User login
Oral Isotretinoin for Acne in the US Military: How Accelerated Courses and Teledermatology Can Minimize the Duty-Limiting Impacts of Treatment
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
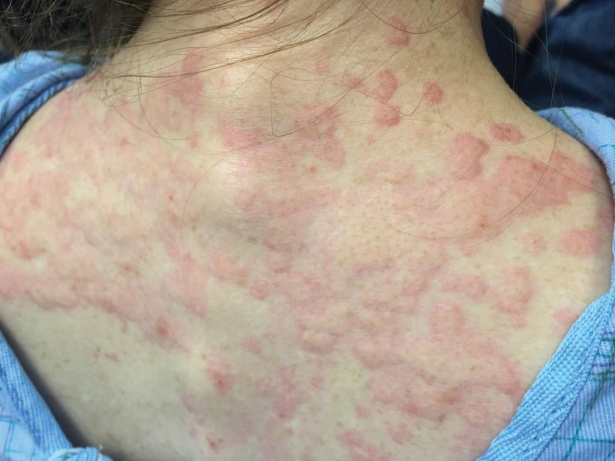
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
Practice Points
- Acne is a common skin disease with a high prevalence in the active-duty US Military population.
- Oral isotretinoin is a commonly utilized acne medication that can limit the ability for military service members to deploy and is considered disqualifying for some special duty assignments.
- High daily- and cumulative-dose oral isotretinoin therapy as well as teledermatology can minimize the duty-limiting impact of isotretinoin therapy for military service members.
Questions about optimal dosing of isotretinoin persist, expert says
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
FROM ODAC 2022
More than a month after launch, iPLEDGE glitches persist
which mandates the program to prevent fetal exposure to the teratogenic effects of isotretinoin, and by the American Academy of Dermatology Association, whose members have repeatedly asked the FDA for meetings to discuss solutions. The AADA is the legislative and advocacy arm of AAD.
When the new program launched Dec. 13, 2021, the website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they tried to follow instructions to enter information. Hold times to talk to a live person stretched to hours.
The latest improvement attempt, announced Jan. 14 by the FDA, is a tool created by the Isotretinoin Products Manufacturers Group, the manufacturers responsible for the FDA-mandated REMS program. It is meant to allow prescribers and designees to send log-in links directly to patients’ email accounts through the iPLEDGE REMS portal, bypassing the troublesome call center.
And it’s not the answer, dermatologists said.
“The new tool does not solve issues such as prescribers or pharmacies not being able to access the site, unacceptably long call center wait times, inefficiencies caused by frequent attestation requirements for those who cannot become pregnant, patients becoming ‘locked out’ because they missed a window period through no fault of their own, among others,” said John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital and instructor in dermatology at Harvard Medical School, both in Boston.
The day after the FDA update about the new tool, Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., tweeted: “Lip service and empty words.” He noted that the situation has been “disastrous from the start” as the new platform launched.
Under the iPLEDGE program in place for the acne drug, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The aim of the new gender-neutral approach to the risk mitigation program is to make the experience more inclusive for transgender patients. The previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) are now reduced to just two (those capable of getting pregnant and those not capable of getting pregnant).
The problem is the execution of the new platform. The transition from the old website to the new was done quickly. By most accounts, the Dec. 13 rollout was chaotic, a failure, and disastrous, triggering numerous expressions of frustration on Twitter and other social media, with some calling for the program to be halted until the bugs could be worked out.
“While the new gender-neutral categories are a welcome improvement to the system, the new categorization approach was not the underlying reason for the new platform and its failed rollout, which was instead due to a change in vendor,” Dr. Barbieri told this news organization.
AADA: More recent efforts to improve the system
“We have a letter to the FDA asking for a stakeholders meeting to include us, the IPMG, and pharmacists because there are ongoing problems, though there have been some improvement in terms of certain elements,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco, said in an interview shortly after the FDA posted the update on the new tool. “That said, there are many patients who have not gotten isotretinoin during the 1 month since the roll-out of the new platform.”
What still needs to be fixed? “We have ongoing concerns about the lack of transparency of the IPMG, about call center wait times, actual number of prescriptions on the hands of patients compared to the previous month, and those patients who can get pregnant who – despite complying with all of the REMS requirements – are being locked out because of the lack of timely attestation to their negative pregnancy status due to the website, not the patients themselves,” Dr. Frieden told this news organization.
“We are continuing to advocate to have decreased attestation requirements for individuals who cannot become pregnant – because this will improve the efficiency of the system for those patients for whom the REMS program goals are truly intended – those who can become pregnant, since the primary aim of the REMS program is to minimize fetal exposure.”
An AADA spokesperson said that the IPMG has invited the AADA to a joint stakeholders meeting on Jan. 26, along with representatives from the FDA and pharmacy industry.
Spotty progress
“The iPLEDGE situation is as frustrating as ever,” said Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, after the FDA’s Jan. 14 update was released. “It’s like they never tested the new website before deploying it.”
Among the issues he has experienced in his practice, he said, is an instance in which iPLEDGE swapped the first names of a mother and daughter, so it was impossible to fill the prescription. “It happened twice in the same day,” Dr. Goldberg said. The patient had to call iPLEDGE to fix this, but the call center wasn’t taking calls.
In today’s technology environment, he said, it’s hard to believe that “we have to put up with this.”
Some have seen success. ‘’The tool is working fine on our end,” said Mitesh Patel, PharmD, pharmacy manager at Sunshine Pharmacy in White Plains, N.Y. However, he added that some doctors and patients are still having issues. He encourages dermatologists still having issues with the system to reach out to independent pharmacies that have processed iPLEDGE prescriptions and ‘’lean on them to assist.”
This news organization contacted CVS and Walgreens about how the system is working at their locations, but has not yet received a response.
Dr. Goldberg, Dr. Frieden, Dr. Barbieri, and Dr. Peebles have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This story was updated on 1/24/22.
which mandates the program to prevent fetal exposure to the teratogenic effects of isotretinoin, and by the American Academy of Dermatology Association, whose members have repeatedly asked the FDA for meetings to discuss solutions. The AADA is the legislative and advocacy arm of AAD.
When the new program launched Dec. 13, 2021, the website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they tried to follow instructions to enter information. Hold times to talk to a live person stretched to hours.
The latest improvement attempt, announced Jan. 14 by the FDA, is a tool created by the Isotretinoin Products Manufacturers Group, the manufacturers responsible for the FDA-mandated REMS program. It is meant to allow prescribers and designees to send log-in links directly to patients’ email accounts through the iPLEDGE REMS portal, bypassing the troublesome call center.
And it’s not the answer, dermatologists said.
“The new tool does not solve issues such as prescribers or pharmacies not being able to access the site, unacceptably long call center wait times, inefficiencies caused by frequent attestation requirements for those who cannot become pregnant, patients becoming ‘locked out’ because they missed a window period through no fault of their own, among others,” said John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital and instructor in dermatology at Harvard Medical School, both in Boston.
The day after the FDA update about the new tool, Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., tweeted: “Lip service and empty words.” He noted that the situation has been “disastrous from the start” as the new platform launched.
Under the iPLEDGE program in place for the acne drug, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The aim of the new gender-neutral approach to the risk mitigation program is to make the experience more inclusive for transgender patients. The previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) are now reduced to just two (those capable of getting pregnant and those not capable of getting pregnant).
The problem is the execution of the new platform. The transition from the old website to the new was done quickly. By most accounts, the Dec. 13 rollout was chaotic, a failure, and disastrous, triggering numerous expressions of frustration on Twitter and other social media, with some calling for the program to be halted until the bugs could be worked out.
“While the new gender-neutral categories are a welcome improvement to the system, the new categorization approach was not the underlying reason for the new platform and its failed rollout, which was instead due to a change in vendor,” Dr. Barbieri told this news organization.
AADA: More recent efforts to improve the system
“We have a letter to the FDA asking for a stakeholders meeting to include us, the IPMG, and pharmacists because there are ongoing problems, though there have been some improvement in terms of certain elements,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco, said in an interview shortly after the FDA posted the update on the new tool. “That said, there are many patients who have not gotten isotretinoin during the 1 month since the roll-out of the new platform.”
What still needs to be fixed? “We have ongoing concerns about the lack of transparency of the IPMG, about call center wait times, actual number of prescriptions on the hands of patients compared to the previous month, and those patients who can get pregnant who – despite complying with all of the REMS requirements – are being locked out because of the lack of timely attestation to their negative pregnancy status due to the website, not the patients themselves,” Dr. Frieden told this news organization.
“We are continuing to advocate to have decreased attestation requirements for individuals who cannot become pregnant – because this will improve the efficiency of the system for those patients for whom the REMS program goals are truly intended – those who can become pregnant, since the primary aim of the REMS program is to minimize fetal exposure.”
An AADA spokesperson said that the IPMG has invited the AADA to a joint stakeholders meeting on Jan. 26, along with representatives from the FDA and pharmacy industry.
Spotty progress
“The iPLEDGE situation is as frustrating as ever,” said Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, after the FDA’s Jan. 14 update was released. “It’s like they never tested the new website before deploying it.”
Among the issues he has experienced in his practice, he said, is an instance in which iPLEDGE swapped the first names of a mother and daughter, so it was impossible to fill the prescription. “It happened twice in the same day,” Dr. Goldberg said. The patient had to call iPLEDGE to fix this, but the call center wasn’t taking calls.
In today’s technology environment, he said, it’s hard to believe that “we have to put up with this.”
Some have seen success. ‘’The tool is working fine on our end,” said Mitesh Patel, PharmD, pharmacy manager at Sunshine Pharmacy in White Plains, N.Y. However, he added that some doctors and patients are still having issues. He encourages dermatologists still having issues with the system to reach out to independent pharmacies that have processed iPLEDGE prescriptions and ‘’lean on them to assist.”
This news organization contacted CVS and Walgreens about how the system is working at their locations, but has not yet received a response.
Dr. Goldberg, Dr. Frieden, Dr. Barbieri, and Dr. Peebles have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This story was updated on 1/24/22.
which mandates the program to prevent fetal exposure to the teratogenic effects of isotretinoin, and by the American Academy of Dermatology Association, whose members have repeatedly asked the FDA for meetings to discuss solutions. The AADA is the legislative and advocacy arm of AAD.
When the new program launched Dec. 13, 2021, the website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they tried to follow instructions to enter information. Hold times to talk to a live person stretched to hours.
The latest improvement attempt, announced Jan. 14 by the FDA, is a tool created by the Isotretinoin Products Manufacturers Group, the manufacturers responsible for the FDA-mandated REMS program. It is meant to allow prescribers and designees to send log-in links directly to patients’ email accounts through the iPLEDGE REMS portal, bypassing the troublesome call center.
And it’s not the answer, dermatologists said.
“The new tool does not solve issues such as prescribers or pharmacies not being able to access the site, unacceptably long call center wait times, inefficiencies caused by frequent attestation requirements for those who cannot become pregnant, patients becoming ‘locked out’ because they missed a window period through no fault of their own, among others,” said John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital and instructor in dermatology at Harvard Medical School, both in Boston.
The day after the FDA update about the new tool, Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., tweeted: “Lip service and empty words.” He noted that the situation has been “disastrous from the start” as the new platform launched.
Under the iPLEDGE program in place for the acne drug, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The aim of the new gender-neutral approach to the risk mitigation program is to make the experience more inclusive for transgender patients. The previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) are now reduced to just two (those capable of getting pregnant and those not capable of getting pregnant).
The problem is the execution of the new platform. The transition from the old website to the new was done quickly. By most accounts, the Dec. 13 rollout was chaotic, a failure, and disastrous, triggering numerous expressions of frustration on Twitter and other social media, with some calling for the program to be halted until the bugs could be worked out.
“While the new gender-neutral categories are a welcome improvement to the system, the new categorization approach was not the underlying reason for the new platform and its failed rollout, which was instead due to a change in vendor,” Dr. Barbieri told this news organization.
AADA: More recent efforts to improve the system
“We have a letter to the FDA asking for a stakeholders meeting to include us, the IPMG, and pharmacists because there are ongoing problems, though there have been some improvement in terms of certain elements,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco, said in an interview shortly after the FDA posted the update on the new tool. “That said, there are many patients who have not gotten isotretinoin during the 1 month since the roll-out of the new platform.”
What still needs to be fixed? “We have ongoing concerns about the lack of transparency of the IPMG, about call center wait times, actual number of prescriptions on the hands of patients compared to the previous month, and those patients who can get pregnant who – despite complying with all of the REMS requirements – are being locked out because of the lack of timely attestation to their negative pregnancy status due to the website, not the patients themselves,” Dr. Frieden told this news organization.
“We are continuing to advocate to have decreased attestation requirements for individuals who cannot become pregnant – because this will improve the efficiency of the system for those patients for whom the REMS program goals are truly intended – those who can become pregnant, since the primary aim of the REMS program is to minimize fetal exposure.”
An AADA spokesperson said that the IPMG has invited the AADA to a joint stakeholders meeting on Jan. 26, along with representatives from the FDA and pharmacy industry.
Spotty progress
“The iPLEDGE situation is as frustrating as ever,” said Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, after the FDA’s Jan. 14 update was released. “It’s like they never tested the new website before deploying it.”
Among the issues he has experienced in his practice, he said, is an instance in which iPLEDGE swapped the first names of a mother and daughter, so it was impossible to fill the prescription. “It happened twice in the same day,” Dr. Goldberg said. The patient had to call iPLEDGE to fix this, but the call center wasn’t taking calls.
In today’s technology environment, he said, it’s hard to believe that “we have to put up with this.”
Some have seen success. ‘’The tool is working fine on our end,” said Mitesh Patel, PharmD, pharmacy manager at Sunshine Pharmacy in White Plains, N.Y. However, he added that some doctors and patients are still having issues. He encourages dermatologists still having issues with the system to reach out to independent pharmacies that have processed iPLEDGE prescriptions and ‘’lean on them to assist.”
This news organization contacted CVS and Walgreens about how the system is working at their locations, but has not yet received a response.
Dr. Goldberg, Dr. Frieden, Dr. Barbieri, and Dr. Peebles have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This story was updated on 1/24/22.
FDA updates status of iPLEDGE access problems
The, one month after a modified program was launched, the Food and Drug Administration announced on Jan. 14.
The IPMG has “created a new tool within the system to help resolve account access for some user groups without using the call center. This tool is intended to allow prescribers and designees to send login links directly to their patients’ desired email address through the Manage Patients page of the iPLEDGE REMS portal,” the FDA statement said.
“Prescribers can also send login links to their designees still having difficulty accessing their iPLEDGE account,” and users should check their emails for messages from iPLEDGE, including spam folders, the FDA advises. The iPLEDGE strategy is designed to prevent fetal exposure to isotretinoin, which is highly teratogenic.
Days after the new, gender-neutral approach to the isotretinoin risk mitigation program was launched on Dec. 13, the FDA convened an emergency meeting with representatives from the American Academy of Dermatology Association (AADA) to discuss the problematic rollout of the program, which was described as disastrous, chaotic, and a failure, with dermatologists on Twitter and elsewhere expressing anger and frustration over not being able to access the program or reach the call center.
A statement by the FDA on Dec. 23 followed, urging manufacturers to develop solutions for the website and to work with the AADA and pharmacy organizations to find solutions that would minimize treatment interruptions during the transition.
The modified REMS, launched on Dec. 13, is designed to make it more inclusive for transgender patients prescribed isotretinoin. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), patients who are prescribed isotretinoin for acne are assigned to one of two risk categories: those who can get pregnant and those who cannot get pregnant.
In the Jan. 14 statement, the FDA notes that the agency is continuing to work with the IPMG regarding the problems clinicians, pharmacists, and patients have had with accessing iPLEDGE over the last month.
“Although there has been progress, there is a significant amount of work still to be done,” the FDA acknowledged. “While we consider potential steps within the scope of FDA’s authorities, we will continue to meet with the IPMG for updates on the status of the problems with the iPLEDGE REMS and their progress towards having the system work as intended for all users.”
The, one month after a modified program was launched, the Food and Drug Administration announced on Jan. 14.
The IPMG has “created a new tool within the system to help resolve account access for some user groups without using the call center. This tool is intended to allow prescribers and designees to send login links directly to their patients’ desired email address through the Manage Patients page of the iPLEDGE REMS portal,” the FDA statement said.
“Prescribers can also send login links to their designees still having difficulty accessing their iPLEDGE account,” and users should check their emails for messages from iPLEDGE, including spam folders, the FDA advises. The iPLEDGE strategy is designed to prevent fetal exposure to isotretinoin, which is highly teratogenic.
Days after the new, gender-neutral approach to the isotretinoin risk mitigation program was launched on Dec. 13, the FDA convened an emergency meeting with representatives from the American Academy of Dermatology Association (AADA) to discuss the problematic rollout of the program, which was described as disastrous, chaotic, and a failure, with dermatologists on Twitter and elsewhere expressing anger and frustration over not being able to access the program or reach the call center.
A statement by the FDA on Dec. 23 followed, urging manufacturers to develop solutions for the website and to work with the AADA and pharmacy organizations to find solutions that would minimize treatment interruptions during the transition.
The modified REMS, launched on Dec. 13, is designed to make it more inclusive for transgender patients prescribed isotretinoin. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), patients who are prescribed isotretinoin for acne are assigned to one of two risk categories: those who can get pregnant and those who cannot get pregnant.
In the Jan. 14 statement, the FDA notes that the agency is continuing to work with the IPMG regarding the problems clinicians, pharmacists, and patients have had with accessing iPLEDGE over the last month.
“Although there has been progress, there is a significant amount of work still to be done,” the FDA acknowledged. “While we consider potential steps within the scope of FDA’s authorities, we will continue to meet with the IPMG for updates on the status of the problems with the iPLEDGE REMS and their progress towards having the system work as intended for all users.”
The, one month after a modified program was launched, the Food and Drug Administration announced on Jan. 14.
The IPMG has “created a new tool within the system to help resolve account access for some user groups without using the call center. This tool is intended to allow prescribers and designees to send login links directly to their patients’ desired email address through the Manage Patients page of the iPLEDGE REMS portal,” the FDA statement said.
“Prescribers can also send login links to their designees still having difficulty accessing their iPLEDGE account,” and users should check their emails for messages from iPLEDGE, including spam folders, the FDA advises. The iPLEDGE strategy is designed to prevent fetal exposure to isotretinoin, which is highly teratogenic.
Days after the new, gender-neutral approach to the isotretinoin risk mitigation program was launched on Dec. 13, the FDA convened an emergency meeting with representatives from the American Academy of Dermatology Association (AADA) to discuss the problematic rollout of the program, which was described as disastrous, chaotic, and a failure, with dermatologists on Twitter and elsewhere expressing anger and frustration over not being able to access the program or reach the call center.
A statement by the FDA on Dec. 23 followed, urging manufacturers to develop solutions for the website and to work with the AADA and pharmacy organizations to find solutions that would minimize treatment interruptions during the transition.
The modified REMS, launched on Dec. 13, is designed to make it more inclusive for transgender patients prescribed isotretinoin. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), patients who are prescribed isotretinoin for acne are assigned to one of two risk categories: those who can get pregnant and those who cannot get pregnant.
In the Jan. 14 statement, the FDA notes that the agency is continuing to work with the IPMG regarding the problems clinicians, pharmacists, and patients have had with accessing iPLEDGE over the last month.
“Although there has been progress, there is a significant amount of work still to be done,” the FDA acknowledged. “While we consider potential steps within the scope of FDA’s authorities, we will continue to meet with the IPMG for updates on the status of the problems with the iPLEDGE REMS and their progress towards having the system work as intended for all users.”
iPLEDGE rollout: As frustration mounts, FDA agrees to help solve issues
, according to dermatologists, pharmacists, and patients.
When the new website and call center launched Dec. 13, hours-long hold times and repeated crashing of the website were reported as the norm, not the exception, triggering the American Academy of Dermatology Association (AADA) to request – and get – an emergency meeting on Dec. 16 with the U.S. Food and Drug Administration, which mandates the risk evaluation and mitigation strategy (REMS) for isotretinoin due to the teratogenicity of the acne medication.
At that meeting, ‘’the FDA and HHS [U.S. Department of Health and Human Services] acknowledged the concerns of dermatologists and the need for stakeholders to work collaboratively to find a solution,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE workgroup and professor of dermatology at the University of California, San Francisco, said in an email interview. At the meeting, the AADA representatives described the severe impact on patient access to treatment that is resulting from the issues. The AADA also ‘’reiterated our call for a temporary pause to the program while stakeholders work to resolve the urgent issues with the platform,” she said.
The new approach, which is intended to make the experience more inclusive for transgender patients, reduces the previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) to just two (those capable of getting pregnant and those not capable). The program requires physicians, patients, and pharmacists who prescribe, use, or dispense the drug to be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by patients capable of becoming pregnant.
With reduced or no access during the technology glitches, access to the medicine was delayed for some patients. And dermatologists, pharmacists, and their staffs reported grueling hold times trying to reach the call center when the website had issues.
While the FDA agreed to help find a solution, it noted that the solution ‘’was to be found with dermatologists and pharmacists who are on the ground living the program every day,” Dr. Frieden said. No timeline for solving the issues was provided, so on Dec. 21, the AADA asked the FDA for a constructive dialogue among stakeholders within the next 24 hours, Dr. Frieden told this news organization.
While Dr. Frieden sees progress, ‘’we are disappointed that this situation continues to drag on for more than a week later, with more patients losing access to their needed medication each day.” While some prescribers have been able to log onto the portal and enter the information required, confirming some patients, large gaps remain, she said. Patients and pharmacists still report difficulties logging on. When that happens and they try to reach the call center, there are often hours-long hold times, dropped calls, or a message saying to call back.
The iPLEDGE administrator is Syneos Health, but a spokesperson for Syneos, Gary Gatyas, said the company does not maintain the system or the contact center.
So who does manage the call center and website? “The AADA has asked stakeholders, including Syneos Health, for clarification on who manages the call center and website but has not received a response,” Dr. Frieden said. “In the meeting [Dec. 16], representatives from the FDA made clear that the iPLEDGE sponsors are ultimately responsible for this REMS program,” Dr. Frieden said.
According to the FDA, isotretinoin manufacturers are part of the iPLEDGE program. On the iPLEDGE website, 12 isotretinoin products are listed, made by eight different companies.
One dermatologist maneuvering the new website who registered successfully as a provider told this news organization that he received a follow-up survey from United BioSource about the new website. This news organization contacted that company to confirm it runs the website but has not yet received a response.
Meanwhile, dermatologists continue to help frustrated patients cope with the new website and registration details. Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, heard from two mothers who helped their teen daughters complete the forms by attesting they would use abstinence as contraception but then couldn’t figure out how to answer another question. As a result, their answers were interpreted as the patients saying they were using abstinence but didn’t commit to not having sexual contact with a partner capable of impregnating them. So Dr. Goldberg got an automated message back from the iPLEDGE program that the answers were a mismatch.
And in the comments section following a previous story on the problematic rollout, one reader offered a suggestion for reducing hold times to the call center: choose the Spanish option.
Dr. Frieden and Dr. Goldberg have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, according to dermatologists, pharmacists, and patients.
When the new website and call center launched Dec. 13, hours-long hold times and repeated crashing of the website were reported as the norm, not the exception, triggering the American Academy of Dermatology Association (AADA) to request – and get – an emergency meeting on Dec. 16 with the U.S. Food and Drug Administration, which mandates the risk evaluation and mitigation strategy (REMS) for isotretinoin due to the teratogenicity of the acne medication.
At that meeting, ‘’the FDA and HHS [U.S. Department of Health and Human Services] acknowledged the concerns of dermatologists and the need for stakeholders to work collaboratively to find a solution,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE workgroup and professor of dermatology at the University of California, San Francisco, said in an email interview. At the meeting, the AADA representatives described the severe impact on patient access to treatment that is resulting from the issues. The AADA also ‘’reiterated our call for a temporary pause to the program while stakeholders work to resolve the urgent issues with the platform,” she said.
The new approach, which is intended to make the experience more inclusive for transgender patients, reduces the previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) to just two (those capable of getting pregnant and those not capable). The program requires physicians, patients, and pharmacists who prescribe, use, or dispense the drug to be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by patients capable of becoming pregnant.
With reduced or no access during the technology glitches, access to the medicine was delayed for some patients. And dermatologists, pharmacists, and their staffs reported grueling hold times trying to reach the call center when the website had issues.
While the FDA agreed to help find a solution, it noted that the solution ‘’was to be found with dermatologists and pharmacists who are on the ground living the program every day,” Dr. Frieden said. No timeline for solving the issues was provided, so on Dec. 21, the AADA asked the FDA for a constructive dialogue among stakeholders within the next 24 hours, Dr. Frieden told this news organization.
While Dr. Frieden sees progress, ‘’we are disappointed that this situation continues to drag on for more than a week later, with more patients losing access to their needed medication each day.” While some prescribers have been able to log onto the portal and enter the information required, confirming some patients, large gaps remain, she said. Patients and pharmacists still report difficulties logging on. When that happens and they try to reach the call center, there are often hours-long hold times, dropped calls, or a message saying to call back.
The iPLEDGE administrator is Syneos Health, but a spokesperson for Syneos, Gary Gatyas, said the company does not maintain the system or the contact center.
So who does manage the call center and website? “The AADA has asked stakeholders, including Syneos Health, for clarification on who manages the call center and website but has not received a response,” Dr. Frieden said. “In the meeting [Dec. 16], representatives from the FDA made clear that the iPLEDGE sponsors are ultimately responsible for this REMS program,” Dr. Frieden said.
According to the FDA, isotretinoin manufacturers are part of the iPLEDGE program. On the iPLEDGE website, 12 isotretinoin products are listed, made by eight different companies.
One dermatologist maneuvering the new website who registered successfully as a provider told this news organization that he received a follow-up survey from United BioSource about the new website. This news organization contacted that company to confirm it runs the website but has not yet received a response.
Meanwhile, dermatologists continue to help frustrated patients cope with the new website and registration details. Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, heard from two mothers who helped their teen daughters complete the forms by attesting they would use abstinence as contraception but then couldn’t figure out how to answer another question. As a result, their answers were interpreted as the patients saying they were using abstinence but didn’t commit to not having sexual contact with a partner capable of impregnating them. So Dr. Goldberg got an automated message back from the iPLEDGE program that the answers were a mismatch.
And in the comments section following a previous story on the problematic rollout, one reader offered a suggestion for reducing hold times to the call center: choose the Spanish option.
Dr. Frieden and Dr. Goldberg have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, according to dermatologists, pharmacists, and patients.
When the new website and call center launched Dec. 13, hours-long hold times and repeated crashing of the website were reported as the norm, not the exception, triggering the American Academy of Dermatology Association (AADA) to request – and get – an emergency meeting on Dec. 16 with the U.S. Food and Drug Administration, which mandates the risk evaluation and mitigation strategy (REMS) for isotretinoin due to the teratogenicity of the acne medication.
At that meeting, ‘’the FDA and HHS [U.S. Department of Health and Human Services] acknowledged the concerns of dermatologists and the need for stakeholders to work collaboratively to find a solution,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE workgroup and professor of dermatology at the University of California, San Francisco, said in an email interview. At the meeting, the AADA representatives described the severe impact on patient access to treatment that is resulting from the issues. The AADA also ‘’reiterated our call for a temporary pause to the program while stakeholders work to resolve the urgent issues with the platform,” she said.
The new approach, which is intended to make the experience more inclusive for transgender patients, reduces the previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) to just two (those capable of getting pregnant and those not capable). The program requires physicians, patients, and pharmacists who prescribe, use, or dispense the drug to be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by patients capable of becoming pregnant.
With reduced or no access during the technology glitches, access to the medicine was delayed for some patients. And dermatologists, pharmacists, and their staffs reported grueling hold times trying to reach the call center when the website had issues.
While the FDA agreed to help find a solution, it noted that the solution ‘’was to be found with dermatologists and pharmacists who are on the ground living the program every day,” Dr. Frieden said. No timeline for solving the issues was provided, so on Dec. 21, the AADA asked the FDA for a constructive dialogue among stakeholders within the next 24 hours, Dr. Frieden told this news organization.
While Dr. Frieden sees progress, ‘’we are disappointed that this situation continues to drag on for more than a week later, with more patients losing access to their needed medication each day.” While some prescribers have been able to log onto the portal and enter the information required, confirming some patients, large gaps remain, she said. Patients and pharmacists still report difficulties logging on. When that happens and they try to reach the call center, there are often hours-long hold times, dropped calls, or a message saying to call back.
The iPLEDGE administrator is Syneos Health, but a spokesperson for Syneos, Gary Gatyas, said the company does not maintain the system or the contact center.
So who does manage the call center and website? “The AADA has asked stakeholders, including Syneos Health, for clarification on who manages the call center and website but has not received a response,” Dr. Frieden said. “In the meeting [Dec. 16], representatives from the FDA made clear that the iPLEDGE sponsors are ultimately responsible for this REMS program,” Dr. Frieden said.
According to the FDA, isotretinoin manufacturers are part of the iPLEDGE program. On the iPLEDGE website, 12 isotretinoin products are listed, made by eight different companies.
One dermatologist maneuvering the new website who registered successfully as a provider told this news organization that he received a follow-up survey from United BioSource about the new website. This news organization contacted that company to confirm it runs the website but has not yet received a response.
Meanwhile, dermatologists continue to help frustrated patients cope with the new website and registration details. Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, heard from two mothers who helped their teen daughters complete the forms by attesting they would use abstinence as contraception but then couldn’t figure out how to answer another question. As a result, their answers were interpreted as the patients saying they were using abstinence but didn’t commit to not having sexual contact with a partner capable of impregnating them. So Dr. Goldberg got an automated message back from the iPLEDGE program that the answers were a mismatch.
And in the comments section following a previous story on the problematic rollout, one reader offered a suggestion for reducing hold times to the call center: choose the Spanish option.
Dr. Frieden and Dr. Goldberg have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Dermatologists take to TikTok to share their own ‘hacks’
A young woman is having her lip swabbed with an unknown substance, smiling, on the TikTok video. Seconds later, another young woman, wearing gloves, pushes a hyaluron pen against the first woman’s lips, who, in the next cut, is smiling, happy. “My first syringe down and already 1,000x more confident,” the caption reads.
That video is one of thousands showing hyaluron pen use on TikTok. The pens are sold online and are unapproved – which led to a Food and Drug Administration warning in October 2021 that use could cause bleeding, infection, blood vessel occlusion that could result in blindness or stroke, allergic reactions, and other injuries.
The warning has not stopped many TikTokkers, who also use the medium to promote all sorts of skin and aesthetic products and procedures, a large number unproven, unapproved, or ill advised. which, more often than not, comes from “skinfluencers,” aestheticians, and other laypeople, not board-certified dermatologists.
The suggested “hacks” can be harmless or ineffective, but they also can be misleading, fraudulent, or even dangerous.
Skinfluencers take the lead
TikTok has a reported 1 billion monthly users. Two-thirds are aged 10-29 years, according to data reported in February 2021 in the Journal of the American Academy of Dermatology by David X. Zheng, BA, and colleagues at Case Western Reserve University, Cleveland, and the department of dermatology, Johns Hopkins University, Baltimore.
Visitors consume information in video bits that run from 15 seconds to up to 3 minutes and can follow their favorite TikTokkers, browse for people or hashtags with a search function, or click on content recommended by the platform, which uses algorithms based on the user’s viewing habits to determine what might be of interest.
Some of the biggest “skinfluencers” have millions of followers: Hyram Yarbro, (@skincarebyhyram) for instance, has 6.6 million followers and his own line of skin care products at Sephora. Mr. Yarbro is seen as a no-nonsense debunker of skin care myths, as is British influencer James Welsh, who has 124,000 followers.
“The reason why people trust your average influencer person who’s not a doctor is because they’re relatable,” said Muneeb Shah, MD, a dermatology resident at Atlantic Dermatology in Wilmington, N.C. – known to his 11.4 million TikTok followers as @dermdoctor.
To Sandra Lee, MD, the popularity of nonprofessionals is easy to explain. “You have to think about the fact that a lot of people can’t see dermatologists – they don’t have the money, they don’t have the time to travel there, they don’t have health insurance, or they’re scared of doctors, so they’re willing to try to find an answer, and one of the easiest ways, one of the more entertaining ways to get information, is on social media.”
Dr. Lee is in private practice in Upland, Calif., but is better known as “Dr. Pimple Popper,” through her television show of the same name and her social media accounts, including on TikTok, where she has 14.4 million followers after having started in 2020.
“We’re all looking for that no-down-time, no-expense, no-lines, no-wrinkles, stay-young-forever magic bullet,” said Dr. Lee.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, agreed that people are looking for a quick fix. They don’t want to wait 12 weeks for an acne medication or 16 weeks for a biologic to work. “They want something simple, easy, do-it-yourself,” and “natural,” he said.
Laypeople are still the dominant producers – and have the most views – of dermatology content.
Morgan Nguyen, BA, at Northwestern University, Chicago, and colleagues looked at hashtags for the top 10 dermatologic diagnoses and procedures and analyzed the content of the first 40 TikTok videos in each category. About half the videos were produced by an individual, and 39% by a health care provider, according to the study, published in the International Journal of Women’s Dermatology. About 40% of the videos were educational, focusing on skin care, procedures, and disease treatment.
Viewership was highest for videos by laypeople, followed by those produced by business or industry accounts. Those produced by health care providers received only 18% of the views.
The most popular videos were about dermatologic diagnoses, with 2.5 billion views, followed by dermatologic procedures, with 708 million views.
Ms. Nguyen noted in the study that the most liked and most viewed posts were related to #skincare but that board-certified dermatologists produced only 2.5% of the #skincare videos.
Dermatologists take to TikTok
Some dermatologists have started their own TikTok accounts, seeking both to counteract misinformation and provide education.
Dr. Shah has become one of the top influencers on the platform. In a year-end wrap, TikTok put Dr. Shah at No. 7 on its top creators list for 2021.
The dermatology resident said that TikTok is a good tool for reaching patients who might not otherwise interact with dermatologists. He recounted the story of an individual who came into his office with the idea that they had hidradenitis suppurativa.
The person had self-diagnosed after seeing one of Dr. Shah’s TikTok videos on the condition. It was a pleasant surprise, said Dr. Shah. People with hidradenitis suppurativa often avoid treatment, and it’s underdiagnosed and improperly treated, despite an American Academy of Dermatology awareness campaign.
“Dermatologists on social media are almost like the communications department for dermatology,” Dr. Shah commented.
A key to making TikTok work to advance dermatologists’ goals is knowing what makes it unique.
Dr. Lee said she prefers it to Instagram, because TikTok’s algorithms and its younger-skewing audience help her reach a more specific audience.
The algorithm “creates a positive feedback loop in which popular content creators or viral trends are prioritized on the users’ homepages, in turn providing the creators of these videos with an even larger audience,” Mr. Zheng, of University Hospitals Cleveland Medical Center, and coauthors noted in their letter in the Journal of the American Academy of Dermatology.
TikTok also celebrates the everyday – someone doesn’t have to be a celebrity to make something go viral, said Dr. Lee. She believes that TikTok users are more accepting of average people with real problems – which helps when someone is TikTokking about a skin condition.
Doris Day, MD, who goes by @drdorisday on TikTok, agreed with Dr. Lee. “There are so many creative ways you can convey information with it that’s different than what you have on Instagram,” said Dr. Day, who is in private practice in New York. And, she added, “it does really lend itself to getting points out super-fast.”
Dermatologists on TikTok also said they like the “duets” and the “stitch” features, which allow users to add on to an existing video, essentially chiming in or responding to what might have already been posted, in a side-by-side format.
Dr. Shah said he often duets videos that have questionable content. “It allows me to directly respond to people. A lot of times, if something is going really viral and it’s not accurate, you’ll have a response from me or one of the other doctors” within hours or days.
Dr. Shah’s duets are labeled with “DermDoctor Reacts” or “DermDoctor Explains.” In one duet, with more than 2.8 million views, the upper half of the video is someone squeezing a blackhead, while Dr. Shah, in the bottom half, in green scrubs, opines over some hip-hop music: “This is just a blackhead. But once it gets to this point, they do need to be extracted because topical treatments won’t help.”
Dr. Lee – whose TikTok and other accounts capitalize on teens’ obsession with popping pimples – has a duet in which she advised that although popping will leave scars, there are more ideal times to pop, if they must. The duet has at least 21 million views.
Sometimes a TikTok video effectively takes on a trend without being a duet. Nurse practitioner Uy Dam (@uy.np) has a video that demonstrates the dangers of hyaluron pens. He uses both a pen and a needle to inject fluid into a block of jello. The pen delivers a scattershot load of differing depths, while the needle is exact. It’s visual and easy to understand and has at least 1.3 million views.
Still, TikTok, like other forms of social media, is full of misinformation and false accounts, including people who claim to be doctors. “It’s hard for the regular person, myself included, sometimes to be able to root through that and find out whether something is real or not,” said Dr. Lee.
Dr. Friedman said he’s concerned about the lack of accountability. A doctor could lose his or her license for promoting unproven cures, especially if they are harmful. But for influencers, “there’s no accountability for posting information that can actually hurt people.”
TikTok trends gone bad
And some people are being hurt by emulating what they see on TikTok.
Dr. Friedman had a patient with extreme irritant contact dermatitis, “almost like chemical burns to her underarms,” he said. He determined that she saw a video “hack” that recommended using baking soda to stop hyperhidrosis. The patient used so much that it burned her skin.
In 2020, do-it-yourself freckles – with henna or sewing needles impregnated with ink – went viral. Tilly Whitfeld, a 21-year-old reality TV star on Australia’s Big Brother show, told the New York Times that she tried it at home after seeing a TikTok video. She ordered brown tattoo ink online and later found out that it was contaminated with lead, according to the Times. Ms. Whitfeld developed an infection and temporary vision loss and has permanent scarring.
She has since put out a cautionary TikTok video that’s been viewed some 300,000 times.
TikTokkers have also flocked to the idea of using sunscreen to “contour” the face. Selected areas are left without sunscreen to burn or tan. In a duet, a plastic surgeon shakes his head as a young woman explains that “it works.”
Scalp-popping – in which the hair is yanked so hard that it pulls the galea off the skull – has been mostly shut down by TikTok. A search of “scalp popping” brings up the message: “Learn how to recognize harmful challenges and hoaxes.” At-home mole and skin tag removal, pimple-popping, and supposed acne cures such as drinking chlorophyll are all avidly documented and shared on TikTok.
Dr. Shah had a back-and-forth video dialog with someone who had stubbed a toe and then drilled a hole into the nail to drain the hematoma. In a reaction video, Dr. Shah said it was likely to turn into an infection. When it did, the man revealed the infection in a video where he tagged Dr. Shah and later posted a video at the podiatrist’s office having his nail removed, again tagging Dr. Shah.
“I think that pretty much no procedure for skin is good to do at home,” said Dr. Shah, who repeatedly admonishes against mole removal by a nonphysician. He tells followers that “it’s extremely dangerous – not only is it going to cause scarring, but you are potentially discarding a cancerous lesion.”
Unfortunately, most will not follow the advice, said Dr. Shah. That’s especially true of pimple-popping. Aiming for the least harm, he suggests in some TikTok videos that poppers keep the area clean, wear gloves, and consult a physician to get an antibiotic prescription. “You might as well at least guide them in the right direction,” he added.
Dr. Lee believes that lack of access to physicians, insurance, or money may play into how TikTok trends evolve. “Probably those people who injected their lips with this air gun thing, maybe they didn’t have the money necessarily to get filler,” she said.
Also, she noted, while TikTok may try to police its content, creators are incentivized to be outrageous. “The more inflammatory your post is, the more engagement you get.”
Dr. Shah thinks TikTok is self-correcting. “If you’re not being ethical or contradicting yourself, putting out information that’s not accurate, people are going to catch on very quickly,” he said. “The only value, the only currency you have on social media is the trust that you build with people that follow you.”
What it takes to be a TikTokker
For dermatologists, conveying their credentials and experience is one way to build that currency. Dr. Lee advised fellow doctors on TikTok to “showcase your training and how many years it took to become a dermatologist.”
Plunging into TikTok is not for everyone, though. It’s time consuming, said Dr. Lee, who now devotes most of her nonclinical time to TikTok. She creates her own content, leaving others to manage her Instagram account.
Many of those in the medical field who have dived into TikTok are residents, like Dr. Shah. “They are attuned to it and understand it more,” said Dr. Lee. “It’s harder for a lot of us who are older, who really weren’t involved that much in social media at all. It’s very hard to jump in.” There’s a learning curve, and it takes hours to create a single video. “You have to enjoy it and it has to be a part of your life,” she said.
Dr. Shah started experimenting with TikTok at the beginning of the pandemic in 2020 and has never turned back. Fast-talking, curious, and with an infectious sense of fun, he shares tidbits about his personal life – putting his wife in some of his videos – and always seems upbeat.
He said that, as his following grew, users began to see him as an authority figure and started “tagging” him more often, seeking his opinion on other videos. Although still a resident, he believes he has specialized knowledge to share. “Even if you’re not the world’s leading expert in a particular topic, you’re still adding value for the person who doesn’t know much.”
Dr. Shah also occasionally does promotional TikToks, identified as sponsored content. He said he only works with companies that he believes have legitimate products. “You do have to monetize at some point,” he said, noting that many dermatologists, himself included, are trading clinic time for TikTok. “There’s no universe where they can do this for free.”
Product endorsements are likely more rewarding for influencers and other users like Dr. Shah than the remuneration from TikTok, the company. The platform pays user accounts $20 per 1 million views, Dr. Shah said. “Financially, it’s not a big winner for a practicing dermatologist, but the educational outreach is worthwhile.”
To be successful also means understanding what drives viewership.
Using “trending” sounds has “been shown to increase the likelihood of a video amassing millions of views” and may increase engagement with dermatologists’ TikTok videos, wrote Bina Kassamali, BA, and colleagues at the Brigham and Women’s Hospital in Boston and the Ponce Health Science University School of Medicine in Ponce, Puerto Rico, in a letter published in the Journal of the American Academy of Dermatology in July 2021.
Certain content is more likely to engage viewers. In their analysis of top trending dermatologic hashtags, acne-related content was viewed 6.7 billion times, followed by alopecia, with 1.1 billion views. Psoriasis content had 84 million views, putting it eighth on the list of topics.
Dermatologists are still cracking TikTok. They are accumulating more followers on TikTok than on Instagram but have greater engagement on Instagram reels, wrote Mindy D. Szeto, MS, and colleagues at the University of Colorado at Denver, Aurora, and Rocky Vista University in Parker, Colo., in the Journal of the American Academy of Dermatology in April 2021.
Dr. Lee and Dr. Shah had the highest engagement rate on TikTok, according to Ms. Szeto. The engagement rate is calculated as (likes + comments per post)/(total followers) x 100.
“TikTok may currently be the leading avenue for audience education by dermatologist influencers,” they wrote, urging dermatologists to use the platform to answer the call as more of the public “continues to turn to social media for medical advice.”
Dr. Day said she will keep trying to build her TikTok audience. She has just 239 followers, compared with her 44,500 on Instagram. “The more I do TikTok, the more I do any of these mediums, the better I get at it,” she said. “We just have to put a little time and effort into it and try to get more followers and just keep sharing the information.”
Dr. Friedman sees it as a positive that some dermatologists have taken to TikTok to dispel myths and put “good information out there in small bites.” But to be more effective, they need more followers.
“The truth is that 14-year-old is probably going to listen more to a Hyram than a dermatologist,” he said. “Maybe we need to work with these other individuals who know how to take these messages and convert them to a language that can be digested by a 14-year-old, by a 12-year-old, by a 23-year-old. We need to come to the table together and not fight.”
A version of this article first appeared on Medscape.com.
A young woman is having her lip swabbed with an unknown substance, smiling, on the TikTok video. Seconds later, another young woman, wearing gloves, pushes a hyaluron pen against the first woman’s lips, who, in the next cut, is smiling, happy. “My first syringe down and already 1,000x more confident,” the caption reads.
That video is one of thousands showing hyaluron pen use on TikTok. The pens are sold online and are unapproved – which led to a Food and Drug Administration warning in October 2021 that use could cause bleeding, infection, blood vessel occlusion that could result in blindness or stroke, allergic reactions, and other injuries.
The warning has not stopped many TikTokkers, who also use the medium to promote all sorts of skin and aesthetic products and procedures, a large number unproven, unapproved, or ill advised. which, more often than not, comes from “skinfluencers,” aestheticians, and other laypeople, not board-certified dermatologists.
The suggested “hacks” can be harmless or ineffective, but they also can be misleading, fraudulent, or even dangerous.
Skinfluencers take the lead
TikTok has a reported 1 billion monthly users. Two-thirds are aged 10-29 years, according to data reported in February 2021 in the Journal of the American Academy of Dermatology by David X. Zheng, BA, and colleagues at Case Western Reserve University, Cleveland, and the department of dermatology, Johns Hopkins University, Baltimore.
Visitors consume information in video bits that run from 15 seconds to up to 3 minutes and can follow their favorite TikTokkers, browse for people or hashtags with a search function, or click on content recommended by the platform, which uses algorithms based on the user’s viewing habits to determine what might be of interest.
Some of the biggest “skinfluencers” have millions of followers: Hyram Yarbro, (@skincarebyhyram) for instance, has 6.6 million followers and his own line of skin care products at Sephora. Mr. Yarbro is seen as a no-nonsense debunker of skin care myths, as is British influencer James Welsh, who has 124,000 followers.
“The reason why people trust your average influencer person who’s not a doctor is because they’re relatable,” said Muneeb Shah, MD, a dermatology resident at Atlantic Dermatology in Wilmington, N.C. – known to his 11.4 million TikTok followers as @dermdoctor.
To Sandra Lee, MD, the popularity of nonprofessionals is easy to explain. “You have to think about the fact that a lot of people can’t see dermatologists – they don’t have the money, they don’t have the time to travel there, they don’t have health insurance, or they’re scared of doctors, so they’re willing to try to find an answer, and one of the easiest ways, one of the more entertaining ways to get information, is on social media.”
Dr. Lee is in private practice in Upland, Calif., but is better known as “Dr. Pimple Popper,” through her television show of the same name and her social media accounts, including on TikTok, where she has 14.4 million followers after having started in 2020.
“We’re all looking for that no-down-time, no-expense, no-lines, no-wrinkles, stay-young-forever magic bullet,” said Dr. Lee.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, agreed that people are looking for a quick fix. They don’t want to wait 12 weeks for an acne medication or 16 weeks for a biologic to work. “They want something simple, easy, do-it-yourself,” and “natural,” he said.
Laypeople are still the dominant producers – and have the most views – of dermatology content.
Morgan Nguyen, BA, at Northwestern University, Chicago, and colleagues looked at hashtags for the top 10 dermatologic diagnoses and procedures and analyzed the content of the first 40 TikTok videos in each category. About half the videos were produced by an individual, and 39% by a health care provider, according to the study, published in the International Journal of Women’s Dermatology. About 40% of the videos were educational, focusing on skin care, procedures, and disease treatment.
Viewership was highest for videos by laypeople, followed by those produced by business or industry accounts. Those produced by health care providers received only 18% of the views.
The most popular videos were about dermatologic diagnoses, with 2.5 billion views, followed by dermatologic procedures, with 708 million views.
Ms. Nguyen noted in the study that the most liked and most viewed posts were related to #skincare but that board-certified dermatologists produced only 2.5% of the #skincare videos.
Dermatologists take to TikTok
Some dermatologists have started their own TikTok accounts, seeking both to counteract misinformation and provide education.
Dr. Shah has become one of the top influencers on the platform. In a year-end wrap, TikTok put Dr. Shah at No. 7 on its top creators list for 2021.
The dermatology resident said that TikTok is a good tool for reaching patients who might not otherwise interact with dermatologists. He recounted the story of an individual who came into his office with the idea that they had hidradenitis suppurativa.
The person had self-diagnosed after seeing one of Dr. Shah’s TikTok videos on the condition. It was a pleasant surprise, said Dr. Shah. People with hidradenitis suppurativa often avoid treatment, and it’s underdiagnosed and improperly treated, despite an American Academy of Dermatology awareness campaign.
“Dermatologists on social media are almost like the communications department for dermatology,” Dr. Shah commented.
A key to making TikTok work to advance dermatologists’ goals is knowing what makes it unique.
Dr. Lee said she prefers it to Instagram, because TikTok’s algorithms and its younger-skewing audience help her reach a more specific audience.
The algorithm “creates a positive feedback loop in which popular content creators or viral trends are prioritized on the users’ homepages, in turn providing the creators of these videos with an even larger audience,” Mr. Zheng, of University Hospitals Cleveland Medical Center, and coauthors noted in their letter in the Journal of the American Academy of Dermatology.
TikTok also celebrates the everyday – someone doesn’t have to be a celebrity to make something go viral, said Dr. Lee. She believes that TikTok users are more accepting of average people with real problems – which helps when someone is TikTokking about a skin condition.
Doris Day, MD, who goes by @drdorisday on TikTok, agreed with Dr. Lee. “There are so many creative ways you can convey information with it that’s different than what you have on Instagram,” said Dr. Day, who is in private practice in New York. And, she added, “it does really lend itself to getting points out super-fast.”
Dermatologists on TikTok also said they like the “duets” and the “stitch” features, which allow users to add on to an existing video, essentially chiming in or responding to what might have already been posted, in a side-by-side format.
Dr. Shah said he often duets videos that have questionable content. “It allows me to directly respond to people. A lot of times, if something is going really viral and it’s not accurate, you’ll have a response from me or one of the other doctors” within hours or days.
Dr. Shah’s duets are labeled with “DermDoctor Reacts” or “DermDoctor Explains.” In one duet, with more than 2.8 million views, the upper half of the video is someone squeezing a blackhead, while Dr. Shah, in the bottom half, in green scrubs, opines over some hip-hop music: “This is just a blackhead. But once it gets to this point, they do need to be extracted because topical treatments won’t help.”
Dr. Lee – whose TikTok and other accounts capitalize on teens’ obsession with popping pimples – has a duet in which she advised that although popping will leave scars, there are more ideal times to pop, if they must. The duet has at least 21 million views.
Sometimes a TikTok video effectively takes on a trend without being a duet. Nurse practitioner Uy Dam (@uy.np) has a video that demonstrates the dangers of hyaluron pens. He uses both a pen and a needle to inject fluid into a block of jello. The pen delivers a scattershot load of differing depths, while the needle is exact. It’s visual and easy to understand and has at least 1.3 million views.
Still, TikTok, like other forms of social media, is full of misinformation and false accounts, including people who claim to be doctors. “It’s hard for the regular person, myself included, sometimes to be able to root through that and find out whether something is real or not,” said Dr. Lee.
Dr. Friedman said he’s concerned about the lack of accountability. A doctor could lose his or her license for promoting unproven cures, especially if they are harmful. But for influencers, “there’s no accountability for posting information that can actually hurt people.”
TikTok trends gone bad
And some people are being hurt by emulating what they see on TikTok.
Dr. Friedman had a patient with extreme irritant contact dermatitis, “almost like chemical burns to her underarms,” he said. He determined that she saw a video “hack” that recommended using baking soda to stop hyperhidrosis. The patient used so much that it burned her skin.
In 2020, do-it-yourself freckles – with henna or sewing needles impregnated with ink – went viral. Tilly Whitfeld, a 21-year-old reality TV star on Australia’s Big Brother show, told the New York Times that she tried it at home after seeing a TikTok video. She ordered brown tattoo ink online and later found out that it was contaminated with lead, according to the Times. Ms. Whitfeld developed an infection and temporary vision loss and has permanent scarring.
She has since put out a cautionary TikTok video that’s been viewed some 300,000 times.
TikTokkers have also flocked to the idea of using sunscreen to “contour” the face. Selected areas are left without sunscreen to burn or tan. In a duet, a plastic surgeon shakes his head as a young woman explains that “it works.”
Scalp-popping – in which the hair is yanked so hard that it pulls the galea off the skull – has been mostly shut down by TikTok. A search of “scalp popping” brings up the message: “Learn how to recognize harmful challenges and hoaxes.” At-home mole and skin tag removal, pimple-popping, and supposed acne cures such as drinking chlorophyll are all avidly documented and shared on TikTok.
Dr. Shah had a back-and-forth video dialog with someone who had stubbed a toe and then drilled a hole into the nail to drain the hematoma. In a reaction video, Dr. Shah said it was likely to turn into an infection. When it did, the man revealed the infection in a video where he tagged Dr. Shah and later posted a video at the podiatrist’s office having his nail removed, again tagging Dr. Shah.
“I think that pretty much no procedure for skin is good to do at home,” said Dr. Shah, who repeatedly admonishes against mole removal by a nonphysician. He tells followers that “it’s extremely dangerous – not only is it going to cause scarring, but you are potentially discarding a cancerous lesion.”
Unfortunately, most will not follow the advice, said Dr. Shah. That’s especially true of pimple-popping. Aiming for the least harm, he suggests in some TikTok videos that poppers keep the area clean, wear gloves, and consult a physician to get an antibiotic prescription. “You might as well at least guide them in the right direction,” he added.
Dr. Lee believes that lack of access to physicians, insurance, or money may play into how TikTok trends evolve. “Probably those people who injected their lips with this air gun thing, maybe they didn’t have the money necessarily to get filler,” she said.
Also, she noted, while TikTok may try to police its content, creators are incentivized to be outrageous. “The more inflammatory your post is, the more engagement you get.”
Dr. Shah thinks TikTok is self-correcting. “If you’re not being ethical or contradicting yourself, putting out information that’s not accurate, people are going to catch on very quickly,” he said. “The only value, the only currency you have on social media is the trust that you build with people that follow you.”
What it takes to be a TikTokker
For dermatologists, conveying their credentials and experience is one way to build that currency. Dr. Lee advised fellow doctors on TikTok to “showcase your training and how many years it took to become a dermatologist.”
Plunging into TikTok is not for everyone, though. It’s time consuming, said Dr. Lee, who now devotes most of her nonclinical time to TikTok. She creates her own content, leaving others to manage her Instagram account.
Many of those in the medical field who have dived into TikTok are residents, like Dr. Shah. “They are attuned to it and understand it more,” said Dr. Lee. “It’s harder for a lot of us who are older, who really weren’t involved that much in social media at all. It’s very hard to jump in.” There’s a learning curve, and it takes hours to create a single video. “You have to enjoy it and it has to be a part of your life,” she said.
Dr. Shah started experimenting with TikTok at the beginning of the pandemic in 2020 and has never turned back. Fast-talking, curious, and with an infectious sense of fun, he shares tidbits about his personal life – putting his wife in some of his videos – and always seems upbeat.
He said that, as his following grew, users began to see him as an authority figure and started “tagging” him more often, seeking his opinion on other videos. Although still a resident, he believes he has specialized knowledge to share. “Even if you’re not the world’s leading expert in a particular topic, you’re still adding value for the person who doesn’t know much.”
Dr. Shah also occasionally does promotional TikToks, identified as sponsored content. He said he only works with companies that he believes have legitimate products. “You do have to monetize at some point,” he said, noting that many dermatologists, himself included, are trading clinic time for TikTok. “There’s no universe where they can do this for free.”
Product endorsements are likely more rewarding for influencers and other users like Dr. Shah than the remuneration from TikTok, the company. The platform pays user accounts $20 per 1 million views, Dr. Shah said. “Financially, it’s not a big winner for a practicing dermatologist, but the educational outreach is worthwhile.”
To be successful also means understanding what drives viewership.
Using “trending” sounds has “been shown to increase the likelihood of a video amassing millions of views” and may increase engagement with dermatologists’ TikTok videos, wrote Bina Kassamali, BA, and colleagues at the Brigham and Women’s Hospital in Boston and the Ponce Health Science University School of Medicine in Ponce, Puerto Rico, in a letter published in the Journal of the American Academy of Dermatology in July 2021.
Certain content is more likely to engage viewers. In their analysis of top trending dermatologic hashtags, acne-related content was viewed 6.7 billion times, followed by alopecia, with 1.1 billion views. Psoriasis content had 84 million views, putting it eighth on the list of topics.
Dermatologists are still cracking TikTok. They are accumulating more followers on TikTok than on Instagram but have greater engagement on Instagram reels, wrote Mindy D. Szeto, MS, and colleagues at the University of Colorado at Denver, Aurora, and Rocky Vista University in Parker, Colo., in the Journal of the American Academy of Dermatology in April 2021.
Dr. Lee and Dr. Shah had the highest engagement rate on TikTok, according to Ms. Szeto. The engagement rate is calculated as (likes + comments per post)/(total followers) x 100.
“TikTok may currently be the leading avenue for audience education by dermatologist influencers,” they wrote, urging dermatologists to use the platform to answer the call as more of the public “continues to turn to social media for medical advice.”
Dr. Day said she will keep trying to build her TikTok audience. She has just 239 followers, compared with her 44,500 on Instagram. “The more I do TikTok, the more I do any of these mediums, the better I get at it,” she said. “We just have to put a little time and effort into it and try to get more followers and just keep sharing the information.”
Dr. Friedman sees it as a positive that some dermatologists have taken to TikTok to dispel myths and put “good information out there in small bites.” But to be more effective, they need more followers.
“The truth is that 14-year-old is probably going to listen more to a Hyram than a dermatologist,” he said. “Maybe we need to work with these other individuals who know how to take these messages and convert them to a language that can be digested by a 14-year-old, by a 12-year-old, by a 23-year-old. We need to come to the table together and not fight.”
A version of this article first appeared on Medscape.com.
A young woman is having her lip swabbed with an unknown substance, smiling, on the TikTok video. Seconds later, another young woman, wearing gloves, pushes a hyaluron pen against the first woman’s lips, who, in the next cut, is smiling, happy. “My first syringe down and already 1,000x more confident,” the caption reads.
That video is one of thousands showing hyaluron pen use on TikTok. The pens are sold online and are unapproved – which led to a Food and Drug Administration warning in October 2021 that use could cause bleeding, infection, blood vessel occlusion that could result in blindness or stroke, allergic reactions, and other injuries.
The warning has not stopped many TikTokkers, who also use the medium to promote all sorts of skin and aesthetic products and procedures, a large number unproven, unapproved, or ill advised. which, more often than not, comes from “skinfluencers,” aestheticians, and other laypeople, not board-certified dermatologists.
The suggested “hacks” can be harmless or ineffective, but they also can be misleading, fraudulent, or even dangerous.
Skinfluencers take the lead
TikTok has a reported 1 billion monthly users. Two-thirds are aged 10-29 years, according to data reported in February 2021 in the Journal of the American Academy of Dermatology by David X. Zheng, BA, and colleagues at Case Western Reserve University, Cleveland, and the department of dermatology, Johns Hopkins University, Baltimore.
Visitors consume information in video bits that run from 15 seconds to up to 3 minutes and can follow their favorite TikTokkers, browse for people or hashtags with a search function, or click on content recommended by the platform, which uses algorithms based on the user’s viewing habits to determine what might be of interest.
Some of the biggest “skinfluencers” have millions of followers: Hyram Yarbro, (@skincarebyhyram) for instance, has 6.6 million followers and his own line of skin care products at Sephora. Mr. Yarbro is seen as a no-nonsense debunker of skin care myths, as is British influencer James Welsh, who has 124,000 followers.
“The reason why people trust your average influencer person who’s not a doctor is because they’re relatable,” said Muneeb Shah, MD, a dermatology resident at Atlantic Dermatology in Wilmington, N.C. – known to his 11.4 million TikTok followers as @dermdoctor.
To Sandra Lee, MD, the popularity of nonprofessionals is easy to explain. “You have to think about the fact that a lot of people can’t see dermatologists – they don’t have the money, they don’t have the time to travel there, they don’t have health insurance, or they’re scared of doctors, so they’re willing to try to find an answer, and one of the easiest ways, one of the more entertaining ways to get information, is on social media.”
Dr. Lee is in private practice in Upland, Calif., but is better known as “Dr. Pimple Popper,” through her television show of the same name and her social media accounts, including on TikTok, where she has 14.4 million followers after having started in 2020.
“We’re all looking for that no-down-time, no-expense, no-lines, no-wrinkles, stay-young-forever magic bullet,” said Dr. Lee.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, agreed that people are looking for a quick fix. They don’t want to wait 12 weeks for an acne medication or 16 weeks for a biologic to work. “They want something simple, easy, do-it-yourself,” and “natural,” he said.
Laypeople are still the dominant producers – and have the most views – of dermatology content.
Morgan Nguyen, BA, at Northwestern University, Chicago, and colleagues looked at hashtags for the top 10 dermatologic diagnoses and procedures and analyzed the content of the first 40 TikTok videos in each category. About half the videos were produced by an individual, and 39% by a health care provider, according to the study, published in the International Journal of Women’s Dermatology. About 40% of the videos were educational, focusing on skin care, procedures, and disease treatment.
Viewership was highest for videos by laypeople, followed by those produced by business or industry accounts. Those produced by health care providers received only 18% of the views.
The most popular videos were about dermatologic diagnoses, with 2.5 billion views, followed by dermatologic procedures, with 708 million views.
Ms. Nguyen noted in the study that the most liked and most viewed posts were related to #skincare but that board-certified dermatologists produced only 2.5% of the #skincare videos.
Dermatologists take to TikTok
Some dermatologists have started their own TikTok accounts, seeking both to counteract misinformation and provide education.
Dr. Shah has become one of the top influencers on the platform. In a year-end wrap, TikTok put Dr. Shah at No. 7 on its top creators list for 2021.
The dermatology resident said that TikTok is a good tool for reaching patients who might not otherwise interact with dermatologists. He recounted the story of an individual who came into his office with the idea that they had hidradenitis suppurativa.
The person had self-diagnosed after seeing one of Dr. Shah’s TikTok videos on the condition. It was a pleasant surprise, said Dr. Shah. People with hidradenitis suppurativa often avoid treatment, and it’s underdiagnosed and improperly treated, despite an American Academy of Dermatology awareness campaign.
“Dermatologists on social media are almost like the communications department for dermatology,” Dr. Shah commented.
A key to making TikTok work to advance dermatologists’ goals is knowing what makes it unique.
Dr. Lee said she prefers it to Instagram, because TikTok’s algorithms and its younger-skewing audience help her reach a more specific audience.
The algorithm “creates a positive feedback loop in which popular content creators or viral trends are prioritized on the users’ homepages, in turn providing the creators of these videos with an even larger audience,” Mr. Zheng, of University Hospitals Cleveland Medical Center, and coauthors noted in their letter in the Journal of the American Academy of Dermatology.
TikTok also celebrates the everyday – someone doesn’t have to be a celebrity to make something go viral, said Dr. Lee. She believes that TikTok users are more accepting of average people with real problems – which helps when someone is TikTokking about a skin condition.
Doris Day, MD, who goes by @drdorisday on TikTok, agreed with Dr. Lee. “There are so many creative ways you can convey information with it that’s different than what you have on Instagram,” said Dr. Day, who is in private practice in New York. And, she added, “it does really lend itself to getting points out super-fast.”
Dermatologists on TikTok also said they like the “duets” and the “stitch” features, which allow users to add on to an existing video, essentially chiming in or responding to what might have already been posted, in a side-by-side format.
Dr. Shah said he often duets videos that have questionable content. “It allows me to directly respond to people. A lot of times, if something is going really viral and it’s not accurate, you’ll have a response from me or one of the other doctors” within hours or days.
Dr. Shah’s duets are labeled with “DermDoctor Reacts” or “DermDoctor Explains.” In one duet, with more than 2.8 million views, the upper half of the video is someone squeezing a blackhead, while Dr. Shah, in the bottom half, in green scrubs, opines over some hip-hop music: “This is just a blackhead. But once it gets to this point, they do need to be extracted because topical treatments won’t help.”
Dr. Lee – whose TikTok and other accounts capitalize on teens’ obsession with popping pimples – has a duet in which she advised that although popping will leave scars, there are more ideal times to pop, if they must. The duet has at least 21 million views.
Sometimes a TikTok video effectively takes on a trend without being a duet. Nurse practitioner Uy Dam (@uy.np) has a video that demonstrates the dangers of hyaluron pens. He uses both a pen and a needle to inject fluid into a block of jello. The pen delivers a scattershot load of differing depths, while the needle is exact. It’s visual and easy to understand and has at least 1.3 million views.
Still, TikTok, like other forms of social media, is full of misinformation and false accounts, including people who claim to be doctors. “It’s hard for the regular person, myself included, sometimes to be able to root through that and find out whether something is real or not,” said Dr. Lee.
Dr. Friedman said he’s concerned about the lack of accountability. A doctor could lose his or her license for promoting unproven cures, especially if they are harmful. But for influencers, “there’s no accountability for posting information that can actually hurt people.”
TikTok trends gone bad
And some people are being hurt by emulating what they see on TikTok.
Dr. Friedman had a patient with extreme irritant contact dermatitis, “almost like chemical burns to her underarms,” he said. He determined that she saw a video “hack” that recommended using baking soda to stop hyperhidrosis. The patient used so much that it burned her skin.
In 2020, do-it-yourself freckles – with henna or sewing needles impregnated with ink – went viral. Tilly Whitfeld, a 21-year-old reality TV star on Australia’s Big Brother show, told the New York Times that she tried it at home after seeing a TikTok video. She ordered brown tattoo ink online and later found out that it was contaminated with lead, according to the Times. Ms. Whitfeld developed an infection and temporary vision loss and has permanent scarring.
She has since put out a cautionary TikTok video that’s been viewed some 300,000 times.
TikTokkers have also flocked to the idea of using sunscreen to “contour” the face. Selected areas are left without sunscreen to burn or tan. In a duet, a plastic surgeon shakes his head as a young woman explains that “it works.”
Scalp-popping – in which the hair is yanked so hard that it pulls the galea off the skull – has been mostly shut down by TikTok. A search of “scalp popping” brings up the message: “Learn how to recognize harmful challenges and hoaxes.” At-home mole and skin tag removal, pimple-popping, and supposed acne cures such as drinking chlorophyll are all avidly documented and shared on TikTok.
Dr. Shah had a back-and-forth video dialog with someone who had stubbed a toe and then drilled a hole into the nail to drain the hematoma. In a reaction video, Dr. Shah said it was likely to turn into an infection. When it did, the man revealed the infection in a video where he tagged Dr. Shah and later posted a video at the podiatrist’s office having his nail removed, again tagging Dr. Shah.
“I think that pretty much no procedure for skin is good to do at home,” said Dr. Shah, who repeatedly admonishes against mole removal by a nonphysician. He tells followers that “it’s extremely dangerous – not only is it going to cause scarring, but you are potentially discarding a cancerous lesion.”
Unfortunately, most will not follow the advice, said Dr. Shah. That’s especially true of pimple-popping. Aiming for the least harm, he suggests in some TikTok videos that poppers keep the area clean, wear gloves, and consult a physician to get an antibiotic prescription. “You might as well at least guide them in the right direction,” he added.
Dr. Lee believes that lack of access to physicians, insurance, or money may play into how TikTok trends evolve. “Probably those people who injected their lips with this air gun thing, maybe they didn’t have the money necessarily to get filler,” she said.
Also, she noted, while TikTok may try to police its content, creators are incentivized to be outrageous. “The more inflammatory your post is, the more engagement you get.”
Dr. Shah thinks TikTok is self-correcting. “If you’re not being ethical or contradicting yourself, putting out information that’s not accurate, people are going to catch on very quickly,” he said. “The only value, the only currency you have on social media is the trust that you build with people that follow you.”
What it takes to be a TikTokker
For dermatologists, conveying their credentials and experience is one way to build that currency. Dr. Lee advised fellow doctors on TikTok to “showcase your training and how many years it took to become a dermatologist.”
Plunging into TikTok is not for everyone, though. It’s time consuming, said Dr. Lee, who now devotes most of her nonclinical time to TikTok. She creates her own content, leaving others to manage her Instagram account.
Many of those in the medical field who have dived into TikTok are residents, like Dr. Shah. “They are attuned to it and understand it more,” said Dr. Lee. “It’s harder for a lot of us who are older, who really weren’t involved that much in social media at all. It’s very hard to jump in.” There’s a learning curve, and it takes hours to create a single video. “You have to enjoy it and it has to be a part of your life,” she said.
Dr. Shah started experimenting with TikTok at the beginning of the pandemic in 2020 and has never turned back. Fast-talking, curious, and with an infectious sense of fun, he shares tidbits about his personal life – putting his wife in some of his videos – and always seems upbeat.
He said that, as his following grew, users began to see him as an authority figure and started “tagging” him more often, seeking his opinion on other videos. Although still a resident, he believes he has specialized knowledge to share. “Even if you’re not the world’s leading expert in a particular topic, you’re still adding value for the person who doesn’t know much.”
Dr. Shah also occasionally does promotional TikToks, identified as sponsored content. He said he only works with companies that he believes have legitimate products. “You do have to monetize at some point,” he said, noting that many dermatologists, himself included, are trading clinic time for TikTok. “There’s no universe where they can do this for free.”
Product endorsements are likely more rewarding for influencers and other users like Dr. Shah than the remuneration from TikTok, the company. The platform pays user accounts $20 per 1 million views, Dr. Shah said. “Financially, it’s not a big winner for a practicing dermatologist, but the educational outreach is worthwhile.”
To be successful also means understanding what drives viewership.
Using “trending” sounds has “been shown to increase the likelihood of a video amassing millions of views” and may increase engagement with dermatologists’ TikTok videos, wrote Bina Kassamali, BA, and colleagues at the Brigham and Women’s Hospital in Boston and the Ponce Health Science University School of Medicine in Ponce, Puerto Rico, in a letter published in the Journal of the American Academy of Dermatology in July 2021.
Certain content is more likely to engage viewers. In their analysis of top trending dermatologic hashtags, acne-related content was viewed 6.7 billion times, followed by alopecia, with 1.1 billion views. Psoriasis content had 84 million views, putting it eighth on the list of topics.
Dermatologists are still cracking TikTok. They are accumulating more followers on TikTok than on Instagram but have greater engagement on Instagram reels, wrote Mindy D. Szeto, MS, and colleagues at the University of Colorado at Denver, Aurora, and Rocky Vista University in Parker, Colo., in the Journal of the American Academy of Dermatology in April 2021.
Dr. Lee and Dr. Shah had the highest engagement rate on TikTok, according to Ms. Szeto. The engagement rate is calculated as (likes + comments per post)/(total followers) x 100.
“TikTok may currently be the leading avenue for audience education by dermatologist influencers,” they wrote, urging dermatologists to use the platform to answer the call as more of the public “continues to turn to social media for medical advice.”
Dr. Day said she will keep trying to build her TikTok audience. She has just 239 followers, compared with her 44,500 on Instagram. “The more I do TikTok, the more I do any of these mediums, the better I get at it,” she said. “We just have to put a little time and effort into it and try to get more followers and just keep sharing the information.”
Dr. Friedman sees it as a positive that some dermatologists have taken to TikTok to dispel myths and put “good information out there in small bites.” But to be more effective, they need more followers.
“The truth is that 14-year-old is probably going to listen more to a Hyram than a dermatologist,” he said. “Maybe we need to work with these other individuals who know how to take these messages and convert them to a language that can be digested by a 14-year-old, by a 12-year-old, by a 23-year-old. We need to come to the table together and not fight.”
A version of this article first appeared on Medscape.com.
iPLEDGE rollout described as a failure, chaotic, and a disaster
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
International panel backs energy-based devices as first-line treatment of acne scars
International consensus .
Peter R. Shumaker, MD, a dermatologist and dermatologic surgeon at the VA San Diego Healthcare System and one of the authors of the paper, noted that a panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the management of acne scarring.
“The advent of fractional laser technology in the mid-2000s ushered in an exciting new period of exploration and advances in scar treatment with EBDs,” Dr. Shumaker said in an interview. “Despite intense interest and a wealth of available literature, international treatment guidelines and patient access to these potentially life-changing treatments are currently lagging behind our capabilities.”
One of the key recommendations of the paper is that EBDs should have an expanded role in the treatment of acne scars, according to Dr. Shumaker, associate clinical professor of dermatology at the University of California, San Diego. “Panel members were unanimous in their view that EBDs, particularly ablative and nonablative fractional lasers, vascular lasers, and fractional radiofrequency devices, have an important role in the management of acne scars and should be considered a first-line treatment for a variety of scar types,” he said.
The process leading to the recommendations included developing clinical questions, based on input from the panelists and a literature review, and using a two-step modified Delphi method, “an iterative process used to achieve consensus for a defined clinical problem where there is little or conflicting published evidence and where expert opinion is decisive,” the authors wrote. This involved email questionnaires highlighting different topics, including the role of EBDs in mitigating and treating acne scars in patients with active acne, the use of different EBDs for treating different types of acne scars, and considerations in treating skin of color.
The panel noted considerations in the treatment of acne scars in skin of color. “Regardless of the platform, patients with darker skin types may require treatment modifications including: a reduction in fluence/pulse energy; decreased microcolumn density; greater intervals between treatments; longer pulse durations; epidermal cooling with fastidious technique to ensure appropriate cooling, additional cooling in between passes to decrease bulk heating; and pretreatment and posttreatment topical regimens (e.g., retinoids, bleaching creams, etc.) and strict sun precautions,” wrote the authors.
Panelists agreed that there is an absence of large, well-controlled, multicenter comparative trials of various laser and energy treatments for acne scars. “Such trials would be helpful in establishing the relative utility and persistence of benefit of various laser treatments and also in comparing their effectiveness versus that of nonenergy treatments,” the authors noted.
Asked to comment on the paper, Andrei Metelitsa, MD, a dermatologist in Calgary, Alta., and clinical associate professor at the University of Calgary, said the consensus recommendations on EBDs in acne scarring are “providing an international expert perspective, potentially changing a long-perceived paradigm of treatments.”
Dr. Metelitsa pointed out that the authors are taking a solid position with respect to reducing the delay to initiation of laser treatment following isotretinoin therapy. “The authors take a strong stance against the old dogma of postponing laser resurfacing for at least 6 months post isotretinoin,” he said. “According to the authors, there is sufficient evidence to support the idea of safely starting laser therapies, including fractional ablative and nonablative, within 1 month post isotretinoin, much sooner than previously suggested.”
He added that the authors point to the fact most experts utilize vascular lasers, such as pulsed-dye, to treat active acne in combination with medical therapy, thus reducing duration and severity of inflammation and potentially reducing further scar formation. “According to this published consensus, such laser therapies can even be used while the patient is actively treated with isotretinoin,” he said.
Dr. Metelitsa noted that the consensus recommendations outline how the choice of device should be guided by the clinical subtype of acne scars.
Dr. Shumaker, Dr. Metelitsa, and the authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
International consensus .
Peter R. Shumaker, MD, a dermatologist and dermatologic surgeon at the VA San Diego Healthcare System and one of the authors of the paper, noted that a panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the management of acne scarring.
“The advent of fractional laser technology in the mid-2000s ushered in an exciting new period of exploration and advances in scar treatment with EBDs,” Dr. Shumaker said in an interview. “Despite intense interest and a wealth of available literature, international treatment guidelines and patient access to these potentially life-changing treatments are currently lagging behind our capabilities.”
One of the key recommendations of the paper is that EBDs should have an expanded role in the treatment of acne scars, according to Dr. Shumaker, associate clinical professor of dermatology at the University of California, San Diego. “Panel members were unanimous in their view that EBDs, particularly ablative and nonablative fractional lasers, vascular lasers, and fractional radiofrequency devices, have an important role in the management of acne scars and should be considered a first-line treatment for a variety of scar types,” he said.
The process leading to the recommendations included developing clinical questions, based on input from the panelists and a literature review, and using a two-step modified Delphi method, “an iterative process used to achieve consensus for a defined clinical problem where there is little or conflicting published evidence and where expert opinion is decisive,” the authors wrote. This involved email questionnaires highlighting different topics, including the role of EBDs in mitigating and treating acne scars in patients with active acne, the use of different EBDs for treating different types of acne scars, and considerations in treating skin of color.
The panel noted considerations in the treatment of acne scars in skin of color. “Regardless of the platform, patients with darker skin types may require treatment modifications including: a reduction in fluence/pulse energy; decreased microcolumn density; greater intervals between treatments; longer pulse durations; epidermal cooling with fastidious technique to ensure appropriate cooling, additional cooling in between passes to decrease bulk heating; and pretreatment and posttreatment topical regimens (e.g., retinoids, bleaching creams, etc.) and strict sun precautions,” wrote the authors.
Panelists agreed that there is an absence of large, well-controlled, multicenter comparative trials of various laser and energy treatments for acne scars. “Such trials would be helpful in establishing the relative utility and persistence of benefit of various laser treatments and also in comparing their effectiveness versus that of nonenergy treatments,” the authors noted.
Asked to comment on the paper, Andrei Metelitsa, MD, a dermatologist in Calgary, Alta., and clinical associate professor at the University of Calgary, said the consensus recommendations on EBDs in acne scarring are “providing an international expert perspective, potentially changing a long-perceived paradigm of treatments.”
Dr. Metelitsa pointed out that the authors are taking a solid position with respect to reducing the delay to initiation of laser treatment following isotretinoin therapy. “The authors take a strong stance against the old dogma of postponing laser resurfacing for at least 6 months post isotretinoin,” he said. “According to the authors, there is sufficient evidence to support the idea of safely starting laser therapies, including fractional ablative and nonablative, within 1 month post isotretinoin, much sooner than previously suggested.”
He added that the authors point to the fact most experts utilize vascular lasers, such as pulsed-dye, to treat active acne in combination with medical therapy, thus reducing duration and severity of inflammation and potentially reducing further scar formation. “According to this published consensus, such laser therapies can even be used while the patient is actively treated with isotretinoin,” he said.
Dr. Metelitsa noted that the consensus recommendations outline how the choice of device should be guided by the clinical subtype of acne scars.
Dr. Shumaker, Dr. Metelitsa, and the authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
International consensus .
Peter R. Shumaker, MD, a dermatologist and dermatologic surgeon at the VA San Diego Healthcare System and one of the authors of the paper, noted that a panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the management of acne scarring.
“The advent of fractional laser technology in the mid-2000s ushered in an exciting new period of exploration and advances in scar treatment with EBDs,” Dr. Shumaker said in an interview. “Despite intense interest and a wealth of available literature, international treatment guidelines and patient access to these potentially life-changing treatments are currently lagging behind our capabilities.”
One of the key recommendations of the paper is that EBDs should have an expanded role in the treatment of acne scars, according to Dr. Shumaker, associate clinical professor of dermatology at the University of California, San Diego. “Panel members were unanimous in their view that EBDs, particularly ablative and nonablative fractional lasers, vascular lasers, and fractional radiofrequency devices, have an important role in the management of acne scars and should be considered a first-line treatment for a variety of scar types,” he said.
The process leading to the recommendations included developing clinical questions, based on input from the panelists and a literature review, and using a two-step modified Delphi method, “an iterative process used to achieve consensus for a defined clinical problem where there is little or conflicting published evidence and where expert opinion is decisive,” the authors wrote. This involved email questionnaires highlighting different topics, including the role of EBDs in mitigating and treating acne scars in patients with active acne, the use of different EBDs for treating different types of acne scars, and considerations in treating skin of color.
The panel noted considerations in the treatment of acne scars in skin of color. “Regardless of the platform, patients with darker skin types may require treatment modifications including: a reduction in fluence/pulse energy; decreased microcolumn density; greater intervals between treatments; longer pulse durations; epidermal cooling with fastidious technique to ensure appropriate cooling, additional cooling in between passes to decrease bulk heating; and pretreatment and posttreatment topical regimens (e.g., retinoids, bleaching creams, etc.) and strict sun precautions,” wrote the authors.
Panelists agreed that there is an absence of large, well-controlled, multicenter comparative trials of various laser and energy treatments for acne scars. “Such trials would be helpful in establishing the relative utility and persistence of benefit of various laser treatments and also in comparing their effectiveness versus that of nonenergy treatments,” the authors noted.
Asked to comment on the paper, Andrei Metelitsa, MD, a dermatologist in Calgary, Alta., and clinical associate professor at the University of Calgary, said the consensus recommendations on EBDs in acne scarring are “providing an international expert perspective, potentially changing a long-perceived paradigm of treatments.”
Dr. Metelitsa pointed out that the authors are taking a solid position with respect to reducing the delay to initiation of laser treatment following isotretinoin therapy. “The authors take a strong stance against the old dogma of postponing laser resurfacing for at least 6 months post isotretinoin,” he said. “According to the authors, there is sufficient evidence to support the idea of safely starting laser therapies, including fractional ablative and nonablative, within 1 month post isotretinoin, much sooner than previously suggested.”
He added that the authors point to the fact most experts utilize vascular lasers, such as pulsed-dye, to treat active acne in combination with medical therapy, thus reducing duration and severity of inflammation and potentially reducing further scar formation. “According to this published consensus, such laser therapies can even be used while the patient is actively treated with isotretinoin,” he said.
Dr. Metelitsa noted that the consensus recommendations outline how the choice of device should be guided by the clinical subtype of acne scars.
Dr. Shumaker, Dr. Metelitsa, and the authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Acute Severe Urticaria From Minocycline
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.
The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.
The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.
The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
Practice Points
- Minocycline is a commonly prescribed long-term treatment for acne vulgaris.
- Minocycline-induced acute urticaria and anaphylaxis are rare adverse events.
Topical options for acne patients continue to expand
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR