User login
Care Team Identification
Patient‐centered communication is a strategy that is used to promote shared understanding of the plan of care among providers and patients.[1, 2, 3] Caring for hospitalized patients is a collaborative effort that requires seamless patient‐centered communication among a rapidly changing care team to safely progress a patient from admission through discharge. Yet, hospitals continue to struggle with improving the complex and increasingly electronic conversation patterns among care team members and patients to achieve effective patient‐centered communication.[4, 5] When members of the care team operate in this environment, patients often receive conflicting information regarding their plan of care, medications, and test results. Ineffective communication can lead to a suboptimal patient experience, additional costs, medical errors, and preventable adverse events.[6, 7, 8, 9, 10]
A critical first step to improving patient‐centered communication is identifying the care team.[11, 12] Accurate and reliable identification of all care team members is a pressing information need; it is fundamental to efficiently conveying information about the plan of care to those who know the patient the best, must make timely decisions, or will assume care once the patient leaves the hospital.[13] Furthermore, it has implications for engaging patients more meaningfully in their care.[14, 15, 16, 17] Ideally, the process of identifying an individual caring for the patient in a specific role is quickly and reliably determined from the electronic health record, the single source of truth where any provider can quickly identify other team members. This source of truth can be updated manually when individual members assign and remove themselves from the care team, or automatically when accessing the patient's record, writing a note, placing an order, or adding a patient to a coverage list. When providers correctly identify other team members in this way, hospital paging directories and secure messaging tools that link to the electronic health record become more effective at supporting care team communication.[18]
In general, the process of identifying care teams is difficult,[19] and maintaining role assignments in the electronic health record is equally challenging. Vawdrey et al. previously reported that care team lists are inaccurate and cannot be used to reliably identify other members at any given moment.[18] The inability to identify team members often leads to incorrectly routed pages, e‐mail messages, and phone calls.[20] Consequently, the potential to reliably manage the care team and improve electronic communication remains untapped, rendering team collaboration and care coordination less effective.[18, 21, 22]
In recent years, the trend toward restructuring inpatient teamsgeographical localization, structured communication interventions, teamwork training, and interdisciplinary roundswould seem to diminish the need for electronic care team identification, as those efforts have already made a positive impact with regard to interprofessional communication and collaboration, team satisfaction, and adverse events.[23, 24, 25, 26] Nonetheless, interdisciplinary teamwork, though critically important for patient‐centered communication, does not completely obviate the need for accurate and reliable care team identification.[26] Although care teams are statically located on units, the plan of care is dynamic; it evolves when the patient's status changes, when new information becomes available, and when key longitudinal providers (eg, primary care physician, subspecialty consultant) make recommendations. Thus, information conveyed as a team on rounds quickly becomes out of date, requiring additional forms of communication. Furthermore, due to frequent ad hoc coverage among team members, the identity of providers covering the patient at any given moment is often not clear.[27] This is particularly problematic for nonunit‐based providers who try to communicate with unit‐based care team members. These providers, in particular, have valuable knowledge and insight that can aid the primary team in decision making.[28, 29] However, they typically do not participate in rounds, often waste time identifying responsible providers,[20] and may communicate their recommendations directly with the patient without discussing with the primary team. These factors in part explain why geographic localization has shown limited improvement in shared understanding of the plan of care.[23]
From the perspective of patients and caregivers, identification of the providers entering and leaving their room is also challenging; only 11% to 51% of patients identify their providers correctly.[30] This adds to confusion regarding who is responsible for which aspects of the patient's care and can negatively affect the perception of the quality of care received.[31] Use of whiteboards has been shown to improve the proportion of patients who could identify key providers,[32] but these are not reliably updated and generally cannot accommodate all team members. When face cards are used, patients and caregivers report that they are more likely to identify their providers correctly.[14, 33, 34] However, potential confusion may ensue when another provider assumes care of the patient in the same role. Finally, use of technology to display team members at the bedside is typically a feature that patients like and can improve identification of care team members.[14, 15, 16] Yet, patient engagement technologies are not readily available in the hospital setting,[35] and ideally should be linked to the electronic health record, which again must be reliably updated.[11, 12, 15, 16]
If care team identification is so critical for delivering effective patient‐centered communication, why is maintaining role assignment problematic? At the individual level, reasons include discontinuity of the care team due to changing clinical rotations and intrateam coverage, shift‐based schedules, and lack of awareness and underutilization of functionality. Additionally, clinicians may have different ways to maintain lists of patients. At the institutional level, functionality to enforce role assignment when accessing patient records may be disabled (to avoid perceived burdens on clinical staff or nonclinical personnel who require access for administrative functions). Finally, electronic health record vendors currently have no incentive to adopt functionality that supports more effective care coordination across settings.[22]
However, more than technical solutions and policy changes are required; care team identification in the electronic health record requires a change in institutional culture. Maintaining an accurate relationship to each patient requires work without tangible benefitsthe benefits accrue only when everyone else identifies their role on the teama tragedy of the commons. This can be illustrated by our own experience. We conducted a quality improvement initiative (Table 1) as a part of 2 concurrent research initiatives that serve to promote patient‐centered communication:[12] PCORI (Patient‐Centered Outcomes Research Institute Transitions), the goal of which is to improve care transitions within the Partners' Pioneer Accountable Care Organization; and the PROSPECT (Promoting Respect and Ongoing Safety Through Patient‐Centeredness, Engagement, Communication, and Technology) project, an initiative funded by the Gordon and Betty Moore Foundation to eliminate preventable harms in the acute care environment.[29] Our goal was to electronically manage the care team with a high degree of fidelity. We enhanced a home‐grown application, which was developed to improve management of team lists for inpatient providers, accessible from our electronic health record, to facilitate role assignment. Specifically, we leveraged existing care processes (eg, nursing log‐on to the electronic medication administration system) to automatically assign certain providers to the care team at change of shift, added functionality to make it easy to assign a provider to all patients on a list for a defined period of time, and encouraged providers to assign their role by demonstrating benefits including quick access to patient‐specific group e‐mail and secure messaging tools (Table 1, Key Facets). The initiative was well‐received by most disciplines, but uptake was suboptimal. Our research assistants routinely assigned residents and others to the care team because our proactive attempts at advertising and reinforcing use of the application failed to reach a critical mass. Most did not see immediate benefits because it was an added step to their busy day, had other methods of managing team lists, and only saw benefit if everyone else participated. Key facets of our care team identification initiative, successes, and challenges are outlined in Table 1.
| Key Facets | Successes | Challenges |
|---|---|---|
| Linked electronic role assignment to administrative processes and clinical workflows | Leveraged existing processes to identify attending provider by routinely reviewing online schedules Linked role assignment to electronic medication administration system sign‐in process for nurses at the start of their shift |
Difficult to generate buy‐in from administrators and specific clinician groups to incorporate routine use of role assignment functionality into existing and/or new workflows No institutional policy mandating role assignments for members of extended care team |
| Incorporated default functionality to specify length of role assignment (eg, stop date) | Used by trainees (residents, fellows) to automate team list role assignments for a prespecified period of time according to online schedules | Underutilized by subspecialty consultants, many of who were unaware or did not fully appreciate the added value of this functionality Research assistants regularly verified that default role assignments were accurately maintained for trainees |
| Linked role assignment to patient‐specific group e‐mail and messaging tools | Clinicians acknowledged clear efficiency benefits (eg, automated patient identification within messages, correct routing of e‐mails) Used by specific members of the care team tasked with facilitating coordination of care (eg, nurse practitioner trained as discharge advocate for research study) |
Difficult to promote use of patient‐specific messaging, particularly for nonunit‐based providers (eg, consultants, primary care physicians) Required access to an application not typically used for clinical messaging Difficult to change culture of network e‐mail use for clinical messaging |
| Advertised new functionality and demonstrated potential efficiencies for care team communication | Unit‐based clinicians (hospitalists, nurses, housestaff) typically understood benefits when demonstrated and were easier to engage | Some nonunit‐based clinicians (eg, consulting attendings, primary care physicians) did not see benefits and/or were difficult to engage |
| Some nonunit‐based provider groups (eg, social workers, nutritionists, subspecialty fellows) considered the initiative worthwhile, and were open to learning about new functionality to improve communication | Clinicians had several options for managing team lists prior to implementation of new electronic health record | |
| Institutional effort toward implementing new electronic health record detracted from efforts at demonstrating enhanced functionality of existing applications |
There were a few glimmers of hope, however. On several PROSPECT units, we displayed team members on a tablet‐based patient portal so that patients would recognize their providers.[11, 17, 36] Similar to recent work by O'Leary et al.,[14] patients on PROSPECT units were able to correctly identify several care team members, but regularly asked why other providers (eg, consulting fellow) were not listed. Those providers asked the same question, and some eventually learned to assign their role via the application. As part of PROSEPCT, we visited other institutions and learned of an effort to display team members on high‐definition televisions in the patient's room. Several providers, wondering why they were not listed, learned to assign their role and their picture then appeared. Social pressure was the driving force.
Coincidently, we recently implemented a new electronic health record at our institution. Anecdotally, although no formal policy was established, many providers (eg, attendings, first responders, nurses, care coordinators, and other unit‐based providers) appear to be assigning their roles. Other providers (eg, dieticians, physical therapists, residents) also assign their role, but often fail to end role assignments upon completing their rotation or when the patient transfers to another service. Finally, even when actively involved, most subspecialists still do not designate their role. Despite these gaps and inconsistencies, we have made progress toward improving care team identification. The reasons for this progress are straightforward; during required training for the new electronic health record, all inpatient providers were taught to assign their role on the treatment team when assuming care of patients and now have 1 option for managing team lists. However, most providers were not trained to end their role assignments, and many have learned that role assignment is not required to access the patient's record; functionality to enforce this was disabled. Based on lessons learned from our experience,[12] we offer several strategies that hospitalists can employ to improve care team identification in the electronic health record (Table 2).
| Goal | Strategies to Achieve Goal |
|---|---|
| Identify and/or establish reliable processes that administrative staff can use to ensure accurate care team role assignments | Identify databases that serve as the source of truth for provider schedules and routinely access those databases |
| Access resident scheduling application (eg, Amion) that is routinely updated by training program staff | |
| Work with clinical and administrative staff to maintain care team role assignments | |
| Engage affiliated ambulatory practices to ensure patient's primary care physician is updated in the electronic health record | |
| Engage admissions office to improve reliability of attending assignments based on online clinical schedules when patients are admitted | |
| Integrate role assignment into established workflows for specific provider groups when administrative processes not feasible | Link routine care processes to care team role assignment |
| Train nurses, interns, physician assistants to assign role on care team when assuming care of patient at shift change | |
| Train residents, fellows to use default functionality to automatically assign their role on care team at the beginning of a clinical rotation | |
| Demonstrate value of maintaining role assignments in the electronic health record to the unit‐based care team | Emphasize how accurate and reliable care team role assignment can facilitate correct routing of information (eg, test results, discharge summaries) |
| Helps to maintain patient coverage lists (eg, fellows, consultants, social workers) | |
| Facilitates patient‐specific communication (eg, via group email and messaging tools linked to the electronic health record's care team functionality) | |
| Align with concurrent institutional initiatives that enforce or incentivize care team role assignment | Mandate role assignment when writing a note, placing an order, or adding a patient to a coverage list in the electronic health record |
| Provide patients and caregivers the ability to identify the care team via patient portalcreates social pressure for those providers who do not identify themselves on the care team | |
| Incentivize providers to maintain role assignments during patient's hospitalization in order to receive notifications if patients are readmitted | |
| Automate role assignments for all members of the care team whenever possible | Work with clinical informatics/emnformation system staff to determine feasibility of linking online scheduling systems or log‐in process to other systems routinely accessed by specific providers to automatically assign/unassign specific providers at the beginning/end of a shift (eg, nurses automatically assigned to care team when they access the electronic medication administration record system at beginning of shift) |
| Explore availability of default functionality to assign and unassign providers to and from the care team in a specific role by team, service, or unit‐based patient lists | |
| Require a stop time/date for role assignments or set a default if none entered |
In the future, care team identification in the electronic health record can be automated by integrating directly with electronic workflows, online scheduling applications, and provider directories. Hospitals could then leverage care team lists to facilitate patient‐centered communication via secure web‐based and mobile messaging applications configured to simultaneously update all team members (eg, group messaging apps, microblogs).[11, 37, 38] By synchronizing with the electronic health record, role assignments can be automatically updated via these applications, further increasing fidelity of care team identification.[12] Finally, as hospitals implement acute care patient portals, team lists can be leveraged to display all care team members correctly so that patients and caregivers can communicate more easily with providers.[17] The potential ramifications for patient‐centered communication are tremendous.
Disclosures
This work was funded by the Patient‐Centered Outcomes Research Institute and the Gordon and Betty Moore Foundation (GBMF3914). The authors report no conflicts of interest.
- . Patient‐centered communication. Annu Rev Nurs Res. 1999;17:85–104.
- , , , . Facilitating patient‐centered cancer communication: a road map. Patient Educ Couns. 2009;77:319–321.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH Publication No. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7:277–286.
- . Communication systems in healthcare. Clin Biochem Rev. 2006;27:89–98.
- , , , et al. The nature of adverse events in hospitalized patients. results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384.
- , , , et al. A look into the nature and causes of human errors in the intensive care unit. Crit Care Med. 1995;23:294–300.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79:186–194.
- , . Interdisciplinary communication: an uncharted source of medical error? J Crit Care. 2006;21:236–242; discussion 242.
- , , . Quantifying the economic impact of communication inefficiencies in U.S. hospitals. J Healthc Manag. 2010;55:265–281; discussion 281–282.
- , , , . Transforming the acute care environment: a web‐based patient‐centered toolkit [abstract]. J Hosp Med. 2014;9(suppl 2):694.
- , , , et al. Creating a culture of patient‐centered care team communication at a large academic medical center [Abstract]. J Hosp Med. 2015;10 (suppl 2). Available at: http://www.shmabstracts.com/abstract/creating‐a‐culture‐of‐patient‐centered‐care‐team‐communication‐at‐a‐large‐academic‐medical‐center. Accessed April 24, 2015.
- , , , , . Perceived information needs and communication difficulties of inpatient physicians and nurses. Proc AMIA Symp. 2001:453–457.
- , , , , , . The effect of tablet computers with a mobile patient portal application on hospitalized patients' knowledge and activation [published online June 15, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv058.
- , , , , . Bedside information technology to support patient‐centered care. Int J Med Inform. 2012;81(7):442–451.
- , , , et al. Building and testing a patient‐centric electronic bedside communication center. J Gerontol Nurs. 2013;39:15–19.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Awareness of the care team in electronic health records. Appl Clin Inform. 2011;2:395–405.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19:117–121.
- , , , et al. Frequency and clinical importance of pages sent to the wrong physician. Arch Intern Med. 2009;169:1072–1073.
- , . Patient experiences with coordination of care: the benefit of continuity and primary care physician as referral source. J Gen Intern Med. 2009;24:170–177.
- , , , , . Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2010;25:177–185.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24:1223–1227.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6:88–93.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171:678–684.
- , , , ; High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7:48–54.
- , , . Intrateam coverage is common, intrateam handoffs are not. J Hosp Med. 2014;9:734–736.
- . A primary care physician's ideal transitions of care? Where's the evidence? J Hosp Med. 2013;8:472–477.
- , , , , . Primary care physician communication at hospital discharge reduces medication discrepancies. J Hosp Med. 2013;8:672–677.
- , . Let's “face” it: time to introduce yourself to patients. J Hosp Med. 2014;9:199–200.
- , , . Patient perceptions of coordinated care: the importance of organized communication in hospitals. J Healthc Qual. 1999;21:18–23.
- , , , . Patient whiteboards to improve patient‐centred care in the hospital. Postgrad Med J. 2013;89:604–609.
- , , , , , . Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9:186–188.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9:137–141.
- , , , et al. Patient engagement in the inpatient setting: a systematic review. J Am Med Inform Assoc. 2014;21:742–750.
- PROSPECT: Promoting Respect and Ongoing Safety Through Patient‐centeredness, Engagement, Communication, and Technology. Available at: http://www.partners.org/cird/PROSPECT/Index.htm. Accessed May 3, 2015.
- , , , , , . Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9:573–578.
- , , , et al. Engaging patients, providers, and institutional stakeholders in developing a patient‐centered microblog. Paper presented at: Proceeding of the American Medical Informatics Association Annual Fall Symposium; November 16–19, 2014; Washington, DC.
Patient‐centered communication is a strategy that is used to promote shared understanding of the plan of care among providers and patients.[1, 2, 3] Caring for hospitalized patients is a collaborative effort that requires seamless patient‐centered communication among a rapidly changing care team to safely progress a patient from admission through discharge. Yet, hospitals continue to struggle with improving the complex and increasingly electronic conversation patterns among care team members and patients to achieve effective patient‐centered communication.[4, 5] When members of the care team operate in this environment, patients often receive conflicting information regarding their plan of care, medications, and test results. Ineffective communication can lead to a suboptimal patient experience, additional costs, medical errors, and preventable adverse events.[6, 7, 8, 9, 10]
A critical first step to improving patient‐centered communication is identifying the care team.[11, 12] Accurate and reliable identification of all care team members is a pressing information need; it is fundamental to efficiently conveying information about the plan of care to those who know the patient the best, must make timely decisions, or will assume care once the patient leaves the hospital.[13] Furthermore, it has implications for engaging patients more meaningfully in their care.[14, 15, 16, 17] Ideally, the process of identifying an individual caring for the patient in a specific role is quickly and reliably determined from the electronic health record, the single source of truth where any provider can quickly identify other team members. This source of truth can be updated manually when individual members assign and remove themselves from the care team, or automatically when accessing the patient's record, writing a note, placing an order, or adding a patient to a coverage list. When providers correctly identify other team members in this way, hospital paging directories and secure messaging tools that link to the electronic health record become more effective at supporting care team communication.[18]
In general, the process of identifying care teams is difficult,[19] and maintaining role assignments in the electronic health record is equally challenging. Vawdrey et al. previously reported that care team lists are inaccurate and cannot be used to reliably identify other members at any given moment.[18] The inability to identify team members often leads to incorrectly routed pages, e‐mail messages, and phone calls.[20] Consequently, the potential to reliably manage the care team and improve electronic communication remains untapped, rendering team collaboration and care coordination less effective.[18, 21, 22]
In recent years, the trend toward restructuring inpatient teamsgeographical localization, structured communication interventions, teamwork training, and interdisciplinary roundswould seem to diminish the need for electronic care team identification, as those efforts have already made a positive impact with regard to interprofessional communication and collaboration, team satisfaction, and adverse events.[23, 24, 25, 26] Nonetheless, interdisciplinary teamwork, though critically important for patient‐centered communication, does not completely obviate the need for accurate and reliable care team identification.[26] Although care teams are statically located on units, the plan of care is dynamic; it evolves when the patient's status changes, when new information becomes available, and when key longitudinal providers (eg, primary care physician, subspecialty consultant) make recommendations. Thus, information conveyed as a team on rounds quickly becomes out of date, requiring additional forms of communication. Furthermore, due to frequent ad hoc coverage among team members, the identity of providers covering the patient at any given moment is often not clear.[27] This is particularly problematic for nonunit‐based providers who try to communicate with unit‐based care team members. These providers, in particular, have valuable knowledge and insight that can aid the primary team in decision making.[28, 29] However, they typically do not participate in rounds, often waste time identifying responsible providers,[20] and may communicate their recommendations directly with the patient without discussing with the primary team. These factors in part explain why geographic localization has shown limited improvement in shared understanding of the plan of care.[23]
From the perspective of patients and caregivers, identification of the providers entering and leaving their room is also challenging; only 11% to 51% of patients identify their providers correctly.[30] This adds to confusion regarding who is responsible for which aspects of the patient's care and can negatively affect the perception of the quality of care received.[31] Use of whiteboards has been shown to improve the proportion of patients who could identify key providers,[32] but these are not reliably updated and generally cannot accommodate all team members. When face cards are used, patients and caregivers report that they are more likely to identify their providers correctly.[14, 33, 34] However, potential confusion may ensue when another provider assumes care of the patient in the same role. Finally, use of technology to display team members at the bedside is typically a feature that patients like and can improve identification of care team members.[14, 15, 16] Yet, patient engagement technologies are not readily available in the hospital setting,[35] and ideally should be linked to the electronic health record, which again must be reliably updated.[11, 12, 15, 16]
If care team identification is so critical for delivering effective patient‐centered communication, why is maintaining role assignment problematic? At the individual level, reasons include discontinuity of the care team due to changing clinical rotations and intrateam coverage, shift‐based schedules, and lack of awareness and underutilization of functionality. Additionally, clinicians may have different ways to maintain lists of patients. At the institutional level, functionality to enforce role assignment when accessing patient records may be disabled (to avoid perceived burdens on clinical staff or nonclinical personnel who require access for administrative functions). Finally, electronic health record vendors currently have no incentive to adopt functionality that supports more effective care coordination across settings.[22]
However, more than technical solutions and policy changes are required; care team identification in the electronic health record requires a change in institutional culture. Maintaining an accurate relationship to each patient requires work without tangible benefitsthe benefits accrue only when everyone else identifies their role on the teama tragedy of the commons. This can be illustrated by our own experience. We conducted a quality improvement initiative (Table 1) as a part of 2 concurrent research initiatives that serve to promote patient‐centered communication:[12] PCORI (Patient‐Centered Outcomes Research Institute Transitions), the goal of which is to improve care transitions within the Partners' Pioneer Accountable Care Organization; and the PROSPECT (Promoting Respect and Ongoing Safety Through Patient‐Centeredness, Engagement, Communication, and Technology) project, an initiative funded by the Gordon and Betty Moore Foundation to eliminate preventable harms in the acute care environment.[29] Our goal was to electronically manage the care team with a high degree of fidelity. We enhanced a home‐grown application, which was developed to improve management of team lists for inpatient providers, accessible from our electronic health record, to facilitate role assignment. Specifically, we leveraged existing care processes (eg, nursing log‐on to the electronic medication administration system) to automatically assign certain providers to the care team at change of shift, added functionality to make it easy to assign a provider to all patients on a list for a defined period of time, and encouraged providers to assign their role by demonstrating benefits including quick access to patient‐specific group e‐mail and secure messaging tools (Table 1, Key Facets). The initiative was well‐received by most disciplines, but uptake was suboptimal. Our research assistants routinely assigned residents and others to the care team because our proactive attempts at advertising and reinforcing use of the application failed to reach a critical mass. Most did not see immediate benefits because it was an added step to their busy day, had other methods of managing team lists, and only saw benefit if everyone else participated. Key facets of our care team identification initiative, successes, and challenges are outlined in Table 1.
| Key Facets | Successes | Challenges |
|---|---|---|
| Linked electronic role assignment to administrative processes and clinical workflows | Leveraged existing processes to identify attending provider by routinely reviewing online schedules Linked role assignment to electronic medication administration system sign‐in process for nurses at the start of their shift |
Difficult to generate buy‐in from administrators and specific clinician groups to incorporate routine use of role assignment functionality into existing and/or new workflows No institutional policy mandating role assignments for members of extended care team |
| Incorporated default functionality to specify length of role assignment (eg, stop date) | Used by trainees (residents, fellows) to automate team list role assignments for a prespecified period of time according to online schedules | Underutilized by subspecialty consultants, many of who were unaware or did not fully appreciate the added value of this functionality Research assistants regularly verified that default role assignments were accurately maintained for trainees |
| Linked role assignment to patient‐specific group e‐mail and messaging tools | Clinicians acknowledged clear efficiency benefits (eg, automated patient identification within messages, correct routing of e‐mails) Used by specific members of the care team tasked with facilitating coordination of care (eg, nurse practitioner trained as discharge advocate for research study) |
Difficult to promote use of patient‐specific messaging, particularly for nonunit‐based providers (eg, consultants, primary care physicians) Required access to an application not typically used for clinical messaging Difficult to change culture of network e‐mail use for clinical messaging |
| Advertised new functionality and demonstrated potential efficiencies for care team communication | Unit‐based clinicians (hospitalists, nurses, housestaff) typically understood benefits when demonstrated and were easier to engage | Some nonunit‐based clinicians (eg, consulting attendings, primary care physicians) did not see benefits and/or were difficult to engage |
| Some nonunit‐based provider groups (eg, social workers, nutritionists, subspecialty fellows) considered the initiative worthwhile, and were open to learning about new functionality to improve communication | Clinicians had several options for managing team lists prior to implementation of new electronic health record | |
| Institutional effort toward implementing new electronic health record detracted from efforts at demonstrating enhanced functionality of existing applications |
There were a few glimmers of hope, however. On several PROSPECT units, we displayed team members on a tablet‐based patient portal so that patients would recognize their providers.[11, 17, 36] Similar to recent work by O'Leary et al.,[14] patients on PROSPECT units were able to correctly identify several care team members, but regularly asked why other providers (eg, consulting fellow) were not listed. Those providers asked the same question, and some eventually learned to assign their role via the application. As part of PROSEPCT, we visited other institutions and learned of an effort to display team members on high‐definition televisions in the patient's room. Several providers, wondering why they were not listed, learned to assign their role and their picture then appeared. Social pressure was the driving force.
Coincidently, we recently implemented a new electronic health record at our institution. Anecdotally, although no formal policy was established, many providers (eg, attendings, first responders, nurses, care coordinators, and other unit‐based providers) appear to be assigning their roles. Other providers (eg, dieticians, physical therapists, residents) also assign their role, but often fail to end role assignments upon completing their rotation or when the patient transfers to another service. Finally, even when actively involved, most subspecialists still do not designate their role. Despite these gaps and inconsistencies, we have made progress toward improving care team identification. The reasons for this progress are straightforward; during required training for the new electronic health record, all inpatient providers were taught to assign their role on the treatment team when assuming care of patients and now have 1 option for managing team lists. However, most providers were not trained to end their role assignments, and many have learned that role assignment is not required to access the patient's record; functionality to enforce this was disabled. Based on lessons learned from our experience,[12] we offer several strategies that hospitalists can employ to improve care team identification in the electronic health record (Table 2).
| Goal | Strategies to Achieve Goal |
|---|---|
| Identify and/or establish reliable processes that administrative staff can use to ensure accurate care team role assignments | Identify databases that serve as the source of truth for provider schedules and routinely access those databases |
| Access resident scheduling application (eg, Amion) that is routinely updated by training program staff | |
| Work with clinical and administrative staff to maintain care team role assignments | |
| Engage affiliated ambulatory practices to ensure patient's primary care physician is updated in the electronic health record | |
| Engage admissions office to improve reliability of attending assignments based on online clinical schedules when patients are admitted | |
| Integrate role assignment into established workflows for specific provider groups when administrative processes not feasible | Link routine care processes to care team role assignment |
| Train nurses, interns, physician assistants to assign role on care team when assuming care of patient at shift change | |
| Train residents, fellows to use default functionality to automatically assign their role on care team at the beginning of a clinical rotation | |
| Demonstrate value of maintaining role assignments in the electronic health record to the unit‐based care team | Emphasize how accurate and reliable care team role assignment can facilitate correct routing of information (eg, test results, discharge summaries) |
| Helps to maintain patient coverage lists (eg, fellows, consultants, social workers) | |
| Facilitates patient‐specific communication (eg, via group email and messaging tools linked to the electronic health record's care team functionality) | |
| Align with concurrent institutional initiatives that enforce or incentivize care team role assignment | Mandate role assignment when writing a note, placing an order, or adding a patient to a coverage list in the electronic health record |
| Provide patients and caregivers the ability to identify the care team via patient portalcreates social pressure for those providers who do not identify themselves on the care team | |
| Incentivize providers to maintain role assignments during patient's hospitalization in order to receive notifications if patients are readmitted | |
| Automate role assignments for all members of the care team whenever possible | Work with clinical informatics/emnformation system staff to determine feasibility of linking online scheduling systems or log‐in process to other systems routinely accessed by specific providers to automatically assign/unassign specific providers at the beginning/end of a shift (eg, nurses automatically assigned to care team when they access the electronic medication administration record system at beginning of shift) |
| Explore availability of default functionality to assign and unassign providers to and from the care team in a specific role by team, service, or unit‐based patient lists | |
| Require a stop time/date for role assignments or set a default if none entered |
In the future, care team identification in the electronic health record can be automated by integrating directly with electronic workflows, online scheduling applications, and provider directories. Hospitals could then leverage care team lists to facilitate patient‐centered communication via secure web‐based and mobile messaging applications configured to simultaneously update all team members (eg, group messaging apps, microblogs).[11, 37, 38] By synchronizing with the electronic health record, role assignments can be automatically updated via these applications, further increasing fidelity of care team identification.[12] Finally, as hospitals implement acute care patient portals, team lists can be leveraged to display all care team members correctly so that patients and caregivers can communicate more easily with providers.[17] The potential ramifications for patient‐centered communication are tremendous.
Disclosures
This work was funded by the Patient‐Centered Outcomes Research Institute and the Gordon and Betty Moore Foundation (GBMF3914). The authors report no conflicts of interest.
Patient‐centered communication is a strategy that is used to promote shared understanding of the plan of care among providers and patients.[1, 2, 3] Caring for hospitalized patients is a collaborative effort that requires seamless patient‐centered communication among a rapidly changing care team to safely progress a patient from admission through discharge. Yet, hospitals continue to struggle with improving the complex and increasingly electronic conversation patterns among care team members and patients to achieve effective patient‐centered communication.[4, 5] When members of the care team operate in this environment, patients often receive conflicting information regarding their plan of care, medications, and test results. Ineffective communication can lead to a suboptimal patient experience, additional costs, medical errors, and preventable adverse events.[6, 7, 8, 9, 10]
A critical first step to improving patient‐centered communication is identifying the care team.[11, 12] Accurate and reliable identification of all care team members is a pressing information need; it is fundamental to efficiently conveying information about the plan of care to those who know the patient the best, must make timely decisions, or will assume care once the patient leaves the hospital.[13] Furthermore, it has implications for engaging patients more meaningfully in their care.[14, 15, 16, 17] Ideally, the process of identifying an individual caring for the patient in a specific role is quickly and reliably determined from the electronic health record, the single source of truth where any provider can quickly identify other team members. This source of truth can be updated manually when individual members assign and remove themselves from the care team, or automatically when accessing the patient's record, writing a note, placing an order, or adding a patient to a coverage list. When providers correctly identify other team members in this way, hospital paging directories and secure messaging tools that link to the electronic health record become more effective at supporting care team communication.[18]
In general, the process of identifying care teams is difficult,[19] and maintaining role assignments in the electronic health record is equally challenging. Vawdrey et al. previously reported that care team lists are inaccurate and cannot be used to reliably identify other members at any given moment.[18] The inability to identify team members often leads to incorrectly routed pages, e‐mail messages, and phone calls.[20] Consequently, the potential to reliably manage the care team and improve electronic communication remains untapped, rendering team collaboration and care coordination less effective.[18, 21, 22]
In recent years, the trend toward restructuring inpatient teamsgeographical localization, structured communication interventions, teamwork training, and interdisciplinary roundswould seem to diminish the need for electronic care team identification, as those efforts have already made a positive impact with regard to interprofessional communication and collaboration, team satisfaction, and adverse events.[23, 24, 25, 26] Nonetheless, interdisciplinary teamwork, though critically important for patient‐centered communication, does not completely obviate the need for accurate and reliable care team identification.[26] Although care teams are statically located on units, the plan of care is dynamic; it evolves when the patient's status changes, when new information becomes available, and when key longitudinal providers (eg, primary care physician, subspecialty consultant) make recommendations. Thus, information conveyed as a team on rounds quickly becomes out of date, requiring additional forms of communication. Furthermore, due to frequent ad hoc coverage among team members, the identity of providers covering the patient at any given moment is often not clear.[27] This is particularly problematic for nonunit‐based providers who try to communicate with unit‐based care team members. These providers, in particular, have valuable knowledge and insight that can aid the primary team in decision making.[28, 29] However, they typically do not participate in rounds, often waste time identifying responsible providers,[20] and may communicate their recommendations directly with the patient without discussing with the primary team. These factors in part explain why geographic localization has shown limited improvement in shared understanding of the plan of care.[23]
From the perspective of patients and caregivers, identification of the providers entering and leaving their room is also challenging; only 11% to 51% of patients identify their providers correctly.[30] This adds to confusion regarding who is responsible for which aspects of the patient's care and can negatively affect the perception of the quality of care received.[31] Use of whiteboards has been shown to improve the proportion of patients who could identify key providers,[32] but these are not reliably updated and generally cannot accommodate all team members. When face cards are used, patients and caregivers report that they are more likely to identify their providers correctly.[14, 33, 34] However, potential confusion may ensue when another provider assumes care of the patient in the same role. Finally, use of technology to display team members at the bedside is typically a feature that patients like and can improve identification of care team members.[14, 15, 16] Yet, patient engagement technologies are not readily available in the hospital setting,[35] and ideally should be linked to the electronic health record, which again must be reliably updated.[11, 12, 15, 16]
If care team identification is so critical for delivering effective patient‐centered communication, why is maintaining role assignment problematic? At the individual level, reasons include discontinuity of the care team due to changing clinical rotations and intrateam coverage, shift‐based schedules, and lack of awareness and underutilization of functionality. Additionally, clinicians may have different ways to maintain lists of patients. At the institutional level, functionality to enforce role assignment when accessing patient records may be disabled (to avoid perceived burdens on clinical staff or nonclinical personnel who require access for administrative functions). Finally, electronic health record vendors currently have no incentive to adopt functionality that supports more effective care coordination across settings.[22]
However, more than technical solutions and policy changes are required; care team identification in the electronic health record requires a change in institutional culture. Maintaining an accurate relationship to each patient requires work without tangible benefitsthe benefits accrue only when everyone else identifies their role on the teama tragedy of the commons. This can be illustrated by our own experience. We conducted a quality improvement initiative (Table 1) as a part of 2 concurrent research initiatives that serve to promote patient‐centered communication:[12] PCORI (Patient‐Centered Outcomes Research Institute Transitions), the goal of which is to improve care transitions within the Partners' Pioneer Accountable Care Organization; and the PROSPECT (Promoting Respect and Ongoing Safety Through Patient‐Centeredness, Engagement, Communication, and Technology) project, an initiative funded by the Gordon and Betty Moore Foundation to eliminate preventable harms in the acute care environment.[29] Our goal was to electronically manage the care team with a high degree of fidelity. We enhanced a home‐grown application, which was developed to improve management of team lists for inpatient providers, accessible from our electronic health record, to facilitate role assignment. Specifically, we leveraged existing care processes (eg, nursing log‐on to the electronic medication administration system) to automatically assign certain providers to the care team at change of shift, added functionality to make it easy to assign a provider to all patients on a list for a defined period of time, and encouraged providers to assign their role by demonstrating benefits including quick access to patient‐specific group e‐mail and secure messaging tools (Table 1, Key Facets). The initiative was well‐received by most disciplines, but uptake was suboptimal. Our research assistants routinely assigned residents and others to the care team because our proactive attempts at advertising and reinforcing use of the application failed to reach a critical mass. Most did not see immediate benefits because it was an added step to their busy day, had other methods of managing team lists, and only saw benefit if everyone else participated. Key facets of our care team identification initiative, successes, and challenges are outlined in Table 1.
| Key Facets | Successes | Challenges |
|---|---|---|
| Linked electronic role assignment to administrative processes and clinical workflows | Leveraged existing processes to identify attending provider by routinely reviewing online schedules Linked role assignment to electronic medication administration system sign‐in process for nurses at the start of their shift |
Difficult to generate buy‐in from administrators and specific clinician groups to incorporate routine use of role assignment functionality into existing and/or new workflows No institutional policy mandating role assignments for members of extended care team |
| Incorporated default functionality to specify length of role assignment (eg, stop date) | Used by trainees (residents, fellows) to automate team list role assignments for a prespecified period of time according to online schedules | Underutilized by subspecialty consultants, many of who were unaware or did not fully appreciate the added value of this functionality Research assistants regularly verified that default role assignments were accurately maintained for trainees |
| Linked role assignment to patient‐specific group e‐mail and messaging tools | Clinicians acknowledged clear efficiency benefits (eg, automated patient identification within messages, correct routing of e‐mails) Used by specific members of the care team tasked with facilitating coordination of care (eg, nurse practitioner trained as discharge advocate for research study) |
Difficult to promote use of patient‐specific messaging, particularly for nonunit‐based providers (eg, consultants, primary care physicians) Required access to an application not typically used for clinical messaging Difficult to change culture of network e‐mail use for clinical messaging |
| Advertised new functionality and demonstrated potential efficiencies for care team communication | Unit‐based clinicians (hospitalists, nurses, housestaff) typically understood benefits when demonstrated and were easier to engage | Some nonunit‐based clinicians (eg, consulting attendings, primary care physicians) did not see benefits and/or were difficult to engage |
| Some nonunit‐based provider groups (eg, social workers, nutritionists, subspecialty fellows) considered the initiative worthwhile, and were open to learning about new functionality to improve communication | Clinicians had several options for managing team lists prior to implementation of new electronic health record | |
| Institutional effort toward implementing new electronic health record detracted from efforts at demonstrating enhanced functionality of existing applications |
There were a few glimmers of hope, however. On several PROSPECT units, we displayed team members on a tablet‐based patient portal so that patients would recognize their providers.[11, 17, 36] Similar to recent work by O'Leary et al.,[14] patients on PROSPECT units were able to correctly identify several care team members, but regularly asked why other providers (eg, consulting fellow) were not listed. Those providers asked the same question, and some eventually learned to assign their role via the application. As part of PROSEPCT, we visited other institutions and learned of an effort to display team members on high‐definition televisions in the patient's room. Several providers, wondering why they were not listed, learned to assign their role and their picture then appeared. Social pressure was the driving force.
Coincidently, we recently implemented a new electronic health record at our institution. Anecdotally, although no formal policy was established, many providers (eg, attendings, first responders, nurses, care coordinators, and other unit‐based providers) appear to be assigning their roles. Other providers (eg, dieticians, physical therapists, residents) also assign their role, but often fail to end role assignments upon completing their rotation or when the patient transfers to another service. Finally, even when actively involved, most subspecialists still do not designate their role. Despite these gaps and inconsistencies, we have made progress toward improving care team identification. The reasons for this progress are straightforward; during required training for the new electronic health record, all inpatient providers were taught to assign their role on the treatment team when assuming care of patients and now have 1 option for managing team lists. However, most providers were not trained to end their role assignments, and many have learned that role assignment is not required to access the patient's record; functionality to enforce this was disabled. Based on lessons learned from our experience,[12] we offer several strategies that hospitalists can employ to improve care team identification in the electronic health record (Table 2).
| Goal | Strategies to Achieve Goal |
|---|---|
| Identify and/or establish reliable processes that administrative staff can use to ensure accurate care team role assignments | Identify databases that serve as the source of truth for provider schedules and routinely access those databases |
| Access resident scheduling application (eg, Amion) that is routinely updated by training program staff | |
| Work with clinical and administrative staff to maintain care team role assignments | |
| Engage affiliated ambulatory practices to ensure patient's primary care physician is updated in the electronic health record | |
| Engage admissions office to improve reliability of attending assignments based on online clinical schedules when patients are admitted | |
| Integrate role assignment into established workflows for specific provider groups when administrative processes not feasible | Link routine care processes to care team role assignment |
| Train nurses, interns, physician assistants to assign role on care team when assuming care of patient at shift change | |
| Train residents, fellows to use default functionality to automatically assign their role on care team at the beginning of a clinical rotation | |
| Demonstrate value of maintaining role assignments in the electronic health record to the unit‐based care team | Emphasize how accurate and reliable care team role assignment can facilitate correct routing of information (eg, test results, discharge summaries) |
| Helps to maintain patient coverage lists (eg, fellows, consultants, social workers) | |
| Facilitates patient‐specific communication (eg, via group email and messaging tools linked to the electronic health record's care team functionality) | |
| Align with concurrent institutional initiatives that enforce or incentivize care team role assignment | Mandate role assignment when writing a note, placing an order, or adding a patient to a coverage list in the electronic health record |
| Provide patients and caregivers the ability to identify the care team via patient portalcreates social pressure for those providers who do not identify themselves on the care team | |
| Incentivize providers to maintain role assignments during patient's hospitalization in order to receive notifications if patients are readmitted | |
| Automate role assignments for all members of the care team whenever possible | Work with clinical informatics/emnformation system staff to determine feasibility of linking online scheduling systems or log‐in process to other systems routinely accessed by specific providers to automatically assign/unassign specific providers at the beginning/end of a shift (eg, nurses automatically assigned to care team when they access the electronic medication administration record system at beginning of shift) |
| Explore availability of default functionality to assign and unassign providers to and from the care team in a specific role by team, service, or unit‐based patient lists | |
| Require a stop time/date for role assignments or set a default if none entered |
In the future, care team identification in the electronic health record can be automated by integrating directly with electronic workflows, online scheduling applications, and provider directories. Hospitals could then leverage care team lists to facilitate patient‐centered communication via secure web‐based and mobile messaging applications configured to simultaneously update all team members (eg, group messaging apps, microblogs).[11, 37, 38] By synchronizing with the electronic health record, role assignments can be automatically updated via these applications, further increasing fidelity of care team identification.[12] Finally, as hospitals implement acute care patient portals, team lists can be leveraged to display all care team members correctly so that patients and caregivers can communicate more easily with providers.[17] The potential ramifications for patient‐centered communication are tremendous.
Disclosures
This work was funded by the Patient‐Centered Outcomes Research Institute and the Gordon and Betty Moore Foundation (GBMF3914). The authors report no conflicts of interest.
- . Patient‐centered communication. Annu Rev Nurs Res. 1999;17:85–104.
- , , , . Facilitating patient‐centered cancer communication: a road map. Patient Educ Couns. 2009;77:319–321.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH Publication No. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7:277–286.
- . Communication systems in healthcare. Clin Biochem Rev. 2006;27:89–98.
- , , , et al. The nature of adverse events in hospitalized patients. results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384.
- , , , et al. A look into the nature and causes of human errors in the intensive care unit. Crit Care Med. 1995;23:294–300.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79:186–194.
- , . Interdisciplinary communication: an uncharted source of medical error? J Crit Care. 2006;21:236–242; discussion 242.
- , , . Quantifying the economic impact of communication inefficiencies in U.S. hospitals. J Healthc Manag. 2010;55:265–281; discussion 281–282.
- , , , . Transforming the acute care environment: a web‐based patient‐centered toolkit [abstract]. J Hosp Med. 2014;9(suppl 2):694.
- , , , et al. Creating a culture of patient‐centered care team communication at a large academic medical center [Abstract]. J Hosp Med. 2015;10 (suppl 2). Available at: http://www.shmabstracts.com/abstract/creating‐a‐culture‐of‐patient‐centered‐care‐team‐communication‐at‐a‐large‐academic‐medical‐center. Accessed April 24, 2015.
- , , , , . Perceived information needs and communication difficulties of inpatient physicians and nurses. Proc AMIA Symp. 2001:453–457.
- , , , , , . The effect of tablet computers with a mobile patient portal application on hospitalized patients' knowledge and activation [published online June 15, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv058.
- , , , , . Bedside information technology to support patient‐centered care. Int J Med Inform. 2012;81(7):442–451.
- , , , et al. Building and testing a patient‐centric electronic bedside communication center. J Gerontol Nurs. 2013;39:15–19.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Awareness of the care team in electronic health records. Appl Clin Inform. 2011;2:395–405.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19:117–121.
- , , , et al. Frequency and clinical importance of pages sent to the wrong physician. Arch Intern Med. 2009;169:1072–1073.
- , . Patient experiences with coordination of care: the benefit of continuity and primary care physician as referral source. J Gen Intern Med. 2009;24:170–177.
- , , , , . Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2010;25:177–185.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24:1223–1227.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6:88–93.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171:678–684.
- , , , ; High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7:48–54.
- , , . Intrateam coverage is common, intrateam handoffs are not. J Hosp Med. 2014;9:734–736.
- . A primary care physician's ideal transitions of care? Where's the evidence? J Hosp Med. 2013;8:472–477.
- , , , , . Primary care physician communication at hospital discharge reduces medication discrepancies. J Hosp Med. 2013;8:672–677.
- , . Let's “face” it: time to introduce yourself to patients. J Hosp Med. 2014;9:199–200.
- , , . Patient perceptions of coordinated care: the importance of organized communication in hospitals. J Healthc Qual. 1999;21:18–23.
- , , , . Patient whiteboards to improve patient‐centred care in the hospital. Postgrad Med J. 2013;89:604–609.
- , , , , , . Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9:186–188.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9:137–141.
- , , , et al. Patient engagement in the inpatient setting: a systematic review. J Am Med Inform Assoc. 2014;21:742–750.
- PROSPECT: Promoting Respect and Ongoing Safety Through Patient‐centeredness, Engagement, Communication, and Technology. Available at: http://www.partners.org/cird/PROSPECT/Index.htm. Accessed May 3, 2015.
- , , , , , . Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9:573–578.
- , , , et al. Engaging patients, providers, and institutional stakeholders in developing a patient‐centered microblog. Paper presented at: Proceeding of the American Medical Informatics Association Annual Fall Symposium; November 16–19, 2014; Washington, DC.
- . Patient‐centered communication. Annu Rev Nurs Res. 1999;17:85–104.
- , , , . Facilitating patient‐centered cancer communication: a road map. Patient Educ Couns. 2009;77:319–321.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH Publication No. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7:277–286.
- . Communication systems in healthcare. Clin Biochem Rev. 2006;27:89–98.
- , , , et al. The nature of adverse events in hospitalized patients. results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384.
- , , , et al. A look into the nature and causes of human errors in the intensive care unit. Crit Care Med. 1995;23:294–300.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79:186–194.
- , . Interdisciplinary communication: an uncharted source of medical error? J Crit Care. 2006;21:236–242; discussion 242.
- , , . Quantifying the economic impact of communication inefficiencies in U.S. hospitals. J Healthc Manag. 2010;55:265–281; discussion 281–282.
- , , , . Transforming the acute care environment: a web‐based patient‐centered toolkit [abstract]. J Hosp Med. 2014;9(suppl 2):694.
- , , , et al. Creating a culture of patient‐centered care team communication at a large academic medical center [Abstract]. J Hosp Med. 2015;10 (suppl 2). Available at: http://www.shmabstracts.com/abstract/creating‐a‐culture‐of‐patient‐centered‐care‐team‐communication‐at‐a‐large‐academic‐medical‐center. Accessed April 24, 2015.
- , , , , . Perceived information needs and communication difficulties of inpatient physicians and nurses. Proc AMIA Symp. 2001:453–457.
- , , , , , . The effect of tablet computers with a mobile patient portal application on hospitalized patients' knowledge and activation [published online June 15, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv058.
- , , , , . Bedside information technology to support patient‐centered care. Int J Med Inform. 2012;81(7):442–451.
- , , , et al. Building and testing a patient‐centric electronic bedside communication center. J Gerontol Nurs. 2013;39:15–19.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Awareness of the care team in electronic health records. Appl Clin Inform. 2011;2:395–405.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19:117–121.
- , , , et al. Frequency and clinical importance of pages sent to the wrong physician. Arch Intern Med. 2009;169:1072–1073.
- , . Patient experiences with coordination of care: the benefit of continuity and primary care physician as referral source. J Gen Intern Med. 2009;24:170–177.
- , , , , . Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2010;25:177–185.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24:1223–1227.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6:88–93.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171:678–684.
- , , , ; High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7:48–54.
- , , . Intrateam coverage is common, intrateam handoffs are not. J Hosp Med. 2014;9:734–736.
- . A primary care physician's ideal transitions of care? Where's the evidence? J Hosp Med. 2013;8:472–477.
- , , , , . Primary care physician communication at hospital discharge reduces medication discrepancies. J Hosp Med. 2013;8:672–677.
- , . Let's “face” it: time to introduce yourself to patients. J Hosp Med. 2014;9:199–200.
- , , . Patient perceptions of coordinated care: the importance of organized communication in hospitals. J Healthc Qual. 1999;21:18–23.
- , , , . Patient whiteboards to improve patient‐centred care in the hospital. Postgrad Med J. 2013;89:604–609.
- , , , , , . Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9:186–188.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9:137–141.
- , , , et al. Patient engagement in the inpatient setting: a systematic review. J Am Med Inform Assoc. 2014;21:742–750.
- PROSPECT: Promoting Respect and Ongoing Safety Through Patient‐centeredness, Engagement, Communication, and Technology. Available at: http://www.partners.org/cird/PROSPECT/Index.htm. Accessed May 3, 2015.
- , , , , , . Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9:573–578.
- , , , et al. Engaging patients, providers, and institutional stakeholders in developing a patient‐centered microblog. Paper presented at: Proceeding of the American Medical Informatics Association Annual Fall Symposium; November 16–19, 2014; Washington, DC.
PICC and Venous Catheter Appropriateness
Vascular access devices (VADs), including peripherally inserted central venous catheters (PICCs) and traditional central venous catheters (CVCs), remain a cornerstone for the delivery of necessary therapy. VADs are used routinely to treat inpatients and increasingly outpatients too. PICCs possess characteristics that are often favorable in a variety of clinical settings when compared to traditional CVCs. However, a paucity of evidence regarding the indication, selection, application, duration, and risks associated with these devices exists. PICCs are often used in situations when peripheral venous catheters (PIVsincluding ultrasound‐guided peripheral intravenous catheters and midline catheters [midlines]) would meet patient needs and confer a lower risk of complications. An unmet need to define indications and promote utilization that conforms to optimal use currently exists. The purpose of this article was to highlight for hospitalists the methodology and subsequent key recommendations published recently[1] regarding appropriateness of PICCs as they pertain to other vascular access device use.
BACKGROUND
Greater utilization of PICCs to meet a variety of clinical needs has recently emerged in hospital‐based medicine.[2, 3] This phenomenon is likely a function of favorable characteristics when comparing PICCs with traditional CVCs. PICCs are often favored because of safety with insertion in the arm, compatibility with inpatient and outpatient therapies, ease of protocolization for insertion by vascular access nursing services, patient tolerability, and cost savings.[4, 5, 6, 7, 8] Yet limitations of PICCs exist and complications including malpositioning, dislodgement, and luminal occlusion[9, 10, 11] affect patient safety and outcomes. Most notably, PICCs are strongly associated with risk for thrombosis and infection, complications that are most frequent in hospitalized and critically ill patients.[12, 13, 14, 15, 16]
Vascular access devices and particularly PICCs pose a substantial risk for thrombosis.[16, 17, 18, 19, 20] PICCs represent the greatest risk factor for upper extremity deep vein thrombosis (DVT), and in one study, PICC‐associated DVT risk was double that with traditional CVCs.[17] Risk factors for the development of PICC‐associated DVT include ipsilateral paresis,[21] infection,[22] PICC diameter,[19, 20] and prolonged surgery (procedure duration >1 hour) with a PICC in place.[23] Recently, PICCs placed in the upper extremity have been described as a possible risk factor for lower extremity venous thrombosis as well.[24, 25]
Infection complicating CVCs is well described,[12, 15] and guidelines for the prevention of catheter‐associated blood stream infections exist.[26, 27] However, the magnitude of the risk of infection associated with PICCs compared with traditional CVCs remains uncertain. Some reports suggest a decrease risk for infection with the utilization of PICCs[28]; others suggest a similar risk.[29] Existing guidelines, however, do not recommend substituting PICCs for CVCs as a technique to reduce infection, especially in general medical patients.[30]
It is not surprising that variability in the clinical use of PICCs and inappropriate PICC utilization has been described[31, 32] given the heterogeneity of patients and clinical situations in which PICCs are used. Simple awareness of medical devices in place is central to optimizing care. Important to the hospitalist physician is a recent study that found that 1 in 5 physicians were unaware of a CVC being present in their patient.[33] Indeed, emphasis has been placed on optimizing the use of PICC lines nationally through the Choosing Wisely initiative.[34, 35]
A panel of experts was convened at the University of Michigan in an effort to further clarify the appropriate use of VADs. Panelists engaged in a RAND Corporation/University of California Los Angeles (RAND/UCLA) Appropriateness Methodology review[36] to provide guidance regarding VAD use. The RAND/UCLA methodology is a validated way to assess the appropriateness of medical and surgical resource utilization, and details of this methodology are published elsewhere.[1] In brief, each panelist was provided a series of clinical scenarios associated with the use of central venous catheters purposefully including areas of consensus, controversy, and ambiguity. Using a standardized method for rating appropriateness, whereby median ratings on opposite ends of a 1 to 9 scale were used to indicate preference of one device over another (for example 9 reflected appropriate and 13 reflected inappropriate), the methodology classified consensus results into three levels of appropriateness. These three levels are: appropriate when the panel median is between 7 and 9 and without disagreement, uncertain/neutral when the panel median is between 4 and 6 or disagreement exists regardless of the median, or inappropriate when the panel median is between 1 and 3 without disagreement.
RESULTS
Comprehensive results regarding appropriateness ratings are reported elsewhere.[1] Results especially key to hospital‐based practitioners are summarized below. Table 1 highlights common scenarios when PICC placement is considered appropriate and inappropriate.
|
| A. Appropriate indications for PICC use |
| Delivery of peripherally compatible infusates when the proposed duration is 6 or more days* |
| Delivery of nonperipherally compatible infusates (eg, irritants/vesicants) regardless of proposed duration of use |
| Delivery of cyclical or episodic chemotherapy that can be administered through a peripheral vein in patients with active cancer, provided the proposed duration of such treatment is 3 or more months |
| Invasive hemodynamic monitoring or necessary central venous access in a critically ill patient, provided the proposed duration is 15 or more days |
| Frequent phlebotomy (every 8 hours) in a hospitalized patient provided the proposed duration is 6 or more days |
| Intermittent infusions or infrequent phlebotomy in patients with poor/difficult peripheral venous access, provided that the proposed duration is 6 or more days |
| Intermittent infusions or infrequent phlebotomy in patients with poor/difficult peripheral venous access, provided that the proposed duration is 6 or more days |
| For infusions or palliative treatment during end‐of‐life care∥ |
| Delivery of peripherally compatible infusates for patients residing in skilled nursing facilities or transitioning from hospital to home, provided that the proposed duration is at least 15 or more days |
| B. Inappropriate indications for PICC use |
| Placement for any indication other than infusion of nonperipherally compatible infusates (eg, irritants/vesicants) when the proposed duration is 5 or fewer days |
| Placement in a patient with active cancer for cyclical chemotherapy that can be administered through a peripheral vein, when the proposed duration of treatment is 3 or fewer months and peripheral veins are available |
| Placement in a patient with stage 3b or greater chronic kidney disease (estimated glomerular filtration rate <44 mL/min) or in patients currently receiving renal replacement therapy via any modality |
| Insertion for nonfrequent phlebotomy if the proposed duration is 5 or fewer days |
| Patient or family request in a patient that is not actively dying/on hospice for comfort from daily lab draws |
| Medical or nursing provider request in the absence of other appropriate criteria for PICC use |
Appropriateness of PICCs in General Hospitalized Medical Patients
The appropriateness of PICCs when compared to other VADs among hospitalized medical patients can be broadly characterized based upon the planned infusate and the anticipated duration of use. PICCs were the preferred VAD when the anticipated duration of infusion was greater than 15 days or for any duration if the infusion was an irritant/vesicant (such as parenteral nutrition or chemotherapy). PICCs were considered appropriate if the proposed duration of use was 6 to 14 days, though preference for a midline or an ultrasound‐guided PIV was noted for this time‐frame. Tunneled catheters were considered appropriate only for the infusion of an irritant/vesicant when the anticipated duration was 15 days; similarly, implanted ports were rated as appropriate when an irritant/vesicant infusion was planned for 31 days. Both tunneled catheters and ports were rated as appropriate when episodic infusion over the duration of several months was necessary. Disagreement existed between the panelists regarding the appropriateness of PICC placement for the indication of frequent blood draws (3 phlebotomies per day) and among patients with difficult venous access, when phlebotomy would be needed for 5 days. In these cases an individualized patient‐centered approach was recommended. PICC placement was considered appropriate in these situations if venous access was required 6 days, but ultrasound‐guided and midline PIVs were again preferred to PICCs when the expected duration of use was <14 days.
Appropriateness of PICCs in Patients With Chronic Kidney Disease
The appropriateness of PICC use among patients with chronic kidney disease (CKD) takes into consideration disease stage as defined by the Kidney Disease: Improving Global Outcomes workgroup.[37] Although panelist recommendations did not differ for patients with stage 1 to 3a CKD (estimated GFR 45 mL/min) from those noted above, for patient's stage 3b or greater CKD, insertion of devices into an arm vein was rated as inappropriate (valuing the preservation of peripheral and central veins for possible hemodialysis/creation of arteriovenous fistulae and grafts). Among patients with stage 3b or greater CKD, PIV access in the dorsum of the hand was recommended for an expected duration of use 5 days. In consultation with a nephrologist, the use of a tunneled small‐bore central catheter (4 French or 5 French) inserted into the jugular vein was rated as appropriate in stage 3b or greater CKD patients requiring venous access for a longer duration.
Appropriateness of PICC Use in Patients with Cancer
The panelists' acknowledged the heterogeneity of thrombosis risk based on cancer type; recommendations reflect the assumption of cancer as a solid tumor. Vascular access choice among cancer patients is complicated by the cyclic nature of therapy frequently administered, the diversity of infusate (eg, nonirritant or nonvesicant versus irritant/vesicant), and uncertainties surrounding duration of therapy. To address this, the panelists chose a pragmatic approach considering the infusate (irritant/vesicant or not), and dichotomized treatment duration (3 months or not). Among cancer patients requiring nonvesicant/nonirritant chemotherapy for a duration 3 months, interval placement of PIVs was rated as appropriate, and disagreement existed among the panelists regarding the appropriateness of PICCs. If 3 months of chemotherapy was necessary, then PICCs or tunneled‐cuffed catheters were considered appropriate. Ports were rated as appropriate if the expected use was 6 months. Among cancer patients requiring vesicant/emrritant chemotherapy, PICCs and tunneled‐cuffed catheters were rated as appropriate for all time intervals, and ports were rated as neutral for 3‐ to 6‐month durations of infusion, and appropriate for durations greater than 6 months. When acceptable, PICCs were favored over tunneled‐cuffed catheters among cancer patients with coagulopathy (eg, severe thrombocytopenia, elevated international normalized ratios).
Appropriateness of PICCs in Patients With Critical Illness
Among critically ill patients, PIVs and midline catheters were rated as appropriate for infusion of 5 days, and 6 to 14 days, respectively, whereas PICCs were considered appropriate only when use 15 days was anticipated. Although both CVCs and PICCs were rated as appropriate among hemodynamically unstable patients in scenarios where invasive cardiovascular monitoring is necessary for durations of 14 days and 15 days, respectively, CVCs were favored over PICCs among patients who are hemodynamically unstable or requiring vasopressors.
Appropriateness of PICC Use In Special Populations
The existence of patients who require lifelong, often intermittent, intravenous access (eg, sickle cell anemia, short‐gut syndrome, cystic fibrosis) necessitates distinct recommendations for venous access. In this population, recommendations were categorized based on frequency of hospitalization. In patients that were hospitalized infrequently (<5 hospitalizations per year), use of midlines was preferred to PICCs when the hospitalization was expected to last 5 days; PICCs were rated as appropriate for a duration of use 15 days. However, in patients who require frequent hospitalization (6 hospitalizations annually), tunneled‐cuffed catheters were rated as appropriate and preferred over PICCs when the expected duration of use was 15 days per session.
For long‐term residents in skilled nursing facilities, PICCs were rated as appropriate for an expected duration of use 15 days, but uncertain for a duration of 6 to 14 days (when midlines were rated as appropriate). For venous access of 5 days, PIVs were rated as most appropriate.
How, When, by Whom, and Which PICCs Should Be Inserted
Societal recommendations[26] and guidelines[38] for routine placement and positioning of PICCs by dedicated nursing services exist.[39, 40] Panelists favored consultation with the specialists ordering vascular access devices (eg, infectious disease, nephrology, hematology, oncology) within the first few days of admission for optimal device selection and timing of insertion. For example, PICCs were rated as appropriate to be placed within 2 to 3 days of hospital admission for patients requiring longterm antimicrobial infusion (in the absence of bacteremia). Preferential PICC placement by interventional radiology was rated as appropriate if portable ultrasound did not identify a suitable target vein, the catheter fails to advance over the guidewire during a bedside attempt, or the patient requires sedation not appropriate for bedside placement. Interventional radiology insertion was also preferred in patients with bilateral mastectomy, altered chest anatomy, and for patients with permanent pacemakers or defibrillators if the contralateral arm is was not amenable for insertion. PICCs are generally placed at the bedside (with radiographic confirmation of catheter position, or with electrocardiography guidance when proficiency with this technique exists) or under direct visualization in the interventional radiology suite. As recommended elsewhere,[21, 26, 41] panelists rated the placement of the PICC catheter tip in the lower one‐third of the superior vena cava, at the cavoatrial junction, or in the right atrium as being appropriate. Nuanced recommendations surrounding PICC adjustment under varying circumstances can be found in the parent document.[1] Single‐lumen devices, which are associated with fewer complications, were rated as the appropriate default lumen of choice in the absence of a documented rationale for a multilumen PICC as a mechanism to decrease possible complications.[19, 20, 42] The insertion of multilumen PICCs for separating blood draws from infusions or ensuring a backup lumen is available was rated as inappropriate. Consistent with recent recommendations,[43, 44] normal saline rather than heparin was rated as appropriate to maintain catheter patency. The advancement of a migrated PICC was rated as inappropriate under all circumstances.
CONCLUSIONS
In‐hospital healthcare providers are routinely confronted with dilemmas surrounding choice of VAD. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative is a multidisciplinary effort to clarify decision‐making related to VAD use. The systematic literature review and RAND/UCLA appropriateness method applied by the MAGIC panelists identifies areas of broad consensus surrounding the use of PICCs in relation to other VADs, and highlights uncertainties regarding the best practice to guide clinical care. Appropriateness statements facilitate standardization for the use, care, and discontinuation of VADs. These recommendations may be important to healthcare quality officers and payers as they allow for measurement of, and adherence to, standardized practice. In an era of electronic medical records and embedded clinical decision support, these recommendations may facilitate a just‐in‐time resource for optimal VAD management, outcomes measurement, and patient follow‐up. In addition to directing clinical care, these recommendations may serve as a lattice for the formation of future randomized clinical trials to further clarify important areas of the uncertainty surrounding VAD use.
Disclosures: Drs. Woller and Stevens disclose financial support paid to their institution of employment (Intermountain Medical Center) for conducting clinical research (with no financial support paid to either investigator). Dr. Woller discloses serving as an expert panelist for the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative. The authors report no other conflicts of interest.
- , , , et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , , . Peripherally inserted central catheters may lower the incidence of catheter‐related blood stream infections in patients in surgical intensive care units. Surg Infect (Larchmt). 2011;12(4):279–282.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;35(1):34–42.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , . Facility wide benefits of radiology vascular access teams, part 2. Radiol Manage. 2010;32(3):39–43.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 3–4.
- , , , . Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol. 2013;52(5):886–892.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , . Malposition of peripherally inserted central catheter: experience from 3,012 patients with cancer. Exp Ther Med. 2013;6(4):891–893.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , , et al. A randomised, controlled trial comparing the long‐term effects of peripherally inserted central catheter placement in chemotherapy patients using B‐mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94–103.
- , , , , . A retrospective study on the long‐term placement of peripherally inserted central catheters and the importance of nursing care and education. Cancer Nurs. 2011;34(1):E25–E30.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis. Chest. 2013;143(3):627–633.
- , , , , , . Risk factors associated with peripherally inserted central venous catheter‐related large vein thrombosis in neurological intensive care patients. Intensive Care Med. 2012;38(2):272–278.
- , , , , . Upper extremity venous thrombosis in patients with cancer with peripherally inserted central venous catheters: a retrospective analysis of risk factors. J Oncol Pract. 2013;9(1):e8–e12.
- , , , et al. 2008 Standards, Options and Recommendations (SOR) guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol. 2009;20(9):1459–1471.
- , , , , . The association between picc use and venous thromboembolism in upper and lower extremities. Am J Med. 2015;128(9):986–993.e1.
- , , , et al. VTE Incidence and risk factors in patients with severe sepsis and septic shock. Chest. 2015;148(5):1224–1230.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S).
- , , , , , , et al. Healthcare Infection Control Practices Advisory Committee (HICPAC) (Appendix 1). Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter‐related Infections. Clin Infect Dis. 2011;52:1087–1099.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2011;52(9):e162–e193.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- Choosing Wisely. American Society of Nephrology. Don't place peripherally inserted central catheters (PICC) in stage III‐V CKD patients without consulting nephrology. Available at: http://www.choosingwisely.org/clinician‐lists/american‐society‐nephrology‐peripherally‐inserted‐central‐catheters‐in‐stage‐iii‐iv‐ckd‐patients. Accessed November 3, 2015.
- Society of General Internal Medicine. Don't place, or leave in place, peripherally inserted central catheters for patient or provider convenience. Available at: http://www.choosingwisely.org/clinician‐lists/society‐general‐internal‐medicine‐peripherally‐inserted‐central‐catheters‐for‐patient‐provider‐convenience. Accessed November 3, 2015.
- , , , et al. The RAND/UCLA appropriateness method user's manual. Santa Monica, CA: RAND; 2001. Available at: http://www.rand.org/pubs/monograph_reports/MR1269.html.
- National Kidney Foundation/Kidney Disease Outcomes Quality Initiative. KDOQI 2012 clinical practice guidelines for chronic kidney disease. Kidney Inter. 2013;(suppl 3):1–150. Accessed November 3, 2015.
- , , , et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539–573.
- , , , , . Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enteral Nutr. 2005;29(5):374–379.
- , , , . Analysis of tip malposition and correction in peripherally inserted central catheters placed at bedside by a dedicated nursing team. J Vasc Interv Radiol. 2007;18(4):513–518.
- Food and Drug Administration Task Force. Precautions necessary with central venous catheters. FDA Drug Bull. 1989:15–16.
- , , , . Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864–868.
- , , , et al. Flushing the central venous catheter: is heparin necessary? J Vasc Access. 2014;15(4):241–248.
- , , , , , . Heparin versus 0.9% sodium chloride intermittent flushing for prevention of occlusion in central venous catheters in adults. Cochrane Database Syst Rev. 2014;10:CD008462.
Vascular access devices (VADs), including peripherally inserted central venous catheters (PICCs) and traditional central venous catheters (CVCs), remain a cornerstone for the delivery of necessary therapy. VADs are used routinely to treat inpatients and increasingly outpatients too. PICCs possess characteristics that are often favorable in a variety of clinical settings when compared to traditional CVCs. However, a paucity of evidence regarding the indication, selection, application, duration, and risks associated with these devices exists. PICCs are often used in situations when peripheral venous catheters (PIVsincluding ultrasound‐guided peripheral intravenous catheters and midline catheters [midlines]) would meet patient needs and confer a lower risk of complications. An unmet need to define indications and promote utilization that conforms to optimal use currently exists. The purpose of this article was to highlight for hospitalists the methodology and subsequent key recommendations published recently[1] regarding appropriateness of PICCs as they pertain to other vascular access device use.
BACKGROUND
Greater utilization of PICCs to meet a variety of clinical needs has recently emerged in hospital‐based medicine.[2, 3] This phenomenon is likely a function of favorable characteristics when comparing PICCs with traditional CVCs. PICCs are often favored because of safety with insertion in the arm, compatibility with inpatient and outpatient therapies, ease of protocolization for insertion by vascular access nursing services, patient tolerability, and cost savings.[4, 5, 6, 7, 8] Yet limitations of PICCs exist and complications including malpositioning, dislodgement, and luminal occlusion[9, 10, 11] affect patient safety and outcomes. Most notably, PICCs are strongly associated with risk for thrombosis and infection, complications that are most frequent in hospitalized and critically ill patients.[12, 13, 14, 15, 16]
Vascular access devices and particularly PICCs pose a substantial risk for thrombosis.[16, 17, 18, 19, 20] PICCs represent the greatest risk factor for upper extremity deep vein thrombosis (DVT), and in one study, PICC‐associated DVT risk was double that with traditional CVCs.[17] Risk factors for the development of PICC‐associated DVT include ipsilateral paresis,[21] infection,[22] PICC diameter,[19, 20] and prolonged surgery (procedure duration >1 hour) with a PICC in place.[23] Recently, PICCs placed in the upper extremity have been described as a possible risk factor for lower extremity venous thrombosis as well.[24, 25]
Infection complicating CVCs is well described,[12, 15] and guidelines for the prevention of catheter‐associated blood stream infections exist.[26, 27] However, the magnitude of the risk of infection associated with PICCs compared with traditional CVCs remains uncertain. Some reports suggest a decrease risk for infection with the utilization of PICCs[28]; others suggest a similar risk.[29] Existing guidelines, however, do not recommend substituting PICCs for CVCs as a technique to reduce infection, especially in general medical patients.[30]
It is not surprising that variability in the clinical use of PICCs and inappropriate PICC utilization has been described[31, 32] given the heterogeneity of patients and clinical situations in which PICCs are used. Simple awareness of medical devices in place is central to optimizing care. Important to the hospitalist physician is a recent study that found that 1 in 5 physicians were unaware of a CVC being present in their patient.[33] Indeed, emphasis has been placed on optimizing the use of PICC lines nationally through the Choosing Wisely initiative.[34, 35]
A panel of experts was convened at the University of Michigan in an effort to further clarify the appropriate use of VADs. Panelists engaged in a RAND Corporation/University of California Los Angeles (RAND/UCLA) Appropriateness Methodology review[36] to provide guidance regarding VAD use. The RAND/UCLA methodology is a validated way to assess the appropriateness of medical and surgical resource utilization, and details of this methodology are published elsewhere.[1] In brief, each panelist was provided a series of clinical scenarios associated with the use of central venous catheters purposefully including areas of consensus, controversy, and ambiguity. Using a standardized method for rating appropriateness, whereby median ratings on opposite ends of a 1 to 9 scale were used to indicate preference of one device over another (for example 9 reflected appropriate and 13 reflected inappropriate), the methodology classified consensus results into three levels of appropriateness. These three levels are: appropriate when the panel median is between 7 and 9 and without disagreement, uncertain/neutral when the panel median is between 4 and 6 or disagreement exists regardless of the median, or inappropriate when the panel median is between 1 and 3 without disagreement.
RESULTS
Comprehensive results regarding appropriateness ratings are reported elsewhere.[1] Results especially key to hospital‐based practitioners are summarized below. Table 1 highlights common scenarios when PICC placement is considered appropriate and inappropriate.
|
| A. Appropriate indications for PICC use |
| Delivery of peripherally compatible infusates when the proposed duration is 6 or more days* |
| Delivery of nonperipherally compatible infusates (eg, irritants/vesicants) regardless of proposed duration of use |
| Delivery of cyclical or episodic chemotherapy that can be administered through a peripheral vein in patients with active cancer, provided the proposed duration of such treatment is 3 or more months |
| Invasive hemodynamic monitoring or necessary central venous access in a critically ill patient, provided the proposed duration is 15 or more days |
| Frequent phlebotomy (every 8 hours) in a hospitalized patient provided the proposed duration is 6 or more days |
| Intermittent infusions or infrequent phlebotomy in patients with poor/difficult peripheral venous access, provided that the proposed duration is 6 or more days |
| Intermittent infusions or infrequent phlebotomy in patients with poor/difficult peripheral venous access, provided that the proposed duration is 6 or more days |
| For infusions or palliative treatment during end‐of‐life care∥ |
| Delivery of peripherally compatible infusates for patients residing in skilled nursing facilities or transitioning from hospital to home, provided that the proposed duration is at least 15 or more days |
| B. Inappropriate indications for PICC use |
| Placement for any indication other than infusion of nonperipherally compatible infusates (eg, irritants/vesicants) when the proposed duration is 5 or fewer days |
| Placement in a patient with active cancer for cyclical chemotherapy that can be administered through a peripheral vein, when the proposed duration of treatment is 3 or fewer months and peripheral veins are available |
| Placement in a patient with stage 3b or greater chronic kidney disease (estimated glomerular filtration rate <44 mL/min) or in patients currently receiving renal replacement therapy via any modality |
| Insertion for nonfrequent phlebotomy if the proposed duration is 5 or fewer days |
| Patient or family request in a patient that is not actively dying/on hospice for comfort from daily lab draws |
| Medical or nursing provider request in the absence of other appropriate criteria for PICC use |
Appropriateness of PICCs in General Hospitalized Medical Patients
The appropriateness of PICCs when compared to other VADs among hospitalized medical patients can be broadly characterized based upon the planned infusate and the anticipated duration of use. PICCs were the preferred VAD when the anticipated duration of infusion was greater than 15 days or for any duration if the infusion was an irritant/vesicant (such as parenteral nutrition or chemotherapy). PICCs were considered appropriate if the proposed duration of use was 6 to 14 days, though preference for a midline or an ultrasound‐guided PIV was noted for this time‐frame. Tunneled catheters were considered appropriate only for the infusion of an irritant/vesicant when the anticipated duration was 15 days; similarly, implanted ports were rated as appropriate when an irritant/vesicant infusion was planned for 31 days. Both tunneled catheters and ports were rated as appropriate when episodic infusion over the duration of several months was necessary. Disagreement existed between the panelists regarding the appropriateness of PICC placement for the indication of frequent blood draws (3 phlebotomies per day) and among patients with difficult venous access, when phlebotomy would be needed for 5 days. In these cases an individualized patient‐centered approach was recommended. PICC placement was considered appropriate in these situations if venous access was required 6 days, but ultrasound‐guided and midline PIVs were again preferred to PICCs when the expected duration of use was <14 days.
Appropriateness of PICCs in Patients With Chronic Kidney Disease
The appropriateness of PICC use among patients with chronic kidney disease (CKD) takes into consideration disease stage as defined by the Kidney Disease: Improving Global Outcomes workgroup.[37] Although panelist recommendations did not differ for patients with stage 1 to 3a CKD (estimated GFR 45 mL/min) from those noted above, for patient's stage 3b or greater CKD, insertion of devices into an arm vein was rated as inappropriate (valuing the preservation of peripheral and central veins for possible hemodialysis/creation of arteriovenous fistulae and grafts). Among patients with stage 3b or greater CKD, PIV access in the dorsum of the hand was recommended for an expected duration of use 5 days. In consultation with a nephrologist, the use of a tunneled small‐bore central catheter (4 French or 5 French) inserted into the jugular vein was rated as appropriate in stage 3b or greater CKD patients requiring venous access for a longer duration.
Appropriateness of PICC Use in Patients with Cancer
The panelists' acknowledged the heterogeneity of thrombosis risk based on cancer type; recommendations reflect the assumption of cancer as a solid tumor. Vascular access choice among cancer patients is complicated by the cyclic nature of therapy frequently administered, the diversity of infusate (eg, nonirritant or nonvesicant versus irritant/vesicant), and uncertainties surrounding duration of therapy. To address this, the panelists chose a pragmatic approach considering the infusate (irritant/vesicant or not), and dichotomized treatment duration (3 months or not). Among cancer patients requiring nonvesicant/nonirritant chemotherapy for a duration 3 months, interval placement of PIVs was rated as appropriate, and disagreement existed among the panelists regarding the appropriateness of PICCs. If 3 months of chemotherapy was necessary, then PICCs or tunneled‐cuffed catheters were considered appropriate. Ports were rated as appropriate if the expected use was 6 months. Among cancer patients requiring vesicant/emrritant chemotherapy, PICCs and tunneled‐cuffed catheters were rated as appropriate for all time intervals, and ports were rated as neutral for 3‐ to 6‐month durations of infusion, and appropriate for durations greater than 6 months. When acceptable, PICCs were favored over tunneled‐cuffed catheters among cancer patients with coagulopathy (eg, severe thrombocytopenia, elevated international normalized ratios).
Appropriateness of PICCs in Patients With Critical Illness
Among critically ill patients, PIVs and midline catheters were rated as appropriate for infusion of 5 days, and 6 to 14 days, respectively, whereas PICCs were considered appropriate only when use 15 days was anticipated. Although both CVCs and PICCs were rated as appropriate among hemodynamically unstable patients in scenarios where invasive cardiovascular monitoring is necessary for durations of 14 days and 15 days, respectively, CVCs were favored over PICCs among patients who are hemodynamically unstable or requiring vasopressors.
Appropriateness of PICC Use In Special Populations
The existence of patients who require lifelong, often intermittent, intravenous access (eg, sickle cell anemia, short‐gut syndrome, cystic fibrosis) necessitates distinct recommendations for venous access. In this population, recommendations were categorized based on frequency of hospitalization. In patients that were hospitalized infrequently (<5 hospitalizations per year), use of midlines was preferred to PICCs when the hospitalization was expected to last 5 days; PICCs were rated as appropriate for a duration of use 15 days. However, in patients who require frequent hospitalization (6 hospitalizations annually), tunneled‐cuffed catheters were rated as appropriate and preferred over PICCs when the expected duration of use was 15 days per session.
For long‐term residents in skilled nursing facilities, PICCs were rated as appropriate for an expected duration of use 15 days, but uncertain for a duration of 6 to 14 days (when midlines were rated as appropriate). For venous access of 5 days, PIVs were rated as most appropriate.
How, When, by Whom, and Which PICCs Should Be Inserted
Societal recommendations[26] and guidelines[38] for routine placement and positioning of PICCs by dedicated nursing services exist.[39, 40] Panelists favored consultation with the specialists ordering vascular access devices (eg, infectious disease, nephrology, hematology, oncology) within the first few days of admission for optimal device selection and timing of insertion. For example, PICCs were rated as appropriate to be placed within 2 to 3 days of hospital admission for patients requiring longterm antimicrobial infusion (in the absence of bacteremia). Preferential PICC placement by interventional radiology was rated as appropriate if portable ultrasound did not identify a suitable target vein, the catheter fails to advance over the guidewire during a bedside attempt, or the patient requires sedation not appropriate for bedside placement. Interventional radiology insertion was also preferred in patients with bilateral mastectomy, altered chest anatomy, and for patients with permanent pacemakers or defibrillators if the contralateral arm is was not amenable for insertion. PICCs are generally placed at the bedside (with radiographic confirmation of catheter position, or with electrocardiography guidance when proficiency with this technique exists) or under direct visualization in the interventional radiology suite. As recommended elsewhere,[21, 26, 41] panelists rated the placement of the PICC catheter tip in the lower one‐third of the superior vena cava, at the cavoatrial junction, or in the right atrium as being appropriate. Nuanced recommendations surrounding PICC adjustment under varying circumstances can be found in the parent document.[1] Single‐lumen devices, which are associated with fewer complications, were rated as the appropriate default lumen of choice in the absence of a documented rationale for a multilumen PICC as a mechanism to decrease possible complications.[19, 20, 42] The insertion of multilumen PICCs for separating blood draws from infusions or ensuring a backup lumen is available was rated as inappropriate. Consistent with recent recommendations,[43, 44] normal saline rather than heparin was rated as appropriate to maintain catheter patency. The advancement of a migrated PICC was rated as inappropriate under all circumstances.
CONCLUSIONS
In‐hospital healthcare providers are routinely confronted with dilemmas surrounding choice of VAD. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative is a multidisciplinary effort to clarify decision‐making related to VAD use. The systematic literature review and RAND/UCLA appropriateness method applied by the MAGIC panelists identifies areas of broad consensus surrounding the use of PICCs in relation to other VADs, and highlights uncertainties regarding the best practice to guide clinical care. Appropriateness statements facilitate standardization for the use, care, and discontinuation of VADs. These recommendations may be important to healthcare quality officers and payers as they allow for measurement of, and adherence to, standardized practice. In an era of electronic medical records and embedded clinical decision support, these recommendations may facilitate a just‐in‐time resource for optimal VAD management, outcomes measurement, and patient follow‐up. In addition to directing clinical care, these recommendations may serve as a lattice for the formation of future randomized clinical trials to further clarify important areas of the uncertainty surrounding VAD use.
Disclosures: Drs. Woller and Stevens disclose financial support paid to their institution of employment (Intermountain Medical Center) for conducting clinical research (with no financial support paid to either investigator). Dr. Woller discloses serving as an expert panelist for the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative. The authors report no other conflicts of interest.
Vascular access devices (VADs), including peripherally inserted central venous catheters (PICCs) and traditional central venous catheters (CVCs), remain a cornerstone for the delivery of necessary therapy. VADs are used routinely to treat inpatients and increasingly outpatients too. PICCs possess characteristics that are often favorable in a variety of clinical settings when compared to traditional CVCs. However, a paucity of evidence regarding the indication, selection, application, duration, and risks associated with these devices exists. PICCs are often used in situations when peripheral venous catheters (PIVsincluding ultrasound‐guided peripheral intravenous catheters and midline catheters [midlines]) would meet patient needs and confer a lower risk of complications. An unmet need to define indications and promote utilization that conforms to optimal use currently exists. The purpose of this article was to highlight for hospitalists the methodology and subsequent key recommendations published recently[1] regarding appropriateness of PICCs as they pertain to other vascular access device use.
BACKGROUND
Greater utilization of PICCs to meet a variety of clinical needs has recently emerged in hospital‐based medicine.[2, 3] This phenomenon is likely a function of favorable characteristics when comparing PICCs with traditional CVCs. PICCs are often favored because of safety with insertion in the arm, compatibility with inpatient and outpatient therapies, ease of protocolization for insertion by vascular access nursing services, patient tolerability, and cost savings.[4, 5, 6, 7, 8] Yet limitations of PICCs exist and complications including malpositioning, dislodgement, and luminal occlusion[9, 10, 11] affect patient safety and outcomes. Most notably, PICCs are strongly associated with risk for thrombosis and infection, complications that are most frequent in hospitalized and critically ill patients.[12, 13, 14, 15, 16]
Vascular access devices and particularly PICCs pose a substantial risk for thrombosis.[16, 17, 18, 19, 20] PICCs represent the greatest risk factor for upper extremity deep vein thrombosis (DVT), and in one study, PICC‐associated DVT risk was double that with traditional CVCs.[17] Risk factors for the development of PICC‐associated DVT include ipsilateral paresis,[21] infection,[22] PICC diameter,[19, 20] and prolonged surgery (procedure duration >1 hour) with a PICC in place.[23] Recently, PICCs placed in the upper extremity have been described as a possible risk factor for lower extremity venous thrombosis as well.[24, 25]
Infection complicating CVCs is well described,[12, 15] and guidelines for the prevention of catheter‐associated blood stream infections exist.[26, 27] However, the magnitude of the risk of infection associated with PICCs compared with traditional CVCs remains uncertain. Some reports suggest a decrease risk for infection with the utilization of PICCs[28]; others suggest a similar risk.[29] Existing guidelines, however, do not recommend substituting PICCs for CVCs as a technique to reduce infection, especially in general medical patients.[30]
It is not surprising that variability in the clinical use of PICCs and inappropriate PICC utilization has been described[31, 32] given the heterogeneity of patients and clinical situations in which PICCs are used. Simple awareness of medical devices in place is central to optimizing care. Important to the hospitalist physician is a recent study that found that 1 in 5 physicians were unaware of a CVC being present in their patient.[33] Indeed, emphasis has been placed on optimizing the use of PICC lines nationally through the Choosing Wisely initiative.[34, 35]
A panel of experts was convened at the University of Michigan in an effort to further clarify the appropriate use of VADs. Panelists engaged in a RAND Corporation/University of California Los Angeles (RAND/UCLA) Appropriateness Methodology review[36] to provide guidance regarding VAD use. The RAND/UCLA methodology is a validated way to assess the appropriateness of medical and surgical resource utilization, and details of this methodology are published elsewhere.[1] In brief, each panelist was provided a series of clinical scenarios associated with the use of central venous catheters purposefully including areas of consensus, controversy, and ambiguity. Using a standardized method for rating appropriateness, whereby median ratings on opposite ends of a 1 to 9 scale were used to indicate preference of one device over another (for example 9 reflected appropriate and 13 reflected inappropriate), the methodology classified consensus results into three levels of appropriateness. These three levels are: appropriate when the panel median is between 7 and 9 and without disagreement, uncertain/neutral when the panel median is between 4 and 6 or disagreement exists regardless of the median, or inappropriate when the panel median is between 1 and 3 without disagreement.
RESULTS
Comprehensive results regarding appropriateness ratings are reported elsewhere.[1] Results especially key to hospital‐based practitioners are summarized below. Table 1 highlights common scenarios when PICC placement is considered appropriate and inappropriate.
|
| A. Appropriate indications for PICC use |
| Delivery of peripherally compatible infusates when the proposed duration is 6 or more days* |
| Delivery of nonperipherally compatible infusates (eg, irritants/vesicants) regardless of proposed duration of use |
| Delivery of cyclical or episodic chemotherapy that can be administered through a peripheral vein in patients with active cancer, provided the proposed duration of such treatment is 3 or more months |
| Invasive hemodynamic monitoring or necessary central venous access in a critically ill patient, provided the proposed duration is 15 or more days |
| Frequent phlebotomy (every 8 hours) in a hospitalized patient provided the proposed duration is 6 or more days |
| Intermittent infusions or infrequent phlebotomy in patients with poor/difficult peripheral venous access, provided that the proposed duration is 6 or more days |
| Intermittent infusions or infrequent phlebotomy in patients with poor/difficult peripheral venous access, provided that the proposed duration is 6 or more days |
| For infusions or palliative treatment during end‐of‐life care∥ |
| Delivery of peripherally compatible infusates for patients residing in skilled nursing facilities or transitioning from hospital to home, provided that the proposed duration is at least 15 or more days |
| B. Inappropriate indications for PICC use |
| Placement for any indication other than infusion of nonperipherally compatible infusates (eg, irritants/vesicants) when the proposed duration is 5 or fewer days |
| Placement in a patient with active cancer for cyclical chemotherapy that can be administered through a peripheral vein, when the proposed duration of treatment is 3 or fewer months and peripheral veins are available |
| Placement in a patient with stage 3b or greater chronic kidney disease (estimated glomerular filtration rate <44 mL/min) or in patients currently receiving renal replacement therapy via any modality |
| Insertion for nonfrequent phlebotomy if the proposed duration is 5 or fewer days |
| Patient or family request in a patient that is not actively dying/on hospice for comfort from daily lab draws |
| Medical or nursing provider request in the absence of other appropriate criteria for PICC use |
Appropriateness of PICCs in General Hospitalized Medical Patients
The appropriateness of PICCs when compared to other VADs among hospitalized medical patients can be broadly characterized based upon the planned infusate and the anticipated duration of use. PICCs were the preferred VAD when the anticipated duration of infusion was greater than 15 days or for any duration if the infusion was an irritant/vesicant (such as parenteral nutrition or chemotherapy). PICCs were considered appropriate if the proposed duration of use was 6 to 14 days, though preference for a midline or an ultrasound‐guided PIV was noted for this time‐frame. Tunneled catheters were considered appropriate only for the infusion of an irritant/vesicant when the anticipated duration was 15 days; similarly, implanted ports were rated as appropriate when an irritant/vesicant infusion was planned for 31 days. Both tunneled catheters and ports were rated as appropriate when episodic infusion over the duration of several months was necessary. Disagreement existed between the panelists regarding the appropriateness of PICC placement for the indication of frequent blood draws (3 phlebotomies per day) and among patients with difficult venous access, when phlebotomy would be needed for 5 days. In these cases an individualized patient‐centered approach was recommended. PICC placement was considered appropriate in these situations if venous access was required 6 days, but ultrasound‐guided and midline PIVs were again preferred to PICCs when the expected duration of use was <14 days.
Appropriateness of PICCs in Patients With Chronic Kidney Disease
The appropriateness of PICC use among patients with chronic kidney disease (CKD) takes into consideration disease stage as defined by the Kidney Disease: Improving Global Outcomes workgroup.[37] Although panelist recommendations did not differ for patients with stage 1 to 3a CKD (estimated GFR 45 mL/min) from those noted above, for patient's stage 3b or greater CKD, insertion of devices into an arm vein was rated as inappropriate (valuing the preservation of peripheral and central veins for possible hemodialysis/creation of arteriovenous fistulae and grafts). Among patients with stage 3b or greater CKD, PIV access in the dorsum of the hand was recommended for an expected duration of use 5 days. In consultation with a nephrologist, the use of a tunneled small‐bore central catheter (4 French or 5 French) inserted into the jugular vein was rated as appropriate in stage 3b or greater CKD patients requiring venous access for a longer duration.
Appropriateness of PICC Use in Patients with Cancer
The panelists' acknowledged the heterogeneity of thrombosis risk based on cancer type; recommendations reflect the assumption of cancer as a solid tumor. Vascular access choice among cancer patients is complicated by the cyclic nature of therapy frequently administered, the diversity of infusate (eg, nonirritant or nonvesicant versus irritant/vesicant), and uncertainties surrounding duration of therapy. To address this, the panelists chose a pragmatic approach considering the infusate (irritant/vesicant or not), and dichotomized treatment duration (3 months or not). Among cancer patients requiring nonvesicant/nonirritant chemotherapy for a duration 3 months, interval placement of PIVs was rated as appropriate, and disagreement existed among the panelists regarding the appropriateness of PICCs. If 3 months of chemotherapy was necessary, then PICCs or tunneled‐cuffed catheters were considered appropriate. Ports were rated as appropriate if the expected use was 6 months. Among cancer patients requiring vesicant/emrritant chemotherapy, PICCs and tunneled‐cuffed catheters were rated as appropriate for all time intervals, and ports were rated as neutral for 3‐ to 6‐month durations of infusion, and appropriate for durations greater than 6 months. When acceptable, PICCs were favored over tunneled‐cuffed catheters among cancer patients with coagulopathy (eg, severe thrombocytopenia, elevated international normalized ratios).
Appropriateness of PICCs in Patients With Critical Illness
Among critically ill patients, PIVs and midline catheters were rated as appropriate for infusion of 5 days, and 6 to 14 days, respectively, whereas PICCs were considered appropriate only when use 15 days was anticipated. Although both CVCs and PICCs were rated as appropriate among hemodynamically unstable patients in scenarios where invasive cardiovascular monitoring is necessary for durations of 14 days and 15 days, respectively, CVCs were favored over PICCs among patients who are hemodynamically unstable or requiring vasopressors.
Appropriateness of PICC Use In Special Populations
The existence of patients who require lifelong, often intermittent, intravenous access (eg, sickle cell anemia, short‐gut syndrome, cystic fibrosis) necessitates distinct recommendations for venous access. In this population, recommendations were categorized based on frequency of hospitalization. In patients that were hospitalized infrequently (<5 hospitalizations per year), use of midlines was preferred to PICCs when the hospitalization was expected to last 5 days; PICCs were rated as appropriate for a duration of use 15 days. However, in patients who require frequent hospitalization (6 hospitalizations annually), tunneled‐cuffed catheters were rated as appropriate and preferred over PICCs when the expected duration of use was 15 days per session.
For long‐term residents in skilled nursing facilities, PICCs were rated as appropriate for an expected duration of use 15 days, but uncertain for a duration of 6 to 14 days (when midlines were rated as appropriate). For venous access of 5 days, PIVs were rated as most appropriate.
How, When, by Whom, and Which PICCs Should Be Inserted
Societal recommendations[26] and guidelines[38] for routine placement and positioning of PICCs by dedicated nursing services exist.[39, 40] Panelists favored consultation with the specialists ordering vascular access devices (eg, infectious disease, nephrology, hematology, oncology) within the first few days of admission for optimal device selection and timing of insertion. For example, PICCs were rated as appropriate to be placed within 2 to 3 days of hospital admission for patients requiring longterm antimicrobial infusion (in the absence of bacteremia). Preferential PICC placement by interventional radiology was rated as appropriate if portable ultrasound did not identify a suitable target vein, the catheter fails to advance over the guidewire during a bedside attempt, or the patient requires sedation not appropriate for bedside placement. Interventional radiology insertion was also preferred in patients with bilateral mastectomy, altered chest anatomy, and for patients with permanent pacemakers or defibrillators if the contralateral arm is was not amenable for insertion. PICCs are generally placed at the bedside (with radiographic confirmation of catheter position, or with electrocardiography guidance when proficiency with this technique exists) or under direct visualization in the interventional radiology suite. As recommended elsewhere,[21, 26, 41] panelists rated the placement of the PICC catheter tip in the lower one‐third of the superior vena cava, at the cavoatrial junction, or in the right atrium as being appropriate. Nuanced recommendations surrounding PICC adjustment under varying circumstances can be found in the parent document.[1] Single‐lumen devices, which are associated with fewer complications, were rated as the appropriate default lumen of choice in the absence of a documented rationale for a multilumen PICC as a mechanism to decrease possible complications.[19, 20, 42] The insertion of multilumen PICCs for separating blood draws from infusions or ensuring a backup lumen is available was rated as inappropriate. Consistent with recent recommendations,[43, 44] normal saline rather than heparin was rated as appropriate to maintain catheter patency. The advancement of a migrated PICC was rated as inappropriate under all circumstances.
CONCLUSIONS
In‐hospital healthcare providers are routinely confronted with dilemmas surrounding choice of VAD. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative is a multidisciplinary effort to clarify decision‐making related to VAD use. The systematic literature review and RAND/UCLA appropriateness method applied by the MAGIC panelists identifies areas of broad consensus surrounding the use of PICCs in relation to other VADs, and highlights uncertainties regarding the best practice to guide clinical care. Appropriateness statements facilitate standardization for the use, care, and discontinuation of VADs. These recommendations may be important to healthcare quality officers and payers as they allow for measurement of, and adherence to, standardized practice. In an era of electronic medical records and embedded clinical decision support, these recommendations may facilitate a just‐in‐time resource for optimal VAD management, outcomes measurement, and patient follow‐up. In addition to directing clinical care, these recommendations may serve as a lattice for the formation of future randomized clinical trials to further clarify important areas of the uncertainty surrounding VAD use.
Disclosures: Drs. Woller and Stevens disclose financial support paid to their institution of employment (Intermountain Medical Center) for conducting clinical research (with no financial support paid to either investigator). Dr. Woller discloses serving as an expert panelist for the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative. The authors report no other conflicts of interest.
- , , , et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , , . Peripherally inserted central catheters may lower the incidence of catheter‐related blood stream infections in patients in surgical intensive care units. Surg Infect (Larchmt). 2011;12(4):279–282.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;35(1):34–42.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , . Facility wide benefits of radiology vascular access teams, part 2. Radiol Manage. 2010;32(3):39–43.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 3–4.
- , , , . Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol. 2013;52(5):886–892.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , . Malposition of peripherally inserted central catheter: experience from 3,012 patients with cancer. Exp Ther Med. 2013;6(4):891–893.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , , et al. A randomised, controlled trial comparing the long‐term effects of peripherally inserted central catheter placement in chemotherapy patients using B‐mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94–103.
- , , , , . A retrospective study on the long‐term placement of peripherally inserted central catheters and the importance of nursing care and education. Cancer Nurs. 2011;34(1):E25–E30.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis. Chest. 2013;143(3):627–633.
- , , , , , . Risk factors associated with peripherally inserted central venous catheter‐related large vein thrombosis in neurological intensive care patients. Intensive Care Med. 2012;38(2):272–278.
- , , , , . Upper extremity venous thrombosis in patients with cancer with peripherally inserted central venous catheters: a retrospective analysis of risk factors. J Oncol Pract. 2013;9(1):e8–e12.
- , , , et al. 2008 Standards, Options and Recommendations (SOR) guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol. 2009;20(9):1459–1471.
- , , , , . The association between picc use and venous thromboembolism in upper and lower extremities. Am J Med. 2015;128(9):986–993.e1.
- , , , et al. VTE Incidence and risk factors in patients with severe sepsis and septic shock. Chest. 2015;148(5):1224–1230.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S).
- , , , , , , et al. Healthcare Infection Control Practices Advisory Committee (HICPAC) (Appendix 1). Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter‐related Infections. Clin Infect Dis. 2011;52:1087–1099.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2011;52(9):e162–e193.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- Choosing Wisely. American Society of Nephrology. Don't place peripherally inserted central catheters (PICC) in stage III‐V CKD patients without consulting nephrology. Available at: http://www.choosingwisely.org/clinician‐lists/american‐society‐nephrology‐peripherally‐inserted‐central‐catheters‐in‐stage‐iii‐iv‐ckd‐patients. Accessed November 3, 2015.
- Society of General Internal Medicine. Don't place, or leave in place, peripherally inserted central catheters for patient or provider convenience. Available at: http://www.choosingwisely.org/clinician‐lists/society‐general‐internal‐medicine‐peripherally‐inserted‐central‐catheters‐for‐patient‐provider‐convenience. Accessed November 3, 2015.
- , , , et al. The RAND/UCLA appropriateness method user's manual. Santa Monica, CA: RAND; 2001. Available at: http://www.rand.org/pubs/monograph_reports/MR1269.html.
- National Kidney Foundation/Kidney Disease Outcomes Quality Initiative. KDOQI 2012 clinical practice guidelines for chronic kidney disease. Kidney Inter. 2013;(suppl 3):1–150. Accessed November 3, 2015.
- , , , et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539–573.
- , , , , . Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enteral Nutr. 2005;29(5):374–379.
- , , , . Analysis of tip malposition and correction in peripherally inserted central catheters placed at bedside by a dedicated nursing team. J Vasc Interv Radiol. 2007;18(4):513–518.
- Food and Drug Administration Task Force. Precautions necessary with central venous catheters. FDA Drug Bull. 1989:15–16.
- , , , . Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864–868.
- , , , et al. Flushing the central venous catheter: is heparin necessary? J Vasc Access. 2014;15(4):241–248.
- , , , , , . Heparin versus 0.9% sodium chloride intermittent flushing for prevention of occlusion in central venous catheters in adults. Cochrane Database Syst Rev. 2014;10:CD008462.
- , , , et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , , . Peripherally inserted central catheters may lower the incidence of catheter‐related blood stream infections in patients in surgical intensive care units. Surg Infect (Larchmt). 2011;12(4):279–282.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;35(1):34–42.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , . Facility wide benefits of radiology vascular access teams, part 2. Radiol Manage. 2010;32(3):39–43.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 3–4.
- , , , . Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol. 2013;52(5):886–892.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , . Malposition of peripherally inserted central catheter: experience from 3,012 patients with cancer. Exp Ther Med. 2013;6(4):891–893.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , , et al. A randomised, controlled trial comparing the long‐term effects of peripherally inserted central catheter placement in chemotherapy patients using B‐mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94–103.
- , , , , . A retrospective study on the long‐term placement of peripherally inserted central catheters and the importance of nursing care and education. Cancer Nurs. 2011;34(1):E25–E30.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis. Chest. 2013;143(3):627–633.
- , , , , , . Risk factors associated with peripherally inserted central venous catheter‐related large vein thrombosis in neurological intensive care patients. Intensive Care Med. 2012;38(2):272–278.
- , , , , . Upper extremity venous thrombosis in patients with cancer with peripherally inserted central venous catheters: a retrospective analysis of risk factors. J Oncol Pract. 2013;9(1):e8–e12.
- , , , et al. 2008 Standards, Options and Recommendations (SOR) guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol. 2009;20(9):1459–1471.
- , , , , . The association between picc use and venous thromboembolism in upper and lower extremities. Am J Med. 2015;128(9):986–993.e1.
- , , , et al. VTE Incidence and risk factors in patients with severe sepsis and septic shock. Chest. 2015;148(5):1224–1230.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S).
- , , , , , , et al. Healthcare Infection Control Practices Advisory Committee (HICPAC) (Appendix 1). Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter‐related Infections. Clin Infect Dis. 2011;52:1087–1099.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2011;52(9):e162–e193.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- Choosing Wisely. American Society of Nephrology. Don't place peripherally inserted central catheters (PICC) in stage III‐V CKD patients without consulting nephrology. Available at: http://www.choosingwisely.org/clinician‐lists/american‐society‐nephrology‐peripherally‐inserted‐central‐catheters‐in‐stage‐iii‐iv‐ckd‐patients. Accessed November 3, 2015.
- Society of General Internal Medicine. Don't place, or leave in place, peripherally inserted central catheters for patient or provider convenience. Available at: http://www.choosingwisely.org/clinician‐lists/society‐general‐internal‐medicine‐peripherally‐inserted‐central‐catheters‐for‐patient‐provider‐convenience. Accessed November 3, 2015.
- , , , et al. The RAND/UCLA appropriateness method user's manual. Santa Monica, CA: RAND; 2001. Available at: http://www.rand.org/pubs/monograph_reports/MR1269.html.
- National Kidney Foundation/Kidney Disease Outcomes Quality Initiative. KDOQI 2012 clinical practice guidelines for chronic kidney disease. Kidney Inter. 2013;(suppl 3):1–150. Accessed November 3, 2015.
- , , , et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539–573.
- , , , , . Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enteral Nutr. 2005;29(5):374–379.
- , , , . Analysis of tip malposition and correction in peripherally inserted central catheters placed at bedside by a dedicated nursing team. J Vasc Interv Radiol. 2007;18(4):513–518.
- Food and Drug Administration Task Force. Precautions necessary with central venous catheters. FDA Drug Bull. 1989:15–16.
- , , , . Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864–868.
- , , , et al. Flushing the central venous catheter: is heparin necessary? J Vasc Access. 2014;15(4):241–248.
- , , , , , . Heparin versus 0.9% sodium chloride intermittent flushing for prevention of occlusion in central venous catheters in adults. Cochrane Database Syst Rev. 2014;10:CD008462.
Direct Admissions to the Hospital
Increasing use of emergency departments (EDs) throughout the United States has become a focus of national healthcare policy and reform efforts. ED growth continues to outpace population growth, with the Institute of Medicine describing our ED systems as fragmented, overburdened, and at the breaking point.[1] Associations between ED crowding and patient dissatisfaction, delays in treatment, medical errors, and patient mortality speak to the urgency of systems improvements.[2] One major factor contributing to ED volumes is the growing number of hospital admissions that begin in EDs. From 1993 to 2006, the proportion of hospitalizations originating in EDs increased from 33.5% to 43.8%, with more than 17 million hospital admissions originating in EDs annually.[3, 4] Despite these challenges, discussions about alternative approaches to hospital admission remain at the periphery of healthcare policy conversations.
Direct admission to the hospital, defined as hospitalization without first receiving care in the hospital's ED, is an alternative approach to hospital admission, and may be a vehicle to both observation and inpatient hospital stays. Direct admissions account for 25% of all nonelective pediatric hospitalizations and 15% of nonelective adult hospitalizations in the United States.[5, 6] This admission approach was considerably more common in the past, facilitated by primary care providers (PCPs) or specialists who provided both outpatient and hospital‐based care for their patients.[4] However, as the number of hospitalists in the United States has grown over the last 30 years, the number of direct admissions has decreased concurrently. In fact, from 2003 to 2009, the number of direct admissions from clinics and physicians' offices decreased by a total of 1.6 million.[4] Although this decline is undoubtedly multifactorial, hospitalists may have contributed, both deliberately and inadvertently, to the shifting epidemiology of hospital admissions. Although many factors influence the source of hospital admissions and admission processes, direct admission has 2 important prerequisites: patients require timely access to outpatient providers for acute care, and hospitals, in partnership with outpatient‐based clinics and practices, require systems to safely and efficiently facilitate admissions without ED involvement. However, we know little about hospital admission systems, developed in the era of hospital medicine, to facilitate admissions independent of the ED.
Direct admission offers a number of potential benefits for both patients and healthcare delivery systems including reductions in the number of sites and providers of care, improved communication and coordination between outpatient and hospital‐based healthcare providers, greater patient and referring physician satisfaction, and reduced ED volumes and subsequent costs.[7] However, there are also risks and potential harms associated with direct admission, including potential delays in initial evaluation and management, inconsistent admission processes, and difficulties determining direct admission appropriateness, all of which could adversely impact patient safety and quality of care.[7, 8, 9] One study of adults with sepsis found that direct admission was associated with increased mortality compared to ED admission, which the authors speculated to be related to less timely care.[9] Similarly, a study of unscheduled adult hospitalizations found that patients admitted directly had higher mortality for time‐sensitive conditions such as acute myocardial infarction and sepsis than patients admitted through EDs, differences not observed among adults admitted with pneumonia, asthma, cellulitis, and several other common, yet frequently less emergent, reasons for hospitalization.[8] Among children with pneumonia, the most common reason for pediatric hospitalization, direct admission has been associated with significantly lower costs than admissions originating in the ED, with no significant differences in rates of transfer to the intensive care unit or hospital readmission.[10]
There is significant variation across both diagnoses and hospitals in rates of direct admission, raising questions about the contextual factors unique to hospital medicine programs that perform a substantial proportion of direct admissions.[5] This variation also highlights opportunities to identify the populations, conditions, and systems that facilitate safe and effective direct admissions. Certainly, direct admission is unlikely to be appropriate for all populations or conditions. Patients requiring emergent care or rapid diagnostic imaging are likely to receive more timely care in the ED; sepsis, acute myocardial infarction, and trauma are but a few examples of conditions for which rapid ED care decreases morbidity and mortality. Similarly, patients for whom the need for hospitalization is uncertainfor example, dehydration, asthmamay be more appropriate for initial ED management followed by re‐evaluation to inform the need for hospitalization. Finally, patients for whom the admitting diagnosis is uncertain and who require consultation for several subspecialists may be more efficiently evaluated in EDs. In our national survey of pediatric direct admission guidelines, less than one‐third of hospitals reported having formal criteria to assess the appropriateness of direct admissions, and respondents' perspectives regarding populations and diagnoses appropriate for this admission approach varied considerably.[7] These results point to the need for further research and quality‐improvement initiatives to inform the development of direct admission guidelines and protocols.
During the last decade, hospitals' discharge processes have been the focus of tremendous research, policy, and quality improvement efforts. The phrase transition of care is now widely understood to describe the changes in patient care that begin with discharge planning and conclude when patients' have established care at home or another healthcare facility. Transitions of care have been a focus of the Journal of Hospital Medicine since its inception, including publication of the Transitions of Care Consensus Policy Statement in 2009, as well as numerous other studies highlighting both risks associated with transitions of care as well as methods to address these.[11, 12, 13, 14, 15, 16] Similar to hospital discharge, hospital admission is an inherent feature of every hospitalization, and admission and discharge processes share many commonalities. Both involve transitions in sites of care, and handoffs between healthcare providers. Most involve changes in medical therapies, including both the addition of new medications and changes to existing treatments. Moreover, both are associated with significant stress to patients and their families. As a result, hospital admissions expose patients to many of same risks that have been the focus of hospital discharge reform: unstructured patient handoffs, poor communication between healthcare providers, and costly, inefficient care. The Society of Hospital Medicine has been a leader in articulating the importance of patient‐centered, clinically relevant medication reconciliation across the healthcare continuum.[17] However, with the exception of this important work, research and policy focused on understanding and improving transitions of care into the hospital have received disproportionately little attention.
To facilitate research and quality improvement efforts focused on hospital admission, we suggest that the transitions of care framework, typically discussed in the context of hospital discharge, be expanded to reflect the different origins of hospitalizations and multiple transitions that can be experienced by patients as they enter the hospital. A broadening of the transitions of care framework to incorporate hospital admissions brings numerous questions previously addressed in hospital‐to‐home transitions to the forefront. How do transitions into the hospital impact patients and healthcare systems? When is direct admission safe and effective, and how does this vary across conditions and hospital settings? What protocols and tools might optimize the associated transitions and reduce the risks of error and harm? There are numerous stakeholders who will undoubtedly bring diverse perspectives to these questionspatients and their families, hospital‐based healthcare providers, PCPs and specialists, ED physicians, and payors.
Increasing ED volumes, long wait times, and rising ED costs speak to the importance of better understanding hospital admission alternatives and the associated risks and benefits. Encouraging more direct admissions may be a solution, but evidence to guide best practices must precede this. The growing presence of round‐the‐clock pediatric and adult hospitalists across the country creates unique opportunities to transform hospital admission systems for the vast number of patients who do not require emergent care. The Affordable Care Act's expansion of insurance coverage and incentivized coordinated care within patient‐centered medical homes creates a unique opportunity for this broadened view of transitions of care. This suggests that the time is ripe for pursuing strategies that will both improve patients' transitions from outpatient to inpatient care and reduce stress on our overburdened emergency departments.
Disclosure: Dr. Lagu was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. She has received consulting fees from the Institute for Healthcare Improvement, under contract to the Centers for Medicare and Medicaid Services (CMS), for her work on a project to help health systems achieve disability competence, and from the Island Peer Review Organization, under contract to CMS, for her work on development of episodes of care for CMS payment purposes (both unrelated to the current work). Dr. Leyenaar was supported by grant number K08HS024133 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The authors report no conflicts of interest.
- Institute of Medicine. Hospital‐based emergency care: At the breaking point. Washington, DC: National Academies Press; 2006. Available at: http://www.nap.edu/openbook.php?record_id=11621. Accessed September 13, 2015.
- , , , . National trends in emergency department occupancy, 2001 to 2008: effect of inpatient admissions versus emergency department practice intensity. Ann Emerg Med. 2012;60(6):679–686.e3.
- , . The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;367(5):391–393.
- , , , et al. The Evolving Role of Emergency Departments in the United States. Santa Monica, CA: RAND Corp.; 2013:1–79.
- , , , , . Direct admission to hospitals among children in the United States. JAMA Pediatr. 2015;169(5):500–502.
- Healthcare Cost and Utilization Project. National Inpatient Sample. 2012. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed October 11, 2014.
- , , , , . Direct admission to hospital: a mixed methods survey of pediatric practices, benefits, and challenges [published online August 17, 2015]. Acad Pediatr.
- , , . Changes in the source of unscheduled hospitalizations in the United States. Med Care. 2013;51(8):689–698.
- , , , . Lower mortality in sepsis patients admitted through the ED vs direct admission. Am J Emerg Med. 2012;30(3):432–439.
- , , , , . Variation and outcomes associated with direct admission among children with pneumonia in the United States. JAMA Pediatr. 2014;168(9):829–836.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- . Safety in numbers: physicians joining forces to seal the cracks during transitions. J Hosp Med. 2009;4(6):329–330.
- , , , et al. Development of a checklist of safe discharge practices for hospital patients. J Hosp Med. 2013;8(8):444–449.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421–427.
- , , . The successes and challenges of hospital to home transitions. J Hosp Med. 2014;9(4):271–273.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
Increasing use of emergency departments (EDs) throughout the United States has become a focus of national healthcare policy and reform efforts. ED growth continues to outpace population growth, with the Institute of Medicine describing our ED systems as fragmented, overburdened, and at the breaking point.[1] Associations between ED crowding and patient dissatisfaction, delays in treatment, medical errors, and patient mortality speak to the urgency of systems improvements.[2] One major factor contributing to ED volumes is the growing number of hospital admissions that begin in EDs. From 1993 to 2006, the proportion of hospitalizations originating in EDs increased from 33.5% to 43.8%, with more than 17 million hospital admissions originating in EDs annually.[3, 4] Despite these challenges, discussions about alternative approaches to hospital admission remain at the periphery of healthcare policy conversations.
Direct admission to the hospital, defined as hospitalization without first receiving care in the hospital's ED, is an alternative approach to hospital admission, and may be a vehicle to both observation and inpatient hospital stays. Direct admissions account for 25% of all nonelective pediatric hospitalizations and 15% of nonelective adult hospitalizations in the United States.[5, 6] This admission approach was considerably more common in the past, facilitated by primary care providers (PCPs) or specialists who provided both outpatient and hospital‐based care for their patients.[4] However, as the number of hospitalists in the United States has grown over the last 30 years, the number of direct admissions has decreased concurrently. In fact, from 2003 to 2009, the number of direct admissions from clinics and physicians' offices decreased by a total of 1.6 million.[4] Although this decline is undoubtedly multifactorial, hospitalists may have contributed, both deliberately and inadvertently, to the shifting epidemiology of hospital admissions. Although many factors influence the source of hospital admissions and admission processes, direct admission has 2 important prerequisites: patients require timely access to outpatient providers for acute care, and hospitals, in partnership with outpatient‐based clinics and practices, require systems to safely and efficiently facilitate admissions without ED involvement. However, we know little about hospital admission systems, developed in the era of hospital medicine, to facilitate admissions independent of the ED.
Direct admission offers a number of potential benefits for both patients and healthcare delivery systems including reductions in the number of sites and providers of care, improved communication and coordination between outpatient and hospital‐based healthcare providers, greater patient and referring physician satisfaction, and reduced ED volumes and subsequent costs.[7] However, there are also risks and potential harms associated with direct admission, including potential delays in initial evaluation and management, inconsistent admission processes, and difficulties determining direct admission appropriateness, all of which could adversely impact patient safety and quality of care.[7, 8, 9] One study of adults with sepsis found that direct admission was associated with increased mortality compared to ED admission, which the authors speculated to be related to less timely care.[9] Similarly, a study of unscheduled adult hospitalizations found that patients admitted directly had higher mortality for time‐sensitive conditions such as acute myocardial infarction and sepsis than patients admitted through EDs, differences not observed among adults admitted with pneumonia, asthma, cellulitis, and several other common, yet frequently less emergent, reasons for hospitalization.[8] Among children with pneumonia, the most common reason for pediatric hospitalization, direct admission has been associated with significantly lower costs than admissions originating in the ED, with no significant differences in rates of transfer to the intensive care unit or hospital readmission.[10]
There is significant variation across both diagnoses and hospitals in rates of direct admission, raising questions about the contextual factors unique to hospital medicine programs that perform a substantial proportion of direct admissions.[5] This variation also highlights opportunities to identify the populations, conditions, and systems that facilitate safe and effective direct admissions. Certainly, direct admission is unlikely to be appropriate for all populations or conditions. Patients requiring emergent care or rapid diagnostic imaging are likely to receive more timely care in the ED; sepsis, acute myocardial infarction, and trauma are but a few examples of conditions for which rapid ED care decreases morbidity and mortality. Similarly, patients for whom the need for hospitalization is uncertainfor example, dehydration, asthmamay be more appropriate for initial ED management followed by re‐evaluation to inform the need for hospitalization. Finally, patients for whom the admitting diagnosis is uncertain and who require consultation for several subspecialists may be more efficiently evaluated in EDs. In our national survey of pediatric direct admission guidelines, less than one‐third of hospitals reported having formal criteria to assess the appropriateness of direct admissions, and respondents' perspectives regarding populations and diagnoses appropriate for this admission approach varied considerably.[7] These results point to the need for further research and quality‐improvement initiatives to inform the development of direct admission guidelines and protocols.
During the last decade, hospitals' discharge processes have been the focus of tremendous research, policy, and quality improvement efforts. The phrase transition of care is now widely understood to describe the changes in patient care that begin with discharge planning and conclude when patients' have established care at home or another healthcare facility. Transitions of care have been a focus of the Journal of Hospital Medicine since its inception, including publication of the Transitions of Care Consensus Policy Statement in 2009, as well as numerous other studies highlighting both risks associated with transitions of care as well as methods to address these.[11, 12, 13, 14, 15, 16] Similar to hospital discharge, hospital admission is an inherent feature of every hospitalization, and admission and discharge processes share many commonalities. Both involve transitions in sites of care, and handoffs between healthcare providers. Most involve changes in medical therapies, including both the addition of new medications and changes to existing treatments. Moreover, both are associated with significant stress to patients and their families. As a result, hospital admissions expose patients to many of same risks that have been the focus of hospital discharge reform: unstructured patient handoffs, poor communication between healthcare providers, and costly, inefficient care. The Society of Hospital Medicine has been a leader in articulating the importance of patient‐centered, clinically relevant medication reconciliation across the healthcare continuum.[17] However, with the exception of this important work, research and policy focused on understanding and improving transitions of care into the hospital have received disproportionately little attention.
To facilitate research and quality improvement efforts focused on hospital admission, we suggest that the transitions of care framework, typically discussed in the context of hospital discharge, be expanded to reflect the different origins of hospitalizations and multiple transitions that can be experienced by patients as they enter the hospital. A broadening of the transitions of care framework to incorporate hospital admissions brings numerous questions previously addressed in hospital‐to‐home transitions to the forefront. How do transitions into the hospital impact patients and healthcare systems? When is direct admission safe and effective, and how does this vary across conditions and hospital settings? What protocols and tools might optimize the associated transitions and reduce the risks of error and harm? There are numerous stakeholders who will undoubtedly bring diverse perspectives to these questionspatients and their families, hospital‐based healthcare providers, PCPs and specialists, ED physicians, and payors.
Increasing ED volumes, long wait times, and rising ED costs speak to the importance of better understanding hospital admission alternatives and the associated risks and benefits. Encouraging more direct admissions may be a solution, but evidence to guide best practices must precede this. The growing presence of round‐the‐clock pediatric and adult hospitalists across the country creates unique opportunities to transform hospital admission systems for the vast number of patients who do not require emergent care. The Affordable Care Act's expansion of insurance coverage and incentivized coordinated care within patient‐centered medical homes creates a unique opportunity for this broadened view of transitions of care. This suggests that the time is ripe for pursuing strategies that will both improve patients' transitions from outpatient to inpatient care and reduce stress on our overburdened emergency departments.
Disclosure: Dr. Lagu was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. She has received consulting fees from the Institute for Healthcare Improvement, under contract to the Centers for Medicare and Medicaid Services (CMS), for her work on a project to help health systems achieve disability competence, and from the Island Peer Review Organization, under contract to CMS, for her work on development of episodes of care for CMS payment purposes (both unrelated to the current work). Dr. Leyenaar was supported by grant number K08HS024133 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The authors report no conflicts of interest.
Increasing use of emergency departments (EDs) throughout the United States has become a focus of national healthcare policy and reform efforts. ED growth continues to outpace population growth, with the Institute of Medicine describing our ED systems as fragmented, overburdened, and at the breaking point.[1] Associations between ED crowding and patient dissatisfaction, delays in treatment, medical errors, and patient mortality speak to the urgency of systems improvements.[2] One major factor contributing to ED volumes is the growing number of hospital admissions that begin in EDs. From 1993 to 2006, the proportion of hospitalizations originating in EDs increased from 33.5% to 43.8%, with more than 17 million hospital admissions originating in EDs annually.[3, 4] Despite these challenges, discussions about alternative approaches to hospital admission remain at the periphery of healthcare policy conversations.
Direct admission to the hospital, defined as hospitalization without first receiving care in the hospital's ED, is an alternative approach to hospital admission, and may be a vehicle to both observation and inpatient hospital stays. Direct admissions account for 25% of all nonelective pediatric hospitalizations and 15% of nonelective adult hospitalizations in the United States.[5, 6] This admission approach was considerably more common in the past, facilitated by primary care providers (PCPs) or specialists who provided both outpatient and hospital‐based care for their patients.[4] However, as the number of hospitalists in the United States has grown over the last 30 years, the number of direct admissions has decreased concurrently. In fact, from 2003 to 2009, the number of direct admissions from clinics and physicians' offices decreased by a total of 1.6 million.[4] Although this decline is undoubtedly multifactorial, hospitalists may have contributed, both deliberately and inadvertently, to the shifting epidemiology of hospital admissions. Although many factors influence the source of hospital admissions and admission processes, direct admission has 2 important prerequisites: patients require timely access to outpatient providers for acute care, and hospitals, in partnership with outpatient‐based clinics and practices, require systems to safely and efficiently facilitate admissions without ED involvement. However, we know little about hospital admission systems, developed in the era of hospital medicine, to facilitate admissions independent of the ED.
Direct admission offers a number of potential benefits for both patients and healthcare delivery systems including reductions in the number of sites and providers of care, improved communication and coordination between outpatient and hospital‐based healthcare providers, greater patient and referring physician satisfaction, and reduced ED volumes and subsequent costs.[7] However, there are also risks and potential harms associated with direct admission, including potential delays in initial evaluation and management, inconsistent admission processes, and difficulties determining direct admission appropriateness, all of which could adversely impact patient safety and quality of care.[7, 8, 9] One study of adults with sepsis found that direct admission was associated with increased mortality compared to ED admission, which the authors speculated to be related to less timely care.[9] Similarly, a study of unscheduled adult hospitalizations found that patients admitted directly had higher mortality for time‐sensitive conditions such as acute myocardial infarction and sepsis than patients admitted through EDs, differences not observed among adults admitted with pneumonia, asthma, cellulitis, and several other common, yet frequently less emergent, reasons for hospitalization.[8] Among children with pneumonia, the most common reason for pediatric hospitalization, direct admission has been associated with significantly lower costs than admissions originating in the ED, with no significant differences in rates of transfer to the intensive care unit or hospital readmission.[10]
There is significant variation across both diagnoses and hospitals in rates of direct admission, raising questions about the contextual factors unique to hospital medicine programs that perform a substantial proportion of direct admissions.[5] This variation also highlights opportunities to identify the populations, conditions, and systems that facilitate safe and effective direct admissions. Certainly, direct admission is unlikely to be appropriate for all populations or conditions. Patients requiring emergent care or rapid diagnostic imaging are likely to receive more timely care in the ED; sepsis, acute myocardial infarction, and trauma are but a few examples of conditions for which rapid ED care decreases morbidity and mortality. Similarly, patients for whom the need for hospitalization is uncertainfor example, dehydration, asthmamay be more appropriate for initial ED management followed by re‐evaluation to inform the need for hospitalization. Finally, patients for whom the admitting diagnosis is uncertain and who require consultation for several subspecialists may be more efficiently evaluated in EDs. In our national survey of pediatric direct admission guidelines, less than one‐third of hospitals reported having formal criteria to assess the appropriateness of direct admissions, and respondents' perspectives regarding populations and diagnoses appropriate for this admission approach varied considerably.[7] These results point to the need for further research and quality‐improvement initiatives to inform the development of direct admission guidelines and protocols.
During the last decade, hospitals' discharge processes have been the focus of tremendous research, policy, and quality improvement efforts. The phrase transition of care is now widely understood to describe the changes in patient care that begin with discharge planning and conclude when patients' have established care at home or another healthcare facility. Transitions of care have been a focus of the Journal of Hospital Medicine since its inception, including publication of the Transitions of Care Consensus Policy Statement in 2009, as well as numerous other studies highlighting both risks associated with transitions of care as well as methods to address these.[11, 12, 13, 14, 15, 16] Similar to hospital discharge, hospital admission is an inherent feature of every hospitalization, and admission and discharge processes share many commonalities. Both involve transitions in sites of care, and handoffs between healthcare providers. Most involve changes in medical therapies, including both the addition of new medications and changes to existing treatments. Moreover, both are associated with significant stress to patients and their families. As a result, hospital admissions expose patients to many of same risks that have been the focus of hospital discharge reform: unstructured patient handoffs, poor communication between healthcare providers, and costly, inefficient care. The Society of Hospital Medicine has been a leader in articulating the importance of patient‐centered, clinically relevant medication reconciliation across the healthcare continuum.[17] However, with the exception of this important work, research and policy focused on understanding and improving transitions of care into the hospital have received disproportionately little attention.
To facilitate research and quality improvement efforts focused on hospital admission, we suggest that the transitions of care framework, typically discussed in the context of hospital discharge, be expanded to reflect the different origins of hospitalizations and multiple transitions that can be experienced by patients as they enter the hospital. A broadening of the transitions of care framework to incorporate hospital admissions brings numerous questions previously addressed in hospital‐to‐home transitions to the forefront. How do transitions into the hospital impact patients and healthcare systems? When is direct admission safe and effective, and how does this vary across conditions and hospital settings? What protocols and tools might optimize the associated transitions and reduce the risks of error and harm? There are numerous stakeholders who will undoubtedly bring diverse perspectives to these questionspatients and their families, hospital‐based healthcare providers, PCPs and specialists, ED physicians, and payors.
Increasing ED volumes, long wait times, and rising ED costs speak to the importance of better understanding hospital admission alternatives and the associated risks and benefits. Encouraging more direct admissions may be a solution, but evidence to guide best practices must precede this. The growing presence of round‐the‐clock pediatric and adult hospitalists across the country creates unique opportunities to transform hospital admission systems for the vast number of patients who do not require emergent care. The Affordable Care Act's expansion of insurance coverage and incentivized coordinated care within patient‐centered medical homes creates a unique opportunity for this broadened view of transitions of care. This suggests that the time is ripe for pursuing strategies that will both improve patients' transitions from outpatient to inpatient care and reduce stress on our overburdened emergency departments.
Disclosure: Dr. Lagu was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. She has received consulting fees from the Institute for Healthcare Improvement, under contract to the Centers for Medicare and Medicaid Services (CMS), for her work on a project to help health systems achieve disability competence, and from the Island Peer Review Organization, under contract to CMS, for her work on development of episodes of care for CMS payment purposes (both unrelated to the current work). Dr. Leyenaar was supported by grant number K08HS024133 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The authors report no conflicts of interest.
- Institute of Medicine. Hospital‐based emergency care: At the breaking point. Washington, DC: National Academies Press; 2006. Available at: http://www.nap.edu/openbook.php?record_id=11621. Accessed September 13, 2015.
- , , , . National trends in emergency department occupancy, 2001 to 2008: effect of inpatient admissions versus emergency department practice intensity. Ann Emerg Med. 2012;60(6):679–686.e3.
- , . The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;367(5):391–393.
- , , , et al. The Evolving Role of Emergency Departments in the United States. Santa Monica, CA: RAND Corp.; 2013:1–79.
- , , , , . Direct admission to hospitals among children in the United States. JAMA Pediatr. 2015;169(5):500–502.
- Healthcare Cost and Utilization Project. National Inpatient Sample. 2012. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed October 11, 2014.
- , , , , . Direct admission to hospital: a mixed methods survey of pediatric practices, benefits, and challenges [published online August 17, 2015]. Acad Pediatr.
- , , . Changes in the source of unscheduled hospitalizations in the United States. Med Care. 2013;51(8):689–698.
- , , , . Lower mortality in sepsis patients admitted through the ED vs direct admission. Am J Emerg Med. 2012;30(3):432–439.
- , , , , . Variation and outcomes associated with direct admission among children with pneumonia in the United States. JAMA Pediatr. 2014;168(9):829–836.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- . Safety in numbers: physicians joining forces to seal the cracks during transitions. J Hosp Med. 2009;4(6):329–330.
- , , , et al. Development of a checklist of safe discharge practices for hospital patients. J Hosp Med. 2013;8(8):444–449.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421–427.
- , , . The successes and challenges of hospital to home transitions. J Hosp Med. 2014;9(4):271–273.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- Institute of Medicine. Hospital‐based emergency care: At the breaking point. Washington, DC: National Academies Press; 2006. Available at: http://www.nap.edu/openbook.php?record_id=11621. Accessed September 13, 2015.
- , , , . National trends in emergency department occupancy, 2001 to 2008: effect of inpatient admissions versus emergency department practice intensity. Ann Emerg Med. 2012;60(6):679–686.e3.
- , . The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;367(5):391–393.
- , , , et al. The Evolving Role of Emergency Departments in the United States. Santa Monica, CA: RAND Corp.; 2013:1–79.
- , , , , . Direct admission to hospitals among children in the United States. JAMA Pediatr. 2015;169(5):500–502.
- Healthcare Cost and Utilization Project. National Inpatient Sample. 2012. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed October 11, 2014.
- , , , , . Direct admission to hospital: a mixed methods survey of pediatric practices, benefits, and challenges [published online August 17, 2015]. Acad Pediatr.
- , , . Changes in the source of unscheduled hospitalizations in the United States. Med Care. 2013;51(8):689–698.
- , , , . Lower mortality in sepsis patients admitted through the ED vs direct admission. Am J Emerg Med. 2012;30(3):432–439.
- , , , , . Variation and outcomes associated with direct admission among children with pneumonia in the United States. JAMA Pediatr. 2014;168(9):829–836.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- . Safety in numbers: physicians joining forces to seal the cracks during transitions. J Hosp Med. 2009;4(6):329–330.
- , , , et al. Development of a checklist of safe discharge practices for hospital patients. J Hosp Med. 2013;8(8):444–449.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421–427.
- , , . The successes and challenges of hospital to home transitions. J Hosp Med. 2014;9(4):271–273.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
Metacognition to Reduce Medical Error
A 71‐year‐old man with widely metastatic nonsmall cell lung cancer presented to an emergency department of a teaching hospital at 7 pm with a chief complaint of severe chest pain relieved by sitting upright and leaning forward. A senior cardiologist, with expertise in echocardiography, assessed the patient and performed a bedside echocardiogram. He found a large pericardial effusion but concluded there was no cardiac tamponade. Given the patient's other medical problems, he referred him to internal medicine for admission to their service. The attending internist agreed to admit the patient, suggesting close cardiac monitoring and reevaluation with a formal echocardiogram in the morning. At 9 am, the team and the cardiologist were urgently summoned to the echo lab by the technician who now diagnosed tamponade. After looking at the images, the cardiologist disagreed with the technician's interpretation and declared that there was no sign of tamponade.
After leaving the echo lab, the attending internist led a team discussion on the phenomenon of and reasons for interobserver variation. The residents initially focused on the difference in expertise between the cardiologist and technician. The attending, who felt this was unlikely because the technician was very experienced, introduced the possibility of a cognitive misstep. Having staked out an opinion on the lack of tamponade the night before and acting on that interpretation by declining admission to his service, the cardiologist was susceptible to anchoring bias, where adjustments to a preliminary diagnosis are insufficient because of the influence of the initial interpretation.[1] The following day, the cardiologist performed a pericardiocentesis and reported that the fluid came out under pressure. In the face of this definitive information, he concluded that his prior assessment was incorrect and that tamponade had been present from the start.
The origins of medical error reduction lie in the practice of using autopsies to determine the cause of death spearheaded by Karl Rokitansky at the Vienna Medical School in the 1800s.[2] Ernest Amory Codman expanded the effort through the linkage of treatment decisions to subsequent outcomes by following patients after hospital discharge.[3] The advent of modern imaging techniques coupled with interventional methods of obtaining pathological specimens has dramatically improved diagnostic accuracy over the past 40 years. As a result, the practice of using autopsies to improve clinical acumen and reduce diagnostic error has virtually disappeared, while the focus on medical error has actually increased. The forum for reducing error shifted to morbidity and mortality rounds (MMRs), which have been relabeled quality‐improvement rounds in many hospitals.
In these regularly scheduled meetings, interprofessional clinicians discuss errors and adverse outcomes. Because deaths are rarely unexpected and often occur outside of the acute care setting, the focus is usually on errors in the execution of complex clinical plans that combine the wide array of modern laboratory, imaging, pharmaceutical, interventional, surgical, and pathological tools available to clinicians today. In the era of patient safety and quality improvement, errors are mostly blamed on systems‐based issues that lead to hospital complications, despite evidence that cognitive factors play a large role.[4] Systems‐based analysis was popularized by the landmark report of the Institute of Medicine.[5] In our local institutions (the University of Toronto teaching hospitals), improving diagnostic accuracy is almost never on the agenda. We suspect the same is true elsewhere. Common themes include mistakes in medication administration and dosing, communication, and physician handover. The Swiss cheese model[6] is often invoked to diffuse blame across a number of individuals, processes, and even machines. However, as Wachter and Pronovost point out, reengineering of systems has limited capacity for solving all safety and quality improvement issues when people are involved; human error can still sabotage the effort.[7]
Discussions centered on a physician's raw thinking ability have become a third rail, even though clinical reasoning lies at the core of patient safety. Human error is rarely discussed, in part because it is mistakenly believed to be uncommon and felt to be the result of deficits in knowledge or incompetence. Furthermore, the fear of assigning blame to individuals in front of their peers may be counterproductive, discouraging identification of future errors. However, the fields of cognitive psychology and medical decision making have clearly established that cognitive errors occur predictably and often, especially at times of high cognitive load (eg, when many high stakes complex decisions need to be made in a short period of time). Errors do not usually result from a lack of knowledge (although they can), but rather because people rely on instincts that include common biases called heuristics.[8] Most of the time, heuristics are a helpful and necessary evolutionary adaptation of the human thought process, but by their inherent nature, they can lead to predictable and repeatable errors. Because the effects of cognitive biases are inherent to all decision makers, using this framework for discussing individual error may be a method of decreasing the second victim effect[9] and avoid demoralizing the individual.
MMRs thus represent fertile ground for introducing cognitive psychology into medical education and quality improvement. The existing format is useful for teaching cognitive psychology because it is an open forum where discussions center on errors of omission and commission, many of which are a result of both systems issues and decision making heuristics. Several studies have attempted to describe methods for improving MMRs[10, 11, 12]; however, none have incorporated concepts from cognitive psychology. This type of analysis has penetrated several cases in the WebM&M series created by the Agency of Healthcare Quality Research, which can be used as a model for hospital‐based MMRs.[13] For the vignette described above, a MMR that considers systems‐based approaches might discuss how a busy emergency room, limitations of capacity on the cardiology service, and closure of the echo lab at night, played a role in this story. However, although it is difficult to replay another person's mental processing, ignoring the possibility that the cardiologist in this case may have fallen prey to a common cognitive error would be a missed opportunity to learn how frequently heuristics can be faulty. A cognitive approach applied to this example would explore explanations such as anchoring, ego, and hassle biases. Front‐line clinicians in busy hospital settings will recognize the interaction between workload pressures and cognitive mistakes common to examples like this one.
Cognitive heuristics should first be introduced to MMRs by experienced clinicians, well respected for their clinical acumen, by telling specific personal stories where heuristics led to errors in their practices and why the shortcut in thinking occurred. Thereafter, the traditional MMR format can be used: presenting a case, describing how an experienced clinician might manage the case, and then asking the audience members for comment. Incorporating discussions of cognitive missteps, in medical and nonmedical contexts, would help normalize the understanding that even the most experienced and smartest people fall prey to them. The tone must be positive.
Attendees could be encouraged to review their own thought processes through diagnostic verification for cases where their initial diagnosis was incorrect. This would involve assessment for adequacy, ensuring that potential diagnoses account for all abnormal and normal clinical findings, and coherency, ensuring that the diagnoses are pathophysiologically consistent with all clinical findings. Another strategy may be to illustrate cognitive forcing strategies for particular biases.[14] For example, in the case of anchoring bias, trainees may be encouraged to replay the clinical scenario with a different priming stem and evaluate if they would come to the same clinical conclusion. A challenge for all MMRs is how best to select cases; given the difficulties in replaying one's cognitive processes, this problem may be magnified. Potential selection methods could utilize anonymous reporting systems or patient complaints; however, the optimal strategy is yet to be determined.
Graber et al. have summarized the limited research on attempts to improve cognitive processes through educational interventions and illustrate its mixed results.[15] The most positive study was a randomized control trial using combined pattern recognition and deliberative reasoning to improve diagnostic accuracy in the face of biasing information.[16] Despite positive results, others have suggested that cognitive biases are impossible to teach due to their subconscious nature.[17] They argue that training physicians to avoid heuristics will simply lead to over investigation. These polarizing views highlight the need for research to evaluate interventions like the cognitive autopsy suggested here.
Trainees recognize early that their knowledge base is limited. However, it takes more internal analysis to realize that their brains' decision‐making capacity is similarly limited. Utilizing these regularly scheduled clinical meetings in the manner described above may build improved metacognition, cognition about cognition or more colloquially thinking about thinking. Clinicians understand that bias can easily occur in research and accept mechanisms to protect studies from those potential threats to validity such as double blinding of outcome assessments. Supplementing MMRs with cognitive discussions represents an analogous intent to reduce biases, introducing metacognition as the next frontier in advancing clinical care. Errors are inevitable,[18] and recognition of our cognitive blind spots will provide physicians with an improved framework for analysis of these errors. Building metacognition is a difficult task; however, this is not a reason to stop trying. In the spirit of innovation begun by pioneers like Rokitansky and Codman, and renewed focus on diagnostic errors generated by the recent report of the National Academy of Sciences[19], it is time for the cognitive autopsy to be built into the quality improvement and patient safety map.
Acknowledgements
The authors thank Donald A. Redelemeier, MD, MSc, University of Toronto, and Gurpreet Dhaliwal, MD, University of California, San Francisco, for providing comments on an earlier draft of this article. Neither was compensated for their contributions.
Disclosure: Nothing to report.
- , . Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–1131.
- . Doctors: The Biography of Medicine. New York, NY: Vintage Books; 1995.
- . The classic: a study in hospital efficiency: as demonstrated by the case report of first five years of private hospital. Clin Orthop Relat Res. 2013;471(6):1778–1783.
- , , . Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493–1499.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 1999.
- . The contribution of latent human failures to the breakdown of complex systems. Philos Trans R Soc Lond B Biol Sci. 1990;327(1241):475–484.
- , . Balancing “no blame” with accountability in patient safety. N Engl J Med. 2009;361(14):1401–1406.
- . From mindless to mindful practice—cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445–2448.
- . Medical error: the second victim. The doctor who makes the mistake needs help too. BMJ. 2000;320(7237):726–727.
- , , , et al. Impact of morbidity and mortality conferences on analysis of mortality and critical events in intensive care practice. Am J Crit Care. 2010;19(2):135–145.
- , , , . Using patient safety morbidity and mortality conferences to promote transparency and a culture of safety. Jt Comm J Qual Patient Saf. 2010;36(1):3–9.
- , , , et al. Enhancing the quality of morbidity and mortality rounds: the Ottawa M21(3):314–321.
- Agency for Healthcare Research and Quality. AHRQ WebM41(1):110–120.
- , , , et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21(7):535–557.
- , , , . Teaching from the clinical reasoning literature: combined reasoning strategies help novice diagnosticians overcome misleading information. Med Educ. 2007;41(12):1152–1158.
- , . Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94–100.
- , . Everyone's a little bit biased (even physicians). JAMA. 2008;299(24):2893–2895.
- , , . Improving Diagnosis in Health Care. Washington, DC: National Academies Press; 2015.
A 71‐year‐old man with widely metastatic nonsmall cell lung cancer presented to an emergency department of a teaching hospital at 7 pm with a chief complaint of severe chest pain relieved by sitting upright and leaning forward. A senior cardiologist, with expertise in echocardiography, assessed the patient and performed a bedside echocardiogram. He found a large pericardial effusion but concluded there was no cardiac tamponade. Given the patient's other medical problems, he referred him to internal medicine for admission to their service. The attending internist agreed to admit the patient, suggesting close cardiac monitoring and reevaluation with a formal echocardiogram in the morning. At 9 am, the team and the cardiologist were urgently summoned to the echo lab by the technician who now diagnosed tamponade. After looking at the images, the cardiologist disagreed with the technician's interpretation and declared that there was no sign of tamponade.
After leaving the echo lab, the attending internist led a team discussion on the phenomenon of and reasons for interobserver variation. The residents initially focused on the difference in expertise between the cardiologist and technician. The attending, who felt this was unlikely because the technician was very experienced, introduced the possibility of a cognitive misstep. Having staked out an opinion on the lack of tamponade the night before and acting on that interpretation by declining admission to his service, the cardiologist was susceptible to anchoring bias, where adjustments to a preliminary diagnosis are insufficient because of the influence of the initial interpretation.[1] The following day, the cardiologist performed a pericardiocentesis and reported that the fluid came out under pressure. In the face of this definitive information, he concluded that his prior assessment was incorrect and that tamponade had been present from the start.
The origins of medical error reduction lie in the practice of using autopsies to determine the cause of death spearheaded by Karl Rokitansky at the Vienna Medical School in the 1800s.[2] Ernest Amory Codman expanded the effort through the linkage of treatment decisions to subsequent outcomes by following patients after hospital discharge.[3] The advent of modern imaging techniques coupled with interventional methods of obtaining pathological specimens has dramatically improved diagnostic accuracy over the past 40 years. As a result, the practice of using autopsies to improve clinical acumen and reduce diagnostic error has virtually disappeared, while the focus on medical error has actually increased. The forum for reducing error shifted to morbidity and mortality rounds (MMRs), which have been relabeled quality‐improvement rounds in many hospitals.
In these regularly scheduled meetings, interprofessional clinicians discuss errors and adverse outcomes. Because deaths are rarely unexpected and often occur outside of the acute care setting, the focus is usually on errors in the execution of complex clinical plans that combine the wide array of modern laboratory, imaging, pharmaceutical, interventional, surgical, and pathological tools available to clinicians today. In the era of patient safety and quality improvement, errors are mostly blamed on systems‐based issues that lead to hospital complications, despite evidence that cognitive factors play a large role.[4] Systems‐based analysis was popularized by the landmark report of the Institute of Medicine.[5] In our local institutions (the University of Toronto teaching hospitals), improving diagnostic accuracy is almost never on the agenda. We suspect the same is true elsewhere. Common themes include mistakes in medication administration and dosing, communication, and physician handover. The Swiss cheese model[6] is often invoked to diffuse blame across a number of individuals, processes, and even machines. However, as Wachter and Pronovost point out, reengineering of systems has limited capacity for solving all safety and quality improvement issues when people are involved; human error can still sabotage the effort.[7]
Discussions centered on a physician's raw thinking ability have become a third rail, even though clinical reasoning lies at the core of patient safety. Human error is rarely discussed, in part because it is mistakenly believed to be uncommon and felt to be the result of deficits in knowledge or incompetence. Furthermore, the fear of assigning blame to individuals in front of their peers may be counterproductive, discouraging identification of future errors. However, the fields of cognitive psychology and medical decision making have clearly established that cognitive errors occur predictably and often, especially at times of high cognitive load (eg, when many high stakes complex decisions need to be made in a short period of time). Errors do not usually result from a lack of knowledge (although they can), but rather because people rely on instincts that include common biases called heuristics.[8] Most of the time, heuristics are a helpful and necessary evolutionary adaptation of the human thought process, but by their inherent nature, they can lead to predictable and repeatable errors. Because the effects of cognitive biases are inherent to all decision makers, using this framework for discussing individual error may be a method of decreasing the second victim effect[9] and avoid demoralizing the individual.
MMRs thus represent fertile ground for introducing cognitive psychology into medical education and quality improvement. The existing format is useful for teaching cognitive psychology because it is an open forum where discussions center on errors of omission and commission, many of which are a result of both systems issues and decision making heuristics. Several studies have attempted to describe methods for improving MMRs[10, 11, 12]; however, none have incorporated concepts from cognitive psychology. This type of analysis has penetrated several cases in the WebM&M series created by the Agency of Healthcare Quality Research, which can be used as a model for hospital‐based MMRs.[13] For the vignette described above, a MMR that considers systems‐based approaches might discuss how a busy emergency room, limitations of capacity on the cardiology service, and closure of the echo lab at night, played a role in this story. However, although it is difficult to replay another person's mental processing, ignoring the possibility that the cardiologist in this case may have fallen prey to a common cognitive error would be a missed opportunity to learn how frequently heuristics can be faulty. A cognitive approach applied to this example would explore explanations such as anchoring, ego, and hassle biases. Front‐line clinicians in busy hospital settings will recognize the interaction between workload pressures and cognitive mistakes common to examples like this one.
Cognitive heuristics should first be introduced to MMRs by experienced clinicians, well respected for their clinical acumen, by telling specific personal stories where heuristics led to errors in their practices and why the shortcut in thinking occurred. Thereafter, the traditional MMR format can be used: presenting a case, describing how an experienced clinician might manage the case, and then asking the audience members for comment. Incorporating discussions of cognitive missteps, in medical and nonmedical contexts, would help normalize the understanding that even the most experienced and smartest people fall prey to them. The tone must be positive.
Attendees could be encouraged to review their own thought processes through diagnostic verification for cases where their initial diagnosis was incorrect. This would involve assessment for adequacy, ensuring that potential diagnoses account for all abnormal and normal clinical findings, and coherency, ensuring that the diagnoses are pathophysiologically consistent with all clinical findings. Another strategy may be to illustrate cognitive forcing strategies for particular biases.[14] For example, in the case of anchoring bias, trainees may be encouraged to replay the clinical scenario with a different priming stem and evaluate if they would come to the same clinical conclusion. A challenge for all MMRs is how best to select cases; given the difficulties in replaying one's cognitive processes, this problem may be magnified. Potential selection methods could utilize anonymous reporting systems or patient complaints; however, the optimal strategy is yet to be determined.
Graber et al. have summarized the limited research on attempts to improve cognitive processes through educational interventions and illustrate its mixed results.[15] The most positive study was a randomized control trial using combined pattern recognition and deliberative reasoning to improve diagnostic accuracy in the face of biasing information.[16] Despite positive results, others have suggested that cognitive biases are impossible to teach due to their subconscious nature.[17] They argue that training physicians to avoid heuristics will simply lead to over investigation. These polarizing views highlight the need for research to evaluate interventions like the cognitive autopsy suggested here.
Trainees recognize early that their knowledge base is limited. However, it takes more internal analysis to realize that their brains' decision‐making capacity is similarly limited. Utilizing these regularly scheduled clinical meetings in the manner described above may build improved metacognition, cognition about cognition or more colloquially thinking about thinking. Clinicians understand that bias can easily occur in research and accept mechanisms to protect studies from those potential threats to validity such as double blinding of outcome assessments. Supplementing MMRs with cognitive discussions represents an analogous intent to reduce biases, introducing metacognition as the next frontier in advancing clinical care. Errors are inevitable,[18] and recognition of our cognitive blind spots will provide physicians with an improved framework for analysis of these errors. Building metacognition is a difficult task; however, this is not a reason to stop trying. In the spirit of innovation begun by pioneers like Rokitansky and Codman, and renewed focus on diagnostic errors generated by the recent report of the National Academy of Sciences[19], it is time for the cognitive autopsy to be built into the quality improvement and patient safety map.
Acknowledgements
The authors thank Donald A. Redelemeier, MD, MSc, University of Toronto, and Gurpreet Dhaliwal, MD, University of California, San Francisco, for providing comments on an earlier draft of this article. Neither was compensated for their contributions.
Disclosure: Nothing to report.
A 71‐year‐old man with widely metastatic nonsmall cell lung cancer presented to an emergency department of a teaching hospital at 7 pm with a chief complaint of severe chest pain relieved by sitting upright and leaning forward. A senior cardiologist, with expertise in echocardiography, assessed the patient and performed a bedside echocardiogram. He found a large pericardial effusion but concluded there was no cardiac tamponade. Given the patient's other medical problems, he referred him to internal medicine for admission to their service. The attending internist agreed to admit the patient, suggesting close cardiac monitoring and reevaluation with a formal echocardiogram in the morning. At 9 am, the team and the cardiologist were urgently summoned to the echo lab by the technician who now diagnosed tamponade. After looking at the images, the cardiologist disagreed with the technician's interpretation and declared that there was no sign of tamponade.
After leaving the echo lab, the attending internist led a team discussion on the phenomenon of and reasons for interobserver variation. The residents initially focused on the difference in expertise between the cardiologist and technician. The attending, who felt this was unlikely because the technician was very experienced, introduced the possibility of a cognitive misstep. Having staked out an opinion on the lack of tamponade the night before and acting on that interpretation by declining admission to his service, the cardiologist was susceptible to anchoring bias, where adjustments to a preliminary diagnosis are insufficient because of the influence of the initial interpretation.[1] The following day, the cardiologist performed a pericardiocentesis and reported that the fluid came out under pressure. In the face of this definitive information, he concluded that his prior assessment was incorrect and that tamponade had been present from the start.
The origins of medical error reduction lie in the practice of using autopsies to determine the cause of death spearheaded by Karl Rokitansky at the Vienna Medical School in the 1800s.[2] Ernest Amory Codman expanded the effort through the linkage of treatment decisions to subsequent outcomes by following patients after hospital discharge.[3] The advent of modern imaging techniques coupled with interventional methods of obtaining pathological specimens has dramatically improved diagnostic accuracy over the past 40 years. As a result, the practice of using autopsies to improve clinical acumen and reduce diagnostic error has virtually disappeared, while the focus on medical error has actually increased. The forum for reducing error shifted to morbidity and mortality rounds (MMRs), which have been relabeled quality‐improvement rounds in many hospitals.
In these regularly scheduled meetings, interprofessional clinicians discuss errors and adverse outcomes. Because deaths are rarely unexpected and often occur outside of the acute care setting, the focus is usually on errors in the execution of complex clinical plans that combine the wide array of modern laboratory, imaging, pharmaceutical, interventional, surgical, and pathological tools available to clinicians today. In the era of patient safety and quality improvement, errors are mostly blamed on systems‐based issues that lead to hospital complications, despite evidence that cognitive factors play a large role.[4] Systems‐based analysis was popularized by the landmark report of the Institute of Medicine.[5] In our local institutions (the University of Toronto teaching hospitals), improving diagnostic accuracy is almost never on the agenda. We suspect the same is true elsewhere. Common themes include mistakes in medication administration and dosing, communication, and physician handover. The Swiss cheese model[6] is often invoked to diffuse blame across a number of individuals, processes, and even machines. However, as Wachter and Pronovost point out, reengineering of systems has limited capacity for solving all safety and quality improvement issues when people are involved; human error can still sabotage the effort.[7]
Discussions centered on a physician's raw thinking ability have become a third rail, even though clinical reasoning lies at the core of patient safety. Human error is rarely discussed, in part because it is mistakenly believed to be uncommon and felt to be the result of deficits in knowledge or incompetence. Furthermore, the fear of assigning blame to individuals in front of their peers may be counterproductive, discouraging identification of future errors. However, the fields of cognitive psychology and medical decision making have clearly established that cognitive errors occur predictably and often, especially at times of high cognitive load (eg, when many high stakes complex decisions need to be made in a short period of time). Errors do not usually result from a lack of knowledge (although they can), but rather because people rely on instincts that include common biases called heuristics.[8] Most of the time, heuristics are a helpful and necessary evolutionary adaptation of the human thought process, but by their inherent nature, they can lead to predictable and repeatable errors. Because the effects of cognitive biases are inherent to all decision makers, using this framework for discussing individual error may be a method of decreasing the second victim effect[9] and avoid demoralizing the individual.
MMRs thus represent fertile ground for introducing cognitive psychology into medical education and quality improvement. The existing format is useful for teaching cognitive psychology because it is an open forum where discussions center on errors of omission and commission, many of which are a result of both systems issues and decision making heuristics. Several studies have attempted to describe methods for improving MMRs[10, 11, 12]; however, none have incorporated concepts from cognitive psychology. This type of analysis has penetrated several cases in the WebM&M series created by the Agency of Healthcare Quality Research, which can be used as a model for hospital‐based MMRs.[13] For the vignette described above, a MMR that considers systems‐based approaches might discuss how a busy emergency room, limitations of capacity on the cardiology service, and closure of the echo lab at night, played a role in this story. However, although it is difficult to replay another person's mental processing, ignoring the possibility that the cardiologist in this case may have fallen prey to a common cognitive error would be a missed opportunity to learn how frequently heuristics can be faulty. A cognitive approach applied to this example would explore explanations such as anchoring, ego, and hassle biases. Front‐line clinicians in busy hospital settings will recognize the interaction between workload pressures and cognitive mistakes common to examples like this one.
Cognitive heuristics should first be introduced to MMRs by experienced clinicians, well respected for their clinical acumen, by telling specific personal stories where heuristics led to errors in their practices and why the shortcut in thinking occurred. Thereafter, the traditional MMR format can be used: presenting a case, describing how an experienced clinician might manage the case, and then asking the audience members for comment. Incorporating discussions of cognitive missteps, in medical and nonmedical contexts, would help normalize the understanding that even the most experienced and smartest people fall prey to them. The tone must be positive.
Attendees could be encouraged to review their own thought processes through diagnostic verification for cases where their initial diagnosis was incorrect. This would involve assessment for adequacy, ensuring that potential diagnoses account for all abnormal and normal clinical findings, and coherency, ensuring that the diagnoses are pathophysiologically consistent with all clinical findings. Another strategy may be to illustrate cognitive forcing strategies for particular biases.[14] For example, in the case of anchoring bias, trainees may be encouraged to replay the clinical scenario with a different priming stem and evaluate if they would come to the same clinical conclusion. A challenge for all MMRs is how best to select cases; given the difficulties in replaying one's cognitive processes, this problem may be magnified. Potential selection methods could utilize anonymous reporting systems or patient complaints; however, the optimal strategy is yet to be determined.
Graber et al. have summarized the limited research on attempts to improve cognitive processes through educational interventions and illustrate its mixed results.[15] The most positive study was a randomized control trial using combined pattern recognition and deliberative reasoning to improve diagnostic accuracy in the face of biasing information.[16] Despite positive results, others have suggested that cognitive biases are impossible to teach due to their subconscious nature.[17] They argue that training physicians to avoid heuristics will simply lead to over investigation. These polarizing views highlight the need for research to evaluate interventions like the cognitive autopsy suggested here.
Trainees recognize early that their knowledge base is limited. However, it takes more internal analysis to realize that their brains' decision‐making capacity is similarly limited. Utilizing these regularly scheduled clinical meetings in the manner described above may build improved metacognition, cognition about cognition or more colloquially thinking about thinking. Clinicians understand that bias can easily occur in research and accept mechanisms to protect studies from those potential threats to validity such as double blinding of outcome assessments. Supplementing MMRs with cognitive discussions represents an analogous intent to reduce biases, introducing metacognition as the next frontier in advancing clinical care. Errors are inevitable,[18] and recognition of our cognitive blind spots will provide physicians with an improved framework for analysis of these errors. Building metacognition is a difficult task; however, this is not a reason to stop trying. In the spirit of innovation begun by pioneers like Rokitansky and Codman, and renewed focus on diagnostic errors generated by the recent report of the National Academy of Sciences[19], it is time for the cognitive autopsy to be built into the quality improvement and patient safety map.
Acknowledgements
The authors thank Donald A. Redelemeier, MD, MSc, University of Toronto, and Gurpreet Dhaliwal, MD, University of California, San Francisco, for providing comments on an earlier draft of this article. Neither was compensated for their contributions.
Disclosure: Nothing to report.
- , . Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–1131.
- . Doctors: The Biography of Medicine. New York, NY: Vintage Books; 1995.
- . The classic: a study in hospital efficiency: as demonstrated by the case report of first five years of private hospital. Clin Orthop Relat Res. 2013;471(6):1778–1783.
- , , . Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493–1499.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 1999.
- . The contribution of latent human failures to the breakdown of complex systems. Philos Trans R Soc Lond B Biol Sci. 1990;327(1241):475–484.
- , . Balancing “no blame” with accountability in patient safety. N Engl J Med. 2009;361(14):1401–1406.
- . From mindless to mindful practice—cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445–2448.
- . Medical error: the second victim. The doctor who makes the mistake needs help too. BMJ. 2000;320(7237):726–727.
- , , , et al. Impact of morbidity and mortality conferences on analysis of mortality and critical events in intensive care practice. Am J Crit Care. 2010;19(2):135–145.
- , , , . Using patient safety morbidity and mortality conferences to promote transparency and a culture of safety. Jt Comm J Qual Patient Saf. 2010;36(1):3–9.
- , , , et al. Enhancing the quality of morbidity and mortality rounds: the Ottawa M21(3):314–321.
- Agency for Healthcare Research and Quality. AHRQ WebM41(1):110–120.
- , , , et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21(7):535–557.
- , , , . Teaching from the clinical reasoning literature: combined reasoning strategies help novice diagnosticians overcome misleading information. Med Educ. 2007;41(12):1152–1158.
- , . Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94–100.
- , . Everyone's a little bit biased (even physicians). JAMA. 2008;299(24):2893–2895.
- , , . Improving Diagnosis in Health Care. Washington, DC: National Academies Press; 2015.
- , . Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–1131.
- . Doctors: The Biography of Medicine. New York, NY: Vintage Books; 1995.
- . The classic: a study in hospital efficiency: as demonstrated by the case report of first five years of private hospital. Clin Orthop Relat Res. 2013;471(6):1778–1783.
- , , . Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493–1499.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 1999.
- . The contribution of latent human failures to the breakdown of complex systems. Philos Trans R Soc Lond B Biol Sci. 1990;327(1241):475–484.
- , . Balancing “no blame” with accountability in patient safety. N Engl J Med. 2009;361(14):1401–1406.
- . From mindless to mindful practice—cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445–2448.
- . Medical error: the second victim. The doctor who makes the mistake needs help too. BMJ. 2000;320(7237):726–727.
- , , , et al. Impact of morbidity and mortality conferences on analysis of mortality and critical events in intensive care practice. Am J Crit Care. 2010;19(2):135–145.
- , , , . Using patient safety morbidity and mortality conferences to promote transparency and a culture of safety. Jt Comm J Qual Patient Saf. 2010;36(1):3–9.
- , , , et al. Enhancing the quality of morbidity and mortality rounds: the Ottawa M21(3):314–321.
- Agency for Healthcare Research and Quality. AHRQ WebM41(1):110–120.
- , , , et al. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21(7):535–557.
- , , , . Teaching from the clinical reasoning literature: combined reasoning strategies help novice diagnosticians overcome misleading information. Med Educ. 2007;41(12):1152–1158.
- , . Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94–100.
- , . Everyone's a little bit biased (even physicians). JAMA. 2008;299(24):2893–2895.
- , , . Improving Diagnosis in Health Care. Washington, DC: National Academies Press; 2015.
Value‐Based Payment
The United States is moving aggressively toward value‐based payment. The Department of Health and Human Services recently announced a goal to link 85% of Medicare's fee‐for‐service payments to quality or value by 2016.[1] Despite the inherent logic of paying providers for their results, evidence of the effectiveness of value‐based payment has been mixed and underwhelming. Recent reviews of pay‐for‐performancereflecting the emerging understanding of the complexities of designing successful programshave painted a more negative picture of their overall effectiveness.[2, 3] One study of over 6 million patients found that the Medicare Premier Hospital Quality Incentive Demonstration had no effect on long‐term patient outcomes including 30‐day mortality.[4] At the same time, research suggests that lower performing providers tend to have a disproportionate number of poor patients, many of whom are racial and ethnic minorities. Value‐based payment risks the dual failure of not improving health outcomes while exacerbating health inequities.
We have seen this movie before. In 2001, No Child Left Behind was enacted to improve quality and reduce inequities in K12 education in the United States. Much like healthcare, education suffers from uneven quality and wide socioeconomic disparities.[5] No Child Left Behind attempted to address these problems with new accountability measures. Based on the results from standardized tests, No Child Left Behind rewarded the highest performing schools with more funding while penalizing poor performing schools with reduced funding, and in some cases, forcing failing schools to cede control to outside operators.
In the aftermath of its implementation, however, it became clear that these incentives had not worked as intended. No Child Left Behind did not improve reading performance and was associated with improvements in math performance only for younger students.[6] These modest gains came at a high cost; consistent with teaching to the test, No Child Left Behind led to a shifting of instructional time toward math and reading and away from other subjects. It also led to widespread cheating, challenging the validity of observed performance improvements. Before No Child Left Behind was rolled out, the wealthiest school districts in the country spent as much as 10 times more than the poorest districts.[5] By penalizing the lowest performers, these gaps persisted. Schools were not given the support that they needed to improve performance.
The parallels to healthcare are striking (Table 1). Early results from Medicare's Hospital Value‐Based Purchasing and Readmission Reduction Program show that hospitals caring for more disadvantaged patients have been disproportionately penalized.[7] Similar reverse Robin Hood effects have been observed in incentive programs for physician practices.[8] Over time, financial incentive programs may substantially decrease operating revenue for hospitals and physicians caring for low‐income and minority communities. This could perpetuate the already large disparities in quality and health outcomes facing these populations. Although risk‐adjusting for socioeconomic status may alleviate these concerns in the short term, allowing low‐income or minority patients to have poorer health outcomes simply accepts that disparities exist rather than trying to reduce them.
| Healthcare | Education | ||
|---|---|---|---|
| National incentive programs | Examples | Hospital Value‐Based Purchasing Hospital Readmission‐Reduction Program | No Child Left Behind |
| Hospital‐Acquired Conditions Penalty Program | |||
| Physician Value‐Based Payment Modifier | |||
| Approach toward improving performance | Reimbursements are tied to quality and cost. | Test‐based accountability: Results of standardized tests are used to determine levels of federal funding. Schools failing to meet testing goals are penalized with reductions in funding. | |
| Bonuses are given to hospitals and providers that perform well on performance metrics. Low performers are penalized with lower reimbursements. | Takeover of failing districts: Districts failing to make adequate yearly progress for 5 years in a row must implement a restructuring plan that may involve changing the school's governance arrangement, converting the school to a charter, or turning the school over to a private management company. | ||
| Unintended consequences | Gaming | Cheating to boost test scores. | |
| Ignoring or neglecting areas of care that are unincentivized. | Shift of instruction time toward math and reading. | ||
| Avoiding high‐risk or disadvantaged patients. | States intentionally making assessment tools easier. | ||
| Stress among administrators, teachers, and students due to high‐stakes testing. | |||
| Collaboration‐based programs | Examples | Quality collaboratives | Shanghai school system |
| Hospital engagement networks | |||
| Approach toward improving performance | Improvement networks: High performing hospitals or providers are identified and work with other groups to improve patient treatment and the care process. | Pairing of districts: High‐performing districts are paired with lower performing districts to exchange education development plans, curricula, and teaching materials. | |
| Data sharing: Facilities collect and share data to monitor quality improvements and better identify best practices. | Commissioned administration: A high‐performing school partners with low performers by sending experienced teachers and administrators to share successful practices and turn around their performance. | ||
| Example of success | The Michigan Surgical Quality Collaborative was associated with a 2.6% drop in general and vascular surgery complications. Hospitals participating in the programs made improvements at a faster rate than those outside of the program. | Zhabei District No. 8 School, located in an area with high crime rates and low student performance, was transformed from one of the lowest performing schools in its district to ranking 15 out of 30. Approximately 80% of the school's graduates go on to study at universities compared to the municipal average of 56%. | |
How then is it possible to improve the quality of care at lower performing hospitals without simultaneously designing an incentive system that hurts them? Lessons from the education policy are again instructive. Every 3 years the Organization for Economic Cooperation and Development ranks countries by the performance of their 15‐year olds on a standardized test called the Program for International Student Assessment.[9] For the past 2 sets of rankings, Shanghai, China has topped the list. Like many attempts to generate international rankings, this one has its flaws, and Shanghai's top position has not been without controversy. For one, China is not ranked at the country‐level like other nations; yet, due to the city's status as a wealthy business and financial center, Shanghai certainly cannot be considered representative of the Chinese education system. Nevertheless, the story of how Shanghai reformed its education system and achieved its high position has important implications.
Prior to implementing reforms, Shanghai's rural outer districts struggled with less funding, high teacher turnover rates, and low test scores compared to wealthier urban districts. To reduce education disparities within the city's schools, the government of Shanghai enacted a number of policies aimed at bringing lower performers up to the same level as schools with the highest degree of student achievement.[10] The government gives schools a grade of A, B, C, or D based on the quality of their infrastructure and student performance. It then uses several programs to facilitate the exchange of staff and ideas among schools at different levels. One program pairs high‐performing districts with low‐performing districts to share education development plans, curricula, teaching materials, and best practices. Another strategycalled commissioned administrationinvolves temporary contracts between schools to exchange both administrators and experienced teachers. In addition to these approaches, the government sets a minimum level of spending for schools and transfers public funds to indigent districts to provide them with assistance to reach this level.
The notion that the very best can help the weak requires a sense of solidarity. This solidarity may falter in environments in which hospitals and physicians are in cutthroat competition. Though there will always be some tension between competition and collaboration, in most markets, competition between hospitals does not rule out collaboration. Policies can either relieve or reinforce the natural tension between competition and collaboration. This suggests that adopting reforms with the same intent as the Shanghai system is still possible in healthcare, especially through physician and other provider networks. The healthcare workforce has a rich history of cross‐organizational collaboration through mentorships, the publication of research, and participation in continuing medical education courses. The Centers for Medicare and Medicaid Services' Hospital Engagement Networks, a program in which leading organizations have helped to disseminate interventions to reduce hospital acquired conditions, are an example of this approach. Quality collaborativesgroups of providers who collaborate across institutions to identify problems and best practices for improvementhave similarly shown great promise.[11] Similar approaches have been used by the Institute for Healthcare Improvement in many of their quality improvement initiatives.
Such collaboration‐based programs could be harnessed and tied to financial incentives for quality improvement. For instance, top‐performing hospitals could be incentivized to participate in a venue where they share their best practices with the lower performers in their field. Low performers, in turn, could be provided with financial assistance to implement the appropriate changes. Over time, financial assistance could be made contingent on quality improvements. By providing physicians and other providers with examples of what success looks like and assisting them with garnering the resources to reach this level, improvement would not only be incentivized, it might also become more tangible.
Although some hospitals and physicians may welcome changes to incentive systems, implementation of collaboration‐based programs would not be possible without a facilitator that is willing to underwrite program costs, provide financial incentivizes to providers, and develop a platform for collaboration. Large insurers are the most likely group to have the financial resources and widespread network to develop such programs, but that does not mean that they would be willing to experiment with this approach. This may especially be the case if cost savings and measurable improvements in quality are not immediate. Even though the results of collaboration‐based efforts have been promising, the implementation of these programs has been limited, and adoption in different contexts may not yield the same results. Collaboration‐based programs that have already shown success can serve as models, but they may need significant adaptations to meet the needs of providers in a given area.
Despite its promise, collaboration‐based strategies alone will not be enough to improve certain aspects of quality and value. Although providing physicians with knowledge on how to reduce unnecessary care, for example, could help limit overutilization, it is not sufficient to overcome the incentives of fee‐for‐service payment. In this case, broader payment reform and population‐based accountability can be paired with programs to encourage collaboration. For instance, the Blue Cross and Blue Shield of Massachusetts' Alternative Contract has used a combination of technical assistance, shared savings, and large quality bonuses to improve quality and reduce medical spending growth.[12] Collaboration‐based strategies should be seen as a complement to these broad, thoughtful reforms and a substitute for narrow incentives that encourage myopia and destructive competition.
Evidence from education and healthcare shows that penalizing the worst and rewarding the best will not shift the bell curve of performance. Such approaches are more likely to entrench and expand disparities. Instead, policy should encourage and incentivize collaboration to expand best practices that improve patient outcomes. Lessons from education provide both cautionary tales and novel solutions that might improve healthcare.
Disclosure: Nothing to report.
- . Setting value‐based payment goals—HHS efforts to improve US health care. N Engl J Med. 2015;372:897–899.
- , , , , , . Systematic review: effects, design choices, and context of pay‐for‐performance in health care. BMC Health Serv Res. 2010;10:247.
- , , , , . Does performance‐based remuneration for individual health care practitioners affect patient care?: a systematic review. Ann Intern Med. 2012;157(12):889–899.
- , , , . The long‐term effect of premier pay‐for‐ performance on patient outcomes. N Engl J Med. 2012;366:1606–1615.
- Race, inequality and education accountability: the irony of ‘no child left behind.’ Race Ethn Educ. 2007;10:245–260.
- , . The impact of no child left behind on students, teachers, and schools. In: Brookings Paper on Economic Activity. Washington, DC: The Brookings Institution; 2010:149–194.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369:2472–2474.
- , , , et al. Do physician organizations located in lower socioeconomic status areas score lower on pay‐for‐performance measures? J Gen Intern Med. 2012;27:548–554.
- . Brown Center Chalkboard. Attention OECD‐PISA: your silence on China is wrong. Washington, DC: The Brookings Institute; 2013:48.
- Organisation for Economic Cooperation and Development. Shanghai and Hong Kong: two distinct examples of education reform in China. In: Strong Performers and Successful Reformers in Education: Lessons from PISA for the United States. Paris, France: OECD Publishing; 2010:83–115.
- , , , et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff. 2011;30:636–645.
- , , , , , . Changes in health care spending and quality 4 years into global payment. N Engl J Med. 2014;371:1704–1714.
The United States is moving aggressively toward value‐based payment. The Department of Health and Human Services recently announced a goal to link 85% of Medicare's fee‐for‐service payments to quality or value by 2016.[1] Despite the inherent logic of paying providers for their results, evidence of the effectiveness of value‐based payment has been mixed and underwhelming. Recent reviews of pay‐for‐performancereflecting the emerging understanding of the complexities of designing successful programshave painted a more negative picture of their overall effectiveness.[2, 3] One study of over 6 million patients found that the Medicare Premier Hospital Quality Incentive Demonstration had no effect on long‐term patient outcomes including 30‐day mortality.[4] At the same time, research suggests that lower performing providers tend to have a disproportionate number of poor patients, many of whom are racial and ethnic minorities. Value‐based payment risks the dual failure of not improving health outcomes while exacerbating health inequities.
We have seen this movie before. In 2001, No Child Left Behind was enacted to improve quality and reduce inequities in K12 education in the United States. Much like healthcare, education suffers from uneven quality and wide socioeconomic disparities.[5] No Child Left Behind attempted to address these problems with new accountability measures. Based on the results from standardized tests, No Child Left Behind rewarded the highest performing schools with more funding while penalizing poor performing schools with reduced funding, and in some cases, forcing failing schools to cede control to outside operators.
In the aftermath of its implementation, however, it became clear that these incentives had not worked as intended. No Child Left Behind did not improve reading performance and was associated with improvements in math performance only for younger students.[6] These modest gains came at a high cost; consistent with teaching to the test, No Child Left Behind led to a shifting of instructional time toward math and reading and away from other subjects. It also led to widespread cheating, challenging the validity of observed performance improvements. Before No Child Left Behind was rolled out, the wealthiest school districts in the country spent as much as 10 times more than the poorest districts.[5] By penalizing the lowest performers, these gaps persisted. Schools were not given the support that they needed to improve performance.
The parallels to healthcare are striking (Table 1). Early results from Medicare's Hospital Value‐Based Purchasing and Readmission Reduction Program show that hospitals caring for more disadvantaged patients have been disproportionately penalized.[7] Similar reverse Robin Hood effects have been observed in incentive programs for physician practices.[8] Over time, financial incentive programs may substantially decrease operating revenue for hospitals and physicians caring for low‐income and minority communities. This could perpetuate the already large disparities in quality and health outcomes facing these populations. Although risk‐adjusting for socioeconomic status may alleviate these concerns in the short term, allowing low‐income or minority patients to have poorer health outcomes simply accepts that disparities exist rather than trying to reduce them.
| Healthcare | Education | ||
|---|---|---|---|
| National incentive programs | Examples | Hospital Value‐Based Purchasing Hospital Readmission‐Reduction Program | No Child Left Behind |
| Hospital‐Acquired Conditions Penalty Program | |||
| Physician Value‐Based Payment Modifier | |||
| Approach toward improving performance | Reimbursements are tied to quality and cost. | Test‐based accountability: Results of standardized tests are used to determine levels of federal funding. Schools failing to meet testing goals are penalized with reductions in funding. | |
| Bonuses are given to hospitals and providers that perform well on performance metrics. Low performers are penalized with lower reimbursements. | Takeover of failing districts: Districts failing to make adequate yearly progress for 5 years in a row must implement a restructuring plan that may involve changing the school's governance arrangement, converting the school to a charter, or turning the school over to a private management company. | ||
| Unintended consequences | Gaming | Cheating to boost test scores. | |
| Ignoring or neglecting areas of care that are unincentivized. | Shift of instruction time toward math and reading. | ||
| Avoiding high‐risk or disadvantaged patients. | States intentionally making assessment tools easier. | ||
| Stress among administrators, teachers, and students due to high‐stakes testing. | |||
| Collaboration‐based programs | Examples | Quality collaboratives | Shanghai school system |
| Hospital engagement networks | |||
| Approach toward improving performance | Improvement networks: High performing hospitals or providers are identified and work with other groups to improve patient treatment and the care process. | Pairing of districts: High‐performing districts are paired with lower performing districts to exchange education development plans, curricula, and teaching materials. | |
| Data sharing: Facilities collect and share data to monitor quality improvements and better identify best practices. | Commissioned administration: A high‐performing school partners with low performers by sending experienced teachers and administrators to share successful practices and turn around their performance. | ||
| Example of success | The Michigan Surgical Quality Collaborative was associated with a 2.6% drop in general and vascular surgery complications. Hospitals participating in the programs made improvements at a faster rate than those outside of the program. | Zhabei District No. 8 School, located in an area with high crime rates and low student performance, was transformed from one of the lowest performing schools in its district to ranking 15 out of 30. Approximately 80% of the school's graduates go on to study at universities compared to the municipal average of 56%. | |
How then is it possible to improve the quality of care at lower performing hospitals without simultaneously designing an incentive system that hurts them? Lessons from the education policy are again instructive. Every 3 years the Organization for Economic Cooperation and Development ranks countries by the performance of their 15‐year olds on a standardized test called the Program for International Student Assessment.[9] For the past 2 sets of rankings, Shanghai, China has topped the list. Like many attempts to generate international rankings, this one has its flaws, and Shanghai's top position has not been without controversy. For one, China is not ranked at the country‐level like other nations; yet, due to the city's status as a wealthy business and financial center, Shanghai certainly cannot be considered representative of the Chinese education system. Nevertheless, the story of how Shanghai reformed its education system and achieved its high position has important implications.
Prior to implementing reforms, Shanghai's rural outer districts struggled with less funding, high teacher turnover rates, and low test scores compared to wealthier urban districts. To reduce education disparities within the city's schools, the government of Shanghai enacted a number of policies aimed at bringing lower performers up to the same level as schools with the highest degree of student achievement.[10] The government gives schools a grade of A, B, C, or D based on the quality of their infrastructure and student performance. It then uses several programs to facilitate the exchange of staff and ideas among schools at different levels. One program pairs high‐performing districts with low‐performing districts to share education development plans, curricula, teaching materials, and best practices. Another strategycalled commissioned administrationinvolves temporary contracts between schools to exchange both administrators and experienced teachers. In addition to these approaches, the government sets a minimum level of spending for schools and transfers public funds to indigent districts to provide them with assistance to reach this level.
The notion that the very best can help the weak requires a sense of solidarity. This solidarity may falter in environments in which hospitals and physicians are in cutthroat competition. Though there will always be some tension between competition and collaboration, in most markets, competition between hospitals does not rule out collaboration. Policies can either relieve or reinforce the natural tension between competition and collaboration. This suggests that adopting reforms with the same intent as the Shanghai system is still possible in healthcare, especially through physician and other provider networks. The healthcare workforce has a rich history of cross‐organizational collaboration through mentorships, the publication of research, and participation in continuing medical education courses. The Centers for Medicare and Medicaid Services' Hospital Engagement Networks, a program in which leading organizations have helped to disseminate interventions to reduce hospital acquired conditions, are an example of this approach. Quality collaborativesgroups of providers who collaborate across institutions to identify problems and best practices for improvementhave similarly shown great promise.[11] Similar approaches have been used by the Institute for Healthcare Improvement in many of their quality improvement initiatives.
Such collaboration‐based programs could be harnessed and tied to financial incentives for quality improvement. For instance, top‐performing hospitals could be incentivized to participate in a venue where they share their best practices with the lower performers in their field. Low performers, in turn, could be provided with financial assistance to implement the appropriate changes. Over time, financial assistance could be made contingent on quality improvements. By providing physicians and other providers with examples of what success looks like and assisting them with garnering the resources to reach this level, improvement would not only be incentivized, it might also become more tangible.
Although some hospitals and physicians may welcome changes to incentive systems, implementation of collaboration‐based programs would not be possible without a facilitator that is willing to underwrite program costs, provide financial incentivizes to providers, and develop a platform for collaboration. Large insurers are the most likely group to have the financial resources and widespread network to develop such programs, but that does not mean that they would be willing to experiment with this approach. This may especially be the case if cost savings and measurable improvements in quality are not immediate. Even though the results of collaboration‐based efforts have been promising, the implementation of these programs has been limited, and adoption in different contexts may not yield the same results. Collaboration‐based programs that have already shown success can serve as models, but they may need significant adaptations to meet the needs of providers in a given area.
Despite its promise, collaboration‐based strategies alone will not be enough to improve certain aspects of quality and value. Although providing physicians with knowledge on how to reduce unnecessary care, for example, could help limit overutilization, it is not sufficient to overcome the incentives of fee‐for‐service payment. In this case, broader payment reform and population‐based accountability can be paired with programs to encourage collaboration. For instance, the Blue Cross and Blue Shield of Massachusetts' Alternative Contract has used a combination of technical assistance, shared savings, and large quality bonuses to improve quality and reduce medical spending growth.[12] Collaboration‐based strategies should be seen as a complement to these broad, thoughtful reforms and a substitute for narrow incentives that encourage myopia and destructive competition.
Evidence from education and healthcare shows that penalizing the worst and rewarding the best will not shift the bell curve of performance. Such approaches are more likely to entrench and expand disparities. Instead, policy should encourage and incentivize collaboration to expand best practices that improve patient outcomes. Lessons from education provide both cautionary tales and novel solutions that might improve healthcare.
Disclosure: Nothing to report.
The United States is moving aggressively toward value‐based payment. The Department of Health and Human Services recently announced a goal to link 85% of Medicare's fee‐for‐service payments to quality or value by 2016.[1] Despite the inherent logic of paying providers for their results, evidence of the effectiveness of value‐based payment has been mixed and underwhelming. Recent reviews of pay‐for‐performancereflecting the emerging understanding of the complexities of designing successful programshave painted a more negative picture of their overall effectiveness.[2, 3] One study of over 6 million patients found that the Medicare Premier Hospital Quality Incentive Demonstration had no effect on long‐term patient outcomes including 30‐day mortality.[4] At the same time, research suggests that lower performing providers tend to have a disproportionate number of poor patients, many of whom are racial and ethnic minorities. Value‐based payment risks the dual failure of not improving health outcomes while exacerbating health inequities.
We have seen this movie before. In 2001, No Child Left Behind was enacted to improve quality and reduce inequities in K12 education in the United States. Much like healthcare, education suffers from uneven quality and wide socioeconomic disparities.[5] No Child Left Behind attempted to address these problems with new accountability measures. Based on the results from standardized tests, No Child Left Behind rewarded the highest performing schools with more funding while penalizing poor performing schools with reduced funding, and in some cases, forcing failing schools to cede control to outside operators.
In the aftermath of its implementation, however, it became clear that these incentives had not worked as intended. No Child Left Behind did not improve reading performance and was associated with improvements in math performance only for younger students.[6] These modest gains came at a high cost; consistent with teaching to the test, No Child Left Behind led to a shifting of instructional time toward math and reading and away from other subjects. It also led to widespread cheating, challenging the validity of observed performance improvements. Before No Child Left Behind was rolled out, the wealthiest school districts in the country spent as much as 10 times more than the poorest districts.[5] By penalizing the lowest performers, these gaps persisted. Schools were not given the support that they needed to improve performance.
The parallels to healthcare are striking (Table 1). Early results from Medicare's Hospital Value‐Based Purchasing and Readmission Reduction Program show that hospitals caring for more disadvantaged patients have been disproportionately penalized.[7] Similar reverse Robin Hood effects have been observed in incentive programs for physician practices.[8] Over time, financial incentive programs may substantially decrease operating revenue for hospitals and physicians caring for low‐income and minority communities. This could perpetuate the already large disparities in quality and health outcomes facing these populations. Although risk‐adjusting for socioeconomic status may alleviate these concerns in the short term, allowing low‐income or minority patients to have poorer health outcomes simply accepts that disparities exist rather than trying to reduce them.
| Healthcare | Education | ||
|---|---|---|---|
| National incentive programs | Examples | Hospital Value‐Based Purchasing Hospital Readmission‐Reduction Program | No Child Left Behind |
| Hospital‐Acquired Conditions Penalty Program | |||
| Physician Value‐Based Payment Modifier | |||
| Approach toward improving performance | Reimbursements are tied to quality and cost. | Test‐based accountability: Results of standardized tests are used to determine levels of federal funding. Schools failing to meet testing goals are penalized with reductions in funding. | |
| Bonuses are given to hospitals and providers that perform well on performance metrics. Low performers are penalized with lower reimbursements. | Takeover of failing districts: Districts failing to make adequate yearly progress for 5 years in a row must implement a restructuring plan that may involve changing the school's governance arrangement, converting the school to a charter, or turning the school over to a private management company. | ||
| Unintended consequences | Gaming | Cheating to boost test scores. | |
| Ignoring or neglecting areas of care that are unincentivized. | Shift of instruction time toward math and reading. | ||
| Avoiding high‐risk or disadvantaged patients. | States intentionally making assessment tools easier. | ||
| Stress among administrators, teachers, and students due to high‐stakes testing. | |||
| Collaboration‐based programs | Examples | Quality collaboratives | Shanghai school system |
| Hospital engagement networks | |||
| Approach toward improving performance | Improvement networks: High performing hospitals or providers are identified and work with other groups to improve patient treatment and the care process. | Pairing of districts: High‐performing districts are paired with lower performing districts to exchange education development plans, curricula, and teaching materials. | |
| Data sharing: Facilities collect and share data to monitor quality improvements and better identify best practices. | Commissioned administration: A high‐performing school partners with low performers by sending experienced teachers and administrators to share successful practices and turn around their performance. | ||
| Example of success | The Michigan Surgical Quality Collaborative was associated with a 2.6% drop in general and vascular surgery complications. Hospitals participating in the programs made improvements at a faster rate than those outside of the program. | Zhabei District No. 8 School, located in an area with high crime rates and low student performance, was transformed from one of the lowest performing schools in its district to ranking 15 out of 30. Approximately 80% of the school's graduates go on to study at universities compared to the municipal average of 56%. | |
How then is it possible to improve the quality of care at lower performing hospitals without simultaneously designing an incentive system that hurts them? Lessons from the education policy are again instructive. Every 3 years the Organization for Economic Cooperation and Development ranks countries by the performance of their 15‐year olds on a standardized test called the Program for International Student Assessment.[9] For the past 2 sets of rankings, Shanghai, China has topped the list. Like many attempts to generate international rankings, this one has its flaws, and Shanghai's top position has not been without controversy. For one, China is not ranked at the country‐level like other nations; yet, due to the city's status as a wealthy business and financial center, Shanghai certainly cannot be considered representative of the Chinese education system. Nevertheless, the story of how Shanghai reformed its education system and achieved its high position has important implications.
Prior to implementing reforms, Shanghai's rural outer districts struggled with less funding, high teacher turnover rates, and low test scores compared to wealthier urban districts. To reduce education disparities within the city's schools, the government of Shanghai enacted a number of policies aimed at bringing lower performers up to the same level as schools with the highest degree of student achievement.[10] The government gives schools a grade of A, B, C, or D based on the quality of their infrastructure and student performance. It then uses several programs to facilitate the exchange of staff and ideas among schools at different levels. One program pairs high‐performing districts with low‐performing districts to share education development plans, curricula, teaching materials, and best practices. Another strategycalled commissioned administrationinvolves temporary contracts between schools to exchange both administrators and experienced teachers. In addition to these approaches, the government sets a minimum level of spending for schools and transfers public funds to indigent districts to provide them with assistance to reach this level.
The notion that the very best can help the weak requires a sense of solidarity. This solidarity may falter in environments in which hospitals and physicians are in cutthroat competition. Though there will always be some tension between competition and collaboration, in most markets, competition between hospitals does not rule out collaboration. Policies can either relieve or reinforce the natural tension between competition and collaboration. This suggests that adopting reforms with the same intent as the Shanghai system is still possible in healthcare, especially through physician and other provider networks. The healthcare workforce has a rich history of cross‐organizational collaboration through mentorships, the publication of research, and participation in continuing medical education courses. The Centers for Medicare and Medicaid Services' Hospital Engagement Networks, a program in which leading organizations have helped to disseminate interventions to reduce hospital acquired conditions, are an example of this approach. Quality collaborativesgroups of providers who collaborate across institutions to identify problems and best practices for improvementhave similarly shown great promise.[11] Similar approaches have been used by the Institute for Healthcare Improvement in many of their quality improvement initiatives.
Such collaboration‐based programs could be harnessed and tied to financial incentives for quality improvement. For instance, top‐performing hospitals could be incentivized to participate in a venue where they share their best practices with the lower performers in their field. Low performers, in turn, could be provided with financial assistance to implement the appropriate changes. Over time, financial assistance could be made contingent on quality improvements. By providing physicians and other providers with examples of what success looks like and assisting them with garnering the resources to reach this level, improvement would not only be incentivized, it might also become more tangible.
Although some hospitals and physicians may welcome changes to incentive systems, implementation of collaboration‐based programs would not be possible without a facilitator that is willing to underwrite program costs, provide financial incentivizes to providers, and develop a platform for collaboration. Large insurers are the most likely group to have the financial resources and widespread network to develop such programs, but that does not mean that they would be willing to experiment with this approach. This may especially be the case if cost savings and measurable improvements in quality are not immediate. Even though the results of collaboration‐based efforts have been promising, the implementation of these programs has been limited, and adoption in different contexts may not yield the same results. Collaboration‐based programs that have already shown success can serve as models, but they may need significant adaptations to meet the needs of providers in a given area.
Despite its promise, collaboration‐based strategies alone will not be enough to improve certain aspects of quality and value. Although providing physicians with knowledge on how to reduce unnecessary care, for example, could help limit overutilization, it is not sufficient to overcome the incentives of fee‐for‐service payment. In this case, broader payment reform and population‐based accountability can be paired with programs to encourage collaboration. For instance, the Blue Cross and Blue Shield of Massachusetts' Alternative Contract has used a combination of technical assistance, shared savings, and large quality bonuses to improve quality and reduce medical spending growth.[12] Collaboration‐based strategies should be seen as a complement to these broad, thoughtful reforms and a substitute for narrow incentives that encourage myopia and destructive competition.
Evidence from education and healthcare shows that penalizing the worst and rewarding the best will not shift the bell curve of performance. Such approaches are more likely to entrench and expand disparities. Instead, policy should encourage and incentivize collaboration to expand best practices that improve patient outcomes. Lessons from education provide both cautionary tales and novel solutions that might improve healthcare.
Disclosure: Nothing to report.
- . Setting value‐based payment goals—HHS efforts to improve US health care. N Engl J Med. 2015;372:897–899.
- , , , , , . Systematic review: effects, design choices, and context of pay‐for‐performance in health care. BMC Health Serv Res. 2010;10:247.
- , , , , . Does performance‐based remuneration for individual health care practitioners affect patient care?: a systematic review. Ann Intern Med. 2012;157(12):889–899.
- , , , . The long‐term effect of premier pay‐for‐ performance on patient outcomes. N Engl J Med. 2012;366:1606–1615.
- Race, inequality and education accountability: the irony of ‘no child left behind.’ Race Ethn Educ. 2007;10:245–260.
- , . The impact of no child left behind on students, teachers, and schools. In: Brookings Paper on Economic Activity. Washington, DC: The Brookings Institution; 2010:149–194.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369:2472–2474.
- , , , et al. Do physician organizations located in lower socioeconomic status areas score lower on pay‐for‐performance measures? J Gen Intern Med. 2012;27:548–554.
- . Brown Center Chalkboard. Attention OECD‐PISA: your silence on China is wrong. Washington, DC: The Brookings Institute; 2013:48.
- Organisation for Economic Cooperation and Development. Shanghai and Hong Kong: two distinct examples of education reform in China. In: Strong Performers and Successful Reformers in Education: Lessons from PISA for the United States. Paris, France: OECD Publishing; 2010:83–115.
- , , , et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff. 2011;30:636–645.
- , , , , , . Changes in health care spending and quality 4 years into global payment. N Engl J Med. 2014;371:1704–1714.
- . Setting value‐based payment goals—HHS efforts to improve US health care. N Engl J Med. 2015;372:897–899.
- , , , , , . Systematic review: effects, design choices, and context of pay‐for‐performance in health care. BMC Health Serv Res. 2010;10:247.
- , , , , . Does performance‐based remuneration for individual health care practitioners affect patient care?: a systematic review. Ann Intern Med. 2012;157(12):889–899.
- , , , . The long‐term effect of premier pay‐for‐ performance on patient outcomes. N Engl J Med. 2012;366:1606–1615.
- Race, inequality and education accountability: the irony of ‘no child left behind.’ Race Ethn Educ. 2007;10:245–260.
- , . The impact of no child left behind on students, teachers, and schools. In: Brookings Paper on Economic Activity. Washington, DC: The Brookings Institution; 2010:149–194.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369:2472–2474.
- , , , et al. Do physician organizations located in lower socioeconomic status areas score lower on pay‐for‐performance measures? J Gen Intern Med. 2012;27:548–554.
- . Brown Center Chalkboard. Attention OECD‐PISA: your silence on China is wrong. Washington, DC: The Brookings Institute; 2013:48.
- Organisation for Economic Cooperation and Development. Shanghai and Hong Kong: two distinct examples of education reform in China. In: Strong Performers and Successful Reformers in Education: Lessons from PISA for the United States. Paris, France: OECD Publishing; 2010:83–115.
- , , , et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff. 2011;30:636–645.
- , , , , , . Changes in health care spending and quality 4 years into global payment. N Engl J Med. 2014;371:1704–1714.
Parent and Stakeholder Engagement
We believe that patients, families, and other stakeholders can provide meaningful contributions throughout the research process. Involvement of a diverse group of stakeholders is also encouraged by the Patient Centered Outcomes Research Institute (PCORI), which emphasizes research focused on patient‐ and family‐centered outcomes.[1] Patient and family engagement in healthcare, however, has generally focused on children and adults with chronic conditions.[1, 2] Engagement of families of children with serious acute illnesses is infrequent, and no studies have documented the feasibility or acceptability of different methods of family engagement.[3] Furthermore, stakeholders, such as nurses, may participate in study execution but rarely receive opportunities to inform the research process. In this Perspective, we describe our experiences with family engagement using a novel approach of serial, focused, short‐term engagement of stakeholders.
PRESTUDY WORK
In 2012, our institution introduced a nurse‐led transitional home‐visit program, an approach associated with reduced healthcare utilization in adults.[4] Patients hospitalized for acute illness received a 1‐time transitional home visit 24 to 72 hours after hospital discharge. We formed a multidisciplinary team, consisting of physicians, nurse scientists, home healthcare (HHC) nursing staff, patient families, and research staff to design a mixed‐methods study of the transitional home visit, which was funded by PCORI in 2014. This study, the Hospital‐to‐Home Outcomes (H2O) study, has 3 aims: (1) identify barriers to successful transitions home and outcomes of such transitions that are meaningful to families, (2) optimize the transitional home visits to address family‐identified barriers and outcomes, and (3) determine the efficacy of transitional home visits through a randomized control trial.
Two parents joined the study team during study development. Both had children hospitalized for acute illnesses, received a transitional home visit, and participated in a pilot focus group to provide insight into barriers families encounter during care transitions. These parents made valuable contributions, including recommending strategies for patient enrollment and retention. They also committed to participating in regularly scheduled study meetings and ad hoc discussions. However, feedback from the pilot focus group also highlighted a potential research engagement challenge; specifically, once the acute illness resolved, many families were primarily focused on the return to their normal routine and may not be easily engaged in research.
Based on family input, we included several mechanisms to engage caregivers of children with acute illness in the study design of H2O. Each design element allowed families and other stakeholders to contribute via short‐term focused approaches (eg, focus groups, phone surveys). These short‐term interactions drove iterative changes in study processes and approaches, including how to measure outcomes important to families. Rather than a small group of stakeholders making a series of recommendations over a long period of time, we had dozens of individual stakeholders make a few recommendations apiece that were quickly implemented and subsequently tested via feedback from the next few stakeholders (Figure 1).
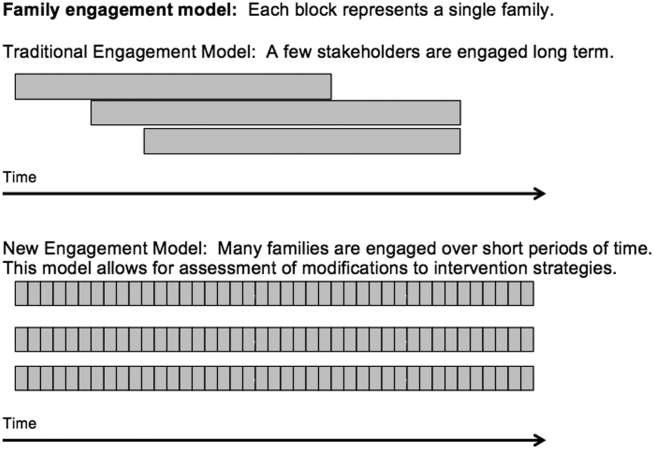
PATIENT AND STAKEHOLDER ENGAGEMENT IN THE H2O STUDY
Having the short‐term, focused engagement strategy built into the study proved beneficial, when the 2 parents who were part of the initial design team and had planned to participate longitudinally were no longer able to participate. Over time, their circumstances changed. One parent moved out of the area to pursue a professional opportunity, and the second parent became increasingly difficult to reach and unable to join planned study meetings, a situation anticipated by the pilot focus group participants. These 2 instances illustrate challenges with long‐term engagement of families in research when the potential primary driver of their engagement, their child's acute illness, has resolved.
Short‐Term Focused Engagement Via Focus Groups With Parents/Caregivers
The first aim of the H2O study used 15 focus groups and semistructured interviews with parents/caregivers of recently discharged patients to identify barriers to and metrics of successful transitions of care from the hospital to home. The focus group question guide was developed by the research team and adapted as the focus groups progressed to incorporate new issues raised by participants. Analysis of focus group data revealed opportunities to improve the transitional home visit and identified outcomes important to families, including the need for emotional reassurance in the immediate period after discharge and the impact on family finances.
Short‐Term Focused Engagement Via Phone Calls With Parents/Caregivers
To continuously improve study processes and the transitional home visit during the second aim of H2O, we relied on short‐term focused engagement from 2 stakeholder groups, families and field nurses. We completed 107 phone calls with families who received a transitional home visit during the visit optimization period. These calls, completed 3 to 7 days after the visit, assessed parental perceptions of the effect of recent visit modifications through a standardized survey documented in an electronic database. These data were utilized in plan‐do‐study‐act cycles,[5] every 1 to 2 weeks, to determine if additional modifications to the visits were necessary. A cycle ended when the calls no longer provided new information. The questions asked on the calls also changed over time as different interventions were tested.
As an example, in aim 1, families highlighted the lack of clarity of discharge instructions, particularly regarding when and why to return for medical care. Thus, we developed condition‐specific red flag reminder cards to be shared at transitional home visits to help families remember and recognize concerning signs and symptoms and understand when additional evaluation may be warranted (Figure 2). Families in postvisit calls endorsed the concept of red flags, but sometimes preferred electronic rather than paper versions of the red flag cards to facilitate sharing with family members. Thus, we tested and refined texting the red flag information to families. Subsequent calls strongly supported this practice, so we will continue to use it during the third aim, the randomized trial of the transitional home visit.

The remaining calls (N=72) were completed 14 days after the visit to mirror the time frame for follow‐up calls in the planned randomized trial. These calls allowed us to test measurement of family‐identified outcomes and determine their usability in the trial. We used family feedback to shorten the survey and reorder questions. We also used feedback from these calls to develop an optimal call‐back strategy to maximize family contacts.
Short‐Term Focused Engagement Via Discussions With Nurses
We also incorporated feedback from HHC nurses on 60 visits to ensure that the visit modifications were feasible to implement. HHC nurse feedback, which aligned with aim 1 data from families, highlighted the potential benefits of standardizing the transitional home visit to be more condition specific. The nurses also provided ongoing ad hoc feedback on other changes to the transitional home visit, which indicated both when tests were successful and when they were challenging to implement. The study team wanted to ensure that the nurses performing the visits were involved in the modification process.
ONGOING H2O WORK AND CONCLUSION
The third aim, with ongoing patient enrollment, involves a randomized trial to determine the efficacy of the revised transitional home visit compared with standard of care as measured by subsequent healthcare utilization and outcomes suggested in aim 1 and refined during aim 2, such as parental coping, stress, and confidence in care. We have engaged 1 parent to provide longitudinal feedback during regularly scheduled meetings.
We believe that our short‐term, focused engagement strategies have allowed integration of the invaluable perspective of families and other stakeholders into our research questions, intervention design, outcome measurement, and study execution. Our approach combined short‐term engagement from many stakeholders, blending qualitative techniques with rapid‐cycle implementation methods to quickly react to stakeholder input. Given the challenge of sustaining longitudinal engagement of families in research focused on acute care questions, and the tendency for many families interested in such engagement to be well versed in the care system due to chronic conditions, we propose this short‐term focused approach to include the unique viewpoints of families and patients whose care experience is confined to an acute period. Similarly, we propose that such an approach can efficiently include and rapidly react to input from other hard‐to‐engage key stakeholders such as field nurses.
Disclosures
This work was supported by the Patient Centered Outcomes Research Institute(HIS‐1306‐0081, SSS). The Patient Centered Outcomes Research Institute had no role in the design, preparation, review, or approval of the manuscript or in the decision to submit the manuscript for publication. The authors have no financial relationships relevant to this article to disclose. The authors report no potential conflicts of interest. The H2O study team members include the following: Katherine A. Auger, MD, MSc, JoAnne Bachus, BSN, Andrew F. Beck, MD, MPH, Monica L. Borell, BSN, Stephanie A. Brunswick, BS, Lenisa Chang, MA, PhD, Jennifer M. Gold, BSN, Judy A. Heilman, RN, Joseph A. Jabour, BS, Jane C. Khoury, PhD, Margo J. Moore, BSN, CCRP, Rita H. Pickler, PNP, PhD, Susan N. Sherman, DPA, Lauren G. Solan, MD, MEd, Angela M. Statile, MD, MEd, Heidi J. Sucharew, PhD, Karen P. Sullivan, BSN, Heather L. Tubbs‐Cooley, RN, PhD, Susan Wade‐Murphy, MSN, and Christine M. White, MD, MAT.
- , , , et al. Conceptual and practical foundations of patient engagement in research at the patient‐centered outcomes research institute. Qual Life Res. 2015;24(5):1033–1041.
- , . A review of parent participation engagement in child and family mental health treatment. Clin Child Fam Psychol Rev. 2015;18(2):133–150.
- , , , et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89.
- , , , . Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251–260.
- , , , , , . The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA: Jossey‐Bass; 2009.
We believe that patients, families, and other stakeholders can provide meaningful contributions throughout the research process. Involvement of a diverse group of stakeholders is also encouraged by the Patient Centered Outcomes Research Institute (PCORI), which emphasizes research focused on patient‐ and family‐centered outcomes.[1] Patient and family engagement in healthcare, however, has generally focused on children and adults with chronic conditions.[1, 2] Engagement of families of children with serious acute illnesses is infrequent, and no studies have documented the feasibility or acceptability of different methods of family engagement.[3] Furthermore, stakeholders, such as nurses, may participate in study execution but rarely receive opportunities to inform the research process. In this Perspective, we describe our experiences with family engagement using a novel approach of serial, focused, short‐term engagement of stakeholders.
PRESTUDY WORK
In 2012, our institution introduced a nurse‐led transitional home‐visit program, an approach associated with reduced healthcare utilization in adults.[4] Patients hospitalized for acute illness received a 1‐time transitional home visit 24 to 72 hours after hospital discharge. We formed a multidisciplinary team, consisting of physicians, nurse scientists, home healthcare (HHC) nursing staff, patient families, and research staff to design a mixed‐methods study of the transitional home visit, which was funded by PCORI in 2014. This study, the Hospital‐to‐Home Outcomes (H2O) study, has 3 aims: (1) identify barriers to successful transitions home and outcomes of such transitions that are meaningful to families, (2) optimize the transitional home visits to address family‐identified barriers and outcomes, and (3) determine the efficacy of transitional home visits through a randomized control trial.
Two parents joined the study team during study development. Both had children hospitalized for acute illnesses, received a transitional home visit, and participated in a pilot focus group to provide insight into barriers families encounter during care transitions. These parents made valuable contributions, including recommending strategies for patient enrollment and retention. They also committed to participating in regularly scheduled study meetings and ad hoc discussions. However, feedback from the pilot focus group also highlighted a potential research engagement challenge; specifically, once the acute illness resolved, many families were primarily focused on the return to their normal routine and may not be easily engaged in research.
Based on family input, we included several mechanisms to engage caregivers of children with acute illness in the study design of H2O. Each design element allowed families and other stakeholders to contribute via short‐term focused approaches (eg, focus groups, phone surveys). These short‐term interactions drove iterative changes in study processes and approaches, including how to measure outcomes important to families. Rather than a small group of stakeholders making a series of recommendations over a long period of time, we had dozens of individual stakeholders make a few recommendations apiece that were quickly implemented and subsequently tested via feedback from the next few stakeholders (Figure 1).

PATIENT AND STAKEHOLDER ENGAGEMENT IN THE H2O STUDY
Having the short‐term, focused engagement strategy built into the study proved beneficial, when the 2 parents who were part of the initial design team and had planned to participate longitudinally were no longer able to participate. Over time, their circumstances changed. One parent moved out of the area to pursue a professional opportunity, and the second parent became increasingly difficult to reach and unable to join planned study meetings, a situation anticipated by the pilot focus group participants. These 2 instances illustrate challenges with long‐term engagement of families in research when the potential primary driver of their engagement, their child's acute illness, has resolved.
Short‐Term Focused Engagement Via Focus Groups With Parents/Caregivers
The first aim of the H2O study used 15 focus groups and semistructured interviews with parents/caregivers of recently discharged patients to identify barriers to and metrics of successful transitions of care from the hospital to home. The focus group question guide was developed by the research team and adapted as the focus groups progressed to incorporate new issues raised by participants. Analysis of focus group data revealed opportunities to improve the transitional home visit and identified outcomes important to families, including the need for emotional reassurance in the immediate period after discharge and the impact on family finances.
Short‐Term Focused Engagement Via Phone Calls With Parents/Caregivers
To continuously improve study processes and the transitional home visit during the second aim of H2O, we relied on short‐term focused engagement from 2 stakeholder groups, families and field nurses. We completed 107 phone calls with families who received a transitional home visit during the visit optimization period. These calls, completed 3 to 7 days after the visit, assessed parental perceptions of the effect of recent visit modifications through a standardized survey documented in an electronic database. These data were utilized in plan‐do‐study‐act cycles,[5] every 1 to 2 weeks, to determine if additional modifications to the visits were necessary. A cycle ended when the calls no longer provided new information. The questions asked on the calls also changed over time as different interventions were tested.
As an example, in aim 1, families highlighted the lack of clarity of discharge instructions, particularly regarding when and why to return for medical care. Thus, we developed condition‐specific red flag reminder cards to be shared at transitional home visits to help families remember and recognize concerning signs and symptoms and understand when additional evaluation may be warranted (Figure 2). Families in postvisit calls endorsed the concept of red flags, but sometimes preferred electronic rather than paper versions of the red flag cards to facilitate sharing with family members. Thus, we tested and refined texting the red flag information to families. Subsequent calls strongly supported this practice, so we will continue to use it during the third aim, the randomized trial of the transitional home visit.

The remaining calls (N=72) were completed 14 days after the visit to mirror the time frame for follow‐up calls in the planned randomized trial. These calls allowed us to test measurement of family‐identified outcomes and determine their usability in the trial. We used family feedback to shorten the survey and reorder questions. We also used feedback from these calls to develop an optimal call‐back strategy to maximize family contacts.
Short‐Term Focused Engagement Via Discussions With Nurses
We also incorporated feedback from HHC nurses on 60 visits to ensure that the visit modifications were feasible to implement. HHC nurse feedback, which aligned with aim 1 data from families, highlighted the potential benefits of standardizing the transitional home visit to be more condition specific. The nurses also provided ongoing ad hoc feedback on other changes to the transitional home visit, which indicated both when tests were successful and when they were challenging to implement. The study team wanted to ensure that the nurses performing the visits were involved in the modification process.
ONGOING H2O WORK AND CONCLUSION
The third aim, with ongoing patient enrollment, involves a randomized trial to determine the efficacy of the revised transitional home visit compared with standard of care as measured by subsequent healthcare utilization and outcomes suggested in aim 1 and refined during aim 2, such as parental coping, stress, and confidence in care. We have engaged 1 parent to provide longitudinal feedback during regularly scheduled meetings.
We believe that our short‐term, focused engagement strategies have allowed integration of the invaluable perspective of families and other stakeholders into our research questions, intervention design, outcome measurement, and study execution. Our approach combined short‐term engagement from many stakeholders, blending qualitative techniques with rapid‐cycle implementation methods to quickly react to stakeholder input. Given the challenge of sustaining longitudinal engagement of families in research focused on acute care questions, and the tendency for many families interested in such engagement to be well versed in the care system due to chronic conditions, we propose this short‐term focused approach to include the unique viewpoints of families and patients whose care experience is confined to an acute period. Similarly, we propose that such an approach can efficiently include and rapidly react to input from other hard‐to‐engage key stakeholders such as field nurses.
Disclosures
This work was supported by the Patient Centered Outcomes Research Institute(HIS‐1306‐0081, SSS). The Patient Centered Outcomes Research Institute had no role in the design, preparation, review, or approval of the manuscript or in the decision to submit the manuscript for publication. The authors have no financial relationships relevant to this article to disclose. The authors report no potential conflicts of interest. The H2O study team members include the following: Katherine A. Auger, MD, MSc, JoAnne Bachus, BSN, Andrew F. Beck, MD, MPH, Monica L. Borell, BSN, Stephanie A. Brunswick, BS, Lenisa Chang, MA, PhD, Jennifer M. Gold, BSN, Judy A. Heilman, RN, Joseph A. Jabour, BS, Jane C. Khoury, PhD, Margo J. Moore, BSN, CCRP, Rita H. Pickler, PNP, PhD, Susan N. Sherman, DPA, Lauren G. Solan, MD, MEd, Angela M. Statile, MD, MEd, Heidi J. Sucharew, PhD, Karen P. Sullivan, BSN, Heather L. Tubbs‐Cooley, RN, PhD, Susan Wade‐Murphy, MSN, and Christine M. White, MD, MAT.
We believe that patients, families, and other stakeholders can provide meaningful contributions throughout the research process. Involvement of a diverse group of stakeholders is also encouraged by the Patient Centered Outcomes Research Institute (PCORI), which emphasizes research focused on patient‐ and family‐centered outcomes.[1] Patient and family engagement in healthcare, however, has generally focused on children and adults with chronic conditions.[1, 2] Engagement of families of children with serious acute illnesses is infrequent, and no studies have documented the feasibility or acceptability of different methods of family engagement.[3] Furthermore, stakeholders, such as nurses, may participate in study execution but rarely receive opportunities to inform the research process. In this Perspective, we describe our experiences with family engagement using a novel approach of serial, focused, short‐term engagement of stakeholders.
PRESTUDY WORK
In 2012, our institution introduced a nurse‐led transitional home‐visit program, an approach associated with reduced healthcare utilization in adults.[4] Patients hospitalized for acute illness received a 1‐time transitional home visit 24 to 72 hours after hospital discharge. We formed a multidisciplinary team, consisting of physicians, nurse scientists, home healthcare (HHC) nursing staff, patient families, and research staff to design a mixed‐methods study of the transitional home visit, which was funded by PCORI in 2014. This study, the Hospital‐to‐Home Outcomes (H2O) study, has 3 aims: (1) identify barriers to successful transitions home and outcomes of such transitions that are meaningful to families, (2) optimize the transitional home visits to address family‐identified barriers and outcomes, and (3) determine the efficacy of transitional home visits through a randomized control trial.
Two parents joined the study team during study development. Both had children hospitalized for acute illnesses, received a transitional home visit, and participated in a pilot focus group to provide insight into barriers families encounter during care transitions. These parents made valuable contributions, including recommending strategies for patient enrollment and retention. They also committed to participating in regularly scheduled study meetings and ad hoc discussions. However, feedback from the pilot focus group also highlighted a potential research engagement challenge; specifically, once the acute illness resolved, many families were primarily focused on the return to their normal routine and may not be easily engaged in research.
Based on family input, we included several mechanisms to engage caregivers of children with acute illness in the study design of H2O. Each design element allowed families and other stakeholders to contribute via short‐term focused approaches (eg, focus groups, phone surveys). These short‐term interactions drove iterative changes in study processes and approaches, including how to measure outcomes important to families. Rather than a small group of stakeholders making a series of recommendations over a long period of time, we had dozens of individual stakeholders make a few recommendations apiece that were quickly implemented and subsequently tested via feedback from the next few stakeholders (Figure 1).

PATIENT AND STAKEHOLDER ENGAGEMENT IN THE H2O STUDY
Having the short‐term, focused engagement strategy built into the study proved beneficial, when the 2 parents who were part of the initial design team and had planned to participate longitudinally were no longer able to participate. Over time, their circumstances changed. One parent moved out of the area to pursue a professional opportunity, and the second parent became increasingly difficult to reach and unable to join planned study meetings, a situation anticipated by the pilot focus group participants. These 2 instances illustrate challenges with long‐term engagement of families in research when the potential primary driver of their engagement, their child's acute illness, has resolved.
Short‐Term Focused Engagement Via Focus Groups With Parents/Caregivers
The first aim of the H2O study used 15 focus groups and semistructured interviews with parents/caregivers of recently discharged patients to identify barriers to and metrics of successful transitions of care from the hospital to home. The focus group question guide was developed by the research team and adapted as the focus groups progressed to incorporate new issues raised by participants. Analysis of focus group data revealed opportunities to improve the transitional home visit and identified outcomes important to families, including the need for emotional reassurance in the immediate period after discharge and the impact on family finances.
Short‐Term Focused Engagement Via Phone Calls With Parents/Caregivers
To continuously improve study processes and the transitional home visit during the second aim of H2O, we relied on short‐term focused engagement from 2 stakeholder groups, families and field nurses. We completed 107 phone calls with families who received a transitional home visit during the visit optimization period. These calls, completed 3 to 7 days after the visit, assessed parental perceptions of the effect of recent visit modifications through a standardized survey documented in an electronic database. These data were utilized in plan‐do‐study‐act cycles,[5] every 1 to 2 weeks, to determine if additional modifications to the visits were necessary. A cycle ended when the calls no longer provided new information. The questions asked on the calls also changed over time as different interventions were tested.
As an example, in aim 1, families highlighted the lack of clarity of discharge instructions, particularly regarding when and why to return for medical care. Thus, we developed condition‐specific red flag reminder cards to be shared at transitional home visits to help families remember and recognize concerning signs and symptoms and understand when additional evaluation may be warranted (Figure 2). Families in postvisit calls endorsed the concept of red flags, but sometimes preferred electronic rather than paper versions of the red flag cards to facilitate sharing with family members. Thus, we tested and refined texting the red flag information to families. Subsequent calls strongly supported this practice, so we will continue to use it during the third aim, the randomized trial of the transitional home visit.

The remaining calls (N=72) were completed 14 days after the visit to mirror the time frame for follow‐up calls in the planned randomized trial. These calls allowed us to test measurement of family‐identified outcomes and determine their usability in the trial. We used family feedback to shorten the survey and reorder questions. We also used feedback from these calls to develop an optimal call‐back strategy to maximize family contacts.
Short‐Term Focused Engagement Via Discussions With Nurses
We also incorporated feedback from HHC nurses on 60 visits to ensure that the visit modifications were feasible to implement. HHC nurse feedback, which aligned with aim 1 data from families, highlighted the potential benefits of standardizing the transitional home visit to be more condition specific. The nurses also provided ongoing ad hoc feedback on other changes to the transitional home visit, which indicated both when tests were successful and when they were challenging to implement. The study team wanted to ensure that the nurses performing the visits were involved in the modification process.
ONGOING H2O WORK AND CONCLUSION
The third aim, with ongoing patient enrollment, involves a randomized trial to determine the efficacy of the revised transitional home visit compared with standard of care as measured by subsequent healthcare utilization and outcomes suggested in aim 1 and refined during aim 2, such as parental coping, stress, and confidence in care. We have engaged 1 parent to provide longitudinal feedback during regularly scheduled meetings.
We believe that our short‐term, focused engagement strategies have allowed integration of the invaluable perspective of families and other stakeholders into our research questions, intervention design, outcome measurement, and study execution. Our approach combined short‐term engagement from many stakeholders, blending qualitative techniques with rapid‐cycle implementation methods to quickly react to stakeholder input. Given the challenge of sustaining longitudinal engagement of families in research focused on acute care questions, and the tendency for many families interested in such engagement to be well versed in the care system due to chronic conditions, we propose this short‐term focused approach to include the unique viewpoints of families and patients whose care experience is confined to an acute period. Similarly, we propose that such an approach can efficiently include and rapidly react to input from other hard‐to‐engage key stakeholders such as field nurses.
Disclosures
This work was supported by the Patient Centered Outcomes Research Institute(HIS‐1306‐0081, SSS). The Patient Centered Outcomes Research Institute had no role in the design, preparation, review, or approval of the manuscript or in the decision to submit the manuscript for publication. The authors have no financial relationships relevant to this article to disclose. The authors report no potential conflicts of interest. The H2O study team members include the following: Katherine A. Auger, MD, MSc, JoAnne Bachus, BSN, Andrew F. Beck, MD, MPH, Monica L. Borell, BSN, Stephanie A. Brunswick, BS, Lenisa Chang, MA, PhD, Jennifer M. Gold, BSN, Judy A. Heilman, RN, Joseph A. Jabour, BS, Jane C. Khoury, PhD, Margo J. Moore, BSN, CCRP, Rita H. Pickler, PNP, PhD, Susan N. Sherman, DPA, Lauren G. Solan, MD, MEd, Angela M. Statile, MD, MEd, Heidi J. Sucharew, PhD, Karen P. Sullivan, BSN, Heather L. Tubbs‐Cooley, RN, PhD, Susan Wade‐Murphy, MSN, and Christine M. White, MD, MAT.
- , , , et al. Conceptual and practical foundations of patient engagement in research at the patient‐centered outcomes research institute. Qual Life Res. 2015;24(5):1033–1041.
- , . A review of parent participation engagement in child and family mental health treatment. Clin Child Fam Psychol Rev. 2015;18(2):133–150.
- , , , et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89.
- , , , . Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251–260.
- , , , , , . The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA: Jossey‐Bass; 2009.
- , , , et al. Conceptual and practical foundations of patient engagement in research at the patient‐centered outcomes research institute. Qual Life Res. 2015;24(5):1033–1041.
- , . A review of parent participation engagement in child and family mental health treatment. Clin Child Fam Psychol Rev. 2015;18(2):133–150.
- , , , et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89.
- , , , . Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9:251–260.
- , , , , , . The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA: Jossey‐Bass; 2009.
The Puzzle of Posthospital Recovery
Admission to a hospital for acute care is often a puzzling and traumatic experience for patients.[1, 2] Even after overcoming important hurdles such as receiving the right diagnosis, being treated with appropriate therapies, and experiencing initial improvement, the ultimate goal of complete recovery after discharge remains elusive for many. Dozens of interventions have been tested to reduce failed recoveries and readmissions with mixed results. Most of these have relied on system‐level changes such as improved medication reconciliation and postdischarge phone calls.[3, 4] Physicians have largely been ignored in such efforts. Most systems leave it up to individual physicians to decide how much time and effort to invest in postdischarge care, and patient outcomes are often highly dependent on a physician's skill, interest, and experience.
We are both hospitalists who attend regularly on general internal medicine services in the United States and Canada. In that capacity, we have experienced many successes and failures in helping patients recover after discharge. This Perspective frames the problem of engaging both hospitalists and office‐based physicians in transitions of care within the current context of readmission reduction efforts, and proposes a more structured approach for integrating those physicians into postdischarge care to promote recovery. Although we also consider broader policy efforts to reduce fragmentation and misaligned incentives such as electronic health records (EHRs), accountable care organizations (ACOs), and the patient‐centered medical home (PCMH), our focus is on how these may (or may not) help front‐line physicians to solve the puzzle of posthospital recovery in the current state of affairs.
THE PROBLEMLACK OF TIME, VARIABLE ENGAGEMENT, SILOED COMMUNICATION
Perhaps the most important barrier to engaging physicians in the posthospital recovery phase is their limited time and energy. Today's rapid throughput and the complexity of acute care leave little bandwidth for issues that are not right in front of hospitalists. Once discharged, patients are often out of sight, out of mind.[5] Office‐based physicians face similar time constraints.[6] In both settings, physicians find themselves operating in silos with significant communication barriers that are time consuming and difficult to overcome.
There are many current policy efforts to break down these silos, a prominent example being recent incentives to speed the widespread use of EHRs. Although EHR implementation progress has been steady, nearly half of US hospitals still do not have a basic EHR, and more advanced functions required for sharing care summaries and allowing patients to access their EHR are not in place at most hospitals that have implemented basic EHRs already.[7] Furthermore, the state of implementation in office‐based settings lags even farther behind hospitals.[8] Finally, our personal experience working in systems with fully integrated EHR systems has suggested to us that sometimes more shared information simply becomes part of the problem, as it is far too easy to include too many complex details of hospitalization in discharge summaries.
Moreover, as front‐line hospitalists, we generally want to help with transitional issues that occur after patients have left our hospital, and we are very mindful of the tradition of the physician who takes responsibility for all aspects of their patients' care in all settings. Yet this tradition may be more representative of the 20th century ideal of continuity than the new continuity that we see emerging in the 21st century.[9] Thus, the question at hand now is how individual physicians should prioritize and execute these tasks without overreaching.
EFFECTS OF THE PROBLEM IN PRACTICEVARIATIONS IN PHYSICIAN ENGAGEMENT
Patient needs after discharge are not uniform, and risk prediction is still imprecise despite many studies.[10] Some patients need no help; others need only targeted help with specific gaps; still others need full‐time navigators to meaningfully reduce their risk of ending up back in the emergency department.[11] The goal is to piece together the resources required to create a complete picture of patient support; much like the way ones solves a jigsaw puzzle (Figure 1A). Despite best efforts, the gaps in careor missing pieces[12]may only become apparent after discharge. Recent research suggests physicians do not see the same gaps as patients do and agree on causes for readmission less than 50% of the time.[13, 14] Often, these gaps come to light when an outside pharmacist, home health nurse, or case manager reaches out to the hospital or primary care physician to address a new problem (Figure 1B). As frequent recipients of those calls for help, we are conflicted in our reaction. On the one hand, we want to know when our carefully crafted plans fall apart. On the other hand, neither of us looks forward to voice mail messages informing us that the specialist to whom we referred the patient for follow‐up never called with an appointment. Micromanaging this kind of care can be very frustrating, both when we are the first person called or resource of last resort.
Even when physicians do not feel burdened by postdischarge care, they may be ineffective due to a lack of experience or resources. These efforts can leave them feeling demoralized, which in turn may further discourage them from future engagement, solidifying a pattern of missing (or perhaps lost) pieces (Figure 1B). Too often, a well‐intentioned but underpowered effort becomes a solution crushed by the weight of the problem. Successful physician models for care coordination must balance competing ideals of the 1 doctor, 1 patient strategy that preserve continuity,[15] with the need to focus individual physicians' time on those postdischarge tasks in which their engagement is clearly needed.
Certain payment models, such as ACOs, may help catalyze specific solutions to these problems by creating incentives for better coordination at the organizational level (eg, hospitals, skilled nursing facilities, and clinics), but these incentives may not necessarily translate into changes in physician practice, particularly as physicians payments are not yet part of bundled hospital care payments.[16] Likewise, innovative practice models such as the PCMH have promise to reshape the way healthcare is delivered, particularly by fortifying the role of primary care providers; but again, we note the lack of specific guidance for providers, particularly hospitalists. The Agency for Healthcare Research and Quality defines care coordination as 1 of the 5 pillars of the PCMH, but notes considerable uncertainty about how to operationalize coordination around transitions from hospital care: A clearer understanding of, and research on, the optimal role of the PCMH in terms of leadership and care coordination in inpatient care is needed. Specifically, a better understanding of the possible approaches and the tradeoffs involved with eachin terms of access, quality, cost, and patient experiencewould be useful.[17] Early studies of these outcomes from both ACOs and PCMHs suggest improvements in some areas of patient and provider experience but not in others.[18, 19, 20, 21] Thus, we believe that although EHRs, ACOs, and PCMHs provide laudable and fundamentally necessary organizational changes to spur innovation and quality in transitions, more discussion about the specific roles for physicians is still needed. Though certainly not a definitive or exhaustive list, we provide a few specific suggestions for more effective physician engagement below.
ENABLING STRUCTURESAPPROACHES FOR MORE EFFECTIVE POSTDISCHARGE ENGAGEMENT
One approach for structuring physician participation is to create new roles for physicians as transitionalists,[22] extensivists,[23] or comprehensive‐care physicians[24] to help patients migrate from the volatile postacute period into a more stable state of recovery. Much as hospital‐based rapid response teams add a layer of additional expertise and availability without replacing the role of the attending physician, in this model, transitionalist or extensivist teams could respond to postacute issues in concert with inpatient and outpatient physicians of record.
Another approach could be to integrate the patients' hospitalists or primary care physicians into interprofessional teams modeled after hospital transfer centers, robust interdisciplinary teams that manage intense care‐coordination issues for complex inpatients. A similar approach could be used to elevate care transitions from hospital to homea postdischarge recovery center. In the same way that transfer centers develop ongoing relationships with referring hospitals and communities, postdischarge recovery centers will also need to develop working relationships with community resources like senior centers, transportation services, and the patients' physicians that provide ongoing care to be effective. A recent study of a similar concept (a virtual ward) [25] provides both a framework for this type of interprofessional collaboration and also caution in underestimating the dose or intensity of such interventions needed for those interventions to succeed. In that study, the interprofessional team was not fully integrated into the ecosystem in which patients lived, and providers frequently had difficulty communicating with the patients' ongoing caregivers, including both physicians and personal support workers.
Certainly, there are many other approaches that could be imagined, and there are pros and cons for those suggested here. Although some of these roles may seem like new types of physicians, which could worsen fragmentation, what we are suggesting is more akin to hybridization of current hospitalist and primary care provider roles. A first step could be just giving a name to the additional effort asked of these providers, and paying for time spent when they are not acting in either the inpatient attending or outpatient attending role but in the coordinating role. Fortunately, Medicare's new initiative to pay for chronic‐care management will allow physicians, clinics, and hospitals more flexibility to bill for such services that are not based on face‐to‐face encounters in the hospital or clinic.[26]
Moreover, although solving the puzzle of posthospital recovery cannot be fixed with hospitalist‐centric solutions alone, we believe more discourse is needed to define contributions from these physicians. Current policies, such as the PCMH, focus on the clinic and primary‐care providers, whereas the Medicare Readmission Reduction Program focuses on the hospital but not the hospitalist. Thus, there is a specific gap in engaging hospitalists in ongoing efforts to solve this puzzle and answer important questions about the specific role(s) of the hospitalist[27] as well as the primary care provider[28] in preventing readmissions and facilitating recovery. Certainly, integration of any new roles is needed to avoid fragmentation by default, and our suggestion of roles such as transitionalists or transfer center physicians are intended as examples to facilitate broader discussion about individual physician roles. As is often the case in healthcare, a 1 size fits all solution is unlikely, and a variety of complimentary roles may be needed to accommodate the diversity of patients and providers as well as the delivery systems where they interact.
CONCLUSION
Although the emphasis on interdisciplinary care and systems approaches in promoting recovery is welcome, individual physicians are usually overlooked in these discussions. Most physicians want to help but cannot simply do more in the absence of more creative and structured approaches. As a recent commentary on care transitions suggested, It's the how, not just the what.[29] We agree but would add, It's also about who. Thus, the time has come to engage physicians within care‐delivery models specifically designed to solve this puzzle. Although interprofessional teams are clearly needed, patients look to individuals who know them, not teams, when they run into trouble, and their first move is often to call the doctor. Because physicians play such an important role in the acute phase of illness, their struggles and efforts in the postacute phase need to be recognized and streamlined if we are to improve our patients' chances of full recovery.
Disclosure: Nothing to report.
- . Post‐hospital syndrome‐an acquired transient condition of generalized risk. N Engl J Med. 2013;368:2169–2170.
- , . Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169–2170.
- , , , et al. Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:433–440.
- , , , et al. Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , . “Out of sight, out of mind”: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
- . Instant replay—a quarterback's view of care coordination. N Engl J Med. 2014;371:489–491.
- , , , et al. More than half of US hospitals have at least a basic EHR, but stage 2 criteria remain challenging for most. Health Aff (Millwood). 2014;33(9):1664–1671.
- , , , , , . Despite substantial progress In EHR adoption, health information exchange and patient engagement remain low in office settings. Health Aff (Millwood). 2014;33(9):1672–1679.
- , . Understanding the value of continuity in the 21st century [published online May 18, 2015]. JAMA Intern Med. doi: 10.1001/jamainternmed.2015.1345.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , , . Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709–712.
- , , , et al. “Missing pieces”—functional, social, and environmental barriers to recovery for vulnerable older adults transitioning from hospital to home. J Am Geriatr Soc. 2014;62:1556–1561.
- , , , et al. Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870–1872.
- , , , et al. Challenges faced by patients with low socioeconomic status during the post‐hospital transition. J Gen Intern Med. 2014;29(2):283–289.
- , . Teaching physicians to care amid chaos. JAMA. 2013;309(10):987–988.
- , . Including physicians in bundled hospital care payments: time to revisit an old idea? JAMA. 2015;313(19):1907–1908.
- Agency for Healthcare Research and Quality. Coordinating care for adults with complex care needs in the patient‐centered medical home: challenges and solutions. Available at: http://www.pcmh.ahrq.gov/sites/default/files/attachments/Coordinating%20Care%20for%20Adults%20with%20Complex%20Care%20Needs.pdf. Accessed June 8, 2015.
- , , , . Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372(20):1927–1936.
- , , , . Changes in patients' experiences in Medicare Accountable Care Organizations. N Engl J Med. 2014;371(18):1715–1724.
- , , , , . Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311(8):815–825.
- , , , et al. Patient‐centered medical home intervention at an internal medicine resident safety‐net clinic. JAMA Intern Med. 2013;173(18):1694–1701.
- . Walking the walk in transitional care: the “hospitalist” role expands far beyond hospital walls. Today's Hospitalist. Available at: http://www.todayshospitalist.com/index.php?b=articles_read33(5):770–777.
- , , , et al. Effect of a post‐discharge virtual ward on readmission or death for high‐risk patients: a randomized clinical trial. JAMA. 2014;312:1305–1312.
- , , . Medicare and care coordination: expanding the clinician's toolbox. JAMA. 2015;313(8):797–798.
- . Hospitalists' responsibility, role in readmission prevention. The Hospitalist. Available at: http://www.the‐hospitalist.org/article/hospitalists‐responsibility‐role‐in‐readmission‐prevention. Published April 3, 2015. Accessed July 7, 2015.
- , . Bridging the hospitalist‐primary care divide through collaborative care. N Engl J Med. 2015;372(4):308–309.
- , . Care transitions: it's the how, not just the what. J Gen Intern Med. 2015;30(5):539–540.
Admission to a hospital for acute care is often a puzzling and traumatic experience for patients.[1, 2] Even after overcoming important hurdles such as receiving the right diagnosis, being treated with appropriate therapies, and experiencing initial improvement, the ultimate goal of complete recovery after discharge remains elusive for many. Dozens of interventions have been tested to reduce failed recoveries and readmissions with mixed results. Most of these have relied on system‐level changes such as improved medication reconciliation and postdischarge phone calls.[3, 4] Physicians have largely been ignored in such efforts. Most systems leave it up to individual physicians to decide how much time and effort to invest in postdischarge care, and patient outcomes are often highly dependent on a physician's skill, interest, and experience.
We are both hospitalists who attend regularly on general internal medicine services in the United States and Canada. In that capacity, we have experienced many successes and failures in helping patients recover after discharge. This Perspective frames the problem of engaging both hospitalists and office‐based physicians in transitions of care within the current context of readmission reduction efforts, and proposes a more structured approach for integrating those physicians into postdischarge care to promote recovery. Although we also consider broader policy efforts to reduce fragmentation and misaligned incentives such as electronic health records (EHRs), accountable care organizations (ACOs), and the patient‐centered medical home (PCMH), our focus is on how these may (or may not) help front‐line physicians to solve the puzzle of posthospital recovery in the current state of affairs.
THE PROBLEMLACK OF TIME, VARIABLE ENGAGEMENT, SILOED COMMUNICATION
Perhaps the most important barrier to engaging physicians in the posthospital recovery phase is their limited time and energy. Today's rapid throughput and the complexity of acute care leave little bandwidth for issues that are not right in front of hospitalists. Once discharged, patients are often out of sight, out of mind.[5] Office‐based physicians face similar time constraints.[6] In both settings, physicians find themselves operating in silos with significant communication barriers that are time consuming and difficult to overcome.
There are many current policy efforts to break down these silos, a prominent example being recent incentives to speed the widespread use of EHRs. Although EHR implementation progress has been steady, nearly half of US hospitals still do not have a basic EHR, and more advanced functions required for sharing care summaries and allowing patients to access their EHR are not in place at most hospitals that have implemented basic EHRs already.[7] Furthermore, the state of implementation in office‐based settings lags even farther behind hospitals.[8] Finally, our personal experience working in systems with fully integrated EHR systems has suggested to us that sometimes more shared information simply becomes part of the problem, as it is far too easy to include too many complex details of hospitalization in discharge summaries.
Moreover, as front‐line hospitalists, we generally want to help with transitional issues that occur after patients have left our hospital, and we are very mindful of the tradition of the physician who takes responsibility for all aspects of their patients' care in all settings. Yet this tradition may be more representative of the 20th century ideal of continuity than the new continuity that we see emerging in the 21st century.[9] Thus, the question at hand now is how individual physicians should prioritize and execute these tasks without overreaching.
EFFECTS OF THE PROBLEM IN PRACTICEVARIATIONS IN PHYSICIAN ENGAGEMENT
Patient needs after discharge are not uniform, and risk prediction is still imprecise despite many studies.[10] Some patients need no help; others need only targeted help with specific gaps; still others need full‐time navigators to meaningfully reduce their risk of ending up back in the emergency department.[11] The goal is to piece together the resources required to create a complete picture of patient support; much like the way ones solves a jigsaw puzzle (Figure 1A). Despite best efforts, the gaps in careor missing pieces[12]may only become apparent after discharge. Recent research suggests physicians do not see the same gaps as patients do and agree on causes for readmission less than 50% of the time.[13, 14] Often, these gaps come to light when an outside pharmacist, home health nurse, or case manager reaches out to the hospital or primary care physician to address a new problem (Figure 1B). As frequent recipients of those calls for help, we are conflicted in our reaction. On the one hand, we want to know when our carefully crafted plans fall apart. On the other hand, neither of us looks forward to voice mail messages informing us that the specialist to whom we referred the patient for follow‐up never called with an appointment. Micromanaging this kind of care can be very frustrating, both when we are the first person called or resource of last resort.

Even when physicians do not feel burdened by postdischarge care, they may be ineffective due to a lack of experience or resources. These efforts can leave them feeling demoralized, which in turn may further discourage them from future engagement, solidifying a pattern of missing (or perhaps lost) pieces (Figure 1B). Too often, a well‐intentioned but underpowered effort becomes a solution crushed by the weight of the problem. Successful physician models for care coordination must balance competing ideals of the 1 doctor, 1 patient strategy that preserve continuity,[15] with the need to focus individual physicians' time on those postdischarge tasks in which their engagement is clearly needed.
Certain payment models, such as ACOs, may help catalyze specific solutions to these problems by creating incentives for better coordination at the organizational level (eg, hospitals, skilled nursing facilities, and clinics), but these incentives may not necessarily translate into changes in physician practice, particularly as physicians payments are not yet part of bundled hospital care payments.[16] Likewise, innovative practice models such as the PCMH have promise to reshape the way healthcare is delivered, particularly by fortifying the role of primary care providers; but again, we note the lack of specific guidance for providers, particularly hospitalists. The Agency for Healthcare Research and Quality defines care coordination as 1 of the 5 pillars of the PCMH, but notes considerable uncertainty about how to operationalize coordination around transitions from hospital care: A clearer understanding of, and research on, the optimal role of the PCMH in terms of leadership and care coordination in inpatient care is needed. Specifically, a better understanding of the possible approaches and the tradeoffs involved with eachin terms of access, quality, cost, and patient experiencewould be useful.[17] Early studies of these outcomes from both ACOs and PCMHs suggest improvements in some areas of patient and provider experience but not in others.[18, 19, 20, 21] Thus, we believe that although EHRs, ACOs, and PCMHs provide laudable and fundamentally necessary organizational changes to spur innovation and quality in transitions, more discussion about the specific roles for physicians is still needed. Though certainly not a definitive or exhaustive list, we provide a few specific suggestions for more effective physician engagement below.
ENABLING STRUCTURESAPPROACHES FOR MORE EFFECTIVE POSTDISCHARGE ENGAGEMENT
One approach for structuring physician participation is to create new roles for physicians as transitionalists,[22] extensivists,[23] or comprehensive‐care physicians[24] to help patients migrate from the volatile postacute period into a more stable state of recovery. Much as hospital‐based rapid response teams add a layer of additional expertise and availability without replacing the role of the attending physician, in this model, transitionalist or extensivist teams could respond to postacute issues in concert with inpatient and outpatient physicians of record.
Another approach could be to integrate the patients' hospitalists or primary care physicians into interprofessional teams modeled after hospital transfer centers, robust interdisciplinary teams that manage intense care‐coordination issues for complex inpatients. A similar approach could be used to elevate care transitions from hospital to homea postdischarge recovery center. In the same way that transfer centers develop ongoing relationships with referring hospitals and communities, postdischarge recovery centers will also need to develop working relationships with community resources like senior centers, transportation services, and the patients' physicians that provide ongoing care to be effective. A recent study of a similar concept (a virtual ward) [25] provides both a framework for this type of interprofessional collaboration and also caution in underestimating the dose or intensity of such interventions needed for those interventions to succeed. In that study, the interprofessional team was not fully integrated into the ecosystem in which patients lived, and providers frequently had difficulty communicating with the patients' ongoing caregivers, including both physicians and personal support workers.
Certainly, there are many other approaches that could be imagined, and there are pros and cons for those suggested here. Although some of these roles may seem like new types of physicians, which could worsen fragmentation, what we are suggesting is more akin to hybridization of current hospitalist and primary care provider roles. A first step could be just giving a name to the additional effort asked of these providers, and paying for time spent when they are not acting in either the inpatient attending or outpatient attending role but in the coordinating role. Fortunately, Medicare's new initiative to pay for chronic‐care management will allow physicians, clinics, and hospitals more flexibility to bill for such services that are not based on face‐to‐face encounters in the hospital or clinic.[26]
Moreover, although solving the puzzle of posthospital recovery cannot be fixed with hospitalist‐centric solutions alone, we believe more discourse is needed to define contributions from these physicians. Current policies, such as the PCMH, focus on the clinic and primary‐care providers, whereas the Medicare Readmission Reduction Program focuses on the hospital but not the hospitalist. Thus, there is a specific gap in engaging hospitalists in ongoing efforts to solve this puzzle and answer important questions about the specific role(s) of the hospitalist[27] as well as the primary care provider[28] in preventing readmissions and facilitating recovery. Certainly, integration of any new roles is needed to avoid fragmentation by default, and our suggestion of roles such as transitionalists or transfer center physicians are intended as examples to facilitate broader discussion about individual physician roles. As is often the case in healthcare, a 1 size fits all solution is unlikely, and a variety of complimentary roles may be needed to accommodate the diversity of patients and providers as well as the delivery systems where they interact.
CONCLUSION
Although the emphasis on interdisciplinary care and systems approaches in promoting recovery is welcome, individual physicians are usually overlooked in these discussions. Most physicians want to help but cannot simply do more in the absence of more creative and structured approaches. As a recent commentary on care transitions suggested, It's the how, not just the what.[29] We agree but would add, It's also about who. Thus, the time has come to engage physicians within care‐delivery models specifically designed to solve this puzzle. Although interprofessional teams are clearly needed, patients look to individuals who know them, not teams, when they run into trouble, and their first move is often to call the doctor. Because physicians play such an important role in the acute phase of illness, their struggles and efforts in the postacute phase need to be recognized and streamlined if we are to improve our patients' chances of full recovery.
Disclosure: Nothing to report.
Admission to a hospital for acute care is often a puzzling and traumatic experience for patients.[1, 2] Even after overcoming important hurdles such as receiving the right diagnosis, being treated with appropriate therapies, and experiencing initial improvement, the ultimate goal of complete recovery after discharge remains elusive for many. Dozens of interventions have been tested to reduce failed recoveries and readmissions with mixed results. Most of these have relied on system‐level changes such as improved medication reconciliation and postdischarge phone calls.[3, 4] Physicians have largely been ignored in such efforts. Most systems leave it up to individual physicians to decide how much time and effort to invest in postdischarge care, and patient outcomes are often highly dependent on a physician's skill, interest, and experience.
We are both hospitalists who attend regularly on general internal medicine services in the United States and Canada. In that capacity, we have experienced many successes and failures in helping patients recover after discharge. This Perspective frames the problem of engaging both hospitalists and office‐based physicians in transitions of care within the current context of readmission reduction efforts, and proposes a more structured approach for integrating those physicians into postdischarge care to promote recovery. Although we also consider broader policy efforts to reduce fragmentation and misaligned incentives such as electronic health records (EHRs), accountable care organizations (ACOs), and the patient‐centered medical home (PCMH), our focus is on how these may (or may not) help front‐line physicians to solve the puzzle of posthospital recovery in the current state of affairs.
THE PROBLEMLACK OF TIME, VARIABLE ENGAGEMENT, SILOED COMMUNICATION
Perhaps the most important barrier to engaging physicians in the posthospital recovery phase is their limited time and energy. Today's rapid throughput and the complexity of acute care leave little bandwidth for issues that are not right in front of hospitalists. Once discharged, patients are often out of sight, out of mind.[5] Office‐based physicians face similar time constraints.[6] In both settings, physicians find themselves operating in silos with significant communication barriers that are time consuming and difficult to overcome.
There are many current policy efforts to break down these silos, a prominent example being recent incentives to speed the widespread use of EHRs. Although EHR implementation progress has been steady, nearly half of US hospitals still do not have a basic EHR, and more advanced functions required for sharing care summaries and allowing patients to access their EHR are not in place at most hospitals that have implemented basic EHRs already.[7] Furthermore, the state of implementation in office‐based settings lags even farther behind hospitals.[8] Finally, our personal experience working in systems with fully integrated EHR systems has suggested to us that sometimes more shared information simply becomes part of the problem, as it is far too easy to include too many complex details of hospitalization in discharge summaries.
Moreover, as front‐line hospitalists, we generally want to help with transitional issues that occur after patients have left our hospital, and we are very mindful of the tradition of the physician who takes responsibility for all aspects of their patients' care in all settings. Yet this tradition may be more representative of the 20th century ideal of continuity than the new continuity that we see emerging in the 21st century.[9] Thus, the question at hand now is how individual physicians should prioritize and execute these tasks without overreaching.
EFFECTS OF THE PROBLEM IN PRACTICEVARIATIONS IN PHYSICIAN ENGAGEMENT
Patient needs after discharge are not uniform, and risk prediction is still imprecise despite many studies.[10] Some patients need no help; others need only targeted help with specific gaps; still others need full‐time navigators to meaningfully reduce their risk of ending up back in the emergency department.[11] The goal is to piece together the resources required to create a complete picture of patient support; much like the way ones solves a jigsaw puzzle (Figure 1A). Despite best efforts, the gaps in careor missing pieces[12]may only become apparent after discharge. Recent research suggests physicians do not see the same gaps as patients do and agree on causes for readmission less than 50% of the time.[13, 14] Often, these gaps come to light when an outside pharmacist, home health nurse, or case manager reaches out to the hospital or primary care physician to address a new problem (Figure 1B). As frequent recipients of those calls for help, we are conflicted in our reaction. On the one hand, we want to know when our carefully crafted plans fall apart. On the other hand, neither of us looks forward to voice mail messages informing us that the specialist to whom we referred the patient for follow‐up never called with an appointment. Micromanaging this kind of care can be very frustrating, both when we are the first person called or resource of last resort.

Even when physicians do not feel burdened by postdischarge care, they may be ineffective due to a lack of experience or resources. These efforts can leave them feeling demoralized, which in turn may further discourage them from future engagement, solidifying a pattern of missing (or perhaps lost) pieces (Figure 1B). Too often, a well‐intentioned but underpowered effort becomes a solution crushed by the weight of the problem. Successful physician models for care coordination must balance competing ideals of the 1 doctor, 1 patient strategy that preserve continuity,[15] with the need to focus individual physicians' time on those postdischarge tasks in which their engagement is clearly needed.
Certain payment models, such as ACOs, may help catalyze specific solutions to these problems by creating incentives for better coordination at the organizational level (eg, hospitals, skilled nursing facilities, and clinics), but these incentives may not necessarily translate into changes in physician practice, particularly as physicians payments are not yet part of bundled hospital care payments.[16] Likewise, innovative practice models such as the PCMH have promise to reshape the way healthcare is delivered, particularly by fortifying the role of primary care providers; but again, we note the lack of specific guidance for providers, particularly hospitalists. The Agency for Healthcare Research and Quality defines care coordination as 1 of the 5 pillars of the PCMH, but notes considerable uncertainty about how to operationalize coordination around transitions from hospital care: A clearer understanding of, and research on, the optimal role of the PCMH in terms of leadership and care coordination in inpatient care is needed. Specifically, a better understanding of the possible approaches and the tradeoffs involved with eachin terms of access, quality, cost, and patient experiencewould be useful.[17] Early studies of these outcomes from both ACOs and PCMHs suggest improvements in some areas of patient and provider experience but not in others.[18, 19, 20, 21] Thus, we believe that although EHRs, ACOs, and PCMHs provide laudable and fundamentally necessary organizational changes to spur innovation and quality in transitions, more discussion about the specific roles for physicians is still needed. Though certainly not a definitive or exhaustive list, we provide a few specific suggestions for more effective physician engagement below.
ENABLING STRUCTURESAPPROACHES FOR MORE EFFECTIVE POSTDISCHARGE ENGAGEMENT
One approach for structuring physician participation is to create new roles for physicians as transitionalists,[22] extensivists,[23] or comprehensive‐care physicians[24] to help patients migrate from the volatile postacute period into a more stable state of recovery. Much as hospital‐based rapid response teams add a layer of additional expertise and availability without replacing the role of the attending physician, in this model, transitionalist or extensivist teams could respond to postacute issues in concert with inpatient and outpatient physicians of record.
Another approach could be to integrate the patients' hospitalists or primary care physicians into interprofessional teams modeled after hospital transfer centers, robust interdisciplinary teams that manage intense care‐coordination issues for complex inpatients. A similar approach could be used to elevate care transitions from hospital to homea postdischarge recovery center. In the same way that transfer centers develop ongoing relationships with referring hospitals and communities, postdischarge recovery centers will also need to develop working relationships with community resources like senior centers, transportation services, and the patients' physicians that provide ongoing care to be effective. A recent study of a similar concept (a virtual ward) [25] provides both a framework for this type of interprofessional collaboration and also caution in underestimating the dose or intensity of such interventions needed for those interventions to succeed. In that study, the interprofessional team was not fully integrated into the ecosystem in which patients lived, and providers frequently had difficulty communicating with the patients' ongoing caregivers, including both physicians and personal support workers.
Certainly, there are many other approaches that could be imagined, and there are pros and cons for those suggested here. Although some of these roles may seem like new types of physicians, which could worsen fragmentation, what we are suggesting is more akin to hybridization of current hospitalist and primary care provider roles. A first step could be just giving a name to the additional effort asked of these providers, and paying for time spent when they are not acting in either the inpatient attending or outpatient attending role but in the coordinating role. Fortunately, Medicare's new initiative to pay for chronic‐care management will allow physicians, clinics, and hospitals more flexibility to bill for such services that are not based on face‐to‐face encounters in the hospital or clinic.[26]
Moreover, although solving the puzzle of posthospital recovery cannot be fixed with hospitalist‐centric solutions alone, we believe more discourse is needed to define contributions from these physicians. Current policies, such as the PCMH, focus on the clinic and primary‐care providers, whereas the Medicare Readmission Reduction Program focuses on the hospital but not the hospitalist. Thus, there is a specific gap in engaging hospitalists in ongoing efforts to solve this puzzle and answer important questions about the specific role(s) of the hospitalist[27] as well as the primary care provider[28] in preventing readmissions and facilitating recovery. Certainly, integration of any new roles is needed to avoid fragmentation by default, and our suggestion of roles such as transitionalists or transfer center physicians are intended as examples to facilitate broader discussion about individual physician roles. As is often the case in healthcare, a 1 size fits all solution is unlikely, and a variety of complimentary roles may be needed to accommodate the diversity of patients and providers as well as the delivery systems where they interact.
CONCLUSION
Although the emphasis on interdisciplinary care and systems approaches in promoting recovery is welcome, individual physicians are usually overlooked in these discussions. Most physicians want to help but cannot simply do more in the absence of more creative and structured approaches. As a recent commentary on care transitions suggested, It's the how, not just the what.[29] We agree but would add, It's also about who. Thus, the time has come to engage physicians within care‐delivery models specifically designed to solve this puzzle. Although interprofessional teams are clearly needed, patients look to individuals who know them, not teams, when they run into trouble, and their first move is often to call the doctor. Because physicians play such an important role in the acute phase of illness, their struggles and efforts in the postacute phase need to be recognized and streamlined if we are to improve our patients' chances of full recovery.
Disclosure: Nothing to report.
- . Post‐hospital syndrome‐an acquired transient condition of generalized risk. N Engl J Med. 2013;368:2169–2170.
- , . Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169–2170.
- , , , et al. Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:433–440.
- , , , et al. Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , . “Out of sight, out of mind”: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
- . Instant replay—a quarterback's view of care coordination. N Engl J Med. 2014;371:489–491.
- , , , et al. More than half of US hospitals have at least a basic EHR, but stage 2 criteria remain challenging for most. Health Aff (Millwood). 2014;33(9):1664–1671.
- , , , , , . Despite substantial progress In EHR adoption, health information exchange and patient engagement remain low in office settings. Health Aff (Millwood). 2014;33(9):1672–1679.
- , . Understanding the value of continuity in the 21st century [published online May 18, 2015]. JAMA Intern Med. doi: 10.1001/jamainternmed.2015.1345.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , , . Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709–712.
- , , , et al. “Missing pieces”—functional, social, and environmental barriers to recovery for vulnerable older adults transitioning from hospital to home. J Am Geriatr Soc. 2014;62:1556–1561.
- , , , et al. Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870–1872.
- , , , et al. Challenges faced by patients with low socioeconomic status during the post‐hospital transition. J Gen Intern Med. 2014;29(2):283–289.
- , . Teaching physicians to care amid chaos. JAMA. 2013;309(10):987–988.
- , . Including physicians in bundled hospital care payments: time to revisit an old idea? JAMA. 2015;313(19):1907–1908.
- Agency for Healthcare Research and Quality. Coordinating care for adults with complex care needs in the patient‐centered medical home: challenges and solutions. Available at: http://www.pcmh.ahrq.gov/sites/default/files/attachments/Coordinating%20Care%20for%20Adults%20with%20Complex%20Care%20Needs.pdf. Accessed June 8, 2015.
- , , , . Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372(20):1927–1936.
- , , , . Changes in patients' experiences in Medicare Accountable Care Organizations. N Engl J Med. 2014;371(18):1715–1724.
- , , , , . Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311(8):815–825.
- , , , et al. Patient‐centered medical home intervention at an internal medicine resident safety‐net clinic. JAMA Intern Med. 2013;173(18):1694–1701.
- . Walking the walk in transitional care: the “hospitalist” role expands far beyond hospital walls. Today's Hospitalist. Available at: http://www.todayshospitalist.com/index.php?b=articles_read33(5):770–777.
- , , , et al. Effect of a post‐discharge virtual ward on readmission or death for high‐risk patients: a randomized clinical trial. JAMA. 2014;312:1305–1312.
- , , . Medicare and care coordination: expanding the clinician's toolbox. JAMA. 2015;313(8):797–798.
- . Hospitalists' responsibility, role in readmission prevention. The Hospitalist. Available at: http://www.the‐hospitalist.org/article/hospitalists‐responsibility‐role‐in‐readmission‐prevention. Published April 3, 2015. Accessed July 7, 2015.
- , . Bridging the hospitalist‐primary care divide through collaborative care. N Engl J Med. 2015;372(4):308–309.
- , . Care transitions: it's the how, not just the what. J Gen Intern Med. 2015;30(5):539–540.
- . Post‐hospital syndrome‐an acquired transient condition of generalized risk. N Engl J Med. 2013;368:2169–2170.
- , . Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169–2170.
- , , , et al. Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:433–440.
- , , , et al. Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , . “Out of sight, out of mind”: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
- . Instant replay—a quarterback's view of care coordination. N Engl J Med. 2014;371:489–491.
- , , , et al. More than half of US hospitals have at least a basic EHR, but stage 2 criteria remain challenging for most. Health Aff (Millwood). 2014;33(9):1664–1671.
- , , , , , . Despite substantial progress In EHR adoption, health information exchange and patient engagement remain low in office settings. Health Aff (Millwood). 2014;33(9):1672–1679.
- , . Understanding the value of continuity in the 21st century [published online May 18, 2015]. JAMA Intern Med. doi: 10.1001/jamainternmed.2015.1345.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , , . Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709–712.
- , , , et al. “Missing pieces”—functional, social, and environmental barriers to recovery for vulnerable older adults transitioning from hospital to home. J Am Geriatr Soc. 2014;62:1556–1561.
- , , , et al. Readmissions in the era of patient engagement. JAMA Intern Med. 2014;174(11):1870–1872.
- , , , et al. Challenges faced by patients with low socioeconomic status during the post‐hospital transition. J Gen Intern Med. 2014;29(2):283–289.
- , . Teaching physicians to care amid chaos. JAMA. 2013;309(10):987–988.
- , . Including physicians in bundled hospital care payments: time to revisit an old idea? JAMA. 2015;313(19):1907–1908.
- Agency for Healthcare Research and Quality. Coordinating care for adults with complex care needs in the patient‐centered medical home: challenges and solutions. Available at: http://www.pcmh.ahrq.gov/sites/default/files/attachments/Coordinating%20Care%20for%20Adults%20with%20Complex%20Care%20Needs.pdf. Accessed June 8, 2015.
- , , , . Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372(20):1927–1936.
- , , , . Changes in patients' experiences in Medicare Accountable Care Organizations. N Engl J Med. 2014;371(18):1715–1724.
- , , , , . Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311(8):815–825.
- , , , et al. Patient‐centered medical home intervention at an internal medicine resident safety‐net clinic. JAMA Intern Med. 2013;173(18):1694–1701.
- . Walking the walk in transitional care: the “hospitalist” role expands far beyond hospital walls. Today's Hospitalist. Available at: http://www.todayshospitalist.com/index.php?b=articles_read33(5):770–777.
- , , , et al. Effect of a post‐discharge virtual ward on readmission or death for high‐risk patients: a randomized clinical trial. JAMA. 2014;312:1305–1312.
- , , . Medicare and care coordination: expanding the clinician's toolbox. JAMA. 2015;313(8):797–798.
- . Hospitalists' responsibility, role in readmission prevention. The Hospitalist. Available at: http://www.the‐hospitalist.org/article/hospitalists‐responsibility‐role‐in‐readmission‐prevention. Published April 3, 2015. Accessed July 7, 2015.
- , . Bridging the hospitalist‐primary care divide through collaborative care. N Engl J Med. 2015;372(4):308–309.
- , . Care transitions: it's the how, not just the what. J Gen Intern Med. 2015;30(5):539–540.
Hospitalists' Role in the Ebola Response
Concern and fear over an infection and how best to contain its spread is a well‐known storyline dating back centuries before the germ theory was hypothesized. During the Plague of Athens in the fourth century bc, upon noticing that physicians and caregivers of the sick were most at risk of dying, the Greek historian and philosopher Thucydides wrote that thwarting the disease likely required more practical measures than simple prayer to the gods.[1] To this day, the fears of contracting disease often outstripor worse, overrideour practical or scientific understanding.
Ebola has rekindled past concerns about disease transmission, whether real or imagined. In this article we will describe the history of Ebola outside the United States as well as recent events in US hospitals. We will review guidelines for how to prepare hospitals to treat potential Ebola patients and highlight the key role that hospitalists can have in ensuring the safety of their patients and coworkers. We will also describe the emerging role of global health hospitalists, whose numbers are growing and whose expertise is ideally suited to improving hospital care in developing countries.
EBOLA EPIDEMICS AND HEALTH EQUITY
In the first identified Ebola outbreak in 1976 in Zaire, now the Democratic Republic of the Congo, patients arrived at local hospitals with symptoms that resembled common endemic illnesses like malaria, typhoid, and yellow fever. The ensuing deaths of 11 of the 17 staff members at 1 hospital were only an introduction to the deadly consequences of Ebola and the necessity of vigilant infection control. Unfortunately, not much has changed with each subsequent outbreak; to this day the heralding of an Ebola outbreak denotes the death of healthcare workers. Since the discovery of the Ebola virus 38 years ago, there have been nearly 30 recorded Ebola outbreaks. The mortality rates of prior outbreaks range widely between 25% and 90%; reports of the current outbreak's mortality falls within that range, between 50% and 70%. It is important to note that despite this high mortality, there had been no more than 1600 Ebola deaths worldwide before the 2014 West African pandemic.[2] In comparison, the number of deaths in Guinea, Sierra Leone, and Liberia approaches 6000.[3]
Although these disturbing figures have fueled public fear and panic in the United States, we wonder how many of these deaths were actually preventable. At the epicenter of the current Ebola virus disease (EVD) outbreak are 3 countries especially vulnerable to epidemics, with armed conflict recently crippling the health systems that have now just started to rebuild. When Liberia emerged from its second civil war in 2003, they had barely 50 physicians caring for a country of nearly 4 million.[4] In contrast with the devastating death toll in Liberia, it perhaps should not come as a surprise that out of the 10 known cases of Ebola that were treated in the United States and caught early, none have resulted in death. With an advanced healthcare infrastructure in place, the mortality rate for EVD in the United States has been close to zero. Although the true unpreventable case fatality rate in West Africa is unknown, the positive outcomes in the United States cases illustrate that hospitals can treat EVD successfully, provided that they are well staffed, supplied, and prepared.
Furthermore, the stark difference in access to quality healthcare between nations suggests that combating the virus should not be our only concern. In fact, EVD itself is only an acute symptom of a much larger, chronic problem with the health systems in Guinea, Sierra Leone, and Liberia. Western countries must direct more resources and attention to improving the overall quality of care in these countries, for although the underlying inequities may be limited to places like Guinea, Liberia, and Sierra Leone, the resulting threat is a global one.
PREPARING FOR THE FIGHT AGAINST EBOLA IN THE UNITED STATES
On September 25, 2014, Thomas Eric Duncan presented to Texas Health Presbyterian Hospital with fever, abdominal pain, and headache 11 days after transporting a pregnant neighbor in his home country of Liberia who later died of EVD. Duncan was discharged from the emergency room without admission. Three days later, on September 28, 2014, an ambulance was called and transported him to Texas Health where he was admitted. He was isolated and the hospital followed existing Centers for Disease Control and Prevention (CDC) guidelines for infection control. He was confirmed to have EVD on September 30, 2014. He was cared for by the healthcare providers at Texas Health Presbyterian Hospital, but despite their care, his condition deteriorated. Eric Duncan died on October 8, 2014. About 120 healthcare workers came into contact with the patient. None of his 48 contacts prior to admission to the hospital contracted the disease. However, 2 nurses who cared for him during his admission developed symptoms and were confirmed to have Ebola. An alarm was sounded across the United States, awakening hospitals to the reality of their vulnerability and need for preparedness.[5]
For any healthcare worker treating EVD, whether working in a hospital here in the United States or an Ebola treatment unit (ETU) in West Africa, intensive training is necessary to keep oneself safe. Although infection control is not a novel concept, the stakes have undoubtedly been raised, as even the smallest misstep can be deadly when dealing with the Ebola virus. We were participants in a 3‐day infection control training program conducted by the CDC in Anniston, Alabama, which aimed to prepare healthcare workers to assist with the Ebola response in West Africa. Throughout the training, we repeatedly donned and doffed personal protective equipment (PPE), following protocols established by the World Health Organization and Doctors Without Borders (Mdecins Sans Frontires):
- Use a combination of contact, droplet, and standard precautions, ensuring no area of skin is left uncovered.
- Enter and exit with a buddy; inspect one another for breaches at each step of donning, caring for the patient, and doffing.
- Wash gloved hands with 0.5% chlorine bleach between tasks and patients.
- Exit at the slightest breach of infection protocol.
- Doff PPE per protocol and under supervision; take great care not to contaminate oneself.
Doffing is considered the most difficult and also the most important part, with 7 pieces to remove and no less than 20 steps to do so. After 3 full days of training, although we felt more confident with the carefully choreographed movements of donning and doffing, we were also more aware of the many opportunities during which a breach could occur. Despite our repeated practice, the training staff was firm in telling us that we were still not prepared to work in an ETU. We had only undergone what could be called a cold training. To work in the hot zone of the ETUs, it is essential to undergo additional mentorship and training once in the field. We believe a similar approach to extensive training should be employed for all healthcare workers on the front lines of an Ebola response, both in the United States and abroad.
Given the complexity of the infection control practices above, questions have appropriately been raised about US healthcare facilities' aptitude at providing care for patients with EVD. Although hospitals have historically been focal point(s) for dissemination of infection of Ebola, this does not have to be the case.[6] With appropriate preparedness, training, and understanding of facility limitations, hospitals can also be places of confidence and healing.
Both Emory University and the University of Nebraska have successfully treated multiple Ebola patients without incurring further transmission.[7, 8] Much of their success comes from the work of longitudinal teams that are trained in infectious disease response on a regular basis, despite the rare occurrence of a serious outbreak. When Emory scaled up this team upon receiving a confirmed EVD patient, new members were required to pass a proficiency test prior to providing care. Emory's strict adherence to infection control and advanced preparation serve as a model for other institutions.
It is important to recognize that not all hospitals should be fully equipped and staffed for safe care of a patient with EVD. Much like in cases of high‐level trauma, regional centers should be identified, prepared, and available to be called upon in a time of need (Figure 1). At the writing of this article, guidelines for hospitals are evolving; most states, with input from federal and local officials, are moving toward designating regional referral centers. Although the number of designated facilities and standards for each vary by state, general preparedness for care of a patient with suspected or confirmed EVD should include: (1) a designated and trained care team, (2) appropriate and vetted operational protocols, (3) an assigned isolation unit with adequate space for the necessary specialized precautions, (4) separate laboratory and diagnostic capabilities, and (5) a working waste management plan.[9] Although not all facilities throughout the United States will have the capability to care for a patient with EVD, all hospitals must have an action plan.
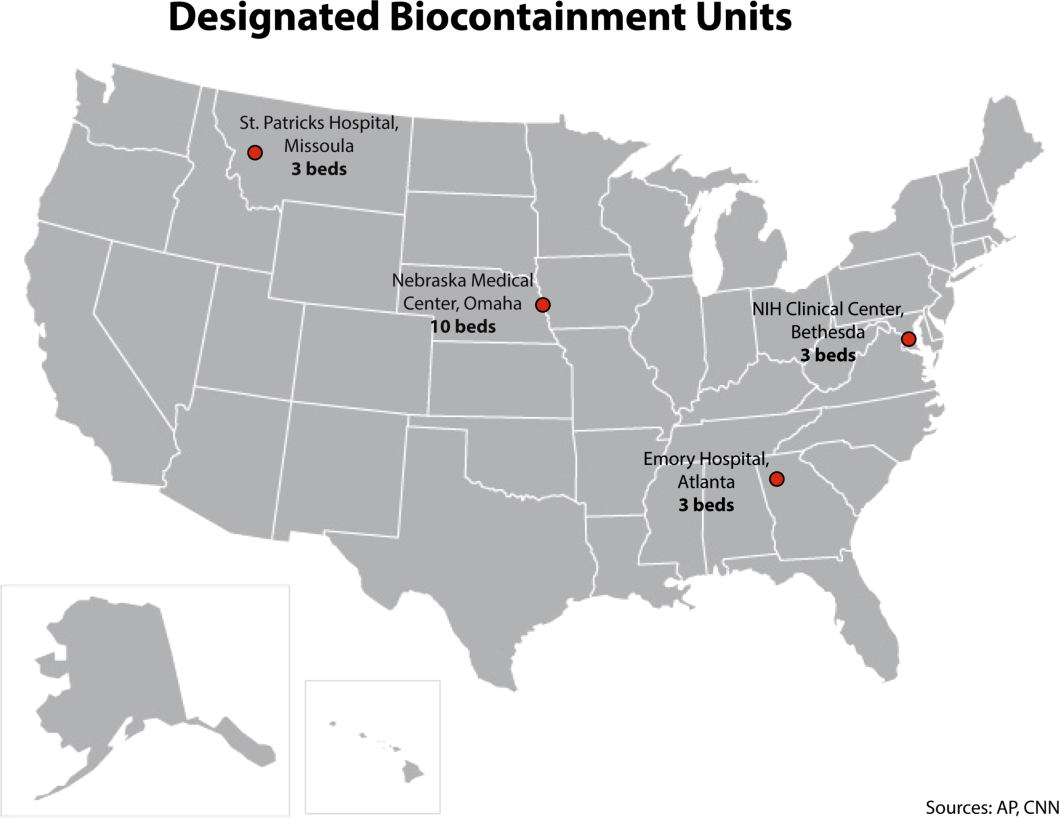
Triage is the first step; facilities should have well‐informed and trained staff prepared to perform assessments of potential cases safely (Figure 2). The ability to temporarily isolate and then efficiently transfer suspected or confirmed EVD patients to an appropriate facility also requires careful planning. Prompt identification, constant vigilance, and accurate histories all serve as stepping stones to a successful outcome and can protect employees and other patients in the process.
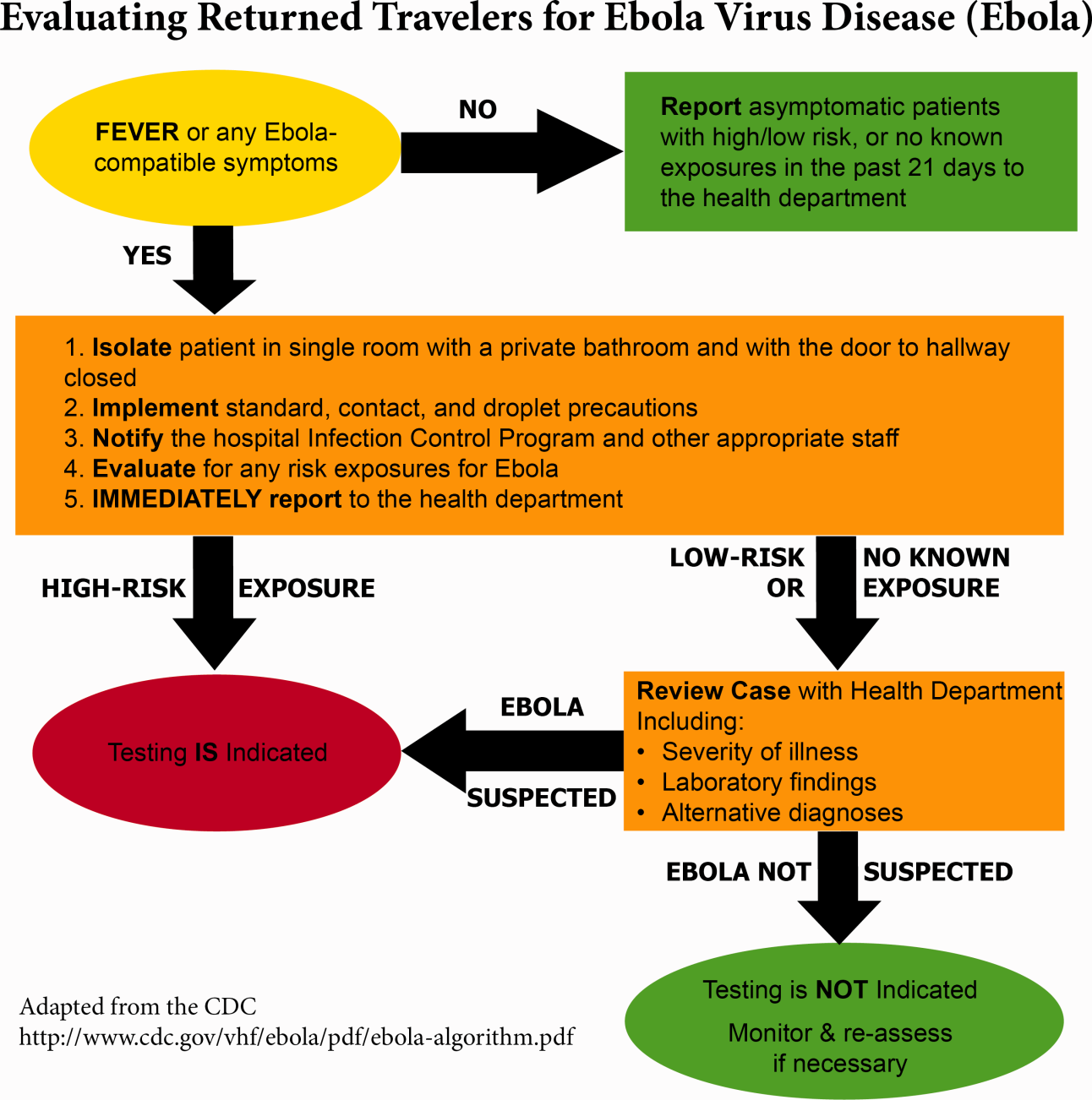
Within this proposed structure, hospitalists are uniquely equipped to play a leading role in EVD response. First, hospitalists are frequently responsible for interfacility transfer. Their intimate knowledge of this process and the collegiality they have fostered through its use are both assets in navigating the tiered referral system outlined above. Furthermore, as point people in the acute‐care setting who accept patients from emergency medicine colleagues, discuss cases with various consultants, and coordinate discharges with multidisciplinary teams on a daily basis, hospitalists have honed communication and leadership skills that are highly valuable in collaborative efforts. Finally, many hospitalists are also champions of quality and safety in their institutions. The focus on the process of continuous improvement is critical in the face of evolving challenges such as EVD.
GLOBAL HEALTH HOSPITALISTS
The Ebola outbreak is a global crisis that clearly illustrates the challenges of addressing highly infectious diseases in modern times. We have outlined the role of hospitalists and defined the steps toward adequate preparedness within our facilities. By following the measured and practical actions Thucydides advised, we can further strengthen our health systems and work toward the control of this outbreak in the United States. The important role of hospitalists can and should be extended to serve those in poor countries.
Over the last decade, health systems strengthening, health workforce training, and patient safety have come to the forefront of global health priorities, the same priorities that are also at the forefront of many hospitalists' aspirations. Shoeb et al. recently conducted a survey of global health hospitalists. They found that within the framework of global health, hospitalists are uniquely positioned to contribute to this growing field, particularly in areas such as quality improvement, safety, systems thinking, and medical education, all strengths of the hospital medicine model that could be translated to resource‐limited settings.[10]
We believe that hospitalists, with their unique positions and skills, are natural and necessary agents of action in this time of need. Our ultimate aim is for hospitalists to become actively engaged in addressing not only the current Ebola outbreak, but also the extreme inequity of healthcare globallyan inequity that lies at the heart of the current devastation in West Africa. We call upon hospital medicine leaders, from division chiefs to chairs of medicine to chief medical officers to support, encourage, and incentivize their staff and faculty to join the ranks of global health hospitalists as they work to both end gross inequities in healthcare abroad and protect our patients and ourselves back home.
Acknowledgements
The authors are grateful for Brett Lewis' assistance in the preparation of this article.
Disclosure
Nothing to report.
- . Infection control throughout history. Lancet Infect Dis. 2014;14(4):280.
- , . Ebola then and now. N Engl J Med. 2014;371:1663–1666.
- World Health Organization. Ebola response roadmap—situation report. Available at: http://www.who.int/csr/disease/ebola/situation‐reports/en/. Accessed November 2014.
- , . Effect of civil war on medical education in Liberia. Int J Emerg Med. 2011;4:6.
- , . Is the U.S. prepared for an Ebola outbreak? New York Times. October 10, 2014. Available at: http://www.nytimes.com/interactive/2014/10/09/us/is-the-us-prepared-for-an-ebola-outbreak.html?module=Search61(6):997–1003.
- . With good hospital practices, Emory rises to Ebola challenge. Kaiser Health News. U.S. News 8(3):162–163.
Concern and fear over an infection and how best to contain its spread is a well‐known storyline dating back centuries before the germ theory was hypothesized. During the Plague of Athens in the fourth century bc, upon noticing that physicians and caregivers of the sick were most at risk of dying, the Greek historian and philosopher Thucydides wrote that thwarting the disease likely required more practical measures than simple prayer to the gods.[1] To this day, the fears of contracting disease often outstripor worse, overrideour practical or scientific understanding.
Ebola has rekindled past concerns about disease transmission, whether real or imagined. In this article we will describe the history of Ebola outside the United States as well as recent events in US hospitals. We will review guidelines for how to prepare hospitals to treat potential Ebola patients and highlight the key role that hospitalists can have in ensuring the safety of their patients and coworkers. We will also describe the emerging role of global health hospitalists, whose numbers are growing and whose expertise is ideally suited to improving hospital care in developing countries.
EBOLA EPIDEMICS AND HEALTH EQUITY
In the first identified Ebola outbreak in 1976 in Zaire, now the Democratic Republic of the Congo, patients arrived at local hospitals with symptoms that resembled common endemic illnesses like malaria, typhoid, and yellow fever. The ensuing deaths of 11 of the 17 staff members at 1 hospital were only an introduction to the deadly consequences of Ebola and the necessity of vigilant infection control. Unfortunately, not much has changed with each subsequent outbreak; to this day the heralding of an Ebola outbreak denotes the death of healthcare workers. Since the discovery of the Ebola virus 38 years ago, there have been nearly 30 recorded Ebola outbreaks. The mortality rates of prior outbreaks range widely between 25% and 90%; reports of the current outbreak's mortality falls within that range, between 50% and 70%. It is important to note that despite this high mortality, there had been no more than 1600 Ebola deaths worldwide before the 2014 West African pandemic.[2] In comparison, the number of deaths in Guinea, Sierra Leone, and Liberia approaches 6000.[3]
Although these disturbing figures have fueled public fear and panic in the United States, we wonder how many of these deaths were actually preventable. At the epicenter of the current Ebola virus disease (EVD) outbreak are 3 countries especially vulnerable to epidemics, with armed conflict recently crippling the health systems that have now just started to rebuild. When Liberia emerged from its second civil war in 2003, they had barely 50 physicians caring for a country of nearly 4 million.[4] In contrast with the devastating death toll in Liberia, it perhaps should not come as a surprise that out of the 10 known cases of Ebola that were treated in the United States and caught early, none have resulted in death. With an advanced healthcare infrastructure in place, the mortality rate for EVD in the United States has been close to zero. Although the true unpreventable case fatality rate in West Africa is unknown, the positive outcomes in the United States cases illustrate that hospitals can treat EVD successfully, provided that they are well staffed, supplied, and prepared.
Furthermore, the stark difference in access to quality healthcare between nations suggests that combating the virus should not be our only concern. In fact, EVD itself is only an acute symptom of a much larger, chronic problem with the health systems in Guinea, Sierra Leone, and Liberia. Western countries must direct more resources and attention to improving the overall quality of care in these countries, for although the underlying inequities may be limited to places like Guinea, Liberia, and Sierra Leone, the resulting threat is a global one.
PREPARING FOR THE FIGHT AGAINST EBOLA IN THE UNITED STATES
On September 25, 2014, Thomas Eric Duncan presented to Texas Health Presbyterian Hospital with fever, abdominal pain, and headache 11 days after transporting a pregnant neighbor in his home country of Liberia who later died of EVD. Duncan was discharged from the emergency room without admission. Three days later, on September 28, 2014, an ambulance was called and transported him to Texas Health where he was admitted. He was isolated and the hospital followed existing Centers for Disease Control and Prevention (CDC) guidelines for infection control. He was confirmed to have EVD on September 30, 2014. He was cared for by the healthcare providers at Texas Health Presbyterian Hospital, but despite their care, his condition deteriorated. Eric Duncan died on October 8, 2014. About 120 healthcare workers came into contact with the patient. None of his 48 contacts prior to admission to the hospital contracted the disease. However, 2 nurses who cared for him during his admission developed symptoms and were confirmed to have Ebola. An alarm was sounded across the United States, awakening hospitals to the reality of their vulnerability and need for preparedness.[5]
For any healthcare worker treating EVD, whether working in a hospital here in the United States or an Ebola treatment unit (ETU) in West Africa, intensive training is necessary to keep oneself safe. Although infection control is not a novel concept, the stakes have undoubtedly been raised, as even the smallest misstep can be deadly when dealing with the Ebola virus. We were participants in a 3‐day infection control training program conducted by the CDC in Anniston, Alabama, which aimed to prepare healthcare workers to assist with the Ebola response in West Africa. Throughout the training, we repeatedly donned and doffed personal protective equipment (PPE), following protocols established by the World Health Organization and Doctors Without Borders (Mdecins Sans Frontires):
- Use a combination of contact, droplet, and standard precautions, ensuring no area of skin is left uncovered.
- Enter and exit with a buddy; inspect one another for breaches at each step of donning, caring for the patient, and doffing.
- Wash gloved hands with 0.5% chlorine bleach between tasks and patients.
- Exit at the slightest breach of infection protocol.
- Doff PPE per protocol and under supervision; take great care not to contaminate oneself.
Doffing is considered the most difficult and also the most important part, with 7 pieces to remove and no less than 20 steps to do so. After 3 full days of training, although we felt more confident with the carefully choreographed movements of donning and doffing, we were also more aware of the many opportunities during which a breach could occur. Despite our repeated practice, the training staff was firm in telling us that we were still not prepared to work in an ETU. We had only undergone what could be called a cold training. To work in the hot zone of the ETUs, it is essential to undergo additional mentorship and training once in the field. We believe a similar approach to extensive training should be employed for all healthcare workers on the front lines of an Ebola response, both in the United States and abroad.
Given the complexity of the infection control practices above, questions have appropriately been raised about US healthcare facilities' aptitude at providing care for patients with EVD. Although hospitals have historically been focal point(s) for dissemination of infection of Ebola, this does not have to be the case.[6] With appropriate preparedness, training, and understanding of facility limitations, hospitals can also be places of confidence and healing.
Both Emory University and the University of Nebraska have successfully treated multiple Ebola patients without incurring further transmission.[7, 8] Much of their success comes from the work of longitudinal teams that are trained in infectious disease response on a regular basis, despite the rare occurrence of a serious outbreak. When Emory scaled up this team upon receiving a confirmed EVD patient, new members were required to pass a proficiency test prior to providing care. Emory's strict adherence to infection control and advanced preparation serve as a model for other institutions.
It is important to recognize that not all hospitals should be fully equipped and staffed for safe care of a patient with EVD. Much like in cases of high‐level trauma, regional centers should be identified, prepared, and available to be called upon in a time of need (Figure 1). At the writing of this article, guidelines for hospitals are evolving; most states, with input from federal and local officials, are moving toward designating regional referral centers. Although the number of designated facilities and standards for each vary by state, general preparedness for care of a patient with suspected or confirmed EVD should include: (1) a designated and trained care team, (2) appropriate and vetted operational protocols, (3) an assigned isolation unit with adequate space for the necessary specialized precautions, (4) separate laboratory and diagnostic capabilities, and (5) a working waste management plan.[9] Although not all facilities throughout the United States will have the capability to care for a patient with EVD, all hospitals must have an action plan.

Triage is the first step; facilities should have well‐informed and trained staff prepared to perform assessments of potential cases safely (Figure 2). The ability to temporarily isolate and then efficiently transfer suspected or confirmed EVD patients to an appropriate facility also requires careful planning. Prompt identification, constant vigilance, and accurate histories all serve as stepping stones to a successful outcome and can protect employees and other patients in the process.

Within this proposed structure, hospitalists are uniquely equipped to play a leading role in EVD response. First, hospitalists are frequently responsible for interfacility transfer. Their intimate knowledge of this process and the collegiality they have fostered through its use are both assets in navigating the tiered referral system outlined above. Furthermore, as point people in the acute‐care setting who accept patients from emergency medicine colleagues, discuss cases with various consultants, and coordinate discharges with multidisciplinary teams on a daily basis, hospitalists have honed communication and leadership skills that are highly valuable in collaborative efforts. Finally, many hospitalists are also champions of quality and safety in their institutions. The focus on the process of continuous improvement is critical in the face of evolving challenges such as EVD.
GLOBAL HEALTH HOSPITALISTS
The Ebola outbreak is a global crisis that clearly illustrates the challenges of addressing highly infectious diseases in modern times. We have outlined the role of hospitalists and defined the steps toward adequate preparedness within our facilities. By following the measured and practical actions Thucydides advised, we can further strengthen our health systems and work toward the control of this outbreak in the United States. The important role of hospitalists can and should be extended to serve those in poor countries.
Over the last decade, health systems strengthening, health workforce training, and patient safety have come to the forefront of global health priorities, the same priorities that are also at the forefront of many hospitalists' aspirations. Shoeb et al. recently conducted a survey of global health hospitalists. They found that within the framework of global health, hospitalists are uniquely positioned to contribute to this growing field, particularly in areas such as quality improvement, safety, systems thinking, and medical education, all strengths of the hospital medicine model that could be translated to resource‐limited settings.[10]
We believe that hospitalists, with their unique positions and skills, are natural and necessary agents of action in this time of need. Our ultimate aim is for hospitalists to become actively engaged in addressing not only the current Ebola outbreak, but also the extreme inequity of healthcare globallyan inequity that lies at the heart of the current devastation in West Africa. We call upon hospital medicine leaders, from division chiefs to chairs of medicine to chief medical officers to support, encourage, and incentivize their staff and faculty to join the ranks of global health hospitalists as they work to both end gross inequities in healthcare abroad and protect our patients and ourselves back home.
Acknowledgements
The authors are grateful for Brett Lewis' assistance in the preparation of this article.
Disclosure
Nothing to report.
Concern and fear over an infection and how best to contain its spread is a well‐known storyline dating back centuries before the germ theory was hypothesized. During the Plague of Athens in the fourth century bc, upon noticing that physicians and caregivers of the sick were most at risk of dying, the Greek historian and philosopher Thucydides wrote that thwarting the disease likely required more practical measures than simple prayer to the gods.[1] To this day, the fears of contracting disease often outstripor worse, overrideour practical or scientific understanding.
Ebola has rekindled past concerns about disease transmission, whether real or imagined. In this article we will describe the history of Ebola outside the United States as well as recent events in US hospitals. We will review guidelines for how to prepare hospitals to treat potential Ebola patients and highlight the key role that hospitalists can have in ensuring the safety of their patients and coworkers. We will also describe the emerging role of global health hospitalists, whose numbers are growing and whose expertise is ideally suited to improving hospital care in developing countries.
EBOLA EPIDEMICS AND HEALTH EQUITY
In the first identified Ebola outbreak in 1976 in Zaire, now the Democratic Republic of the Congo, patients arrived at local hospitals with symptoms that resembled common endemic illnesses like malaria, typhoid, and yellow fever. The ensuing deaths of 11 of the 17 staff members at 1 hospital were only an introduction to the deadly consequences of Ebola and the necessity of vigilant infection control. Unfortunately, not much has changed with each subsequent outbreak; to this day the heralding of an Ebola outbreak denotes the death of healthcare workers. Since the discovery of the Ebola virus 38 years ago, there have been nearly 30 recorded Ebola outbreaks. The mortality rates of prior outbreaks range widely between 25% and 90%; reports of the current outbreak's mortality falls within that range, between 50% and 70%. It is important to note that despite this high mortality, there had been no more than 1600 Ebola deaths worldwide before the 2014 West African pandemic.[2] In comparison, the number of deaths in Guinea, Sierra Leone, and Liberia approaches 6000.[3]
Although these disturbing figures have fueled public fear and panic in the United States, we wonder how many of these deaths were actually preventable. At the epicenter of the current Ebola virus disease (EVD) outbreak are 3 countries especially vulnerable to epidemics, with armed conflict recently crippling the health systems that have now just started to rebuild. When Liberia emerged from its second civil war in 2003, they had barely 50 physicians caring for a country of nearly 4 million.[4] In contrast with the devastating death toll in Liberia, it perhaps should not come as a surprise that out of the 10 known cases of Ebola that were treated in the United States and caught early, none have resulted in death. With an advanced healthcare infrastructure in place, the mortality rate for EVD in the United States has been close to zero. Although the true unpreventable case fatality rate in West Africa is unknown, the positive outcomes in the United States cases illustrate that hospitals can treat EVD successfully, provided that they are well staffed, supplied, and prepared.
Furthermore, the stark difference in access to quality healthcare between nations suggests that combating the virus should not be our only concern. In fact, EVD itself is only an acute symptom of a much larger, chronic problem with the health systems in Guinea, Sierra Leone, and Liberia. Western countries must direct more resources and attention to improving the overall quality of care in these countries, for although the underlying inequities may be limited to places like Guinea, Liberia, and Sierra Leone, the resulting threat is a global one.
PREPARING FOR THE FIGHT AGAINST EBOLA IN THE UNITED STATES
On September 25, 2014, Thomas Eric Duncan presented to Texas Health Presbyterian Hospital with fever, abdominal pain, and headache 11 days after transporting a pregnant neighbor in his home country of Liberia who later died of EVD. Duncan was discharged from the emergency room without admission. Three days later, on September 28, 2014, an ambulance was called and transported him to Texas Health where he was admitted. He was isolated and the hospital followed existing Centers for Disease Control and Prevention (CDC) guidelines for infection control. He was confirmed to have EVD on September 30, 2014. He was cared for by the healthcare providers at Texas Health Presbyterian Hospital, but despite their care, his condition deteriorated. Eric Duncan died on October 8, 2014. About 120 healthcare workers came into contact with the patient. None of his 48 contacts prior to admission to the hospital contracted the disease. However, 2 nurses who cared for him during his admission developed symptoms and were confirmed to have Ebola. An alarm was sounded across the United States, awakening hospitals to the reality of their vulnerability and need for preparedness.[5]
For any healthcare worker treating EVD, whether working in a hospital here in the United States or an Ebola treatment unit (ETU) in West Africa, intensive training is necessary to keep oneself safe. Although infection control is not a novel concept, the stakes have undoubtedly been raised, as even the smallest misstep can be deadly when dealing with the Ebola virus. We were participants in a 3‐day infection control training program conducted by the CDC in Anniston, Alabama, which aimed to prepare healthcare workers to assist with the Ebola response in West Africa. Throughout the training, we repeatedly donned and doffed personal protective equipment (PPE), following protocols established by the World Health Organization and Doctors Without Borders (Mdecins Sans Frontires):
- Use a combination of contact, droplet, and standard precautions, ensuring no area of skin is left uncovered.
- Enter and exit with a buddy; inspect one another for breaches at each step of donning, caring for the patient, and doffing.
- Wash gloved hands with 0.5% chlorine bleach between tasks and patients.
- Exit at the slightest breach of infection protocol.
- Doff PPE per protocol and under supervision; take great care not to contaminate oneself.
Doffing is considered the most difficult and also the most important part, with 7 pieces to remove and no less than 20 steps to do so. After 3 full days of training, although we felt more confident with the carefully choreographed movements of donning and doffing, we were also more aware of the many opportunities during which a breach could occur. Despite our repeated practice, the training staff was firm in telling us that we were still not prepared to work in an ETU. We had only undergone what could be called a cold training. To work in the hot zone of the ETUs, it is essential to undergo additional mentorship and training once in the field. We believe a similar approach to extensive training should be employed for all healthcare workers on the front lines of an Ebola response, both in the United States and abroad.
Given the complexity of the infection control practices above, questions have appropriately been raised about US healthcare facilities' aptitude at providing care for patients with EVD. Although hospitals have historically been focal point(s) for dissemination of infection of Ebola, this does not have to be the case.[6] With appropriate preparedness, training, and understanding of facility limitations, hospitals can also be places of confidence and healing.
Both Emory University and the University of Nebraska have successfully treated multiple Ebola patients without incurring further transmission.[7, 8] Much of their success comes from the work of longitudinal teams that are trained in infectious disease response on a regular basis, despite the rare occurrence of a serious outbreak. When Emory scaled up this team upon receiving a confirmed EVD patient, new members were required to pass a proficiency test prior to providing care. Emory's strict adherence to infection control and advanced preparation serve as a model for other institutions.
It is important to recognize that not all hospitals should be fully equipped and staffed for safe care of a patient with EVD. Much like in cases of high‐level trauma, regional centers should be identified, prepared, and available to be called upon in a time of need (Figure 1). At the writing of this article, guidelines for hospitals are evolving; most states, with input from federal and local officials, are moving toward designating regional referral centers. Although the number of designated facilities and standards for each vary by state, general preparedness for care of a patient with suspected or confirmed EVD should include: (1) a designated and trained care team, (2) appropriate and vetted operational protocols, (3) an assigned isolation unit with adequate space for the necessary specialized precautions, (4) separate laboratory and diagnostic capabilities, and (5) a working waste management plan.[9] Although not all facilities throughout the United States will have the capability to care for a patient with EVD, all hospitals must have an action plan.

Triage is the first step; facilities should have well‐informed and trained staff prepared to perform assessments of potential cases safely (Figure 2). The ability to temporarily isolate and then efficiently transfer suspected or confirmed EVD patients to an appropriate facility also requires careful planning. Prompt identification, constant vigilance, and accurate histories all serve as stepping stones to a successful outcome and can protect employees and other patients in the process.

Within this proposed structure, hospitalists are uniquely equipped to play a leading role in EVD response. First, hospitalists are frequently responsible for interfacility transfer. Their intimate knowledge of this process and the collegiality they have fostered through its use are both assets in navigating the tiered referral system outlined above. Furthermore, as point people in the acute‐care setting who accept patients from emergency medicine colleagues, discuss cases with various consultants, and coordinate discharges with multidisciplinary teams on a daily basis, hospitalists have honed communication and leadership skills that are highly valuable in collaborative efforts. Finally, many hospitalists are also champions of quality and safety in their institutions. The focus on the process of continuous improvement is critical in the face of evolving challenges such as EVD.
GLOBAL HEALTH HOSPITALISTS
The Ebola outbreak is a global crisis that clearly illustrates the challenges of addressing highly infectious diseases in modern times. We have outlined the role of hospitalists and defined the steps toward adequate preparedness within our facilities. By following the measured and practical actions Thucydides advised, we can further strengthen our health systems and work toward the control of this outbreak in the United States. The important role of hospitalists can and should be extended to serve those in poor countries.
Over the last decade, health systems strengthening, health workforce training, and patient safety have come to the forefront of global health priorities, the same priorities that are also at the forefront of many hospitalists' aspirations. Shoeb et al. recently conducted a survey of global health hospitalists. They found that within the framework of global health, hospitalists are uniquely positioned to contribute to this growing field, particularly in areas such as quality improvement, safety, systems thinking, and medical education, all strengths of the hospital medicine model that could be translated to resource‐limited settings.[10]
We believe that hospitalists, with their unique positions and skills, are natural and necessary agents of action in this time of need. Our ultimate aim is for hospitalists to become actively engaged in addressing not only the current Ebola outbreak, but also the extreme inequity of healthcare globallyan inequity that lies at the heart of the current devastation in West Africa. We call upon hospital medicine leaders, from division chiefs to chairs of medicine to chief medical officers to support, encourage, and incentivize their staff and faculty to join the ranks of global health hospitalists as they work to both end gross inequities in healthcare abroad and protect our patients and ourselves back home.
Acknowledgements
The authors are grateful for Brett Lewis' assistance in the preparation of this article.
Disclosure
Nothing to report.
- . Infection control throughout history. Lancet Infect Dis. 2014;14(4):280.
- , . Ebola then and now. N Engl J Med. 2014;371:1663–1666.
- World Health Organization. Ebola response roadmap—situation report. Available at: http://www.who.int/csr/disease/ebola/situation‐reports/en/. Accessed November 2014.
- , . Effect of civil war on medical education in Liberia. Int J Emerg Med. 2011;4:6.
- , . Is the U.S. prepared for an Ebola outbreak? New York Times. October 10, 2014. Available at: http://www.nytimes.com/interactive/2014/10/09/us/is-the-us-prepared-for-an-ebola-outbreak.html?module=Search61(6):997–1003.
- . With good hospital practices, Emory rises to Ebola challenge. Kaiser Health News. U.S. News 8(3):162–163.
- . Infection control throughout history. Lancet Infect Dis. 2014;14(4):280.
- , . Ebola then and now. N Engl J Med. 2014;371:1663–1666.
- World Health Organization. Ebola response roadmap—situation report. Available at: http://www.who.int/csr/disease/ebola/situation‐reports/en/. Accessed November 2014.
- , . Effect of civil war on medical education in Liberia. Int J Emerg Med. 2011;4:6.
- , . Is the U.S. prepared for an Ebola outbreak? New York Times. October 10, 2014. Available at: http://www.nytimes.com/interactive/2014/10/09/us/is-the-us-prepared-for-an-ebola-outbreak.html?module=Search61(6):997–1003.
- . With good hospital practices, Emory rises to Ebola challenge. Kaiser Health News. U.S. News 8(3):162–163.
© 2014 Society of Hospital Medicine
Public Quality Reporting
Few consumers would choose to dine at a restaurant if they knew the kitchen was infested with cockroaches. Few patients would choose to undergo a liver transplant in a hospital that was performing the procedure for the first time. In most sectors, consumers gather information about quality (and price) from the marketplace, where economic theory predicts that rational behavior and competition will lead to continuous improvement over time. However, for some goods and services, information is sparse and asymmetric between consumers and suppliers. In sectors where consumer health is at risk, society has often intervened to assure minimum standards. Yet sometimes these efforts have fallen short. In healthcare, physician licensure and hospital accreditation (eg, through the Joint Commission), although providing an important foundation to assure safety, have not come close to solving the widespread quality problems.[1] Basic regulatory requirements for restaurants have also proven inadequate to prevent food‐borne illness. Consumer trust, without information, can be a recipe (or prescription) for trouble.
In response, high‐profile efforts have been introduced to publicize the quality and safety of service providers. One example is Hospital Compare, Medicare's national quality reporting program for US hospitals.[2] The New York City sanitary grade inspection program is a parallel effort for restaurants. Although customers can judge how much they like the food from a restaurantor look up reviews at
The aims of Hospital Compare and the New York City sanitary inspection program are fundamentally similar. Both initiatives seek to address a common market failure resulting in the consumer's lack of information on quality and safety. By infusing the market with information, these programs enable consumers to make better choices and encourage service providers to improve quality and safety.[3] Despite the promise of these programs, a copious literature about the effects of public quality reporting in healthcare has found mixed results.[4, 5] Although the performance measures in any public reporting program must be valid and reliable, good measures are not sufficient to achieve the goals of public reporting. To engage patients, reported results must also be accessible, understandable, and meaningful. Both patients' lack of knowledge about the reports[6] and patients' inability to effectively use these data to make better decisions[7] are some reasons why public quality reporting has fallen short of its expectations. This article argues that the New York City program is much better structured to positively affect patient choice, and holds important lessons for public quality reporting in US hospitals.
CONTRASTS BETWEEN HOSPITAL COMPARE AND THE NEW YORK CITY RESTAURANT SANITARY INSPECTION PROGRAM
Hospital Compare reports performance for 108 separate quality indicators related to quality and patient safety for US hospitals (Table 1). These are a combination of structure measures (eg, hospital participation in a systematic database for cardiac surgery), process of care measures (eg, acute myocardial infarction patients receiving fibrinolytic therapy within 30 minutes of hospital arrival), outcomes (eg, 30‐day mortality and readmission), and patient experience measures (eg, how you would rate your communication with your physician). Hospital Compare data, frequently based on hospital quality performance 1 to 3 years prior to publication, are displayed on a website. Hospitals do not receive a summary measure of quality or safety.[8] Hospitals face financial incentives that are tied to measure reporting[9] and performance for some of the measures on Hospital Compare.[10, 11] Hospital accreditation is only loosely related to performance on these measures.
| Attribute | Hospital Compare | New York City Sanitary Inspection Program |
|---|---|---|
| Display of information | On a website ( |
On the front of the restaurant, with additional information also available on a website ( |
| Frequency of information update | Quarterly; data often lag by between 1 and 3 years. | Unannounced inspections occur at least annually. Grades are posted immediately after inspection. |
| Quality measures | Mix of measures pertaining to quality improvement activities (eg, hospital participation in a cardiac surgery registry or a quality improvement initiative), rates of adherence with evidence‐based medicine (eg, heart failure patients receiving discharge instructions, acute myocardial infarction patients receiving ‐blocker at arrival), and patient outcomes (eg, 30‐day mortality and 30‐day readmission for acute myocardial infarction, heart failure, and pneumonia). | Mix of measures pertaining to conditions of the facility (eg, improper sewage disposal system, improper food contact surface, evidence of live rats in the facility) and the treatment and handling of food (eg, food is unwrapped, appropriate thermometer not used to measure temperature of potentially hazardous foods, food not prepared to sufficiently high temperature). |
| Clarity and simplicity of information | 108 individual measures. No summary measure. | Single summary letter grade displayed on front of restaurant. Detailed data on individual violations (ie, measures) available on website. |
| Consequences of poor performance and mechanisms for enforcement | Hospitals are subject to financial penalties for not reporting certain measures and face financial incentives for performance on a subset of measures. | Restaurants are fined for violations, are subject to repeated inspections for poor performance, and are subject to closure for severe violations. |
| Consumer awareness | Limited | Widespread |
The New York City sanitation program regularly inspects restaurants and scores them on a standard set of indicators that correspond to critical violations (eg, food is contaminated by mouse droppings) or general violations (eg, garbage is not adequately covered).[12] Points are assigned to each type and severity of violation, and the sum of the points are converted into a summary grade of A, B, or C. Restaurants can dispute the grades, receiving a grade pending designation until the dispute is adjudicated. After inspection, sanitation grades are immediately posted on restaurants' front door or window, providing current information that is clearly visible to consumers before entering. More detailed information on sanitation violations is also available on a website. If restaurants receive an A grade, they face no additional inspections for 1 year, but poorly graded restaurants may receive monthly inspections. Restaurants face fines from violations and are subject to closure from severe violations. Recently proposed changes would decrease fines and give restaurants greater opportunities to appeal grades, but leave the program otherwise intact.[13]
IMPLICATIONS FOR PUBLIC QUALITY REPORTING IN HOSPITALS
Along with value‐based payment reforms, public quality reporting is one of the few major system‐level approaches that is being implemented in the US to improve quality and safety in healthcare. However, without a simple and understandable display of information that is available when a patient needs it, quality and safety information will likely go unused.[14] Hospital Compare leaves it up the patient to find the quality and safety information and does little to help patients understand and use the information effectively. Hospital Compare asks patients to do far more work, which is perhaps why it has been largely ignored by patients.[2, 15] The New York City sanitation inspection program evaluates restaurants, prominently displays an understandable summary result, and puts the scoring details in the background. Although peer‐reviewed evaluations of the New York City sanitation inspection program have not yet been published, internal data show that the program has decreased customer concern about getting sick, improved sanitary practices, and decreased salmonella.[16] Evidence from a similar program in Los Angeles County found that hygiene grades steered consumers toward restaurants with better sanitary conditions and decreased food‐borne illness.[17]
The nature of choice in healthcare, particularly the choice of hospital, is much different than it is for restaurants. In some areas, a single hospital may serve a large geographical area, severely limiting choice. Even when patients have the ability to receive care at different hospitals, choice may be limited because patients are referred to a specific hospital by their outpatient physician or are brought to a hospital during an emergency.[18] In these cases, quality grades on the front doors of hospitals would not affect patient decisions, at least for that admission. Nonetheless, if quality grades were posted on the front doors of hospitals, patients receiving both inpatient and outpatient care would see the grades, and could use the information to make future decisions. Posted grades may also lead patients to review more in‐depth quality information related to their condition on the Hospital Compare website. Posted quality grades would also increase the visibility of the grades for other stakeholdersincluding the media and boards of directorsmagnifying their salience and impact.
How quality information is displayed and summarized can make or break public reporting programs. The New York City sanitation inspection program displays summarized, composite measures in the form of widely understood letter grades. Hospital Compare, however, displays myriad, unrelated performance measures that are not summarized into a global quality or safety measure. This information display is at odds with best practice. Patients find it difficult to synthesize data from multiple performance indicators to determine the relative quality of healthcare providers or insurance plans.7 In many cases, more information can lead to worse decision making.[19] Patients' difficulty making optimal choices has been noted in numerous healthcare settings, including purchasing Medicare Part D plans[20] and choosing health plans.[21] Recent evidence suggests that Nursing Home Compare's shift from an unsummarized collection of disparate performance measures to a 5‐star rating system has led patients to choose higher‐ranked facilities.[22] The fact that commercial providers of product quality information, such as Consumer Reports[23] and US News and World Report,[24] publish global summary scores, in addition to component scores, is a hint that this style of reporting is more appealing to consumers. Reports suggest that Medicare is moving toward a 5‐star quality rating system for hospitals,[8] which is a welcome development.
Different types of patients may demand different types of quality information, and a single summary measure for Hospital Compare may not meet the needs of a diverse set of patients. Nonetheless, the benefits from an actionable, understandable, comprehensive, and appropriate summary measure likely outweigh the costs of a potential mismatch for certain types of patients. Many of the performance measures on Hospital Compare already apply broadly to diverse sets of patients (eg, the structure measures, patient experience, and surgical safety) and are not specific to certain disease areas. Global summary measures could be complemented by separate component scores (eg, by disease area or domain of quality) for patients who wanted information on different aspects of care.
The inspection regime that underlies the New York City sanitary inspection program has parallels in healthcare that could be extended to Hospital Compare. For instance, the Joint Commission performs surprise inspections of hospitals as part of its accreditation process. The publicly reported 5‐star ratings for nursing homes are also based, in part, on inspection results.[25] Results from these types of inspections can capture up‐to‐date information on important dimensions of quality and safety that are not available in standard administrative data sources. Incorporating inspection results into Hospital Compare could increase both the timeliness and validity of the reporting.
The New York City sanitation inspection program is not a panacea: the indicators may not capture all relevant aspects of restaurant sanitation, some research suggests that past sanitary grades do not predict future grades,[26] and sanitary grade inflation over time has the potential to mask meaningful differences in sanitary conditions that are related to food‐borne illness.[16, 26] However, by providing understandable and meaningful reports at the point of service, the New York City program is well designed to encourage sanitation improvement through both consumer and supplier behavior.
Where the New York City sanitation inspection program succeeds, Hospital Compare fails. Hospital Compare is not patient centered, and it is not working for patients. Medicare can learn from the New York City restaurant sanitation inspection program to enhance the effects of public reporting by presenting information to consumers that is relevant, easy to access and interpret, and up to date. The greater complexity of hospital product lines should not deter these efforts. Patients' lives, not just the health of their gastrointestinal tracts, are at stake.
ACKNOWLEDGEMENTS
The authors thank Kaveh G. Shojania, MD, and Edward E. Etchells, MD, MSc, University of Toronto, and Martin Roland, DM, University of Oxford and RAND Europe for their comments on an earlier draft of the manuscript. None were compensated for their contributions.
Disclosures: Nothing to report.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , . Medicare's public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood). 2012;31(3):585–592.
- , . Public reporting of hospital hand hygiene compliance—helpful or harmful? JAMA. 2010;304(10):1116–1117.
- . Do cardiac surgery report cards reduce mortality? Assessing the evidence. Med Care Res Rev. 2006;63(4):403–426.
- , . Quality and consumer decision making in the market for health insurance and health care services. Med Care Res Rev. 2009;66(1 suppl):28S–52S.
- , . Use of public performance reports: a survey of patients undergoing cardiac surgery. JAMA. 1998;279(20):1638–1642.
- , , . Informing consumer decisions in health care: implications from decision‐making research. Milbank Q. 1997;75(3):395–414.
- Centers for Medicare hospital inpatient prospective payment systems for acute care hospitals and the long‐term care hospital prospective payment system and proposed fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation. Fed Regist. 2013:27486–27823.
- , . Relationship between Medicare's hospital compare performance measures and mortality rates. JAMA. 2006;296(22):2694–2702.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369(26):2472–2474.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177.
- New York City Department of Health and Mental Hygiene. What to expect when you're inspected: a guide for food service operators. New York, NY: New York City Department of Health and Mental Hygiene; 2010.
- . In reprieve for restaurant industry, New York proposes changes to grading system. New York Times. March 22, 2014:A15.
- . Thinking, Fast and Slow. New York, NY: Farrar, Straus and Giroux; 2011.
- , , . Public hospital quality report awareness: evidence from National and Californian Internet searches and social media mentions, 2012. BMJ Open. 2014;4(3):e004417.
- New York City Department of Health and Mental Hygiene. Restaurant Grading in New York City at 18 Months. New York, NY: New York City Department of Health and Mental Hygiene; 2013.
- , . The effect of information on product quality: evidence from restaurant hygiene grade cards. Q J Econ. 2003;118(2):409–451.
- , , , . Do high‐cost hospitals deliver better care? Evidence from ambulance referral patterns. National Bureau of Economic Research. Working paper no. 17936. Available at: http://www.nber.org/papers/w17936.pdf. Published March 2012. Accessed November 18, 2014.
- , , , , . Less is more in presenting quality information to consumers. Med Care Res Rev. 2007;64(2)169–190.
- and . Choice inconsistencies among the elderly: evidence from plan choice in the Medicare Part D program. Amer Econ Rev. 2011;101(4)1180–1210.
- , , , . Strategies for reporting health plan performance information to consumers: evidence from controlled studies. Health Serv Res. 2002;37(2):291–313.
- , . Quality reporting and private prices: evidence from the nursing home industry. Paper presented at: American Society of Health Economists Annual Meeting; June 23, 2014; Los Angeles, CA.
- Consumer Reports. Best new care values. Available at: http://consumerreports.org/cro/2012/05/best-new-car-values/index.htm. Updated February 2014. Accessed November 18, 2014.
- . Best value schools methodology. US News and World Report. September 8, 2014. Available at: http://www.usnews.com/education/best-colleges/articles/2013/09/09/best-value-schools-methodology. Accessed November 18, 2014.
- Centers for Medicare 122:574–677.
Few consumers would choose to dine at a restaurant if they knew the kitchen was infested with cockroaches. Few patients would choose to undergo a liver transplant in a hospital that was performing the procedure for the first time. In most sectors, consumers gather information about quality (and price) from the marketplace, where economic theory predicts that rational behavior and competition will lead to continuous improvement over time. However, for some goods and services, information is sparse and asymmetric between consumers and suppliers. In sectors where consumer health is at risk, society has often intervened to assure minimum standards. Yet sometimes these efforts have fallen short. In healthcare, physician licensure and hospital accreditation (eg, through the Joint Commission), although providing an important foundation to assure safety, have not come close to solving the widespread quality problems.[1] Basic regulatory requirements for restaurants have also proven inadequate to prevent food‐borne illness. Consumer trust, without information, can be a recipe (or prescription) for trouble.
In response, high‐profile efforts have been introduced to publicize the quality and safety of service providers. One example is Hospital Compare, Medicare's national quality reporting program for US hospitals.[2] The New York City sanitary grade inspection program is a parallel effort for restaurants. Although customers can judge how much they like the food from a restaurantor look up reviews at
The aims of Hospital Compare and the New York City sanitary inspection program are fundamentally similar. Both initiatives seek to address a common market failure resulting in the consumer's lack of information on quality and safety. By infusing the market with information, these programs enable consumers to make better choices and encourage service providers to improve quality and safety.[3] Despite the promise of these programs, a copious literature about the effects of public quality reporting in healthcare has found mixed results.[4, 5] Although the performance measures in any public reporting program must be valid and reliable, good measures are not sufficient to achieve the goals of public reporting. To engage patients, reported results must also be accessible, understandable, and meaningful. Both patients' lack of knowledge about the reports[6] and patients' inability to effectively use these data to make better decisions[7] are some reasons why public quality reporting has fallen short of its expectations. This article argues that the New York City program is much better structured to positively affect patient choice, and holds important lessons for public quality reporting in US hospitals.
CONTRASTS BETWEEN HOSPITAL COMPARE AND THE NEW YORK CITY RESTAURANT SANITARY INSPECTION PROGRAM
Hospital Compare reports performance for 108 separate quality indicators related to quality and patient safety for US hospitals (Table 1). These are a combination of structure measures (eg, hospital participation in a systematic database for cardiac surgery), process of care measures (eg, acute myocardial infarction patients receiving fibrinolytic therapy within 30 minutes of hospital arrival), outcomes (eg, 30‐day mortality and readmission), and patient experience measures (eg, how you would rate your communication with your physician). Hospital Compare data, frequently based on hospital quality performance 1 to 3 years prior to publication, are displayed on a website. Hospitals do not receive a summary measure of quality or safety.[8] Hospitals face financial incentives that are tied to measure reporting[9] and performance for some of the measures on Hospital Compare.[10, 11] Hospital accreditation is only loosely related to performance on these measures.
| Attribute | Hospital Compare | New York City Sanitary Inspection Program |
|---|---|---|
| Display of information | On a website ( |
On the front of the restaurant, with additional information also available on a website ( |
| Frequency of information update | Quarterly; data often lag by between 1 and 3 years. | Unannounced inspections occur at least annually. Grades are posted immediately after inspection. |
| Quality measures | Mix of measures pertaining to quality improvement activities (eg, hospital participation in a cardiac surgery registry or a quality improvement initiative), rates of adherence with evidence‐based medicine (eg, heart failure patients receiving discharge instructions, acute myocardial infarction patients receiving ‐blocker at arrival), and patient outcomes (eg, 30‐day mortality and 30‐day readmission for acute myocardial infarction, heart failure, and pneumonia). | Mix of measures pertaining to conditions of the facility (eg, improper sewage disposal system, improper food contact surface, evidence of live rats in the facility) and the treatment and handling of food (eg, food is unwrapped, appropriate thermometer not used to measure temperature of potentially hazardous foods, food not prepared to sufficiently high temperature). |
| Clarity and simplicity of information | 108 individual measures. No summary measure. | Single summary letter grade displayed on front of restaurant. Detailed data on individual violations (ie, measures) available on website. |
| Consequences of poor performance and mechanisms for enforcement | Hospitals are subject to financial penalties for not reporting certain measures and face financial incentives for performance on a subset of measures. | Restaurants are fined for violations, are subject to repeated inspections for poor performance, and are subject to closure for severe violations. |
| Consumer awareness | Limited | Widespread |
The New York City sanitation program regularly inspects restaurants and scores them on a standard set of indicators that correspond to critical violations (eg, food is contaminated by mouse droppings) or general violations (eg, garbage is not adequately covered).[12] Points are assigned to each type and severity of violation, and the sum of the points are converted into a summary grade of A, B, or C. Restaurants can dispute the grades, receiving a grade pending designation until the dispute is adjudicated. After inspection, sanitation grades are immediately posted on restaurants' front door or window, providing current information that is clearly visible to consumers before entering. More detailed information on sanitation violations is also available on a website. If restaurants receive an A grade, they face no additional inspections for 1 year, but poorly graded restaurants may receive monthly inspections. Restaurants face fines from violations and are subject to closure from severe violations. Recently proposed changes would decrease fines and give restaurants greater opportunities to appeal grades, but leave the program otherwise intact.[13]
IMPLICATIONS FOR PUBLIC QUALITY REPORTING IN HOSPITALS
Along with value‐based payment reforms, public quality reporting is one of the few major system‐level approaches that is being implemented in the US to improve quality and safety in healthcare. However, without a simple and understandable display of information that is available when a patient needs it, quality and safety information will likely go unused.[14] Hospital Compare leaves it up the patient to find the quality and safety information and does little to help patients understand and use the information effectively. Hospital Compare asks patients to do far more work, which is perhaps why it has been largely ignored by patients.[2, 15] The New York City sanitation inspection program evaluates restaurants, prominently displays an understandable summary result, and puts the scoring details in the background. Although peer‐reviewed evaluations of the New York City sanitation inspection program have not yet been published, internal data show that the program has decreased customer concern about getting sick, improved sanitary practices, and decreased salmonella.[16] Evidence from a similar program in Los Angeles County found that hygiene grades steered consumers toward restaurants with better sanitary conditions and decreased food‐borne illness.[17]
The nature of choice in healthcare, particularly the choice of hospital, is much different than it is for restaurants. In some areas, a single hospital may serve a large geographical area, severely limiting choice. Even when patients have the ability to receive care at different hospitals, choice may be limited because patients are referred to a specific hospital by their outpatient physician or are brought to a hospital during an emergency.[18] In these cases, quality grades on the front doors of hospitals would not affect patient decisions, at least for that admission. Nonetheless, if quality grades were posted on the front doors of hospitals, patients receiving both inpatient and outpatient care would see the grades, and could use the information to make future decisions. Posted grades may also lead patients to review more in‐depth quality information related to their condition on the Hospital Compare website. Posted quality grades would also increase the visibility of the grades for other stakeholdersincluding the media and boards of directorsmagnifying their salience and impact.
How quality information is displayed and summarized can make or break public reporting programs. The New York City sanitation inspection program displays summarized, composite measures in the form of widely understood letter grades. Hospital Compare, however, displays myriad, unrelated performance measures that are not summarized into a global quality or safety measure. This information display is at odds with best practice. Patients find it difficult to synthesize data from multiple performance indicators to determine the relative quality of healthcare providers or insurance plans.7 In many cases, more information can lead to worse decision making.[19] Patients' difficulty making optimal choices has been noted in numerous healthcare settings, including purchasing Medicare Part D plans[20] and choosing health plans.[21] Recent evidence suggests that Nursing Home Compare's shift from an unsummarized collection of disparate performance measures to a 5‐star rating system has led patients to choose higher‐ranked facilities.[22] The fact that commercial providers of product quality information, such as Consumer Reports[23] and US News and World Report,[24] publish global summary scores, in addition to component scores, is a hint that this style of reporting is more appealing to consumers. Reports suggest that Medicare is moving toward a 5‐star quality rating system for hospitals,[8] which is a welcome development.
Different types of patients may demand different types of quality information, and a single summary measure for Hospital Compare may not meet the needs of a diverse set of patients. Nonetheless, the benefits from an actionable, understandable, comprehensive, and appropriate summary measure likely outweigh the costs of a potential mismatch for certain types of patients. Many of the performance measures on Hospital Compare already apply broadly to diverse sets of patients (eg, the structure measures, patient experience, and surgical safety) and are not specific to certain disease areas. Global summary measures could be complemented by separate component scores (eg, by disease area or domain of quality) for patients who wanted information on different aspects of care.
The inspection regime that underlies the New York City sanitary inspection program has parallels in healthcare that could be extended to Hospital Compare. For instance, the Joint Commission performs surprise inspections of hospitals as part of its accreditation process. The publicly reported 5‐star ratings for nursing homes are also based, in part, on inspection results.[25] Results from these types of inspections can capture up‐to‐date information on important dimensions of quality and safety that are not available in standard administrative data sources. Incorporating inspection results into Hospital Compare could increase both the timeliness and validity of the reporting.
The New York City sanitation inspection program is not a panacea: the indicators may not capture all relevant aspects of restaurant sanitation, some research suggests that past sanitary grades do not predict future grades,[26] and sanitary grade inflation over time has the potential to mask meaningful differences in sanitary conditions that are related to food‐borne illness.[16, 26] However, by providing understandable and meaningful reports at the point of service, the New York City program is well designed to encourage sanitation improvement through both consumer and supplier behavior.
Where the New York City sanitation inspection program succeeds, Hospital Compare fails. Hospital Compare is not patient centered, and it is not working for patients. Medicare can learn from the New York City restaurant sanitation inspection program to enhance the effects of public reporting by presenting information to consumers that is relevant, easy to access and interpret, and up to date. The greater complexity of hospital product lines should not deter these efforts. Patients' lives, not just the health of their gastrointestinal tracts, are at stake.
ACKNOWLEDGEMENTS
The authors thank Kaveh G. Shojania, MD, and Edward E. Etchells, MD, MSc, University of Toronto, and Martin Roland, DM, University of Oxford and RAND Europe for their comments on an earlier draft of the manuscript. None were compensated for their contributions.
Disclosures: Nothing to report.
Few consumers would choose to dine at a restaurant if they knew the kitchen was infested with cockroaches. Few patients would choose to undergo a liver transplant in a hospital that was performing the procedure for the first time. In most sectors, consumers gather information about quality (and price) from the marketplace, where economic theory predicts that rational behavior and competition will lead to continuous improvement over time. However, for some goods and services, information is sparse and asymmetric between consumers and suppliers. In sectors where consumer health is at risk, society has often intervened to assure minimum standards. Yet sometimes these efforts have fallen short. In healthcare, physician licensure and hospital accreditation (eg, through the Joint Commission), although providing an important foundation to assure safety, have not come close to solving the widespread quality problems.[1] Basic regulatory requirements for restaurants have also proven inadequate to prevent food‐borne illness. Consumer trust, without information, can be a recipe (or prescription) for trouble.
In response, high‐profile efforts have been introduced to publicize the quality and safety of service providers. One example is Hospital Compare, Medicare's national quality reporting program for US hospitals.[2] The New York City sanitary grade inspection program is a parallel effort for restaurants. Although customers can judge how much they like the food from a restaurantor look up reviews at
The aims of Hospital Compare and the New York City sanitary inspection program are fundamentally similar. Both initiatives seek to address a common market failure resulting in the consumer's lack of information on quality and safety. By infusing the market with information, these programs enable consumers to make better choices and encourage service providers to improve quality and safety.[3] Despite the promise of these programs, a copious literature about the effects of public quality reporting in healthcare has found mixed results.[4, 5] Although the performance measures in any public reporting program must be valid and reliable, good measures are not sufficient to achieve the goals of public reporting. To engage patients, reported results must also be accessible, understandable, and meaningful. Both patients' lack of knowledge about the reports[6] and patients' inability to effectively use these data to make better decisions[7] are some reasons why public quality reporting has fallen short of its expectations. This article argues that the New York City program is much better structured to positively affect patient choice, and holds important lessons for public quality reporting in US hospitals.
CONTRASTS BETWEEN HOSPITAL COMPARE AND THE NEW YORK CITY RESTAURANT SANITARY INSPECTION PROGRAM
Hospital Compare reports performance for 108 separate quality indicators related to quality and patient safety for US hospitals (Table 1). These are a combination of structure measures (eg, hospital participation in a systematic database for cardiac surgery), process of care measures (eg, acute myocardial infarction patients receiving fibrinolytic therapy within 30 minutes of hospital arrival), outcomes (eg, 30‐day mortality and readmission), and patient experience measures (eg, how you would rate your communication with your physician). Hospital Compare data, frequently based on hospital quality performance 1 to 3 years prior to publication, are displayed on a website. Hospitals do not receive a summary measure of quality or safety.[8] Hospitals face financial incentives that are tied to measure reporting[9] and performance for some of the measures on Hospital Compare.[10, 11] Hospital accreditation is only loosely related to performance on these measures.
| Attribute | Hospital Compare | New York City Sanitary Inspection Program |
|---|---|---|
| Display of information | On a website ( |
On the front of the restaurant, with additional information also available on a website ( |
| Frequency of information update | Quarterly; data often lag by between 1 and 3 years. | Unannounced inspections occur at least annually. Grades are posted immediately after inspection. |
| Quality measures | Mix of measures pertaining to quality improvement activities (eg, hospital participation in a cardiac surgery registry or a quality improvement initiative), rates of adherence with evidence‐based medicine (eg, heart failure patients receiving discharge instructions, acute myocardial infarction patients receiving ‐blocker at arrival), and patient outcomes (eg, 30‐day mortality and 30‐day readmission for acute myocardial infarction, heart failure, and pneumonia). | Mix of measures pertaining to conditions of the facility (eg, improper sewage disposal system, improper food contact surface, evidence of live rats in the facility) and the treatment and handling of food (eg, food is unwrapped, appropriate thermometer not used to measure temperature of potentially hazardous foods, food not prepared to sufficiently high temperature). |
| Clarity and simplicity of information | 108 individual measures. No summary measure. | Single summary letter grade displayed on front of restaurant. Detailed data on individual violations (ie, measures) available on website. |
| Consequences of poor performance and mechanisms for enforcement | Hospitals are subject to financial penalties for not reporting certain measures and face financial incentives for performance on a subset of measures. | Restaurants are fined for violations, are subject to repeated inspections for poor performance, and are subject to closure for severe violations. |
| Consumer awareness | Limited | Widespread |
The New York City sanitation program regularly inspects restaurants and scores them on a standard set of indicators that correspond to critical violations (eg, food is contaminated by mouse droppings) or general violations (eg, garbage is not adequately covered).[12] Points are assigned to each type and severity of violation, and the sum of the points are converted into a summary grade of A, B, or C. Restaurants can dispute the grades, receiving a grade pending designation until the dispute is adjudicated. After inspection, sanitation grades are immediately posted on restaurants' front door or window, providing current information that is clearly visible to consumers before entering. More detailed information on sanitation violations is also available on a website. If restaurants receive an A grade, they face no additional inspections for 1 year, but poorly graded restaurants may receive monthly inspections. Restaurants face fines from violations and are subject to closure from severe violations. Recently proposed changes would decrease fines and give restaurants greater opportunities to appeal grades, but leave the program otherwise intact.[13]
IMPLICATIONS FOR PUBLIC QUALITY REPORTING IN HOSPITALS
Along with value‐based payment reforms, public quality reporting is one of the few major system‐level approaches that is being implemented in the US to improve quality and safety in healthcare. However, without a simple and understandable display of information that is available when a patient needs it, quality and safety information will likely go unused.[14] Hospital Compare leaves it up the patient to find the quality and safety information and does little to help patients understand and use the information effectively. Hospital Compare asks patients to do far more work, which is perhaps why it has been largely ignored by patients.[2, 15] The New York City sanitation inspection program evaluates restaurants, prominently displays an understandable summary result, and puts the scoring details in the background. Although peer‐reviewed evaluations of the New York City sanitation inspection program have not yet been published, internal data show that the program has decreased customer concern about getting sick, improved sanitary practices, and decreased salmonella.[16] Evidence from a similar program in Los Angeles County found that hygiene grades steered consumers toward restaurants with better sanitary conditions and decreased food‐borne illness.[17]
The nature of choice in healthcare, particularly the choice of hospital, is much different than it is for restaurants. In some areas, a single hospital may serve a large geographical area, severely limiting choice. Even when patients have the ability to receive care at different hospitals, choice may be limited because patients are referred to a specific hospital by their outpatient physician or are brought to a hospital during an emergency.[18] In these cases, quality grades on the front doors of hospitals would not affect patient decisions, at least for that admission. Nonetheless, if quality grades were posted on the front doors of hospitals, patients receiving both inpatient and outpatient care would see the grades, and could use the information to make future decisions. Posted grades may also lead patients to review more in‐depth quality information related to their condition on the Hospital Compare website. Posted quality grades would also increase the visibility of the grades for other stakeholdersincluding the media and boards of directorsmagnifying their salience and impact.
How quality information is displayed and summarized can make or break public reporting programs. The New York City sanitation inspection program displays summarized, composite measures in the form of widely understood letter grades. Hospital Compare, however, displays myriad, unrelated performance measures that are not summarized into a global quality or safety measure. This information display is at odds with best practice. Patients find it difficult to synthesize data from multiple performance indicators to determine the relative quality of healthcare providers or insurance plans.7 In many cases, more information can lead to worse decision making.[19] Patients' difficulty making optimal choices has been noted in numerous healthcare settings, including purchasing Medicare Part D plans[20] and choosing health plans.[21] Recent evidence suggests that Nursing Home Compare's shift from an unsummarized collection of disparate performance measures to a 5‐star rating system has led patients to choose higher‐ranked facilities.[22] The fact that commercial providers of product quality information, such as Consumer Reports[23] and US News and World Report,[24] publish global summary scores, in addition to component scores, is a hint that this style of reporting is more appealing to consumers. Reports suggest that Medicare is moving toward a 5‐star quality rating system for hospitals,[8] which is a welcome development.
Different types of patients may demand different types of quality information, and a single summary measure for Hospital Compare may not meet the needs of a diverse set of patients. Nonetheless, the benefits from an actionable, understandable, comprehensive, and appropriate summary measure likely outweigh the costs of a potential mismatch for certain types of patients. Many of the performance measures on Hospital Compare already apply broadly to diverse sets of patients (eg, the structure measures, patient experience, and surgical safety) and are not specific to certain disease areas. Global summary measures could be complemented by separate component scores (eg, by disease area or domain of quality) for patients who wanted information on different aspects of care.
The inspection regime that underlies the New York City sanitary inspection program has parallels in healthcare that could be extended to Hospital Compare. For instance, the Joint Commission performs surprise inspections of hospitals as part of its accreditation process. The publicly reported 5‐star ratings for nursing homes are also based, in part, on inspection results.[25] Results from these types of inspections can capture up‐to‐date information on important dimensions of quality and safety that are not available in standard administrative data sources. Incorporating inspection results into Hospital Compare could increase both the timeliness and validity of the reporting.
The New York City sanitation inspection program is not a panacea: the indicators may not capture all relevant aspects of restaurant sanitation, some research suggests that past sanitary grades do not predict future grades,[26] and sanitary grade inflation over time has the potential to mask meaningful differences in sanitary conditions that are related to food‐borne illness.[16, 26] However, by providing understandable and meaningful reports at the point of service, the New York City program is well designed to encourage sanitation improvement through both consumer and supplier behavior.
Where the New York City sanitation inspection program succeeds, Hospital Compare fails. Hospital Compare is not patient centered, and it is not working for patients. Medicare can learn from the New York City restaurant sanitation inspection program to enhance the effects of public reporting by presenting information to consumers that is relevant, easy to access and interpret, and up to date. The greater complexity of hospital product lines should not deter these efforts. Patients' lives, not just the health of their gastrointestinal tracts, are at stake.
ACKNOWLEDGEMENTS
The authors thank Kaveh G. Shojania, MD, and Edward E. Etchells, MD, MSc, University of Toronto, and Martin Roland, DM, University of Oxford and RAND Europe for their comments on an earlier draft of the manuscript. None were compensated for their contributions.
Disclosures: Nothing to report.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , . Medicare's public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood). 2012;31(3):585–592.
- , . Public reporting of hospital hand hygiene compliance—helpful or harmful? JAMA. 2010;304(10):1116–1117.
- . Do cardiac surgery report cards reduce mortality? Assessing the evidence. Med Care Res Rev. 2006;63(4):403–426.
- , . Quality and consumer decision making in the market for health insurance and health care services. Med Care Res Rev. 2009;66(1 suppl):28S–52S.
- , . Use of public performance reports: a survey of patients undergoing cardiac surgery. JAMA. 1998;279(20):1638–1642.
- , , . Informing consumer decisions in health care: implications from decision‐making research. Milbank Q. 1997;75(3):395–414.
- Centers for Medicare hospital inpatient prospective payment systems for acute care hospitals and the long‐term care hospital prospective payment system and proposed fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation. Fed Regist. 2013:27486–27823.
- , . Relationship between Medicare's hospital compare performance measures and mortality rates. JAMA. 2006;296(22):2694–2702.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369(26):2472–2474.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177.
- New York City Department of Health and Mental Hygiene. What to expect when you're inspected: a guide for food service operators. New York, NY: New York City Department of Health and Mental Hygiene; 2010.
- . In reprieve for restaurant industry, New York proposes changes to grading system. New York Times. March 22, 2014:A15.
- . Thinking, Fast and Slow. New York, NY: Farrar, Straus and Giroux; 2011.
- , , . Public hospital quality report awareness: evidence from National and Californian Internet searches and social media mentions, 2012. BMJ Open. 2014;4(3):e004417.
- New York City Department of Health and Mental Hygiene. Restaurant Grading in New York City at 18 Months. New York, NY: New York City Department of Health and Mental Hygiene; 2013.
- , . The effect of information on product quality: evidence from restaurant hygiene grade cards. Q J Econ. 2003;118(2):409–451.
- , , , . Do high‐cost hospitals deliver better care? Evidence from ambulance referral patterns. National Bureau of Economic Research. Working paper no. 17936. Available at: http://www.nber.org/papers/w17936.pdf. Published March 2012. Accessed November 18, 2014.
- , , , , . Less is more in presenting quality information to consumers. Med Care Res Rev. 2007;64(2)169–190.
- and . Choice inconsistencies among the elderly: evidence from plan choice in the Medicare Part D program. Amer Econ Rev. 2011;101(4)1180–1210.
- , , , . Strategies for reporting health plan performance information to consumers: evidence from controlled studies. Health Serv Res. 2002;37(2):291–313.
- , . Quality reporting and private prices: evidence from the nursing home industry. Paper presented at: American Society of Health Economists Annual Meeting; June 23, 2014; Los Angeles, CA.
- Consumer Reports. Best new care values. Available at: http://consumerreports.org/cro/2012/05/best-new-car-values/index.htm. Updated February 2014. Accessed November 18, 2014.
- . Best value schools methodology. US News and World Report. September 8, 2014. Available at: http://www.usnews.com/education/best-colleges/articles/2013/09/09/best-value-schools-methodology. Accessed November 18, 2014.
- Centers for Medicare 122:574–677.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , . Medicare's public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood). 2012;31(3):585–592.
- , . Public reporting of hospital hand hygiene compliance—helpful or harmful? JAMA. 2010;304(10):1116–1117.
- . Do cardiac surgery report cards reduce mortality? Assessing the evidence. Med Care Res Rev. 2006;63(4):403–426.
- , . Quality and consumer decision making in the market for health insurance and health care services. Med Care Res Rev. 2009;66(1 suppl):28S–52S.
- , . Use of public performance reports: a survey of patients undergoing cardiac surgery. JAMA. 1998;279(20):1638–1642.
- , , . Informing consumer decisions in health care: implications from decision‐making research. Milbank Q. 1997;75(3):395–414.
- Centers for Medicare hospital inpatient prospective payment systems for acute care hospitals and the long‐term care hospital prospective payment system and proposed fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation. Fed Regist. 2013:27486–27823.
- , . Relationship between Medicare's hospital compare performance measures and mortality rates. JAMA. 2006;296(22):2694–2702.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369(26):2472–2474.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177.
- New York City Department of Health and Mental Hygiene. What to expect when you're inspected: a guide for food service operators. New York, NY: New York City Department of Health and Mental Hygiene; 2010.
- . In reprieve for restaurant industry, New York proposes changes to grading system. New York Times. March 22, 2014:A15.
- . Thinking, Fast and Slow. New York, NY: Farrar, Straus and Giroux; 2011.
- , , . Public hospital quality report awareness: evidence from National and Californian Internet searches and social media mentions, 2012. BMJ Open. 2014;4(3):e004417.
- New York City Department of Health and Mental Hygiene. Restaurant Grading in New York City at 18 Months. New York, NY: New York City Department of Health and Mental Hygiene; 2013.
- , . The effect of information on product quality: evidence from restaurant hygiene grade cards. Q J Econ. 2003;118(2):409–451.
- , , , . Do high‐cost hospitals deliver better care? Evidence from ambulance referral patterns. National Bureau of Economic Research. Working paper no. 17936. Available at: http://www.nber.org/papers/w17936.pdf. Published March 2012. Accessed November 18, 2014.
- , , , , . Less is more in presenting quality information to consumers. Med Care Res Rev. 2007;64(2)169–190.
- and . Choice inconsistencies among the elderly: evidence from plan choice in the Medicare Part D program. Amer Econ Rev. 2011;101(4)1180–1210.
- , , , . Strategies for reporting health plan performance information to consumers: evidence from controlled studies. Health Serv Res. 2002;37(2):291–313.
- , . Quality reporting and private prices: evidence from the nursing home industry. Paper presented at: American Society of Health Economists Annual Meeting; June 23, 2014; Los Angeles, CA.
- Consumer Reports. Best new care values. Available at: http://consumerreports.org/cro/2012/05/best-new-car-values/index.htm. Updated February 2014. Accessed November 18, 2014.
- . Best value schools methodology. US News and World Report. September 8, 2014. Available at: http://www.usnews.com/education/best-colleges/articles/2013/09/09/best-value-schools-methodology. Accessed November 18, 2014.
- Centers for Medicare 122:574–677.
ECMO in Adults
As the distribution and utilization of technology in critical care medicine expands, patients experiencing respiratory failure, heart failure, or cardiac arrest are increasingly being treated with extracorporeal membrane oxygenation (ECMO). Although not customarily responsible for managing ECMO, hospitalists need to understand the rudiments of this technology and its associated ethical issues to assure that ECMO use is consistent with patient preferences and goals of care. This review aims to help prepare hospitalists for these clinical responsibilities. Following a brief review of modern‐day ECMO, including both venoarterial extracorporeal membrane oxygenation (VA‐ECMO) and venovenous extracorporeal membrane oxygenation (VV‐ECMO), we highlight special ethical considerations that may arise with VA‐ECMO and present an ethically grounded approach to the initiation, continuation, and discontinuation of treatment.
Many of the questions regarding the use of ECMO will be familiar. Certainly, similar questions arise with other life‐sustaining therapies; however, the general hospitalist may be a bit unfamiliar with ECMO and its unique ethical challenges. For example, ECMO is only provided transiently and generally while patients are in an intensive care unit. Unlike mechanical ventilation, which may be provided long‐term via tracheostomy, there is no comparable, enduring form of ECMO. Next, patients requiring ECMO are utterly dependent on the machine for their survival. If they do not recover and are not candidates for a ventricular assist device (VAD) or transplantation, there are no other therapies to offer. In this scenario, terminal discontinuation is the only option.
Informed hospitalists, who bring to counseling sessions both an understanding of the patient and family, and technical knowledge and background information on ECMO, will be far better equipped to help patients and families facing these difficult choices. As the use of ECMO becomes more prevalent, hospitalists must be prepared to address questions related to this evolving technology.
TECHNICAL AND HISTORICAL BACKGROUND
Extracorporeal life support (ECLS) involves the use of mechanical devices when native organ function fails.[1] ECMO involves the application of ECLS to provide a replacement form of cardiac and/or pulmonary function. An illustrative figure of the ECMO circuit may be seen at The Extracorporeal Life Support Organization (ELSO) (
Encouraging outcomes of clinical trials have ushered in enthusiasm for adult ECMO in the United States.[9] For example, the Conventional Ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial, a prospective study of adult VV‐ECMO for respiratory failure conducted in the United Kingdom from 2001 to 2006, demonstrated a measurable survival benefit. Patients with severe adult respiratory failure randomized to an ECMO center (75% received ECMO) had a 63% 6‐month survival without severe disability, versus 47% for patients managed conventionally at a tertiary care center.[10] Similarly, data from the 2009 H1N1 flu virus epidemic in Australia and New Zealand suggested a benefit when patients with acute respiratory distress syndrome, who had failed mechanical ventilation, were treated with ECMO; 76% survived, which was an improvement over previously reported mortality rates of 30% to 48%.[11]
With respect to VA‐ECMO, recent studies and case reports out of Taiwan, Germany, and France propose a survival benefit when ECMO is used in patients with cardiac failure.[12, 13, 14, 15] Patients with in‐hospital cardiac arrest refractory to cardiopulmonary resuscitation (CPR) in Taiwan had close to a 20% increase in survival to hospital discharge when treated with VA‐ECMO.[12] A retrospective study of 1764 patients who had cardiac surgery from 2002 to 2006 in Taiwan demonstrated that, of the nearly 3% who required ECMO for postoperative cardiogenic shock, 53% were successfully weaned from ECMO and had a 1‐year survival approaching 30%.[13] A 2003 to 2006 study of 5750 patients undergoing cardiac surgery in Germany found that of the 0.8% of patients requiring VA‐ECMO for refractory cardiogenic shock, 29% survived to discharge, and 22% were alive at 1 year.[14] In France, among 81 patients who received ECMO for refractory cardiogenic shock from 2002 to 2006, 42% survived to hospital discharge.[15]
The survival benefit associated with adult ECMO is thought to stem both from improvements in circuit design (advancements in the pump and oxygenator), as well as from better patient selection. Further, antithrombotic circuit tubing has allowed for lower levels of anticoagulation and less risk of fatal bleeding.[16] According to the ELSO, a group that maintains an active registry of data from medical centers providing ECMO, in 2013 there were approximately 223 ECMO centers, a significant increase from the 83 centers present in 1990; there were nearly 4400 ECMO cases (all ages) in 2013.[17]
Although the number of physicians, patients, and families who consider ECMO as a treatment option have all expanded considerably in recent years and continue to rise, the use of the technology is often discretionary, and decisions as to whether and when to initiate and discontinue ECMO are not always clear‐cut either clinically or ethically.
TREATMENT WITH ECMO
Typically ECMO is initiated not as a treatment itself, but rather as a means to support a patient with cardiopulmonary failure, in order to buy time. Time for an intervention that may serve to fix the underlying organ defect, or time to allow the organ to heal on its own. As such, ECMO is often considered either a bridge to recovery or a bridge to a definitive and longer‐term treatment option (ie, VAD, heart or lung transplantation).[16, 18] ECMO is especially valuable given that the mechanical oxygenation and perfusion provide time for additional workup and intervention, which would not otherwise be feasible for a patient suffering from acute cardiopulmonary collapse.
There are 3 possible clinical outcomes for patients treated with ECMO: (1) native cardiopulmonary recovery and successful weaning off ECMO; (2) failure to recover, with ECMO serving as a bridge to a longer‐term circulatory support device or heart or lung transplantation; or (3) death.
Presently, ECMO may only be provided in an intensive care setting and only temporarily. Patients on VV‐ECMO may be maintained on the machine for weeks to months in some cases, and may be awake, walking, and talking, potentially allowing for these individuals to directly participate in discussions about goals of care.[19, 20] In contrast, adult patients on VA‐ECMO historically have only been maintained for days to weeks on the machine, intubated and typically sedated, making their participation in goals of care discussions generally more difficult, if not impossible.[7] As collective expertise in adult VA‐ECMO grows, however, patients awaiting heart or heart/lung transplants are similarly finding support for longer periods of time, enabling wakefulness and the ability to participate in decision making. Generally speaking, if a patient on ECMO neither recovers nor is a candidate for a longer‐term support device or transplantation, the risks of thromboembolic and infectious complications from continuing the treatment will eventually outweigh any real benefit. Accordingly, ELSO recommends that ECMO should be discontinued promptly if there is no hope for healthy survival (severe brain damage, no hope of heart or lung recovery, and no hope of organ replacement by VAD or transplant).[21]
Given that approximately 32% of adults treated with ECMO for cardiac failure and 47% treated for respiratory failure will survive to hospital discharge, many patients and families will be forced to make difficult, end‐of‐life decisions with ECMO.[22] ECMO is different from other life‐sustaining therapy (LST), such as mechanical ventilation, in that it may only be provided in an intensive care setting. Furthermore, unlike patients who cannot wean from a ventilator and thus are transitioned to a tracheostomy, there is no long‐term treatment option with ECMO. Terminal discontinuation is the sole option for patients on VA‐ECMO who do not recover and are not candidates for VAD or transplantation.
The remainder of this article will examine the ethical issues that emerge with ECMO. We will focus more specifically on VA‐ECMO, although certainly issues described and the guidance offered are relevant to VV‐ECMO. VA‐ECMO presents some unique issues, however, as patients are generally (although not uniformly) intubated, sedated, and thus incapacitated and unable to participate in goals of care discussions once treatment is initiated. Thus, the hospitalist can help ensure, preemptively, that the provision of VA‐ECMO is consistent with patient preferences and goals of care. In addition, VA‐ECMO is also unique in that some patients suffering from cardiac arrest refractory to cardiopulmonary resuscitation and advanced cardiac life support may be successfully oxygenated and perfused with VA‐ECMO; thus, VA‐ECMO extends the boundaries of what we commonly consider to be the limits of cardiac resuscitation, perhaps suggesting a need to reframe do not resuscitate (DNR) discussions.
VA‐ECMO: ETHICAL CONSIDERATIONS
Ethical concerns and difficult decisions may arise at any time during treatment with VA‐ECMO. For teaching purposes, we have conceptualized the treatment trajectory as consisting of 3 phases: (1) initiation, (2) continuation, and (3) discontinuation, each with its own set of issues (Table 1). Clinically, however, each phase of treatment is intrinsically linked to the others, and in reality clinicians must look forward, anticipate upcoming decisions to the extent possible, and prepare families for what lies ahead. Before we attend to each phase, we will briefly review who makes these decisions.
| Treatment Phase | Ethical Issues | Suggested Ethical Theories |
|---|---|---|
| Initiation | Informed consent | Emergency presumption |
| Goals of Care | ||
| Proportionality | ||
| Religious or cultural objection to terminal discontinuation of life‐sustaining therapy | Preventive ethics | |
| Justice | ||
| Proportionality | ||
| Goals of care | ||
| Continuation | On‐going consent | Proportionality |
| Autonomy | ||
| Goals of care | ||
| Discontinuation | Informed consent | Goals of care |
| Autonomy | ||
| Futility disputes | Preventive Ethics | |
| Respect for persons | ||
| Mediation | ||
| Religious or cultural objection to terminal discontinuation of life‐sustaining therapy | Proportionality | |
| Goals of care |
Who Decides?
Central to contemporary Western medicine is the principle of autonomy, manifested in most medical encounters as allowing patients to decide for themselves what should be done to and for them.[23] When patients are incapacitated, however, others must decide for them. Physicians must be prepared to guide families, with limited knowledge and familiarity with VA‐ECMO, through this process, providing information so that they truly can make informed decisions.[24]
In the absence of a patient‐designated healthcare agent or proxy, we turn to the surrogate of highest priority to assist with decision making. Although this may vary by jurisdiction, the typical hierarchy for surrogate decision making is as follows from highest to lowest priority: a court‐ appointed guardian or committee, a spouse or domestic partner, an adult son or daughter (>18 years old), a parent, a sibling, and then other relatives or close friends.[25] It should be noted that all adult children, regardless of age or birth order, should have equal standing as surrogate decision makers. In addition, if the surrogate of highest priority is unavailable or unwilling to make decisions, he or she may not simply delegate decision making to another person; we instead turn to the next individual in the hierarchy presented above.
Initiation of VA‐ECMO
VA‐ECMO is often initiated in emergencies, leaving little time for customary informed consent prior to treatment. Given that the need for VA‐ECMO might be anticipated earlier in the course of illness, however, in patients with chronic heart failure, those undergoing heart surgery, or those at risk for myocardial infarction, there may be an opportunity to initiate the consent process earlier. When possible, for patients or for families/surrogates, the consent process should include a full discussion of the risks, benefits, and goals of the VA‐ECMO, to allow for consideration of both the benefits and burdens of this treatment. This process should occur in conjunction with an exploration of goals of care and current or prior expressed wishes about medical and/or end‐of‐life care. As such, the hospitalist, particularly a hospitalist who may have had a longitudinal relationship with the patient, is integral to this process.
The hospitalist can help patients to clarify goals of care and elucidate whether a trial of VA‐ECMO, should it be medically indicated, is consistent with goals and wishes. Anticipating the need for ECMO and discussing it in advance will be advantageous, regardless of the ultimate decision, for if the patient loses capacity at any point during the course of treatment, documentation from these prior discussions about goals of care and attitudes toward various treatment modalities may serve as an advance directive to guide treatment decisions. Looking forward, as the use of VA‐ECMO becomes increasingly more commonplace, discussions about advance directives may expand accordingly, routinely integrating discussions of VA‐ECMO as a vital topic for consideration and reflection.
Continuation of VA‐ECMO
Once a patient is stabilized on VA‐ECMO, an opportunity emerges to engage in more comprehensive discussions about prognosis, treatment benefit and burdens, and goals of care. If VA‐ECMO was started emergently, there may not have been an opportunity to obtain informed consent prior to treatment initiation, and this vital task must now be assumed. Regardless of the circumstances, once VA‐ECMO is underway, we recommend that physicians regularly engage in discussions of on‐going consent.
We find this term to be helpful as a reminder that, although the patient is already receiving treatment, frequent discussions regarding prognosis, burdens and benefits of treatment, and goals of care remain essential. Clinically, it is important to monitor cardiopulmonary recovery and also renal function and neurologic status. As previously discussed, VA‐ECMO will not serve to fix the underlying cardiopulmonary pathology, and in fact, complications related to VA‐ECMO may be expected to grow over time.[7] Proportionality, a careful analysis of the benefits of continuing treatment, balanced with the risks and burdens imposed, will allow for thoughtful consideration about whether continuation is in the patient's best interest and consistent with the goals of care.
Discontinuation of VA‐ECMO
Three primary clinical indications may prompt the recommendation to discontinue VA‐ECMO: (1) there may be sufficient recovery and cardiopulmonary support is no longer needed, (2) there may be insufficent recovery with plans to transition to a VAD or transplantation, (3) or there may be insufficient recovery and recommendation for terminal discontinuation.
The procedure for discontinuing VA‐ECMO may vary with the clinical circumstances and institution. To anticipate the likely outcome with VA‐ECMO removed, prior to decannulation (the removal of the ECMO cannulas), support might be weaned weaned down with echocardiography used to assess cardiac function. Should indications point to decannulation, this process may take place in the operating room or catheters may be removed at the bedside.[7] In cases of terminal discontinuation, the VA‐ECMO may be stopped (assuming the patient is adequately sedated), the patient will then be allowed to die, with the cannulas subsequently removed.
Analogous to discontinuation of other cardiac devices, such as a pacemaker or defibrillator, ceasing VA‐ECMO may result in: (1) no clinical consequences, as the patient has recovered sufficiently; (2) immediate declaration of death; or (3) the emergence of new symptoms, for example symptoms of heart failure, which may precede death.[26] So as to prospectively account for this variability, a full discussion of the rationale behind discontinuation, as well as the range of expected outcomes, should precede cessation. Similarly, clinicians should implement a plan for symptom management and palliation. In cases of expected recovery, a contingency plan should be developed in case the patient unexpectedly decompensates upon or shortly after cessation. In sum, it remains essential to understand the prospective course, as the lack of anticipatory planning may precipitate confusion, distress, and conflict for patients, family members, and the clinical team.
DNR on VA‐ECMO?
Hospitalists accustomed to writing DNR orders may be distressed to find that, in our opinion, DNR orders are not appropriate for patients who are maintained on VA‐ECMO.[9] (It should also be noted that patients on VV‐ECMO, a device that only provides pulmonary function, could suffer a cardiac arrest necessitating CPR; thus, DNR may be relevant in this clinical context.) VA‐ECMO provides more effective oxygenation and perfusion than traditional advanced cardiac life support with CPR. Thus patients on VA‐ECMO will generally not receive CPR and, consequently, there is effectively no clinical meaning to a DNR order for a patient on VA‐ECMO. That said, when discontinuing VA‐ECMO (and at times VV‐ECMO), depending on the goals of care, a DNR may be useful to prevent further aggressive treatment should the patient arrest following cessation of ECMO.
The clinician will be wise to recognize that if families request a DNR order for a patient on VA‐ECMO, they are asking for something. Although a request not to resuscitate may not make medical sense in this context, clinicians must take the time to explore what is intended by this request. For many families, DNR is a stepping stone toward de‐escalation of treatment and a first move toward withdrawal of life‐sustaining therapies.[27, 28] A nuanced understanding of what a family hopes to accomplish by the suggested order, and specifically whether and how goals of care may have changed, is vital toward the maintenance of an appropriate, timely, and evolving treatment plan.
Terminal Discontinuation of VA‐ECMO
Among clinical ethicists, some of the most distressing conversations and meetings we have had with families have emerged in the context of terminal discontinuation of VA‐ECMO. Unlike mechanical ventilation, which theoretically may be continued indefinitely via tracheostomy, VA‐ECMO is only a temporary measure and, according to ELSO, should be discontinued promptly if healthy survival is not anticipated with the possibility of stopping for futility explained to the family before ECLS is begun.[21] Given the time constraints for what may have been an emergency procedure, and given the frequent reluctance of families and surrogates to discontinue life‐sustaining therapies, how does a clinician or institution ethically enact these guidelines? With respect to practical guidance, we offer 3 suggestions for directing these conversations.
First, we suggest physicians discuss the possibility and potential rationales of terminal discontinuation early and often, ideally as part of the initial consent process. Second, informed consent conversations should address potential complications (stroke, hemorrhage, and thrombosis) and their sequelae alongside discussions with patients and surrogates about their wishes in the context of such an event. Finally, we also recommend frequently revisiting the goals of care with the surrogate throughout the course of treatment.[28] Thus, when goals of care can no longer be achieved by continuing VA‐ECMO, either: (1) because the patient has no chance for recovery; (2) because VA‐ECMO no longer serves its intended purpose; or (3) owing to harm from complications, families may be able to appreciate that continuation of the intervention has become ethically disproportionate, and ECMO is now more burdensome than beneficial. Continuous and open dialogue should build a strong foundation of trust and knowledge that allows the surrogate to understand and accept the rationale behind a recommendation to terminally discontinue treatment, should the clinical course necessitate such.[29]
CONCLUSION
With indications for and utilization of ECMO in adult patients expanding, hospitalists may be expected to encounter these technologies with greater frequency and guide patients and families with medical decision‐making. Although the ethical issues reviewed are certainly not exclusive to ECMO, specific facets of ECMO, as discussed, may precipitate unique challenges or exacerbate common ones. Hospitalists can help to uphold patient autonomy by providing information that enables patients and surrogates to actively participate in goal setting and decision‐making. As the utilization of this technology grows, further research will need to address decision‐making in the context of ECMO to ensure that the process remains optimally patient‐ and family‐centered.
Acknowledgment
Disclosures: All work was performed at Weill Cornell Medical College of Cornell University, New York Presbyterian Weill Cornell Medical Center, and University of Pennsylvania. The authors report no conflicts of interest.
- , . Current status of extracorporeal life support (ECMO) for cardiopulmonary failure. Minerva Anestesiol. 2010;76(7):534–540.
- . . The first 20 years of the heart‐lung machine. Tex Heart Inst J. 1997;24(1):1–8.
- , , , , . Venovenous extracorporeal membrane oxygenation in adults: practical aspects of circuits, cannulae, and procedures. J Cardiothorac Vasc Anesth. 2012;26(5):893–909.
- , , , et al. Extracorporeal life support for adult cardiorespiratory failure. Surgery. 1993;114(2):161–172; discussion 172–163.
- , . Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–1914.
- , , , , . Extracorporeal life support. BMJ. 2010;341:c5317.
- , , , , , . Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2‐technical considerations. J Cardiothorac Vasc Anesth. 2010;24(1):164–172.
- , . Mechanical circulatory support for bridge to decision: which device and when to decide. J Card Surg. 2010;25(4):425–433.
- , , . DNR on ECMO: a paradox worth exploring. J Clin Ethics. 2013;25(1):13–19.
- , , , et al. Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR). Health Technol Assess. 2010;14(35):1–46.
- , , , et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895.
- , , , et al. Cardiopulmonary resuscitation with assisted extracorporeal life‐support versus conventional cardiopulmonary resuscitation in adults with in‐hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372(9638):554–561.
- , , , et al. Extracorporeal membrane oxygenation for refractory cardiogenic shock after cardiac surgery: predictors of early mortality and outcome from 51 adult patients. Eur J Cardiothorac Surg. 2010;37(2):328–333.
- , , , et al. Venoarterial extracorporeal membrane oxygenation for treatment of cardiogenic shock: clinical experiences in 45 adult patients. J Thorac Cardiovasc Surg. 2008;135(2):382–388.
- , , , et al. Outcomes and long‐term quality‐of‐life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36(5):1404–1411.
- , . Extracorporeal life support as a bridge to lung transplantation. Clin Chest Med. 2011;32(2):245–251.
- Extracoporeal Life Support Organization. General Guidelines for all ECLS Cases. 2012; http://www.elsonet.org/. Accessed August 5, 2014.
- , , , et al. Impact of extracorporeal life support on outcome in patients with idiopathic pulmonary arterial hypertension awaiting lung transplantation. J Heart Lung Transplant. 2011;30(9):997–1002.
- , , , . Ambulatory extracorporeal membrane oxygenation: a new approach for bridge‐to‐lung transplantation. J Thorac Cardiovasc Surg. 2010;139(6):e137–e139.
- , , , et al. Ambulatory veno‐venous extracorporeal membrane oxygenation: innovation and pitfalls. J Thorac Cardiovasc Surg. 2011;142(4):755–761.
- Extracoporeal Life Support Organization. General Guidelines for all ECLS Cases. 2012; http://www.elsonet.org/. Accessed August 5, 2014.
- Extracorporeal Life Support Organization. ECLS Registry Report United States Summary. August 2014; http://www.elsonet.org/. Accessed August 5, 2014.
- , . Principles of Biomedical Ethics. 6th ed. New York, NY: Oxford University Press; 2009.
- , , , et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125(15):1928–1952.
- , , . Ethics in Clinical Practice. 2nd ed. Sudbury, MA: Jones and Bartlett; 2005.
- . Pacemaker and defibrillator deactivation in competent hospice patients: an ethical consideration. Am J Hosp Palliat Care. 2005;22(1):14–19.
- , , , . Limitation of medical care: an ethnographic analysis. J Clin Ethics. 1993;4(2):134–145.
- . A Palliative Ethic of Care: Clinical Wisdom at Life's End. Sudbury, MA: Jones and Bartlett; 2006.
- , , , , , . Extracorporeal membrane oxygenation as a bridge to chemotherapy in an orthodox Jewish patient. Oncologist. 2014;19(9):985–989.
As the distribution and utilization of technology in critical care medicine expands, patients experiencing respiratory failure, heart failure, or cardiac arrest are increasingly being treated with extracorporeal membrane oxygenation (ECMO). Although not customarily responsible for managing ECMO, hospitalists need to understand the rudiments of this technology and its associated ethical issues to assure that ECMO use is consistent with patient preferences and goals of care. This review aims to help prepare hospitalists for these clinical responsibilities. Following a brief review of modern‐day ECMO, including both venoarterial extracorporeal membrane oxygenation (VA‐ECMO) and venovenous extracorporeal membrane oxygenation (VV‐ECMO), we highlight special ethical considerations that may arise with VA‐ECMO and present an ethically grounded approach to the initiation, continuation, and discontinuation of treatment.
Many of the questions regarding the use of ECMO will be familiar. Certainly, similar questions arise with other life‐sustaining therapies; however, the general hospitalist may be a bit unfamiliar with ECMO and its unique ethical challenges. For example, ECMO is only provided transiently and generally while patients are in an intensive care unit. Unlike mechanical ventilation, which may be provided long‐term via tracheostomy, there is no comparable, enduring form of ECMO. Next, patients requiring ECMO are utterly dependent on the machine for their survival. If they do not recover and are not candidates for a ventricular assist device (VAD) or transplantation, there are no other therapies to offer. In this scenario, terminal discontinuation is the only option.
Informed hospitalists, who bring to counseling sessions both an understanding of the patient and family, and technical knowledge and background information on ECMO, will be far better equipped to help patients and families facing these difficult choices. As the use of ECMO becomes more prevalent, hospitalists must be prepared to address questions related to this evolving technology.
TECHNICAL AND HISTORICAL BACKGROUND
Extracorporeal life support (ECLS) involves the use of mechanical devices when native organ function fails.[1] ECMO involves the application of ECLS to provide a replacement form of cardiac and/or pulmonary function. An illustrative figure of the ECMO circuit may be seen at The Extracorporeal Life Support Organization (ELSO) (
Encouraging outcomes of clinical trials have ushered in enthusiasm for adult ECMO in the United States.[9] For example, the Conventional Ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial, a prospective study of adult VV‐ECMO for respiratory failure conducted in the United Kingdom from 2001 to 2006, demonstrated a measurable survival benefit. Patients with severe adult respiratory failure randomized to an ECMO center (75% received ECMO) had a 63% 6‐month survival without severe disability, versus 47% for patients managed conventionally at a tertiary care center.[10] Similarly, data from the 2009 H1N1 flu virus epidemic in Australia and New Zealand suggested a benefit when patients with acute respiratory distress syndrome, who had failed mechanical ventilation, were treated with ECMO; 76% survived, which was an improvement over previously reported mortality rates of 30% to 48%.[11]
With respect to VA‐ECMO, recent studies and case reports out of Taiwan, Germany, and France propose a survival benefit when ECMO is used in patients with cardiac failure.[12, 13, 14, 15] Patients with in‐hospital cardiac arrest refractory to cardiopulmonary resuscitation (CPR) in Taiwan had close to a 20% increase in survival to hospital discharge when treated with VA‐ECMO.[12] A retrospective study of 1764 patients who had cardiac surgery from 2002 to 2006 in Taiwan demonstrated that, of the nearly 3% who required ECMO for postoperative cardiogenic shock, 53% were successfully weaned from ECMO and had a 1‐year survival approaching 30%.[13] A 2003 to 2006 study of 5750 patients undergoing cardiac surgery in Germany found that of the 0.8% of patients requiring VA‐ECMO for refractory cardiogenic shock, 29% survived to discharge, and 22% were alive at 1 year.[14] In France, among 81 patients who received ECMO for refractory cardiogenic shock from 2002 to 2006, 42% survived to hospital discharge.[15]
The survival benefit associated with adult ECMO is thought to stem both from improvements in circuit design (advancements in the pump and oxygenator), as well as from better patient selection. Further, antithrombotic circuit tubing has allowed for lower levels of anticoagulation and less risk of fatal bleeding.[16] According to the ELSO, a group that maintains an active registry of data from medical centers providing ECMO, in 2013 there were approximately 223 ECMO centers, a significant increase from the 83 centers present in 1990; there were nearly 4400 ECMO cases (all ages) in 2013.[17]
Although the number of physicians, patients, and families who consider ECMO as a treatment option have all expanded considerably in recent years and continue to rise, the use of the technology is often discretionary, and decisions as to whether and when to initiate and discontinue ECMO are not always clear‐cut either clinically or ethically.
TREATMENT WITH ECMO
Typically ECMO is initiated not as a treatment itself, but rather as a means to support a patient with cardiopulmonary failure, in order to buy time. Time for an intervention that may serve to fix the underlying organ defect, or time to allow the organ to heal on its own. As such, ECMO is often considered either a bridge to recovery or a bridge to a definitive and longer‐term treatment option (ie, VAD, heart or lung transplantation).[16, 18] ECMO is especially valuable given that the mechanical oxygenation and perfusion provide time for additional workup and intervention, which would not otherwise be feasible for a patient suffering from acute cardiopulmonary collapse.
There are 3 possible clinical outcomes for patients treated with ECMO: (1) native cardiopulmonary recovery and successful weaning off ECMO; (2) failure to recover, with ECMO serving as a bridge to a longer‐term circulatory support device or heart or lung transplantation; or (3) death.
Presently, ECMO may only be provided in an intensive care setting and only temporarily. Patients on VV‐ECMO may be maintained on the machine for weeks to months in some cases, and may be awake, walking, and talking, potentially allowing for these individuals to directly participate in discussions about goals of care.[19, 20] In contrast, adult patients on VA‐ECMO historically have only been maintained for days to weeks on the machine, intubated and typically sedated, making their participation in goals of care discussions generally more difficult, if not impossible.[7] As collective expertise in adult VA‐ECMO grows, however, patients awaiting heart or heart/lung transplants are similarly finding support for longer periods of time, enabling wakefulness and the ability to participate in decision making. Generally speaking, if a patient on ECMO neither recovers nor is a candidate for a longer‐term support device or transplantation, the risks of thromboembolic and infectious complications from continuing the treatment will eventually outweigh any real benefit. Accordingly, ELSO recommends that ECMO should be discontinued promptly if there is no hope for healthy survival (severe brain damage, no hope of heart or lung recovery, and no hope of organ replacement by VAD or transplant).[21]
Given that approximately 32% of adults treated with ECMO for cardiac failure and 47% treated for respiratory failure will survive to hospital discharge, many patients and families will be forced to make difficult, end‐of‐life decisions with ECMO.[22] ECMO is different from other life‐sustaining therapy (LST), such as mechanical ventilation, in that it may only be provided in an intensive care setting. Furthermore, unlike patients who cannot wean from a ventilator and thus are transitioned to a tracheostomy, there is no long‐term treatment option with ECMO. Terminal discontinuation is the sole option for patients on VA‐ECMO who do not recover and are not candidates for VAD or transplantation.
The remainder of this article will examine the ethical issues that emerge with ECMO. We will focus more specifically on VA‐ECMO, although certainly issues described and the guidance offered are relevant to VV‐ECMO. VA‐ECMO presents some unique issues, however, as patients are generally (although not uniformly) intubated, sedated, and thus incapacitated and unable to participate in goals of care discussions once treatment is initiated. Thus, the hospitalist can help ensure, preemptively, that the provision of VA‐ECMO is consistent with patient preferences and goals of care. In addition, VA‐ECMO is also unique in that some patients suffering from cardiac arrest refractory to cardiopulmonary resuscitation and advanced cardiac life support may be successfully oxygenated and perfused with VA‐ECMO; thus, VA‐ECMO extends the boundaries of what we commonly consider to be the limits of cardiac resuscitation, perhaps suggesting a need to reframe do not resuscitate (DNR) discussions.
VA‐ECMO: ETHICAL CONSIDERATIONS
Ethical concerns and difficult decisions may arise at any time during treatment with VA‐ECMO. For teaching purposes, we have conceptualized the treatment trajectory as consisting of 3 phases: (1) initiation, (2) continuation, and (3) discontinuation, each with its own set of issues (Table 1). Clinically, however, each phase of treatment is intrinsically linked to the others, and in reality clinicians must look forward, anticipate upcoming decisions to the extent possible, and prepare families for what lies ahead. Before we attend to each phase, we will briefly review who makes these decisions.
| Treatment Phase | Ethical Issues | Suggested Ethical Theories |
|---|---|---|
| Initiation | Informed consent | Emergency presumption |
| Goals of Care | ||
| Proportionality | ||
| Religious or cultural objection to terminal discontinuation of life‐sustaining therapy | Preventive ethics | |
| Justice | ||
| Proportionality | ||
| Goals of care | ||
| Continuation | On‐going consent | Proportionality |
| Autonomy | ||
| Goals of care | ||
| Discontinuation | Informed consent | Goals of care |
| Autonomy | ||
| Futility disputes | Preventive Ethics | |
| Respect for persons | ||
| Mediation | ||
| Religious or cultural objection to terminal discontinuation of life‐sustaining therapy | Proportionality | |
| Goals of care |
Who Decides?
Central to contemporary Western medicine is the principle of autonomy, manifested in most medical encounters as allowing patients to decide for themselves what should be done to and for them.[23] When patients are incapacitated, however, others must decide for them. Physicians must be prepared to guide families, with limited knowledge and familiarity with VA‐ECMO, through this process, providing information so that they truly can make informed decisions.[24]
In the absence of a patient‐designated healthcare agent or proxy, we turn to the surrogate of highest priority to assist with decision making. Although this may vary by jurisdiction, the typical hierarchy for surrogate decision making is as follows from highest to lowest priority: a court‐ appointed guardian or committee, a spouse or domestic partner, an adult son or daughter (>18 years old), a parent, a sibling, and then other relatives or close friends.[25] It should be noted that all adult children, regardless of age or birth order, should have equal standing as surrogate decision makers. In addition, if the surrogate of highest priority is unavailable or unwilling to make decisions, he or she may not simply delegate decision making to another person; we instead turn to the next individual in the hierarchy presented above.
Initiation of VA‐ECMO
VA‐ECMO is often initiated in emergencies, leaving little time for customary informed consent prior to treatment. Given that the need for VA‐ECMO might be anticipated earlier in the course of illness, however, in patients with chronic heart failure, those undergoing heart surgery, or those at risk for myocardial infarction, there may be an opportunity to initiate the consent process earlier. When possible, for patients or for families/surrogates, the consent process should include a full discussion of the risks, benefits, and goals of the VA‐ECMO, to allow for consideration of both the benefits and burdens of this treatment. This process should occur in conjunction with an exploration of goals of care and current or prior expressed wishes about medical and/or end‐of‐life care. As such, the hospitalist, particularly a hospitalist who may have had a longitudinal relationship with the patient, is integral to this process.
The hospitalist can help patients to clarify goals of care and elucidate whether a trial of VA‐ECMO, should it be medically indicated, is consistent with goals and wishes. Anticipating the need for ECMO and discussing it in advance will be advantageous, regardless of the ultimate decision, for if the patient loses capacity at any point during the course of treatment, documentation from these prior discussions about goals of care and attitudes toward various treatment modalities may serve as an advance directive to guide treatment decisions. Looking forward, as the use of VA‐ECMO becomes increasingly more commonplace, discussions about advance directives may expand accordingly, routinely integrating discussions of VA‐ECMO as a vital topic for consideration and reflection.
Continuation of VA‐ECMO
Once a patient is stabilized on VA‐ECMO, an opportunity emerges to engage in more comprehensive discussions about prognosis, treatment benefit and burdens, and goals of care. If VA‐ECMO was started emergently, there may not have been an opportunity to obtain informed consent prior to treatment initiation, and this vital task must now be assumed. Regardless of the circumstances, once VA‐ECMO is underway, we recommend that physicians regularly engage in discussions of on‐going consent.
We find this term to be helpful as a reminder that, although the patient is already receiving treatment, frequent discussions regarding prognosis, burdens and benefits of treatment, and goals of care remain essential. Clinically, it is important to monitor cardiopulmonary recovery and also renal function and neurologic status. As previously discussed, VA‐ECMO will not serve to fix the underlying cardiopulmonary pathology, and in fact, complications related to VA‐ECMO may be expected to grow over time.[7] Proportionality, a careful analysis of the benefits of continuing treatment, balanced with the risks and burdens imposed, will allow for thoughtful consideration about whether continuation is in the patient's best interest and consistent with the goals of care.
Discontinuation of VA‐ECMO
Three primary clinical indications may prompt the recommendation to discontinue VA‐ECMO: (1) there may be sufficient recovery and cardiopulmonary support is no longer needed, (2) there may be insufficent recovery with plans to transition to a VAD or transplantation, (3) or there may be insufficient recovery and recommendation for terminal discontinuation.
The procedure for discontinuing VA‐ECMO may vary with the clinical circumstances and institution. To anticipate the likely outcome with VA‐ECMO removed, prior to decannulation (the removal of the ECMO cannulas), support might be weaned weaned down with echocardiography used to assess cardiac function. Should indications point to decannulation, this process may take place in the operating room or catheters may be removed at the bedside.[7] In cases of terminal discontinuation, the VA‐ECMO may be stopped (assuming the patient is adequately sedated), the patient will then be allowed to die, with the cannulas subsequently removed.
Analogous to discontinuation of other cardiac devices, such as a pacemaker or defibrillator, ceasing VA‐ECMO may result in: (1) no clinical consequences, as the patient has recovered sufficiently; (2) immediate declaration of death; or (3) the emergence of new symptoms, for example symptoms of heart failure, which may precede death.[26] So as to prospectively account for this variability, a full discussion of the rationale behind discontinuation, as well as the range of expected outcomes, should precede cessation. Similarly, clinicians should implement a plan for symptom management and palliation. In cases of expected recovery, a contingency plan should be developed in case the patient unexpectedly decompensates upon or shortly after cessation. In sum, it remains essential to understand the prospective course, as the lack of anticipatory planning may precipitate confusion, distress, and conflict for patients, family members, and the clinical team.
DNR on VA‐ECMO?
Hospitalists accustomed to writing DNR orders may be distressed to find that, in our opinion, DNR orders are not appropriate for patients who are maintained on VA‐ECMO.[9] (It should also be noted that patients on VV‐ECMO, a device that only provides pulmonary function, could suffer a cardiac arrest necessitating CPR; thus, DNR may be relevant in this clinical context.) VA‐ECMO provides more effective oxygenation and perfusion than traditional advanced cardiac life support with CPR. Thus patients on VA‐ECMO will generally not receive CPR and, consequently, there is effectively no clinical meaning to a DNR order for a patient on VA‐ECMO. That said, when discontinuing VA‐ECMO (and at times VV‐ECMO), depending on the goals of care, a DNR may be useful to prevent further aggressive treatment should the patient arrest following cessation of ECMO.
The clinician will be wise to recognize that if families request a DNR order for a patient on VA‐ECMO, they are asking for something. Although a request not to resuscitate may not make medical sense in this context, clinicians must take the time to explore what is intended by this request. For many families, DNR is a stepping stone toward de‐escalation of treatment and a first move toward withdrawal of life‐sustaining therapies.[27, 28] A nuanced understanding of what a family hopes to accomplish by the suggested order, and specifically whether and how goals of care may have changed, is vital toward the maintenance of an appropriate, timely, and evolving treatment plan.
Terminal Discontinuation of VA‐ECMO
Among clinical ethicists, some of the most distressing conversations and meetings we have had with families have emerged in the context of terminal discontinuation of VA‐ECMO. Unlike mechanical ventilation, which theoretically may be continued indefinitely via tracheostomy, VA‐ECMO is only a temporary measure and, according to ELSO, should be discontinued promptly if healthy survival is not anticipated with the possibility of stopping for futility explained to the family before ECLS is begun.[21] Given the time constraints for what may have been an emergency procedure, and given the frequent reluctance of families and surrogates to discontinue life‐sustaining therapies, how does a clinician or institution ethically enact these guidelines? With respect to practical guidance, we offer 3 suggestions for directing these conversations.
First, we suggest physicians discuss the possibility and potential rationales of terminal discontinuation early and often, ideally as part of the initial consent process. Second, informed consent conversations should address potential complications (stroke, hemorrhage, and thrombosis) and their sequelae alongside discussions with patients and surrogates about their wishes in the context of such an event. Finally, we also recommend frequently revisiting the goals of care with the surrogate throughout the course of treatment.[28] Thus, when goals of care can no longer be achieved by continuing VA‐ECMO, either: (1) because the patient has no chance for recovery; (2) because VA‐ECMO no longer serves its intended purpose; or (3) owing to harm from complications, families may be able to appreciate that continuation of the intervention has become ethically disproportionate, and ECMO is now more burdensome than beneficial. Continuous and open dialogue should build a strong foundation of trust and knowledge that allows the surrogate to understand and accept the rationale behind a recommendation to terminally discontinue treatment, should the clinical course necessitate such.[29]
CONCLUSION
With indications for and utilization of ECMO in adult patients expanding, hospitalists may be expected to encounter these technologies with greater frequency and guide patients and families with medical decision‐making. Although the ethical issues reviewed are certainly not exclusive to ECMO, specific facets of ECMO, as discussed, may precipitate unique challenges or exacerbate common ones. Hospitalists can help to uphold patient autonomy by providing information that enables patients and surrogates to actively participate in goal setting and decision‐making. As the utilization of this technology grows, further research will need to address decision‐making in the context of ECMO to ensure that the process remains optimally patient‐ and family‐centered.
Acknowledgment
Disclosures: All work was performed at Weill Cornell Medical College of Cornell University, New York Presbyterian Weill Cornell Medical Center, and University of Pennsylvania. The authors report no conflicts of interest.
As the distribution and utilization of technology in critical care medicine expands, patients experiencing respiratory failure, heart failure, or cardiac arrest are increasingly being treated with extracorporeal membrane oxygenation (ECMO). Although not customarily responsible for managing ECMO, hospitalists need to understand the rudiments of this technology and its associated ethical issues to assure that ECMO use is consistent with patient preferences and goals of care. This review aims to help prepare hospitalists for these clinical responsibilities. Following a brief review of modern‐day ECMO, including both venoarterial extracorporeal membrane oxygenation (VA‐ECMO) and venovenous extracorporeal membrane oxygenation (VV‐ECMO), we highlight special ethical considerations that may arise with VA‐ECMO and present an ethically grounded approach to the initiation, continuation, and discontinuation of treatment.
Many of the questions regarding the use of ECMO will be familiar. Certainly, similar questions arise with other life‐sustaining therapies; however, the general hospitalist may be a bit unfamiliar with ECMO and its unique ethical challenges. For example, ECMO is only provided transiently and generally while patients are in an intensive care unit. Unlike mechanical ventilation, which may be provided long‐term via tracheostomy, there is no comparable, enduring form of ECMO. Next, patients requiring ECMO are utterly dependent on the machine for their survival. If they do not recover and are not candidates for a ventricular assist device (VAD) or transplantation, there are no other therapies to offer. In this scenario, terminal discontinuation is the only option.
Informed hospitalists, who bring to counseling sessions both an understanding of the patient and family, and technical knowledge and background information on ECMO, will be far better equipped to help patients and families facing these difficult choices. As the use of ECMO becomes more prevalent, hospitalists must be prepared to address questions related to this evolving technology.
TECHNICAL AND HISTORICAL BACKGROUND
Extracorporeal life support (ECLS) involves the use of mechanical devices when native organ function fails.[1] ECMO involves the application of ECLS to provide a replacement form of cardiac and/or pulmonary function. An illustrative figure of the ECMO circuit may be seen at The Extracorporeal Life Support Organization (ELSO) (
Encouraging outcomes of clinical trials have ushered in enthusiasm for adult ECMO in the United States.[9] For example, the Conventional Ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial, a prospective study of adult VV‐ECMO for respiratory failure conducted in the United Kingdom from 2001 to 2006, demonstrated a measurable survival benefit. Patients with severe adult respiratory failure randomized to an ECMO center (75% received ECMO) had a 63% 6‐month survival without severe disability, versus 47% for patients managed conventionally at a tertiary care center.[10] Similarly, data from the 2009 H1N1 flu virus epidemic in Australia and New Zealand suggested a benefit when patients with acute respiratory distress syndrome, who had failed mechanical ventilation, were treated with ECMO; 76% survived, which was an improvement over previously reported mortality rates of 30% to 48%.[11]
With respect to VA‐ECMO, recent studies and case reports out of Taiwan, Germany, and France propose a survival benefit when ECMO is used in patients with cardiac failure.[12, 13, 14, 15] Patients with in‐hospital cardiac arrest refractory to cardiopulmonary resuscitation (CPR) in Taiwan had close to a 20% increase in survival to hospital discharge when treated with VA‐ECMO.[12] A retrospective study of 1764 patients who had cardiac surgery from 2002 to 2006 in Taiwan demonstrated that, of the nearly 3% who required ECMO for postoperative cardiogenic shock, 53% were successfully weaned from ECMO and had a 1‐year survival approaching 30%.[13] A 2003 to 2006 study of 5750 patients undergoing cardiac surgery in Germany found that of the 0.8% of patients requiring VA‐ECMO for refractory cardiogenic shock, 29% survived to discharge, and 22% were alive at 1 year.[14] In France, among 81 patients who received ECMO for refractory cardiogenic shock from 2002 to 2006, 42% survived to hospital discharge.[15]
The survival benefit associated with adult ECMO is thought to stem both from improvements in circuit design (advancements in the pump and oxygenator), as well as from better patient selection. Further, antithrombotic circuit tubing has allowed for lower levels of anticoagulation and less risk of fatal bleeding.[16] According to the ELSO, a group that maintains an active registry of data from medical centers providing ECMO, in 2013 there were approximately 223 ECMO centers, a significant increase from the 83 centers present in 1990; there were nearly 4400 ECMO cases (all ages) in 2013.[17]
Although the number of physicians, patients, and families who consider ECMO as a treatment option have all expanded considerably in recent years and continue to rise, the use of the technology is often discretionary, and decisions as to whether and when to initiate and discontinue ECMO are not always clear‐cut either clinically or ethically.
TREATMENT WITH ECMO
Typically ECMO is initiated not as a treatment itself, but rather as a means to support a patient with cardiopulmonary failure, in order to buy time. Time for an intervention that may serve to fix the underlying organ defect, or time to allow the organ to heal on its own. As such, ECMO is often considered either a bridge to recovery or a bridge to a definitive and longer‐term treatment option (ie, VAD, heart or lung transplantation).[16, 18] ECMO is especially valuable given that the mechanical oxygenation and perfusion provide time for additional workup and intervention, which would not otherwise be feasible for a patient suffering from acute cardiopulmonary collapse.
There are 3 possible clinical outcomes for patients treated with ECMO: (1) native cardiopulmonary recovery and successful weaning off ECMO; (2) failure to recover, with ECMO serving as a bridge to a longer‐term circulatory support device or heart or lung transplantation; or (3) death.
Presently, ECMO may only be provided in an intensive care setting and only temporarily. Patients on VV‐ECMO may be maintained on the machine for weeks to months in some cases, and may be awake, walking, and talking, potentially allowing for these individuals to directly participate in discussions about goals of care.[19, 20] In contrast, adult patients on VA‐ECMO historically have only been maintained for days to weeks on the machine, intubated and typically sedated, making their participation in goals of care discussions generally more difficult, if not impossible.[7] As collective expertise in adult VA‐ECMO grows, however, patients awaiting heart or heart/lung transplants are similarly finding support for longer periods of time, enabling wakefulness and the ability to participate in decision making. Generally speaking, if a patient on ECMO neither recovers nor is a candidate for a longer‐term support device or transplantation, the risks of thromboembolic and infectious complications from continuing the treatment will eventually outweigh any real benefit. Accordingly, ELSO recommends that ECMO should be discontinued promptly if there is no hope for healthy survival (severe brain damage, no hope of heart or lung recovery, and no hope of organ replacement by VAD or transplant).[21]
Given that approximately 32% of adults treated with ECMO for cardiac failure and 47% treated for respiratory failure will survive to hospital discharge, many patients and families will be forced to make difficult, end‐of‐life decisions with ECMO.[22] ECMO is different from other life‐sustaining therapy (LST), such as mechanical ventilation, in that it may only be provided in an intensive care setting. Furthermore, unlike patients who cannot wean from a ventilator and thus are transitioned to a tracheostomy, there is no long‐term treatment option with ECMO. Terminal discontinuation is the sole option for patients on VA‐ECMO who do not recover and are not candidates for VAD or transplantation.
The remainder of this article will examine the ethical issues that emerge with ECMO. We will focus more specifically on VA‐ECMO, although certainly issues described and the guidance offered are relevant to VV‐ECMO. VA‐ECMO presents some unique issues, however, as patients are generally (although not uniformly) intubated, sedated, and thus incapacitated and unable to participate in goals of care discussions once treatment is initiated. Thus, the hospitalist can help ensure, preemptively, that the provision of VA‐ECMO is consistent with patient preferences and goals of care. In addition, VA‐ECMO is also unique in that some patients suffering from cardiac arrest refractory to cardiopulmonary resuscitation and advanced cardiac life support may be successfully oxygenated and perfused with VA‐ECMO; thus, VA‐ECMO extends the boundaries of what we commonly consider to be the limits of cardiac resuscitation, perhaps suggesting a need to reframe do not resuscitate (DNR) discussions.
VA‐ECMO: ETHICAL CONSIDERATIONS
Ethical concerns and difficult decisions may arise at any time during treatment with VA‐ECMO. For teaching purposes, we have conceptualized the treatment trajectory as consisting of 3 phases: (1) initiation, (2) continuation, and (3) discontinuation, each with its own set of issues (Table 1). Clinically, however, each phase of treatment is intrinsically linked to the others, and in reality clinicians must look forward, anticipate upcoming decisions to the extent possible, and prepare families for what lies ahead. Before we attend to each phase, we will briefly review who makes these decisions.
| Treatment Phase | Ethical Issues | Suggested Ethical Theories |
|---|---|---|
| Initiation | Informed consent | Emergency presumption |
| Goals of Care | ||
| Proportionality | ||
| Religious or cultural objection to terminal discontinuation of life‐sustaining therapy | Preventive ethics | |
| Justice | ||
| Proportionality | ||
| Goals of care | ||
| Continuation | On‐going consent | Proportionality |
| Autonomy | ||
| Goals of care | ||
| Discontinuation | Informed consent | Goals of care |
| Autonomy | ||
| Futility disputes | Preventive Ethics | |
| Respect for persons | ||
| Mediation | ||
| Religious or cultural objection to terminal discontinuation of life‐sustaining therapy | Proportionality | |
| Goals of care |
Who Decides?
Central to contemporary Western medicine is the principle of autonomy, manifested in most medical encounters as allowing patients to decide for themselves what should be done to and for them.[23] When patients are incapacitated, however, others must decide for them. Physicians must be prepared to guide families, with limited knowledge and familiarity with VA‐ECMO, through this process, providing information so that they truly can make informed decisions.[24]
In the absence of a patient‐designated healthcare agent or proxy, we turn to the surrogate of highest priority to assist with decision making. Although this may vary by jurisdiction, the typical hierarchy for surrogate decision making is as follows from highest to lowest priority: a court‐ appointed guardian or committee, a spouse or domestic partner, an adult son or daughter (>18 years old), a parent, a sibling, and then other relatives or close friends.[25] It should be noted that all adult children, regardless of age or birth order, should have equal standing as surrogate decision makers. In addition, if the surrogate of highest priority is unavailable or unwilling to make decisions, he or she may not simply delegate decision making to another person; we instead turn to the next individual in the hierarchy presented above.
Initiation of VA‐ECMO
VA‐ECMO is often initiated in emergencies, leaving little time for customary informed consent prior to treatment. Given that the need for VA‐ECMO might be anticipated earlier in the course of illness, however, in patients with chronic heart failure, those undergoing heart surgery, or those at risk for myocardial infarction, there may be an opportunity to initiate the consent process earlier. When possible, for patients or for families/surrogates, the consent process should include a full discussion of the risks, benefits, and goals of the VA‐ECMO, to allow for consideration of both the benefits and burdens of this treatment. This process should occur in conjunction with an exploration of goals of care and current or prior expressed wishes about medical and/or end‐of‐life care. As such, the hospitalist, particularly a hospitalist who may have had a longitudinal relationship with the patient, is integral to this process.
The hospitalist can help patients to clarify goals of care and elucidate whether a trial of VA‐ECMO, should it be medically indicated, is consistent with goals and wishes. Anticipating the need for ECMO and discussing it in advance will be advantageous, regardless of the ultimate decision, for if the patient loses capacity at any point during the course of treatment, documentation from these prior discussions about goals of care and attitudes toward various treatment modalities may serve as an advance directive to guide treatment decisions. Looking forward, as the use of VA‐ECMO becomes increasingly more commonplace, discussions about advance directives may expand accordingly, routinely integrating discussions of VA‐ECMO as a vital topic for consideration and reflection.
Continuation of VA‐ECMO
Once a patient is stabilized on VA‐ECMO, an opportunity emerges to engage in more comprehensive discussions about prognosis, treatment benefit and burdens, and goals of care. If VA‐ECMO was started emergently, there may not have been an opportunity to obtain informed consent prior to treatment initiation, and this vital task must now be assumed. Regardless of the circumstances, once VA‐ECMO is underway, we recommend that physicians regularly engage in discussions of on‐going consent.
We find this term to be helpful as a reminder that, although the patient is already receiving treatment, frequent discussions regarding prognosis, burdens and benefits of treatment, and goals of care remain essential. Clinically, it is important to monitor cardiopulmonary recovery and also renal function and neurologic status. As previously discussed, VA‐ECMO will not serve to fix the underlying cardiopulmonary pathology, and in fact, complications related to VA‐ECMO may be expected to grow over time.[7] Proportionality, a careful analysis of the benefits of continuing treatment, balanced with the risks and burdens imposed, will allow for thoughtful consideration about whether continuation is in the patient's best interest and consistent with the goals of care.
Discontinuation of VA‐ECMO
Three primary clinical indications may prompt the recommendation to discontinue VA‐ECMO: (1) there may be sufficient recovery and cardiopulmonary support is no longer needed, (2) there may be insufficent recovery with plans to transition to a VAD or transplantation, (3) or there may be insufficient recovery and recommendation for terminal discontinuation.
The procedure for discontinuing VA‐ECMO may vary with the clinical circumstances and institution. To anticipate the likely outcome with VA‐ECMO removed, prior to decannulation (the removal of the ECMO cannulas), support might be weaned weaned down with echocardiography used to assess cardiac function. Should indications point to decannulation, this process may take place in the operating room or catheters may be removed at the bedside.[7] In cases of terminal discontinuation, the VA‐ECMO may be stopped (assuming the patient is adequately sedated), the patient will then be allowed to die, with the cannulas subsequently removed.
Analogous to discontinuation of other cardiac devices, such as a pacemaker or defibrillator, ceasing VA‐ECMO may result in: (1) no clinical consequences, as the patient has recovered sufficiently; (2) immediate declaration of death; or (3) the emergence of new symptoms, for example symptoms of heart failure, which may precede death.[26] So as to prospectively account for this variability, a full discussion of the rationale behind discontinuation, as well as the range of expected outcomes, should precede cessation. Similarly, clinicians should implement a plan for symptom management and palliation. In cases of expected recovery, a contingency plan should be developed in case the patient unexpectedly decompensates upon or shortly after cessation. In sum, it remains essential to understand the prospective course, as the lack of anticipatory planning may precipitate confusion, distress, and conflict for patients, family members, and the clinical team.
DNR on VA‐ECMO?
Hospitalists accustomed to writing DNR orders may be distressed to find that, in our opinion, DNR orders are not appropriate for patients who are maintained on VA‐ECMO.[9] (It should also be noted that patients on VV‐ECMO, a device that only provides pulmonary function, could suffer a cardiac arrest necessitating CPR; thus, DNR may be relevant in this clinical context.) VA‐ECMO provides more effective oxygenation and perfusion than traditional advanced cardiac life support with CPR. Thus patients on VA‐ECMO will generally not receive CPR and, consequently, there is effectively no clinical meaning to a DNR order for a patient on VA‐ECMO. That said, when discontinuing VA‐ECMO (and at times VV‐ECMO), depending on the goals of care, a DNR may be useful to prevent further aggressive treatment should the patient arrest following cessation of ECMO.
The clinician will be wise to recognize that if families request a DNR order for a patient on VA‐ECMO, they are asking for something. Although a request not to resuscitate may not make medical sense in this context, clinicians must take the time to explore what is intended by this request. For many families, DNR is a stepping stone toward de‐escalation of treatment and a first move toward withdrawal of life‐sustaining therapies.[27, 28] A nuanced understanding of what a family hopes to accomplish by the suggested order, and specifically whether and how goals of care may have changed, is vital toward the maintenance of an appropriate, timely, and evolving treatment plan.
Terminal Discontinuation of VA‐ECMO
Among clinical ethicists, some of the most distressing conversations and meetings we have had with families have emerged in the context of terminal discontinuation of VA‐ECMO. Unlike mechanical ventilation, which theoretically may be continued indefinitely via tracheostomy, VA‐ECMO is only a temporary measure and, according to ELSO, should be discontinued promptly if healthy survival is not anticipated with the possibility of stopping for futility explained to the family before ECLS is begun.[21] Given the time constraints for what may have been an emergency procedure, and given the frequent reluctance of families and surrogates to discontinue life‐sustaining therapies, how does a clinician or institution ethically enact these guidelines? With respect to practical guidance, we offer 3 suggestions for directing these conversations.
First, we suggest physicians discuss the possibility and potential rationales of terminal discontinuation early and often, ideally as part of the initial consent process. Second, informed consent conversations should address potential complications (stroke, hemorrhage, and thrombosis) and their sequelae alongside discussions with patients and surrogates about their wishes in the context of such an event. Finally, we also recommend frequently revisiting the goals of care with the surrogate throughout the course of treatment.[28] Thus, when goals of care can no longer be achieved by continuing VA‐ECMO, either: (1) because the patient has no chance for recovery; (2) because VA‐ECMO no longer serves its intended purpose; or (3) owing to harm from complications, families may be able to appreciate that continuation of the intervention has become ethically disproportionate, and ECMO is now more burdensome than beneficial. Continuous and open dialogue should build a strong foundation of trust and knowledge that allows the surrogate to understand and accept the rationale behind a recommendation to terminally discontinue treatment, should the clinical course necessitate such.[29]
CONCLUSION
With indications for and utilization of ECMO in adult patients expanding, hospitalists may be expected to encounter these technologies with greater frequency and guide patients and families with medical decision‐making. Although the ethical issues reviewed are certainly not exclusive to ECMO, specific facets of ECMO, as discussed, may precipitate unique challenges or exacerbate common ones. Hospitalists can help to uphold patient autonomy by providing information that enables patients and surrogates to actively participate in goal setting and decision‐making. As the utilization of this technology grows, further research will need to address decision‐making in the context of ECMO to ensure that the process remains optimally patient‐ and family‐centered.
Acknowledgment
Disclosures: All work was performed at Weill Cornell Medical College of Cornell University, New York Presbyterian Weill Cornell Medical Center, and University of Pennsylvania. The authors report no conflicts of interest.
- , . Current status of extracorporeal life support (ECMO) for cardiopulmonary failure. Minerva Anestesiol. 2010;76(7):534–540.
- . . The first 20 years of the heart‐lung machine. Tex Heart Inst J. 1997;24(1):1–8.
- , , , , . Venovenous extracorporeal membrane oxygenation in adults: practical aspects of circuits, cannulae, and procedures. J Cardiothorac Vasc Anesth. 2012;26(5):893–909.
- , , , et al. Extracorporeal life support for adult cardiorespiratory failure. Surgery. 1993;114(2):161–172; discussion 172–163.
- , . Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–1914.
- , , , , . Extracorporeal life support. BMJ. 2010;341:c5317.
- , , , , , . Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2‐technical considerations. J Cardiothorac Vasc Anesth. 2010;24(1):164–172.
- , . Mechanical circulatory support for bridge to decision: which device and when to decide. J Card Surg. 2010;25(4):425–433.
- , , . DNR on ECMO: a paradox worth exploring. J Clin Ethics. 2013;25(1):13–19.
- , , , et al. Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR). Health Technol Assess. 2010;14(35):1–46.
- , , , et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895.
- , , , et al. Cardiopulmonary resuscitation with assisted extracorporeal life‐support versus conventional cardiopulmonary resuscitation in adults with in‐hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372(9638):554–561.
- , , , et al. Extracorporeal membrane oxygenation for refractory cardiogenic shock after cardiac surgery: predictors of early mortality and outcome from 51 adult patients. Eur J Cardiothorac Surg. 2010;37(2):328–333.
- , , , et al. Venoarterial extracorporeal membrane oxygenation for treatment of cardiogenic shock: clinical experiences in 45 adult patients. J Thorac Cardiovasc Surg. 2008;135(2):382–388.
- , , , et al. Outcomes and long‐term quality‐of‐life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36(5):1404–1411.
- , . Extracorporeal life support as a bridge to lung transplantation. Clin Chest Med. 2011;32(2):245–251.
- Extracoporeal Life Support Organization. General Guidelines for all ECLS Cases. 2012; http://www.elsonet.org/. Accessed August 5, 2014.
- , , , et al. Impact of extracorporeal life support on outcome in patients with idiopathic pulmonary arterial hypertension awaiting lung transplantation. J Heart Lung Transplant. 2011;30(9):997–1002.
- , , , . Ambulatory extracorporeal membrane oxygenation: a new approach for bridge‐to‐lung transplantation. J Thorac Cardiovasc Surg. 2010;139(6):e137–e139.
- , , , et al. Ambulatory veno‐venous extracorporeal membrane oxygenation: innovation and pitfalls. J Thorac Cardiovasc Surg. 2011;142(4):755–761.
- Extracoporeal Life Support Organization. General Guidelines for all ECLS Cases. 2012; http://www.elsonet.org/. Accessed August 5, 2014.
- Extracorporeal Life Support Organization. ECLS Registry Report United States Summary. August 2014; http://www.elsonet.org/. Accessed August 5, 2014.
- , . Principles of Biomedical Ethics. 6th ed. New York, NY: Oxford University Press; 2009.
- , , , et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125(15):1928–1952.
- , , . Ethics in Clinical Practice. 2nd ed. Sudbury, MA: Jones and Bartlett; 2005.
- . Pacemaker and defibrillator deactivation in competent hospice patients: an ethical consideration. Am J Hosp Palliat Care. 2005;22(1):14–19.
- , , , . Limitation of medical care: an ethnographic analysis. J Clin Ethics. 1993;4(2):134–145.
- . A Palliative Ethic of Care: Clinical Wisdom at Life's End. Sudbury, MA: Jones and Bartlett; 2006.
- , , , , , . Extracorporeal membrane oxygenation as a bridge to chemotherapy in an orthodox Jewish patient. Oncologist. 2014;19(9):985–989.
- , . Current status of extracorporeal life support (ECMO) for cardiopulmonary failure. Minerva Anestesiol. 2010;76(7):534–540.
- . . The first 20 years of the heart‐lung machine. Tex Heart Inst J. 1997;24(1):1–8.
- , , , , . Venovenous extracorporeal membrane oxygenation in adults: practical aspects of circuits, cannulae, and procedures. J Cardiothorac Vasc Anesth. 2012;26(5):893–909.
- , , , et al. Extracorporeal life support for adult cardiorespiratory failure. Surgery. 1993;114(2):161–172; discussion 172–163.
- , . Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–1914.
- , , , , . Extracorporeal life support. BMJ. 2010;341:c5317.
- , , , , , . Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2‐technical considerations. J Cardiothorac Vasc Anesth. 2010;24(1):164–172.
- , . Mechanical circulatory support for bridge to decision: which device and when to decide. J Card Surg. 2010;25(4):425–433.
- , , . DNR on ECMO: a paradox worth exploring. J Clin Ethics. 2013;25(1):13–19.
- , , , et al. Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR). Health Technol Assess. 2010;14(35):1–46.
- , , , et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895.
- , , , et al. Cardiopulmonary resuscitation with assisted extracorporeal life‐support versus conventional cardiopulmonary resuscitation in adults with in‐hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372(9638):554–561.
- , , , et al. Extracorporeal membrane oxygenation for refractory cardiogenic shock after cardiac surgery: predictors of early mortality and outcome from 51 adult patients. Eur J Cardiothorac Surg. 2010;37(2):328–333.
- , , , et al. Venoarterial extracorporeal membrane oxygenation for treatment of cardiogenic shock: clinical experiences in 45 adult patients. J Thorac Cardiovasc Surg. 2008;135(2):382–388.
- , , , et al. Outcomes and long‐term quality‐of‐life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36(5):1404–1411.
- , . Extracorporeal life support as a bridge to lung transplantation. Clin Chest Med. 2011;32(2):245–251.
- Extracoporeal Life Support Organization. General Guidelines for all ECLS Cases. 2012; http://www.elsonet.org/. Accessed August 5, 2014.
- , , , et al. Impact of extracorporeal life support on outcome in patients with idiopathic pulmonary arterial hypertension awaiting lung transplantation. J Heart Lung Transplant. 2011;30(9):997–1002.
- , , , . Ambulatory extracorporeal membrane oxygenation: a new approach for bridge‐to‐lung transplantation. J Thorac Cardiovasc Surg. 2010;139(6):e137–e139.
- , , , et al. Ambulatory veno‐venous extracorporeal membrane oxygenation: innovation and pitfalls. J Thorac Cardiovasc Surg. 2011;142(4):755–761.
- Extracoporeal Life Support Organization. General Guidelines for all ECLS Cases. 2012; http://www.elsonet.org/. Accessed August 5, 2014.
- Extracorporeal Life Support Organization. ECLS Registry Report United States Summary. August 2014; http://www.elsonet.org/. Accessed August 5, 2014.
- , . Principles of Biomedical Ethics. 6th ed. New York, NY: Oxford University Press; 2009.
- , , , et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125(15):1928–1952.
- , , . Ethics in Clinical Practice. 2nd ed. Sudbury, MA: Jones and Bartlett; 2005.
- . Pacemaker and defibrillator deactivation in competent hospice patients: an ethical consideration. Am J Hosp Palliat Care. 2005;22(1):14–19.
- , , , . Limitation of medical care: an ethnographic analysis. J Clin Ethics. 1993;4(2):134–145.
- . A Palliative Ethic of Care: Clinical Wisdom at Life's End. Sudbury, MA: Jones and Bartlett; 2006.
- , , , , , . Extracorporeal membrane oxygenation as a bridge to chemotherapy in an orthodox Jewish patient. Oncologist. 2014;19(9):985–989.