User login
52-year-old man • intermittent fevers • recently received second dose of COVID-19 vaccine • tremors in all 4 extremities • Dx?
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; Leara@ccf.org
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; Leara@ccf.org
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; Leara@ccf.org
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
► Intermittent fevers
► Recently received second dose of COVID-19 vaccine
► Tremors in all 4 extremities
Syracuse Hemoglobinopathy Presenting With Tophaceous Gout: A Case Report
Hemoglobinopathies are inherited disorders of hemoglobin that alter oxygen binding capacity by affecting the production of a specific subset of globin chains or their structure.1 A lesser-known subtype, Syracuse hemoglobinopathy (SH), was first identified in 4 generations of a family in the 1970s.2 As with other disorders of hemoglobin structure, there is an inherent risk of increased cell breakdown and turnover. This case discusses the presentation of gout in a patient with a history of SH.
Case presentation
A 44-year-old man with known SH, tobacco use disorder, and shoulder osteoarthritis presented with pain and palpable nodular masses on bilateral elbows, metacarpophalangeal joints, and feet progressively over 5 years. Of note, he was initially misdiagnosed with polycythemia vera after an incidental finding of elevated hematocrit more than 10 years prior. His mother, maternal aunt, and maternal grandmother have all been treated for polycythemia vera.
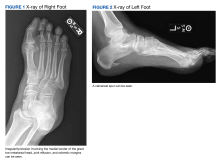
On examination, there were irregular palpable masses of varying sizes, erythema, and tenderness over the second metacarpophalangeal joint of the left hand, bilateral elbows, and bilateral metatarsophalangeal joints. Laboratory studies were remarkable for 19.8 g/dL hemoglobin (reference range, 12.0-16.0 g/dL); 63.4% hematocrit (reference range, 37.0%-47.0%); 219 × 103 µL platelets (reference range, 150-450 × 103 µL); 79.3 fL mean corpuscular volume (reference range, 81.0-99.0 fL); 14 mg/dL blood urea nitrogen (reference range, 8-27 mg/dL); 1.18 mg/dL creatinine (reference range, 0.60-1.60 mg/dL); 3 mmol/h erythrocyte sedimentation rate (reference range, 0-30 mmol/h); 88 IU/L alkaline phosphatase (reference range, 34-130 IU/L); and 11.3 mg/dL uric acid (reference range, 2.4-7.9 mg/dL). Hemoglobin electrophoresis studies showed a 49% hemoglobin A1 (reference range, 95%-98%); 3.0% hemoglobin A2 (reference range, 2%-3%); 3.1% hemoglobin F (reference range, < 0.6%); and 44.9% hemoglobin Syracuse (reference range, absent). It was negative for JAK2 V617F mutation. An X-ray of the bilateral feet showed irregularity/erosion involving the medial border of the great toe metatarsal head, joint effusions, and sclerotic margins (Figure 1). A prominent plantar calcaneal spur was present (Figure 2). Synovial fluid analysis detected the presence of negatively birefringent needle-shaped urate crystals.
Per the Clinical Gout Diagnosis tool, which has a sensitivity of 97%, this patient scored high given the findings of greater than one attack of acute arthritis, mono/oligoarthritic attacks, podagra, erythema, probable tophi, and hyperuricemia. This raised the likelihood of his presentation being an acute flare of tophaceous gout.3 He was treated with colchicine and prednisone for acute exacerbation. Once the exacerbation subsided, the colchicine was discontinued, and allopurinol was added. The uric acid goal was < 6 mg/dL and was consistently maintained. Over the subsequent months, he reported mild joint pain if he stopped taking allopurinol but did not report a recurrence in disease exacerbation.
Discussion
Hemoglobin Syracuse was first identified in the early 1970s after the discovery of similar familial hemoglobinopathies unique for their high oxygen affinity hemoglobin.1 High oxygen affinity hemoglobin functions by causing a leftward shift in the hemoglobin dissociation curve and therefore slower off-loading of oxygen into tissues.4 The hypoxic state at the tissue level created by the hemoglobin binding tightly to oxygen promotes the production of erythropoietin, increasing red blood cell and hemoglobin production.5 A study looking at uric acid levels in patients living at high altitudes (which can imitate the low-oxygen state seen in high affinity hemoglobinopathy) theorized that increased erythroblast turnover in the setting of polycythemia involves increased purine metabolism and consequently, uric acid as a breakdown product.6 Uric acid levels have also been used as a marker for hypoxia in studies regarding sleep apnea. Tissue hypoxia can increase adenosine triphosphate breakdown. One byproduct of this breakdown is hypoxanthine, which is further metabolized by xanthine oxidase, which, in turn, produces uric acid.7
The relationship between elevated uric acid and gout was first studied in the mid-nineteenth century after Alfred Barring Garrod identified urate deposits in the articular cartilage of patients with gout.1 These urate deposits garner a proinflammatory response with the activation of the complement cascade, resulting in the recruitment of neutrophils, macrophages, and lymphocytes. Recurrent gout flares eventually result in a chronic granulomatous inflammatory response to the deposited crystals resulting in the classic tophi.8 A study looking at patients with thalassemia showed that while elevated serum uric acid levels were common in these patients, only 6% developed gout. Significant risk factors were noted to be intact spleen and inefficient urinary excretion of urea due to chronic kidney disease.9
Current treatment of gout flares consistsof pain control in the acute phase and prevention in the long-term setting. The first-line treatment for acute gout attack is colchicine, prednisone, or nonsteroidal anti-inflammatory drugs. Clinicians can consider switching or combining these therapies if ineffective or in the event of severe exacerbation. Prophylactic therapy involves urate-lowering agents, such as allopurinol and febuxostat.10
Conclusions
This case illustrates how a rare disorder of high oxygen affinity hemoglobin, SH, can present itself with findings of elevated serum uric acid and tophaceous gout. Most patients with hyperuricemia never develop gout, but having a condition that increases their serum levels of uric acid can increase their chances.11 It is important for clinicians to consider this increased risk when a patient with hemoglobinopathy presents with joint pain.
1. Garrod AB. The Nature and Treatment of Gout and Rheumatic Gout. 2nd ed. Walton and Maberly; 1859.
2. Jensen M, Oski FA, Nathan DG, Bunn HF. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J Clin Invest. 1975;55(3):469-477. doi:10.1172/JCI107953
3. Vázquez-Mellado J, Hernández-Cuevas CB, Alvarez-Hernández E, et al. The diagnostic value of the proposal for clinical gout diagnosis (CGD). Clin Rheumatol. 2012;31(3):429-434. doi:10.1007/s10067-011-1873-4
4. Kaufman DP, Kandle PF, Murray IV, et al. Physiology, Oxyhemoglobin Dissociation Curve. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499818/
5. Yudin J, Verhovsek M. How we diagnose and manage altered oxygen affinity hemoglobin variants. Am J Hematol. 2019;94(5):597-603. doi:10.1002/ajh.25425
6. Jefferson JA, Escudero E, Hurtado ME, et al. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis. 2002;39(6):1135-1142. doi:10.1053/ajkd.2002.33380
7. Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PLoS One. 2013;8(6):e66891. Published 2013 Jun 24. doi:10.1371/journal.pone.0066891
8. Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis. 2018;77(7):1048-1052. doi:10.1136/annrheumdis-2017-212288
9. Ballou SP, Khan MA, Kushner I, Harris JW. Secondary gout in hemoglobinopathies: report of two cases and review of the literature. Am J Hematol. 1977;2(4):397-402. doi:10.1002/ajh.2830020410
10. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. doi:10.1002/acr.21773
11. Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. Published 2019 Sep 26. doi:10.1038/s41572-019-0115-y
Hemoglobinopathies are inherited disorders of hemoglobin that alter oxygen binding capacity by affecting the production of a specific subset of globin chains or their structure.1 A lesser-known subtype, Syracuse hemoglobinopathy (SH), was first identified in 4 generations of a family in the 1970s.2 As with other disorders of hemoglobin structure, there is an inherent risk of increased cell breakdown and turnover. This case discusses the presentation of gout in a patient with a history of SH.
Case presentation
A 44-year-old man with known SH, tobacco use disorder, and shoulder osteoarthritis presented with pain and palpable nodular masses on bilateral elbows, metacarpophalangeal joints, and feet progressively over 5 years. Of note, he was initially misdiagnosed with polycythemia vera after an incidental finding of elevated hematocrit more than 10 years prior. His mother, maternal aunt, and maternal grandmother have all been treated for polycythemia vera.
On examination, there were irregular palpable masses of varying sizes, erythema, and tenderness over the second metacarpophalangeal joint of the left hand, bilateral elbows, and bilateral metatarsophalangeal joints. Laboratory studies were remarkable for 19.8 g/dL hemoglobin (reference range, 12.0-16.0 g/dL); 63.4% hematocrit (reference range, 37.0%-47.0%); 219 × 103 µL platelets (reference range, 150-450 × 103 µL); 79.3 fL mean corpuscular volume (reference range, 81.0-99.0 fL); 14 mg/dL blood urea nitrogen (reference range, 8-27 mg/dL); 1.18 mg/dL creatinine (reference range, 0.60-1.60 mg/dL); 3 mmol/h erythrocyte sedimentation rate (reference range, 0-30 mmol/h); 88 IU/L alkaline phosphatase (reference range, 34-130 IU/L); and 11.3 mg/dL uric acid (reference range, 2.4-7.9 mg/dL). Hemoglobin electrophoresis studies showed a 49% hemoglobin A1 (reference range, 95%-98%); 3.0% hemoglobin A2 (reference range, 2%-3%); 3.1% hemoglobin F (reference range, < 0.6%); and 44.9% hemoglobin Syracuse (reference range, absent). It was negative for JAK2 V617F mutation. An X-ray of the bilateral feet showed irregularity/erosion involving the medial border of the great toe metatarsal head, joint effusions, and sclerotic margins (Figure 1). A prominent plantar calcaneal spur was present (Figure 2). Synovial fluid analysis detected the presence of negatively birefringent needle-shaped urate crystals.
Per the Clinical Gout Diagnosis tool, which has a sensitivity of 97%, this patient scored high given the findings of greater than one attack of acute arthritis, mono/oligoarthritic attacks, podagra, erythema, probable tophi, and hyperuricemia. This raised the likelihood of his presentation being an acute flare of tophaceous gout.3 He was treated with colchicine and prednisone for acute exacerbation. Once the exacerbation subsided, the colchicine was discontinued, and allopurinol was added. The uric acid goal was < 6 mg/dL and was consistently maintained. Over the subsequent months, he reported mild joint pain if he stopped taking allopurinol but did not report a recurrence in disease exacerbation.
Discussion
Hemoglobin Syracuse was first identified in the early 1970s after the discovery of similar familial hemoglobinopathies unique for their high oxygen affinity hemoglobin.1 High oxygen affinity hemoglobin functions by causing a leftward shift in the hemoglobin dissociation curve and therefore slower off-loading of oxygen into tissues.4 The hypoxic state at the tissue level created by the hemoglobin binding tightly to oxygen promotes the production of erythropoietin, increasing red blood cell and hemoglobin production.5 A study looking at uric acid levels in patients living at high altitudes (which can imitate the low-oxygen state seen in high affinity hemoglobinopathy) theorized that increased erythroblast turnover in the setting of polycythemia involves increased purine metabolism and consequently, uric acid as a breakdown product.6 Uric acid levels have also been used as a marker for hypoxia in studies regarding sleep apnea. Tissue hypoxia can increase adenosine triphosphate breakdown. One byproduct of this breakdown is hypoxanthine, which is further metabolized by xanthine oxidase, which, in turn, produces uric acid.7
The relationship between elevated uric acid and gout was first studied in the mid-nineteenth century after Alfred Barring Garrod identified urate deposits in the articular cartilage of patients with gout.1 These urate deposits garner a proinflammatory response with the activation of the complement cascade, resulting in the recruitment of neutrophils, macrophages, and lymphocytes. Recurrent gout flares eventually result in a chronic granulomatous inflammatory response to the deposited crystals resulting in the classic tophi.8 A study looking at patients with thalassemia showed that while elevated serum uric acid levels were common in these patients, only 6% developed gout. Significant risk factors were noted to be intact spleen and inefficient urinary excretion of urea due to chronic kidney disease.9
Current treatment of gout flares consistsof pain control in the acute phase and prevention in the long-term setting. The first-line treatment for acute gout attack is colchicine, prednisone, or nonsteroidal anti-inflammatory drugs. Clinicians can consider switching or combining these therapies if ineffective or in the event of severe exacerbation. Prophylactic therapy involves urate-lowering agents, such as allopurinol and febuxostat.10
Conclusions
This case illustrates how a rare disorder of high oxygen affinity hemoglobin, SH, can present itself with findings of elevated serum uric acid and tophaceous gout. Most patients with hyperuricemia never develop gout, but having a condition that increases their serum levels of uric acid can increase their chances.11 It is important for clinicians to consider this increased risk when a patient with hemoglobinopathy presents with joint pain.
Hemoglobinopathies are inherited disorders of hemoglobin that alter oxygen binding capacity by affecting the production of a specific subset of globin chains or their structure.1 A lesser-known subtype, Syracuse hemoglobinopathy (SH), was first identified in 4 generations of a family in the 1970s.2 As with other disorders of hemoglobin structure, there is an inherent risk of increased cell breakdown and turnover. This case discusses the presentation of gout in a patient with a history of SH.
Case presentation
A 44-year-old man with known SH, tobacco use disorder, and shoulder osteoarthritis presented with pain and palpable nodular masses on bilateral elbows, metacarpophalangeal joints, and feet progressively over 5 years. Of note, he was initially misdiagnosed with polycythemia vera after an incidental finding of elevated hematocrit more than 10 years prior. His mother, maternal aunt, and maternal grandmother have all been treated for polycythemia vera.
On examination, there were irregular palpable masses of varying sizes, erythema, and tenderness over the second metacarpophalangeal joint of the left hand, bilateral elbows, and bilateral metatarsophalangeal joints. Laboratory studies were remarkable for 19.8 g/dL hemoglobin (reference range, 12.0-16.0 g/dL); 63.4% hematocrit (reference range, 37.0%-47.0%); 219 × 103 µL platelets (reference range, 150-450 × 103 µL); 79.3 fL mean corpuscular volume (reference range, 81.0-99.0 fL); 14 mg/dL blood urea nitrogen (reference range, 8-27 mg/dL); 1.18 mg/dL creatinine (reference range, 0.60-1.60 mg/dL); 3 mmol/h erythrocyte sedimentation rate (reference range, 0-30 mmol/h); 88 IU/L alkaline phosphatase (reference range, 34-130 IU/L); and 11.3 mg/dL uric acid (reference range, 2.4-7.9 mg/dL). Hemoglobin electrophoresis studies showed a 49% hemoglobin A1 (reference range, 95%-98%); 3.0% hemoglobin A2 (reference range, 2%-3%); 3.1% hemoglobin F (reference range, < 0.6%); and 44.9% hemoglobin Syracuse (reference range, absent). It was negative for JAK2 V617F mutation. An X-ray of the bilateral feet showed irregularity/erosion involving the medial border of the great toe metatarsal head, joint effusions, and sclerotic margins (Figure 1). A prominent plantar calcaneal spur was present (Figure 2). Synovial fluid analysis detected the presence of negatively birefringent needle-shaped urate crystals.
Per the Clinical Gout Diagnosis tool, which has a sensitivity of 97%, this patient scored high given the findings of greater than one attack of acute arthritis, mono/oligoarthritic attacks, podagra, erythema, probable tophi, and hyperuricemia. This raised the likelihood of his presentation being an acute flare of tophaceous gout.3 He was treated with colchicine and prednisone for acute exacerbation. Once the exacerbation subsided, the colchicine was discontinued, and allopurinol was added. The uric acid goal was < 6 mg/dL and was consistently maintained. Over the subsequent months, he reported mild joint pain if he stopped taking allopurinol but did not report a recurrence in disease exacerbation.
Discussion
Hemoglobin Syracuse was first identified in the early 1970s after the discovery of similar familial hemoglobinopathies unique for their high oxygen affinity hemoglobin.1 High oxygen affinity hemoglobin functions by causing a leftward shift in the hemoglobin dissociation curve and therefore slower off-loading of oxygen into tissues.4 The hypoxic state at the tissue level created by the hemoglobin binding tightly to oxygen promotes the production of erythropoietin, increasing red blood cell and hemoglobin production.5 A study looking at uric acid levels in patients living at high altitudes (which can imitate the low-oxygen state seen in high affinity hemoglobinopathy) theorized that increased erythroblast turnover in the setting of polycythemia involves increased purine metabolism and consequently, uric acid as a breakdown product.6 Uric acid levels have also been used as a marker for hypoxia in studies regarding sleep apnea. Tissue hypoxia can increase adenosine triphosphate breakdown. One byproduct of this breakdown is hypoxanthine, which is further metabolized by xanthine oxidase, which, in turn, produces uric acid.7
The relationship between elevated uric acid and gout was first studied in the mid-nineteenth century after Alfred Barring Garrod identified urate deposits in the articular cartilage of patients with gout.1 These urate deposits garner a proinflammatory response with the activation of the complement cascade, resulting in the recruitment of neutrophils, macrophages, and lymphocytes. Recurrent gout flares eventually result in a chronic granulomatous inflammatory response to the deposited crystals resulting in the classic tophi.8 A study looking at patients with thalassemia showed that while elevated serum uric acid levels were common in these patients, only 6% developed gout. Significant risk factors were noted to be intact spleen and inefficient urinary excretion of urea due to chronic kidney disease.9
Current treatment of gout flares consistsof pain control in the acute phase and prevention in the long-term setting. The first-line treatment for acute gout attack is colchicine, prednisone, or nonsteroidal anti-inflammatory drugs. Clinicians can consider switching or combining these therapies if ineffective or in the event of severe exacerbation. Prophylactic therapy involves urate-lowering agents, such as allopurinol and febuxostat.10
Conclusions
This case illustrates how a rare disorder of high oxygen affinity hemoglobin, SH, can present itself with findings of elevated serum uric acid and tophaceous gout. Most patients with hyperuricemia never develop gout, but having a condition that increases their serum levels of uric acid can increase their chances.11 It is important for clinicians to consider this increased risk when a patient with hemoglobinopathy presents with joint pain.
1. Garrod AB. The Nature and Treatment of Gout and Rheumatic Gout. 2nd ed. Walton and Maberly; 1859.
2. Jensen M, Oski FA, Nathan DG, Bunn HF. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J Clin Invest. 1975;55(3):469-477. doi:10.1172/JCI107953
3. Vázquez-Mellado J, Hernández-Cuevas CB, Alvarez-Hernández E, et al. The diagnostic value of the proposal for clinical gout diagnosis (CGD). Clin Rheumatol. 2012;31(3):429-434. doi:10.1007/s10067-011-1873-4
4. Kaufman DP, Kandle PF, Murray IV, et al. Physiology, Oxyhemoglobin Dissociation Curve. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499818/
5. Yudin J, Verhovsek M. How we diagnose and manage altered oxygen affinity hemoglobin variants. Am J Hematol. 2019;94(5):597-603. doi:10.1002/ajh.25425
6. Jefferson JA, Escudero E, Hurtado ME, et al. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis. 2002;39(6):1135-1142. doi:10.1053/ajkd.2002.33380
7. Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PLoS One. 2013;8(6):e66891. Published 2013 Jun 24. doi:10.1371/journal.pone.0066891
8. Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis. 2018;77(7):1048-1052. doi:10.1136/annrheumdis-2017-212288
9. Ballou SP, Khan MA, Kushner I, Harris JW. Secondary gout in hemoglobinopathies: report of two cases and review of the literature. Am J Hematol. 1977;2(4):397-402. doi:10.1002/ajh.2830020410
10. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. doi:10.1002/acr.21773
11. Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. Published 2019 Sep 26. doi:10.1038/s41572-019-0115-y
1. Garrod AB. The Nature and Treatment of Gout and Rheumatic Gout. 2nd ed. Walton and Maberly; 1859.
2. Jensen M, Oski FA, Nathan DG, Bunn HF. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J Clin Invest. 1975;55(3):469-477. doi:10.1172/JCI107953
3. Vázquez-Mellado J, Hernández-Cuevas CB, Alvarez-Hernández E, et al. The diagnostic value of the proposal for clinical gout diagnosis (CGD). Clin Rheumatol. 2012;31(3):429-434. doi:10.1007/s10067-011-1873-4
4. Kaufman DP, Kandle PF, Murray IV, et al. Physiology, Oxyhemoglobin Dissociation Curve. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499818/
5. Yudin J, Verhovsek M. How we diagnose and manage altered oxygen affinity hemoglobin variants. Am J Hematol. 2019;94(5):597-603. doi:10.1002/ajh.25425
6. Jefferson JA, Escudero E, Hurtado ME, et al. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis. 2002;39(6):1135-1142. doi:10.1053/ajkd.2002.33380
7. Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PLoS One. 2013;8(6):e66891. Published 2013 Jun 24. doi:10.1371/journal.pone.0066891
8. Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis. 2018;77(7):1048-1052. doi:10.1136/annrheumdis-2017-212288
9. Ballou SP, Khan MA, Kushner I, Harris JW. Secondary gout in hemoglobinopathies: report of two cases and review of the literature. Am J Hematol. 1977;2(4):397-402. doi:10.1002/ajh.2830020410
10. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. doi:10.1002/acr.21773
11. Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. Published 2019 Sep 26. doi:10.1038/s41572-019-0115-y
FLOTCH Syndrome: A Case of Leukonychia Totalis and Multiple Pilar Cysts
FLOTCH (leukonychia totalis-trichilemmal cysts-ciliary dystrophy syndrome) syndrome is a rare genetic cutaneous disorder primarily characterized by multiple recurrent trichilemmal pilar cysts and leukonychia. It may be associated with ciliary dystrophy, koilonychia, and/or less frequently renal calculi and pancreatitis. This disorder often presents in an autosomal-dominant pattern of inheritance. Leukonychia and associated pilar cysts originally were termed Bauer syndrome in 1920 and later described in 1986 as FLOTCH syndrome secondary to the association with ciliary dystrophy. 1,2 The term FLOTCH was coined by Friedel et al 1 to describe a combination of diagnoses experienced by a family in which several members had multiple pilar cysts, leukonychia, and ciliary dystrophy. We present a 25-year-old Black woman with suspected FLOTCH syndrome who was seen in our clinic for enlarging cysts.
Case Report
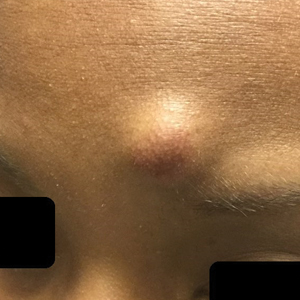
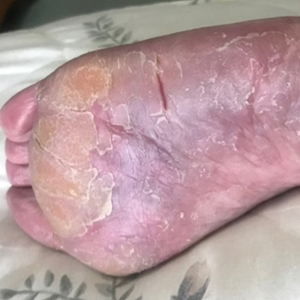
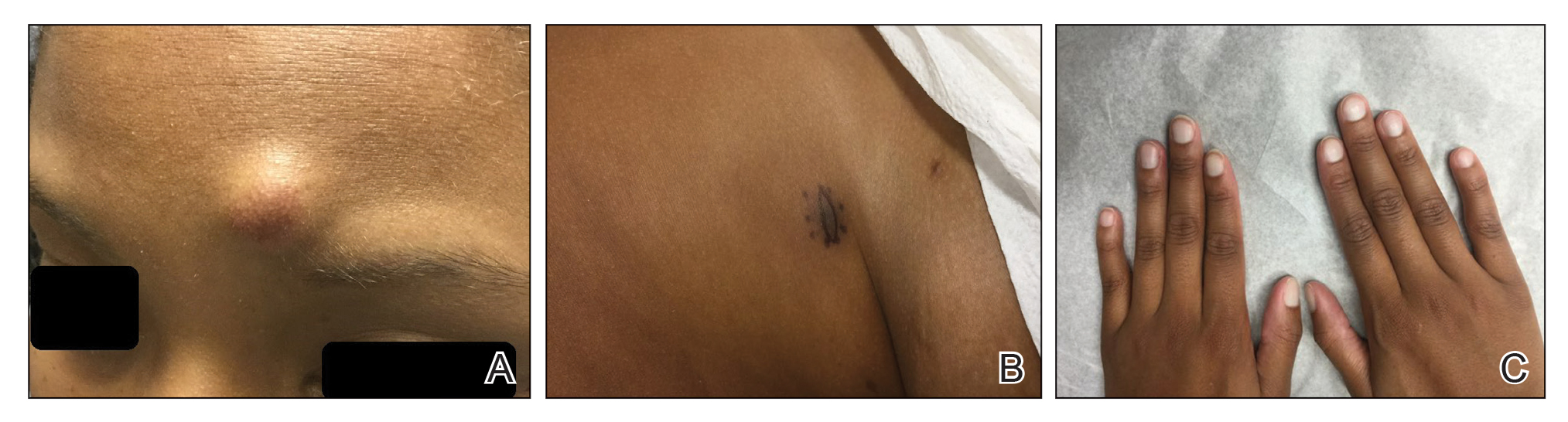
A 25-year-old Black woman with no notable medical history presented to the clinic for a surgical evaluation of cysts of several years’ duration that were enlarging and tender. Physical examination revealed multiple firm, fixed, tender nodules on the left superior parietal scalp, left inferior frontal scalp (Figure 1A), right inferior parietal scalp, right central postauricular skin, and right inferior occipital scalp. Similar-appearing cysts measuring 1.5 to 2 cm were seen on the left rib cage (Figure 1B) and left lateral forearm. Upon further examination, there was homogeneous, nonblanchable, white discoloration of all 10 fingernails consistent with true leukonychia (Figure 1C). When questioned about the nails, the patient stated they had been this color her whole life. Moreover, the patient confirmed that her brother’s nails had a similar appearance.
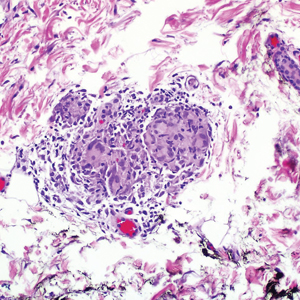
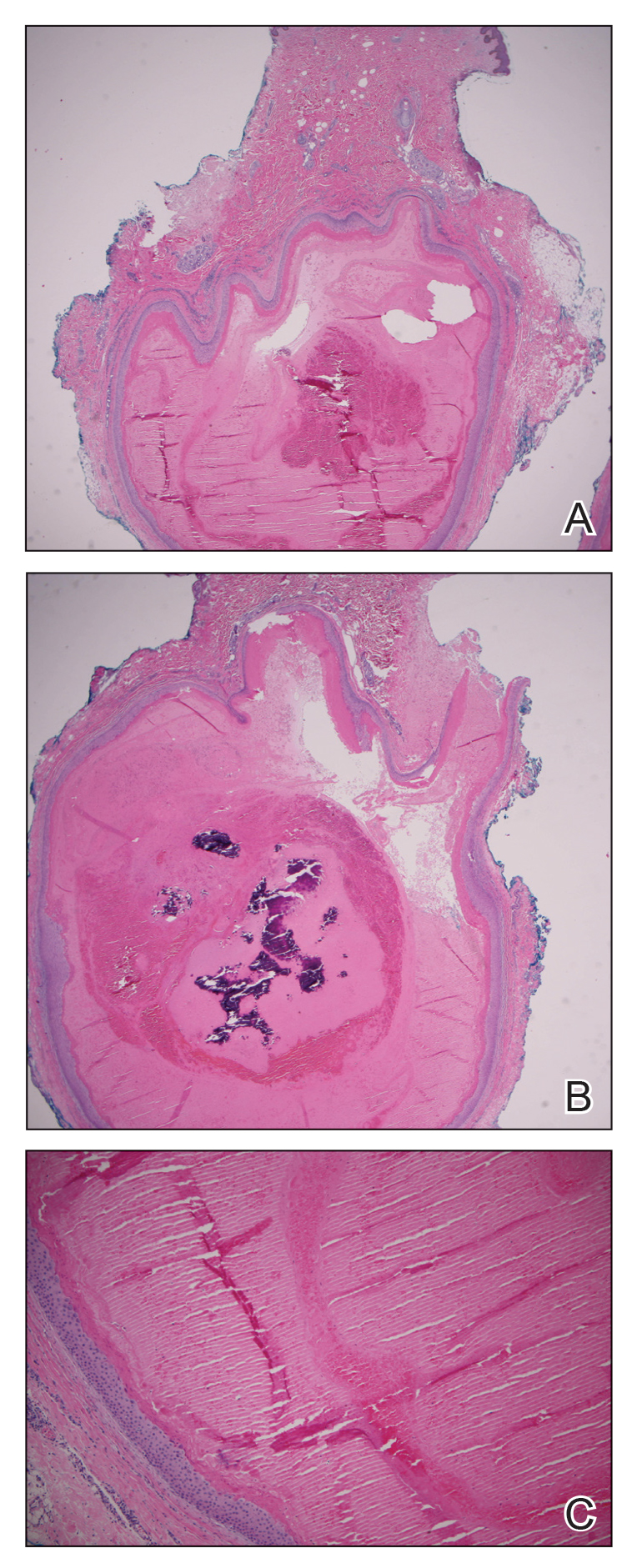
The patient subsequently underwent elliptical excision of the cysts located on the left medial forehead and left rib cage, and histopathology revealed trichilemmal pilar cysts with dystrophic calcification, dermal fibrosis, and mild chronic inflammation (Figure 2). The pathology report also noted that the anatomic site was somewhat unusual; however, the features were otherwise typical and diagnostic. Given the presentation of multiple pilar cysts throughout the body, leukonychia totalis, and positive family history, the patient was diagnosed with FLOTCH syndrome. Unfortunately, the patient was lost to follow-up following the excision, and no further management could be provided.
Comment
Leukonychia is an abnormality of the nail that results in a visible distribution of white color across the nail plate. It can be classified as totalis when covering the entire nail or partialis when covering localized areas of the nail. The disease also is categorized as acquired or inherited. Acquired leukonychia may appear after damage to a particular area of the nail or secondary to an underlying systemic disease, clinically appearing as white puncta or transverse striae. Hereditary leukonychia is rare, primarily covering the entire nail (totalis), and often is inherited in an autosomal-dominant pattern.3,4 The appearance of this disease can be an isolated occurrence or may be a component of a condition such as FLOTCH syndrome, as proposed in this case.
Pilar cysts (also known as trichilemmal cysts) are benign, slowly growing, firm, subcutaneous nodules that are similar to epidermoid cysts but arise from the root sheaths of hair follicles. Pilar cysts are inherited in an autosomal-dominant pattern and are caused by a mutation involving a 2-hit mechanism of variants of the phospholipase C delta 1 gene, PLCD1. Patients typically present with multiple cysts,5 as in our case.
This association of leukonychia and multiple pilar cysts previously has been reported in 7 family lines.1-3,6-9 The molecular basis of FLOTCH syndrome is unknown, and these combined diagnoses may be of syndromic nature. Histologic observations of leukonychia and the mechanism of the creation of pilar cysts suggest derivation from similar abnormal keratinization in the nail beds and hair follicles, respectively.6
The first familial association between leukonychia totalis and sebaceous cysts was described by Bauer2 in 1920. In 1975, Bushkell and Gorlin7 reported a similar inherited association with the addition of a history of renal calculi. In 1986, Friedel et al1 coined the term FLOTCH syndrome when reporting a case of an affected family presenting with leukonychia, recurrent cysts, and ciliary dystrophy. Slee et al8 reported 2 cases of pancreatitis experienced by patients presenting with these cysts and leukonychia. The etiology of the pancreatitis was unknown, leading researchers to believe it may be a complication associated with the spectrum of diseases.8 In 2008, Morin et al6 proposed that those with linked leukonychia and trichilemmal cysts may be at risk for neuromas or spinal tumors and suggested systematic screening after observing a family member with an ependymoma and bilateral multiple acoustic tumors. Rodríguez-Lojo et al3 described a 5-generation family with leukonychia totalis and numerous pilar cysts. Mutoh et al9 reported another 5-generation family with associated leukonychia and multiple pilar cysts as well as koilonychia. One family member had a reported history of renal calculus.9
In our case, FLOTCH syndrome was suspected given the patient’s concurrent pilar and follicular infundibular cysts. No specific treatment was indicated; however, as seen in prior cases and in ours, many patients prefer to have the cysts excised. A more comprehensive investigation could have revealed other associations, such as ciliary dystrophy, renal calculi, or pancreatitis. It is possible that in conjunction with the syndrome, patients could develop other such clinical manifestations. Pilar cysts most frequently are found on the scalp, yet in patients with concurrent leukonychia, the cysts have been shown to also develop in other regions of the body, as seen in our patient and in the case reported by Mutoh et al.9 Given the autosomal-dominant nature of this disease and the keratinizing structures affected, we confer with the hypotheses that a general keratin dysfunction is suspected. Further investigation is needed to determine the exact altered genetic mechanism or deficiency that may be causing this abnormal keratinization as well as a more extensive examination of patients to confirm if other described symptoms may be related.
- Friedel J, Heid E, Grosshans E. The FLOTCH syndrome. familial occurrence of total leukonychia, trichilemmal cysts and ciliary dystrophy with dominant autosomal heredity [in French]. Ann Dermatol Venereol. 1986;113:549-553.
- Bauer AW. Beiträge zur klinischen Konstitutionspathologie, V. heredofamiliäre leukonychie und multiple atherombilderung der kopfhaut. Z Menschl Vererb. Konstitutitionslehre. 1920;5:47-48.
- Rodríguez-Lojo R, Del Pozo J, Sacristán F, et al. Leukonychia totalis associated with multiple pilar cysts: report of a five-generation family: FLOTCH syndrome? Eur J Dermatol. 2011;21:484-486.
- Claudel CD, Zic JA, Boyd AS. Idiopathic leukonychia totalis and partialis in a 12-year-old patient. J Am Acad Dermatol. 2001;44:379-380.
- Hörer S, Marrakchi S, Radner FPW, et al. A monoallelic two-hit mechanism in PLCD1 explains the genetic pathogenesis of hereditary trichilemmal cyst formation. J Invest Dermatol. 2019;139:2154-2163.e5.
- Morin G, Desenclos C, Jeanpetit C, et al. Additional familial case of subtotal leukonychia and sebaceous cysts (Bauer syndrome): belong the nervous tumours to the phenotype? Eur J Med Genet. 2008;51:436-443.
- Bushkell LL, Gorlin RJ. Leukonychia totalis, multiple sebaceous cysts, and renal calculi. Arch Dermatol. 1975;111:899-901.
- Slee JJ, Wallman IS, Goldblatt J. A syndrome or leukonychia totalis and multiple sebaceous cysts. Clin Dysmorphol. 1997;6:229-233.
- Mutoh M, Niiyama S, Nishikawa S, et al. A syndrome of leukonychia, koilonychia and multiple pilar cysts. Acta Derm Venereol. 2015;95:249-250. doi:10.2340/00015555-1893
FLOTCH (leukonychia totalis-trichilemmal cysts-ciliary dystrophy syndrome) syndrome is a rare genetic cutaneous disorder primarily characterized by multiple recurrent trichilemmal pilar cysts and leukonychia. It may be associated with ciliary dystrophy, koilonychia, and/or less frequently renal calculi and pancreatitis. This disorder often presents in an autosomal-dominant pattern of inheritance. Leukonychia and associated pilar cysts originally were termed Bauer syndrome in 1920 and later described in 1986 as FLOTCH syndrome secondary to the association with ciliary dystrophy. 1,2 The term FLOTCH was coined by Friedel et al 1 to describe a combination of diagnoses experienced by a family in which several members had multiple pilar cysts, leukonychia, and ciliary dystrophy. We present a 25-year-old Black woman with suspected FLOTCH syndrome who was seen in our clinic for enlarging cysts.
Case Report
A 25-year-old Black woman with no notable medical history presented to the clinic for a surgical evaluation of cysts of several years’ duration that were enlarging and tender. Physical examination revealed multiple firm, fixed, tender nodules on the left superior parietal scalp, left inferior frontal scalp (Figure 1A), right inferior parietal scalp, right central postauricular skin, and right inferior occipital scalp. Similar-appearing cysts measuring 1.5 to 2 cm were seen on the left rib cage (Figure 1B) and left lateral forearm. Upon further examination, there was homogeneous, nonblanchable, white discoloration of all 10 fingernails consistent with true leukonychia (Figure 1C). When questioned about the nails, the patient stated they had been this color her whole life. Moreover, the patient confirmed that her brother’s nails had a similar appearance.

The patient subsequently underwent elliptical excision of the cysts located on the left medial forehead and left rib cage, and histopathology revealed trichilemmal pilar cysts with dystrophic calcification, dermal fibrosis, and mild chronic inflammation (Figure 2). The pathology report also noted that the anatomic site was somewhat unusual; however, the features were otherwise typical and diagnostic. Given the presentation of multiple pilar cysts throughout the body, leukonychia totalis, and positive family history, the patient was diagnosed with FLOTCH syndrome. Unfortunately, the patient was lost to follow-up following the excision, and no further management could be provided.

Comment
Leukonychia is an abnormality of the nail that results in a visible distribution of white color across the nail plate. It can be classified as totalis when covering the entire nail or partialis when covering localized areas of the nail. The disease also is categorized as acquired or inherited. Acquired leukonychia may appear after damage to a particular area of the nail or secondary to an underlying systemic disease, clinically appearing as white puncta or transverse striae. Hereditary leukonychia is rare, primarily covering the entire nail (totalis), and often is inherited in an autosomal-dominant pattern.3,4 The appearance of this disease can be an isolated occurrence or may be a component of a condition such as FLOTCH syndrome, as proposed in this case.
Pilar cysts (also known as trichilemmal cysts) are benign, slowly growing, firm, subcutaneous nodules that are similar to epidermoid cysts but arise from the root sheaths of hair follicles. Pilar cysts are inherited in an autosomal-dominant pattern and are caused by a mutation involving a 2-hit mechanism of variants of the phospholipase C delta 1 gene, PLCD1. Patients typically present with multiple cysts,5 as in our case.
This association of leukonychia and multiple pilar cysts previously has been reported in 7 family lines.1-3,6-9 The molecular basis of FLOTCH syndrome is unknown, and these combined diagnoses may be of syndromic nature. Histologic observations of leukonychia and the mechanism of the creation of pilar cysts suggest derivation from similar abnormal keratinization in the nail beds and hair follicles, respectively.6
The first familial association between leukonychia totalis and sebaceous cysts was described by Bauer2 in 1920. In 1975, Bushkell and Gorlin7 reported a similar inherited association with the addition of a history of renal calculi. In 1986, Friedel et al1 coined the term FLOTCH syndrome when reporting a case of an affected family presenting with leukonychia, recurrent cysts, and ciliary dystrophy. Slee et al8 reported 2 cases of pancreatitis experienced by patients presenting with these cysts and leukonychia. The etiology of the pancreatitis was unknown, leading researchers to believe it may be a complication associated with the spectrum of diseases.8 In 2008, Morin et al6 proposed that those with linked leukonychia and trichilemmal cysts may be at risk for neuromas or spinal tumors and suggested systematic screening after observing a family member with an ependymoma and bilateral multiple acoustic tumors. Rodríguez-Lojo et al3 described a 5-generation family with leukonychia totalis and numerous pilar cysts. Mutoh et al9 reported another 5-generation family with associated leukonychia and multiple pilar cysts as well as koilonychia. One family member had a reported history of renal calculus.9
In our case, FLOTCH syndrome was suspected given the patient’s concurrent pilar and follicular infundibular cysts. No specific treatment was indicated; however, as seen in prior cases and in ours, many patients prefer to have the cysts excised. A more comprehensive investigation could have revealed other associations, such as ciliary dystrophy, renal calculi, or pancreatitis. It is possible that in conjunction with the syndrome, patients could develop other such clinical manifestations. Pilar cysts most frequently are found on the scalp, yet in patients with concurrent leukonychia, the cysts have been shown to also develop in other regions of the body, as seen in our patient and in the case reported by Mutoh et al.9 Given the autosomal-dominant nature of this disease and the keratinizing structures affected, we confer with the hypotheses that a general keratin dysfunction is suspected. Further investigation is needed to determine the exact altered genetic mechanism or deficiency that may be causing this abnormal keratinization as well as a more extensive examination of patients to confirm if other described symptoms may be related.
FLOTCH (leukonychia totalis-trichilemmal cysts-ciliary dystrophy syndrome) syndrome is a rare genetic cutaneous disorder primarily characterized by multiple recurrent trichilemmal pilar cysts and leukonychia. It may be associated with ciliary dystrophy, koilonychia, and/or less frequently renal calculi and pancreatitis. This disorder often presents in an autosomal-dominant pattern of inheritance. Leukonychia and associated pilar cysts originally were termed Bauer syndrome in 1920 and later described in 1986 as FLOTCH syndrome secondary to the association with ciliary dystrophy. 1,2 The term FLOTCH was coined by Friedel et al 1 to describe a combination of diagnoses experienced by a family in which several members had multiple pilar cysts, leukonychia, and ciliary dystrophy. We present a 25-year-old Black woman with suspected FLOTCH syndrome who was seen in our clinic for enlarging cysts.
Case Report
A 25-year-old Black woman with no notable medical history presented to the clinic for a surgical evaluation of cysts of several years’ duration that were enlarging and tender. Physical examination revealed multiple firm, fixed, tender nodules on the left superior parietal scalp, left inferior frontal scalp (Figure 1A), right inferior parietal scalp, right central postauricular skin, and right inferior occipital scalp. Similar-appearing cysts measuring 1.5 to 2 cm were seen on the left rib cage (Figure 1B) and left lateral forearm. Upon further examination, there was homogeneous, nonblanchable, white discoloration of all 10 fingernails consistent with true leukonychia (Figure 1C). When questioned about the nails, the patient stated they had been this color her whole life. Moreover, the patient confirmed that her brother’s nails had a similar appearance.

The patient subsequently underwent elliptical excision of the cysts located on the left medial forehead and left rib cage, and histopathology revealed trichilemmal pilar cysts with dystrophic calcification, dermal fibrosis, and mild chronic inflammation (Figure 2). The pathology report also noted that the anatomic site was somewhat unusual; however, the features were otherwise typical and diagnostic. Given the presentation of multiple pilar cysts throughout the body, leukonychia totalis, and positive family history, the patient was diagnosed with FLOTCH syndrome. Unfortunately, the patient was lost to follow-up following the excision, and no further management could be provided.

Comment
Leukonychia is an abnormality of the nail that results in a visible distribution of white color across the nail plate. It can be classified as totalis when covering the entire nail or partialis when covering localized areas of the nail. The disease also is categorized as acquired or inherited. Acquired leukonychia may appear after damage to a particular area of the nail or secondary to an underlying systemic disease, clinically appearing as white puncta or transverse striae. Hereditary leukonychia is rare, primarily covering the entire nail (totalis), and often is inherited in an autosomal-dominant pattern.3,4 The appearance of this disease can be an isolated occurrence or may be a component of a condition such as FLOTCH syndrome, as proposed in this case.
Pilar cysts (also known as trichilemmal cysts) are benign, slowly growing, firm, subcutaneous nodules that are similar to epidermoid cysts but arise from the root sheaths of hair follicles. Pilar cysts are inherited in an autosomal-dominant pattern and are caused by a mutation involving a 2-hit mechanism of variants of the phospholipase C delta 1 gene, PLCD1. Patients typically present with multiple cysts,5 as in our case.
This association of leukonychia and multiple pilar cysts previously has been reported in 7 family lines.1-3,6-9 The molecular basis of FLOTCH syndrome is unknown, and these combined diagnoses may be of syndromic nature. Histologic observations of leukonychia and the mechanism of the creation of pilar cysts suggest derivation from similar abnormal keratinization in the nail beds and hair follicles, respectively.6
The first familial association between leukonychia totalis and sebaceous cysts was described by Bauer2 in 1920. In 1975, Bushkell and Gorlin7 reported a similar inherited association with the addition of a history of renal calculi. In 1986, Friedel et al1 coined the term FLOTCH syndrome when reporting a case of an affected family presenting with leukonychia, recurrent cysts, and ciliary dystrophy. Slee et al8 reported 2 cases of pancreatitis experienced by patients presenting with these cysts and leukonychia. The etiology of the pancreatitis was unknown, leading researchers to believe it may be a complication associated with the spectrum of diseases.8 In 2008, Morin et al6 proposed that those with linked leukonychia and trichilemmal cysts may be at risk for neuromas or spinal tumors and suggested systematic screening after observing a family member with an ependymoma and bilateral multiple acoustic tumors. Rodríguez-Lojo et al3 described a 5-generation family with leukonychia totalis and numerous pilar cysts. Mutoh et al9 reported another 5-generation family with associated leukonychia and multiple pilar cysts as well as koilonychia. One family member had a reported history of renal calculus.9
In our case, FLOTCH syndrome was suspected given the patient’s concurrent pilar and follicular infundibular cysts. No specific treatment was indicated; however, as seen in prior cases and in ours, many patients prefer to have the cysts excised. A more comprehensive investigation could have revealed other associations, such as ciliary dystrophy, renal calculi, or pancreatitis. It is possible that in conjunction with the syndrome, patients could develop other such clinical manifestations. Pilar cysts most frequently are found on the scalp, yet in patients with concurrent leukonychia, the cysts have been shown to also develop in other regions of the body, as seen in our patient and in the case reported by Mutoh et al.9 Given the autosomal-dominant nature of this disease and the keratinizing structures affected, we confer with the hypotheses that a general keratin dysfunction is suspected. Further investigation is needed to determine the exact altered genetic mechanism or deficiency that may be causing this abnormal keratinization as well as a more extensive examination of patients to confirm if other described symptoms may be related.
- Friedel J, Heid E, Grosshans E. The FLOTCH syndrome. familial occurrence of total leukonychia, trichilemmal cysts and ciliary dystrophy with dominant autosomal heredity [in French]. Ann Dermatol Venereol. 1986;113:549-553.
- Bauer AW. Beiträge zur klinischen Konstitutionspathologie, V. heredofamiliäre leukonychie und multiple atherombilderung der kopfhaut. Z Menschl Vererb. Konstitutitionslehre. 1920;5:47-48.
- Rodríguez-Lojo R, Del Pozo J, Sacristán F, et al. Leukonychia totalis associated with multiple pilar cysts: report of a five-generation family: FLOTCH syndrome? Eur J Dermatol. 2011;21:484-486.
- Claudel CD, Zic JA, Boyd AS. Idiopathic leukonychia totalis and partialis in a 12-year-old patient. J Am Acad Dermatol. 2001;44:379-380.
- Hörer S, Marrakchi S, Radner FPW, et al. A monoallelic two-hit mechanism in PLCD1 explains the genetic pathogenesis of hereditary trichilemmal cyst formation. J Invest Dermatol. 2019;139:2154-2163.e5.
- Morin G, Desenclos C, Jeanpetit C, et al. Additional familial case of subtotal leukonychia and sebaceous cysts (Bauer syndrome): belong the nervous tumours to the phenotype? Eur J Med Genet. 2008;51:436-443.
- Bushkell LL, Gorlin RJ. Leukonychia totalis, multiple sebaceous cysts, and renal calculi. Arch Dermatol. 1975;111:899-901.
- Slee JJ, Wallman IS, Goldblatt J. A syndrome or leukonychia totalis and multiple sebaceous cysts. Clin Dysmorphol. 1997;6:229-233.
- Mutoh M, Niiyama S, Nishikawa S, et al. A syndrome of leukonychia, koilonychia and multiple pilar cysts. Acta Derm Venereol. 2015;95:249-250. doi:10.2340/00015555-1893
- Friedel J, Heid E, Grosshans E. The FLOTCH syndrome. familial occurrence of total leukonychia, trichilemmal cysts and ciliary dystrophy with dominant autosomal heredity [in French]. Ann Dermatol Venereol. 1986;113:549-553.
- Bauer AW. Beiträge zur klinischen Konstitutionspathologie, V. heredofamiliäre leukonychie und multiple atherombilderung der kopfhaut. Z Menschl Vererb. Konstitutitionslehre. 1920;5:47-48.
- Rodríguez-Lojo R, Del Pozo J, Sacristán F, et al. Leukonychia totalis associated with multiple pilar cysts: report of a five-generation family: FLOTCH syndrome? Eur J Dermatol. 2011;21:484-486.
- Claudel CD, Zic JA, Boyd AS. Idiopathic leukonychia totalis and partialis in a 12-year-old patient. J Am Acad Dermatol. 2001;44:379-380.
- Hörer S, Marrakchi S, Radner FPW, et al. A monoallelic two-hit mechanism in PLCD1 explains the genetic pathogenesis of hereditary trichilemmal cyst formation. J Invest Dermatol. 2019;139:2154-2163.e5.
- Morin G, Desenclos C, Jeanpetit C, et al. Additional familial case of subtotal leukonychia and sebaceous cysts (Bauer syndrome): belong the nervous tumours to the phenotype? Eur J Med Genet. 2008;51:436-443.
- Bushkell LL, Gorlin RJ. Leukonychia totalis, multiple sebaceous cysts, and renal calculi. Arch Dermatol. 1975;111:899-901.
- Slee JJ, Wallman IS, Goldblatt J. A syndrome or leukonychia totalis and multiple sebaceous cysts. Clin Dysmorphol. 1997;6:229-233.
- Mutoh M, Niiyama S, Nishikawa S, et al. A syndrome of leukonychia, koilonychia and multiple pilar cysts. Acta Derm Venereol. 2015;95:249-250. doi:10.2340/00015555-1893
PRACTICE POINTS
- FLOTCH (leukonychia totalis-trichilemmal cysts-ciliary dystrophy syndrome) syndrome is an extremely rare condition that presents with multiple pilar cysts and leukonychia totalis. Pilar cysts in unusual locations along with distinct nail changes should prompt clinicians to consider further investigation for conditions such as FLOTCH syndrome.
- Although FLOTCH syndrome has been associated with other conditions such as ciliary dystrophy, renal calculi, pancreatitis, and central nervous system tumors, this does not preclude an extensive workup. Rather, careful family history may be the best predictor of clinical manifestations along the spectrum of this disease.
Granulomatous Dermatitis in a Patient With Cholangiocarcinoma Treated With BRAF and MEK Inhibitors
To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.
A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.
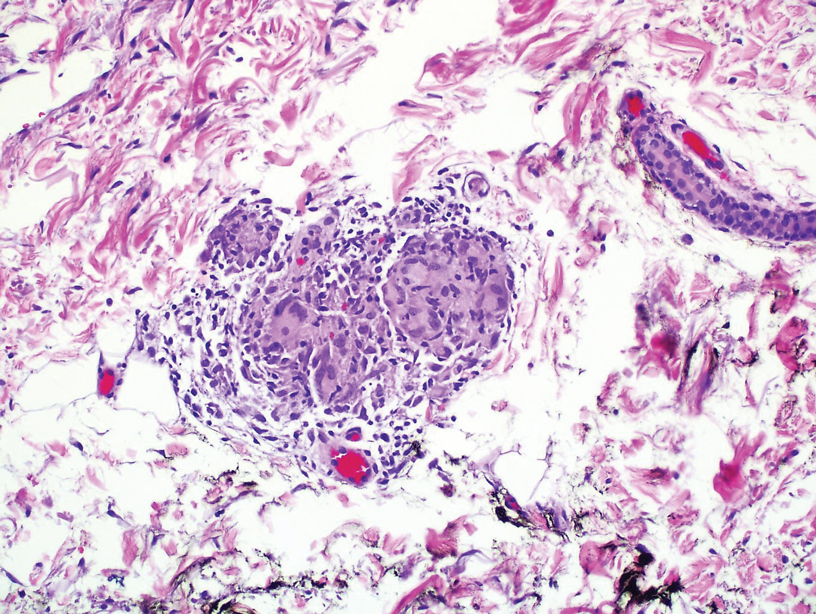
BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20
Drug-induced GD most likely is caused by vascular insults that lead to deposition of immune complexes in vessels causing inflammation and a consequent granulomatous infiltrate.21,22 Although cordlike lesions in the subcutaneous tissue on the trunk commonly are reported, the presentation of GD can vary considerably. Other presentations include areas of violaceous or erythematous patches or plaques on the limbs, intertriginous areas, and upper trunk. Diffuse macular erythema or small flesh-colored papules also can be observed.23
Granulomatous dermatitis secondary to drug reactions can have varying morphologies. The infiltrate often can have an interstitial appearance with the presence of lymphocytes, plasma cells, histiocytes, eosinophils, and multinucleated giant cells.24 These findings can be confused with interstitial granuloma annulare. Other cases, such as in our patient, can have discrete granulomata formation with a sarcoidlike appearance. These naked granulomas lack surrounding inflammation and suggest a differential diagnosis of sarcoidosis and infection. Use of immune checkpoint inhibitors (CIs) and kinase inhibitors has been proven to cause sarcoidosislike reactions.25 The development of granulomatous/sarcoidlike lesions associated with the use of BRAF and MEK inhibitors may clinically and radiographically mimic disease recurrence. An awareness of this type of reaction by clinicians and pathologists is important to ensure appropriate management in patients who develop GD.26
Checkpoint inhibitor–induced GD that remains asymptomatic does not necessarily warrant treatment; however, corticosteroid use and elimination of CI therapies have resolved GD in prior cases. Responsiveness of the cancer to CI therapy and severity of GD symptoms should be considered before discontinuation of a CI trial.25
One case report described complete resolution of a GD eruption without interruption of the scheduled BRAF and MEK inhibitor therapies for the treatment of metastatic melanoma. There was no reported use of a steroidal cream or other topical medication to aid in controlling the eruption.27 The exact mechanism of how GD resolves while continuing therapy is unknown; however, it has been suggested that a GD eruption may be the consequence of a BRAF and MEK inhibitor–mediated immune response against a subclinical area of metastatic melanoma.28 If the immune response successfully eliminates the subclinical tumor, one could postulate that the inflammatory response and granulomatous eruption would resolve. Future studies are necessary to further elucidate the exact mechanisms involved.
There have been several case reports of GD with vemurafenib treatment,29,30 1 report of GD and erythema induratum with vemurafenib and cobimetinib treatment,31 2 reports of GD with dabrafenib treatment,27,30 and a few reports of GD with the BRAF inhibitor dabrafenib combined with the MEK inhibitor trametinib,28,32,33 all for the treatment of metastatic melanoma. Additionally, a report described a 3-year-old boy who developed GD secondary to vemurafenib for the treatment of Langerhans cell histiocytosis.34 We present a unique case of BRAF and MEK inhibitor therapy–induced GD in the treatment of metastatic cholangiocarcinoma with vemurafenib and cobimetinib.
BRAF and MEK inhibitor therapy is used in patients with metastatic melanomas with a positive BRAF V600E mutation. Due to advancements in next-generation DNA sequencing, these therapies also are being tested in clinical trials for use in the treatment of other cancers with the same checkpoint mutation, such as metastatic cholangiocarcinoma. Cutaneous reactions frequently are documented side effects that occur during treatment with BRAF and MEK inhibitors; GD is an uncommon finding. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of other cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation.
- Mackiewicz J, Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Comtemp Oncol (Pozn). 2018;22:68-72.
- Jovanovic B, Krockel D, Linden D, et al. Lack of cytoplasmic ERK activation is an independent adverse prognostic factor in primary cutaneous melanoma. J Invest Dermatol. 2008;128:2696-2704.
- Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10:1103-1114.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Casey D, Demko S, Sinha A, et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin Cancer Res. 2021;27;4142-4146
- Flaherty K, Davies MA, Grob JJ, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) fl trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. P9502.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Karaszewska B, Schachter J, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(suppl 6):vi552-vi587.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advance BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Once. 2016;17:1248-1260.
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8:E32-E38.
- Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study [published online July 11, 2018]. JCO Precis Oncol. doi:10.1200/PO.18.00122
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331.
- Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16INK4A alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727.
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244.
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180.
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712.
- Bunyatov T, Zhao A, Kovalenko J, et al. Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV. J Gastrointest Oncol. 2019;10:815-820.
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102.
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479.
- Rosenbach M, English JC. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33:373-387.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol 2012;166:775-783.
- Calonje JE, Brenn T, Lazar A, Billings S. Lichenoid and interface dermatitis. In: McKee’s Pathology of the Skin. 5th ed. China: Elsevier Limited: 2018;7:241-282.
- Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13:1076-1082.
- Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6:14.
- Garrido MC, Gutiérrez C, Riveiro-Falkenbach E, et al. BRAF inhibitor-induced antitumoral granulomatous dermatitis eruption in advanced melanoma. Am J Dermatopathol. 2015;37:795-798.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307‐311.
- Ong ELH, Sinha R, Jmor S, et al. BRAF inhibitor-associated granulomatous dermatitis: a report of 3 cases. Am J of Dermatopathol. 2019;41:214-217.
- Wali GN, Stonard C, Espinosa O, et al. Persistent granulomatous cutaneous drug eruption to a BRAF inhibitor. J Am Acad Dermatol. 2017;76(suppl 1):AB195.
- Aj lafolla M, Ramsay J, Wismer J, et al. Cobimetinib- and vemurafenib-induced granulomatous dermatitis and erythema induratum: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847358
- Jansen YJ, Janssens P, Hoorens A, et al. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550‐554.
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Chen L, His A, Kothari A, et al. Granulomatous dermatitis secondary to vemurafenib in a child with Langerhans cell histiocytosis. Pediatr Dermatol. 2018;35:E402-E403.
To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.

A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.

BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20
Drug-induced GD most likely is caused by vascular insults that lead to deposition of immune complexes in vessels causing inflammation and a consequent granulomatous infiltrate.21,22 Although cordlike lesions in the subcutaneous tissue on the trunk commonly are reported, the presentation of GD can vary considerably. Other presentations include areas of violaceous or erythematous patches or plaques on the limbs, intertriginous areas, and upper trunk. Diffuse macular erythema or small flesh-colored papules also can be observed.23
Granulomatous dermatitis secondary to drug reactions can have varying morphologies. The infiltrate often can have an interstitial appearance with the presence of lymphocytes, plasma cells, histiocytes, eosinophils, and multinucleated giant cells.24 These findings can be confused with interstitial granuloma annulare. Other cases, such as in our patient, can have discrete granulomata formation with a sarcoidlike appearance. These naked granulomas lack surrounding inflammation and suggest a differential diagnosis of sarcoidosis and infection. Use of immune checkpoint inhibitors (CIs) and kinase inhibitors has been proven to cause sarcoidosislike reactions.25 The development of granulomatous/sarcoidlike lesions associated with the use of BRAF and MEK inhibitors may clinically and radiographically mimic disease recurrence. An awareness of this type of reaction by clinicians and pathologists is important to ensure appropriate management in patients who develop GD.26
Checkpoint inhibitor–induced GD that remains asymptomatic does not necessarily warrant treatment; however, corticosteroid use and elimination of CI therapies have resolved GD in prior cases. Responsiveness of the cancer to CI therapy and severity of GD symptoms should be considered before discontinuation of a CI trial.25
One case report described complete resolution of a GD eruption without interruption of the scheduled BRAF and MEK inhibitor therapies for the treatment of metastatic melanoma. There was no reported use of a steroidal cream or other topical medication to aid in controlling the eruption.27 The exact mechanism of how GD resolves while continuing therapy is unknown; however, it has been suggested that a GD eruption may be the consequence of a BRAF and MEK inhibitor–mediated immune response against a subclinical area of metastatic melanoma.28 If the immune response successfully eliminates the subclinical tumor, one could postulate that the inflammatory response and granulomatous eruption would resolve. Future studies are necessary to further elucidate the exact mechanisms involved.
There have been several case reports of GD with vemurafenib treatment,29,30 1 report of GD and erythema induratum with vemurafenib and cobimetinib treatment,31 2 reports of GD with dabrafenib treatment,27,30 and a few reports of GD with the BRAF inhibitor dabrafenib combined with the MEK inhibitor trametinib,28,32,33 all for the treatment of metastatic melanoma. Additionally, a report described a 3-year-old boy who developed GD secondary to vemurafenib for the treatment of Langerhans cell histiocytosis.34 We present a unique case of BRAF and MEK inhibitor therapy–induced GD in the treatment of metastatic cholangiocarcinoma with vemurafenib and cobimetinib.
BRAF and MEK inhibitor therapy is used in patients with metastatic melanomas with a positive BRAF V600E mutation. Due to advancements in next-generation DNA sequencing, these therapies also are being tested in clinical trials for use in the treatment of other cancers with the same checkpoint mutation, such as metastatic cholangiocarcinoma. Cutaneous reactions frequently are documented side effects that occur during treatment with BRAF and MEK inhibitors; GD is an uncommon finding. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of other cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation.
To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.

A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.

BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20
Drug-induced GD most likely is caused by vascular insults that lead to deposition of immune complexes in vessels causing inflammation and a consequent granulomatous infiltrate.21,22 Although cordlike lesions in the subcutaneous tissue on the trunk commonly are reported, the presentation of GD can vary considerably. Other presentations include areas of violaceous or erythematous patches or plaques on the limbs, intertriginous areas, and upper trunk. Diffuse macular erythema or small flesh-colored papules also can be observed.23
Granulomatous dermatitis secondary to drug reactions can have varying morphologies. The infiltrate often can have an interstitial appearance with the presence of lymphocytes, plasma cells, histiocytes, eosinophils, and multinucleated giant cells.24 These findings can be confused with interstitial granuloma annulare. Other cases, such as in our patient, can have discrete granulomata formation with a sarcoidlike appearance. These naked granulomas lack surrounding inflammation and suggest a differential diagnosis of sarcoidosis and infection. Use of immune checkpoint inhibitors (CIs) and kinase inhibitors has been proven to cause sarcoidosislike reactions.25 The development of granulomatous/sarcoidlike lesions associated with the use of BRAF and MEK inhibitors may clinically and radiographically mimic disease recurrence. An awareness of this type of reaction by clinicians and pathologists is important to ensure appropriate management in patients who develop GD.26
Checkpoint inhibitor–induced GD that remains asymptomatic does not necessarily warrant treatment; however, corticosteroid use and elimination of CI therapies have resolved GD in prior cases. Responsiveness of the cancer to CI therapy and severity of GD symptoms should be considered before discontinuation of a CI trial.25
One case report described complete resolution of a GD eruption without interruption of the scheduled BRAF and MEK inhibitor therapies for the treatment of metastatic melanoma. There was no reported use of a steroidal cream or other topical medication to aid in controlling the eruption.27 The exact mechanism of how GD resolves while continuing therapy is unknown; however, it has been suggested that a GD eruption may be the consequence of a BRAF and MEK inhibitor–mediated immune response against a subclinical area of metastatic melanoma.28 If the immune response successfully eliminates the subclinical tumor, one could postulate that the inflammatory response and granulomatous eruption would resolve. Future studies are necessary to further elucidate the exact mechanisms involved.
There have been several case reports of GD with vemurafenib treatment,29,30 1 report of GD and erythema induratum with vemurafenib and cobimetinib treatment,31 2 reports of GD with dabrafenib treatment,27,30 and a few reports of GD with the BRAF inhibitor dabrafenib combined with the MEK inhibitor trametinib,28,32,33 all for the treatment of metastatic melanoma. Additionally, a report described a 3-year-old boy who developed GD secondary to vemurafenib for the treatment of Langerhans cell histiocytosis.34 We present a unique case of BRAF and MEK inhibitor therapy–induced GD in the treatment of metastatic cholangiocarcinoma with vemurafenib and cobimetinib.
BRAF and MEK inhibitor therapy is used in patients with metastatic melanomas with a positive BRAF V600E mutation. Due to advancements in next-generation DNA sequencing, these therapies also are being tested in clinical trials for use in the treatment of other cancers with the same checkpoint mutation, such as metastatic cholangiocarcinoma. Cutaneous reactions frequently are documented side effects that occur during treatment with BRAF and MEK inhibitors; GD is an uncommon finding. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of other cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation.
- Mackiewicz J, Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Comtemp Oncol (Pozn). 2018;22:68-72.
- Jovanovic B, Krockel D, Linden D, et al. Lack of cytoplasmic ERK activation is an independent adverse prognostic factor in primary cutaneous melanoma. J Invest Dermatol. 2008;128:2696-2704.
- Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10:1103-1114.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Casey D, Demko S, Sinha A, et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin Cancer Res. 2021;27;4142-4146
- Flaherty K, Davies MA, Grob JJ, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) fl trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. P9502.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Karaszewska B, Schachter J, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(suppl 6):vi552-vi587.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advance BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Once. 2016;17:1248-1260.
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8:E32-E38.
- Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study [published online July 11, 2018]. JCO Precis Oncol. doi:10.1200/PO.18.00122
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331.
- Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16INK4A alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727.
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244.
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180.
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712.
- Bunyatov T, Zhao A, Kovalenko J, et al. Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV. J Gastrointest Oncol. 2019;10:815-820.
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102.
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479.
- Rosenbach M, English JC. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33:373-387.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol 2012;166:775-783.
- Calonje JE, Brenn T, Lazar A, Billings S. Lichenoid and interface dermatitis. In: McKee’s Pathology of the Skin. 5th ed. China: Elsevier Limited: 2018;7:241-282.
- Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13:1076-1082.
- Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6:14.
- Garrido MC, Gutiérrez C, Riveiro-Falkenbach E, et al. BRAF inhibitor-induced antitumoral granulomatous dermatitis eruption in advanced melanoma. Am J Dermatopathol. 2015;37:795-798.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307‐311.
- Ong ELH, Sinha R, Jmor S, et al. BRAF inhibitor-associated granulomatous dermatitis: a report of 3 cases. Am J of Dermatopathol. 2019;41:214-217.
- Wali GN, Stonard C, Espinosa O, et al. Persistent granulomatous cutaneous drug eruption to a BRAF inhibitor. J Am Acad Dermatol. 2017;76(suppl 1):AB195.
- Aj lafolla M, Ramsay J, Wismer J, et al. Cobimetinib- and vemurafenib-induced granulomatous dermatitis and erythema induratum: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847358
- Jansen YJ, Janssens P, Hoorens A, et al. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550‐554.
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Chen L, His A, Kothari A, et al. Granulomatous dermatitis secondary to vemurafenib in a child with Langerhans cell histiocytosis. Pediatr Dermatol. 2018;35:E402-E403.
- Mackiewicz J, Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Comtemp Oncol (Pozn). 2018;22:68-72.
- Jovanovic B, Krockel D, Linden D, et al. Lack of cytoplasmic ERK activation is an independent adverse prognostic factor in primary cutaneous melanoma. J Invest Dermatol. 2008;128:2696-2704.
- Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10:1103-1114.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Casey D, Demko S, Sinha A, et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin Cancer Res. 2021;27;4142-4146
- Flaherty K, Davies MA, Grob JJ, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) fl trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. P9502.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Karaszewska B, Schachter J, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(suppl 6):vi552-vi587.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advance BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Once. 2016;17:1248-1260.
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8:E32-E38.
- Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study [published online July 11, 2018]. JCO Precis Oncol. doi:10.1200/PO.18.00122
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331.
- Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16INK4A alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727.
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244.
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180.
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712.
- Bunyatov T, Zhao A, Kovalenko J, et al. Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV. J Gastrointest Oncol. 2019;10:815-820.
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102.
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479.
- Rosenbach M, English JC. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33:373-387.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol 2012;166:775-783.
- Calonje JE, Brenn T, Lazar A, Billings S. Lichenoid and interface dermatitis. In: McKee’s Pathology of the Skin. 5th ed. China: Elsevier Limited: 2018;7:241-282.
- Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13:1076-1082.
- Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6:14.
- Garrido MC, Gutiérrez C, Riveiro-Falkenbach E, et al. BRAF inhibitor-induced antitumoral granulomatous dermatitis eruption in advanced melanoma. Am J Dermatopathol. 2015;37:795-798.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307‐311.
- Ong ELH, Sinha R, Jmor S, et al. BRAF inhibitor-associated granulomatous dermatitis: a report of 3 cases. Am J of Dermatopathol. 2019;41:214-217.
- Wali GN, Stonard C, Espinosa O, et al. Persistent granulomatous cutaneous drug eruption to a BRAF inhibitor. J Am Acad Dermatol. 2017;76(suppl 1):AB195.
- Aj lafolla M, Ramsay J, Wismer J, et al. Cobimetinib- and vemurafenib-induced granulomatous dermatitis and erythema induratum: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847358
- Jansen YJ, Janssens P, Hoorens A, et al. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550‐554.
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Chen L, His A, Kothari A, et al. Granulomatous dermatitis secondary to vemurafenib in a child with Langerhans cell histiocytosis. Pediatr Dermatol. 2018;35:E402-E403.
Practice Points
- Granulomatous dermatitis (GD) is a potential rare side effect of the use of BRAF and MEK inhibitors for the treatment of BRAF V600 mutation–positive cancers, including metastatic cholangiocarcinoma.
- Granulomatous dermatitis can resolve despite continuation of BRAF and MEK inhibitor therapies.
- Histologically, GD can appear similar to disease recurrence. It is imperative that clinicians and pathologists recognize the cutaneous manifestations of BRAF and MEK inhibitors.
Concurrent Atopic Dermatitis and Psoriasis Successfully Treated With Dual Biologic Therapy
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.
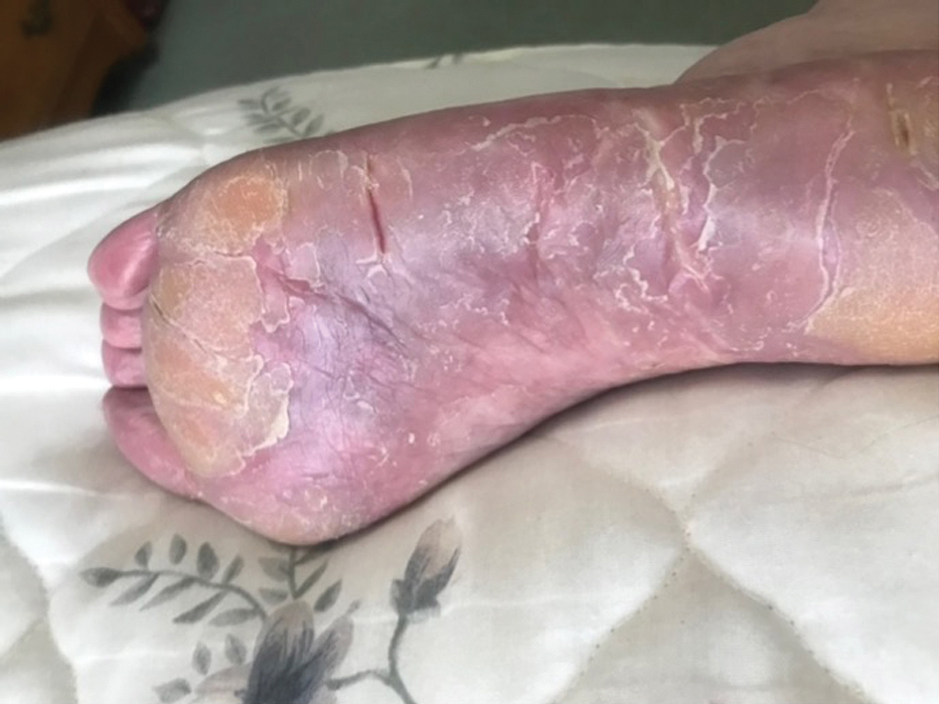
Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.
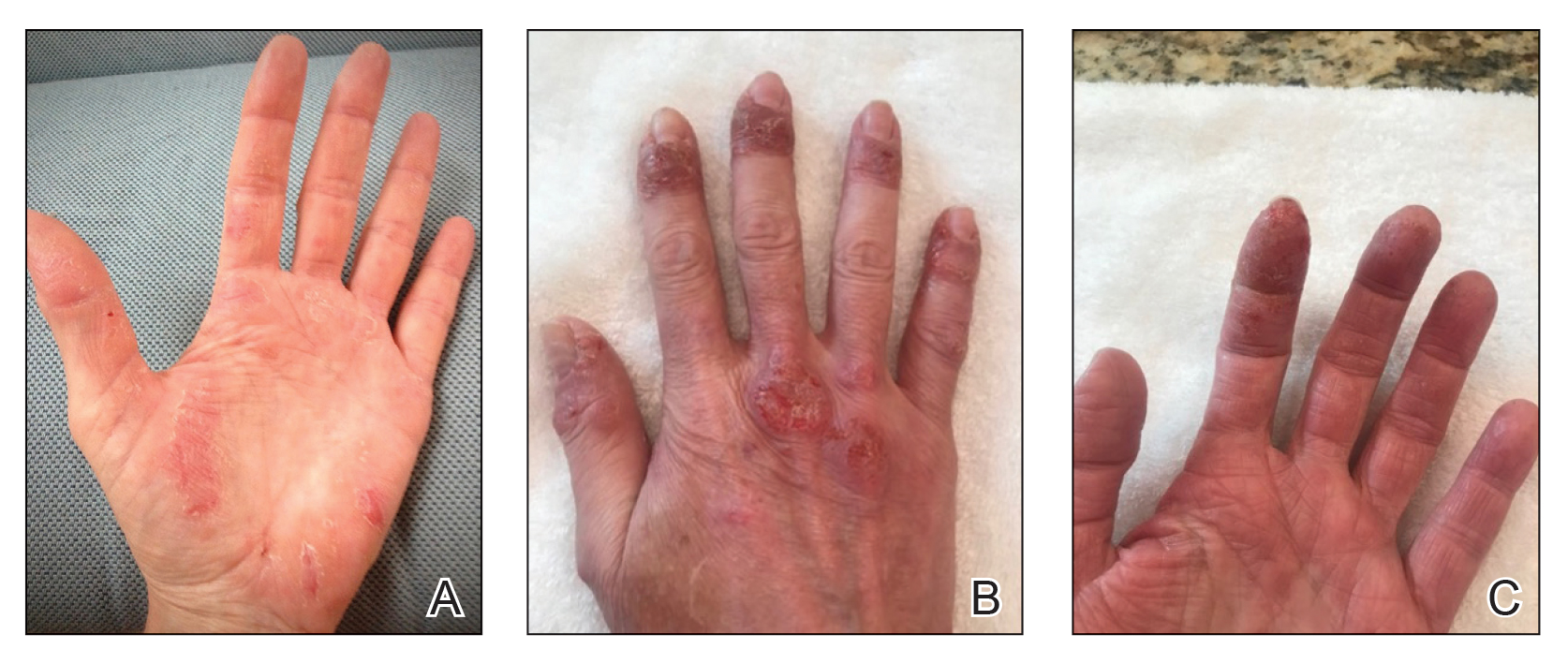
After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.
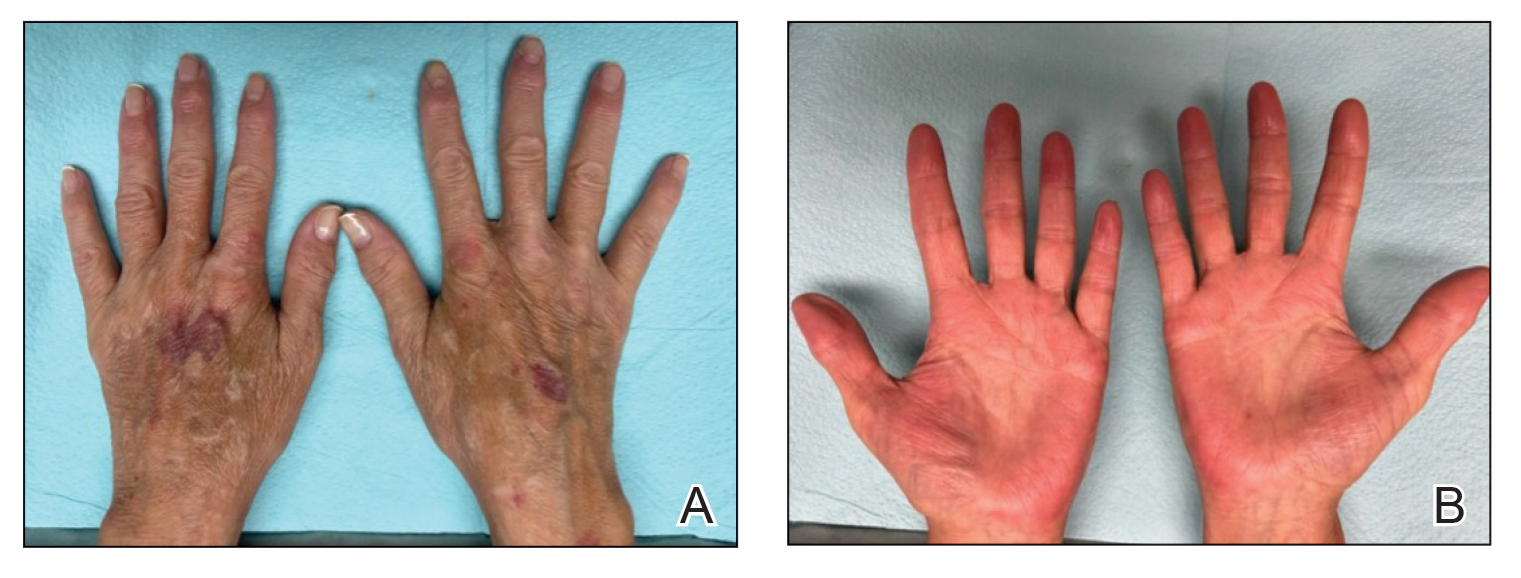
Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.

Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.

After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.

Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.

Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.

After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.

Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
Practice Points
- Atopic dermatitis and psoriasis can share clinical and histopathologic features, which represents their underlying immunopathologic spectrum.
- Atopic dermatitis and psoriasis can coexist in a single patient, which may be suspected from a clinical picture of treatment-resistant disease, a partial response to targeted therapies, or extensive hand involvement.
Idiopathic Granulomatous Lobular Mastitis: A Mimicker of Inflammatory Breast Cancer
Idiopathic granulomatous lobular mastitis (IGLM) is a rare, chronic inflammatory breast disease first described in 1972.1 IGLM usually affects women during reproductive years and has similar clinical features to breast cancer.2 Ultrasonography and mammography yield nonspecific results and cannot adequately differentiate between malignancy and inflammation.3 Magnetic resonance imaging (MRI) is known to be more sensitive in detecting lesions in dense breasts; however, it does not differentiate between granulomatous lesions and other disorders.4,5 Histopathology is the gold standard for diagnosis.1-12
Infectious and autoimmune causes of granulomatous mastitis must be excluded before establishing an IGLM diagnosis. The clinical quandary that remains is how to adequately manage the disease. Although there are no defined treatment guidelines, current literature has proposed a multimodal strategy.6,9 In this report, we describe a case of IGLM successfully treated with surgical excision after failed medical therapy.
Case Presentation
A 43-year-old gravida 5, para 4 White woman presented with a 2-week history of right breast tenderness, heaviness, warmth, and redness that was refractory to cephalexin and dicloxacillin. She had no personal or family history of breast cancer; never had breast surgery and breastfed all 4 children.
An examination of the right breast demonstrated erythema and an 8-cm tender mass in the right lower outer quadrant but no skin retraction or dimpling (Figure 1). The mammography, concerning for inflammatory breast cancer, was category BI-RADS 4 and demonstrated a suspicious right axillary lymph node (Figure 2).
A core needle breast biopsy revealed granulomatous mastitis (Figure 3A), without evidence of malignancy. Rheumatology and endocrinology excluded secondary causes of granulomatous mastitis (ie, sarcoidosis, tuberculosis, granulomatosis with polyangiitis, and other autoimmune conditions). A pituitary MRI to assess an elevated serum prolactin level showed no evidence of microadenoma.
After a prolonged course of 8 months of unsuccessful therapy with prednisone and methotrexate, the patient was referred for surgical excision. Culture and special stains (Gram stain, periodic acid-Schiff stain, acid-fast Bacillus culture, Fite stain, and Brown and Benn stain) of the breast tissue were negative for organisms (Figure 3B). Seven months after excision the patient was doing well and had no evidence of recurrence.
Discussion
IGLM is a rare, chronic benign inflammatory breast disease of unknown etiology and more commonly reported in individuals of Mediterranean descent.13 It is believed that hyperprolactinemia causing extravasation of fat and protein during milk letdown leads to lymphocyte and macrophage migration, resulting in a localized autoimmune response in the breast ducts.10,14
There are 2 types of granulomatous mastitis: idiopathic and specific. Infectious, autoimmune, and malignant causes of granulomatous mastitis (ie, tuberculosis, sarcoidosis, Corynebacterium spp, granulomatosis with polyangiitis, systemic lupus erythematosus, Behçet disease, ductal ectasia, or granulomatous reaction in a carcinoma) must be excluded prior to establishing an IGLM diagnosis, as these can be fatal if left untreated.15 The most frequent findings on ultrasound and mammography are hypoechoic masses and focal asymmetric densities, respectively.3,5 MRI has been proposed more for surveillance in patients with chronic IGLM.4,5 Histopathology—featuring lobular noncaseating granulomas with epithelioid histiocytes; and multinucleated giant cells in a background of neutrophils, lymphocytes, plasma cells, and eosinophils—is the gold standard for diagnosing IGLM.1-12
There are currently no universal treatment guidelines and management usually consists of observation, systemic and topical steroids, or surgery.3,13 Topical and injectable steroids have been effective in treating both initial and recurrent IGLM in patients who are unable to be treated with systemic steroids.16-18 Due to reported high recurrence rates with steroid tapers, adjunctive therapy with methotrexate, azathioprine, colchicine, and hydroxychloroquine have been proposed.1,3-6,10-12
Additionally, antibiotics are recommended only in the management of IGLM when microbial co-infection is concerning, such as with Corynebacterium spp.9,11,19-22 Histologically, this bacterium is distinct from IGLM and demonstrates granulomatous, neutrophilic inflammation within cystic spaces.19-21 Wide surgical excision with negative margins is the only definitive treatment to reduce recurrence and expedite recovery time.2,3,7-10 Notably, surgical excision has been associated with poor wound healing and occasional recurrence compared with medication alone.5,11
Although IGLM is normally a benign process, chronic disease has been related (without causality) to infiltrating breast carcinoma.4 A proposed theory for the development of malignancy suggests that chronic inflammation leading to free radical formation can result in cellular dysplasia and cancer.23
Conclusions
Fifty years after its first description, IGLM is still a poorly understood disease. There remains no consensus behind its etiology or management. In our case, we demonstrated a stepwise treatment progression, beginning with medical therapy before proceeding to surgical cure. Given concerns for poor wound healing and postsurgical infections, monitoring the response and recurrence to an initial trial of conservative medical treatment is not unreasonable. Because of possible risk for malignancy with chronic IGLM, patients should not delay surgical excision if their condition remains refractory to medical therapy alone.
1. Garcia-Rodiguez JA, Pattullo A. Idiopathic granulomatous mastitis: a mimicking disease in a pregnant woman: a case report. BMC Res Notes. 2013;6:95. doi.10.1186/1756-0500-6-95
2. Gurleyik G, Aktekin A, Aker F, Karagulle H, Saglamc A. Medical and surgical treatment of idiopathic granulomatous lobular mastitis: a benign inflammatory disease mimicking invasive carcinoma. J Breast Cancer. 2012;15(1):119-123. doi:10.4048/jbc.2012.15.1.119
3. Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009;193(2):574-581. doi:10.2214/AJR.08.1528
4. Mazlan L, Suhaimi SN, Jasmin SJ, Latar NH, Adzman S, Muhammad R. Breast carcinoma occurring from chronic granulomatous mastitis. Malays J Med Sci. 2012;19(2):82-85.
5. Patel RA, Strickland P, Sankara IR, Pinkston G, Many W Jr, Rodriguez M. Idiopathic granulomatous mastitis: case reports and review of literature. J Gen Intern Med. 2010;25(3):270-273. doi:10.1007/s11606-009-1207-2
6. Akbulut S, Yilmaz D, Bakir S. Methotrexate in the management of idiopathic granulomatous mastitis: review of 108 published cases and report of four cases. Breast J. 2011;17(6):661-668. doi:10.1111/j.1524-4741.2011.01162.x
7. Ergin AB, Cristofanilli M, Daw H, Tahan G, Gong Y. Recurrent granulomatous mastitis mimicking inflammatory breast cancer. BMJ Case Rep. 2011;2011:bcr0720103156. doi:10.1136/bcr.07.2010.3156
8. Hladik M, Schoeller T, Ensat F, Wechselberger G. Idiopathic granulomatous mastitis: successful treatment by mastectomy and immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2011;64(12):1604-1607. doi:10.1016/j.bjps.2011.07.01
9. Hur SM, Cho DH, Lee SK, et al. Experience of treatment of patients with granulomatous lobular mastitis. J Korean Surg Soc. 2013;85(1):1-6. doi:10.4174/jkss.2013.85.1.
10. Kayahan M, Kadioglu H, Muslumanoglu M. Management of patients with granulomatous mastitis: analysis of 31 cases. Breast Care (Basel). 2012;7(3):226-230. doi:10.1159/000337758
11. Neel A, Hello M, Cottereau A, et al. Long-term outcome in idiopathic granulomatous mastitis: a western multicentre study. QJM. 2013;106(5):433-441. doi:10.1093/qjmed/hct040
12. Seo HR, Na KY, Yim HE, et al. Differential diagnosis in idiopathic granulomatous mastitis and tuberculous mastitis. J Breast Cancer. 2012;15(1):111-118. doi:10.4048/jbc.2012.15.1.111
13. Martinez-Ramos D, Simon-Monterde L, Suelves-Piqueres C, et al. Idiopathic granulomatous mastitis: a systematic review of 3060 patients. Breast J. 2019;25(6):1245-1250. doi:10.1111/tbj.13446
14. Lin CH, Hsu CW, Tsao TY, Chou J. Idiopathic granulomatous mastitis associated with risperidone-induced hyperprolactinemia. Diagn Pathol. 2012;7:2. doi:10.1186/1746-1596-7-2
15. Goulabchand R, Hafidi A, Van de Perre P, et al. Mastitis in autoimmune diseases: review of the literature, diagnostic pathway, and pathophysiological key players. J Clin Med. 2020;9(4):958. doi:10.3390/jcm9040958
16. Altintoprak F. Topical steroids to treat granulomatous mastitis: a case report. Korean J Intern Med. 2011;26(3):356-359. doi:10.3904/kjim.2011.26.3.356
17. Tang A, Dominguez DA, Edquilang JK, et al. Granulomatous mastitis: comparison of novel treatment of steroid injection and current management. J Surg Res. 2020;254:300-305. doi:10.1016/j.jss.2020.04.018
18. Toktas O, Toprak N. Treatment results of intralesional steroid injection and topical steroid administration in pregnant women with idiopathic granulomatous mastitis. Eur J Breast Health. 2021;17(3):283-287. doi:10.4274/ejbh.galenos.2021.2021-2-4
19. Bercot B, Kannengiesser C, Oudin C, et al. First description of NOD2 variant associated with defective neutrophil responses in a woman with granulomatous mastitis related to corynebacteria. J Clin Microbiol. 2009;47(9):3034-3037. doi:10.1128/JCM.00561-09
20. Renshaw AA, Derhagopian RP, Gould EW. Cystic neutrophilic granulomatous mastitis: an underappreciated pattern strongly associated with gram-positive bacilli. Am J Clin Pathol. 2011;136(3):424-427. doi:10.1309/AJCP1W9JBRYOQSNZ
21. Stary CM, Lee YS, Balfour J. Idiopathic granulomatous mastitis associated with corynebacterium sp. Infection. Hawaii Med J. 2011;70(5):99-101.
22. Taylor GB, Paviour SD, Musaad S, Jones WO, Holland DJ. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathology. 2003;35(2):109-119.
23. Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med. 2006;79(3-4):123-130.
Idiopathic granulomatous lobular mastitis (IGLM) is a rare, chronic inflammatory breast disease first described in 1972.1 IGLM usually affects women during reproductive years and has similar clinical features to breast cancer.2 Ultrasonography and mammography yield nonspecific results and cannot adequately differentiate between malignancy and inflammation.3 Magnetic resonance imaging (MRI) is known to be more sensitive in detecting lesions in dense breasts; however, it does not differentiate between granulomatous lesions and other disorders.4,5 Histopathology is the gold standard for diagnosis.1-12
Infectious and autoimmune causes of granulomatous mastitis must be excluded before establishing an IGLM diagnosis. The clinical quandary that remains is how to adequately manage the disease. Although there are no defined treatment guidelines, current literature has proposed a multimodal strategy.6,9 In this report, we describe a case of IGLM successfully treated with surgical excision after failed medical therapy.
Case Presentation
A 43-year-old gravida 5, para 4 White woman presented with a 2-week history of right breast tenderness, heaviness, warmth, and redness that was refractory to cephalexin and dicloxacillin. She had no personal or family history of breast cancer; never had breast surgery and breastfed all 4 children.
An examination of the right breast demonstrated erythema and an 8-cm tender mass in the right lower outer quadrant but no skin retraction or dimpling (Figure 1). The mammography, concerning for inflammatory breast cancer, was category BI-RADS 4 and demonstrated a suspicious right axillary lymph node (Figure 2).
A core needle breast biopsy revealed granulomatous mastitis (Figure 3A), without evidence of malignancy. Rheumatology and endocrinology excluded secondary causes of granulomatous mastitis (ie, sarcoidosis, tuberculosis, granulomatosis with polyangiitis, and other autoimmune conditions). A pituitary MRI to assess an elevated serum prolactin level showed no evidence of microadenoma.
After a prolonged course of 8 months of unsuccessful therapy with prednisone and methotrexate, the patient was referred for surgical excision. Culture and special stains (Gram stain, periodic acid-Schiff stain, acid-fast Bacillus culture, Fite stain, and Brown and Benn stain) of the breast tissue were negative for organisms (Figure 3B). Seven months after excision the patient was doing well and had no evidence of recurrence.
Discussion
IGLM is a rare, chronic benign inflammatory breast disease of unknown etiology and more commonly reported in individuals of Mediterranean descent.13 It is believed that hyperprolactinemia causing extravasation of fat and protein during milk letdown leads to lymphocyte and macrophage migration, resulting in a localized autoimmune response in the breast ducts.10,14
There are 2 types of granulomatous mastitis: idiopathic and specific. Infectious, autoimmune, and malignant causes of granulomatous mastitis (ie, tuberculosis, sarcoidosis, Corynebacterium spp, granulomatosis with polyangiitis, systemic lupus erythematosus, Behçet disease, ductal ectasia, or granulomatous reaction in a carcinoma) must be excluded prior to establishing an IGLM diagnosis, as these can be fatal if left untreated.15 The most frequent findings on ultrasound and mammography are hypoechoic masses and focal asymmetric densities, respectively.3,5 MRI has been proposed more for surveillance in patients with chronic IGLM.4,5 Histopathology—featuring lobular noncaseating granulomas with epithelioid histiocytes; and multinucleated giant cells in a background of neutrophils, lymphocytes, plasma cells, and eosinophils—is the gold standard for diagnosing IGLM.1-12
There are currently no universal treatment guidelines and management usually consists of observation, systemic and topical steroids, or surgery.3,13 Topical and injectable steroids have been effective in treating both initial and recurrent IGLM in patients who are unable to be treated with systemic steroids.16-18 Due to reported high recurrence rates with steroid tapers, adjunctive therapy with methotrexate, azathioprine, colchicine, and hydroxychloroquine have been proposed.1,3-6,10-12
Additionally, antibiotics are recommended only in the management of IGLM when microbial co-infection is concerning, such as with Corynebacterium spp.9,11,19-22 Histologically, this bacterium is distinct from IGLM and demonstrates granulomatous, neutrophilic inflammation within cystic spaces.19-21 Wide surgical excision with negative margins is the only definitive treatment to reduce recurrence and expedite recovery time.2,3,7-10 Notably, surgical excision has been associated with poor wound healing and occasional recurrence compared with medication alone.5,11
Although IGLM is normally a benign process, chronic disease has been related (without causality) to infiltrating breast carcinoma.4 A proposed theory for the development of malignancy suggests that chronic inflammation leading to free radical formation can result in cellular dysplasia and cancer.23
Conclusions
Fifty years after its first description, IGLM is still a poorly understood disease. There remains no consensus behind its etiology or management. In our case, we demonstrated a stepwise treatment progression, beginning with medical therapy before proceeding to surgical cure. Given concerns for poor wound healing and postsurgical infections, monitoring the response and recurrence to an initial trial of conservative medical treatment is not unreasonable. Because of possible risk for malignancy with chronic IGLM, patients should not delay surgical excision if their condition remains refractory to medical therapy alone.
Idiopathic granulomatous lobular mastitis (IGLM) is a rare, chronic inflammatory breast disease first described in 1972.1 IGLM usually affects women during reproductive years and has similar clinical features to breast cancer.2 Ultrasonography and mammography yield nonspecific results and cannot adequately differentiate between malignancy and inflammation.3 Magnetic resonance imaging (MRI) is known to be more sensitive in detecting lesions in dense breasts; however, it does not differentiate between granulomatous lesions and other disorders.4,5 Histopathology is the gold standard for diagnosis.1-12
Infectious and autoimmune causes of granulomatous mastitis must be excluded before establishing an IGLM diagnosis. The clinical quandary that remains is how to adequately manage the disease. Although there are no defined treatment guidelines, current literature has proposed a multimodal strategy.6,9 In this report, we describe a case of IGLM successfully treated with surgical excision after failed medical therapy.
Case Presentation
A 43-year-old gravida 5, para 4 White woman presented with a 2-week history of right breast tenderness, heaviness, warmth, and redness that was refractory to cephalexin and dicloxacillin. She had no personal or family history of breast cancer; never had breast surgery and breastfed all 4 children.
An examination of the right breast demonstrated erythema and an 8-cm tender mass in the right lower outer quadrant but no skin retraction or dimpling (Figure 1). The mammography, concerning for inflammatory breast cancer, was category BI-RADS 4 and demonstrated a suspicious right axillary lymph node (Figure 2).
A core needle breast biopsy revealed granulomatous mastitis (Figure 3A), without evidence of malignancy. Rheumatology and endocrinology excluded secondary causes of granulomatous mastitis (ie, sarcoidosis, tuberculosis, granulomatosis with polyangiitis, and other autoimmune conditions). A pituitary MRI to assess an elevated serum prolactin level showed no evidence of microadenoma.
After a prolonged course of 8 months of unsuccessful therapy with prednisone and methotrexate, the patient was referred for surgical excision. Culture and special stains (Gram stain, periodic acid-Schiff stain, acid-fast Bacillus culture, Fite stain, and Brown and Benn stain) of the breast tissue were negative for organisms (Figure 3B). Seven months after excision the patient was doing well and had no evidence of recurrence.
Discussion
IGLM is a rare, chronic benign inflammatory breast disease of unknown etiology and more commonly reported in individuals of Mediterranean descent.13 It is believed that hyperprolactinemia causing extravasation of fat and protein during milk letdown leads to lymphocyte and macrophage migration, resulting in a localized autoimmune response in the breast ducts.10,14
There are 2 types of granulomatous mastitis: idiopathic and specific. Infectious, autoimmune, and malignant causes of granulomatous mastitis (ie, tuberculosis, sarcoidosis, Corynebacterium spp, granulomatosis with polyangiitis, systemic lupus erythematosus, Behçet disease, ductal ectasia, or granulomatous reaction in a carcinoma) must be excluded prior to establishing an IGLM diagnosis, as these can be fatal if left untreated.15 The most frequent findings on ultrasound and mammography are hypoechoic masses and focal asymmetric densities, respectively.3,5 MRI has been proposed more for surveillance in patients with chronic IGLM.4,5 Histopathology—featuring lobular noncaseating granulomas with epithelioid histiocytes; and multinucleated giant cells in a background of neutrophils, lymphocytes, plasma cells, and eosinophils—is the gold standard for diagnosing IGLM.1-12
There are currently no universal treatment guidelines and management usually consists of observation, systemic and topical steroids, or surgery.3,13 Topical and injectable steroids have been effective in treating both initial and recurrent IGLM in patients who are unable to be treated with systemic steroids.16-18 Due to reported high recurrence rates with steroid tapers, adjunctive therapy with methotrexate, azathioprine, colchicine, and hydroxychloroquine have been proposed.1,3-6,10-12
Additionally, antibiotics are recommended only in the management of IGLM when microbial co-infection is concerning, such as with Corynebacterium spp.9,11,19-22 Histologically, this bacterium is distinct from IGLM and demonstrates granulomatous, neutrophilic inflammation within cystic spaces.19-21 Wide surgical excision with negative margins is the only definitive treatment to reduce recurrence and expedite recovery time.2,3,7-10 Notably, surgical excision has been associated with poor wound healing and occasional recurrence compared with medication alone.5,11
Although IGLM is normally a benign process, chronic disease has been related (without causality) to infiltrating breast carcinoma.4 A proposed theory for the development of malignancy suggests that chronic inflammation leading to free radical formation can result in cellular dysplasia and cancer.23
Conclusions
Fifty years after its first description, IGLM is still a poorly understood disease. There remains no consensus behind its etiology or management. In our case, we demonstrated a stepwise treatment progression, beginning with medical therapy before proceeding to surgical cure. Given concerns for poor wound healing and postsurgical infections, monitoring the response and recurrence to an initial trial of conservative medical treatment is not unreasonable. Because of possible risk for malignancy with chronic IGLM, patients should not delay surgical excision if their condition remains refractory to medical therapy alone.
1. Garcia-Rodiguez JA, Pattullo A. Idiopathic granulomatous mastitis: a mimicking disease in a pregnant woman: a case report. BMC Res Notes. 2013;6:95. doi.10.1186/1756-0500-6-95
2. Gurleyik G, Aktekin A, Aker F, Karagulle H, Saglamc A. Medical and surgical treatment of idiopathic granulomatous lobular mastitis: a benign inflammatory disease mimicking invasive carcinoma. J Breast Cancer. 2012;15(1):119-123. doi:10.4048/jbc.2012.15.1.119
3. Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009;193(2):574-581. doi:10.2214/AJR.08.1528
4. Mazlan L, Suhaimi SN, Jasmin SJ, Latar NH, Adzman S, Muhammad R. Breast carcinoma occurring from chronic granulomatous mastitis. Malays J Med Sci. 2012;19(2):82-85.
5. Patel RA, Strickland P, Sankara IR, Pinkston G, Many W Jr, Rodriguez M. Idiopathic granulomatous mastitis: case reports and review of literature. J Gen Intern Med. 2010;25(3):270-273. doi:10.1007/s11606-009-1207-2
6. Akbulut S, Yilmaz D, Bakir S. Methotrexate in the management of idiopathic granulomatous mastitis: review of 108 published cases and report of four cases. Breast J. 2011;17(6):661-668. doi:10.1111/j.1524-4741.2011.01162.x
7. Ergin AB, Cristofanilli M, Daw H, Tahan G, Gong Y. Recurrent granulomatous mastitis mimicking inflammatory breast cancer. BMJ Case Rep. 2011;2011:bcr0720103156. doi:10.1136/bcr.07.2010.3156
8. Hladik M, Schoeller T, Ensat F, Wechselberger G. Idiopathic granulomatous mastitis: successful treatment by mastectomy and immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2011;64(12):1604-1607. doi:10.1016/j.bjps.2011.07.01
9. Hur SM, Cho DH, Lee SK, et al. Experience of treatment of patients with granulomatous lobular mastitis. J Korean Surg Soc. 2013;85(1):1-6. doi:10.4174/jkss.2013.85.1.
10. Kayahan M, Kadioglu H, Muslumanoglu M. Management of patients with granulomatous mastitis: analysis of 31 cases. Breast Care (Basel). 2012;7(3):226-230. doi:10.1159/000337758
11. Neel A, Hello M, Cottereau A, et al. Long-term outcome in idiopathic granulomatous mastitis: a western multicentre study. QJM. 2013;106(5):433-441. doi:10.1093/qjmed/hct040
12. Seo HR, Na KY, Yim HE, et al. Differential diagnosis in idiopathic granulomatous mastitis and tuberculous mastitis. J Breast Cancer. 2012;15(1):111-118. doi:10.4048/jbc.2012.15.1.111
13. Martinez-Ramos D, Simon-Monterde L, Suelves-Piqueres C, et al. Idiopathic granulomatous mastitis: a systematic review of 3060 patients. Breast J. 2019;25(6):1245-1250. doi:10.1111/tbj.13446
14. Lin CH, Hsu CW, Tsao TY, Chou J. Idiopathic granulomatous mastitis associated with risperidone-induced hyperprolactinemia. Diagn Pathol. 2012;7:2. doi:10.1186/1746-1596-7-2
15. Goulabchand R, Hafidi A, Van de Perre P, et al. Mastitis in autoimmune diseases: review of the literature, diagnostic pathway, and pathophysiological key players. J Clin Med. 2020;9(4):958. doi:10.3390/jcm9040958
16. Altintoprak F. Topical steroids to treat granulomatous mastitis: a case report. Korean J Intern Med. 2011;26(3):356-359. doi:10.3904/kjim.2011.26.3.356
17. Tang A, Dominguez DA, Edquilang JK, et al. Granulomatous mastitis: comparison of novel treatment of steroid injection and current management. J Surg Res. 2020;254:300-305. doi:10.1016/j.jss.2020.04.018
18. Toktas O, Toprak N. Treatment results of intralesional steroid injection and topical steroid administration in pregnant women with idiopathic granulomatous mastitis. Eur J Breast Health. 2021;17(3):283-287. doi:10.4274/ejbh.galenos.2021.2021-2-4
19. Bercot B, Kannengiesser C, Oudin C, et al. First description of NOD2 variant associated with defective neutrophil responses in a woman with granulomatous mastitis related to corynebacteria. J Clin Microbiol. 2009;47(9):3034-3037. doi:10.1128/JCM.00561-09
20. Renshaw AA, Derhagopian RP, Gould EW. Cystic neutrophilic granulomatous mastitis: an underappreciated pattern strongly associated with gram-positive bacilli. Am J Clin Pathol. 2011;136(3):424-427. doi:10.1309/AJCP1W9JBRYOQSNZ
21. Stary CM, Lee YS, Balfour J. Idiopathic granulomatous mastitis associated with corynebacterium sp. Infection. Hawaii Med J. 2011;70(5):99-101.
22. Taylor GB, Paviour SD, Musaad S, Jones WO, Holland DJ. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathology. 2003;35(2):109-119.
23. Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med. 2006;79(3-4):123-130.
1. Garcia-Rodiguez JA, Pattullo A. Idiopathic granulomatous mastitis: a mimicking disease in a pregnant woman: a case report. BMC Res Notes. 2013;6:95. doi.10.1186/1756-0500-6-95
2. Gurleyik G, Aktekin A, Aker F, Karagulle H, Saglamc A. Medical and surgical treatment of idiopathic granulomatous lobular mastitis: a benign inflammatory disease mimicking invasive carcinoma. J Breast Cancer. 2012;15(1):119-123. doi:10.4048/jbc.2012.15.1.119
3. Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009;193(2):574-581. doi:10.2214/AJR.08.1528
4. Mazlan L, Suhaimi SN, Jasmin SJ, Latar NH, Adzman S, Muhammad R. Breast carcinoma occurring from chronic granulomatous mastitis. Malays J Med Sci. 2012;19(2):82-85.
5. Patel RA, Strickland P, Sankara IR, Pinkston G, Many W Jr, Rodriguez M. Idiopathic granulomatous mastitis: case reports and review of literature. J Gen Intern Med. 2010;25(3):270-273. doi:10.1007/s11606-009-1207-2
6. Akbulut S, Yilmaz D, Bakir S. Methotrexate in the management of idiopathic granulomatous mastitis: review of 108 published cases and report of four cases. Breast J. 2011;17(6):661-668. doi:10.1111/j.1524-4741.2011.01162.x
7. Ergin AB, Cristofanilli M, Daw H, Tahan G, Gong Y. Recurrent granulomatous mastitis mimicking inflammatory breast cancer. BMJ Case Rep. 2011;2011:bcr0720103156. doi:10.1136/bcr.07.2010.3156
8. Hladik M, Schoeller T, Ensat F, Wechselberger G. Idiopathic granulomatous mastitis: successful treatment by mastectomy and immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2011;64(12):1604-1607. doi:10.1016/j.bjps.2011.07.01
9. Hur SM, Cho DH, Lee SK, et al. Experience of treatment of patients with granulomatous lobular mastitis. J Korean Surg Soc. 2013;85(1):1-6. doi:10.4174/jkss.2013.85.1.
10. Kayahan M, Kadioglu H, Muslumanoglu M. Management of patients with granulomatous mastitis: analysis of 31 cases. Breast Care (Basel). 2012;7(3):226-230. doi:10.1159/000337758
11. Neel A, Hello M, Cottereau A, et al. Long-term outcome in idiopathic granulomatous mastitis: a western multicentre study. QJM. 2013;106(5):433-441. doi:10.1093/qjmed/hct040
12. Seo HR, Na KY, Yim HE, et al. Differential diagnosis in idiopathic granulomatous mastitis and tuberculous mastitis. J Breast Cancer. 2012;15(1):111-118. doi:10.4048/jbc.2012.15.1.111
13. Martinez-Ramos D, Simon-Monterde L, Suelves-Piqueres C, et al. Idiopathic granulomatous mastitis: a systematic review of 3060 patients. Breast J. 2019;25(6):1245-1250. doi:10.1111/tbj.13446
14. Lin CH, Hsu CW, Tsao TY, Chou J. Idiopathic granulomatous mastitis associated with risperidone-induced hyperprolactinemia. Diagn Pathol. 2012;7:2. doi:10.1186/1746-1596-7-2
15. Goulabchand R, Hafidi A, Van de Perre P, et al. Mastitis in autoimmune diseases: review of the literature, diagnostic pathway, and pathophysiological key players. J Clin Med. 2020;9(4):958. doi:10.3390/jcm9040958
16. Altintoprak F. Topical steroids to treat granulomatous mastitis: a case report. Korean J Intern Med. 2011;26(3):356-359. doi:10.3904/kjim.2011.26.3.356
17. Tang A, Dominguez DA, Edquilang JK, et al. Granulomatous mastitis: comparison of novel treatment of steroid injection and current management. J Surg Res. 2020;254:300-305. doi:10.1016/j.jss.2020.04.018
18. Toktas O, Toprak N. Treatment results of intralesional steroid injection and topical steroid administration in pregnant women with idiopathic granulomatous mastitis. Eur J Breast Health. 2021;17(3):283-287. doi:10.4274/ejbh.galenos.2021.2021-2-4
19. Bercot B, Kannengiesser C, Oudin C, et al. First description of NOD2 variant associated with defective neutrophil responses in a woman with granulomatous mastitis related to corynebacteria. J Clin Microbiol. 2009;47(9):3034-3037. doi:10.1128/JCM.00561-09
20. Renshaw AA, Derhagopian RP, Gould EW. Cystic neutrophilic granulomatous mastitis: an underappreciated pattern strongly associated with gram-positive bacilli. Am J Clin Pathol. 2011;136(3):424-427. doi:10.1309/AJCP1W9JBRYOQSNZ
21. Stary CM, Lee YS, Balfour J. Idiopathic granulomatous mastitis associated with corynebacterium sp. Infection. Hawaii Med J. 2011;70(5):99-101.
22. Taylor GB, Paviour SD, Musaad S, Jones WO, Holland DJ. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathology. 2003;35(2):109-119.
23. Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med. 2006;79(3-4):123-130.
Supplements Are Not a Synonym for Safe: Suspected Liver Injury From Ashwagandha
Many patients take herbals as alternative supplements to boost energy and mood. There are increasing reports of unintended adverse effects related to these supplements, particularly to the liver.1-3 A study by the Drug-Induced Liver Injury Network found that liver injury caused by herbals and dietary supplements has increased from 7% in 2004 to 20% in 2013.4
The supplement ashwagandha has become increasingly popular. Ashwagandha is extracted from the root of Withania somnifera (
To date, the factors defining the population at risk for ashwagandha toxicity are unclear, and an understanding of how to diagnose drug-induced liver injury is still immature in clinical practice. The regulation and study of the herbal and dietary supplement industry remain challenging. While many so-called natural substances are well tolerated, others can have unanticipated and harmful adverse effects and drug interactions. Future research should not only identify potentially harmful substances, but also which patients may be at greatest risk.
Case Presentation
A 48-year-old man with a history of severe alcohol use disorder (AUD) complicated by fatty liver and withdrawal seizures and delirium tremens, hypertension, depression, and anxiety presented to the emergency department (ED) after 4 days of having jaundice, epigastric abdominal pain, dark urine, and pale stools. In the preceding months, he had increased his alcohol use to as many as 12 drinks daily due to depression. After experiencing a blackout, he stopped drinking 7 days before presenting to the ED. He felt withdrawal symptoms, including tremors, diaphoresis, abdominal pain, nausea, and vomiting. On the third day of withdrawals, he reported that he had started taking an over-the-counter testosterone-boosting supplement to increase his energy, which he referred to as TestBoost—a mix of 8 ingredients, including ashwagandha, eleuthero root, Hawthorn berry, longjack, ginseng root, mushroom extract, bindii, and horny goat weed. After taking the supplement for 2 days, he noticed that his urine darkened, his stools became paler, his abdominal pain worsened, and he became jaundiced. After 2 additional days without improvement, and still taking the supplement, he presented to the ED. He reported having no fever, chills, recent illness, chest pain, shortness of breath, melena, lower extremity swelling, recent travel, or any changes in medications.
The patient had a 100.1 °F temperature, 102 beats per minute pulse; 129/94 mm Hg blood pressure, 18 beats per minute respiratory rate, and 97% oxygen saturation on room air on admission. He was in no acute distress, though his examination was notable for generalized jaundice and scleral icterus. He was mildly tender to palpation in the epigastric and right upper quadrant region. He was alert and oriented without confusion. He did not have any asterixis or spider angiomas, though he had scattered bruises on his left flank and left calf. His laboratory results were notable for mildly elevated aspartate aminotransferase (AST), 58 U/L (reference range, 13-35); alanine transaminase (ALT), 49 U/L (reference range, 7-45); and alkaline phosphatase (ALP), 98 U/L (reference range 33-94); total bilirubin, 13.6 mg/dL (reference range, 0.2-1.0); direct bilirubin, 8.4 mg/dL (reference range, 0.2-1); and international normalized ratio (INR), 1.11 (reference range, 2-3). His white blood cell and platelet counts were not remarkable at 9790/μL (reference range, 4500-11,000) and 337,000/μL (reference range, 150,000-440,000), respectively. Abdominal ultrasound and computed tomography (CT) revealed fatty liver with contracted gallbladder and no biliary dilatation. Urine ethanol levels were negative. The gastrointestinal (GI) service was consulted and agreed that his cholestatic injury was nonobstructive and likely related to the ashwagandha component of his supplement. The recommendation was cessation with close outpatient follow-up.
The patient was not prescribed any additional medications, such as steroids or ursodiol. He ceased supplement use following hospitalization; but relapsed into alcohol use 1 month after his discharge. Within 3 weeks, his total bilirubin had improved to 2.87 mg/dL, though AST, ALT, and ALP worsened to 127 U/L, 152 U/L, and 140 U/L, respectively. According to the notes of his psychiatrist who saw him at the time the laboratory tests were drawn, he had remained sober since discharge. His acute hepatitis panel drawn on admission was negative, and he demonstrated immunity to hepatitis A and B. Urine toxicology was negative. Antinuclear antibody (ANA) test was negative 1 year prior to discharge. Epstein-Barr virus (EBV), cytomegalovirus (CMV), ANA, antismooth muscle antibody, and immunoglobulins were not checked as suspicion for these etiologies was low. The Roussel Uclaf Causality Assessment Method (RUCAM) score was calculated as 6 (+1 for timing, +2 for drop in total bilirubin, +1 for ethanol risk factor, 0 for no other drugs, 0 for rule out of other diseases, +2 for known hepatotoxicity, 0 no repeat administration) for this patient indicating probable adverse drug reaction liver injury (Tables 1 and 2). However, we acknowledge that CMV, EBV, and herpes simplex virus status were not tested.
The 8 ingredients contained in TestBoost aside from ashwagandha did not have any major known liver adverse effects per a major database of medications. The other ingredients include eleuthero root, Hawthorn berry (crataegus laevigata), longjack (eurycoma longifolla) root, American ginseng root (American panax ginseng—panax quinquefolius), and Cordyceps mycelium (mushroom) extract, bindii (Tribulus terrestris), and epimedium grandiflorum (horny goat weed).6 No assays were performed to confirm purity of the ingredients in the patient’s supplement container.
Alcoholic hepatitis is an important consideration in this patient with AUD, though the timing of symptoms with supplement use and the cholestatic injury pattern with normal INR seems more consistent with drug-induced injury. Viral, infectious, and obstructive etiologies also were investigated. Acute viral hepatitis was ruled out based on bloodwork. The normal hepatobiliary tree on both ultrasound and CT effectively ruled out acute cholecystitis, cholangitis, and choledocholithiasis and there was no further indication for magnetic resonance cholangiopancreatography. There was no hepatic vein clot suggestive of Budd-Chiari syndrome. Autoimmune hepatitis was thought to be unlikely given that the etiology of injury seemed cholestatic in nature. Given the timing of the liver injury relative to supplement use it is likely that ashwagandha was a causative factor of this patient’s liver injury overlaid on an already strained liver from increased alcohol abuse.
The patient did not follow up with the GI service as an outpatient. There are no reports that the patient continued using the testosterone booster. His bilirubin improved dramatically within 1.5 months while his liver enzymes peaked 3 weeks later, with ALT ≥ AST. During his next admission 3 months later, he had relapsed, and his liver enzymes had the classic 2:1 AST to ALT ratio.
Discussion
Generally, ashwagandha has been thought to be well tolerated and possibly hepatoprotective.7-10 However, recent studies suggest potential for hepatotoxicity, though without clear guidance about which patients are most at risk.5,11,12 A study by Inagaki and colleagues suggests the potential for dose-dependent mechanism of liver injury, and this is supported by in vitro CYP450 inhibition with high doses of W Somnifera extract.11,13 We hypothesize that there may be a multihit process that makes some patients more susceptible to supplement harm, particularly those with repeated exposures and with ongoing exposure to hepatic toxins, such as AUD.14 Supplements should be used with more caution in these individuals.
Additionally, although there are no validated guidelines to confirm the diagnosis of drug-induced liver injury (DILI) from a manufactured medication or herbal remedy, the Council for International Organizations of Medical Sciences (CIOMS) developed RUCAM, a set of diagnostic criteria for DILI, which can be used to determine the probability of DILI based on pattern of injury.15 Although not widely used in clinical practice, RUCAM can help identify the possibility of DILI outside of expert consensus.16 It seems to have better discriminative ability than the Maria and Victorino scale, also used to identify DILI.16,17 While there is no replacement for clinical judgment, these scales may aid in identifying potential causes of DILI. The National Institutes of Health also has a LiverTox online tool that can assist health care professionals in identifying potentially hepatotoxic substances.6
Conclusions
We present a patient with AUD who developed cholestatic liver injury after ashwagandha use. Crucial to the diagnostic process is quantifying the amount ingested before presentation and the presence of contaminants, which is currently difficult to quantify given the lack of mechanisms to test supplements expediently in this manner in the clinical setting, which also requires the patient to bring in the supplements directly. There is also a lack of regulation and uniformity in these products. A clinician may be inclined to measure ashwagandha serum levels; however, such a test is not available to our knowledge. Nonetheless, using clinical tools such as RUCAM and utilizing databases, such as LiverTox, may help clinicians identify and remove potentially unsafe supplements. While there are many possible synergies between current medical practice and herbal remedies, practitioners must take care to first do no harm, as outlined in our Hippocratic Oath.
1. Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373-382. doi:10.1055/s-0029-1240006
2. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380-1387. doi:10.1038/ajg.2012.138
3. Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156(8):2230-2241.e11. doi:10.1053/j.gastro.2019.02.002
4. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317
5. Björnsson HK, Björnsson, Avula B, et al. (2020). Ashwagandha‐induced liver injury: a case series from Iceland and the US Drug‐Induced Liver Injury Network. Liver Int. 2020;40(4):825-829. doi:10.1111/liv.14393
6. National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury [internet]. Ashwagandha. Updated May 2, 2019. Accessed August 7, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548536
7. Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK. Efficacy and safety evaluation of Ayurvedic treatment (ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res. 2015;141(1):100-106. doi:10.4103/0971-5916.154510
8. Kumar G, Srivastava A, Sharma SK, Gupta YK. Safety and efficacy evaluation of Ayurvedic treatment (arjuna powder and Arogyavardhini Vati) in dyslipidemia patients: a pilot prospective cohort clinical study. 2012;33(2):197-201. doi:10.4103/0974-8520.105238
9. Sultana N, Shimmi S, Parash MT, Akhtar J. Effects of ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiologist. 2012;7(1): 1-7. doi:10.3329/JBSP.V7I1.11152
10. Patel DP, Yan T, Kim D, et al. Withaferin A improves nonalcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371(2):360-374. doi:10.1124/jpet.119.256792
11. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8):448-454. doi:10.2957/kanzo.58.448
12. Alali F, Hermez K, Ullah N. Acute hepatitis induced by a unique combination of herbal supplements. Am J Gastroenterol. 2018;113:S1661.
13. Sava J, Varghese A, Pandita N. Lack of the cytochrome P450 3A interaction of methanolic extract of Withania somnifera, Withaferin A, Withanolide A and Withanoside IV. J Pharm Negative Results. 2013;4(1):26.
14. Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474-485. doi:10.1056/NEJMra021844.
15. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1333. doi:10.1016/0895-4356(93)90101-6
16. Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29(4):348-356. doi.10.1002/cld.615
17. Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33(1):123-130. doi:10.1053/jhep.2001.20645
Many patients take herbals as alternative supplements to boost energy and mood. There are increasing reports of unintended adverse effects related to these supplements, particularly to the liver.1-3 A study by the Drug-Induced Liver Injury Network found that liver injury caused by herbals and dietary supplements has increased from 7% in 2004 to 20% in 2013.4
The supplement ashwagandha has become increasingly popular. Ashwagandha is extracted from the root of Withania somnifera (
To date, the factors defining the population at risk for ashwagandha toxicity are unclear, and an understanding of how to diagnose drug-induced liver injury is still immature in clinical practice. The regulation and study of the herbal and dietary supplement industry remain challenging. While many so-called natural substances are well tolerated, others can have unanticipated and harmful adverse effects and drug interactions. Future research should not only identify potentially harmful substances, but also which patients may be at greatest risk.
Case Presentation
A 48-year-old man with a history of severe alcohol use disorder (AUD) complicated by fatty liver and withdrawal seizures and delirium tremens, hypertension, depression, and anxiety presented to the emergency department (ED) after 4 days of having jaundice, epigastric abdominal pain, dark urine, and pale stools. In the preceding months, he had increased his alcohol use to as many as 12 drinks daily due to depression. After experiencing a blackout, he stopped drinking 7 days before presenting to the ED. He felt withdrawal symptoms, including tremors, diaphoresis, abdominal pain, nausea, and vomiting. On the third day of withdrawals, he reported that he had started taking an over-the-counter testosterone-boosting supplement to increase his energy, which he referred to as TestBoost—a mix of 8 ingredients, including ashwagandha, eleuthero root, Hawthorn berry, longjack, ginseng root, mushroom extract, bindii, and horny goat weed. After taking the supplement for 2 days, he noticed that his urine darkened, his stools became paler, his abdominal pain worsened, and he became jaundiced. After 2 additional days without improvement, and still taking the supplement, he presented to the ED. He reported having no fever, chills, recent illness, chest pain, shortness of breath, melena, lower extremity swelling, recent travel, or any changes in medications.
The patient had a 100.1 °F temperature, 102 beats per minute pulse; 129/94 mm Hg blood pressure, 18 beats per minute respiratory rate, and 97% oxygen saturation on room air on admission. He was in no acute distress, though his examination was notable for generalized jaundice and scleral icterus. He was mildly tender to palpation in the epigastric and right upper quadrant region. He was alert and oriented without confusion. He did not have any asterixis or spider angiomas, though he had scattered bruises on his left flank and left calf. His laboratory results were notable for mildly elevated aspartate aminotransferase (AST), 58 U/L (reference range, 13-35); alanine transaminase (ALT), 49 U/L (reference range, 7-45); and alkaline phosphatase (ALP), 98 U/L (reference range 33-94); total bilirubin, 13.6 mg/dL (reference range, 0.2-1.0); direct bilirubin, 8.4 mg/dL (reference range, 0.2-1); and international normalized ratio (INR), 1.11 (reference range, 2-3). His white blood cell and platelet counts were not remarkable at 9790/μL (reference range, 4500-11,000) and 337,000/μL (reference range, 150,000-440,000), respectively. Abdominal ultrasound and computed tomography (CT) revealed fatty liver with contracted gallbladder and no biliary dilatation. Urine ethanol levels were negative. The gastrointestinal (GI) service was consulted and agreed that his cholestatic injury was nonobstructive and likely related to the ashwagandha component of his supplement. The recommendation was cessation with close outpatient follow-up.
The patient was not prescribed any additional medications, such as steroids or ursodiol. He ceased supplement use following hospitalization; but relapsed into alcohol use 1 month after his discharge. Within 3 weeks, his total bilirubin had improved to 2.87 mg/dL, though AST, ALT, and ALP worsened to 127 U/L, 152 U/L, and 140 U/L, respectively. According to the notes of his psychiatrist who saw him at the time the laboratory tests were drawn, he had remained sober since discharge. His acute hepatitis panel drawn on admission was negative, and he demonstrated immunity to hepatitis A and B. Urine toxicology was negative. Antinuclear antibody (ANA) test was negative 1 year prior to discharge. Epstein-Barr virus (EBV), cytomegalovirus (CMV), ANA, antismooth muscle antibody, and immunoglobulins were not checked as suspicion for these etiologies was low. The Roussel Uclaf Causality Assessment Method (RUCAM) score was calculated as 6 (+1 for timing, +2 for drop in total bilirubin, +1 for ethanol risk factor, 0 for no other drugs, 0 for rule out of other diseases, +2 for known hepatotoxicity, 0 no repeat administration) for this patient indicating probable adverse drug reaction liver injury (Tables 1 and 2). However, we acknowledge that CMV, EBV, and herpes simplex virus status were not tested.
The 8 ingredients contained in TestBoost aside from ashwagandha did not have any major known liver adverse effects per a major database of medications. The other ingredients include eleuthero root, Hawthorn berry (crataegus laevigata), longjack (eurycoma longifolla) root, American ginseng root (American panax ginseng—panax quinquefolius), and Cordyceps mycelium (mushroom) extract, bindii (Tribulus terrestris), and epimedium grandiflorum (horny goat weed).6 No assays were performed to confirm purity of the ingredients in the patient’s supplement container.
Alcoholic hepatitis is an important consideration in this patient with AUD, though the timing of symptoms with supplement use and the cholestatic injury pattern with normal INR seems more consistent with drug-induced injury. Viral, infectious, and obstructive etiologies also were investigated. Acute viral hepatitis was ruled out based on bloodwork. The normal hepatobiliary tree on both ultrasound and CT effectively ruled out acute cholecystitis, cholangitis, and choledocholithiasis and there was no further indication for magnetic resonance cholangiopancreatography. There was no hepatic vein clot suggestive of Budd-Chiari syndrome. Autoimmune hepatitis was thought to be unlikely given that the etiology of injury seemed cholestatic in nature. Given the timing of the liver injury relative to supplement use it is likely that ashwagandha was a causative factor of this patient’s liver injury overlaid on an already strained liver from increased alcohol abuse.
The patient did not follow up with the GI service as an outpatient. There are no reports that the patient continued using the testosterone booster. His bilirubin improved dramatically within 1.5 months while his liver enzymes peaked 3 weeks later, with ALT ≥ AST. During his next admission 3 months later, he had relapsed, and his liver enzymes had the classic 2:1 AST to ALT ratio.
Discussion
Generally, ashwagandha has been thought to be well tolerated and possibly hepatoprotective.7-10 However, recent studies suggest potential for hepatotoxicity, though without clear guidance about which patients are most at risk.5,11,12 A study by Inagaki and colleagues suggests the potential for dose-dependent mechanism of liver injury, and this is supported by in vitro CYP450 inhibition with high doses of W Somnifera extract.11,13 We hypothesize that there may be a multihit process that makes some patients more susceptible to supplement harm, particularly those with repeated exposures and with ongoing exposure to hepatic toxins, such as AUD.14 Supplements should be used with more caution in these individuals.
Additionally, although there are no validated guidelines to confirm the diagnosis of drug-induced liver injury (DILI) from a manufactured medication or herbal remedy, the Council for International Organizations of Medical Sciences (CIOMS) developed RUCAM, a set of diagnostic criteria for DILI, which can be used to determine the probability of DILI based on pattern of injury.15 Although not widely used in clinical practice, RUCAM can help identify the possibility of DILI outside of expert consensus.16 It seems to have better discriminative ability than the Maria and Victorino scale, also used to identify DILI.16,17 While there is no replacement for clinical judgment, these scales may aid in identifying potential causes of DILI. The National Institutes of Health also has a LiverTox online tool that can assist health care professionals in identifying potentially hepatotoxic substances.6
Conclusions
We present a patient with AUD who developed cholestatic liver injury after ashwagandha use. Crucial to the diagnostic process is quantifying the amount ingested before presentation and the presence of contaminants, which is currently difficult to quantify given the lack of mechanisms to test supplements expediently in this manner in the clinical setting, which also requires the patient to bring in the supplements directly. There is also a lack of regulation and uniformity in these products. A clinician may be inclined to measure ashwagandha serum levels; however, such a test is not available to our knowledge. Nonetheless, using clinical tools such as RUCAM and utilizing databases, such as LiverTox, may help clinicians identify and remove potentially unsafe supplements. While there are many possible synergies between current medical practice and herbal remedies, practitioners must take care to first do no harm, as outlined in our Hippocratic Oath.
Many patients take herbals as alternative supplements to boost energy and mood. There are increasing reports of unintended adverse effects related to these supplements, particularly to the liver.1-3 A study by the Drug-Induced Liver Injury Network found that liver injury caused by herbals and dietary supplements has increased from 7% in 2004 to 20% in 2013.4
The supplement ashwagandha has become increasingly popular. Ashwagandha is extracted from the root of Withania somnifera (
To date, the factors defining the population at risk for ashwagandha toxicity are unclear, and an understanding of how to diagnose drug-induced liver injury is still immature in clinical practice. The regulation and study of the herbal and dietary supplement industry remain challenging. While many so-called natural substances are well tolerated, others can have unanticipated and harmful adverse effects and drug interactions. Future research should not only identify potentially harmful substances, but also which patients may be at greatest risk.
Case Presentation
A 48-year-old man with a history of severe alcohol use disorder (AUD) complicated by fatty liver and withdrawal seizures and delirium tremens, hypertension, depression, and anxiety presented to the emergency department (ED) after 4 days of having jaundice, epigastric abdominal pain, dark urine, and pale stools. In the preceding months, he had increased his alcohol use to as many as 12 drinks daily due to depression. After experiencing a blackout, he stopped drinking 7 days before presenting to the ED. He felt withdrawal symptoms, including tremors, diaphoresis, abdominal pain, nausea, and vomiting. On the third day of withdrawals, he reported that he had started taking an over-the-counter testosterone-boosting supplement to increase his energy, which he referred to as TestBoost—a mix of 8 ingredients, including ashwagandha, eleuthero root, Hawthorn berry, longjack, ginseng root, mushroom extract, bindii, and horny goat weed. After taking the supplement for 2 days, he noticed that his urine darkened, his stools became paler, his abdominal pain worsened, and he became jaundiced. After 2 additional days without improvement, and still taking the supplement, he presented to the ED. He reported having no fever, chills, recent illness, chest pain, shortness of breath, melena, lower extremity swelling, recent travel, or any changes in medications.
The patient had a 100.1 °F temperature, 102 beats per minute pulse; 129/94 mm Hg blood pressure, 18 beats per minute respiratory rate, and 97% oxygen saturation on room air on admission. He was in no acute distress, though his examination was notable for generalized jaundice and scleral icterus. He was mildly tender to palpation in the epigastric and right upper quadrant region. He was alert and oriented without confusion. He did not have any asterixis or spider angiomas, though he had scattered bruises on his left flank and left calf. His laboratory results were notable for mildly elevated aspartate aminotransferase (AST), 58 U/L (reference range, 13-35); alanine transaminase (ALT), 49 U/L (reference range, 7-45); and alkaline phosphatase (ALP), 98 U/L (reference range 33-94); total bilirubin, 13.6 mg/dL (reference range, 0.2-1.0); direct bilirubin, 8.4 mg/dL (reference range, 0.2-1); and international normalized ratio (INR), 1.11 (reference range, 2-3). His white blood cell and platelet counts were not remarkable at 9790/μL (reference range, 4500-11,000) and 337,000/μL (reference range, 150,000-440,000), respectively. Abdominal ultrasound and computed tomography (CT) revealed fatty liver with contracted gallbladder and no biliary dilatation. Urine ethanol levels were negative. The gastrointestinal (GI) service was consulted and agreed that his cholestatic injury was nonobstructive and likely related to the ashwagandha component of his supplement. The recommendation was cessation with close outpatient follow-up.
The patient was not prescribed any additional medications, such as steroids or ursodiol. He ceased supplement use following hospitalization; but relapsed into alcohol use 1 month after his discharge. Within 3 weeks, his total bilirubin had improved to 2.87 mg/dL, though AST, ALT, and ALP worsened to 127 U/L, 152 U/L, and 140 U/L, respectively. According to the notes of his psychiatrist who saw him at the time the laboratory tests were drawn, he had remained sober since discharge. His acute hepatitis panel drawn on admission was negative, and he demonstrated immunity to hepatitis A and B. Urine toxicology was negative. Antinuclear antibody (ANA) test was negative 1 year prior to discharge. Epstein-Barr virus (EBV), cytomegalovirus (CMV), ANA, antismooth muscle antibody, and immunoglobulins were not checked as suspicion for these etiologies was low. The Roussel Uclaf Causality Assessment Method (RUCAM) score was calculated as 6 (+1 for timing, +2 for drop in total bilirubin, +1 for ethanol risk factor, 0 for no other drugs, 0 for rule out of other diseases, +2 for known hepatotoxicity, 0 no repeat administration) for this patient indicating probable adverse drug reaction liver injury (Tables 1 and 2). However, we acknowledge that CMV, EBV, and herpes simplex virus status were not tested.
The 8 ingredients contained in TestBoost aside from ashwagandha did not have any major known liver adverse effects per a major database of medications. The other ingredients include eleuthero root, Hawthorn berry (crataegus laevigata), longjack (eurycoma longifolla) root, American ginseng root (American panax ginseng—panax quinquefolius), and Cordyceps mycelium (mushroom) extract, bindii (Tribulus terrestris), and epimedium grandiflorum (horny goat weed).6 No assays were performed to confirm purity of the ingredients in the patient’s supplement container.
Alcoholic hepatitis is an important consideration in this patient with AUD, though the timing of symptoms with supplement use and the cholestatic injury pattern with normal INR seems more consistent with drug-induced injury. Viral, infectious, and obstructive etiologies also were investigated. Acute viral hepatitis was ruled out based on bloodwork. The normal hepatobiliary tree on both ultrasound and CT effectively ruled out acute cholecystitis, cholangitis, and choledocholithiasis and there was no further indication for magnetic resonance cholangiopancreatography. There was no hepatic vein clot suggestive of Budd-Chiari syndrome. Autoimmune hepatitis was thought to be unlikely given that the etiology of injury seemed cholestatic in nature. Given the timing of the liver injury relative to supplement use it is likely that ashwagandha was a causative factor of this patient’s liver injury overlaid on an already strained liver from increased alcohol abuse.
The patient did not follow up with the GI service as an outpatient. There are no reports that the patient continued using the testosterone booster. His bilirubin improved dramatically within 1.5 months while his liver enzymes peaked 3 weeks later, with ALT ≥ AST. During his next admission 3 months later, he had relapsed, and his liver enzymes had the classic 2:1 AST to ALT ratio.
Discussion
Generally, ashwagandha has been thought to be well tolerated and possibly hepatoprotective.7-10 However, recent studies suggest potential for hepatotoxicity, though without clear guidance about which patients are most at risk.5,11,12 A study by Inagaki and colleagues suggests the potential for dose-dependent mechanism of liver injury, and this is supported by in vitro CYP450 inhibition with high doses of W Somnifera extract.11,13 We hypothesize that there may be a multihit process that makes some patients more susceptible to supplement harm, particularly those with repeated exposures and with ongoing exposure to hepatic toxins, such as AUD.14 Supplements should be used with more caution in these individuals.
Additionally, although there are no validated guidelines to confirm the diagnosis of drug-induced liver injury (DILI) from a manufactured medication or herbal remedy, the Council for International Organizations of Medical Sciences (CIOMS) developed RUCAM, a set of diagnostic criteria for DILI, which can be used to determine the probability of DILI based on pattern of injury.15 Although not widely used in clinical practice, RUCAM can help identify the possibility of DILI outside of expert consensus.16 It seems to have better discriminative ability than the Maria and Victorino scale, also used to identify DILI.16,17 While there is no replacement for clinical judgment, these scales may aid in identifying potential causes of DILI. The National Institutes of Health also has a LiverTox online tool that can assist health care professionals in identifying potentially hepatotoxic substances.6
Conclusions
We present a patient with AUD who developed cholestatic liver injury after ashwagandha use. Crucial to the diagnostic process is quantifying the amount ingested before presentation and the presence of contaminants, which is currently difficult to quantify given the lack of mechanisms to test supplements expediently in this manner in the clinical setting, which also requires the patient to bring in the supplements directly. There is also a lack of regulation and uniformity in these products. A clinician may be inclined to measure ashwagandha serum levels; however, such a test is not available to our knowledge. Nonetheless, using clinical tools such as RUCAM and utilizing databases, such as LiverTox, may help clinicians identify and remove potentially unsafe supplements. While there are many possible synergies between current medical practice and herbal remedies, practitioners must take care to first do no harm, as outlined in our Hippocratic Oath.
1. Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373-382. doi:10.1055/s-0029-1240006
2. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380-1387. doi:10.1038/ajg.2012.138
3. Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156(8):2230-2241.e11. doi:10.1053/j.gastro.2019.02.002
4. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317
5. Björnsson HK, Björnsson, Avula B, et al. (2020). Ashwagandha‐induced liver injury: a case series from Iceland and the US Drug‐Induced Liver Injury Network. Liver Int. 2020;40(4):825-829. doi:10.1111/liv.14393
6. National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury [internet]. Ashwagandha. Updated May 2, 2019. Accessed August 7, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548536
7. Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK. Efficacy and safety evaluation of Ayurvedic treatment (ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res. 2015;141(1):100-106. doi:10.4103/0971-5916.154510
8. Kumar G, Srivastava A, Sharma SK, Gupta YK. Safety and efficacy evaluation of Ayurvedic treatment (arjuna powder and Arogyavardhini Vati) in dyslipidemia patients: a pilot prospective cohort clinical study. 2012;33(2):197-201. doi:10.4103/0974-8520.105238
9. Sultana N, Shimmi S, Parash MT, Akhtar J. Effects of ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiologist. 2012;7(1): 1-7. doi:10.3329/JBSP.V7I1.11152
10. Patel DP, Yan T, Kim D, et al. Withaferin A improves nonalcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371(2):360-374. doi:10.1124/jpet.119.256792
11. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8):448-454. doi:10.2957/kanzo.58.448
12. Alali F, Hermez K, Ullah N. Acute hepatitis induced by a unique combination of herbal supplements. Am J Gastroenterol. 2018;113:S1661.
13. Sava J, Varghese A, Pandita N. Lack of the cytochrome P450 3A interaction of methanolic extract of Withania somnifera, Withaferin A, Withanolide A and Withanoside IV. J Pharm Negative Results. 2013;4(1):26.
14. Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474-485. doi:10.1056/NEJMra021844.
15. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1333. doi:10.1016/0895-4356(93)90101-6
16. Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29(4):348-356. doi.10.1002/cld.615
17. Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33(1):123-130. doi:10.1053/jhep.2001.20645
1. Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373-382. doi:10.1055/s-0029-1240006
2. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380-1387. doi:10.1038/ajg.2012.138
3. Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156(8):2230-2241.e11. doi:10.1053/j.gastro.2019.02.002
4. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317
5. Björnsson HK, Björnsson, Avula B, et al. (2020). Ashwagandha‐induced liver injury: a case series from Iceland and the US Drug‐Induced Liver Injury Network. Liver Int. 2020;40(4):825-829. doi:10.1111/liv.14393
6. National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury [internet]. Ashwagandha. Updated May 2, 2019. Accessed August 7, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548536
7. Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK. Efficacy and safety evaluation of Ayurvedic treatment (ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res. 2015;141(1):100-106. doi:10.4103/0971-5916.154510
8. Kumar G, Srivastava A, Sharma SK, Gupta YK. Safety and efficacy evaluation of Ayurvedic treatment (arjuna powder and Arogyavardhini Vati) in dyslipidemia patients: a pilot prospective cohort clinical study. 2012;33(2):197-201. doi:10.4103/0974-8520.105238
9. Sultana N, Shimmi S, Parash MT, Akhtar J. Effects of ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiologist. 2012;7(1): 1-7. doi:10.3329/JBSP.V7I1.11152
10. Patel DP, Yan T, Kim D, et al. Withaferin A improves nonalcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371(2):360-374. doi:10.1124/jpet.119.256792
11. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8):448-454. doi:10.2957/kanzo.58.448
12. Alali F, Hermez K, Ullah N. Acute hepatitis induced by a unique combination of herbal supplements. Am J Gastroenterol. 2018;113:S1661.
13. Sava J, Varghese A, Pandita N. Lack of the cytochrome P450 3A interaction of methanolic extract of Withania somnifera, Withaferin A, Withanolide A and Withanoside IV. J Pharm Negative Results. 2013;4(1):26.
14. Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474-485. doi:10.1056/NEJMra021844.
15. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1333. doi:10.1016/0895-4356(93)90101-6
16. Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29(4):348-356. doi.10.1002/cld.615
17. Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33(1):123-130. doi:10.1053/jhep.2001.20645
Role of Prophylactic Cranial Irradiation in Small Cell Carcinoma of Urinary Bladder: Case Report and Literature Review
INTRODUCTION
Urinary bladder is an extremely rare site of extrapulmonary small cell cancer (EPSCC). Unlike small cell lung cancer (SCLC), there is no clear guideline for prophylactic cranial irradiation (PCI) for EPSCC. In this case report and literature review, we discuss small cell cancer of urinary bladder (SCCUB) and the role of PCI in SCCUB.
CASE PRESENTATION
A 74-year-old male presented with gross hematuria and an unremarkable physical examination. CT showed 1.7 cm right anterolateral bladder wall thickening. Cystoscopy revealed a 2-3 cm high-grade bladder lesion. Pathology from transurethral resection of the tumor was consistent with T1N0M0 small cell carcinoma. MRI brain and FDG-PET showed no extravesical disease. Patient received four cycles of neoadjuvant carboplatin/etoposide per his preference as he wanted to protect his hearing due to his profession followed by radical cystoprostatectomy. Post-op pathology showed clear margins. We decided to forego PCI in favor of interval surveillance with MRI and follow- up images remain negative for distant metastases.
DISCUSSION
EPSCC accounts for 2.5-5% of all SCC, very rare in male genitourinary tract. Treatment approach is derived from SCLC, guided by extent of disease and patient’s functional status. Role of PCI in EPSCC has not been clearly described, and even less evidence is available for SCCUB. From a review of eleven studies in PubMed for the role of PCI in SCCUB or EPSCC, we found that SCCUB has lower incidence of brain metastases than SCLC. One study suggested that SCCUB arises from totipotent cells in the submucosa, unlike Kulchitsky cell origin of SCLC. This difference might explain the difference in their metastatic behavior. With this background, PCI is not routinely recommended for limited- stage SCCUB. There might still be a role for PCI in extensive SCCUB with high metastatic burden. More studies are needed to update the guidelines for the role of PCI for these tumors.
CONCLUSIONS
Per this literature review, PCI is not routinely recommended for SCCUB, likely due to different cells of origin compared to SCLC. Future studies should focus on characterizing differences in their metastatic behavior and updating guidelines for PCI for SCCUB.
INTRODUCTION
Urinary bladder is an extremely rare site of extrapulmonary small cell cancer (EPSCC). Unlike small cell lung cancer (SCLC), there is no clear guideline for prophylactic cranial irradiation (PCI) for EPSCC. In this case report and literature review, we discuss small cell cancer of urinary bladder (SCCUB) and the role of PCI in SCCUB.
CASE PRESENTATION
A 74-year-old male presented with gross hematuria and an unremarkable physical examination. CT showed 1.7 cm right anterolateral bladder wall thickening. Cystoscopy revealed a 2-3 cm high-grade bladder lesion. Pathology from transurethral resection of the tumor was consistent with T1N0M0 small cell carcinoma. MRI brain and FDG-PET showed no extravesical disease. Patient received four cycles of neoadjuvant carboplatin/etoposide per his preference as he wanted to protect his hearing due to his profession followed by radical cystoprostatectomy. Post-op pathology showed clear margins. We decided to forego PCI in favor of interval surveillance with MRI and follow- up images remain negative for distant metastases.
DISCUSSION
EPSCC accounts for 2.5-5% of all SCC, very rare in male genitourinary tract. Treatment approach is derived from SCLC, guided by extent of disease and patient’s functional status. Role of PCI in EPSCC has not been clearly described, and even less evidence is available for SCCUB. From a review of eleven studies in PubMed for the role of PCI in SCCUB or EPSCC, we found that SCCUB has lower incidence of brain metastases than SCLC. One study suggested that SCCUB arises from totipotent cells in the submucosa, unlike Kulchitsky cell origin of SCLC. This difference might explain the difference in their metastatic behavior. With this background, PCI is not routinely recommended for limited- stage SCCUB. There might still be a role for PCI in extensive SCCUB with high metastatic burden. More studies are needed to update the guidelines for the role of PCI for these tumors.
CONCLUSIONS
Per this literature review, PCI is not routinely recommended for SCCUB, likely due to different cells of origin compared to SCLC. Future studies should focus on characterizing differences in their metastatic behavior and updating guidelines for PCI for SCCUB.
INTRODUCTION
Urinary bladder is an extremely rare site of extrapulmonary small cell cancer (EPSCC). Unlike small cell lung cancer (SCLC), there is no clear guideline for prophylactic cranial irradiation (PCI) for EPSCC. In this case report and literature review, we discuss small cell cancer of urinary bladder (SCCUB) and the role of PCI in SCCUB.
CASE PRESENTATION
A 74-year-old male presented with gross hematuria and an unremarkable physical examination. CT showed 1.7 cm right anterolateral bladder wall thickening. Cystoscopy revealed a 2-3 cm high-grade bladder lesion. Pathology from transurethral resection of the tumor was consistent with T1N0M0 small cell carcinoma. MRI brain and FDG-PET showed no extravesical disease. Patient received four cycles of neoadjuvant carboplatin/etoposide per his preference as he wanted to protect his hearing due to his profession followed by radical cystoprostatectomy. Post-op pathology showed clear margins. We decided to forego PCI in favor of interval surveillance with MRI and follow- up images remain negative for distant metastases.
DISCUSSION
EPSCC accounts for 2.5-5% of all SCC, very rare in male genitourinary tract. Treatment approach is derived from SCLC, guided by extent of disease and patient’s functional status. Role of PCI in EPSCC has not been clearly described, and even less evidence is available for SCCUB. From a review of eleven studies in PubMed for the role of PCI in SCCUB or EPSCC, we found that SCCUB has lower incidence of brain metastases than SCLC. One study suggested that SCCUB arises from totipotent cells in the submucosa, unlike Kulchitsky cell origin of SCLC. This difference might explain the difference in their metastatic behavior. With this background, PCI is not routinely recommended for limited- stage SCCUB. There might still be a role for PCI in extensive SCCUB with high metastatic burden. More studies are needed to update the guidelines for the role of PCI for these tumors.
CONCLUSIONS
Per this literature review, PCI is not routinely recommended for SCCUB, likely due to different cells of origin compared to SCLC. Future studies should focus on characterizing differences in their metastatic behavior and updating guidelines for PCI for SCCUB.