User login
Eyeglasses May Offer Periocular AK Protection
RALEIGH, N.C. – Wearing glasses was associated with a significantly lower rate of developing actinic keratoses on periocular skin, according to the results of a large multicenter clinical trial.
During an average 4-year prospective follow-up conducted at 6-month intervals in the Veterans Affairs Topical Tretinoin Chemoprevention Trial, the 1,131 elderly participants, most of whom were male, developed 3,291 periocular actinic keratoses.
Patients who didn’t regularly wear eyeglasses had a 40% higher rate of developing AKs on periocular skin. This is consistent with reports in the ophthalmologic literature that eyeglasses can reduce the flux of UV radiation filtered to the periocular area, according to Dr. Kachiu C. Lee, a dermatology resident at Brown University in Providence, R.I.
Her hypothesis was that eyeglasses wearers would also have a lower rate of periocular nonmelanoma skin cancer. However, this wasn’t borne out, possibly because too few of the malignancies occurred to be able to demonstrate a protective effect, she noted. Although all suspected skin cancers were biopsied for histologic diagnosis, only 19 periocular squamous cell carcinomas and 71 periocular basal cell carcinomas occurred during follow-up.
A significant study limitation was that patients weren’t surveyed as to their use of sunglasses. "That’s a huge potential confounding variable," Dr. Lee said.
The study was sponsored by the U.S. Department of Veterans Affairs. Dr. Lee reported having no financial conflicts.
RALEIGH, N.C. – Wearing glasses was associated with a significantly lower rate of developing actinic keratoses on periocular skin, according to the results of a large multicenter clinical trial.
During an average 4-year prospective follow-up conducted at 6-month intervals in the Veterans Affairs Topical Tretinoin Chemoprevention Trial, the 1,131 elderly participants, most of whom were male, developed 3,291 periocular actinic keratoses.
Patients who didn’t regularly wear eyeglasses had a 40% higher rate of developing AKs on periocular skin. This is consistent with reports in the ophthalmologic literature that eyeglasses can reduce the flux of UV radiation filtered to the periocular area, according to Dr. Kachiu C. Lee, a dermatology resident at Brown University in Providence, R.I.
Her hypothesis was that eyeglasses wearers would also have a lower rate of periocular nonmelanoma skin cancer. However, this wasn’t borne out, possibly because too few of the malignancies occurred to be able to demonstrate a protective effect, she noted. Although all suspected skin cancers were biopsied for histologic diagnosis, only 19 periocular squamous cell carcinomas and 71 periocular basal cell carcinomas occurred during follow-up.
A significant study limitation was that patients weren’t surveyed as to their use of sunglasses. "That’s a huge potential confounding variable," Dr. Lee said.
The study was sponsored by the U.S. Department of Veterans Affairs. Dr. Lee reported having no financial conflicts.
RALEIGH, N.C. – Wearing glasses was associated with a significantly lower rate of developing actinic keratoses on periocular skin, according to the results of a large multicenter clinical trial.
During an average 4-year prospective follow-up conducted at 6-month intervals in the Veterans Affairs Topical Tretinoin Chemoprevention Trial, the 1,131 elderly participants, most of whom were male, developed 3,291 periocular actinic keratoses.
Patients who didn’t regularly wear eyeglasses had a 40% higher rate of developing AKs on periocular skin. This is consistent with reports in the ophthalmologic literature that eyeglasses can reduce the flux of UV radiation filtered to the periocular area, according to Dr. Kachiu C. Lee, a dermatology resident at Brown University in Providence, R.I.
Her hypothesis was that eyeglasses wearers would also have a lower rate of periocular nonmelanoma skin cancer. However, this wasn’t borne out, possibly because too few of the malignancies occurred to be able to demonstrate a protective effect, she noted. Although all suspected skin cancers were biopsied for histologic diagnosis, only 19 periocular squamous cell carcinomas and 71 periocular basal cell carcinomas occurred during follow-up.
A significant study limitation was that patients weren’t surveyed as to their use of sunglasses. "That’s a huge potential confounding variable," Dr. Lee said.
The study was sponsored by the U.S. Department of Veterans Affairs. Dr. Lee reported having no financial conflicts.
AT THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: Patients who didn’t regularly wear eyeglasses had a 40% higher rate of developing AKs on periocular skin.
Data Source: A 4-year prospective study of 1,131 elderly participants, most of whom were male, who developed 3,291 periocular actinic keratoses.
Disclosures: The study was sponsored by the U.S. Department of Veterans Affairs. Dr. Lee reported having no financial conflicts.
Psoriatic Arthritis Screening Tests Deemed Inadequate
RALEIGH, N.C. – Current screening instruments that are designed to identify the 20%-30% of psoriasis patients who have psoriatic arthritis leave much to be desired, Dr. Jessica A. Walsh asserted at the annual meeting of the Society for Investigative Dermatology.
She asked 189 psoriasis patients who are enrolled in the Utah Psoriasis Initiative to take three of the most popular psoriatic arthritis self-administered screening questionnaires. The tests’ sensitivities and specificities proved markedly lower than previously reported in the instruments’ validation studies.
Moreover, all three tests – the Psoriatic Arthritis Screening and Evaluation (PASE), the Psoriasis Epidemiology Screening Project (PEST), and the Toronto Psoriatic Arthritis Screen (ToPAS) – differentiated only poorly among psoriatic arthritis, other types of arthritis, and fibromyalgia.
And the tests had other weak points: "The instruments were less sensitive in patients with lower psoriatic arthritis disease activity, fewer disease features, and shorter disease duration. This is suboptimal because these instruments were designed to help capture early disease," said Dr. Walsh, a rheumatologist at the University of Utah, Salt Lake City.
She is involved in a project aimed at reducing delays in diagnosis of psoriatic arthritis, an underdiagnosed condition in psoriasis patients. According to a 2011 National Psoriasis Foundation survey, 29% of psoriatic arthritis patients don’t receive their diagnosis until 2 years or more after onset of symptoms. That’s bad news, because early diagnosis and treatment help minimize symptoms, enhance quality of life, and prevent joint damage. Studies show that nearly one-half of psoriatic arthritis patients develop radiographic evidence of erosive damage within 2 years of symptom onset. Treatment can slow that progressive erosion.
All 189 psoriasis patients in Dr. Walsh’s study had musculoskeletal complaints of sufficient magnitude that they were willing to take the three screening tests in order to receive a free rheumatologic evaluation. A total of 137 patients had psoriatic arthritis, and two-thirds of them had already been diagnosed with the disease prior to participation in her study.
The gold standard to which the screening tests were compared was Dr. Walsh’s diagnostic evaluation, which included laboratory testing and imaging as warranted. When there was a discrepancy between any of the screening tests and her diagnosis, as occurred in 138 cases, a second rheumatologist with expertise in psoriatic arthritis was called in as a tiebreaker.
The sensitivities of PACE, PEST, and ToPAS were 68%, 85%, and 75%, respectively. The specificities were worse, at 50%, 45%, and 55%. These are in sharp contrast to previous studies, in which the test sensitivities were 88%-97%, with reported specificities of 79%-95%, she noted.
The frequency of discrepancies between patient responses to specific screening questions and the rheumatologic exam findings varied among the test instruments in a way that caused Dr. Walsh to believe that combining selected questions from each test and, in some cases, modifying the wording might result in a hybrid-screening instrument with improved performance. She plans to test that hypothesis.
Dr. Abrar A. Qureshi, one of the developers of PASE, said he and his coworkers have been thinking along the same lines.
"We’re working on a shorter version of PASE that might work better. Nine of the 15 questions on PASE seem to track well with inflammatory arthritis," commented Dr. Qureshi, vice chairman of the department of dermatology at Brigham and Women’s Hospital, Boston.
The Utah Psoriasis Initiative is funded by the University of Utah department of dermatology. Dr. Walsh reported having no financial conflicts.
RALEIGH, N.C. – Current screening instruments that are designed to identify the 20%-30% of psoriasis patients who have psoriatic arthritis leave much to be desired, Dr. Jessica A. Walsh asserted at the annual meeting of the Society for Investigative Dermatology.
She asked 189 psoriasis patients who are enrolled in the Utah Psoriasis Initiative to take three of the most popular psoriatic arthritis self-administered screening questionnaires. The tests’ sensitivities and specificities proved markedly lower than previously reported in the instruments’ validation studies.
Moreover, all three tests – the Psoriatic Arthritis Screening and Evaluation (PASE), the Psoriasis Epidemiology Screening Project (PEST), and the Toronto Psoriatic Arthritis Screen (ToPAS) – differentiated only poorly among psoriatic arthritis, other types of arthritis, and fibromyalgia.
And the tests had other weak points: "The instruments were less sensitive in patients with lower psoriatic arthritis disease activity, fewer disease features, and shorter disease duration. This is suboptimal because these instruments were designed to help capture early disease," said Dr. Walsh, a rheumatologist at the University of Utah, Salt Lake City.
She is involved in a project aimed at reducing delays in diagnosis of psoriatic arthritis, an underdiagnosed condition in psoriasis patients. According to a 2011 National Psoriasis Foundation survey, 29% of psoriatic arthritis patients don’t receive their diagnosis until 2 years or more after onset of symptoms. That’s bad news, because early diagnosis and treatment help minimize symptoms, enhance quality of life, and prevent joint damage. Studies show that nearly one-half of psoriatic arthritis patients develop radiographic evidence of erosive damage within 2 years of symptom onset. Treatment can slow that progressive erosion.
All 189 psoriasis patients in Dr. Walsh’s study had musculoskeletal complaints of sufficient magnitude that they were willing to take the three screening tests in order to receive a free rheumatologic evaluation. A total of 137 patients had psoriatic arthritis, and two-thirds of them had already been diagnosed with the disease prior to participation in her study.
The gold standard to which the screening tests were compared was Dr. Walsh’s diagnostic evaluation, which included laboratory testing and imaging as warranted. When there was a discrepancy between any of the screening tests and her diagnosis, as occurred in 138 cases, a second rheumatologist with expertise in psoriatic arthritis was called in as a tiebreaker.
The sensitivities of PACE, PEST, and ToPAS were 68%, 85%, and 75%, respectively. The specificities were worse, at 50%, 45%, and 55%. These are in sharp contrast to previous studies, in which the test sensitivities were 88%-97%, with reported specificities of 79%-95%, she noted.
The frequency of discrepancies between patient responses to specific screening questions and the rheumatologic exam findings varied among the test instruments in a way that caused Dr. Walsh to believe that combining selected questions from each test and, in some cases, modifying the wording might result in a hybrid-screening instrument with improved performance. She plans to test that hypothesis.
Dr. Abrar A. Qureshi, one of the developers of PASE, said he and his coworkers have been thinking along the same lines.
"We’re working on a shorter version of PASE that might work better. Nine of the 15 questions on PASE seem to track well with inflammatory arthritis," commented Dr. Qureshi, vice chairman of the department of dermatology at Brigham and Women’s Hospital, Boston.
The Utah Psoriasis Initiative is funded by the University of Utah department of dermatology. Dr. Walsh reported having no financial conflicts.
RALEIGH, N.C. – Current screening instruments that are designed to identify the 20%-30% of psoriasis patients who have psoriatic arthritis leave much to be desired, Dr. Jessica A. Walsh asserted at the annual meeting of the Society for Investigative Dermatology.
She asked 189 psoriasis patients who are enrolled in the Utah Psoriasis Initiative to take three of the most popular psoriatic arthritis self-administered screening questionnaires. The tests’ sensitivities and specificities proved markedly lower than previously reported in the instruments’ validation studies.
Moreover, all three tests – the Psoriatic Arthritis Screening and Evaluation (PASE), the Psoriasis Epidemiology Screening Project (PEST), and the Toronto Psoriatic Arthritis Screen (ToPAS) – differentiated only poorly among psoriatic arthritis, other types of arthritis, and fibromyalgia.
And the tests had other weak points: "The instruments were less sensitive in patients with lower psoriatic arthritis disease activity, fewer disease features, and shorter disease duration. This is suboptimal because these instruments were designed to help capture early disease," said Dr. Walsh, a rheumatologist at the University of Utah, Salt Lake City.
She is involved in a project aimed at reducing delays in diagnosis of psoriatic arthritis, an underdiagnosed condition in psoriasis patients. According to a 2011 National Psoriasis Foundation survey, 29% of psoriatic arthritis patients don’t receive their diagnosis until 2 years or more after onset of symptoms. That’s bad news, because early diagnosis and treatment help minimize symptoms, enhance quality of life, and prevent joint damage. Studies show that nearly one-half of psoriatic arthritis patients develop radiographic evidence of erosive damage within 2 years of symptom onset. Treatment can slow that progressive erosion.
All 189 psoriasis patients in Dr. Walsh’s study had musculoskeletal complaints of sufficient magnitude that they were willing to take the three screening tests in order to receive a free rheumatologic evaluation. A total of 137 patients had psoriatic arthritis, and two-thirds of them had already been diagnosed with the disease prior to participation in her study.
The gold standard to which the screening tests were compared was Dr. Walsh’s diagnostic evaluation, which included laboratory testing and imaging as warranted. When there was a discrepancy between any of the screening tests and her diagnosis, as occurred in 138 cases, a second rheumatologist with expertise in psoriatic arthritis was called in as a tiebreaker.
The sensitivities of PACE, PEST, and ToPAS were 68%, 85%, and 75%, respectively. The specificities were worse, at 50%, 45%, and 55%. These are in sharp contrast to previous studies, in which the test sensitivities were 88%-97%, with reported specificities of 79%-95%, she noted.
The frequency of discrepancies between patient responses to specific screening questions and the rheumatologic exam findings varied among the test instruments in a way that caused Dr. Walsh to believe that combining selected questions from each test and, in some cases, modifying the wording might result in a hybrid-screening instrument with improved performance. She plans to test that hypothesis.
Dr. Abrar A. Qureshi, one of the developers of PASE, said he and his coworkers have been thinking along the same lines.
"We’re working on a shorter version of PASE that might work better. Nine of the 15 questions on PASE seem to track well with inflammatory arthritis," commented Dr. Qureshi, vice chairman of the department of dermatology at Brigham and Women’s Hospital, Boston.
The Utah Psoriasis Initiative is funded by the University of Utah department of dermatology. Dr. Walsh reported having no financial conflicts.
AT THE ANNUAL MEETING OF SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: Three popular screening tests for psoriatic arthritis in patients with psoriasis displayed suboptimal specificities of 45%-55% in a prospective study. Those rates are considerably lower than those in previous studies.
Data Source: This was a prospective study involving 189 psoriasis patients, all with musculoskeletal complaints.
Disclosures: The presenter reported having no financial disclosures.
Eczema Therapy Response Varies by Filaggrin Mutation
RALEIGH, N.C. – Atopic dermatitis patients who possess a filaggrin loss-of-function mutation are not likely to be clear of the skin disease for longer than 5 months at a time, according to study results.
The prospective cohort PEER (Pediatric Eczema Elective Registry) study also found that the four most prevalent filaggrin mutations in white patients do not respond similarly to atopic dermatitis medications.
For example, patients with at least one null allele for R501X – the most prevalent filaggrin mutation in the PEER cohort – were 55% less likely than the group as a whole to report being symptom free for 6 months while off all medications for atopic dermatitis. In contrast, those with an S3247X mutation were 3.7-fold more likely to be both symptom and medication free for a 6-month period, according to Dr. David J. Margolis, professor of dermatology at the University of Pennsylvania, Philadelphia.
He presented a study involving 857 PEER participants who underwent DNA analysis screening for the four most common filaggrin mutations in white patients (R501X, 2282del4, R2247X, and S3247X). A total of 28% of white patients had at least one of the mutations, as did 5% of black patients. An R501X mutation was present in 12.2% of white patients and 3.3% of black patients. The second most common mutation (2282del4) was noted in 11.8% of white patients and 0.6% of black patients.
The ongoing Valeant Pharmaceutical–funded PEER study involves children with atopic dermatitis who used topical pimecrolimus (Elidel) for at least 6 weeks during the 6 months immediately prior to enrollment. Their average age at diagnosis was 2.1 years. Participants have been prospectively followed in the registry for a mean of roughly 5 years. They can use any form of treatment they choose. Data on the status of their skin disease are collected at 6-month intervals, allowing for the characteristic waxing and waning of atopic dermatitis.
In all, 81% of PEER participants have been symptom free for at least 6 months on at least one occasion during the 5 years of follow-up. However, only 25.6% were both symptom free and not using an eczema medication.
In a cross-sectional snapshot obtained at 3 years of follow-up, 62% of patients were still using a topical calcineurin inhibitor, 58% were on topical steroids, and 35% were on both forms of therapy.
Patients with any of the four filaggrin mutations were 50% as likely as those without a mutation to be symptom free for a 6-month period, after adjustment for age, sex, and ancestry.
Participants with an R501X mutation were an adjusted 55% less likely than the overall PEER cohort to be symptom free for 6 months while off medications; hence, they could be said to have significantly more persistent or severe disease, Dr. Margolis noted.
In contrast, patients with a 2282del4 mutation were 1.7-fold more likely than the overall group to be symptom free while off therapy for 6 months, whereas those with an R2247X mutation were about equally likely as the patients with none of the filaggrin mutations to enjoy such a period.
The filaggrin gene, which is located on chromosome 1, codes for filament-aggregating protein. It is thought that filaggrin loss-of-function mutations promote breakdown of the skin barrier, allowing a more aggressive immunologic response to antigens and irritants. In 2006, it was demonstrated that filaggrin loss-of-function mutations increase an individual’s risk of developing atopic dermatitis threefold.
That being said, the PEER analysis demonstrates that different filaggrin mutations vary considerably in the degree to which their associated skin disease is treatment-refractory. One reasonable hypothesis is that these differences in treatment responsiveness are related to mutation penetrance.
A limitation of this PEER analysis is that it screened only for the four most prevalent filaggrin mutations, he noted. Roughly 30-40 mutations have been reported thus far.
This study was funded by the National Institutes of Health. Dr. Margolis reported having no financial conflicts.
PEER study, Pediatric Eczema Elective Registry study, R501X, S3247X mutation, Dr. David J. Margolis, Valeant Pharmaceuticals, topical pimecrolimus, Elidel,
RALEIGH, N.C. – Atopic dermatitis patients who possess a filaggrin loss-of-function mutation are not likely to be clear of the skin disease for longer than 5 months at a time, according to study results.
The prospective cohort PEER (Pediatric Eczema Elective Registry) study also found that the four most prevalent filaggrin mutations in white patients do not respond similarly to atopic dermatitis medications.
For example, patients with at least one null allele for R501X – the most prevalent filaggrin mutation in the PEER cohort – were 55% less likely than the group as a whole to report being symptom free for 6 months while off all medications for atopic dermatitis. In contrast, those with an S3247X mutation were 3.7-fold more likely to be both symptom and medication free for a 6-month period, according to Dr. David J. Margolis, professor of dermatology at the University of Pennsylvania, Philadelphia.
He presented a study involving 857 PEER participants who underwent DNA analysis screening for the four most common filaggrin mutations in white patients (R501X, 2282del4, R2247X, and S3247X). A total of 28% of white patients had at least one of the mutations, as did 5% of black patients. An R501X mutation was present in 12.2% of white patients and 3.3% of black patients. The second most common mutation (2282del4) was noted in 11.8% of white patients and 0.6% of black patients.
The ongoing Valeant Pharmaceutical–funded PEER study involves children with atopic dermatitis who used topical pimecrolimus (Elidel) for at least 6 weeks during the 6 months immediately prior to enrollment. Their average age at diagnosis was 2.1 years. Participants have been prospectively followed in the registry for a mean of roughly 5 years. They can use any form of treatment they choose. Data on the status of their skin disease are collected at 6-month intervals, allowing for the characteristic waxing and waning of atopic dermatitis.
In all, 81% of PEER participants have been symptom free for at least 6 months on at least one occasion during the 5 years of follow-up. However, only 25.6% were both symptom free and not using an eczema medication.
In a cross-sectional snapshot obtained at 3 years of follow-up, 62% of patients were still using a topical calcineurin inhibitor, 58% were on topical steroids, and 35% were on both forms of therapy.
Patients with any of the four filaggrin mutations were 50% as likely as those without a mutation to be symptom free for a 6-month period, after adjustment for age, sex, and ancestry.
Participants with an R501X mutation were an adjusted 55% less likely than the overall PEER cohort to be symptom free for 6 months while off medications; hence, they could be said to have significantly more persistent or severe disease, Dr. Margolis noted.
In contrast, patients with a 2282del4 mutation were 1.7-fold more likely than the overall group to be symptom free while off therapy for 6 months, whereas those with an R2247X mutation were about equally likely as the patients with none of the filaggrin mutations to enjoy such a period.
The filaggrin gene, which is located on chromosome 1, codes for filament-aggregating protein. It is thought that filaggrin loss-of-function mutations promote breakdown of the skin barrier, allowing a more aggressive immunologic response to antigens and irritants. In 2006, it was demonstrated that filaggrin loss-of-function mutations increase an individual’s risk of developing atopic dermatitis threefold.
That being said, the PEER analysis demonstrates that different filaggrin mutations vary considerably in the degree to which their associated skin disease is treatment-refractory. One reasonable hypothesis is that these differences in treatment responsiveness are related to mutation penetrance.
A limitation of this PEER analysis is that it screened only for the four most prevalent filaggrin mutations, he noted. Roughly 30-40 mutations have been reported thus far.
This study was funded by the National Institutes of Health. Dr. Margolis reported having no financial conflicts.
RALEIGH, N.C. – Atopic dermatitis patients who possess a filaggrin loss-of-function mutation are not likely to be clear of the skin disease for longer than 5 months at a time, according to study results.
The prospective cohort PEER (Pediatric Eczema Elective Registry) study also found that the four most prevalent filaggrin mutations in white patients do not respond similarly to atopic dermatitis medications.
For example, patients with at least one null allele for R501X – the most prevalent filaggrin mutation in the PEER cohort – were 55% less likely than the group as a whole to report being symptom free for 6 months while off all medications for atopic dermatitis. In contrast, those with an S3247X mutation were 3.7-fold more likely to be both symptom and medication free for a 6-month period, according to Dr. David J. Margolis, professor of dermatology at the University of Pennsylvania, Philadelphia.
He presented a study involving 857 PEER participants who underwent DNA analysis screening for the four most common filaggrin mutations in white patients (R501X, 2282del4, R2247X, and S3247X). A total of 28% of white patients had at least one of the mutations, as did 5% of black patients. An R501X mutation was present in 12.2% of white patients and 3.3% of black patients. The second most common mutation (2282del4) was noted in 11.8% of white patients and 0.6% of black patients.
The ongoing Valeant Pharmaceutical–funded PEER study involves children with atopic dermatitis who used topical pimecrolimus (Elidel) for at least 6 weeks during the 6 months immediately prior to enrollment. Their average age at diagnosis was 2.1 years. Participants have been prospectively followed in the registry for a mean of roughly 5 years. They can use any form of treatment they choose. Data on the status of their skin disease are collected at 6-month intervals, allowing for the characteristic waxing and waning of atopic dermatitis.
In all, 81% of PEER participants have been symptom free for at least 6 months on at least one occasion during the 5 years of follow-up. However, only 25.6% were both symptom free and not using an eczema medication.
In a cross-sectional snapshot obtained at 3 years of follow-up, 62% of patients were still using a topical calcineurin inhibitor, 58% were on topical steroids, and 35% were on both forms of therapy.
Patients with any of the four filaggrin mutations were 50% as likely as those without a mutation to be symptom free for a 6-month period, after adjustment for age, sex, and ancestry.
Participants with an R501X mutation were an adjusted 55% less likely than the overall PEER cohort to be symptom free for 6 months while off medications; hence, they could be said to have significantly more persistent or severe disease, Dr. Margolis noted.
In contrast, patients with a 2282del4 mutation were 1.7-fold more likely than the overall group to be symptom free while off therapy for 6 months, whereas those with an R2247X mutation were about equally likely as the patients with none of the filaggrin mutations to enjoy such a period.
The filaggrin gene, which is located on chromosome 1, codes for filament-aggregating protein. It is thought that filaggrin loss-of-function mutations promote breakdown of the skin barrier, allowing a more aggressive immunologic response to antigens and irritants. In 2006, it was demonstrated that filaggrin loss-of-function mutations increase an individual’s risk of developing atopic dermatitis threefold.
That being said, the PEER analysis demonstrates that different filaggrin mutations vary considerably in the degree to which their associated skin disease is treatment-refractory. One reasonable hypothesis is that these differences in treatment responsiveness are related to mutation penetrance.
A limitation of this PEER analysis is that it screened only for the four most prevalent filaggrin mutations, he noted. Roughly 30-40 mutations have been reported thus far.
This study was funded by the National Institutes of Health. Dr. Margolis reported having no financial conflicts.
PEER study, Pediatric Eczema Elective Registry study, R501X, S3247X mutation, Dr. David J. Margolis, Valeant Pharmaceuticals, topical pimecrolimus, Elidel,
PEER study, Pediatric Eczema Elective Registry study, R501X, S3247X mutation, Dr. David J. Margolis, Valeant Pharmaceuticals, topical pimecrolimus, Elidel,
AT THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: Patients with at least one null allele for R501X were 55% less likely than the group as a whole to report being symptom free for 6 months while off all medications for atopic dermatitis.
Data Source: Data are from a study involving 857 PEER participants who underwent DNA analysis screening for the four most common filaggrin mutations in white patients.
Disclosures: This study was funded by the National Institutes of Health. Dr. Margolis reported having no financial conflicts.
Ixekizumab Normalizes Mutant Psoriasis Genes
RALEIGH, N.C. – At 2 weeks after psoriasis patients received their first dose of the investigational interleukin-17 inhibitor ixekizumab, their gene expression pattern resembled that of healthy controls.
At the annual meeting of the Society for Investigative Dermatology, Dr. James G. Krueger presented a translational medicine study aimed at defining the impact that turning off IL-17 signaling has on pathological tissue structures, molecular pathways, and biomarkers.
His conclusion: "I think these data suggest IL-17 is the central cytokine driving pathogenesis in psoriasis," said Dr. Krueger of Rockefeller University in New York.
Eight psoriasis patients received a subcutaneous 150-mg dose of ixekizumab at weeks 0, 2, and 4 and underwent skin biopsies at weeks 0, 2, and 6. He compared their inflammatory gene expression profiles 2 weeks into treatment vs. those obtained from 15 psoriasis patients 2 weeks after beginning treatment with etanercept (at 50 mg twice weekly for 12 weeks).
IL-17 blockade with ixekizumab proved to have a greater impact on the expression of inflammatory genes known to be associated with psoriasis than did tumor necrosis factor inhibition via etanercept. Ixekizumab resulted in greater-than-75% normalization of expression of 643 of these inflammatory genes, compared with 104 with etanercept.
The structural result of IL-17 blockade was reversal by week 6 of the epidermal hyperplasia that is the hallmark of psoriasis, he added.
Two of the eight patients on the ixekizumab 150-mg regimen achieved PASI 75 (that is, a 75% improvement from baseline on the Psoriasis Area and Severity Index) in 2 weeks. That’s remarkably fast improvement, Dr. Krueger noted. By week 6, all eight patients had achieved PASI 75.
Another point in favor of IL-17’s being a central cytokine driving psoriasis is that ixekizumab shut down not only the IL-17A target genes downstream, but also genes controlling the production of key cytokines located upstream, including interferon-gamma and IL-22, he noted.
Phase III trials investigating ixekizumab for psoriasis are underway. The drug is also being investigated for the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
Dr. Krueger is a consultant to Eli Lilly, which funded the study and is developing ixekizumab as a treatment for psoriasis.
RALEIGH, N.C. – At 2 weeks after psoriasis patients received their first dose of the investigational interleukin-17 inhibitor ixekizumab, their gene expression pattern resembled that of healthy controls.
At the annual meeting of the Society for Investigative Dermatology, Dr. James G. Krueger presented a translational medicine study aimed at defining the impact that turning off IL-17 signaling has on pathological tissue structures, molecular pathways, and biomarkers.
His conclusion: "I think these data suggest IL-17 is the central cytokine driving pathogenesis in psoriasis," said Dr. Krueger of Rockefeller University in New York.
Eight psoriasis patients received a subcutaneous 150-mg dose of ixekizumab at weeks 0, 2, and 4 and underwent skin biopsies at weeks 0, 2, and 6. He compared their inflammatory gene expression profiles 2 weeks into treatment vs. those obtained from 15 psoriasis patients 2 weeks after beginning treatment with etanercept (at 50 mg twice weekly for 12 weeks).
IL-17 blockade with ixekizumab proved to have a greater impact on the expression of inflammatory genes known to be associated with psoriasis than did tumor necrosis factor inhibition via etanercept. Ixekizumab resulted in greater-than-75% normalization of expression of 643 of these inflammatory genes, compared with 104 with etanercept.
The structural result of IL-17 blockade was reversal by week 6 of the epidermal hyperplasia that is the hallmark of psoriasis, he added.
Two of the eight patients on the ixekizumab 150-mg regimen achieved PASI 75 (that is, a 75% improvement from baseline on the Psoriasis Area and Severity Index) in 2 weeks. That’s remarkably fast improvement, Dr. Krueger noted. By week 6, all eight patients had achieved PASI 75.
Another point in favor of IL-17’s being a central cytokine driving psoriasis is that ixekizumab shut down not only the IL-17A target genes downstream, but also genes controlling the production of key cytokines located upstream, including interferon-gamma and IL-22, he noted.
Phase III trials investigating ixekizumab for psoriasis are underway. The drug is also being investigated for the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
Dr. Krueger is a consultant to Eli Lilly, which funded the study and is developing ixekizumab as a treatment for psoriasis.
RALEIGH, N.C. – At 2 weeks after psoriasis patients received their first dose of the investigational interleukin-17 inhibitor ixekizumab, their gene expression pattern resembled that of healthy controls.
At the annual meeting of the Society for Investigative Dermatology, Dr. James G. Krueger presented a translational medicine study aimed at defining the impact that turning off IL-17 signaling has on pathological tissue structures, molecular pathways, and biomarkers.
His conclusion: "I think these data suggest IL-17 is the central cytokine driving pathogenesis in psoriasis," said Dr. Krueger of Rockefeller University in New York.
Eight psoriasis patients received a subcutaneous 150-mg dose of ixekizumab at weeks 0, 2, and 4 and underwent skin biopsies at weeks 0, 2, and 6. He compared their inflammatory gene expression profiles 2 weeks into treatment vs. those obtained from 15 psoriasis patients 2 weeks after beginning treatment with etanercept (at 50 mg twice weekly for 12 weeks).
IL-17 blockade with ixekizumab proved to have a greater impact on the expression of inflammatory genes known to be associated with psoriasis than did tumor necrosis factor inhibition via etanercept. Ixekizumab resulted in greater-than-75% normalization of expression of 643 of these inflammatory genes, compared with 104 with etanercept.
The structural result of IL-17 blockade was reversal by week 6 of the epidermal hyperplasia that is the hallmark of psoriasis, he added.
Two of the eight patients on the ixekizumab 150-mg regimen achieved PASI 75 (that is, a 75% improvement from baseline on the Psoriasis Area and Severity Index) in 2 weeks. That’s remarkably fast improvement, Dr. Krueger noted. By week 6, all eight patients had achieved PASI 75.
Another point in favor of IL-17’s being a central cytokine driving psoriasis is that ixekizumab shut down not only the IL-17A target genes downstream, but also genes controlling the production of key cytokines located upstream, including interferon-gamma and IL-22, he noted.
Phase III trials investigating ixekizumab for psoriasis are underway. The drug is also being investigated for the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
Dr. Krueger is a consultant to Eli Lilly, which funded the study and is developing ixekizumab as a treatment for psoriasis.
AT THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: Ixekizumab resulted in a greater-than-75% normalization of expression of 643 inflammatory genes, compared with 104 with etanercept.
Data Source: Eight psoriasis patients who received a subcutaneous 150-mg dose of ixekizumab were compared with 15 psoriasis patients treated with etanercept.
Disclosures: Dr. Krueger is a consultant to Eli Lilly, which funded the study and is developing ixekizumab as a treatment for psoriasis.
Cyst Removal: Punch Incision Leaves Smaller Scar
RALEIGH, N.C. – Punch incision epidermal inclusion cysts located on the trunk leaves a significantly smaller scar than does elliptical excision with a similarly low recurrence rate, according to the results of a randomized trail.
Procedure time was essentially the same for the two techniques, at around 13 minutes. Although punch incision and its wound closure can be easier, it took a fair amount of time to squeeze the cyst contents through the small punch opening and remove the cyst lining using a curette, Dr. Justin T. Cheeley explained at the annual meeting of the Society for Investigative Dermatology.
He reported on 40 consecutive patients with one or more truncal epidermal inclusion cysts 1-3 cm in diameter who were randomized to elliptical excision or punch incision in a head-to-head comparative trial.
The primary study end point – cyst recurrence during 16 months of prospective follow-up – occurred in three patients in the punch incision group and two in the elliptical excision group. Predictors of cyst recurrence were sought, but none could be identified, according to Dr. Cheeley of Emory University, Atlanta.
Most secondary end points were similar for the two study arms, including early and late complication rates, as well as improvement in skin-specific quality of life and patient satisfaction as measured by change in Skindex-16 scores.
There was, however, a significant difference between the two study groups in terms of average scar length. In the punch incision group, average scar length was 1.1 cm, compared with 1.8 cm in the elliptical excision group.
The investigators employed a 4-mm punch for the most part, although they turned to a 6-mm punch in treating larger cysts. Punch incision wounds were closed with a single nylon suture. Closure of the elliptical excision sites required more extensive suturing.
Audience member Dr. Eric L. Simpson complimented Dr. Cheeley and his coinvestigators for conducting a study with important cost implications given how often epidermal inclusion cysts are encountered in practice.
“The difference between punch incision and elliptical excision with an intermediate-level repair is probably 10-fold in terms of cost,” said Dr. Simpson of Oregon Health and Science University, Portland.
Dr. Cheeley reported having no financial conflicts.
RALEIGH, N.C. – Punch incision epidermal inclusion cysts located on the trunk leaves a significantly smaller scar than does elliptical excision with a similarly low recurrence rate, according to the results of a randomized trail.
Procedure time was essentially the same for the two techniques, at around 13 minutes. Although punch incision and its wound closure can be easier, it took a fair amount of time to squeeze the cyst contents through the small punch opening and remove the cyst lining using a curette, Dr. Justin T. Cheeley explained at the annual meeting of the Society for Investigative Dermatology.
He reported on 40 consecutive patients with one or more truncal epidermal inclusion cysts 1-3 cm in diameter who were randomized to elliptical excision or punch incision in a head-to-head comparative trial.
The primary study end point – cyst recurrence during 16 months of prospective follow-up – occurred in three patients in the punch incision group and two in the elliptical excision group. Predictors of cyst recurrence were sought, but none could be identified, according to Dr. Cheeley of Emory University, Atlanta.
Most secondary end points were similar for the two study arms, including early and late complication rates, as well as improvement in skin-specific quality of life and patient satisfaction as measured by change in Skindex-16 scores.
There was, however, a significant difference between the two study groups in terms of average scar length. In the punch incision group, average scar length was 1.1 cm, compared with 1.8 cm in the elliptical excision group.
The investigators employed a 4-mm punch for the most part, although they turned to a 6-mm punch in treating larger cysts. Punch incision wounds were closed with a single nylon suture. Closure of the elliptical excision sites required more extensive suturing.
Audience member Dr. Eric L. Simpson complimented Dr. Cheeley and his coinvestigators for conducting a study with important cost implications given how often epidermal inclusion cysts are encountered in practice.
“The difference between punch incision and elliptical excision with an intermediate-level repair is probably 10-fold in terms of cost,” said Dr. Simpson of Oregon Health and Science University, Portland.
Dr. Cheeley reported having no financial conflicts.
RALEIGH, N.C. – Punch incision epidermal inclusion cysts located on the trunk leaves a significantly smaller scar than does elliptical excision with a similarly low recurrence rate, according to the results of a randomized trail.
Procedure time was essentially the same for the two techniques, at around 13 minutes. Although punch incision and its wound closure can be easier, it took a fair amount of time to squeeze the cyst contents through the small punch opening and remove the cyst lining using a curette, Dr. Justin T. Cheeley explained at the annual meeting of the Society for Investigative Dermatology.
He reported on 40 consecutive patients with one or more truncal epidermal inclusion cysts 1-3 cm in diameter who were randomized to elliptical excision or punch incision in a head-to-head comparative trial.
The primary study end point – cyst recurrence during 16 months of prospective follow-up – occurred in three patients in the punch incision group and two in the elliptical excision group. Predictors of cyst recurrence were sought, but none could be identified, according to Dr. Cheeley of Emory University, Atlanta.
Most secondary end points were similar for the two study arms, including early and late complication rates, as well as improvement in skin-specific quality of life and patient satisfaction as measured by change in Skindex-16 scores.
There was, however, a significant difference between the two study groups in terms of average scar length. In the punch incision group, average scar length was 1.1 cm, compared with 1.8 cm in the elliptical excision group.
The investigators employed a 4-mm punch for the most part, although they turned to a 6-mm punch in treating larger cysts. Punch incision wounds were closed with a single nylon suture. Closure of the elliptical excision sites required more extensive suturing.
Audience member Dr. Eric L. Simpson complimented Dr. Cheeley and his coinvestigators for conducting a study with important cost implications given how often epidermal inclusion cysts are encountered in practice.
“The difference between punch incision and elliptical excision with an intermediate-level repair is probably 10-fold in terms of cost,” said Dr. Simpson of Oregon Health and Science University, Portland.
Dr. Cheeley reported having no financial conflicts.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: In the punch incision group, average scar length was 1.1 cm, compared with 1.8 cm in the elliptical excision group.
Data Source: This was a randomized trial of 40 consecutive patients.
Disclosures: Dr. Cheeley reported having no financial conflicts.
Ziana Proves Less Irritating Than Epiduo for Acne
RALEIGH, N.C. - Topical clindamycin phosphate 1.2%/tretinoin 0.025% gel was found to be significantly less irritating than benzoyl peroxide 2.5%/adapalene 0.1% gel was during the first 3 weeks of acne therapy, according to a double-blind, randomized, split-face comparative trial.
However, it should be noted that at all times, study participants scored their irritation as moderate or less with both medications. “Both products are pretty well tolerated. Nevertheless, looking at patient outcomes, clindamycin/tretinoin [Ziana]does seem to be less irritating than benzoyl peroxide/adapalene [Epiduo], which could be of clinical significance because it might [affect] patient compliance,” noted Dr. Renato Goreshi of the department of dermatology at the Oregon Health and Science University, Portland.
He presented a double-blind study involving 24 patients with mild to moderate facial acne at the annual meeting of the Society for Investigative Dermatology. All were white, and most were in their mid-20s.
Participants applied clindamycin/tretinoin to one side of their face and benzoyl peroxide/adapalene to the other side once daily for 3 weeks. They kept a daily record scoring burning/stinging and itching on a 0-3 scale. In addition, investigators measured transepidermal water loss and conducted Investigator’s Global Assessments of dryness/scaling and erythema at baseline and weekly thereafter.
The primary study outcome was transepidermal water loss, a reliable and validated measure of epidermal barrier disruption. Transepidermal water loss was significantly greater at weeks 1, 2, and 3 on the benzoyl peroxide/adapalene–treated side of the face.
The benzoyl peroxide/adapalene–treated side also scored threefold higher on the patients’ self-assessed rating of burning/stinging, and twice as high on itching.
“This isn’t entirely surprising, since in studies in atopic dermatitis we also see that there’s a correlation between higher transepidermal water-loss scores and increased pruritus, which is what we saw here,” Dr. Goreshi said.
The difference in composite irritancy scores was larger in the first half of the study than in the second half, when scores headed toward convergence.
Investigators found no significant difference between the two products in erythema or dryness/scaling.
There was also no significant difference between the two topical agents in terms of reduction of acne lesion counts. However, one wouldn’t expect to see significant differences during this short of a study, which was designed to look at irritancy, not efficacy, Dr. Goreshi noted.
The study was funded by Medicis, which markets Ziana.
RALEIGH, N.C. - Topical clindamycin phosphate 1.2%/tretinoin 0.025% gel was found to be significantly less irritating than benzoyl peroxide 2.5%/adapalene 0.1% gel was during the first 3 weeks of acne therapy, according to a double-blind, randomized, split-face comparative trial.
However, it should be noted that at all times, study participants scored their irritation as moderate or less with both medications. “Both products are pretty well tolerated. Nevertheless, looking at patient outcomes, clindamycin/tretinoin [Ziana]does seem to be less irritating than benzoyl peroxide/adapalene [Epiduo], which could be of clinical significance because it might [affect] patient compliance,” noted Dr. Renato Goreshi of the department of dermatology at the Oregon Health and Science University, Portland.
He presented a double-blind study involving 24 patients with mild to moderate facial acne at the annual meeting of the Society for Investigative Dermatology. All were white, and most were in their mid-20s.
Participants applied clindamycin/tretinoin to one side of their face and benzoyl peroxide/adapalene to the other side once daily for 3 weeks. They kept a daily record scoring burning/stinging and itching on a 0-3 scale. In addition, investigators measured transepidermal water loss and conducted Investigator’s Global Assessments of dryness/scaling and erythema at baseline and weekly thereafter.
The primary study outcome was transepidermal water loss, a reliable and validated measure of epidermal barrier disruption. Transepidermal water loss was significantly greater at weeks 1, 2, and 3 on the benzoyl peroxide/adapalene–treated side of the face.
The benzoyl peroxide/adapalene–treated side also scored threefold higher on the patients’ self-assessed rating of burning/stinging, and twice as high on itching.
“This isn’t entirely surprising, since in studies in atopic dermatitis we also see that there’s a correlation between higher transepidermal water-loss scores and increased pruritus, which is what we saw here,” Dr. Goreshi said.
The difference in composite irritancy scores was larger in the first half of the study than in the second half, when scores headed toward convergence.
Investigators found no significant difference between the two products in erythema or dryness/scaling.
There was also no significant difference between the two topical agents in terms of reduction of acne lesion counts. However, one wouldn’t expect to see significant differences during this short of a study, which was designed to look at irritancy, not efficacy, Dr. Goreshi noted.
The study was funded by Medicis, which markets Ziana.
RALEIGH, N.C. - Topical clindamycin phosphate 1.2%/tretinoin 0.025% gel was found to be significantly less irritating than benzoyl peroxide 2.5%/adapalene 0.1% gel was during the first 3 weeks of acne therapy, according to a double-blind, randomized, split-face comparative trial.
However, it should be noted that at all times, study participants scored their irritation as moderate or less with both medications. “Both products are pretty well tolerated. Nevertheless, looking at patient outcomes, clindamycin/tretinoin [Ziana]does seem to be less irritating than benzoyl peroxide/adapalene [Epiduo], which could be of clinical significance because it might [affect] patient compliance,” noted Dr. Renato Goreshi of the department of dermatology at the Oregon Health and Science University, Portland.
He presented a double-blind study involving 24 patients with mild to moderate facial acne at the annual meeting of the Society for Investigative Dermatology. All were white, and most were in their mid-20s.
Participants applied clindamycin/tretinoin to one side of their face and benzoyl peroxide/adapalene to the other side once daily for 3 weeks. They kept a daily record scoring burning/stinging and itching on a 0-3 scale. In addition, investigators measured transepidermal water loss and conducted Investigator’s Global Assessments of dryness/scaling and erythema at baseline and weekly thereafter.
The primary study outcome was transepidermal water loss, a reliable and validated measure of epidermal barrier disruption. Transepidermal water loss was significantly greater at weeks 1, 2, and 3 on the benzoyl peroxide/adapalene–treated side of the face.
The benzoyl peroxide/adapalene–treated side also scored threefold higher on the patients’ self-assessed rating of burning/stinging, and twice as high on itching.
“This isn’t entirely surprising, since in studies in atopic dermatitis we also see that there’s a correlation between higher transepidermal water-loss scores and increased pruritus, which is what we saw here,” Dr. Goreshi said.
The difference in composite irritancy scores was larger in the first half of the study than in the second half, when scores headed toward convergence.
Investigators found no significant difference between the two products in erythema or dryness/scaling.
There was also no significant difference between the two topical agents in terms of reduction of acne lesion counts. However, one wouldn’t expect to see significant differences during this short of a study, which was designed to look at irritancy, not efficacy, Dr. Goreshi noted.
The study was funded by Medicis, which markets Ziana.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: Transepidermal water loss was significantly greater at weeks 1, 2, and 3 on the benzoyl peroxide/adapalene–treated side of the face.
Data Source: Results were taken from a double-blind, randomized, split-face comparative trial involving 24 patients with mild to moderate facial acne.
Disclosures: The study was sponsored by Medicis.
Onion Extract Improved Scars by 36%
RALEIGH, N.C. – A new once-daily topical gel containing a proprietary onion extract resulted in a 36% improvement in the appearance of recent postsurgical dermal scars at 8 weeks, according to the results of a randomized, controlled trial.
The over-the-counter product, Merz Pharmaceuticals’ Mederma Advanced Scar Gel, was studied in 44 adults, each of whom underwent surgical shave removal of two similar-size seborrheic keratoses on the chest. At 2 weeks, after the wounds had reepithelialized, patients were randomly assigned to apply the nonprescription onion extract gel once daily to one scar and no treatment to the other.
Blinded investigator assessment was carried out after 2, 4, and 8 weeks of once-daily therapy. Each scar was graded on a 0-3 scale for improvement over baseline for overall appearance and for more specific individual domains of texture, redness, and softness. Patients independently carried out the same assessments, explained Dr. Zoe D. Draelos, a clinical dermatologist and researcher in High Point, N.C.
At week 8, investigators rated the onion extract–treated scars as demonstrating a mean 2.6-point improvement over baseline in terms of overall appearance, with comparable improvements noted in texture, redness, and scar softness. These were significantly better outcomes than was the mean 2.1-point improvement in the overall appearance of untreated control scars, she noted.
The patients rated the onion extract gel–treated scars as showing a mean 2.0-point improvement at week 8, significantly better than the 1.5-point improvement noted in the control scars.
Although optimal results were seen at week 8, the topical gel–treated scars showed a significant advantage in appearance scores, compared with control scars, as early as week 4, with a nonsignificant favorable trend noted at week 2.
The chief advantage that the transparent onion extract gel offers over other scar treatment products is the convenience of once-daily application, noted Dr. Draelos.
Merz announced the launch of Mederma Advanced Scar Gel in the spring. It is available in the first-aid section of pharmacies nationwide at a retail price of about $20 for a 20-g tube and $32 for 50 g, according to the company.
Other Merz products containing Cepalin, the proprietary onion extract, include Mederma Scar Cream plus SPF 30, Mederma for Kids, and Mederma Stretch Marks Therapy.
Dr. Draelos received research funding from Merz to conduct the clinical trial.
RALEIGH, N.C. – A new once-daily topical gel containing a proprietary onion extract resulted in a 36% improvement in the appearance of recent postsurgical dermal scars at 8 weeks, according to the results of a randomized, controlled trial.
The over-the-counter product, Merz Pharmaceuticals’ Mederma Advanced Scar Gel, was studied in 44 adults, each of whom underwent surgical shave removal of two similar-size seborrheic keratoses on the chest. At 2 weeks, after the wounds had reepithelialized, patients were randomly assigned to apply the nonprescription onion extract gel once daily to one scar and no treatment to the other.
Blinded investigator assessment was carried out after 2, 4, and 8 weeks of once-daily therapy. Each scar was graded on a 0-3 scale for improvement over baseline for overall appearance and for more specific individual domains of texture, redness, and softness. Patients independently carried out the same assessments, explained Dr. Zoe D. Draelos, a clinical dermatologist and researcher in High Point, N.C.
At week 8, investigators rated the onion extract–treated scars as demonstrating a mean 2.6-point improvement over baseline in terms of overall appearance, with comparable improvements noted in texture, redness, and scar softness. These were significantly better outcomes than was the mean 2.1-point improvement in the overall appearance of untreated control scars, she noted.
The patients rated the onion extract gel–treated scars as showing a mean 2.0-point improvement at week 8, significantly better than the 1.5-point improvement noted in the control scars.
Although optimal results were seen at week 8, the topical gel–treated scars showed a significant advantage in appearance scores, compared with control scars, as early as week 4, with a nonsignificant favorable trend noted at week 2.
The chief advantage that the transparent onion extract gel offers over other scar treatment products is the convenience of once-daily application, noted Dr. Draelos.
Merz announced the launch of Mederma Advanced Scar Gel in the spring. It is available in the first-aid section of pharmacies nationwide at a retail price of about $20 for a 20-g tube and $32 for 50 g, according to the company.
Other Merz products containing Cepalin, the proprietary onion extract, include Mederma Scar Cream plus SPF 30, Mederma for Kids, and Mederma Stretch Marks Therapy.
Dr. Draelos received research funding from Merz to conduct the clinical trial.
RALEIGH, N.C. – A new once-daily topical gel containing a proprietary onion extract resulted in a 36% improvement in the appearance of recent postsurgical dermal scars at 8 weeks, according to the results of a randomized, controlled trial.
The over-the-counter product, Merz Pharmaceuticals’ Mederma Advanced Scar Gel, was studied in 44 adults, each of whom underwent surgical shave removal of two similar-size seborrheic keratoses on the chest. At 2 weeks, after the wounds had reepithelialized, patients were randomly assigned to apply the nonprescription onion extract gel once daily to one scar and no treatment to the other.
Blinded investigator assessment was carried out after 2, 4, and 8 weeks of once-daily therapy. Each scar was graded on a 0-3 scale for improvement over baseline for overall appearance and for more specific individual domains of texture, redness, and softness. Patients independently carried out the same assessments, explained Dr. Zoe D. Draelos, a clinical dermatologist and researcher in High Point, N.C.
At week 8, investigators rated the onion extract–treated scars as demonstrating a mean 2.6-point improvement over baseline in terms of overall appearance, with comparable improvements noted in texture, redness, and scar softness. These were significantly better outcomes than was the mean 2.1-point improvement in the overall appearance of untreated control scars, she noted.
The patients rated the onion extract gel–treated scars as showing a mean 2.0-point improvement at week 8, significantly better than the 1.5-point improvement noted in the control scars.
Although optimal results were seen at week 8, the topical gel–treated scars showed a significant advantage in appearance scores, compared with control scars, as early as week 4, with a nonsignificant favorable trend noted at week 2.
The chief advantage that the transparent onion extract gel offers over other scar treatment products is the convenience of once-daily application, noted Dr. Draelos.
Merz announced the launch of Mederma Advanced Scar Gel in the spring. It is available in the first-aid section of pharmacies nationwide at a retail price of about $20 for a 20-g tube and $32 for 50 g, according to the company.
Other Merz products containing Cepalin, the proprietary onion extract, include Mederma Scar Cream plus SPF 30, Mederma for Kids, and Mederma Stretch Marks Therapy.
Dr. Draelos received research funding from Merz to conduct the clinical trial.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: An 8-week regimen of an onion extract–based topical gel led to a 36% greater improvement in the overall appearance of new postsurgical dermal scars, compared with no treatment.
Data Source: A randomized, controlled study of 44 patients who underwent surgical removal of two similar-sized seborrheic keratoses on their chest.
Disclosures: Dr. Draelos received research funding from Merz to conduct the clinical trial.
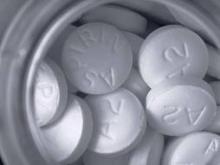
Aspirin Found to Protect Against Melanoma
RALEIGH, N.C. – Postmenopausal women who used aspirin regularly had a significantly reduced risk of developing melanoma during long-term prospective follow-up, according to a Women’s Health Initiative observational study analysis.
The magnitude of this risk reduction grew with greater duration of aspirin use, reported Christina A. Gamba, a medical student at Stanford (Calif.) University.
During 12 years of prospective follow-up, women who at the time of study enrollment reported they had been taking aspirin regularly for less than a year had roughly an 11% reduction in the likelihood of developing incident melanoma, compared with aspirin nonusers. Those who had been using aspirin regularly for 1-4 years at enrollment went on to have a 20% risk reduction. And women on aspirin for 5 years or longer at enrollment were 30% less likely to develop melanoma than nonusers.
These relative risk figures were fully adjusted for age, education, income, skin type, melanoma risk factors, physical activity, vitamin D intake, smoking status, body mass index, sunburn history, time spent outdoors, sunscreen use, and medical indications for aspirin use, such as cardiovascular disease, she noted.
The findings suggest a possible chemopreventive effect for aspirin against the development of melanoma. As such, these data are consistent with other studies that have linked NSAIDs with decreased risks of breast, colorectal, and gastric cancer. The specific mechanism of benefit is thought to involve inhibition of cyclo-oxygenase-2, which is proinflammatory.
Ms. Gamba explained that her Women’s Health Initiative observational study analysis included 59,806 postmenopausal white women for whom complete pertinent data were available. During nearly 12 years of follow-up, 548 incident melanomas occurred among the study population: 255 were invasive; the rest were in situ.
At enrollment, 25% of women reported using aspirin regularly; their melanoma incidence during follow-up was 0.76%. Fifteen percent of subjects used nonaspirin NSAIDs; their melanoma incidence was 0.97%, which was identical to the rate in the 60% of women who were nonusers of any NSAIDs. Regular users of acetaminophen, a drug that relieves pain but doesn’t inhibit cyclo-oxygenase-2, had the same melanoma incidence as nonusers of aspirin and other NSAIDs.
Three-quarters of the aspirin users were on regular- or extrastrength formulations. There were too few women on low-dose aspirin to be able to draw any conclusions as to whether it, too, protected against melanoma, she said at the annual meeting of the Society of Investigative Dermatology.
The study included one measure of consistency: At 3 years of follow-up, 60% of women who reported taking aspirin regularly at baseline were still doing so, as were 35% of those who had been on nonaspirin NSAIDs at enrollment.
Audience members posed this question: If aspirin’s presumed chemoprevention mechanism involves the inhibition of cyclooxygenase-2, why didn’t users of nonaspirin NSAIDs enjoy a similar benefit? Ms. Gamba offered two possible theories. One is that users of nonaspirin NSAIDs took the medication less frequently, in contrast to aspirin users who generally took the drug on a daily basis.
The other possibility, she continued, is that aspirin curbs tumorigenesis through cyclo-oxygenase-independent mechanisms – perhaps by promoting apoptotic genes and/or inhibiting tumor-promoting genes.
All cases of melanoma in the Women’s Health Initiative were pathologically confirmed. Ms. Gamba and her investigators are now going back and examining the pathology reports to learn what specific types of melanoma were involved. They are also reanalyzing their aspirin/NSAID usage data, this time restricting the analysis to cases of in situ melanoma.
The Women’s Health Initiative Observational Study was sponsored by the National Institutes of Health. Ms. Gamba reported having no relevant financial conflicts.
RALEIGH, N.C. – Postmenopausal women who used aspirin regularly had a significantly reduced risk of developing melanoma during long-term prospective follow-up, according to a Women’s Health Initiative observational study analysis.
The magnitude of this risk reduction grew with greater duration of aspirin use, reported Christina A. Gamba, a medical student at Stanford (Calif.) University.
During 12 years of prospective follow-up, women who at the time of study enrollment reported they had been taking aspirin regularly for less than a year had roughly an 11% reduction in the likelihood of developing incident melanoma, compared with aspirin nonusers. Those who had been using aspirin regularly for 1-4 years at enrollment went on to have a 20% risk reduction. And women on aspirin for 5 years or longer at enrollment were 30% less likely to develop melanoma than nonusers.
These relative risk figures were fully adjusted for age, education, income, skin type, melanoma risk factors, physical activity, vitamin D intake, smoking status, body mass index, sunburn history, time spent outdoors, sunscreen use, and medical indications for aspirin use, such as cardiovascular disease, she noted.
The findings suggest a possible chemopreventive effect for aspirin against the development of melanoma. As such, these data are consistent with other studies that have linked NSAIDs with decreased risks of breast, colorectal, and gastric cancer. The specific mechanism of benefit is thought to involve inhibition of cyclo-oxygenase-2, which is proinflammatory.
Ms. Gamba explained that her Women’s Health Initiative observational study analysis included 59,806 postmenopausal white women for whom complete pertinent data were available. During nearly 12 years of follow-up, 548 incident melanomas occurred among the study population: 255 were invasive; the rest were in situ.
At enrollment, 25% of women reported using aspirin regularly; their melanoma incidence during follow-up was 0.76%. Fifteen percent of subjects used nonaspirin NSAIDs; their melanoma incidence was 0.97%, which was identical to the rate in the 60% of women who were nonusers of any NSAIDs. Regular users of acetaminophen, a drug that relieves pain but doesn’t inhibit cyclo-oxygenase-2, had the same melanoma incidence as nonusers of aspirin and other NSAIDs.
Three-quarters of the aspirin users were on regular- or extrastrength formulations. There were too few women on low-dose aspirin to be able to draw any conclusions as to whether it, too, protected against melanoma, she said at the annual meeting of the Society of Investigative Dermatology.
The study included one measure of consistency: At 3 years of follow-up, 60% of women who reported taking aspirin regularly at baseline were still doing so, as were 35% of those who had been on nonaspirin NSAIDs at enrollment.
Audience members posed this question: If aspirin’s presumed chemoprevention mechanism involves the inhibition of cyclooxygenase-2, why didn’t users of nonaspirin NSAIDs enjoy a similar benefit? Ms. Gamba offered two possible theories. One is that users of nonaspirin NSAIDs took the medication less frequently, in contrast to aspirin users who generally took the drug on a daily basis.
The other possibility, she continued, is that aspirin curbs tumorigenesis through cyclo-oxygenase-independent mechanisms – perhaps by promoting apoptotic genes and/or inhibiting tumor-promoting genes.
All cases of melanoma in the Women’s Health Initiative were pathologically confirmed. Ms. Gamba and her investigators are now going back and examining the pathology reports to learn what specific types of melanoma were involved. They are also reanalyzing their aspirin/NSAID usage data, this time restricting the analysis to cases of in situ melanoma.
The Women’s Health Initiative Observational Study was sponsored by the National Institutes of Health. Ms. Gamba reported having no relevant financial conflicts.
RALEIGH, N.C. – Postmenopausal women who used aspirin regularly had a significantly reduced risk of developing melanoma during long-term prospective follow-up, according to a Women’s Health Initiative observational study analysis.
The magnitude of this risk reduction grew with greater duration of aspirin use, reported Christina A. Gamba, a medical student at Stanford (Calif.) University.
During 12 years of prospective follow-up, women who at the time of study enrollment reported they had been taking aspirin regularly for less than a year had roughly an 11% reduction in the likelihood of developing incident melanoma, compared with aspirin nonusers. Those who had been using aspirin regularly for 1-4 years at enrollment went on to have a 20% risk reduction. And women on aspirin for 5 years or longer at enrollment were 30% less likely to develop melanoma than nonusers.
These relative risk figures were fully adjusted for age, education, income, skin type, melanoma risk factors, physical activity, vitamin D intake, smoking status, body mass index, sunburn history, time spent outdoors, sunscreen use, and medical indications for aspirin use, such as cardiovascular disease, she noted.
The findings suggest a possible chemopreventive effect for aspirin against the development of melanoma. As such, these data are consistent with other studies that have linked NSAIDs with decreased risks of breast, colorectal, and gastric cancer. The specific mechanism of benefit is thought to involve inhibition of cyclo-oxygenase-2, which is proinflammatory.
Ms. Gamba explained that her Women’s Health Initiative observational study analysis included 59,806 postmenopausal white women for whom complete pertinent data were available. During nearly 12 years of follow-up, 548 incident melanomas occurred among the study population: 255 were invasive; the rest were in situ.
At enrollment, 25% of women reported using aspirin regularly; their melanoma incidence during follow-up was 0.76%. Fifteen percent of subjects used nonaspirin NSAIDs; their melanoma incidence was 0.97%, which was identical to the rate in the 60% of women who were nonusers of any NSAIDs. Regular users of acetaminophen, a drug that relieves pain but doesn’t inhibit cyclo-oxygenase-2, had the same melanoma incidence as nonusers of aspirin and other NSAIDs.
Three-quarters of the aspirin users were on regular- or extrastrength formulations. There were too few women on low-dose aspirin to be able to draw any conclusions as to whether it, too, protected against melanoma, she said at the annual meeting of the Society of Investigative Dermatology.
The study included one measure of consistency: At 3 years of follow-up, 60% of women who reported taking aspirin regularly at baseline were still doing so, as were 35% of those who had been on nonaspirin NSAIDs at enrollment.
Audience members posed this question: If aspirin’s presumed chemoprevention mechanism involves the inhibition of cyclooxygenase-2, why didn’t users of nonaspirin NSAIDs enjoy a similar benefit? Ms. Gamba offered two possible theories. One is that users of nonaspirin NSAIDs took the medication less frequently, in contrast to aspirin users who generally took the drug on a daily basis.
The other possibility, she continued, is that aspirin curbs tumorigenesis through cyclo-oxygenase-independent mechanisms – perhaps by promoting apoptotic genes and/or inhibiting tumor-promoting genes.
All cases of melanoma in the Women’s Health Initiative were pathologically confirmed. Ms. Gamba and her investigators are now going back and examining the pathology reports to learn what specific types of melanoma were involved. They are also reanalyzing their aspirin/NSAID usage data, this time restricting the analysis to cases of in situ melanoma.
The Women’s Health Initiative Observational Study was sponsored by the National Institutes of Health. Ms. Gamba reported having no relevant financial conflicts.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: Postmenopausal women who reported using aspirin regularly at baseline went on to have an 11%-30% reduction in the risk for developing melanoma, compared with aspirin nonusers.
Data Source: Data were from an analysis of nearly 60,000 postmenopausal white participants in the Women’s Health Initiative observational study.
Disclosures: The Women’s Health Initiative study was funded by the National Institutes of Health. Ms. Gamba reported having no relevant financial conflicts.
Stanford Experience Shows Long Metastatic BCC Survival
RALEIGH, N.C. – The prognosis for patients with metastatic basal cell carcinoma may be better than previous reports have suggested, according to Christina Danial.
A single-center case series found a median overall survival of 7.8 years from diagnosis of BCC metastasis to all-cause mortality, which was significantly better than the 10- to 14-month overall survival described in meta-analyses of roughly 300 case reports of metastatic BCC published since the 1890s, noted Ms. Danial, a medical student at Stanford (Calif.) University.
The Stanford series involved 17 patients with metastatic BCC treated there from 1998 to 2011. Although 17 patients may not sound like a lot, it is in fact the largest single-center experience ever reported – a fact that’s testimony to the rarity of metastatic BCC. The BCC metastasis rate has been estimated at 0.003%-0.55%, Ms. Danial said.
The mean overall survival from the date of diagnosis of the primary tumor to death from any cause was 15.96 years. The mean progression-free survival from diagnosis of the metastasis to disease progression based on physician assessment or radiologic findings was 2.4 years.
The most important predictor of metastasis identified by Ms. Danial and her coinvestigators was having a primary tumor of the basosquamous histologic subtype. This was associated with a 6.5-fold increased risk of metastasis.
Eleven patients experienced metastasis to the lung. The second most common site was bone, in eight patients. All metastases were pathologically confirmed by tissue diagnosis.
The explanation for the markedly better overall survival in the Stanford series than in earlier reports is unclear. Improvements in treatment are one possibility. Another is that reporting bias may have been a factor in previous reports, with more aggressive cases of metastatic BCC having a greater likelihood of being published. Yet another possibility is that patients who are referred to Stanford for treatment are in better overall health than those featured in prior case reports, since they are able to travel to the academic medical center, Ms. Danial noted.
None of the various forms of chemotherapy, surgery, and radiation treatment utilized in Stanford patients with metastatic BCC was associated with a significant increase in overall survival. That being said, it’s too soon to know whether the Stanford patients involved in a clinical trial of vismodegib (Erivedge), the novel Hedgehog signaling pathway inhibitor, will experience a significant survival advantage over those who didn’t receive the drug.
The Stanford series presented by Ms. Danial also included 32 patients with locally advanced BCC. Their mean overall survival from the time of diagnosis of the primary tumor was 10.1 years. The primary tumor was located on the head or neck in 22 of the 32 patients, as was the case in 8 of 17 patients with metastatic BCC.
Ms. Danial reported having no relevant financial conflicts.
RALEIGH, N.C. – The prognosis for patients with metastatic basal cell carcinoma may be better than previous reports have suggested, according to Christina Danial.
A single-center case series found a median overall survival of 7.8 years from diagnosis of BCC metastasis to all-cause mortality, which was significantly better than the 10- to 14-month overall survival described in meta-analyses of roughly 300 case reports of metastatic BCC published since the 1890s, noted Ms. Danial, a medical student at Stanford (Calif.) University.
The Stanford series involved 17 patients with metastatic BCC treated there from 1998 to 2011. Although 17 patients may not sound like a lot, it is in fact the largest single-center experience ever reported – a fact that’s testimony to the rarity of metastatic BCC. The BCC metastasis rate has been estimated at 0.003%-0.55%, Ms. Danial said.
The mean overall survival from the date of diagnosis of the primary tumor to death from any cause was 15.96 years. The mean progression-free survival from diagnosis of the metastasis to disease progression based on physician assessment or radiologic findings was 2.4 years.
The most important predictor of metastasis identified by Ms. Danial and her coinvestigators was having a primary tumor of the basosquamous histologic subtype. This was associated with a 6.5-fold increased risk of metastasis.
Eleven patients experienced metastasis to the lung. The second most common site was bone, in eight patients. All metastases were pathologically confirmed by tissue diagnosis.
The explanation for the markedly better overall survival in the Stanford series than in earlier reports is unclear. Improvements in treatment are one possibility. Another is that reporting bias may have been a factor in previous reports, with more aggressive cases of metastatic BCC having a greater likelihood of being published. Yet another possibility is that patients who are referred to Stanford for treatment are in better overall health than those featured in prior case reports, since they are able to travel to the academic medical center, Ms. Danial noted.
None of the various forms of chemotherapy, surgery, and radiation treatment utilized in Stanford patients with metastatic BCC was associated with a significant increase in overall survival. That being said, it’s too soon to know whether the Stanford patients involved in a clinical trial of vismodegib (Erivedge), the novel Hedgehog signaling pathway inhibitor, will experience a significant survival advantage over those who didn’t receive the drug.
The Stanford series presented by Ms. Danial also included 32 patients with locally advanced BCC. Their mean overall survival from the time of diagnosis of the primary tumor was 10.1 years. The primary tumor was located on the head or neck in 22 of the 32 patients, as was the case in 8 of 17 patients with metastatic BCC.
Ms. Danial reported having no relevant financial conflicts.
RALEIGH, N.C. – The prognosis for patients with metastatic basal cell carcinoma may be better than previous reports have suggested, according to Christina Danial.
A single-center case series found a median overall survival of 7.8 years from diagnosis of BCC metastasis to all-cause mortality, which was significantly better than the 10- to 14-month overall survival described in meta-analyses of roughly 300 case reports of metastatic BCC published since the 1890s, noted Ms. Danial, a medical student at Stanford (Calif.) University.
The Stanford series involved 17 patients with metastatic BCC treated there from 1998 to 2011. Although 17 patients may not sound like a lot, it is in fact the largest single-center experience ever reported – a fact that’s testimony to the rarity of metastatic BCC. The BCC metastasis rate has been estimated at 0.003%-0.55%, Ms. Danial said.
The mean overall survival from the date of diagnosis of the primary tumor to death from any cause was 15.96 years. The mean progression-free survival from diagnosis of the metastasis to disease progression based on physician assessment or radiologic findings was 2.4 years.
The most important predictor of metastasis identified by Ms. Danial and her coinvestigators was having a primary tumor of the basosquamous histologic subtype. This was associated with a 6.5-fold increased risk of metastasis.
Eleven patients experienced metastasis to the lung. The second most common site was bone, in eight patients. All metastases were pathologically confirmed by tissue diagnosis.
The explanation for the markedly better overall survival in the Stanford series than in earlier reports is unclear. Improvements in treatment are one possibility. Another is that reporting bias may have been a factor in previous reports, with more aggressive cases of metastatic BCC having a greater likelihood of being published. Yet another possibility is that patients who are referred to Stanford for treatment are in better overall health than those featured in prior case reports, since they are able to travel to the academic medical center, Ms. Danial noted.
None of the various forms of chemotherapy, surgery, and radiation treatment utilized in Stanford patients with metastatic BCC was associated with a significant increase in overall survival. That being said, it’s too soon to know whether the Stanford patients involved in a clinical trial of vismodegib (Erivedge), the novel Hedgehog signaling pathway inhibitor, will experience a significant survival advantage over those who didn’t receive the drug.
The Stanford series presented by Ms. Danial also included 32 patients with locally advanced BCC. Their mean overall survival from the time of diagnosis of the primary tumor was 10.1 years. The primary tumor was located on the head or neck in 22 of the 32 patients, as was the case in 8 of 17 patients with metastatic BCC.
Ms. Danial reported having no relevant financial conflicts.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: The median overall survival from the time of diagnosis of metastatic basal cell carcinoma was 7.8 years in a single-center series, compared with 10-14 months reported in prior studies.
Data Source: Data came from a series of 17 cases of metastatic basal cell carcinoma managed at Stanford University from1998 to 2011.
Disclosures: Ms. Danial reported having no relevant financial conflicts.
Poor Dental Health Noted in Children With Atopy
RALEIGH, N.C. – Pediatric atopic dermatitis has been linked to several previously unreported comorbidities – including increased rates of recurrent ear infections, bleeding gums and other dental problems, and visual difficulties – in a large, national population-based study.
While the dental findings need confirmation, the study provided supportive evidence on behalf of previously reported associations between atopic dermatitis and comorbid asthma and respiratory and food allergies. The study also broke new ground by demonstrating that the more severe the eczema, the greater the severity of the comorbid conditions, according to Dr. Jonathan I. Silverberg of St Luke’s–Roosevelt Hospital and Beth Israel Medical Center, New York.
"In general, food allergy, respiratory allergy, and asthma are more severe in children with concomitant eczema. In addition, the severity of these comorbidities correlates with the severity of the skin disease. Thus, aggressive treatment or prevention of atopic dermatitis might modify the risk of developing these comorbidities," he said.
He presented an analysis of the 2007 National Survey of Children’s Health, a Department of Health and Human Services–funded survey involving in-depth telephone interviews with parents in 91,642 households having one or more children under age 18 years.
The prevalence of pediatric eczema in this national study was 13%. The mean age of affected children was 7.5 years. Parents of kids with eczema characterized the skin disease as mild in 67% of cases, moderate in 26%, and severe in 7%.
Among the notable study findings:
– Among all children without eczema, 13.5% were black. However, black children accounted for 22.2% of all children with eczema.
– Children diagnosed with atopic dermatitis in the previous 12 months were significantly more likely than were children without eczema to have had food allergies in the past 12 months, by a margin of 15.1% to 3.6%. They also were more likely to have had asthma in the past 12 months (19.8% versus 7.9%), as well as respiratory allergies during the same time period (34.4%, compared with a 14.3% rate in children without eczema).
– Among children with asthma and mild or moderate atopic dermatitis, 5.5% had severe asthma in the past 12 months. But among those with comorbid severe atopic dermatitis, 36.1% had severe asthma. The same trend pertained to children with atopic dermatitis and comorbid hay fever or food allergies.
– Only 7.6% of children without eczema had impaired sleep for an average of 4 or more nights per week, compared with 10% of those with mild or moderate eczema and 22.2% of those with severe eczema.
– Recurrent ear infections within the past 12 months were reported for 6% of children with no eczema and 10.4% of those with eczema. Visual problems occurred in 1% of kids without eczema and 2.2% with eczema. These differences were significant; however, the rates of recurrent ear infections and visual problems didn’t rise with increasing eczema severity.
– In contrast, bleeding gums within the past 12 months occurred in 2.9% of children without atopic dermatitis, 4.3% with mild or moderate atopic dermatitis, and 12.6% of those with severe atopic dermatitis. Similarly, broken teeth within the last year were reported in 3.8% of subjects with no eczema, 5.1% with mild eczema, and 10.3% with severe eczema.
– Children with eczema were found to have poorer overall health and used a greater number of health-related services. Thirty-seven percent of them had seen a specialist within the past 12 months, compared with 21% of children without eczema.
Dr. Silverberg reported having no relevant financial disclosures.
RALEIGH, N.C. – Pediatric atopic dermatitis has been linked to several previously unreported comorbidities – including increased rates of recurrent ear infections, bleeding gums and other dental problems, and visual difficulties – in a large, national population-based study.
While the dental findings need confirmation, the study provided supportive evidence on behalf of previously reported associations between atopic dermatitis and comorbid asthma and respiratory and food allergies. The study also broke new ground by demonstrating that the more severe the eczema, the greater the severity of the comorbid conditions, according to Dr. Jonathan I. Silverberg of St Luke’s–Roosevelt Hospital and Beth Israel Medical Center, New York.
"In general, food allergy, respiratory allergy, and asthma are more severe in children with concomitant eczema. In addition, the severity of these comorbidities correlates with the severity of the skin disease. Thus, aggressive treatment or prevention of atopic dermatitis might modify the risk of developing these comorbidities," he said.
He presented an analysis of the 2007 National Survey of Children’s Health, a Department of Health and Human Services–funded survey involving in-depth telephone interviews with parents in 91,642 households having one or more children under age 18 years.
The prevalence of pediatric eczema in this national study was 13%. The mean age of affected children was 7.5 years. Parents of kids with eczema characterized the skin disease as mild in 67% of cases, moderate in 26%, and severe in 7%.
Among the notable study findings:
– Among all children without eczema, 13.5% were black. However, black children accounted for 22.2% of all children with eczema.
– Children diagnosed with atopic dermatitis in the previous 12 months were significantly more likely than were children without eczema to have had food allergies in the past 12 months, by a margin of 15.1% to 3.6%. They also were more likely to have had asthma in the past 12 months (19.8% versus 7.9%), as well as respiratory allergies during the same time period (34.4%, compared with a 14.3% rate in children without eczema).
– Among children with asthma and mild or moderate atopic dermatitis, 5.5% had severe asthma in the past 12 months. But among those with comorbid severe atopic dermatitis, 36.1% had severe asthma. The same trend pertained to children with atopic dermatitis and comorbid hay fever or food allergies.
– Only 7.6% of children without eczema had impaired sleep for an average of 4 or more nights per week, compared with 10% of those with mild or moderate eczema and 22.2% of those with severe eczema.
– Recurrent ear infections within the past 12 months were reported for 6% of children with no eczema and 10.4% of those with eczema. Visual problems occurred in 1% of kids without eczema and 2.2% with eczema. These differences were significant; however, the rates of recurrent ear infections and visual problems didn’t rise with increasing eczema severity.
– In contrast, bleeding gums within the past 12 months occurred in 2.9% of children without atopic dermatitis, 4.3% with mild or moderate atopic dermatitis, and 12.6% of those with severe atopic dermatitis. Similarly, broken teeth within the last year were reported in 3.8% of subjects with no eczema, 5.1% with mild eczema, and 10.3% with severe eczema.
– Children with eczema were found to have poorer overall health and used a greater number of health-related services. Thirty-seven percent of them had seen a specialist within the past 12 months, compared with 21% of children without eczema.
Dr. Silverberg reported having no relevant financial disclosures.
RALEIGH, N.C. – Pediatric atopic dermatitis has been linked to several previously unreported comorbidities – including increased rates of recurrent ear infections, bleeding gums and other dental problems, and visual difficulties – in a large, national population-based study.
While the dental findings need confirmation, the study provided supportive evidence on behalf of previously reported associations between atopic dermatitis and comorbid asthma and respiratory and food allergies. The study also broke new ground by demonstrating that the more severe the eczema, the greater the severity of the comorbid conditions, according to Dr. Jonathan I. Silverberg of St Luke’s–Roosevelt Hospital and Beth Israel Medical Center, New York.
"In general, food allergy, respiratory allergy, and asthma are more severe in children with concomitant eczema. In addition, the severity of these comorbidities correlates with the severity of the skin disease. Thus, aggressive treatment or prevention of atopic dermatitis might modify the risk of developing these comorbidities," he said.
He presented an analysis of the 2007 National Survey of Children’s Health, a Department of Health and Human Services–funded survey involving in-depth telephone interviews with parents in 91,642 households having one or more children under age 18 years.
The prevalence of pediatric eczema in this national study was 13%. The mean age of affected children was 7.5 years. Parents of kids with eczema characterized the skin disease as mild in 67% of cases, moderate in 26%, and severe in 7%.
Among the notable study findings:
– Among all children without eczema, 13.5% were black. However, black children accounted for 22.2% of all children with eczema.
– Children diagnosed with atopic dermatitis in the previous 12 months were significantly more likely than were children without eczema to have had food allergies in the past 12 months, by a margin of 15.1% to 3.6%. They also were more likely to have had asthma in the past 12 months (19.8% versus 7.9%), as well as respiratory allergies during the same time period (34.4%, compared with a 14.3% rate in children without eczema).
– Among children with asthma and mild or moderate atopic dermatitis, 5.5% had severe asthma in the past 12 months. But among those with comorbid severe atopic dermatitis, 36.1% had severe asthma. The same trend pertained to children with atopic dermatitis and comorbid hay fever or food allergies.
– Only 7.6% of children without eczema had impaired sleep for an average of 4 or more nights per week, compared with 10% of those with mild or moderate eczema and 22.2% of those with severe eczema.
– Recurrent ear infections within the past 12 months were reported for 6% of children with no eczema and 10.4% of those with eczema. Visual problems occurred in 1% of kids without eczema and 2.2% with eczema. These differences were significant; however, the rates of recurrent ear infections and visual problems didn’t rise with increasing eczema severity.
– In contrast, bleeding gums within the past 12 months occurred in 2.9% of children without atopic dermatitis, 4.3% with mild or moderate atopic dermatitis, and 12.6% of those with severe atopic dermatitis. Similarly, broken teeth within the last year were reported in 3.8% of subjects with no eczema, 5.1% with mild eczema, and 10.3% with severe eczema.
– Children with eczema were found to have poorer overall health and used a greater number of health-related services. Thirty-seven percent of them had seen a specialist within the past 12 months, compared with 21% of children without eczema.
Dr. Silverberg reported having no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: Bleeding gums within the past 12 months occurred in 2.9% of children without atopic dermatitis, 4.3% of those with mild or moderate atopic dermatitis, and 12.6% of those with severe atopic dermatitis.
Data Source: Researchers conducted a population-based study using data from the 2007 National Survey of Children’s Health, which entailed in-depth telephone interviews with parents in 91,642 households having one or more children under age 18 years.
Disclosures: Dr. Silverberg reported having no relevant financial disclosures.