User login
American Association for the Study of Liver Disease (AASLD): The Liver Meeting
The Liver Meeting 2017 NAFLD debrief – key abstracts
WASHINGTON – Nonalcoholic fatty liver disease (NAFLD) is a complex disease that involves multiple systems, and several standout abstracts at the annual meeting of the American Association for the Study of Liver Diseases emphasized the importance of multisystem management and the potential of combination therapies, Kymberly D. Watt, MD, said during the final debrief.
“The actual underlying mechanisms and the underlying processes that are going on are way more complicated than just inflammation and scarring,” said Dr. Watt, associate professor of medicine and medical director of liver transplantation at the Mayo Clinic, Rochester, Minn. “We have numerous areas to target, including insulin resistance, lipid metabolism, oxidative stress, inflammation, immune modulation, cell death, etc.”
She noted several studies that evaluated the prevalence of NAFLD, including a study that found that “about one-third of patients walking through the door of the clinic had nonalcoholic steatohepatitis [NASH],” suggesting physicians should consider screening at-risk patients (abstract 58). A Korean study found about 18% of asymptomatic lean individuals (body mass index less than 23 kg/m2) had NAFLD and identified sarcopenia as a significant risk factor for NAFLD in these lean patients (abstract 59). “Sarcopenia is something that we really need to pay a lot more attention to,” Dr. Watt said.
Other studies better outlined the increasing association between NAFLD and hepatocellular carcinoma, Dr. Watt noted (abstracts 2119 and 2102). Another study confirmed that men with NAFLD/NASH have almost twice the incidence of hepatocellular carcinoma (HCC) as women — 0.43%-0.5% vs. 0.22%-0.28%, with both groups significantly higher than the general population (abstract 2116). “And looking further, we can actually quote an HCC incidence in NASH of 0.009%,” she added.
Again emphasizing the multisystem impact of NAFLD, Dr. Watt cited a study that calculated the cardiovascular risks incumbent with liver disease. Researchers reported that men and women at the time of NAFLD diagnosis had significantly higher rates of either angina/ischemic heart disease or heart failure (abstract 55). Women, specifically, had a higher risk for cardiovascular events earlier than men and overall are at equal risk to men, unlike in the general population where women are at lower risk. “We need to start looking at screening and prevention of other diseases in our patients with NASH,” Dr. Watt said. “In addition, we need to be more aware of the elevated risk in these patients and not just approach them in the same way as the general population.”
Physicians may be tempted to discontinue statin therapy in patients with chronic liver disease, but Dr. Watt cited a poster that showed that this results in worse outcomes (abstract 2106). The researchers found that continued statin use was associated with a lower risk of death with compensated and decompensated liver function. “These data help to educate certain patients of their risk of decompensation over time,” Dr. Watt said.
An international study determined that the severity of advanced compensated liver disease is a key determinant in outcomes, finding that those with bridging fibrosis are at greater risk of vascular events, but those with cirrhosis and Child-Turcotte-Pugh A5 and A6 disease have much higher risks of hepatic decompensation and HCC out to 14 years (abstract 60). “The reason to look at these is to be able to tell your patients that they probably have a 30% increased risk of decompensation by 4 years,” Dr. Watt said.
Dr. Watt pointed out three studies that shed more light on important biomarkers of NAFLD. One study reported that three biomarkers – alpha-2-macroglobulin, hyaluronic acid, and tissue inhibitor of metalloproteinase-1 – have a high sensitivity for differentiating low-stage and stage F3-F4 disease (abstract 95). Another study found that a measure using Pro-C3 and other clinical markers were predictive of F3 or F4 fibrosis in NAFLD (abstract 93). And, other researchers found that a HepQuant-STAT measure of greater than 0.50 microM in patients who ingested deuterated cholic acid (d4-CA) solution may be a minimally invasive alternative to biopsy for diagnosing NASH (abstract 96).
Management studies focusing on varying targets were also presented. A trial of fibroblast growth factor–21 for treatment of NAFLD found that patients in the 10- and 20-mg dose arms showed improvement in MRI hepatic fat fraction, ALT, AST, and liver stiffness at 16 weeks vs. placebo. A few patients had some mild elevation to their liver enzymes on treatment (abstract 182). “So I think we need to remain cautious and watch these patients closely, but overall it seems to be reasonably safe data,” she said. Another drug trial of the acetyl-CoA carboxylase inhibitor GS-0976 also showed promise for overall improvement in MRI steatosis measures (abstract LB-9).
Three preclinical studies of dual-agent therapies in animals have demonstrated improvement in inflammatory and fibrosis scores, Dr. Watt noted (abstracts 2,000, 2,002 and 2,052). “There’s no one drug that’s going to be likely the magic cure,” Dr. Watt said. “There will likely be a lot more focus and data coming out on dual-action agents.” Another animal study addressed the burning question if decaffeinated coffee has the same protective effect against NASH as caffeinated coffee (abstract 2093). Said Dr. Watt: “If you are interested in the potential benefits of coffee but really can’t handle the caffeine, this study suggests, you may still be OK.”
Finally, Dr. Watt noted an early study of three-dimensional printing has shown potential for replicating NASH tissue for bench studies (abstract 1963). “3-D printing is certainly a wave of the future,” she said, pointing out that researchers have created a 3-D model that has some metabolic equivalency to NASH, with the inflammatory cytokine release, hepatic stellate cell activation, “and all of the features that we see in NASH. This may be of potential use down the road to avoid relying on animal models in preclinical studies.”
The Liver Meeting next convenes Nov. 9-13, 2018, in San Francisco.
Dr. Watt disclosed ties to Bristol-Myers Squibb, Exelixis, and Seattle Genetics.
WASHINGTON – Nonalcoholic fatty liver disease (NAFLD) is a complex disease that involves multiple systems, and several standout abstracts at the annual meeting of the American Association for the Study of Liver Diseases emphasized the importance of multisystem management and the potential of combination therapies, Kymberly D. Watt, MD, said during the final debrief.
“The actual underlying mechanisms and the underlying processes that are going on are way more complicated than just inflammation and scarring,” said Dr. Watt, associate professor of medicine and medical director of liver transplantation at the Mayo Clinic, Rochester, Minn. “We have numerous areas to target, including insulin resistance, lipid metabolism, oxidative stress, inflammation, immune modulation, cell death, etc.”
She noted several studies that evaluated the prevalence of NAFLD, including a study that found that “about one-third of patients walking through the door of the clinic had nonalcoholic steatohepatitis [NASH],” suggesting physicians should consider screening at-risk patients (abstract 58). A Korean study found about 18% of asymptomatic lean individuals (body mass index less than 23 kg/m2) had NAFLD and identified sarcopenia as a significant risk factor for NAFLD in these lean patients (abstract 59). “Sarcopenia is something that we really need to pay a lot more attention to,” Dr. Watt said.
Other studies better outlined the increasing association between NAFLD and hepatocellular carcinoma, Dr. Watt noted (abstracts 2119 and 2102). Another study confirmed that men with NAFLD/NASH have almost twice the incidence of hepatocellular carcinoma (HCC) as women — 0.43%-0.5% vs. 0.22%-0.28%, with both groups significantly higher than the general population (abstract 2116). “And looking further, we can actually quote an HCC incidence in NASH of 0.009%,” she added.
Again emphasizing the multisystem impact of NAFLD, Dr. Watt cited a study that calculated the cardiovascular risks incumbent with liver disease. Researchers reported that men and women at the time of NAFLD diagnosis had significantly higher rates of either angina/ischemic heart disease or heart failure (abstract 55). Women, specifically, had a higher risk for cardiovascular events earlier than men and overall are at equal risk to men, unlike in the general population where women are at lower risk. “We need to start looking at screening and prevention of other diseases in our patients with NASH,” Dr. Watt said. “In addition, we need to be more aware of the elevated risk in these patients and not just approach them in the same way as the general population.”
Physicians may be tempted to discontinue statin therapy in patients with chronic liver disease, but Dr. Watt cited a poster that showed that this results in worse outcomes (abstract 2106). The researchers found that continued statin use was associated with a lower risk of death with compensated and decompensated liver function. “These data help to educate certain patients of their risk of decompensation over time,” Dr. Watt said.
An international study determined that the severity of advanced compensated liver disease is a key determinant in outcomes, finding that those with bridging fibrosis are at greater risk of vascular events, but those with cirrhosis and Child-Turcotte-Pugh A5 and A6 disease have much higher risks of hepatic decompensation and HCC out to 14 years (abstract 60). “The reason to look at these is to be able to tell your patients that they probably have a 30% increased risk of decompensation by 4 years,” Dr. Watt said.
Dr. Watt pointed out three studies that shed more light on important biomarkers of NAFLD. One study reported that three biomarkers – alpha-2-macroglobulin, hyaluronic acid, and tissue inhibitor of metalloproteinase-1 – have a high sensitivity for differentiating low-stage and stage F3-F4 disease (abstract 95). Another study found that a measure using Pro-C3 and other clinical markers were predictive of F3 or F4 fibrosis in NAFLD (abstract 93). And, other researchers found that a HepQuant-STAT measure of greater than 0.50 microM in patients who ingested deuterated cholic acid (d4-CA) solution may be a minimally invasive alternative to biopsy for diagnosing NASH (abstract 96).
Management studies focusing on varying targets were also presented. A trial of fibroblast growth factor–21 for treatment of NAFLD found that patients in the 10- and 20-mg dose arms showed improvement in MRI hepatic fat fraction, ALT, AST, and liver stiffness at 16 weeks vs. placebo. A few patients had some mild elevation to their liver enzymes on treatment (abstract 182). “So I think we need to remain cautious and watch these patients closely, but overall it seems to be reasonably safe data,” she said. Another drug trial of the acetyl-CoA carboxylase inhibitor GS-0976 also showed promise for overall improvement in MRI steatosis measures (abstract LB-9).
Three preclinical studies of dual-agent therapies in animals have demonstrated improvement in inflammatory and fibrosis scores, Dr. Watt noted (abstracts 2,000, 2,002 and 2,052). “There’s no one drug that’s going to be likely the magic cure,” Dr. Watt said. “There will likely be a lot more focus and data coming out on dual-action agents.” Another animal study addressed the burning question if decaffeinated coffee has the same protective effect against NASH as caffeinated coffee (abstract 2093). Said Dr. Watt: “If you are interested in the potential benefits of coffee but really can’t handle the caffeine, this study suggests, you may still be OK.”
Finally, Dr. Watt noted an early study of three-dimensional printing has shown potential for replicating NASH tissue for bench studies (abstract 1963). “3-D printing is certainly a wave of the future,” she said, pointing out that researchers have created a 3-D model that has some metabolic equivalency to NASH, with the inflammatory cytokine release, hepatic stellate cell activation, “and all of the features that we see in NASH. This may be of potential use down the road to avoid relying on animal models in preclinical studies.”
The Liver Meeting next convenes Nov. 9-13, 2018, in San Francisco.
Dr. Watt disclosed ties to Bristol-Myers Squibb, Exelixis, and Seattle Genetics.
WASHINGTON – Nonalcoholic fatty liver disease (NAFLD) is a complex disease that involves multiple systems, and several standout abstracts at the annual meeting of the American Association for the Study of Liver Diseases emphasized the importance of multisystem management and the potential of combination therapies, Kymberly D. Watt, MD, said during the final debrief.
“The actual underlying mechanisms and the underlying processes that are going on are way more complicated than just inflammation and scarring,” said Dr. Watt, associate professor of medicine and medical director of liver transplantation at the Mayo Clinic, Rochester, Minn. “We have numerous areas to target, including insulin resistance, lipid metabolism, oxidative stress, inflammation, immune modulation, cell death, etc.”
She noted several studies that evaluated the prevalence of NAFLD, including a study that found that “about one-third of patients walking through the door of the clinic had nonalcoholic steatohepatitis [NASH],” suggesting physicians should consider screening at-risk patients (abstract 58). A Korean study found about 18% of asymptomatic lean individuals (body mass index less than 23 kg/m2) had NAFLD and identified sarcopenia as a significant risk factor for NAFLD in these lean patients (abstract 59). “Sarcopenia is something that we really need to pay a lot more attention to,” Dr. Watt said.
Other studies better outlined the increasing association between NAFLD and hepatocellular carcinoma, Dr. Watt noted (abstracts 2119 and 2102). Another study confirmed that men with NAFLD/NASH have almost twice the incidence of hepatocellular carcinoma (HCC) as women — 0.43%-0.5% vs. 0.22%-0.28%, with both groups significantly higher than the general population (abstract 2116). “And looking further, we can actually quote an HCC incidence in NASH of 0.009%,” she added.
Again emphasizing the multisystem impact of NAFLD, Dr. Watt cited a study that calculated the cardiovascular risks incumbent with liver disease. Researchers reported that men and women at the time of NAFLD diagnosis had significantly higher rates of either angina/ischemic heart disease or heart failure (abstract 55). Women, specifically, had a higher risk for cardiovascular events earlier than men and overall are at equal risk to men, unlike in the general population where women are at lower risk. “We need to start looking at screening and prevention of other diseases in our patients with NASH,” Dr. Watt said. “In addition, we need to be more aware of the elevated risk in these patients and not just approach them in the same way as the general population.”
Physicians may be tempted to discontinue statin therapy in patients with chronic liver disease, but Dr. Watt cited a poster that showed that this results in worse outcomes (abstract 2106). The researchers found that continued statin use was associated with a lower risk of death with compensated and decompensated liver function. “These data help to educate certain patients of their risk of decompensation over time,” Dr. Watt said.
An international study determined that the severity of advanced compensated liver disease is a key determinant in outcomes, finding that those with bridging fibrosis are at greater risk of vascular events, but those with cirrhosis and Child-Turcotte-Pugh A5 and A6 disease have much higher risks of hepatic decompensation and HCC out to 14 years (abstract 60). “The reason to look at these is to be able to tell your patients that they probably have a 30% increased risk of decompensation by 4 years,” Dr. Watt said.
Dr. Watt pointed out three studies that shed more light on important biomarkers of NAFLD. One study reported that three biomarkers – alpha-2-macroglobulin, hyaluronic acid, and tissue inhibitor of metalloproteinase-1 – have a high sensitivity for differentiating low-stage and stage F3-F4 disease (abstract 95). Another study found that a measure using Pro-C3 and other clinical markers were predictive of F3 or F4 fibrosis in NAFLD (abstract 93). And, other researchers found that a HepQuant-STAT measure of greater than 0.50 microM in patients who ingested deuterated cholic acid (d4-CA) solution may be a minimally invasive alternative to biopsy for diagnosing NASH (abstract 96).
Management studies focusing on varying targets were also presented. A trial of fibroblast growth factor–21 for treatment of NAFLD found that patients in the 10- and 20-mg dose arms showed improvement in MRI hepatic fat fraction, ALT, AST, and liver stiffness at 16 weeks vs. placebo. A few patients had some mild elevation to their liver enzymes on treatment (abstract 182). “So I think we need to remain cautious and watch these patients closely, but overall it seems to be reasonably safe data,” she said. Another drug trial of the acetyl-CoA carboxylase inhibitor GS-0976 also showed promise for overall improvement in MRI steatosis measures (abstract LB-9).
Three preclinical studies of dual-agent therapies in animals have demonstrated improvement in inflammatory and fibrosis scores, Dr. Watt noted (abstracts 2,000, 2,002 and 2,052). “There’s no one drug that’s going to be likely the magic cure,” Dr. Watt said. “There will likely be a lot more focus and data coming out on dual-action agents.” Another animal study addressed the burning question if decaffeinated coffee has the same protective effect against NASH as caffeinated coffee (abstract 2093). Said Dr. Watt: “If you are interested in the potential benefits of coffee but really can’t handle the caffeine, this study suggests, you may still be OK.”
Finally, Dr. Watt noted an early study of three-dimensional printing has shown potential for replicating NASH tissue for bench studies (abstract 1963). “3-D printing is certainly a wave of the future,” she said, pointing out that researchers have created a 3-D model that has some metabolic equivalency to NASH, with the inflammatory cytokine release, hepatic stellate cell activation, “and all of the features that we see in NASH. This may be of potential use down the road to avoid relying on animal models in preclinical studies.”
The Liver Meeting next convenes Nov. 9-13, 2018, in San Francisco.
Dr. Watt disclosed ties to Bristol-Myers Squibb, Exelixis, and Seattle Genetics.
AT THE LIVER MEETING 2017
Key clinical point: Nonalcoholic fatty liver disease involves treatment and management of multiple systems.
Major finding: Physicians managing NAFLD must target insulin resistance, lipid metabolism, oxidative stress, and more.
Data source: Debrief of key abstracts on NAFLD presented at the Liver Meeting 2017.
Disclosures: Dr. Watt reported having relationships with Bristol-Myers Squibb, Exelixis, and Seattle Genetics.
Clinical hepatology debrief wraps up 2017 Liver Meeting
WASHINGTON – Research into alcoholic liver disease, drug-induced liver injury, the complications of chronic liver disease, and cholestatic liver diseases were among the clinical hepatology highlights presented at the annual meeting of the American Association for the Study of Liver Diseases.
This year’s debrief was given by Kris V. Kowdley, MD, of Swedish Medical Center in Seattle.
Over a median of 1.6 years of follow-up, 27% of patients resumed alcohol consumption post transplant with a median time to alcohol of 160 days, according to Brian Lee, MD, of the University of California, San Francisco. Younger age and lack of complete acceptance of their alcoholic hepatitis diagnosis were significant predictors of alcohol use post transplant while factors such as length of abstinence, race/ethnicity, insurance status, history of illicit drug use, and history of failed rehab attempts were not. Further, heavy drinking at presentation (more than 10 drinks per day), any alcohol use post transplant, and sustained alcohol use post transplant were significant predictors of posttransplant death.
Alcoholic hepatitis now “appears to be affecting more and more younger women, who present with a higher level of acuity,” Dr. Kowdley noted. He added that, while recent advances have decreased the absolute number of hepatitis C patients with decompensated liver disease who are listed for liver transplant, “the number is increasing rapidly in alcoholic liver disease, approaching the rate of patients being listed for hepatitis C.”
Unknown ingredients in herbal and dietary supplements continue to be of concern, Dr. Kowdley noted, as highlighted by Victor J. Navarro, MD, of Einstein Healthcare Network, Philadelphia, and his colleagues at the Drug-Induced Liver Injury Network (DILIN).
Investigators collected herbal and dietary supplements from patients enrolled in the DILIN prospective study and had chemical analysis performed by an outside laboratory. Labeled contents could not be verified in over half of the supplements collected and several unlabeled hepatotoxic ingredients were identified, Dr. Navarro and colleagues found (abstract 264).
“Even though we collect the supplements and review them with the patients, it’s not clear that we even know what it is that they are taking,” Dr. Kowdley commented.
Another DILIN study, this one presented by Jawad Ahmad, MD, of the Icahn School of Medicine at Mount Sinai, New York, “provided an opportunity for pause,” Dr. Kowdley said.
Dr. Ahmad and colleagues looked at hepatitis C virus (HCV) testing in DILIN patients and were able to correlate anti-HCV test results with HCV RNA tests results in more than 95% of 1,500 patients (abstract 16). About 7% of patients were HCV positive, and 23 cases of acute hepatitis were identified (16 with anti-HCV antibodies and HCV RNA, 7 with HCV RNA alone, and none with anti-HCV antibodies alone).
“So the take-home message here for me is, even if we think the patient has drug-induced liver injury, if they have not been tested for hepatitis C, especially if in the hospitalized setting … it is important to check not only the antibody test but also the RNA test,” Dr. Kowdley said.
Finally, in children, minocycline and valproate were the most commonly indicated agents in pediatric drug-induced liver injury, according to Frank DiPaola, MD, of the University of Michigan, and colleagues, on behalf of DILIN (abstract 13).
Dr. Kowdley also highlighted a couple of studies that addressed the complications of chronic liver disease.
The ADAPT-1 and ADAPT-2 trials (abstract 217) studied the use of avatrombopag, a thrombopoietin (TPO)–receptor agonist, to reduce severe thrombocytopenia in patients with chronic liver disease. Platelet transfusion is the current standard of care to reduce the risk of bleeding during invasive procedures in these patients; currently there are no drugs approved for this indication, Dr. Kowdley said.
Avatrombopag is an oral, small molecule TPO-receptor agonist, he said. “Because it binds to a different site on the TPO receptor than endogenous TPO, the effects are additive.”
In the phase 3 ADAPT-1 and ADAPT-2, the proportion of patients who did not require platelet transfusion or any rescue procedure for bleeding was significantly less in avatrombopag-treated patients than those receiving placebo. The effect was the same for patients with a low baseline platelet count (less than 40,000 platelets per mcL) as well as those with a high baseline platelet count (between 40,000/mcL and 50,000/mcL). Further, the proportion of patients who by procedure day achieved platelet count of at least 50,000/mcL was significantly higher in patients on the study drug.
Data on lusutrombopag, another TPO-receptor agonist, was presented as a late-breaker at the meeting, with very similar results in avoiding platelet transfusion, Dr. Kowdley noted.Two abstracts (502 and 219) focused on reducing ammonia levels in hospitalized cirrhosis patients with hepatic encephalitis.
Patients in the STOP-HE trial were randomized to either physician’s choice for standard of care or standard of care plus continuous infusion of ornithine phenylacetate for up to 5 days. Patients were assigned to one of three dosing groups (20 g, 15 g, or 10 g), based on severity of underlying liver disease; those with the most severe disease received the lowest dose.
Reduction in plasma ammonia levels correlated significantly with clinical improvement. At 48 hours, meaningful clinical improvement occurred in 84% of patients on ornithine phenylacetate, compared with 58% of placebo patients, according to Robert S. Rahimi, MD, of Baylor University, Dallas, and his colleagues.
“So, this may be an option for our hepatic encephalopathy patients who are admitted to the hospital and need acute treatment,” Dr. Kowdley said.
Dr. Kowdley finished up with two studies on primary biliary cholangitis (PBC).
Carla Murillo Perez, MD, of Toronto General Hospital and her colleagues in the Global PBC Study Group investigated the role of serum bilirubin in predicting transplant-free survival in patients with PBC (abstract 70).
When serum bilirubin levels from a previous study were input into a Cox regression analysis as a cubic spline function, then adjusted for factors such as age, sex, treatment with ursodeoxycholic acid, and year of diagnosis, the investigators found that patients with serum bilirubin levels of 0.7 times the upper limit of normal had a significantly increased risk of liver transplantation or death.
“We may want to be more sensitive in looking at bilirubin levels,” Dr. Kowdley said.
Another small but notable study presented by Gideon M. Hirschfield, MD, of the University of Birmingham (England), looked into whether a lower dose of seladelpar would safely and effectively lower alkaline phosphatase (AP) levels in PBC patients. A previous study of seladelpar at 50 mg and 200 mg doses indicated the drug’s effectiveness; however, the study was stopped because of the development of grade 3 alanine aminotransferase increases in a number of patients (Lancet Gastroenterol Hepatol. 2017;2;716-26).
Dr. Hirschfield and colleagues enrolled 24 patients and randomized 12 to seladelpar 5 mg and another 12 to 10 mg. The study cohort was mostly female, with an average age of 58 years. Most were either intolerant of or inadequately treated by ursodeoxycholic acid. AP levels were reduced significantly over time in both groups; however, differences between the groups were not significant, the investigators noted.
The Liver Meeting will be held in San Francisco in 2018, taking place Nov. 9-13. Many investigators in these trials reported relevant conflicts of interest; information is available (open access) in a supplement to Hepatology.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
WASHINGTON – Research into alcoholic liver disease, drug-induced liver injury, the complications of chronic liver disease, and cholestatic liver diseases were among the clinical hepatology highlights presented at the annual meeting of the American Association for the Study of Liver Diseases.
This year’s debrief was given by Kris V. Kowdley, MD, of Swedish Medical Center in Seattle.
Over a median of 1.6 years of follow-up, 27% of patients resumed alcohol consumption post transplant with a median time to alcohol of 160 days, according to Brian Lee, MD, of the University of California, San Francisco. Younger age and lack of complete acceptance of their alcoholic hepatitis diagnosis were significant predictors of alcohol use post transplant while factors such as length of abstinence, race/ethnicity, insurance status, history of illicit drug use, and history of failed rehab attempts were not. Further, heavy drinking at presentation (more than 10 drinks per day), any alcohol use post transplant, and sustained alcohol use post transplant were significant predictors of posttransplant death.
Alcoholic hepatitis now “appears to be affecting more and more younger women, who present with a higher level of acuity,” Dr. Kowdley noted. He added that, while recent advances have decreased the absolute number of hepatitis C patients with decompensated liver disease who are listed for liver transplant, “the number is increasing rapidly in alcoholic liver disease, approaching the rate of patients being listed for hepatitis C.”
Unknown ingredients in herbal and dietary supplements continue to be of concern, Dr. Kowdley noted, as highlighted by Victor J. Navarro, MD, of Einstein Healthcare Network, Philadelphia, and his colleagues at the Drug-Induced Liver Injury Network (DILIN).
Investigators collected herbal and dietary supplements from patients enrolled in the DILIN prospective study and had chemical analysis performed by an outside laboratory. Labeled contents could not be verified in over half of the supplements collected and several unlabeled hepatotoxic ingredients were identified, Dr. Navarro and colleagues found (abstract 264).
“Even though we collect the supplements and review them with the patients, it’s not clear that we even know what it is that they are taking,” Dr. Kowdley commented.
Another DILIN study, this one presented by Jawad Ahmad, MD, of the Icahn School of Medicine at Mount Sinai, New York, “provided an opportunity for pause,” Dr. Kowdley said.
Dr. Ahmad and colleagues looked at hepatitis C virus (HCV) testing in DILIN patients and were able to correlate anti-HCV test results with HCV RNA tests results in more than 95% of 1,500 patients (abstract 16). About 7% of patients were HCV positive, and 23 cases of acute hepatitis were identified (16 with anti-HCV antibodies and HCV RNA, 7 with HCV RNA alone, and none with anti-HCV antibodies alone).
“So the take-home message here for me is, even if we think the patient has drug-induced liver injury, if they have not been tested for hepatitis C, especially if in the hospitalized setting … it is important to check not only the antibody test but also the RNA test,” Dr. Kowdley said.
Finally, in children, minocycline and valproate were the most commonly indicated agents in pediatric drug-induced liver injury, according to Frank DiPaola, MD, of the University of Michigan, and colleagues, on behalf of DILIN (abstract 13).
Dr. Kowdley also highlighted a couple of studies that addressed the complications of chronic liver disease.
The ADAPT-1 and ADAPT-2 trials (abstract 217) studied the use of avatrombopag, a thrombopoietin (TPO)–receptor agonist, to reduce severe thrombocytopenia in patients with chronic liver disease. Platelet transfusion is the current standard of care to reduce the risk of bleeding during invasive procedures in these patients; currently there are no drugs approved for this indication, Dr. Kowdley said.
Avatrombopag is an oral, small molecule TPO-receptor agonist, he said. “Because it binds to a different site on the TPO receptor than endogenous TPO, the effects are additive.”
In the phase 3 ADAPT-1 and ADAPT-2, the proportion of patients who did not require platelet transfusion or any rescue procedure for bleeding was significantly less in avatrombopag-treated patients than those receiving placebo. The effect was the same for patients with a low baseline platelet count (less than 40,000 platelets per mcL) as well as those with a high baseline platelet count (between 40,000/mcL and 50,000/mcL). Further, the proportion of patients who by procedure day achieved platelet count of at least 50,000/mcL was significantly higher in patients on the study drug.
Data on lusutrombopag, another TPO-receptor agonist, was presented as a late-breaker at the meeting, with very similar results in avoiding platelet transfusion, Dr. Kowdley noted.Two abstracts (502 and 219) focused on reducing ammonia levels in hospitalized cirrhosis patients with hepatic encephalitis.
Patients in the STOP-HE trial were randomized to either physician’s choice for standard of care or standard of care plus continuous infusion of ornithine phenylacetate for up to 5 days. Patients were assigned to one of three dosing groups (20 g, 15 g, or 10 g), based on severity of underlying liver disease; those with the most severe disease received the lowest dose.
Reduction in plasma ammonia levels correlated significantly with clinical improvement. At 48 hours, meaningful clinical improvement occurred in 84% of patients on ornithine phenylacetate, compared with 58% of placebo patients, according to Robert S. Rahimi, MD, of Baylor University, Dallas, and his colleagues.
“So, this may be an option for our hepatic encephalopathy patients who are admitted to the hospital and need acute treatment,” Dr. Kowdley said.
Dr. Kowdley finished up with two studies on primary biliary cholangitis (PBC).
Carla Murillo Perez, MD, of Toronto General Hospital and her colleagues in the Global PBC Study Group investigated the role of serum bilirubin in predicting transplant-free survival in patients with PBC (abstract 70).
When serum bilirubin levels from a previous study were input into a Cox regression analysis as a cubic spline function, then adjusted for factors such as age, sex, treatment with ursodeoxycholic acid, and year of diagnosis, the investigators found that patients with serum bilirubin levels of 0.7 times the upper limit of normal had a significantly increased risk of liver transplantation or death.
“We may want to be more sensitive in looking at bilirubin levels,” Dr. Kowdley said.
Another small but notable study presented by Gideon M. Hirschfield, MD, of the University of Birmingham (England), looked into whether a lower dose of seladelpar would safely and effectively lower alkaline phosphatase (AP) levels in PBC patients. A previous study of seladelpar at 50 mg and 200 mg doses indicated the drug’s effectiveness; however, the study was stopped because of the development of grade 3 alanine aminotransferase increases in a number of patients (Lancet Gastroenterol Hepatol. 2017;2;716-26).
Dr. Hirschfield and colleagues enrolled 24 patients and randomized 12 to seladelpar 5 mg and another 12 to 10 mg. The study cohort was mostly female, with an average age of 58 years. Most were either intolerant of or inadequately treated by ursodeoxycholic acid. AP levels were reduced significantly over time in both groups; however, differences between the groups were not significant, the investigators noted.
The Liver Meeting will be held in San Francisco in 2018, taking place Nov. 9-13. Many investigators in these trials reported relevant conflicts of interest; information is available (open access) in a supplement to Hepatology.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
WASHINGTON – Research into alcoholic liver disease, drug-induced liver injury, the complications of chronic liver disease, and cholestatic liver diseases were among the clinical hepatology highlights presented at the annual meeting of the American Association for the Study of Liver Diseases.
This year’s debrief was given by Kris V. Kowdley, MD, of Swedish Medical Center in Seattle.
Over a median of 1.6 years of follow-up, 27% of patients resumed alcohol consumption post transplant with a median time to alcohol of 160 days, according to Brian Lee, MD, of the University of California, San Francisco. Younger age and lack of complete acceptance of their alcoholic hepatitis diagnosis were significant predictors of alcohol use post transplant while factors such as length of abstinence, race/ethnicity, insurance status, history of illicit drug use, and history of failed rehab attempts were not. Further, heavy drinking at presentation (more than 10 drinks per day), any alcohol use post transplant, and sustained alcohol use post transplant were significant predictors of posttransplant death.
Alcoholic hepatitis now “appears to be affecting more and more younger women, who present with a higher level of acuity,” Dr. Kowdley noted. He added that, while recent advances have decreased the absolute number of hepatitis C patients with decompensated liver disease who are listed for liver transplant, “the number is increasing rapidly in alcoholic liver disease, approaching the rate of patients being listed for hepatitis C.”
Unknown ingredients in herbal and dietary supplements continue to be of concern, Dr. Kowdley noted, as highlighted by Victor J. Navarro, MD, of Einstein Healthcare Network, Philadelphia, and his colleagues at the Drug-Induced Liver Injury Network (DILIN).
Investigators collected herbal and dietary supplements from patients enrolled in the DILIN prospective study and had chemical analysis performed by an outside laboratory. Labeled contents could not be verified in over half of the supplements collected and several unlabeled hepatotoxic ingredients were identified, Dr. Navarro and colleagues found (abstract 264).
“Even though we collect the supplements and review them with the patients, it’s not clear that we even know what it is that they are taking,” Dr. Kowdley commented.
Another DILIN study, this one presented by Jawad Ahmad, MD, of the Icahn School of Medicine at Mount Sinai, New York, “provided an opportunity for pause,” Dr. Kowdley said.
Dr. Ahmad and colleagues looked at hepatitis C virus (HCV) testing in DILIN patients and were able to correlate anti-HCV test results with HCV RNA tests results in more than 95% of 1,500 patients (abstract 16). About 7% of patients were HCV positive, and 23 cases of acute hepatitis were identified (16 with anti-HCV antibodies and HCV RNA, 7 with HCV RNA alone, and none with anti-HCV antibodies alone).
“So the take-home message here for me is, even if we think the patient has drug-induced liver injury, if they have not been tested for hepatitis C, especially if in the hospitalized setting … it is important to check not only the antibody test but also the RNA test,” Dr. Kowdley said.
Finally, in children, minocycline and valproate were the most commonly indicated agents in pediatric drug-induced liver injury, according to Frank DiPaola, MD, of the University of Michigan, and colleagues, on behalf of DILIN (abstract 13).
Dr. Kowdley also highlighted a couple of studies that addressed the complications of chronic liver disease.
The ADAPT-1 and ADAPT-2 trials (abstract 217) studied the use of avatrombopag, a thrombopoietin (TPO)–receptor agonist, to reduce severe thrombocytopenia in patients with chronic liver disease. Platelet transfusion is the current standard of care to reduce the risk of bleeding during invasive procedures in these patients; currently there are no drugs approved for this indication, Dr. Kowdley said.
Avatrombopag is an oral, small molecule TPO-receptor agonist, he said. “Because it binds to a different site on the TPO receptor than endogenous TPO, the effects are additive.”
In the phase 3 ADAPT-1 and ADAPT-2, the proportion of patients who did not require platelet transfusion or any rescue procedure for bleeding was significantly less in avatrombopag-treated patients than those receiving placebo. The effect was the same for patients with a low baseline platelet count (less than 40,000 platelets per mcL) as well as those with a high baseline platelet count (between 40,000/mcL and 50,000/mcL). Further, the proportion of patients who by procedure day achieved platelet count of at least 50,000/mcL was significantly higher in patients on the study drug.
Data on lusutrombopag, another TPO-receptor agonist, was presented as a late-breaker at the meeting, with very similar results in avoiding platelet transfusion, Dr. Kowdley noted.Two abstracts (502 and 219) focused on reducing ammonia levels in hospitalized cirrhosis patients with hepatic encephalitis.
Patients in the STOP-HE trial were randomized to either physician’s choice for standard of care or standard of care plus continuous infusion of ornithine phenylacetate for up to 5 days. Patients were assigned to one of three dosing groups (20 g, 15 g, or 10 g), based on severity of underlying liver disease; those with the most severe disease received the lowest dose.
Reduction in plasma ammonia levels correlated significantly with clinical improvement. At 48 hours, meaningful clinical improvement occurred in 84% of patients on ornithine phenylacetate, compared with 58% of placebo patients, according to Robert S. Rahimi, MD, of Baylor University, Dallas, and his colleagues.
“So, this may be an option for our hepatic encephalopathy patients who are admitted to the hospital and need acute treatment,” Dr. Kowdley said.
Dr. Kowdley finished up with two studies on primary biliary cholangitis (PBC).
Carla Murillo Perez, MD, of Toronto General Hospital and her colleagues in the Global PBC Study Group investigated the role of serum bilirubin in predicting transplant-free survival in patients with PBC (abstract 70).
When serum bilirubin levels from a previous study were input into a Cox regression analysis as a cubic spline function, then adjusted for factors such as age, sex, treatment with ursodeoxycholic acid, and year of diagnosis, the investigators found that patients with serum bilirubin levels of 0.7 times the upper limit of normal had a significantly increased risk of liver transplantation or death.
“We may want to be more sensitive in looking at bilirubin levels,” Dr. Kowdley said.
Another small but notable study presented by Gideon M. Hirschfield, MD, of the University of Birmingham (England), looked into whether a lower dose of seladelpar would safely and effectively lower alkaline phosphatase (AP) levels in PBC patients. A previous study of seladelpar at 50 mg and 200 mg doses indicated the drug’s effectiveness; however, the study was stopped because of the development of grade 3 alanine aminotransferase increases in a number of patients (Lancet Gastroenterol Hepatol. 2017;2;716-26).
Dr. Hirschfield and colleagues enrolled 24 patients and randomized 12 to seladelpar 5 mg and another 12 to 10 mg. The study cohort was mostly female, with an average age of 58 years. Most were either intolerant of or inadequately treated by ursodeoxycholic acid. AP levels were reduced significantly over time in both groups; however, differences between the groups were not significant, the investigators noted.
The Liver Meeting will be held in San Francisco in 2018, taking place Nov. 9-13. Many investigators in these trials reported relevant conflicts of interest; information is available (open access) in a supplement to Hepatology.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
AT THE LIVER MEETING 2017
Study examines intestinal microbiota role post liver transplant
WASHINGTON – During and after liver transplant, the reaction of the intestinal microbiota may be a critical determinant of outcomes; preliminary data from a cohort study may provide some clarification of what modulates gut microbiota post transplantation and shed light on predictive factors.
Anna-Catrin Uhlemann, MD, PhD, of Columbia University Medical Center, New York, noted that several studies in recent years sought to clarify influences on gut microbiota in people receiving liver transplants, but “there are still a number of important gaps in knowledge, including what exactly is the longitudinal evolution of the host transplant microbiome and what is the predictive value of pre- and early posttransplant dysbiosis on outcomes and complications.”
The researchers collected more than 1,000 samples to screen for colonization by the following MDR organisms: carbapenem-resistant Enterobacteriaceae (CRE), Enterobacteriaceae resistant to third-generation cephalosporins (ESBL), and vancomycin-resistant enterococci (VRE). Over the 1-year follow-up period, 19% (P =.031) of patients had CRE colonization associated with subsequent infection, 41% (P = .003) had ESBL colonization, and 46% (P = .021) had VRE colonization, Dr. Uhlemann said at the annual meeting of the American Association for the Study of Liver Diseases. The researchers then selected 484 samples for sequencing of the 16S ribosomal RNA gene to determine the composition of gut microbiota.
The study used two indexes to determine the alpha diversity of microbiota: the Chao index to estimate richness and the Shannon diversity index to determine the abundance of species in different settings. “We observed dynamic temporal evolution of alpha diversity and taxa abundance over the 1-year follow-up period,” Dr. Uhlemann said. “The diagnosis, the Child-Pugh class, and changes in perioperative antibiotics were important predictors of posttransplant alpha diversity.”
The study also found that Enterobacteriaceae and enterococci increased post transplant in general and as MDR organisms, and that a patient’s MDR status was an important modulator of the posttransplant microbiome, as was the lack of protective operational taxonomic units (OTUs).
The researchers evaluated the relative abundance of taxa and beta diversity. For example, pretransplant patients with a Model for End-stage Liver Disease (MELD) score greater than 25 showed enrichment of Enterobacteriaceae as well as different taxa of the Bacteroidiaceae, while those with MELD scores below 25 showed enrichment of Veillonellaceae. “The significance of this is not clear yet,” Dr. Uhlemann said.
Liver disease severity can also influence gut microbes. Those with Child-Pugh class C disease have the highest numbers in terms of richness and lowest in terms of diversity, Dr. Uhlemann said. “However, at the moment when we are looking at the differential abundance of the taxa, we don’t see quite as clear a pattern, although we noticed in the high group a higher abundance of Bacteroidiaceae,” she said.
Hepatitis B and C patients also presented divergent microbiota profiles. Hepatitis B virus patients “in general are always relatively healthy, and we actually see that these indices are relatively preserved,” Dr. Uhlemann said. “When we look at hepatitis C, however, we see that these patients are starting off quite low and then have an increase in alpha-diversity measures at around month 6.” A subset of patients with alcoholic liver disease also didn’t reach higher Chao and Shannon levels until 6 months after transplant.
“We also find that adjustment of periodic antibiotics for allergy or history of prior infection is significantly associated with a decrease in alpha diversity several months into the posttransplant course,” said Dr. Uhlemann. This is driven by an increase in the abundance of Enterococcaceae and Enterobacteriaceae. “And when we look at MDR colonization as a predictor of alpha diversity, we see that those who have MDR colonization, irrespective of the species, also have the lower alpha diversity.”
The researchers also started to look at pretransplant alpha diversity as a predictor of transplant outcomes, and while the analysis is still in progress, the Shannon indices were significantly different between patients who died and those who survived a year. “There was a trend for significant differences for posttransplant infection and the length of the hospital stay,” Dr. Uhlemann said. “However, we did not see any association with posttransplant ICU readmission, rejection, or VRE complications.”
She added that future analyses are needed to further evaluate the interaction between the clinical comorbidities in the microbiome and vice versa.
Dr. Uhlemann disclosed links to Merck.
WASHINGTON – During and after liver transplant, the reaction of the intestinal microbiota may be a critical determinant of outcomes; preliminary data from a cohort study may provide some clarification of what modulates gut microbiota post transplantation and shed light on predictive factors.
Anna-Catrin Uhlemann, MD, PhD, of Columbia University Medical Center, New York, noted that several studies in recent years sought to clarify influences on gut microbiota in people receiving liver transplants, but “there are still a number of important gaps in knowledge, including what exactly is the longitudinal evolution of the host transplant microbiome and what is the predictive value of pre- and early posttransplant dysbiosis on outcomes and complications.”
The researchers collected more than 1,000 samples to screen for colonization by the following MDR organisms: carbapenem-resistant Enterobacteriaceae (CRE), Enterobacteriaceae resistant to third-generation cephalosporins (ESBL), and vancomycin-resistant enterococci (VRE). Over the 1-year follow-up period, 19% (P =.031) of patients had CRE colonization associated with subsequent infection, 41% (P = .003) had ESBL colonization, and 46% (P = .021) had VRE colonization, Dr. Uhlemann said at the annual meeting of the American Association for the Study of Liver Diseases. The researchers then selected 484 samples for sequencing of the 16S ribosomal RNA gene to determine the composition of gut microbiota.
The study used two indexes to determine the alpha diversity of microbiota: the Chao index to estimate richness and the Shannon diversity index to determine the abundance of species in different settings. “We observed dynamic temporal evolution of alpha diversity and taxa abundance over the 1-year follow-up period,” Dr. Uhlemann said. “The diagnosis, the Child-Pugh class, and changes in perioperative antibiotics were important predictors of posttransplant alpha diversity.”
The study also found that Enterobacteriaceae and enterococci increased post transplant in general and as MDR organisms, and that a patient’s MDR status was an important modulator of the posttransplant microbiome, as was the lack of protective operational taxonomic units (OTUs).
The researchers evaluated the relative abundance of taxa and beta diversity. For example, pretransplant patients with a Model for End-stage Liver Disease (MELD) score greater than 25 showed enrichment of Enterobacteriaceae as well as different taxa of the Bacteroidiaceae, while those with MELD scores below 25 showed enrichment of Veillonellaceae. “The significance of this is not clear yet,” Dr. Uhlemann said.
Liver disease severity can also influence gut microbes. Those with Child-Pugh class C disease have the highest numbers in terms of richness and lowest in terms of diversity, Dr. Uhlemann said. “However, at the moment when we are looking at the differential abundance of the taxa, we don’t see quite as clear a pattern, although we noticed in the high group a higher abundance of Bacteroidiaceae,” she said.
Hepatitis B and C patients also presented divergent microbiota profiles. Hepatitis B virus patients “in general are always relatively healthy, and we actually see that these indices are relatively preserved,” Dr. Uhlemann said. “When we look at hepatitis C, however, we see that these patients are starting off quite low and then have an increase in alpha-diversity measures at around month 6.” A subset of patients with alcoholic liver disease also didn’t reach higher Chao and Shannon levels until 6 months after transplant.
“We also find that adjustment of periodic antibiotics for allergy or history of prior infection is significantly associated with a decrease in alpha diversity several months into the posttransplant course,” said Dr. Uhlemann. This is driven by an increase in the abundance of Enterococcaceae and Enterobacteriaceae. “And when we look at MDR colonization as a predictor of alpha diversity, we see that those who have MDR colonization, irrespective of the species, also have the lower alpha diversity.”
The researchers also started to look at pretransplant alpha diversity as a predictor of transplant outcomes, and while the analysis is still in progress, the Shannon indices were significantly different between patients who died and those who survived a year. “There was a trend for significant differences for posttransplant infection and the length of the hospital stay,” Dr. Uhlemann said. “However, we did not see any association with posttransplant ICU readmission, rejection, or VRE complications.”
She added that future analyses are needed to further evaluate the interaction between the clinical comorbidities in the microbiome and vice versa.
Dr. Uhlemann disclosed links to Merck.
WASHINGTON – During and after liver transplant, the reaction of the intestinal microbiota may be a critical determinant of outcomes; preliminary data from a cohort study may provide some clarification of what modulates gut microbiota post transplantation and shed light on predictive factors.
Anna-Catrin Uhlemann, MD, PhD, of Columbia University Medical Center, New York, noted that several studies in recent years sought to clarify influences on gut microbiota in people receiving liver transplants, but “there are still a number of important gaps in knowledge, including what exactly is the longitudinal evolution of the host transplant microbiome and what is the predictive value of pre- and early posttransplant dysbiosis on outcomes and complications.”
The researchers collected more than 1,000 samples to screen for colonization by the following MDR organisms: carbapenem-resistant Enterobacteriaceae (CRE), Enterobacteriaceae resistant to third-generation cephalosporins (ESBL), and vancomycin-resistant enterococci (VRE). Over the 1-year follow-up period, 19% (P =.031) of patients had CRE colonization associated with subsequent infection, 41% (P = .003) had ESBL colonization, and 46% (P = .021) had VRE colonization, Dr. Uhlemann said at the annual meeting of the American Association for the Study of Liver Diseases. The researchers then selected 484 samples for sequencing of the 16S ribosomal RNA gene to determine the composition of gut microbiota.
The study used two indexes to determine the alpha diversity of microbiota: the Chao index to estimate richness and the Shannon diversity index to determine the abundance of species in different settings. “We observed dynamic temporal evolution of alpha diversity and taxa abundance over the 1-year follow-up period,” Dr. Uhlemann said. “The diagnosis, the Child-Pugh class, and changes in perioperative antibiotics were important predictors of posttransplant alpha diversity.”
The study also found that Enterobacteriaceae and enterococci increased post transplant in general and as MDR organisms, and that a patient’s MDR status was an important modulator of the posttransplant microbiome, as was the lack of protective operational taxonomic units (OTUs).
The researchers evaluated the relative abundance of taxa and beta diversity. For example, pretransplant patients with a Model for End-stage Liver Disease (MELD) score greater than 25 showed enrichment of Enterobacteriaceae as well as different taxa of the Bacteroidiaceae, while those with MELD scores below 25 showed enrichment of Veillonellaceae. “The significance of this is not clear yet,” Dr. Uhlemann said.
Liver disease severity can also influence gut microbes. Those with Child-Pugh class C disease have the highest numbers in terms of richness and lowest in terms of diversity, Dr. Uhlemann said. “However, at the moment when we are looking at the differential abundance of the taxa, we don’t see quite as clear a pattern, although we noticed in the high group a higher abundance of Bacteroidiaceae,” she said.
Hepatitis B and C patients also presented divergent microbiota profiles. Hepatitis B virus patients “in general are always relatively healthy, and we actually see that these indices are relatively preserved,” Dr. Uhlemann said. “When we look at hepatitis C, however, we see that these patients are starting off quite low and then have an increase in alpha-diversity measures at around month 6.” A subset of patients with alcoholic liver disease also didn’t reach higher Chao and Shannon levels until 6 months after transplant.
“We also find that adjustment of periodic antibiotics for allergy or history of prior infection is significantly associated with a decrease in alpha diversity several months into the posttransplant course,” said Dr. Uhlemann. This is driven by an increase in the abundance of Enterococcaceae and Enterobacteriaceae. “And when we look at MDR colonization as a predictor of alpha diversity, we see that those who have MDR colonization, irrespective of the species, also have the lower alpha diversity.”
The researchers also started to look at pretransplant alpha diversity as a predictor of transplant outcomes, and while the analysis is still in progress, the Shannon indices were significantly different between patients who died and those who survived a year. “There was a trend for significant differences for posttransplant infection and the length of the hospital stay,” Dr. Uhlemann said. “However, we did not see any association with posttransplant ICU readmission, rejection, or VRE complications.”
She added that future analyses are needed to further evaluate the interaction between the clinical comorbidities in the microbiome and vice versa.
Dr. Uhlemann disclosed links to Merck.
AT THE LIVER MEETING 2017
Key clinical point: The presence or lack of specific modulators of gut microbiota may influence outcomes of liver transplantation.
Major finding: Over a 1-year follow-up period, 19% of patients had colonization with carbapenem-resistant Enterobacteriaceae, 41% had Enterobacteriaceae resistant to third-generation cephalosporins, and 46% had vancomycin-resistant enterococci associated with subsequent infections.
Data source: A prospective longitudinal cohort study of 323 patients, 125 of whom completed 1 year of follow-up.
Disclosures: Dr. Uhlemann disclosed receiving research funding from Merck.
Nivolumab may extend survival in HCC patients
WASHINGTON – A multinational clinical trial has found that the metastatic cancer agent nivolumab can improve long-term survival and durable tumor responses in patients with advanced hepatocellular carcinoma (HCC) whether or not they’ve had previous treatment with a chemotherapy agent already approved for advanced primary liver cancer, a principal investigator reported at the annual meeting of the American Association for the Study of Liver Diseases.
“Nivolumab has demonstrated clinically meaningful efficacy across etiologies in sorafenib-naive and -experienced patients with extended follow-up,” Bruno Sangro, MD, of the University of Navarra in Pamplona, Spain, said in reporting results of the CheckMate-040 trial. “The median overall survival is 15 and 15.6 months in patients who were sorafenib-experienced in both the dose-escalation and expansion cohorts.”
The dose-escalation cohort received 0.1 to 10 mg/kg of nivolumab (Opdivo) while the dose-expansion group received a steady dose of 3 mg/kg. In all, 262 patients participated in the trial, 80 of whom had never been on sorafenib (Nexavar) therapy. The survival outcome in these subgroups, Dr. Sangro said, “really speaks for the consistency and the robustness of the results.”
Trial participants had inoperable, usually metastatic HCC, with Child-Pugh scores up to and including 7 in the escalation group or up to and including 6 in the expansion group. Most of them were progressing to treatment with one or more prior systemic therapies, including sorafenib. Their aspartate aminotransferase and alanine aminotransferase scores were in the upper limits of normal, and bilirubin was less than or equal to 3 mg/dL. If they had hepatitis B (HBV), their viral load had to be less than 100 IU/mL and they had to be on effective antiviral therapy. Any history of hepatic encephalopathy or clinically significant ascites and an active HBV and hepatitis C (HCV) coinfection were grounds for exclusion.
“Most patients had to discontinue nivolumab because of disease progression,” Dr. Sangro noted, so that only 36 patients, or 14%, were continuing treatment at the time of this analysis. Thirteen patients in the total population that discontinued nivolumab did so because of toxicity, he said.
“Around 20% of patients achieved an objective remission that included complete responses in all subgroups of patients; 15% of progressors and 23% of sorafenib-intolerant patients had an objective response,” Dr. Sangro said. In terms of overall response, about half of all patients in the sorafenib-experienced subgroups had a complete or partial response or stable disease: 51% in the dose-escalation subgroup and 54% in the dose-expansion subgroup.
Although tumor responses were associated with declines in alpha-fetoprotein levels, “it’s unlikely that these biomarkers will be useful either for monitoring or selecting patients for treatment,” he added. “Indeed, baseline alpha-fetoprotein levels were comparable between responders and nonresponders to nivolumab” Dr. Sangro said.
“We also showed there was some impact on HCV viral kinetics in infected individuals,” Dr. Sangro noted. “The overall safety profile for the HCC population is consistent with other tumor types in which nivolumab is approved; these include patients who are infected with hepatitis B or C viruses.”
The study showed that 36% (19/53) of HCV infected patients had a greater than 1 log decrease in viral load. No signs of additional antiviral activity were detected among HBV-infected patients already on effective antiviral treatment: only 5% (3/59) posted a up to 1 log decrease in HB surface antigen levels, and 11% (7/64) of patients had increases in viral load. “These increases occurred in the setting of low-level viremia.” Dr. Sangro said. “They were asymptomatic and [nivolumab] did not result in changes in hepatic parameters or other serious adverse events.”
With regard to adverse events (AEs), 77% of all patients had some treatment-related AEs, ranging from fatigue to rash to dry mouth to increased lab levels, but only 20% were grade 3 or 4, and 88% of those resolved in an average of 8 weeks, Dr. Sangro said.
More research into nivolumab for HCC is needed, Dr. Sangro said. “Ongoing and future studies in patients with advanced tumors will evaluate nivolumab in the first-line setting or in combination with other agents,” he said.
Dr. Sangro disclosed relationships with Bayer Schering Pharma, Onxeo, Astra Zeneca, and Bristol-Myers Squibb. Bristol-Myers Squibb funded the trial, and Chrysalis Medical Communications assisted in reporting the study results.
WASHINGTON – A multinational clinical trial has found that the metastatic cancer agent nivolumab can improve long-term survival and durable tumor responses in patients with advanced hepatocellular carcinoma (HCC) whether or not they’ve had previous treatment with a chemotherapy agent already approved for advanced primary liver cancer, a principal investigator reported at the annual meeting of the American Association for the Study of Liver Diseases.
“Nivolumab has demonstrated clinically meaningful efficacy across etiologies in sorafenib-naive and -experienced patients with extended follow-up,” Bruno Sangro, MD, of the University of Navarra in Pamplona, Spain, said in reporting results of the CheckMate-040 trial. “The median overall survival is 15 and 15.6 months in patients who were sorafenib-experienced in both the dose-escalation and expansion cohorts.”
The dose-escalation cohort received 0.1 to 10 mg/kg of nivolumab (Opdivo) while the dose-expansion group received a steady dose of 3 mg/kg. In all, 262 patients participated in the trial, 80 of whom had never been on sorafenib (Nexavar) therapy. The survival outcome in these subgroups, Dr. Sangro said, “really speaks for the consistency and the robustness of the results.”
Trial participants had inoperable, usually metastatic HCC, with Child-Pugh scores up to and including 7 in the escalation group or up to and including 6 in the expansion group. Most of them were progressing to treatment with one or more prior systemic therapies, including sorafenib. Their aspartate aminotransferase and alanine aminotransferase scores were in the upper limits of normal, and bilirubin was less than or equal to 3 mg/dL. If they had hepatitis B (HBV), their viral load had to be less than 100 IU/mL and they had to be on effective antiviral therapy. Any history of hepatic encephalopathy or clinically significant ascites and an active HBV and hepatitis C (HCV) coinfection were grounds for exclusion.
“Most patients had to discontinue nivolumab because of disease progression,” Dr. Sangro noted, so that only 36 patients, or 14%, were continuing treatment at the time of this analysis. Thirteen patients in the total population that discontinued nivolumab did so because of toxicity, he said.
“Around 20% of patients achieved an objective remission that included complete responses in all subgroups of patients; 15% of progressors and 23% of sorafenib-intolerant patients had an objective response,” Dr. Sangro said. In terms of overall response, about half of all patients in the sorafenib-experienced subgroups had a complete or partial response or stable disease: 51% in the dose-escalation subgroup and 54% in the dose-expansion subgroup.
Although tumor responses were associated with declines in alpha-fetoprotein levels, “it’s unlikely that these biomarkers will be useful either for monitoring or selecting patients for treatment,” he added. “Indeed, baseline alpha-fetoprotein levels were comparable between responders and nonresponders to nivolumab” Dr. Sangro said.
“We also showed there was some impact on HCV viral kinetics in infected individuals,” Dr. Sangro noted. “The overall safety profile for the HCC population is consistent with other tumor types in which nivolumab is approved; these include patients who are infected with hepatitis B or C viruses.”
The study showed that 36% (19/53) of HCV infected patients had a greater than 1 log decrease in viral load. No signs of additional antiviral activity were detected among HBV-infected patients already on effective antiviral treatment: only 5% (3/59) posted a up to 1 log decrease in HB surface antigen levels, and 11% (7/64) of patients had increases in viral load. “These increases occurred in the setting of low-level viremia.” Dr. Sangro said. “They were asymptomatic and [nivolumab] did not result in changes in hepatic parameters or other serious adverse events.”
With regard to adverse events (AEs), 77% of all patients had some treatment-related AEs, ranging from fatigue to rash to dry mouth to increased lab levels, but only 20% were grade 3 or 4, and 88% of those resolved in an average of 8 weeks, Dr. Sangro said.
More research into nivolumab for HCC is needed, Dr. Sangro said. “Ongoing and future studies in patients with advanced tumors will evaluate nivolumab in the first-line setting or in combination with other agents,” he said.
Dr. Sangro disclosed relationships with Bayer Schering Pharma, Onxeo, Astra Zeneca, and Bristol-Myers Squibb. Bristol-Myers Squibb funded the trial, and Chrysalis Medical Communications assisted in reporting the study results.
WASHINGTON – A multinational clinical trial has found that the metastatic cancer agent nivolumab can improve long-term survival and durable tumor responses in patients with advanced hepatocellular carcinoma (HCC) whether or not they’ve had previous treatment with a chemotherapy agent already approved for advanced primary liver cancer, a principal investigator reported at the annual meeting of the American Association for the Study of Liver Diseases.
“Nivolumab has demonstrated clinically meaningful efficacy across etiologies in sorafenib-naive and -experienced patients with extended follow-up,” Bruno Sangro, MD, of the University of Navarra in Pamplona, Spain, said in reporting results of the CheckMate-040 trial. “The median overall survival is 15 and 15.6 months in patients who were sorafenib-experienced in both the dose-escalation and expansion cohorts.”
The dose-escalation cohort received 0.1 to 10 mg/kg of nivolumab (Opdivo) while the dose-expansion group received a steady dose of 3 mg/kg. In all, 262 patients participated in the trial, 80 of whom had never been on sorafenib (Nexavar) therapy. The survival outcome in these subgroups, Dr. Sangro said, “really speaks for the consistency and the robustness of the results.”
Trial participants had inoperable, usually metastatic HCC, with Child-Pugh scores up to and including 7 in the escalation group or up to and including 6 in the expansion group. Most of them were progressing to treatment with one or more prior systemic therapies, including sorafenib. Their aspartate aminotransferase and alanine aminotransferase scores were in the upper limits of normal, and bilirubin was less than or equal to 3 mg/dL. If they had hepatitis B (HBV), their viral load had to be less than 100 IU/mL and they had to be on effective antiviral therapy. Any history of hepatic encephalopathy or clinically significant ascites and an active HBV and hepatitis C (HCV) coinfection were grounds for exclusion.
“Most patients had to discontinue nivolumab because of disease progression,” Dr. Sangro noted, so that only 36 patients, or 14%, were continuing treatment at the time of this analysis. Thirteen patients in the total population that discontinued nivolumab did so because of toxicity, he said.
“Around 20% of patients achieved an objective remission that included complete responses in all subgroups of patients; 15% of progressors and 23% of sorafenib-intolerant patients had an objective response,” Dr. Sangro said. In terms of overall response, about half of all patients in the sorafenib-experienced subgroups had a complete or partial response or stable disease: 51% in the dose-escalation subgroup and 54% in the dose-expansion subgroup.
Although tumor responses were associated with declines in alpha-fetoprotein levels, “it’s unlikely that these biomarkers will be useful either for monitoring or selecting patients for treatment,” he added. “Indeed, baseline alpha-fetoprotein levels were comparable between responders and nonresponders to nivolumab” Dr. Sangro said.
“We also showed there was some impact on HCV viral kinetics in infected individuals,” Dr. Sangro noted. “The overall safety profile for the HCC population is consistent with other tumor types in which nivolumab is approved; these include patients who are infected with hepatitis B or C viruses.”
The study showed that 36% (19/53) of HCV infected patients had a greater than 1 log decrease in viral load. No signs of additional antiviral activity were detected among HBV-infected patients already on effective antiviral treatment: only 5% (3/59) posted a up to 1 log decrease in HB surface antigen levels, and 11% (7/64) of patients had increases in viral load. “These increases occurred in the setting of low-level viremia.” Dr. Sangro said. “They were asymptomatic and [nivolumab] did not result in changes in hepatic parameters or other serious adverse events.”
With regard to adverse events (AEs), 77% of all patients had some treatment-related AEs, ranging from fatigue to rash to dry mouth to increased lab levels, but only 20% were grade 3 or 4, and 88% of those resolved in an average of 8 weeks, Dr. Sangro said.
More research into nivolumab for HCC is needed, Dr. Sangro said. “Ongoing and future studies in patients with advanced tumors will evaluate nivolumab in the first-line setting or in combination with other agents,” he said.
Dr. Sangro disclosed relationships with Bayer Schering Pharma, Onxeo, Astra Zeneca, and Bristol-Myers Squibb. Bristol-Myers Squibb funded the trial, and Chrysalis Medical Communications assisted in reporting the study results.
AT THE LIVER MEETING 2017
Key clinical point: Nivolumab demonstrated long-term survival, durable tumor responses, and manageable overall and hepatic safety profiles, regardless of prior sorafenib treatment, in patients with advanced hepatocellular carcinoma.
Major finding: The 18-month overall survival rate was 57% in sorafenib-naive patients and 46% (dose-escalation) and 44% (dose-expansion) in sorafenib-experienced patients.
Data source: CheckMate-040 phase 1/2 dose-escalation and -expansion trial of 262 patients.
Disclosures: Dr. Sangro disclosed relationships with Bayer Schering Pharma, Onxeo, Astra Zeneca, and Bristol-Myers Squibb. Bristol-Myers Squibb funded the trial, and Chrysalis Medical Communications assisted in reporting the study results.
Emerging oral agent reduces ALT in NAFLD
WASHINGTON – Limited treatment options for nonalcoholic steatohepatitis (NASH) in nonalcoholic fatty liver disease mean that NASH is the fastest-growing reason for liver transplants in the United States, but preclinical and, now, phase 2 clinical results have shown that treatment with 24-nor-ursodeoxycholic acid, otherwise known as norUDCA, can improve steatosis and liver stiffness in selected patients, a principal investigator in a European study of the treatment reported at the 2017 annual meeting of the American Association for the Study of Liver Diseases.
“The norUDCA dose of 1,500 mg resulted in significant reduction of ALT [alanine aminotransferase] within 12 weeks,” said Michael Trauner, MD, head of the division of gastroenterology and hepatology at the Medical University of Vienna, a coinventor of the drug. “The results are supported by improvement in liver stiffness and steatosis in the subsets analyzed.”
The 1,500-mg group had an average reduction in ALT of 17.4% whereas those in the 500-mg group only had a 4.2% reduction and placebo actually had an increase of 10.4%. “The reduction of the 500-mg dose was not significant,” Dr. Trauner said. “And this was emphasized in the proportion of patients reaching ALT less than 0.8 x ULN (upper limits of normal) at the end of treatment, with about 17% of patients reaching this endpoint in the higher dose group.” Among patients in the 500-mg group, 15% achieved that level, as did 5% in the placebo group.
The therapy also had an effect on lipid levels, Dr. Trauner noted. “Surprisingly, we saw a slight increase in LDL levels, with the highest in the 1,500-mg dose,” he said. “There were no significant changes in triglycerides and HDL levels, although there were some trends for reduced triglycerides and increased HDL.” Triglycerides decreased 14.6 mg/dL on average and HDL increased 2.8 mg/dL. The slight rise in LDL, 14.6 mg/dL on average, occurred in the first 2 weeks of treatment and continued through the treatment period, but then receded after discontinuation of therapy, said Dr. Trauner. “Please note that HDL cholesterol also increased in time, and the HDL-LDL ratio remained unchanged in these patients,” he added.
During the discussion, Dr. Trauner offered a possible explanation for the change in lipid levels. “One possibility could be that a slight repression of endogenous bile acid biosynthesis and subsequent upregulation of the LDL receptor,” he said, “but the changes are really very mild and subtle.”
He also noted that liver stiffness improved in a higher proportion of patients in the treatment groups than in the placebo group – 25% and 21% of patients in the 1,500- and 500-mg groups vs. 9% under placebo. Hepatic fat fraction values also improved from 21.3% to 16.3% (relative reduction of 23.5%) from baseline to end of treatment in the 1,500-mg group in a subset of patients undergoing more extensive MRI and spectroscopy studies – a degree of reduction that other studies have shown to be predictive of histologic improvement, Dr. Trauner said. Patients in this exploratory study did not have liver biopsies.
Overall, the drug was well tolerated, Dr. Trauner said. “There were slightly higher potentially adverse drug reactions in the 1,500-mg group, mainly due to higher rate of headache, nausea, and rash,” he said. Based on these results, a phase 2b study with histologic endpoints is underway, he added.
Dr. Trauner disclosed relationships with Gilead, Albireo, Takeda, Falk Pharma, Genfit, Intercept, MSD, Novartis, Roche, and Phenex.
WASHINGTON – Limited treatment options for nonalcoholic steatohepatitis (NASH) in nonalcoholic fatty liver disease mean that NASH is the fastest-growing reason for liver transplants in the United States, but preclinical and, now, phase 2 clinical results have shown that treatment with 24-nor-ursodeoxycholic acid, otherwise known as norUDCA, can improve steatosis and liver stiffness in selected patients, a principal investigator in a European study of the treatment reported at the 2017 annual meeting of the American Association for the Study of Liver Diseases.
“The norUDCA dose of 1,500 mg resulted in significant reduction of ALT [alanine aminotransferase] within 12 weeks,” said Michael Trauner, MD, head of the division of gastroenterology and hepatology at the Medical University of Vienna, a coinventor of the drug. “The results are supported by improvement in liver stiffness and steatosis in the subsets analyzed.”
The 1,500-mg group had an average reduction in ALT of 17.4% whereas those in the 500-mg group only had a 4.2% reduction and placebo actually had an increase of 10.4%. “The reduction of the 500-mg dose was not significant,” Dr. Trauner said. “And this was emphasized in the proportion of patients reaching ALT less than 0.8 x ULN (upper limits of normal) at the end of treatment, with about 17% of patients reaching this endpoint in the higher dose group.” Among patients in the 500-mg group, 15% achieved that level, as did 5% in the placebo group.
The therapy also had an effect on lipid levels, Dr. Trauner noted. “Surprisingly, we saw a slight increase in LDL levels, with the highest in the 1,500-mg dose,” he said. “There were no significant changes in triglycerides and HDL levels, although there were some trends for reduced triglycerides and increased HDL.” Triglycerides decreased 14.6 mg/dL on average and HDL increased 2.8 mg/dL. The slight rise in LDL, 14.6 mg/dL on average, occurred in the first 2 weeks of treatment and continued through the treatment period, but then receded after discontinuation of therapy, said Dr. Trauner. “Please note that HDL cholesterol also increased in time, and the HDL-LDL ratio remained unchanged in these patients,” he added.
During the discussion, Dr. Trauner offered a possible explanation for the change in lipid levels. “One possibility could be that a slight repression of endogenous bile acid biosynthesis and subsequent upregulation of the LDL receptor,” he said, “but the changes are really very mild and subtle.”
He also noted that liver stiffness improved in a higher proportion of patients in the treatment groups than in the placebo group – 25% and 21% of patients in the 1,500- and 500-mg groups vs. 9% under placebo. Hepatic fat fraction values also improved from 21.3% to 16.3% (relative reduction of 23.5%) from baseline to end of treatment in the 1,500-mg group in a subset of patients undergoing more extensive MRI and spectroscopy studies – a degree of reduction that other studies have shown to be predictive of histologic improvement, Dr. Trauner said. Patients in this exploratory study did not have liver biopsies.
Overall, the drug was well tolerated, Dr. Trauner said. “There were slightly higher potentially adverse drug reactions in the 1,500-mg group, mainly due to higher rate of headache, nausea, and rash,” he said. Based on these results, a phase 2b study with histologic endpoints is underway, he added.
Dr. Trauner disclosed relationships with Gilead, Albireo, Takeda, Falk Pharma, Genfit, Intercept, MSD, Novartis, Roche, and Phenex.
WASHINGTON – Limited treatment options for nonalcoholic steatohepatitis (NASH) in nonalcoholic fatty liver disease mean that NASH is the fastest-growing reason for liver transplants in the United States, but preclinical and, now, phase 2 clinical results have shown that treatment with 24-nor-ursodeoxycholic acid, otherwise known as norUDCA, can improve steatosis and liver stiffness in selected patients, a principal investigator in a European study of the treatment reported at the 2017 annual meeting of the American Association for the Study of Liver Diseases.
“The norUDCA dose of 1,500 mg resulted in significant reduction of ALT [alanine aminotransferase] within 12 weeks,” said Michael Trauner, MD, head of the division of gastroenterology and hepatology at the Medical University of Vienna, a coinventor of the drug. “The results are supported by improvement in liver stiffness and steatosis in the subsets analyzed.”
The 1,500-mg group had an average reduction in ALT of 17.4% whereas those in the 500-mg group only had a 4.2% reduction and placebo actually had an increase of 10.4%. “The reduction of the 500-mg dose was not significant,” Dr. Trauner said. “And this was emphasized in the proportion of patients reaching ALT less than 0.8 x ULN (upper limits of normal) at the end of treatment, with about 17% of patients reaching this endpoint in the higher dose group.” Among patients in the 500-mg group, 15% achieved that level, as did 5% in the placebo group.
The therapy also had an effect on lipid levels, Dr. Trauner noted. “Surprisingly, we saw a slight increase in LDL levels, with the highest in the 1,500-mg dose,” he said. “There were no significant changes in triglycerides and HDL levels, although there were some trends for reduced triglycerides and increased HDL.” Triglycerides decreased 14.6 mg/dL on average and HDL increased 2.8 mg/dL. The slight rise in LDL, 14.6 mg/dL on average, occurred in the first 2 weeks of treatment and continued through the treatment period, but then receded after discontinuation of therapy, said Dr. Trauner. “Please note that HDL cholesterol also increased in time, and the HDL-LDL ratio remained unchanged in these patients,” he added.
During the discussion, Dr. Trauner offered a possible explanation for the change in lipid levels. “One possibility could be that a slight repression of endogenous bile acid biosynthesis and subsequent upregulation of the LDL receptor,” he said, “but the changes are really very mild and subtle.”
He also noted that liver stiffness improved in a higher proportion of patients in the treatment groups than in the placebo group – 25% and 21% of patients in the 1,500- and 500-mg groups vs. 9% under placebo. Hepatic fat fraction values also improved from 21.3% to 16.3% (relative reduction of 23.5%) from baseline to end of treatment in the 1,500-mg group in a subset of patients undergoing more extensive MRI and spectroscopy studies – a degree of reduction that other studies have shown to be predictive of histologic improvement, Dr. Trauner said. Patients in this exploratory study did not have liver biopsies.
Overall, the drug was well tolerated, Dr. Trauner said. “There were slightly higher potentially adverse drug reactions in the 1,500-mg group, mainly due to higher rate of headache, nausea, and rash,” he said. Based on these results, a phase 2b study with histologic endpoints is underway, he added.
Dr. Trauner disclosed relationships with Gilead, Albireo, Takeda, Falk Pharma, Genfit, Intercept, MSD, Novartis, Roche, and Phenex.
AT THE LIVER MEETING 2017
Key clinical point: Treatment with oral 24-nor-ursodeoxycholic acid (norUDCA) resulted in a significant reduction of alanine aminotransferase (ALT) levels in nonalcoholic fatty liver disease.
Major finding: Mean value of hepatic fat fraction decreased 23.5% in a subset of patients treated with 1,500 mg norUDCA.
Data source: Double-blind, randomized, placebo-controlled, proof-of-concept phase 2 dose-finding study of 198 patients receiving treatment over 12 weeks.
Disclosures: Dr. Trauner disclosed relationships with Gilead, Albireo, Takeda, Falk Pharma, Genfit, Intercept, MSD, Novartis, Roche, and Phenex, and is listed as a coinventor on patents filed for the medical use of 24-nor-ursodeoxycholic acid.
Novel metabolite may be key in NAFLD
WASHINGTON – A newly identified metabolite of the gut microbiome may be a potentially useful biomarker in determining the severity of nonalcoholic fatty liver disease (NAFLD) and may provide a new treatment target, according to results of an analysis of serum metabolites isolated from 100 pairs of twins presented at the annual meeting of the American Association for the Study of Liver Diseases.
The researchers at the NAFLD Research Center at the University of California at San Diego isolated a metabolite derived from the gut microbiome, known as 3-(4-hydroxyphenyl)lactate, from 713 serum metabolites they analyzed, 440 of which were identified as heritable, said Cyrielle Caussy, MD, PhD. The researchers further winnowed that pool down to 94 associated with fibrosis alone and 170 associated with hepatic steatosis alone, 56 of which overlapped to have a shared gene effect with both hepatic steatosis and fibrosis, six of which derived from the gut microbiome.
Of the four heritable serum metabolites the researchers found to be significantly associated with NAFLD after adjustment for age, sex and Hispanic ethnicity, 3-(4-hydroxyphenyl)lactate had the highest odds ratio (95% confidence interval): 4.29 (1.87-9.81, P = .0006) vs. phenyllactate (OR 2.12, 1.09-4.10, P = .0258), palmitic acid (2.58, 1.31-5.17, P = .0065) and gamma-glutamylisoleucine (2.98, 1.36-6.51, P = .0062).
Dr. Caussy noted that previous studies have found a strong correlation between bacterial species in the gut and advanced fibrosis in NAFLD (Cell Metab. 2017;25:1054-62). This latest research takes those findings to the next level, Dr. Caussy said. “This metabolite could be a useful biomarker of the severity of NAFLD and may be a target for future treatment of NAFLD and could be used to monitor a treatment response,” she added.
The goal of the study was to determine if any serum metabolites have a shared genetic effect with hepatic steatosis and fibrosis, Dr. Caussy said. “The heritability of serum metabolites associated with NAFLD and their shared gene effect with hepatic steatosis and fibrosis have not been assessed yet,” she noted. The researchers isolated the serum metabolites from a cohort of 100 pairs of twins and 56 other relatives in the Southern California Twins Register, and validated the data in a cohort of 156 patients who had biopsy-proven NAFLD.
Dr. Caussy reported having no financial disclosures.
WASHINGTON – A newly identified metabolite of the gut microbiome may be a potentially useful biomarker in determining the severity of nonalcoholic fatty liver disease (NAFLD) and may provide a new treatment target, according to results of an analysis of serum metabolites isolated from 100 pairs of twins presented at the annual meeting of the American Association for the Study of Liver Diseases.
The researchers at the NAFLD Research Center at the University of California at San Diego isolated a metabolite derived from the gut microbiome, known as 3-(4-hydroxyphenyl)lactate, from 713 serum metabolites they analyzed, 440 of which were identified as heritable, said Cyrielle Caussy, MD, PhD. The researchers further winnowed that pool down to 94 associated with fibrosis alone and 170 associated with hepatic steatosis alone, 56 of which overlapped to have a shared gene effect with both hepatic steatosis and fibrosis, six of which derived from the gut microbiome.
Of the four heritable serum metabolites the researchers found to be significantly associated with NAFLD after adjustment for age, sex and Hispanic ethnicity, 3-(4-hydroxyphenyl)lactate had the highest odds ratio (95% confidence interval): 4.29 (1.87-9.81, P = .0006) vs. phenyllactate (OR 2.12, 1.09-4.10, P = .0258), palmitic acid (2.58, 1.31-5.17, P = .0065) and gamma-glutamylisoleucine (2.98, 1.36-6.51, P = .0062).
Dr. Caussy noted that previous studies have found a strong correlation between bacterial species in the gut and advanced fibrosis in NAFLD (Cell Metab. 2017;25:1054-62). This latest research takes those findings to the next level, Dr. Caussy said. “This metabolite could be a useful biomarker of the severity of NAFLD and may be a target for future treatment of NAFLD and could be used to monitor a treatment response,” she added.
The goal of the study was to determine if any serum metabolites have a shared genetic effect with hepatic steatosis and fibrosis, Dr. Caussy said. “The heritability of serum metabolites associated with NAFLD and their shared gene effect with hepatic steatosis and fibrosis have not been assessed yet,” she noted. The researchers isolated the serum metabolites from a cohort of 100 pairs of twins and 56 other relatives in the Southern California Twins Register, and validated the data in a cohort of 156 patients who had biopsy-proven NAFLD.
Dr. Caussy reported having no financial disclosures.
WASHINGTON – A newly identified metabolite of the gut microbiome may be a potentially useful biomarker in determining the severity of nonalcoholic fatty liver disease (NAFLD) and may provide a new treatment target, according to results of an analysis of serum metabolites isolated from 100 pairs of twins presented at the annual meeting of the American Association for the Study of Liver Diseases.
The researchers at the NAFLD Research Center at the University of California at San Diego isolated a metabolite derived from the gut microbiome, known as 3-(4-hydroxyphenyl)lactate, from 713 serum metabolites they analyzed, 440 of which were identified as heritable, said Cyrielle Caussy, MD, PhD. The researchers further winnowed that pool down to 94 associated with fibrosis alone and 170 associated with hepatic steatosis alone, 56 of which overlapped to have a shared gene effect with both hepatic steatosis and fibrosis, six of which derived from the gut microbiome.
Of the four heritable serum metabolites the researchers found to be significantly associated with NAFLD after adjustment for age, sex and Hispanic ethnicity, 3-(4-hydroxyphenyl)lactate had the highest odds ratio (95% confidence interval): 4.29 (1.87-9.81, P = .0006) vs. phenyllactate (OR 2.12, 1.09-4.10, P = .0258), palmitic acid (2.58, 1.31-5.17, P = .0065) and gamma-glutamylisoleucine (2.98, 1.36-6.51, P = .0062).
Dr. Caussy noted that previous studies have found a strong correlation between bacterial species in the gut and advanced fibrosis in NAFLD (Cell Metab. 2017;25:1054-62). This latest research takes those findings to the next level, Dr. Caussy said. “This metabolite could be a useful biomarker of the severity of NAFLD and may be a target for future treatment of NAFLD and could be used to monitor a treatment response,” she added.
The goal of the study was to determine if any serum metabolites have a shared genetic effect with hepatic steatosis and fibrosis, Dr. Caussy said. “The heritability of serum metabolites associated with NAFLD and their shared gene effect with hepatic steatosis and fibrosis have not been assessed yet,” she noted. The researchers isolated the serum metabolites from a cohort of 100 pairs of twins and 56 other relatives in the Southern California Twins Register, and validated the data in a cohort of 156 patients who had biopsy-proven NAFLD.
Dr. Caussy reported having no financial disclosures.
AT THE LIVER MEETING 2017
Key clinical point: Researchers have identified a novel serum metabolite that may be the key to linking the role of the gut microbiome with the presence of nonalcoholic fatty liver disease (NAFLD)–related fibrosis.
Major finding: An analysis of 713 serum metabolites identified a novel, gut microbiome–derived serum metabolite known as 3-(4-hydroxyphenyl)lactate that had an odds ratio of 4.29 for NAFLD.
Data source: Cross-sectional analysis of a prospective cohort of 156 subjects in the Southern California Twin Study Cohort.
Disclosures: Dr. Caussy reported having no financial disclosures.
Hispanics bear brunt of NAFLD disease burden
WASHINGTON — Significant racial and ethnic disparities exist in nonalcoholic fatty liver disease prevalence and severity in the United States, with Hispanics at highest risk and blacks at the lowest, but the risk of death from NAFLD is highest in whites, according to a meta-analysis of 34 studies presented at the annual meeting of the American Association for the Study of Liver Diseases.
The findings are based on a meta-analysis of studies in the MEDLINE, EMBASE, and Cochrane databases from inception through August 2016 that included 368,569 unique patients that characterized disparities in NAFLD prevalence, severity, or prognosis, Dr. Rich said.
When the researchers drilled down into the data, they found the disparities dissipated somewhat. “When we looked at the severity of NAFLD we looked at two things: whether there was NASH (nonalcoholic steatohepatitis) present, or if there was the presence of advanced fibrosis,” Dr. Rich said. “We found there was no significant difference in the risk of NASH in Hispanic patients, compared to white patients; however, the proportion of Hispanic NAFLD patients that had NASH was 45.4%, compared to 32.2% in whites.” The pooled relative risk was 1.09.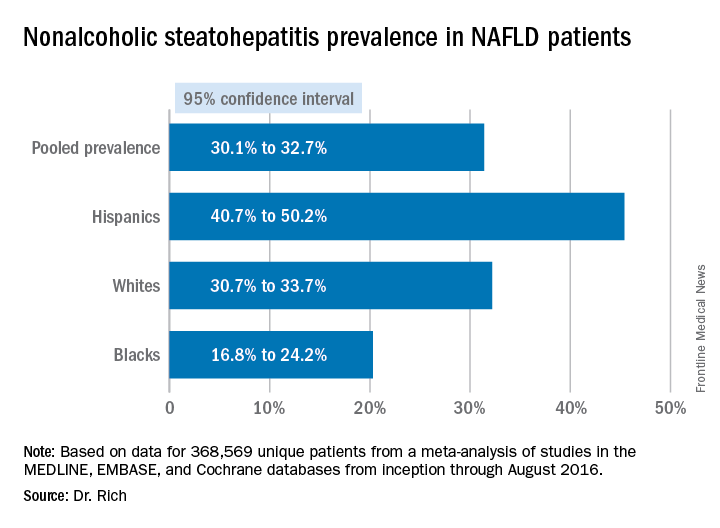
The meta-analysis tended to attenuate the disparities found in disease severity, compared with the disparities the researchers found in prevalence, Dr. Rich said. “Data are limited and discordant on racial and ethnic differences in NAFLD prognosis and outcomes,” she said. “In the current literature the studies have notable limitations, highlighting the need for future high-quality data in this area, and further studies are needed to determine the need and pathways to reduce NAFLD disparities in the future,” she said.
Dr. Rich had no financial relationships to disclose.
WASHINGTON — Significant racial and ethnic disparities exist in nonalcoholic fatty liver disease prevalence and severity in the United States, with Hispanics at highest risk and blacks at the lowest, but the risk of death from NAFLD is highest in whites, according to a meta-analysis of 34 studies presented at the annual meeting of the American Association for the Study of Liver Diseases.
The findings are based on a meta-analysis of studies in the MEDLINE, EMBASE, and Cochrane databases from inception through August 2016 that included 368,569 unique patients that characterized disparities in NAFLD prevalence, severity, or prognosis, Dr. Rich said.
When the researchers drilled down into the data, they found the disparities dissipated somewhat. “When we looked at the severity of NAFLD we looked at two things: whether there was NASH (nonalcoholic steatohepatitis) present, or if there was the presence of advanced fibrosis,” Dr. Rich said. “We found there was no significant difference in the risk of NASH in Hispanic patients, compared to white patients; however, the proportion of Hispanic NAFLD patients that had NASH was 45.4%, compared to 32.2% in whites.” The pooled relative risk was 1.09.
The meta-analysis tended to attenuate the disparities found in disease severity, compared with the disparities the researchers found in prevalence, Dr. Rich said. “Data are limited and discordant on racial and ethnic differences in NAFLD prognosis and outcomes,” she said. “In the current literature the studies have notable limitations, highlighting the need for future high-quality data in this area, and further studies are needed to determine the need and pathways to reduce NAFLD disparities in the future,” she said.
Dr. Rich had no financial relationships to disclose.
WASHINGTON — Significant racial and ethnic disparities exist in nonalcoholic fatty liver disease prevalence and severity in the United States, with Hispanics at highest risk and blacks at the lowest, but the risk of death from NAFLD is highest in whites, according to a meta-analysis of 34 studies presented at the annual meeting of the American Association for the Study of Liver Diseases.
The findings are based on a meta-analysis of studies in the MEDLINE, EMBASE, and Cochrane databases from inception through August 2016 that included 368,569 unique patients that characterized disparities in NAFLD prevalence, severity, or prognosis, Dr. Rich said.
When the researchers drilled down into the data, they found the disparities dissipated somewhat. “When we looked at the severity of NAFLD we looked at two things: whether there was NASH (nonalcoholic steatohepatitis) present, or if there was the presence of advanced fibrosis,” Dr. Rich said. “We found there was no significant difference in the risk of NASH in Hispanic patients, compared to white patients; however, the proportion of Hispanic NAFLD patients that had NASH was 45.4%, compared to 32.2% in whites.” The pooled relative risk was 1.09.
The meta-analysis tended to attenuate the disparities found in disease severity, compared with the disparities the researchers found in prevalence, Dr. Rich said. “Data are limited and discordant on racial and ethnic differences in NAFLD prognosis and outcomes,” she said. “In the current literature the studies have notable limitations, highlighting the need for future high-quality data in this area, and further studies are needed to determine the need and pathways to reduce NAFLD disparities in the future,” she said.
Dr. Rich had no financial relationships to disclose.
AT THE LIVER MEETING 2017
Key clinical point: Hispanics have a much higher incidence of fatty liver disease than other ethnic groups.
Major finding: NAFLD prevalence is highest in Hispanics and lower in blacks, compared with whites, although differences between groups are smaller in high-risk cohorts (range, 47.6%-55.5%) than in population-based cohorts (range, 13.0%-22.9%).
Data source: Meta-analysis of 34 studies in the MEDLINE, EMBASE, and Cochrane databases from inception through August 2016 that involved 368,569 patients.
Disclosures: Dr. Rich had no financial relationships to disclose.
VIDEO: Metabolic regulator FGF21 improves fibrosis in NASH patients
WASHINGTON – Fibroblast growth factor 21 (FGF21), a nonmitogenic hormone, improved fibrosis, liver injury, and steatosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the American Association for the Study of Liver Disease’s annual meeting.
There is no drug therapy currently available for NASH, the most advanced form of nonalcoholic fatty liver disease (NAFLD), creating a strong need for effective treatments, according to Arun Sanyal, MD, of the Virginia Commonwealth University, Richmond, said in a video interview.
This treatment “relative to placebo was associated with improvements in biomarkers of fibrosis, metabolic parameters, and markers of hepatic injury,” said Dr. Sanyal. “These results suggest BMS-986036 [FGF21] has beneficial effects on steatosis, liver injury, and fibrosis in NASH.”
Investigators conducted a phase 2 multicenter, double-blind, placebo-controlled study of 74 NASH patients to test BMS-986036, a pegylated version of FGF21.
Patients were an average of 51 years old, most were women (64%), who were predominantly white (96%), with a mean hepatic fat fraction of 19%.
Patients received either a 10-mg treatment daily, a 20-mg treatment weekly, or placebo, over the course of 16 weeks, with patients distributed equally among the three arms.
Overall hepatic fat fraction among the daily and weekly treatment groups reduced by 6.8% and 5.2%, respectively, compared with the placebo group, which reduced by 1.3% (P less than .001).
Patients in the treatment arms also saw improvement in average adiponectin levels, growing 15.3% in the daily arm and 15.7% in the weekly arm. Meanwhile, adiponectin levels dropped by an average of 3.5% in the placebo group.
In investigating serum Pro-C3 levels, which are associated with fibrosis, patients in the daily and weekly treatment group saw an average drop of 29% and 19%, respectively, as opposed to an increase of 2% in the placebo group (P less than .0001).
Patients in the treatment groups saw no serious adverse effects, and no patients died during the study.
Dr. Sanyal received funding for this study from Bristol-Myers Squibb and reported receiving financial compensation from Pfizer, Nimbus, Novartis, AstraZeneca, and other similar companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – Fibroblast growth factor 21 (FGF21), a nonmitogenic hormone, improved fibrosis, liver injury, and steatosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the American Association for the Study of Liver Disease’s annual meeting.
There is no drug therapy currently available for NASH, the most advanced form of nonalcoholic fatty liver disease (NAFLD), creating a strong need for effective treatments, according to Arun Sanyal, MD, of the Virginia Commonwealth University, Richmond, said in a video interview.
This treatment “relative to placebo was associated with improvements in biomarkers of fibrosis, metabolic parameters, and markers of hepatic injury,” said Dr. Sanyal. “These results suggest BMS-986036 [FGF21] has beneficial effects on steatosis, liver injury, and fibrosis in NASH.”
Investigators conducted a phase 2 multicenter, double-blind, placebo-controlled study of 74 NASH patients to test BMS-986036, a pegylated version of FGF21.
Patients were an average of 51 years old, most were women (64%), who were predominantly white (96%), with a mean hepatic fat fraction of 19%.
Patients received either a 10-mg treatment daily, a 20-mg treatment weekly, or placebo, over the course of 16 weeks, with patients distributed equally among the three arms.
Overall hepatic fat fraction among the daily and weekly treatment groups reduced by 6.8% and 5.2%, respectively, compared with the placebo group, which reduced by 1.3% (P less than .001).
Patients in the treatment arms also saw improvement in average adiponectin levels, growing 15.3% in the daily arm and 15.7% in the weekly arm. Meanwhile, adiponectin levels dropped by an average of 3.5% in the placebo group.
In investigating serum Pro-C3 levels, which are associated with fibrosis, patients in the daily and weekly treatment group saw an average drop of 29% and 19%, respectively, as opposed to an increase of 2% in the placebo group (P less than .0001).
Patients in the treatment groups saw no serious adverse effects, and no patients died during the study.
Dr. Sanyal received funding for this study from Bristol-Myers Squibb and reported receiving financial compensation from Pfizer, Nimbus, Novartis, AstraZeneca, and other similar companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – Fibroblast growth factor 21 (FGF21), a nonmitogenic hormone, improved fibrosis, liver injury, and steatosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the American Association for the Study of Liver Disease’s annual meeting.
There is no drug therapy currently available for NASH, the most advanced form of nonalcoholic fatty liver disease (NAFLD), creating a strong need for effective treatments, according to Arun Sanyal, MD, of the Virginia Commonwealth University, Richmond, said in a video interview.
This treatment “relative to placebo was associated with improvements in biomarkers of fibrosis, metabolic parameters, and markers of hepatic injury,” said Dr. Sanyal. “These results suggest BMS-986036 [FGF21] has beneficial effects on steatosis, liver injury, and fibrosis in NASH.”
Investigators conducted a phase 2 multicenter, double-blind, placebo-controlled study of 74 NASH patients to test BMS-986036, a pegylated version of FGF21.
Patients were an average of 51 years old, most were women (64%), who were predominantly white (96%), with a mean hepatic fat fraction of 19%.
Patients received either a 10-mg treatment daily, a 20-mg treatment weekly, or placebo, over the course of 16 weeks, with patients distributed equally among the three arms.
Overall hepatic fat fraction among the daily and weekly treatment groups reduced by 6.8% and 5.2%, respectively, compared with the placebo group, which reduced by 1.3% (P less than .001).
Patients in the treatment arms also saw improvement in average adiponectin levels, growing 15.3% in the daily arm and 15.7% in the weekly arm. Meanwhile, adiponectin levels dropped by an average of 3.5% in the placebo group.
In investigating serum Pro-C3 levels, which are associated with fibrosis, patients in the daily and weekly treatment group saw an average drop of 29% and 19%, respectively, as opposed to an increase of 2% in the placebo group (P less than .0001).
Patients in the treatment groups saw no serious adverse effects, and no patients died during the study.
Dr. Sanyal received funding for this study from Bristol-Myers Squibb and reported receiving financial compensation from Pfizer, Nimbus, Novartis, AstraZeneca, and other similar companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
AT THE LIVER MEETING 2017
Vascular risks heightened in fibrotic NAFLD
WASHINGTON – The true impact of advanced fibrosis and cirrhosis advancing to serious hepatic and nonhepatic complications in nonalcoholic fatty liver disease remains somewhat a mystery, but a multinational prospective cohort study has found that patients with bridging fibrosis are at risk of nonhepatic malignancies and vascular problems while patients with cirrhosis are prone to predominantly liver-related complications that result in higher rates of death and liver transplant, according to a report presented at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD patients with biopsy-proven cirrhosis have a higher mortality and liver-related complications than those with bridging fibrosis, whereas vascular events and nonhepatic malignancies are the commonest complications in those with bridging fibrosis,” said Eduardo Vilar-Gomez, MD, PhD, of Indiana University, Indianapolis. “Among compensated cirrhotic patients, those with Child-Pugh (CP) A6 are at highest risk of liver-related outcomes and mortality.”
Dr. Vilar-Gomez reported on a multicenter, international prospective cohort study of 458 NAFLD patients, 159 with biopsy-proven bridging fibrosis and 299 with compensated cirrhosis (222 with CP A5 and 77 with CP A6) who were followed at six academic centers in four different countries between April 1995 and December 2016.
During a mean follow-up of 5.7 years, 41 patients died, 43 had liver transplants, 90 had initial hepatic decompensation events, 42 were diagnosed with hepatocellular carcinoma (HCC), 14 had major vascular events, and 30 were diagnosed with nonhepatic cancers. The overall death or transplant rates were 3% (four) in the bridging fibrosis group, 14% (32) in the cirrhosis and CP A5 group, and 62% (48) in the cirrhosis and CP A6 group.
Liver-related death rates were also highest among patients with cirrhosis, Dr. Vilar-Gomez said. While the four deaths in the fibrosis patients were evenly split between liver- and non–liver-related causes, 79% of deaths (15) in the cirrhosis and CP A5 patients were liver related, as were all 18 deaths in the cirrhosis and CP A6 group.
“Cirrhotic patients displayed the highest rates of hepatic outcomes overall as compared to those with bridging fibrosis,” Dr. Vilar-Gomez said. Rates of hepatic decompensation were 3% (5) for bridging fibrosis, 18% (40) for cirrhosis and CP A5, and 58% (48) for cirrhosis and CP A6. Likewise, HCC rates were also highest in cirrhotic patients: 9% (21) and 25% (19) for those with CP A5 and CP A6 disease, respectively, vs. 1% (2) for those with bridging fibrosis.
But nonhepatic outcomes were highest among the bridging fibrosis patients, Dr. Vilar-Gomez noted. “These include higher rates of major vascular events – 5% (eight) for those with bridging fibrosis vs. 2% (five) and 1% (one) for those with cirrhosis and CP A5 or CP A6 disease, respectively,” he said. “Rates of nonhepatic malignancies were comparable: 8% (13) for those with bridging fibrosis, 5% (10) for those with cirrhosis and CP5 disease and 9% (7) for those with cirrhosis and CP6 disease.
“Cirrhosis increased the risk of death/transplant, decompensation, and hepatocellular carcinoma by 4.3-, 4.5-, and 3.9-fold, respectively,” Dr. Vilar-Gomez said, “but was associated with a lower risk of vascular events.”
He noted that the study identified a number of risk factors associated with overall mortality and severity of liver disease in cirrhosis compared with bridging fibrosis. Cirrhosis patients tended to be 2-3 years older, had lower body mass index and blood pressure, had higher rates of type 2 diabetes, had lower lipid levels, and had worse liver and renal function.
“Type 2 diabetes and steatosis severity on liver histology, among other factors, were independent predictors for liver-related outcomes,” Dr. Vilar-Gomez said. The findings have implications in assessing patients with advanced NAFLD and for the design and interpretation of clinical trials, he added.
Dr. Vilar-Gomez had no financial relationships to disclose.
WASHINGTON – The true impact of advanced fibrosis and cirrhosis advancing to serious hepatic and nonhepatic complications in nonalcoholic fatty liver disease remains somewhat a mystery, but a multinational prospective cohort study has found that patients with bridging fibrosis are at risk of nonhepatic malignancies and vascular problems while patients with cirrhosis are prone to predominantly liver-related complications that result in higher rates of death and liver transplant, according to a report presented at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD patients with biopsy-proven cirrhosis have a higher mortality and liver-related complications than those with bridging fibrosis, whereas vascular events and nonhepatic malignancies are the commonest complications in those with bridging fibrosis,” said Eduardo Vilar-Gomez, MD, PhD, of Indiana University, Indianapolis. “Among compensated cirrhotic patients, those with Child-Pugh (CP) A6 are at highest risk of liver-related outcomes and mortality.”
Dr. Vilar-Gomez reported on a multicenter, international prospective cohort study of 458 NAFLD patients, 159 with biopsy-proven bridging fibrosis and 299 with compensated cirrhosis (222 with CP A5 and 77 with CP A6) who were followed at six academic centers in four different countries between April 1995 and December 2016.
During a mean follow-up of 5.7 years, 41 patients died, 43 had liver transplants, 90 had initial hepatic decompensation events, 42 were diagnosed with hepatocellular carcinoma (HCC), 14 had major vascular events, and 30 were diagnosed with nonhepatic cancers. The overall death or transplant rates were 3% (four) in the bridging fibrosis group, 14% (32) in the cirrhosis and CP A5 group, and 62% (48) in the cirrhosis and CP A6 group.
Liver-related death rates were also highest among patients with cirrhosis, Dr. Vilar-Gomez said. While the four deaths in the fibrosis patients were evenly split between liver- and non–liver-related causes, 79% of deaths (15) in the cirrhosis and CP A5 patients were liver related, as were all 18 deaths in the cirrhosis and CP A6 group.
“Cirrhotic patients displayed the highest rates of hepatic outcomes overall as compared to those with bridging fibrosis,” Dr. Vilar-Gomez said. Rates of hepatic decompensation were 3% (5) for bridging fibrosis, 18% (40) for cirrhosis and CP A5, and 58% (48) for cirrhosis and CP A6. Likewise, HCC rates were also highest in cirrhotic patients: 9% (21) and 25% (19) for those with CP A5 and CP A6 disease, respectively, vs. 1% (2) for those with bridging fibrosis.
But nonhepatic outcomes were highest among the bridging fibrosis patients, Dr. Vilar-Gomez noted. “These include higher rates of major vascular events – 5% (eight) for those with bridging fibrosis vs. 2% (five) and 1% (one) for those with cirrhosis and CP A5 or CP A6 disease, respectively,” he said. “Rates of nonhepatic malignancies were comparable: 8% (13) for those with bridging fibrosis, 5% (10) for those with cirrhosis and CP5 disease and 9% (7) for those with cirrhosis and CP6 disease.
“Cirrhosis increased the risk of death/transplant, decompensation, and hepatocellular carcinoma by 4.3-, 4.5-, and 3.9-fold, respectively,” Dr. Vilar-Gomez said, “but was associated with a lower risk of vascular events.”
He noted that the study identified a number of risk factors associated with overall mortality and severity of liver disease in cirrhosis compared with bridging fibrosis. Cirrhosis patients tended to be 2-3 years older, had lower body mass index and blood pressure, had higher rates of type 2 diabetes, had lower lipid levels, and had worse liver and renal function.
“Type 2 diabetes and steatosis severity on liver histology, among other factors, were independent predictors for liver-related outcomes,” Dr. Vilar-Gomez said. The findings have implications in assessing patients with advanced NAFLD and for the design and interpretation of clinical trials, he added.
Dr. Vilar-Gomez had no financial relationships to disclose.
WASHINGTON – The true impact of advanced fibrosis and cirrhosis advancing to serious hepatic and nonhepatic complications in nonalcoholic fatty liver disease remains somewhat a mystery, but a multinational prospective cohort study has found that patients with bridging fibrosis are at risk of nonhepatic malignancies and vascular problems while patients with cirrhosis are prone to predominantly liver-related complications that result in higher rates of death and liver transplant, according to a report presented at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD patients with biopsy-proven cirrhosis have a higher mortality and liver-related complications than those with bridging fibrosis, whereas vascular events and nonhepatic malignancies are the commonest complications in those with bridging fibrosis,” said Eduardo Vilar-Gomez, MD, PhD, of Indiana University, Indianapolis. “Among compensated cirrhotic patients, those with Child-Pugh (CP) A6 are at highest risk of liver-related outcomes and mortality.”
Dr. Vilar-Gomez reported on a multicenter, international prospective cohort study of 458 NAFLD patients, 159 with biopsy-proven bridging fibrosis and 299 with compensated cirrhosis (222 with CP A5 and 77 with CP A6) who were followed at six academic centers in four different countries between April 1995 and December 2016.
During a mean follow-up of 5.7 years, 41 patients died, 43 had liver transplants, 90 had initial hepatic decompensation events, 42 were diagnosed with hepatocellular carcinoma (HCC), 14 had major vascular events, and 30 were diagnosed with nonhepatic cancers. The overall death or transplant rates were 3% (four) in the bridging fibrosis group, 14% (32) in the cirrhosis and CP A5 group, and 62% (48) in the cirrhosis and CP A6 group.
Liver-related death rates were also highest among patients with cirrhosis, Dr. Vilar-Gomez said. While the four deaths in the fibrosis patients were evenly split between liver- and non–liver-related causes, 79% of deaths (15) in the cirrhosis and CP A5 patients were liver related, as were all 18 deaths in the cirrhosis and CP A6 group.
“Cirrhotic patients displayed the highest rates of hepatic outcomes overall as compared to those with bridging fibrosis,” Dr. Vilar-Gomez said. Rates of hepatic decompensation were 3% (5) for bridging fibrosis, 18% (40) for cirrhosis and CP A5, and 58% (48) for cirrhosis and CP A6. Likewise, HCC rates were also highest in cirrhotic patients: 9% (21) and 25% (19) for those with CP A5 and CP A6 disease, respectively, vs. 1% (2) for those with bridging fibrosis.
But nonhepatic outcomes were highest among the bridging fibrosis patients, Dr. Vilar-Gomez noted. “These include higher rates of major vascular events – 5% (eight) for those with bridging fibrosis vs. 2% (five) and 1% (one) for those with cirrhosis and CP A5 or CP A6 disease, respectively,” he said. “Rates of nonhepatic malignancies were comparable: 8% (13) for those with bridging fibrosis, 5% (10) for those with cirrhosis and CP5 disease and 9% (7) for those with cirrhosis and CP6 disease.
“Cirrhosis increased the risk of death/transplant, decompensation, and hepatocellular carcinoma by 4.3-, 4.5-, and 3.9-fold, respectively,” Dr. Vilar-Gomez said, “but was associated with a lower risk of vascular events.”
He noted that the study identified a number of risk factors associated with overall mortality and severity of liver disease in cirrhosis compared with bridging fibrosis. Cirrhosis patients tended to be 2-3 years older, had lower body mass index and blood pressure, had higher rates of type 2 diabetes, had lower lipid levels, and had worse liver and renal function.
“Type 2 diabetes and steatosis severity on liver histology, among other factors, were independent predictors for liver-related outcomes,” Dr. Vilar-Gomez said. The findings have implications in assessing patients with advanced NAFLD and for the design and interpretation of clinical trials, he added.
Dr. Vilar-Gomez had no financial relationships to disclose.
AT THE LIVER MEETING 2017
Key clinical point: Cirrhotic patients with advanced nonalcoholic fatty liver disease are at higher risk for liver-related adverse outcomes whereas patients with bridging fibrosis are more prone to nonhepatic malignancies and vascular events.
Major finding: Overall death or transplant rates were 3% in the bridging fibrosis group, 14% in the cirrhosis and Child-Pugh (CP) A5 group, and 62% in the cirrhosis and CP A6 group
Data source: Multicenter, international prospective cohort study of 458 NAFLD patients with biopsy-proven bridging fibrosis or compensated cirrhosis.
Disclosures: Dr. Vilar-Gomez had no financial relationships to disclose.
Middle-aged hepatocellular carcinoma patients increasingly ineligible for transplant
WASHINGTON – Fewer than half of studied hepatocellular carcinoma patients born between 1945 and 1965 were eligible for transplant, despite a 58% increase in HCC rate during the past decade, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases 2017.
This disparity is a cause for concern given that this cohort constitutes nearly 75% of hepatitis C virus (HCV) infections in the United States.
“Understanding hepatocellular carcinoma trends among the 1945-1965 birth cohort is particularly important given the increasing number of chronic liver diseases in that group,” said presenter Ann Robinson, MD, of Highland Hospital, Oakland, Calif.
In a retrospective study, researchers evaluated 38,045 patients born between 1945 and 1965 and who were on the Surveillance, Epidemiology, and End Results (SEER) registry and diagnosed with HCC between 2004 and 2014.
Patients were predominantly male (81.6%), white (50%), insured by Medicare or private insurance (66.2%), and diagnosed with localized tumors (52%).
White and Hispanic patients displayed the largest increase in HCC diagnoses during the study period, growing by 67.6% and 66.1%, respectively, followed by Native American and African American patients, whose HCC diagnoses increased by 61% and 57.2%, respectively.
Overall, 57.2% of patients studied did not meet the Milan criteria, according to Dr. Robinson.
Disparities in patients’ meeting the Milan criteria were apparent once researchers adjusted for patients’ sex, race, insurance status, or cancer subtype.
The largest disparity was seen among patients who were uninsured or on Medicaid, who were half as likely to meet Milan criteria at time of diagnosis, compared with insured patients (odds ratio, less than 0.5; P less than .001).
African Americans also saw lower odds of eligibility for transplantation (OR, less than 0.75; P less than .001), compared with white patients.
While the difference between men and women was statistically significant (OR, 0.875; P = .022), the difference in odds was not as prominent as that of uninsured patients or African American patients was.
These disparities may have to do with a lack of patient knowledge or less frequent screening among these patients, as well as an overall rise in nonalcoholic fatty liver disease, according to Dr. Robinson and her fellow investigators.
“It’s been well documented in prior studies that there is an underutilization of screenings both for one-time hepatitis and baby boomer population, despite recommendations by the CDC [Centers for Disease Control and Prevention]” said Dr. Robinson. Other factors may include whether patients know they should be receive these screenings, whether providers have educated their patients about this, and how much the provider knows about the screening guidelines.
The number of patients who meet the Milan criteria are growing, however, according to investigators. In 2013-2014, 46.3% of baby boomers met the Milan criteria, compared with 36.4% in 2004-2006.
Identifying vulnerabilities within these cohorts and increasing education for both providers and patients will help narrow the gap even further, explained Dr. Robinson.
“Looking at etiology-specific differences to know which populations are not receiving screening, [focusing on] things that can help us communicate this with patients, as well as distribute this information among care providers, and breaking down barriers to treatment,” are all important factors, according to Dr. Robinson.
Investigators were limited by SEER’s exclusion of etiology of HCC and comorbidities. Additionally, the researchers were unaware whether patients were receiving surveillance that was within practice guidelines.
Presenters reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – Fewer than half of studied hepatocellular carcinoma patients born between 1945 and 1965 were eligible for transplant, despite a 58% increase in HCC rate during the past decade, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases 2017.
This disparity is a cause for concern given that this cohort constitutes nearly 75% of hepatitis C virus (HCV) infections in the United States.
“Understanding hepatocellular carcinoma trends among the 1945-1965 birth cohort is particularly important given the increasing number of chronic liver diseases in that group,” said presenter Ann Robinson, MD, of Highland Hospital, Oakland, Calif.
In a retrospective study, researchers evaluated 38,045 patients born between 1945 and 1965 and who were on the Surveillance, Epidemiology, and End Results (SEER) registry and diagnosed with HCC between 2004 and 2014.
Patients were predominantly male (81.6%), white (50%), insured by Medicare or private insurance (66.2%), and diagnosed with localized tumors (52%).
White and Hispanic patients displayed the largest increase in HCC diagnoses during the study period, growing by 67.6% and 66.1%, respectively, followed by Native American and African American patients, whose HCC diagnoses increased by 61% and 57.2%, respectively.
Overall, 57.2% of patients studied did not meet the Milan criteria, according to Dr. Robinson.
Disparities in patients’ meeting the Milan criteria were apparent once researchers adjusted for patients’ sex, race, insurance status, or cancer subtype.
The largest disparity was seen among patients who were uninsured or on Medicaid, who were half as likely to meet Milan criteria at time of diagnosis, compared with insured patients (odds ratio, less than 0.5; P less than .001).
African Americans also saw lower odds of eligibility for transplantation (OR, less than 0.75; P less than .001), compared with white patients.
While the difference between men and women was statistically significant (OR, 0.875; P = .022), the difference in odds was not as prominent as that of uninsured patients or African American patients was.
These disparities may have to do with a lack of patient knowledge or less frequent screening among these patients, as well as an overall rise in nonalcoholic fatty liver disease, according to Dr. Robinson and her fellow investigators.
“It’s been well documented in prior studies that there is an underutilization of screenings both for one-time hepatitis and baby boomer population, despite recommendations by the CDC [Centers for Disease Control and Prevention]” said Dr. Robinson. Other factors may include whether patients know they should be receive these screenings, whether providers have educated their patients about this, and how much the provider knows about the screening guidelines.
The number of patients who meet the Milan criteria are growing, however, according to investigators. In 2013-2014, 46.3% of baby boomers met the Milan criteria, compared with 36.4% in 2004-2006.
Identifying vulnerabilities within these cohorts and increasing education for both providers and patients will help narrow the gap even further, explained Dr. Robinson.
“Looking at etiology-specific differences to know which populations are not receiving screening, [focusing on] things that can help us communicate this with patients, as well as distribute this information among care providers, and breaking down barriers to treatment,” are all important factors, according to Dr. Robinson.
Investigators were limited by SEER’s exclusion of etiology of HCC and comorbidities. Additionally, the researchers were unaware whether patients were receiving surveillance that was within practice guidelines.
Presenters reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – Fewer than half of studied hepatocellular carcinoma patients born between 1945 and 1965 were eligible for transplant, despite a 58% increase in HCC rate during the past decade, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases 2017.
This disparity is a cause for concern given that this cohort constitutes nearly 75% of hepatitis C virus (HCV) infections in the United States.
“Understanding hepatocellular carcinoma trends among the 1945-1965 birth cohort is particularly important given the increasing number of chronic liver diseases in that group,” said presenter Ann Robinson, MD, of Highland Hospital, Oakland, Calif.
In a retrospective study, researchers evaluated 38,045 patients born between 1945 and 1965 and who were on the Surveillance, Epidemiology, and End Results (SEER) registry and diagnosed with HCC between 2004 and 2014.
Patients were predominantly male (81.6%), white (50%), insured by Medicare or private insurance (66.2%), and diagnosed with localized tumors (52%).
White and Hispanic patients displayed the largest increase in HCC diagnoses during the study period, growing by 67.6% and 66.1%, respectively, followed by Native American and African American patients, whose HCC diagnoses increased by 61% and 57.2%, respectively.
Overall, 57.2% of patients studied did not meet the Milan criteria, according to Dr. Robinson.
Disparities in patients’ meeting the Milan criteria were apparent once researchers adjusted for patients’ sex, race, insurance status, or cancer subtype.
The largest disparity was seen among patients who were uninsured or on Medicaid, who were half as likely to meet Milan criteria at time of diagnosis, compared with insured patients (odds ratio, less than 0.5; P less than .001).
African Americans also saw lower odds of eligibility for transplantation (OR, less than 0.75; P less than .001), compared with white patients.
While the difference between men and women was statistically significant (OR, 0.875; P = .022), the difference in odds was not as prominent as that of uninsured patients or African American patients was.
These disparities may have to do with a lack of patient knowledge or less frequent screening among these patients, as well as an overall rise in nonalcoholic fatty liver disease, according to Dr. Robinson and her fellow investigators.
“It’s been well documented in prior studies that there is an underutilization of screenings both for one-time hepatitis and baby boomer population, despite recommendations by the CDC [Centers for Disease Control and Prevention]” said Dr. Robinson. Other factors may include whether patients know they should be receive these screenings, whether providers have educated their patients about this, and how much the provider knows about the screening guidelines.
The number of patients who meet the Milan criteria are growing, however, according to investigators. In 2013-2014, 46.3% of baby boomers met the Milan criteria, compared with 36.4% in 2004-2006.
Identifying vulnerabilities within these cohorts and increasing education for both providers and patients will help narrow the gap even further, explained Dr. Robinson.
“Looking at etiology-specific differences to know which populations are not receiving screening, [focusing on] things that can help us communicate this with patients, as well as distribute this information among care providers, and breaking down barriers to treatment,” are all important factors, according to Dr. Robinson.
Investigators were limited by SEER’s exclusion of etiology of HCC and comorbidities. Additionally, the researchers were unaware whether patients were receiving surveillance that was within practice guidelines.
Presenters reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
AT THE LIVER MEETING 2017
Key clinical point:
Major finding: Of HCC patients born between 1945 and 1965, 57.2% did not meet the Milan criteria.
Data source: Retrospective study of 38,045 patients born between 1945 and 1965 who were diagnosed with HCC during 2004-2014 and who were added to the SEER registry.
Disclosures: Presenters reported no relevant financial disclosures.














