User login
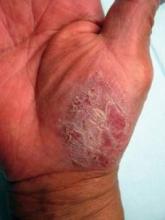
Vismodegib Proves Promising for Operable BCCs
RALEIGH, N.C. – Vismodegib, the Hedgehog pathway inhibitor approved earlier this year for the treatment of advanced basal cell carcinomas, is now being studied for the treatment of newly diagnosed, operable, nodular BCCs.
In the just-completed first part of an ongoing three-part phase II study, 23 of 24 patients (96%) showed a clinical response to 12 weeks of treatment with vismodegib (Erivedge) at the approved dosage of 150 mg once daily, Dr. Ivor Caro reported at the annual meeting of the Society for Investigative Dermatology.
Ten patients (42%) demonstrated complete histologic clearance at 12 weeks as assessed through independent review by two dermatopathologists after Mohs surgery, noted Dr. Caro, a dermatologist employed by Genentech in San Francisco.
Of note, complete histologic clearance didn’t correlate with the investigator-assessed clinical response at the 12-week time point. That is, of the 10 patients with complete histologic clearance of their tumor, only 5 were rated by clinicians as having a complete response. Another four were characterized by clinicians as having a partial response, and one was scored as having stable disease.
Adverse events were common, and rates were consistent with those seen in other studies of vismodegib. Muscle spasms occurred in 19 of 24 patients, dysgeusia or ageusia in 19, alopecia in 9, fatigue in 5, and nausea in 5 patients. Seven patients collectively experienced nine grade 3 adverse events, mostly muscle spasms.
The second part of the ongoing phase II study is aimed at evaluating the durability of vismodegib-induced complete histologic clearances. Twenty-five patients with newly diagnosed operable nodular BCCs are undergoing 12 weeks of once-daily therapy, followed by 24 weeks of observation, then excision for pathologic review and Mohs surgery for margin assessment.
The third part of the study, just now underway, will evaluate a dosing regimen designed to boost treatment efficacy through longer drug exposure while also improving tolerability. Twenty-five patients will receive once-daily vismodegib for 8 weeks, then 4 weeks off, followed by another 8 weeks of vismodegib, then excision and Mohs surgery.
"This approach is based upon the observation that when the drug is discontinued, adverse events recover very rapidly," Dr. Caro explained.
Roughly 80% of the 2.1 million nonmelanoma skin cancers diagnosed annually in the United States are BCCs, and about 60% of them are nodular BCCs. More than 90% of all BCCs have abnormalities in the Hedgehog signaling pathway that promote carcinogenesis and are hence potentially susceptible to vismodegib.
Dr. Caro is an employee of Genentech, sponsor of the phase II study.
RALEIGH, N.C. – Vismodegib, the Hedgehog pathway inhibitor approved earlier this year for the treatment of advanced basal cell carcinomas, is now being studied for the treatment of newly diagnosed, operable, nodular BCCs.
In the just-completed first part of an ongoing three-part phase II study, 23 of 24 patients (96%) showed a clinical response to 12 weeks of treatment with vismodegib (Erivedge) at the approved dosage of 150 mg once daily, Dr. Ivor Caro reported at the annual meeting of the Society for Investigative Dermatology.
Ten patients (42%) demonstrated complete histologic clearance at 12 weeks as assessed through independent review by two dermatopathologists after Mohs surgery, noted Dr. Caro, a dermatologist employed by Genentech in San Francisco.
Of note, complete histologic clearance didn’t correlate with the investigator-assessed clinical response at the 12-week time point. That is, of the 10 patients with complete histologic clearance of their tumor, only 5 were rated by clinicians as having a complete response. Another four were characterized by clinicians as having a partial response, and one was scored as having stable disease.
Adverse events were common, and rates were consistent with those seen in other studies of vismodegib. Muscle spasms occurred in 19 of 24 patients, dysgeusia or ageusia in 19, alopecia in 9, fatigue in 5, and nausea in 5 patients. Seven patients collectively experienced nine grade 3 adverse events, mostly muscle spasms.
The second part of the ongoing phase II study is aimed at evaluating the durability of vismodegib-induced complete histologic clearances. Twenty-five patients with newly diagnosed operable nodular BCCs are undergoing 12 weeks of once-daily therapy, followed by 24 weeks of observation, then excision for pathologic review and Mohs surgery for margin assessment.
The third part of the study, just now underway, will evaluate a dosing regimen designed to boost treatment efficacy through longer drug exposure while also improving tolerability. Twenty-five patients will receive once-daily vismodegib for 8 weeks, then 4 weeks off, followed by another 8 weeks of vismodegib, then excision and Mohs surgery.
"This approach is based upon the observation that when the drug is discontinued, adverse events recover very rapidly," Dr. Caro explained.
Roughly 80% of the 2.1 million nonmelanoma skin cancers diagnosed annually in the United States are BCCs, and about 60% of them are nodular BCCs. More than 90% of all BCCs have abnormalities in the Hedgehog signaling pathway that promote carcinogenesis and are hence potentially susceptible to vismodegib.
Dr. Caro is an employee of Genentech, sponsor of the phase II study.
RALEIGH, N.C. – Vismodegib, the Hedgehog pathway inhibitor approved earlier this year for the treatment of advanced basal cell carcinomas, is now being studied for the treatment of newly diagnosed, operable, nodular BCCs.
In the just-completed first part of an ongoing three-part phase II study, 23 of 24 patients (96%) showed a clinical response to 12 weeks of treatment with vismodegib (Erivedge) at the approved dosage of 150 mg once daily, Dr. Ivor Caro reported at the annual meeting of the Society for Investigative Dermatology.
Ten patients (42%) demonstrated complete histologic clearance at 12 weeks as assessed through independent review by two dermatopathologists after Mohs surgery, noted Dr. Caro, a dermatologist employed by Genentech in San Francisco.
Of note, complete histologic clearance didn’t correlate with the investigator-assessed clinical response at the 12-week time point. That is, of the 10 patients with complete histologic clearance of their tumor, only 5 were rated by clinicians as having a complete response. Another four were characterized by clinicians as having a partial response, and one was scored as having stable disease.
Adverse events were common, and rates were consistent with those seen in other studies of vismodegib. Muscle spasms occurred in 19 of 24 patients, dysgeusia or ageusia in 19, alopecia in 9, fatigue in 5, and nausea in 5 patients. Seven patients collectively experienced nine grade 3 adverse events, mostly muscle spasms.
The second part of the ongoing phase II study is aimed at evaluating the durability of vismodegib-induced complete histologic clearances. Twenty-five patients with newly diagnosed operable nodular BCCs are undergoing 12 weeks of once-daily therapy, followed by 24 weeks of observation, then excision for pathologic review and Mohs surgery for margin assessment.
The third part of the study, just now underway, will evaluate a dosing regimen designed to boost treatment efficacy through longer drug exposure while also improving tolerability. Twenty-five patients will receive once-daily vismodegib for 8 weeks, then 4 weeks off, followed by another 8 weeks of vismodegib, then excision and Mohs surgery.
"This approach is based upon the observation that when the drug is discontinued, adverse events recover very rapidly," Dr. Caro explained.
Roughly 80% of the 2.1 million nonmelanoma skin cancers diagnosed annually in the United States are BCCs, and about 60% of them are nodular BCCs. More than 90% of all BCCs have abnormalities in the Hedgehog signaling pathway that promote carcinogenesis and are hence potentially susceptible to vismodegib.
Dr. Caro is an employee of Genentech, sponsor of the phase II study.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: Twelve weeks of once-daily oral vismodegib resulted in complete histologic clearance in 10 of 24 patients with newly diagnosed, operable, nodular BCCs.
Data Source: An open-label phase II study involving 24 patients.
Disclosures: Dr. Caro is an employee of Genentech, sponsor of the study.
High-Dose Ustekinumab Stomps Out Palmoplantar Psoriasis
RALEIGH, N.C. – Ustekinumab proved effective for the clearance of palmoplantar psoriasis in an open-label pilot study, but only at the higher 90-mg dose reserved under current labeling for psoriasis patients weighing at least 100 kg.
"Higher or more frequent dosing may be required to achieve clinical clearance in patients with palmoplantar psoriasis compared to current recommendations for plaque-type psoriasis," said Dr. Shiu-Chung Au of Tufts Medical Center, Boston.
Dr. Au presented an open-label, 24-week study of 20 patients with moderate to severe palmoplantar psoriasis that was refractory to topical corticosteroids. Dosing of ustekinumab (Stelara) was as for plaque-type psoriasis: The 11 patients weighing less than 100 kg received 45 mg subcutaneously at weeks 0, 4, and 16, whereas the 9 patients weighing 100 kg or more received 90 mg on the same schedule.
The primary study end point was a Palm-Sole PGA (Physician’s Global Assessment) score of 0 or 1 at week 16. The average baseline score on this 0-5 measure of induration, erythema, and scaling was 4. The primary end point was achieved in 1 of 11 patients on the 45-mg dose, compared with 6 of 9 on the 90-mg dose (J. Dermatolog. Treat. 2012 May 8. [Epub ahead of print]). Of 20 patients, 12 improved at least 2 points on the Palm-Sole PGA at week 16.
In addition, significant improvements were documented with respect to the secondary end points for pain and quality of life. The mean pain score on a 100-point visual analog scale improved from 51 at week 0 to 29 at week 16, and held steady with a score of 30 at 24 weeks. Moreover, the mean score on the Dermatology Life Quality Index improved from 13.9 at baseline to 6.05 at week 16 and to 6.18 at week 24.
Ustekinumab was well tolerated with no serious adverse events.
Palmoplantar psoriasis is an uncommon variant of psoriasis that constitutes a therapeutic challenge, noted Dr. Au. It can be disfiguring and disabling, even though affected patients don’t necessarily have a large area of body surface involvement. The condition is characterized by fissures and sterile inflammatory pustules, in addition to classic well-demarcated psoriasis plaques. Affected patients experience considerable skin pain, often accompanied by a burning sensation. Some patients are unable to walk.
Clinically, palmoplantar psoriasis can look like other diseases, including palmoplantar pustulosis, dyshidrotic eczema, tinea pedis, and contact dermatitis, he explained. To help eliminate other possible diagnoses in the differential diagnosis, participants in the ustekinumab study had to have at least one classic psoriasis plaque somewhere other than the palms and soles.
Current treatment of palmoplantar psoriasis has been unsatisfactory, noted Dr. Au. No standard therapy is recognized, which was the impetus for studying ustekinumab. Future studies will look at employing the 90-mg dose in patients who weigh less than 100 kg and/or administering the 45-mg dose more frequently than the current standard every 3 months, he added.
In response to an audience question, he noted that responsiveness to the 90-mg dose was not weight related. The average weight in the high-dose group was 250 pounds, and the three nonresponders weighed in similarly to the six responders.
This investigator-initiated ustekinumab study was funded by a grant from Janssen Pharmaceuticals. Dr. Au reported having no financial conflicts. Senior investigator Dr. Alice B. Gottlieb reported having numerous industry relationships.
RALEIGH, N.C. – Ustekinumab proved effective for the clearance of palmoplantar psoriasis in an open-label pilot study, but only at the higher 90-mg dose reserved under current labeling for psoriasis patients weighing at least 100 kg.
"Higher or more frequent dosing may be required to achieve clinical clearance in patients with palmoplantar psoriasis compared to current recommendations for plaque-type psoriasis," said Dr. Shiu-Chung Au of Tufts Medical Center, Boston.
Dr. Au presented an open-label, 24-week study of 20 patients with moderate to severe palmoplantar psoriasis that was refractory to topical corticosteroids. Dosing of ustekinumab (Stelara) was as for plaque-type psoriasis: The 11 patients weighing less than 100 kg received 45 mg subcutaneously at weeks 0, 4, and 16, whereas the 9 patients weighing 100 kg or more received 90 mg on the same schedule.
The primary study end point was a Palm-Sole PGA (Physician’s Global Assessment) score of 0 or 1 at week 16. The average baseline score on this 0-5 measure of induration, erythema, and scaling was 4. The primary end point was achieved in 1 of 11 patients on the 45-mg dose, compared with 6 of 9 on the 90-mg dose (J. Dermatolog. Treat. 2012 May 8. [Epub ahead of print]). Of 20 patients, 12 improved at least 2 points on the Palm-Sole PGA at week 16.
In addition, significant improvements were documented with respect to the secondary end points for pain and quality of life. The mean pain score on a 100-point visual analog scale improved from 51 at week 0 to 29 at week 16, and held steady with a score of 30 at 24 weeks. Moreover, the mean score on the Dermatology Life Quality Index improved from 13.9 at baseline to 6.05 at week 16 and to 6.18 at week 24.
Ustekinumab was well tolerated with no serious adverse events.
Palmoplantar psoriasis is an uncommon variant of psoriasis that constitutes a therapeutic challenge, noted Dr. Au. It can be disfiguring and disabling, even though affected patients don’t necessarily have a large area of body surface involvement. The condition is characterized by fissures and sterile inflammatory pustules, in addition to classic well-demarcated psoriasis plaques. Affected patients experience considerable skin pain, often accompanied by a burning sensation. Some patients are unable to walk.
Clinically, palmoplantar psoriasis can look like other diseases, including palmoplantar pustulosis, dyshidrotic eczema, tinea pedis, and contact dermatitis, he explained. To help eliminate other possible diagnoses in the differential diagnosis, participants in the ustekinumab study had to have at least one classic psoriasis plaque somewhere other than the palms and soles.
Current treatment of palmoplantar psoriasis has been unsatisfactory, noted Dr. Au. No standard therapy is recognized, which was the impetus for studying ustekinumab. Future studies will look at employing the 90-mg dose in patients who weigh less than 100 kg and/or administering the 45-mg dose more frequently than the current standard every 3 months, he added.
In response to an audience question, he noted that responsiveness to the 90-mg dose was not weight related. The average weight in the high-dose group was 250 pounds, and the three nonresponders weighed in similarly to the six responders.
This investigator-initiated ustekinumab study was funded by a grant from Janssen Pharmaceuticals. Dr. Au reported having no financial conflicts. Senior investigator Dr. Alice B. Gottlieb reported having numerous industry relationships.
RALEIGH, N.C. – Ustekinumab proved effective for the clearance of palmoplantar psoriasis in an open-label pilot study, but only at the higher 90-mg dose reserved under current labeling for psoriasis patients weighing at least 100 kg.
"Higher or more frequent dosing may be required to achieve clinical clearance in patients with palmoplantar psoriasis compared to current recommendations for plaque-type psoriasis," said Dr. Shiu-Chung Au of Tufts Medical Center, Boston.
Dr. Au presented an open-label, 24-week study of 20 patients with moderate to severe palmoplantar psoriasis that was refractory to topical corticosteroids. Dosing of ustekinumab (Stelara) was as for plaque-type psoriasis: The 11 patients weighing less than 100 kg received 45 mg subcutaneously at weeks 0, 4, and 16, whereas the 9 patients weighing 100 kg or more received 90 mg on the same schedule.
The primary study end point was a Palm-Sole PGA (Physician’s Global Assessment) score of 0 or 1 at week 16. The average baseline score on this 0-5 measure of induration, erythema, and scaling was 4. The primary end point was achieved in 1 of 11 patients on the 45-mg dose, compared with 6 of 9 on the 90-mg dose (J. Dermatolog. Treat. 2012 May 8. [Epub ahead of print]). Of 20 patients, 12 improved at least 2 points on the Palm-Sole PGA at week 16.
In addition, significant improvements were documented with respect to the secondary end points for pain and quality of life. The mean pain score on a 100-point visual analog scale improved from 51 at week 0 to 29 at week 16, and held steady with a score of 30 at 24 weeks. Moreover, the mean score on the Dermatology Life Quality Index improved from 13.9 at baseline to 6.05 at week 16 and to 6.18 at week 24.
Ustekinumab was well tolerated with no serious adverse events.
Palmoplantar psoriasis is an uncommon variant of psoriasis that constitutes a therapeutic challenge, noted Dr. Au. It can be disfiguring and disabling, even though affected patients don’t necessarily have a large area of body surface involvement. The condition is characterized by fissures and sterile inflammatory pustules, in addition to classic well-demarcated psoriasis plaques. Affected patients experience considerable skin pain, often accompanied by a burning sensation. Some patients are unable to walk.
Clinically, palmoplantar psoriasis can look like other diseases, including palmoplantar pustulosis, dyshidrotic eczema, tinea pedis, and contact dermatitis, he explained. To help eliminate other possible diagnoses in the differential diagnosis, participants in the ustekinumab study had to have at least one classic psoriasis plaque somewhere other than the palms and soles.
Current treatment of palmoplantar psoriasis has been unsatisfactory, noted Dr. Au. No standard therapy is recognized, which was the impetus for studying ustekinumab. Future studies will look at employing the 90-mg dose in patients who weigh less than 100 kg and/or administering the 45-mg dose more frequently than the current standard every 3 months, he added.
In response to an audience question, he noted that responsiveness to the 90-mg dose was not weight related. The average weight in the high-dose group was 250 pounds, and the three nonresponders weighed in similarly to the six responders.
This investigator-initiated ustekinumab study was funded by a grant from Janssen Pharmaceuticals. Dr. Au reported having no financial conflicts. Senior investigator Dr. Alice B. Gottlieb reported having numerous industry relationships.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: The primary endpoint of a palm-sole PGA score of 0 or 1 was achieved in 1 of 11 patients on the 45-mg dose, compared with 6 of 9 on the 90-mg dose
Data Source: An open-label, 24-week study of 20 patients treated with ustekinumab for moderate-to-severe palmoplantar psoriasis refractory to topical corticosteroids.
Disclosures: This investigator-initiated study was funded by a grant from Janssen Pharmaceuticals. Dr. Au reported having no financial conflicts. Senior investigator, Dr. Alice B. Gottlieb, reported having numerous industry relationships.
Psoriasis Boosts Crohn's Risk Fourfold
RALEIGH, N.C. – Psoriasis patients are at a nearly fourfold increased risk of developing Crohn’s disease, and the risk is higher in psoriatic arthritis patients.
These findings from 174,646 prospectively followed participants in the Nurses’ Health Study and the Nurses’ Health Study II are consistent with the results of genomewide association studies which have found susceptibility genes common to both psoriasis and inflammatory bowel disease, especially genes in the interleukin-23 pathway, Dr. Wenqing Li said at the annual meeting of the Society for Investigative Dermatology.
"Further understanding of the mechanisms that mediate both psoriasis and Crohn’s disease could eventually lead to elucidation of new targets for interventions that may modulate the incidence or activity of both diseases," added Dr. Li of Harvard Medical School, Boston.
Of note, the psoriasis patients were not at significantly increased risk for developing ulcerative colitis. This suggests that psoriasis may share fewer overlapping pathways with ulcerative colitis than it does with Crohn’s disease, he continued.
Women in the Nurses’ Health Study were prospectively followed from 1996 to 2008, whereas those in the NHS II were followed from 1991 to 2005. During follow-up, there were 188 incident cases of Crohn’s disease and 240 of ulcerative colitis in the study population. All diagnoses of inflammatory bowel disease were confirmed by two gastroenterologists blinded as to whether or not the affected patients also had psoriasis, Dr. Li noted.
The combined analysis of the two studies included 47,618 person-years of prospective follow-up of psoriasis patients and 2,401,883 person-years of follow-up of participants without psoriasis. The psoriasis patients had an age-adjusted 3.74-fold increased risk of developing Crohn’s disease.
Having psoriasis was still an independent risk factor for Crohn’s disease, with an associated 3.5-fold relative risk, in a multivariate analysis that was adjusted for body mass index, physical activity, smoking status, alcohol consumption, use of oral contraceptives, and postmenopausal hormone therapy as well as age.
Based upon 5,661 person-years of prospective follow-up of subjects with psoriasis and comorbid psoriatic arthritis, affected patients had a 6.8-fold increased of Crohn’s disease in a multivariate analysis.
Multivariate analysis was appropriate because patients with psoriasis had a higher BMI, tended to be older, consumed more alcohol, and were less physically active than were those without psoriasis. The psoriasis patients were also more likely to be current smokers, users of oral contraceptives, and current users of postmenopausal hormone therapy.
The risk of new-onset Crohn’s disease was significantly greater among psoriasis patients whose dermatologic disease was diagnosed when they were younger than 40 years than it was among those diagnosed later in life. The risk was also greater in those with at least a 10-year history of active psoriasis. However, as 87% of patients with psoriasis had mild skin disease based upon the involved body surface area, this study didn’t have sufficient power to determine if the risk of Crohn’s disease was greater in individuals with more severe psoriasis, according to Dr. Li.
To ensure that the increased risk of inflammatory bowel disease associated with having psoriasis wasn’t in some way affected by the use of tumor necrosis factor inhibitors to treat psoriasis, Dr. Li and coinvestigators conducted a separate analysis restricting follow-up through 2004, the year the biologic therapies were approved for psoriasis. This didn’t change the results.
The studies were sponsored by the National Institutes of Health. Dr. Li reported having no financial conflicts.
RALEIGH, N.C. – Psoriasis patients are at a nearly fourfold increased risk of developing Crohn’s disease, and the risk is higher in psoriatic arthritis patients.
These findings from 174,646 prospectively followed participants in the Nurses’ Health Study and the Nurses’ Health Study II are consistent with the results of genomewide association studies which have found susceptibility genes common to both psoriasis and inflammatory bowel disease, especially genes in the interleukin-23 pathway, Dr. Wenqing Li said at the annual meeting of the Society for Investigative Dermatology.
"Further understanding of the mechanisms that mediate both psoriasis and Crohn’s disease could eventually lead to elucidation of new targets for interventions that may modulate the incidence or activity of both diseases," added Dr. Li of Harvard Medical School, Boston.
Of note, the psoriasis patients were not at significantly increased risk for developing ulcerative colitis. This suggests that psoriasis may share fewer overlapping pathways with ulcerative colitis than it does with Crohn’s disease, he continued.
Women in the Nurses’ Health Study were prospectively followed from 1996 to 2008, whereas those in the NHS II were followed from 1991 to 2005. During follow-up, there were 188 incident cases of Crohn’s disease and 240 of ulcerative colitis in the study population. All diagnoses of inflammatory bowel disease were confirmed by two gastroenterologists blinded as to whether or not the affected patients also had psoriasis, Dr. Li noted.
The combined analysis of the two studies included 47,618 person-years of prospective follow-up of psoriasis patients and 2,401,883 person-years of follow-up of participants without psoriasis. The psoriasis patients had an age-adjusted 3.74-fold increased risk of developing Crohn’s disease.
Having psoriasis was still an independent risk factor for Crohn’s disease, with an associated 3.5-fold relative risk, in a multivariate analysis that was adjusted for body mass index, physical activity, smoking status, alcohol consumption, use of oral contraceptives, and postmenopausal hormone therapy as well as age.
Based upon 5,661 person-years of prospective follow-up of subjects with psoriasis and comorbid psoriatic arthritis, affected patients had a 6.8-fold increased of Crohn’s disease in a multivariate analysis.
Multivariate analysis was appropriate because patients with psoriasis had a higher BMI, tended to be older, consumed more alcohol, and were less physically active than were those without psoriasis. The psoriasis patients were also more likely to be current smokers, users of oral contraceptives, and current users of postmenopausal hormone therapy.
The risk of new-onset Crohn’s disease was significantly greater among psoriasis patients whose dermatologic disease was diagnosed when they were younger than 40 years than it was among those diagnosed later in life. The risk was also greater in those with at least a 10-year history of active psoriasis. However, as 87% of patients with psoriasis had mild skin disease based upon the involved body surface area, this study didn’t have sufficient power to determine if the risk of Crohn’s disease was greater in individuals with more severe psoriasis, according to Dr. Li.
To ensure that the increased risk of inflammatory bowel disease associated with having psoriasis wasn’t in some way affected by the use of tumor necrosis factor inhibitors to treat psoriasis, Dr. Li and coinvestigators conducted a separate analysis restricting follow-up through 2004, the year the biologic therapies were approved for psoriasis. This didn’t change the results.
The studies were sponsored by the National Institutes of Health. Dr. Li reported having no financial conflicts.
RALEIGH, N.C. – Psoriasis patients are at a nearly fourfold increased risk of developing Crohn’s disease, and the risk is higher in psoriatic arthritis patients.
These findings from 174,646 prospectively followed participants in the Nurses’ Health Study and the Nurses’ Health Study II are consistent with the results of genomewide association studies which have found susceptibility genes common to both psoriasis and inflammatory bowel disease, especially genes in the interleukin-23 pathway, Dr. Wenqing Li said at the annual meeting of the Society for Investigative Dermatology.
"Further understanding of the mechanisms that mediate both psoriasis and Crohn’s disease could eventually lead to elucidation of new targets for interventions that may modulate the incidence or activity of both diseases," added Dr. Li of Harvard Medical School, Boston.
Of note, the psoriasis patients were not at significantly increased risk for developing ulcerative colitis. This suggests that psoriasis may share fewer overlapping pathways with ulcerative colitis than it does with Crohn’s disease, he continued.
Women in the Nurses’ Health Study were prospectively followed from 1996 to 2008, whereas those in the NHS II were followed from 1991 to 2005. During follow-up, there were 188 incident cases of Crohn’s disease and 240 of ulcerative colitis in the study population. All diagnoses of inflammatory bowel disease were confirmed by two gastroenterologists blinded as to whether or not the affected patients also had psoriasis, Dr. Li noted.
The combined analysis of the two studies included 47,618 person-years of prospective follow-up of psoriasis patients and 2,401,883 person-years of follow-up of participants without psoriasis. The psoriasis patients had an age-adjusted 3.74-fold increased risk of developing Crohn’s disease.
Having psoriasis was still an independent risk factor for Crohn’s disease, with an associated 3.5-fold relative risk, in a multivariate analysis that was adjusted for body mass index, physical activity, smoking status, alcohol consumption, use of oral contraceptives, and postmenopausal hormone therapy as well as age.
Based upon 5,661 person-years of prospective follow-up of subjects with psoriasis and comorbid psoriatic arthritis, affected patients had a 6.8-fold increased of Crohn’s disease in a multivariate analysis.
Multivariate analysis was appropriate because patients with psoriasis had a higher BMI, tended to be older, consumed more alcohol, and were less physically active than were those without psoriasis. The psoriasis patients were also more likely to be current smokers, users of oral contraceptives, and current users of postmenopausal hormone therapy.
The risk of new-onset Crohn’s disease was significantly greater among psoriasis patients whose dermatologic disease was diagnosed when they were younger than 40 years than it was among those diagnosed later in life. The risk was also greater in those with at least a 10-year history of active psoriasis. However, as 87% of patients with psoriasis had mild skin disease based upon the involved body surface area, this study didn’t have sufficient power to determine if the risk of Crohn’s disease was greater in individuals with more severe psoriasis, according to Dr. Li.
To ensure that the increased risk of inflammatory bowel disease associated with having psoriasis wasn’t in some way affected by the use of tumor necrosis factor inhibitors to treat psoriasis, Dr. Li and coinvestigators conducted a separate analysis restricting follow-up through 2004, the year the biologic therapies were approved for psoriasis. This didn’t change the results.
The studies were sponsored by the National Institutes of Health. Dr. Li reported having no financial conflicts.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: Psoriasis was an independent risk factor for the development of Crohn’s disease, with an associated age-adjusted 3.74-fold increased risk.
Data Source: Data were from an analysis that included 174,646 participants in the prospective, longitudinal Nurses’ Health Study and Nurses’ Health Study II.
Disclosures: The studies were sponsored by the National Institutes of Health. Dr. Li reported having no financial conflicts.
Gene Shields Psoriasis Patients from Heart Disease
RALEIGH, N.C. – Psoriasis patients who possess the HLA-Cw6 allele appear to be protected against the increased risk of cardiovascular and metabolic comorbidities associated with the disease.
In a study that included 199 psoriasis patients under the age of 40, those who carried the histocompatibility antigen HLA-Cw6 allele had a 66% reduction in the prevalence of comorbid hypertension and a 72% reduction in dyslipidemia, compared with HLA-Cw6-negative psoriasis patients, according to Dr. Trilokraj Tejasvi of the University of Michigan, Ann Arbor.
This is a particularly intriguing finding, he noted, because previous studies have demonstrated that HLA-Cw6-positive psoriasis patients tend to have more severe skin disease, an earlier age at disease onset, higher rates of the guttate and eruptive forms of psoriasis as well as Koebner phenomenon, and more extensive disease (J. Invest. Dermatol. 2002;118:362-5).
Moreover, other studies have shown that patients with more severe psoriasis tend to have higher rates of comorbid cardiovascular and metabolic disorders. For example, a large recent study that included 4,065 psoriasis patients aged 45-65 years and nearly 41,000 age- and physician practice-matched controls showed that the risk of comorbid metabolic syndrome increased from 1.2-fold in patients with mild psoriasis to twofold in those with severe psoriasis, compared with controls (J. Invest. Dermatol. 2012;132:556-62).
A strikingly similar twofold increased risk of the metabolic syndrome in psoriasis patients was recently reported based upon data from the National Health and Nutrition Examination Survey for 2003-2006 (Arch. Dermatol. 2011;147:419-24).
Since neither the NHANES psoriasis patients nor those in the U.K. study underwent genotyping, there is no way of knowing what proportion of those who were HLA-Cw6-positive had the metabolic syndrome, Dr. Tejasvi noted.
In a recent, still-to-be-published genome-wide association study, he and his colleagues found that all known psoriasis-associated single nucleotide polymorphisms explained roughly 22% of the disease’s heritability – and nearly half of were because of genes located at HLA-C.
The study included 1,134 psoriasis patients and 1,174 unaffected controls who underwent genomic DNA analysis. He and his coinvestigators examined the rates of hypertension, dyslipidemia, acute MI, and diabetes mellitus in the two groups.
One analysis was restricted to the 199 psoriasis patients and 392 controls under age 40, since the comorbid conditions under scrutiny tend to less frequently in younger people. In this under-40 analysis, the prevalence of diabetes wasn’t significantly different between psoriasis patients and controls. Neither was a history of MI.
However, 15% of the younger psoriasis patients carried the diagnosis of hypertension, compared with 6.8% of controls, and 29% of the psoriasis patients were dyslipidemic, compared with 10.5% of controls. Thus, psoriasis patients under age 40 were 2.2-fold more likely than controls to be hypertensive and 2.75-fold more likely to be dyslipidemic. Both differences were highly significant.
The prevalence of hypertension among 80 HLA-Cw6-positive psoriasis patients under age 40 was 7.5%, compared with 22% in HLA-Cw6-negative psoriasis patients under age 40. And the prevalence of dyslipidemia in the HLA-Cw6-positive group was 12.5% vs. 44% in the HLA-Cw6-negative psoriasis patients.
The under-40 psoriatic HLA-Cw6 carriers and noncarriers were essentially the same in terms of body mass index – a mean of 28 kg/m2 in both groups – and in mean age at evaluation, which was 28 years in the HLa-Cw6-positive patients and 29 years in the HLA-Cw6-negative cohort, Dr. Tejasvi reported.
Several key findings stood out in the analysis of the overall study population comprised of 1,134 psoriasis patients and 1,174 controls. One was that among control patients without psoriasis, HLA-Cw6 status was unrelated to comorbid hypertension, dyslipidemia, diabetes, or a positive history for MI. And in the full group of psoriasis patients, hypertension and dyslipidemia remained significantly less common among those who were HLA-Cw6-negative, although the association was less robust than in the below-40 subgroup. Specifically, HLA-Cw6 carriers with psoriasis were 24% less likely to be hypertensive and 25% less likely to have dyslipidemia than psoriatic HLAL-Cw6 noncarriers.
Dr. Tejasvi commented that an HLA-Cw6 protective effect against hypertension and dyslipidemia isn’t the only possible explanation for the lower rates of these two components of the metabolic syndrome found in HLA-Cw6-positive psoriasis patients. The alternate possibility is that HLA-Cw6 noncarrier status in psoriasis patients is associated with other genetic loci that increase the risk of dyslipidemia and hypertension. Sorting this out will require a prospective study with a large sample size.
The study was sponsored by the National Institutes of Health. Dr. Tejasvi reported having no financial conflicts.
RALEIGH, N.C. – Psoriasis patients who possess the HLA-Cw6 allele appear to be protected against the increased risk of cardiovascular and metabolic comorbidities associated with the disease.
In a study that included 199 psoriasis patients under the age of 40, those who carried the histocompatibility antigen HLA-Cw6 allele had a 66% reduction in the prevalence of comorbid hypertension and a 72% reduction in dyslipidemia, compared with HLA-Cw6-negative psoriasis patients, according to Dr. Trilokraj Tejasvi of the University of Michigan, Ann Arbor.
This is a particularly intriguing finding, he noted, because previous studies have demonstrated that HLA-Cw6-positive psoriasis patients tend to have more severe skin disease, an earlier age at disease onset, higher rates of the guttate and eruptive forms of psoriasis as well as Koebner phenomenon, and more extensive disease (J. Invest. Dermatol. 2002;118:362-5).
Moreover, other studies have shown that patients with more severe psoriasis tend to have higher rates of comorbid cardiovascular and metabolic disorders. For example, a large recent study that included 4,065 psoriasis patients aged 45-65 years and nearly 41,000 age- and physician practice-matched controls showed that the risk of comorbid metabolic syndrome increased from 1.2-fold in patients with mild psoriasis to twofold in those with severe psoriasis, compared with controls (J. Invest. Dermatol. 2012;132:556-62).
A strikingly similar twofold increased risk of the metabolic syndrome in psoriasis patients was recently reported based upon data from the National Health and Nutrition Examination Survey for 2003-2006 (Arch. Dermatol. 2011;147:419-24).
Since neither the NHANES psoriasis patients nor those in the U.K. study underwent genotyping, there is no way of knowing what proportion of those who were HLA-Cw6-positive had the metabolic syndrome, Dr. Tejasvi noted.
In a recent, still-to-be-published genome-wide association study, he and his colleagues found that all known psoriasis-associated single nucleotide polymorphisms explained roughly 22% of the disease’s heritability – and nearly half of were because of genes located at HLA-C.
The study included 1,134 psoriasis patients and 1,174 unaffected controls who underwent genomic DNA analysis. He and his coinvestigators examined the rates of hypertension, dyslipidemia, acute MI, and diabetes mellitus in the two groups.
One analysis was restricted to the 199 psoriasis patients and 392 controls under age 40, since the comorbid conditions under scrutiny tend to less frequently in younger people. In this under-40 analysis, the prevalence of diabetes wasn’t significantly different between psoriasis patients and controls. Neither was a history of MI.
However, 15% of the younger psoriasis patients carried the diagnosis of hypertension, compared with 6.8% of controls, and 29% of the psoriasis patients were dyslipidemic, compared with 10.5% of controls. Thus, psoriasis patients under age 40 were 2.2-fold more likely than controls to be hypertensive and 2.75-fold more likely to be dyslipidemic. Both differences were highly significant.
The prevalence of hypertension among 80 HLA-Cw6-positive psoriasis patients under age 40 was 7.5%, compared with 22% in HLA-Cw6-negative psoriasis patients under age 40. And the prevalence of dyslipidemia in the HLA-Cw6-positive group was 12.5% vs. 44% in the HLA-Cw6-negative psoriasis patients.
The under-40 psoriatic HLA-Cw6 carriers and noncarriers were essentially the same in terms of body mass index – a mean of 28 kg/m2 in both groups – and in mean age at evaluation, which was 28 years in the HLa-Cw6-positive patients and 29 years in the HLA-Cw6-negative cohort, Dr. Tejasvi reported.
Several key findings stood out in the analysis of the overall study population comprised of 1,134 psoriasis patients and 1,174 controls. One was that among control patients without psoriasis, HLA-Cw6 status was unrelated to comorbid hypertension, dyslipidemia, diabetes, or a positive history for MI. And in the full group of psoriasis patients, hypertension and dyslipidemia remained significantly less common among those who were HLA-Cw6-negative, although the association was less robust than in the below-40 subgroup. Specifically, HLA-Cw6 carriers with psoriasis were 24% less likely to be hypertensive and 25% less likely to have dyslipidemia than psoriatic HLAL-Cw6 noncarriers.
Dr. Tejasvi commented that an HLA-Cw6 protective effect against hypertension and dyslipidemia isn’t the only possible explanation for the lower rates of these two components of the metabolic syndrome found in HLA-Cw6-positive psoriasis patients. The alternate possibility is that HLA-Cw6 noncarrier status in psoriasis patients is associated with other genetic loci that increase the risk of dyslipidemia and hypertension. Sorting this out will require a prospective study with a large sample size.
The study was sponsored by the National Institutes of Health. Dr. Tejasvi reported having no financial conflicts.
RALEIGH, N.C. – Psoriasis patients who possess the HLA-Cw6 allele appear to be protected against the increased risk of cardiovascular and metabolic comorbidities associated with the disease.
In a study that included 199 psoriasis patients under the age of 40, those who carried the histocompatibility antigen HLA-Cw6 allele had a 66% reduction in the prevalence of comorbid hypertension and a 72% reduction in dyslipidemia, compared with HLA-Cw6-negative psoriasis patients, according to Dr. Trilokraj Tejasvi of the University of Michigan, Ann Arbor.
This is a particularly intriguing finding, he noted, because previous studies have demonstrated that HLA-Cw6-positive psoriasis patients tend to have more severe skin disease, an earlier age at disease onset, higher rates of the guttate and eruptive forms of psoriasis as well as Koebner phenomenon, and more extensive disease (J. Invest. Dermatol. 2002;118:362-5).
Moreover, other studies have shown that patients with more severe psoriasis tend to have higher rates of comorbid cardiovascular and metabolic disorders. For example, a large recent study that included 4,065 psoriasis patients aged 45-65 years and nearly 41,000 age- and physician practice-matched controls showed that the risk of comorbid metabolic syndrome increased from 1.2-fold in patients with mild psoriasis to twofold in those with severe psoriasis, compared with controls (J. Invest. Dermatol. 2012;132:556-62).
A strikingly similar twofold increased risk of the metabolic syndrome in psoriasis patients was recently reported based upon data from the National Health and Nutrition Examination Survey for 2003-2006 (Arch. Dermatol. 2011;147:419-24).
Since neither the NHANES psoriasis patients nor those in the U.K. study underwent genotyping, there is no way of knowing what proportion of those who were HLA-Cw6-positive had the metabolic syndrome, Dr. Tejasvi noted.
In a recent, still-to-be-published genome-wide association study, he and his colleagues found that all known psoriasis-associated single nucleotide polymorphisms explained roughly 22% of the disease’s heritability – and nearly half of were because of genes located at HLA-C.
The study included 1,134 psoriasis patients and 1,174 unaffected controls who underwent genomic DNA analysis. He and his coinvestigators examined the rates of hypertension, dyslipidemia, acute MI, and diabetes mellitus in the two groups.
One analysis was restricted to the 199 psoriasis patients and 392 controls under age 40, since the comorbid conditions under scrutiny tend to less frequently in younger people. In this under-40 analysis, the prevalence of diabetes wasn’t significantly different between psoriasis patients and controls. Neither was a history of MI.
However, 15% of the younger psoriasis patients carried the diagnosis of hypertension, compared with 6.8% of controls, and 29% of the psoriasis patients were dyslipidemic, compared with 10.5% of controls. Thus, psoriasis patients under age 40 were 2.2-fold more likely than controls to be hypertensive and 2.75-fold more likely to be dyslipidemic. Both differences were highly significant.
The prevalence of hypertension among 80 HLA-Cw6-positive psoriasis patients under age 40 was 7.5%, compared with 22% in HLA-Cw6-negative psoriasis patients under age 40. And the prevalence of dyslipidemia in the HLA-Cw6-positive group was 12.5% vs. 44% in the HLA-Cw6-negative psoriasis patients.
The under-40 psoriatic HLA-Cw6 carriers and noncarriers were essentially the same in terms of body mass index – a mean of 28 kg/m2 in both groups – and in mean age at evaluation, which was 28 years in the HLa-Cw6-positive patients and 29 years in the HLA-Cw6-negative cohort, Dr. Tejasvi reported.
Several key findings stood out in the analysis of the overall study population comprised of 1,134 psoriasis patients and 1,174 controls. One was that among control patients without psoriasis, HLA-Cw6 status was unrelated to comorbid hypertension, dyslipidemia, diabetes, or a positive history for MI. And in the full group of psoriasis patients, hypertension and dyslipidemia remained significantly less common among those who were HLA-Cw6-negative, although the association was less robust than in the below-40 subgroup. Specifically, HLA-Cw6 carriers with psoriasis were 24% less likely to be hypertensive and 25% less likely to have dyslipidemia than psoriatic HLAL-Cw6 noncarriers.
Dr. Tejasvi commented that an HLA-Cw6 protective effect against hypertension and dyslipidemia isn’t the only possible explanation for the lower rates of these two components of the metabolic syndrome found in HLA-Cw6-positive psoriasis patients. The alternate possibility is that HLA-Cw6 noncarrier status in psoriasis patients is associated with other genetic loci that increase the risk of dyslipidemia and hypertension. Sorting this out will require a prospective study with a large sample size.
The study was sponsored by the National Institutes of Health. Dr. Tejasvi reported having no financial conflicts.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: The prevalence of hypertension among 80 HLA-Cw6-positive psoriasis patients under age 40 was 7.5%, compared with 22% in HLA-Cw6-negative psoriasis patients under age 40.
Data Source: A genomic DNA analysis of 1,134 psoriasis patients and 1,174 unaffected controls, which included 591 participants under age 40.
Disclosures: The study was sponsored by the National Institutes of Health. Dr. Tejasvi reported having no financial conflicts.
SCREEN: Melanoma Deaths Reduced by Half in Largest Study Ever
RALEIGH, N.C. – A population-based total-body skin cancer screening program reduces melanoma mortality, according to the results of a landmark German project presented at the annual meeting of the Society for Investigative Dermatology.
"I would argue that this is the most important presentation anywhere at this meeting," Dr. Martin A. Weinstock said during his presentation of the SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) project.
"The reason I make that argument is very simply because melanoma accounts for more deaths than anything else in the dermatology world. If we want to reduce that number of deaths, the best way is [through] early detection – and SCREEN provides the best evidence to date that we can do that," explained Dr. Weinstock, professor of dermatology and epidemiology at Brown University, Providence, R.I., and a SCREEN project organizer.
SCREEN was a population-based skin cancer screening program in which all residents over age 20 in the German federal state of Schleswig-Holstein were encouraged to undergo a standardized whole-body skin examination between July 2003 and June 2004. Nineteen percent of the state’s eligible population – 360,288 individuals – participated. Half of the 1,169 melanomas diagnosed in Schleswig-Holstein during the study period were detected via the SCREEN program.
Before the skin cancer screening period (1998-1999), the melanoma mortality rate was 1.9 per 100,000 men and 1.4 per 100,000 women. The key study finding was that melanoma mortality fell by 48% in Schleswig-Holstein by 2008-2009 to 1.0 per 100,000 men to 0.7 per 100,000 women.
Nothing parallel occurred in the rest of Germany, or in Schleswig-Holstein’s neighbor to the north, Denmark.
The observed melanoma mortality rate in Schleswig-Holstein during 2008-2009 was 1.0 per 100,000 for men and 0.7 per 100,000 for women, compared with 1.8 and 1.2, respectively, in Germany as a whole excluding Schleswig-Holstein.
During 2000-2009, the most recent 10-year period for which official German mortality statistics are available, melanoma mortality in Schleswig-Holstein declined by 7.4% annually. In contrast, melanoma mortality rates were stable over time in each of the four adjacent states to the north, south, east, and west, none of which had a skin cancer screening program.
A bump in the incidence of melanoma was recorded in Schleswig-Holstein during the screening year, but not elsewhere, according to Dr. Weinstock.
The standardized total-body skin examinations were performed by physicians, who had to complete an 8-hour, day-long training course in order to participate. Of note, 116 of the 118 dermatologists practicing in Schleswig-Holstein participated in SCREEN, as did 64% of primary care physicians. Physicians were paid to perform the screens, and the public was encouraged to undergo screening via an extensive multimedia campaign.
The screening had a two-tiered structure. More than three-quarters of individuals were initially screened by primary care physicians. Participants with suspicious findings were sent to a dermatologist, who performed a second whole-body skin examination and performed biopsies as warranted.
Total-body skin examination as a means of reducing melanoma mortality has long been a controversial issue. The U.S. Preventive Services Task Force has recommended that there is not enough evidence to recommend screening the general adult population (Ann. Intern. Med. 2009;150:188-93). However, according to Dr. Weinstock, the recommendation will need to be revisited in light of the new evidence from Germany.
He observed that although SCREEN was an observational study, and, hence, doesn’t constitute absolute proof that a skin cancer screening program saves lives, it was the most ambitious effort to screen for melanoma ever conducted anywhere in the world. And it provides what is probably the strongest evidence that will ever be available, in his view, given the great expense and many years of follow-up required for a randomized controlled trial of skin cancer screening.
As he and his coauthors wrote in a new report from the SCREEN project, "In the public health arena, absolute proof is not necessarily required when lives are at stake" (Cancer 2012 April 19 [doi: 10.1002/cncr.27566]).
German dermatologists, flush with the SCREEN success, had proposed to follow-up the project with a definitive randomized controlled trial of melanoma screening, but were overruled. Federal health officials found the SCREEN results so persuasive that they launched an ongoing national skin cancer screening program. All 45 million Germans aged 35 years and older are now eligible for a total-body skin examination every 2 years.
The SCREEN investigators ruled out improvements in melanoma therapy as a potential explanation for the observed reduction in mortality, since there were none during the study years. Nor were there any changes in coding practices in the Schleswig-Holstein statistics office. And no major melanoma primary prevention programs were introduced.
Melanoma mortality rates in Schleswig-Holstein were fairly constant from 1990 to 2003, then dropped during and immediately after introduction of the statewide SCREEN program. All of which points to the skin cancer screening program as the almost-certain explanation for the melanoma mortality reduction, he said.
Dr. Weinstock promised there will be much more information and analysis to come from the SCREEN project, including tumor thickness–specific incidence rates. Also, by examining the incidence of melanoma in Schleswig-Holstein in the years following the screening program, it will be possible to create models that provide an idea of the optimal screening interval.
In a presentation at the Hawaii Dermatology Seminar sponsored by the Skin Disease Education Foundation (SDEF) in Waikoloa, Hawaii, Dr. Andreas Blum noted that while the main purpose of whole-body skin examination is to save lives through early detection of melanoma, the SCREEN project also detected basal cell carcinomas at a rate of 5.4 malignancies per 1,000 persons screened, and squamous cell carcinomas at a rate of 1.1 per 1,000.
Five lesion excisions had to be performed in order to detect one malignancy, according to Dr. Blum, professor of dermatology at the University of Tübingen (Germany).
He predicted that the cost involved in routine total-body skin examinations is likely to be a critical source of controversy. Using the U.S. National Cancer Institute’s estimate that 12.5% of melanomas are fatal, and assuming the cost of a total-body skin exam to be $50 per person, he estimated that routine total-body skin exams in the SCREEN project cost $240,000 per melanoma death avoided.
Meanwhile, in a recently reported multicenter study in which more than 14,000 subjects underwent a total-body skin exam, 400 patients had to be screened in order to find 1 melanoma (J. Am. Acad. Dermatol. 2012;66:212-9). Again, assuming a cost of $50 per total-body skin exam, that would work out to roughly $140,000 per melanoma death avoided, Dr. Blum said.
He added that in his own specialized skin cancer clinic, where he and his colleagues see a relatively select patient population, routine total-body skin exams cost an estimated $65,000 per melanoma death avoided. And when he plugged in the numbers provided by session chair Dr. Ashfaq A. Margoob from the skin cancer clinic at Memorial Sloan-Kettering Cancer Center in New York, Dr. Blum once again came up with a figure of roughly $65,000 per melanoma death avoided, which is close to one-fourth of the estimated cost per melanoma death avoided in the SCREEN project.
"The range is quite large. I think the cost debate will continue," Dr. Blum said.
Dr. Weinstock and Dr. Blum reported having no financial conflicts.
SDEF and this news organization are owned by Elsevier.
RALEIGH, N.C. – A population-based total-body skin cancer screening program reduces melanoma mortality, according to the results of a landmark German project presented at the annual meeting of the Society for Investigative Dermatology.
"I would argue that this is the most important presentation anywhere at this meeting," Dr. Martin A. Weinstock said during his presentation of the SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) project.
"The reason I make that argument is very simply because melanoma accounts for more deaths than anything else in the dermatology world. If we want to reduce that number of deaths, the best way is [through] early detection – and SCREEN provides the best evidence to date that we can do that," explained Dr. Weinstock, professor of dermatology and epidemiology at Brown University, Providence, R.I., and a SCREEN project organizer.
SCREEN was a population-based skin cancer screening program in which all residents over age 20 in the German federal state of Schleswig-Holstein were encouraged to undergo a standardized whole-body skin examination between July 2003 and June 2004. Nineteen percent of the state’s eligible population – 360,288 individuals – participated. Half of the 1,169 melanomas diagnosed in Schleswig-Holstein during the study period were detected via the SCREEN program.
Before the skin cancer screening period (1998-1999), the melanoma mortality rate was 1.9 per 100,000 men and 1.4 per 100,000 women. The key study finding was that melanoma mortality fell by 48% in Schleswig-Holstein by 2008-2009 to 1.0 per 100,000 men to 0.7 per 100,000 women.
Nothing parallel occurred in the rest of Germany, or in Schleswig-Holstein’s neighbor to the north, Denmark.
The observed melanoma mortality rate in Schleswig-Holstein during 2008-2009 was 1.0 per 100,000 for men and 0.7 per 100,000 for women, compared with 1.8 and 1.2, respectively, in Germany as a whole excluding Schleswig-Holstein.
During 2000-2009, the most recent 10-year period for which official German mortality statistics are available, melanoma mortality in Schleswig-Holstein declined by 7.4% annually. In contrast, melanoma mortality rates were stable over time in each of the four adjacent states to the north, south, east, and west, none of which had a skin cancer screening program.
A bump in the incidence of melanoma was recorded in Schleswig-Holstein during the screening year, but not elsewhere, according to Dr. Weinstock.
The standardized total-body skin examinations were performed by physicians, who had to complete an 8-hour, day-long training course in order to participate. Of note, 116 of the 118 dermatologists practicing in Schleswig-Holstein participated in SCREEN, as did 64% of primary care physicians. Physicians were paid to perform the screens, and the public was encouraged to undergo screening via an extensive multimedia campaign.
The screening had a two-tiered structure. More than three-quarters of individuals were initially screened by primary care physicians. Participants with suspicious findings were sent to a dermatologist, who performed a second whole-body skin examination and performed biopsies as warranted.
Total-body skin examination as a means of reducing melanoma mortality has long been a controversial issue. The U.S. Preventive Services Task Force has recommended that there is not enough evidence to recommend screening the general adult population (Ann. Intern. Med. 2009;150:188-93). However, according to Dr. Weinstock, the recommendation will need to be revisited in light of the new evidence from Germany.
He observed that although SCREEN was an observational study, and, hence, doesn’t constitute absolute proof that a skin cancer screening program saves lives, it was the most ambitious effort to screen for melanoma ever conducted anywhere in the world. And it provides what is probably the strongest evidence that will ever be available, in his view, given the great expense and many years of follow-up required for a randomized controlled trial of skin cancer screening.
As he and his coauthors wrote in a new report from the SCREEN project, "In the public health arena, absolute proof is not necessarily required when lives are at stake" (Cancer 2012 April 19 [doi: 10.1002/cncr.27566]).
German dermatologists, flush with the SCREEN success, had proposed to follow-up the project with a definitive randomized controlled trial of melanoma screening, but were overruled. Federal health officials found the SCREEN results so persuasive that they launched an ongoing national skin cancer screening program. All 45 million Germans aged 35 years and older are now eligible for a total-body skin examination every 2 years.
The SCREEN investigators ruled out improvements in melanoma therapy as a potential explanation for the observed reduction in mortality, since there were none during the study years. Nor were there any changes in coding practices in the Schleswig-Holstein statistics office. And no major melanoma primary prevention programs were introduced.
Melanoma mortality rates in Schleswig-Holstein were fairly constant from 1990 to 2003, then dropped during and immediately after introduction of the statewide SCREEN program. All of which points to the skin cancer screening program as the almost-certain explanation for the melanoma mortality reduction, he said.
Dr. Weinstock promised there will be much more information and analysis to come from the SCREEN project, including tumor thickness–specific incidence rates. Also, by examining the incidence of melanoma in Schleswig-Holstein in the years following the screening program, it will be possible to create models that provide an idea of the optimal screening interval.
In a presentation at the Hawaii Dermatology Seminar sponsored by the Skin Disease Education Foundation (SDEF) in Waikoloa, Hawaii, Dr. Andreas Blum noted that while the main purpose of whole-body skin examination is to save lives through early detection of melanoma, the SCREEN project also detected basal cell carcinomas at a rate of 5.4 malignancies per 1,000 persons screened, and squamous cell carcinomas at a rate of 1.1 per 1,000.
Five lesion excisions had to be performed in order to detect one malignancy, according to Dr. Blum, professor of dermatology at the University of Tübingen (Germany).
He predicted that the cost involved in routine total-body skin examinations is likely to be a critical source of controversy. Using the U.S. National Cancer Institute’s estimate that 12.5% of melanomas are fatal, and assuming the cost of a total-body skin exam to be $50 per person, he estimated that routine total-body skin exams in the SCREEN project cost $240,000 per melanoma death avoided.
Meanwhile, in a recently reported multicenter study in which more than 14,000 subjects underwent a total-body skin exam, 400 patients had to be screened in order to find 1 melanoma (J. Am. Acad. Dermatol. 2012;66:212-9). Again, assuming a cost of $50 per total-body skin exam, that would work out to roughly $140,000 per melanoma death avoided, Dr. Blum said.
He added that in his own specialized skin cancer clinic, where he and his colleagues see a relatively select patient population, routine total-body skin exams cost an estimated $65,000 per melanoma death avoided. And when he plugged in the numbers provided by session chair Dr. Ashfaq A. Margoob from the skin cancer clinic at Memorial Sloan-Kettering Cancer Center in New York, Dr. Blum once again came up with a figure of roughly $65,000 per melanoma death avoided, which is close to one-fourth of the estimated cost per melanoma death avoided in the SCREEN project.
"The range is quite large. I think the cost debate will continue," Dr. Blum said.
Dr. Weinstock and Dr. Blum reported having no financial conflicts.
SDEF and this news organization are owned by Elsevier.
RALEIGH, N.C. – A population-based total-body skin cancer screening program reduces melanoma mortality, according to the results of a landmark German project presented at the annual meeting of the Society for Investigative Dermatology.
"I would argue that this is the most important presentation anywhere at this meeting," Dr. Martin A. Weinstock said during his presentation of the SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) project.
"The reason I make that argument is very simply because melanoma accounts for more deaths than anything else in the dermatology world. If we want to reduce that number of deaths, the best way is [through] early detection – and SCREEN provides the best evidence to date that we can do that," explained Dr. Weinstock, professor of dermatology and epidemiology at Brown University, Providence, R.I., and a SCREEN project organizer.
SCREEN was a population-based skin cancer screening program in which all residents over age 20 in the German federal state of Schleswig-Holstein were encouraged to undergo a standardized whole-body skin examination between July 2003 and June 2004. Nineteen percent of the state’s eligible population – 360,288 individuals – participated. Half of the 1,169 melanomas diagnosed in Schleswig-Holstein during the study period were detected via the SCREEN program.
Before the skin cancer screening period (1998-1999), the melanoma mortality rate was 1.9 per 100,000 men and 1.4 per 100,000 women. The key study finding was that melanoma mortality fell by 48% in Schleswig-Holstein by 2008-2009 to 1.0 per 100,000 men to 0.7 per 100,000 women.
Nothing parallel occurred in the rest of Germany, or in Schleswig-Holstein’s neighbor to the north, Denmark.
The observed melanoma mortality rate in Schleswig-Holstein during 2008-2009 was 1.0 per 100,000 for men and 0.7 per 100,000 for women, compared with 1.8 and 1.2, respectively, in Germany as a whole excluding Schleswig-Holstein.
During 2000-2009, the most recent 10-year period for which official German mortality statistics are available, melanoma mortality in Schleswig-Holstein declined by 7.4% annually. In contrast, melanoma mortality rates were stable over time in each of the four adjacent states to the north, south, east, and west, none of which had a skin cancer screening program.
A bump in the incidence of melanoma was recorded in Schleswig-Holstein during the screening year, but not elsewhere, according to Dr. Weinstock.
The standardized total-body skin examinations were performed by physicians, who had to complete an 8-hour, day-long training course in order to participate. Of note, 116 of the 118 dermatologists practicing in Schleswig-Holstein participated in SCREEN, as did 64% of primary care physicians. Physicians were paid to perform the screens, and the public was encouraged to undergo screening via an extensive multimedia campaign.
The screening had a two-tiered structure. More than three-quarters of individuals were initially screened by primary care physicians. Participants with suspicious findings were sent to a dermatologist, who performed a second whole-body skin examination and performed biopsies as warranted.
Total-body skin examination as a means of reducing melanoma mortality has long been a controversial issue. The U.S. Preventive Services Task Force has recommended that there is not enough evidence to recommend screening the general adult population (Ann. Intern. Med. 2009;150:188-93). However, according to Dr. Weinstock, the recommendation will need to be revisited in light of the new evidence from Germany.
He observed that although SCREEN was an observational study, and, hence, doesn’t constitute absolute proof that a skin cancer screening program saves lives, it was the most ambitious effort to screen for melanoma ever conducted anywhere in the world. And it provides what is probably the strongest evidence that will ever be available, in his view, given the great expense and many years of follow-up required for a randomized controlled trial of skin cancer screening.
As he and his coauthors wrote in a new report from the SCREEN project, "In the public health arena, absolute proof is not necessarily required when lives are at stake" (Cancer 2012 April 19 [doi: 10.1002/cncr.27566]).
German dermatologists, flush with the SCREEN success, had proposed to follow-up the project with a definitive randomized controlled trial of melanoma screening, but were overruled. Federal health officials found the SCREEN results so persuasive that they launched an ongoing national skin cancer screening program. All 45 million Germans aged 35 years and older are now eligible for a total-body skin examination every 2 years.
The SCREEN investigators ruled out improvements in melanoma therapy as a potential explanation for the observed reduction in mortality, since there were none during the study years. Nor were there any changes in coding practices in the Schleswig-Holstein statistics office. And no major melanoma primary prevention programs were introduced.
Melanoma mortality rates in Schleswig-Holstein were fairly constant from 1990 to 2003, then dropped during and immediately after introduction of the statewide SCREEN program. All of which points to the skin cancer screening program as the almost-certain explanation for the melanoma mortality reduction, he said.
Dr. Weinstock promised there will be much more information and analysis to come from the SCREEN project, including tumor thickness–specific incidence rates. Also, by examining the incidence of melanoma in Schleswig-Holstein in the years following the screening program, it will be possible to create models that provide an idea of the optimal screening interval.
In a presentation at the Hawaii Dermatology Seminar sponsored by the Skin Disease Education Foundation (SDEF) in Waikoloa, Hawaii, Dr. Andreas Blum noted that while the main purpose of whole-body skin examination is to save lives through early detection of melanoma, the SCREEN project also detected basal cell carcinomas at a rate of 5.4 malignancies per 1,000 persons screened, and squamous cell carcinomas at a rate of 1.1 per 1,000.
Five lesion excisions had to be performed in order to detect one malignancy, according to Dr. Blum, professor of dermatology at the University of Tübingen (Germany).
He predicted that the cost involved in routine total-body skin examinations is likely to be a critical source of controversy. Using the U.S. National Cancer Institute’s estimate that 12.5% of melanomas are fatal, and assuming the cost of a total-body skin exam to be $50 per person, he estimated that routine total-body skin exams in the SCREEN project cost $240,000 per melanoma death avoided.
Meanwhile, in a recently reported multicenter study in which more than 14,000 subjects underwent a total-body skin exam, 400 patients had to be screened in order to find 1 melanoma (J. Am. Acad. Dermatol. 2012;66:212-9). Again, assuming a cost of $50 per total-body skin exam, that would work out to roughly $140,000 per melanoma death avoided, Dr. Blum said.
He added that in his own specialized skin cancer clinic, where he and his colleagues see a relatively select patient population, routine total-body skin exams cost an estimated $65,000 per melanoma death avoided. And when he plugged in the numbers provided by session chair Dr. Ashfaq A. Margoob from the skin cancer clinic at Memorial Sloan-Kettering Cancer Center in New York, Dr. Blum once again came up with a figure of roughly $65,000 per melanoma death avoided, which is close to one-fourth of the estimated cost per melanoma death avoided in the SCREEN project.
"The range is quite large. I think the cost debate will continue," Dr. Blum said.
Dr. Weinstock and Dr. Blum reported having no financial conflicts.
SDEF and this news organization are owned by Elsevier.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Daily Emollient Prevented Eczema in 67% of High-Risk Infants
RALEIGH, N.C. – Once-daily application of an emollient from birth through age 6 months has shown considerable promise as a means of preventing atopic dermatitis, according to the Barrier Enhancement for Eczema Prevention study.
BEEP was a multicenter, international, randomized controlled pilot study assessing the feasibility, safety, and effectiveness of a novel approach to the prevention of atopic dermatitis. The study hypothesis was that protecting the skin barrier early in life can prevent this common skin disease, explained Dr. Eric L. Simpson of Oregon Health and Science University, Portland.
The rationale for this approach lies in previous work demonstrating that skin barrier dysfunction precedes eczema development. And emollients can be effective in treating mild disease and preventing flares, he said at the annual meeting of the Society for Investigative Dermatology.
BEEP involved 124 infants in Portland and at four medical centers in the United Kingdom. All were deemed high risk for atopic dermatitis because they had one or more first-degree relatives with a history of asthma, hay fever, or atopic dermatitis. Participating families were randomized to either once-daily application of an emollient to the baby’s entire body except the scalp and diaper area starting before age 3 weeks and continuing for 6 months, or to a control group that agreed to refrain from regular use of emollients. All families received advice on best-practice skin care, namely to minimize the use of harsh cleansers and hot water bathing.
The 6-month cumulative incidence of investigator-diagnosed eczema was 21.8% in the daily emollient group, compared with 43.3% in controls, for a 67% reduction in risk. It was a considerably more dramatic effect than what the investigators had anticipated.
"This was kind of a surprising finding to us," Dr. Simpson admitted. Patients will be followed up at 1 and 2 years to learn whether the early-life treatment actually prevented or simply delayed onset of atopic dermatitis.
In a subanalysis, skin barrier function studies were carried out in 15 patients divided between a control and intervention arm. Children in the control arm showed favorable albeit nonsignificant trends for less transepidermal water loss and a lower skin pH.
Parents in the intervention arm were given a choice of three emollients of various viscosities: sunflower seed oil; Cetaphil cream in the United States or Doublebase gel in the United Kingdom; or Aquaphor in the United States or 50-50 ointment, a white soft paraffin/liquid paraffin product marketed in the United Kingdom. More than two-thirds of families opted for Cetaphil cream or Doublebase gel.
Ninety-six percent of families in the intervention arm found their emollient acceptable, and 80% indicated they used it at least 5 days per week.
No cases of irritant or contact dermatitis occurred in the emollient group. Three mild skin infections occurred in each study arm.
Dr. Simpson’s BEEP coinvestigators included atopic dermatitis experts such as Dr. Hywel C. Williams, professor of dermatology at the University of Nottingham (England) and Dr. Jon M. Hanifin, professor emeritus of dermatology at Oregon Health and Science University.
The researchers are now planning a larger, definitive, randomized controlled trial of emollient therapy early in life as a means of preventing the development of atopic dermatitis. This study will be powered to look at the relative efficacy of different emollients. Also, it will include objective measures of adherence, such as volume of emollient used, rather than simply relying upon parental report.
Audience members expressed enthusiasm over the BEEP findings. The prevalence of atopic dermatitis has been rising for decades; the disease exacts a steep toll in terms of quality of life; and to date, there has been no established eczema prevention strategy. Moreover, there is the prospect that by preventing eczema via a simple topical therapy it will be possible to halt the "atopic march" to asthma and other comorbidities.
Dr. Simpson noted that BEEP was a small-scale feasibility study carried out because investigators were initially unsure if families would be willing to participate in a clinical trial where they could be randomized to avoiding emollients. But 28%-59% of the families approached at the participating centers agreed to enroll.
BEEP was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Oregon Clinical and Translational Research Institute, and the U.K. National Institute for Health Research.
Dr. Simpson reported having no financial conflicts of interest.
RALEIGH, N.C. – Once-daily application of an emollient from birth through age 6 months has shown considerable promise as a means of preventing atopic dermatitis, according to the Barrier Enhancement for Eczema Prevention study.
BEEP was a multicenter, international, randomized controlled pilot study assessing the feasibility, safety, and effectiveness of a novel approach to the prevention of atopic dermatitis. The study hypothesis was that protecting the skin barrier early in life can prevent this common skin disease, explained Dr. Eric L. Simpson of Oregon Health and Science University, Portland.
The rationale for this approach lies in previous work demonstrating that skin barrier dysfunction precedes eczema development. And emollients can be effective in treating mild disease and preventing flares, he said at the annual meeting of the Society for Investigative Dermatology.
BEEP involved 124 infants in Portland and at four medical centers in the United Kingdom. All were deemed high risk for atopic dermatitis because they had one or more first-degree relatives with a history of asthma, hay fever, or atopic dermatitis. Participating families were randomized to either once-daily application of an emollient to the baby’s entire body except the scalp and diaper area starting before age 3 weeks and continuing for 6 months, or to a control group that agreed to refrain from regular use of emollients. All families received advice on best-practice skin care, namely to minimize the use of harsh cleansers and hot water bathing.
The 6-month cumulative incidence of investigator-diagnosed eczema was 21.8% in the daily emollient group, compared with 43.3% in controls, for a 67% reduction in risk. It was a considerably more dramatic effect than what the investigators had anticipated.
"This was kind of a surprising finding to us," Dr. Simpson admitted. Patients will be followed up at 1 and 2 years to learn whether the early-life treatment actually prevented or simply delayed onset of atopic dermatitis.
In a subanalysis, skin barrier function studies were carried out in 15 patients divided between a control and intervention arm. Children in the control arm showed favorable albeit nonsignificant trends for less transepidermal water loss and a lower skin pH.
Parents in the intervention arm were given a choice of three emollients of various viscosities: sunflower seed oil; Cetaphil cream in the United States or Doublebase gel in the United Kingdom; or Aquaphor in the United States or 50-50 ointment, a white soft paraffin/liquid paraffin product marketed in the United Kingdom. More than two-thirds of families opted for Cetaphil cream or Doublebase gel.
Ninety-six percent of families in the intervention arm found their emollient acceptable, and 80% indicated they used it at least 5 days per week.
No cases of irritant or contact dermatitis occurred in the emollient group. Three mild skin infections occurred in each study arm.
Dr. Simpson’s BEEP coinvestigators included atopic dermatitis experts such as Dr. Hywel C. Williams, professor of dermatology at the University of Nottingham (England) and Dr. Jon M. Hanifin, professor emeritus of dermatology at Oregon Health and Science University.
The researchers are now planning a larger, definitive, randomized controlled trial of emollient therapy early in life as a means of preventing the development of atopic dermatitis. This study will be powered to look at the relative efficacy of different emollients. Also, it will include objective measures of adherence, such as volume of emollient used, rather than simply relying upon parental report.
Audience members expressed enthusiasm over the BEEP findings. The prevalence of atopic dermatitis has been rising for decades; the disease exacts a steep toll in terms of quality of life; and to date, there has been no established eczema prevention strategy. Moreover, there is the prospect that by preventing eczema via a simple topical therapy it will be possible to halt the "atopic march" to asthma and other comorbidities.
Dr. Simpson noted that BEEP was a small-scale feasibility study carried out because investigators were initially unsure if families would be willing to participate in a clinical trial where they could be randomized to avoiding emollients. But 28%-59% of the families approached at the participating centers agreed to enroll.
BEEP was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Oregon Clinical and Translational Research Institute, and the U.K. National Institute for Health Research.
Dr. Simpson reported having no financial conflicts of interest.
RALEIGH, N.C. – Once-daily application of an emollient from birth through age 6 months has shown considerable promise as a means of preventing atopic dermatitis, according to the Barrier Enhancement for Eczema Prevention study.
BEEP was a multicenter, international, randomized controlled pilot study assessing the feasibility, safety, and effectiveness of a novel approach to the prevention of atopic dermatitis. The study hypothesis was that protecting the skin barrier early in life can prevent this common skin disease, explained Dr. Eric L. Simpson of Oregon Health and Science University, Portland.
The rationale for this approach lies in previous work demonstrating that skin barrier dysfunction precedes eczema development. And emollients can be effective in treating mild disease and preventing flares, he said at the annual meeting of the Society for Investigative Dermatology.
BEEP involved 124 infants in Portland and at four medical centers in the United Kingdom. All were deemed high risk for atopic dermatitis because they had one or more first-degree relatives with a history of asthma, hay fever, or atopic dermatitis. Participating families were randomized to either once-daily application of an emollient to the baby’s entire body except the scalp and diaper area starting before age 3 weeks and continuing for 6 months, or to a control group that agreed to refrain from regular use of emollients. All families received advice on best-practice skin care, namely to minimize the use of harsh cleansers and hot water bathing.
The 6-month cumulative incidence of investigator-diagnosed eczema was 21.8% in the daily emollient group, compared with 43.3% in controls, for a 67% reduction in risk. It was a considerably more dramatic effect than what the investigators had anticipated.
"This was kind of a surprising finding to us," Dr. Simpson admitted. Patients will be followed up at 1 and 2 years to learn whether the early-life treatment actually prevented or simply delayed onset of atopic dermatitis.
In a subanalysis, skin barrier function studies were carried out in 15 patients divided between a control and intervention arm. Children in the control arm showed favorable albeit nonsignificant trends for less transepidermal water loss and a lower skin pH.
Parents in the intervention arm were given a choice of three emollients of various viscosities: sunflower seed oil; Cetaphil cream in the United States or Doublebase gel in the United Kingdom; or Aquaphor in the United States or 50-50 ointment, a white soft paraffin/liquid paraffin product marketed in the United Kingdom. More than two-thirds of families opted for Cetaphil cream or Doublebase gel.
Ninety-six percent of families in the intervention arm found their emollient acceptable, and 80% indicated they used it at least 5 days per week.
No cases of irritant or contact dermatitis occurred in the emollient group. Three mild skin infections occurred in each study arm.
Dr. Simpson’s BEEP coinvestigators included atopic dermatitis experts such as Dr. Hywel C. Williams, professor of dermatology at the University of Nottingham (England) and Dr. Jon M. Hanifin, professor emeritus of dermatology at Oregon Health and Science University.
The researchers are now planning a larger, definitive, randomized controlled trial of emollient therapy early in life as a means of preventing the development of atopic dermatitis. This study will be powered to look at the relative efficacy of different emollients. Also, it will include objective measures of adherence, such as volume of emollient used, rather than simply relying upon parental report.
Audience members expressed enthusiasm over the BEEP findings. The prevalence of atopic dermatitis has been rising for decades; the disease exacts a steep toll in terms of quality of life; and to date, there has been no established eczema prevention strategy. Moreover, there is the prospect that by preventing eczema via a simple topical therapy it will be possible to halt the "atopic march" to asthma and other comorbidities.
Dr. Simpson noted that BEEP was a small-scale feasibility study carried out because investigators were initially unsure if families would be willing to participate in a clinical trial where they could be randomized to avoiding emollients. But 28%-59% of the families approached at the participating centers agreed to enroll.
BEEP was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Oregon Clinical and Translational Research Institute, and the U.K. National Institute for Health Research.
Dr. Simpson reported having no financial conflicts of interest.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: The 6-month cumulative incidence of investigator-diagnosed eczema was 21.8% in the daily emollient group, compared with 43.3% in controls, for a 67% reduction in risk.
Data Source: BEEP involved 124 infants in Portland and at four medical centers in the United Kingdom deemed to be at high risk for atopic dermatitis.
Disclosures: BEEP was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Oregon Clinical and Translational Research Institute, and the U.K. National Institute for Health Research. Dr. Simpson reported having no financial conflicts of interest.
For Infants With Bronchiolitis, Nasogastric Feeds Have Advantages
BOSTON – Nasogastric feeding is safe and feasible for infants with viral bronchiolitis.
Infants who received nutrition by NG tube did not experience any exacerbation of their respiratory illness and recovered just as quickly as those who received intravenous fluids, Dr. Amir Kugelman said at the annual meeting of the Pediatric Academic Societies.
Although his randomized controlled study wasn’t designed to evaluate nutritional outcomes, Dr. Kugelman, director of the pediatric pulmonary unit at Bnai Zion Medical Center, Haifa, Israel, said that NG feeding offers some advantages over intravenous fluids.
Intravenous fluids provide limited calories and no lipids or proteins, which can lead to a catabolic state during a time of increased nutritional needs. "Gastric tube feeding is more physiologic and allows mothers to continue breast-feeding" while their infant is unable to tolerate oral intake, said Dr. Kugelman. "There is also the possibility that better nutrition might enhance recovery."
His study included 51 infants (mean age, 2.5 months) who had moderate viral bronchiolitis. They could not sustain oral feeding because of an increased respiratory rate of at least 60 breaths per minute. Most of them (41) were positive for respiratory syncytial virus; 11 had developed pneumonia. There were no significant baseline differences except for length of illness – those randomized to the intravenous group had been ill an average of 4 days, compared with 3 days in the NG group.
The IV group received a continuous infusion of a standard 5% dextrose in normal saline solution. The NG group received a slow drip of breast milk or infant formula. Three infants on NG feeds were switched to IV because of vomiting, and one on an IV was switched to NG feeds because of poor IV access and fluid extravasation.
There were no significant differences in the duration of oxygen needed, the time to full oral feeds, or the length of stay, Dr. Kugelman said. There were no incidences of aspiration. Infants in the IV group had a trend toward a shorter length of stay (100 hours vs. 120 hours), but this is probably because they had a longer duration of illness before hospitalization, he said.
Dr. Kugelman had no financial disclosures.
BOSTON – Nasogastric feeding is safe and feasible for infants with viral bronchiolitis.
Infants who received nutrition by NG tube did not experience any exacerbation of their respiratory illness and recovered just as quickly as those who received intravenous fluids, Dr. Amir Kugelman said at the annual meeting of the Pediatric Academic Societies.
Although his randomized controlled study wasn’t designed to evaluate nutritional outcomes, Dr. Kugelman, director of the pediatric pulmonary unit at Bnai Zion Medical Center, Haifa, Israel, said that NG feeding offers some advantages over intravenous fluids.
Intravenous fluids provide limited calories and no lipids or proteins, which can lead to a catabolic state during a time of increased nutritional needs. "Gastric tube feeding is more physiologic and allows mothers to continue breast-feeding" while their infant is unable to tolerate oral intake, said Dr. Kugelman. "There is also the possibility that better nutrition might enhance recovery."
His study included 51 infants (mean age, 2.5 months) who had moderate viral bronchiolitis. They could not sustain oral feeding because of an increased respiratory rate of at least 60 breaths per minute. Most of them (41) were positive for respiratory syncytial virus; 11 had developed pneumonia. There were no significant baseline differences except for length of illness – those randomized to the intravenous group had been ill an average of 4 days, compared with 3 days in the NG group.
The IV group received a continuous infusion of a standard 5% dextrose in normal saline solution. The NG group received a slow drip of breast milk or infant formula. Three infants on NG feeds were switched to IV because of vomiting, and one on an IV was switched to NG feeds because of poor IV access and fluid extravasation.
There were no significant differences in the duration of oxygen needed, the time to full oral feeds, or the length of stay, Dr. Kugelman said. There were no incidences of aspiration. Infants in the IV group had a trend toward a shorter length of stay (100 hours vs. 120 hours), but this is probably because they had a longer duration of illness before hospitalization, he said.
Dr. Kugelman had no financial disclosures.
BOSTON – Nasogastric feeding is safe and feasible for infants with viral bronchiolitis.
Infants who received nutrition by NG tube did not experience any exacerbation of their respiratory illness and recovered just as quickly as those who received intravenous fluids, Dr. Amir Kugelman said at the annual meeting of the Pediatric Academic Societies.
Although his randomized controlled study wasn’t designed to evaluate nutritional outcomes, Dr. Kugelman, director of the pediatric pulmonary unit at Bnai Zion Medical Center, Haifa, Israel, said that NG feeding offers some advantages over intravenous fluids.
Intravenous fluids provide limited calories and no lipids or proteins, which can lead to a catabolic state during a time of increased nutritional needs. "Gastric tube feeding is more physiologic and allows mothers to continue breast-feeding" while their infant is unable to tolerate oral intake, said Dr. Kugelman. "There is also the possibility that better nutrition might enhance recovery."
His study included 51 infants (mean age, 2.5 months) who had moderate viral bronchiolitis. They could not sustain oral feeding because of an increased respiratory rate of at least 60 breaths per minute. Most of them (41) were positive for respiratory syncytial virus; 11 had developed pneumonia. There were no significant baseline differences except for length of illness – those randomized to the intravenous group had been ill an average of 4 days, compared with 3 days in the NG group.
The IV group received a continuous infusion of a standard 5% dextrose in normal saline solution. The NG group received a slow drip of breast milk or infant formula. Three infants on NG feeds were switched to IV because of vomiting, and one on an IV was switched to NG feeds because of poor IV access and fluid extravasation.
There were no significant differences in the duration of oxygen needed, the time to full oral feeds, or the length of stay, Dr. Kugelman said. There were no incidences of aspiration. Infants in the IV group had a trend toward a shorter length of stay (100 hours vs. 120 hours), but this is probably because they had a longer duration of illness before hospitalization, he said.
Dr. Kugelman had no financial disclosures.
FROM THE ANNUAL MEETING OF THE PEDIATRIC ACADEMIC SOCIETIES
Major Finding: Infants with moderate bronchiolitis who received nasogastric feeding recovered as well as did those with intravenous fluids.
Data Source: This randomized controlled trial included 51 infants.
Disclosures: Dr. Kugelman had no financial disclosures.