User login
Heart transplantation: Preop LVAD erases adverse impact of pulmonary hypertension
COLORADO SPRINGS – Reconsideration of the role of pulmonary hypertension in heart transplant outcomes is appropriate in the emerging era of the use of left ventricular assist devices (LVADs) as bridge to transplant, according to Ann C. Gaffey, MD, of the University of Pennsylvania, Philadelphia.
“Pulmonary hypertension secondary to congestive heart failure more than likely can be reversed to the values acceptable for heart transplant by the use of an LVAD. For bridge-to-transplant patients, pretransplant pulmonary hypertension does not affect recipient outcomes post transplantation,” she said at the annual meeting of the Western Thoracic Surgical Association.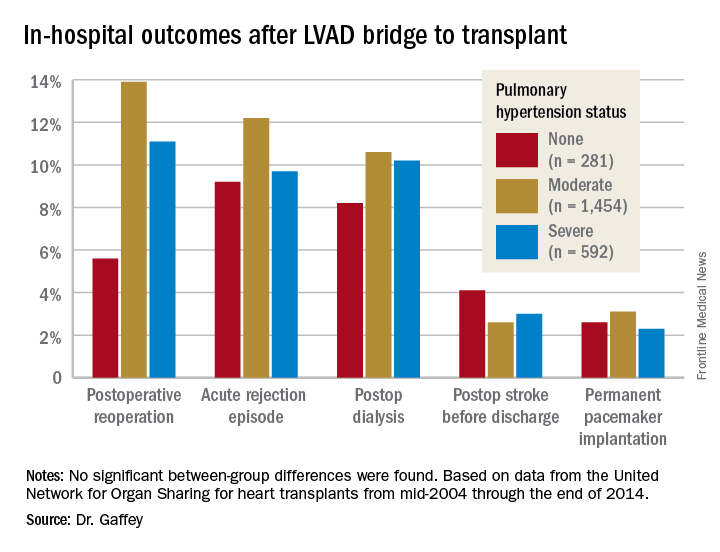
Vasodilators are prescribed in an effort to reduce PH; however, 40% of patients with PH are unresponsive to the medications and have therefore been excluded from consideration as potential candidates for a donor heart.
But the growing use of LVADs as a bridge to transplant has changed all that, Dr. Gaffey said. As supporting evidence, she presented a retrospective analysis of the United Network for Organ Sharing database on adult heart transplants from mid-2004 through the end of 2014.
The review turned up 3,951 heart transplant recipients who had been bridged to transplant with an LVAD. Dr. Gaffey and her coinvestigators divided them into three groups: 281 patients without pretransplant PH; 1,454 with moderate PH as defined by 1-3 Wood units; and 592 with severe PH and more than 3 Wood units.
The three groups didn’t differ in terms of age, sex, wait-list time, or the prevalence of diabetes or renal, liver, or cerebrovascular disease. Nor did their donors differ in age, sex, left ventricular function, or allograft ischemic time.
Key in-hospital outcomes were similar between the groups with no, mild, and severe PH.
Moreover, there was no between-group difference in the rate of rejection at 1 year. Five-year survival rates were closely similar in the three groups, in the mid-70s.
Audience member Nahush A. Mokadam, MD, rose to praise Dr. Gaffey’s report.
“This is a great and important study. I think as a group we have been too conservative with pulmonary hypertension, so thank you for shining a good light on it,” said Dr. Mokadam of the University of Washington, Seattle.
Dr. Gaffey reported having no financial conflicts regarding the study, which was conducted free of commercial support.
COLORADO SPRINGS – Reconsideration of the role of pulmonary hypertension in heart transplant outcomes is appropriate in the emerging era of the use of left ventricular assist devices (LVADs) as bridge to transplant, according to Ann C. Gaffey, MD, of the University of Pennsylvania, Philadelphia.
“Pulmonary hypertension secondary to congestive heart failure more than likely can be reversed to the values acceptable for heart transplant by the use of an LVAD. For bridge-to-transplant patients, pretransplant pulmonary hypertension does not affect recipient outcomes post transplantation,” she said at the annual meeting of the Western Thoracic Surgical Association.
Vasodilators are prescribed in an effort to reduce PH; however, 40% of patients with PH are unresponsive to the medications and have therefore been excluded from consideration as potential candidates for a donor heart.
But the growing use of LVADs as a bridge to transplant has changed all that, Dr. Gaffey said. As supporting evidence, she presented a retrospective analysis of the United Network for Organ Sharing database on adult heart transplants from mid-2004 through the end of 2014.
The review turned up 3,951 heart transplant recipients who had been bridged to transplant with an LVAD. Dr. Gaffey and her coinvestigators divided them into three groups: 281 patients without pretransplant PH; 1,454 with moderate PH as defined by 1-3 Wood units; and 592 with severe PH and more than 3 Wood units.
The three groups didn’t differ in terms of age, sex, wait-list time, or the prevalence of diabetes or renal, liver, or cerebrovascular disease. Nor did their donors differ in age, sex, left ventricular function, or allograft ischemic time.
Key in-hospital outcomes were similar between the groups with no, mild, and severe PH.
Moreover, there was no between-group difference in the rate of rejection at 1 year. Five-year survival rates were closely similar in the three groups, in the mid-70s.
Audience member Nahush A. Mokadam, MD, rose to praise Dr. Gaffey’s report.
“This is a great and important study. I think as a group we have been too conservative with pulmonary hypertension, so thank you for shining a good light on it,” said Dr. Mokadam of the University of Washington, Seattle.
Dr. Gaffey reported having no financial conflicts regarding the study, which was conducted free of commercial support.
COLORADO SPRINGS – Reconsideration of the role of pulmonary hypertension in heart transplant outcomes is appropriate in the emerging era of the use of left ventricular assist devices (LVADs) as bridge to transplant, according to Ann C. Gaffey, MD, of the University of Pennsylvania, Philadelphia.
“Pulmonary hypertension secondary to congestive heart failure more than likely can be reversed to the values acceptable for heart transplant by the use of an LVAD. For bridge-to-transplant patients, pretransplant pulmonary hypertension does not affect recipient outcomes post transplantation,” she said at the annual meeting of the Western Thoracic Surgical Association.
Vasodilators are prescribed in an effort to reduce PH; however, 40% of patients with PH are unresponsive to the medications and have therefore been excluded from consideration as potential candidates for a donor heart.
But the growing use of LVADs as a bridge to transplant has changed all that, Dr. Gaffey said. As supporting evidence, she presented a retrospective analysis of the United Network for Organ Sharing database on adult heart transplants from mid-2004 through the end of 2014.
The review turned up 3,951 heart transplant recipients who had been bridged to transplant with an LVAD. Dr. Gaffey and her coinvestigators divided them into three groups: 281 patients without pretransplant PH; 1,454 with moderate PH as defined by 1-3 Wood units; and 592 with severe PH and more than 3 Wood units.
The three groups didn’t differ in terms of age, sex, wait-list time, or the prevalence of diabetes or renal, liver, or cerebrovascular disease. Nor did their donors differ in age, sex, left ventricular function, or allograft ischemic time.
Key in-hospital outcomes were similar between the groups with no, mild, and severe PH.
Moreover, there was no between-group difference in the rate of rejection at 1 year. Five-year survival rates were closely similar in the three groups, in the mid-70s.
Audience member Nahush A. Mokadam, MD, rose to praise Dr. Gaffey’s report.
“This is a great and important study. I think as a group we have been too conservative with pulmonary hypertension, so thank you for shining a good light on it,” said Dr. Mokadam of the University of Washington, Seattle.
Dr. Gaffey reported having no financial conflicts regarding the study, which was conducted free of commercial support.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: It’s time to reconsider the practice of excluding patients with pulmonary hypertension from consideration for a donor heart.
Data source: A retrospective analysis of the United Network for Organ Sharing database including outcomes out to 5 years on 3,951 heart transplant recipients who had been bridged to transplant with an LVAD, most of whom had moderate or severe pulmonary hypertension before transplant.
Disclosures: This study was conducted free of commercial support. The presenter reported having no relevant financial conflicts of interest.
BIMA’s benefits extend to high-risk CABG patients
COLORADO SPRINGS – The survival advantage of bilateral internal over left internal mammary artery grafts persists even among multivessel CABG patients perceived to be at high surgical risk, Nishant Saran, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
Many surgeons hesitate to perform bilateral internal mammary artery (BIMA) grafting in high-risk patients on the presumption that BIMA might not benefit them. It’s a concern that appears to be without merit, however, based on a retrospective analysis of the 6,468 multivessel CABG procedures performed at the Mayo Clinic during 2000-2015, said Dr. Saran of the Mayo Clinic in Rochester, Minn.
The BIMA patients were as a whole significantly younger, primarily men, and less likely to have diabetes or to be obese than the LIMA patients. Also, LIMA patients were fourfold more likely to have baseline heart failure, twice as likely to have a history of stroke, and had a twofold greater prevalence of chronic lung disease.
“The unmatched comparison shows the clear treatment selection bias we have: BIMA goes to the healthier patients,” Dr. Saran observed.
But is that bias justified? To find out, he and his coinvestigators performed extensive propensity score matching using several dozen baseline variables in order to identify 1,011 closely matched patient pairs. In this propensity score-matched analysis, 5- and 10-year survival rates were significantly better in the BIMA group. The gap between the two survival curves widened after about 7 years and continued to expand steadily through year 10. Incision time averaged 298 minutes in the BIMA group and 254 minutes in the propensity-matched LIMA group.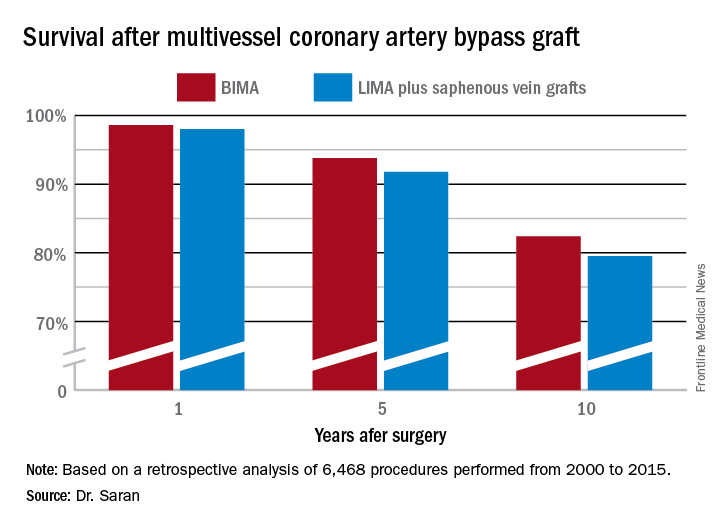
Discussant Eric J. Lehr, MD, a cardiac surgeon at Swedish Medical Center in Seattle, noted that the impressive survival benefit for BIMA in the retrospective Mayo Clinic study came at what he termed “a modest cost”: a doubled incidence of sternal site infections, from 1.4% in the LIMA group to 3% with BIMA. Importantly, though, there was no significant difference in the more serious deep sternal wound infections.
He agreed with Dr. Saran that BIMA is seriously underutilized, noting that only one cardiothoracic surgery program in the state of Washington uses BIMA more than 10% of the time in multivessel CABG.
Dr. Lehr then posed a provocative question: “Should BIMA grafting be considered a quality metric in coronary revascularization surgery, despite the small increase in sternal site infections, even though sternal wound infections have been declared a ‘never’ event and are tied to reimbursement?”
“I think BIMA should be a gold standard,” Dr. Saran replied. “The first thing that a cardiac surgeon should always think of when a patient is going to have CABG is ‘BIMA first,’ and only then look into reasons for not doing it. But I guess in current real-world practice, things are different.”
Howard K. Song, MD, commented, “I think a study like this doesn’t necessarily show that every surgeon should be using BIMA liberally, it shows that surgeons in your practice who do that have excellent outcomes.”
Dr. Song, professor of surgery and chief of the division of cardiothoracic surgery at Oregon Health and Science University, Portland, added that he believes extensive use of BIMA is actually a surrogate marker for a highly skilled subspecialist who would be expected to have very good outcomes as a matter of course.
“That may be one way of looking at it; however, I do think that even very skilled surgeons still have an inherent resistance to doing BIMA,” Dr. Saran responded.
“In the current era, the surgeon is pressured to achieve improved short-term outcomes and improved OR turnover times. An extra half hour for BIMA tends to push the surgeon away,” he added.
Dr. Saran reported having no financial conflicts of interest.
COLORADO SPRINGS – The survival advantage of bilateral internal over left internal mammary artery grafts persists even among multivessel CABG patients perceived to be at high surgical risk, Nishant Saran, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
Many surgeons hesitate to perform bilateral internal mammary artery (BIMA) grafting in high-risk patients on the presumption that BIMA might not benefit them. It’s a concern that appears to be without merit, however, based on a retrospective analysis of the 6,468 multivessel CABG procedures performed at the Mayo Clinic during 2000-2015, said Dr. Saran of the Mayo Clinic in Rochester, Minn.
The BIMA patients were as a whole significantly younger, primarily men, and less likely to have diabetes or to be obese than the LIMA patients. Also, LIMA patients were fourfold more likely to have baseline heart failure, twice as likely to have a history of stroke, and had a twofold greater prevalence of chronic lung disease.
“The unmatched comparison shows the clear treatment selection bias we have: BIMA goes to the healthier patients,” Dr. Saran observed.
But is that bias justified? To find out, he and his coinvestigators performed extensive propensity score matching using several dozen baseline variables in order to identify 1,011 closely matched patient pairs. In this propensity score-matched analysis, 5- and 10-year survival rates were significantly better in the BIMA group. The gap between the two survival curves widened after about 7 years and continued to expand steadily through year 10. Incision time averaged 298 minutes in the BIMA group and 254 minutes in the propensity-matched LIMA group.
Discussant Eric J. Lehr, MD, a cardiac surgeon at Swedish Medical Center in Seattle, noted that the impressive survival benefit for BIMA in the retrospective Mayo Clinic study came at what he termed “a modest cost”: a doubled incidence of sternal site infections, from 1.4% in the LIMA group to 3% with BIMA. Importantly, though, there was no significant difference in the more serious deep sternal wound infections.
He agreed with Dr. Saran that BIMA is seriously underutilized, noting that only one cardiothoracic surgery program in the state of Washington uses BIMA more than 10% of the time in multivessel CABG.
Dr. Lehr then posed a provocative question: “Should BIMA grafting be considered a quality metric in coronary revascularization surgery, despite the small increase in sternal site infections, even though sternal wound infections have been declared a ‘never’ event and are tied to reimbursement?”
“I think BIMA should be a gold standard,” Dr. Saran replied. “The first thing that a cardiac surgeon should always think of when a patient is going to have CABG is ‘BIMA first,’ and only then look into reasons for not doing it. But I guess in current real-world practice, things are different.”
Howard K. Song, MD, commented, “I think a study like this doesn’t necessarily show that every surgeon should be using BIMA liberally, it shows that surgeons in your practice who do that have excellent outcomes.”
Dr. Song, professor of surgery and chief of the division of cardiothoracic surgery at Oregon Health and Science University, Portland, added that he believes extensive use of BIMA is actually a surrogate marker for a highly skilled subspecialist who would be expected to have very good outcomes as a matter of course.
“That may be one way of looking at it; however, I do think that even very skilled surgeons still have an inherent resistance to doing BIMA,” Dr. Saran responded.
“In the current era, the surgeon is pressured to achieve improved short-term outcomes and improved OR turnover times. An extra half hour for BIMA tends to push the surgeon away,” he added.
Dr. Saran reported having no financial conflicts of interest.
COLORADO SPRINGS – The survival advantage of bilateral internal over left internal mammary artery grafts persists even among multivessel CABG patients perceived to be at high surgical risk, Nishant Saran, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
Many surgeons hesitate to perform bilateral internal mammary artery (BIMA) grafting in high-risk patients on the presumption that BIMA might not benefit them. It’s a concern that appears to be without merit, however, based on a retrospective analysis of the 6,468 multivessel CABG procedures performed at the Mayo Clinic during 2000-2015, said Dr. Saran of the Mayo Clinic in Rochester, Minn.
The BIMA patients were as a whole significantly younger, primarily men, and less likely to have diabetes or to be obese than the LIMA patients. Also, LIMA patients were fourfold more likely to have baseline heart failure, twice as likely to have a history of stroke, and had a twofold greater prevalence of chronic lung disease.
“The unmatched comparison shows the clear treatment selection bias we have: BIMA goes to the healthier patients,” Dr. Saran observed.
But is that bias justified? To find out, he and his coinvestigators performed extensive propensity score matching using several dozen baseline variables in order to identify 1,011 closely matched patient pairs. In this propensity score-matched analysis, 5- and 10-year survival rates were significantly better in the BIMA group. The gap between the two survival curves widened after about 7 years and continued to expand steadily through year 10. Incision time averaged 298 minutes in the BIMA group and 254 minutes in the propensity-matched LIMA group.
Discussant Eric J. Lehr, MD, a cardiac surgeon at Swedish Medical Center in Seattle, noted that the impressive survival benefit for BIMA in the retrospective Mayo Clinic study came at what he termed “a modest cost”: a doubled incidence of sternal site infections, from 1.4% in the LIMA group to 3% with BIMA. Importantly, though, there was no significant difference in the more serious deep sternal wound infections.
He agreed with Dr. Saran that BIMA is seriously underutilized, noting that only one cardiothoracic surgery program in the state of Washington uses BIMA more than 10% of the time in multivessel CABG.
Dr. Lehr then posed a provocative question: “Should BIMA grafting be considered a quality metric in coronary revascularization surgery, despite the small increase in sternal site infections, even though sternal wound infections have been declared a ‘never’ event and are tied to reimbursement?”
“I think BIMA should be a gold standard,” Dr. Saran replied. “The first thing that a cardiac surgeon should always think of when a patient is going to have CABG is ‘BIMA first,’ and only then look into reasons for not doing it. But I guess in current real-world practice, things are different.”
Howard K. Song, MD, commented, “I think a study like this doesn’t necessarily show that every surgeon should be using BIMA liberally, it shows that surgeons in your practice who do that have excellent outcomes.”
Dr. Song, professor of surgery and chief of the division of cardiothoracic surgery at Oregon Health and Science University, Portland, added that he believes extensive use of BIMA is actually a surrogate marker for a highly skilled subspecialist who would be expected to have very good outcomes as a matter of course.
“That may be one way of looking at it; however, I do think that even very skilled surgeons still have an inherent resistance to doing BIMA,” Dr. Saran responded.
“In the current era, the surgeon is pressured to achieve improved short-term outcomes and improved OR turnover times. An extra half hour for BIMA tends to push the surgeon away,” he added.
Dr. Saran reported having no financial conflicts of interest.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: Ten-year survival following multivessel CABG using bilateral internal mammary artery grafting was 82.4%, significantly better than the 79.5% rate with left internal mammary artery grafting plus saphenous vein grafts.
Data source: This retrospective observational single-center included 6,468 patients who underwent multivessel CABG during 2000-2015.
Disclosures: Dr. Saran reported having no financial conflicts of interest.
Minimally invasive esophagectomy may mean less major morbidity
COLORADO SPRINGS – Minimally invasive esophagectomy was associated with a significantly lower rate of postoperative major morbidity as well as a mean 1-day briefer length of stay than open esophagectomy in a propensity-matched analysis of the real-world American College of Surgeons-National Quality Improvement Program database, Mark F. Berry, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
However, both of the study’s discussants questioned whether the reported modest absolute reduction in major morbidity was really attributable to the minimally invasive approach or could instead have resulted from one of several potential confounders that couldn’t be fully adjusted for, given inherent limitations of the ACS-NSQIP database.
“There was a statistically significant difference in morbidity,” replied Dr. Berry of Stanford (Calif.) University. “It was a 4% absolute difference, which I think is probably clinically meaningful, but certainly it’s not really, really dramatic.”
“What I think we found is that it’s safe to do a minimally invasive esophagectomy and safe for people to introduce it into their practice. But it’s not necessarily something that’s a game changer, unlike what’s been seen with minimally invasive approaches for some other things,” said Dr. Berry, who added that he didn’t wish to overstate the importance of the observed difference in morbidity.
Studies from high-volume centers show that minimally-invasive esophagectomy (MIE) reduces length of stay, postoperative major morbidity, and features equivalent or even slightly lower mortality than traditional open esophagectomy, the generalizability of these findings beyond such centers is questionable. That’s why Dr. Berry and his coinvestigators turned to the ACS-NSQIP database, which includes all esophagectomies performed for esophageal cancer at roughly 700 U.S. hospitals, not just those done by board-certified thoracic surgeons.
He presented a retrospective cohort study of 3,901 esophagectomy patients during 2005-2013 who met study criteria, 16.4% of whom had MIE. The use of this approach increased steadily from 6.5% of all esophagectomies in 2005 to 22.3% in 2013. A propensity-matched analysis designed to neutralize potentially confounding differences included 638 MIE and 1,914 open esophagectomy patients.
The primary outcome was the 30-day rate of composite major morbidity in the realms of various wound, respiratory, renal, and cardiovascular complications. The rate was 36.1% in the MIE group and 40.5% with open esophagectomy in the propensity-matched analysis, an absolute risk reduction of 4.4% and a relative risk reduction of 17%. Although rates were consistently slightly lower in each of the categories of major morbidity, those individual differences didn’t achieve statistical significance. The difference in major morbidity became significant only when major morbidity was considered as a whole.
Mean length of stay was 9 days with MIE and 10 days with open surgery.
There was no significant difference between the two study groups in 30-day rates of readmission, reoperation, or mortality.
Discussant Donald E. Low said “esophagectomy is being analysed regarding its place in all sorts of presentations, stages, and situations, so the aspect of making sure that we’re delivering the services as efficiently as possible is going to become more important, not less important.”
That being said, he noted that there is no specific CPT code for MIE. That raises the possibility of an uncertain amount of procedural misclassification in the ACS-NSQIP database.
Also, the only significant difference in major morbidity between the two study groups was in the subcategory of intra- or postoperative bleeding requiring transfusion, which occurred in 10.8% of the MIE and 16.7% of the open esophagectomy groups, observed Dr. Low, director of the Esophageal Center of Excellence at Virginia Mason Medical Center, Seattle.
“Some of us believe that blood utilization and transfusion requirement is really a quality measure and not a complication,” the surgeon said. And if that outcome is excluded from consideration, then there is no significant difference in major morbidity.
Discussant Douglas E. Wood, MD, professor and chair of the department of surgery at the University of Washington, Seattle, took the opportunity to share a self-described “pet peeve” about analyses of national surgical databases: these databases typically don’t contain key details necessary to correct for provider and hospital characteristics.
“The small differences that you demonstrate could easily have been completely driven by providers who choose to do minimally invasive esophagectomy and are in higher-volume, more specialized centers,” he said. “I’m not convinced of your conclusion that MIE produces less morbidity based on a 4% difference and no analysis of provider characteristics.”
COLORADO SPRINGS – Minimally invasive esophagectomy was associated with a significantly lower rate of postoperative major morbidity as well as a mean 1-day briefer length of stay than open esophagectomy in a propensity-matched analysis of the real-world American College of Surgeons-National Quality Improvement Program database, Mark F. Berry, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
However, both of the study’s discussants questioned whether the reported modest absolute reduction in major morbidity was really attributable to the minimally invasive approach or could instead have resulted from one of several potential confounders that couldn’t be fully adjusted for, given inherent limitations of the ACS-NSQIP database.
“There was a statistically significant difference in morbidity,” replied Dr. Berry of Stanford (Calif.) University. “It was a 4% absolute difference, which I think is probably clinically meaningful, but certainly it’s not really, really dramatic.”
“What I think we found is that it’s safe to do a minimally invasive esophagectomy and safe for people to introduce it into their practice. But it’s not necessarily something that’s a game changer, unlike what’s been seen with minimally invasive approaches for some other things,” said Dr. Berry, who added that he didn’t wish to overstate the importance of the observed difference in morbidity.
Studies from high-volume centers show that minimally-invasive esophagectomy (MIE) reduces length of stay, postoperative major morbidity, and features equivalent or even slightly lower mortality than traditional open esophagectomy, the generalizability of these findings beyond such centers is questionable. That’s why Dr. Berry and his coinvestigators turned to the ACS-NSQIP database, which includes all esophagectomies performed for esophageal cancer at roughly 700 U.S. hospitals, not just those done by board-certified thoracic surgeons.
He presented a retrospective cohort study of 3,901 esophagectomy patients during 2005-2013 who met study criteria, 16.4% of whom had MIE. The use of this approach increased steadily from 6.5% of all esophagectomies in 2005 to 22.3% in 2013. A propensity-matched analysis designed to neutralize potentially confounding differences included 638 MIE and 1,914 open esophagectomy patients.
The primary outcome was the 30-day rate of composite major morbidity in the realms of various wound, respiratory, renal, and cardiovascular complications. The rate was 36.1% in the MIE group and 40.5% with open esophagectomy in the propensity-matched analysis, an absolute risk reduction of 4.4% and a relative risk reduction of 17%. Although rates were consistently slightly lower in each of the categories of major morbidity, those individual differences didn’t achieve statistical significance. The difference in major morbidity became significant only when major morbidity was considered as a whole.
Mean length of stay was 9 days with MIE and 10 days with open surgery.
There was no significant difference between the two study groups in 30-day rates of readmission, reoperation, or mortality.
Discussant Donald E. Low said “esophagectomy is being analysed regarding its place in all sorts of presentations, stages, and situations, so the aspect of making sure that we’re delivering the services as efficiently as possible is going to become more important, not less important.”
That being said, he noted that there is no specific CPT code for MIE. That raises the possibility of an uncertain amount of procedural misclassification in the ACS-NSQIP database.
Also, the only significant difference in major morbidity between the two study groups was in the subcategory of intra- or postoperative bleeding requiring transfusion, which occurred in 10.8% of the MIE and 16.7% of the open esophagectomy groups, observed Dr. Low, director of the Esophageal Center of Excellence at Virginia Mason Medical Center, Seattle.
“Some of us believe that blood utilization and transfusion requirement is really a quality measure and not a complication,” the surgeon said. And if that outcome is excluded from consideration, then there is no significant difference in major morbidity.
Discussant Douglas E. Wood, MD, professor and chair of the department of surgery at the University of Washington, Seattle, took the opportunity to share a self-described “pet peeve” about analyses of national surgical databases: these databases typically don’t contain key details necessary to correct for provider and hospital characteristics.
“The small differences that you demonstrate could easily have been completely driven by providers who choose to do minimally invasive esophagectomy and are in higher-volume, more specialized centers,” he said. “I’m not convinced of your conclusion that MIE produces less morbidity based on a 4% difference and no analysis of provider characteristics.”
COLORADO SPRINGS – Minimally invasive esophagectomy was associated with a significantly lower rate of postoperative major morbidity as well as a mean 1-day briefer length of stay than open esophagectomy in a propensity-matched analysis of the real-world American College of Surgeons-National Quality Improvement Program database, Mark F. Berry, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
However, both of the study’s discussants questioned whether the reported modest absolute reduction in major morbidity was really attributable to the minimally invasive approach or could instead have resulted from one of several potential confounders that couldn’t be fully adjusted for, given inherent limitations of the ACS-NSQIP database.
“There was a statistically significant difference in morbidity,” replied Dr. Berry of Stanford (Calif.) University. “It was a 4% absolute difference, which I think is probably clinically meaningful, but certainly it’s not really, really dramatic.”
“What I think we found is that it’s safe to do a minimally invasive esophagectomy and safe for people to introduce it into their practice. But it’s not necessarily something that’s a game changer, unlike what’s been seen with minimally invasive approaches for some other things,” said Dr. Berry, who added that he didn’t wish to overstate the importance of the observed difference in morbidity.
Studies from high-volume centers show that minimally-invasive esophagectomy (MIE) reduces length of stay, postoperative major morbidity, and features equivalent or even slightly lower mortality than traditional open esophagectomy, the generalizability of these findings beyond such centers is questionable. That’s why Dr. Berry and his coinvestigators turned to the ACS-NSQIP database, which includes all esophagectomies performed for esophageal cancer at roughly 700 U.S. hospitals, not just those done by board-certified thoracic surgeons.
He presented a retrospective cohort study of 3,901 esophagectomy patients during 2005-2013 who met study criteria, 16.4% of whom had MIE. The use of this approach increased steadily from 6.5% of all esophagectomies in 2005 to 22.3% in 2013. A propensity-matched analysis designed to neutralize potentially confounding differences included 638 MIE and 1,914 open esophagectomy patients.
The primary outcome was the 30-day rate of composite major morbidity in the realms of various wound, respiratory, renal, and cardiovascular complications. The rate was 36.1% in the MIE group and 40.5% with open esophagectomy in the propensity-matched analysis, an absolute risk reduction of 4.4% and a relative risk reduction of 17%. Although rates were consistently slightly lower in each of the categories of major morbidity, those individual differences didn’t achieve statistical significance. The difference in major morbidity became significant only when major morbidity was considered as a whole.
Mean length of stay was 9 days with MIE and 10 days with open surgery.
There was no significant difference between the two study groups in 30-day rates of readmission, reoperation, or mortality.
Discussant Donald E. Low said “esophagectomy is being analysed regarding its place in all sorts of presentations, stages, and situations, so the aspect of making sure that we’re delivering the services as efficiently as possible is going to become more important, not less important.”
That being said, he noted that there is no specific CPT code for MIE. That raises the possibility of an uncertain amount of procedural misclassification in the ACS-NSQIP database.
Also, the only significant difference in major morbidity between the two study groups was in the subcategory of intra- or postoperative bleeding requiring transfusion, which occurred in 10.8% of the MIE and 16.7% of the open esophagectomy groups, observed Dr. Low, director of the Esophageal Center of Excellence at Virginia Mason Medical Center, Seattle.
“Some of us believe that blood utilization and transfusion requirement is really a quality measure and not a complication,” the surgeon said. And if that outcome is excluded from consideration, then there is no significant difference in major morbidity.
Discussant Douglas E. Wood, MD, professor and chair of the department of surgery at the University of Washington, Seattle, took the opportunity to share a self-described “pet peeve” about analyses of national surgical databases: these databases typically don’t contain key details necessary to correct for provider and hospital characteristics.
“The small differences that you demonstrate could easily have been completely driven by providers who choose to do minimally invasive esophagectomy and are in higher-volume, more specialized centers,” he said. “I’m not convinced of your conclusion that MIE produces less morbidity based on a 4% difference and no analysis of provider characteristics.”
AT WTSA 2017
Key clinical point:
Major finding: The 30-day rate of major morbidity was 36.1% in patients who underwent minimally invasive esophagectomy, significantly lower than the 40.5% rate with open esophagectomy in a propensity-matched analysis.
Data source: This retrospective cohort study included 3,901 patients who underwent esophagectomy for esophageal cancer as recorded in the American College of Surgeons-National Quality Improvement Program database for 2005-2013.
Disclosures: The study presenter reported having no financial conflicts of interest.
Preop atrial fib in CABG patients spells trouble
COLORADO SPRINGS – Preoperative atrial fibrillation is present in more than 10% of patients undergoing isolated coronary artery bypass graft (CABG) surgery, and if not subjected to concomitant surgical ablation it’s associated with increased perioperative and long-term major morbidity and mortality, S. Chris Malaisrie, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
The increased early and late risks posed by preoperative atrial fibrillation (AF) that go unaddressed remain significant even after adjusting for the numerous comorbid conditions more prevalent in CABG patients with preoperative AF than in those without the arrhythmia, added Dr. Malaisrie, a cardiac surgeon at Northwestern University in Chicago.
The unadjusted operative mortality rate was 1.8% in the no-AF group and 4.0% in patients with preoperative AF. Unadjusted in-hospital rates of permanent stroke, prolonged ventilation, reoperation, and new renal failure were also significantly higher in the preoperative AF group.
Not surprisingly, the preoperative AF group was older. They also had significantly higher baseline rates of numerous comorbid conditions, including diabetes, peripheral vascular disease, renal failure, and prior stroke, as well as a lower mean left ventricular ejection fraction. However, after adjustment for the many comorbidities in multivariate regression analysis, the risks of all in-hospital adverse outcomes remained significantly higher in the preoperative AF group. For example, their adjusted risk of operative mortality was 1.5-fold greater than in the no-AF patients.
In the long-term follow-up analysis, the unadjusted risk of mortality in the first 5 years after CABG was 2.5-fold greater in the preoperative AF group. Their 5-year risk of stroke or systemic embolization was 1.5-fold greater, too. Upon adjustment for potentially confounding comorbid conditions, preoperative AF was associated with a 1.5-fold increased 5-year risk of mortality and a 1.2-fold increase in stroke or systemic embolism.
In an effort to identify a particularly high-risk group of CABG patients with preoperative AF, Dr. Malaisrie and his coinvestigators stratified the group’s long-term stroke and mortality risks by their CHA2DS2-VASc score at the time of surgery. The results were revealing: the unadjusted 5-year risk of stroke or systemic embolization was 7.9% in those with a CHA2DS2-VASc score of 1-3, 12.2% with a score of 4-6, and 15.4% with a score of 7-9. The 5-year survival rate was 74.8% with a score of 1-3, 56.5% with a score of 4-6, and 41.2% with a score of 7-9.
“That’s really a striking finding,” Dr. Malaisrie observed. “When you consider a patient who’s, say, 72-75 years old, who is undergoing isolated CABG with preoperative atrial fibrillation and who has a high CHA2DS2-VASc score of 7-9, 5-year survival is only 41%, with a 15% risk of stroke or systemic embolization.”
Discussant William T. Caine, MD, found the study results unsettling.
“I was surprised to see that in this day and age, fully two-thirds of the patients who had preoperative atrial fibrillation had no attempt at any ablation procedure to treat their atrial fibrillation,” declared Dr. Caine of Intermountain Medical Center in Salt Lake City.
In reply, Dr. Malaisrie noted that other, smaller studies have also found that only about 30% of CABG patients with preoperative AF undergo surgical AF ablation through a maze procedure or some other method.
“Probably most of us in this room would go ahead and perform surgical ablation, but the STS database represents all isolated CABG procedures done throughout the United States,” Dr. Malaisrie said. “I think this dataset should help convince the other 70% of surgeons out there that there is a high cost for preoperative AF – in particular, in patients with very high CHA2DS2-VASc scores. If you can identify a group of patients at increased risk for stroke and mortality, you’d certainly want to bend their survival curve.”
The maze procedure has been convincingly shown to be very safe, with no associated increased risk of perioperative morbidity and mortality. The downside is cost. But while it’s true that adding surgical ablation to an isolated CABG procedure boosts OR time and procedural costs, a successful ablation is likely to pay dividends through reduced downstream rates of major morbidity and mortality.
“I look forward to the second part of our analysis, where we’ll look at the comparative data for the patients who did in fact have surgical ablation. That dataset is pending from the Duke Clinical Research Institute,” according to Dr. Malaisrie.
He cited as study limitations the inability to complete linkage to the Medicare database in about 37% of CABG patients in the STS database, or more than 200,000 people. Also, the Medicare database is an administrative dataset reliant upon medical record coding. The mortality data are probably quite accurate, but the stroke and systemic embolization rates cited in this analysis likely underestimate the true rates.
He reported serving as a consultant to Edwards Lifesciences, Abbott Vascular, and Baxter, and serving on speakers’ bureaus for Bolton and Abiomed. However, the STS analysis was funded exclusively by philanthropy.
COLORADO SPRINGS – Preoperative atrial fibrillation is present in more than 10% of patients undergoing isolated coronary artery bypass graft (CABG) surgery, and if not subjected to concomitant surgical ablation it’s associated with increased perioperative and long-term major morbidity and mortality, S. Chris Malaisrie, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
The increased early and late risks posed by preoperative atrial fibrillation (AF) that go unaddressed remain significant even after adjusting for the numerous comorbid conditions more prevalent in CABG patients with preoperative AF than in those without the arrhythmia, added Dr. Malaisrie, a cardiac surgeon at Northwestern University in Chicago.
The unadjusted operative mortality rate was 1.8% in the no-AF group and 4.0% in patients with preoperative AF. Unadjusted in-hospital rates of permanent stroke, prolonged ventilation, reoperation, and new renal failure were also significantly higher in the preoperative AF group.
Not surprisingly, the preoperative AF group was older. They also had significantly higher baseline rates of numerous comorbid conditions, including diabetes, peripheral vascular disease, renal failure, and prior stroke, as well as a lower mean left ventricular ejection fraction. However, after adjustment for the many comorbidities in multivariate regression analysis, the risks of all in-hospital adverse outcomes remained significantly higher in the preoperative AF group. For example, their adjusted risk of operative mortality was 1.5-fold greater than in the no-AF patients.
In the long-term follow-up analysis, the unadjusted risk of mortality in the first 5 years after CABG was 2.5-fold greater in the preoperative AF group. Their 5-year risk of stroke or systemic embolization was 1.5-fold greater, too. Upon adjustment for potentially confounding comorbid conditions, preoperative AF was associated with a 1.5-fold increased 5-year risk of mortality and a 1.2-fold increase in stroke or systemic embolism.
In an effort to identify a particularly high-risk group of CABG patients with preoperative AF, Dr. Malaisrie and his coinvestigators stratified the group’s long-term stroke and mortality risks by their CHA2DS2-VASc score at the time of surgery. The results were revealing: the unadjusted 5-year risk of stroke or systemic embolization was 7.9% in those with a CHA2DS2-VASc score of 1-3, 12.2% with a score of 4-6, and 15.4% with a score of 7-9. The 5-year survival rate was 74.8% with a score of 1-3, 56.5% with a score of 4-6, and 41.2% with a score of 7-9.
“That’s really a striking finding,” Dr. Malaisrie observed. “When you consider a patient who’s, say, 72-75 years old, who is undergoing isolated CABG with preoperative atrial fibrillation and who has a high CHA2DS2-VASc score of 7-9, 5-year survival is only 41%, with a 15% risk of stroke or systemic embolization.”
Discussant William T. Caine, MD, found the study results unsettling.
“I was surprised to see that in this day and age, fully two-thirds of the patients who had preoperative atrial fibrillation had no attempt at any ablation procedure to treat their atrial fibrillation,” declared Dr. Caine of Intermountain Medical Center in Salt Lake City.
In reply, Dr. Malaisrie noted that other, smaller studies have also found that only about 30% of CABG patients with preoperative AF undergo surgical AF ablation through a maze procedure or some other method.
“Probably most of us in this room would go ahead and perform surgical ablation, but the STS database represents all isolated CABG procedures done throughout the United States,” Dr. Malaisrie said. “I think this dataset should help convince the other 70% of surgeons out there that there is a high cost for preoperative AF – in particular, in patients with very high CHA2DS2-VASc scores. If you can identify a group of patients at increased risk for stroke and mortality, you’d certainly want to bend their survival curve.”
The maze procedure has been convincingly shown to be very safe, with no associated increased risk of perioperative morbidity and mortality. The downside is cost. But while it’s true that adding surgical ablation to an isolated CABG procedure boosts OR time and procedural costs, a successful ablation is likely to pay dividends through reduced downstream rates of major morbidity and mortality.
“I look forward to the second part of our analysis, where we’ll look at the comparative data for the patients who did in fact have surgical ablation. That dataset is pending from the Duke Clinical Research Institute,” according to Dr. Malaisrie.
He cited as study limitations the inability to complete linkage to the Medicare database in about 37% of CABG patients in the STS database, or more than 200,000 people. Also, the Medicare database is an administrative dataset reliant upon medical record coding. The mortality data are probably quite accurate, but the stroke and systemic embolization rates cited in this analysis likely underestimate the true rates.
He reported serving as a consultant to Edwards Lifesciences, Abbott Vascular, and Baxter, and serving on speakers’ bureaus for Bolton and Abiomed. However, the STS analysis was funded exclusively by philanthropy.
COLORADO SPRINGS – Preoperative atrial fibrillation is present in more than 10% of patients undergoing isolated coronary artery bypass graft (CABG) surgery, and if not subjected to concomitant surgical ablation it’s associated with increased perioperative and long-term major morbidity and mortality, S. Chris Malaisrie, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
The increased early and late risks posed by preoperative atrial fibrillation (AF) that go unaddressed remain significant even after adjusting for the numerous comorbid conditions more prevalent in CABG patients with preoperative AF than in those without the arrhythmia, added Dr. Malaisrie, a cardiac surgeon at Northwestern University in Chicago.
The unadjusted operative mortality rate was 1.8% in the no-AF group and 4.0% in patients with preoperative AF. Unadjusted in-hospital rates of permanent stroke, prolonged ventilation, reoperation, and new renal failure were also significantly higher in the preoperative AF group.
Not surprisingly, the preoperative AF group was older. They also had significantly higher baseline rates of numerous comorbid conditions, including diabetes, peripheral vascular disease, renal failure, and prior stroke, as well as a lower mean left ventricular ejection fraction. However, after adjustment for the many comorbidities in multivariate regression analysis, the risks of all in-hospital adverse outcomes remained significantly higher in the preoperative AF group. For example, their adjusted risk of operative mortality was 1.5-fold greater than in the no-AF patients.
In the long-term follow-up analysis, the unadjusted risk of mortality in the first 5 years after CABG was 2.5-fold greater in the preoperative AF group. Their 5-year risk of stroke or systemic embolization was 1.5-fold greater, too. Upon adjustment for potentially confounding comorbid conditions, preoperative AF was associated with a 1.5-fold increased 5-year risk of mortality and a 1.2-fold increase in stroke or systemic embolism.
In an effort to identify a particularly high-risk group of CABG patients with preoperative AF, Dr. Malaisrie and his coinvestigators stratified the group’s long-term stroke and mortality risks by their CHA2DS2-VASc score at the time of surgery. The results were revealing: the unadjusted 5-year risk of stroke or systemic embolization was 7.9% in those with a CHA2DS2-VASc score of 1-3, 12.2% with a score of 4-6, and 15.4% with a score of 7-9. The 5-year survival rate was 74.8% with a score of 1-3, 56.5% with a score of 4-6, and 41.2% with a score of 7-9.
“That’s really a striking finding,” Dr. Malaisrie observed. “When you consider a patient who’s, say, 72-75 years old, who is undergoing isolated CABG with preoperative atrial fibrillation and who has a high CHA2DS2-VASc score of 7-9, 5-year survival is only 41%, with a 15% risk of stroke or systemic embolization.”
Discussant William T. Caine, MD, found the study results unsettling.
“I was surprised to see that in this day and age, fully two-thirds of the patients who had preoperative atrial fibrillation had no attempt at any ablation procedure to treat their atrial fibrillation,” declared Dr. Caine of Intermountain Medical Center in Salt Lake City.
In reply, Dr. Malaisrie noted that other, smaller studies have also found that only about 30% of CABG patients with preoperative AF undergo surgical AF ablation through a maze procedure or some other method.
“Probably most of us in this room would go ahead and perform surgical ablation, but the STS database represents all isolated CABG procedures done throughout the United States,” Dr. Malaisrie said. “I think this dataset should help convince the other 70% of surgeons out there that there is a high cost for preoperative AF – in particular, in patients with very high CHA2DS2-VASc scores. If you can identify a group of patients at increased risk for stroke and mortality, you’d certainly want to bend their survival curve.”
The maze procedure has been convincingly shown to be very safe, with no associated increased risk of perioperative morbidity and mortality. The downside is cost. But while it’s true that adding surgical ablation to an isolated CABG procedure boosts OR time and procedural costs, a successful ablation is likely to pay dividends through reduced downstream rates of major morbidity and mortality.
“I look forward to the second part of our analysis, where we’ll look at the comparative data for the patients who did in fact have surgical ablation. That dataset is pending from the Duke Clinical Research Institute,” according to Dr. Malaisrie.
He cited as study limitations the inability to complete linkage to the Medicare database in about 37% of CABG patients in the STS database, or more than 200,000 people. Also, the Medicare database is an administrative dataset reliant upon medical record coding. The mortality data are probably quite accurate, but the stroke and systemic embolization rates cited in this analysis likely underestimate the true rates.
He reported serving as a consultant to Edwards Lifesciences, Abbott Vascular, and Baxter, and serving on speakers’ bureaus for Bolton and Abiomed. However, the STS analysis was funded exclusively by philanthropy.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: Preoperative AF in patients undergoing isolated CABG was tied to an adjusted 45% greater 5-year mortality and 25% increase in stroke and systemic embolization risk, compared with CABG patients without the preoperative arrhythmia.
Data source: This retrospective study compared perioperative and long-term morbidity and mortality in nearly 350,000 patients in the Society of Thoracic Surgeons database who underwent isolated CABG, including more than 24,000 who had preoperative atrial fibrillation that wasn’t addressed surgically.
Disclosures: The study presenter reported serving as a consultant to Edwards Lifesciences, Abbott Vascular, and Baxter, and serving on speakers’ bureaus for Bolton and Abiomed. However, the STS analysis was funded exclusively by philanthropy.
Wide variability found in invasive mediastinal staging rates for lung cancer
COLORADO SPRINGS – Significant variability exists between hospitals in Washington state in their rates of invasive mediastinal staging for lung cancer, Farhood Farjah, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
“We found evidence of a fivefold variation in hospital-level rates of invasive mediastinal staging not explained by chance or case mix,” according to Dr. Farjah of the University of Washington, Seattle.
“This has led to substantial concerns about quality of thoracic surgical care in the community at large,” he noted.
The Washington study is the first to show hospital-by-hospital variation in rates of invasive mediastinal staging.
Invasive mediastinal staging for lung cancer is considered important because imaging is known to have a substantial false-negative rate, and staging results have a profound impact on treatment recommendations, which can range from surgery alone to additional chemoradiation therapy.
Yet the meaning of the hospital-level huge variability in practice observed in the Washington study remains unclear.
“Our understanding of the underutilization of invasive mediastinal staging is further complicated by the fact that patterns of invasive mediastinal staging are highly variable across hospitals staffed by at least one board-certified thoracic surgeon with a noncardiac practice,” Dr. Farjah explained. “This variability could be a marker of poor-quality care. However, because the guidelines are not supported by level 1 evidence, it’s equally plausible that this variability might represent uncertainty or even disagreement with the practice guidelines – and specifically about the appropriate indication for invasive staging.”
He presented a retrospective cohort study of 406 patients whose non–small cell lung cancer was resected during July 2011–December 2013 at one of five Washington hospitals, each with at least one board-certified thoracic surgeon with a noncardiac practice on staff. The four participating community hospitals and one academic medical center were involved in a National Cancer Institute–funded, physician-led quality improvement initiative.
Overall, 66% of the 406 patients underwent any form of invasive mediastinal staging: 85% by mediastinoscopy only; 12% by mediastinoscopy plus endobronchial ultrasound-guided nodal aspiration (EBUS); 3% by EBUS only; and the remaining handful by mediastinoscopy, EBUS, and esophageal ultrasound-guided nodal aspiration. The invasive staging was performed at the time of resection in 64% of cases. A median of three nodal stations were sampled.
After statistical adjustment for random variation and between-hospital differences in clinical stage, rates of invasive staging were all over the map. While an overall mean of 66% of the lung cancer patients underwent invasive mediastinal staging, the rates at the five hospitals were 94%, 84%, 31%, 80%, and 17%.
Dr. Farjah and his coinvestigators are now conducting provider interviews and focus groups in an effort to understand what drove the participating surgeons’ wide variability in performing invasive mediastinal staging.
Discussant Jane Yanagawa, MD, of the University of California, Los Angeles, commented, “I think this is a really interesting study because, historically, lower rates of mediastinoscopy are assumed to be a reflection of low-quality care – and you suggest that might not be the case, that it might be more complicated than that.”
Dr. Yanagawa sketched one fairly common scenario that might represent a surgeon’s reasonable avoidance of guideline-recommended invasive mediastinal staging: a patient who by all preoperative imaging appears to have stage IA lung cancer and wishes to avoid the morbidity, time, and cost of needle biopsy, instead choosing to go straight to the operating room for a diagnosis by wedge resection, followed by a completion lobectomy based upon the frozen section results. Could such a pathway account for the variability seen in the Washington study?
“I think it could have,” Dr. Farjah replied. “I would say that’s probably one driver of variability.”
As for the generalizability of the findings of a five-hospital study carried out in a single state, Dr. Farjah said he thinks the results are applicable to any academic or community hospital with at least one board-certified thoracic surgeon with a noncardiac practice.
He reported having no financial conflicts of interest regarding the study.
M. Patricia Rivera, MD, FCCP, comments: Staging of lung cancer is essential to select the best treatment strategy for a given patient. However, despite multiple guideline recommendations
M. Patricia Rivera, MD, FCCP, comments: Staging of lung cancer is essential to select the best treatment strategy for a given patient. However, despite multiple guideline recommendations
M. Patricia Rivera, MD, FCCP, comments: Staging of lung cancer is essential to select the best treatment strategy for a given patient. However, despite multiple guideline recommendations
COLORADO SPRINGS – Significant variability exists between hospitals in Washington state in their rates of invasive mediastinal staging for lung cancer, Farhood Farjah, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
“We found evidence of a fivefold variation in hospital-level rates of invasive mediastinal staging not explained by chance or case mix,” according to Dr. Farjah of the University of Washington, Seattle.
“This has led to substantial concerns about quality of thoracic surgical care in the community at large,” he noted.
The Washington study is the first to show hospital-by-hospital variation in rates of invasive mediastinal staging.
Invasive mediastinal staging for lung cancer is considered important because imaging is known to have a substantial false-negative rate, and staging results have a profound impact on treatment recommendations, which can range from surgery alone to additional chemoradiation therapy.
Yet the meaning of the hospital-level huge variability in practice observed in the Washington study remains unclear.
“Our understanding of the underutilization of invasive mediastinal staging is further complicated by the fact that patterns of invasive mediastinal staging are highly variable across hospitals staffed by at least one board-certified thoracic surgeon with a noncardiac practice,” Dr. Farjah explained. “This variability could be a marker of poor-quality care. However, because the guidelines are not supported by level 1 evidence, it’s equally plausible that this variability might represent uncertainty or even disagreement with the practice guidelines – and specifically about the appropriate indication for invasive staging.”
He presented a retrospective cohort study of 406 patients whose non–small cell lung cancer was resected during July 2011–December 2013 at one of five Washington hospitals, each with at least one board-certified thoracic surgeon with a noncardiac practice on staff. The four participating community hospitals and one academic medical center were involved in a National Cancer Institute–funded, physician-led quality improvement initiative.
Overall, 66% of the 406 patients underwent any form of invasive mediastinal staging: 85% by mediastinoscopy only; 12% by mediastinoscopy plus endobronchial ultrasound-guided nodal aspiration (EBUS); 3% by EBUS only; and the remaining handful by mediastinoscopy, EBUS, and esophageal ultrasound-guided nodal aspiration. The invasive staging was performed at the time of resection in 64% of cases. A median of three nodal stations were sampled.
After statistical adjustment for random variation and between-hospital differences in clinical stage, rates of invasive staging were all over the map. While an overall mean of 66% of the lung cancer patients underwent invasive mediastinal staging, the rates at the five hospitals were 94%, 84%, 31%, 80%, and 17%.
Dr. Farjah and his coinvestigators are now conducting provider interviews and focus groups in an effort to understand what drove the participating surgeons’ wide variability in performing invasive mediastinal staging.
Discussant Jane Yanagawa, MD, of the University of California, Los Angeles, commented, “I think this is a really interesting study because, historically, lower rates of mediastinoscopy are assumed to be a reflection of low-quality care – and you suggest that might not be the case, that it might be more complicated than that.”
Dr. Yanagawa sketched one fairly common scenario that might represent a surgeon’s reasonable avoidance of guideline-recommended invasive mediastinal staging: a patient who by all preoperative imaging appears to have stage IA lung cancer and wishes to avoid the morbidity, time, and cost of needle biopsy, instead choosing to go straight to the operating room for a diagnosis by wedge resection, followed by a completion lobectomy based upon the frozen section results. Could such a pathway account for the variability seen in the Washington study?
“I think it could have,” Dr. Farjah replied. “I would say that’s probably one driver of variability.”
As for the generalizability of the findings of a five-hospital study carried out in a single state, Dr. Farjah said he thinks the results are applicable to any academic or community hospital with at least one board-certified thoracic surgeon with a noncardiac practice.
He reported having no financial conflicts of interest regarding the study.
COLORADO SPRINGS – Significant variability exists between hospitals in Washington state in their rates of invasive mediastinal staging for lung cancer, Farhood Farjah, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
“We found evidence of a fivefold variation in hospital-level rates of invasive mediastinal staging not explained by chance or case mix,” according to Dr. Farjah of the University of Washington, Seattle.
“This has led to substantial concerns about quality of thoracic surgical care in the community at large,” he noted.
The Washington study is the first to show hospital-by-hospital variation in rates of invasive mediastinal staging.
Invasive mediastinal staging for lung cancer is considered important because imaging is known to have a substantial false-negative rate, and staging results have a profound impact on treatment recommendations, which can range from surgery alone to additional chemoradiation therapy.
Yet the meaning of the hospital-level huge variability in practice observed in the Washington study remains unclear.
“Our understanding of the underutilization of invasive mediastinal staging is further complicated by the fact that patterns of invasive mediastinal staging are highly variable across hospitals staffed by at least one board-certified thoracic surgeon with a noncardiac practice,” Dr. Farjah explained. “This variability could be a marker of poor-quality care. However, because the guidelines are not supported by level 1 evidence, it’s equally plausible that this variability might represent uncertainty or even disagreement with the practice guidelines – and specifically about the appropriate indication for invasive staging.”
He presented a retrospective cohort study of 406 patients whose non–small cell lung cancer was resected during July 2011–December 2013 at one of five Washington hospitals, each with at least one board-certified thoracic surgeon with a noncardiac practice on staff. The four participating community hospitals and one academic medical center were involved in a National Cancer Institute–funded, physician-led quality improvement initiative.
Overall, 66% of the 406 patients underwent any form of invasive mediastinal staging: 85% by mediastinoscopy only; 12% by mediastinoscopy plus endobronchial ultrasound-guided nodal aspiration (EBUS); 3% by EBUS only; and the remaining handful by mediastinoscopy, EBUS, and esophageal ultrasound-guided nodal aspiration. The invasive staging was performed at the time of resection in 64% of cases. A median of three nodal stations were sampled.
After statistical adjustment for random variation and between-hospital differences in clinical stage, rates of invasive staging were all over the map. While an overall mean of 66% of the lung cancer patients underwent invasive mediastinal staging, the rates at the five hospitals were 94%, 84%, 31%, 80%, and 17%.
Dr. Farjah and his coinvestigators are now conducting provider interviews and focus groups in an effort to understand what drove the participating surgeons’ wide variability in performing invasive mediastinal staging.
Discussant Jane Yanagawa, MD, of the University of California, Los Angeles, commented, “I think this is a really interesting study because, historically, lower rates of mediastinoscopy are assumed to be a reflection of low-quality care – and you suggest that might not be the case, that it might be more complicated than that.”
Dr. Yanagawa sketched one fairly common scenario that might represent a surgeon’s reasonable avoidance of guideline-recommended invasive mediastinal staging: a patient who by all preoperative imaging appears to have stage IA lung cancer and wishes to avoid the morbidity, time, and cost of needle biopsy, instead choosing to go straight to the operating room for a diagnosis by wedge resection, followed by a completion lobectomy based upon the frozen section results. Could such a pathway account for the variability seen in the Washington study?
“I think it could have,” Dr. Farjah replied. “I would say that’s probably one driver of variability.”
As for the generalizability of the findings of a five-hospital study carried out in a single state, Dr. Farjah said he thinks the results are applicable to any academic or community hospital with at least one board-certified thoracic surgeon with a noncardiac practice.
He reported having no financial conflicts of interest regarding the study.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: Rates of invasive mediastinal staging after adjustment for clinical stage ranged from a low of 17% at one hospital to as high as 94% at another.
Data source: This retrospective cohort study included 406 patients.
Disclosures: Dr. Farjah reported having no financial conflicts of interest.
Hidden CABG costs will disrupt bundled payment systems
COLORADO SPRINGS – With bundled payment models for coronary artery bypass graft surgery looming ahead, it’s vital that cardiac surgeons take a hard look at the procedure’s hidden costs – namely, the steep price tag for postoperative complications, James H. Mehaffey, MD, said at the annual meeting of the Western Thoracic Surgical Association.
He presented a retrospective study of the 30-day hospital costs for all 36,588 patients who underwent isolated CABG during 2006-2015 at the 19 Virginia centers where the surgery is performed. This was a typical CABG population, with an average predicted risk of mortality of 1.9%. The actual 30-day mortality was 0.6%, so the surgical performance was better than expected.
“The population of patients experiencing one or more major comorbidities demonstrated a significant and dramatic increase in total hospital costs. It was an exponential increase with each additional major morbidity,” reported Dr. Mehaffey of the University of Virginia, Charlottesville.
Indeed, the average cost jumped from $36,580 for uncomplicated surgery to $64,542 with one major complication, $111,239 with two, and $194,043 with three.
The two most frequent major complications were postoperative atrial fibrillation, which occurred in 18.4% of patients, and prolonged ventilation for longer than 24 hours, which occurred in 9%. Over the course of the decade-long study period, the 19 medical centers in the Virginia Cardiac Surgery Quality Initiative collectively spent roughly $59 million on prolonged ventilation and $27 million for postoperative atrial fibrillation.
The cost of CABG during the study years outpaced the CMS health care–specific inflation rate, and this escalating cost was driven primarily by postoperative complications.
For the Virginia cardiac surgery collaborative, these data on the cost of postoperative complications will be utilized to prioritize quality improvement projects.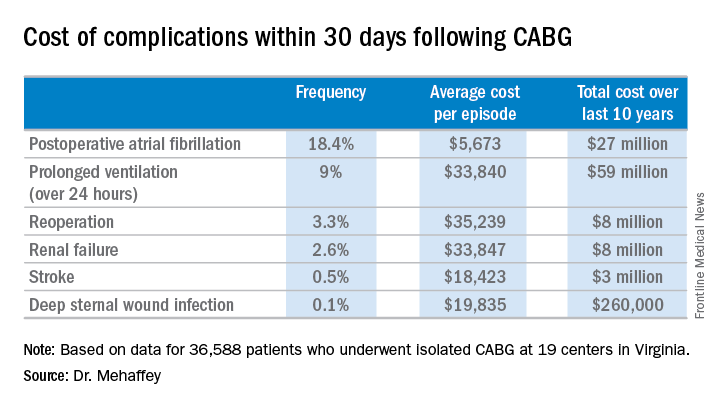
For example, during the past decade, the Virginia collaborative made reduction in the rate of postoperative atrial fibrillation a priority. Toward that end, the collaborative developed a protocol for routine perioperative prophylactic amiodarone therapy.
“At the beginning of the study decade we had postoperative atrial fibrillation rates above 25%. The average for the entire decade was just over 18%, and in the last couple years we’ve been in the 15%-16% range. So I think we are moving the needle on this. We are making a meaningful impact,” Dr. Mehaffey said.
“We’ve already used the complication cost data to do a cost-effectiveness analysis of our prophylactic amiodarone innovation. We showed we saved an average of $250 per patient, even though we’re treating a bunch of patients who’d never get that complication,” he continued.
This sort of data on the cost of adverse events is also critical to accurately risk-adjust bundled payment models.
Discussant Richard J. Shemin, MD, asked if there was much variability in postoperative complication costs between the CABG centers in the Virginia collaborative.
The variability is enormous, Dr. Mehaffey replied. Investigators recently plugged the last 5 years worth of hospital cost and complication rate data into a proposed CABG bundled payment model and extrapolated what that would mean over the next 5 years.
“There were some institutions that would be positive by a couple million dollars from this payment system and some that were losing more than $20 million, just because of the cost variability,” said Dr. Mehaffey.
Dr. Shemin also noted that the Virginia collaborative was able to collect 30-day outcome data only through the STS database, yet the bundled payment programs are based on the 90-day postoperative experience.
“How do we capture the costs in that full 90 days that we’ll be responsible for?” asked Dr. Shemin, professor of surgery and codirector of the UCLA Cardiovascular Center.
Dr. Mehaffey said that’s indeed an important question, since a major complication such as stroke or deep sternal wound infection typically entails considerable long-term costs and repeated hospital admissions beyond the 30-day window. In Virginia, the cardiac surgery collaborative is working with payers to gain access to the 90 days worth of patient data.
He reported having no financial conflicts regarding his study.
COLORADO SPRINGS – With bundled payment models for coronary artery bypass graft surgery looming ahead, it’s vital that cardiac surgeons take a hard look at the procedure’s hidden costs – namely, the steep price tag for postoperative complications, James H. Mehaffey, MD, said at the annual meeting of the Western Thoracic Surgical Association.
He presented a retrospective study of the 30-day hospital costs for all 36,588 patients who underwent isolated CABG during 2006-2015 at the 19 Virginia centers where the surgery is performed. This was a typical CABG population, with an average predicted risk of mortality of 1.9%. The actual 30-day mortality was 0.6%, so the surgical performance was better than expected.
“The population of patients experiencing one or more major comorbidities demonstrated a significant and dramatic increase in total hospital costs. It was an exponential increase with each additional major morbidity,” reported Dr. Mehaffey of the University of Virginia, Charlottesville.
Indeed, the average cost jumped from $36,580 for uncomplicated surgery to $64,542 with one major complication, $111,239 with two, and $194,043 with three.
The two most frequent major complications were postoperative atrial fibrillation, which occurred in 18.4% of patients, and prolonged ventilation for longer than 24 hours, which occurred in 9%. Over the course of the decade-long study period, the 19 medical centers in the Virginia Cardiac Surgery Quality Initiative collectively spent roughly $59 million on prolonged ventilation and $27 million for postoperative atrial fibrillation.
The cost of CABG during the study years outpaced the CMS health care–specific inflation rate, and this escalating cost was driven primarily by postoperative complications.
For the Virginia cardiac surgery collaborative, these data on the cost of postoperative complications will be utilized to prioritize quality improvement projects.
For example, during the past decade, the Virginia collaborative made reduction in the rate of postoperative atrial fibrillation a priority. Toward that end, the collaborative developed a protocol for routine perioperative prophylactic amiodarone therapy.
“At the beginning of the study decade we had postoperative atrial fibrillation rates above 25%. The average for the entire decade was just over 18%, and in the last couple years we’ve been in the 15%-16% range. So I think we are moving the needle on this. We are making a meaningful impact,” Dr. Mehaffey said.
“We’ve already used the complication cost data to do a cost-effectiveness analysis of our prophylactic amiodarone innovation. We showed we saved an average of $250 per patient, even though we’re treating a bunch of patients who’d never get that complication,” he continued.
This sort of data on the cost of adverse events is also critical to accurately risk-adjust bundled payment models.
Discussant Richard J. Shemin, MD, asked if there was much variability in postoperative complication costs between the CABG centers in the Virginia collaborative.
The variability is enormous, Dr. Mehaffey replied. Investigators recently plugged the last 5 years worth of hospital cost and complication rate data into a proposed CABG bundled payment model and extrapolated what that would mean over the next 5 years.
“There were some institutions that would be positive by a couple million dollars from this payment system and some that were losing more than $20 million, just because of the cost variability,” said Dr. Mehaffey.
Dr. Shemin also noted that the Virginia collaborative was able to collect 30-day outcome data only through the STS database, yet the bundled payment programs are based on the 90-day postoperative experience.
“How do we capture the costs in that full 90 days that we’ll be responsible for?” asked Dr. Shemin, professor of surgery and codirector of the UCLA Cardiovascular Center.
Dr. Mehaffey said that’s indeed an important question, since a major complication such as stroke or deep sternal wound infection typically entails considerable long-term costs and repeated hospital admissions beyond the 30-day window. In Virginia, the cardiac surgery collaborative is working with payers to gain access to the 90 days worth of patient data.
He reported having no financial conflicts regarding his study.
COLORADO SPRINGS – With bundled payment models for coronary artery bypass graft surgery looming ahead, it’s vital that cardiac surgeons take a hard look at the procedure’s hidden costs – namely, the steep price tag for postoperative complications, James H. Mehaffey, MD, said at the annual meeting of the Western Thoracic Surgical Association.
He presented a retrospective study of the 30-day hospital costs for all 36,588 patients who underwent isolated CABG during 2006-2015 at the 19 Virginia centers where the surgery is performed. This was a typical CABG population, with an average predicted risk of mortality of 1.9%. The actual 30-day mortality was 0.6%, so the surgical performance was better than expected.
“The population of patients experiencing one or more major comorbidities demonstrated a significant and dramatic increase in total hospital costs. It was an exponential increase with each additional major morbidity,” reported Dr. Mehaffey of the University of Virginia, Charlottesville.
Indeed, the average cost jumped from $36,580 for uncomplicated surgery to $64,542 with one major complication, $111,239 with two, and $194,043 with three.
The two most frequent major complications were postoperative atrial fibrillation, which occurred in 18.4% of patients, and prolonged ventilation for longer than 24 hours, which occurred in 9%. Over the course of the decade-long study period, the 19 medical centers in the Virginia Cardiac Surgery Quality Initiative collectively spent roughly $59 million on prolonged ventilation and $27 million for postoperative atrial fibrillation.
The cost of CABG during the study years outpaced the CMS health care–specific inflation rate, and this escalating cost was driven primarily by postoperative complications.
For the Virginia cardiac surgery collaborative, these data on the cost of postoperative complications will be utilized to prioritize quality improvement projects.
For example, during the past decade, the Virginia collaborative made reduction in the rate of postoperative atrial fibrillation a priority. Toward that end, the collaborative developed a protocol for routine perioperative prophylactic amiodarone therapy.
“At the beginning of the study decade we had postoperative atrial fibrillation rates above 25%. The average for the entire decade was just over 18%, and in the last couple years we’ve been in the 15%-16% range. So I think we are moving the needle on this. We are making a meaningful impact,” Dr. Mehaffey said.
“We’ve already used the complication cost data to do a cost-effectiveness analysis of our prophylactic amiodarone innovation. We showed we saved an average of $250 per patient, even though we’re treating a bunch of patients who’d never get that complication,” he continued.
This sort of data on the cost of adverse events is also critical to accurately risk-adjust bundled payment models.
Discussant Richard J. Shemin, MD, asked if there was much variability in postoperative complication costs between the CABG centers in the Virginia collaborative.
The variability is enormous, Dr. Mehaffey replied. Investigators recently plugged the last 5 years worth of hospital cost and complication rate data into a proposed CABG bundled payment model and extrapolated what that would mean over the next 5 years.
“There were some institutions that would be positive by a couple million dollars from this payment system and some that were losing more than $20 million, just because of the cost variability,” said Dr. Mehaffey.
Dr. Shemin also noted that the Virginia collaborative was able to collect 30-day outcome data only through the STS database, yet the bundled payment programs are based on the 90-day postoperative experience.
“How do we capture the costs in that full 90 days that we’ll be responsible for?” asked Dr. Shemin, professor of surgery and codirector of the UCLA Cardiovascular Center.
Dr. Mehaffey said that’s indeed an important question, since a major complication such as stroke or deep sternal wound infection typically entails considerable long-term costs and repeated hospital admissions beyond the 30-day window. In Virginia, the cardiac surgery collaborative is working with payers to gain access to the 90 days worth of patient data.
He reported having no financial conflicts regarding his study.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: The average 30-day total hospital cost of an isolated CABG procedure during 2006-2015 in Virginia was $36,580 if there were no postoperative complications, jumping to $64,542 with one major complication and $111,239 with two.
Data source: A retrospective study of the 30-day total hospital costs for all isolated CABG procedures performed in Virginia during 2006-2015.
Disclosures: The study presenter reported having no financial conflicts.
How to pump up the donor heart pool
COLORADO SPRINGS – Diminished left ventricular systolic function alone should not be used as a basis for declining a donor heart for transplantation, Agustin Sibona, MD, asserted at the annual meeting of the Western Thoracic Surgical Association.
“Expansion of the donor pool to include more of these organs is appropriate,” said Dr. Sibona of Loma Linda (Calif.) University.
He presented an analysis of the United Network for Organ Sharing database that encompassed all adult isolated first-time heart transplants in the United States from 2000 through March 2016.
“Carefully selected potential donor hearts with LVEF of 30% or higher should not be excluded from consideration of transplantation on the basis of depressed LVEF alone,” he concluded. “We’re not saying we should use every heart that has an EF of 35% or 45%. We say you should thoroughly evaluate those patients and those hearts and consider them.”
Roughly 500,000 people develop new end-stage heart failure each year. Heart transplantation has long been considered the definitive therapy for this condition. However, heart transplantation rates have remained static at 2,000-2,500 per year in the United States for the past 15 years because of the shortage of donor organs.
Previous work by Dr. Sibona’s senior coinvestigators has documented that 19% of potential donor hearts are not utilized for transplant solely based upon the presence of left ventricular dysfunction. That’s about 1,300 hearts per year.
“About 60% of those hearts had an LVEF greater than 40%. That’s 785 hearts. If only half of those are used, that still represents an increase in the domestic transplant rate of almost 20%,” he observed.
Twenty-one patients in the study received a heart with an LVEF of 20%-29.9%. They had an unacceptably high perioperative mortality.
There was no significant difference between the LVEF groups in terms of race, cause of death, or ischemic time.
Mean transplantation hospital length of stay varied inversely with donor heart LVEF, from 20.3 days in patients with a normal LVEF, to 23.9 days with an LVEF of 40%-49.9%, and 31.1 days with an LVEF of 30%-39.9%.
Dr. Sibona replied that unfortunately the UNOS database is not informative on that score.
Dr. Kwon offered a practical reservation about embracing the use of compromised donor hearts: “Ninety-one percent of programs in the U.S. do less than 30 heart transplants per year, and 76% do less than 20. Smaller programs won’t necessarily have the luxury of 6,000 days to see if their survival statistics bear out. If they have two or three deaths per year, that’s enough to get a notice from UNOS and CMS and private payers. So I would note some caution in that regard.”
He also posed a question: In this new era of highly effective left ventricular assist devices serving as a long-term bridge to transplant, does it make sense to turn to dysfunctional donor hearts?
“Ventricular assist devices are an evolving technology,” Dr. Sibona responded. “Short-term outcomes are equivalent to transplant, but the devices often have complications: GI bleed, stroke, thrombosis, and infections. So we still believe that heart transplantation is the gold standard for treatment. Remember, these patients have end-stage heart failure. Many can’t get out of bed without shortness of breath. So, yes, I would take those hearts.”
He reported having no financial conflicts regarding his study, which was supported by Loma Linda and Stanford universities.
COLORADO SPRINGS – Diminished left ventricular systolic function alone should not be used as a basis for declining a donor heart for transplantation, Agustin Sibona, MD, asserted at the annual meeting of the Western Thoracic Surgical Association.
“Expansion of the donor pool to include more of these organs is appropriate,” said Dr. Sibona of Loma Linda (Calif.) University.
He presented an analysis of the United Network for Organ Sharing database that encompassed all adult isolated first-time heart transplants in the United States from 2000 through March 2016.
“Carefully selected potential donor hearts with LVEF of 30% or higher should not be excluded from consideration of transplantation on the basis of depressed LVEF alone,” he concluded. “We’re not saying we should use every heart that has an EF of 35% or 45%. We say you should thoroughly evaluate those patients and those hearts and consider them.”
Roughly 500,000 people develop new end-stage heart failure each year. Heart transplantation has long been considered the definitive therapy for this condition. However, heart transplantation rates have remained static at 2,000-2,500 per year in the United States for the past 15 years because of the shortage of donor organs.
Previous work by Dr. Sibona’s senior coinvestigators has documented that 19% of potential donor hearts are not utilized for transplant solely based upon the presence of left ventricular dysfunction. That’s about 1,300 hearts per year.
“About 60% of those hearts had an LVEF greater than 40%. That’s 785 hearts. If only half of those are used, that still represents an increase in the domestic transplant rate of almost 20%,” he observed.
Twenty-one patients in the study received a heart with an LVEF of 20%-29.9%. They had an unacceptably high perioperative mortality.
There was no significant difference between the LVEF groups in terms of race, cause of death, or ischemic time.
Mean transplantation hospital length of stay varied inversely with donor heart LVEF, from 20.3 days in patients with a normal LVEF, to 23.9 days with an LVEF of 40%-49.9%, and 31.1 days with an LVEF of 30%-39.9%.
Dr. Sibona replied that unfortunately the UNOS database is not informative on that score.
Dr. Kwon offered a practical reservation about embracing the use of compromised donor hearts: “Ninety-one percent of programs in the U.S. do less than 30 heart transplants per year, and 76% do less than 20. Smaller programs won’t necessarily have the luxury of 6,000 days to see if their survival statistics bear out. If they have two or three deaths per year, that’s enough to get a notice from UNOS and CMS and private payers. So I would note some caution in that regard.”
He also posed a question: In this new era of highly effective left ventricular assist devices serving as a long-term bridge to transplant, does it make sense to turn to dysfunctional donor hearts?
“Ventricular assist devices are an evolving technology,” Dr. Sibona responded. “Short-term outcomes are equivalent to transplant, but the devices often have complications: GI bleed, stroke, thrombosis, and infections. So we still believe that heart transplantation is the gold standard for treatment. Remember, these patients have end-stage heart failure. Many can’t get out of bed without shortness of breath. So, yes, I would take those hearts.”
He reported having no financial conflicts regarding his study, which was supported by Loma Linda and Stanford universities.
COLORADO SPRINGS – Diminished left ventricular systolic function alone should not be used as a basis for declining a donor heart for transplantation, Agustin Sibona, MD, asserted at the annual meeting of the Western Thoracic Surgical Association.
“Expansion of the donor pool to include more of these organs is appropriate,” said Dr. Sibona of Loma Linda (Calif.) University.
He presented an analysis of the United Network for Organ Sharing database that encompassed all adult isolated first-time heart transplants in the United States from 2000 through March 2016.
“Carefully selected potential donor hearts with LVEF of 30% or higher should not be excluded from consideration of transplantation on the basis of depressed LVEF alone,” he concluded. “We’re not saying we should use every heart that has an EF of 35% or 45%. We say you should thoroughly evaluate those patients and those hearts and consider them.”
Roughly 500,000 people develop new end-stage heart failure each year. Heart transplantation has long been considered the definitive therapy for this condition. However, heart transplantation rates have remained static at 2,000-2,500 per year in the United States for the past 15 years because of the shortage of donor organs.
Previous work by Dr. Sibona’s senior coinvestigators has documented that 19% of potential donor hearts are not utilized for transplant solely based upon the presence of left ventricular dysfunction. That’s about 1,300 hearts per year.
“About 60% of those hearts had an LVEF greater than 40%. That’s 785 hearts. If only half of those are used, that still represents an increase in the domestic transplant rate of almost 20%,” he observed.
Twenty-one patients in the study received a heart with an LVEF of 20%-29.9%. They had an unacceptably high perioperative mortality.
There was no significant difference between the LVEF groups in terms of race, cause of death, or ischemic time.
Mean transplantation hospital length of stay varied inversely with donor heart LVEF, from 20.3 days in patients with a normal LVEF, to 23.9 days with an LVEF of 40%-49.9%, and 31.1 days with an LVEF of 30%-39.9%.
Dr. Sibona replied that unfortunately the UNOS database is not informative on that score.
Dr. Kwon offered a practical reservation about embracing the use of compromised donor hearts: “Ninety-one percent of programs in the U.S. do less than 30 heart transplants per year, and 76% do less than 20. Smaller programs won’t necessarily have the luxury of 6,000 days to see if their survival statistics bear out. If they have two or three deaths per year, that’s enough to get a notice from UNOS and CMS and private payers. So I would note some caution in that regard.”
He also posed a question: In this new era of highly effective left ventricular assist devices serving as a long-term bridge to transplant, does it make sense to turn to dysfunctional donor hearts?
“Ventricular assist devices are an evolving technology,” Dr. Sibona responded. “Short-term outcomes are equivalent to transplant, but the devices often have complications: GI bleed, stroke, thrombosis, and infections. So we still believe that heart transplantation is the gold standard for treatment. Remember, these patients have end-stage heart failure. Many can’t get out of bed without shortness of breath. So, yes, I would take those hearts.”
He reported having no financial conflicts regarding his study, which was supported by Loma Linda and Stanford universities.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: Survival of heart transplant recipients whose donor organ had left ventricular systolic dysfunction with an LVEF as low as 30%-39% was not significantly less than for those with a normal donor heart.
Data source: A retrospective study of all of the nearly 31,000 isolated first-time adult heart transplants performed in the U.S. during 2000-March 2016.
Disclosures: Loma Linda and Stanford universities supported the study. The presenter reported having no financial conflicts.












