User login
VIDEO: Genetic tests, clinical data sharpen pigmented lesion diagnosis
WAIKOLOA, HAWAII – While many pigmented lesions analyzed in the dermatopathology lab are easily classifiable as benign or malignant, a certain percentage of cases are “very challenging to all examiners” – and in those cases, newer testing methods such as immunostaining or genetic testing can provide useful information, according to Dr. Whitney High.
Morphologic analysis of pigmented lesions under the microscope to determine whether a lesion is benign or malignant has limitations, said Dr. High, director of dermatopathology at the University of Colorado at Denver, Aurora. “We don’t really actually know. We haven’t genetically queried the cells.”
In a video interview at the Hawaii Dermatology Seminar, Dr. High emphasized, however, that no test is 100% sensitive and 100% specific. In fact, such tests should be considered adjunctive – requiring “some type of physician oversight or guidance to decide what is a significant result, what is an insignificant result, what is a confounded result, [and] what is a discrepant result.”
Clinical information is also useful, Dr. High said, noting that when this information is not provided, “I don’t really have any feeling from the clinician as to whether the lesion is new, growing, changing, [or] doing anything suspicious.”
The Hawaii Dermatology Seminar is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company.
Dr. High had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – While many pigmented lesions analyzed in the dermatopathology lab are easily classifiable as benign or malignant, a certain percentage of cases are “very challenging to all examiners” – and in those cases, newer testing methods such as immunostaining or genetic testing can provide useful information, according to Dr. Whitney High.
Morphologic analysis of pigmented lesions under the microscope to determine whether a lesion is benign or malignant has limitations, said Dr. High, director of dermatopathology at the University of Colorado at Denver, Aurora. “We don’t really actually know. We haven’t genetically queried the cells.”
In a video interview at the Hawaii Dermatology Seminar, Dr. High emphasized, however, that no test is 100% sensitive and 100% specific. In fact, such tests should be considered adjunctive – requiring “some type of physician oversight or guidance to decide what is a significant result, what is an insignificant result, what is a confounded result, [and] what is a discrepant result.”
Clinical information is also useful, Dr. High said, noting that when this information is not provided, “I don’t really have any feeling from the clinician as to whether the lesion is new, growing, changing, [or] doing anything suspicious.”
The Hawaii Dermatology Seminar is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company.
Dr. High had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – While many pigmented lesions analyzed in the dermatopathology lab are easily classifiable as benign or malignant, a certain percentage of cases are “very challenging to all examiners” – and in those cases, newer testing methods such as immunostaining or genetic testing can provide useful information, according to Dr. Whitney High.
Morphologic analysis of pigmented lesions under the microscope to determine whether a lesion is benign or malignant has limitations, said Dr. High, director of dermatopathology at the University of Colorado at Denver, Aurora. “We don’t really actually know. We haven’t genetically queried the cells.”
In a video interview at the Hawaii Dermatology Seminar, Dr. High emphasized, however, that no test is 100% sensitive and 100% specific. In fact, such tests should be considered adjunctive – requiring “some type of physician oversight or guidance to decide what is a significant result, what is an insignificant result, what is a confounded result, [and] what is a discrepant result.”
Clinical information is also useful, Dr. High said, noting that when this information is not provided, “I don’t really have any feeling from the clinician as to whether the lesion is new, growing, changing, [or] doing anything suspicious.”
The Hawaii Dermatology Seminar is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company.
Dr. High had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: New microscopy tools improve melanoma detection
WAIKOLOA, HAWAII – Armed with dermoscopy and reflective confocal microscopy, dermatologists have been “pushing the envelope and becoming better and better at detecting melanoma and limiting the number of benign lesions being removed,” said Dr. Ashfaq A. Marghoob.
Dermoscopy – now used by about 75% of dermatologists in the United States – has lowered the benign-to-malignant ratio to about 5:1, Dr. Marghoob explained. That’s five benign nevi removed for every one melanoma found.
In an interview at the Hawaii Dermatology Seminar, Dr. Marghoob of Memorial Sloan Kettering Cancer Center, New York, discussed the impact that new technologies such as reflectance confocal microscopy are having on finding melanomas and differentiating them from benign nevi.
The Hawaii Dermatology Seminar is provided by the Global Academy for Medical Education/Skin Disease Education Foundation. The SDEF and this news organization are owned by the same parent company.
Dr. Marghoob had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Armed with dermoscopy and reflective confocal microscopy, dermatologists have been “pushing the envelope and becoming better and better at detecting melanoma and limiting the number of benign lesions being removed,” said Dr. Ashfaq A. Marghoob.
Dermoscopy – now used by about 75% of dermatologists in the United States – has lowered the benign-to-malignant ratio to about 5:1, Dr. Marghoob explained. That’s five benign nevi removed for every one melanoma found.
In an interview at the Hawaii Dermatology Seminar, Dr. Marghoob of Memorial Sloan Kettering Cancer Center, New York, discussed the impact that new technologies such as reflectance confocal microscopy are having on finding melanomas and differentiating them from benign nevi.
The Hawaii Dermatology Seminar is provided by the Global Academy for Medical Education/Skin Disease Education Foundation. The SDEF and this news organization are owned by the same parent company.
Dr. Marghoob had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Armed with dermoscopy and reflective confocal microscopy, dermatologists have been “pushing the envelope and becoming better and better at detecting melanoma and limiting the number of benign lesions being removed,” said Dr. Ashfaq A. Marghoob.
Dermoscopy – now used by about 75% of dermatologists in the United States – has lowered the benign-to-malignant ratio to about 5:1, Dr. Marghoob explained. That’s five benign nevi removed for every one melanoma found.
In an interview at the Hawaii Dermatology Seminar, Dr. Marghoob of Memorial Sloan Kettering Cancer Center, New York, discussed the impact that new technologies such as reflectance confocal microscopy are having on finding melanomas and differentiating them from benign nevi.
The Hawaii Dermatology Seminar is provided by the Global Academy for Medical Education/Skin Disease Education Foundation. The SDEF and this news organization are owned by the same parent company.
Dr. Marghoob had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
NNTs show once-unimaginable psoriasis outcomes now readily attainable
WAIKOLOA, HAWAII – Scrutiny of the number needed to treat with various systemic drugs to achieve a Psoriasis Area and Severity Index–90 (PASI-90) response in psoriasis highlights the folly of current stepwise treatment strategies imposed upon dermatologists by many payers, Dr. Craig L. Leonardi asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“It’s time to rethink our goals,” declared Dr. Leonardi, a dermatologist at Saint Louis University and a noted clinical trialist.
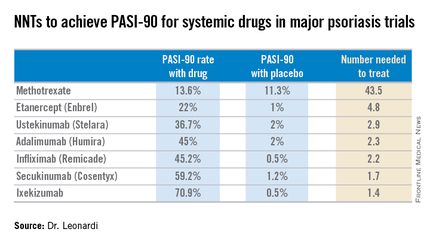
Most health plans insist that patients with moderate to severe psoriasis must have tried methotrexate and failed to achieve an adequate response before moving on to costlier biologic agents. But a PASI-90 response rate, indicative of 90% improvement in psoriasis area and severity, was achieved in only 13.6% of patients on methotrexate in the CHAMPION trial, compared with 59.2% and 70.9% of patients in phase III randomized trials of the interleukin-17 antagonists secukinumab (Cosentyx) and ixekizumab, respectively.
In other words, it is now routinely possible using highly effective medications to achieve a PASI-90 response in the majority of psoriasis patients, he noted.
Although statisticians say it isn’t appropriate to compare outcomes across clinical trials because study populations may differ, Dr. Leonardi decided it nevertheless would be illuminating to compare NNTs (the number of patients who needed to be treated with a medication instead of placebo to achieve one additional responder in a defined time period). In this analysis, he used data from phase III randomized, placebo-controlled clinical trials and Food and Drug Administration–regulated package inserts to calculate NNTs for systemic medications for psoriasis for the 10- to 16-week duration of phase III trials. The NNTs for a PASI-90 response ranged from a whopping 43.5 for methotrexate to 1.7 with secukinumab and 1.4 for ixekizumab.
“You can see immediately that methotrexate is outed as a weak and ineffective drug. And yet which drug are we usually asked to use first? Methotrexate. This is a structural problem that we have to solve in our specialty. We have to get with the insurance industry, we have to get with our guidelines of care and rewrite them. There is no reason that this drug should be placed in front of any of the other drugs, which are much more effective,” Dr. Leonardi said.
Viewing the data another way, the proportion of patients who achieved a PASI-75 response at the time of a major placebo-controlled clinical trial’s primary endpoint ranged from a low of 35.5% with methotrexate to 81.6% with secukinumab and 89.1% with ixekizumab. The NNTs to get a PASI-75 improvement with secukinumab and ixekizumab were, respectively, 1.3 and 1.2, compared to 6.0 for methotrexate.
“With an NNT of 1.2, if you treat 12 patients with ixekizumab, 10 of them are going to achieve PASI-75. This is a very different world than we had at the beginning of this adventure back in the early 2000s,” he observed.
In the modern era of highly effective biologic therapies for psoriasis, it makes sense to push as hard as possible in an effort to try to clear patients, according to Dr. Leonardi. That’s in part because the closer patients come to that once-nearly-unattainable goal, the better they actually feel, as underscored in the phase III results for ixekizumab.
In that study, the proportion of patients with a Dermatology Life Quality Index (DLQI) score of 0 or 1 at week 12 rose stepwise with the size of their PASI response. Among patients with a week-12 PASI response of 50 to less than 75, 18.8% had a DLQI of 0 or 1. For patients with a PASI-75 to less than PASI-90, it jumped to 52.3%. Among subjects with a PASI-90 to less than 100, 66.9% had a DLQI of 0 or 1. And among the 35.3% of ixekizumab-treated patients who had a PASI-100 response, the likelihood of a DLQI of 0 or 1 rose to 71.1%.
Noting that etanercept (Enbrel) is the only biologic on the market that achieves a PASI-75 response in less than half of treated patients, Dr. Leonardi said, “I’m over using etanercept as a first-line biologic. It’s the weakest biologic we can pick right now. I really like the highly efficacious drugs. I like adalimumab. I like the efficacy I see with ustekinumab. I like secukinumab. They are all preferred first-line drugs if I can get them, but it’s an insurance company world. I can’t tell you how many times I’ve heard, ‘Well, we’re not saying you can’t prescribe it, Dr. Leonardi, we’re just not going to pay for it.’ ”
Ixekizumab is under FDA review for psoriasis and a decision is expected within the first quarter of 2016, according to a spokesperson for Eli Lilly.
Dr. Leonardi is a recipient of research grants from well over a dozen pharmaceutical companies and is a consultant to and/or member of the speakers bureaus for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Janssen, Eli Lilly, Leo, Novartis, Pfizer, Sandoz, UCB, and Vitae.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Scrutiny of the number needed to treat with various systemic drugs to achieve a Psoriasis Area and Severity Index–90 (PASI-90) response in psoriasis highlights the folly of current stepwise treatment strategies imposed upon dermatologists by many payers, Dr. Craig L. Leonardi asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“It’s time to rethink our goals,” declared Dr. Leonardi, a dermatologist at Saint Louis University and a noted clinical trialist.
Most health plans insist that patients with moderate to severe psoriasis must have tried methotrexate and failed to achieve an adequate response before moving on to costlier biologic agents. But a PASI-90 response rate, indicative of 90% improvement in psoriasis area and severity, was achieved in only 13.6% of patients on methotrexate in the CHAMPION trial, compared with 59.2% and 70.9% of patients in phase III randomized trials of the interleukin-17 antagonists secukinumab (Cosentyx) and ixekizumab, respectively.
In other words, it is now routinely possible using highly effective medications to achieve a PASI-90 response in the majority of psoriasis patients, he noted.
Although statisticians say it isn’t appropriate to compare outcomes across clinical trials because study populations may differ, Dr. Leonardi decided it nevertheless would be illuminating to compare NNTs (the number of patients who needed to be treated with a medication instead of placebo to achieve one additional responder in a defined time period). In this analysis, he used data from phase III randomized, placebo-controlled clinical trials and Food and Drug Administration–regulated package inserts to calculate NNTs for systemic medications for psoriasis for the 10- to 16-week duration of phase III trials. The NNTs for a PASI-90 response ranged from a whopping 43.5 for methotrexate to 1.7 with secukinumab and 1.4 for ixekizumab.
“You can see immediately that methotrexate is outed as a weak and ineffective drug. And yet which drug are we usually asked to use first? Methotrexate. This is a structural problem that we have to solve in our specialty. We have to get with the insurance industry, we have to get with our guidelines of care and rewrite them. There is no reason that this drug should be placed in front of any of the other drugs, which are much more effective,” Dr. Leonardi said.

Viewing the data another way, the proportion of patients who achieved a PASI-75 response at the time of a major placebo-controlled clinical trial’s primary endpoint ranged from a low of 35.5% with methotrexate to 81.6% with secukinumab and 89.1% with ixekizumab. The NNTs to get a PASI-75 improvement with secukinumab and ixekizumab were, respectively, 1.3 and 1.2, compared to 6.0 for methotrexate.
“With an NNT of 1.2, if you treat 12 patients with ixekizumab, 10 of them are going to achieve PASI-75. This is a very different world than we had at the beginning of this adventure back in the early 2000s,” he observed.
In the modern era of highly effective biologic therapies for psoriasis, it makes sense to push as hard as possible in an effort to try to clear patients, according to Dr. Leonardi. That’s in part because the closer patients come to that once-nearly-unattainable goal, the better they actually feel, as underscored in the phase III results for ixekizumab.
In that study, the proportion of patients with a Dermatology Life Quality Index (DLQI) score of 0 or 1 at week 12 rose stepwise with the size of their PASI response. Among patients with a week-12 PASI response of 50 to less than 75, 18.8% had a DLQI of 0 or 1. For patients with a PASI-75 to less than PASI-90, it jumped to 52.3%. Among subjects with a PASI-90 to less than 100, 66.9% had a DLQI of 0 or 1. And among the 35.3% of ixekizumab-treated patients who had a PASI-100 response, the likelihood of a DLQI of 0 or 1 rose to 71.1%.
Noting that etanercept (Enbrel) is the only biologic on the market that achieves a PASI-75 response in less than half of treated patients, Dr. Leonardi said, “I’m over using etanercept as a first-line biologic. It’s the weakest biologic we can pick right now. I really like the highly efficacious drugs. I like adalimumab. I like the efficacy I see with ustekinumab. I like secukinumab. They are all preferred first-line drugs if I can get them, but it’s an insurance company world. I can’t tell you how many times I’ve heard, ‘Well, we’re not saying you can’t prescribe it, Dr. Leonardi, we’re just not going to pay for it.’ ”
Ixekizumab is under FDA review for psoriasis and a decision is expected within the first quarter of 2016, according to a spokesperson for Eli Lilly.
Dr. Leonardi is a recipient of research grants from well over a dozen pharmaceutical companies and is a consultant to and/or member of the speakers bureaus for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Janssen, Eli Lilly, Leo, Novartis, Pfizer, Sandoz, UCB, and Vitae.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Scrutiny of the number needed to treat with various systemic drugs to achieve a Psoriasis Area and Severity Index–90 (PASI-90) response in psoriasis highlights the folly of current stepwise treatment strategies imposed upon dermatologists by many payers, Dr. Craig L. Leonardi asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“It’s time to rethink our goals,” declared Dr. Leonardi, a dermatologist at Saint Louis University and a noted clinical trialist.
Most health plans insist that patients with moderate to severe psoriasis must have tried methotrexate and failed to achieve an adequate response before moving on to costlier biologic agents. But a PASI-90 response rate, indicative of 90% improvement in psoriasis area and severity, was achieved in only 13.6% of patients on methotrexate in the CHAMPION trial, compared with 59.2% and 70.9% of patients in phase III randomized trials of the interleukin-17 antagonists secukinumab (Cosentyx) and ixekizumab, respectively.
In other words, it is now routinely possible using highly effective medications to achieve a PASI-90 response in the majority of psoriasis patients, he noted.
Although statisticians say it isn’t appropriate to compare outcomes across clinical trials because study populations may differ, Dr. Leonardi decided it nevertheless would be illuminating to compare NNTs (the number of patients who needed to be treated with a medication instead of placebo to achieve one additional responder in a defined time period). In this analysis, he used data from phase III randomized, placebo-controlled clinical trials and Food and Drug Administration–regulated package inserts to calculate NNTs for systemic medications for psoriasis for the 10- to 16-week duration of phase III trials. The NNTs for a PASI-90 response ranged from a whopping 43.5 for methotrexate to 1.7 with secukinumab and 1.4 for ixekizumab.
“You can see immediately that methotrexate is outed as a weak and ineffective drug. And yet which drug are we usually asked to use first? Methotrexate. This is a structural problem that we have to solve in our specialty. We have to get with the insurance industry, we have to get with our guidelines of care and rewrite them. There is no reason that this drug should be placed in front of any of the other drugs, which are much more effective,” Dr. Leonardi said.

Viewing the data another way, the proportion of patients who achieved a PASI-75 response at the time of a major placebo-controlled clinical trial’s primary endpoint ranged from a low of 35.5% with methotrexate to 81.6% with secukinumab and 89.1% with ixekizumab. The NNTs to get a PASI-75 improvement with secukinumab and ixekizumab were, respectively, 1.3 and 1.2, compared to 6.0 for methotrexate.
“With an NNT of 1.2, if you treat 12 patients with ixekizumab, 10 of them are going to achieve PASI-75. This is a very different world than we had at the beginning of this adventure back in the early 2000s,” he observed.
In the modern era of highly effective biologic therapies for psoriasis, it makes sense to push as hard as possible in an effort to try to clear patients, according to Dr. Leonardi. That’s in part because the closer patients come to that once-nearly-unattainable goal, the better they actually feel, as underscored in the phase III results for ixekizumab.
In that study, the proportion of patients with a Dermatology Life Quality Index (DLQI) score of 0 or 1 at week 12 rose stepwise with the size of their PASI response. Among patients with a week-12 PASI response of 50 to less than 75, 18.8% had a DLQI of 0 or 1. For patients with a PASI-75 to less than PASI-90, it jumped to 52.3%. Among subjects with a PASI-90 to less than 100, 66.9% had a DLQI of 0 or 1. And among the 35.3% of ixekizumab-treated patients who had a PASI-100 response, the likelihood of a DLQI of 0 or 1 rose to 71.1%.
Noting that etanercept (Enbrel) is the only biologic on the market that achieves a PASI-75 response in less than half of treated patients, Dr. Leonardi said, “I’m over using etanercept as a first-line biologic. It’s the weakest biologic we can pick right now. I really like the highly efficacious drugs. I like adalimumab. I like the efficacy I see with ustekinumab. I like secukinumab. They are all preferred first-line drugs if I can get them, but it’s an insurance company world. I can’t tell you how many times I’ve heard, ‘Well, we’re not saying you can’t prescribe it, Dr. Leonardi, we’re just not going to pay for it.’ ”
Ixekizumab is under FDA review for psoriasis and a decision is expected within the first quarter of 2016, according to a spokesperson for Eli Lilly.
Dr. Leonardi is a recipient of research grants from well over a dozen pharmaceutical companies and is a consultant to and/or member of the speakers bureaus for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Janssen, Eli Lilly, Leo, Novartis, Pfizer, Sandoz, UCB, and Vitae.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Registry shows no increased cancer risk with biologics for psoriasis
WAIKOLOA, HAWAII – The latest update from the ongoing PSOLAR registry provides “very reassuring” evidence that the use of biologic agents to treat moderate to severe psoriasis doesn’t significantly increase malignancy risk other than for skin cancer, according to Dr. Kristina Callis Duffin.
Dr. Duffin of the department of dermatology at the University of Utah, Salt Lake City, cited a report presented by Dr. David Fiorentino, professor of dermatology at Stanford (Calif.) University, at the annual meeting of the European Academy of Dermatology and Venereology last October in Copenhagen. This update from the prospective international Psoriasis Longitudinal Assessment and Registry (PSOLAR) included 12,093 psoriasis patients deemed candidates for biologics, including 2,084 who did not go on a biologic agent while the rest did.
During 40,388 patient-years of prospective follow-up, or an average of 3.3 years, 455 patients were diagnosed with a malignancy other than skin cancer. The cumulative malignancy rate was 0.75 cases per 100 patient-years in patients on nonbiologic therapies, which was not significantly different from the rates of 0.51 per 100 patient-years in participants who started on ustekinumab (Stelara) at enrollment, 0.81 in patients on infliximab (Remicade), or 0.73 per 100 patient-years in those on other biologics, Dr. Duffin said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“These are all very low rates,” commented Dr. Duffin.
The PSOLAR data are particularly valuable because the registry was set up specifically to prospectively examine the long-term safety and efficacy of biologic agents in psoriasis patients. In contrast, the landmark epidemiologic study led by Dr. Joel M. Gelfand of the University of Pennsylvania, Philadelphia, which concluded that mild psoriasis was associated with a 34% increased risk of lymphoma and that severe psoriasis carried a 59% greater risk than in nonpsoriatic controls (J Invest Dermatol. 2006 Oct;126[10]:2194-201), involved a retrospective analysis of the U.K. General Practice Research Database. And while that study had strength in numbers – it included more than 153,000 British psoriasis patients and nearly 800,000 controls – it wasn’t designed to look specifically at psoriasis patients.
It’s reassuring that the lymphoma rate of 0.47 cases per 100 patient-years in patients with severe psoriasis in the U.K. registry during the prebiologics era is virtually identical to the rates associated with biologic agents in PSOLAR to date, which ranged from 0.3 to 0.5 cases per 100 patient-years, Dr. Duffin said.
The PSOLAR findings are worth sharing with patients. As a result of direct-to-consumer advertising by pharmaceutical companies, psoriasis patients are typically quite concerned about the risk of cancer associated with biologic agents, she added.
“They hear the comment in the ad that serious infections and malignancies have been reported in patients on these drugs as ‘these drugs increase the risk of malignancy.’ So where I start this conversation is, ‘Actually, patients with psoriasis do have some increased risk of malignancy, but those malignancies are mostly nonmelanoma skin cancers and lymphoproliferative diseases,’” the dermatologist explained.
Much of this risk is probably related to the fact that patients with moderate to severe psoriasis often have an extensive history of exposure to immunosuppressive agents such as cyclosporine as well as UV light therapies, which increase the risk of skin cancer.
“You also have to consider the fact that psoriasis patients tend to have a lot of smoking behaviors and alcohol behaviors that increase cancer risk,” Dr. Duffin continued.
In shared decision making regarding the option of biologic therapy in psoriasis patients having a history of cancer or who develop cancer while on a biologic, she likes to pose a question: What scares you more: the risk of your cancer coming back or not being able to have a good quality of life?
“That gets them thinking,” she said.
She stressed that as part of discussions regarding the risk/benefit profile of biologic therapy in an individual with a history of cancer, or of continuing a biologic in someone diagnosed with a malignancy while on treatment, it’s important for the dermatologist to talk with the patient’s oncologist, who is best positioned to provide insight into the risk of cancer recurrence.
Dr. Duffin is a recipient of research grants from and a consultant to Janssen, which sponsors the PSOLAR registry, as well as to more than half a dozen other pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The latest update from the ongoing PSOLAR registry provides “very reassuring” evidence that the use of biologic agents to treat moderate to severe psoriasis doesn’t significantly increase malignancy risk other than for skin cancer, according to Dr. Kristina Callis Duffin.
Dr. Duffin of the department of dermatology at the University of Utah, Salt Lake City, cited a report presented by Dr. David Fiorentino, professor of dermatology at Stanford (Calif.) University, at the annual meeting of the European Academy of Dermatology and Venereology last October in Copenhagen. This update from the prospective international Psoriasis Longitudinal Assessment and Registry (PSOLAR) included 12,093 psoriasis patients deemed candidates for biologics, including 2,084 who did not go on a biologic agent while the rest did.
During 40,388 patient-years of prospective follow-up, or an average of 3.3 years, 455 patients were diagnosed with a malignancy other than skin cancer. The cumulative malignancy rate was 0.75 cases per 100 patient-years in patients on nonbiologic therapies, which was not significantly different from the rates of 0.51 per 100 patient-years in participants who started on ustekinumab (Stelara) at enrollment, 0.81 in patients on infliximab (Remicade), or 0.73 per 100 patient-years in those on other biologics, Dr. Duffin said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“These are all very low rates,” commented Dr. Duffin.
The PSOLAR data are particularly valuable because the registry was set up specifically to prospectively examine the long-term safety and efficacy of biologic agents in psoriasis patients. In contrast, the landmark epidemiologic study led by Dr. Joel M. Gelfand of the University of Pennsylvania, Philadelphia, which concluded that mild psoriasis was associated with a 34% increased risk of lymphoma and that severe psoriasis carried a 59% greater risk than in nonpsoriatic controls (J Invest Dermatol. 2006 Oct;126[10]:2194-201), involved a retrospective analysis of the U.K. General Practice Research Database. And while that study had strength in numbers – it included more than 153,000 British psoriasis patients and nearly 800,000 controls – it wasn’t designed to look specifically at psoriasis patients.
It’s reassuring that the lymphoma rate of 0.47 cases per 100 patient-years in patients with severe psoriasis in the U.K. registry during the prebiologics era is virtually identical to the rates associated with biologic agents in PSOLAR to date, which ranged from 0.3 to 0.5 cases per 100 patient-years, Dr. Duffin said.
The PSOLAR findings are worth sharing with patients. As a result of direct-to-consumer advertising by pharmaceutical companies, psoriasis patients are typically quite concerned about the risk of cancer associated with biologic agents, she added.
“They hear the comment in the ad that serious infections and malignancies have been reported in patients on these drugs as ‘these drugs increase the risk of malignancy.’ So where I start this conversation is, ‘Actually, patients with psoriasis do have some increased risk of malignancy, but those malignancies are mostly nonmelanoma skin cancers and lymphoproliferative diseases,’” the dermatologist explained.
Much of this risk is probably related to the fact that patients with moderate to severe psoriasis often have an extensive history of exposure to immunosuppressive agents such as cyclosporine as well as UV light therapies, which increase the risk of skin cancer.
“You also have to consider the fact that psoriasis patients tend to have a lot of smoking behaviors and alcohol behaviors that increase cancer risk,” Dr. Duffin continued.
In shared decision making regarding the option of biologic therapy in psoriasis patients having a history of cancer or who develop cancer while on a biologic, she likes to pose a question: What scares you more: the risk of your cancer coming back or not being able to have a good quality of life?
“That gets them thinking,” she said.
She stressed that as part of discussions regarding the risk/benefit profile of biologic therapy in an individual with a history of cancer, or of continuing a biologic in someone diagnosed with a malignancy while on treatment, it’s important for the dermatologist to talk with the patient’s oncologist, who is best positioned to provide insight into the risk of cancer recurrence.
Dr. Duffin is a recipient of research grants from and a consultant to Janssen, which sponsors the PSOLAR registry, as well as to more than half a dozen other pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The latest update from the ongoing PSOLAR registry provides “very reassuring” evidence that the use of biologic agents to treat moderate to severe psoriasis doesn’t significantly increase malignancy risk other than for skin cancer, according to Dr. Kristina Callis Duffin.
Dr. Duffin of the department of dermatology at the University of Utah, Salt Lake City, cited a report presented by Dr. David Fiorentino, professor of dermatology at Stanford (Calif.) University, at the annual meeting of the European Academy of Dermatology and Venereology last October in Copenhagen. This update from the prospective international Psoriasis Longitudinal Assessment and Registry (PSOLAR) included 12,093 psoriasis patients deemed candidates for biologics, including 2,084 who did not go on a biologic agent while the rest did.
During 40,388 patient-years of prospective follow-up, or an average of 3.3 years, 455 patients were diagnosed with a malignancy other than skin cancer. The cumulative malignancy rate was 0.75 cases per 100 patient-years in patients on nonbiologic therapies, which was not significantly different from the rates of 0.51 per 100 patient-years in participants who started on ustekinumab (Stelara) at enrollment, 0.81 in patients on infliximab (Remicade), or 0.73 per 100 patient-years in those on other biologics, Dr. Duffin said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“These are all very low rates,” commented Dr. Duffin.
The PSOLAR data are particularly valuable because the registry was set up specifically to prospectively examine the long-term safety and efficacy of biologic agents in psoriasis patients. In contrast, the landmark epidemiologic study led by Dr. Joel M. Gelfand of the University of Pennsylvania, Philadelphia, which concluded that mild psoriasis was associated with a 34% increased risk of lymphoma and that severe psoriasis carried a 59% greater risk than in nonpsoriatic controls (J Invest Dermatol. 2006 Oct;126[10]:2194-201), involved a retrospective analysis of the U.K. General Practice Research Database. And while that study had strength in numbers – it included more than 153,000 British psoriasis patients and nearly 800,000 controls – it wasn’t designed to look specifically at psoriasis patients.
It’s reassuring that the lymphoma rate of 0.47 cases per 100 patient-years in patients with severe psoriasis in the U.K. registry during the prebiologics era is virtually identical to the rates associated with biologic agents in PSOLAR to date, which ranged from 0.3 to 0.5 cases per 100 patient-years, Dr. Duffin said.
The PSOLAR findings are worth sharing with patients. As a result of direct-to-consumer advertising by pharmaceutical companies, psoriasis patients are typically quite concerned about the risk of cancer associated with biologic agents, she added.
“They hear the comment in the ad that serious infections and malignancies have been reported in patients on these drugs as ‘these drugs increase the risk of malignancy.’ So where I start this conversation is, ‘Actually, patients with psoriasis do have some increased risk of malignancy, but those malignancies are mostly nonmelanoma skin cancers and lymphoproliferative diseases,’” the dermatologist explained.
Much of this risk is probably related to the fact that patients with moderate to severe psoriasis often have an extensive history of exposure to immunosuppressive agents such as cyclosporine as well as UV light therapies, which increase the risk of skin cancer.
“You also have to consider the fact that psoriasis patients tend to have a lot of smoking behaviors and alcohol behaviors that increase cancer risk,” Dr. Duffin continued.
In shared decision making regarding the option of biologic therapy in psoriasis patients having a history of cancer or who develop cancer while on a biologic, she likes to pose a question: What scares you more: the risk of your cancer coming back or not being able to have a good quality of life?
“That gets them thinking,” she said.
She stressed that as part of discussions regarding the risk/benefit profile of biologic therapy in an individual with a history of cancer, or of continuing a biologic in someone diagnosed with a malignancy while on treatment, it’s important for the dermatologist to talk with the patient’s oncologist, who is best positioned to provide insight into the risk of cancer recurrence.
Dr. Duffin is a recipient of research grants from and a consultant to Janssen, which sponsors the PSOLAR registry, as well as to more than half a dozen other pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Which Patients Are Best Candidates for New Onychomycosis Topicals?
WAIKOLOA, HAWAII – Two new topical treatments for nail fungal infections are more effective than previous topical therapies, but the key to successful results is picking the right onychomycosis patient, according to Dr. Theodore Rosen.
The two new agents, tavaborole and efinaconazole, “are both better than what we had previously, especially considering topical agents don’t do quite as well as oral agents do,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
The new topicals are “very convenient, in that it’s an easy-to-do regimen, once a day,” Dr. Rosen noted. But “they are inconvenient, in that they both have to be used about 48 weeks. So, that’s about a year’s worth of therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Rosen discussed approaches to achieving the best outcomes with the new agents, and he outlined other practical steps patients can take to prevent the return of nail fungal infections.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Two new topical treatments for nail fungal infections are more effective than previous topical therapies, but the key to successful results is picking the right onychomycosis patient, according to Dr. Theodore Rosen.
The two new agents, tavaborole and efinaconazole, “are both better than what we had previously, especially considering topical agents don’t do quite as well as oral agents do,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
The new topicals are “very convenient, in that it’s an easy-to-do regimen, once a day,” Dr. Rosen noted. But “they are inconvenient, in that they both have to be used about 48 weeks. So, that’s about a year’s worth of therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Rosen discussed approaches to achieving the best outcomes with the new agents, and he outlined other practical steps patients can take to prevent the return of nail fungal infections.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Two new topical treatments for nail fungal infections are more effective than previous topical therapies, but the key to successful results is picking the right onychomycosis patient, according to Dr. Theodore Rosen.
The two new agents, tavaborole and efinaconazole, “are both better than what we had previously, especially considering topical agents don’t do quite as well as oral agents do,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
The new topicals are “very convenient, in that it’s an easy-to-do regimen, once a day,” Dr. Rosen noted. But “they are inconvenient, in that they both have to be used about 48 weeks. So, that’s about a year’s worth of therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Rosen discussed approaches to achieving the best outcomes with the new agents, and he outlined other practical steps patients can take to prevent the return of nail fungal infections.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: Which patients are best for new onychomycosis topicals?
WAIKOLOA, HAWAII – Two new topical treatments for nail fungal infections are more effective than previous topical therapies, but the key to successful results is picking the right onychomycosis patient, according to Dr. Theodore Rosen.
The two new agents, tavaborole and efinaconazole, “are both better than what we had previously, especially considering topical agents don’t do quite as well as oral agents do,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
The new topicals are “very convenient, in that it’s an easy-to-do regimen, once a day,” Dr. Rosen noted. But “they are inconvenient, in that they both have to be used about 48 weeks. So, that’s about a year’s worth of therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Rosen discussed approaches to achieving the best outcomes with the new agents, and he outlined other practical steps patients can take to prevent the return of nail fungal infections.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Two new topical treatments for nail fungal infections are more effective than previous topical therapies, but the key to successful results is picking the right onychomycosis patient, according to Dr. Theodore Rosen.
The two new agents, tavaborole and efinaconazole, “are both better than what we had previously, especially considering topical agents don’t do quite as well as oral agents do,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
The new topicals are “very convenient, in that it’s an easy-to-do regimen, once a day,” Dr. Rosen noted. But “they are inconvenient, in that they both have to be used about 48 weeks. So, that’s about a year’s worth of therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Rosen discussed approaches to achieving the best outcomes with the new agents, and he outlined other practical steps patients can take to prevent the return of nail fungal infections.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Two new topical treatments for nail fungal infections are more effective than previous topical therapies, but the key to successful results is picking the right onychomycosis patient, according to Dr. Theodore Rosen.
The two new agents, tavaborole and efinaconazole, “are both better than what we had previously, especially considering topical agents don’t do quite as well as oral agents do,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston.
The new topicals are “very convenient, in that it’s an easy-to-do regimen, once a day,” Dr. Rosen noted. But “they are inconvenient, in that they both have to be used about 48 weeks. So, that’s about a year’s worth of therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Rosen discussed approaches to achieving the best outcomes with the new agents, and he outlined other practical steps patients can take to prevent the return of nail fungal infections.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
New Topical Acne Therapies Will Target Sebum
WAIKOLOA, HAWAII – Three new approaches to topical treatment of acne are on the horizon, and they all share a common foe: sebum.
“One exciting new avenue for topical therapy are drugs that actually target the production of sebum,” explained Dr. Linda F. Stein Gold, director of dermatology research at Henry Ford Health System, Detroit. “For the first time, we have a drug that potentially targets sebum with a topical mechanism. In the past, we’ve only been able to do that with oral therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Stein Gold discussed three topical, sebum-focused drugs in clinical trials and outlined their differing mechanisms of action.
SDEF and this news organization are owned by the same parent company.

WAIKOLOA, HAWAII – Three new approaches to topical treatment of acne are on the horizon, and they all share a common foe: sebum.
“One exciting new avenue for topical therapy are drugs that actually target the production of sebum,” explained Dr. Linda F. Stein Gold, director of dermatology research at Henry Ford Health System, Detroit. “For the first time, we have a drug that potentially targets sebum with a topical mechanism. In the past, we’ve only been able to do that with oral therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Stein Gold discussed three topical, sebum-focused drugs in clinical trials and outlined their differing mechanisms of action.
SDEF and this news organization are owned by the same parent company.

WAIKOLOA, HAWAII – Three new approaches to topical treatment of acne are on the horizon, and they all share a common foe: sebum.
“One exciting new avenue for topical therapy are drugs that actually target the production of sebum,” explained Dr. Linda F. Stein Gold, director of dermatology research at Henry Ford Health System, Detroit. “For the first time, we have a drug that potentially targets sebum with a topical mechanism. In the past, we’ve only been able to do that with oral therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Stein Gold discussed three topical, sebum-focused drugs in clinical trials and outlined their differing mechanisms of action.
SDEF and this news organization are owned by the same parent company.

AT SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: New topical acne therapies will target sebum
WAIKOLOA, HAWAII – Three new approaches to topical treatment of acne are on the horizon, and they all share a common foe: sebum.
“One exciting new avenue for topical therapy are drugs that actually target the production of sebum,” explained Dr. Linda F. Stein Gold, director of dermatology research at Henry Ford Health System, Detroit. “For the first time, we have a drug that potentially targets sebum with a topical mechanism. In the past, we’ve only been able to do that with oral therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Stein Gold discussed three topical, sebum-focused drugs in clinical trials and outlined their differing mechanisms of action.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Three new approaches to topical treatment of acne are on the horizon, and they all share a common foe: sebum.
“One exciting new avenue for topical therapy are drugs that actually target the production of sebum,” explained Dr. Linda F. Stein Gold, director of dermatology research at Henry Ford Health System, Detroit. “For the first time, we have a drug that potentially targets sebum with a topical mechanism. In the past, we’ve only been able to do that with oral therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Stein Gold discussed three topical, sebum-focused drugs in clinical trials and outlined their differing mechanisms of action.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Three new approaches to topical treatment of acne are on the horizon, and they all share a common foe: sebum.
“One exciting new avenue for topical therapy are drugs that actually target the production of sebum,” explained Dr. Linda F. Stein Gold, director of dermatology research at Henry Ford Health System, Detroit. “For the first time, we have a drug that potentially targets sebum with a topical mechanism. In the past, we’ve only been able to do that with oral therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Stein Gold discussed three topical, sebum-focused drugs in clinical trials and outlined their differing mechanisms of action.
SDEF and this news organization are owned by the same parent company.
AT SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: What’s new on atopic dermatitis drugs and cancer concerns?
WAIKOLOA, HAWAII – Topical calcineurin inhibitors’ boxed warnings give many patients and physicians pause over cancer concerns – but a new database analysis may put some minds at ease about the drugs’ use for atopic dermatitis.
“Pimecrolimus and tacrolimus are given topically, not internally – very little absorption occurs. So, it was hoped that ... we wouldn’t see cancer increases in these patients,” explained Dr. Joseph F. Fowler Jr., clinical professor of dermatology at the University of Louisville (Ky.). “And in fact, that’s exactly what was shown in this large study.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Fowler discussed the data from new research examining cancer incidence and calcineurin inhibitor use.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Topical calcineurin inhibitors’ boxed warnings give many patients and physicians pause over cancer concerns – but a new database analysis may put some minds at ease about the drugs’ use for atopic dermatitis.
“Pimecrolimus and tacrolimus are given topically, not internally – very little absorption occurs. So, it was hoped that ... we wouldn’t see cancer increases in these patients,” explained Dr. Joseph F. Fowler Jr., clinical professor of dermatology at the University of Louisville (Ky.). “And in fact, that’s exactly what was shown in this large study.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Fowler discussed the data from new research examining cancer incidence and calcineurin inhibitor use.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Topical calcineurin inhibitors’ boxed warnings give many patients and physicians pause over cancer concerns – but a new database analysis may put some minds at ease about the drugs’ use for atopic dermatitis.
“Pimecrolimus and tacrolimus are given topically, not internally – very little absorption occurs. So, it was hoped that ... we wouldn’t see cancer increases in these patients,” explained Dr. Joseph F. Fowler Jr., clinical professor of dermatology at the University of Louisville (Ky.). “And in fact, that’s exactly what was shown in this large study.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Fowler discussed the data from new research examining cancer incidence and calcineurin inhibitor use.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
SDEF: Severe acne responds to fixed-combo gel
A convenient, once-daily fixed combination of 0.3% adapalene plus 2.5% benzoyl peroxide gel significantly improved lesion counts over the course of 12 weeks in patients aged 12 years and older with moderate or severe acne.
Investigators enrolled just over 500 patients from 31 sites in the United States and Canada. About half of patients were rated as having severe acne and half as having moderate acne on the investigator’s global assessment (IGA) scale, Dr. Linda F. Stein Gold said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Patients were randomized to three treatment groups: adapalene 0.3%/benzoyl peroxide 2.5% gel (A-BPO-0.3%), adapalene 0.1%/benzoyl peroxide 2.5% (A-BPO-0.1%), or vehicle. Patients in each group had approximately the same total lesion count, and about half in each group had truncal acne lesions, said Dr. Stein Gold, director of clinical research in the department of dermatology at Henry Ford Hospital, Detroit.
Patients were instructed to use their study medications once daily at night after washing with a provided cleanser. They were provided with a standardized moisturizer and cleaners.
Treatment with A-BPO-0.3% was judged as successful (IGA of 1 or almost clear) at 12 weeks in 31% of patients with severe acne. By contrast, 13.3% of patients with severe acne were judged as almost clear. In patients with severe acne, A-BPO-1% was not statistically superior to vehicle (J Drugs Dermatol. 2015 Dec 1;14[12]:1427-35).
“Topical treatment is still the cornerstone of acne therapy, and it is great to have additional options, especially for our more severe acne patients,” Dr. Stein Gold said.
Patients noted dryness, scaling, erythema, and stinging/burning with A-BPO-0.3%, especially between weeks 1 and 2.
Dr. Stein Gold disclosed that she serves as a consultant and scientific advisory board member to Galderma, which markets A-BPO-0.3% as Epiduo Forte.
SDEF and this news organization are owned by the same parent company.
On Twitter @denisefulton
A convenient, once-daily fixed combination of 0.3% adapalene plus 2.5% benzoyl peroxide gel significantly improved lesion counts over the course of 12 weeks in patients aged 12 years and older with moderate or severe acne.
Investigators enrolled just over 500 patients from 31 sites in the United States and Canada. About half of patients were rated as having severe acne and half as having moderate acne on the investigator’s global assessment (IGA) scale, Dr. Linda F. Stein Gold said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Patients were randomized to three treatment groups: adapalene 0.3%/benzoyl peroxide 2.5% gel (A-BPO-0.3%), adapalene 0.1%/benzoyl peroxide 2.5% (A-BPO-0.1%), or vehicle. Patients in each group had approximately the same total lesion count, and about half in each group had truncal acne lesions, said Dr. Stein Gold, director of clinical research in the department of dermatology at Henry Ford Hospital, Detroit.
Patients were instructed to use their study medications once daily at night after washing with a provided cleanser. They were provided with a standardized moisturizer and cleaners.
Treatment with A-BPO-0.3% was judged as successful (IGA of 1 or almost clear) at 12 weeks in 31% of patients with severe acne. By contrast, 13.3% of patients with severe acne were judged as almost clear. In patients with severe acne, A-BPO-1% was not statistically superior to vehicle (J Drugs Dermatol. 2015 Dec 1;14[12]:1427-35).
“Topical treatment is still the cornerstone of acne therapy, and it is great to have additional options, especially for our more severe acne patients,” Dr. Stein Gold said.
Patients noted dryness, scaling, erythema, and stinging/burning with A-BPO-0.3%, especially between weeks 1 and 2.
Dr. Stein Gold disclosed that she serves as a consultant and scientific advisory board member to Galderma, which markets A-BPO-0.3% as Epiduo Forte.
SDEF and this news organization are owned by the same parent company.
On Twitter @denisefulton
A convenient, once-daily fixed combination of 0.3% adapalene plus 2.5% benzoyl peroxide gel significantly improved lesion counts over the course of 12 weeks in patients aged 12 years and older with moderate or severe acne.
Investigators enrolled just over 500 patients from 31 sites in the United States and Canada. About half of patients were rated as having severe acne and half as having moderate acne on the investigator’s global assessment (IGA) scale, Dr. Linda F. Stein Gold said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Patients were randomized to three treatment groups: adapalene 0.3%/benzoyl peroxide 2.5% gel (A-BPO-0.3%), adapalene 0.1%/benzoyl peroxide 2.5% (A-BPO-0.1%), or vehicle. Patients in each group had approximately the same total lesion count, and about half in each group had truncal acne lesions, said Dr. Stein Gold, director of clinical research in the department of dermatology at Henry Ford Hospital, Detroit.
Patients were instructed to use their study medications once daily at night after washing with a provided cleanser. They were provided with a standardized moisturizer and cleaners.
Treatment with A-BPO-0.3% was judged as successful (IGA of 1 or almost clear) at 12 weeks in 31% of patients with severe acne. By contrast, 13.3% of patients with severe acne were judged as almost clear. In patients with severe acne, A-BPO-1% was not statistically superior to vehicle (J Drugs Dermatol. 2015 Dec 1;14[12]:1427-35).
“Topical treatment is still the cornerstone of acne therapy, and it is great to have additional options, especially for our more severe acne patients,” Dr. Stein Gold said.
Patients noted dryness, scaling, erythema, and stinging/burning with A-BPO-0.3%, especially between weeks 1 and 2.
Dr. Stein Gold disclosed that she serves as a consultant and scientific advisory board member to Galderma, which markets A-BPO-0.3% as Epiduo Forte.
SDEF and this news organization are owned by the same parent company.
On Twitter @denisefulton
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR












