User login
In geriatric urinary incontinence, think DIAPERS mnemonic
NEW ORLEANS – Neil M. Resnick, MD, has devoted more than 30 years of his career to refining the diagnosis and management of geriatric urinary incontinence. He has found it to be a deeply rewarding area of his medical practice. And he wants primary care physicians to share the joy.
Once you get the hang of it, you’re going to love it,” he promised at the annual meeting of the American College of Physicians.
“There is so much you have to offer, and it’s going to make you one happy, fulfilled, non–burned-out physician,” added Dr. Resnick, professor of medicine and chief of the division of geriatric medicine at the University of Pittsburgh.
He insisted that geriatric urinary incontinence belongs squarely in the province of primary care physicians, not just urologic surgeons. That’s because the condition is typically caused or exacerbated by medical diseases and drugs.
“These are things for which we are the experts, because they are conditions outside the bladder that our surgical colleagues aren’t always expert in,” the internist emphasized.
The seven reversible causes of geriatric urinary incontinence, which are categorized as transient urinary incontinence, can easily be remembered by busy primary care practitioners with the aid of a mnemonic of Dr. Resnick’s own devising: DIAPERS. It stands for Delirium, Infection, Atrophic urethritis/vaginitis, Pharmaceuticals, Excess urine output, Restricted mobility, and Stool impaction.
“Treatable causes of urinary incontinence are much more common in older people than in the young,” Dr. Resnick said. “If you just pay attention to these, and you can’t even spell ‘bladder,’ you can cure one-third of older patients. It’s pretty dramatic. And it improves the incontinence in all of the people in whom it’s still persistent, and that means improved responsiveness to further treatment addressing the urinary tract, improvement of other problems related to the incontinence, better quality of life, and it just makes patients better overall. This is really the joy and glory of geriatrics.”
He emphasized that urinary incontinence is never normal, no matter how advanced the patient’s age. The basic geriatric principle is that aging reduces resilience. Bladder sensation and contractility decrease with age. The prostate enlarges. Sphincter strength and urethral length decrease in older women. Involuntary bladder contractions occur in half of all elderly individuals. Nocturnal urine excretion increases. Postvoid urine volume creeps up to 50-100 mL. These are normal changes, but they predispose to tipping over into urinary incontinence in the setting of any additional challenges created by DIAPERS.
The scope of the problem
More than one-third of elderly individuals experience urinary incontinence with daily to weekly frequency. The associated morbidity includes cellulitis, perineal rashes, pressure ulcers, falls, fractures, anxiety, depression, and sexual dysfunction. The economic cost of geriatric urinary incontinence is believed to exceed that of coronary artery bypass surgery and renal dialysis combined.
“The morbidity is huge and the costs are astonishing,” the geriatrician declared.
Fewer than one-fifth and perhaps as few as one-tenth of affected patients actually require surgery.
Less than 20% of elderly patients with urinary incontinence volunteer that information to their primary care physician because of the stigma involved. So, it’s important to ask about it, he noted.
The lowdown on DIAPERS
- Delirium. “The last thing you want to do is refer a patient with urinary incontinence and delirium to a urologist for cystoscopy or urodynamic testing,” according to Dr. Resnick. “It misses the point: The problem is their brain is not working. If you address the causes of delirium, once the delirium subsides, the incontinence will abate.” However, addressing the cause of the acute confusional state can be challenging, he conceded, because delirium can result from virtually any drug or disease anywhere in the body.
- Infection. Acute urinary tract infection (UTI) is the cause of about only 3% of geriatric urinary incontinence. But when present, it’s simple enough to diagnosis and treat. Far more common is asymptomatic bacteriuria, which is present in about 20% of elderly men and 40% of elderly women but does not cause incontinence.
- “The key symptom is dysuria: If the patient [with bacteriuria] has new-onset urinary incontinence or worsened urinary incontinence that’s happened for only the last couple days, that’s an acute UTI that needs to be treated,” Dr. Resnick advised. “Other than that, don’t treat. All you’ll do is select for more virulent organisms, so when the patient does get an acute UTI, it’s tougher to treat.”
- Atrophic vaginitis/urethritis. A common condition when endogenous estrogen goes down. It is characterized by vaginal and urethral erosions and tissue friability. When an affected woman urinates, the acid urine gains exposure to the underlying subendothelial tissue, causing inflammation and irritation that prevent the urethra from closing properly. This condition, frequently mistaken for a UTI, responds well to low-dose topical estrogen in the form of either an easily implantable ring that lasts for 3 months or a topical estrogen cream applied once daily, after establishing the absence of breast or uterine cancer.
- “It takes weeks to months for this condition to remit,” he said. “So, if they’re doing cream, they do it every day for a month. Then every month, they pull back by one day. Eventually, they get to the point where they can be maintained with once- or twice-weekly application.”
- Pharmaceuticals. The list of potential offenders is lengthy. Dr. Resnick focused on six types of medications that are most often linked to increased risk of geriatric urinary incontinence. Those six include long-acting sedative hypnotics, including diazepam (Valium); loop diuretics; and anticholinergic agents, including sedating antihistamines, antipsychotics, tricyclic antidepressants, and tiotropium bromide (Spiriva).
- They also include adrenergic agents, with alpha-adrenergic blockers causing or contributing to urinary incontinence in women and alpha-adrenergic agonists – present in a vast number of OTC cold, sleep, and cough medications – being responsible for problems in men; drugs causing fluid accumulation, including the dihydropyridine calcium channel blockers, NSAIDs, some Parkinson’s agents, and gabapentin/pregabalin; and ACE inhibitors because of their side effect of cough.
- “The most common problem drugs in my practice are calcium channel blockers and gabapentin or pregabalin,” according to the geriatrician.
- Excess urine output. Older people have smaller bladders. Dr. Resnick loathes the popular advice to drink 8 glasses of water per day. Every time that so-called health tip appears in the mass media, he sees a flurry of patients with new-onset geriatric urinary incontinence. Other causes of excess urine output include alcohol, caffeine, metabolic disorders including hyperglycemia, and peripheral edema attributable to heart failure or venous insufficiency.
- Restricted mobility. This often results from overlooked correctable conditions that bedevil older people, including poorly fitting shoes, calluses, bunions, and deformed toenails, as well as readily treatable disorders including depression, orthostatic or postprandial hypotension, and arthritis pain.
- Stool impaction. “The clinical key is new onset of double incontinence associated with bladder distension. One gloved finger will disimpact and cure both,” Dr. Resnick said.
- In patients whose urinary incontinence persists after systematic attention to the DIAPERS details, there are only four possible mechanisms, according to Dr. Resnick: an overactive detrusor or stress incontinence, which can be categorized as storage problems, or an underactive detrusor or a urethral obstruction, which can be considered emptying problems.
Dr. Resnick reported having no financial conflicts of interest regarding his presentation.
NEW ORLEANS – Neil M. Resnick, MD, has devoted more than 30 years of his career to refining the diagnosis and management of geriatric urinary incontinence. He has found it to be a deeply rewarding area of his medical practice. And he wants primary care physicians to share the joy.
Once you get the hang of it, you’re going to love it,” he promised at the annual meeting of the American College of Physicians.
“There is so much you have to offer, and it’s going to make you one happy, fulfilled, non–burned-out physician,” added Dr. Resnick, professor of medicine and chief of the division of geriatric medicine at the University of Pittsburgh.
He insisted that geriatric urinary incontinence belongs squarely in the province of primary care physicians, not just urologic surgeons. That’s because the condition is typically caused or exacerbated by medical diseases and drugs.
“These are things for which we are the experts, because they are conditions outside the bladder that our surgical colleagues aren’t always expert in,” the internist emphasized.
The seven reversible causes of geriatric urinary incontinence, which are categorized as transient urinary incontinence, can easily be remembered by busy primary care practitioners with the aid of a mnemonic of Dr. Resnick’s own devising: DIAPERS. It stands for Delirium, Infection, Atrophic urethritis/vaginitis, Pharmaceuticals, Excess urine output, Restricted mobility, and Stool impaction.
“Treatable causes of urinary incontinence are much more common in older people than in the young,” Dr. Resnick said. “If you just pay attention to these, and you can’t even spell ‘bladder,’ you can cure one-third of older patients. It’s pretty dramatic. And it improves the incontinence in all of the people in whom it’s still persistent, and that means improved responsiveness to further treatment addressing the urinary tract, improvement of other problems related to the incontinence, better quality of life, and it just makes patients better overall. This is really the joy and glory of geriatrics.”
He emphasized that urinary incontinence is never normal, no matter how advanced the patient’s age. The basic geriatric principle is that aging reduces resilience. Bladder sensation and contractility decrease with age. The prostate enlarges. Sphincter strength and urethral length decrease in older women. Involuntary bladder contractions occur in half of all elderly individuals. Nocturnal urine excretion increases. Postvoid urine volume creeps up to 50-100 mL. These are normal changes, but they predispose to tipping over into urinary incontinence in the setting of any additional challenges created by DIAPERS.
The scope of the problem
More than one-third of elderly individuals experience urinary incontinence with daily to weekly frequency. The associated morbidity includes cellulitis, perineal rashes, pressure ulcers, falls, fractures, anxiety, depression, and sexual dysfunction. The economic cost of geriatric urinary incontinence is believed to exceed that of coronary artery bypass surgery and renal dialysis combined.
“The morbidity is huge and the costs are astonishing,” the geriatrician declared.
Fewer than one-fifth and perhaps as few as one-tenth of affected patients actually require surgery.
Less than 20% of elderly patients with urinary incontinence volunteer that information to their primary care physician because of the stigma involved. So, it’s important to ask about it, he noted.
The lowdown on DIAPERS
- Delirium. “The last thing you want to do is refer a patient with urinary incontinence and delirium to a urologist for cystoscopy or urodynamic testing,” according to Dr. Resnick. “It misses the point: The problem is their brain is not working. If you address the causes of delirium, once the delirium subsides, the incontinence will abate.” However, addressing the cause of the acute confusional state can be challenging, he conceded, because delirium can result from virtually any drug or disease anywhere in the body.
- Infection. Acute urinary tract infection (UTI) is the cause of about only 3% of geriatric urinary incontinence. But when present, it’s simple enough to diagnosis and treat. Far more common is asymptomatic bacteriuria, which is present in about 20% of elderly men and 40% of elderly women but does not cause incontinence.
- “The key symptom is dysuria: If the patient [with bacteriuria] has new-onset urinary incontinence or worsened urinary incontinence that’s happened for only the last couple days, that’s an acute UTI that needs to be treated,” Dr. Resnick advised. “Other than that, don’t treat. All you’ll do is select for more virulent organisms, so when the patient does get an acute UTI, it’s tougher to treat.”
- Atrophic vaginitis/urethritis. A common condition when endogenous estrogen goes down. It is characterized by vaginal and urethral erosions and tissue friability. When an affected woman urinates, the acid urine gains exposure to the underlying subendothelial tissue, causing inflammation and irritation that prevent the urethra from closing properly. This condition, frequently mistaken for a UTI, responds well to low-dose topical estrogen in the form of either an easily implantable ring that lasts for 3 months or a topical estrogen cream applied once daily, after establishing the absence of breast or uterine cancer.
- “It takes weeks to months for this condition to remit,” he said. “So, if they’re doing cream, they do it every day for a month. Then every month, they pull back by one day. Eventually, they get to the point where they can be maintained with once- or twice-weekly application.”
- Pharmaceuticals. The list of potential offenders is lengthy. Dr. Resnick focused on six types of medications that are most often linked to increased risk of geriatric urinary incontinence. Those six include long-acting sedative hypnotics, including diazepam (Valium); loop diuretics; and anticholinergic agents, including sedating antihistamines, antipsychotics, tricyclic antidepressants, and tiotropium bromide (Spiriva).
- They also include adrenergic agents, with alpha-adrenergic blockers causing or contributing to urinary incontinence in women and alpha-adrenergic agonists – present in a vast number of OTC cold, sleep, and cough medications – being responsible for problems in men; drugs causing fluid accumulation, including the dihydropyridine calcium channel blockers, NSAIDs, some Parkinson’s agents, and gabapentin/pregabalin; and ACE inhibitors because of their side effect of cough.
- “The most common problem drugs in my practice are calcium channel blockers and gabapentin or pregabalin,” according to the geriatrician.
- Excess urine output. Older people have smaller bladders. Dr. Resnick loathes the popular advice to drink 8 glasses of water per day. Every time that so-called health tip appears in the mass media, he sees a flurry of patients with new-onset geriatric urinary incontinence. Other causes of excess urine output include alcohol, caffeine, metabolic disorders including hyperglycemia, and peripheral edema attributable to heart failure or venous insufficiency.
- Restricted mobility. This often results from overlooked correctable conditions that bedevil older people, including poorly fitting shoes, calluses, bunions, and deformed toenails, as well as readily treatable disorders including depression, orthostatic or postprandial hypotension, and arthritis pain.
- Stool impaction. “The clinical key is new onset of double incontinence associated with bladder distension. One gloved finger will disimpact and cure both,” Dr. Resnick said.
- In patients whose urinary incontinence persists after systematic attention to the DIAPERS details, there are only four possible mechanisms, according to Dr. Resnick: an overactive detrusor or stress incontinence, which can be categorized as storage problems, or an underactive detrusor or a urethral obstruction, which can be considered emptying problems.
Dr. Resnick reported having no financial conflicts of interest regarding his presentation.
NEW ORLEANS – Neil M. Resnick, MD, has devoted more than 30 years of his career to refining the diagnosis and management of geriatric urinary incontinence. He has found it to be a deeply rewarding area of his medical practice. And he wants primary care physicians to share the joy.
Once you get the hang of it, you’re going to love it,” he promised at the annual meeting of the American College of Physicians.
“There is so much you have to offer, and it’s going to make you one happy, fulfilled, non–burned-out physician,” added Dr. Resnick, professor of medicine and chief of the division of geriatric medicine at the University of Pittsburgh.
He insisted that geriatric urinary incontinence belongs squarely in the province of primary care physicians, not just urologic surgeons. That’s because the condition is typically caused or exacerbated by medical diseases and drugs.
“These are things for which we are the experts, because they are conditions outside the bladder that our surgical colleagues aren’t always expert in,” the internist emphasized.
The seven reversible causes of geriatric urinary incontinence, which are categorized as transient urinary incontinence, can easily be remembered by busy primary care practitioners with the aid of a mnemonic of Dr. Resnick’s own devising: DIAPERS. It stands for Delirium, Infection, Atrophic urethritis/vaginitis, Pharmaceuticals, Excess urine output, Restricted mobility, and Stool impaction.
“Treatable causes of urinary incontinence are much more common in older people than in the young,” Dr. Resnick said. “If you just pay attention to these, and you can’t even spell ‘bladder,’ you can cure one-third of older patients. It’s pretty dramatic. And it improves the incontinence in all of the people in whom it’s still persistent, and that means improved responsiveness to further treatment addressing the urinary tract, improvement of other problems related to the incontinence, better quality of life, and it just makes patients better overall. This is really the joy and glory of geriatrics.”
He emphasized that urinary incontinence is never normal, no matter how advanced the patient’s age. The basic geriatric principle is that aging reduces resilience. Bladder sensation and contractility decrease with age. The prostate enlarges. Sphincter strength and urethral length decrease in older women. Involuntary bladder contractions occur in half of all elderly individuals. Nocturnal urine excretion increases. Postvoid urine volume creeps up to 50-100 mL. These are normal changes, but they predispose to tipping over into urinary incontinence in the setting of any additional challenges created by DIAPERS.
The scope of the problem
More than one-third of elderly individuals experience urinary incontinence with daily to weekly frequency. The associated morbidity includes cellulitis, perineal rashes, pressure ulcers, falls, fractures, anxiety, depression, and sexual dysfunction. The economic cost of geriatric urinary incontinence is believed to exceed that of coronary artery bypass surgery and renal dialysis combined.
“The morbidity is huge and the costs are astonishing,” the geriatrician declared.
Fewer than one-fifth and perhaps as few as one-tenth of affected patients actually require surgery.
Less than 20% of elderly patients with urinary incontinence volunteer that information to their primary care physician because of the stigma involved. So, it’s important to ask about it, he noted.
The lowdown on DIAPERS
- Delirium. “The last thing you want to do is refer a patient with urinary incontinence and delirium to a urologist for cystoscopy or urodynamic testing,” according to Dr. Resnick. “It misses the point: The problem is their brain is not working. If you address the causes of delirium, once the delirium subsides, the incontinence will abate.” However, addressing the cause of the acute confusional state can be challenging, he conceded, because delirium can result from virtually any drug or disease anywhere in the body.
- Infection. Acute urinary tract infection (UTI) is the cause of about only 3% of geriatric urinary incontinence. But when present, it’s simple enough to diagnosis and treat. Far more common is asymptomatic bacteriuria, which is present in about 20% of elderly men and 40% of elderly women but does not cause incontinence.
- “The key symptom is dysuria: If the patient [with bacteriuria] has new-onset urinary incontinence or worsened urinary incontinence that’s happened for only the last couple days, that’s an acute UTI that needs to be treated,” Dr. Resnick advised. “Other than that, don’t treat. All you’ll do is select for more virulent organisms, so when the patient does get an acute UTI, it’s tougher to treat.”
- Atrophic vaginitis/urethritis. A common condition when endogenous estrogen goes down. It is characterized by vaginal and urethral erosions and tissue friability. When an affected woman urinates, the acid urine gains exposure to the underlying subendothelial tissue, causing inflammation and irritation that prevent the urethra from closing properly. This condition, frequently mistaken for a UTI, responds well to low-dose topical estrogen in the form of either an easily implantable ring that lasts for 3 months or a topical estrogen cream applied once daily, after establishing the absence of breast or uterine cancer.
- “It takes weeks to months for this condition to remit,” he said. “So, if they’re doing cream, they do it every day for a month. Then every month, they pull back by one day. Eventually, they get to the point where they can be maintained with once- or twice-weekly application.”
- Pharmaceuticals. The list of potential offenders is lengthy. Dr. Resnick focused on six types of medications that are most often linked to increased risk of geriatric urinary incontinence. Those six include long-acting sedative hypnotics, including diazepam (Valium); loop diuretics; and anticholinergic agents, including sedating antihistamines, antipsychotics, tricyclic antidepressants, and tiotropium bromide (Spiriva).
- They also include adrenergic agents, with alpha-adrenergic blockers causing or contributing to urinary incontinence in women and alpha-adrenergic agonists – present in a vast number of OTC cold, sleep, and cough medications – being responsible for problems in men; drugs causing fluid accumulation, including the dihydropyridine calcium channel blockers, NSAIDs, some Parkinson’s agents, and gabapentin/pregabalin; and ACE inhibitors because of their side effect of cough.
- “The most common problem drugs in my practice are calcium channel blockers and gabapentin or pregabalin,” according to the geriatrician.
- Excess urine output. Older people have smaller bladders. Dr. Resnick loathes the popular advice to drink 8 glasses of water per day. Every time that so-called health tip appears in the mass media, he sees a flurry of patients with new-onset geriatric urinary incontinence. Other causes of excess urine output include alcohol, caffeine, metabolic disorders including hyperglycemia, and peripheral edema attributable to heart failure or venous insufficiency.
- Restricted mobility. This often results from overlooked correctable conditions that bedevil older people, including poorly fitting shoes, calluses, bunions, and deformed toenails, as well as readily treatable disorders including depression, orthostatic or postprandial hypotension, and arthritis pain.
- Stool impaction. “The clinical key is new onset of double incontinence associated with bladder distension. One gloved finger will disimpact and cure both,” Dr. Resnick said.
- In patients whose urinary incontinence persists after systematic attention to the DIAPERS details, there are only four possible mechanisms, according to Dr. Resnick: an overactive detrusor or stress incontinence, which can be categorized as storage problems, or an underactive detrusor or a urethral obstruction, which can be considered emptying problems.
Dr. Resnick reported having no financial conflicts of interest regarding his presentation.
REPORTING FROM ACP INTERNAL MEDICINE
New Valsalva maneuver for SVT beats all others
NEW ORLEANS – , according to Jeet Mehta, MD, a resident in the combined medicine/pediatrics program at the University of Kansas, Wichita.
The 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommended vagal maneuvers as first-line treatment of supraventricular tachycardia, but added that there was no gold standard method. Since then, the situation has changed. Two well-conducted randomized clinical trials have been published that bring clarity as to the vagal maneuver of choice, Dr. Mehta reported at the annual meeting of the American College of Physicians.
He and his coinvestigators performed a meta-analysis of the three pre-2000 randomized controlled trials that compared the standard Valsalva maneuver to carotid sinus massage plus the two newer studies, both of which systematically compared a modified Valsalva maneuver with the standard version.
The clear winner in terms of efficacy was the modified Valsalva maneuver, in which patients with supraventricular tachycardia (SVT) performed a standardized strain while in a semirecumbent position, then immediately laid flat and had their legs raised to 45 degrees for 15 seconds before returning to the semirecumbent position. The purpose of this postural modification is to boost relaxation phase venous return and vagal stimulation.
In the 433-patient multicenter REVERT trial in the United Kingdom, 43% of those assigned to the modified Valsalva maneuver returned to sinus rhythm 1 minute after completing the task, compared with 17% of those randomized to the standard semirecumbent Valsalva maneuver. This resulted in significantly less need for adenosine and other treatments. Although REVERT investigators had the patients blow into a manometer at 40 mm Hg for 15 seconds, they noted that the same intensity of strain can be achieved more practically by blowing into a 10-mL syringe sufficient to just move the plunger (Lancet. 2015 Oct 31;386[10005]:1747-53).
The REVERT findings were confirmed by a second trial conducted by Turkish investigators, in which the modified Valsalva maneuver was successful in 43% of patients, compared with 11% in the standard Valsalva maneuver group (Am J Emerg Med. 2017 Nov;35[11]:1662-5).
Extrapolating from the published evidence, including a Cochrane Collaboration review (Cochrane Database Syst Rev. 2015 Feb 18;[2]:CD009502. doi: 10.1002/14651858.CD009502.pub3), Dr. Mehta and his coinvestigators ranked the likelihood of successful conversion of SVT to sinus rhythm from a high of 48% for the modified Valsalva maneuver, descending to 43% for a supine Valsalva maneuver, 36% for a standard semirecumbent Valsalva, 21% for a seated Valsalva, 19% for a standing one, and just 11% for carotid sinus massage.
“Based on evidence of high quality, we encourage that the modified Valsalva maneuver be done due its safety and low cost,” Dr. Mehta concluded.
He reported having no financial conflicts regarding his study, conducted free of commercial support.
NEW ORLEANS – , according to Jeet Mehta, MD, a resident in the combined medicine/pediatrics program at the University of Kansas, Wichita.
The 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommended vagal maneuvers as first-line treatment of supraventricular tachycardia, but added that there was no gold standard method. Since then, the situation has changed. Two well-conducted randomized clinical trials have been published that bring clarity as to the vagal maneuver of choice, Dr. Mehta reported at the annual meeting of the American College of Physicians.
He and his coinvestigators performed a meta-analysis of the three pre-2000 randomized controlled trials that compared the standard Valsalva maneuver to carotid sinus massage plus the two newer studies, both of which systematically compared a modified Valsalva maneuver with the standard version.
The clear winner in terms of efficacy was the modified Valsalva maneuver, in which patients with supraventricular tachycardia (SVT) performed a standardized strain while in a semirecumbent position, then immediately laid flat and had their legs raised to 45 degrees for 15 seconds before returning to the semirecumbent position. The purpose of this postural modification is to boost relaxation phase venous return and vagal stimulation.
In the 433-patient multicenter REVERT trial in the United Kingdom, 43% of those assigned to the modified Valsalva maneuver returned to sinus rhythm 1 minute after completing the task, compared with 17% of those randomized to the standard semirecumbent Valsalva maneuver. This resulted in significantly less need for adenosine and other treatments. Although REVERT investigators had the patients blow into a manometer at 40 mm Hg for 15 seconds, they noted that the same intensity of strain can be achieved more practically by blowing into a 10-mL syringe sufficient to just move the plunger (Lancet. 2015 Oct 31;386[10005]:1747-53).
The REVERT findings were confirmed by a second trial conducted by Turkish investigators, in which the modified Valsalva maneuver was successful in 43% of patients, compared with 11% in the standard Valsalva maneuver group (Am J Emerg Med. 2017 Nov;35[11]:1662-5).
Extrapolating from the published evidence, including a Cochrane Collaboration review (Cochrane Database Syst Rev. 2015 Feb 18;[2]:CD009502. doi: 10.1002/14651858.CD009502.pub3), Dr. Mehta and his coinvestigators ranked the likelihood of successful conversion of SVT to sinus rhythm from a high of 48% for the modified Valsalva maneuver, descending to 43% for a supine Valsalva maneuver, 36% for a standard semirecumbent Valsalva, 21% for a seated Valsalva, 19% for a standing one, and just 11% for carotid sinus massage.
“Based on evidence of high quality, we encourage that the modified Valsalva maneuver be done due its safety and low cost,” Dr. Mehta concluded.
He reported having no financial conflicts regarding his study, conducted free of commercial support.
NEW ORLEANS – , according to Jeet Mehta, MD, a resident in the combined medicine/pediatrics program at the University of Kansas, Wichita.
The 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommended vagal maneuvers as first-line treatment of supraventricular tachycardia, but added that there was no gold standard method. Since then, the situation has changed. Two well-conducted randomized clinical trials have been published that bring clarity as to the vagal maneuver of choice, Dr. Mehta reported at the annual meeting of the American College of Physicians.
He and his coinvestigators performed a meta-analysis of the three pre-2000 randomized controlled trials that compared the standard Valsalva maneuver to carotid sinus massage plus the two newer studies, both of which systematically compared a modified Valsalva maneuver with the standard version.
The clear winner in terms of efficacy was the modified Valsalva maneuver, in which patients with supraventricular tachycardia (SVT) performed a standardized strain while in a semirecumbent position, then immediately laid flat and had their legs raised to 45 degrees for 15 seconds before returning to the semirecumbent position. The purpose of this postural modification is to boost relaxation phase venous return and vagal stimulation.
In the 433-patient multicenter REVERT trial in the United Kingdom, 43% of those assigned to the modified Valsalva maneuver returned to sinus rhythm 1 minute after completing the task, compared with 17% of those randomized to the standard semirecumbent Valsalva maneuver. This resulted in significantly less need for adenosine and other treatments. Although REVERT investigators had the patients blow into a manometer at 40 mm Hg for 15 seconds, they noted that the same intensity of strain can be achieved more practically by blowing into a 10-mL syringe sufficient to just move the plunger (Lancet. 2015 Oct 31;386[10005]:1747-53).
The REVERT findings were confirmed by a second trial conducted by Turkish investigators, in which the modified Valsalva maneuver was successful in 43% of patients, compared with 11% in the standard Valsalva maneuver group (Am J Emerg Med. 2017 Nov;35[11]:1662-5).
Extrapolating from the published evidence, including a Cochrane Collaboration review (Cochrane Database Syst Rev. 2015 Feb 18;[2]:CD009502. doi: 10.1002/14651858.CD009502.pub3), Dr. Mehta and his coinvestigators ranked the likelihood of successful conversion of SVT to sinus rhythm from a high of 48% for the modified Valsalva maneuver, descending to 43% for a supine Valsalva maneuver, 36% for a standard semirecumbent Valsalva, 21% for a seated Valsalva, 19% for a standing one, and just 11% for carotid sinus massage.
“Based on evidence of high quality, we encourage that the modified Valsalva maneuver be done due its safety and low cost,” Dr. Mehta concluded.
He reported having no financial conflicts regarding his study, conducted free of commercial support.
REPORTING FROM ACP INTERNAL MEDICINE
Key clinical point: A simple postural modification to the standard Valsalva maneuver boosts conversion rate.
Major finding: Nearly half of patients in SVT converted to sinus rhythm in response to a modified Valsalva maneuver.
Study details: This was a meta-analysis of five randomized clinical trials of vagal maneuvers for conversion of supraventricular tachycardia to sinus rhythm.
Disclosures: The presenter reported having no financial conflicts regarding the study, conducted free of commercial support.
A new, simple, inexpensive DVT diagnostic aid
NEW ORLEANS – Both the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio proved to be better predictors of the presence or absence of deep vein thrombosis than the ubiquitous D-dimer test in a retrospective study, Jason Mouabbi, MD, reported at the annual meeting of the American College of Physicians.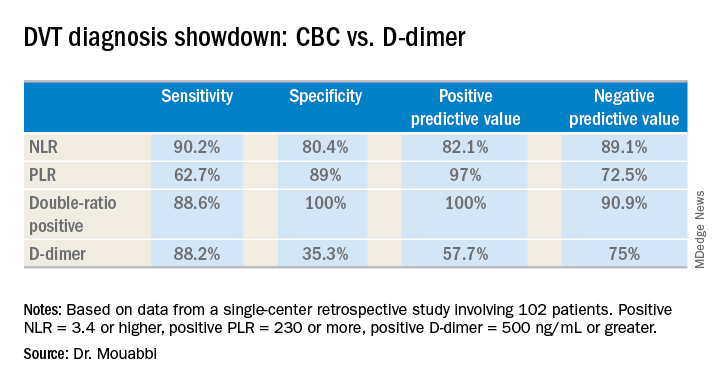
What’s more, both the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) can be readily calculated from the readout of a complete blood count (CBC) with differential. A CBC costs an average of $16, and everybody that comes through a hospital emergency department gets one. In contrast, the average charge for a D-dimer test is about $231 nationwide, and depending upon the specific test used the results can take up to a couple of hours to come back, noted Dr. Mouabbi of St. John Hospital and Medical Center in Detroit.
“The NLR and PLR ratios offer a new, powerful, affordable, simple, and readily available tool in the hands of clinicians to help them in the diagnosis of DVT,” he said. “The NLR can be useful to rule out DVT when it’s negative, whereas PLR can be useful in ruling DVT when positive.”
Investigators in a variety of fields are looking at the NLR and PLR as emerging practical, easily obtainable biomarkers for systemic inflammation. And DVT is thought to be an inflammatory process, he explained.
Dr. Mouabbi presented a single-center retrospective study of 102 matched patients who presented with lower extremity swelling and had a CBC drawn, as well as a D-dimer test, on the same day they underwent a lower extremity Doppler ultrasound evaluation. In 51 patients, the ultrasound revealed the presence of DVT and anticoagulation was started. In the other 51 patients, the ultrasound exam was negative and they weren’t anticoagulated. Since the study purpose was to assess the implications of a primary elevation of NLR and/or PLR, patients with rheumatic diseases, inflammatory bowel disease, recent surgery, chronic renal or liver disease, inherited thrombophilia, infection, or other possible secondary causes of altered ratios were excluded from the study.
A positive NLR was considered 3.4 or higher, a positive PLR was a ratio of 230 or more, and a positive D-dimer level was 500 ng/mL or greater. The NLR and PLR collectively outperformed the D-dimer test in terms of sensitivity, specificity, positive predictive value, and negative predictive value.
In addition, 89% of the DVT group were classified as “double-positive,” meaning they were both NLR and PLR positive. That combination provided the best diagnostic value of all, since none of the controls were double-positive and only 2% were PLR positive.
While the results are encouraging, before NLR and PLR can supplant D-dimer in patients with suspected DVT in clinical practice a confirmatory prospective study should be carried out, according to Dr. Mouabbi. Ideally it should include the use of the Wells score, which is part of most diagnostic algorithms as a preliminary means of categorizing DVT probability as low, moderate, or high. However, the popularity of the Wells score has fallen off in the face of reports that the results are subjective and variable. Indeed, the Wells score was included in the electronic medical record of so few participants in Dr. Mouabbi’s study that he couldn’t evaluate its utility.
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
NEW ORLEANS – Both the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio proved to be better predictors of the presence or absence of deep vein thrombosis than the ubiquitous D-dimer test in a retrospective study, Jason Mouabbi, MD, reported at the annual meeting of the American College of Physicians.
What’s more, both the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) can be readily calculated from the readout of a complete blood count (CBC) with differential. A CBC costs an average of $16, and everybody that comes through a hospital emergency department gets one. In contrast, the average charge for a D-dimer test is about $231 nationwide, and depending upon the specific test used the results can take up to a couple of hours to come back, noted Dr. Mouabbi of St. John Hospital and Medical Center in Detroit.
“The NLR and PLR ratios offer a new, powerful, affordable, simple, and readily available tool in the hands of clinicians to help them in the diagnosis of DVT,” he said. “The NLR can be useful to rule out DVT when it’s negative, whereas PLR can be useful in ruling DVT when positive.”
Investigators in a variety of fields are looking at the NLR and PLR as emerging practical, easily obtainable biomarkers for systemic inflammation. And DVT is thought to be an inflammatory process, he explained.
Dr. Mouabbi presented a single-center retrospective study of 102 matched patients who presented with lower extremity swelling and had a CBC drawn, as well as a D-dimer test, on the same day they underwent a lower extremity Doppler ultrasound evaluation. In 51 patients, the ultrasound revealed the presence of DVT and anticoagulation was started. In the other 51 patients, the ultrasound exam was negative and they weren’t anticoagulated. Since the study purpose was to assess the implications of a primary elevation of NLR and/or PLR, patients with rheumatic diseases, inflammatory bowel disease, recent surgery, chronic renal or liver disease, inherited thrombophilia, infection, or other possible secondary causes of altered ratios were excluded from the study.
A positive NLR was considered 3.4 or higher, a positive PLR was a ratio of 230 or more, and a positive D-dimer level was 500 ng/mL or greater. The NLR and PLR collectively outperformed the D-dimer test in terms of sensitivity, specificity, positive predictive value, and negative predictive value.
In addition, 89% of the DVT group were classified as “double-positive,” meaning they were both NLR and PLR positive. That combination provided the best diagnostic value of all, since none of the controls were double-positive and only 2% were PLR positive.
While the results are encouraging, before NLR and PLR can supplant D-dimer in patients with suspected DVT in clinical practice a confirmatory prospective study should be carried out, according to Dr. Mouabbi. Ideally it should include the use of the Wells score, which is part of most diagnostic algorithms as a preliminary means of categorizing DVT probability as low, moderate, or high. However, the popularity of the Wells score has fallen off in the face of reports that the results are subjective and variable. Indeed, the Wells score was included in the electronic medical record of so few participants in Dr. Mouabbi’s study that he couldn’t evaluate its utility.
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
NEW ORLEANS – Both the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio proved to be better predictors of the presence or absence of deep vein thrombosis than the ubiquitous D-dimer test in a retrospective study, Jason Mouabbi, MD, reported at the annual meeting of the American College of Physicians.
What’s more, both the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) can be readily calculated from the readout of a complete blood count (CBC) with differential. A CBC costs an average of $16, and everybody that comes through a hospital emergency department gets one. In contrast, the average charge for a D-dimer test is about $231 nationwide, and depending upon the specific test used the results can take up to a couple of hours to come back, noted Dr. Mouabbi of St. John Hospital and Medical Center in Detroit.
“The NLR and PLR ratios offer a new, powerful, affordable, simple, and readily available tool in the hands of clinicians to help them in the diagnosis of DVT,” he said. “The NLR can be useful to rule out DVT when it’s negative, whereas PLR can be useful in ruling DVT when positive.”
Investigators in a variety of fields are looking at the NLR and PLR as emerging practical, easily obtainable biomarkers for systemic inflammation. And DVT is thought to be an inflammatory process, he explained.
Dr. Mouabbi presented a single-center retrospective study of 102 matched patients who presented with lower extremity swelling and had a CBC drawn, as well as a D-dimer test, on the same day they underwent a lower extremity Doppler ultrasound evaluation. In 51 patients, the ultrasound revealed the presence of DVT and anticoagulation was started. In the other 51 patients, the ultrasound exam was negative and they weren’t anticoagulated. Since the study purpose was to assess the implications of a primary elevation of NLR and/or PLR, patients with rheumatic diseases, inflammatory bowel disease, recent surgery, chronic renal or liver disease, inherited thrombophilia, infection, or other possible secondary causes of altered ratios were excluded from the study.
A positive NLR was considered 3.4 or higher, a positive PLR was a ratio of 230 or more, and a positive D-dimer level was 500 ng/mL or greater. The NLR and PLR collectively outperformed the D-dimer test in terms of sensitivity, specificity, positive predictive value, and negative predictive value.
In addition, 89% of the DVT group were classified as “double-positive,” meaning they were both NLR and PLR positive. That combination provided the best diagnostic value of all, since none of the controls were double-positive and only 2% were PLR positive.
While the results are encouraging, before NLR and PLR can supplant D-dimer in patients with suspected DVT in clinical practice a confirmatory prospective study should be carried out, according to Dr. Mouabbi. Ideally it should include the use of the Wells score, which is part of most diagnostic algorithms as a preliminary means of categorizing DVT probability as low, moderate, or high. However, the popularity of the Wells score has fallen off in the face of reports that the results are subjective and variable. Indeed, the Wells score was included in the electronic medical record of so few participants in Dr. Mouabbi’s study that he couldn’t evaluate its utility.
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
REPORTING FROM ACP INTERNAL MEDICINE
Key clinical point:
Major finding: The neutrophil-to-lymphocyte ratio was better than the D-dimer test at helping to rule out DVT, while the platelet-to-lymphocyte ratio bested the D-dimer at ruling in DVT.
Study details: A retrospective study of 102 patients with suspected DVT.
Disclosures: Dr. Mouabbi reported no financial conflicts regarding his study, which was conducted free of commercial support.
Prepare for ‘the coming tsunami’ of NAFLD
NEW ORLEANS – Zobair M. Younossi, MD, declared at the annual meeting of the American College of Physicians.
The massive growth in nonalcoholic fatty liver disease (NAFLD) is being fueled to a great extent by the related epidemics of obesity and type 2 diabetes mellitus. While the overall prevalence of NAFLD worldwide is 24%, almost three-quarters of patients with NAFLD are obese. And the prevalence of NAFLD in individuals with T2DM was 58% in a recent meta-analysis of studies from 20 countries conducted by Dr. Younossi and his coinvestigators.
“The prevalence of NAFLD in U.S. kids is about 10%. This is of course part of the coming tsunami because our kids are getting obese, diabetic, and they’re going to have problems with NASH [nonalcoholic steatohepatitis],” said Dr. Younossi, a gastroenterologist who is professor and chairman of the department of medicine at the Inova Fairfax (Va.) campus of Virginia Commonwealth University.
NASH is the form of NAFLD that has the strongest prognostic implications. It can progress to cirrhosis, liver failure, or hepatocellular carcinoma. As Dr. Younossi and his coworkers have shown (Hepat Commun. 2017 Jun 6;1[5]:421-8), it is associated with a significantly greater risk of both liver-related and all-cause mortality than that of non-NASH NAFLD, although NAFLD also carries an increased risk of cardiovascular disease, the leading cause of death in that population.
In addition to highlighting the enormous clinical, economic, and quality-of-life implications of the NAFLD epidemic, Dr. Younossi offered practical tips on how busy primary care physicians can identify patients in their practice who have high-risk NAFLD. They have not done a very good job of this to date. That’s possibly due to lack of incentive, since in 2018 there is no approved drug for the treatment of NASH. He cited one representative retrospective study in which only about 15% of patients identified as having NAFLD received a recommendation for lifestyle modification involving diet and exercise, which is the standard evidence-based treatment, albeit admittedly difficult to sustain. And only 3% of patients with advanced liver fibrosis were referred to a specialist for management.
“So NAFLD is common, but its recognition and doing something about it is quite a challenge,” Dr. Younossi observed.
He argued that patients who have NASH deserve to know it because of its prognostic implications and also so they can have the chance to participate in one of the roughly two dozen ongoing clinical trials of potential therapies, some of which look quite promising. All of the trials required a liver biopsy as a condition for enrollment. Plus, once a patient is known to have stage 3 fibrosis, it’s time to start screening for hepatocellular carcinoma and esophageal varices.
The scope of the epidemic
NASH is the most rapidly growing indication for liver transplantation in the United States, with most of the increase coming from the baby boomer population. NASH is now the second most common indication for placement on the wait list. Meanwhile, liver transplantation due to the consequences of hepatitis C, the No. 1 indication, is declining as a result of the spectacular advances in medical treatment introduced a few years ago. It’s likely that in coming years NASH will take over the top spot, according to Dr. Younossi.
He was coauthor of a recent study that modeled the estimated trends for the NAFLD epidemic in the United States through 2030. The forecast is that the prevalence of NAFLD among adults will climb to 33.5% and the proportion of NAFLD categorized as NASH will increase from 20% at present to 27%. Moreover, this will result in a 168% jump in the incidence of decompensated cirrhosis, a 137% increase in the incidence of hepatocellular carcinoma, and a 178% increase in liver-related mortality, which will account for an estimated 78,300 deaths in 2030 (Hepatology. 2018 Jan;67[1]:123-33).
Practical ways to identify high-risk patients
The best noninvasive means of detecting NAFLD is by ultrasound showing a fatty liver. Often the condition is detected as an incidental finding on abdominal ultrasound ordered for another reason. Elevated liver enzymes can be a tipoff as well. Of course, alcoholic liver disease and other causes must be excluded.
But what’s most important is to identify patients with NASH. It’s a diagnosis made by biopsy. However, it is unthinkable to perform liver biopsies in the entire vast population with NAFLD, so there is a great deal of interest in developing noninvasive diagnostic modalities that can help zero in on the subset of high-risk NAFLD patients who should be considered for referral for liver biopsy.
One useful clue is the presence of comorbid metabolic syndrome in patients with NAFLD. It confers a substantially higher mortality risk – especially cardiovascular mortality – than does NAFLD without metabolic syndrome. Dr. Younossi and his coinvestigators have shown in a study of 3,613 NAFLD patients followed long-term that those with one component of the metabolic syndrome – either hypertension, central obesity, increased fasting plasma glucose, or hyperlipidemia – had 8- and 16-year all-cause mortality rates of 4.7% and 11.9%, nearly double the 2.6% and 6% rates in NAFLD patients with no elements of the metabolic syndrome.
Moreover, the magnitude of risk increased with each additional metabolic syndrome condition: a 3.57-fold increased mortality risk in NAFLD patients with two components of metabolic syndrome, a 5.87-fold increase in those with three, and a 13.09-fold increase in NAFLD patients with all four elements of metabolic syndrome (Medicine [Baltimore]. 2018 Mar;97[13]:e0214. doi: 10.1097/MD.0000000000010214).
Dr. Younossi was a member of the American Association for the Study of Liver Disease expert panel that developed the latest practice guidance regarding the diagnosis and management of NAFLD (Hepatology. 2018 Jan;67[1]:328-57). He said that probably the best simple noninvasive scoring system for the detection of NASH with advanced fibrosis is the NAFLD fibrosis score, which is easily calculated using laboratory values and clinical parameters already in a patient’s chart.
A more sophisticated serum biomarker test known as ELF, or the Enhanced Liver Fibrosis test, combines serum levels of hyaluronic acid, tissue inhibitor of metalloproteinase I, and procollagen amino terminal peptide.
“ELF is a very, very good test. It’s approved in Europe and I suspect it will be in the U.S. within the next year or so,” said Dr. Younossi.
The most exciting noninvasive tests, however, involve imaging that measures liver stiffness, which provides a fairly accurate indication of the degree of scarring in the organ. There are two methods available: vibration wave transient elastography and magnetic resonance elastography.
Transient elastography using the FibroScan device is commercially available in the United States. “It’s a good test, very easy to do, noninvasive. I have a couple of these machines, and we use them all the time,” the gastroenterologist said.
MR elastography provides superior accuracy, but access is an issue.
“At our institution you sometimes have to wait for weeks to get an outpatient MRI, so if you have hundreds of patients with fatty liver disease it makes things difficult. So in our practice we use transient elastography,” he explained.
Both imaging modalities also measure the amount of fat in the liver.
Dr. Younossi uses transient elastography in patients who don’t have type 2 diabetes or frank insulin resistance. If the FibroScan score is 7 kiloPascals or more, he considers liver biopsy, since that’s the threshold for detection of earlier, potentially reversible stage 2 fibrosis. If, however, a patient has diabetes or insulin resistance along with a NAFLD fibrosis score suggesting a high possibility of fibrosis, he sends that patient for liver biopsy, since those endocrinologic disorders are known to be independent risk factors for mortality in the setting of NAFLD.
Dr. Younossi reported having no financial conflicts of interest regarding his presentation.
NEW ORLEANS – Zobair M. Younossi, MD, declared at the annual meeting of the American College of Physicians.
The massive growth in nonalcoholic fatty liver disease (NAFLD) is being fueled to a great extent by the related epidemics of obesity and type 2 diabetes mellitus. While the overall prevalence of NAFLD worldwide is 24%, almost three-quarters of patients with NAFLD are obese. And the prevalence of NAFLD in individuals with T2DM was 58% in a recent meta-analysis of studies from 20 countries conducted by Dr. Younossi and his coinvestigators.
“The prevalence of NAFLD in U.S. kids is about 10%. This is of course part of the coming tsunami because our kids are getting obese, diabetic, and they’re going to have problems with NASH [nonalcoholic steatohepatitis],” said Dr. Younossi, a gastroenterologist who is professor and chairman of the department of medicine at the Inova Fairfax (Va.) campus of Virginia Commonwealth University.
NASH is the form of NAFLD that has the strongest prognostic implications. It can progress to cirrhosis, liver failure, or hepatocellular carcinoma. As Dr. Younossi and his coworkers have shown (Hepat Commun. 2017 Jun 6;1[5]:421-8), it is associated with a significantly greater risk of both liver-related and all-cause mortality than that of non-NASH NAFLD, although NAFLD also carries an increased risk of cardiovascular disease, the leading cause of death in that population.
In addition to highlighting the enormous clinical, economic, and quality-of-life implications of the NAFLD epidemic, Dr. Younossi offered practical tips on how busy primary care physicians can identify patients in their practice who have high-risk NAFLD. They have not done a very good job of this to date. That’s possibly due to lack of incentive, since in 2018 there is no approved drug for the treatment of NASH. He cited one representative retrospective study in which only about 15% of patients identified as having NAFLD received a recommendation for lifestyle modification involving diet and exercise, which is the standard evidence-based treatment, albeit admittedly difficult to sustain. And only 3% of patients with advanced liver fibrosis were referred to a specialist for management.
“So NAFLD is common, but its recognition and doing something about it is quite a challenge,” Dr. Younossi observed.
He argued that patients who have NASH deserve to know it because of its prognostic implications and also so they can have the chance to participate in one of the roughly two dozen ongoing clinical trials of potential therapies, some of which look quite promising. All of the trials required a liver biopsy as a condition for enrollment. Plus, once a patient is known to have stage 3 fibrosis, it’s time to start screening for hepatocellular carcinoma and esophageal varices.
The scope of the epidemic
NASH is the most rapidly growing indication for liver transplantation in the United States, with most of the increase coming from the baby boomer population. NASH is now the second most common indication for placement on the wait list. Meanwhile, liver transplantation due to the consequences of hepatitis C, the No. 1 indication, is declining as a result of the spectacular advances in medical treatment introduced a few years ago. It’s likely that in coming years NASH will take over the top spot, according to Dr. Younossi.
He was coauthor of a recent study that modeled the estimated trends for the NAFLD epidemic in the United States through 2030. The forecast is that the prevalence of NAFLD among adults will climb to 33.5% and the proportion of NAFLD categorized as NASH will increase from 20% at present to 27%. Moreover, this will result in a 168% jump in the incidence of decompensated cirrhosis, a 137% increase in the incidence of hepatocellular carcinoma, and a 178% increase in liver-related mortality, which will account for an estimated 78,300 deaths in 2030 (Hepatology. 2018 Jan;67[1]:123-33).
Practical ways to identify high-risk patients
The best noninvasive means of detecting NAFLD is by ultrasound showing a fatty liver. Often the condition is detected as an incidental finding on abdominal ultrasound ordered for another reason. Elevated liver enzymes can be a tipoff as well. Of course, alcoholic liver disease and other causes must be excluded.
But what’s most important is to identify patients with NASH. It’s a diagnosis made by biopsy. However, it is unthinkable to perform liver biopsies in the entire vast population with NAFLD, so there is a great deal of interest in developing noninvasive diagnostic modalities that can help zero in on the subset of high-risk NAFLD patients who should be considered for referral for liver biopsy.
One useful clue is the presence of comorbid metabolic syndrome in patients with NAFLD. It confers a substantially higher mortality risk – especially cardiovascular mortality – than does NAFLD without metabolic syndrome. Dr. Younossi and his coinvestigators have shown in a study of 3,613 NAFLD patients followed long-term that those with one component of the metabolic syndrome – either hypertension, central obesity, increased fasting plasma glucose, or hyperlipidemia – had 8- and 16-year all-cause mortality rates of 4.7% and 11.9%, nearly double the 2.6% and 6% rates in NAFLD patients with no elements of the metabolic syndrome.
Moreover, the magnitude of risk increased with each additional metabolic syndrome condition: a 3.57-fold increased mortality risk in NAFLD patients with two components of metabolic syndrome, a 5.87-fold increase in those with three, and a 13.09-fold increase in NAFLD patients with all four elements of metabolic syndrome (Medicine [Baltimore]. 2018 Mar;97[13]:e0214. doi: 10.1097/MD.0000000000010214).
Dr. Younossi was a member of the American Association for the Study of Liver Disease expert panel that developed the latest practice guidance regarding the diagnosis and management of NAFLD (Hepatology. 2018 Jan;67[1]:328-57). He said that probably the best simple noninvasive scoring system for the detection of NASH with advanced fibrosis is the NAFLD fibrosis score, which is easily calculated using laboratory values and clinical parameters already in a patient’s chart.
A more sophisticated serum biomarker test known as ELF, or the Enhanced Liver Fibrosis test, combines serum levels of hyaluronic acid, tissue inhibitor of metalloproteinase I, and procollagen amino terminal peptide.
“ELF is a very, very good test. It’s approved in Europe and I suspect it will be in the U.S. within the next year or so,” said Dr. Younossi.
The most exciting noninvasive tests, however, involve imaging that measures liver stiffness, which provides a fairly accurate indication of the degree of scarring in the organ. There are two methods available: vibration wave transient elastography and magnetic resonance elastography.
Transient elastography using the FibroScan device is commercially available in the United States. “It’s a good test, very easy to do, noninvasive. I have a couple of these machines, and we use them all the time,” the gastroenterologist said.
MR elastography provides superior accuracy, but access is an issue.
“At our institution you sometimes have to wait for weeks to get an outpatient MRI, so if you have hundreds of patients with fatty liver disease it makes things difficult. So in our practice we use transient elastography,” he explained.
Both imaging modalities also measure the amount of fat in the liver.
Dr. Younossi uses transient elastography in patients who don’t have type 2 diabetes or frank insulin resistance. If the FibroScan score is 7 kiloPascals or more, he considers liver biopsy, since that’s the threshold for detection of earlier, potentially reversible stage 2 fibrosis. If, however, a patient has diabetes or insulin resistance along with a NAFLD fibrosis score suggesting a high possibility of fibrosis, he sends that patient for liver biopsy, since those endocrinologic disorders are known to be independent risk factors for mortality in the setting of NAFLD.
Dr. Younossi reported having no financial conflicts of interest regarding his presentation.
NEW ORLEANS – Zobair M. Younossi, MD, declared at the annual meeting of the American College of Physicians.
The massive growth in nonalcoholic fatty liver disease (NAFLD) is being fueled to a great extent by the related epidemics of obesity and type 2 diabetes mellitus. While the overall prevalence of NAFLD worldwide is 24%, almost three-quarters of patients with NAFLD are obese. And the prevalence of NAFLD in individuals with T2DM was 58% in a recent meta-analysis of studies from 20 countries conducted by Dr. Younossi and his coinvestigators.
“The prevalence of NAFLD in U.S. kids is about 10%. This is of course part of the coming tsunami because our kids are getting obese, diabetic, and they’re going to have problems with NASH [nonalcoholic steatohepatitis],” said Dr. Younossi, a gastroenterologist who is professor and chairman of the department of medicine at the Inova Fairfax (Va.) campus of Virginia Commonwealth University.
NASH is the form of NAFLD that has the strongest prognostic implications. It can progress to cirrhosis, liver failure, or hepatocellular carcinoma. As Dr. Younossi and his coworkers have shown (Hepat Commun. 2017 Jun 6;1[5]:421-8), it is associated with a significantly greater risk of both liver-related and all-cause mortality than that of non-NASH NAFLD, although NAFLD also carries an increased risk of cardiovascular disease, the leading cause of death in that population.
In addition to highlighting the enormous clinical, economic, and quality-of-life implications of the NAFLD epidemic, Dr. Younossi offered practical tips on how busy primary care physicians can identify patients in their practice who have high-risk NAFLD. They have not done a very good job of this to date. That’s possibly due to lack of incentive, since in 2018 there is no approved drug for the treatment of NASH. He cited one representative retrospective study in which only about 15% of patients identified as having NAFLD received a recommendation for lifestyle modification involving diet and exercise, which is the standard evidence-based treatment, albeit admittedly difficult to sustain. And only 3% of patients with advanced liver fibrosis were referred to a specialist for management.
“So NAFLD is common, but its recognition and doing something about it is quite a challenge,” Dr. Younossi observed.
He argued that patients who have NASH deserve to know it because of its prognostic implications and also so they can have the chance to participate in one of the roughly two dozen ongoing clinical trials of potential therapies, some of which look quite promising. All of the trials required a liver biopsy as a condition for enrollment. Plus, once a patient is known to have stage 3 fibrosis, it’s time to start screening for hepatocellular carcinoma and esophageal varices.
The scope of the epidemic
NASH is the most rapidly growing indication for liver transplantation in the United States, with most of the increase coming from the baby boomer population. NASH is now the second most common indication for placement on the wait list. Meanwhile, liver transplantation due to the consequences of hepatitis C, the No. 1 indication, is declining as a result of the spectacular advances in medical treatment introduced a few years ago. It’s likely that in coming years NASH will take over the top spot, according to Dr. Younossi.
He was coauthor of a recent study that modeled the estimated trends for the NAFLD epidemic in the United States through 2030. The forecast is that the prevalence of NAFLD among adults will climb to 33.5% and the proportion of NAFLD categorized as NASH will increase from 20% at present to 27%. Moreover, this will result in a 168% jump in the incidence of decompensated cirrhosis, a 137% increase in the incidence of hepatocellular carcinoma, and a 178% increase in liver-related mortality, which will account for an estimated 78,300 deaths in 2030 (Hepatology. 2018 Jan;67[1]:123-33).
Practical ways to identify high-risk patients
The best noninvasive means of detecting NAFLD is by ultrasound showing a fatty liver. Often the condition is detected as an incidental finding on abdominal ultrasound ordered for another reason. Elevated liver enzymes can be a tipoff as well. Of course, alcoholic liver disease and other causes must be excluded.
But what’s most important is to identify patients with NASH. It’s a diagnosis made by biopsy. However, it is unthinkable to perform liver biopsies in the entire vast population with NAFLD, so there is a great deal of interest in developing noninvasive diagnostic modalities that can help zero in on the subset of high-risk NAFLD patients who should be considered for referral for liver biopsy.
One useful clue is the presence of comorbid metabolic syndrome in patients with NAFLD. It confers a substantially higher mortality risk – especially cardiovascular mortality – than does NAFLD without metabolic syndrome. Dr. Younossi and his coinvestigators have shown in a study of 3,613 NAFLD patients followed long-term that those with one component of the metabolic syndrome – either hypertension, central obesity, increased fasting plasma glucose, or hyperlipidemia – had 8- and 16-year all-cause mortality rates of 4.7% and 11.9%, nearly double the 2.6% and 6% rates in NAFLD patients with no elements of the metabolic syndrome.
Moreover, the magnitude of risk increased with each additional metabolic syndrome condition: a 3.57-fold increased mortality risk in NAFLD patients with two components of metabolic syndrome, a 5.87-fold increase in those with three, and a 13.09-fold increase in NAFLD patients with all four elements of metabolic syndrome (Medicine [Baltimore]. 2018 Mar;97[13]:e0214. doi: 10.1097/MD.0000000000010214).
Dr. Younossi was a member of the American Association for the Study of Liver Disease expert panel that developed the latest practice guidance regarding the diagnosis and management of NAFLD (Hepatology. 2018 Jan;67[1]:328-57). He said that probably the best simple noninvasive scoring system for the detection of NASH with advanced fibrosis is the NAFLD fibrosis score, which is easily calculated using laboratory values and clinical parameters already in a patient’s chart.
A more sophisticated serum biomarker test known as ELF, or the Enhanced Liver Fibrosis test, combines serum levels of hyaluronic acid, tissue inhibitor of metalloproteinase I, and procollagen amino terminal peptide.
“ELF is a very, very good test. It’s approved in Europe and I suspect it will be in the U.S. within the next year or so,” said Dr. Younossi.
The most exciting noninvasive tests, however, involve imaging that measures liver stiffness, which provides a fairly accurate indication of the degree of scarring in the organ. There are two methods available: vibration wave transient elastography and magnetic resonance elastography.
Transient elastography using the FibroScan device is commercially available in the United States. “It’s a good test, very easy to do, noninvasive. I have a couple of these machines, and we use them all the time,” the gastroenterologist said.
MR elastography provides superior accuracy, but access is an issue.
“At our institution you sometimes have to wait for weeks to get an outpatient MRI, so if you have hundreds of patients with fatty liver disease it makes things difficult. So in our practice we use transient elastography,” he explained.
Both imaging modalities also measure the amount of fat in the liver.
Dr. Younossi uses transient elastography in patients who don’t have type 2 diabetes or frank insulin resistance. If the FibroScan score is 7 kiloPascals or more, he considers liver biopsy, since that’s the threshold for detection of earlier, potentially reversible stage 2 fibrosis. If, however, a patient has diabetes or insulin resistance along with a NAFLD fibrosis score suggesting a high possibility of fibrosis, he sends that patient for liver biopsy, since those endocrinologic disorders are known to be independent risk factors for mortality in the setting of NAFLD.
Dr. Younossi reported having no financial conflicts of interest regarding his presentation.
REPORTING FROM ACP INTERNAL MEDICINE
Next-gen sputum PCR panel boosts CAP diagnostics
NEW ORLEANS – A next-generation lower respiratory tract sputum polymerase chain reaction (PCR) film array panel identified etiologic pathogens in 100% of a group of patients hospitalized for community-acquired pneumonia, Kathryn Hendrickson, MD, reported at the annual meeting of the American College of Physicians.
The investigational new diagnostic assay, the BioFire Pneumonia Panel, is now under Food and Drug Administration review for marketing clearance. (CAP), observed Dr. Hendrickson, an internal medicine resident at Providence Portland (Ore.) Medical Center. The new product is designed to complement the currently available respiratory panels from BioFire.
“Rapid-detection results in less empiric antibiotic use in hospitalized patients. When it’s FDA approved, this investigational sputum PCR panel will simplify the diagnostic bundle while improving antibiotic stewardship,” she observed.
She presented a prospective study of 63 patients with CAP hospitalized at the medical center, all of whom were evaluated by two laboratory methods: the hospital’s standard bundle of diagnostic tests and the new BioFire film array panel. The purpose was to determine if there was a difference between the two tests in the detection rate of viral and/or bacterial pathogens as well as the clinical significance of any such differences; that is, was there an impact on days of treatment and length of hospital stay?
Traditional diagnostic methods detect an etiologic pathogen in at best half of hospitalized CAP patients, and the results take too much time. So Providence Portland Medical Center adopted as its standard diagnostic bundle a nasopharyngeal swab and a BioFire film array PCR that’s currently on the market and can detect nine viruses and three bacteria, along with urine antigens for Legionella sp. and Streptococcus pneumoniae, nucleic acid amplification testing for S. pneumoniae and Staphylococcus aureus, and blood and sputum cultures. In contrast, the investigational panel probes for 17 viruses, 18 bacterial pathogens, and seven antibiotic-resistant genes; it also measures procalcitonin levels in order to distinguish between bacterial colonization and invasion.
The new BioFire Pneumonia Panel detected a mean of 1.4 species of pathogenic bacteria in 79% of patients, while the standard diagnostic bundle detected 0.7 species in 59% of patients. The investigational panel identified a mean of 1.0 species of viral pathogens in 86% of the CAP patients; the standard bundle detected a mean of 0.6 species in 56%.
All told, any CAP pathogen was detected in 100% of patients using the new panel, with a mean of 2.5 different pathogens identified. The standard bundle detected any pathogen in 84% of patients, with half as many different pathogens found, according to Dr. Hendrickson.
A peak procalcitonin level of 0.25 ng/mL or less, which was defined as bacterial colonization, was associated with a mean 7 days of treatment, while a level above that threshold was associated with 11.3 days of treatment. Patients with a peak procalcitonin of 0.25 ng/mL or less had an average hospital length of stay of 5.9 days, versus 7.8 days for those with a higher procalcitonin indicative of bacterial invasion.
The new biofilm assay reports information about the abundance of 15 of the 18 bacterial targets in the sample. However, in contrast to peak procalcitonin, Dr. Hendrickson and her coinvestigators didn’t find this bacterial quantitation feature to be substantially more useful than a coin flip in distinguishing bacterial colonization from invasion.
She reported having no financial conflicts regarding the head-to-head comparative study, which was supported by BioFire Diagnostics.
NEW ORLEANS – A next-generation lower respiratory tract sputum polymerase chain reaction (PCR) film array panel identified etiologic pathogens in 100% of a group of patients hospitalized for community-acquired pneumonia, Kathryn Hendrickson, MD, reported at the annual meeting of the American College of Physicians.
The investigational new diagnostic assay, the BioFire Pneumonia Panel, is now under Food and Drug Administration review for marketing clearance. (CAP), observed Dr. Hendrickson, an internal medicine resident at Providence Portland (Ore.) Medical Center. The new product is designed to complement the currently available respiratory panels from BioFire.
“Rapid-detection results in less empiric antibiotic use in hospitalized patients. When it’s FDA approved, this investigational sputum PCR panel will simplify the diagnostic bundle while improving antibiotic stewardship,” she observed.
She presented a prospective study of 63 patients with CAP hospitalized at the medical center, all of whom were evaluated by two laboratory methods: the hospital’s standard bundle of diagnostic tests and the new BioFire film array panel. The purpose was to determine if there was a difference between the two tests in the detection rate of viral and/or bacterial pathogens as well as the clinical significance of any such differences; that is, was there an impact on days of treatment and length of hospital stay?
Traditional diagnostic methods detect an etiologic pathogen in at best half of hospitalized CAP patients, and the results take too much time. So Providence Portland Medical Center adopted as its standard diagnostic bundle a nasopharyngeal swab and a BioFire film array PCR that’s currently on the market and can detect nine viruses and three bacteria, along with urine antigens for Legionella sp. and Streptococcus pneumoniae, nucleic acid amplification testing for S. pneumoniae and Staphylococcus aureus, and blood and sputum cultures. In contrast, the investigational panel probes for 17 viruses, 18 bacterial pathogens, and seven antibiotic-resistant genes; it also measures procalcitonin levels in order to distinguish between bacterial colonization and invasion.
The new BioFire Pneumonia Panel detected a mean of 1.4 species of pathogenic bacteria in 79% of patients, while the standard diagnostic bundle detected 0.7 species in 59% of patients. The investigational panel identified a mean of 1.0 species of viral pathogens in 86% of the CAP patients; the standard bundle detected a mean of 0.6 species in 56%.
All told, any CAP pathogen was detected in 100% of patients using the new panel, with a mean of 2.5 different pathogens identified. The standard bundle detected any pathogen in 84% of patients, with half as many different pathogens found, according to Dr. Hendrickson.
A peak procalcitonin level of 0.25 ng/mL or less, which was defined as bacterial colonization, was associated with a mean 7 days of treatment, while a level above that threshold was associated with 11.3 days of treatment. Patients with a peak procalcitonin of 0.25 ng/mL or less had an average hospital length of stay of 5.9 days, versus 7.8 days for those with a higher procalcitonin indicative of bacterial invasion.
The new biofilm assay reports information about the abundance of 15 of the 18 bacterial targets in the sample. However, in contrast to peak procalcitonin, Dr. Hendrickson and her coinvestigators didn’t find this bacterial quantitation feature to be substantially more useful than a coin flip in distinguishing bacterial colonization from invasion.
She reported having no financial conflicts regarding the head-to-head comparative study, which was supported by BioFire Diagnostics.
NEW ORLEANS – A next-generation lower respiratory tract sputum polymerase chain reaction (PCR) film array panel identified etiologic pathogens in 100% of a group of patients hospitalized for community-acquired pneumonia, Kathryn Hendrickson, MD, reported at the annual meeting of the American College of Physicians.
The investigational new diagnostic assay, the BioFire Pneumonia Panel, is now under Food and Drug Administration review for marketing clearance. (CAP), observed Dr. Hendrickson, an internal medicine resident at Providence Portland (Ore.) Medical Center. The new product is designed to complement the currently available respiratory panels from BioFire.
“Rapid-detection results in less empiric antibiotic use in hospitalized patients. When it’s FDA approved, this investigational sputum PCR panel will simplify the diagnostic bundle while improving antibiotic stewardship,” she observed.
She presented a prospective study of 63 patients with CAP hospitalized at the medical center, all of whom were evaluated by two laboratory methods: the hospital’s standard bundle of diagnostic tests and the new BioFire film array panel. The purpose was to determine if there was a difference between the two tests in the detection rate of viral and/or bacterial pathogens as well as the clinical significance of any such differences; that is, was there an impact on days of treatment and length of hospital stay?
Traditional diagnostic methods detect an etiologic pathogen in at best half of hospitalized CAP patients, and the results take too much time. So Providence Portland Medical Center adopted as its standard diagnostic bundle a nasopharyngeal swab and a BioFire film array PCR that’s currently on the market and can detect nine viruses and three bacteria, along with urine antigens for Legionella sp. and Streptococcus pneumoniae, nucleic acid amplification testing for S. pneumoniae and Staphylococcus aureus, and blood and sputum cultures. In contrast, the investigational panel probes for 17 viruses, 18 bacterial pathogens, and seven antibiotic-resistant genes; it also measures procalcitonin levels in order to distinguish between bacterial colonization and invasion.
The new BioFire Pneumonia Panel detected a mean of 1.4 species of pathogenic bacteria in 79% of patients, while the standard diagnostic bundle detected 0.7 species in 59% of patients. The investigational panel identified a mean of 1.0 species of viral pathogens in 86% of the CAP patients; the standard bundle detected a mean of 0.6 species in 56%.
All told, any CAP pathogen was detected in 100% of patients using the new panel, with a mean of 2.5 different pathogens identified. The standard bundle detected any pathogen in 84% of patients, with half as many different pathogens found, according to Dr. Hendrickson.
A peak procalcitonin level of 0.25 ng/mL or less, which was defined as bacterial colonization, was associated with a mean 7 days of treatment, while a level above that threshold was associated with 11.3 days of treatment. Patients with a peak procalcitonin of 0.25 ng/mL or less had an average hospital length of stay of 5.9 days, versus 7.8 days for those with a higher procalcitonin indicative of bacterial invasion.
The new biofilm assay reports information about the abundance of 15 of the 18 bacterial targets in the sample. However, in contrast to peak procalcitonin, Dr. Hendrickson and her coinvestigators didn’t find this bacterial quantitation feature to be substantially more useful than a coin flip in distinguishing bacterial colonization from invasion.
She reported having no financial conflicts regarding the head-to-head comparative study, which was supported by BioFire Diagnostics.
REPORTING FROM ACP INTERNAL MEDICINE
Key clinical point: A new CAP diagnostic panel represents a significant advance in clinical care.
Major finding: The investigational BioFire Pneumonia Panel identified specific pathogens in 100% of patients hospitalized for CAP, compared with 84% using the hospital’s standard test bundle.
Study details: This was a prospective head-to-head study comparing two approaches to identification of specific pathogens in 63 patients hospitalized for CAP.
Disclosures: The study was supported by BioFire Diagnostics. The presenter reported having no financial conflicts.
ACP roadmap to raise adult immunization rates
NEW ORLEANS – carried out in seven rural southwestern Georgia counties.
“We saw increases of 52%-93% in our pneumococcal vaccination rates in our nine adult medicine clinics,” Frances E. Ferguson, MD, said at the annual meeting of the American College of Physicians.
The project, conducted through the ACP’s quality improvement program, known as Quality Connect, with funding from the Centers for Disease Control and Prevention, has as its ultimate goal making adult immunization standard practice across the country in general internal medicine and primary care. One of the initial pilot projects was conducted at Albany (Ga.) Area Primary Health Care, a federally qualified community health center with 28 service delivery sites in seven mainly rural southwestern Georgia counties. The majority of the center’s patients are at or below the poverty level. The lessons learned from this and other successful local projects will be disseminated nationally, according to Dr. Ferguson, a general internist at the health center.
The project utilizes the ACP Quality Connect “Plan, Do, Study, Act” approach to implementing constructive changes in medical practice, coupled with an abundance of resources readily available from the nonprofit Immunization Action Coalition, which has been funded by the CDC for more than 20 years.
The coalition’s website includes easily downloadable and custom-modifiable standing orders, plain-language information handouts on pneumococcal pneumonia and other vaccine-preventable illnesses and possible vaccine side effects for patients to read in the waiting room before their office visit, concise CDC-standardized talking points for providers to use in convincing patients to get vaccinated, as well as a detailed 10-point action plan for medical practice leadership to make it all happen.
“You don’t have to reinvent the wheel with this. You just go to their website,” Dr. Ferguson explained.
She cited her personal experience as representative of that of her fellow general internists who got on board with the project’s goal. As of April 2016, just before rollout of the pilot project, Dr. Ferguson’s pneumococcal vaccination rate stood at 22.5%. One month into the project, her vaccination rate had zoomed to 60%. When the formal pilot project ended in February 2017, her rate was 88.2%, an absolute 65.7% increase in 10 months. And since the pilot project’s conclusion, adult pneumococcal vaccination rates across Albany Area Primary Health Care have continued to climb.
“We have continued to improve because our champions are still in place, and our standing orders are, too,” she noted.
Standing orders are at the core of the project’s success, according to Dr. Ferguson. They provide nurses with the legal authority to determine if a patient is eligible for a vaccine and to go ahead and give it before seeing the physician.
“The most important thing for me is that nurses are an essential and invaluable asset to your practice, especially when you empower them to function fully within the scope of their practice. They can do amazing things. I just feel like the nurses are the winners here. We are winners because of our nurses,” she said.
It wasn’t easy at first, Dr. Ferguson recalled.
“We had pushback from nurses because they were afraid to do anything without the doctor directly telling them to do it. And we had physicians who didn’t want to have standing orders because they didn’t want anybody to do anything until they told them to. But we managed to get all that straightened out in the first month,” according to the internist.
Other keys to the program’s success included designating champions of the project at every clinic. Often this was the director of nursing, who was appointed to be in charge of the standing orders program. This “champions” concept is detailed in the ACP Quality Connect website. The champion – Dr. Ferguson was one – gets staff buy in on promoting the importance of immunizations, teaches strategies, and engages in community outreach.
“They lead the practice in the quality improvement project. We found that was very important, to have someone at each site who could keep the fire under the staff, keep the project going even when it’s very busy and everybody’s running around. There has to be someone there who’s encouraging people to remember to keep giving immunizations in spite of everything that’s going on that day,” she explained.
A central principle of successful quality improvement programs is accurate performance data gathering and dissemination. “Doctors are very competitive people,” Dr. Ferguson observed. “When I tell you you’re not doing as well as your colleagues and I can show you a graph that shows how far you are lagging behind, you get the fire under you and get moving.”
Audience members were agog at the community health center’s stratospheric vaccination rates. What about all the vaccine skeptics? they asked.
“I think for us it’s a matter of trust,” she replied. “We are a community health center and many of our patients and our providers have been with us for a long time. The nursing staff live in those communities, so the patients know them well. The CDC’s cards with talking points are a big help. I take the time to talk to patients and explain how the vaccine is going to build an army of immunologic protection. There are always going to be the diehards who say, ‘My cousin’s foot fell off when he got the pneumonia vaccine,’ though. You can’t get past those people. There’s just nothing you can say to them that will change their mind.”
She reported having no financial conflicts regarding her presentation or the adult immunization initiative.
bjancin@mdedge.com
NEW ORLEANS – carried out in seven rural southwestern Georgia counties.
“We saw increases of 52%-93% in our pneumococcal vaccination rates in our nine adult medicine clinics,” Frances E. Ferguson, MD, said at the annual meeting of the American College of Physicians.
The project, conducted through the ACP’s quality improvement program, known as Quality Connect, with funding from the Centers for Disease Control and Prevention, has as its ultimate goal making adult immunization standard practice across the country in general internal medicine and primary care. One of the initial pilot projects was conducted at Albany (Ga.) Area Primary Health Care, a federally qualified community health center with 28 service delivery sites in seven mainly rural southwestern Georgia counties. The majority of the center’s patients are at or below the poverty level. The lessons learned from this and other successful local projects will be disseminated nationally, according to Dr. Ferguson, a general internist at the health center.
The project utilizes the ACP Quality Connect “Plan, Do, Study, Act” approach to implementing constructive changes in medical practice, coupled with an abundance of resources readily available from the nonprofit Immunization Action Coalition, which has been funded by the CDC for more than 20 years.
The coalition’s website includes easily downloadable and custom-modifiable standing orders, plain-language information handouts on pneumococcal pneumonia and other vaccine-preventable illnesses and possible vaccine side effects for patients to read in the waiting room before their office visit, concise CDC-standardized talking points for providers to use in convincing patients to get vaccinated, as well as a detailed 10-point action plan for medical practice leadership to make it all happen.
“You don’t have to reinvent the wheel with this. You just go to their website,” Dr. Ferguson explained.
She cited her personal experience as representative of that of her fellow general internists who got on board with the project’s goal. As of April 2016, just before rollout of the pilot project, Dr. Ferguson’s pneumococcal vaccination rate stood at 22.5%. One month into the project, her vaccination rate had zoomed to 60%. When the formal pilot project ended in February 2017, her rate was 88.2%, an absolute 65.7% increase in 10 months. And since the pilot project’s conclusion, adult pneumococcal vaccination rates across Albany Area Primary Health Care have continued to climb.
“We have continued to improve because our champions are still in place, and our standing orders are, too,” she noted.
Standing orders are at the core of the project’s success, according to Dr. Ferguson. They provide nurses with the legal authority to determine if a patient is eligible for a vaccine and to go ahead and give it before seeing the physician.
“The most important thing for me is that nurses are an essential and invaluable asset to your practice, especially when you empower them to function fully within the scope of their practice. They can do amazing things. I just feel like the nurses are the winners here. We are winners because of our nurses,” she said.
It wasn’t easy at first, Dr. Ferguson recalled.
“We had pushback from nurses because they were afraid to do anything without the doctor directly telling them to do it. And we had physicians who didn’t want to have standing orders because they didn’t want anybody to do anything until they told them to. But we managed to get all that straightened out in the first month,” according to the internist.
Other keys to the program’s success included designating champions of the project at every clinic. Often this was the director of nursing, who was appointed to be in charge of the standing orders program. This “champions” concept is detailed in the ACP Quality Connect website. The champion – Dr. Ferguson was one – gets staff buy in on promoting the importance of immunizations, teaches strategies, and engages in community outreach.
“They lead the practice in the quality improvement project. We found that was very important, to have someone at each site who could keep the fire under the staff, keep the project going even when it’s very busy and everybody’s running around. There has to be someone there who’s encouraging people to remember to keep giving immunizations in spite of everything that’s going on that day,” she explained.
A central principle of successful quality improvement programs is accurate performance data gathering and dissemination. “Doctors are very competitive people,” Dr. Ferguson observed. “When I tell you you’re not doing as well as your colleagues and I can show you a graph that shows how far you are lagging behind, you get the fire under you and get moving.”
Audience members were agog at the community health center’s stratospheric vaccination rates. What about all the vaccine skeptics? they asked.
“I think for us it’s a matter of trust,” she replied. “We are a community health center and many of our patients and our providers have been with us for a long time. The nursing staff live in those communities, so the patients know them well. The CDC’s cards with talking points are a big help. I take the time to talk to patients and explain how the vaccine is going to build an army of immunologic protection. There are always going to be the diehards who say, ‘My cousin’s foot fell off when he got the pneumonia vaccine,’ though. You can’t get past those people. There’s just nothing you can say to them that will change their mind.”
She reported having no financial conflicts regarding her presentation or the adult immunization initiative.
bjancin@mdedge.com
NEW ORLEANS – carried out in seven rural southwestern Georgia counties.
“We saw increases of 52%-93% in our pneumococcal vaccination rates in our nine adult medicine clinics,” Frances E. Ferguson, MD, said at the annual meeting of the American College of Physicians.
The project, conducted through the ACP’s quality improvement program, known as Quality Connect, with funding from the Centers for Disease Control and Prevention, has as its ultimate goal making adult immunization standard practice across the country in general internal medicine and primary care. One of the initial pilot projects was conducted at Albany (Ga.) Area Primary Health Care, a federally qualified community health center with 28 service delivery sites in seven mainly rural southwestern Georgia counties. The majority of the center’s patients are at or below the poverty level. The lessons learned from this and other successful local projects will be disseminated nationally, according to Dr. Ferguson, a general internist at the health center.
The project utilizes the ACP Quality Connect “Plan, Do, Study, Act” approach to implementing constructive changes in medical practice, coupled with an abundance of resources readily available from the nonprofit Immunization Action Coalition, which has been funded by the CDC for more than 20 years.
The coalition’s website includes easily downloadable and custom-modifiable standing orders, plain-language information handouts on pneumococcal pneumonia and other vaccine-preventable illnesses and possible vaccine side effects for patients to read in the waiting room before their office visit, concise CDC-standardized talking points for providers to use in convincing patients to get vaccinated, as well as a detailed 10-point action plan for medical practice leadership to make it all happen.
“You don’t have to reinvent the wheel with this. You just go to their website,” Dr. Ferguson explained.
She cited her personal experience as representative of that of her fellow general internists who got on board with the project’s goal. As of April 2016, just before rollout of the pilot project, Dr. Ferguson’s pneumococcal vaccination rate stood at 22.5%. One month into the project, her vaccination rate had zoomed to 60%. When the formal pilot project ended in February 2017, her rate was 88.2%, an absolute 65.7% increase in 10 months. And since the pilot project’s conclusion, adult pneumococcal vaccination rates across Albany Area Primary Health Care have continued to climb.
“We have continued to improve because our champions are still in place, and our standing orders are, too,” she noted.
Standing orders are at the core of the project’s success, according to Dr. Ferguson. They provide nurses with the legal authority to determine if a patient is eligible for a vaccine and to go ahead and give it before seeing the physician.
“The most important thing for me is that nurses are an essential and invaluable asset to your practice, especially when you empower them to function fully within the scope of their practice. They can do amazing things. I just feel like the nurses are the winners here. We are winners because of our nurses,” she said.
It wasn’t easy at first, Dr. Ferguson recalled.
“We had pushback from nurses because they were afraid to do anything without the doctor directly telling them to do it. And we had physicians who didn’t want to have standing orders because they didn’t want anybody to do anything until they told them to. But we managed to get all that straightened out in the first month,” according to the internist.
Other keys to the program’s success included designating champions of the project at every clinic. Often this was the director of nursing, who was appointed to be in charge of the standing orders program. This “champions” concept is detailed in the ACP Quality Connect website. The champion – Dr. Ferguson was one – gets staff buy in on promoting the importance of immunizations, teaches strategies, and engages in community outreach.
“They lead the practice in the quality improvement project. We found that was very important, to have someone at each site who could keep the fire under the staff, keep the project going even when it’s very busy and everybody’s running around. There has to be someone there who’s encouraging people to remember to keep giving immunizations in spite of everything that’s going on that day,” she explained.
A central principle of successful quality improvement programs is accurate performance data gathering and dissemination. “Doctors are very competitive people,” Dr. Ferguson observed. “When I tell you you’re not doing as well as your colleagues and I can show you a graph that shows how far you are lagging behind, you get the fire under you and get moving.”
Audience members were agog at the community health center’s stratospheric vaccination rates. What about all the vaccine skeptics? they asked.
“I think for us it’s a matter of trust,” she replied. “We are a community health center and many of our patients and our providers have been with us for a long time. The nursing staff live in those communities, so the patients know them well. The CDC’s cards with talking points are a big help. I take the time to talk to patients and explain how the vaccine is going to build an army of immunologic protection. There are always going to be the diehards who say, ‘My cousin’s foot fell off when he got the pneumonia vaccine,’ though. You can’t get past those people. There’s just nothing you can say to them that will change their mind.”
She reported having no financial conflicts regarding her presentation or the adult immunization initiative.
bjancin@mdedge.com
REPORTING FROM ACP INTERNAL MEDICINE
How to prescribe effectively for opioid use disorder
NEW ORLEANS – Physicians committed to fighting the national opioid epidemic really need to take the 8-hour training course on addiction treatment required to obtain a Drug Enforcement Administration ‘X’ number, because it will enable them to prescribe buprenorphine, a drug with unique advantages for many affected patients, Ellie Grossman, MD, asserted at the annual meeting of the American College of Physicians.
Buprenorphine (Subutex) is one of the three medications approved for treatment of opioid use disorder (OUD), along with methadone and naltrexone (Revia). And for certain patients, it’s clearly the best choice, according to Dr. Grossman, a general internist at Harvard Medical School, Boston, and the primary care lead for behavioral health integration at the Cambridge (Mass.) Health Alliance.
The DEA X number certification process, which entails obtaining a waiver through SAMHSA – the Substance Abuse and Mental Health Services Administration – is bureaucratic. It’s unpopular with many physicians. But it’s well worth 8 hours of an internist’s time to get the waiver and gain the ability to prescribe buprenorphine.
“The requirement is admittedly clunky, and many people have strong feelings about whether this is a regulation that should exist,” according to Dr. Grossman. “I myself didn’t need to have special training to prescribe methadone, a full opioid agonist that my patients could easily die from. But I did have to undergo an 8-hour training to prescribe buprenorphine, and it’s much harder to die from that drug.”
She addressed which of the three medications for OUD is the best fit in a given patient, the appropriate treatment duration, and the role of adjunctive counseling, which – spoiler alert – has been cast into question by the results of a major government-funded randomized trial.
Dr. Grossman’s overriding message: “You are saving lives by getting people on medication.”
Indeed, studies have shown that patients with OUD who receive no treatment have a sixfold increase in the standardized mortality ratio, compared with the general population. Contrast that with the less than 2-fold increased risk with medication-assisted treatment and roughly a 2.5-fold increased risk when medication is given short term to cover withdrawal and then tapered and discontinued.
Other documented benefits of long-term medication-assisted treatment of patients with OUD as described in a 2014 Cochrane review include reductions in injection drug use, crime days, HIV-related risk behaviors and seroconversion, and improved health and social functioning.
Of note, those well-documented benefits apply only to methadone, a full opioid agonist, and buprenorphine, a partial agonist, because those two drugs have been around long enough to generate long-term outcome data. Naltrexone, which has a completely different mechanism of action – it’s a full opioid antagonist – has not as of yet.
Individualizing medical therapy for OUD
Physicians can’t write a prescription for methadone. The drug must be administered at a certified opioid treatment program, or OTP, otherwise known as a methadone clinic. Those clinics are highly regulated at both the federal and state levels, with lots of minutia involved. Patient counseling and drug screening are required.
In contrast, a physician with a DEA X number can write a prescription for buprenorphine and have a patient fill it at a pharmacy. There is inherently less structure surrounding buprenorphine therapy than that of methadone, Dr. Grossman noted. There are no hard and fast rules about how often a physician has to see the patient or do drug screens or counseling. Buprenorphine is available as once-daily oral sublingual therapy and, more recently, in long-acting injectable and implantable formulations, although Dr. Grossman believes the jury is still out about how these nonoral agents are best utilized.
“I’m often asked, ‘Which is better, methadone or buprenorphine?’ Really, the answer is they’re both pretty darn good,” according to Dr. Grossman.
The Cochrane review concluded that, in the studies that have used real-world dosing – that is, higher doses than in the initial studies – high-dose buprenorphine and high-dose methadone have similar rates of retention in treatment.
“What I tell patients is that a lot hinges on the structure of the treatment delivery system,” Dr. Grossman said. “If it’s methadone, they’re going to the OTP every day. Some people need more structure; they need a set of eyes on them every day. Or if they are at high risk for medication diversion – for example, someone else in their household might want to steal their medications – going to a methadone program gets around that. Also, when somebody has been on methadone in the past and did well on it and wants to go on it again, I’m likely to say, ‘That sounds like a good fit.’”
Buprenorphine is a good option for patients who don’t require close, structured supervision. It has fewer drug interactions than does methadone and is less prone to cause QTc prolongation. Also, it’s a more realistic option for patients who live so far from an OTP that daily attendance is impractical. And ob.gyns increasingly favor buprenorphine, because the problem of neonatal abstinence syndrome is less severe than when mothers are on methadone.
As for extended-release naltrexone (Vivitrol), the pivotal double-blind Russian trial that won FDA approval for treatment of OUD showed a dramatic improvement in opioid-free weeks (Lancet. 2011 Apr 30;377[9776]:1506-13).
More recently, the 24-week, multicenter, open-label X:BOT trial randomized 570 U.S. patients with OUD to once-monthly extended-release naltrexone or daily sublingual buprenorphine-naloxone (Suboxone). The dropout rate was higher in the extended-release naltrexone arm because patients had to be opioid free for 2 weeks before starting on the opioid antagonist. As a practical matter, that can be difficult to achieve unless a patient has just been released from jail or prison. But the per-protocol relapse rates were similar (Lancet. 2018 Jan 27;391[10118]:309-18).
“Many people interpret this study as saying, with the right patient who can get into an opioid-free state or, if you inherit an opioid-free state, the choice between extended-release naltrexone and buprenorphine-naloxone may be a bit of a wash in terms of clinical effectiveness, as best we can detect,” Dr. Grossman explained. “That said, they’re very different experiences: One is a shot in your butt once a month, the other is something you put in your mouth once a day. Patients typically have a strong point of view regarding what they’re up for.”
Extended-release naltrexone doesn’t require a DEA waiver or attendance at an OTP. But it costs roughly $800 per injection, although many insurers do cover it after additional paperwork is completed. While Dr. Grossman does use extended-release naltrexone in her own practice, it comes with some baggage. The drug comes in a powder, which is mixed with a diluent in the office, creating a thick, frothy substance that’s slow to inject. It has to be kept refrigerated, then warmed up in time for the patient visit.
“If you live somewhere where there’s no OTP and you don’t have a DEA X number, and you have a patient with OUD who’s interested in extended-release naltrexone, it’s not crazy to think about,” she noted.
Duration of medical therapy
Study after study demonstrates that, when treatment stops, the risk of relapse goes up.
“We as health care providers are used to the mentality of chronic diseases, like diabetes, where you’re probably on medicine for the rest of your life,” Dr. Grossman said. “OUD is another chronic disease where you might have a patient on medication for the rest of their life, although you may not want to drum that into their head right up front. It’s kind of scary. I don’t usually talk that way with my diabetic patients when I give them their diagnosis. So, I don’t push it.
“But the reality is, to give them the best chance of health, they should be on medication for a good long time,” she added. “And that’s true for all of the OUD medications.”
The role of counseling
The best evidence of the utility of adjunctive counseling in the treatment of OUD comes from the landmark Prescription Opioid Addiction Treatment Study (POATS), a 653-patient multicenter trial conducted by the National Drug Abuse Treatment Network and funded by the National Institute on Drug Abuse. Participants were randomized to standard medical management including medication and a meeting with a physician every 1-2 weeks, or to standard therapy plus individual counseling with a trained substance use counselor.
To the surprise of many, given that SAMHSA guidance strongly recommends counseling and other forms of behavioral therapy, there was no difference in outcomes between the two groups (Drug Alcohol Depend. 2015 May 1;150:112-9).
Subsequent parsing of the POATS data showed that the subgroup of people who were using heroin rather than prescription pills and who actually attended at least 60% of their counseling sessions did better than if they were randomized to no counseling.
“There’s still room for quibbling about the study, but many people would say, ‘You know, it’s not a slam dunk that everybody needs counseling,’ ” the internist commented.
“So, how do we pick the right treatment for our patients with OUD? It’s what feels right for them,” Dr. Grossman cautioned. “This gets back to what we do every day in managing chronic diseases: We nudge, we encourage, we use our motivational interviewing skills to help people figure out how they can change their lives and get healthier. There’s a long list of things going on in our patients’ lives that are going to help guide that decision.
“The message here: Medication is better than no medication, but it’s not a slam dunk which medication or how,” she concluded.
NEW ORLEANS – Physicians committed to fighting the national opioid epidemic really need to take the 8-hour training course on addiction treatment required to obtain a Drug Enforcement Administration ‘X’ number, because it will enable them to prescribe buprenorphine, a drug with unique advantages for many affected patients, Ellie Grossman, MD, asserted at the annual meeting of the American College of Physicians.
Buprenorphine (Subutex) is one of the three medications approved for treatment of opioid use disorder (OUD), along with methadone and naltrexone (Revia). And for certain patients, it’s clearly the best choice, according to Dr. Grossman, a general internist at Harvard Medical School, Boston, and the primary care lead for behavioral health integration at the Cambridge (Mass.) Health Alliance.
The DEA X number certification process, which entails obtaining a waiver through SAMHSA – the Substance Abuse and Mental Health Services Administration – is bureaucratic. It’s unpopular with many physicians. But it’s well worth 8 hours of an internist’s time to get the waiver and gain the ability to prescribe buprenorphine.
“The requirement is admittedly clunky, and many people have strong feelings about whether this is a regulation that should exist,” according to Dr. Grossman. “I myself didn’t need to have special training to prescribe methadone, a full opioid agonist that my patients could easily die from. But I did have to undergo an 8-hour training to prescribe buprenorphine, and it’s much harder to die from that drug.”
She addressed which of the three medications for OUD is the best fit in a given patient, the appropriate treatment duration, and the role of adjunctive counseling, which – spoiler alert – has been cast into question by the results of a major government-funded randomized trial.
Dr. Grossman’s overriding message: “You are saving lives by getting people on medication.”
Indeed, studies have shown that patients with OUD who receive no treatment have a sixfold increase in the standardized mortality ratio, compared with the general population. Contrast that with the less than 2-fold increased risk with medication-assisted treatment and roughly a 2.5-fold increased risk when medication is given short term to cover withdrawal and then tapered and discontinued.
Other documented benefits of long-term medication-assisted treatment of patients with OUD as described in a 2014 Cochrane review include reductions in injection drug use, crime days, HIV-related risk behaviors and seroconversion, and improved health and social functioning.
Of note, those well-documented benefits apply only to methadone, a full opioid agonist, and buprenorphine, a partial agonist, because those two drugs have been around long enough to generate long-term outcome data. Naltrexone, which has a completely different mechanism of action – it’s a full opioid antagonist – has not as of yet.
Individualizing medical therapy for OUD
Physicians can’t write a prescription for methadone. The drug must be administered at a certified opioid treatment program, or OTP, otherwise known as a methadone clinic. Those clinics are highly regulated at both the federal and state levels, with lots of minutia involved. Patient counseling and drug screening are required.
In contrast, a physician with a DEA X number can write a prescription for buprenorphine and have a patient fill it at a pharmacy. There is inherently less structure surrounding buprenorphine therapy than that of methadone, Dr. Grossman noted. There are no hard and fast rules about how often a physician has to see the patient or do drug screens or counseling. Buprenorphine is available as once-daily oral sublingual therapy and, more recently, in long-acting injectable and implantable formulations, although Dr. Grossman believes the jury is still out about how these nonoral agents are best utilized.
“I’m often asked, ‘Which is better, methadone or buprenorphine?’ Really, the answer is they’re both pretty darn good,” according to Dr. Grossman.
The Cochrane review concluded that, in the studies that have used real-world dosing – that is, higher doses than in the initial studies – high-dose buprenorphine and high-dose methadone have similar rates of retention in treatment.
“What I tell patients is that a lot hinges on the structure of the treatment delivery system,” Dr. Grossman said. “If it’s methadone, they’re going to the OTP every day. Some people need more structure; they need a set of eyes on them every day. Or if they are at high risk for medication diversion – for example, someone else in their household might want to steal their medications – going to a methadone program gets around that. Also, when somebody has been on methadone in the past and did well on it and wants to go on it again, I’m likely to say, ‘That sounds like a good fit.’”
Buprenorphine is a good option for patients who don’t require close, structured supervision. It has fewer drug interactions than does methadone and is less prone to cause QTc prolongation. Also, it’s a more realistic option for patients who live so far from an OTP that daily attendance is impractical. And ob.gyns increasingly favor buprenorphine, because the problem of neonatal abstinence syndrome is less severe than when mothers are on methadone.
As for extended-release naltrexone (Vivitrol), the pivotal double-blind Russian trial that won FDA approval for treatment of OUD showed a dramatic improvement in opioid-free weeks (Lancet. 2011 Apr 30;377[9776]:1506-13).
More recently, the 24-week, multicenter, open-label X:BOT trial randomized 570 U.S. patients with OUD to once-monthly extended-release naltrexone or daily sublingual buprenorphine-naloxone (Suboxone). The dropout rate was higher in the extended-release naltrexone arm because patients had to be opioid free for 2 weeks before starting on the opioid antagonist. As a practical matter, that can be difficult to achieve unless a patient has just been released from jail or prison. But the per-protocol relapse rates were similar (Lancet. 2018 Jan 27;391[10118]:309-18).
“Many people interpret this study as saying, with the right patient who can get into an opioid-free state or, if you inherit an opioid-free state, the choice between extended-release naltrexone and buprenorphine-naloxone may be a bit of a wash in terms of clinical effectiveness, as best we can detect,” Dr. Grossman explained. “That said, they’re very different experiences: One is a shot in your butt once a month, the other is something you put in your mouth once a day. Patients typically have a strong point of view regarding what they’re up for.”
Extended-release naltrexone doesn’t require a DEA waiver or attendance at an OTP. But it costs roughly $800 per injection, although many insurers do cover it after additional paperwork is completed. While Dr. Grossman does use extended-release naltrexone in her own practice, it comes with some baggage. The drug comes in a powder, which is mixed with a diluent in the office, creating a thick, frothy substance that’s slow to inject. It has to be kept refrigerated, then warmed up in time for the patient visit.
“If you live somewhere where there’s no OTP and you don’t have a DEA X number, and you have a patient with OUD who’s interested in extended-release naltrexone, it’s not crazy to think about,” she noted.
Duration of medical therapy
Study after study demonstrates that, when treatment stops, the risk of relapse goes up.
“We as health care providers are used to the mentality of chronic diseases, like diabetes, where you’re probably on medicine for the rest of your life,” Dr. Grossman said. “OUD is another chronic disease where you might have a patient on medication for the rest of their life, although you may not want to drum that into their head right up front. It’s kind of scary. I don’t usually talk that way with my diabetic patients when I give them their diagnosis. So, I don’t push it.
“But the reality is, to give them the best chance of health, they should be on medication for a good long time,” she added. “And that’s true for all of the OUD medications.”
The role of counseling
The best evidence of the utility of adjunctive counseling in the treatment of OUD comes from the landmark Prescription Opioid Addiction Treatment Study (POATS), a 653-patient multicenter trial conducted by the National Drug Abuse Treatment Network and funded by the National Institute on Drug Abuse. Participants were randomized to standard medical management including medication and a meeting with a physician every 1-2 weeks, or to standard therapy plus individual counseling with a trained substance use counselor.
To the surprise of many, given that SAMHSA guidance strongly recommends counseling and other forms of behavioral therapy, there was no difference in outcomes between the two groups (Drug Alcohol Depend. 2015 May 1;150:112-9).
Subsequent parsing of the POATS data showed that the subgroup of people who were using heroin rather than prescription pills and who actually attended at least 60% of their counseling sessions did better than if they were randomized to no counseling.
“There’s still room for quibbling about the study, but many people would say, ‘You know, it’s not a slam dunk that everybody needs counseling,’ ” the internist commented.
“So, how do we pick the right treatment for our patients with OUD? It’s what feels right for them,” Dr. Grossman cautioned. “This gets back to what we do every day in managing chronic diseases: We nudge, we encourage, we use our motivational interviewing skills to help people figure out how they can change their lives and get healthier. There’s a long list of things going on in our patients’ lives that are going to help guide that decision.
“The message here: Medication is better than no medication, but it’s not a slam dunk which medication or how,” she concluded.
NEW ORLEANS – Physicians committed to fighting the national opioid epidemic really need to take the 8-hour training course on addiction treatment required to obtain a Drug Enforcement Administration ‘X’ number, because it will enable them to prescribe buprenorphine, a drug with unique advantages for many affected patients, Ellie Grossman, MD, asserted at the annual meeting of the American College of Physicians.
Buprenorphine (Subutex) is one of the three medications approved for treatment of opioid use disorder (OUD), along with methadone and naltrexone (Revia). And for certain patients, it’s clearly the best choice, according to Dr. Grossman, a general internist at Harvard Medical School, Boston, and the primary care lead for behavioral health integration at the Cambridge (Mass.) Health Alliance.
The DEA X number certification process, which entails obtaining a waiver through SAMHSA – the Substance Abuse and Mental Health Services Administration – is bureaucratic. It’s unpopular with many physicians. But it’s well worth 8 hours of an internist’s time to get the waiver and gain the ability to prescribe buprenorphine.
“The requirement is admittedly clunky, and many people have strong feelings about whether this is a regulation that should exist,” according to Dr. Grossman. “I myself didn’t need to have special training to prescribe methadone, a full opioid agonist that my patients could easily die from. But I did have to undergo an 8-hour training to prescribe buprenorphine, and it’s much harder to die from that drug.”
She addressed which of the three medications for OUD is the best fit in a given patient, the appropriate treatment duration, and the role of adjunctive counseling, which – spoiler alert – has been cast into question by the results of a major government-funded randomized trial.
Dr. Grossman’s overriding message: “You are saving lives by getting people on medication.”
Indeed, studies have shown that patients with OUD who receive no treatment have a sixfold increase in the standardized mortality ratio, compared with the general population. Contrast that with the less than 2-fold increased risk with medication-assisted treatment and roughly a 2.5-fold increased risk when medication is given short term to cover withdrawal and then tapered and discontinued.
Other documented benefits of long-term medication-assisted treatment of patients with OUD as described in a 2014 Cochrane review include reductions in injection drug use, crime days, HIV-related risk behaviors and seroconversion, and improved health and social functioning.
Of note, those well-documented benefits apply only to methadone, a full opioid agonist, and buprenorphine, a partial agonist, because those two drugs have been around long enough to generate long-term outcome data. Naltrexone, which has a completely different mechanism of action – it’s a full opioid antagonist – has not as of yet.
Individualizing medical therapy for OUD
Physicians can’t write a prescription for methadone. The drug must be administered at a certified opioid treatment program, or OTP, otherwise known as a methadone clinic. Those clinics are highly regulated at both the federal and state levels, with lots of minutia involved. Patient counseling and drug screening are required.
In contrast, a physician with a DEA X number can write a prescription for buprenorphine and have a patient fill it at a pharmacy. There is inherently less structure surrounding buprenorphine therapy than that of methadone, Dr. Grossman noted. There are no hard and fast rules about how often a physician has to see the patient or do drug screens or counseling. Buprenorphine is available as once-daily oral sublingual therapy and, more recently, in long-acting injectable and implantable formulations, although Dr. Grossman believes the jury is still out about how these nonoral agents are best utilized.
“I’m often asked, ‘Which is better, methadone or buprenorphine?’ Really, the answer is they’re both pretty darn good,” according to Dr. Grossman.
The Cochrane review concluded that, in the studies that have used real-world dosing – that is, higher doses than in the initial studies – high-dose buprenorphine and high-dose methadone have similar rates of retention in treatment.
“What I tell patients is that a lot hinges on the structure of the treatment delivery system,” Dr. Grossman said. “If it’s methadone, they’re going to the OTP every day. Some people need more structure; they need a set of eyes on them every day. Or if they are at high risk for medication diversion – for example, someone else in their household might want to steal their medications – going to a methadone program gets around that. Also, when somebody has been on methadone in the past and did well on it and wants to go on it again, I’m likely to say, ‘That sounds like a good fit.’”
Buprenorphine is a good option for patients who don’t require close, structured supervision. It has fewer drug interactions than does methadone and is less prone to cause QTc prolongation. Also, it’s a more realistic option for patients who live so far from an OTP that daily attendance is impractical. And ob.gyns increasingly favor buprenorphine, because the problem of neonatal abstinence syndrome is less severe than when mothers are on methadone.
As for extended-release naltrexone (Vivitrol), the pivotal double-blind Russian trial that won FDA approval for treatment of OUD showed a dramatic improvement in opioid-free weeks (Lancet. 2011 Apr 30;377[9776]:1506-13).
More recently, the 24-week, multicenter, open-label X:BOT trial randomized 570 U.S. patients with OUD to once-monthly extended-release naltrexone or daily sublingual buprenorphine-naloxone (Suboxone). The dropout rate was higher in the extended-release naltrexone arm because patients had to be opioid free for 2 weeks before starting on the opioid antagonist. As a practical matter, that can be difficult to achieve unless a patient has just been released from jail or prison. But the per-protocol relapse rates were similar (Lancet. 2018 Jan 27;391[10118]:309-18).
“Many people interpret this study as saying, with the right patient who can get into an opioid-free state or, if you inherit an opioid-free state, the choice between extended-release naltrexone and buprenorphine-naloxone may be a bit of a wash in terms of clinical effectiveness, as best we can detect,” Dr. Grossman explained. “That said, they’re very different experiences: One is a shot in your butt once a month, the other is something you put in your mouth once a day. Patients typically have a strong point of view regarding what they’re up for.”
Extended-release naltrexone doesn’t require a DEA waiver or attendance at an OTP. But it costs roughly $800 per injection, although many insurers do cover it after additional paperwork is completed. While Dr. Grossman does use extended-release naltrexone in her own practice, it comes with some baggage. The drug comes in a powder, which is mixed with a diluent in the office, creating a thick, frothy substance that’s slow to inject. It has to be kept refrigerated, then warmed up in time for the patient visit.
“If you live somewhere where there’s no OTP and you don’t have a DEA X number, and you have a patient with OUD who’s interested in extended-release naltrexone, it’s not crazy to think about,” she noted.
Duration of medical therapy
Study after study demonstrates that, when treatment stops, the risk of relapse goes up.
“We as health care providers are used to the mentality of chronic diseases, like diabetes, where you’re probably on medicine for the rest of your life,” Dr. Grossman said. “OUD is another chronic disease where you might have a patient on medication for the rest of their life, although you may not want to drum that into their head right up front. It’s kind of scary. I don’t usually talk that way with my diabetic patients when I give them their diagnosis. So, I don’t push it.
“But the reality is, to give them the best chance of health, they should be on medication for a good long time,” she added. “And that’s true for all of the OUD medications.”
The role of counseling
The best evidence of the utility of adjunctive counseling in the treatment of OUD comes from the landmark Prescription Opioid Addiction Treatment Study (POATS), a 653-patient multicenter trial conducted by the National Drug Abuse Treatment Network and funded by the National Institute on Drug Abuse. Participants were randomized to standard medical management including medication and a meeting with a physician every 1-2 weeks, or to standard therapy plus individual counseling with a trained substance use counselor.
To the surprise of many, given that SAMHSA guidance strongly recommends counseling and other forms of behavioral therapy, there was no difference in outcomes between the two groups (Drug Alcohol Depend. 2015 May 1;150:112-9).
Subsequent parsing of the POATS data showed that the subgroup of people who were using heroin rather than prescription pills and who actually attended at least 60% of their counseling sessions did better than if they were randomized to no counseling.
“There’s still room for quibbling about the study, but many people would say, ‘You know, it’s not a slam dunk that everybody needs counseling,’ ” the internist commented.
“So, how do we pick the right treatment for our patients with OUD? It’s what feels right for them,” Dr. Grossman cautioned. “This gets back to what we do every day in managing chronic diseases: We nudge, we encourage, we use our motivational interviewing skills to help people figure out how they can change their lives and get healthier. There’s a long list of things going on in our patients’ lives that are going to help guide that decision.
“The message here: Medication is better than no medication, but it’s not a slam dunk which medication or how,” she concluded.
REPORTING FROM ACP INTERNAL MEDICINE
Internist comanagement of orthopedic inpatients boosts outcomes
NEW ORLEANS – Comanagement of orthopedic inpatients by an internist or hospitalist can improve outcomes in myriad ways, Mary Anderson Wallace, MD, said at the annual meeting of the American College of Physicians.
She focused on patients undergoing total hip or knee arthroplasty (THA/TKA). In 2014, there were 400,000 of them under Medicare alone, accounting for $7 billion in hospitalization costs and nearly as much again in the cost of related postdischarge care.
So, changes in management that improve key outcomes in this population by even a small increment reap huge benefits when spread across this enormous patient population, noted Dr. Wallace, an internist and hospitalist at the University of Colorado, Denver.
Among the examples she highlighted where comanagement can have a favorable impact were optimization of perioperative pain management pathways; how to handle the use of disease-modifying antirheumatic drugs (DMARDs) in patients undergoing THA/TKA; the latest thinking on the appropriateness of low-dose aspirin for deep vein thrombosis (DVT) prophylaxis; a simple way to predict postop delirium in older individuals without known dementia; how to decide which postoperative fevers warrant a costly infectious disease workup; and the optimal wait time from arrival at the hospital with a fractured hip and THA.
These are all issues where a well-informed internist/hospitalist can be of enormous assistance to a busy orthopedic surgeon in providing high-value care, she explained.
Optimizing perioperative pain management pathways
As of 2015, orthopedists ranked as the third highest prescribers of opioids. Impressively, a retrospective cohort study of 641,941 opioid-naive, privately insured patients undergoing 1 of 11 types of surgery demonstrated that TKA was associated with a 5.1-fold increased risk for subsequent chronic opioid use in the first year after surgery, compared with 18 million opioid-naive nonsurgical controls. Indeed, TKA was the highest-risk of the 11 surgical procedures examined (JAMA Intern Med. 2016 Sep 1;176[9]:1286-93).
Another study that points to a need to develop best practices for opioid prescribing in orthopedic surgery – and other types of surgery as well – was a systematic review of six studies of patients who received prescription opioid analgesics in conjunction with seven types of surgery.
Opioid oversupply was identified as a clear problem: 67%-92% of patients in the six studies reported unused opioids. Up to 71% of opioid tablets went unused, mainly because patients felt they’d achieved adequate pain control and didn’t need them. Rates of safe disposal of unused opioids were in the single digits, suggesting that overprescribing provides a large potential reservoir of opioids that can be diverted to nonmedical use (JAMA Surg. 2017 Nov 1;152[11]:1066-71).
Moreover, a recent retrospective study of more than 1 million opioid-naive patients undergoing surgery showed that 56% of them received postoperative opioids. And each additional week of use was associated with a 44% increase in the relative risk of the composite endpoint of opioid dependence, abuse, or overdose. Duration of opioid use was a stronger predictor of this adverse outcome than was dosage (BMJ. 2018 Jan 17;360:j5790).
Other studies have shown that multimodal analgesia is utilized in only 25%-50% of surgical patients, even though it is considered the standard of care. Only 20% of patients undergoing THA/TKA receive peripheral nerve and neuraxial blocks. So, there is an opportunity for optimization of perioperative pain management pathways in orthopedic surgery patients, including avoidance of unnecessary p.r.n. prescribing, to favorably impact the national opioid epidemic, Dr. Wallace observed.
A surprise benefit of multimodal pain management that includes acetaminophen and a nonsteroidal anti-inflammatory agent is that it markedly reduces the incidence of postoperative fevers after total joint arthroplasty, compared with opioid-based pain management.
That was demonstrated in a retrospective study of 2,417 THA/TKAs in which multimodal pain management was used, and 1,484 procedures that relied on opioid-based pain relief. All of the operations were performed by the same three orthopedic surgeons. Only 5% of patients in the multimodal pain management group developed a fever greater than 38.5 degrees Celsius during the first 5 postoperative days, compared with 25% of those in the opioid-based analgesia group.
Moreover, an infectious disease workup was ordered in 2% of the multimodal analgesia group, versus 10% in the opioid-based pain management cohort, with no difference in the positive workup rates between the two groups (Clin Orthop Relat Res. 2014 May;472[5]:1489-95).
“It’s interesting that multimodal pain management has the side effect of putting you in a better position to practice high-value care, with less fever and fewer infectious disease workups,” Dr. Wallace said.
Perioperative management of DMARDs
Recent joint guidelines from the American College of Rheumatology and American Association of Hip and Knee Surgeons specifically address this issue in patients undergoing elective joint replacement (Arthritis Rheumatol. 2017 Aug;69[8]:1538-51).
“The quality of evidence isn’t high, but at least it’s a starting point,” Dr. Wallace said.
Patients with rheumatoid arthritis, systemic lupus erythematosus, spondyloarthropathies, and other rheumatic diseases who are on methotrexate or other nonbiologic DMARDs should be maintained on their current dose, according to the guidelines.
In contrast, all biologic agents should be withheld prior to surgery, which should be scheduled to coincide with the end of the dosing cycle for that specific biologic. The biologic agent should be resumed only once adequate wound healing has occurred, typically about 14 days post-THA/TKA.
Patients on daily glucocorticoids should continue on their current dose; supraphysiologic stress dosing is to be avoided.
Low-dose aspirin for VTE prophylaxis
“It seems like nothing has been such an enduring controversy in the comanagement literature as the question of whether aspirin is an effective prophylactic agent for prevention of DVT post THA/TKA,” according to Dr. Wallace.
She noted that in the space of just 4 years between the eighth and ninth editions of the American College of Chest Physician guidelines, that organization underwent a 180-degree reversal on the issue – whipsawing from a grade 1A recommendation against aspirin in 2008 to a 1B recommendation for it in 2012.
The literature is increasingly supportive of the use of aspirin for venous thromboembolism (VTE) prophylaxis in low-risk THA/TKA patients. Separate guidelines from the American Academy of Orthopaedic Surgeons (AAOS) and the Surgical Care Improvement Project, as well as the chest physicians, support the practice.
The hitch is that there is as yet no single validated risk-stratification protocol. AAOS recommends 325 mg of aspirin twice daily for 6 weeks. But a prospective crossover study of more than 4,600 total joint arthroplasty patients conducted by investigators at Thomas Jefferson University in Philadelphia showed that 81 mg BID for 4 weeks was just as effective as was 325 mg b.i.d., albeit with an incidence of GI bleeding that to their surprise wasn’t significantly lower (J Bone Joint Surg Am. 2017 Jan 18;99[2]:91-8).
Dr. Wallace anticipates definitive answers on VTE prophylaxis to come from the ongoing Patient-Centered Outcomes Research Institute-supported PEPPER (Comparative Effectiveness of Pulmonary Embolism Prevention After Hip and Knee Replacement) trial. In that study, roughly 25,000 patients undergoing THA/TKA are being randomized to prophylactic aspirin at 81 mg b.i.d., warfarin at an International Normalized Ratio of 2.0, or rivaroxaban (Xarelto). Endpoints include mortality, VTE, and bleeding. Results are expected in 2021.
Preoperative prediction of postop delirium
Unrecognized preoperative cognitive impairment in older patients without dementia who are undergoing THA/TKA is a common and powerful risk factor for postop delirium and other complications, as demonstrated recently by investigators at Harvard University and affiliated hospitals.
They had 211 patients aged 65 or older, none with known dementia, take the Mini-Cog screening test prior to their surgery. Fully 24% had probable cognitive impairment as reflected in a score of 2 or less out of a possible 5 points on this simple test, which consists of a three-word recall and clock drawing.
“I was very surprised at this high rate. These are patients who are at risk for delirium in the hospital when you’re taking care of them,” Dr. Wallace observed.
In the Harvard study, the incidence of postop delirium was 21% in patients with a Mini-Cog score of 2 or less, compared with 7% who scored 3-5, for an odds ratio of 4.5 in an age-adjusted multivariate analysis. Moreover, 67% of the low scorers were discharged somewhere other than home, in contrast to 34% of patients with a preop Mini-Cog score of 3-5, for an adjusted 3.9-fold increased risk. The group with a Mini-Cog score of 2 or less also had a significantly longer hospital length of stay (Anesthesiology. 2017 Nov;127[5]:765-74).
Perioperative gabapentin is often added to the medication regimen of older surgical patients to reduce postop delirium. The latest evidence indicates that doesn’t work, as demonstrated in a recent 697-patient randomized trial. The incidence of delirium during the first 3 days post surgery as measured by the Confusion Assessment Method was 22.4% in patients randomized to 900 mg of gabapentin per day, and 20.8% with placebo. Nearly 200 participants had THA or TKA, and in that subgroup, there was an even stronger – albeit still not statistically significant – trend for a higher delirium rate with gabapentin than with placebo (Anesthesiology. 2017 Oct.127[4]:633-44).
“Think twice about adding gabapentin to the pain regimen for THA/TKA/spine patients for the purpose of preventing postop delirium,” she advised.
When is postop fever a concern?
Up to half of patients develop fever early after THA/TKA. In most cases, this is a self-limited ancillary effect of cytokine release, with the temperature peaking on postop day 1-2.
Three strong predictors of a positive infectious disease workup are fever after postop day 3, with an associated 23.3-fold increased risk; multiple days of fever, with an odds ratio of 8.6; and a maximum temperature greater than 39.0 degrees Celsius, with a 2.4-fold increased risk. In this 7-year-old study, the cost of infectious disease workup per change in patient management was a hefty $8,209 (J Arthroplasty. 2010 Sep;25[6 Suppl]:43-8).
A retrospective study of nearly 125,000 THA/TKA patients in the American College of Surgeons National Surgical Quality Improvement Program database has important implications for clinical surveillance for postop adverse events. Stroke occurred early, on median postop day 1. The median time of acute MI and pulmonary embolism was postop day 3, and pneumonia day 4.
The key take-home message was that the median time to DVT was postop day 6, by which point most patients had been discharged. Thus, 60% of postoperative DVTs occurred after discharge. And the time to diagnosis of DVT differed markedly by surgical procedure: The median day of diagnosis was day 5 in TKA patients, compared with day 13 for THA patients. Sixty-eight percent of urinary tract infections occurred post discharge. Sepsis occurred on median day 10 post surgery, surgical site infections on day 17 (Clin Orthop Relat Res. 2017 Dec;475[12]:2952-9).
In light of ever-shortening hospital lengths of stay, Dr. Wallace noted, the findings underscore the importance of comprehensive predischarge patient counseling.
Optimal time window for hip fracture surgery
AAOS guidelines recommend that hip fracture surgery should take place within 48 hours, assuming medical comorbidities are stabilized, because complication rates go up with longer wait times.
But that is controversial. A University of Toronto retrospective cohort study of 42,430 adults with hip fracture treated at 72 Canadian hospitals during 2009-2014 found that the inflection point was 24 hours. Among 13,731 patients whose elapsed time from hospital arrival to surgery was 24 hours or less, 30-day mortality was 5.8%, significantly less than the 6.5% rate in an equal number of propensity score–matched patients with a longer wait time.
The 90- and 365-day mortality rates in the patients who received surgery within 24 hours were 10.7% and 19.3%, both significantly lower than the 12.0% and 21.6% figures in patients with longer wait times.
For the 30-day composite outcome of death, myocardial infarction, pulmonary embolism, DVT, or pneumonia, the rates were 10.1% and 12.2% – again, statistically significant and clinically meaningful. The 90- and 365-day composite outcomes followed suit (JAMA. 2017 Nov 28;318[20]:1994-2003).
But the Canadian study won’t be the final word. The ongoing international multicenter HIP ATTACK (Hip Fracture Accelerated Surgical Treatment and Care Track) trial is comparing outcomes in 3,000 patients randomized to hip fracture surgery within 6 hours versus 24 hours. Endpoints include mortality, myocardial infarction, pulmonary embolism, pneumonia, stroke, sepsis, and life-threatening and major bleeding.
Dr. Wallace reported having no financial conflicts regarding her presentation.
NEW ORLEANS – Comanagement of orthopedic inpatients by an internist or hospitalist can improve outcomes in myriad ways, Mary Anderson Wallace, MD, said at the annual meeting of the American College of Physicians.
She focused on patients undergoing total hip or knee arthroplasty (THA/TKA). In 2014, there were 400,000 of them under Medicare alone, accounting for $7 billion in hospitalization costs and nearly as much again in the cost of related postdischarge care.
So, changes in management that improve key outcomes in this population by even a small increment reap huge benefits when spread across this enormous patient population, noted Dr. Wallace, an internist and hospitalist at the University of Colorado, Denver.
Among the examples she highlighted where comanagement can have a favorable impact were optimization of perioperative pain management pathways; how to handle the use of disease-modifying antirheumatic drugs (DMARDs) in patients undergoing THA/TKA; the latest thinking on the appropriateness of low-dose aspirin for deep vein thrombosis (DVT) prophylaxis; a simple way to predict postop delirium in older individuals without known dementia; how to decide which postoperative fevers warrant a costly infectious disease workup; and the optimal wait time from arrival at the hospital with a fractured hip and THA.
These are all issues where a well-informed internist/hospitalist can be of enormous assistance to a busy orthopedic surgeon in providing high-value care, she explained.
Optimizing perioperative pain management pathways
As of 2015, orthopedists ranked as the third highest prescribers of opioids. Impressively, a retrospective cohort study of 641,941 opioid-naive, privately insured patients undergoing 1 of 11 types of surgery demonstrated that TKA was associated with a 5.1-fold increased risk for subsequent chronic opioid use in the first year after surgery, compared with 18 million opioid-naive nonsurgical controls. Indeed, TKA was the highest-risk of the 11 surgical procedures examined (JAMA Intern Med. 2016 Sep 1;176[9]:1286-93).
Another study that points to a need to develop best practices for opioid prescribing in orthopedic surgery – and other types of surgery as well – was a systematic review of six studies of patients who received prescription opioid analgesics in conjunction with seven types of surgery.
Opioid oversupply was identified as a clear problem: 67%-92% of patients in the six studies reported unused opioids. Up to 71% of opioid tablets went unused, mainly because patients felt they’d achieved adequate pain control and didn’t need them. Rates of safe disposal of unused opioids were in the single digits, suggesting that overprescribing provides a large potential reservoir of opioids that can be diverted to nonmedical use (JAMA Surg. 2017 Nov 1;152[11]:1066-71).
Moreover, a recent retrospective study of more than 1 million opioid-naive patients undergoing surgery showed that 56% of them received postoperative opioids. And each additional week of use was associated with a 44% increase in the relative risk of the composite endpoint of opioid dependence, abuse, or overdose. Duration of opioid use was a stronger predictor of this adverse outcome than was dosage (BMJ. 2018 Jan 17;360:j5790).
Other studies have shown that multimodal analgesia is utilized in only 25%-50% of surgical patients, even though it is considered the standard of care. Only 20% of patients undergoing THA/TKA receive peripheral nerve and neuraxial blocks. So, there is an opportunity for optimization of perioperative pain management pathways in orthopedic surgery patients, including avoidance of unnecessary p.r.n. prescribing, to favorably impact the national opioid epidemic, Dr. Wallace observed.
A surprise benefit of multimodal pain management that includes acetaminophen and a nonsteroidal anti-inflammatory agent is that it markedly reduces the incidence of postoperative fevers after total joint arthroplasty, compared with opioid-based pain management.
That was demonstrated in a retrospective study of 2,417 THA/TKAs in which multimodal pain management was used, and 1,484 procedures that relied on opioid-based pain relief. All of the operations were performed by the same three orthopedic surgeons. Only 5% of patients in the multimodal pain management group developed a fever greater than 38.5 degrees Celsius during the first 5 postoperative days, compared with 25% of those in the opioid-based analgesia group.
Moreover, an infectious disease workup was ordered in 2% of the multimodal analgesia group, versus 10% in the opioid-based pain management cohort, with no difference in the positive workup rates between the two groups (Clin Orthop Relat Res. 2014 May;472[5]:1489-95).
“It’s interesting that multimodal pain management has the side effect of putting you in a better position to practice high-value care, with less fever and fewer infectious disease workups,” Dr. Wallace said.
Perioperative management of DMARDs
Recent joint guidelines from the American College of Rheumatology and American Association of Hip and Knee Surgeons specifically address this issue in patients undergoing elective joint replacement (Arthritis Rheumatol. 2017 Aug;69[8]:1538-51).
“The quality of evidence isn’t high, but at least it’s a starting point,” Dr. Wallace said.
Patients with rheumatoid arthritis, systemic lupus erythematosus, spondyloarthropathies, and other rheumatic diseases who are on methotrexate or other nonbiologic DMARDs should be maintained on their current dose, according to the guidelines.
In contrast, all biologic agents should be withheld prior to surgery, which should be scheduled to coincide with the end of the dosing cycle for that specific biologic. The biologic agent should be resumed only once adequate wound healing has occurred, typically about 14 days post-THA/TKA.
Patients on daily glucocorticoids should continue on their current dose; supraphysiologic stress dosing is to be avoided.
Low-dose aspirin for VTE prophylaxis
“It seems like nothing has been such an enduring controversy in the comanagement literature as the question of whether aspirin is an effective prophylactic agent for prevention of DVT post THA/TKA,” according to Dr. Wallace.
She noted that in the space of just 4 years between the eighth and ninth editions of the American College of Chest Physician guidelines, that organization underwent a 180-degree reversal on the issue – whipsawing from a grade 1A recommendation against aspirin in 2008 to a 1B recommendation for it in 2012.
The literature is increasingly supportive of the use of aspirin for venous thromboembolism (VTE) prophylaxis in low-risk THA/TKA patients. Separate guidelines from the American Academy of Orthopaedic Surgeons (AAOS) and the Surgical Care Improvement Project, as well as the chest physicians, support the practice.
The hitch is that there is as yet no single validated risk-stratification protocol. AAOS recommends 325 mg of aspirin twice daily for 6 weeks. But a prospective crossover study of more than 4,600 total joint arthroplasty patients conducted by investigators at Thomas Jefferson University in Philadelphia showed that 81 mg BID for 4 weeks was just as effective as was 325 mg b.i.d., albeit with an incidence of GI bleeding that to their surprise wasn’t significantly lower (J Bone Joint Surg Am. 2017 Jan 18;99[2]:91-8).
Dr. Wallace anticipates definitive answers on VTE prophylaxis to come from the ongoing Patient-Centered Outcomes Research Institute-supported PEPPER (Comparative Effectiveness of Pulmonary Embolism Prevention After Hip and Knee Replacement) trial. In that study, roughly 25,000 patients undergoing THA/TKA are being randomized to prophylactic aspirin at 81 mg b.i.d., warfarin at an International Normalized Ratio of 2.0, or rivaroxaban (Xarelto). Endpoints include mortality, VTE, and bleeding. Results are expected in 2021.
Preoperative prediction of postop delirium
Unrecognized preoperative cognitive impairment in older patients without dementia who are undergoing THA/TKA is a common and powerful risk factor for postop delirium and other complications, as demonstrated recently by investigators at Harvard University and affiliated hospitals.
They had 211 patients aged 65 or older, none with known dementia, take the Mini-Cog screening test prior to their surgery. Fully 24% had probable cognitive impairment as reflected in a score of 2 or less out of a possible 5 points on this simple test, which consists of a three-word recall and clock drawing.
“I was very surprised at this high rate. These are patients who are at risk for delirium in the hospital when you’re taking care of them,” Dr. Wallace observed.
In the Harvard study, the incidence of postop delirium was 21% in patients with a Mini-Cog score of 2 or less, compared with 7% who scored 3-5, for an odds ratio of 4.5 in an age-adjusted multivariate analysis. Moreover, 67% of the low scorers were discharged somewhere other than home, in contrast to 34% of patients with a preop Mini-Cog score of 3-5, for an adjusted 3.9-fold increased risk. The group with a Mini-Cog score of 2 or less also had a significantly longer hospital length of stay (Anesthesiology. 2017 Nov;127[5]:765-74).
Perioperative gabapentin is often added to the medication regimen of older surgical patients to reduce postop delirium. The latest evidence indicates that doesn’t work, as demonstrated in a recent 697-patient randomized trial. The incidence of delirium during the first 3 days post surgery as measured by the Confusion Assessment Method was 22.4% in patients randomized to 900 mg of gabapentin per day, and 20.8% with placebo. Nearly 200 participants had THA or TKA, and in that subgroup, there was an even stronger – albeit still not statistically significant – trend for a higher delirium rate with gabapentin than with placebo (Anesthesiology. 2017 Oct.127[4]:633-44).
“Think twice about adding gabapentin to the pain regimen for THA/TKA/spine patients for the purpose of preventing postop delirium,” she advised.
When is postop fever a concern?
Up to half of patients develop fever early after THA/TKA. In most cases, this is a self-limited ancillary effect of cytokine release, with the temperature peaking on postop day 1-2.
Three strong predictors of a positive infectious disease workup are fever after postop day 3, with an associated 23.3-fold increased risk; multiple days of fever, with an odds ratio of 8.6; and a maximum temperature greater than 39.0 degrees Celsius, with a 2.4-fold increased risk. In this 7-year-old study, the cost of infectious disease workup per change in patient management was a hefty $8,209 (J Arthroplasty. 2010 Sep;25[6 Suppl]:43-8).
A retrospective study of nearly 125,000 THA/TKA patients in the American College of Surgeons National Surgical Quality Improvement Program database has important implications for clinical surveillance for postop adverse events. Stroke occurred early, on median postop day 1. The median time of acute MI and pulmonary embolism was postop day 3, and pneumonia day 4.
The key take-home message was that the median time to DVT was postop day 6, by which point most patients had been discharged. Thus, 60% of postoperative DVTs occurred after discharge. And the time to diagnosis of DVT differed markedly by surgical procedure: The median day of diagnosis was day 5 in TKA patients, compared with day 13 for THA patients. Sixty-eight percent of urinary tract infections occurred post discharge. Sepsis occurred on median day 10 post surgery, surgical site infections on day 17 (Clin Orthop Relat Res. 2017 Dec;475[12]:2952-9).
In light of ever-shortening hospital lengths of stay, Dr. Wallace noted, the findings underscore the importance of comprehensive predischarge patient counseling.
Optimal time window for hip fracture surgery
AAOS guidelines recommend that hip fracture surgery should take place within 48 hours, assuming medical comorbidities are stabilized, because complication rates go up with longer wait times.
But that is controversial. A University of Toronto retrospective cohort study of 42,430 adults with hip fracture treated at 72 Canadian hospitals during 2009-2014 found that the inflection point was 24 hours. Among 13,731 patients whose elapsed time from hospital arrival to surgery was 24 hours or less, 30-day mortality was 5.8%, significantly less than the 6.5% rate in an equal number of propensity score–matched patients with a longer wait time.
The 90- and 365-day mortality rates in the patients who received surgery within 24 hours were 10.7% and 19.3%, both significantly lower than the 12.0% and 21.6% figures in patients with longer wait times.
For the 30-day composite outcome of death, myocardial infarction, pulmonary embolism, DVT, or pneumonia, the rates were 10.1% and 12.2% – again, statistically significant and clinically meaningful. The 90- and 365-day composite outcomes followed suit (JAMA. 2017 Nov 28;318[20]:1994-2003).
But the Canadian study won’t be the final word. The ongoing international multicenter HIP ATTACK (Hip Fracture Accelerated Surgical Treatment and Care Track) trial is comparing outcomes in 3,000 patients randomized to hip fracture surgery within 6 hours versus 24 hours. Endpoints include mortality, myocardial infarction, pulmonary embolism, pneumonia, stroke, sepsis, and life-threatening and major bleeding.
Dr. Wallace reported having no financial conflicts regarding her presentation.
NEW ORLEANS – Comanagement of orthopedic inpatients by an internist or hospitalist can improve outcomes in myriad ways, Mary Anderson Wallace, MD, said at the annual meeting of the American College of Physicians.
She focused on patients undergoing total hip or knee arthroplasty (THA/TKA). In 2014, there were 400,000 of them under Medicare alone, accounting for $7 billion in hospitalization costs and nearly as much again in the cost of related postdischarge care.
So, changes in management that improve key outcomes in this population by even a small increment reap huge benefits when spread across this enormous patient population, noted Dr. Wallace, an internist and hospitalist at the University of Colorado, Denver.
Among the examples she highlighted where comanagement can have a favorable impact were optimization of perioperative pain management pathways; how to handle the use of disease-modifying antirheumatic drugs (DMARDs) in patients undergoing THA/TKA; the latest thinking on the appropriateness of low-dose aspirin for deep vein thrombosis (DVT) prophylaxis; a simple way to predict postop delirium in older individuals without known dementia; how to decide which postoperative fevers warrant a costly infectious disease workup; and the optimal wait time from arrival at the hospital with a fractured hip and THA.
These are all issues where a well-informed internist/hospitalist can be of enormous assistance to a busy orthopedic surgeon in providing high-value care, she explained.
Optimizing perioperative pain management pathways
As of 2015, orthopedists ranked as the third highest prescribers of opioids. Impressively, a retrospective cohort study of 641,941 opioid-naive, privately insured patients undergoing 1 of 11 types of surgery demonstrated that TKA was associated with a 5.1-fold increased risk for subsequent chronic opioid use in the first year after surgery, compared with 18 million opioid-naive nonsurgical controls. Indeed, TKA was the highest-risk of the 11 surgical procedures examined (JAMA Intern Med. 2016 Sep 1;176[9]:1286-93).
Another study that points to a need to develop best practices for opioid prescribing in orthopedic surgery – and other types of surgery as well – was a systematic review of six studies of patients who received prescription opioid analgesics in conjunction with seven types of surgery.
Opioid oversupply was identified as a clear problem: 67%-92% of patients in the six studies reported unused opioids. Up to 71% of opioid tablets went unused, mainly because patients felt they’d achieved adequate pain control and didn’t need them. Rates of safe disposal of unused opioids were in the single digits, suggesting that overprescribing provides a large potential reservoir of opioids that can be diverted to nonmedical use (JAMA Surg. 2017 Nov 1;152[11]:1066-71).
Moreover, a recent retrospective study of more than 1 million opioid-naive patients undergoing surgery showed that 56% of them received postoperative opioids. And each additional week of use was associated with a 44% increase in the relative risk of the composite endpoint of opioid dependence, abuse, or overdose. Duration of opioid use was a stronger predictor of this adverse outcome than was dosage (BMJ. 2018 Jan 17;360:j5790).
Other studies have shown that multimodal analgesia is utilized in only 25%-50% of surgical patients, even though it is considered the standard of care. Only 20% of patients undergoing THA/TKA receive peripheral nerve and neuraxial blocks. So, there is an opportunity for optimization of perioperative pain management pathways in orthopedic surgery patients, including avoidance of unnecessary p.r.n. prescribing, to favorably impact the national opioid epidemic, Dr. Wallace observed.
A surprise benefit of multimodal pain management that includes acetaminophen and a nonsteroidal anti-inflammatory agent is that it markedly reduces the incidence of postoperative fevers after total joint arthroplasty, compared with opioid-based pain management.
That was demonstrated in a retrospective study of 2,417 THA/TKAs in which multimodal pain management was used, and 1,484 procedures that relied on opioid-based pain relief. All of the operations were performed by the same three orthopedic surgeons. Only 5% of patients in the multimodal pain management group developed a fever greater than 38.5 degrees Celsius during the first 5 postoperative days, compared with 25% of those in the opioid-based analgesia group.
Moreover, an infectious disease workup was ordered in 2% of the multimodal analgesia group, versus 10% in the opioid-based pain management cohort, with no difference in the positive workup rates between the two groups (Clin Orthop Relat Res. 2014 May;472[5]:1489-95).
“It’s interesting that multimodal pain management has the side effect of putting you in a better position to practice high-value care, with less fever and fewer infectious disease workups,” Dr. Wallace said.
Perioperative management of DMARDs
Recent joint guidelines from the American College of Rheumatology and American Association of Hip and Knee Surgeons specifically address this issue in patients undergoing elective joint replacement (Arthritis Rheumatol. 2017 Aug;69[8]:1538-51).
“The quality of evidence isn’t high, but at least it’s a starting point,” Dr. Wallace said.
Patients with rheumatoid arthritis, systemic lupus erythematosus, spondyloarthropathies, and other rheumatic diseases who are on methotrexate or other nonbiologic DMARDs should be maintained on their current dose, according to the guidelines.
In contrast, all biologic agents should be withheld prior to surgery, which should be scheduled to coincide with the end of the dosing cycle for that specific biologic. The biologic agent should be resumed only once adequate wound healing has occurred, typically about 14 days post-THA/TKA.
Patients on daily glucocorticoids should continue on their current dose; supraphysiologic stress dosing is to be avoided.
Low-dose aspirin for VTE prophylaxis
“It seems like nothing has been such an enduring controversy in the comanagement literature as the question of whether aspirin is an effective prophylactic agent for prevention of DVT post THA/TKA,” according to Dr. Wallace.
She noted that in the space of just 4 years between the eighth and ninth editions of the American College of Chest Physician guidelines, that organization underwent a 180-degree reversal on the issue – whipsawing from a grade 1A recommendation against aspirin in 2008 to a 1B recommendation for it in 2012.
The literature is increasingly supportive of the use of aspirin for venous thromboembolism (VTE) prophylaxis in low-risk THA/TKA patients. Separate guidelines from the American Academy of Orthopaedic Surgeons (AAOS) and the Surgical Care Improvement Project, as well as the chest physicians, support the practice.
The hitch is that there is as yet no single validated risk-stratification protocol. AAOS recommends 325 mg of aspirin twice daily for 6 weeks. But a prospective crossover study of more than 4,600 total joint arthroplasty patients conducted by investigators at Thomas Jefferson University in Philadelphia showed that 81 mg BID for 4 weeks was just as effective as was 325 mg b.i.d., albeit with an incidence of GI bleeding that to their surprise wasn’t significantly lower (J Bone Joint Surg Am. 2017 Jan 18;99[2]:91-8).
Dr. Wallace anticipates definitive answers on VTE prophylaxis to come from the ongoing Patient-Centered Outcomes Research Institute-supported PEPPER (Comparative Effectiveness of Pulmonary Embolism Prevention After Hip and Knee Replacement) trial. In that study, roughly 25,000 patients undergoing THA/TKA are being randomized to prophylactic aspirin at 81 mg b.i.d., warfarin at an International Normalized Ratio of 2.0, or rivaroxaban (Xarelto). Endpoints include mortality, VTE, and bleeding. Results are expected in 2021.
Preoperative prediction of postop delirium
Unrecognized preoperative cognitive impairment in older patients without dementia who are undergoing THA/TKA is a common and powerful risk factor for postop delirium and other complications, as demonstrated recently by investigators at Harvard University and affiliated hospitals.
They had 211 patients aged 65 or older, none with known dementia, take the Mini-Cog screening test prior to their surgery. Fully 24% had probable cognitive impairment as reflected in a score of 2 or less out of a possible 5 points on this simple test, which consists of a three-word recall and clock drawing.
“I was very surprised at this high rate. These are patients who are at risk for delirium in the hospital when you’re taking care of them,” Dr. Wallace observed.
In the Harvard study, the incidence of postop delirium was 21% in patients with a Mini-Cog score of 2 or less, compared with 7% who scored 3-5, for an odds ratio of 4.5 in an age-adjusted multivariate analysis. Moreover, 67% of the low scorers were discharged somewhere other than home, in contrast to 34% of patients with a preop Mini-Cog score of 3-5, for an adjusted 3.9-fold increased risk. The group with a Mini-Cog score of 2 or less also had a significantly longer hospital length of stay (Anesthesiology. 2017 Nov;127[5]:765-74).
Perioperative gabapentin is often added to the medication regimen of older surgical patients to reduce postop delirium. The latest evidence indicates that doesn’t work, as demonstrated in a recent 697-patient randomized trial. The incidence of delirium during the first 3 days post surgery as measured by the Confusion Assessment Method was 22.4% in patients randomized to 900 mg of gabapentin per day, and 20.8% with placebo. Nearly 200 participants had THA or TKA, and in that subgroup, there was an even stronger – albeit still not statistically significant – trend for a higher delirium rate with gabapentin than with placebo (Anesthesiology. 2017 Oct.127[4]:633-44).
“Think twice about adding gabapentin to the pain regimen for THA/TKA/spine patients for the purpose of preventing postop delirium,” she advised.
When is postop fever a concern?
Up to half of patients develop fever early after THA/TKA. In most cases, this is a self-limited ancillary effect of cytokine release, with the temperature peaking on postop day 1-2.
Three strong predictors of a positive infectious disease workup are fever after postop day 3, with an associated 23.3-fold increased risk; multiple days of fever, with an odds ratio of 8.6; and a maximum temperature greater than 39.0 degrees Celsius, with a 2.4-fold increased risk. In this 7-year-old study, the cost of infectious disease workup per change in patient management was a hefty $8,209 (J Arthroplasty. 2010 Sep;25[6 Suppl]:43-8).
A retrospective study of nearly 125,000 THA/TKA patients in the American College of Surgeons National Surgical Quality Improvement Program database has important implications for clinical surveillance for postop adverse events. Stroke occurred early, on median postop day 1. The median time of acute MI and pulmonary embolism was postop day 3, and pneumonia day 4.
The key take-home message was that the median time to DVT was postop day 6, by which point most patients had been discharged. Thus, 60% of postoperative DVTs occurred after discharge. And the time to diagnosis of DVT differed markedly by surgical procedure: The median day of diagnosis was day 5 in TKA patients, compared with day 13 for THA patients. Sixty-eight percent of urinary tract infections occurred post discharge. Sepsis occurred on median day 10 post surgery, surgical site infections on day 17 (Clin Orthop Relat Res. 2017 Dec;475[12]:2952-9).
In light of ever-shortening hospital lengths of stay, Dr. Wallace noted, the findings underscore the importance of comprehensive predischarge patient counseling.
Optimal time window for hip fracture surgery
AAOS guidelines recommend that hip fracture surgery should take place within 48 hours, assuming medical comorbidities are stabilized, because complication rates go up with longer wait times.
But that is controversial. A University of Toronto retrospective cohort study of 42,430 adults with hip fracture treated at 72 Canadian hospitals during 2009-2014 found that the inflection point was 24 hours. Among 13,731 patients whose elapsed time from hospital arrival to surgery was 24 hours or less, 30-day mortality was 5.8%, significantly less than the 6.5% rate in an equal number of propensity score–matched patients with a longer wait time.
The 90- and 365-day mortality rates in the patients who received surgery within 24 hours were 10.7% and 19.3%, both significantly lower than the 12.0% and 21.6% figures in patients with longer wait times.
For the 30-day composite outcome of death, myocardial infarction, pulmonary embolism, DVT, or pneumonia, the rates were 10.1% and 12.2% – again, statistically significant and clinically meaningful. The 90- and 365-day composite outcomes followed suit (JAMA. 2017 Nov 28;318[20]:1994-2003).
But the Canadian study won’t be the final word. The ongoing international multicenter HIP ATTACK (Hip Fracture Accelerated Surgical Treatment and Care Track) trial is comparing outcomes in 3,000 patients randomized to hip fracture surgery within 6 hours versus 24 hours. Endpoints include mortality, myocardial infarction, pulmonary embolism, pneumonia, stroke, sepsis, and life-threatening and major bleeding.
Dr. Wallace reported having no financial conflicts regarding her presentation.
REPORTING FROM ACP INTERNAL MEDICINE
Make adult immunization a profit center
NEW ORLEANS – It’s a widespread misconception among internists: Implementing an office-based adult immunization program is a potential financial sinkhole and just isn’t worth the hassle.
That’s utterly wrong, Jason M. Goldman, MD, declared at the annual meeting of the American College of Physicians.
“But it is virtually impossible to lose money giving vaccines,” he countered. “You may not be able to retire on it, but you’re certainly not going to break the bank – and you’re not going to lose money. And more importantly, you’re doing what’s best for the patient. This is one of the few times where the payers and the government recognize that doing what’s best for the patient can actually be profitable in running a practice.”
At the annual meeting of the American College of Physicians, he detailed how to create a successful immunization program, offering money-saving tips on vaccine purchasing and proper storage, as well as wading into the complexities of coding and billing – which, by the way, he insisted actually is not daunting.
“The vaccine schedule is not nearly as complicated as it appears,” according to Dr. Goldman. “Read through it. Look at it. As automatically as you say, ‘You’re over 50, get a colonoscopy,’ you can very quickly learn to look at a patient and say, ‘These are your diseases, this is your age, these are the vaccines you need.’
“This is not difficult. If I can do it, anyone can do it,” Dr. Goldman noted. “Start simple with one or two vaccines until you hit your comfort level; then you can get more advanced. I do the travel vaccines – yellow fever, typhus, the whole gamut. And it’s just as easy vaccinating for that as for any of the others.”
Why implement adult immunization?
Many internists send patients off to a pharmacy for their vaccinations. That’s simply not good medical care, Dr. Goldman said.
“We are the primary care doctors,” he said. “We are the ones who should be vaccinating our patients, for several reasons: It’s the standard of care. It’s good medical practice.”
And Dr. Goldman frequently doesn’t receive any reports from the pharmacies. That means patients come to his office and have no idea what vaccines they received.
“That’s not good documentation,” he cautioned. “And when patients go into the hospital, they all get Pneumovax every single week because the hospital isn’t keeping documentation.”
The bottom line with vaccinating: “Whether you’re in a small group, a solo practitioner, or in a large health system, the vaccine programs work. They prevent disease and save lives. It’s easy to incorporate into your practice. And it is profitable.”
How profitable?
Dr. Goldman has the answer. For a great many different vaccines, he has calculated his average cost for the needle, syringe, medical assistant, time in the room, and other factors involved in running his practice. He also knows from experience the average purchase price paid for a given vaccine, the typical reimbursement for that vaccine, plus the reimbursement for its administration, which is a separate yet necessary coding/billing item.
The typical net profit ranges from $21.50 for high-dose influenza vaccine to, at the top end, $47.41 for meningococcal group B vaccine (Bexsero) and $49.58 for recombinant human papillomavirus 9-valent vaccine (Gardasil-9).
Purchasing and storage considerations
Always buy vaccines directly from the manufacturer; it’s a better deal than going through a middleman, who’ll invariably take a cut out of what should be the physician’s profit.
Each of the major vaccine makers has a dedicated vaccine purchase website where a physician can sign up for an account and order the company’s vaccines. These include Merck (www.merckvaccines.com), Aventis (www.vaccineshoppe.com), Pfizer (www.pfizerprime.com), and GlaxoSmithKline (www.gskdirect.com).
You’ll get a discount by buying multiple different vaccines on the same order.
“You can defer payment of your invoice for several months,” Dr. Goldman explained. “You purchase the vaccines now, but you don’t have to pay for them until 3-4 months later. By then, hopefully, you’ll have received reimbursement. So, your cost is covered, and you have profit on the side.”
For paying promptly on the due date, the manufacturer will provide an additional discount. The easiest way to do that is to have the money automatically charged to a credit card on that date.
Also, the vaccine manufacturers’ staff are happy to provide reliably expert reimbursement guidance.
With a little experience, it’s easy to predict how many vaccines will be used per month, Dr. Goldman said. Order what’s needed, so there aren’t a bunch of vaccines expiring in the office.
“However, even if that does happen, all is not lost,” he noted. “You can call up the manufacturer, and many of them will take back unused or even expired vaccines for full credit to the account. So, again, you really can’t lose money.”
With regard to vaccine storage, don’t skimp on the refrigerator and/or freezer. Get a professional model. And follow the best practices as described in the Centers for Disease Control and Prevention toolkit.
“It’s really common sense: Don’t use a dorm-type refrigerator; don’t put food or beverages in there; make sure the vaccines are appropriately stored; check the temperature every day; make sure if you lose power, your building has a backup generator,” he explained. “If you train your staff the right way, they’ll be able to handle it so you don’t have to worry about it. You just have to look at the logs and make sure they’re doing it.”
Use standing orders
Studies show that standing orders result in higher vaccination rates.
“You’re empowering the nurses or other staff members to act within the full extent of their license,” Dr. Goldman said. “It takes the burden off the physician to have to do anything that can be delegated to other individuals to make sure patients get vaccinated.”
Coding and billing for commercially insured patients
All vaccines have the same ICD-10 diagnostic code: Z23. And each vaccine has its own CPT code. For example, 90750 for Shingrix, the new herpes zoster vaccine; 90715 for Tdap; and 90686 for quadrivalent influenza.
But there are two components to the CPT code for a vaccination: the individual vaccine code and the administration code.
If you give one vaccination to a non-Medicare patient, the administration code is 90471. If you give a second vaccination during the same visit, its administration code is 90472. If you give a patient, say, four vaccines during one visit, you would bill the first using the administration code 90471, and the others as 90472 times three units.
If the vaccines are being given during a legitimate office visit, the physician can bill for both by employing modifiers 25 and 59. Modifier 25 goes with the appropriate E/M code for the office visit; it serves to tell the coding system that other things are going on in addition to the billable office visit. Modifier 59 needs to be attached to both the specific vaccine code and the vaccine administration code for reimbursement to occur.
Billing for vaccines for all commercially insured patients go through the office’s normal claims process.
Immunizing Medicare patients
For patients under Medicare Part B, vaccines for influenza, pneumonia, and hepatitis B have their own individual G codes: G0008 for influenza, G0009 for a pneumonia vaccine, and G0010 for hepatitis B. If a Medicare patient also gets an additional vaccine other than one of those three during the visit, administration code 90472 is applied to it. Those G-code bills are also submitted through the office’s normal claims process.
Under Medicare, vaccines for herpes zoster, hepatitis A, and Tdap are a special case. They are considered drugs and are covered under Medicare Part D.
“To bill that, you have to tell Medicare that you’re acting as a pharmacy,” Dr. Goldman explained. “You go to www.mytransactRX.com. You request there to be seen as a pharmacy billing for a drug. You will then be able to receive direct payment into your bank account from your Medicare payer. It will also allow you to check out patient coverage, print out proof of coverage, and submit the claim through the portal.”
If the Medicare patient doesn’t have a drug plan for those vaccines, or if the information in the system isn’t up to date, it’s a good idea to download the Advanced Beneficiary Notice of Noncoverage from the Medicare website and have the patient sign it. It spells out what the patient’s financial responsibility could be.
“The ABN also protects you as a provider, because it shows you’re not trying to balance-bill the patient,” he noted.
Dr. Goldman implored his internist colleagues to stand up and become the stewards of adult immunization.
“Remember: Keep calm and vaccinate,” he urged.
He reported having no relevant financial conflicts.
NEW ORLEANS – It’s a widespread misconception among internists: Implementing an office-based adult immunization program is a potential financial sinkhole and just isn’t worth the hassle.
That’s utterly wrong, Jason M. Goldman, MD, declared at the annual meeting of the American College of Physicians.
“But it is virtually impossible to lose money giving vaccines,” he countered. “You may not be able to retire on it, but you’re certainly not going to break the bank – and you’re not going to lose money. And more importantly, you’re doing what’s best for the patient. This is one of the few times where the payers and the government recognize that doing what’s best for the patient can actually be profitable in running a practice.”
At the annual meeting of the American College of Physicians, he detailed how to create a successful immunization program, offering money-saving tips on vaccine purchasing and proper storage, as well as wading into the complexities of coding and billing – which, by the way, he insisted actually is not daunting.
“The vaccine schedule is not nearly as complicated as it appears,” according to Dr. Goldman. “Read through it. Look at it. As automatically as you say, ‘You’re over 50, get a colonoscopy,’ you can very quickly learn to look at a patient and say, ‘These are your diseases, this is your age, these are the vaccines you need.’
“This is not difficult. If I can do it, anyone can do it,” Dr. Goldman noted. “Start simple with one or two vaccines until you hit your comfort level; then you can get more advanced. I do the travel vaccines – yellow fever, typhus, the whole gamut. And it’s just as easy vaccinating for that as for any of the others.”
Why implement adult immunization?
Many internists send patients off to a pharmacy for their vaccinations. That’s simply not good medical care, Dr. Goldman said.
“We are the primary care doctors,” he said. “We are the ones who should be vaccinating our patients, for several reasons: It’s the standard of care. It’s good medical practice.”
And Dr. Goldman frequently doesn’t receive any reports from the pharmacies. That means patients come to his office and have no idea what vaccines they received.
“That’s not good documentation,” he cautioned. “And when patients go into the hospital, they all get Pneumovax every single week because the hospital isn’t keeping documentation.”
The bottom line with vaccinating: “Whether you’re in a small group, a solo practitioner, or in a large health system, the vaccine programs work. They prevent disease and save lives. It’s easy to incorporate into your practice. And it is profitable.”
How profitable?
Dr. Goldman has the answer. For a great many different vaccines, he has calculated his average cost for the needle, syringe, medical assistant, time in the room, and other factors involved in running his practice. He also knows from experience the average purchase price paid for a given vaccine, the typical reimbursement for that vaccine, plus the reimbursement for its administration, which is a separate yet necessary coding/billing item.
The typical net profit ranges from $21.50 for high-dose influenza vaccine to, at the top end, $47.41 for meningococcal group B vaccine (Bexsero) and $49.58 for recombinant human papillomavirus 9-valent vaccine (Gardasil-9).
Purchasing and storage considerations
Always buy vaccines directly from the manufacturer; it’s a better deal than going through a middleman, who’ll invariably take a cut out of what should be the physician’s profit.
Each of the major vaccine makers has a dedicated vaccine purchase website where a physician can sign up for an account and order the company’s vaccines. These include Merck (www.merckvaccines.com), Aventis (www.vaccineshoppe.com), Pfizer (www.pfizerprime.com), and GlaxoSmithKline (www.gskdirect.com).
You’ll get a discount by buying multiple different vaccines on the same order.
“You can defer payment of your invoice for several months,” Dr. Goldman explained. “You purchase the vaccines now, but you don’t have to pay for them until 3-4 months later. By then, hopefully, you’ll have received reimbursement. So, your cost is covered, and you have profit on the side.”
For paying promptly on the due date, the manufacturer will provide an additional discount. The easiest way to do that is to have the money automatically charged to a credit card on that date.
Also, the vaccine manufacturers’ staff are happy to provide reliably expert reimbursement guidance.
With a little experience, it’s easy to predict how many vaccines will be used per month, Dr. Goldman said. Order what’s needed, so there aren’t a bunch of vaccines expiring in the office.
“However, even if that does happen, all is not lost,” he noted. “You can call up the manufacturer, and many of them will take back unused or even expired vaccines for full credit to the account. So, again, you really can’t lose money.”
With regard to vaccine storage, don’t skimp on the refrigerator and/or freezer. Get a professional model. And follow the best practices as described in the Centers for Disease Control and Prevention toolkit.
“It’s really common sense: Don’t use a dorm-type refrigerator; don’t put food or beverages in there; make sure the vaccines are appropriately stored; check the temperature every day; make sure if you lose power, your building has a backup generator,” he explained. “If you train your staff the right way, they’ll be able to handle it so you don’t have to worry about it. You just have to look at the logs and make sure they’re doing it.”
Use standing orders
Studies show that standing orders result in higher vaccination rates.
“You’re empowering the nurses or other staff members to act within the full extent of their license,” Dr. Goldman said. “It takes the burden off the physician to have to do anything that can be delegated to other individuals to make sure patients get vaccinated.”
Coding and billing for commercially insured patients
All vaccines have the same ICD-10 diagnostic code: Z23. And each vaccine has its own CPT code. For example, 90750 for Shingrix, the new herpes zoster vaccine; 90715 for Tdap; and 90686 for quadrivalent influenza.
But there are two components to the CPT code for a vaccination: the individual vaccine code and the administration code.
If you give one vaccination to a non-Medicare patient, the administration code is 90471. If you give a second vaccination during the same visit, its administration code is 90472. If you give a patient, say, four vaccines during one visit, you would bill the first using the administration code 90471, and the others as 90472 times three units.
If the vaccines are being given during a legitimate office visit, the physician can bill for both by employing modifiers 25 and 59. Modifier 25 goes with the appropriate E/M code for the office visit; it serves to tell the coding system that other things are going on in addition to the billable office visit. Modifier 59 needs to be attached to both the specific vaccine code and the vaccine administration code for reimbursement to occur.
Billing for vaccines for all commercially insured patients go through the office’s normal claims process.
Immunizing Medicare patients
For patients under Medicare Part B, vaccines for influenza, pneumonia, and hepatitis B have their own individual G codes: G0008 for influenza, G0009 for a pneumonia vaccine, and G0010 for hepatitis B. If a Medicare patient also gets an additional vaccine other than one of those three during the visit, administration code 90472 is applied to it. Those G-code bills are also submitted through the office’s normal claims process.
Under Medicare, vaccines for herpes zoster, hepatitis A, and Tdap are a special case. They are considered drugs and are covered under Medicare Part D.
“To bill that, you have to tell Medicare that you’re acting as a pharmacy,” Dr. Goldman explained. “You go to www.mytransactRX.com. You request there to be seen as a pharmacy billing for a drug. You will then be able to receive direct payment into your bank account from your Medicare payer. It will also allow you to check out patient coverage, print out proof of coverage, and submit the claim through the portal.”
If the Medicare patient doesn’t have a drug plan for those vaccines, or if the information in the system isn’t up to date, it’s a good idea to download the Advanced Beneficiary Notice of Noncoverage from the Medicare website and have the patient sign it. It spells out what the patient’s financial responsibility could be.
“The ABN also protects you as a provider, because it shows you’re not trying to balance-bill the patient,” he noted.
Dr. Goldman implored his internist colleagues to stand up and become the stewards of adult immunization.
“Remember: Keep calm and vaccinate,” he urged.
He reported having no relevant financial conflicts.
NEW ORLEANS – It’s a widespread misconception among internists: Implementing an office-based adult immunization program is a potential financial sinkhole and just isn’t worth the hassle.
That’s utterly wrong, Jason M. Goldman, MD, declared at the annual meeting of the American College of Physicians.
“But it is virtually impossible to lose money giving vaccines,” he countered. “You may not be able to retire on it, but you’re certainly not going to break the bank – and you’re not going to lose money. And more importantly, you’re doing what’s best for the patient. This is one of the few times where the payers and the government recognize that doing what’s best for the patient can actually be profitable in running a practice.”
At the annual meeting of the American College of Physicians, he detailed how to create a successful immunization program, offering money-saving tips on vaccine purchasing and proper storage, as well as wading into the complexities of coding and billing – which, by the way, he insisted actually is not daunting.
“The vaccine schedule is not nearly as complicated as it appears,” according to Dr. Goldman. “Read through it. Look at it. As automatically as you say, ‘You’re over 50, get a colonoscopy,’ you can very quickly learn to look at a patient and say, ‘These are your diseases, this is your age, these are the vaccines you need.’
“This is not difficult. If I can do it, anyone can do it,” Dr. Goldman noted. “Start simple with one or two vaccines until you hit your comfort level; then you can get more advanced. I do the travel vaccines – yellow fever, typhus, the whole gamut. And it’s just as easy vaccinating for that as for any of the others.”
Why implement adult immunization?
Many internists send patients off to a pharmacy for their vaccinations. That’s simply not good medical care, Dr. Goldman said.
“We are the primary care doctors,” he said. “We are the ones who should be vaccinating our patients, for several reasons: It’s the standard of care. It’s good medical practice.”
And Dr. Goldman frequently doesn’t receive any reports from the pharmacies. That means patients come to his office and have no idea what vaccines they received.
“That’s not good documentation,” he cautioned. “And when patients go into the hospital, they all get Pneumovax every single week because the hospital isn’t keeping documentation.”
The bottom line with vaccinating: “Whether you’re in a small group, a solo practitioner, or in a large health system, the vaccine programs work. They prevent disease and save lives. It’s easy to incorporate into your practice. And it is profitable.”
How profitable?
Dr. Goldman has the answer. For a great many different vaccines, he has calculated his average cost for the needle, syringe, medical assistant, time in the room, and other factors involved in running his practice. He also knows from experience the average purchase price paid for a given vaccine, the typical reimbursement for that vaccine, plus the reimbursement for its administration, which is a separate yet necessary coding/billing item.
The typical net profit ranges from $21.50 for high-dose influenza vaccine to, at the top end, $47.41 for meningococcal group B vaccine (Bexsero) and $49.58 for recombinant human papillomavirus 9-valent vaccine (Gardasil-9).
Purchasing and storage considerations
Always buy vaccines directly from the manufacturer; it’s a better deal than going through a middleman, who’ll invariably take a cut out of what should be the physician’s profit.
Each of the major vaccine makers has a dedicated vaccine purchase website where a physician can sign up for an account and order the company’s vaccines. These include Merck (www.merckvaccines.com), Aventis (www.vaccineshoppe.com), Pfizer (www.pfizerprime.com), and GlaxoSmithKline (www.gskdirect.com).
You’ll get a discount by buying multiple different vaccines on the same order.
“You can defer payment of your invoice for several months,” Dr. Goldman explained. “You purchase the vaccines now, but you don’t have to pay for them until 3-4 months later. By then, hopefully, you’ll have received reimbursement. So, your cost is covered, and you have profit on the side.”
For paying promptly on the due date, the manufacturer will provide an additional discount. The easiest way to do that is to have the money automatically charged to a credit card on that date.
Also, the vaccine manufacturers’ staff are happy to provide reliably expert reimbursement guidance.
With a little experience, it’s easy to predict how many vaccines will be used per month, Dr. Goldman said. Order what’s needed, so there aren’t a bunch of vaccines expiring in the office.
“However, even if that does happen, all is not lost,” he noted. “You can call up the manufacturer, and many of them will take back unused or even expired vaccines for full credit to the account. So, again, you really can’t lose money.”
With regard to vaccine storage, don’t skimp on the refrigerator and/or freezer. Get a professional model. And follow the best practices as described in the Centers for Disease Control and Prevention toolkit.
“It’s really common sense: Don’t use a dorm-type refrigerator; don’t put food or beverages in there; make sure the vaccines are appropriately stored; check the temperature every day; make sure if you lose power, your building has a backup generator,” he explained. “If you train your staff the right way, they’ll be able to handle it so you don’t have to worry about it. You just have to look at the logs and make sure they’re doing it.”
Use standing orders
Studies show that standing orders result in higher vaccination rates.
“You’re empowering the nurses or other staff members to act within the full extent of their license,” Dr. Goldman said. “It takes the burden off the physician to have to do anything that can be delegated to other individuals to make sure patients get vaccinated.”
Coding and billing for commercially insured patients
All vaccines have the same ICD-10 diagnostic code: Z23. And each vaccine has its own CPT code. For example, 90750 for Shingrix, the new herpes zoster vaccine; 90715 for Tdap; and 90686 for quadrivalent influenza.
But there are two components to the CPT code for a vaccination: the individual vaccine code and the administration code.
If you give one vaccination to a non-Medicare patient, the administration code is 90471. If you give a second vaccination during the same visit, its administration code is 90472. If you give a patient, say, four vaccines during one visit, you would bill the first using the administration code 90471, and the others as 90472 times three units.
If the vaccines are being given during a legitimate office visit, the physician can bill for both by employing modifiers 25 and 59. Modifier 25 goes with the appropriate E/M code for the office visit; it serves to tell the coding system that other things are going on in addition to the billable office visit. Modifier 59 needs to be attached to both the specific vaccine code and the vaccine administration code for reimbursement to occur.
Billing for vaccines for all commercially insured patients go through the office’s normal claims process.
Immunizing Medicare patients
For patients under Medicare Part B, vaccines for influenza, pneumonia, and hepatitis B have their own individual G codes: G0008 for influenza, G0009 for a pneumonia vaccine, and G0010 for hepatitis B. If a Medicare patient also gets an additional vaccine other than one of those three during the visit, administration code 90472 is applied to it. Those G-code bills are also submitted through the office’s normal claims process.
Under Medicare, vaccines for herpes zoster, hepatitis A, and Tdap are a special case. They are considered drugs and are covered under Medicare Part D.
“To bill that, you have to tell Medicare that you’re acting as a pharmacy,” Dr. Goldman explained. “You go to www.mytransactRX.com. You request there to be seen as a pharmacy billing for a drug. You will then be able to receive direct payment into your bank account from your Medicare payer. It will also allow you to check out patient coverage, print out proof of coverage, and submit the claim through the portal.”
If the Medicare patient doesn’t have a drug plan for those vaccines, or if the information in the system isn’t up to date, it’s a good idea to download the Advanced Beneficiary Notice of Noncoverage from the Medicare website and have the patient sign it. It spells out what the patient’s financial responsibility could be.
“The ABN also protects you as a provider, because it shows you’re not trying to balance-bill the patient,” he noted.
Dr. Goldman implored his internist colleagues to stand up and become the stewards of adult immunization.
“Remember: Keep calm and vaccinate,” he urged.
He reported having no relevant financial conflicts.
REPORTING FROM ACP INTERNAL MEDICINE
Vestibular/oculomotor component of concussion warrants more attention
NEW ORLEANS – Vestibular and oculomotor impairment is increasingly recognized as a common, underappreciated, and yet treatable aspect of sports concussions, Gary W. Dorshimer, MD, said at the annual meeting of the American College of Physicians.
A major advance in the diagnosis and treatment of this form of impairment has been achieved by researchers at the University of Pittsburgh Medical Center sports medicine concussion program.
“The Pitt group has come up with a nice exam to assess this part of the concussion injury, which doesn’t affect your memory, it doesn’t affect your cognition, it affects what I’ve found to be the thing that takes the longest to get better: the oculomotor/vestibular mechanism,” explained Dr. Dorshimer, chief of general internal medicine at Penn Medicine, Philadelphia, and team physician for the Philadelphia Flyers professional ice hockey team.
The exam, which the Pitt group has described in full detail (Am J Sports Med. 2014;42(10):2479-86), is known as the Vestibular/Ocular Motor Screening assessment, or VOMS. The tool has filled an unmet need in sports medicine, he said. It takes only a few minutes for a physician to perform. The rating scale assesses visual motion sensitivity, smooth eye pursuits, horizontal and vertical saccades, the vestibular ocular reflex, and convergence. Positive findings warrant specialized referral for targeted rehabilitation using visual-ocular and vestibular therapies.
The symptoms of sports concussion–related oculomotor/vestibular impairment may include nausea, vertigo, dizziness, blurred or double vision, difficulty tracking a moving target, and discomfort in busy environments. These symptoms often translate to difficulty reading and academic problems, which historically often were misinterpreted as cognitive impairments.
It’s estimated that oculomotor/vestibular impairment occurs in roughly 60% of sports concussions. These vestibular and/or vision symptoms are associated with protracted recovery. And preliminary evidence demonstrates that targeted physical therapies are effective in speeding recovery.
“It’s so important to be able to find this [impairment] because it’s something you can do something about. We find that when these things are off and people work on them, they get better. That’s why so many people in the field are now saying that if a patient works hard, does the rehabilitation, the majority of them are going to get better. And they won’t get better unless they press forward,” the internist said.
The VOMS screen is simple to perform. It entails tasks such as convergence testing, in which the physician moves a finger or pen steadily closer to the patient’s face; if the patient reports that the single object has turned into two at a distance of more than 6 cm, that’s a positive result indicative of convergence insufficiency.
In another task, the physician hold his two index fingers apart and has the patients move their eyes from finger to finger while holding their heads still.
“I’m not that interested in whether they’re catching the tips of fingers, I’m interested in if they can go fast, and can they go faster if I challenge them, or do they stop doing it? When people with ocular vestibular dysfunction start doing this task, they’re going to slow down. They can’t keep it up because it’s so unpleasant. It really bothers them a lot,” Dr. Dorshimer observed.
In his experience, another key element in a smooth and successful recovery from sports concussions, in addition to getting skilled help for vestibular/oculomotor impairment, if present, is to encourage a positive attitude.
“If you think you’re going to get CTE [chronic traumatic encephalopathy] when you get older because you got waffled a bit in sport, that’s just such a negative attitude. I mean, you can’t lie to them: We don’t know. But I take care of a ton of retired athletes who don’t have CTE. Maybe they’re going to have some tangles in their brains, but they don’t have it clinically. So you want them to keep a positive attitude,” he emphasized.
“CTE was around when your parents and grandparents were jocks. They went out on the playground and pummeled each other every day after school. There are probably all kinds of factors involved in CTE: the number of concussions, hereditary factors, alcohol, drugs. No one really knows yet,” he said.
Dr. Dorshimer reported having no financial conflicts regarding his presentation on the athlete as patient.
NEW ORLEANS – Vestibular and oculomotor impairment is increasingly recognized as a common, underappreciated, and yet treatable aspect of sports concussions, Gary W. Dorshimer, MD, said at the annual meeting of the American College of Physicians.
A major advance in the diagnosis and treatment of this form of impairment has been achieved by researchers at the University of Pittsburgh Medical Center sports medicine concussion program.
“The Pitt group has come up with a nice exam to assess this part of the concussion injury, which doesn’t affect your memory, it doesn’t affect your cognition, it affects what I’ve found to be the thing that takes the longest to get better: the oculomotor/vestibular mechanism,” explained Dr. Dorshimer, chief of general internal medicine at Penn Medicine, Philadelphia, and team physician for the Philadelphia Flyers professional ice hockey team.
The exam, which the Pitt group has described in full detail (Am J Sports Med. 2014;42(10):2479-86), is known as the Vestibular/Ocular Motor Screening assessment, or VOMS. The tool has filled an unmet need in sports medicine, he said. It takes only a few minutes for a physician to perform. The rating scale assesses visual motion sensitivity, smooth eye pursuits, horizontal and vertical saccades, the vestibular ocular reflex, and convergence. Positive findings warrant specialized referral for targeted rehabilitation using visual-ocular and vestibular therapies.
The symptoms of sports concussion–related oculomotor/vestibular impairment may include nausea, vertigo, dizziness, blurred or double vision, difficulty tracking a moving target, and discomfort in busy environments. These symptoms often translate to difficulty reading and academic problems, which historically often were misinterpreted as cognitive impairments.
It’s estimated that oculomotor/vestibular impairment occurs in roughly 60% of sports concussions. These vestibular and/or vision symptoms are associated with protracted recovery. And preliminary evidence demonstrates that targeted physical therapies are effective in speeding recovery.
“It’s so important to be able to find this [impairment] because it’s something you can do something about. We find that when these things are off and people work on them, they get better. That’s why so many people in the field are now saying that if a patient works hard, does the rehabilitation, the majority of them are going to get better. And they won’t get better unless they press forward,” the internist said.
The VOMS screen is simple to perform. It entails tasks such as convergence testing, in which the physician moves a finger or pen steadily closer to the patient’s face; if the patient reports that the single object has turned into two at a distance of more than 6 cm, that’s a positive result indicative of convergence insufficiency.
In another task, the physician hold his two index fingers apart and has the patients move their eyes from finger to finger while holding their heads still.
“I’m not that interested in whether they’re catching the tips of fingers, I’m interested in if they can go fast, and can they go faster if I challenge them, or do they stop doing it? When people with ocular vestibular dysfunction start doing this task, they’re going to slow down. They can’t keep it up because it’s so unpleasant. It really bothers them a lot,” Dr. Dorshimer observed.
In his experience, another key element in a smooth and successful recovery from sports concussions, in addition to getting skilled help for vestibular/oculomotor impairment, if present, is to encourage a positive attitude.
“If you think you’re going to get CTE [chronic traumatic encephalopathy] when you get older because you got waffled a bit in sport, that’s just such a negative attitude. I mean, you can’t lie to them: We don’t know. But I take care of a ton of retired athletes who don’t have CTE. Maybe they’re going to have some tangles in their brains, but they don’t have it clinically. So you want them to keep a positive attitude,” he emphasized.
“CTE was around when your parents and grandparents were jocks. They went out on the playground and pummeled each other every day after school. There are probably all kinds of factors involved in CTE: the number of concussions, hereditary factors, alcohol, drugs. No one really knows yet,” he said.
Dr. Dorshimer reported having no financial conflicts regarding his presentation on the athlete as patient.
NEW ORLEANS – Vestibular and oculomotor impairment is increasingly recognized as a common, underappreciated, and yet treatable aspect of sports concussions, Gary W. Dorshimer, MD, said at the annual meeting of the American College of Physicians.
A major advance in the diagnosis and treatment of this form of impairment has been achieved by researchers at the University of Pittsburgh Medical Center sports medicine concussion program.
“The Pitt group has come up with a nice exam to assess this part of the concussion injury, which doesn’t affect your memory, it doesn’t affect your cognition, it affects what I’ve found to be the thing that takes the longest to get better: the oculomotor/vestibular mechanism,” explained Dr. Dorshimer, chief of general internal medicine at Penn Medicine, Philadelphia, and team physician for the Philadelphia Flyers professional ice hockey team.
The exam, which the Pitt group has described in full detail (Am J Sports Med. 2014;42(10):2479-86), is known as the Vestibular/Ocular Motor Screening assessment, or VOMS. The tool has filled an unmet need in sports medicine, he said. It takes only a few minutes for a physician to perform. The rating scale assesses visual motion sensitivity, smooth eye pursuits, horizontal and vertical saccades, the vestibular ocular reflex, and convergence. Positive findings warrant specialized referral for targeted rehabilitation using visual-ocular and vestibular therapies.
The symptoms of sports concussion–related oculomotor/vestibular impairment may include nausea, vertigo, dizziness, blurred or double vision, difficulty tracking a moving target, and discomfort in busy environments. These symptoms often translate to difficulty reading and academic problems, which historically often were misinterpreted as cognitive impairments.
It’s estimated that oculomotor/vestibular impairment occurs in roughly 60% of sports concussions. These vestibular and/or vision symptoms are associated with protracted recovery. And preliminary evidence demonstrates that targeted physical therapies are effective in speeding recovery.
“It’s so important to be able to find this [impairment] because it’s something you can do something about. We find that when these things are off and people work on them, they get better. That’s why so many people in the field are now saying that if a patient works hard, does the rehabilitation, the majority of them are going to get better. And they won’t get better unless they press forward,” the internist said.
The VOMS screen is simple to perform. It entails tasks such as convergence testing, in which the physician moves a finger or pen steadily closer to the patient’s face; if the patient reports that the single object has turned into two at a distance of more than 6 cm, that’s a positive result indicative of convergence insufficiency.
In another task, the physician hold his two index fingers apart and has the patients move their eyes from finger to finger while holding their heads still.
“I’m not that interested in whether they’re catching the tips of fingers, I’m interested in if they can go fast, and can they go faster if I challenge them, or do they stop doing it? When people with ocular vestibular dysfunction start doing this task, they’re going to slow down. They can’t keep it up because it’s so unpleasant. It really bothers them a lot,” Dr. Dorshimer observed.
In his experience, another key element in a smooth and successful recovery from sports concussions, in addition to getting skilled help for vestibular/oculomotor impairment, if present, is to encourage a positive attitude.
“If you think you’re going to get CTE [chronic traumatic encephalopathy] when you get older because you got waffled a bit in sport, that’s just such a negative attitude. I mean, you can’t lie to them: We don’t know. But I take care of a ton of retired athletes who don’t have CTE. Maybe they’re going to have some tangles in their brains, but they don’t have it clinically. So you want them to keep a positive attitude,” he emphasized.
“CTE was around when your parents and grandparents were jocks. They went out on the playground and pummeled each other every day after school. There are probably all kinds of factors involved in CTE: the number of concussions, hereditary factors, alcohol, drugs. No one really knows yet,” he said.
Dr. Dorshimer reported having no financial conflicts regarding his presentation on the athlete as patient.
REPORTING FROM ACP INTERNAL MEDICINE