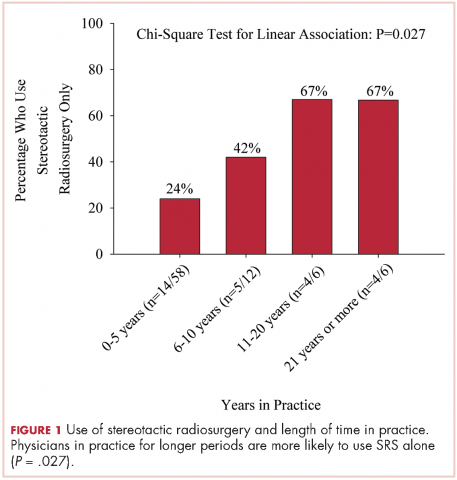
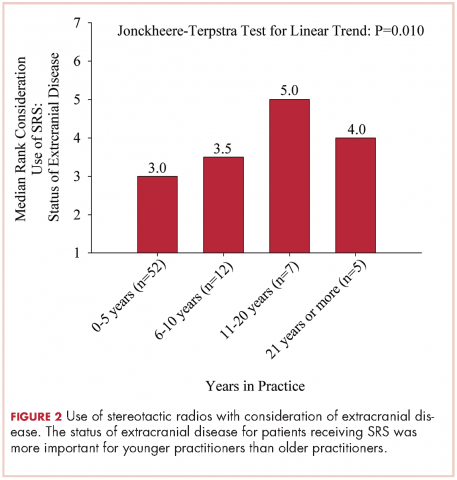
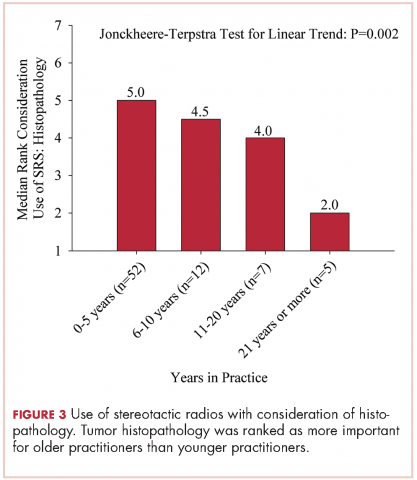
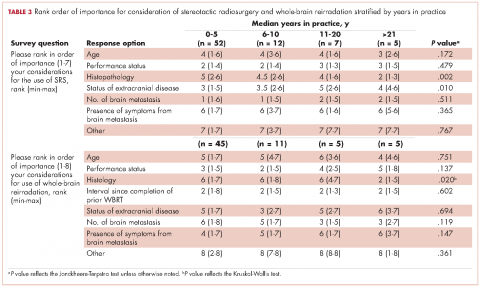
User login
Research and Reviews for the Practicing Oncologist
Prevention and treatment options for mTOR inhibitor-associated stomatitis
Mammalian target of rapamycin (mTOR), a serine–threonine protein kinase, operates in the phosphoinositide 3-kinase (PI3K)–protein kinase B (AKT)–mTOR signal transduction pathway regulating both normal and cancer cellular processes, including cell growth, proliferation, motility, survival, and protein and lipid synthesis.1 Genetic alterations affecting this pathway, including mutations in receptor tyrosine kinases PI3K and AKT, occur frequently in human cancers,2 supporting the rationale to develop drugs that target pathway components, such as mTOR inhibitors.
Two mTOR inhibitors are currently approved by the US Food and Drug Administration for cancer treatment: temsirolimus, for advanced renal cell carcinoma (RCC; approved 2007)3 and everolimus, for advanced RCC (approved 2009), advanced pancreatic neuroendocrine tumors (pNET; approved 2011), and hormone receptor-positive (HR-positive), human epidermal growth factor receptor-2 (HER2)-negative advanced breast cancer (approved 2012).4 Another mTOR inhibitor, sirolimus, is approved for use as an immunosuppressive agent and prophylactic against organ rejection after kidney transplant.5
Stomatitis, inflammation of the oral mucosa with contributing factors of genetic predisposition, nutritional deficiencies, infections, and immunological or hematologic dysfunction,6 occurs frequently as a side effect associated with mTOR inhibitor treatment.7-9 Left untreated or managed unsatisfactorily, mTOR inhibitor-associated stomatitis (mIAS) may cause patients discomfort and trouble with maintaining adequate nutritional intake and proper oral hygiene, as well as strict adherence to cancer treatment. It is therefore important for health care providers of cancer patients receiving mTOR inhibitor treatment to be knowledgeable about this side effect. The purpose of the present systematic review of published literature is to provide a better understanding of the differential diagnosis of mIAS, the pathophysiology of mIAS, preventive strategies for patients initiating mTOR inhibitor treatment, and treatment options available to manage mIAS.
Method
The PubMed database was searched with the terms mTOR inhibitor and stomatitis (no date restriction); 79 articles were retrieved, and all abstracts were reviewed to select those relevant to the aims of this review article. To understand future directions for management and prevention of mIAS, a search of clinicaltrials.gov was performed with the terms temsirolimus everolimus stomatitis yielding 12 clinical trials, of which 4 were excluded: 1 trial was terminated due to slow accrual, the status of 1 trial had not been verified in >2 years, and 2 studies focused on efficacy outcomes. A search of the American Society of Clinical Oncology (ASCO) meeting abstracts database was performed to assess the availability of clinical trial data; the search was limited to 2011-2016 and terms were stomatitis in the title and mTOR in the abstract or title. Seven abstracts were retrieved; 2 discussed stomatitis prevention (1 as a “trial-in-progress” and 1 presented results of the trial); the other 5 abstracts presented meta-analyses or reviews of previous clinical studies to assess the risk, incidence, management, and resolution of mIAS.
Review findings
Incidence of mIAS in patients treated for cancer
Two recent meta-analyses quantified the rate of mIAS in patients receiving mTOR inhibitors. Shameem and colleagues10 identified 9 randomized studies of everolimus (8 phase 3, 1 phase 2) and 2 of temsirolimus (1 each phase 2 and 3) involving a total of 4752 patients with a variety of tumor types including angiomyolipoma, breast, gastric, giant cell astrocytoma, pNET, and RCC. Patients received everolimus monotherapy (n = 1,075) or in combination with exemestane (n = 485), tamoxifen (n = 54), letrozole (n = 137), or octreotide (n = 216). Temsirolimus was administered as monotherapy (n = 208) or in combination with interferon
(n = 210) or letrozole (n = 550). The incidence of all-grade stomatitis in the 11 studies ranged from 11%-63%, and the overall incidence of any grade stomatitis was 33.5% (95% confidence interval [CI], 21.9%-47.6%). The concurrent use of a second agent may have confounded these findings because, for example, stomatitis has been reported in pooled analyses and in postmarketing experience with letrozole.11
Rugo and colleagues12 evaluated the incidence of stomatitis in 1455 patients participating in 5 phase 3 randomized clinical trials of everolimus in breast cancer, carcinoid tumor, pNET, and RCC. Patients received everolimus monotherapy
(n = 478) or in combination with exemestane (n = 482), trastuzumab plus vinorelbine (n = 280), or octreotide
(n = 215). The incidence of stomatitis in patients receiving everolimus was 59%-71%, compared with 19%-29% in 1,071 patients of the comparator arms (placebo, and placebo–trastuzumab–vinorelbine). The overall incidence of any grade stomatitis was 67%; most events were mild (grade 1/2); 9% of stomatitis events were moderate to severe (grade 3/4).
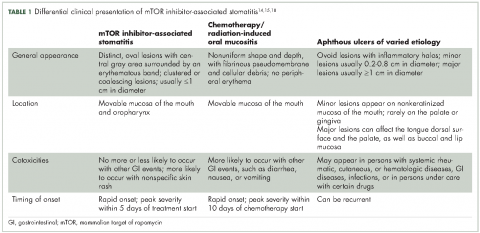
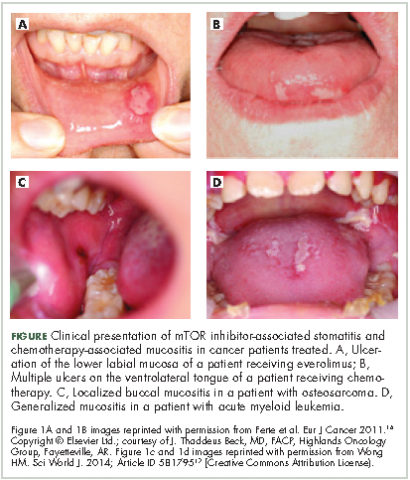
Differential clinical presentation of mIAS and severity
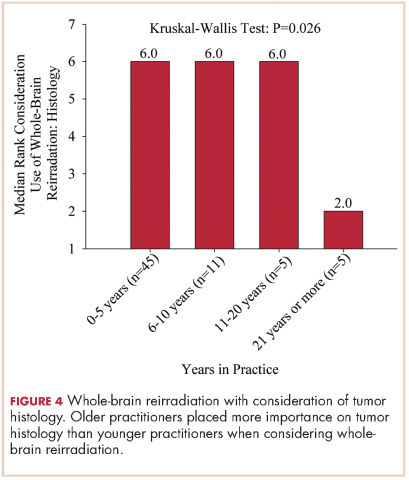
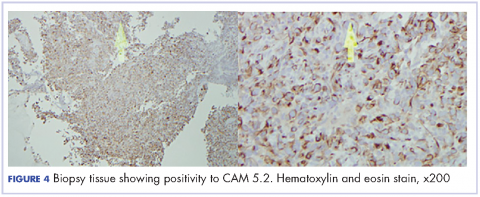
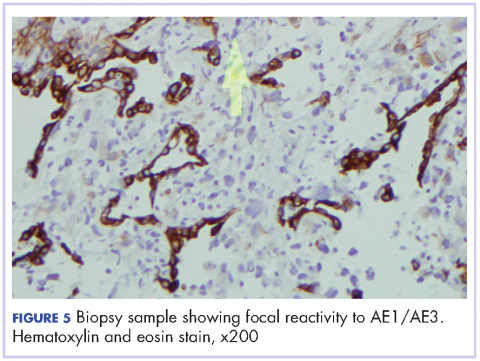
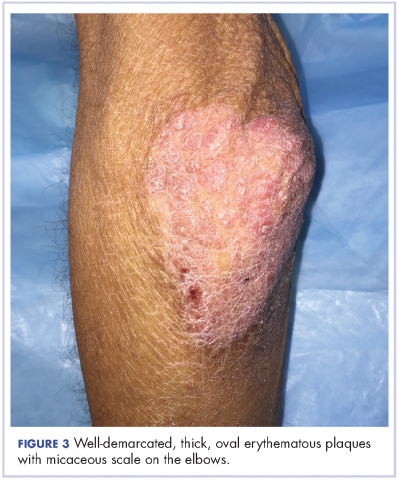
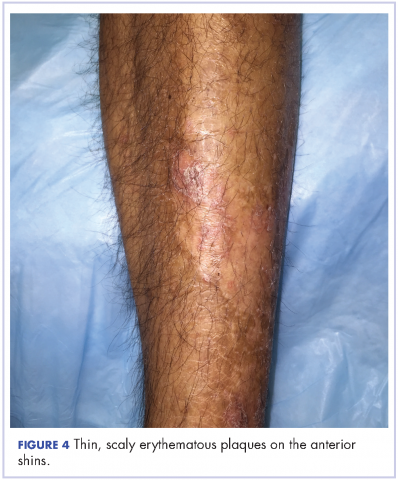
Oral mucositis is a common significant adverse event (AE) that occurs in patients with cancer who receive standard chemotherapy regimens and/or radiation therapy,13 so it is important to recognize that the clinical presentation of mIAS differs from that of oral mucositis (Table 1, Figure 14,15).16 mIAS shares some similarities with aphthous ulcers (also referred to as canker sores), a common oral condition with varied causes related to systemic disorders, gastrointestinal disorders, and infections, among others .17 In general, mIAS ulcers develop with a median onset of 10 days (range, 4-25 days) after initiation of mTOR inhibitor treatment and resolve in about 1-3 weeks after dose interruption/reduction of everolimus.16,18,19 mIAS ulcers appear as distinct, oval lesions with a central gray area surrounded by peripheral erythema. They are usually localized to the movable mucosa of the mouth and oropharynx. Although mIAS lesions are usually small, they are quite painful and may cluster.
Differential diagnosis of mIAS should be made based on physical examination and medical history, with consideration given to appearance of lesions (number, size, and location), current infection status, and current medications. Specific diagnostic testing should be conducted to confirm a coexisting or alternative cause of oral lesions.17
Although there are many different scales for grading mIAS severity, the most commonly used are the National Cancer Institute Common Terminology Criteria for Adverse Events (based on patient function, symptoms, and intervention needs) and the World Health Organization oral mucositis scales (based on symptoms, clinical presentation, and interference with patient function).20-22 These scales distinguish between mild lesions (grade 1/2) and moderate to severe lesions (grade 3/4) that cause significant pain or interfere with oral intake.
Pathophysiology of mIAS
The pathophysiology mIAS is incompletely understood. The ubiquitous role of the PI3K-AKT-mTOR pathway in regulating broad cellular functions suggests that mTOR inhibition is likely to have wide-ranging effects on many biological processes. It is not known whether disruption of one or more processes – or upsetting the balance of mTOR activities – underlies the formation of mIAS.
Differences between mIAS and oral mucositis, including clinical presentation and concomitant toxicities,16,23 suggest that the two types of oral lesions are fundamentally distinct. This distinction is supported by animal studies in which mTOR inhibition was found to almost completely prevent the appearance of oral mucositis in irradiated mice. The protective effect of mTOR inhibition is mediated through suppression of oxidative stress generated by radiation therapy.24
Although mIAS and recurrent aphthous ulcers share some similarities, it is not clear whether they also share a common pathophysiology. Recent studies suggest that patients with recurrent aphthous ulcers have immune dysfunction that leads to excessive immune response to normally innocuous substrates in the oral mucosa.25 mTOR inhibition can have proinflammatory activity by promoting autophagy, a process that stimulates antigen presentation and activation of T cells that produce proinflammatory cytokines.26 It is interesting to note that the incidence of stomatitis in patients receiving sirolimus after kidney transplant is relatively low, 3%-20%.5 Sirolimus is administered in combination with other immunosuppressants, namely cyclosporine and corticosteroids, so it suggests that concomitant use of a steroid-based regimen may have a preventive or therapeutic effect. However, posttransplant sirolimus is typically administered at relatively low doses, which might account in part for the lower incidence of mIAS observed. Ongoing clinical studies of steroid-based mouthwashes in patients receiving everolimus should shed light on this.
Other study findings have shown that inhibition of the PI3K-AKT-mTOR signaling pathway affects skin wound healing,27,28 which raises the possibility that mIAS may stem from a diminished capacity to repair physical injuries to the oral mucosa. More research is needed to elucidate the pathophysiology of mIAS.
Preventive measures for patients initiating mTOR inhibitor treatment
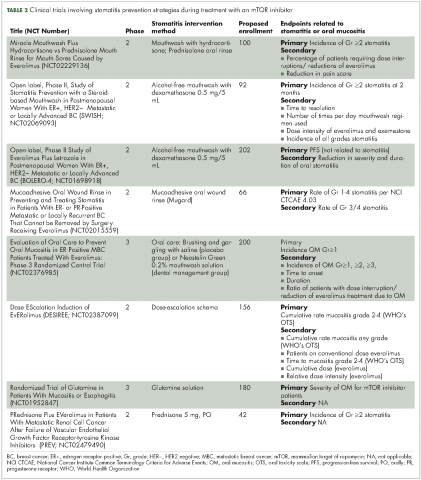
There are preventive measures for mIAS that have not yet been backed up with evidence-based findings, although several clinical studies that are underway aim to address this gap (Table 2). The hypotheses about the pathophysiology of mIAS suggest that certain preventive and therapeutic interventions might be effective against mIAS. For example, two studies are evaluating the use of steroid-based mouthwashes in patients receiving everolimus, based on the hypothesis that mIAS may arise from an inflammatory process; another study will evaluate a mucoadhesive oral wound rinse, based on the hypothesis that wound protection might prevent mIAS. Glutamine suspension is also under evaluation as it is understood to have wound-preventative and tissue-repair properties, and another study is focused on dentist-guided oral management. Recent results of one of these trials (SWISH),29 reported that preventative care with a dexamethasone mouthwash 3-4 times a day significantly minimized or prevented the incidence of all grades of stomatitis in women receiving everolimus plus exemestane therapy for advanced/metastatic breast cancer compared with the incidence of stomatitis observed in a previously published phase 3 trial (BOLERO-2)30,31 of everolimus plus exemestane in the same patient population. Results from several other studies are expected soon.
Current approaches to mIAS prevention are based largely on clinical experience with chemotherapy- or radiation-induced oral mucositis (Table 3).13,32 Preventive measures use three main strategies: establish and maintain good routine oral care; modify diet to avoid potentially damaging foods; and improve patient education about mIAS. In regard to patient education, numerous studies have reported that establishing an institutional protocol for oral care helped reduce the incidence of chemotherapy- or radiation-induced oral mucositis.33-40 An ongoing clinical study that will randomize patients to receive oral care education from oral surgeons or instruction on brushing only (NCT02376985) is investigating whether having an oral care protocol holds for patients with mIAS. The hypothesis is that focusing attention on oral care and educating patients to recognize the onset of mIAS facilitates early detection and promotes early intervention.
Therapeutic measures for patients with mIAS
Therapeutic measures for mIAS are based largely on experience with chemotherapy- or radiation-induced oral mucositis or recurrent aphthous ulcers (Table 3) and vary in part by the severity of lesions. Treatments for mild mIAS aim to ameliorate symptoms (eg, topical analgesics for pain), protect the oral mucosa (eg, mucoadhesive gels or viscous solutions that coat the oral cavity), prevent potential sequelae (eg, prophylactic antibiotics to avoid secondary infections), and reduce inflammation/immune response (eg, steroid-based mouth rinses, topical steroids, or topical anti-inflammatory agents). Treatments for mild mIAS are generally local rather than systemic.
Treatment options for moderate to severe mIAS include systemic approaches that generally carry increased risk of AEs and, therefore, should be reserved for patients with multiple lesions, uncontrolled or poorly controlled pain, or greatly diminished oral food intake (Table 3).41 When mIAS cannot be controlled with the interventions described, the dose of the mTOR inhibitor can be reduced with the recognition that dose modification of anticancer therapy may affect disease outcomes.29 The experience of reduction or interruption of treatment with everolimus in the BOLERO-2 trial as a strategy for management of AEs is discussed in a recent review.29 Prescribing information for both temsirolimus and everolimus specify that grade 3 AEs be treated with temporary dose interruption, and with resolution (temsirolimus: grade ≤2; everolimus: grade ≤1), treatment may be resumed at lower doses (temsirolimus: reduce by 5 mg/week; no lower than 15 mg/week; everolimus: reduce by half the previously administered dose).3,4 Grade 4 events due to treatment with temsirolimus may also be treated with dose interruption/reduction; the everolimus prescribing information advises treatment discontinuation for grade 4 stomatitis.
Summary and discussion
mTOR inhibitors can be effective treatments for patients with advanced cancer, specifically for advanced RCC, advanced pNET, and HR+, HER2-negative advanced breast cancer. Although mIAS may occur in many patients, it is usually grade 1 or 2 in severity. mIAS has an early onset, usually within the first 2 weeks of treatment16,19,42 and a relatively rapid resolution, usually within 3 weeks.16,19 Thus, most cases of mIAS are self-limiting.
The relatively recent emergence of mIAS poses short-term challenges regarding diagnosis, assessment, prevention, and treatment. Several clinical studies are underway to evaluate a range of interventions for their preventive and therapeutic efficacy in mIAS. Furthermore, our growing understanding of the underlying pathophysiology of mIAS can guide how mIAS is managed and what interventions patients receive.
Although mIAS is believed to differ from chemotherapy- or radiation-induced oral mucositis and aphthous ulcers, much can be learned from the treatment of both of these. Several strategies have been proposed to limit the occurrence of mIAS (Table 3). First, establish an oral care protocol. Educate patients who are initiating treatment with an mTOR inhibitor on implementation of the oral care protocol and emphasize adherence. Second, educate patients on the symptoms and timing of mIAS. Patients may hesitate to report mild symptoms or assume they are innocuous, so be clear that reporting all symptoms is important to allow timely clinical evaluation. Early recognition of mIAS facilitates early intervention and can prevent dose modification and interruption. Third, implement the preventive and treatment measures described. Many of the preventive measures can be incorporated into an oral care protocol.
The advent of mTOR inhibitors has clinically benefited many patients with cancer. Although side effects, like mIAS, may develop during treatment, they should not be considered insurmountable. Through education, vigilance, and aggressive management, health care providers and patients can work together to help patients maintain their quality of life while continuing to optimally address their disease.
Acknowledgment
The authors thank Anna Lau, PhD, and Patricia Segarini, PhD, of Percolation Communications LLC, for their editorial assistance. Funding for manuscript development was provided by Novartis Pharmaceuticals Corp.
1. Lauring J, Park BH, Wolff AC. The phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target in breast cancer. J Natl Compr Canc Netw. 2013;11:670-678.
2. Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140-156.
3. Torisel (temsirolimus) [prescribing information]. Philadelphia, PA: Wyeth Pharmaceuticals; 2014.
4. Afinitor (everolimus) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
5. Rapamune (sirolimus) [prescribing information]. Philadelphia, PA: Wyeth Pharmaceuticals; 2012.
6. Peterson DE, Boers-Doets CB, Bensadoun RJ, Herrstedt J, ESMO Guidelines Committee. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2015;26 Suppl 5:v139-151.
7. Hidalgo M, Buckner JC, Erlichman C, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12:5755-5763.
8. Martins F, de Oliveira MA, Wang Q, et al. A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol. 2013;49:293-298.
9. O’Donnell A, Faivre S, Burris HA, 3rd, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588-1595.
10. Shameem R, Lacouture M, Wu S. Incidence and risk of high-grade stomatitis with mTOR inhibitors in cancer patients. Cancer Invest. 2015;33:70-77.
11. Femara (letrozole) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
12. Rugo HS, Hortobagyi GN, Yao J, et al. Meta-analysis of stomatitis in clinical studies of everolimus: incidence and relationship with efficacy. Ann Oncol. 2016;27:519-525.
13. Keefe DM, Schubert MM, Elting LS, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109:820-831.
14. Sonis S, Treister N, Chawla S, Demetri G, Haluska F. Preliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patients. Cancer. 2010;116:210-215.
15. Scully C. Clinical practice. Aphthous ulceration. N Engl J Med. 2006;355:165-172.
16. Ferte C, Paci A, Zizi M, et al. Natural history, management and pharmacokinetics of everolimus-induced-oral ulcers: insights into compliance issues. Eur J Cancer. 2011;47:2249-2255.
17. Wong HM. Oral complications and management strategies for patients undergoing cancer therapy ScienceWorldJournal. 2014;581795.
18. de Oliveira MA, Martins EMF, Wang Q, et al. Clinical presentation and management of mTOR inhibitor-associated stomatitis. Oral Oncol. 2011;47:998-1003.
19. Rugo HS, Pritchard KI, Gnant M, et al. Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: insights from BOLERO-2. Ann Oncol. 2014;25:808-815.
20. National Cancer Institute. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed February 13, 2017.
21. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed February 13, 2017.
22. World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: World Health Organization (WHO Offset Publication No. 48); 1979.
23. Epstein JB, Thariat J, Bensadoun RJ, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012;62:400-422.
24. Iglesias-Bartolome R, Patel V, Cotrim A, et al. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell. 2012;11:401-414.
25. Lewkowicz N, Lewkowicz P, Dzitko K, et al. Dysfunction of CD4+CD25high T regulatory cells in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2008;37:454-461.
26. Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767-777.
27. Jin Y, Tymen SD, Chen D, et al. MicroRNA-99 family targets AKT/mTOR signaling pathway in dermal wound healing. PLoS One. 2013;8:e64434.
28. Rosselli-Murai LK, Almeida LO, Zagni C, et al. Periostin responds to mechanical stress and tension by activating the MTOR signaling pathway. PLoS One. 2013;8:e83580.
29. Rugo HS. Dosing and safety implications for oncologists when administering everolimus to patients with hormone receptor-positive breast cancer. Clin Breast Cancer. 2016;16:18-22.
30. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
31. Yardley DA, Noguchi S, Pritchard KI, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870-884.
32. Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100:2026-2046.
33. Borowski B, Benhamou E, Pico JL, Laplanche A, Margainaud JP, Hayat M. Prevention of oral mucositis in patients treated with high-dose chemotherapy and bone marrow transplantation: a randomised controlled trial comparing two protocols of dental care. Eur J Cancer B Oral Oncol. 1994;30B:93-97.
34. Cheng KK, Molassiotis A, Chang AM, Wai WC, Cheung SS. Evaluation of an oral care protocol intervention in the prevention of chemotherapy-induced oral mucositis in paediatric cancer patients. Eur J Cancer. 2001;37:2056-2063.
35. Dudjak LA. Mouth care for mucositis due to radiation therapy. Cancer Nurs. 1987;10:131-140.
36. Graham KM, Pecoraro DA, Ventura M, Meyer CC. Reducing the incidence of stomatitis using a quality assessment and improvement approach. Cancer Nurs. 1993;16:117-122.
37. Kenny SA. Effect of two oral care protocols on the incidence of stomatitis in hematology patients. Cancer Nurs. 1990;13:345-353.
38. Larson PJ, Miaskowski C, MacPhail L, et al. The PRO-SELF Mouth Aware program: an effective approach for reducing chemotherapy-induced mucositis. Cancer Nurs. 1998;21:263-268.
39. Levy-Polack MP, Sebelli P, Polack NL. Incidence of oral complications and application of a preventive protocol in children with acute leukemia. Spec Care Dentist. 1998;18:189-193.
40. Yeager KA, Webster J, Crain M, Kasow J, McGuire DB. Implementation of an oral care standard for leukemia and transplantation patients. Cancer Nurs. 2000;23:40-47; quiz 47-48.
41. Pilotte AP, Hohos MB, Polson KM, Huftalen TM, Treister N. Managing stomatitis in patients treated with mammalian target of rapamycin inhibitors. Clin J Oncol Nurs. 2011;15:E83-89.
42. Gomez-Fernandez C, Garden BC, Wu S, Feldman DR, Lacouture ME. The risk of skin rash and stomatitis with the mammalian target of rapamycin inhibitor temsirolimus: a systematic review of the literature and meta-analysis. Eur J Cancer. 2012;48:340-346.
43. Bonnaure-Mallet M, Bunetel L, Tricot-Doleux S, Guerin J, Bergeron C, LeGall E. Oral complications during treatment of malignant diseases in childhood: effects of tooth brushing. Eur J Cancer. 1998;34:1588-1591.
44. Chuang P, Langone AJ. Clobetasol ameliorates aphthous ulceration in renal transplant patients on sirolimus. Am J Transplant. 2007;7:714-717.
45. Femiano F, Buonaiuto C, Gombos F, Lanza A, Cirillo N. Pilot study on recurrent aphthous stomatitis (RAS): a randomized placebo-controlled trial for the comparative therapeutic effects of systemic prednisone and systemic montelukast in subjects unresponsive to topical therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:402-407.
Mammalian target of rapamycin (mTOR), a serine–threonine protein kinase, operates in the phosphoinositide 3-kinase (PI3K)–protein kinase B (AKT)–mTOR signal transduction pathway regulating both normal and cancer cellular processes, including cell growth, proliferation, motility, survival, and protein and lipid synthesis.1 Genetic alterations affecting this pathway, including mutations in receptor tyrosine kinases PI3K and AKT, occur frequently in human cancers,2 supporting the rationale to develop drugs that target pathway components, such as mTOR inhibitors.
Two mTOR inhibitors are currently approved by the US Food and Drug Administration for cancer treatment: temsirolimus, for advanced renal cell carcinoma (RCC; approved 2007)3 and everolimus, for advanced RCC (approved 2009), advanced pancreatic neuroendocrine tumors (pNET; approved 2011), and hormone receptor-positive (HR-positive), human epidermal growth factor receptor-2 (HER2)-negative advanced breast cancer (approved 2012).4 Another mTOR inhibitor, sirolimus, is approved for use as an immunosuppressive agent and prophylactic against organ rejection after kidney transplant.5
Stomatitis, inflammation of the oral mucosa with contributing factors of genetic predisposition, nutritional deficiencies, infections, and immunological or hematologic dysfunction,6 occurs frequently as a side effect associated with mTOR inhibitor treatment.7-9 Left untreated or managed unsatisfactorily, mTOR inhibitor-associated stomatitis (mIAS) may cause patients discomfort and trouble with maintaining adequate nutritional intake and proper oral hygiene, as well as strict adherence to cancer treatment. It is therefore important for health care providers of cancer patients receiving mTOR inhibitor treatment to be knowledgeable about this side effect. The purpose of the present systematic review of published literature is to provide a better understanding of the differential diagnosis of mIAS, the pathophysiology of mIAS, preventive strategies for patients initiating mTOR inhibitor treatment, and treatment options available to manage mIAS.
Method
The PubMed database was searched with the terms mTOR inhibitor and stomatitis (no date restriction); 79 articles were retrieved, and all abstracts were reviewed to select those relevant to the aims of this review article. To understand future directions for management and prevention of mIAS, a search of clinicaltrials.gov was performed with the terms temsirolimus everolimus stomatitis yielding 12 clinical trials, of which 4 were excluded: 1 trial was terminated due to slow accrual, the status of 1 trial had not been verified in >2 years, and 2 studies focused on efficacy outcomes. A search of the American Society of Clinical Oncology (ASCO) meeting abstracts database was performed to assess the availability of clinical trial data; the search was limited to 2011-2016 and terms were stomatitis in the title and mTOR in the abstract or title. Seven abstracts were retrieved; 2 discussed stomatitis prevention (1 as a “trial-in-progress” and 1 presented results of the trial); the other 5 abstracts presented meta-analyses or reviews of previous clinical studies to assess the risk, incidence, management, and resolution of mIAS.
Review findings
Incidence of mIAS in patients treated for cancer
Two recent meta-analyses quantified the rate of mIAS in patients receiving mTOR inhibitors. Shameem and colleagues10 identified 9 randomized studies of everolimus (8 phase 3, 1 phase 2) and 2 of temsirolimus (1 each phase 2 and 3) involving a total of 4752 patients with a variety of tumor types including angiomyolipoma, breast, gastric, giant cell astrocytoma, pNET, and RCC. Patients received everolimus monotherapy (n = 1,075) or in combination with exemestane (n = 485), tamoxifen (n = 54), letrozole (n = 137), or octreotide (n = 216). Temsirolimus was administered as monotherapy (n = 208) or in combination with interferon
(n = 210) or letrozole (n = 550). The incidence of all-grade stomatitis in the 11 studies ranged from 11%-63%, and the overall incidence of any grade stomatitis was 33.5% (95% confidence interval [CI], 21.9%-47.6%). The concurrent use of a second agent may have confounded these findings because, for example, stomatitis has been reported in pooled analyses and in postmarketing experience with letrozole.11
Rugo and colleagues12 evaluated the incidence of stomatitis in 1455 patients participating in 5 phase 3 randomized clinical trials of everolimus in breast cancer, carcinoid tumor, pNET, and RCC. Patients received everolimus monotherapy
(n = 478) or in combination with exemestane (n = 482), trastuzumab plus vinorelbine (n = 280), or octreotide
(n = 215). The incidence of stomatitis in patients receiving everolimus was 59%-71%, compared with 19%-29% in 1,071 patients of the comparator arms (placebo, and placebo–trastuzumab–vinorelbine). The overall incidence of any grade stomatitis was 67%; most events were mild (grade 1/2); 9% of stomatitis events were moderate to severe (grade 3/4).
Differential clinical presentation of mIAS and severity
Oral mucositis is a common significant adverse event (AE) that occurs in patients with cancer who receive standard chemotherapy regimens and/or radiation therapy,13 so it is important to recognize that the clinical presentation of mIAS differs from that of oral mucositis (Table 1, Figure 14,15).16 mIAS shares some similarities with aphthous ulcers (also referred to as canker sores), a common oral condition with varied causes related to systemic disorders, gastrointestinal disorders, and infections, among others .17 In general, mIAS ulcers develop with a median onset of 10 days (range, 4-25 days) after initiation of mTOR inhibitor treatment and resolve in about 1-3 weeks after dose interruption/reduction of everolimus.16,18,19 mIAS ulcers appear as distinct, oval lesions with a central gray area surrounded by peripheral erythema. They are usually localized to the movable mucosa of the mouth and oropharynx. Although mIAS lesions are usually small, they are quite painful and may cluster.
Differential diagnosis of mIAS should be made based on physical examination and medical history, with consideration given to appearance of lesions (number, size, and location), current infection status, and current medications. Specific diagnostic testing should be conducted to confirm a coexisting or alternative cause of oral lesions.17
Although there are many different scales for grading mIAS severity, the most commonly used are the National Cancer Institute Common Terminology Criteria for Adverse Events (based on patient function, symptoms, and intervention needs) and the World Health Organization oral mucositis scales (based on symptoms, clinical presentation, and interference with patient function).20-22 These scales distinguish between mild lesions (grade 1/2) and moderate to severe lesions (grade 3/4) that cause significant pain or interfere with oral intake.
Pathophysiology of mIAS
The pathophysiology mIAS is incompletely understood. The ubiquitous role of the PI3K-AKT-mTOR pathway in regulating broad cellular functions suggests that mTOR inhibition is likely to have wide-ranging effects on many biological processes. It is not known whether disruption of one or more processes – or upsetting the balance of mTOR activities – underlies the formation of mIAS.
Differences between mIAS and oral mucositis, including clinical presentation and concomitant toxicities,16,23 suggest that the two types of oral lesions are fundamentally distinct. This distinction is supported by animal studies in which mTOR inhibition was found to almost completely prevent the appearance of oral mucositis in irradiated mice. The protective effect of mTOR inhibition is mediated through suppression of oxidative stress generated by radiation therapy.24
Although mIAS and recurrent aphthous ulcers share some similarities, it is not clear whether they also share a common pathophysiology. Recent studies suggest that patients with recurrent aphthous ulcers have immune dysfunction that leads to excessive immune response to normally innocuous substrates in the oral mucosa.25 mTOR inhibition can have proinflammatory activity by promoting autophagy, a process that stimulates antigen presentation and activation of T cells that produce proinflammatory cytokines.26 It is interesting to note that the incidence of stomatitis in patients receiving sirolimus after kidney transplant is relatively low, 3%-20%.5 Sirolimus is administered in combination with other immunosuppressants, namely cyclosporine and corticosteroids, so it suggests that concomitant use of a steroid-based regimen may have a preventive or therapeutic effect. However, posttransplant sirolimus is typically administered at relatively low doses, which might account in part for the lower incidence of mIAS observed. Ongoing clinical studies of steroid-based mouthwashes in patients receiving everolimus should shed light on this.
Other study findings have shown that inhibition of the PI3K-AKT-mTOR signaling pathway affects skin wound healing,27,28 which raises the possibility that mIAS may stem from a diminished capacity to repair physical injuries to the oral mucosa. More research is needed to elucidate the pathophysiology of mIAS.
Preventive measures for patients initiating mTOR inhibitor treatment
There are preventive measures for mIAS that have not yet been backed up with evidence-based findings, although several clinical studies that are underway aim to address this gap (Table 2). The hypotheses about the pathophysiology of mIAS suggest that certain preventive and therapeutic interventions might be effective against mIAS. For example, two studies are evaluating the use of steroid-based mouthwashes in patients receiving everolimus, based on the hypothesis that mIAS may arise from an inflammatory process; another study will evaluate a mucoadhesive oral wound rinse, based on the hypothesis that wound protection might prevent mIAS. Glutamine suspension is also under evaluation as it is understood to have wound-preventative and tissue-repair properties, and another study is focused on dentist-guided oral management. Recent results of one of these trials (SWISH),29 reported that preventative care with a dexamethasone mouthwash 3-4 times a day significantly minimized or prevented the incidence of all grades of stomatitis in women receiving everolimus plus exemestane therapy for advanced/metastatic breast cancer compared with the incidence of stomatitis observed in a previously published phase 3 trial (BOLERO-2)30,31 of everolimus plus exemestane in the same patient population. Results from several other studies are expected soon.
Current approaches to mIAS prevention are based largely on clinical experience with chemotherapy- or radiation-induced oral mucositis (Table 3).13,32 Preventive measures use three main strategies: establish and maintain good routine oral care; modify diet to avoid potentially damaging foods; and improve patient education about mIAS. In regard to patient education, numerous studies have reported that establishing an institutional protocol for oral care helped reduce the incidence of chemotherapy- or radiation-induced oral mucositis.33-40 An ongoing clinical study that will randomize patients to receive oral care education from oral surgeons or instruction on brushing only (NCT02376985) is investigating whether having an oral care protocol holds for patients with mIAS. The hypothesis is that focusing attention on oral care and educating patients to recognize the onset of mIAS facilitates early detection and promotes early intervention.
Therapeutic measures for patients with mIAS
Therapeutic measures for mIAS are based largely on experience with chemotherapy- or radiation-induced oral mucositis or recurrent aphthous ulcers (Table 3) and vary in part by the severity of lesions. Treatments for mild mIAS aim to ameliorate symptoms (eg, topical analgesics for pain), protect the oral mucosa (eg, mucoadhesive gels or viscous solutions that coat the oral cavity), prevent potential sequelae (eg, prophylactic antibiotics to avoid secondary infections), and reduce inflammation/immune response (eg, steroid-based mouth rinses, topical steroids, or topical anti-inflammatory agents). Treatments for mild mIAS are generally local rather than systemic.
Treatment options for moderate to severe mIAS include systemic approaches that generally carry increased risk of AEs and, therefore, should be reserved for patients with multiple lesions, uncontrolled or poorly controlled pain, or greatly diminished oral food intake (Table 3).41 When mIAS cannot be controlled with the interventions described, the dose of the mTOR inhibitor can be reduced with the recognition that dose modification of anticancer therapy may affect disease outcomes.29 The experience of reduction or interruption of treatment with everolimus in the BOLERO-2 trial as a strategy for management of AEs is discussed in a recent review.29 Prescribing information for both temsirolimus and everolimus specify that grade 3 AEs be treated with temporary dose interruption, and with resolution (temsirolimus: grade ≤2; everolimus: grade ≤1), treatment may be resumed at lower doses (temsirolimus: reduce by 5 mg/week; no lower than 15 mg/week; everolimus: reduce by half the previously administered dose).3,4 Grade 4 events due to treatment with temsirolimus may also be treated with dose interruption/reduction; the everolimus prescribing information advises treatment discontinuation for grade 4 stomatitis.
Summary and discussion
mTOR inhibitors can be effective treatments for patients with advanced cancer, specifically for advanced RCC, advanced pNET, and HR+, HER2-negative advanced breast cancer. Although mIAS may occur in many patients, it is usually grade 1 or 2 in severity. mIAS has an early onset, usually within the first 2 weeks of treatment16,19,42 and a relatively rapid resolution, usually within 3 weeks.16,19 Thus, most cases of mIAS are self-limiting.
The relatively recent emergence of mIAS poses short-term challenges regarding diagnosis, assessment, prevention, and treatment. Several clinical studies are underway to evaluate a range of interventions for their preventive and therapeutic efficacy in mIAS. Furthermore, our growing understanding of the underlying pathophysiology of mIAS can guide how mIAS is managed and what interventions patients receive.
Although mIAS is believed to differ from chemotherapy- or radiation-induced oral mucositis and aphthous ulcers, much can be learned from the treatment of both of these. Several strategies have been proposed to limit the occurrence of mIAS (Table 3). First, establish an oral care protocol. Educate patients who are initiating treatment with an mTOR inhibitor on implementation of the oral care protocol and emphasize adherence. Second, educate patients on the symptoms and timing of mIAS. Patients may hesitate to report mild symptoms or assume they are innocuous, so be clear that reporting all symptoms is important to allow timely clinical evaluation. Early recognition of mIAS facilitates early intervention and can prevent dose modification and interruption. Third, implement the preventive and treatment measures described. Many of the preventive measures can be incorporated into an oral care protocol.
The advent of mTOR inhibitors has clinically benefited many patients with cancer. Although side effects, like mIAS, may develop during treatment, they should not be considered insurmountable. Through education, vigilance, and aggressive management, health care providers and patients can work together to help patients maintain their quality of life while continuing to optimally address their disease.
Acknowledgment
The authors thank Anna Lau, PhD, and Patricia Segarini, PhD, of Percolation Communications LLC, for their editorial assistance. Funding for manuscript development was provided by Novartis Pharmaceuticals Corp.
Mammalian target of rapamycin (mTOR), a serine–threonine protein kinase, operates in the phosphoinositide 3-kinase (PI3K)–protein kinase B (AKT)–mTOR signal transduction pathway regulating both normal and cancer cellular processes, including cell growth, proliferation, motility, survival, and protein and lipid synthesis.1 Genetic alterations affecting this pathway, including mutations in receptor tyrosine kinases PI3K and AKT, occur frequently in human cancers,2 supporting the rationale to develop drugs that target pathway components, such as mTOR inhibitors.
Two mTOR inhibitors are currently approved by the US Food and Drug Administration for cancer treatment: temsirolimus, for advanced renal cell carcinoma (RCC; approved 2007)3 and everolimus, for advanced RCC (approved 2009), advanced pancreatic neuroendocrine tumors (pNET; approved 2011), and hormone receptor-positive (HR-positive), human epidermal growth factor receptor-2 (HER2)-negative advanced breast cancer (approved 2012).4 Another mTOR inhibitor, sirolimus, is approved for use as an immunosuppressive agent and prophylactic against organ rejection after kidney transplant.5
Stomatitis, inflammation of the oral mucosa with contributing factors of genetic predisposition, nutritional deficiencies, infections, and immunological or hematologic dysfunction,6 occurs frequently as a side effect associated with mTOR inhibitor treatment.7-9 Left untreated or managed unsatisfactorily, mTOR inhibitor-associated stomatitis (mIAS) may cause patients discomfort and trouble with maintaining adequate nutritional intake and proper oral hygiene, as well as strict adherence to cancer treatment. It is therefore important for health care providers of cancer patients receiving mTOR inhibitor treatment to be knowledgeable about this side effect. The purpose of the present systematic review of published literature is to provide a better understanding of the differential diagnosis of mIAS, the pathophysiology of mIAS, preventive strategies for patients initiating mTOR inhibitor treatment, and treatment options available to manage mIAS.
Method
The PubMed database was searched with the terms mTOR inhibitor and stomatitis (no date restriction); 79 articles were retrieved, and all abstracts were reviewed to select those relevant to the aims of this review article. To understand future directions for management and prevention of mIAS, a search of clinicaltrials.gov was performed with the terms temsirolimus everolimus stomatitis yielding 12 clinical trials, of which 4 were excluded: 1 trial was terminated due to slow accrual, the status of 1 trial had not been verified in >2 years, and 2 studies focused on efficacy outcomes. A search of the American Society of Clinical Oncology (ASCO) meeting abstracts database was performed to assess the availability of clinical trial data; the search was limited to 2011-2016 and terms were stomatitis in the title and mTOR in the abstract or title. Seven abstracts were retrieved; 2 discussed stomatitis prevention (1 as a “trial-in-progress” and 1 presented results of the trial); the other 5 abstracts presented meta-analyses or reviews of previous clinical studies to assess the risk, incidence, management, and resolution of mIAS.
Review findings
Incidence of mIAS in patients treated for cancer
Two recent meta-analyses quantified the rate of mIAS in patients receiving mTOR inhibitors. Shameem and colleagues10 identified 9 randomized studies of everolimus (8 phase 3, 1 phase 2) and 2 of temsirolimus (1 each phase 2 and 3) involving a total of 4752 patients with a variety of tumor types including angiomyolipoma, breast, gastric, giant cell astrocytoma, pNET, and RCC. Patients received everolimus monotherapy (n = 1,075) or in combination with exemestane (n = 485), tamoxifen (n = 54), letrozole (n = 137), or octreotide (n = 216). Temsirolimus was administered as monotherapy (n = 208) or in combination with interferon
(n = 210) or letrozole (n = 550). The incidence of all-grade stomatitis in the 11 studies ranged from 11%-63%, and the overall incidence of any grade stomatitis was 33.5% (95% confidence interval [CI], 21.9%-47.6%). The concurrent use of a second agent may have confounded these findings because, for example, stomatitis has been reported in pooled analyses and in postmarketing experience with letrozole.11
Rugo and colleagues12 evaluated the incidence of stomatitis in 1455 patients participating in 5 phase 3 randomized clinical trials of everolimus in breast cancer, carcinoid tumor, pNET, and RCC. Patients received everolimus monotherapy
(n = 478) or in combination with exemestane (n = 482), trastuzumab plus vinorelbine (n = 280), or octreotide
(n = 215). The incidence of stomatitis in patients receiving everolimus was 59%-71%, compared with 19%-29% in 1,071 patients of the comparator arms (placebo, and placebo–trastuzumab–vinorelbine). The overall incidence of any grade stomatitis was 67%; most events were mild (grade 1/2); 9% of stomatitis events were moderate to severe (grade 3/4).
Differential clinical presentation of mIAS and severity
Oral mucositis is a common significant adverse event (AE) that occurs in patients with cancer who receive standard chemotherapy regimens and/or radiation therapy,13 so it is important to recognize that the clinical presentation of mIAS differs from that of oral mucositis (Table 1, Figure 14,15).16 mIAS shares some similarities with aphthous ulcers (also referred to as canker sores), a common oral condition with varied causes related to systemic disorders, gastrointestinal disorders, and infections, among others .17 In general, mIAS ulcers develop with a median onset of 10 days (range, 4-25 days) after initiation of mTOR inhibitor treatment and resolve in about 1-3 weeks after dose interruption/reduction of everolimus.16,18,19 mIAS ulcers appear as distinct, oval lesions with a central gray area surrounded by peripheral erythema. They are usually localized to the movable mucosa of the mouth and oropharynx. Although mIAS lesions are usually small, they are quite painful and may cluster.
Differential diagnosis of mIAS should be made based on physical examination and medical history, with consideration given to appearance of lesions (number, size, and location), current infection status, and current medications. Specific diagnostic testing should be conducted to confirm a coexisting or alternative cause of oral lesions.17
Although there are many different scales for grading mIAS severity, the most commonly used are the National Cancer Institute Common Terminology Criteria for Adverse Events (based on patient function, symptoms, and intervention needs) and the World Health Organization oral mucositis scales (based on symptoms, clinical presentation, and interference with patient function).20-22 These scales distinguish between mild lesions (grade 1/2) and moderate to severe lesions (grade 3/4) that cause significant pain or interfere with oral intake.
Pathophysiology of mIAS
The pathophysiology mIAS is incompletely understood. The ubiquitous role of the PI3K-AKT-mTOR pathway in regulating broad cellular functions suggests that mTOR inhibition is likely to have wide-ranging effects on many biological processes. It is not known whether disruption of one or more processes – or upsetting the balance of mTOR activities – underlies the formation of mIAS.
Differences between mIAS and oral mucositis, including clinical presentation and concomitant toxicities,16,23 suggest that the two types of oral lesions are fundamentally distinct. This distinction is supported by animal studies in which mTOR inhibition was found to almost completely prevent the appearance of oral mucositis in irradiated mice. The protective effect of mTOR inhibition is mediated through suppression of oxidative stress generated by radiation therapy.24
Although mIAS and recurrent aphthous ulcers share some similarities, it is not clear whether they also share a common pathophysiology. Recent studies suggest that patients with recurrent aphthous ulcers have immune dysfunction that leads to excessive immune response to normally innocuous substrates in the oral mucosa.25 mTOR inhibition can have proinflammatory activity by promoting autophagy, a process that stimulates antigen presentation and activation of T cells that produce proinflammatory cytokines.26 It is interesting to note that the incidence of stomatitis in patients receiving sirolimus after kidney transplant is relatively low, 3%-20%.5 Sirolimus is administered in combination with other immunosuppressants, namely cyclosporine and corticosteroids, so it suggests that concomitant use of a steroid-based regimen may have a preventive or therapeutic effect. However, posttransplant sirolimus is typically administered at relatively low doses, which might account in part for the lower incidence of mIAS observed. Ongoing clinical studies of steroid-based mouthwashes in patients receiving everolimus should shed light on this.
Other study findings have shown that inhibition of the PI3K-AKT-mTOR signaling pathway affects skin wound healing,27,28 which raises the possibility that mIAS may stem from a diminished capacity to repair physical injuries to the oral mucosa. More research is needed to elucidate the pathophysiology of mIAS.
Preventive measures for patients initiating mTOR inhibitor treatment
There are preventive measures for mIAS that have not yet been backed up with evidence-based findings, although several clinical studies that are underway aim to address this gap (Table 2). The hypotheses about the pathophysiology of mIAS suggest that certain preventive and therapeutic interventions might be effective against mIAS. For example, two studies are evaluating the use of steroid-based mouthwashes in patients receiving everolimus, based on the hypothesis that mIAS may arise from an inflammatory process; another study will evaluate a mucoadhesive oral wound rinse, based on the hypothesis that wound protection might prevent mIAS. Glutamine suspension is also under evaluation as it is understood to have wound-preventative and tissue-repair properties, and another study is focused on dentist-guided oral management. Recent results of one of these trials (SWISH),29 reported that preventative care with a dexamethasone mouthwash 3-4 times a day significantly minimized or prevented the incidence of all grades of stomatitis in women receiving everolimus plus exemestane therapy for advanced/metastatic breast cancer compared with the incidence of stomatitis observed in a previously published phase 3 trial (BOLERO-2)30,31 of everolimus plus exemestane in the same patient population. Results from several other studies are expected soon.
Current approaches to mIAS prevention are based largely on clinical experience with chemotherapy- or radiation-induced oral mucositis (Table 3).13,32 Preventive measures use three main strategies: establish and maintain good routine oral care; modify diet to avoid potentially damaging foods; and improve patient education about mIAS. In regard to patient education, numerous studies have reported that establishing an institutional protocol for oral care helped reduce the incidence of chemotherapy- or radiation-induced oral mucositis.33-40 An ongoing clinical study that will randomize patients to receive oral care education from oral surgeons or instruction on brushing only (NCT02376985) is investigating whether having an oral care protocol holds for patients with mIAS. The hypothesis is that focusing attention on oral care and educating patients to recognize the onset of mIAS facilitates early detection and promotes early intervention.
Therapeutic measures for patients with mIAS
Therapeutic measures for mIAS are based largely on experience with chemotherapy- or radiation-induced oral mucositis or recurrent aphthous ulcers (Table 3) and vary in part by the severity of lesions. Treatments for mild mIAS aim to ameliorate symptoms (eg, topical analgesics for pain), protect the oral mucosa (eg, mucoadhesive gels or viscous solutions that coat the oral cavity), prevent potential sequelae (eg, prophylactic antibiotics to avoid secondary infections), and reduce inflammation/immune response (eg, steroid-based mouth rinses, topical steroids, or topical anti-inflammatory agents). Treatments for mild mIAS are generally local rather than systemic.
Treatment options for moderate to severe mIAS include systemic approaches that generally carry increased risk of AEs and, therefore, should be reserved for patients with multiple lesions, uncontrolled or poorly controlled pain, or greatly diminished oral food intake (Table 3).41 When mIAS cannot be controlled with the interventions described, the dose of the mTOR inhibitor can be reduced with the recognition that dose modification of anticancer therapy may affect disease outcomes.29 The experience of reduction or interruption of treatment with everolimus in the BOLERO-2 trial as a strategy for management of AEs is discussed in a recent review.29 Prescribing information for both temsirolimus and everolimus specify that grade 3 AEs be treated with temporary dose interruption, and with resolution (temsirolimus: grade ≤2; everolimus: grade ≤1), treatment may be resumed at lower doses (temsirolimus: reduce by 5 mg/week; no lower than 15 mg/week; everolimus: reduce by half the previously administered dose).3,4 Grade 4 events due to treatment with temsirolimus may also be treated with dose interruption/reduction; the everolimus prescribing information advises treatment discontinuation for grade 4 stomatitis.
Summary and discussion
mTOR inhibitors can be effective treatments for patients with advanced cancer, specifically for advanced RCC, advanced pNET, and HR+, HER2-negative advanced breast cancer. Although mIAS may occur in many patients, it is usually grade 1 or 2 in severity. mIAS has an early onset, usually within the first 2 weeks of treatment16,19,42 and a relatively rapid resolution, usually within 3 weeks.16,19 Thus, most cases of mIAS are self-limiting.
The relatively recent emergence of mIAS poses short-term challenges regarding diagnosis, assessment, prevention, and treatment. Several clinical studies are underway to evaluate a range of interventions for their preventive and therapeutic efficacy in mIAS. Furthermore, our growing understanding of the underlying pathophysiology of mIAS can guide how mIAS is managed and what interventions patients receive.
Although mIAS is believed to differ from chemotherapy- or radiation-induced oral mucositis and aphthous ulcers, much can be learned from the treatment of both of these. Several strategies have been proposed to limit the occurrence of mIAS (Table 3). First, establish an oral care protocol. Educate patients who are initiating treatment with an mTOR inhibitor on implementation of the oral care protocol and emphasize adherence. Second, educate patients on the symptoms and timing of mIAS. Patients may hesitate to report mild symptoms or assume they are innocuous, so be clear that reporting all symptoms is important to allow timely clinical evaluation. Early recognition of mIAS facilitates early intervention and can prevent dose modification and interruption. Third, implement the preventive and treatment measures described. Many of the preventive measures can be incorporated into an oral care protocol.
The advent of mTOR inhibitors has clinically benefited many patients with cancer. Although side effects, like mIAS, may develop during treatment, they should not be considered insurmountable. Through education, vigilance, and aggressive management, health care providers and patients can work together to help patients maintain their quality of life while continuing to optimally address their disease.
Acknowledgment
The authors thank Anna Lau, PhD, and Patricia Segarini, PhD, of Percolation Communications LLC, for their editorial assistance. Funding for manuscript development was provided by Novartis Pharmaceuticals Corp.
1. Lauring J, Park BH, Wolff AC. The phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target in breast cancer. J Natl Compr Canc Netw. 2013;11:670-678.
2. Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140-156.
3. Torisel (temsirolimus) [prescribing information]. Philadelphia, PA: Wyeth Pharmaceuticals; 2014.
4. Afinitor (everolimus) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
5. Rapamune (sirolimus) [prescribing information]. Philadelphia, PA: Wyeth Pharmaceuticals; 2012.
6. Peterson DE, Boers-Doets CB, Bensadoun RJ, Herrstedt J, ESMO Guidelines Committee. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2015;26 Suppl 5:v139-151.
7. Hidalgo M, Buckner JC, Erlichman C, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12:5755-5763.
8. Martins F, de Oliveira MA, Wang Q, et al. A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol. 2013;49:293-298.
9. O’Donnell A, Faivre S, Burris HA, 3rd, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588-1595.
10. Shameem R, Lacouture M, Wu S. Incidence and risk of high-grade stomatitis with mTOR inhibitors in cancer patients. Cancer Invest. 2015;33:70-77.
11. Femara (letrozole) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
12. Rugo HS, Hortobagyi GN, Yao J, et al. Meta-analysis of stomatitis in clinical studies of everolimus: incidence and relationship with efficacy. Ann Oncol. 2016;27:519-525.
13. Keefe DM, Schubert MM, Elting LS, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109:820-831.
14. Sonis S, Treister N, Chawla S, Demetri G, Haluska F. Preliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patients. Cancer. 2010;116:210-215.
15. Scully C. Clinical practice. Aphthous ulceration. N Engl J Med. 2006;355:165-172.
16. Ferte C, Paci A, Zizi M, et al. Natural history, management and pharmacokinetics of everolimus-induced-oral ulcers: insights into compliance issues. Eur J Cancer. 2011;47:2249-2255.
17. Wong HM. Oral complications and management strategies for patients undergoing cancer therapy ScienceWorldJournal. 2014;581795.
18. de Oliveira MA, Martins EMF, Wang Q, et al. Clinical presentation and management of mTOR inhibitor-associated stomatitis. Oral Oncol. 2011;47:998-1003.
19. Rugo HS, Pritchard KI, Gnant M, et al. Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: insights from BOLERO-2. Ann Oncol. 2014;25:808-815.
20. National Cancer Institute. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed February 13, 2017.
21. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed February 13, 2017.
22. World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: World Health Organization (WHO Offset Publication No. 48); 1979.
23. Epstein JB, Thariat J, Bensadoun RJ, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012;62:400-422.
24. Iglesias-Bartolome R, Patel V, Cotrim A, et al. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell. 2012;11:401-414.
25. Lewkowicz N, Lewkowicz P, Dzitko K, et al. Dysfunction of CD4+CD25high T regulatory cells in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2008;37:454-461.
26. Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767-777.
27. Jin Y, Tymen SD, Chen D, et al. MicroRNA-99 family targets AKT/mTOR signaling pathway in dermal wound healing. PLoS One. 2013;8:e64434.
28. Rosselli-Murai LK, Almeida LO, Zagni C, et al. Periostin responds to mechanical stress and tension by activating the MTOR signaling pathway. PLoS One. 2013;8:e83580.
29. Rugo HS. Dosing and safety implications for oncologists when administering everolimus to patients with hormone receptor-positive breast cancer. Clin Breast Cancer. 2016;16:18-22.
30. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
31. Yardley DA, Noguchi S, Pritchard KI, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870-884.
32. Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100:2026-2046.
33. Borowski B, Benhamou E, Pico JL, Laplanche A, Margainaud JP, Hayat M. Prevention of oral mucositis in patients treated with high-dose chemotherapy and bone marrow transplantation: a randomised controlled trial comparing two protocols of dental care. Eur J Cancer B Oral Oncol. 1994;30B:93-97.
34. Cheng KK, Molassiotis A, Chang AM, Wai WC, Cheung SS. Evaluation of an oral care protocol intervention in the prevention of chemotherapy-induced oral mucositis in paediatric cancer patients. Eur J Cancer. 2001;37:2056-2063.
35. Dudjak LA. Mouth care for mucositis due to radiation therapy. Cancer Nurs. 1987;10:131-140.
36. Graham KM, Pecoraro DA, Ventura M, Meyer CC. Reducing the incidence of stomatitis using a quality assessment and improvement approach. Cancer Nurs. 1993;16:117-122.
37. Kenny SA. Effect of two oral care protocols on the incidence of stomatitis in hematology patients. Cancer Nurs. 1990;13:345-353.
38. Larson PJ, Miaskowski C, MacPhail L, et al. The PRO-SELF Mouth Aware program: an effective approach for reducing chemotherapy-induced mucositis. Cancer Nurs. 1998;21:263-268.
39. Levy-Polack MP, Sebelli P, Polack NL. Incidence of oral complications and application of a preventive protocol in children with acute leukemia. Spec Care Dentist. 1998;18:189-193.
40. Yeager KA, Webster J, Crain M, Kasow J, McGuire DB. Implementation of an oral care standard for leukemia and transplantation patients. Cancer Nurs. 2000;23:40-47; quiz 47-48.
41. Pilotte AP, Hohos MB, Polson KM, Huftalen TM, Treister N. Managing stomatitis in patients treated with mammalian target of rapamycin inhibitors. Clin J Oncol Nurs. 2011;15:E83-89.
42. Gomez-Fernandez C, Garden BC, Wu S, Feldman DR, Lacouture ME. The risk of skin rash and stomatitis with the mammalian target of rapamycin inhibitor temsirolimus: a systematic review of the literature and meta-analysis. Eur J Cancer. 2012;48:340-346.
43. Bonnaure-Mallet M, Bunetel L, Tricot-Doleux S, Guerin J, Bergeron C, LeGall E. Oral complications during treatment of malignant diseases in childhood: effects of tooth brushing. Eur J Cancer. 1998;34:1588-1591.
44. Chuang P, Langone AJ. Clobetasol ameliorates aphthous ulceration in renal transplant patients on sirolimus. Am J Transplant. 2007;7:714-717.
45. Femiano F, Buonaiuto C, Gombos F, Lanza A, Cirillo N. Pilot study on recurrent aphthous stomatitis (RAS): a randomized placebo-controlled trial for the comparative therapeutic effects of systemic prednisone and systemic montelukast in subjects unresponsive to topical therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:402-407.
1. Lauring J, Park BH, Wolff AC. The phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target in breast cancer. J Natl Compr Canc Netw. 2013;11:670-678.
2. Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140-156.
3. Torisel (temsirolimus) [prescribing information]. Philadelphia, PA: Wyeth Pharmaceuticals; 2014.
4. Afinitor (everolimus) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
5. Rapamune (sirolimus) [prescribing information]. Philadelphia, PA: Wyeth Pharmaceuticals; 2012.
6. Peterson DE, Boers-Doets CB, Bensadoun RJ, Herrstedt J, ESMO Guidelines Committee. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2015;26 Suppl 5:v139-151.
7. Hidalgo M, Buckner JC, Erlichman C, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12:5755-5763.
8. Martins F, de Oliveira MA, Wang Q, et al. A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol. 2013;49:293-298.
9. O’Donnell A, Faivre S, Burris HA, 3rd, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588-1595.
10. Shameem R, Lacouture M, Wu S. Incidence and risk of high-grade stomatitis with mTOR inhibitors in cancer patients. Cancer Invest. 2015;33:70-77.
11. Femara (letrozole) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
12. Rugo HS, Hortobagyi GN, Yao J, et al. Meta-analysis of stomatitis in clinical studies of everolimus: incidence and relationship with efficacy. Ann Oncol. 2016;27:519-525.
13. Keefe DM, Schubert MM, Elting LS, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109:820-831.
14. Sonis S, Treister N, Chawla S, Demetri G, Haluska F. Preliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patients. Cancer. 2010;116:210-215.
15. Scully C. Clinical practice. Aphthous ulceration. N Engl J Med. 2006;355:165-172.
16. Ferte C, Paci A, Zizi M, et al. Natural history, management and pharmacokinetics of everolimus-induced-oral ulcers: insights into compliance issues. Eur J Cancer. 2011;47:2249-2255.
17. Wong HM. Oral complications and management strategies for patients undergoing cancer therapy ScienceWorldJournal. 2014;581795.
18. de Oliveira MA, Martins EMF, Wang Q, et al. Clinical presentation and management of mTOR inhibitor-associated stomatitis. Oral Oncol. 2011;47:998-1003.
19. Rugo HS, Pritchard KI, Gnant M, et al. Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: insights from BOLERO-2. Ann Oncol. 2014;25:808-815.
20. National Cancer Institute. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed February 13, 2017.
21. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed February 13, 2017.
22. World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: World Health Organization (WHO Offset Publication No. 48); 1979.
23. Epstein JB, Thariat J, Bensadoun RJ, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012;62:400-422.
24. Iglesias-Bartolome R, Patel V, Cotrim A, et al. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell. 2012;11:401-414.
25. Lewkowicz N, Lewkowicz P, Dzitko K, et al. Dysfunction of CD4+CD25high T regulatory cells in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2008;37:454-461.
26. Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767-777.
27. Jin Y, Tymen SD, Chen D, et al. MicroRNA-99 family targets AKT/mTOR signaling pathway in dermal wound healing. PLoS One. 2013;8:e64434.
28. Rosselli-Murai LK, Almeida LO, Zagni C, et al. Periostin responds to mechanical stress and tension by activating the MTOR signaling pathway. PLoS One. 2013;8:e83580.
29. Rugo HS. Dosing and safety implications for oncologists when administering everolimus to patients with hormone receptor-positive breast cancer. Clin Breast Cancer. 2016;16:18-22.
30. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
31. Yardley DA, Noguchi S, Pritchard KI, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870-884.
32. Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100:2026-2046.
33. Borowski B, Benhamou E, Pico JL, Laplanche A, Margainaud JP, Hayat M. Prevention of oral mucositis in patients treated with high-dose chemotherapy and bone marrow transplantation: a randomised controlled trial comparing two protocols of dental care. Eur J Cancer B Oral Oncol. 1994;30B:93-97.
34. Cheng KK, Molassiotis A, Chang AM, Wai WC, Cheung SS. Evaluation of an oral care protocol intervention in the prevention of chemotherapy-induced oral mucositis in paediatric cancer patients. Eur J Cancer. 2001;37:2056-2063.
35. Dudjak LA. Mouth care for mucositis due to radiation therapy. Cancer Nurs. 1987;10:131-140.
36. Graham KM, Pecoraro DA, Ventura M, Meyer CC. Reducing the incidence of stomatitis using a quality assessment and improvement approach. Cancer Nurs. 1993;16:117-122.
37. Kenny SA. Effect of two oral care protocols on the incidence of stomatitis in hematology patients. Cancer Nurs. 1990;13:345-353.
38. Larson PJ, Miaskowski C, MacPhail L, et al. The PRO-SELF Mouth Aware program: an effective approach for reducing chemotherapy-induced mucositis. Cancer Nurs. 1998;21:263-268.
39. Levy-Polack MP, Sebelli P, Polack NL. Incidence of oral complications and application of a preventive protocol in children with acute leukemia. Spec Care Dentist. 1998;18:189-193.
40. Yeager KA, Webster J, Crain M, Kasow J, McGuire DB. Implementation of an oral care standard for leukemia and transplantation patients. Cancer Nurs. 2000;23:40-47; quiz 47-48.
41. Pilotte AP, Hohos MB, Polson KM, Huftalen TM, Treister N. Managing stomatitis in patients treated with mammalian target of rapamycin inhibitors. Clin J Oncol Nurs. 2011;15:E83-89.
42. Gomez-Fernandez C, Garden BC, Wu S, Feldman DR, Lacouture ME. The risk of skin rash and stomatitis with the mammalian target of rapamycin inhibitor temsirolimus: a systematic review of the literature and meta-analysis. Eur J Cancer. 2012;48:340-346.
43. Bonnaure-Mallet M, Bunetel L, Tricot-Doleux S, Guerin J, Bergeron C, LeGall E. Oral complications during treatment of malignant diseases in childhood: effects of tooth brushing. Eur J Cancer. 1998;34:1588-1591.
44. Chuang P, Langone AJ. Clobetasol ameliorates aphthous ulceration in renal transplant patients on sirolimus. Am J Transplant. 2007;7:714-717.
45. Femiano F, Buonaiuto C, Gombos F, Lanza A, Cirillo N. Pilot study on recurrent aphthous stomatitis (RAS): a randomized placebo-controlled trial for the comparative therapeutic effects of systemic prednisone and systemic montelukast in subjects unresponsive to topical therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:402-407.
APF530 for nausea and vomiting prevention following cisplatin: phase 3 MAGIC trial analysis
Despite available antiemetic therapies, chemotherapy-induced nausea and vomiting (CINV) following highly emetogenic chemotherapy (HEC), particularly in the delayed phase (>24-120 h after chemotherapy), continues to impair patient quality of life and chemotherapy compliance.1 Cisplatin-based chemotherapy, classified as HEC at any dose,2 is widely used to treat cancers such as non–small-cell and small
5-hydroxytryptamine type 3 (5-HT3) receptor antagonists (RAs; eg, granisetron, ondansetron, dolasetron, and palonosetron) have been the cornerstone of CINV therapy for decades and remain an integral part of contemporary antiemetic treatment regimens. Most current antiemetic guidelines for HEC recommend a 3-drug regimen, comprising a 5-HT3 RA, a neurokinin 1 (NK-1) RA, and a corticosteroid (dexamethasone).2,6,7 A regimen of olanzapine (antipsychotic), palonosetron (5-HT3 RA), and dexamethasone (corticosteroid) has been recommended as an alternative option. Recently, the oral fixed-dose combination of netupitant and palonosetron (NEPA) was approved and has shown efficacy in the cisplatin setting.8,9 However, the administration of oral medication to patients experiencing CINV and those with head and neck cancer may be difficult.10 Alternative antiemetic treatments that provide CINV control into the delayed phase and with a convenient route of administration, are needed.
APF530 is a novel extended-release granisetron formulation that provides sustained release of therapeutic concentrations for ≥5 days. The Biochronomer tri(ethylene glycol) poly(orthoester) (TEG-POE) vehicle releases granisetron slowly by polymer hydrolysis after it has been injected subcutaneously (SC) into the abdomen or upper arm.11,12 In 2016, the US Food and Drug Administration approved APF530 in combination with other antiemetics for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy (MEC) or anthracycline plus cyclophosphamide (AC) combination chemotherapy regimens based on data from 2 pivotal phase 3 trials.13
A phase 3 trial demonstrated noninferiority of APF530 (500 mg, SC) to palonosetron (0.25 mg, intravenously [IV]), each with dexamethasone (corticosteroid), in the control of acute-phase CINV after MEC or HEC, and delayed-phase CINV after MEC (classified by Hesketh criteria).14,15 Furthermore, APF530 provided sustained CINV control over multiple cycles of chemotherapy.16 Numerically higher complete response (CR: no emesis, no rescue medication use) rates were observed with APF530, compared with palonosetron, in the delayed phase after HEC (APF530 500 mg, 67.1%; palonosetron 0.25 mg, 64.3%).15
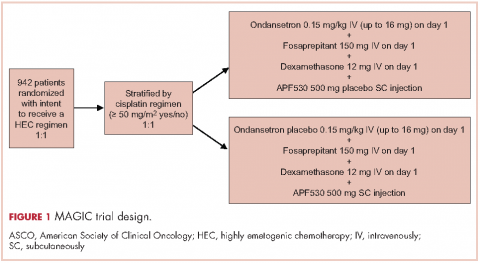
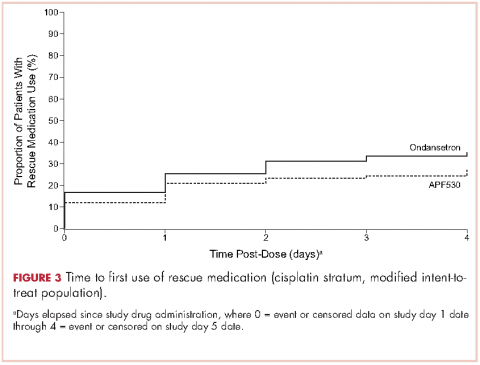
A reanalysis of study endpoints by newer emetogenicity classification guidelines from the American Society of Clinical Oncology (ASCO)7 maintained overall study conclusions.17 Notably, the numerically higher CR rates with APF530 in the delayed phase following HEC were enhanced (APF530 500 mg, 55.8%; palonosetron 0.25 mg, 50.5%), suggesting a need for further examination in this setting. The subsequent APF530 phase 3 MAGIC trial (Modified Absorption of Granisetron In the prevention of CINV; NCT02106494), compared APF530 (500 mg, SC) with ondansetron (0.15 mg/kg, IV), each with fosaprepitant (NK-1 RA) and dexamethasone in patients receiving HEC. The primary endpoint was met: the APF530 regimen demonstrated superior delayed-phase CR compared with the ondansetron regimen (64.7% vs 56.6%; 95% confidence interval [CI]:
1.7-14.4; P = .014; 8.0% absolute improvement).18
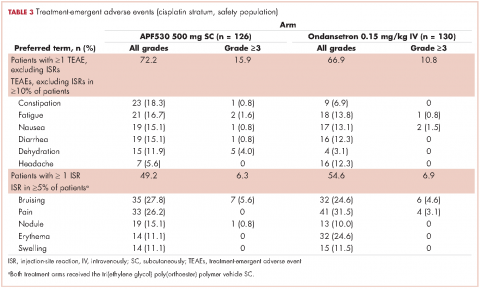
APF530 also demonstrated a significant benefit over ondansetron for other endpoints including nausea control, rescue medication use, and satisfaction with antiemetic therapy.18 APF530 is the first and only 5-HT3 RA to demonstrate superiority over another in a phase 3 efficacy trial using a guideline-recommended 3-drug regimen for both arms.
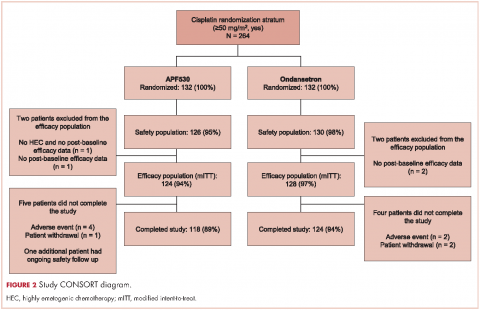
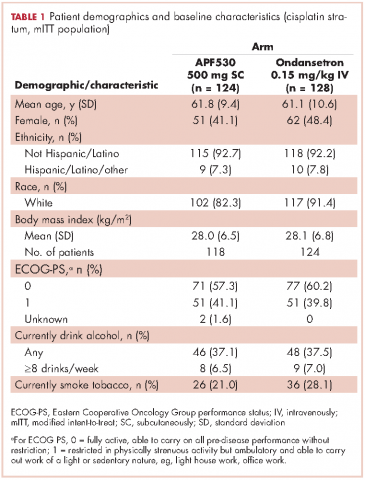
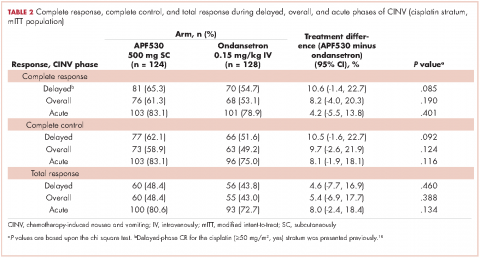
A prespecified MAGIC trial analysis of the primary endpoint by intent to receive cisplatin (≥50 mg/m2, Yes/No) demonstrated a pronounced treatment benefit in terms of delayed-phase CR rates with the APF530 regimen among patients in the cisplatin (≥50 mg/m2, Yes) stratum (CR: 65.3% vs 54.7%; 95% CI: -1.4-22.7; 10.6% absolute improvement).18 These results are compelling, since cisplatin represents a particularly emetogenic class of chemotherapy; a more in-depth analysis of additional MAGIC trial endpoints for these patients would be of clinical interest, and is presented here. Efficacy endpoints in this analysis include CR in the overall and acute phases, complete control (CC) and total response (TR) rates, rescue medication use, nausea frequency, and safety.
Methods
Study design and patients
The MAGIC trial was a prospective, randomized, multicenter, placebo-controlled, double-blind, double-dummy phase 3 trial conducted at 77 centers across the United States. The study protocol was reviewed and approved by the institutional review board at each participating center, and conducted according to the Declaration of Helsinki. The study design, previously presented in detail,18 is reviewed briefly here.
Eligible men and women were 18-80 years of age with histologically or cytologically confirmed malignancy (cancer type information was not captured) and were entering the first cycle of their single-day HEC treatment (defined by ASCO 2011 emetogenicity criteria).7 Patients had Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0 or 1, no history or presence of significant cardiac disease or QT interval prolongation, and adequate bone marrow, kidney, and liver function. All patients provided written informed consent.
Procedures
Patients were stratified by planned receipt of the cisplatin regimen ≥50 mg/m2 (Yes/No), randomized 1:1 to receive APF530 500 mg SC (granisetron 10 mg) or ondansetron 0.15 mg/kg IV (up to a maximum of 16 mg as a single dose) on day 1 (Figure 1). The APF530 arm received the ondansetron saline placebo, and the ondansetron arm received the APF530 SC placebo containing the TEG-POE vehicle. All patients were scheduled to receive fosaprepitant 150 mg IV and dexamethasone 12 mg IV on day 1, then oral dexamethasone 8 mg once daily on day 2 and 8 mg twice daily on days 3 and 4. Rescue medication was allowed at the investigator’s discretion.
Outcomes
The primary objective of the trial was to demonstrate the superiority of APF530 500 mg SC compared with ondansetron 0.15 mg/kg IV, as part of the current guideline-recommended 3-drug regimen, in preventing delayed-phase CINV after HEC. The primary endpoint was delayed-phase (24-120 h) CR (no emetic episodes [vomit or retch] and no rescue medication use). In addition, a prespecified analysis of delayed-phase CR by randomization strata (planned use of cisplatin) was performed.
Secondary and other endpoints included overall-phase CR
(0-120 h); delayed-, overall-, and acute-phase complete control (CC: CR and no more than mild nausea); delayed-, overall-, and acute-phase total response (TR; CR and no nausea); and rescue medication use. A post hoc analysis of nausea severity was also conducted. Safety assessments included treatment-emergent adverse events (TEAEs), injection-site reactions (ISRs), laboratory parameters, and vital signs. TEAEs were assessed by type, duration, severity, and relationship to study drug. ISR timing and severity were captured in patient diaries.
Statistical analysis
All efficacy analyses were conducted using the modified intent-to-treat population (mITT; randomized patients who received study drug and a HEC regimen and had post-baseline efficacy data). Safety assessments were performed on the safety population (randomized patients who received study drug).
This analysis conducted on the subgroup of patients with intent to receive cisplatin (cisplatin randomization stratum, ≥50 mg/m2, Yes) was exploratory and was not powered to detect treatment differences. Preplanned analyses compared CR, CC, and TR rates across treatment arms using 95% CIs.
Post hoc analyses of time to first rescue medication use, proportion of patients with rescue medication use, and less frequent nausea were performed. All P values were calculated using the Cochran-Mantel-Haenszel chi square test. Rescue medication use results were based on observed data, without imputation for missing results (ie, calculated from the number of patients with a response). Further analyses of efficacy endpoints CR, CC, and TR in the subset of female patients in the cisplatin randomization stratum were performed. Safety assessments were summarized descriptively.
Results
A total of 942 patients were randomized across 77 US centers during March 31, 2014 and May 15, 2015 (471 APF530, 471 ondansetron). Among those, 264 had intent to receive cisplatin and were included in the cisplatin randomization stratum (≥50 mg/m2, Yes) (Figure 2). A total of 256 patients in the cisplatin stratum received study drug and were included in the safety population (126 APF530, 130 ondansetron); 252 patients were included in the mITT population (124 APF530, 128 ondansetron).
Baseline demographics were generally balanced between treatment arms (Table 1). The proportion of female patients was 41.1% (51/124) and 48.4% (62/128) in the APF530 and ondansetron arms, respectively. The majority of patients had an ECOG PS of 0 (57.3% [71/124] APF530; 60.2% [77/128] ondansetron). The most common cisplatin-based chemotherapy regimen in both treatment arms was cisplatin and gemcitabine (25.0% [31/124] APF530; 28.9% [37/128] ondansetron) (Suppl Table 1). Two patients in the APF530 arm and 3 patients in the ondansetron arm either received a lower cisplatin dose (<50 mg/m2) or did not go on to receive cisplatin as intended at randomization (Suppl Table 1). As previously reported, in the cisplatin stratum (Table 2), delayed-phase CR was numerically higher in the APF530 arm versus the ondansetron arm, with a corresponding treatment difference of 10.6% (65.3% [81/124] APF530; 54.7% [70/128] ondansetron; 95% CI [-1.4, 22.7]; P = .085). Although the CI contains 0, the result is consistent with the significant benefit observed in the overall study population (64.7% [291/450] APF530; 56.6% [256/452] ondansetron; 95% CI [1.7, 14.4];
P = .014).18 This more in-depth analysis found similar trends favoring the APF530 over the ondansetron regimen across overall- and acute-phase CR (Table 2).
A significantly greater proportion of patients in the APF530 arm, compared with the ondansetron arm, reported no rescue medication use during the delayed phase (74.4% [90/121] APF530; 62.6% [77/123] ondansetron; P = .048). Trends in favor of APF530 were observed in the overall phase (71.1% [86/121] APF530; 61.8% [76/123] ondansetron; P = .125) and acute phase (86.9% [106/122] APF530; 81.9% [104/127] ondansetron; P = .278). Time to first rescue medication use was consistently longer in the APF530 arm, compared with the ondansetron arm, although not statistically significantly (P = .150) (Figure 3).
The APF530 regimen was generally well tolerated in the cisplatin subgroup, and no new safety signals were identified (Table 3). Most patients experienced at least one TEAE. Excluding ISRs, TEAE incidences were 72.2% and 66.9% in the APF530 and ondansetron arms, respectively; most common were constipation, fatigue, nausea, diarrhea, dehydration, and headache. Excluding ISRs, the most common treatment-related TEAEs in the APF530 and ondansetron arms were constipation (2.4% and 2.3%, respectively and headache (3.2% and 4.6%).
Discussion
The MAGIC trial is the first phase 3 efficacy trial in the prevention of CINV in patients receiving HEC using the current guideline-recommended 3-drug antiemetic regimen in both treatment arms.18 Ondansetron was chosen as the appropriate 5-HT3 RA comparator because no other 5-HT3 RA has shown superiority to ondansetron in delayed-phase CINV following HEC. Furthermore, ondansetron is indicated for prevention of nausea and vomiting associated with initial and repeat courses of chemotherapy, including high-dose cisplatin.19 The MAGIC trial primary endpoint was met for the overall study population; in the context of a 3-drug regimen, APF530 demonstrated superior control of delayed-phase CINV following HEC compared with standard-of-care ondansetron.18 As reported previously, significant benefits were also observed with the APF530 regimen over the ondansetron regimen in terms of rescue medication use, patient satisfaction with antiemetic therapy, and nausea frequency in the overall study population.18
Cisplatin is generally regarded as one of the most emetogenic chemotherapeutic agents. For this reason, cisplatin is often evaluated separately in clinical trials, and was a stratification factor in the MAGIC trial. Consistent with the previously reported significant results,18 trends in the cisplatin stratum analysis favored the APF530 regimen, compared with the ondansetron regimen, in delayed- and overall-phase CR (treatment difference: 10.6%).18 Numerical trends presented here favoring the APF530 regimen over the ondansetron regimen were observed in CC and TR, two more stringent measures of CINV control that account for incidence of nausea. Furthermore, among women in the cisplatin stratum, a population at increased risk for CINV, the numerically higher CR, CC, and TR persisted in the APF530 arm, compared with the ondansetron arm.
The APF530 regimen was generally well tolerated in the cisplatin stratum, and no new safety signals were identified. The most common TEAEs were ISRs, mostly mild or moderate and resolving by study end. The double-dummy design resulted in ISRs in the ondansetron arm due to TEG-POE vehicle as the dummy APF530 injection. Transient ISRs have been observed with other agents administered SC, and are expected.20,21 Excluding ISRs, TEAEs were generally consistent with those observed for the 5-HT3 RA class.22
This analysis of patients randomized to receive cisplatin-based HEC in the MAGIC trial is exploratory and was not sufficiently powered to detect between-arm differences. Five total patients did not go on to receive cisplatin ≥50 mg/m2 as intended at randomization (2 APF530, 3 ondansetron); however, this is not uncommon in large clinical trials and represents less than 2% of patients in this analysis.
Recent phase 3 studies in patients receiving cisplatin-based HEC showed significant improvement in CINV prevention with the current guideline-recommended 3-drug regimen over the traditional 2-drug regimen (5-HT3 RA + dexamethasone).8,23 Results presented here, in a similar population receiving cisplatin-based HEC, suggest that in the context of a 3-drug antiemetic regimen in both treatment arms, APF530 provides additional benefit in CINV prevention compared with the standard of care, ondansetron. Furthermore, a recent phase 3 trial in patients receiving cisplatin or AC-based HEC demonstrated significant improvement in nausea when olanzapine was added to a traditional 3-drug regimen of a 5-HT3 RA, NK-1 RA, and dexamethasone.24 These compelling data support the addition of olanzapine as a fourth agent to the CINV treatment regimen to provide further control of nausea, which has been one of the more difficult components of CINV to control to date.
APF530 is the only 5-HT3 RA to demonstrate superiority over another as part of the guideline-recommended regimen in a 3-drug versus 3-drug phase 3 efficacy trial examining antiemetic efficacy following HEC. Results from the MAGIC trial, this exploratory analysis, and previous studies in MEC and HEC provide clinically meaningful benefits in preventing both acute- and delayed-phase CINV following guideline-specified MEC or HEC regimens. Consequently, APF530 was approved for use in combination with other antiemetics for prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of MEC or AC combination chemotherapy regimens.13 Both the superior control of delayed-phase CINV following HEC demonstrated by the MAGIC trial18 and the consistent trends in the cisplatin stratum indicate a particular benefit for high-risk patients receiving high doses of cisplatin.
Acknowledgments
Joanna K Sandilos Rega, PhD, of SciStrategy Communications provided medical writing assistance, supported by Heron Therapeutics Inc, the maker of the study drug.
1. Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer. 2012;20:107-117.
2. NCCN clinical practice guidelines in oncology: antiemesis—version 1.2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#antiemesis. Accessed March 1, 2017.
3. Platinol (cisplatin for injection, USP) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018057s079lbl.pdf. Updated May 2010. Accessed September 12, 2016.
4. Pollera CF, Giannarelli D. Prognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic model. Cancer. 1989;64:1117-1122.
5. Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol. 1991;14:238-242.
6. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119-v133.
7. Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-4198.
8. Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340-1346.
9. Akynzeo (netupitant and palonosetron capsules) [prescribing information]. Woodcliff, NJ: Eisai; 2015. http://www.akynzeo.com. Revised April 2015. Accessed September 12, 2016.
10. Tsukahara K, Nakamura K, Motohashi R, et al. Antiemetic therapy of fosaprepitant, palonosetron, and dexamethasone combined with cisplatin-based chemotherapy for head and neck carcinomas. Acta Otolaryngol. 2014;134:1198-1204.
11. Ottoboni. Biochronomer technology and the development of APF530, a sustained release formulation of granisetron. J Exp Pharmacol. 2014;6:15-21.
12. Gabrail N, Yanagihara R, Spaczynski M, et al. Pharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two phase II trials. Cancer Manag Res. 2015;7:83-92.
13. Sustol (granisetron) extended-release injection, for subcutaneous use [prescribing information]. Redwood City, CA; Heron Therapeutics; 2016. http://sustol.com/hcp/healthcare-professionals. Updated August 2016. Accessed September 12, 2016.
14. Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15:103-109.
15. Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. 2015;23:723-732.
16. Boccia RV, Cooper W, O’Boyle E. Sustained antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of emetogenic chemotherapy. J Community Support Oncol. 2015;13:38-46.
17. Raftopoulos H, Boccia R, Cooper W, O’Boyle E, Gralla RJ. Slow-release granisetron (APF530) versus palonosetron for chemotherapy-induced nausea/vomiting: analysis by American Society of Clinical Oncology emetogenicity criteria. Future Oncol. 2015;11:2541-2551.
18. Schnadig ID, Agajanian R, Dakhil C, et al. APF530 (Granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol. 2016;12:1469-1481.
19. Zofran (ondansetron hydrochloride) injection for intravenous use. Research Triangle Park, NC: GlaxoSmithKline; 2014. http://www.pharma.us.novartis.com/product/pi/pdf/zofran_inj.pdf. Revised September 2014. Accessed September 12, 2016.
20. Eligard (luprolide acetate) kit for subcutaneous use [prescribing information]. Fort Collins, CO: Tolmar Pharmaceuticals Inc; 2017. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d. Revised January 2017. Accessed March 1, 2017.
21. Sandostatin LAR Depot (octreotide acetate for injectable suspension) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. https://www.pharma.us.novartis.com/product/pi/pdf/sandostatin_lar.pdf. Revised July 2016. Accessed September 12, 2016.
22. Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73:249-262.
23. Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16:1079-1089.
24. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134-142.
Despite available antiemetic therapies, chemotherapy-induced nausea and vomiting (CINV) following highly emetogenic chemotherapy (HEC), particularly in the delayed phase (>24-120 h after chemotherapy), continues to impair patient quality of life and chemotherapy compliance.1 Cisplatin-based chemotherapy, classified as HEC at any dose,2 is widely used to treat cancers such as non–small-cell and small
5-hydroxytryptamine type 3 (5-HT3) receptor antagonists (RAs; eg, granisetron, ondansetron, dolasetron, and palonosetron) have been the cornerstone of CINV therapy for decades and remain an integral part of contemporary antiemetic treatment regimens. Most current antiemetic guidelines for HEC recommend a 3-drug regimen, comprising a 5-HT3 RA, a neurokinin 1 (NK-1) RA, and a corticosteroid (dexamethasone).2,6,7 A regimen of olanzapine (antipsychotic), palonosetron (5-HT3 RA), and dexamethasone (corticosteroid) has been recommended as an alternative option. Recently, the oral fixed-dose combination of netupitant and palonosetron (NEPA) was approved and has shown efficacy in the cisplatin setting.8,9 However, the administration of oral medication to patients experiencing CINV and those with head and neck cancer may be difficult.10 Alternative antiemetic treatments that provide CINV control into the delayed phase and with a convenient route of administration, are needed.
APF530 is a novel extended-release granisetron formulation that provides sustained release of therapeutic concentrations for ≥5 days. The Biochronomer tri(ethylene glycol) poly(orthoester) (TEG-POE) vehicle releases granisetron slowly by polymer hydrolysis after it has been injected subcutaneously (SC) into the abdomen or upper arm.11,12 In 2016, the US Food and Drug Administration approved APF530 in combination with other antiemetics for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy (MEC) or anthracycline plus cyclophosphamide (AC) combination chemotherapy regimens based on data from 2 pivotal phase 3 trials.13
A phase 3 trial demonstrated noninferiority of APF530 (500 mg, SC) to palonosetron (0.25 mg, intravenously [IV]), each with dexamethasone (corticosteroid), in the control of acute-phase CINV after MEC or HEC, and delayed-phase CINV after MEC (classified by Hesketh criteria).14,15 Furthermore, APF530 provided sustained CINV control over multiple cycles of chemotherapy.16 Numerically higher complete response (CR: no emesis, no rescue medication use) rates were observed with APF530, compared with palonosetron, in the delayed phase after HEC (APF530 500 mg, 67.1%; palonosetron 0.25 mg, 64.3%).15
A reanalysis of study endpoints by newer emetogenicity classification guidelines from the American Society of Clinical Oncology (ASCO)7 maintained overall study conclusions.17 Notably, the numerically higher CR rates with APF530 in the delayed phase following HEC were enhanced (APF530 500 mg, 55.8%; palonosetron 0.25 mg, 50.5%), suggesting a need for further examination in this setting. The subsequent APF530 phase 3 MAGIC trial (Modified Absorption of Granisetron In the prevention of CINV; NCT02106494), compared APF530 (500 mg, SC) with ondansetron (0.15 mg/kg, IV), each with fosaprepitant (NK-1 RA) and dexamethasone in patients receiving HEC. The primary endpoint was met: the APF530 regimen demonstrated superior delayed-phase CR compared with the ondansetron regimen (64.7% vs 56.6%; 95% confidence interval [CI]:
1.7-14.4; P = .014; 8.0% absolute improvement).18
APF530 also demonstrated a significant benefit over ondansetron for other endpoints including nausea control, rescue medication use, and satisfaction with antiemetic therapy.18 APF530 is the first and only 5-HT3 RA to demonstrate superiority over another in a phase 3 efficacy trial using a guideline-recommended 3-drug regimen for both arms.
A prespecified MAGIC trial analysis of the primary endpoint by intent to receive cisplatin (≥50 mg/m2, Yes/No) demonstrated a pronounced treatment benefit in terms of delayed-phase CR rates with the APF530 regimen among patients in the cisplatin (≥50 mg/m2, Yes) stratum (CR: 65.3% vs 54.7%; 95% CI: -1.4-22.7; 10.6% absolute improvement).18 These results are compelling, since cisplatin represents a particularly emetogenic class of chemotherapy; a more in-depth analysis of additional MAGIC trial endpoints for these patients would be of clinical interest, and is presented here. Efficacy endpoints in this analysis include CR in the overall and acute phases, complete control (CC) and total response (TR) rates, rescue medication use, nausea frequency, and safety.
Methods
Study design and patients
The MAGIC trial was a prospective, randomized, multicenter, placebo-controlled, double-blind, double-dummy phase 3 trial conducted at 77 centers across the United States. The study protocol was reviewed and approved by the institutional review board at each participating center, and conducted according to the Declaration of Helsinki. The study design, previously presented in detail,18 is reviewed briefly here.
Eligible men and women were 18-80 years of age with histologically or cytologically confirmed malignancy (cancer type information was not captured) and were entering the first cycle of their single-day HEC treatment (defined by ASCO 2011 emetogenicity criteria).7 Patients had Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0 or 1, no history or presence of significant cardiac disease or QT interval prolongation, and adequate bone marrow, kidney, and liver function. All patients provided written informed consent.
Procedures
Patients were stratified by planned receipt of the cisplatin regimen ≥50 mg/m2 (Yes/No), randomized 1:1 to receive APF530 500 mg SC (granisetron 10 mg) or ondansetron 0.15 mg/kg IV (up to a maximum of 16 mg as a single dose) on day 1 (Figure 1). The APF530 arm received the ondansetron saline placebo, and the ondansetron arm received the APF530 SC placebo containing the TEG-POE vehicle. All patients were scheduled to receive fosaprepitant 150 mg IV and dexamethasone 12 mg IV on day 1, then oral dexamethasone 8 mg once daily on day 2 and 8 mg twice daily on days 3 and 4. Rescue medication was allowed at the investigator’s discretion.
Outcomes
The primary objective of the trial was to demonstrate the superiority of APF530 500 mg SC compared with ondansetron 0.15 mg/kg IV, as part of the current guideline-recommended 3-drug regimen, in preventing delayed-phase CINV after HEC. The primary endpoint was delayed-phase (24-120 h) CR (no emetic episodes [vomit or retch] and no rescue medication use). In addition, a prespecified analysis of delayed-phase CR by randomization strata (planned use of cisplatin) was performed.
Secondary and other endpoints included overall-phase CR
(0-120 h); delayed-, overall-, and acute-phase complete control (CC: CR and no more than mild nausea); delayed-, overall-, and acute-phase total response (TR; CR and no nausea); and rescue medication use. A post hoc analysis of nausea severity was also conducted. Safety assessments included treatment-emergent adverse events (TEAEs), injection-site reactions (ISRs), laboratory parameters, and vital signs. TEAEs were assessed by type, duration, severity, and relationship to study drug. ISR timing and severity were captured in patient diaries.
Statistical analysis
All efficacy analyses were conducted using the modified intent-to-treat population (mITT; randomized patients who received study drug and a HEC regimen and had post-baseline efficacy data). Safety assessments were performed on the safety population (randomized patients who received study drug).
This analysis conducted on the subgroup of patients with intent to receive cisplatin (cisplatin randomization stratum, ≥50 mg/m2, Yes) was exploratory and was not powered to detect treatment differences. Preplanned analyses compared CR, CC, and TR rates across treatment arms using 95% CIs.
Post hoc analyses of time to first rescue medication use, proportion of patients with rescue medication use, and less frequent nausea were performed. All P values were calculated using the Cochran-Mantel-Haenszel chi square test. Rescue medication use results were based on observed data, without imputation for missing results (ie, calculated from the number of patients with a response). Further analyses of efficacy endpoints CR, CC, and TR in the subset of female patients in the cisplatin randomization stratum were performed. Safety assessments were summarized descriptively.
Results
A total of 942 patients were randomized across 77 US centers during March 31, 2014 and May 15, 2015 (471 APF530, 471 ondansetron). Among those, 264 had intent to receive cisplatin and were included in the cisplatin randomization stratum (≥50 mg/m2, Yes) (Figure 2). A total of 256 patients in the cisplatin stratum received study drug and were included in the safety population (126 APF530, 130 ondansetron); 252 patients were included in the mITT population (124 APF530, 128 ondansetron).
Baseline demographics were generally balanced between treatment arms (Table 1). The proportion of female patients was 41.1% (51/124) and 48.4% (62/128) in the APF530 and ondansetron arms, respectively. The majority of patients had an ECOG PS of 0 (57.3% [71/124] APF530; 60.2% [77/128] ondansetron). The most common cisplatin-based chemotherapy regimen in both treatment arms was cisplatin and gemcitabine (25.0% [31/124] APF530; 28.9% [37/128] ondansetron) (Suppl Table 1). Two patients in the APF530 arm and 3 patients in the ondansetron arm either received a lower cisplatin dose (<50 mg/m2) or did not go on to receive cisplatin as intended at randomization (Suppl Table 1). As previously reported, in the cisplatin stratum (Table 2), delayed-phase CR was numerically higher in the APF530 arm versus the ondansetron arm, with a corresponding treatment difference of 10.6% (65.3% [81/124] APF530; 54.7% [70/128] ondansetron; 95% CI [-1.4, 22.7]; P = .085). Although the CI contains 0, the result is consistent with the significant benefit observed in the overall study population (64.7% [291/450] APF530; 56.6% [256/452] ondansetron; 95% CI [1.7, 14.4];
P = .014).18 This more in-depth analysis found similar trends favoring the APF530 over the ondansetron regimen across overall- and acute-phase CR (Table 2).
A significantly greater proportion of patients in the APF530 arm, compared with the ondansetron arm, reported no rescue medication use during the delayed phase (74.4% [90/121] APF530; 62.6% [77/123] ondansetron; P = .048). Trends in favor of APF530 were observed in the overall phase (71.1% [86/121] APF530; 61.8% [76/123] ondansetron; P = .125) and acute phase (86.9% [106/122] APF530; 81.9% [104/127] ondansetron; P = .278). Time to first rescue medication use was consistently longer in the APF530 arm, compared with the ondansetron arm, although not statistically significantly (P = .150) (Figure 3).
The APF530 regimen was generally well tolerated in the cisplatin subgroup, and no new safety signals were identified (Table 3). Most patients experienced at least one TEAE. Excluding ISRs, TEAE incidences were 72.2% and 66.9% in the APF530 and ondansetron arms, respectively; most common were constipation, fatigue, nausea, diarrhea, dehydration, and headache. Excluding ISRs, the most common treatment-related TEAEs in the APF530 and ondansetron arms were constipation (2.4% and 2.3%, respectively and headache (3.2% and 4.6%).
Discussion
The MAGIC trial is the first phase 3 efficacy trial in the prevention of CINV in patients receiving HEC using the current guideline-recommended 3-drug antiemetic regimen in both treatment arms.18 Ondansetron was chosen as the appropriate 5-HT3 RA comparator because no other 5-HT3 RA has shown superiority to ondansetron in delayed-phase CINV following HEC. Furthermore, ondansetron is indicated for prevention of nausea and vomiting associated with initial and repeat courses of chemotherapy, including high-dose cisplatin.19 The MAGIC trial primary endpoint was met for the overall study population; in the context of a 3-drug regimen, APF530 demonstrated superior control of delayed-phase CINV following HEC compared with standard-of-care ondansetron.18 As reported previously, significant benefits were also observed with the APF530 regimen over the ondansetron regimen in terms of rescue medication use, patient satisfaction with antiemetic therapy, and nausea frequency in the overall study population.18
Cisplatin is generally regarded as one of the most emetogenic chemotherapeutic agents. For this reason, cisplatin is often evaluated separately in clinical trials, and was a stratification factor in the MAGIC trial. Consistent with the previously reported significant results,18 trends in the cisplatin stratum analysis favored the APF530 regimen, compared with the ondansetron regimen, in delayed- and overall-phase CR (treatment difference: 10.6%).18 Numerical trends presented here favoring the APF530 regimen over the ondansetron regimen were observed in CC and TR, two more stringent measures of CINV control that account for incidence of nausea. Furthermore, among women in the cisplatin stratum, a population at increased risk for CINV, the numerically higher CR, CC, and TR persisted in the APF530 arm, compared with the ondansetron arm.
The APF530 regimen was generally well tolerated in the cisplatin stratum, and no new safety signals were identified. The most common TEAEs were ISRs, mostly mild or moderate and resolving by study end. The double-dummy design resulted in ISRs in the ondansetron arm due to TEG-POE vehicle as the dummy APF530 injection. Transient ISRs have been observed with other agents administered SC, and are expected.20,21 Excluding ISRs, TEAEs were generally consistent with those observed for the 5-HT3 RA class.22
This analysis of patients randomized to receive cisplatin-based HEC in the MAGIC trial is exploratory and was not sufficiently powered to detect between-arm differences. Five total patients did not go on to receive cisplatin ≥50 mg/m2 as intended at randomization (2 APF530, 3 ondansetron); however, this is not uncommon in large clinical trials and represents less than 2% of patients in this analysis.
Recent phase 3 studies in patients receiving cisplatin-based HEC showed significant improvement in CINV prevention with the current guideline-recommended 3-drug regimen over the traditional 2-drug regimen (5-HT3 RA + dexamethasone).8,23 Results presented here, in a similar population receiving cisplatin-based HEC, suggest that in the context of a 3-drug antiemetic regimen in both treatment arms, APF530 provides additional benefit in CINV prevention compared with the standard of care, ondansetron. Furthermore, a recent phase 3 trial in patients receiving cisplatin or AC-based HEC demonstrated significant improvement in nausea when olanzapine was added to a traditional 3-drug regimen of a 5-HT3 RA, NK-1 RA, and dexamethasone.24 These compelling data support the addition of olanzapine as a fourth agent to the CINV treatment regimen to provide further control of nausea, which has been one of the more difficult components of CINV to control to date.
APF530 is the only 5-HT3 RA to demonstrate superiority over another as part of the guideline-recommended regimen in a 3-drug versus 3-drug phase 3 efficacy trial examining antiemetic efficacy following HEC. Results from the MAGIC trial, this exploratory analysis, and previous studies in MEC and HEC provide clinically meaningful benefits in preventing both acute- and delayed-phase CINV following guideline-specified MEC or HEC regimens. Consequently, APF530 was approved for use in combination with other antiemetics for prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of MEC or AC combination chemotherapy regimens.13 Both the superior control of delayed-phase CINV following HEC demonstrated by the MAGIC trial18 and the consistent trends in the cisplatin stratum indicate a particular benefit for high-risk patients receiving high doses of cisplatin.
Acknowledgments
Joanna K Sandilos Rega, PhD, of SciStrategy Communications provided medical writing assistance, supported by Heron Therapeutics Inc, the maker of the study drug.
Despite available antiemetic therapies, chemotherapy-induced nausea and vomiting (CINV) following highly emetogenic chemotherapy (HEC), particularly in the delayed phase (>24-120 h after chemotherapy), continues to impair patient quality of life and chemotherapy compliance.1 Cisplatin-based chemotherapy, classified as HEC at any dose,2 is widely used to treat cancers such as non–small-cell and small
5-hydroxytryptamine type 3 (5-HT3) receptor antagonists (RAs; eg, granisetron, ondansetron, dolasetron, and palonosetron) have been the cornerstone of CINV therapy for decades and remain an integral part of contemporary antiemetic treatment regimens. Most current antiemetic guidelines for HEC recommend a 3-drug regimen, comprising a 5-HT3 RA, a neurokinin 1 (NK-1) RA, and a corticosteroid (dexamethasone).2,6,7 A regimen of olanzapine (antipsychotic), palonosetron (5-HT3 RA), and dexamethasone (corticosteroid) has been recommended as an alternative option. Recently, the oral fixed-dose combination of netupitant and palonosetron (NEPA) was approved and has shown efficacy in the cisplatin setting.8,9 However, the administration of oral medication to patients experiencing CINV and those with head and neck cancer may be difficult.10 Alternative antiemetic treatments that provide CINV control into the delayed phase and with a convenient route of administration, are needed.
APF530 is a novel extended-release granisetron formulation that provides sustained release of therapeutic concentrations for ≥5 days. The Biochronomer tri(ethylene glycol) poly(orthoester) (TEG-POE) vehicle releases granisetron slowly by polymer hydrolysis after it has been injected subcutaneously (SC) into the abdomen or upper arm.11,12 In 2016, the US Food and Drug Administration approved APF530 in combination with other antiemetics for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy (MEC) or anthracycline plus cyclophosphamide (AC) combination chemotherapy regimens based on data from 2 pivotal phase 3 trials.13
A phase 3 trial demonstrated noninferiority of APF530 (500 mg, SC) to palonosetron (0.25 mg, intravenously [IV]), each with dexamethasone (corticosteroid), in the control of acute-phase CINV after MEC or HEC, and delayed-phase CINV after MEC (classified by Hesketh criteria).14,15 Furthermore, APF530 provided sustained CINV control over multiple cycles of chemotherapy.16 Numerically higher complete response (CR: no emesis, no rescue medication use) rates were observed with APF530, compared with palonosetron, in the delayed phase after HEC (APF530 500 mg, 67.1%; palonosetron 0.25 mg, 64.3%).15
A reanalysis of study endpoints by newer emetogenicity classification guidelines from the American Society of Clinical Oncology (ASCO)7 maintained overall study conclusions.17 Notably, the numerically higher CR rates with APF530 in the delayed phase following HEC were enhanced (APF530 500 mg, 55.8%; palonosetron 0.25 mg, 50.5%), suggesting a need for further examination in this setting. The subsequent APF530 phase 3 MAGIC trial (Modified Absorption of Granisetron In the prevention of CINV; NCT02106494), compared APF530 (500 mg, SC) with ondansetron (0.15 mg/kg, IV), each with fosaprepitant (NK-1 RA) and dexamethasone in patients receiving HEC. The primary endpoint was met: the APF530 regimen demonstrated superior delayed-phase CR compared with the ondansetron regimen (64.7% vs 56.6%; 95% confidence interval [CI]:
1.7-14.4; P = .014; 8.0% absolute improvement).18
APF530 also demonstrated a significant benefit over ondansetron for other endpoints including nausea control, rescue medication use, and satisfaction with antiemetic therapy.18 APF530 is the first and only 5-HT3 RA to demonstrate superiority over another in a phase 3 efficacy trial using a guideline-recommended 3-drug regimen for both arms.
A prespecified MAGIC trial analysis of the primary endpoint by intent to receive cisplatin (≥50 mg/m2, Yes/No) demonstrated a pronounced treatment benefit in terms of delayed-phase CR rates with the APF530 regimen among patients in the cisplatin (≥50 mg/m2, Yes) stratum (CR: 65.3% vs 54.7%; 95% CI: -1.4-22.7; 10.6% absolute improvement).18 These results are compelling, since cisplatin represents a particularly emetogenic class of chemotherapy; a more in-depth analysis of additional MAGIC trial endpoints for these patients would be of clinical interest, and is presented here. Efficacy endpoints in this analysis include CR in the overall and acute phases, complete control (CC) and total response (TR) rates, rescue medication use, nausea frequency, and safety.
Methods
Study design and patients
The MAGIC trial was a prospective, randomized, multicenter, placebo-controlled, double-blind, double-dummy phase 3 trial conducted at 77 centers across the United States. The study protocol was reviewed and approved by the institutional review board at each participating center, and conducted according to the Declaration of Helsinki. The study design, previously presented in detail,18 is reviewed briefly here.
Eligible men and women were 18-80 years of age with histologically or cytologically confirmed malignancy (cancer type information was not captured) and were entering the first cycle of their single-day HEC treatment (defined by ASCO 2011 emetogenicity criteria).7 Patients had Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0 or 1, no history or presence of significant cardiac disease or QT interval prolongation, and adequate bone marrow, kidney, and liver function. All patients provided written informed consent.
Procedures
Patients were stratified by planned receipt of the cisplatin regimen ≥50 mg/m2 (Yes/No), randomized 1:1 to receive APF530 500 mg SC (granisetron 10 mg) or ondansetron 0.15 mg/kg IV (up to a maximum of 16 mg as a single dose) on day 1 (Figure 1). The APF530 arm received the ondansetron saline placebo, and the ondansetron arm received the APF530 SC placebo containing the TEG-POE vehicle. All patients were scheduled to receive fosaprepitant 150 mg IV and dexamethasone 12 mg IV on day 1, then oral dexamethasone 8 mg once daily on day 2 and 8 mg twice daily on days 3 and 4. Rescue medication was allowed at the investigator’s discretion.
Outcomes
The primary objective of the trial was to demonstrate the superiority of APF530 500 mg SC compared with ondansetron 0.15 mg/kg IV, as part of the current guideline-recommended 3-drug regimen, in preventing delayed-phase CINV after HEC. The primary endpoint was delayed-phase (24-120 h) CR (no emetic episodes [vomit or retch] and no rescue medication use). In addition, a prespecified analysis of delayed-phase CR by randomization strata (planned use of cisplatin) was performed.
Secondary and other endpoints included overall-phase CR
(0-120 h); delayed-, overall-, and acute-phase complete control (CC: CR and no more than mild nausea); delayed-, overall-, and acute-phase total response (TR; CR and no nausea); and rescue medication use. A post hoc analysis of nausea severity was also conducted. Safety assessments included treatment-emergent adverse events (TEAEs), injection-site reactions (ISRs), laboratory parameters, and vital signs. TEAEs were assessed by type, duration, severity, and relationship to study drug. ISR timing and severity were captured in patient diaries.
Statistical analysis
All efficacy analyses were conducted using the modified intent-to-treat population (mITT; randomized patients who received study drug and a HEC regimen and had post-baseline efficacy data). Safety assessments were performed on the safety population (randomized patients who received study drug).
This analysis conducted on the subgroup of patients with intent to receive cisplatin (cisplatin randomization stratum, ≥50 mg/m2, Yes) was exploratory and was not powered to detect treatment differences. Preplanned analyses compared CR, CC, and TR rates across treatment arms using 95% CIs.
Post hoc analyses of time to first rescue medication use, proportion of patients with rescue medication use, and less frequent nausea were performed. All P values were calculated using the Cochran-Mantel-Haenszel chi square test. Rescue medication use results were based on observed data, without imputation for missing results (ie, calculated from the number of patients with a response). Further analyses of efficacy endpoints CR, CC, and TR in the subset of female patients in the cisplatin randomization stratum were performed. Safety assessments were summarized descriptively.
Results
A total of 942 patients were randomized across 77 US centers during March 31, 2014 and May 15, 2015 (471 APF530, 471 ondansetron). Among those, 264 had intent to receive cisplatin and were included in the cisplatin randomization stratum (≥50 mg/m2, Yes) (Figure 2). A total of 256 patients in the cisplatin stratum received study drug and were included in the safety population (126 APF530, 130 ondansetron); 252 patients were included in the mITT population (124 APF530, 128 ondansetron).
Baseline demographics were generally balanced between treatment arms (Table 1). The proportion of female patients was 41.1% (51/124) and 48.4% (62/128) in the APF530 and ondansetron arms, respectively. The majority of patients had an ECOG PS of 0 (57.3% [71/124] APF530; 60.2% [77/128] ondansetron). The most common cisplatin-based chemotherapy regimen in both treatment arms was cisplatin and gemcitabine (25.0% [31/124] APF530; 28.9% [37/128] ondansetron) (Suppl Table 1). Two patients in the APF530 arm and 3 patients in the ondansetron arm either received a lower cisplatin dose (<50 mg/m2) or did not go on to receive cisplatin as intended at randomization (Suppl Table 1). As previously reported, in the cisplatin stratum (Table 2), delayed-phase CR was numerically higher in the APF530 arm versus the ondansetron arm, with a corresponding treatment difference of 10.6% (65.3% [81/124] APF530; 54.7% [70/128] ondansetron; 95% CI [-1.4, 22.7]; P = .085). Although the CI contains 0, the result is consistent with the significant benefit observed in the overall study population (64.7% [291/450] APF530; 56.6% [256/452] ondansetron; 95% CI [1.7, 14.4];
P = .014).18 This more in-depth analysis found similar trends favoring the APF530 over the ondansetron regimen across overall- and acute-phase CR (Table 2).
A significantly greater proportion of patients in the APF530 arm, compared with the ondansetron arm, reported no rescue medication use during the delayed phase (74.4% [90/121] APF530; 62.6% [77/123] ondansetron; P = .048). Trends in favor of APF530 were observed in the overall phase (71.1% [86/121] APF530; 61.8% [76/123] ondansetron; P = .125) and acute phase (86.9% [106/122] APF530; 81.9% [104/127] ondansetron; P = .278). Time to first rescue medication use was consistently longer in the APF530 arm, compared with the ondansetron arm, although not statistically significantly (P = .150) (Figure 3).
The APF530 regimen was generally well tolerated in the cisplatin subgroup, and no new safety signals were identified (Table 3). Most patients experienced at least one TEAE. Excluding ISRs, TEAE incidences were 72.2% and 66.9% in the APF530 and ondansetron arms, respectively; most common were constipation, fatigue, nausea, diarrhea, dehydration, and headache. Excluding ISRs, the most common treatment-related TEAEs in the APF530 and ondansetron arms were constipation (2.4% and 2.3%, respectively and headache (3.2% and 4.6%).
Discussion
The MAGIC trial is the first phase 3 efficacy trial in the prevention of CINV in patients receiving HEC using the current guideline-recommended 3-drug antiemetic regimen in both treatment arms.18 Ondansetron was chosen as the appropriate 5-HT3 RA comparator because no other 5-HT3 RA has shown superiority to ondansetron in delayed-phase CINV following HEC. Furthermore, ondansetron is indicated for prevention of nausea and vomiting associated with initial and repeat courses of chemotherapy, including high-dose cisplatin.19 The MAGIC trial primary endpoint was met for the overall study population; in the context of a 3-drug regimen, APF530 demonstrated superior control of delayed-phase CINV following HEC compared with standard-of-care ondansetron.18 As reported previously, significant benefits were also observed with the APF530 regimen over the ondansetron regimen in terms of rescue medication use, patient satisfaction with antiemetic therapy, and nausea frequency in the overall study population.18
Cisplatin is generally regarded as one of the most emetogenic chemotherapeutic agents. For this reason, cisplatin is often evaluated separately in clinical trials, and was a stratification factor in the MAGIC trial. Consistent with the previously reported significant results,18 trends in the cisplatin stratum analysis favored the APF530 regimen, compared with the ondansetron regimen, in delayed- and overall-phase CR (treatment difference: 10.6%).18 Numerical trends presented here favoring the APF530 regimen over the ondansetron regimen were observed in CC and TR, two more stringent measures of CINV control that account for incidence of nausea. Furthermore, among women in the cisplatin stratum, a population at increased risk for CINV, the numerically higher CR, CC, and TR persisted in the APF530 arm, compared with the ondansetron arm.
The APF530 regimen was generally well tolerated in the cisplatin stratum, and no new safety signals were identified. The most common TEAEs were ISRs, mostly mild or moderate and resolving by study end. The double-dummy design resulted in ISRs in the ondansetron arm due to TEG-POE vehicle as the dummy APF530 injection. Transient ISRs have been observed with other agents administered SC, and are expected.20,21 Excluding ISRs, TEAEs were generally consistent with those observed for the 5-HT3 RA class.22
This analysis of patients randomized to receive cisplatin-based HEC in the MAGIC trial is exploratory and was not sufficiently powered to detect between-arm differences. Five total patients did not go on to receive cisplatin ≥50 mg/m2 as intended at randomization (2 APF530, 3 ondansetron); however, this is not uncommon in large clinical trials and represents less than 2% of patients in this analysis.
Recent phase 3 studies in patients receiving cisplatin-based HEC showed significant improvement in CINV prevention with the current guideline-recommended 3-drug regimen over the traditional 2-drug regimen (5-HT3 RA + dexamethasone).8,23 Results presented here, in a similar population receiving cisplatin-based HEC, suggest that in the context of a 3-drug antiemetic regimen in both treatment arms, APF530 provides additional benefit in CINV prevention compared with the standard of care, ondansetron. Furthermore, a recent phase 3 trial in patients receiving cisplatin or AC-based HEC demonstrated significant improvement in nausea when olanzapine was added to a traditional 3-drug regimen of a 5-HT3 RA, NK-1 RA, and dexamethasone.24 These compelling data support the addition of olanzapine as a fourth agent to the CINV treatment regimen to provide further control of nausea, which has been one of the more difficult components of CINV to control to date.
APF530 is the only 5-HT3 RA to demonstrate superiority over another as part of the guideline-recommended regimen in a 3-drug versus 3-drug phase 3 efficacy trial examining antiemetic efficacy following HEC. Results from the MAGIC trial, this exploratory analysis, and previous studies in MEC and HEC provide clinically meaningful benefits in preventing both acute- and delayed-phase CINV following guideline-specified MEC or HEC regimens. Consequently, APF530 was approved for use in combination with other antiemetics for prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of MEC or AC combination chemotherapy regimens.13 Both the superior control of delayed-phase CINV following HEC demonstrated by the MAGIC trial18 and the consistent trends in the cisplatin stratum indicate a particular benefit for high-risk patients receiving high doses of cisplatin.
Acknowledgments
Joanna K Sandilos Rega, PhD, of SciStrategy Communications provided medical writing assistance, supported by Heron Therapeutics Inc, the maker of the study drug.
1. Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer. 2012;20:107-117.
2. NCCN clinical practice guidelines in oncology: antiemesis—version 1.2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#antiemesis. Accessed March 1, 2017.
3. Platinol (cisplatin for injection, USP) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018057s079lbl.pdf. Updated May 2010. Accessed September 12, 2016.
4. Pollera CF, Giannarelli D. Prognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic model. Cancer. 1989;64:1117-1122.
5. Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol. 1991;14:238-242.
6. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119-v133.
7. Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-4198.
8. Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340-1346.
9. Akynzeo (netupitant and palonosetron capsules) [prescribing information]. Woodcliff, NJ: Eisai; 2015. http://www.akynzeo.com. Revised April 2015. Accessed September 12, 2016.
10. Tsukahara K, Nakamura K, Motohashi R, et al. Antiemetic therapy of fosaprepitant, palonosetron, and dexamethasone combined with cisplatin-based chemotherapy for head and neck carcinomas. Acta Otolaryngol. 2014;134:1198-1204.
11. Ottoboni. Biochronomer technology and the development of APF530, a sustained release formulation of granisetron. J Exp Pharmacol. 2014;6:15-21.
12. Gabrail N, Yanagihara R, Spaczynski M, et al. Pharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two phase II trials. Cancer Manag Res. 2015;7:83-92.
13. Sustol (granisetron) extended-release injection, for subcutaneous use [prescribing information]. Redwood City, CA; Heron Therapeutics; 2016. http://sustol.com/hcp/healthcare-professionals. Updated August 2016. Accessed September 12, 2016.
14. Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15:103-109.
15. Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. 2015;23:723-732.
16. Boccia RV, Cooper W, O’Boyle E. Sustained antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of emetogenic chemotherapy. J Community Support Oncol. 2015;13:38-46.
17. Raftopoulos H, Boccia R, Cooper W, O’Boyle E, Gralla RJ. Slow-release granisetron (APF530) versus palonosetron for chemotherapy-induced nausea/vomiting: analysis by American Society of Clinical Oncology emetogenicity criteria. Future Oncol. 2015;11:2541-2551.
18. Schnadig ID, Agajanian R, Dakhil C, et al. APF530 (Granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol. 2016;12:1469-1481.
19. Zofran (ondansetron hydrochloride) injection for intravenous use. Research Triangle Park, NC: GlaxoSmithKline; 2014. http://www.pharma.us.novartis.com/product/pi/pdf/zofran_inj.pdf. Revised September 2014. Accessed September 12, 2016.
20. Eligard (luprolide acetate) kit for subcutaneous use [prescribing information]. Fort Collins, CO: Tolmar Pharmaceuticals Inc; 2017. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d. Revised January 2017. Accessed March 1, 2017.
21. Sandostatin LAR Depot (octreotide acetate for injectable suspension) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. https://www.pharma.us.novartis.com/product/pi/pdf/sandostatin_lar.pdf. Revised July 2016. Accessed September 12, 2016.
22. Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73:249-262.
23. Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16:1079-1089.
24. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134-142.
1. Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer. 2012;20:107-117.
2. NCCN clinical practice guidelines in oncology: antiemesis—version 1.2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#antiemesis. Accessed March 1, 2017.
3. Platinol (cisplatin for injection, USP) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018057s079lbl.pdf. Updated May 2010. Accessed September 12, 2016.
4. Pollera CF, Giannarelli D. Prognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic model. Cancer. 1989;64:1117-1122.
5. Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol. 1991;14:238-242.
6. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119-v133.
7. Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-4198.
8. Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340-1346.
9. Akynzeo (netupitant and palonosetron capsules) [prescribing information]. Woodcliff, NJ: Eisai; 2015. http://www.akynzeo.com. Revised April 2015. Accessed September 12, 2016.
10. Tsukahara K, Nakamura K, Motohashi R, et al. Antiemetic therapy of fosaprepitant, palonosetron, and dexamethasone combined with cisplatin-based chemotherapy for head and neck carcinomas. Acta Otolaryngol. 2014;134:1198-1204.
11. Ottoboni. Biochronomer technology and the development of APF530, a sustained release formulation of granisetron. J Exp Pharmacol. 2014;6:15-21.
12. Gabrail N, Yanagihara R, Spaczynski M, et al. Pharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two phase II trials. Cancer Manag Res. 2015;7:83-92.
13. Sustol (granisetron) extended-release injection, for subcutaneous use [prescribing information]. Redwood City, CA; Heron Therapeutics; 2016. http://sustol.com/hcp/healthcare-professionals. Updated August 2016. Accessed September 12, 2016.
14. Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15:103-109.
15. Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. 2015;23:723-732.
16. Boccia RV, Cooper W, O’Boyle E. Sustained antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of emetogenic chemotherapy. J Community Support Oncol. 2015;13:38-46.
17. Raftopoulos H, Boccia R, Cooper W, O’Boyle E, Gralla RJ. Slow-release granisetron (APF530) versus palonosetron for chemotherapy-induced nausea/vomiting: analysis by American Society of Clinical Oncology emetogenicity criteria. Future Oncol. 2015;11:2541-2551.
18. Schnadig ID, Agajanian R, Dakhil C, et al. APF530 (Granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol. 2016;12:1469-1481.
19. Zofran (ondansetron hydrochloride) injection for intravenous use. Research Triangle Park, NC: GlaxoSmithKline; 2014. http://www.pharma.us.novartis.com/product/pi/pdf/zofran_inj.pdf. Revised September 2014. Accessed September 12, 2016.
20. Eligard (luprolide acetate) kit for subcutaneous use [prescribing information]. Fort Collins, CO: Tolmar Pharmaceuticals Inc; 2017. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d. Revised January 2017. Accessed March 1, 2017.
21. Sandostatin LAR Depot (octreotide acetate for injectable suspension) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. https://www.pharma.us.novartis.com/product/pi/pdf/sandostatin_lar.pdf. Revised July 2016. Accessed September 12, 2016.
22. Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73:249-262.
23. Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16:1079-1089.
24. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134-142.
Patterns of care with regard to whole-brain radiotherapy technique and delivery among academic centers in the United States
Despite the recent advances in systemic therapy, metastatic spread to the brain continues to be the most common neurologic complication of many cancers. The clinical incidence of brain metastases varies with primary cancer diagnosis, with estimates ranging from 1.2%-19.8%.1,2 Metastatic spread to the brain is even more prevalent at autopsy, with evidence of intracranial tumor being found in 26% of patients in some series.3 It is possible that the clinical incidence of metastatic disease to the brain will continue to increase as newer therapeutic agents improve survival and imaging techniques continue to improve.
The management of brain metastases has changed rapidly as technological improvements have made treatment increasingly safe and efficacious. Traditionally, treatment consisted of radiotherapy to the whole brain, with or without surgical resection.4,5 More recently, stereotactic radiosurgery (SRS) has been adopted on the basis of evidence that it is safe and efficacious alone or in combination with radiotherapy to the whole brain.6 Further evidence is emerging that neurocognitive outcomes are improved when whole-brain radiotherapy (WBRT) is omitted, which possibly contributes to improved patient quality of life.7 Taking into account this and other data, the American Society for Radiation Oncology’s Choosing Wisely campaign now recommends not routinely adding WBRT to radiosurgery in patients with limited brain metastases.8
Despite this recommendation, many patients continue to benefit from WBRT, and it remains a common treatment in radiation oncology clinics across the US for several reasons. Many patients present with multiple brain metastases and are ineligible for radiosurgery. Even for technically eligible patients, WBRT has been shown to improve local control and decrease the rate of distant brain failure over radiosurgery alone.6 With higher rates of subsequent failures, patients receiving radiosurgery alone must adhere to more rigorous follow-up and imaging schedules, which can be difficult for many rural patients who have to travel long distances to centers. Furthermore, there is some suggestion that this decreased failure rate may result in improved survival in highly selected patients with excellent disease and performance status.9 Controversies exist, however, and strong institutional biases persist, contributing to significant differences in practice. We surveyed academic radiation oncologists and in an effort to identify and describe practice patterns in the delivery of WBRT at academic centers.
Methods
We conducted a thorough review of available literature on radiation for brain metastases and based on our findings, devised a survey 19 questions to ascertain practice patterns and treatment delivery among US academic physicians (Table 1). After obtaining institutional review board approval to do the study, we sent the survey to program coordinators at radiation oncology programs that are accredited by the Accreditation Council for Graduate Medical Education. We instructed coordinators to e-mail the survey to their practicing resident and attending physicians. The surveys were created using SurveyMonkey software. We obtained informed consent from the providers. A total of 3 follow-up e-mails were sent to each recipient of the survey to solicit responses, similar to the Dillman Total Design Survey Method.10
SPSS version 22.0 was used to analyze the data in an exploratory fashion. Statistical methods were used to assess the association of demographic data with SRS and WBRT delivery and treatment technique items when the analyses involved percentages that included the Pearson chi-square statistic and the chi-square test for linear trend. When the analysis focused on ranking data, the Kruskal-Wallis test, Mann-Whitney U test, the Jonckheere-Terpstra and the Kendall tau-b rank correlation were used as appropriate. If there were small sample sizes within some groups, then exact significant levels were assessed. Statistical significance was set by convention at P < .05.
Results
We received 95 responses of which 87 were considered complete for analysis. Forty-seven percent of the 87 respondents were not board-certified, and the remainder had passed their radiobiology and physics boards exams. A majority of respondents (70%, 61 of 87) were physicians who had been in practice for ≤5 years. Fifty-four percent of respondents were located in the Northeast US, 22% in the South, 14% in the West, and 10% in the Midwest and Hawaii (Table 2).
We used the chi-square test for linear trends to assess for a relationship between years of practice and whether respondents deviated from their typical method of WBRT therapy when treating more radioresistant tumors (melanoma, renal cell carcinoma). Respondents were classified by years in practice: 0-5, 6-10, 11-20, and >21 years. The results showed a linear association, with those in practice for longer periods more likely to use SRS alone, P = .027 (Figure 1).
Discussion
The incidence of brain metastases is increasing because of improvements in diagnostic imaging techniques and advancements in systemic therapy control of extracranial disease but not of intracranial disease or metastasis, because therapies do not cross the blood-brain barrier.11,12 Brain metastases are the most common type of brain tumor. Given that most chemotherapeutic agents cannot cross the blood-brain barrier, radiotherapy is considered a means of treatment and of controlling brain metastases. Early data from the 1950s13 and 1960s14 have suggested clinical improvement with brain radiation, making radiotherapy the cornerstone for treatment of brain metastases.
The Radiation Therapy Oncology Group (RTOG) has evaluated several fractionation schedules, with 5 schemas evaluated by the RTOG 6901 and 7361 studies: 30 Gy in 10 fractions, 30 Gy in 15 fractions, 40 Gy in 15 fractions, 40 Gy in 20 fractions, and 20 Gy in 5 fractions. The combined results from these two trials showed that outcomes were similar for patients treated with a shorter regimen than for those treated with a more protracted schedule. In our study, respondents reported that they most frequently treated brain metastases to a total dose of 30 Gy in 10 fractions. Given the results of the aforementioned RTOG trials and practice patterns among academic physicians, we recommend all practitioners consider a shorter hypofractioned course when treating brain metastases with WBRT. This will also reduce delays for patients who are likely to benefit greatly from earlier enrollment into hospice care, because protracted radiation schedules typically are not covered while a patient is in hospice.
Pharmacologic management for patients with brain metastases is important for symptomatic improvement. Glucocorticoids are important for palliation of symptoms from edema and increased intracranial pressure.15 However, steroids have a multitude of side effects and their use in asymptomatic patients is unnecessary. Improvements in imaging and detection11 have allowed us to find smaller and asymptomatic brain tumors. In our survey, it was promising to see a change in former practice patterns, with only 8% of academic practitioners regularly prescribing steroids to all of their patients receiving whole-brain radiation.
Diminished cognitive function and short-term memory loss are troublesome side effects of WBRT. As cancer patients live longer, such cognitive dysfunction will become more than just a nuisance. The RTOG has investigated the use of prophylactic memantine for patients receiving whole-brain radiation to determine if it would aid in the preservation of cognition. It found that patients who received memantine did better and had delayed time to cognitive decline and a reduced rate of memory decline, executive function, and processing speed.16 In our study, about a third of practitioners prescribed memantine and it was reserved for patients who had an otherwise favorable prognosis.
The RTOG has also investigated adjusting treatment technique for patients who receive WBRT. RTOG 0933 was a phase 2 trial that evaluated hippocampal avoidance during deliverance of WBRT with intensity-modulated radiation therapy (IMRT). Results showed that avoiding the hippocampus during WBRT was associated with improved memory preservation and patient quality of life.17 In a survey of practicing radiation oncologists in the US, most reported that they did not use memantine or IMRT for hippocampal sparing when delivering whole-brain radiation.18 Given the positive results of RTOG 0933 and 0614, the NRG Oncology research organization is conducting a phase 3 randomized trial that compares memantine use for patients receiving whole-brain radiation with or without hippocampal sparing to determine if patients will have reduced cognitive decline. All patients receiving WBRT should be considered for enrolment on this trial if they are eligible.
The delivery of brain radiation has continued to change, especially with the introduction of SRS. Recent publication of a meta-analysis of three phase 3 trials evaluating SRS with or without WBRT for 1-4 brain metastases showed that patients aged 50 years or younger experienced a survival benefit with SRS, and the omission of whole-brain radiation did not affect distant brain relapse rates. 19 The authors recommended that for this population, SRS alone is the preferred treatment. In our study, physicians who had been in practice for a longer time were more likely to treat using SRS alone. The results showed a linear association, with those in practice for a longer time being more likely to use SRS alone compared with those practicing for a shorter time (P = .027). Accordingly, 67% of respondents (8 of 12) who had been in practice for 11 or more years used SRS alone, whereas 24% (14 of 58) who had practiced for 0-5 years and 42% (5 of 12) who had practice from 6-10 years used SRS alone (Figure 1). When treating with SRS, younger practitioners placed more importance on the status of extracranial disease, whereas older practitioners placed more importance on tumor histopathology.
The use of repeat whole-brain reirradiation is more controversial among practitioners.20-22 Son and colleagues evaluated patients who needed whole-brain reirradiation after intracranial disease progression.22 The authors noted that patients with stable extracranial disease benefited from reirradiation. In our study, we found that when considering whole-brain reirradiation, older practitioners placed more importance on tumor histology than other factors.
As far as we know, this is the first study evaluating the practices and patterns of care with regard to the delivery of brain radiation in academic centers in the US. We found that time in practice was the most significant predictor of treatment technique and delivery. We also found that older practitioners place more importance on tumor histopathology compared with younger practitioners. A limitation of this study is that we had contact information only for program coordinators at ACGME-accredited programs. As such, we were not able to assess practice patterns among community practitioners. In addition, it seemed that residents and junior faculty were more likely to respond to this survey, likely because of the dissemination pattern. Given the evolution and diversity of treatment regimens for brain metastases, we believe that patients with brain metastases should be managed individually using a multidisciplinary approach.
1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872.
2. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698-2705.
3. Takakura K. Metastatic tumors of the central nervous system. Tokyo: Igaku-Shoin; 1982.
4. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
5. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New Engl J Med. 1990;322(8):494-500.
6. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91(4):710-717.
7. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
8. Choosing Wisely [ASTRO]. Don’t routinely add adjuvant whole-brain radiation therapy to stereotactic radiosurgery for limited brain metastases. http://www.choosingwisely.org/clinician-lists/american-society-radiation-oncology-adjunct-whole-brain-radiation-therapy/. Updated June 21, 2016. Accessed November 10, 2016.
9. Aoyama H, Tago M, Shirato H, Japanese Radiation Oncology Study Group I. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457-464.
10. Hoddinott SN, Bass MJ. The Dillman total design survey method. Can Fam Physician. 1986;32:2366-2368.
11. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54.
12. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5-14.
13. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7(4):682-689.
14. Nieder C, Niewald M, Schnabel K. Treatment of brain metastases from hypernephroma. Urol Int. 1996;57(1):17-20.
15. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
16. Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429-1437.
17. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810-3816.
18. Slade AN, Stanic S. The impact of RTOG 0614 and RTOG 0933 trials in routine clinical practice: The US Survey of Utilization of Memantine and IMRT planning for hippocampus sparing in patients receiving whole-brain radiotherapy for brain metastases. Contemp Clin Trials. 2016;47:74-77.
19. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2015;91(4):710-717.
20. Hazuka MB, Kinzie JJ. Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys. 1988;15(2):433-437.
21. Sadikov E, Bezjak A, Yi QL, et al. Value of whole-brain re-irradiation for brain metastases — single centre experience. Clin Oncol (R Coll Radiol). 2007;19(7):532-538.
22. Son CH, Jimenez R, Niemierko A, Loeffler JS, Oh KS, Shih HA. outcomes after whole-brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(2):e167-e172.
Despite the recent advances in systemic therapy, metastatic spread to the brain continues to be the most common neurologic complication of many cancers. The clinical incidence of brain metastases varies with primary cancer diagnosis, with estimates ranging from 1.2%-19.8%.1,2 Metastatic spread to the brain is even more prevalent at autopsy, with evidence of intracranial tumor being found in 26% of patients in some series.3 It is possible that the clinical incidence of metastatic disease to the brain will continue to increase as newer therapeutic agents improve survival and imaging techniques continue to improve.
The management of brain metastases has changed rapidly as technological improvements have made treatment increasingly safe and efficacious. Traditionally, treatment consisted of radiotherapy to the whole brain, with or without surgical resection.4,5 More recently, stereotactic radiosurgery (SRS) has been adopted on the basis of evidence that it is safe and efficacious alone or in combination with radiotherapy to the whole brain.6 Further evidence is emerging that neurocognitive outcomes are improved when whole-brain radiotherapy (WBRT) is omitted, which possibly contributes to improved patient quality of life.7 Taking into account this and other data, the American Society for Radiation Oncology’s Choosing Wisely campaign now recommends not routinely adding WBRT to radiosurgery in patients with limited brain metastases.8
Despite this recommendation, many patients continue to benefit from WBRT, and it remains a common treatment in radiation oncology clinics across the US for several reasons. Many patients present with multiple brain metastases and are ineligible for radiosurgery. Even for technically eligible patients, WBRT has been shown to improve local control and decrease the rate of distant brain failure over radiosurgery alone.6 With higher rates of subsequent failures, patients receiving radiosurgery alone must adhere to more rigorous follow-up and imaging schedules, which can be difficult for many rural patients who have to travel long distances to centers. Furthermore, there is some suggestion that this decreased failure rate may result in improved survival in highly selected patients with excellent disease and performance status.9 Controversies exist, however, and strong institutional biases persist, contributing to significant differences in practice. We surveyed academic radiation oncologists and in an effort to identify and describe practice patterns in the delivery of WBRT at academic centers.
Methods
We conducted a thorough review of available literature on radiation for brain metastases and based on our findings, devised a survey 19 questions to ascertain practice patterns and treatment delivery among US academic physicians (Table 1). After obtaining institutional review board approval to do the study, we sent the survey to program coordinators at radiation oncology programs that are accredited by the Accreditation Council for Graduate Medical Education. We instructed coordinators to e-mail the survey to their practicing resident and attending physicians. The surveys were created using SurveyMonkey software. We obtained informed consent from the providers. A total of 3 follow-up e-mails were sent to each recipient of the survey to solicit responses, similar to the Dillman Total Design Survey Method.10
SPSS version 22.0 was used to analyze the data in an exploratory fashion. Statistical methods were used to assess the association of demographic data with SRS and WBRT delivery and treatment technique items when the analyses involved percentages that included the Pearson chi-square statistic and the chi-square test for linear trend. When the analysis focused on ranking data, the Kruskal-Wallis test, Mann-Whitney U test, the Jonckheere-Terpstra and the Kendall tau-b rank correlation were used as appropriate. If there were small sample sizes within some groups, then exact significant levels were assessed. Statistical significance was set by convention at P < .05.
Results
We received 95 responses of which 87 were considered complete for analysis. Forty-seven percent of the 87 respondents were not board-certified, and the remainder had passed their radiobiology and physics boards exams. A majority of respondents (70%, 61 of 87) were physicians who had been in practice for ≤5 years. Fifty-four percent of respondents were located in the Northeast US, 22% in the South, 14% in the West, and 10% in the Midwest and Hawaii (Table 2).
We used the chi-square test for linear trends to assess for a relationship between years of practice and whether respondents deviated from their typical method of WBRT therapy when treating more radioresistant tumors (melanoma, renal cell carcinoma). Respondents were classified by years in practice: 0-5, 6-10, 11-20, and >21 years. The results showed a linear association, with those in practice for longer periods more likely to use SRS alone, P = .027 (Figure 1).
Discussion
The incidence of brain metastases is increasing because of improvements in diagnostic imaging techniques and advancements in systemic therapy control of extracranial disease but not of intracranial disease or metastasis, because therapies do not cross the blood-brain barrier.11,12 Brain metastases are the most common type of brain tumor. Given that most chemotherapeutic agents cannot cross the blood-brain barrier, radiotherapy is considered a means of treatment and of controlling brain metastases. Early data from the 1950s13 and 1960s14 have suggested clinical improvement with brain radiation, making radiotherapy the cornerstone for treatment of brain metastases.
The Radiation Therapy Oncology Group (RTOG) has evaluated several fractionation schedules, with 5 schemas evaluated by the RTOG 6901 and 7361 studies: 30 Gy in 10 fractions, 30 Gy in 15 fractions, 40 Gy in 15 fractions, 40 Gy in 20 fractions, and 20 Gy in 5 fractions. The combined results from these two trials showed that outcomes were similar for patients treated with a shorter regimen than for those treated with a more protracted schedule. In our study, respondents reported that they most frequently treated brain metastases to a total dose of 30 Gy in 10 fractions. Given the results of the aforementioned RTOG trials and practice patterns among academic physicians, we recommend all practitioners consider a shorter hypofractioned course when treating brain metastases with WBRT. This will also reduce delays for patients who are likely to benefit greatly from earlier enrollment into hospice care, because protracted radiation schedules typically are not covered while a patient is in hospice.
Pharmacologic management for patients with brain metastases is important for symptomatic improvement. Glucocorticoids are important for palliation of symptoms from edema and increased intracranial pressure.15 However, steroids have a multitude of side effects and their use in asymptomatic patients is unnecessary. Improvements in imaging and detection11 have allowed us to find smaller and asymptomatic brain tumors. In our survey, it was promising to see a change in former practice patterns, with only 8% of academic practitioners regularly prescribing steroids to all of their patients receiving whole-brain radiation.
Diminished cognitive function and short-term memory loss are troublesome side effects of WBRT. As cancer patients live longer, such cognitive dysfunction will become more than just a nuisance. The RTOG has investigated the use of prophylactic memantine for patients receiving whole-brain radiation to determine if it would aid in the preservation of cognition. It found that patients who received memantine did better and had delayed time to cognitive decline and a reduced rate of memory decline, executive function, and processing speed.16 In our study, about a third of practitioners prescribed memantine and it was reserved for patients who had an otherwise favorable prognosis.
The RTOG has also investigated adjusting treatment technique for patients who receive WBRT. RTOG 0933 was a phase 2 trial that evaluated hippocampal avoidance during deliverance of WBRT with intensity-modulated radiation therapy (IMRT). Results showed that avoiding the hippocampus during WBRT was associated with improved memory preservation and patient quality of life.17 In a survey of practicing radiation oncologists in the US, most reported that they did not use memantine or IMRT for hippocampal sparing when delivering whole-brain radiation.18 Given the positive results of RTOG 0933 and 0614, the NRG Oncology research organization is conducting a phase 3 randomized trial that compares memantine use for patients receiving whole-brain radiation with or without hippocampal sparing to determine if patients will have reduced cognitive decline. All patients receiving WBRT should be considered for enrolment on this trial if they are eligible.
The delivery of brain radiation has continued to change, especially with the introduction of SRS. Recent publication of a meta-analysis of three phase 3 trials evaluating SRS with or without WBRT for 1-4 brain metastases showed that patients aged 50 years or younger experienced a survival benefit with SRS, and the omission of whole-brain radiation did not affect distant brain relapse rates. 19 The authors recommended that for this population, SRS alone is the preferred treatment. In our study, physicians who had been in practice for a longer time were more likely to treat using SRS alone. The results showed a linear association, with those in practice for a longer time being more likely to use SRS alone compared with those practicing for a shorter time (P = .027). Accordingly, 67% of respondents (8 of 12) who had been in practice for 11 or more years used SRS alone, whereas 24% (14 of 58) who had practiced for 0-5 years and 42% (5 of 12) who had practice from 6-10 years used SRS alone (Figure 1). When treating with SRS, younger practitioners placed more importance on the status of extracranial disease, whereas older practitioners placed more importance on tumor histopathology.
The use of repeat whole-brain reirradiation is more controversial among practitioners.20-22 Son and colleagues evaluated patients who needed whole-brain reirradiation after intracranial disease progression.22 The authors noted that patients with stable extracranial disease benefited from reirradiation. In our study, we found that when considering whole-brain reirradiation, older practitioners placed more importance on tumor histology than other factors.
As far as we know, this is the first study evaluating the practices and patterns of care with regard to the delivery of brain radiation in academic centers in the US. We found that time in practice was the most significant predictor of treatment technique and delivery. We also found that older practitioners place more importance on tumor histopathology compared with younger practitioners. A limitation of this study is that we had contact information only for program coordinators at ACGME-accredited programs. As such, we were not able to assess practice patterns among community practitioners. In addition, it seemed that residents and junior faculty were more likely to respond to this survey, likely because of the dissemination pattern. Given the evolution and diversity of treatment regimens for brain metastases, we believe that patients with brain metastases should be managed individually using a multidisciplinary approach.
Despite the recent advances in systemic therapy, metastatic spread to the brain continues to be the most common neurologic complication of many cancers. The clinical incidence of brain metastases varies with primary cancer diagnosis, with estimates ranging from 1.2%-19.8%.1,2 Metastatic spread to the brain is even more prevalent at autopsy, with evidence of intracranial tumor being found in 26% of patients in some series.3 It is possible that the clinical incidence of metastatic disease to the brain will continue to increase as newer therapeutic agents improve survival and imaging techniques continue to improve.
The management of brain metastases has changed rapidly as technological improvements have made treatment increasingly safe and efficacious. Traditionally, treatment consisted of radiotherapy to the whole brain, with or without surgical resection.4,5 More recently, stereotactic radiosurgery (SRS) has been adopted on the basis of evidence that it is safe and efficacious alone or in combination with radiotherapy to the whole brain.6 Further evidence is emerging that neurocognitive outcomes are improved when whole-brain radiotherapy (WBRT) is omitted, which possibly contributes to improved patient quality of life.7 Taking into account this and other data, the American Society for Radiation Oncology’s Choosing Wisely campaign now recommends not routinely adding WBRT to radiosurgery in patients with limited brain metastases.8
Despite this recommendation, many patients continue to benefit from WBRT, and it remains a common treatment in radiation oncology clinics across the US for several reasons. Many patients present with multiple brain metastases and are ineligible for radiosurgery. Even for technically eligible patients, WBRT has been shown to improve local control and decrease the rate of distant brain failure over radiosurgery alone.6 With higher rates of subsequent failures, patients receiving radiosurgery alone must adhere to more rigorous follow-up and imaging schedules, which can be difficult for many rural patients who have to travel long distances to centers. Furthermore, there is some suggestion that this decreased failure rate may result in improved survival in highly selected patients with excellent disease and performance status.9 Controversies exist, however, and strong institutional biases persist, contributing to significant differences in practice. We surveyed academic radiation oncologists and in an effort to identify and describe practice patterns in the delivery of WBRT at academic centers.
Methods
We conducted a thorough review of available literature on radiation for brain metastases and based on our findings, devised a survey 19 questions to ascertain practice patterns and treatment delivery among US academic physicians (Table 1). After obtaining institutional review board approval to do the study, we sent the survey to program coordinators at radiation oncology programs that are accredited by the Accreditation Council for Graduate Medical Education. We instructed coordinators to e-mail the survey to their practicing resident and attending physicians. The surveys were created using SurveyMonkey software. We obtained informed consent from the providers. A total of 3 follow-up e-mails were sent to each recipient of the survey to solicit responses, similar to the Dillman Total Design Survey Method.10
SPSS version 22.0 was used to analyze the data in an exploratory fashion. Statistical methods were used to assess the association of demographic data with SRS and WBRT delivery and treatment technique items when the analyses involved percentages that included the Pearson chi-square statistic and the chi-square test for linear trend. When the analysis focused on ranking data, the Kruskal-Wallis test, Mann-Whitney U test, the Jonckheere-Terpstra and the Kendall tau-b rank correlation were used as appropriate. If there were small sample sizes within some groups, then exact significant levels were assessed. Statistical significance was set by convention at P < .05.
Results
We received 95 responses of which 87 were considered complete for analysis. Forty-seven percent of the 87 respondents were not board-certified, and the remainder had passed their radiobiology and physics boards exams. A majority of respondents (70%, 61 of 87) were physicians who had been in practice for ≤5 years. Fifty-four percent of respondents were located in the Northeast US, 22% in the South, 14% in the West, and 10% in the Midwest and Hawaii (Table 2).
We used the chi-square test for linear trends to assess for a relationship between years of practice and whether respondents deviated from their typical method of WBRT therapy when treating more radioresistant tumors (melanoma, renal cell carcinoma). Respondents were classified by years in practice: 0-5, 6-10, 11-20, and >21 years. The results showed a linear association, with those in practice for longer periods more likely to use SRS alone, P = .027 (Figure 1).
Discussion
The incidence of brain metastases is increasing because of improvements in diagnostic imaging techniques and advancements in systemic therapy control of extracranial disease but not of intracranial disease or metastasis, because therapies do not cross the blood-brain barrier.11,12 Brain metastases are the most common type of brain tumor. Given that most chemotherapeutic agents cannot cross the blood-brain barrier, radiotherapy is considered a means of treatment and of controlling brain metastases. Early data from the 1950s13 and 1960s14 have suggested clinical improvement with brain radiation, making radiotherapy the cornerstone for treatment of brain metastases.
The Radiation Therapy Oncology Group (RTOG) has evaluated several fractionation schedules, with 5 schemas evaluated by the RTOG 6901 and 7361 studies: 30 Gy in 10 fractions, 30 Gy in 15 fractions, 40 Gy in 15 fractions, 40 Gy in 20 fractions, and 20 Gy in 5 fractions. The combined results from these two trials showed that outcomes were similar for patients treated with a shorter regimen than for those treated with a more protracted schedule. In our study, respondents reported that they most frequently treated brain metastases to a total dose of 30 Gy in 10 fractions. Given the results of the aforementioned RTOG trials and practice patterns among academic physicians, we recommend all practitioners consider a shorter hypofractioned course when treating brain metastases with WBRT. This will also reduce delays for patients who are likely to benefit greatly from earlier enrollment into hospice care, because protracted radiation schedules typically are not covered while a patient is in hospice.
Pharmacologic management for patients with brain metastases is important for symptomatic improvement. Glucocorticoids are important for palliation of symptoms from edema and increased intracranial pressure.15 However, steroids have a multitude of side effects and their use in asymptomatic patients is unnecessary. Improvements in imaging and detection11 have allowed us to find smaller and asymptomatic brain tumors. In our survey, it was promising to see a change in former practice patterns, with only 8% of academic practitioners regularly prescribing steroids to all of their patients receiving whole-brain radiation.
Diminished cognitive function and short-term memory loss are troublesome side effects of WBRT. As cancer patients live longer, such cognitive dysfunction will become more than just a nuisance. The RTOG has investigated the use of prophylactic memantine for patients receiving whole-brain radiation to determine if it would aid in the preservation of cognition. It found that patients who received memantine did better and had delayed time to cognitive decline and a reduced rate of memory decline, executive function, and processing speed.16 In our study, about a third of practitioners prescribed memantine and it was reserved for patients who had an otherwise favorable prognosis.
The RTOG has also investigated adjusting treatment technique for patients who receive WBRT. RTOG 0933 was a phase 2 trial that evaluated hippocampal avoidance during deliverance of WBRT with intensity-modulated radiation therapy (IMRT). Results showed that avoiding the hippocampus during WBRT was associated with improved memory preservation and patient quality of life.17 In a survey of practicing radiation oncologists in the US, most reported that they did not use memantine or IMRT for hippocampal sparing when delivering whole-brain radiation.18 Given the positive results of RTOG 0933 and 0614, the NRG Oncology research organization is conducting a phase 3 randomized trial that compares memantine use for patients receiving whole-brain radiation with or without hippocampal sparing to determine if patients will have reduced cognitive decline. All patients receiving WBRT should be considered for enrolment on this trial if they are eligible.
The delivery of brain radiation has continued to change, especially with the introduction of SRS. Recent publication of a meta-analysis of three phase 3 trials evaluating SRS with or without WBRT for 1-4 brain metastases showed that patients aged 50 years or younger experienced a survival benefit with SRS, and the omission of whole-brain radiation did not affect distant brain relapse rates. 19 The authors recommended that for this population, SRS alone is the preferred treatment. In our study, physicians who had been in practice for a longer time were more likely to treat using SRS alone. The results showed a linear association, with those in practice for a longer time being more likely to use SRS alone compared with those practicing for a shorter time (P = .027). Accordingly, 67% of respondents (8 of 12) who had been in practice for 11 or more years used SRS alone, whereas 24% (14 of 58) who had practiced for 0-5 years and 42% (5 of 12) who had practice from 6-10 years used SRS alone (Figure 1). When treating with SRS, younger practitioners placed more importance on the status of extracranial disease, whereas older practitioners placed more importance on tumor histopathology.
The use of repeat whole-brain reirradiation is more controversial among practitioners.20-22 Son and colleagues evaluated patients who needed whole-brain reirradiation after intracranial disease progression.22 The authors noted that patients with stable extracranial disease benefited from reirradiation. In our study, we found that when considering whole-brain reirradiation, older practitioners placed more importance on tumor histology than other factors.
As far as we know, this is the first study evaluating the practices and patterns of care with regard to the delivery of brain radiation in academic centers in the US. We found that time in practice was the most significant predictor of treatment technique and delivery. We also found that older practitioners place more importance on tumor histopathology compared with younger practitioners. A limitation of this study is that we had contact information only for program coordinators at ACGME-accredited programs. As such, we were not able to assess practice patterns among community practitioners. In addition, it seemed that residents and junior faculty were more likely to respond to this survey, likely because of the dissemination pattern. Given the evolution and diversity of treatment regimens for brain metastases, we believe that patients with brain metastases should be managed individually using a multidisciplinary approach.
1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872.
2. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698-2705.
3. Takakura K. Metastatic tumors of the central nervous system. Tokyo: Igaku-Shoin; 1982.
4. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
5. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New Engl J Med. 1990;322(8):494-500.
6. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91(4):710-717.
7. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
8. Choosing Wisely [ASTRO]. Don’t routinely add adjuvant whole-brain radiation therapy to stereotactic radiosurgery for limited brain metastases. http://www.choosingwisely.org/clinician-lists/american-society-radiation-oncology-adjunct-whole-brain-radiation-therapy/. Updated June 21, 2016. Accessed November 10, 2016.
9. Aoyama H, Tago M, Shirato H, Japanese Radiation Oncology Study Group I. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457-464.
10. Hoddinott SN, Bass MJ. The Dillman total design survey method. Can Fam Physician. 1986;32:2366-2368.
11. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54.
12. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5-14.
13. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7(4):682-689.
14. Nieder C, Niewald M, Schnabel K. Treatment of brain metastases from hypernephroma. Urol Int. 1996;57(1):17-20.
15. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
16. Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429-1437.
17. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810-3816.
18. Slade AN, Stanic S. The impact of RTOG 0614 and RTOG 0933 trials in routine clinical practice: The US Survey of Utilization of Memantine and IMRT planning for hippocampus sparing in patients receiving whole-brain radiotherapy for brain metastases. Contemp Clin Trials. 2016;47:74-77.
19. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2015;91(4):710-717.
20. Hazuka MB, Kinzie JJ. Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys. 1988;15(2):433-437.
21. Sadikov E, Bezjak A, Yi QL, et al. Value of whole-brain re-irradiation for brain metastases — single centre experience. Clin Oncol (R Coll Radiol). 2007;19(7):532-538.
22. Son CH, Jimenez R, Niemierko A, Loeffler JS, Oh KS, Shih HA. outcomes after whole-brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(2):e167-e172.
1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872.
2. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698-2705.
3. Takakura K. Metastatic tumors of the central nervous system. Tokyo: Igaku-Shoin; 1982.
4. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
5. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New Engl J Med. 1990;322(8):494-500.
6. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91(4):710-717.
7. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
8. Choosing Wisely [ASTRO]. Don’t routinely add adjuvant whole-brain radiation therapy to stereotactic radiosurgery for limited brain metastases. http://www.choosingwisely.org/clinician-lists/american-society-radiation-oncology-adjunct-whole-brain-radiation-therapy/. Updated June 21, 2016. Accessed November 10, 2016.
9. Aoyama H, Tago M, Shirato H, Japanese Radiation Oncology Study Group I. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457-464.
10. Hoddinott SN, Bass MJ. The Dillman total design survey method. Can Fam Physician. 1986;32:2366-2368.
11. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54.
12. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5-14.
13. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7(4):682-689.
14. Nieder C, Niewald M, Schnabel K. Treatment of brain metastases from hypernephroma. Urol Int. 1996;57(1):17-20.
15. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
16. Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429-1437.
17. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810-3816.
18. Slade AN, Stanic S. The impact of RTOG 0614 and RTOG 0933 trials in routine clinical practice: The US Survey of Utilization of Memantine and IMRT planning for hippocampus sparing in patients receiving whole-brain radiotherapy for brain metastases. Contemp Clin Trials. 2016;47:74-77.
19. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2015;91(4):710-717.
20. Hazuka MB, Kinzie JJ. Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys. 1988;15(2):433-437.
21. Sadikov E, Bezjak A, Yi QL, et al. Value of whole-brain re-irradiation for brain metastases — single centre experience. Clin Oncol (R Coll Radiol). 2007;19(7):532-538.
22. Son CH, Jimenez R, Niemierko A, Loeffler JS, Oh KS, Shih HA. outcomes after whole-brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(2):e167-e172.
Distress management in cancer patients in Puerto Rico
A comprehensive, patient-centered approach is required to accomplish cancer best standards of care.1 This approach reflects the holistic conceptualization of health in which the physical, emotional, and social dimensions of the human being are considered when providing medical care. As a result, to look after all patient needs, interdisciplinary and well-coordinated interventions are recommended. Cancer patients should be provided not only with diagnostic, treatment, and follow-up clinical service, but also with the supportive assistance that may positively influence all aspects of their health.
To appraise physical, social, emotional and spiritual issues and to develop supportive interventional action plans, the National Comprehensive Cancer Network (NCCN) recommends screening all cancer patients for distress.2 In particular, screening the emotional component of distress occupies a prominent place in this process because it is now recognized as the sixth vital sign in oncology.3 Even though the influence of emotional distress over cancer mortality rates and disease progression is still under scrutiny,4 its plausible implications over treatment compliance have been pointed out. Patients with higher levels of emotional distress show lower adherence to treatment and poorer health outcomes.5 Furthermore, prevalence rates of emotional distress in cancer patients from ambulatory settings6 and oncology surgical units have been studied and have provided justification for distress management.7 Studies have shown low ability among oncologists to identify patients in distress and oncologists’ tendency to judge distress higher than the patients themselves.8 As a consequence, to achieve systematic distress evaluations and appropriate referrals for care, guidelines for distress management should be implemented in clinical settings. It is recommended that tests are conducted to find brief screening instruments and procedures to assure accurate interventions according to patient specific needs.
This article presents the process of implementing a distress management program at HIMA-San Pablo Oncologic Hospital in Caguas, Puerto Rico, with particular emphasis on the management of emotional distress, which has been defined as the feeling of suffering that cancer patients may experience after diagnosis. In addition, we have included data from a pilot study that was completed for content validation of the Patient Health Questionnaire (PHQ-9) to estimate depression levels in Puerto Rican cancer patients.
Methods
HIMA-San Pablo operates a group of privately owned hospitals in Puerto Rico. It established a cancer center in Caguas in 2007, recruiting a multispecialty medical faculty to provide cancer care and bone marrow transplants for adult and pediatric patients. The cancer center, currently named HIMA-San Pablo Oncologic Hospital (HSPOH), is a hospital within a hospital licensed by the Puerto Rico Department of Health. In 2007, a cancer committee was established as the steering committee to ensure the delivery of cancer care according to best standards of care. The committee took responsibility for developing all activities needed to achieve the American College of Surgeons’ Commission on Cancer (CoC) accreditation under the category of Comprehensive Community Cancer Center. The committee established a psychosocial team to develop a protocol for the delivery of distress management for adult patients. (The psychosocial needs of pediatric patients are assessed through other procedures.)
To develop the protocol, principles of input-output model of research and quality analysis in health care were applied.9 The input-output model, with its origin in engineering, helped map systematic activities to transform empirical data on cancer psychosocial care into operational procedures. Focus was given to data gathering (input), information organization and analysis (throughput), and the schematization of emotional distress management care (output).
The input phase
In the input phase, elements of psychosocial care and operational definitions related to distress management in general were identified through literature review (Table 1). Basic parameters for distress management were clarified, resulting in a conceptual framework based in four remarks: First, according to NCCN, distress is a multifactorial unpleasant emotional experience of a psychological, social, and spiritual nature. It may interfere with the ability to cope effectively with cancer, its symptoms and its treatment. Its intensity may fluctuate from feelings of suffering and fear to incapacitating manifestations of anxiety and depression2 and its severity may hamper patient quality of life and treatment compliance.
Second, distress management requires the intervention of an interdisciplinary team with both medical and allied health professionals. This may include mental health specialists and other professionals with training and experience in cancer-related issues, who work with reciprocal channels of communication for the exchange of patient information.
Third, NCCN recommends using the Distress Thermometer for patient initial distress screening.10-12 It consists of a numeric scale ranging from 0 (no distress) to 10 (severe distress) in which patients classify their level of distress. The numeric scale is followed by a section in which patients identify areas of practical, familiar, emotional, spiritual/ religious, and physical concerns. Based on responses, interviews may follow to set distress management interventions.
Fourth, screening and assessment are different but sequential and complementary stages of distress management. Screening is viewed as a rapid strategy to identify cancer patients in distress. Assessment looks out for a broader appraisal and documentation of factors with repercussions over patient distress level and resiliency capability.13 In many instances, the patient’s emotional distress is better understood in the assessment phase.
The throughput phase
Within the throughput phase of information organization and analysis, an inventory of health professionals and other in-house consultants needed for distress management was completed. Roles and procedures for information sharing were determined, and we established collaborative agreements with professionals in the community who could contribute to distress management. Members of the psychosocial team held workshops to discuss elements of NCCN guidelines for distress management and to create an action plan for the implementation of the protocol. Data analyses were performed to create a demographic profile of the oncology population at the hospital and assess patient willingness to receive emotional support services,16 which led to the implementation of support group meetings at which additional substantive information was collected about issues affecting cancer patients’ emotions.
The NCCN Distress Thermometer for measuring distress was translated to Spanish. Its format was adapted, and it was identified as a distress screening tool (DST), which we named Distress Assessment Tool for Oncology Patients (Figure 1). The instrument helps for rapid screening of patient needs and proper determination of initial interventions. In addition, psychometric properties of several instruments were reviewed for instances when patient emotional distress could not be clearly determined. We decided to proceed with the validation of the 9-item Patient Health Questionnaire (PHQ-9) to estimate patient depression level. A proposal for content validation of the PHQ-9 was approved by the University of Puerto Rico institutional review board, and patients were recruited to participate in the pilot study.
The PHQ-9. The PHQ-9 is a self-report version of the PRIME-MD instrument developed to assess mental disorders in clinical settings. It is based on DSM-IV diagnostic criteria.17 The PHQ-9 is the depression module with nine depression symptoms to check off if they become the cause of emotional impairment. Respondents categorized depression symptoms in four frequency degrees representing numeric values: 0 (not at all), 1 (several days), 2 (more than half the days), 3 (nearly every day). Measures of depression severity are subsequently determined in a Likert-type scale according to numeric calculations of responses: 0-4 (none severe depression), 5-9 (mild), 10-14 (moderate), 15-19 (moderately severe), and 20-27 (severe-major depression).
The instrument is widely used because of its validity in small and large populations. It showed adequate reliability and validity in a small sample of head and neck cancer patients, with a Cronbach’s alpha of 0.80 and a correlation coefficient of 0.71.18 Similarly, it showed good performance in identifying major depression in 4264 cancer outpatients, with sensitivity of 93%, specificity of 81%, and a positive predictive value (PPV) of 25% and negative predictive value (NPV) of 99%.19 Even when administered on a touch screen computer, the instrument showed valid data of depression from patients in treatment.20
The Beck Depression Inventory. We used the Beck Depression Inventory (BDI-II) Spanish version as the gold standard measure for the validation study. It is a 22-items inventory that measures attitudes and symptoms of depression.21 It can be administered in 10 minutes and has shown good psychometric measures when administered in Spain and Puerto Rico.22, 23
The pilot study. In all, 44 cancer patients who were receiving outpatient treatment at the radiotherapy unit agreed to participate in the study. The participants signed a consent form after the confidentiality protection measures and the main objectives of the study had been explained to them. Patients were interviewed individually during November and December 2012, with the Spanish versions of the PHQ-9 and BDI-II administered by one of two interviewers. At the beginning of each interview, the patient was asked 10 questions so that we could gather demographic data and confirm participant eligibility: aged 21 years or older, born and raised in Puerto Rico, being a Spanish speaker, and having a primary cancer diagnosis with no previous disease. Three patients were excluded from the sample because they either had cancer previously or had a recurrence or metastasis. The final sample consisted of 41 outpatients (N = 41).
Data analysis for demographics was completed with STATA v.12 software. Measures of central tendency and dispersion as well as PHQ-9 internal consistency analysis were made through Cronbach alpha with SPSS.
From a total of 41 patients surveyed, 22 (54%) were men and 19 (46%) were women, with an overall median age of 61 years. Among the men, 15 (68%) had a prostate cancer diagnosis and among women, 9 (47.4%) had a breast cancer diagnosis. In regard to health insurance, 19 (46%) had Medicare or Veterans/federal insurance coverage, and 13 (32%) had Reforma, the Puerto Rican government health insurance program partially funded by Medicaid funds. In addition, 8 participants (20%) were unemployed or disabled. As previously stated, all of the patients were in ambulatory care. Only 3 (7%) were participating in support groups.
Of all the respondents, 16 (39%) reported some level of depression. In particular, 2 (5%) showed severe-major depression, 4 (10%) moderately severe depression, and 10 (24%) moderate depression. Of those with depression, 8 (50%) were women, 8 (50%) were men. All 6 of the patients with head and neck cancer showed moderate or moderately severe depression (Table 2).
When respondent PHQ-9 scoring reflected moderate to severe depression (>10), a letter was sent to the patient’s radio-oncologist for referral to counseling and clinical psychological evaluation. All participants had access to the support group program, to a radiotherapy education program meeting weekly, and written information about their cancer diagnosis and treatment. They also were interviewed by the psychosocial coordinator or patient navigator for further assessment.
The output phase
In the output phase, a graphic representing the process of emotional assessment at the institution was created and then modified. PHQ-9 was added to the process when it was found suitable to assess level of depression contributing to the identification of patients requiring psychological and psychiatric assistance which by other means would be missed. PHQ-9 was useful in the busy clinical setting as it was completed, scored and interpreted in minutes. It showed the potential for routine evaluations when looking to identify improvement or deterioration in depression levels thus helping to monitor responses to treatment and providing insights for follow up interventions. As stated by NCCN guidelines, distress should be monitored, documented and managed at all stages of the cancer continuum.
Results and discussion
The protocol for distress management at HSPOH is based on the 2013 NCCN guidelines. Cancer patients are screened for levels of distress in all settings (inpatients and outpatients). Screening is held with the DST Spanish translation at the moment of diagnosis or as soon as possible after a diagnosis is made. Screening for distress is also done before or after surgery, in recurrence or progression, and when clinically indicated. Patients are informed that distress management is an essential part of their care and are encouraged to provide information so that we can make a proper need assessment.
Patients are screened by the psychosocial coordinator or patient navigator who administers the DST followed by in-depth interviews for additional appraisal. An action plan is designed based on patient needs, which include their intervention and the intervention of other members of the psychosocial team from the institution and/or from the community. Additional in-house health professionals contributing in distress management include, but are not limited to: physicians; clinical psychologists; health educators; social workers; dietitians; chaplains; and physical, respiratory, speech, and/or swallow therapists. Follow-up and rescreening sessions are scheduled to assure coordination of services between those health professionals as well as to secure continuity of distress management during all stages of the cancer continuum.
The results of the DST are filed in patient medical records. Members of the psychosocial team also document their interventions in the patient medical record, which helps in the exchange of information among the cancer care team. The psychosocial team meets once a month – or as required for extraordinary cases – to review and discuss the cases, determine the best options for distress management, and identify areas for psychosocial care improvement. Those findings and the results of distress management in patient level of satisfaction are then reported and discussed quarterly by the psychosocial coordinator and the cancer committee.
Figure 2 shows in what phase of emotional distress assessment the PHQ-9 was included. Patients reporting four or more of the six areas of concern related to emotional distress in the DST (Figure 1) are automatically referred to a mental health specialist. But when patients report three areas of concern with no clear data on their specific level of depression, PHQ-9 is administered to differentiate those who need a mental health specialist from those who could be adequately supported by health education and support group interventions. In this way detrimental outcomes such as duplicity and over or underuse of services and resources are reduced. In addition, it is recognized that using an interview after the administration of the DST to determine distress management actions does not always provide enough information about a patient’s emotional circumstances and previous comorbidities. Patient responses during interviews may be influenced by the patient’s level of literacy, verbal comprehension, and communication style,24 so emotional distress can go unrecognized during interviews, resulting in delays for treatment and supportive care.
National guidelines in oncology consider such socio-ecological models emphasizing the delivery of patient-centered, interdisciplinary, and evidence-based care. That does not mean that institutions should apply protocols of psychosocial care as previously developed, but that they should test, review, adapt, and improve them during the implementation of the care. In fact, NCCN encourages conducting trials to examine protocols, screening instruments, and models of intervention to determine applicability to particular settings.2
Findings from a study by NCCN member institutions to evaluate progress of implementing distress management guidelines found that 53% (n = 8) of respondent institutions conducted routine distress screening. Of those, 37.5% (3) relied only on interviews. That finding is of concern because if interviews are not standardized and have not been systematically evaluated, then their sensitivity and specificity in identifying distressed patients is unknown.26 Accordingly, the process described in this article and the PHQ-9 validation was an effort to standardize emotional distress management, and was underlined as an achievement during the CoC accreditation visit to the cancer center in December 2013. The hospital was accredited as a comprehensive community cancer center with gold commendations, becoming the first privately owned hospital in Puerto Rico to achieve the accreditation.
1. Commission on Cancer, American College of Surgeons. Cancer Programs Standards 2012: Ensuring Patient-Centered Care. Version 1.2.1. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Published 2012. Accessed March 5, 2013.
2. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN guidelines): Distress management. Version I. 2012. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Accessed March 5, 2013.
3. Bultz BD, Groff SL. Screening for distress, the 6th vital sign in oncology: from theory to practice: http://www.oncologyex.com/issue/2009/vol8_no1/8_comment2_1.html. Published February 2009. Accessed February 16, 2017.
4. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349-5361.
5. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Int Med. 2000;160:2101-2107.
6. Jadoon NA, Munir W, Shahzad MA, Choudhry ZS. Assessment of depression and anxiety in adult cancer outpatients: a cross sectional study. BMC Cancer. 2010;10:594.
7. Fisher D, Wedel B. Anxiety and depression disorders in cancer patients: incidence, diagnosis and therapy. Mag Eur Med Oncol. 2012;5:52-54.
8. Sollner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and in need for psychosocial counselling? Br J Cancer. 2001;84:179-185.
9. Scott RD, Solomon SL, McGowan JE. Applying economic principles to health care: special issue. Emerg Infect Dis. 2001;7:282-285.
10. Adler NE, Page AEK. A model for delivering psychosocial health services. In: Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press (US); 2008.
11. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8:4-12.
12. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494-1502.
13. Maihoff SE. Assessment. In Washington CM, Leaver D, eds. Principles and practice of radiation therapy. St Louis, MO: Mosby Elsevier; 2004:243-264.
14. National Academy of Sciences. Adler NE, Page AEK, eds. Cancer care for the whole patient: meeting psychosocial health needs. https://www.ncbi.nlm.nih.gov/books/NBK4015/. Published 2008. Accessed February 22, 2012.
15. Nancarrow SA, Booth A, Ariss
16. Baker-Glenn EA, Park B, Granger L, Symonds P, Mitchell AJ. Desire for psychological support in cancer patients with depression or distress: validation of a simple help question. Psychooncology. 2011;20:525-531.
17. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
18. Omoro SA, Fann JR, Weymuller EA, Macharia IM, Yueh B. Swahili translation and validation of the Patient Health Questionnaire-9 depression scale in the Kenyan head and neck cancer patient population. Int J Psychiatry Med. 2006;36:367-381.
19. Thekkumpurath P, Walker J, Butcher I, et al. Screening for major depression on cancer outpatients: the diagnostic accuracy of the 9-item Patient Health Questionnaire. Cancer. 2011;117:218-227.
20. Fann JR, Berry DL, Wolpin S, et al. Depression screening using the Patient Health Questionnaire-9 administered on a touch screen computer. Psychooncology. 2009;18:14-22.
21. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:651-571.
22. Sanz J, Perdigón AL, Vázquez C. The Spanish adaptation of Beck’s Depression Inventory–II (BDI-II): psychometric properties in the general population. Clínica y Salud. 2003;14:249-280.
23. Bonilla J, Bernal G, Santos A, Santos D. A revised Spanish version of the Beck Depression Inventory: psychometric properties with a Puerto Rican sample of college students. J Clin Psychol. 2004;60:119-130.
24. Alcántara C, Gone JP. Multicultural issues in the clinical interview and diagnostic process. In Leong FTL, ed. APA handbook of multicultural psychology. Vol 2. Applications and training. Washington, DC: American Psychological Association; 2014:153-163.
25. Sharma M, Romas JA. Theoretical foundations of health education and health promotion. 2nd ed. Burlington, MA: Jones & Barlett Learning; 2012.
26. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5:99-103.
A comprehensive, patient-centered approach is required to accomplish cancer best standards of care.1 This approach reflects the holistic conceptualization of health in which the physical, emotional, and social dimensions of the human being are considered when providing medical care. As a result, to look after all patient needs, interdisciplinary and well-coordinated interventions are recommended. Cancer patients should be provided not only with diagnostic, treatment, and follow-up clinical service, but also with the supportive assistance that may positively influence all aspects of their health.
To appraise physical, social, emotional and spiritual issues and to develop supportive interventional action plans, the National Comprehensive Cancer Network (NCCN) recommends screening all cancer patients for distress.2 In particular, screening the emotional component of distress occupies a prominent place in this process because it is now recognized as the sixth vital sign in oncology.3 Even though the influence of emotional distress over cancer mortality rates and disease progression is still under scrutiny,4 its plausible implications over treatment compliance have been pointed out. Patients with higher levels of emotional distress show lower adherence to treatment and poorer health outcomes.5 Furthermore, prevalence rates of emotional distress in cancer patients from ambulatory settings6 and oncology surgical units have been studied and have provided justification for distress management.7 Studies have shown low ability among oncologists to identify patients in distress and oncologists’ tendency to judge distress higher than the patients themselves.8 As a consequence, to achieve systematic distress evaluations and appropriate referrals for care, guidelines for distress management should be implemented in clinical settings. It is recommended that tests are conducted to find brief screening instruments and procedures to assure accurate interventions according to patient specific needs.
This article presents the process of implementing a distress management program at HIMA-San Pablo Oncologic Hospital in Caguas, Puerto Rico, with particular emphasis on the management of emotional distress, which has been defined as the feeling of suffering that cancer patients may experience after diagnosis. In addition, we have included data from a pilot study that was completed for content validation of the Patient Health Questionnaire (PHQ-9) to estimate depression levels in Puerto Rican cancer patients.
Methods
HIMA-San Pablo operates a group of privately owned hospitals in Puerto Rico. It established a cancer center in Caguas in 2007, recruiting a multispecialty medical faculty to provide cancer care and bone marrow transplants for adult and pediatric patients. The cancer center, currently named HIMA-San Pablo Oncologic Hospital (HSPOH), is a hospital within a hospital licensed by the Puerto Rico Department of Health. In 2007, a cancer committee was established as the steering committee to ensure the delivery of cancer care according to best standards of care. The committee took responsibility for developing all activities needed to achieve the American College of Surgeons’ Commission on Cancer (CoC) accreditation under the category of Comprehensive Community Cancer Center. The committee established a psychosocial team to develop a protocol for the delivery of distress management for adult patients. (The psychosocial needs of pediatric patients are assessed through other procedures.)
To develop the protocol, principles of input-output model of research and quality analysis in health care were applied.9 The input-output model, with its origin in engineering, helped map systematic activities to transform empirical data on cancer psychosocial care into operational procedures. Focus was given to data gathering (input), information organization and analysis (throughput), and the schematization of emotional distress management care (output).
The input phase
In the input phase, elements of psychosocial care and operational definitions related to distress management in general were identified through literature review (Table 1). Basic parameters for distress management were clarified, resulting in a conceptual framework based in four remarks: First, according to NCCN, distress is a multifactorial unpleasant emotional experience of a psychological, social, and spiritual nature. It may interfere with the ability to cope effectively with cancer, its symptoms and its treatment. Its intensity may fluctuate from feelings of suffering and fear to incapacitating manifestations of anxiety and depression2 and its severity may hamper patient quality of life and treatment compliance.
Second, distress management requires the intervention of an interdisciplinary team with both medical and allied health professionals. This may include mental health specialists and other professionals with training and experience in cancer-related issues, who work with reciprocal channels of communication for the exchange of patient information.
Third, NCCN recommends using the Distress Thermometer for patient initial distress screening.10-12 It consists of a numeric scale ranging from 0 (no distress) to 10 (severe distress) in which patients classify their level of distress. The numeric scale is followed by a section in which patients identify areas of practical, familiar, emotional, spiritual/ religious, and physical concerns. Based on responses, interviews may follow to set distress management interventions.
Fourth, screening and assessment are different but sequential and complementary stages of distress management. Screening is viewed as a rapid strategy to identify cancer patients in distress. Assessment looks out for a broader appraisal and documentation of factors with repercussions over patient distress level and resiliency capability.13 In many instances, the patient’s emotional distress is better understood in the assessment phase.
The throughput phase
Within the throughput phase of information organization and analysis, an inventory of health professionals and other in-house consultants needed for distress management was completed. Roles and procedures for information sharing were determined, and we established collaborative agreements with professionals in the community who could contribute to distress management. Members of the psychosocial team held workshops to discuss elements of NCCN guidelines for distress management and to create an action plan for the implementation of the protocol. Data analyses were performed to create a demographic profile of the oncology population at the hospital and assess patient willingness to receive emotional support services,16 which led to the implementation of support group meetings at which additional substantive information was collected about issues affecting cancer patients’ emotions.
The NCCN Distress Thermometer for measuring distress was translated to Spanish. Its format was adapted, and it was identified as a distress screening tool (DST), which we named Distress Assessment Tool for Oncology Patients (Figure 1). The instrument helps for rapid screening of patient needs and proper determination of initial interventions. In addition, psychometric properties of several instruments were reviewed for instances when patient emotional distress could not be clearly determined. We decided to proceed with the validation of the 9-item Patient Health Questionnaire (PHQ-9) to estimate patient depression level. A proposal for content validation of the PHQ-9 was approved by the University of Puerto Rico institutional review board, and patients were recruited to participate in the pilot study.
The PHQ-9. The PHQ-9 is a self-report version of the PRIME-MD instrument developed to assess mental disorders in clinical settings. It is based on DSM-IV diagnostic criteria.17 The PHQ-9 is the depression module with nine depression symptoms to check off if they become the cause of emotional impairment. Respondents categorized depression symptoms in four frequency degrees representing numeric values: 0 (not at all), 1 (several days), 2 (more than half the days), 3 (nearly every day). Measures of depression severity are subsequently determined in a Likert-type scale according to numeric calculations of responses: 0-4 (none severe depression), 5-9 (mild), 10-14 (moderate), 15-19 (moderately severe), and 20-27 (severe-major depression).
The instrument is widely used because of its validity in small and large populations. It showed adequate reliability and validity in a small sample of head and neck cancer patients, with a Cronbach’s alpha of 0.80 and a correlation coefficient of 0.71.18 Similarly, it showed good performance in identifying major depression in 4264 cancer outpatients, with sensitivity of 93%, specificity of 81%, and a positive predictive value (PPV) of 25% and negative predictive value (NPV) of 99%.19 Even when administered on a touch screen computer, the instrument showed valid data of depression from patients in treatment.20
The Beck Depression Inventory. We used the Beck Depression Inventory (BDI-II) Spanish version as the gold standard measure for the validation study. It is a 22-items inventory that measures attitudes and symptoms of depression.21 It can be administered in 10 minutes and has shown good psychometric measures when administered in Spain and Puerto Rico.22, 23
The pilot study. In all, 44 cancer patients who were receiving outpatient treatment at the radiotherapy unit agreed to participate in the study. The participants signed a consent form after the confidentiality protection measures and the main objectives of the study had been explained to them. Patients were interviewed individually during November and December 2012, with the Spanish versions of the PHQ-9 and BDI-II administered by one of two interviewers. At the beginning of each interview, the patient was asked 10 questions so that we could gather demographic data and confirm participant eligibility: aged 21 years or older, born and raised in Puerto Rico, being a Spanish speaker, and having a primary cancer diagnosis with no previous disease. Three patients were excluded from the sample because they either had cancer previously or had a recurrence or metastasis. The final sample consisted of 41 outpatients (N = 41).
Data analysis for demographics was completed with STATA v.12 software. Measures of central tendency and dispersion as well as PHQ-9 internal consistency analysis were made through Cronbach alpha with SPSS.
From a total of 41 patients surveyed, 22 (54%) were men and 19 (46%) were women, with an overall median age of 61 years. Among the men, 15 (68%) had a prostate cancer diagnosis and among women, 9 (47.4%) had a breast cancer diagnosis. In regard to health insurance, 19 (46%) had Medicare or Veterans/federal insurance coverage, and 13 (32%) had Reforma, the Puerto Rican government health insurance program partially funded by Medicaid funds. In addition, 8 participants (20%) were unemployed or disabled. As previously stated, all of the patients were in ambulatory care. Only 3 (7%) were participating in support groups.
Of all the respondents, 16 (39%) reported some level of depression. In particular, 2 (5%) showed severe-major depression, 4 (10%) moderately severe depression, and 10 (24%) moderate depression. Of those with depression, 8 (50%) were women, 8 (50%) were men. All 6 of the patients with head and neck cancer showed moderate or moderately severe depression (Table 2).
When respondent PHQ-9 scoring reflected moderate to severe depression (>10), a letter was sent to the patient’s radio-oncologist for referral to counseling and clinical psychological evaluation. All participants had access to the support group program, to a radiotherapy education program meeting weekly, and written information about their cancer diagnosis and treatment. They also were interviewed by the psychosocial coordinator or patient navigator for further assessment.
The output phase
In the output phase, a graphic representing the process of emotional assessment at the institution was created and then modified. PHQ-9 was added to the process when it was found suitable to assess level of depression contributing to the identification of patients requiring psychological and psychiatric assistance which by other means would be missed. PHQ-9 was useful in the busy clinical setting as it was completed, scored and interpreted in minutes. It showed the potential for routine evaluations when looking to identify improvement or deterioration in depression levels thus helping to monitor responses to treatment and providing insights for follow up interventions. As stated by NCCN guidelines, distress should be monitored, documented and managed at all stages of the cancer continuum.
Results and discussion
The protocol for distress management at HSPOH is based on the 2013 NCCN guidelines. Cancer patients are screened for levels of distress in all settings (inpatients and outpatients). Screening is held with the DST Spanish translation at the moment of diagnosis or as soon as possible after a diagnosis is made. Screening for distress is also done before or after surgery, in recurrence or progression, and when clinically indicated. Patients are informed that distress management is an essential part of their care and are encouraged to provide information so that we can make a proper need assessment.
Patients are screened by the psychosocial coordinator or patient navigator who administers the DST followed by in-depth interviews for additional appraisal. An action plan is designed based on patient needs, which include their intervention and the intervention of other members of the psychosocial team from the institution and/or from the community. Additional in-house health professionals contributing in distress management include, but are not limited to: physicians; clinical psychologists; health educators; social workers; dietitians; chaplains; and physical, respiratory, speech, and/or swallow therapists. Follow-up and rescreening sessions are scheduled to assure coordination of services between those health professionals as well as to secure continuity of distress management during all stages of the cancer continuum.
The results of the DST are filed in patient medical records. Members of the psychosocial team also document their interventions in the patient medical record, which helps in the exchange of information among the cancer care team. The psychosocial team meets once a month – or as required for extraordinary cases – to review and discuss the cases, determine the best options for distress management, and identify areas for psychosocial care improvement. Those findings and the results of distress management in patient level of satisfaction are then reported and discussed quarterly by the psychosocial coordinator and the cancer committee.
Figure 2 shows in what phase of emotional distress assessment the PHQ-9 was included. Patients reporting four or more of the six areas of concern related to emotional distress in the DST (Figure 1) are automatically referred to a mental health specialist. But when patients report three areas of concern with no clear data on their specific level of depression, PHQ-9 is administered to differentiate those who need a mental health specialist from those who could be adequately supported by health education and support group interventions. In this way detrimental outcomes such as duplicity and over or underuse of services and resources are reduced. In addition, it is recognized that using an interview after the administration of the DST to determine distress management actions does not always provide enough information about a patient’s emotional circumstances and previous comorbidities. Patient responses during interviews may be influenced by the patient’s level of literacy, verbal comprehension, and communication style,24 so emotional distress can go unrecognized during interviews, resulting in delays for treatment and supportive care.
National guidelines in oncology consider such socio-ecological models emphasizing the delivery of patient-centered, interdisciplinary, and evidence-based care. That does not mean that institutions should apply protocols of psychosocial care as previously developed, but that they should test, review, adapt, and improve them during the implementation of the care. In fact, NCCN encourages conducting trials to examine protocols, screening instruments, and models of intervention to determine applicability to particular settings.2
Findings from a study by NCCN member institutions to evaluate progress of implementing distress management guidelines found that 53% (n = 8) of respondent institutions conducted routine distress screening. Of those, 37.5% (3) relied only on interviews. That finding is of concern because if interviews are not standardized and have not been systematically evaluated, then their sensitivity and specificity in identifying distressed patients is unknown.26 Accordingly, the process described in this article and the PHQ-9 validation was an effort to standardize emotional distress management, and was underlined as an achievement during the CoC accreditation visit to the cancer center in December 2013. The hospital was accredited as a comprehensive community cancer center with gold commendations, becoming the first privately owned hospital in Puerto Rico to achieve the accreditation.
A comprehensive, patient-centered approach is required to accomplish cancer best standards of care.1 This approach reflects the holistic conceptualization of health in which the physical, emotional, and social dimensions of the human being are considered when providing medical care. As a result, to look after all patient needs, interdisciplinary and well-coordinated interventions are recommended. Cancer patients should be provided not only with diagnostic, treatment, and follow-up clinical service, but also with the supportive assistance that may positively influence all aspects of their health.
To appraise physical, social, emotional and spiritual issues and to develop supportive interventional action plans, the National Comprehensive Cancer Network (NCCN) recommends screening all cancer patients for distress.2 In particular, screening the emotional component of distress occupies a prominent place in this process because it is now recognized as the sixth vital sign in oncology.3 Even though the influence of emotional distress over cancer mortality rates and disease progression is still under scrutiny,4 its plausible implications over treatment compliance have been pointed out. Patients with higher levels of emotional distress show lower adherence to treatment and poorer health outcomes.5 Furthermore, prevalence rates of emotional distress in cancer patients from ambulatory settings6 and oncology surgical units have been studied and have provided justification for distress management.7 Studies have shown low ability among oncologists to identify patients in distress and oncologists’ tendency to judge distress higher than the patients themselves.8 As a consequence, to achieve systematic distress evaluations and appropriate referrals for care, guidelines for distress management should be implemented in clinical settings. It is recommended that tests are conducted to find brief screening instruments and procedures to assure accurate interventions according to patient specific needs.
This article presents the process of implementing a distress management program at HIMA-San Pablo Oncologic Hospital in Caguas, Puerto Rico, with particular emphasis on the management of emotional distress, which has been defined as the feeling of suffering that cancer patients may experience after diagnosis. In addition, we have included data from a pilot study that was completed for content validation of the Patient Health Questionnaire (PHQ-9) to estimate depression levels in Puerto Rican cancer patients.
Methods
HIMA-San Pablo operates a group of privately owned hospitals in Puerto Rico. It established a cancer center in Caguas in 2007, recruiting a multispecialty medical faculty to provide cancer care and bone marrow transplants for adult and pediatric patients. The cancer center, currently named HIMA-San Pablo Oncologic Hospital (HSPOH), is a hospital within a hospital licensed by the Puerto Rico Department of Health. In 2007, a cancer committee was established as the steering committee to ensure the delivery of cancer care according to best standards of care. The committee took responsibility for developing all activities needed to achieve the American College of Surgeons’ Commission on Cancer (CoC) accreditation under the category of Comprehensive Community Cancer Center. The committee established a psychosocial team to develop a protocol for the delivery of distress management for adult patients. (The psychosocial needs of pediatric patients are assessed through other procedures.)
To develop the protocol, principles of input-output model of research and quality analysis in health care were applied.9 The input-output model, with its origin in engineering, helped map systematic activities to transform empirical data on cancer psychosocial care into operational procedures. Focus was given to data gathering (input), information organization and analysis (throughput), and the schematization of emotional distress management care (output).
The input phase
In the input phase, elements of psychosocial care and operational definitions related to distress management in general were identified through literature review (Table 1). Basic parameters for distress management were clarified, resulting in a conceptual framework based in four remarks: First, according to NCCN, distress is a multifactorial unpleasant emotional experience of a psychological, social, and spiritual nature. It may interfere with the ability to cope effectively with cancer, its symptoms and its treatment. Its intensity may fluctuate from feelings of suffering and fear to incapacitating manifestations of anxiety and depression2 and its severity may hamper patient quality of life and treatment compliance.
Second, distress management requires the intervention of an interdisciplinary team with both medical and allied health professionals. This may include mental health specialists and other professionals with training and experience in cancer-related issues, who work with reciprocal channels of communication for the exchange of patient information.
Third, NCCN recommends using the Distress Thermometer for patient initial distress screening.10-12 It consists of a numeric scale ranging from 0 (no distress) to 10 (severe distress) in which patients classify their level of distress. The numeric scale is followed by a section in which patients identify areas of practical, familiar, emotional, spiritual/ religious, and physical concerns. Based on responses, interviews may follow to set distress management interventions.
Fourth, screening and assessment are different but sequential and complementary stages of distress management. Screening is viewed as a rapid strategy to identify cancer patients in distress. Assessment looks out for a broader appraisal and documentation of factors with repercussions over patient distress level and resiliency capability.13 In many instances, the patient’s emotional distress is better understood in the assessment phase.
The throughput phase
Within the throughput phase of information organization and analysis, an inventory of health professionals and other in-house consultants needed for distress management was completed. Roles and procedures for information sharing were determined, and we established collaborative agreements with professionals in the community who could contribute to distress management. Members of the psychosocial team held workshops to discuss elements of NCCN guidelines for distress management and to create an action plan for the implementation of the protocol. Data analyses were performed to create a demographic profile of the oncology population at the hospital and assess patient willingness to receive emotional support services,16 which led to the implementation of support group meetings at which additional substantive information was collected about issues affecting cancer patients’ emotions.
The NCCN Distress Thermometer for measuring distress was translated to Spanish. Its format was adapted, and it was identified as a distress screening tool (DST), which we named Distress Assessment Tool for Oncology Patients (Figure 1). The instrument helps for rapid screening of patient needs and proper determination of initial interventions. In addition, psychometric properties of several instruments were reviewed for instances when patient emotional distress could not be clearly determined. We decided to proceed with the validation of the 9-item Patient Health Questionnaire (PHQ-9) to estimate patient depression level. A proposal for content validation of the PHQ-9 was approved by the University of Puerto Rico institutional review board, and patients were recruited to participate in the pilot study.
The PHQ-9. The PHQ-9 is a self-report version of the PRIME-MD instrument developed to assess mental disorders in clinical settings. It is based on DSM-IV diagnostic criteria.17 The PHQ-9 is the depression module with nine depression symptoms to check off if they become the cause of emotional impairment. Respondents categorized depression symptoms in four frequency degrees representing numeric values: 0 (not at all), 1 (several days), 2 (more than half the days), 3 (nearly every day). Measures of depression severity are subsequently determined in a Likert-type scale according to numeric calculations of responses: 0-4 (none severe depression), 5-9 (mild), 10-14 (moderate), 15-19 (moderately severe), and 20-27 (severe-major depression).
The instrument is widely used because of its validity in small and large populations. It showed adequate reliability and validity in a small sample of head and neck cancer patients, with a Cronbach’s alpha of 0.80 and a correlation coefficient of 0.71.18 Similarly, it showed good performance in identifying major depression in 4264 cancer outpatients, with sensitivity of 93%, specificity of 81%, and a positive predictive value (PPV) of 25% and negative predictive value (NPV) of 99%.19 Even when administered on a touch screen computer, the instrument showed valid data of depression from patients in treatment.20
The Beck Depression Inventory. We used the Beck Depression Inventory (BDI-II) Spanish version as the gold standard measure for the validation study. It is a 22-items inventory that measures attitudes and symptoms of depression.21 It can be administered in 10 minutes and has shown good psychometric measures when administered in Spain and Puerto Rico.22, 23
The pilot study. In all, 44 cancer patients who were receiving outpatient treatment at the radiotherapy unit agreed to participate in the study. The participants signed a consent form after the confidentiality protection measures and the main objectives of the study had been explained to them. Patients were interviewed individually during November and December 2012, with the Spanish versions of the PHQ-9 and BDI-II administered by one of two interviewers. At the beginning of each interview, the patient was asked 10 questions so that we could gather demographic data and confirm participant eligibility: aged 21 years or older, born and raised in Puerto Rico, being a Spanish speaker, and having a primary cancer diagnosis with no previous disease. Three patients were excluded from the sample because they either had cancer previously or had a recurrence or metastasis. The final sample consisted of 41 outpatients (N = 41).
Data analysis for demographics was completed with STATA v.12 software. Measures of central tendency and dispersion as well as PHQ-9 internal consistency analysis were made through Cronbach alpha with SPSS.
From a total of 41 patients surveyed, 22 (54%) were men and 19 (46%) were women, with an overall median age of 61 years. Among the men, 15 (68%) had a prostate cancer diagnosis and among women, 9 (47.4%) had a breast cancer diagnosis. In regard to health insurance, 19 (46%) had Medicare or Veterans/federal insurance coverage, and 13 (32%) had Reforma, the Puerto Rican government health insurance program partially funded by Medicaid funds. In addition, 8 participants (20%) were unemployed or disabled. As previously stated, all of the patients were in ambulatory care. Only 3 (7%) were participating in support groups.
Of all the respondents, 16 (39%) reported some level of depression. In particular, 2 (5%) showed severe-major depression, 4 (10%) moderately severe depression, and 10 (24%) moderate depression. Of those with depression, 8 (50%) were women, 8 (50%) were men. All 6 of the patients with head and neck cancer showed moderate or moderately severe depression (Table 2).
When respondent PHQ-9 scoring reflected moderate to severe depression (>10), a letter was sent to the patient’s radio-oncologist for referral to counseling and clinical psychological evaluation. All participants had access to the support group program, to a radiotherapy education program meeting weekly, and written information about their cancer diagnosis and treatment. They also were interviewed by the psychosocial coordinator or patient navigator for further assessment.
The output phase
In the output phase, a graphic representing the process of emotional assessment at the institution was created and then modified. PHQ-9 was added to the process when it was found suitable to assess level of depression contributing to the identification of patients requiring psychological and psychiatric assistance which by other means would be missed. PHQ-9 was useful in the busy clinical setting as it was completed, scored and interpreted in minutes. It showed the potential for routine evaluations when looking to identify improvement or deterioration in depression levels thus helping to monitor responses to treatment and providing insights for follow up interventions. As stated by NCCN guidelines, distress should be monitored, documented and managed at all stages of the cancer continuum.
Results and discussion
The protocol for distress management at HSPOH is based on the 2013 NCCN guidelines. Cancer patients are screened for levels of distress in all settings (inpatients and outpatients). Screening is held with the DST Spanish translation at the moment of diagnosis or as soon as possible after a diagnosis is made. Screening for distress is also done before or after surgery, in recurrence or progression, and when clinically indicated. Patients are informed that distress management is an essential part of their care and are encouraged to provide information so that we can make a proper need assessment.
Patients are screened by the psychosocial coordinator or patient navigator who administers the DST followed by in-depth interviews for additional appraisal. An action plan is designed based on patient needs, which include their intervention and the intervention of other members of the psychosocial team from the institution and/or from the community. Additional in-house health professionals contributing in distress management include, but are not limited to: physicians; clinical psychologists; health educators; social workers; dietitians; chaplains; and physical, respiratory, speech, and/or swallow therapists. Follow-up and rescreening sessions are scheduled to assure coordination of services between those health professionals as well as to secure continuity of distress management during all stages of the cancer continuum.
The results of the DST are filed in patient medical records. Members of the psychosocial team also document their interventions in the patient medical record, which helps in the exchange of information among the cancer care team. The psychosocial team meets once a month – or as required for extraordinary cases – to review and discuss the cases, determine the best options for distress management, and identify areas for psychosocial care improvement. Those findings and the results of distress management in patient level of satisfaction are then reported and discussed quarterly by the psychosocial coordinator and the cancer committee.
Figure 2 shows in what phase of emotional distress assessment the PHQ-9 was included. Patients reporting four or more of the six areas of concern related to emotional distress in the DST (Figure 1) are automatically referred to a mental health specialist. But when patients report three areas of concern with no clear data on their specific level of depression, PHQ-9 is administered to differentiate those who need a mental health specialist from those who could be adequately supported by health education and support group interventions. In this way detrimental outcomes such as duplicity and over or underuse of services and resources are reduced. In addition, it is recognized that using an interview after the administration of the DST to determine distress management actions does not always provide enough information about a patient’s emotional circumstances and previous comorbidities. Patient responses during interviews may be influenced by the patient’s level of literacy, verbal comprehension, and communication style,24 so emotional distress can go unrecognized during interviews, resulting in delays for treatment and supportive care.
National guidelines in oncology consider such socio-ecological models emphasizing the delivery of patient-centered, interdisciplinary, and evidence-based care. That does not mean that institutions should apply protocols of psychosocial care as previously developed, but that they should test, review, adapt, and improve them during the implementation of the care. In fact, NCCN encourages conducting trials to examine protocols, screening instruments, and models of intervention to determine applicability to particular settings.2
Findings from a study by NCCN member institutions to evaluate progress of implementing distress management guidelines found that 53% (n = 8) of respondent institutions conducted routine distress screening. Of those, 37.5% (3) relied only on interviews. That finding is of concern because if interviews are not standardized and have not been systematically evaluated, then their sensitivity and specificity in identifying distressed patients is unknown.26 Accordingly, the process described in this article and the PHQ-9 validation was an effort to standardize emotional distress management, and was underlined as an achievement during the CoC accreditation visit to the cancer center in December 2013. The hospital was accredited as a comprehensive community cancer center with gold commendations, becoming the first privately owned hospital in Puerto Rico to achieve the accreditation.
1. Commission on Cancer, American College of Surgeons. Cancer Programs Standards 2012: Ensuring Patient-Centered Care. Version 1.2.1. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Published 2012. Accessed March 5, 2013.
2. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN guidelines): Distress management. Version I. 2012. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Accessed March 5, 2013.
3. Bultz BD, Groff SL. Screening for distress, the 6th vital sign in oncology: from theory to practice: http://www.oncologyex.com/issue/2009/vol8_no1/8_comment2_1.html. Published February 2009. Accessed February 16, 2017.
4. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349-5361.
5. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Int Med. 2000;160:2101-2107.
6. Jadoon NA, Munir W, Shahzad MA, Choudhry ZS. Assessment of depression and anxiety in adult cancer outpatients: a cross sectional study. BMC Cancer. 2010;10:594.
7. Fisher D, Wedel B. Anxiety and depression disorders in cancer patients: incidence, diagnosis and therapy. Mag Eur Med Oncol. 2012;5:52-54.
8. Sollner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and in need for psychosocial counselling? Br J Cancer. 2001;84:179-185.
9. Scott RD, Solomon SL, McGowan JE. Applying economic principles to health care: special issue. Emerg Infect Dis. 2001;7:282-285.
10. Adler NE, Page AEK. A model for delivering psychosocial health services. In: Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press (US); 2008.
11. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8:4-12.
12. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494-1502.
13. Maihoff SE. Assessment. In Washington CM, Leaver D, eds. Principles and practice of radiation therapy. St Louis, MO: Mosby Elsevier; 2004:243-264.
14. National Academy of Sciences. Adler NE, Page AEK, eds. Cancer care for the whole patient: meeting psychosocial health needs. https://www.ncbi.nlm.nih.gov/books/NBK4015/. Published 2008. Accessed February 22, 2012.
15. Nancarrow SA, Booth A, Ariss
16. Baker-Glenn EA, Park B, Granger L, Symonds P, Mitchell AJ. Desire for psychological support in cancer patients with depression or distress: validation of a simple help question. Psychooncology. 2011;20:525-531.
17. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
18. Omoro SA, Fann JR, Weymuller EA, Macharia IM, Yueh B. Swahili translation and validation of the Patient Health Questionnaire-9 depression scale in the Kenyan head and neck cancer patient population. Int J Psychiatry Med. 2006;36:367-381.
19. Thekkumpurath P, Walker J, Butcher I, et al. Screening for major depression on cancer outpatients: the diagnostic accuracy of the 9-item Patient Health Questionnaire. Cancer. 2011;117:218-227.
20. Fann JR, Berry DL, Wolpin S, et al. Depression screening using the Patient Health Questionnaire-9 administered on a touch screen computer. Psychooncology. 2009;18:14-22.
21. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:651-571.
22. Sanz J, Perdigón AL, Vázquez C. The Spanish adaptation of Beck’s Depression Inventory–II (BDI-II): psychometric properties in the general population. Clínica y Salud. 2003;14:249-280.
23. Bonilla J, Bernal G, Santos A, Santos D. A revised Spanish version of the Beck Depression Inventory: psychometric properties with a Puerto Rican sample of college students. J Clin Psychol. 2004;60:119-130.
24. Alcántara C, Gone JP. Multicultural issues in the clinical interview and diagnostic process. In Leong FTL, ed. APA handbook of multicultural psychology. Vol 2. Applications and training. Washington, DC: American Psychological Association; 2014:153-163.
25. Sharma M, Romas JA. Theoretical foundations of health education and health promotion. 2nd ed. Burlington, MA: Jones & Barlett Learning; 2012.
26. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5:99-103.
1. Commission on Cancer, American College of Surgeons. Cancer Programs Standards 2012: Ensuring Patient-Centered Care. Version 1.2.1. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Published 2012. Accessed March 5, 2013.
2. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN guidelines): Distress management. Version I. 2012. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Accessed March 5, 2013.
3. Bultz BD, Groff SL. Screening for distress, the 6th vital sign in oncology: from theory to practice: http://www.oncologyex.com/issue/2009/vol8_no1/8_comment2_1.html. Published February 2009. Accessed February 16, 2017.
4. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349-5361.
5. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Int Med. 2000;160:2101-2107.
6. Jadoon NA, Munir W, Shahzad MA, Choudhry ZS. Assessment of depression and anxiety in adult cancer outpatients: a cross sectional study. BMC Cancer. 2010;10:594.
7. Fisher D, Wedel B. Anxiety and depression disorders in cancer patients: incidence, diagnosis and therapy. Mag Eur Med Oncol. 2012;5:52-54.
8. Sollner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and in need for psychosocial counselling? Br J Cancer. 2001;84:179-185.
9. Scott RD, Solomon SL, McGowan JE. Applying economic principles to health care: special issue. Emerg Infect Dis. 2001;7:282-285.
10. Adler NE, Page AEK. A model for delivering psychosocial health services. In: Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press (US); 2008.
11. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8:4-12.
12. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494-1502.
13. Maihoff SE. Assessment. In Washington CM, Leaver D, eds. Principles and practice of radiation therapy. St Louis, MO: Mosby Elsevier; 2004:243-264.
14. National Academy of Sciences. Adler NE, Page AEK, eds. Cancer care for the whole patient: meeting psychosocial health needs. https://www.ncbi.nlm.nih.gov/books/NBK4015/. Published 2008. Accessed February 22, 2012.
15. Nancarrow SA, Booth A, Ariss
16. Baker-Glenn EA, Park B, Granger L, Symonds P, Mitchell AJ. Desire for psychological support in cancer patients with depression or distress: validation of a simple help question. Psychooncology. 2011;20:525-531.
17. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
18. Omoro SA, Fann JR, Weymuller EA, Macharia IM, Yueh B. Swahili translation and validation of the Patient Health Questionnaire-9 depression scale in the Kenyan head and neck cancer patient population. Int J Psychiatry Med. 2006;36:367-381.
19. Thekkumpurath P, Walker J, Butcher I, et al. Screening for major depression on cancer outpatients: the diagnostic accuracy of the 9-item Patient Health Questionnaire. Cancer. 2011;117:218-227.
20. Fann JR, Berry DL, Wolpin S, et al. Depression screening using the Patient Health Questionnaire-9 administered on a touch screen computer. Psychooncology. 2009;18:14-22.
21. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:651-571.
22. Sanz J, Perdigón AL, Vázquez C. The Spanish adaptation of Beck’s Depression Inventory–II (BDI-II): psychometric properties in the general population. Clínica y Salud. 2003;14:249-280.
23. Bonilla J, Bernal G, Santos A, Santos D. A revised Spanish version of the Beck Depression Inventory: psychometric properties with a Puerto Rican sample of college students. J Clin Psychol. 2004;60:119-130.
24. Alcántara C, Gone JP. Multicultural issues in the clinical interview and diagnostic process. In Leong FTL, ed. APA handbook of multicultural psychology. Vol 2. Applications and training. Washington, DC: American Psychological Association; 2014:153-163.
25. Sharma M, Romas JA. Theoretical foundations of health education and health promotion. 2nd ed. Burlington, MA: Jones & Barlett Learning; 2012.
26. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5:99-103.
Repeal and replace? How about retain, review, and refine?
A suggestion for Congress: keep what’s working in the Patient Protection and Affordable Care Act (PPACA), adjust what isn’t working – just make the whole thing better and call it what you will.
A good thing, but needing work
The PPACA, which is also referred to as Obama care, had a lot in it that any reasonable person would consider good. Let’s take a look. As Dr Valerie Arkoosh wrote in our journal in 2012,2 the law attempted to expand access to health care to the embarrassingly large 30 million or more Americans who were not insured. How would it do this? By expanding Medicaid, enhancing consumer protections in the private health insurance market, requiring large employers to offer insurance or pay a fine, giving tax credits to increase affordability of insurance for small businesses, creating state-based competitive market places, and requiring individuals to purchase health insurance plans (the so-called insurance mandate), thereby creating a pool of large numbers of healthy people who would help defray the costs of those not so fortunate.The law also guaranteed insurability despite any preexisting condition, surely a step in the right direction. Likewise, the need for employers to provide health insurance, the state-based health insurance exchanges, and especially the individual mandate to buy insurance or pay a fine, were all steps in the right direction.
And the law went further – it also addressed preventive care. Medicare and all new insurance plans would have to cover, without copay, co-insurance, or deductible, high-certainty preventive services such as screening for breast, cervical, colorectal, lung, and skin cancers, the annual well-woman visit, breast cancer preventative medications, and many others.3 Medicare recipients would be eligible for one non-copay annual wellness visit to their caregiver. Beyond providing increased access to health care, the PPACA added incentives to caregivers who were coming out of training programs to serve in underserved areas and benefit from a decrease in their med school loans or in their loan repayments.
Finally, and especially important, under the PPACA, our age-old insurance system of fee for service, which tends to incentivize more care, would change to incentivizing high-quality, outcomes-based care , thus replacing “quantity of care” with quality of care. So what’s wrong with the features of the law outlined in the preceding paragraphs? Well, of course, for every 100 ideas, only a few will be implemented and actually pay off. Certainly some of the PPACA could have been better implemented, and perhaps the task now facing Congress, if it could ever abandon its current pitched-camp approach, should be to take the ideas that health care policy scientists have established as being valid and find a way to make them work. Surely that would be best for all players, rather than carping about the repeal-replace approach versus staying with the PPACA.
So my response to the repeal-replace assertion? Retain, review, and refine.
Practitioner-friendly content
Health care calamities notwithstanding, we have a line-up of articles in this issue that uniformly address some of the pressing needs many of us face in our daily practice. Barry and colleagues examined the patterns of care with regard to whole brain radiotherapy technique and delivery at US-based academic centers. Their results show some interesting differences in the way younger and older practitioners deliver that care, with older practitioners placing more importance on tumor histopathology when considering brain irradiation. Speaking of access to care in the context of health reform, how often do our cancer patients use the emergency department? Lash and colleagues looked at the ED-use numbers from two databases in California and found that patients go to the ED at higher rates than previously reported and with notable variability by cancer type. Now we need to examine the reasons for those visits and establish ways to identify predictors of ED use to improve patient quality of care and rein in the higher costs of ED use.
In regard to symptom management, we can never have enough about nausea and vomiting prevention. Schwartzberg and colleagues report on a trial in which they evaluated the clinical benefits of APF530, a subcutaneous formulation of granisetron, compared with ondansetron in patients who had received cisplatin therapy. This longer-acting formulation of granisetron performed very well against a standard of care and might give our patients another option in the clinic for highly emetogenic chemotherapy.
Still on the topic of symptom management, preventing and treating mTOR-inhibitor–associated stomatitis (mIAS) is the subject of a review by Ramchandran and colleagues. The inhibitors have been approved for treatment in renal cell, neuroendocrine, and breast cancers, but of course, many of our newer molecules have some associated toxicity. Based on their literature scan, the authors report that management of mIAS should focus on three major approaches: prevention, early aggressive treatment, and, when needed, more aggressive pain management. Early recognition and diagnosis of mIAS facilitate early intervention to limit potential sequelae of mIAS and minimize the need for mTOR inhibitor dose reduction and interruption.
In a way, stress management could also fall under the symptom management category. I often remember being told during my training that we should always discuss with your patients their level of anxiety and depression. But I think sometimes we are so busy addressing the cancer, its treatment, and treatment side effects, we overlook the fact that the patient is suffering psychologically and might need additional intervention in the form of talk therapy and/or medication. Ramírez-Solá and colleagues describe in our How We Do It section the process of developing and implementing a psychosocial distress management program at their institution in Puerto Rico. The authors also summarize the results of a pilot study to validate the Patient Health Questionnaire (PHQ-9) as a measure to improve the process of emotional distress management in particular.
In recent years, the number of approvals and new indications for therapies for different cancer types has increased significantly. We highlight two such approvals in this issue. One is the PARP inhibitor, rucaparib, which was approved in both the platinum-sensitive and -resistant settings for BRCA1- and BRCA2-mutant patients with ovarian cancer. The other is the new CD38 antibody daratumumab, which was originally approved as a single-agent therapy for relapsed myeloma and which has now received a second approval with demonstrated improvement of progression-free survival when given with the lenalidomide-dexamethasone or bortezomib-dexamethasone combinations.
When it comes to new therapies, immunotherapies are at the cutting edge. Who hasn’t heard of the new checkpoint inhibitor drugs for a range of cancers that have either been approved or are in trial? Until now, we have used these immunotherapies as single agents, but Jane de Lartigue writes of the potential of combining more than one immunotherapy drug and/or combining an immune checkpoint inhibitor with a chemotherapy drug. The key behind this concept is that the more antigenic differentiation and tumor infiltrating lymphocytes in the system, the better the immunotherapy might work.
In the previous issue of the journal, one of our Editors, Thomas Strouse, discussed the issue of physician aid in dying (PAD)4 and asserted he had come to view “active non-participation” in legal PAD as a “toxic form of patient abandonment.” This is, of course, a very challenging and complex topic, and one that we likely have to address on a weekly basis with some of our cancer patients: if palliative care and end-of-life is the goal, how can we most humanely achieve that ethically and legally in concert with our patients’ wishes? Is it right or wrong to aid in some way in the dying process? Dr Alva Weir responds to Dr Strouse’s editorial, taking the view point that physician-assisted suicide is toxic abandonment. Dr Strauss responds, and I encourage you to read this very interesting exchange that highlights the point-counterpoint views of physician involvement in the dying process.
We round off the issue with a bumper crop of Case Reports. They include two that document diagnostic challenges: one in a patient with pulmonary sarcomatoid carcinoma presenting as a necrotizing cavitary lung lesion and another in which atraumatic splenic rupture is the initial presentation of CML. Also included is a report on a case of primary cardiac prosthetic valve-associated lymphoma and another on how a collaborative effort between oncologists and dermatologists contributed to the resolution of palmoplantar exacerbation of psoriasis in a patient who had been treated with nivolumab.
Going digital
I will close by remarking that the Journal of Community and Supportive Oncology, or JCSO, will be going digital only after this print issue. We will continue publishing the same content as a bimonthly digital issue, posting articles directly to our website, and mailing out our regular electronic newsletters. So visit the website, www.jcso-online.com, where you can read the articles as soon as they are posted and also find instructions for downloading the app for the digital edition – it’s quick, easy, and free, in case you were wondering. For a shortcut to the download the app, you can also use http://bit.ly/2nCEPIa.
Finally, if you would like to submit a paper to us for consideration for publication, you can do so by going to www.editorialmanager.com/jso/. We will consider submissions in original research, reviews, How We Do It, case reports, and tumor board summaries – you’ll find all the information you need to submit a paper at the EditorialManager platform. And let’s not forget social media – we’re on Twitter where our handle is @jcs_onc, my personal Twitter handle is @davidhenrymd, so connect with us – follow us, like us, and retweet us.
1. Pear R, Kelly K. Trump concedes health law overhaul is ‘unbelievably complex.’ https://www.nytimes.com/2017/02/27/us/politics/trump-concedes-health-law-overhaul-is-unbelievably-complex.html?_r=0. New York Times. February 27, 2017. Accessed April 4, 2017.
2. Arkoosh VA. The Patient Protection and Affordable Care Act: no rhetoric, just the facts. Commun Oncol. 2012;9(6):206-209.
3. USPSTF A and B Recommendations. US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/. January 2017. Accessed April 4, 2017.
4. Strouse T. End-of-life options and the legal pathways to physician aid in dying. J Community Support Oncol. 2017;15(1):1-3.
A suggestion for Congress: keep what’s working in the Patient Protection and Affordable Care Act (PPACA), adjust what isn’t working – just make the whole thing better and call it what you will.
A good thing, but needing work
The PPACA, which is also referred to as Obama care, had a lot in it that any reasonable person would consider good. Let’s take a look. As Dr Valerie Arkoosh wrote in our journal in 2012,2 the law attempted to expand access to health care to the embarrassingly large 30 million or more Americans who were not insured. How would it do this? By expanding Medicaid, enhancing consumer protections in the private health insurance market, requiring large employers to offer insurance or pay a fine, giving tax credits to increase affordability of insurance for small businesses, creating state-based competitive market places, and requiring individuals to purchase health insurance plans (the so-called insurance mandate), thereby creating a pool of large numbers of healthy people who would help defray the costs of those not so fortunate.The law also guaranteed insurability despite any preexisting condition, surely a step in the right direction. Likewise, the need for employers to provide health insurance, the state-based health insurance exchanges, and especially the individual mandate to buy insurance or pay a fine, were all steps in the right direction.
And the law went further – it also addressed preventive care. Medicare and all new insurance plans would have to cover, without copay, co-insurance, or deductible, high-certainty preventive services such as screening for breast, cervical, colorectal, lung, and skin cancers, the annual well-woman visit, breast cancer preventative medications, and many others.3 Medicare recipients would be eligible for one non-copay annual wellness visit to their caregiver. Beyond providing increased access to health care, the PPACA added incentives to caregivers who were coming out of training programs to serve in underserved areas and benefit from a decrease in their med school loans or in their loan repayments.
Finally, and especially important, under the PPACA, our age-old insurance system of fee for service, which tends to incentivize more care, would change to incentivizing high-quality, outcomes-based care , thus replacing “quantity of care” with quality of care. So what’s wrong with the features of the law outlined in the preceding paragraphs? Well, of course, for every 100 ideas, only a few will be implemented and actually pay off. Certainly some of the PPACA could have been better implemented, and perhaps the task now facing Congress, if it could ever abandon its current pitched-camp approach, should be to take the ideas that health care policy scientists have established as being valid and find a way to make them work. Surely that would be best for all players, rather than carping about the repeal-replace approach versus staying with the PPACA.
So my response to the repeal-replace assertion? Retain, review, and refine.
Practitioner-friendly content
Health care calamities notwithstanding, we have a line-up of articles in this issue that uniformly address some of the pressing needs many of us face in our daily practice. Barry and colleagues examined the patterns of care with regard to whole brain radiotherapy technique and delivery at US-based academic centers. Their results show some interesting differences in the way younger and older practitioners deliver that care, with older practitioners placing more importance on tumor histopathology when considering brain irradiation. Speaking of access to care in the context of health reform, how often do our cancer patients use the emergency department? Lash and colleagues looked at the ED-use numbers from two databases in California and found that patients go to the ED at higher rates than previously reported and with notable variability by cancer type. Now we need to examine the reasons for those visits and establish ways to identify predictors of ED use to improve patient quality of care and rein in the higher costs of ED use.
In regard to symptom management, we can never have enough about nausea and vomiting prevention. Schwartzberg and colleagues report on a trial in which they evaluated the clinical benefits of APF530, a subcutaneous formulation of granisetron, compared with ondansetron in patients who had received cisplatin therapy. This longer-acting formulation of granisetron performed very well against a standard of care and might give our patients another option in the clinic for highly emetogenic chemotherapy.
Still on the topic of symptom management, preventing and treating mTOR-inhibitor–associated stomatitis (mIAS) is the subject of a review by Ramchandran and colleagues. The inhibitors have been approved for treatment in renal cell, neuroendocrine, and breast cancers, but of course, many of our newer molecules have some associated toxicity. Based on their literature scan, the authors report that management of mIAS should focus on three major approaches: prevention, early aggressive treatment, and, when needed, more aggressive pain management. Early recognition and diagnosis of mIAS facilitate early intervention to limit potential sequelae of mIAS and minimize the need for mTOR inhibitor dose reduction and interruption.
In a way, stress management could also fall under the symptom management category. I often remember being told during my training that we should always discuss with your patients their level of anxiety and depression. But I think sometimes we are so busy addressing the cancer, its treatment, and treatment side effects, we overlook the fact that the patient is suffering psychologically and might need additional intervention in the form of talk therapy and/or medication. Ramírez-Solá and colleagues describe in our How We Do It section the process of developing and implementing a psychosocial distress management program at their institution in Puerto Rico. The authors also summarize the results of a pilot study to validate the Patient Health Questionnaire (PHQ-9) as a measure to improve the process of emotional distress management in particular.
In recent years, the number of approvals and new indications for therapies for different cancer types has increased significantly. We highlight two such approvals in this issue. One is the PARP inhibitor, rucaparib, which was approved in both the platinum-sensitive and -resistant settings for BRCA1- and BRCA2-mutant patients with ovarian cancer. The other is the new CD38 antibody daratumumab, which was originally approved as a single-agent therapy for relapsed myeloma and which has now received a second approval with demonstrated improvement of progression-free survival when given with the lenalidomide-dexamethasone or bortezomib-dexamethasone combinations.
When it comes to new therapies, immunotherapies are at the cutting edge. Who hasn’t heard of the new checkpoint inhibitor drugs for a range of cancers that have either been approved or are in trial? Until now, we have used these immunotherapies as single agents, but Jane de Lartigue writes of the potential of combining more than one immunotherapy drug and/or combining an immune checkpoint inhibitor with a chemotherapy drug. The key behind this concept is that the more antigenic differentiation and tumor infiltrating lymphocytes in the system, the better the immunotherapy might work.
In the previous issue of the journal, one of our Editors, Thomas Strouse, discussed the issue of physician aid in dying (PAD)4 and asserted he had come to view “active non-participation” in legal PAD as a “toxic form of patient abandonment.” This is, of course, a very challenging and complex topic, and one that we likely have to address on a weekly basis with some of our cancer patients: if palliative care and end-of-life is the goal, how can we most humanely achieve that ethically and legally in concert with our patients’ wishes? Is it right or wrong to aid in some way in the dying process? Dr Alva Weir responds to Dr Strouse’s editorial, taking the view point that physician-assisted suicide is toxic abandonment. Dr Strauss responds, and I encourage you to read this very interesting exchange that highlights the point-counterpoint views of physician involvement in the dying process.
We round off the issue with a bumper crop of Case Reports. They include two that document diagnostic challenges: one in a patient with pulmonary sarcomatoid carcinoma presenting as a necrotizing cavitary lung lesion and another in which atraumatic splenic rupture is the initial presentation of CML. Also included is a report on a case of primary cardiac prosthetic valve-associated lymphoma and another on how a collaborative effort between oncologists and dermatologists contributed to the resolution of palmoplantar exacerbation of psoriasis in a patient who had been treated with nivolumab.
Going digital
I will close by remarking that the Journal of Community and Supportive Oncology, or JCSO, will be going digital only after this print issue. We will continue publishing the same content as a bimonthly digital issue, posting articles directly to our website, and mailing out our regular electronic newsletters. So visit the website, www.jcso-online.com, where you can read the articles as soon as they are posted and also find instructions for downloading the app for the digital edition – it’s quick, easy, and free, in case you were wondering. For a shortcut to the download the app, you can also use http://bit.ly/2nCEPIa.
Finally, if you would like to submit a paper to us for consideration for publication, you can do so by going to www.editorialmanager.com/jso/. We will consider submissions in original research, reviews, How We Do It, case reports, and tumor board summaries – you’ll find all the information you need to submit a paper at the EditorialManager platform. And let’s not forget social media – we’re on Twitter where our handle is @jcs_onc, my personal Twitter handle is @davidhenrymd, so connect with us – follow us, like us, and retweet us.
A suggestion for Congress: keep what’s working in the Patient Protection and Affordable Care Act (PPACA), adjust what isn’t working – just make the whole thing better and call it what you will.
A good thing, but needing work
The PPACA, which is also referred to as Obama care, had a lot in it that any reasonable person would consider good. Let’s take a look. As Dr Valerie Arkoosh wrote in our journal in 2012,2 the law attempted to expand access to health care to the embarrassingly large 30 million or more Americans who were not insured. How would it do this? By expanding Medicaid, enhancing consumer protections in the private health insurance market, requiring large employers to offer insurance or pay a fine, giving tax credits to increase affordability of insurance for small businesses, creating state-based competitive market places, and requiring individuals to purchase health insurance plans (the so-called insurance mandate), thereby creating a pool of large numbers of healthy people who would help defray the costs of those not so fortunate.The law also guaranteed insurability despite any preexisting condition, surely a step in the right direction. Likewise, the need for employers to provide health insurance, the state-based health insurance exchanges, and especially the individual mandate to buy insurance or pay a fine, were all steps in the right direction.
And the law went further – it also addressed preventive care. Medicare and all new insurance plans would have to cover, without copay, co-insurance, or deductible, high-certainty preventive services such as screening for breast, cervical, colorectal, lung, and skin cancers, the annual well-woman visit, breast cancer preventative medications, and many others.3 Medicare recipients would be eligible for one non-copay annual wellness visit to their caregiver. Beyond providing increased access to health care, the PPACA added incentives to caregivers who were coming out of training programs to serve in underserved areas and benefit from a decrease in their med school loans or in their loan repayments.
Finally, and especially important, under the PPACA, our age-old insurance system of fee for service, which tends to incentivize more care, would change to incentivizing high-quality, outcomes-based care , thus replacing “quantity of care” with quality of care. So what’s wrong with the features of the law outlined in the preceding paragraphs? Well, of course, for every 100 ideas, only a few will be implemented and actually pay off. Certainly some of the PPACA could have been better implemented, and perhaps the task now facing Congress, if it could ever abandon its current pitched-camp approach, should be to take the ideas that health care policy scientists have established as being valid and find a way to make them work. Surely that would be best for all players, rather than carping about the repeal-replace approach versus staying with the PPACA.
So my response to the repeal-replace assertion? Retain, review, and refine.
Practitioner-friendly content
Health care calamities notwithstanding, we have a line-up of articles in this issue that uniformly address some of the pressing needs many of us face in our daily practice. Barry and colleagues examined the patterns of care with regard to whole brain radiotherapy technique and delivery at US-based academic centers. Their results show some interesting differences in the way younger and older practitioners deliver that care, with older practitioners placing more importance on tumor histopathology when considering brain irradiation. Speaking of access to care in the context of health reform, how often do our cancer patients use the emergency department? Lash and colleagues looked at the ED-use numbers from two databases in California and found that patients go to the ED at higher rates than previously reported and with notable variability by cancer type. Now we need to examine the reasons for those visits and establish ways to identify predictors of ED use to improve patient quality of care and rein in the higher costs of ED use.
In regard to symptom management, we can never have enough about nausea and vomiting prevention. Schwartzberg and colleagues report on a trial in which they evaluated the clinical benefits of APF530, a subcutaneous formulation of granisetron, compared with ondansetron in patients who had received cisplatin therapy. This longer-acting formulation of granisetron performed very well against a standard of care and might give our patients another option in the clinic for highly emetogenic chemotherapy.
Still on the topic of symptom management, preventing and treating mTOR-inhibitor–associated stomatitis (mIAS) is the subject of a review by Ramchandran and colleagues. The inhibitors have been approved for treatment in renal cell, neuroendocrine, and breast cancers, but of course, many of our newer molecules have some associated toxicity. Based on their literature scan, the authors report that management of mIAS should focus on three major approaches: prevention, early aggressive treatment, and, when needed, more aggressive pain management. Early recognition and diagnosis of mIAS facilitate early intervention to limit potential sequelae of mIAS and minimize the need for mTOR inhibitor dose reduction and interruption.
In a way, stress management could also fall under the symptom management category. I often remember being told during my training that we should always discuss with your patients their level of anxiety and depression. But I think sometimes we are so busy addressing the cancer, its treatment, and treatment side effects, we overlook the fact that the patient is suffering psychologically and might need additional intervention in the form of talk therapy and/or medication. Ramírez-Solá and colleagues describe in our How We Do It section the process of developing and implementing a psychosocial distress management program at their institution in Puerto Rico. The authors also summarize the results of a pilot study to validate the Patient Health Questionnaire (PHQ-9) as a measure to improve the process of emotional distress management in particular.
In recent years, the number of approvals and new indications for therapies for different cancer types has increased significantly. We highlight two such approvals in this issue. One is the PARP inhibitor, rucaparib, which was approved in both the platinum-sensitive and -resistant settings for BRCA1- and BRCA2-mutant patients with ovarian cancer. The other is the new CD38 antibody daratumumab, which was originally approved as a single-agent therapy for relapsed myeloma and which has now received a second approval with demonstrated improvement of progression-free survival when given with the lenalidomide-dexamethasone or bortezomib-dexamethasone combinations.
When it comes to new therapies, immunotherapies are at the cutting edge. Who hasn’t heard of the new checkpoint inhibitor drugs for a range of cancers that have either been approved or are in trial? Until now, we have used these immunotherapies as single agents, but Jane de Lartigue writes of the potential of combining more than one immunotherapy drug and/or combining an immune checkpoint inhibitor with a chemotherapy drug. The key behind this concept is that the more antigenic differentiation and tumor infiltrating lymphocytes in the system, the better the immunotherapy might work.
In the previous issue of the journal, one of our Editors, Thomas Strouse, discussed the issue of physician aid in dying (PAD)4 and asserted he had come to view “active non-participation” in legal PAD as a “toxic form of patient abandonment.” This is, of course, a very challenging and complex topic, and one that we likely have to address on a weekly basis with some of our cancer patients: if palliative care and end-of-life is the goal, how can we most humanely achieve that ethically and legally in concert with our patients’ wishes? Is it right or wrong to aid in some way in the dying process? Dr Alva Weir responds to Dr Strouse’s editorial, taking the view point that physician-assisted suicide is toxic abandonment. Dr Strauss responds, and I encourage you to read this very interesting exchange that highlights the point-counterpoint views of physician involvement in the dying process.
We round off the issue with a bumper crop of Case Reports. They include two that document diagnostic challenges: one in a patient with pulmonary sarcomatoid carcinoma presenting as a necrotizing cavitary lung lesion and another in which atraumatic splenic rupture is the initial presentation of CML. Also included is a report on a case of primary cardiac prosthetic valve-associated lymphoma and another on how a collaborative effort between oncologists and dermatologists contributed to the resolution of palmoplantar exacerbation of psoriasis in a patient who had been treated with nivolumab.
Going digital
I will close by remarking that the Journal of Community and Supportive Oncology, or JCSO, will be going digital only after this print issue. We will continue publishing the same content as a bimonthly digital issue, posting articles directly to our website, and mailing out our regular electronic newsletters. So visit the website, www.jcso-online.com, where you can read the articles as soon as they are posted and also find instructions for downloading the app for the digital edition – it’s quick, easy, and free, in case you were wondering. For a shortcut to the download the app, you can also use http://bit.ly/2nCEPIa.
Finally, if you would like to submit a paper to us for consideration for publication, you can do so by going to www.editorialmanager.com/jso/. We will consider submissions in original research, reviews, How We Do It, case reports, and tumor board summaries – you’ll find all the information you need to submit a paper at the EditorialManager platform. And let’s not forget social media – we’re on Twitter where our handle is @jcs_onc, my personal Twitter handle is @davidhenrymd, so connect with us – follow us, like us, and retweet us.
1. Pear R, Kelly K. Trump concedes health law overhaul is ‘unbelievably complex.’ https://www.nytimes.com/2017/02/27/us/politics/trump-concedes-health-law-overhaul-is-unbelievably-complex.html?_r=0. New York Times. February 27, 2017. Accessed April 4, 2017.
2. Arkoosh VA. The Patient Protection and Affordable Care Act: no rhetoric, just the facts. Commun Oncol. 2012;9(6):206-209.
3. USPSTF A and B Recommendations. US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/. January 2017. Accessed April 4, 2017.
4. Strouse T. End-of-life options and the legal pathways to physician aid in dying. J Community Support Oncol. 2017;15(1):1-3.
1. Pear R, Kelly K. Trump concedes health law overhaul is ‘unbelievably complex.’ https://www.nytimes.com/2017/02/27/us/politics/trump-concedes-health-law-overhaul-is-unbelievably-complex.html?_r=0. New York Times. February 27, 2017. Accessed April 4, 2017.
2. Arkoosh VA. The Patient Protection and Affordable Care Act: no rhetoric, just the facts. Commun Oncol. 2012;9(6):206-209.
3. USPSTF A and B Recommendations. US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/. January 2017. Accessed April 4, 2017.
4. Strouse T. End-of-life options and the legal pathways to physician aid in dying. J Community Support Oncol. 2017;15(1):1-3.
Toxic abandonment: a case for non-participation in physician-assisted suicide
I recently read with interest Dr Thomas Strouse’s article written to support physician aid in dying. Within the article he made the following statement: “I have come to view ‘active non-participation’ in legal PAD [physician aid in dying] – that is, decisions by individual physicians and/or health systems not only to not provide, but also not refer patients to possibly willing providers and systems without regard for specific clinical contexts – as a toxic form of patient abandonment.”1 Within the article, Dr Strouse lays out for us thoughtful precautions in the aid-in-dying laws, attempting to demonstrate that no vulnerable population is abused. Such precautions are important but provide the same result for all participants: the death of a patient. This is the central problem with aid in dying. Certainly there is nothing wrong with dying, and we all will have that opportunity. Though most of us would choose to put that moment off a while, for some, the suffering in this life makes death seem a welcome relief.
What is a physician’s central responsibility in the care of his or her patients near the end of their lives?
As program director for the hematology and oncology fellowship at my institution, I impress upon my fellows the importance of goal-oriented decision-making. I specifically teach them that there are only four goals worth achieving in any therapeutic or diagnostic decision making: to cure the disease; to help patients live longer despite the disease; to maximize the patient’s quality of life, and to prevent impending disasters. I know of no other worthwhile goal in any decision we are to make for our patients. I can point to none of these goals that physician aid in dying achieves. When it comes to physician-assisted suicide, some would argue that selecting an early death is a way of “maximizing quality of life.” And certainly our task is to make life the best it can be for our patients while they live through the dying process, but I am unaware of any published quality of life formula that calculates the end of life as a positive measure.
The question for us is the role of the doctor. Dr Strouse raises two issues with those whom he accuses of toxic abandonment. The first is whether physicians should provide aid in death, and the second is whether physicians should refer for the same service if they believe it is wrong for their patients.
It certainly has not been well established that physician-assisted suicide is a good thing rather than a tragic thing. A 2012 statement from the Ethics, Professionalism and Human Rights Committee of the American College of Physicians suggests otherwise: “After much consideration, the College concluded that making physician-assisted suicide legal raised serious ethical, clinical and social concerns and that the practice might undermine patient trust; distract from reform in end-of-life care; and be used in vulnerable patients, including those who are poor, are disabled, or are unable to speak for themselves or minority groups who have experienced discrimination.”2 The disability rights group, Not Dead Yet, has agreed with the ACP: “It cannot be seriously maintained that assisted suicide laws can or do limit assisted suicide to people who are imminently dying, and voluntarily request and consume a lethal dose, free of inappropriate pressures from family or society. Rather, assisted suicide laws ensure legal immunity for physicians who already devalue the lives of older and disabled people and have significant economic incentives to at least agree with their suicides, if not encourage them, or worse.”3
Such statements sound prophetic within both our present cost containment health care culture and in the real world of personal family economic pressures that can lead a patient toward the understanding that a right to die is actually a “duty to die.”
As society is driving physicians to be technicians to carry out their bidding, physicians should be clinging tightly to their role as trusted advocates for their patients. Certainly our patients have fears and pain that would at times lead them to prefer death to living, but a patient’s move to nonexistence is not the task of the physician. Our task as physicians was well described recently by Yang and Curlin: “Many patients with terminal illnesses fear unbearable pain or other symptoms. The physician’s role is to care for them in their illness so as to relieve pain or otherwise help them bear up under the symptoms they endure. Many patients loathe the prospect of abject debility. The physician’s role is to maintain solidarity with those whose health is diminished, not to not to imply that debility renders a patient’s life not worth living.”4
Statements such as these by reasoned people suggest we, as a country, have no consensus for the question whether aid in dying is possibly good or seriously bad for our patients. So it is quite reasonable for compassionate physicians to refuse to administer lethal medicines to their patients in order to “do no harm.”
The second question Dr Strouse explores is whether physicians who disapprove of physician-assisted suicide are abandoning their patients because they do not refer them to a provider who will provide such services. Dr Edmund Pelligrino, a well-respected medical ethicist, in his discussion of moral absolutes in medicine establishes the moral absolute, “Do not kill” and then addresses the ethical problem of complicity in killing. “Formal cooperation is absolutely and always, forbidden. This is the case when the physician shares the evil intent, partakes directly and freely, or in any way facilitates an intrinsically evil act like abortion or assisted suicide.”5 Though personally I would not use the word, “evil,” as he does, since evil implies motive; I would substitute the word “harm” and suggest that we should never be complicit in an act that we feel brings the harm of death to our patients. I would suggest that the expectation that physicians referring for aid in dying is analogous with the patient who comes to me demanding a chemotherapy that I know would cause her harm. I would refuse to give it to her and refuse to send her to a doctor who would be willing to give to her. Referral to produce harm is complicity with causing the harm itself. Our society should never go there. Our society should never ask a physician to cross the boundary line of conscience that is the ultimate protection for vulnerable patients.
I know what it is like to watch our patients suffer. I know what it is like to watch our loved ones suffer. I pushed the morphine at my father’s bedside until he quit screaming in pain. But I did not kill him. I cared for him. Such is the physician’s role. If society decides to allow patients the autonomy to end their lives early and wishes to provide skilled technical help in doing so, let it do so at their peril. But let it choose and train technicians to do it. Do not compromise the one person whom our patients should trust totally to never do them harm.
Alva B Weir, III, MD, FACP (alweir@WESTCLINIC.com)
West Cancer Center, Memphis, Tennessee
1. Strouse T. End-of-life options and the legal pathways to physician aid in dying. J Commun and Support Oncol. 2017;15(1):1-3.
2. Snyder L. American College of Physicians ethics manual: sixth edition. Ann Int Med. 2012;156(1, part 2)73-104.
3. Coleman D. Assisted suicide laws create discriminatory double standard for who gets suicide prevention and who gets suicide assistance: Not Dead Yet Responds to Autonomy Inc. Disabil Health. http://www.disabilityandhealthjnl.com/article/S1936-6574(09)00089-2/fulltext. Published January 2010. Accessed on March 12, 2017.
4. Yang YT, Curlin FA. Why physicians should oppose assisted suicide. JAMA 2016;315(3):247-248.
5. Pelligrino E. Some things ought never be done: moral absolutes in clinical ethics. Theo Med Bioeth. 2005;26:469-486.
I recently read with interest Dr Thomas Strouse’s article written to support physician aid in dying. Within the article he made the following statement: “I have come to view ‘active non-participation’ in legal PAD [physician aid in dying] – that is, decisions by individual physicians and/or health systems not only to not provide, but also not refer patients to possibly willing providers and systems without regard for specific clinical contexts – as a toxic form of patient abandonment.”1 Within the article, Dr Strouse lays out for us thoughtful precautions in the aid-in-dying laws, attempting to demonstrate that no vulnerable population is abused. Such precautions are important but provide the same result for all participants: the death of a patient. This is the central problem with aid in dying. Certainly there is nothing wrong with dying, and we all will have that opportunity. Though most of us would choose to put that moment off a while, for some, the suffering in this life makes death seem a welcome relief.
What is a physician’s central responsibility in the care of his or her patients near the end of their lives?
As program director for the hematology and oncology fellowship at my institution, I impress upon my fellows the importance of goal-oriented decision-making. I specifically teach them that there are only four goals worth achieving in any therapeutic or diagnostic decision making: to cure the disease; to help patients live longer despite the disease; to maximize the patient’s quality of life, and to prevent impending disasters. I know of no other worthwhile goal in any decision we are to make for our patients. I can point to none of these goals that physician aid in dying achieves. When it comes to physician-assisted suicide, some would argue that selecting an early death is a way of “maximizing quality of life.” And certainly our task is to make life the best it can be for our patients while they live through the dying process, but I am unaware of any published quality of life formula that calculates the end of life as a positive measure.
The question for us is the role of the doctor. Dr Strouse raises two issues with those whom he accuses of toxic abandonment. The first is whether physicians should provide aid in death, and the second is whether physicians should refer for the same service if they believe it is wrong for their patients.
It certainly has not been well established that physician-assisted suicide is a good thing rather than a tragic thing. A 2012 statement from the Ethics, Professionalism and Human Rights Committee of the American College of Physicians suggests otherwise: “After much consideration, the College concluded that making physician-assisted suicide legal raised serious ethical, clinical and social concerns and that the practice might undermine patient trust; distract from reform in end-of-life care; and be used in vulnerable patients, including those who are poor, are disabled, or are unable to speak for themselves or minority groups who have experienced discrimination.”2 The disability rights group, Not Dead Yet, has agreed with the ACP: “It cannot be seriously maintained that assisted suicide laws can or do limit assisted suicide to people who are imminently dying, and voluntarily request and consume a lethal dose, free of inappropriate pressures from family or society. Rather, assisted suicide laws ensure legal immunity for physicians who already devalue the lives of older and disabled people and have significant economic incentives to at least agree with their suicides, if not encourage them, or worse.”3
Such statements sound prophetic within both our present cost containment health care culture and in the real world of personal family economic pressures that can lead a patient toward the understanding that a right to die is actually a “duty to die.”
As society is driving physicians to be technicians to carry out their bidding, physicians should be clinging tightly to their role as trusted advocates for their patients. Certainly our patients have fears and pain that would at times lead them to prefer death to living, but a patient’s move to nonexistence is not the task of the physician. Our task as physicians was well described recently by Yang and Curlin: “Many patients with terminal illnesses fear unbearable pain or other symptoms. The physician’s role is to care for them in their illness so as to relieve pain or otherwise help them bear up under the symptoms they endure. Many patients loathe the prospect of abject debility. The physician’s role is to maintain solidarity with those whose health is diminished, not to not to imply that debility renders a patient’s life not worth living.”4
Statements such as these by reasoned people suggest we, as a country, have no consensus for the question whether aid in dying is possibly good or seriously bad for our patients. So it is quite reasonable for compassionate physicians to refuse to administer lethal medicines to their patients in order to “do no harm.”
The second question Dr Strouse explores is whether physicians who disapprove of physician-assisted suicide are abandoning their patients because they do not refer them to a provider who will provide such services. Dr Edmund Pelligrino, a well-respected medical ethicist, in his discussion of moral absolutes in medicine establishes the moral absolute, “Do not kill” and then addresses the ethical problem of complicity in killing. “Formal cooperation is absolutely and always, forbidden. This is the case when the physician shares the evil intent, partakes directly and freely, or in any way facilitates an intrinsically evil act like abortion or assisted suicide.”5 Though personally I would not use the word, “evil,” as he does, since evil implies motive; I would substitute the word “harm” and suggest that we should never be complicit in an act that we feel brings the harm of death to our patients. I would suggest that the expectation that physicians referring for aid in dying is analogous with the patient who comes to me demanding a chemotherapy that I know would cause her harm. I would refuse to give it to her and refuse to send her to a doctor who would be willing to give to her. Referral to produce harm is complicity with causing the harm itself. Our society should never go there. Our society should never ask a physician to cross the boundary line of conscience that is the ultimate protection for vulnerable patients.
I know what it is like to watch our patients suffer. I know what it is like to watch our loved ones suffer. I pushed the morphine at my father’s bedside until he quit screaming in pain. But I did not kill him. I cared for him. Such is the physician’s role. If society decides to allow patients the autonomy to end their lives early and wishes to provide skilled technical help in doing so, let it do so at their peril. But let it choose and train technicians to do it. Do not compromise the one person whom our patients should trust totally to never do them harm.
Alva B Weir, III, MD, FACP (alweir@WESTCLINIC.com)
West Cancer Center, Memphis, Tennessee
I recently read with interest Dr Thomas Strouse’s article written to support physician aid in dying. Within the article he made the following statement: “I have come to view ‘active non-participation’ in legal PAD [physician aid in dying] – that is, decisions by individual physicians and/or health systems not only to not provide, but also not refer patients to possibly willing providers and systems without regard for specific clinical contexts – as a toxic form of patient abandonment.”1 Within the article, Dr Strouse lays out for us thoughtful precautions in the aid-in-dying laws, attempting to demonstrate that no vulnerable population is abused. Such precautions are important but provide the same result for all participants: the death of a patient. This is the central problem with aid in dying. Certainly there is nothing wrong with dying, and we all will have that opportunity. Though most of us would choose to put that moment off a while, for some, the suffering in this life makes death seem a welcome relief.
What is a physician’s central responsibility in the care of his or her patients near the end of their lives?
As program director for the hematology and oncology fellowship at my institution, I impress upon my fellows the importance of goal-oriented decision-making. I specifically teach them that there are only four goals worth achieving in any therapeutic or diagnostic decision making: to cure the disease; to help patients live longer despite the disease; to maximize the patient’s quality of life, and to prevent impending disasters. I know of no other worthwhile goal in any decision we are to make for our patients. I can point to none of these goals that physician aid in dying achieves. When it comes to physician-assisted suicide, some would argue that selecting an early death is a way of “maximizing quality of life.” And certainly our task is to make life the best it can be for our patients while they live through the dying process, but I am unaware of any published quality of life formula that calculates the end of life as a positive measure.
The question for us is the role of the doctor. Dr Strouse raises two issues with those whom he accuses of toxic abandonment. The first is whether physicians should provide aid in death, and the second is whether physicians should refer for the same service if they believe it is wrong for their patients.
It certainly has not been well established that physician-assisted suicide is a good thing rather than a tragic thing. A 2012 statement from the Ethics, Professionalism and Human Rights Committee of the American College of Physicians suggests otherwise: “After much consideration, the College concluded that making physician-assisted suicide legal raised serious ethical, clinical and social concerns and that the practice might undermine patient trust; distract from reform in end-of-life care; and be used in vulnerable patients, including those who are poor, are disabled, or are unable to speak for themselves or minority groups who have experienced discrimination.”2 The disability rights group, Not Dead Yet, has agreed with the ACP: “It cannot be seriously maintained that assisted suicide laws can or do limit assisted suicide to people who are imminently dying, and voluntarily request and consume a lethal dose, free of inappropriate pressures from family or society. Rather, assisted suicide laws ensure legal immunity for physicians who already devalue the lives of older and disabled people and have significant economic incentives to at least agree with their suicides, if not encourage them, or worse.”3
Such statements sound prophetic within both our present cost containment health care culture and in the real world of personal family economic pressures that can lead a patient toward the understanding that a right to die is actually a “duty to die.”
As society is driving physicians to be technicians to carry out their bidding, physicians should be clinging tightly to their role as trusted advocates for their patients. Certainly our patients have fears and pain that would at times lead them to prefer death to living, but a patient’s move to nonexistence is not the task of the physician. Our task as physicians was well described recently by Yang and Curlin: “Many patients with terminal illnesses fear unbearable pain or other symptoms. The physician’s role is to care for them in their illness so as to relieve pain or otherwise help them bear up under the symptoms they endure. Many patients loathe the prospect of abject debility. The physician’s role is to maintain solidarity with those whose health is diminished, not to not to imply that debility renders a patient’s life not worth living.”4
Statements such as these by reasoned people suggest we, as a country, have no consensus for the question whether aid in dying is possibly good or seriously bad for our patients. So it is quite reasonable for compassionate physicians to refuse to administer lethal medicines to their patients in order to “do no harm.”
The second question Dr Strouse explores is whether physicians who disapprove of physician-assisted suicide are abandoning their patients because they do not refer them to a provider who will provide such services. Dr Edmund Pelligrino, a well-respected medical ethicist, in his discussion of moral absolutes in medicine establishes the moral absolute, “Do not kill” and then addresses the ethical problem of complicity in killing. “Formal cooperation is absolutely and always, forbidden. This is the case when the physician shares the evil intent, partakes directly and freely, or in any way facilitates an intrinsically evil act like abortion or assisted suicide.”5 Though personally I would not use the word, “evil,” as he does, since evil implies motive; I would substitute the word “harm” and suggest that we should never be complicit in an act that we feel brings the harm of death to our patients. I would suggest that the expectation that physicians referring for aid in dying is analogous with the patient who comes to me demanding a chemotherapy that I know would cause her harm. I would refuse to give it to her and refuse to send her to a doctor who would be willing to give to her. Referral to produce harm is complicity with causing the harm itself. Our society should never go there. Our society should never ask a physician to cross the boundary line of conscience that is the ultimate protection for vulnerable patients.
I know what it is like to watch our patients suffer. I know what it is like to watch our loved ones suffer. I pushed the morphine at my father’s bedside until he quit screaming in pain. But I did not kill him. I cared for him. Such is the physician’s role. If society decides to allow patients the autonomy to end their lives early and wishes to provide skilled technical help in doing so, let it do so at their peril. But let it choose and train technicians to do it. Do not compromise the one person whom our patients should trust totally to never do them harm.
Alva B Weir, III, MD, FACP (alweir@WESTCLINIC.com)
West Cancer Center, Memphis, Tennessee
1. Strouse T. End-of-life options and the legal pathways to physician aid in dying. J Commun and Support Oncol. 2017;15(1):1-3.
2. Snyder L. American College of Physicians ethics manual: sixth edition. Ann Int Med. 2012;156(1, part 2)73-104.
3. Coleman D. Assisted suicide laws create discriminatory double standard for who gets suicide prevention and who gets suicide assistance: Not Dead Yet Responds to Autonomy Inc. Disabil Health. http://www.disabilityandhealthjnl.com/article/S1936-6574(09)00089-2/fulltext. Published January 2010. Accessed on March 12, 2017.
4. Yang YT, Curlin FA. Why physicians should oppose assisted suicide. JAMA 2016;315(3):247-248.
5. Pelligrino E. Some things ought never be done: moral absolutes in clinical ethics. Theo Med Bioeth. 2005;26:469-486.
1. Strouse T. End-of-life options and the legal pathways to physician aid in dying. J Commun and Support Oncol. 2017;15(1):1-3.
2. Snyder L. American College of Physicians ethics manual: sixth edition. Ann Int Med. 2012;156(1, part 2)73-104.
3. Coleman D. Assisted suicide laws create discriminatory double standard for who gets suicide prevention and who gets suicide assistance: Not Dead Yet Responds to Autonomy Inc. Disabil Health. http://www.disabilityandhealthjnl.com/article/S1936-6574(09)00089-2/fulltext. Published January 2010. Accessed on March 12, 2017.
4. Yang YT, Curlin FA. Why physicians should oppose assisted suicide. JAMA 2016;315(3):247-248.
5. Pelligrino E. Some things ought never be done: moral absolutes in clinical ethics. Theo Med Bioeth. 2005;26:469-486.
Pulmonary sarcomatoid carcinoma presenting as a necrotizing cavitary lung lesion: diagnostic dilemma
Pulmonary sarcomatoid carcinoma (PSC) is a rare histological subtype that has an aggressive course with average survival of 11-13 months.1 In clinical practice, the possible presentations of this rare cancer are not widely known, resulting in a misdiagnosis. That is what happened with our patient, who presented with necrotizing cavitary lung lesion and soft tissue necrotizing lymphadenitis. The clinical picture was reminiscent of tuberculosis or granulomatosis with polyangiitis and was further confounded by negative computed-tomography (CT)-guided biopsy and bronchoscopy findings, which added to the delay in diagnosis. With the currently available knowledge, the diagnosis of PSC depends largely on evaluation of the surgically resected specimen, which in most cases is avoided until there is a high suspicion of PSC. Biopsy is not useful due to extensive necrosis, as will be seen in our case. Consequently, most of the data in the literature is based on case series of autopsy specimen, and the clinical characteristics of PSC remain unclear. The rarity of PSC has prevented its characterization in literature. We report here a rare presentation of PSC with necrotizing lung lesion, to add to the paucity of the current data.
Case presentation and summary
A 58-year-old homeless man presented to the Upstate University Hospital, Syracuse, New York, with a 25-pound weight loss during the previous month and associated productive cough and hemoptysis for a week and a painful mass in the nape of his neck. He denied any fever, chest pain, sick contacts, or joint pain. He had a history of about 40 pack-years of smoking, and his brother had recently been diagnosed with lung cancer. A tender fluctuant mass was detected in the nape of his neck on examination (Figure 1).
The patient had presented 9 months earlier with persistent cough and hemoptysis, and at that visit was found to have a cavitary lesion in the right lung measuring 2 cm (0.8 in). He had undergone a computed-tomograpghy (CT)-guided biopsy of the lesion, which had shown acute and chronic inflammation with fibrosis, and he had negative bronchoscopy findings. The patient tested negative for tuberculosis during the first visit but he left the hospital against the medical advice of the physicians and he was lost to follow-up until his re-presentation.
On physical examination at his re-presentation, the patient seemed cachectic, with a blood pressure of 94/62 mm of Hg. The mass in the nape of his neck was about 3 cm (1.2 in) long, with erythema of the surrounding skin (Figure 1). Bronchial breath sounds were heard in the right upper lobe of the lung, likely due to the underlying cavitary lesion (Figure 2).
Given the patient’s advanced disease, he was started on palliative radiotherapy with radiosensitizing chemotherapy with carboplatin (target AUC 6) and paclitaxel (135 mg/m2 over 24 hours). His symptoms of hemoptysis improved transiently after the first cycle, but he became hypotensive and drowsy during the second cycle of therapy, and the family decided to make the patient comfort care and withdraw all further treatment. He was discharged to hospice.
Discussion
PSC is a rare variant of non-small-cell carcinoma lung cancer, accounting for up to 0.4% of lung malignancy.1 It was recently subtyped by the World Health Organization as a non-small cell lung carcinoma with certain amount of differentiation resembling sarcoma or containing elements of sarcoma.2-4 It is not known why both elements co-exist in the tumor, but Franks and colleagues some theories have been postulated in the literature, including possible origin from a single, aberrant stem cell with progenies differentiating in two separate pathways.3
Sarcomatoid carcinoma consists of spectrum of tumors including pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and blastoma.3,4 It usually shows male preponderance, and association with smoking.3 The diagnosis commonly occurs in the sixth decade of life, except for pulmonary blastoma, which is more common in the fourth decade and with equal gender distribution.4
The presenting symptoms can be variable and nonspecific, but predominantly include chest pain, cough, hemoptysis, and/or weight loss.5 Radiologically, pulmonary sarcomatoid cancer presenting as a necrotizing cavitary lesion in the lung is a rare finding, seldom reported in the past.6,7 The presentation in our case, with necrotizing lymphadenitis, was reminiscent of an infectious or autoimmune etiology such as tuberculosis or granulomatosis with polyangiitis. The presence of extensive necrosis in the lesion and the characteristic heterogeneity of the tumor had resulted in inconclusive biopsy findings during the previous presentation. In clinical practice, there is over-reliance on biopsy findings to make the distinction between cancer and other mimicking conditions. This is especially true for rare tumors such as PSC, which often results in misdiagnosis and a delay in administering the proper treatment.
Transbronchial biopsy in cases such as the present case, carries little benefit because the diagnosis depends on the site from which the biopsy is taken and whether the biopsied tissue is representative of the entire mass. The diagnosis can be suspected based on the clinical and radiological findings but confirmation requires a surgical resection to delineate the accurate cytology and architecture.5,6,8 Huang and colleagues showed a misdiagnosis rate of PSC of >70% preoperatively.4 Resective surgery is feasible only in patients with high index of suspicion for a malignancy, which in most cases requires previous confirmation with a biopsy. The rarity of this cancer, its unusual presentations, and the lack of specific testing preclude early diagnosis and timely treatment of this fatal condition.
Initial treatment options for localized or with limited spread disease is resective surgery. The role of chemo- or radiation therapy is not known, but they have not previously shown promising results,6,8 except in some cases when they are used as postoperative adjuvant chemotherapy4 or in bulky, locally invasive tumors.1 The recurrence rate after surgery is very high, resulting in a poor 5-year survival rate.1,8 Experimental therapies, such as antibodies that target epidermal growth factor receptor mutations, have not shown much success either.8 In conclusion, the outlook for patients with PSC with the current available knowledge and treatment protocols, is dismal.
Most of the current knowledge and data in the literature is based on cases from autopsy or early-stage surgical resections rather than on patients with advanced cancer.5 Moreover, the role of surgical resection in PSC is questionable, given the high recurrence rate. Subsequently, the clinical and pathological manifestations have yet to be well characterized.4 There has been advance with the publication of more studies recently. Cytokeratin markers such as CAM 5.2 and AE1/AE3 are commonly useful to support the diagnosis when suspected.3 Other markers, including the carcinoembryonic antigen, CD15, and thyroid transcription factor-1 may be variably positive, based on the differentiation of the cancer. Other exciting prospects in the study of PSC include the suggestion of a modified vimentin histologic score for better characterization of the cancer and the discovery of high platelet-derived growth factor receptor beta immunohistochemistry expression in PSC as a potential target for future therapy.
Conclusion
Pulmonary sarcomatoid lung cancer can present with a predominant necrotizing picture that mimics diseases such as tuberculosis. In such case, transbronchial biopsy carries little benefit because the diagnosis depends on whether the biopsied tissue is representative of the entire mass, often confounded by the extensive necrosis. More data is needed to determine prognostic factors and appropriate therapeutic strategies.
1. Martin LW, Correa AM, Ordonez NG, et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg. 2007;84(3):973-980.
2. Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18(6):1059-1068.
3. Franks TJ, Galvin JR. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134(1):49-54.
4. Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. http://wjso.biomedcentral.com/articles/10.1186/1477-7819-11-252. Published 2013. Accessed March 12, 2017.
5. Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med. 2010;134(11):1645-1658.
6. Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol. 2010;18(2):103-120.
7. Chang YL, Lee YC, Shih JY, Wu CT. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer. 2001;34(1):91-97.
8. Park JS, Lee Y, Han J, et al. Clinicopathologic outcomes of curative resection for sarcomatoid carcinoma of the lung. Oncology. 2011;81(3-4):206-213.
Pulmonary sarcomatoid carcinoma (PSC) is a rare histological subtype that has an aggressive course with average survival of 11-13 months.1 In clinical practice, the possible presentations of this rare cancer are not widely known, resulting in a misdiagnosis. That is what happened with our patient, who presented with necrotizing cavitary lung lesion and soft tissue necrotizing lymphadenitis. The clinical picture was reminiscent of tuberculosis or granulomatosis with polyangiitis and was further confounded by negative computed-tomography (CT)-guided biopsy and bronchoscopy findings, which added to the delay in diagnosis. With the currently available knowledge, the diagnosis of PSC depends largely on evaluation of the surgically resected specimen, which in most cases is avoided until there is a high suspicion of PSC. Biopsy is not useful due to extensive necrosis, as will be seen in our case. Consequently, most of the data in the literature is based on case series of autopsy specimen, and the clinical characteristics of PSC remain unclear. The rarity of PSC has prevented its characterization in literature. We report here a rare presentation of PSC with necrotizing lung lesion, to add to the paucity of the current data.
Case presentation and summary
A 58-year-old homeless man presented to the Upstate University Hospital, Syracuse, New York, with a 25-pound weight loss during the previous month and associated productive cough and hemoptysis for a week and a painful mass in the nape of his neck. He denied any fever, chest pain, sick contacts, or joint pain. He had a history of about 40 pack-years of smoking, and his brother had recently been diagnosed with lung cancer. A tender fluctuant mass was detected in the nape of his neck on examination (Figure 1).
The patient had presented 9 months earlier with persistent cough and hemoptysis, and at that visit was found to have a cavitary lesion in the right lung measuring 2 cm (0.8 in). He had undergone a computed-tomograpghy (CT)-guided biopsy of the lesion, which had shown acute and chronic inflammation with fibrosis, and he had negative bronchoscopy findings. The patient tested negative for tuberculosis during the first visit but he left the hospital against the medical advice of the physicians and he was lost to follow-up until his re-presentation.
On physical examination at his re-presentation, the patient seemed cachectic, with a blood pressure of 94/62 mm of Hg. The mass in the nape of his neck was about 3 cm (1.2 in) long, with erythema of the surrounding skin (Figure 1). Bronchial breath sounds were heard in the right upper lobe of the lung, likely due to the underlying cavitary lesion (Figure 2).
Given the patient’s advanced disease, he was started on palliative radiotherapy with radiosensitizing chemotherapy with carboplatin (target AUC 6) and paclitaxel (135 mg/m2 over 24 hours). His symptoms of hemoptysis improved transiently after the first cycle, but he became hypotensive and drowsy during the second cycle of therapy, and the family decided to make the patient comfort care and withdraw all further treatment. He was discharged to hospice.
Discussion
PSC is a rare variant of non-small-cell carcinoma lung cancer, accounting for up to 0.4% of lung malignancy.1 It was recently subtyped by the World Health Organization as a non-small cell lung carcinoma with certain amount of differentiation resembling sarcoma or containing elements of sarcoma.2-4 It is not known why both elements co-exist in the tumor, but Franks and colleagues some theories have been postulated in the literature, including possible origin from a single, aberrant stem cell with progenies differentiating in two separate pathways.3
Sarcomatoid carcinoma consists of spectrum of tumors including pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and blastoma.3,4 It usually shows male preponderance, and association with smoking.3 The diagnosis commonly occurs in the sixth decade of life, except for pulmonary blastoma, which is more common in the fourth decade and with equal gender distribution.4
The presenting symptoms can be variable and nonspecific, but predominantly include chest pain, cough, hemoptysis, and/or weight loss.5 Radiologically, pulmonary sarcomatoid cancer presenting as a necrotizing cavitary lesion in the lung is a rare finding, seldom reported in the past.6,7 The presentation in our case, with necrotizing lymphadenitis, was reminiscent of an infectious or autoimmune etiology such as tuberculosis or granulomatosis with polyangiitis. The presence of extensive necrosis in the lesion and the characteristic heterogeneity of the tumor had resulted in inconclusive biopsy findings during the previous presentation. In clinical practice, there is over-reliance on biopsy findings to make the distinction between cancer and other mimicking conditions. This is especially true for rare tumors such as PSC, which often results in misdiagnosis and a delay in administering the proper treatment.
Transbronchial biopsy in cases such as the present case, carries little benefit because the diagnosis depends on the site from which the biopsy is taken and whether the biopsied tissue is representative of the entire mass. The diagnosis can be suspected based on the clinical and radiological findings but confirmation requires a surgical resection to delineate the accurate cytology and architecture.5,6,8 Huang and colleagues showed a misdiagnosis rate of PSC of >70% preoperatively.4 Resective surgery is feasible only in patients with high index of suspicion for a malignancy, which in most cases requires previous confirmation with a biopsy. The rarity of this cancer, its unusual presentations, and the lack of specific testing preclude early diagnosis and timely treatment of this fatal condition.
Initial treatment options for localized or with limited spread disease is resective surgery. The role of chemo- or radiation therapy is not known, but they have not previously shown promising results,6,8 except in some cases when they are used as postoperative adjuvant chemotherapy4 or in bulky, locally invasive tumors.1 The recurrence rate after surgery is very high, resulting in a poor 5-year survival rate.1,8 Experimental therapies, such as antibodies that target epidermal growth factor receptor mutations, have not shown much success either.8 In conclusion, the outlook for patients with PSC with the current available knowledge and treatment protocols, is dismal.
Most of the current knowledge and data in the literature is based on cases from autopsy or early-stage surgical resections rather than on patients with advanced cancer.5 Moreover, the role of surgical resection in PSC is questionable, given the high recurrence rate. Subsequently, the clinical and pathological manifestations have yet to be well characterized.4 There has been advance with the publication of more studies recently. Cytokeratin markers such as CAM 5.2 and AE1/AE3 are commonly useful to support the diagnosis when suspected.3 Other markers, including the carcinoembryonic antigen, CD15, and thyroid transcription factor-1 may be variably positive, based on the differentiation of the cancer. Other exciting prospects in the study of PSC include the suggestion of a modified vimentin histologic score for better characterization of the cancer and the discovery of high platelet-derived growth factor receptor beta immunohistochemistry expression in PSC as a potential target for future therapy.
Conclusion
Pulmonary sarcomatoid lung cancer can present with a predominant necrotizing picture that mimics diseases such as tuberculosis. In such case, transbronchial biopsy carries little benefit because the diagnosis depends on whether the biopsied tissue is representative of the entire mass, often confounded by the extensive necrosis. More data is needed to determine prognostic factors and appropriate therapeutic strategies.
Pulmonary sarcomatoid carcinoma (PSC) is a rare histological subtype that has an aggressive course with average survival of 11-13 months.1 In clinical practice, the possible presentations of this rare cancer are not widely known, resulting in a misdiagnosis. That is what happened with our patient, who presented with necrotizing cavitary lung lesion and soft tissue necrotizing lymphadenitis. The clinical picture was reminiscent of tuberculosis or granulomatosis with polyangiitis and was further confounded by negative computed-tomography (CT)-guided biopsy and bronchoscopy findings, which added to the delay in diagnosis. With the currently available knowledge, the diagnosis of PSC depends largely on evaluation of the surgically resected specimen, which in most cases is avoided until there is a high suspicion of PSC. Biopsy is not useful due to extensive necrosis, as will be seen in our case. Consequently, most of the data in the literature is based on case series of autopsy specimen, and the clinical characteristics of PSC remain unclear. The rarity of PSC has prevented its characterization in literature. We report here a rare presentation of PSC with necrotizing lung lesion, to add to the paucity of the current data.
Case presentation and summary
A 58-year-old homeless man presented to the Upstate University Hospital, Syracuse, New York, with a 25-pound weight loss during the previous month and associated productive cough and hemoptysis for a week and a painful mass in the nape of his neck. He denied any fever, chest pain, sick contacts, or joint pain. He had a history of about 40 pack-years of smoking, and his brother had recently been diagnosed with lung cancer. A tender fluctuant mass was detected in the nape of his neck on examination (Figure 1).
The patient had presented 9 months earlier with persistent cough and hemoptysis, and at that visit was found to have a cavitary lesion in the right lung measuring 2 cm (0.8 in). He had undergone a computed-tomograpghy (CT)-guided biopsy of the lesion, which had shown acute and chronic inflammation with fibrosis, and he had negative bronchoscopy findings. The patient tested negative for tuberculosis during the first visit but he left the hospital against the medical advice of the physicians and he was lost to follow-up until his re-presentation.
On physical examination at his re-presentation, the patient seemed cachectic, with a blood pressure of 94/62 mm of Hg. The mass in the nape of his neck was about 3 cm (1.2 in) long, with erythema of the surrounding skin (Figure 1). Bronchial breath sounds were heard in the right upper lobe of the lung, likely due to the underlying cavitary lesion (Figure 2).
Given the patient’s advanced disease, he was started on palliative radiotherapy with radiosensitizing chemotherapy with carboplatin (target AUC 6) and paclitaxel (135 mg/m2 over 24 hours). His symptoms of hemoptysis improved transiently after the first cycle, but he became hypotensive and drowsy during the second cycle of therapy, and the family decided to make the patient comfort care and withdraw all further treatment. He was discharged to hospice.
Discussion
PSC is a rare variant of non-small-cell carcinoma lung cancer, accounting for up to 0.4% of lung malignancy.1 It was recently subtyped by the World Health Organization as a non-small cell lung carcinoma with certain amount of differentiation resembling sarcoma or containing elements of sarcoma.2-4 It is not known why both elements co-exist in the tumor, but Franks and colleagues some theories have been postulated in the literature, including possible origin from a single, aberrant stem cell with progenies differentiating in two separate pathways.3
Sarcomatoid carcinoma consists of spectrum of tumors including pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and blastoma.3,4 It usually shows male preponderance, and association with smoking.3 The diagnosis commonly occurs in the sixth decade of life, except for pulmonary blastoma, which is more common in the fourth decade and with equal gender distribution.4
The presenting symptoms can be variable and nonspecific, but predominantly include chest pain, cough, hemoptysis, and/or weight loss.5 Radiologically, pulmonary sarcomatoid cancer presenting as a necrotizing cavitary lesion in the lung is a rare finding, seldom reported in the past.6,7 The presentation in our case, with necrotizing lymphadenitis, was reminiscent of an infectious or autoimmune etiology such as tuberculosis or granulomatosis with polyangiitis. The presence of extensive necrosis in the lesion and the characteristic heterogeneity of the tumor had resulted in inconclusive biopsy findings during the previous presentation. In clinical practice, there is over-reliance on biopsy findings to make the distinction between cancer and other mimicking conditions. This is especially true for rare tumors such as PSC, which often results in misdiagnosis and a delay in administering the proper treatment.
Transbronchial biopsy in cases such as the present case, carries little benefit because the diagnosis depends on the site from which the biopsy is taken and whether the biopsied tissue is representative of the entire mass. The diagnosis can be suspected based on the clinical and radiological findings but confirmation requires a surgical resection to delineate the accurate cytology and architecture.5,6,8 Huang and colleagues showed a misdiagnosis rate of PSC of >70% preoperatively.4 Resective surgery is feasible only in patients with high index of suspicion for a malignancy, which in most cases requires previous confirmation with a biopsy. The rarity of this cancer, its unusual presentations, and the lack of specific testing preclude early diagnosis and timely treatment of this fatal condition.
Initial treatment options for localized or with limited spread disease is resective surgery. The role of chemo- or radiation therapy is not known, but they have not previously shown promising results,6,8 except in some cases when they are used as postoperative adjuvant chemotherapy4 or in bulky, locally invasive tumors.1 The recurrence rate after surgery is very high, resulting in a poor 5-year survival rate.1,8 Experimental therapies, such as antibodies that target epidermal growth factor receptor mutations, have not shown much success either.8 In conclusion, the outlook for patients with PSC with the current available knowledge and treatment protocols, is dismal.
Most of the current knowledge and data in the literature is based on cases from autopsy or early-stage surgical resections rather than on patients with advanced cancer.5 Moreover, the role of surgical resection in PSC is questionable, given the high recurrence rate. Subsequently, the clinical and pathological manifestations have yet to be well characterized.4 There has been advance with the publication of more studies recently. Cytokeratin markers such as CAM 5.2 and AE1/AE3 are commonly useful to support the diagnosis when suspected.3 Other markers, including the carcinoembryonic antigen, CD15, and thyroid transcription factor-1 may be variably positive, based on the differentiation of the cancer. Other exciting prospects in the study of PSC include the suggestion of a modified vimentin histologic score for better characterization of the cancer and the discovery of high platelet-derived growth factor receptor beta immunohistochemistry expression in PSC as a potential target for future therapy.
Conclusion
Pulmonary sarcomatoid lung cancer can present with a predominant necrotizing picture that mimics diseases such as tuberculosis. In such case, transbronchial biopsy carries little benefit because the diagnosis depends on whether the biopsied tissue is representative of the entire mass, often confounded by the extensive necrosis. More data is needed to determine prognostic factors and appropriate therapeutic strategies.
1. Martin LW, Correa AM, Ordonez NG, et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg. 2007;84(3):973-980.
2. Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18(6):1059-1068.
3. Franks TJ, Galvin JR. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134(1):49-54.
4. Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. http://wjso.biomedcentral.com/articles/10.1186/1477-7819-11-252. Published 2013. Accessed March 12, 2017.
5. Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med. 2010;134(11):1645-1658.
6. Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol. 2010;18(2):103-120.
7. Chang YL, Lee YC, Shih JY, Wu CT. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer. 2001;34(1):91-97.
8. Park JS, Lee Y, Han J, et al. Clinicopathologic outcomes of curative resection for sarcomatoid carcinoma of the lung. Oncology. 2011;81(3-4):206-213.
1. Martin LW, Correa AM, Ordonez NG, et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg. 2007;84(3):973-980.
2. Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18(6):1059-1068.
3. Franks TJ, Galvin JR. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134(1):49-54.
4. Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. http://wjso.biomedcentral.com/articles/10.1186/1477-7819-11-252. Published 2013. Accessed March 12, 2017.
5. Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med. 2010;134(11):1645-1658.
6. Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol. 2010;18(2):103-120.
7. Chang YL, Lee YC, Shih JY, Wu CT. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer. 2001;34(1):91-97.
8. Park JS, Lee Y, Han J, et al. Clinicopathologic outcomes of curative resection for sarcomatoid carcinoma of the lung. Oncology. 2011;81(3-4):206-213.
Palmoplantar exacerbation of psoriasis after nivolumab for lung cancer
Nivolumab is a full human immunoglobulin antibody to the programmed cell death 1 (PD-1) immune checkpoint receptor on T cells. This programmed cell death inhibitor is a targeted immunotherapy used to treat patients with melanoma, among other malignancies.1 More recently, nivolumab has been used for advanced non-small-cell lung cancer (NSCLC) after failure of previous chemotherapeutic agents. It was approved by the US Food and Drug Administration for the NSCLC indication in 2015.2
PD-1 inhibitors are efficacious in treating advanced malignancies, although their immune-mediated functions can lead to undesirable side effects. Patients treated with nivolumab have been reported to develop thyroid disease,1,3,4 diabetes,3 hypophysitis,1,3 hypopituitarism,3 and pneumonitis,4,2 as well as other autoimmune conditions.3 Although nivolumab is often used to treat skin diseases such as melanoma, it can have many cutaneous side effects including pruritus,1,3-6 rash,1,3,4,6,7 vitiligo,1,3,7,6 mouth sores,3 injection site reactions,3,6 and alopecia.5 Herein, we describe a patient who was treated with nivolumab and developed an exacerbation of pre-existing psoriasis.
Case presentation and summary
A 57-year-old man with metastatic NSCLC and a history of plaque psoriasis presented to the dermatology clinic for evaluation of new lesions on his palms and soles. The patient had been previously treated with numerous therapies for NSCLC, including chemotherapy and radiation. Previous chemotherapeutic agents included the cisplatin plus etoposide combination, with doxetaxel and pemetrexed. The patient was not able to tolerate the chemotherapy and instead opted for hospice care. After several months, he chose to restart therapy, and was started on the programmed cell death (PD)-1 inhibitor, nivolumab, at a dose of 3 mg/kg for a total of 6 cycles. He received his first dose 5 weeks before his current presentation to the clinic, and his second dose 2 weeks before.
The patient reported a 20-year history of plaque psoriasis, characterized by psoriatic plaques on the elbows and shins and for which he was treated with topical therapies with good effect. Every few months, he would develop one or two small plaques of psoriasis on his palms and soles. The lesions were inconsequential to the patient, as he never experienced more than one or two small palmoplantar lesions at a time. One week after his second cycle of nivolumab, the patient developed an eruption of lesions on his palms and soles. He observed that the lesions seemed to be similar to his previous palmoplantar psoriatic plaques but with significantly greater skin involvement. The patient denied any new-onset joint pain.
The results of a physical examination revealed a cachectic man in no acute distress, with more than 30 erythematous circular to oval circumscribed plaques with yellow to whitish scales on the bilateral palms (Figure 1) and soles (Figure 2).
The patient also had well-demarcated, thick oval erythematous plaques with micaceous scales on the bilateral elbows (Figure 3), and thin scaly erythematous plaques on the anterior shins (Figure 4). There were no psoriatic plaques on the remainder of the trunk or extremities. Mucosal surfaces, scalp, and nails were uninvolved.
A clinical diagnosis of exacerbation of pre-existing psoriasis owing to nivolumab therapy was made. The patient was started on clobetasol 0.05% ointment twice daily under occlusion with plastic wrap to the affected areas, and he was continued on nivolumab for his NSCLC.
Discussion
Treatment with nivolumab can lead to a range of autoimmune side effects, and as shown in this case, psoriasis is one of the cutaneous findings that could be exacerbated by treatment with nivolumab. To date, two cases of exacerbation of psoriasis in patients treated with nivolumab for melanoma have been reported in the literature.8,9 In the first case, the patient had well-controlled plaque psoriasis at baseline and he subsequently developed psoriatic plaques on the trunk and extremities after the second infusion of nivolumab for metastatic melanoma. A biopsy showed regular acanthosis with hyperkeratosis and parakeratosis in addition to dilated vessels in the papillary dermis.8 In the second case, the patient had a history of psoriasis vulgaris with no active lesions. Three weeks after his first course of nivolumab for metastatic oral mucosal melanoma, he developed new, well-circumscribed erythematous scaly plaques on the trunk and extremities that were clinically diagnosed as psoriasis.9 In a third case, a patient without a prior history of psoriasis experienced a psoriasiform eruption on the trunk and extremities after the fourth dose of nivolumab for oral mucosal melanoma.10 Thus, our case is the third reported case of exacerbation of preexisting psoriasis in a patient treated with nivolumab. Furthermore, our patient is the first reported case of a patient treated with nivolumab for NSCLC to develop this adverse event. Whereas the previously reported cases were characterized by widespread trunk and extremity involvement, our patient developed focal exacerbation of the palmoplantar areas.
Additional studies are needed to more clearly characterize the specific cutaneous toxicities of nivolumab and to determine if particular skin reactions may indicate a better response to the anticancer agent. Side effects such as psoriasis can often be managed with topical therapies and may not require withdrawal of the medication. We encourage the collaboration of dermatologists and oncologists to enhance the diagnosis and management of these cutaneous side effects in cancer patients.
1. Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of Nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433-440.
2. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004-2012.
3. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
4. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257-265.
5. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384.
6. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311-4318.
7. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber J. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2015.
8. Matsumura N, Ohtsuka M, Kikuchi N, Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm Venereol. 2016;96(2):259-260.
9. Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol. 2016;30(10):e89-e91.
10. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
Nivolumab is a full human immunoglobulin antibody to the programmed cell death 1 (PD-1) immune checkpoint receptor on T cells. This programmed cell death inhibitor is a targeted immunotherapy used to treat patients with melanoma, among other malignancies.1 More recently, nivolumab has been used for advanced non-small-cell lung cancer (NSCLC) after failure of previous chemotherapeutic agents. It was approved by the US Food and Drug Administration for the NSCLC indication in 2015.2
PD-1 inhibitors are efficacious in treating advanced malignancies, although their immune-mediated functions can lead to undesirable side effects. Patients treated with nivolumab have been reported to develop thyroid disease,1,3,4 diabetes,3 hypophysitis,1,3 hypopituitarism,3 and pneumonitis,4,2 as well as other autoimmune conditions.3 Although nivolumab is often used to treat skin diseases such as melanoma, it can have many cutaneous side effects including pruritus,1,3-6 rash,1,3,4,6,7 vitiligo,1,3,7,6 mouth sores,3 injection site reactions,3,6 and alopecia.5 Herein, we describe a patient who was treated with nivolumab and developed an exacerbation of pre-existing psoriasis.
Case presentation and summary
A 57-year-old man with metastatic NSCLC and a history of plaque psoriasis presented to the dermatology clinic for evaluation of new lesions on his palms and soles. The patient had been previously treated with numerous therapies for NSCLC, including chemotherapy and radiation. Previous chemotherapeutic agents included the cisplatin plus etoposide combination, with doxetaxel and pemetrexed. The patient was not able to tolerate the chemotherapy and instead opted for hospice care. After several months, he chose to restart therapy, and was started on the programmed cell death (PD)-1 inhibitor, nivolumab, at a dose of 3 mg/kg for a total of 6 cycles. He received his first dose 5 weeks before his current presentation to the clinic, and his second dose 2 weeks before.
The patient reported a 20-year history of plaque psoriasis, characterized by psoriatic plaques on the elbows and shins and for which he was treated with topical therapies with good effect. Every few months, he would develop one or two small plaques of psoriasis on his palms and soles. The lesions were inconsequential to the patient, as he never experienced more than one or two small palmoplantar lesions at a time. One week after his second cycle of nivolumab, the patient developed an eruption of lesions on his palms and soles. He observed that the lesions seemed to be similar to his previous palmoplantar psoriatic plaques but with significantly greater skin involvement. The patient denied any new-onset joint pain.
The results of a physical examination revealed a cachectic man in no acute distress, with more than 30 erythematous circular to oval circumscribed plaques with yellow to whitish scales on the bilateral palms (Figure 1) and soles (Figure 2).
The patient also had well-demarcated, thick oval erythematous plaques with micaceous scales on the bilateral elbows (Figure 3), and thin scaly erythematous plaques on the anterior shins (Figure 4). There were no psoriatic plaques on the remainder of the trunk or extremities. Mucosal surfaces, scalp, and nails were uninvolved.
A clinical diagnosis of exacerbation of pre-existing psoriasis owing to nivolumab therapy was made. The patient was started on clobetasol 0.05% ointment twice daily under occlusion with plastic wrap to the affected areas, and he was continued on nivolumab for his NSCLC.
Discussion
Treatment with nivolumab can lead to a range of autoimmune side effects, and as shown in this case, psoriasis is one of the cutaneous findings that could be exacerbated by treatment with nivolumab. To date, two cases of exacerbation of psoriasis in patients treated with nivolumab for melanoma have been reported in the literature.8,9 In the first case, the patient had well-controlled plaque psoriasis at baseline and he subsequently developed psoriatic plaques on the trunk and extremities after the second infusion of nivolumab for metastatic melanoma. A biopsy showed regular acanthosis with hyperkeratosis and parakeratosis in addition to dilated vessels in the papillary dermis.8 In the second case, the patient had a history of psoriasis vulgaris with no active lesions. Three weeks after his first course of nivolumab for metastatic oral mucosal melanoma, he developed new, well-circumscribed erythematous scaly plaques on the trunk and extremities that were clinically diagnosed as psoriasis.9 In a third case, a patient without a prior history of psoriasis experienced a psoriasiform eruption on the trunk and extremities after the fourth dose of nivolumab for oral mucosal melanoma.10 Thus, our case is the third reported case of exacerbation of preexisting psoriasis in a patient treated with nivolumab. Furthermore, our patient is the first reported case of a patient treated with nivolumab for NSCLC to develop this adverse event. Whereas the previously reported cases were characterized by widespread trunk and extremity involvement, our patient developed focal exacerbation of the palmoplantar areas.
Additional studies are needed to more clearly characterize the specific cutaneous toxicities of nivolumab and to determine if particular skin reactions may indicate a better response to the anticancer agent. Side effects such as psoriasis can often be managed with topical therapies and may not require withdrawal of the medication. We encourage the collaboration of dermatologists and oncologists to enhance the diagnosis and management of these cutaneous side effects in cancer patients.
Nivolumab is a full human immunoglobulin antibody to the programmed cell death 1 (PD-1) immune checkpoint receptor on T cells. This programmed cell death inhibitor is a targeted immunotherapy used to treat patients with melanoma, among other malignancies.1 More recently, nivolumab has been used for advanced non-small-cell lung cancer (NSCLC) after failure of previous chemotherapeutic agents. It was approved by the US Food and Drug Administration for the NSCLC indication in 2015.2
PD-1 inhibitors are efficacious in treating advanced malignancies, although their immune-mediated functions can lead to undesirable side effects. Patients treated with nivolumab have been reported to develop thyroid disease,1,3,4 diabetes,3 hypophysitis,1,3 hypopituitarism,3 and pneumonitis,4,2 as well as other autoimmune conditions.3 Although nivolumab is often used to treat skin diseases such as melanoma, it can have many cutaneous side effects including pruritus,1,3-6 rash,1,3,4,6,7 vitiligo,1,3,7,6 mouth sores,3 injection site reactions,3,6 and alopecia.5 Herein, we describe a patient who was treated with nivolumab and developed an exacerbation of pre-existing psoriasis.
Case presentation and summary
A 57-year-old man with metastatic NSCLC and a history of plaque psoriasis presented to the dermatology clinic for evaluation of new lesions on his palms and soles. The patient had been previously treated with numerous therapies for NSCLC, including chemotherapy and radiation. Previous chemotherapeutic agents included the cisplatin plus etoposide combination, with doxetaxel and pemetrexed. The patient was not able to tolerate the chemotherapy and instead opted for hospice care. After several months, he chose to restart therapy, and was started on the programmed cell death (PD)-1 inhibitor, nivolumab, at a dose of 3 mg/kg for a total of 6 cycles. He received his first dose 5 weeks before his current presentation to the clinic, and his second dose 2 weeks before.
The patient reported a 20-year history of plaque psoriasis, characterized by psoriatic plaques on the elbows and shins and for which he was treated with topical therapies with good effect. Every few months, he would develop one or two small plaques of psoriasis on his palms and soles. The lesions were inconsequential to the patient, as he never experienced more than one or two small palmoplantar lesions at a time. One week after his second cycle of nivolumab, the patient developed an eruption of lesions on his palms and soles. He observed that the lesions seemed to be similar to his previous palmoplantar psoriatic plaques but with significantly greater skin involvement. The patient denied any new-onset joint pain.
The results of a physical examination revealed a cachectic man in no acute distress, with more than 30 erythematous circular to oval circumscribed plaques with yellow to whitish scales on the bilateral palms (Figure 1) and soles (Figure 2).
The patient also had well-demarcated, thick oval erythematous plaques with micaceous scales on the bilateral elbows (Figure 3), and thin scaly erythematous plaques on the anterior shins (Figure 4). There were no psoriatic plaques on the remainder of the trunk or extremities. Mucosal surfaces, scalp, and nails were uninvolved.
A clinical diagnosis of exacerbation of pre-existing psoriasis owing to nivolumab therapy was made. The patient was started on clobetasol 0.05% ointment twice daily under occlusion with plastic wrap to the affected areas, and he was continued on nivolumab for his NSCLC.
Discussion
Treatment with nivolumab can lead to a range of autoimmune side effects, and as shown in this case, psoriasis is one of the cutaneous findings that could be exacerbated by treatment with nivolumab. To date, two cases of exacerbation of psoriasis in patients treated with nivolumab for melanoma have been reported in the literature.8,9 In the first case, the patient had well-controlled plaque psoriasis at baseline and he subsequently developed psoriatic plaques on the trunk and extremities after the second infusion of nivolumab for metastatic melanoma. A biopsy showed regular acanthosis with hyperkeratosis and parakeratosis in addition to dilated vessels in the papillary dermis.8 In the second case, the patient had a history of psoriasis vulgaris with no active lesions. Three weeks after his first course of nivolumab for metastatic oral mucosal melanoma, he developed new, well-circumscribed erythematous scaly plaques on the trunk and extremities that were clinically diagnosed as psoriasis.9 In a third case, a patient without a prior history of psoriasis experienced a psoriasiform eruption on the trunk and extremities after the fourth dose of nivolumab for oral mucosal melanoma.10 Thus, our case is the third reported case of exacerbation of preexisting psoriasis in a patient treated with nivolumab. Furthermore, our patient is the first reported case of a patient treated with nivolumab for NSCLC to develop this adverse event. Whereas the previously reported cases were characterized by widespread trunk and extremity involvement, our patient developed focal exacerbation of the palmoplantar areas.
Additional studies are needed to more clearly characterize the specific cutaneous toxicities of nivolumab and to determine if particular skin reactions may indicate a better response to the anticancer agent. Side effects such as psoriasis can often be managed with topical therapies and may not require withdrawal of the medication. We encourage the collaboration of dermatologists and oncologists to enhance the diagnosis and management of these cutaneous side effects in cancer patients.
1. Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of Nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433-440.
2. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004-2012.
3. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
4. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257-265.
5. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384.
6. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311-4318.
7. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber J. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2015.
8. Matsumura N, Ohtsuka M, Kikuchi N, Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm Venereol. 2016;96(2):259-260.
9. Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol. 2016;30(10):e89-e91.
10. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
1. Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of Nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433-440.
2. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004-2012.
3. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
4. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257-265.
5. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384.
6. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311-4318.
7. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber J. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2015.
8. Matsumura N, Ohtsuka M, Kikuchi N, Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm Venereol. 2016;96(2):259-260.
9. Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol. 2016;30(10):e89-e91.
10. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
Rucaparib – second PARP inhibitor hits the market for ovarian cancer
Rucaparib was granted accelerated approval by the US Food and Drug Administration for the treatment of patients with BRCA1/2 mutant advanced ovarian cancer in January this year, making it the second drug in its class for this indication. It is a poly(ADP-ribose) polymerase inhibitor that works by blocking the repair of damaged DNA in cancer cells and triggering cell death.
The approval was based on findings from 2 single-arm clinical trials in which rucaparib led to complete or partial tumor shrinkage in more than half of the patients enrolled. A pooled analysis included 106 patients from the phase 2 trials, Study 10 (NCT01482715; N = 42) and ARIEL2 (NCT01891344; N = 64), in which patients with BRCA1/2 mutation-positive ovarian cancer who had progressed on 2 or more previous chemotherapy regimens, received 600 mg rucaparib twice daily.
Study 10 included only patients with platinum-sensitive disease and eligible patients were aged 18 years or older, with a known deleterious BRCA mutation, evidence of measurable disease as defined by Response Evaluation Criteria in Solid Tumors (version 1.1), sufficient archival tumor tissue, histologically confirmed high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer and relapsed disease confirmed by radiologic assessment. Meanwhile, ARIEL2 had similar eligibility criteria, except that patients with platinum-sensitive, resistant, and refractory disease were included.
Both studies excluded patients with active second malignancies, and for those with a history of prior cancer that had been curatively treated, no evidence of current disease was required and chemotherapy should have been completed more than 6 months or bone marrow transplant more than 2 years before the first dose of rucaparib. Patients who had previously been treated with a PARP inhibitor, with symptomatic and/or untreated central nervous system metastases, or who had been hospitalized for bowel obstruction within the previous 3 months, were also ineligible.
Across the 2 trials, the median age of trial participants was 59 years, 78% were white, and all had an Eastern Cooperative Oncology Group performance status of 0 (fully active, able to carry on all pre-disease performance without restriction) or 1 (restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature). Both trials used a surrogate endpoint for approval, measuring the percentage of patients who experienced complete or partial tumor shrinkage, the overall response rate (ORR), while taking rucaparib.
In Study 10, the ORR was 60%, including a complete response (CR) rate of 10% and a partial response (PR) rate of 50%, over a median duration of response (DoR) of 7.9 months, while in ARIEL2, the ORR was 50%, including a CR of 8% and a PR of 42%, over a median DoR of 11.6 months. The pooled analysis demonstrated an ORR of 54%, CR of 9% and PR of 45%, over a median DoR of 9.2 months. In separate data reported in the prescribing information, the ORR as assessed by independent radiology review was 42%, with a median DoR of 6.7 months, while ORR according to investigator assessment was 66%. In all analyses, the response rate was similar for patients having BRCA1 versus BRCA2 gene mutations.
Safety analyses were performed in 377 patients across the 2 studies who received 600 mg rucaparib twice daily. The most common adverse events (AEs) of any grade included nausea, fatigue, vomiting, anemia, abdominal pain, dysgeusia, constipation, decreased appetite, diarrhea, thrombocytopenia, and dyspnea. The most common serious AEs (grade 3 or 4) were anemia (25%), fatigue/asthenia (11%), and increased alanine aminotransferase or aspartate aminotransferase levels (11%). Overall, 8% of patients discontinued treatment because of AEs.
The recommended dose according to the prescribing information is 600 mg, in the form of two 300-mg tablets taken orally twice daily with or without food. Phy
If hematologic toxicities occur while taking rucaparib, treatment should be interrupted and blood counts monitored until recovery and failure to recover to grade 1 or higher after 4 weeks should prompt referral to a hematologist for further investigation, while confirmed diagnosis of myelodysplastic syndromes or acute myeloid leukemia should lead to discontinuation of rucaparib. Pregnant women and those of reproductive potential should be advised of the potential risk to a fetus or the need for effective contraception during treatment and for 6 months after the last dose of rucaparib.
Rucaparib is indicated only for the treatment of patients with confirmed BRCA1/2 mutations, so the drug was approved in conjunction with a companion diagnostic. FoundationFocus CDxBRCA is the first next-generation sequencing-based test to receive FDA approval and detects the presence of deleterious BRCA gene mutations in tumor tissue samples. Rucaparib is marketed as Rubraca by Clovis Oncology Inc, and the companion diagnostic by Foundation Medicine Inc.
1. Rubraca (rucaparib) capsules, for oral use. Prescribing information. Clovis Oncology Inc. http://clovisoncology.com/files/rubraca-prescribing-info.pdf. Released December 2016. Accessed January 8th, 2017.
2. FDA grants accelerated approval to new treatment for advanced ovarian cancer. FDA News Release. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm533873.htm. Last updated December 19, 2016. Accessed January 8, 2017.
3. [No author listed.] Rucaparib approved for ovarian cancer. Cancer Discov. Epub ahead of print. January 5, 2017. doi: 10.1158/2159-8290. CD-NB2016-164.
Rucaparib was granted accelerated approval by the US Food and Drug Administration for the treatment of patients with BRCA1/2 mutant advanced ovarian cancer in January this year, making it the second drug in its class for this indication. It is a poly(ADP-ribose) polymerase inhibitor that works by blocking the repair of damaged DNA in cancer cells and triggering cell death.
The approval was based on findings from 2 single-arm clinical trials in which rucaparib led to complete or partial tumor shrinkage in more than half of the patients enrolled. A pooled analysis included 106 patients from the phase 2 trials, Study 10 (NCT01482715; N = 42) and ARIEL2 (NCT01891344; N = 64), in which patients with BRCA1/2 mutation-positive ovarian cancer who had progressed on 2 or more previous chemotherapy regimens, received 600 mg rucaparib twice daily.
Study 10 included only patients with platinum-sensitive disease and eligible patients were aged 18 years or older, with a known deleterious BRCA mutation, evidence of measurable disease as defined by Response Evaluation Criteria in Solid Tumors (version 1.1), sufficient archival tumor tissue, histologically confirmed high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer and relapsed disease confirmed by radiologic assessment. Meanwhile, ARIEL2 had similar eligibility criteria, except that patients with platinum-sensitive, resistant, and refractory disease were included.
Both studies excluded patients with active second malignancies, and for those with a history of prior cancer that had been curatively treated, no evidence of current disease was required and chemotherapy should have been completed more than 6 months or bone marrow transplant more than 2 years before the first dose of rucaparib. Patients who had previously been treated with a PARP inhibitor, with symptomatic and/or untreated central nervous system metastases, or who had been hospitalized for bowel obstruction within the previous 3 months, were also ineligible.
Across the 2 trials, the median age of trial participants was 59 years, 78% were white, and all had an Eastern Cooperative Oncology Group performance status of 0 (fully active, able to carry on all pre-disease performance without restriction) or 1 (restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature). Both trials used a surrogate endpoint for approval, measuring the percentage of patients who experienced complete or partial tumor shrinkage, the overall response rate (ORR), while taking rucaparib.
In Study 10, the ORR was 60%, including a complete response (CR) rate of 10% and a partial response (PR) rate of 50%, over a median duration of response (DoR) of 7.9 months, while in ARIEL2, the ORR was 50%, including a CR of 8% and a PR of 42%, over a median DoR of 11.6 months. The pooled analysis demonstrated an ORR of 54%, CR of 9% and PR of 45%, over a median DoR of 9.2 months. In separate data reported in the prescribing information, the ORR as assessed by independent radiology review was 42%, with a median DoR of 6.7 months, while ORR according to investigator assessment was 66%. In all analyses, the response rate was similar for patients having BRCA1 versus BRCA2 gene mutations.
Safety analyses were performed in 377 patients across the 2 studies who received 600 mg rucaparib twice daily. The most common adverse events (AEs) of any grade included nausea, fatigue, vomiting, anemia, abdominal pain, dysgeusia, constipation, decreased appetite, diarrhea, thrombocytopenia, and dyspnea. The most common serious AEs (grade 3 or 4) were anemia (25%), fatigue/asthenia (11%), and increased alanine aminotransferase or aspartate aminotransferase levels (11%). Overall, 8% of patients discontinued treatment because of AEs.
The recommended dose according to the prescribing information is 600 mg, in the form of two 300-mg tablets taken orally twice daily with or without food. Phy
If hematologic toxicities occur while taking rucaparib, treatment should be interrupted and blood counts monitored until recovery and failure to recover to grade 1 or higher after 4 weeks should prompt referral to a hematologist for further investigation, while confirmed diagnosis of myelodysplastic syndromes or acute myeloid leukemia should lead to discontinuation of rucaparib. Pregnant women and those of reproductive potential should be advised of the potential risk to a fetus or the need for effective contraception during treatment and for 6 months after the last dose of rucaparib.
Rucaparib is indicated only for the treatment of patients with confirmed BRCA1/2 mutations, so the drug was approved in conjunction with a companion diagnostic. FoundationFocus CDxBRCA is the first next-generation sequencing-based test to receive FDA approval and detects the presence of deleterious BRCA gene mutations in tumor tissue samples. Rucaparib is marketed as Rubraca by Clovis Oncology Inc, and the companion diagnostic by Foundation Medicine Inc.
Rucaparib was granted accelerated approval by the US Food and Drug Administration for the treatment of patients with BRCA1/2 mutant advanced ovarian cancer in January this year, making it the second drug in its class for this indication. It is a poly(ADP-ribose) polymerase inhibitor that works by blocking the repair of damaged DNA in cancer cells and triggering cell death.
The approval was based on findings from 2 single-arm clinical trials in which rucaparib led to complete or partial tumor shrinkage in more than half of the patients enrolled. A pooled analysis included 106 patients from the phase 2 trials, Study 10 (NCT01482715; N = 42) and ARIEL2 (NCT01891344; N = 64), in which patients with BRCA1/2 mutation-positive ovarian cancer who had progressed on 2 or more previous chemotherapy regimens, received 600 mg rucaparib twice daily.
Study 10 included only patients with platinum-sensitive disease and eligible patients were aged 18 years or older, with a known deleterious BRCA mutation, evidence of measurable disease as defined by Response Evaluation Criteria in Solid Tumors (version 1.1), sufficient archival tumor tissue, histologically confirmed high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer and relapsed disease confirmed by radiologic assessment. Meanwhile, ARIEL2 had similar eligibility criteria, except that patients with platinum-sensitive, resistant, and refractory disease were included.
Both studies excluded patients with active second malignancies, and for those with a history of prior cancer that had been curatively treated, no evidence of current disease was required and chemotherapy should have been completed more than 6 months or bone marrow transplant more than 2 years before the first dose of rucaparib. Patients who had previously been treated with a PARP inhibitor, with symptomatic and/or untreated central nervous system metastases, or who had been hospitalized for bowel obstruction within the previous 3 months, were also ineligible.
Across the 2 trials, the median age of trial participants was 59 years, 78% were white, and all had an Eastern Cooperative Oncology Group performance status of 0 (fully active, able to carry on all pre-disease performance without restriction) or 1 (restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature). Both trials used a surrogate endpoint for approval, measuring the percentage of patients who experienced complete or partial tumor shrinkage, the overall response rate (ORR), while taking rucaparib.
In Study 10, the ORR was 60%, including a complete response (CR) rate of 10% and a partial response (PR) rate of 50%, over a median duration of response (DoR) of 7.9 months, while in ARIEL2, the ORR was 50%, including a CR of 8% and a PR of 42%, over a median DoR of 11.6 months. The pooled analysis demonstrated an ORR of 54%, CR of 9% and PR of 45%, over a median DoR of 9.2 months. In separate data reported in the prescribing information, the ORR as assessed by independent radiology review was 42%, with a median DoR of 6.7 months, while ORR according to investigator assessment was 66%. In all analyses, the response rate was similar for patients having BRCA1 versus BRCA2 gene mutations.
Safety analyses were performed in 377 patients across the 2 studies who received 600 mg rucaparib twice daily. The most common adverse events (AEs) of any grade included nausea, fatigue, vomiting, anemia, abdominal pain, dysgeusia, constipation, decreased appetite, diarrhea, thrombocytopenia, and dyspnea. The most common serious AEs (grade 3 or 4) were anemia (25%), fatigue/asthenia (11%), and increased alanine aminotransferase or aspartate aminotransferase levels (11%). Overall, 8% of patients discontinued treatment because of AEs.
The recommended dose according to the prescribing information is 600 mg, in the form of two 300-mg tablets taken orally twice daily with or without food. Phy
If hematologic toxicities occur while taking rucaparib, treatment should be interrupted and blood counts monitored until recovery and failure to recover to grade 1 or higher after 4 weeks should prompt referral to a hematologist for further investigation, while confirmed diagnosis of myelodysplastic syndromes or acute myeloid leukemia should lead to discontinuation of rucaparib. Pregnant women and those of reproductive potential should be advised of the potential risk to a fetus or the need for effective contraception during treatment and for 6 months after the last dose of rucaparib.
Rucaparib is indicated only for the treatment of patients with confirmed BRCA1/2 mutations, so the drug was approved in conjunction with a companion diagnostic. FoundationFocus CDxBRCA is the first next-generation sequencing-based test to receive FDA approval and detects the presence of deleterious BRCA gene mutations in tumor tissue samples. Rucaparib is marketed as Rubraca by Clovis Oncology Inc, and the companion diagnostic by Foundation Medicine Inc.
1. Rubraca (rucaparib) capsules, for oral use. Prescribing information. Clovis Oncology Inc. http://clovisoncology.com/files/rubraca-prescribing-info.pdf. Released December 2016. Accessed January 8th, 2017.
2. FDA grants accelerated approval to new treatment for advanced ovarian cancer. FDA News Release. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm533873.htm. Last updated December 19, 2016. Accessed January 8, 2017.
3. [No author listed.] Rucaparib approved for ovarian cancer. Cancer Discov. Epub ahead of print. January 5, 2017. doi: 10.1158/2159-8290. CD-NB2016-164.
1. Rubraca (rucaparib) capsules, for oral use. Prescribing information. Clovis Oncology Inc. http://clovisoncology.com/files/rubraca-prescribing-info.pdf. Released December 2016. Accessed January 8th, 2017.
2. FDA grants accelerated approval to new treatment for advanced ovarian cancer. FDA News Release. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm533873.htm. Last updated December 19, 2016. Accessed January 8, 2017.
3. [No author listed.] Rucaparib approved for ovarian cancer. Cancer Discov. Epub ahead of print. January 5, 2017. doi: 10.1158/2159-8290. CD-NB2016-164.
End-of-life options and the legal pathways to physician aid in dying
By early 2017, roughly 18% of all US citizens will reside in a state with a legal pathway to physician aid in dying via lethal prescription. When the End of Life Options Act (EOLOA) went into effect in in California in June 2016, it became the fourth state with laws allowing physician aid in dying (PAD). Oregon (1997), Washington (2009), and Vermont (2009) had preceded it, and Montana (2009) operates similarly as a result of a Supreme Court decision there. However, California’s law also legalized PAD in a state that is much larger and more socioeconomically diverse than the other four states – with its 39 million residents, California more than triples the number of Americans who live in PAD legal states. Together, these 5 states represent 16% of the entire US population (roughly 321 million according to the 2015 Census). Most recently, in December 2016, they were joined by Colorado, adding a state population of 5.5 million.
The state laws have much in common: to “qualify” for legal access to a lethal prescription, a patient must make an in-person verbal request to his/her attending physician. The patient must also: be an adult (aged 18 years or older); be a resident of that state; have a terminal illness the course of which is expected to lead to natural death within 6 months; be making a noncoerced, voluntary request; repeat the verbal request no sooner than 15 days after the first request, followed by a witnessed, formal written request; and have the capacity to self-administer the lethal prescription in a private setting.1
In California, as in the other states, additional safeguards are built in: the terminal diagnosis and the patient’s capacity to make the request must be verified by a second, independent consultant physician. If either the attending or the consultant physician finds evidence of a “mental disorder,” they are obligated under the law to refer the patient to a psychiatrist or psychologist for an evaluation. The psychological expert is charged with verifying the patient’s mental capacity and ability to make a voluntary end-of-life choice, with determining whether a mental disorder is in fact present, and if it is, whether that mental disorder is impairing the patient’s judgment. A finding of impaired judgment due to mental disorder halts the legal process until the disorder is rectified by treatment, the passage of time, or other factors.
Many of the themes and concepts outlined in these laws are familiar to oncology clinicians simply because we take care of seriously ill and dying patients. Indeed, access to the Medicare Hospice Benefit requires certification – often by an oncologist – that a patient has a terminal diagnosis with a maximum 6-month expected survival. In addition, oncologists encounter many patients who wish to talk about quality of life while they weigh various treatment options, and it is normative for patients (though often anxiety producing for clinicians) to broach topics related to end of life, symptom management, and even aid in dying. Many patients fear poor quality of life, intractable symptom burden, dependency on others, and loss of control more than they fear their cancers. Their efforts to initiate this discussion often fit into a much larger and more durable set of personal values and ideals about suffering, dependency, futility, and personal autonomy.
Weighing the evidence
And yet there is vigorous objection to PAD laws from many corners. Some religious organizations and faith-based health care delivery systems oppose the laws and, in opting-out of the voluntary legal pathways for participation, prohibit their employed and affiliated physicians and other professionals from doing so as well.2 Some physician organizations, and individual physicians, claim that involvement in aid in dying – such as by providing a legal lethal prescription – violates the Hippocratic oath and that (in effect) there is no circumstance under which it could be ethically permissible.
There are also bioethicists, including physician ethicists, who sincerely reach similar conclusions and warn of the “slippery slope” that might lead beyond aid in dying as currently legalized in the US to assisting in the deaths of those with disabilities, those with depression or other treatable psychiatric illness, and even to active euthanasia, including euthanasia of nonconsenting or incapable individuals.3 These objectors generally remain adamant and cite what we would all agree are excesses in certain European countries, despite the absence of evidence that the European measures could be approved in the United States under current laws and practices.
The largest amount of publicly available evidence to inform this discussion in the US comes from Oregon, which has nearly 20 years of experience with the law and its reporting requirements.4 Very broadly, the Oregon experience supports the view that PAD is pursued and completed by a very small percentage of the population: in 2015 (the most recent year for which data is available) 218 people possessed lethal prescriptions; 132 of them ingested the medications and died. Thus about 61% of those who received the prescription used it for its intended purpose, resulting in a Death with Dignity Act death rate of 0.39% (132 of 34,160 deaths in Oregon) in 2015. Since the law’s inception in 1997, 991 patients are known to have died from lethal ingestion of 1545 prescriptions written (a 64% “use” rate).
Equally important is the evidence from Oregon describing those who seek to use the law. In 2015, as in previous years, most patients were older than the general population (78% aged 65 years or older; median age at death, 73). Of those patients, 93% were white and well educated (43% had at least a college degree), compared with the population at large. In all, 72%-78% of patients had cancer; 6%-8% had ALS (amyotrophic lateral sclerosis); and end-stage heart disease seemed to be increasing, trending up from 2% to 6% in recent years.
In addition, 90% died at home, with 92% on hospice, and more than 99% had health insurance of some kind. These figures provide strong evidence that PAD is not being inappropriately used among historically vulnerable or disempowered ethnic/racial minorities, socioeconomically or educationally disadvantaged groups, or disabled individuals. On the contrary, “uptake” or use of PAD by the disadvantaged in Oregon seems, perhaps not surprisingly, to occur at rates significantly below their representation in the general population of the state.
Intractable symptom burden (or fear of it) was rated as a minor contributor to the decision to pursue PAD, ranking sixth out of the 7 options and endorsed by about a quarter of patients. The three most frequently cited end-of-life concerns were: decreasing ability to participate in activities that made life enjoyable (96%), loss of autonomy (92%), and loss of dignity (75%).
A broader range of choice
I have worked for nearly 30 years in California oncology clinical settings as a palliative care physician and psychiatrist. During that time I have been involved in the care of two patients who committed violent suicide (self-inflicted gunshot). Both events took place before the passage of the California EOLOA, both patients were educated, professional older white men who were fiercely independent and who saw their progressive cancers as rapidly worsening their quality of life and intolerably increasing their dependency on beloved others (although their judgments about this did not take into account how the others actually felt); neither had a primary psychiatric illness, and neither had intractable symptom burden. Both men had expressed interest in and were denied access to lethal prescription. Sadly, neither had the kind of long-term, trusting relationship with a physician that appears to have provided access to non-legally sanctioned PAD for decades before the first state laws allowing it – and therefore each apparently decided to exert his autonomy in the ultimate act of self-determination. In both cases, it seemed to me that violent suicide was a bad, last recourse – clearly, each man regarded continued living in his intolerable state as even worse – but also the worst possible outcome for their surviving families, for their traumatized clinicians, and for the bystanders who witnessed these deaths and the first responders who were called to the scenes. We cannot know that the availability of lethal prescription would have pre-empted these violent suicides, but I suspect it might have given each man a much broader range of choice about how to deal with circumstances he found entirely unacceptable, and which he simply could not and would not tolerate.
An informed, person-centered approach
It is in the context of these experiences that I have come to view “active non-participation” in legal PAD – that is, decisions by individual physicians and/or health systems not only to not provide, but also not refer patients to possibly willing providers and systems without regard for specific clinical contexts – as a toxic form of patient abandonment. I am also concerned that this rigid stance (like many rigid stances in the service of alleged moral absolutes) may lead to greater suffering and harm – such as the violent suicides I have described – than a more moderate, contextually informed, person-centered approach that does not outlaw certain clinical topics. Indeed, in my participating institution in California, it has become clear that a request for PAD leads (as a result of a carefully and comprehensively constructed “navigator” process) to a level of patient and family care that should be provided to every patient with terminal illness in this country. While that statement is a sad reflection on our society’s general commitment to caring for the dying, it seems that the extra attention required by the process leading to a PAD, and the revelations that emerge in that process, often lead to a withdrawal of the request for a lethal prescription, and/or allows the drug to go unused if provided.
Many leading bioethical treatises, including those emerging from faith-based academic and university settings, also support the view that PAD can be and is morally justified under a certain set of circumstances. Not surprisingly, those circumstances encompass most of what is written into the state laws permitting PAD. They include, according to Beauchamp and Childress:5
- A voluntary request by a competent patient
- An ongoing physician-patient relationship
- Mutual and informed decision-making by patient and physician
- A supportive yet critical and probing environment of decision making
- A considered rejection of alternatives
- Structured consultation with other parties in medicine
- A patient’s expression of a durable preference for death
- Unacceptable suffering by the patient
- Use of a means that is as painless and comfortable as possible
We tell many of our patients that cancer is now treated as a chronic illness. In the context of treating that chronic illness we have the profound opportunity – some would say the obligation – to come to know our patients as whole individuals who often have long-held health values, ideas about what a life worth living looks like, and very personal fears and hopes. We may well come to know them more intimately while serving as their cancer clinicians than any other health professionals do – and even as do any other individuals with whom they will ever interact.
The hours in the infusion chair afford many opportunities for us to understand (and, ideally, document) a patient’s advance care plans, health values, goals, views about end-of-life measures such as artificial ventilation and resuscitation. No one reasonably disputes the “rightness” of learning these things. The evidence shows us that under very rare circumstances, knowing and respecting our patients may include understanding their wishes about physician aid in dying, which requires us to build upon the profound trust that has been established by being able to hear and understand their requests. It seems to me that the end of life is the most inappropriate time for any of us to tell patients they must look elsewhere.
The opinions expressed here are those of the author alone, and do not reflect the view of other individuals, institutions, or professional organizations with which Dr Strouse is affiliated.
1. Gostin LO, Roberts AE. Physician assisted dying: a turning point? JAMA 2016;315;249-250.
2. Buck C. With barbiturates and martini, Sonoma man among first Californians to die under end-of-life law. http://www.sacbee.com/news/local/health-and-medicine/article95676342.html. Published August 16, 2016. Accessed January 17, 2017.
3. Snyder L, Sulmasy DP. Physician-assisted suicide. http://annals.org/aim/article/714672/physician-assisted-suicide. Published August 7, 2001. Accessed January 17, 2017.
4. Oregan Public Health Division. Oregan Death With Dignity Act: 2015 data summary. https://public.health.oregon.gov/ProviderPartnerResources/EvaluationResearch/DeathwithDignityAct/Documents/year18.pdf. Published February 4, 2016. Accessed January 17, 2017.
5. Beauchamp TL, Childress JF. Nonmaleficence. In Principles of biomedical ethics. 7th ed. New York, NY: Oxford University Press; 2013:184.
By early 2017, roughly 18% of all US citizens will reside in a state with a legal pathway to physician aid in dying via lethal prescription. When the End of Life Options Act (EOLOA) went into effect in in California in June 2016, it became the fourth state with laws allowing physician aid in dying (PAD). Oregon (1997), Washington (2009), and Vermont (2009) had preceded it, and Montana (2009) operates similarly as a result of a Supreme Court decision there. However, California’s law also legalized PAD in a state that is much larger and more socioeconomically diverse than the other four states – with its 39 million residents, California more than triples the number of Americans who live in PAD legal states. Together, these 5 states represent 16% of the entire US population (roughly 321 million according to the 2015 Census). Most recently, in December 2016, they were joined by Colorado, adding a state population of 5.5 million.
The state laws have much in common: to “qualify” for legal access to a lethal prescription, a patient must make an in-person verbal request to his/her attending physician. The patient must also: be an adult (aged 18 years or older); be a resident of that state; have a terminal illness the course of which is expected to lead to natural death within 6 months; be making a noncoerced, voluntary request; repeat the verbal request no sooner than 15 days after the first request, followed by a witnessed, formal written request; and have the capacity to self-administer the lethal prescription in a private setting.1
In California, as in the other states, additional safeguards are built in: the terminal diagnosis and the patient’s capacity to make the request must be verified by a second, independent consultant physician. If either the attending or the consultant physician finds evidence of a “mental disorder,” they are obligated under the law to refer the patient to a psychiatrist or psychologist for an evaluation. The psychological expert is charged with verifying the patient’s mental capacity and ability to make a voluntary end-of-life choice, with determining whether a mental disorder is in fact present, and if it is, whether that mental disorder is impairing the patient’s judgment. A finding of impaired judgment due to mental disorder halts the legal process until the disorder is rectified by treatment, the passage of time, or other factors.
Many of the themes and concepts outlined in these laws are familiar to oncology clinicians simply because we take care of seriously ill and dying patients. Indeed, access to the Medicare Hospice Benefit requires certification – often by an oncologist – that a patient has a terminal diagnosis with a maximum 6-month expected survival. In addition, oncologists encounter many patients who wish to talk about quality of life while they weigh various treatment options, and it is normative for patients (though often anxiety producing for clinicians) to broach topics related to end of life, symptom management, and even aid in dying. Many patients fear poor quality of life, intractable symptom burden, dependency on others, and loss of control more than they fear their cancers. Their efforts to initiate this discussion often fit into a much larger and more durable set of personal values and ideals about suffering, dependency, futility, and personal autonomy.
Weighing the evidence
And yet there is vigorous objection to PAD laws from many corners. Some religious organizations and faith-based health care delivery systems oppose the laws and, in opting-out of the voluntary legal pathways for participation, prohibit their employed and affiliated physicians and other professionals from doing so as well.2 Some physician organizations, and individual physicians, claim that involvement in aid in dying – such as by providing a legal lethal prescription – violates the Hippocratic oath and that (in effect) there is no circumstance under which it could be ethically permissible.
There are also bioethicists, including physician ethicists, who sincerely reach similar conclusions and warn of the “slippery slope” that might lead beyond aid in dying as currently legalized in the US to assisting in the deaths of those with disabilities, those with depression or other treatable psychiatric illness, and even to active euthanasia, including euthanasia of nonconsenting or incapable individuals.3 These objectors generally remain adamant and cite what we would all agree are excesses in certain European countries, despite the absence of evidence that the European measures could be approved in the United States under current laws and practices.
The largest amount of publicly available evidence to inform this discussion in the US comes from Oregon, which has nearly 20 years of experience with the law and its reporting requirements.4 Very broadly, the Oregon experience supports the view that PAD is pursued and completed by a very small percentage of the population: in 2015 (the most recent year for which data is available) 218 people possessed lethal prescriptions; 132 of them ingested the medications and died. Thus about 61% of those who received the prescription used it for its intended purpose, resulting in a Death with Dignity Act death rate of 0.39% (132 of 34,160 deaths in Oregon) in 2015. Since the law’s inception in 1997, 991 patients are known to have died from lethal ingestion of 1545 prescriptions written (a 64% “use” rate).
Equally important is the evidence from Oregon describing those who seek to use the law. In 2015, as in previous years, most patients were older than the general population (78% aged 65 years or older; median age at death, 73). Of those patients, 93% were white and well educated (43% had at least a college degree), compared with the population at large. In all, 72%-78% of patients had cancer; 6%-8% had ALS (amyotrophic lateral sclerosis); and end-stage heart disease seemed to be increasing, trending up from 2% to 6% in recent years.
In addition, 90% died at home, with 92% on hospice, and more than 99% had health insurance of some kind. These figures provide strong evidence that PAD is not being inappropriately used among historically vulnerable or disempowered ethnic/racial minorities, socioeconomically or educationally disadvantaged groups, or disabled individuals. On the contrary, “uptake” or use of PAD by the disadvantaged in Oregon seems, perhaps not surprisingly, to occur at rates significantly below their representation in the general population of the state.
Intractable symptom burden (or fear of it) was rated as a minor contributor to the decision to pursue PAD, ranking sixth out of the 7 options and endorsed by about a quarter of patients. The three most frequently cited end-of-life concerns were: decreasing ability to participate in activities that made life enjoyable (96%), loss of autonomy (92%), and loss of dignity (75%).
A broader range of choice
I have worked for nearly 30 years in California oncology clinical settings as a palliative care physician and psychiatrist. During that time I have been involved in the care of two patients who committed violent suicide (self-inflicted gunshot). Both events took place before the passage of the California EOLOA, both patients were educated, professional older white men who were fiercely independent and who saw their progressive cancers as rapidly worsening their quality of life and intolerably increasing their dependency on beloved others (although their judgments about this did not take into account how the others actually felt); neither had a primary psychiatric illness, and neither had intractable symptom burden. Both men had expressed interest in and were denied access to lethal prescription. Sadly, neither had the kind of long-term, trusting relationship with a physician that appears to have provided access to non-legally sanctioned PAD for decades before the first state laws allowing it – and therefore each apparently decided to exert his autonomy in the ultimate act of self-determination. In both cases, it seemed to me that violent suicide was a bad, last recourse – clearly, each man regarded continued living in his intolerable state as even worse – but also the worst possible outcome for their surviving families, for their traumatized clinicians, and for the bystanders who witnessed these deaths and the first responders who were called to the scenes. We cannot know that the availability of lethal prescription would have pre-empted these violent suicides, but I suspect it might have given each man a much broader range of choice about how to deal with circumstances he found entirely unacceptable, and which he simply could not and would not tolerate.
An informed, person-centered approach
It is in the context of these experiences that I have come to view “active non-participation” in legal PAD – that is, decisions by individual physicians and/or health systems not only to not provide, but also not refer patients to possibly willing providers and systems without regard for specific clinical contexts – as a toxic form of patient abandonment. I am also concerned that this rigid stance (like many rigid stances in the service of alleged moral absolutes) may lead to greater suffering and harm – such as the violent suicides I have described – than a more moderate, contextually informed, person-centered approach that does not outlaw certain clinical topics. Indeed, in my participating institution in California, it has become clear that a request for PAD leads (as a result of a carefully and comprehensively constructed “navigator” process) to a level of patient and family care that should be provided to every patient with terminal illness in this country. While that statement is a sad reflection on our society’s general commitment to caring for the dying, it seems that the extra attention required by the process leading to a PAD, and the revelations that emerge in that process, often lead to a withdrawal of the request for a lethal prescription, and/or allows the drug to go unused if provided.
Many leading bioethical treatises, including those emerging from faith-based academic and university settings, also support the view that PAD can be and is morally justified under a certain set of circumstances. Not surprisingly, those circumstances encompass most of what is written into the state laws permitting PAD. They include, according to Beauchamp and Childress:5
- A voluntary request by a competent patient
- An ongoing physician-patient relationship
- Mutual and informed decision-making by patient and physician
- A supportive yet critical and probing environment of decision making
- A considered rejection of alternatives
- Structured consultation with other parties in medicine
- A patient’s expression of a durable preference for death
- Unacceptable suffering by the patient
- Use of a means that is as painless and comfortable as possible
We tell many of our patients that cancer is now treated as a chronic illness. In the context of treating that chronic illness we have the profound opportunity – some would say the obligation – to come to know our patients as whole individuals who often have long-held health values, ideas about what a life worth living looks like, and very personal fears and hopes. We may well come to know them more intimately while serving as their cancer clinicians than any other health professionals do – and even as do any other individuals with whom they will ever interact.
The hours in the infusion chair afford many opportunities for us to understand (and, ideally, document) a patient’s advance care plans, health values, goals, views about end-of-life measures such as artificial ventilation and resuscitation. No one reasonably disputes the “rightness” of learning these things. The evidence shows us that under very rare circumstances, knowing and respecting our patients may include understanding their wishes about physician aid in dying, which requires us to build upon the profound trust that has been established by being able to hear and understand their requests. It seems to me that the end of life is the most inappropriate time for any of us to tell patients they must look elsewhere.
The opinions expressed here are those of the author alone, and do not reflect the view of other individuals, institutions, or professional organizations with which Dr Strouse is affiliated.
By early 2017, roughly 18% of all US citizens will reside in a state with a legal pathway to physician aid in dying via lethal prescription. When the End of Life Options Act (EOLOA) went into effect in in California in June 2016, it became the fourth state with laws allowing physician aid in dying (PAD). Oregon (1997), Washington (2009), and Vermont (2009) had preceded it, and Montana (2009) operates similarly as a result of a Supreme Court decision there. However, California’s law also legalized PAD in a state that is much larger and more socioeconomically diverse than the other four states – with its 39 million residents, California more than triples the number of Americans who live in PAD legal states. Together, these 5 states represent 16% of the entire US population (roughly 321 million according to the 2015 Census). Most recently, in December 2016, they were joined by Colorado, adding a state population of 5.5 million.
The state laws have much in common: to “qualify” for legal access to a lethal prescription, a patient must make an in-person verbal request to his/her attending physician. The patient must also: be an adult (aged 18 years or older); be a resident of that state; have a terminal illness the course of which is expected to lead to natural death within 6 months; be making a noncoerced, voluntary request; repeat the verbal request no sooner than 15 days after the first request, followed by a witnessed, formal written request; and have the capacity to self-administer the lethal prescription in a private setting.1
In California, as in the other states, additional safeguards are built in: the terminal diagnosis and the patient’s capacity to make the request must be verified by a second, independent consultant physician. If either the attending or the consultant physician finds evidence of a “mental disorder,” they are obligated under the law to refer the patient to a psychiatrist or psychologist for an evaluation. The psychological expert is charged with verifying the patient’s mental capacity and ability to make a voluntary end-of-life choice, with determining whether a mental disorder is in fact present, and if it is, whether that mental disorder is impairing the patient’s judgment. A finding of impaired judgment due to mental disorder halts the legal process until the disorder is rectified by treatment, the passage of time, or other factors.
Many of the themes and concepts outlined in these laws are familiar to oncology clinicians simply because we take care of seriously ill and dying patients. Indeed, access to the Medicare Hospice Benefit requires certification – often by an oncologist – that a patient has a terminal diagnosis with a maximum 6-month expected survival. In addition, oncologists encounter many patients who wish to talk about quality of life while they weigh various treatment options, and it is normative for patients (though often anxiety producing for clinicians) to broach topics related to end of life, symptom management, and even aid in dying. Many patients fear poor quality of life, intractable symptom burden, dependency on others, and loss of control more than they fear their cancers. Their efforts to initiate this discussion often fit into a much larger and more durable set of personal values and ideals about suffering, dependency, futility, and personal autonomy.
Weighing the evidence
And yet there is vigorous objection to PAD laws from many corners. Some religious organizations and faith-based health care delivery systems oppose the laws and, in opting-out of the voluntary legal pathways for participation, prohibit their employed and affiliated physicians and other professionals from doing so as well.2 Some physician organizations, and individual physicians, claim that involvement in aid in dying – such as by providing a legal lethal prescription – violates the Hippocratic oath and that (in effect) there is no circumstance under which it could be ethically permissible.
There are also bioethicists, including physician ethicists, who sincerely reach similar conclusions and warn of the “slippery slope” that might lead beyond aid in dying as currently legalized in the US to assisting in the deaths of those with disabilities, those with depression or other treatable psychiatric illness, and even to active euthanasia, including euthanasia of nonconsenting or incapable individuals.3 These objectors generally remain adamant and cite what we would all agree are excesses in certain European countries, despite the absence of evidence that the European measures could be approved in the United States under current laws and practices.
The largest amount of publicly available evidence to inform this discussion in the US comes from Oregon, which has nearly 20 years of experience with the law and its reporting requirements.4 Very broadly, the Oregon experience supports the view that PAD is pursued and completed by a very small percentage of the population: in 2015 (the most recent year for which data is available) 218 people possessed lethal prescriptions; 132 of them ingested the medications and died. Thus about 61% of those who received the prescription used it for its intended purpose, resulting in a Death with Dignity Act death rate of 0.39% (132 of 34,160 deaths in Oregon) in 2015. Since the law’s inception in 1997, 991 patients are known to have died from lethal ingestion of 1545 prescriptions written (a 64% “use” rate).
Equally important is the evidence from Oregon describing those who seek to use the law. In 2015, as in previous years, most patients were older than the general population (78% aged 65 years or older; median age at death, 73). Of those patients, 93% were white and well educated (43% had at least a college degree), compared with the population at large. In all, 72%-78% of patients had cancer; 6%-8% had ALS (amyotrophic lateral sclerosis); and end-stage heart disease seemed to be increasing, trending up from 2% to 6% in recent years.
In addition, 90% died at home, with 92% on hospice, and more than 99% had health insurance of some kind. These figures provide strong evidence that PAD is not being inappropriately used among historically vulnerable or disempowered ethnic/racial minorities, socioeconomically or educationally disadvantaged groups, or disabled individuals. On the contrary, “uptake” or use of PAD by the disadvantaged in Oregon seems, perhaps not surprisingly, to occur at rates significantly below their representation in the general population of the state.
Intractable symptom burden (or fear of it) was rated as a minor contributor to the decision to pursue PAD, ranking sixth out of the 7 options and endorsed by about a quarter of patients. The three most frequently cited end-of-life concerns were: decreasing ability to participate in activities that made life enjoyable (96%), loss of autonomy (92%), and loss of dignity (75%).
A broader range of choice
I have worked for nearly 30 years in California oncology clinical settings as a palliative care physician and psychiatrist. During that time I have been involved in the care of two patients who committed violent suicide (self-inflicted gunshot). Both events took place before the passage of the California EOLOA, both patients were educated, professional older white men who were fiercely independent and who saw their progressive cancers as rapidly worsening their quality of life and intolerably increasing their dependency on beloved others (although their judgments about this did not take into account how the others actually felt); neither had a primary psychiatric illness, and neither had intractable symptom burden. Both men had expressed interest in and were denied access to lethal prescription. Sadly, neither had the kind of long-term, trusting relationship with a physician that appears to have provided access to non-legally sanctioned PAD for decades before the first state laws allowing it – and therefore each apparently decided to exert his autonomy in the ultimate act of self-determination. In both cases, it seemed to me that violent suicide was a bad, last recourse – clearly, each man regarded continued living in his intolerable state as even worse – but also the worst possible outcome for their surviving families, for their traumatized clinicians, and for the bystanders who witnessed these deaths and the first responders who were called to the scenes. We cannot know that the availability of lethal prescription would have pre-empted these violent suicides, but I suspect it might have given each man a much broader range of choice about how to deal with circumstances he found entirely unacceptable, and which he simply could not and would not tolerate.
An informed, person-centered approach
It is in the context of these experiences that I have come to view “active non-participation” in legal PAD – that is, decisions by individual physicians and/or health systems not only to not provide, but also not refer patients to possibly willing providers and systems without regard for specific clinical contexts – as a toxic form of patient abandonment. I am also concerned that this rigid stance (like many rigid stances in the service of alleged moral absolutes) may lead to greater suffering and harm – such as the violent suicides I have described – than a more moderate, contextually informed, person-centered approach that does not outlaw certain clinical topics. Indeed, in my participating institution in California, it has become clear that a request for PAD leads (as a result of a carefully and comprehensively constructed “navigator” process) to a level of patient and family care that should be provided to every patient with terminal illness in this country. While that statement is a sad reflection on our society’s general commitment to caring for the dying, it seems that the extra attention required by the process leading to a PAD, and the revelations that emerge in that process, often lead to a withdrawal of the request for a lethal prescription, and/or allows the drug to go unused if provided.
Many leading bioethical treatises, including those emerging from faith-based academic and university settings, also support the view that PAD can be and is morally justified under a certain set of circumstances. Not surprisingly, those circumstances encompass most of what is written into the state laws permitting PAD. They include, according to Beauchamp and Childress:5
- A voluntary request by a competent patient
- An ongoing physician-patient relationship
- Mutual and informed decision-making by patient and physician
- A supportive yet critical and probing environment of decision making
- A considered rejection of alternatives
- Structured consultation with other parties in medicine
- A patient’s expression of a durable preference for death
- Unacceptable suffering by the patient
- Use of a means that is as painless and comfortable as possible
We tell many of our patients that cancer is now treated as a chronic illness. In the context of treating that chronic illness we have the profound opportunity – some would say the obligation – to come to know our patients as whole individuals who often have long-held health values, ideas about what a life worth living looks like, and very personal fears and hopes. We may well come to know them more intimately while serving as their cancer clinicians than any other health professionals do – and even as do any other individuals with whom they will ever interact.
The hours in the infusion chair afford many opportunities for us to understand (and, ideally, document) a patient’s advance care plans, health values, goals, views about end-of-life measures such as artificial ventilation and resuscitation. No one reasonably disputes the “rightness” of learning these things. The evidence shows us that under very rare circumstances, knowing and respecting our patients may include understanding their wishes about physician aid in dying, which requires us to build upon the profound trust that has been established by being able to hear and understand their requests. It seems to me that the end of life is the most inappropriate time for any of us to tell patients they must look elsewhere.
The opinions expressed here are those of the author alone, and do not reflect the view of other individuals, institutions, or professional organizations with which Dr Strouse is affiliated.
1. Gostin LO, Roberts AE. Physician assisted dying: a turning point? JAMA 2016;315;249-250.
2. Buck C. With barbiturates and martini, Sonoma man among first Californians to die under end-of-life law. http://www.sacbee.com/news/local/health-and-medicine/article95676342.html. Published August 16, 2016. Accessed January 17, 2017.
3. Snyder L, Sulmasy DP. Physician-assisted suicide. http://annals.org/aim/article/714672/physician-assisted-suicide. Published August 7, 2001. Accessed January 17, 2017.
4. Oregan Public Health Division. Oregan Death With Dignity Act: 2015 data summary. https://public.health.oregon.gov/ProviderPartnerResources/EvaluationResearch/DeathwithDignityAct/Documents/year18.pdf. Published February 4, 2016. Accessed January 17, 2017.
5. Beauchamp TL, Childress JF. Nonmaleficence. In Principles of biomedical ethics. 7th ed. New York, NY: Oxford University Press; 2013:184.
1. Gostin LO, Roberts AE. Physician assisted dying: a turning point? JAMA 2016;315;249-250.
2. Buck C. With barbiturates and martini, Sonoma man among first Californians to die under end-of-life law. http://www.sacbee.com/news/local/health-and-medicine/article95676342.html. Published August 16, 2016. Accessed January 17, 2017.
3. Snyder L, Sulmasy DP. Physician-assisted suicide. http://annals.org/aim/article/714672/physician-assisted-suicide. Published August 7, 2001. Accessed January 17, 2017.
4. Oregan Public Health Division. Oregan Death With Dignity Act: 2015 data summary. https://public.health.oregon.gov/ProviderPartnerResources/EvaluationResearch/DeathwithDignityAct/Documents/year18.pdf. Published February 4, 2016. Accessed January 17, 2017.
5. Beauchamp TL, Childress JF. Nonmaleficence. In Principles of biomedical ethics. 7th ed. New York, NY: Oxford University Press; 2013:184.