respondents and to calculate the mean score for each concern for the total sample and then for each category of time since treatment. Because of the large number of respondents with breast cancer, the respondents were stratified into two groups, one of breast cancer the other of nonbreast cancer respondents. Then, the Mann-Whitney test was performed for each concern to examine differences between respondents with and without breast cancer.
To identify the most prevalent concerns, ratings for concerns were recoded into no concern (rated as 0), low concern (1 or 2), and moderate/high concern (3, 4, or 5). Since our interest was in the moderate and high concerns, the responses were dichotomized into moderate/high concerns and all other levels. Logistic regression models were then used to identify associations between a set of survivor characteristics or covariates (age, sex, living status, marital status, employment status, cancer type, and time since treatment) with the 12 most highly rated moderate/high concerns. All the analyses were performed using statistical software SPSS 20 and Stata 13.0
Results
Respondents
A total of 1,005 surveys were returned for a 37% response rate. Forty-two patients responded by telephone. The mean age of respondents was 64.9 years (range, 22-98; SD, 12.8). The typical respondent was female, white, and married (Table 1). Twenty-four percent of the respondents (n = 240) reported living alone. Although about 47% of respondents (n = 473) reported a breast cancer diagnosis, more than 17 cancers were identified, and 14% of respondents (n = 145) listed multiple diagnoses. About a third of respondents were receiving treatment when they completed the survey.
Just under half of the respondents (n = 498) reported using community resources for support and information about cancer, and 29.5% (n = 296) sought information on the internet during their cancer experience. The most commonly used community resources were The Gathering Place, a local organization offering free supportive programs and services to individuals with cancer and their families (n = 167), and the American Cancer Society (n = 138). Of the 496 respondents who reported accessing hospital resources, most (n = 322) said they used information that their health care team recommended. Other supportive options were used to a lesser degree: support groups (n = 92), chemotherapy and radiation therapy classes (n = 129), and supportive/educational programs offered by the cancer center (n = 27). Most of the respondents (n = 822, 88.6%) preferred to have their follow-up care remain with their cancer care team 1 year after treatments are completed. Almost two-thirds of respondents (n = 601, 64%) cited being seen at the cancer center for follow-up care as the most important factor in considering follow-up care.
Concerns In determining whether the large proportion of respondents with breast cancer skewed the study results, it was determined that median scores differed significantly in only four concerns. Compared with respondents without breast cancer, respondents with breast cancer were more likely to have significantly lower scores for concerns related to fatigue (P <.001) and sexual issues/intimacy (P = .001). Respondents with breast cancer were more likely to have significantly higher scores than respondents without breast cancer for concerns related to genetic counseling (P = .001) and fear of developing a new cancer (P = .010).
Fears of the cancer returning and developing a new cancer were the two most prevalent concerns, identified by 51% (n = 486) and 47.5% (n = 459), respectively (Table 2). Physical concerns, rated as moderate/high concerns by at least 25% of the sample, were fatigue (n = 336, 34.8%), changes in [the] body after cancer (n = 323, 33.7%), trouble sleeping (n = 302, 31.0%), sexual issues/intimacy (n = 263, 28.0%), memory and concentration (n = 261, 26.7%), and weight changes (n = 248, 25.5%). The most prevalent moderate/high social concerns were related to finances (n = 265, 27.5%) and debt from medical bills (n = 232, 25.1%). Managing stress (n = 279, 29.2%) and difficult emotions (n = 244, 25.1%) were prevalent moderate/high emotional concerns. Spiritual concerns were less often rated as moderate/high concerns. Having a breast cancer diagnosis was not significantly related to the number of reported moderate to high concerns (P = 1.00).
Variables associated with the 12 most frequent moderate/high concerns are shown in Tables 3 and 4. Age was associated with the most moderate/high concerns. With every decade of age, the odds of having the following moderate/high concerns decreased: bodily changes after cancer (odds ratio [OR], 0.75), sexual intimacy (OR, 0.81), memory and concentration (OR, 0.83), weight changes (OR, 0.77), financial (OR, 0.75), debt (OR, 0.71), cancer returning (OR, 0.66), developing a new cancer (OR, 0.67), managing stress (OR, 0.67), and managing difficult emotions (OR, 0.67).
Female sex was associated with lower odds of having a concern about sexual intimacy (OR, 0.30) and increased odds of having concerns related to memory and concentration (OR, 1.78), managing stress (OR, 2.35), and managing difficult emotions (OR, 1.77). Race was another demographic characteristic statistically associated with numerous moderate/high concerns. Survivors who identified white, were more likely than other people of other races to have fewer moderate/high concerns regarding bodily changes after cancer (OR, 0.46), weight change (OR, 0.46), finances (OR, 0.46), debt (OR, 0.40), managing stress (OR, 0.55), and managing difficult emotions (OR, 0.49). The odds of having a moderate/high concern regarding debt was 2.25 times higher given widowed marital status compared with those survivors who were single. Unemployment status, when compared with full-time employment, was significantly associated with increased odds of having moderate/high concerns related to fatigue (OR, 2.08), bodily changes after cancer (OR, 1.72), memory and concentration (OR, 2.45), weight changes (OR, 2.17), finances (OR, 1.93), developing a new cancer (OR, 1.91), and managing difficult emotions (OR, 1.80).
As expected, respondents who had completed treatment were less likely to have many of the moderate/high concerns as those still undergoing treatment. Survivors who were up to 2 years posttreatment were significantly more likely than those survivors receiving treatment to have fewer moderate/high concerns regarding fatigue (OR, 0.56), sexual intimacy (OR, 0.54), weight change (OR, 0.55), fears of the cancer returning (OR, 0.48), developing a new cancer (OR, 0.35), managing stress (OR, 0.43), and managing difficult emotions (OR, 0.49).
However, those improved odds were not sustained over the cancer trajectory. Compared with survivors who were receiving treatment, survivors who were between 2-5 years posttreatment did not have significantly reduced odds for moderate/high concerns related to fatigue, sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress, and managing difficult emotions. They did have significantly reduced odds for having concerns only related to finances (OR, 0.61) and debt (OR, 0.52).
Long-term survivors, who were beyond 5 years posttreatment, had significantly reduced odds for having moderate/high concerns related to fatigue (OR, 0.45), finances (OR, 0.52), debt (OR, 0.47), and managing difficult emotions (OR, 0.54), compared with survivors receiving treatment. Moderate/high concerns related to sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress did not have improved odds for these long-term survivors.
Attention to needs
The health care teams were rated highly for their attention to the patients’ physical needs. Most respondents (n = 845, 92.4%) viewed the health care team’s attention their physical needs as important and 763 (77.6%) survivors rated the team’s attention to these needs as excellent. The importance of addressing emotional needs was affirmed by 723 (78.5%) respondents, and although 454 (46.8%) viewed the team’s attention to these needs as excellent, 119 (12.3%) reported that the health care team did not ask about emotional needs. In addition, 566 respondents (60%) viewed having the health care team address their social needs as important, and most (n = 715, 74.2%) rated the team’s attention to social needs as good or excellent. Yet, 162 (16.8%) respondents reported that team did not ask about their social needs. The health care team’s addressing of spiritual needs was viewed as important by 346 (37.5%) respondents and ratings for how well the team attended to spiritual needs were: 148 (15.6%) poor or fair, 204 (21.5%) good, and 150 (15.8%) excellent. However, 448 (47.2%) respondents reported that the health care team did not ask about their spiritual needs.
Discussion
The primary purpose of this project was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs and to assess the perceived importance of these needs and the extent to which the cancer center staff were attentive to those needs. The overall goal of this assessment was to inform the development of survivorship and supportive care programs by highlighting common concerns, demographic and medical factors associated with specific concerns, and timing of moderate/high level concerns along the cancer trajectory. There were 3 main findings.
First, the results support the need for enhancing supportive care services to meet emotional concerns of survivors beyond the treatment phase. Similar to other studies,8,9 emotional concerns ranked higher than all other concerns in this study with about 50% of the sample rating “fear the cancer will return” and “fear of developing a new cancer” as moderate/high concern. Although the odds of not having these emotional concerns improved up to 2 years posttreatment, these concerns are likely to resurface, as odds for survivors beyond 2 years were not significantly different from those receiving treatment. A recent systematic review reported that fear of cancer recurrence is experienced by about 73% of cancer survivors, with 49% reporting a moderate to high degree.10 It can have a chronic, stable trajectory for some survivors and is strongly associated with higher levels of anxiety, distress, and depression, and less global, emotional/mental, physical, role, social, and cognitive quality of life.10 In this sample, managing stress and difficult emotions were also rated as moderate/high concerns by at least 25% of the sample.
Second, the findings identified patients at risk for cancer-related concerns throughout the cancer trajectory. As demonstrated in other studies, younger age was associated with greater odds of having multiple greater moderate/high concerns.11-13 Unemployment was the second most common demographic factor associated with multiple moderate/high concerns related to physical symptoms, finances and emotions. Similarly, identifying as black, Asian, American Indian/Alaskan Native, or other was also associated with greater odds of having numerous physical, financial, and emotional concerns. Women had greater concerns related to memory, sexual intimacy, coping with difficult emotions, and stress.
Third, the results helped to identify gaps in supportive care at our cancer center. Although spiritual concerns were not prevalent as being moderate/high, they were still viewed by about a third of survivors as being an important area for the health care team to address. Yet, consistent with other need assessments, spiritual concerns in this study were least often addressed by staff.1 Assessment of spiritual care needs, screening for spiritual distress, and providing spiritual care are essential components of a clinician-patient relationship that supports healing.14 The importance of attending to spiritual care needs was underscored by a recent systematic review that found a positive association between overall spiritual well-being and quality of life in patients with cancer, with the meaning/peace factor consistently and positively associated with physical and mental health.15 Another identified gap was the health care team’s lack of attention to the patient’s social needs, which included concerns related to finances and debt from medical bills. In all, 46% of the respondents reported having financial concerns, with the odds of having moderate/high financial concerns being greatest during treatment to 2 years posttreatment. Attention to the financial burden of cancer patients is critical because the magnitude of cancer-related financial concerns is a significant, strong predictor of quality of life and adverse psychological issues such as depression, anxiety, and distress.16,17
There were several program implications based on the results. A periodic audit of the concerns of survivors and their views on how well their needs were being met was a relatively low cost endeavor. Although the findings were consistent with the literature, the results, when shared with administrators and clinicians, were instrumental in effecting change because they represented the concerns of survivors at the cancer center. Another program directive, based on the results, was to extend the routine screening of patients’ needs during treatment to posttreatment survivorship. Patients who are young, unemployed, do not identify as white, and female warrant more thorough assessment of needs and concerns along the cancer trajectory. Integral to these screenings is the need for patient-centered communication, with discussion of how cancer is affecting the different domains of quality of life within the context of the patient’s life. Lastly, the results clearly indicated the need for additional training of health care providers on how to assess and address spiritual well-being in cancer survivors.
There were limitations to this study, including use of a nonvalidated survey and cross-sectional approach that limited our ability to explore how concerns might change over the trajectory. Also, it was not possible to clarify medical information of the respondents, such as cancer stage. Although the response rate of this study was not high, we are confident in the results because of the large sample size and the finding that the large proportion of respondents with breast cancer was not influential. Despite these limitations, this needs assessment of cancer survivors over the trajectory of care provided insight into the scope of their concerns, identified vulnerable groups of survivors, and highlighted gaps in addressing those concerns. A quality- of-life framework for assessing needs assured a comprehensive focus and generated practice changes to strengthen holistic, comprehensive oncology care.
1. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
2. Paterson C, Robertson A, Smith A, Nabi G. Identifying the unmet supportive care needs of men living with and beyond prostate cancer: A systematic review. Eur J Oncol Nurs. 2015;19:405-418.
3. Fiszer C, Dolbeault S, Sultan S, Bredart A. Prevalence, intensity, and predictors of the supportive care needs of women diagnosed with breast cancer: A systematic review. Psychooncology. 2014;23:361-374.
4. Maguire R, Kotronoulas G, Simpson M, Paterson C. A systematic review of the supportive care needs of women living with and beyond cervical cancer. Gynecol Oncol. 2015;136:478-490.
5. Hall A, Lynagh M, Bryant J, Sanson-Fisher R. Supportive care needs of hematological cancer survivors: A critical review of the literature. Crit Rev Oncol Hematol. 2013;88:102-116.
6. Maguire R, Papadopoulou C, Kotronoulas G, Simpson MF, McPhelim J, Irvine L. A systematic review of supportive care needs of people living with lung cancer. Eur J Oncol Nurs. 2013;17:449-464.
7. Adler NE, Page EK. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press; Institute of Medicine, 2008.
8. Ness S, Kokal J, Fee-Schroeder K, Novotny P, Satele D, Barton D. Concerns across the survivorship trajectory: results from a survey of cancer survivors. Oncol Nurs Forum. 2013;40:35-42.
9. Swash B, Hulbert-Williams N, Bramwell R. Unmet psychosocial needs in haematological cancer: A systematic review. Support Care Cancer. 2014;22:1131-1141.
10. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J Cancer Surviv. 2013;7:300-322.
11. Choi KH, Park JH, Park JH, Park JS. Psychosocial needs of cancer patients and related factors: A multi-center, cross-sectional study in Korea. Psychooncology. 2013;22:1073-1080.
12. Pauwels EE, Charlier C, De Bourdeaudhuij I, Lechner L, Van Hoof E. Care needs after primary breast cancer treatment. Survivors’ associated sociodemographic and medical characteristics. Psychooncology. 2013;22:125-132.
13. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
14. Puchalski CM, Blatt B, Kogan M, Butler A. Spirituality and health: The development of a field. Academic Medicine. 2014;89:10-16.
15. Bai M, Lazenby M. A systematic review of associations between spiritual well-being and quality of life at the scale and factor levels in studies among patients with cancer. J Palliat Med. 2015;18:286-298.
16. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332-338.
17. Sharp L, Carsin AE, Timmons A. Associations between cancer-related financial stress and strain and psychological wellbeing among individuals living with cancer. Psychooncology. 2013;22:745-755.
1. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
2. Paterson C, Robertson A, Smith A, Nabi G. Identifying the unmet supportive care needs of men living with and beyond prostate cancer: A systematic review. Eur J Oncol Nurs. 2015;19:405-418.
3. Fiszer C, Dolbeault S, Sultan S, Bredart A. Prevalence, intensity, and predictors of the supportive care needs of women diagnosed with breast cancer: A systematic review. Psychooncology. 2014;23:361-374.
4. Maguire R, Kotronoulas G, Simpson M, Paterson C. A systematic review of the supportive care needs of women living with and beyond cervical cancer. Gynecol Oncol. 2015;136:478-490.
5. Hall A, Lynagh M, Bryant J, Sanson-Fisher R. Supportive care needs of hematological cancer survivors: A critical review of the literature. Crit Rev Oncol Hematol. 2013;88:102-116.
6. Maguire R, Papadopoulou C, Kotronoulas G, Simpson MF, McPhelim J, Irvine L. A systematic review of supportive care needs of people living with lung cancer. Eur J Oncol Nurs. 2013;17:449-464.
7. Adler NE, Page EK. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press; Institute of Medicine, 2008.
8. Ness S, Kokal J, Fee-Schroeder K, Novotny P, Satele D, Barton D. Concerns across the survivorship trajectory: results from a survey of cancer survivors. Oncol Nurs Forum. 2013;40:35-42.
9. Swash B, Hulbert-Williams N, Bramwell R. Unmet psychosocial needs in haematological cancer: A systematic review. Support Care Cancer. 2014;22:1131-1141.
10. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J Cancer Surviv. 2013;7:300-322.
11. Choi KH, Park JH, Park JH, Park JS. Psychosocial needs of cancer patients and related factors: A multi-center, cross-sectional study in Korea. Psychooncology. 2013;22:1073-1080.
12. Pauwels EE, Charlier C, De Bourdeaudhuij I, Lechner L, Van Hoof E. Care needs after primary breast cancer treatment. Survivors’ associated sociodemographic and medical characteristics. Psychooncology. 2013;22:125-132.
13. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
14. Puchalski CM, Blatt B, Kogan M, Butler A. Spirituality and health: The development of a field. Academic Medicine. 2014;89:10-16.
15. Bai M, Lazenby M. A systematic review of associations between spiritual well-being and quality of life at the scale and factor levels in studies among patients with cancer. J Palliat Med. 2015;18:286-298.
16. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332-338.
17. Sharp L, Carsin AE, Timmons A. Associations between cancer-related financial stress and strain and psychological wellbeing among individuals living with cancer. Psychooncology. 2013;22:745-755.
Bilateral chylothorax in an AIDS patient with newly diagnosed Kaposi sarcoma
Kaposi sarcoma is an angioproliferative tumor that is associated with human herpes virus-8 (HHV-8). Mucocutaneous disease is the most common site for manifestation of AIDS-related Kaposi sarcoma, commonly affecting the lower extremities, oral mucosa, face, and genitalia. Pleural effusions can occur in 36%-60% of patients with Kaposi sarcoma, and it has been documented that chylothorax is a rare, but plausible presentation in patients with Kaposi sarcoma.1 We present here a case of bilateral chylothorax in a patient with AIDS-related Kaposi sarcoma.
Case presentation and summary
A 52-year-old MSM male with AIDS (CD4, <20 mm3; viral load, 58 copies/ml) presented to the emergency department with complaints of shortness of breath, productive cough, and diarrhea for 2 days prior to presentation. His medical history also included chronic obstructive pulmonary disease, coronary artery disease, and hyperlipidemia. The patient was not on HAART because of his history of noncompliance. The results of a chest X-ray and computed-tomography (CT) scan showed that the patient had bilateral pleural effusion and a spiculated 14-mm nodule in the left upper lobe.
The patient underwent ultrasound-guided placement of a 12-French left-sided chest catheter, and a milky white fluid was aspirated from the left pleural space. Laboratory analysis of the pleural fluid confirmed an exudate with an elevated triglyceride level of 120 mg/dL (chylous, >110 mg/dL) indicating chylothorax.
On close physical examination, the patient was found to have multiple irregular plaques on the back and lower extremities. As described by dermatology, there was a violaceous indurated plaque on the left axillae, violaceous indurated plaques with superficial scale grouped on the left midlateral back, and hyperpigmented lichenified plaques and papules on bilateral shins, with some with plate-like scale. Two punch biopsies were taken of the skin lesions, which confirmed Kaposi sarcoma, plaque stage from the lesion biopsied on the back, and patch stage from the lesion biopsied in the left axilla. Cytology of the pleural fluid was negative for malignant cells. On review by the radiologist of the CT scan of the chest, there was no indication of gross distention of the thoracic duct. Treatment options were offered to the patient, and the patient was considering options for chemotherapy and home hospice given his advanced disease state at the time of discharge.
Discussion
Chylothorax occurs with a thoracic duct obstruction, which results in leakage of lymphatic fluid into the pleural cavity. The two leading causes of chylothorax are trauma and malignancy, with lymphoma being the most common cause of chylothorax among those with malignancy.2 Chylothorax, however, is a rare but documented complication of Kaposi sarcoma. Marais and colleagues reported the case of a 3-year-old HIV-positive patient with newly diagnosed Kaposi sarcoma who was found to have tumor infiltration in the thoracic duct leading to bilateral chylothorax.3 Maradona and colleagues described a 40-year-old man with AIDS-related Kaposi sarcoma who was found to have pleural and pericardial Kaposi sarcoma with chylothorax.4 Priest and colleagues wrote about a 32-year-old patient with AIDS with biopsy-proven Kaposi sarcoma who required multiple therapeutic thoracenteses for rapidly recurrent left chylothorax effusions.5
There are two leading discussions as to the pathophysiology of chylothorax that is related to Kaposi sarcoma: chylothorax developing secondary to metastatic disease or the development of chylothorax secondary to primary Kaposi sarcoma arising from the pleural region.6 One case report examined pleural and lung biopsies in a 34-year-old patient with AIDS-related Kaposi sarcoma that showed immunohistochemical staining that was suggestive of early-stage Kaposi sarcoma of lymphatic endothelial origin. The authors were attempting to illustrate that Kaposi sarcoma may have a stem-cell origin which can differentiate into lymph cells. Kontantinopoulos and colleagues postulated that in situ Kaposi sarcoma can arise from the lymphatic system with a resultant clinical presentation of chylothorax.7 The more mainstream thought however, is that chylothorax has been found to develop secondary to metastatic disease. The present case, therefore, illustrates an unusual presentation of cytology negative chylothorax in a patient with AIDS-related Kaposi sarcoma.
1. Sridar S, Garza EG, Cox J, Rumbak MJ. Serosanguineous pleural effusions in a patient with HIV and Kaposi sarcoma: pleuroscopic findings. J Bronchology Interv Pulmonol. 2011;18(4):337-339.
2. Light RW. Chylothorax and pseudochylothorax. In: Light RW, ed. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:412-426.
3. Marais BJ, Pienaar J, Gie RP. Kaposi sarcoma with upper airway obstruction and bilateral chylothoraces. Pediatr Infect Dis J. 2003;22:926-928.
4. Maradona JA, Carton JA, Asensi V, Rodriguez-Guardado A. AIDS-related Kaposi sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS. 2002;16(5):806.
5. Priest ER, Weiss R. Chylothorax with Kaposi sarcoma. South Med J. 1991;84:806-807.
6. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190.
7. Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in situ Kaposi sarcoma. BMC Clin Pathol. 2006;30:6:7.
Kaposi sarcoma is an angioproliferative tumor that is associated with human herpes virus-8 (HHV-8). Mucocutaneous disease is the most common site for manifestation of AIDS-related Kaposi sarcoma, commonly affecting the lower extremities, oral mucosa, face, and genitalia. Pleural effusions can occur in 36%-60% of patients with Kaposi sarcoma, and it has been documented that chylothorax is a rare, but plausible presentation in patients with Kaposi sarcoma.1 We present here a case of bilateral chylothorax in a patient with AIDS-related Kaposi sarcoma.
Case presentation and summary
A 52-year-old MSM male with AIDS (CD4, <20 mm3; viral load, 58 copies/ml) presented to the emergency department with complaints of shortness of breath, productive cough, and diarrhea for 2 days prior to presentation. His medical history also included chronic obstructive pulmonary disease, coronary artery disease, and hyperlipidemia. The patient was not on HAART because of his history of noncompliance. The results of a chest X-ray and computed-tomography (CT) scan showed that the patient had bilateral pleural effusion and a spiculated 14-mm nodule in the left upper lobe.
The patient underwent ultrasound-guided placement of a 12-French left-sided chest catheter, and a milky white fluid was aspirated from the left pleural space. Laboratory analysis of the pleural fluid confirmed an exudate with an elevated triglyceride level of 120 mg/dL (chylous, >110 mg/dL) indicating chylothorax.
On close physical examination, the patient was found to have multiple irregular plaques on the back and lower extremities. As described by dermatology, there was a violaceous indurated plaque on the left axillae, violaceous indurated plaques with superficial scale grouped on the left midlateral back, and hyperpigmented lichenified plaques and papules on bilateral shins, with some with plate-like scale. Two punch biopsies were taken of the skin lesions, which confirmed Kaposi sarcoma, plaque stage from the lesion biopsied on the back, and patch stage from the lesion biopsied in the left axilla. Cytology of the pleural fluid was negative for malignant cells. On review by the radiologist of the CT scan of the chest, there was no indication of gross distention of the thoracic duct. Treatment options were offered to the patient, and the patient was considering options for chemotherapy and home hospice given his advanced disease state at the time of discharge.
Discussion
Chylothorax occurs with a thoracic duct obstruction, which results in leakage of lymphatic fluid into the pleural cavity. The two leading causes of chylothorax are trauma and malignancy, with lymphoma being the most common cause of chylothorax among those with malignancy.2 Chylothorax, however, is a rare but documented complication of Kaposi sarcoma. Marais and colleagues reported the case of a 3-year-old HIV-positive patient with newly diagnosed Kaposi sarcoma who was found to have tumor infiltration in the thoracic duct leading to bilateral chylothorax.3 Maradona and colleagues described a 40-year-old man with AIDS-related Kaposi sarcoma who was found to have pleural and pericardial Kaposi sarcoma with chylothorax.4 Priest and colleagues wrote about a 32-year-old patient with AIDS with biopsy-proven Kaposi sarcoma who required multiple therapeutic thoracenteses for rapidly recurrent left chylothorax effusions.5
There are two leading discussions as to the pathophysiology of chylothorax that is related to Kaposi sarcoma: chylothorax developing secondary to metastatic disease or the development of chylothorax secondary to primary Kaposi sarcoma arising from the pleural region.6 One case report examined pleural and lung biopsies in a 34-year-old patient with AIDS-related Kaposi sarcoma that showed immunohistochemical staining that was suggestive of early-stage Kaposi sarcoma of lymphatic endothelial origin. The authors were attempting to illustrate that Kaposi sarcoma may have a stem-cell origin which can differentiate into lymph cells. Kontantinopoulos and colleagues postulated that in situ Kaposi sarcoma can arise from the lymphatic system with a resultant clinical presentation of chylothorax.7 The more mainstream thought however, is that chylothorax has been found to develop secondary to metastatic disease. The present case, therefore, illustrates an unusual presentation of cytology negative chylothorax in a patient with AIDS-related Kaposi sarcoma.
Kaposi sarcoma is an angioproliferative tumor that is associated with human herpes virus-8 (HHV-8). Mucocutaneous disease is the most common site for manifestation of AIDS-related Kaposi sarcoma, commonly affecting the lower extremities, oral mucosa, face, and genitalia. Pleural effusions can occur in 36%-60% of patients with Kaposi sarcoma, and it has been documented that chylothorax is a rare, but plausible presentation in patients with Kaposi sarcoma.1 We present here a case of bilateral chylothorax in a patient with AIDS-related Kaposi sarcoma.
Case presentation and summary
A 52-year-old MSM male with AIDS (CD4, <20 mm3; viral load, 58 copies/ml) presented to the emergency department with complaints of shortness of breath, productive cough, and diarrhea for 2 days prior to presentation. His medical history also included chronic obstructive pulmonary disease, coronary artery disease, and hyperlipidemia. The patient was not on HAART because of his history of noncompliance. The results of a chest X-ray and computed-tomography (CT) scan showed that the patient had bilateral pleural effusion and a spiculated 14-mm nodule in the left upper lobe.
The patient underwent ultrasound-guided placement of a 12-French left-sided chest catheter, and a milky white fluid was aspirated from the left pleural space. Laboratory analysis of the pleural fluid confirmed an exudate with an elevated triglyceride level of 120 mg/dL (chylous, >110 mg/dL) indicating chylothorax.
On close physical examination, the patient was found to have multiple irregular plaques on the back and lower extremities. As described by dermatology, there was a violaceous indurated plaque on the left axillae, violaceous indurated plaques with superficial scale grouped on the left midlateral back, and hyperpigmented lichenified plaques and papules on bilateral shins, with some with plate-like scale. Two punch biopsies were taken of the skin lesions, which confirmed Kaposi sarcoma, plaque stage from the lesion biopsied on the back, and patch stage from the lesion biopsied in the left axilla. Cytology of the pleural fluid was negative for malignant cells. On review by the radiologist of the CT scan of the chest, there was no indication of gross distention of the thoracic duct. Treatment options were offered to the patient, and the patient was considering options for chemotherapy and home hospice given his advanced disease state at the time of discharge.
Discussion
Chylothorax occurs with a thoracic duct obstruction, which results in leakage of lymphatic fluid into the pleural cavity. The two leading causes of chylothorax are trauma and malignancy, with lymphoma being the most common cause of chylothorax among those with malignancy.2 Chylothorax, however, is a rare but documented complication of Kaposi sarcoma. Marais and colleagues reported the case of a 3-year-old HIV-positive patient with newly diagnosed Kaposi sarcoma who was found to have tumor infiltration in the thoracic duct leading to bilateral chylothorax.3 Maradona and colleagues described a 40-year-old man with AIDS-related Kaposi sarcoma who was found to have pleural and pericardial Kaposi sarcoma with chylothorax.4 Priest and colleagues wrote about a 32-year-old patient with AIDS with biopsy-proven Kaposi sarcoma who required multiple therapeutic thoracenteses for rapidly recurrent left chylothorax effusions.5
There are two leading discussions as to the pathophysiology of chylothorax that is related to Kaposi sarcoma: chylothorax developing secondary to metastatic disease or the development of chylothorax secondary to primary Kaposi sarcoma arising from the pleural region.6 One case report examined pleural and lung biopsies in a 34-year-old patient with AIDS-related Kaposi sarcoma that showed immunohistochemical staining that was suggestive of early-stage Kaposi sarcoma of lymphatic endothelial origin. The authors were attempting to illustrate that Kaposi sarcoma may have a stem-cell origin which can differentiate into lymph cells. Kontantinopoulos and colleagues postulated that in situ Kaposi sarcoma can arise from the lymphatic system with a resultant clinical presentation of chylothorax.7 The more mainstream thought however, is that chylothorax has been found to develop secondary to metastatic disease. The present case, therefore, illustrates an unusual presentation of cytology negative chylothorax in a patient with AIDS-related Kaposi sarcoma.
1. Sridar S, Garza EG, Cox J, Rumbak MJ. Serosanguineous pleural effusions in a patient with HIV and Kaposi sarcoma: pleuroscopic findings. J Bronchology Interv Pulmonol. 2011;18(4):337-339.
2. Light RW. Chylothorax and pseudochylothorax. In: Light RW, ed. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:412-426.
3. Marais BJ, Pienaar J, Gie RP. Kaposi sarcoma with upper airway obstruction and bilateral chylothoraces. Pediatr Infect Dis J. 2003;22:926-928.
4. Maradona JA, Carton JA, Asensi V, Rodriguez-Guardado A. AIDS-related Kaposi sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS. 2002;16(5):806.
5. Priest ER, Weiss R. Chylothorax with Kaposi sarcoma. South Med J. 1991;84:806-807.
6. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190.
7. Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in situ Kaposi sarcoma. BMC Clin Pathol. 2006;30:6:7.
1. Sridar S, Garza EG, Cox J, Rumbak MJ. Serosanguineous pleural effusions in a patient with HIV and Kaposi sarcoma: pleuroscopic findings. J Bronchology Interv Pulmonol. 2011;18(4):337-339.
2. Light RW. Chylothorax and pseudochylothorax. In: Light RW, ed. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:412-426.
3. Marais BJ, Pienaar J, Gie RP. Kaposi sarcoma with upper airway obstruction and bilateral chylothoraces. Pediatr Infect Dis J. 2003;22:926-928.
4. Maradona JA, Carton JA, Asensi V, Rodriguez-Guardado A. AIDS-related Kaposi sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS. 2002;16(5):806.
5. Priest ER, Weiss R. Chylothorax with Kaposi sarcoma. South Med J. 1991;84:806-807.
6. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190.
7. Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in situ Kaposi sarcoma. BMC Clin Pathol. 2006;30:6:7.
Improving cancer care through modern portfolio theory
We struggle daily to improve cancer care – to improve our therapeutic outcomes in cancer – as individual physicians and as researchers. We work collectively to disseminate information and collaborate, and there are welcome calls for open data sharing to accelerate progress.1 We enroll patients on clinical trials, or we work in a basic science lab to discover mechanisms of carcinogenesis and potential therapeutic targets. We discuss “n of 1” trials and the “paradigm shift of precision oncology,” and we are optimistic about the future of cancer care.
Leaving the world of biology and clinical trials for a minute, we also can apply economic theory in our never-ending quest to improve cancer outcomes. One area of interest may be modern portfolio theory (MPT), which the economist Harry Markowitz introduced in an essay in 1952 and later won the Nobel Prize for his work.
At least 71 billionaires live in the San Francisco Bay Area, where I live, but 14,000 children (13%) in the area live below the poverty line.3 When there is a range of asset allocations in health care, results can vary not on the basis of the underlying disease state or the quality of the provider, but on access to care. As an example, most pediatric cancers are curable, yet a recent retrospective analysis of data in the SEER-Medicare registry showed that mortality within 1 month of diagnosis of childhood cancer related in part to socioeconomic factors – those patients with a lower socioeconomic status (which correlates with being an ethnic minority in the United States) were more likely to die within a month of diagnosis of their cancer than were patients with a higher socioeconomic status.3 Here is where MPT can transform the cancer outcomes landscape at no additional investment in basic science or costly precision medicine5: by triaging these patients according to their disease state rather than their ability to pay, they could be administered curative chemotherapy, placed on the appropriate clinical trial, and be cured of their cancer like other children of higher socioeconomic status.
My colleagues and I observed a similar trend when we looked at treatment of diffuse large-cell non-Hodgkin lymphoma in Medicare recipients.6 Although the cure rate is as high as 60%-80% with the use of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or R (rituxin)-CHOP chemotherapy, we found that many patients had received suboptimal chemotherapy. Upon closer examination, we found that there were variations in care by socioeconomic status even in a single-payer system. Thus aspects of cultural literacy and additional efforts for triage need to be developed, but again, application of MPT could be instrumental in improving cancer cure rates by reducing disparities in care by allocating assets to solve access-to-care issues, and curing these patients of their non-Hodgkin lymphoma.
A physician at a Bay Area health care system notes that the open slots in his schedule are triaged by his employer by the patient’s ability to pay – well-insured patients are seen within a few days, but there are very few slots for Medicaid patients, who have to wait weeks or longer to be seen. During this time, their malignancies have time to grow, and potentially metastasize. This may provide suboptimal outcomes for some patients in his community.
We solved this problem at a local hospital where all patients were on Medicaid or uninsured. We triaged patients according to severity of illness, with patients with rapidly growing cancers, particularly curable ones, were brought in as soon as possible and patients with stable benign hematologic conditions seen on a less urgent basis. A social worker and I saw patients together. She would find them resources such as transportation, food, copay assistance to help them through their treatment, and I would optimize their cancer care clinically. On a small scale, this application of MPT (or asset allocation) worked quite well. Perhaps it can be reproduced on a much larger scale. Return on investment relates largely to how you allocate your assets. What’s nice about these applications of MPT is that the return on investment – increasing the cure rate of cancer - is quite large for just a minimal change in asset allocation.
1. Bertagnolli M, Sartor O, Chabner BA, et al. Advantages of a truly open-access data-sharing model. N Engl J Med. 2017;376(12):1178-1181.
2. Baum M. Justice. In: The scepticaemic surgeon: how not to win friends and influence people. New York: Nova Science Pubkishers; November 30, 2014.
3. Glaeser E. Gentfrification and its discontents. Wall Street Journal. May 5, 2017.
4. Green AL, Furutani E, Riberio KB, Galindo CR. Death within 1 month of diagnosis in childhood cancer : analysis of risk factors and scope of the problem. J Clin Oncol. 2017;35(12):1320-1327.
5. McCartney M. Are we too captivated by precision medicine? http://www.bmj.com/content/356/bmj.j1168.long. Published March 9, 2017. Accessed May 12, 2017.
6. Griffiths R, Gleeson M, Knopf K, Danese M. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2995801/. Published November 12, 2010. Accessed May 12, 2017.
We struggle daily to improve cancer care – to improve our therapeutic outcomes in cancer – as individual physicians and as researchers. We work collectively to disseminate information and collaborate, and there are welcome calls for open data sharing to accelerate progress.1 We enroll patients on clinical trials, or we work in a basic science lab to discover mechanisms of carcinogenesis and potential therapeutic targets. We discuss “n of 1” trials and the “paradigm shift of precision oncology,” and we are optimistic about the future of cancer care.
Leaving the world of biology and clinical trials for a minute, we also can apply economic theory in our never-ending quest to improve cancer outcomes. One area of interest may be modern portfolio theory (MPT), which the economist Harry Markowitz introduced in an essay in 1952 and later won the Nobel Prize for his work.
At least 71 billionaires live in the San Francisco Bay Area, where I live, but 14,000 children (13%) in the area live below the poverty line.3 When there is a range of asset allocations in health care, results can vary not on the basis of the underlying disease state or the quality of the provider, but on access to care. As an example, most pediatric cancers are curable, yet a recent retrospective analysis of data in the SEER-Medicare registry showed that mortality within 1 month of diagnosis of childhood cancer related in part to socioeconomic factors – those patients with a lower socioeconomic status (which correlates with being an ethnic minority in the United States) were more likely to die within a month of diagnosis of their cancer than were patients with a higher socioeconomic status.3 Here is where MPT can transform the cancer outcomes landscape at no additional investment in basic science or costly precision medicine5: by triaging these patients according to their disease state rather than their ability to pay, they could be administered curative chemotherapy, placed on the appropriate clinical trial, and be cured of their cancer like other children of higher socioeconomic status.
My colleagues and I observed a similar trend when we looked at treatment of diffuse large-cell non-Hodgkin lymphoma in Medicare recipients.6 Although the cure rate is as high as 60%-80% with the use of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or R (rituxin)-CHOP chemotherapy, we found that many patients had received suboptimal chemotherapy. Upon closer examination, we found that there were variations in care by socioeconomic status even in a single-payer system. Thus aspects of cultural literacy and additional efforts for triage need to be developed, but again, application of MPT could be instrumental in improving cancer cure rates by reducing disparities in care by allocating assets to solve access-to-care issues, and curing these patients of their non-Hodgkin lymphoma.
A physician at a Bay Area health care system notes that the open slots in his schedule are triaged by his employer by the patient’s ability to pay – well-insured patients are seen within a few days, but there are very few slots for Medicaid patients, who have to wait weeks or longer to be seen. During this time, their malignancies have time to grow, and potentially metastasize. This may provide suboptimal outcomes for some patients in his community.
We solved this problem at a local hospital where all patients were on Medicaid or uninsured. We triaged patients according to severity of illness, with patients with rapidly growing cancers, particularly curable ones, were brought in as soon as possible and patients with stable benign hematologic conditions seen on a less urgent basis. A social worker and I saw patients together. She would find them resources such as transportation, food, copay assistance to help them through their treatment, and I would optimize their cancer care clinically. On a small scale, this application of MPT (or asset allocation) worked quite well. Perhaps it can be reproduced on a much larger scale. Return on investment relates largely to how you allocate your assets. What’s nice about these applications of MPT is that the return on investment – increasing the cure rate of cancer - is quite large for just a minimal change in asset allocation.
We struggle daily to improve cancer care – to improve our therapeutic outcomes in cancer – as individual physicians and as researchers. We work collectively to disseminate information and collaborate, and there are welcome calls for open data sharing to accelerate progress.1 We enroll patients on clinical trials, or we work in a basic science lab to discover mechanisms of carcinogenesis and potential therapeutic targets. We discuss “n of 1” trials and the “paradigm shift of precision oncology,” and we are optimistic about the future of cancer care.
Leaving the world of biology and clinical trials for a minute, we also can apply economic theory in our never-ending quest to improve cancer outcomes. One area of interest may be modern portfolio theory (MPT), which the economist Harry Markowitz introduced in an essay in 1952 and later won the Nobel Prize for his work.
At least 71 billionaires live in the San Francisco Bay Area, where I live, but 14,000 children (13%) in the area live below the poverty line.3 When there is a range of asset allocations in health care, results can vary not on the basis of the underlying disease state or the quality of the provider, but on access to care. As an example, most pediatric cancers are curable, yet a recent retrospective analysis of data in the SEER-Medicare registry showed that mortality within 1 month of diagnosis of childhood cancer related in part to socioeconomic factors – those patients with a lower socioeconomic status (which correlates with being an ethnic minority in the United States) were more likely to die within a month of diagnosis of their cancer than were patients with a higher socioeconomic status.3 Here is where MPT can transform the cancer outcomes landscape at no additional investment in basic science or costly precision medicine5: by triaging these patients according to their disease state rather than their ability to pay, they could be administered curative chemotherapy, placed on the appropriate clinical trial, and be cured of their cancer like other children of higher socioeconomic status.
My colleagues and I observed a similar trend when we looked at treatment of diffuse large-cell non-Hodgkin lymphoma in Medicare recipients.6 Although the cure rate is as high as 60%-80% with the use of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or R (rituxin)-CHOP chemotherapy, we found that many patients had received suboptimal chemotherapy. Upon closer examination, we found that there were variations in care by socioeconomic status even in a single-payer system. Thus aspects of cultural literacy and additional efforts for triage need to be developed, but again, application of MPT could be instrumental in improving cancer cure rates by reducing disparities in care by allocating assets to solve access-to-care issues, and curing these patients of their non-Hodgkin lymphoma.
A physician at a Bay Area health care system notes that the open slots in his schedule are triaged by his employer by the patient’s ability to pay – well-insured patients are seen within a few days, but there are very few slots for Medicaid patients, who have to wait weeks or longer to be seen. During this time, their malignancies have time to grow, and potentially metastasize. This may provide suboptimal outcomes for some patients in his community.
We solved this problem at a local hospital where all patients were on Medicaid or uninsured. We triaged patients according to severity of illness, with patients with rapidly growing cancers, particularly curable ones, were brought in as soon as possible and patients with stable benign hematologic conditions seen on a less urgent basis. A social worker and I saw patients together. She would find them resources such as transportation, food, copay assistance to help them through their treatment, and I would optimize their cancer care clinically. On a small scale, this application of MPT (or asset allocation) worked quite well. Perhaps it can be reproduced on a much larger scale. Return on investment relates largely to how you allocate your assets. What’s nice about these applications of MPT is that the return on investment – increasing the cure rate of cancer - is quite large for just a minimal change in asset allocation.
1. Bertagnolli M, Sartor O, Chabner BA, et al. Advantages of a truly open-access data-sharing model. N Engl J Med. 2017;376(12):1178-1181.
2. Baum M. Justice. In: The scepticaemic surgeon: how not to win friends and influence people. New York: Nova Science Pubkishers; November 30, 2014.
3. Glaeser E. Gentfrification and its discontents. Wall Street Journal. May 5, 2017.
4. Green AL, Furutani E, Riberio KB, Galindo CR. Death within 1 month of diagnosis in childhood cancer : analysis of risk factors and scope of the problem. J Clin Oncol. 2017;35(12):1320-1327.
5. McCartney M. Are we too captivated by precision medicine? http://www.bmj.com/content/356/bmj.j1168.long. Published March 9, 2017. Accessed May 12, 2017.
6. Griffiths R, Gleeson M, Knopf K, Danese M. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2995801/. Published November 12, 2010. Accessed May 12, 2017.
1. Bertagnolli M, Sartor O, Chabner BA, et al. Advantages of a truly open-access data-sharing model. N Engl J Med. 2017;376(12):1178-1181.
2. Baum M. Justice. In: The scepticaemic surgeon: how not to win friends and influence people. New York: Nova Science Pubkishers; November 30, 2014.
3. Glaeser E. Gentfrification and its discontents. Wall Street Journal. May 5, 2017.
4. Green AL, Furutani E, Riberio KB, Galindo CR. Death within 1 month of diagnosis in childhood cancer : analysis of risk factors and scope of the problem. J Clin Oncol. 2017;35(12):1320-1327.
5. McCartney M. Are we too captivated by precision medicine? http://www.bmj.com/content/356/bmj.j1168.long. Published March 9, 2017. Accessed May 12, 2017.
6. Griffiths R, Gleeson M, Knopf K, Danese M. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2995801/. Published November 12, 2010. Accessed May 12, 2017.
Metastatic Kaposi sarcoma with osseous involvement in a patient with AIDS
Kaposi sarcoma is an AIDS-defining illness associated with human herpes virus-8 (HHV-8) co-infection. It was described in 1872 by the Hungarian dermatologist Mortiz Kaposi, and was an isolated and sporadic occurrence before the emergence of HIV infection and AIDS.1 It was first affiliated as an AIDS-associated neoplasm in 1981.1 Kaposi sarcoma is a systemic disease that can present with cutaneous lesions with or without internal involvement. There are four subtypes: Classic, African endemic, AIDS-related (CD4 count, <200), and Kaposi sarcoma in iatrogenically immunosuppressed patients. The disease has the propensity to manifest in the skin and gastro-intestinal and respiratory tracts, and osseous involvement is rarely encountered. We present here the case of an AIDS-positive man with generalized bone pain as a result of metastasis from Kaposi sarcoma. Our discussion includes the epidemiological, clinical, pathological, and radiological facets of AIDS-related Kaposi sarcoma, and the anomaly of osseous involvement.
Case presentation and summary
He restarted his previous HAART regimen in March 2016, and was subsequently started on chemotherapy with liposomal doxorubicin (50 mg [20 mg/m2] in 250 ml D5W IV every 2 weeks) because of his extensive disease.2 He completed 6 cycles by June 2016. However, he returned in July 2016 with worsening back pain. A repeat CT scan revealed significant improvement in the disseminated lymphadenopathy, but worsening osseous metastatic disease was seen in the lumbar, thoracic, and pelvic regions. A pelvic lytic lesion biopsy revealed Kaposi sarcoma; pathology showed spindle cells positive for CD34, CD31, and HHV-8 (Figure 2). The patient received palliative radiation to the spine, aiding in pain management and ambulatory dysfunction. He continued with his noncompliance with all medications and outpatient follow-ups, and succumbed to his disease burden.
Discussion
Kaposi sarcoma is a low-grade mesenchymal tumor that involves the blood and lymphatic vessels.3 Its association with AIDS was revealed in the early 1980s at the start of the HIV epidemic in the United States. In 1994, Chang and colleagues discovered the association between Karposi sarcoma and HHV-8 by isolating DNA fragments of HHV in Kaposi sarcoma tumors from AIDS patients.4 The mode of transmission of HHV-8 has not been fully decoded. It has been presumed that adult homosexual contact continues to be an important route of transmission, inferring a common route of infection. In 1990, the overall risk of developing Kaposi sarcoma in AIDS patients was 20,000 times greater than it was in the general population, and 300 times greater than in other immunosuppressed patients.5 This suggests an increase in incidence, in direct relation, with a decrease in the CD4 count.
Kaposi sarcoma can present with a range of clinical features, from negligible cutaneous lesions to a hastily progressing neoplasm. Involvement in the musculoskeletal system is infrequent, but encountered increasingly in the AIDS-related subtype. Moreover, it is recurrently observed in the African population.6 In one of the largest reviews to date exploring Kaposi sarcoma involving the musculoskeletal system, Caponetti and colleagues observed the greatest osseous involvement distinctly in patients with CD4 and T-cell counts below 100 cells/mm3.6
Kaposi sarcoma musculoskeletal involvement, specifically bone, is atypical. If it does occur, it usually manifests as a result of contiguous invasion from an adjacent nonosseous lesion. Caponetti and colleagues that isolated osseous Kaposi sarcoma lesions (with no overlying skin lesion) were found to be more likely to be associated with AIDS in the review by Caponetti and colleagues.6 As in our patient, it is also typically a manifestation of more widely disseminated disease.7
Most of the osseous lytic lesions in AIDS patients are located in the axial skeleton. Radiological features of musculoskeletal Kaposi sarcoma are variable. As observed by Caponetti and colleagues, Kaposi sarcoma lesions can appear as a periosteal reaction, cortical erosions, osteolysis, or osseous destruction, with irregular-shaped cortical erosions being most typical.6 Despite their osteolytic features, Kaposi sarcoma lesions are often not visualized by conventional radiography.6 The preferred imaging for identification of lytic bone changes is CT (Figure 3). Magnetic resonance imaging can also help distinguish marrow abnormalities as well as adjacent soft tissues masses. Radiologically, Kaposi sarcoma osseous lesions have parallel features to bacillary angiomatosis, tuberculosis, or lymphoma.8 Therefore, biopsy of the lesion is essential in establishing the diagnosis of Kaposi sarcoma.
In theory, there should be clinical improvement in Kaposi sarcoma when immunity is restored. Cancers caused by the Epstein-Barr virus and Kaposi sarcoma-associated herpes virus may eventually also be preventable with vaccines.10
There is rarely bone involvement without the foreshadowing of a poor prognosis. Erroneous patient care may inevitably arise from Kaposi sarcoma in uncharacteristic sites. A differential of Kaposi sarcoma should be included if a patient with AIDS presents with osteolytic lesions on imaging. Biopsying the lesion cements the diagnosis and eliminates the possibility of mimicry conditions such as bacillary angiomatosis, benign vascular lesions, and angiosarcoma. As of today, a HAART regimen remains the standard initial care for patients with Kaposi sarcoma.
1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
2. Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445-2451.
3. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. 2006;26:1169-1185.
4. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi sarcoma. Science. 1994;266:1865-1869.
5. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
6. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz I. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007;109(6):1040-1052.
7. Krishna G, Chitkara RK. Osseous Kaposi sarcoma. JAMA. 2003;286(9):1106.
8. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004;33(4):241-243.
9. Guiholt A, Dupin N, Marcelin AG, et al. Low T-cell response to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078-1088.
10. Gopal S, Achenbach CJ, Yanik EL, Dither DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876-880.
Kaposi sarcoma is an AIDS-defining illness associated with human herpes virus-8 (HHV-8) co-infection. It was described in 1872 by the Hungarian dermatologist Mortiz Kaposi, and was an isolated and sporadic occurrence before the emergence of HIV infection and AIDS.1 It was first affiliated as an AIDS-associated neoplasm in 1981.1 Kaposi sarcoma is a systemic disease that can present with cutaneous lesions with or without internal involvement. There are four subtypes: Classic, African endemic, AIDS-related (CD4 count, <200), and Kaposi sarcoma in iatrogenically immunosuppressed patients. The disease has the propensity to manifest in the skin and gastro-intestinal and respiratory tracts, and osseous involvement is rarely encountered. We present here the case of an AIDS-positive man with generalized bone pain as a result of metastasis from Kaposi sarcoma. Our discussion includes the epidemiological, clinical, pathological, and radiological facets of AIDS-related Kaposi sarcoma, and the anomaly of osseous involvement.
Case presentation and summary
He restarted his previous HAART regimen in March 2016, and was subsequently started on chemotherapy with liposomal doxorubicin (50 mg [20 mg/m2] in 250 ml D5W IV every 2 weeks) because of his extensive disease.2 He completed 6 cycles by June 2016. However, he returned in July 2016 with worsening back pain. A repeat CT scan revealed significant improvement in the disseminated lymphadenopathy, but worsening osseous metastatic disease was seen in the lumbar, thoracic, and pelvic regions. A pelvic lytic lesion biopsy revealed Kaposi sarcoma; pathology showed spindle cells positive for CD34, CD31, and HHV-8 (Figure 2). The patient received palliative radiation to the spine, aiding in pain management and ambulatory dysfunction. He continued with his noncompliance with all medications and outpatient follow-ups, and succumbed to his disease burden.
Discussion
Kaposi sarcoma is a low-grade mesenchymal tumor that involves the blood and lymphatic vessels.3 Its association with AIDS was revealed in the early 1980s at the start of the HIV epidemic in the United States. In 1994, Chang and colleagues discovered the association between Karposi sarcoma and HHV-8 by isolating DNA fragments of HHV in Kaposi sarcoma tumors from AIDS patients.4 The mode of transmission of HHV-8 has not been fully decoded. It has been presumed that adult homosexual contact continues to be an important route of transmission, inferring a common route of infection. In 1990, the overall risk of developing Kaposi sarcoma in AIDS patients was 20,000 times greater than it was in the general population, and 300 times greater than in other immunosuppressed patients.5 This suggests an increase in incidence, in direct relation, with a decrease in the CD4 count.
Kaposi sarcoma can present with a range of clinical features, from negligible cutaneous lesions to a hastily progressing neoplasm. Involvement in the musculoskeletal system is infrequent, but encountered increasingly in the AIDS-related subtype. Moreover, it is recurrently observed in the African population.6 In one of the largest reviews to date exploring Kaposi sarcoma involving the musculoskeletal system, Caponetti and colleagues observed the greatest osseous involvement distinctly in patients with CD4 and T-cell counts below 100 cells/mm3.6
Kaposi sarcoma musculoskeletal involvement, specifically bone, is atypical. If it does occur, it usually manifests as a result of contiguous invasion from an adjacent nonosseous lesion. Caponetti and colleagues that isolated osseous Kaposi sarcoma lesions (with no overlying skin lesion) were found to be more likely to be associated with AIDS in the review by Caponetti and colleagues.6 As in our patient, it is also typically a manifestation of more widely disseminated disease.7
Most of the osseous lytic lesions in AIDS patients are located in the axial skeleton. Radiological features of musculoskeletal Kaposi sarcoma are variable. As observed by Caponetti and colleagues, Kaposi sarcoma lesions can appear as a periosteal reaction, cortical erosions, osteolysis, or osseous destruction, with irregular-shaped cortical erosions being most typical.6 Despite their osteolytic features, Kaposi sarcoma lesions are often not visualized by conventional radiography.6 The preferred imaging for identification of lytic bone changes is CT (Figure 3). Magnetic resonance imaging can also help distinguish marrow abnormalities as well as adjacent soft tissues masses. Radiologically, Kaposi sarcoma osseous lesions have parallel features to bacillary angiomatosis, tuberculosis, or lymphoma.8 Therefore, biopsy of the lesion is essential in establishing the diagnosis of Kaposi sarcoma.
In theory, there should be clinical improvement in Kaposi sarcoma when immunity is restored. Cancers caused by the Epstein-Barr virus and Kaposi sarcoma-associated herpes virus may eventually also be preventable with vaccines.10
There is rarely bone involvement without the foreshadowing of a poor prognosis. Erroneous patient care may inevitably arise from Kaposi sarcoma in uncharacteristic sites. A differential of Kaposi sarcoma should be included if a patient with AIDS presents with osteolytic lesions on imaging. Biopsying the lesion cements the diagnosis and eliminates the possibility of mimicry conditions such as bacillary angiomatosis, benign vascular lesions, and angiosarcoma. As of today, a HAART regimen remains the standard initial care for patients with Kaposi sarcoma.
Kaposi sarcoma is an AIDS-defining illness associated with human herpes virus-8 (HHV-8) co-infection. It was described in 1872 by the Hungarian dermatologist Mortiz Kaposi, and was an isolated and sporadic occurrence before the emergence of HIV infection and AIDS.1 It was first affiliated as an AIDS-associated neoplasm in 1981.1 Kaposi sarcoma is a systemic disease that can present with cutaneous lesions with or without internal involvement. There are four subtypes: Classic, African endemic, AIDS-related (CD4 count, <200), and Kaposi sarcoma in iatrogenically immunosuppressed patients. The disease has the propensity to manifest in the skin and gastro-intestinal and respiratory tracts, and osseous involvement is rarely encountered. We present here the case of an AIDS-positive man with generalized bone pain as a result of metastasis from Kaposi sarcoma. Our discussion includes the epidemiological, clinical, pathological, and radiological facets of AIDS-related Kaposi sarcoma, and the anomaly of osseous involvement.
Case presentation and summary
He restarted his previous HAART regimen in March 2016, and was subsequently started on chemotherapy with liposomal doxorubicin (50 mg [20 mg/m2] in 250 ml D5W IV every 2 weeks) because of his extensive disease.2 He completed 6 cycles by June 2016. However, he returned in July 2016 with worsening back pain. A repeat CT scan revealed significant improvement in the disseminated lymphadenopathy, but worsening osseous metastatic disease was seen in the lumbar, thoracic, and pelvic regions. A pelvic lytic lesion biopsy revealed Kaposi sarcoma; pathology showed spindle cells positive for CD34, CD31, and HHV-8 (Figure 2). The patient received palliative radiation to the spine, aiding in pain management and ambulatory dysfunction. He continued with his noncompliance with all medications and outpatient follow-ups, and succumbed to his disease burden.
Discussion
Kaposi sarcoma is a low-grade mesenchymal tumor that involves the blood and lymphatic vessels.3 Its association with AIDS was revealed in the early 1980s at the start of the HIV epidemic in the United States. In 1994, Chang and colleagues discovered the association between Karposi sarcoma and HHV-8 by isolating DNA fragments of HHV in Kaposi sarcoma tumors from AIDS patients.4 The mode of transmission of HHV-8 has not been fully decoded. It has been presumed that adult homosexual contact continues to be an important route of transmission, inferring a common route of infection. In 1990, the overall risk of developing Kaposi sarcoma in AIDS patients was 20,000 times greater than it was in the general population, and 300 times greater than in other immunosuppressed patients.5 This suggests an increase in incidence, in direct relation, with a decrease in the CD4 count.
Kaposi sarcoma can present with a range of clinical features, from negligible cutaneous lesions to a hastily progressing neoplasm. Involvement in the musculoskeletal system is infrequent, but encountered increasingly in the AIDS-related subtype. Moreover, it is recurrently observed in the African population.6 In one of the largest reviews to date exploring Kaposi sarcoma involving the musculoskeletal system, Caponetti and colleagues observed the greatest osseous involvement distinctly in patients with CD4 and T-cell counts below 100 cells/mm3.6
Kaposi sarcoma musculoskeletal involvement, specifically bone, is atypical. If it does occur, it usually manifests as a result of contiguous invasion from an adjacent nonosseous lesion. Caponetti and colleagues that isolated osseous Kaposi sarcoma lesions (with no overlying skin lesion) were found to be more likely to be associated with AIDS in the review by Caponetti and colleagues.6 As in our patient, it is also typically a manifestation of more widely disseminated disease.7
Most of the osseous lytic lesions in AIDS patients are located in the axial skeleton. Radiological features of musculoskeletal Kaposi sarcoma are variable. As observed by Caponetti and colleagues, Kaposi sarcoma lesions can appear as a periosteal reaction, cortical erosions, osteolysis, or osseous destruction, with irregular-shaped cortical erosions being most typical.6 Despite their osteolytic features, Kaposi sarcoma lesions are often not visualized by conventional radiography.6 The preferred imaging for identification of lytic bone changes is CT (Figure 3). Magnetic resonance imaging can also help distinguish marrow abnormalities as well as adjacent soft tissues masses. Radiologically, Kaposi sarcoma osseous lesions have parallel features to bacillary angiomatosis, tuberculosis, or lymphoma.8 Therefore, biopsy of the lesion is essential in establishing the diagnosis of Kaposi sarcoma.
In theory, there should be clinical improvement in Kaposi sarcoma when immunity is restored. Cancers caused by the Epstein-Barr virus and Kaposi sarcoma-associated herpes virus may eventually also be preventable with vaccines.10
There is rarely bone involvement without the foreshadowing of a poor prognosis. Erroneous patient care may inevitably arise from Kaposi sarcoma in uncharacteristic sites. A differential of Kaposi sarcoma should be included if a patient with AIDS presents with osteolytic lesions on imaging. Biopsying the lesion cements the diagnosis and eliminates the possibility of mimicry conditions such as bacillary angiomatosis, benign vascular lesions, and angiosarcoma. As of today, a HAART regimen remains the standard initial care for patients with Kaposi sarcoma.
1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
2. Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445-2451.
3. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. 2006;26:1169-1185.
4. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi sarcoma. Science. 1994;266:1865-1869.
5. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
6. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz I. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007;109(6):1040-1052.
7. Krishna G, Chitkara RK. Osseous Kaposi sarcoma. JAMA. 2003;286(9):1106.
8. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004;33(4):241-243.
9. Guiholt A, Dupin N, Marcelin AG, et al. Low T-cell response to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078-1088.
10. Gopal S, Achenbach CJ, Yanik EL, Dither DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876-880.
1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
2. Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445-2451.
3. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. 2006;26:1169-1185.
4. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi sarcoma. Science. 1994;266:1865-1869.
5. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
6. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz I. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007;109(6):1040-1052.
7. Krishna G, Chitkara RK. Osseous Kaposi sarcoma. JAMA. 2003;286(9):1106.
8. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004;33(4):241-243.
9. Guiholt A, Dupin N, Marcelin AG, et al. Low T-cell response to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078-1088.
10. Gopal S, Achenbach CJ, Yanik EL, Dither DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876-880.
A rare case of hypoglycemia induced by a classic gastrointestinal stromal tumor
Hypoglycemia, a frequently encountered medical emergency, is usually seen in patients with diabetes, most commonly as a result of iatrogenesis. However, it can also be encountered in nondiabetic patients. Various causes, such as pancreatic islet cell tumors producing insulin, primary or secondary adrenal insufficiency, advanced liver disease, pheochromocytoma and hypothyroidism, have been found to contribute to the condition in the nondiabetic population.1 In rare cases, an excessive production of insulin-like growth factor (IGF-2) – a condition known as nonislet cell tumor-induced hypoglycemia (NICTH) – has also been found to cause hypoglycemia. Hypoinsulinemic hypoglycemia, with low IGF-1 levels and an IGF-2-IgF1 ratio of greater than 10, is found to be suggestive of NICTH.
Case presentation and summary
An 81-year-old man with a history of diabetes mellitus, systolic heart failure, chronic kidney disease, and metastatic classical gastrointestinal spindle cell sarcoma presented to the emergency department with an acute change in mental status resulting from a new onset hypoglycemia. He was admitted, and during his hospital stay, he experienced severe hypoglycemic episodes with symptomatic presentations of diaphoresis on multiple occasions. A detailed history revealed that for diabetes, the patient had been on insulin for the first 12 years after his diagnosis, after which he was switched to metformin 500 mg twice daily for about 2 years, and as a satisfactory glycemic control was attained, eventually metformin had also been stopped 3 years prior to the current presentation.
The patient’s past medical records were obtained from the hospital at which he had been diagnosed gastrointestinal spindle cell sarcoma. Patient had not received treatment for the cancer as the disease was too widespread to be treated. The gastrointestinal spindle cell sarcoma, which had initially been surgically resected 7 years before the current presentation, had a recurrence 3 years later with abdominal and pulmonary metastasis, but no liver metastasis. No further intervention was carried out because the widely metastasized disease would not have benefited from any more surgical intervention and chemotherapy was not initiated because of the patient’s comorbid illnesses.
A blood sample drawn from the patient at the time of one hypoglycemic event, revealed low serum insulin <0.1 U/ml (normal, 2-19.6 U/ml); low C-peptide level, 0.59 ng/ml (0.8-3.85 ng/ml); low IGF-1, 16 ng/ml (5-4 ng/ml); and IGF-3, 0.9 ng/ml (2.2-4.5 ng/ml). IGF-2 levels were found to be markedly elevated at 945 ng/ml (47-350 ng/ml). The calculated IGF-2-IGF-1 ratio was 59.06 (normal, <10), suggesting NICTH as the etiology for the patient’s hypoglycemia.
The hypoglycemic episodes were initially treated with a continuous dextrose infusion followed by diazoxide treatment. However, diazoxide did not prevent his hypoglycemic episodes, so dexamethasone was considered as an alternative for his condition. The dexamethasone treatment resulted in the normalization of the patient’s serum glucose levels and resolution of his symptoms. The patient was discharged in a satisfactory state few days later and followed up thereafter. No recurrence of hypoglycemic episodes was found, and he was continued on dexamethasone therapy.
Discussion
Hypoglycemia due to NICTH is rare, with a prevalence of four times less than that of insulinoma.3 In most cases, NICTH occurs in patients with solid tumors of mesenchymal and epithelial origins such as hepatocellular carcinoma, gastric carcinoma or mesothelioma.4 In NICTH, the serum levels of insulin, C-peptide, and IGF-1 are usually decreased or undetectable. However, the circulating levels of total IGF2 may be increased, decreased, or normal. Concurrent normal to high morning cortisol and normal response to cosyntropin stimulation can rule out adrenal insufficiency and suggest NICTH. An IGF-2: IGF-1 ratio of >10 is considered to be clinically significant and highly suggestive of NICTH.5 Hypoglycemia in NICTH can be managed by administration of oral glucose, intravenous dextrose or glucagon. In some cases, diazoxide, a potent inhibitor of insulin secretion, has been found to be useful.6 Diazoxide directly inhibits the release of insulin through stimulation of adrenergic receptors and also has an extra pancreatic hyperglycemic effect, probably by inhibiting cyclic adenosine monophosphate phosphodiesterase, resulting in higher plasma levels of cyclic AMP and enhanced glycogenolysis.
Glucocorticoid therapy has been shown to suppress IGF-2 in a dose dependent manner and also by increasing gluconeogenesis.7 Surgical resection of the tumor whenever possible is the treatment of choice followed by radiotherapy and chemotherapy for inoperable disease and if successful, usually results in resolution of hypoglycemia. Imatinib, is the chemotherapeutic drug of choice for metastatic GIST, but many case reports have suggested worsening of hypoglycemia in advanced GIST with the use of the drug.8 The patient described in our report was not on any chemotherapy, hence hypoglycemia could not be attributed to it. On the basis of findings among 24 patients with GIST, Rikhof and colleagues have recommended monitoring plasma levels of pro-IGF-IIE to identify patients at high risk for developing hypoglycemia, especially those with progressive disease.9 Furthermore, over expression of IGF-2 as a predictor of potential relapse may be an area for potential research and further study.10
1. Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes Metab Rev. 1991;7:79-91.
2. Dutta P, Aggarwal A, Gogate Y, Nahar U, Shah VN, Singla M. Non-islet cell tumor-induced hypoglycemia: a report of five cases and brief review of the literature. Endocrinol Diabetes Metab Case Rep. 2013;2013:130046
3. de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14:979-93.
4. Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor II producing non-islet-cell tumor hypoglycemia
5. Marks V, Teale JD: Tumours producing hypoglycaemia. Endocr Relat Cancer. 1998;5:111-129.
6. Le Roith D. Tumor-induced hypoglycemia. N Engl J Med. 1999;341:757-758.
7. Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol .1998;49:491-498.
8. Hamberg P, De Jong FA, Boonstra JG, et al. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J Clin Oncol. 2006;24:e30-e31.
9. Rikhof B, van Doorn J, Suurmeijer AJ, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins in relation to disease status and incidence of hypoglycaemia in patients with a gastrointestinal stromal tumour. Ann Oncol. 2009;20:1582-1588.
10. Braconi C, Bracci R, Bearzi I, et al. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293-1298.
Hypoglycemia, a frequently encountered medical emergency, is usually seen in patients with diabetes, most commonly as a result of iatrogenesis. However, it can also be encountered in nondiabetic patients. Various causes, such as pancreatic islet cell tumors producing insulin, primary or secondary adrenal insufficiency, advanced liver disease, pheochromocytoma and hypothyroidism, have been found to contribute to the condition in the nondiabetic population.1 In rare cases, an excessive production of insulin-like growth factor (IGF-2) – a condition known as nonislet cell tumor-induced hypoglycemia (NICTH) – has also been found to cause hypoglycemia. Hypoinsulinemic hypoglycemia, with low IGF-1 levels and an IGF-2-IgF1 ratio of greater than 10, is found to be suggestive of NICTH.
Case presentation and summary
An 81-year-old man with a history of diabetes mellitus, systolic heart failure, chronic kidney disease, and metastatic classical gastrointestinal spindle cell sarcoma presented to the emergency department with an acute change in mental status resulting from a new onset hypoglycemia. He was admitted, and during his hospital stay, he experienced severe hypoglycemic episodes with symptomatic presentations of diaphoresis on multiple occasions. A detailed history revealed that for diabetes, the patient had been on insulin for the first 12 years after his diagnosis, after which he was switched to metformin 500 mg twice daily for about 2 years, and as a satisfactory glycemic control was attained, eventually metformin had also been stopped 3 years prior to the current presentation.
The patient’s past medical records were obtained from the hospital at which he had been diagnosed gastrointestinal spindle cell sarcoma. Patient had not received treatment for the cancer as the disease was too widespread to be treated. The gastrointestinal spindle cell sarcoma, which had initially been surgically resected 7 years before the current presentation, had a recurrence 3 years later with abdominal and pulmonary metastasis, but no liver metastasis. No further intervention was carried out because the widely metastasized disease would not have benefited from any more surgical intervention and chemotherapy was not initiated because of the patient’s comorbid illnesses.
A blood sample drawn from the patient at the time of one hypoglycemic event, revealed low serum insulin <0.1 U/ml (normal, 2-19.6 U/ml); low C-peptide level, 0.59 ng/ml (0.8-3.85 ng/ml); low IGF-1, 16 ng/ml (5-4 ng/ml); and IGF-3, 0.9 ng/ml (2.2-4.5 ng/ml). IGF-2 levels were found to be markedly elevated at 945 ng/ml (47-350 ng/ml). The calculated IGF-2-IGF-1 ratio was 59.06 (normal, <10), suggesting NICTH as the etiology for the patient’s hypoglycemia.
The hypoglycemic episodes were initially treated with a continuous dextrose infusion followed by diazoxide treatment. However, diazoxide did not prevent his hypoglycemic episodes, so dexamethasone was considered as an alternative for his condition. The dexamethasone treatment resulted in the normalization of the patient’s serum glucose levels and resolution of his symptoms. The patient was discharged in a satisfactory state few days later and followed up thereafter. No recurrence of hypoglycemic episodes was found, and he was continued on dexamethasone therapy.
Discussion
Hypoglycemia due to NICTH is rare, with a prevalence of four times less than that of insulinoma.3 In most cases, NICTH occurs in patients with solid tumors of mesenchymal and epithelial origins such as hepatocellular carcinoma, gastric carcinoma or mesothelioma.4 In NICTH, the serum levels of insulin, C-peptide, and IGF-1 are usually decreased or undetectable. However, the circulating levels of total IGF2 may be increased, decreased, or normal. Concurrent normal to high morning cortisol and normal response to cosyntropin stimulation can rule out adrenal insufficiency and suggest NICTH. An IGF-2: IGF-1 ratio of >10 is considered to be clinically significant and highly suggestive of NICTH.5 Hypoglycemia in NICTH can be managed by administration of oral glucose, intravenous dextrose or glucagon. In some cases, diazoxide, a potent inhibitor of insulin secretion, has been found to be useful.6 Diazoxide directly inhibits the release of insulin through stimulation of adrenergic receptors and also has an extra pancreatic hyperglycemic effect, probably by inhibiting cyclic adenosine monophosphate phosphodiesterase, resulting in higher plasma levels of cyclic AMP and enhanced glycogenolysis.
Glucocorticoid therapy has been shown to suppress IGF-2 in a dose dependent manner and also by increasing gluconeogenesis.7 Surgical resection of the tumor whenever possible is the treatment of choice followed by radiotherapy and chemotherapy for inoperable disease and if successful, usually results in resolution of hypoglycemia. Imatinib, is the chemotherapeutic drug of choice for metastatic GIST, but many case reports have suggested worsening of hypoglycemia in advanced GIST with the use of the drug.8 The patient described in our report was not on any chemotherapy, hence hypoglycemia could not be attributed to it. On the basis of findings among 24 patients with GIST, Rikhof and colleagues have recommended monitoring plasma levels of pro-IGF-IIE to identify patients at high risk for developing hypoglycemia, especially those with progressive disease.9 Furthermore, over expression of IGF-2 as a predictor of potential relapse may be an area for potential research and further study.10
Hypoglycemia, a frequently encountered medical emergency, is usually seen in patients with diabetes, most commonly as a result of iatrogenesis. However, it can also be encountered in nondiabetic patients. Various causes, such as pancreatic islet cell tumors producing insulin, primary or secondary adrenal insufficiency, advanced liver disease, pheochromocytoma and hypothyroidism, have been found to contribute to the condition in the nondiabetic population.1 In rare cases, an excessive production of insulin-like growth factor (IGF-2) – a condition known as nonislet cell tumor-induced hypoglycemia (NICTH) – has also been found to cause hypoglycemia. Hypoinsulinemic hypoglycemia, with low IGF-1 levels and an IGF-2-IgF1 ratio of greater than 10, is found to be suggestive of NICTH.
Case presentation and summary
An 81-year-old man with a history of diabetes mellitus, systolic heart failure, chronic kidney disease, and metastatic classical gastrointestinal spindle cell sarcoma presented to the emergency department with an acute change in mental status resulting from a new onset hypoglycemia. He was admitted, and during his hospital stay, he experienced severe hypoglycemic episodes with symptomatic presentations of diaphoresis on multiple occasions. A detailed history revealed that for diabetes, the patient had been on insulin for the first 12 years after his diagnosis, after which he was switched to metformin 500 mg twice daily for about 2 years, and as a satisfactory glycemic control was attained, eventually metformin had also been stopped 3 years prior to the current presentation.
The patient’s past medical records were obtained from the hospital at which he had been diagnosed gastrointestinal spindle cell sarcoma. Patient had not received treatment for the cancer as the disease was too widespread to be treated. The gastrointestinal spindle cell sarcoma, which had initially been surgically resected 7 years before the current presentation, had a recurrence 3 years later with abdominal and pulmonary metastasis, but no liver metastasis. No further intervention was carried out because the widely metastasized disease would not have benefited from any more surgical intervention and chemotherapy was not initiated because of the patient’s comorbid illnesses.
A blood sample drawn from the patient at the time of one hypoglycemic event, revealed low serum insulin <0.1 U/ml (normal, 2-19.6 U/ml); low C-peptide level, 0.59 ng/ml (0.8-3.85 ng/ml); low IGF-1, 16 ng/ml (5-4 ng/ml); and IGF-3, 0.9 ng/ml (2.2-4.5 ng/ml). IGF-2 levels were found to be markedly elevated at 945 ng/ml (47-350 ng/ml). The calculated IGF-2-IGF-1 ratio was 59.06 (normal, <10), suggesting NICTH as the etiology for the patient’s hypoglycemia.
The hypoglycemic episodes were initially treated with a continuous dextrose infusion followed by diazoxide treatment. However, diazoxide did not prevent his hypoglycemic episodes, so dexamethasone was considered as an alternative for his condition. The dexamethasone treatment resulted in the normalization of the patient’s serum glucose levels and resolution of his symptoms. The patient was discharged in a satisfactory state few days later and followed up thereafter. No recurrence of hypoglycemic episodes was found, and he was continued on dexamethasone therapy.
Discussion
Hypoglycemia due to NICTH is rare, with a prevalence of four times less than that of insulinoma.3 In most cases, NICTH occurs in patients with solid tumors of mesenchymal and epithelial origins such as hepatocellular carcinoma, gastric carcinoma or mesothelioma.4 In NICTH, the serum levels of insulin, C-peptide, and IGF-1 are usually decreased or undetectable. However, the circulating levels of total IGF2 may be increased, decreased, or normal. Concurrent normal to high morning cortisol and normal response to cosyntropin stimulation can rule out adrenal insufficiency and suggest NICTH. An IGF-2: IGF-1 ratio of >10 is considered to be clinically significant and highly suggestive of NICTH.5 Hypoglycemia in NICTH can be managed by administration of oral glucose, intravenous dextrose or glucagon. In some cases, diazoxide, a potent inhibitor of insulin secretion, has been found to be useful.6 Diazoxide directly inhibits the release of insulin through stimulation of adrenergic receptors and also has an extra pancreatic hyperglycemic effect, probably by inhibiting cyclic adenosine monophosphate phosphodiesterase, resulting in higher plasma levels of cyclic AMP and enhanced glycogenolysis.
Glucocorticoid therapy has been shown to suppress IGF-2 in a dose dependent manner and also by increasing gluconeogenesis.7 Surgical resection of the tumor whenever possible is the treatment of choice followed by radiotherapy and chemotherapy for inoperable disease and if successful, usually results in resolution of hypoglycemia. Imatinib, is the chemotherapeutic drug of choice for metastatic GIST, but many case reports have suggested worsening of hypoglycemia in advanced GIST with the use of the drug.8 The patient described in our report was not on any chemotherapy, hence hypoglycemia could not be attributed to it. On the basis of findings among 24 patients with GIST, Rikhof and colleagues have recommended monitoring plasma levels of pro-IGF-IIE to identify patients at high risk for developing hypoglycemia, especially those with progressive disease.9 Furthermore, over expression of IGF-2 as a predictor of potential relapse may be an area for potential research and further study.10
1. Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes Metab Rev. 1991;7:79-91.
2. Dutta P, Aggarwal A, Gogate Y, Nahar U, Shah VN, Singla M. Non-islet cell tumor-induced hypoglycemia: a report of five cases and brief review of the literature. Endocrinol Diabetes Metab Case Rep. 2013;2013:130046
3. de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14:979-93.
4. Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor II producing non-islet-cell tumor hypoglycemia
5. Marks V, Teale JD: Tumours producing hypoglycaemia. Endocr Relat Cancer. 1998;5:111-129.
6. Le Roith D. Tumor-induced hypoglycemia. N Engl J Med. 1999;341:757-758.
7. Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol .1998;49:491-498.
8. Hamberg P, De Jong FA, Boonstra JG, et al. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J Clin Oncol. 2006;24:e30-e31.
9. Rikhof B, van Doorn J, Suurmeijer AJ, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins in relation to disease status and incidence of hypoglycaemia in patients with a gastrointestinal stromal tumour. Ann Oncol. 2009;20:1582-1588.
10. Braconi C, Bracci R, Bearzi I, et al. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293-1298.
1. Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes Metab Rev. 1991;7:79-91.
2. Dutta P, Aggarwal A, Gogate Y, Nahar U, Shah VN, Singla M. Non-islet cell tumor-induced hypoglycemia: a report of five cases and brief review of the literature. Endocrinol Diabetes Metab Case Rep. 2013;2013:130046
3. de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14:979-93.
4. Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor II producing non-islet-cell tumor hypoglycemia
5. Marks V, Teale JD: Tumours producing hypoglycaemia. Endocr Relat Cancer. 1998;5:111-129.
6. Le Roith D. Tumor-induced hypoglycemia. N Engl J Med. 1999;341:757-758.
7. Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol .1998;49:491-498.
8. Hamberg P, De Jong FA, Boonstra JG, et al. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J Clin Oncol. 2006;24:e30-e31.
9. Rikhof B, van Doorn J, Suurmeijer AJ, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins in relation to disease status and incidence of hypoglycaemia in patients with a gastrointestinal stromal tumour. Ann Oncol. 2009;20:1582-1588.
10. Braconi C, Bracci R, Bearzi I, et al. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293-1298.
David Henry's JCSO podcast, May-June 2017
For the May-June issue of the Journal of Community and Supportive Oncology, the Editor in Chief, Dr David Henry, discusses an editorial by Kevin Knopf, a JCSO editor, about drawing on modern portfolio theory to improve cancer care. Side effects come under scrutiny this issue, with a How We Do It article on prehabilitation for lymphedema in head and neck cancer patients, a Review article that examines pancreatitis associated with newer classes of antineoplastic therapies, and a research article that looks at prescriber adherence to antiemetic guidelines with trifluridine-tipiracil. In other research articles, investigators report on physician attitudes and prevalence of molecular testing in lung cancer; a comprehensive assessment of cancer survivors’ concerns to inform program development; and perceived financial hardship among patients with advanced cancer. Two Case Reports address the treatment of Kaposi sarcoma in patients with AIDS, and a third describes a rare case of hypoglycemia induced by a classic gastrointestinal stromal tumor. Finally, Dr Henry summarizes an in-depth interview on cardiotoxicity, which he did with his colleague, Dr Joseph Carver.
Listen to the podcast below.
For the May-June issue of the Journal of Community and Supportive Oncology, the Editor in Chief, Dr David Henry, discusses an editorial by Kevin Knopf, a JCSO editor, about drawing on modern portfolio theory to improve cancer care. Side effects come under scrutiny this issue, with a How We Do It article on prehabilitation for lymphedema in head and neck cancer patients, a Review article that examines pancreatitis associated with newer classes of antineoplastic therapies, and a research article that looks at prescriber adherence to antiemetic guidelines with trifluridine-tipiracil. In other research articles, investigators report on physician attitudes and prevalence of molecular testing in lung cancer; a comprehensive assessment of cancer survivors’ concerns to inform program development; and perceived financial hardship among patients with advanced cancer. Two Case Reports address the treatment of Kaposi sarcoma in patients with AIDS, and a third describes a rare case of hypoglycemia induced by a classic gastrointestinal stromal tumor. Finally, Dr Henry summarizes an in-depth interview on cardiotoxicity, which he did with his colleague, Dr Joseph Carver.
Listen to the podcast below.
For the May-June issue of the Journal of Community and Supportive Oncology, the Editor in Chief, Dr David Henry, discusses an editorial by Kevin Knopf, a JCSO editor, about drawing on modern portfolio theory to improve cancer care. Side effects come under scrutiny this issue, with a How We Do It article on prehabilitation for lymphedema in head and neck cancer patients, a Review article that examines pancreatitis associated with newer classes of antineoplastic therapies, and a research article that looks at prescriber adherence to antiemetic guidelines with trifluridine-tipiracil. In other research articles, investigators report on physician attitudes and prevalence of molecular testing in lung cancer; a comprehensive assessment of cancer survivors’ concerns to inform program development; and perceived financial hardship among patients with advanced cancer. Two Case Reports address the treatment of Kaposi sarcoma in patients with AIDS, and a third describes a rare case of hypoglycemia induced by a classic gastrointestinal stromal tumor. Finally, Dr Henry summarizes an in-depth interview on cardiotoxicity, which he did with his colleague, Dr Joseph Carver.
Listen to the podcast below.
Emergency department use by recently diagnosed cancer patients in California
In 2017 there will be nearly 1.7 million new cancer cases diagnosed, and over 600,000 cancer deaths in the Unites States.1 A 2013 Institute of Medicine report highlighted problems with the current quality of cancer care, including high costs and fragmentation of care.2 Other national reports have called for improvements in the overall quality of care and for reducing costly and possibly avoidable use of health services such as emergency department (ED) visits.3-6 Reduction of avoidable ED visits is often cited as a pathway to reduce costs by avoiding unnecessary tests and treatments that occur in the ED and subsequent hospital admissions.7,8
ED crowding, long waits, and unpredictable treatment environments can also make an ED visit an unpleasant experience for the patient. ED visits during cancer treatment can be particularly troubling and present health concerns for patients who are immunocompromised. In particular, cancer patients in the ED have been found to experience delays in the administration of analgesics, antiemetics, or antibiotics.9
Few studies have examined ED use or its associated predictors among cancer patients. Reports to date that have described ED use have focused on different cancers, which makes comparisons across studies difficult.10 Moreover, the time frames of interest and the type of event after which ED use is evaluated (ie, diagnosis or treatment) are inconsistent in the existing literature.10 Some studies quantifying ED use excluded patients admitted to the hospital after an ED visit.11,12 Taken together, these studies do not provide a clear overview of the extent of ED use by cancer patients or the amount of cancer-related care provided by EDs.
Patterns of ED use among cancer patients derived from large and generalizable samples may help inform providers about true risk factors for ED use. In addition, prioritizing new interventions and focusing future research on groups of patients who are at higher risk for preventable ED use could also improve overall care. To address these issues, accurate estimates of ED use among cancer patients are required.
To our knowledge, this is the first study to describe ED use across a range of cancers in a large population-based sample and to consider the timing of ED visits in relation to initial diagnosis. The findings could provide benchmark comparison data to inform future efforts to identify the subset of possibly preventable ED visits and to design interventions to address preventable ED use.
Material and methods
Data source
California’s Office of Statewide Health Planning and Development (OSHPD) manages the patient discharge dataset (PDD) and the emergency department use (EDU) dataset, providing a high-quality source of information on inpatient and ED use in the state.13 A principal diagnosis and up to 24 secondary diagnoses are recorded in OSHPD datasets. The EDU dataset was used to identify treat-and-release ED visits, and the PDD was used to identify hospitalizations initiated in the ED. The California Cancer Registry (CCR) obtains demographic and diagnosis information for every new invasive cancer diagnosed in California, and data collected by the registry are considered to be complete.14 CCR-OSPHD-linked data provide high-quality health care use information for cancer patients in California.15,16 Using an encrypted version of the social security number called the record linkage number (RLN), we linked the CCR records to the corresponding OSHPD files from 2009-2010.
Institutional review board approval for this study was obtained from the University of California, Davis, Human Subjects Committee and the State of California Committee for the Protection of Human Subjects.
Analysis
ED visits. Visits were included if they occurred on or up to 365 days after the date of cancer diagnosis recorded in the CCR. The visits were coded in mutually exclusive groups as occurring within 30, 31-180, and 181-365 days of diagnosis. Subsequently, we flagged each person as having any ED visit (Yes/No) within 180 days and within 365 days of diagnosis, and we tallied the total number of visits occurring within these time frames for each person.
Cancer type. We used relevant site and histology codes to classify cancer type into 24 mutually exclusive categories using the Surveillance Epidemiology and End Result‘s International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) Recode Definitions17-19 (Suppl Figure 1).
Individual-level variables. Sociodemographic information for each person was collected from the CCR including gender, age, race/ethnicity, marital status, health insurance status, rural residence, survival time in months, neighborhood socio-economic (SES) status based on the Yang index, and the American Joint Committee on Cancer (AJCC) stage.20,21
Data analysis
Demographic information was analyzed for the cohort using descriptive statistics (frequencies, proportions, means, standard deviations, and ranges) and evaluated for correlations. Fewer than 20 observations had missing data and we removed those observations from our analyses on an item-specific basis.
We tabulated ED visits by cancer type and time from diagnosis and then collapsed visit-level data by RLN to determine the number of ED visits for each person in the sample. The number of days from diagnosis to first ED visit was also tabulated. The cohort was stratified by cancer type and cumulative rates of ED visits were tabulated for individuals with ED visits within 0-180 and 0-365 days from diagnosis. To test the robustness of the findings adjusting for confounding factors known to impact ED use, we used logistic regression to model any ED use (Yes/No) as a function of age, gender, race/ethnicity, cancer stage, insurance status, marital status, urban residence, and Yang SES. After model estimation, we used the method of recycled predictions controlling for the confounding variables to compute the marginal probabilities of ED use by cancer type.22 To adjust for the possible impact of survival on ED use, we performed sensitivity analyses and estimated predicted probabilities adjusting for survival. Separate analyses were performed first adjusting for whether the patient died during the course of each month after diagnosis and then adjusting for whether or not the patient died within 180 days of diagnosis. All analyses were conducted using Stata 13.1.23
Results
The CCR identified 222,087 adults with a new primary cancer diagnosis in 2009-2010. After excluding those with Stage 0 cancer (n = 21,154) and nonmelanoma skin cancer (n = 1,031), for whom data are inconsistently collected by CCR, a total of 199,872 individuals were included in the analytic sample. Of those patients (Table 1), most were white non-Hispanic (62%), women (51%), holders of private insurance (53%), married (56%), and urban residents (86%). Most were older than 50 years and had either Stage I or Stage II cancer. The most common cancer types were breast (17%), prostate (16%), lung (11%), and colon (9%; results not shown). In unadjusted comparisons, the incidence of ED use was significantly higher among those who were older, of non-Hispanic black race/ethnicity, uninsured, in the lowest SES group, widowed, or diagnosed with Stage IV cancer (Table 1).
ED visits
Within 365 days after initial cancer diagnosis, 87,025 cancer patients made a total of 197,886 ED visits (not shown in tables). Of those visits, 68% (n = 134,556) occurred within 180 days of diagnosis, with 22% (n = 43,535) occurring within the first 30 days and 46% (n = 91,027) occurring within 31-180 days after diagnosis (Figure). Given that most of the visits occurred within 180 days of diagnosis, we used that time frame in subsequent analyses. Among all ED visits within 180 days of diagnosis (Table 2), the largest proportions of visits were made by those with lung cancer (16%), breast cancer (11%), and colon cancer (10%).
About 51% of visits resulted in admission to the hospital and 45% in discharge (Table 2). For some cancers (lung, colon, non-Hodgkin lymphoma, pancreatic, digestive, liver, stomach, leukemia, and myeloma) most of the visits resulted in admission to the hospital (Table 2). Among visits resulting in admission, the top three principal diagnoses were: septicemia (8%), cardiovascular problems (7%), and complications from surgery (5%) (not shown in tables). Among visits resulting in a discharge home, the three top principal diagnoses were abdominal pain (7%), cardiovascular problems (6%), and urinary, kidney, and bladder complaints other than a urinary tract infection (5%) (results not shown).
Individuals
The cumulative incidence of at least one ED visit was 35% (n = 70,813) within 180 days after diagnosis (Table 3). Visit rates varied by cancer type: individuals with pancreatic (62%), brain (60%), and lung (55%) cancers had the highest cumulative incidences of ED use within 180 days of diagnosis (Table 3). Those with melanoma (14%), prostate (17%), and eye (18%) cancers had the lowest cumulative incidences of ED visits (Table 3).
Recycled predictions from logistic regression models, accounting for potential confounding factors, yielded substantively similar results for the cumulative incidence of ED use across cancer types (Table 4). Results did not differ substantially after accounting for survival. Differences in the predicted probability of an ED visits adjusting for death within 180 days of diagnosis were noted to be 2% or greater from estimates reported in Table 4 for only four cancers. Estimates of having any ED visits for those with lung cancer decreased from 46% to 44% (95% CI: 43.0-44.4%), pancreatic cancer from 53% to 49% (95% CI: 48-51%), liver cancer from 51% to 47% (95% CI: 49-53%), and those with eye cancers increased from 20% to 22% (95% CI: 18-26%) (not shown in tables).
For patients with certain cancers (eg, lung, pancreas, leukemia) the proportion of individuals with an initial ED visit was highest in the first 30 days after diagnosis (Table 3). For individuals with other cancers (eg, breast, prostate, melanoma) the proportion of individuals with an initial ED visit increased by more than 5% during the 31-181–day time period. Those with the remaining cancers had less than 5% change in cumulative ED use between the two time periods.
The number of visits per person ranged from 0-44 during the first 180 days after diagnosis (results not shown in tables). Of all patients diagnosed with cancer, 20% (n = 39,429) had one ED visit, 8% (n = 16,238) had two visits, and 7% (n = 14,760) had three or more visits. Of those patients having at least one ED visit within 180 days of diagnosis, 44% (n = 31,080) had two or more visits and 21% (n = 14,760) had 3 or more visits.
Discussion
This study extends previous research by describing ED use for more than 20 cancer types by time from diagnosis in a large, heterogeneous and population-based sample of recently diagnosed adults in California. We found that 16% of newly diagnosed individuals with cancer used the ED within 30 days of diagnosis, 35% within 6 months of diagnosis, and 44% within 1 year of diagnosis. These findings suggest that ED use by cancer patients is more than double that of the US general population and is higher than previously estimated for cancer patients.10,24 In 2010, about 21% of the US population visited the ED, compared with 44% of cancer patients in the same time period.24 Although persons with greater medical need, such as those with cancer, inevitably require more health services, new approaches are needed to explore the extent to which some of these visits by cancer patients could be prevented by providing care in other settings.
Few studies have examined ED use by cancer patients, but previous findings suggest that 1%-12% of cancer patients use the ED within 30 days of diagnosis, and 15%-25% use the ED within a year of diagnosis.10,25,26 One study did report higher rates of ED use by cancer patients, but attributed the increased use to changes in Medicaid copayments.27 The finding that ED use is higher among cancer patients than previously considered is important for several reasons. First, high rates of ED use may reflect excessive fragmentation in cancer care, or patients’ inability to access providers when acute concerns arise. Furthermore, providers and policymakers may be particularly interested in populations with high ED use because reducing potentially preventable ED use is often cited as one of the goals of care coordination and alternative health care model programs.2,28,29
The number of newly diagnosed cancer patients with multiple ED visits is also substantial. We found 15% of recently diagnosed cancer patients had two or more ED visits within 180 days of diagnosis, compared with 8% of the general US population having two or more ED visits in all of 2010.24 Among cancer patients with at least one ED visit, 44% visited more than once. Repeat visits may represent worsening health status, continued unmet health needs, or new complications that might have been prevented or treated in other health care settings. In addition, there may be opportunities to identify cancer patients at risk for multiple visits at their initial ED visit. A better understanding of the reasons for ED visits and the factors driving unmet need – such as inadequate patient education, limited access to specialty services, or failure to admit a patient to resolve a problem appropriately (eg, pain, infection) – may help to identify which visits are potentially preventable. Ultimately, failure to adequately describe the number of cancer patients that visit the ED and the number of times they visit may result in a lost opportunity for improvement in care, the patient experience and cost reduction in cancer care.
The distinction between cancer types that account for the most ED visits and cancer types with the highest cumulative incidences of ED use is informative. For instance, lung cancer patients accounted for the largest number of ED visits and over half of those with lung cancer visited the ED within 180 days of diagnosis. However, although more than 60% of individuals with pancreatic cancer visited an ED within 180 days of diagnosis; they accounted for only 5% of all ED visits by cancer patients during the same time period. This in part reflects the relative frequency of these cancers. However, prostate cancer, which has a high incidence rate, represents about 8% of all ED visits by cancer patients, yet only 17% of all prostate cancer patients visit the ED within 180 days of diagnosis.
One approach to reduce the absolute number of ED visits by cancer patients would be to target the most frequent users of the ED such as lung, breast, prostate, and colon cancer patients. These cancers are the most common in the general population, so proportionate reduction in ED visits in these groups would have a large overall impact on ED use. Alternatively, patients with cancers that have high rates of ED use could be targeted with interventions to better address their needs. Additional studies of ED use among cancer patients, including understudied cancers, are needed to determine whether care provided in the ED could be provided in alternate clinical settings. Such research can also support training of emergency department staff to manage the full range of cancer-related conditions presenting to the ED.
Another approach to identifying potentially avoidable ED visits is to explore visits that result in admission to the hospital compared with those that result in discharge from the ED. In some circumstances, visits that result in discharge home may not have been true medical emergencies, and therefore might have been preventable. It is also true that even an acute problem requiring admission may have been preventable with timely outpatient management. While we found that 45% of visits ended in discharge home, over half of cancer patients who visit the ED are admitted to the hospital. This is higher than admission rates from the ED for the U.S. population overall (11%-15%),30-32and even higher than the estimated rate of individuals with chronic conditions, such as diabetes (42%), who visit the ED and then are subsequently admitted to the hospital.33
Relatively high rates of admissions may indicate that cancer patients seeking care in the ED require increased medical attention; however, it is possible that other explanations exist. For instance, ED physicians may be uncomfortable with complex cancer cases and may admit patients to be evaluated by a specialist. As such, it is possible that some of these admissions could have been appropriate for outpatient follow up. It is also possible that patients are referred to the ED for admission to the hospital. In these situations the ED visit may be entirely preventable through a direct admission process, although such processes are not available at all institutions and may vary by the admitting provider. For instance, if a hospitalist is overseeing the hospital stay, they may prefer the admission to occur through the ED, whereas a primary care provider or oncologist may be more likely to facilitate a direct admission. Future research could address the extent to which admissions from the ED may be avoidable by examining reasons for and length of admission following an ED visit. While this study found top reasons for admission (principal diagnosis) to be septicemia, cardiovascular complaints and complications from surgery, cumulatively these diagnosis accounted for less than 20% of admissions from the ED. Examining frequent diagnoses by cancer type will also provide insight into potentially avoidable ED use, which may vary by disease course and treatment regimen.
The distribution of days from diagnosis to the first ED visit also varied by cancer type. This variation is likely attributable, at least in part, to differences in condition-specific treatment regimens, severity of illness, and stage at diagnosis. For example, patients with ED visits within 30 days of diagnosis may be those with advanced stage cancers who are at higher risk of complications, or they may be visiting the ED for post-surgical problems. Likewise, individuals who incur visits during later time periods may be undergoing longer treatment regimens. Further research is warranted to explore site-specific predictors of ED use and high-risk periods, accounting for cancer treatment and the timing of treatments.
In summary, ED use among cancer patients is substantial and higher than previously reported. Most ED visits occur within the first 180 days after diagnosis, suggesting focus on the first 30 days after hospital discharge may be misguided. Time frames for ED measurement in future research should be selected with careful attention to cancer-specific periods within which most ED use occurs and the outcomes of interest. Furthermore, better models identifying cancer-specific predictors of ED use, which account for treatment and comorbidities, will facilitate the development of interventions focused on high-risk segments of this population. Research is needed to explore cancer-specific reasons for ED visits and which ED admission diagnoses may be potentially preventable.
Limitations
The limitations of this study include those common to use of administrative and registry data and the CCR and OSHPD data in particular. While CCR data are known to be complete with respect to demographic and cancer information, treatment data is less robust and specific treatment dates are not available.14,34 As a result, we were unable to analyze ED use in relation to receipt of outpatient treatment. As we included all ED visits on or up to a year after the day of diagnosis, it is possible that our analysis includes diagnoses that occurred in conjunction with an ED visits. However, it is unlikely a reporting hospital would report a cancer diagnosis to the CCR without a corresponding hospital admission. Therefore, we assume such cases to be rare.
Lastly, California had lower prevalence of health insurance coverage and higher market penetration by health maintenance organizations, relative to the national average, which may limit the generalizability of the results to other states.35 At the same time, CCR-OSHPD linked data offer the advantage of providing complete data to enumerate ED visits among patients whether they were discharged home or subsequently admitted to hospital.
1. American Cancer Society. Facts & Figures 2017. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. Published 2017. Accessed March 16, 2017.
2. Levit L, Balogh E, Nass S, Ganz PA. Delivering high-quality cancer care: charting a new course for a system in crisis. https://www.nap.edu/read/18359/chapter/1. Published 2013. Accessed March 16, 2017.
3. Readmissions Reduction Program (HRRP). CMS website. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Updated April 8, 2016. Accessed March 16, 2017.
4. Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Clin Oncol. 2007;3(2):79-86.
5. Guadagnolo B, Dohan D, Raich P. Metrics for evaluating patient navigation during cancer diagnosis and treatment: crafting a policy-relevant research agenda for patient navigation in cancer care. Cancer. 2011;117(15 Suppl):3565-3574.
6. Medicare Patient Access to Cancer Treatment Act of 2013, H.R.2869, 113th Cong.(2013). https://www.congress.gov/bill/113th-congress/house-bill/2869/text?format=txt. Introduced July 31, 2013; latest action, referred to the Subcommittee on Health, August 2, 2013. Accessed March 16, 2017.
7. Smulowitz P, Honigman L, Landon B. A novel approach to identifying targets for cost reduction in the emergency department. Ann Emerg Med. 2013;61(3):293-300.
8. Agrawal S, Conway P. Integrating emergency care into a patient- and outcome-centered health care system. Ann Emerg Med. 2013;61(3):301-302.
9. Swenson K, Rose M, Ritz L, Murray CL, Adlis S. Recognition and evaluation of oncology-related symptoms in the emergency department. Ann Emerg Med.1995;26(1):12-17.
10. Lash R, Bell J, Reed S, et al. A systematic review of emergency department use among cancer patients. Cancer Nurs. 2017;40(2):135-144.
11. Sanoff H, Carpenter W, Freburger J, et al. Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: a population-based analysis. Cancer. 2012;118(17):4309-4320.
12. Hansen D, Fox J, Gross C, Bruun J. Hospital readmissions and emergency department visits following laparoscopic and open colon resection for cancer. Dis Colon Rectum. 2013;56(9):1053-1061.
13. Office of Statewide Health Planning and Development. Hospital Data Products. http://www.oshpd.ca.gov/HID/DataFlow/HospData.html. Last updated September 6, 2016. Accessed March 16, 2017.
14. California Cancer Registry. Overview. http://www.ccrcal.org/Inside_CCR/About_Us.shtml. Published 2009. Accessed April 15, 2015.
15. Patel M, Ma Y, Mitchell B, Rhoads K. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
16. Parikh-Patel A, White R, Allen M, Cress R. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control. 2009;20(6):1001-1010.
17. National Cancer Institute: Surveillance, Epidemiology and End Results Program. Site recode ICD-O-3/WHO 2008 definition. https://seer.cancer.gov/siterecode/icdo3_dwhoheme/. Published 2008. Accessed March 16, 2017.
18. Washington State Cancer Registry. Cancer Codes Used in Reports. https://fortress. wa.gov/doh/wscr/WSCR/CancerCode.mvc/CancerCode. Data updated, January 2016; report updated, March 2016. Accessed March 16, 2017.
19. National Cancer Institute: Surveillance, Epidemiology and End Results Program. ICD-O-3 SEER Site/Histology Validation List. https://seer.cancer.gov/icd-o-3/. Published 2012, updated September 2015. Accessed March 16, 2017.
20. American Joint Committee on Cancer. What is Cancer Staging? https://cancerstaging.org/references-tools/Pages/What-is-Cancer-Staging.aspx. Published 2010. Accessed March 16, 2017.
21. Yang J S, Harrati A, Clarke C, Keegan T, Gomez S. Cancer Prevention Institute of California. Developing an area based socioeconomic measures from American Community Survey data. http://www.cpic.org/files/PDF/Research_Files/Reports/CPIC_ACS_SES_Index_Documentation_3-102014.pdf. Published March 10, 2014. Accessed March 16, 2017.
22. Basu A, Rathouz P. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6(1):93-109.
23. Stata Statistical Software [computer program]. Version 13 College Station, TX: StataCorp LP. 2013.
24. National Center for Health Statistics. Health, United States, 2013 – with a special feature on prescription drugs. https://www.cdc.gov/nchs/data/hus/hus13.pdf. Updated May 2014. Accessed March 16, 2017.
25. Goyal R, Wheeler S, Kohler R, et al. Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014;75(4):231-238.
26. Hassett M, O’Malley A, Pakes J, Newhouse J, Earle C. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98(16):1108-1117.
27. Subramanian S. Impact of Medicaid copayments on patients with cancer: lessons for Medicaid expansion under health reform. Med Care. 2011;49(9):842-847.
28. Coyle Y, Miller A, Paulson R. Model for the cost-efficient delivery of continuous quality cancer care: a hospital and private-practice collaboration. Proc (Bayl Univ Med Cent). 2013;26(2):95-99.
29. Agency for Healthcare Research and Quality. 2011 National Healthcare Disparities Report. https://archive.ahrq.gov/research/findings/nhqrdr/nhdr11/index.html. Last reviewed October 2014. Accessed March 16, 2017.
30. Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Emergency Department Sample (NEDS) 2010. https://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2010.jsp. Issued November 2012, updated November 2015. Accessed March 16, 2017.
31. Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Emergency Department Sample (NEDS) 2013. https://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2013.jsp. Published November 2015. Accessed March 16, 2017.
32. Weiss AJ, Wier LM, Stocks C, Blanchard J. Overview of emergency department visits in the United States, 2011. Statistical Brief #174. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb174-Emergency-Department-Visits-Overview.pdf. Published June 2014. Accessed March 16, 2017.
33. Washington R, Andrews R, Mutter, R. Emergency department visits for adults with diabetes, 2010. Statistical Brief #167. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb167.jsp. Published November 2013. Accessed March 16, 2017.
34. Penberthy L, Petkov V, McClish D, et al. The value of billing data from oncology practice to supplement treatment information for cancer surveillance. Journal of registry management. 2014;41(2):57-64.
35. National Center for Health Statistics. Health, United States, 2014 – with a special feature on adults aged 55-64. https://www.cdc.gov/nchs/data/hus/hus14.pdf. Published May 2015. Accessed March 16, 2017.
In 2017 there will be nearly 1.7 million new cancer cases diagnosed, and over 600,000 cancer deaths in the Unites States.1 A 2013 Institute of Medicine report highlighted problems with the current quality of cancer care, including high costs and fragmentation of care.2 Other national reports have called for improvements in the overall quality of care and for reducing costly and possibly avoidable use of health services such as emergency department (ED) visits.3-6 Reduction of avoidable ED visits is often cited as a pathway to reduce costs by avoiding unnecessary tests and treatments that occur in the ED and subsequent hospital admissions.7,8
ED crowding, long waits, and unpredictable treatment environments can also make an ED visit an unpleasant experience for the patient. ED visits during cancer treatment can be particularly troubling and present health concerns for patients who are immunocompromised. In particular, cancer patients in the ED have been found to experience delays in the administration of analgesics, antiemetics, or antibiotics.9
Few studies have examined ED use or its associated predictors among cancer patients. Reports to date that have described ED use have focused on different cancers, which makes comparisons across studies difficult.10 Moreover, the time frames of interest and the type of event after which ED use is evaluated (ie, diagnosis or treatment) are inconsistent in the existing literature.10 Some studies quantifying ED use excluded patients admitted to the hospital after an ED visit.11,12 Taken together, these studies do not provide a clear overview of the extent of ED use by cancer patients or the amount of cancer-related care provided by EDs.
Patterns of ED use among cancer patients derived from large and generalizable samples may help inform providers about true risk factors for ED use. In addition, prioritizing new interventions and focusing future research on groups of patients who are at higher risk for preventable ED use could also improve overall care. To address these issues, accurate estimates of ED use among cancer patients are required.
To our knowledge, this is the first study to describe ED use across a range of cancers in a large population-based sample and to consider the timing of ED visits in relation to initial diagnosis. The findings could provide benchmark comparison data to inform future efforts to identify the subset of possibly preventable ED visits and to design interventions to address preventable ED use.
Material and methods
Data source
California’s Office of Statewide Health Planning and Development (OSHPD) manages the patient discharge dataset (PDD) and the emergency department use (EDU) dataset, providing a high-quality source of information on inpatient and ED use in the state.13 A principal diagnosis and up to 24 secondary diagnoses are recorded in OSHPD datasets. The EDU dataset was used to identify treat-and-release ED visits, and the PDD was used to identify hospitalizations initiated in the ED. The California Cancer Registry (CCR) obtains demographic and diagnosis information for every new invasive cancer diagnosed in California, and data collected by the registry are considered to be complete.14 CCR-OSPHD-linked data provide high-quality health care use information for cancer patients in California.15,16 Using an encrypted version of the social security number called the record linkage number (RLN), we linked the CCR records to the corresponding OSHPD files from 2009-2010.
Institutional review board approval for this study was obtained from the University of California, Davis, Human Subjects Committee and the State of California Committee for the Protection of Human Subjects.
Analysis
ED visits. Visits were included if they occurred on or up to 365 days after the date of cancer diagnosis recorded in the CCR. The visits were coded in mutually exclusive groups as occurring within 30, 31-180, and 181-365 days of diagnosis. Subsequently, we flagged each person as having any ED visit (Yes/No) within 180 days and within 365 days of diagnosis, and we tallied the total number of visits occurring within these time frames for each person.
Cancer type. We used relevant site and histology codes to classify cancer type into 24 mutually exclusive categories using the Surveillance Epidemiology and End Result‘s International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) Recode Definitions17-19 (Suppl Figure 1).
Individual-level variables. Sociodemographic information for each person was collected from the CCR including gender, age, race/ethnicity, marital status, health insurance status, rural residence, survival time in months, neighborhood socio-economic (SES) status based on the Yang index, and the American Joint Committee on Cancer (AJCC) stage.20,21
Data analysis
Demographic information was analyzed for the cohort using descriptive statistics (frequencies, proportions, means, standard deviations, and ranges) and evaluated for correlations. Fewer than 20 observations had missing data and we removed those observations from our analyses on an item-specific basis.
We tabulated ED visits by cancer type and time from diagnosis and then collapsed visit-level data by RLN to determine the number of ED visits for each person in the sample. The number of days from diagnosis to first ED visit was also tabulated. The cohort was stratified by cancer type and cumulative rates of ED visits were tabulated for individuals with ED visits within 0-180 and 0-365 days from diagnosis. To test the robustness of the findings adjusting for confounding factors known to impact ED use, we used logistic regression to model any ED use (Yes/No) as a function of age, gender, race/ethnicity, cancer stage, insurance status, marital status, urban residence, and Yang SES. After model estimation, we used the method of recycled predictions controlling for the confounding variables to compute the marginal probabilities of ED use by cancer type.22 To adjust for the possible impact of survival on ED use, we performed sensitivity analyses and estimated predicted probabilities adjusting for survival. Separate analyses were performed first adjusting for whether the patient died during the course of each month after diagnosis and then adjusting for whether or not the patient died within 180 days of diagnosis. All analyses were conducted using Stata 13.1.23
Results
The CCR identified 222,087 adults with a new primary cancer diagnosis in 2009-2010. After excluding those with Stage 0 cancer (n = 21,154) and nonmelanoma skin cancer (n = 1,031), for whom data are inconsistently collected by CCR, a total of 199,872 individuals were included in the analytic sample. Of those patients (Table 1), most were white non-Hispanic (62%), women (51%), holders of private insurance (53%), married (56%), and urban residents (86%). Most were older than 50 years and had either Stage I or Stage II cancer. The most common cancer types were breast (17%), prostate (16%), lung (11%), and colon (9%; results not shown). In unadjusted comparisons, the incidence of ED use was significantly higher among those who were older, of non-Hispanic black race/ethnicity, uninsured, in the lowest SES group, widowed, or diagnosed with Stage IV cancer (Table 1).
ED visits
Within 365 days after initial cancer diagnosis, 87,025 cancer patients made a total of 197,886 ED visits (not shown in tables). Of those visits, 68% (n = 134,556) occurred within 180 days of diagnosis, with 22% (n = 43,535) occurring within the first 30 days and 46% (n = 91,027) occurring within 31-180 days after diagnosis (Figure). Given that most of the visits occurred within 180 days of diagnosis, we used that time frame in subsequent analyses. Among all ED visits within 180 days of diagnosis (Table 2), the largest proportions of visits were made by those with lung cancer (16%), breast cancer (11%), and colon cancer (10%).
About 51% of visits resulted in admission to the hospital and 45% in discharge (Table 2). For some cancers (lung, colon, non-Hodgkin lymphoma, pancreatic, digestive, liver, stomach, leukemia, and myeloma) most of the visits resulted in admission to the hospital (Table 2). Among visits resulting in admission, the top three principal diagnoses were: septicemia (8%), cardiovascular problems (7%), and complications from surgery (5%) (not shown in tables). Among visits resulting in a discharge home, the three top principal diagnoses were abdominal pain (7%), cardiovascular problems (6%), and urinary, kidney, and bladder complaints other than a urinary tract infection (5%) (results not shown).
Individuals
The cumulative incidence of at least one ED visit was 35% (n = 70,813) within 180 days after diagnosis (Table 3). Visit rates varied by cancer type: individuals with pancreatic (62%), brain (60%), and lung (55%) cancers had the highest cumulative incidences of ED use within 180 days of diagnosis (Table 3). Those with melanoma (14%), prostate (17%), and eye (18%) cancers had the lowest cumulative incidences of ED visits (Table 3).
Recycled predictions from logistic regression models, accounting for potential confounding factors, yielded substantively similar results for the cumulative incidence of ED use across cancer types (Table 4). Results did not differ substantially after accounting for survival. Differences in the predicted probability of an ED visits adjusting for death within 180 days of diagnosis were noted to be 2% or greater from estimates reported in Table 4 for only four cancers. Estimates of having any ED visits for those with lung cancer decreased from 46% to 44% (95% CI: 43.0-44.4%), pancreatic cancer from 53% to 49% (95% CI: 48-51%), liver cancer from 51% to 47% (95% CI: 49-53%), and those with eye cancers increased from 20% to 22% (95% CI: 18-26%) (not shown in tables).
For patients with certain cancers (eg, lung, pancreas, leukemia) the proportion of individuals with an initial ED visit was highest in the first 30 days after diagnosis (Table 3). For individuals with other cancers (eg, breast, prostate, melanoma) the proportion of individuals with an initial ED visit increased by more than 5% during the 31-181–day time period. Those with the remaining cancers had less than 5% change in cumulative ED use between the two time periods.
The number of visits per person ranged from 0-44 during the first 180 days after diagnosis (results not shown in tables). Of all patients diagnosed with cancer, 20% (n = 39,429) had one ED visit, 8% (n = 16,238) had two visits, and 7% (n = 14,760) had three or more visits. Of those patients having at least one ED visit within 180 days of diagnosis, 44% (n = 31,080) had two or more visits and 21% (n = 14,760) had 3 or more visits.
Discussion
This study extends previous research by describing ED use for more than 20 cancer types by time from diagnosis in a large, heterogeneous and population-based sample of recently diagnosed adults in California. We found that 16% of newly diagnosed individuals with cancer used the ED within 30 days of diagnosis, 35% within 6 months of diagnosis, and 44% within 1 year of diagnosis. These findings suggest that ED use by cancer patients is more than double that of the US general population and is higher than previously estimated for cancer patients.10,24 In 2010, about 21% of the US population visited the ED, compared with 44% of cancer patients in the same time period.24 Although persons with greater medical need, such as those with cancer, inevitably require more health services, new approaches are needed to explore the extent to which some of these visits by cancer patients could be prevented by providing care in other settings.
Few studies have examined ED use by cancer patients, but previous findings suggest that 1%-12% of cancer patients use the ED within 30 days of diagnosis, and 15%-25% use the ED within a year of diagnosis.10,25,26 One study did report higher rates of ED use by cancer patients, but attributed the increased use to changes in Medicaid copayments.27 The finding that ED use is higher among cancer patients than previously considered is important for several reasons. First, high rates of ED use may reflect excessive fragmentation in cancer care, or patients’ inability to access providers when acute concerns arise. Furthermore, providers and policymakers may be particularly interested in populations with high ED use because reducing potentially preventable ED use is often cited as one of the goals of care coordination and alternative health care model programs.2,28,29
The number of newly diagnosed cancer patients with multiple ED visits is also substantial. We found 15% of recently diagnosed cancer patients had two or more ED visits within 180 days of diagnosis, compared with 8% of the general US population having two or more ED visits in all of 2010.24 Among cancer patients with at least one ED visit, 44% visited more than once. Repeat visits may represent worsening health status, continued unmet health needs, or new complications that might have been prevented or treated in other health care settings. In addition, there may be opportunities to identify cancer patients at risk for multiple visits at their initial ED visit. A better understanding of the reasons for ED visits and the factors driving unmet need – such as inadequate patient education, limited access to specialty services, or failure to admit a patient to resolve a problem appropriately (eg, pain, infection) – may help to identify which visits are potentially preventable. Ultimately, failure to adequately describe the number of cancer patients that visit the ED and the number of times they visit may result in a lost opportunity for improvement in care, the patient experience and cost reduction in cancer care.
The distinction between cancer types that account for the most ED visits and cancer types with the highest cumulative incidences of ED use is informative. For instance, lung cancer patients accounted for the largest number of ED visits and over half of those with lung cancer visited the ED within 180 days of diagnosis. However, although more than 60% of individuals with pancreatic cancer visited an ED within 180 days of diagnosis; they accounted for only 5% of all ED visits by cancer patients during the same time period. This in part reflects the relative frequency of these cancers. However, prostate cancer, which has a high incidence rate, represents about 8% of all ED visits by cancer patients, yet only 17% of all prostate cancer patients visit the ED within 180 days of diagnosis.
One approach to reduce the absolute number of ED visits by cancer patients would be to target the most frequent users of the ED such as lung, breast, prostate, and colon cancer patients. These cancers are the most common in the general population, so proportionate reduction in ED visits in these groups would have a large overall impact on ED use. Alternatively, patients with cancers that have high rates of ED use could be targeted with interventions to better address their needs. Additional studies of ED use among cancer patients, including understudied cancers, are needed to determine whether care provided in the ED could be provided in alternate clinical settings. Such research can also support training of emergency department staff to manage the full range of cancer-related conditions presenting to the ED.
Another approach to identifying potentially avoidable ED visits is to explore visits that result in admission to the hospital compared with those that result in discharge from the ED. In some circumstances, visits that result in discharge home may not have been true medical emergencies, and therefore might have been preventable. It is also true that even an acute problem requiring admission may have been preventable with timely outpatient management. While we found that 45% of visits ended in discharge home, over half of cancer patients who visit the ED are admitted to the hospital. This is higher than admission rates from the ED for the U.S. population overall (11%-15%),30-32and even higher than the estimated rate of individuals with chronic conditions, such as diabetes (42%), who visit the ED and then are subsequently admitted to the hospital.33
Relatively high rates of admissions may indicate that cancer patients seeking care in the ED require increased medical attention; however, it is possible that other explanations exist. For instance, ED physicians may be uncomfortable with complex cancer cases and may admit patients to be evaluated by a specialist. As such, it is possible that some of these admissions could have been appropriate for outpatient follow up. It is also possible that patients are referred to the ED for admission to the hospital. In these situations the ED visit may be entirely preventable through a direct admission process, although such processes are not available at all institutions and may vary by the admitting provider. For instance, if a hospitalist is overseeing the hospital stay, they may prefer the admission to occur through the ED, whereas a primary care provider or oncologist may be more likely to facilitate a direct admission. Future research could address the extent to which admissions from the ED may be avoidable by examining reasons for and length of admission following an ED visit. While this study found top reasons for admission (principal diagnosis) to be septicemia, cardiovascular complaints and complications from surgery, cumulatively these diagnosis accounted for less than 20% of admissions from the ED. Examining frequent diagnoses by cancer type will also provide insight into potentially avoidable ED use, which may vary by disease course and treatment regimen.
The distribution of days from diagnosis to the first ED visit also varied by cancer type. This variation is likely attributable, at least in part, to differences in condition-specific treatment regimens, severity of illness, and stage at diagnosis. For example, patients with ED visits within 30 days of diagnosis may be those with advanced stage cancers who are at higher risk of complications, or they may be visiting the ED for post-surgical problems. Likewise, individuals who incur visits during later time periods may be undergoing longer treatment regimens. Further research is warranted to explore site-specific predictors of ED use and high-risk periods, accounting for cancer treatment and the timing of treatments.
In summary, ED use among cancer patients is substantial and higher than previously reported. Most ED visits occur within the first 180 days after diagnosis, suggesting focus on the first 30 days after hospital discharge may be misguided. Time frames for ED measurement in future research should be selected with careful attention to cancer-specific periods within which most ED use occurs and the outcomes of interest. Furthermore, better models identifying cancer-specific predictors of ED use, which account for treatment and comorbidities, will facilitate the development of interventions focused on high-risk segments of this population. Research is needed to explore cancer-specific reasons for ED visits and which ED admission diagnoses may be potentially preventable.
Limitations
The limitations of this study include those common to use of administrative and registry data and the CCR and OSHPD data in particular. While CCR data are known to be complete with respect to demographic and cancer information, treatment data is less robust and specific treatment dates are not available.14,34 As a result, we were unable to analyze ED use in relation to receipt of outpatient treatment. As we included all ED visits on or up to a year after the day of diagnosis, it is possible that our analysis includes diagnoses that occurred in conjunction with an ED visits. However, it is unlikely a reporting hospital would report a cancer diagnosis to the CCR without a corresponding hospital admission. Therefore, we assume such cases to be rare.
Lastly, California had lower prevalence of health insurance coverage and higher market penetration by health maintenance organizations, relative to the national average, which may limit the generalizability of the results to other states.35 At the same time, CCR-OSHPD linked data offer the advantage of providing complete data to enumerate ED visits among patients whether they were discharged home or subsequently admitted to hospital.
In 2017 there will be nearly 1.7 million new cancer cases diagnosed, and over 600,000 cancer deaths in the Unites States.1 A 2013 Institute of Medicine report highlighted problems with the current quality of cancer care, including high costs and fragmentation of care.2 Other national reports have called for improvements in the overall quality of care and for reducing costly and possibly avoidable use of health services such as emergency department (ED) visits.3-6 Reduction of avoidable ED visits is often cited as a pathway to reduce costs by avoiding unnecessary tests and treatments that occur in the ED and subsequent hospital admissions.7,8
ED crowding, long waits, and unpredictable treatment environments can also make an ED visit an unpleasant experience for the patient. ED visits during cancer treatment can be particularly troubling and present health concerns for patients who are immunocompromised. In particular, cancer patients in the ED have been found to experience delays in the administration of analgesics, antiemetics, or antibiotics.9
Few studies have examined ED use or its associated predictors among cancer patients. Reports to date that have described ED use have focused on different cancers, which makes comparisons across studies difficult.10 Moreover, the time frames of interest and the type of event after which ED use is evaluated (ie, diagnosis or treatment) are inconsistent in the existing literature.10 Some studies quantifying ED use excluded patients admitted to the hospital after an ED visit.11,12 Taken together, these studies do not provide a clear overview of the extent of ED use by cancer patients or the amount of cancer-related care provided by EDs.
Patterns of ED use among cancer patients derived from large and generalizable samples may help inform providers about true risk factors for ED use. In addition, prioritizing new interventions and focusing future research on groups of patients who are at higher risk for preventable ED use could also improve overall care. To address these issues, accurate estimates of ED use among cancer patients are required.
To our knowledge, this is the first study to describe ED use across a range of cancers in a large population-based sample and to consider the timing of ED visits in relation to initial diagnosis. The findings could provide benchmark comparison data to inform future efforts to identify the subset of possibly preventable ED visits and to design interventions to address preventable ED use.
Material and methods
Data source
California’s Office of Statewide Health Planning and Development (OSHPD) manages the patient discharge dataset (PDD) and the emergency department use (EDU) dataset, providing a high-quality source of information on inpatient and ED use in the state.13 A principal diagnosis and up to 24 secondary diagnoses are recorded in OSHPD datasets. The EDU dataset was used to identify treat-and-release ED visits, and the PDD was used to identify hospitalizations initiated in the ED. The California Cancer Registry (CCR) obtains demographic and diagnosis information for every new invasive cancer diagnosed in California, and data collected by the registry are considered to be complete.14 CCR-OSPHD-linked data provide high-quality health care use information for cancer patients in California.15,16 Using an encrypted version of the social security number called the record linkage number (RLN), we linked the CCR records to the corresponding OSHPD files from 2009-2010.
Institutional review board approval for this study was obtained from the University of California, Davis, Human Subjects Committee and the State of California Committee for the Protection of Human Subjects.
Analysis
ED visits. Visits were included if they occurred on or up to 365 days after the date of cancer diagnosis recorded in the CCR. The visits were coded in mutually exclusive groups as occurring within 30, 31-180, and 181-365 days of diagnosis. Subsequently, we flagged each person as having any ED visit (Yes/No) within 180 days and within 365 days of diagnosis, and we tallied the total number of visits occurring within these time frames for each person.
Cancer type. We used relevant site and histology codes to classify cancer type into 24 mutually exclusive categories using the Surveillance Epidemiology and End Result‘s International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) Recode Definitions17-19 (Suppl Figure 1).
Individual-level variables. Sociodemographic information for each person was collected from the CCR including gender, age, race/ethnicity, marital status, health insurance status, rural residence, survival time in months, neighborhood socio-economic (SES) status based on the Yang index, and the American Joint Committee on Cancer (AJCC) stage.20,21
Data analysis
Demographic information was analyzed for the cohort using descriptive statistics (frequencies, proportions, means, standard deviations, and ranges) and evaluated for correlations. Fewer than 20 observations had missing data and we removed those observations from our analyses on an item-specific basis.
We tabulated ED visits by cancer type and time from diagnosis and then collapsed visit-level data by RLN to determine the number of ED visits for each person in the sample. The number of days from diagnosis to first ED visit was also tabulated. The cohort was stratified by cancer type and cumulative rates of ED visits were tabulated for individuals with ED visits within 0-180 and 0-365 days from diagnosis. To test the robustness of the findings adjusting for confounding factors known to impact ED use, we used logistic regression to model any ED use (Yes/No) as a function of age, gender, race/ethnicity, cancer stage, insurance status, marital status, urban residence, and Yang SES. After model estimation, we used the method of recycled predictions controlling for the confounding variables to compute the marginal probabilities of ED use by cancer type.22 To adjust for the possible impact of survival on ED use, we performed sensitivity analyses and estimated predicted probabilities adjusting for survival. Separate analyses were performed first adjusting for whether the patient died during the course of each month after diagnosis and then adjusting for whether or not the patient died within 180 days of diagnosis. All analyses were conducted using Stata 13.1.23
Results
The CCR identified 222,087 adults with a new primary cancer diagnosis in 2009-2010. After excluding those with Stage 0 cancer (n = 21,154) and nonmelanoma skin cancer (n = 1,031), for whom data are inconsistently collected by CCR, a total of 199,872 individuals were included in the analytic sample. Of those patients (Table 1), most were white non-Hispanic (62%), women (51%), holders of private insurance (53%), married (56%), and urban residents (86%). Most were older than 50 years and had either Stage I or Stage II cancer. The most common cancer types were breast (17%), prostate (16%), lung (11%), and colon (9%; results not shown). In unadjusted comparisons, the incidence of ED use was significantly higher among those who were older, of non-Hispanic black race/ethnicity, uninsured, in the lowest SES group, widowed, or diagnosed with Stage IV cancer (Table 1).
ED visits
Within 365 days after initial cancer diagnosis, 87,025 cancer patients made a total of 197,886 ED visits (not shown in tables). Of those visits, 68% (n = 134,556) occurred within 180 days of diagnosis, with 22% (n = 43,535) occurring within the first 30 days and 46% (n = 91,027) occurring within 31-180 days after diagnosis (Figure). Given that most of the visits occurred within 180 days of diagnosis, we used that time frame in subsequent analyses. Among all ED visits within 180 days of diagnosis (Table 2), the largest proportions of visits were made by those with lung cancer (16%), breast cancer (11%), and colon cancer (10%).
About 51% of visits resulted in admission to the hospital and 45% in discharge (Table 2). For some cancers (lung, colon, non-Hodgkin lymphoma, pancreatic, digestive, liver, stomach, leukemia, and myeloma) most of the visits resulted in admission to the hospital (Table 2). Among visits resulting in admission, the top three principal diagnoses were: septicemia (8%), cardiovascular problems (7%), and complications from surgery (5%) (not shown in tables). Among visits resulting in a discharge home, the three top principal diagnoses were abdominal pain (7%), cardiovascular problems (6%), and urinary, kidney, and bladder complaints other than a urinary tract infection (5%) (results not shown).
Individuals
The cumulative incidence of at least one ED visit was 35% (n = 70,813) within 180 days after diagnosis (Table 3). Visit rates varied by cancer type: individuals with pancreatic (62%), brain (60%), and lung (55%) cancers had the highest cumulative incidences of ED use within 180 days of diagnosis (Table 3). Those with melanoma (14%), prostate (17%), and eye (18%) cancers had the lowest cumulative incidences of ED visits (Table 3).
Recycled predictions from logistic regression models, accounting for potential confounding factors, yielded substantively similar results for the cumulative incidence of ED use across cancer types (Table 4). Results did not differ substantially after accounting for survival. Differences in the predicted probability of an ED visits adjusting for death within 180 days of diagnosis were noted to be 2% or greater from estimates reported in Table 4 for only four cancers. Estimates of having any ED visits for those with lung cancer decreased from 46% to 44% (95% CI: 43.0-44.4%), pancreatic cancer from 53% to 49% (95% CI: 48-51%), liver cancer from 51% to 47% (95% CI: 49-53%), and those with eye cancers increased from 20% to 22% (95% CI: 18-26%) (not shown in tables).
For patients with certain cancers (eg, lung, pancreas, leukemia) the proportion of individuals with an initial ED visit was highest in the first 30 days after diagnosis (Table 3). For individuals with other cancers (eg, breast, prostate, melanoma) the proportion of individuals with an initial ED visit increased by more than 5% during the 31-181–day time period. Those with the remaining cancers had less than 5% change in cumulative ED use between the two time periods.
The number of visits per person ranged from 0-44 during the first 180 days after diagnosis (results not shown in tables). Of all patients diagnosed with cancer, 20% (n = 39,429) had one ED visit, 8% (n = 16,238) had two visits, and 7% (n = 14,760) had three or more visits. Of those patients having at least one ED visit within 180 days of diagnosis, 44% (n = 31,080) had two or more visits and 21% (n = 14,760) had 3 or more visits.
Discussion
This study extends previous research by describing ED use for more than 20 cancer types by time from diagnosis in a large, heterogeneous and population-based sample of recently diagnosed adults in California. We found that 16% of newly diagnosed individuals with cancer used the ED within 30 days of diagnosis, 35% within 6 months of diagnosis, and 44% within 1 year of diagnosis. These findings suggest that ED use by cancer patients is more than double that of the US general population and is higher than previously estimated for cancer patients.10,24 In 2010, about 21% of the US population visited the ED, compared with 44% of cancer patients in the same time period.24 Although persons with greater medical need, such as those with cancer, inevitably require more health services, new approaches are needed to explore the extent to which some of these visits by cancer patients could be prevented by providing care in other settings.
Few studies have examined ED use by cancer patients, but previous findings suggest that 1%-12% of cancer patients use the ED within 30 days of diagnosis, and 15%-25% use the ED within a year of diagnosis.10,25,26 One study did report higher rates of ED use by cancer patients, but attributed the increased use to changes in Medicaid copayments.27 The finding that ED use is higher among cancer patients than previously considered is important for several reasons. First, high rates of ED use may reflect excessive fragmentation in cancer care, or patients’ inability to access providers when acute concerns arise. Furthermore, providers and policymakers may be particularly interested in populations with high ED use because reducing potentially preventable ED use is often cited as one of the goals of care coordination and alternative health care model programs.2,28,29
The number of newly diagnosed cancer patients with multiple ED visits is also substantial. We found 15% of recently diagnosed cancer patients had two or more ED visits within 180 days of diagnosis, compared with 8% of the general US population having two or more ED visits in all of 2010.24 Among cancer patients with at least one ED visit, 44% visited more than once. Repeat visits may represent worsening health status, continued unmet health needs, or new complications that might have been prevented or treated in other health care settings. In addition, there may be opportunities to identify cancer patients at risk for multiple visits at their initial ED visit. A better understanding of the reasons for ED visits and the factors driving unmet need – such as inadequate patient education, limited access to specialty services, or failure to admit a patient to resolve a problem appropriately (eg, pain, infection) – may help to identify which visits are potentially preventable. Ultimately, failure to adequately describe the number of cancer patients that visit the ED and the number of times they visit may result in a lost opportunity for improvement in care, the patient experience and cost reduction in cancer care.
The distinction between cancer types that account for the most ED visits and cancer types with the highest cumulative incidences of ED use is informative. For instance, lung cancer patients accounted for the largest number of ED visits and over half of those with lung cancer visited the ED within 180 days of diagnosis. However, although more than 60% of individuals with pancreatic cancer visited an ED within 180 days of diagnosis; they accounted for only 5% of all ED visits by cancer patients during the same time period. This in part reflects the relative frequency of these cancers. However, prostate cancer, which has a high incidence rate, represents about 8% of all ED visits by cancer patients, yet only 17% of all prostate cancer patients visit the ED within 180 days of diagnosis.
One approach to reduce the absolute number of ED visits by cancer patients would be to target the most frequent users of the ED such as lung, breast, prostate, and colon cancer patients. These cancers are the most common in the general population, so proportionate reduction in ED visits in these groups would have a large overall impact on ED use. Alternatively, patients with cancers that have high rates of ED use could be targeted with interventions to better address their needs. Additional studies of ED use among cancer patients, including understudied cancers, are needed to determine whether care provided in the ED could be provided in alternate clinical settings. Such research can also support training of emergency department staff to manage the full range of cancer-related conditions presenting to the ED.
Another approach to identifying potentially avoidable ED visits is to explore visits that result in admission to the hospital compared with those that result in discharge from the ED. In some circumstances, visits that result in discharge home may not have been true medical emergencies, and therefore might have been preventable. It is also true that even an acute problem requiring admission may have been preventable with timely outpatient management. While we found that 45% of visits ended in discharge home, over half of cancer patients who visit the ED are admitted to the hospital. This is higher than admission rates from the ED for the U.S. population overall (11%-15%),30-32and even higher than the estimated rate of individuals with chronic conditions, such as diabetes (42%), who visit the ED and then are subsequently admitted to the hospital.33
Relatively high rates of admissions may indicate that cancer patients seeking care in the ED require increased medical attention; however, it is possible that other explanations exist. For instance, ED physicians may be uncomfortable with complex cancer cases and may admit patients to be evaluated by a specialist. As such, it is possible that some of these admissions could have been appropriate for outpatient follow up. It is also possible that patients are referred to the ED for admission to the hospital. In these situations the ED visit may be entirely preventable through a direct admission process, although such processes are not available at all institutions and may vary by the admitting provider. For instance, if a hospitalist is overseeing the hospital stay, they may prefer the admission to occur through the ED, whereas a primary care provider or oncologist may be more likely to facilitate a direct admission. Future research could address the extent to which admissions from the ED may be avoidable by examining reasons for and length of admission following an ED visit. While this study found top reasons for admission (principal diagnosis) to be septicemia, cardiovascular complaints and complications from surgery, cumulatively these diagnosis accounted for less than 20% of admissions from the ED. Examining frequent diagnoses by cancer type will also provide insight into potentially avoidable ED use, which may vary by disease course and treatment regimen.
The distribution of days from diagnosis to the first ED visit also varied by cancer type. This variation is likely attributable, at least in part, to differences in condition-specific treatment regimens, severity of illness, and stage at diagnosis. For example, patients with ED visits within 30 days of diagnosis may be those with advanced stage cancers who are at higher risk of complications, or they may be visiting the ED for post-surgical problems. Likewise, individuals who incur visits during later time periods may be undergoing longer treatment regimens. Further research is warranted to explore site-specific predictors of ED use and high-risk periods, accounting for cancer treatment and the timing of treatments.
In summary, ED use among cancer patients is substantial and higher than previously reported. Most ED visits occur within the first 180 days after diagnosis, suggesting focus on the first 30 days after hospital discharge may be misguided. Time frames for ED measurement in future research should be selected with careful attention to cancer-specific periods within which most ED use occurs and the outcomes of interest. Furthermore, better models identifying cancer-specific predictors of ED use, which account for treatment and comorbidities, will facilitate the development of interventions focused on high-risk segments of this population. Research is needed to explore cancer-specific reasons for ED visits and which ED admission diagnoses may be potentially preventable.
Limitations
The limitations of this study include those common to use of administrative and registry data and the CCR and OSHPD data in particular. While CCR data are known to be complete with respect to demographic and cancer information, treatment data is less robust and specific treatment dates are not available.14,34 As a result, we were unable to analyze ED use in relation to receipt of outpatient treatment. As we included all ED visits on or up to a year after the day of diagnosis, it is possible that our analysis includes diagnoses that occurred in conjunction with an ED visits. However, it is unlikely a reporting hospital would report a cancer diagnosis to the CCR without a corresponding hospital admission. Therefore, we assume such cases to be rare.
Lastly, California had lower prevalence of health insurance coverage and higher market penetration by health maintenance organizations, relative to the national average, which may limit the generalizability of the results to other states.35 At the same time, CCR-OSHPD linked data offer the advantage of providing complete data to enumerate ED visits among patients whether they were discharged home or subsequently admitted to hospital.
1. American Cancer Society. Facts & Figures 2017. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. Published 2017. Accessed March 16, 2017.
2. Levit L, Balogh E, Nass S, Ganz PA. Delivering high-quality cancer care: charting a new course for a system in crisis. https://www.nap.edu/read/18359/chapter/1. Published 2013. Accessed March 16, 2017.
3. Readmissions Reduction Program (HRRP). CMS website. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Updated April 8, 2016. Accessed March 16, 2017.
4. Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Clin Oncol. 2007;3(2):79-86.
5. Guadagnolo B, Dohan D, Raich P. Metrics for evaluating patient navigation during cancer diagnosis and treatment: crafting a policy-relevant research agenda for patient navigation in cancer care. Cancer. 2011;117(15 Suppl):3565-3574.
6. Medicare Patient Access to Cancer Treatment Act of 2013, H.R.2869, 113th Cong.(2013). https://www.congress.gov/bill/113th-congress/house-bill/2869/text?format=txt. Introduced July 31, 2013; latest action, referred to the Subcommittee on Health, August 2, 2013. Accessed March 16, 2017.
7. Smulowitz P, Honigman L, Landon B. A novel approach to identifying targets for cost reduction in the emergency department. Ann Emerg Med. 2013;61(3):293-300.
8. Agrawal S, Conway P. Integrating emergency care into a patient- and outcome-centered health care system. Ann Emerg Med. 2013;61(3):301-302.
9. Swenson K, Rose M, Ritz L, Murray CL, Adlis S. Recognition and evaluation of oncology-related symptoms in the emergency department. Ann Emerg Med.1995;26(1):12-17.
10. Lash R, Bell J, Reed S, et al. A systematic review of emergency department use among cancer patients. Cancer Nurs. 2017;40(2):135-144.
11. Sanoff H, Carpenter W, Freburger J, et al. Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: a population-based analysis. Cancer. 2012;118(17):4309-4320.
12. Hansen D, Fox J, Gross C, Bruun J. Hospital readmissions and emergency department visits following laparoscopic and open colon resection for cancer. Dis Colon Rectum. 2013;56(9):1053-1061.
13. Office of Statewide Health Planning and Development. Hospital Data Products. http://www.oshpd.ca.gov/HID/DataFlow/HospData.html. Last updated September 6, 2016. Accessed March 16, 2017.
14. California Cancer Registry. Overview. http://www.ccrcal.org/Inside_CCR/About_Us.shtml. Published 2009. Accessed April 15, 2015.
15. Patel M, Ma Y, Mitchell B, Rhoads K. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
16. Parikh-Patel A, White R, Allen M, Cress R. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control. 2009;20(6):1001-1010.
17. National Cancer Institute: Surveillance, Epidemiology and End Results Program. Site recode ICD-O-3/WHO 2008 definition. https://seer.cancer.gov/siterecode/icdo3_dwhoheme/. Published 2008. Accessed March 16, 2017.
18. Washington State Cancer Registry. Cancer Codes Used in Reports. https://fortress. wa.gov/doh/wscr/WSCR/CancerCode.mvc/CancerCode. Data updated, January 2016; report updated, March 2016. Accessed March 16, 2017.
19. National Cancer Institute: Surveillance, Epidemiology and End Results Program. ICD-O-3 SEER Site/Histology Validation List. https://seer.cancer.gov/icd-o-3/. Published 2012, updated September 2015. Accessed March 16, 2017.
20. American Joint Committee on Cancer. What is Cancer Staging? https://cancerstaging.org/references-tools/Pages/What-is-Cancer-Staging.aspx. Published 2010. Accessed March 16, 2017.
21. Yang J S, Harrati A, Clarke C, Keegan T, Gomez S. Cancer Prevention Institute of California. Developing an area based socioeconomic measures from American Community Survey data. http://www.cpic.org/files/PDF/Research_Files/Reports/CPIC_ACS_SES_Index_Documentation_3-102014.pdf. Published March 10, 2014. Accessed March 16, 2017.
22. Basu A, Rathouz P. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6(1):93-109.
23. Stata Statistical Software [computer program]. Version 13 College Station, TX: StataCorp LP. 2013.
24. National Center for Health Statistics. Health, United States, 2013 – with a special feature on prescription drugs. https://www.cdc.gov/nchs/data/hus/hus13.pdf. Updated May 2014. Accessed March 16, 2017.
25. Goyal R, Wheeler S, Kohler R, et al. Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014;75(4):231-238.
26. Hassett M, O’Malley A, Pakes J, Newhouse J, Earle C. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98(16):1108-1117.
27. Subramanian S. Impact of Medicaid copayments on patients with cancer: lessons for Medicaid expansion under health reform. Med Care. 2011;49(9):842-847.
28. Coyle Y, Miller A, Paulson R. Model for the cost-efficient delivery of continuous quality cancer care: a hospital and private-practice collaboration. Proc (Bayl Univ Med Cent). 2013;26(2):95-99.
29. Agency for Healthcare Research and Quality. 2011 National Healthcare Disparities Report. https://archive.ahrq.gov/research/findings/nhqrdr/nhdr11/index.html. Last reviewed October 2014. Accessed March 16, 2017.
30. Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Emergency Department Sample (NEDS) 2010. https://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2010.jsp. Issued November 2012, updated November 2015. Accessed March 16, 2017.
31. Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Emergency Department Sample (NEDS) 2013. https://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2013.jsp. Published November 2015. Accessed March 16, 2017.
32. Weiss AJ, Wier LM, Stocks C, Blanchard J. Overview of emergency department visits in the United States, 2011. Statistical Brief #174. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb174-Emergency-Department-Visits-Overview.pdf. Published June 2014. Accessed March 16, 2017.
33. Washington R, Andrews R, Mutter, R. Emergency department visits for adults with diabetes, 2010. Statistical Brief #167. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb167.jsp. Published November 2013. Accessed March 16, 2017.
34. Penberthy L, Petkov V, McClish D, et al. The value of billing data from oncology practice to supplement treatment information for cancer surveillance. Journal of registry management. 2014;41(2):57-64.
35. National Center for Health Statistics. Health, United States, 2014 – with a special feature on adults aged 55-64. https://www.cdc.gov/nchs/data/hus/hus14.pdf. Published May 2015. Accessed March 16, 2017.
1. American Cancer Society. Facts & Figures 2017. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. Published 2017. Accessed March 16, 2017.
2. Levit L, Balogh E, Nass S, Ganz PA. Delivering high-quality cancer care: charting a new course for a system in crisis. https://www.nap.edu/read/18359/chapter/1. Published 2013. Accessed March 16, 2017.
3. Readmissions Reduction Program (HRRP). CMS website. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Updated April 8, 2016. Accessed March 16, 2017.
4. Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Clin Oncol. 2007;3(2):79-86.
5. Guadagnolo B, Dohan D, Raich P. Metrics for evaluating patient navigation during cancer diagnosis and treatment: crafting a policy-relevant research agenda for patient navigation in cancer care. Cancer. 2011;117(15 Suppl):3565-3574.
6. Medicare Patient Access to Cancer Treatment Act of 2013, H.R.2869, 113th Cong.(2013). https://www.congress.gov/bill/113th-congress/house-bill/2869/text?format=txt. Introduced July 31, 2013; latest action, referred to the Subcommittee on Health, August 2, 2013. Accessed March 16, 2017.
7. Smulowitz P, Honigman L, Landon B. A novel approach to identifying targets for cost reduction in the emergency department. Ann Emerg Med. 2013;61(3):293-300.
8. Agrawal S, Conway P. Integrating emergency care into a patient- and outcome-centered health care system. Ann Emerg Med. 2013;61(3):301-302.
9. Swenson K, Rose M, Ritz L, Murray CL, Adlis S. Recognition and evaluation of oncology-related symptoms in the emergency department. Ann Emerg Med.1995;26(1):12-17.
10. Lash R, Bell J, Reed S, et al. A systematic review of emergency department use among cancer patients. Cancer Nurs. 2017;40(2):135-144.
11. Sanoff H, Carpenter W, Freburger J, et al. Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: a population-based analysis. Cancer. 2012;118(17):4309-4320.
12. Hansen D, Fox J, Gross C, Bruun J. Hospital readmissions and emergency department visits following laparoscopic and open colon resection for cancer. Dis Colon Rectum. 2013;56(9):1053-1061.
13. Office of Statewide Health Planning and Development. Hospital Data Products. http://www.oshpd.ca.gov/HID/DataFlow/HospData.html. Last updated September 6, 2016. Accessed March 16, 2017.
14. California Cancer Registry. Overview. http://www.ccrcal.org/Inside_CCR/About_Us.shtml. Published 2009. Accessed April 15, 2015.
15. Patel M, Ma Y, Mitchell B, Rhoads K. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
16. Parikh-Patel A, White R, Allen M, Cress R. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control. 2009;20(6):1001-1010.
17. National Cancer Institute: Surveillance, Epidemiology and End Results Program. Site recode ICD-O-3/WHO 2008 definition. https://seer.cancer.gov/siterecode/icdo3_dwhoheme/. Published 2008. Accessed March 16, 2017.
18. Washington State Cancer Registry. Cancer Codes Used in Reports. https://fortress. wa.gov/doh/wscr/WSCR/CancerCode.mvc/CancerCode. Data updated, January 2016; report updated, March 2016. Accessed March 16, 2017.
19. National Cancer Institute: Surveillance, Epidemiology and End Results Program. ICD-O-3 SEER Site/Histology Validation List. https://seer.cancer.gov/icd-o-3/. Published 2012, updated September 2015. Accessed March 16, 2017.
20. American Joint Committee on Cancer. What is Cancer Staging? https://cancerstaging.org/references-tools/Pages/What-is-Cancer-Staging.aspx. Published 2010. Accessed March 16, 2017.
21. Yang J S, Harrati A, Clarke C, Keegan T, Gomez S. Cancer Prevention Institute of California. Developing an area based socioeconomic measures from American Community Survey data. http://www.cpic.org/files/PDF/Research_Files/Reports/CPIC_ACS_SES_Index_Documentation_3-102014.pdf. Published March 10, 2014. Accessed March 16, 2017.
22. Basu A, Rathouz P. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6(1):93-109.
23. Stata Statistical Software [computer program]. Version 13 College Station, TX: StataCorp LP. 2013.
24. National Center for Health Statistics. Health, United States, 2013 – with a special feature on prescription drugs. https://www.cdc.gov/nchs/data/hus/hus13.pdf. Updated May 2014. Accessed March 16, 2017.
25. Goyal R, Wheeler S, Kohler R, et al. Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014;75(4):231-238.
26. Hassett M, O’Malley A, Pakes J, Newhouse J, Earle C. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98(16):1108-1117.
27. Subramanian S. Impact of Medicaid copayments on patients with cancer: lessons for Medicaid expansion under health reform. Med Care. 2011;49(9):842-847.
28. Coyle Y, Miller A, Paulson R. Model for the cost-efficient delivery of continuous quality cancer care: a hospital and private-practice collaboration. Proc (Bayl Univ Med Cent). 2013;26(2):95-99.
29. Agency for Healthcare Research and Quality. 2011 National Healthcare Disparities Report. https://archive.ahrq.gov/research/findings/nhqrdr/nhdr11/index.html. Last reviewed October 2014. Accessed March 16, 2017.
30. Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Emergency Department Sample (NEDS) 2010. https://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2010.jsp. Issued November 2012, updated November 2015. Accessed March 16, 2017.
31. Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Emergency Department Sample (NEDS) 2013. https://www.hcup-us.ahrq.gov/db/nation/neds/NEDS_Introduction_2013.jsp. Published November 2015. Accessed March 16, 2017.
32. Weiss AJ, Wier LM, Stocks C, Blanchard J. Overview of emergency department visits in the United States, 2011. Statistical Brief #174. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb174-Emergency-Department-Visits-Overview.pdf. Published June 2014. Accessed March 16, 2017.
33. Washington R, Andrews R, Mutter, R. Emergency department visits for adults with diabetes, 2010. Statistical Brief #167. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb167.jsp. Published November 2013. Accessed March 16, 2017.
34. Penberthy L, Petkov V, McClish D, et al. The value of billing data from oncology practice to supplement treatment information for cancer surveillance. Journal of registry management. 2014;41(2):57-64.
35. National Center for Health Statistics. Health, United States, 2014 – with a special feature on adults aged 55-64. https://www.cdc.gov/nchs/data/hus/hus14.pdf. Published May 2015. Accessed March 16, 2017.