User login
ACC 21 looks to repeat success despite pandemic headwinds
The American College of Cardiology pulled off an impressive all-virtual meeting in March 2020, less than 3 weeks after canceling its in-person event and just 2 weeks after COVID-19 was declared a national emergency.
Optimistic plans for the annual scientific sessions of the American College of Cardiology (ACC 2021) to be a March hybrid affair in Atlanta pivoted not once, but twice, as the pandemic evolved, with the date pushed back 2 full months, to May 15-17, and the format revised to fully virtual.
“While this meeting is being delivered virtually, I think you’ll see there have been benefits in the time to plan and also the lessons that ACC has learned in virtual education over the past year. This has come together to really create a robust educational and scientific agenda,” ACC 2021 chair Pamela B. Morris, MD, said in a press conference focused on the upcoming meeting.
Over the 3 days, there will be more than 200 education sessions, 10 guideline-specific sessions, and 11 learning pathways that include core areas, but also special topics, such as COVID-19 and the emerging cardio-obstetrics subspecialty.
The meeting will be delivered through a new virtual education program built to optimize real-time interaction between faculty members and attendees, she said. A dedicated portal on the platform will allow attendees to interact virtually, for example, with presenters of the nearly 3,000 ePosters and 420 moderated posters.
For those suffering from Zoom fatigue, the increasingly popular Heart2Heart stage talks have also been converted to podcasts, which cover topics like gender equity in cardiology, the evolving role of advanced practice professionals, and “one of my favorites: art as a tool for healing,” said Dr. Morris, from the Medical University of South Carolina, Charleston. “Those sessions are really not to be missed.”
Reconnecting is an underlying theme of the meeting but the great divider will not be ignored. COVID-19 will be the focus of two 90-minute Intensive Sessions on Saturday, May 15, the first kicking off at 10:30 a.m. ET, with the Bishop Keynote lecture on bringing health equity to the frontline of cardiovascular care, followed by lessons learned during the pandemic, how to conduct clinical trials, and vaccine development.
The second session, set for 12:15 p.m., continues the “silver linings” theme, with case presentations on advances in telehealth, myocardial involvement, and thrombosis in COVID. For those wanting more, 18 abstracts are on tap in a 2-hour Spotlight on Special Topics session beginning at 2:30 p.m.
Asked about the pandemic’s effect on bringing science to fruition this past year, Dr. Morris said there’s no question it’s slowed some of the progress the cardiology community had made but, like clinical practice, “we’ve also surmounted many of those obstacles.”
“I think research has rebounded,” she said. “Just in terms of the number of abstracts and the quality of abstracts that were submitted this year, I don’t think there’s any question that we are right on par with previous years.”
Indeed, 5,258 abstracts from 76 countries were submitted, with more than 3,400 chosen for oral and poster presentation, including 25 late-breaking clinical trials to be presented in five sessions.
The late-breaking presentations and discussions will be prerecorded but speakers and panelists have been invited to be present during the streaming to answer live any questions that may arise in the chat box, ACC 2021 vice chair Douglas Drachman, MD, Massachusetts General Hospital, Boston, said in an interview.
Late-breaking clinical trials
The Joint ACC/JACC Late-Breaking Clinical Trials I (Saturday, May 15, 9:00 a.m.–-10:00 a.m.) kicks off with PARADISE-MI, the first head-to-head comparison of an angiotensin receptor neprilysin inhibitor (ARNI) and an ACE inhibitor in patients with reduced ejection fractions (EFs) after MI but no history of heart failure (HF), studying 200 mg sacubitril/valsartan (Entresto) versus 5 mg of ramipril, both twice daily, in 5,669 patients.
Sacubitril/valsartan was initially approved for HF with reduced EF and added a new indication to treat some HF patients with preserved EF. Novartis, however, recently told investors that although numerical trends consistently favored the ARNI over the ACE inhibitor ramipril, the phase 3 study failed to meet the primary endpoint for efficacy superiority of reducing the risk for cardiovascular (CV) death and HF events after an acute MI.
Second up is ADAPTABLE, which looks to close a surprising evidence gap over whether 81 mg or 325 mg daily is the optimal dose of the ubiquitously prescribed aspirin for secondary prevention in high-risk patients with established atherosclerotic CV disease.
The open-label, randomized study will look at efficacy and major bleeding over roughly 4 years in 15,000 patients within PCORnet, the National Patient-centered Clinical Research Network, a partnership of clinical research, health plan research, and patient-powered networks created to streamline patient-reported outcomes research.
“This study will not only give important clinical information for us, practically speaking, whether we should prescribe lower- or higher-dose aspirin, but it may also serve as a template for future pragmatic clinical trial design in the real world,” Dr. Drachman said during the press conference.
Up next is the 4,812-patient Canadian LAAOS III, the largest trial to examine the efficacy of left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation (AFib) already undergoing cardiac surgery. The primary outcome is the first occurrence of stroke or systemic arterial embolism over an average follow-up of 4 years.
Percutaneous closure of the left atrial appendage (LAA) has been shown to reduce stroke in AFib patients at high-risk of bleeding on systemic anticoagulation. But these devices can be expensive and studies haven’t included patients who also have valvular heart disease, a group that actually comprises more than half of patients undergoing cardiac surgery who also have AFib, he noted.
At the same time, surgical LAA closure studies have been small and have had very mixed results. “There isn’t a large-scale rigorous assessment out there for these patients undergoing surgery, so I think this is going to be fascinating to see,” Dr. Drachman said.
The session closes with ATLANTIS, which looks to shed some light on the role of anticoagulation therapy in patients after transcatheter aortic valve replacement (TAVR or TAVI). POPular TAVI, presented at ACC 2020, showed aspirin alone was the preferred antithrombotic therapy over aspirin plus clopidogrel (Plavix) in patients not on oral anticoagulants, but the optimal anticoagulation regimen remains unsettled.
The French open-label, 1,510-patient ATLANTIS trial examined whether the novel oral anticoagulant apixaban (Eliquis) is superior in preventing CV events after TAVR, compared with antiplatelet therapy in patients without an indication for anticoagulation and compared with vitamin K antagonists in those receiving anticoagulants.
An ATLANTIS 4D CT substudy of valve thrombosis is also slated for Saturday’s Featured Clinical Research 1 session at 12:15 p.m. to 1:45 p.m..
Sunday LBCTs
Dr. Drachman highlighted a series of other late-breaking studies, including the global DARE-19 trial testing the diabetes and HF drug dapagliflozin (Farxiga) given with local standard-of-care therapy for 30 days in hospitalized COVID-19 patients with CV, metabolic, or renal risk factors.
Although sodium-glucose cotransporter-2 inhibitors have been white-hot of late, top-line results reported last month show dapagliflozin failed to achieve statistical significance for the primary endpoints of reducing organ dysfunction and all-cause mortality and for improving recovery. Details will be presented in the Joint ACC/JAMA Late-Breaking Clinical Trials II (Sunday, May 16, 8:00 a.m.-9:30 a.m.).
Two trials, FLOWER-MI and RADIANCE-HTN TRIO, were singled out in the Joint ACC/New England Journal of Medicine Late-Breaking Clinical Trials III (Sunday, May 16, 10:45 a.m.-12:00 p.m.). FLOWER-MI examines whether fractional flow reserve (FFR) is better than angiography to guide complete multivessel revascularization in ST-elevation MI patients with at least 50% stenosis in at least one nonculprit lesion requiring percutaneous coronary intervention (PCI). Recent studies have shown the superiority of FFR-guided PCI for nonculprit lesions, compared with culprit lesion treatment-only, but this is the first time FFR- and angiography-guided PCI have been compared in STEMI patients.
RADIANCE-HTN TRIO already tipped its hand, with top-line results reported in late 2020 showing that the trial met its primary efficacy endpoint of greater reduction in daytime blood pressure over 2 months with the Paradise endovascular ultrasound renal denervation system, compared with a sham procedure, in 136 patients with resistant hypertension, importantly, after being given a single pill containing a calcium channel blocker, angiotensin II receptor blocker, and diuretic.
Renal denervation for hypertension has been making something of a comeback, with the 2018 RADIANCE-HTN SOLO reporting better ambulatory blood pressure control with the Paradise system than with a sham procedure in the absence of antihypertensive agents. The device has been granted breakthrough device designation from the Food and Drug Administration for the treatment of hypertensive patients who are unable to sufficiently respond to or are intolerant of antihypertensive therapy.
Monday LBCTs
In the Late-Breaking Clinical Trials IV session (Monday, May 17, 8 a.m.–9:30 a.m.), Drachman called out a secondary analysis from GALATIC-HF looking at the impact of EF on the therapeutic effect of omecamtiv mecarbil. In last year’s primary analysis, the selective cardiac myosin activator produced a modest but significant reduction in HF events or CV death in 8,232 patients with HF and an EF of 35% or less.
Rounding out the list is the Canadian CAPITAL CHILL study of moderate versus mild therapeutic hypothermia in out-of-hospital cardiac arrest, to be presented in the final Late-Breaking Clinical Trials V session (Monday, May 17, 10:45 a.m.–12:00 p.m.).
The double-blind trial sought to determine whether neurologic outcomes at 6 months are improved by targeting a core temperature of 31 ˚C versus 34 ˚C after the return of spontaneous circulation in comatose survivors of out-of-hospital cardiac arrest.
“For me, I think this could really change practice and has personal relevance from experience with cardiac arrest survivors that I’ve known and care for very deeply,” Dr. Drachman said in an interview. “I think that there’s a lot of opportunity here as well.”
Asked what other trials have the potential to change practice, Dr. Drachman said FLOWER-MI holds particular interest because it looks at how to manage patients with STEMI with multiple lesions at the point of care.
“We’ve gained a lot of clarity from several other prior clinical trials, but this will help to answer the question in a slightly different way of saying: can you eyeball it, can you look at the angiogram and say whether or not that other, nonculprit lesion ought to be treated in the same hospitalization or should you really be using a pressure wire,” he said. “For me as an interventionalist, this is really important because when you finish up doing an intervention on a patient it might be the middle of the night and the patient may be more or less stable, but you’ve already exposed them to the risk of a procedure, should you then move on and do another aspect of the procedure to interrogate with a pressure wire a remaining narrowing? I think that’s very important; that’ll help me make decisions on a day-to-day basis.”
Dr. Drachman also cited RADIANCE-HTN TRIO because it employs an endovascular technique to control blood pressure in patients with hypertension, specifically those resistant to multiple drugs.
During the press conference, Dr. Morris, a preventive cardiologist, put her money on the ADAPTABLE study of aspirin dosing, reiterating that the unique trial design could inform future research, and on Sunday’s 8:45 a.m. late-breaking post hoc analysis from the STRENGTH trial that looks to pick up where the controversy over omega-3 fatty acid preparations left off at last year’s American Heart Association meeting.
A lack of benefit on CV event rates reported with Epanova, a high-dose combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid, led to a contentious debate over how to reconcile STRENGTH with the findings from REDUCE-IT, which showed a 25% relative risk reduction in major CV events with the EPA product icosapent ethyl (Vascepa).
STRENGTH investigator Steven Nissen, MD, Cleveland Clinic, and REDUCE-IT investigator and session panelist Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, will share the virtual stage at ACC 2021, but Dr. Morris said the “good news” is both researchers know one another very well and “will really be focusing on no political issues, just the omega-3 fatty levels in the bloodstream and what does that mean in either trial.
“This is not designed to be a debate, point counterpoint,” she added.
For that, as all cardiologists and journalists know, there will be the wild and woolly #CardioTwitter sphere.
A version of this article first appeared on Medscape.com.
The American College of Cardiology pulled off an impressive all-virtual meeting in March 2020, less than 3 weeks after canceling its in-person event and just 2 weeks after COVID-19 was declared a national emergency.
Optimistic plans for the annual scientific sessions of the American College of Cardiology (ACC 2021) to be a March hybrid affair in Atlanta pivoted not once, but twice, as the pandemic evolved, with the date pushed back 2 full months, to May 15-17, and the format revised to fully virtual.
“While this meeting is being delivered virtually, I think you’ll see there have been benefits in the time to plan and also the lessons that ACC has learned in virtual education over the past year. This has come together to really create a robust educational and scientific agenda,” ACC 2021 chair Pamela B. Morris, MD, said in a press conference focused on the upcoming meeting.
Over the 3 days, there will be more than 200 education sessions, 10 guideline-specific sessions, and 11 learning pathways that include core areas, but also special topics, such as COVID-19 and the emerging cardio-obstetrics subspecialty.
The meeting will be delivered through a new virtual education program built to optimize real-time interaction between faculty members and attendees, she said. A dedicated portal on the platform will allow attendees to interact virtually, for example, with presenters of the nearly 3,000 ePosters and 420 moderated posters.
For those suffering from Zoom fatigue, the increasingly popular Heart2Heart stage talks have also been converted to podcasts, which cover topics like gender equity in cardiology, the evolving role of advanced practice professionals, and “one of my favorites: art as a tool for healing,” said Dr. Morris, from the Medical University of South Carolina, Charleston. “Those sessions are really not to be missed.”
Reconnecting is an underlying theme of the meeting but the great divider will not be ignored. COVID-19 will be the focus of two 90-minute Intensive Sessions on Saturday, May 15, the first kicking off at 10:30 a.m. ET, with the Bishop Keynote lecture on bringing health equity to the frontline of cardiovascular care, followed by lessons learned during the pandemic, how to conduct clinical trials, and vaccine development.
The second session, set for 12:15 p.m., continues the “silver linings” theme, with case presentations on advances in telehealth, myocardial involvement, and thrombosis in COVID. For those wanting more, 18 abstracts are on tap in a 2-hour Spotlight on Special Topics session beginning at 2:30 p.m.
Asked about the pandemic’s effect on bringing science to fruition this past year, Dr. Morris said there’s no question it’s slowed some of the progress the cardiology community had made but, like clinical practice, “we’ve also surmounted many of those obstacles.”
“I think research has rebounded,” she said. “Just in terms of the number of abstracts and the quality of abstracts that were submitted this year, I don’t think there’s any question that we are right on par with previous years.”
Indeed, 5,258 abstracts from 76 countries were submitted, with more than 3,400 chosen for oral and poster presentation, including 25 late-breaking clinical trials to be presented in five sessions.
The late-breaking presentations and discussions will be prerecorded but speakers and panelists have been invited to be present during the streaming to answer live any questions that may arise in the chat box, ACC 2021 vice chair Douglas Drachman, MD, Massachusetts General Hospital, Boston, said in an interview.
Late-breaking clinical trials
The Joint ACC/JACC Late-Breaking Clinical Trials I (Saturday, May 15, 9:00 a.m.–-10:00 a.m.) kicks off with PARADISE-MI, the first head-to-head comparison of an angiotensin receptor neprilysin inhibitor (ARNI) and an ACE inhibitor in patients with reduced ejection fractions (EFs) after MI but no history of heart failure (HF), studying 200 mg sacubitril/valsartan (Entresto) versus 5 mg of ramipril, both twice daily, in 5,669 patients.
Sacubitril/valsartan was initially approved for HF with reduced EF and added a new indication to treat some HF patients with preserved EF. Novartis, however, recently told investors that although numerical trends consistently favored the ARNI over the ACE inhibitor ramipril, the phase 3 study failed to meet the primary endpoint for efficacy superiority of reducing the risk for cardiovascular (CV) death and HF events after an acute MI.
Second up is ADAPTABLE, which looks to close a surprising evidence gap over whether 81 mg or 325 mg daily is the optimal dose of the ubiquitously prescribed aspirin for secondary prevention in high-risk patients with established atherosclerotic CV disease.
The open-label, randomized study will look at efficacy and major bleeding over roughly 4 years in 15,000 patients within PCORnet, the National Patient-centered Clinical Research Network, a partnership of clinical research, health plan research, and patient-powered networks created to streamline patient-reported outcomes research.
“This study will not only give important clinical information for us, practically speaking, whether we should prescribe lower- or higher-dose aspirin, but it may also serve as a template for future pragmatic clinical trial design in the real world,” Dr. Drachman said during the press conference.
Up next is the 4,812-patient Canadian LAAOS III, the largest trial to examine the efficacy of left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation (AFib) already undergoing cardiac surgery. The primary outcome is the first occurrence of stroke or systemic arterial embolism over an average follow-up of 4 years.
Percutaneous closure of the left atrial appendage (LAA) has been shown to reduce stroke in AFib patients at high-risk of bleeding on systemic anticoagulation. But these devices can be expensive and studies haven’t included patients who also have valvular heart disease, a group that actually comprises more than half of patients undergoing cardiac surgery who also have AFib, he noted.
At the same time, surgical LAA closure studies have been small and have had very mixed results. “There isn’t a large-scale rigorous assessment out there for these patients undergoing surgery, so I think this is going to be fascinating to see,” Dr. Drachman said.
The session closes with ATLANTIS, which looks to shed some light on the role of anticoagulation therapy in patients after transcatheter aortic valve replacement (TAVR or TAVI). POPular TAVI, presented at ACC 2020, showed aspirin alone was the preferred antithrombotic therapy over aspirin plus clopidogrel (Plavix) in patients not on oral anticoagulants, but the optimal anticoagulation regimen remains unsettled.
The French open-label, 1,510-patient ATLANTIS trial examined whether the novel oral anticoagulant apixaban (Eliquis) is superior in preventing CV events after TAVR, compared with antiplatelet therapy in patients without an indication for anticoagulation and compared with vitamin K antagonists in those receiving anticoagulants.
An ATLANTIS 4D CT substudy of valve thrombosis is also slated for Saturday’s Featured Clinical Research 1 session at 12:15 p.m. to 1:45 p.m..
Sunday LBCTs
Dr. Drachman highlighted a series of other late-breaking studies, including the global DARE-19 trial testing the diabetes and HF drug dapagliflozin (Farxiga) given with local standard-of-care therapy for 30 days in hospitalized COVID-19 patients with CV, metabolic, or renal risk factors.
Although sodium-glucose cotransporter-2 inhibitors have been white-hot of late, top-line results reported last month show dapagliflozin failed to achieve statistical significance for the primary endpoints of reducing organ dysfunction and all-cause mortality and for improving recovery. Details will be presented in the Joint ACC/JAMA Late-Breaking Clinical Trials II (Sunday, May 16, 8:00 a.m.-9:30 a.m.).
Two trials, FLOWER-MI and RADIANCE-HTN TRIO, were singled out in the Joint ACC/New England Journal of Medicine Late-Breaking Clinical Trials III (Sunday, May 16, 10:45 a.m.-12:00 p.m.). FLOWER-MI examines whether fractional flow reserve (FFR) is better than angiography to guide complete multivessel revascularization in ST-elevation MI patients with at least 50% stenosis in at least one nonculprit lesion requiring percutaneous coronary intervention (PCI). Recent studies have shown the superiority of FFR-guided PCI for nonculprit lesions, compared with culprit lesion treatment-only, but this is the first time FFR- and angiography-guided PCI have been compared in STEMI patients.
RADIANCE-HTN TRIO already tipped its hand, with top-line results reported in late 2020 showing that the trial met its primary efficacy endpoint of greater reduction in daytime blood pressure over 2 months with the Paradise endovascular ultrasound renal denervation system, compared with a sham procedure, in 136 patients with resistant hypertension, importantly, after being given a single pill containing a calcium channel blocker, angiotensin II receptor blocker, and diuretic.
Renal denervation for hypertension has been making something of a comeback, with the 2018 RADIANCE-HTN SOLO reporting better ambulatory blood pressure control with the Paradise system than with a sham procedure in the absence of antihypertensive agents. The device has been granted breakthrough device designation from the Food and Drug Administration for the treatment of hypertensive patients who are unable to sufficiently respond to or are intolerant of antihypertensive therapy.
Monday LBCTs
In the Late-Breaking Clinical Trials IV session (Monday, May 17, 8 a.m.–9:30 a.m.), Drachman called out a secondary analysis from GALATIC-HF looking at the impact of EF on the therapeutic effect of omecamtiv mecarbil. In last year’s primary analysis, the selective cardiac myosin activator produced a modest but significant reduction in HF events or CV death in 8,232 patients with HF and an EF of 35% or less.
Rounding out the list is the Canadian CAPITAL CHILL study of moderate versus mild therapeutic hypothermia in out-of-hospital cardiac arrest, to be presented in the final Late-Breaking Clinical Trials V session (Monday, May 17, 10:45 a.m.–12:00 p.m.).
The double-blind trial sought to determine whether neurologic outcomes at 6 months are improved by targeting a core temperature of 31 ˚C versus 34 ˚C after the return of spontaneous circulation in comatose survivors of out-of-hospital cardiac arrest.
“For me, I think this could really change practice and has personal relevance from experience with cardiac arrest survivors that I’ve known and care for very deeply,” Dr. Drachman said in an interview. “I think that there’s a lot of opportunity here as well.”
Asked what other trials have the potential to change practice, Dr. Drachman said FLOWER-MI holds particular interest because it looks at how to manage patients with STEMI with multiple lesions at the point of care.
“We’ve gained a lot of clarity from several other prior clinical trials, but this will help to answer the question in a slightly different way of saying: can you eyeball it, can you look at the angiogram and say whether or not that other, nonculprit lesion ought to be treated in the same hospitalization or should you really be using a pressure wire,” he said. “For me as an interventionalist, this is really important because when you finish up doing an intervention on a patient it might be the middle of the night and the patient may be more or less stable, but you’ve already exposed them to the risk of a procedure, should you then move on and do another aspect of the procedure to interrogate with a pressure wire a remaining narrowing? I think that’s very important; that’ll help me make decisions on a day-to-day basis.”
Dr. Drachman also cited RADIANCE-HTN TRIO because it employs an endovascular technique to control blood pressure in patients with hypertension, specifically those resistant to multiple drugs.
During the press conference, Dr. Morris, a preventive cardiologist, put her money on the ADAPTABLE study of aspirin dosing, reiterating that the unique trial design could inform future research, and on Sunday’s 8:45 a.m. late-breaking post hoc analysis from the STRENGTH trial that looks to pick up where the controversy over omega-3 fatty acid preparations left off at last year’s American Heart Association meeting.
A lack of benefit on CV event rates reported with Epanova, a high-dose combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid, led to a contentious debate over how to reconcile STRENGTH with the findings from REDUCE-IT, which showed a 25% relative risk reduction in major CV events with the EPA product icosapent ethyl (Vascepa).
STRENGTH investigator Steven Nissen, MD, Cleveland Clinic, and REDUCE-IT investigator and session panelist Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, will share the virtual stage at ACC 2021, but Dr. Morris said the “good news” is both researchers know one another very well and “will really be focusing on no political issues, just the omega-3 fatty levels in the bloodstream and what does that mean in either trial.
“This is not designed to be a debate, point counterpoint,” she added.
For that, as all cardiologists and journalists know, there will be the wild and woolly #CardioTwitter sphere.
A version of this article first appeared on Medscape.com.
The American College of Cardiology pulled off an impressive all-virtual meeting in March 2020, less than 3 weeks after canceling its in-person event and just 2 weeks after COVID-19 was declared a national emergency.
Optimistic plans for the annual scientific sessions of the American College of Cardiology (ACC 2021) to be a March hybrid affair in Atlanta pivoted not once, but twice, as the pandemic evolved, with the date pushed back 2 full months, to May 15-17, and the format revised to fully virtual.
“While this meeting is being delivered virtually, I think you’ll see there have been benefits in the time to plan and also the lessons that ACC has learned in virtual education over the past year. This has come together to really create a robust educational and scientific agenda,” ACC 2021 chair Pamela B. Morris, MD, said in a press conference focused on the upcoming meeting.
Over the 3 days, there will be more than 200 education sessions, 10 guideline-specific sessions, and 11 learning pathways that include core areas, but also special topics, such as COVID-19 and the emerging cardio-obstetrics subspecialty.
The meeting will be delivered through a new virtual education program built to optimize real-time interaction between faculty members and attendees, she said. A dedicated portal on the platform will allow attendees to interact virtually, for example, with presenters of the nearly 3,000 ePosters and 420 moderated posters.
For those suffering from Zoom fatigue, the increasingly popular Heart2Heart stage talks have also been converted to podcasts, which cover topics like gender equity in cardiology, the evolving role of advanced practice professionals, and “one of my favorites: art as a tool for healing,” said Dr. Morris, from the Medical University of South Carolina, Charleston. “Those sessions are really not to be missed.”
Reconnecting is an underlying theme of the meeting but the great divider will not be ignored. COVID-19 will be the focus of two 90-minute Intensive Sessions on Saturday, May 15, the first kicking off at 10:30 a.m. ET, with the Bishop Keynote lecture on bringing health equity to the frontline of cardiovascular care, followed by lessons learned during the pandemic, how to conduct clinical trials, and vaccine development.
The second session, set for 12:15 p.m., continues the “silver linings” theme, with case presentations on advances in telehealth, myocardial involvement, and thrombosis in COVID. For those wanting more, 18 abstracts are on tap in a 2-hour Spotlight on Special Topics session beginning at 2:30 p.m.
Asked about the pandemic’s effect on bringing science to fruition this past year, Dr. Morris said there’s no question it’s slowed some of the progress the cardiology community had made but, like clinical practice, “we’ve also surmounted many of those obstacles.”
“I think research has rebounded,” she said. “Just in terms of the number of abstracts and the quality of abstracts that were submitted this year, I don’t think there’s any question that we are right on par with previous years.”
Indeed, 5,258 abstracts from 76 countries were submitted, with more than 3,400 chosen for oral and poster presentation, including 25 late-breaking clinical trials to be presented in five sessions.
The late-breaking presentations and discussions will be prerecorded but speakers and panelists have been invited to be present during the streaming to answer live any questions that may arise in the chat box, ACC 2021 vice chair Douglas Drachman, MD, Massachusetts General Hospital, Boston, said in an interview.
Late-breaking clinical trials
The Joint ACC/JACC Late-Breaking Clinical Trials I (Saturday, May 15, 9:00 a.m.–-10:00 a.m.) kicks off with PARADISE-MI, the first head-to-head comparison of an angiotensin receptor neprilysin inhibitor (ARNI) and an ACE inhibitor in patients with reduced ejection fractions (EFs) after MI but no history of heart failure (HF), studying 200 mg sacubitril/valsartan (Entresto) versus 5 mg of ramipril, both twice daily, in 5,669 patients.
Sacubitril/valsartan was initially approved for HF with reduced EF and added a new indication to treat some HF patients with preserved EF. Novartis, however, recently told investors that although numerical trends consistently favored the ARNI over the ACE inhibitor ramipril, the phase 3 study failed to meet the primary endpoint for efficacy superiority of reducing the risk for cardiovascular (CV) death and HF events after an acute MI.
Second up is ADAPTABLE, which looks to close a surprising evidence gap over whether 81 mg or 325 mg daily is the optimal dose of the ubiquitously prescribed aspirin for secondary prevention in high-risk patients with established atherosclerotic CV disease.
The open-label, randomized study will look at efficacy and major bleeding over roughly 4 years in 15,000 patients within PCORnet, the National Patient-centered Clinical Research Network, a partnership of clinical research, health plan research, and patient-powered networks created to streamline patient-reported outcomes research.
“This study will not only give important clinical information for us, practically speaking, whether we should prescribe lower- or higher-dose aspirin, but it may also serve as a template for future pragmatic clinical trial design in the real world,” Dr. Drachman said during the press conference.
Up next is the 4,812-patient Canadian LAAOS III, the largest trial to examine the efficacy of left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation (AFib) already undergoing cardiac surgery. The primary outcome is the first occurrence of stroke or systemic arterial embolism over an average follow-up of 4 years.
Percutaneous closure of the left atrial appendage (LAA) has been shown to reduce stroke in AFib patients at high-risk of bleeding on systemic anticoagulation. But these devices can be expensive and studies haven’t included patients who also have valvular heart disease, a group that actually comprises more than half of patients undergoing cardiac surgery who also have AFib, he noted.
At the same time, surgical LAA closure studies have been small and have had very mixed results. “There isn’t a large-scale rigorous assessment out there for these patients undergoing surgery, so I think this is going to be fascinating to see,” Dr. Drachman said.
The session closes with ATLANTIS, which looks to shed some light on the role of anticoagulation therapy in patients after transcatheter aortic valve replacement (TAVR or TAVI). POPular TAVI, presented at ACC 2020, showed aspirin alone was the preferred antithrombotic therapy over aspirin plus clopidogrel (Plavix) in patients not on oral anticoagulants, but the optimal anticoagulation regimen remains unsettled.
The French open-label, 1,510-patient ATLANTIS trial examined whether the novel oral anticoagulant apixaban (Eliquis) is superior in preventing CV events after TAVR, compared with antiplatelet therapy in patients without an indication for anticoagulation and compared with vitamin K antagonists in those receiving anticoagulants.
An ATLANTIS 4D CT substudy of valve thrombosis is also slated for Saturday’s Featured Clinical Research 1 session at 12:15 p.m. to 1:45 p.m..
Sunday LBCTs
Dr. Drachman highlighted a series of other late-breaking studies, including the global DARE-19 trial testing the diabetes and HF drug dapagliflozin (Farxiga) given with local standard-of-care therapy for 30 days in hospitalized COVID-19 patients with CV, metabolic, or renal risk factors.
Although sodium-glucose cotransporter-2 inhibitors have been white-hot of late, top-line results reported last month show dapagliflozin failed to achieve statistical significance for the primary endpoints of reducing organ dysfunction and all-cause mortality and for improving recovery. Details will be presented in the Joint ACC/JAMA Late-Breaking Clinical Trials II (Sunday, May 16, 8:00 a.m.-9:30 a.m.).
Two trials, FLOWER-MI and RADIANCE-HTN TRIO, were singled out in the Joint ACC/New England Journal of Medicine Late-Breaking Clinical Trials III (Sunday, May 16, 10:45 a.m.-12:00 p.m.). FLOWER-MI examines whether fractional flow reserve (FFR) is better than angiography to guide complete multivessel revascularization in ST-elevation MI patients with at least 50% stenosis in at least one nonculprit lesion requiring percutaneous coronary intervention (PCI). Recent studies have shown the superiority of FFR-guided PCI for nonculprit lesions, compared with culprit lesion treatment-only, but this is the first time FFR- and angiography-guided PCI have been compared in STEMI patients.
RADIANCE-HTN TRIO already tipped its hand, with top-line results reported in late 2020 showing that the trial met its primary efficacy endpoint of greater reduction in daytime blood pressure over 2 months with the Paradise endovascular ultrasound renal denervation system, compared with a sham procedure, in 136 patients with resistant hypertension, importantly, after being given a single pill containing a calcium channel blocker, angiotensin II receptor blocker, and diuretic.
Renal denervation for hypertension has been making something of a comeback, with the 2018 RADIANCE-HTN SOLO reporting better ambulatory blood pressure control with the Paradise system than with a sham procedure in the absence of antihypertensive agents. The device has been granted breakthrough device designation from the Food and Drug Administration for the treatment of hypertensive patients who are unable to sufficiently respond to or are intolerant of antihypertensive therapy.
Monday LBCTs
In the Late-Breaking Clinical Trials IV session (Monday, May 17, 8 a.m.–9:30 a.m.), Drachman called out a secondary analysis from GALATIC-HF looking at the impact of EF on the therapeutic effect of omecamtiv mecarbil. In last year’s primary analysis, the selective cardiac myosin activator produced a modest but significant reduction in HF events or CV death in 8,232 patients with HF and an EF of 35% or less.
Rounding out the list is the Canadian CAPITAL CHILL study of moderate versus mild therapeutic hypothermia in out-of-hospital cardiac arrest, to be presented in the final Late-Breaking Clinical Trials V session (Monday, May 17, 10:45 a.m.–12:00 p.m.).
The double-blind trial sought to determine whether neurologic outcomes at 6 months are improved by targeting a core temperature of 31 ˚C versus 34 ˚C after the return of spontaneous circulation in comatose survivors of out-of-hospital cardiac arrest.
“For me, I think this could really change practice and has personal relevance from experience with cardiac arrest survivors that I’ve known and care for very deeply,” Dr. Drachman said in an interview. “I think that there’s a lot of opportunity here as well.”
Asked what other trials have the potential to change practice, Dr. Drachman said FLOWER-MI holds particular interest because it looks at how to manage patients with STEMI with multiple lesions at the point of care.
“We’ve gained a lot of clarity from several other prior clinical trials, but this will help to answer the question in a slightly different way of saying: can you eyeball it, can you look at the angiogram and say whether or not that other, nonculprit lesion ought to be treated in the same hospitalization or should you really be using a pressure wire,” he said. “For me as an interventionalist, this is really important because when you finish up doing an intervention on a patient it might be the middle of the night and the patient may be more or less stable, but you’ve already exposed them to the risk of a procedure, should you then move on and do another aspect of the procedure to interrogate with a pressure wire a remaining narrowing? I think that’s very important; that’ll help me make decisions on a day-to-day basis.”
Dr. Drachman also cited RADIANCE-HTN TRIO because it employs an endovascular technique to control blood pressure in patients with hypertension, specifically those resistant to multiple drugs.
During the press conference, Dr. Morris, a preventive cardiologist, put her money on the ADAPTABLE study of aspirin dosing, reiterating that the unique trial design could inform future research, and on Sunday’s 8:45 a.m. late-breaking post hoc analysis from the STRENGTH trial that looks to pick up where the controversy over omega-3 fatty acid preparations left off at last year’s American Heart Association meeting.
A lack of benefit on CV event rates reported with Epanova, a high-dose combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid, led to a contentious debate over how to reconcile STRENGTH with the findings from REDUCE-IT, which showed a 25% relative risk reduction in major CV events with the EPA product icosapent ethyl (Vascepa).
STRENGTH investigator Steven Nissen, MD, Cleveland Clinic, and REDUCE-IT investigator and session panelist Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, will share the virtual stage at ACC 2021, but Dr. Morris said the “good news” is both researchers know one another very well and “will really be focusing on no political issues, just the omega-3 fatty levels in the bloodstream and what does that mean in either trial.
“This is not designed to be a debate, point counterpoint,” she added.
For that, as all cardiologists and journalists know, there will be the wild and woolly #CardioTwitter sphere.
A version of this article first appeared on Medscape.com.
Fresh look at ISCHEMIA bolsters conservative message in stable CAD
The more complicated a primary endpoint, the greater a puzzle it can be for clinicians to interpret the results. It’s likely even tougher for patients, who don’t help choose the events studied in clinical trials yet are increasingly sharing in the management decisions they influence.
That creates an opening for a more patient-centered take on one of cardiology’s most influential recent studies, ISCHEMIA, which bolsters the case for conservative, med-oriented management over a more invasive initial strategy for patients with stable coronary artery disease (CAD) and positive stress tests, researchers said.
The new, prespecified analysis replaced the trial’s conventional primary endpoint of major adverse cardiac events (MACE) with one based on “days alive out of hospital” (DAOH) and found an early advantage for the conservative approach, with caveats.
Those assigned to the conservative arm benefited with more out-of-hospital days throughout the next 2 years than those in the invasive-management group, owing to the latter’s protocol-mandated early cath-lab work-up with possible revascularization. The difference averaged more than 6 days for much of that time.
But DAOH evened out for the two groups by the fourth year in the analysis of more than 5,000 patients.
Protocol-determined cath procedures accounted for 61% of hospitalizations in the invasively managed group. A secondary DAOH analysis that excluded such required hospital days, also prespecified, showed no meaningful difference between the two strategies over the 4 years, noted the report published online May 3 in JAMA Cardiology.
DOAH is ‘very, very important’
The DAOH metric has been a far less common consideration in clinical trials, compared with clinical events, yet in some ways it is as “hard” a metric as mortality, encompasses a broader range of outcomes, and may matter more to patients, it’s been argued.
“The thing patients most value is time at home. So they don’t want to be in the hospital, they don’t want to be away from friends, they want to do recreation, or they may want to work,” lead author Harvey D. White, DSc, Green Lane Cardiovascular Services, Auckland (New Zealand) City Hospital, University of Auckland, told this news organization.
“When we need to talk to patients – and we do need to talk to patients – to have a days-out-of-hospital metric is very, very important,” he said. It is not only patient focused, it’s “meaningful in terms of the seriousness of events,” in that length of hospitalization tracks with clinical severity, observed Dr. White, who is slated to present the analysis May 17 during the virtual American College of Cardiology 2021 scientific sessions.
As previously reported, ISCHEMIA showed no significant effect on the primary endpoint of cardiovascular mortality, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest by assignment group over a median 3.2 years. Angina and quality of life measures were improved for patients in the invasive arm.
With an invasive initial strategy, “What we know now is that you get nothing of an advantage in terms of the composite endpoint, and you’re going to spend 6 days more in the hospital in the first 2 years, for largely no benefit,” Dr. White said.
That outlook may apply out to 4 years, the analysis suggests, but could conceivably change if DAOH is reassessed later as the ISCHEMIA follow-up continues for what is now a planned total of 10 years.
Meanwhile, the current findings could enhance doctor-patient discussions about the trade-offs between the two strategies for individuals whose considerations will vary.
“This is a very helpful measure to understand the burden of an approach to the patient,” observed E. Magnus Ohman, MD, an interventional cardiologist at Duke University, Durham, N.C., who was not involved in the trial.
With DAOH as an endpoint, “you as a clinician get another aspect of understanding of a treatment’s impact on a multitude of endpoints.” Days out of hospital, he noted, encompasses the effects of clinical events that often go into composite clinical endpoints – not death, but including nonfatal MI, stroke, need for revascularization, and cardiovascular hospitalization.
To patients with stable CAD who ask whether the invasive approach has merits in their case, the DAOH finding “helps you to say, well, at the end of the day, you will probably be spending an equal amount of time in the hospital. Your price up front is a little bit higher, but over time, the group who gets conservative treatment will catch up.”
The DAOH outcome also avoids the limitations of an endpoint based on time to first event, “not the least of which,” said Dr. White, is that it counts only the first of what might be multiple events of varying clinical impact. Misleadingly, “you can have an event that’s a small troponin rise, but that becomes more important in a person than dying the next day.”
The DAOH analysis was based on 5,179 patients from 37 countries who averaged 64 years of age and of whom 23% were women. The endpoint considered only overnight stays in hospitals, skilled nursing facilities, rehabilitation centers, and nursing homes.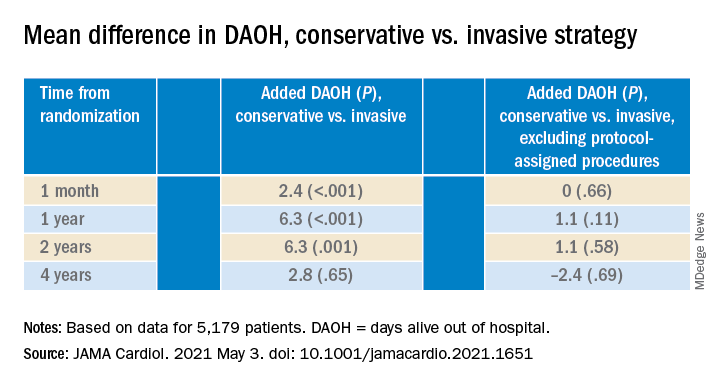
There were many more hospital or extended care facility stays overall in the invasive-management group, 4,002 versus 1,897 for those following the conservative strategy (P < .001), but the numbers flipped after excluding protocol-assigned procedures: 1,568 stays in the invasive group, compared with 1,897 (P = .001)
There were no associations between DAOH and Seattle Angina Questionnaire 7–Angina Frequency scores or DAOH interactions by age, sex, geographic region, or whether the patient had diabetes, prior MI, or heart failure, the report notes.
The primary ISCHEMIA analysis hinted at a possible long-term advantage for the invasive initial strategy in that event curves for the two arms crossed after 2-3 years, Dr. Ohman observed.
Based on that, for younger patients with stable CAD and ischemia at stress testing, “an investment of more hospital days early on might be worth it in the long run.” But ISCHEMIA, he said, “only suggests it, it doesn’t confirm it.”
The study was supported in part by grants from Arbor Pharmaceuticals and AstraZeneca. Devices or medications were provided by Abbott Vascular, Amgen, Arbor, AstraZeneca, Esperion, Medtronic, Merck Sharp & Dohme, Phillips, Omron Healthcare, and Sunovion. Dr. White disclosed receiving grants paid to his institution and fees for serving on a steering committee from Sanofi-Aventis, Regeneron, Eli Lilly, Omthera, American Regent, Eisai, DalCor, CSL Behring, Sanofi-Aventis Australia, and Esperion Therapeutics, and personal fees from Genentech and AstraZeneca. Dr. Ohman reported receiving grants from Abiomed and Cheisi USA, and consulting for Abiomed, Cara Therapeutics, Chiesi USA, Cytokinetics, Imbria Pharmaceuticals, Otsuka Pharmaceuticals, Milestone Pharmaceuticals, and XyloCor Therapeutics.
A version of this article first appeared on Medscape.com.
The more complicated a primary endpoint, the greater a puzzle it can be for clinicians to interpret the results. It’s likely even tougher for patients, who don’t help choose the events studied in clinical trials yet are increasingly sharing in the management decisions they influence.
That creates an opening for a more patient-centered take on one of cardiology’s most influential recent studies, ISCHEMIA, which bolsters the case for conservative, med-oriented management over a more invasive initial strategy for patients with stable coronary artery disease (CAD) and positive stress tests, researchers said.
The new, prespecified analysis replaced the trial’s conventional primary endpoint of major adverse cardiac events (MACE) with one based on “days alive out of hospital” (DAOH) and found an early advantage for the conservative approach, with caveats.
Those assigned to the conservative arm benefited with more out-of-hospital days throughout the next 2 years than those in the invasive-management group, owing to the latter’s protocol-mandated early cath-lab work-up with possible revascularization. The difference averaged more than 6 days for much of that time.
But DAOH evened out for the two groups by the fourth year in the analysis of more than 5,000 patients.
Protocol-determined cath procedures accounted for 61% of hospitalizations in the invasively managed group. A secondary DAOH analysis that excluded such required hospital days, also prespecified, showed no meaningful difference between the two strategies over the 4 years, noted the report published online May 3 in JAMA Cardiology.
DOAH is ‘very, very important’
The DAOH metric has been a far less common consideration in clinical trials, compared with clinical events, yet in some ways it is as “hard” a metric as mortality, encompasses a broader range of outcomes, and may matter more to patients, it’s been argued.
“The thing patients most value is time at home. So they don’t want to be in the hospital, they don’t want to be away from friends, they want to do recreation, or they may want to work,” lead author Harvey D. White, DSc, Green Lane Cardiovascular Services, Auckland (New Zealand) City Hospital, University of Auckland, told this news organization.
“When we need to talk to patients – and we do need to talk to patients – to have a days-out-of-hospital metric is very, very important,” he said. It is not only patient focused, it’s “meaningful in terms of the seriousness of events,” in that length of hospitalization tracks with clinical severity, observed Dr. White, who is slated to present the analysis May 17 during the virtual American College of Cardiology 2021 scientific sessions.
As previously reported, ISCHEMIA showed no significant effect on the primary endpoint of cardiovascular mortality, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest by assignment group over a median 3.2 years. Angina and quality of life measures were improved for patients in the invasive arm.
With an invasive initial strategy, “What we know now is that you get nothing of an advantage in terms of the composite endpoint, and you’re going to spend 6 days more in the hospital in the first 2 years, for largely no benefit,” Dr. White said.
That outlook may apply out to 4 years, the analysis suggests, but could conceivably change if DAOH is reassessed later as the ISCHEMIA follow-up continues for what is now a planned total of 10 years.
Meanwhile, the current findings could enhance doctor-patient discussions about the trade-offs between the two strategies for individuals whose considerations will vary.
“This is a very helpful measure to understand the burden of an approach to the patient,” observed E. Magnus Ohman, MD, an interventional cardiologist at Duke University, Durham, N.C., who was not involved in the trial.
With DAOH as an endpoint, “you as a clinician get another aspect of understanding of a treatment’s impact on a multitude of endpoints.” Days out of hospital, he noted, encompasses the effects of clinical events that often go into composite clinical endpoints – not death, but including nonfatal MI, stroke, need for revascularization, and cardiovascular hospitalization.
To patients with stable CAD who ask whether the invasive approach has merits in their case, the DAOH finding “helps you to say, well, at the end of the day, you will probably be spending an equal amount of time in the hospital. Your price up front is a little bit higher, but over time, the group who gets conservative treatment will catch up.”
The DAOH outcome also avoids the limitations of an endpoint based on time to first event, “not the least of which,” said Dr. White, is that it counts only the first of what might be multiple events of varying clinical impact. Misleadingly, “you can have an event that’s a small troponin rise, but that becomes more important in a person than dying the next day.”
The DAOH analysis was based on 5,179 patients from 37 countries who averaged 64 years of age and of whom 23% were women. The endpoint considered only overnight stays in hospitals, skilled nursing facilities, rehabilitation centers, and nursing homes.
There were many more hospital or extended care facility stays overall in the invasive-management group, 4,002 versus 1,897 for those following the conservative strategy (P < .001), but the numbers flipped after excluding protocol-assigned procedures: 1,568 stays in the invasive group, compared with 1,897 (P = .001)
There were no associations between DAOH and Seattle Angina Questionnaire 7–Angina Frequency scores or DAOH interactions by age, sex, geographic region, or whether the patient had diabetes, prior MI, or heart failure, the report notes.
The primary ISCHEMIA analysis hinted at a possible long-term advantage for the invasive initial strategy in that event curves for the two arms crossed after 2-3 years, Dr. Ohman observed.
Based on that, for younger patients with stable CAD and ischemia at stress testing, “an investment of more hospital days early on might be worth it in the long run.” But ISCHEMIA, he said, “only suggests it, it doesn’t confirm it.”
The study was supported in part by grants from Arbor Pharmaceuticals and AstraZeneca. Devices or medications were provided by Abbott Vascular, Amgen, Arbor, AstraZeneca, Esperion, Medtronic, Merck Sharp & Dohme, Phillips, Omron Healthcare, and Sunovion. Dr. White disclosed receiving grants paid to his institution and fees for serving on a steering committee from Sanofi-Aventis, Regeneron, Eli Lilly, Omthera, American Regent, Eisai, DalCor, CSL Behring, Sanofi-Aventis Australia, and Esperion Therapeutics, and personal fees from Genentech and AstraZeneca. Dr. Ohman reported receiving grants from Abiomed and Cheisi USA, and consulting for Abiomed, Cara Therapeutics, Chiesi USA, Cytokinetics, Imbria Pharmaceuticals, Otsuka Pharmaceuticals, Milestone Pharmaceuticals, and XyloCor Therapeutics.
A version of this article first appeared on Medscape.com.
The more complicated a primary endpoint, the greater a puzzle it can be for clinicians to interpret the results. It’s likely even tougher for patients, who don’t help choose the events studied in clinical trials yet are increasingly sharing in the management decisions they influence.
That creates an opening for a more patient-centered take on one of cardiology’s most influential recent studies, ISCHEMIA, which bolsters the case for conservative, med-oriented management over a more invasive initial strategy for patients with stable coronary artery disease (CAD) and positive stress tests, researchers said.
The new, prespecified analysis replaced the trial’s conventional primary endpoint of major adverse cardiac events (MACE) with one based on “days alive out of hospital” (DAOH) and found an early advantage for the conservative approach, with caveats.
Those assigned to the conservative arm benefited with more out-of-hospital days throughout the next 2 years than those in the invasive-management group, owing to the latter’s protocol-mandated early cath-lab work-up with possible revascularization. The difference averaged more than 6 days for much of that time.
But DAOH evened out for the two groups by the fourth year in the analysis of more than 5,000 patients.
Protocol-determined cath procedures accounted for 61% of hospitalizations in the invasively managed group. A secondary DAOH analysis that excluded such required hospital days, also prespecified, showed no meaningful difference between the two strategies over the 4 years, noted the report published online May 3 in JAMA Cardiology.
DOAH is ‘very, very important’
The DAOH metric has been a far less common consideration in clinical trials, compared with clinical events, yet in some ways it is as “hard” a metric as mortality, encompasses a broader range of outcomes, and may matter more to patients, it’s been argued.
“The thing patients most value is time at home. So they don’t want to be in the hospital, they don’t want to be away from friends, they want to do recreation, or they may want to work,” lead author Harvey D. White, DSc, Green Lane Cardiovascular Services, Auckland (New Zealand) City Hospital, University of Auckland, told this news organization.
“When we need to talk to patients – and we do need to talk to patients – to have a days-out-of-hospital metric is very, very important,” he said. It is not only patient focused, it’s “meaningful in terms of the seriousness of events,” in that length of hospitalization tracks with clinical severity, observed Dr. White, who is slated to present the analysis May 17 during the virtual American College of Cardiology 2021 scientific sessions.
As previously reported, ISCHEMIA showed no significant effect on the primary endpoint of cardiovascular mortality, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest by assignment group over a median 3.2 years. Angina and quality of life measures were improved for patients in the invasive arm.
With an invasive initial strategy, “What we know now is that you get nothing of an advantage in terms of the composite endpoint, and you’re going to spend 6 days more in the hospital in the first 2 years, for largely no benefit,” Dr. White said.
That outlook may apply out to 4 years, the analysis suggests, but could conceivably change if DAOH is reassessed later as the ISCHEMIA follow-up continues for what is now a planned total of 10 years.
Meanwhile, the current findings could enhance doctor-patient discussions about the trade-offs between the two strategies for individuals whose considerations will vary.
“This is a very helpful measure to understand the burden of an approach to the patient,” observed E. Magnus Ohman, MD, an interventional cardiologist at Duke University, Durham, N.C., who was not involved in the trial.
With DAOH as an endpoint, “you as a clinician get another aspect of understanding of a treatment’s impact on a multitude of endpoints.” Days out of hospital, he noted, encompasses the effects of clinical events that often go into composite clinical endpoints – not death, but including nonfatal MI, stroke, need for revascularization, and cardiovascular hospitalization.
To patients with stable CAD who ask whether the invasive approach has merits in their case, the DAOH finding “helps you to say, well, at the end of the day, you will probably be spending an equal amount of time in the hospital. Your price up front is a little bit higher, but over time, the group who gets conservative treatment will catch up.”
The DAOH outcome also avoids the limitations of an endpoint based on time to first event, “not the least of which,” said Dr. White, is that it counts only the first of what might be multiple events of varying clinical impact. Misleadingly, “you can have an event that’s a small troponin rise, but that becomes more important in a person than dying the next day.”
The DAOH analysis was based on 5,179 patients from 37 countries who averaged 64 years of age and of whom 23% were women. The endpoint considered only overnight stays in hospitals, skilled nursing facilities, rehabilitation centers, and nursing homes.
There were many more hospital or extended care facility stays overall in the invasive-management group, 4,002 versus 1,897 for those following the conservative strategy (P < .001), but the numbers flipped after excluding protocol-assigned procedures: 1,568 stays in the invasive group, compared with 1,897 (P = .001)
There were no associations between DAOH and Seattle Angina Questionnaire 7–Angina Frequency scores or DAOH interactions by age, sex, geographic region, or whether the patient had diabetes, prior MI, or heart failure, the report notes.
The primary ISCHEMIA analysis hinted at a possible long-term advantage for the invasive initial strategy in that event curves for the two arms crossed after 2-3 years, Dr. Ohman observed.
Based on that, for younger patients with stable CAD and ischemia at stress testing, “an investment of more hospital days early on might be worth it in the long run.” But ISCHEMIA, he said, “only suggests it, it doesn’t confirm it.”
The study was supported in part by grants from Arbor Pharmaceuticals and AstraZeneca. Devices or medications were provided by Abbott Vascular, Amgen, Arbor, AstraZeneca, Esperion, Medtronic, Merck Sharp & Dohme, Phillips, Omron Healthcare, and Sunovion. Dr. White disclosed receiving grants paid to his institution and fees for serving on a steering committee from Sanofi-Aventis, Regeneron, Eli Lilly, Omthera, American Regent, Eisai, DalCor, CSL Behring, Sanofi-Aventis Australia, and Esperion Therapeutics, and personal fees from Genentech and AstraZeneca. Dr. Ohman reported receiving grants from Abiomed and Cheisi USA, and consulting for Abiomed, Cara Therapeutics, Chiesi USA, Cytokinetics, Imbria Pharmaceuticals, Otsuka Pharmaceuticals, Milestone Pharmaceuticals, and XyloCor Therapeutics.
A version of this article first appeared on Medscape.com.
Hypertension worsened by commonly used prescription meds
Nearly half of these American adults had hypertension, and in this subgroup, 18.5% reported using a prescription drug known to increase blood pressure. The most widely used class of agents with this effect was antidepressants, used by 8.7%; followed by nonsteroidal anti-inflammatory drugs (NSAIDs), used by 6.5%; steroids, 1.9%; estrogens, 1.7%; and several other agents each used by fewer than 1% of the study cohort, John Vitarello, MD, said during a press briefing on reports from the upcoming annual scientific sessions of the American College of Cardiology.
He and his associates estimated that this use of prescription drugs known to raise blood pressure could be what stands in the way of some 560,000-2.2 million Americans from having their hypertension under control, depending on the exact blood pressure impact that various pressure-increasing drugs have and presuming that half of those on blood pressure increasing agents could stop them and switch to alternative agents, said Dr. Vitarello, a researcher at Beth Israel Deaconess Medical Center in Boston.
He also highlighted that the study assessed only prescription drugs and did not examine OTC drug use, which may be especially relevant for the many people who regularly take NSAIDs.
“Clinicians should review the prescription and OTC drug use of patients with hypertension and consider stopping drugs that increase blood pressure or switching the patient to alternatives” that are blood pressure neutral, Dr. Vitarello said during the briefing. He cautioned that maintaining hypertensive patients on agents that raise their blood pressure can result in “prescribing cascades” where taking drugs that boost blood pressure results in need for intensified antihypertensive treatment.
An opportunity for NSAID alternatives
“This study hopefully raises awareness that there is a very high use of medications that increase blood pressure, and use of OTC agents could increase the rate even higher” said Eugene Yang, MD, a cardiologist and codirector of the Cardiovascular Wellness and Prevention Program of the University of Washington, Seattle. Substituting for certain antidepressant agents may often not be realistic, but an opportunity exists for reducing NSAID use, a class also linked with an increased risk for bleeding and other adverse effects, Dr. Yang said during the briefing. Minimizing use of NSAIDs including ibuprofen and naproxen use “is something to think about,” he suggested.
“The effect of NSAIDs on blood pressure is not well studied and can vary from person to person” noted Dr. Vitarello, who added that higher NSAID dosages and more prolonged use likely increase the risk for an adverse effect on blood pressure. One reasonable option is to encourage patients to use an alternative class of pain reliever such as acetaminophen.
It remains “a challenge” to discern differences in adverse blood pressure effects, and in all adverse cardiovascular effects among different NSAIDs, said Dr. Yang. Results from “some studies show that certain NSAIDs may be safer, but other studies did not. We need to be very careful using NSAIDs because, on average, they increase blood pressure by about 3 mm Hg. We need to be mindful to try to prescribe alternative agents, like acetaminophen.”
A decade of data from NHANES
The analysis run by Dr. Vitarello and associates used data from 27,599 American adults included in the NHANES during 2009-2018, and focused on the 44% who either had an average blood pressure measurement of at least 130/80 mm Hg or reported having ever been told by a clinician that they had hypertension. The NHANES assessments included the prescription medications taken by each participant. The prevalence of using at least one prescription drug known to raise blood pressure was 24% among women and 14% among men, and 4% of those with hypertension were on two or more pressure-increasing agents.
The researchers based their identification of pressure-increasing prescription drugs on the list included in the 2017 guideline for managing high blood pressure from the American College of Cardiology and American Heart Association. This list specifies that the antidepressants that raise blood pressure are the monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic antidepressants.
Dr. Vitarello and Dr. Yang had no disclosures.
Nearly half of these American adults had hypertension, and in this subgroup, 18.5% reported using a prescription drug known to increase blood pressure. The most widely used class of agents with this effect was antidepressants, used by 8.7%; followed by nonsteroidal anti-inflammatory drugs (NSAIDs), used by 6.5%; steroids, 1.9%; estrogens, 1.7%; and several other agents each used by fewer than 1% of the study cohort, John Vitarello, MD, said during a press briefing on reports from the upcoming annual scientific sessions of the American College of Cardiology.
He and his associates estimated that this use of prescription drugs known to raise blood pressure could be what stands in the way of some 560,000-2.2 million Americans from having their hypertension under control, depending on the exact blood pressure impact that various pressure-increasing drugs have and presuming that half of those on blood pressure increasing agents could stop them and switch to alternative agents, said Dr. Vitarello, a researcher at Beth Israel Deaconess Medical Center in Boston.
He also highlighted that the study assessed only prescription drugs and did not examine OTC drug use, which may be especially relevant for the many people who regularly take NSAIDs.
“Clinicians should review the prescription and OTC drug use of patients with hypertension and consider stopping drugs that increase blood pressure or switching the patient to alternatives” that are blood pressure neutral, Dr. Vitarello said during the briefing. He cautioned that maintaining hypertensive patients on agents that raise their blood pressure can result in “prescribing cascades” where taking drugs that boost blood pressure results in need for intensified antihypertensive treatment.
An opportunity for NSAID alternatives
“This study hopefully raises awareness that there is a very high use of medications that increase blood pressure, and use of OTC agents could increase the rate even higher” said Eugene Yang, MD, a cardiologist and codirector of the Cardiovascular Wellness and Prevention Program of the University of Washington, Seattle. Substituting for certain antidepressant agents may often not be realistic, but an opportunity exists for reducing NSAID use, a class also linked with an increased risk for bleeding and other adverse effects, Dr. Yang said during the briefing. Minimizing use of NSAIDs including ibuprofen and naproxen use “is something to think about,” he suggested.
“The effect of NSAIDs on blood pressure is not well studied and can vary from person to person” noted Dr. Vitarello, who added that higher NSAID dosages and more prolonged use likely increase the risk for an adverse effect on blood pressure. One reasonable option is to encourage patients to use an alternative class of pain reliever such as acetaminophen.
It remains “a challenge” to discern differences in adverse blood pressure effects, and in all adverse cardiovascular effects among different NSAIDs, said Dr. Yang. Results from “some studies show that certain NSAIDs may be safer, but other studies did not. We need to be very careful using NSAIDs because, on average, they increase blood pressure by about 3 mm Hg. We need to be mindful to try to prescribe alternative agents, like acetaminophen.”
A decade of data from NHANES
The analysis run by Dr. Vitarello and associates used data from 27,599 American adults included in the NHANES during 2009-2018, and focused on the 44% who either had an average blood pressure measurement of at least 130/80 mm Hg or reported having ever been told by a clinician that they had hypertension. The NHANES assessments included the prescription medications taken by each participant. The prevalence of using at least one prescription drug known to raise blood pressure was 24% among women and 14% among men, and 4% of those with hypertension were on two or more pressure-increasing agents.
The researchers based their identification of pressure-increasing prescription drugs on the list included in the 2017 guideline for managing high blood pressure from the American College of Cardiology and American Heart Association. This list specifies that the antidepressants that raise blood pressure are the monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic antidepressants.
Dr. Vitarello and Dr. Yang had no disclosures.
Nearly half of these American adults had hypertension, and in this subgroup, 18.5% reported using a prescription drug known to increase blood pressure. The most widely used class of agents with this effect was antidepressants, used by 8.7%; followed by nonsteroidal anti-inflammatory drugs (NSAIDs), used by 6.5%; steroids, 1.9%; estrogens, 1.7%; and several other agents each used by fewer than 1% of the study cohort, John Vitarello, MD, said during a press briefing on reports from the upcoming annual scientific sessions of the American College of Cardiology.
He and his associates estimated that this use of prescription drugs known to raise blood pressure could be what stands in the way of some 560,000-2.2 million Americans from having their hypertension under control, depending on the exact blood pressure impact that various pressure-increasing drugs have and presuming that half of those on blood pressure increasing agents could stop them and switch to alternative agents, said Dr. Vitarello, a researcher at Beth Israel Deaconess Medical Center in Boston.
He also highlighted that the study assessed only prescription drugs and did not examine OTC drug use, which may be especially relevant for the many people who regularly take NSAIDs.
“Clinicians should review the prescription and OTC drug use of patients with hypertension and consider stopping drugs that increase blood pressure or switching the patient to alternatives” that are blood pressure neutral, Dr. Vitarello said during the briefing. He cautioned that maintaining hypertensive patients on agents that raise their blood pressure can result in “prescribing cascades” where taking drugs that boost blood pressure results in need for intensified antihypertensive treatment.
An opportunity for NSAID alternatives
“This study hopefully raises awareness that there is a very high use of medications that increase blood pressure, and use of OTC agents could increase the rate even higher” said Eugene Yang, MD, a cardiologist and codirector of the Cardiovascular Wellness and Prevention Program of the University of Washington, Seattle. Substituting for certain antidepressant agents may often not be realistic, but an opportunity exists for reducing NSAID use, a class also linked with an increased risk for bleeding and other adverse effects, Dr. Yang said during the briefing. Minimizing use of NSAIDs including ibuprofen and naproxen use “is something to think about,” he suggested.
“The effect of NSAIDs on blood pressure is not well studied and can vary from person to person” noted Dr. Vitarello, who added that higher NSAID dosages and more prolonged use likely increase the risk for an adverse effect on blood pressure. One reasonable option is to encourage patients to use an alternative class of pain reliever such as acetaminophen.
It remains “a challenge” to discern differences in adverse blood pressure effects, and in all adverse cardiovascular effects among different NSAIDs, said Dr. Yang. Results from “some studies show that certain NSAIDs may be safer, but other studies did not. We need to be very careful using NSAIDs because, on average, they increase blood pressure by about 3 mm Hg. We need to be mindful to try to prescribe alternative agents, like acetaminophen.”
A decade of data from NHANES
The analysis run by Dr. Vitarello and associates used data from 27,599 American adults included in the NHANES during 2009-2018, and focused on the 44% who either had an average blood pressure measurement of at least 130/80 mm Hg or reported having ever been told by a clinician that they had hypertension. The NHANES assessments included the prescription medications taken by each participant. The prevalence of using at least one prescription drug known to raise blood pressure was 24% among women and 14% among men, and 4% of those with hypertension were on two or more pressure-increasing agents.
The researchers based their identification of pressure-increasing prescription drugs on the list included in the 2017 guideline for managing high blood pressure from the American College of Cardiology and American Heart Association. This list specifies that the antidepressants that raise blood pressure are the monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic antidepressants.
Dr. Vitarello and Dr. Yang had no disclosures.
FROM ACC 2021

