User login
Expert shares top five atopic dermatitis–related questions he fields
Will my child outgrow the eczema?
That is perhaps the No. 1 atopic dermatitis–related question that Lawrence F. Eichenfield, MD, fields from parents in his role as chief of pediatric and adolescent dermatology at Rady’s Children’s Hospital, San Diego.
The answer “is pretty tricky,” he said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We used to say, ‘yeah, your kid will probably outgrow the disease,’ but we now have good data that show there are variable courses.”
Using data from the birth study cohort known as the Avon Longitudinal Study of Parents and Children, researchers in the United Kingdom investigated the existence of different longitudinal phenotypes of AD among 9,894 children. They found that 58% of the children in the cohort were unaffected or had transient AD, while 12.9% had early-onset/early-resolving AD. The remaining AD phenotypes consisted of 7%-8% patients each (early-onset persistent, early-onset late-resolving, mid-onset resolving, and late-onset resolving).
“There have been several studies that looked at the natural course of AD,” said Dr. Eichenfield, distinguished professor of dermatology and pediatrics at the University of California, San Diego. “A cohort study from Thailand showed that 50% of patients with childhood AD lost their AD diagnosis about 5 years into it, while there was an increase in allergic rhino-conjunctivitis and asthma, similar to what’s been seen in atopic march studies,” he noted.
A separate group of investigators analyzed records from The Health Improvement Network in the UK to determine the prevalence of AD among more than 8 million patients seen in primary care between 1994 and 2013. They found that the cumulative lifetime prevalence of atopic eczema was 9.9% and the highest rates of active disease were among children and older adults. “The takeaway was markedly inconsistent in terms of whether AD went away over time or increased over time, so it’s really not especially helpful prevalence data,” Dr. Eichenfield said. “Overall, you have a high prevalence in the first years of life, it decreases, and it may increase again when people are 60 years and older. Whether that’s truly AD or xerotic eczema isn’t known in this data set.”
A separate meta-analysis of 17 studies reported that 26% of adults with AD said they had adult-onset disease, which is characterized by more atopy, more foot dermatitis, and less flexural involvement.
Dr. Eichenfield tells parents, “there’s a really good chance (depending on disease severity) that 60% to 70% of children will outgrow their eczema or most of it,” he said. “If you ask me when, I won’t tell you. The important thing is to treat it to minimize its impact. We want minimal rash, minimal itch, and minimal sleep disturbance. Sometimes I say, ‘that might improve the chance of the eczema getting better over time.’ ”
Following are four other common questions parents and patients ask him:
Can we figure out the allergies causing the eczema? “This is probably one of the most unnerving questions I get asked,” he said. “It’s a loaded question. My answer is that allergies are intertwined with AD. Searching for the secret allergy causing the atopic dermatitis is rarely successful.” Sensitization is much more common with AD, he added, meaning specific IgE testing, whether it be blood testing or skin prick testing. “The more severe your eczema is, the more chance you’ll have of real food allergy,” he said. “About 15% of milder eczema patients will have at least one food allergy, but when you get to the more moderate to severe cases, about 40% will have a true food allergy.”
Food reactions may not cause eczema, though. Food reactions can cause urticaria, angioedema, eczematous dermatitis, allergic contact dermatitis, contact urticaria, and respiratory findings. According to National Institutes of Health guidelines for food allergy, skin prick tests and serum IgE tests are recommended to assist in identification of foods that may be provoking IgE-mediated food reactions, but are not diagnostic of food allergy.
“There’s a huge literature showing that there’s a lot of food allergy testing that’s just not helpful,” he said. In one study, 89% of food challenges administered in patients who were listed as being allergic based on skin prick tests or serum IgE tests did not have a true food allergy.
“Empiric elimination diets aren’t especially useful. However, we occasionally see children who do have AD exacerbated by food allergies in the first year of life,” he said. NIH guidelines suggest that children younger than 5 years of age with moderate to severe AD be considered for food allergy evaluation for milk, egg, peanut, wheat, and soy, if at least one of the following conditions is met: the child has persistent AD in spite of optimized management and topical therapy, and/or the child has a reliable history of an immediate reaction after ingestion of a specific food.
“We do know that there are high rates of comorbid allergic processes, besides food allergy, associated with atopic dermatitis, including allergic rhinitis and asthma both in children and adults,” Dr. Eichenfield said. “I do discuss allergy triggers and their importance in the life of the individual, though not necessarily as factors in AD. There are a variety of environmental allergens and/or environmental triggers that can significantly impact AD. Recently, we have seen studies discussing air pollution and wildfires as exacerbators of AD.”
How should I bathe and moisturize? There are no standard guidelines for the frequency, type, or duration of bathing in patients with AD, he said, though in more severe disease, frequent bathing can be helpful along with standard anti-inflammatory topical medicines. “I keep my general recommendations vague,” Dr. Eichenfield said. “I do explain that we don’t want to use harsh soaps; we want to be gentle in our washing. I usually recommend daily to every other day bathing. It’s important to pat the skin dry and then apply a moisturizer. Applying a moisturizer 2-3 minutes after bathing is important and limited significant cleanser use can be helpful.”
Moisturizers and emollients are a standard of care in U.S. guidelines published in 2013 and 2014, and international guidelines, and are steroid-sparing and useful for both prevention and maintenance. “I tell parents and patients that there is no reason to avoid bathing because of AD as long as you moisturize after,” he said.
Do I have to use topical [name of drug]? “I try to explain that there is skin barrier dysfunction that stimulates the inflammatory milieu, and that inflammation in the skin or blood in AD negatively impacts skin barrier function,” Dr. Eichenfield said. “I explain that if inflammation doesn’t get better with good skin care, moisturizers, and avoidance of triggers, we need anti-inflammatory medication. Then we discuss what the options are, the significant variation in strengths of topical corticosteroids, and topical nonsteroid options.”
When he counsels parents and patients on the use of topical corticosteroids, he tells them that cortisone is a naturally-occurring metabolite, and that “we can work together to let you know how much medicine to use, and how a safe amount is a powerful tool to fix the eczema.” He often says that topical steroids “are like hammers. We have tiny hammers, like over-the-counter hydrocortisone, and sledgehammers like clobetasol. We also have ‘screwdrivers’ and ‘pliers’ with nonsteroidal topical calcineurin and PDE-4 inhibitors, which are especially useful for maintenance therapy. Topical ruxolitinib is a new medicine that we may use for patients as well. The label includes discussion of side effects from oral JAK inhibitors as well as from the drug development program, so it takes some time to talk through.”
Is it time for a stronger systemic medicine? Any conversation about this topic should support the concept that the AD is multifactorial. “We have the rash of eczema,” he said. “We have the itch. We have impact on sleep disturbance. We have the comorbidities. We have other physical changes, which can happen with bacterial infections and other immune system or cardiovascular changes. We have the impact on quality of life and impact on school and work. When we recognize that if patients have significant enough disease that it is not getting better with topicals and is having a negative impact on their lives, we can move our discussion to systemic therapy.”
When counseling patients about systemic therapy, Dr. Eichenfield will conduct a body surface area assessment and document how bad the itch is. “But I’m not just recording the information; I’m bringing it out in the room,” he said. “I’ll do a BSA assessment and say, for example, ‘oh, you have 32% of your body involved with eczema.’ I ask about sleep disturbance, to get the answer ‘out in the room.’ ” He also asks questions such as: “When was the last time your skin was last totally clear? Are there activities that you or your family don’t do because of your eczema, or that you’re living your life around it? Is there anxiety or depression?” Documenting both the impact on quality of life and the severity of disease “makes it easier to discuss systemic therapy,” Dr. Eichenfield said. “Meanwhile, as the provider, I am trying to figure out if the patient should ‘go into the topical therapy bucket’ or into the ‘systemic therapy bucket.’ ”
Counseling about systemic therapy includes shared decision-making regarding the choice of biologics versus oral JAK inhibitors versus traditional systemic agent or phototherapy. Factors to consider in the decision making include patient age, sex, severity, comorbidities, prior therapy, risk aversion, duration, medication access, and desired efficacy. “Evolving therapies can change the conversation, the questions, and the outcomes, but the overarching desired outcome is long-term disease control, minimal eczematous rash, minimal pruritus, and minimal sleep disturbance,” he said.
Dr. Eichenfield disclosed that he has served as a consultant to or investigator for AbbVie; Almirall; Arcutis; Arena; Asana; Termagant; Dermira; Forte Biosciences; Galderma Laboratories; Glenmark/Chinos; Incyte; Kyowa Kirin; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Otsuka; Pfizer; Freestone; Regeneron, and Sanofi Genzyme.
MedscapeLive and this news organization are owned by the same parent company.
Will my child outgrow the eczema?
That is perhaps the No. 1 atopic dermatitis–related question that Lawrence F. Eichenfield, MD, fields from parents in his role as chief of pediatric and adolescent dermatology at Rady’s Children’s Hospital, San Diego.
The answer “is pretty tricky,” he said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We used to say, ‘yeah, your kid will probably outgrow the disease,’ but we now have good data that show there are variable courses.”
Using data from the birth study cohort known as the Avon Longitudinal Study of Parents and Children, researchers in the United Kingdom investigated the existence of different longitudinal phenotypes of AD among 9,894 children. They found that 58% of the children in the cohort were unaffected or had transient AD, while 12.9% had early-onset/early-resolving AD. The remaining AD phenotypes consisted of 7%-8% patients each (early-onset persistent, early-onset late-resolving, mid-onset resolving, and late-onset resolving).
“There have been several studies that looked at the natural course of AD,” said Dr. Eichenfield, distinguished professor of dermatology and pediatrics at the University of California, San Diego. “A cohort study from Thailand showed that 50% of patients with childhood AD lost their AD diagnosis about 5 years into it, while there was an increase in allergic rhino-conjunctivitis and asthma, similar to what’s been seen in atopic march studies,” he noted.
A separate group of investigators analyzed records from The Health Improvement Network in the UK to determine the prevalence of AD among more than 8 million patients seen in primary care between 1994 and 2013. They found that the cumulative lifetime prevalence of atopic eczema was 9.9% and the highest rates of active disease were among children and older adults. “The takeaway was markedly inconsistent in terms of whether AD went away over time or increased over time, so it’s really not especially helpful prevalence data,” Dr. Eichenfield said. “Overall, you have a high prevalence in the first years of life, it decreases, and it may increase again when people are 60 years and older. Whether that’s truly AD or xerotic eczema isn’t known in this data set.”
A separate meta-analysis of 17 studies reported that 26% of adults with AD said they had adult-onset disease, which is characterized by more atopy, more foot dermatitis, and less flexural involvement.
Dr. Eichenfield tells parents, “there’s a really good chance (depending on disease severity) that 60% to 70% of children will outgrow their eczema or most of it,” he said. “If you ask me when, I won’t tell you. The important thing is to treat it to minimize its impact. We want minimal rash, minimal itch, and minimal sleep disturbance. Sometimes I say, ‘that might improve the chance of the eczema getting better over time.’ ”
Following are four other common questions parents and patients ask him:
Can we figure out the allergies causing the eczema? “This is probably one of the most unnerving questions I get asked,” he said. “It’s a loaded question. My answer is that allergies are intertwined with AD. Searching for the secret allergy causing the atopic dermatitis is rarely successful.” Sensitization is much more common with AD, he added, meaning specific IgE testing, whether it be blood testing or skin prick testing. “The more severe your eczema is, the more chance you’ll have of real food allergy,” he said. “About 15% of milder eczema patients will have at least one food allergy, but when you get to the more moderate to severe cases, about 40% will have a true food allergy.”
Food reactions may not cause eczema, though. Food reactions can cause urticaria, angioedema, eczematous dermatitis, allergic contact dermatitis, contact urticaria, and respiratory findings. According to National Institutes of Health guidelines for food allergy, skin prick tests and serum IgE tests are recommended to assist in identification of foods that may be provoking IgE-mediated food reactions, but are not diagnostic of food allergy.
“There’s a huge literature showing that there’s a lot of food allergy testing that’s just not helpful,” he said. In one study, 89% of food challenges administered in patients who were listed as being allergic based on skin prick tests or serum IgE tests did not have a true food allergy.
“Empiric elimination diets aren’t especially useful. However, we occasionally see children who do have AD exacerbated by food allergies in the first year of life,” he said. NIH guidelines suggest that children younger than 5 years of age with moderate to severe AD be considered for food allergy evaluation for milk, egg, peanut, wheat, and soy, if at least one of the following conditions is met: the child has persistent AD in spite of optimized management and topical therapy, and/or the child has a reliable history of an immediate reaction after ingestion of a specific food.
“We do know that there are high rates of comorbid allergic processes, besides food allergy, associated with atopic dermatitis, including allergic rhinitis and asthma both in children and adults,” Dr. Eichenfield said. “I do discuss allergy triggers and their importance in the life of the individual, though not necessarily as factors in AD. There are a variety of environmental allergens and/or environmental triggers that can significantly impact AD. Recently, we have seen studies discussing air pollution and wildfires as exacerbators of AD.”
How should I bathe and moisturize? There are no standard guidelines for the frequency, type, or duration of bathing in patients with AD, he said, though in more severe disease, frequent bathing can be helpful along with standard anti-inflammatory topical medicines. “I keep my general recommendations vague,” Dr. Eichenfield said. “I do explain that we don’t want to use harsh soaps; we want to be gentle in our washing. I usually recommend daily to every other day bathing. It’s important to pat the skin dry and then apply a moisturizer. Applying a moisturizer 2-3 minutes after bathing is important and limited significant cleanser use can be helpful.”
Moisturizers and emollients are a standard of care in U.S. guidelines published in 2013 and 2014, and international guidelines, and are steroid-sparing and useful for both prevention and maintenance. “I tell parents and patients that there is no reason to avoid bathing because of AD as long as you moisturize after,” he said.
Do I have to use topical [name of drug]? “I try to explain that there is skin barrier dysfunction that stimulates the inflammatory milieu, and that inflammation in the skin or blood in AD negatively impacts skin barrier function,” Dr. Eichenfield said. “I explain that if inflammation doesn’t get better with good skin care, moisturizers, and avoidance of triggers, we need anti-inflammatory medication. Then we discuss what the options are, the significant variation in strengths of topical corticosteroids, and topical nonsteroid options.”
When he counsels parents and patients on the use of topical corticosteroids, he tells them that cortisone is a naturally-occurring metabolite, and that “we can work together to let you know how much medicine to use, and how a safe amount is a powerful tool to fix the eczema.” He often says that topical steroids “are like hammers. We have tiny hammers, like over-the-counter hydrocortisone, and sledgehammers like clobetasol. We also have ‘screwdrivers’ and ‘pliers’ with nonsteroidal topical calcineurin and PDE-4 inhibitors, which are especially useful for maintenance therapy. Topical ruxolitinib is a new medicine that we may use for patients as well. The label includes discussion of side effects from oral JAK inhibitors as well as from the drug development program, so it takes some time to talk through.”
Is it time for a stronger systemic medicine? Any conversation about this topic should support the concept that the AD is multifactorial. “We have the rash of eczema,” he said. “We have the itch. We have impact on sleep disturbance. We have the comorbidities. We have other physical changes, which can happen with bacterial infections and other immune system or cardiovascular changes. We have the impact on quality of life and impact on school and work. When we recognize that if patients have significant enough disease that it is not getting better with topicals and is having a negative impact on their lives, we can move our discussion to systemic therapy.”
When counseling patients about systemic therapy, Dr. Eichenfield will conduct a body surface area assessment and document how bad the itch is. “But I’m not just recording the information; I’m bringing it out in the room,” he said. “I’ll do a BSA assessment and say, for example, ‘oh, you have 32% of your body involved with eczema.’ I ask about sleep disturbance, to get the answer ‘out in the room.’ ” He also asks questions such as: “When was the last time your skin was last totally clear? Are there activities that you or your family don’t do because of your eczema, or that you’re living your life around it? Is there anxiety or depression?” Documenting both the impact on quality of life and the severity of disease “makes it easier to discuss systemic therapy,” Dr. Eichenfield said. “Meanwhile, as the provider, I am trying to figure out if the patient should ‘go into the topical therapy bucket’ or into the ‘systemic therapy bucket.’ ”
Counseling about systemic therapy includes shared decision-making regarding the choice of biologics versus oral JAK inhibitors versus traditional systemic agent or phototherapy. Factors to consider in the decision making include patient age, sex, severity, comorbidities, prior therapy, risk aversion, duration, medication access, and desired efficacy. “Evolving therapies can change the conversation, the questions, and the outcomes, but the overarching desired outcome is long-term disease control, minimal eczematous rash, minimal pruritus, and minimal sleep disturbance,” he said.
Dr. Eichenfield disclosed that he has served as a consultant to or investigator for AbbVie; Almirall; Arcutis; Arena; Asana; Termagant; Dermira; Forte Biosciences; Galderma Laboratories; Glenmark/Chinos; Incyte; Kyowa Kirin; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Otsuka; Pfizer; Freestone; Regeneron, and Sanofi Genzyme.
MedscapeLive and this news organization are owned by the same parent company.
Will my child outgrow the eczema?
That is perhaps the No. 1 atopic dermatitis–related question that Lawrence F. Eichenfield, MD, fields from parents in his role as chief of pediatric and adolescent dermatology at Rady’s Children’s Hospital, San Diego.
The answer “is pretty tricky,” he said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We used to say, ‘yeah, your kid will probably outgrow the disease,’ but we now have good data that show there are variable courses.”
Using data from the birth study cohort known as the Avon Longitudinal Study of Parents and Children, researchers in the United Kingdom investigated the existence of different longitudinal phenotypes of AD among 9,894 children. They found that 58% of the children in the cohort were unaffected or had transient AD, while 12.9% had early-onset/early-resolving AD. The remaining AD phenotypes consisted of 7%-8% patients each (early-onset persistent, early-onset late-resolving, mid-onset resolving, and late-onset resolving).
“There have been several studies that looked at the natural course of AD,” said Dr. Eichenfield, distinguished professor of dermatology and pediatrics at the University of California, San Diego. “A cohort study from Thailand showed that 50% of patients with childhood AD lost their AD diagnosis about 5 years into it, while there was an increase in allergic rhino-conjunctivitis and asthma, similar to what’s been seen in atopic march studies,” he noted.
A separate group of investigators analyzed records from The Health Improvement Network in the UK to determine the prevalence of AD among more than 8 million patients seen in primary care between 1994 and 2013. They found that the cumulative lifetime prevalence of atopic eczema was 9.9% and the highest rates of active disease were among children and older adults. “The takeaway was markedly inconsistent in terms of whether AD went away over time or increased over time, so it’s really not especially helpful prevalence data,” Dr. Eichenfield said. “Overall, you have a high prevalence in the first years of life, it decreases, and it may increase again when people are 60 years and older. Whether that’s truly AD or xerotic eczema isn’t known in this data set.”
A separate meta-analysis of 17 studies reported that 26% of adults with AD said they had adult-onset disease, which is characterized by more atopy, more foot dermatitis, and less flexural involvement.
Dr. Eichenfield tells parents, “there’s a really good chance (depending on disease severity) that 60% to 70% of children will outgrow their eczema or most of it,” he said. “If you ask me when, I won’t tell you. The important thing is to treat it to minimize its impact. We want minimal rash, minimal itch, and minimal sleep disturbance. Sometimes I say, ‘that might improve the chance of the eczema getting better over time.’ ”
Following are four other common questions parents and patients ask him:
Can we figure out the allergies causing the eczema? “This is probably one of the most unnerving questions I get asked,” he said. “It’s a loaded question. My answer is that allergies are intertwined with AD. Searching for the secret allergy causing the atopic dermatitis is rarely successful.” Sensitization is much more common with AD, he added, meaning specific IgE testing, whether it be blood testing or skin prick testing. “The more severe your eczema is, the more chance you’ll have of real food allergy,” he said. “About 15% of milder eczema patients will have at least one food allergy, but when you get to the more moderate to severe cases, about 40% will have a true food allergy.”
Food reactions may not cause eczema, though. Food reactions can cause urticaria, angioedema, eczematous dermatitis, allergic contact dermatitis, contact urticaria, and respiratory findings. According to National Institutes of Health guidelines for food allergy, skin prick tests and serum IgE tests are recommended to assist in identification of foods that may be provoking IgE-mediated food reactions, but are not diagnostic of food allergy.
“There’s a huge literature showing that there’s a lot of food allergy testing that’s just not helpful,” he said. In one study, 89% of food challenges administered in patients who were listed as being allergic based on skin prick tests or serum IgE tests did not have a true food allergy.
“Empiric elimination diets aren’t especially useful. However, we occasionally see children who do have AD exacerbated by food allergies in the first year of life,” he said. NIH guidelines suggest that children younger than 5 years of age with moderate to severe AD be considered for food allergy evaluation for milk, egg, peanut, wheat, and soy, if at least one of the following conditions is met: the child has persistent AD in spite of optimized management and topical therapy, and/or the child has a reliable history of an immediate reaction after ingestion of a specific food.
“We do know that there are high rates of comorbid allergic processes, besides food allergy, associated with atopic dermatitis, including allergic rhinitis and asthma both in children and adults,” Dr. Eichenfield said. “I do discuss allergy triggers and their importance in the life of the individual, though not necessarily as factors in AD. There are a variety of environmental allergens and/or environmental triggers that can significantly impact AD. Recently, we have seen studies discussing air pollution and wildfires as exacerbators of AD.”
How should I bathe and moisturize? There are no standard guidelines for the frequency, type, or duration of bathing in patients with AD, he said, though in more severe disease, frequent bathing can be helpful along with standard anti-inflammatory topical medicines. “I keep my general recommendations vague,” Dr. Eichenfield said. “I do explain that we don’t want to use harsh soaps; we want to be gentle in our washing. I usually recommend daily to every other day bathing. It’s important to pat the skin dry and then apply a moisturizer. Applying a moisturizer 2-3 minutes after bathing is important and limited significant cleanser use can be helpful.”
Moisturizers and emollients are a standard of care in U.S. guidelines published in 2013 and 2014, and international guidelines, and are steroid-sparing and useful for both prevention and maintenance. “I tell parents and patients that there is no reason to avoid bathing because of AD as long as you moisturize after,” he said.
Do I have to use topical [name of drug]? “I try to explain that there is skin barrier dysfunction that stimulates the inflammatory milieu, and that inflammation in the skin or blood in AD negatively impacts skin barrier function,” Dr. Eichenfield said. “I explain that if inflammation doesn’t get better with good skin care, moisturizers, and avoidance of triggers, we need anti-inflammatory medication. Then we discuss what the options are, the significant variation in strengths of topical corticosteroids, and topical nonsteroid options.”
When he counsels parents and patients on the use of topical corticosteroids, he tells them that cortisone is a naturally-occurring metabolite, and that “we can work together to let you know how much medicine to use, and how a safe amount is a powerful tool to fix the eczema.” He often says that topical steroids “are like hammers. We have tiny hammers, like over-the-counter hydrocortisone, and sledgehammers like clobetasol. We also have ‘screwdrivers’ and ‘pliers’ with nonsteroidal topical calcineurin and PDE-4 inhibitors, which are especially useful for maintenance therapy. Topical ruxolitinib is a new medicine that we may use for patients as well. The label includes discussion of side effects from oral JAK inhibitors as well as from the drug development program, so it takes some time to talk through.”
Is it time for a stronger systemic medicine? Any conversation about this topic should support the concept that the AD is multifactorial. “We have the rash of eczema,” he said. “We have the itch. We have impact on sleep disturbance. We have the comorbidities. We have other physical changes, which can happen with bacterial infections and other immune system or cardiovascular changes. We have the impact on quality of life and impact on school and work. When we recognize that if patients have significant enough disease that it is not getting better with topicals and is having a negative impact on their lives, we can move our discussion to systemic therapy.”
When counseling patients about systemic therapy, Dr. Eichenfield will conduct a body surface area assessment and document how bad the itch is. “But I’m not just recording the information; I’m bringing it out in the room,” he said. “I’ll do a BSA assessment and say, for example, ‘oh, you have 32% of your body involved with eczema.’ I ask about sleep disturbance, to get the answer ‘out in the room.’ ” He also asks questions such as: “When was the last time your skin was last totally clear? Are there activities that you or your family don’t do because of your eczema, or that you’re living your life around it? Is there anxiety or depression?” Documenting both the impact on quality of life and the severity of disease “makes it easier to discuss systemic therapy,” Dr. Eichenfield said. “Meanwhile, as the provider, I am trying to figure out if the patient should ‘go into the topical therapy bucket’ or into the ‘systemic therapy bucket.’ ”
Counseling about systemic therapy includes shared decision-making regarding the choice of biologics versus oral JAK inhibitors versus traditional systemic agent or phototherapy. Factors to consider in the decision making include patient age, sex, severity, comorbidities, prior therapy, risk aversion, duration, medication access, and desired efficacy. “Evolving therapies can change the conversation, the questions, and the outcomes, but the overarching desired outcome is long-term disease control, minimal eczematous rash, minimal pruritus, and minimal sleep disturbance,” he said.
Dr. Eichenfield disclosed that he has served as a consultant to or investigator for AbbVie; Almirall; Arcutis; Arena; Asana; Termagant; Dermira; Forte Biosciences; Galderma Laboratories; Glenmark/Chinos; Incyte; Kyowa Kirin; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Otsuka; Pfizer; Freestone; Regeneron, and Sanofi Genzyme.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Two questions can help establish a diagnosis of hidradenitis suppurativa
According to Iltefat H. Hamzavi, MD,
If the answer to the first question is “yes” and the patient has had at least two boils in intertriginous areas, that person likely has HS, a disease of apocrine gland–bearing skin that occurs in 1%-4% of people, has a higher prevalence in Blacks, compared with Whites, and affects more women than men by a 3:1 ratio.
“Current treatments offer limited efficacy, and the disease is chronic and recurrent,” Dr. Hamzavi, of the department of dermatology at Henry Ford Health System, Detroit, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “You often see nodules, abscesses, fistulae, and scarring,” with all different skin types represented in the majority of patients.
Typical HS lesions appear as inflamed nodules, abscesses, draining fistulas, and scars as well as double-headed “tombstone” comedones, he said. These are typically located in the axilla, intermammary folds, in the groin, around the genitals, and on the buttocks. Atypical lesions can also occur – often folliculitis and open comedones in locations such as the waistline, the neck, and behind the ears.
The differential diagnosis is wide-ranging and includes bacterial abscess, inflamed cyst, folliculitis, pilonidal sinus, cellulitis, and cutaneous Crohn’s disease. Pain may appear out of proportion to the physical examination.
“There is a window of opportunity to treat HS, early in the disease process,” Dr. Hamzavi said. “There are no definitive cures for HS but lots of treatment options.”
According to clinical management guidelines published by the United States and Canadian Hidradenitis Suppurativa Foundations, options for moderate stage disease include antibiotics, antiandrogens, retinoids, immunosuppression/biologics, deroofing, and limited excision with primary closure. Options for severe disease include radical excision.
“HS requires a mix of medical and procedural treatments based on the number of nodules,” Dr. Hamzavi said. “Because the disease has so many different phases, there is no perfect outcome measure yet, but progress is being made.”
In 2018, an effort to develop a consensus core outcome set of domains regarding what to measure in clinical trials of HS was launched; it is known as the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HISTORIC). It was formed as a collaboration between the International Dermatology Outcome Measures (IDEOM) initiative, the Cochrane Skin Group – Core Outcome Set Initiative (CSG-COUSIN), and Zealand University Hospital, Roskilde.
HISTORIC is now part of the partnership with CSG-COUSIN and this work continues onward. Core domains as defined by the group include pain, physical signs, HS-specific quality of life, global assessment, and disease progression. “For now, we are mostly using some objective measures and some patient-reported outcomes with the addition of ultrasound in some centers,” Dr. Hamzavi said.
He underscored the importance of lifestyle modifications in patients with HS, including smoking cessation and weight loss, as well as decreasing pressure/friction on lesions, using warm compresses, and modifying diet. “This generally involves a low-inflammatory diet: Low carbohydrate, low dairy, and higher protein content, but there is much work needed to understand the role of diet in HS,” he said.
“This is a tough disease, but the compassion you offer these patients will be paid back to you a thousandfold. They tend to be some of the happiest and most appreciative patients you will ever have in your practice.”
Dr. Hamzavi disclosed that he has been a clinical investigator for Clinuvel, Incyte, Pfizer, Avita, and Ferndale Labs. He has also been a consultant for Pfizer, AbbVie, Novartis, and Aclaris, and has received a grant from Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
According to Iltefat H. Hamzavi, MD,
If the answer to the first question is “yes” and the patient has had at least two boils in intertriginous areas, that person likely has HS, a disease of apocrine gland–bearing skin that occurs in 1%-4% of people, has a higher prevalence in Blacks, compared with Whites, and affects more women than men by a 3:1 ratio.
“Current treatments offer limited efficacy, and the disease is chronic and recurrent,” Dr. Hamzavi, of the department of dermatology at Henry Ford Health System, Detroit, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “You often see nodules, abscesses, fistulae, and scarring,” with all different skin types represented in the majority of patients.
Typical HS lesions appear as inflamed nodules, abscesses, draining fistulas, and scars as well as double-headed “tombstone” comedones, he said. These are typically located in the axilla, intermammary folds, in the groin, around the genitals, and on the buttocks. Atypical lesions can also occur – often folliculitis and open comedones in locations such as the waistline, the neck, and behind the ears.
The differential diagnosis is wide-ranging and includes bacterial abscess, inflamed cyst, folliculitis, pilonidal sinus, cellulitis, and cutaneous Crohn’s disease. Pain may appear out of proportion to the physical examination.
“There is a window of opportunity to treat HS, early in the disease process,” Dr. Hamzavi said. “There are no definitive cures for HS but lots of treatment options.”
According to clinical management guidelines published by the United States and Canadian Hidradenitis Suppurativa Foundations, options for moderate stage disease include antibiotics, antiandrogens, retinoids, immunosuppression/biologics, deroofing, and limited excision with primary closure. Options for severe disease include radical excision.
“HS requires a mix of medical and procedural treatments based on the number of nodules,” Dr. Hamzavi said. “Because the disease has so many different phases, there is no perfect outcome measure yet, but progress is being made.”
In 2018, an effort to develop a consensus core outcome set of domains regarding what to measure in clinical trials of HS was launched; it is known as the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HISTORIC). It was formed as a collaboration between the International Dermatology Outcome Measures (IDEOM) initiative, the Cochrane Skin Group – Core Outcome Set Initiative (CSG-COUSIN), and Zealand University Hospital, Roskilde.
HISTORIC is now part of the partnership with CSG-COUSIN and this work continues onward. Core domains as defined by the group include pain, physical signs, HS-specific quality of life, global assessment, and disease progression. “For now, we are mostly using some objective measures and some patient-reported outcomes with the addition of ultrasound in some centers,” Dr. Hamzavi said.
He underscored the importance of lifestyle modifications in patients with HS, including smoking cessation and weight loss, as well as decreasing pressure/friction on lesions, using warm compresses, and modifying diet. “This generally involves a low-inflammatory diet: Low carbohydrate, low dairy, and higher protein content, but there is much work needed to understand the role of diet in HS,” he said.
“This is a tough disease, but the compassion you offer these patients will be paid back to you a thousandfold. They tend to be some of the happiest and most appreciative patients you will ever have in your practice.”
Dr. Hamzavi disclosed that he has been a clinical investigator for Clinuvel, Incyte, Pfizer, Avita, and Ferndale Labs. He has also been a consultant for Pfizer, AbbVie, Novartis, and Aclaris, and has received a grant from Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
According to Iltefat H. Hamzavi, MD,
If the answer to the first question is “yes” and the patient has had at least two boils in intertriginous areas, that person likely has HS, a disease of apocrine gland–bearing skin that occurs in 1%-4% of people, has a higher prevalence in Blacks, compared with Whites, and affects more women than men by a 3:1 ratio.
“Current treatments offer limited efficacy, and the disease is chronic and recurrent,” Dr. Hamzavi, of the department of dermatology at Henry Ford Health System, Detroit, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “You often see nodules, abscesses, fistulae, and scarring,” with all different skin types represented in the majority of patients.
Typical HS lesions appear as inflamed nodules, abscesses, draining fistulas, and scars as well as double-headed “tombstone” comedones, he said. These are typically located in the axilla, intermammary folds, in the groin, around the genitals, and on the buttocks. Atypical lesions can also occur – often folliculitis and open comedones in locations such as the waistline, the neck, and behind the ears.
The differential diagnosis is wide-ranging and includes bacterial abscess, inflamed cyst, folliculitis, pilonidal sinus, cellulitis, and cutaneous Crohn’s disease. Pain may appear out of proportion to the physical examination.
“There is a window of opportunity to treat HS, early in the disease process,” Dr. Hamzavi said. “There are no definitive cures for HS but lots of treatment options.”
According to clinical management guidelines published by the United States and Canadian Hidradenitis Suppurativa Foundations, options for moderate stage disease include antibiotics, antiandrogens, retinoids, immunosuppression/biologics, deroofing, and limited excision with primary closure. Options for severe disease include radical excision.
“HS requires a mix of medical and procedural treatments based on the number of nodules,” Dr. Hamzavi said. “Because the disease has so many different phases, there is no perfect outcome measure yet, but progress is being made.”
In 2018, an effort to develop a consensus core outcome set of domains regarding what to measure in clinical trials of HS was launched; it is known as the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HISTORIC). It was formed as a collaboration between the International Dermatology Outcome Measures (IDEOM) initiative, the Cochrane Skin Group – Core Outcome Set Initiative (CSG-COUSIN), and Zealand University Hospital, Roskilde.
HISTORIC is now part of the partnership with CSG-COUSIN and this work continues onward. Core domains as defined by the group include pain, physical signs, HS-specific quality of life, global assessment, and disease progression. “For now, we are mostly using some objective measures and some patient-reported outcomes with the addition of ultrasound in some centers,” Dr. Hamzavi said.
He underscored the importance of lifestyle modifications in patients with HS, including smoking cessation and weight loss, as well as decreasing pressure/friction on lesions, using warm compresses, and modifying diet. “This generally involves a low-inflammatory diet: Low carbohydrate, low dairy, and higher protein content, but there is much work needed to understand the role of diet in HS,” he said.
“This is a tough disease, but the compassion you offer these patients will be paid back to you a thousandfold. They tend to be some of the happiest and most appreciative patients you will ever have in your practice.”
Dr. Hamzavi disclosed that he has been a clinical investigator for Clinuvel, Incyte, Pfizer, Avita, and Ferndale Labs. He has also been a consultant for Pfizer, AbbVie, Novartis, and Aclaris, and has received a grant from Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Certain opioids hold promise for treating itch
Certain opioids are proving to be effective in treating a variety of itch conditions, according to Brian S. Kim, MD.
“We know that opioids or opiates do cause itch in a significant number of patients,” Dr. Kim, a dermatologist who is codirector of the Center for the Study of Itch & Sensory Disorders at Washington University, St. Louis, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It’s thought to do this by way of acting as a pruritogen at times and stimulating sensory neurons [that] then activate the itch cascade. But it’s also been well known that endogenous kappa opioids can activate sensory neurons that can then suppress itch and gate out signals from these opiates, but perhaps other pruritogens as well.”
Multiple drugs differentially target kappa-opioid receptor (KOR) and mu-opioid receptor (MOR) pathways, he continued. For example, oral naltrexone is a MOR antagonist, oral nalfurafine and intravenous difelikefalin are KOR agonists, while intranasal butorphanol and oral nalbuphine have a dual mechanism.
Difelikefalin is the first Food and Drug Administration–approved treatment for uremic pruritus associated with dialysis, approved in August 2021 for moderate-to-severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis; it is administered intravenously. During the 2021 annual congress of the European Academy of Dermatology and Venereology, Dr. Kim and colleagues presented findings from a phase 2 trial of 401 people with atopic dermatitis (AD) and moderate to severe pruritus, who were randomized to receive oral difelikefalin at a dose of 0.25 mg, 0.5 mg, or 1.0 mg, or placebo over a 12-week treatment period. The primary endpoint, change from baseline in Itch Numerical Rating Scale score, was not met in any of the difelikefalin dose groups in the overall study population, but patients with a body surface area of less than 10% experienced a significant improvement in itch at week 12 in the combined difelikefalin dose group in (P = .039). A significant reduction in itch with difelikefalin was seen in this group of patients with itch-dominant AD, as early as the second day of treatment.
In another trial, 373 hemodialysis patients with moderate or severe uremic pruritus were randomized in a 1: 1:1 ratio to nalbuphine extended-release tablets 120 mg, 60 mg, or placebo and treated for 8 weeks. The researchers found that nalbuphine 120 mg significantly reduced the itching intensity. Specifically, from a baseline numerical rate scale (NRS) of 6.9, the mean NRS declined by 3.5 and by 2.8 in the nalbuphine 120-mg and the placebo groups, respectively (P = .017).
In a separate, unpublished multicenter, randomized, phase 2/3 trial, researchers evaluated the safety and antipruritic efficacy of nalbuphine extended-release tablets dosed twice daily at 90 mg and 180 mg in 62 patients in the United States and Europe. The proportion of patients in the nalbuphine 180-mg arm who met 50% responder criteria at week 10 or last observed visit approached statistical significance (P = .083), and this arm met statistical significance for patients who completed treatment (P = .028).
Dr. Kim disclosed that he has served as a consultant for AbbVie, AstraZeneca, Cara Therapeutics, Galderma, GlaxoSmithKline, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi, Trevi Therapeutics. He also has conducted contracted research for Cara Therapeutics and LEO Pharma.
MedscapeLive and this news organization are owned by the same parent company.
Certain opioids are proving to be effective in treating a variety of itch conditions, according to Brian S. Kim, MD.
“We know that opioids or opiates do cause itch in a significant number of patients,” Dr. Kim, a dermatologist who is codirector of the Center for the Study of Itch & Sensory Disorders at Washington University, St. Louis, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It’s thought to do this by way of acting as a pruritogen at times and stimulating sensory neurons [that] then activate the itch cascade. But it’s also been well known that endogenous kappa opioids can activate sensory neurons that can then suppress itch and gate out signals from these opiates, but perhaps other pruritogens as well.”
Multiple drugs differentially target kappa-opioid receptor (KOR) and mu-opioid receptor (MOR) pathways, he continued. For example, oral naltrexone is a MOR antagonist, oral nalfurafine and intravenous difelikefalin are KOR agonists, while intranasal butorphanol and oral nalbuphine have a dual mechanism.
Difelikefalin is the first Food and Drug Administration–approved treatment for uremic pruritus associated with dialysis, approved in August 2021 for moderate-to-severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis; it is administered intravenously. During the 2021 annual congress of the European Academy of Dermatology and Venereology, Dr. Kim and colleagues presented findings from a phase 2 trial of 401 people with atopic dermatitis (AD) and moderate to severe pruritus, who were randomized to receive oral difelikefalin at a dose of 0.25 mg, 0.5 mg, or 1.0 mg, or placebo over a 12-week treatment period. The primary endpoint, change from baseline in Itch Numerical Rating Scale score, was not met in any of the difelikefalin dose groups in the overall study population, but patients with a body surface area of less than 10% experienced a significant improvement in itch at week 12 in the combined difelikefalin dose group in (P = .039). A significant reduction in itch with difelikefalin was seen in this group of patients with itch-dominant AD, as early as the second day of treatment.
In another trial, 373 hemodialysis patients with moderate or severe uremic pruritus were randomized in a 1: 1:1 ratio to nalbuphine extended-release tablets 120 mg, 60 mg, or placebo and treated for 8 weeks. The researchers found that nalbuphine 120 mg significantly reduced the itching intensity. Specifically, from a baseline numerical rate scale (NRS) of 6.9, the mean NRS declined by 3.5 and by 2.8 in the nalbuphine 120-mg and the placebo groups, respectively (P = .017).
In a separate, unpublished multicenter, randomized, phase 2/3 trial, researchers evaluated the safety and antipruritic efficacy of nalbuphine extended-release tablets dosed twice daily at 90 mg and 180 mg in 62 patients in the United States and Europe. The proportion of patients in the nalbuphine 180-mg arm who met 50% responder criteria at week 10 or last observed visit approached statistical significance (P = .083), and this arm met statistical significance for patients who completed treatment (P = .028).
Dr. Kim disclosed that he has served as a consultant for AbbVie, AstraZeneca, Cara Therapeutics, Galderma, GlaxoSmithKline, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi, Trevi Therapeutics. He also has conducted contracted research for Cara Therapeutics and LEO Pharma.
MedscapeLive and this news organization are owned by the same parent company.
Certain opioids are proving to be effective in treating a variety of itch conditions, according to Brian S. Kim, MD.
“We know that opioids or opiates do cause itch in a significant number of patients,” Dr. Kim, a dermatologist who is codirector of the Center for the Study of Itch & Sensory Disorders at Washington University, St. Louis, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It’s thought to do this by way of acting as a pruritogen at times and stimulating sensory neurons [that] then activate the itch cascade. But it’s also been well known that endogenous kappa opioids can activate sensory neurons that can then suppress itch and gate out signals from these opiates, but perhaps other pruritogens as well.”
Multiple drugs differentially target kappa-opioid receptor (KOR) and mu-opioid receptor (MOR) pathways, he continued. For example, oral naltrexone is a MOR antagonist, oral nalfurafine and intravenous difelikefalin are KOR agonists, while intranasal butorphanol and oral nalbuphine have a dual mechanism.
Difelikefalin is the first Food and Drug Administration–approved treatment for uremic pruritus associated with dialysis, approved in August 2021 for moderate-to-severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis; it is administered intravenously. During the 2021 annual congress of the European Academy of Dermatology and Venereology, Dr. Kim and colleagues presented findings from a phase 2 trial of 401 people with atopic dermatitis (AD) and moderate to severe pruritus, who were randomized to receive oral difelikefalin at a dose of 0.25 mg, 0.5 mg, or 1.0 mg, or placebo over a 12-week treatment period. The primary endpoint, change from baseline in Itch Numerical Rating Scale score, was not met in any of the difelikefalin dose groups in the overall study population, but patients with a body surface area of less than 10% experienced a significant improvement in itch at week 12 in the combined difelikefalin dose group in (P = .039). A significant reduction in itch with difelikefalin was seen in this group of patients with itch-dominant AD, as early as the second day of treatment.
In another trial, 373 hemodialysis patients with moderate or severe uremic pruritus were randomized in a 1: 1:1 ratio to nalbuphine extended-release tablets 120 mg, 60 mg, or placebo and treated for 8 weeks. The researchers found that nalbuphine 120 mg significantly reduced the itching intensity. Specifically, from a baseline numerical rate scale (NRS) of 6.9, the mean NRS declined by 3.5 and by 2.8 in the nalbuphine 120-mg and the placebo groups, respectively (P = .017).
In a separate, unpublished multicenter, randomized, phase 2/3 trial, researchers evaluated the safety and antipruritic efficacy of nalbuphine extended-release tablets dosed twice daily at 90 mg and 180 mg in 62 patients in the United States and Europe. The proportion of patients in the nalbuphine 180-mg arm who met 50% responder criteria at week 10 or last observed visit approached statistical significance (P = .083), and this arm met statistical significance for patients who completed treatment (P = .028).
Dr. Kim disclosed that he has served as a consultant for AbbVie, AstraZeneca, Cara Therapeutics, Galderma, GlaxoSmithKline, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi, Trevi Therapeutics. He also has conducted contracted research for Cara Therapeutics and LEO Pharma.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Validity of commercial serologic tests for dermatomyositis still questionable
, according to Jeffrey P. Callen, MD.
That’s because the validity and reproducibility of testing in commercial laboratories remain questionable, Dr. Callen, professor of medicine and chief of the division of dermatology at the University of Louisville, Ky., said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “The testing in research laboratories is not widely available and the results are often delayed by weeks to months,” he said.
In addition, while the associations between antibody results and risks of malignancy or pulmonary disease are “statistically valid,” he said, “there are patients with disease in whom antibodies are not present and those without associated disease in whom the testing was positive.” For example, there are patients positive for anti–transition initiation factor (TIF)-1gamma but don’t have a malignancy, “and the ones with anti-MDA-5 tend to have pulmonary disease, but there are patients with anti-MDA-5 who don’t have pulmonary disease.”
Compared with patients with systemic lupus erythematosus, patients with dermatomyositis tend to have more itching and they tend of have fewer serologic abnormalities, such as anti-Ro/SS-A antibody, “but there is overlap,” Dr. Callen said. “The reason to differentiate cutaneous lupus erythematosus from dermatomyositis is because we think that patients who have amyopathic dermatomyositis still have an increased risk of having or developing an internal malignancy,” he added. Another differentiating point that is substantive is the presence of Gottron papules.
In a recent development related to antibody testing, researchers demonstrated that the IgG2 isotype of anti-TIF-1gamma antibodies is a biomarker of cancer and mortality in adult dermatomyositis.
According to population-based studies, about 20%-25% of dermatomyositis patients have had, have, or will develop a cancer (Lancet 2001;357: 96-100). Amyopathic dermatomyositis patients may also have cancer. Polymyositis patients generally have lower rates and their risk of subsequent malignancy is much closer to that of the general population, suggesting that the presence of the association is due to a “diagnostic suspicion bias,” Dr. Callen said.
A large-scale multicenter cohort study that set out to identify the risk factors and prognosis of patients with cancer-associated myositis found that ovarian cancer seems to be overrepresented. The only serologic abnormality that was statistically significant was anti-TIF-1gamma antibody (P less than .001). Patients with cancer-associated myositis also have less overall survival compared with those with non–cancer-associated myositis (P = .004), with malignancy being the primary cause of death (P less than .001).
In what is believed to be the largest study of its kind, Dr. Callen and colleagues retrospectively examined the prevalence of malignancy and screening practices in 400 dermatomyositis patients. Of the 400 patients, 48 (12%) had malignancies, and 21 cancers (40%) were diagnosed within 1 year of the dermatomyositis diagnosis. Both classic dermatomyositis and amyopathic dermatomyositis were associated with cancer, and 27 patients (6.8%) had a cancer at the time of diagnosis. Of those, 59% were asymptomatic; their cancers were discovered with CT scans, suggesting that “blind” screening is effective in identifying cancers in DM patients.
Dr. Callen’s malignancy evaluation includes chest x-ray, CT of the chest and abdomen, stool Hematest in all dermatomyositis patients; a mammogram, pelvic ultrasound and/or CT of the pelvis in women; and age, race or ethnicity-related testing. “I generally reevaluate patients annually for 3 years, because data from epidemiologic studies suggest that after 3 years [from the initial diagnosis], the rates of malignancy return toward normal,” he said. “I also evaluate any new symptom that might be suggestive of malignancy. The remaining issue is how to handle a patient in remission for several years, but who develops a relapse. What I do is perform another malignancy assessment.”
According to results from a meta-analysis of risk factors and systematic review of screening approaches, factors that increase malignancy risk include dermatomyositis subtype (risk ratio, 2.21), older age (weighted mean difference 11.19), male gender (RR, 1.53), dysphagia (RR, 2.09), cutaneous necrosis (RR, 2.73), and positive anti-TIF-1gamma (RR, 4.41).
Factors associated with a decreased risk of malignancy include polymyositis (RR, 0.49), clinically amyopathic dermatomyositis subtypes (RR, 0.44), Raynaud’s phenomenon (RR, 0.61), interstitial lung disease (RR, 0.49), very high serum creatine kinase (WMD –1189.96) or lactate dehydrogenase levels (WMD –336.53), and anti-Jo1 (RR, 0.45) or anti-EJ (RR, 0.17) positivity.
The analysis also found that CT scanning of the thorax, abdomen and pelvis appeared to yield a high proportion of underlying asymptomatic cancers. Limited evidence relating to the utility of tumor markers and 18F-FDG PET/CT was available.
As for treatment, the use of tofacitinib for cutaneous lesions of dermatomyositis has been suggested in various studies. In a recent open-label study of 10 patients with dermatomyositis who took extended release the JAK inhibitor tofacitinib 11 mg daily for 12 weeks, half experienced moderate improvement in disease activity, and the other half experienced minimal improvement. JAK inhibitors have been used in patients with juvenile dermatomyositis.
Dr. Callen’s treatment approach with dermatomyositis patients includes recommendations for sunscreens and protective clothing, plus assessment of vitamin D levels. “I will use topical emollients, corticosteroids, and calcineurin inhibitors,” he said. “Antimalarials might be used. I generally reach for methotrexate or mycophenolate mofetil relatively early. IVIG has also been studied.” Off-label therapies that have been used include dapsone, thalidomide, leflunomide, sirolimus, chlorambucil, etanercept, infliximab, rituximab, apremilast, tofacitinib, lenabasum, and low-dose naltrexone.
Dr. Callen disclosed that he is a consultant to Genentech and is a member of the safety monitoring committee for Principia Biopharma. He holds equity in Celgene, Pfizer, 3M, Johnson & Johnson, Merck, Abbott Laboratories, AbbVie, Procter & Gamble, Gilead, Allergen, and Amgen.
MedscapeLive and this news organization are owned by the same parent company.
, according to Jeffrey P. Callen, MD.
That’s because the validity and reproducibility of testing in commercial laboratories remain questionable, Dr. Callen, professor of medicine and chief of the division of dermatology at the University of Louisville, Ky., said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “The testing in research laboratories is not widely available and the results are often delayed by weeks to months,” he said.
In addition, while the associations between antibody results and risks of malignancy or pulmonary disease are “statistically valid,” he said, “there are patients with disease in whom antibodies are not present and those without associated disease in whom the testing was positive.” For example, there are patients positive for anti–transition initiation factor (TIF)-1gamma but don’t have a malignancy, “and the ones with anti-MDA-5 tend to have pulmonary disease, but there are patients with anti-MDA-5 who don’t have pulmonary disease.”
Compared with patients with systemic lupus erythematosus, patients with dermatomyositis tend to have more itching and they tend of have fewer serologic abnormalities, such as anti-Ro/SS-A antibody, “but there is overlap,” Dr. Callen said. “The reason to differentiate cutaneous lupus erythematosus from dermatomyositis is because we think that patients who have amyopathic dermatomyositis still have an increased risk of having or developing an internal malignancy,” he added. Another differentiating point that is substantive is the presence of Gottron papules.
In a recent development related to antibody testing, researchers demonstrated that the IgG2 isotype of anti-TIF-1gamma antibodies is a biomarker of cancer and mortality in adult dermatomyositis.
According to population-based studies, about 20%-25% of dermatomyositis patients have had, have, or will develop a cancer (Lancet 2001;357: 96-100). Amyopathic dermatomyositis patients may also have cancer. Polymyositis patients generally have lower rates and their risk of subsequent malignancy is much closer to that of the general population, suggesting that the presence of the association is due to a “diagnostic suspicion bias,” Dr. Callen said.
A large-scale multicenter cohort study that set out to identify the risk factors and prognosis of patients with cancer-associated myositis found that ovarian cancer seems to be overrepresented. The only serologic abnormality that was statistically significant was anti-TIF-1gamma antibody (P less than .001). Patients with cancer-associated myositis also have less overall survival compared with those with non–cancer-associated myositis (P = .004), with malignancy being the primary cause of death (P less than .001).
In what is believed to be the largest study of its kind, Dr. Callen and colleagues retrospectively examined the prevalence of malignancy and screening practices in 400 dermatomyositis patients. Of the 400 patients, 48 (12%) had malignancies, and 21 cancers (40%) were diagnosed within 1 year of the dermatomyositis diagnosis. Both classic dermatomyositis and amyopathic dermatomyositis were associated with cancer, and 27 patients (6.8%) had a cancer at the time of diagnosis. Of those, 59% were asymptomatic; their cancers were discovered with CT scans, suggesting that “blind” screening is effective in identifying cancers in DM patients.
Dr. Callen’s malignancy evaluation includes chest x-ray, CT of the chest and abdomen, stool Hematest in all dermatomyositis patients; a mammogram, pelvic ultrasound and/or CT of the pelvis in women; and age, race or ethnicity-related testing. “I generally reevaluate patients annually for 3 years, because data from epidemiologic studies suggest that after 3 years [from the initial diagnosis], the rates of malignancy return toward normal,” he said. “I also evaluate any new symptom that might be suggestive of malignancy. The remaining issue is how to handle a patient in remission for several years, but who develops a relapse. What I do is perform another malignancy assessment.”
According to results from a meta-analysis of risk factors and systematic review of screening approaches, factors that increase malignancy risk include dermatomyositis subtype (risk ratio, 2.21), older age (weighted mean difference 11.19), male gender (RR, 1.53), dysphagia (RR, 2.09), cutaneous necrosis (RR, 2.73), and positive anti-TIF-1gamma (RR, 4.41).
Factors associated with a decreased risk of malignancy include polymyositis (RR, 0.49), clinically amyopathic dermatomyositis subtypes (RR, 0.44), Raynaud’s phenomenon (RR, 0.61), interstitial lung disease (RR, 0.49), very high serum creatine kinase (WMD –1189.96) or lactate dehydrogenase levels (WMD –336.53), and anti-Jo1 (RR, 0.45) or anti-EJ (RR, 0.17) positivity.
The analysis also found that CT scanning of the thorax, abdomen and pelvis appeared to yield a high proportion of underlying asymptomatic cancers. Limited evidence relating to the utility of tumor markers and 18F-FDG PET/CT was available.
As for treatment, the use of tofacitinib for cutaneous lesions of dermatomyositis has been suggested in various studies. In a recent open-label study of 10 patients with dermatomyositis who took extended release the JAK inhibitor tofacitinib 11 mg daily for 12 weeks, half experienced moderate improvement in disease activity, and the other half experienced minimal improvement. JAK inhibitors have been used in patients with juvenile dermatomyositis.
Dr. Callen’s treatment approach with dermatomyositis patients includes recommendations for sunscreens and protective clothing, plus assessment of vitamin D levels. “I will use topical emollients, corticosteroids, and calcineurin inhibitors,” he said. “Antimalarials might be used. I generally reach for methotrexate or mycophenolate mofetil relatively early. IVIG has also been studied.” Off-label therapies that have been used include dapsone, thalidomide, leflunomide, sirolimus, chlorambucil, etanercept, infliximab, rituximab, apremilast, tofacitinib, lenabasum, and low-dose naltrexone.
Dr. Callen disclosed that he is a consultant to Genentech and is a member of the safety monitoring committee for Principia Biopharma. He holds equity in Celgene, Pfizer, 3M, Johnson & Johnson, Merck, Abbott Laboratories, AbbVie, Procter & Gamble, Gilead, Allergen, and Amgen.
MedscapeLive and this news organization are owned by the same parent company.
, according to Jeffrey P. Callen, MD.
That’s because the validity and reproducibility of testing in commercial laboratories remain questionable, Dr. Callen, professor of medicine and chief of the division of dermatology at the University of Louisville, Ky., said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “The testing in research laboratories is not widely available and the results are often delayed by weeks to months,” he said.
In addition, while the associations between antibody results and risks of malignancy or pulmonary disease are “statistically valid,” he said, “there are patients with disease in whom antibodies are not present and those without associated disease in whom the testing was positive.” For example, there are patients positive for anti–transition initiation factor (TIF)-1gamma but don’t have a malignancy, “and the ones with anti-MDA-5 tend to have pulmonary disease, but there are patients with anti-MDA-5 who don’t have pulmonary disease.”
Compared with patients with systemic lupus erythematosus, patients with dermatomyositis tend to have more itching and they tend of have fewer serologic abnormalities, such as anti-Ro/SS-A antibody, “but there is overlap,” Dr. Callen said. “The reason to differentiate cutaneous lupus erythematosus from dermatomyositis is because we think that patients who have amyopathic dermatomyositis still have an increased risk of having or developing an internal malignancy,” he added. Another differentiating point that is substantive is the presence of Gottron papules.
In a recent development related to antibody testing, researchers demonstrated that the IgG2 isotype of anti-TIF-1gamma antibodies is a biomarker of cancer and mortality in adult dermatomyositis.
According to population-based studies, about 20%-25% of dermatomyositis patients have had, have, or will develop a cancer (Lancet 2001;357: 96-100). Amyopathic dermatomyositis patients may also have cancer. Polymyositis patients generally have lower rates and their risk of subsequent malignancy is much closer to that of the general population, suggesting that the presence of the association is due to a “diagnostic suspicion bias,” Dr. Callen said.
A large-scale multicenter cohort study that set out to identify the risk factors and prognosis of patients with cancer-associated myositis found that ovarian cancer seems to be overrepresented. The only serologic abnormality that was statistically significant was anti-TIF-1gamma antibody (P less than .001). Patients with cancer-associated myositis also have less overall survival compared with those with non–cancer-associated myositis (P = .004), with malignancy being the primary cause of death (P less than .001).
In what is believed to be the largest study of its kind, Dr. Callen and colleagues retrospectively examined the prevalence of malignancy and screening practices in 400 dermatomyositis patients. Of the 400 patients, 48 (12%) had malignancies, and 21 cancers (40%) were diagnosed within 1 year of the dermatomyositis diagnosis. Both classic dermatomyositis and amyopathic dermatomyositis were associated with cancer, and 27 patients (6.8%) had a cancer at the time of diagnosis. Of those, 59% were asymptomatic; their cancers were discovered with CT scans, suggesting that “blind” screening is effective in identifying cancers in DM patients.
Dr. Callen’s malignancy evaluation includes chest x-ray, CT of the chest and abdomen, stool Hematest in all dermatomyositis patients; a mammogram, pelvic ultrasound and/or CT of the pelvis in women; and age, race or ethnicity-related testing. “I generally reevaluate patients annually for 3 years, because data from epidemiologic studies suggest that after 3 years [from the initial diagnosis], the rates of malignancy return toward normal,” he said. “I also evaluate any new symptom that might be suggestive of malignancy. The remaining issue is how to handle a patient in remission for several years, but who develops a relapse. What I do is perform another malignancy assessment.”
According to results from a meta-analysis of risk factors and systematic review of screening approaches, factors that increase malignancy risk include dermatomyositis subtype (risk ratio, 2.21), older age (weighted mean difference 11.19), male gender (RR, 1.53), dysphagia (RR, 2.09), cutaneous necrosis (RR, 2.73), and positive anti-TIF-1gamma (RR, 4.41).
Factors associated with a decreased risk of malignancy include polymyositis (RR, 0.49), clinically amyopathic dermatomyositis subtypes (RR, 0.44), Raynaud’s phenomenon (RR, 0.61), interstitial lung disease (RR, 0.49), very high serum creatine kinase (WMD –1189.96) or lactate dehydrogenase levels (WMD –336.53), and anti-Jo1 (RR, 0.45) or anti-EJ (RR, 0.17) positivity.
The analysis also found that CT scanning of the thorax, abdomen and pelvis appeared to yield a high proportion of underlying asymptomatic cancers. Limited evidence relating to the utility of tumor markers and 18F-FDG PET/CT was available.
As for treatment, the use of tofacitinib for cutaneous lesions of dermatomyositis has been suggested in various studies. In a recent open-label study of 10 patients with dermatomyositis who took extended release the JAK inhibitor tofacitinib 11 mg daily for 12 weeks, half experienced moderate improvement in disease activity, and the other half experienced minimal improvement. JAK inhibitors have been used in patients with juvenile dermatomyositis.
Dr. Callen’s treatment approach with dermatomyositis patients includes recommendations for sunscreens and protective clothing, plus assessment of vitamin D levels. “I will use topical emollients, corticosteroids, and calcineurin inhibitors,” he said. “Antimalarials might be used. I generally reach for methotrexate or mycophenolate mofetil relatively early. IVIG has also been studied.” Off-label therapies that have been used include dapsone, thalidomide, leflunomide, sirolimus, chlorambucil, etanercept, infliximab, rituximab, apremilast, tofacitinib, lenabasum, and low-dose naltrexone.
Dr. Callen disclosed that he is a consultant to Genentech and is a member of the safety monitoring committee for Principia Biopharma. He holds equity in Celgene, Pfizer, 3M, Johnson & Johnson, Merck, Abbott Laboratories, AbbVie, Procter & Gamble, Gilead, Allergen, and Amgen.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
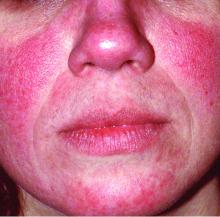
Rosacea is in the eye of the beholder, expert says
In the clinical experience of Emmy Graber, MD, MBA, rosacea is in the eye of the beholder.
“It’s not really up to us as the providers as to what’s important to the patient or how bad their rosacea is,” she said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It really is up to the patient,” added Dr. Graber, president of The Dermatology Institute of Boston, who recommends asking patients about how severe they consider their rosacea to be, and what about rosacea bothers them most. Their responses may be surprising.
A study published in 2017 showed that complete resolution of even mild rosacea prolongs remission of rosacea, and most importantly, improves the quality of life for patients. “So, don’t discount what you consider to be mild rosacea in patients,” she said.
Skin care recommendations
“And don’t forget about basic skin care,” she advised. A recently published Chinese study of 999 rosacea patients and 1,010 controls with healthy skin found that a high frequency of cleansing and expansive use of cleansers were positively correlated with rosacea occurrence, suggesting that overcleansing can be a risk factor for rosacea. “Ask your patient, ‘how often are you cleaning your face?’ ” Dr. Graber suggested. “You might find that they’re overdoing it by washing three or four times a day. Several studies have shown that basic skin care alone improves rosacea.”
Skin care recommendations for patients with rosacea include avoiding chemical or physical exfoliants and alcohol-based topical products, and moisturizing and washing their faces with mild, synthetic detergent-based products rather than traditional soaps, which may further alkalinize and irritate the skin. “Patients should also be counseled to use physical-based sunscreens rather than chemical-based sunscreens,” she said.
Treating erythema
For treating erythema with topicals, a systematic review published in 2019 found the most evidence for brimonidine 0.33% gel, an alpha2-adrenergic agonist, and oxymetazoline 1% cream, an alpha1-adrenergic agonist. “Both of these products functionally constrict facial blood vessels,” and are Food and Drug Administration approved for treating persistent erythema, Dr. Graber said. “These products improve erythema within 3 hours of and up to 12 hours after application and overall, they are well tolerated.”
Based on clinical trial results, about 15% of patients on brimonidine report adverse reactions such as dermatitis, burning, pruritus, and erythema, compared with 8% of patients on oxymetazoline. At the same time, up to 20% of individuals on brimonidine report rebound erythema, compared with fewer than 1% of those using oxymetazoline. Laser and light therapies such as pulse-dye lasers, potassium-titanyl-phosphate lasers, and intense-pulse light devices are also effective in treating persistent erythema but are less effective for transient flushing.
Treatment of papules and pustules
For treating papules and pustules, the 2019 systemic review also found high-certainty evidence for using azelaic acid and topical ivermectin, and moderate-certainty evidence for using topical metronidazole and topical minocycline. “Topical ivermectin was demonstrated to be the most effective topical treatment for papulopustular rosacea and to provide the greatest psychological benefit to these patients,” Dr. Graber said.
In a double-blind, multicenter 15-week trial comparing azelaic acid 15% gel with metronidazole 0.75% gel in patients with papulopustular rosacea, both agents were found to be effective. But those treated with azelaic acid 15% gel had a greater reduction in lesion counts and erythema, and improvement in global assessments, compared with metronidazole 0.75% gel. However, the azelaic acid 15% gel was associated with more stinging compared with metronidazole 0.75% gel, although it was usually transient.
Another study, a double-blind, single-center, 15-week trial, compared the efficacy of azelaic acid 20% cream with metronidazole 0.75% cream. Both agents were found to be effective and had similar levels of reductions in papules and pustules. However, patients in the azelaic acid 20% cream arm had significantly higher physician ratings of global improvement, as well as overall higher patient satisfaction.
More recently, a phase 3 study of 962 patients found that ivermectin 1% cream once daily improved quality of life slightly more than metronidazole 0.75% cream twice daily. No difference in adverse events were noted between the two agents.
Other options for treating papules and pustules include topical minocycline 1.5% foam, which is FDA approved for rosacea, as well as second-line agents topical sodium sulfacetamide with sulfur cleanser (cream or lotion), and permethrin, Dr. Graber said.
As for treating papules and pustules with oral agents, the strongest evidence favors oral tetracyclines and isotretinoin, she noted.
Doxycycline, minocycline, tetracycline, and sarecycline can be used as monotherapy or coadministered with topical agents. “The addition of topical agents may also help to shorten the duration of antibiotic use, which is very important,” Dr. Graber said.
She noted that oral beta-blockers might be useful to treat persistent erythema and flushing because they antagonize the effects of sympathetic nerve stimulation and circulating catecholamines at b-adrenoceptors. Carvedilol and propranolol have been the most studied. The most common potential side effects are hypotension and bradycardia.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
In the clinical experience of Emmy Graber, MD, MBA, rosacea is in the eye of the beholder.
“It’s not really up to us as the providers as to what’s important to the patient or how bad their rosacea is,” she said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It really is up to the patient,” added Dr. Graber, president of The Dermatology Institute of Boston, who recommends asking patients about how severe they consider their rosacea to be, and what about rosacea bothers them most. Their responses may be surprising.
A study published in 2017 showed that complete resolution of even mild rosacea prolongs remission of rosacea, and most importantly, improves the quality of life for patients. “So, don’t discount what you consider to be mild rosacea in patients,” she said.
Skin care recommendations
“And don’t forget about basic skin care,” she advised. A recently published Chinese study of 999 rosacea patients and 1,010 controls with healthy skin found that a high frequency of cleansing and expansive use of cleansers were positively correlated with rosacea occurrence, suggesting that overcleansing can be a risk factor for rosacea. “Ask your patient, ‘how often are you cleaning your face?’ ” Dr. Graber suggested. “You might find that they’re overdoing it by washing three or four times a day. Several studies have shown that basic skin care alone improves rosacea.”
Skin care recommendations for patients with rosacea include avoiding chemical or physical exfoliants and alcohol-based topical products, and moisturizing and washing their faces with mild, synthetic detergent-based products rather than traditional soaps, which may further alkalinize and irritate the skin. “Patients should also be counseled to use physical-based sunscreens rather than chemical-based sunscreens,” she said.
Treating erythema
For treating erythema with topicals, a systematic review published in 2019 found the most evidence for brimonidine 0.33% gel, an alpha2-adrenergic agonist, and oxymetazoline 1% cream, an alpha1-adrenergic agonist. “Both of these products functionally constrict facial blood vessels,” and are Food and Drug Administration approved for treating persistent erythema, Dr. Graber said. “These products improve erythema within 3 hours of and up to 12 hours after application and overall, they are well tolerated.”
Based on clinical trial results, about 15% of patients on brimonidine report adverse reactions such as dermatitis, burning, pruritus, and erythema, compared with 8% of patients on oxymetazoline. At the same time, up to 20% of individuals on brimonidine report rebound erythema, compared with fewer than 1% of those using oxymetazoline. Laser and light therapies such as pulse-dye lasers, potassium-titanyl-phosphate lasers, and intense-pulse light devices are also effective in treating persistent erythema but are less effective for transient flushing.
Treatment of papules and pustules
For treating papules and pustules, the 2019 systemic review also found high-certainty evidence for using azelaic acid and topical ivermectin, and moderate-certainty evidence for using topical metronidazole and topical minocycline. “Topical ivermectin was demonstrated to be the most effective topical treatment for papulopustular rosacea and to provide the greatest psychological benefit to these patients,” Dr. Graber said.
In a double-blind, multicenter 15-week trial comparing azelaic acid 15% gel with metronidazole 0.75% gel in patients with papulopustular rosacea, both agents were found to be effective. But those treated with azelaic acid 15% gel had a greater reduction in lesion counts and erythema, and improvement in global assessments, compared with metronidazole 0.75% gel. However, the azelaic acid 15% gel was associated with more stinging compared with metronidazole 0.75% gel, although it was usually transient.
Another study, a double-blind, single-center, 15-week trial, compared the efficacy of azelaic acid 20% cream with metronidazole 0.75% cream. Both agents were found to be effective and had similar levels of reductions in papules and pustules. However, patients in the azelaic acid 20% cream arm had significantly higher physician ratings of global improvement, as well as overall higher patient satisfaction.
More recently, a phase 3 study of 962 patients found that ivermectin 1% cream once daily improved quality of life slightly more than metronidazole 0.75% cream twice daily. No difference in adverse events were noted between the two agents.
Other options for treating papules and pustules include topical minocycline 1.5% foam, which is FDA approved for rosacea, as well as second-line agents topical sodium sulfacetamide with sulfur cleanser (cream or lotion), and permethrin, Dr. Graber said.
As for treating papules and pustules with oral agents, the strongest evidence favors oral tetracyclines and isotretinoin, she noted.
Doxycycline, minocycline, tetracycline, and sarecycline can be used as monotherapy or coadministered with topical agents. “The addition of topical agents may also help to shorten the duration of antibiotic use, which is very important,” Dr. Graber said.
She noted that oral beta-blockers might be useful to treat persistent erythema and flushing because they antagonize the effects of sympathetic nerve stimulation and circulating catecholamines at b-adrenoceptors. Carvedilol and propranolol have been the most studied. The most common potential side effects are hypotension and bradycardia.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
In the clinical experience of Emmy Graber, MD, MBA, rosacea is in the eye of the beholder.
“It’s not really up to us as the providers as to what’s important to the patient or how bad their rosacea is,” she said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It really is up to the patient,” added Dr. Graber, president of The Dermatology Institute of Boston, who recommends asking patients about how severe they consider their rosacea to be, and what about rosacea bothers them most. Their responses may be surprising.
A study published in 2017 showed that complete resolution of even mild rosacea prolongs remission of rosacea, and most importantly, improves the quality of life for patients. “So, don’t discount what you consider to be mild rosacea in patients,” she said.
Skin care recommendations
“And don’t forget about basic skin care,” she advised. A recently published Chinese study of 999 rosacea patients and 1,010 controls with healthy skin found that a high frequency of cleansing and expansive use of cleansers were positively correlated with rosacea occurrence, suggesting that overcleansing can be a risk factor for rosacea. “Ask your patient, ‘how often are you cleaning your face?’ ” Dr. Graber suggested. “You might find that they’re overdoing it by washing three or four times a day. Several studies have shown that basic skin care alone improves rosacea.”
Skin care recommendations for patients with rosacea include avoiding chemical or physical exfoliants and alcohol-based topical products, and moisturizing and washing their faces with mild, synthetic detergent-based products rather than traditional soaps, which may further alkalinize and irritate the skin. “Patients should also be counseled to use physical-based sunscreens rather than chemical-based sunscreens,” she said.
Treating erythema
For treating erythema with topicals, a systematic review published in 2019 found the most evidence for brimonidine 0.33% gel, an alpha2-adrenergic agonist, and oxymetazoline 1% cream, an alpha1-adrenergic agonist. “Both of these products functionally constrict facial blood vessels,” and are Food and Drug Administration approved for treating persistent erythema, Dr. Graber said. “These products improve erythema within 3 hours of and up to 12 hours after application and overall, they are well tolerated.”
Based on clinical trial results, about 15% of patients on brimonidine report adverse reactions such as dermatitis, burning, pruritus, and erythema, compared with 8% of patients on oxymetazoline. At the same time, up to 20% of individuals on brimonidine report rebound erythema, compared with fewer than 1% of those using oxymetazoline. Laser and light therapies such as pulse-dye lasers, potassium-titanyl-phosphate lasers, and intense-pulse light devices are also effective in treating persistent erythema but are less effective for transient flushing.
Treatment of papules and pustules
For treating papules and pustules, the 2019 systemic review also found high-certainty evidence for using azelaic acid and topical ivermectin, and moderate-certainty evidence for using topical metronidazole and topical minocycline. “Topical ivermectin was demonstrated to be the most effective topical treatment for papulopustular rosacea and to provide the greatest psychological benefit to these patients,” Dr. Graber said.
In a double-blind, multicenter 15-week trial comparing azelaic acid 15% gel with metronidazole 0.75% gel in patients with papulopustular rosacea, both agents were found to be effective. But those treated with azelaic acid 15% gel had a greater reduction in lesion counts and erythema, and improvement in global assessments, compared with metronidazole 0.75% gel. However, the azelaic acid 15% gel was associated with more stinging compared with metronidazole 0.75% gel, although it was usually transient.
Another study, a double-blind, single-center, 15-week trial, compared the efficacy of azelaic acid 20% cream with metronidazole 0.75% cream. Both agents were found to be effective and had similar levels of reductions in papules and pustules. However, patients in the azelaic acid 20% cream arm had significantly higher physician ratings of global improvement, as well as overall higher patient satisfaction.
More recently, a phase 3 study of 962 patients found that ivermectin 1% cream once daily improved quality of life slightly more than metronidazole 0.75% cream twice daily. No difference in adverse events were noted between the two agents.
Other options for treating papules and pustules include topical minocycline 1.5% foam, which is FDA approved for rosacea, as well as second-line agents topical sodium sulfacetamide with sulfur cleanser (cream or lotion), and permethrin, Dr. Graber said.
As for treating papules and pustules with oral agents, the strongest evidence favors oral tetracyclines and isotretinoin, she noted.
Doxycycline, minocycline, tetracycline, and sarecycline can be used as monotherapy or coadministered with topical agents. “The addition of topical agents may also help to shorten the duration of antibiotic use, which is very important,” Dr. Graber said.
She noted that oral beta-blockers might be useful to treat persistent erythema and flushing because they antagonize the effects of sympathetic nerve stimulation and circulating catecholamines at b-adrenoceptors. Carvedilol and propranolol have been the most studied. The most common potential side effects are hypotension and bradycardia.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Topical options for acne patients continue to expand
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR