User login
Rapid assay distinguishes viral from bacterial infection
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
REPORTING FROM ESPID 2019
Key clinical point: A novel real-time single-gene–expression PCR test quickly distinguishes viral from bacterial infection in febrile children.
Major finding: The expression signature of the IFI44L gene rapidly distinguished bacterial from viral infection in febrile children with 91% sensitivity and 93% specificity.
Study details: This translational study included 25 febrile children with definite bacterial or viral infections and 10 healthy controls.
Disclosures: The presenter reported having no financial conflicts regarding her study, supported by institutional funding.
How to have ‘the talk’ with vaccine skeptics
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
EXPERT ANALYSIS FROM ESPID 2019
Reducing pediatric RSV burden is top priority
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.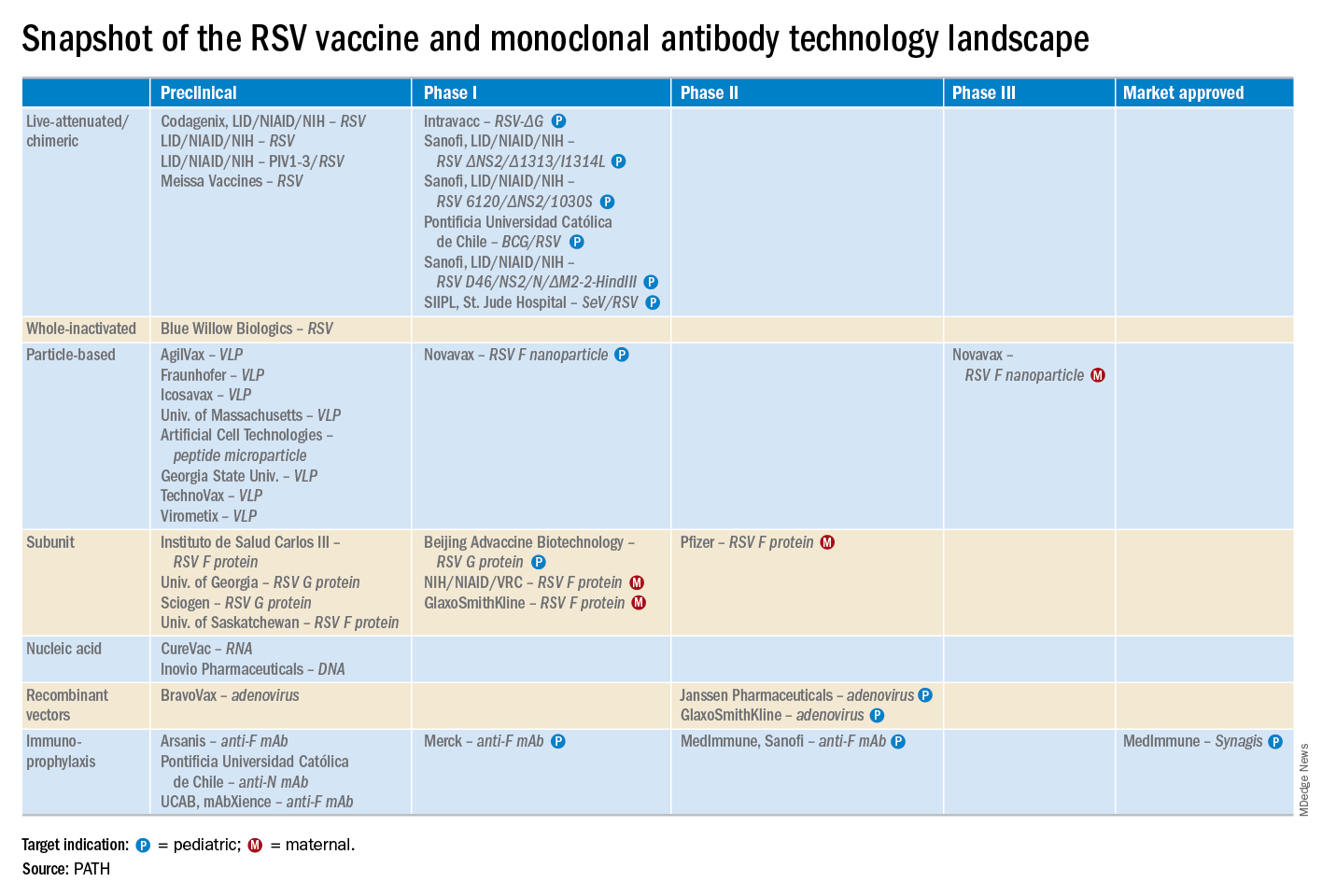
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
EXPERT ANALYSIS FROM ESPID 2019
Consider measles vaccine booster in HIV-positive patients
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
REPORTING FROM ESPID 2019
Breastfeeding protects against intussusception
LJUBLJANA, SLOVENIA – in a German case-control study.
Two other potent risk factors for intussusception in children less than 1 year old were identified: a family history of intussusception, and an episode of gastroenteritis, Doris F. Oberle, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Oberle, of the Paul Ehrlich Institute in Langen, Germany, presented a retrospective study of 116 meticulously validated cases of intussusception in infancy treated at 19 German pediatric centers during 2010-2014 and 272 controls matched by birth month, sex, and location. A standardized interview was conducted with the parents of all study participants.
Rotavirus vaccine was added to the German national vaccination schedule in 2013. In a multivariate logistic regression analysis, the risk of intussusception was increased by 5.4-fold following the first dose of the vaccine, compared with nonrecipients. However, subsequent doses of rotavirus vaccine were not associated with any excess risk.
In addition, a family history of intussusception was linked to a 4.2-fold increased risk, while an episode of gastroenteritis during the first year of life was associated with a 4.7-fold elevated risk.
In a novel finding, breastfeeding was independently associated with a 44% reduction in the risk of intussusception, compared with that of bottle-fed babies.
The most common presenting signs and symptoms of intussusception were vomiting, abdominal pain, hematochezia, pallor, and reduced appetite, each present in at least half of affected infants.
Dr. Oberle reported having no financial conflicts regarding her study, supported by the Paul Ehrlich Institute.
LJUBLJANA, SLOVENIA – in a German case-control study.
Two other potent risk factors for intussusception in children less than 1 year old were identified: a family history of intussusception, and an episode of gastroenteritis, Doris F. Oberle, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Oberle, of the Paul Ehrlich Institute in Langen, Germany, presented a retrospective study of 116 meticulously validated cases of intussusception in infancy treated at 19 German pediatric centers during 2010-2014 and 272 controls matched by birth month, sex, and location. A standardized interview was conducted with the parents of all study participants.
Rotavirus vaccine was added to the German national vaccination schedule in 2013. In a multivariate logistic regression analysis, the risk of intussusception was increased by 5.4-fold following the first dose of the vaccine, compared with nonrecipients. However, subsequent doses of rotavirus vaccine were not associated with any excess risk.
In addition, a family history of intussusception was linked to a 4.2-fold increased risk, while an episode of gastroenteritis during the first year of life was associated with a 4.7-fold elevated risk.
In a novel finding, breastfeeding was independently associated with a 44% reduction in the risk of intussusception, compared with that of bottle-fed babies.
The most common presenting signs and symptoms of intussusception were vomiting, abdominal pain, hematochezia, pallor, and reduced appetite, each present in at least half of affected infants.
Dr. Oberle reported having no financial conflicts regarding her study, supported by the Paul Ehrlich Institute.
LJUBLJANA, SLOVENIA – in a German case-control study.
Two other potent risk factors for intussusception in children less than 1 year old were identified: a family history of intussusception, and an episode of gastroenteritis, Doris F. Oberle, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Oberle, of the Paul Ehrlich Institute in Langen, Germany, presented a retrospective study of 116 meticulously validated cases of intussusception in infancy treated at 19 German pediatric centers during 2010-2014 and 272 controls matched by birth month, sex, and location. A standardized interview was conducted with the parents of all study participants.
Rotavirus vaccine was added to the German national vaccination schedule in 2013. In a multivariate logistic regression analysis, the risk of intussusception was increased by 5.4-fold following the first dose of the vaccine, compared with nonrecipients. However, subsequent doses of rotavirus vaccine were not associated with any excess risk.
In addition, a family history of intussusception was linked to a 4.2-fold increased risk, while an episode of gastroenteritis during the first year of life was associated with a 4.7-fold elevated risk.
In a novel finding, breastfeeding was independently associated with a 44% reduction in the risk of intussusception, compared with that of bottle-fed babies.
The most common presenting signs and symptoms of intussusception were vomiting, abdominal pain, hematochezia, pallor, and reduced appetite, each present in at least half of affected infants.
Dr. Oberle reported having no financial conflicts regarding her study, supported by the Paul Ehrlich Institute.
REPORTING FROM ESPID 2019
Expanded indication being considered for meningococcal group B vaccine
LJUBLJANA, SLOVENIA – under the agency’s Breakthrough Therapy designation.
Breakthrough Therapy status is reserved for accelerated review of therapies considered to show substantial preliminary promise of effectively targeting a major unmet medical need.
The unmet need here is that there is no meningococcal group B vaccine approved for use in children under age 10 years. Yet infants and children under 5 years of age are at greatest risk of invasive meningococcal B disease, with reported case fatality rates of 8%-9%, Jason D. Maguire, MD, noted at the annual meeting of the European Society for Paediatric Infectious Diseases.
Trumenba has been approved in the United States for patients aged 10-25 years and in the European Union for individuals aged 10 years or older.
Dr. Maguire, of Pfizer’s vaccine clinical research and development program, presented the results of the two phase 2 randomized safety and immunogenicity trials conducted in patients aged 1- 9 years that the company has submitted to the FDA in support of the expanded indication. One study was carried out in 352 1-year-old toddlers, the other in 400 children aged 2-9 years, whose mean age was 4 years. The studies were carried out in Australia, Finland, Poland, and the Czech Republic.
In a pooled analysis of the vaccine’s immunogenicity when administered in a three-dose schedule of 120 mcg at 0, 2, and 6 months to 193 toddlers and 274 of the children aged 2-9 years, robust bactericidal antibody responses were seen against the four major Neisseria meningitidis group B strains that cause invasive disease. In fact, at least a fourfold rise in titers from baseline to 1 month after dose three was documented in the same high proportion of 1- to 9-year-olds as previously seen in the phase 3 trials that led to vaccine licensure in adolescents and young adults.
“These results support that the use of Trumenba, when given to children ages 1 to less than 10 years at the same dose and schedule that is currently approved in adolescents and young adults, can afford a high degree of protective antibody responses that correlate with immunity in this population,” Dr. Maguire said.
The safety and tolerability analysis included all 752 children in the two phase 2 studies, including the 110 toddlers randomized to three 60-mcg doses of the vaccine, although it has subsequently become clear that 120 mcg is the dose that provides the best immunogenicity with an acceptable safety profile, according to the physician.
Across the age groups, local reactions, including redness and swelling, were more common in Trumenba recipients than in controls who received hepatitis A vaccine and saline injections. So were systemic adverse events. Fever – a systemic event of particular interest to parents and clinicians – occurred in 37% of toddlers after vaccination, compared with 25% of 2- to 9-year-olds and 10%-12% of controls. Of note, prophylactic antipyretics weren’t allowed in the study.
“There’s somewhat of an inverse relationship between age and temperature. So as we go down in age, the rate of fever rises. But after each subsequent dose, regardless of age, there’s a reduction in the incidence of fever,” Dr. Maguire observed.
Most fevers were less than 39.0° C. Only 3 of 752 (less than 1%) patients experienced fever in excess of 40.0° C.
Two children withdrew from the study after developing hip synovitis, which was transient. Another withdrew because of prolonged irritability, fatigue, and decreased appetite.
“Although Trumenba had an acceptable safety and tolerability profile in 1- to 9-year-olds, this analysis wasn’t powered enough to detect uncommon adverse events, so we’ll continue to monitor safety for things like synovitis,” he said.
In 10- to 25-year-olds, the meningococcal vaccine can be given concomitantly with other vaccines without interference. There are plans to study concurrent vaccination with MMR and pneumococcal vaccines in 1- to 9-year-olds as well, according to Dr. Maguire.
Pfizer also now is planning clinical trials of the vaccine in infants, another important group currently unprotected against meningococcal group B disease, he added.
Dr. Maguire is an employee of Pfizer, who funded the studies.
LJUBLJANA, SLOVENIA – under the agency’s Breakthrough Therapy designation.
Breakthrough Therapy status is reserved for accelerated review of therapies considered to show substantial preliminary promise of effectively targeting a major unmet medical need.
The unmet need here is that there is no meningococcal group B vaccine approved for use in children under age 10 years. Yet infants and children under 5 years of age are at greatest risk of invasive meningococcal B disease, with reported case fatality rates of 8%-9%, Jason D. Maguire, MD, noted at the annual meeting of the European Society for Paediatric Infectious Diseases.
Trumenba has been approved in the United States for patients aged 10-25 years and in the European Union for individuals aged 10 years or older.
Dr. Maguire, of Pfizer’s vaccine clinical research and development program, presented the results of the two phase 2 randomized safety and immunogenicity trials conducted in patients aged 1- 9 years that the company has submitted to the FDA in support of the expanded indication. One study was carried out in 352 1-year-old toddlers, the other in 400 children aged 2-9 years, whose mean age was 4 years. The studies were carried out in Australia, Finland, Poland, and the Czech Republic.
In a pooled analysis of the vaccine’s immunogenicity when administered in a three-dose schedule of 120 mcg at 0, 2, and 6 months to 193 toddlers and 274 of the children aged 2-9 years, robust bactericidal antibody responses were seen against the four major Neisseria meningitidis group B strains that cause invasive disease. In fact, at least a fourfold rise in titers from baseline to 1 month after dose three was documented in the same high proportion of 1- to 9-year-olds as previously seen in the phase 3 trials that led to vaccine licensure in adolescents and young adults.
“These results support that the use of Trumenba, when given to children ages 1 to less than 10 years at the same dose and schedule that is currently approved in adolescents and young adults, can afford a high degree of protective antibody responses that correlate with immunity in this population,” Dr. Maguire said.
The safety and tolerability analysis included all 752 children in the two phase 2 studies, including the 110 toddlers randomized to three 60-mcg doses of the vaccine, although it has subsequently become clear that 120 mcg is the dose that provides the best immunogenicity with an acceptable safety profile, according to the physician.
Across the age groups, local reactions, including redness and swelling, were more common in Trumenba recipients than in controls who received hepatitis A vaccine and saline injections. So were systemic adverse events. Fever – a systemic event of particular interest to parents and clinicians – occurred in 37% of toddlers after vaccination, compared with 25% of 2- to 9-year-olds and 10%-12% of controls. Of note, prophylactic antipyretics weren’t allowed in the study.
“There’s somewhat of an inverse relationship between age and temperature. So as we go down in age, the rate of fever rises. But after each subsequent dose, regardless of age, there’s a reduction in the incidence of fever,” Dr. Maguire observed.
Most fevers were less than 39.0° C. Only 3 of 752 (less than 1%) patients experienced fever in excess of 40.0° C.
Two children withdrew from the study after developing hip synovitis, which was transient. Another withdrew because of prolonged irritability, fatigue, and decreased appetite.
“Although Trumenba had an acceptable safety and tolerability profile in 1- to 9-year-olds, this analysis wasn’t powered enough to detect uncommon adverse events, so we’ll continue to monitor safety for things like synovitis,” he said.
In 10- to 25-year-olds, the meningococcal vaccine can be given concomitantly with other vaccines without interference. There are plans to study concurrent vaccination with MMR and pneumococcal vaccines in 1- to 9-year-olds as well, according to Dr. Maguire.
Pfizer also now is planning clinical trials of the vaccine in infants, another important group currently unprotected against meningococcal group B disease, he added.
Dr. Maguire is an employee of Pfizer, who funded the studies.
LJUBLJANA, SLOVENIA – under the agency’s Breakthrough Therapy designation.
Breakthrough Therapy status is reserved for accelerated review of therapies considered to show substantial preliminary promise of effectively targeting a major unmet medical need.
The unmet need here is that there is no meningococcal group B vaccine approved for use in children under age 10 years. Yet infants and children under 5 years of age are at greatest risk of invasive meningococcal B disease, with reported case fatality rates of 8%-9%, Jason D. Maguire, MD, noted at the annual meeting of the European Society for Paediatric Infectious Diseases.
Trumenba has been approved in the United States for patients aged 10-25 years and in the European Union for individuals aged 10 years or older.
Dr. Maguire, of Pfizer’s vaccine clinical research and development program, presented the results of the two phase 2 randomized safety and immunogenicity trials conducted in patients aged 1- 9 years that the company has submitted to the FDA in support of the expanded indication. One study was carried out in 352 1-year-old toddlers, the other in 400 children aged 2-9 years, whose mean age was 4 years. The studies were carried out in Australia, Finland, Poland, and the Czech Republic.
In a pooled analysis of the vaccine’s immunogenicity when administered in a three-dose schedule of 120 mcg at 0, 2, and 6 months to 193 toddlers and 274 of the children aged 2-9 years, robust bactericidal antibody responses were seen against the four major Neisseria meningitidis group B strains that cause invasive disease. In fact, at least a fourfold rise in titers from baseline to 1 month after dose three was documented in the same high proportion of 1- to 9-year-olds as previously seen in the phase 3 trials that led to vaccine licensure in adolescents and young adults.
“These results support that the use of Trumenba, when given to children ages 1 to less than 10 years at the same dose and schedule that is currently approved in adolescents and young adults, can afford a high degree of protective antibody responses that correlate with immunity in this population,” Dr. Maguire said.
The safety and tolerability analysis included all 752 children in the two phase 2 studies, including the 110 toddlers randomized to three 60-mcg doses of the vaccine, although it has subsequently become clear that 120 mcg is the dose that provides the best immunogenicity with an acceptable safety profile, according to the physician.
Across the age groups, local reactions, including redness and swelling, were more common in Trumenba recipients than in controls who received hepatitis A vaccine and saline injections. So were systemic adverse events. Fever – a systemic event of particular interest to parents and clinicians – occurred in 37% of toddlers after vaccination, compared with 25% of 2- to 9-year-olds and 10%-12% of controls. Of note, prophylactic antipyretics weren’t allowed in the study.
“There’s somewhat of an inverse relationship between age and temperature. So as we go down in age, the rate of fever rises. But after each subsequent dose, regardless of age, there’s a reduction in the incidence of fever,” Dr. Maguire observed.
Most fevers were less than 39.0° C. Only 3 of 752 (less than 1%) patients experienced fever in excess of 40.0° C.
Two children withdrew from the study after developing hip synovitis, which was transient. Another withdrew because of prolonged irritability, fatigue, and decreased appetite.
“Although Trumenba had an acceptable safety and tolerability profile in 1- to 9-year-olds, this analysis wasn’t powered enough to detect uncommon adverse events, so we’ll continue to monitor safety for things like synovitis,” he said.
In 10- to 25-year-olds, the meningococcal vaccine can be given concomitantly with other vaccines without interference. There are plans to study concurrent vaccination with MMR and pneumococcal vaccines in 1- to 9-year-olds as well, according to Dr. Maguire.
Pfizer also now is planning clinical trials of the vaccine in infants, another important group currently unprotected against meningococcal group B disease, he added.
Dr. Maguire is an employee of Pfizer, who funded the studies.
EXPERT ANALYSIS FROM ESPID 2019
Obesity doesn’t hamper flu vaccine response in pregnancy
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
REPORTING FROM ESPID 2019
Key clinical point: High BMI doesn’t impair influenza vaccine responses in pregnant women.
Major finding: Protective antibody levels against all three vaccine antigens were documented 1 month post vaccination in 92% of the obese and 74% of the nonobese mothers.
Study details: This was a prospective observational study of 90 women vaccinated against influenza during pregnancy, 24 of whom were obese.
Disclosures: The study was supported by the University of Adelaide Women’s and Children’s Hospital Research Foundation.
Some Brits snuff out TORCH screen to raise awareness of congenital syphilis
LJUBLJANA, SLOVENIA – Pediatricians in the south of England are so concerned about the recent national increase in the diagnosis of syphilis in adults and its ramifications for neonates that they’ve ditched the traditional TORCH newborn screen because the acronym doesn’t specifically remind clinicians to think about congenital syphilis, Mildred A. Iro, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“ explained Dr. Iro of the University of Southampton (England).
She highlighted salient features of three recent cases of congenital syphilis managed at Southampton Children’s Hospital.
“The key message that we’d like to share is that we just need to be more aware about congenital syphilis. Retest mothers if their risk factor status changes, and test suspected infants and children,” Dr. Iro said.
As a practical matter, however, even though current guidelines recommend retesting mothers whose risk factor status becomes heightened following an initial negative syphilis serology result early in pregnancy, clinicians often are unaware that a mother’s risk status has changed. And retesting all mothers during pregnancy isn’t attractive from a cost-benefit standpoint. This makes scrupulous screening of newborns all the more important. And yet TORCH, which stands for Toxoplasmosis, Other, Rubella, Cytomegalovirus, and Herpes infections, isn’t an acronym that promotes awareness of congenital syphilis, a disease which occupies an obscure position in TORCH under the “O” for “Other” heading. That’s why the term “congenital infection screen” has become the new norm in the south of England, she explained.
However, one pediatrician who didn’t consider congenital infection screen to be an improvement in terminology over TORCH had an alternative suggestion, which struck a favorable chord with his fellow audience members: Simply change the acronym to TORCHS, with the S standing for syphilis.
Dr. Iro noted that two of the three affected children were diagnosed at age 7-8 weeks. The third wasn’t diagnosed until age 15 months, when the mother tested positive for syphilis in a subsequent pregnancy. As is typical of the disease known as “the great masquerader,” while all three of the affected children were unwell early in infancy, they presented with a wide range of symptoms. Among the more prominent features were prolonged irritability, respiratory distress, odd rashes, anemia, hepatomegaly, and tachypnea. One infant had reduced movement and pain in one arm.
All three children underwent extensive testing. None had neurosyphilis. All achieved good outcomes on standard guideline-directed therapy.
As for the mothers, they were aged 19, 21, and 23 years when diagnosed with syphilis. All were Caucasian, and antenatal blood testing was negative in all three. None were retested during pregnancy, even though two of them had a male partner or former partner who was positive for syphilis, and the partner of the third disclosed to her that he had sex with men.
At diagnosis, all three women had a strongly positive Treponema pallidum particle agglutination assay, a high rapid plasma reagin, and a positive syphilis IgM assay.
Dr. Iro reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – Pediatricians in the south of England are so concerned about the recent national increase in the diagnosis of syphilis in adults and its ramifications for neonates that they’ve ditched the traditional TORCH newborn screen because the acronym doesn’t specifically remind clinicians to think about congenital syphilis, Mildred A. Iro, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“ explained Dr. Iro of the University of Southampton (England).
She highlighted salient features of three recent cases of congenital syphilis managed at Southampton Children’s Hospital.
“The key message that we’d like to share is that we just need to be more aware about congenital syphilis. Retest mothers if their risk factor status changes, and test suspected infants and children,” Dr. Iro said.
As a practical matter, however, even though current guidelines recommend retesting mothers whose risk factor status becomes heightened following an initial negative syphilis serology result early in pregnancy, clinicians often are unaware that a mother’s risk status has changed. And retesting all mothers during pregnancy isn’t attractive from a cost-benefit standpoint. This makes scrupulous screening of newborns all the more important. And yet TORCH, which stands for Toxoplasmosis, Other, Rubella, Cytomegalovirus, and Herpes infections, isn’t an acronym that promotes awareness of congenital syphilis, a disease which occupies an obscure position in TORCH under the “O” for “Other” heading. That’s why the term “congenital infection screen” has become the new norm in the south of England, she explained.
However, one pediatrician who didn’t consider congenital infection screen to be an improvement in terminology over TORCH had an alternative suggestion, which struck a favorable chord with his fellow audience members: Simply change the acronym to TORCHS, with the S standing for syphilis.
Dr. Iro noted that two of the three affected children were diagnosed at age 7-8 weeks. The third wasn’t diagnosed until age 15 months, when the mother tested positive for syphilis in a subsequent pregnancy. As is typical of the disease known as “the great masquerader,” while all three of the affected children were unwell early in infancy, they presented with a wide range of symptoms. Among the more prominent features were prolonged irritability, respiratory distress, odd rashes, anemia, hepatomegaly, and tachypnea. One infant had reduced movement and pain in one arm.
All three children underwent extensive testing. None had neurosyphilis. All achieved good outcomes on standard guideline-directed therapy.
As for the mothers, they were aged 19, 21, and 23 years when diagnosed with syphilis. All were Caucasian, and antenatal blood testing was negative in all three. None were retested during pregnancy, even though two of them had a male partner or former partner who was positive for syphilis, and the partner of the third disclosed to her that he had sex with men.
At diagnosis, all three women had a strongly positive Treponema pallidum particle agglutination assay, a high rapid plasma reagin, and a positive syphilis IgM assay.
Dr. Iro reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – Pediatricians in the south of England are so concerned about the recent national increase in the diagnosis of syphilis in adults and its ramifications for neonates that they’ve ditched the traditional TORCH newborn screen because the acronym doesn’t specifically remind clinicians to think about congenital syphilis, Mildred A. Iro, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“ explained Dr. Iro of the University of Southampton (England).
She highlighted salient features of three recent cases of congenital syphilis managed at Southampton Children’s Hospital.
“The key message that we’d like to share is that we just need to be more aware about congenital syphilis. Retest mothers if their risk factor status changes, and test suspected infants and children,” Dr. Iro said.
As a practical matter, however, even though current guidelines recommend retesting mothers whose risk factor status becomes heightened following an initial negative syphilis serology result early in pregnancy, clinicians often are unaware that a mother’s risk status has changed. And retesting all mothers during pregnancy isn’t attractive from a cost-benefit standpoint. This makes scrupulous screening of newborns all the more important. And yet TORCH, which stands for Toxoplasmosis, Other, Rubella, Cytomegalovirus, and Herpes infections, isn’t an acronym that promotes awareness of congenital syphilis, a disease which occupies an obscure position in TORCH under the “O” for “Other” heading. That’s why the term “congenital infection screen” has become the new norm in the south of England, she explained.
However, one pediatrician who didn’t consider congenital infection screen to be an improvement in terminology over TORCH had an alternative suggestion, which struck a favorable chord with his fellow audience members: Simply change the acronym to TORCHS, with the S standing for syphilis.
Dr. Iro noted that two of the three affected children were diagnosed at age 7-8 weeks. The third wasn’t diagnosed until age 15 months, when the mother tested positive for syphilis in a subsequent pregnancy. As is typical of the disease known as “the great masquerader,” while all three of the affected children were unwell early in infancy, they presented with a wide range of symptoms. Among the more prominent features were prolonged irritability, respiratory distress, odd rashes, anemia, hepatomegaly, and tachypnea. One infant had reduced movement and pain in one arm.
All three children underwent extensive testing. None had neurosyphilis. All achieved good outcomes on standard guideline-directed therapy.
As for the mothers, they were aged 19, 21, and 23 years when diagnosed with syphilis. All were Caucasian, and antenatal blood testing was negative in all three. None were retested during pregnancy, even though two of them had a male partner or former partner who was positive for syphilis, and the partner of the third disclosed to her that he had sex with men.
At diagnosis, all three women had a strongly positive Treponema pallidum particle agglutination assay, a high rapid plasma reagin, and a positive syphilis IgM assay.
Dr. Iro reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM ESPID 2019
C-section linked to serious infection in preschoolers
LJUBLJANA, SLOVENIA – Delivery by C-section – especially when elective – carries a significantly higher hospitalization risk for severe infection in the first 5 years of life than vaginal delivery in a study of nearly 7.3 million singleton deliveries in four asset-rich countries, David Burgner, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“This is something that obstetricians might need to consider when discussing with the family the pros and cons for an elective C-section, particularly one that isn’t otherwise indicated for the baby or the mother,” said Dr. Burgner of the Murdoch Children’s Research Institute in Melbourne.
He presented an observational study of 7.29 million singleton births in Denmark, Great Britain, Scotland, and two Australian states during 1996-2015. C-section rates ranged from a low of 17.5% in Denmark to 29.4% in Western Australia, all of which are greater than the 10%-15% rate endorsed by the World Health Organization. Elective C-section rates varied by country from 39% to 57%. Of note, pediatric hospital care in all four countries is free, so economic considerations didn’t drive admission.
The impetus for this international collaboration was to gain new insight into the differential susceptibility to childhood infection, he explained.
“We know from our clinical practice that pretty much all of the children are exposed to pretty much all potentially serious pathogens during early life. And yet it’s only a minority that develop severe infection. It’s an extremely interesting scientific question and an extremely important clinical question as to what’s driving that differential susceptibility,” according to the pediatric infectious disease specialist.
There are a number of established risk factors for infection-related hospitalization in children, including parental smoking, maternal antibiotic exposure during pregnancy, and growth measurements at birth. Dr. Burgner and coinvestigators hypothesized that another important risk factor is the nature of the microbiome transmitted from mother to baby during delivery. This postnatal microbiome varies depending upon mode of delivery: Vaginal delivery transmits the maternal enteric microbiome, which they reasoned might be through direct immunomodulation that sets up protective immune responses early in life, especially against respiratory and gastrointestinal tract infections. In contrast, delivery by C-section causes the baby to pick up the maternal skin and hospital environment microbiomes, but not the maternal enteric microbiome.
Thus, the investigators hypothesized that C-section poses a greater risk of infection-related hospitalization during the first 5 years of life than does vaginal delivery, and that elective C-section poses a higher risk than does emergency C-section because it is more likely to involve rupture of membranes.
The center-specific rates of C-section and infection-related pediatric infection, when combined into a meta-analysis, bore out the study hypothesis. Emergency C-section was associated with a 9% greater risk of infection-related hospitalization through 5 years of age than was vaginal delivery, while elective C-section was associated with a 13% increased risk, both of which were statistically significant and clinically important.
“We were quite taken with these results. We think they provide evidence that C-section is consistently associated with infection-related hospitalization. It’s an association study that can’t prove causality, but the results implicate the postnatal microbiome as the most plausible explanation in terms of what’s driving this association,” according to Dr. Burgner.
The association between C-section and infection-related hospitalization was persistent throughout the preschool years. For example, the increased risk associated with elective C-section was 16% during age 0-3 months, 20% during months 4-6, 14% in months 7-12, 13% during ages 1-2 years, and 11% among 2- to 5-year-olds, he continued.
The increased risk of severe preschool infection was highest for upper and lower respiratory tract and gastrointestinal infections, which involve the organ systems most likely to experience direct inoculation of the maternal microbiome, he noted.
Because the investigators recognized that the study results were potentially vulnerable to confounding by indication – that is, that the reason for doing a C-section might itself confer increased risk of subsequent preschool infection-related hospitalization – they repeated their analysis in a predefined low-risk subpopulation. The results closely mirrored those in the overall study population: an 8% increased risk in the emergency C-section group and a 14% increased risk with elective C-section.
Results of this large multinational study should provide further support for ongoing research aimed at supporting the infant microbiome after delivery by C-section via vaginal microbial transfer and other methods, he observed.
Dr. Burgner reported having no financial conflicts regarding the study, which was cosponsored by the National Health and Medical Research Council of Australia, the Danish Council for Independent Research, and nonprofit foundations.
LJUBLJANA, SLOVENIA – Delivery by C-section – especially when elective – carries a significantly higher hospitalization risk for severe infection in the first 5 years of life than vaginal delivery in a study of nearly 7.3 million singleton deliveries in four asset-rich countries, David Burgner, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“This is something that obstetricians might need to consider when discussing with the family the pros and cons for an elective C-section, particularly one that isn’t otherwise indicated for the baby or the mother,” said Dr. Burgner of the Murdoch Children’s Research Institute in Melbourne.
He presented an observational study of 7.29 million singleton births in Denmark, Great Britain, Scotland, and two Australian states during 1996-2015. C-section rates ranged from a low of 17.5% in Denmark to 29.4% in Western Australia, all of which are greater than the 10%-15% rate endorsed by the World Health Organization. Elective C-section rates varied by country from 39% to 57%. Of note, pediatric hospital care in all four countries is free, so economic considerations didn’t drive admission.
The impetus for this international collaboration was to gain new insight into the differential susceptibility to childhood infection, he explained.
“We know from our clinical practice that pretty much all of the children are exposed to pretty much all potentially serious pathogens during early life. And yet it’s only a minority that develop severe infection. It’s an extremely interesting scientific question and an extremely important clinical question as to what’s driving that differential susceptibility,” according to the pediatric infectious disease specialist.
There are a number of established risk factors for infection-related hospitalization in children, including parental smoking, maternal antibiotic exposure during pregnancy, and growth measurements at birth. Dr. Burgner and coinvestigators hypothesized that another important risk factor is the nature of the microbiome transmitted from mother to baby during delivery. This postnatal microbiome varies depending upon mode of delivery: Vaginal delivery transmits the maternal enteric microbiome, which they reasoned might be through direct immunomodulation that sets up protective immune responses early in life, especially against respiratory and gastrointestinal tract infections. In contrast, delivery by C-section causes the baby to pick up the maternal skin and hospital environment microbiomes, but not the maternal enteric microbiome.
Thus, the investigators hypothesized that C-section poses a greater risk of infection-related hospitalization during the first 5 years of life than does vaginal delivery, and that elective C-section poses a higher risk than does emergency C-section because it is more likely to involve rupture of membranes.
The center-specific rates of C-section and infection-related pediatric infection, when combined into a meta-analysis, bore out the study hypothesis. Emergency C-section was associated with a 9% greater risk of infection-related hospitalization through 5 years of age than was vaginal delivery, while elective C-section was associated with a 13% increased risk, both of which were statistically significant and clinically important.
“We were quite taken with these results. We think they provide evidence that C-section is consistently associated with infection-related hospitalization. It’s an association study that can’t prove causality, but the results implicate the postnatal microbiome as the most plausible explanation in terms of what’s driving this association,” according to Dr. Burgner.
The association between C-section and infection-related hospitalization was persistent throughout the preschool years. For example, the increased risk associated with elective C-section was 16% during age 0-3 months, 20% during months 4-6, 14% in months 7-12, 13% during ages 1-2 years, and 11% among 2- to 5-year-olds, he continued.
The increased risk of severe preschool infection was highest for upper and lower respiratory tract and gastrointestinal infections, which involve the organ systems most likely to experience direct inoculation of the maternal microbiome, he noted.
Because the investigators recognized that the study results were potentially vulnerable to confounding by indication – that is, that the reason for doing a C-section might itself confer increased risk of subsequent preschool infection-related hospitalization – they repeated their analysis in a predefined low-risk subpopulation. The results closely mirrored those in the overall study population: an 8% increased risk in the emergency C-section group and a 14% increased risk with elective C-section.
Results of this large multinational study should provide further support for ongoing research aimed at supporting the infant microbiome after delivery by C-section via vaginal microbial transfer and other methods, he observed.
Dr. Burgner reported having no financial conflicts regarding the study, which was cosponsored by the National Health and Medical Research Council of Australia, the Danish Council for Independent Research, and nonprofit foundations.
LJUBLJANA, SLOVENIA – Delivery by C-section – especially when elective – carries a significantly higher hospitalization risk for severe infection in the first 5 years of life than vaginal delivery in a study of nearly 7.3 million singleton deliveries in four asset-rich countries, David Burgner, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“This is something that obstetricians might need to consider when discussing with the family the pros and cons for an elective C-section, particularly one that isn’t otherwise indicated for the baby or the mother,” said Dr. Burgner of the Murdoch Children’s Research Institute in Melbourne.
He presented an observational study of 7.29 million singleton births in Denmark, Great Britain, Scotland, and two Australian states during 1996-2015. C-section rates ranged from a low of 17.5% in Denmark to 29.4% in Western Australia, all of which are greater than the 10%-15% rate endorsed by the World Health Organization. Elective C-section rates varied by country from 39% to 57%. Of note, pediatric hospital care in all four countries is free, so economic considerations didn’t drive admission.
The impetus for this international collaboration was to gain new insight into the differential susceptibility to childhood infection, he explained.
“We know from our clinical practice that pretty much all of the children are exposed to pretty much all potentially serious pathogens during early life. And yet it’s only a minority that develop severe infection. It’s an extremely interesting scientific question and an extremely important clinical question as to what’s driving that differential susceptibility,” according to the pediatric infectious disease specialist.
There are a number of established risk factors for infection-related hospitalization in children, including parental smoking, maternal antibiotic exposure during pregnancy, and growth measurements at birth. Dr. Burgner and coinvestigators hypothesized that another important risk factor is the nature of the microbiome transmitted from mother to baby during delivery. This postnatal microbiome varies depending upon mode of delivery: Vaginal delivery transmits the maternal enteric microbiome, which they reasoned might be through direct immunomodulation that sets up protective immune responses early in life, especially against respiratory and gastrointestinal tract infections. In contrast, delivery by C-section causes the baby to pick up the maternal skin and hospital environment microbiomes, but not the maternal enteric microbiome.
Thus, the investigators hypothesized that C-section poses a greater risk of infection-related hospitalization during the first 5 years of life than does vaginal delivery, and that elective C-section poses a higher risk than does emergency C-section because it is more likely to involve rupture of membranes.
The center-specific rates of C-section and infection-related pediatric infection, when combined into a meta-analysis, bore out the study hypothesis. Emergency C-section was associated with a 9% greater risk of infection-related hospitalization through 5 years of age than was vaginal delivery, while elective C-section was associated with a 13% increased risk, both of which were statistically significant and clinically important.
“We were quite taken with these results. We think they provide evidence that C-section is consistently associated with infection-related hospitalization. It’s an association study that can’t prove causality, but the results implicate the postnatal microbiome as the most plausible explanation in terms of what’s driving this association,” according to Dr. Burgner.
The association between C-section and infection-related hospitalization was persistent throughout the preschool years. For example, the increased risk associated with elective C-section was 16% during age 0-3 months, 20% during months 4-6, 14% in months 7-12, 13% during ages 1-2 years, and 11% among 2- to 5-year-olds, he continued.
The increased risk of severe preschool infection was highest for upper and lower respiratory tract and gastrointestinal infections, which involve the organ systems most likely to experience direct inoculation of the maternal microbiome, he noted.
Because the investigators recognized that the study results were potentially vulnerable to confounding by indication – that is, that the reason for doing a C-section might itself confer increased risk of subsequent preschool infection-related hospitalization – they repeated their analysis in a predefined low-risk subpopulation. The results closely mirrored those in the overall study population: an 8% increased risk in the emergency C-section group and a 14% increased risk with elective C-section.
Results of this large multinational study should provide further support for ongoing research aimed at supporting the infant microbiome after delivery by C-section via vaginal microbial transfer and other methods, he observed.
Dr. Burgner reported having no financial conflicts regarding the study, which was cosponsored by the National Health and Medical Research Council of Australia, the Danish Council for Independent Research, and nonprofit foundations.
REPORTING FROM ESPID 2019
HPV vaccine: Is one dose enough?
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM ESPID 2019