User login
Disruptive Physician Behavior: The Importance of Recognition and Intervention and Its Impact on Patient Safety
Dramatic stories of disruptive physician behavior (DPB) appear occasionally in the news, such as the physician who shot and killed a colleague within hospital confines or the gynecologist who secretly took photographs using a camera disguised as a pen during pelvic examinations. More common in hospitals, however, are incidents of inappropriate behavior that may generate complaints from patients or other providers and at times snowball into administrative or legal challenges.
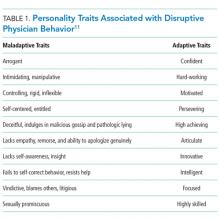
“Professionalism” is one of the six competencies listed by the Accreditation Council for Graduate Medical Education (ACGME)1 and the American Board of Medical Specialties. Unfortunately, incidents of disruptive behavior can result in violation of the tenets of professionalism in the healthcare environment. These behaviors fall along a continuum ranging from outwardly aggressive and uncivil to overly passive and insidious. Although these behaviors can occur across all healthcare disciplines and settings and are not just limited to physicians, the behaviors of physicians often have a much greater impact on the healthcare system as a whole because of their positions of relative “power” within the system.2 Hence, this problem requires greater awareness and education. In this context, the aim of this article is to discuss disruptive behaviors in physicians
The AMA defines DPB as “personal conduct, verbal or physical that has the potential to negatively affect patient care or the ability to work with other members of the healthcare team.”3 The definition of DPB by the Joint Commission includes “all behaviors that undermine a culture of safety.”4 Both the Joint Commission and the AMA recognize the significance and patient safety implications of such behavior. Policy statements by both these organizations underscore the importance of confronting and remedying these potentially dangerous interpersonal behaviors.
Data regarding the prevalence of DPB have been inconsistent. One study estimated that 3%–5% of physicians demonstrate this behavior,5 whereas another study reported a DPB prevalence of 97% among physicians and nurses in the workplace.6 According to a 2004 survey of physician executives, more than 95% of them reported regular encounters of DPB.7
The etiology of such disruptive behaviors is multifactorial and complex. Explanations associated with ‘nature versus nurture’ have ranged from physician psychopathology to unhealthy modeling during training. Both extrinsic and intrinsic factors may also contribute to DPB. External stressors and negative experiences–professional and/or personal–can provoke disruptive behaviors. Overwork, fatigue, strife, and a dysfunctional environment that can arise in both work and home environments can contribute to the development of mental health problems. Stress, burnout, and depression have increasingly become prevalent among physicians and can play a significant role in causing impaired patterns of professional conduct.8, 9 These mental health problems can cause physicians to acquire maladaptive coping strategies such as substance abuse and drug or alcohol dependence. However, it is important to note that physician impairment and substance abuse are not the most frequent causes of DPB. In fact, fewer than 10% of physician behavior issues have been related to substance abuse.2, 5
Psychiatric disorders such as major depression and bipolar and anxiety disorders may also contribute to DPB.10 Most of these disorders (except for schizophrenia) are likely as common among physicians as among the general public.9 An essential clarification is that although DPB can be a manifestation of personality disorders or psychiatric disorders, it does not always stem from underlying psychopathology. Clarifying these distinctions is important for managing the problem and calls for expert professional evaluation in some cases.10
A person’s behavior is shaped by character, values, perceptions, and attitudes. Individuals who engage in DPB typically lack insight and justify their behaviors as a means to achieve a goal. Disrespectful behavior is rooted, in part, in characteristics such as insecurity, immaturity, and aggressiveness; however, it can also be learned, tolerated, and reinforced in the hierarchical hospital culture.11
Other intrinsic factors that may contribute to DPB include lack of emotional intelligence, poor social skills, cultural and ethnic issues, and generation and gender bias.12 Identifying the root causes of DPB can be challenging due to the complexity of the interaction between the healthcare environment and the key players within it; nevertheless, awareness of the contributing factors and early recognition are important. Those who take on the mantle of leadership within hospitals should be educated in this regard.
Repercussions of Disruptive Physician Behavior
An institution’s organizational culture often has an impact on how DPB is addressed. Tolerance of such behavior can have far-reaching consequences. The central tenets of a “culture of safety and respect”–teamwork across disciplines and a blame-free environment in which every member of the healthcare team feels equally empowered to report errors and openly discuss safety issues–would be negatively impacted.
DPB can diminish the quality of care provided, increase the risk of medical errors, and adversely affect patient safety and satisfaction.11-13 Such behavior can cause erosion of relationships and communication between individuals and contribute to a hostile work environment. For instance, nurses or trainees may be afraid to question a physician because of the fear of getting yelled at or being humiliated. Consequently, improperly written orders may be overlooked or a potentially “wrong-site” surgical procedure may not be questioned for fear of provoking a hostile response.
DPB can increase litigation risk and financial costs to institutions. Provider retention may be adversely affected; valued staff may leave hospitals and need to be replaced, and productivity may suffer. When physicians in training observe how their superiors model disruptive behaviors with impunity, a concerning problem that arises is that DPB becomes normalized in the workplace culture, especially if such behaviors are tolerated and result in a perceived gain.
Proposed Interventions
Confrontation of DPB can be challenging without appropriate infrastructure. Healthcare facilities should have a fair system in place for reliable reporting and monitoring of DPB, including a complaints’ verification process, appeals process, and an option for fair hearing.
It is best to initially address the issue in a direct, timely, yet informal manner through counseling or a verbal warning. In several situations, such informal counseling opportunities create a mindful awareness of the problem and the problematic behavior ceases without the need for further action.
When informal intervention is either not appropriate (eg, if the alleged event involved an assault or other illegal behavior) or has already been offered in the past, more formal intervention is required. Institutional progressive disciplinary polices should be in place and adhered to. For example, repeat offenders may be issued written warnings or even temporary suspension of privileges.
Institutional resources such as human resources departments, office of general counsel, office of medical affairs, and the hospital’s medical board may be consulted. Some medical centers have “employee assistance programs” staffed with clinicians skilled in dealing with DPB. Individuals diagnosed with substance abuse or a mental health disorder may require consultation with mental health professionals.14
Special “Professionalism Committees” can be instituted and tasked with investigating complaints and making recommendations for the involvement of resources outside the institution, such as a state medical society.15
Conclusion
Although the vast majority of physicians are well-behaved, it is important to acknowledge that disruptive behaviors can occur in the healthcare environment. Such behaviors have a major impact on workplace culture and patient safety and must be recognized early. Hospital executives and leaders must ensure that appropriate interventions are undertaken—before the quality of patient care is affected and before lives are endangered.
Acknowledgment
The authors would like to thank Ansu John for providing editorial assistance with the manuscript.
Disclosures
The authors have nothing to disclose (Conflict of Interest Form submitted as separate PDF document). Dr. Heitt consults with local hospitals, medical practices, and licensing boards regarding physicians and other healthcare practitioners who have been accused of engaging in disruptive behavior. In these situations he may be paid by the board, medical society, hospital, practice or the professional (patient).
1. Accreditation Council for Graduate Medical Education. Common program requirements: general competencies. https://www.acgme.org/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed July 25, 2017.
2. Porto G, Lauve R. Disruptive clinician behavior: a persistent threat to patient safety. Patient safety and quality healthcare. Lionheart Publishing, Inc. 2006;3:16-24 https://www.psqh.com/julaug06/disruptive.html. Accessed October 1, 2017.
3. American Medical Association. Opinion E- 9.045–Physicians with disruptive behavior. Chicago, IL American Medical Association 2008.
4. Joint Commission: Behaviors that undermine a culture of safety. Sentinel event alert, July 9, 2008:40. http://www.jointcommission.org/sentinel_event_alert_issue_40_behaviors_that_undermine_a_culture_of_safety/. Accessed October 1, 2017.
5. Leape LL, Fromson JA. Problem doctors: is there a system-level solution? Ann Int Med. 2006;144:107-115. PubMed
6. Rosenstein AH, O’Daniel M. A survey of the impact of disruptive behaviors and communication defects on patient safety. Jt Comm J Qual Patient Saf. 2008;34(8):464-471. PubMed
7. Weber DO. Poll Results: Doctors’ disruptive behavior disturbs physician leaders. The Physician Executive. 2004;30(5):6. PubMed
8. Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289(23):3161-3166. PubMed
9. Brown S, Goske M, Johnson C. Beyond substance abuse: stress, burnout and depression as causes of physician impairment and disruptive behavior. J Am Coll Radiol. 2009 6;(7):479-485. PubMed
10. Reynolds NT. Disruptive physician behavior: use and misuse of the label. J Med Regulation. 2012;98(1):8-19.
11. Leape LL, Shore MF, Dienstag JL, et al. Perspective: a culture of respect, part 1: the nature and causes of disrespectful behavior by physicians. Acad Med. 2012;87(7):845-852. PubMed
12. Rosenstein AH, O’Daniel M. Impact and implications of disruptive behavior in the perioperative arena. J Am Coll Surg. 2006;203(1):96-105. PubMed
13. Patient Safety Primer: Disruptive and unprofessional behavior. Available at AHRQ Patient Safety Network: https://psnet.ahrq.gov/primers/primer/15/disruptive-and-unprofessional-behavior(Accessed October 1, 2017.
14. Williams BW, Williams MV. The disruptive physician: conceptual organization. JMed Licensure Discipline. 2008;94(3):12-19.
15. Speck R, Foster J, Mulhem V, et al. Development of a professionalism committee approach to address unprofessional medical staff behavior at an academic medical center. Jt Comm J Qual Patient Saf. 2004;40(4):161-167. PubMed
Dramatic stories of disruptive physician behavior (DPB) appear occasionally in the news, such as the physician who shot and killed a colleague within hospital confines or the gynecologist who secretly took photographs using a camera disguised as a pen during pelvic examinations. More common in hospitals, however, are incidents of inappropriate behavior that may generate complaints from patients or other providers and at times snowball into administrative or legal challenges.
“Professionalism” is one of the six competencies listed by the Accreditation Council for Graduate Medical Education (ACGME)1 and the American Board of Medical Specialties. Unfortunately, incidents of disruptive behavior can result in violation of the tenets of professionalism in the healthcare environment. These behaviors fall along a continuum ranging from outwardly aggressive and uncivil to overly passive and insidious. Although these behaviors can occur across all healthcare disciplines and settings and are not just limited to physicians, the behaviors of physicians often have a much greater impact on the healthcare system as a whole because of their positions of relative “power” within the system.2 Hence, this problem requires greater awareness and education. In this context, the aim of this article is to discuss disruptive behaviors in physicians
The AMA defines DPB as “personal conduct, verbal or physical that has the potential to negatively affect patient care or the ability to work with other members of the healthcare team.”3 The definition of DPB by the Joint Commission includes “all behaviors that undermine a culture of safety.”4 Both the Joint Commission and the AMA recognize the significance and patient safety implications of such behavior. Policy statements by both these organizations underscore the importance of confronting and remedying these potentially dangerous interpersonal behaviors.
Data regarding the prevalence of DPB have been inconsistent. One study estimated that 3%–5% of physicians demonstrate this behavior,5 whereas another study reported a DPB prevalence of 97% among physicians and nurses in the workplace.6 According to a 2004 survey of physician executives, more than 95% of them reported regular encounters of DPB.7
The etiology of such disruptive behaviors is multifactorial and complex. Explanations associated with ‘nature versus nurture’ have ranged from physician psychopathology to unhealthy modeling during training. Both extrinsic and intrinsic factors may also contribute to DPB. External stressors and negative experiences–professional and/or personal–can provoke disruptive behaviors. Overwork, fatigue, strife, and a dysfunctional environment that can arise in both work and home environments can contribute to the development of mental health problems. Stress, burnout, and depression have increasingly become prevalent among physicians and can play a significant role in causing impaired patterns of professional conduct.8, 9 These mental health problems can cause physicians to acquire maladaptive coping strategies such as substance abuse and drug or alcohol dependence. However, it is important to note that physician impairment and substance abuse are not the most frequent causes of DPB. In fact, fewer than 10% of physician behavior issues have been related to substance abuse.2, 5
Psychiatric disorders such as major depression and bipolar and anxiety disorders may also contribute to DPB.10 Most of these disorders (except for schizophrenia) are likely as common among physicians as among the general public.9 An essential clarification is that although DPB can be a manifestation of personality disorders or psychiatric disorders, it does not always stem from underlying psychopathology. Clarifying these distinctions is important for managing the problem and calls for expert professional evaluation in some cases.10
A person’s behavior is shaped by character, values, perceptions, and attitudes. Individuals who engage in DPB typically lack insight and justify their behaviors as a means to achieve a goal. Disrespectful behavior is rooted, in part, in characteristics such as insecurity, immaturity, and aggressiveness; however, it can also be learned, tolerated, and reinforced in the hierarchical hospital culture.11
Other intrinsic factors that may contribute to DPB include lack of emotional intelligence, poor social skills, cultural and ethnic issues, and generation and gender bias.12 Identifying the root causes of DPB can be challenging due to the complexity of the interaction between the healthcare environment and the key players within it; nevertheless, awareness of the contributing factors and early recognition are important. Those who take on the mantle of leadership within hospitals should be educated in this regard.
Repercussions of Disruptive Physician Behavior
An institution’s organizational culture often has an impact on how DPB is addressed. Tolerance of such behavior can have far-reaching consequences. The central tenets of a “culture of safety and respect”–teamwork across disciplines and a blame-free environment in which every member of the healthcare team feels equally empowered to report errors and openly discuss safety issues–would be negatively impacted.
DPB can diminish the quality of care provided, increase the risk of medical errors, and adversely affect patient safety and satisfaction.11-13 Such behavior can cause erosion of relationships and communication between individuals and contribute to a hostile work environment. For instance, nurses or trainees may be afraid to question a physician because of the fear of getting yelled at or being humiliated. Consequently, improperly written orders may be overlooked or a potentially “wrong-site” surgical procedure may not be questioned for fear of provoking a hostile response.
DPB can increase litigation risk and financial costs to institutions. Provider retention may be adversely affected; valued staff may leave hospitals and need to be replaced, and productivity may suffer. When physicians in training observe how their superiors model disruptive behaviors with impunity, a concerning problem that arises is that DPB becomes normalized in the workplace culture, especially if such behaviors are tolerated and result in a perceived gain.
Proposed Interventions
Confrontation of DPB can be challenging without appropriate infrastructure. Healthcare facilities should have a fair system in place for reliable reporting and monitoring of DPB, including a complaints’ verification process, appeals process, and an option for fair hearing.
It is best to initially address the issue in a direct, timely, yet informal manner through counseling or a verbal warning. In several situations, such informal counseling opportunities create a mindful awareness of the problem and the problematic behavior ceases without the need for further action.
When informal intervention is either not appropriate (eg, if the alleged event involved an assault or other illegal behavior) or has already been offered in the past, more formal intervention is required. Institutional progressive disciplinary polices should be in place and adhered to. For example, repeat offenders may be issued written warnings or even temporary suspension of privileges.
Institutional resources such as human resources departments, office of general counsel, office of medical affairs, and the hospital’s medical board may be consulted. Some medical centers have “employee assistance programs” staffed with clinicians skilled in dealing with DPB. Individuals diagnosed with substance abuse or a mental health disorder may require consultation with mental health professionals.14
Special “Professionalism Committees” can be instituted and tasked with investigating complaints and making recommendations for the involvement of resources outside the institution, such as a state medical society.15
Conclusion
Although the vast majority of physicians are well-behaved, it is important to acknowledge that disruptive behaviors can occur in the healthcare environment. Such behaviors have a major impact on workplace culture and patient safety and must be recognized early. Hospital executives and leaders must ensure that appropriate interventions are undertaken—before the quality of patient care is affected and before lives are endangered.
Acknowledgment
The authors would like to thank Ansu John for providing editorial assistance with the manuscript.
Disclosures
The authors have nothing to disclose (Conflict of Interest Form submitted as separate PDF document). Dr. Heitt consults with local hospitals, medical practices, and licensing boards regarding physicians and other healthcare practitioners who have been accused of engaging in disruptive behavior. In these situations he may be paid by the board, medical society, hospital, practice or the professional (patient).
Dramatic stories of disruptive physician behavior (DPB) appear occasionally in the news, such as the physician who shot and killed a colleague within hospital confines or the gynecologist who secretly took photographs using a camera disguised as a pen during pelvic examinations. More common in hospitals, however, are incidents of inappropriate behavior that may generate complaints from patients or other providers and at times snowball into administrative or legal challenges.
“Professionalism” is one of the six competencies listed by the Accreditation Council for Graduate Medical Education (ACGME)1 and the American Board of Medical Specialties. Unfortunately, incidents of disruptive behavior can result in violation of the tenets of professionalism in the healthcare environment. These behaviors fall along a continuum ranging from outwardly aggressive and uncivil to overly passive and insidious. Although these behaviors can occur across all healthcare disciplines and settings and are not just limited to physicians, the behaviors of physicians often have a much greater impact on the healthcare system as a whole because of their positions of relative “power” within the system.2 Hence, this problem requires greater awareness and education. In this context, the aim of this article is to discuss disruptive behaviors in physicians
The AMA defines DPB as “personal conduct, verbal or physical that has the potential to negatively affect patient care or the ability to work with other members of the healthcare team.”3 The definition of DPB by the Joint Commission includes “all behaviors that undermine a culture of safety.”4 Both the Joint Commission and the AMA recognize the significance and patient safety implications of such behavior. Policy statements by both these organizations underscore the importance of confronting and remedying these potentially dangerous interpersonal behaviors.
Data regarding the prevalence of DPB have been inconsistent. One study estimated that 3%–5% of physicians demonstrate this behavior,5 whereas another study reported a DPB prevalence of 97% among physicians and nurses in the workplace.6 According to a 2004 survey of physician executives, more than 95% of them reported regular encounters of DPB.7
The etiology of such disruptive behaviors is multifactorial and complex. Explanations associated with ‘nature versus nurture’ have ranged from physician psychopathology to unhealthy modeling during training. Both extrinsic and intrinsic factors may also contribute to DPB. External stressors and negative experiences–professional and/or personal–can provoke disruptive behaviors. Overwork, fatigue, strife, and a dysfunctional environment that can arise in both work and home environments can contribute to the development of mental health problems. Stress, burnout, and depression have increasingly become prevalent among physicians and can play a significant role in causing impaired patterns of professional conduct.8, 9 These mental health problems can cause physicians to acquire maladaptive coping strategies such as substance abuse and drug or alcohol dependence. However, it is important to note that physician impairment and substance abuse are not the most frequent causes of DPB. In fact, fewer than 10% of physician behavior issues have been related to substance abuse.2, 5
Psychiatric disorders such as major depression and bipolar and anxiety disorders may also contribute to DPB.10 Most of these disorders (except for schizophrenia) are likely as common among physicians as among the general public.9 An essential clarification is that although DPB can be a manifestation of personality disorders or psychiatric disorders, it does not always stem from underlying psychopathology. Clarifying these distinctions is important for managing the problem and calls for expert professional evaluation in some cases.10
A person’s behavior is shaped by character, values, perceptions, and attitudes. Individuals who engage in DPB typically lack insight and justify their behaviors as a means to achieve a goal. Disrespectful behavior is rooted, in part, in characteristics such as insecurity, immaturity, and aggressiveness; however, it can also be learned, tolerated, and reinforced in the hierarchical hospital culture.11
Other intrinsic factors that may contribute to DPB include lack of emotional intelligence, poor social skills, cultural and ethnic issues, and generation and gender bias.12 Identifying the root causes of DPB can be challenging due to the complexity of the interaction between the healthcare environment and the key players within it; nevertheless, awareness of the contributing factors and early recognition are important. Those who take on the mantle of leadership within hospitals should be educated in this regard.
Repercussions of Disruptive Physician Behavior
An institution’s organizational culture often has an impact on how DPB is addressed. Tolerance of such behavior can have far-reaching consequences. The central tenets of a “culture of safety and respect”–teamwork across disciplines and a blame-free environment in which every member of the healthcare team feels equally empowered to report errors and openly discuss safety issues–would be negatively impacted.
DPB can diminish the quality of care provided, increase the risk of medical errors, and adversely affect patient safety and satisfaction.11-13 Such behavior can cause erosion of relationships and communication between individuals and contribute to a hostile work environment. For instance, nurses or trainees may be afraid to question a physician because of the fear of getting yelled at or being humiliated. Consequently, improperly written orders may be overlooked or a potentially “wrong-site” surgical procedure may not be questioned for fear of provoking a hostile response.
DPB can increase litigation risk and financial costs to institutions. Provider retention may be adversely affected; valued staff may leave hospitals and need to be replaced, and productivity may suffer. When physicians in training observe how their superiors model disruptive behaviors with impunity, a concerning problem that arises is that DPB becomes normalized in the workplace culture, especially if such behaviors are tolerated and result in a perceived gain.
Proposed Interventions
Confrontation of DPB can be challenging without appropriate infrastructure. Healthcare facilities should have a fair system in place for reliable reporting and monitoring of DPB, including a complaints’ verification process, appeals process, and an option for fair hearing.
It is best to initially address the issue in a direct, timely, yet informal manner through counseling or a verbal warning. In several situations, such informal counseling opportunities create a mindful awareness of the problem and the problematic behavior ceases without the need for further action.
When informal intervention is either not appropriate (eg, if the alleged event involved an assault or other illegal behavior) or has already been offered in the past, more formal intervention is required. Institutional progressive disciplinary polices should be in place and adhered to. For example, repeat offenders may be issued written warnings or even temporary suspension of privileges.
Institutional resources such as human resources departments, office of general counsel, office of medical affairs, and the hospital’s medical board may be consulted. Some medical centers have “employee assistance programs” staffed with clinicians skilled in dealing with DPB. Individuals diagnosed with substance abuse or a mental health disorder may require consultation with mental health professionals.14
Special “Professionalism Committees” can be instituted and tasked with investigating complaints and making recommendations for the involvement of resources outside the institution, such as a state medical society.15
Conclusion
Although the vast majority of physicians are well-behaved, it is important to acknowledge that disruptive behaviors can occur in the healthcare environment. Such behaviors have a major impact on workplace culture and patient safety and must be recognized early. Hospital executives and leaders must ensure that appropriate interventions are undertaken—before the quality of patient care is affected and before lives are endangered.
Acknowledgment
The authors would like to thank Ansu John for providing editorial assistance with the manuscript.
Disclosures
The authors have nothing to disclose (Conflict of Interest Form submitted as separate PDF document). Dr. Heitt consults with local hospitals, medical practices, and licensing boards regarding physicians and other healthcare practitioners who have been accused of engaging in disruptive behavior. In these situations he may be paid by the board, medical society, hospital, practice or the professional (patient).
1. Accreditation Council for Graduate Medical Education. Common program requirements: general competencies. https://www.acgme.org/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed July 25, 2017.
2. Porto G, Lauve R. Disruptive clinician behavior: a persistent threat to patient safety. Patient safety and quality healthcare. Lionheart Publishing, Inc. 2006;3:16-24 https://www.psqh.com/julaug06/disruptive.html. Accessed October 1, 2017.
3. American Medical Association. Opinion E- 9.045–Physicians with disruptive behavior. Chicago, IL American Medical Association 2008.
4. Joint Commission: Behaviors that undermine a culture of safety. Sentinel event alert, July 9, 2008:40. http://www.jointcommission.org/sentinel_event_alert_issue_40_behaviors_that_undermine_a_culture_of_safety/. Accessed October 1, 2017.
5. Leape LL, Fromson JA. Problem doctors: is there a system-level solution? Ann Int Med. 2006;144:107-115. PubMed
6. Rosenstein AH, O’Daniel M. A survey of the impact of disruptive behaviors and communication defects on patient safety. Jt Comm J Qual Patient Saf. 2008;34(8):464-471. PubMed
7. Weber DO. Poll Results: Doctors’ disruptive behavior disturbs physician leaders. The Physician Executive. 2004;30(5):6. PubMed
8. Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289(23):3161-3166. PubMed
9. Brown S, Goske M, Johnson C. Beyond substance abuse: stress, burnout and depression as causes of physician impairment and disruptive behavior. J Am Coll Radiol. 2009 6;(7):479-485. PubMed
10. Reynolds NT. Disruptive physician behavior: use and misuse of the label. J Med Regulation. 2012;98(1):8-19.
11. Leape LL, Shore MF, Dienstag JL, et al. Perspective: a culture of respect, part 1: the nature and causes of disrespectful behavior by physicians. Acad Med. 2012;87(7):845-852. PubMed
12. Rosenstein AH, O’Daniel M. Impact and implications of disruptive behavior in the perioperative arena. J Am Coll Surg. 2006;203(1):96-105. PubMed
13. Patient Safety Primer: Disruptive and unprofessional behavior. Available at AHRQ Patient Safety Network: https://psnet.ahrq.gov/primers/primer/15/disruptive-and-unprofessional-behavior(Accessed October 1, 2017.
14. Williams BW, Williams MV. The disruptive physician: conceptual organization. JMed Licensure Discipline. 2008;94(3):12-19.
15. Speck R, Foster J, Mulhem V, et al. Development of a professionalism committee approach to address unprofessional medical staff behavior at an academic medical center. Jt Comm J Qual Patient Saf. 2004;40(4):161-167. PubMed
1. Accreditation Council for Graduate Medical Education. Common program requirements: general competencies. https://www.acgme.org/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed July 25, 2017.
2. Porto G, Lauve R. Disruptive clinician behavior: a persistent threat to patient safety. Patient safety and quality healthcare. Lionheart Publishing, Inc. 2006;3:16-24 https://www.psqh.com/julaug06/disruptive.html. Accessed October 1, 2017.
3. American Medical Association. Opinion E- 9.045–Physicians with disruptive behavior. Chicago, IL American Medical Association 2008.
4. Joint Commission: Behaviors that undermine a culture of safety. Sentinel event alert, July 9, 2008:40. http://www.jointcommission.org/sentinel_event_alert_issue_40_behaviors_that_undermine_a_culture_of_safety/. Accessed October 1, 2017.
5. Leape LL, Fromson JA. Problem doctors: is there a system-level solution? Ann Int Med. 2006;144:107-115. PubMed
6. Rosenstein AH, O’Daniel M. A survey of the impact of disruptive behaviors and communication defects on patient safety. Jt Comm J Qual Patient Saf. 2008;34(8):464-471. PubMed
7. Weber DO. Poll Results: Doctors’ disruptive behavior disturbs physician leaders. The Physician Executive. 2004;30(5):6. PubMed
8. Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289(23):3161-3166. PubMed
9. Brown S, Goske M, Johnson C. Beyond substance abuse: stress, burnout and depression as causes of physician impairment and disruptive behavior. J Am Coll Radiol. 2009 6;(7):479-485. PubMed
10. Reynolds NT. Disruptive physician behavior: use and misuse of the label. J Med Regulation. 2012;98(1):8-19.
11. Leape LL, Shore MF, Dienstag JL, et al. Perspective: a culture of respect, part 1: the nature and causes of disrespectful behavior by physicians. Acad Med. 2012;87(7):845-852. PubMed
12. Rosenstein AH, O’Daniel M. Impact and implications of disruptive behavior in the perioperative arena. J Am Coll Surg. 2006;203(1):96-105. PubMed
13. Patient Safety Primer: Disruptive and unprofessional behavior. Available at AHRQ Patient Safety Network: https://psnet.ahrq.gov/primers/primer/15/disruptive-and-unprofessional-behavior(Accessed October 1, 2017.
14. Williams BW, Williams MV. The disruptive physician: conceptual organization. JMed Licensure Discipline. 2008;94(3):12-19.
15. Speck R, Foster J, Mulhem V, et al. Development of a professionalism committee approach to address unprofessional medical staff behavior at an academic medical center. Jt Comm J Qual Patient Saf. 2004;40(4):161-167. PubMed
© 2018 Society of Hospital Medicine
The Harm We Do: The Environmental Impact of Medicine
Healthcare is a “dirty” business with widespread effects on the environment. In the US, healthcare is estimated to generate 9.8% of our greenhouse gases and 9% of our particulate matter emissions.1 Hazardous wastes must be incinerated, emitting carbon dioxide, nitrogen oxides, and volatile substances into the atmosphere.2 Similarly, hospitals are responsible for 7% of commercial water use in the US.3 Conventional water treatment systems are not designed to remove heavy metals, pharmaceuticals, and disinfectants in hospital wastewaters; these compounds have been detected in rivers and streams throughout the US.4,5 Furthermore, pharmaceutical compounds such as antibiotics, anti-epileptics, and narcotics have even been isolated in our drinking water.5
As hospitalists, we are the directors of inpatient care, yet we only witness brief moments in the lives of our patients and the products we use for their care. For example, we are unaware of particulate matter emissions needed to power an extra imaging study or the contribution of unused materials to a growing landfill. However, pollution, including that from our clinical practice, is detrimental to human health in many ways. Exposure to particulate matter and toxic wastes has been linked to increased rates of reproductive and developmental disorders, cancer, and respiratory disease. 6 Particles <2.5 µm in diameter can diffuse through alveoli into the bloodstream, contributing to heart disease, stroke, and lung disease.7 Climate change has been linked to a wide range of adverse cardiovascular, respiratory, infectious, and mental health outcomes.8,9 These examples of the health impacts of pollution are illustrative but not exhaustive.
The environmental impact of US healthcare accounts for an estimated 470,000 disability-adjusted life years lost; this figure is on par with the burden of preventable medical errors.1 Clearly, change is necessary at all levels in the healthcare system to address our impact on human health. Fortunately, healthcare systems and hospital administrators have begun to address this issue. This perspective describes sustainability efforts in hospitals and healthcare systems and seeks to motivate hospitalists to build upon these efforts.
EFFORTS BY HOSPITALS AND HEALTHCARE SYSTEMS
With the ability to affect change from the top down, health systems are playing an important role in healthcare’s environmental sustainability. Ambitiously, Kaiser Permanente outlined eight environmental stewardship goals, which include becoming net carbon positive and recycling, reusing, or composting 100% of their non-hazardous waste by 2025.10 The Cleveland Clinic has pledged to become carbon neutral within the next 10 years.11 Other healthcare systems may follow suite. Many “green” interventions aimed at reducing waste and pollution also protect population health and reduce hospital operating costs.
From 2011 to 2015, a group of Boston Hospitals decreased energy use by 9.4% compared with a historical growth of 1.5% per year and saved over 15 million dollars.12 Similarly, Virginia Mason reduced landfill waste by reprocessing single-use medical devices, thereby decreasing purchasing costs by $3 million.13 As part of a regional campaign to protect the St. Croix River, Hudson Hospital and Clinic in Wisconsin saved over $20,000 with new recycling and waste reduction programs.13 Notably, these programs not only benefit hospitals but also patients and payers by reducing costs of care.
ROLE OF THE HOSPITALIST
These examples illustrate that a greener healthcare industry is achievable. Despite the potential benefits, sustainability efforts in US hospitals are the exception, not the rule, and the diffusion of such innovations must be encouraged from within.
In addition to the moral case for environmentally sustainable healthcare,14,15 such efforts can also improve our quality of care. The conversation around healthcare waste has focused on costs. Yet, examining our waste from a new perspective may reveal new ways to increase the value of patient care while protecting population health. Our communities and families are not immune to the health impacts of pollution, including that generated by our industry. However, predicted effects of climate change including altered patterns of vector-borne disease and frequent hurricanes and forest fires are upon us, affecting our communities, hospitals, and health delivery enterprise today. These challenges represent educational, academic, and economic opportunities that hospitalists should embrace.
RECOMMENDATIONS FOR ACTION
Education and Awareness
The first step to engagement is to promote awareness of the effects of healthcare waste. Physicians remain one of the most trusted sources of information about the health impacts of climate change.16 By educating ourselves, we can spread accurate knowledge to our patients and communities. Furthermore, we have the ability to advocate for our hospitals to follow institutions such as Kaiser Permanente and the Cleveland Clinic.
Given that hospitalists play a key role in educating students and residents, they are ideal vehicles for such dissemination. Education should begin in medical and nursing schools, where curricula detailing the importance and impact of healthcare pollution may be introduced. As hospitalists, we should champion such efforts.
Measurement and Amelioration
Second, resource use, waste production, and areas for improvement must be systematically quantified. At a national level, the Sustainable Development Unit of the National Health System (NHS) measures and reports water use, waste production, and energy consumption of the UK’s healthcare sector. Consequently, the NHS has surpassed their 2015 goal of reducing their carbon footprint by 10%.17 By establishing a baseline understanding of our carbon emissions, waste production, and water consumption, areas where physicians and hospitals can target improvement can similarly be identified.
Hospitalists appreciate the practical tradeoffs between clinical work and change efforts; thus, they are critical in establishing pragmatic policies. Physicians, often in collaboration with environmental engineers, have used evidence-based methods such as life-cycle analysis (LCA) to evaluate the environmental impacts of the pharmaceuticals and procedures that they use.18-20 An LCA is a cost-benefit analysis that examines multiple parameters of a product, namely, emissions, water use, costs, and waste production, from production to disposal. For example, an LCA of disposable custom packs for hysterectomies, vaginal deliveries, and laryngeal masks found costs savings and environmental benefits from choosing reusable over single-use items and removing unnecessary materials such as extra towels in this setting. 18-20 By considering the full life cycle of a procedure, LCAs reveal important information about the value and safety of care. LCAs, along with other sustainable design strategies, are tools that can provide hospitalists with new insights for quality improvement.
Public Reporting
Numerous physicians are known for educating their communities about the impacts of pollution on health. Recently, a pediatrician brought the presence of lead in Flint’s water supply to the public’s attention, instigating government action and policy change.21 A group called Utah Physicians for a Healthy Environment publishes online summaries of peer-reviewed information on air pollution and health. The Huma Lung Foundation led by a pulmonologist in Chennai, India, is working with a local radio station to report daily air quality measurements along with health advisories for the city.
We must now extend this paradigm to encompass transparency about healthcare’s practices and their impact on health. Indeed, the public is comfortable with this idea: a survey of 1011 respondents in the UK found that 92% indicated that the healthcare system should be environmentally sustainable.22 One idea may be a public-facing scorecard for hospitals, akin to publicly reported quality metrics. We can look to the example of the SDU and corporations such as Apple, which publicly report their carbon emissions, waste production, water use, and other metrics of their environmental impact. By galvanizing efforts to quantify and report our impact, hospitalists have the opportunity to be a role model for the industry and increase trust within their communities.
Individual Actions
What can a hospitalist do today? First, simple measures, like turning off idle electronics, recycling appropriately, or avoiding the use of unnecessary supplies or tests, are behavioral steps in the right direction. Second, just as education, goal setting, and feedback have met success in improving hand hygiene,23 we must begin the hard work of developing programs to monitor our environmental impact. Individual hospitalist carbon scores may help intensify efforts and spur improvement. Finally, we should learn and celebrate each other’s success. Renewed focus on this topic with increased reporting of interventions and outcomes is needed.
CONCLUSIONS
As hospitalists, we must look within ourselves to protect our planet and advocate for solutions that assure a sustainable future. By recognizing that a healthy environment is crucial to human health, we can set an example for other industries and create a safer world for our patients. Eliminating the harm we do is the first step in this process.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Chopra is supported by a Career Development Award from the Agency for Healthcare Quality and Research (1-K08-HS-022835-01).
1. Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. Ahmad S, ed. PLoS One. 2016;11(6):e0157014. doi:10.1371/journal.pone.0157014. PubMed
2. Windfeld ES, Brooks MS-L. Medical waste management–A review. J Environ Manage. 2015;163:98-108. doi:10.1016/j.jenvman.2015.08.013. PubMed
3. Environmental Protection Agency. Saving Water in Hospitals. Available at: https://www.epa.gov/sites/production/files/2017-01/documents/ws-commercial-factsheet-hospitals.pdf. Accessed December 9, 2017.
4. Kolpin DW, Furlong ET, Meyer MT, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: A national reconnaissance. Environ Sci & Technol 2002;36(6):1202-1211. PubMed
5. Deo, RP, Halden, RU. Pharmaceuticals in the built and natural water environment of the United States. Water. 2013;5(3):1346-1365. doi:10.3390/w5031346.
6. Lim SS, Vos T, Flaxman AD, Danaei G, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013; 380: 2224-60. PubMed
7. Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American heart association. Circulation. 2010;121(21):2331-2378. doi:10.1161/CIR.0b013e3181dbece1. PubMed
8. Watts N, Adger WN, Ayeb-Karlsson S, et al. The Lancet countdown: tracking progress on health and climate change. Lancet. 2017;389(10074):1151-1164. doi:10.1016/S0140-6736(16)32124-9. PubMed
9. Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet. 2015;386(10007):1973–2028. PubMed
10. Kaiser Permanente. Environmental Stewardship. Available at: https://share.kaiserpermanente.org/article/environmental-stewardship-overview/. Accessed December 2, 2017.
11. Health Facilities Management Magazine. Cleveland Clinic makes carbon-neutrality its newest sustainability goal. Available at: https://www.hfmmagazine.com/articles/3210-cleveland-clinic-makes-carbon-neutrality-its-newest-sustainability-goal?lipi=urn%3Ali%3Apage%3Ad_flagship3_feed%3BHXuZOUrpQUu0OQ3RcUQqEg%3D%3D. Accessed December 2, 2017.
12. Healthcare without Harm. Metropolitan Boston Health Care Energy & Greenhouse Gas Profile: 2011 through 2015, and 2020 Projection. Available at: https://noharm-uscanada.org/sites/default/files/documents-files/4723/Report-Boston%20Health%20Care%20Energy%20Profile-May%202017.pdf Accessed December 9, 2017.
13. Practice Greenhealth. Advancing sustainability in healthcare: a collection of special case studies. Available at: https://practicegreenhealth.org/sites/default/files/upload-files/hhi.case_.studies.pdf. Accessed July 22, 2017.
14. Macpherson C, Hill J. Are physicians obliged to lead environmental sustainability efforts in health care organizations? AMA J Ethics. 2017;19(12):1164-1173. doi:10.1001/journalofethics.2017.19.12.ecas2-1712. PubMed
15. American Nurses Association. ANA’s principles of environmental health for nursing practice with implementation strategies. Available at: http://www.nursingworld.org/MainMenuCategories/WorkplaceSafety/Healthy-Nurse/ANAsPrinciplesofEnvironmentalHealthforNursingPractice.pd. Accessed December 9, 2017.
16. Maibach EW, Kreslake JM, Roser-Renouf C, et al. Do Americans understand that global warming is harmful to human health? Evidence from a national survey. Ann Glob Health. 2015;81(3):396-409. doi:10.1016/j.aogh.2015.08.010. PubMed
17. Healthcare without Harm. Reducing Healthcare’s Climate Footprint: opportunities for European Hospitals & Health Systems. Available at: https://noharm-europe.org/sites/default/files/documents-files/4746/HCWHEurope_Climate_Report_Dec2016.pdf. Accessed May 22, 2017.
18. Campion, N, Thiel, CL, Woods, et al. Sustainable healthcare and environmental life-cycle impacts of disposable supplies: a focus on disposable custom packs. J Clean Prod. 2015;94:46-55. doi:10.1016/j.jclepro.2015.01.076.
19. Eckelman M, Mosher M, Gonzalez A, et al. Comparative life cycle assessment of disposable and reusable laryngeal mask airways: Anesth Analg. 2012;114(5):1067-1072. doi:10.1213/ANE.0b013e31824f6959. PubMed
20. Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci & Technol. 2015;49(3):1779-1786. doi:10.1021/es504719g. PubMed
21. Hanna-Attisha M, LaChance J, Sadler RC, et al. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. PubMed
22. Sustainable Development Unit. Sustainability and the NHS, Public Health and Social Care system–Ipsos Mori survey. Available at: https://www.sduhealth.org.uk/policy-strategy/reporting/ipsos-mori.aspx. Accessed December 9, 2017.
23. Luangasanatip N, Hongsuwan M, Limmathurotsakul D, et al. Comparative efficacy of interventions to promote hand hygiene in hospital: systematic review and network meta-analysis. BMJ. 2015;351:h3728. doi:10.1136/bmj.h3728. PubMed
Healthcare is a “dirty” business with widespread effects on the environment. In the US, healthcare is estimated to generate 9.8% of our greenhouse gases and 9% of our particulate matter emissions.1 Hazardous wastes must be incinerated, emitting carbon dioxide, nitrogen oxides, and volatile substances into the atmosphere.2 Similarly, hospitals are responsible for 7% of commercial water use in the US.3 Conventional water treatment systems are not designed to remove heavy metals, pharmaceuticals, and disinfectants in hospital wastewaters; these compounds have been detected in rivers and streams throughout the US.4,5 Furthermore, pharmaceutical compounds such as antibiotics, anti-epileptics, and narcotics have even been isolated in our drinking water.5
As hospitalists, we are the directors of inpatient care, yet we only witness brief moments in the lives of our patients and the products we use for their care. For example, we are unaware of particulate matter emissions needed to power an extra imaging study or the contribution of unused materials to a growing landfill. However, pollution, including that from our clinical practice, is detrimental to human health in many ways. Exposure to particulate matter and toxic wastes has been linked to increased rates of reproductive and developmental disorders, cancer, and respiratory disease. 6 Particles <2.5 µm in diameter can diffuse through alveoli into the bloodstream, contributing to heart disease, stroke, and lung disease.7 Climate change has been linked to a wide range of adverse cardiovascular, respiratory, infectious, and mental health outcomes.8,9 These examples of the health impacts of pollution are illustrative but not exhaustive.
The environmental impact of US healthcare accounts for an estimated 470,000 disability-adjusted life years lost; this figure is on par with the burden of preventable medical errors.1 Clearly, change is necessary at all levels in the healthcare system to address our impact on human health. Fortunately, healthcare systems and hospital administrators have begun to address this issue. This perspective describes sustainability efforts in hospitals and healthcare systems and seeks to motivate hospitalists to build upon these efforts.
EFFORTS BY HOSPITALS AND HEALTHCARE SYSTEMS
With the ability to affect change from the top down, health systems are playing an important role in healthcare’s environmental sustainability. Ambitiously, Kaiser Permanente outlined eight environmental stewardship goals, which include becoming net carbon positive and recycling, reusing, or composting 100% of their non-hazardous waste by 2025.10 The Cleveland Clinic has pledged to become carbon neutral within the next 10 years.11 Other healthcare systems may follow suite. Many “green” interventions aimed at reducing waste and pollution also protect population health and reduce hospital operating costs.
From 2011 to 2015, a group of Boston Hospitals decreased energy use by 9.4% compared with a historical growth of 1.5% per year and saved over 15 million dollars.12 Similarly, Virginia Mason reduced landfill waste by reprocessing single-use medical devices, thereby decreasing purchasing costs by $3 million.13 As part of a regional campaign to protect the St. Croix River, Hudson Hospital and Clinic in Wisconsin saved over $20,000 with new recycling and waste reduction programs.13 Notably, these programs not only benefit hospitals but also patients and payers by reducing costs of care.
ROLE OF THE HOSPITALIST
These examples illustrate that a greener healthcare industry is achievable. Despite the potential benefits, sustainability efforts in US hospitals are the exception, not the rule, and the diffusion of such innovations must be encouraged from within.
In addition to the moral case for environmentally sustainable healthcare,14,15 such efforts can also improve our quality of care. The conversation around healthcare waste has focused on costs. Yet, examining our waste from a new perspective may reveal new ways to increase the value of patient care while protecting population health. Our communities and families are not immune to the health impacts of pollution, including that generated by our industry. However, predicted effects of climate change including altered patterns of vector-borne disease and frequent hurricanes and forest fires are upon us, affecting our communities, hospitals, and health delivery enterprise today. These challenges represent educational, academic, and economic opportunities that hospitalists should embrace.
RECOMMENDATIONS FOR ACTION
Education and Awareness
The first step to engagement is to promote awareness of the effects of healthcare waste. Physicians remain one of the most trusted sources of information about the health impacts of climate change.16 By educating ourselves, we can spread accurate knowledge to our patients and communities. Furthermore, we have the ability to advocate for our hospitals to follow institutions such as Kaiser Permanente and the Cleveland Clinic.
Given that hospitalists play a key role in educating students and residents, they are ideal vehicles for such dissemination. Education should begin in medical and nursing schools, where curricula detailing the importance and impact of healthcare pollution may be introduced. As hospitalists, we should champion such efforts.
Measurement and Amelioration
Second, resource use, waste production, and areas for improvement must be systematically quantified. At a national level, the Sustainable Development Unit of the National Health System (NHS) measures and reports water use, waste production, and energy consumption of the UK’s healthcare sector. Consequently, the NHS has surpassed their 2015 goal of reducing their carbon footprint by 10%.17 By establishing a baseline understanding of our carbon emissions, waste production, and water consumption, areas where physicians and hospitals can target improvement can similarly be identified.
Hospitalists appreciate the practical tradeoffs between clinical work and change efforts; thus, they are critical in establishing pragmatic policies. Physicians, often in collaboration with environmental engineers, have used evidence-based methods such as life-cycle analysis (LCA) to evaluate the environmental impacts of the pharmaceuticals and procedures that they use.18-20 An LCA is a cost-benefit analysis that examines multiple parameters of a product, namely, emissions, water use, costs, and waste production, from production to disposal. For example, an LCA of disposable custom packs for hysterectomies, vaginal deliveries, and laryngeal masks found costs savings and environmental benefits from choosing reusable over single-use items and removing unnecessary materials such as extra towels in this setting. 18-20 By considering the full life cycle of a procedure, LCAs reveal important information about the value and safety of care. LCAs, along with other sustainable design strategies, are tools that can provide hospitalists with new insights for quality improvement.
Public Reporting
Numerous physicians are known for educating their communities about the impacts of pollution on health. Recently, a pediatrician brought the presence of lead in Flint’s water supply to the public’s attention, instigating government action and policy change.21 A group called Utah Physicians for a Healthy Environment publishes online summaries of peer-reviewed information on air pollution and health. The Huma Lung Foundation led by a pulmonologist in Chennai, India, is working with a local radio station to report daily air quality measurements along with health advisories for the city.
We must now extend this paradigm to encompass transparency about healthcare’s practices and their impact on health. Indeed, the public is comfortable with this idea: a survey of 1011 respondents in the UK found that 92% indicated that the healthcare system should be environmentally sustainable.22 One idea may be a public-facing scorecard for hospitals, akin to publicly reported quality metrics. We can look to the example of the SDU and corporations such as Apple, which publicly report their carbon emissions, waste production, water use, and other metrics of their environmental impact. By galvanizing efforts to quantify and report our impact, hospitalists have the opportunity to be a role model for the industry and increase trust within their communities.
Individual Actions
What can a hospitalist do today? First, simple measures, like turning off idle electronics, recycling appropriately, or avoiding the use of unnecessary supplies or tests, are behavioral steps in the right direction. Second, just as education, goal setting, and feedback have met success in improving hand hygiene,23 we must begin the hard work of developing programs to monitor our environmental impact. Individual hospitalist carbon scores may help intensify efforts and spur improvement. Finally, we should learn and celebrate each other’s success. Renewed focus on this topic with increased reporting of interventions and outcomes is needed.
CONCLUSIONS
As hospitalists, we must look within ourselves to protect our planet and advocate for solutions that assure a sustainable future. By recognizing that a healthy environment is crucial to human health, we can set an example for other industries and create a safer world for our patients. Eliminating the harm we do is the first step in this process.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Chopra is supported by a Career Development Award from the Agency for Healthcare Quality and Research (1-K08-HS-022835-01).
Healthcare is a “dirty” business with widespread effects on the environment. In the US, healthcare is estimated to generate 9.8% of our greenhouse gases and 9% of our particulate matter emissions.1 Hazardous wastes must be incinerated, emitting carbon dioxide, nitrogen oxides, and volatile substances into the atmosphere.2 Similarly, hospitals are responsible for 7% of commercial water use in the US.3 Conventional water treatment systems are not designed to remove heavy metals, pharmaceuticals, and disinfectants in hospital wastewaters; these compounds have been detected in rivers and streams throughout the US.4,5 Furthermore, pharmaceutical compounds such as antibiotics, anti-epileptics, and narcotics have even been isolated in our drinking water.5
As hospitalists, we are the directors of inpatient care, yet we only witness brief moments in the lives of our patients and the products we use for their care. For example, we are unaware of particulate matter emissions needed to power an extra imaging study or the contribution of unused materials to a growing landfill. However, pollution, including that from our clinical practice, is detrimental to human health in many ways. Exposure to particulate matter and toxic wastes has been linked to increased rates of reproductive and developmental disorders, cancer, and respiratory disease. 6 Particles <2.5 µm in diameter can diffuse through alveoli into the bloodstream, contributing to heart disease, stroke, and lung disease.7 Climate change has been linked to a wide range of adverse cardiovascular, respiratory, infectious, and mental health outcomes.8,9 These examples of the health impacts of pollution are illustrative but not exhaustive.
The environmental impact of US healthcare accounts for an estimated 470,000 disability-adjusted life years lost; this figure is on par with the burden of preventable medical errors.1 Clearly, change is necessary at all levels in the healthcare system to address our impact on human health. Fortunately, healthcare systems and hospital administrators have begun to address this issue. This perspective describes sustainability efforts in hospitals and healthcare systems and seeks to motivate hospitalists to build upon these efforts.
EFFORTS BY HOSPITALS AND HEALTHCARE SYSTEMS
With the ability to affect change from the top down, health systems are playing an important role in healthcare’s environmental sustainability. Ambitiously, Kaiser Permanente outlined eight environmental stewardship goals, which include becoming net carbon positive and recycling, reusing, or composting 100% of their non-hazardous waste by 2025.10 The Cleveland Clinic has pledged to become carbon neutral within the next 10 years.11 Other healthcare systems may follow suite. Many “green” interventions aimed at reducing waste and pollution also protect population health and reduce hospital operating costs.
From 2011 to 2015, a group of Boston Hospitals decreased energy use by 9.4% compared with a historical growth of 1.5% per year and saved over 15 million dollars.12 Similarly, Virginia Mason reduced landfill waste by reprocessing single-use medical devices, thereby decreasing purchasing costs by $3 million.13 As part of a regional campaign to protect the St. Croix River, Hudson Hospital and Clinic in Wisconsin saved over $20,000 with new recycling and waste reduction programs.13 Notably, these programs not only benefit hospitals but also patients and payers by reducing costs of care.
ROLE OF THE HOSPITALIST
These examples illustrate that a greener healthcare industry is achievable. Despite the potential benefits, sustainability efforts in US hospitals are the exception, not the rule, and the diffusion of such innovations must be encouraged from within.
In addition to the moral case for environmentally sustainable healthcare,14,15 such efforts can also improve our quality of care. The conversation around healthcare waste has focused on costs. Yet, examining our waste from a new perspective may reveal new ways to increase the value of patient care while protecting population health. Our communities and families are not immune to the health impacts of pollution, including that generated by our industry. However, predicted effects of climate change including altered patterns of vector-borne disease and frequent hurricanes and forest fires are upon us, affecting our communities, hospitals, and health delivery enterprise today. These challenges represent educational, academic, and economic opportunities that hospitalists should embrace.
RECOMMENDATIONS FOR ACTION
Education and Awareness
The first step to engagement is to promote awareness of the effects of healthcare waste. Physicians remain one of the most trusted sources of information about the health impacts of climate change.16 By educating ourselves, we can spread accurate knowledge to our patients and communities. Furthermore, we have the ability to advocate for our hospitals to follow institutions such as Kaiser Permanente and the Cleveland Clinic.
Given that hospitalists play a key role in educating students and residents, they are ideal vehicles for such dissemination. Education should begin in medical and nursing schools, where curricula detailing the importance and impact of healthcare pollution may be introduced. As hospitalists, we should champion such efforts.
Measurement and Amelioration
Second, resource use, waste production, and areas for improvement must be systematically quantified. At a national level, the Sustainable Development Unit of the National Health System (NHS) measures and reports water use, waste production, and energy consumption of the UK’s healthcare sector. Consequently, the NHS has surpassed their 2015 goal of reducing their carbon footprint by 10%.17 By establishing a baseline understanding of our carbon emissions, waste production, and water consumption, areas where physicians and hospitals can target improvement can similarly be identified.
Hospitalists appreciate the practical tradeoffs between clinical work and change efforts; thus, they are critical in establishing pragmatic policies. Physicians, often in collaboration with environmental engineers, have used evidence-based methods such as life-cycle analysis (LCA) to evaluate the environmental impacts of the pharmaceuticals and procedures that they use.18-20 An LCA is a cost-benefit analysis that examines multiple parameters of a product, namely, emissions, water use, costs, and waste production, from production to disposal. For example, an LCA of disposable custom packs for hysterectomies, vaginal deliveries, and laryngeal masks found costs savings and environmental benefits from choosing reusable over single-use items and removing unnecessary materials such as extra towels in this setting. 18-20 By considering the full life cycle of a procedure, LCAs reveal important information about the value and safety of care. LCAs, along with other sustainable design strategies, are tools that can provide hospitalists with new insights for quality improvement.
Public Reporting
Numerous physicians are known for educating their communities about the impacts of pollution on health. Recently, a pediatrician brought the presence of lead in Flint’s water supply to the public’s attention, instigating government action and policy change.21 A group called Utah Physicians for a Healthy Environment publishes online summaries of peer-reviewed information on air pollution and health. The Huma Lung Foundation led by a pulmonologist in Chennai, India, is working with a local radio station to report daily air quality measurements along with health advisories for the city.
We must now extend this paradigm to encompass transparency about healthcare’s practices and their impact on health. Indeed, the public is comfortable with this idea: a survey of 1011 respondents in the UK found that 92% indicated that the healthcare system should be environmentally sustainable.22 One idea may be a public-facing scorecard for hospitals, akin to publicly reported quality metrics. We can look to the example of the SDU and corporations such as Apple, which publicly report their carbon emissions, waste production, water use, and other metrics of their environmental impact. By galvanizing efforts to quantify and report our impact, hospitalists have the opportunity to be a role model for the industry and increase trust within their communities.
Individual Actions
What can a hospitalist do today? First, simple measures, like turning off idle electronics, recycling appropriately, or avoiding the use of unnecessary supplies or tests, are behavioral steps in the right direction. Second, just as education, goal setting, and feedback have met success in improving hand hygiene,23 we must begin the hard work of developing programs to monitor our environmental impact. Individual hospitalist carbon scores may help intensify efforts and spur improvement. Finally, we should learn and celebrate each other’s success. Renewed focus on this topic with increased reporting of interventions and outcomes is needed.
CONCLUSIONS
As hospitalists, we must look within ourselves to protect our planet and advocate for solutions that assure a sustainable future. By recognizing that a healthy environment is crucial to human health, we can set an example for other industries and create a safer world for our patients. Eliminating the harm we do is the first step in this process.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Chopra is supported by a Career Development Award from the Agency for Healthcare Quality and Research (1-K08-HS-022835-01).
1. Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. Ahmad S, ed. PLoS One. 2016;11(6):e0157014. doi:10.1371/journal.pone.0157014. PubMed
2. Windfeld ES, Brooks MS-L. Medical waste management–A review. J Environ Manage. 2015;163:98-108. doi:10.1016/j.jenvman.2015.08.013. PubMed
3. Environmental Protection Agency. Saving Water in Hospitals. Available at: https://www.epa.gov/sites/production/files/2017-01/documents/ws-commercial-factsheet-hospitals.pdf. Accessed December 9, 2017.
4. Kolpin DW, Furlong ET, Meyer MT, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: A national reconnaissance. Environ Sci & Technol 2002;36(6):1202-1211. PubMed
5. Deo, RP, Halden, RU. Pharmaceuticals in the built and natural water environment of the United States. Water. 2013;5(3):1346-1365. doi:10.3390/w5031346.
6. Lim SS, Vos T, Flaxman AD, Danaei G, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013; 380: 2224-60. PubMed
7. Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American heart association. Circulation. 2010;121(21):2331-2378. doi:10.1161/CIR.0b013e3181dbece1. PubMed
8. Watts N, Adger WN, Ayeb-Karlsson S, et al. The Lancet countdown: tracking progress on health and climate change. Lancet. 2017;389(10074):1151-1164. doi:10.1016/S0140-6736(16)32124-9. PubMed
9. Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet. 2015;386(10007):1973–2028. PubMed
10. Kaiser Permanente. Environmental Stewardship. Available at: https://share.kaiserpermanente.org/article/environmental-stewardship-overview/. Accessed December 2, 2017.
11. Health Facilities Management Magazine. Cleveland Clinic makes carbon-neutrality its newest sustainability goal. Available at: https://www.hfmmagazine.com/articles/3210-cleveland-clinic-makes-carbon-neutrality-its-newest-sustainability-goal?lipi=urn%3Ali%3Apage%3Ad_flagship3_feed%3BHXuZOUrpQUu0OQ3RcUQqEg%3D%3D. Accessed December 2, 2017.
12. Healthcare without Harm. Metropolitan Boston Health Care Energy & Greenhouse Gas Profile: 2011 through 2015, and 2020 Projection. Available at: https://noharm-uscanada.org/sites/default/files/documents-files/4723/Report-Boston%20Health%20Care%20Energy%20Profile-May%202017.pdf Accessed December 9, 2017.
13. Practice Greenhealth. Advancing sustainability in healthcare: a collection of special case studies. Available at: https://practicegreenhealth.org/sites/default/files/upload-files/hhi.case_.studies.pdf. Accessed July 22, 2017.
14. Macpherson C, Hill J. Are physicians obliged to lead environmental sustainability efforts in health care organizations? AMA J Ethics. 2017;19(12):1164-1173. doi:10.1001/journalofethics.2017.19.12.ecas2-1712. PubMed
15. American Nurses Association. ANA’s principles of environmental health for nursing practice with implementation strategies. Available at: http://www.nursingworld.org/MainMenuCategories/WorkplaceSafety/Healthy-Nurse/ANAsPrinciplesofEnvironmentalHealthforNursingPractice.pd. Accessed December 9, 2017.
16. Maibach EW, Kreslake JM, Roser-Renouf C, et al. Do Americans understand that global warming is harmful to human health? Evidence from a national survey. Ann Glob Health. 2015;81(3):396-409. doi:10.1016/j.aogh.2015.08.010. PubMed
17. Healthcare without Harm. Reducing Healthcare’s Climate Footprint: opportunities for European Hospitals & Health Systems. Available at: https://noharm-europe.org/sites/default/files/documents-files/4746/HCWHEurope_Climate_Report_Dec2016.pdf. Accessed May 22, 2017.
18. Campion, N, Thiel, CL, Woods, et al. Sustainable healthcare and environmental life-cycle impacts of disposable supplies: a focus on disposable custom packs. J Clean Prod. 2015;94:46-55. doi:10.1016/j.jclepro.2015.01.076.
19. Eckelman M, Mosher M, Gonzalez A, et al. Comparative life cycle assessment of disposable and reusable laryngeal mask airways: Anesth Analg. 2012;114(5):1067-1072. doi:10.1213/ANE.0b013e31824f6959. PubMed
20. Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci & Technol. 2015;49(3):1779-1786. doi:10.1021/es504719g. PubMed
21. Hanna-Attisha M, LaChance J, Sadler RC, et al. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. PubMed
22. Sustainable Development Unit. Sustainability and the NHS, Public Health and Social Care system–Ipsos Mori survey. Available at: https://www.sduhealth.org.uk/policy-strategy/reporting/ipsos-mori.aspx. Accessed December 9, 2017.
23. Luangasanatip N, Hongsuwan M, Limmathurotsakul D, et al. Comparative efficacy of interventions to promote hand hygiene in hospital: systematic review and network meta-analysis. BMJ. 2015;351:h3728. doi:10.1136/bmj.h3728. PubMed
1. Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. Ahmad S, ed. PLoS One. 2016;11(6):e0157014. doi:10.1371/journal.pone.0157014. PubMed
2. Windfeld ES, Brooks MS-L. Medical waste management–A review. J Environ Manage. 2015;163:98-108. doi:10.1016/j.jenvman.2015.08.013. PubMed
3. Environmental Protection Agency. Saving Water in Hospitals. Available at: https://www.epa.gov/sites/production/files/2017-01/documents/ws-commercial-factsheet-hospitals.pdf. Accessed December 9, 2017.
4. Kolpin DW, Furlong ET, Meyer MT, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: A national reconnaissance. Environ Sci & Technol 2002;36(6):1202-1211. PubMed
5. Deo, RP, Halden, RU. Pharmaceuticals in the built and natural water environment of the United States. Water. 2013;5(3):1346-1365. doi:10.3390/w5031346.
6. Lim SS, Vos T, Flaxman AD, Danaei G, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013; 380: 2224-60. PubMed
7. Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American heart association. Circulation. 2010;121(21):2331-2378. doi:10.1161/CIR.0b013e3181dbece1. PubMed
8. Watts N, Adger WN, Ayeb-Karlsson S, et al. The Lancet countdown: tracking progress on health and climate change. Lancet. 2017;389(10074):1151-1164. doi:10.1016/S0140-6736(16)32124-9. PubMed
9. Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet. 2015;386(10007):1973–2028. PubMed
10. Kaiser Permanente. Environmental Stewardship. Available at: https://share.kaiserpermanente.org/article/environmental-stewardship-overview/. Accessed December 2, 2017.
11. Health Facilities Management Magazine. Cleveland Clinic makes carbon-neutrality its newest sustainability goal. Available at: https://www.hfmmagazine.com/articles/3210-cleveland-clinic-makes-carbon-neutrality-its-newest-sustainability-goal?lipi=urn%3Ali%3Apage%3Ad_flagship3_feed%3BHXuZOUrpQUu0OQ3RcUQqEg%3D%3D. Accessed December 2, 2017.
12. Healthcare without Harm. Metropolitan Boston Health Care Energy & Greenhouse Gas Profile: 2011 through 2015, and 2020 Projection. Available at: https://noharm-uscanada.org/sites/default/files/documents-files/4723/Report-Boston%20Health%20Care%20Energy%20Profile-May%202017.pdf Accessed December 9, 2017.
13. Practice Greenhealth. Advancing sustainability in healthcare: a collection of special case studies. Available at: https://practicegreenhealth.org/sites/default/files/upload-files/hhi.case_.studies.pdf. Accessed July 22, 2017.
14. Macpherson C, Hill J. Are physicians obliged to lead environmental sustainability efforts in health care organizations? AMA J Ethics. 2017;19(12):1164-1173. doi:10.1001/journalofethics.2017.19.12.ecas2-1712. PubMed
15. American Nurses Association. ANA’s principles of environmental health for nursing practice with implementation strategies. Available at: http://www.nursingworld.org/MainMenuCategories/WorkplaceSafety/Healthy-Nurse/ANAsPrinciplesofEnvironmentalHealthforNursingPractice.pd. Accessed December 9, 2017.
16. Maibach EW, Kreslake JM, Roser-Renouf C, et al. Do Americans understand that global warming is harmful to human health? Evidence from a national survey. Ann Glob Health. 2015;81(3):396-409. doi:10.1016/j.aogh.2015.08.010. PubMed
17. Healthcare without Harm. Reducing Healthcare’s Climate Footprint: opportunities for European Hospitals & Health Systems. Available at: https://noharm-europe.org/sites/default/files/documents-files/4746/HCWHEurope_Climate_Report_Dec2016.pdf. Accessed May 22, 2017.
18. Campion, N, Thiel, CL, Woods, et al. Sustainable healthcare and environmental life-cycle impacts of disposable supplies: a focus on disposable custom packs. J Clean Prod. 2015;94:46-55. doi:10.1016/j.jclepro.2015.01.076.
19. Eckelman M, Mosher M, Gonzalez A, et al. Comparative life cycle assessment of disposable and reusable laryngeal mask airways: Anesth Analg. 2012;114(5):1067-1072. doi:10.1213/ANE.0b013e31824f6959. PubMed
20. Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci & Technol. 2015;49(3):1779-1786. doi:10.1021/es504719g. PubMed
21. Hanna-Attisha M, LaChance J, Sadler RC, et al. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. PubMed
22. Sustainable Development Unit. Sustainability and the NHS, Public Health and Social Care system–Ipsos Mori survey. Available at: https://www.sduhealth.org.uk/policy-strategy/reporting/ipsos-mori.aspx. Accessed December 9, 2017.
23. Luangasanatip N, Hongsuwan M, Limmathurotsakul D, et al. Comparative efficacy of interventions to promote hand hygiene in hospital: systematic review and network meta-analysis. BMJ. 2015;351:h3728. doi:10.1136/bmj.h3728. PubMed
© 2018 Society of Hospital Medicine
Opportunities and Challenges for Improving the Patient Experience in the Acute and Post–Acute Care Setting Using Patient Portals: The Patient’s Perspective
To realize the vision of patient-centered care, efforts are focusing on engaging patients and “care partners,” often a family caregiver, by using patient-facing technologies.1-4 Web-based patient portals linked to the electronic health record (EHR) provide patients and care partners with the ability to access personal health information online and to communicate with clinicians. In recent years, institutions have been increasing patient portal offerings to improve the patient experience, promote safety, and optimize healthcare delivery.5-7
DRIVERS OF ADOPTION
The adoption of patient portals has been driven by federal incentive programs (Meaningful Use), efforts by the Center for Medicare and Medicaid Services, and the Office of the National Coordinator for Health Information Technology to improve patient outcomes and the transition toward value-based reimbursement.2,8,9 The vast majority of use has been in ambulatory settings; use for acute care is nascent at best.10 Among hospitalized patients, few bring an internet-enabled computer or mobile device to access personal health records online.11 However, evidence suggests that care partners will use portals on behalf of acutely ill patients.4 As the Caregiver Advise, Record, Enable Act is implemented, hospitals will be required to identify patients’ care partners during hospitalization, inform them when the patient is ready for discharge, and provide self-management instructions during the transition home.12 In this context, understanding how best to leverage acute care patient portals will be important to institutions, clinicians, and vendors.
CURRENT KNOWLEDGE
The literature regarding acute care patient portals is rapidly growing.4,10 Hospitalized patients have unmet information and communication needs, and hospital-based clinicians struggle to meet these needs in a timely manner.13-15 In general, patients feel that using a mobile device to access personal health records has the potential to improve their experience.11 Early studies suggest that acute care patient portals can promote patient-centered communication and collaboration during hospitalization, including in intensive care settings.4,16,17 Furthermore, the use of acute care patient portals can improve perception of safety and quality, decrease anxiety, and increase understanding of health conditions.3,14 Although early evidence is promising, considerable knowledge gaps exist regarding patient outcomes over the acute episode of care.10,18
OUTSTANDING QUESTIONS
A clear area of interest is accessing acute care patient portals via mobile technology to engage patients during recovery from hospitalization.4,11 Although we do not yet know whether use during care transitions will favorably impact outcomes, given the high rate of harm after discharge, this seems likely.19 The few studies evaluating the effect on validated measures of engagement (Patient Activation Measure) and hospital readmissions have not shown demonstrable improvement to date.20,21 Clearly, optimizing acute care patient portals with regard to patient-clinician communication, as well as the type, timing, and format of information delivered, will be necessary to maximize value.4,22
From the patient’s perspective, there is much we can learn.23 Is the information that is presented pertinent, timely, and easy to understand? Will the use of portals detract from face-to-face interactions? Does greater transparency foster more accountability? Achieving an appropriate balance of digital health-information sharing for hospitalized patients is challenging given the sensitivity of patient data when diagnoses are uncertain and treatments are in flux.4,24 These questions must be answered as hospitals implement acute care patient portals.
ACUTE CARE PATIENT PORTAL TASK FORCE
To start addressing knowledge gaps, we established a task force of 21 leading researchers, informatics and policy experts, and clinical leaders. The Acute Care Patient Portal Task Force was a subgroup of the Libretto Consortium, a collaboration of 4 academic medical centers established by the Gordon and Betty Moore Foundation to design, develop, and implement technologies to engage patients, care partners, and providers in preventing harm in hospital settings. Initially, we were challenged with assessing stakeholders’ perspectives from early adopter institutions. We learned that acute care patient portals must offer an integrated experience across care settings, humanize the patient-clinician relationship, enable equitable access, and align with institutional strategy to promote sustainability.19
Cognitive Support
The opportunities identified include acclimatizing and assimilating to the hospital environment (reviewing policies and patient rights) and facilitating self-education and preparation by linking to personal health information and providing structured guidance at transitions.4 For example, a care partner of an incapacitated patient may watch a video to orient to the intensive care unit, navigate educational content linked to the patient’s admission diagnosis (pneumonia) entered in the EHR, view the timing of an upcoming imaging study (chest computed tomography scan), and complete a standardized checklist prior to discharge.
The main challenges we identified include ensuring accuracy of hospital-, unit-, and patient-level information, addressing information overload, configuring notification and display settings to optimize the user experience, presenting information at an appropriate health literacy level,4,21 and addressing security and privacy concerns when expanding access to family members.24
Respect and Boundaries
Opportunities identified include supporting individual learning styles by using interactive features of mobile devices to improve comprehension for visual, auditory, and tactile learners and reinforcing learning through the use of various types of digital media.25-27 For example, a visual learner may view a video tutorial for a newly prescribed medication. A tactile learner may prefer to use interactive graphical displays that exploit multidimensional touch capabilities of mobile devices to learn about active conditions or an upcoming procedure. An auditory learner may choose to use intelligent personal assistants to navigate their plan of care (“Hey Siri, what is my schedule for today?”). By addressing the learning preferences of patients and time constraints of clinicians, institutions can use acute care patient portals to promote more respectful interactions and collaborative decision-making during important care processes, such as obtaining surgical consent.28,29
We also identified opportunities to facilitate personalization by tailoring educational content and by enabling the use of patient-generated health data collected from wearable devices. For example, patients may prefer to interact with a virtual advocate to review discharge instructions (“Louis” in Project Re-Engineered Discharge) when personalized to their demographics and health literacy level.30-32 Patients may choose to upload step counts from wearable devices so that clinicians can monitor activity goals in preparation for discharge and while recovering afterwards. When supported in these ways, acute care patient portals allow patients to have more meaningful interactions with clinicians about diagnoses, treatments, prognosis, and goals for recovery.
The main challenges we identified include balancing interactions with technology and clinicians, ensuring clinicians understand how patients from different socioeconomic backgrounds use existing and newer technology to enhance self-management, assessing health and technology literacy, and understanding individual preferences for sharing patient-generated health data. Importantly, we must remain vigilant that patients will express concern about overdependence on technology, especially if it detracts from in-person interaction; our panelists emphasized that technology should never replace “human touch.”
Patient and Family Empowerment
The opportunities identified include promoting patient-centered communication by supporting a real-time and asynchronous dialogue among patients, care partners, and care team members (including ambulatory clinicians) while minimizing conversational silos4,33; displaying names, roles, and pictures of all care team members4,34; fostering transparency by sharing clinician documentation in progress notes and sign-outs35; ensuring accountability for a single plan of care spanning shift changes and handoffs, and providing a mechanism to enable real-time feedback.
Hospitalization can be a vulnerable and isolating experience, perpetuated by a lack of timely and coordinated communication with the care team. We identified opportunities to mitigate anxiety by promoting shared understanding when questions require input from multiple clinicians, when team members change, or when patients wish to communicate with their longitudinal ambulatory providers.4,34 For example, inviting patients to review clinicians’ progress notes should stimulate more open and meaningful communication.35 Furthermore, requesting that patients state their wishes, preferences, and goals could improve overall concordance with care team members.36,37 Empowering patients and care partners to voice their concerns, particularly those related to miscommunication, may mitigate harm propagated by handoffs, shift work, and weekend coverage.38,39 While reporting safety concerns represents a novel mechanism to augment medical-error reporting by clinicians alone,23,40 this strategy will be most effective when aligned with standardized communication initiatives (I-PASS) that have been proven to reduce medical errors and preventable adverse events and are being implemented nationally.41 Finally, by leveraging tools that facilitate instantaneous feedback, patients can be empowered to react to their plan (ranking skilled nursing facility options) as it is developed.
The main challenges we identified include managing expectations regarding the use of communication tools, accurately and reliably identifying care team members in the EHR,34 acknowledging patients as equal partners, ensuring patients receive a consistent message about diagnoses and therapies during handoffs and when multiple consultants have conflicting opinions about the plan,37 and addressing patient concerns fairly and respectfully.
RECOMMENDATIONS AND CONCLUSIONS
In summary, the patient-centered themes we identified serve as guiding principles for institutions, clinicians, and vendors who wish to use patient portals to improve the acute and postacute care patient experience. One central message resonates: Patients do not simply want access to their health information and the ability to communicate with the clinicians who furnish this information; they want to feel supported, respected, and empowered when doing so. It is only through partnership with patients and their advocates that we can fully realize the impact of digital technologies when patients are in their most vulnerable state.
Acknowledgments
The authors thank their colleagues and the patient and family advocates who contributed to this body of work as part of the Acute Care Patient Portal Task Force and conference: Brittany Couture; Ronen Rozenblum, PhD, MPH; Jennifer Prey, MPhil, MS, PhD; Kristin O’Reilly, RN, BSN, MPH; Patricia Q. Bourie, RN, MS, Cindy Dwyer, RN, BSN,S; Ryan Greysen, MD, MHS, MA; Jeffery Smith, MPP; Michael Gropper, MD, PhD; Patricia Dykes, RN, PhD; Martha B. Carnie; Jeffrey W. Mello; and Jane Webster.
Disclosure
Anuj K. Dalal, MD, David W. Bates, MD, MSc, and Sarah Collins, RN, PhD, are responsible for the conception or design of the work; acquisition, analysis, or interpretation of data; drafting the work or revising it critically for important intellectual content; and final approval of the version to be published. The authors agree to be accountable for all aspects of the work and to ensure that questions related to the accuracy or integrity of the work are appropriately investigated and resolved. This work was supported by a grant from the Gordon and Betty Moore Foundation ([GBMF] #4993). GBMF had no role in the design or conduct of the study; the collection, analysis, or interpretation of data; or preparation or review of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of GBMF. The authors report no conflicts of interest.
1. Sarkar U, Bates DW. Care partners and online patient portals. JAMA. 2014;311(4):357-358. PubMed
2. Grando MA, Rozenblum R, Bates DW, eds. Information Technology for Patient Empowerment in Healthcare, 1st Edition. Berlin: Walter de Gruyter Inc.; 2015.
3. Kelly MM, Hoonakker PLT, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. J Am Med Inform Assoc. 2016;24(1):153-161. PubMed
4. Dalal AK, Dykes PC, Collins S, et al. A web-based, patient-centered toolkit to engage patients and caregivers in the acute care setting: A preliminary evaluation. J Am Med Inform Assoc. 2016;23(1):80-87. PubMed
5. Prey JE, Restaino S, Vawdrey DK. Providing hospital patients with access to their medical records. AMIA Annu Symp Proc. 2014;2014:1884-1893. PubMed
6. Herrin J, Harris KG, Kenward K, Hines S, Joshi MS, Frosch DL. Patient and family engagement: A survey of US hospital practices. BMJ Qual Saf. 2016;25(3):182-189. PubMed
7. Tom JO, Mangione-Smith R, Solomon C, Grossman DC. Integrated personal health record use: Association with parent-reported care experiences. Pediatrics. 2012;130(1):e183-e190. PubMed
8. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Stage 2. Federal Register Final Rule. Sect. 170; 2012. https://www.federalregister.gov/documents/2012/03/07/2012-4443/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-2. Accessed March 1, 2017.
9. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; merit-based incentive payment system (MIPS) and alternative payment model (APM) incentive under the physician fee schedule, and criteria for physician-focused payment models. Final rule with comment period. Fed Regist. 2016;81(214):77008-77831. PubMed
10. Prey JE, Woollen J, Wilcox L, et al. Patient engagement in the inpatient setting: A systematic review. J Am Med Informat Assoc. 2014;21(4):742-750. PubMed
11. Ludwin S, Greysen SR. Use of smartphones and mobile devices in hospitalized patients: Untapped opportunities for inpatient engagement. J Hosp Med. 2015;10(7):459-461. PubMed
12. Coleman EA. Family caregivers as partners in care transitions: The caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. PubMed
13. Kaziunas E, Hanauer DA, Ackerman MS, Choi SW. Identifying unmet informational needs in the inpatient setting to increase patient and caregiver engagement in the context of pediatric hematopoietic stem cell transplantation. J Am Med Inform Assoc. 2016;23(1):94-104. PubMed
14. Woollen J, Prey J, Wilcox L, et al. Patient experiences using an inpatient personal health record. Appl Clin Inform. 2016;7(2):446-460. PubMed
15. Irizarry T, DeVito Dabbs A, Curran CR. Patient portals and patient engagement: A state of the science review. J Med Internet Res. 2015;17(6):e148. doi:10.2196/jmir.4255. PubMed
16. Vawdrey DK, Wilcox LG, Collins SA, et al. A tablet computer application for patients to participate in their hospital care. AMIA Annu Symp Proc. 2011;2011:1428-1435. PubMed
17. Collins SA, Rozenblum R, Leung WY, et al. Acute care patient portals: A qualitative study of stakeholder perspectives on current practices. J Am Med Inform Assoc. 2016;24(e1):e9-e17. PubMed
18. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: A systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
19. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
20. Griffin A, Skinner A, Thornhill J, Weinberger M. Patient Portals: Who uses them? What features do they use? And do they reduce hospital readmissions? Appl Clin Inform. 2016;7(2):489-501. PubMed
21. O’Leary KJ, Lohman ME, Culver E, Killarney A, Randy Smith G Jr, Liebovitz DM. The effect of tablet computers with a mobile patient portal application on hospitalized patients’ knowledge and activation. J Am Med Inform Assoc. 2016;23(1):159-165. PubMed
22. O’Leary KJ, Sharma RK, Killarney A, et al. Patients’ and Healthcare Providers’ Perceptions of a Mobile Portal Application for Hospitalized Patients. BMC Med Inform Decis Mak. 2016;16(1):123. PubMed
23. Pell JM, Mancuso M, Limon S, Oman K, Lin CT. Patient access to electronic health records during hospitalization. JAMA Intern Med. 2015;175(5):856-858. PubMed
24. Brown SM, Aboumatar HJ, Francis L, et al. Balancing digital information-sharing and patient privacy when engaging families in the intensive care unit. J Am Med Inform Assoc. 2016;23(5):995-1000. PubMed
25. Krishna S, Francisco BD, Balas EA, et al. Internet-enabled interactive multimedia asthma education program: A randomized trial. Pediatrics. 2003;111(3):503-510. PubMed
26. Fox MP. A systematic review of the literature reporting on studies that examined the impact of interactive, computer-based patient education programs. Patient Educ Couns. 2009;77(1):6-13. PubMed
27. Morgan ER, Laing K, McCarthy J, McCrate F, Seal MD. Using tablet-based technology in patient education about systemic therapy options for early-stage breast cancer: A pilot study. Curr Oncol. 2015;22(5):e364-e369. PubMed
28. Nehme J, El-Khani U, Chow A, Hakky S, Ahmed AR, Purkayastha S. The use of multimedia consent programs for surgical procedures: A systematic review. Surg Innov. 2013;20(1):13-23. PubMed
29. Waller A, Forshaw K, Carey M, et al. Optimizing patient preparation and surgical experience using eHealth technology. JMIR Med Inform. 2015;3(3):e29. PubMed
30. Abbott MB, Shaw P. Virtual nursing avatars: Nurse roles and evolving concepts of care. Online J Issues Nurs. 2016;21(3):7. PubMed
31. Cawthon C, Walia S, Osborn CY, Niesner KJ, Schnipper JL, Kripalani S. Improving care transitions: The patient perspective. J Health Commun. 2012;17 Suppl 3:312-324. PubMed
32. Bickmore TW, Pfeifer LM, Byron D, et al. Usability of conversational agents by patients with inadequate health literacy: Evidence from two clinical trials. J Health Commun. 2010;15 Suppl 2:197-210. PubMed
33. 2017;376(20):1905-1907. N Engl J Med.42. Mandl KD, Kohane IS. A 21st-century health IT system—creating a real-world information economy. PubMed
34. 2014;371(19):1803-1812.N Engl J Med41. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. PubMed
35. 2016;24(1):153-161.J Am Med Inform Assoc.40. Kelly MM, Hoonakker PLT, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. PubMed
36. 2017;171(4):372-381.JAMA Pediatr.39. Khan A, Coffey M, Litterer KP, et al. Families as partners in hospital error and adverse event surveillance. PubMed
37. 2017;17(4):389-402.Acad Pediatr.38. Khan A, Baird J, Rogers JE, et al. Parent and provider experience and shared understanding after a family-centered nighttime communication intervention. PubMed
38. 2016;6(6):319-329.Hosp Pediatr. 37. Khan A, Rogers JE, Forster CS, Furtak SL, Schuster MA, Landrigan CP. Communication and shared understanding between parents and resident-physicians at night. PubMed
39. 2016;11(9):615-619.J Hosp Med36. Figueroa JF, Schnipper JL, McNally K, Stade D, Lipsitz SR, Dalal AK. How often are hospitalized patients and providers on the same page with regard to the patient’s primary recovery goal for hospitalization? PubMed
40. 2013;8(7):414-417.J Hosp Med.35. Feldman HJ, Walker J, Li J, Delbanco T. OpenNotes: Hospitalists’ challenge and opportunity. PubMed
41. 2016;11(5):381-385.J Hosp Med.34. Dalal AK, Schnipper JL. Care team identification in the electronic health record: A critical first step for patient-centered communication.PubMed
42. 2016;24(e1):e178-e184.J Am Med Inform Assoc.33. Dalal AK, Schnipper J, Massaro A, et al. A web-based and mobile patient-centered “microblog” messaging platform to improve care team communication in acute care. PubMed
To realize the vision of patient-centered care, efforts are focusing on engaging patients and “care partners,” often a family caregiver, by using patient-facing technologies.1-4 Web-based patient portals linked to the electronic health record (EHR) provide patients and care partners with the ability to access personal health information online and to communicate with clinicians. In recent years, institutions have been increasing patient portal offerings to improve the patient experience, promote safety, and optimize healthcare delivery.5-7
DRIVERS OF ADOPTION
The adoption of patient portals has been driven by federal incentive programs (Meaningful Use), efforts by the Center for Medicare and Medicaid Services, and the Office of the National Coordinator for Health Information Technology to improve patient outcomes and the transition toward value-based reimbursement.2,8,9 The vast majority of use has been in ambulatory settings; use for acute care is nascent at best.10 Among hospitalized patients, few bring an internet-enabled computer or mobile device to access personal health records online.11 However, evidence suggests that care partners will use portals on behalf of acutely ill patients.4 As the Caregiver Advise, Record, Enable Act is implemented, hospitals will be required to identify patients’ care partners during hospitalization, inform them when the patient is ready for discharge, and provide self-management instructions during the transition home.12 In this context, understanding how best to leverage acute care patient portals will be important to institutions, clinicians, and vendors.
CURRENT KNOWLEDGE
The literature regarding acute care patient portals is rapidly growing.4,10 Hospitalized patients have unmet information and communication needs, and hospital-based clinicians struggle to meet these needs in a timely manner.13-15 In general, patients feel that using a mobile device to access personal health records has the potential to improve their experience.11 Early studies suggest that acute care patient portals can promote patient-centered communication and collaboration during hospitalization, including in intensive care settings.4,16,17 Furthermore, the use of acute care patient portals can improve perception of safety and quality, decrease anxiety, and increase understanding of health conditions.3,14 Although early evidence is promising, considerable knowledge gaps exist regarding patient outcomes over the acute episode of care.10,18
OUTSTANDING QUESTIONS
A clear area of interest is accessing acute care patient portals via mobile technology to engage patients during recovery from hospitalization.4,11 Although we do not yet know whether use during care transitions will favorably impact outcomes, given the high rate of harm after discharge, this seems likely.19 The few studies evaluating the effect on validated measures of engagement (Patient Activation Measure) and hospital readmissions have not shown demonstrable improvement to date.20,21 Clearly, optimizing acute care patient portals with regard to patient-clinician communication, as well as the type, timing, and format of information delivered, will be necessary to maximize value.4,22
From the patient’s perspective, there is much we can learn.23 Is the information that is presented pertinent, timely, and easy to understand? Will the use of portals detract from face-to-face interactions? Does greater transparency foster more accountability? Achieving an appropriate balance of digital health-information sharing for hospitalized patients is challenging given the sensitivity of patient data when diagnoses are uncertain and treatments are in flux.4,24 These questions must be answered as hospitals implement acute care patient portals.
ACUTE CARE PATIENT PORTAL TASK FORCE
To start addressing knowledge gaps, we established a task force of 21 leading researchers, informatics and policy experts, and clinical leaders. The Acute Care Patient Portal Task Force was a subgroup of the Libretto Consortium, a collaboration of 4 academic medical centers established by the Gordon and Betty Moore Foundation to design, develop, and implement technologies to engage patients, care partners, and providers in preventing harm in hospital settings. Initially, we were challenged with assessing stakeholders’ perspectives from early adopter institutions. We learned that acute care patient portals must offer an integrated experience across care settings, humanize the patient-clinician relationship, enable equitable access, and align with institutional strategy to promote sustainability.19
Cognitive Support
The opportunities identified include acclimatizing and assimilating to the hospital environment (reviewing policies and patient rights) and facilitating self-education and preparation by linking to personal health information and providing structured guidance at transitions.4 For example, a care partner of an incapacitated patient may watch a video to orient to the intensive care unit, navigate educational content linked to the patient’s admission diagnosis (pneumonia) entered in the EHR, view the timing of an upcoming imaging study (chest computed tomography scan), and complete a standardized checklist prior to discharge.
The main challenges we identified include ensuring accuracy of hospital-, unit-, and patient-level information, addressing information overload, configuring notification and display settings to optimize the user experience, presenting information at an appropriate health literacy level,4,21 and addressing security and privacy concerns when expanding access to family members.24
Respect and Boundaries
Opportunities identified include supporting individual learning styles by using interactive features of mobile devices to improve comprehension for visual, auditory, and tactile learners and reinforcing learning through the use of various types of digital media.25-27 For example, a visual learner may view a video tutorial for a newly prescribed medication. A tactile learner may prefer to use interactive graphical displays that exploit multidimensional touch capabilities of mobile devices to learn about active conditions or an upcoming procedure. An auditory learner may choose to use intelligent personal assistants to navigate their plan of care (“Hey Siri, what is my schedule for today?”). By addressing the learning preferences of patients and time constraints of clinicians, institutions can use acute care patient portals to promote more respectful interactions and collaborative decision-making during important care processes, such as obtaining surgical consent.28,29
We also identified opportunities to facilitate personalization by tailoring educational content and by enabling the use of patient-generated health data collected from wearable devices. For example, patients may prefer to interact with a virtual advocate to review discharge instructions (“Louis” in Project Re-Engineered Discharge) when personalized to their demographics and health literacy level.30-32 Patients may choose to upload step counts from wearable devices so that clinicians can monitor activity goals in preparation for discharge and while recovering afterwards. When supported in these ways, acute care patient portals allow patients to have more meaningful interactions with clinicians about diagnoses, treatments, prognosis, and goals for recovery.
The main challenges we identified include balancing interactions with technology and clinicians, ensuring clinicians understand how patients from different socioeconomic backgrounds use existing and newer technology to enhance self-management, assessing health and technology literacy, and understanding individual preferences for sharing patient-generated health data. Importantly, we must remain vigilant that patients will express concern about overdependence on technology, especially if it detracts from in-person interaction; our panelists emphasized that technology should never replace “human touch.”
Patient and Family Empowerment
The opportunities identified include promoting patient-centered communication by supporting a real-time and asynchronous dialogue among patients, care partners, and care team members (including ambulatory clinicians) while minimizing conversational silos4,33; displaying names, roles, and pictures of all care team members4,34; fostering transparency by sharing clinician documentation in progress notes and sign-outs35; ensuring accountability for a single plan of care spanning shift changes and handoffs, and providing a mechanism to enable real-time feedback.
Hospitalization can be a vulnerable and isolating experience, perpetuated by a lack of timely and coordinated communication with the care team. We identified opportunities to mitigate anxiety by promoting shared understanding when questions require input from multiple clinicians, when team members change, or when patients wish to communicate with their longitudinal ambulatory providers.4,34 For example, inviting patients to review clinicians’ progress notes should stimulate more open and meaningful communication.35 Furthermore, requesting that patients state their wishes, preferences, and goals could improve overall concordance with care team members.36,37 Empowering patients and care partners to voice their concerns, particularly those related to miscommunication, may mitigate harm propagated by handoffs, shift work, and weekend coverage.38,39 While reporting safety concerns represents a novel mechanism to augment medical-error reporting by clinicians alone,23,40 this strategy will be most effective when aligned with standardized communication initiatives (I-PASS) that have been proven to reduce medical errors and preventable adverse events and are being implemented nationally.41 Finally, by leveraging tools that facilitate instantaneous feedback, patients can be empowered to react to their plan (ranking skilled nursing facility options) as it is developed.
The main challenges we identified include managing expectations regarding the use of communication tools, accurately and reliably identifying care team members in the EHR,34 acknowledging patients as equal partners, ensuring patients receive a consistent message about diagnoses and therapies during handoffs and when multiple consultants have conflicting opinions about the plan,37 and addressing patient concerns fairly and respectfully.
RECOMMENDATIONS AND CONCLUSIONS
In summary, the patient-centered themes we identified serve as guiding principles for institutions, clinicians, and vendors who wish to use patient portals to improve the acute and postacute care patient experience. One central message resonates: Patients do not simply want access to their health information and the ability to communicate with the clinicians who furnish this information; they want to feel supported, respected, and empowered when doing so. It is only through partnership with patients and their advocates that we can fully realize the impact of digital technologies when patients are in their most vulnerable state.
Acknowledgments
The authors thank their colleagues and the patient and family advocates who contributed to this body of work as part of the Acute Care Patient Portal Task Force and conference: Brittany Couture; Ronen Rozenblum, PhD, MPH; Jennifer Prey, MPhil, MS, PhD; Kristin O’Reilly, RN, BSN, MPH; Patricia Q. Bourie, RN, MS, Cindy Dwyer, RN, BSN,S; Ryan Greysen, MD, MHS, MA; Jeffery Smith, MPP; Michael Gropper, MD, PhD; Patricia Dykes, RN, PhD; Martha B. Carnie; Jeffrey W. Mello; and Jane Webster.
Disclosure
Anuj K. Dalal, MD, David W. Bates, MD, MSc, and Sarah Collins, RN, PhD, are responsible for the conception or design of the work; acquisition, analysis, or interpretation of data; drafting the work or revising it critically for important intellectual content; and final approval of the version to be published. The authors agree to be accountable for all aspects of the work and to ensure that questions related to the accuracy or integrity of the work are appropriately investigated and resolved. This work was supported by a grant from the Gordon and Betty Moore Foundation ([GBMF] #4993). GBMF had no role in the design or conduct of the study; the collection, analysis, or interpretation of data; or preparation or review of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of GBMF. The authors report no conflicts of interest.
To realize the vision of patient-centered care, efforts are focusing on engaging patients and “care partners,” often a family caregiver, by using patient-facing technologies.1-4 Web-based patient portals linked to the electronic health record (EHR) provide patients and care partners with the ability to access personal health information online and to communicate with clinicians. In recent years, institutions have been increasing patient portal offerings to improve the patient experience, promote safety, and optimize healthcare delivery.5-7
DRIVERS OF ADOPTION
The adoption of patient portals has been driven by federal incentive programs (Meaningful Use), efforts by the Center for Medicare and Medicaid Services, and the Office of the National Coordinator for Health Information Technology to improve patient outcomes and the transition toward value-based reimbursement.2,8,9 The vast majority of use has been in ambulatory settings; use for acute care is nascent at best.10 Among hospitalized patients, few bring an internet-enabled computer or mobile device to access personal health records online.11 However, evidence suggests that care partners will use portals on behalf of acutely ill patients.4 As the Caregiver Advise, Record, Enable Act is implemented, hospitals will be required to identify patients’ care partners during hospitalization, inform them when the patient is ready for discharge, and provide self-management instructions during the transition home.12 In this context, understanding how best to leverage acute care patient portals will be important to institutions, clinicians, and vendors.
CURRENT KNOWLEDGE
The literature regarding acute care patient portals is rapidly growing.4,10 Hospitalized patients have unmet information and communication needs, and hospital-based clinicians struggle to meet these needs in a timely manner.13-15 In general, patients feel that using a mobile device to access personal health records has the potential to improve their experience.11 Early studies suggest that acute care patient portals can promote patient-centered communication and collaboration during hospitalization, including in intensive care settings.4,16,17 Furthermore, the use of acute care patient portals can improve perception of safety and quality, decrease anxiety, and increase understanding of health conditions.3,14 Although early evidence is promising, considerable knowledge gaps exist regarding patient outcomes over the acute episode of care.10,18
OUTSTANDING QUESTIONS
A clear area of interest is accessing acute care patient portals via mobile technology to engage patients during recovery from hospitalization.4,11 Although we do not yet know whether use during care transitions will favorably impact outcomes, given the high rate of harm after discharge, this seems likely.19 The few studies evaluating the effect on validated measures of engagement (Patient Activation Measure) and hospital readmissions have not shown demonstrable improvement to date.20,21 Clearly, optimizing acute care patient portals with regard to patient-clinician communication, as well as the type, timing, and format of information delivered, will be necessary to maximize value.4,22
From the patient’s perspective, there is much we can learn.23 Is the information that is presented pertinent, timely, and easy to understand? Will the use of portals detract from face-to-face interactions? Does greater transparency foster more accountability? Achieving an appropriate balance of digital health-information sharing for hospitalized patients is challenging given the sensitivity of patient data when diagnoses are uncertain and treatments are in flux.4,24 These questions must be answered as hospitals implement acute care patient portals.
ACUTE CARE PATIENT PORTAL TASK FORCE
To start addressing knowledge gaps, we established a task force of 21 leading researchers, informatics and policy experts, and clinical leaders. The Acute Care Patient Portal Task Force was a subgroup of the Libretto Consortium, a collaboration of 4 academic medical centers established by the Gordon and Betty Moore Foundation to design, develop, and implement technologies to engage patients, care partners, and providers in preventing harm in hospital settings. Initially, we were challenged with assessing stakeholders’ perspectives from early adopter institutions. We learned that acute care patient portals must offer an integrated experience across care settings, humanize the patient-clinician relationship, enable equitable access, and align with institutional strategy to promote sustainability.19
Cognitive Support
The opportunities identified include acclimatizing and assimilating to the hospital environment (reviewing policies and patient rights) and facilitating self-education and preparation by linking to personal health information and providing structured guidance at transitions.4 For example, a care partner of an incapacitated patient may watch a video to orient to the intensive care unit, navigate educational content linked to the patient’s admission diagnosis (pneumonia) entered in the EHR, view the timing of an upcoming imaging study (chest computed tomography scan), and complete a standardized checklist prior to discharge.
The main challenges we identified include ensuring accuracy of hospital-, unit-, and patient-level information, addressing information overload, configuring notification and display settings to optimize the user experience, presenting information at an appropriate health literacy level,4,21 and addressing security and privacy concerns when expanding access to family members.24
Respect and Boundaries
Opportunities identified include supporting individual learning styles by using interactive features of mobile devices to improve comprehension for visual, auditory, and tactile learners and reinforcing learning through the use of various types of digital media.25-27 For example, a visual learner may view a video tutorial for a newly prescribed medication. A tactile learner may prefer to use interactive graphical displays that exploit multidimensional touch capabilities of mobile devices to learn about active conditions or an upcoming procedure. An auditory learner may choose to use intelligent personal assistants to navigate their plan of care (“Hey Siri, what is my schedule for today?”). By addressing the learning preferences of patients and time constraints of clinicians, institutions can use acute care patient portals to promote more respectful interactions and collaborative decision-making during important care processes, such as obtaining surgical consent.28,29
We also identified opportunities to facilitate personalization by tailoring educational content and by enabling the use of patient-generated health data collected from wearable devices. For example, patients may prefer to interact with a virtual advocate to review discharge instructions (“Louis” in Project Re-Engineered Discharge) when personalized to their demographics and health literacy level.30-32 Patients may choose to upload step counts from wearable devices so that clinicians can monitor activity goals in preparation for discharge and while recovering afterwards. When supported in these ways, acute care patient portals allow patients to have more meaningful interactions with clinicians about diagnoses, treatments, prognosis, and goals for recovery.
The main challenges we identified include balancing interactions with technology and clinicians, ensuring clinicians understand how patients from different socioeconomic backgrounds use existing and newer technology to enhance self-management, assessing health and technology literacy, and understanding individual preferences for sharing patient-generated health data. Importantly, we must remain vigilant that patients will express concern about overdependence on technology, especially if it detracts from in-person interaction; our panelists emphasized that technology should never replace “human touch.”
Patient and Family Empowerment
The opportunities identified include promoting patient-centered communication by supporting a real-time and asynchronous dialogue among patients, care partners, and care team members (including ambulatory clinicians) while minimizing conversational silos4,33; displaying names, roles, and pictures of all care team members4,34; fostering transparency by sharing clinician documentation in progress notes and sign-outs35; ensuring accountability for a single plan of care spanning shift changes and handoffs, and providing a mechanism to enable real-time feedback.
Hospitalization can be a vulnerable and isolating experience, perpetuated by a lack of timely and coordinated communication with the care team. We identified opportunities to mitigate anxiety by promoting shared understanding when questions require input from multiple clinicians, when team members change, or when patients wish to communicate with their longitudinal ambulatory providers.4,34 For example, inviting patients to review clinicians’ progress notes should stimulate more open and meaningful communication.35 Furthermore, requesting that patients state their wishes, preferences, and goals could improve overall concordance with care team members.36,37 Empowering patients and care partners to voice their concerns, particularly those related to miscommunication, may mitigate harm propagated by handoffs, shift work, and weekend coverage.38,39 While reporting safety concerns represents a novel mechanism to augment medical-error reporting by clinicians alone,23,40 this strategy will be most effective when aligned with standardized communication initiatives (I-PASS) that have been proven to reduce medical errors and preventable adverse events and are being implemented nationally.41 Finally, by leveraging tools that facilitate instantaneous feedback, patients can be empowered to react to their plan (ranking skilled nursing facility options) as it is developed.
The main challenges we identified include managing expectations regarding the use of communication tools, accurately and reliably identifying care team members in the EHR,34 acknowledging patients as equal partners, ensuring patients receive a consistent message about diagnoses and therapies during handoffs and when multiple consultants have conflicting opinions about the plan,37 and addressing patient concerns fairly and respectfully.
RECOMMENDATIONS AND CONCLUSIONS
In summary, the patient-centered themes we identified serve as guiding principles for institutions, clinicians, and vendors who wish to use patient portals to improve the acute and postacute care patient experience. One central message resonates: Patients do not simply want access to their health information and the ability to communicate with the clinicians who furnish this information; they want to feel supported, respected, and empowered when doing so. It is only through partnership with patients and their advocates that we can fully realize the impact of digital technologies when patients are in their most vulnerable state.
Acknowledgments
The authors thank their colleagues and the patient and family advocates who contributed to this body of work as part of the Acute Care Patient Portal Task Force and conference: Brittany Couture; Ronen Rozenblum, PhD, MPH; Jennifer Prey, MPhil, MS, PhD; Kristin O’Reilly, RN, BSN, MPH; Patricia Q. Bourie, RN, MS, Cindy Dwyer, RN, BSN,S; Ryan Greysen, MD, MHS, MA; Jeffery Smith, MPP; Michael Gropper, MD, PhD; Patricia Dykes, RN, PhD; Martha B. Carnie; Jeffrey W. Mello; and Jane Webster.
Disclosure
Anuj K. Dalal, MD, David W. Bates, MD, MSc, and Sarah Collins, RN, PhD, are responsible for the conception or design of the work; acquisition, analysis, or interpretation of data; drafting the work or revising it critically for important intellectual content; and final approval of the version to be published. The authors agree to be accountable for all aspects of the work and to ensure that questions related to the accuracy or integrity of the work are appropriately investigated and resolved. This work was supported by a grant from the Gordon and Betty Moore Foundation ([GBMF] #4993). GBMF had no role in the design or conduct of the study; the collection, analysis, or interpretation of data; or preparation or review of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of GBMF. The authors report no conflicts of interest.
1. Sarkar U, Bates DW. Care partners and online patient portals. JAMA. 2014;311(4):357-358. PubMed
2. Grando MA, Rozenblum R, Bates DW, eds. Information Technology for Patient Empowerment in Healthcare, 1st Edition. Berlin: Walter de Gruyter Inc.; 2015.
3. Kelly MM, Hoonakker PLT, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. J Am Med Inform Assoc. 2016;24(1):153-161. PubMed
4. Dalal AK, Dykes PC, Collins S, et al. A web-based, patient-centered toolkit to engage patients and caregivers in the acute care setting: A preliminary evaluation. J Am Med Inform Assoc. 2016;23(1):80-87. PubMed
5. Prey JE, Restaino S, Vawdrey DK. Providing hospital patients with access to their medical records. AMIA Annu Symp Proc. 2014;2014:1884-1893. PubMed
6. Herrin J, Harris KG, Kenward K, Hines S, Joshi MS, Frosch DL. Patient and family engagement: A survey of US hospital practices. BMJ Qual Saf. 2016;25(3):182-189. PubMed
7. Tom JO, Mangione-Smith R, Solomon C, Grossman DC. Integrated personal health record use: Association with parent-reported care experiences. Pediatrics. 2012;130(1):e183-e190. PubMed
8. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Stage 2. Federal Register Final Rule. Sect. 170; 2012. https://www.federalregister.gov/documents/2012/03/07/2012-4443/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-2. Accessed March 1, 2017.
9. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; merit-based incentive payment system (MIPS) and alternative payment model (APM) incentive under the physician fee schedule, and criteria for physician-focused payment models. Final rule with comment period. Fed Regist. 2016;81(214):77008-77831. PubMed
10. Prey JE, Woollen J, Wilcox L, et al. Patient engagement in the inpatient setting: A systematic review. J Am Med Informat Assoc. 2014;21(4):742-750. PubMed
11. Ludwin S, Greysen SR. Use of smartphones and mobile devices in hospitalized patients: Untapped opportunities for inpatient engagement. J Hosp Med. 2015;10(7):459-461. PubMed
12. Coleman EA. Family caregivers as partners in care transitions: The caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. PubMed
13. Kaziunas E, Hanauer DA, Ackerman MS, Choi SW. Identifying unmet informational needs in the inpatient setting to increase patient and caregiver engagement in the context of pediatric hematopoietic stem cell transplantation. J Am Med Inform Assoc. 2016;23(1):94-104. PubMed
14. Woollen J, Prey J, Wilcox L, et al. Patient experiences using an inpatient personal health record. Appl Clin Inform. 2016;7(2):446-460. PubMed
15. Irizarry T, DeVito Dabbs A, Curran CR. Patient portals and patient engagement: A state of the science review. J Med Internet Res. 2015;17(6):e148. doi:10.2196/jmir.4255. PubMed
16. Vawdrey DK, Wilcox LG, Collins SA, et al. A tablet computer application for patients to participate in their hospital care. AMIA Annu Symp Proc. 2011;2011:1428-1435. PubMed
17. Collins SA, Rozenblum R, Leung WY, et al. Acute care patient portals: A qualitative study of stakeholder perspectives on current practices. J Am Med Inform Assoc. 2016;24(e1):e9-e17. PubMed
18. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: A systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
19. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
20. Griffin A, Skinner A, Thornhill J, Weinberger M. Patient Portals: Who uses them? What features do they use? And do they reduce hospital readmissions? Appl Clin Inform. 2016;7(2):489-501. PubMed
21. O’Leary KJ, Lohman ME, Culver E, Killarney A, Randy Smith G Jr, Liebovitz DM. The effect of tablet computers with a mobile patient portal application on hospitalized patients’ knowledge and activation. J Am Med Inform Assoc. 2016;23(1):159-165. PubMed
22. O’Leary KJ, Sharma RK, Killarney A, et al. Patients’ and Healthcare Providers’ Perceptions of a Mobile Portal Application for Hospitalized Patients. BMC Med Inform Decis Mak. 2016;16(1):123. PubMed
23. Pell JM, Mancuso M, Limon S, Oman K, Lin CT. Patient access to electronic health records during hospitalization. JAMA Intern Med. 2015;175(5):856-858. PubMed
24. Brown SM, Aboumatar HJ, Francis L, et al. Balancing digital information-sharing and patient privacy when engaging families in the intensive care unit. J Am Med Inform Assoc. 2016;23(5):995-1000. PubMed
25. Krishna S, Francisco BD, Balas EA, et al. Internet-enabled interactive multimedia asthma education program: A randomized trial. Pediatrics. 2003;111(3):503-510. PubMed
26. Fox MP. A systematic review of the literature reporting on studies that examined the impact of interactive, computer-based patient education programs. Patient Educ Couns. 2009;77(1):6-13. PubMed
27. Morgan ER, Laing K, McCarthy J, McCrate F, Seal MD. Using tablet-based technology in patient education about systemic therapy options for early-stage breast cancer: A pilot study. Curr Oncol. 2015;22(5):e364-e369. PubMed
28. Nehme J, El-Khani U, Chow A, Hakky S, Ahmed AR, Purkayastha S. The use of multimedia consent programs for surgical procedures: A systematic review. Surg Innov. 2013;20(1):13-23. PubMed
29. Waller A, Forshaw K, Carey M, et al. Optimizing patient preparation and surgical experience using eHealth technology. JMIR Med Inform. 2015;3(3):e29. PubMed
30. Abbott MB, Shaw P. Virtual nursing avatars: Nurse roles and evolving concepts of care. Online J Issues Nurs. 2016;21(3):7. PubMed
31. Cawthon C, Walia S, Osborn CY, Niesner KJ, Schnipper JL, Kripalani S. Improving care transitions: The patient perspective. J Health Commun. 2012;17 Suppl 3:312-324. PubMed
32. Bickmore TW, Pfeifer LM, Byron D, et al. Usability of conversational agents by patients with inadequate health literacy: Evidence from two clinical trials. J Health Commun. 2010;15 Suppl 2:197-210. PubMed
33. 2017;376(20):1905-1907. N Engl J Med.42. Mandl KD, Kohane IS. A 21st-century health IT system—creating a real-world information economy. PubMed
34. 2014;371(19):1803-1812.N Engl J Med41. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. PubMed
35. 2016;24(1):153-161.J Am Med Inform Assoc.40. Kelly MM, Hoonakker PLT, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. PubMed
36. 2017;171(4):372-381.JAMA Pediatr.39. Khan A, Coffey M, Litterer KP, et al. Families as partners in hospital error and adverse event surveillance. PubMed
37. 2017;17(4):389-402.Acad Pediatr.38. Khan A, Baird J, Rogers JE, et al. Parent and provider experience and shared understanding after a family-centered nighttime communication intervention. PubMed
38. 2016;6(6):319-329.Hosp Pediatr. 37. Khan A, Rogers JE, Forster CS, Furtak SL, Schuster MA, Landrigan CP. Communication and shared understanding between parents and resident-physicians at night. PubMed
39. 2016;11(9):615-619.J Hosp Med36. Figueroa JF, Schnipper JL, McNally K, Stade D, Lipsitz SR, Dalal AK. How often are hospitalized patients and providers on the same page with regard to the patient’s primary recovery goal for hospitalization? PubMed
40. 2013;8(7):414-417.J Hosp Med.35. Feldman HJ, Walker J, Li J, Delbanco T. OpenNotes: Hospitalists’ challenge and opportunity. PubMed
41. 2016;11(5):381-385.J Hosp Med.34. Dalal AK, Schnipper JL. Care team identification in the electronic health record: A critical first step for patient-centered communication.PubMed
42. 2016;24(e1):e178-e184.J Am Med Inform Assoc.33. Dalal AK, Schnipper J, Massaro A, et al. A web-based and mobile patient-centered “microblog” messaging platform to improve care team communication in acute care. PubMed
1. Sarkar U, Bates DW. Care partners and online patient portals. JAMA. 2014;311(4):357-358. PubMed
2. Grando MA, Rozenblum R, Bates DW, eds. Information Technology for Patient Empowerment in Healthcare, 1st Edition. Berlin: Walter de Gruyter Inc.; 2015.
3. Kelly MM, Hoonakker PLT, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. J Am Med Inform Assoc. 2016;24(1):153-161. PubMed
4. Dalal AK, Dykes PC, Collins S, et al. A web-based, patient-centered toolkit to engage patients and caregivers in the acute care setting: A preliminary evaluation. J Am Med Inform Assoc. 2016;23(1):80-87. PubMed
5. Prey JE, Restaino S, Vawdrey DK. Providing hospital patients with access to their medical records. AMIA Annu Symp Proc. 2014;2014:1884-1893. PubMed
6. Herrin J, Harris KG, Kenward K, Hines S, Joshi MS, Frosch DL. Patient and family engagement: A survey of US hospital practices. BMJ Qual Saf. 2016;25(3):182-189. PubMed
7. Tom JO, Mangione-Smith R, Solomon C, Grossman DC. Integrated personal health record use: Association with parent-reported care experiences. Pediatrics. 2012;130(1):e183-e190. PubMed
8. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Stage 2. Federal Register Final Rule. Sect. 170; 2012. https://www.federalregister.gov/documents/2012/03/07/2012-4443/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-2. Accessed March 1, 2017.
9. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; merit-based incentive payment system (MIPS) and alternative payment model (APM) incentive under the physician fee schedule, and criteria for physician-focused payment models. Final rule with comment period. Fed Regist. 2016;81(214):77008-77831. PubMed
10. Prey JE, Woollen J, Wilcox L, et al. Patient engagement in the inpatient setting: A systematic review. J Am Med Informat Assoc. 2014;21(4):742-750. PubMed
11. Ludwin S, Greysen SR. Use of smartphones and mobile devices in hospitalized patients: Untapped opportunities for inpatient engagement. J Hosp Med. 2015;10(7):459-461. PubMed
12. Coleman EA. Family caregivers as partners in care transitions: The caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. PubMed
13. Kaziunas E, Hanauer DA, Ackerman MS, Choi SW. Identifying unmet informational needs in the inpatient setting to increase patient and caregiver engagement in the context of pediatric hematopoietic stem cell transplantation. J Am Med Inform Assoc. 2016;23(1):94-104. PubMed
14. Woollen J, Prey J, Wilcox L, et al. Patient experiences using an inpatient personal health record. Appl Clin Inform. 2016;7(2):446-460. PubMed
15. Irizarry T, DeVito Dabbs A, Curran CR. Patient portals and patient engagement: A state of the science review. J Med Internet Res. 2015;17(6):e148. doi:10.2196/jmir.4255. PubMed
16. Vawdrey DK, Wilcox LG, Collins SA, et al. A tablet computer application for patients to participate in their hospital care. AMIA Annu Symp Proc. 2011;2011:1428-1435. PubMed
17. Collins SA, Rozenblum R, Leung WY, et al. Acute care patient portals: A qualitative study of stakeholder perspectives on current practices. J Am Med Inform Assoc. 2016;24(e1):e9-e17. PubMed
18. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: A systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
19. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
20. Griffin A, Skinner A, Thornhill J, Weinberger M. Patient Portals: Who uses them? What features do they use? And do they reduce hospital readmissions? Appl Clin Inform. 2016;7(2):489-501. PubMed
21. O’Leary KJ, Lohman ME, Culver E, Killarney A, Randy Smith G Jr, Liebovitz DM. The effect of tablet computers with a mobile patient portal application on hospitalized patients’ knowledge and activation. J Am Med Inform Assoc. 2016;23(1):159-165. PubMed
22. O’Leary KJ, Sharma RK, Killarney A, et al. Patients’ and Healthcare Providers’ Perceptions of a Mobile Portal Application for Hospitalized Patients. BMC Med Inform Decis Mak. 2016;16(1):123. PubMed
23. Pell JM, Mancuso M, Limon S, Oman K, Lin CT. Patient access to electronic health records during hospitalization. JAMA Intern Med. 2015;175(5):856-858. PubMed
24. Brown SM, Aboumatar HJ, Francis L, et al. Balancing digital information-sharing and patient privacy when engaging families in the intensive care unit. J Am Med Inform Assoc. 2016;23(5):995-1000. PubMed
25. Krishna S, Francisco BD, Balas EA, et al. Internet-enabled interactive multimedia asthma education program: A randomized trial. Pediatrics. 2003;111(3):503-510. PubMed
26. Fox MP. A systematic review of the literature reporting on studies that examined the impact of interactive, computer-based patient education programs. Patient Educ Couns. 2009;77(1):6-13. PubMed
27. Morgan ER, Laing K, McCarthy J, McCrate F, Seal MD. Using tablet-based technology in patient education about systemic therapy options for early-stage breast cancer: A pilot study. Curr Oncol. 2015;22(5):e364-e369. PubMed
28. Nehme J, El-Khani U, Chow A, Hakky S, Ahmed AR, Purkayastha S. The use of multimedia consent programs for surgical procedures: A systematic review. Surg Innov. 2013;20(1):13-23. PubMed
29. Waller A, Forshaw K, Carey M, et al. Optimizing patient preparation and surgical experience using eHealth technology. JMIR Med Inform. 2015;3(3):e29. PubMed
30. Abbott MB, Shaw P. Virtual nursing avatars: Nurse roles and evolving concepts of care. Online J Issues Nurs. 2016;21(3):7. PubMed
31. Cawthon C, Walia S, Osborn CY, Niesner KJ, Schnipper JL, Kripalani S. Improving care transitions: The patient perspective. J Health Commun. 2012;17 Suppl 3:312-324. PubMed
32. Bickmore TW, Pfeifer LM, Byron D, et al. Usability of conversational agents by patients with inadequate health literacy: Evidence from two clinical trials. J Health Commun. 2010;15 Suppl 2:197-210. PubMed
33. 2017;376(20):1905-1907. N Engl J Med.42. Mandl KD, Kohane IS. A 21st-century health IT system—creating a real-world information economy. PubMed
34. 2014;371(19):1803-1812.N Engl J Med41. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. PubMed
35. 2016;24(1):153-161.J Am Med Inform Assoc.40. Kelly MM, Hoonakker PLT, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. PubMed
36. 2017;171(4):372-381.JAMA Pediatr.39. Khan A, Coffey M, Litterer KP, et al. Families as partners in hospital error and adverse event surveillance. PubMed
37. 2017;17(4):389-402.Acad Pediatr.38. Khan A, Baird J, Rogers JE, et al. Parent and provider experience and shared understanding after a family-centered nighttime communication intervention. PubMed
38. 2016;6(6):319-329.Hosp Pediatr. 37. Khan A, Rogers JE, Forster CS, Furtak SL, Schuster MA, Landrigan CP. Communication and shared understanding between parents and resident-physicians at night. PubMed
39. 2016;11(9):615-619.J Hosp Med36. Figueroa JF, Schnipper JL, McNally K, Stade D, Lipsitz SR, Dalal AK. How often are hospitalized patients and providers on the same page with regard to the patient’s primary recovery goal for hospitalization? PubMed
40. 2013;8(7):414-417.J Hosp Med.35. Feldman HJ, Walker J, Li J, Delbanco T. OpenNotes: Hospitalists’ challenge and opportunity. PubMed
41. 2016;11(5):381-385.J Hosp Med.34. Dalal AK, Schnipper JL. Care team identification in the electronic health record: A critical first step for patient-centered communication.PubMed
42. 2016;24(e1):e178-e184.J Am Med Inform Assoc.33. Dalal AK, Schnipper J, Massaro A, et al. A web-based and mobile patient-centered “microblog” messaging platform to improve care team communication in acute care. PubMed
© 2017 Society of Hospital Medicine
Reconsidering Hospital Readmission Measures
Hospital readmission rates are a consequential and contentious measure of hospital quality. Readmissions within 30 days of hospital discharge are part of the Centers for Medicare & Medicaid Services (CMS) Value-Based Purchasing Program and are publicly reported. Hospital-wide readmissions and condition-specific readmissions are heavily weighted by US News & World Report in its hospital rankings and in the new CMS Five-Star Quality Rating System.1 However, clinicians and researchers question the construct validity of current readmission measures.2,3
The focus on readmissions began in 2009 when Jencks et al.4 reported that 20% of Medicare patients were readmitted within 30 days after hospital discharge. Policy makers embraced readmission reduction, assuming that a hospital readmission so soon after discharge reflected poor quality of hospital care and that, with focused efforts, hospitals could reduce readmissions and save CMS money. In 2010, the Affordable Care Act introduced an initiative to reduce readmissions and, in 2012, the Hospital Readmission Reduction Program was implemented, financially penalizing hospitals with higher-than-expected readmission rates for patients hospitalized with principal diagnoses of heart failure, myocardial infarction, and pneumonia.5 Readmission measures have since proliferated and now include pay-for-performance metrics for hospitalizations for chronic obstructive pulmonary disease (COPD), coronary artery bypass grafting, and total hip or knee arthroplasty. Measures are also reported for stroke patients and for “hospital-wide readmissions,” a catch-all measure intended to capture readmission rates across most diagnoses, with various exclusions intended to prevent counting planned readmissions (eg, hospitalization for cholecystectomy following a hospitalization for cholecystitis). These measures use claims data to construct hierarchical regression models at the patient and hospital levels, assuming that variation among readmission rates are due to hospital quality effects. The goal of this approach is to level the playing field to avoid penalizing hospitals for caring for sicker patients who are at higher risk for readmission for reasons unrelated to hospital care. Yet hospital readmissions are influenced by a complex set of variables that go well beyond hospital care, some of which may be better captured by existing models than others. Below we review several potential biases in the hospital readmission measures and offer policy recommendations to improve the accuracy of these measures.
Variation in a quality measure is influenced by the quality of the underlying data, the mix of patients served, bias in the performance measure, and the degree of systemic or random error.6 Hospital readmission rates are subject to multiple sources of variation, and true differences in the quality of care are often a much smaller source of this variation. A recent analysis of patient readmissions following general surgery found that the majority were unrelated to suboptimal medical care.7 Consider 3 scenarios in which a patient with COPD is readmitted 22 days after discharge. In hospital 1, the patient was discharged without a prescription for a steroid inhaler. In hospital 2, the patient was discharged on a steroid inhaler, filled the prescription, and elected not to use it. In hospital 3, the patient was discharged on a steroid inhaler and was provided medical assistance to fill the prescription but still could not afford the $15 copay. In all 3 scenarios, the hospital would be equally culpable under the current readmission measures, suffering financial and reputational penalties.
Yet the hospitals in these scenarios are not equally culpable. Variation in the mix of patients and bias in the measure impacted performance. Hospital 1 should clearly be held accountable for the readmission. In the cases of hospitals 2 and 3, the situations are more nuanced. More education about COPD, financial investment by the hospital to cover a copay, or a different transitional care approach may have increased the likelihood of patient compliance, but, ultimately, hospitals 2 and 3 were impacted by personal health behaviors and access to public health services and financial assistance, and the readmissions were less within their control.8
To be valid, hospital readmission measures would need to ensure that all hospitals are similar in patient characteristics and in the need for an availability of public health services. Yet these factors vary among hospitals and cannot be accounted for by models that rely exclusively on patient-level variables, such as the nature and severity of illness. As a result, the existing readmission measures are biased against certain types of hospitals. Hospitals that treat a greater proportion of patients who are socioeconomically disadvantaged; who lack access to primary care, medical assistance, or public health programs; and who have substance abuse and mental health issues will have higher readmission rates. Hospitals that care for patients who fail initial treatments and require referral for complex care will also have higher readmission rates. These types of patients are not randomly distributed throughout our healthcare system. They are clustered at rural hospitals in underserved areas, certain urban health systems, safety net hospitals, and academic health centers. It is not surprising that readmission penalties have most severely impacted large academic hospitals that care for disadvantaged populations.2 These penalties may have unintended consequences, reducing a hospital’s willingness to care for disadvantaged populations.
While these biases may unfairly harm hospitals caring for disadvantaged patients, the readmission measures may also indirectly harm patients. Low hospital readmission rates are not associated with reduced mortality and, in some instances, track with higher mortality.9-11 This may result from measurement factors (patients who die cannot be readmitted), from neighborhood socioeconomic status (SES) factors that may impact readmissions more,12 or from actual patient harm (some patients need acute care following discharge and may have worse outcomes if that care is delayed).11 Doctors have long recognized this potential risk; empiric evidence now supports them. While mortality measures may also be impacted by sociodemographic variables,13 whether to adjust for SES should be defined by the purpose of the measure. If the measure is meant to evaluate hospital quality (or utilization in the case of readmissions), adjusting for SES is appropriate because it is unrealistic to expect a health system to reduce income inequality and provide safe housing. Failure to adjust for SES, which has a large impact on outcomes, may mask a quality of care issue. Conversely, if the purpose of a measure is for a community to improve population health, then it should not be adjusted for SES because the community could adjust for income inequality.
Despite the complex ethical challenges created by the efforts to reduce readmissions, there has been virtually no public dialogue with patients, physicians, and policy makers regarding how to balance the trade-offs between reducing readmission and maintaining safety. Patients would likely value increased survival more than reduced readmissions, yet the current CMS Five-Star Rating System for hospital quality weighs readmissions equally with mortality in its hospital rankings, potentially misinforming patients. For example, many well-known academic medical centers score well (4 or 5 stars) on mortality and poorly (1 or 2 stars) on readmissions, resulting in a low or average overall score, calling into question face validity and confounding consumers struggling to make decisions about where to seek care. The Medicare Payment Advisory Commission’s Report to the Congress14 highlights the multiple significant systematic and random errors with the hospital readmission data.
Revisiting the Hospital Readmission Measures
Given significant bias in the hospital readmission measures and the ethical challenges imposed by reducing readmissions, potentially at the expense of survival, we believe CMS needs to take action to remedy the problem. First, CMS should drop hospital readmissions as a quality measure from its hospital rankings. Other hospital-rating groups and insurers should do the same. When included in payment schemes, readmissions should not be construed as a quality measure but as a utilization measure, like length of stay.
Second, the Department of Health & Human Services (HHS) should invest in maturing the hospital readmission measures to ensure construct, content, and criterion validity and reliability. No doubt the risk adjustment is complex and may be inherently limited using Medicare claims data. In the case of SES adjustment, for example, limited numbers of SES measures can be constructed from current data sources.8,13 There are other approaches to address this recommendation. For example, HHS could define a preventable readmission as one linked to some process or outcome of hospital care, such as whether the patient was discharged on an inhaler. The National Quality Forum used this approach to define a preventable venous thromboembolic event as one occurring when a patient did not receive appropriate prophylaxis. In this way, only hospital 1 in the 3 scenarios for the patient with COPD would be penalized. However, we recognize that it is not always simple to define specific process measures (eg, prescribing an inhaler) that link to readmission outcomes and that there may be other important yet hard-to-measure interventions (eg, patient and family education) that are important components of patient-centered care and readmission prevention. This is why readmissions are so challenging as a quality measure. If experts cannot define clinician behaviors that have a strong theory of change or are causally related to reduced readmissions, it is hard to call readmissions a modifiable quality measure. Another potential strategy to level the playing field would be to compare readmission rates across peer institutions only. For instance, tertiary-care safety net hospitals would be compared to one another and rural community hospitals would be compared to one another.14 Lastly, new data sources could be added to account for the social, community-level, public health, and personal health factors that heavily influence a patient’s risk for readmission, in addition to hospital-level factors. Appropriate methods will be needed to develop statistical models for risk adjustment; however, this is a complex topic and beyond the scope of the current paper.
Third, HHS could continue to use the current readmission measures as population health measures while supporting multistakeholder teams to better understand how people and their communities, public health agencies, insurers, and healthcare providers can collaborate to help patients thrive and avoid readmissions by addressing true defects in care and care coordination.
While it is understandable why policy makers chose to focus on hospital readmissions, and while we recognize that concerns about the measures were unknown when they were created, emerging evidence demonstrates that the current readmission measures (particularly when used as a quality metric) lack construct validity, contain significant bias and systematic errors, and create ethical tension by rewarding hospitals both financially and reputationally for turning away sick and socially disadvantaged patients who may, consequently, have adverse outcomes. Current readmission measures need to be reconsidered.
Acknowledgments
The authors thank Christine G. Holzmueller, BLA, with the Armstrong Institute for Patient Safety and Quality, Johns Hopkins Medicine, for her assistance in editing the manuscript and preparing it for journal submission.
Disclosure
Dr. Pronovost errs on the side of full disclosure and reports receiving grant or contract support from the Agency for Healthcare Research and Quality, the Gordon and Betty Moore Foundation (research related to patient safety and quality of care), the National Institutes of Health (acute lung injury research), and the American Medical Association Inc. (improve blood pressure control); honoraria from various healthcare organizations for speaking on patient safety and quality (the Leigh Bureau manages engagements); book royalties from the Penguin Group for his book Safe Patients, Smart Hospitals; and was receiving stock and fees to serve as a director for Cantel Medical up until 24 months ago. Dr. Pronovost is a founder of Patient Doctor Technologies, a startup company that seeks to enhance the partnership between patients and clinicians with an application called Doctella. Dr. Brotman, Dr. Hoyer, and Ms. Deutschendorf report no relevant conflicts of interest.
1. Centers for Medicare & Medicaid Services. Five-star quality rating system. https://www.cms.gov/medicare/provider-enrollment-and-certification/certificationandcomplianc/fsqrs.html. Accessed October 11, 2016.
2. Joynt KE, Jha AK. Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342-343. PubMed
3. Boozary AS, Manchin J, 3rd, Wicker RF. The Medicare Hospital Readmissions Reduction Program: time for reform. JAMA. 2015;314(4):347-348. PubMed
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. PubMed
5. Centers for Medicare & Medicaid Services. Readmissions Reduction Program (HRRP). https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Accessed April 12, 2017.
6. Parker C, Schwamm LH, Fonarow GC, Smith EE, Reeves MJ. Stroke quality metrics: systematic reviews of the relationships to patient-centered outcomes and impact of public reporting. Stroke. 2012;43(1):155-162. PubMed
7. McIntyre LK, Arbabi S, Robinson EF, Maier RV. Analysis of risk factors for patient readmission 30 days following discharge from general surgery. JAMA Surg. 2016;151(9):855-861. PubMed
8. Sheingold SH, Zuckerman R, Shartzer A. Understanding Medicare hospital readmission rates and differing penalties between safety-net and other hospitals. Health Aff (Millwood). 2016;35(1):124-131. PubMed
9. Brotman DJ, Hoyer EH, Leung C, Lepley D, Deutschendorf A. Associations between hospital-wide readmission rates and mortality measures at the hospital level: are hospital-wide readmissions a measure of quality? J Hosp Med. 2016;11(9):650-651. PubMed
10. Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587-593. PubMed
11. Fan VS, Gaziano JM, Lew R, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156(10):673-683. PubMed
12. Bikdeli B, Wayda B, Bao H, et al. Place of residence and outcomes of patients with heart failure: analysis from the Telemonitoring to Improve Heart Failure Outcomes Trial. Circ Cardiovasc Qual Outcomes. 2014;7(5):749-756. PubMed
13. Bernheim SM, Parzynski CS, Horwitz L, et al. Accounting for patients’ socioeconomic status does not change hospital readmission rates. Health Aff (Millwood). 2016;35(8):1461-1470. PubMed
14. Medicare Payment Advisory Commission. Refining the Hospital Readmissions Reduction Program. In: Report to the Congress: Medicare and the Health Care Delivery System, Chapter 4. June 2013. PubMed
Hospital readmission rates are a consequential and contentious measure of hospital quality. Readmissions within 30 days of hospital discharge are part of the Centers for Medicare & Medicaid Services (CMS) Value-Based Purchasing Program and are publicly reported. Hospital-wide readmissions and condition-specific readmissions are heavily weighted by US News & World Report in its hospital rankings and in the new CMS Five-Star Quality Rating System.1 However, clinicians and researchers question the construct validity of current readmission measures.2,3
The focus on readmissions began in 2009 when Jencks et al.4 reported that 20% of Medicare patients were readmitted within 30 days after hospital discharge. Policy makers embraced readmission reduction, assuming that a hospital readmission so soon after discharge reflected poor quality of hospital care and that, with focused efforts, hospitals could reduce readmissions and save CMS money. In 2010, the Affordable Care Act introduced an initiative to reduce readmissions and, in 2012, the Hospital Readmission Reduction Program was implemented, financially penalizing hospitals with higher-than-expected readmission rates for patients hospitalized with principal diagnoses of heart failure, myocardial infarction, and pneumonia.5 Readmission measures have since proliferated and now include pay-for-performance metrics for hospitalizations for chronic obstructive pulmonary disease (COPD), coronary artery bypass grafting, and total hip or knee arthroplasty. Measures are also reported for stroke patients and for “hospital-wide readmissions,” a catch-all measure intended to capture readmission rates across most diagnoses, with various exclusions intended to prevent counting planned readmissions (eg, hospitalization for cholecystectomy following a hospitalization for cholecystitis). These measures use claims data to construct hierarchical regression models at the patient and hospital levels, assuming that variation among readmission rates are due to hospital quality effects. The goal of this approach is to level the playing field to avoid penalizing hospitals for caring for sicker patients who are at higher risk for readmission for reasons unrelated to hospital care. Yet hospital readmissions are influenced by a complex set of variables that go well beyond hospital care, some of which may be better captured by existing models than others. Below we review several potential biases in the hospital readmission measures and offer policy recommendations to improve the accuracy of these measures.
Variation in a quality measure is influenced by the quality of the underlying data, the mix of patients served, bias in the performance measure, and the degree of systemic or random error.6 Hospital readmission rates are subject to multiple sources of variation, and true differences in the quality of care are often a much smaller source of this variation. A recent analysis of patient readmissions following general surgery found that the majority were unrelated to suboptimal medical care.7 Consider 3 scenarios in which a patient with COPD is readmitted 22 days after discharge. In hospital 1, the patient was discharged without a prescription for a steroid inhaler. In hospital 2, the patient was discharged on a steroid inhaler, filled the prescription, and elected not to use it. In hospital 3, the patient was discharged on a steroid inhaler and was provided medical assistance to fill the prescription but still could not afford the $15 copay. In all 3 scenarios, the hospital would be equally culpable under the current readmission measures, suffering financial and reputational penalties.
Yet the hospitals in these scenarios are not equally culpable. Variation in the mix of patients and bias in the measure impacted performance. Hospital 1 should clearly be held accountable for the readmission. In the cases of hospitals 2 and 3, the situations are more nuanced. More education about COPD, financial investment by the hospital to cover a copay, or a different transitional care approach may have increased the likelihood of patient compliance, but, ultimately, hospitals 2 and 3 were impacted by personal health behaviors and access to public health services and financial assistance, and the readmissions were less within their control.8
To be valid, hospital readmission measures would need to ensure that all hospitals are similar in patient characteristics and in the need for an availability of public health services. Yet these factors vary among hospitals and cannot be accounted for by models that rely exclusively on patient-level variables, such as the nature and severity of illness. As a result, the existing readmission measures are biased against certain types of hospitals. Hospitals that treat a greater proportion of patients who are socioeconomically disadvantaged; who lack access to primary care, medical assistance, or public health programs; and who have substance abuse and mental health issues will have higher readmission rates. Hospitals that care for patients who fail initial treatments and require referral for complex care will also have higher readmission rates. These types of patients are not randomly distributed throughout our healthcare system. They are clustered at rural hospitals in underserved areas, certain urban health systems, safety net hospitals, and academic health centers. It is not surprising that readmission penalties have most severely impacted large academic hospitals that care for disadvantaged populations.2 These penalties may have unintended consequences, reducing a hospital’s willingness to care for disadvantaged populations.
While these biases may unfairly harm hospitals caring for disadvantaged patients, the readmission measures may also indirectly harm patients. Low hospital readmission rates are not associated with reduced mortality and, in some instances, track with higher mortality.9-11 This may result from measurement factors (patients who die cannot be readmitted), from neighborhood socioeconomic status (SES) factors that may impact readmissions more,12 or from actual patient harm (some patients need acute care following discharge and may have worse outcomes if that care is delayed).11 Doctors have long recognized this potential risk; empiric evidence now supports them. While mortality measures may also be impacted by sociodemographic variables,13 whether to adjust for SES should be defined by the purpose of the measure. If the measure is meant to evaluate hospital quality (or utilization in the case of readmissions), adjusting for SES is appropriate because it is unrealistic to expect a health system to reduce income inequality and provide safe housing. Failure to adjust for SES, which has a large impact on outcomes, may mask a quality of care issue. Conversely, if the purpose of a measure is for a community to improve population health, then it should not be adjusted for SES because the community could adjust for income inequality.
Despite the complex ethical challenges created by the efforts to reduce readmissions, there has been virtually no public dialogue with patients, physicians, and policy makers regarding how to balance the trade-offs between reducing readmission and maintaining safety. Patients would likely value increased survival more than reduced readmissions, yet the current CMS Five-Star Rating System for hospital quality weighs readmissions equally with mortality in its hospital rankings, potentially misinforming patients. For example, many well-known academic medical centers score well (4 or 5 stars) on mortality and poorly (1 or 2 stars) on readmissions, resulting in a low or average overall score, calling into question face validity and confounding consumers struggling to make decisions about where to seek care. The Medicare Payment Advisory Commission’s Report to the Congress14 highlights the multiple significant systematic and random errors with the hospital readmission data.
Revisiting the Hospital Readmission Measures
Given significant bias in the hospital readmission measures and the ethical challenges imposed by reducing readmissions, potentially at the expense of survival, we believe CMS needs to take action to remedy the problem. First, CMS should drop hospital readmissions as a quality measure from its hospital rankings. Other hospital-rating groups and insurers should do the same. When included in payment schemes, readmissions should not be construed as a quality measure but as a utilization measure, like length of stay.
Second, the Department of Health & Human Services (HHS) should invest in maturing the hospital readmission measures to ensure construct, content, and criterion validity and reliability. No doubt the risk adjustment is complex and may be inherently limited using Medicare claims data. In the case of SES adjustment, for example, limited numbers of SES measures can be constructed from current data sources.8,13 There are other approaches to address this recommendation. For example, HHS could define a preventable readmission as one linked to some process or outcome of hospital care, such as whether the patient was discharged on an inhaler. The National Quality Forum used this approach to define a preventable venous thromboembolic event as one occurring when a patient did not receive appropriate prophylaxis. In this way, only hospital 1 in the 3 scenarios for the patient with COPD would be penalized. However, we recognize that it is not always simple to define specific process measures (eg, prescribing an inhaler) that link to readmission outcomes and that there may be other important yet hard-to-measure interventions (eg, patient and family education) that are important components of patient-centered care and readmission prevention. This is why readmissions are so challenging as a quality measure. If experts cannot define clinician behaviors that have a strong theory of change or are causally related to reduced readmissions, it is hard to call readmissions a modifiable quality measure. Another potential strategy to level the playing field would be to compare readmission rates across peer institutions only. For instance, tertiary-care safety net hospitals would be compared to one another and rural community hospitals would be compared to one another.14 Lastly, new data sources could be added to account for the social, community-level, public health, and personal health factors that heavily influence a patient’s risk for readmission, in addition to hospital-level factors. Appropriate methods will be needed to develop statistical models for risk adjustment; however, this is a complex topic and beyond the scope of the current paper.
Third, HHS could continue to use the current readmission measures as population health measures while supporting multistakeholder teams to better understand how people and their communities, public health agencies, insurers, and healthcare providers can collaborate to help patients thrive and avoid readmissions by addressing true defects in care and care coordination.
While it is understandable why policy makers chose to focus on hospital readmissions, and while we recognize that concerns about the measures were unknown when they were created, emerging evidence demonstrates that the current readmission measures (particularly when used as a quality metric) lack construct validity, contain significant bias and systematic errors, and create ethical tension by rewarding hospitals both financially and reputationally for turning away sick and socially disadvantaged patients who may, consequently, have adverse outcomes. Current readmission measures need to be reconsidered.
Acknowledgments
The authors thank Christine G. Holzmueller, BLA, with the Armstrong Institute for Patient Safety and Quality, Johns Hopkins Medicine, for her assistance in editing the manuscript and preparing it for journal submission.
Disclosure
Dr. Pronovost errs on the side of full disclosure and reports receiving grant or contract support from the Agency for Healthcare Research and Quality, the Gordon and Betty Moore Foundation (research related to patient safety and quality of care), the National Institutes of Health (acute lung injury research), and the American Medical Association Inc. (improve blood pressure control); honoraria from various healthcare organizations for speaking on patient safety and quality (the Leigh Bureau manages engagements); book royalties from the Penguin Group for his book Safe Patients, Smart Hospitals; and was receiving stock and fees to serve as a director for Cantel Medical up until 24 months ago. Dr. Pronovost is a founder of Patient Doctor Technologies, a startup company that seeks to enhance the partnership between patients and clinicians with an application called Doctella. Dr. Brotman, Dr. Hoyer, and Ms. Deutschendorf report no relevant conflicts of interest.
Hospital readmission rates are a consequential and contentious measure of hospital quality. Readmissions within 30 days of hospital discharge are part of the Centers for Medicare & Medicaid Services (CMS) Value-Based Purchasing Program and are publicly reported. Hospital-wide readmissions and condition-specific readmissions are heavily weighted by US News & World Report in its hospital rankings and in the new CMS Five-Star Quality Rating System.1 However, clinicians and researchers question the construct validity of current readmission measures.2,3
The focus on readmissions began in 2009 when Jencks et al.4 reported that 20% of Medicare patients were readmitted within 30 days after hospital discharge. Policy makers embraced readmission reduction, assuming that a hospital readmission so soon after discharge reflected poor quality of hospital care and that, with focused efforts, hospitals could reduce readmissions and save CMS money. In 2010, the Affordable Care Act introduced an initiative to reduce readmissions and, in 2012, the Hospital Readmission Reduction Program was implemented, financially penalizing hospitals with higher-than-expected readmission rates for patients hospitalized with principal diagnoses of heart failure, myocardial infarction, and pneumonia.5 Readmission measures have since proliferated and now include pay-for-performance metrics for hospitalizations for chronic obstructive pulmonary disease (COPD), coronary artery bypass grafting, and total hip or knee arthroplasty. Measures are also reported for stroke patients and for “hospital-wide readmissions,” a catch-all measure intended to capture readmission rates across most diagnoses, with various exclusions intended to prevent counting planned readmissions (eg, hospitalization for cholecystectomy following a hospitalization for cholecystitis). These measures use claims data to construct hierarchical regression models at the patient and hospital levels, assuming that variation among readmission rates are due to hospital quality effects. The goal of this approach is to level the playing field to avoid penalizing hospitals for caring for sicker patients who are at higher risk for readmission for reasons unrelated to hospital care. Yet hospital readmissions are influenced by a complex set of variables that go well beyond hospital care, some of which may be better captured by existing models than others. Below we review several potential biases in the hospital readmission measures and offer policy recommendations to improve the accuracy of these measures.
Variation in a quality measure is influenced by the quality of the underlying data, the mix of patients served, bias in the performance measure, and the degree of systemic or random error.6 Hospital readmission rates are subject to multiple sources of variation, and true differences in the quality of care are often a much smaller source of this variation. A recent analysis of patient readmissions following general surgery found that the majority were unrelated to suboptimal medical care.7 Consider 3 scenarios in which a patient with COPD is readmitted 22 days after discharge. In hospital 1, the patient was discharged without a prescription for a steroid inhaler. In hospital 2, the patient was discharged on a steroid inhaler, filled the prescription, and elected not to use it. In hospital 3, the patient was discharged on a steroid inhaler and was provided medical assistance to fill the prescription but still could not afford the $15 copay. In all 3 scenarios, the hospital would be equally culpable under the current readmission measures, suffering financial and reputational penalties.
Yet the hospitals in these scenarios are not equally culpable. Variation in the mix of patients and bias in the measure impacted performance. Hospital 1 should clearly be held accountable for the readmission. In the cases of hospitals 2 and 3, the situations are more nuanced. More education about COPD, financial investment by the hospital to cover a copay, or a different transitional care approach may have increased the likelihood of patient compliance, but, ultimately, hospitals 2 and 3 were impacted by personal health behaviors and access to public health services and financial assistance, and the readmissions were less within their control.8
To be valid, hospital readmission measures would need to ensure that all hospitals are similar in patient characteristics and in the need for an availability of public health services. Yet these factors vary among hospitals and cannot be accounted for by models that rely exclusively on patient-level variables, such as the nature and severity of illness. As a result, the existing readmission measures are biased against certain types of hospitals. Hospitals that treat a greater proportion of patients who are socioeconomically disadvantaged; who lack access to primary care, medical assistance, or public health programs; and who have substance abuse and mental health issues will have higher readmission rates. Hospitals that care for patients who fail initial treatments and require referral for complex care will also have higher readmission rates. These types of patients are not randomly distributed throughout our healthcare system. They are clustered at rural hospitals in underserved areas, certain urban health systems, safety net hospitals, and academic health centers. It is not surprising that readmission penalties have most severely impacted large academic hospitals that care for disadvantaged populations.2 These penalties may have unintended consequences, reducing a hospital’s willingness to care for disadvantaged populations.
While these biases may unfairly harm hospitals caring for disadvantaged patients, the readmission measures may also indirectly harm patients. Low hospital readmission rates are not associated with reduced mortality and, in some instances, track with higher mortality.9-11 This may result from measurement factors (patients who die cannot be readmitted), from neighborhood socioeconomic status (SES) factors that may impact readmissions more,12 or from actual patient harm (some patients need acute care following discharge and may have worse outcomes if that care is delayed).11 Doctors have long recognized this potential risk; empiric evidence now supports them. While mortality measures may also be impacted by sociodemographic variables,13 whether to adjust for SES should be defined by the purpose of the measure. If the measure is meant to evaluate hospital quality (or utilization in the case of readmissions), adjusting for SES is appropriate because it is unrealistic to expect a health system to reduce income inequality and provide safe housing. Failure to adjust for SES, which has a large impact on outcomes, may mask a quality of care issue. Conversely, if the purpose of a measure is for a community to improve population health, then it should not be adjusted for SES because the community could adjust for income inequality.
Despite the complex ethical challenges created by the efforts to reduce readmissions, there has been virtually no public dialogue with patients, physicians, and policy makers regarding how to balance the trade-offs between reducing readmission and maintaining safety. Patients would likely value increased survival more than reduced readmissions, yet the current CMS Five-Star Rating System for hospital quality weighs readmissions equally with mortality in its hospital rankings, potentially misinforming patients. For example, many well-known academic medical centers score well (4 or 5 stars) on mortality and poorly (1 or 2 stars) on readmissions, resulting in a low or average overall score, calling into question face validity and confounding consumers struggling to make decisions about where to seek care. The Medicare Payment Advisory Commission’s Report to the Congress14 highlights the multiple significant systematic and random errors with the hospital readmission data.
Revisiting the Hospital Readmission Measures
Given significant bias in the hospital readmission measures and the ethical challenges imposed by reducing readmissions, potentially at the expense of survival, we believe CMS needs to take action to remedy the problem. First, CMS should drop hospital readmissions as a quality measure from its hospital rankings. Other hospital-rating groups and insurers should do the same. When included in payment schemes, readmissions should not be construed as a quality measure but as a utilization measure, like length of stay.
Second, the Department of Health & Human Services (HHS) should invest in maturing the hospital readmission measures to ensure construct, content, and criterion validity and reliability. No doubt the risk adjustment is complex and may be inherently limited using Medicare claims data. In the case of SES adjustment, for example, limited numbers of SES measures can be constructed from current data sources.8,13 There are other approaches to address this recommendation. For example, HHS could define a preventable readmission as one linked to some process or outcome of hospital care, such as whether the patient was discharged on an inhaler. The National Quality Forum used this approach to define a preventable venous thromboembolic event as one occurring when a patient did not receive appropriate prophylaxis. In this way, only hospital 1 in the 3 scenarios for the patient with COPD would be penalized. However, we recognize that it is not always simple to define specific process measures (eg, prescribing an inhaler) that link to readmission outcomes and that there may be other important yet hard-to-measure interventions (eg, patient and family education) that are important components of patient-centered care and readmission prevention. This is why readmissions are so challenging as a quality measure. If experts cannot define clinician behaviors that have a strong theory of change or are causally related to reduced readmissions, it is hard to call readmissions a modifiable quality measure. Another potential strategy to level the playing field would be to compare readmission rates across peer institutions only. For instance, tertiary-care safety net hospitals would be compared to one another and rural community hospitals would be compared to one another.14 Lastly, new data sources could be added to account for the social, community-level, public health, and personal health factors that heavily influence a patient’s risk for readmission, in addition to hospital-level factors. Appropriate methods will be needed to develop statistical models for risk adjustment; however, this is a complex topic and beyond the scope of the current paper.
Third, HHS could continue to use the current readmission measures as population health measures while supporting multistakeholder teams to better understand how people and their communities, public health agencies, insurers, and healthcare providers can collaborate to help patients thrive and avoid readmissions by addressing true defects in care and care coordination.
While it is understandable why policy makers chose to focus on hospital readmissions, and while we recognize that concerns about the measures were unknown when they were created, emerging evidence demonstrates that the current readmission measures (particularly when used as a quality metric) lack construct validity, contain significant bias and systematic errors, and create ethical tension by rewarding hospitals both financially and reputationally for turning away sick and socially disadvantaged patients who may, consequently, have adverse outcomes. Current readmission measures need to be reconsidered.
Acknowledgments
The authors thank Christine G. Holzmueller, BLA, with the Armstrong Institute for Patient Safety and Quality, Johns Hopkins Medicine, for her assistance in editing the manuscript and preparing it for journal submission.
Disclosure
Dr. Pronovost errs on the side of full disclosure and reports receiving grant or contract support from the Agency for Healthcare Research and Quality, the Gordon and Betty Moore Foundation (research related to patient safety and quality of care), the National Institutes of Health (acute lung injury research), and the American Medical Association Inc. (improve blood pressure control); honoraria from various healthcare organizations for speaking on patient safety and quality (the Leigh Bureau manages engagements); book royalties from the Penguin Group for his book Safe Patients, Smart Hospitals; and was receiving stock and fees to serve as a director for Cantel Medical up until 24 months ago. Dr. Pronovost is a founder of Patient Doctor Technologies, a startup company that seeks to enhance the partnership between patients and clinicians with an application called Doctella. Dr. Brotman, Dr. Hoyer, and Ms. Deutschendorf report no relevant conflicts of interest.
1. Centers for Medicare & Medicaid Services. Five-star quality rating system. https://www.cms.gov/medicare/provider-enrollment-and-certification/certificationandcomplianc/fsqrs.html. Accessed October 11, 2016.
2. Joynt KE, Jha AK. Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342-343. PubMed
3. Boozary AS, Manchin J, 3rd, Wicker RF. The Medicare Hospital Readmissions Reduction Program: time for reform. JAMA. 2015;314(4):347-348. PubMed
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. PubMed
5. Centers for Medicare & Medicaid Services. Readmissions Reduction Program (HRRP). https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Accessed April 12, 2017.
6. Parker C, Schwamm LH, Fonarow GC, Smith EE, Reeves MJ. Stroke quality metrics: systematic reviews of the relationships to patient-centered outcomes and impact of public reporting. Stroke. 2012;43(1):155-162. PubMed
7. McIntyre LK, Arbabi S, Robinson EF, Maier RV. Analysis of risk factors for patient readmission 30 days following discharge from general surgery. JAMA Surg. 2016;151(9):855-861. PubMed
8. Sheingold SH, Zuckerman R, Shartzer A. Understanding Medicare hospital readmission rates and differing penalties between safety-net and other hospitals. Health Aff (Millwood). 2016;35(1):124-131. PubMed
9. Brotman DJ, Hoyer EH, Leung C, Lepley D, Deutschendorf A. Associations between hospital-wide readmission rates and mortality measures at the hospital level: are hospital-wide readmissions a measure of quality? J Hosp Med. 2016;11(9):650-651. PubMed
10. Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587-593. PubMed
11. Fan VS, Gaziano JM, Lew R, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156(10):673-683. PubMed
12. Bikdeli B, Wayda B, Bao H, et al. Place of residence and outcomes of patients with heart failure: analysis from the Telemonitoring to Improve Heart Failure Outcomes Trial. Circ Cardiovasc Qual Outcomes. 2014;7(5):749-756. PubMed
13. Bernheim SM, Parzynski CS, Horwitz L, et al. Accounting for patients’ socioeconomic status does not change hospital readmission rates. Health Aff (Millwood). 2016;35(8):1461-1470. PubMed
14. Medicare Payment Advisory Commission. Refining the Hospital Readmissions Reduction Program. In: Report to the Congress: Medicare and the Health Care Delivery System, Chapter 4. June 2013. PubMed
1. Centers for Medicare & Medicaid Services. Five-star quality rating system. https://www.cms.gov/medicare/provider-enrollment-and-certification/certificationandcomplianc/fsqrs.html. Accessed October 11, 2016.
2. Joynt KE, Jha AK. Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342-343. PubMed
3. Boozary AS, Manchin J, 3rd, Wicker RF. The Medicare Hospital Readmissions Reduction Program: time for reform. JAMA. 2015;314(4):347-348. PubMed
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. PubMed
5. Centers for Medicare & Medicaid Services. Readmissions Reduction Program (HRRP). https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Accessed April 12, 2017.
6. Parker C, Schwamm LH, Fonarow GC, Smith EE, Reeves MJ. Stroke quality metrics: systematic reviews of the relationships to patient-centered outcomes and impact of public reporting. Stroke. 2012;43(1):155-162. PubMed
7. McIntyre LK, Arbabi S, Robinson EF, Maier RV. Analysis of risk factors for patient readmission 30 days following discharge from general surgery. JAMA Surg. 2016;151(9):855-861. PubMed
8. Sheingold SH, Zuckerman R, Shartzer A. Understanding Medicare hospital readmission rates and differing penalties between safety-net and other hospitals. Health Aff (Millwood). 2016;35(1):124-131. PubMed
9. Brotman DJ, Hoyer EH, Leung C, Lepley D, Deutschendorf A. Associations between hospital-wide readmission rates and mortality measures at the hospital level: are hospital-wide readmissions a measure of quality? J Hosp Med. 2016;11(9):650-651. PubMed
10. Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587-593. PubMed
11. Fan VS, Gaziano JM, Lew R, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156(10):673-683. PubMed
12. Bikdeli B, Wayda B, Bao H, et al. Place of residence and outcomes of patients with heart failure: analysis from the Telemonitoring to Improve Heart Failure Outcomes Trial. Circ Cardiovasc Qual Outcomes. 2014;7(5):749-756. PubMed
13. Bernheim SM, Parzynski CS, Horwitz L, et al. Accounting for patients’ socioeconomic status does not change hospital readmission rates. Health Aff (Millwood). 2016;35(8):1461-1470. PubMed
14. Medicare Payment Advisory Commission. Refining the Hospital Readmissions Reduction Program. In: Report to the Congress: Medicare and the Health Care Delivery System, Chapter 4. June 2013. PubMed
© 2017 Society of Hospital Medicine
Expanding Treatment Opportunities for Hospitalized Patients with Opioid Use Disorders
The United States is facing an epidemic of prescription opioid and heroin use, which has been linked to the escalating prescribing of opioid analgesics. Though opioid prescriptions appear to be reaching a plateau, estimates suggest there are at least 900,000 active heroin users in the United States, and this number continues to grow.1 One response to this epidemic (through state legislation and medical society guidelines) has been a move to reduce opioid prescribing in order to diminish the potential for diversion and misuse.2 However, the treatment of pain is not the sole driver of heroin epidemiology, and new strategies are also needed to better engage patients with existing opioid use disorders (OUDs) to begin treatment. These patients are increasingly hospitalized for infectious comorbidities of injection drug use, trauma, or pregnancy, and this may present a unique opportunity to initiate these patients on maintenance opioid agonist therapy, the most effective option for medication-assisted treatment (MAT) for addiction.
MISSED OPPORTUNITIES
Patients with OUDs comprise an estimated 2% to 4% of hospitalized patients, representing a disproportionately large number of inpatients.3-6 According to a recent analysis of data from the National (Nationwide) Inpatient Sample, the estimated annual number of hospitalizations associated with OUDs in the United States increased from approximately 300,000 to more than 500,000 in the decade from 2002 to 2012.7 Severe bacterial infections associated with intravenous administration of opioids (including endocarditis, osteomyelitis, septic arthritis, and epidural abscess) increased substantially at an estimated cost of more than $700 million in 2012.7 Over a similar period, the prevalence of opioid use among women in labor increased from 13.7 to 22.0 per 10,000 live births,8 and there was a corresponding rise in admissions to neonatal intensive care units for neonatal abstinence syndrome.9 As the prevalence of prescription drug and heroin dependence continues to rise across the United States, hospitals and clinicians find themselves on the front lines of this epidemic, creating potential opportunities to engage patients in recovery, a “treatable moment” for this vulnerable population.10
Currently, a common approach in the hospitalized patient is to attempt medically assisted withdrawal using a rapid taper of long-acting opioids. This process may appeal to healthcare providers who hope to guide their patients in transitioning to opioid abstinence. However, tapering an opioid regimen, even over a period of months, results in unacceptably high rates of relapse (as high as 70% to 90% in some studies), especially when a patient is acutely ill and symptomatic from a concurrent medical issue.11-13 In the hospital setting, this treatment failure can manifest as pain and undertreated withdrawal symptoms (such as agitation, arthralgias, and gastrointestinal distress), which may hinder some patients from completing their treatment or drive some to leave against medical advice.14 Further harm may occur when an inpatient rapid taper is accomplished, putting patients at increased risk of a fatal relapse after discharge due to loss of tolerance.15Maintenance opioid agonist therapy with buprenorphine or methadone, in which a long-acting opioid is titrated until craving and withdrawal symptoms are well controlled, is the first-line modality for MAT among patients with OUDs in outpatient settings and is associated with reduced risk of fatal overdose and all-cause mortality.16 Initiation and dose stabilization of agonist therapy with these agents during acute medical hospitalization has been shown to be feasible in a variety of inpatient settings.17-20 In one trial, patients randomized to buprenorphine induction and linkage to office-based therapy during their inpatient stay were more than 5 times as likely to enter and remain in treatment after discharge when compared with those in whom buprenorphine was tapered.20 International guidelines support the use of maintenance agonist therapy in this context, but this remains an underutilized strategy in recent efforts to treat OUDs in the United States.21,22 A few key barriers currently prevent this strategy from being applied broadly within our healthcare system.
TOWARD EVIDENCE-BASED INPATIENT MANAGEMENT
First, there is a common misconception that regulations prohibit the use of methadone and buprenorphine for opioid agonist therapy by inpatient medical providers without special certification. Title 42 of the Code of Federal Regulations (CFR) provides extensive guidance regarding the use of opioid medications by registered outpatient opioid treatment programs. However, it also contains an exemption from these rules for hospitals treating patients with emergent medical needs (21 CFR § 1306.07[c]) allowing hospital-based clinicians “to maintain or detoxify a person as an incidental adjunct to medical or surgical treatment of conditions other than addiction” without restriction. According to guidelines from the Substance Abuse and Mental Health Services Administration, this exemption applies to the use of both methadone and buprenorphine.23
Many clinicians and hospital pharmacy departments interpret this law to limit the use of maintenance therapy in patients already enrolled in outpatient programs or to require a rapid taper over the first 3 days of hospitalization. However, these interpretations may in part be rooted in confusion with an adjacent section of the regulations (21 CFR § 1306.07[b]) directed at outpatient physicians providing time-limited, emergency treatment for withdrawal in an office setting. The application of this time limit to hospitalized patients has not been supported by communication from the Drug Enforcement Agency.24 There is no case law or other regulation requiring an opioid regimen to be time limited for patients during medical hospitalization, and hospital policies need not place undue constraints on the ability of clinicians to stabilize patients on maintenance therapy and transition them to outpatient treatment.
Second, the limited capacity of existing opioid maintenance programs can lead to a gap in treatment upon hospital discharge for patients in whom methadone or buprenorphine is initiated. Health delivery systems can play a role in mitigating the impact of this resource gap. Integrating the model of screening, brief intervention, and referral to treatment into hospital admission processes and engaging social workers, addiction consult services (where available), and other supports early in the course of hospitalization can help facilitate appropriate follow-up care.25,26 Hospitals may also be eligible for federal funding to strengthen local referral networks for outpatient MAT programs under Section 103 of the Comprehensive Addiction and Recovery Act passed into law in July 2016. Innovative delivery models designed to enhance integration across community stakeholders in healthcare, social services, and criminal justice have recently been developed, such as Vermont’s “Hub and Spoke” model,27 Boston Medical Center’s Faster Paths opioid urgent care center,28 and the police-led Angel Program in Gloucester, Massachusetts.29 Implementation science studies will be needed to identify the most effective ways to engage inpatient medical teams in such efforts.
Currently, individual providers can already play a central role in providing a bridge for patients in whom a delay in beginning MAT cannot be avoided upon discharge. Interim buprenorphine maintenance treatment has been shown to dramatically decrease the use of illicit opioids among those awaiting initiation of comprehensive MAT programs and substantially increase retention in long-term treatment.20,30,31 With the recent expansion of the limits on buprenorphine prescriptions to 275 patients per provider (part of the waiver required under the Drug Addiction Treatment Act [DATA] of 2000 to provide outpatient buprenorphine treatment, also known as a DATA waiver), this may be an increasingly promising option for hospital discharge.
Obtaining a waiver to prescribe buprenorphine is not required for the inpatient initiation of buprenorphine therapy. However, doing so is relatively simple (requiring an online, 8-hour training [https://www.samhsa.gov/medication-assisted-treatment/training-resources/buprenorphine-physician-training]) and allows hospital-based providers not only to ensure optimal management of OUDs during hospitalization but also to help their patients with the next steps toward recovery after discharge. The use of buprenorphine may be challenging in some patients with significant pain as a component of their medical condition. For these patients, methadone will likely be better tolerated.
Additional funding is also urgently needed to expand the capacity of existing opioid treatment programs and create specialized discharge-transition clinics that can provide structured interim opioid therapy while patients are on waitlists for traditional MAT programs. Requiring patients who are not ready or able to begin long-term maintenance agonist therapy to rapidly taper an inpatient opioid regimen unnecessarily puts them at risk for overdose after discharge.15 Regardless of the available resources for long-term treatment within the community, hospital discharge planning should include a naloxone prescription and brief training for patients and their loved ones.32 The long-acting opioid antagonist, depot naltrexone, is another effective, alternative MAT option and is increasingly used in community settings among patients who are motivated to achieve opioid abstinence.33,34 It has not yet been studied among hospitalized patients, and further research is needed to determine if it could be a viable option for discharge. However, the requirement that a patient be abstinent from opioids for 7 to 10 days prior to administering the first dose of depot naltrexone may serve as a significant barrier to its use for most hospitalized patients.
Finally, healthcare providers must be trained in the appropriate use of opioid agonist therapy. Medical schools, residency programs, and schools of pharmacy and nursing should develop curricula to expand the capacity of nonspecialists to care for patients with OUDs and to focus on judicious analgesic prescribing to prevent chronic opioid use. This curriculum should address the appropriate titration of methadone and buprenorphine for agonist therapy and address the stigma faced by patients with substance use disorders. Other important topics include the management of overdose and withdrawal symptoms, structured approaches to pain management in patients with OUDs, harm-reduction methods, and multidisciplinary care for the psychosocial and psychiatric comorbidities of addiction. Though international guidelines have been developed for the inpatient management of patients with OUDs,21,22 hospitals and professional societies should take a leadership role in facilitating continuing education to disseminate them among current medical providers.
There is great potential for the leadership and front-line staff of hospital systems, with a few key changes in policy and practice, to become advocates for patients with OUDs to access treatment. As perspectives about opioid prescribing change amid efforts to limit the escalation of the current heroin epidemic, it is vital to identify opportunities to reduce opioid exposure for opioid-naïve patients and enhance the engagement of patients diagnosed with OUDs in treatment.
Disclosure
The authors have no conflicts of interest to declare.
1. Longo DL, Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med. 2016;374(2):154-163. doi:10.1056/NEJMra1508490. PubMed
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. doi:10.15585/mmwr.rr6501e1. PubMed
3. Dans PE, Matricciani RM, Otter SE, Reuland DS. Intravenous drug abuse and one academic health center. JAMA. 1990;263(23):3173-3176. PubMed
4. Stein MD, Wilkinson J, Berglas N, O’Sullivan P. Prevalence and detection of illicit drug disorders among hospitalized patients. Am J Drug Alcohol Abuse. 1996;22(3):463-471. PubMed
5. Brown RL, Leonard T, Saunders LA, Papasouliotis O. The prevalence and detection of substance use disorders among inpatients ages 18 to 49: an opportunity for prevention. Prev Med. 1998;27(1):101-110. doi:10.1006/pmed.1997.0250. PubMed
6. McNeely J, Gourevitch MN, Paone D, Shah S, Wright S, Heller D. Estimating the prevalence of illicit opioid use in New York City using multiple data sources. BMC Public Health. 2012;12:443. doi:10.1186/1471-2458-12-443. PubMed
7. Ronan MV, Herzig SJ. Hospitalizations Related To Opioid Abuse/Dependence And Associated Serious Infections Increased Sharply, 2002-12. Health Aff. 2016;35(5):832-837. doi:10.1377/hlthaff.2015.1424. PubMed
8. Pan I-J, Yi H. Prevalence of hospitalized live births affected by alcohol and drugs and parturient women diagnosed with substance abuse at liveborn delivery: United States, 1999-2008. Matern Child Health J. 2013;17(4):667-676. doi:10.1007/s10995-012-1046-3. PubMed
9. Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372(22):2118-2126. doi:10.1056/NEJMsa1500439. PubMed
10. O’Toole TP, Pollini RA, Ford DE, Bigelow G. The health encounter as a treatable moment for homeless substance-using adults: the role of homelessness, health seeking behavior, readiness for behavior change and motivation for treatment. Addict Behav. 2008;33(9):1239-1243. doi:10.1016/j.addbeh.2008.04.015. PubMed
11. Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database Syst Rev. 2016;(5):CD011117. doi:10.1002/14651858.CD011117.pub2. PubMed
12. Gossop M, Green L, Phillips G, Bradley B. Lapse, relapse and survival among opiate addicts after treatment. A prospective follow-up study. Br J Psychiatry. 1989;154:348-353. PubMed
13. Smyth BP, Barry J, Keenan E, Ducray K. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010;103(6):176-179. PubMed
14. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. doi:10.1016/j.socscimed.2014.01.010. PubMed
15. Strang J. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. doi:10.1136/bmj.326.7396.959. PubMed
16. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. PubMed
17. Persico AM, Di Giannantonio M, Tempesta E. A prospective assessment of opiate addiction treatment protocols for inpatients with HIV-related syndromes. Drug Alcohol Depend. 1991;27(1):79-86. PubMed
18. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. doi:10.1007/s11606-010-1311-3. PubMed
19. Morozova O, Dvoryak S, Altice FL. Methadone treatment improves tuberculosis treatment among hospitalized opioid dependent patients in Ukraine. Int J Drug Policy. 2013;24(6):e91-e98. doi:10.1016/j.drugpo.2013.09.001. PubMed
20. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine Treatment for Hospitalized, Opioid-Dependent Patients: A Randomized Clinical Trial. JAMA Intern Med. 2014;174(8):1369. doi:10.1001/jamainternmed.2014.2556. PubMed
21. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284-1293. doi:10.1016/S0140-6736(09)61036-9. PubMed
22. Donroe JH, Holt SR, Tetrault JM. Caring for patients with opioid use disorder in the hospital. CMAJ. 2016;188(17-18):1232-1239. doi:10.1503/cmaj.160290. PubMed
23. Substance Abuse and Mental Health Services Administration. Special Circumstances for Providing Buprenorphine. https://www.samhsa.gov/medication-assisted-treatment/legislation-regulations-guidelines/special-circumstances-providing-buprenorphine. Accessed October 8, 2016.
24. Noska A, Mohan A, Wakeman S, Rich J, Boutwell A. Managing Opioid Use Disorder During and After Acute Hospitalization: A Case-Based Review Clarifying Methadone Regulation for Acute Care Settings. J Addict Behav Ther Rehabil. 2015;4(2). pii: 1000138. doi:10.4172/2324-9005.1000138. PubMed
25. InSight Project Research Group. SBIRT outcomes in Houston: final report on InSight, a hospital district-based program for patients at risk for alcohol or drug use problems. Alcohol Clin Exp Res. 2009;33(8):1374-1381. doi:10.1111/j.1530-0277.2009.00967.x. PubMed
26. Estee S, Wickizer T, He L, Shah MF, Mancuso D. Evaluation of the Washington state screening, brief intervention, and referral to treatment project: cost outcomes for Medicaid patients screened in hospital emergency departments. Med Care. 2010;48(1):18-24. doi:10.1097/MLR.0b013e3181bd498f. PubMed
27. Simpatico TA. Vermont responds to its opioid crisis. Prev Med. 2015;80:10-11. doi:10.1016/j.ypmed.2015.04.002. PubMed
28. Boston University Medical Center. Boston medical center launches new opioid urgent care center. https://www.eurekalert.org/pub_releases/2016-10/bumc-bmc101716.php. Published on October 17, 2016. Accessed December 29, 2016.
29. Schiff DM, Drainoni M-L, Bair-Merritt M, Weinstein Z, Rosenbloom D. A Police-Led Addiction Treatment Referral Program in Massachusetts. N Engl J Med. 2016;375(25):2502-2503. doi:10.1056/NEJMc1611640. PubMed
30. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474. PubMed
31. Sigmon SC, Ochalek TA, Meyer AC, et al. Interim Buprenorphine vs. Waiting List for Opioid Dependence. N Engl J Med. 2016;375(25):2504-2505. doi:10.1056/NEJMc1610047. PubMed
32. McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction. 2016;111(7):1177-1187. doi:10.1111/add.13326. . 2015;9(3):238-243. doi:10.1097/ADM.0000000000000125.J Addict Med PubMed
34. Nunes EV, Krupitsky E, Ling W, et al. Treating Opioid Dependence With Injectable Extended-Release Naltrexone (XR-NTX): Who Will Respond? . 2011;377(9776):1506-1513. doi:10.1016/S0140-6736(11)60358-9.Lancet PubMed
33. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. PubMed
The United States is facing an epidemic of prescription opioid and heroin use, which has been linked to the escalating prescribing of opioid analgesics. Though opioid prescriptions appear to be reaching a plateau, estimates suggest there are at least 900,000 active heroin users in the United States, and this number continues to grow.1 One response to this epidemic (through state legislation and medical society guidelines) has been a move to reduce opioid prescribing in order to diminish the potential for diversion and misuse.2 However, the treatment of pain is not the sole driver of heroin epidemiology, and new strategies are also needed to better engage patients with existing opioid use disorders (OUDs) to begin treatment. These patients are increasingly hospitalized for infectious comorbidities of injection drug use, trauma, or pregnancy, and this may present a unique opportunity to initiate these patients on maintenance opioid agonist therapy, the most effective option for medication-assisted treatment (MAT) for addiction.
MISSED OPPORTUNITIES
Patients with OUDs comprise an estimated 2% to 4% of hospitalized patients, representing a disproportionately large number of inpatients.3-6 According to a recent analysis of data from the National (Nationwide) Inpatient Sample, the estimated annual number of hospitalizations associated with OUDs in the United States increased from approximately 300,000 to more than 500,000 in the decade from 2002 to 2012.7 Severe bacterial infections associated with intravenous administration of opioids (including endocarditis, osteomyelitis, septic arthritis, and epidural abscess) increased substantially at an estimated cost of more than $700 million in 2012.7 Over a similar period, the prevalence of opioid use among women in labor increased from 13.7 to 22.0 per 10,000 live births,8 and there was a corresponding rise in admissions to neonatal intensive care units for neonatal abstinence syndrome.9 As the prevalence of prescription drug and heroin dependence continues to rise across the United States, hospitals and clinicians find themselves on the front lines of this epidemic, creating potential opportunities to engage patients in recovery, a “treatable moment” for this vulnerable population.10
Currently, a common approach in the hospitalized patient is to attempt medically assisted withdrawal using a rapid taper of long-acting opioids. This process may appeal to healthcare providers who hope to guide their patients in transitioning to opioid abstinence. However, tapering an opioid regimen, even over a period of months, results in unacceptably high rates of relapse (as high as 70% to 90% in some studies), especially when a patient is acutely ill and symptomatic from a concurrent medical issue.11-13 In the hospital setting, this treatment failure can manifest as pain and undertreated withdrawal symptoms (such as agitation, arthralgias, and gastrointestinal distress), which may hinder some patients from completing their treatment or drive some to leave against medical advice.14 Further harm may occur when an inpatient rapid taper is accomplished, putting patients at increased risk of a fatal relapse after discharge due to loss of tolerance.15Maintenance opioid agonist therapy with buprenorphine or methadone, in which a long-acting opioid is titrated until craving and withdrawal symptoms are well controlled, is the first-line modality for MAT among patients with OUDs in outpatient settings and is associated with reduced risk of fatal overdose and all-cause mortality.16 Initiation and dose stabilization of agonist therapy with these agents during acute medical hospitalization has been shown to be feasible in a variety of inpatient settings.17-20 In one trial, patients randomized to buprenorphine induction and linkage to office-based therapy during their inpatient stay were more than 5 times as likely to enter and remain in treatment after discharge when compared with those in whom buprenorphine was tapered.20 International guidelines support the use of maintenance agonist therapy in this context, but this remains an underutilized strategy in recent efforts to treat OUDs in the United States.21,22 A few key barriers currently prevent this strategy from being applied broadly within our healthcare system.
TOWARD EVIDENCE-BASED INPATIENT MANAGEMENT
First, there is a common misconception that regulations prohibit the use of methadone and buprenorphine for opioid agonist therapy by inpatient medical providers without special certification. Title 42 of the Code of Federal Regulations (CFR) provides extensive guidance regarding the use of opioid medications by registered outpatient opioid treatment programs. However, it also contains an exemption from these rules for hospitals treating patients with emergent medical needs (21 CFR § 1306.07[c]) allowing hospital-based clinicians “to maintain or detoxify a person as an incidental adjunct to medical or surgical treatment of conditions other than addiction” without restriction. According to guidelines from the Substance Abuse and Mental Health Services Administration, this exemption applies to the use of both methadone and buprenorphine.23
Many clinicians and hospital pharmacy departments interpret this law to limit the use of maintenance therapy in patients already enrolled in outpatient programs or to require a rapid taper over the first 3 days of hospitalization. However, these interpretations may in part be rooted in confusion with an adjacent section of the regulations (21 CFR § 1306.07[b]) directed at outpatient physicians providing time-limited, emergency treatment for withdrawal in an office setting. The application of this time limit to hospitalized patients has not been supported by communication from the Drug Enforcement Agency.24 There is no case law or other regulation requiring an opioid regimen to be time limited for patients during medical hospitalization, and hospital policies need not place undue constraints on the ability of clinicians to stabilize patients on maintenance therapy and transition them to outpatient treatment.
Second, the limited capacity of existing opioid maintenance programs can lead to a gap in treatment upon hospital discharge for patients in whom methadone or buprenorphine is initiated. Health delivery systems can play a role in mitigating the impact of this resource gap. Integrating the model of screening, brief intervention, and referral to treatment into hospital admission processes and engaging social workers, addiction consult services (where available), and other supports early in the course of hospitalization can help facilitate appropriate follow-up care.25,26 Hospitals may also be eligible for federal funding to strengthen local referral networks for outpatient MAT programs under Section 103 of the Comprehensive Addiction and Recovery Act passed into law in July 2016. Innovative delivery models designed to enhance integration across community stakeholders in healthcare, social services, and criminal justice have recently been developed, such as Vermont’s “Hub and Spoke” model,27 Boston Medical Center’s Faster Paths opioid urgent care center,28 and the police-led Angel Program in Gloucester, Massachusetts.29 Implementation science studies will be needed to identify the most effective ways to engage inpatient medical teams in such efforts.
Currently, individual providers can already play a central role in providing a bridge for patients in whom a delay in beginning MAT cannot be avoided upon discharge. Interim buprenorphine maintenance treatment has been shown to dramatically decrease the use of illicit opioids among those awaiting initiation of comprehensive MAT programs and substantially increase retention in long-term treatment.20,30,31 With the recent expansion of the limits on buprenorphine prescriptions to 275 patients per provider (part of the waiver required under the Drug Addiction Treatment Act [DATA] of 2000 to provide outpatient buprenorphine treatment, also known as a DATA waiver), this may be an increasingly promising option for hospital discharge.
Obtaining a waiver to prescribe buprenorphine is not required for the inpatient initiation of buprenorphine therapy. However, doing so is relatively simple (requiring an online, 8-hour training [https://www.samhsa.gov/medication-assisted-treatment/training-resources/buprenorphine-physician-training]) and allows hospital-based providers not only to ensure optimal management of OUDs during hospitalization but also to help their patients with the next steps toward recovery after discharge. The use of buprenorphine may be challenging in some patients with significant pain as a component of their medical condition. For these patients, methadone will likely be better tolerated.
Additional funding is also urgently needed to expand the capacity of existing opioid treatment programs and create specialized discharge-transition clinics that can provide structured interim opioid therapy while patients are on waitlists for traditional MAT programs. Requiring patients who are not ready or able to begin long-term maintenance agonist therapy to rapidly taper an inpatient opioid regimen unnecessarily puts them at risk for overdose after discharge.15 Regardless of the available resources for long-term treatment within the community, hospital discharge planning should include a naloxone prescription and brief training for patients and their loved ones.32 The long-acting opioid antagonist, depot naltrexone, is another effective, alternative MAT option and is increasingly used in community settings among patients who are motivated to achieve opioid abstinence.33,34 It has not yet been studied among hospitalized patients, and further research is needed to determine if it could be a viable option for discharge. However, the requirement that a patient be abstinent from opioids for 7 to 10 days prior to administering the first dose of depot naltrexone may serve as a significant barrier to its use for most hospitalized patients.
Finally, healthcare providers must be trained in the appropriate use of opioid agonist therapy. Medical schools, residency programs, and schools of pharmacy and nursing should develop curricula to expand the capacity of nonspecialists to care for patients with OUDs and to focus on judicious analgesic prescribing to prevent chronic opioid use. This curriculum should address the appropriate titration of methadone and buprenorphine for agonist therapy and address the stigma faced by patients with substance use disorders. Other important topics include the management of overdose and withdrawal symptoms, structured approaches to pain management in patients with OUDs, harm-reduction methods, and multidisciplinary care for the psychosocial and psychiatric comorbidities of addiction. Though international guidelines have been developed for the inpatient management of patients with OUDs,21,22 hospitals and professional societies should take a leadership role in facilitating continuing education to disseminate them among current medical providers.
There is great potential for the leadership and front-line staff of hospital systems, with a few key changes in policy and practice, to become advocates for patients with OUDs to access treatment. As perspectives about opioid prescribing change amid efforts to limit the escalation of the current heroin epidemic, it is vital to identify opportunities to reduce opioid exposure for opioid-naïve patients and enhance the engagement of patients diagnosed with OUDs in treatment.
Disclosure
The authors have no conflicts of interest to declare.
The United States is facing an epidemic of prescription opioid and heroin use, which has been linked to the escalating prescribing of opioid analgesics. Though opioid prescriptions appear to be reaching a plateau, estimates suggest there are at least 900,000 active heroin users in the United States, and this number continues to grow.1 One response to this epidemic (through state legislation and medical society guidelines) has been a move to reduce opioid prescribing in order to diminish the potential for diversion and misuse.2 However, the treatment of pain is not the sole driver of heroin epidemiology, and new strategies are also needed to better engage patients with existing opioid use disorders (OUDs) to begin treatment. These patients are increasingly hospitalized for infectious comorbidities of injection drug use, trauma, or pregnancy, and this may present a unique opportunity to initiate these patients on maintenance opioid agonist therapy, the most effective option for medication-assisted treatment (MAT) for addiction.
MISSED OPPORTUNITIES
Patients with OUDs comprise an estimated 2% to 4% of hospitalized patients, representing a disproportionately large number of inpatients.3-6 According to a recent analysis of data from the National (Nationwide) Inpatient Sample, the estimated annual number of hospitalizations associated with OUDs in the United States increased from approximately 300,000 to more than 500,000 in the decade from 2002 to 2012.7 Severe bacterial infections associated with intravenous administration of opioids (including endocarditis, osteomyelitis, septic arthritis, and epidural abscess) increased substantially at an estimated cost of more than $700 million in 2012.7 Over a similar period, the prevalence of opioid use among women in labor increased from 13.7 to 22.0 per 10,000 live births,8 and there was a corresponding rise in admissions to neonatal intensive care units for neonatal abstinence syndrome.9 As the prevalence of prescription drug and heroin dependence continues to rise across the United States, hospitals and clinicians find themselves on the front lines of this epidemic, creating potential opportunities to engage patients in recovery, a “treatable moment” for this vulnerable population.10
Currently, a common approach in the hospitalized patient is to attempt medically assisted withdrawal using a rapid taper of long-acting opioids. This process may appeal to healthcare providers who hope to guide their patients in transitioning to opioid abstinence. However, tapering an opioid regimen, even over a period of months, results in unacceptably high rates of relapse (as high as 70% to 90% in some studies), especially when a patient is acutely ill and symptomatic from a concurrent medical issue.11-13 In the hospital setting, this treatment failure can manifest as pain and undertreated withdrawal symptoms (such as agitation, arthralgias, and gastrointestinal distress), which may hinder some patients from completing their treatment or drive some to leave against medical advice.14 Further harm may occur when an inpatient rapid taper is accomplished, putting patients at increased risk of a fatal relapse after discharge due to loss of tolerance.15Maintenance opioid agonist therapy with buprenorphine or methadone, in which a long-acting opioid is titrated until craving and withdrawal symptoms are well controlled, is the first-line modality for MAT among patients with OUDs in outpatient settings and is associated with reduced risk of fatal overdose and all-cause mortality.16 Initiation and dose stabilization of agonist therapy with these agents during acute medical hospitalization has been shown to be feasible in a variety of inpatient settings.17-20 In one trial, patients randomized to buprenorphine induction and linkage to office-based therapy during their inpatient stay were more than 5 times as likely to enter and remain in treatment after discharge when compared with those in whom buprenorphine was tapered.20 International guidelines support the use of maintenance agonist therapy in this context, but this remains an underutilized strategy in recent efforts to treat OUDs in the United States.21,22 A few key barriers currently prevent this strategy from being applied broadly within our healthcare system.
TOWARD EVIDENCE-BASED INPATIENT MANAGEMENT
First, there is a common misconception that regulations prohibit the use of methadone and buprenorphine for opioid agonist therapy by inpatient medical providers without special certification. Title 42 of the Code of Federal Regulations (CFR) provides extensive guidance regarding the use of opioid medications by registered outpatient opioid treatment programs. However, it also contains an exemption from these rules for hospitals treating patients with emergent medical needs (21 CFR § 1306.07[c]) allowing hospital-based clinicians “to maintain or detoxify a person as an incidental adjunct to medical or surgical treatment of conditions other than addiction” without restriction. According to guidelines from the Substance Abuse and Mental Health Services Administration, this exemption applies to the use of both methadone and buprenorphine.23
Many clinicians and hospital pharmacy departments interpret this law to limit the use of maintenance therapy in patients already enrolled in outpatient programs or to require a rapid taper over the first 3 days of hospitalization. However, these interpretations may in part be rooted in confusion with an adjacent section of the regulations (21 CFR § 1306.07[b]) directed at outpatient physicians providing time-limited, emergency treatment for withdrawal in an office setting. The application of this time limit to hospitalized patients has not been supported by communication from the Drug Enforcement Agency.24 There is no case law or other regulation requiring an opioid regimen to be time limited for patients during medical hospitalization, and hospital policies need not place undue constraints on the ability of clinicians to stabilize patients on maintenance therapy and transition them to outpatient treatment.
Second, the limited capacity of existing opioid maintenance programs can lead to a gap in treatment upon hospital discharge for patients in whom methadone or buprenorphine is initiated. Health delivery systems can play a role in mitigating the impact of this resource gap. Integrating the model of screening, brief intervention, and referral to treatment into hospital admission processes and engaging social workers, addiction consult services (where available), and other supports early in the course of hospitalization can help facilitate appropriate follow-up care.25,26 Hospitals may also be eligible for federal funding to strengthen local referral networks for outpatient MAT programs under Section 103 of the Comprehensive Addiction and Recovery Act passed into law in July 2016. Innovative delivery models designed to enhance integration across community stakeholders in healthcare, social services, and criminal justice have recently been developed, such as Vermont’s “Hub and Spoke” model,27 Boston Medical Center’s Faster Paths opioid urgent care center,28 and the police-led Angel Program in Gloucester, Massachusetts.29 Implementation science studies will be needed to identify the most effective ways to engage inpatient medical teams in such efforts.
Currently, individual providers can already play a central role in providing a bridge for patients in whom a delay in beginning MAT cannot be avoided upon discharge. Interim buprenorphine maintenance treatment has been shown to dramatically decrease the use of illicit opioids among those awaiting initiation of comprehensive MAT programs and substantially increase retention in long-term treatment.20,30,31 With the recent expansion of the limits on buprenorphine prescriptions to 275 patients per provider (part of the waiver required under the Drug Addiction Treatment Act [DATA] of 2000 to provide outpatient buprenorphine treatment, also known as a DATA waiver), this may be an increasingly promising option for hospital discharge.
Obtaining a waiver to prescribe buprenorphine is not required for the inpatient initiation of buprenorphine therapy. However, doing so is relatively simple (requiring an online, 8-hour training [https://www.samhsa.gov/medication-assisted-treatment/training-resources/buprenorphine-physician-training]) and allows hospital-based providers not only to ensure optimal management of OUDs during hospitalization but also to help their patients with the next steps toward recovery after discharge. The use of buprenorphine may be challenging in some patients with significant pain as a component of their medical condition. For these patients, methadone will likely be better tolerated.
Additional funding is also urgently needed to expand the capacity of existing opioid treatment programs and create specialized discharge-transition clinics that can provide structured interim opioid therapy while patients are on waitlists for traditional MAT programs. Requiring patients who are not ready or able to begin long-term maintenance agonist therapy to rapidly taper an inpatient opioid regimen unnecessarily puts them at risk for overdose after discharge.15 Regardless of the available resources for long-term treatment within the community, hospital discharge planning should include a naloxone prescription and brief training for patients and their loved ones.32 The long-acting opioid antagonist, depot naltrexone, is another effective, alternative MAT option and is increasingly used in community settings among patients who are motivated to achieve opioid abstinence.33,34 It has not yet been studied among hospitalized patients, and further research is needed to determine if it could be a viable option for discharge. However, the requirement that a patient be abstinent from opioids for 7 to 10 days prior to administering the first dose of depot naltrexone may serve as a significant barrier to its use for most hospitalized patients.
Finally, healthcare providers must be trained in the appropriate use of opioid agonist therapy. Medical schools, residency programs, and schools of pharmacy and nursing should develop curricula to expand the capacity of nonspecialists to care for patients with OUDs and to focus on judicious analgesic prescribing to prevent chronic opioid use. This curriculum should address the appropriate titration of methadone and buprenorphine for agonist therapy and address the stigma faced by patients with substance use disorders. Other important topics include the management of overdose and withdrawal symptoms, structured approaches to pain management in patients with OUDs, harm-reduction methods, and multidisciplinary care for the psychosocial and psychiatric comorbidities of addiction. Though international guidelines have been developed for the inpatient management of patients with OUDs,21,22 hospitals and professional societies should take a leadership role in facilitating continuing education to disseminate them among current medical providers.
There is great potential for the leadership and front-line staff of hospital systems, with a few key changes in policy and practice, to become advocates for patients with OUDs to access treatment. As perspectives about opioid prescribing change amid efforts to limit the escalation of the current heroin epidemic, it is vital to identify opportunities to reduce opioid exposure for opioid-naïve patients and enhance the engagement of patients diagnosed with OUDs in treatment.
Disclosure
The authors have no conflicts of interest to declare.
1. Longo DL, Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med. 2016;374(2):154-163. doi:10.1056/NEJMra1508490. PubMed
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. doi:10.15585/mmwr.rr6501e1. PubMed
3. Dans PE, Matricciani RM, Otter SE, Reuland DS. Intravenous drug abuse and one academic health center. JAMA. 1990;263(23):3173-3176. PubMed
4. Stein MD, Wilkinson J, Berglas N, O’Sullivan P. Prevalence and detection of illicit drug disorders among hospitalized patients. Am J Drug Alcohol Abuse. 1996;22(3):463-471. PubMed
5. Brown RL, Leonard T, Saunders LA, Papasouliotis O. The prevalence and detection of substance use disorders among inpatients ages 18 to 49: an opportunity for prevention. Prev Med. 1998;27(1):101-110. doi:10.1006/pmed.1997.0250. PubMed
6. McNeely J, Gourevitch MN, Paone D, Shah S, Wright S, Heller D. Estimating the prevalence of illicit opioid use in New York City using multiple data sources. BMC Public Health. 2012;12:443. doi:10.1186/1471-2458-12-443. PubMed
7. Ronan MV, Herzig SJ. Hospitalizations Related To Opioid Abuse/Dependence And Associated Serious Infections Increased Sharply, 2002-12. Health Aff. 2016;35(5):832-837. doi:10.1377/hlthaff.2015.1424. PubMed
8. Pan I-J, Yi H. Prevalence of hospitalized live births affected by alcohol and drugs and parturient women diagnosed with substance abuse at liveborn delivery: United States, 1999-2008. Matern Child Health J. 2013;17(4):667-676. doi:10.1007/s10995-012-1046-3. PubMed
9. Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372(22):2118-2126. doi:10.1056/NEJMsa1500439. PubMed
10. O’Toole TP, Pollini RA, Ford DE, Bigelow G. The health encounter as a treatable moment for homeless substance-using adults: the role of homelessness, health seeking behavior, readiness for behavior change and motivation for treatment. Addict Behav. 2008;33(9):1239-1243. doi:10.1016/j.addbeh.2008.04.015. PubMed
11. Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database Syst Rev. 2016;(5):CD011117. doi:10.1002/14651858.CD011117.pub2. PubMed
12. Gossop M, Green L, Phillips G, Bradley B. Lapse, relapse and survival among opiate addicts after treatment. A prospective follow-up study. Br J Psychiatry. 1989;154:348-353. PubMed
13. Smyth BP, Barry J, Keenan E, Ducray K. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010;103(6):176-179. PubMed
14. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. doi:10.1016/j.socscimed.2014.01.010. PubMed
15. Strang J. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. doi:10.1136/bmj.326.7396.959. PubMed
16. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. PubMed
17. Persico AM, Di Giannantonio M, Tempesta E. A prospective assessment of opiate addiction treatment protocols for inpatients with HIV-related syndromes. Drug Alcohol Depend. 1991;27(1):79-86. PubMed
18. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. doi:10.1007/s11606-010-1311-3. PubMed
19. Morozova O, Dvoryak S, Altice FL. Methadone treatment improves tuberculosis treatment among hospitalized opioid dependent patients in Ukraine. Int J Drug Policy. 2013;24(6):e91-e98. doi:10.1016/j.drugpo.2013.09.001. PubMed
20. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine Treatment for Hospitalized, Opioid-Dependent Patients: A Randomized Clinical Trial. JAMA Intern Med. 2014;174(8):1369. doi:10.1001/jamainternmed.2014.2556. PubMed
21. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284-1293. doi:10.1016/S0140-6736(09)61036-9. PubMed
22. Donroe JH, Holt SR, Tetrault JM. Caring for patients with opioid use disorder in the hospital. CMAJ. 2016;188(17-18):1232-1239. doi:10.1503/cmaj.160290. PubMed
23. Substance Abuse and Mental Health Services Administration. Special Circumstances for Providing Buprenorphine. https://www.samhsa.gov/medication-assisted-treatment/legislation-regulations-guidelines/special-circumstances-providing-buprenorphine. Accessed October 8, 2016.
24. Noska A, Mohan A, Wakeman S, Rich J, Boutwell A. Managing Opioid Use Disorder During and After Acute Hospitalization: A Case-Based Review Clarifying Methadone Regulation for Acute Care Settings. J Addict Behav Ther Rehabil. 2015;4(2). pii: 1000138. doi:10.4172/2324-9005.1000138. PubMed
25. InSight Project Research Group. SBIRT outcomes in Houston: final report on InSight, a hospital district-based program for patients at risk for alcohol or drug use problems. Alcohol Clin Exp Res. 2009;33(8):1374-1381. doi:10.1111/j.1530-0277.2009.00967.x. PubMed
26. Estee S, Wickizer T, He L, Shah MF, Mancuso D. Evaluation of the Washington state screening, brief intervention, and referral to treatment project: cost outcomes for Medicaid patients screened in hospital emergency departments. Med Care. 2010;48(1):18-24. doi:10.1097/MLR.0b013e3181bd498f. PubMed
27. Simpatico TA. Vermont responds to its opioid crisis. Prev Med. 2015;80:10-11. doi:10.1016/j.ypmed.2015.04.002. PubMed
28. Boston University Medical Center. Boston medical center launches new opioid urgent care center. https://www.eurekalert.org/pub_releases/2016-10/bumc-bmc101716.php. Published on October 17, 2016. Accessed December 29, 2016.
29. Schiff DM, Drainoni M-L, Bair-Merritt M, Weinstein Z, Rosenbloom D. A Police-Led Addiction Treatment Referral Program in Massachusetts. N Engl J Med. 2016;375(25):2502-2503. doi:10.1056/NEJMc1611640. PubMed
30. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474. PubMed
31. Sigmon SC, Ochalek TA, Meyer AC, et al. Interim Buprenorphine vs. Waiting List for Opioid Dependence. N Engl J Med. 2016;375(25):2504-2505. doi:10.1056/NEJMc1610047. PubMed
32. McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction. 2016;111(7):1177-1187. doi:10.1111/add.13326. . 2015;9(3):238-243. doi:10.1097/ADM.0000000000000125.J Addict Med PubMed
34. Nunes EV, Krupitsky E, Ling W, et al. Treating Opioid Dependence With Injectable Extended-Release Naltrexone (XR-NTX): Who Will Respond? . 2011;377(9776):1506-1513. doi:10.1016/S0140-6736(11)60358-9.Lancet PubMed
33. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. PubMed
1. Longo DL, Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med. 2016;374(2):154-163. doi:10.1056/NEJMra1508490. PubMed
2. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. doi:10.15585/mmwr.rr6501e1. PubMed
3. Dans PE, Matricciani RM, Otter SE, Reuland DS. Intravenous drug abuse and one academic health center. JAMA. 1990;263(23):3173-3176. PubMed
4. Stein MD, Wilkinson J, Berglas N, O’Sullivan P. Prevalence and detection of illicit drug disorders among hospitalized patients. Am J Drug Alcohol Abuse. 1996;22(3):463-471. PubMed
5. Brown RL, Leonard T, Saunders LA, Papasouliotis O. The prevalence and detection of substance use disorders among inpatients ages 18 to 49: an opportunity for prevention. Prev Med. 1998;27(1):101-110. doi:10.1006/pmed.1997.0250. PubMed
6. McNeely J, Gourevitch MN, Paone D, Shah S, Wright S, Heller D. Estimating the prevalence of illicit opioid use in New York City using multiple data sources. BMC Public Health. 2012;12:443. doi:10.1186/1471-2458-12-443. PubMed
7. Ronan MV, Herzig SJ. Hospitalizations Related To Opioid Abuse/Dependence And Associated Serious Infections Increased Sharply, 2002-12. Health Aff. 2016;35(5):832-837. doi:10.1377/hlthaff.2015.1424. PubMed
8. Pan I-J, Yi H. Prevalence of hospitalized live births affected by alcohol and drugs and parturient women diagnosed with substance abuse at liveborn delivery: United States, 1999-2008. Matern Child Health J. 2013;17(4):667-676. doi:10.1007/s10995-012-1046-3. PubMed
9. Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372(22):2118-2126. doi:10.1056/NEJMsa1500439. PubMed
10. O’Toole TP, Pollini RA, Ford DE, Bigelow G. The health encounter as a treatable moment for homeless substance-using adults: the role of homelessness, health seeking behavior, readiness for behavior change and motivation for treatment. Addict Behav. 2008;33(9):1239-1243. doi:10.1016/j.addbeh.2008.04.015. PubMed
11. Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database Syst Rev. 2016;(5):CD011117. doi:10.1002/14651858.CD011117.pub2. PubMed
12. Gossop M, Green L, Phillips G, Bradley B. Lapse, relapse and survival among opiate addicts after treatment. A prospective follow-up study. Br J Psychiatry. 1989;154:348-353. PubMed
13. Smyth BP, Barry J, Keenan E, Ducray K. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010;103(6):176-179. PubMed
14. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. doi:10.1016/j.socscimed.2014.01.010. PubMed
15. Strang J. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. doi:10.1136/bmj.326.7396.959. PubMed
16. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. PubMed
17. Persico AM, Di Giannantonio M, Tempesta E. A prospective assessment of opiate addiction treatment protocols for inpatients with HIV-related syndromes. Drug Alcohol Depend. 1991;27(1):79-86. PubMed
18. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. doi:10.1007/s11606-010-1311-3. PubMed
19. Morozova O, Dvoryak S, Altice FL. Methadone treatment improves tuberculosis treatment among hospitalized opioid dependent patients in Ukraine. Int J Drug Policy. 2013;24(6):e91-e98. doi:10.1016/j.drugpo.2013.09.001. PubMed
20. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine Treatment for Hospitalized, Opioid-Dependent Patients: A Randomized Clinical Trial. JAMA Intern Med. 2014;174(8):1369. doi:10.1001/jamainternmed.2014.2556. PubMed
21. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284-1293. doi:10.1016/S0140-6736(09)61036-9. PubMed
22. Donroe JH, Holt SR, Tetrault JM. Caring for patients with opioid use disorder in the hospital. CMAJ. 2016;188(17-18):1232-1239. doi:10.1503/cmaj.160290. PubMed
23. Substance Abuse and Mental Health Services Administration. Special Circumstances for Providing Buprenorphine. https://www.samhsa.gov/medication-assisted-treatment/legislation-regulations-guidelines/special-circumstances-providing-buprenorphine. Accessed October 8, 2016.
24. Noska A, Mohan A, Wakeman S, Rich J, Boutwell A. Managing Opioid Use Disorder During and After Acute Hospitalization: A Case-Based Review Clarifying Methadone Regulation for Acute Care Settings. J Addict Behav Ther Rehabil. 2015;4(2). pii: 1000138. doi:10.4172/2324-9005.1000138. PubMed
25. InSight Project Research Group. SBIRT outcomes in Houston: final report on InSight, a hospital district-based program for patients at risk for alcohol or drug use problems. Alcohol Clin Exp Res. 2009;33(8):1374-1381. doi:10.1111/j.1530-0277.2009.00967.x. PubMed
26. Estee S, Wickizer T, He L, Shah MF, Mancuso D. Evaluation of the Washington state screening, brief intervention, and referral to treatment project: cost outcomes for Medicaid patients screened in hospital emergency departments. Med Care. 2010;48(1):18-24. doi:10.1097/MLR.0b013e3181bd498f. PubMed
27. Simpatico TA. Vermont responds to its opioid crisis. Prev Med. 2015;80:10-11. doi:10.1016/j.ypmed.2015.04.002. PubMed
28. Boston University Medical Center. Boston medical center launches new opioid urgent care center. https://www.eurekalert.org/pub_releases/2016-10/bumc-bmc101716.php. Published on October 17, 2016. Accessed December 29, 2016.
29. Schiff DM, Drainoni M-L, Bair-Merritt M, Weinstein Z, Rosenbloom D. A Police-Led Addiction Treatment Referral Program in Massachusetts. N Engl J Med. 2016;375(25):2502-2503. doi:10.1056/NEJMc1611640. PubMed
30. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474. PubMed
31. Sigmon SC, Ochalek TA, Meyer AC, et al. Interim Buprenorphine vs. Waiting List for Opioid Dependence. N Engl J Med. 2016;375(25):2504-2505. doi:10.1056/NEJMc1610047. PubMed
32. McDonald R, Strang J. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction. 2016;111(7):1177-1187. doi:10.1111/add.13326. . 2015;9(3):238-243. doi:10.1097/ADM.0000000000000125.J Addict Med PubMed
34. Nunes EV, Krupitsky E, Ling W, et al. Treating Opioid Dependence With Injectable Extended-Release Naltrexone (XR-NTX): Who Will Respond? . 2011;377(9776):1506-1513. doi:10.1016/S0140-6736(11)60358-9.Lancet PubMed
33. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. PubMed
© 2017 Society of Hospital Medicine
Hospital Medicine Point of Care Ultrasound Credentialing: An Example Protocol
Ultrasound has been used for decades by radiology, obstetrics-gynecology, and cardiology departments within a comprehensive paradigm in which a physician enters an order, then a trained sonographer performs the study, followed by a physician evaluating and interpreting the images.1 Unlike the traditional comprehensive paradigm, point-of-care ultrasound (POCUS) is a focused study that is both performed and interpreted by the bedside provider.2 POCUS has been demonstrated to improve diagnosis and clinical management in multiple studies.3-15
The scope of practice in POCUS differs by specialty, as POCUS is done to achieve specific procedural aims (eg, direct the needle to the correct location) or answer focused questions (eg, does the patient have a distended bladder?) related to the specialty. POCUS in hospital medicine (HM) provides immediate answers, without the delay and potential risk of transportation to other hospital areas. It may be used to diagnose pleural effusion, pneumonia, hydronephrosis, heart failure, deep vein thrombosis, and many other pathologies.5-15 It is important to understand that POCUS performed by HM is a limited study and is not a substitute for more complete ultrasound examinations conducted in the radiology suite or in the echocardiography lab.
POCUS should not be used exclusively in medical decision making, but rather in conjunction with the greater clinical context of each patient, building on established principles of diagnosis and management.
DEFINITIONS
- Credentialing: An umbrella term, which incorporates licensure, education, and certification.
- Privileging: Used to define the scope authorized for a provider by a healthcare organization based on an evaluation of the individual’s credentials and performance.
- Competency: An observable ability of a provider, integrating multiple components, such as knowledge and skills. Since competencies are observable, they can be measured and assessed to ensure their acquisition.
- Certification: The process by which an association grants recognition to a provider who has met certain predetermined qualifications specified by the association. Competence is distinguished from certification, which is defined as the process by which competence is recognized by an external agency.
All of the above mechanisms work together to provide the highest quality of reliability that a practitioner is providing safe, competent care.16-18
STATEMENTS FROM MAJOR SPECIALTY SOCIETIES
Acknowledging that there are no published guidelines in the realm of HM POCUS, the development of the credentialing process at our institution is consistent with published guidelines by Emergency Medicine societies (the most established physician users of POCUS) and the American Medical Association (AMA).19-21
The use of emergency ultrasound by physicians in the emergency department is endorsed by the American College of Emergency Physicians (ACEP).19 ACEP, along with the Society of Academic Emergency Medicine (SAEM), recommends that training in the performance and interpretation of ultrasound imaging be included during residency.20 ACEP and SAEM add that the availability of equivalent training should be made available to practicing physicians. The American Society of Echocardiography has supported the use of POCUS and sees this modality as part of the continuum of care.23,24
The AMA has also recognized that POCUS is within the scope of practice of trained physicians.22 The AMA further recommended hospital staff create their own criteria for granting ultrasound privileges based on the background and training of the physician and in accordance with the standards set within specific specialties.22,23
LOCAL POLICY AND PROCEDURE
The provision of clinical privileges in HM is governed by the rules and regulations of the department and institution for which privileges are sought. In detailing our policies and procedures above, we intend to provide an example for HM departments at other institutions that are attempting to create a POCUS credentialing program.
An interdisciplinary approach was created by our institution to address training, competency, and ongoing quality assurance (QA) concerns due to the increasing popularity of POCUS and variability in its use. We developed a hospital-wide POCUS committee with, among others, members from HM, emergency medicine, critical care, radiology, and cardiology, with a charter to standardize POCUS across departments. After review of the literature,16-18, 20, 21, 23-74 baseline training requirements were established for credentialing and developing a unified delineation of privileges for hospital-wide POCUS. The data support the use of a variety of assessments to ensure a provider has developed competence (portfolio development, knowledge-based examination, skills-based assessment, ongoing QA process). The POCUS committee identified which exams could be performed at bedside for credentialed providers, delineated imaging requirements for each exam, and set up the information technology infrastructure to support ordering and reporting through electronic health records (EHR). While the POCUS committee delineated this process for all hospital providers, we will focus our discussion on the credentialing policy and procedure in HM.
STEP 1: PATHWAY TO POCUS CREDENTIALING IN HM: COMPLETE MINIMAL FORMAL REQUIREMENTS
The credentialing requirements at our institution include one of the the following basic education pathways and minimal formal training:
Residency/Fellowship Based Pathway
Completed training in an Accreditation Council for Graduate Medical Education–approved program that provided opportunities for 20 hours of POCUS training with at least 6 hours of hands-on ultrasound scanning, 5 proctored limited cardiac ultrasound cases and portfolio development.
Practice Based Pathway
Completed 20 hours of POCUS continuing medical education (CME) with at least 6 hours of hands-on ultrasound scanning and has completed 5 proctored limited cardiac ultrasound cases (as part of CME).
The majority of HM providers had little formal residency training in POCUS, so a training program needed to be developed. Our training program, modeled after the American College of Chest Physicians’ CHEST certificate of completion,86 utilizes didactic training, hands-on instruction, and portfolio development that fulfills the minimal formal requirements in the practice-based pathway.
STEP 2: PATHWAY TO POCUS CREDENTIALING IN HM: COMPLETE PORTFOLIO AND FINAL ASSESSMENTS (KNOWLEDGE AND SKILLS–BASED)
After satisfactory completion of the minimal formal training, applicants need to provide documentation of a set number of cases. To aid this requirement, our HM department developed the portfolio guidelines in the Table. These are minimum requirements, and because of the varying training curves of learning,76-80 1 hospitalist may need to submit 300 files for review to meet the standards, while another may need to submit 500 files. Submissions are not accepted unless they yield high-quality video files with meticulous attention to gain, depth, and appropriate topographic planes. The portfolio development monitors hospitalists’ progression during their deliberate practice, providing objective assessments, feedback, and mentorship.81,82
A final knowledge exam with case-based image interpretation and hands-on examination is also provided. The passing score for the written examination is 85% and was based on the Angoff methodology.75 Providers who meet these requirements are then able to apply for POCUS credentialing in HM. Providers who do not pass the final assessments are required to participate in further training before they reattempt the assessments. There is uniformity in training outcomes but diversity in training time for POCUS providers.
Candidates who complete the portfolio and satisfactorily pass the final assessments are credentialed after review by the POCUS committee. Credentialed physicians are then able to perform POCUS and to integrate the findings into patient care.
MAINTENANCE OF CREDENTIALS
Documentation
After credentialing is obtained, all POCUS studies used in patient care are included in the EHR following a clearly defined workflow. The study is ordered through the EHR and is retrieved wirelessly on the ultrasound machine. After performing the ultrasound, all images are wirelessly transferred to the radiology Picture Archiving and Communication System server. Standardized text reports are used to distinguish focused POCUS from traditional diagnostic ultrasound studies. Documentation is optimized using electronic drop-down menus for documenting ultrasound findings in the EHR.
Minimum Number of Examinations
Maintenance of credentials will require that each hospitalist perform 10 documented ultrasounds per year for each cardiac and noncardiac application for which credentials are requested. If these numbers are not met, then all the studies performed during the previous year will be reviewed by the ultrasound committee, and providers will be provided with opportunities to meet the minimum benchmark (supervised scanning sessions).
Quality Assurance
Establishing scope of practice, developing curricula, and credentialing criteria are important steps toward assuring provider competence.16,17,22,74 To be confident that providers are using POCUS appropriately, there must also be a development of standards of periodic assessment that encompass both examination performance and interpretation. The objective of a QA process is to evaluate the POCUS cases for technical competence and the interpretations for clinical accuracy, and to provide feedback to improve performance of providers.
QA is maintained through the interdisciplinary POCUS committee and is described in the Figure.
After initial credentialing, continued QA of HM POCUS is done for a proportion of ongoing exams (10% as per recommendations by ACEP) to document continued competency.2 Credentialed POCUS providers perform and document their exam and interpretations. Ultrasound interpretations are reviewed by the POCUS committee (every case by 2 physicians, 1 hospitalist, and 1 radiologist or cardiologist depending on the study type) at appropriate intervals based on volume (at minimum, quarterly). A standardized review form is used to grade images and interpretations. This is the same general rubric used with the portfolio for initial credentialing. Each case is scored on a scale of 1 to 6, with 1 representing high image quality and support for diagnosis and 6 representing studies limited by patient factors. All scores rated 4 or 5 are reviewed at the larger quarterly POCUS committee meetings. For any provider scoring a 4 or 5, the ultrasound committee will recommend a focused professional practice evaluation as it pertains to POCUS. The committee will also make recommendations on a physician’s continued privileges to the department leaders.83
BILLING
Coding, billing, and reimbursement for focused ultrasound has been supported through the AMA Physicians’ Current Procedural Terminology (CPT) 2011 codes, which includes CPT code modifiers for POCUS.84 There are significant costs associated with building a HM ultrasound program, including the education of hospitalists, ultrasound equipment purchase and maintenance, as well as image archiving and QA. The development of a HM ultrasound billing program can help justify and fund these costs.19,85
To appropriately bill for POCUS, permanently retrievable images and an interpretation document need to be available for review. HM coders are instructed to only bill if both components are available. Because most insurers will not pay for 2 of the same type of study performed within a 24-hour period, coders do not bill for ultrasounds when a comprehensive ultrasound of the same body region is performed within a 24-hour period. The workflow that we have developed, including ordering, performing, and documenting, allows for easy coding and billing.
BARRIERS AND LIMITATIONS
While POCUS has a well-established literature base in other specialties like emergency medicine, it has been a relatively recent addition to the HM specialty. As such, there exists a paucity of evidence-based medicine to support its use of POCUS in HM. While it is tempting to extrapolate from the literature of other specialties, this may not be a valid approach.
Training curves in which novice users of ultrasound become competent in specific applications are incompletely understood. Little research describes the rate of progression of learners in ultrasound towards competency. We have recently started the QA process and hope that the data will further guide feedback to the process.
Additionally, with the portfolios, the raters’ expertise may not be stable (develops through experience). We aim to mitigate this by having a group of raters reviewing each file, particularly if there is a question about if a submission is of high image quality. A notable barrier that groups face is support from their leadership regarding POCUS. Our group has had support from the chief medical officer who helped mandate the development of POCUS standards.
LESSONS LEARNED
We have developed a robust collaborative HM POCUS program. We have noted challenges in motivating all providers to work through this protocol. Development of a POCUS program takes dedicated time, and without a champion, it is at risk for failing. HM departments would be advised to seek out willing collaborators at their institutions. We have seen that it is useful to partner with some experienced emergency medicine providers. Additionally, portfolio development and feedback has been key to demonstrating growth in image acquisition. Deliberate longitudinal practice with feedback and successive refinements with POCUS obtain the highest yield towards competency. We hope our QA data will provide further feedback into the credentialing policy and procedure.
SUMMARY
It is important that POCUS users work together to recognize its potential and limitations, teach current and future care providers’ best practices, and create an infrastructure that maximizes quality of care while minimizing patient risk.
We are hopeful that this document will prove beneficial to other HM departments in the development of successful POCUS programs. We feel that it is important to make available to other HM departments a concise protocol that has successfully passed through the credentialing process at a large tertiary care medical system.
Acknowledgments
The authors would like to acknowledge Susan Truman, MD, for her contributions to the success of the POCUS committee at Regions Hospital. The authors would like to acknowledge Kreegan Reierson, MD, Ankit Mehta, MBBS, and Khuong Vuong, MD for their contributions to the success of POCUS within hospital medicine at HealthPartners. The authors would like to acknowledge Sandi Wewerka, MPH, for her review and input of this manuscript.
Disclosure
The authors do not have any relevant financial disclosures to report.
1. Soni NJ, Nilam J, Arntfield R, Kory P. Point of Care Ultrasound. Philadelphia:
Elsevier; 2015.
2. Moore CL, Copel JA. Point-of-Care Ultrasonography. N Engl J Med.
2011;364(8):749-757. PubMed
3. Randolph AG, Cook DJ, Gonzales CA, et al. Ultrasound guidance for placement
of central venous catheters: A meta-analysis of the literature. Crit Care Med.
1996;24:2053-2058. PubMed
4. Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis:
A systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339. PubMed
5. Soni NJ, Nilam J, Franco R, et al. Ultrasound in the diagnosis and management of
pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
6. Nazerian P, Volpicelli G, Gigli C, et al. Diagnostic performance of Wells score
combined with point-of-care lung and venous ultrasound in suspected pulmonary
embolism. Acad Emerg Med. 2017;24(3):270-280. PubMed
7. Chatziantoniou A, Nazerian P, Vanni S, et al. A combination of the Wells score
with multiorgan ultrasound to stratify patients with suspected pulmonary embolism.
Eur Respir J. 2015;46:OA493; DOI:10.1183/13993003.congress-2015.
OA493.
8. Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid
management. Crit Care. 2016; DOI:10.1186/s13054-016-1407-1. PubMed
9. Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-Care Multi-Organ Ultrasound
Improves Diagnostic Accuracy in Adults Presenting to the Emergency
Department with Acute Dyspnea. West J Emerg Med. 2016;17(1):46-53. PubMed
10. Glockner E, Christ M, Geier F, et al. Accuracy of Point-of-Care B-Line Lung
Ultrasound in Comparison to NT-ProBNP for Screening Acute Heart Failure.
Ultrasound Int Open. 2016;2(3):e90-e92. PubMed
11. Bhagra A, Tierney DM, Sekiguchi H, Soni NJ. Point-of-Care Ultrasonography
for Primary Care Physicians and General Internists. Mayo Clin Proc.
2016;91(12):1811-1827. PubMed
12. Crisp JG, Lovato LM, and Jang TB. Compression ultrasonography of the lower extremity
with portable vascular ultrasonography can accurately detect deep venous
thrombosis in the emergency department. Ann Emerg Med. 2010;56:601-610. PubMed
13. Squire BT, Fox JC, and Anderson C. ABSCESS: Applied bedside sonography
for convenient. Evaluation of superficial soft tissue infections. Acad Emerg Med.
2005;12:601-606. PubMed
14. Narasimhan M, Koenig SJ, Mayo PH. A Whole-Body Approach to Point of Care
Ultrasound. Chest. 2016;150(4):772-776. PubMed
15. Oks M, Cleven KL, Cardenas-Garcia J, et al. The effect of point-of-care ultrasonography
on imaging studies in the medical ICU: a comparative study. Chest.
2014;146(6):1574-1577. PubMed
16. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/
La Société de Réanimation de Langue Française Statement on Competence in
Critical Care Ultrasonography. Chest. 2009;135(4):1050-1060. PubMed
17. Frank JR, Snell LS, Ten Cate O, et al. Competency-based medical education:
Theory to practice. Med Teach. 2010;32:638-645. PubMed
18. The Who, What, When, and Where’s of Credentialing and Privileging. The
Joint Commission. http://www.jointcommission.org/assets/1/6/AHC_who_what_
when_and_where_credentialing_booklet.pdf. Accessed December 21, 2016.
19. American College of Emergency Physicians Policy Statement: Emergency Ultrasound
Guidelines. 2016. https://www.acep.org/Clinical---Practice-Management/
ACEP-Ultrasound-Guidelines/. Accessed October 26, 2016.
20. Society for Academic Emergency Medicine. Ultrasound Position Statement. Annual
Meeting 1996.
21. American Medical Association. Privileging for ultrasound imaging. 2001; Policy
H-230.960. www.ama-assn.org. Accessed July 28, 2017 PubMed
22. Stein JC, Nobay F. Emergency Department Ultrasound Credentialing: a sample
policy and procedure. J Emerg Med. 2009;37(2):153-159. PubMed
23. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused
Cardiac Ultrasound: Recommendations from the American Society of Echocardiography.
J Am Soc Echocardiogr. 2013;26(6):567-581. PubMed
24. Wiegers S. The Point of Care. J Am Soc Echocardiogr. 2016;29(4):19. PubMed
25. Mandavia D, Aragona J, Chan L, et al. Ultrasound training for emergency physicians—
a prospective study. Acad Emerg Med. 2000;7:1008-1014. PubMed
26. American College of Radiology Practice Parameters and Technical Standards.
https://www.acr.org/quality-safety/standards-guidelines. Accessed December 21, 2016.
27. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can
Fam Physician. 2012;58(3):e172-e178. PubMed
28. Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic review: emergency
department bedside ultrasonography for diagnosing suspected abdominal
aortic aneurysm. Acad Emerg Med. 2013;20:128-138. PubMed
29. Dijos M, Pucheux Y, Lafitte M, et al. Fast track echo of abdominal aortic aneurysm
using a real pocket-ultrasound device at bedside. Echocardiography. PubMed
2012;29(3):285-290.
30. Cox C, MacDonald S, Henneberry R, Atkinson PR. My patient has abdominal
and flank pain: Identifying renal causes. Ultrasound. 2015;23(4):242-250. PubMed
31. Gaspari R, Horst K. Emergency ultrasound and urinalysis in the evaluation of
patients with flank pain. Acad Emerg Med. 2005;12:1180-1184. PubMed
32. Kartal M, Eray O, Erdogru T, et al. Prospective validation of a current algorithm
including bedside US performed by emergency physicians for patients with acute
flank pain suspected for renal colic. Emerg Med J. 2006;23(5):341-344. PubMed
33. Noble VE, Brown DF. Renal ultrasound. Emerg Med Clin North Am. 2004;22:641-659. PubMed
34. Surange R, Jeygopal NS, Chowdhury SD, et al. Bedside ultrasound: a useful tool
for the on call urologist? Int Urol Nephrol. 2001;32:591-596. PubMed
35. Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency physician-performed
ultrasonography in the diagnosis of deep-vein thrombosis. Thromb Haemost.
2013;109(1):137-145. PubMed
36. Bernardi E, Camporese G, Buller HR, et al. Erasmus Study Group. Serial 2-Point
Ultrasonography Plus D-Dimer vs Whole-Leg Color-Coded Doppler Ultrasonography
for Diagnosing Suspected Symptomatic Deep Vein Thrombosis: A Randomized
Controlled Trial. JAMA. 2008;300(14):1653-1659. PubMed
37. Burnside PR, Brown MD, Kline JA. Systematic Review of Emergency Physician–
performed Ultrasonography for Lower-Extremity Deep Vein Thrombosis. Acad
Emerg Med. 2008;15:493-498. PubMed
38. Magazzini S, Vanni S, Toccafondi S, et al. Duplex ultrasound in the emergency
department for the diagnostic management of clinically suspected deep vein
thrombosis. Acad Emerg Med. 2007;14:216-220. PubMed
39. Jacoby J, Cesta M, Axelband J, Melanson S, Heller M, Reed J. Can emergency
medicine residents detect acute deep venous thrombosis with a limited, two-site
ultrasound examination? J Emerg Med. 2007;32:197-200. PubMed
40. Jang T, Docherty M, Aubin C, Polites G. Resident-performed compression ultrasonography
for the detection of proximal deep vein thrombosis: fast and accurate.
Acad Emerg Med. 2004;11:319-322. PubMed
41. Frazee BW, Snoey ER, Levitt A. Emergency Department compression ultrasound
to diagnose proximal deep vein thrombosis. J Emerg Med. 2001;20:107-112. PubMed
42. Blaivas M, Lambert MJ, Harwood RA, Wood JP, Konicki J. Lower-extremity Doppler
for deep venous thrombosis--can emergency physicians be accurate and fast?
Acad Emerg Med. 2000;7:120-126. PubMed
43. Koenig SJ, Narasimhan M, Mayo PH. Thoracic ultrasonography for the pulmonary
specialist. Chest. 2011;140(5):1332-1341. PubMed
44. Lichtenstein, DA. A bedside ultrasound sign ruling out pneumothorax in the critically
ill. Lung sliding. Chest. 1995;108(5):1345-1348. PubMed
45. Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact.
An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care
Med. 1997;156(5):1640-1646. PubMed
46. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate
acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc
Ultrasound. 2008;6:16. PubMed
47. Agricola E, Bove T, Oppizzi M, et al. Ultrasound comet-tail images: a marker
of pulmonary edema: a comparative study with wedge pressure and extravascular
lung water. Chest. 2005;127(5):1690-1695. PubMed
48. Lichtenstein DA, Meziere GA, Laqoueyte JF, Biderman P, Goldstein I, Gepner A.
A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary
artery occlusion pressure in the critically ill. Chest. 2009;136(4):1014-1020. PubMed
49. Lichtenstein DA, Lascols N, Meziere G, Gepner A. Ultrasound diagnosis of alveolar
consolidation in the critically ill. Intensive Care Med. 2004;30(2):276-281. PubMed
50. Lichtenstein D, Mezière G, Seitz J. The dynamic air bronchogram. A lung
ultrasound sign of alveolar consolidation ruling out atelectasis. Chest.
2009;135(6):1421–1425. PubMed
51. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative
diagnostic performances of auscultation, chest radiography, and lung ultrasonography
in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9-15. PubMed
52. Lichtenstein D, Meziere G. Relevance of lung ultrasound in the diagnosis of acute
respiratory failure: the BLUE protocol. Chest. 2008;134(1):117-125. PubMed
53. Mayo P, Doelken P. Pleural ultrasonography. Clin Chest Med. 2006;27(2):215-227. PubMed
54. Galderisi M, Santoro A, Versiero M, et al. Improved cardiovascular diagnostic accuracy
by pocket size imaging device in non-cardiologic outpatients: the NaUSi-
Ca (Naples Ultrasound Stethoscope in Cardiology) study. Cardiovasc Ultrasound.
2010;8:51. PubMed
55. DeCara JM, Lang RM, Koch R, Bala R, Penzotti J, Spencer KT. The use of small
personal ultrasound devices by internists without formal training in echocardiography.
Eur J Echocardiography. 2002;4:141-147. PubMed
56. Martin LD, Howell EE, Ziegelstein RC, Martire C, Shapiro EP, Hellmann DB.
Hospitalist performance of cardiac hand-carried ultrasound after focused training.
Am J Med. 2007;120:1000-1004. PubMed
57. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound performed
by hospitalists: does it improve the cardiac physical examination? Am J Med.
2009;122:35-41. PubMed
58. Perez-Avraham G, Kobal SL, Etzion O, et al. Left ventricular geometric abnormality
screening in hypertensive patients using a hand-carried ultrasound device.
J Clin Hypertens. 2010;12:181-186. PubMed
59. Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of hospitalist-performed
hand-carried ultrasound echocardiography after a brief training program. J
Hosp Med. 2009;4:340-349. PubMed
60. Kimura BJ, Fowler SJ, Fergus TS, et al. Detection of left atrial enlargement using
hand-carried ultrasound devices to screen for cardiac abnormalities. Am J Med.
2005;118:912-916. PubMed
61. Brennan JM, Blair JE, Goonewardena S, et al. A comparison by medicine residents of physical examination versus hand-carried ultrasound for estimation of
right atrial pressure. Am J Cardiol. 2007;99:1614-1616. PubMed
62. Blair JE, Brennan JM, Goonewardena SN, Shah D, Vasaiwala S, Spencer KT.
Usefulness of hand-carried ultrasound to predict elevated left ventricular filling
pressure. Am J Cardiol. 2009;103:246-247. PubMed
63. Stawicki SP, Braslow BM, Panebianco NL, et al. Intensivist use of hand-carried
ultrasonography to measure IVC collapsibility in estimating intravascular volume
status: correlations with CVP. J Am Coll Surg. 2009;209:55-61. PubMed
64. Gunst M, Ghaemmaghami V, Sperry J, et al. Accuracy of cardiac function and volume
status estimates using the bedside echocardiographic assessment in trauma/
critical care. J Trauma. 2008;65:509-515. PubMed
65. Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal
medicine residents versus traditional clinical assessment for the identification
of systolic dysfunction in patients admitted with decompensated heart failure. J
Am Soc Echocardiogr. 2011;24:1319-1324. PubMed
66. Croft LB, Duvall WL, Goldman ME. A pilot study of the clinical impact
of hand-carried cardiac ultrasound in the medical clinic. Echocardiography.
2006;23:439-446. PubMed
67. Vignon P, Dugard A, Abraham J, et al. Focused training for goal-oriented handheld
echocardiography performed by noncardiologist residents in the intensive
care unit. Intensive Care Med. 2007;33:1795-1799. PubMed
68. Melamed R, Sprenkle MD, Ulstad VK, Herzog CA, Leatherman JW. Assessment
of left ventricular function by intensivists using hand-held echocardiography.
Chest. 2009;135:1416-1420. PubMed
69. Mark DG, Hayden GE, Ky B, et al. Hand-carried echocardiography for assessment
of left ventricular filling and ejection fraction in the surgical intensive care unit. J
Crit Care. 2009;24(3):470.e1-470.e7. PubMed
70. Kirkpatrick JN, Davis A, Decara JM, et al. Hand-carried cardiac ultrasound as a
tool to screen for important cardiovascular disease in an underserved minority
health care clinic. J Am Soc Echocardiogr. 2004;17:399-403. PubMed
71. Fedson S, Neithardt G, Thomas P, et al. Unsuspected clinically important findings
detected with a small portable ultrasound device in patients admitted to a general
medicine service. J Am Soc Echocardiogr. 2003;16:901-905. PubMed
72. Ghani SN, Kirkpatrick JN, Spencer, KT, et al. Rapid assessment of left ventricular
systolic function in a pacemaker clinic using a hand-carried ultrasound device.
J Interv Card Electrophysiol. 2006;16:39-43. PubMed
73. Kirkpatrick JN, Ghani SN, Spencer KT. Hand carried echocardiography
screening for LV systolic dysfunction in a pulmonary function laboratory.
Eur J Echocardiogr. 2008;9:381-383. PubMed
74. Alexander JH, Peterson ED, Chen AY, Harding TM, Adams DB, Kisslo JA Jr.
Feasibility of point-of-care echocardiography by internal medicine house staff. Am
Heart J. 2004;147:476-481. PubMed
75. Angoff WH. Scales, norms and equivalent Scores. Washington, DC: American
Council on Education; 1971.
76. Hellmann DB, Whiting-O’Keefe Q, Shapiro EP, Martin LD, Martire C, Ziegelstein
RC. The rate at which residents learn to use hand-held echocardiography at
the bedside. Am J Med. 2005;118:1010-1018. PubMed
77. Kimura BJ, Amundson SA, Phan JN, Agan DL, Shaw DJ. Observations during
development of an internal medicine residency training program in cardiovascular
limited ultrasound examination. J Hosp Med. 2012;7:537-542. PubMed
78. Akhtar S, Theodoro D, Gaspari R, et al. Resident training in emergency ultrasound:
consensus recommendations from the 2008 Council of Emergency Medicine
Residency Directors Conference. Acad Emerg Med. 2009;16(s2):S32-S36. PubMed
79. Ma OJ, Gaddis G, Norvell JG, Subramanian S. How fast is the focused assessment
with sonography for trauma examination learning curve? Emerg Med Australas.
2008;20(1):32-37. PubMed
80. Gaspari RJ, Dickman E, Blehar D. Learning curve of bedside ultrasound of the gallbladder. J Emerg Med. 2009;37(1):51-56. DOI:10.1016/j.jemermed.2007.10.070. PubMed
81. Ericsson KA, Lehmann AC. Expert and exceptional performance: Evidence of
maximal adaptation to task constraints. Ann Rev Psychol. 1996;47:273-305. PubMed
82. Ericcson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the
acquisition of expert performance. Psychol Rev. 1993;100:363-406.
83. OPPE and FPPE: Tools to help make privileging decisions. The Joint Commission.
2013. http://www.jointcommission.org/jc_physician_blog/oppe_fppe_tools_privileging_
decisions/ Accessed October 26, 2016.
84. American Medical Association. Physicians’ Current Procedural Terminology (CPT)
2011. American Medical Association, Chicago; 2011.
85. Moore CL, Gregg S, Lambert M. Performance, training, quality assurance, and
reimbursement of emergency physician-performed ultrasonography at academic
medical centers. J Ultrasound Med. 2004;23(4):459-466. PubMed
86. Critical Care Ultrasonography Certificate of Completion Program. CHEST.
American College of Chest Physicians. http://www.chestnet.org/Education/Advanced-
Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography.
Accessed July 28, 2017.
Ultrasound has been used for decades by radiology, obstetrics-gynecology, and cardiology departments within a comprehensive paradigm in which a physician enters an order, then a trained sonographer performs the study, followed by a physician evaluating and interpreting the images.1 Unlike the traditional comprehensive paradigm, point-of-care ultrasound (POCUS) is a focused study that is both performed and interpreted by the bedside provider.2 POCUS has been demonstrated to improve diagnosis and clinical management in multiple studies.3-15
The scope of practice in POCUS differs by specialty, as POCUS is done to achieve specific procedural aims (eg, direct the needle to the correct location) or answer focused questions (eg, does the patient have a distended bladder?) related to the specialty. POCUS in hospital medicine (HM) provides immediate answers, without the delay and potential risk of transportation to other hospital areas. It may be used to diagnose pleural effusion, pneumonia, hydronephrosis, heart failure, deep vein thrombosis, and many other pathologies.5-15 It is important to understand that POCUS performed by HM is a limited study and is not a substitute for more complete ultrasound examinations conducted in the radiology suite or in the echocardiography lab.
POCUS should not be used exclusively in medical decision making, but rather in conjunction with the greater clinical context of each patient, building on established principles of diagnosis and management.
DEFINITIONS
- Credentialing: An umbrella term, which incorporates licensure, education, and certification.
- Privileging: Used to define the scope authorized for a provider by a healthcare organization based on an evaluation of the individual’s credentials and performance.
- Competency: An observable ability of a provider, integrating multiple components, such as knowledge and skills. Since competencies are observable, they can be measured and assessed to ensure their acquisition.
- Certification: The process by which an association grants recognition to a provider who has met certain predetermined qualifications specified by the association. Competence is distinguished from certification, which is defined as the process by which competence is recognized by an external agency.
All of the above mechanisms work together to provide the highest quality of reliability that a practitioner is providing safe, competent care.16-18
STATEMENTS FROM MAJOR SPECIALTY SOCIETIES
Acknowledging that there are no published guidelines in the realm of HM POCUS, the development of the credentialing process at our institution is consistent with published guidelines by Emergency Medicine societies (the most established physician users of POCUS) and the American Medical Association (AMA).19-21
The use of emergency ultrasound by physicians in the emergency department is endorsed by the American College of Emergency Physicians (ACEP).19 ACEP, along with the Society of Academic Emergency Medicine (SAEM), recommends that training in the performance and interpretation of ultrasound imaging be included during residency.20 ACEP and SAEM add that the availability of equivalent training should be made available to practicing physicians. The American Society of Echocardiography has supported the use of POCUS and sees this modality as part of the continuum of care.23,24
The AMA has also recognized that POCUS is within the scope of practice of trained physicians.22 The AMA further recommended hospital staff create their own criteria for granting ultrasound privileges based on the background and training of the physician and in accordance with the standards set within specific specialties.22,23
LOCAL POLICY AND PROCEDURE
The provision of clinical privileges in HM is governed by the rules and regulations of the department and institution for which privileges are sought. In detailing our policies and procedures above, we intend to provide an example for HM departments at other institutions that are attempting to create a POCUS credentialing program.
An interdisciplinary approach was created by our institution to address training, competency, and ongoing quality assurance (QA) concerns due to the increasing popularity of POCUS and variability in its use. We developed a hospital-wide POCUS committee with, among others, members from HM, emergency medicine, critical care, radiology, and cardiology, with a charter to standardize POCUS across departments. After review of the literature,16-18, 20, 21, 23-74 baseline training requirements were established for credentialing and developing a unified delineation of privileges for hospital-wide POCUS. The data support the use of a variety of assessments to ensure a provider has developed competence (portfolio development, knowledge-based examination, skills-based assessment, ongoing QA process). The POCUS committee identified which exams could be performed at bedside for credentialed providers, delineated imaging requirements for each exam, and set up the information technology infrastructure to support ordering and reporting through electronic health records (EHR). While the POCUS committee delineated this process for all hospital providers, we will focus our discussion on the credentialing policy and procedure in HM.
STEP 1: PATHWAY TO POCUS CREDENTIALING IN HM: COMPLETE MINIMAL FORMAL REQUIREMENTS
The credentialing requirements at our institution include one of the the following basic education pathways and minimal formal training:
Residency/Fellowship Based Pathway
Completed training in an Accreditation Council for Graduate Medical Education–approved program that provided opportunities for 20 hours of POCUS training with at least 6 hours of hands-on ultrasound scanning, 5 proctored limited cardiac ultrasound cases and portfolio development.
Practice Based Pathway
Completed 20 hours of POCUS continuing medical education (CME) with at least 6 hours of hands-on ultrasound scanning and has completed 5 proctored limited cardiac ultrasound cases (as part of CME).
The majority of HM providers had little formal residency training in POCUS, so a training program needed to be developed. Our training program, modeled after the American College of Chest Physicians’ CHEST certificate of completion,86 utilizes didactic training, hands-on instruction, and portfolio development that fulfills the minimal formal requirements in the practice-based pathway.
STEP 2: PATHWAY TO POCUS CREDENTIALING IN HM: COMPLETE PORTFOLIO AND FINAL ASSESSMENTS (KNOWLEDGE AND SKILLS–BASED)
After satisfactory completion of the minimal formal training, applicants need to provide documentation of a set number of cases. To aid this requirement, our HM department developed the portfolio guidelines in the Table. These are minimum requirements, and because of the varying training curves of learning,76-80 1 hospitalist may need to submit 300 files for review to meet the standards, while another may need to submit 500 files. Submissions are not accepted unless they yield high-quality video files with meticulous attention to gain, depth, and appropriate topographic planes. The portfolio development monitors hospitalists’ progression during their deliberate practice, providing objective assessments, feedback, and mentorship.81,82
A final knowledge exam with case-based image interpretation and hands-on examination is also provided. The passing score for the written examination is 85% and was based on the Angoff methodology.75 Providers who meet these requirements are then able to apply for POCUS credentialing in HM. Providers who do not pass the final assessments are required to participate in further training before they reattempt the assessments. There is uniformity in training outcomes but diversity in training time for POCUS providers.
Candidates who complete the portfolio and satisfactorily pass the final assessments are credentialed after review by the POCUS committee. Credentialed physicians are then able to perform POCUS and to integrate the findings into patient care.
MAINTENANCE OF CREDENTIALS
Documentation
After credentialing is obtained, all POCUS studies used in patient care are included in the EHR following a clearly defined workflow. The study is ordered through the EHR and is retrieved wirelessly on the ultrasound machine. After performing the ultrasound, all images are wirelessly transferred to the radiology Picture Archiving and Communication System server. Standardized text reports are used to distinguish focused POCUS from traditional diagnostic ultrasound studies. Documentation is optimized using electronic drop-down menus for documenting ultrasound findings in the EHR.
Minimum Number of Examinations
Maintenance of credentials will require that each hospitalist perform 10 documented ultrasounds per year for each cardiac and noncardiac application for which credentials are requested. If these numbers are not met, then all the studies performed during the previous year will be reviewed by the ultrasound committee, and providers will be provided with opportunities to meet the minimum benchmark (supervised scanning sessions).
Quality Assurance
Establishing scope of practice, developing curricula, and credentialing criteria are important steps toward assuring provider competence.16,17,22,74 To be confident that providers are using POCUS appropriately, there must also be a development of standards of periodic assessment that encompass both examination performance and interpretation. The objective of a QA process is to evaluate the POCUS cases for technical competence and the interpretations for clinical accuracy, and to provide feedback to improve performance of providers.
QA is maintained through the interdisciplinary POCUS committee and is described in the Figure.
After initial credentialing, continued QA of HM POCUS is done for a proportion of ongoing exams (10% as per recommendations by ACEP) to document continued competency.2 Credentialed POCUS providers perform and document their exam and interpretations. Ultrasound interpretations are reviewed by the POCUS committee (every case by 2 physicians, 1 hospitalist, and 1 radiologist or cardiologist depending on the study type) at appropriate intervals based on volume (at minimum, quarterly). A standardized review form is used to grade images and interpretations. This is the same general rubric used with the portfolio for initial credentialing. Each case is scored on a scale of 1 to 6, with 1 representing high image quality and support for diagnosis and 6 representing studies limited by patient factors. All scores rated 4 or 5 are reviewed at the larger quarterly POCUS committee meetings. For any provider scoring a 4 or 5, the ultrasound committee will recommend a focused professional practice evaluation as it pertains to POCUS. The committee will also make recommendations on a physician’s continued privileges to the department leaders.83
BILLING
Coding, billing, and reimbursement for focused ultrasound has been supported through the AMA Physicians’ Current Procedural Terminology (CPT) 2011 codes, which includes CPT code modifiers for POCUS.84 There are significant costs associated with building a HM ultrasound program, including the education of hospitalists, ultrasound equipment purchase and maintenance, as well as image archiving and QA. The development of a HM ultrasound billing program can help justify and fund these costs.19,85
To appropriately bill for POCUS, permanently retrievable images and an interpretation document need to be available for review. HM coders are instructed to only bill if both components are available. Because most insurers will not pay for 2 of the same type of study performed within a 24-hour period, coders do not bill for ultrasounds when a comprehensive ultrasound of the same body region is performed within a 24-hour period. The workflow that we have developed, including ordering, performing, and documenting, allows for easy coding and billing.
BARRIERS AND LIMITATIONS
While POCUS has a well-established literature base in other specialties like emergency medicine, it has been a relatively recent addition to the HM specialty. As such, there exists a paucity of evidence-based medicine to support its use of POCUS in HM. While it is tempting to extrapolate from the literature of other specialties, this may not be a valid approach.
Training curves in which novice users of ultrasound become competent in specific applications are incompletely understood. Little research describes the rate of progression of learners in ultrasound towards competency. We have recently started the QA process and hope that the data will further guide feedback to the process.
Additionally, with the portfolios, the raters’ expertise may not be stable (develops through experience). We aim to mitigate this by having a group of raters reviewing each file, particularly if there is a question about if a submission is of high image quality. A notable barrier that groups face is support from their leadership regarding POCUS. Our group has had support from the chief medical officer who helped mandate the development of POCUS standards.
LESSONS LEARNED
We have developed a robust collaborative HM POCUS program. We have noted challenges in motivating all providers to work through this protocol. Development of a POCUS program takes dedicated time, and without a champion, it is at risk for failing. HM departments would be advised to seek out willing collaborators at their institutions. We have seen that it is useful to partner with some experienced emergency medicine providers. Additionally, portfolio development and feedback has been key to demonstrating growth in image acquisition. Deliberate longitudinal practice with feedback and successive refinements with POCUS obtain the highest yield towards competency. We hope our QA data will provide further feedback into the credentialing policy and procedure.
SUMMARY
It is important that POCUS users work together to recognize its potential and limitations, teach current and future care providers’ best practices, and create an infrastructure that maximizes quality of care while minimizing patient risk.
We are hopeful that this document will prove beneficial to other HM departments in the development of successful POCUS programs. We feel that it is important to make available to other HM departments a concise protocol that has successfully passed through the credentialing process at a large tertiary care medical system.
Acknowledgments
The authors would like to acknowledge Susan Truman, MD, for her contributions to the success of the POCUS committee at Regions Hospital. The authors would like to acknowledge Kreegan Reierson, MD, Ankit Mehta, MBBS, and Khuong Vuong, MD for their contributions to the success of POCUS within hospital medicine at HealthPartners. The authors would like to acknowledge Sandi Wewerka, MPH, for her review and input of this manuscript.
Disclosure
The authors do not have any relevant financial disclosures to report.
Ultrasound has been used for decades by radiology, obstetrics-gynecology, and cardiology departments within a comprehensive paradigm in which a physician enters an order, then a trained sonographer performs the study, followed by a physician evaluating and interpreting the images.1 Unlike the traditional comprehensive paradigm, point-of-care ultrasound (POCUS) is a focused study that is both performed and interpreted by the bedside provider.2 POCUS has been demonstrated to improve diagnosis and clinical management in multiple studies.3-15
The scope of practice in POCUS differs by specialty, as POCUS is done to achieve specific procedural aims (eg, direct the needle to the correct location) or answer focused questions (eg, does the patient have a distended bladder?) related to the specialty. POCUS in hospital medicine (HM) provides immediate answers, without the delay and potential risk of transportation to other hospital areas. It may be used to diagnose pleural effusion, pneumonia, hydronephrosis, heart failure, deep vein thrombosis, and many other pathologies.5-15 It is important to understand that POCUS performed by HM is a limited study and is not a substitute for more complete ultrasound examinations conducted in the radiology suite or in the echocardiography lab.
POCUS should not be used exclusively in medical decision making, but rather in conjunction with the greater clinical context of each patient, building on established principles of diagnosis and management.
DEFINITIONS
- Credentialing: An umbrella term, which incorporates licensure, education, and certification.
- Privileging: Used to define the scope authorized for a provider by a healthcare organization based on an evaluation of the individual’s credentials and performance.
- Competency: An observable ability of a provider, integrating multiple components, such as knowledge and skills. Since competencies are observable, they can be measured and assessed to ensure their acquisition.
- Certification: The process by which an association grants recognition to a provider who has met certain predetermined qualifications specified by the association. Competence is distinguished from certification, which is defined as the process by which competence is recognized by an external agency.
All of the above mechanisms work together to provide the highest quality of reliability that a practitioner is providing safe, competent care.16-18
STATEMENTS FROM MAJOR SPECIALTY SOCIETIES
Acknowledging that there are no published guidelines in the realm of HM POCUS, the development of the credentialing process at our institution is consistent with published guidelines by Emergency Medicine societies (the most established physician users of POCUS) and the American Medical Association (AMA).19-21
The use of emergency ultrasound by physicians in the emergency department is endorsed by the American College of Emergency Physicians (ACEP).19 ACEP, along with the Society of Academic Emergency Medicine (SAEM), recommends that training in the performance and interpretation of ultrasound imaging be included during residency.20 ACEP and SAEM add that the availability of equivalent training should be made available to practicing physicians. The American Society of Echocardiography has supported the use of POCUS and sees this modality as part of the continuum of care.23,24
The AMA has also recognized that POCUS is within the scope of practice of trained physicians.22 The AMA further recommended hospital staff create their own criteria for granting ultrasound privileges based on the background and training of the physician and in accordance with the standards set within specific specialties.22,23
LOCAL POLICY AND PROCEDURE
The provision of clinical privileges in HM is governed by the rules and regulations of the department and institution for which privileges are sought. In detailing our policies and procedures above, we intend to provide an example for HM departments at other institutions that are attempting to create a POCUS credentialing program.
An interdisciplinary approach was created by our institution to address training, competency, and ongoing quality assurance (QA) concerns due to the increasing popularity of POCUS and variability in its use. We developed a hospital-wide POCUS committee with, among others, members from HM, emergency medicine, critical care, radiology, and cardiology, with a charter to standardize POCUS across departments. After review of the literature,16-18, 20, 21, 23-74 baseline training requirements were established for credentialing and developing a unified delineation of privileges for hospital-wide POCUS. The data support the use of a variety of assessments to ensure a provider has developed competence (portfolio development, knowledge-based examination, skills-based assessment, ongoing QA process). The POCUS committee identified which exams could be performed at bedside for credentialed providers, delineated imaging requirements for each exam, and set up the information technology infrastructure to support ordering and reporting through electronic health records (EHR). While the POCUS committee delineated this process for all hospital providers, we will focus our discussion on the credentialing policy and procedure in HM.
STEP 1: PATHWAY TO POCUS CREDENTIALING IN HM: COMPLETE MINIMAL FORMAL REQUIREMENTS
The credentialing requirements at our institution include one of the the following basic education pathways and minimal formal training:
Residency/Fellowship Based Pathway
Completed training in an Accreditation Council for Graduate Medical Education–approved program that provided opportunities for 20 hours of POCUS training with at least 6 hours of hands-on ultrasound scanning, 5 proctored limited cardiac ultrasound cases and portfolio development.
Practice Based Pathway
Completed 20 hours of POCUS continuing medical education (CME) with at least 6 hours of hands-on ultrasound scanning and has completed 5 proctored limited cardiac ultrasound cases (as part of CME).
The majority of HM providers had little formal residency training in POCUS, so a training program needed to be developed. Our training program, modeled after the American College of Chest Physicians’ CHEST certificate of completion,86 utilizes didactic training, hands-on instruction, and portfolio development that fulfills the minimal formal requirements in the practice-based pathway.
STEP 2: PATHWAY TO POCUS CREDENTIALING IN HM: COMPLETE PORTFOLIO AND FINAL ASSESSMENTS (KNOWLEDGE AND SKILLS–BASED)
After satisfactory completion of the minimal formal training, applicants need to provide documentation of a set number of cases. To aid this requirement, our HM department developed the portfolio guidelines in the Table. These are minimum requirements, and because of the varying training curves of learning,76-80 1 hospitalist may need to submit 300 files for review to meet the standards, while another may need to submit 500 files. Submissions are not accepted unless they yield high-quality video files with meticulous attention to gain, depth, and appropriate topographic planes. The portfolio development monitors hospitalists’ progression during their deliberate practice, providing objective assessments, feedback, and mentorship.81,82
A final knowledge exam with case-based image interpretation and hands-on examination is also provided. The passing score for the written examination is 85% and was based on the Angoff methodology.75 Providers who meet these requirements are then able to apply for POCUS credentialing in HM. Providers who do not pass the final assessments are required to participate in further training before they reattempt the assessments. There is uniformity in training outcomes but diversity in training time for POCUS providers.
Candidates who complete the portfolio and satisfactorily pass the final assessments are credentialed after review by the POCUS committee. Credentialed physicians are then able to perform POCUS and to integrate the findings into patient care.
MAINTENANCE OF CREDENTIALS
Documentation
After credentialing is obtained, all POCUS studies used in patient care are included in the EHR following a clearly defined workflow. The study is ordered through the EHR and is retrieved wirelessly on the ultrasound machine. After performing the ultrasound, all images are wirelessly transferred to the radiology Picture Archiving and Communication System server. Standardized text reports are used to distinguish focused POCUS from traditional diagnostic ultrasound studies. Documentation is optimized using electronic drop-down menus for documenting ultrasound findings in the EHR.
Minimum Number of Examinations
Maintenance of credentials will require that each hospitalist perform 10 documented ultrasounds per year for each cardiac and noncardiac application for which credentials are requested. If these numbers are not met, then all the studies performed during the previous year will be reviewed by the ultrasound committee, and providers will be provided with opportunities to meet the minimum benchmark (supervised scanning sessions).
Quality Assurance
Establishing scope of practice, developing curricula, and credentialing criteria are important steps toward assuring provider competence.16,17,22,74 To be confident that providers are using POCUS appropriately, there must also be a development of standards of periodic assessment that encompass both examination performance and interpretation. The objective of a QA process is to evaluate the POCUS cases for technical competence and the interpretations for clinical accuracy, and to provide feedback to improve performance of providers.
QA is maintained through the interdisciplinary POCUS committee and is described in the Figure.
After initial credentialing, continued QA of HM POCUS is done for a proportion of ongoing exams (10% as per recommendations by ACEP) to document continued competency.2 Credentialed POCUS providers perform and document their exam and interpretations. Ultrasound interpretations are reviewed by the POCUS committee (every case by 2 physicians, 1 hospitalist, and 1 radiologist or cardiologist depending on the study type) at appropriate intervals based on volume (at minimum, quarterly). A standardized review form is used to grade images and interpretations. This is the same general rubric used with the portfolio for initial credentialing. Each case is scored on a scale of 1 to 6, with 1 representing high image quality and support for diagnosis and 6 representing studies limited by patient factors. All scores rated 4 or 5 are reviewed at the larger quarterly POCUS committee meetings. For any provider scoring a 4 or 5, the ultrasound committee will recommend a focused professional practice evaluation as it pertains to POCUS. The committee will also make recommendations on a physician’s continued privileges to the department leaders.83
BILLING
Coding, billing, and reimbursement for focused ultrasound has been supported through the AMA Physicians’ Current Procedural Terminology (CPT) 2011 codes, which includes CPT code modifiers for POCUS.84 There are significant costs associated with building a HM ultrasound program, including the education of hospitalists, ultrasound equipment purchase and maintenance, as well as image archiving and QA. The development of a HM ultrasound billing program can help justify and fund these costs.19,85
To appropriately bill for POCUS, permanently retrievable images and an interpretation document need to be available for review. HM coders are instructed to only bill if both components are available. Because most insurers will not pay for 2 of the same type of study performed within a 24-hour period, coders do not bill for ultrasounds when a comprehensive ultrasound of the same body region is performed within a 24-hour period. The workflow that we have developed, including ordering, performing, and documenting, allows for easy coding and billing.
BARRIERS AND LIMITATIONS
While POCUS has a well-established literature base in other specialties like emergency medicine, it has been a relatively recent addition to the HM specialty. As such, there exists a paucity of evidence-based medicine to support its use of POCUS in HM. While it is tempting to extrapolate from the literature of other specialties, this may not be a valid approach.
Training curves in which novice users of ultrasound become competent in specific applications are incompletely understood. Little research describes the rate of progression of learners in ultrasound towards competency. We have recently started the QA process and hope that the data will further guide feedback to the process.
Additionally, with the portfolios, the raters’ expertise may not be stable (develops through experience). We aim to mitigate this by having a group of raters reviewing each file, particularly if there is a question about if a submission is of high image quality. A notable barrier that groups face is support from their leadership regarding POCUS. Our group has had support from the chief medical officer who helped mandate the development of POCUS standards.
LESSONS LEARNED
We have developed a robust collaborative HM POCUS program. We have noted challenges in motivating all providers to work through this protocol. Development of a POCUS program takes dedicated time, and without a champion, it is at risk for failing. HM departments would be advised to seek out willing collaborators at their institutions. We have seen that it is useful to partner with some experienced emergency medicine providers. Additionally, portfolio development and feedback has been key to demonstrating growth in image acquisition. Deliberate longitudinal practice with feedback and successive refinements with POCUS obtain the highest yield towards competency. We hope our QA data will provide further feedback into the credentialing policy and procedure.
SUMMARY
It is important that POCUS users work together to recognize its potential and limitations, teach current and future care providers’ best practices, and create an infrastructure that maximizes quality of care while minimizing patient risk.
We are hopeful that this document will prove beneficial to other HM departments in the development of successful POCUS programs. We feel that it is important to make available to other HM departments a concise protocol that has successfully passed through the credentialing process at a large tertiary care medical system.
Acknowledgments
The authors would like to acknowledge Susan Truman, MD, for her contributions to the success of the POCUS committee at Regions Hospital. The authors would like to acknowledge Kreegan Reierson, MD, Ankit Mehta, MBBS, and Khuong Vuong, MD for their contributions to the success of POCUS within hospital medicine at HealthPartners. The authors would like to acknowledge Sandi Wewerka, MPH, for her review and input of this manuscript.
Disclosure
The authors do not have any relevant financial disclosures to report.
1. Soni NJ, Nilam J, Arntfield R, Kory P. Point of Care Ultrasound. Philadelphia:
Elsevier; 2015.
2. Moore CL, Copel JA. Point-of-Care Ultrasonography. N Engl J Med.
2011;364(8):749-757. PubMed
3. Randolph AG, Cook DJ, Gonzales CA, et al. Ultrasound guidance for placement
of central venous catheters: A meta-analysis of the literature. Crit Care Med.
1996;24:2053-2058. PubMed
4. Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis:
A systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339. PubMed
5. Soni NJ, Nilam J, Franco R, et al. Ultrasound in the diagnosis and management of
pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
6. Nazerian P, Volpicelli G, Gigli C, et al. Diagnostic performance of Wells score
combined with point-of-care lung and venous ultrasound in suspected pulmonary
embolism. Acad Emerg Med. 2017;24(3):270-280. PubMed
7. Chatziantoniou A, Nazerian P, Vanni S, et al. A combination of the Wells score
with multiorgan ultrasound to stratify patients with suspected pulmonary embolism.
Eur Respir J. 2015;46:OA493; DOI:10.1183/13993003.congress-2015.
OA493.
8. Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid
management. Crit Care. 2016; DOI:10.1186/s13054-016-1407-1. PubMed
9. Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-Care Multi-Organ Ultrasound
Improves Diagnostic Accuracy in Adults Presenting to the Emergency
Department with Acute Dyspnea. West J Emerg Med. 2016;17(1):46-53. PubMed
10. Glockner E, Christ M, Geier F, et al. Accuracy of Point-of-Care B-Line Lung
Ultrasound in Comparison to NT-ProBNP for Screening Acute Heart Failure.
Ultrasound Int Open. 2016;2(3):e90-e92. PubMed
11. Bhagra A, Tierney DM, Sekiguchi H, Soni NJ. Point-of-Care Ultrasonography
for Primary Care Physicians and General Internists. Mayo Clin Proc.
2016;91(12):1811-1827. PubMed
12. Crisp JG, Lovato LM, and Jang TB. Compression ultrasonography of the lower extremity
with portable vascular ultrasonography can accurately detect deep venous
thrombosis in the emergency department. Ann Emerg Med. 2010;56:601-610. PubMed
13. Squire BT, Fox JC, and Anderson C. ABSCESS: Applied bedside sonography
for convenient. Evaluation of superficial soft tissue infections. Acad Emerg Med.
2005;12:601-606. PubMed
14. Narasimhan M, Koenig SJ, Mayo PH. A Whole-Body Approach to Point of Care
Ultrasound. Chest. 2016;150(4):772-776. PubMed
15. Oks M, Cleven KL, Cardenas-Garcia J, et al. The effect of point-of-care ultrasonography
on imaging studies in the medical ICU: a comparative study. Chest.
2014;146(6):1574-1577. PubMed
16. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/
La Société de Réanimation de Langue Française Statement on Competence in
Critical Care Ultrasonography. Chest. 2009;135(4):1050-1060. PubMed
17. Frank JR, Snell LS, Ten Cate O, et al. Competency-based medical education:
Theory to practice. Med Teach. 2010;32:638-645. PubMed
18. The Who, What, When, and Where’s of Credentialing and Privileging. The
Joint Commission. http://www.jointcommission.org/assets/1/6/AHC_who_what_
when_and_where_credentialing_booklet.pdf. Accessed December 21, 2016.
19. American College of Emergency Physicians Policy Statement: Emergency Ultrasound
Guidelines. 2016. https://www.acep.org/Clinical---Practice-Management/
ACEP-Ultrasound-Guidelines/. Accessed October 26, 2016.
20. Society for Academic Emergency Medicine. Ultrasound Position Statement. Annual
Meeting 1996.
21. American Medical Association. Privileging for ultrasound imaging. 2001; Policy
H-230.960. www.ama-assn.org. Accessed July 28, 2017 PubMed
22. Stein JC, Nobay F. Emergency Department Ultrasound Credentialing: a sample
policy and procedure. J Emerg Med. 2009;37(2):153-159. PubMed
23. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused
Cardiac Ultrasound: Recommendations from the American Society of Echocardiography.
J Am Soc Echocardiogr. 2013;26(6):567-581. PubMed
24. Wiegers S. The Point of Care. J Am Soc Echocardiogr. 2016;29(4):19. PubMed
25. Mandavia D, Aragona J, Chan L, et al. Ultrasound training for emergency physicians—
a prospective study. Acad Emerg Med. 2000;7:1008-1014. PubMed
26. American College of Radiology Practice Parameters and Technical Standards.
https://www.acr.org/quality-safety/standards-guidelines. Accessed December 21, 2016.
27. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can
Fam Physician. 2012;58(3):e172-e178. PubMed
28. Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic review: emergency
department bedside ultrasonography for diagnosing suspected abdominal
aortic aneurysm. Acad Emerg Med. 2013;20:128-138. PubMed
29. Dijos M, Pucheux Y, Lafitte M, et al. Fast track echo of abdominal aortic aneurysm
using a real pocket-ultrasound device at bedside. Echocardiography. PubMed
2012;29(3):285-290.
30. Cox C, MacDonald S, Henneberry R, Atkinson PR. My patient has abdominal
and flank pain: Identifying renal causes. Ultrasound. 2015;23(4):242-250. PubMed
31. Gaspari R, Horst K. Emergency ultrasound and urinalysis in the evaluation of
patients with flank pain. Acad Emerg Med. 2005;12:1180-1184. PubMed
32. Kartal M, Eray O, Erdogru T, et al. Prospective validation of a current algorithm
including bedside US performed by emergency physicians for patients with acute
flank pain suspected for renal colic. Emerg Med J. 2006;23(5):341-344. PubMed
33. Noble VE, Brown DF. Renal ultrasound. Emerg Med Clin North Am. 2004;22:641-659. PubMed
34. Surange R, Jeygopal NS, Chowdhury SD, et al. Bedside ultrasound: a useful tool
for the on call urologist? Int Urol Nephrol. 2001;32:591-596. PubMed
35. Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency physician-performed
ultrasonography in the diagnosis of deep-vein thrombosis. Thromb Haemost.
2013;109(1):137-145. PubMed
36. Bernardi E, Camporese G, Buller HR, et al. Erasmus Study Group. Serial 2-Point
Ultrasonography Plus D-Dimer vs Whole-Leg Color-Coded Doppler Ultrasonography
for Diagnosing Suspected Symptomatic Deep Vein Thrombosis: A Randomized
Controlled Trial. JAMA. 2008;300(14):1653-1659. PubMed
37. Burnside PR, Brown MD, Kline JA. Systematic Review of Emergency Physician–
performed Ultrasonography for Lower-Extremity Deep Vein Thrombosis. Acad
Emerg Med. 2008;15:493-498. PubMed
38. Magazzini S, Vanni S, Toccafondi S, et al. Duplex ultrasound in the emergency
department for the diagnostic management of clinically suspected deep vein
thrombosis. Acad Emerg Med. 2007;14:216-220. PubMed
39. Jacoby J, Cesta M, Axelband J, Melanson S, Heller M, Reed J. Can emergency
medicine residents detect acute deep venous thrombosis with a limited, two-site
ultrasound examination? J Emerg Med. 2007;32:197-200. PubMed
40. Jang T, Docherty M, Aubin C, Polites G. Resident-performed compression ultrasonography
for the detection of proximal deep vein thrombosis: fast and accurate.
Acad Emerg Med. 2004;11:319-322. PubMed
41. Frazee BW, Snoey ER, Levitt A. Emergency Department compression ultrasound
to diagnose proximal deep vein thrombosis. J Emerg Med. 2001;20:107-112. PubMed
42. Blaivas M, Lambert MJ, Harwood RA, Wood JP, Konicki J. Lower-extremity Doppler
for deep venous thrombosis--can emergency physicians be accurate and fast?
Acad Emerg Med. 2000;7:120-126. PubMed
43. Koenig SJ, Narasimhan M, Mayo PH. Thoracic ultrasonography for the pulmonary
specialist. Chest. 2011;140(5):1332-1341. PubMed
44. Lichtenstein, DA. A bedside ultrasound sign ruling out pneumothorax in the critically
ill. Lung sliding. Chest. 1995;108(5):1345-1348. PubMed
45. Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact.
An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care
Med. 1997;156(5):1640-1646. PubMed
46. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate
acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc
Ultrasound. 2008;6:16. PubMed
47. Agricola E, Bove T, Oppizzi M, et al. Ultrasound comet-tail images: a marker
of pulmonary edema: a comparative study with wedge pressure and extravascular
lung water. Chest. 2005;127(5):1690-1695. PubMed
48. Lichtenstein DA, Meziere GA, Laqoueyte JF, Biderman P, Goldstein I, Gepner A.
A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary
artery occlusion pressure in the critically ill. Chest. 2009;136(4):1014-1020. PubMed
49. Lichtenstein DA, Lascols N, Meziere G, Gepner A. Ultrasound diagnosis of alveolar
consolidation in the critically ill. Intensive Care Med. 2004;30(2):276-281. PubMed
50. Lichtenstein D, Mezière G, Seitz J. The dynamic air bronchogram. A lung
ultrasound sign of alveolar consolidation ruling out atelectasis. Chest.
2009;135(6):1421–1425. PubMed
51. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative
diagnostic performances of auscultation, chest radiography, and lung ultrasonography
in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9-15. PubMed
52. Lichtenstein D, Meziere G. Relevance of lung ultrasound in the diagnosis of acute
respiratory failure: the BLUE protocol. Chest. 2008;134(1):117-125. PubMed
53. Mayo P, Doelken P. Pleural ultrasonography. Clin Chest Med. 2006;27(2):215-227. PubMed
54. Galderisi M, Santoro A, Versiero M, et al. Improved cardiovascular diagnostic accuracy
by pocket size imaging device in non-cardiologic outpatients: the NaUSi-
Ca (Naples Ultrasound Stethoscope in Cardiology) study. Cardiovasc Ultrasound.
2010;8:51. PubMed
55. DeCara JM, Lang RM, Koch R, Bala R, Penzotti J, Spencer KT. The use of small
personal ultrasound devices by internists without formal training in echocardiography.
Eur J Echocardiography. 2002;4:141-147. PubMed
56. Martin LD, Howell EE, Ziegelstein RC, Martire C, Shapiro EP, Hellmann DB.
Hospitalist performance of cardiac hand-carried ultrasound after focused training.
Am J Med. 2007;120:1000-1004. PubMed
57. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound performed
by hospitalists: does it improve the cardiac physical examination? Am J Med.
2009;122:35-41. PubMed
58. Perez-Avraham G, Kobal SL, Etzion O, et al. Left ventricular geometric abnormality
screening in hypertensive patients using a hand-carried ultrasound device.
J Clin Hypertens. 2010;12:181-186. PubMed
59. Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of hospitalist-performed
hand-carried ultrasound echocardiography after a brief training program. J
Hosp Med. 2009;4:340-349. PubMed
60. Kimura BJ, Fowler SJ, Fergus TS, et al. Detection of left atrial enlargement using
hand-carried ultrasound devices to screen for cardiac abnormalities. Am J Med.
2005;118:912-916. PubMed
61. Brennan JM, Blair JE, Goonewardena S, et al. A comparison by medicine residents of physical examination versus hand-carried ultrasound for estimation of
right atrial pressure. Am J Cardiol. 2007;99:1614-1616. PubMed
62. Blair JE, Brennan JM, Goonewardena SN, Shah D, Vasaiwala S, Spencer KT.
Usefulness of hand-carried ultrasound to predict elevated left ventricular filling
pressure. Am J Cardiol. 2009;103:246-247. PubMed
63. Stawicki SP, Braslow BM, Panebianco NL, et al. Intensivist use of hand-carried
ultrasonography to measure IVC collapsibility in estimating intravascular volume
status: correlations with CVP. J Am Coll Surg. 2009;209:55-61. PubMed
64. Gunst M, Ghaemmaghami V, Sperry J, et al. Accuracy of cardiac function and volume
status estimates using the bedside echocardiographic assessment in trauma/
critical care. J Trauma. 2008;65:509-515. PubMed
65. Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal
medicine residents versus traditional clinical assessment for the identification
of systolic dysfunction in patients admitted with decompensated heart failure. J
Am Soc Echocardiogr. 2011;24:1319-1324. PubMed
66. Croft LB, Duvall WL, Goldman ME. A pilot study of the clinical impact
of hand-carried cardiac ultrasound in the medical clinic. Echocardiography.
2006;23:439-446. PubMed
67. Vignon P, Dugard A, Abraham J, et al. Focused training for goal-oriented handheld
echocardiography performed by noncardiologist residents in the intensive
care unit. Intensive Care Med. 2007;33:1795-1799. PubMed
68. Melamed R, Sprenkle MD, Ulstad VK, Herzog CA, Leatherman JW. Assessment
of left ventricular function by intensivists using hand-held echocardiography.
Chest. 2009;135:1416-1420. PubMed
69. Mark DG, Hayden GE, Ky B, et al. Hand-carried echocardiography for assessment
of left ventricular filling and ejection fraction in the surgical intensive care unit. J
Crit Care. 2009;24(3):470.e1-470.e7. PubMed
70. Kirkpatrick JN, Davis A, Decara JM, et al. Hand-carried cardiac ultrasound as a
tool to screen for important cardiovascular disease in an underserved minority
health care clinic. J Am Soc Echocardiogr. 2004;17:399-403. PubMed
71. Fedson S, Neithardt G, Thomas P, et al. Unsuspected clinically important findings
detected with a small portable ultrasound device in patients admitted to a general
medicine service. J Am Soc Echocardiogr. 2003;16:901-905. PubMed
72. Ghani SN, Kirkpatrick JN, Spencer, KT, et al. Rapid assessment of left ventricular
systolic function in a pacemaker clinic using a hand-carried ultrasound device.
J Interv Card Electrophysiol. 2006;16:39-43. PubMed
73. Kirkpatrick JN, Ghani SN, Spencer KT. Hand carried echocardiography
screening for LV systolic dysfunction in a pulmonary function laboratory.
Eur J Echocardiogr. 2008;9:381-383. PubMed
74. Alexander JH, Peterson ED, Chen AY, Harding TM, Adams DB, Kisslo JA Jr.
Feasibility of point-of-care echocardiography by internal medicine house staff. Am
Heart J. 2004;147:476-481. PubMed
75. Angoff WH. Scales, norms and equivalent Scores. Washington, DC: American
Council on Education; 1971.
76. Hellmann DB, Whiting-O’Keefe Q, Shapiro EP, Martin LD, Martire C, Ziegelstein
RC. The rate at which residents learn to use hand-held echocardiography at
the bedside. Am J Med. 2005;118:1010-1018. PubMed
77. Kimura BJ, Amundson SA, Phan JN, Agan DL, Shaw DJ. Observations during
development of an internal medicine residency training program in cardiovascular
limited ultrasound examination. J Hosp Med. 2012;7:537-542. PubMed
78. Akhtar S, Theodoro D, Gaspari R, et al. Resident training in emergency ultrasound:
consensus recommendations from the 2008 Council of Emergency Medicine
Residency Directors Conference. Acad Emerg Med. 2009;16(s2):S32-S36. PubMed
79. Ma OJ, Gaddis G, Norvell JG, Subramanian S. How fast is the focused assessment
with sonography for trauma examination learning curve? Emerg Med Australas.
2008;20(1):32-37. PubMed
80. Gaspari RJ, Dickman E, Blehar D. Learning curve of bedside ultrasound of the gallbladder. J Emerg Med. 2009;37(1):51-56. DOI:10.1016/j.jemermed.2007.10.070. PubMed
81. Ericsson KA, Lehmann AC. Expert and exceptional performance: Evidence of
maximal adaptation to task constraints. Ann Rev Psychol. 1996;47:273-305. PubMed
82. Ericcson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the
acquisition of expert performance. Psychol Rev. 1993;100:363-406.
83. OPPE and FPPE: Tools to help make privileging decisions. The Joint Commission.
2013. http://www.jointcommission.org/jc_physician_blog/oppe_fppe_tools_privileging_
decisions/ Accessed October 26, 2016.
84. American Medical Association. Physicians’ Current Procedural Terminology (CPT)
2011. American Medical Association, Chicago; 2011.
85. Moore CL, Gregg S, Lambert M. Performance, training, quality assurance, and
reimbursement of emergency physician-performed ultrasonography at academic
medical centers. J Ultrasound Med. 2004;23(4):459-466. PubMed
86. Critical Care Ultrasonography Certificate of Completion Program. CHEST.
American College of Chest Physicians. http://www.chestnet.org/Education/Advanced-
Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography.
Accessed July 28, 2017.
1. Soni NJ, Nilam J, Arntfield R, Kory P. Point of Care Ultrasound. Philadelphia:
Elsevier; 2015.
2. Moore CL, Copel JA. Point-of-Care Ultrasonography. N Engl J Med.
2011;364(8):749-757. PubMed
3. Randolph AG, Cook DJ, Gonzales CA, et al. Ultrasound guidance for placement
of central venous catheters: A meta-analysis of the literature. Crit Care Med.
1996;24:2053-2058. PubMed
4. Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis:
A systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339. PubMed
5. Soni NJ, Nilam J, Franco R, et al. Ultrasound in the diagnosis and management of
pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
6. Nazerian P, Volpicelli G, Gigli C, et al. Diagnostic performance of Wells score
combined with point-of-care lung and venous ultrasound in suspected pulmonary
embolism. Acad Emerg Med. 2017;24(3):270-280. PubMed
7. Chatziantoniou A, Nazerian P, Vanni S, et al. A combination of the Wells score
with multiorgan ultrasound to stratify patients with suspected pulmonary embolism.
Eur Respir J. 2015;46:OA493; DOI:10.1183/13993003.congress-2015.
OA493.
8. Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid
management. Crit Care. 2016; DOI:10.1186/s13054-016-1407-1. PubMed
9. Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-Care Multi-Organ Ultrasound
Improves Diagnostic Accuracy in Adults Presenting to the Emergency
Department with Acute Dyspnea. West J Emerg Med. 2016;17(1):46-53. PubMed
10. Glockner E, Christ M, Geier F, et al. Accuracy of Point-of-Care B-Line Lung
Ultrasound in Comparison to NT-ProBNP for Screening Acute Heart Failure.
Ultrasound Int Open. 2016;2(3):e90-e92. PubMed
11. Bhagra A, Tierney DM, Sekiguchi H, Soni NJ. Point-of-Care Ultrasonography
for Primary Care Physicians and General Internists. Mayo Clin Proc.
2016;91(12):1811-1827. PubMed
12. Crisp JG, Lovato LM, and Jang TB. Compression ultrasonography of the lower extremity
with portable vascular ultrasonography can accurately detect deep venous
thrombosis in the emergency department. Ann Emerg Med. 2010;56:601-610. PubMed
13. Squire BT, Fox JC, and Anderson C. ABSCESS: Applied bedside sonography
for convenient. Evaluation of superficial soft tissue infections. Acad Emerg Med.
2005;12:601-606. PubMed
14. Narasimhan M, Koenig SJ, Mayo PH. A Whole-Body Approach to Point of Care
Ultrasound. Chest. 2016;150(4):772-776. PubMed
15. Oks M, Cleven KL, Cardenas-Garcia J, et al. The effect of point-of-care ultrasonography
on imaging studies in the medical ICU: a comparative study. Chest.
2014;146(6):1574-1577. PubMed
16. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/
La Société de Réanimation de Langue Française Statement on Competence in
Critical Care Ultrasonography. Chest. 2009;135(4):1050-1060. PubMed
17. Frank JR, Snell LS, Ten Cate O, et al. Competency-based medical education:
Theory to practice. Med Teach. 2010;32:638-645. PubMed
18. The Who, What, When, and Where’s of Credentialing and Privileging. The
Joint Commission. http://www.jointcommission.org/assets/1/6/AHC_who_what_
when_and_where_credentialing_booklet.pdf. Accessed December 21, 2016.
19. American College of Emergency Physicians Policy Statement: Emergency Ultrasound
Guidelines. 2016. https://www.acep.org/Clinical---Practice-Management/
ACEP-Ultrasound-Guidelines/. Accessed October 26, 2016.
20. Society for Academic Emergency Medicine. Ultrasound Position Statement. Annual
Meeting 1996.
21. American Medical Association. Privileging for ultrasound imaging. 2001; Policy
H-230.960. www.ama-assn.org. Accessed July 28, 2017 PubMed
22. Stein JC, Nobay F. Emergency Department Ultrasound Credentialing: a sample
policy and procedure. J Emerg Med. 2009;37(2):153-159. PubMed
23. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused
Cardiac Ultrasound: Recommendations from the American Society of Echocardiography.
J Am Soc Echocardiogr. 2013;26(6):567-581. PubMed
24. Wiegers S. The Point of Care. J Am Soc Echocardiogr. 2016;29(4):19. PubMed
25. Mandavia D, Aragona J, Chan L, et al. Ultrasound training for emergency physicians—
a prospective study. Acad Emerg Med. 2000;7:1008-1014. PubMed
26. American College of Radiology Practice Parameters and Technical Standards.
https://www.acr.org/quality-safety/standards-guidelines. Accessed December 21, 2016.
27. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can
Fam Physician. 2012;58(3):e172-e178. PubMed
28. Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic review: emergency
department bedside ultrasonography for diagnosing suspected abdominal
aortic aneurysm. Acad Emerg Med. 2013;20:128-138. PubMed
29. Dijos M, Pucheux Y, Lafitte M, et al. Fast track echo of abdominal aortic aneurysm
using a real pocket-ultrasound device at bedside. Echocardiography. PubMed
2012;29(3):285-290.
30. Cox C, MacDonald S, Henneberry R, Atkinson PR. My patient has abdominal
and flank pain: Identifying renal causes. Ultrasound. 2015;23(4):242-250. PubMed
31. Gaspari R, Horst K. Emergency ultrasound and urinalysis in the evaluation of
patients with flank pain. Acad Emerg Med. 2005;12:1180-1184. PubMed
32. Kartal M, Eray O, Erdogru T, et al. Prospective validation of a current algorithm
including bedside US performed by emergency physicians for patients with acute
flank pain suspected for renal colic. Emerg Med J. 2006;23(5):341-344. PubMed
33. Noble VE, Brown DF. Renal ultrasound. Emerg Med Clin North Am. 2004;22:641-659. PubMed
34. Surange R, Jeygopal NS, Chowdhury SD, et al. Bedside ultrasound: a useful tool
for the on call urologist? Int Urol Nephrol. 2001;32:591-596. PubMed
35. Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency physician-performed
ultrasonography in the diagnosis of deep-vein thrombosis. Thromb Haemost.
2013;109(1):137-145. PubMed
36. Bernardi E, Camporese G, Buller HR, et al. Erasmus Study Group. Serial 2-Point
Ultrasonography Plus D-Dimer vs Whole-Leg Color-Coded Doppler Ultrasonography
for Diagnosing Suspected Symptomatic Deep Vein Thrombosis: A Randomized
Controlled Trial. JAMA. 2008;300(14):1653-1659. PubMed
37. Burnside PR, Brown MD, Kline JA. Systematic Review of Emergency Physician–
performed Ultrasonography for Lower-Extremity Deep Vein Thrombosis. Acad
Emerg Med. 2008;15:493-498. PubMed
38. Magazzini S, Vanni S, Toccafondi S, et al. Duplex ultrasound in the emergency
department for the diagnostic management of clinically suspected deep vein
thrombosis. Acad Emerg Med. 2007;14:216-220. PubMed
39. Jacoby J, Cesta M, Axelband J, Melanson S, Heller M, Reed J. Can emergency
medicine residents detect acute deep venous thrombosis with a limited, two-site
ultrasound examination? J Emerg Med. 2007;32:197-200. PubMed
40. Jang T, Docherty M, Aubin C, Polites G. Resident-performed compression ultrasonography
for the detection of proximal deep vein thrombosis: fast and accurate.
Acad Emerg Med. 2004;11:319-322. PubMed
41. Frazee BW, Snoey ER, Levitt A. Emergency Department compression ultrasound
to diagnose proximal deep vein thrombosis. J Emerg Med. 2001;20:107-112. PubMed
42. Blaivas M, Lambert MJ, Harwood RA, Wood JP, Konicki J. Lower-extremity Doppler
for deep venous thrombosis--can emergency physicians be accurate and fast?
Acad Emerg Med. 2000;7:120-126. PubMed
43. Koenig SJ, Narasimhan M, Mayo PH. Thoracic ultrasonography for the pulmonary
specialist. Chest. 2011;140(5):1332-1341. PubMed
44. Lichtenstein, DA. A bedside ultrasound sign ruling out pneumothorax in the critically
ill. Lung sliding. Chest. 1995;108(5):1345-1348. PubMed
45. Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact.
An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care
Med. 1997;156(5):1640-1646. PubMed
46. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate
acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc
Ultrasound. 2008;6:16. PubMed
47. Agricola E, Bove T, Oppizzi M, et al. Ultrasound comet-tail images: a marker
of pulmonary edema: a comparative study with wedge pressure and extravascular
lung water. Chest. 2005;127(5):1690-1695. PubMed
48. Lichtenstein DA, Meziere GA, Laqoueyte JF, Biderman P, Goldstein I, Gepner A.
A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary
artery occlusion pressure in the critically ill. Chest. 2009;136(4):1014-1020. PubMed
49. Lichtenstein DA, Lascols N, Meziere G, Gepner A. Ultrasound diagnosis of alveolar
consolidation in the critically ill. Intensive Care Med. 2004;30(2):276-281. PubMed
50. Lichtenstein D, Mezière G, Seitz J. The dynamic air bronchogram. A lung
ultrasound sign of alveolar consolidation ruling out atelectasis. Chest.
2009;135(6):1421–1425. PubMed
51. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative
diagnostic performances of auscultation, chest radiography, and lung ultrasonography
in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9-15. PubMed
52. Lichtenstein D, Meziere G. Relevance of lung ultrasound in the diagnosis of acute
respiratory failure: the BLUE protocol. Chest. 2008;134(1):117-125. PubMed
53. Mayo P, Doelken P. Pleural ultrasonography. Clin Chest Med. 2006;27(2):215-227. PubMed
54. Galderisi M, Santoro A, Versiero M, et al. Improved cardiovascular diagnostic accuracy
by pocket size imaging device in non-cardiologic outpatients: the NaUSi-
Ca (Naples Ultrasound Stethoscope in Cardiology) study. Cardiovasc Ultrasound.
2010;8:51. PubMed
55. DeCara JM, Lang RM, Koch R, Bala R, Penzotti J, Spencer KT. The use of small
personal ultrasound devices by internists without formal training in echocardiography.
Eur J Echocardiography. 2002;4:141-147. PubMed
56. Martin LD, Howell EE, Ziegelstein RC, Martire C, Shapiro EP, Hellmann DB.
Hospitalist performance of cardiac hand-carried ultrasound after focused training.
Am J Med. 2007;120:1000-1004. PubMed
57. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound performed
by hospitalists: does it improve the cardiac physical examination? Am J Med.
2009;122:35-41. PubMed
58. Perez-Avraham G, Kobal SL, Etzion O, et al. Left ventricular geometric abnormality
screening in hypertensive patients using a hand-carried ultrasound device.
J Clin Hypertens. 2010;12:181-186. PubMed
59. Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of hospitalist-performed
hand-carried ultrasound echocardiography after a brief training program. J
Hosp Med. 2009;4:340-349. PubMed
60. Kimura BJ, Fowler SJ, Fergus TS, et al. Detection of left atrial enlargement using
hand-carried ultrasound devices to screen for cardiac abnormalities. Am J Med.
2005;118:912-916. PubMed
61. Brennan JM, Blair JE, Goonewardena S, et al. A comparison by medicine residents of physical examination versus hand-carried ultrasound for estimation of
right atrial pressure. Am J Cardiol. 2007;99:1614-1616. PubMed
62. Blair JE, Brennan JM, Goonewardena SN, Shah D, Vasaiwala S, Spencer KT.
Usefulness of hand-carried ultrasound to predict elevated left ventricular filling
pressure. Am J Cardiol. 2009;103:246-247. PubMed
63. Stawicki SP, Braslow BM, Panebianco NL, et al. Intensivist use of hand-carried
ultrasonography to measure IVC collapsibility in estimating intravascular volume
status: correlations with CVP. J Am Coll Surg. 2009;209:55-61. PubMed
64. Gunst M, Ghaemmaghami V, Sperry J, et al. Accuracy of cardiac function and volume
status estimates using the bedside echocardiographic assessment in trauma/
critical care. J Trauma. 2008;65:509-515. PubMed
65. Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal
medicine residents versus traditional clinical assessment for the identification
of systolic dysfunction in patients admitted with decompensated heart failure. J
Am Soc Echocardiogr. 2011;24:1319-1324. PubMed
66. Croft LB, Duvall WL, Goldman ME. A pilot study of the clinical impact
of hand-carried cardiac ultrasound in the medical clinic. Echocardiography.
2006;23:439-446. PubMed
67. Vignon P, Dugard A, Abraham J, et al. Focused training for goal-oriented handheld
echocardiography performed by noncardiologist residents in the intensive
care unit. Intensive Care Med. 2007;33:1795-1799. PubMed
68. Melamed R, Sprenkle MD, Ulstad VK, Herzog CA, Leatherman JW. Assessment
of left ventricular function by intensivists using hand-held echocardiography.
Chest. 2009;135:1416-1420. PubMed
69. Mark DG, Hayden GE, Ky B, et al. Hand-carried echocardiography for assessment
of left ventricular filling and ejection fraction in the surgical intensive care unit. J
Crit Care. 2009;24(3):470.e1-470.e7. PubMed
70. Kirkpatrick JN, Davis A, Decara JM, et al. Hand-carried cardiac ultrasound as a
tool to screen for important cardiovascular disease in an underserved minority
health care clinic. J Am Soc Echocardiogr. 2004;17:399-403. PubMed
71. Fedson S, Neithardt G, Thomas P, et al. Unsuspected clinically important findings
detected with a small portable ultrasound device in patients admitted to a general
medicine service. J Am Soc Echocardiogr. 2003;16:901-905. PubMed
72. Ghani SN, Kirkpatrick JN, Spencer, KT, et al. Rapid assessment of left ventricular
systolic function in a pacemaker clinic using a hand-carried ultrasound device.
J Interv Card Electrophysiol. 2006;16:39-43. PubMed
73. Kirkpatrick JN, Ghani SN, Spencer KT. Hand carried echocardiography
screening for LV systolic dysfunction in a pulmonary function laboratory.
Eur J Echocardiogr. 2008;9:381-383. PubMed
74. Alexander JH, Peterson ED, Chen AY, Harding TM, Adams DB, Kisslo JA Jr.
Feasibility of point-of-care echocardiography by internal medicine house staff. Am
Heart J. 2004;147:476-481. PubMed
75. Angoff WH. Scales, norms and equivalent Scores. Washington, DC: American
Council on Education; 1971.
76. Hellmann DB, Whiting-O’Keefe Q, Shapiro EP, Martin LD, Martire C, Ziegelstein
RC. The rate at which residents learn to use hand-held echocardiography at
the bedside. Am J Med. 2005;118:1010-1018. PubMed
77. Kimura BJ, Amundson SA, Phan JN, Agan DL, Shaw DJ. Observations during
development of an internal medicine residency training program in cardiovascular
limited ultrasound examination. J Hosp Med. 2012;7:537-542. PubMed
78. Akhtar S, Theodoro D, Gaspari R, et al. Resident training in emergency ultrasound:
consensus recommendations from the 2008 Council of Emergency Medicine
Residency Directors Conference. Acad Emerg Med. 2009;16(s2):S32-S36. PubMed
79. Ma OJ, Gaddis G, Norvell JG, Subramanian S. How fast is the focused assessment
with sonography for trauma examination learning curve? Emerg Med Australas.
2008;20(1):32-37. PubMed
80. Gaspari RJ, Dickman E, Blehar D. Learning curve of bedside ultrasound of the gallbladder. J Emerg Med. 2009;37(1):51-56. DOI:10.1016/j.jemermed.2007.10.070. PubMed
81. Ericsson KA, Lehmann AC. Expert and exceptional performance: Evidence of
maximal adaptation to task constraints. Ann Rev Psychol. 1996;47:273-305. PubMed
82. Ericcson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the
acquisition of expert performance. Psychol Rev. 1993;100:363-406.
83. OPPE and FPPE: Tools to help make privileging decisions. The Joint Commission.
2013. http://www.jointcommission.org/jc_physician_blog/oppe_fppe_tools_privileging_
decisions/ Accessed October 26, 2016.
84. American Medical Association. Physicians’ Current Procedural Terminology (CPT)
2011. American Medical Association, Chicago; 2011.
85. Moore CL, Gregg S, Lambert M. Performance, training, quality assurance, and
reimbursement of emergency physician-performed ultrasonography at academic
medical centers. J Ultrasound Med. 2004;23(4):459-466. PubMed
86. Critical Care Ultrasonography Certificate of Completion Program. CHEST.
American College of Chest Physicians. http://www.chestnet.org/Education/Advanced-
Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography.
Accessed July 28, 2017.
© 2017 Society of Hospital Medicine
Caregiver Partners in Care Transitions
Under the national leadership of AARP, 42 states and territories have introduced the Caregiver Advise Record and Enable (CARE) Act, 32 have passed it, and the following 30 have enacted it into law: Arkansas, California, Colorado, Connecticut, District of Columbia, Illinois, Indiana, Louisiana, Maine, Maryland, Michigan, Minnesota, Mississippi, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Utah, Virginia, Virgin Islands, West Virginia, Washington and Wyoming (as of June 6, 2016). The CARE Act requires hospitals to: (1) record the name of the family caregiver in the medical record, (2) inform the family caregiver when the patient is to be discharged, and (3) provide the family caregiver with education and instruction on the medical tasks that he or she will need to perform for the patient upon return home.[1, 2]
The family caregiver is to be identified by the patient. Because the patient is the source of the information, Health Insurance Portability and Accountability Act concerns are minimal. A family caregiver need not be related to the patient by blood or marriage; a friend, neighbor, partner, or paid caregiver could be identified by the patient as serving in this role. If the patient does not identify a family caregiver (due to the absence of such an individual, concern for potential burden on a loved one, desire for confidentiality, transient or progressive cognitive impairment, or other reasons), this also needs to be documented, though the additional provisions of the CARE Act would not be applicable. As some states have made additions or individual modifications to the CARE Act, the reader is encouraged to learn more about state‐specific differences that might impact implementation.[2, 3]
The impetus for the CARE Act emerges from challenges faced by both family caregivers and healthcare professionals. The 3 care elements included in the Act appear to have considerable face validity for what would constitute good transitional care. To further explore why this is necessary, we need to begin by asking why are these 3 care elements not routine, and why did an advocacy organization resort to a legislative solution to formally recognize and include family caregivers in discharge preparation?
Family caregivers, when able and willing, often play an instrumental role in the care of their loved ones, particularly during the vulnerable time of transitions out of the hospital.[4, 5] They are often the first line of defense for detecting lapses in quality or safety as care is transitioned from the hospital. Family caregivers frequently take on a primary or secondary role in operationalizing and executing the discharge plan. Nearly half of family caregivers perform skilled medical or nursing tasks for their loved ones (eg, wound care, injections, complex medication management, operating specialized medical equipment) often with insufficient assistance or training from healthcare professionals.[6]
Lack of sufficient time might be a major reason why the 3 care elements identified in the CARE Act are not routinely addressed by the discharging team, which may include hospitalists, nurses, pharmacists, social workers, and other clinicians. However, there may be other reasons as well, including a lack of knowledge, confidence, or tools for how to best prepare the patient and family caregiver. This is compounded by the absence of routine feedback loops for gauging the effectiveness of discharge preparation beyond a patient's readmission to the same facility. If hospital‐based clinicians were asked to rank order their daily tasks from greatest sense of professional gratification to lowest, discharge preparation would likely appear toward the bottom of the list.[7, 8]
Meanwhile, hospitalists and hospital clinical leaders are struggling to keep pace with a confluence of new demands that include value‐based purchasing initiatives and population health efforts, to name but a few. Although current Centers for Medicare and Medicaid Services' (CMS) Hospital Conditions of Participation for Discharge Planning do not require recognition or preparation of family caregivers, CMS' newly proposed revisions emphasize better preparation of family caregivers to be active partners upon hospital discharge.[9] Thus, although it might be reflexive to view the CARE Act in isolation as yet 1 more initiative requiring new effort and resources to address, widening the lens may confirm that the contributions of family caregivers are integral and aligned across nearly all efforts aimed at promoting greater value, and in this light could be viewed as complementary rather than competitive.
Innovation or new resources may be needed to implement the CARE Act. In the absence of a step‐by‐step user's guide, hospitals may wish to take advantage of valuable publicly available resources that encourage more effective collaboration between family caregivers and healthcare professionals (Table 1).
| Organization (URL) | Relevant Resources for Implementing the CARE Act |
|---|---|
| |
| AARP ( |
Family caregiver video guides to managing medications |
| Alzheimer's Association ( |
Addresses the unique needs of persons with dementia: |
| Ensuring that all treating physicians and medical professions are aware of the diagnosis of Alzheimer's or other dementia | |
| If the person with dementia has difficulty communicating, the family caregiver may help medical staff by offering suggestions about what the person may want or need | |
| The family caregiver may alert medical staff of triggers that may cause unpredictable behavior | |
| Considerations for discharge to a residential facility or assisted living | |
| Care Transitions Program ( |
Provides a wide range of resources for professionals, patients, and family caregivers: |
| The FCAT tool | |
| Hospital discharge checklist | |
| Tips for managing care at home | |
| Tips for recognizing and responding to red flags | |
| Tips for effective medication management | |
| Institute for Patient‐ and Family‐Centered Care ( |
Offers practical advice for establishing patient and family advisory councils: |
| Qualities and skills of patient/family advisors | |
| Recruitment | |
| Development of bylaws | |
| Meeting schedule | |
| National Transitions of Care Coalition ( |
Provides a wide variety of tools and resources: |
| Taking Care of MY Health Care developed as a guide to help patients and family caregivers feel better prepared | |
| My Medicine List helps patients and family caregivers gather important information about medications | |
| Cultural competence tool provides strategies and resources to enhance professionals' capacity to deliver culturally competent services to patients and family caregivers during transitions of care | |
| Next Step in Care ( |
The most comprehensive site supporting both family caregivers and health professionals; includes: |
| A toolkit for working with family caregivers | |
| HIPAA considerations for family caregivers | |
| Tips on identifying the family caregiver | |
| Assessment tool for family caregivers' needs | |
| Tips for referring patients and family caregivers to community‐based services | |
|
Project BOOST( |
Extensive toolkit includes: |
| Self‐assessment questions to promote planning for how to include family | |
| Return on investment calculator that includes patient as well as family satisfaction | |
| Teach back approach applicable to patient and family caregivers | |
| Patient and family caregiver preparedness tool | |
|
Project RED( |
Extensive toolkit includes: |
| Five steps to integrating family caregivers into the discharge plan | |
| Understanding and enhancing the role of family caregivers in RED | |
Operationalizing the CARE Act may initially appear simple but in practice will not be easy. The first care element focuses on identifying the family caregiver. Next, Step in Care offers a practical guide for how to identify the family caregiver in a busy hospital environment (Table 1). The guide advises health professionals on how to identify the person most likely to assume responsibility for care after discharge by asking a series of questions: Who assists you at home? Who do you call in case of emergency? Who helps with medications or doctor appointments? The guide cautions health professionals not to assume that individuals encountered at the patient's bedside are necessarily the family caregivers. They may be covering for the family caregiver, who has other duties (eg, job, child care).
The second CARE Act element entails informing the family caregiver when the patient will be discharged. At present there is no standardization of this practice. Many hospitals conduct interdisciplinary rounds, during which a discharge date is frequently estimated. A designated member of the inpatient team (eg, primary nurse, social worker, care manager) might be tasked with notifying the family caregiver of this estimated date (either in person, by telephone, or using other approved mode[s] of communication). Ideally, this notification should be conveyed as soon as the inpatient care team can foresee a discharge date, as it would be preferable to give the family caregiver an estimate that turns out to be a day or 2 off and needs to be revised than to inform the family caregiver at the last minute. The white board in the patient's room may serve as a reminder to both the patient and family caregiver as well as to other members of the inpatient care team.
The third CARE Act element could be facilitated with the Family Caregiver Activation in Transitions (FCAT) tool, a self‐efficacy measure of transition specific tasks. The FCAT tool is designed to facilitate more productive interactions and guide the care team in understanding what common transition‐related areas family caregivers would like to feel more prepared for or confident with. The FCAT tool can be administered by a health professional or self‐administered by a family caregiver and takes approximately 2 minutes to complete[10] (Table 1).
Hospital leaders might consider creating an interdisciplinary team charged with facilitating the implementation of the CARE Act. Specifically, this team might develop guidelines and serve as a forum whereby clinicians might share particularly challenging cases. Similarly, for ongoing input and suggestions for how to further improve all aspects of hospital care, including the discharge experience, hospitals are encouraged to form and foster patient and family advisory councils (Table 1).
Finally, when it comes to improving the hospital discharge experience for family caregivers, there is no us and them. Despite our professional advantages, each of has had or will likely have an opportunity to overcome the many gaps in hospital discharge planning, not just as healthcare professionals but also in our roles as adult children, spouses, and parents. In this regard, we are all invested in improving the discharge experience.
Disclosures
Support for this work was provided by the Gordon and Betty Moore Foundation. The sponsor had no role in the preparation, review, or approval of this article. The author reports no conflicts of interest.
- . One caregiver's regret: how the CARE Act could have helped. Available at: http://blog.aarp.org/2016/04/18/one‐caregivers‐regret‐how‐the‐care‐act‐could‐have‐helped. Published April 18, 2016. Accessed June 7, 2016.
- , . Stepping up to support family caregivers. Available at: http://blog.aarp.org/2016/06/07/stepping‐up‐to‐support‐family‐caregivers. Published June 7, 2016. Accessed June 7, 2016.
- . New state laws support millions of Americans who minister to aging relatives and form the backbone of the nation's long‐term care system. Available at: http://www.ncsl.org/research/human‐services/helping‐the‐helpers.aspx. Published February 1, 2015. Accessed June 7, 2016.
- , . Family caregivers' experiences during transitions out of the hospital. J Healthc Qual. 2015;37:12–21.
- . The critical role of caregivers in achieving patient‐centered care. JAMA. 2013;310:575–576.
- , , . Home alone: family caregivers providing complex chronic care, 2012. Available at: http://www.aarp.org/home‐family/caregiving/info‐10‐2012/home‐alone‐family‐caregivers‐providing‐complex‐chronic‐care.html. Accessed June 7, 2016.
- , , , et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1:354–360.
- , , , , . Out of sight, out of mind: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012:7:376–381.
- Centers for Medicare and Medicaid Services. Proposed revisions to requirements for discharge planning for hospitals, critical access hospitals, and home health agencies. Fed Regist. 2015;80:68125–68155.
- , , . The Family Caregiver Activation in Transitions tool (FCAT): a new measure of family caregiver self‐efficacy. Jt Comm J Qual Patient Saf. 2015;41:502–507.
Under the national leadership of AARP, 42 states and territories have introduced the Caregiver Advise Record and Enable (CARE) Act, 32 have passed it, and the following 30 have enacted it into law: Arkansas, California, Colorado, Connecticut, District of Columbia, Illinois, Indiana, Louisiana, Maine, Maryland, Michigan, Minnesota, Mississippi, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Utah, Virginia, Virgin Islands, West Virginia, Washington and Wyoming (as of June 6, 2016). The CARE Act requires hospitals to: (1) record the name of the family caregiver in the medical record, (2) inform the family caregiver when the patient is to be discharged, and (3) provide the family caregiver with education and instruction on the medical tasks that he or she will need to perform for the patient upon return home.[1, 2]
The family caregiver is to be identified by the patient. Because the patient is the source of the information, Health Insurance Portability and Accountability Act concerns are minimal. A family caregiver need not be related to the patient by blood or marriage; a friend, neighbor, partner, or paid caregiver could be identified by the patient as serving in this role. If the patient does not identify a family caregiver (due to the absence of such an individual, concern for potential burden on a loved one, desire for confidentiality, transient or progressive cognitive impairment, or other reasons), this also needs to be documented, though the additional provisions of the CARE Act would not be applicable. As some states have made additions or individual modifications to the CARE Act, the reader is encouraged to learn more about state‐specific differences that might impact implementation.[2, 3]
The impetus for the CARE Act emerges from challenges faced by both family caregivers and healthcare professionals. The 3 care elements included in the Act appear to have considerable face validity for what would constitute good transitional care. To further explore why this is necessary, we need to begin by asking why are these 3 care elements not routine, and why did an advocacy organization resort to a legislative solution to formally recognize and include family caregivers in discharge preparation?
Family caregivers, when able and willing, often play an instrumental role in the care of their loved ones, particularly during the vulnerable time of transitions out of the hospital.[4, 5] They are often the first line of defense for detecting lapses in quality or safety as care is transitioned from the hospital. Family caregivers frequently take on a primary or secondary role in operationalizing and executing the discharge plan. Nearly half of family caregivers perform skilled medical or nursing tasks for their loved ones (eg, wound care, injections, complex medication management, operating specialized medical equipment) often with insufficient assistance or training from healthcare professionals.[6]
Lack of sufficient time might be a major reason why the 3 care elements identified in the CARE Act are not routinely addressed by the discharging team, which may include hospitalists, nurses, pharmacists, social workers, and other clinicians. However, there may be other reasons as well, including a lack of knowledge, confidence, or tools for how to best prepare the patient and family caregiver. This is compounded by the absence of routine feedback loops for gauging the effectiveness of discharge preparation beyond a patient's readmission to the same facility. If hospital‐based clinicians were asked to rank order their daily tasks from greatest sense of professional gratification to lowest, discharge preparation would likely appear toward the bottom of the list.[7, 8]
Meanwhile, hospitalists and hospital clinical leaders are struggling to keep pace with a confluence of new demands that include value‐based purchasing initiatives and population health efforts, to name but a few. Although current Centers for Medicare and Medicaid Services' (CMS) Hospital Conditions of Participation for Discharge Planning do not require recognition or preparation of family caregivers, CMS' newly proposed revisions emphasize better preparation of family caregivers to be active partners upon hospital discharge.[9] Thus, although it might be reflexive to view the CARE Act in isolation as yet 1 more initiative requiring new effort and resources to address, widening the lens may confirm that the contributions of family caregivers are integral and aligned across nearly all efforts aimed at promoting greater value, and in this light could be viewed as complementary rather than competitive.
Innovation or new resources may be needed to implement the CARE Act. In the absence of a step‐by‐step user's guide, hospitals may wish to take advantage of valuable publicly available resources that encourage more effective collaboration between family caregivers and healthcare professionals (Table 1).
| Organization (URL) | Relevant Resources for Implementing the CARE Act |
|---|---|
| |
| AARP ( |
Family caregiver video guides to managing medications |
| Alzheimer's Association ( |
Addresses the unique needs of persons with dementia: |
| Ensuring that all treating physicians and medical professions are aware of the diagnosis of Alzheimer's or other dementia | |
| If the person with dementia has difficulty communicating, the family caregiver may help medical staff by offering suggestions about what the person may want or need | |
| The family caregiver may alert medical staff of triggers that may cause unpredictable behavior | |
| Considerations for discharge to a residential facility or assisted living | |
| Care Transitions Program ( |
Provides a wide range of resources for professionals, patients, and family caregivers: |
| The FCAT tool | |
| Hospital discharge checklist | |
| Tips for managing care at home | |
| Tips for recognizing and responding to red flags | |
| Tips for effective medication management | |
| Institute for Patient‐ and Family‐Centered Care ( |
Offers practical advice for establishing patient and family advisory councils: |
| Qualities and skills of patient/family advisors | |
| Recruitment | |
| Development of bylaws | |
| Meeting schedule | |
| National Transitions of Care Coalition ( |
Provides a wide variety of tools and resources: |
| Taking Care of MY Health Care developed as a guide to help patients and family caregivers feel better prepared | |
| My Medicine List helps patients and family caregivers gather important information about medications | |
| Cultural competence tool provides strategies and resources to enhance professionals' capacity to deliver culturally competent services to patients and family caregivers during transitions of care | |
| Next Step in Care ( |
The most comprehensive site supporting both family caregivers and health professionals; includes: |
| A toolkit for working with family caregivers | |
| HIPAA considerations for family caregivers | |
| Tips on identifying the family caregiver | |
| Assessment tool for family caregivers' needs | |
| Tips for referring patients and family caregivers to community‐based services | |
|
Project BOOST( |
Extensive toolkit includes: |
| Self‐assessment questions to promote planning for how to include family | |
| Return on investment calculator that includes patient as well as family satisfaction | |
| Teach back approach applicable to patient and family caregivers | |
| Patient and family caregiver preparedness tool | |
|
Project RED( |
Extensive toolkit includes: |
| Five steps to integrating family caregivers into the discharge plan | |
| Understanding and enhancing the role of family caregivers in RED | |
Operationalizing the CARE Act may initially appear simple but in practice will not be easy. The first care element focuses on identifying the family caregiver. Next, Step in Care offers a practical guide for how to identify the family caregiver in a busy hospital environment (Table 1). The guide advises health professionals on how to identify the person most likely to assume responsibility for care after discharge by asking a series of questions: Who assists you at home? Who do you call in case of emergency? Who helps with medications or doctor appointments? The guide cautions health professionals not to assume that individuals encountered at the patient's bedside are necessarily the family caregivers. They may be covering for the family caregiver, who has other duties (eg, job, child care).
The second CARE Act element entails informing the family caregiver when the patient will be discharged. At present there is no standardization of this practice. Many hospitals conduct interdisciplinary rounds, during which a discharge date is frequently estimated. A designated member of the inpatient team (eg, primary nurse, social worker, care manager) might be tasked with notifying the family caregiver of this estimated date (either in person, by telephone, or using other approved mode[s] of communication). Ideally, this notification should be conveyed as soon as the inpatient care team can foresee a discharge date, as it would be preferable to give the family caregiver an estimate that turns out to be a day or 2 off and needs to be revised than to inform the family caregiver at the last minute. The white board in the patient's room may serve as a reminder to both the patient and family caregiver as well as to other members of the inpatient care team.
The third CARE Act element could be facilitated with the Family Caregiver Activation in Transitions (FCAT) tool, a self‐efficacy measure of transition specific tasks. The FCAT tool is designed to facilitate more productive interactions and guide the care team in understanding what common transition‐related areas family caregivers would like to feel more prepared for or confident with. The FCAT tool can be administered by a health professional or self‐administered by a family caregiver and takes approximately 2 minutes to complete[10] (Table 1).
Hospital leaders might consider creating an interdisciplinary team charged with facilitating the implementation of the CARE Act. Specifically, this team might develop guidelines and serve as a forum whereby clinicians might share particularly challenging cases. Similarly, for ongoing input and suggestions for how to further improve all aspects of hospital care, including the discharge experience, hospitals are encouraged to form and foster patient and family advisory councils (Table 1).
Finally, when it comes to improving the hospital discharge experience for family caregivers, there is no us and them. Despite our professional advantages, each of has had or will likely have an opportunity to overcome the many gaps in hospital discharge planning, not just as healthcare professionals but also in our roles as adult children, spouses, and parents. In this regard, we are all invested in improving the discharge experience.
Disclosures
Support for this work was provided by the Gordon and Betty Moore Foundation. The sponsor had no role in the preparation, review, or approval of this article. The author reports no conflicts of interest.
Under the national leadership of AARP, 42 states and territories have introduced the Caregiver Advise Record and Enable (CARE) Act, 32 have passed it, and the following 30 have enacted it into law: Arkansas, California, Colorado, Connecticut, District of Columbia, Illinois, Indiana, Louisiana, Maine, Maryland, Michigan, Minnesota, Mississippi, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Utah, Virginia, Virgin Islands, West Virginia, Washington and Wyoming (as of June 6, 2016). The CARE Act requires hospitals to: (1) record the name of the family caregiver in the medical record, (2) inform the family caregiver when the patient is to be discharged, and (3) provide the family caregiver with education and instruction on the medical tasks that he or she will need to perform for the patient upon return home.[1, 2]
The family caregiver is to be identified by the patient. Because the patient is the source of the information, Health Insurance Portability and Accountability Act concerns are minimal. A family caregiver need not be related to the patient by blood or marriage; a friend, neighbor, partner, or paid caregiver could be identified by the patient as serving in this role. If the patient does not identify a family caregiver (due to the absence of such an individual, concern for potential burden on a loved one, desire for confidentiality, transient or progressive cognitive impairment, or other reasons), this also needs to be documented, though the additional provisions of the CARE Act would not be applicable. As some states have made additions or individual modifications to the CARE Act, the reader is encouraged to learn more about state‐specific differences that might impact implementation.[2, 3]
The impetus for the CARE Act emerges from challenges faced by both family caregivers and healthcare professionals. The 3 care elements included in the Act appear to have considerable face validity for what would constitute good transitional care. To further explore why this is necessary, we need to begin by asking why are these 3 care elements not routine, and why did an advocacy organization resort to a legislative solution to formally recognize and include family caregivers in discharge preparation?
Family caregivers, when able and willing, often play an instrumental role in the care of their loved ones, particularly during the vulnerable time of transitions out of the hospital.[4, 5] They are often the first line of defense for detecting lapses in quality or safety as care is transitioned from the hospital. Family caregivers frequently take on a primary or secondary role in operationalizing and executing the discharge plan. Nearly half of family caregivers perform skilled medical or nursing tasks for their loved ones (eg, wound care, injections, complex medication management, operating specialized medical equipment) often with insufficient assistance or training from healthcare professionals.[6]
Lack of sufficient time might be a major reason why the 3 care elements identified in the CARE Act are not routinely addressed by the discharging team, which may include hospitalists, nurses, pharmacists, social workers, and other clinicians. However, there may be other reasons as well, including a lack of knowledge, confidence, or tools for how to best prepare the patient and family caregiver. This is compounded by the absence of routine feedback loops for gauging the effectiveness of discharge preparation beyond a patient's readmission to the same facility. If hospital‐based clinicians were asked to rank order their daily tasks from greatest sense of professional gratification to lowest, discharge preparation would likely appear toward the bottom of the list.[7, 8]
Meanwhile, hospitalists and hospital clinical leaders are struggling to keep pace with a confluence of new demands that include value‐based purchasing initiatives and population health efforts, to name but a few. Although current Centers for Medicare and Medicaid Services' (CMS) Hospital Conditions of Participation for Discharge Planning do not require recognition or preparation of family caregivers, CMS' newly proposed revisions emphasize better preparation of family caregivers to be active partners upon hospital discharge.[9] Thus, although it might be reflexive to view the CARE Act in isolation as yet 1 more initiative requiring new effort and resources to address, widening the lens may confirm that the contributions of family caregivers are integral and aligned across nearly all efforts aimed at promoting greater value, and in this light could be viewed as complementary rather than competitive.
Innovation or new resources may be needed to implement the CARE Act. In the absence of a step‐by‐step user's guide, hospitals may wish to take advantage of valuable publicly available resources that encourage more effective collaboration between family caregivers and healthcare professionals (Table 1).
| Organization (URL) | Relevant Resources for Implementing the CARE Act |
|---|---|
| |
| AARP ( |
Family caregiver video guides to managing medications |
| Alzheimer's Association ( |
Addresses the unique needs of persons with dementia: |
| Ensuring that all treating physicians and medical professions are aware of the diagnosis of Alzheimer's or other dementia | |
| If the person with dementia has difficulty communicating, the family caregiver may help medical staff by offering suggestions about what the person may want or need | |
| The family caregiver may alert medical staff of triggers that may cause unpredictable behavior | |
| Considerations for discharge to a residential facility or assisted living | |
| Care Transitions Program ( |
Provides a wide range of resources for professionals, patients, and family caregivers: |
| The FCAT tool | |
| Hospital discharge checklist | |
| Tips for managing care at home | |
| Tips for recognizing and responding to red flags | |
| Tips for effective medication management | |
| Institute for Patient‐ and Family‐Centered Care ( |
Offers practical advice for establishing patient and family advisory councils: |
| Qualities and skills of patient/family advisors | |
| Recruitment | |
| Development of bylaws | |
| Meeting schedule | |
| National Transitions of Care Coalition ( |
Provides a wide variety of tools and resources: |
| Taking Care of MY Health Care developed as a guide to help patients and family caregivers feel better prepared | |
| My Medicine List helps patients and family caregivers gather important information about medications | |
| Cultural competence tool provides strategies and resources to enhance professionals' capacity to deliver culturally competent services to patients and family caregivers during transitions of care | |
| Next Step in Care ( |
The most comprehensive site supporting both family caregivers and health professionals; includes: |
| A toolkit for working with family caregivers | |
| HIPAA considerations for family caregivers | |
| Tips on identifying the family caregiver | |
| Assessment tool for family caregivers' needs | |
| Tips for referring patients and family caregivers to community‐based services | |
|
Project BOOST( |
Extensive toolkit includes: |
| Self‐assessment questions to promote planning for how to include family | |
| Return on investment calculator that includes patient as well as family satisfaction | |
| Teach back approach applicable to patient and family caregivers | |
| Patient and family caregiver preparedness tool | |
|
Project RED( |
Extensive toolkit includes: |
| Five steps to integrating family caregivers into the discharge plan | |
| Understanding and enhancing the role of family caregivers in RED | |
Operationalizing the CARE Act may initially appear simple but in practice will not be easy. The first care element focuses on identifying the family caregiver. Next, Step in Care offers a practical guide for how to identify the family caregiver in a busy hospital environment (Table 1). The guide advises health professionals on how to identify the person most likely to assume responsibility for care after discharge by asking a series of questions: Who assists you at home? Who do you call in case of emergency? Who helps with medications or doctor appointments? The guide cautions health professionals not to assume that individuals encountered at the patient's bedside are necessarily the family caregivers. They may be covering for the family caregiver, who has other duties (eg, job, child care).
The second CARE Act element entails informing the family caregiver when the patient will be discharged. At present there is no standardization of this practice. Many hospitals conduct interdisciplinary rounds, during which a discharge date is frequently estimated. A designated member of the inpatient team (eg, primary nurse, social worker, care manager) might be tasked with notifying the family caregiver of this estimated date (either in person, by telephone, or using other approved mode[s] of communication). Ideally, this notification should be conveyed as soon as the inpatient care team can foresee a discharge date, as it would be preferable to give the family caregiver an estimate that turns out to be a day or 2 off and needs to be revised than to inform the family caregiver at the last minute. The white board in the patient's room may serve as a reminder to both the patient and family caregiver as well as to other members of the inpatient care team.
The third CARE Act element could be facilitated with the Family Caregiver Activation in Transitions (FCAT) tool, a self‐efficacy measure of transition specific tasks. The FCAT tool is designed to facilitate more productive interactions and guide the care team in understanding what common transition‐related areas family caregivers would like to feel more prepared for or confident with. The FCAT tool can be administered by a health professional or self‐administered by a family caregiver and takes approximately 2 minutes to complete[10] (Table 1).
Hospital leaders might consider creating an interdisciplinary team charged with facilitating the implementation of the CARE Act. Specifically, this team might develop guidelines and serve as a forum whereby clinicians might share particularly challenging cases. Similarly, for ongoing input and suggestions for how to further improve all aspects of hospital care, including the discharge experience, hospitals are encouraged to form and foster patient and family advisory councils (Table 1).
Finally, when it comes to improving the hospital discharge experience for family caregivers, there is no us and them. Despite our professional advantages, each of has had or will likely have an opportunity to overcome the many gaps in hospital discharge planning, not just as healthcare professionals but also in our roles as adult children, spouses, and parents. In this regard, we are all invested in improving the discharge experience.
Disclosures
Support for this work was provided by the Gordon and Betty Moore Foundation. The sponsor had no role in the preparation, review, or approval of this article. The author reports no conflicts of interest.
- . One caregiver's regret: how the CARE Act could have helped. Available at: http://blog.aarp.org/2016/04/18/one‐caregivers‐regret‐how‐the‐care‐act‐could‐have‐helped. Published April 18, 2016. Accessed June 7, 2016.
- , . Stepping up to support family caregivers. Available at: http://blog.aarp.org/2016/06/07/stepping‐up‐to‐support‐family‐caregivers. Published June 7, 2016. Accessed June 7, 2016.
- . New state laws support millions of Americans who minister to aging relatives and form the backbone of the nation's long‐term care system. Available at: http://www.ncsl.org/research/human‐services/helping‐the‐helpers.aspx. Published February 1, 2015. Accessed June 7, 2016.
- , . Family caregivers' experiences during transitions out of the hospital. J Healthc Qual. 2015;37:12–21.
- . The critical role of caregivers in achieving patient‐centered care. JAMA. 2013;310:575–576.
- , , . Home alone: family caregivers providing complex chronic care, 2012. Available at: http://www.aarp.org/home‐family/caregiving/info‐10‐2012/home‐alone‐family‐caregivers‐providing‐complex‐chronic‐care.html. Accessed June 7, 2016.
- , , , et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1:354–360.
- , , , , . Out of sight, out of mind: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012:7:376–381.
- Centers for Medicare and Medicaid Services. Proposed revisions to requirements for discharge planning for hospitals, critical access hospitals, and home health agencies. Fed Regist. 2015;80:68125–68155.
- , , . The Family Caregiver Activation in Transitions tool (FCAT): a new measure of family caregiver self‐efficacy. Jt Comm J Qual Patient Saf. 2015;41:502–507.
- . One caregiver's regret: how the CARE Act could have helped. Available at: http://blog.aarp.org/2016/04/18/one‐caregivers‐regret‐how‐the‐care‐act‐could‐have‐helped. Published April 18, 2016. Accessed June 7, 2016.
- , . Stepping up to support family caregivers. Available at: http://blog.aarp.org/2016/06/07/stepping‐up‐to‐support‐family‐caregivers. Published June 7, 2016. Accessed June 7, 2016.
- . New state laws support millions of Americans who minister to aging relatives and form the backbone of the nation's long‐term care system. Available at: http://www.ncsl.org/research/human‐services/helping‐the‐helpers.aspx. Published February 1, 2015. Accessed June 7, 2016.
- , . Family caregivers' experiences during transitions out of the hospital. J Healthc Qual. 2015;37:12–21.
- . The critical role of caregivers in achieving patient‐centered care. JAMA. 2013;310:575–576.
- , , . Home alone: family caregivers providing complex chronic care, 2012. Available at: http://www.aarp.org/home‐family/caregiving/info‐10‐2012/home‐alone‐family‐caregivers‐providing‐complex‐chronic‐care.html. Accessed June 7, 2016.
- , , , et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1:354–360.
- , , , , . Out of sight, out of mind: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012:7:376–381.
- Centers for Medicare and Medicaid Services. Proposed revisions to requirements for discharge planning for hospitals, critical access hospitals, and home health agencies. Fed Regist. 2015;80:68125–68155.
- , , . The Family Caregiver Activation in Transitions tool (FCAT): a new measure of family caregiver self‐efficacy. Jt Comm J Qual Patient Saf. 2015;41:502–507.