User login
ACC/AHA issue updated atrial fibrillation guideline
The American College of Cardiology (ACC), the American Heart Association (AHA), the American College of Chest Physicians (ACCP), and the Heart Rhythm Society (HRS) have issued an updated guideline for preventing and optimally managing atrial fibrillation (AF).
The 2023 ACC/AHA/ACCP/HRS Guideline for Diagnosis and Management of Atrial Fibrillation was published online in the Journal of the American College of Cardiology and Circulation.
“The new guideline has important changes,” including a new way to classify AF, Jose Joglar, MD, professor of cardiac electrophysiology at UT Southwestern Medical Center in Dallas, Texas, and chair of the writing committee, said in an interview.
The previous classification was largely based only on arrhythmia duration and tended to emphasize specific therapeutic interventions rather than a more holistic and multidisciplinary management approach, Dr. Joglar explained.
, from prevention, lifestyle and risk factor modification, screening, and therapy.
Stage 1: At risk for AF due to the presence of risk factors
Stage 2: Pre-AF, with evidence of structural or electrical findings predisposing to AF
Stage 3: AF, including paroxysmal (3A), persistent (3B), long-standing persistent (3C), successful AF ablation (3D)
Stage 4: Permanent AF
The updated guideline recognizes lifestyle and risk factor modification as a “pillar” of AF management and offers “more prescriptive” recommendations, including management of obesity, weight loss, physical activity, smoking cessation, alcohol moderation, hypertension, and other comorbidities.
“We should not only be telling patients they need to be healthy, which doesn’t mean much to a patient, we need to tell them precisely what they need to do. For example, how much exercise to do or how much weight to lose to have a benefit,” Dr. Joglar said in an interview.
The good news for many people, he noted, is that coffee, which has had a “bad reputation,” is okay, as the latest data show it doesn’t seem to exacerbate AF.
The new guideline continues to endorse use of the CHA2DS2-VASc score as the predictor of choice to determine the risk of stroke, but it also allows for flexibility to use other calculators when uncertainty exists or when other risk factors, such as kidney disease, need to be included.
With the emergence of “new and consistent” evidence, the guideline also emphasizes the importance of early and continued management of patients with AF with a focus on maintaining sinus rhythm and minimizing AF burden.
Catheter ablation of AF is given a class 1 indication as first-line therapy in selected patients, including those with heart failure with reduced ejection fraction.
That’s based on recent randomized studies that have shown catheter ablation to be “superior to pharmacological therapy” for rhythm control in appropriately selected patients, Dr. Joglar told this news organization.
“There’s no need to try pharmacological therapies after a discussion between the patient and doctor and they decide that they want to proceed with the most effective intervention,” he added.
The new guideline also upgrades the class of recommendation for left atrial appendage occlusion devices to 2a, compared with the 2019 AF Focused Update, for use of these devices in patients with long-term contraindications to anticoagulation.
It also provides updated recommendations for AF detected via implantable devices and wearables as well as recommendations for patients with AF identified during medical illness or surgery.
Development of the guideline had no commercial funding. Disclosures for the writing group are available with the original articles.
A version of this article appeared on Medscape.com.
The American College of Cardiology (ACC), the American Heart Association (AHA), the American College of Chest Physicians (ACCP), and the Heart Rhythm Society (HRS) have issued an updated guideline for preventing and optimally managing atrial fibrillation (AF).
The 2023 ACC/AHA/ACCP/HRS Guideline for Diagnosis and Management of Atrial Fibrillation was published online in the Journal of the American College of Cardiology and Circulation.
“The new guideline has important changes,” including a new way to classify AF, Jose Joglar, MD, professor of cardiac electrophysiology at UT Southwestern Medical Center in Dallas, Texas, and chair of the writing committee, said in an interview.
The previous classification was largely based only on arrhythmia duration and tended to emphasize specific therapeutic interventions rather than a more holistic and multidisciplinary management approach, Dr. Joglar explained.
, from prevention, lifestyle and risk factor modification, screening, and therapy.
Stage 1: At risk for AF due to the presence of risk factors
Stage 2: Pre-AF, with evidence of structural or electrical findings predisposing to AF
Stage 3: AF, including paroxysmal (3A), persistent (3B), long-standing persistent (3C), successful AF ablation (3D)
Stage 4: Permanent AF
The updated guideline recognizes lifestyle and risk factor modification as a “pillar” of AF management and offers “more prescriptive” recommendations, including management of obesity, weight loss, physical activity, smoking cessation, alcohol moderation, hypertension, and other comorbidities.
“We should not only be telling patients they need to be healthy, which doesn’t mean much to a patient, we need to tell them precisely what they need to do. For example, how much exercise to do or how much weight to lose to have a benefit,” Dr. Joglar said in an interview.
The good news for many people, he noted, is that coffee, which has had a “bad reputation,” is okay, as the latest data show it doesn’t seem to exacerbate AF.
The new guideline continues to endorse use of the CHA2DS2-VASc score as the predictor of choice to determine the risk of stroke, but it also allows for flexibility to use other calculators when uncertainty exists or when other risk factors, such as kidney disease, need to be included.
With the emergence of “new and consistent” evidence, the guideline also emphasizes the importance of early and continued management of patients with AF with a focus on maintaining sinus rhythm and minimizing AF burden.
Catheter ablation of AF is given a class 1 indication as first-line therapy in selected patients, including those with heart failure with reduced ejection fraction.
That’s based on recent randomized studies that have shown catheter ablation to be “superior to pharmacological therapy” for rhythm control in appropriately selected patients, Dr. Joglar told this news organization.
“There’s no need to try pharmacological therapies after a discussion between the patient and doctor and they decide that they want to proceed with the most effective intervention,” he added.
The new guideline also upgrades the class of recommendation for left atrial appendage occlusion devices to 2a, compared with the 2019 AF Focused Update, for use of these devices in patients with long-term contraindications to anticoagulation.
It also provides updated recommendations for AF detected via implantable devices and wearables as well as recommendations for patients with AF identified during medical illness or surgery.
Development of the guideline had no commercial funding. Disclosures for the writing group are available with the original articles.
A version of this article appeared on Medscape.com.
The American College of Cardiology (ACC), the American Heart Association (AHA), the American College of Chest Physicians (ACCP), and the Heart Rhythm Society (HRS) have issued an updated guideline for preventing and optimally managing atrial fibrillation (AF).
The 2023 ACC/AHA/ACCP/HRS Guideline for Diagnosis and Management of Atrial Fibrillation was published online in the Journal of the American College of Cardiology and Circulation.
“The new guideline has important changes,” including a new way to classify AF, Jose Joglar, MD, professor of cardiac electrophysiology at UT Southwestern Medical Center in Dallas, Texas, and chair of the writing committee, said in an interview.
The previous classification was largely based only on arrhythmia duration and tended to emphasize specific therapeutic interventions rather than a more holistic and multidisciplinary management approach, Dr. Joglar explained.
, from prevention, lifestyle and risk factor modification, screening, and therapy.
Stage 1: At risk for AF due to the presence of risk factors
Stage 2: Pre-AF, with evidence of structural or electrical findings predisposing to AF
Stage 3: AF, including paroxysmal (3A), persistent (3B), long-standing persistent (3C), successful AF ablation (3D)
Stage 4: Permanent AF
The updated guideline recognizes lifestyle and risk factor modification as a “pillar” of AF management and offers “more prescriptive” recommendations, including management of obesity, weight loss, physical activity, smoking cessation, alcohol moderation, hypertension, and other comorbidities.
“We should not only be telling patients they need to be healthy, which doesn’t mean much to a patient, we need to tell them precisely what they need to do. For example, how much exercise to do or how much weight to lose to have a benefit,” Dr. Joglar said in an interview.
The good news for many people, he noted, is that coffee, which has had a “bad reputation,” is okay, as the latest data show it doesn’t seem to exacerbate AF.
The new guideline continues to endorse use of the CHA2DS2-VASc score as the predictor of choice to determine the risk of stroke, but it also allows for flexibility to use other calculators when uncertainty exists or when other risk factors, such as kidney disease, need to be included.
With the emergence of “new and consistent” evidence, the guideline also emphasizes the importance of early and continued management of patients with AF with a focus on maintaining sinus rhythm and minimizing AF burden.
Catheter ablation of AF is given a class 1 indication as first-line therapy in selected patients, including those with heart failure with reduced ejection fraction.
That’s based on recent randomized studies that have shown catheter ablation to be “superior to pharmacological therapy” for rhythm control in appropriately selected patients, Dr. Joglar told this news organization.
“There’s no need to try pharmacological therapies after a discussion between the patient and doctor and they decide that they want to proceed with the most effective intervention,” he added.
The new guideline also upgrades the class of recommendation for left atrial appendage occlusion devices to 2a, compared with the 2019 AF Focused Update, for use of these devices in patients with long-term contraindications to anticoagulation.
It also provides updated recommendations for AF detected via implantable devices and wearables as well as recommendations for patients with AF identified during medical illness or surgery.
Development of the guideline had no commercial funding. Disclosures for the writing group are available with the original articles.
A version of this article appeared on Medscape.com.
Conditional recommendations rule in new SARD-associated interstitial lung disease guidelines
SAN DIEGO – In the spring of 2024, the American College of Rheumatology is expected to release guidelines to help inform the screening, monitoring, and treatment of interstitial lung disease (ILD) in people with systemic autoimmune rheumatic diseases (SARDs).
The guidelines, which were previewed during a session at the ACR’s annual meeting, will include 50 recommendations, 3 of which met criteria for a strong rating:
- For people with SARDs at increased risk of developing ILD, the authors strongly recommend against screening with surgical lung biopsy.
- For people with systemic sclerosis (SSc)-related ILD, the authors strongly recommend against glucocorticoids as a first-line ILD treatment.
- For people with SSc-related ILD progression despite an initial ILD treatment, the authors strongly recommend against using long-term glucocorticoids.
Elana J. Bernstein, MD, MSc, a rheumatologist who directs the Columbia/New York-Presbyterian Scleroderma Center, and Sindhu R. Johnson, MD, a rheumatologist who directs the Toronto Scleroderma Program at the University of Toronto, provided a sneak peek of the recommendations to attendees before anticipated publication in Arthritis & Rheumatology and Arthritis Care & Research. For now, guideline summaries for screening and monitoring and treatment are currently available, and three manuscripts are under peer review: one about screening and monitoring, one about treatment, and one about the patient panel that participated in the effort.
“ILD is a significant cause of morbidity and mortality in people with SARDs,” said Dr. Bernstein, who is co-first author of the guidelines. “People with systemic sclerosis, rheumatoid arthritis, idiopathic inflammatory myopathies, mixed connective tissue disease, and Sjögren’s disease are at greatest risk of developing ILD.”
Pediatric patients with SARDs excluded
The guidelines’ population of interest was people 17 years of age and older who were diagnosed with SARDs with a high risk of ILD. Pediatric patients with SARDs were excluded from the endeavor, as were those with systemic lupus erythematosus, antineutrophil cytoplasmic antibody–associated vasculitis, sarcoidosis, ankylosing spondylitis, undifferentiated connective tissue disease, interstitial pneumonia with autoimmune features, and those with unclassifiable ILD.
In the realm of screening, the guideline authors conditionally recommend two screening tests for patients considered at increased risk of ILD: pulmonary function tests and high-resolution chest CT (HRCT). Pulmonary function tests should include spirometry, lung volumes, and diffusion capacity. “Office spirometry alone is insufficient,” said Dr. Johnson, who served as lead author of the guidelines. And while a HRCT scan is recommended, “some patients may present to the emergency room with acute onset shortness of breath, and they may receive a CT angiogram to screen for pulmonary embolism,” she said. “It’s important to note that CT angiograms are performed in incomplete inspiration to maximize pulmonary artery enhancement. This may produce atelectasis that may obscure or mimic ILD. As a result, CTA studies are often inadequate to screen for ILD.”
Once a patient is diagnosed with ILD, three tests are recommended for monitoring: pulmonary function testing (every 3-6 months the first year in patients with IIM and SSc, then less frequently once stable, and every 3-12 months in the first year in patients with RA, SjD, and MCTD, then less frequently once stable); ambulatory desaturation testing every 3-12 months; and HRCT as needed. Dr. Johnson noted that while that the screening of ILD lies within the realm of rheumatologists, “once a patient is diagnosed, we are encouraged to comanage these patients with pulmonologists,” she said. “Ambulatory desaturation testing is not an infrequent test in the hands of pulmonologists. This is where co-management can be helpful.” She characterized a 6-minute walk test with continuous oximetry as “insufficient and is not synonymous with ambulatory desaturation testing. Ambulatory desaturation testing includes up titration of oxygen if a patient desaturates.”
The guidelines conditionally recommend against using chest radiography, 6-minute walk test distance, ambulatory desaturation testing, and bronchoscopy for ILD screening, and there is a strong recommendation against surgical lung biopsy. “However, there are unique circumstances where these tests may be considered,” Dr. Johnson said. “For example, ambulatory desaturation testing may be helpful if a patient is unable to perform a pulmonary function test. Bronchoscopy may be used to rule out infection, sarcoidosis, lymphoma, or alveolar hemorrhage, and surgical lung biopsy may be considered if you’re trying to rule out a malignancy.”
Similarly, several tests are conditionally recommended against for the monitoring of ILD, including chest radiography, the 6-minute walk test distance, and bronchoscopy. “But there are unique circumstances where they may be considered,” she said. “The 6-minute walk test may be used if a patient is unable to perform a pulmonary function test or if they’re being assessed for lung transplantation. Bronchoscopy may be used to rule out infection or alveolar hemorrhage.”
Preferred treatment options described
First-line treatment recommendations for ILD were based on the best available published evidence, voting panel expertise, and patient preferences. For SSc, the preferred treatment options include mycophenolate (CellCept), tocilizumab (Actemra), or rituximab (Rituxan and biosimilars), while additional options include cyclophosphamide, nintedanib (Ofev), and azathioprine. For myositis, the preferred treatment options include mycophenolate, azathioprine, rituximab, or calcineurin inhibitors, while additional options include a Janus kinase (JAK) inhibitor or cyclophosphamide. For MCTD, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include tocilizumab or cyclophosphamide. For RA and Sjögren’s, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include cyclophosphamide. Dr. Johnson emphasized that there was low certainty evidence to recommend one treatment over another. “Many situations might lead a provider to choose a different option for ILD treatment, such as the presence of comorbidities or extra-pulmonary disease,” she said. “So, while our guidelines were focused on effectiveness for ILD, providers may choose therapies that will help ILD and other disease manifestations.”
The guidelines conditionally recommend a short course of glucocorticoids as a bridging therapy or for treatment of a flare of ILD in patients with myositis, MCTD, RA, and Sjögren’s. The panel strongly recommends against the use of glucocorticoids in patients with SSc due to the concern for inducing a scleroderma renal crisis. “While this may be common knowledge for rheumatologists, it may not be common knowledge for pulmonologists,” she said. “So here is an opportunity to educate our pulmonology colleagues in our consultation notes.”
The guidelines also include recommendations for progression of ILD, which was defined using the INBUILD trial criteria. Mycophenolate is conditionally recommended to be the first ILD treatment for all SARDs when progression occurs, if it wasn’t the first ILD treatment used. “If it was, then other medications that rheumatologists are used to can be considered as the next ILD treatment in the face of progression: rituximab, nintedanib, tocilizumab, and cyclophosphamide,” she said. The guidelines include a conditional recommendation against long-term glucocorticoid use in myositis, MCTD, RA, and Sjögren’s, plus a strong recommendation against long-term glucocorticoid use in SSc. Finally, there is a conditional recommendation of referral for lung transplant evaluation at the appropriate time at experienced centers.
Another group of recommendations has to do with cases of rapidly progressive ILD, which is characterized by rapid progression from no oxygen or a patient’s baseline oxygen requirement to a high oxygen requirement or intubation usually within days to weeks without a documented cause, such as infection or heart failure. “In cases of rapidly progressive ILD, which typically occurs in the setting of anti-MDA5 antibodies, there is a conditional recommendation for IV glucocorticoids plus two additional therapies: traditionally rituximab and mycophenolate,” Dr. Johnson said. “However, what may be new to some clinicians is combination IVIG [intravenous immunoglobulin] and a calcineurin inhibitor, notably tacrolimus,” she said. “This is the situation where experience at expert centers is influencing our guidelines in advance of data.”
A patient panel provided input
For the undertaking, a core team that included six rheumatologists; one pulmonologist; one thoracic radiologist; one expert on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology; and two literature review experts developed clinically relevant population, intervention, comparator, and outcomes (PICO) questions. The literature review team included 13 rheumatologists, 8 pulmonologists, and 3 methodologists. Finally, a 21-member patient panel was convened to share their values and preferences regarding screening, monitoring, and treatment of SARD-related ILD. Of these, Dr. Bernstein said that 4 were at risk for ILD and 17 had been diagnosed with ILD. Next, the literature review team conducted a systematic review and used the GRADE methodology to rate the available evidence as high, moderate, low, or very low. Then, a voting panel comprising 13 rheumatologists, 10 pulmonologists, 1 radiologist, and 3 patients from the patient panel cast votes for each PICO question and made final recommendations.
The review of evidence left the guidelines authors with 241 PICO questions, “which is a lot,” Dr. Bernstein said. “To put this in perspective, some guidelines address only 10 or 15 PICO questions. Fortunately, we had a dedicated group of experts who were up to the challenge.” Dr. Johnson emphasized that the forthcoming guidelines should not be used by insurers to mandate a specific order of prescribing. “Clinicians must retain the latitude to prescribe medications based on individual patient factors and preferences,” she said.
Dr. Bernstein disclosed that she is an adviser to, a consultant for, and has received grant or research support from Boehringer Ingelheim and has also received grant or research support from Kadmon and Pfizer. Dr. Johnson disclosed that she has received research support from the American College of Rheumatology to develop these guidelines. She has also been an investigator for trials sponsored by Bristol-Myers Squibb, Roche, and Boehringer Ingelheim and has mitigated these relevant conflicts of interest 1 year prior to the development of these guidelines, and will continue to do so for the foreseeable future.
SAN DIEGO – In the spring of 2024, the American College of Rheumatology is expected to release guidelines to help inform the screening, monitoring, and treatment of interstitial lung disease (ILD) in people with systemic autoimmune rheumatic diseases (SARDs).
The guidelines, which were previewed during a session at the ACR’s annual meeting, will include 50 recommendations, 3 of which met criteria for a strong rating:
- For people with SARDs at increased risk of developing ILD, the authors strongly recommend against screening with surgical lung biopsy.
- For people with systemic sclerosis (SSc)-related ILD, the authors strongly recommend against glucocorticoids as a first-line ILD treatment.
- For people with SSc-related ILD progression despite an initial ILD treatment, the authors strongly recommend against using long-term glucocorticoids.
Elana J. Bernstein, MD, MSc, a rheumatologist who directs the Columbia/New York-Presbyterian Scleroderma Center, and Sindhu R. Johnson, MD, a rheumatologist who directs the Toronto Scleroderma Program at the University of Toronto, provided a sneak peek of the recommendations to attendees before anticipated publication in Arthritis & Rheumatology and Arthritis Care & Research. For now, guideline summaries for screening and monitoring and treatment are currently available, and three manuscripts are under peer review: one about screening and monitoring, one about treatment, and one about the patient panel that participated in the effort.
“ILD is a significant cause of morbidity and mortality in people with SARDs,” said Dr. Bernstein, who is co-first author of the guidelines. “People with systemic sclerosis, rheumatoid arthritis, idiopathic inflammatory myopathies, mixed connective tissue disease, and Sjögren’s disease are at greatest risk of developing ILD.”
Pediatric patients with SARDs excluded
The guidelines’ population of interest was people 17 years of age and older who were diagnosed with SARDs with a high risk of ILD. Pediatric patients with SARDs were excluded from the endeavor, as were those with systemic lupus erythematosus, antineutrophil cytoplasmic antibody–associated vasculitis, sarcoidosis, ankylosing spondylitis, undifferentiated connective tissue disease, interstitial pneumonia with autoimmune features, and those with unclassifiable ILD.
In the realm of screening, the guideline authors conditionally recommend two screening tests for patients considered at increased risk of ILD: pulmonary function tests and high-resolution chest CT (HRCT). Pulmonary function tests should include spirometry, lung volumes, and diffusion capacity. “Office spirometry alone is insufficient,” said Dr. Johnson, who served as lead author of the guidelines. And while a HRCT scan is recommended, “some patients may present to the emergency room with acute onset shortness of breath, and they may receive a CT angiogram to screen for pulmonary embolism,” she said. “It’s important to note that CT angiograms are performed in incomplete inspiration to maximize pulmonary artery enhancement. This may produce atelectasis that may obscure or mimic ILD. As a result, CTA studies are often inadequate to screen for ILD.”
Once a patient is diagnosed with ILD, three tests are recommended for monitoring: pulmonary function testing (every 3-6 months the first year in patients with IIM and SSc, then less frequently once stable, and every 3-12 months in the first year in patients with RA, SjD, and MCTD, then less frequently once stable); ambulatory desaturation testing every 3-12 months; and HRCT as needed. Dr. Johnson noted that while that the screening of ILD lies within the realm of rheumatologists, “once a patient is diagnosed, we are encouraged to comanage these patients with pulmonologists,” she said. “Ambulatory desaturation testing is not an infrequent test in the hands of pulmonologists. This is where co-management can be helpful.” She characterized a 6-minute walk test with continuous oximetry as “insufficient and is not synonymous with ambulatory desaturation testing. Ambulatory desaturation testing includes up titration of oxygen if a patient desaturates.”
The guidelines conditionally recommend against using chest radiography, 6-minute walk test distance, ambulatory desaturation testing, and bronchoscopy for ILD screening, and there is a strong recommendation against surgical lung biopsy. “However, there are unique circumstances where these tests may be considered,” Dr. Johnson said. “For example, ambulatory desaturation testing may be helpful if a patient is unable to perform a pulmonary function test. Bronchoscopy may be used to rule out infection, sarcoidosis, lymphoma, or alveolar hemorrhage, and surgical lung biopsy may be considered if you’re trying to rule out a malignancy.”
Similarly, several tests are conditionally recommended against for the monitoring of ILD, including chest radiography, the 6-minute walk test distance, and bronchoscopy. “But there are unique circumstances where they may be considered,” she said. “The 6-minute walk test may be used if a patient is unable to perform a pulmonary function test or if they’re being assessed for lung transplantation. Bronchoscopy may be used to rule out infection or alveolar hemorrhage.”
Preferred treatment options described
First-line treatment recommendations for ILD were based on the best available published evidence, voting panel expertise, and patient preferences. For SSc, the preferred treatment options include mycophenolate (CellCept), tocilizumab (Actemra), or rituximab (Rituxan and biosimilars), while additional options include cyclophosphamide, nintedanib (Ofev), and azathioprine. For myositis, the preferred treatment options include mycophenolate, azathioprine, rituximab, or calcineurin inhibitors, while additional options include a Janus kinase (JAK) inhibitor or cyclophosphamide. For MCTD, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include tocilizumab or cyclophosphamide. For RA and Sjögren’s, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include cyclophosphamide. Dr. Johnson emphasized that there was low certainty evidence to recommend one treatment over another. “Many situations might lead a provider to choose a different option for ILD treatment, such as the presence of comorbidities or extra-pulmonary disease,” she said. “So, while our guidelines were focused on effectiveness for ILD, providers may choose therapies that will help ILD and other disease manifestations.”
The guidelines conditionally recommend a short course of glucocorticoids as a bridging therapy or for treatment of a flare of ILD in patients with myositis, MCTD, RA, and Sjögren’s. The panel strongly recommends against the use of glucocorticoids in patients with SSc due to the concern for inducing a scleroderma renal crisis. “While this may be common knowledge for rheumatologists, it may not be common knowledge for pulmonologists,” she said. “So here is an opportunity to educate our pulmonology colleagues in our consultation notes.”
The guidelines also include recommendations for progression of ILD, which was defined using the INBUILD trial criteria. Mycophenolate is conditionally recommended to be the first ILD treatment for all SARDs when progression occurs, if it wasn’t the first ILD treatment used. “If it was, then other medications that rheumatologists are used to can be considered as the next ILD treatment in the face of progression: rituximab, nintedanib, tocilizumab, and cyclophosphamide,” she said. The guidelines include a conditional recommendation against long-term glucocorticoid use in myositis, MCTD, RA, and Sjögren’s, plus a strong recommendation against long-term glucocorticoid use in SSc. Finally, there is a conditional recommendation of referral for lung transplant evaluation at the appropriate time at experienced centers.
Another group of recommendations has to do with cases of rapidly progressive ILD, which is characterized by rapid progression from no oxygen or a patient’s baseline oxygen requirement to a high oxygen requirement or intubation usually within days to weeks without a documented cause, such as infection or heart failure. “In cases of rapidly progressive ILD, which typically occurs in the setting of anti-MDA5 antibodies, there is a conditional recommendation for IV glucocorticoids plus two additional therapies: traditionally rituximab and mycophenolate,” Dr. Johnson said. “However, what may be new to some clinicians is combination IVIG [intravenous immunoglobulin] and a calcineurin inhibitor, notably tacrolimus,” she said. “This is the situation where experience at expert centers is influencing our guidelines in advance of data.”
A patient panel provided input
For the undertaking, a core team that included six rheumatologists; one pulmonologist; one thoracic radiologist; one expert on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology; and two literature review experts developed clinically relevant population, intervention, comparator, and outcomes (PICO) questions. The literature review team included 13 rheumatologists, 8 pulmonologists, and 3 methodologists. Finally, a 21-member patient panel was convened to share their values and preferences regarding screening, monitoring, and treatment of SARD-related ILD. Of these, Dr. Bernstein said that 4 were at risk for ILD and 17 had been diagnosed with ILD. Next, the literature review team conducted a systematic review and used the GRADE methodology to rate the available evidence as high, moderate, low, or very low. Then, a voting panel comprising 13 rheumatologists, 10 pulmonologists, 1 radiologist, and 3 patients from the patient panel cast votes for each PICO question and made final recommendations.
The review of evidence left the guidelines authors with 241 PICO questions, “which is a lot,” Dr. Bernstein said. “To put this in perspective, some guidelines address only 10 or 15 PICO questions. Fortunately, we had a dedicated group of experts who were up to the challenge.” Dr. Johnson emphasized that the forthcoming guidelines should not be used by insurers to mandate a specific order of prescribing. “Clinicians must retain the latitude to prescribe medications based on individual patient factors and preferences,” she said.
Dr. Bernstein disclosed that she is an adviser to, a consultant for, and has received grant or research support from Boehringer Ingelheim and has also received grant or research support from Kadmon and Pfizer. Dr. Johnson disclosed that she has received research support from the American College of Rheumatology to develop these guidelines. She has also been an investigator for trials sponsored by Bristol-Myers Squibb, Roche, and Boehringer Ingelheim and has mitigated these relevant conflicts of interest 1 year prior to the development of these guidelines, and will continue to do so for the foreseeable future.
SAN DIEGO – In the spring of 2024, the American College of Rheumatology is expected to release guidelines to help inform the screening, monitoring, and treatment of interstitial lung disease (ILD) in people with systemic autoimmune rheumatic diseases (SARDs).
The guidelines, which were previewed during a session at the ACR’s annual meeting, will include 50 recommendations, 3 of which met criteria for a strong rating:
- For people with SARDs at increased risk of developing ILD, the authors strongly recommend against screening with surgical lung biopsy.
- For people with systemic sclerosis (SSc)-related ILD, the authors strongly recommend against glucocorticoids as a first-line ILD treatment.
- For people with SSc-related ILD progression despite an initial ILD treatment, the authors strongly recommend against using long-term glucocorticoids.
Elana J. Bernstein, MD, MSc, a rheumatologist who directs the Columbia/New York-Presbyterian Scleroderma Center, and Sindhu R. Johnson, MD, a rheumatologist who directs the Toronto Scleroderma Program at the University of Toronto, provided a sneak peek of the recommendations to attendees before anticipated publication in Arthritis & Rheumatology and Arthritis Care & Research. For now, guideline summaries for screening and monitoring and treatment are currently available, and three manuscripts are under peer review: one about screening and monitoring, one about treatment, and one about the patient panel that participated in the effort.
“ILD is a significant cause of morbidity and mortality in people with SARDs,” said Dr. Bernstein, who is co-first author of the guidelines. “People with systemic sclerosis, rheumatoid arthritis, idiopathic inflammatory myopathies, mixed connective tissue disease, and Sjögren’s disease are at greatest risk of developing ILD.”
Pediatric patients with SARDs excluded
The guidelines’ population of interest was people 17 years of age and older who were diagnosed with SARDs with a high risk of ILD. Pediatric patients with SARDs were excluded from the endeavor, as were those with systemic lupus erythematosus, antineutrophil cytoplasmic antibody–associated vasculitis, sarcoidosis, ankylosing spondylitis, undifferentiated connective tissue disease, interstitial pneumonia with autoimmune features, and those with unclassifiable ILD.
In the realm of screening, the guideline authors conditionally recommend two screening tests for patients considered at increased risk of ILD: pulmonary function tests and high-resolution chest CT (HRCT). Pulmonary function tests should include spirometry, lung volumes, and diffusion capacity. “Office spirometry alone is insufficient,” said Dr. Johnson, who served as lead author of the guidelines. And while a HRCT scan is recommended, “some patients may present to the emergency room with acute onset shortness of breath, and they may receive a CT angiogram to screen for pulmonary embolism,” she said. “It’s important to note that CT angiograms are performed in incomplete inspiration to maximize pulmonary artery enhancement. This may produce atelectasis that may obscure or mimic ILD. As a result, CTA studies are often inadequate to screen for ILD.”
Once a patient is diagnosed with ILD, three tests are recommended for monitoring: pulmonary function testing (every 3-6 months the first year in patients with IIM and SSc, then less frequently once stable, and every 3-12 months in the first year in patients with RA, SjD, and MCTD, then less frequently once stable); ambulatory desaturation testing every 3-12 months; and HRCT as needed. Dr. Johnson noted that while that the screening of ILD lies within the realm of rheumatologists, “once a patient is diagnosed, we are encouraged to comanage these patients with pulmonologists,” she said. “Ambulatory desaturation testing is not an infrequent test in the hands of pulmonologists. This is where co-management can be helpful.” She characterized a 6-minute walk test with continuous oximetry as “insufficient and is not synonymous with ambulatory desaturation testing. Ambulatory desaturation testing includes up titration of oxygen if a patient desaturates.”
The guidelines conditionally recommend against using chest radiography, 6-minute walk test distance, ambulatory desaturation testing, and bronchoscopy for ILD screening, and there is a strong recommendation against surgical lung biopsy. “However, there are unique circumstances where these tests may be considered,” Dr. Johnson said. “For example, ambulatory desaturation testing may be helpful if a patient is unable to perform a pulmonary function test. Bronchoscopy may be used to rule out infection, sarcoidosis, lymphoma, or alveolar hemorrhage, and surgical lung biopsy may be considered if you’re trying to rule out a malignancy.”
Similarly, several tests are conditionally recommended against for the monitoring of ILD, including chest radiography, the 6-minute walk test distance, and bronchoscopy. “But there are unique circumstances where they may be considered,” she said. “The 6-minute walk test may be used if a patient is unable to perform a pulmonary function test or if they’re being assessed for lung transplantation. Bronchoscopy may be used to rule out infection or alveolar hemorrhage.”
Preferred treatment options described
First-line treatment recommendations for ILD were based on the best available published evidence, voting panel expertise, and patient preferences. For SSc, the preferred treatment options include mycophenolate (CellCept), tocilizumab (Actemra), or rituximab (Rituxan and biosimilars), while additional options include cyclophosphamide, nintedanib (Ofev), and azathioprine. For myositis, the preferred treatment options include mycophenolate, azathioprine, rituximab, or calcineurin inhibitors, while additional options include a Janus kinase (JAK) inhibitor or cyclophosphamide. For MCTD, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include tocilizumab or cyclophosphamide. For RA and Sjögren’s, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include cyclophosphamide. Dr. Johnson emphasized that there was low certainty evidence to recommend one treatment over another. “Many situations might lead a provider to choose a different option for ILD treatment, such as the presence of comorbidities or extra-pulmonary disease,” she said. “So, while our guidelines were focused on effectiveness for ILD, providers may choose therapies that will help ILD and other disease manifestations.”
The guidelines conditionally recommend a short course of glucocorticoids as a bridging therapy or for treatment of a flare of ILD in patients with myositis, MCTD, RA, and Sjögren’s. The panel strongly recommends against the use of glucocorticoids in patients with SSc due to the concern for inducing a scleroderma renal crisis. “While this may be common knowledge for rheumatologists, it may not be common knowledge for pulmonologists,” she said. “So here is an opportunity to educate our pulmonology colleagues in our consultation notes.”
The guidelines also include recommendations for progression of ILD, which was defined using the INBUILD trial criteria. Mycophenolate is conditionally recommended to be the first ILD treatment for all SARDs when progression occurs, if it wasn’t the first ILD treatment used. “If it was, then other medications that rheumatologists are used to can be considered as the next ILD treatment in the face of progression: rituximab, nintedanib, tocilizumab, and cyclophosphamide,” she said. The guidelines include a conditional recommendation against long-term glucocorticoid use in myositis, MCTD, RA, and Sjögren’s, plus a strong recommendation against long-term glucocorticoid use in SSc. Finally, there is a conditional recommendation of referral for lung transplant evaluation at the appropriate time at experienced centers.
Another group of recommendations has to do with cases of rapidly progressive ILD, which is characterized by rapid progression from no oxygen or a patient’s baseline oxygen requirement to a high oxygen requirement or intubation usually within days to weeks without a documented cause, such as infection or heart failure. “In cases of rapidly progressive ILD, which typically occurs in the setting of anti-MDA5 antibodies, there is a conditional recommendation for IV glucocorticoids plus two additional therapies: traditionally rituximab and mycophenolate,” Dr. Johnson said. “However, what may be new to some clinicians is combination IVIG [intravenous immunoglobulin] and a calcineurin inhibitor, notably tacrolimus,” she said. “This is the situation where experience at expert centers is influencing our guidelines in advance of data.”
A patient panel provided input
For the undertaking, a core team that included six rheumatologists; one pulmonologist; one thoracic radiologist; one expert on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology; and two literature review experts developed clinically relevant population, intervention, comparator, and outcomes (PICO) questions. The literature review team included 13 rheumatologists, 8 pulmonologists, and 3 methodologists. Finally, a 21-member patient panel was convened to share their values and preferences regarding screening, monitoring, and treatment of SARD-related ILD. Of these, Dr. Bernstein said that 4 were at risk for ILD and 17 had been diagnosed with ILD. Next, the literature review team conducted a systematic review and used the GRADE methodology to rate the available evidence as high, moderate, low, or very low. Then, a voting panel comprising 13 rheumatologists, 10 pulmonologists, 1 radiologist, and 3 patients from the patient panel cast votes for each PICO question and made final recommendations.
The review of evidence left the guidelines authors with 241 PICO questions, “which is a lot,” Dr. Bernstein said. “To put this in perspective, some guidelines address only 10 or 15 PICO questions. Fortunately, we had a dedicated group of experts who were up to the challenge.” Dr. Johnson emphasized that the forthcoming guidelines should not be used by insurers to mandate a specific order of prescribing. “Clinicians must retain the latitude to prescribe medications based on individual patient factors and preferences,” she said.
Dr. Bernstein disclosed that she is an adviser to, a consultant for, and has received grant or research support from Boehringer Ingelheim and has also received grant or research support from Kadmon and Pfizer. Dr. Johnson disclosed that she has received research support from the American College of Rheumatology to develop these guidelines. She has also been an investigator for trials sponsored by Bristol-Myers Squibb, Roche, and Boehringer Ingelheim and has mitigated these relevant conflicts of interest 1 year prior to the development of these guidelines, and will continue to do so for the foreseeable future.
AT ACR 2023
First referral guide issued for axial spondyloarthritis
SAN DIEGO – The Spondyloarthritis Research and Treatment Network (SPARTAN) has created the first referral recommendations for axial spondyloarthritis (axSpA).
The draft recommendations use a points scoring system, with the goal that at least one in three patients referred would be diagnosed with axSpA, an inflammatory arthritis that affects the central skeleton and shares a genetic overlap with skin psoriasis, inflammatory bowel disease, and inflammatory eye disease.
Patients with axSpA can wait 10 years after symptom onset to be diagnosed with the condition. There are currently no guidelines to advise clinicians on when to refer to a rheumatologist, and with the rheumatology workforce shortage, “it is impossible for rheumatologists to evaluate the 20% of adults in the U.S. who have chronic back pain,” said Maureen Dubreuil, MD, a rheumatologist at Boston University. She presented the work at the annual meeting of the American College of Rheumatology.
To address this issue, Dr. Dubreuil and colleagues conducted a literature review to determine how predictive different spondyloarthritis features were of eventual axSpA diagnosis. The interdisciplinary team identified 38 studies published before March 2022, and uncovered 28 individual potential features associated with axSpA, including pain sites, family history of axSpA and related conditions, blood markers of inflammation, genetic testing, and imaging findings.
Inflammatory back pain elements had the lower predictive values, with positive likelihood ratios (LR+) ranging from 1.15 to 2.32, while imaging findings were the most predictive (LR+s from 6.40 to 10.02).
Using a Delphi exercise and discrete choice experiments, members narrowed the checklist down to 10 features. These 10 features were assigned points, with a score of 3 points qualifying for a referral of adults 45 years or younger with chronic pain (3 or more months) in the back, hip, or buttock.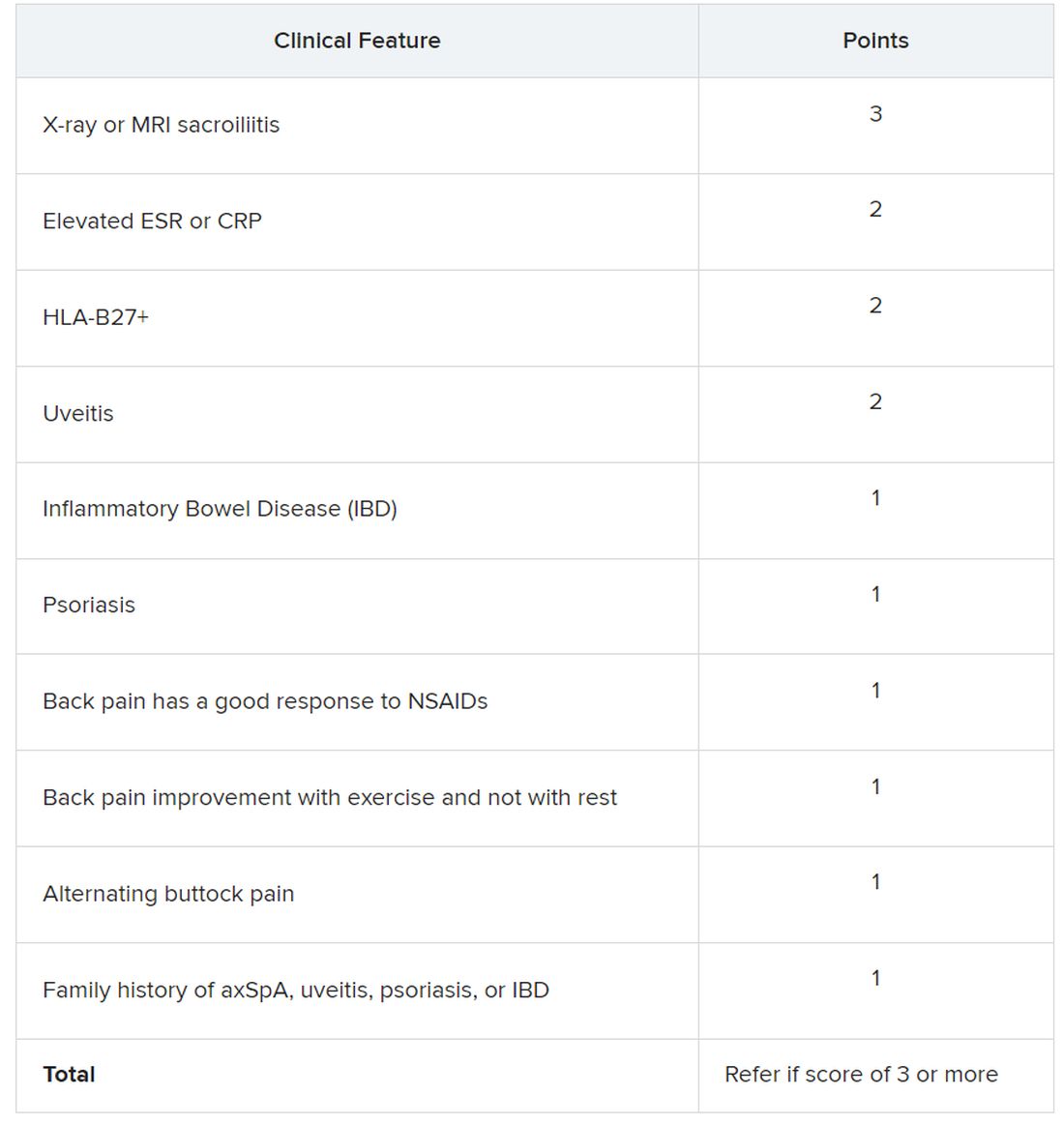
Sacroiliitis seen on imaging, either by x-ray or MRI, received the highest score of 3 points. Dr. Dubreuil emphasized that imaging was not required for a referral, but if a patient has received imaging “that shows sacroiliitis, that is sufficient for referral to a rheumatologist,” she said in her presentation.
Elevated erythrocyte sedimentation rate or C-reactive protein, HLA-B27 positivity, and uveitis score 2 points. Inflammatory bowel disease; psoriasis; back pain with good response to NSAIDs; back pain improvement with exercise and not with rest; alternating buttock pain; and family history of axial spondyloarthritis, uveitis, psoriasis, or IBD score 1 point.
Dr. Dubreuil and colleagues expect that these criteria for referral will result in about one in three referred adults aged 45 years or younger with chronic back pain being diagnosed with axSpA. They also say additional research is necessary to understand if these recommendations increase probability of axSpA diagnosis and reduce diagnostic delays.
“We’re now getting to the stage where we are creating this screening tool, but [testing the] performance of the screening tool is going to be the major next step,” said Mark Hwang, MD, of UTHealth Houston in an interview with this news organization. He is a member of SPARTAN but was not involved with authoring the recommendations. “Will the screening tool enhance the ability on the back end to identify axSpA? We don’t know yet.”
Jon Chan, MD, a rheumatologist at the University of British Columbia, Vancouver, agreed that these recommendations “are a good first step,” but that more awareness about axSpA from nonrheumatologists would also be helpful in identifying new axSpA patients. He is also a member of SPARTAN and comoderated with Dr. Hwang the session where the new recommendations were presented. “I think other diseases like rheumatoid arthritis or lupus have a lot more recognition in the nonrheumatology community,” he told this news organization.
Connecting with other health professionals who see a lot of patients with back pain – physiotherapists, chiropractors, and chronic pain physicians – could also be helpful, he added. “A lot of times, patients go straight to a physio and circumvent the doctor,” he said.
Dr. Chan reports success in educating other departments. “I put up a poster in the emergency department saying, ‘If you’re young with back pain and uveitis, you need to be seen by rheumatology,’ and we’ve identified a ton of axSpA patients that way,” he said. “Maybe their uveitis was very mild, but their back pain was quite severe, and no one really clued in.”
Dr. Dubreuil disclosed financial relationships with Amgen, Pfizer, and UCB Pharma. Her abstract coauthors disclosed financial relationships with multiple pharmaceutical companies. Dr. Hwang consults for UCB and has received research support from Janssen. Dr. Chan has relationships with AbbVie/Abbott, Eli Lilly, Janssen, Novartis, and UCB.
SAN DIEGO – The Spondyloarthritis Research and Treatment Network (SPARTAN) has created the first referral recommendations for axial spondyloarthritis (axSpA).
The draft recommendations use a points scoring system, with the goal that at least one in three patients referred would be diagnosed with axSpA, an inflammatory arthritis that affects the central skeleton and shares a genetic overlap with skin psoriasis, inflammatory bowel disease, and inflammatory eye disease.
Patients with axSpA can wait 10 years after symptom onset to be diagnosed with the condition. There are currently no guidelines to advise clinicians on when to refer to a rheumatologist, and with the rheumatology workforce shortage, “it is impossible for rheumatologists to evaluate the 20% of adults in the U.S. who have chronic back pain,” said Maureen Dubreuil, MD, a rheumatologist at Boston University. She presented the work at the annual meeting of the American College of Rheumatology.
To address this issue, Dr. Dubreuil and colleagues conducted a literature review to determine how predictive different spondyloarthritis features were of eventual axSpA diagnosis. The interdisciplinary team identified 38 studies published before March 2022, and uncovered 28 individual potential features associated with axSpA, including pain sites, family history of axSpA and related conditions, blood markers of inflammation, genetic testing, and imaging findings.
Inflammatory back pain elements had the lower predictive values, with positive likelihood ratios (LR+) ranging from 1.15 to 2.32, while imaging findings were the most predictive (LR+s from 6.40 to 10.02).
Using a Delphi exercise and discrete choice experiments, members narrowed the checklist down to 10 features. These 10 features were assigned points, with a score of 3 points qualifying for a referral of adults 45 years or younger with chronic pain (3 or more months) in the back, hip, or buttock.
Sacroiliitis seen on imaging, either by x-ray or MRI, received the highest score of 3 points. Dr. Dubreuil emphasized that imaging was not required for a referral, but if a patient has received imaging “that shows sacroiliitis, that is sufficient for referral to a rheumatologist,” she said in her presentation.
Elevated erythrocyte sedimentation rate or C-reactive protein, HLA-B27 positivity, and uveitis score 2 points. Inflammatory bowel disease; psoriasis; back pain with good response to NSAIDs; back pain improvement with exercise and not with rest; alternating buttock pain; and family history of axial spondyloarthritis, uveitis, psoriasis, or IBD score 1 point.
Dr. Dubreuil and colleagues expect that these criteria for referral will result in about one in three referred adults aged 45 years or younger with chronic back pain being diagnosed with axSpA. They also say additional research is necessary to understand if these recommendations increase probability of axSpA diagnosis and reduce diagnostic delays.
“We’re now getting to the stage where we are creating this screening tool, but [testing the] performance of the screening tool is going to be the major next step,” said Mark Hwang, MD, of UTHealth Houston in an interview with this news organization. He is a member of SPARTAN but was not involved with authoring the recommendations. “Will the screening tool enhance the ability on the back end to identify axSpA? We don’t know yet.”
Jon Chan, MD, a rheumatologist at the University of British Columbia, Vancouver, agreed that these recommendations “are a good first step,” but that more awareness about axSpA from nonrheumatologists would also be helpful in identifying new axSpA patients. He is also a member of SPARTAN and comoderated with Dr. Hwang the session where the new recommendations were presented. “I think other diseases like rheumatoid arthritis or lupus have a lot more recognition in the nonrheumatology community,” he told this news organization.
Connecting with other health professionals who see a lot of patients with back pain – physiotherapists, chiropractors, and chronic pain physicians – could also be helpful, he added. “A lot of times, patients go straight to a physio and circumvent the doctor,” he said.
Dr. Chan reports success in educating other departments. “I put up a poster in the emergency department saying, ‘If you’re young with back pain and uveitis, you need to be seen by rheumatology,’ and we’ve identified a ton of axSpA patients that way,” he said. “Maybe their uveitis was very mild, but their back pain was quite severe, and no one really clued in.”
Dr. Dubreuil disclosed financial relationships with Amgen, Pfizer, and UCB Pharma. Her abstract coauthors disclosed financial relationships with multiple pharmaceutical companies. Dr. Hwang consults for UCB and has received research support from Janssen. Dr. Chan has relationships with AbbVie/Abbott, Eli Lilly, Janssen, Novartis, and UCB.
SAN DIEGO – The Spondyloarthritis Research and Treatment Network (SPARTAN) has created the first referral recommendations for axial spondyloarthritis (axSpA).
The draft recommendations use a points scoring system, with the goal that at least one in three patients referred would be diagnosed with axSpA, an inflammatory arthritis that affects the central skeleton and shares a genetic overlap with skin psoriasis, inflammatory bowel disease, and inflammatory eye disease.
Patients with axSpA can wait 10 years after symptom onset to be diagnosed with the condition. There are currently no guidelines to advise clinicians on when to refer to a rheumatologist, and with the rheumatology workforce shortage, “it is impossible for rheumatologists to evaluate the 20% of adults in the U.S. who have chronic back pain,” said Maureen Dubreuil, MD, a rheumatologist at Boston University. She presented the work at the annual meeting of the American College of Rheumatology.
To address this issue, Dr. Dubreuil and colleagues conducted a literature review to determine how predictive different spondyloarthritis features were of eventual axSpA diagnosis. The interdisciplinary team identified 38 studies published before March 2022, and uncovered 28 individual potential features associated with axSpA, including pain sites, family history of axSpA and related conditions, blood markers of inflammation, genetic testing, and imaging findings.
Inflammatory back pain elements had the lower predictive values, with positive likelihood ratios (LR+) ranging from 1.15 to 2.32, while imaging findings were the most predictive (LR+s from 6.40 to 10.02).
Using a Delphi exercise and discrete choice experiments, members narrowed the checklist down to 10 features. These 10 features were assigned points, with a score of 3 points qualifying for a referral of adults 45 years or younger with chronic pain (3 or more months) in the back, hip, or buttock.
Sacroiliitis seen on imaging, either by x-ray or MRI, received the highest score of 3 points. Dr. Dubreuil emphasized that imaging was not required for a referral, but if a patient has received imaging “that shows sacroiliitis, that is sufficient for referral to a rheumatologist,” she said in her presentation.
Elevated erythrocyte sedimentation rate or C-reactive protein, HLA-B27 positivity, and uveitis score 2 points. Inflammatory bowel disease; psoriasis; back pain with good response to NSAIDs; back pain improvement with exercise and not with rest; alternating buttock pain; and family history of axial spondyloarthritis, uveitis, psoriasis, or IBD score 1 point.
Dr. Dubreuil and colleagues expect that these criteria for referral will result in about one in three referred adults aged 45 years or younger with chronic back pain being diagnosed with axSpA. They also say additional research is necessary to understand if these recommendations increase probability of axSpA diagnosis and reduce diagnostic delays.
“We’re now getting to the stage where we are creating this screening tool, but [testing the] performance of the screening tool is going to be the major next step,” said Mark Hwang, MD, of UTHealth Houston in an interview with this news organization. He is a member of SPARTAN but was not involved with authoring the recommendations. “Will the screening tool enhance the ability on the back end to identify axSpA? We don’t know yet.”
Jon Chan, MD, a rheumatologist at the University of British Columbia, Vancouver, agreed that these recommendations “are a good first step,” but that more awareness about axSpA from nonrheumatologists would also be helpful in identifying new axSpA patients. He is also a member of SPARTAN and comoderated with Dr. Hwang the session where the new recommendations were presented. “I think other diseases like rheumatoid arthritis or lupus have a lot more recognition in the nonrheumatology community,” he told this news organization.
Connecting with other health professionals who see a lot of patients with back pain – physiotherapists, chiropractors, and chronic pain physicians – could also be helpful, he added. “A lot of times, patients go straight to a physio and circumvent the doctor,” he said.
Dr. Chan reports success in educating other departments. “I put up a poster in the emergency department saying, ‘If you’re young with back pain and uveitis, you need to be seen by rheumatology,’ and we’ve identified a ton of axSpA patients that way,” he said. “Maybe their uveitis was very mild, but their back pain was quite severe, and no one really clued in.”
Dr. Dubreuil disclosed financial relationships with Amgen, Pfizer, and UCB Pharma. Her abstract coauthors disclosed financial relationships with multiple pharmaceutical companies. Dr. Hwang consults for UCB and has received research support from Janssen. Dr. Chan has relationships with AbbVie/Abbott, Eli Lilly, Janssen, Novartis, and UCB.
AT ACR 2023
Experts offer guidance on GLP-1 receptor agonists prior to endoscopy
to support the success of endoscopic procedures, according to a new Clinical Practice Update from the American Gastroenterological Association.
Use of glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1 RAs) has been associated with delayed gastric emptying, which raises a clinical concern about performing endoscopic procedures, especially upper endoscopies in patients using these medications, wrote Jana G. Al Hashash, MD, MSc, of the Mayo Clinic, Jacksonville, Fla., and colleagues.
The Clinical Practice Update (CPU), published in Clinical Gastroenterology and Hepatology, reviews the evidence and provides expert advice for clinicians on the evolving landscape of patients taking GLP-1 receptor agonists prior to endoscopic procedures. The CPU reflects on the most recent literature and the experience of the authors, all experts in bariatric medicine and/or endoscopy.
The American Society of Anesthesiologists (ASA) issued guidance that reflects concerns for the risk of aspiration in sedated patients because of delayed gastric motility from the use of GLP-1 RAs. The ASA advises patients on daily doses of GLP-1 RAs to refrain from taking the medications on the day of a procedure; those on weekly dosing should hold the drugs for a week prior to surgery.
However, the ASA suggestions do not differentiate based on the indication for the drug or for the type of procedure, and questions remain as to whether these changes are necessary and/or effective, the CPU authors said. The ASA’s guidance is based mainly on expert opinion, as not enough published evidence on this topic exists for a robust review and formal guideline, they added.
Recently, a multisociety statement from the AGA, AASLD, ACG, ASGE, and NASPGHAN noted that widespread implementation of the ASA guidance could be associated with unintended harms to patients.
Therefore, the AGA CPU suggests an individualized approach to managing patients on GLP-1 RAs in a pre-endoscopic setting.
For patients on GLP-1 RAs for diabetes management, discontinuing prior to endoscopic may not be worth the potential risk. Also, consider not only the dose and frequency of the GLP-1 RAs but also other comorbidities, medications, and potential gastrointestinal side effects.
“If patients taking GLP-1 RAs solely for weight loss can be identified beforehand, a dose of the medication could be withheld prior to endoscopy with likely little harm, though this should not be considered mandatory or evidence-based,” the CPU authors wrote.
However, withholding a single dose of medication may not be enough for an individual’s gastric motility to return to normal, the authors emphasized.
Additionally, the ASA’s suggestions for holding GLP-1 RAs add complexity to periprocedural medication management, which may strain resources and delay care.
The AGA CPU offers the following guidance for patients on GLP-1 RAs prior to endoscopy:
In general, patients using GLP-1 RAs who have followed the standard perioperative procedures, usually an 8-hour solid-food fast and 2-hour liquid fast, and who do not have symptoms such as ongoing nausea, vomiting, or abdominal distension should proceed with upper and/or lower endoscopy.
For symptomatic patients who may experience negative clinical consequences of endoscopy if delayed, consider rapid-sequence intubation, but the authors acknowledge that this option may not be possible in most ambulatory or office-based endoscopy settings.
Finally, consider placing patients on a liquid diet the day before a sedated procedure instead of stopping GLP-1 RAs; this strategy is “more consistent with the holistic approach to preprocedural management of other similar condi-tions,” the authors said.
The current CPU endorses the multi-society statement that puts patient safety first and encourages AGA members to follow best practices when performing endoscopies on patients who are using GLP-1 RAs, in the absence of actionable data, the authors concluded.
The Clinical Practice Update received no outside funding. Lead author Dr. Al Hashash had no financial conflicts to disclose.
to support the success of endoscopic procedures, according to a new Clinical Practice Update from the American Gastroenterological Association.
Use of glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1 RAs) has been associated with delayed gastric emptying, which raises a clinical concern about performing endoscopic procedures, especially upper endoscopies in patients using these medications, wrote Jana G. Al Hashash, MD, MSc, of the Mayo Clinic, Jacksonville, Fla., and colleagues.
The Clinical Practice Update (CPU), published in Clinical Gastroenterology and Hepatology, reviews the evidence and provides expert advice for clinicians on the evolving landscape of patients taking GLP-1 receptor agonists prior to endoscopic procedures. The CPU reflects on the most recent literature and the experience of the authors, all experts in bariatric medicine and/or endoscopy.
The American Society of Anesthesiologists (ASA) issued guidance that reflects concerns for the risk of aspiration in sedated patients because of delayed gastric motility from the use of GLP-1 RAs. The ASA advises patients on daily doses of GLP-1 RAs to refrain from taking the medications on the day of a procedure; those on weekly dosing should hold the drugs for a week prior to surgery.
However, the ASA suggestions do not differentiate based on the indication for the drug or for the type of procedure, and questions remain as to whether these changes are necessary and/or effective, the CPU authors said. The ASA’s guidance is based mainly on expert opinion, as not enough published evidence on this topic exists for a robust review and formal guideline, they added.
Recently, a multisociety statement from the AGA, AASLD, ACG, ASGE, and NASPGHAN noted that widespread implementation of the ASA guidance could be associated with unintended harms to patients.
Therefore, the AGA CPU suggests an individualized approach to managing patients on GLP-1 RAs in a pre-endoscopic setting.
For patients on GLP-1 RAs for diabetes management, discontinuing prior to endoscopic may not be worth the potential risk. Also, consider not only the dose and frequency of the GLP-1 RAs but also other comorbidities, medications, and potential gastrointestinal side effects.
“If patients taking GLP-1 RAs solely for weight loss can be identified beforehand, a dose of the medication could be withheld prior to endoscopy with likely little harm, though this should not be considered mandatory or evidence-based,” the CPU authors wrote.
However, withholding a single dose of medication may not be enough for an individual’s gastric motility to return to normal, the authors emphasized.
Additionally, the ASA’s suggestions for holding GLP-1 RAs add complexity to periprocedural medication management, which may strain resources and delay care.
The AGA CPU offers the following guidance for patients on GLP-1 RAs prior to endoscopy:
In general, patients using GLP-1 RAs who have followed the standard perioperative procedures, usually an 8-hour solid-food fast and 2-hour liquid fast, and who do not have symptoms such as ongoing nausea, vomiting, or abdominal distension should proceed with upper and/or lower endoscopy.
For symptomatic patients who may experience negative clinical consequences of endoscopy if delayed, consider rapid-sequence intubation, but the authors acknowledge that this option may not be possible in most ambulatory or office-based endoscopy settings.
Finally, consider placing patients on a liquid diet the day before a sedated procedure instead of stopping GLP-1 RAs; this strategy is “more consistent with the holistic approach to preprocedural management of other similar condi-tions,” the authors said.
The current CPU endorses the multi-society statement that puts patient safety first and encourages AGA members to follow best practices when performing endoscopies on patients who are using GLP-1 RAs, in the absence of actionable data, the authors concluded.
The Clinical Practice Update received no outside funding. Lead author Dr. Al Hashash had no financial conflicts to disclose.
to support the success of endoscopic procedures, according to a new Clinical Practice Update from the American Gastroenterological Association.
Use of glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1 RAs) has been associated with delayed gastric emptying, which raises a clinical concern about performing endoscopic procedures, especially upper endoscopies in patients using these medications, wrote Jana G. Al Hashash, MD, MSc, of the Mayo Clinic, Jacksonville, Fla., and colleagues.
The Clinical Practice Update (CPU), published in Clinical Gastroenterology and Hepatology, reviews the evidence and provides expert advice for clinicians on the evolving landscape of patients taking GLP-1 receptor agonists prior to endoscopic procedures. The CPU reflects on the most recent literature and the experience of the authors, all experts in bariatric medicine and/or endoscopy.
The American Society of Anesthesiologists (ASA) issued guidance that reflects concerns for the risk of aspiration in sedated patients because of delayed gastric motility from the use of GLP-1 RAs. The ASA advises patients on daily doses of GLP-1 RAs to refrain from taking the medications on the day of a procedure; those on weekly dosing should hold the drugs for a week prior to surgery.
However, the ASA suggestions do not differentiate based on the indication for the drug or for the type of procedure, and questions remain as to whether these changes are necessary and/or effective, the CPU authors said. The ASA’s guidance is based mainly on expert opinion, as not enough published evidence on this topic exists for a robust review and formal guideline, they added.
Recently, a multisociety statement from the AGA, AASLD, ACG, ASGE, and NASPGHAN noted that widespread implementation of the ASA guidance could be associated with unintended harms to patients.
Therefore, the AGA CPU suggests an individualized approach to managing patients on GLP-1 RAs in a pre-endoscopic setting.
For patients on GLP-1 RAs for diabetes management, discontinuing prior to endoscopic may not be worth the potential risk. Also, consider not only the dose and frequency of the GLP-1 RAs but also other comorbidities, medications, and potential gastrointestinal side effects.
“If patients taking GLP-1 RAs solely for weight loss can be identified beforehand, a dose of the medication could be withheld prior to endoscopy with likely little harm, though this should not be considered mandatory or evidence-based,” the CPU authors wrote.
However, withholding a single dose of medication may not be enough for an individual’s gastric motility to return to normal, the authors emphasized.
Additionally, the ASA’s suggestions for holding GLP-1 RAs add complexity to periprocedural medication management, which may strain resources and delay care.
The AGA CPU offers the following guidance for patients on GLP-1 RAs prior to endoscopy:
In general, patients using GLP-1 RAs who have followed the standard perioperative procedures, usually an 8-hour solid-food fast and 2-hour liquid fast, and who do not have symptoms such as ongoing nausea, vomiting, or abdominal distension should proceed with upper and/or lower endoscopy.
For symptomatic patients who may experience negative clinical consequences of endoscopy if delayed, consider rapid-sequence intubation, but the authors acknowledge that this option may not be possible in most ambulatory or office-based endoscopy settings.
Finally, consider placing patients on a liquid diet the day before a sedated procedure instead of stopping GLP-1 RAs; this strategy is “more consistent with the holistic approach to preprocedural management of other similar condi-tions,” the authors said.
The current CPU endorses the multi-society statement that puts patient safety first and encourages AGA members to follow best practices when performing endoscopies on patients who are using GLP-1 RAs, in the absence of actionable data, the authors concluded.
The Clinical Practice Update received no outside funding. Lead author Dr. Al Hashash had no financial conflicts to disclose.
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
New guidelines for determining brain death released
The consensus practice guideline on brain death, also known as death by neurologic criteria (BD/DNC), was developed by a panel of 20 experts from different specialties, institutions, and medical societies.
As with previous guidelines, the updated version stipulates that brain death should be declared when a patient with a known cause of catastrophic brain injury has permanent loss of function of the brain, including the brain stem, which results in coma, brain stem areflexia, and apnea in the setting of an adequate stimulus.
But the updated version also clarifies questions on neurological examinations and apnea testing and offers new guidance on pre-evaluation targets for blood pressure and body temperature and evaluating brain death in patients who are pregnant, are on extracorporeal membrane oxygenation, or have an injury to the base of the brain.
Also, for the first time, the guidance clarifies that clinicians don’t need to obtain consent before performing a brain death evaluation, unless institutional policy, state laws, or regulations stipulate otherwise.
“The 2023 guidelines will be considered the standard of care in the U.S.,” lead author David M. Greer, MD, chair and chief of neurology, Boston University, and chief of neurology, Boston Medical Center, said in an interview. “Each hospital in the U.S. is responsible for its own policy for BD/DNC determination, and our hope is that they will quickly revise their policies in accordance with this new national standard.”
The guidelines, which are accompanied by a three-page checklist and a free digital app, were published online in Neurology.
Four years in the making
Work on the 85 recommendations in the new report began more than 4 years ago as a collaborative effort by the American Academy of Neurology, the American Academy of Pediatrics, the Child Neurology Society, and the Society of Critical Care Medicine.
A lack of high-quality evidence on brain death determination led panelists to devise an evidence-informed formal consensus process to develop the guidelines, which involved three rounds of anonymous voting on each recommendation and the rationales behind them.
The strength of each recommendation was based on the level of consensus reached through voting, with Level A denoting a recommendation that “must” be followed, Level B one that “should” be followed, and Level C one that “may” be followed.
The majority of recommendations received an A or B rating. Only one recommendation, about whether a second clinical exam is needed in adults, garnered a C rating.
In children, the guidelines recommend that clinicians must perform two clinical examinations and two apnea tests 12 hours apart. In adults, only one exam is required. Both of those recommendations were rated Level A. A recommendation for a second exam in adults received the single Level C rating.
A uniform set of guidelines?
The new guidelines replace adult practice guidance published by AAN in 2010 and guideline for infants and children released in 2011 by AAP, CNS, and SCCM, and for the first time combine brain death guidelines for adult and pediatric patients into one document.
“It is important for clinicians to review the new guideline carefully and ensure their hospital brain death guidelines are updated to be consistent with the new guideline in order to prevent inaccurate determinations of death,” guidelines coauthor Ariane Lewis, MD, NYU Langone Health, New York, said in an interview.
The 1981 Uniform Determination of Death Act (UDDA) is the legal foundation for the declaration of BD/DNC in the United States, but it only stipulates that brain death determination must be made in accordance with accepted medical standards.
There is no single national standard, and states and hospitals are free to adopt their own, which many have done. One goal of the new guidelines was to create a uniform set of guidelines that all institutions follow.
“This is a step toward having a set of guidelines that are accepted by most of the societies and clinical specialties involved in this sort of diagnosis,” that could lead to a national-level policy, Fernando Goldenberg, MD, professor of neurology and director of neuroscience critical care, University of Chicago Medicine, said in an interview.
Dr. Goldenberg was not part of the panel that developed the updated guidelines, but was a coauthor of a consensus statement from the World Brain Death Project in 2020.
Developing a singular global guideline for brain death determination is unlikely, Dr. Goldenberg said. Policies vary widely across the world, and some countries don’t even recognize brain death.
“But this attempts to unify things at the U.S. level, which is very important,” he said.
Permanent vs. irreversible
Dr. Goldenberg said that combining adult and pediatric guidelines into one document will be very helpful for clinicians like him who treat patients from age 16 years and up.
The expanded guidance on apnea testing, recommendations on specific ancillary tests to use or avoid, and inclusion of language stipulating that prior consent is not needed to perform a brain death evaluation are also useful.
He also noted that the section on credentialing and training of clinicians who perform BD/DNC evaluations recognizes advanced practice providers, the first time he recalls seeing these professionals included in brain death guidelines.
However, the panel’s decision to use the term “permanent” to describe loss of brain function instead of “irreversible” gave Dr. Goldenberg pause.
The UDDA provides that an individual is declared legally dead when “circulatory and respiratory functions irreversibly stop; or all functions of the entire brain, including the brain stem, irreversibly stop.”
Earlier in October, the American College of Physicians released a position paper on cardiorespiratory death determination that called for a revision of the UDDA language.
The ACP suggested that “irreversibly” be replaced with “permanently” with regard to the cessation of circulatory and respiratory functions, but that “irreversible” be kept in the description of brain death.
“Permanent means that there is damage that is potentially reversible and irreversible means that the damage is so profound, it cannot be reversed even if an attempt to do so is performed,” Dr. Goldenberg said.
Even though the World Brain Death Project, on which he worked, also used “permanent” to describe brain function loss, Dr. Goldenberg said he aligns with ACP’s position.
“The understanding of brain death is that the damage is so profound, it is irreversible, even if you were to try,” he said. “Therefore, I think that the most appropriate term for brain death should be irreversible as opposed to permanent.”
The report was funded by the American Academy of Neurology. Dr. Greer has received travel funding from Boston University; serves as editor-in-chief for Seminars in Neurology; receives publishing royalties for 50 Studies Every Neurologist Should Know and Successful Leadership in Academic Medicine; has received honoraria from AAN; has received research funding from Becton, Dickinson, and Company; and has served as expert witness in legal proceedings. Dr. Lewis has received honoraria from AAN and Neurodiem, serves as Neurology deputy editor of disputes and debates, and serves as deputy editor of seminars in Neurology. Dr. Goldenberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The consensus practice guideline on brain death, also known as death by neurologic criteria (BD/DNC), was developed by a panel of 20 experts from different specialties, institutions, and medical societies.
As with previous guidelines, the updated version stipulates that brain death should be declared when a patient with a known cause of catastrophic brain injury has permanent loss of function of the brain, including the brain stem, which results in coma, brain stem areflexia, and apnea in the setting of an adequate stimulus.
But the updated version also clarifies questions on neurological examinations and apnea testing and offers new guidance on pre-evaluation targets for blood pressure and body temperature and evaluating brain death in patients who are pregnant, are on extracorporeal membrane oxygenation, or have an injury to the base of the brain.
Also, for the first time, the guidance clarifies that clinicians don’t need to obtain consent before performing a brain death evaluation, unless institutional policy, state laws, or regulations stipulate otherwise.
“The 2023 guidelines will be considered the standard of care in the U.S.,” lead author David M. Greer, MD, chair and chief of neurology, Boston University, and chief of neurology, Boston Medical Center, said in an interview. “Each hospital in the U.S. is responsible for its own policy for BD/DNC determination, and our hope is that they will quickly revise their policies in accordance with this new national standard.”
The guidelines, which are accompanied by a three-page checklist and a free digital app, were published online in Neurology.
Four years in the making
Work on the 85 recommendations in the new report began more than 4 years ago as a collaborative effort by the American Academy of Neurology, the American Academy of Pediatrics, the Child Neurology Society, and the Society of Critical Care Medicine.
A lack of high-quality evidence on brain death determination led panelists to devise an evidence-informed formal consensus process to develop the guidelines, which involved three rounds of anonymous voting on each recommendation and the rationales behind them.
The strength of each recommendation was based on the level of consensus reached through voting, with Level A denoting a recommendation that “must” be followed, Level B one that “should” be followed, and Level C one that “may” be followed.
The majority of recommendations received an A or B rating. Only one recommendation, about whether a second clinical exam is needed in adults, garnered a C rating.
In children, the guidelines recommend that clinicians must perform two clinical examinations and two apnea tests 12 hours apart. In adults, only one exam is required. Both of those recommendations were rated Level A. A recommendation for a second exam in adults received the single Level C rating.
A uniform set of guidelines?
The new guidelines replace adult practice guidance published by AAN in 2010 and guideline for infants and children released in 2011 by AAP, CNS, and SCCM, and for the first time combine brain death guidelines for adult and pediatric patients into one document.
“It is important for clinicians to review the new guideline carefully and ensure their hospital brain death guidelines are updated to be consistent with the new guideline in order to prevent inaccurate determinations of death,” guidelines coauthor Ariane Lewis, MD, NYU Langone Health, New York, said in an interview.
The 1981 Uniform Determination of Death Act (UDDA) is the legal foundation for the declaration of BD/DNC in the United States, but it only stipulates that brain death determination must be made in accordance with accepted medical standards.
There is no single national standard, and states and hospitals are free to adopt their own, which many have done. One goal of the new guidelines was to create a uniform set of guidelines that all institutions follow.
“This is a step toward having a set of guidelines that are accepted by most of the societies and clinical specialties involved in this sort of diagnosis,” that could lead to a national-level policy, Fernando Goldenberg, MD, professor of neurology and director of neuroscience critical care, University of Chicago Medicine, said in an interview.
Dr. Goldenberg was not part of the panel that developed the updated guidelines, but was a coauthor of a consensus statement from the World Brain Death Project in 2020.
Developing a singular global guideline for brain death determination is unlikely, Dr. Goldenberg said. Policies vary widely across the world, and some countries don’t even recognize brain death.
“But this attempts to unify things at the U.S. level, which is very important,” he said.
Permanent vs. irreversible
Dr. Goldenberg said that combining adult and pediatric guidelines into one document will be very helpful for clinicians like him who treat patients from age 16 years and up.
The expanded guidance on apnea testing, recommendations on specific ancillary tests to use or avoid, and inclusion of language stipulating that prior consent is not needed to perform a brain death evaluation are also useful.
He also noted that the section on credentialing and training of clinicians who perform BD/DNC evaluations recognizes advanced practice providers, the first time he recalls seeing these professionals included in brain death guidelines.
However, the panel’s decision to use the term “permanent” to describe loss of brain function instead of “irreversible” gave Dr. Goldenberg pause.
The UDDA provides that an individual is declared legally dead when “circulatory and respiratory functions irreversibly stop; or all functions of the entire brain, including the brain stem, irreversibly stop.”
Earlier in October, the American College of Physicians released a position paper on cardiorespiratory death determination that called for a revision of the UDDA language.
The ACP suggested that “irreversibly” be replaced with “permanently” with regard to the cessation of circulatory and respiratory functions, but that “irreversible” be kept in the description of brain death.
“Permanent means that there is damage that is potentially reversible and irreversible means that the damage is so profound, it cannot be reversed even if an attempt to do so is performed,” Dr. Goldenberg said.
Even though the World Brain Death Project, on which he worked, also used “permanent” to describe brain function loss, Dr. Goldenberg said he aligns with ACP’s position.
“The understanding of brain death is that the damage is so profound, it is irreversible, even if you were to try,” he said. “Therefore, I think that the most appropriate term for brain death should be irreversible as opposed to permanent.”
The report was funded by the American Academy of Neurology. Dr. Greer has received travel funding from Boston University; serves as editor-in-chief for Seminars in Neurology; receives publishing royalties for 50 Studies Every Neurologist Should Know and Successful Leadership in Academic Medicine; has received honoraria from AAN; has received research funding from Becton, Dickinson, and Company; and has served as expert witness in legal proceedings. Dr. Lewis has received honoraria from AAN and Neurodiem, serves as Neurology deputy editor of disputes and debates, and serves as deputy editor of seminars in Neurology. Dr. Goldenberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The consensus practice guideline on brain death, also known as death by neurologic criteria (BD/DNC), was developed by a panel of 20 experts from different specialties, institutions, and medical societies.
As with previous guidelines, the updated version stipulates that brain death should be declared when a patient with a known cause of catastrophic brain injury has permanent loss of function of the brain, including the brain stem, which results in coma, brain stem areflexia, and apnea in the setting of an adequate stimulus.
But the updated version also clarifies questions on neurological examinations and apnea testing and offers new guidance on pre-evaluation targets for blood pressure and body temperature and evaluating brain death in patients who are pregnant, are on extracorporeal membrane oxygenation, or have an injury to the base of the brain.
Also, for the first time, the guidance clarifies that clinicians don’t need to obtain consent before performing a brain death evaluation, unless institutional policy, state laws, or regulations stipulate otherwise.
“The 2023 guidelines will be considered the standard of care in the U.S.,” lead author David M. Greer, MD, chair and chief of neurology, Boston University, and chief of neurology, Boston Medical Center, said in an interview. “Each hospital in the U.S. is responsible for its own policy for BD/DNC determination, and our hope is that they will quickly revise their policies in accordance with this new national standard.”
The guidelines, which are accompanied by a three-page checklist and a free digital app, were published online in Neurology.
Four years in the making
Work on the 85 recommendations in the new report began more than 4 years ago as a collaborative effort by the American Academy of Neurology, the American Academy of Pediatrics, the Child Neurology Society, and the Society of Critical Care Medicine.
A lack of high-quality evidence on brain death determination led panelists to devise an evidence-informed formal consensus process to develop the guidelines, which involved three rounds of anonymous voting on each recommendation and the rationales behind them.
The strength of each recommendation was based on the level of consensus reached through voting, with Level A denoting a recommendation that “must” be followed, Level B one that “should” be followed, and Level C one that “may” be followed.
The majority of recommendations received an A or B rating. Only one recommendation, about whether a second clinical exam is needed in adults, garnered a C rating.
In children, the guidelines recommend that clinicians must perform two clinical examinations and two apnea tests 12 hours apart. In adults, only one exam is required. Both of those recommendations were rated Level A. A recommendation for a second exam in adults received the single Level C rating.
A uniform set of guidelines?
The new guidelines replace adult practice guidance published by AAN in 2010 and guideline for infants and children released in 2011 by AAP, CNS, and SCCM, and for the first time combine brain death guidelines for adult and pediatric patients into one document.
“It is important for clinicians to review the new guideline carefully and ensure their hospital brain death guidelines are updated to be consistent with the new guideline in order to prevent inaccurate determinations of death,” guidelines coauthor Ariane Lewis, MD, NYU Langone Health, New York, said in an interview.
The 1981 Uniform Determination of Death Act (UDDA) is the legal foundation for the declaration of BD/DNC in the United States, but it only stipulates that brain death determination must be made in accordance with accepted medical standards.
There is no single national standard, and states and hospitals are free to adopt their own, which many have done. One goal of the new guidelines was to create a uniform set of guidelines that all institutions follow.
“This is a step toward having a set of guidelines that are accepted by most of the societies and clinical specialties involved in this sort of diagnosis,” that could lead to a national-level policy, Fernando Goldenberg, MD, professor of neurology and director of neuroscience critical care, University of Chicago Medicine, said in an interview.
Dr. Goldenberg was not part of the panel that developed the updated guidelines, but was a coauthor of a consensus statement from the World Brain Death Project in 2020.
Developing a singular global guideline for brain death determination is unlikely, Dr. Goldenberg said. Policies vary widely across the world, and some countries don’t even recognize brain death.
“But this attempts to unify things at the U.S. level, which is very important,” he said.
Permanent vs. irreversible
Dr. Goldenberg said that combining adult and pediatric guidelines into one document will be very helpful for clinicians like him who treat patients from age 16 years and up.
The expanded guidance on apnea testing, recommendations on specific ancillary tests to use or avoid, and inclusion of language stipulating that prior consent is not needed to perform a brain death evaluation are also useful.
He also noted that the section on credentialing and training of clinicians who perform BD/DNC evaluations recognizes advanced practice providers, the first time he recalls seeing these professionals included in brain death guidelines.
However, the panel’s decision to use the term “permanent” to describe loss of brain function instead of “irreversible” gave Dr. Goldenberg pause.
The UDDA provides that an individual is declared legally dead when “circulatory and respiratory functions irreversibly stop; or all functions of the entire brain, including the brain stem, irreversibly stop.”
Earlier in October, the American College of Physicians released a position paper on cardiorespiratory death determination that called for a revision of the UDDA language.
The ACP suggested that “irreversibly” be replaced with “permanently” with regard to the cessation of circulatory and respiratory functions, but that “irreversible” be kept in the description of brain death.
“Permanent means that there is damage that is potentially reversible and irreversible means that the damage is so profound, it cannot be reversed even if an attempt to do so is performed,” Dr. Goldenberg said.
Even though the World Brain Death Project, on which he worked, also used “permanent” to describe brain function loss, Dr. Goldenberg said he aligns with ACP’s position.
“The understanding of brain death is that the damage is so profound, it is irreversible, even if you were to try,” he said. “Therefore, I think that the most appropriate term for brain death should be irreversible as opposed to permanent.”
The report was funded by the American Academy of Neurology. Dr. Greer has received travel funding from Boston University; serves as editor-in-chief for Seminars in Neurology; receives publishing royalties for 50 Studies Every Neurologist Should Know and Successful Leadership in Academic Medicine; has received honoraria from AAN; has received research funding from Becton, Dickinson, and Company; and has served as expert witness in legal proceedings. Dr. Lewis has received honoraria from AAN and Neurodiem, serves as Neurology deputy editor of disputes and debates, and serves as deputy editor of seminars in Neurology. Dr. Goldenberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
AHA updates CPR guidelines on cardiac arrest after poisoning
The update reflects treatment advances and new knowledge, including the use of venoarterial extracorporeal membrane oxygenation (VA-ECMO) for patients whose condition is refractory to poison antidotes and other therapies.
The new guidelines are designed primarily for North American health care professionals who treat adults and children who are critically ill because of poisoning, including intentional and unintentional drug overdose, chemical exposure, and drug-drug interactions, the authors note.
Published online in Circulation, the update was endorsed by the American Academy of Pediatrics.
‘Nearly miraculous’
“It’s been 13 years since the poisoning treatment guidelines had a comprehensive update,” lead author Eric J. Lavonas, MD, professor of emergency medicine at Denver Health and the Rocky Mountain Poison and Drug Center, Colo., told this news organization. “In that time, we’ve learned a lot about how to best use antidotes and other treatments to save the most critically poisoned patients.”
Highlighting a few key points from the update, he said, “For those rare situations when antidotes aren’t enough, the new guidelines include the use of heart-lung machines (VA-ECMO) for patients with beta-blocker, calcium channel blocker, or sodium channel blocker poisoning causing cardiogenic shock.”
Furthermore, he said, “High-dose insulin treatment for patients with beta-blocker and calcium channel blocker poisoning [also recommended in the update] has really become mainstream. The doses are up to 10 times higher than the amount used to treat diabetic emergencies.
“Some excellent science has shown that giving IV lipid emulsion can save the life of someone with an accidental overdose of local anesthetic medications, particularly bupivacaine,” he added. “The result is sometimes nearly miraculous.
“But when this treatment is extended to poisoning from other medications, it often doesn’t work as well, and in some situations may make things worse,” he said. “The issue may be that giving lipids increases absorption of drug from the stomach and intestines, which can be dangerous when the patient took an overdose of pills.”
Low level of evidence
The guidelines were compiled by the Critical Poisoning Writing Group, which includes experts from emergency medicine, pediatrics, medical toxicology, pharmacology, critical care, emergency medical services, education, research, and nursing. Group members were appointed by the AHA Emergency Cardiovascular Care Science Subcommittee and were approved by the AHA Manuscript Oversight Committee.
First and foremost, the group recommends timely consultation with a medical toxicologist, a clinical toxicologist, or a regional poison center to facilitate rapid, effective therapy, because treatment of cardiac arrest and toxicity from poisoning often requires treatments that most clinicians don’t use frequently.
Other key points include the following:
- Naloxone administration may reverse respiratory arrest due to opioid overdose, preventing progression to cardiac arrest.
- Give high-dose insulin therapy early in the treatment of patients with beta-blocker and calcium channel blocker poisoning, Dr. Lavonas noted.
- Standard advanced life support plus sodium bicarbonate is appropriate for life-threatening dysrhythmias caused by cocaine or other sodium channel blockers.
- If cyanide poisoning is suspected, clinicians should not wait for confirmatory testing; treatment should begin immediately with hydroxocobalamin (preferred) or sodium nitrite plus sodium thiosulfate.
- Digoxin-specific immune antibody fragments can reverse life-threatening dysrhythmias from digoxin poisoning.
- Use of 20% intravenous lipid emulsion can be efficacious in the resuscitation of life-threatening local anesthetic toxicity, especially from bupivacaine, Dr. Lavonas indicated.
- Sedation is recommended for patients with severe agitation from sympathomimetic poisoning to manage hyperthermia and acidosis, prevent rhabdomyolysis and injury, and allow evaluation for other life-threatening conditions.
- Although flumazenil reverses central nervous system and respiratory depression from benzodiazepine poisoning, risks and contraindications, provided in the guidelines, limit its use.
- VA-ECMO can be lifesaving for patients with cardiogenic shock or dysrhythmias that are refractory to other treatments.
“Unfortunately, despite improvements in the design and funding support for resuscitation research, the overall certainty of the evidence base for resuscitation science and management of critical poisoning is low,” the group acknowledges.
Of the 73 guideline recommendations, only 2 are supported by level A evidence; 3 are supported by level B-randomized evidence, 12 by level B-nonrandomized evidence, and the rest by level C evidence.
“Accordingly, the strength of recommendations is weaker than optimal,” they write. “Clinical trials in resuscitation and the management of critical poisoning are sorely needed.”
‘Don’t go it alone!’
“Most critical poisonings are pretty uncommon, and each patient is different,” Dr. Lavonas said. “Even in the emergency department or ICU, most physicians will treat a patient who is critically ill with any given poison less than once a year. The antidotes and medication doses needed to effectively treat these patients are often very different than everyday medical practice.
“Don’t try to go it alone!” he urges. “Poisoning cases are complex, and the treatments work best when they are implemented quickly and assertively. A toxicologist can help sort through complex situations and get effective treatment started without delay.”
Every certified poison center has a medical toxicologist or clinical toxicologist on call 24/7 to give advice to physicians and hospitals about patients who are critically ill after being poisoned, he added. “Everyone in the U.S. has access to a poison center by calling one number: 1-800-222-1222.”
Dr. Lavonas has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The update reflects treatment advances and new knowledge, including the use of venoarterial extracorporeal membrane oxygenation (VA-ECMO) for patients whose condition is refractory to poison antidotes and other therapies.
The new guidelines are designed primarily for North American health care professionals who treat adults and children who are critically ill because of poisoning, including intentional and unintentional drug overdose, chemical exposure, and drug-drug interactions, the authors note.
Published online in Circulation, the update was endorsed by the American Academy of Pediatrics.
‘Nearly miraculous’
“It’s been 13 years since the poisoning treatment guidelines had a comprehensive update,” lead author Eric J. Lavonas, MD, professor of emergency medicine at Denver Health and the Rocky Mountain Poison and Drug Center, Colo., told this news organization. “In that time, we’ve learned a lot about how to best use antidotes and other treatments to save the most critically poisoned patients.”
Highlighting a few key points from the update, he said, “For those rare situations when antidotes aren’t enough, the new guidelines include the use of heart-lung machines (VA-ECMO) for patients with beta-blocker, calcium channel blocker, or sodium channel blocker poisoning causing cardiogenic shock.”
Furthermore, he said, “High-dose insulin treatment for patients with beta-blocker and calcium channel blocker poisoning [also recommended in the update] has really become mainstream. The doses are up to 10 times higher than the amount used to treat diabetic emergencies.
“Some excellent science has shown that giving IV lipid emulsion can save the life of someone with an accidental overdose of local anesthetic medications, particularly bupivacaine,” he added. “The result is sometimes nearly miraculous.
“But when this treatment is extended to poisoning from other medications, it often doesn’t work as well, and in some situations may make things worse,” he said. “The issue may be that giving lipids increases absorption of drug from the stomach and intestines, which can be dangerous when the patient took an overdose of pills.”
Low level of evidence
The guidelines were compiled by the Critical Poisoning Writing Group, which includes experts from emergency medicine, pediatrics, medical toxicology, pharmacology, critical care, emergency medical services, education, research, and nursing. Group members were appointed by the AHA Emergency Cardiovascular Care Science Subcommittee and were approved by the AHA Manuscript Oversight Committee.
First and foremost, the group recommends timely consultation with a medical toxicologist, a clinical toxicologist, or a regional poison center to facilitate rapid, effective therapy, because treatment of cardiac arrest and toxicity from poisoning often requires treatments that most clinicians don’t use frequently.
Other key points include the following:
- Naloxone administration may reverse respiratory arrest due to opioid overdose, preventing progression to cardiac arrest.
- Give high-dose insulin therapy early in the treatment of patients with beta-blocker and calcium channel blocker poisoning, Dr. Lavonas noted.
- Standard advanced life support plus sodium bicarbonate is appropriate for life-threatening dysrhythmias caused by cocaine or other sodium channel blockers.
- If cyanide poisoning is suspected, clinicians should not wait for confirmatory testing; treatment should begin immediately with hydroxocobalamin (preferred) or sodium nitrite plus sodium thiosulfate.
- Digoxin-specific immune antibody fragments can reverse life-threatening dysrhythmias from digoxin poisoning.
- Use of 20% intravenous lipid emulsion can be efficacious in the resuscitation of life-threatening local anesthetic toxicity, especially from bupivacaine, Dr. Lavonas indicated.
- Sedation is recommended for patients with severe agitation from sympathomimetic poisoning to manage hyperthermia and acidosis, prevent rhabdomyolysis and injury, and allow evaluation for other life-threatening conditions.
- Although flumazenil reverses central nervous system and respiratory depression from benzodiazepine poisoning, risks and contraindications, provided in the guidelines, limit its use.
- VA-ECMO can be lifesaving for patients with cardiogenic shock or dysrhythmias that are refractory to other treatments.
“Unfortunately, despite improvements in the design and funding support for resuscitation research, the overall certainty of the evidence base for resuscitation science and management of critical poisoning is low,” the group acknowledges.
Of the 73 guideline recommendations, only 2 are supported by level A evidence; 3 are supported by level B-randomized evidence, 12 by level B-nonrandomized evidence, and the rest by level C evidence.
“Accordingly, the strength of recommendations is weaker than optimal,” they write. “Clinical trials in resuscitation and the management of critical poisoning are sorely needed.”
‘Don’t go it alone!’
“Most critical poisonings are pretty uncommon, and each patient is different,” Dr. Lavonas said. “Even in the emergency department or ICU, most physicians will treat a patient who is critically ill with any given poison less than once a year. The antidotes and medication doses needed to effectively treat these patients are often very different than everyday medical practice.
“Don’t try to go it alone!” he urges. “Poisoning cases are complex, and the treatments work best when they are implemented quickly and assertively. A toxicologist can help sort through complex situations and get effective treatment started without delay.”
Every certified poison center has a medical toxicologist or clinical toxicologist on call 24/7 to give advice to physicians and hospitals about patients who are critically ill after being poisoned, he added. “Everyone in the U.S. has access to a poison center by calling one number: 1-800-222-1222.”
Dr. Lavonas has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The update reflects treatment advances and new knowledge, including the use of venoarterial extracorporeal membrane oxygenation (VA-ECMO) for patients whose condition is refractory to poison antidotes and other therapies.
The new guidelines are designed primarily for North American health care professionals who treat adults and children who are critically ill because of poisoning, including intentional and unintentional drug overdose, chemical exposure, and drug-drug interactions, the authors note.
Published online in Circulation, the update was endorsed by the American Academy of Pediatrics.
‘Nearly miraculous’
“It’s been 13 years since the poisoning treatment guidelines had a comprehensive update,” lead author Eric J. Lavonas, MD, professor of emergency medicine at Denver Health and the Rocky Mountain Poison and Drug Center, Colo., told this news organization. “In that time, we’ve learned a lot about how to best use antidotes and other treatments to save the most critically poisoned patients.”
Highlighting a few key points from the update, he said, “For those rare situations when antidotes aren’t enough, the new guidelines include the use of heart-lung machines (VA-ECMO) for patients with beta-blocker, calcium channel blocker, or sodium channel blocker poisoning causing cardiogenic shock.”
Furthermore, he said, “High-dose insulin treatment for patients with beta-blocker and calcium channel blocker poisoning [also recommended in the update] has really become mainstream. The doses are up to 10 times higher than the amount used to treat diabetic emergencies.
“Some excellent science has shown that giving IV lipid emulsion can save the life of someone with an accidental overdose of local anesthetic medications, particularly bupivacaine,” he added. “The result is sometimes nearly miraculous.
“But when this treatment is extended to poisoning from other medications, it often doesn’t work as well, and in some situations may make things worse,” he said. “The issue may be that giving lipids increases absorption of drug from the stomach and intestines, which can be dangerous when the patient took an overdose of pills.”
Low level of evidence
The guidelines were compiled by the Critical Poisoning Writing Group, which includes experts from emergency medicine, pediatrics, medical toxicology, pharmacology, critical care, emergency medical services, education, research, and nursing. Group members were appointed by the AHA Emergency Cardiovascular Care Science Subcommittee and were approved by the AHA Manuscript Oversight Committee.
First and foremost, the group recommends timely consultation with a medical toxicologist, a clinical toxicologist, or a regional poison center to facilitate rapid, effective therapy, because treatment of cardiac arrest and toxicity from poisoning often requires treatments that most clinicians don’t use frequently.
Other key points include the following:
- Naloxone administration may reverse respiratory arrest due to opioid overdose, preventing progression to cardiac arrest.
- Give high-dose insulin therapy early in the treatment of patients with beta-blocker and calcium channel blocker poisoning, Dr. Lavonas noted.
- Standard advanced life support plus sodium bicarbonate is appropriate for life-threatening dysrhythmias caused by cocaine or other sodium channel blockers.
- If cyanide poisoning is suspected, clinicians should not wait for confirmatory testing; treatment should begin immediately with hydroxocobalamin (preferred) or sodium nitrite plus sodium thiosulfate.
- Digoxin-specific immune antibody fragments can reverse life-threatening dysrhythmias from digoxin poisoning.
- Use of 20% intravenous lipid emulsion can be efficacious in the resuscitation of life-threatening local anesthetic toxicity, especially from bupivacaine, Dr. Lavonas indicated.
- Sedation is recommended for patients with severe agitation from sympathomimetic poisoning to manage hyperthermia and acidosis, prevent rhabdomyolysis and injury, and allow evaluation for other life-threatening conditions.
- Although flumazenil reverses central nervous system and respiratory depression from benzodiazepine poisoning, risks and contraindications, provided in the guidelines, limit its use.
- VA-ECMO can be lifesaving for patients with cardiogenic shock or dysrhythmias that are refractory to other treatments.
“Unfortunately, despite improvements in the design and funding support for resuscitation research, the overall certainty of the evidence base for resuscitation science and management of critical poisoning is low,” the group acknowledges.
Of the 73 guideline recommendations, only 2 are supported by level A evidence; 3 are supported by level B-randomized evidence, 12 by level B-nonrandomized evidence, and the rest by level C evidence.
“Accordingly, the strength of recommendations is weaker than optimal,” they write. “Clinical trials in resuscitation and the management of critical poisoning are sorely needed.”
‘Don’t go it alone!’
“Most critical poisonings are pretty uncommon, and each patient is different,” Dr. Lavonas said. “Even in the emergency department or ICU, most physicians will treat a patient who is critically ill with any given poison less than once a year. The antidotes and medication doses needed to effectively treat these patients are often very different than everyday medical practice.
“Don’t try to go it alone!” he urges. “Poisoning cases are complex, and the treatments work best when they are implemented quickly and assertively. A toxicologist can help sort through complex situations and get effective treatment started without delay.”
Every certified poison center has a medical toxicologist or clinical toxicologist on call 24/7 to give advice to physicians and hospitals about patients who are critically ill after being poisoned, he added. “Everyone in the U.S. has access to a poison center by calling one number: 1-800-222-1222.”
Dr. Lavonas has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Thyroid ablation safety addressed by expert consensus
“There are no documents to date in the United States focusing primarily on the safe adoption and implementation of ablation techniques, including learning curve considerations and necessary pre-procedural skillsets,” reports the ATA task force in the consensus statement, which was published in Thyroid.
“Although these emerging technologies hold great promise, they are not without risk and require development of a unique skill set and environment for optimal, safe performance and consistent outcomes,” task force co-author Catherine F. Sinclair, MD, an associate professor at the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
Chemical ablation has long been utilized as a nonsurgical option for benign thyroid nodule ablation. However, the current array of treatment options has expanded with thermal ablation. Techniques such as radiofrequency ablation (RFA), laser ablation, microwave ablation, and high-intensity focused ultrasound have gained favor as minimally invasive alternatives to surgery.
Much has been published on indications and outcomes with the use of the techniques. The multidisciplinary global task force was convened to address key issues regarding safety and utilization. The report is directed toward specialists, including surgeons, endocrinologists, and interventional radiologists.
The recommendations cover three broad categories: safety considerations spanning preprocedural to postprocedural periods; necessary skill sets for optimal, safe performance with the approaches; and the expectations for success in the context of risks and benefits.
Ablation methods can depend on nodule type
Among key issues addressed are which ablation methods are most appropriate for which types of nodules. Recommendations include chemical ablation, typically involving the injection of dehydrated ethanol in a target nodule. In solid nodules, diffusion with chemical ablation can be unpredictable, which makes it more appropriate for cystic nodules.
Thermal ablation is considered best suited for patients with compressive and/or cosmetic complaints that clearly involve a single or dominant nodule, as well as for autonomously functioning thyroid nodules that cause subclinical or overt hyperthyroidism.
While ethanol ablation is recommended as a first-line treatment for benign cystic thyroid nodules, its efficacy decreases when there is an increase of more than 20% of the solid component. In such cases, RFA or a combination of ethanol ablation and RFA may be considered, the task force recommends.
Patient counseling – managing expectations
Another key consideration in treatment with thyroid nodule ablation is managing patients’ expectations.
Patients should be advised of benefits, such as the avoidance of surgery and general anesthesia and less recovery time. Risks can include thermal or chemical injury to the recurrent laryngeal nerve and other vital structures. The task force underscores discussion of alternative options with patients.
Alternative management options to ablation, including observation, radioactive iodine for functioning nodules, and surgery should also be discussed, and “their relative advantages and disadvantages should be presented without bias such that the patient can make an informed, individual treatment decision,” the task force recommends.
Patients should be informed that, in contrast to surgical management, the benefits of ablation are not immediate; rather, they accrue over the course of months. Reduction in nodule size within the first month is often limited.
Pain, soreness, and some swelling of the nodule and surrounding tissues are common in the first week. These symptoms usually peak in the first 3-5 days after the procedure. Importantly, patients rarely require opioid medications, and their use should be avoided, the task force recommends.
Patients should also be informed about the possibilities of nodule regrowth following ablation and the possible need for more than one ablation procedure.
“Although regrowth definitions in the literature vary, risk of regrowth after thermal ablation is 5%-40% and increases the larger the baseline nodule volume,” the task force notes.
Of note, most studies on ablation to date have shown that thermal ablation complication rates are low. Twelve months post procedure, volume reductions are typically greater than 50%.
Follow-up
For long-term monitoring following ablation, follow-up neck ultrasound is typically recommended at 1-3 months and at 6 and 12 months post ablation to assess volume reduction, nodule appearance, nodule vascularity, and areas at risk for regrowth, the authors note.
Prolonged serial biochemical evaluation of thyroid function is only recommended in cases of hyperfunctioning thyroid nodules.
Key considerations for additional ablative sessions for nodules greater than 20-30 mL in volume should include a failure to achieve adequate reduction in volume, nodule regrowth in previously untreated peripheral areas, and/or persistent or new compressive symptoms.
Learning curve
Dr. Sinclair underscored that successful thyroid nodule ablation requires skill – and experience.
“Probably the greatest concern shared by the writing group on this statement was the potential for clinicians to start ablation practices without having an appropriate prior skill set,” she said.
“Ablation is an advanced, ultrasound-guided procedure, and clinicians need to be experienced in performing neck ultrasounds and biopsies,” she added. “To consider performing ablations without this skill set is both unrealistic and dangerous.”
RFA, currently the most commonly used thermal ablation method for benign thyroid nodule ablation in the U.S., “has a good safety profile but can have a steep learning curve initially,” she said.
Among the most important recommendations is that for their first 20-60 ablation procedures, clinicians should consider limiting treatment to small- to medium-sized benign nodules rather than large-volume disease, Dr. Sinclair added.
“In addition, prior to starting thyroid ablation practices, clinicians should be proficient in ultrasound imaging and fine-needle biopsies and can gain valuable experience by practicing on phantoms and having expert proctoring for the first few cases,” she said.
For initial ablative cases, the task force recommends that clinicians select moderate-size (< 20-30 mL), nonvascular nodules with favorable characteristics and location. The final volume reduction should be based not only on baseline nodule characteristics, such as volume and vascularity, but also on the practitioner’s skill.
Clinicians furthermore should be board certified or eligible in an appropriate medical specialty, have extensive background knowledge, and “should have clinical experience in the clinical diagnosis and treatment of thyroid nodules; neck imaging anatomy; thyroid ultrasound imaging and fine needle aspiration biopsy procedures; and ultrasound risk stratification for benign and malignant thyroid tumors,” the group recommends.
Importantly, the statement is designed to reflect a consensus opinion of the panel of experts but is not meant to serve as a formal guideline or a standard of care for the clinical practice of thermal ablation, Dr. Sinclair added.
“It is not the intent of the statement to replace individual decision-making, the wishes of the patient or family, or clinical judgment.”
The authors’ disclosures are detailed in the published report.
A version of this article first appeared on Medscape.com.
“There are no documents to date in the United States focusing primarily on the safe adoption and implementation of ablation techniques, including learning curve considerations and necessary pre-procedural skillsets,” reports the ATA task force in the consensus statement, which was published in Thyroid.
“Although these emerging technologies hold great promise, they are not without risk and require development of a unique skill set and environment for optimal, safe performance and consistent outcomes,” task force co-author Catherine F. Sinclair, MD, an associate professor at the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
Chemical ablation has long been utilized as a nonsurgical option for benign thyroid nodule ablation. However, the current array of treatment options has expanded with thermal ablation. Techniques such as radiofrequency ablation (RFA), laser ablation, microwave ablation, and high-intensity focused ultrasound have gained favor as minimally invasive alternatives to surgery.
Much has been published on indications and outcomes with the use of the techniques. The multidisciplinary global task force was convened to address key issues regarding safety and utilization. The report is directed toward specialists, including surgeons, endocrinologists, and interventional radiologists.
The recommendations cover three broad categories: safety considerations spanning preprocedural to postprocedural periods; necessary skill sets for optimal, safe performance with the approaches; and the expectations for success in the context of risks and benefits.
Ablation methods can depend on nodule type
Among key issues addressed are which ablation methods are most appropriate for which types of nodules. Recommendations include chemical ablation, typically involving the injection of dehydrated ethanol in a target nodule. In solid nodules, diffusion with chemical ablation can be unpredictable, which makes it more appropriate for cystic nodules.
Thermal ablation is considered best suited for patients with compressive and/or cosmetic complaints that clearly involve a single or dominant nodule, as well as for autonomously functioning thyroid nodules that cause subclinical or overt hyperthyroidism.
While ethanol ablation is recommended as a first-line treatment for benign cystic thyroid nodules, its efficacy decreases when there is an increase of more than 20% of the solid component. In such cases, RFA or a combination of ethanol ablation and RFA may be considered, the task force recommends.
Patient counseling – managing expectations
Another key consideration in treatment with thyroid nodule ablation is managing patients’ expectations.
Patients should be advised of benefits, such as the avoidance of surgery and general anesthesia and less recovery time. Risks can include thermal or chemical injury to the recurrent laryngeal nerve and other vital structures. The task force underscores discussion of alternative options with patients.
Alternative management options to ablation, including observation, radioactive iodine for functioning nodules, and surgery should also be discussed, and “their relative advantages and disadvantages should be presented without bias such that the patient can make an informed, individual treatment decision,” the task force recommends.
Patients should be informed that, in contrast to surgical management, the benefits of ablation are not immediate; rather, they accrue over the course of months. Reduction in nodule size within the first month is often limited.
Pain, soreness, and some swelling of the nodule and surrounding tissues are common in the first week. These symptoms usually peak in the first 3-5 days after the procedure. Importantly, patients rarely require opioid medications, and their use should be avoided, the task force recommends.
Patients should also be informed about the possibilities of nodule regrowth following ablation and the possible need for more than one ablation procedure.
“Although regrowth definitions in the literature vary, risk of regrowth after thermal ablation is 5%-40% and increases the larger the baseline nodule volume,” the task force notes.
Of note, most studies on ablation to date have shown that thermal ablation complication rates are low. Twelve months post procedure, volume reductions are typically greater than 50%.
Follow-up
For long-term monitoring following ablation, follow-up neck ultrasound is typically recommended at 1-3 months and at 6 and 12 months post ablation to assess volume reduction, nodule appearance, nodule vascularity, and areas at risk for regrowth, the authors note.
Prolonged serial biochemical evaluation of thyroid function is only recommended in cases of hyperfunctioning thyroid nodules.
Key considerations for additional ablative sessions for nodules greater than 20-30 mL in volume should include a failure to achieve adequate reduction in volume, nodule regrowth in previously untreated peripheral areas, and/or persistent or new compressive symptoms.
Learning curve
Dr. Sinclair underscored that successful thyroid nodule ablation requires skill – and experience.
“Probably the greatest concern shared by the writing group on this statement was the potential for clinicians to start ablation practices without having an appropriate prior skill set,” she said.
“Ablation is an advanced, ultrasound-guided procedure, and clinicians need to be experienced in performing neck ultrasounds and biopsies,” she added. “To consider performing ablations without this skill set is both unrealistic and dangerous.”
RFA, currently the most commonly used thermal ablation method for benign thyroid nodule ablation in the U.S., “has a good safety profile but can have a steep learning curve initially,” she said.
Among the most important recommendations is that for their first 20-60 ablation procedures, clinicians should consider limiting treatment to small- to medium-sized benign nodules rather than large-volume disease, Dr. Sinclair added.
“In addition, prior to starting thyroid ablation practices, clinicians should be proficient in ultrasound imaging and fine-needle biopsies and can gain valuable experience by practicing on phantoms and having expert proctoring for the first few cases,” she said.
For initial ablative cases, the task force recommends that clinicians select moderate-size (< 20-30 mL), nonvascular nodules with favorable characteristics and location. The final volume reduction should be based not only on baseline nodule characteristics, such as volume and vascularity, but also on the practitioner’s skill.
Clinicians furthermore should be board certified or eligible in an appropriate medical specialty, have extensive background knowledge, and “should have clinical experience in the clinical diagnosis and treatment of thyroid nodules; neck imaging anatomy; thyroid ultrasound imaging and fine needle aspiration biopsy procedures; and ultrasound risk stratification for benign and malignant thyroid tumors,” the group recommends.
Importantly, the statement is designed to reflect a consensus opinion of the panel of experts but is not meant to serve as a formal guideline or a standard of care for the clinical practice of thermal ablation, Dr. Sinclair added.
“It is not the intent of the statement to replace individual decision-making, the wishes of the patient or family, or clinical judgment.”
The authors’ disclosures are detailed in the published report.
A version of this article first appeared on Medscape.com.
“There are no documents to date in the United States focusing primarily on the safe adoption and implementation of ablation techniques, including learning curve considerations and necessary pre-procedural skillsets,” reports the ATA task force in the consensus statement, which was published in Thyroid.
“Although these emerging technologies hold great promise, they are not without risk and require development of a unique skill set and environment for optimal, safe performance and consistent outcomes,” task force co-author Catherine F. Sinclair, MD, an associate professor at the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
Chemical ablation has long been utilized as a nonsurgical option for benign thyroid nodule ablation. However, the current array of treatment options has expanded with thermal ablation. Techniques such as radiofrequency ablation (RFA), laser ablation, microwave ablation, and high-intensity focused ultrasound have gained favor as minimally invasive alternatives to surgery.
Much has been published on indications and outcomes with the use of the techniques. The multidisciplinary global task force was convened to address key issues regarding safety and utilization. The report is directed toward specialists, including surgeons, endocrinologists, and interventional radiologists.
The recommendations cover three broad categories: safety considerations spanning preprocedural to postprocedural periods; necessary skill sets for optimal, safe performance with the approaches; and the expectations for success in the context of risks and benefits.
Ablation methods can depend on nodule type
Among key issues addressed are which ablation methods are most appropriate for which types of nodules. Recommendations include chemical ablation, typically involving the injection of dehydrated ethanol in a target nodule. In solid nodules, diffusion with chemical ablation can be unpredictable, which makes it more appropriate for cystic nodules.
Thermal ablation is considered best suited for patients with compressive and/or cosmetic complaints that clearly involve a single or dominant nodule, as well as for autonomously functioning thyroid nodules that cause subclinical or overt hyperthyroidism.
While ethanol ablation is recommended as a first-line treatment for benign cystic thyroid nodules, its efficacy decreases when there is an increase of more than 20% of the solid component. In such cases, RFA or a combination of ethanol ablation and RFA may be considered, the task force recommends.
Patient counseling – managing expectations
Another key consideration in treatment with thyroid nodule ablation is managing patients’ expectations.
Patients should be advised of benefits, such as the avoidance of surgery and general anesthesia and less recovery time. Risks can include thermal or chemical injury to the recurrent laryngeal nerve and other vital structures. The task force underscores discussion of alternative options with patients.
Alternative management options to ablation, including observation, radioactive iodine for functioning nodules, and surgery should also be discussed, and “their relative advantages and disadvantages should be presented without bias such that the patient can make an informed, individual treatment decision,” the task force recommends.
Patients should be informed that, in contrast to surgical management, the benefits of ablation are not immediate; rather, they accrue over the course of months. Reduction in nodule size within the first month is often limited.
Pain, soreness, and some swelling of the nodule and surrounding tissues are common in the first week. These symptoms usually peak in the first 3-5 days after the procedure. Importantly, patients rarely require opioid medications, and their use should be avoided, the task force recommends.
Patients should also be informed about the possibilities of nodule regrowth following ablation and the possible need for more than one ablation procedure.
“Although regrowth definitions in the literature vary, risk of regrowth after thermal ablation is 5%-40% and increases the larger the baseline nodule volume,” the task force notes.
Of note, most studies on ablation to date have shown that thermal ablation complication rates are low. Twelve months post procedure, volume reductions are typically greater than 50%.
Follow-up
For long-term monitoring following ablation, follow-up neck ultrasound is typically recommended at 1-3 months and at 6 and 12 months post ablation to assess volume reduction, nodule appearance, nodule vascularity, and areas at risk for regrowth, the authors note.
Prolonged serial biochemical evaluation of thyroid function is only recommended in cases of hyperfunctioning thyroid nodules.
Key considerations for additional ablative sessions for nodules greater than 20-30 mL in volume should include a failure to achieve adequate reduction in volume, nodule regrowth in previously untreated peripheral areas, and/or persistent or new compressive symptoms.
Learning curve
Dr. Sinclair underscored that successful thyroid nodule ablation requires skill – and experience.
“Probably the greatest concern shared by the writing group on this statement was the potential for clinicians to start ablation practices without having an appropriate prior skill set,” she said.
“Ablation is an advanced, ultrasound-guided procedure, and clinicians need to be experienced in performing neck ultrasounds and biopsies,” she added. “To consider performing ablations without this skill set is both unrealistic and dangerous.”
RFA, currently the most commonly used thermal ablation method for benign thyroid nodule ablation in the U.S., “has a good safety profile but can have a steep learning curve initially,” she said.
Among the most important recommendations is that for their first 20-60 ablation procedures, clinicians should consider limiting treatment to small- to medium-sized benign nodules rather than large-volume disease, Dr. Sinclair added.
“In addition, prior to starting thyroid ablation practices, clinicians should be proficient in ultrasound imaging and fine-needle biopsies and can gain valuable experience by practicing on phantoms and having expert proctoring for the first few cases,” she said.
For initial ablative cases, the task force recommends that clinicians select moderate-size (< 20-30 mL), nonvascular nodules with favorable characteristics and location. The final volume reduction should be based not only on baseline nodule characteristics, such as volume and vascularity, but also on the practitioner’s skill.
Clinicians furthermore should be board certified or eligible in an appropriate medical specialty, have extensive background knowledge, and “should have clinical experience in the clinical diagnosis and treatment of thyroid nodules; neck imaging anatomy; thyroid ultrasound imaging and fine needle aspiration biopsy procedures; and ultrasound risk stratification for benign and malignant thyroid tumors,” the group recommends.
Importantly, the statement is designed to reflect a consensus opinion of the panel of experts but is not meant to serve as a formal guideline or a standard of care for the clinical practice of thermal ablation, Dr. Sinclair added.
“It is not the intent of the statement to replace individual decision-making, the wishes of the patient or family, or clinical judgment.”
The authors’ disclosures are detailed in the published report.
A version of this article first appeared on Medscape.com.
FROM THYROID
ESC issues first comprehensive cardiomyopathy guidelines
The European Society of Cardiology has released new guidelines for cardiomyopathies, their first major comprehensive international guidelines to address diagnosis and treatment of the broad causes of heart muscle dysfunction.
The document was released in conjunction with the annual congress of the European Society of Cardiology and is also available online in the European Heart Journal.
“We have considered cardiomyopathies across the life course from pediatric to adult,” explained Elena Arbelo, MD, PhD, coordinator of the cardiac genetic diseases and sudden arrhythmic death unit, Hospital Clinic de Barcelona. Dr. Arbelo is first author and one of two chairpersons of the ESC task force that brought the guidelines forward.
Not an update, Dr. Arbelo said.
Guidelines organize cardiomyopathy phenotypes
Cardiomyopathy can present at any age. It can have multiple complex etiologies, including genetic predisposition, heart muscle injury caused by disease, or a mix of participating factors. The ESC task force employed several strategies in taking a comprehensive approach to the condition, said Juan Kaski, MD, PhD, professor of pediatric inherited cardiovascular medicine at the University College of London.
“From my point of view, the key innovations include a diagnostic workup that starts with a detailed phenotypic description, including the new phenotype of nondilated left ventricular cardiomyopathy, that then triggers a multiparametric, systematic evaluation,” said Dr. Kaski, cochair of the task force.
As explained in the introduction to the guideline and reiterated by both Dr. Arbelo and Dr. Kaski, the guidelines have been organized around the patient pathway, meaning that focus should be placed on recognizing the presenting phenotype as a critical first step in discerning the underlying etiology and its treatments.
“Central to this approach is not only the individual patient but also the family as a whole,” Dr. Arbelo said. “Clinical findings in relatives are essential for understanding what happens to the patient and vice versa.”
Genetic testing in children described
The new guidelines include specific recommendations about genetic testing of children. They also emphasize the value of cardiovascular magnetic resonance (CMR) imaging in the “diagnosis, screening, monitoring, and prognostication” for patients of all ages, according to Dr. Kaski.
“CMR is recommended at the initial evaluation for every patient with cardiomyopathy,” Dr. Arbelo said. It should be “considered” during follow-up and for many other applications, including the evaluation of “genotype-positive but phenotype-negative relatives.”
Etiologic prediction models have been incorporated into the guidelines, including genotyping for dilated cardiomyopathies and nondilated left ventricular cardiomyopathy, said both Dr. Arbelo and Dr. Kaski, interviewed separately. They both indicated that the task force did their best to make the guidelines user friendly.
Each of the recommendations in the guidelines is provided with an evidence-based classification. In order, these are class I (recommended), class IIa (should be considered), class IIb (may be considered), and class III (not recommended).
Many symptoms are cardiomyopathy related
Dr. Kaski and Dr. Arbelo both emphasized that the guidelines draw attention to the relationship of cardiomyopathy to common cardiovascular conditions, such as heart failure, arrhythmia, and chest pain. Dr. Kaski pointed out that these are the types of problems commonly encountered by general cardiologists and well as primary care physicians.
In 2014, the ESC published guidelines specific to HCM. The new broader guidelines do not overlook this subtype. According to Dr. Kaski, there have been several innovations in HCM since the previous guidelines, such as when to consider cardiac myosin inhibitors for symptomatic left ventricular outflow tract obstruction.
The ESC guidelines place an emphasis on a “coordinated, systematic, and individualized” care pathway based on a multidisciplinary approach, according to Dr. Arbelo. Although the composition of the interdisciplinary team depends on the individual case, the guidelines recognize a key role for general cardiologists in managing the majority of patients. Suggestions of when to refer challenging cases to expert centers are outlined.
32 key messages derived from guidelines
The guidelines include almost 90 pages of recommendations. The task force isolated 32 key messages from 13 sections ranging from descriptions of how the patient pathway is defined to what types of physical activity should be considered for different forms of cardiomyopathy. There is also a section devoted to important gaps in evidence and areas in which there is the most need for further studies.
The guidelines end with a comprehensive list of “what to do” and “what not to do” in the diagnosis and care of cardiomyopathy. These include most of the class I recommendations and summarize some important class III cautions.
“Most of the recommendations in the guideline are new,” the authors wrote in the introduction. Although they acknowledged that they did not attempt to provide detailed recommendations for every cardiomyopathy phenotype, they endeavored to cover general evaluation and management issues supported by relevant evidence.
Dr. Arbelo and Dr. Kaski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology has released new guidelines for cardiomyopathies, their first major comprehensive international guidelines to address diagnosis and treatment of the broad causes of heart muscle dysfunction.
The document was released in conjunction with the annual congress of the European Society of Cardiology and is also available online in the European Heart Journal.
“We have considered cardiomyopathies across the life course from pediatric to adult,” explained Elena Arbelo, MD, PhD, coordinator of the cardiac genetic diseases and sudden arrhythmic death unit, Hospital Clinic de Barcelona. Dr. Arbelo is first author and one of two chairpersons of the ESC task force that brought the guidelines forward.
Not an update, Dr. Arbelo said.
Guidelines organize cardiomyopathy phenotypes
Cardiomyopathy can present at any age. It can have multiple complex etiologies, including genetic predisposition, heart muscle injury caused by disease, or a mix of participating factors. The ESC task force employed several strategies in taking a comprehensive approach to the condition, said Juan Kaski, MD, PhD, professor of pediatric inherited cardiovascular medicine at the University College of London.
“From my point of view, the key innovations include a diagnostic workup that starts with a detailed phenotypic description, including the new phenotype of nondilated left ventricular cardiomyopathy, that then triggers a multiparametric, systematic evaluation,” said Dr. Kaski, cochair of the task force.
As explained in the introduction to the guideline and reiterated by both Dr. Arbelo and Dr. Kaski, the guidelines have been organized around the patient pathway, meaning that focus should be placed on recognizing the presenting phenotype as a critical first step in discerning the underlying etiology and its treatments.
“Central to this approach is not only the individual patient but also the family as a whole,” Dr. Arbelo said. “Clinical findings in relatives are essential for understanding what happens to the patient and vice versa.”
Genetic testing in children described
The new guidelines include specific recommendations about genetic testing of children. They also emphasize the value of cardiovascular magnetic resonance (CMR) imaging in the “diagnosis, screening, monitoring, and prognostication” for patients of all ages, according to Dr. Kaski.
“CMR is recommended at the initial evaluation for every patient with cardiomyopathy,” Dr. Arbelo said. It should be “considered” during follow-up and for many other applications, including the evaluation of “genotype-positive but phenotype-negative relatives.”
Etiologic prediction models have been incorporated into the guidelines, including genotyping for dilated cardiomyopathies and nondilated left ventricular cardiomyopathy, said both Dr. Arbelo and Dr. Kaski, interviewed separately. They both indicated that the task force did their best to make the guidelines user friendly.
Each of the recommendations in the guidelines is provided with an evidence-based classification. In order, these are class I (recommended), class IIa (should be considered), class IIb (may be considered), and class III (not recommended).
Many symptoms are cardiomyopathy related
Dr. Kaski and Dr. Arbelo both emphasized that the guidelines draw attention to the relationship of cardiomyopathy to common cardiovascular conditions, such as heart failure, arrhythmia, and chest pain. Dr. Kaski pointed out that these are the types of problems commonly encountered by general cardiologists and well as primary care physicians.
In 2014, the ESC published guidelines specific to HCM. The new broader guidelines do not overlook this subtype. According to Dr. Kaski, there have been several innovations in HCM since the previous guidelines, such as when to consider cardiac myosin inhibitors for symptomatic left ventricular outflow tract obstruction.
The ESC guidelines place an emphasis on a “coordinated, systematic, and individualized” care pathway based on a multidisciplinary approach, according to Dr. Arbelo. Although the composition of the interdisciplinary team depends on the individual case, the guidelines recognize a key role for general cardiologists in managing the majority of patients. Suggestions of when to refer challenging cases to expert centers are outlined.
32 key messages derived from guidelines
The guidelines include almost 90 pages of recommendations. The task force isolated 32 key messages from 13 sections ranging from descriptions of how the patient pathway is defined to what types of physical activity should be considered for different forms of cardiomyopathy. There is also a section devoted to important gaps in evidence and areas in which there is the most need for further studies.
The guidelines end with a comprehensive list of “what to do” and “what not to do” in the diagnosis and care of cardiomyopathy. These include most of the class I recommendations and summarize some important class III cautions.
“Most of the recommendations in the guideline are new,” the authors wrote in the introduction. Although they acknowledged that they did not attempt to provide detailed recommendations for every cardiomyopathy phenotype, they endeavored to cover general evaluation and management issues supported by relevant evidence.
Dr. Arbelo and Dr. Kaski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology has released new guidelines for cardiomyopathies, their first major comprehensive international guidelines to address diagnosis and treatment of the broad causes of heart muscle dysfunction.
The document was released in conjunction with the annual congress of the European Society of Cardiology and is also available online in the European Heart Journal.
“We have considered cardiomyopathies across the life course from pediatric to adult,” explained Elena Arbelo, MD, PhD, coordinator of the cardiac genetic diseases and sudden arrhythmic death unit, Hospital Clinic de Barcelona. Dr. Arbelo is first author and one of two chairpersons of the ESC task force that brought the guidelines forward.
Not an update, Dr. Arbelo said.
Guidelines organize cardiomyopathy phenotypes
Cardiomyopathy can present at any age. It can have multiple complex etiologies, including genetic predisposition, heart muscle injury caused by disease, or a mix of participating factors. The ESC task force employed several strategies in taking a comprehensive approach to the condition, said Juan Kaski, MD, PhD, professor of pediatric inherited cardiovascular medicine at the University College of London.
“From my point of view, the key innovations include a diagnostic workup that starts with a detailed phenotypic description, including the new phenotype of nondilated left ventricular cardiomyopathy, that then triggers a multiparametric, systematic evaluation,” said Dr. Kaski, cochair of the task force.
As explained in the introduction to the guideline and reiterated by both Dr. Arbelo and Dr. Kaski, the guidelines have been organized around the patient pathway, meaning that focus should be placed on recognizing the presenting phenotype as a critical first step in discerning the underlying etiology and its treatments.
“Central to this approach is not only the individual patient but also the family as a whole,” Dr. Arbelo said. “Clinical findings in relatives are essential for understanding what happens to the patient and vice versa.”
Genetic testing in children described
The new guidelines include specific recommendations about genetic testing of children. They also emphasize the value of cardiovascular magnetic resonance (CMR) imaging in the “diagnosis, screening, monitoring, and prognostication” for patients of all ages, according to Dr. Kaski.
“CMR is recommended at the initial evaluation for every patient with cardiomyopathy,” Dr. Arbelo said. It should be “considered” during follow-up and for many other applications, including the evaluation of “genotype-positive but phenotype-negative relatives.”
Etiologic prediction models have been incorporated into the guidelines, including genotyping for dilated cardiomyopathies and nondilated left ventricular cardiomyopathy, said both Dr. Arbelo and Dr. Kaski, interviewed separately. They both indicated that the task force did their best to make the guidelines user friendly.
Each of the recommendations in the guidelines is provided with an evidence-based classification. In order, these are class I (recommended), class IIa (should be considered), class IIb (may be considered), and class III (not recommended).
Many symptoms are cardiomyopathy related
Dr. Kaski and Dr. Arbelo both emphasized that the guidelines draw attention to the relationship of cardiomyopathy to common cardiovascular conditions, such as heart failure, arrhythmia, and chest pain. Dr. Kaski pointed out that these are the types of problems commonly encountered by general cardiologists and well as primary care physicians.
In 2014, the ESC published guidelines specific to HCM. The new broader guidelines do not overlook this subtype. According to Dr. Kaski, there have been several innovations in HCM since the previous guidelines, such as when to consider cardiac myosin inhibitors for symptomatic left ventricular outflow tract obstruction.
The ESC guidelines place an emphasis on a “coordinated, systematic, and individualized” care pathway based on a multidisciplinary approach, according to Dr. Arbelo. Although the composition of the interdisciplinary team depends on the individual case, the guidelines recognize a key role for general cardiologists in managing the majority of patients. Suggestions of when to refer challenging cases to expert centers are outlined.
32 key messages derived from guidelines
The guidelines include almost 90 pages of recommendations. The task force isolated 32 key messages from 13 sections ranging from descriptions of how the patient pathway is defined to what types of physical activity should be considered for different forms of cardiomyopathy. There is also a section devoted to important gaps in evidence and areas in which there is the most need for further studies.
The guidelines end with a comprehensive list of “what to do” and “what not to do” in the diagnosis and care of cardiomyopathy. These include most of the class I recommendations and summarize some important class III cautions.
“Most of the recommendations in the guideline are new,” the authors wrote in the introduction. Although they acknowledged that they did not attempt to provide detailed recommendations for every cardiomyopathy phenotype, they endeavored to cover general evaluation and management issues supported by relevant evidence.
Dr. Arbelo and Dr. Kaski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
ACR releases guideline for managing ILD in patients with rheumatic disease
The American College of Rheumatology has released a summary of upcoming guidelines on screening, monitoring, and treatment for interstitial lung disease (ILD) in patients with systemic autoimmune rheumatic disease.
The recommendations apply to adults with rheumatic diseases at greater risk for ILD: rheumatoid arthritis, systemic sclerosis (SSc), mixed connective tissue disease (MCTD), Sjögren’s disease (SjD), and idiopathic inflammatory myopathies (IIM).
“Interstitial lung disease is a major cause of morbidity and mortality across several systemic autoimmune rheumatic diseases,” Sindhu R. Johnson, MD, PhD, lead author of the new guidelines and director of the clinical epidemiology and health care research program at the University of Toronto, said in an ACR press release. “Guidance was needed for which tests to use for screening and monitoring this particular disease.”
The two documents are summaries of part of a larger manuscript currently awaiting peer review, according to the ACR, and the final guidelines are anticipated to be published by early 2024.
The recommendations were developed using “the best available evidence and consensus across a range of expert opinions and incorporated patient values and preferences,” according to the press release.
Highlights of recommendations for screening and monitoring ILD are:
- Providers can screen patients at higher risk for ILD with pulmonary function tests (PFTs) and high-resolution CT of the chest.
- PFTs, chest high-resolution CT, and ambulatory desaturation testing are conditionally recommended for monitoring ILD progression.
- It is conditionally recommended that providers do not use 6-minute walk test distance, chest radiography, or bronchoscopy for screening or monitoring disease.
- It is suggested that patients with IIM-ILD and SSc-ILD receive PFTs for monitoring every 3-6 months during the first year, then less frequently once stable.
- It is suggested that patients with RA-ILD, SjD-ILD, and MCTD-ILD receive PFTs every 3-12 months for the first year, then less frequently once stable.
Suggestions on how often to screen for ILD were not present in the summary documents, but will be made available in the larger manuscript, said Elana Bernstein, MD, director of the Columbia University Medical Center/New York–Presbyterian Hospital scleroderma program, New York. She is co–first author of the guidelines.
Nearly all recommendations are conditional, primarily because the certainty of evidence behind many of these recommendations is low or very low, she said in an interview. More clinical data on ILD in patients with rheumatic disease would help strengthen evidence, she said, particularly for best practices in frequency of testing. “We need more research on how often patients should be screened for ILD and how often they should be monitored for ILD progression,” she said. “That would enable us to provide recommendations, rather than just suggestions.”
Highlights of recommendations for ILD treatment are:
- The guidelines strongly recommend against using glucocorticoids for first-line ILD treatment in patients with SSc-ILD.
- Short-term glucocorticoids are conditionally recommended as a first-line ILD treatment for patients with systemic autoimmune rheumatic disease–related ILD (SARD-ILD), excluding SSc-ILD.
- Mycophenolate, azathioprine, rituximab, and cyclophosphamide are all potential first-line ILD treatment options for patients with SARD-ILD.
- It is conditionally recommended that patients with SARD-ILD do not receive leflunomide, methotrexate, tumor necrosis factor inhibitors, or abatacept as first-line ILD treatment.
- If SARD-ILD progresses despite first-line therapy, mycophenolate, rituximab, cyclophosphamide, and nintedanib are potential secondary treatment options.
- If RA-ILD progresses following initial therapy, pirfenidone is a treatment option.
- The guidelines conditionally recommend against pirfenidone as a secondary treatment option for SARD-ILD other than RA-ILD.
These summary guidelines appear “comprehensive,” but there has yet to be information published on the basis of these recommendations, Elizabeth Volkmann, MD, said in an interview.
“It’s important to understand that we don’t know whether most of these recommendations were just driven by expert opinion versus actual evidence from randomized, controlled clinical trials,” said Dr. Volkmann, who codirects the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles. She was not involved with creating the guidelines.
She expects that many of the recommendations for first- and second-line ILD treatment options were based on expert opinion, as there have been no randomized clinical trials looking at that specific topic, she said. For example, nintedanib is conditionally recommended as a first-line treatment option for SSc-ILD, but as a second-line treatment for SjD-ILD, IIM-ILD, and MCTD-ILD. “There’s no literature to support one or the other – whether nintedanib is first-line or second-line [treatment].”
The decision to publish the summary recommendations online prior to peer review is unusual, she said, as these recommendations could be altered during that process; however, Dr. Bernstein noted that was not likely.
By releasing the summary guideline now, the ACR can “get the needed information to clinicians earlier as the manuscript goes through its remaining stages and is finalized,” an ACR representative explained.
Prior to the expected publication of these guidelines in early 2024, Dr. Volkmann noted that the American Thoracic Society will be publishing guidelines on the treatment of SSc-ILD in the American Journal of Respiratory and Critical Care Medicine in September.
Dr. Bernstein reported grants/contracts with the Department of Defense, the Scleroderma Research Foundation, the National Institutes of Health, Eicos, Boehringer Ingelheim, Kadmon, and Pfizer. Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and GlaxoSmithKline and institutional support for performing studies on systemic sclerosis for Kadmon, Boehringer Ingelheim, Horizon, and Prometheus.
A version of this article first appeared on Medscape.com.
The American College of Rheumatology has released a summary of upcoming guidelines on screening, monitoring, and treatment for interstitial lung disease (ILD) in patients with systemic autoimmune rheumatic disease.
The recommendations apply to adults with rheumatic diseases at greater risk for ILD: rheumatoid arthritis, systemic sclerosis (SSc), mixed connective tissue disease (MCTD), Sjögren’s disease (SjD), and idiopathic inflammatory myopathies (IIM).
“Interstitial lung disease is a major cause of morbidity and mortality across several systemic autoimmune rheumatic diseases,” Sindhu R. Johnson, MD, PhD, lead author of the new guidelines and director of the clinical epidemiology and health care research program at the University of Toronto, said in an ACR press release. “Guidance was needed for which tests to use for screening and monitoring this particular disease.”
The two documents are summaries of part of a larger manuscript currently awaiting peer review, according to the ACR, and the final guidelines are anticipated to be published by early 2024.
The recommendations were developed using “the best available evidence and consensus across a range of expert opinions and incorporated patient values and preferences,” according to the press release.
Highlights of recommendations for screening and monitoring ILD are:
- Providers can screen patients at higher risk for ILD with pulmonary function tests (PFTs) and high-resolution CT of the chest.
- PFTs, chest high-resolution CT, and ambulatory desaturation testing are conditionally recommended for monitoring ILD progression.
- It is conditionally recommended that providers do not use 6-minute walk test distance, chest radiography, or bronchoscopy for screening or monitoring disease.
- It is suggested that patients with IIM-ILD and SSc-ILD receive PFTs for monitoring every 3-6 months during the first year, then less frequently once stable.
- It is suggested that patients with RA-ILD, SjD-ILD, and MCTD-ILD receive PFTs every 3-12 months for the first year, then less frequently once stable.
Suggestions on how often to screen for ILD were not present in the summary documents, but will be made available in the larger manuscript, said Elana Bernstein, MD, director of the Columbia University Medical Center/New York–Presbyterian Hospital scleroderma program, New York. She is co–first author of the guidelines.
Nearly all recommendations are conditional, primarily because the certainty of evidence behind many of these recommendations is low or very low, she said in an interview. More clinical data on ILD in patients with rheumatic disease would help strengthen evidence, she said, particularly for best practices in frequency of testing. “We need more research on how often patients should be screened for ILD and how often they should be monitored for ILD progression,” she said. “That would enable us to provide recommendations, rather than just suggestions.”
Highlights of recommendations for ILD treatment are:
- The guidelines strongly recommend against using glucocorticoids for first-line ILD treatment in patients with SSc-ILD.
- Short-term glucocorticoids are conditionally recommended as a first-line ILD treatment for patients with systemic autoimmune rheumatic disease–related ILD (SARD-ILD), excluding SSc-ILD.
- Mycophenolate, azathioprine, rituximab, and cyclophosphamide are all potential first-line ILD treatment options for patients with SARD-ILD.
- It is conditionally recommended that patients with SARD-ILD do not receive leflunomide, methotrexate, tumor necrosis factor inhibitors, or abatacept as first-line ILD treatment.
- If SARD-ILD progresses despite first-line therapy, mycophenolate, rituximab, cyclophosphamide, and nintedanib are potential secondary treatment options.
- If RA-ILD progresses following initial therapy, pirfenidone is a treatment option.
- The guidelines conditionally recommend against pirfenidone as a secondary treatment option for SARD-ILD other than RA-ILD.
These summary guidelines appear “comprehensive,” but there has yet to be information published on the basis of these recommendations, Elizabeth Volkmann, MD, said in an interview.
“It’s important to understand that we don’t know whether most of these recommendations were just driven by expert opinion versus actual evidence from randomized, controlled clinical trials,” said Dr. Volkmann, who codirects the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles. She was not involved with creating the guidelines.
She expects that many of the recommendations for first- and second-line ILD treatment options were based on expert opinion, as there have been no randomized clinical trials looking at that specific topic, she said. For example, nintedanib is conditionally recommended as a first-line treatment option for SSc-ILD, but as a second-line treatment for SjD-ILD, IIM-ILD, and MCTD-ILD. “There’s no literature to support one or the other – whether nintedanib is first-line or second-line [treatment].”
The decision to publish the summary recommendations online prior to peer review is unusual, she said, as these recommendations could be altered during that process; however, Dr. Bernstein noted that was not likely.
By releasing the summary guideline now, the ACR can “get the needed information to clinicians earlier as the manuscript goes through its remaining stages and is finalized,” an ACR representative explained.
Prior to the expected publication of these guidelines in early 2024, Dr. Volkmann noted that the American Thoracic Society will be publishing guidelines on the treatment of SSc-ILD in the American Journal of Respiratory and Critical Care Medicine in September.
Dr. Bernstein reported grants/contracts with the Department of Defense, the Scleroderma Research Foundation, the National Institutes of Health, Eicos, Boehringer Ingelheim, Kadmon, and Pfizer. Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and GlaxoSmithKline and institutional support for performing studies on systemic sclerosis for Kadmon, Boehringer Ingelheim, Horizon, and Prometheus.
A version of this article first appeared on Medscape.com.
The American College of Rheumatology has released a summary of upcoming guidelines on screening, monitoring, and treatment for interstitial lung disease (ILD) in patients with systemic autoimmune rheumatic disease.
The recommendations apply to adults with rheumatic diseases at greater risk for ILD: rheumatoid arthritis, systemic sclerosis (SSc), mixed connective tissue disease (MCTD), Sjögren’s disease (SjD), and idiopathic inflammatory myopathies (IIM).
“Interstitial lung disease is a major cause of morbidity and mortality across several systemic autoimmune rheumatic diseases,” Sindhu R. Johnson, MD, PhD, lead author of the new guidelines and director of the clinical epidemiology and health care research program at the University of Toronto, said in an ACR press release. “Guidance was needed for which tests to use for screening and monitoring this particular disease.”
The two documents are summaries of part of a larger manuscript currently awaiting peer review, according to the ACR, and the final guidelines are anticipated to be published by early 2024.
The recommendations were developed using “the best available evidence and consensus across a range of expert opinions and incorporated patient values and preferences,” according to the press release.
Highlights of recommendations for screening and monitoring ILD are:
- Providers can screen patients at higher risk for ILD with pulmonary function tests (PFTs) and high-resolution CT of the chest.
- PFTs, chest high-resolution CT, and ambulatory desaturation testing are conditionally recommended for monitoring ILD progression.
- It is conditionally recommended that providers do not use 6-minute walk test distance, chest radiography, or bronchoscopy for screening or monitoring disease.
- It is suggested that patients with IIM-ILD and SSc-ILD receive PFTs for monitoring every 3-6 months during the first year, then less frequently once stable.
- It is suggested that patients with RA-ILD, SjD-ILD, and MCTD-ILD receive PFTs every 3-12 months for the first year, then less frequently once stable.
Suggestions on how often to screen for ILD were not present in the summary documents, but will be made available in the larger manuscript, said Elana Bernstein, MD, director of the Columbia University Medical Center/New York–Presbyterian Hospital scleroderma program, New York. She is co–first author of the guidelines.
Nearly all recommendations are conditional, primarily because the certainty of evidence behind many of these recommendations is low or very low, she said in an interview. More clinical data on ILD in patients with rheumatic disease would help strengthen evidence, she said, particularly for best practices in frequency of testing. “We need more research on how often patients should be screened for ILD and how often they should be monitored for ILD progression,” she said. “That would enable us to provide recommendations, rather than just suggestions.”
Highlights of recommendations for ILD treatment are:
- The guidelines strongly recommend against using glucocorticoids for first-line ILD treatment in patients with SSc-ILD.
- Short-term glucocorticoids are conditionally recommended as a first-line ILD treatment for patients with systemic autoimmune rheumatic disease–related ILD (SARD-ILD), excluding SSc-ILD.
- Mycophenolate, azathioprine, rituximab, and cyclophosphamide are all potential first-line ILD treatment options for patients with SARD-ILD.
- It is conditionally recommended that patients with SARD-ILD do not receive leflunomide, methotrexate, tumor necrosis factor inhibitors, or abatacept as first-line ILD treatment.
- If SARD-ILD progresses despite first-line therapy, mycophenolate, rituximab, cyclophosphamide, and nintedanib are potential secondary treatment options.
- If RA-ILD progresses following initial therapy, pirfenidone is a treatment option.
- The guidelines conditionally recommend against pirfenidone as a secondary treatment option for SARD-ILD other than RA-ILD.
These summary guidelines appear “comprehensive,” but there has yet to be information published on the basis of these recommendations, Elizabeth Volkmann, MD, said in an interview.
“It’s important to understand that we don’t know whether most of these recommendations were just driven by expert opinion versus actual evidence from randomized, controlled clinical trials,” said Dr. Volkmann, who codirects the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles. She was not involved with creating the guidelines.
She expects that many of the recommendations for first- and second-line ILD treatment options were based on expert opinion, as there have been no randomized clinical trials looking at that specific topic, she said. For example, nintedanib is conditionally recommended as a first-line treatment option for SSc-ILD, but as a second-line treatment for SjD-ILD, IIM-ILD, and MCTD-ILD. “There’s no literature to support one or the other – whether nintedanib is first-line or second-line [treatment].”
The decision to publish the summary recommendations online prior to peer review is unusual, she said, as these recommendations could be altered during that process; however, Dr. Bernstein noted that was not likely.
By releasing the summary guideline now, the ACR can “get the needed information to clinicians earlier as the manuscript goes through its remaining stages and is finalized,” an ACR representative explained.
Prior to the expected publication of these guidelines in early 2024, Dr. Volkmann noted that the American Thoracic Society will be publishing guidelines on the treatment of SSc-ILD in the American Journal of Respiratory and Critical Care Medicine in September.
Dr. Bernstein reported grants/contracts with the Department of Defense, the Scleroderma Research Foundation, the National Institutes of Health, Eicos, Boehringer Ingelheim, Kadmon, and Pfizer. Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and GlaxoSmithKline and institutional support for performing studies on systemic sclerosis for Kadmon, Boehringer Ingelheim, Horizon, and Prometheus.
A version of this article first appeared on Medscape.com.
ESC issues new guidelines on infective endocarditis
(IE) – a rare and potentially lethal infection of the heart’s lining and valves.
The document revises the 2015 version, based on advances in imaging and a trial of antibiotic prophylaxis, among other new developments.
Cochairpersons of the writing task force, Victoria Delgado, MD, PhD, and Michael A. Borger, MD, PhD, and other members presented and discussed the guidelines in three packed sessions at the annual congress of the European Society of Cardiology, and the document was simultaneously published online in the European Heart Journal.
Endocarditis “can present with so many different clinical scenarios, so making the diagnosis can be very challenging,” Dr. Borger, from the Heart Center of Leipzig, Germany, said in an interview.
Diagnosing a lethal, rare, but not uncommon disease such as endocarditis “is something that clinicians struggle with every day,” he noted, pointing to the large overflowing auditoriums where these guidelines were presented. Dr. Borger identified four main takeaways from the document:
- Increased level of recommendation and a clearer definition of prevention and prophylaxis of endocarditis in higher-risk patients.
- An increasing role of nonechocardiographic, advanced cardiac imaging techniques in the diagnosis of endocarditis. “The advanced cardiac imaging techniques achieved the same level of recommendation as echocardiography,” he noted.
- More precisely defined indications for surgery and the timing for surgery, as well as a couple of new surgical recommendations.
- More precisely defined criteria for diagnosing and managing cardiac electronic implantable device (CIED)–associated endocarditis.
The guidelines identify patients at high risk for IE as those with previous IE and patients with surgically implanted prosthetic valves, certain congenital heart diseases, surgery with prosthetic material, or a ventricular assist device as destination therapy and recommend giving them prophylactic antibiotics before oral or dental procedures.
Patients at intermediate risk for IE include those with rheumatic heart disease, nonrheumatic degenerative valve disease, congenital valve abnormalities, CIEDs, and hypertrophic cardiomyopathy. They should be evaluated on a case-by-case basis for this prophylaxis, the guideline authors write.
Making the diagnosis
Advances in imaging techniques necessitated a revised version of the endocarditis guidelines, Dr. Borger noted.
Patients are classified as having a definite, possible, or rejected diagnosis of IE (where a definite diagnosis requires two major criteria, or one major criterion and at least three minor criteria, or five minor criteria).
The two major criteria are blood cultures positive for IE and imaging positive for IE by transesophageal echocardiography, transthoracic echocardiography, or – what is new – cardiac computed tomography, 18F-fluorodeoxyglucose positron emission tomography, or white blood cell single photon emission tomography/CT.
The five minor criteria are predisposing conditions, fever (temperature > 38° C), embolic vascular dissemination, immunologic phenomena, and microbiological evidence.
Patient education
Patient education is “paramount to early diagnosis and treatment,” Dr. Delgado, from Germans Trias i Pujol Hospital, Barcelona, said in a press release from ESC. “Those with valvular heart disease or previous heart valve surgery should be particularly diligent with regards to prevention and recognizing symptoms.”
IE occurs when bacteria or fungi enter the bloodstream, for example through skin infections, dental procedures, and surgery. Symptoms include fever, night sweats, unexplained weight loss, cough, dizziness, and fainting, the press release notes.
“We have several clinical scenarios that are increasing,” Dr. Delgado said at an Ask the Experts session, including implanted cardiac electronic devices, new transcatheter therapies, and increasing endocarditis in people who use injection drugs, “and we have recommendation of evaluation of these patients at follow up.”
“The guidelines have 34 new recommendations,” she noted in a Guideline Overview session. She drew attention to a central figure, “where we tried to summarize the pathway of the patient who is diagnosed with endocarditis, and where we highlight the role of the endocarditis team,” she said.
The guidelines specify a prophylactic antibiotic regimen for high-risk dental procedures, for children and for adults with or without allergy to penicillin or ampicillin, given as a single dose 30-60 minutes before a procedure, she noted.
A new recommendation is that systemic antibiotic prophylaxis may be considered for high-risk patients undergoing invasive procedures of the respiratory, gastrointestinal, or genitourinary tract; skin; or musculoskeletal system.
“It is very important to have a well-educated population,” Dr. Delgado stressed.
Figure 2 of the guideline depicts what patients should do, she said, such as “maintain good dental hygiene, avoid tattoos and piercings, be mindful of infections, do not self-prescribe antibiotics.” This card can be given to the patient, and they can show it to doctors before interventions.
The main targets for antibiotic prophylaxis are oral streptococci, but the emerging and increasing resistance of these bacteria are reasons why patients should not self-prescribe, Dr. Delgado noted.
“Patients should not be self-medicating in order to try to lower their risk of endocarditis,” Dr. Borger said. “They should be speaking to their physicians and have their physician group them according to their risk category.”
“If they are low risk, there’s no reason to take antibiotic prophylaxis [before oral or dental procedures], but if they are high risk, they should not only be taking antibiotic prophylaxis, they should also be doing things like good dental hygiene – visiting the dentist once or twice a year, avoiding unnecessary procedures such as tattooing and piercings, and quick aseptic management of skin wounds.”
POET Trial: Earlier shift to oral antibiotics at home
“Another very important point is the increasing use of oral outpatient antibiotic therapy based on the Partial Oral Treatment of Endocarditis (POET) randomized trial,” Dr. Borger observed. “That’s a new recommendation,” he said, “with significant implications for the care of patients with this oftentimes life-threatening disease.”
In POET, patients in stable condition who had endocarditis on the left side of the heart caused by streptococci, Enterococcus faecalis, Staphylococcus aureus, or coagulase-negative staphylococci were randomly assigned to continue treatment with intravenous antibiotics (199 patients) or to shift to step-down treatment with oral antibiotics (201 patients) after at least 10 days of initial treatment with IV antibiotics.
The 5-year results were published in 2019 in the New England Journal of Medicine and presented at ESC that year. “We were hoping or expecting to see that oral outpatient therapy would be equivalent to inpatient IV therapy,” Dr. Borger said, “but we were surprised to see that oral outpatient therapy was actually statistically significantly better in terms of survival, a very hard outcome, at 5 years after the randomization.”
“This was an important part of our new guideline document. In select patients who are ‘clinically stable’,” as defined in the guidelines, he said, “they could be successfully managed at home with oral antibiotics, rather than keeping them in hospital the whole 6 weeks.”
“In the U.S. and in Canada, a lot of patients are sent home for intravenous therapy, whereas that practice doesn’t exist in a lot of places in Europe. The patients are sitting in the hospital here for 6 weeks, oftentimes for no other reason – just to get their IV antibiotic therapy. The POET trial has shown us that that is probably the wrong thing to be doing.”
Earlier surgical intervention
The new guidelines also recommend that “once there is an indication to do cardiac surgery, it should be promptly performed,” Dr. Borger noted.
Surgery to remove infected material and drain abscesses is indicated for patients with heart failure or uncontrolled infection and to prevent embolism.
“We have defined emergency indications that should be done within 24 hours; urgent, which should be done within 3-5 days; and nonurgent, more than 5 days but within the same hospitalization,” he elaborated. “We’re basically trying to encourage surgeons and nonsurgeons that once there is an indication for surgery, there’s not a lot of benefit to just waiting. You should proceed with operation in a timely manner” to improve survival.
The guidelines recommend surgery for early prosthetic valve endocarditis, within 6 months of valve surgery, with new valve replacement and complete debridement.
Patients who present with stroke and require surgery are not uncommon, Dr. Borger noted. Ischemic stroke should not be a reason to delay surgery, and patients with hemorrhagic stoke, with favorable features, can undergo surgery.
The guidelines provide a figure for the management of CIED-related infective endocarditis. They also include a new section devoted to patient-centered care and shared decision-making.
The guidelines were endorsed by the European Association for Cardio-Thoracic Surgery and the European Association of Nuclear Medicine. The writing task force included representatives from EACTS, EANM, and the European Society of Clinical Microbiology and Infectious Diseases.
The complete guidelines, as well as pocket guidelines, essential messages, a pocket guidelines app, and an official guidelines slide set, all addressing endocarditis, are available from the ESC website.
The guidelines did not receive any funding. The disclosure forms of all experts involved in their development are available on the ESC website.
A version of this article appeared on Medscape.com.
(IE) – a rare and potentially lethal infection of the heart’s lining and valves.
The document revises the 2015 version, based on advances in imaging and a trial of antibiotic prophylaxis, among other new developments.
Cochairpersons of the writing task force, Victoria Delgado, MD, PhD, and Michael A. Borger, MD, PhD, and other members presented and discussed the guidelines in three packed sessions at the annual congress of the European Society of Cardiology, and the document was simultaneously published online in the European Heart Journal.
Endocarditis “can present with so many different clinical scenarios, so making the diagnosis can be very challenging,” Dr. Borger, from the Heart Center of Leipzig, Germany, said in an interview.
Diagnosing a lethal, rare, but not uncommon disease such as endocarditis “is something that clinicians struggle with every day,” he noted, pointing to the large overflowing auditoriums where these guidelines were presented. Dr. Borger identified four main takeaways from the document:
- Increased level of recommendation and a clearer definition of prevention and prophylaxis of endocarditis in higher-risk patients.
- An increasing role of nonechocardiographic, advanced cardiac imaging techniques in the diagnosis of endocarditis. “The advanced cardiac imaging techniques achieved the same level of recommendation as echocardiography,” he noted.
- More precisely defined indications for surgery and the timing for surgery, as well as a couple of new surgical recommendations.
- More precisely defined criteria for diagnosing and managing cardiac electronic implantable device (CIED)–associated endocarditis.
The guidelines identify patients at high risk for IE as those with previous IE and patients with surgically implanted prosthetic valves, certain congenital heart diseases, surgery with prosthetic material, or a ventricular assist device as destination therapy and recommend giving them prophylactic antibiotics before oral or dental procedures.
Patients at intermediate risk for IE include those with rheumatic heart disease, nonrheumatic degenerative valve disease, congenital valve abnormalities, CIEDs, and hypertrophic cardiomyopathy. They should be evaluated on a case-by-case basis for this prophylaxis, the guideline authors write.
Making the diagnosis
Advances in imaging techniques necessitated a revised version of the endocarditis guidelines, Dr. Borger noted.
Patients are classified as having a definite, possible, or rejected diagnosis of IE (where a definite diagnosis requires two major criteria, or one major criterion and at least three minor criteria, or five minor criteria).
The two major criteria are blood cultures positive for IE and imaging positive for IE by transesophageal echocardiography, transthoracic echocardiography, or – what is new – cardiac computed tomography, 18F-fluorodeoxyglucose positron emission tomography, or white blood cell single photon emission tomography/CT.
The five minor criteria are predisposing conditions, fever (temperature > 38° C), embolic vascular dissemination, immunologic phenomena, and microbiological evidence.
Patient education
Patient education is “paramount to early diagnosis and treatment,” Dr. Delgado, from Germans Trias i Pujol Hospital, Barcelona, said in a press release from ESC. “Those with valvular heart disease or previous heart valve surgery should be particularly diligent with regards to prevention and recognizing symptoms.”
IE occurs when bacteria or fungi enter the bloodstream, for example through skin infections, dental procedures, and surgery. Symptoms include fever, night sweats, unexplained weight loss, cough, dizziness, and fainting, the press release notes.
“We have several clinical scenarios that are increasing,” Dr. Delgado said at an Ask the Experts session, including implanted cardiac electronic devices, new transcatheter therapies, and increasing endocarditis in people who use injection drugs, “and we have recommendation of evaluation of these patients at follow up.”
“The guidelines have 34 new recommendations,” she noted in a Guideline Overview session. She drew attention to a central figure, “where we tried to summarize the pathway of the patient who is diagnosed with endocarditis, and where we highlight the role of the endocarditis team,” she said.
The guidelines specify a prophylactic antibiotic regimen for high-risk dental procedures, for children and for adults with or without allergy to penicillin or ampicillin, given as a single dose 30-60 minutes before a procedure, she noted.
A new recommendation is that systemic antibiotic prophylaxis may be considered for high-risk patients undergoing invasive procedures of the respiratory, gastrointestinal, or genitourinary tract; skin; or musculoskeletal system.
“It is very important to have a well-educated population,” Dr. Delgado stressed.
Figure 2 of the guideline depicts what patients should do, she said, such as “maintain good dental hygiene, avoid tattoos and piercings, be mindful of infections, do not self-prescribe antibiotics.” This card can be given to the patient, and they can show it to doctors before interventions.
The main targets for antibiotic prophylaxis are oral streptococci, but the emerging and increasing resistance of these bacteria are reasons why patients should not self-prescribe, Dr. Delgado noted.
“Patients should not be self-medicating in order to try to lower their risk of endocarditis,” Dr. Borger said. “They should be speaking to their physicians and have their physician group them according to their risk category.”
“If they are low risk, there’s no reason to take antibiotic prophylaxis [before oral or dental procedures], but if they are high risk, they should not only be taking antibiotic prophylaxis, they should also be doing things like good dental hygiene – visiting the dentist once or twice a year, avoiding unnecessary procedures such as tattooing and piercings, and quick aseptic management of skin wounds.”
POET Trial: Earlier shift to oral antibiotics at home
“Another very important point is the increasing use of oral outpatient antibiotic therapy based on the Partial Oral Treatment of Endocarditis (POET) randomized trial,” Dr. Borger observed. “That’s a new recommendation,” he said, “with significant implications for the care of patients with this oftentimes life-threatening disease.”
In POET, patients in stable condition who had endocarditis on the left side of the heart caused by streptococci, Enterococcus faecalis, Staphylococcus aureus, or coagulase-negative staphylococci were randomly assigned to continue treatment with intravenous antibiotics (199 patients) or to shift to step-down treatment with oral antibiotics (201 patients) after at least 10 days of initial treatment with IV antibiotics.
The 5-year results were published in 2019 in the New England Journal of Medicine and presented at ESC that year. “We were hoping or expecting to see that oral outpatient therapy would be equivalent to inpatient IV therapy,” Dr. Borger said, “but we were surprised to see that oral outpatient therapy was actually statistically significantly better in terms of survival, a very hard outcome, at 5 years after the randomization.”
“This was an important part of our new guideline document. In select patients who are ‘clinically stable’,” as defined in the guidelines, he said, “they could be successfully managed at home with oral antibiotics, rather than keeping them in hospital the whole 6 weeks.”
“In the U.S. and in Canada, a lot of patients are sent home for intravenous therapy, whereas that practice doesn’t exist in a lot of places in Europe. The patients are sitting in the hospital here for 6 weeks, oftentimes for no other reason – just to get their IV antibiotic therapy. The POET trial has shown us that that is probably the wrong thing to be doing.”
Earlier surgical intervention
The new guidelines also recommend that “once there is an indication to do cardiac surgery, it should be promptly performed,” Dr. Borger noted.
Surgery to remove infected material and drain abscesses is indicated for patients with heart failure or uncontrolled infection and to prevent embolism.
“We have defined emergency indications that should be done within 24 hours; urgent, which should be done within 3-5 days; and nonurgent, more than 5 days but within the same hospitalization,” he elaborated. “We’re basically trying to encourage surgeons and nonsurgeons that once there is an indication for surgery, there’s not a lot of benefit to just waiting. You should proceed with operation in a timely manner” to improve survival.
The guidelines recommend surgery for early prosthetic valve endocarditis, within 6 months of valve surgery, with new valve replacement and complete debridement.
Patients who present with stroke and require surgery are not uncommon, Dr. Borger noted. Ischemic stroke should not be a reason to delay surgery, and patients with hemorrhagic stoke, with favorable features, can undergo surgery.
The guidelines provide a figure for the management of CIED-related infective endocarditis. They also include a new section devoted to patient-centered care and shared decision-making.
The guidelines were endorsed by the European Association for Cardio-Thoracic Surgery and the European Association of Nuclear Medicine. The writing task force included representatives from EACTS, EANM, and the European Society of Clinical Microbiology and Infectious Diseases.
The complete guidelines, as well as pocket guidelines, essential messages, a pocket guidelines app, and an official guidelines slide set, all addressing endocarditis, are available from the ESC website.
The guidelines did not receive any funding. The disclosure forms of all experts involved in their development are available on the ESC website.
A version of this article appeared on Medscape.com.
(IE) – a rare and potentially lethal infection of the heart’s lining and valves.
The document revises the 2015 version, based on advances in imaging and a trial of antibiotic prophylaxis, among other new developments.
Cochairpersons of the writing task force, Victoria Delgado, MD, PhD, and Michael A. Borger, MD, PhD, and other members presented and discussed the guidelines in three packed sessions at the annual congress of the European Society of Cardiology, and the document was simultaneously published online in the European Heart Journal.
Endocarditis “can present with so many different clinical scenarios, so making the diagnosis can be very challenging,” Dr. Borger, from the Heart Center of Leipzig, Germany, said in an interview.
Diagnosing a lethal, rare, but not uncommon disease such as endocarditis “is something that clinicians struggle with every day,” he noted, pointing to the large overflowing auditoriums where these guidelines were presented. Dr. Borger identified four main takeaways from the document:
- Increased level of recommendation and a clearer definition of prevention and prophylaxis of endocarditis in higher-risk patients.
- An increasing role of nonechocardiographic, advanced cardiac imaging techniques in the diagnosis of endocarditis. “The advanced cardiac imaging techniques achieved the same level of recommendation as echocardiography,” he noted.
- More precisely defined indications for surgery and the timing for surgery, as well as a couple of new surgical recommendations.
- More precisely defined criteria for diagnosing and managing cardiac electronic implantable device (CIED)–associated endocarditis.
The guidelines identify patients at high risk for IE as those with previous IE and patients with surgically implanted prosthetic valves, certain congenital heart diseases, surgery with prosthetic material, or a ventricular assist device as destination therapy and recommend giving them prophylactic antibiotics before oral or dental procedures.
Patients at intermediate risk for IE include those with rheumatic heart disease, nonrheumatic degenerative valve disease, congenital valve abnormalities, CIEDs, and hypertrophic cardiomyopathy. They should be evaluated on a case-by-case basis for this prophylaxis, the guideline authors write.
Making the diagnosis
Advances in imaging techniques necessitated a revised version of the endocarditis guidelines, Dr. Borger noted.
Patients are classified as having a definite, possible, or rejected diagnosis of IE (where a definite diagnosis requires two major criteria, or one major criterion and at least three minor criteria, or five minor criteria).
The two major criteria are blood cultures positive for IE and imaging positive for IE by transesophageal echocardiography, transthoracic echocardiography, or – what is new – cardiac computed tomography, 18F-fluorodeoxyglucose positron emission tomography, or white blood cell single photon emission tomography/CT.
The five minor criteria are predisposing conditions, fever (temperature > 38° C), embolic vascular dissemination, immunologic phenomena, and microbiological evidence.
Patient education
Patient education is “paramount to early diagnosis and treatment,” Dr. Delgado, from Germans Trias i Pujol Hospital, Barcelona, said in a press release from ESC. “Those with valvular heart disease or previous heart valve surgery should be particularly diligent with regards to prevention and recognizing symptoms.”
IE occurs when bacteria or fungi enter the bloodstream, for example through skin infections, dental procedures, and surgery. Symptoms include fever, night sweats, unexplained weight loss, cough, dizziness, and fainting, the press release notes.
“We have several clinical scenarios that are increasing,” Dr. Delgado said at an Ask the Experts session, including implanted cardiac electronic devices, new transcatheter therapies, and increasing endocarditis in people who use injection drugs, “and we have recommendation of evaluation of these patients at follow up.”
“The guidelines have 34 new recommendations,” she noted in a Guideline Overview session. She drew attention to a central figure, “where we tried to summarize the pathway of the patient who is diagnosed with endocarditis, and where we highlight the role of the endocarditis team,” she said.
The guidelines specify a prophylactic antibiotic regimen for high-risk dental procedures, for children and for adults with or without allergy to penicillin or ampicillin, given as a single dose 30-60 minutes before a procedure, she noted.
A new recommendation is that systemic antibiotic prophylaxis may be considered for high-risk patients undergoing invasive procedures of the respiratory, gastrointestinal, or genitourinary tract; skin; or musculoskeletal system.
“It is very important to have a well-educated population,” Dr. Delgado stressed.
Figure 2 of the guideline depicts what patients should do, she said, such as “maintain good dental hygiene, avoid tattoos and piercings, be mindful of infections, do not self-prescribe antibiotics.” This card can be given to the patient, and they can show it to doctors before interventions.
The main targets for antibiotic prophylaxis are oral streptococci, but the emerging and increasing resistance of these bacteria are reasons why patients should not self-prescribe, Dr. Delgado noted.
“Patients should not be self-medicating in order to try to lower their risk of endocarditis,” Dr. Borger said. “They should be speaking to their physicians and have their physician group them according to their risk category.”
“If they are low risk, there’s no reason to take antibiotic prophylaxis [before oral or dental procedures], but if they are high risk, they should not only be taking antibiotic prophylaxis, they should also be doing things like good dental hygiene – visiting the dentist once or twice a year, avoiding unnecessary procedures such as tattooing and piercings, and quick aseptic management of skin wounds.”
POET Trial: Earlier shift to oral antibiotics at home
“Another very important point is the increasing use of oral outpatient antibiotic therapy based on the Partial Oral Treatment of Endocarditis (POET) randomized trial,” Dr. Borger observed. “That’s a new recommendation,” he said, “with significant implications for the care of patients with this oftentimes life-threatening disease.”
In POET, patients in stable condition who had endocarditis on the left side of the heart caused by streptococci, Enterococcus faecalis, Staphylococcus aureus, or coagulase-negative staphylococci were randomly assigned to continue treatment with intravenous antibiotics (199 patients) or to shift to step-down treatment with oral antibiotics (201 patients) after at least 10 days of initial treatment with IV antibiotics.
The 5-year results were published in 2019 in the New England Journal of Medicine and presented at ESC that year. “We were hoping or expecting to see that oral outpatient therapy would be equivalent to inpatient IV therapy,” Dr. Borger said, “but we were surprised to see that oral outpatient therapy was actually statistically significantly better in terms of survival, a very hard outcome, at 5 years after the randomization.”
“This was an important part of our new guideline document. In select patients who are ‘clinically stable’,” as defined in the guidelines, he said, “they could be successfully managed at home with oral antibiotics, rather than keeping them in hospital the whole 6 weeks.”
“In the U.S. and in Canada, a lot of patients are sent home for intravenous therapy, whereas that practice doesn’t exist in a lot of places in Europe. The patients are sitting in the hospital here for 6 weeks, oftentimes for no other reason – just to get their IV antibiotic therapy. The POET trial has shown us that that is probably the wrong thing to be doing.”
Earlier surgical intervention
The new guidelines also recommend that “once there is an indication to do cardiac surgery, it should be promptly performed,” Dr. Borger noted.
Surgery to remove infected material and drain abscesses is indicated for patients with heart failure or uncontrolled infection and to prevent embolism.
“We have defined emergency indications that should be done within 24 hours; urgent, which should be done within 3-5 days; and nonurgent, more than 5 days but within the same hospitalization,” he elaborated. “We’re basically trying to encourage surgeons and nonsurgeons that once there is an indication for surgery, there’s not a lot of benefit to just waiting. You should proceed with operation in a timely manner” to improve survival.
The guidelines recommend surgery for early prosthetic valve endocarditis, within 6 months of valve surgery, with new valve replacement and complete debridement.
Patients who present with stroke and require surgery are not uncommon, Dr. Borger noted. Ischemic stroke should not be a reason to delay surgery, and patients with hemorrhagic stoke, with favorable features, can undergo surgery.
The guidelines provide a figure for the management of CIED-related infective endocarditis. They also include a new section devoted to patient-centered care and shared decision-making.
The guidelines were endorsed by the European Association for Cardio-Thoracic Surgery and the European Association of Nuclear Medicine. The writing task force included representatives from EACTS, EANM, and the European Society of Clinical Microbiology and Infectious Diseases.
The complete guidelines, as well as pocket guidelines, essential messages, a pocket guidelines app, and an official guidelines slide set, all addressing endocarditis, are available from the ESC website.
The guidelines did not receive any funding. The disclosure forms of all experts involved in their development are available on the ESC website.
A version of this article appeared on Medscape.com.
FROM ESC CONGRESS 2023





