User login
‘Reform School’ for Pharmacy Benefit Managers: How Might Legislation Help Patients?
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).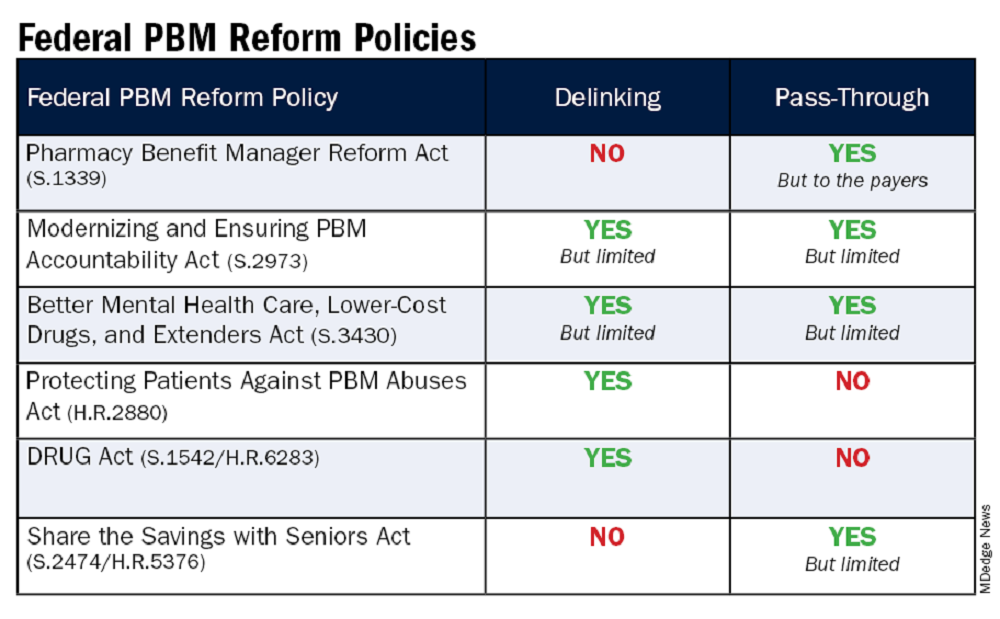
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at rhnews@mdedge.com.
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at rhnews@mdedge.com.
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at rhnews@mdedge.com.
Fed Worker Health Plans Ban Maximizers and Copay Accumulators: Why Not for the Rest of the US?
The escalating costs of medications and the prevalence of medical bankruptcy in our country have drawn criticism from governments, regulators, and the media. Federal and state governments are exploring various strategies to mitigate this issue, including the Inflation Reduction Act (IRA) for drug price negotiations and the establishment of state Pharmaceutical Drug Affordability Boards (PDABs). However, it’s uncertain whether these measures will effectively reduce patients’ medication expenses, given the tendency of pharmacy benefit managers (PBMs) to favor more expensive drugs on their formularies and the implementation challenges faced by PDABs.
The question then arises: How can we promptly assist patients, especially those with multiple chronic conditions, in affording their healthcare? Many of these patients are enrolled in high-deductible plans and struggle to cover all their medical and pharmacy costs.
A significant obstacle to healthcare affordability emerged in 2018 with the introduction of Copay Accumulator Programs by PBMs. These programs prevent patients from applying manufacturer copay cards toward their deductible and maximum out-of-pocket (OOP) costs. The impact of these policies has been devastating, leading to decreased adherence to medications and delayed necessary medical procedures, such as colonoscopies. Copay accumulators do nothing to address the high cost of medical care. They merely shift the burden from insurance companies to patients.
There is a direct solution to help patients, particularly those burdened with high pharmacy bills, afford their medical care. It would be that all payments from patients, including manufacturer copay cards, count toward their deductible and maximum OOP costs. This should apply regardless of whether the insurance plan is fully funded or a self-insured employer plan. This would be an immediate step toward making healthcare more affordable for patients.
Copay Accumulator Programs
How did these detrimental policies, which have been proven to harm patients, originate? It’s interesting that health insurance policies for federal employees do not allow these programs and yet the federal government has done little to protect its citizens from these egregious policies. More on that later.
In 2018, insurance companies and PBMs conceived an idea to introduce what they called copay accumulator adjustment programs. These programs would prevent the use of manufacturer copay cards from counting toward patient deductibles or OOP maximums. They justified this by arguing that manufacturer copay cards encouraged patients to opt for higher-priced brand drugs when lower-cost generics were available.
However, data from IQVIA contradicts this claim. An analysis of copay card usage from 2013 to 2017 revealed that a mere 0.4% of these cards were used for brand-name drugs that had already lost their exclusivity. This indicates that the vast majority of copay cards were not being used to purchase more expensive brand-name drugs when cheaper, generic alternatives were available.
Another argument put forth by one of the large PBMs was that patients with high deductibles don’t have enough “skin in the game” due to their low premiums, and therefore don’t deserve to have their deductible covered by a copay card. This raises the question, “Does a patient with hemophilia or systemic lupus who can’t afford a low deductible plan not have ‘skin in the game’? Is that a fair assessment?” It’s disconcerting to see a multibillion-dollar company dictating who deserves to have their deductible covered. These policies clearly disproportionately harm patients with chronic illnesses, especially those with high deductibles. As a result, many organizations have labeled these policies as discriminatory.
Following the implementation of accumulator programs in 2018 and 2019, many patients were unaware that their copay cards weren’t contributing toward their deductibles. They were taken aback when specialty pharmacies informed them of owing substantial amounts because of unmet deductibles. Consequently, patients discontinued their medications, leading to disease progression and increased costs. The only downside for health insurers and PBMs was the negative publicity associated with patients losing medication access.
Maximizer Programs
By the end of 2019, the three major PBMs had devised a strategy to keep patients on their medication throughout the year, without counting copay cards toward the deductible, and found a way to profit more from these cards, sometimes quadrupling their value. This was the birth of the maximizer programs.
Maximizers exploit a “loophole” in the Affordable Care Act (ACA). The ACA defines Essential Healthcare Benefits (EHB); anything not listed as an EHB is deemed “non-essential.” As a result, neither personal payments nor copay cards count toward deductibles or OOP maximums. Patients were informed that neither their own money nor manufacturer copay cards would count toward their deductible/OOP max.
One of my patients was warned that without enrolling in the maximizer program through SaveOnSP (owned by Express Scripts), she would bear the full cost of the drug, and nothing would count toward her OOP max. Frightened, she enrolled and surrendered her manufacturer copay card to SaveOnSP. Maximizers pocket the maximum value of the copay card, even if it exceeds the insurance plan’s yearly cost share by threefold or more. To do this legally, PBMs increase the patient’s original cost share amount during the plan year to match the value of the manufacturer copay card.
Combating These Programs
Nineteen states, the District of Columbia, and Puerto Rico have outlawed copay accumulators in health plans under state jurisdiction. I personally testified in Louisiana, leading to a ban in our state. CSRO’s award-winning map tool can show if your state has passed the ban on copay accumulator programs. However, many states have not passed bans on copay accumulators and self-insured employer groups, which fall under the Department of Labor and not state regulation, are still unaffected. There is also proposed federal legislation, the “Help Ensure Lower Patient Copays Act,” that would prohibit the use of copay accumulators in exchange plans. Despite having bipartisan support, it is having a hard time getting across the finish line in Congress.
In 2020, the Department of Health and Human Services (HHS) issued a rule prohibiting accumulator programs in all plans if the product was a brand name without a generic alternative. Unfortunately, this rule was rescinded in 2021, allowing copay accumulators even if a lower-cost generic was available.
In a positive turn of events, the US District Court of the District of Columbia overturned the 2021 rule in late 2023, reinstating the 2020 ban on copay accumulators. However, HHS has yet to enforce this ban.
Double Standard
Why is it that our federal government refrains from enforcing bans on copay accumulators for the American public, yet the US Office of Personnel Management (OPM) in its 2024 health plan for federal employees has explicitly stated that it “will decline any arrangements which may manipulate the prescription drug benefit design or incorporate any programs such as copay maximizers, copay optimizers, or other similar programs as these types of benefit designs are not in the best interest of enrollees or the Government.”
If such practices are deemed unsuitable for federal employees, why are they considered acceptable for the rest of the American population? This discrepancy raises important questions about healthcare equity.
In conclusion, the prevalence of medical bankruptcy in our country is a pressing issue that requires immediate attention. The introduction of copay accumulator programs and maximizers by PBMs has led to decreased adherence to needed medications, as well as delay in important medical procedures, exacerbating this situation. An across-the-board ban on these programs would offer immediate relief to many families that no longer can afford needed care.
It is clear that more needs to be done to ensure that all patients, regardless of their financial situation or the nature of their health insurance plan, can afford the healthcare they need. This includes ensuring that patients are not penalized for using manufacturer copay cards to help cover their costs. As we move forward, it is crucial that we continue to advocate for policies that prioritize the health and well-being of all patients.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
The escalating costs of medications and the prevalence of medical bankruptcy in our country have drawn criticism from governments, regulators, and the media. Federal and state governments are exploring various strategies to mitigate this issue, including the Inflation Reduction Act (IRA) for drug price negotiations and the establishment of state Pharmaceutical Drug Affordability Boards (PDABs). However, it’s uncertain whether these measures will effectively reduce patients’ medication expenses, given the tendency of pharmacy benefit managers (PBMs) to favor more expensive drugs on their formularies and the implementation challenges faced by PDABs.
The question then arises: How can we promptly assist patients, especially those with multiple chronic conditions, in affording their healthcare? Many of these patients are enrolled in high-deductible plans and struggle to cover all their medical and pharmacy costs.
A significant obstacle to healthcare affordability emerged in 2018 with the introduction of Copay Accumulator Programs by PBMs. These programs prevent patients from applying manufacturer copay cards toward their deductible and maximum out-of-pocket (OOP) costs. The impact of these policies has been devastating, leading to decreased adherence to medications and delayed necessary medical procedures, such as colonoscopies. Copay accumulators do nothing to address the high cost of medical care. They merely shift the burden from insurance companies to patients.
There is a direct solution to help patients, particularly those burdened with high pharmacy bills, afford their medical care. It would be that all payments from patients, including manufacturer copay cards, count toward their deductible and maximum OOP costs. This should apply regardless of whether the insurance plan is fully funded or a self-insured employer plan. This would be an immediate step toward making healthcare more affordable for patients.
Copay Accumulator Programs
How did these detrimental policies, which have been proven to harm patients, originate? It’s interesting that health insurance policies for federal employees do not allow these programs and yet the federal government has done little to protect its citizens from these egregious policies. More on that later.
In 2018, insurance companies and PBMs conceived an idea to introduce what they called copay accumulator adjustment programs. These programs would prevent the use of manufacturer copay cards from counting toward patient deductibles or OOP maximums. They justified this by arguing that manufacturer copay cards encouraged patients to opt for higher-priced brand drugs when lower-cost generics were available.
However, data from IQVIA contradicts this claim. An analysis of copay card usage from 2013 to 2017 revealed that a mere 0.4% of these cards were used for brand-name drugs that had already lost their exclusivity. This indicates that the vast majority of copay cards were not being used to purchase more expensive brand-name drugs when cheaper, generic alternatives were available.
Another argument put forth by one of the large PBMs was that patients with high deductibles don’t have enough “skin in the game” due to their low premiums, and therefore don’t deserve to have their deductible covered by a copay card. This raises the question, “Does a patient with hemophilia or systemic lupus who can’t afford a low deductible plan not have ‘skin in the game’? Is that a fair assessment?” It’s disconcerting to see a multibillion-dollar company dictating who deserves to have their deductible covered. These policies clearly disproportionately harm patients with chronic illnesses, especially those with high deductibles. As a result, many organizations have labeled these policies as discriminatory.
Following the implementation of accumulator programs in 2018 and 2019, many patients were unaware that their copay cards weren’t contributing toward their deductibles. They were taken aback when specialty pharmacies informed them of owing substantial amounts because of unmet deductibles. Consequently, patients discontinued their medications, leading to disease progression and increased costs. The only downside for health insurers and PBMs was the negative publicity associated with patients losing medication access.
Maximizer Programs
By the end of 2019, the three major PBMs had devised a strategy to keep patients on their medication throughout the year, without counting copay cards toward the deductible, and found a way to profit more from these cards, sometimes quadrupling their value. This was the birth of the maximizer programs.
Maximizers exploit a “loophole” in the Affordable Care Act (ACA). The ACA defines Essential Healthcare Benefits (EHB); anything not listed as an EHB is deemed “non-essential.” As a result, neither personal payments nor copay cards count toward deductibles or OOP maximums. Patients were informed that neither their own money nor manufacturer copay cards would count toward their deductible/OOP max.
One of my patients was warned that without enrolling in the maximizer program through SaveOnSP (owned by Express Scripts), she would bear the full cost of the drug, and nothing would count toward her OOP max. Frightened, she enrolled and surrendered her manufacturer copay card to SaveOnSP. Maximizers pocket the maximum value of the copay card, even if it exceeds the insurance plan’s yearly cost share by threefold or more. To do this legally, PBMs increase the patient’s original cost share amount during the plan year to match the value of the manufacturer copay card.
Combating These Programs
Nineteen states, the District of Columbia, and Puerto Rico have outlawed copay accumulators in health plans under state jurisdiction. I personally testified in Louisiana, leading to a ban in our state. CSRO’s award-winning map tool can show if your state has passed the ban on copay accumulator programs. However, many states have not passed bans on copay accumulators and self-insured employer groups, which fall under the Department of Labor and not state regulation, are still unaffected. There is also proposed federal legislation, the “Help Ensure Lower Patient Copays Act,” that would prohibit the use of copay accumulators in exchange plans. Despite having bipartisan support, it is having a hard time getting across the finish line in Congress.
In 2020, the Department of Health and Human Services (HHS) issued a rule prohibiting accumulator programs in all plans if the product was a brand name without a generic alternative. Unfortunately, this rule was rescinded in 2021, allowing copay accumulators even if a lower-cost generic was available.
In a positive turn of events, the US District Court of the District of Columbia overturned the 2021 rule in late 2023, reinstating the 2020 ban on copay accumulators. However, HHS has yet to enforce this ban.
Double Standard
Why is it that our federal government refrains from enforcing bans on copay accumulators for the American public, yet the US Office of Personnel Management (OPM) in its 2024 health plan for federal employees has explicitly stated that it “will decline any arrangements which may manipulate the prescription drug benefit design or incorporate any programs such as copay maximizers, copay optimizers, or other similar programs as these types of benefit designs are not in the best interest of enrollees or the Government.”
If such practices are deemed unsuitable for federal employees, why are they considered acceptable for the rest of the American population? This discrepancy raises important questions about healthcare equity.
In conclusion, the prevalence of medical bankruptcy in our country is a pressing issue that requires immediate attention. The introduction of copay accumulator programs and maximizers by PBMs has led to decreased adherence to needed medications, as well as delay in important medical procedures, exacerbating this situation. An across-the-board ban on these programs would offer immediate relief to many families that no longer can afford needed care.
It is clear that more needs to be done to ensure that all patients, regardless of their financial situation or the nature of their health insurance plan, can afford the healthcare they need. This includes ensuring that patients are not penalized for using manufacturer copay cards to help cover their costs. As we move forward, it is crucial that we continue to advocate for policies that prioritize the health and well-being of all patients.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
The escalating costs of medications and the prevalence of medical bankruptcy in our country have drawn criticism from governments, regulators, and the media. Federal and state governments are exploring various strategies to mitigate this issue, including the Inflation Reduction Act (IRA) for drug price negotiations and the establishment of state Pharmaceutical Drug Affordability Boards (PDABs). However, it’s uncertain whether these measures will effectively reduce patients’ medication expenses, given the tendency of pharmacy benefit managers (PBMs) to favor more expensive drugs on their formularies and the implementation challenges faced by PDABs.
The question then arises: How can we promptly assist patients, especially those with multiple chronic conditions, in affording their healthcare? Many of these patients are enrolled in high-deductible plans and struggle to cover all their medical and pharmacy costs.
A significant obstacle to healthcare affordability emerged in 2018 with the introduction of Copay Accumulator Programs by PBMs. These programs prevent patients from applying manufacturer copay cards toward their deductible and maximum out-of-pocket (OOP) costs. The impact of these policies has been devastating, leading to decreased adherence to medications and delayed necessary medical procedures, such as colonoscopies. Copay accumulators do nothing to address the high cost of medical care. They merely shift the burden from insurance companies to patients.
There is a direct solution to help patients, particularly those burdened with high pharmacy bills, afford their medical care. It would be that all payments from patients, including manufacturer copay cards, count toward their deductible and maximum OOP costs. This should apply regardless of whether the insurance plan is fully funded or a self-insured employer plan. This would be an immediate step toward making healthcare more affordable for patients.
Copay Accumulator Programs
How did these detrimental policies, which have been proven to harm patients, originate? It’s interesting that health insurance policies for federal employees do not allow these programs and yet the federal government has done little to protect its citizens from these egregious policies. More on that later.
In 2018, insurance companies and PBMs conceived an idea to introduce what they called copay accumulator adjustment programs. These programs would prevent the use of manufacturer copay cards from counting toward patient deductibles or OOP maximums. They justified this by arguing that manufacturer copay cards encouraged patients to opt for higher-priced brand drugs when lower-cost generics were available.
However, data from IQVIA contradicts this claim. An analysis of copay card usage from 2013 to 2017 revealed that a mere 0.4% of these cards were used for brand-name drugs that had already lost their exclusivity. This indicates that the vast majority of copay cards were not being used to purchase more expensive brand-name drugs when cheaper, generic alternatives were available.
Another argument put forth by one of the large PBMs was that patients with high deductibles don’t have enough “skin in the game” due to their low premiums, and therefore don’t deserve to have their deductible covered by a copay card. This raises the question, “Does a patient with hemophilia or systemic lupus who can’t afford a low deductible plan not have ‘skin in the game’? Is that a fair assessment?” It’s disconcerting to see a multibillion-dollar company dictating who deserves to have their deductible covered. These policies clearly disproportionately harm patients with chronic illnesses, especially those with high deductibles. As a result, many organizations have labeled these policies as discriminatory.
Following the implementation of accumulator programs in 2018 and 2019, many patients were unaware that their copay cards weren’t contributing toward their deductibles. They were taken aback when specialty pharmacies informed them of owing substantial amounts because of unmet deductibles. Consequently, patients discontinued their medications, leading to disease progression and increased costs. The only downside for health insurers and PBMs was the negative publicity associated with patients losing medication access.
Maximizer Programs
By the end of 2019, the three major PBMs had devised a strategy to keep patients on their medication throughout the year, without counting copay cards toward the deductible, and found a way to profit more from these cards, sometimes quadrupling their value. This was the birth of the maximizer programs.
Maximizers exploit a “loophole” in the Affordable Care Act (ACA). The ACA defines Essential Healthcare Benefits (EHB); anything not listed as an EHB is deemed “non-essential.” As a result, neither personal payments nor copay cards count toward deductibles or OOP maximums. Patients were informed that neither their own money nor manufacturer copay cards would count toward their deductible/OOP max.
One of my patients was warned that without enrolling in the maximizer program through SaveOnSP (owned by Express Scripts), she would bear the full cost of the drug, and nothing would count toward her OOP max. Frightened, she enrolled and surrendered her manufacturer copay card to SaveOnSP. Maximizers pocket the maximum value of the copay card, even if it exceeds the insurance plan’s yearly cost share by threefold or more. To do this legally, PBMs increase the patient’s original cost share amount during the plan year to match the value of the manufacturer copay card.
Combating These Programs
Nineteen states, the District of Columbia, and Puerto Rico have outlawed copay accumulators in health plans under state jurisdiction. I personally testified in Louisiana, leading to a ban in our state. CSRO’s award-winning map tool can show if your state has passed the ban on copay accumulator programs. However, many states have not passed bans on copay accumulators and self-insured employer groups, which fall under the Department of Labor and not state regulation, are still unaffected. There is also proposed federal legislation, the “Help Ensure Lower Patient Copays Act,” that would prohibit the use of copay accumulators in exchange plans. Despite having bipartisan support, it is having a hard time getting across the finish line in Congress.
In 2020, the Department of Health and Human Services (HHS) issued a rule prohibiting accumulator programs in all plans if the product was a brand name without a generic alternative. Unfortunately, this rule was rescinded in 2021, allowing copay accumulators even if a lower-cost generic was available.
In a positive turn of events, the US District Court of the District of Columbia overturned the 2021 rule in late 2023, reinstating the 2020 ban on copay accumulators. However, HHS has yet to enforce this ban.
Double Standard
Why is it that our federal government refrains from enforcing bans on copay accumulators for the American public, yet the US Office of Personnel Management (OPM) in its 2024 health plan for federal employees has explicitly stated that it “will decline any arrangements which may manipulate the prescription drug benefit design or incorporate any programs such as copay maximizers, copay optimizers, or other similar programs as these types of benefit designs are not in the best interest of enrollees or the Government.”
If such practices are deemed unsuitable for federal employees, why are they considered acceptable for the rest of the American population? This discrepancy raises important questions about healthcare equity.
In conclusion, the prevalence of medical bankruptcy in our country is a pressing issue that requires immediate attention. The introduction of copay accumulator programs and maximizers by PBMs has led to decreased adherence to needed medications, as well as delay in important medical procedures, exacerbating this situation. An across-the-board ban on these programs would offer immediate relief to many families that no longer can afford needed care.
It is clear that more needs to be done to ensure that all patients, regardless of their financial situation or the nature of their health insurance plan, can afford the healthcare they need. This includes ensuring that patients are not penalized for using manufacturer copay cards to help cover their costs. As we move forward, it is crucial that we continue to advocate for policies that prioritize the health and well-being of all patients.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Don’t Leave CVD Risk in RA Undertreated Despite Unresolved Questions
NEW YORK — Patients with rheumatoid arthritis (RA) carry a high risk for cardiovascular events, but mounting clinical evidence suggests they’re being undertreated to manage that risk. Rheumatologists should consider a patient with RA’s cardiovascular disease (CVD) status before deciding on RA treatments, a researcher of cardiometabolic disorders advised.
“The ORAL Surveillance trial suggests that we need to consider cardiovascular risk factors and maybe do additional screening in these patients before we use RA therapies,” Jon T. Giles, MD, PhD, director of the Cedars-Sinai Inflammatory Arthritis Clinical Center at Cedars-Sinai in Los Angeles, told attendees at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
Underuse of Statins
ORAL Surveillance enrolled 4362 patients with RA aged 50 years and older with at least one cardiovascular risk factor. About 23% of all patients were taking statins, as were about half of patients with a history of atherosclerotic CVD (ASCVD).
“A lot of those people should have been on statins,” Dr. Giles said in an interview. “Not because of their RA but because of their risk factors, and then RA brings it up another notch.” In the population with ASCVD, Dr. Giles added, “It should have been more like 70% and 80%. If we’re talking about a disease that has enhanced cardiovascular risk, then the adoption of standard care that you would do for anybody in the general population should be at that standard and maybe above.”
Multiple studies have documented the underlying risk for CVD events, CV mortality, and subclinical atherosclerosis in people with RA, Dr. Giles noted in his presentation. Physiologically, the RA-specific risk factors most linked to CVD risk are systemic inflammation/cytokine excess and specific circulating T-cell and intermediate monocyte subsets, or both, Dr. Giles said.
Disease-Modifying Antirheumatic Drugs (DMARDs) and CVD Risk
Likewise, research in the past decade has linked methotrexate and tumor necrosis factor (TNF) inhibitors to reduced ASCVD events in RA. Another study showed that abatacept had an effect similar to that of etanercept in patients with RA, and the ENTRACTE trial, for which Dr. Giles was the lead author, demonstrated that tocilizumab matched etanercept in reducing CV events.
The ORAL Surveillance investigators also reported that patients with RA who were receiving the Janus kinase (JAK) inhibitor tofacitinib had a higher risk for major adverse cardiovascular events and cancers than those on TNF therapy, Dr. Giles noted. While statins in combination with JAK inhibitors may have the potential to provide a balance for controlling CV risk in patients with RA, he said later that the potential of JAK inhibitors in reducing CVD risk in RA “is still unsettled.”
The ongoing TARGET trial is further evaluating the impact of DMARDs on vascular inflammation in RA, said Dr. Giles, who’s also a trial principal investigator. TARGET is randomizing 115 patients with RA who didn’t respond to methotrexate to a TNF inhibitor or the addition of sulfasalazine and hydroxychloroquine to their methotrexate. Patients can be on low-intensity but not high-intensity statin therapy, Dr. Giles said.
TARGET results reported last year demonstrated an 8% decrease in arterial fluorodeoxyglucose (FDG) uptake on PET-CT in both treatment arms. Previous studies, Dr. Giles noted, have shown a potential link between FDG and histologic markers of inflammation. “An 8% decrease in vascular FDG is in line with what you would expect from statin treatment,” he said.
TARGET results published in April showed that a measure of a cluster of 12 cytokines and other inflammatory mediators, known as the multibiomarker disease activity (MBDA) score and marketed under the brand name Vectra DA, may help determine arterial FDG uptake. “Those who had a low MBDA score at week 24 actually had the greatest reduction in the arterial FDG,” he said.
Those results were driven entirely by low serum amyloid A (SAA) levels, Dr. Giles said. Those same results didn’t hold for patients in whom SAA and C-reactive protein were correlated.
“So, there’s more to come here,” Dr. Giles said. “We’re looking at other, much larger biomarker panels.”
Nonetheless, he said, sufficient evidence exists to conclude that treating RA to target reduces CV events. “The idea is that at every visit that you see an RA patient, you measure their disease activity, and if they’re not at the target of low disease activity or remission, then you change their therapy to improve that,” he said in an interview.
But an evidence-based guideline is needed to improve coverage of CVD risks in patients with RA, Dr. Giles said. “There is a movement afoot” for a guideline, he said. “If you just did what is supposed to happen for a general population, you would make some improvements. The risk-benefit [ratio] for statins for people with RA has been looked at, and it’s very favorable.”
Unanswered Questions
Dr. Giles noted that the ORAL Surveillance trial has left a number of questions unanswered about the role of JAK inhibitors in managing CVD risk in patients with RA. “The issue that we’re trying to ask is, is it just the TNF inhibitors may be better? Is this a subpopulation issue, or was it just bad luck from the purposes of this one trial? Granted, it was a very large trial, but you can still have luck in terms of getting an effect that’s not accurate.”
Dr. Giles’ “gut feeling” on JAK inhibitors is that they’re not causing harm, but that they’re not as effective as TNF inhibitors in ameliorating CV risks in patients with RA.
Michael S. Garshick, MD, who attended the conference and is head of the cardio-rheumatology program at NYU Langone Health, concurred that a number of unanswered questions persist over the treatment of CVD risk in RA — and autoimmune disease in general.
“I think we’re still trying to prove that DMARDs reduce cardiovascular risk in autoimmune conditions,” he said. “The epidemiologic data would suggest, yes, that inflammation prevention is beneficial for cardiovascular disease, but the TARGET trial suggested that vascular inflammation improved by treating RA, but that biologic therapy wasn’t better than traditional triple therapy.”
Other questions remain unanswered, Dr. Garshick said.
“Is there a specific immunotherapy that is most beneficial to reduce heart disease in patients with an autoimmune condition, whether it’s rheumatoid arthritis, psoriasis, or lupus?”
Dr. Garshick said he’s specifically interested in the residual risk that exists after treating the autoimmunity. “Do you still have a higher risk for heart disease, and if so, why? Is there something else going on that we can’t see?”
The biggest unanswered question, he said, is “How can we do a better job of recognizing heart disease risk in these patients? That’s the low-hanging fruit that people are studying, but across many of those studies, patients have higher rates of blood pressure, cholesterol issues, obesity, diabetes, and many times, we’re not adequately treating these comorbidities.”
That, Dr. Garshick said, may be a result of physician fatigue. “And so [treatment of these comorbidities is] kicked down the road for a year or years,” he added.
Dr. Giles disclosed financial relationships with Pfizer, AbbVie, Eli Lilly, and Novartis. Dr. Garshick disclosed relationships with Kiniksa Pharmaceuticals, Agepha Pharma, Bristol Myers Squibb, and Horizon Therapeutics.
A version of this article appeared on Medscape.com.
NEW YORK — Patients with rheumatoid arthritis (RA) carry a high risk for cardiovascular events, but mounting clinical evidence suggests they’re being undertreated to manage that risk. Rheumatologists should consider a patient with RA’s cardiovascular disease (CVD) status before deciding on RA treatments, a researcher of cardiometabolic disorders advised.
“The ORAL Surveillance trial suggests that we need to consider cardiovascular risk factors and maybe do additional screening in these patients before we use RA therapies,” Jon T. Giles, MD, PhD, director of the Cedars-Sinai Inflammatory Arthritis Clinical Center at Cedars-Sinai in Los Angeles, told attendees at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
Underuse of Statins
ORAL Surveillance enrolled 4362 patients with RA aged 50 years and older with at least one cardiovascular risk factor. About 23% of all patients were taking statins, as were about half of patients with a history of atherosclerotic CVD (ASCVD).
“A lot of those people should have been on statins,” Dr. Giles said in an interview. “Not because of their RA but because of their risk factors, and then RA brings it up another notch.” In the population with ASCVD, Dr. Giles added, “It should have been more like 70% and 80%. If we’re talking about a disease that has enhanced cardiovascular risk, then the adoption of standard care that you would do for anybody in the general population should be at that standard and maybe above.”
Multiple studies have documented the underlying risk for CVD events, CV mortality, and subclinical atherosclerosis in people with RA, Dr. Giles noted in his presentation. Physiologically, the RA-specific risk factors most linked to CVD risk are systemic inflammation/cytokine excess and specific circulating T-cell and intermediate monocyte subsets, or both, Dr. Giles said.
Disease-Modifying Antirheumatic Drugs (DMARDs) and CVD Risk
Likewise, research in the past decade has linked methotrexate and tumor necrosis factor (TNF) inhibitors to reduced ASCVD events in RA. Another study showed that abatacept had an effect similar to that of etanercept in patients with RA, and the ENTRACTE trial, for which Dr. Giles was the lead author, demonstrated that tocilizumab matched etanercept in reducing CV events.
The ORAL Surveillance investigators also reported that patients with RA who were receiving the Janus kinase (JAK) inhibitor tofacitinib had a higher risk for major adverse cardiovascular events and cancers than those on TNF therapy, Dr. Giles noted. While statins in combination with JAK inhibitors may have the potential to provide a balance for controlling CV risk in patients with RA, he said later that the potential of JAK inhibitors in reducing CVD risk in RA “is still unsettled.”
The ongoing TARGET trial is further evaluating the impact of DMARDs on vascular inflammation in RA, said Dr. Giles, who’s also a trial principal investigator. TARGET is randomizing 115 patients with RA who didn’t respond to methotrexate to a TNF inhibitor or the addition of sulfasalazine and hydroxychloroquine to their methotrexate. Patients can be on low-intensity but not high-intensity statin therapy, Dr. Giles said.
TARGET results reported last year demonstrated an 8% decrease in arterial fluorodeoxyglucose (FDG) uptake on PET-CT in both treatment arms. Previous studies, Dr. Giles noted, have shown a potential link between FDG and histologic markers of inflammation. “An 8% decrease in vascular FDG is in line with what you would expect from statin treatment,” he said.
TARGET results published in April showed that a measure of a cluster of 12 cytokines and other inflammatory mediators, known as the multibiomarker disease activity (MBDA) score and marketed under the brand name Vectra DA, may help determine arterial FDG uptake. “Those who had a low MBDA score at week 24 actually had the greatest reduction in the arterial FDG,” he said.
Those results were driven entirely by low serum amyloid A (SAA) levels, Dr. Giles said. Those same results didn’t hold for patients in whom SAA and C-reactive protein were correlated.
“So, there’s more to come here,” Dr. Giles said. “We’re looking at other, much larger biomarker panels.”
Nonetheless, he said, sufficient evidence exists to conclude that treating RA to target reduces CV events. “The idea is that at every visit that you see an RA patient, you measure their disease activity, and if they’re not at the target of low disease activity or remission, then you change their therapy to improve that,” he said in an interview.
But an evidence-based guideline is needed to improve coverage of CVD risks in patients with RA, Dr. Giles said. “There is a movement afoot” for a guideline, he said. “If you just did what is supposed to happen for a general population, you would make some improvements. The risk-benefit [ratio] for statins for people with RA has been looked at, and it’s very favorable.”
Unanswered Questions
Dr. Giles noted that the ORAL Surveillance trial has left a number of questions unanswered about the role of JAK inhibitors in managing CVD risk in patients with RA. “The issue that we’re trying to ask is, is it just the TNF inhibitors may be better? Is this a subpopulation issue, or was it just bad luck from the purposes of this one trial? Granted, it was a very large trial, but you can still have luck in terms of getting an effect that’s not accurate.”
Dr. Giles’ “gut feeling” on JAK inhibitors is that they’re not causing harm, but that they’re not as effective as TNF inhibitors in ameliorating CV risks in patients with RA.
Michael S. Garshick, MD, who attended the conference and is head of the cardio-rheumatology program at NYU Langone Health, concurred that a number of unanswered questions persist over the treatment of CVD risk in RA — and autoimmune disease in general.
“I think we’re still trying to prove that DMARDs reduce cardiovascular risk in autoimmune conditions,” he said. “The epidemiologic data would suggest, yes, that inflammation prevention is beneficial for cardiovascular disease, but the TARGET trial suggested that vascular inflammation improved by treating RA, but that biologic therapy wasn’t better than traditional triple therapy.”
Other questions remain unanswered, Dr. Garshick said.
“Is there a specific immunotherapy that is most beneficial to reduce heart disease in patients with an autoimmune condition, whether it’s rheumatoid arthritis, psoriasis, or lupus?”
Dr. Garshick said he’s specifically interested in the residual risk that exists after treating the autoimmunity. “Do you still have a higher risk for heart disease, and if so, why? Is there something else going on that we can’t see?”
The biggest unanswered question, he said, is “How can we do a better job of recognizing heart disease risk in these patients? That’s the low-hanging fruit that people are studying, but across many of those studies, patients have higher rates of blood pressure, cholesterol issues, obesity, diabetes, and many times, we’re not adequately treating these comorbidities.”
That, Dr. Garshick said, may be a result of physician fatigue. “And so [treatment of these comorbidities is] kicked down the road for a year or years,” he added.
Dr. Giles disclosed financial relationships with Pfizer, AbbVie, Eli Lilly, and Novartis. Dr. Garshick disclosed relationships with Kiniksa Pharmaceuticals, Agepha Pharma, Bristol Myers Squibb, and Horizon Therapeutics.
A version of this article appeared on Medscape.com.
NEW YORK — Patients with rheumatoid arthritis (RA) carry a high risk for cardiovascular events, but mounting clinical evidence suggests they’re being undertreated to manage that risk. Rheumatologists should consider a patient with RA’s cardiovascular disease (CVD) status before deciding on RA treatments, a researcher of cardiometabolic disorders advised.
“The ORAL Surveillance trial suggests that we need to consider cardiovascular risk factors and maybe do additional screening in these patients before we use RA therapies,” Jon T. Giles, MD, PhD, director of the Cedars-Sinai Inflammatory Arthritis Clinical Center at Cedars-Sinai in Los Angeles, told attendees at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
Underuse of Statins
ORAL Surveillance enrolled 4362 patients with RA aged 50 years and older with at least one cardiovascular risk factor. About 23% of all patients were taking statins, as were about half of patients with a history of atherosclerotic CVD (ASCVD).
“A lot of those people should have been on statins,” Dr. Giles said in an interview. “Not because of their RA but because of their risk factors, and then RA brings it up another notch.” In the population with ASCVD, Dr. Giles added, “It should have been more like 70% and 80%. If we’re talking about a disease that has enhanced cardiovascular risk, then the adoption of standard care that you would do for anybody in the general population should be at that standard and maybe above.”
Multiple studies have documented the underlying risk for CVD events, CV mortality, and subclinical atherosclerosis in people with RA, Dr. Giles noted in his presentation. Physiologically, the RA-specific risk factors most linked to CVD risk are systemic inflammation/cytokine excess and specific circulating T-cell and intermediate monocyte subsets, or both, Dr. Giles said.
Disease-Modifying Antirheumatic Drugs (DMARDs) and CVD Risk
Likewise, research in the past decade has linked methotrexate and tumor necrosis factor (TNF) inhibitors to reduced ASCVD events in RA. Another study showed that abatacept had an effect similar to that of etanercept in patients with RA, and the ENTRACTE trial, for which Dr. Giles was the lead author, demonstrated that tocilizumab matched etanercept in reducing CV events.
The ORAL Surveillance investigators also reported that patients with RA who were receiving the Janus kinase (JAK) inhibitor tofacitinib had a higher risk for major adverse cardiovascular events and cancers than those on TNF therapy, Dr. Giles noted. While statins in combination with JAK inhibitors may have the potential to provide a balance for controlling CV risk in patients with RA, he said later that the potential of JAK inhibitors in reducing CVD risk in RA “is still unsettled.”
The ongoing TARGET trial is further evaluating the impact of DMARDs on vascular inflammation in RA, said Dr. Giles, who’s also a trial principal investigator. TARGET is randomizing 115 patients with RA who didn’t respond to methotrexate to a TNF inhibitor or the addition of sulfasalazine and hydroxychloroquine to their methotrexate. Patients can be on low-intensity but not high-intensity statin therapy, Dr. Giles said.
TARGET results reported last year demonstrated an 8% decrease in arterial fluorodeoxyglucose (FDG) uptake on PET-CT in both treatment arms. Previous studies, Dr. Giles noted, have shown a potential link between FDG and histologic markers of inflammation. “An 8% decrease in vascular FDG is in line with what you would expect from statin treatment,” he said.
TARGET results published in April showed that a measure of a cluster of 12 cytokines and other inflammatory mediators, known as the multibiomarker disease activity (MBDA) score and marketed under the brand name Vectra DA, may help determine arterial FDG uptake. “Those who had a low MBDA score at week 24 actually had the greatest reduction in the arterial FDG,” he said.
Those results were driven entirely by low serum amyloid A (SAA) levels, Dr. Giles said. Those same results didn’t hold for patients in whom SAA and C-reactive protein were correlated.
“So, there’s more to come here,” Dr. Giles said. “We’re looking at other, much larger biomarker panels.”
Nonetheless, he said, sufficient evidence exists to conclude that treating RA to target reduces CV events. “The idea is that at every visit that you see an RA patient, you measure their disease activity, and if they’re not at the target of low disease activity or remission, then you change their therapy to improve that,” he said in an interview.
But an evidence-based guideline is needed to improve coverage of CVD risks in patients with RA, Dr. Giles said. “There is a movement afoot” for a guideline, he said. “If you just did what is supposed to happen for a general population, you would make some improvements. The risk-benefit [ratio] for statins for people with RA has been looked at, and it’s very favorable.”
Unanswered Questions
Dr. Giles noted that the ORAL Surveillance trial has left a number of questions unanswered about the role of JAK inhibitors in managing CVD risk in patients with RA. “The issue that we’re trying to ask is, is it just the TNF inhibitors may be better? Is this a subpopulation issue, or was it just bad luck from the purposes of this one trial? Granted, it was a very large trial, but you can still have luck in terms of getting an effect that’s not accurate.”
Dr. Giles’ “gut feeling” on JAK inhibitors is that they’re not causing harm, but that they’re not as effective as TNF inhibitors in ameliorating CV risks in patients with RA.
Michael S. Garshick, MD, who attended the conference and is head of the cardio-rheumatology program at NYU Langone Health, concurred that a number of unanswered questions persist over the treatment of CVD risk in RA — and autoimmune disease in general.
“I think we’re still trying to prove that DMARDs reduce cardiovascular risk in autoimmune conditions,” he said. “The epidemiologic data would suggest, yes, that inflammation prevention is beneficial for cardiovascular disease, but the TARGET trial suggested that vascular inflammation improved by treating RA, but that biologic therapy wasn’t better than traditional triple therapy.”
Other questions remain unanswered, Dr. Garshick said.
“Is there a specific immunotherapy that is most beneficial to reduce heart disease in patients with an autoimmune condition, whether it’s rheumatoid arthritis, psoriasis, or lupus?”
Dr. Garshick said he’s specifically interested in the residual risk that exists after treating the autoimmunity. “Do you still have a higher risk for heart disease, and if so, why? Is there something else going on that we can’t see?”
The biggest unanswered question, he said, is “How can we do a better job of recognizing heart disease risk in these patients? That’s the low-hanging fruit that people are studying, but across many of those studies, patients have higher rates of blood pressure, cholesterol issues, obesity, diabetes, and many times, we’re not adequately treating these comorbidities.”
That, Dr. Garshick said, may be a result of physician fatigue. “And so [treatment of these comorbidities is] kicked down the road for a year or years,” he added.
Dr. Giles disclosed financial relationships with Pfizer, AbbVie, Eli Lilly, and Novartis. Dr. Garshick disclosed relationships with Kiniksa Pharmaceuticals, Agepha Pharma, Bristol Myers Squibb, and Horizon Therapeutics.
A version of this article appeared on Medscape.com.
Specialists Are ‘Underwater’ With Some Insurance-Preferred Biosimilars
Editor’s note: This article is adapted from an explanatory statement that Dr. Feldman wrote for the Coalition of State Rheumatology Organizations (CSRO).
According to the Guinness Book of World records, the longest time someone has held their breath underwater voluntarily is 24 minutes and 37.36 seconds. While certainly an amazing feat, UnitedHealthcare, many of the Blues, and other national “payers” are expecting rheumatologists and other specialists to live “underwater” in order to take care of their patients. In other words, these insurance companies are mandating that specialists use certain provider-administered biosimilars whose acquisition cost is higher than what the insurance company is willing to reimburse them. Essentially, the insurance companies expect the rheumatologists to pay them to take care of their patients. Because of the substantial and destabilizing financial losses incurred, many practices and free-standing infusion centers have been forced to cease offering these biosimilars. Most rheumatologists will provide patients with appropriate alternatives when available and permitted by the insurer; otherwise, they must refer patients to hospital-based infusion centers. That results in delayed care and increased costs for patients and the system, because hospital-based infusion typically costs more than twice what office-based infusion costs.
Quantifying the Problem
To help quantify the magnitude of this issue, the Coalition of State Rheumatology Organizations (CSRO) recently conducted a survey of its membership. A shocking 97% of respondents reported that their practice had been affected by reimbursement rates for some biosimilars being lower than acquisition costs, with 91% of respondents stating that this issue is more pronounced for certain biosimilars than others. Across the board, respondents most frequently identified Inflectra (infliximab-dyyb) and Avsola (infliximab-axxq) as being especially affected: Over 88% and over 85% of respondents identified these two products, respectively, as being underwater. These results support the ongoing anecdotal reports CSRO continues to receive from rheumatology practices.
However, the survey results indicated that this issue is by no means confined to those two biosimilars. Truxima (rituximab-abbs) — a biosimilar for Rituxan — was frequently mentioned as well. Notably, respondents almost uniformly identified biosimilars in the infliximab and rituximab families, which illustrates that this issue is no longer confined to one or two early-to-market biosimilars but has almost become a hallmark of this particular biosimilars market. Remarkably, one respondent commented that the brand products are now cheaper to acquire than the biosimilars. Furthermore, the survey included respondents from across the country, indicating that this issue is not confined to a particular region.
How Did This Happen?
Biosimilars held promise for increasing availability and decreasing biologic costs for patients but, thus far, no patients have seen their cost go down. It appears that the only biosimilars that have made it to “preferred” status on the formulary are the ones that have made more money for the middlemen in the drug supply chain, particularly those that construct formularies. Now, we have provider-administered biosimilars whose acquisition cost exceeds the reimbursement for these drugs. This disparity was ultimately created by biosimilar manufacturers “over-rebating” their drugs to health insurance companies to gain “fail-first” status on the formulary.
For example, the manufacturer of Inflectra offered substantial rebates to health insurers for preferred formulary placement. These rebates are factored into the sales price of the medication, which then results in a rapidly declining average sales price (ASP) for the biosimilar. Unfortunately, the acquisition cost for the drug does not experience commensurate reductions, resulting in physicians being reimbursed far less for the drug than it costs to acquire. The financial losses for physicians put them underwater as a result of the acquisition costs for the preferred drugs far surpassing the reimbursement from the health insurance company that constructed the formulary.
While various factors affect ASPs and acquisition costs, this particular consequence of formulary placement based on price concessions is a major driver of the underwater situation in which physicians have found themselves with many biosimilars. Not only does that lead to a lower uptake of biosimilars, but it also results in patients being referred to the hospital outpatient infusion sites to receive this care, as freestanding infusion centers cannot treat these patients either. Hospitals incur higher costs because of facility fees and elevated rates, and this makes private rheumatology in-office infusion centers a much lower-cost option. Similarly, home infusion services, while convenient, are marginally more expensive than private practices and, in cases of biologic infusions, it is important to note that physicians’ offices have a greater safety profile than home infusion of biologics. The overall result of these “fail-first underwater drugs” is delayed and more costly care for the patient and the “system,” particularly self-insured employers.
What Is Being Done to Correct This?
Since ASPs are updated quarterly, it is possible that acquisition costs and reimbursements might stabilize over time, making the drugs affordable again to practices. However, that does not appear to be happening in the near future, so that possibility does not offer immediate relief to struggling practices. It doesn’t promise a favorable outlook for future biosimilar entries of provider-administered medications if formularies continue to prefer the highest-rebated medication.
This dynamic between ASP and acquisition cost does not happen on the pharmacy side because the price concessions on specific drug rebates and fees are proprietary. There appears to be no equivalent to a publicly known ASP on the pharmacy side, which has led to myriad pricing definitions and manipulation on the pharmacy benefit side of medications. In any event, the savings from rebates and other manufacturer price concessions on pharmacy drugs do not influence ASPs of medical benefit drugs.
The Inflation Reduction Act provided a temporary increase in the add-on payment for biosimilars from ASP+6% to ASP+8%, but as long as the biosimilar’s ASP is lower than the reference brand’s ASP, that temporary increase does not appear to make up for the large differential between ASP and acquisition cost. It should be noted that any federal attempt to artificially lower the ASP of a provider-administered drug without a pathway assuring that the acquisition cost for the provider is less than the reimbursement is going to result in loss of access for patients to those medications and/or higher hospital site of care costs.
A Few Partial Fixes, But Most Complaints Go Ignored
Considering the higher costs of hospital-based infusion, insurers should be motivated to keep patients within private practices. Perhaps through insurers’ recognition of that fact, some practices have successfully negotiated exceptions for specific patients by discussing this situation with insurers. From the feedback that CSRO has received from rheumatology practices, it appears that most insurers have been ignoring the complaints from physicians. The few who have responded have resulted in only partial fixes, with some of the biosimilars still left underwater.
Ultimate Solution?
This issue is a direct result of the “rebate game,” whereby price concessions from drug manufacturers drive formulary placement. For provider-administered medications, this results in an artificially lowered ASP, not as a consequence of free-market incentives that benefit the patient, but as a result of misaligned incentives created by Safe Harbor–protected “kickbacks,” distorting the free market and paradoxically reducing access to these medications, delaying care, and increasing prices for patients and the healthcare system.
While federal and state governments are not likely to address this particular situation in the biosimilars market, CSRO is highlighting this issue as a prime example of why the current formulary construction system urgently requires federal reform. At this time, the biosimilars most affected are Inflectra and Avsola, but if nothing changes, more and more biosimilars will fall victim to the short-sighted pricing strategy of aggressive rebating to gain formulary position, with physician purchasers and patients left to navigate the aftermath. The existing system, which necessitates drug companies purchasing formulary access from pharmacy benefit managers, has led to delayed and even denied patient access to certain provider-administered drugs. Moreover, it now appears to be hindering the adoption of biosimilars.
To address this, a multifaceted approach is required. It not only involves reevaluating the rebate system and its impact on formulary construction and ASP, but also ensuring that acquisition costs for providers are aligned with reimbursement rates. Insurers must recognize the economic and clinical value of maintaining infusions within private practices and immediately update their policies to ensure that physician in-office infusion is financially feasible for these “fail-first” biosimilars.
Ultimately, the goal should be to create a sustainable model that promotes the use of affordable biosimilars, enhances patient access to affordable care, and supports the financial viability of medical practices. Concerted efforts to reform the current formulary construction system are required to achieve a healthcare environment that is both cost effective and patient centric.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Editor’s note: This article is adapted from an explanatory statement that Dr. Feldman wrote for the Coalition of State Rheumatology Organizations (CSRO).
According to the Guinness Book of World records, the longest time someone has held their breath underwater voluntarily is 24 minutes and 37.36 seconds. While certainly an amazing feat, UnitedHealthcare, many of the Blues, and other national “payers” are expecting rheumatologists and other specialists to live “underwater” in order to take care of their patients. In other words, these insurance companies are mandating that specialists use certain provider-administered biosimilars whose acquisition cost is higher than what the insurance company is willing to reimburse them. Essentially, the insurance companies expect the rheumatologists to pay them to take care of their patients. Because of the substantial and destabilizing financial losses incurred, many practices and free-standing infusion centers have been forced to cease offering these biosimilars. Most rheumatologists will provide patients with appropriate alternatives when available and permitted by the insurer; otherwise, they must refer patients to hospital-based infusion centers. That results in delayed care and increased costs for patients and the system, because hospital-based infusion typically costs more than twice what office-based infusion costs.
Quantifying the Problem
To help quantify the magnitude of this issue, the Coalition of State Rheumatology Organizations (CSRO) recently conducted a survey of its membership. A shocking 97% of respondents reported that their practice had been affected by reimbursement rates for some biosimilars being lower than acquisition costs, with 91% of respondents stating that this issue is more pronounced for certain biosimilars than others. Across the board, respondents most frequently identified Inflectra (infliximab-dyyb) and Avsola (infliximab-axxq) as being especially affected: Over 88% and over 85% of respondents identified these two products, respectively, as being underwater. These results support the ongoing anecdotal reports CSRO continues to receive from rheumatology practices.
However, the survey results indicated that this issue is by no means confined to those two biosimilars. Truxima (rituximab-abbs) — a biosimilar for Rituxan — was frequently mentioned as well. Notably, respondents almost uniformly identified biosimilars in the infliximab and rituximab families, which illustrates that this issue is no longer confined to one or two early-to-market biosimilars but has almost become a hallmark of this particular biosimilars market. Remarkably, one respondent commented that the brand products are now cheaper to acquire than the biosimilars. Furthermore, the survey included respondents from across the country, indicating that this issue is not confined to a particular region.
How Did This Happen?
Biosimilars held promise for increasing availability and decreasing biologic costs for patients but, thus far, no patients have seen their cost go down. It appears that the only biosimilars that have made it to “preferred” status on the formulary are the ones that have made more money for the middlemen in the drug supply chain, particularly those that construct formularies. Now, we have provider-administered biosimilars whose acquisition cost exceeds the reimbursement for these drugs. This disparity was ultimately created by biosimilar manufacturers “over-rebating” their drugs to health insurance companies to gain “fail-first” status on the formulary.
For example, the manufacturer of Inflectra offered substantial rebates to health insurers for preferred formulary placement. These rebates are factored into the sales price of the medication, which then results in a rapidly declining average sales price (ASP) for the biosimilar. Unfortunately, the acquisition cost for the drug does not experience commensurate reductions, resulting in physicians being reimbursed far less for the drug than it costs to acquire. The financial losses for physicians put them underwater as a result of the acquisition costs for the preferred drugs far surpassing the reimbursement from the health insurance company that constructed the formulary.
While various factors affect ASPs and acquisition costs, this particular consequence of formulary placement based on price concessions is a major driver of the underwater situation in which physicians have found themselves with many biosimilars. Not only does that lead to a lower uptake of biosimilars, but it also results in patients being referred to the hospital outpatient infusion sites to receive this care, as freestanding infusion centers cannot treat these patients either. Hospitals incur higher costs because of facility fees and elevated rates, and this makes private rheumatology in-office infusion centers a much lower-cost option. Similarly, home infusion services, while convenient, are marginally more expensive than private practices and, in cases of biologic infusions, it is important to note that physicians’ offices have a greater safety profile than home infusion of biologics. The overall result of these “fail-first underwater drugs” is delayed and more costly care for the patient and the “system,” particularly self-insured employers.
What Is Being Done to Correct This?
Since ASPs are updated quarterly, it is possible that acquisition costs and reimbursements might stabilize over time, making the drugs affordable again to practices. However, that does not appear to be happening in the near future, so that possibility does not offer immediate relief to struggling practices. It doesn’t promise a favorable outlook for future biosimilar entries of provider-administered medications if formularies continue to prefer the highest-rebated medication.
This dynamic between ASP and acquisition cost does not happen on the pharmacy side because the price concessions on specific drug rebates and fees are proprietary. There appears to be no equivalent to a publicly known ASP on the pharmacy side, which has led to myriad pricing definitions and manipulation on the pharmacy benefit side of medications. In any event, the savings from rebates and other manufacturer price concessions on pharmacy drugs do not influence ASPs of medical benefit drugs.
The Inflation Reduction Act provided a temporary increase in the add-on payment for biosimilars from ASP+6% to ASP+8%, but as long as the biosimilar’s ASP is lower than the reference brand’s ASP, that temporary increase does not appear to make up for the large differential between ASP and acquisition cost. It should be noted that any federal attempt to artificially lower the ASP of a provider-administered drug without a pathway assuring that the acquisition cost for the provider is less than the reimbursement is going to result in loss of access for patients to those medications and/or higher hospital site of care costs.
A Few Partial Fixes, But Most Complaints Go Ignored
Considering the higher costs of hospital-based infusion, insurers should be motivated to keep patients within private practices. Perhaps through insurers’ recognition of that fact, some practices have successfully negotiated exceptions for specific patients by discussing this situation with insurers. From the feedback that CSRO has received from rheumatology practices, it appears that most insurers have been ignoring the complaints from physicians. The few who have responded have resulted in only partial fixes, with some of the biosimilars still left underwater.
Ultimate Solution?
This issue is a direct result of the “rebate game,” whereby price concessions from drug manufacturers drive formulary placement. For provider-administered medications, this results in an artificially lowered ASP, not as a consequence of free-market incentives that benefit the patient, but as a result of misaligned incentives created by Safe Harbor–protected “kickbacks,” distorting the free market and paradoxically reducing access to these medications, delaying care, and increasing prices for patients and the healthcare system.
While federal and state governments are not likely to address this particular situation in the biosimilars market, CSRO is highlighting this issue as a prime example of why the current formulary construction system urgently requires federal reform. At this time, the biosimilars most affected are Inflectra and Avsola, but if nothing changes, more and more biosimilars will fall victim to the short-sighted pricing strategy of aggressive rebating to gain formulary position, with physician purchasers and patients left to navigate the aftermath. The existing system, which necessitates drug companies purchasing formulary access from pharmacy benefit managers, has led to delayed and even denied patient access to certain provider-administered drugs. Moreover, it now appears to be hindering the adoption of biosimilars.
To address this, a multifaceted approach is required. It not only involves reevaluating the rebate system and its impact on formulary construction and ASP, but also ensuring that acquisition costs for providers are aligned with reimbursement rates. Insurers must recognize the economic and clinical value of maintaining infusions within private practices and immediately update their policies to ensure that physician in-office infusion is financially feasible for these “fail-first” biosimilars.
Ultimately, the goal should be to create a sustainable model that promotes the use of affordable biosimilars, enhances patient access to affordable care, and supports the financial viability of medical practices. Concerted efforts to reform the current formulary construction system are required to achieve a healthcare environment that is both cost effective and patient centric.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Editor’s note: This article is adapted from an explanatory statement that Dr. Feldman wrote for the Coalition of State Rheumatology Organizations (CSRO).
According to the Guinness Book of World records, the longest time someone has held their breath underwater voluntarily is 24 minutes and 37.36 seconds. While certainly an amazing feat, UnitedHealthcare, many of the Blues, and other national “payers” are expecting rheumatologists and other specialists to live “underwater” in order to take care of their patients. In other words, these insurance companies are mandating that specialists use certain provider-administered biosimilars whose acquisition cost is higher than what the insurance company is willing to reimburse them. Essentially, the insurance companies expect the rheumatologists to pay them to take care of their patients. Because of the substantial and destabilizing financial losses incurred, many practices and free-standing infusion centers have been forced to cease offering these biosimilars. Most rheumatologists will provide patients with appropriate alternatives when available and permitted by the insurer; otherwise, they must refer patients to hospital-based infusion centers. That results in delayed care and increased costs for patients and the system, because hospital-based infusion typically costs more than twice what office-based infusion costs.
Quantifying the Problem
To help quantify the magnitude of this issue, the Coalition of State Rheumatology Organizations (CSRO) recently conducted a survey of its membership. A shocking 97% of respondents reported that their practice had been affected by reimbursement rates for some biosimilars being lower than acquisition costs, with 91% of respondents stating that this issue is more pronounced for certain biosimilars than others. Across the board, respondents most frequently identified Inflectra (infliximab-dyyb) and Avsola (infliximab-axxq) as being especially affected: Over 88% and over 85% of respondents identified these two products, respectively, as being underwater. These results support the ongoing anecdotal reports CSRO continues to receive from rheumatology practices.
However, the survey results indicated that this issue is by no means confined to those two biosimilars. Truxima (rituximab-abbs) — a biosimilar for Rituxan — was frequently mentioned as well. Notably, respondents almost uniformly identified biosimilars in the infliximab and rituximab families, which illustrates that this issue is no longer confined to one or two early-to-market biosimilars but has almost become a hallmark of this particular biosimilars market. Remarkably, one respondent commented that the brand products are now cheaper to acquire than the biosimilars. Furthermore, the survey included respondents from across the country, indicating that this issue is not confined to a particular region.
How Did This Happen?
Biosimilars held promise for increasing availability and decreasing biologic costs for patients but, thus far, no patients have seen their cost go down. It appears that the only biosimilars that have made it to “preferred” status on the formulary are the ones that have made more money for the middlemen in the drug supply chain, particularly those that construct formularies. Now, we have provider-administered biosimilars whose acquisition cost exceeds the reimbursement for these drugs. This disparity was ultimately created by biosimilar manufacturers “over-rebating” their drugs to health insurance companies to gain “fail-first” status on the formulary.
For example, the manufacturer of Inflectra offered substantial rebates to health insurers for preferred formulary placement. These rebates are factored into the sales price of the medication, which then results in a rapidly declining average sales price (ASP) for the biosimilar. Unfortunately, the acquisition cost for the drug does not experience commensurate reductions, resulting in physicians being reimbursed far less for the drug than it costs to acquire. The financial losses for physicians put them underwater as a result of the acquisition costs for the preferred drugs far surpassing the reimbursement from the health insurance company that constructed the formulary.
While various factors affect ASPs and acquisition costs, this particular consequence of formulary placement based on price concessions is a major driver of the underwater situation in which physicians have found themselves with many biosimilars. Not only does that lead to a lower uptake of biosimilars, but it also results in patients being referred to the hospital outpatient infusion sites to receive this care, as freestanding infusion centers cannot treat these patients either. Hospitals incur higher costs because of facility fees and elevated rates, and this makes private rheumatology in-office infusion centers a much lower-cost option. Similarly, home infusion services, while convenient, are marginally more expensive than private practices and, in cases of biologic infusions, it is important to note that physicians’ offices have a greater safety profile than home infusion of biologics. The overall result of these “fail-first underwater drugs” is delayed and more costly care for the patient and the “system,” particularly self-insured employers.
What Is Being Done to Correct This?
Since ASPs are updated quarterly, it is possible that acquisition costs and reimbursements might stabilize over time, making the drugs affordable again to practices. However, that does not appear to be happening in the near future, so that possibility does not offer immediate relief to struggling practices. It doesn’t promise a favorable outlook for future biosimilar entries of provider-administered medications if formularies continue to prefer the highest-rebated medication.
This dynamic between ASP and acquisition cost does not happen on the pharmacy side because the price concessions on specific drug rebates and fees are proprietary. There appears to be no equivalent to a publicly known ASP on the pharmacy side, which has led to myriad pricing definitions and manipulation on the pharmacy benefit side of medications. In any event, the savings from rebates and other manufacturer price concessions on pharmacy drugs do not influence ASPs of medical benefit drugs.
The Inflation Reduction Act provided a temporary increase in the add-on payment for biosimilars from ASP+6% to ASP+8%, but as long as the biosimilar’s ASP is lower than the reference brand’s ASP, that temporary increase does not appear to make up for the large differential between ASP and acquisition cost. It should be noted that any federal attempt to artificially lower the ASP of a provider-administered drug without a pathway assuring that the acquisition cost for the provider is less than the reimbursement is going to result in loss of access for patients to those medications and/or higher hospital site of care costs.
A Few Partial Fixes, But Most Complaints Go Ignored
Considering the higher costs of hospital-based infusion, insurers should be motivated to keep patients within private practices. Perhaps through insurers’ recognition of that fact, some practices have successfully negotiated exceptions for specific patients by discussing this situation with insurers. From the feedback that CSRO has received from rheumatology practices, it appears that most insurers have been ignoring the complaints from physicians. The few who have responded have resulted in only partial fixes, with some of the biosimilars still left underwater.
Ultimate Solution?
This issue is a direct result of the “rebate game,” whereby price concessions from drug manufacturers drive formulary placement. For provider-administered medications, this results in an artificially lowered ASP, not as a consequence of free-market incentives that benefit the patient, but as a result of misaligned incentives created by Safe Harbor–protected “kickbacks,” distorting the free market and paradoxically reducing access to these medications, delaying care, and increasing prices for patients and the healthcare system.
While federal and state governments are not likely to address this particular situation in the biosimilars market, CSRO is highlighting this issue as a prime example of why the current formulary construction system urgently requires federal reform. At this time, the biosimilars most affected are Inflectra and Avsola, but if nothing changes, more and more biosimilars will fall victim to the short-sighted pricing strategy of aggressive rebating to gain formulary position, with physician purchasers and patients left to navigate the aftermath. The existing system, which necessitates drug companies purchasing formulary access from pharmacy benefit managers, has led to delayed and even denied patient access to certain provider-administered drugs. Moreover, it now appears to be hindering the adoption of biosimilars.
To address this, a multifaceted approach is required. It not only involves reevaluating the rebate system and its impact on formulary construction and ASP, but also ensuring that acquisition costs for providers are aligned with reimbursement rates. Insurers must recognize the economic and clinical value of maintaining infusions within private practices and immediately update their policies to ensure that physician in-office infusion is financially feasible for these “fail-first” biosimilars.
Ultimately, the goal should be to create a sustainable model that promotes the use of affordable biosimilars, enhances patient access to affordable care, and supports the financial viability of medical practices. Concerted efforts to reform the current formulary construction system are required to achieve a healthcare environment that is both cost effective and patient centric.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
ERISA Health Plan Lawsuits: Why Should We Care?
A recently filed lawsuit against Johnson & Johnson can serve as an example to use when advocating for patients who have insurance through their employers that can potentially hurt them physically and financially. When your patient has an employer-funded health insurance plan where the employer directly pays for all medical costs — called an ERISA plan for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act — there are certain accountability, fairness, and fiduciary responsibilities that the employers must meet. These so-called ERISA plans do not have to follow state utilization management legislation that addresses harmful changes in insurers’ formularies and other policies, so when the plans are not properly overseen and do not mandate the delivery of proper care at the lowest cost, both the patient and employer may be losing out.
The J&J lawsuit serves as a bellwether warning to self-insured employers to demand transparency from their third-party administrators so as not to (knowingly or unknowingly) breach their fiduciary duty to their health plans and employees. These duties include ensuring reasonable plan costs as well as acting in the best interest of their employees. There were multiple complaints in the lawsuit by a J&J employee, stating that she paid a much higher price for her multiple sclerosis drug through the plan than the price she eventually found at a lower cost pharmacy. The allegations state that J&J failed to show prudence in its selection of a pharmacy benefit manager (PBM). In addition, the company failed to negotiate better drug pricing terms, and the design of the drug plan steered patients to the PBM specialty pharmacy, resulting in higher prices for the employees. All of these led to higher drug costs and premiums for employees, which, according to the lawsuit, is a breach of J&J’s fiduciary duties.
Why Should Rheumatologists Care About This?
With all insurance plans, it feels as though we are dealing with obstacles every day that keep us from giving the excellent rheumatologic care that our patients deserve. Self-insured employers now account for over 50% of commercial health plans, and as rheumatologists caring for the employees of these companies, we can use those transparency, accountability, and fiduciary responsibilities of the employer to ensure that our patients are getting the proper care at the lowest cost.
Not only is the J&J lawsuit a warning to self-insured employers, but a reminder to rheumatologists to be on the lookout for drug pricing issues and formulary construction that leads to higher pricing for employees and the plan. For example, make note if your patient is forced to fail a much higher priced self-injectable biologic before using a much lower cost infusible medication. Or if the plan mandates the use of the much higher priced adalimumab biosimilars over the lower priced biosimilars or even the highest priced JAK inhibitor over the lowest priced one. Let’s not forget mandated white bagging, which is often much more expensive to the plan than the buy-and-bill model through a rheumatologist’s office.
Recently, we have been able to help rheumatology practices get exemptions from white-bagging mandates that large self-insured employers often have in their plan documents. We have been able to show that the cost of obtaining the medication through specialty pharmacy (SP) is much higher than through the buy-and-bill model. Mandating that the plan spend more money on SP drugs, as opposed to allowing the rheumatologist to buy and bill, could easily be interpreted as a breach of fiduciary duty on the part of the employer by mandating a higher cost model.
CSRO Payer Issue Response Team
I have written about the Coalition of State Rheumatology Organizations (CSRO)’s Payer Issue Response Team (PIRT) in the past. Rheumatologists around the country can send to PIRT any problems that they are having with payers. A recent PIRT submission involved a white-bagging mandate for an employee of a very large international Fortune 500 company. This particular example is important because of the response by the VP of Global Benefits for this company. Express Scripts is the administrator of pharmacy benefits for this company. The rheumatologist was told that he could not buy and bill for an infusible medicine but would have to obtain the drug through Express Scripts’ SP. He then asked Express Scripts for the SP medication’s cost to the health plan in order to compare the SP price versus what the buy-and-bill model would cost this company. Express Scripts would not respond to this simple transparency question; often, PBMs claim that this is proprietary information.
I was able to speak with the company’s VP of Global Benefits regarding this issue. First of all, he stated that his company was not mandating white bagging. I explained to him that the plan documents had white bagging as the only option for acquisition of provider-administered drugs. A rheumatologist would have to apply for an exemption to buy and bill, and in this case, it was denied. This is essentially a mandate.
I gave the VP of Global Benefits an example of another large Fortune 500 company (UPS) that spent over $30,000 per year more on an infusible medication when obtained through SP than what it cost them under a buy-and-bill model. I had hoped that this example would impress upon the VP the importance of transparency in pricing and claims to prevent his company from unknowingly costing the health plan more and its being construed as a breach of fiduciary duty. It was explained to me by the VP of Global Benefits that his company is part of the National Drug Purchasers Coalition and they trust Express Scripts to do the right thing for them. As they say, “You can lead a horse to water, but can’t make it drink.”
Liability of a Plan That Physically Harms an Employee?
A slightly different example of a self-insured employer, presumably unknowingly, allowing its third-party administrator to mishandle the care of an employee was recently brought to me by a rheumatologist in North Carolina. She takes care of an employee who has rheumatoid arthritis with severe interstitial lung disease (ILD). The employee’s pulmonary status was stabilized on several courses of Rituxan (reference product of rituximab). Recently, BlueCross BlueShield of North Carolina, the third-party administrator of this employer’s plan, mandated a switch to a biosimilar of rituximab for the treatment of the ILD. The rheumatologist appealed the nonmedical switch but gave the patient the biosimilar so as not to delay care. Her patient’s condition is now deteriorating with progression of the ILD, and she once again has asked for an exemption to use Rituxan, which had initially stabilized the patient. Her staff told her that the BCBSNC rep said that the patient would have to have a life-threatening infusion reaction (and present the bill for the ambulance) before they would approve a return to the reference product. An employer that knowingly or unknowingly allows a third-party administrator to act in such a way as to endanger the life of an employee could be considered to be breaching its fiduciary duty. (Disclaimer: I am not an attorney — merely a rheumatologist with common sense. Nor am I making any qualitative statement about biosimilars.)
We now have a lawsuit to which you can refer when advocating for our patients who are employed by large, self-insured employers. It is unfortunate that it is not the third-party administrators or PBMs that can be sued, as they are generally not the fiduciaries for the plan. It is the unsuspecting employers who “trust” their brokers/consultants and the third-party administrators to do the right thing. Please continue to send us your payer issues. And if your patient works for a self-insured employer, I will continue to remind the CEO, CFO, and chief compliance officer that an employer with an ERISA health plan can potentially face legal action if the health plan’s actions or decisions cause harm to an employee’s health — physically or in the wallet.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
A recently filed lawsuit against Johnson & Johnson can serve as an example to use when advocating for patients who have insurance through their employers that can potentially hurt them physically and financially. When your patient has an employer-funded health insurance plan where the employer directly pays for all medical costs — called an ERISA plan for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act — there are certain accountability, fairness, and fiduciary responsibilities that the employers must meet. These so-called ERISA plans do not have to follow state utilization management legislation that addresses harmful changes in insurers’ formularies and other policies, so when the plans are not properly overseen and do not mandate the delivery of proper care at the lowest cost, both the patient and employer may be losing out.
The J&J lawsuit serves as a bellwether warning to self-insured employers to demand transparency from their third-party administrators so as not to (knowingly or unknowingly) breach their fiduciary duty to their health plans and employees. These duties include ensuring reasonable plan costs as well as acting in the best interest of their employees. There were multiple complaints in the lawsuit by a J&J employee, stating that she paid a much higher price for her multiple sclerosis drug through the plan than the price she eventually found at a lower cost pharmacy. The allegations state that J&J failed to show prudence in its selection of a pharmacy benefit manager (PBM). In addition, the company failed to negotiate better drug pricing terms, and the design of the drug plan steered patients to the PBM specialty pharmacy, resulting in higher prices for the employees. All of these led to higher drug costs and premiums for employees, which, according to the lawsuit, is a breach of J&J’s fiduciary duties.
Why Should Rheumatologists Care About This?
With all insurance plans, it feels as though we are dealing with obstacles every day that keep us from giving the excellent rheumatologic care that our patients deserve. Self-insured employers now account for over 50% of commercial health plans, and as rheumatologists caring for the employees of these companies, we can use those transparency, accountability, and fiduciary responsibilities of the employer to ensure that our patients are getting the proper care at the lowest cost.
Not only is the J&J lawsuit a warning to self-insured employers, but a reminder to rheumatologists to be on the lookout for drug pricing issues and formulary construction that leads to higher pricing for employees and the plan. For example, make note if your patient is forced to fail a much higher priced self-injectable biologic before using a much lower cost infusible medication. Or if the plan mandates the use of the much higher priced adalimumab biosimilars over the lower priced biosimilars or even the highest priced JAK inhibitor over the lowest priced one. Let’s not forget mandated white bagging, which is often much more expensive to the plan than the buy-and-bill model through a rheumatologist’s office.
Recently, we have been able to help rheumatology practices get exemptions from white-bagging mandates that large self-insured employers often have in their plan documents. We have been able to show that the cost of obtaining the medication through specialty pharmacy (SP) is much higher than through the buy-and-bill model. Mandating that the plan spend more money on SP drugs, as opposed to allowing the rheumatologist to buy and bill, could easily be interpreted as a breach of fiduciary duty on the part of the employer by mandating a higher cost model.
CSRO Payer Issue Response Team
I have written about the Coalition of State Rheumatology Organizations (CSRO)’s Payer Issue Response Team (PIRT) in the past. Rheumatologists around the country can send to PIRT any problems that they are having with payers. A recent PIRT submission involved a white-bagging mandate for an employee of a very large international Fortune 500 company. This particular example is important because of the response by the VP of Global Benefits for this company. Express Scripts is the administrator of pharmacy benefits for this company. The rheumatologist was told that he could not buy and bill for an infusible medicine but would have to obtain the drug through Express Scripts’ SP. He then asked Express Scripts for the SP medication’s cost to the health plan in order to compare the SP price versus what the buy-and-bill model would cost this company. Express Scripts would not respond to this simple transparency question; often, PBMs claim that this is proprietary information.
I was able to speak with the company’s VP of Global Benefits regarding this issue. First of all, he stated that his company was not mandating white bagging. I explained to him that the plan documents had white bagging as the only option for acquisition of provider-administered drugs. A rheumatologist would have to apply for an exemption to buy and bill, and in this case, it was denied. This is essentially a mandate.
I gave the VP of Global Benefits an example of another large Fortune 500 company (UPS) that spent over $30,000 per year more on an infusible medication when obtained through SP than what it cost them under a buy-and-bill model. I had hoped that this example would impress upon the VP the importance of transparency in pricing and claims to prevent his company from unknowingly costing the health plan more and its being construed as a breach of fiduciary duty. It was explained to me by the VP of Global Benefits that his company is part of the National Drug Purchasers Coalition and they trust Express Scripts to do the right thing for them. As they say, “You can lead a horse to water, but can’t make it drink.”
Liability of a Plan That Physically Harms an Employee?
A slightly different example of a self-insured employer, presumably unknowingly, allowing its third-party administrator to mishandle the care of an employee was recently brought to me by a rheumatologist in North Carolina. She takes care of an employee who has rheumatoid arthritis with severe interstitial lung disease (ILD). The employee’s pulmonary status was stabilized on several courses of Rituxan (reference product of rituximab). Recently, BlueCross BlueShield of North Carolina, the third-party administrator of this employer’s plan, mandated a switch to a biosimilar of rituximab for the treatment of the ILD. The rheumatologist appealed the nonmedical switch but gave the patient the biosimilar so as not to delay care. Her patient’s condition is now deteriorating with progression of the ILD, and she once again has asked for an exemption to use Rituxan, which had initially stabilized the patient. Her staff told her that the BCBSNC rep said that the patient would have to have a life-threatening infusion reaction (and present the bill for the ambulance) before they would approve a return to the reference product. An employer that knowingly or unknowingly allows a third-party administrator to act in such a way as to endanger the life of an employee could be considered to be breaching its fiduciary duty. (Disclaimer: I am not an attorney — merely a rheumatologist with common sense. Nor am I making any qualitative statement about biosimilars.)
We now have a lawsuit to which you can refer when advocating for our patients who are employed by large, self-insured employers. It is unfortunate that it is not the third-party administrators or PBMs that can be sued, as they are generally not the fiduciaries for the plan. It is the unsuspecting employers who “trust” their brokers/consultants and the third-party administrators to do the right thing. Please continue to send us your payer issues. And if your patient works for a self-insured employer, I will continue to remind the CEO, CFO, and chief compliance officer that an employer with an ERISA health plan can potentially face legal action if the health plan’s actions or decisions cause harm to an employee’s health — physically or in the wallet.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
A recently filed lawsuit against Johnson & Johnson can serve as an example to use when advocating for patients who have insurance through their employers that can potentially hurt them physically and financially. When your patient has an employer-funded health insurance plan where the employer directly pays for all medical costs — called an ERISA plan for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act — there are certain accountability, fairness, and fiduciary responsibilities that the employers must meet. These so-called ERISA plans do not have to follow state utilization management legislation that addresses harmful changes in insurers’ formularies and other policies, so when the plans are not properly overseen and do not mandate the delivery of proper care at the lowest cost, both the patient and employer may be losing out.
The J&J lawsuit serves as a bellwether warning to self-insured employers to demand transparency from their third-party administrators so as not to (knowingly or unknowingly) breach their fiduciary duty to their health plans and employees. These duties include ensuring reasonable plan costs as well as acting in the best interest of their employees. There were multiple complaints in the lawsuit by a J&J employee, stating that she paid a much higher price for her multiple sclerosis drug through the plan than the price she eventually found at a lower cost pharmacy. The allegations state that J&J failed to show prudence in its selection of a pharmacy benefit manager (PBM). In addition, the company failed to negotiate better drug pricing terms, and the design of the drug plan steered patients to the PBM specialty pharmacy, resulting in higher prices for the employees. All of these led to higher drug costs and premiums for employees, which, according to the lawsuit, is a breach of J&J’s fiduciary duties.
Why Should Rheumatologists Care About This?
With all insurance plans, it feels as though we are dealing with obstacles every day that keep us from giving the excellent rheumatologic care that our patients deserve. Self-insured employers now account for over 50% of commercial health plans, and as rheumatologists caring for the employees of these companies, we can use those transparency, accountability, and fiduciary responsibilities of the employer to ensure that our patients are getting the proper care at the lowest cost.
Not only is the J&J lawsuit a warning to self-insured employers, but a reminder to rheumatologists to be on the lookout for drug pricing issues and formulary construction that leads to higher pricing for employees and the plan. For example, make note if your patient is forced to fail a much higher priced self-injectable biologic before using a much lower cost infusible medication. Or if the plan mandates the use of the much higher priced adalimumab biosimilars over the lower priced biosimilars or even the highest priced JAK inhibitor over the lowest priced one. Let’s not forget mandated white bagging, which is often much more expensive to the plan than the buy-and-bill model through a rheumatologist’s office.
Recently, we have been able to help rheumatology practices get exemptions from white-bagging mandates that large self-insured employers often have in their plan documents. We have been able to show that the cost of obtaining the medication through specialty pharmacy (SP) is much higher than through the buy-and-bill model. Mandating that the plan spend more money on SP drugs, as opposed to allowing the rheumatologist to buy and bill, could easily be interpreted as a breach of fiduciary duty on the part of the employer by mandating a higher cost model.
CSRO Payer Issue Response Team
I have written about the Coalition of State Rheumatology Organizations (CSRO)’s Payer Issue Response Team (PIRT) in the past. Rheumatologists around the country can send to PIRT any problems that they are having with payers. A recent PIRT submission involved a white-bagging mandate for an employee of a very large international Fortune 500 company. This particular example is important because of the response by the VP of Global Benefits for this company. Express Scripts is the administrator of pharmacy benefits for this company. The rheumatologist was told that he could not buy and bill for an infusible medicine but would have to obtain the drug through Express Scripts’ SP. He then asked Express Scripts for the SP medication’s cost to the health plan in order to compare the SP price versus what the buy-and-bill model would cost this company. Express Scripts would not respond to this simple transparency question; often, PBMs claim that this is proprietary information.
I was able to speak with the company’s VP of Global Benefits regarding this issue. First of all, he stated that his company was not mandating white bagging. I explained to him that the plan documents had white bagging as the only option for acquisition of provider-administered drugs. A rheumatologist would have to apply for an exemption to buy and bill, and in this case, it was denied. This is essentially a mandate.
I gave the VP of Global Benefits an example of another large Fortune 500 company (UPS) that spent over $30,000 per year more on an infusible medication when obtained through SP than what it cost them under a buy-and-bill model. I had hoped that this example would impress upon the VP the importance of transparency in pricing and claims to prevent his company from unknowingly costing the health plan more and its being construed as a breach of fiduciary duty. It was explained to me by the VP of Global Benefits that his company is part of the National Drug Purchasers Coalition and they trust Express Scripts to do the right thing for them. As they say, “You can lead a horse to water, but can’t make it drink.”
Liability of a Plan That Physically Harms an Employee?
A slightly different example of a self-insured employer, presumably unknowingly, allowing its third-party administrator to mishandle the care of an employee was recently brought to me by a rheumatologist in North Carolina. She takes care of an employee who has rheumatoid arthritis with severe interstitial lung disease (ILD). The employee’s pulmonary status was stabilized on several courses of Rituxan (reference product of rituximab). Recently, BlueCross BlueShield of North Carolina, the third-party administrator of this employer’s plan, mandated a switch to a biosimilar of rituximab for the treatment of the ILD. The rheumatologist appealed the nonmedical switch but gave the patient the biosimilar so as not to delay care. Her patient’s condition is now deteriorating with progression of the ILD, and she once again has asked for an exemption to use Rituxan, which had initially stabilized the patient. Her staff told her that the BCBSNC rep said that the patient would have to have a life-threatening infusion reaction (and present the bill for the ambulance) before they would approve a return to the reference product. An employer that knowingly or unknowingly allows a third-party administrator to act in such a way as to endanger the life of an employee could be considered to be breaching its fiduciary duty. (Disclaimer: I am not an attorney — merely a rheumatologist with common sense. Nor am I making any qualitative statement about biosimilars.)
We now have a lawsuit to which you can refer when advocating for our patients who are employed by large, self-insured employers. It is unfortunate that it is not the third-party administrators or PBMs that can be sued, as they are generally not the fiduciaries for the plan. It is the unsuspecting employers who “trust” their brokers/consultants and the third-party administrators to do the right thing. Please continue to send us your payer issues. And if your patient works for a self-insured employer, I will continue to remind the CEO, CFO, and chief compliance officer that an employer with an ERISA health plan can potentially face legal action if the health plan’s actions or decisions cause harm to an employee’s health — physically or in the wallet.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Biosimilar Business Deals Keep Up ‘Musical Chairs’ Game of Formulary Construction
As the saying goes, “The more things change, the more they stay the same.” That is particularly true when it comes to the affordability of drugs for our patients even after the launch of so many Humira biosimilars. And we still have the “musical chairs” game of formulary construction — when the music stops, who knows whether your patient’s drug found a chair to sit on. There seems to be only a few chairs available for the many adalimumab biosimilars playing the game.
Nothing has changed since my testimony before the FDA Arthritis Advisory Committee in July 2016 during the approval hearing of the first Humira biosimilar. Below is a quote from that meeting where I was speaking predominantly about the pharmacy side of drugs.
“I’d like to highlight the term ‘access’ because none of us are really naive enough to believe that just approving a biosimilar gives a patient true, hands-on access to the medication, because even if the biosimilar is offered at a 30% discount, I don’t have any patients that can afford it. This means that access is ultimately controlled by third-party payers.”
My prediction, that approving and launching biosimilars with lower prices would not ensure patient access to the drug unless it is paid for by insurance, is now our reality. Today, a drug with an 85% discount on the price of Humira is still unattainable for patients without a “payer.”
Competition and Lower Prices
Lawmakers and some in the media cry for more competition to lower prices. This is the main reason that there has been such a push to get biosimilars to the market as quickly as possible. It is abundantly clear that competition to get on the formulary is fierce. Placement of a medication on a formulary can make or break a manufacturer’s ability to get a return on the R&D and make a profit on that medication. For a small biotech manufacturer, it can be the difference between “life and death” of the company.
Does anyone remember when the first interchangeable biosimilar for the reference insulin glargine product Lantus (insulin glargine-yfgn; Semglee) came to market in 2021? Janet Woodcock, MD, then acting FDA commissioner, called it a “momentous day” and further said, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.” There was a high-priced interchangeable biosimilar and an identical unbranded low-priced interchangeable biosimilar, and the only one that could get formulary placement was the high-priced drug.
Patients pay their cost share on the list price of the drug, and because most pharmacy benefit managers’ (PBMs’) formularies cover only the high-priced biosimilar, patients never share in the savings. So much for the “competitive marketplace” creating lower costs for patients. This is just one of hundreds of examples in which lower-priced drugs are excluded from the formulary. It is unfortunate that the bidding process from manufacturers to PBMs to “win” preferred formulary placement is like an art auction, where the highest bidder wins.
Biosimilars and Formulary Construction
For those of us who have been looking into PBMs for many years, it is no surprise that PBMs’ formulary construction has become a profit center for them. Now, with so many adalimumab biosimilars having entered the market, it has become the Wild West where only those with the most money to fork over to the PBMs get preferred placement. Unfortunately, many of the choices that make money for the PBM cost employers and patients more.
How did we get here? In the 1980s and 90s, the price of medications began to increase to the point that many were not affordable without insurance. And who better to construct the list of drugs that would be covered by insurance (formulary) than the PBMs who were already adjudicating the claims for these drugs. The Federal Trade Commission (FTC) realized the power inherent in constructing this list of medications known as the formulary. So when the manufacturer Merck acquired the PBM Medco in the mid-1990s, the FTC stepped in. The FTC surmised that making the drugs and deciding which ones will be paid for created a “conflict of interest” with anticompetitive ramifications.
So, in 1998, William J. Baer, director of the FTC’s Bureau of Competition, said, “Our investigation into the PBM industry has revealed that Merck’s acquisition of Medco has reduced competition in the market for pharmaceutical products … We have found that Medco has given favorable treatment to Merck drugs. As a result, in some cases, consumers have been denied access to the drugs of competing manufacturers. In addition, the merger has made it possible for Medco to share with Merck sensitive pricing information it gets from Merck’s competitors, which could foster collusion among drug manufacturers.” Wow!
These anticompetitive behaviors and conflicts of interest resulting from the Medco acquisition led the FTC to propose a consent agreement.
The agreement would require Merck-Medco to maintain an “open formulary” — one that includes drugs selected and approved by an independent Pharmacy and Therapeutics Committee regardless of the manufacturer. Medco would have to accept rebates and other price concessions and reflect these in the ranking of the drugs on the formulary. Merck would have to make known the availability of the open formulary to any drug maker with an agreement with Medco.
Let’s hope the FTC of 2024 remembers the stance of the FTC in the 1990s regarding anticompetitive behavior involved in formulary construction.
Conflicts of Interest
But today it is apparent that crafting formularies that pay only for the drugs that make the most money for the PBM is not a conflict of interest. In its policy manual, Cigna directly tells employers and employees that they are collecting and keeping rebates and fees on medical pharmaceuticals, and they are not for the benefit of the employer or the plan.
And now, in August 2023, CVS launched Cordavis, a subsidiary wholly owned by CVS. Cordavis/CVS has partnered with Sandoz, which makes Hyrimoz, an adalimumab biosimilar. There is a high-priced version that is discounted 5% from Humira, a lower-cost unbranded version that is discounted 80% off the list price of Humira, and a co-branded CVS/Sandoz version of Hyrimoz that is lower priced as well.
It isn’t a surprise that CVS’ Standard and Advanced Commercial and Chart formularies are offering only Sandoz adalimumab biosimilar products. While these formularies have excluded Humira, CVS has entered into an agreement with AbbVie to allow Humira on a number of their other formularies. It can be very confusing.
As stated earlier, in the 1990s, the FTC frowned upon manufacturers owning PBMs and allowing them to construct their own formularies. Here we have CVS Health, mothership for the PBM CVS Caremark, owning a company that will be co-producing biosimilars with other manufacturers and then determining which biosimilars are on their formularies. The FTC knew back then that the tendency would be to offer only their own drugs for coverage, thus reducing competition. This is exactly what the CVS-Cordavis-Sandoz partnership has done for their Standard and Advanced Commercial and Chart formularies. It is perhaps anti-competitive but certainly profitable.
Perhaps the FTC should require the same consent agreement that was given to Merck in 1998. CVS Caremark would then have to open their formularies to all competitors of their co-branded, co-produced Sandoz biosimilar.
Summary
It is the same old adage, “The more things change, the more they stay the same.” PBMs are still constructing formularies with biosimilars based on their profitability, with huge differences between gross and net cost. Patients still pay their cost share on the list (gross) price. With the CVS-Cordavis-Sandoz partnership, more vertical integration has led to yet another profit river. Self-funded employers are still getting the wool pulled over their eyes by the big three PBMs who threaten to take away rebates if they don’t choose the preferred formularies. The employers don’t realize that sometimes it is less expensive to choose the lower-priced drugs with no rebates, and that holds true for biosimilars as well.
Let’s hope that the FTC investigates the situation of a PBM partnering with a manufacturer and then choosing only that manufacturer’s drugs for many of their formularies.
We need to continue our advocacy for our patients because the medication that has kept them stable for so long may find itself without a chair the next time the music stops.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
As the saying goes, “The more things change, the more they stay the same.” That is particularly true when it comes to the affordability of drugs for our patients even after the launch of so many Humira biosimilars. And we still have the “musical chairs” game of formulary construction — when the music stops, who knows whether your patient’s drug found a chair to sit on. There seems to be only a few chairs available for the many adalimumab biosimilars playing the game.
Nothing has changed since my testimony before the FDA Arthritis Advisory Committee in July 2016 during the approval hearing of the first Humira biosimilar. Below is a quote from that meeting where I was speaking predominantly about the pharmacy side of drugs.
“I’d like to highlight the term ‘access’ because none of us are really naive enough to believe that just approving a biosimilar gives a patient true, hands-on access to the medication, because even if the biosimilar is offered at a 30% discount, I don’t have any patients that can afford it. This means that access is ultimately controlled by third-party payers.”
My prediction, that approving and launching biosimilars with lower prices would not ensure patient access to the drug unless it is paid for by insurance, is now our reality. Today, a drug with an 85% discount on the price of Humira is still unattainable for patients without a “payer.”
Competition and Lower Prices
Lawmakers and some in the media cry for more competition to lower prices. This is the main reason that there has been such a push to get biosimilars to the market as quickly as possible. It is abundantly clear that competition to get on the formulary is fierce. Placement of a medication on a formulary can make or break a manufacturer’s ability to get a return on the R&D and make a profit on that medication. For a small biotech manufacturer, it can be the difference between “life and death” of the company.
Does anyone remember when the first interchangeable biosimilar for the reference insulin glargine product Lantus (insulin glargine-yfgn; Semglee) came to market in 2021? Janet Woodcock, MD, then acting FDA commissioner, called it a “momentous day” and further said, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.” There was a high-priced interchangeable biosimilar and an identical unbranded low-priced interchangeable biosimilar, and the only one that could get formulary placement was the high-priced drug.
Patients pay their cost share on the list price of the drug, and because most pharmacy benefit managers’ (PBMs’) formularies cover only the high-priced biosimilar, patients never share in the savings. So much for the “competitive marketplace” creating lower costs for patients. This is just one of hundreds of examples in which lower-priced drugs are excluded from the formulary. It is unfortunate that the bidding process from manufacturers to PBMs to “win” preferred formulary placement is like an art auction, where the highest bidder wins.
Biosimilars and Formulary Construction
For those of us who have been looking into PBMs for many years, it is no surprise that PBMs’ formulary construction has become a profit center for them. Now, with so many adalimumab biosimilars having entered the market, it has become the Wild West where only those with the most money to fork over to the PBMs get preferred placement. Unfortunately, many of the choices that make money for the PBM cost employers and patients more.
How did we get here? In the 1980s and 90s, the price of medications began to increase to the point that many were not affordable without insurance. And who better to construct the list of drugs that would be covered by insurance (formulary) than the PBMs who were already adjudicating the claims for these drugs. The Federal Trade Commission (FTC) realized the power inherent in constructing this list of medications known as the formulary. So when the manufacturer Merck acquired the PBM Medco in the mid-1990s, the FTC stepped in. The FTC surmised that making the drugs and deciding which ones will be paid for created a “conflict of interest” with anticompetitive ramifications.
So, in 1998, William J. Baer, director of the FTC’s Bureau of Competition, said, “Our investigation into the PBM industry has revealed that Merck’s acquisition of Medco has reduced competition in the market for pharmaceutical products … We have found that Medco has given favorable treatment to Merck drugs. As a result, in some cases, consumers have been denied access to the drugs of competing manufacturers. In addition, the merger has made it possible for Medco to share with Merck sensitive pricing information it gets from Merck’s competitors, which could foster collusion among drug manufacturers.” Wow!
These anticompetitive behaviors and conflicts of interest resulting from the Medco acquisition led the FTC to propose a consent agreement.
The agreement would require Merck-Medco to maintain an “open formulary” — one that includes drugs selected and approved by an independent Pharmacy and Therapeutics Committee regardless of the manufacturer. Medco would have to accept rebates and other price concessions and reflect these in the ranking of the drugs on the formulary. Merck would have to make known the availability of the open formulary to any drug maker with an agreement with Medco.
Let’s hope the FTC of 2024 remembers the stance of the FTC in the 1990s regarding anticompetitive behavior involved in formulary construction.
Conflicts of Interest
But today it is apparent that crafting formularies that pay only for the drugs that make the most money for the PBM is not a conflict of interest. In its policy manual, Cigna directly tells employers and employees that they are collecting and keeping rebates and fees on medical pharmaceuticals, and they are not for the benefit of the employer or the plan.
And now, in August 2023, CVS launched Cordavis, a subsidiary wholly owned by CVS. Cordavis/CVS has partnered with Sandoz, which makes Hyrimoz, an adalimumab biosimilar. There is a high-priced version that is discounted 5% from Humira, a lower-cost unbranded version that is discounted 80% off the list price of Humira, and a co-branded CVS/Sandoz version of Hyrimoz that is lower priced as well.
It isn’t a surprise that CVS’ Standard and Advanced Commercial and Chart formularies are offering only Sandoz adalimumab biosimilar products. While these formularies have excluded Humira, CVS has entered into an agreement with AbbVie to allow Humira on a number of their other formularies. It can be very confusing.
As stated earlier, in the 1990s, the FTC frowned upon manufacturers owning PBMs and allowing them to construct their own formularies. Here we have CVS Health, mothership for the PBM CVS Caremark, owning a company that will be co-producing biosimilars with other manufacturers and then determining which biosimilars are on their formularies. The FTC knew back then that the tendency would be to offer only their own drugs for coverage, thus reducing competition. This is exactly what the CVS-Cordavis-Sandoz partnership has done for their Standard and Advanced Commercial and Chart formularies. It is perhaps anti-competitive but certainly profitable.
Perhaps the FTC should require the same consent agreement that was given to Merck in 1998. CVS Caremark would then have to open their formularies to all competitors of their co-branded, co-produced Sandoz biosimilar.
Summary
It is the same old adage, “The more things change, the more they stay the same.” PBMs are still constructing formularies with biosimilars based on their profitability, with huge differences between gross and net cost. Patients still pay their cost share on the list (gross) price. With the CVS-Cordavis-Sandoz partnership, more vertical integration has led to yet another profit river. Self-funded employers are still getting the wool pulled over their eyes by the big three PBMs who threaten to take away rebates if they don’t choose the preferred formularies. The employers don’t realize that sometimes it is less expensive to choose the lower-priced drugs with no rebates, and that holds true for biosimilars as well.
Let’s hope that the FTC investigates the situation of a PBM partnering with a manufacturer and then choosing only that manufacturer’s drugs for many of their formularies.
We need to continue our advocacy for our patients because the medication that has kept them stable for so long may find itself without a chair the next time the music stops.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
As the saying goes, “The more things change, the more they stay the same.” That is particularly true when it comes to the affordability of drugs for our patients even after the launch of so many Humira biosimilars. And we still have the “musical chairs” game of formulary construction — when the music stops, who knows whether your patient’s drug found a chair to sit on. There seems to be only a few chairs available for the many adalimumab biosimilars playing the game.
Nothing has changed since my testimony before the FDA Arthritis Advisory Committee in July 2016 during the approval hearing of the first Humira biosimilar. Below is a quote from that meeting where I was speaking predominantly about the pharmacy side of drugs.
“I’d like to highlight the term ‘access’ because none of us are really naive enough to believe that just approving a biosimilar gives a patient true, hands-on access to the medication, because even if the biosimilar is offered at a 30% discount, I don’t have any patients that can afford it. This means that access is ultimately controlled by third-party payers.”
My prediction, that approving and launching biosimilars with lower prices would not ensure patient access to the drug unless it is paid for by insurance, is now our reality. Today, a drug with an 85% discount on the price of Humira is still unattainable for patients without a “payer.”
Competition and Lower Prices
Lawmakers and some in the media cry for more competition to lower prices. This is the main reason that there has been such a push to get biosimilars to the market as quickly as possible. It is abundantly clear that competition to get on the formulary is fierce. Placement of a medication on a formulary can make or break a manufacturer’s ability to get a return on the R&D and make a profit on that medication. For a small biotech manufacturer, it can be the difference between “life and death” of the company.
Does anyone remember when the first interchangeable biosimilar for the reference insulin glargine product Lantus (insulin glargine-yfgn; Semglee) came to market in 2021? Janet Woodcock, MD, then acting FDA commissioner, called it a “momentous day” and further said, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.” There was a high-priced interchangeable biosimilar and an identical unbranded low-priced interchangeable biosimilar, and the only one that could get formulary placement was the high-priced drug.
Patients pay their cost share on the list price of the drug, and because most pharmacy benefit managers’ (PBMs’) formularies cover only the high-priced biosimilar, patients never share in the savings. So much for the “competitive marketplace” creating lower costs for patients. This is just one of hundreds of examples in which lower-priced drugs are excluded from the formulary. It is unfortunate that the bidding process from manufacturers to PBMs to “win” preferred formulary placement is like an art auction, where the highest bidder wins.
Biosimilars and Formulary Construction
For those of us who have been looking into PBMs for many years, it is no surprise that PBMs’ formulary construction has become a profit center for them. Now, with so many adalimumab biosimilars having entered the market, it has become the Wild West where only those with the most money to fork over to the PBMs get preferred placement. Unfortunately, many of the choices that make money for the PBM cost employers and patients more.
How did we get here? In the 1980s and 90s, the price of medications began to increase to the point that many were not affordable without insurance. And who better to construct the list of drugs that would be covered by insurance (formulary) than the PBMs who were already adjudicating the claims for these drugs. The Federal Trade Commission (FTC) realized the power inherent in constructing this list of medications known as the formulary. So when the manufacturer Merck acquired the PBM Medco in the mid-1990s, the FTC stepped in. The FTC surmised that making the drugs and deciding which ones will be paid for created a “conflict of interest” with anticompetitive ramifications.
So, in 1998, William J. Baer, director of the FTC’s Bureau of Competition, said, “Our investigation into the PBM industry has revealed that Merck’s acquisition of Medco has reduced competition in the market for pharmaceutical products … We have found that Medco has given favorable treatment to Merck drugs. As a result, in some cases, consumers have been denied access to the drugs of competing manufacturers. In addition, the merger has made it possible for Medco to share with Merck sensitive pricing information it gets from Merck’s competitors, which could foster collusion among drug manufacturers.” Wow!
These anticompetitive behaviors and conflicts of interest resulting from the Medco acquisition led the FTC to propose a consent agreement.
The agreement would require Merck-Medco to maintain an “open formulary” — one that includes drugs selected and approved by an independent Pharmacy and Therapeutics Committee regardless of the manufacturer. Medco would have to accept rebates and other price concessions and reflect these in the ranking of the drugs on the formulary. Merck would have to make known the availability of the open formulary to any drug maker with an agreement with Medco.
Let’s hope the FTC of 2024 remembers the stance of the FTC in the 1990s regarding anticompetitive behavior involved in formulary construction.
Conflicts of Interest
But today it is apparent that crafting formularies that pay only for the drugs that make the most money for the PBM is not a conflict of interest. In its policy manual, Cigna directly tells employers and employees that they are collecting and keeping rebates and fees on medical pharmaceuticals, and they are not for the benefit of the employer or the plan.
And now, in August 2023, CVS launched Cordavis, a subsidiary wholly owned by CVS. Cordavis/CVS has partnered with Sandoz, which makes Hyrimoz, an adalimumab biosimilar. There is a high-priced version that is discounted 5% from Humira, a lower-cost unbranded version that is discounted 80% off the list price of Humira, and a co-branded CVS/Sandoz version of Hyrimoz that is lower priced as well.
It isn’t a surprise that CVS’ Standard and Advanced Commercial and Chart formularies are offering only Sandoz adalimumab biosimilar products. While these formularies have excluded Humira, CVS has entered into an agreement with AbbVie to allow Humira on a number of their other formularies. It can be very confusing.
As stated earlier, in the 1990s, the FTC frowned upon manufacturers owning PBMs and allowing them to construct their own formularies. Here we have CVS Health, mothership for the PBM CVS Caremark, owning a company that will be co-producing biosimilars with other manufacturers and then determining which biosimilars are on their formularies. The FTC knew back then that the tendency would be to offer only their own drugs for coverage, thus reducing competition. This is exactly what the CVS-Cordavis-Sandoz partnership has done for their Standard and Advanced Commercial and Chart formularies. It is perhaps anti-competitive but certainly profitable.
Perhaps the FTC should require the same consent agreement that was given to Merck in 1998. CVS Caremark would then have to open their formularies to all competitors of their co-branded, co-produced Sandoz biosimilar.
Summary
It is the same old adage, “The more things change, the more they stay the same.” PBMs are still constructing formularies with biosimilars based on their profitability, with huge differences between gross and net cost. Patients still pay their cost share on the list (gross) price. With the CVS-Cordavis-Sandoz partnership, more vertical integration has led to yet another profit river. Self-funded employers are still getting the wool pulled over their eyes by the big three PBMs who threaten to take away rebates if they don’t choose the preferred formularies. The employers don’t realize that sometimes it is less expensive to choose the lower-priced drugs with no rebates, and that holds true for biosimilars as well.
Let’s hope that the FTC investigates the situation of a PBM partnering with a manufacturer and then choosing only that manufacturer’s drugs for many of their formularies.
We need to continue our advocacy for our patients because the medication that has kept them stable for so long may find itself without a chair the next time the music stops.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Prescription drug affordability boards: Another quick fix with unintended consequences?
Making medications more accessible to those who need them is the focus of attention in the media and in all levels of government. For a drug to be accessible, it must be affordable and available. Something may be affordable, but if it isn’t available, no one will have access to it. Think of toilet paper in the first year of the COVID pandemic. The opposite is also true. An item may be available, but if it isn’t affordable, access is lost. While medication affordability is viewed as the major problem for patients, lack of availability has begun to creep into our drug supply chain. We are now experiencing drug shortages for medications that are very affordable. The perverse incentives, inherent in formulary construction, favor higher-priced medications, which decreases the availability of lower-priced – yet still expensive – drugs, thus increasing patient cost share. Formulary placement and patient cost share, important determinants of accessibility, are controlled by health plans and differ considerably even from the same payer. And yet, the price of drugs remains the target of most approaches to increasing patients’ access. And now price negotiations and drug affordability boards enter into the picture.
What are prescription drug affordability boards?
Both state and federal legislatures have placed the affordability of medications front and center on their agendas. However, neither are considering how formulary construction affects patient’s access to medications. The Inflation Reduction Act is Congress’s foray into price setting/negotiation of expensive drugs. Over the last few years, states are also attempting to make drugs more affordable by creating prescription drug affordability boards (PDABs). Governors (or other state leaders) appoint PDAB members who are charged with the task of evaluating the affordability of certain drugs for both the state and its residents. How to do it, and what the limitations are, vary from state to state. In 2019, Maryland was first state to establish a PDAB, charging its members to study commercial insurance and drug pricing and make recommendations on how to make drugs more affordable for Maryland residents. Other states that have passed PDAB legislation are Colorado, Maine, Minnesota, New Hampshire, Ohio, Oregon, and Washington.
Colorado, Minnesota, and Washington – and soon Maryland and Oregon – hope to make drugs more affordable for patients by allowing their PDABs to set an upper payment limit (UPL). A UPL serves as a cap on the sales price and reimbursement for a drug. The Michigan legislature is actively debating legislation that would establish a PDAB and allow it to set UPLs. On the surface, this may appear to be a potential solution to the affordability issue. However, as always, there are many questions as to how this will work and what are the unintended consequences of price setting and establishing UPLs for medications. UPLs have the potential to harm access to provider-administered drugs. With the help of advocacy from the Coalition of State Rheumatology Organizations (CSRO), Washington’s PDAB statute potentially has a carve-out for provider-administered drugs.
Possible unintended consequences for provider-administered drugs
CSRO asked for a meeting with the Colorado PDAB after they announced their list of drugs for which UPLs would be set. We spoke with the PDAB in October, hoping to point out some of the unintended consequences that needed to be considered. One of the big questions we have revolves around the “buy and bill” provider-administered drugs. According to the language of the Colorado statute, providers would not be paid any more than the UPL for a drug administered in their office. CSRO is concerned that this would leave providers uncompensated for the service of administering the drug and associated overhead. This is not to mention that providers may not be able to find a group purchasing organization that would even sell the drug at the UPL, much less a lower price than the UPL. And even if a provider could buy it at the UPL, that would mean there would be no margin to cover the overhead for their infusion suite. Interestingly, while Colorado’s rules for the UPL state that pharmacies can be paid an additional reasonable dispensing fee beyond the UPL, no such allowance is made for providers administering one of these medications. In fact, the Colorado PDAB specifically indicated that the goal of the state’s UPL methodology was to ensure that there was no “delta” between what is paid for the drug by the provider and what is reimbursed to a provider for the drug by the payer. This may cause some providers to be unable to “afford” to administer those drugs with UPLs, which ultimately reduces access for residents of Colorado to that particular medication. This is the exact opposite of what the PDAB is supposed to accomplish.
There are still many questions. What impact will UPLs have on a medication’s placement on a formulary? As we know, preferred formulary placement is often given to drugs with the highest price concession from the manufacturers. Will setting a UPL on payment for specialty pharmacy drugs to pharmacy benefit manager-owned specialty pharmacies affect that drug’s ability to be on the formulary? And again, how will the PDAB resolve the issue of compensating the provider for overhead costs associated with administering the medication?
Even more confusing questions remain. How will the UPL be enforced when a “purchase” or “sale” of the drug is made by an out-of-state entity somewhere along the supply chain? When ultimately the drug is purchased and delivered to a Colorado consumer by a Colorado provider/pharmacy, there are multiple points of the supply chain that may be outside of the jurisdiction of Colorado to enforce the UPL. This would create a misalignment in pricing among various supply chain entities.
While the sentiment behind creating PDABs is noble, it may end up having the unintended consequence of patients losing access to these drugs because of the perverse incentives involved in formulary construction or providers’ inability to afford to offer provider-administered drugs with UPLs.
Remember, expensive specialty pharmacy medications are already discounted greatly by manufacturers, often more than 50% to pharmacy benefit managers; and yet those cost savings are not passed on to the patients. Also, there is no oversight of 340B hospital contracted pharmacies to make sure that they pass those savings on to needy patients. Perhaps PDABs should address those issues, as well, if patient access to expensive medications is the goal.
Clearly, there are no easy answers. But with so many variables in the drug supply chain affecting patient access, concentrating only on one aspect may end up causing more harm than good. If your state is thinking of passing a PDAB, please let your legislators know that there are issues with this type of legislation that perhaps should be worked out before the bill is passed.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Making medications more accessible to those who need them is the focus of attention in the media and in all levels of government. For a drug to be accessible, it must be affordable and available. Something may be affordable, but if it isn’t available, no one will have access to it. Think of toilet paper in the first year of the COVID pandemic. The opposite is also true. An item may be available, but if it isn’t affordable, access is lost. While medication affordability is viewed as the major problem for patients, lack of availability has begun to creep into our drug supply chain. We are now experiencing drug shortages for medications that are very affordable. The perverse incentives, inherent in formulary construction, favor higher-priced medications, which decreases the availability of lower-priced – yet still expensive – drugs, thus increasing patient cost share. Formulary placement and patient cost share, important determinants of accessibility, are controlled by health plans and differ considerably even from the same payer. And yet, the price of drugs remains the target of most approaches to increasing patients’ access. And now price negotiations and drug affordability boards enter into the picture.
What are prescription drug affordability boards?
Both state and federal legislatures have placed the affordability of medications front and center on their agendas. However, neither are considering how formulary construction affects patient’s access to medications. The Inflation Reduction Act is Congress’s foray into price setting/negotiation of expensive drugs. Over the last few years, states are also attempting to make drugs more affordable by creating prescription drug affordability boards (PDABs). Governors (or other state leaders) appoint PDAB members who are charged with the task of evaluating the affordability of certain drugs for both the state and its residents. How to do it, and what the limitations are, vary from state to state. In 2019, Maryland was first state to establish a PDAB, charging its members to study commercial insurance and drug pricing and make recommendations on how to make drugs more affordable for Maryland residents. Other states that have passed PDAB legislation are Colorado, Maine, Minnesota, New Hampshire, Ohio, Oregon, and Washington.
Colorado, Minnesota, and Washington – and soon Maryland and Oregon – hope to make drugs more affordable for patients by allowing their PDABs to set an upper payment limit (UPL). A UPL serves as a cap on the sales price and reimbursement for a drug. The Michigan legislature is actively debating legislation that would establish a PDAB and allow it to set UPLs. On the surface, this may appear to be a potential solution to the affordability issue. However, as always, there are many questions as to how this will work and what are the unintended consequences of price setting and establishing UPLs for medications. UPLs have the potential to harm access to provider-administered drugs. With the help of advocacy from the Coalition of State Rheumatology Organizations (CSRO), Washington’s PDAB statute potentially has a carve-out for provider-administered drugs.
Possible unintended consequences for provider-administered drugs
CSRO asked for a meeting with the Colorado PDAB after they announced their list of drugs for which UPLs would be set. We spoke with the PDAB in October, hoping to point out some of the unintended consequences that needed to be considered. One of the big questions we have revolves around the “buy and bill” provider-administered drugs. According to the language of the Colorado statute, providers would not be paid any more than the UPL for a drug administered in their office. CSRO is concerned that this would leave providers uncompensated for the service of administering the drug and associated overhead. This is not to mention that providers may not be able to find a group purchasing organization that would even sell the drug at the UPL, much less a lower price than the UPL. And even if a provider could buy it at the UPL, that would mean there would be no margin to cover the overhead for their infusion suite. Interestingly, while Colorado’s rules for the UPL state that pharmacies can be paid an additional reasonable dispensing fee beyond the UPL, no such allowance is made for providers administering one of these medications. In fact, the Colorado PDAB specifically indicated that the goal of the state’s UPL methodology was to ensure that there was no “delta” between what is paid for the drug by the provider and what is reimbursed to a provider for the drug by the payer. This may cause some providers to be unable to “afford” to administer those drugs with UPLs, which ultimately reduces access for residents of Colorado to that particular medication. This is the exact opposite of what the PDAB is supposed to accomplish.
There are still many questions. What impact will UPLs have on a medication’s placement on a formulary? As we know, preferred formulary placement is often given to drugs with the highest price concession from the manufacturers. Will setting a UPL on payment for specialty pharmacy drugs to pharmacy benefit manager-owned specialty pharmacies affect that drug’s ability to be on the formulary? And again, how will the PDAB resolve the issue of compensating the provider for overhead costs associated with administering the medication?
Even more confusing questions remain. How will the UPL be enforced when a “purchase” or “sale” of the drug is made by an out-of-state entity somewhere along the supply chain? When ultimately the drug is purchased and delivered to a Colorado consumer by a Colorado provider/pharmacy, there are multiple points of the supply chain that may be outside of the jurisdiction of Colorado to enforce the UPL. This would create a misalignment in pricing among various supply chain entities.
While the sentiment behind creating PDABs is noble, it may end up having the unintended consequence of patients losing access to these drugs because of the perverse incentives involved in formulary construction or providers’ inability to afford to offer provider-administered drugs with UPLs.
Remember, expensive specialty pharmacy medications are already discounted greatly by manufacturers, often more than 50% to pharmacy benefit managers; and yet those cost savings are not passed on to the patients. Also, there is no oversight of 340B hospital contracted pharmacies to make sure that they pass those savings on to needy patients. Perhaps PDABs should address those issues, as well, if patient access to expensive medications is the goal.
Clearly, there are no easy answers. But with so many variables in the drug supply chain affecting patient access, concentrating only on one aspect may end up causing more harm than good. If your state is thinking of passing a PDAB, please let your legislators know that there are issues with this type of legislation that perhaps should be worked out before the bill is passed.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Making medications more accessible to those who need them is the focus of attention in the media and in all levels of government. For a drug to be accessible, it must be affordable and available. Something may be affordable, but if it isn’t available, no one will have access to it. Think of toilet paper in the first year of the COVID pandemic. The opposite is also true. An item may be available, but if it isn’t affordable, access is lost. While medication affordability is viewed as the major problem for patients, lack of availability has begun to creep into our drug supply chain. We are now experiencing drug shortages for medications that are very affordable. The perverse incentives, inherent in formulary construction, favor higher-priced medications, which decreases the availability of lower-priced – yet still expensive – drugs, thus increasing patient cost share. Formulary placement and patient cost share, important determinants of accessibility, are controlled by health plans and differ considerably even from the same payer. And yet, the price of drugs remains the target of most approaches to increasing patients’ access. And now price negotiations and drug affordability boards enter into the picture.
What are prescription drug affordability boards?
Both state and federal legislatures have placed the affordability of medications front and center on their agendas. However, neither are considering how formulary construction affects patient’s access to medications. The Inflation Reduction Act is Congress’s foray into price setting/negotiation of expensive drugs. Over the last few years, states are also attempting to make drugs more affordable by creating prescription drug affordability boards (PDABs). Governors (or other state leaders) appoint PDAB members who are charged with the task of evaluating the affordability of certain drugs for both the state and its residents. How to do it, and what the limitations are, vary from state to state. In 2019, Maryland was first state to establish a PDAB, charging its members to study commercial insurance and drug pricing and make recommendations on how to make drugs more affordable for Maryland residents. Other states that have passed PDAB legislation are Colorado, Maine, Minnesota, New Hampshire, Ohio, Oregon, and Washington.
Colorado, Minnesota, and Washington – and soon Maryland and Oregon – hope to make drugs more affordable for patients by allowing their PDABs to set an upper payment limit (UPL). A UPL serves as a cap on the sales price and reimbursement for a drug. The Michigan legislature is actively debating legislation that would establish a PDAB and allow it to set UPLs. On the surface, this may appear to be a potential solution to the affordability issue. However, as always, there are many questions as to how this will work and what are the unintended consequences of price setting and establishing UPLs for medications. UPLs have the potential to harm access to provider-administered drugs. With the help of advocacy from the Coalition of State Rheumatology Organizations (CSRO), Washington’s PDAB statute potentially has a carve-out for provider-administered drugs.
Possible unintended consequences for provider-administered drugs
CSRO asked for a meeting with the Colorado PDAB after they announced their list of drugs for which UPLs would be set. We spoke with the PDAB in October, hoping to point out some of the unintended consequences that needed to be considered. One of the big questions we have revolves around the “buy and bill” provider-administered drugs. According to the language of the Colorado statute, providers would not be paid any more than the UPL for a drug administered in their office. CSRO is concerned that this would leave providers uncompensated for the service of administering the drug and associated overhead. This is not to mention that providers may not be able to find a group purchasing organization that would even sell the drug at the UPL, much less a lower price than the UPL. And even if a provider could buy it at the UPL, that would mean there would be no margin to cover the overhead for their infusion suite. Interestingly, while Colorado’s rules for the UPL state that pharmacies can be paid an additional reasonable dispensing fee beyond the UPL, no such allowance is made for providers administering one of these medications. In fact, the Colorado PDAB specifically indicated that the goal of the state’s UPL methodology was to ensure that there was no “delta” between what is paid for the drug by the provider and what is reimbursed to a provider for the drug by the payer. This may cause some providers to be unable to “afford” to administer those drugs with UPLs, which ultimately reduces access for residents of Colorado to that particular medication. This is the exact opposite of what the PDAB is supposed to accomplish.
There are still many questions. What impact will UPLs have on a medication’s placement on a formulary? As we know, preferred formulary placement is often given to drugs with the highest price concession from the manufacturers. Will setting a UPL on payment for specialty pharmacy drugs to pharmacy benefit manager-owned specialty pharmacies affect that drug’s ability to be on the formulary? And again, how will the PDAB resolve the issue of compensating the provider for overhead costs associated with administering the medication?
Even more confusing questions remain. How will the UPL be enforced when a “purchase” or “sale” of the drug is made by an out-of-state entity somewhere along the supply chain? When ultimately the drug is purchased and delivered to a Colorado consumer by a Colorado provider/pharmacy, there are multiple points of the supply chain that may be outside of the jurisdiction of Colorado to enforce the UPL. This would create a misalignment in pricing among various supply chain entities.
While the sentiment behind creating PDABs is noble, it may end up having the unintended consequence of patients losing access to these drugs because of the perverse incentives involved in formulary construction or providers’ inability to afford to offer provider-administered drugs with UPLs.
Remember, expensive specialty pharmacy medications are already discounted greatly by manufacturers, often more than 50% to pharmacy benefit managers; and yet those cost savings are not passed on to the patients. Also, there is no oversight of 340B hospital contracted pharmacies to make sure that they pass those savings on to needy patients. Perhaps PDABs should address those issues, as well, if patient access to expensive medications is the goal.
Clearly, there are no easy answers. But with so many variables in the drug supply chain affecting patient access, concentrating only on one aspect may end up causing more harm than good. If your state is thinking of passing a PDAB, please let your legislators know that there are issues with this type of legislation that perhaps should be worked out before the bill is passed.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Insurer’s foray into AI-based ‘shared savings’ program creates ethical problems
Editor’s note: As of this writing, the following proposed health insurance policy from Blue Cross and Blue Shield of North Carolina is still active. The Coalition of State Rheumatology Organizations and other rheumatology advocacy groups are in ongoing discussions with the health insurer and hope to have major changes to this policy implemented.
While AI has been in our world for years, it is expanding by the minute, perhaps by the nanosecond, within the health care sector. The $6.7 billion dollar health care AI market in 2020 is expected to climb to more than $120 billion by 2028. There are many questions regarding the application of AI in our world. Is it a mere instructional algorithm that computes things in a much faster way, or does it create a new story based on the information it has access to? Does it engender excitement or fear ... or both? Remember HAL? As we have seen throughout history with new inventions and technologies, there are risks and rewards. Even the best can have harmful unintended consequences. AI is no different, particularly when it comes to health care. In this case, AI can get a bad name if it is utilized along with biased data input and bad policy.
Shared savings
Here is where “shared savings” comes into play. A shared savings program starts with a baseline cost analysis of a particular care plan and then tracks costs (performance) going forward after certain changes to the original care plan are instituted. If savings are accrued when compared with baseline spending, those savings are shared with the providers of the care. Depending on how the shared savings program is implemented, the optics can be very bad if it appears as though physicians are being paid to reduce care.
‘The volunteer opportunity’
Recently, Blue Cross and Blue Shield of North Carolina, in partnership with Outcomes Matter Innovations, a data analysis company that uses AI/machine-learning technology, offered rheumatologists a new voluntary shared savings, value-based care (VBC) “opportunity.” Rheumatologists would be able to “utilize a web-based machine-learning technology platform that suggests evidence-based care pathways” in the treatment of rheumatoid arthritis and psoriatic arthritis (PsA). The VBC/shared savings model uses the AI platform to propose two different pathways. One model would delay the start of biologics or Janus kinase inhibitors (JAKi), and the second model would taper and/or stop biologics or JAKi altogether.
Delaying the start of biologics/JAKi would be achieved through “methotrexate optimization” and/or the use of triple therapy with methotrexate, sulfasalazine, and hydroxychloroquine. The other model would recommend tapering biologic/JAKi dosing in patients in remission or low disease activity and might even suggest a “medication holiday.”
The intention of this 3-year VBC/shared savings program is to reduce costs and create savings by reducing the use of biologics or JAKi. A tangential question might be, “Reduce costs and create savings for whom?” Apparently, the patients will not reap any of the cost savings, as this is proposed to be a shared savings program with the savings going to the physicians and the insurance company. Perhaps the idea is that patients will benefit by reducing unneeded expensive medications.
How will it work?
A cost baseline will be established on biologic and JAKi use prior to the start of the program. Once started, there will be a calculation of savings based on biologic/JAKi use going forward. It was stated that physicians would receive 22% of the total costs saved. In one flyer, it was estimated that, with methotrexate optimization, rheumatologists could be paid an average of $1,527 a month per patient per month of delay before starting a biologic or JAKi.
The American College of Rheumatology has guidelines for the treatment of RA and PsA, and while optimizing methotrexate and triple therapy is mentioned, tapering or stopping treatment with biologics or JAKi is not. Additionally, after lack of response at 3 months, the standard of care is to change to a more effective treatment, which for most patients is a biologic disease-modifying antirheumatic drug (DMARD). It could be construed that rheumatologists are being monetarily incentivized to reduce the use of expensive medications through ways that are not included in ACR guidelines and are not standard of care.
What if after the medication holiday the patient cannot recapture control of their disease? Is there a liability concern? Remember, there is no institutional review board or informed patient consent for this VBC data gathering model.
How will a patient feel knowing that their physician was paid to withhold care, or even worse, if a patient is not told of this and then finds out later? Not only are the optics for this suboptimal (at best), where does the liability fall if the patient does not do well and it comes out that their rheumatologist was paid to reduce the care, particularly in a way that is not supported in the guideline. Clearly, this appears to be a clinical study without an institutional review board and without patient consent.
There are also the data that are collected from this voluntary “opportunity.” A valid question would be, “What kind of data will this produce if rheumatologists are paid to delay, reduce, or stop the use of biologics/JAKi?” Is it possible that physicians may subconsciously delay putting patients on a biologic and taper more rapidly because of the reimbursement? This could lead to faulty, biased, AI-generated data that erroneously show this type of care is working. It would not be unheard of to wonder whether this once-voluntary opportunity might evolve into mandatory policy because now, they have “data to prove it.” … only this time there is no shared savings.
Low disease activity results in long-term savings
This is not meant to be an indictment of AI in health care, value-based care, or shared savings programs. In reality, AI had very little to do with how poorly this program was presented. Hopefully, it will bring about further discussions on how to achieve savings without sacrificing care. In fact, optimal care in RA and PsA is probably one of the best ways to save money in the long run. Nowhere in this program is there any mention of the high cost associated with uncontrolled disease activity in patients with RA or PsA. The downstream costs can be enormous when long- and short-term sequelae are taken into consideration: joint replacements, cardiovascular disease, certain kinds of malignancies, and all the side effects of increased steroid usage are just a few of the consequences we see with uncontrolled disease activity. It is only recently that we have been able to achieve low disease activity and remission in our patients. The rush to get patients off these medications is not the answer to achieving long-term savings. In addition to the very bad optics of paying rheumatologists to delay, taper, or stop using expensive mediations in their patients, the ultimate data achieved will be biased, and the only real winner will be the health insurance company.
Again, AI machine-learning and shared saving programs are not the guilty parties here. In fact, AI may be helpful in coming up with solutions to long-term health care costs, whether in the realm of economics or scientific research. CSRO and our state member organizations continue to educate the health insurance company on the significant drawbacks to this “volunteer opportunity.” Let’s hope a more reasonable program is put forward with AI-generated data that can be trusted. Hopefully not with a platform named “HAL,” for those of you old enough to remember “2001: A Space Odyssey.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Editor’s note: As of this writing, the following proposed health insurance policy from Blue Cross and Blue Shield of North Carolina is still active. The Coalition of State Rheumatology Organizations and other rheumatology advocacy groups are in ongoing discussions with the health insurer and hope to have major changes to this policy implemented.
While AI has been in our world for years, it is expanding by the minute, perhaps by the nanosecond, within the health care sector. The $6.7 billion dollar health care AI market in 2020 is expected to climb to more than $120 billion by 2028. There are many questions regarding the application of AI in our world. Is it a mere instructional algorithm that computes things in a much faster way, or does it create a new story based on the information it has access to? Does it engender excitement or fear ... or both? Remember HAL? As we have seen throughout history with new inventions and technologies, there are risks and rewards. Even the best can have harmful unintended consequences. AI is no different, particularly when it comes to health care. In this case, AI can get a bad name if it is utilized along with biased data input and bad policy.
Shared savings
Here is where “shared savings” comes into play. A shared savings program starts with a baseline cost analysis of a particular care plan and then tracks costs (performance) going forward after certain changes to the original care plan are instituted. If savings are accrued when compared with baseline spending, those savings are shared with the providers of the care. Depending on how the shared savings program is implemented, the optics can be very bad if it appears as though physicians are being paid to reduce care.
‘The volunteer opportunity’
Recently, Blue Cross and Blue Shield of North Carolina, in partnership with Outcomes Matter Innovations, a data analysis company that uses AI/machine-learning technology, offered rheumatologists a new voluntary shared savings, value-based care (VBC) “opportunity.” Rheumatologists would be able to “utilize a web-based machine-learning technology platform that suggests evidence-based care pathways” in the treatment of rheumatoid arthritis and psoriatic arthritis (PsA). The VBC/shared savings model uses the AI platform to propose two different pathways. One model would delay the start of biologics or Janus kinase inhibitors (JAKi), and the second model would taper and/or stop biologics or JAKi altogether.
Delaying the start of biologics/JAKi would be achieved through “methotrexate optimization” and/or the use of triple therapy with methotrexate, sulfasalazine, and hydroxychloroquine. The other model would recommend tapering biologic/JAKi dosing in patients in remission or low disease activity and might even suggest a “medication holiday.”
The intention of this 3-year VBC/shared savings program is to reduce costs and create savings by reducing the use of biologics or JAKi. A tangential question might be, “Reduce costs and create savings for whom?” Apparently, the patients will not reap any of the cost savings, as this is proposed to be a shared savings program with the savings going to the physicians and the insurance company. Perhaps the idea is that patients will benefit by reducing unneeded expensive medications.
How will it work?
A cost baseline will be established on biologic and JAKi use prior to the start of the program. Once started, there will be a calculation of savings based on biologic/JAKi use going forward. It was stated that physicians would receive 22% of the total costs saved. In one flyer, it was estimated that, with methotrexate optimization, rheumatologists could be paid an average of $1,527 a month per patient per month of delay before starting a biologic or JAKi.
The American College of Rheumatology has guidelines for the treatment of RA and PsA, and while optimizing methotrexate and triple therapy is mentioned, tapering or stopping treatment with biologics or JAKi is not. Additionally, after lack of response at 3 months, the standard of care is to change to a more effective treatment, which for most patients is a biologic disease-modifying antirheumatic drug (DMARD). It could be construed that rheumatologists are being monetarily incentivized to reduce the use of expensive medications through ways that are not included in ACR guidelines and are not standard of care.
What if after the medication holiday the patient cannot recapture control of their disease? Is there a liability concern? Remember, there is no institutional review board or informed patient consent for this VBC data gathering model.
How will a patient feel knowing that their physician was paid to withhold care, or even worse, if a patient is not told of this and then finds out later? Not only are the optics for this suboptimal (at best), where does the liability fall if the patient does not do well and it comes out that their rheumatologist was paid to reduce the care, particularly in a way that is not supported in the guideline. Clearly, this appears to be a clinical study without an institutional review board and without patient consent.
There are also the data that are collected from this voluntary “opportunity.” A valid question would be, “What kind of data will this produce if rheumatologists are paid to delay, reduce, or stop the use of biologics/JAKi?” Is it possible that physicians may subconsciously delay putting patients on a biologic and taper more rapidly because of the reimbursement? This could lead to faulty, biased, AI-generated data that erroneously show this type of care is working. It would not be unheard of to wonder whether this once-voluntary opportunity might evolve into mandatory policy because now, they have “data to prove it.” … only this time there is no shared savings.
Low disease activity results in long-term savings
This is not meant to be an indictment of AI in health care, value-based care, or shared savings programs. In reality, AI had very little to do with how poorly this program was presented. Hopefully, it will bring about further discussions on how to achieve savings without sacrificing care. In fact, optimal care in RA and PsA is probably one of the best ways to save money in the long run. Nowhere in this program is there any mention of the high cost associated with uncontrolled disease activity in patients with RA or PsA. The downstream costs can be enormous when long- and short-term sequelae are taken into consideration: joint replacements, cardiovascular disease, certain kinds of malignancies, and all the side effects of increased steroid usage are just a few of the consequences we see with uncontrolled disease activity. It is only recently that we have been able to achieve low disease activity and remission in our patients. The rush to get patients off these medications is not the answer to achieving long-term savings. In addition to the very bad optics of paying rheumatologists to delay, taper, or stop using expensive mediations in their patients, the ultimate data achieved will be biased, and the only real winner will be the health insurance company.
Again, AI machine-learning and shared saving programs are not the guilty parties here. In fact, AI may be helpful in coming up with solutions to long-term health care costs, whether in the realm of economics or scientific research. CSRO and our state member organizations continue to educate the health insurance company on the significant drawbacks to this “volunteer opportunity.” Let’s hope a more reasonable program is put forward with AI-generated data that can be trusted. Hopefully not with a platform named “HAL,” for those of you old enough to remember “2001: A Space Odyssey.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Editor’s note: As of this writing, the following proposed health insurance policy from Blue Cross and Blue Shield of North Carolina is still active. The Coalition of State Rheumatology Organizations and other rheumatology advocacy groups are in ongoing discussions with the health insurer and hope to have major changes to this policy implemented.
While AI has been in our world for years, it is expanding by the minute, perhaps by the nanosecond, within the health care sector. The $6.7 billion dollar health care AI market in 2020 is expected to climb to more than $120 billion by 2028. There are many questions regarding the application of AI in our world. Is it a mere instructional algorithm that computes things in a much faster way, or does it create a new story based on the information it has access to? Does it engender excitement or fear ... or both? Remember HAL? As we have seen throughout history with new inventions and technologies, there are risks and rewards. Even the best can have harmful unintended consequences. AI is no different, particularly when it comes to health care. In this case, AI can get a bad name if it is utilized along with biased data input and bad policy.
Shared savings
Here is where “shared savings” comes into play. A shared savings program starts with a baseline cost analysis of a particular care plan and then tracks costs (performance) going forward after certain changes to the original care plan are instituted. If savings are accrued when compared with baseline spending, those savings are shared with the providers of the care. Depending on how the shared savings program is implemented, the optics can be very bad if it appears as though physicians are being paid to reduce care.
‘The volunteer opportunity’
Recently, Blue Cross and Blue Shield of North Carolina, in partnership with Outcomes Matter Innovations, a data analysis company that uses AI/machine-learning technology, offered rheumatologists a new voluntary shared savings, value-based care (VBC) “opportunity.” Rheumatologists would be able to “utilize a web-based machine-learning technology platform that suggests evidence-based care pathways” in the treatment of rheumatoid arthritis and psoriatic arthritis (PsA). The VBC/shared savings model uses the AI platform to propose two different pathways. One model would delay the start of biologics or Janus kinase inhibitors (JAKi), and the second model would taper and/or stop biologics or JAKi altogether.
Delaying the start of biologics/JAKi would be achieved through “methotrexate optimization” and/or the use of triple therapy with methotrexate, sulfasalazine, and hydroxychloroquine. The other model would recommend tapering biologic/JAKi dosing in patients in remission or low disease activity and might even suggest a “medication holiday.”
The intention of this 3-year VBC/shared savings program is to reduce costs and create savings by reducing the use of biologics or JAKi. A tangential question might be, “Reduce costs and create savings for whom?” Apparently, the patients will not reap any of the cost savings, as this is proposed to be a shared savings program with the savings going to the physicians and the insurance company. Perhaps the idea is that patients will benefit by reducing unneeded expensive medications.
How will it work?
A cost baseline will be established on biologic and JAKi use prior to the start of the program. Once started, there will be a calculation of savings based on biologic/JAKi use going forward. It was stated that physicians would receive 22% of the total costs saved. In one flyer, it was estimated that, with methotrexate optimization, rheumatologists could be paid an average of $1,527 a month per patient per month of delay before starting a biologic or JAKi.
The American College of Rheumatology has guidelines for the treatment of RA and PsA, and while optimizing methotrexate and triple therapy is mentioned, tapering or stopping treatment with biologics or JAKi is not. Additionally, after lack of response at 3 months, the standard of care is to change to a more effective treatment, which for most patients is a biologic disease-modifying antirheumatic drug (DMARD). It could be construed that rheumatologists are being monetarily incentivized to reduce the use of expensive medications through ways that are not included in ACR guidelines and are not standard of care.
What if after the medication holiday the patient cannot recapture control of their disease? Is there a liability concern? Remember, there is no institutional review board or informed patient consent for this VBC data gathering model.
How will a patient feel knowing that their physician was paid to withhold care, or even worse, if a patient is not told of this and then finds out later? Not only are the optics for this suboptimal (at best), where does the liability fall if the patient does not do well and it comes out that their rheumatologist was paid to reduce the care, particularly in a way that is not supported in the guideline. Clearly, this appears to be a clinical study without an institutional review board and without patient consent.
There are also the data that are collected from this voluntary “opportunity.” A valid question would be, “What kind of data will this produce if rheumatologists are paid to delay, reduce, or stop the use of biologics/JAKi?” Is it possible that physicians may subconsciously delay putting patients on a biologic and taper more rapidly because of the reimbursement? This could lead to faulty, biased, AI-generated data that erroneously show this type of care is working. It would not be unheard of to wonder whether this once-voluntary opportunity might evolve into mandatory policy because now, they have “data to prove it.” … only this time there is no shared savings.
Low disease activity results in long-term savings
This is not meant to be an indictment of AI in health care, value-based care, or shared savings programs. In reality, AI had very little to do with how poorly this program was presented. Hopefully, it will bring about further discussions on how to achieve savings without sacrificing care. In fact, optimal care in RA and PsA is probably one of the best ways to save money in the long run. Nowhere in this program is there any mention of the high cost associated with uncontrolled disease activity in patients with RA or PsA. The downstream costs can be enormous when long- and short-term sequelae are taken into consideration: joint replacements, cardiovascular disease, certain kinds of malignancies, and all the side effects of increased steroid usage are just a few of the consequences we see with uncontrolled disease activity. It is only recently that we have been able to achieve low disease activity and remission in our patients. The rush to get patients off these medications is not the answer to achieving long-term savings. In addition to the very bad optics of paying rheumatologists to delay, taper, or stop using expensive mediations in their patients, the ultimate data achieved will be biased, and the only real winner will be the health insurance company.
Again, AI machine-learning and shared saving programs are not the guilty parties here. In fact, AI may be helpful in coming up with solutions to long-term health care costs, whether in the realm of economics or scientific research. CSRO and our state member organizations continue to educate the health insurance company on the significant drawbacks to this “volunteer opportunity.” Let’s hope a more reasonable program is put forward with AI-generated data that can be trusted. Hopefully not with a platform named “HAL,” for those of you old enough to remember “2001: A Space Odyssey.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
In-office infusions at risk with new Medicare Part B reimbursement recommendation
The Medicare Payment Advisory Commission (MedPAC) is an independent agency to advise Congress on Medicare (MC) policy, much of which pertains to payment issues. The 17 commissioners meet publicly and issue two reports a year with their recommendations to Congress, who then decides whether to enact these recommendations or not.
One MedPAC recommendation in 2023 was quickly introduced in the House of Representatives in May and passed the Energy and Commerce Committee 49-0. That recommendation relates to “site neutrality” payments to MC providers. If passed by Congress, it would result in some “site-neutral” cuts to hospitals. That MedPAC recommendation was acted upon very quickly by Congress. Consequently, it is important to discuss the potential negative ramifications of other MedPAC recommendations released in June regarding reimbursement of Medicare Part B drugs and proactively educate Congress accordingly on those ramifications.
Medicare Part B drugs
Medicare Part B drugs are those administered by providers, unlike the Part D medications which are generally obtained through pharmacies. Presently, MC reimburses providers for the administered Part B medication based on the average sales price (ASP) plus 6%. However, with sequestration, that add-on amount is reduced to ASP plus 4.3%. It has long been touted by MedPAC and other policy makers that physicians choose to infuse higher-priced drugs in order to increase reimbursements. That has not been borne out when it comes to rheumatologists, and, in fact, a retired MedPAC commissioner even stated that premise did not hold true for rheumatologists.
Regardless, it continues to be suggested that MC should reduce its costs for Part B medications by reducing reimbursement to physicians. It should be noted that often the margins on the drugs are already quite thin, and at times the reimbursement amount, compared with the acquisition cost of the drug even leaves the physician “underwater.”
A few years ago, there was a proposed Part B demonstration project that essentially removed the +6% add-on and replaced it with a very low fixed amount that would have left most physicians “underwater” in their Part B drug acquisitions. This was vigorously opposed by physicians around the country, who let Congress know exactly how they felt. We have been told that the Coalition of State Rheumatology Organizations was one of the most vociferous organizations that helped in fighting back this proposal and resulting in its withdrawal.
MedPAC recommendations
That brings us back to MedPAC. In June, MedPAC released recommendations to Congress in an attempt to address the “high price of drugs” covered under MC Part B. Unfortunately, the recommendations do nothing to address the root cause of high drug prices, but once again attempt to balance MC expenditures on the backs of physicians. In this case, it is physicians who infuse Part B drugs in their office to chronically ill patients. In-office infusions have been shown to be the most cost-effective site of care, as well as being safer when compared with home infusion for a number of rheumatologic medications.
One of the MedPAC recommendations gives the Secretary of Health & Human Services the authority to establish a single ASP for drugs with “similar health effects.” The ambiguity of the phrase “similar health effects” should put us all on alert as to the significant unintended consequences that may result. For example, HHS could assign one ASP to all drugs that treat rheumatoid arthritis based on the lowest ASP of the group. This certainly would lead to a number of drugs being out of reach for MC beneficiaries if the artificial ASP of the medication is much lower than the actual acquisition cost of the drug, leaving physicians unable to acquire it. Yet, MedPAC states this recommendation would not affect access to care for MC beneficiaries.
Another recommendation would require HHS to reduce or eliminate the add-on percentage to the ASP for higher-priced drugs and/or put in an added fixed amount. This recommendation is clearly reminiscent of the old ill-conceived Part B demonstration project.
A fixed “add-on amount” might work if it is sufficient to cover the overhead of maintaining a provider’s infusion suite. But if practices are left underwater in their purchases of certain Part B drugs, there may be no choice but to stop offering those infusions to MC beneficiaries or – worst-case scenario – shut the door completely. Yet again, MedPAC stated that this recommendation would not result in a loss of access to these treatments for MC beneficiaries.
Loss of access?
Rheumatologists have gone to great lengths to continue offering care to MC patients in spite of the yearly cuts and threats of more cuts in the future to physician reimbursements. In addition, physicians have no annual inflationary update to their reimbursements. I am not sure how MedPAC concludes that continued cuts to physician fee schedules, along with a decrease in reimbursement for administered drugs, will not affect access to care for MC beneficiaries.
Finally, the timing on these recommendations is confusing, considering that implementation of the Inflation Reduction Act (IRA) has just begun. Next quarter, a number of Part B drugs will be subject to inflationary penalties; there will also be additional Part B biosimilars coming to market, resulting in lower ASPs. And don’t forget, the IRA just instituted an ASP plus 8% reimbursement for biosimilars in an attempt to get physicians to do something that the Centers for Medicare & Medicaid Services has asked them not to do. That is, choose a drug based on its reimbursement, not necessarily the one which is right for the patient.
Overall, with so many variables up in the air, now is not the time to create even more uncertainty for physicians and the Medicare patients that they take care of.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
The Medicare Payment Advisory Commission (MedPAC) is an independent agency to advise Congress on Medicare (MC) policy, much of which pertains to payment issues. The 17 commissioners meet publicly and issue two reports a year with their recommendations to Congress, who then decides whether to enact these recommendations or not.
One MedPAC recommendation in 2023 was quickly introduced in the House of Representatives in May and passed the Energy and Commerce Committee 49-0. That recommendation relates to “site neutrality” payments to MC providers. If passed by Congress, it would result in some “site-neutral” cuts to hospitals. That MedPAC recommendation was acted upon very quickly by Congress. Consequently, it is important to discuss the potential negative ramifications of other MedPAC recommendations released in June regarding reimbursement of Medicare Part B drugs and proactively educate Congress accordingly on those ramifications.
Medicare Part B drugs
Medicare Part B drugs are those administered by providers, unlike the Part D medications which are generally obtained through pharmacies. Presently, MC reimburses providers for the administered Part B medication based on the average sales price (ASP) plus 6%. However, with sequestration, that add-on amount is reduced to ASP plus 4.3%. It has long been touted by MedPAC and other policy makers that physicians choose to infuse higher-priced drugs in order to increase reimbursements. That has not been borne out when it comes to rheumatologists, and, in fact, a retired MedPAC commissioner even stated that premise did not hold true for rheumatologists.
Regardless, it continues to be suggested that MC should reduce its costs for Part B medications by reducing reimbursement to physicians. It should be noted that often the margins on the drugs are already quite thin, and at times the reimbursement amount, compared with the acquisition cost of the drug even leaves the physician “underwater.”
A few years ago, there was a proposed Part B demonstration project that essentially removed the +6% add-on and replaced it with a very low fixed amount that would have left most physicians “underwater” in their Part B drug acquisitions. This was vigorously opposed by physicians around the country, who let Congress know exactly how they felt. We have been told that the Coalition of State Rheumatology Organizations was one of the most vociferous organizations that helped in fighting back this proposal and resulting in its withdrawal.
MedPAC recommendations
That brings us back to MedPAC. In June, MedPAC released recommendations to Congress in an attempt to address the “high price of drugs” covered under MC Part B. Unfortunately, the recommendations do nothing to address the root cause of high drug prices, but once again attempt to balance MC expenditures on the backs of physicians. In this case, it is physicians who infuse Part B drugs in their office to chronically ill patients. In-office infusions have been shown to be the most cost-effective site of care, as well as being safer when compared with home infusion for a number of rheumatologic medications.
One of the MedPAC recommendations gives the Secretary of Health & Human Services the authority to establish a single ASP for drugs with “similar health effects.” The ambiguity of the phrase “similar health effects” should put us all on alert as to the significant unintended consequences that may result. For example, HHS could assign one ASP to all drugs that treat rheumatoid arthritis based on the lowest ASP of the group. This certainly would lead to a number of drugs being out of reach for MC beneficiaries if the artificial ASP of the medication is much lower than the actual acquisition cost of the drug, leaving physicians unable to acquire it. Yet, MedPAC states this recommendation would not affect access to care for MC beneficiaries.
Another recommendation would require HHS to reduce or eliminate the add-on percentage to the ASP for higher-priced drugs and/or put in an added fixed amount. This recommendation is clearly reminiscent of the old ill-conceived Part B demonstration project.
A fixed “add-on amount” might work if it is sufficient to cover the overhead of maintaining a provider’s infusion suite. But if practices are left underwater in their purchases of certain Part B drugs, there may be no choice but to stop offering those infusions to MC beneficiaries or – worst-case scenario – shut the door completely. Yet again, MedPAC stated that this recommendation would not result in a loss of access to these treatments for MC beneficiaries.
Loss of access?
Rheumatologists have gone to great lengths to continue offering care to MC patients in spite of the yearly cuts and threats of more cuts in the future to physician reimbursements. In addition, physicians have no annual inflationary update to their reimbursements. I am not sure how MedPAC concludes that continued cuts to physician fee schedules, along with a decrease in reimbursement for administered drugs, will not affect access to care for MC beneficiaries.
Finally, the timing on these recommendations is confusing, considering that implementation of the Inflation Reduction Act (IRA) has just begun. Next quarter, a number of Part B drugs will be subject to inflationary penalties; there will also be additional Part B biosimilars coming to market, resulting in lower ASPs. And don’t forget, the IRA just instituted an ASP plus 8% reimbursement for biosimilars in an attempt to get physicians to do something that the Centers for Medicare & Medicaid Services has asked them not to do. That is, choose a drug based on its reimbursement, not necessarily the one which is right for the patient.
Overall, with so many variables up in the air, now is not the time to create even more uncertainty for physicians and the Medicare patients that they take care of.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
The Medicare Payment Advisory Commission (MedPAC) is an independent agency to advise Congress on Medicare (MC) policy, much of which pertains to payment issues. The 17 commissioners meet publicly and issue two reports a year with their recommendations to Congress, who then decides whether to enact these recommendations or not.
One MedPAC recommendation in 2023 was quickly introduced in the House of Representatives in May and passed the Energy and Commerce Committee 49-0. That recommendation relates to “site neutrality” payments to MC providers. If passed by Congress, it would result in some “site-neutral” cuts to hospitals. That MedPAC recommendation was acted upon very quickly by Congress. Consequently, it is important to discuss the potential negative ramifications of other MedPAC recommendations released in June regarding reimbursement of Medicare Part B drugs and proactively educate Congress accordingly on those ramifications.
Medicare Part B drugs
Medicare Part B drugs are those administered by providers, unlike the Part D medications which are generally obtained through pharmacies. Presently, MC reimburses providers for the administered Part B medication based on the average sales price (ASP) plus 6%. However, with sequestration, that add-on amount is reduced to ASP plus 4.3%. It has long been touted by MedPAC and other policy makers that physicians choose to infuse higher-priced drugs in order to increase reimbursements. That has not been borne out when it comes to rheumatologists, and, in fact, a retired MedPAC commissioner even stated that premise did not hold true for rheumatologists.
Regardless, it continues to be suggested that MC should reduce its costs for Part B medications by reducing reimbursement to physicians. It should be noted that often the margins on the drugs are already quite thin, and at times the reimbursement amount, compared with the acquisition cost of the drug even leaves the physician “underwater.”
A few years ago, there was a proposed Part B demonstration project that essentially removed the +6% add-on and replaced it with a very low fixed amount that would have left most physicians “underwater” in their Part B drug acquisitions. This was vigorously opposed by physicians around the country, who let Congress know exactly how they felt. We have been told that the Coalition of State Rheumatology Organizations was one of the most vociferous organizations that helped in fighting back this proposal and resulting in its withdrawal.
MedPAC recommendations
That brings us back to MedPAC. In June, MedPAC released recommendations to Congress in an attempt to address the “high price of drugs” covered under MC Part B. Unfortunately, the recommendations do nothing to address the root cause of high drug prices, but once again attempt to balance MC expenditures on the backs of physicians. In this case, it is physicians who infuse Part B drugs in their office to chronically ill patients. In-office infusions have been shown to be the most cost-effective site of care, as well as being safer when compared with home infusion for a number of rheumatologic medications.
One of the MedPAC recommendations gives the Secretary of Health & Human Services the authority to establish a single ASP for drugs with “similar health effects.” The ambiguity of the phrase “similar health effects” should put us all on alert as to the significant unintended consequences that may result. For example, HHS could assign one ASP to all drugs that treat rheumatoid arthritis based on the lowest ASP of the group. This certainly would lead to a number of drugs being out of reach for MC beneficiaries if the artificial ASP of the medication is much lower than the actual acquisition cost of the drug, leaving physicians unable to acquire it. Yet, MedPAC states this recommendation would not affect access to care for MC beneficiaries.
Another recommendation would require HHS to reduce or eliminate the add-on percentage to the ASP for higher-priced drugs and/or put in an added fixed amount. This recommendation is clearly reminiscent of the old ill-conceived Part B demonstration project.
A fixed “add-on amount” might work if it is sufficient to cover the overhead of maintaining a provider’s infusion suite. But if practices are left underwater in their purchases of certain Part B drugs, there may be no choice but to stop offering those infusions to MC beneficiaries or – worst-case scenario – shut the door completely. Yet again, MedPAC stated that this recommendation would not result in a loss of access to these treatments for MC beneficiaries.
Loss of access?
Rheumatologists have gone to great lengths to continue offering care to MC patients in spite of the yearly cuts and threats of more cuts in the future to physician reimbursements. In addition, physicians have no annual inflationary update to their reimbursements. I am not sure how MedPAC concludes that continued cuts to physician fee schedules, along with a decrease in reimbursement for administered drugs, will not affect access to care for MC beneficiaries.
Finally, the timing on these recommendations is confusing, considering that implementation of the Inflation Reduction Act (IRA) has just begun. Next quarter, a number of Part B drugs will be subject to inflationary penalties; there will also be additional Part B biosimilars coming to market, resulting in lower ASPs. And don’t forget, the IRA just instituted an ASP plus 8% reimbursement for biosimilars in an attempt to get physicians to do something that the Centers for Medicare & Medicaid Services has asked them not to do. That is, choose a drug based on its reimbursement, not necessarily the one which is right for the patient.
Overall, with so many variables up in the air, now is not the time to create even more uncertainty for physicians and the Medicare patients that they take care of.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Getting a white-bagging exemption: A win for the patient, employer, and rheumatologist
Whether it’s filling out a prior authorization form or testifying before Congress, it is an action we perform that ultimately helps our patients achieve that care. We are familiar with many of the obstacles that block the path to the best care and interfere with our patient-doctor relationships. Much work has been done to pass legislation in the states to mitigate some of those obstacles, such as unreasonable step therapy regimens, nonmedical switching, and copay accumulators.
Unfortunately, that state legislation does not cover patients who work for companies that are self-insured. Self-insured employers, which account for about 60% of America’s workers, directly pay for the health benefits offered to employees instead of buying “fully funded” insurance plans. Most of those self-funded plans fall under “ERISA” protections and are regulated by the federal Department of Labor. ERISA stands for Employee Retirement Income Security Act. The law, which was enacted in 1974, also covers employee health plans. These plans must act as a fiduciary, meaning they must look after the well-being of the employees, including their finances and those of the plan itself.
The Coalition of State Rheumatology Organizations (CSRO) has learned of a number of issues involving patients who work for self-funded companies, regulated by ERISA. One such issue is that of mandated “white bagging.” White bagging has been discussed in “Rheum for Action” in the past. There is a long list of white-bagging problems, including dosing issues, lack of “chain of custody” with the medications, delays in treatment, mandatory up-front payments by the patient, and wastage of unused medication. However, there is another issue that is of concern not only to the employees (our patients) but to the employer as well.
Employers’ fiduciary responsibility
As mentioned earlier, the employers who self insure are responsible for the financial well-being of their employee and the plan itself. Therefore, if certain practices are mandated within the health plan that harm our patients or the plan financially, the company could be in violation of their fiduciary duty. Rheumatologists have said that buying and billing the drug to the medical side of the health plan in many cases costs much less than white bagging. Conceivably, that could result in breach of an employer’s fiduciary duty to their employee.
Evidence for violating fiduciary duty
CSRO recently received redacted receipts comparing costs between the two models of drug acquisition for a patient in an ERISA plan. White bagging for the patient occurred in 2021, and in 2022 an exemption was granted for the rheumatologist to buy and bill the administered medication. Unfortunately, the exemption to buy and bill in 2023 was denied and continues to be denied (as of this writing). A comparison of the receipts revealed the company was charged over $40,000 for the white-bagged medication in 2021, and the patient’s cost share for that year was $525. Under the traditional buy-and-bill acquisition model in 2022, the company was charged around $12,000 for the medication and the patient’s cost share was $30. There is a clear difference in cost to the employee and plan between the two acquisition models.
Is this major company unknowingly violating its fiduciary duty by mandating white bagging as per their contract with one of the three big pharmacy benefit managers (PBMs)? If so, how does something like this happen with a large national company that has ERISA attorneys looking over the contracts with the PBMs?
Why is white bagging mandated?
Often, white bagging is mandated because the cost of infusions in a hospital outpatient facility can be very high. Nationally, it has been shown that hospitals charge four to five times the cost they paid for the drug, and the 100 most expensive hospitals charge 10-18 times the cost of their drugs. With these up-charges, white bagging could easily be a lower cost for employee and company. But across-the-board mandating of white bagging ignores that physician office–based infusions may offer a much lower cost to employees and the employer.
Another reason large and small self-funded companies may unknowingly sign contracts that are often more profitable to the PBM than to the employer is that the employer pharmacy benefit consultants are paid handsomely by the big PBMs and have been known to “rig” the contract in favor of the PBM, according to Paul Holmes, an ERISA attorney with a focus in pharmacy health plan contracts. Clearly, the PBM profits more with white-bagged medicines billed through the pharmacy (PBM) side of insurance as opposed to buy-and-bill medications that are billed on the medical side of insurance. So mandated white bagging is often included in these contracts, ignoring the lower cost in an infusion suite at a physician’s office.
Suggestions for employers
Employers and employees should be able to obtain the costs of mandated, white-bagged drugs from their PBMs because the Consolidated Appropriations Act of 2021 (CAA) mandates that group health plans ensure access to cost data. The employer should also have access to their consultant’s compensation from the PBM as Section 202 in the CAA states that employer benefit consultants must “disclose actual and anticipated cash and non-cash compensation they expect to earn in connection with the sale, renewal, and extension of group health insurance.”
It would be wise for all self-insured companies to use this section to see how much their consultants are being influenced by the company that they are recommending. Additionally, the companies should consider hiring ERISA attorneys that understand not only the legalese of the contract with a PBM but also the pharmacy lingo, such as the difference between maximum allowable cost, average wholesale price, average sales price, and average manufacturer’s price.
Suggestion for the rheumatologist
This leads to a suggestion to rheumatologists trying to get an exemption from mandated white bagging. If a patient has already had white-bagged medication, have them obtain a receipt from the PBM for their charges to the plan for the medication. If the patient has not gone through the white bagging yet, the PBM should be able to tell the plan the cost of the white-bagged medication and the cost to the patient. Compare those costs with what would be charged through buy and bill, and if it is less, present that evidence to the employer and remind them of their fiduciary responsibility to their employees.
Granted, this process may take more effort than filling out a prior authorization, but getting the white-bag exemption will help the patient, the employer, and the rheumatologist in the long run. A win-win-win!
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Whether it’s filling out a prior authorization form or testifying before Congress, it is an action we perform that ultimately helps our patients achieve that care. We are familiar with many of the obstacles that block the path to the best care and interfere with our patient-doctor relationships. Much work has been done to pass legislation in the states to mitigate some of those obstacles, such as unreasonable step therapy regimens, nonmedical switching, and copay accumulators.
Unfortunately, that state legislation does not cover patients who work for companies that are self-insured. Self-insured employers, which account for about 60% of America’s workers, directly pay for the health benefits offered to employees instead of buying “fully funded” insurance plans. Most of those self-funded plans fall under “ERISA” protections and are regulated by the federal Department of Labor. ERISA stands for Employee Retirement Income Security Act. The law, which was enacted in 1974, also covers employee health plans. These plans must act as a fiduciary, meaning they must look after the well-being of the employees, including their finances and those of the plan itself.
The Coalition of State Rheumatology Organizations (CSRO) has learned of a number of issues involving patients who work for self-funded companies, regulated by ERISA. One such issue is that of mandated “white bagging.” White bagging has been discussed in “Rheum for Action” in the past. There is a long list of white-bagging problems, including dosing issues, lack of “chain of custody” with the medications, delays in treatment, mandatory up-front payments by the patient, and wastage of unused medication. However, there is another issue that is of concern not only to the employees (our patients) but to the employer as well.
Employers’ fiduciary responsibility
As mentioned earlier, the employers who self insure are responsible for the financial well-being of their employee and the plan itself. Therefore, if certain practices are mandated within the health plan that harm our patients or the plan financially, the company could be in violation of their fiduciary duty. Rheumatologists have said that buying and billing the drug to the medical side of the health plan in many cases costs much less than white bagging. Conceivably, that could result in breach of an employer’s fiduciary duty to their employee.
Evidence for violating fiduciary duty
CSRO recently received redacted receipts comparing costs between the two models of drug acquisition for a patient in an ERISA plan. White bagging for the patient occurred in 2021, and in 2022 an exemption was granted for the rheumatologist to buy and bill the administered medication. Unfortunately, the exemption to buy and bill in 2023 was denied and continues to be denied (as of this writing). A comparison of the receipts revealed the company was charged over $40,000 for the white-bagged medication in 2021, and the patient’s cost share for that year was $525. Under the traditional buy-and-bill acquisition model in 2022, the company was charged around $12,000 for the medication and the patient’s cost share was $30. There is a clear difference in cost to the employee and plan between the two acquisition models.
Is this major company unknowingly violating its fiduciary duty by mandating white bagging as per their contract with one of the three big pharmacy benefit managers (PBMs)? If so, how does something like this happen with a large national company that has ERISA attorneys looking over the contracts with the PBMs?
Why is white bagging mandated?
Often, white bagging is mandated because the cost of infusions in a hospital outpatient facility can be very high. Nationally, it has been shown that hospitals charge four to five times the cost they paid for the drug, and the 100 most expensive hospitals charge 10-18 times the cost of their drugs. With these up-charges, white bagging could easily be a lower cost for employee and company. But across-the-board mandating of white bagging ignores that physician office–based infusions may offer a much lower cost to employees and the employer.
Another reason large and small self-funded companies may unknowingly sign contracts that are often more profitable to the PBM than to the employer is that the employer pharmacy benefit consultants are paid handsomely by the big PBMs and have been known to “rig” the contract in favor of the PBM, according to Paul Holmes, an ERISA attorney with a focus in pharmacy health plan contracts. Clearly, the PBM profits more with white-bagged medicines billed through the pharmacy (PBM) side of insurance as opposed to buy-and-bill medications that are billed on the medical side of insurance. So mandated white bagging is often included in these contracts, ignoring the lower cost in an infusion suite at a physician’s office.
Suggestions for employers
Employers and employees should be able to obtain the costs of mandated, white-bagged drugs from their PBMs because the Consolidated Appropriations Act of 2021 (CAA) mandates that group health plans ensure access to cost data. The employer should also have access to their consultant’s compensation from the PBM as Section 202 in the CAA states that employer benefit consultants must “disclose actual and anticipated cash and non-cash compensation they expect to earn in connection with the sale, renewal, and extension of group health insurance.”
It would be wise for all self-insured companies to use this section to see how much their consultants are being influenced by the company that they are recommending. Additionally, the companies should consider hiring ERISA attorneys that understand not only the legalese of the contract with a PBM but also the pharmacy lingo, such as the difference between maximum allowable cost, average wholesale price, average sales price, and average manufacturer’s price.
Suggestion for the rheumatologist
This leads to a suggestion to rheumatologists trying to get an exemption from mandated white bagging. If a patient has already had white-bagged medication, have them obtain a receipt from the PBM for their charges to the plan for the medication. If the patient has not gone through the white bagging yet, the PBM should be able to tell the plan the cost of the white-bagged medication and the cost to the patient. Compare those costs with what would be charged through buy and bill, and if it is less, present that evidence to the employer and remind them of their fiduciary responsibility to their employees.
Granted, this process may take more effort than filling out a prior authorization, but getting the white-bag exemption will help the patient, the employer, and the rheumatologist in the long run. A win-win-win!
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Whether it’s filling out a prior authorization form or testifying before Congress, it is an action we perform that ultimately helps our patients achieve that care. We are familiar with many of the obstacles that block the path to the best care and interfere with our patient-doctor relationships. Much work has been done to pass legislation in the states to mitigate some of those obstacles, such as unreasonable step therapy regimens, nonmedical switching, and copay accumulators.
Unfortunately, that state legislation does not cover patients who work for companies that are self-insured. Self-insured employers, which account for about 60% of America’s workers, directly pay for the health benefits offered to employees instead of buying “fully funded” insurance plans. Most of those self-funded plans fall under “ERISA” protections and are regulated by the federal Department of Labor. ERISA stands for Employee Retirement Income Security Act. The law, which was enacted in 1974, also covers employee health plans. These plans must act as a fiduciary, meaning they must look after the well-being of the employees, including their finances and those of the plan itself.
The Coalition of State Rheumatology Organizations (CSRO) has learned of a number of issues involving patients who work for self-funded companies, regulated by ERISA. One such issue is that of mandated “white bagging.” White bagging has been discussed in “Rheum for Action” in the past. There is a long list of white-bagging problems, including dosing issues, lack of “chain of custody” with the medications, delays in treatment, mandatory up-front payments by the patient, and wastage of unused medication. However, there is another issue that is of concern not only to the employees (our patients) but to the employer as well.
Employers’ fiduciary responsibility
As mentioned earlier, the employers who self insure are responsible for the financial well-being of their employee and the plan itself. Therefore, if certain practices are mandated within the health plan that harm our patients or the plan financially, the company could be in violation of their fiduciary duty. Rheumatologists have said that buying and billing the drug to the medical side of the health plan in many cases costs much less than white bagging. Conceivably, that could result in breach of an employer’s fiduciary duty to their employee.
Evidence for violating fiduciary duty
CSRO recently received redacted receipts comparing costs between the two models of drug acquisition for a patient in an ERISA plan. White bagging for the patient occurred in 2021, and in 2022 an exemption was granted for the rheumatologist to buy and bill the administered medication. Unfortunately, the exemption to buy and bill in 2023 was denied and continues to be denied (as of this writing). A comparison of the receipts revealed the company was charged over $40,000 for the white-bagged medication in 2021, and the patient’s cost share for that year was $525. Under the traditional buy-and-bill acquisition model in 2022, the company was charged around $12,000 for the medication and the patient’s cost share was $30. There is a clear difference in cost to the employee and plan between the two acquisition models.
Is this major company unknowingly violating its fiduciary duty by mandating white bagging as per their contract with one of the three big pharmacy benefit managers (PBMs)? If so, how does something like this happen with a large national company that has ERISA attorneys looking over the contracts with the PBMs?
Why is white bagging mandated?
Often, white bagging is mandated because the cost of infusions in a hospital outpatient facility can be very high. Nationally, it has been shown that hospitals charge four to five times the cost they paid for the drug, and the 100 most expensive hospitals charge 10-18 times the cost of their drugs. With these up-charges, white bagging could easily be a lower cost for employee and company. But across-the-board mandating of white bagging ignores that physician office–based infusions may offer a much lower cost to employees and the employer.
Another reason large and small self-funded companies may unknowingly sign contracts that are often more profitable to the PBM than to the employer is that the employer pharmacy benefit consultants are paid handsomely by the big PBMs and have been known to “rig” the contract in favor of the PBM, according to Paul Holmes, an ERISA attorney with a focus in pharmacy health plan contracts. Clearly, the PBM profits more with white-bagged medicines billed through the pharmacy (PBM) side of insurance as opposed to buy-and-bill medications that are billed on the medical side of insurance. So mandated white bagging is often included in these contracts, ignoring the lower cost in an infusion suite at a physician’s office.
Suggestions for employers
Employers and employees should be able to obtain the costs of mandated, white-bagged drugs from their PBMs because the Consolidated Appropriations Act of 2021 (CAA) mandates that group health plans ensure access to cost data. The employer should also have access to their consultant’s compensation from the PBM as Section 202 in the CAA states that employer benefit consultants must “disclose actual and anticipated cash and non-cash compensation they expect to earn in connection with the sale, renewal, and extension of group health insurance.”
It would be wise for all self-insured companies to use this section to see how much their consultants are being influenced by the company that they are recommending. Additionally, the companies should consider hiring ERISA attorneys that understand not only the legalese of the contract with a PBM but also the pharmacy lingo, such as the difference between maximum allowable cost, average wholesale price, average sales price, and average manufacturer’s price.
Suggestion for the rheumatologist
This leads to a suggestion to rheumatologists trying to get an exemption from mandated white bagging. If a patient has already had white-bagged medication, have them obtain a receipt from the PBM for their charges to the plan for the medication. If the patient has not gone through the white bagging yet, the PBM should be able to tell the plan the cost of the white-bagged medication and the cost to the patient. Compare those costs with what would be charged through buy and bill, and if it is less, present that evidence to the employer and remind them of their fiduciary responsibility to their employees.
Granted, this process may take more effort than filling out a prior authorization, but getting the white-bag exemption will help the patient, the employer, and the rheumatologist in the long run. A win-win-win!
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.






