User login
Targeted therapies forge ahead in multiple breast cancer subtypes
As our understanding of the biology of breast cancer has improved, treatment has become increasingly personalized. Targeted therapies continue to significantly improve patient outcomes in multiple subtypes, with several recent drug approvals. Here, we discuss some of these latest developments.
A disease of many faces
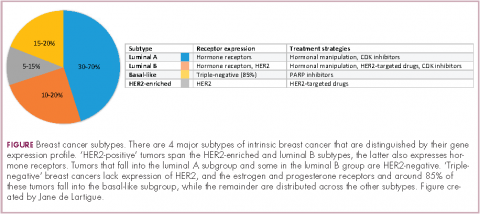
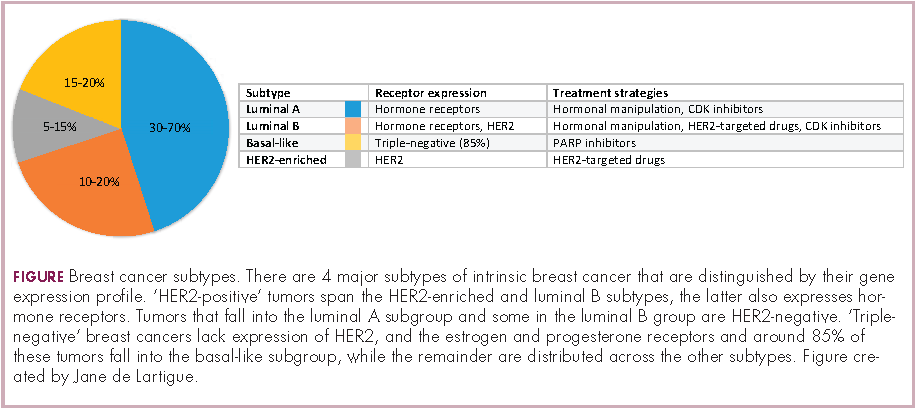
Clinically speaking, breast cancers can be divided into at least 5 subtypes on the basis of the genes they express (Figure 1). The luminal subtypes make up the largest proportion and are characterized by the expression of hormone receptor (HR) genes. Luminal A tumors are negative for human epidermal growth factor receptor 2 (HER2; HER2-negative), whereas luminal B tumors often co-express the HER2 genes.1
The remainder of HER2-positive patients fall into the HER2-enriched category, in which HER2 expression is the defining characteristic. Basal-like tumors, meanwhile, represent the most heterogeneous subtype, overlapping to a large extent with tumors dubbed “triple-negative” because of their lack of either HER2 or ESR1 and PGR gene expression. The fifth subtype is known as normal breast-like and remains poorly characterized.
In recent years, there have been significant advancements in the genomic characterization of breast cancer that have begun to provide a more comprehensive understanding of the driver molecular mechanisms, which has helped to explain some of the limitations of current targeted approaches and to reveal new possible treatments, with a shift toward increasingly personalized strategies.2
HER2: what’s neu?
An estimated 18%-20% of breast tumors are HER2 positive, displaying amplification of the HER2/neu gene or overexpression of its protein product.3 Historically, HER2 positivity correlated with a highly aggressive and metastatic form of disease, conferring poor prognosis.4,5 The HER2-targeted monoclonal antibody (mAb), trastuzumab serves as a prime example of the power of personalized medicine. Evidence suggests that trastuzumab has altered the natural history of HER2-positive breast cancer, such that trastuzumab-treated patients with HER2-positive breast cancer now have a better prognosis than do patients with HER2-negative disease.6,7
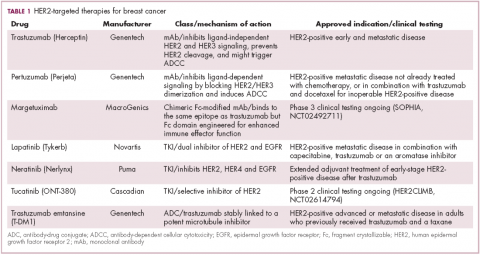
Several additional HER2-targeted drugs have joined trastuzumab on the market, including other mAbs, small molecule tyrosine kinase inhibitors (TKIs), and an antibody–drug conjugate that combines the specificity of a mAb with the anti-tumor potency of a cytotoxic drug. These drugs have further improved patient outcomes in both early and advanced disease settings (Table 1).
The most recent regulatory approval was for neratinib, a potent TKI inhibiting all members of the HER protein family. On the basis of the phase 3 ExteNET study, neratinib was granted approval by the US Food and Drug Administration (FDA) for extended adjuvant treatment of patients with HER2-positive, early-stage breast cancer previously treated with trastuzumab. In a 5-year analysis of the study, invasive disease-free survival (DFS) was 90.4% with neratinib, compared with 87.9% with placebo (hazard ratio [HR], 0.74; P = .017).8,9
The tide of advancements in HER2-targeted therapy looks set to continue in the coming years as potentially practice-changing data emerges from ongoing clinical trials and, as the patent on trastuzumab has expired, a number of biosimilars, such as MYL-1401O have the potential to help patients who may not have access to trastuzumab.10
One of the biggest remaining challenges is identifying drugs that can effectively treat patients with brain metastases because the blood–brain barrier presents an impediment to the delivery of effective concentrations of anticancer drugs. Initially, it was hoped that the small molecule inhibitors lapatinib and neratinib could cross the blood–brain barrier and may be more effective in patients with brain metastases, but that hypothesis has not borne out in randomized clinical trials.11
Tucatinib (ONT-380) has shown significant promise in this respect. In a phase 1 trial, ONT-380 had significant efficacy in patients with and without central nervous system metastases; the overall response rate (ORR) in the CNS was 36%. ONT-380 is also notable for its specificity for HER2, without significant inhibition of HER1 and EGFR, which could translate into a better toxicity profile.12
Doubling down on resistant tumors
Since the success of HER2-targeted therapy is limited by the development of resistance, there has been significant interest in assessing the potential of dual HER2 blockade, exploiting the unique mechanisms of action of different drugs in combination therapy, and ensuring more complete inhibition of the HER2 pathway. Although numerous different combinations have been tested, a double antibody combination has proved most effective.
In fact, dual HER2 targeting with trastuzumab and pertuzumab in combination with chemotherapy has replaced a trastuzumab-chemotherapy regimen as the new standard of care in the metastatic setting. A 6-month improvement in progression-free survival (PFS) sealed FDA approval for the combination and in a recently published final analysis of the trial overall survival (OS) was also improved to a level unprecedented in the first-line setting.13,14The double antibody combination has also been successful in the neoadjuvant setting. Approval followed the results of the phase 2 NeoSphere trial, in which the combination was associated with a significant improvement in pathologic complete response (pCR) rate, a measure that acts as a surrogate for improved survival in the neoadjuvant setting. In a 5-year analysis of the NeoSphere trial, improved pCR did indeed translate into improved PFS and DFS.15,16
The results of the phase 3 APHINITY trial evaluating this combination in the adjuvant setting have been hotly anticipated. In a presentation at the 2017 American Society of Clinical Oncology (ASCO) meeting in June, the study authors reported that in 4,085 patients with operable HER2-positive disease, it significantly reduced the risk of disease recurrence or death compared with trastuzumab and chemotherapy alone.17
There is an ongoing effort to determine if it is possible to de-escalate treatment by removing the chemotherapy component. At least in the neoadjuvant setting, pCR rates in the chemotherapy-free arms of several studies suggest that a proportion of patients might benefit from this strategy15,18,19 and the challenge now is to identify them. To that end, the phase 2 PAMELA trial identified the HER2-enriched subtype as a strong predictor of response to neoadjuvant dual blockade (lapatinib and trastuzumab) without chemotherapy. The pCR rate was 40.6% for the combination in patients with the HER2-enriched subtype of breast cancer and only 10% in patients with non–HER2-enriched tumors.20
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
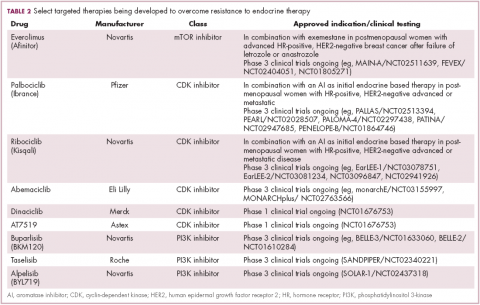
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.
PARP inhibitors make a dent in BRCA1/2-mutated cancers
The most renowned breast cancer genes, BRCA1 and BRCA2 are present in about 5%-10% of all breast cancers. They play a central role in the homologous recombination pathway that fixes double-strand breaks in the DNA. Genome sequencing studies have revealed that the presence of the BRCA1/2 genes and other DNA repair defects is highest among patients with the basal-like subtype of breast cancer, in particular those who have triple-negative disease.34,35
This type of breast cancer has proved stubbornly resistant to efforts to improve patient outcomes with targeted therapies. BRCA1/2 mutations and other DNA repair defects that confer a so-called BRCAness phenotype, render tumor cells dependent on other pathways for DNA repair and there has been considerable interest in therapeutically exploiting this through the development of inhibitors of the poly(ADP-ribose) polymerase (PARP) enzyme, which is involved in the repair of single-strand breaks in the DNA. The double damage to DNA repair mechanisms through PARP inhibition in patients with BRCA1/2-mutant tumors proves overwhelming to cancerous cells.
Despite more than a decade of investigation in breast cancer, PARP inhibitors have yet to yield any FDA-approved treatment options. That may be set to change imminently, following the success of olaparib (Table 3). In the first randomized phase 3 trial of a PARP inhibitor in breast cancer (OlympiAD), olaparib was compared with standard chemotherapy in patients with BRCA1/2-mutated MBC who had received up to 2 previous lines of chemotherapy. Olaparib reduced the risk of disease progression by 42% compared with standard chemotherapy and was well tolerated.36
The novel PARP inhibitor talazoparib, which is the most potent to date, is also demonstrating significant efficacy in clinical trials. The results of the phase 2 ABRAZO trial were presented at the ASCO annual meeting. Two cohorts were treated; the first included 49 patients who had responded to their last platinum-containing regimen for metastatic disease and progressed more than 8 weeks after last platinum dose and the other included 35 patients previously treated with 3 or more nonplatinum regimens for metastatic disease. ORR was 28% across the 2 cohorts; 23% and 33% in BRCA1- and BRCA2-mutant carriers, respectively; and 26% in patients with triple-negative breast cancer.37 PARP inhibition is not faring so well in early-stage triple-negative disease; a phase 3 trial of veliparib in combination with chemotherapy did not meet its primary endpoint.38
As our understanding of the biology of breast cancer has improved, treatment has become increasingly personalized. Targeted therapies continue to significantly improve patient outcomes in multiple subtypes, with several recent drug approvals. Here, we discuss some of these latest developments.
A disease of many faces
Clinically speaking, breast cancers can be divided into at least 5 subtypes on the basis of the genes they express (Figure 1). The luminal subtypes make up the largest proportion and are characterized by the expression of hormone receptor (HR) genes. Luminal A tumors are negative for human epidermal growth factor receptor 2 (HER2; HER2-negative), whereas luminal B tumors often co-express the HER2 genes.1
The remainder of HER2-positive patients fall into the HER2-enriched category, in which HER2 expression is the defining characteristic. Basal-like tumors, meanwhile, represent the most heterogeneous subtype, overlapping to a large extent with tumors dubbed “triple-negative” because of their lack of either HER2 or ESR1 and PGR gene expression. The fifth subtype is known as normal breast-like and remains poorly characterized.
In recent years, there have been significant advancements in the genomic characterization of breast cancer that have begun to provide a more comprehensive understanding of the driver molecular mechanisms, which has helped to explain some of the limitations of current targeted approaches and to reveal new possible treatments, with a shift toward increasingly personalized strategies.2
HER2: what’s neu?
An estimated 18%-20% of breast tumors are HER2 positive, displaying amplification of the HER2/neu gene or overexpression of its protein product.3 Historically, HER2 positivity correlated with a highly aggressive and metastatic form of disease, conferring poor prognosis.4,5 The HER2-targeted monoclonal antibody (mAb), trastuzumab serves as a prime example of the power of personalized medicine. Evidence suggests that trastuzumab has altered the natural history of HER2-positive breast cancer, such that trastuzumab-treated patients with HER2-positive breast cancer now have a better prognosis than do patients with HER2-negative disease.6,7
Several additional HER2-targeted drugs have joined trastuzumab on the market, including other mAbs, small molecule tyrosine kinase inhibitors (TKIs), and an antibody–drug conjugate that combines the specificity of a mAb with the anti-tumor potency of a cytotoxic drug. These drugs have further improved patient outcomes in both early and advanced disease settings (Table 1).
The most recent regulatory approval was for neratinib, a potent TKI inhibiting all members of the HER protein family. On the basis of the phase 3 ExteNET study, neratinib was granted approval by the US Food and Drug Administration (FDA) for extended adjuvant treatment of patients with HER2-positive, early-stage breast cancer previously treated with trastuzumab. In a 5-year analysis of the study, invasive disease-free survival (DFS) was 90.4% with neratinib, compared with 87.9% with placebo (hazard ratio [HR], 0.74; P = .017).8,9
The tide of advancements in HER2-targeted therapy looks set to continue in the coming years as potentially practice-changing data emerges from ongoing clinical trials and, as the patent on trastuzumab has expired, a number of biosimilars, such as MYL-1401O have the potential to help patients who may not have access to trastuzumab.10
One of the biggest remaining challenges is identifying drugs that can effectively treat patients with brain metastases because the blood–brain barrier presents an impediment to the delivery of effective concentrations of anticancer drugs. Initially, it was hoped that the small molecule inhibitors lapatinib and neratinib could cross the blood–brain barrier and may be more effective in patients with brain metastases, but that hypothesis has not borne out in randomized clinical trials.11
Tucatinib (ONT-380) has shown significant promise in this respect. In a phase 1 trial, ONT-380 had significant efficacy in patients with and without central nervous system metastases; the overall response rate (ORR) in the CNS was 36%. ONT-380 is also notable for its specificity for HER2, without significant inhibition of HER1 and EGFR, which could translate into a better toxicity profile.12
Doubling down on resistant tumors
Since the success of HER2-targeted therapy is limited by the development of resistance, there has been significant interest in assessing the potential of dual HER2 blockade, exploiting the unique mechanisms of action of different drugs in combination therapy, and ensuring more complete inhibition of the HER2 pathway. Although numerous different combinations have been tested, a double antibody combination has proved most effective.
In fact, dual HER2 targeting with trastuzumab and pertuzumab in combination with chemotherapy has replaced a trastuzumab-chemotherapy regimen as the new standard of care in the metastatic setting. A 6-month improvement in progression-free survival (PFS) sealed FDA approval for the combination and in a recently published final analysis of the trial overall survival (OS) was also improved to a level unprecedented in the first-line setting.13,14The double antibody combination has also been successful in the neoadjuvant setting. Approval followed the results of the phase 2 NeoSphere trial, in which the combination was associated with a significant improvement in pathologic complete response (pCR) rate, a measure that acts as a surrogate for improved survival in the neoadjuvant setting. In a 5-year analysis of the NeoSphere trial, improved pCR did indeed translate into improved PFS and DFS.15,16
The results of the phase 3 APHINITY trial evaluating this combination in the adjuvant setting have been hotly anticipated. In a presentation at the 2017 American Society of Clinical Oncology (ASCO) meeting in June, the study authors reported that in 4,085 patients with operable HER2-positive disease, it significantly reduced the risk of disease recurrence or death compared with trastuzumab and chemotherapy alone.17
There is an ongoing effort to determine if it is possible to de-escalate treatment by removing the chemotherapy component. At least in the neoadjuvant setting, pCR rates in the chemotherapy-free arms of several studies suggest that a proportion of patients might benefit from this strategy15,18,19 and the challenge now is to identify them. To that end, the phase 2 PAMELA trial identified the HER2-enriched subtype as a strong predictor of response to neoadjuvant dual blockade (lapatinib and trastuzumab) without chemotherapy. The pCR rate was 40.6% for the combination in patients with the HER2-enriched subtype of breast cancer and only 10% in patients with non–HER2-enriched tumors.20
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.
PARP inhibitors make a dent in BRCA1/2-mutated cancers
The most renowned breast cancer genes, BRCA1 and BRCA2 are present in about 5%-10% of all breast cancers. They play a central role in the homologous recombination pathway that fixes double-strand breaks in the DNA. Genome sequencing studies have revealed that the presence of the BRCA1/2 genes and other DNA repair defects is highest among patients with the basal-like subtype of breast cancer, in particular those who have triple-negative disease.34,35
This type of breast cancer has proved stubbornly resistant to efforts to improve patient outcomes with targeted therapies. BRCA1/2 mutations and other DNA repair defects that confer a so-called BRCAness phenotype, render tumor cells dependent on other pathways for DNA repair and there has been considerable interest in therapeutically exploiting this through the development of inhibitors of the poly(ADP-ribose) polymerase (PARP) enzyme, which is involved in the repair of single-strand breaks in the DNA. The double damage to DNA repair mechanisms through PARP inhibition in patients with BRCA1/2-mutant tumors proves overwhelming to cancerous cells.
Despite more than a decade of investigation in breast cancer, PARP inhibitors have yet to yield any FDA-approved treatment options. That may be set to change imminently, following the success of olaparib (Table 3). In the first randomized phase 3 trial of a PARP inhibitor in breast cancer (OlympiAD), olaparib was compared with standard chemotherapy in patients with BRCA1/2-mutated MBC who had received up to 2 previous lines of chemotherapy. Olaparib reduced the risk of disease progression by 42% compared with standard chemotherapy and was well tolerated.36
The novel PARP inhibitor talazoparib, which is the most potent to date, is also demonstrating significant efficacy in clinical trials. The results of the phase 2 ABRAZO trial were presented at the ASCO annual meeting. Two cohorts were treated; the first included 49 patients who had responded to their last platinum-containing regimen for metastatic disease and progressed more than 8 weeks after last platinum dose and the other included 35 patients previously treated with 3 or more nonplatinum regimens for metastatic disease. ORR was 28% across the 2 cohorts; 23% and 33% in BRCA1- and BRCA2-mutant carriers, respectively; and 26% in patients with triple-negative breast cancer.37 PARP inhibition is not faring so well in early-stage triple-negative disease; a phase 3 trial of veliparib in combination with chemotherapy did not meet its primary endpoint.38
As our understanding of the biology of breast cancer has improved, treatment has become increasingly personalized. Targeted therapies continue to significantly improve patient outcomes in multiple subtypes, with several recent drug approvals. Here, we discuss some of these latest developments.
A disease of many faces
Clinically speaking, breast cancers can be divided into at least 5 subtypes on the basis of the genes they express (Figure 1). The luminal subtypes make up the largest proportion and are characterized by the expression of hormone receptor (HR) genes. Luminal A tumors are negative for human epidermal growth factor receptor 2 (HER2; HER2-negative), whereas luminal B tumors often co-express the HER2 genes.1
The remainder of HER2-positive patients fall into the HER2-enriched category, in which HER2 expression is the defining characteristic. Basal-like tumors, meanwhile, represent the most heterogeneous subtype, overlapping to a large extent with tumors dubbed “triple-negative” because of their lack of either HER2 or ESR1 and PGR gene expression. The fifth subtype is known as normal breast-like and remains poorly characterized.
In recent years, there have been significant advancements in the genomic characterization of breast cancer that have begun to provide a more comprehensive understanding of the driver molecular mechanisms, which has helped to explain some of the limitations of current targeted approaches and to reveal new possible treatments, with a shift toward increasingly personalized strategies.2
HER2: what’s neu?
An estimated 18%-20% of breast tumors are HER2 positive, displaying amplification of the HER2/neu gene or overexpression of its protein product.3 Historically, HER2 positivity correlated with a highly aggressive and metastatic form of disease, conferring poor prognosis.4,5 The HER2-targeted monoclonal antibody (mAb), trastuzumab serves as a prime example of the power of personalized medicine. Evidence suggests that trastuzumab has altered the natural history of HER2-positive breast cancer, such that trastuzumab-treated patients with HER2-positive breast cancer now have a better prognosis than do patients with HER2-negative disease.6,7
Several additional HER2-targeted drugs have joined trastuzumab on the market, including other mAbs, small molecule tyrosine kinase inhibitors (TKIs), and an antibody–drug conjugate that combines the specificity of a mAb with the anti-tumor potency of a cytotoxic drug. These drugs have further improved patient outcomes in both early and advanced disease settings (Table 1).
The most recent regulatory approval was for neratinib, a potent TKI inhibiting all members of the HER protein family. On the basis of the phase 3 ExteNET study, neratinib was granted approval by the US Food and Drug Administration (FDA) for extended adjuvant treatment of patients with HER2-positive, early-stage breast cancer previously treated with trastuzumab. In a 5-year analysis of the study, invasive disease-free survival (DFS) was 90.4% with neratinib, compared with 87.9% with placebo (hazard ratio [HR], 0.74; P = .017).8,9
The tide of advancements in HER2-targeted therapy looks set to continue in the coming years as potentially practice-changing data emerges from ongoing clinical trials and, as the patent on trastuzumab has expired, a number of biosimilars, such as MYL-1401O have the potential to help patients who may not have access to trastuzumab.10
One of the biggest remaining challenges is identifying drugs that can effectively treat patients with brain metastases because the blood–brain barrier presents an impediment to the delivery of effective concentrations of anticancer drugs. Initially, it was hoped that the small molecule inhibitors lapatinib and neratinib could cross the blood–brain barrier and may be more effective in patients with brain metastases, but that hypothesis has not borne out in randomized clinical trials.11
Tucatinib (ONT-380) has shown significant promise in this respect. In a phase 1 trial, ONT-380 had significant efficacy in patients with and without central nervous system metastases; the overall response rate (ORR) in the CNS was 36%. ONT-380 is also notable for its specificity for HER2, without significant inhibition of HER1 and EGFR, which could translate into a better toxicity profile.12
Doubling down on resistant tumors
Since the success of HER2-targeted therapy is limited by the development of resistance, there has been significant interest in assessing the potential of dual HER2 blockade, exploiting the unique mechanisms of action of different drugs in combination therapy, and ensuring more complete inhibition of the HER2 pathway. Although numerous different combinations have been tested, a double antibody combination has proved most effective.
In fact, dual HER2 targeting with trastuzumab and pertuzumab in combination with chemotherapy has replaced a trastuzumab-chemotherapy regimen as the new standard of care in the metastatic setting. A 6-month improvement in progression-free survival (PFS) sealed FDA approval for the combination and in a recently published final analysis of the trial overall survival (OS) was also improved to a level unprecedented in the first-line setting.13,14The double antibody combination has also been successful in the neoadjuvant setting. Approval followed the results of the phase 2 NeoSphere trial, in which the combination was associated with a significant improvement in pathologic complete response (pCR) rate, a measure that acts as a surrogate for improved survival in the neoadjuvant setting. In a 5-year analysis of the NeoSphere trial, improved pCR did indeed translate into improved PFS and DFS.15,16
The results of the phase 3 APHINITY trial evaluating this combination in the adjuvant setting have been hotly anticipated. In a presentation at the 2017 American Society of Clinical Oncology (ASCO) meeting in June, the study authors reported that in 4,085 patients with operable HER2-positive disease, it significantly reduced the risk of disease recurrence or death compared with trastuzumab and chemotherapy alone.17
There is an ongoing effort to determine if it is possible to de-escalate treatment by removing the chemotherapy component. At least in the neoadjuvant setting, pCR rates in the chemotherapy-free arms of several studies suggest that a proportion of patients might benefit from this strategy15,18,19 and the challenge now is to identify them. To that end, the phase 2 PAMELA trial identified the HER2-enriched subtype as a strong predictor of response to neoadjuvant dual blockade (lapatinib and trastuzumab) without chemotherapy. The pCR rate was 40.6% for the combination in patients with the HER2-enriched subtype of breast cancer and only 10% in patients with non–HER2-enriched tumors.20
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.
PARP inhibitors make a dent in BRCA1/2-mutated cancers
The most renowned breast cancer genes, BRCA1 and BRCA2 are present in about 5%-10% of all breast cancers. They play a central role in the homologous recombination pathway that fixes double-strand breaks in the DNA. Genome sequencing studies have revealed that the presence of the BRCA1/2 genes and other DNA repair defects is highest among patients with the basal-like subtype of breast cancer, in particular those who have triple-negative disease.34,35
This type of breast cancer has proved stubbornly resistant to efforts to improve patient outcomes with targeted therapies. BRCA1/2 mutations and other DNA repair defects that confer a so-called BRCAness phenotype, render tumor cells dependent on other pathways for DNA repair and there has been considerable interest in therapeutically exploiting this through the development of inhibitors of the poly(ADP-ribose) polymerase (PARP) enzyme, which is involved in the repair of single-strand breaks in the DNA. The double damage to DNA repair mechanisms through PARP inhibition in patients with BRCA1/2-mutant tumors proves overwhelming to cancerous cells.
Despite more than a decade of investigation in breast cancer, PARP inhibitors have yet to yield any FDA-approved treatment options. That may be set to change imminently, following the success of olaparib (Table 3). In the first randomized phase 3 trial of a PARP inhibitor in breast cancer (OlympiAD), olaparib was compared with standard chemotherapy in patients with BRCA1/2-mutated MBC who had received up to 2 previous lines of chemotherapy. Olaparib reduced the risk of disease progression by 42% compared with standard chemotherapy and was well tolerated.36
The novel PARP inhibitor talazoparib, which is the most potent to date, is also demonstrating significant efficacy in clinical trials. The results of the phase 2 ABRAZO trial were presented at the ASCO annual meeting. Two cohorts were treated; the first included 49 patients who had responded to their last platinum-containing regimen for metastatic disease and progressed more than 8 weeks after last platinum dose and the other included 35 patients previously treated with 3 or more nonplatinum regimens for metastatic disease. ORR was 28% across the 2 cohorts; 23% and 33% in BRCA1- and BRCA2-mutant carriers, respectively; and 26% in patients with triple-negative breast cancer.37 PARP inhibition is not faring so well in early-stage triple-negative disease; a phase 3 trial of veliparib in combination with chemotherapy did not meet its primary endpoint.38
Testing the Limits of Dual Antiplatelet Treatment for PCI Patients
What’s the right duration of dual antiplatelet therapy (DAPT) for patients who have had drug-eluting stents implanted? According to researchers from Sungkyunkwan University in Seoul, Korea, long-term clinical outcomes are similar whether patients receive therapy for less than or longer than 12 months.
The researchers conducted a retrospective study of 512 patients who had undergone percutaneous coronary intervention (PCI) for coronary chronic total occlusion (CTO) and were event free at 12 months. They separated the patients into 2 groups: 199 received aspirin and clopidogrel for ≤ 12 months, and 313 for > 12 months. The primary outcome was major adverse cardiac and cerebrovascular event (MACCE) during follow-up. Median follow-up was 64 months.
Related: Statins for the Physically Fit: Do They Help or Hurt?
No significant differences were seen between the groups in the incidence of MACCE: 21.6% of the patients in the ≤ 12-month group and 17.6% of the patients in the > 12-month group developed MACCE. After propensity matching, moderate or severe bleeding rates were also similar (1.6% in the shorter duration group and 2.2% in the longer duration group).
The researchers note that previously published data showed that longer duration DAPT was not associated with improved clinical outcomes in patients with CTO, although other subsets of complex PCI, such as longer stent length, bifurcation stenting, or multiple stents showed better clinical outcomes. To the best of their knowledge, the researchers add, theirs is the first study to directly compare DAPT durations in patients with CTO-PCI.
Related: A Heart Failure Management Program Using Shared Medical Appointments
Source:
Lee SH, Yang JH, Choi SH, et al. PLoS One. 2017;12(5):e0176737.
doi: 10.1371/journal.pone.0176737.
What’s the right duration of dual antiplatelet therapy (DAPT) for patients who have had drug-eluting stents implanted? According to researchers from Sungkyunkwan University in Seoul, Korea, long-term clinical outcomes are similar whether patients receive therapy for less than or longer than 12 months.
The researchers conducted a retrospective study of 512 patients who had undergone percutaneous coronary intervention (PCI) for coronary chronic total occlusion (CTO) and were event free at 12 months. They separated the patients into 2 groups: 199 received aspirin and clopidogrel for ≤ 12 months, and 313 for > 12 months. The primary outcome was major adverse cardiac and cerebrovascular event (MACCE) during follow-up. Median follow-up was 64 months.
Related: Statins for the Physically Fit: Do They Help or Hurt?
No significant differences were seen between the groups in the incidence of MACCE: 21.6% of the patients in the ≤ 12-month group and 17.6% of the patients in the > 12-month group developed MACCE. After propensity matching, moderate or severe bleeding rates were also similar (1.6% in the shorter duration group and 2.2% in the longer duration group).
The researchers note that previously published data showed that longer duration DAPT was not associated with improved clinical outcomes in patients with CTO, although other subsets of complex PCI, such as longer stent length, bifurcation stenting, or multiple stents showed better clinical outcomes. To the best of their knowledge, the researchers add, theirs is the first study to directly compare DAPT durations in patients with CTO-PCI.
Related: A Heart Failure Management Program Using Shared Medical Appointments
Source:
Lee SH, Yang JH, Choi SH, et al. PLoS One. 2017;12(5):e0176737.
doi: 10.1371/journal.pone.0176737.
What’s the right duration of dual antiplatelet therapy (DAPT) for patients who have had drug-eluting stents implanted? According to researchers from Sungkyunkwan University in Seoul, Korea, long-term clinical outcomes are similar whether patients receive therapy for less than or longer than 12 months.
The researchers conducted a retrospective study of 512 patients who had undergone percutaneous coronary intervention (PCI) for coronary chronic total occlusion (CTO) and were event free at 12 months. They separated the patients into 2 groups: 199 received aspirin and clopidogrel for ≤ 12 months, and 313 for > 12 months. The primary outcome was major adverse cardiac and cerebrovascular event (MACCE) during follow-up. Median follow-up was 64 months.
Related: Statins for the Physically Fit: Do They Help or Hurt?
No significant differences were seen between the groups in the incidence of MACCE: 21.6% of the patients in the ≤ 12-month group and 17.6% of the patients in the > 12-month group developed MACCE. After propensity matching, moderate or severe bleeding rates were also similar (1.6% in the shorter duration group and 2.2% in the longer duration group).
The researchers note that previously published data showed that longer duration DAPT was not associated with improved clinical outcomes in patients with CTO, although other subsets of complex PCI, such as longer stent length, bifurcation stenting, or multiple stents showed better clinical outcomes. To the best of their knowledge, the researchers add, theirs is the first study to directly compare DAPT durations in patients with CTO-PCI.
Related: A Heart Failure Management Program Using Shared Medical Appointments
Source:
Lee SH, Yang JH, Choi SH, et al. PLoS One. 2017;12(5):e0176737.
doi: 10.1371/journal.pone.0176737.
Arthritis Is On the Rise—But There Are Ways to Help Reduce the Effects
Arthritis aches and pains are not a normal part of aging. Nonetheless, approximately 54 million American adults who took the CDC’s National Health Survey said their doctor had diagnosed them with arthritis. That’s about 1 in 4 US adults, the majority of whom are of working age.
Related: Taking Steps to Reduce Arthritis Pain
Arthritis can make it hard to lift a cup, let alone a bag of groceries or a heavy briefcase. The percentage of adults with arthritis who have activity limitations grew from 35.9% in 2002 to 42.8% in 2014, a 20% increase independent of the aging of the population.
Research has shown that engaging in physical activity can reduce arthritis symptoms by up to 40%. But one third of adults with arthritis say they don’t engage in physical activity during leisure time. And, while they also can reduce their symptoms by participating in disease management education programs, only 1 in 10 has taken part in such programs.
Related: Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
“It’s extremely important for primary care providers to encourage their patients with arthritis to be physically active,” says CDC epidemiologist Kamil Barbour, PhD. But Barbour adds that it’s just as important to motivate patients to attend education programs. The CDC says adults with arthritis are significantly more likely to attend an education program when a health care provider has recommended it.
Arthritis aches and pains are not a normal part of aging. Nonetheless, approximately 54 million American adults who took the CDC’s National Health Survey said their doctor had diagnosed them with arthritis. That’s about 1 in 4 US adults, the majority of whom are of working age.
Related: Taking Steps to Reduce Arthritis Pain
Arthritis can make it hard to lift a cup, let alone a bag of groceries or a heavy briefcase. The percentage of adults with arthritis who have activity limitations grew from 35.9% in 2002 to 42.8% in 2014, a 20% increase independent of the aging of the population.
Research has shown that engaging in physical activity can reduce arthritis symptoms by up to 40%. But one third of adults with arthritis say they don’t engage in physical activity during leisure time. And, while they also can reduce their symptoms by participating in disease management education programs, only 1 in 10 has taken part in such programs.
Related: Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
“It’s extremely important for primary care providers to encourage their patients with arthritis to be physically active,” says CDC epidemiologist Kamil Barbour, PhD. But Barbour adds that it’s just as important to motivate patients to attend education programs. The CDC says adults with arthritis are significantly more likely to attend an education program when a health care provider has recommended it.
Arthritis aches and pains are not a normal part of aging. Nonetheless, approximately 54 million American adults who took the CDC’s National Health Survey said their doctor had diagnosed them with arthritis. That’s about 1 in 4 US adults, the majority of whom are of working age.
Related: Taking Steps to Reduce Arthritis Pain
Arthritis can make it hard to lift a cup, let alone a bag of groceries or a heavy briefcase. The percentage of adults with arthritis who have activity limitations grew from 35.9% in 2002 to 42.8% in 2014, a 20% increase independent of the aging of the population.
Research has shown that engaging in physical activity can reduce arthritis symptoms by up to 40%. But one third of adults with arthritis say they don’t engage in physical activity during leisure time. And, while they also can reduce their symptoms by participating in disease management education programs, only 1 in 10 has taken part in such programs.
Related: Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
“It’s extremely important for primary care providers to encourage their patients with arthritis to be physically active,” says CDC epidemiologist Kamil Barbour, PhD. But Barbour adds that it’s just as important to motivate patients to attend education programs. The CDC says adults with arthritis are significantly more likely to attend an education program when a health care provider has recommended it.
Testosterone Trial Offers Plusses and Minuses
“Mixed results,” is the current status report on the Testosterone Trials (“T Trials”). In older men with low testosterone, 1 year of testosterone treatment not only improved bone density and corrected anemia, but also increased the volume of coronary artery plaque.
The T Trials were designed to determine whether testosterone treatment could alleviate problems, such as impaired cognition, anemia, cardiovascular disease, diminished sexual function, decreased mobility, and fatigue. The trials were conducted at 12 sites nationwide in 790 men aged ≥ 65 years. Participants were randomly assigned to apply testosterone gel or placebo to the skin daily. Serum testosterone was measured at 1, 2, 3,6, 9, and 12 months. The men were closely monitored for prostate and cardiovascular problems.
Related: Testosterone Replacement Therapy: Playing Catch-up With Patients
After 1 year of testosterone treatment, 54% of men with unexplained anemia and 52% of men with anemia from known causes had clinically significant increases in hemoglobin, compared with 15% and 12% of men in the placebo group. Older men with low testosterone had significantly increased volumetric bone density and estimated bone strength compared with the controls.
However, the testosterone-treated group also had significantly higher levels of coronary artery plaque, although only 170 men had coronary computed tomograph arteriography. The researchers found no significant differences between the 2 groups in cognition in older men with age-associated memory impairment.
Related: Restoring Testosterone Levels May Improve Sexual Function
The results illustrate the need for individualized decisions about testosterone treatment, said Evan Hadley, MD, director of National Institute on Aging’s Division of Geriatrics and Clinical Gerontology. The “diverse outcomes,” he notes, indicate the potential trade-offs between benefits and risks of testosterone treatment in older men. Clarifying the effects of testosterone will take longer, larger scale trials.
“Mixed results,” is the current status report on the Testosterone Trials (“T Trials”). In older men with low testosterone, 1 year of testosterone treatment not only improved bone density and corrected anemia, but also increased the volume of coronary artery plaque.
The T Trials were designed to determine whether testosterone treatment could alleviate problems, such as impaired cognition, anemia, cardiovascular disease, diminished sexual function, decreased mobility, and fatigue. The trials were conducted at 12 sites nationwide in 790 men aged ≥ 65 years. Participants were randomly assigned to apply testosterone gel or placebo to the skin daily. Serum testosterone was measured at 1, 2, 3,6, 9, and 12 months. The men were closely monitored for prostate and cardiovascular problems.
Related: Testosterone Replacement Therapy: Playing Catch-up With Patients
After 1 year of testosterone treatment, 54% of men with unexplained anemia and 52% of men with anemia from known causes had clinically significant increases in hemoglobin, compared with 15% and 12% of men in the placebo group. Older men with low testosterone had significantly increased volumetric bone density and estimated bone strength compared with the controls.
However, the testosterone-treated group also had significantly higher levels of coronary artery plaque, although only 170 men had coronary computed tomograph arteriography. The researchers found no significant differences between the 2 groups in cognition in older men with age-associated memory impairment.
Related: Restoring Testosterone Levels May Improve Sexual Function
The results illustrate the need for individualized decisions about testosterone treatment, said Evan Hadley, MD, director of National Institute on Aging’s Division of Geriatrics and Clinical Gerontology. The “diverse outcomes,” he notes, indicate the potential trade-offs between benefits and risks of testosterone treatment in older men. Clarifying the effects of testosterone will take longer, larger scale trials.
“Mixed results,” is the current status report on the Testosterone Trials (“T Trials”). In older men with low testosterone, 1 year of testosterone treatment not only improved bone density and corrected anemia, but also increased the volume of coronary artery plaque.
The T Trials were designed to determine whether testosterone treatment could alleviate problems, such as impaired cognition, anemia, cardiovascular disease, diminished sexual function, decreased mobility, and fatigue. The trials were conducted at 12 sites nationwide in 790 men aged ≥ 65 years. Participants were randomly assigned to apply testosterone gel or placebo to the skin daily. Serum testosterone was measured at 1, 2, 3,6, 9, and 12 months. The men were closely monitored for prostate and cardiovascular problems.
Related: Testosterone Replacement Therapy: Playing Catch-up With Patients
After 1 year of testosterone treatment, 54% of men with unexplained anemia and 52% of men with anemia from known causes had clinically significant increases in hemoglobin, compared with 15% and 12% of men in the placebo group. Older men with low testosterone had significantly increased volumetric bone density and estimated bone strength compared with the controls.
However, the testosterone-treated group also had significantly higher levels of coronary artery plaque, although only 170 men had coronary computed tomograph arteriography. The researchers found no significant differences between the 2 groups in cognition in older men with age-associated memory impairment.
Related: Restoring Testosterone Levels May Improve Sexual Function
The results illustrate the need for individualized decisions about testosterone treatment, said Evan Hadley, MD, director of National Institute on Aging’s Division of Geriatrics and Clinical Gerontology. The “diverse outcomes,” he notes, indicate the potential trade-offs between benefits and risks of testosterone treatment in older men. Clarifying the effects of testosterone will take longer, larger scale trials.
Liquid gold: blood-based biopsies make headway
Pathologic and, increasingly, molecular analysis of tumor tissue biopsies is the gold standard in initial diagnosis of cancer, but liquid biopsies, which analyze tumor-derived material circulating in the bloodstream are gaining traction. Here, we discuss the current state of development of this complementary and potentially alternative approach to tumor analysis.
Liquid biopsy gaining traction
Biopsies enable oncologists to gather information about a potential or established tumor, including confirmation of the presence of cancerous tissue and determination of its histological characteristics, such as tumor grade and stage, as well as its molecular features, such as the presence of certain gene mutations. Ultimately, this information can be put to use in determining the most appropriate course of treatment.
The current gold standard is a tissue biopsy that typically involves an invasive procedure to permit the collection of a piece of tumor tissue. Yet, tissue biopsies are not always feasible because of the location of the tumor or the poor performance status of many patients with advanced disease. They also provide only a snapshot of the disease at the time at which they were taken and don’t necessarily reflect the genetic heterogeneity or evolution of a tumor over time.
The detection of components that are derived from the tumor circulating in the blood of cancer patients had fueled the idea of blood-based diagnostics in oncology – so-called liquid biopsies. These have rapidly gained traction in the past several decades as a less expensive (the cost of performing genomic analyses on blood samples is at least an order of magnitude less than on tissue samples), less invasive (requiring only a simple blood draw) alternative source of information about tumors.1
As researchers have refined the ability to exploit liquid biopsies, commercial interest has been piqued. More than 35 companies within the United States alone are developing liquid biopsies, and it’s easy to see why with a market projected to be in the many billions of dollars.2
Seeking out tumor clues in the blood
Liquid biopsies consist of a 10-15 mL blood sample drawn into a tube that contains an anticoagulant and it can contain several different types of tumor-associated material. Thus far, two components – circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) – have formed the cornerstone of liquid biopsies. At present, it is not clear whether these components are released randomly, as a by-product of tumor cell death or if they are released as part of a specific biologic process, such as for the colonization of metastatic sites. It reality, it may be a little of both, and active dissemination may be particularly relevant for CTCs, among which are postulated to be a population of cancer stem cells that can initiate distant metastases.3,4
The discovery of CTCs dates back to the 1860s, when cells that were morphologically identical to the tumor were identified in the blood of a patient with metastatic cancer. Their potential significance was not fully realized until a few decades ago, when they were found to exist from early on in the course of disease.3,4
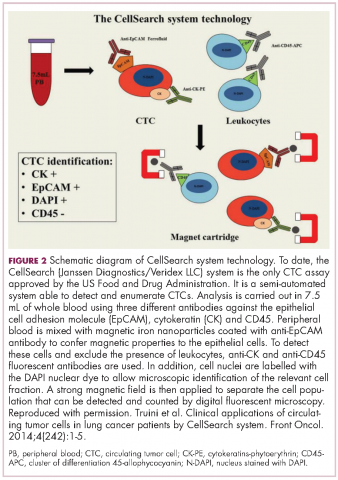
CTCs, which can be either single cells or clusters of cells known as microemboli, have a short half-life in the bloodstream – less than 2 ½ hours – and are also extremely rare (1 mL of blood contains 1-10 CTCs) against a background of many millions of normal cells. Thus the detection and isolation of CTCs presents a significant challenge. More than 40 different platforms are being developed for the isolation and enrichment of CTCs. For the most part, these use a method called positive selection to pick out CTCs.1,3,4
Positive selection exploits the biological or physical properties that are specific to CTCs and absent in normal cells, for example, the presence of a specific tumor-associated antigen on their surface or differences in size, density or electric charge. The limitations of this method are that, not only do you need to know something about CTCs to begin to understand what makes them truly unique and ensure only isolation of CTCs, but their phenotype is also thought to be continually changing.1,3,4
In recent years, the focus has shifted toward technologies that use negative depletion, meaning that they target the other types of cells in the blood sample and filter those away until only the CTCs are left behind. The most advanced are devices that use microfluidic technology to sort the cells, such as the CTC-iChip system being developed by researchers at Massachusetts General Hospital in Boston.5
ctDNA consists of small fragments of nucleic acids that are not contained within a cell or associated with cell fragments and is thought to be present in 50%-90% of patients, depending on the type of cancer they have. ctDNA has a similarly short half-life in the circulation to CTCs and, like CTCs, ctDNA is present at very low levels in the bloodstream. Although levels of ctDNA have been shown to increase with increasing tumor burden, it is still often obscured by the presence of other cell-free DNA derived from non-tumor cells.
ctDNA can be distinguished from other cell-free DNA by the presence of somatic mutations and a number of highly sensitive methods have been developed to detect them, including the amplification-refractory mutation system (ARMS); digital polymerase chain reaction; and the beads, emulsification, amplification, and magnetics (BEAMing) system. Next-generation sequencing technologies, including tagged-amplicon deep sequencing (TAm-Seq), the Safe-Sequencing System (Safe-SeqS), and cancer personalized profiling by deep sequencing (CAPP-seq), can also be used and the race for ever more sensitive analytical tools is ongoing.1,3,4,6
Applying liquid biopsies now and in the future
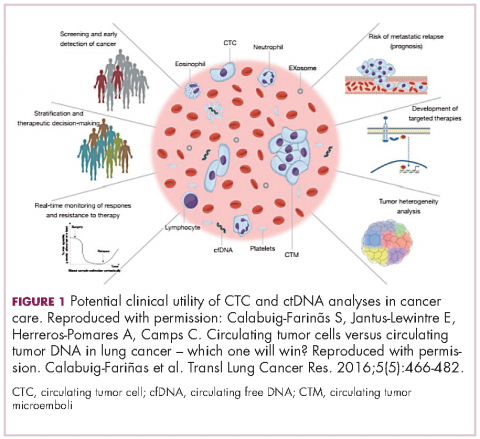
There are a plethora of potential applications for liquid biopsies3,7 (Figure 1), and probably the most exciting among them is the potential for screening for and early detection of cancer. The fact that ctDNA and CTCs have both been shown to be present from the earliest stages of disease has sparked interest in the possibility of developing simple blood tests to identify tumors before they become detectable by other methods and at a point at which they may be curable.
Given that both are present at such low levels within the circulation and are particularly sparse at earlier stages of disease, current technologies may lack the specificity and sensitivity for this application at present. However, numerous clinical trials are ongoing.
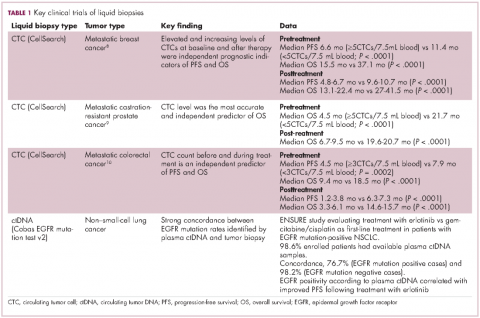
For CTCs, simple enumeration has been the most extensively investigated application to date. Numerous studies have shown that the number of CTCs in the bloodstream has prognostic significance in various different tumor types. Three such studies led to the first regulatory approval for a CTC detection system (Table 1 and Table 2).8-10
One area in which liquid biopsies could really come into their own is in providing more real-time analysis of tumors. This is something that has proven particularly challenging with tissue biopsies because repeating these invasive procedures is problematic. But the ease of repeat blood draws means that serial liquid biopsies could be performed and might offer the possibility of monitoring disease progression and evolution over the course of disease and particularly in response to treatment.
Indeed, studies have shown that in addition to baseline CTC counts, changes in CTC number during treatment are also prognostic. There was improved survival among patients whose CTC counts decreased below a threshold value during treatment and vice versa. This is also an approved use for CellSearch though at present it is not widely clinically implemented.12
Clinical utility remains elusive
The ultimate goal would be for liquid biopsies to have an impact on treatment decisions, allowing oncologists to change management strategy based on predicted sensitivity or resistance to therapy, so-called clinical utility. Thus far, clinical utility has proved elusive, though liquid biopsies using ctDNA to evaluate tumor genotype have come closest.
The Cobas EGFR Mutation Test v2 recently became the first ctDNA-based liquid biopsy to receive regulatory approval. It was approved as a companion diagnostic to identify patients with advanced non–small-cell lung cancer (NSCLC) who have specific mutations in the epidermal growth factor receptor (EGFR) gene and are therefore eligible for treatment with the EGFR inhibitor erlotinib.13
Approval was based on comparison of EGFR mutation identification rates using plasma ctDNA samples and tumor tissue samples from patients enrolled in the phase 3 ENSURE trial, which compared the efficacy of erlotinib with chemotherapy as first-line therapy in patients with advanced NSCLC. Of the 217 patients enrolled in the trial, 98.6% of patients had both tumor biopsy and plasma ctDNA samples available for testing. Concordance between the two types of biopsy in identifying patients with EGFR mutations was high and patients with EGFR positivity according to liquid biopsy results demonstrated improved progression-free survival when treated with erlotinib.14
The results of a large-scale genomic analysis of various different types of tumors using ctDNA were also recently presented at the 2016 American Society of Clinical Oncology meeting. Blood samples from more than 15,000 patients with 50 different tumor types, including advanced lung cancer (37%), breast cancer (14%), and CRC (10%), were collected and compared with either available tumor biopsy samples from the same cases (n = 398) or, in the majority of cases, with The Cancer Genome Atlas database, which uses tumor biopsies to perform genome-wide sequencing studies. Both types of biopsy revealed very similar mutation patterns when the Guardant360 next-generation sequencing test, which targets 70 genes, was applied. In particular, when EGFR, BRAF, KRAS, ALK, RET, and ROS1 mutations were identified by tumor tissue biopsy, the same mutations were reported in 94%-100% of plasma samples.15
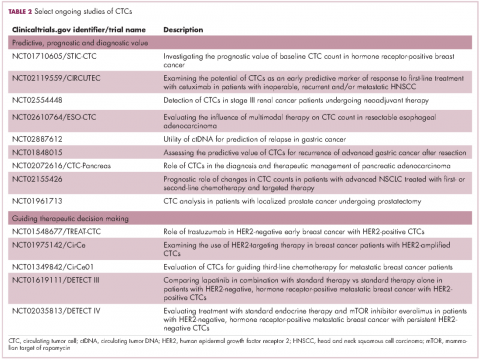
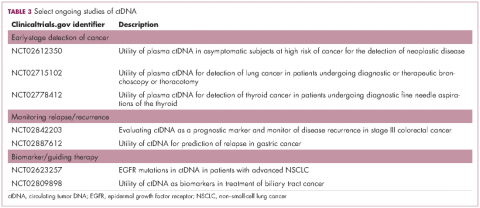
Studies of the clinical utility of ctDNA and CTCs are among ongoing clinical trials of liquid biopsies (Tables 2 and 3). The potential for using CTCs to guide treatment decisions has become particularly relevant in breast cancer in light of results showing that patients with primary tumors that are negative for human epidermal growth factor receptor 2 (HER2) amplification, an important biomarker in breast cancer, may have CTCs that are HER2-positive, in up to 30% of cases. These patients may therefore still benefit from HER2-targeted therapy.16
The DETECT studies are the first phase 3 trials in which treatment decisions are being based on the phenotypic characteristics of CTCs. DETECT III (NCT01619111) is comparing lapatinib in combination with standard therapy with standard therapy alone in patients with HER2-negative metastatic breast cancer who have HER2-positive CTCs, whereas DETECT IV (NCT02035813) is enrolling patients with HER2-negative, hormone receptor-positive metastatic breast cancer and persistent HER2-negative CTCs to receive standard endocrine therapy and the mammalian target of rapamycin inhibitor everolimus.
Other targets and sources for liquid biopsy
Another approach to liquid biopsies that is also beginning to take off is to collect tumor-derived exosomes from the bloodstream. Exosomes are tiny, fluid-filled, membrane-bound sacks that bud off from the surface of a cell to expel waste or to transport cargo from one cell to another. DNA, RNA, and protein can be extracted from tumor-derived exosomes and could also serve as molecular biomarkers relating to the cancer cells from which they came.6,7
Exosome Diagnostics is bringing the first exosome-based diagnostic tests to the market and recently teamed up with Amgen for the development of these liquid biopsies.17 In January 2016, they launched ExoDx Lung (ALK), for detection of EML4-ALK gene fusions in patients with NSCLC, using a proprietary platform for the isolation of RNA from exosomes. Data that was presented at several different conferences in 2015 demonstrated a sensitivity of 88% and specificity of 100% for this diagnostic when compared with tissue ALK status in NSCLC patients receiving a second-generation ALK inhibitor following progression on prior ALK inhibitor therapy.18
In September, they subsequently announced the launch of a test that analyses genetic information from exosomes collected from a urine sample taken from prostate cancer patients. Using a 3-gene signature, in combination with a proprietary algorithm, this diagnostic generates a score assessing a prostate cancer patient’s risk for higher grade, more aggressive disease. It is designed to complement the prostate-specific antigen score and has demonstrated accuracy in ruling out the presence of high-grade cancer before an initial biopsy in more than 1,
1. Lennon NK, Adalsteinsson VA, Gabriel SB. Technological considerations for genome-guided diagnosis and management of cancer. Genome Med. 2016;8:112.
2. MIT Technology Review website. Liquid biopsy: fast DNA-sequencing machines are leading to simple blood tests for cancer. https://www.technologyreview.com/s/534991/liquid-biopsy/. Published 2015. Accessed December 19, 2016.
3. Alix-Panabières C and Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479-491.
4. Calabuig-Farinãs S, Jantus-Lewintre E, Herreros-Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer – which one will win? Transl Lung Cancer Res. 2016;5(5):466-482.
5. Karabacak, NM, Spuhler PS, Fachin F, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9:694-710.
6. Buder A, Tomuta C, and Filipits M. The potential of liquid biopsies. Curr Opin Oncol. 2016;28:130-134.
7. Hofman P, Popper HH. Pathologists and liquid biopsies: to be or not to be? Virchows Arch. 2016;469:601-609.
8. Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumor cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406-414.
9. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302-6309.
10. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213-3221.
11. CellSearch Web site. What is the CELLSEARCH® System? https://www.cellsearchctc.com/product-systems-overview/cellsearch-system-overview. Last updated December 5th, 2016. Accessed online December 19th, 2016.
12. CellSearch Web site [advertisement]. https://www.cellsearchctc.com/clinical-applications/clinical-applications-overview. Last updated December 5, 2016. Accessed December 19, 2016.
13. US Food and Drug Administration. cobas EGFR Mutation Test v2 – P150047. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm519922.htm. Last updated September 9, 2016. Accessed December 19, 2016.
14. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883-1889.
15. Zill OA, Mortimer S, Banks KC, et al. Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol. 2016;34(suppl;abstr LBA11501).
16. Jordan NV, Bardia A, Wittner BS, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102-106.
17. Exosome Diagnostics. Exosome diagnostics enters agreement with Amgen. http://www.exosomedx.com/news-events/press-releases/exosome-diagnostics-enters-agreement-amgen. Published October 3, 2016. Accessed December 19, 2016.
18. Brinkman K, Emenegger J, Tannous B, et al. Exosomal RNA-based liquid biopsy detection of EML4-ALK in plasma from NSCLC patients [2015 World Conference on Lung Cancer, Denver, CO; abstract 2591]. http://library.iaslc.org/search-speaker?search_speaker=30493. Accessed January 6, 2017.
19. Exosome Diagnostics website. Prostate cancer. http://www.exosomedx.com/prostate-cancer-0. Last updated 2017. Accessed online December 19, 2016.
Pathologic and, increasingly, molecular analysis of tumor tissue biopsies is the gold standard in initial diagnosis of cancer, but liquid biopsies, which analyze tumor-derived material circulating in the bloodstream are gaining traction. Here, we discuss the current state of development of this complementary and potentially alternative approach to tumor analysis.
Liquid biopsy gaining traction
Biopsies enable oncologists to gather information about a potential or established tumor, including confirmation of the presence of cancerous tissue and determination of its histological characteristics, such as tumor grade and stage, as well as its molecular features, such as the presence of certain gene mutations. Ultimately, this information can be put to use in determining the most appropriate course of treatment.
The current gold standard is a tissue biopsy that typically involves an invasive procedure to permit the collection of a piece of tumor tissue. Yet, tissue biopsies are not always feasible because of the location of the tumor or the poor performance status of many patients with advanced disease. They also provide only a snapshot of the disease at the time at which they were taken and don’t necessarily reflect the genetic heterogeneity or evolution of a tumor over time.
The detection of components that are derived from the tumor circulating in the blood of cancer patients had fueled the idea of blood-based diagnostics in oncology – so-called liquid biopsies. These have rapidly gained traction in the past several decades as a less expensive (the cost of performing genomic analyses on blood samples is at least an order of magnitude less than on tissue samples), less invasive (requiring only a simple blood draw) alternative source of information about tumors.1
As researchers have refined the ability to exploit liquid biopsies, commercial interest has been piqued. More than 35 companies within the United States alone are developing liquid biopsies, and it’s easy to see why with a market projected to be in the many billions of dollars.2
Seeking out tumor clues in the blood
Liquid biopsies consist of a 10-15 mL blood sample drawn into a tube that contains an anticoagulant and it can contain several different types of tumor-associated material. Thus far, two components – circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) – have formed the cornerstone of liquid biopsies. At present, it is not clear whether these components are released randomly, as a by-product of tumor cell death or if they are released as part of a specific biologic process, such as for the colonization of metastatic sites. It reality, it may be a little of both, and active dissemination may be particularly relevant for CTCs, among which are postulated to be a population of cancer stem cells that can initiate distant metastases.3,4
The discovery of CTCs dates back to the 1860s, when cells that were morphologically identical to the tumor were identified in the blood of a patient with metastatic cancer. Their potential significance was not fully realized until a few decades ago, when they were found to exist from early on in the course of disease.3,4
CTCs, which can be either single cells or clusters of cells known as microemboli, have a short half-life in the bloodstream – less than 2 ½ hours – and are also extremely rare (1 mL of blood contains 1-10 CTCs) against a background of many millions of normal cells. Thus the detection and isolation of CTCs presents a significant challenge. More than 40 different platforms are being developed for the isolation and enrichment of CTCs. For the most part, these use a method called positive selection to pick out CTCs.1,3,4
Positive selection exploits the biological or physical properties that are specific to CTCs and absent in normal cells, for example, the presence of a specific tumor-associated antigen on their surface or differences in size, density or electric charge. The limitations of this method are that, not only do you need to know something about CTCs to begin to understand what makes them truly unique and ensure only isolation of CTCs, but their phenotype is also thought to be continually changing.1,3,4
In recent years, the focus has shifted toward technologies that use negative depletion, meaning that they target the other types of cells in the blood sample and filter those away until only the CTCs are left behind. The most advanced are devices that use microfluidic technology to sort the cells, such as the CTC-iChip system being developed by researchers at Massachusetts General Hospital in Boston.5
ctDNA consists of small fragments of nucleic acids that are not contained within a cell or associated with cell fragments and is thought to be present in 50%-90% of patients, depending on the type of cancer they have. ctDNA has a similarly short half-life in the circulation to CTCs and, like CTCs, ctDNA is present at very low levels in the bloodstream. Although levels of ctDNA have been shown to increase with increasing tumor burden, it is still often obscured by the presence of other cell-free DNA derived from non-tumor cells.
ctDNA can be distinguished from other cell-free DNA by the presence of somatic mutations and a number of highly sensitive methods have been developed to detect them, including the amplification-refractory mutation system (ARMS); digital polymerase chain reaction; and the beads, emulsification, amplification, and magnetics (BEAMing) system. Next-generation sequencing technologies, including tagged-amplicon deep sequencing (TAm-Seq), the Safe-Sequencing System (Safe-SeqS), and cancer personalized profiling by deep sequencing (CAPP-seq), can also be used and the race for ever more sensitive analytical tools is ongoing.1,3,4,6
Applying liquid biopsies now and in the future
There are a plethora of potential applications for liquid biopsies3,7 (Figure 1), and probably the most exciting among them is the potential for screening for and early detection of cancer. The fact that ctDNA and CTCs have both been shown to be present from the earliest stages of disease has sparked interest in the possibility of developing simple blood tests to identify tumors before they become detectable by other methods and at a point at which they may be curable.
Given that both are present at such low levels within the circulation and are particularly sparse at earlier stages of disease, current technologies may lack the specificity and sensitivity for this application at present. However, numerous clinical trials are ongoing.
For CTCs, simple enumeration has been the most extensively investigated application to date. Numerous studies have shown that the number of CTCs in the bloodstream has prognostic significance in various different tumor types. Three such studies led to the first regulatory approval for a CTC detection system (Table 1 and Table 2).8-10
One area in which liquid biopsies could really come into their own is in providing more real-time analysis of tumors. This is something that has proven particularly challenging with tissue biopsies because repeating these invasive procedures is problematic. But the ease of repeat blood draws means that serial liquid biopsies could be performed and might offer the possibility of monitoring disease progression and evolution over the course of disease and particularly in response to treatment.
Indeed, studies have shown that in addition to baseline CTC counts, changes in CTC number during treatment are also prognostic. There was improved survival among patients whose CTC counts decreased below a threshold value during treatment and vice versa. This is also an approved use for CellSearch though at present it is not widely clinically implemented.12
Clinical utility remains elusive
The ultimate goal would be for liquid biopsies to have an impact on treatment decisions, allowing oncologists to change management strategy based on predicted sensitivity or resistance to therapy, so-called clinical utility. Thus far, clinical utility has proved elusive, though liquid biopsies using ctDNA to evaluate tumor genotype have come closest.
The Cobas EGFR Mutation Test v2 recently became the first ctDNA-based liquid biopsy to receive regulatory approval. It was approved as a companion diagnostic to identify patients with advanced non–small-cell lung cancer (NSCLC) who have specific mutations in the epidermal growth factor receptor (EGFR) gene and are therefore eligible for treatment with the EGFR inhibitor erlotinib.13
Approval was based on comparison of EGFR mutation identification rates using plasma ctDNA samples and tumor tissue samples from patients enrolled in the phase 3 ENSURE trial, which compared the efficacy of erlotinib with chemotherapy as first-line therapy in patients with advanced NSCLC. Of the 217 patients enrolled in the trial, 98.6% of patients had both tumor biopsy and plasma ctDNA samples available for testing. Concordance between the two types of biopsy in identifying patients with EGFR mutations was high and patients with EGFR positivity according to liquid biopsy results demonstrated improved progression-free survival when treated with erlotinib.14
The results of a large-scale genomic analysis of various different types of tumors using ctDNA were also recently presented at the 2016 American Society of Clinical Oncology meeting. Blood samples from more than 15,000 patients with 50 different tumor types, including advanced lung cancer (37%), breast cancer (14%), and CRC (10%), were collected and compared with either available tumor biopsy samples from the same cases (n = 398) or, in the majority of cases, with The Cancer Genome Atlas database, which uses tumor biopsies to perform genome-wide sequencing studies. Both types of biopsy revealed very similar mutation patterns when the Guardant360 next-generation sequencing test, which targets 70 genes, was applied. In particular, when EGFR, BRAF, KRAS, ALK, RET, and ROS1 mutations were identified by tumor tissue biopsy, the same mutations were reported in 94%-100% of plasma samples.15
Studies of the clinical utility of ctDNA and CTCs are among ongoing clinical trials of liquid biopsies (Tables 2 and 3). The potential for using CTCs to guide treatment decisions has become particularly relevant in breast cancer in light of results showing that patients with primary tumors that are negative for human epidermal growth factor receptor 2 (HER2) amplification, an important biomarker in breast cancer, may have CTCs that are HER2-positive, in up to 30% of cases. These patients may therefore still benefit from HER2-targeted therapy.16
The DETECT studies are the first phase 3 trials in which treatment decisions are being based on the phenotypic characteristics of CTCs. DETECT III (NCT01619111) is comparing lapatinib in combination with standard therapy with standard therapy alone in patients with HER2-negative metastatic breast cancer who have HER2-positive CTCs, whereas DETECT IV (NCT02035813) is enrolling patients with HER2-negative, hormone receptor-positive metastatic breast cancer and persistent HER2-negative CTCs to receive standard endocrine therapy and the mammalian target of rapamycin inhibitor everolimus.
Other targets and sources for liquid biopsy
Another approach to liquid biopsies that is also beginning to take off is to collect tumor-derived exosomes from the bloodstream. Exosomes are tiny, fluid-filled, membrane-bound sacks that bud off from the surface of a cell to expel waste or to transport cargo from one cell to another. DNA, RNA, and protein can be extracted from tumor-derived exosomes and could also serve as molecular biomarkers relating to the cancer cells from which they came.6,7
Exosome Diagnostics is bringing the first exosome-based diagnostic tests to the market and recently teamed up with Amgen for the development of these liquid biopsies.17 In January 2016, they launched ExoDx Lung (ALK), for detection of EML4-ALK gene fusions in patients with NSCLC, using a proprietary platform for the isolation of RNA from exosomes. Data that was presented at several different conferences in 2015 demonstrated a sensitivity of 88% and specificity of 100% for this diagnostic when compared with tissue ALK status in NSCLC patients receiving a second-generation ALK inhibitor following progression on prior ALK inhibitor therapy.18
In September, they subsequently announced the launch of a test that analyses genetic information from exosomes collected from a urine sample taken from prostate cancer patients. Using a 3-gene signature, in combination with a proprietary algorithm, this diagnostic generates a score assessing a prostate cancer patient’s risk for higher grade, more aggressive disease. It is designed to complement the prostate-specific antigen score and has demonstrated accuracy in ruling out the presence of high-grade cancer before an initial biopsy in more than 1,
Pathologic and, increasingly, molecular analysis of tumor tissue biopsies is the gold standard in initial diagnosis of cancer, but liquid biopsies, which analyze tumor-derived material circulating in the bloodstream are gaining traction. Here, we discuss the current state of development of this complementary and potentially alternative approach to tumor analysis.
Liquid biopsy gaining traction
Biopsies enable oncologists to gather information about a potential or established tumor, including confirmation of the presence of cancerous tissue and determination of its histological characteristics, such as tumor grade and stage, as well as its molecular features, such as the presence of certain gene mutations. Ultimately, this information can be put to use in determining the most appropriate course of treatment.
The current gold standard is a tissue biopsy that typically involves an invasive procedure to permit the collection of a piece of tumor tissue. Yet, tissue biopsies are not always feasible because of the location of the tumor or the poor performance status of many patients with advanced disease. They also provide only a snapshot of the disease at the time at which they were taken and don’t necessarily reflect the genetic heterogeneity or evolution of a tumor over time.
The detection of components that are derived from the tumor circulating in the blood of cancer patients had fueled the idea of blood-based diagnostics in oncology – so-called liquid biopsies. These have rapidly gained traction in the past several decades as a less expensive (the cost of performing genomic analyses on blood samples is at least an order of magnitude less than on tissue samples), less invasive (requiring only a simple blood draw) alternative source of information about tumors.1
As researchers have refined the ability to exploit liquid biopsies, commercial interest has been piqued. More than 35 companies within the United States alone are developing liquid biopsies, and it’s easy to see why with a market projected to be in the many billions of dollars.2
Seeking out tumor clues in the blood
Liquid biopsies consist of a 10-15 mL blood sample drawn into a tube that contains an anticoagulant and it can contain several different types of tumor-associated material. Thus far, two components – circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) – have formed the cornerstone of liquid biopsies. At present, it is not clear whether these components are released randomly, as a by-product of tumor cell death or if they are released as part of a specific biologic process, such as for the colonization of metastatic sites. It reality, it may be a little of both, and active dissemination may be particularly relevant for CTCs, among which are postulated to be a population of cancer stem cells that can initiate distant metastases.3,4
The discovery of CTCs dates back to the 1860s, when cells that were morphologically identical to the tumor were identified in the blood of a patient with metastatic cancer. Their potential significance was not fully realized until a few decades ago, when they were found to exist from early on in the course of disease.3,4
CTCs, which can be either single cells or clusters of cells known as microemboli, have a short half-life in the bloodstream – less than 2 ½ hours – and are also extremely rare (1 mL of blood contains 1-10 CTCs) against a background of many millions of normal cells. Thus the detection and isolation of CTCs presents a significant challenge. More than 40 different platforms are being developed for the isolation and enrichment of CTCs. For the most part, these use a method called positive selection to pick out CTCs.1,3,4
Positive selection exploits the biological or physical properties that are specific to CTCs and absent in normal cells, for example, the presence of a specific tumor-associated antigen on their surface or differences in size, density or electric charge. The limitations of this method are that, not only do you need to know something about CTCs to begin to understand what makes them truly unique and ensure only isolation of CTCs, but their phenotype is also thought to be continually changing.1,3,4
In recent years, the focus has shifted toward technologies that use negative depletion, meaning that they target the other types of cells in the blood sample and filter those away until only the CTCs are left behind. The most advanced are devices that use microfluidic technology to sort the cells, such as the CTC-iChip system being developed by researchers at Massachusetts General Hospital in Boston.5
ctDNA consists of small fragments of nucleic acids that are not contained within a cell or associated with cell fragments and is thought to be present in 50%-90% of patients, depending on the type of cancer they have. ctDNA has a similarly short half-life in the circulation to CTCs and, like CTCs, ctDNA is present at very low levels in the bloodstream. Although levels of ctDNA have been shown to increase with increasing tumor burden, it is still often obscured by the presence of other cell-free DNA derived from non-tumor cells.
ctDNA can be distinguished from other cell-free DNA by the presence of somatic mutations and a number of highly sensitive methods have been developed to detect them, including the amplification-refractory mutation system (ARMS); digital polymerase chain reaction; and the beads, emulsification, amplification, and magnetics (BEAMing) system. Next-generation sequencing technologies, including tagged-amplicon deep sequencing (TAm-Seq), the Safe-Sequencing System (Safe-SeqS), and cancer personalized profiling by deep sequencing (CAPP-seq), can also be used and the race for ever more sensitive analytical tools is ongoing.1,3,4,6
Applying liquid biopsies now and in the future
There are a plethora of potential applications for liquid biopsies3,7 (Figure 1), and probably the most exciting among them is the potential for screening for and early detection of cancer. The fact that ctDNA and CTCs have both been shown to be present from the earliest stages of disease has sparked interest in the possibility of developing simple blood tests to identify tumors before they become detectable by other methods and at a point at which they may be curable.
Given that both are present at such low levels within the circulation and are particularly sparse at earlier stages of disease, current technologies may lack the specificity and sensitivity for this application at present. However, numerous clinical trials are ongoing.
For CTCs, simple enumeration has been the most extensively investigated application to date. Numerous studies have shown that the number of CTCs in the bloodstream has prognostic significance in various different tumor types. Three such studies led to the first regulatory approval for a CTC detection system (Table 1 and Table 2).8-10
One area in which liquid biopsies could really come into their own is in providing more real-time analysis of tumors. This is something that has proven particularly challenging with tissue biopsies because repeating these invasive procedures is problematic. But the ease of repeat blood draws means that serial liquid biopsies could be performed and might offer the possibility of monitoring disease progression and evolution over the course of disease and particularly in response to treatment.
Indeed, studies have shown that in addition to baseline CTC counts, changes in CTC number during treatment are also prognostic. There was improved survival among patients whose CTC counts decreased below a threshold value during treatment and vice versa. This is also an approved use for CellSearch though at present it is not widely clinically implemented.12
Clinical utility remains elusive
The ultimate goal would be for liquid biopsies to have an impact on treatment decisions, allowing oncologists to change management strategy based on predicted sensitivity or resistance to therapy, so-called clinical utility. Thus far, clinical utility has proved elusive, though liquid biopsies using ctDNA to evaluate tumor genotype have come closest.
The Cobas EGFR Mutation Test v2 recently became the first ctDNA-based liquid biopsy to receive regulatory approval. It was approved as a companion diagnostic to identify patients with advanced non–small-cell lung cancer (NSCLC) who have specific mutations in the epidermal growth factor receptor (EGFR) gene and are therefore eligible for treatment with the EGFR inhibitor erlotinib.13
Approval was based on comparison of EGFR mutation identification rates using plasma ctDNA samples and tumor tissue samples from patients enrolled in the phase 3 ENSURE trial, which compared the efficacy of erlotinib with chemotherapy as first-line therapy in patients with advanced NSCLC. Of the 217 patients enrolled in the trial, 98.6% of patients had both tumor biopsy and plasma ctDNA samples available for testing. Concordance between the two types of biopsy in identifying patients with EGFR mutations was high and patients with EGFR positivity according to liquid biopsy results demonstrated improved progression-free survival when treated with erlotinib.14
The results of a large-scale genomic analysis of various different types of tumors using ctDNA were also recently presented at the 2016 American Society of Clinical Oncology meeting. Blood samples from more than 15,000 patients with 50 different tumor types, including advanced lung cancer (37%), breast cancer (14%), and CRC (10%), were collected and compared with either available tumor biopsy samples from the same cases (n = 398) or, in the majority of cases, with The Cancer Genome Atlas database, which uses tumor biopsies to perform genome-wide sequencing studies. Both types of biopsy revealed very similar mutation patterns when the Guardant360 next-generation sequencing test, which targets 70 genes, was applied. In particular, when EGFR, BRAF, KRAS, ALK, RET, and ROS1 mutations were identified by tumor tissue biopsy, the same mutations were reported in 94%-100% of plasma samples.15
Studies of the clinical utility of ctDNA and CTCs are among ongoing clinical trials of liquid biopsies (Tables 2 and 3). The potential for using CTCs to guide treatment decisions has become particularly relevant in breast cancer in light of results showing that patients with primary tumors that are negative for human epidermal growth factor receptor 2 (HER2) amplification, an important biomarker in breast cancer, may have CTCs that are HER2-positive, in up to 30% of cases. These patients may therefore still benefit from HER2-targeted therapy.16
The DETECT studies are the first phase 3 trials in which treatment decisions are being based on the phenotypic characteristics of CTCs. DETECT III (NCT01619111) is comparing lapatinib in combination with standard therapy with standard therapy alone in patients with HER2-negative metastatic breast cancer who have HER2-positive CTCs, whereas DETECT IV (NCT02035813) is enrolling patients with HER2-negative, hormone receptor-positive metastatic breast cancer and persistent HER2-negative CTCs to receive standard endocrine therapy and the mammalian target of rapamycin inhibitor everolimus.
Other targets and sources for liquid biopsy
Another approach to liquid biopsies that is also beginning to take off is to collect tumor-derived exosomes from the bloodstream. Exosomes are tiny, fluid-filled, membrane-bound sacks that bud off from the surface of a cell to expel waste or to transport cargo from one cell to another. DNA, RNA, and protein can be extracted from tumor-derived exosomes and could also serve as molecular biomarkers relating to the cancer cells from which they came.6,7
Exosome Diagnostics is bringing the first exosome-based diagnostic tests to the market and recently teamed up with Amgen for the development of these liquid biopsies.17 In January 2016, they launched ExoDx Lung (ALK), for detection of EML4-ALK gene fusions in patients with NSCLC, using a proprietary platform for the isolation of RNA from exosomes. Data that was presented at several different conferences in 2015 demonstrated a sensitivity of 88% and specificity of 100% for this diagnostic when compared with tissue ALK status in NSCLC patients receiving a second-generation ALK inhibitor following progression on prior ALK inhibitor therapy.18
In September, they subsequently announced the launch of a test that analyses genetic information from exosomes collected from a urine sample taken from prostate cancer patients. Using a 3-gene signature, in combination with a proprietary algorithm, this diagnostic generates a score assessing a prostate cancer patient’s risk for higher grade, more aggressive disease. It is designed to complement the prostate-specific antigen score and has demonstrated accuracy in ruling out the presence of high-grade cancer before an initial biopsy in more than 1,
1. Lennon NK, Adalsteinsson VA, Gabriel SB. Technological considerations for genome-guided diagnosis and management of cancer. Genome Med. 2016;8:112.
2. MIT Technology Review website. Liquid biopsy: fast DNA-sequencing machines are leading to simple blood tests for cancer. https://www.technologyreview.com/s/534991/liquid-biopsy/. Published 2015. Accessed December 19, 2016.
3. Alix-Panabières C and Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479-491.
4. Calabuig-Farinãs S, Jantus-Lewintre E, Herreros-Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer – which one will win? Transl Lung Cancer Res. 2016;5(5):466-482.
5. Karabacak, NM, Spuhler PS, Fachin F, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9:694-710.
6. Buder A, Tomuta C, and Filipits M. The potential of liquid biopsies. Curr Opin Oncol. 2016;28:130-134.
7. Hofman P, Popper HH. Pathologists and liquid biopsies: to be or not to be? Virchows Arch. 2016;469:601-609.
8. Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumor cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406-414.
9. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302-6309.
10. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213-3221.
11. CellSearch Web site. What is the CELLSEARCH® System? https://www.cellsearchctc.com/product-systems-overview/cellsearch-system-overview. Last updated December 5th, 2016. Accessed online December 19th, 2016.
12. CellSearch Web site [advertisement]. https://www.cellsearchctc.com/clinical-applications/clinical-applications-overview. Last updated December 5, 2016. Accessed December 19, 2016.
13. US Food and Drug Administration. cobas EGFR Mutation Test v2 – P150047. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm519922.htm. Last updated September 9, 2016. Accessed December 19, 2016.
14. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883-1889.
15. Zill OA, Mortimer S, Banks KC, et al. Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol. 2016;34(suppl;abstr LBA11501).
16. Jordan NV, Bardia A, Wittner BS, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102-106.
17. Exosome Diagnostics. Exosome diagnostics enters agreement with Amgen. http://www.exosomedx.com/news-events/press-releases/exosome-diagnostics-enters-agreement-amgen. Published October 3, 2016. Accessed December 19, 2016.
18. Brinkman K, Emenegger J, Tannous B, et al. Exosomal RNA-based liquid biopsy detection of EML4-ALK in plasma from NSCLC patients [2015 World Conference on Lung Cancer, Denver, CO; abstract 2591]. http://library.iaslc.org/search-speaker?search_speaker=30493. Accessed January 6, 2017.
19. Exosome Diagnostics website. Prostate cancer. http://www.exosomedx.com/prostate-cancer-0. Last updated 2017. Accessed online December 19, 2016.
1. Lennon NK, Adalsteinsson VA, Gabriel SB. Technological considerations for genome-guided diagnosis and management of cancer. Genome Med. 2016;8:112.
2. MIT Technology Review website. Liquid biopsy: fast DNA-sequencing machines are leading to simple blood tests for cancer. https://www.technologyreview.com/s/534991/liquid-biopsy/. Published 2015. Accessed December 19, 2016.
3. Alix-Panabières C and Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479-491.
4. Calabuig-Farinãs S, Jantus-Lewintre E, Herreros-Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer – which one will win? Transl Lung Cancer Res. 2016;5(5):466-482.
5. Karabacak, NM, Spuhler PS, Fachin F, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9:694-710.
6. Buder A, Tomuta C, and Filipits M. The potential of liquid biopsies. Curr Opin Oncol. 2016;28:130-134.
7. Hofman P, Popper HH. Pathologists and liquid biopsies: to be or not to be? Virchows Arch. 2016;469:601-609.
8. Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumor cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406-414.
9. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302-6309.
10. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213-3221.
11. CellSearch Web site. What is the CELLSEARCH® System? https://www.cellsearchctc.com/product-systems-overview/cellsearch-system-overview. Last updated December 5th, 2016. Accessed online December 19th, 2016.
12. CellSearch Web site [advertisement]. https://www.cellsearchctc.com/clinical-applications/clinical-applications-overview. Last updated December 5, 2016. Accessed December 19, 2016.
13. US Food and Drug Administration. cobas EGFR Mutation Test v2 – P150047. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm519922.htm. Last updated September 9, 2016. Accessed December 19, 2016.
14. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883-1889.
15. Zill OA, Mortimer S, Banks KC, et al. Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol. 2016;34(suppl;abstr LBA11501).
16. Jordan NV, Bardia A, Wittner BS, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102-106.
17. Exosome Diagnostics. Exosome diagnostics enters agreement with Amgen. http://www.exosomedx.com/news-events/press-releases/exosome-diagnostics-enters-agreement-amgen. Published October 3, 2016. Accessed December 19, 2016.
18. Brinkman K, Emenegger J, Tannous B, et al. Exosomal RNA-based liquid biopsy detection of EML4-ALK in plasma from NSCLC patients [2015 World Conference on Lung Cancer, Denver, CO; abstract 2591]. http://library.iaslc.org/search-speaker?search_speaker=30493. Accessed January 6, 2017.
19. Exosome Diagnostics website. Prostate cancer. http://www.exosomedx.com/prostate-cancer-0. Last updated 2017. Accessed online December 19, 2016.
Onetime, Nondrug Treatment May Be Better for Some MS Patients
High-dose immunosuppressive therapy and autologous hematopoietic cell transplant (HDIT/HCT) has had “highly promising” results for patients with relapsing-remitting multiple sclerosis (MS), according to researchers from the HALT-MS trial. In the 5-year study, 69% of 24 participants survived without progression of disability, relapse, or new brain lesions, despite not taking MS medications.
Findings published at the 3-year mark were encouraging. The event-free survival rate was 78%. The extended findings suggest that “onetime treatment with HDIT/HCT may be substantially more effective than long-term treatment with the best available medications” for these patients, said NIAID Director Anthony Fauci, MD.
The treatment “resets” the immune system, the researchers say. First, doctors collect the patient’s blood-forming stem cells, then give the patient chemotherapy to deplete the immune system. Finally, the doctors return the patient’s stem cells to rebuild the immune system.
Adverse events were consistent with those routinely observed after HDIT/HCT. Adverse effects recorded at 4 and 5 years were not related to the transplant and were not considered severe. Three patients died, but their deaths were not related to the study treatments.
Five years later, most trial participants remained in remission and stabilized, and some showed improvements, such as recovering mobility.
High-dose immunosuppressive therapy and autologous hematopoietic cell transplant (HDIT/HCT) has had “highly promising” results for patients with relapsing-remitting multiple sclerosis (MS), according to researchers from the HALT-MS trial. In the 5-year study, 69% of 24 participants survived without progression of disability, relapse, or new brain lesions, despite not taking MS medications.
Findings published at the 3-year mark were encouraging. The event-free survival rate was 78%. The extended findings suggest that “onetime treatment with HDIT/HCT may be substantially more effective than long-term treatment with the best available medications” for these patients, said NIAID Director Anthony Fauci, MD.
The treatment “resets” the immune system, the researchers say. First, doctors collect the patient’s blood-forming stem cells, then give the patient chemotherapy to deplete the immune system. Finally, the doctors return the patient’s stem cells to rebuild the immune system.
Adverse events were consistent with those routinely observed after HDIT/HCT. Adverse effects recorded at 4 and 5 years were not related to the transplant and were not considered severe. Three patients died, but their deaths were not related to the study treatments.
Five years later, most trial participants remained in remission and stabilized, and some showed improvements, such as recovering mobility.
High-dose immunosuppressive therapy and autologous hematopoietic cell transplant (HDIT/HCT) has had “highly promising” results for patients with relapsing-remitting multiple sclerosis (MS), according to researchers from the HALT-MS trial. In the 5-year study, 69% of 24 participants survived without progression of disability, relapse, or new brain lesions, despite not taking MS medications.
Findings published at the 3-year mark were encouraging. The event-free survival rate was 78%. The extended findings suggest that “onetime treatment with HDIT/HCT may be substantially more effective than long-term treatment with the best available medications” for these patients, said NIAID Director Anthony Fauci, MD.
The treatment “resets” the immune system, the researchers say. First, doctors collect the patient’s blood-forming stem cells, then give the patient chemotherapy to deplete the immune system. Finally, the doctors return the patient’s stem cells to rebuild the immune system.
Adverse events were consistent with those routinely observed after HDIT/HCT. Adverse effects recorded at 4 and 5 years were not related to the transplant and were not considered severe. Three patients died, but their deaths were not related to the study treatments.
Five years later, most trial participants remained in remission and stabilized, and some showed improvements, such as recovering mobility.
Artificial Pancreas Moves Closer to Real-Life Option
Roughly 25% of veterans have diabetes mellitus (DM) as opposed to 9% of the general public. A small percentage of veterans have type 1 DM, which according to research, can be caused by both physical and mental trauma that affects the pancreas.
“Managing type 1 diabetes currently requires a constant juggling act between checking bloodglucose levels frequently and delivering just the right amount of insulin while taking into account meals, physical activity, and other aspects of daily life, where a missed or wrong delivery could lead to potential complications,” said Dr. Andrew Bremer, of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). But that may change soon as we draw near to a functional “artificial pancreas,” a fully automated system that will sense rising glucose levels and adjust insulin automatically.
The FDA approved a hybrid model of an artificial pancreas in 2016, which still required users to adjust insulin intake. Now, 4 separate projects are designed to be the “potential last steps” toward requesting regulatory approval for permanent use of a fully automated system, according to NIDDK. The research studies beginning this year will look at safety, efficacy, user-friendliness, physical and emotional health of participants, and cost. The participants will live at home and monitor themselves with remote monitoring by study staff.
“Nearly 100 years since the discovery of insulin,” said NIDDK Director Dr. Griffin P. Rodgers, “a successful artificial pancreas would mark another huge step toward better health for people with type 1 diabetes.”
Roughly 25% of veterans have diabetes mellitus (DM) as opposed to 9% of the general public. A small percentage of veterans have type 1 DM, which according to research, can be caused by both physical and mental trauma that affects the pancreas.
“Managing type 1 diabetes currently requires a constant juggling act between checking bloodglucose levels frequently and delivering just the right amount of insulin while taking into account meals, physical activity, and other aspects of daily life, where a missed or wrong delivery could lead to potential complications,” said Dr. Andrew Bremer, of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). But that may change soon as we draw near to a functional “artificial pancreas,” a fully automated system that will sense rising glucose levels and adjust insulin automatically.
The FDA approved a hybrid model of an artificial pancreas in 2016, which still required users to adjust insulin intake. Now, 4 separate projects are designed to be the “potential last steps” toward requesting regulatory approval for permanent use of a fully automated system, according to NIDDK. The research studies beginning this year will look at safety, efficacy, user-friendliness, physical and emotional health of participants, and cost. The participants will live at home and monitor themselves with remote monitoring by study staff.
“Nearly 100 years since the discovery of insulin,” said NIDDK Director Dr. Griffin P. Rodgers, “a successful artificial pancreas would mark another huge step toward better health for people with type 1 diabetes.”
Roughly 25% of veterans have diabetes mellitus (DM) as opposed to 9% of the general public. A small percentage of veterans have type 1 DM, which according to research, can be caused by both physical and mental trauma that affects the pancreas.
“Managing type 1 diabetes currently requires a constant juggling act between checking bloodglucose levels frequently and delivering just the right amount of insulin while taking into account meals, physical activity, and other aspects of daily life, where a missed or wrong delivery could lead to potential complications,” said Dr. Andrew Bremer, of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). But that may change soon as we draw near to a functional “artificial pancreas,” a fully automated system that will sense rising glucose levels and adjust insulin automatically.
The FDA approved a hybrid model of an artificial pancreas in 2016, which still required users to adjust insulin intake. Now, 4 separate projects are designed to be the “potential last steps” toward requesting regulatory approval for permanent use of a fully automated system, according to NIDDK. The research studies beginning this year will look at safety, efficacy, user-friendliness, physical and emotional health of participants, and cost. The participants will live at home and monitor themselves with remote monitoring by study staff.
“Nearly 100 years since the discovery of insulin,” said NIDDK Director Dr. Griffin P. Rodgers, “a successful artificial pancreas would mark another huge step toward better health for people with type 1 diabetes.”
Mindfulness in the Workplace
Mindfulness practices, such as meditation, yoga, tai chi, and qigong, are on the rise in the workplace, as more studies show how they can improve health and reduce the costs of stress.
Researchers who analyzed data on 85,004 respondents to the National Health Interview Survey say that their findings are “encouraging”; about 1 in 7 workers report engaging in some form of mindfulness-based activity. From 2002 to 2012, the number of yoga practitioners nearly doubled from 6% to 11%. From 2002 to 2007, the number of people who began meditating increased from 8% to 9.9%.
Related: Mindfulness to Reduce Stress
The rise in activity was seen across different groups of workers. In 2002, 2.2% of farm workers reported engaging in at least 1 of the practices studied, as did 18.2% of white-collar workers in 2007. Mindfulness found a foothold among 9% to 12% of the unemployed as well.
Still the trend is mostly seen among white-collar workers, attributable mainly to differences in household income and education level, the researchers say. After they controlled for those 2 factors, blue-collar workers were still less likely to engage in meditation or yoga, and farm workers were less likely to engage in any of the 4 practices.
Related: Preventing Burnout With Cognitive Empathy
But the lack of engagement among blue-collar and farm workers can’t be explained by sociodemographic factors alone, the researchers say. White-collar workers may have more time, access, and opportunity to practice mindfulness and might have different beliefs about the value of these practices.
Lack of engagement could be ameliorated in part by developing interventions to target those occupational groups, the researchers suggest. They found no studies that focused on blue-collar or farm workers—the low prevalence of mindfulness practices in those groups indicates a “pressing need” for interventions, even though those workplace settings may present “unique implementation challenges.”
Mindfulness practices, such as meditation, yoga, tai chi, and qigong, are on the rise in the workplace, as more studies show how they can improve health and reduce the costs of stress.
Researchers who analyzed data on 85,004 respondents to the National Health Interview Survey say that their findings are “encouraging”; about 1 in 7 workers report engaging in some form of mindfulness-based activity. From 2002 to 2012, the number of yoga practitioners nearly doubled from 6% to 11%. From 2002 to 2007, the number of people who began meditating increased from 8% to 9.9%.
Related: Mindfulness to Reduce Stress
The rise in activity was seen across different groups of workers. In 2002, 2.2% of farm workers reported engaging in at least 1 of the practices studied, as did 18.2% of white-collar workers in 2007. Mindfulness found a foothold among 9% to 12% of the unemployed as well.
Still the trend is mostly seen among white-collar workers, attributable mainly to differences in household income and education level, the researchers say. After they controlled for those 2 factors, blue-collar workers were still less likely to engage in meditation or yoga, and farm workers were less likely to engage in any of the 4 practices.
Related: Preventing Burnout With Cognitive Empathy
But the lack of engagement among blue-collar and farm workers can’t be explained by sociodemographic factors alone, the researchers say. White-collar workers may have more time, access, and opportunity to practice mindfulness and might have different beliefs about the value of these practices.
Lack of engagement could be ameliorated in part by developing interventions to target those occupational groups, the researchers suggest. They found no studies that focused on blue-collar or farm workers—the low prevalence of mindfulness practices in those groups indicates a “pressing need” for interventions, even though those workplace settings may present “unique implementation challenges.”
Mindfulness practices, such as meditation, yoga, tai chi, and qigong, are on the rise in the workplace, as more studies show how they can improve health and reduce the costs of stress.
Researchers who analyzed data on 85,004 respondents to the National Health Interview Survey say that their findings are “encouraging”; about 1 in 7 workers report engaging in some form of mindfulness-based activity. From 2002 to 2012, the number of yoga practitioners nearly doubled from 6% to 11%. From 2002 to 2007, the number of people who began meditating increased from 8% to 9.9%.
Related: Mindfulness to Reduce Stress
The rise in activity was seen across different groups of workers. In 2002, 2.2% of farm workers reported engaging in at least 1 of the practices studied, as did 18.2% of white-collar workers in 2007. Mindfulness found a foothold among 9% to 12% of the unemployed as well.
Still the trend is mostly seen among white-collar workers, attributable mainly to differences in household income and education level, the researchers say. After they controlled for those 2 factors, blue-collar workers were still less likely to engage in meditation or yoga, and farm workers were less likely to engage in any of the 4 practices.
Related: Preventing Burnout With Cognitive Empathy
But the lack of engagement among blue-collar and farm workers can’t be explained by sociodemographic factors alone, the researchers say. White-collar workers may have more time, access, and opportunity to practice mindfulness and might have different beliefs about the value of these practices.
Lack of engagement could be ameliorated in part by developing interventions to target those occupational groups, the researchers suggest. They found no studies that focused on blue-collar or farm workers—the low prevalence of mindfulness practices in those groups indicates a “pressing need” for interventions, even though those workplace settings may present “unique implementation challenges.”
Ginseng Derivatives May Protect Against Flu
Ginsenosides are pharmacologically active components of ginseng, which often is used to relieve coughs and colds. They also have been found to have antineoplastic, antioxidant, antimicrobial, and antifungal properties; other studies suggest neuroprotective properties as well. Ginsenosides may act against coxsackievirus B3, enterovirus 71, human rhinovirus 3, and hemagglutinating virus of Japan (HVJ) infection. But do they have an antiviral effect on influenza?
Related: A New Kind of Flu Drug
Researchers from University Health Network & Shantou University Medical College and Guangdong Provincial Key Laboratory of Infectious Diseases and Molecular Immunopathology, both in China, and University of Toronto in Canada conducted a study in mice of the anti-influenza properties of ginseng and ginseng-derived compounds both in vitro and in vivo. They found that ginsenosides exerted “strong antiviral activity” to 2009 pandemic H1N1 virus. Ginsenoside protected the animals from infection and lowered viral titers in their lungs.
Sugars were the key to the effectiveness of the ginsenosides, which are composed of a steroid skeleton with various sugar groups attached. The researchers note that previous studies have shown that ginsenosides’ anticancer activity and antioxidant activity are related to the type and position of sugar moieties.
Related: How Common is Flu Without Fever?
The pilot experiment did not have negative or toxic effects on the animals or in cell proliferation in vitro, thus “defining the nontoxic nature and therapeutic value of these compounds,” the researchers say. They also point out that in phase 2 randomized clinical trials in children, oral consumption of ginseng extract as an alternative influenza treatment did not result in severe adverse effects. They suggest that their findings could spur other research into a novel antiviral drug for influenza.
Source:
Dong W, Farooqui A, Leon AJ, Kelvin DJ. PloS One. 2017;12(2):e0171936.
doi: 10.1371/journal.pone.0171936.
Ginsenosides are pharmacologically active components of ginseng, which often is used to relieve coughs and colds. They also have been found to have antineoplastic, antioxidant, antimicrobial, and antifungal properties; other studies suggest neuroprotective properties as well. Ginsenosides may act against coxsackievirus B3, enterovirus 71, human rhinovirus 3, and hemagglutinating virus of Japan (HVJ) infection. But do they have an antiviral effect on influenza?
Related: A New Kind of Flu Drug
Researchers from University Health Network & Shantou University Medical College and Guangdong Provincial Key Laboratory of Infectious Diseases and Molecular Immunopathology, both in China, and University of Toronto in Canada conducted a study in mice of the anti-influenza properties of ginseng and ginseng-derived compounds both in vitro and in vivo. They found that ginsenosides exerted “strong antiviral activity” to 2009 pandemic H1N1 virus. Ginsenoside protected the animals from infection and lowered viral titers in their lungs.
Sugars were the key to the effectiveness of the ginsenosides, which are composed of a steroid skeleton with various sugar groups attached. The researchers note that previous studies have shown that ginsenosides’ anticancer activity and antioxidant activity are related to the type and position of sugar moieties.
Related: How Common is Flu Without Fever?
The pilot experiment did not have negative or toxic effects on the animals or in cell proliferation in vitro, thus “defining the nontoxic nature and therapeutic value of these compounds,” the researchers say. They also point out that in phase 2 randomized clinical trials in children, oral consumption of ginseng extract as an alternative influenza treatment did not result in severe adverse effects. They suggest that their findings could spur other research into a novel antiviral drug for influenza.
Source:
Dong W, Farooqui A, Leon AJ, Kelvin DJ. PloS One. 2017;12(2):e0171936.
doi: 10.1371/journal.pone.0171936.
Ginsenosides are pharmacologically active components of ginseng, which often is used to relieve coughs and colds. They also have been found to have antineoplastic, antioxidant, antimicrobial, and antifungal properties; other studies suggest neuroprotective properties as well. Ginsenosides may act against coxsackievirus B3, enterovirus 71, human rhinovirus 3, and hemagglutinating virus of Japan (HVJ) infection. But do they have an antiviral effect on influenza?
Related: A New Kind of Flu Drug
Researchers from University Health Network & Shantou University Medical College and Guangdong Provincial Key Laboratory of Infectious Diseases and Molecular Immunopathology, both in China, and University of Toronto in Canada conducted a study in mice of the anti-influenza properties of ginseng and ginseng-derived compounds both in vitro and in vivo. They found that ginsenosides exerted “strong antiviral activity” to 2009 pandemic H1N1 virus. Ginsenoside protected the animals from infection and lowered viral titers in their lungs.
Sugars were the key to the effectiveness of the ginsenosides, which are composed of a steroid skeleton with various sugar groups attached. The researchers note that previous studies have shown that ginsenosides’ anticancer activity and antioxidant activity are related to the type and position of sugar moieties.
Related: How Common is Flu Without Fever?
The pilot experiment did not have negative or toxic effects on the animals or in cell proliferation in vitro, thus “defining the nontoxic nature and therapeutic value of these compounds,” the researchers say. They also point out that in phase 2 randomized clinical trials in children, oral consumption of ginseng extract as an alternative influenza treatment did not result in severe adverse effects. They suggest that their findings could spur other research into a novel antiviral drug for influenza.
Source:
Dong W, Farooqui A, Leon AJ, Kelvin DJ. PloS One. 2017;12(2):e0171936.
doi: 10.1371/journal.pone.0171936.
Novel Treatment Shows Promise for Acute Lymphoblastic Leukemia
Thanks to gene-editing treatment, 2 babies who had leukemia have a longer lease on life. Researchers from University College London, Great Ormond Street Hospital National Health Service Trust, King’s College, and Sheffield Children’s Hospital, in the United Kingdom used genetically engineered white blood cells from healthy individuals to target the cancer cells.
Related: New Treatments for Chronic Lymphocytic Leukemia
One infant was 11 months old, and the second was 16 months old. Both had refractory relapsed B-cell acute lymphoblastic leukemia and had undergone multidrug treatments. Although the potential of the method has been demonstrated in autologous and human-leukocyte-antigen–matched allogeneic settings, the researchers say, “the infrastructure and expertise required to produce personalized cell products present challenges, and low T cell counts in heavily treated individuals may preclude autologous approaches.”
The treatment involves lymphodepleting chemotherapy and anti-CD52 serotherapy, followed by a single-dose infusion of universal CAR19 cells (autologous T cells engineered to express chimeric antigen receptor against the B-cell antigen CD19). This “bridge-to-transplantation strategy” demonstrates the therapeutic potential of gene-editing technology, the researchers say.
Related: Six Open Clinical Trials That Are Expanding Our Understanding of Immunotherapies
Just 28 days after the treatment, molecular markers showed remission in both infants. The treatments were well tolerated. The first infant had an immune reaction (cytopenia and graft-vs-host disease [GVHD] in skin and marrow) in the 2 months after the infusion and was treated with steroids and bone marrow transplantation. The other baby had no adverse reactions apart from mild skin GVHD that reversed “promptly” with topical steroids and an episode of “unexplained irritability” in the 3 weeks after infusion.
The first and second infant remained cancer free 18 and 12 months later.
Source:
Qasim W, Zhan H, Samarasinghe S, et al. Sci Transl Med. 2017;9(374):pii: eaaj2013.
doi: 10.1126/scitranslmed.aaj2013.
Thanks to gene-editing treatment, 2 babies who had leukemia have a longer lease on life. Researchers from University College London, Great Ormond Street Hospital National Health Service Trust, King’s College, and Sheffield Children’s Hospital, in the United Kingdom used genetically engineered white blood cells from healthy individuals to target the cancer cells.
Related: New Treatments for Chronic Lymphocytic Leukemia
One infant was 11 months old, and the second was 16 months old. Both had refractory relapsed B-cell acute lymphoblastic leukemia and had undergone multidrug treatments. Although the potential of the method has been demonstrated in autologous and human-leukocyte-antigen–matched allogeneic settings, the researchers say, “the infrastructure and expertise required to produce personalized cell products present challenges, and low T cell counts in heavily treated individuals may preclude autologous approaches.”
The treatment involves lymphodepleting chemotherapy and anti-CD52 serotherapy, followed by a single-dose infusion of universal CAR19 cells (autologous T cells engineered to express chimeric antigen receptor against the B-cell antigen CD19). This “bridge-to-transplantation strategy” demonstrates the therapeutic potential of gene-editing technology, the researchers say.
Related: Six Open Clinical Trials That Are Expanding Our Understanding of Immunotherapies
Just 28 days after the treatment, molecular markers showed remission in both infants. The treatments were well tolerated. The first infant had an immune reaction (cytopenia and graft-vs-host disease [GVHD] in skin and marrow) in the 2 months after the infusion and was treated with steroids and bone marrow transplantation. The other baby had no adverse reactions apart from mild skin GVHD that reversed “promptly” with topical steroids and an episode of “unexplained irritability” in the 3 weeks after infusion.
The first and second infant remained cancer free 18 and 12 months later.
Source:
Qasim W, Zhan H, Samarasinghe S, et al. Sci Transl Med. 2017;9(374):pii: eaaj2013.
doi: 10.1126/scitranslmed.aaj2013.
Thanks to gene-editing treatment, 2 babies who had leukemia have a longer lease on life. Researchers from University College London, Great Ormond Street Hospital National Health Service Trust, King’s College, and Sheffield Children’s Hospital, in the United Kingdom used genetically engineered white blood cells from healthy individuals to target the cancer cells.
Related: New Treatments for Chronic Lymphocytic Leukemia
One infant was 11 months old, and the second was 16 months old. Both had refractory relapsed B-cell acute lymphoblastic leukemia and had undergone multidrug treatments. Although the potential of the method has been demonstrated in autologous and human-leukocyte-antigen–matched allogeneic settings, the researchers say, “the infrastructure and expertise required to produce personalized cell products present challenges, and low T cell counts in heavily treated individuals may preclude autologous approaches.”
The treatment involves lymphodepleting chemotherapy and anti-CD52 serotherapy, followed by a single-dose infusion of universal CAR19 cells (autologous T cells engineered to express chimeric antigen receptor against the B-cell antigen CD19). This “bridge-to-transplantation strategy” demonstrates the therapeutic potential of gene-editing technology, the researchers say.
Related: Six Open Clinical Trials That Are Expanding Our Understanding of Immunotherapies
Just 28 days after the treatment, molecular markers showed remission in both infants. The treatments were well tolerated. The first infant had an immune reaction (cytopenia and graft-vs-host disease [GVHD] in skin and marrow) in the 2 months after the infusion and was treated with steroids and bone marrow transplantation. The other baby had no adverse reactions apart from mild skin GVHD that reversed “promptly” with topical steroids and an episode of “unexplained irritability” in the 3 weeks after infusion.
The first and second infant remained cancer free 18 and 12 months later.
Source:
Qasim W, Zhan H, Samarasinghe S, et al. Sci Transl Med. 2017;9(374):pii: eaaj2013.
doi: 10.1126/scitranslmed.aaj2013.