User login
Parity for prompt and staged STEMI complete revascularization: MULTISTARS-AMI
The conclusion comes from a randomized outcomes trial of 840 patients that compared prompt same-session CR with a staged procedure carried out weeks later.
That CR is a worthy goal in such cases is largely settled, unlike the question of when to pursue revascularization of nonculprit lesions for best results. So the timing varies in practice, often depending on the patient’s clinical stability, risk status, or practical issues like cath lab resources or personnel.
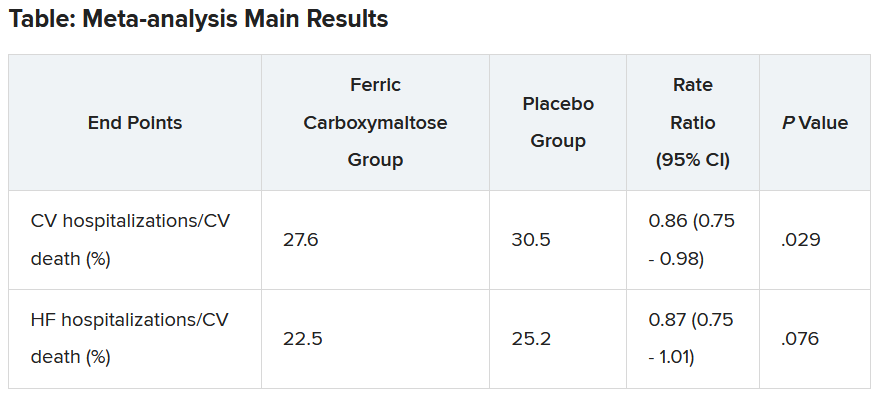
The new trial, MULTISTARS-AMI, supports that kind of flexibility as safe in practice. Immediate, same-session CR led to a 48% drop in risk for a broad composite primary endpoint at 1 year, compared with staged CR an average of 37 days later. The finding was significant for noninferiority in the study’s primary analysis and secondarily, was significant for superiority (P < .001 in both cases).
The composite endpoint included death from any cause, MI, stroke, unplanned ischemia-driven revascularization, or heart failure (HF) hospitalization.
CR’s immediate effect on outcomes was numerically pronounced, but because it was prespecified as a noninferiority trial, MULTISTARS-AMI could show only that the strategy is comparable to staged CR, emphasized Barbara E. Stähli, MD, MPH, MBA, at a press conference.
Still, it appears to be the first trial of its kind to show that operators and patients can safely choose either percutaneous coronary intervention (PCI) approach and attain similar outcomes, said Dr. Stähli, University Hospital Zurich, MULTISTARS-AMI’s principal investigator.
Not only were the two approaches similar with respect to safety, she noted, but immediate CR seemed to require less use of contrast agent and fluoroscopy. “And you have only one procedure, so there’s only one arterial puncture.”
Dr. Stähli formally presented MULTISTARS-AMI at the annual congress of the European Society of Cardiology, held in Amsterdam. She is also lead author on its publication in the New England Journal of Medicine.
After her presentation, invited discussant Robert A. Byrne, PhD, MD, MB, BCh, said that the trial’s central message is that STEMI patients with MVD having primary PCI “should undergo complete revascularization within the first 45 days, with the timing of the non–infarct-related artery procedure individualized according to clinical risk and logistical considerations.”
Still, the trial “provides evidence, but not strong evidence, of benefits with routine immediate PCI during the index procedure as compared with staged outpatient PCI,” said Dr. Byrne, Mater Private Hospital and RCSI University of Medicine and Health Sciences, Dublin.
MULTISTARS-AMI randomly assigned patients at 37 European sites to undergo same-session CR (418 patients) or CR staged 19-45 days later (422 patients).
Rates for the primary endpoint at 1 year were 8.5% and 16.3%, respectively, for a risk ratio of 0.52 (95% confidence interval, 0.38-0.72). The difference was driven by fewer instances of nonfatal MI, 0.36 (95% CI, 0.16-0.80), and unplanned ischemia-driven revascularization, 0.42 (95% CI, 0.24-0.74), in the immediate-CR group.
The wee hours
Sunil V. Rao, MD, director of interventional cardiology at NYU Langone Health System, New York, said that, in general at his center, patients who are stable with a good primary PCI outcome and whose lesions aren’t very high risk are often discharged to return later for the staged CR procedure.
But after the new insights from MULTISTARS-AMI, Dr. Rao, who is not connected to the study, said in an interview that immediate same-session CR is indeed likely preferable to staged CR performed weeks later.
Same-session CR, however, may not always be practical or wise, he observed. For example, some patients with STEMI can be pretty sick with MVD that involves very complex lesions that might be better handled later.
“The wee hours of the night may not be the best time to tackle such complex lesions,” Dr. Rao observed. If confronted with, for example, a bifurcation that requires a complex procedure, “2 o’clock in the morning is probably not the best time to address something like that.”
So the trial’s more realistic translational message, he proposed, may be to perform CR after the primary PCI but during the same hospitalization, “unless there are some real mitigating circumstances.”
The trial was supported by Boston Scientific. Dr. Stähli had no disclosures. Dr. Byrne discloses research funding to his institution from Abbott Vascular, Translumina, Biosensors, and Boston Scientific. Dr. Rao had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The conclusion comes from a randomized outcomes trial of 840 patients that compared prompt same-session CR with a staged procedure carried out weeks later.
That CR is a worthy goal in such cases is largely settled, unlike the question of when to pursue revascularization of nonculprit lesions for best results. So the timing varies in practice, often depending on the patient’s clinical stability, risk status, or practical issues like cath lab resources or personnel.
The new trial, MULTISTARS-AMI, supports that kind of flexibility as safe in practice. Immediate, same-session CR led to a 48% drop in risk for a broad composite primary endpoint at 1 year, compared with staged CR an average of 37 days later. The finding was significant for noninferiority in the study’s primary analysis and secondarily, was significant for superiority (P < .001 in both cases).
The composite endpoint included death from any cause, MI, stroke, unplanned ischemia-driven revascularization, or heart failure (HF) hospitalization.
CR’s immediate effect on outcomes was numerically pronounced, but because it was prespecified as a noninferiority trial, MULTISTARS-AMI could show only that the strategy is comparable to staged CR, emphasized Barbara E. Stähli, MD, MPH, MBA, at a press conference.
Still, it appears to be the first trial of its kind to show that operators and patients can safely choose either percutaneous coronary intervention (PCI) approach and attain similar outcomes, said Dr. Stähli, University Hospital Zurich, MULTISTARS-AMI’s principal investigator.
Not only were the two approaches similar with respect to safety, she noted, but immediate CR seemed to require less use of contrast agent and fluoroscopy. “And you have only one procedure, so there’s only one arterial puncture.”
Dr. Stähli formally presented MULTISTARS-AMI at the annual congress of the European Society of Cardiology, held in Amsterdam. She is also lead author on its publication in the New England Journal of Medicine.
After her presentation, invited discussant Robert A. Byrne, PhD, MD, MB, BCh, said that the trial’s central message is that STEMI patients with MVD having primary PCI “should undergo complete revascularization within the first 45 days, with the timing of the non–infarct-related artery procedure individualized according to clinical risk and logistical considerations.”
Still, the trial “provides evidence, but not strong evidence, of benefits with routine immediate PCI during the index procedure as compared with staged outpatient PCI,” said Dr. Byrne, Mater Private Hospital and RCSI University of Medicine and Health Sciences, Dublin.
MULTISTARS-AMI randomly assigned patients at 37 European sites to undergo same-session CR (418 patients) or CR staged 19-45 days later (422 patients).
Rates for the primary endpoint at 1 year were 8.5% and 16.3%, respectively, for a risk ratio of 0.52 (95% confidence interval, 0.38-0.72). The difference was driven by fewer instances of nonfatal MI, 0.36 (95% CI, 0.16-0.80), and unplanned ischemia-driven revascularization, 0.42 (95% CI, 0.24-0.74), in the immediate-CR group.
The wee hours
Sunil V. Rao, MD, director of interventional cardiology at NYU Langone Health System, New York, said that, in general at his center, patients who are stable with a good primary PCI outcome and whose lesions aren’t very high risk are often discharged to return later for the staged CR procedure.
But after the new insights from MULTISTARS-AMI, Dr. Rao, who is not connected to the study, said in an interview that immediate same-session CR is indeed likely preferable to staged CR performed weeks later.
Same-session CR, however, may not always be practical or wise, he observed. For example, some patients with STEMI can be pretty sick with MVD that involves very complex lesions that might be better handled later.
“The wee hours of the night may not be the best time to tackle such complex lesions,” Dr. Rao observed. If confronted with, for example, a bifurcation that requires a complex procedure, “2 o’clock in the morning is probably not the best time to address something like that.”
So the trial’s more realistic translational message, he proposed, may be to perform CR after the primary PCI but during the same hospitalization, “unless there are some real mitigating circumstances.”
The trial was supported by Boston Scientific. Dr. Stähli had no disclosures. Dr. Byrne discloses research funding to his institution from Abbott Vascular, Translumina, Biosensors, and Boston Scientific. Dr. Rao had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The conclusion comes from a randomized outcomes trial of 840 patients that compared prompt same-session CR with a staged procedure carried out weeks later.
That CR is a worthy goal in such cases is largely settled, unlike the question of when to pursue revascularization of nonculprit lesions for best results. So the timing varies in practice, often depending on the patient’s clinical stability, risk status, or practical issues like cath lab resources or personnel.
The new trial, MULTISTARS-AMI, supports that kind of flexibility as safe in practice. Immediate, same-session CR led to a 48% drop in risk for a broad composite primary endpoint at 1 year, compared with staged CR an average of 37 days later. The finding was significant for noninferiority in the study’s primary analysis and secondarily, was significant for superiority (P < .001 in both cases).
The composite endpoint included death from any cause, MI, stroke, unplanned ischemia-driven revascularization, or heart failure (HF) hospitalization.
CR’s immediate effect on outcomes was numerically pronounced, but because it was prespecified as a noninferiority trial, MULTISTARS-AMI could show only that the strategy is comparable to staged CR, emphasized Barbara E. Stähli, MD, MPH, MBA, at a press conference.
Still, it appears to be the first trial of its kind to show that operators and patients can safely choose either percutaneous coronary intervention (PCI) approach and attain similar outcomes, said Dr. Stähli, University Hospital Zurich, MULTISTARS-AMI’s principal investigator.
Not only were the two approaches similar with respect to safety, she noted, but immediate CR seemed to require less use of contrast agent and fluoroscopy. “And you have only one procedure, so there’s only one arterial puncture.”
Dr. Stähli formally presented MULTISTARS-AMI at the annual congress of the European Society of Cardiology, held in Amsterdam. She is also lead author on its publication in the New England Journal of Medicine.
After her presentation, invited discussant Robert A. Byrne, PhD, MD, MB, BCh, said that the trial’s central message is that STEMI patients with MVD having primary PCI “should undergo complete revascularization within the first 45 days, with the timing of the non–infarct-related artery procedure individualized according to clinical risk and logistical considerations.”
Still, the trial “provides evidence, but not strong evidence, of benefits with routine immediate PCI during the index procedure as compared with staged outpatient PCI,” said Dr. Byrne, Mater Private Hospital and RCSI University of Medicine and Health Sciences, Dublin.
MULTISTARS-AMI randomly assigned patients at 37 European sites to undergo same-session CR (418 patients) or CR staged 19-45 days later (422 patients).
Rates for the primary endpoint at 1 year were 8.5% and 16.3%, respectively, for a risk ratio of 0.52 (95% confidence interval, 0.38-0.72). The difference was driven by fewer instances of nonfatal MI, 0.36 (95% CI, 0.16-0.80), and unplanned ischemia-driven revascularization, 0.42 (95% CI, 0.24-0.74), in the immediate-CR group.
The wee hours
Sunil V. Rao, MD, director of interventional cardiology at NYU Langone Health System, New York, said that, in general at his center, patients who are stable with a good primary PCI outcome and whose lesions aren’t very high risk are often discharged to return later for the staged CR procedure.
But after the new insights from MULTISTARS-AMI, Dr. Rao, who is not connected to the study, said in an interview that immediate same-session CR is indeed likely preferable to staged CR performed weeks later.
Same-session CR, however, may not always be practical or wise, he observed. For example, some patients with STEMI can be pretty sick with MVD that involves very complex lesions that might be better handled later.
“The wee hours of the night may not be the best time to tackle such complex lesions,” Dr. Rao observed. If confronted with, for example, a bifurcation that requires a complex procedure, “2 o’clock in the morning is probably not the best time to address something like that.”
So the trial’s more realistic translational message, he proposed, may be to perform CR after the primary PCI but during the same hospitalization, “unless there are some real mitigating circumstances.”
The trial was supported by Boston Scientific. Dr. Stähli had no disclosures. Dr. Byrne discloses research funding to his institution from Abbott Vascular, Translumina, Biosensors, and Boston Scientific. Dr. Rao had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
Dietary nitrates reduce contrast-induced nephropathy in ACS
AMSTERDAM –
In the NITRATE-CIN Study, non-ST segment elevation myocardial infarction acute coronary syndrome (ACS) patients at risk of renal injury from coronary angiography who received dietary inorganic nitrates had a 70% reduction in CIN compared with those given placebo.
The nitrate group also showed an impressive reduction in periprocedural myocardial infarction (MI) and improved renal function at 3 months, as well as a halving of major adverse cardiovascular events and major adverse kidney events at 1 year.
The trial was presented by Dan Jones, MD, Barts Health NHS Trust, London, at the annual congress of the European Society of Cardiology.
“Currently, aside from intravenous hydration, there is no proven treatment that reduces contrast-induced nephropathy. We feel that dietary inorganic nitrate shows huge promise in this study, and these findings could have important implications in reducing this serious complication of coronary angiography,” Dr. Jones concluded.
He explained that the product used was a formulation of dietary inorganic nitrates given as potassium nitrate capsules, which the study investigators produced specifically for this trial.
At this point, “the only way to get inorganic nitrate is in the diet – specifically by consuming beetroot juice or green leafy vegetables such as spinach and rocket. From a clinician perspective, while these results suggest this is an effective therapy and has great potential, it is not currently possible to prescribe the medication we used in our study, although we are working on producing a commercial product,” he said in an interview.
However, Dr. Jones noted that it is possible to buy beetroot shots, which contain 7 mmol of potassium nitrate in each shot, from health food shops and websites, and two such shots per day for 5 days would give a dose similar to that used in this study, starting the day before angiography.
“While we need a larger multicenter study to confirm these results, studies so far suggest no signal at all that there is any harm in this approach, and there could be a great deal of benefit in taking a couple of beetroot shots prior to and for a few days after an angiogram,” he said.
Dietary nitrates “make sense”
Designated discussant of the NITRATE-CIN trial at the ESC Hotline session, Roxanna Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, said the study was well designed, and the “interesting and plausible” hypothesis to raise nitric oxide levels by dietary nitrates “makes sense.”
On the main findings of a major significant 50% reduction in acute kidney injury, Dr. Mehran said, “It is difficult to imagine such a reduction is possible.”
She pointed out that the large reduction in major adverse cardiac events and major adverse kidney events at 1 year also suggests that there is a sustained benefit in protecting the kidney.
“We’re all going to get on beet juice after this,” she quipped.
Still, Dr. Mehran questioned whether the results were “too good to be true,” adding that a larger trial actually powered for longer term outcome events is needed, as well as a better understanding of whether CIN has a causative role in mortality.
Responding to questions about whether such a large effect could actually be achieved with dietary nitrates, Dr. Jones said he thought there would definitely be some benefits, but maybe not quite as large as those seen in this study.
“From our pilot data we thought nitrate may be effective in preventing CIN,” he said in an interview. “We recruited a higher risk group than we thought, which is why the control event rates were higher than we expected, but the acute kidney injury reduction is roughly what we had estimated, and makes sense biologically.”
Dr. Jones acknowledged that the large reductions in long-term major adverse cardiovascular and kidney events were unexpected.
“The trial was not powered to see reductions in these outcomes, so we need to see if those event reductions can be replicated in larger multicenter trials,” he said. “But this was a double-blind placebo-controlled trial so in this trial the effects are real, and I think the effect size in this trial is too large for there not to be a beneficial effect.
“But I’m not so sure that we would see the same magnitude of effect when we have a larger study with tighter confidence intervals but perhaps a 20%-25% reduction in cardiovascular and kidney may be more realistic, which would still be amazing for such an easy and cost-effective intervention,” Dr. Jones added.
A larger trial is now being planned.
The researchers are also working on the development of a commercial form of dietary inorganic nitrate that would be needed for larger multicenter studies and would then be generally available. “We want this to be a low-cost product that would be available to all,” Dr. Jones said.
He noted that other studies have shown that dietary inorganic nitrates in the form of beetroot juice lower blood pressure; there are suggestions it may also lower cholesterol and prevent stent restenosis, and athletes sometimes take it to increase their aerobic capacity.
“There appears to be many benefits of dietary nitrates, and the one thing we can do at this time is to encourage people to increase their dietary nitrate consumption by eating large quantities of green leafy vegetables and beetroot,” Dr. Jones said.
Replacing lost nitric oxide
In his presentation, Dr. Jones noted that CIN is a serious complication after coronary angiography and is associated with longer hospital stays, worse long-term kidney function, and increased risk of MI and death.
The incidence varies depending on patient risk and definitions used, but it can affect up to 50% of high-risk ACS patients – older patients and/or those with heart failure, chronic kidney disease, or diabetes.
“We don’t really understand the mechanisms that cause CIN, but multiple proposed mechanisms exist, and we know from previous studies that a deficiency of nitric oxide is crucial to the development of CIN,” he explained. “We also know that [nitric oxide] is crucial for normal renal hemostasis. Therefore, a potential therapeutic target to prevent CIN would be to replace this lost nitric oxide.”
The inorganic nitrate evaluated in this trial is found in the diet, is produced endogenously, and is different from medicinally synthesized organic nitrates such as isosorbide mononitrate, he said.
“Isosorbide mononitrate/dinitrate tablets contain organic nitrates and while they are good for angina, we know that they do not have the same beneficial effects on the sustained generation of nitric oxide as inorganic nitrates,” Dr. Jones added.
NITRATE-CIN study
NITRATE-CIN was a double-blind, randomized, placebo-controlled trial conducted at Queen Mary University of London and St. Bartholomew’s Hospital in London, which tested the effectiveness of inorganic nitrate in preventing contrast-induced nephropathy in 640 patients with non-ST elevation ACS referred for invasive coronary angiography.
To be eligible for the trial, patients had to be at risk of contrast-induced nephropathy with an estimated glomerular filtration rate (eGFR) less than 60 mL/min per 1.73 m2 or have two of the following significant risk factors: diabetes, liver failure, over 70 years of age, exposure to contrast within 7 days, heart failure, or on concomitant renally acting drugs.
Patients were randomly assigned to a formulation of potassium nitrate (12 mmol/744 mg nitrate) per day given as capsules for a 5-day course with the first dose administered prior to angiography or to a control group that received potassium chloride with a matched potassium concentration.
The patient population had a mean age of 71 years, 73% were male, 75% were White, 46% had diabetes, and 56% had chronic kidney disease. There was a 13% loss to follow-up, which was attributed to the COVID pandemic.
The amount of contrast administration was 180 mL in the placebo and 170 mL in the nitrate arm, with 50% of patients undergoing some sort of revascularization.
The primary endpoint was the incidence of CIN as defined by KDIGO criteria – a series of stages of acute kidney injury defined by changes in serum creatinine within 72 hours and up to 1 week.
Results showed that this primary CIN endpoint was reduced significantly from 30% in the placebo arm to 9.1% in the nitrate group, a 70% relative risk reduction (P < .0001). The majority (90%) of this CIN was stage 1, but 10% was stage 2.
Consistent results were seen when an alternative definition of CIN (Mehran) was used, although the rates in both arms were lower than when the KDIGO definition was used.
The benefit was seen across prespecified subgroups including diabetes status, troponin positivity, and Mehran risk. But the benefit seemed to be attenuated in patients on preexisting organic nitrate therapy, although the numbers in these groups were too small to draw definitive conclusions.
As would be expected, there were significant elevations in both systemic nitrate and nitrite levels both up to 72 hours after the procedure, which was consistent with the 5-day course. This was associated with reductions in systolic and diastolic blood pressure, but not associated with any adverse events, Dr. Jones reported.
Rates of procedural MI, a prespecified secondary endpoint, were reduced from 12.5% to 4.1% in those on inorganic nitrates (P = .003).
Looking at longer term outcomes, kidney function was improved at 3 months as measured by change in eGFR, which showed a 10% relative improvement of 5.2 mL/min per 1.73 m2 (10%) in the nitrate group vs. the placebo group. Serum creatinine levels were also significantly increased in the nitrate group.
At 12 months, there was a significant 50% relative reduction in major adverse cardiovascular events – including all-cause mortality, recurrent MI, and recurrent revascularization – which were reduced from 18.1% in the placebo group to 9.1% in the nitrate group, with a reduction in all three of the constituent components of the composite endpoint including all-cause mortality.
Major adverse kidney events (all-cause mortality, renal replacement therapy, or persistent renal dysfunction) were also reduced at 12 months from 28.4% in the placebo group to 10.7% in the nitrate group (P < .0001), a 60% relative reduction. This was driven by lower rates of all-cause mortality and persistent renal dysfunction.
While Dr. Jones said these results on major cardiovascular and kidney outcomes should be viewed as hypothesis-generating at the present time, he said there were biological mechanisms that could explain these benefits.
“We saw a reduction in procedural MI, and we know there is a lot of similar biology in preventing procedural MI and subsequent cardiac events in the acute phase. This, in combination with the large reduction in acute kidney injury, could explain why there’s improved outcomes out to 12 months.”
In her comments, Dr. Mehran congratulated the investigators on having conducted the first study to have shown benefit in the prevention of contrast-associated acute kidney injury as well as major adverse cardiovascular and kidney events associated with the condition.
She used the term “contrast-associated acute kidney injury” rather than “contrast-induced nephropathy” because, she said, it has not been proven that the acute kidney injury seen after angiography is actually caused by the contrast and “so many other things are occurring during procedures when these patients are presenting with different syndromes.”
Dr. Mehran pointed out some weaknesses in the NITRATE-CIN study including the single-center design, the large volume of contrast administered, 13% of patients missing the primary endpoint blood draw, and an imbalance in relevant baseline characteristics despite randomization.
The NITRATE-CIN study was funded by Heart Research UK. Dr. Jones has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AMSTERDAM –
In the NITRATE-CIN Study, non-ST segment elevation myocardial infarction acute coronary syndrome (ACS) patients at risk of renal injury from coronary angiography who received dietary inorganic nitrates had a 70% reduction in CIN compared with those given placebo.
The nitrate group also showed an impressive reduction in periprocedural myocardial infarction (MI) and improved renal function at 3 months, as well as a halving of major adverse cardiovascular events and major adverse kidney events at 1 year.
The trial was presented by Dan Jones, MD, Barts Health NHS Trust, London, at the annual congress of the European Society of Cardiology.
“Currently, aside from intravenous hydration, there is no proven treatment that reduces contrast-induced nephropathy. We feel that dietary inorganic nitrate shows huge promise in this study, and these findings could have important implications in reducing this serious complication of coronary angiography,” Dr. Jones concluded.
He explained that the product used was a formulation of dietary inorganic nitrates given as potassium nitrate capsules, which the study investigators produced specifically for this trial.
At this point, “the only way to get inorganic nitrate is in the diet – specifically by consuming beetroot juice or green leafy vegetables such as spinach and rocket. From a clinician perspective, while these results suggest this is an effective therapy and has great potential, it is not currently possible to prescribe the medication we used in our study, although we are working on producing a commercial product,” he said in an interview.
However, Dr. Jones noted that it is possible to buy beetroot shots, which contain 7 mmol of potassium nitrate in each shot, from health food shops and websites, and two such shots per day for 5 days would give a dose similar to that used in this study, starting the day before angiography.
“While we need a larger multicenter study to confirm these results, studies so far suggest no signal at all that there is any harm in this approach, and there could be a great deal of benefit in taking a couple of beetroot shots prior to and for a few days after an angiogram,” he said.
Dietary nitrates “make sense”
Designated discussant of the NITRATE-CIN trial at the ESC Hotline session, Roxanna Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, said the study was well designed, and the “interesting and plausible” hypothesis to raise nitric oxide levels by dietary nitrates “makes sense.”
On the main findings of a major significant 50% reduction in acute kidney injury, Dr. Mehran said, “It is difficult to imagine such a reduction is possible.”
She pointed out that the large reduction in major adverse cardiac events and major adverse kidney events at 1 year also suggests that there is a sustained benefit in protecting the kidney.
“We’re all going to get on beet juice after this,” she quipped.
Still, Dr. Mehran questioned whether the results were “too good to be true,” adding that a larger trial actually powered for longer term outcome events is needed, as well as a better understanding of whether CIN has a causative role in mortality.
Responding to questions about whether such a large effect could actually be achieved with dietary nitrates, Dr. Jones said he thought there would definitely be some benefits, but maybe not quite as large as those seen in this study.
“From our pilot data we thought nitrate may be effective in preventing CIN,” he said in an interview. “We recruited a higher risk group than we thought, which is why the control event rates were higher than we expected, but the acute kidney injury reduction is roughly what we had estimated, and makes sense biologically.”
Dr. Jones acknowledged that the large reductions in long-term major adverse cardiovascular and kidney events were unexpected.
“The trial was not powered to see reductions in these outcomes, so we need to see if those event reductions can be replicated in larger multicenter trials,” he said. “But this was a double-blind placebo-controlled trial so in this trial the effects are real, and I think the effect size in this trial is too large for there not to be a beneficial effect.
“But I’m not so sure that we would see the same magnitude of effect when we have a larger study with tighter confidence intervals but perhaps a 20%-25% reduction in cardiovascular and kidney may be more realistic, which would still be amazing for such an easy and cost-effective intervention,” Dr. Jones added.
A larger trial is now being planned.
The researchers are also working on the development of a commercial form of dietary inorganic nitrate that would be needed for larger multicenter studies and would then be generally available. “We want this to be a low-cost product that would be available to all,” Dr. Jones said.
He noted that other studies have shown that dietary inorganic nitrates in the form of beetroot juice lower blood pressure; there are suggestions it may also lower cholesterol and prevent stent restenosis, and athletes sometimes take it to increase their aerobic capacity.
“There appears to be many benefits of dietary nitrates, and the one thing we can do at this time is to encourage people to increase their dietary nitrate consumption by eating large quantities of green leafy vegetables and beetroot,” Dr. Jones said.
Replacing lost nitric oxide
In his presentation, Dr. Jones noted that CIN is a serious complication after coronary angiography and is associated with longer hospital stays, worse long-term kidney function, and increased risk of MI and death.
The incidence varies depending on patient risk and definitions used, but it can affect up to 50% of high-risk ACS patients – older patients and/or those with heart failure, chronic kidney disease, or diabetes.
“We don’t really understand the mechanisms that cause CIN, but multiple proposed mechanisms exist, and we know from previous studies that a deficiency of nitric oxide is crucial to the development of CIN,” he explained. “We also know that [nitric oxide] is crucial for normal renal hemostasis. Therefore, a potential therapeutic target to prevent CIN would be to replace this lost nitric oxide.”
The inorganic nitrate evaluated in this trial is found in the diet, is produced endogenously, and is different from medicinally synthesized organic nitrates such as isosorbide mononitrate, he said.
“Isosorbide mononitrate/dinitrate tablets contain organic nitrates and while they are good for angina, we know that they do not have the same beneficial effects on the sustained generation of nitric oxide as inorganic nitrates,” Dr. Jones added.
NITRATE-CIN study
NITRATE-CIN was a double-blind, randomized, placebo-controlled trial conducted at Queen Mary University of London and St. Bartholomew’s Hospital in London, which tested the effectiveness of inorganic nitrate in preventing contrast-induced nephropathy in 640 patients with non-ST elevation ACS referred for invasive coronary angiography.
To be eligible for the trial, patients had to be at risk of contrast-induced nephropathy with an estimated glomerular filtration rate (eGFR) less than 60 mL/min per 1.73 m2 or have two of the following significant risk factors: diabetes, liver failure, over 70 years of age, exposure to contrast within 7 days, heart failure, or on concomitant renally acting drugs.
Patients were randomly assigned to a formulation of potassium nitrate (12 mmol/744 mg nitrate) per day given as capsules for a 5-day course with the first dose administered prior to angiography or to a control group that received potassium chloride with a matched potassium concentration.
The patient population had a mean age of 71 years, 73% were male, 75% were White, 46% had diabetes, and 56% had chronic kidney disease. There was a 13% loss to follow-up, which was attributed to the COVID pandemic.
The amount of contrast administration was 180 mL in the placebo and 170 mL in the nitrate arm, with 50% of patients undergoing some sort of revascularization.
The primary endpoint was the incidence of CIN as defined by KDIGO criteria – a series of stages of acute kidney injury defined by changes in serum creatinine within 72 hours and up to 1 week.
Results showed that this primary CIN endpoint was reduced significantly from 30% in the placebo arm to 9.1% in the nitrate group, a 70% relative risk reduction (P < .0001). The majority (90%) of this CIN was stage 1, but 10% was stage 2.
Consistent results were seen when an alternative definition of CIN (Mehran) was used, although the rates in both arms were lower than when the KDIGO definition was used.
The benefit was seen across prespecified subgroups including diabetes status, troponin positivity, and Mehran risk. But the benefit seemed to be attenuated in patients on preexisting organic nitrate therapy, although the numbers in these groups were too small to draw definitive conclusions.
As would be expected, there were significant elevations in both systemic nitrate and nitrite levels both up to 72 hours after the procedure, which was consistent with the 5-day course. This was associated with reductions in systolic and diastolic blood pressure, but not associated with any adverse events, Dr. Jones reported.
Rates of procedural MI, a prespecified secondary endpoint, were reduced from 12.5% to 4.1% in those on inorganic nitrates (P = .003).
Looking at longer term outcomes, kidney function was improved at 3 months as measured by change in eGFR, which showed a 10% relative improvement of 5.2 mL/min per 1.73 m2 (10%) in the nitrate group vs. the placebo group. Serum creatinine levels were also significantly increased in the nitrate group.
At 12 months, there was a significant 50% relative reduction in major adverse cardiovascular events – including all-cause mortality, recurrent MI, and recurrent revascularization – which were reduced from 18.1% in the placebo group to 9.1% in the nitrate group, with a reduction in all three of the constituent components of the composite endpoint including all-cause mortality.
Major adverse kidney events (all-cause mortality, renal replacement therapy, or persistent renal dysfunction) were also reduced at 12 months from 28.4% in the placebo group to 10.7% in the nitrate group (P < .0001), a 60% relative reduction. This was driven by lower rates of all-cause mortality and persistent renal dysfunction.
While Dr. Jones said these results on major cardiovascular and kidney outcomes should be viewed as hypothesis-generating at the present time, he said there were biological mechanisms that could explain these benefits.
“We saw a reduction in procedural MI, and we know there is a lot of similar biology in preventing procedural MI and subsequent cardiac events in the acute phase. This, in combination with the large reduction in acute kidney injury, could explain why there’s improved outcomes out to 12 months.”
In her comments, Dr. Mehran congratulated the investigators on having conducted the first study to have shown benefit in the prevention of contrast-associated acute kidney injury as well as major adverse cardiovascular and kidney events associated with the condition.
She used the term “contrast-associated acute kidney injury” rather than “contrast-induced nephropathy” because, she said, it has not been proven that the acute kidney injury seen after angiography is actually caused by the contrast and “so many other things are occurring during procedures when these patients are presenting with different syndromes.”
Dr. Mehran pointed out some weaknesses in the NITRATE-CIN study including the single-center design, the large volume of contrast administered, 13% of patients missing the primary endpoint blood draw, and an imbalance in relevant baseline characteristics despite randomization.
The NITRATE-CIN study was funded by Heart Research UK. Dr. Jones has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AMSTERDAM –
In the NITRATE-CIN Study, non-ST segment elevation myocardial infarction acute coronary syndrome (ACS) patients at risk of renal injury from coronary angiography who received dietary inorganic nitrates had a 70% reduction in CIN compared with those given placebo.
The nitrate group also showed an impressive reduction in periprocedural myocardial infarction (MI) and improved renal function at 3 months, as well as a halving of major adverse cardiovascular events and major adverse kidney events at 1 year.
The trial was presented by Dan Jones, MD, Barts Health NHS Trust, London, at the annual congress of the European Society of Cardiology.
“Currently, aside from intravenous hydration, there is no proven treatment that reduces contrast-induced nephropathy. We feel that dietary inorganic nitrate shows huge promise in this study, and these findings could have important implications in reducing this serious complication of coronary angiography,” Dr. Jones concluded.
He explained that the product used was a formulation of dietary inorganic nitrates given as potassium nitrate capsules, which the study investigators produced specifically for this trial.
At this point, “the only way to get inorganic nitrate is in the diet – specifically by consuming beetroot juice or green leafy vegetables such as spinach and rocket. From a clinician perspective, while these results suggest this is an effective therapy and has great potential, it is not currently possible to prescribe the medication we used in our study, although we are working on producing a commercial product,” he said in an interview.
However, Dr. Jones noted that it is possible to buy beetroot shots, which contain 7 mmol of potassium nitrate in each shot, from health food shops and websites, and two such shots per day for 5 days would give a dose similar to that used in this study, starting the day before angiography.
“While we need a larger multicenter study to confirm these results, studies so far suggest no signal at all that there is any harm in this approach, and there could be a great deal of benefit in taking a couple of beetroot shots prior to and for a few days after an angiogram,” he said.
Dietary nitrates “make sense”
Designated discussant of the NITRATE-CIN trial at the ESC Hotline session, Roxanna Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, said the study was well designed, and the “interesting and plausible” hypothesis to raise nitric oxide levels by dietary nitrates “makes sense.”
On the main findings of a major significant 50% reduction in acute kidney injury, Dr. Mehran said, “It is difficult to imagine such a reduction is possible.”
She pointed out that the large reduction in major adverse cardiac events and major adverse kidney events at 1 year also suggests that there is a sustained benefit in protecting the kidney.
“We’re all going to get on beet juice after this,” she quipped.
Still, Dr. Mehran questioned whether the results were “too good to be true,” adding that a larger trial actually powered for longer term outcome events is needed, as well as a better understanding of whether CIN has a causative role in mortality.
Responding to questions about whether such a large effect could actually be achieved with dietary nitrates, Dr. Jones said he thought there would definitely be some benefits, but maybe not quite as large as those seen in this study.
“From our pilot data we thought nitrate may be effective in preventing CIN,” he said in an interview. “We recruited a higher risk group than we thought, which is why the control event rates were higher than we expected, but the acute kidney injury reduction is roughly what we had estimated, and makes sense biologically.”
Dr. Jones acknowledged that the large reductions in long-term major adverse cardiovascular and kidney events were unexpected.
“The trial was not powered to see reductions in these outcomes, so we need to see if those event reductions can be replicated in larger multicenter trials,” he said. “But this was a double-blind placebo-controlled trial so in this trial the effects are real, and I think the effect size in this trial is too large for there not to be a beneficial effect.
“But I’m not so sure that we would see the same magnitude of effect when we have a larger study with tighter confidence intervals but perhaps a 20%-25% reduction in cardiovascular and kidney may be more realistic, which would still be amazing for such an easy and cost-effective intervention,” Dr. Jones added.
A larger trial is now being planned.
The researchers are also working on the development of a commercial form of dietary inorganic nitrate that would be needed for larger multicenter studies and would then be generally available. “We want this to be a low-cost product that would be available to all,” Dr. Jones said.
He noted that other studies have shown that dietary inorganic nitrates in the form of beetroot juice lower blood pressure; there are suggestions it may also lower cholesterol and prevent stent restenosis, and athletes sometimes take it to increase their aerobic capacity.
“There appears to be many benefits of dietary nitrates, and the one thing we can do at this time is to encourage people to increase their dietary nitrate consumption by eating large quantities of green leafy vegetables and beetroot,” Dr. Jones said.
Replacing lost nitric oxide
In his presentation, Dr. Jones noted that CIN is a serious complication after coronary angiography and is associated with longer hospital stays, worse long-term kidney function, and increased risk of MI and death.
The incidence varies depending on patient risk and definitions used, but it can affect up to 50% of high-risk ACS patients – older patients and/or those with heart failure, chronic kidney disease, or diabetes.
“We don’t really understand the mechanisms that cause CIN, but multiple proposed mechanisms exist, and we know from previous studies that a deficiency of nitric oxide is crucial to the development of CIN,” he explained. “We also know that [nitric oxide] is crucial for normal renal hemostasis. Therefore, a potential therapeutic target to prevent CIN would be to replace this lost nitric oxide.”
The inorganic nitrate evaluated in this trial is found in the diet, is produced endogenously, and is different from medicinally synthesized organic nitrates such as isosorbide mononitrate, he said.
“Isosorbide mononitrate/dinitrate tablets contain organic nitrates and while they are good for angina, we know that they do not have the same beneficial effects on the sustained generation of nitric oxide as inorganic nitrates,” Dr. Jones added.
NITRATE-CIN study
NITRATE-CIN was a double-blind, randomized, placebo-controlled trial conducted at Queen Mary University of London and St. Bartholomew’s Hospital in London, which tested the effectiveness of inorganic nitrate in preventing contrast-induced nephropathy in 640 patients with non-ST elevation ACS referred for invasive coronary angiography.
To be eligible for the trial, patients had to be at risk of contrast-induced nephropathy with an estimated glomerular filtration rate (eGFR) less than 60 mL/min per 1.73 m2 or have two of the following significant risk factors: diabetes, liver failure, over 70 years of age, exposure to contrast within 7 days, heart failure, or on concomitant renally acting drugs.
Patients were randomly assigned to a formulation of potassium nitrate (12 mmol/744 mg nitrate) per day given as capsules for a 5-day course with the first dose administered prior to angiography or to a control group that received potassium chloride with a matched potassium concentration.
The patient population had a mean age of 71 years, 73% were male, 75% were White, 46% had diabetes, and 56% had chronic kidney disease. There was a 13% loss to follow-up, which was attributed to the COVID pandemic.
The amount of contrast administration was 180 mL in the placebo and 170 mL in the nitrate arm, with 50% of patients undergoing some sort of revascularization.
The primary endpoint was the incidence of CIN as defined by KDIGO criteria – a series of stages of acute kidney injury defined by changes in serum creatinine within 72 hours and up to 1 week.
Results showed that this primary CIN endpoint was reduced significantly from 30% in the placebo arm to 9.1% in the nitrate group, a 70% relative risk reduction (P < .0001). The majority (90%) of this CIN was stage 1, but 10% was stage 2.
Consistent results were seen when an alternative definition of CIN (Mehran) was used, although the rates in both arms were lower than when the KDIGO definition was used.
The benefit was seen across prespecified subgroups including diabetes status, troponin positivity, and Mehran risk. But the benefit seemed to be attenuated in patients on preexisting organic nitrate therapy, although the numbers in these groups were too small to draw definitive conclusions.
As would be expected, there were significant elevations in both systemic nitrate and nitrite levels both up to 72 hours after the procedure, which was consistent with the 5-day course. This was associated with reductions in systolic and diastolic blood pressure, but not associated with any adverse events, Dr. Jones reported.
Rates of procedural MI, a prespecified secondary endpoint, were reduced from 12.5% to 4.1% in those on inorganic nitrates (P = .003).
Looking at longer term outcomes, kidney function was improved at 3 months as measured by change in eGFR, which showed a 10% relative improvement of 5.2 mL/min per 1.73 m2 (10%) in the nitrate group vs. the placebo group. Serum creatinine levels were also significantly increased in the nitrate group.
At 12 months, there was a significant 50% relative reduction in major adverse cardiovascular events – including all-cause mortality, recurrent MI, and recurrent revascularization – which were reduced from 18.1% in the placebo group to 9.1% in the nitrate group, with a reduction in all three of the constituent components of the composite endpoint including all-cause mortality.
Major adverse kidney events (all-cause mortality, renal replacement therapy, or persistent renal dysfunction) were also reduced at 12 months from 28.4% in the placebo group to 10.7% in the nitrate group (P < .0001), a 60% relative reduction. This was driven by lower rates of all-cause mortality and persistent renal dysfunction.
While Dr. Jones said these results on major cardiovascular and kidney outcomes should be viewed as hypothesis-generating at the present time, he said there were biological mechanisms that could explain these benefits.
“We saw a reduction in procedural MI, and we know there is a lot of similar biology in preventing procedural MI and subsequent cardiac events in the acute phase. This, in combination with the large reduction in acute kidney injury, could explain why there’s improved outcomes out to 12 months.”
In her comments, Dr. Mehran congratulated the investigators on having conducted the first study to have shown benefit in the prevention of contrast-associated acute kidney injury as well as major adverse cardiovascular and kidney events associated with the condition.
She used the term “contrast-associated acute kidney injury” rather than “contrast-induced nephropathy” because, she said, it has not been proven that the acute kidney injury seen after angiography is actually caused by the contrast and “so many other things are occurring during procedures when these patients are presenting with different syndromes.”
Dr. Mehran pointed out some weaknesses in the NITRATE-CIN study including the single-center design, the large volume of contrast administered, 13% of patients missing the primary endpoint blood draw, and an imbalance in relevant baseline characteristics despite randomization.
The NITRATE-CIN study was funded by Heart Research UK. Dr. Jones has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT THE ESC CONGRESS 2023
Screening finds high rates of CVD in diabetes, COPD patients
AMSTERDAM – , compared with usual care, in a Dutch study involving more than 1,200 people and 25 primary care practices.
Scaling up this program to larger populations could potentially uncover huge numbers of currently unrecognized people with CVD given the large number of adults with type 2 diabetes plus those with COPD, Amy Groenewegen, MD, said at the annual congress of the European Society of Cardiology.
“I think this screening is ready for routine use, but it could be followed by prospective studies that investigate whether it produces more benefits in patient-centered outcomes,” Dr. Groenewegen said in a press briefing. She stressed that it has not yet been clearly proven that patients with these chronic diseases are better off long term when their CVD is detected sooner using the tested approach.
“We need simple ways to identify relevant patients for additional screening and potential treatment” of CVD, commented Lars Kober, MD, designated discussant at the Congress and a cardiologist and professor at Rigshospitalet, Copenhagen University Hospital.
A ‘very simple’ symptom questionnaire
The study is important because it tested a “very simple” symptom questionnaire as the initial screening phase, yet resulted in a CVD diagnostic rate that was two- to threefold higher than in the control patients managed with usual care, Dr. Kober noted.
The Reviving the Early Diagnosis of CVD (RED-CVD) trial randomized 14 primary care practices in the Netherlands to apply a structured screening protocol to adults with type 2 diabetes or COPD, and another 11 practices that served as controls and provided their patients with usual care.
The study included 624 people in the screening arm and 592 in the usual-care arm. Their average age was about 68 years. In the screening arm, 87% had type 2 diabetes and 20% had COPD, including 6.3% with both. In the usual-care arm, 86% had type 2 diabetes, 21% had COPD, with 7.4% having both.
About a quarter of the study cohort had a history of a CVD diagnosis, but they were included for their potential for developing another form of CVD. The study considered three types of CVD: coronary artery disease, heart failure, and atrial fibrillation.
The CVD screening protocol began with an 11-question survey, completed by patients, that asked about their symptoms. The survey was devised by a research team at the University Medical Center Groningen, the Netherlands, who collaborated on the study.
The second phase for people who had suggestive symptoms was a physical examination, measurement of serum N-terminal pro-brain natriuretic peptide (elevated levels signal incident heart failure), and an ECG. People who continued to show findings consistent with CVD in this phase were then referred on a discretionary basis by the attending physician to a specialist.
More than doubling the CVD diagnosis rate
The screening program produced a total of 50 new CVD diagnoses in the screening cohort (8%) and 18 in the control, usual-care arm (3%), for the study’s primary endpoint. The greatest number of events involved heart failure, followed by coronary disease.
The screening questionnaire identified 70% of the people who completed it with suggestive symptoms, such as shortness of breath, claudication, or palpitations. The follow-up assessments of phase two narrowed the group with possible new CVD down to 44% of the people in this arm, and the participating physicians referred 39% to a specialist.
An analysis that adjusted for several demographic and clinical variables and excluded nonobstructive coronary disease as a new CVD diagnosis showed that the systematic screening approach resulted in 2.4-fold more new diagnoses than usual care, reported Dr. Groenewegen, an epidemiologist at University Medical Center Utrecht, the Netherlands.
RED-CVD received no commercial funding. Dr. Groenewegen disclosed no relevant financial relationships. Dr. Kober has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , compared with usual care, in a Dutch study involving more than 1,200 people and 25 primary care practices.
Scaling up this program to larger populations could potentially uncover huge numbers of currently unrecognized people with CVD given the large number of adults with type 2 diabetes plus those with COPD, Amy Groenewegen, MD, said at the annual congress of the European Society of Cardiology.
“I think this screening is ready for routine use, but it could be followed by prospective studies that investigate whether it produces more benefits in patient-centered outcomes,” Dr. Groenewegen said in a press briefing. She stressed that it has not yet been clearly proven that patients with these chronic diseases are better off long term when their CVD is detected sooner using the tested approach.
“We need simple ways to identify relevant patients for additional screening and potential treatment” of CVD, commented Lars Kober, MD, designated discussant at the Congress and a cardiologist and professor at Rigshospitalet, Copenhagen University Hospital.
A ‘very simple’ symptom questionnaire
The study is important because it tested a “very simple” symptom questionnaire as the initial screening phase, yet resulted in a CVD diagnostic rate that was two- to threefold higher than in the control patients managed with usual care, Dr. Kober noted.
The Reviving the Early Diagnosis of CVD (RED-CVD) trial randomized 14 primary care practices in the Netherlands to apply a structured screening protocol to adults with type 2 diabetes or COPD, and another 11 practices that served as controls and provided their patients with usual care.
The study included 624 people in the screening arm and 592 in the usual-care arm. Their average age was about 68 years. In the screening arm, 87% had type 2 diabetes and 20% had COPD, including 6.3% with both. In the usual-care arm, 86% had type 2 diabetes, 21% had COPD, with 7.4% having both.
About a quarter of the study cohort had a history of a CVD diagnosis, but they were included for their potential for developing another form of CVD. The study considered three types of CVD: coronary artery disease, heart failure, and atrial fibrillation.
The CVD screening protocol began with an 11-question survey, completed by patients, that asked about their symptoms. The survey was devised by a research team at the University Medical Center Groningen, the Netherlands, who collaborated on the study.
The second phase for people who had suggestive symptoms was a physical examination, measurement of serum N-terminal pro-brain natriuretic peptide (elevated levels signal incident heart failure), and an ECG. People who continued to show findings consistent with CVD in this phase were then referred on a discretionary basis by the attending physician to a specialist.
More than doubling the CVD diagnosis rate
The screening program produced a total of 50 new CVD diagnoses in the screening cohort (8%) and 18 in the control, usual-care arm (3%), for the study’s primary endpoint. The greatest number of events involved heart failure, followed by coronary disease.
The screening questionnaire identified 70% of the people who completed it with suggestive symptoms, such as shortness of breath, claudication, or palpitations. The follow-up assessments of phase two narrowed the group with possible new CVD down to 44% of the people in this arm, and the participating physicians referred 39% to a specialist.
An analysis that adjusted for several demographic and clinical variables and excluded nonobstructive coronary disease as a new CVD diagnosis showed that the systematic screening approach resulted in 2.4-fold more new diagnoses than usual care, reported Dr. Groenewegen, an epidemiologist at University Medical Center Utrecht, the Netherlands.
RED-CVD received no commercial funding. Dr. Groenewegen disclosed no relevant financial relationships. Dr. Kober has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , compared with usual care, in a Dutch study involving more than 1,200 people and 25 primary care practices.
Scaling up this program to larger populations could potentially uncover huge numbers of currently unrecognized people with CVD given the large number of adults with type 2 diabetes plus those with COPD, Amy Groenewegen, MD, said at the annual congress of the European Society of Cardiology.
“I think this screening is ready for routine use, but it could be followed by prospective studies that investigate whether it produces more benefits in patient-centered outcomes,” Dr. Groenewegen said in a press briefing. She stressed that it has not yet been clearly proven that patients with these chronic diseases are better off long term when their CVD is detected sooner using the tested approach.
“We need simple ways to identify relevant patients for additional screening and potential treatment” of CVD, commented Lars Kober, MD, designated discussant at the Congress and a cardiologist and professor at Rigshospitalet, Copenhagen University Hospital.
A ‘very simple’ symptom questionnaire
The study is important because it tested a “very simple” symptom questionnaire as the initial screening phase, yet resulted in a CVD diagnostic rate that was two- to threefold higher than in the control patients managed with usual care, Dr. Kober noted.
The Reviving the Early Diagnosis of CVD (RED-CVD) trial randomized 14 primary care practices in the Netherlands to apply a structured screening protocol to adults with type 2 diabetes or COPD, and another 11 practices that served as controls and provided their patients with usual care.
The study included 624 people in the screening arm and 592 in the usual-care arm. Their average age was about 68 years. In the screening arm, 87% had type 2 diabetes and 20% had COPD, including 6.3% with both. In the usual-care arm, 86% had type 2 diabetes, 21% had COPD, with 7.4% having both.
About a quarter of the study cohort had a history of a CVD diagnosis, but they were included for their potential for developing another form of CVD. The study considered three types of CVD: coronary artery disease, heart failure, and atrial fibrillation.
The CVD screening protocol began with an 11-question survey, completed by patients, that asked about their symptoms. The survey was devised by a research team at the University Medical Center Groningen, the Netherlands, who collaborated on the study.
The second phase for people who had suggestive symptoms was a physical examination, measurement of serum N-terminal pro-brain natriuretic peptide (elevated levels signal incident heart failure), and an ECG. People who continued to show findings consistent with CVD in this phase were then referred on a discretionary basis by the attending physician to a specialist.
More than doubling the CVD diagnosis rate
The screening program produced a total of 50 new CVD diagnoses in the screening cohort (8%) and 18 in the control, usual-care arm (3%), for the study’s primary endpoint. The greatest number of events involved heart failure, followed by coronary disease.
The screening questionnaire identified 70% of the people who completed it with suggestive symptoms, such as shortness of breath, claudication, or palpitations. The follow-up assessments of phase two narrowed the group with possible new CVD down to 44% of the people in this arm, and the participating physicians referred 39% to a specialist.
An analysis that adjusted for several demographic and clinical variables and excluded nonobstructive coronary disease as a new CVD diagnosis showed that the systematic screening approach resulted in 2.4-fold more new diagnoses than usual care, reported Dr. Groenewegen, an epidemiologist at University Medical Center Utrecht, the Netherlands.
RED-CVD received no commercial funding. Dr. Groenewegen disclosed no relevant financial relationships. Dr. Kober has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
AT ESC CONGRESS 2023
More weight loss linked with more benefit in STEP-HFpEF
AMSTERDAM – , including symptoms and physical limitations, exercise capacity, and inflammation, new analyses from the trial show.
At the annual congress of the European Society of Cardiology where he presented these new findings, Mikhail N. Kosiborod, MD, also posited that weight loss produced by weekly subcutaneous injections of 2.4 mg semaglutide (Wegovy) for 52 weeks in the study does not fully explain the multiple mechanisms that may be involved in producing this intervention’s effects in the STEP-HFpEF trial.
His report earlier at the congress and in a simultaneously published report of the trial’s primary outcomes established a role for medically induced weight loss in managing patients with obesity-phenotype HFpEF in a total of 529 randomized individuals with HFpEF and obesity but without diabetes.
The new analyses showed that for one of the two primary endpoints – the change from baseline in patients’ assessment on the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ), the placebo-adjusted average change was a 16.1-point improvement in the 51 people with a 5%-10% weight loss during the 1-year study, and a 21.6-point improvement in the 58 who had at least a 20% weight loss, a between-group average 5.5 point difference that represents a clinically meaningful incremental improvement in this validated metric of symptoms and functional limitations.
Similar weight-related differences in benefit also occurred for the secondary outcomes of changes from baseline in 6-minute walk distance and in levels of C-reactive protein (CRP), a measure of systemic inflammation.
In an adjusted regression model, every 10% drop from baseline body weight was significantly linked with a 6.4-point improvement in KCCQ score, a 14.4 meter improvement in 6-minute walk distance, and a 28% relative reduction from baseline in CRP, reported Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
These new, prespecified analyses also showed that people with obesity and HFpEF responded roughly the same to semaglutide treatment compared with placebo-treated controls regardless of their starting body mass index, including people with class 1 (30-34 kg/m2), class 2 (35-39 kg/m2), and class 3 (≥ 40 kg/m2) obesity.
Simultaneously with Dr. Kosiborod’s report at the congress, these findings appeared in a report posted online in Nature Medicine.
Not every benefit was fully mediated by weight loss
These analyses “do not tell us how much of the benefit was mediated by weight loss, but the data do say that the more weight a person lost, the more benefit they got,” Dr. Kosiborod explained in an interview. “That is not the same as saying that everything is mediated by weight. It doesn’t say that nothing beyond weight loss matters.”
He and his associates are planning a mediation analysis of data from STEP-HFpEF that will more directly address this issue.
“It’s likely that people who lost more weight with semaglutide also had greater benefits from other effects of semaglutide at the same time. Weight loss is a good surrogate marker” for the range of effects that a person receives from treatment with semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, Dr. Kosiborod said.
“GLP-1 receptor agonists may have direct effects on atherosclerosis, as well as other effects that are uncoupled from weight loss,” such as proven anti-inflammatory effects, he added.
Another exploratory effect from semaglutide treatment in the study and reported by Dr. Kosiborod was a significant reduction in serum levels of N-terminal pro brain natriuretic peptide, an association never previously seen with weight loss in people with heart failure.
“The outcomes we’ve already seen in STEP-HFpEF were largely symptomatic, which are extraordinarily important, but there may be a completely different relationship between weight and clinical events,” said John E. Deanfield, PhD, a professor of cardiology at University College Hospital, London, who was not involved in the study.
Dr. Deanfield noted that important prognostic markers such as cholesterol levels and blood pressure reductions are usually not temporally related to weight loss. “The idea that [the benefits seen in STEP-HFpEF] are purely from weight loss is something we need to be careful about,” he said.
“My gut feeling is that at least 75% of the effect [in STEP-HFpEF} was due to weight loss,” said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow, who was not associated with the research.
STEP-HFpEF was funded by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Kosiborod has been a consultant and adviser to, and has received honoraria from, Novo Nordisk. He has been a consultant to numerous other companies, received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, honoraria from AstraZeneca, and is a stockholder in Artera Health and Saghmos Therapeutics. Dr. Deanfield has been a consultant to Novo Nordisk as well as to Aegerion, Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Sanofi, and Takeda, and has received research funding from Aegerion, Colgate, MSD, Pfizer, and Roche. Dr. Sattar has been a consultant to Novo Nordisk as well as to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Pfizer, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , including symptoms and physical limitations, exercise capacity, and inflammation, new analyses from the trial show.
At the annual congress of the European Society of Cardiology where he presented these new findings, Mikhail N. Kosiborod, MD, also posited that weight loss produced by weekly subcutaneous injections of 2.4 mg semaglutide (Wegovy) for 52 weeks in the study does not fully explain the multiple mechanisms that may be involved in producing this intervention’s effects in the STEP-HFpEF trial.
His report earlier at the congress and in a simultaneously published report of the trial’s primary outcomes established a role for medically induced weight loss in managing patients with obesity-phenotype HFpEF in a total of 529 randomized individuals with HFpEF and obesity but without diabetes.
The new analyses showed that for one of the two primary endpoints – the change from baseline in patients’ assessment on the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ), the placebo-adjusted average change was a 16.1-point improvement in the 51 people with a 5%-10% weight loss during the 1-year study, and a 21.6-point improvement in the 58 who had at least a 20% weight loss, a between-group average 5.5 point difference that represents a clinically meaningful incremental improvement in this validated metric of symptoms and functional limitations.
Similar weight-related differences in benefit also occurred for the secondary outcomes of changes from baseline in 6-minute walk distance and in levels of C-reactive protein (CRP), a measure of systemic inflammation.
In an adjusted regression model, every 10% drop from baseline body weight was significantly linked with a 6.4-point improvement in KCCQ score, a 14.4 meter improvement in 6-minute walk distance, and a 28% relative reduction from baseline in CRP, reported Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
These new, prespecified analyses also showed that people with obesity and HFpEF responded roughly the same to semaglutide treatment compared with placebo-treated controls regardless of their starting body mass index, including people with class 1 (30-34 kg/m2), class 2 (35-39 kg/m2), and class 3 (≥ 40 kg/m2) obesity.
Simultaneously with Dr. Kosiborod’s report at the congress, these findings appeared in a report posted online in Nature Medicine.
Not every benefit was fully mediated by weight loss
These analyses “do not tell us how much of the benefit was mediated by weight loss, but the data do say that the more weight a person lost, the more benefit they got,” Dr. Kosiborod explained in an interview. “That is not the same as saying that everything is mediated by weight. It doesn’t say that nothing beyond weight loss matters.”
He and his associates are planning a mediation analysis of data from STEP-HFpEF that will more directly address this issue.
“It’s likely that people who lost more weight with semaglutide also had greater benefits from other effects of semaglutide at the same time. Weight loss is a good surrogate marker” for the range of effects that a person receives from treatment with semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, Dr. Kosiborod said.
“GLP-1 receptor agonists may have direct effects on atherosclerosis, as well as other effects that are uncoupled from weight loss,” such as proven anti-inflammatory effects, he added.
Another exploratory effect from semaglutide treatment in the study and reported by Dr. Kosiborod was a significant reduction in serum levels of N-terminal pro brain natriuretic peptide, an association never previously seen with weight loss in people with heart failure.
“The outcomes we’ve already seen in STEP-HFpEF were largely symptomatic, which are extraordinarily important, but there may be a completely different relationship between weight and clinical events,” said John E. Deanfield, PhD, a professor of cardiology at University College Hospital, London, who was not involved in the study.
Dr. Deanfield noted that important prognostic markers such as cholesterol levels and blood pressure reductions are usually not temporally related to weight loss. “The idea that [the benefits seen in STEP-HFpEF] are purely from weight loss is something we need to be careful about,” he said.
“My gut feeling is that at least 75% of the effect [in STEP-HFpEF} was due to weight loss,” said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow, who was not associated with the research.
STEP-HFpEF was funded by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Kosiborod has been a consultant and adviser to, and has received honoraria from, Novo Nordisk. He has been a consultant to numerous other companies, received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, honoraria from AstraZeneca, and is a stockholder in Artera Health and Saghmos Therapeutics. Dr. Deanfield has been a consultant to Novo Nordisk as well as to Aegerion, Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Sanofi, and Takeda, and has received research funding from Aegerion, Colgate, MSD, Pfizer, and Roche. Dr. Sattar has been a consultant to Novo Nordisk as well as to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Pfizer, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , including symptoms and physical limitations, exercise capacity, and inflammation, new analyses from the trial show.
At the annual congress of the European Society of Cardiology where he presented these new findings, Mikhail N. Kosiborod, MD, also posited that weight loss produced by weekly subcutaneous injections of 2.4 mg semaglutide (Wegovy) for 52 weeks in the study does not fully explain the multiple mechanisms that may be involved in producing this intervention’s effects in the STEP-HFpEF trial.
His report earlier at the congress and in a simultaneously published report of the trial’s primary outcomes established a role for medically induced weight loss in managing patients with obesity-phenotype HFpEF in a total of 529 randomized individuals with HFpEF and obesity but without diabetes.
The new analyses showed that for one of the two primary endpoints – the change from baseline in patients’ assessment on the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ), the placebo-adjusted average change was a 16.1-point improvement in the 51 people with a 5%-10% weight loss during the 1-year study, and a 21.6-point improvement in the 58 who had at least a 20% weight loss, a between-group average 5.5 point difference that represents a clinically meaningful incremental improvement in this validated metric of symptoms and functional limitations.
Similar weight-related differences in benefit also occurred for the secondary outcomes of changes from baseline in 6-minute walk distance and in levels of C-reactive protein (CRP), a measure of systemic inflammation.
In an adjusted regression model, every 10% drop from baseline body weight was significantly linked with a 6.4-point improvement in KCCQ score, a 14.4 meter improvement in 6-minute walk distance, and a 28% relative reduction from baseline in CRP, reported Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
These new, prespecified analyses also showed that people with obesity and HFpEF responded roughly the same to semaglutide treatment compared with placebo-treated controls regardless of their starting body mass index, including people with class 1 (30-34 kg/m2), class 2 (35-39 kg/m2), and class 3 (≥ 40 kg/m2) obesity.
Simultaneously with Dr. Kosiborod’s report at the congress, these findings appeared in a report posted online in Nature Medicine.
Not every benefit was fully mediated by weight loss
These analyses “do not tell us how much of the benefit was mediated by weight loss, but the data do say that the more weight a person lost, the more benefit they got,” Dr. Kosiborod explained in an interview. “That is not the same as saying that everything is mediated by weight. It doesn’t say that nothing beyond weight loss matters.”
He and his associates are planning a mediation analysis of data from STEP-HFpEF that will more directly address this issue.
“It’s likely that people who lost more weight with semaglutide also had greater benefits from other effects of semaglutide at the same time. Weight loss is a good surrogate marker” for the range of effects that a person receives from treatment with semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, Dr. Kosiborod said.
“GLP-1 receptor agonists may have direct effects on atherosclerosis, as well as other effects that are uncoupled from weight loss,” such as proven anti-inflammatory effects, he added.
Another exploratory effect from semaglutide treatment in the study and reported by Dr. Kosiborod was a significant reduction in serum levels of N-terminal pro brain natriuretic peptide, an association never previously seen with weight loss in people with heart failure.
“The outcomes we’ve already seen in STEP-HFpEF were largely symptomatic, which are extraordinarily important, but there may be a completely different relationship between weight and clinical events,” said John E. Deanfield, PhD, a professor of cardiology at University College Hospital, London, who was not involved in the study.
Dr. Deanfield noted that important prognostic markers such as cholesterol levels and blood pressure reductions are usually not temporally related to weight loss. “The idea that [the benefits seen in STEP-HFpEF] are purely from weight loss is something we need to be careful about,” he said.
“My gut feeling is that at least 75% of the effect [in STEP-HFpEF} was due to weight loss,” said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow, who was not associated with the research.
STEP-HFpEF was funded by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Kosiborod has been a consultant and adviser to, and has received honoraria from, Novo Nordisk. He has been a consultant to numerous other companies, received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, honoraria from AstraZeneca, and is a stockholder in Artera Health and Saghmos Therapeutics. Dr. Deanfield has been a consultant to Novo Nordisk as well as to Aegerion, Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Sanofi, and Takeda, and has received research funding from Aegerion, Colgate, MSD, Pfizer, and Roche. Dr. Sattar has been a consultant to Novo Nordisk as well as to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Pfizer, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
AT THE ESC CONGRESS 2023
IV iron shows only modest benefit in HF: HEART-FID
AMSTERDAM – , but the study failed to meet the specified more rigorous definition of significance (P = .01) on the primary hierarchical composite of death, hospitalizations for heart failure, or 6-minute walk distance.
The trial, which investigated intravenous ferric carboxymaltose treatment vs. placebo, also showed no statistical difference in the main secondary endpoint: time to cardiovascular death or first heart failure hospitalization.
It was hoped that HEART-FID, the largest study to date to look at intravenous iron supplementation in heart failure, would confirm benefits suggested in previous smaller studies, but its modest results seem to have, if anything, caused more uncertainly on whether supplementing iron is actually worthwhile.
The HEART-FID trial was presented at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Another presentation at the ESC Congress reported a pooled meta-analysis of all the intravenous iron supplementation studies, including HEART-FID. This showed a significant reduction in one coprimary endpoint (cardiovascular hospitalization/CV death) but not in the other (heart failure hospitalization/CV death), which is the more traditional and well-recognized endpoint in heart failure trials.
The meta-analysis was also published online in the European Heart Journal.
HEART-FID lead investigator, Robert J. Mentz, MD, Duke University, Durham, N.C., said the totality of the evidence showed clinical benefits of intravenous iron supplementation with intravenous ferric carboxymaltose.
“I worry that people will focus on a P value rather than the actual clinical benefits seen across all the studies,” Dr. Mentz said in an interview. “Technically, this study was neutral in respect to the primary endpoint, but when we look at all the evidence with respect to ferric carboxymaltose, including this new pooled analysis, this does support clinical benefits.”
Comoderator of the ESC Hotline session at which the trial was presented, John McMurray, MD, University of Glasgow (Scotland), thought the trial had “muddied the waters a bit” on the issue of iron supplementation in heart failure.
“I would say we are in a less clear position on iron supplementation now than we were a few months ago. Those clinicians who have believed that checking iron levels and supplementing iron in those who are low is the right thing to do may now be wondering about that,” he told this news organization.
Dr. McMurray noted that initial impressions of the data from both HEART-FID and the meta-analysis suggested some benefit of intravenous iron on CV death/heart failure hospitalization in the first year, but on longer term follow-up, that benefit was less evident.
“We need to look further into why there is that discrepancy,” he said. “This could be a statistical phenomenon or could be something to do with the frequency of redosing over the longer term.”
He explained that several previous studies of intravenous iron supplementation in heart failure have reported apparent convincing benefits on quality of life and functional capacity, but there has been some uncertainty on this because of the difficulty in producing a placebo for intravenous iron.
“So, it would have been great to have some additional confirmation of these benefits and on harder endpoints,” he said, “but even in HEART-FID, there was only a small nonsignificant benefit in walking distance.”
HEART-FID
The HEART-FID trial randomly assigned 3,065 ambulatory patients with heart failure, a left ventricular ejection fraction of 40% or less, and iron deficiency to intravenous ferric carboxymaltose or placebo, given every 6 months as needed on the basis of iron indexes and hemoglobin levels, in addition to standard therapy for heart failure.
The primary outcome was a hierarchical composite of death within 12 months after randomization, hospitalizations for heart failure within 12 months after randomization, or change from baseline to 6 months in the 6-minute walk distance. The significance level was set at .01.
Results showed that death by month 12 occurred in 8.6% of the ferric carboxymaltose group and 10.3% of the placebo group; a total of 297 and 332 hospitalizations for heart failure, respectively, occurred by month 12; and the mean change from baseline to 6 months in the 6-minute walk distance was 8 meters in the ferric carboxymaltose group and 4 meters with placebo. The P value for the primary composite was .02.
The trial also used another method (unmatched win ratio) to analyze the hierarchical composite outcome in the ferric carboxymaltose group as compared with the placebo group that gave a result of 1.10 (99% confidence interval, 0.99-1.23).
During the follow-up period, CV death or hospitalization for heart failure (the main secondary outcome) occurred in 31.0% of the ferric carboxymaltose group and in 32.2% of the placebo group (hazard ratio, 0.93; 96% CI, 0.81-1.06).
Repeated dosing of ferric carboxymaltose appeared to be safe, with an acceptable adverse-event profile in most patients. The number of patients with serious adverse events occurring during the treatment period was similar in the two groups (27.0% in the ferric carboxymaltose group and 26.2% in the placebo group).
‘It’s hard to argue that we are not disappointed’
Designated discussant of the HEART-FID study at the ESC HOTLINE session, Scott Solomon, MD, Brigham and Women’s Hospital, Boston, described HEART-FID as “an extremely important and well-conducted trial.”
He noted that iron deficiency is extremely common in patients with heart failure, affecting at least about a third of patients, and is associated with reduced New York Heart Association class and reduced survival. Previous smaller studies have suggested benefit but have narrowly missed their primary endpoints. HEART-FID was a larger and sufficiently well-powered trial to test the hypothesis that iron supplementation can improve harder clinical endpoints.
Dr. Solomon said that the primary endpoint could be difficult to interpret, with a hierarchical composite, and a win ratio. “But I think it’s fair to say that the results are modest at best,” he added.
“When we look at the traditional cardiovascular death/heart failure hospitalization endpoint, one of the hard endpoints that we care about most in heart failure, it’s hard to argue that we are not disappointed,” he commented.
Referring to the P value of .01 threshold set for significance, which is based on new U.S. Food and Drug Administration regulatory standards, Dr. Solomon noted, “If they had used a standard P = .05 threshold, then they would be able to claim that this trial had met its primary endpoint. But, nevertheless, whatever threshold for significance we look at, the benefit was clearly modest.”
“As with all trials that show modest results, it will be useful to look at subgroups that are most likely to respond to the greatest extent to this therapy, and I look forward to learning more on this from further analyses,” Dr. Solomon concluded.
In an accompanying editorial in the New England Journal of Medicine, Pieter Martens, MD, and Wilfried Mullens, MD, PhD, Ziekenhuis Oost-Limburg, Genk, Belgium, and Hasselt (Belgium) University, point out that analyses from previous trials have suggested that intravenous iron did not have a treatment effect in patients with a transferrin saturation of more than 20%.
They note that, in the ferric carboxymaltose group in the HEART-FID trial, the mean transferrin saturation was 23.9% at baseline, higher than in previous studies.
Future analyses should assess the importance of the transferrin saturation value at baseline, which “could help redefine the definition of iron deficiency in patients with heart failure and, we hope, help clinicians determine which patients might benefit from intravenous iron supplementation,” they write.
Meta-analysis of trials
The meta-analysis of intravenous iron supplementation trials in heart failure was presented by Piotr Ponikowski, MD, Medical University Wroclaw (Poland).
The analysis pooled individual patient data from three double-blind, placebo-controlled trials – CONFIRM-HF 2, AFFIRM-AHF 3, and HEART-FID – giving a total of 4,475 patients, with 2,241 receiving ferric carboxymaltose and 2,234 receiving placebo.
The two prespecified composite primary endpoints were CV hospitalizations/CV death and heart failure hospitalizations/CV death.
These showed similar 13%-14% relative risk reductions with ferric carboxymaltose, but only the former was statistically significant.
Similar results were seen when a fourth trial – IRONMAN (an open-label trial) – was included. In this case, the heart failure hospitalization/CV death endpoint was also nonsignificantly reduced with ferric carboxymaltose (rate ratio, 0.82; 95% CI, 0.58-1.07).
Subgroup analysis suggested that patients with higher transferrin saturation levels appeared to have a lack of treatment effect, whereas those with lower transferrin saturation (< 15%) showed significant treatment benefits.
A higher 6-month cumulative dose of ferric carboxymaltose – likely the result of redosing – may be associated with a slightly greater treatment effect after 6 months, Dr. Ponikowski reported.
He concluded: “These data support the use of intravenous ferric carboxymaltose to treat iron deficiency among patients with heart failure with reduced/mildly reduced LVEF [left ventricular ejection fraction] to reduce the risk of future hospitalization.”
“Our findings support additional research to challenge the current definition of iron deficiency in heart failure as an indication for IV iron therapy and to identify eligibility criteria for optimal redosing strategy,” Dr. Ponikowski added.
Discussant of the meta-analysis presentation at the ESC Hotline session, Pardeep Jhund, MD, University of Glasgow, suggested that the endpoint of most interest would be heart failure hospitalization/CV death in the analysis that included the IRONMAN trial, “which unfortunately did not meet statistical significance.”
In answer to the question “Where does this leave clinicians when treating patients?”Dr. Jhund said, “After yet another meta-analysis, I think the role of IV iron in reducing morbidity and mortality outcomes in heart failure remains questionable.”
“While the absence of evidence is not evidence of absence, the wide confidence intervals of the treatment effect on heart failure hospitalization/CV death leaves a lot of room for doubt about the efficacy of IV iron for reducing HF hospitalizations,” he concluded.
The HEART-FID trial was funded by American Regent, a Daiichi Sankyo Group company. Dr. Mentz reports receiving research support from American Regent and honoraria from American Regent, Vifor, and Pharmacosmos. Dr. Ponikowski reports consultancy fees/honoraria from Vifor Pharma, Boehringer Ingelheim, AstraZeneca, Servier, Novartis, Bayer, MSD, Pfizer, Moderna, Sanofi, and Radcliffe Group.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , but the study failed to meet the specified more rigorous definition of significance (P = .01) on the primary hierarchical composite of death, hospitalizations for heart failure, or 6-minute walk distance.
The trial, which investigated intravenous ferric carboxymaltose treatment vs. placebo, also showed no statistical difference in the main secondary endpoint: time to cardiovascular death or first heart failure hospitalization.
It was hoped that HEART-FID, the largest study to date to look at intravenous iron supplementation in heart failure, would confirm benefits suggested in previous smaller studies, but its modest results seem to have, if anything, caused more uncertainly on whether supplementing iron is actually worthwhile.
The HEART-FID trial was presented at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Another presentation at the ESC Congress reported a pooled meta-analysis of all the intravenous iron supplementation studies, including HEART-FID. This showed a significant reduction in one coprimary endpoint (cardiovascular hospitalization/CV death) but not in the other (heart failure hospitalization/CV death), which is the more traditional and well-recognized endpoint in heart failure trials.
The meta-analysis was also published online in the European Heart Journal.
HEART-FID lead investigator, Robert J. Mentz, MD, Duke University, Durham, N.C., said the totality of the evidence showed clinical benefits of intravenous iron supplementation with intravenous ferric carboxymaltose.
“I worry that people will focus on a P value rather than the actual clinical benefits seen across all the studies,” Dr. Mentz said in an interview. “Technically, this study was neutral in respect to the primary endpoint, but when we look at all the evidence with respect to ferric carboxymaltose, including this new pooled analysis, this does support clinical benefits.”
Comoderator of the ESC Hotline session at which the trial was presented, John McMurray, MD, University of Glasgow (Scotland), thought the trial had “muddied the waters a bit” on the issue of iron supplementation in heart failure.
“I would say we are in a less clear position on iron supplementation now than we were a few months ago. Those clinicians who have believed that checking iron levels and supplementing iron in those who are low is the right thing to do may now be wondering about that,” he told this news organization.
Dr. McMurray noted that initial impressions of the data from both HEART-FID and the meta-analysis suggested some benefit of intravenous iron on CV death/heart failure hospitalization in the first year, but on longer term follow-up, that benefit was less evident.
“We need to look further into why there is that discrepancy,” he said. “This could be a statistical phenomenon or could be something to do with the frequency of redosing over the longer term.”
He explained that several previous studies of intravenous iron supplementation in heart failure have reported apparent convincing benefits on quality of life and functional capacity, but there has been some uncertainty on this because of the difficulty in producing a placebo for intravenous iron.
“So, it would have been great to have some additional confirmation of these benefits and on harder endpoints,” he said, “but even in HEART-FID, there was only a small nonsignificant benefit in walking distance.”
HEART-FID
The HEART-FID trial randomly assigned 3,065 ambulatory patients with heart failure, a left ventricular ejection fraction of 40% or less, and iron deficiency to intravenous ferric carboxymaltose or placebo, given every 6 months as needed on the basis of iron indexes and hemoglobin levels, in addition to standard therapy for heart failure.
The primary outcome was a hierarchical composite of death within 12 months after randomization, hospitalizations for heart failure within 12 months after randomization, or change from baseline to 6 months in the 6-minute walk distance. The significance level was set at .01.
Results showed that death by month 12 occurred in 8.6% of the ferric carboxymaltose group and 10.3% of the placebo group; a total of 297 and 332 hospitalizations for heart failure, respectively, occurred by month 12; and the mean change from baseline to 6 months in the 6-minute walk distance was 8 meters in the ferric carboxymaltose group and 4 meters with placebo. The P value for the primary composite was .02.
The trial also used another method (unmatched win ratio) to analyze the hierarchical composite outcome in the ferric carboxymaltose group as compared with the placebo group that gave a result of 1.10 (99% confidence interval, 0.99-1.23).
During the follow-up period, CV death or hospitalization for heart failure (the main secondary outcome) occurred in 31.0% of the ferric carboxymaltose group and in 32.2% of the placebo group (hazard ratio, 0.93; 96% CI, 0.81-1.06).
Repeated dosing of ferric carboxymaltose appeared to be safe, with an acceptable adverse-event profile in most patients. The number of patients with serious adverse events occurring during the treatment period was similar in the two groups (27.0% in the ferric carboxymaltose group and 26.2% in the placebo group).
‘It’s hard to argue that we are not disappointed’
Designated discussant of the HEART-FID study at the ESC HOTLINE session, Scott Solomon, MD, Brigham and Women’s Hospital, Boston, described HEART-FID as “an extremely important and well-conducted trial.”
He noted that iron deficiency is extremely common in patients with heart failure, affecting at least about a third of patients, and is associated with reduced New York Heart Association class and reduced survival. Previous smaller studies have suggested benefit but have narrowly missed their primary endpoints. HEART-FID was a larger and sufficiently well-powered trial to test the hypothesis that iron supplementation can improve harder clinical endpoints.
Dr. Solomon said that the primary endpoint could be difficult to interpret, with a hierarchical composite, and a win ratio. “But I think it’s fair to say that the results are modest at best,” he added.
“When we look at the traditional cardiovascular death/heart failure hospitalization endpoint, one of the hard endpoints that we care about most in heart failure, it’s hard to argue that we are not disappointed,” he commented.
Referring to the P value of .01 threshold set for significance, which is based on new U.S. Food and Drug Administration regulatory standards, Dr. Solomon noted, “If they had used a standard P = .05 threshold, then they would be able to claim that this trial had met its primary endpoint. But, nevertheless, whatever threshold for significance we look at, the benefit was clearly modest.”
“As with all trials that show modest results, it will be useful to look at subgroups that are most likely to respond to the greatest extent to this therapy, and I look forward to learning more on this from further analyses,” Dr. Solomon concluded.
In an accompanying editorial in the New England Journal of Medicine, Pieter Martens, MD, and Wilfried Mullens, MD, PhD, Ziekenhuis Oost-Limburg, Genk, Belgium, and Hasselt (Belgium) University, point out that analyses from previous trials have suggested that intravenous iron did not have a treatment effect in patients with a transferrin saturation of more than 20%.
They note that, in the ferric carboxymaltose group in the HEART-FID trial, the mean transferrin saturation was 23.9% at baseline, higher than in previous studies.
Future analyses should assess the importance of the transferrin saturation value at baseline, which “could help redefine the definition of iron deficiency in patients with heart failure and, we hope, help clinicians determine which patients might benefit from intravenous iron supplementation,” they write.
Meta-analysis of trials
The meta-analysis of intravenous iron supplementation trials in heart failure was presented by Piotr Ponikowski, MD, Medical University Wroclaw (Poland).
The analysis pooled individual patient data from three double-blind, placebo-controlled trials – CONFIRM-HF 2, AFFIRM-AHF 3, and HEART-FID – giving a total of 4,475 patients, with 2,241 receiving ferric carboxymaltose and 2,234 receiving placebo.
The two prespecified composite primary endpoints were CV hospitalizations/CV death and heart failure hospitalizations/CV death.
These showed similar 13%-14% relative risk reductions with ferric carboxymaltose, but only the former was statistically significant.
Similar results were seen when a fourth trial – IRONMAN (an open-label trial) – was included. In this case, the heart failure hospitalization/CV death endpoint was also nonsignificantly reduced with ferric carboxymaltose (rate ratio, 0.82; 95% CI, 0.58-1.07).
Subgroup analysis suggested that patients with higher transferrin saturation levels appeared to have a lack of treatment effect, whereas those with lower transferrin saturation (< 15%) showed significant treatment benefits.
A higher 6-month cumulative dose of ferric carboxymaltose – likely the result of redosing – may be associated with a slightly greater treatment effect after 6 months, Dr. Ponikowski reported.
He concluded: “These data support the use of intravenous ferric carboxymaltose to treat iron deficiency among patients with heart failure with reduced/mildly reduced LVEF [left ventricular ejection fraction] to reduce the risk of future hospitalization.”
“Our findings support additional research to challenge the current definition of iron deficiency in heart failure as an indication for IV iron therapy and to identify eligibility criteria for optimal redosing strategy,” Dr. Ponikowski added.
Discussant of the meta-analysis presentation at the ESC Hotline session, Pardeep Jhund, MD, University of Glasgow, suggested that the endpoint of most interest would be heart failure hospitalization/CV death in the analysis that included the IRONMAN trial, “which unfortunately did not meet statistical significance.”
In answer to the question “Where does this leave clinicians when treating patients?”Dr. Jhund said, “After yet another meta-analysis, I think the role of IV iron in reducing morbidity and mortality outcomes in heart failure remains questionable.”
“While the absence of evidence is not evidence of absence, the wide confidence intervals of the treatment effect on heart failure hospitalization/CV death leaves a lot of room for doubt about the efficacy of IV iron for reducing HF hospitalizations,” he concluded.
The HEART-FID trial was funded by American Regent, a Daiichi Sankyo Group company. Dr. Mentz reports receiving research support from American Regent and honoraria from American Regent, Vifor, and Pharmacosmos. Dr. Ponikowski reports consultancy fees/honoraria from Vifor Pharma, Boehringer Ingelheim, AstraZeneca, Servier, Novartis, Bayer, MSD, Pfizer, Moderna, Sanofi, and Radcliffe Group.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , but the study failed to meet the specified more rigorous definition of significance (P = .01) on the primary hierarchical composite of death, hospitalizations for heart failure, or 6-minute walk distance.
The trial, which investigated intravenous ferric carboxymaltose treatment vs. placebo, also showed no statistical difference in the main secondary endpoint: time to cardiovascular death or first heart failure hospitalization.
It was hoped that HEART-FID, the largest study to date to look at intravenous iron supplementation in heart failure, would confirm benefits suggested in previous smaller studies, but its modest results seem to have, if anything, caused more uncertainly on whether supplementing iron is actually worthwhile.
The HEART-FID trial was presented at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Another presentation at the ESC Congress reported a pooled meta-analysis of all the intravenous iron supplementation studies, including HEART-FID. This showed a significant reduction in one coprimary endpoint (cardiovascular hospitalization/CV death) but not in the other (heart failure hospitalization/CV death), which is the more traditional and well-recognized endpoint in heart failure trials.
The meta-analysis was also published online in the European Heart Journal.
HEART-FID lead investigator, Robert J. Mentz, MD, Duke University, Durham, N.C., said the totality of the evidence showed clinical benefits of intravenous iron supplementation with intravenous ferric carboxymaltose.
“I worry that people will focus on a P value rather than the actual clinical benefits seen across all the studies,” Dr. Mentz said in an interview. “Technically, this study was neutral in respect to the primary endpoint, but when we look at all the evidence with respect to ferric carboxymaltose, including this new pooled analysis, this does support clinical benefits.”
Comoderator of the ESC Hotline session at which the trial was presented, John McMurray, MD, University of Glasgow (Scotland), thought the trial had “muddied the waters a bit” on the issue of iron supplementation in heart failure.
“I would say we are in a less clear position on iron supplementation now than we were a few months ago. Those clinicians who have believed that checking iron levels and supplementing iron in those who are low is the right thing to do may now be wondering about that,” he told this news organization.
Dr. McMurray noted that initial impressions of the data from both HEART-FID and the meta-analysis suggested some benefit of intravenous iron on CV death/heart failure hospitalization in the first year, but on longer term follow-up, that benefit was less evident.
“We need to look further into why there is that discrepancy,” he said. “This could be a statistical phenomenon or could be something to do with the frequency of redosing over the longer term.”
He explained that several previous studies of intravenous iron supplementation in heart failure have reported apparent convincing benefits on quality of life and functional capacity, but there has been some uncertainty on this because of the difficulty in producing a placebo for intravenous iron.
“So, it would have been great to have some additional confirmation of these benefits and on harder endpoints,” he said, “but even in HEART-FID, there was only a small nonsignificant benefit in walking distance.”
HEART-FID
The HEART-FID trial randomly assigned 3,065 ambulatory patients with heart failure, a left ventricular ejection fraction of 40% or less, and iron deficiency to intravenous ferric carboxymaltose or placebo, given every 6 months as needed on the basis of iron indexes and hemoglobin levels, in addition to standard therapy for heart failure.
The primary outcome was a hierarchical composite of death within 12 months after randomization, hospitalizations for heart failure within 12 months after randomization, or change from baseline to 6 months in the 6-minute walk distance. The significance level was set at .01.
Results showed that death by month 12 occurred in 8.6% of the ferric carboxymaltose group and 10.3% of the placebo group; a total of 297 and 332 hospitalizations for heart failure, respectively, occurred by month 12; and the mean change from baseline to 6 months in the 6-minute walk distance was 8 meters in the ferric carboxymaltose group and 4 meters with placebo. The P value for the primary composite was .02.
The trial also used another method (unmatched win ratio) to analyze the hierarchical composite outcome in the ferric carboxymaltose group as compared with the placebo group that gave a result of 1.10 (99% confidence interval, 0.99-1.23).
During the follow-up period, CV death or hospitalization for heart failure (the main secondary outcome) occurred in 31.0% of the ferric carboxymaltose group and in 32.2% of the placebo group (hazard ratio, 0.93; 96% CI, 0.81-1.06).
Repeated dosing of ferric carboxymaltose appeared to be safe, with an acceptable adverse-event profile in most patients. The number of patients with serious adverse events occurring during the treatment period was similar in the two groups (27.0% in the ferric carboxymaltose group and 26.2% in the placebo group).
‘It’s hard to argue that we are not disappointed’
Designated discussant of the HEART-FID study at the ESC HOTLINE session, Scott Solomon, MD, Brigham and Women’s Hospital, Boston, described HEART-FID as “an extremely important and well-conducted trial.”
He noted that iron deficiency is extremely common in patients with heart failure, affecting at least about a third of patients, and is associated with reduced New York Heart Association class and reduced survival. Previous smaller studies have suggested benefit but have narrowly missed their primary endpoints. HEART-FID was a larger and sufficiently well-powered trial to test the hypothesis that iron supplementation can improve harder clinical endpoints.
Dr. Solomon said that the primary endpoint could be difficult to interpret, with a hierarchical composite, and a win ratio. “But I think it’s fair to say that the results are modest at best,” he added.
“When we look at the traditional cardiovascular death/heart failure hospitalization endpoint, one of the hard endpoints that we care about most in heart failure, it’s hard to argue that we are not disappointed,” he commented.
Referring to the P value of .01 threshold set for significance, which is based on new U.S. Food and Drug Administration regulatory standards, Dr. Solomon noted, “If they had used a standard P = .05 threshold, then they would be able to claim that this trial had met its primary endpoint. But, nevertheless, whatever threshold for significance we look at, the benefit was clearly modest.”
“As with all trials that show modest results, it will be useful to look at subgroups that are most likely to respond to the greatest extent to this therapy, and I look forward to learning more on this from further analyses,” Dr. Solomon concluded.
In an accompanying editorial in the New England Journal of Medicine, Pieter Martens, MD, and Wilfried Mullens, MD, PhD, Ziekenhuis Oost-Limburg, Genk, Belgium, and Hasselt (Belgium) University, point out that analyses from previous trials have suggested that intravenous iron did not have a treatment effect in patients with a transferrin saturation of more than 20%.
They note that, in the ferric carboxymaltose group in the HEART-FID trial, the mean transferrin saturation was 23.9% at baseline, higher than in previous studies.
Future analyses should assess the importance of the transferrin saturation value at baseline, which “could help redefine the definition of iron deficiency in patients with heart failure and, we hope, help clinicians determine which patients might benefit from intravenous iron supplementation,” they write.
Meta-analysis of trials
The meta-analysis of intravenous iron supplementation trials in heart failure was presented by Piotr Ponikowski, MD, Medical University Wroclaw (Poland).
The analysis pooled individual patient data from three double-blind, placebo-controlled trials – CONFIRM-HF 2, AFFIRM-AHF 3, and HEART-FID – giving a total of 4,475 patients, with 2,241 receiving ferric carboxymaltose and 2,234 receiving placebo.
The two prespecified composite primary endpoints were CV hospitalizations/CV death and heart failure hospitalizations/CV death.
These showed similar 13%-14% relative risk reductions with ferric carboxymaltose, but only the former was statistically significant.
Similar results were seen when a fourth trial – IRONMAN (an open-label trial) – was included. In this case, the heart failure hospitalization/CV death endpoint was also nonsignificantly reduced with ferric carboxymaltose (rate ratio, 0.82; 95% CI, 0.58-1.07).
Subgroup analysis suggested that patients with higher transferrin saturation levels appeared to have a lack of treatment effect, whereas those with lower transferrin saturation (< 15%) showed significant treatment benefits.
A higher 6-month cumulative dose of ferric carboxymaltose – likely the result of redosing – may be associated with a slightly greater treatment effect after 6 months, Dr. Ponikowski reported.
He concluded: “These data support the use of intravenous ferric carboxymaltose to treat iron deficiency among patients with heart failure with reduced/mildly reduced LVEF [left ventricular ejection fraction] to reduce the risk of future hospitalization.”
“Our findings support additional research to challenge the current definition of iron deficiency in heart failure as an indication for IV iron therapy and to identify eligibility criteria for optimal redosing strategy,” Dr. Ponikowski added.
Discussant of the meta-analysis presentation at the ESC Hotline session, Pardeep Jhund, MD, University of Glasgow, suggested that the endpoint of most interest would be heart failure hospitalization/CV death in the analysis that included the IRONMAN trial, “which unfortunately did not meet statistical significance.”
In answer to the question “Where does this leave clinicians when treating patients?”Dr. Jhund said, “After yet another meta-analysis, I think the role of IV iron in reducing morbidity and mortality outcomes in heart failure remains questionable.”
“While the absence of evidence is not evidence of absence, the wide confidence intervals of the treatment effect on heart failure hospitalization/CV death leaves a lot of room for doubt about the efficacy of IV iron for reducing HF hospitalizations,” he concluded.
The HEART-FID trial was funded by American Regent, a Daiichi Sankyo Group company. Dr. Mentz reports receiving research support from American Regent and honoraria from American Regent, Vifor, and Pharmacosmos. Dr. Ponikowski reports consultancy fees/honoraria from Vifor Pharma, Boehringer Ingelheim, AstraZeneca, Servier, Novartis, Bayer, MSD, Pfizer, Moderna, Sanofi, and Radcliffe Group.
A version of this article first appeared on Medscape.com.
AT THE ESC CONGRESS 2023
Traditional Chinese medicine improves outcomes in HFrEF
When added to guideline-directed therapies for heart failure with reduced ejection fraction (HFrEF), a traditional Chinese medicine called qiliqiangxin reduced the composite endpoint of cardiovascular death and heart failure hospitalization by more than 20%, results of a large placebo-controlled trial show.
reported Xinli Li, MD, PhD, First Affiliated Hospital, Nanjing Medical University, China.
Qiliqiangxin, a commonly used therapy in China for cardiovascular disease, is not a single chemical entity but a treatment composed of 11 plant-based substances that together are associated with diuretic effects, vasodilation, and “cardiotonic” activity, Dr. Li said. He also cited studies showing an upregulation effect on peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC1-beta).
The results were presented at the annual congress of the European Society of Cardiology.
Hard endpoints pursued in rigorous design
There have been numerous studies of qiliqiangxin for cardiovascular diseases, including a double-blind study that associated this agent with a greater than 30% reduction in the surrogate endpoint of N-terminal pro–B-type natriuretic peptide (NT-proBNP).
In the newly completed multicenter trial, called QUEST, the goal was to determine whether this therapy could reduce hard endpoints relative to placebo in a rigorously conducted trial enrolling patients receiving an optimized triple-therapy heart failure regimen.
Few patients in the study received a sodium glucose cotransporter-2 (SGLT-2 inhibitor), which was not a standard at the time the study was designed but is now part of the quadruple guideline-directed medical therapy in most European and North American guidelines.
In this trial, 3,119 patients were randomly assigned at 133 centers in China to take four capsules of qiliqiangxin or placebo three times per day. At a median follow-up of 18.3 months, outcomes were evaluable in nearly all 1,561 patients randomly assigned to the experimental therapy and 1,555 patients randomly assigned to placebo.
The key inclusion criteria were a left ventricular ejection fraction of 40% or less and a serum NT-proBNP level of at least 450 pg/mL. Patients in New York Heart Association class IV heart failure were excluded.
At enrollment, more than 80% of patients in both arms were receiving a renin-angiotensin system (RAS) inhibitor (angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or angiotensin receptor neprilysin inhibitor), more than 80% were receiving a mineralocorticoid receptor antagonist, and more than 85% were receiving a beta-blocker.
Death and hospitalization reduced 22%
By hazard ratio, the primary composite endpoint of CV death and heart failure hospitalization was reduced by 22% relative to placebo (HR, 0.78; P < .001). When evaluated separately, the relative reductions in these respective endpoints were 17% (HR, 0.83; P = .045) and 24% (HR, 0.76; P = .002).
The risk reduction was robust (HR, 0.76; P < .001) in patients with an ischemic cause but nonsignificant in those without (HR, 0.92; P = .575). A significant benefit was sustained in patients receiving an angiotensin receptor neprilysin inhibitor (HR, 0.84; P = .041), as well as those who did not receive this class of drug (HR, 0.77; P = .012).
However, the benefit of qiliqiangxin among patients receiving all components of guideline-directed triple therapy (RAS inhibitor, beta-blocker, and mineralocorticoid antagonist) was only a trend (HR, 0.86; P = .079).
All-cause mortality, a secondary endpoint, was lower among patients randomly assigned to qiliqiangxin than to those assigned to placebo, but this difference fell just short of statistical significance (14.21% vs. 16.85%; P = .058).
Qiliqiangxin was well tolerated. The proportion of patients with a serious adverse event was numerically lower with qiliqiangxin than with placebo (17.43% vs. 19.74%), whereas discontinuations associated with an adverse event were numerically higher in the qiliqiangxin group (1.03% vs. 0.58%), albeit still very low in both study arms.
Overlap of drug benefits suspected
Given the safety of this drug and its highly significant reduction in a composite endpoint used in other major HFrEF trials, the ESC-invited discussant, Carolyn S.P. Lam, MBBS, PhD, National Heart Centre, Singapore, called the outcome “remarkable” and a validation for “the millions of people” who are already taking qiliqiangxin in China and other Asian countries.
Using the DAPA-HF trial as a point of reference, Dr. Lam noted that relative reduction in the composite endpoint of cardiovascular death for the SGLT-2 inhibitor dapagliflozin relative to placebo on top of triple guideline-directed medical therapy was lower (17% vs. 24%), but there were significant reductions in each of the components, as well as a nonsignificant signal of a mortality benefit.
However, Dr. Lam pointed out that there does seem to be more of an overlap for the benefits of qiliqiangxin than dapagliflozin relative to other components of triple therapy based on the lower rate of benefit when patients were optimized on triple therapy.
“The subgroup analysis [of this study] is very important,” Dr. Lam said. Qiliqiangxin may be best in patients who cannot take one or more of the components of triple therapy, she suggested, even though she called for further studies to test this theory. She also cautioned that the pill burden of four capsules taken three times per day might be onerous for some patients.
Of the many questions still to be answered, Dr. Lam noted that the low rate of enrollment for patients (< 10%) taking SGLT-2 inhibitors makes the contribution of qiliqiangxin unclear among those receiving the current standard of quadruple guideline-directed medical therapy.
She also suggested that it will be important to dissect the relative contribution of the different active ingredients of qiliqiangxin.
“This is not a purified compound that we are used to in Western medicine,” Dr. Lam said. While she praised the study as “scientifically rigorous” and indicated that the results support a clinical benefit from qiliqiangxin, she thinks an exploration of the mechanism or mechanisms of benefit is a next step in understanding where this therapy fits in HFrEF management.
Dr. Li reports financial relationships with AstraZeneca, Bayer, Novartis, Roche, and Yiling. Dr. Lam reports financial relationships with more than 25 pharmaceutical or device manufacturers, many of which produce therapies for heart failure, as well as with Medscape/WebMD Global LLC. The study was supported by the Chinese National Key Research and Development Project and Yiling Pharmaceuticals.
A version of this article appeared on Medscape.com.
When added to guideline-directed therapies for heart failure with reduced ejection fraction (HFrEF), a traditional Chinese medicine called qiliqiangxin reduced the composite endpoint of cardiovascular death and heart failure hospitalization by more than 20%, results of a large placebo-controlled trial show.
reported Xinli Li, MD, PhD, First Affiliated Hospital, Nanjing Medical University, China.
Qiliqiangxin, a commonly used therapy in China for cardiovascular disease, is not a single chemical entity but a treatment composed of 11 plant-based substances that together are associated with diuretic effects, vasodilation, and “cardiotonic” activity, Dr. Li said. He also cited studies showing an upregulation effect on peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC1-beta).
The results were presented at the annual congress of the European Society of Cardiology.
Hard endpoints pursued in rigorous design
There have been numerous studies of qiliqiangxin for cardiovascular diseases, including a double-blind study that associated this agent with a greater than 30% reduction in the surrogate endpoint of N-terminal pro–B-type natriuretic peptide (NT-proBNP).
In the newly completed multicenter trial, called QUEST, the goal was to determine whether this therapy could reduce hard endpoints relative to placebo in a rigorously conducted trial enrolling patients receiving an optimized triple-therapy heart failure regimen.
Few patients in the study received a sodium glucose cotransporter-2 (SGLT-2 inhibitor), which was not a standard at the time the study was designed but is now part of the quadruple guideline-directed medical therapy in most European and North American guidelines.
In this trial, 3,119 patients were randomly assigned at 133 centers in China to take four capsules of qiliqiangxin or placebo three times per day. At a median follow-up of 18.3 months, outcomes were evaluable in nearly all 1,561 patients randomly assigned to the experimental therapy and 1,555 patients randomly assigned to placebo.
The key inclusion criteria were a left ventricular ejection fraction of 40% or less and a serum NT-proBNP level of at least 450 pg/mL. Patients in New York Heart Association class IV heart failure were excluded.
At enrollment, more than 80% of patients in both arms were receiving a renin-angiotensin system (RAS) inhibitor (angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or angiotensin receptor neprilysin inhibitor), more than 80% were receiving a mineralocorticoid receptor antagonist, and more than 85% were receiving a beta-blocker.
Death and hospitalization reduced 22%
By hazard ratio, the primary composite endpoint of CV death and heart failure hospitalization was reduced by 22% relative to placebo (HR, 0.78; P < .001). When evaluated separately, the relative reductions in these respective endpoints were 17% (HR, 0.83; P = .045) and 24% (HR, 0.76; P = .002).
The risk reduction was robust (HR, 0.76; P < .001) in patients with an ischemic cause but nonsignificant in those without (HR, 0.92; P = .575). A significant benefit was sustained in patients receiving an angiotensin receptor neprilysin inhibitor (HR, 0.84; P = .041), as well as those who did not receive this class of drug (HR, 0.77; P = .012).
However, the benefit of qiliqiangxin among patients receiving all components of guideline-directed triple therapy (RAS inhibitor, beta-blocker, and mineralocorticoid antagonist) was only a trend (HR, 0.86; P = .079).
All-cause mortality, a secondary endpoint, was lower among patients randomly assigned to qiliqiangxin than to those assigned to placebo, but this difference fell just short of statistical significance (14.21% vs. 16.85%; P = .058).
Qiliqiangxin was well tolerated. The proportion of patients with a serious adverse event was numerically lower with qiliqiangxin than with placebo (17.43% vs. 19.74%), whereas discontinuations associated with an adverse event were numerically higher in the qiliqiangxin group (1.03% vs. 0.58%), albeit still very low in both study arms.
Overlap of drug benefits suspected
Given the safety of this drug and its highly significant reduction in a composite endpoint used in other major HFrEF trials, the ESC-invited discussant, Carolyn S.P. Lam, MBBS, PhD, National Heart Centre, Singapore, called the outcome “remarkable” and a validation for “the millions of people” who are already taking qiliqiangxin in China and other Asian countries.
Using the DAPA-HF trial as a point of reference, Dr. Lam noted that relative reduction in the composite endpoint of cardiovascular death for the SGLT-2 inhibitor dapagliflozin relative to placebo on top of triple guideline-directed medical therapy was lower (17% vs. 24%), but there were significant reductions in each of the components, as well as a nonsignificant signal of a mortality benefit.
However, Dr. Lam pointed out that there does seem to be more of an overlap for the benefits of qiliqiangxin than dapagliflozin relative to other components of triple therapy based on the lower rate of benefit when patients were optimized on triple therapy.
“The subgroup analysis [of this study] is very important,” Dr. Lam said. Qiliqiangxin may be best in patients who cannot take one or more of the components of triple therapy, she suggested, even though she called for further studies to test this theory. She also cautioned that the pill burden of four capsules taken three times per day might be onerous for some patients.
Of the many questions still to be answered, Dr. Lam noted that the low rate of enrollment for patients (< 10%) taking SGLT-2 inhibitors makes the contribution of qiliqiangxin unclear among those receiving the current standard of quadruple guideline-directed medical therapy.
She also suggested that it will be important to dissect the relative contribution of the different active ingredients of qiliqiangxin.
“This is not a purified compound that we are used to in Western medicine,” Dr. Lam said. While she praised the study as “scientifically rigorous” and indicated that the results support a clinical benefit from qiliqiangxin, she thinks an exploration of the mechanism or mechanisms of benefit is a next step in understanding where this therapy fits in HFrEF management.
Dr. Li reports financial relationships with AstraZeneca, Bayer, Novartis, Roche, and Yiling. Dr. Lam reports financial relationships with more than 25 pharmaceutical or device manufacturers, many of which produce therapies for heart failure, as well as with Medscape/WebMD Global LLC. The study was supported by the Chinese National Key Research and Development Project and Yiling Pharmaceuticals.
A version of this article appeared on Medscape.com.
When added to guideline-directed therapies for heart failure with reduced ejection fraction (HFrEF), a traditional Chinese medicine called qiliqiangxin reduced the composite endpoint of cardiovascular death and heart failure hospitalization by more than 20%, results of a large placebo-controlled trial show.
reported Xinli Li, MD, PhD, First Affiliated Hospital, Nanjing Medical University, China.
Qiliqiangxin, a commonly used therapy in China for cardiovascular disease, is not a single chemical entity but a treatment composed of 11 plant-based substances that together are associated with diuretic effects, vasodilation, and “cardiotonic” activity, Dr. Li said. He also cited studies showing an upregulation effect on peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC1-beta).
The results were presented at the annual congress of the European Society of Cardiology.
Hard endpoints pursued in rigorous design
There have been numerous studies of qiliqiangxin for cardiovascular diseases, including a double-blind study that associated this agent with a greater than 30% reduction in the surrogate endpoint of N-terminal pro–B-type natriuretic peptide (NT-proBNP).
In the newly completed multicenter trial, called QUEST, the goal was to determine whether this therapy could reduce hard endpoints relative to placebo in a rigorously conducted trial enrolling patients receiving an optimized triple-therapy heart failure regimen.
Few patients in the study received a sodium glucose cotransporter-2 (SGLT-2 inhibitor), which was not a standard at the time the study was designed but is now part of the quadruple guideline-directed medical therapy in most European and North American guidelines.
In this trial, 3,119 patients were randomly assigned at 133 centers in China to take four capsules of qiliqiangxin or placebo three times per day. At a median follow-up of 18.3 months, outcomes were evaluable in nearly all 1,561 patients randomly assigned to the experimental therapy and 1,555 patients randomly assigned to placebo.
The key inclusion criteria were a left ventricular ejection fraction of 40% or less and a serum NT-proBNP level of at least 450 pg/mL. Patients in New York Heart Association class IV heart failure were excluded.
At enrollment, more than 80% of patients in both arms were receiving a renin-angiotensin system (RAS) inhibitor (angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or angiotensin receptor neprilysin inhibitor), more than 80% were receiving a mineralocorticoid receptor antagonist, and more than 85% were receiving a beta-blocker.
Death and hospitalization reduced 22%
By hazard ratio, the primary composite endpoint of CV death and heart failure hospitalization was reduced by 22% relative to placebo (HR, 0.78; P < .001). When evaluated separately, the relative reductions in these respective endpoints were 17% (HR, 0.83; P = .045) and 24% (HR, 0.76; P = .002).
The risk reduction was robust (HR, 0.76; P < .001) in patients with an ischemic cause but nonsignificant in those without (HR, 0.92; P = .575). A significant benefit was sustained in patients receiving an angiotensin receptor neprilysin inhibitor (HR, 0.84; P = .041), as well as those who did not receive this class of drug (HR, 0.77; P = .012).
However, the benefit of qiliqiangxin among patients receiving all components of guideline-directed triple therapy (RAS inhibitor, beta-blocker, and mineralocorticoid antagonist) was only a trend (HR, 0.86; P = .079).
All-cause mortality, a secondary endpoint, was lower among patients randomly assigned to qiliqiangxin than to those assigned to placebo, but this difference fell just short of statistical significance (14.21% vs. 16.85%; P = .058).
Qiliqiangxin was well tolerated. The proportion of patients with a serious adverse event was numerically lower with qiliqiangxin than with placebo (17.43% vs. 19.74%), whereas discontinuations associated with an adverse event were numerically higher in the qiliqiangxin group (1.03% vs. 0.58%), albeit still very low in both study arms.
Overlap of drug benefits suspected
Given the safety of this drug and its highly significant reduction in a composite endpoint used in other major HFrEF trials, the ESC-invited discussant, Carolyn S.P. Lam, MBBS, PhD, National Heart Centre, Singapore, called the outcome “remarkable” and a validation for “the millions of people” who are already taking qiliqiangxin in China and other Asian countries.
Using the DAPA-HF trial as a point of reference, Dr. Lam noted that relative reduction in the composite endpoint of cardiovascular death for the SGLT-2 inhibitor dapagliflozin relative to placebo on top of triple guideline-directed medical therapy was lower (17% vs. 24%), but there were significant reductions in each of the components, as well as a nonsignificant signal of a mortality benefit.
However, Dr. Lam pointed out that there does seem to be more of an overlap for the benefits of qiliqiangxin than dapagliflozin relative to other components of triple therapy based on the lower rate of benefit when patients were optimized on triple therapy.
“The subgroup analysis [of this study] is very important,” Dr. Lam said. Qiliqiangxin may be best in patients who cannot take one or more of the components of triple therapy, she suggested, even though she called for further studies to test this theory. She also cautioned that the pill burden of four capsules taken three times per day might be onerous for some patients.
Of the many questions still to be answered, Dr. Lam noted that the low rate of enrollment for patients (< 10%) taking SGLT-2 inhibitors makes the contribution of qiliqiangxin unclear among those receiving the current standard of quadruple guideline-directed medical therapy.
She also suggested that it will be important to dissect the relative contribution of the different active ingredients of qiliqiangxin.
“This is not a purified compound that we are used to in Western medicine,” Dr. Lam said. While she praised the study as “scientifically rigorous” and indicated that the results support a clinical benefit from qiliqiangxin, she thinks an exploration of the mechanism or mechanisms of benefit is a next step in understanding where this therapy fits in HFrEF management.
Dr. Li reports financial relationships with AstraZeneca, Bayer, Novartis, Roche, and Yiling. Dr. Lam reports financial relationships with more than 25 pharmaceutical or device manufacturers, many of which produce therapies for heart failure, as well as with Medscape/WebMD Global LLC. The study was supported by the Chinese National Key Research and Development Project and Yiling Pharmaceuticals.
A version of this article appeared on Medscape.com.
FROM ESC CONGRESS 2023
Anticoagulation no benefit in presumed AFib detected by cardiac devices
AMSTERDAM – Among patients with atrial high-rate episodes detected by implantable devices, anticoagulation with edoxaban did not significantly reduce the incidence of a composite outcome of cardiovascular death, stroke, or systemic embolism in comparison with placebo but was associated with a higher bleeding risk in the NOAH-AFNET 6 trial.
“ They do not need to be anticoagulated. That is a relief,” the lead investigator of the trial, Paulus Kirchhof, MD, University Heart and Vascular Center Hamburg (Germany), said in an interview.
Dr. Kirchhof pointed out that this result was unexpected. “Many of us thought that because atrial high-rate episodes look like AF[ib] when they occur, then they are an indication for anticoagulation. But based on these results from the first-ever randomized trial on this population, there is no need for anticoagulation in these patients.”
Dr. Kirchhof presented the NOAH-AFNET 6 trial at the annual congress of the European Society of Cardiology. The study was also simultaneously published in the New England Journal of Medicine.
The trial recruited patients with implanted devices that enable continuous monitoring of atrial rhythm, such as pacemakers and defibrillators. “Because we can record the rhythm day and night with these devices, they pick up small abnormalities. About 20% of these patients experience these occasional atrial high-rate episodes – short episodes that look like AF[ib], but they are rare and brief,” Dr. Kirchhof noted.
He explained that whether the occurrence of these atrial high-rate episodes in patients without AFib, as documented on a conventional electrocardiogram, justifies the initiation of anticoagulants has been unclear. “But this trial tells us that these episodes are different to AF[ib] that is diagnosed on ECG,” he added.
Another finding in the trial was that among these patients, there was an unexpectedly low rate of stroke, despite the patients’ having a CHADSVASC score of 4.
“Based on the result of this trial, these occasional atrial high-rate episodes do not appear to be associated with stroke. It appears quite benign,” Dr. Kirchhof said.
Implications for wearable technology?
He said the results may also have implications for wearable devices that pick up abnormal heart rhythm, such as smartwatches.
“We don’t know exactly what these wearable technologies are picking up, but most likely it is these atrial high-rate episodes. But we need more research on the value of these wearable technologies; we need randomized trials in this particular patient population before we consider anticoagulation in these patients,” Dr. Kirchhof stated.
The NOAH-AFNET 6 study was an event-driven, double-blind, double-dummy, randomized trial involving 2,536 patients aged 65 years or older who had atrial high-rate episodes that lasted for at least 6 minutes and who had at least one additional risk factor for stroke.
Patients were randomly assigned in a 1:1 ratio to receive edoxaban or placebo. The primary efficacy outcome was a composite of cardiovascular death, stroke, or systemic embolism, evaluated in a time-to-event analysis. The safety outcome was a composite of death from any cause or major bleeding.
The mean age of the patients was 78 years, 37.4% were women, and the median duration of atrial high-rate episodes was 2.8 hours. The trial was terminated early, at a median follow-up of 21 months, on the basis of safety concerns and the results of an independent, informal assessment of futility for the efficacy of edoxaban; at termination, the planned enrollment had been completed.
Results showed that a primary efficacy outcome event occurred in 83 patients (3.2% per patient-year) in the edoxaban group and in 101 patients (4.0% per patient-year) in the placebo group (hazard ratio, 0.81; 95% confidence interval, 0.60-1.08; P = .15). The incidence of stroke was approximately 1% per patient-year in both groups.
A safety outcome event occurred in 149 patients (5.9% per patient-year) in the edoxaban group and in 114 patients (4.5% per patient-year) in the placebo group (HR, 1.31; 95% CI, 1.02-1.67; P = .03).
ECG-diagnosed AFib developed in 462 of 2,536 patients (18.2% total, 8.7% per patient-year).
In the NEJM article, the authors wrote that the findings of this trial – the low incidence of stroke that was not further reduced by treatment with edoxaban – may make it appropriate to withhold anticoagulant therapy for patients with atrial high-rate episodes.
The main difference between the population studied in this trial and patients with AFib, as documented on an ECG, appears to be the paucity and brevity of atrial arrhythmias in patients with atrial high-rate episodes (termed low arrhythmia burden). Published reports show that a low arrhythmia burden contributes to a low incidence of stroke among patients with AFib, the study authors wrote.
They added that the low rate of stroke in this trial suggests that in addition to clinical risk prediction formulas for stroke, methods to improve the estimation of stroke risk among patients with infrequent atrial arrhythmias detected by long-term monitoring are needed to guide decision-making on the use of anticoagulation.
Commenting on the NOAH-AFNET 6 results, the comoderator of the ESC HOTLINE session at which they were presented, Barbara Casadei, MD, John Radcliffe Hospital, Oxford, England, said: “Finally we know what to with these patients. Before we just had a variety of opinions with no evidence. I think that the trial really highlights that patients who come to the doctor with symptoms of AF[ib] or who have ECG-documented AF[ib] have a much higher risk of cardioembolic stroke than patients in whom this presumed AF[ib] is picked up incidentally from implanted devices.”
She added: “The stroke rates are very low in this trial, so anticoagulation was never going to work. But this is an important finding. We know that anticoagulants are not a free lunch. There is a significant bleeding risk. These results suggest that unless a patient has clinical AF[ib] that shows up on an ECG then we need to more cautious in prescribing anticoagulation.”
Also commenting on the study, immediate past president of the American College of Cardiology Ed Fry, MD, Ascension Indiana St. Vincent Heart Center, Indianapolis, said the management of patients with implanted cardiac devices or personal wearable technology that has picked up an abnormal rhythm suggestive of AFib was a big question in clinical practice.
“These episodes could be AF[ib], which comes with an increased stroke risk, but it could also be something else like atrial tachycardia or supraventricular tachycardia, which do not confer an increased stroke risk,” he explained.
“This study shows that without a firm diagnosis of AF[ib] on an ECG or some sort of continuous AF[ib] monitoring device, we are going to be anticoagulating people who don’t need it. They were exposed to the risk of bleeding without getting the benefit of a reduction in stroke risk,” Dr. Fry noted.
“The important outcome from this trial is that it gives comfort in we can be more confident in withholding anticoagulation until we get a firm diagnosis of AF[ib]. If we have a high index of suspicion that this could be AF[ib], then we can arrange for a further testing,” he added.
Second trial reporting soon
A trial similar to NOAH-AFNET 6 is currently underway – the ARTESIA trial, which is expected to be reported later in 2023.
“We are in close contact with the leadership of that trial, and we hope to do some meta-analysis,” Dr. Kirchhof said. “But I think today we’ve gone from no evidence to one outcome-based trial which shows there is no reason to use anticoagulation in these patients with atrial high-rate episodes. I think this is reason to change practice now, but yes, of course we need to look at the data in totality once the second trial has reported.”
But the lead investigator of the ARTESIA trial, Stuart Connolly, MD, McMaster University, Hamilton, Ont., does not believe the NOAH-AFNET 6 trial should change practice at this time.
“This trial fails to adequately address the critical issue that drives clinical decision-making in these patients because it is underpowered for the most important endpoint of stroke,” he said in an interview.
“The key question is whether anticoagulation reduces stroke in these patients,” he added. “To answer that, a clinical trial needs to have a lot of strokes, and this trial had very few. The trial was stopped early and had way too few strokes to properly answer this key question.”
The NOAH-AFNET 6 trial was an investigator-initiated trial funded by the German Center for Cardiovascular Research and Daiichi Sankyo Europe. Dr. Kirchhof reported research support from several drug and device companies active in AFib. He is also listed as an inventor on two patents held by the University of Hamburg on AFib therapy and AFib markers.
A version of this article first appeared on Medscape.com.
AMSTERDAM – Among patients with atrial high-rate episodes detected by implantable devices, anticoagulation with edoxaban did not significantly reduce the incidence of a composite outcome of cardiovascular death, stroke, or systemic embolism in comparison with placebo but was associated with a higher bleeding risk in the NOAH-AFNET 6 trial.
“ They do not need to be anticoagulated. That is a relief,” the lead investigator of the trial, Paulus Kirchhof, MD, University Heart and Vascular Center Hamburg (Germany), said in an interview.
Dr. Kirchhof pointed out that this result was unexpected. “Many of us thought that because atrial high-rate episodes look like AF[ib] when they occur, then they are an indication for anticoagulation. But based on these results from the first-ever randomized trial on this population, there is no need for anticoagulation in these patients.”
Dr. Kirchhof presented the NOAH-AFNET 6 trial at the annual congress of the European Society of Cardiology. The study was also simultaneously published in the New England Journal of Medicine.
The trial recruited patients with implanted devices that enable continuous monitoring of atrial rhythm, such as pacemakers and defibrillators. “Because we can record the rhythm day and night with these devices, they pick up small abnormalities. About 20% of these patients experience these occasional atrial high-rate episodes – short episodes that look like AF[ib], but they are rare and brief,” Dr. Kirchhof noted.
He explained that whether the occurrence of these atrial high-rate episodes in patients without AFib, as documented on a conventional electrocardiogram, justifies the initiation of anticoagulants has been unclear. “But this trial tells us that these episodes are different to AF[ib] that is diagnosed on ECG,” he added.
Another finding in the trial was that among these patients, there was an unexpectedly low rate of stroke, despite the patients’ having a CHADSVASC score of 4.
“Based on the result of this trial, these occasional atrial high-rate episodes do not appear to be associated with stroke. It appears quite benign,” Dr. Kirchhof said.
Implications for wearable technology?
He said the results may also have implications for wearable devices that pick up abnormal heart rhythm, such as smartwatches.
“We don’t know exactly what these wearable technologies are picking up, but most likely it is these atrial high-rate episodes. But we need more research on the value of these wearable technologies; we need randomized trials in this particular patient population before we consider anticoagulation in these patients,” Dr. Kirchhof stated.
The NOAH-AFNET 6 study was an event-driven, double-blind, double-dummy, randomized trial involving 2,536 patients aged 65 years or older who had atrial high-rate episodes that lasted for at least 6 minutes and who had at least one additional risk factor for stroke.
Patients were randomly assigned in a 1:1 ratio to receive edoxaban or placebo. The primary efficacy outcome was a composite of cardiovascular death, stroke, or systemic embolism, evaluated in a time-to-event analysis. The safety outcome was a composite of death from any cause or major bleeding.
The mean age of the patients was 78 years, 37.4% were women, and the median duration of atrial high-rate episodes was 2.8 hours. The trial was terminated early, at a median follow-up of 21 months, on the basis of safety concerns and the results of an independent, informal assessment of futility for the efficacy of edoxaban; at termination, the planned enrollment had been completed.
Results showed that a primary efficacy outcome event occurred in 83 patients (3.2% per patient-year) in the edoxaban group and in 101 patients (4.0% per patient-year) in the placebo group (hazard ratio, 0.81; 95% confidence interval, 0.60-1.08; P = .15). The incidence of stroke was approximately 1% per patient-year in both groups.
A safety outcome event occurred in 149 patients (5.9% per patient-year) in the edoxaban group and in 114 patients (4.5% per patient-year) in the placebo group (HR, 1.31; 95% CI, 1.02-1.67; P = .03).
ECG-diagnosed AFib developed in 462 of 2,536 patients (18.2% total, 8.7% per patient-year).
In the NEJM article, the authors wrote that the findings of this trial – the low incidence of stroke that was not further reduced by treatment with edoxaban – may make it appropriate to withhold anticoagulant therapy for patients with atrial high-rate episodes.
The main difference between the population studied in this trial and patients with AFib, as documented on an ECG, appears to be the paucity and brevity of atrial arrhythmias in patients with atrial high-rate episodes (termed low arrhythmia burden). Published reports show that a low arrhythmia burden contributes to a low incidence of stroke among patients with AFib, the study authors wrote.
They added that the low rate of stroke in this trial suggests that in addition to clinical risk prediction formulas for stroke, methods to improve the estimation of stroke risk among patients with infrequent atrial arrhythmias detected by long-term monitoring are needed to guide decision-making on the use of anticoagulation.
Commenting on the NOAH-AFNET 6 results, the comoderator of the ESC HOTLINE session at which they were presented, Barbara Casadei, MD, John Radcliffe Hospital, Oxford, England, said: “Finally we know what to with these patients. Before we just had a variety of opinions with no evidence. I think that the trial really highlights that patients who come to the doctor with symptoms of AF[ib] or who have ECG-documented AF[ib] have a much higher risk of cardioembolic stroke than patients in whom this presumed AF[ib] is picked up incidentally from implanted devices.”
She added: “The stroke rates are very low in this trial, so anticoagulation was never going to work. But this is an important finding. We know that anticoagulants are not a free lunch. There is a significant bleeding risk. These results suggest that unless a patient has clinical AF[ib] that shows up on an ECG then we need to more cautious in prescribing anticoagulation.”
Also commenting on the study, immediate past president of the American College of Cardiology Ed Fry, MD, Ascension Indiana St. Vincent Heart Center, Indianapolis, said the management of patients with implanted cardiac devices or personal wearable technology that has picked up an abnormal rhythm suggestive of AFib was a big question in clinical practice.
“These episodes could be AF[ib], which comes with an increased stroke risk, but it could also be something else like atrial tachycardia or supraventricular tachycardia, which do not confer an increased stroke risk,” he explained.
“This study shows that without a firm diagnosis of AF[ib] on an ECG or some sort of continuous AF[ib] monitoring device, we are going to be anticoagulating people who don’t need it. They were exposed to the risk of bleeding without getting the benefit of a reduction in stroke risk,” Dr. Fry noted.
“The important outcome from this trial is that it gives comfort in we can be more confident in withholding anticoagulation until we get a firm diagnosis of AF[ib]. If we have a high index of suspicion that this could be AF[ib], then we can arrange for a further testing,” he added.
Second trial reporting soon
A trial similar to NOAH-AFNET 6 is currently underway – the ARTESIA trial, which is expected to be reported later in 2023.
“We are in close contact with the leadership of that trial, and we hope to do some meta-analysis,” Dr. Kirchhof said. “But I think today we’ve gone from no evidence to one outcome-based trial which shows there is no reason to use anticoagulation in these patients with atrial high-rate episodes. I think this is reason to change practice now, but yes, of course we need to look at the data in totality once the second trial has reported.”
But the lead investigator of the ARTESIA trial, Stuart Connolly, MD, McMaster University, Hamilton, Ont., does not believe the NOAH-AFNET 6 trial should change practice at this time.
“This trial fails to adequately address the critical issue that drives clinical decision-making in these patients because it is underpowered for the most important endpoint of stroke,” he said in an interview.
“The key question is whether anticoagulation reduces stroke in these patients,” he added. “To answer that, a clinical trial needs to have a lot of strokes, and this trial had very few. The trial was stopped early and had way too few strokes to properly answer this key question.”
The NOAH-AFNET 6 trial was an investigator-initiated trial funded by the German Center for Cardiovascular Research and Daiichi Sankyo Europe. Dr. Kirchhof reported research support from several drug and device companies active in AFib. He is also listed as an inventor on two patents held by the University of Hamburg on AFib therapy and AFib markers.
A version of this article first appeared on Medscape.com.
AMSTERDAM – Among patients with atrial high-rate episodes detected by implantable devices, anticoagulation with edoxaban did not significantly reduce the incidence of a composite outcome of cardiovascular death, stroke, or systemic embolism in comparison with placebo but was associated with a higher bleeding risk in the NOAH-AFNET 6 trial.
“ They do not need to be anticoagulated. That is a relief,” the lead investigator of the trial, Paulus Kirchhof, MD, University Heart and Vascular Center Hamburg (Germany), said in an interview.
Dr. Kirchhof pointed out that this result was unexpected. “Many of us thought that because atrial high-rate episodes look like AF[ib] when they occur, then they are an indication for anticoagulation. But based on these results from the first-ever randomized trial on this population, there is no need for anticoagulation in these patients.”
Dr. Kirchhof presented the NOAH-AFNET 6 trial at the annual congress of the European Society of Cardiology. The study was also simultaneously published in the New England Journal of Medicine.
The trial recruited patients with implanted devices that enable continuous monitoring of atrial rhythm, such as pacemakers and defibrillators. “Because we can record the rhythm day and night with these devices, they pick up small abnormalities. About 20% of these patients experience these occasional atrial high-rate episodes – short episodes that look like AF[ib], but they are rare and brief,” Dr. Kirchhof noted.
He explained that whether the occurrence of these atrial high-rate episodes in patients without AFib, as documented on a conventional electrocardiogram, justifies the initiation of anticoagulants has been unclear. “But this trial tells us that these episodes are different to AF[ib] that is diagnosed on ECG,” he added.
Another finding in the trial was that among these patients, there was an unexpectedly low rate of stroke, despite the patients’ having a CHADSVASC score of 4.
“Based on the result of this trial, these occasional atrial high-rate episodes do not appear to be associated with stroke. It appears quite benign,” Dr. Kirchhof said.
Implications for wearable technology?
He said the results may also have implications for wearable devices that pick up abnormal heart rhythm, such as smartwatches.
“We don’t know exactly what these wearable technologies are picking up, but most likely it is these atrial high-rate episodes. But we need more research on the value of these wearable technologies; we need randomized trials in this particular patient population before we consider anticoagulation in these patients,” Dr. Kirchhof stated.
The NOAH-AFNET 6 study was an event-driven, double-blind, double-dummy, randomized trial involving 2,536 patients aged 65 years or older who had atrial high-rate episodes that lasted for at least 6 minutes and who had at least one additional risk factor for stroke.
Patients were randomly assigned in a 1:1 ratio to receive edoxaban or placebo. The primary efficacy outcome was a composite of cardiovascular death, stroke, or systemic embolism, evaluated in a time-to-event analysis. The safety outcome was a composite of death from any cause or major bleeding.
The mean age of the patients was 78 years, 37.4% were women, and the median duration of atrial high-rate episodes was 2.8 hours. The trial was terminated early, at a median follow-up of 21 months, on the basis of safety concerns and the results of an independent, informal assessment of futility for the efficacy of edoxaban; at termination, the planned enrollment had been completed.
Results showed that a primary efficacy outcome event occurred in 83 patients (3.2% per patient-year) in the edoxaban group and in 101 patients (4.0% per patient-year) in the placebo group (hazard ratio, 0.81; 95% confidence interval, 0.60-1.08; P = .15). The incidence of stroke was approximately 1% per patient-year in both groups.
A safety outcome event occurred in 149 patients (5.9% per patient-year) in the edoxaban group and in 114 patients (4.5% per patient-year) in the placebo group (HR, 1.31; 95% CI, 1.02-1.67; P = .03).
ECG-diagnosed AFib developed in 462 of 2,536 patients (18.2% total, 8.7% per patient-year).
In the NEJM article, the authors wrote that the findings of this trial – the low incidence of stroke that was not further reduced by treatment with edoxaban – may make it appropriate to withhold anticoagulant therapy for patients with atrial high-rate episodes.
The main difference between the population studied in this trial and patients with AFib, as documented on an ECG, appears to be the paucity and brevity of atrial arrhythmias in patients with atrial high-rate episodes (termed low arrhythmia burden). Published reports show that a low arrhythmia burden contributes to a low incidence of stroke among patients with AFib, the study authors wrote.
They added that the low rate of stroke in this trial suggests that in addition to clinical risk prediction formulas for stroke, methods to improve the estimation of stroke risk among patients with infrequent atrial arrhythmias detected by long-term monitoring are needed to guide decision-making on the use of anticoagulation.
Commenting on the NOAH-AFNET 6 results, the comoderator of the ESC HOTLINE session at which they were presented, Barbara Casadei, MD, John Radcliffe Hospital, Oxford, England, said: “Finally we know what to with these patients. Before we just had a variety of opinions with no evidence. I think that the trial really highlights that patients who come to the doctor with symptoms of AF[ib] or who have ECG-documented AF[ib] have a much higher risk of cardioembolic stroke than patients in whom this presumed AF[ib] is picked up incidentally from implanted devices.”
She added: “The stroke rates are very low in this trial, so anticoagulation was never going to work. But this is an important finding. We know that anticoagulants are not a free lunch. There is a significant bleeding risk. These results suggest that unless a patient has clinical AF[ib] that shows up on an ECG then we need to more cautious in prescribing anticoagulation.”
Also commenting on the study, immediate past president of the American College of Cardiology Ed Fry, MD, Ascension Indiana St. Vincent Heart Center, Indianapolis, said the management of patients with implanted cardiac devices or personal wearable technology that has picked up an abnormal rhythm suggestive of AFib was a big question in clinical practice.
“These episodes could be AF[ib], which comes with an increased stroke risk, but it could also be something else like atrial tachycardia or supraventricular tachycardia, which do not confer an increased stroke risk,” he explained.
“This study shows that without a firm diagnosis of AF[ib] on an ECG or some sort of continuous AF[ib] monitoring device, we are going to be anticoagulating people who don’t need it. They were exposed to the risk of bleeding without getting the benefit of a reduction in stroke risk,” Dr. Fry noted.
“The important outcome from this trial is that it gives comfort in we can be more confident in withholding anticoagulation until we get a firm diagnosis of AF[ib]. If we have a high index of suspicion that this could be AF[ib], then we can arrange for a further testing,” he added.
Second trial reporting soon
A trial similar to NOAH-AFNET 6 is currently underway – the ARTESIA trial, which is expected to be reported later in 2023.
“We are in close contact with the leadership of that trial, and we hope to do some meta-analysis,” Dr. Kirchhof said. “But I think today we’ve gone from no evidence to one outcome-based trial which shows there is no reason to use anticoagulation in these patients with atrial high-rate episodes. I think this is reason to change practice now, but yes, of course we need to look at the data in totality once the second trial has reported.”
But the lead investigator of the ARTESIA trial, Stuart Connolly, MD, McMaster University, Hamilton, Ont., does not believe the NOAH-AFNET 6 trial should change practice at this time.
“This trial fails to adequately address the critical issue that drives clinical decision-making in these patients because it is underpowered for the most important endpoint of stroke,” he said in an interview.
“The key question is whether anticoagulation reduces stroke in these patients,” he added. “To answer that, a clinical trial needs to have a lot of strokes, and this trial had very few. The trial was stopped early and had way too few strokes to properly answer this key question.”
The NOAH-AFNET 6 trial was an investigator-initiated trial funded by the German Center for Cardiovascular Research and Daiichi Sankyo Europe. Dr. Kirchhof reported research support from several drug and device companies active in AFib. He is also listed as an inventor on two patents held by the University of Hamburg on AFib therapy and AFib markers.
A version of this article first appeared on Medscape.com.
AT THE ESC CONGRESS 2023
Cardiac arrest centers no benefit in OHCA without STEMI
Survivors of out-of-hospital cardiac arrest (OHCA) without ST-segment elevation who were transported to the nearest hospital emergency department had outcomes similar to those of patients transported to specialist cardiac arrest centers, in the ARREST trial.
Both groups had the same 30-day survival, the primary outcome, as well as 3-month survival and neurologic outcomes.
senior author Simon R. Redwood, MD, principal investigator of ARREST, from Guy’s and St. Thomas’ NHS Trust Hospitals and King’s College, London, said during a press briefing. “These results may allow better resource allocation elsewhere.”
Importantly, this study excluded patients who clearly had myocardial infarction (MI), he stressed. Cardiac arrest can result from cardiac causes or from other events, including trauma, overdose, drowning, or electrocution, he noted.
On the other hand, patients with MI “will benefit from going straight to a heart attack center and having an attempt at reopening the artery,” he emphasized.
Tiffany Patterson, PhD, clinical lead of ARREST, with the same affiliations as Dr. Redwood, presented the trial findings at the annual congress of the European Society of Cardiology in Amsterdam, on Aug. 27. The study was simultaneously published online in The Lancet.
Observational studies of registry data suggest that postarrest care for patients resuscitated after cardiac arrest, without ST-segment elevation, may be best delivered in a specialized center, she noted.
The International Liaison Committee on Resuscitation called for a randomized clinical trial of patients resuscitated after cardiac arrest without ST-segment elevation to clarify this.
In the ARREST trial, among 800 patients with return of spontaneous circulation following OHCA without ST-segment elevation who were randomly assigned to be transported to specialized centers or an emergency department, there was no survival benefit, she summarized.
ARREST was “not simply a negative trial, but a new evidence-based starting point,” according to the trial discussant Lia Crotti, MD, PhD, IRCCS Istituto Auxologico Italiano and University Milano-Bicocca, Italy.
She drew attention to two findings: First, among the 862 patients who were enrolled, whom paramedics judged as being without an obvious noncardiac cause of the cardiac arrest, “only 60% ended up having a cardiac cause for their cardiac arrest and only around one quarter of the total had coronary artery disease.”
The small number of patients who could have benefited from early access to a catheterization laboratory probably contributed to the negative result obtained in this trial, with the loss of statistical power, she said.
Second, London is a dense urban area with high-quality acute care hospitals, so the standard of care in the nearest emergency department may be not so different from that in cardiac arrest centers, she noted. Furthermore, four of the seven cardiac arrest centers have an emergency department, and some of the standard care patients may have been transported there.
“If the clinical trial would be extended to the entire country, including rural areas, maybe the result would be different,” she said.
The study authors acknowledge that the main limitation of this study was that “it was done across London with a dense population in a small geographic area,” and “the London Ambulance Service has rapid response times and short transit times and delivers high quality prehospital care, which could limit generalizability.”
Asked during the press conference here why the results were so different from the registry study findings, Dr. Redwood said, “We’ve seen time and time again that registry data think they are telling us the answer. They’re actually not.”
The session cochairs, Rudolf de Boer, MD, PhD, of Erasmus University Medical Centre, Rotterdam, the Netherlands, and Faiez Zannad, MD, PhD, from University of Lorraine–Vandoeuvre-lès-Nancy, France, each congratulated the researchers on a well-done study.
Dr. de Boer wanted to know whether, for example, 100% of these resuscitated OHCA patients without ST-segment elevation had a cardiac cause, “Would results differ? Or is this just real life?” he asked. Dr. Patterson replied that the paramedics excluded obvious noncardiac causes and the findings were based on current facilities.
“Does this trial provide a definitive answer?” Dr. de Boer asked. Dr. Patterson replied that for the moment, subgroup analysis did not identify any subgroup that might benefit from expedited transport to a cardiac arrest center.
Dr. Zannad wanted to know how informed consent was obtained. Dr. Patterson noted that they have an excellent ethical committee that allowed them to undertake this research in vulnerable patients. Written informed consent was obtained from the patient once the initial emergency had passed if they had regained capacity.
Rationale and trial findings
“It’s very well established that early bystander CPR [cardiopulmonary resuscitation], early defibrillation, and advanced in-hospital care improves survival,” Dr. Redwood noted. “Despite this, only 1 in 10 survive to leave the hospital.”
Therefore, “a cardiac arrest center has been proposed as a way of improving outcome.” These centers have a catheterization laboratory, open 24 hours a day, 7 days a week, advanced critical care including advanced ventilation, temperature management of the patient, hemodynamic support, and neuroprognostication and rehab “because often these patients will have brain injury.
“There’s quite overwhelming registry data to suggest that these cardiac arrest centers improve outcome,” he said, “but these are limited by bias.”
Between January 2018 and December 2022, London Ambulance paramedics randomly assigned 862 patients who were successfully resuscitated and without a confirmed MI to be transported the nearest hospital emergency department or the catheterization laboratory in a cardiac arrest center.
Data were available for 822 participants. They had a mean age of 63 years, and 68% were male.
The primary endpoint, 30-day mortality, occurred in 258 (63%) of 411 participants in the cardiac arrest center group and in 258 (63%) of 412 in the standard care group (unadjusted risk ratio for survival, 1.00; 95% confidence interval [CI], 0.90-1.11; P = 0.96).
Mortality at 3 months was similar in both groups: 64% in the standard care group and 65% in the cardiac arrest center group.
Neurologic outcomes at discharge and 3 months were similar in both groups.
Eight (2%) of 414 patients in the cardiac arrest center group and three (1%) of 413 in the standard care group had serious adverse events, none of which were deemed related to the trial intervention.
A cardiac cause of arrest was identified in roughly 60% of patients in each group, and of these, roughly 42% were coronary causes, 33% were arrhythmia, and 17% were cardiomyopathy.
The median time from cardiac arrest to hospital arrival was 84 minutes in the cardiac arrest center group and 77 minutes in the standard care group.
“Surprising and important RCT evidence”
In an accompanying editorial, Carolina Malta Hansen, MD, PhD, University of Copenhagen, and colleagues wrote that “this study provides randomized trial evidence that in urban settings such as London, there is no survival advantage of a strategy of transporting patients who have been resuscitated to centres with specialty expertise in care of cardiac arrest.
“This result is surprising and important, since this complex and critically ill population would be expected to benefit from centres with more expertise.”
However, “it would be a mistake to conclude that the trial results apply to regions where local hospitals provide a lower quality of care than those in this trial,” they cautioned.
“Where does this leave the medical community, researchers, and society in general?” they asked rhetorically. “Prioritising a minimum standard of care at local hospitals caring for this population is at least as important as ensuring high-quality care or advanced treatment at tertiary centres.
“This trial also calls for more focus on the basics, including efforts to increase bystander cardiopulmonary resuscitation and early defibrillation, aspects of care that are currently being assessed in two ongoing clinical trials (NCT04660526 and NCT03835403) and are most strongly associated with improved survival, when coupled with high-quality prehospital care with trained staff and short response times,” they concluded.
The study was fully funded by the British Heart Foundation. The authors reported that they have no relevant financial disclosures. The financial disclosures of the editorialists are listed with the editorial.
A version of this article first appeared on Medscape.com.
Survivors of out-of-hospital cardiac arrest (OHCA) without ST-segment elevation who were transported to the nearest hospital emergency department had outcomes similar to those of patients transported to specialist cardiac arrest centers, in the ARREST trial.
Both groups had the same 30-day survival, the primary outcome, as well as 3-month survival and neurologic outcomes.
senior author Simon R. Redwood, MD, principal investigator of ARREST, from Guy’s and St. Thomas’ NHS Trust Hospitals and King’s College, London, said during a press briefing. “These results may allow better resource allocation elsewhere.”
Importantly, this study excluded patients who clearly had myocardial infarction (MI), he stressed. Cardiac arrest can result from cardiac causes or from other events, including trauma, overdose, drowning, or electrocution, he noted.
On the other hand, patients with MI “will benefit from going straight to a heart attack center and having an attempt at reopening the artery,” he emphasized.
Tiffany Patterson, PhD, clinical lead of ARREST, with the same affiliations as Dr. Redwood, presented the trial findings at the annual congress of the European Society of Cardiology in Amsterdam, on Aug. 27. The study was simultaneously published online in The Lancet.
Observational studies of registry data suggest that postarrest care for patients resuscitated after cardiac arrest, without ST-segment elevation, may be best delivered in a specialized center, she noted.
The International Liaison Committee on Resuscitation called for a randomized clinical trial of patients resuscitated after cardiac arrest without ST-segment elevation to clarify this.
In the ARREST trial, among 800 patients with return of spontaneous circulation following OHCA without ST-segment elevation who were randomly assigned to be transported to specialized centers or an emergency department, there was no survival benefit, she summarized.
ARREST was “not simply a negative trial, but a new evidence-based starting point,” according to the trial discussant Lia Crotti, MD, PhD, IRCCS Istituto Auxologico Italiano and University Milano-Bicocca, Italy.
She drew attention to two findings: First, among the 862 patients who were enrolled, whom paramedics judged as being without an obvious noncardiac cause of the cardiac arrest, “only 60% ended up having a cardiac cause for their cardiac arrest and only around one quarter of the total had coronary artery disease.”
The small number of patients who could have benefited from early access to a catheterization laboratory probably contributed to the negative result obtained in this trial, with the loss of statistical power, she said.
Second, London is a dense urban area with high-quality acute care hospitals, so the standard of care in the nearest emergency department may be not so different from that in cardiac arrest centers, she noted. Furthermore, four of the seven cardiac arrest centers have an emergency department, and some of the standard care patients may have been transported there.
“If the clinical trial would be extended to the entire country, including rural areas, maybe the result would be different,” she said.
The study authors acknowledge that the main limitation of this study was that “it was done across London with a dense population in a small geographic area,” and “the London Ambulance Service has rapid response times and short transit times and delivers high quality prehospital care, which could limit generalizability.”
Asked during the press conference here why the results were so different from the registry study findings, Dr. Redwood said, “We’ve seen time and time again that registry data think they are telling us the answer. They’re actually not.”
The session cochairs, Rudolf de Boer, MD, PhD, of Erasmus University Medical Centre, Rotterdam, the Netherlands, and Faiez Zannad, MD, PhD, from University of Lorraine–Vandoeuvre-lès-Nancy, France, each congratulated the researchers on a well-done study.
Dr. de Boer wanted to know whether, for example, 100% of these resuscitated OHCA patients without ST-segment elevation had a cardiac cause, “Would results differ? Or is this just real life?” he asked. Dr. Patterson replied that the paramedics excluded obvious noncardiac causes and the findings were based on current facilities.
“Does this trial provide a definitive answer?” Dr. de Boer asked. Dr. Patterson replied that for the moment, subgroup analysis did not identify any subgroup that might benefit from expedited transport to a cardiac arrest center.
Dr. Zannad wanted to know how informed consent was obtained. Dr. Patterson noted that they have an excellent ethical committee that allowed them to undertake this research in vulnerable patients. Written informed consent was obtained from the patient once the initial emergency had passed if they had regained capacity.
Rationale and trial findings
“It’s very well established that early bystander CPR [cardiopulmonary resuscitation], early defibrillation, and advanced in-hospital care improves survival,” Dr. Redwood noted. “Despite this, only 1 in 10 survive to leave the hospital.”
Therefore, “a cardiac arrest center has been proposed as a way of improving outcome.” These centers have a catheterization laboratory, open 24 hours a day, 7 days a week, advanced critical care including advanced ventilation, temperature management of the patient, hemodynamic support, and neuroprognostication and rehab “because often these patients will have brain injury.
“There’s quite overwhelming registry data to suggest that these cardiac arrest centers improve outcome,” he said, “but these are limited by bias.”
Between January 2018 and December 2022, London Ambulance paramedics randomly assigned 862 patients who were successfully resuscitated and without a confirmed MI to be transported the nearest hospital emergency department or the catheterization laboratory in a cardiac arrest center.
Data were available for 822 participants. They had a mean age of 63 years, and 68% were male.
The primary endpoint, 30-day mortality, occurred in 258 (63%) of 411 participants in the cardiac arrest center group and in 258 (63%) of 412 in the standard care group (unadjusted risk ratio for survival, 1.00; 95% confidence interval [CI], 0.90-1.11; P = 0.96).
Mortality at 3 months was similar in both groups: 64% in the standard care group and 65% in the cardiac arrest center group.
Neurologic outcomes at discharge and 3 months were similar in both groups.
Eight (2%) of 414 patients in the cardiac arrest center group and three (1%) of 413 in the standard care group had serious adverse events, none of which were deemed related to the trial intervention.
A cardiac cause of arrest was identified in roughly 60% of patients in each group, and of these, roughly 42% were coronary causes, 33% were arrhythmia, and 17% were cardiomyopathy.
The median time from cardiac arrest to hospital arrival was 84 minutes in the cardiac arrest center group and 77 minutes in the standard care group.
“Surprising and important RCT evidence”
In an accompanying editorial, Carolina Malta Hansen, MD, PhD, University of Copenhagen, and colleagues wrote that “this study provides randomized trial evidence that in urban settings such as London, there is no survival advantage of a strategy of transporting patients who have been resuscitated to centres with specialty expertise in care of cardiac arrest.
“This result is surprising and important, since this complex and critically ill population would be expected to benefit from centres with more expertise.”
However, “it would be a mistake to conclude that the trial results apply to regions where local hospitals provide a lower quality of care than those in this trial,” they cautioned.
“Where does this leave the medical community, researchers, and society in general?” they asked rhetorically. “Prioritising a minimum standard of care at local hospitals caring for this population is at least as important as ensuring high-quality care or advanced treatment at tertiary centres.
“This trial also calls for more focus on the basics, including efforts to increase bystander cardiopulmonary resuscitation and early defibrillation, aspects of care that are currently being assessed in two ongoing clinical trials (NCT04660526 and NCT03835403) and are most strongly associated with improved survival, when coupled with high-quality prehospital care with trained staff and short response times,” they concluded.
The study was fully funded by the British Heart Foundation. The authors reported that they have no relevant financial disclosures. The financial disclosures of the editorialists are listed with the editorial.
A version of this article first appeared on Medscape.com.
Survivors of out-of-hospital cardiac arrest (OHCA) without ST-segment elevation who were transported to the nearest hospital emergency department had outcomes similar to those of patients transported to specialist cardiac arrest centers, in the ARREST trial.
Both groups had the same 30-day survival, the primary outcome, as well as 3-month survival and neurologic outcomes.
senior author Simon R. Redwood, MD, principal investigator of ARREST, from Guy’s and St. Thomas’ NHS Trust Hospitals and King’s College, London, said during a press briefing. “These results may allow better resource allocation elsewhere.”
Importantly, this study excluded patients who clearly had myocardial infarction (MI), he stressed. Cardiac arrest can result from cardiac causes or from other events, including trauma, overdose, drowning, or electrocution, he noted.
On the other hand, patients with MI “will benefit from going straight to a heart attack center and having an attempt at reopening the artery,” he emphasized.
Tiffany Patterson, PhD, clinical lead of ARREST, with the same affiliations as Dr. Redwood, presented the trial findings at the annual congress of the European Society of Cardiology in Amsterdam, on Aug. 27. The study was simultaneously published online in The Lancet.
Observational studies of registry data suggest that postarrest care for patients resuscitated after cardiac arrest, without ST-segment elevation, may be best delivered in a specialized center, she noted.
The International Liaison Committee on Resuscitation called for a randomized clinical trial of patients resuscitated after cardiac arrest without ST-segment elevation to clarify this.
In the ARREST trial, among 800 patients with return of spontaneous circulation following OHCA without ST-segment elevation who were randomly assigned to be transported to specialized centers or an emergency department, there was no survival benefit, she summarized.
ARREST was “not simply a negative trial, but a new evidence-based starting point,” according to the trial discussant Lia Crotti, MD, PhD, IRCCS Istituto Auxologico Italiano and University Milano-Bicocca, Italy.
She drew attention to two findings: First, among the 862 patients who were enrolled, whom paramedics judged as being without an obvious noncardiac cause of the cardiac arrest, “only 60% ended up having a cardiac cause for their cardiac arrest and only around one quarter of the total had coronary artery disease.”
The small number of patients who could have benefited from early access to a catheterization laboratory probably contributed to the negative result obtained in this trial, with the loss of statistical power, she said.
Second, London is a dense urban area with high-quality acute care hospitals, so the standard of care in the nearest emergency department may be not so different from that in cardiac arrest centers, she noted. Furthermore, four of the seven cardiac arrest centers have an emergency department, and some of the standard care patients may have been transported there.
“If the clinical trial would be extended to the entire country, including rural areas, maybe the result would be different,” she said.
The study authors acknowledge that the main limitation of this study was that “it was done across London with a dense population in a small geographic area,” and “the London Ambulance Service has rapid response times and short transit times and delivers high quality prehospital care, which could limit generalizability.”
Asked during the press conference here why the results were so different from the registry study findings, Dr. Redwood said, “We’ve seen time and time again that registry data think they are telling us the answer. They’re actually not.”
The session cochairs, Rudolf de Boer, MD, PhD, of Erasmus University Medical Centre, Rotterdam, the Netherlands, and Faiez Zannad, MD, PhD, from University of Lorraine–Vandoeuvre-lès-Nancy, France, each congratulated the researchers on a well-done study.
Dr. de Boer wanted to know whether, for example, 100% of these resuscitated OHCA patients without ST-segment elevation had a cardiac cause, “Would results differ? Or is this just real life?” he asked. Dr. Patterson replied that the paramedics excluded obvious noncardiac causes and the findings were based on current facilities.
“Does this trial provide a definitive answer?” Dr. de Boer asked. Dr. Patterson replied that for the moment, subgroup analysis did not identify any subgroup that might benefit from expedited transport to a cardiac arrest center.
Dr. Zannad wanted to know how informed consent was obtained. Dr. Patterson noted that they have an excellent ethical committee that allowed them to undertake this research in vulnerable patients. Written informed consent was obtained from the patient once the initial emergency had passed if they had regained capacity.
Rationale and trial findings
“It’s very well established that early bystander CPR [cardiopulmonary resuscitation], early defibrillation, and advanced in-hospital care improves survival,” Dr. Redwood noted. “Despite this, only 1 in 10 survive to leave the hospital.”
Therefore, “a cardiac arrest center has been proposed as a way of improving outcome.” These centers have a catheterization laboratory, open 24 hours a day, 7 days a week, advanced critical care including advanced ventilation, temperature management of the patient, hemodynamic support, and neuroprognostication and rehab “because often these patients will have brain injury.
“There’s quite overwhelming registry data to suggest that these cardiac arrest centers improve outcome,” he said, “but these are limited by bias.”
Between January 2018 and December 2022, London Ambulance paramedics randomly assigned 862 patients who were successfully resuscitated and without a confirmed MI to be transported the nearest hospital emergency department or the catheterization laboratory in a cardiac arrest center.
Data were available for 822 participants. They had a mean age of 63 years, and 68% were male.
The primary endpoint, 30-day mortality, occurred in 258 (63%) of 411 participants in the cardiac arrest center group and in 258 (63%) of 412 in the standard care group (unadjusted risk ratio for survival, 1.00; 95% confidence interval [CI], 0.90-1.11; P = 0.96).
Mortality at 3 months was similar in both groups: 64% in the standard care group and 65% in the cardiac arrest center group.
Neurologic outcomes at discharge and 3 months were similar in both groups.
Eight (2%) of 414 patients in the cardiac arrest center group and three (1%) of 413 in the standard care group had serious adverse events, none of which were deemed related to the trial intervention.
A cardiac cause of arrest was identified in roughly 60% of patients in each group, and of these, roughly 42% were coronary causes, 33% were arrhythmia, and 17% were cardiomyopathy.
The median time from cardiac arrest to hospital arrival was 84 minutes in the cardiac arrest center group and 77 minutes in the standard care group.
“Surprising and important RCT evidence”
In an accompanying editorial, Carolina Malta Hansen, MD, PhD, University of Copenhagen, and colleagues wrote that “this study provides randomized trial evidence that in urban settings such as London, there is no survival advantage of a strategy of transporting patients who have been resuscitated to centres with specialty expertise in care of cardiac arrest.
“This result is surprising and important, since this complex and critically ill population would be expected to benefit from centres with more expertise.”
However, “it would be a mistake to conclude that the trial results apply to regions where local hospitals provide a lower quality of care than those in this trial,” they cautioned.
“Where does this leave the medical community, researchers, and society in general?” they asked rhetorically. “Prioritising a minimum standard of care at local hospitals caring for this population is at least as important as ensuring high-quality care or advanced treatment at tertiary centres.
“This trial also calls for more focus on the basics, including efforts to increase bystander cardiopulmonary resuscitation and early defibrillation, aspects of care that are currently being assessed in two ongoing clinical trials (NCT04660526 and NCT03835403) and are most strongly associated with improved survival, when coupled with high-quality prehospital care with trained staff and short response times,” they concluded.
The study was fully funded by the British Heart Foundation. The authors reported that they have no relevant financial disclosures. The financial disclosures of the editorialists are listed with the editorial.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
No reduction in AFib after noncardiac surgery with colchicine: COP-AF
Trends were seen with reductions in events, but these did not reach significance. However, benefit was seen in a post-hoc analysis looking at a composite of both of those endpoints, the researchers note, as well as a composite of vascular death, nonfatal MINS, nonfatal stroke, and clinically important perioperative AFib, the researchers report.
“We interpret that as there is a trend that is promising, a trend that needs to be further explored,” lead author David Conen, MD, Population Health Research Institute, Hamilton, Ont., said in an interview. “We think that further studies are needed to tease out which patients can benefit from colchicine and in what setting it can be used.”
Treatment was safe, with no effect on the risk for sepsis or infection, but it did cause an increase in noninfectious diarrhea. “These events were mostly benign and did not increase length of stay, and only one patient was readmitted because of diarrhea,” Dr. Conen noted.
Results of the COP-AF trial were presented at the annual congress of the European Society of Cardiology, Amsterdam, and published online in The Lancet .
Inflammation and perioperative AFib
AFib and MINS are common complications in patients undergoing major thoracic surgery, Dr. Conen explained. The literature suggests AFib occurs in about 10% and MINS in about 20% of these patients, “and patients with these complications have a much higher risk of additional complications, such as stroke or MI [myocardial infarction],” Dr. Conen said.
Both disorders are associated with high levels of inflammatory biomarkers, so they set out to test colchicine, a well-known anti-inflammatory drug used in higher doses to treat common clinical disorders, such as gout and pericarditis. Small, randomized trials had shown it reduced the incidence of perioperative AFib after cardiac surgery, he noted.
Low-dose colchicine (LoDoCo, Agepha Pharma) was recently approved by the U.S. Food and Drug Administration to reduce the risk for MI, stroke, coronary revascularization, or death in patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease. It was approved on the basis of the LoDoCo 2 trial in patients with stable coronary artery disease and the COLCOT trial in patients with recent MI.
COP-AF was a randomized trial, conducted at 45 sites in 11 countries, and enrolled 3,209 patients aged 55 years or older (51.6% male) undergoing major noncardiac thoracic surgery. Patients were excluded if they had previous AFib, had any contraindications to colchicine, or required colchicine on a clinical basis.
Patients were randomly assigned 1:1 to receive oral colchicine at a dose of 0.5 mg twice daily (1,608 patients) or placebo (1,601 patients). Treatment was begun within 4 hours before surgery and continued for 10 days. Health care providers and patients, as well as data collectors and adjudicators, were blinded to treatment assignment.
The co-primary outcomes were clinically important perioperative AFib or MINS over 14 days of follow-up. The trial was originally looking only at clinically important AFib, Dr. Conen noted, but after the publication of LoDoCo 2 and COLCOT, “MINS was added as an independent co-primary outcome,” requiring more patients to achieve adequate power.
The main safety outcomes were a composite of sepsis or infection, along with noninfectious diarrhea.
Clinically important AFib was defined as AFib that results in angina, heart failure, or symptomatic hypotension or required treatment with a rate-controlling drug, antiarrhythmic drug, or electrical cardioversion. “This definition was chosen because of its prognostic relevance, and to avoid adding short, asymptomatic AFib episodes of uncertain clinical relevance to the primary outcome,” Dr. Conen said during his presentation.
MINS was defined as an MI or any postoperative troponin elevation that was judged by an adjudication panel to be of ischemic origin.
At 14 days, there was no significant difference between groups on either of the co-primary end points.
No significant differences but positive trends were similarly seen in secondary outcomes of a composite of all-cause death, nonfatal MINS, and nonfatal stroke; the composite of all-cause death, nonfatal MI, and nonfatal stroke; MINS not fulfilling the fourth universal definition of MI; or MI.
There were no differences in time to chest tube removal, days in hospital, nights in the step-down unit, or nights in the intensive care unit.
In terms of safety, there was no difference between groups on sepsis or infection, which occurred in 6.4% of patients in the colchicine group and 5.2% of those in the placebo group (hazard ratio, 1.24; 95% confidence interval, 0.93-1.66).
Noninfectious diarrhea was more common with colchicine, with 134 events (8.3%) versus 38 with placebo (2.4%), for an HR of 3.64 (95% CI, 2.54-5.22).
“In two post hoc analyses, colchicine significantly reduced the composite of the two co-primary outcomes,” Dr. Conen noted in his presentation. Clinically important perioperative AFib or MINS occurred in 22.4% in the colchicine group and 25.9% in the placebo group (HR, 0.84; 95% CI, 0.73-0.97; P = .02).
“Colchicine also significantly reduced the composite of vascular mortality, nonfatal MINS, nonfatal stroke, and clinically important AFib,” he said; 22.6% of patients in the colchicine group had one of these events versus 26.4% of those in the placebo group (HR, 0.83; 95% CI, 0.72-0.96; P = .01).
The researchers also reported significant interactions on both co-primary outcomes for the type of incision, “suggesting that stronger and statistically significant effects among patients undergoing thoracoscopic surgery as opposed to nonthoracoscopic surgery,” Dr. Conen said.
Patients undergoing thoracoscopic surgery treated with colchicine had a reduced risk for clinically important AFib (n = 2,397; HR, 0.53; 95% CI, 0.36-0.77), but colchicine treatment increased the risk in patients having open surgery (n = 784; HR, 1.59; 95% CI, 1.07-2.35; P for interaction < .0001).
There was a beneficial effect on MINS with colchicine among patients undergoing thoracoscopic surgery (HR, 0.80; 95% CI, 0.66-0.98), but no effect was seen among those having open surgery (HR, 1.15; 95% CI, 0.87-1.53; P for interaction = .041).
Low-risk patients
Jean-Claude Tardif, MD, Montreal Heart Institute and Université de Montréal, was the invited discussant for the COP-AF presentation and congratulated the researchers on “a job well done.”
He made the point that the risk for perioperative AFib has decreased substantially with the greater use of thoracoscopic rather than open surgical approaches. The population of this trial was relatively young, with an average age of 68 years; the presence of concomitant CVD was low, at about 9%; by design, patients with previous AFib were excluded; and only about 20% of patients had surgery with an open approach.
“So that population of patients were probably at relatively low risk of atrial fibrillation, and sure enough, the incidence of perioperative AFib in that population at 7.5% was lower than the assumed rate in the statistical powering of the study at 9%,” Dr. Tardif noted.
The post-hoc analyses showed a “nominally significant effect on the composite of MINS and AFib; however, that combination is fairly difficult to justify given the different pathophysiology and clinical consequences of both outcomes,” he pointed out.
The incidence of postoperative MI as a secondary outcome was low, less than 1%, as was the incidence of postoperative stroke in that study, Dr. Tardif added. “Given the link between presence of blood in the pericardium as a trigger for AFib, it would be interesting to know the incidence of perioperative pericarditis in COP-AF.”
In conclusion, he said, “when trying to put these results into the bigger picture of colchicine in cardiovascular disease, I believe we need large, well-powered clinical trials to determine the value of colchicine to reduce the risk of AFib after cardiac surgery and after catheter ablation,” Dr. Tardif said.
“We all know that colchicine represents the first line of therapy for the treatment of acute and recurrent pericarditis, and finally, low-dose colchicine, at a lower dose than was used in COP-AF, 0.5 mg once daily, is the first anti-inflammatory agent approved by both U.S. FDA and Health Canada to reduce the risk of atherothombotic events in patients with ASCVD [atherosclerotic cardiovascular disease], I believe offering a new pillar of treatment for the prevention of ischemic events in such patients.”
Session co-moderator Franz Weidinger, MD, Landstrasse Clinic, Vienna, Austria, called the COP-AF results “very important” but also noted that they show “the challenge of doing well-powered randomized trials these days when we have patients so well treated for a wide array of cardiovascular disease.”
The study was supported by the Canadian Institutes of Health Research (CIHR); Accelerating Clinical Trials Consortium; Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario; Population Health Research Institute; Hamilton Health Sciences; Division of Cardiology at McMaster University, Canada; Hanela Foundation, Switzerland; and General Research Fund, Research Grants Council, Hong Kong. Dr. Conen reports receiving research grants from CIHR, speaker fees from Servier outside the current study, and advisory board fees from Roche Diagnostics and Trimedics outside the current study.
A version of this article appeared on Medscape.com.
Trends were seen with reductions in events, but these did not reach significance. However, benefit was seen in a post-hoc analysis looking at a composite of both of those endpoints, the researchers note, as well as a composite of vascular death, nonfatal MINS, nonfatal stroke, and clinically important perioperative AFib, the researchers report.
“We interpret that as there is a trend that is promising, a trend that needs to be further explored,” lead author David Conen, MD, Population Health Research Institute, Hamilton, Ont., said in an interview. “We think that further studies are needed to tease out which patients can benefit from colchicine and in what setting it can be used.”
Treatment was safe, with no effect on the risk for sepsis or infection, but it did cause an increase in noninfectious diarrhea. “These events were mostly benign and did not increase length of stay, and only one patient was readmitted because of diarrhea,” Dr. Conen noted.
Results of the COP-AF trial were presented at the annual congress of the European Society of Cardiology, Amsterdam, and published online in The Lancet .
Inflammation and perioperative AFib
AFib and MINS are common complications in patients undergoing major thoracic surgery, Dr. Conen explained. The literature suggests AFib occurs in about 10% and MINS in about 20% of these patients, “and patients with these complications have a much higher risk of additional complications, such as stroke or MI [myocardial infarction],” Dr. Conen said.
Both disorders are associated with high levels of inflammatory biomarkers, so they set out to test colchicine, a well-known anti-inflammatory drug used in higher doses to treat common clinical disorders, such as gout and pericarditis. Small, randomized trials had shown it reduced the incidence of perioperative AFib after cardiac surgery, he noted.
Low-dose colchicine (LoDoCo, Agepha Pharma) was recently approved by the U.S. Food and Drug Administration to reduce the risk for MI, stroke, coronary revascularization, or death in patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease. It was approved on the basis of the LoDoCo 2 trial in patients with stable coronary artery disease and the COLCOT trial in patients with recent MI.
COP-AF was a randomized trial, conducted at 45 sites in 11 countries, and enrolled 3,209 patients aged 55 years or older (51.6% male) undergoing major noncardiac thoracic surgery. Patients were excluded if they had previous AFib, had any contraindications to colchicine, or required colchicine on a clinical basis.
Patients were randomly assigned 1:1 to receive oral colchicine at a dose of 0.5 mg twice daily (1,608 patients) or placebo (1,601 patients). Treatment was begun within 4 hours before surgery and continued for 10 days. Health care providers and patients, as well as data collectors and adjudicators, were blinded to treatment assignment.
The co-primary outcomes were clinically important perioperative AFib or MINS over 14 days of follow-up. The trial was originally looking only at clinically important AFib, Dr. Conen noted, but after the publication of LoDoCo 2 and COLCOT, “MINS was added as an independent co-primary outcome,” requiring more patients to achieve adequate power.
The main safety outcomes were a composite of sepsis or infection, along with noninfectious diarrhea.
Clinically important AFib was defined as AFib that results in angina, heart failure, or symptomatic hypotension or required treatment with a rate-controlling drug, antiarrhythmic drug, or electrical cardioversion. “This definition was chosen because of its prognostic relevance, and to avoid adding short, asymptomatic AFib episodes of uncertain clinical relevance to the primary outcome,” Dr. Conen said during his presentation.
MINS was defined as an MI or any postoperative troponin elevation that was judged by an adjudication panel to be of ischemic origin.
At 14 days, there was no significant difference between groups on either of the co-primary end points.
No significant differences but positive trends were similarly seen in secondary outcomes of a composite of all-cause death, nonfatal MINS, and nonfatal stroke; the composite of all-cause death, nonfatal MI, and nonfatal stroke; MINS not fulfilling the fourth universal definition of MI; or MI.
There were no differences in time to chest tube removal, days in hospital, nights in the step-down unit, or nights in the intensive care unit.
In terms of safety, there was no difference between groups on sepsis or infection, which occurred in 6.4% of patients in the colchicine group and 5.2% of those in the placebo group (hazard ratio, 1.24; 95% confidence interval, 0.93-1.66).
Noninfectious diarrhea was more common with colchicine, with 134 events (8.3%) versus 38 with placebo (2.4%), for an HR of 3.64 (95% CI, 2.54-5.22).
“In two post hoc analyses, colchicine significantly reduced the composite of the two co-primary outcomes,” Dr. Conen noted in his presentation. Clinically important perioperative AFib or MINS occurred in 22.4% in the colchicine group and 25.9% in the placebo group (HR, 0.84; 95% CI, 0.73-0.97; P = .02).
“Colchicine also significantly reduced the composite of vascular mortality, nonfatal MINS, nonfatal stroke, and clinically important AFib,” he said; 22.6% of patients in the colchicine group had one of these events versus 26.4% of those in the placebo group (HR, 0.83; 95% CI, 0.72-0.96; P = .01).
The researchers also reported significant interactions on both co-primary outcomes for the type of incision, “suggesting that stronger and statistically significant effects among patients undergoing thoracoscopic surgery as opposed to nonthoracoscopic surgery,” Dr. Conen said.
Patients undergoing thoracoscopic surgery treated with colchicine had a reduced risk for clinically important AFib (n = 2,397; HR, 0.53; 95% CI, 0.36-0.77), but colchicine treatment increased the risk in patients having open surgery (n = 784; HR, 1.59; 95% CI, 1.07-2.35; P for interaction < .0001).
There was a beneficial effect on MINS with colchicine among patients undergoing thoracoscopic surgery (HR, 0.80; 95% CI, 0.66-0.98), but no effect was seen among those having open surgery (HR, 1.15; 95% CI, 0.87-1.53; P for interaction = .041).
Low-risk patients
Jean-Claude Tardif, MD, Montreal Heart Institute and Université de Montréal, was the invited discussant for the COP-AF presentation and congratulated the researchers on “a job well done.”
He made the point that the risk for perioperative AFib has decreased substantially with the greater use of thoracoscopic rather than open surgical approaches. The population of this trial was relatively young, with an average age of 68 years; the presence of concomitant CVD was low, at about 9%; by design, patients with previous AFib were excluded; and only about 20% of patients had surgery with an open approach.
“So that population of patients were probably at relatively low risk of atrial fibrillation, and sure enough, the incidence of perioperative AFib in that population at 7.5% was lower than the assumed rate in the statistical powering of the study at 9%,” Dr. Tardif noted.
The post-hoc analyses showed a “nominally significant effect on the composite of MINS and AFib; however, that combination is fairly difficult to justify given the different pathophysiology and clinical consequences of both outcomes,” he pointed out.
The incidence of postoperative MI as a secondary outcome was low, less than 1%, as was the incidence of postoperative stroke in that study, Dr. Tardif added. “Given the link between presence of blood in the pericardium as a trigger for AFib, it would be interesting to know the incidence of perioperative pericarditis in COP-AF.”
In conclusion, he said, “when trying to put these results into the bigger picture of colchicine in cardiovascular disease, I believe we need large, well-powered clinical trials to determine the value of colchicine to reduce the risk of AFib after cardiac surgery and after catheter ablation,” Dr. Tardif said.
“We all know that colchicine represents the first line of therapy for the treatment of acute and recurrent pericarditis, and finally, low-dose colchicine, at a lower dose than was used in COP-AF, 0.5 mg once daily, is the first anti-inflammatory agent approved by both U.S. FDA and Health Canada to reduce the risk of atherothombotic events in patients with ASCVD [atherosclerotic cardiovascular disease], I believe offering a new pillar of treatment for the prevention of ischemic events in such patients.”
Session co-moderator Franz Weidinger, MD, Landstrasse Clinic, Vienna, Austria, called the COP-AF results “very important” but also noted that they show “the challenge of doing well-powered randomized trials these days when we have patients so well treated for a wide array of cardiovascular disease.”
The study was supported by the Canadian Institutes of Health Research (CIHR); Accelerating Clinical Trials Consortium; Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario; Population Health Research Institute; Hamilton Health Sciences; Division of Cardiology at McMaster University, Canada; Hanela Foundation, Switzerland; and General Research Fund, Research Grants Council, Hong Kong. Dr. Conen reports receiving research grants from CIHR, speaker fees from Servier outside the current study, and advisory board fees from Roche Diagnostics and Trimedics outside the current study.
A version of this article appeared on Medscape.com.
Trends were seen with reductions in events, but these did not reach significance. However, benefit was seen in a post-hoc analysis looking at a composite of both of those endpoints, the researchers note, as well as a composite of vascular death, nonfatal MINS, nonfatal stroke, and clinically important perioperative AFib, the researchers report.
“We interpret that as there is a trend that is promising, a trend that needs to be further explored,” lead author David Conen, MD, Population Health Research Institute, Hamilton, Ont., said in an interview. “We think that further studies are needed to tease out which patients can benefit from colchicine and in what setting it can be used.”
Treatment was safe, with no effect on the risk for sepsis or infection, but it did cause an increase in noninfectious diarrhea. “These events were mostly benign and did not increase length of stay, and only one patient was readmitted because of diarrhea,” Dr. Conen noted.
Results of the COP-AF trial were presented at the annual congress of the European Society of Cardiology, Amsterdam, and published online in The Lancet .
Inflammation and perioperative AFib
AFib and MINS are common complications in patients undergoing major thoracic surgery, Dr. Conen explained. The literature suggests AFib occurs in about 10% and MINS in about 20% of these patients, “and patients with these complications have a much higher risk of additional complications, such as stroke or MI [myocardial infarction],” Dr. Conen said.
Both disorders are associated with high levels of inflammatory biomarkers, so they set out to test colchicine, a well-known anti-inflammatory drug used in higher doses to treat common clinical disorders, such as gout and pericarditis. Small, randomized trials had shown it reduced the incidence of perioperative AFib after cardiac surgery, he noted.
Low-dose colchicine (LoDoCo, Agepha Pharma) was recently approved by the U.S. Food and Drug Administration to reduce the risk for MI, stroke, coronary revascularization, or death in patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease. It was approved on the basis of the LoDoCo 2 trial in patients with stable coronary artery disease and the COLCOT trial in patients with recent MI.
COP-AF was a randomized trial, conducted at 45 sites in 11 countries, and enrolled 3,209 patients aged 55 years or older (51.6% male) undergoing major noncardiac thoracic surgery. Patients were excluded if they had previous AFib, had any contraindications to colchicine, or required colchicine on a clinical basis.
Patients were randomly assigned 1:1 to receive oral colchicine at a dose of 0.5 mg twice daily (1,608 patients) or placebo (1,601 patients). Treatment was begun within 4 hours before surgery and continued for 10 days. Health care providers and patients, as well as data collectors and adjudicators, were blinded to treatment assignment.
The co-primary outcomes were clinically important perioperative AFib or MINS over 14 days of follow-up. The trial was originally looking only at clinically important AFib, Dr. Conen noted, but after the publication of LoDoCo 2 and COLCOT, “MINS was added as an independent co-primary outcome,” requiring more patients to achieve adequate power.
The main safety outcomes were a composite of sepsis or infection, along with noninfectious diarrhea.
Clinically important AFib was defined as AFib that results in angina, heart failure, or symptomatic hypotension or required treatment with a rate-controlling drug, antiarrhythmic drug, or electrical cardioversion. “This definition was chosen because of its prognostic relevance, and to avoid adding short, asymptomatic AFib episodes of uncertain clinical relevance to the primary outcome,” Dr. Conen said during his presentation.
MINS was defined as an MI or any postoperative troponin elevation that was judged by an adjudication panel to be of ischemic origin.
At 14 days, there was no significant difference between groups on either of the co-primary end points.
No significant differences but positive trends were similarly seen in secondary outcomes of a composite of all-cause death, nonfatal MINS, and nonfatal stroke; the composite of all-cause death, nonfatal MI, and nonfatal stroke; MINS not fulfilling the fourth universal definition of MI; or MI.
There were no differences in time to chest tube removal, days in hospital, nights in the step-down unit, or nights in the intensive care unit.
In terms of safety, there was no difference between groups on sepsis or infection, which occurred in 6.4% of patients in the colchicine group and 5.2% of those in the placebo group (hazard ratio, 1.24; 95% confidence interval, 0.93-1.66).
Noninfectious diarrhea was more common with colchicine, with 134 events (8.3%) versus 38 with placebo (2.4%), for an HR of 3.64 (95% CI, 2.54-5.22).
“In two post hoc analyses, colchicine significantly reduced the composite of the two co-primary outcomes,” Dr. Conen noted in his presentation. Clinically important perioperative AFib or MINS occurred in 22.4% in the colchicine group and 25.9% in the placebo group (HR, 0.84; 95% CI, 0.73-0.97; P = .02).
“Colchicine also significantly reduced the composite of vascular mortality, nonfatal MINS, nonfatal stroke, and clinically important AFib,” he said; 22.6% of patients in the colchicine group had one of these events versus 26.4% of those in the placebo group (HR, 0.83; 95% CI, 0.72-0.96; P = .01).
The researchers also reported significant interactions on both co-primary outcomes for the type of incision, “suggesting that stronger and statistically significant effects among patients undergoing thoracoscopic surgery as opposed to nonthoracoscopic surgery,” Dr. Conen said.
Patients undergoing thoracoscopic surgery treated with colchicine had a reduced risk for clinically important AFib (n = 2,397; HR, 0.53; 95% CI, 0.36-0.77), but colchicine treatment increased the risk in patients having open surgery (n = 784; HR, 1.59; 95% CI, 1.07-2.35; P for interaction < .0001).
There was a beneficial effect on MINS with colchicine among patients undergoing thoracoscopic surgery (HR, 0.80; 95% CI, 0.66-0.98), but no effect was seen among those having open surgery (HR, 1.15; 95% CI, 0.87-1.53; P for interaction = .041).
Low-risk patients
Jean-Claude Tardif, MD, Montreal Heart Institute and Université de Montréal, was the invited discussant for the COP-AF presentation and congratulated the researchers on “a job well done.”
He made the point that the risk for perioperative AFib has decreased substantially with the greater use of thoracoscopic rather than open surgical approaches. The population of this trial was relatively young, with an average age of 68 years; the presence of concomitant CVD was low, at about 9%; by design, patients with previous AFib were excluded; and only about 20% of patients had surgery with an open approach.
“So that population of patients were probably at relatively low risk of atrial fibrillation, and sure enough, the incidence of perioperative AFib in that population at 7.5% was lower than the assumed rate in the statistical powering of the study at 9%,” Dr. Tardif noted.
The post-hoc analyses showed a “nominally significant effect on the composite of MINS and AFib; however, that combination is fairly difficult to justify given the different pathophysiology and clinical consequences of both outcomes,” he pointed out.
The incidence of postoperative MI as a secondary outcome was low, less than 1%, as was the incidence of postoperative stroke in that study, Dr. Tardif added. “Given the link between presence of blood in the pericardium as a trigger for AFib, it would be interesting to know the incidence of perioperative pericarditis in COP-AF.”
In conclusion, he said, “when trying to put these results into the bigger picture of colchicine in cardiovascular disease, I believe we need large, well-powered clinical trials to determine the value of colchicine to reduce the risk of AFib after cardiac surgery and after catheter ablation,” Dr. Tardif said.
“We all know that colchicine represents the first line of therapy for the treatment of acute and recurrent pericarditis, and finally, low-dose colchicine, at a lower dose than was used in COP-AF, 0.5 mg once daily, is the first anti-inflammatory agent approved by both U.S. FDA and Health Canada to reduce the risk of atherothombotic events in patients with ASCVD [atherosclerotic cardiovascular disease], I believe offering a new pillar of treatment for the prevention of ischemic events in such patients.”
Session co-moderator Franz Weidinger, MD, Landstrasse Clinic, Vienna, Austria, called the COP-AF results “very important” but also noted that they show “the challenge of doing well-powered randomized trials these days when we have patients so well treated for a wide array of cardiovascular disease.”
The study was supported by the Canadian Institutes of Health Research (CIHR); Accelerating Clinical Trials Consortium; Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario; Population Health Research Institute; Hamilton Health Sciences; Division of Cardiology at McMaster University, Canada; Hanela Foundation, Switzerland; and General Research Fund, Research Grants Council, Hong Kong. Dr. Conen reports receiving research grants from CIHR, speaker fees from Servier outside the current study, and advisory board fees from Roche Diagnostics and Trimedics outside the current study.
A version of this article appeared on Medscape.com.
FROM ESC CONGRESS 2023
ESC issues new guidelines on infective endocarditis
(IE) – a rare and potentially lethal infection of the heart’s lining and valves.
The document revises the 2015 version, based on advances in imaging and a trial of antibiotic prophylaxis, among other new developments.
Cochairpersons of the writing task force, Victoria Delgado, MD, PhD, and Michael A. Borger, MD, PhD, and other members presented and discussed the guidelines in three packed sessions at the annual congress of the European Society of Cardiology, and the document was simultaneously published online in the European Heart Journal.
Endocarditis “can present with so many different clinical scenarios, so making the diagnosis can be very challenging,” Dr. Borger, from the Heart Center of Leipzig, Germany, said in an interview.
Diagnosing a lethal, rare, but not uncommon disease such as endocarditis “is something that clinicians struggle with every day,” he noted, pointing to the large overflowing auditoriums where these guidelines were presented. Dr. Borger identified four main takeaways from the document:
- Increased level of recommendation and a clearer definition of prevention and prophylaxis of endocarditis in higher-risk patients.
- An increasing role of nonechocardiographic, advanced cardiac imaging techniques in the diagnosis of endocarditis. “The advanced cardiac imaging techniques achieved the same level of recommendation as echocardiography,” he noted.
- More precisely defined indications for surgery and the timing for surgery, as well as a couple of new surgical recommendations.
- More precisely defined criteria for diagnosing and managing cardiac electronic implantable device (CIED)–associated endocarditis.
The guidelines identify patients at high risk for IE as those with previous IE and patients with surgically implanted prosthetic valves, certain congenital heart diseases, surgery with prosthetic material, or a ventricular assist device as destination therapy and recommend giving them prophylactic antibiotics before oral or dental procedures.
Patients at intermediate risk for IE include those with rheumatic heart disease, nonrheumatic degenerative valve disease, congenital valve abnormalities, CIEDs, and hypertrophic cardiomyopathy. They should be evaluated on a case-by-case basis for this prophylaxis, the guideline authors write.
Making the diagnosis
Advances in imaging techniques necessitated a revised version of the endocarditis guidelines, Dr. Borger noted.
Patients are classified as having a definite, possible, or rejected diagnosis of IE (where a definite diagnosis requires two major criteria, or one major criterion and at least three minor criteria, or five minor criteria).
The two major criteria are blood cultures positive for IE and imaging positive for IE by transesophageal echocardiography, transthoracic echocardiography, or – what is new – cardiac computed tomography, 18F-fluorodeoxyglucose positron emission tomography, or white blood cell single photon emission tomography/CT.
The five minor criteria are predisposing conditions, fever (temperature > 38° C), embolic vascular dissemination, immunologic phenomena, and microbiological evidence.
Patient education
Patient education is “paramount to early diagnosis and treatment,” Dr. Delgado, from Germans Trias i Pujol Hospital, Barcelona, said in a press release from ESC. “Those with valvular heart disease or previous heart valve surgery should be particularly diligent with regards to prevention and recognizing symptoms.”
IE occurs when bacteria or fungi enter the bloodstream, for example through skin infections, dental procedures, and surgery. Symptoms include fever, night sweats, unexplained weight loss, cough, dizziness, and fainting, the press release notes.
“We have several clinical scenarios that are increasing,” Dr. Delgado said at an Ask the Experts session, including implanted cardiac electronic devices, new transcatheter therapies, and increasing endocarditis in people who use injection drugs, “and we have recommendation of evaluation of these patients at follow up.”
“The guidelines have 34 new recommendations,” she noted in a Guideline Overview session. She drew attention to a central figure, “where we tried to summarize the pathway of the patient who is diagnosed with endocarditis, and where we highlight the role of the endocarditis team,” she said.
The guidelines specify a prophylactic antibiotic regimen for high-risk dental procedures, for children and for adults with or without allergy to penicillin or ampicillin, given as a single dose 30-60 minutes before a procedure, she noted.
A new recommendation is that systemic antibiotic prophylaxis may be considered for high-risk patients undergoing invasive procedures of the respiratory, gastrointestinal, or genitourinary tract; skin; or musculoskeletal system.
“It is very important to have a well-educated population,” Dr. Delgado stressed.
Figure 2 of the guideline depicts what patients should do, she said, such as “maintain good dental hygiene, avoid tattoos and piercings, be mindful of infections, do not self-prescribe antibiotics.” This card can be given to the patient, and they can show it to doctors before interventions.
The main targets for antibiotic prophylaxis are oral streptococci, but the emerging and increasing resistance of these bacteria are reasons why patients should not self-prescribe, Dr. Delgado noted.
“Patients should not be self-medicating in order to try to lower their risk of endocarditis,” Dr. Borger said. “They should be speaking to their physicians and have their physician group them according to their risk category.”
“If they are low risk, there’s no reason to take antibiotic prophylaxis [before oral or dental procedures], but if they are high risk, they should not only be taking antibiotic prophylaxis, they should also be doing things like good dental hygiene – visiting the dentist once or twice a year, avoiding unnecessary procedures such as tattooing and piercings, and quick aseptic management of skin wounds.”
POET Trial: Earlier shift to oral antibiotics at home
“Another very important point is the increasing use of oral outpatient antibiotic therapy based on the Partial Oral Treatment of Endocarditis (POET) randomized trial,” Dr. Borger observed. “That’s a new recommendation,” he said, “with significant implications for the care of patients with this oftentimes life-threatening disease.”
In POET, patients in stable condition who had endocarditis on the left side of the heart caused by streptococci, Enterococcus faecalis, Staphylococcus aureus, or coagulase-negative staphylococci were randomly assigned to continue treatment with intravenous antibiotics (199 patients) or to shift to step-down treatment with oral antibiotics (201 patients) after at least 10 days of initial treatment with IV antibiotics.
The 5-year results were published in 2019 in the New England Journal of Medicine and presented at ESC that year. “We were hoping or expecting to see that oral outpatient therapy would be equivalent to inpatient IV therapy,” Dr. Borger said, “but we were surprised to see that oral outpatient therapy was actually statistically significantly better in terms of survival, a very hard outcome, at 5 years after the randomization.”
“This was an important part of our new guideline document. In select patients who are ‘clinically stable’,” as defined in the guidelines, he said, “they could be successfully managed at home with oral antibiotics, rather than keeping them in hospital the whole 6 weeks.”
“In the U.S. and in Canada, a lot of patients are sent home for intravenous therapy, whereas that practice doesn’t exist in a lot of places in Europe. The patients are sitting in the hospital here for 6 weeks, oftentimes for no other reason – just to get their IV antibiotic therapy. The POET trial has shown us that that is probably the wrong thing to be doing.”
Earlier surgical intervention
The new guidelines also recommend that “once there is an indication to do cardiac surgery, it should be promptly performed,” Dr. Borger noted.
Surgery to remove infected material and drain abscesses is indicated for patients with heart failure or uncontrolled infection and to prevent embolism.
“We have defined emergency indications that should be done within 24 hours; urgent, which should be done within 3-5 days; and nonurgent, more than 5 days but within the same hospitalization,” he elaborated. “We’re basically trying to encourage surgeons and nonsurgeons that once there is an indication for surgery, there’s not a lot of benefit to just waiting. You should proceed with operation in a timely manner” to improve survival.
The guidelines recommend surgery for early prosthetic valve endocarditis, within 6 months of valve surgery, with new valve replacement and complete debridement.
Patients who present with stroke and require surgery are not uncommon, Dr. Borger noted. Ischemic stroke should not be a reason to delay surgery, and patients with hemorrhagic stoke, with favorable features, can undergo surgery.
The guidelines provide a figure for the management of CIED-related infective endocarditis. They also include a new section devoted to patient-centered care and shared decision-making.
The guidelines were endorsed by the European Association for Cardio-Thoracic Surgery and the European Association of Nuclear Medicine. The writing task force included representatives from EACTS, EANM, and the European Society of Clinical Microbiology and Infectious Diseases.
The complete guidelines, as well as pocket guidelines, essential messages, a pocket guidelines app, and an official guidelines slide set, all addressing endocarditis, are available from the ESC website.
The guidelines did not receive any funding. The disclosure forms of all experts involved in their development are available on the ESC website.
A version of this article appeared on Medscape.com.
(IE) – a rare and potentially lethal infection of the heart’s lining and valves.
The document revises the 2015 version, based on advances in imaging and a trial of antibiotic prophylaxis, among other new developments.
Cochairpersons of the writing task force, Victoria Delgado, MD, PhD, and Michael A. Borger, MD, PhD, and other members presented and discussed the guidelines in three packed sessions at the annual congress of the European Society of Cardiology, and the document was simultaneously published online in the European Heart Journal.
Endocarditis “can present with so many different clinical scenarios, so making the diagnosis can be very challenging,” Dr. Borger, from the Heart Center of Leipzig, Germany, said in an interview.
Diagnosing a lethal, rare, but not uncommon disease such as endocarditis “is something that clinicians struggle with every day,” he noted, pointing to the large overflowing auditoriums where these guidelines were presented. Dr. Borger identified four main takeaways from the document:
- Increased level of recommendation and a clearer definition of prevention and prophylaxis of endocarditis in higher-risk patients.
- An increasing role of nonechocardiographic, advanced cardiac imaging techniques in the diagnosis of endocarditis. “The advanced cardiac imaging techniques achieved the same level of recommendation as echocardiography,” he noted.
- More precisely defined indications for surgery and the timing for surgery, as well as a couple of new surgical recommendations.
- More precisely defined criteria for diagnosing and managing cardiac electronic implantable device (CIED)–associated endocarditis.
The guidelines identify patients at high risk for IE as those with previous IE and patients with surgically implanted prosthetic valves, certain congenital heart diseases, surgery with prosthetic material, or a ventricular assist device as destination therapy and recommend giving them prophylactic antibiotics before oral or dental procedures.
Patients at intermediate risk for IE include those with rheumatic heart disease, nonrheumatic degenerative valve disease, congenital valve abnormalities, CIEDs, and hypertrophic cardiomyopathy. They should be evaluated on a case-by-case basis for this prophylaxis, the guideline authors write.
Making the diagnosis
Advances in imaging techniques necessitated a revised version of the endocarditis guidelines, Dr. Borger noted.
Patients are classified as having a definite, possible, or rejected diagnosis of IE (where a definite diagnosis requires two major criteria, or one major criterion and at least three minor criteria, or five minor criteria).
The two major criteria are blood cultures positive for IE and imaging positive for IE by transesophageal echocardiography, transthoracic echocardiography, or – what is new – cardiac computed tomography, 18F-fluorodeoxyglucose positron emission tomography, or white blood cell single photon emission tomography/CT.
The five minor criteria are predisposing conditions, fever (temperature > 38° C), embolic vascular dissemination, immunologic phenomena, and microbiological evidence.
Patient education
Patient education is “paramount to early diagnosis and treatment,” Dr. Delgado, from Germans Trias i Pujol Hospital, Barcelona, said in a press release from ESC. “Those with valvular heart disease or previous heart valve surgery should be particularly diligent with regards to prevention and recognizing symptoms.”
IE occurs when bacteria or fungi enter the bloodstream, for example through skin infections, dental procedures, and surgery. Symptoms include fever, night sweats, unexplained weight loss, cough, dizziness, and fainting, the press release notes.
“We have several clinical scenarios that are increasing,” Dr. Delgado said at an Ask the Experts session, including implanted cardiac electronic devices, new transcatheter therapies, and increasing endocarditis in people who use injection drugs, “and we have recommendation of evaluation of these patients at follow up.”
“The guidelines have 34 new recommendations,” she noted in a Guideline Overview session. She drew attention to a central figure, “where we tried to summarize the pathway of the patient who is diagnosed with endocarditis, and where we highlight the role of the endocarditis team,” she said.
The guidelines specify a prophylactic antibiotic regimen for high-risk dental procedures, for children and for adults with or without allergy to penicillin or ampicillin, given as a single dose 30-60 minutes before a procedure, she noted.
A new recommendation is that systemic antibiotic prophylaxis may be considered for high-risk patients undergoing invasive procedures of the respiratory, gastrointestinal, or genitourinary tract; skin; or musculoskeletal system.
“It is very important to have a well-educated population,” Dr. Delgado stressed.
Figure 2 of the guideline depicts what patients should do, she said, such as “maintain good dental hygiene, avoid tattoos and piercings, be mindful of infections, do not self-prescribe antibiotics.” This card can be given to the patient, and they can show it to doctors before interventions.
The main targets for antibiotic prophylaxis are oral streptococci, but the emerging and increasing resistance of these bacteria are reasons why patients should not self-prescribe, Dr. Delgado noted.
“Patients should not be self-medicating in order to try to lower their risk of endocarditis,” Dr. Borger said. “They should be speaking to their physicians and have their physician group them according to their risk category.”
“If they are low risk, there’s no reason to take antibiotic prophylaxis [before oral or dental procedures], but if they are high risk, they should not only be taking antibiotic prophylaxis, they should also be doing things like good dental hygiene – visiting the dentist once or twice a year, avoiding unnecessary procedures such as tattooing and piercings, and quick aseptic management of skin wounds.”
POET Trial: Earlier shift to oral antibiotics at home
“Another very important point is the increasing use of oral outpatient antibiotic therapy based on the Partial Oral Treatment of Endocarditis (POET) randomized trial,” Dr. Borger observed. “That’s a new recommendation,” he said, “with significant implications for the care of patients with this oftentimes life-threatening disease.”
In POET, patients in stable condition who had endocarditis on the left side of the heart caused by streptococci, Enterococcus faecalis, Staphylococcus aureus, or coagulase-negative staphylococci were randomly assigned to continue treatment with intravenous antibiotics (199 patients) or to shift to step-down treatment with oral antibiotics (201 patients) after at least 10 days of initial treatment with IV antibiotics.
The 5-year results were published in 2019 in the New England Journal of Medicine and presented at ESC that year. “We were hoping or expecting to see that oral outpatient therapy would be equivalent to inpatient IV therapy,” Dr. Borger said, “but we were surprised to see that oral outpatient therapy was actually statistically significantly better in terms of survival, a very hard outcome, at 5 years after the randomization.”
“This was an important part of our new guideline document. In select patients who are ‘clinically stable’,” as defined in the guidelines, he said, “they could be successfully managed at home with oral antibiotics, rather than keeping them in hospital the whole 6 weeks.”
“In the U.S. and in Canada, a lot of patients are sent home for intravenous therapy, whereas that practice doesn’t exist in a lot of places in Europe. The patients are sitting in the hospital here for 6 weeks, oftentimes for no other reason – just to get their IV antibiotic therapy. The POET trial has shown us that that is probably the wrong thing to be doing.”
Earlier surgical intervention
The new guidelines also recommend that “once there is an indication to do cardiac surgery, it should be promptly performed,” Dr. Borger noted.
Surgery to remove infected material and drain abscesses is indicated for patients with heart failure or uncontrolled infection and to prevent embolism.
“We have defined emergency indications that should be done within 24 hours; urgent, which should be done within 3-5 days; and nonurgent, more than 5 days but within the same hospitalization,” he elaborated. “We’re basically trying to encourage surgeons and nonsurgeons that once there is an indication for surgery, there’s not a lot of benefit to just waiting. You should proceed with operation in a timely manner” to improve survival.
The guidelines recommend surgery for early prosthetic valve endocarditis, within 6 months of valve surgery, with new valve replacement and complete debridement.
Patients who present with stroke and require surgery are not uncommon, Dr. Borger noted. Ischemic stroke should not be a reason to delay surgery, and patients with hemorrhagic stoke, with favorable features, can undergo surgery.
The guidelines provide a figure for the management of CIED-related infective endocarditis. They also include a new section devoted to patient-centered care and shared decision-making.
The guidelines were endorsed by the European Association for Cardio-Thoracic Surgery and the European Association of Nuclear Medicine. The writing task force included representatives from EACTS, EANM, and the European Society of Clinical Microbiology and Infectious Diseases.
The complete guidelines, as well as pocket guidelines, essential messages, a pocket guidelines app, and an official guidelines slide set, all addressing endocarditis, are available from the ESC website.
The guidelines did not receive any funding. The disclosure forms of all experts involved in their development are available on the ESC website.
A version of this article appeared on Medscape.com.
(IE) – a rare and potentially lethal infection of the heart’s lining and valves.
The document revises the 2015 version, based on advances in imaging and a trial of antibiotic prophylaxis, among other new developments.
Cochairpersons of the writing task force, Victoria Delgado, MD, PhD, and Michael A. Borger, MD, PhD, and other members presented and discussed the guidelines in three packed sessions at the annual congress of the European Society of Cardiology, and the document was simultaneously published online in the European Heart Journal.
Endocarditis “can present with so many different clinical scenarios, so making the diagnosis can be very challenging,” Dr. Borger, from the Heart Center of Leipzig, Germany, said in an interview.
Diagnosing a lethal, rare, but not uncommon disease such as endocarditis “is something that clinicians struggle with every day,” he noted, pointing to the large overflowing auditoriums where these guidelines were presented. Dr. Borger identified four main takeaways from the document:
- Increased level of recommendation and a clearer definition of prevention and prophylaxis of endocarditis in higher-risk patients.
- An increasing role of nonechocardiographic, advanced cardiac imaging techniques in the diagnosis of endocarditis. “The advanced cardiac imaging techniques achieved the same level of recommendation as echocardiography,” he noted.
- More precisely defined indications for surgery and the timing for surgery, as well as a couple of new surgical recommendations.
- More precisely defined criteria for diagnosing and managing cardiac electronic implantable device (CIED)–associated endocarditis.
The guidelines identify patients at high risk for IE as those with previous IE and patients with surgically implanted prosthetic valves, certain congenital heart diseases, surgery with prosthetic material, or a ventricular assist device as destination therapy and recommend giving them prophylactic antibiotics before oral or dental procedures.
Patients at intermediate risk for IE include those with rheumatic heart disease, nonrheumatic degenerative valve disease, congenital valve abnormalities, CIEDs, and hypertrophic cardiomyopathy. They should be evaluated on a case-by-case basis for this prophylaxis, the guideline authors write.
Making the diagnosis
Advances in imaging techniques necessitated a revised version of the endocarditis guidelines, Dr. Borger noted.
Patients are classified as having a definite, possible, or rejected diagnosis of IE (where a definite diagnosis requires two major criteria, or one major criterion and at least three minor criteria, or five minor criteria).
The two major criteria are blood cultures positive for IE and imaging positive for IE by transesophageal echocardiography, transthoracic echocardiography, or – what is new – cardiac computed tomography, 18F-fluorodeoxyglucose positron emission tomography, or white blood cell single photon emission tomography/CT.
The five minor criteria are predisposing conditions, fever (temperature > 38° C), embolic vascular dissemination, immunologic phenomena, and microbiological evidence.
Patient education
Patient education is “paramount to early diagnosis and treatment,” Dr. Delgado, from Germans Trias i Pujol Hospital, Barcelona, said in a press release from ESC. “Those with valvular heart disease or previous heart valve surgery should be particularly diligent with regards to prevention and recognizing symptoms.”
IE occurs when bacteria or fungi enter the bloodstream, for example through skin infections, dental procedures, and surgery. Symptoms include fever, night sweats, unexplained weight loss, cough, dizziness, and fainting, the press release notes.
“We have several clinical scenarios that are increasing,” Dr. Delgado said at an Ask the Experts session, including implanted cardiac electronic devices, new transcatheter therapies, and increasing endocarditis in people who use injection drugs, “and we have recommendation of evaluation of these patients at follow up.”
“The guidelines have 34 new recommendations,” she noted in a Guideline Overview session. She drew attention to a central figure, “where we tried to summarize the pathway of the patient who is diagnosed with endocarditis, and where we highlight the role of the endocarditis team,” she said.
The guidelines specify a prophylactic antibiotic regimen for high-risk dental procedures, for children and for adults with or without allergy to penicillin or ampicillin, given as a single dose 30-60 minutes before a procedure, she noted.
A new recommendation is that systemic antibiotic prophylaxis may be considered for high-risk patients undergoing invasive procedures of the respiratory, gastrointestinal, or genitourinary tract; skin; or musculoskeletal system.
“It is very important to have a well-educated population,” Dr. Delgado stressed.
Figure 2 of the guideline depicts what patients should do, she said, such as “maintain good dental hygiene, avoid tattoos and piercings, be mindful of infections, do not self-prescribe antibiotics.” This card can be given to the patient, and they can show it to doctors before interventions.
The main targets for antibiotic prophylaxis are oral streptococci, but the emerging and increasing resistance of these bacteria are reasons why patients should not self-prescribe, Dr. Delgado noted.
“Patients should not be self-medicating in order to try to lower their risk of endocarditis,” Dr. Borger said. “They should be speaking to their physicians and have their physician group them according to their risk category.”
“If they are low risk, there’s no reason to take antibiotic prophylaxis [before oral or dental procedures], but if they are high risk, they should not only be taking antibiotic prophylaxis, they should also be doing things like good dental hygiene – visiting the dentist once or twice a year, avoiding unnecessary procedures such as tattooing and piercings, and quick aseptic management of skin wounds.”
POET Trial: Earlier shift to oral antibiotics at home
“Another very important point is the increasing use of oral outpatient antibiotic therapy based on the Partial Oral Treatment of Endocarditis (POET) randomized trial,” Dr. Borger observed. “That’s a new recommendation,” he said, “with significant implications for the care of patients with this oftentimes life-threatening disease.”
In POET, patients in stable condition who had endocarditis on the left side of the heart caused by streptococci, Enterococcus faecalis, Staphylococcus aureus, or coagulase-negative staphylococci were randomly assigned to continue treatment with intravenous antibiotics (199 patients) or to shift to step-down treatment with oral antibiotics (201 patients) after at least 10 days of initial treatment with IV antibiotics.
The 5-year results were published in 2019 in the New England Journal of Medicine and presented at ESC that year. “We were hoping or expecting to see that oral outpatient therapy would be equivalent to inpatient IV therapy,” Dr. Borger said, “but we were surprised to see that oral outpatient therapy was actually statistically significantly better in terms of survival, a very hard outcome, at 5 years after the randomization.”
“This was an important part of our new guideline document. In select patients who are ‘clinically stable’,” as defined in the guidelines, he said, “they could be successfully managed at home with oral antibiotics, rather than keeping them in hospital the whole 6 weeks.”
“In the U.S. and in Canada, a lot of patients are sent home for intravenous therapy, whereas that practice doesn’t exist in a lot of places in Europe. The patients are sitting in the hospital here for 6 weeks, oftentimes for no other reason – just to get their IV antibiotic therapy. The POET trial has shown us that that is probably the wrong thing to be doing.”
Earlier surgical intervention
The new guidelines also recommend that “once there is an indication to do cardiac surgery, it should be promptly performed,” Dr. Borger noted.
Surgery to remove infected material and drain abscesses is indicated for patients with heart failure or uncontrolled infection and to prevent embolism.
“We have defined emergency indications that should be done within 24 hours; urgent, which should be done within 3-5 days; and nonurgent, more than 5 days but within the same hospitalization,” he elaborated. “We’re basically trying to encourage surgeons and nonsurgeons that once there is an indication for surgery, there’s not a lot of benefit to just waiting. You should proceed with operation in a timely manner” to improve survival.
The guidelines recommend surgery for early prosthetic valve endocarditis, within 6 months of valve surgery, with new valve replacement and complete debridement.
Patients who present with stroke and require surgery are not uncommon, Dr. Borger noted. Ischemic stroke should not be a reason to delay surgery, and patients with hemorrhagic stoke, with favorable features, can undergo surgery.
The guidelines provide a figure for the management of CIED-related infective endocarditis. They also include a new section devoted to patient-centered care and shared decision-making.
The guidelines were endorsed by the European Association for Cardio-Thoracic Surgery and the European Association of Nuclear Medicine. The writing task force included representatives from EACTS, EANM, and the European Society of Clinical Microbiology and Infectious Diseases.
The complete guidelines, as well as pocket guidelines, essential messages, a pocket guidelines app, and an official guidelines slide set, all addressing endocarditis, are available from the ESC website.
The guidelines did not receive any funding. The disclosure forms of all experts involved in their development are available on the ESC website.
A version of this article appeared on Medscape.com.
FROM ESC CONGRESS 2023