User login
Adult ADHD: 6 studies of nonpharmacologic interventions
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
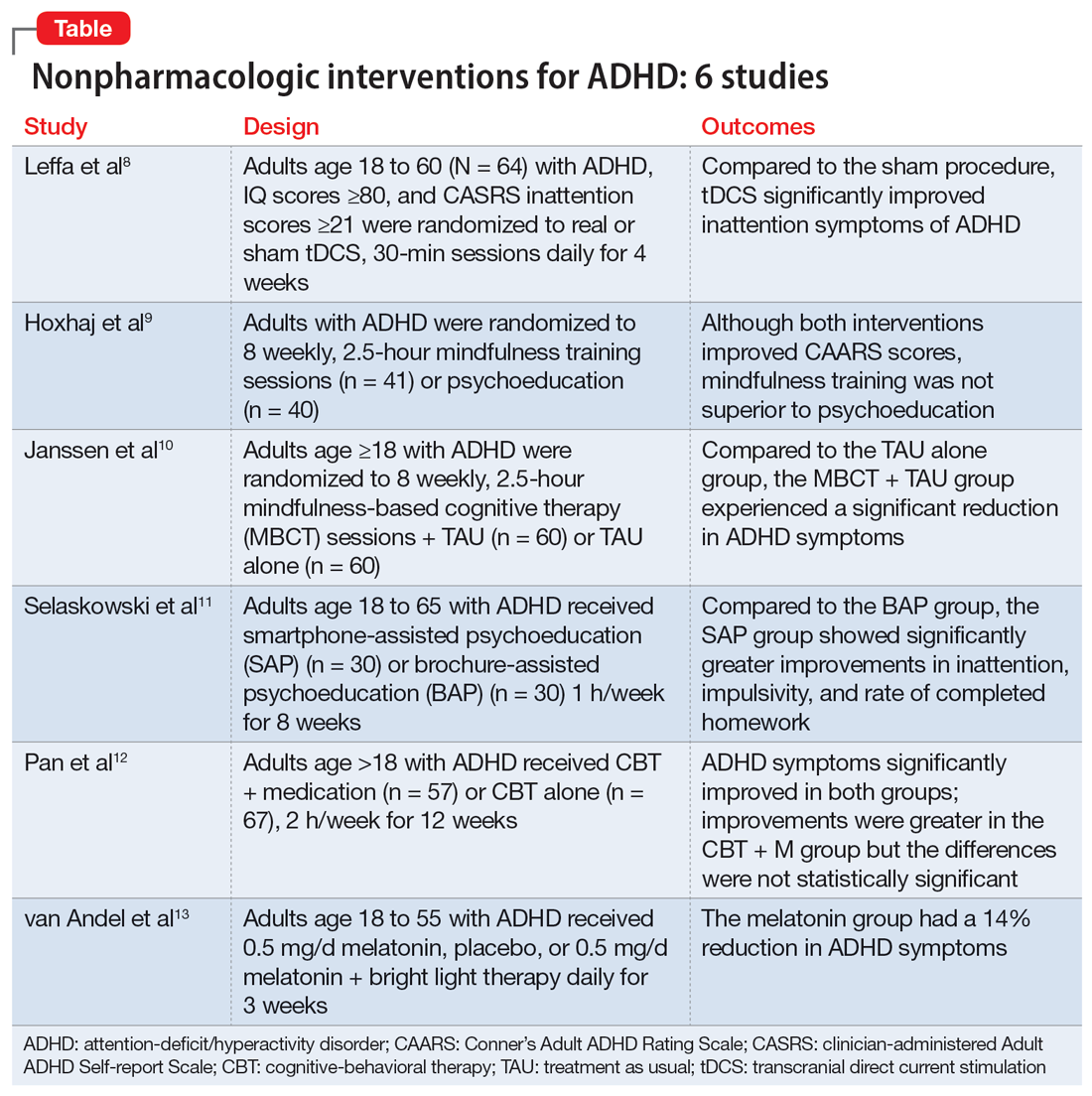
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).
1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).

1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).

1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
Surge in pediatric ADHD med errors prompts call for prevention
according to results of a study published in the journal Pediatrics.
The dramatic jump is likely attributable to an increase in the prescribing of ADHD medications for children. According to the study authors, in 2019, nearly 10% of children in the United States had been diagnosed with ADHD, and some 3.3 million – or about 5% of all children in the country – had received a prescription for an ADHD medication.
“Because therapeutic errors are preventable, more attention should be given to patient and caregiver education and development of improved child-resistant medication dispensing and tracking systems,” the authors commented.
The investigators analyzed data from the National Poison Data System from 2000 through 2021 for therapeutic errors associated with ADHD medication among patients younger than 20 years.
“As medicine changes, it’s nice to look back at some of these things and see how some of these problems have changed,” said Natalie I. Rine, PharmD, a coauthor of the study and director of the Central Ohio Poison Center at Nationwide Children’s Hospital in Columbus.
The researchers identified 124,383 such errors reported to U.S. poison centers during the study period. The frequency increased by 299%.
Two-thirds (66.6%) of the exposures involved children aged 6-12 years, three-fourths (76.4%) were among males, and half (50.5%) involved amphetamines and related compounds. Most (79.7%) therapeutic errors were linked to exposure to a single substance. Nearly 83% of patients did not receive treatment at a health care facility; however, 2.3% were admitted to the hospital, and 4.2% had a “serious medical outcome,” the researchers found.
The most common scenarios were “inadvertently took or given medication twice” (53.9%), followed by “inadvertently took or given someone else’s medication” (13.4%) and “wrong medication taken or given” (12.9%), according to the researchers. Two percent involved mistakes by a pharmacist or nurse.
Easily preventable
Dr. Rine attributed the errors to simple mistakes and said they were likely the product of busy households and distracted caregivers. She added that the errors are easily avoided by storing the medication properly, keeping a sheet with the medication to document what was taken and when, and using a pillbox or one of many apps that can assist in documenting the dispensing of medications.
“I think the biggest thing is that a lot of these errors are preventable, more than anything else,” Dr. Rine said.
The increase in ADHD diagnoses among children and the subsequent prescribing of medications are reasons for the nearly 300% increase in poison control calls. A 2018 study showed that the estimated prevalence of ADHD diagnoses among U.S. children and adolescents increased from 6.1% in 1997-1998 to 10.2% in 2015-2016. The Centers for Disease Control and Prevention states that 6 million children and adolescents aged 3-17 years have been diagnosed with ADHD, and 62% have received ADHD medication.
Colleen Kraft, MD, a pediatrician at Children’s Hospital Los Angeles, said she was not surprised by the reported increase in errors. In addition to the simple uptick in ADHD diagnoses and prescriptions in the past 2 decades, Dr. Kraft said the growing variety of ADHD medication is a cause for more errors.
“Because we have so many more different types of these medications, it’s easy to confuse them, and it’s easy to make an error when you give this to a child,” she said in an interview.
Dr. Kraft also hypothesized that because ADHD can have a genetic component, some parents with undiagnosed and untreated ADHD are responsible for their child’s medication, a scenario ripe for mistakes.
Potential dangers
Not all ADHD medicinal overdosing is created equal, Dr. Kraft pointed out. Doubling up on a stimulant such as methylphenidate (Ritalin) or the combination of amphetamine and dextroamphetamine (Adderall) may cause headaches, suppress appetite, and cause an upset stomach, although those symptoms usually clear up in a few hours.
However, she noted, the use of alpha-1 adrenergic blockers is more concerning. Also used to treat high blood pressure, medications such as guanfacine and clonidine cause sedation. A double dose can cause blood pressure to decrease to dangerous levels.
The study’s primary limitation was bias in self-reporting, which may have led to underreporting of incidences, according to the researchers. Not every case in which an error occurs that involves a child’s taking ADHD medication gets reported to poison control, because some will take a wait-and-see approach and may not call if their child is asymptomatic.
“Our data is only as good as what the callers report to us,” Dr. Rine said.
A version of this article appeared on Medscape.com.
according to results of a study published in the journal Pediatrics.
The dramatic jump is likely attributable to an increase in the prescribing of ADHD medications for children. According to the study authors, in 2019, nearly 10% of children in the United States had been diagnosed with ADHD, and some 3.3 million – or about 5% of all children in the country – had received a prescription for an ADHD medication.
“Because therapeutic errors are preventable, more attention should be given to patient and caregiver education and development of improved child-resistant medication dispensing and tracking systems,” the authors commented.
The investigators analyzed data from the National Poison Data System from 2000 through 2021 for therapeutic errors associated with ADHD medication among patients younger than 20 years.
“As medicine changes, it’s nice to look back at some of these things and see how some of these problems have changed,” said Natalie I. Rine, PharmD, a coauthor of the study and director of the Central Ohio Poison Center at Nationwide Children’s Hospital in Columbus.
The researchers identified 124,383 such errors reported to U.S. poison centers during the study period. The frequency increased by 299%.
Two-thirds (66.6%) of the exposures involved children aged 6-12 years, three-fourths (76.4%) were among males, and half (50.5%) involved amphetamines and related compounds. Most (79.7%) therapeutic errors were linked to exposure to a single substance. Nearly 83% of patients did not receive treatment at a health care facility; however, 2.3% were admitted to the hospital, and 4.2% had a “serious medical outcome,” the researchers found.
The most common scenarios were “inadvertently took or given medication twice” (53.9%), followed by “inadvertently took or given someone else’s medication” (13.4%) and “wrong medication taken or given” (12.9%), according to the researchers. Two percent involved mistakes by a pharmacist or nurse.
Easily preventable
Dr. Rine attributed the errors to simple mistakes and said they were likely the product of busy households and distracted caregivers. She added that the errors are easily avoided by storing the medication properly, keeping a sheet with the medication to document what was taken and when, and using a pillbox or one of many apps that can assist in documenting the dispensing of medications.
“I think the biggest thing is that a lot of these errors are preventable, more than anything else,” Dr. Rine said.
The increase in ADHD diagnoses among children and the subsequent prescribing of medications are reasons for the nearly 300% increase in poison control calls. A 2018 study showed that the estimated prevalence of ADHD diagnoses among U.S. children and adolescents increased from 6.1% in 1997-1998 to 10.2% in 2015-2016. The Centers for Disease Control and Prevention states that 6 million children and adolescents aged 3-17 years have been diagnosed with ADHD, and 62% have received ADHD medication.
Colleen Kraft, MD, a pediatrician at Children’s Hospital Los Angeles, said she was not surprised by the reported increase in errors. In addition to the simple uptick in ADHD diagnoses and prescriptions in the past 2 decades, Dr. Kraft said the growing variety of ADHD medication is a cause for more errors.
“Because we have so many more different types of these medications, it’s easy to confuse them, and it’s easy to make an error when you give this to a child,” she said in an interview.
Dr. Kraft also hypothesized that because ADHD can have a genetic component, some parents with undiagnosed and untreated ADHD are responsible for their child’s medication, a scenario ripe for mistakes.
Potential dangers
Not all ADHD medicinal overdosing is created equal, Dr. Kraft pointed out. Doubling up on a stimulant such as methylphenidate (Ritalin) or the combination of amphetamine and dextroamphetamine (Adderall) may cause headaches, suppress appetite, and cause an upset stomach, although those symptoms usually clear up in a few hours.
However, she noted, the use of alpha-1 adrenergic blockers is more concerning. Also used to treat high blood pressure, medications such as guanfacine and clonidine cause sedation. A double dose can cause blood pressure to decrease to dangerous levels.
The study’s primary limitation was bias in self-reporting, which may have led to underreporting of incidences, according to the researchers. Not every case in which an error occurs that involves a child’s taking ADHD medication gets reported to poison control, because some will take a wait-and-see approach and may not call if their child is asymptomatic.
“Our data is only as good as what the callers report to us,” Dr. Rine said.
A version of this article appeared on Medscape.com.
according to results of a study published in the journal Pediatrics.
The dramatic jump is likely attributable to an increase in the prescribing of ADHD medications for children. According to the study authors, in 2019, nearly 10% of children in the United States had been diagnosed with ADHD, and some 3.3 million – or about 5% of all children in the country – had received a prescription for an ADHD medication.
“Because therapeutic errors are preventable, more attention should be given to patient and caregiver education and development of improved child-resistant medication dispensing and tracking systems,” the authors commented.
The investigators analyzed data from the National Poison Data System from 2000 through 2021 for therapeutic errors associated with ADHD medication among patients younger than 20 years.
“As medicine changes, it’s nice to look back at some of these things and see how some of these problems have changed,” said Natalie I. Rine, PharmD, a coauthor of the study and director of the Central Ohio Poison Center at Nationwide Children’s Hospital in Columbus.
The researchers identified 124,383 such errors reported to U.S. poison centers during the study period. The frequency increased by 299%.
Two-thirds (66.6%) of the exposures involved children aged 6-12 years, three-fourths (76.4%) were among males, and half (50.5%) involved amphetamines and related compounds. Most (79.7%) therapeutic errors were linked to exposure to a single substance. Nearly 83% of patients did not receive treatment at a health care facility; however, 2.3% were admitted to the hospital, and 4.2% had a “serious medical outcome,” the researchers found.
The most common scenarios were “inadvertently took or given medication twice” (53.9%), followed by “inadvertently took or given someone else’s medication” (13.4%) and “wrong medication taken or given” (12.9%), according to the researchers. Two percent involved mistakes by a pharmacist or nurse.
Easily preventable
Dr. Rine attributed the errors to simple mistakes and said they were likely the product of busy households and distracted caregivers. She added that the errors are easily avoided by storing the medication properly, keeping a sheet with the medication to document what was taken and when, and using a pillbox or one of many apps that can assist in documenting the dispensing of medications.
“I think the biggest thing is that a lot of these errors are preventable, more than anything else,” Dr. Rine said.
The increase in ADHD diagnoses among children and the subsequent prescribing of medications are reasons for the nearly 300% increase in poison control calls. A 2018 study showed that the estimated prevalence of ADHD diagnoses among U.S. children and adolescents increased from 6.1% in 1997-1998 to 10.2% in 2015-2016. The Centers for Disease Control and Prevention states that 6 million children and adolescents aged 3-17 years have been diagnosed with ADHD, and 62% have received ADHD medication.
Colleen Kraft, MD, a pediatrician at Children’s Hospital Los Angeles, said she was not surprised by the reported increase in errors. In addition to the simple uptick in ADHD diagnoses and prescriptions in the past 2 decades, Dr. Kraft said the growing variety of ADHD medication is a cause for more errors.
“Because we have so many more different types of these medications, it’s easy to confuse them, and it’s easy to make an error when you give this to a child,” she said in an interview.
Dr. Kraft also hypothesized that because ADHD can have a genetic component, some parents with undiagnosed and untreated ADHD are responsible for their child’s medication, a scenario ripe for mistakes.
Potential dangers
Not all ADHD medicinal overdosing is created equal, Dr. Kraft pointed out. Doubling up on a stimulant such as methylphenidate (Ritalin) or the combination of amphetamine and dextroamphetamine (Adderall) may cause headaches, suppress appetite, and cause an upset stomach, although those symptoms usually clear up in a few hours.
However, she noted, the use of alpha-1 adrenergic blockers is more concerning. Also used to treat high blood pressure, medications such as guanfacine and clonidine cause sedation. A double dose can cause blood pressure to decrease to dangerous levels.
The study’s primary limitation was bias in self-reporting, which may have led to underreporting of incidences, according to the researchers. Not every case in which an error occurs that involves a child’s taking ADHD medication gets reported to poison control, because some will take a wait-and-see approach and may not call if their child is asymptomatic.
“Our data is only as good as what the callers report to us,” Dr. Rine said.
A version of this article appeared on Medscape.com.
FROM PEDIATRICS
Can a decrease in dopamine lead to binge eating?
In medical school, we were repeatedly advised that there is both a science and an art to the practice of medicine. In these days of doc-in-a-box online consultations for obesity, it’s tempting to think that there’s a one-size-fits-all purely scientific approach for these new weight loss medications. Yet, for every nine patients who lose weight seemingly effortlessly on this class of medication, there is always one whose body stubbornly refuses to submit.
Adam is a 58-year-old man who came to me recently because he was having difficulty losing weight. Over the past 20 years, he’d been steadily gaining weight and now, technically has morbid obesity (a term which should arguably be obsolete). His weight gain is complicated by high blood pressure, high cholesterol, and obstructive sleep apnea. His sleep apnea has caused such profound exhaustion that he no longer has the energy to work out. He also has significant ADHD, which has been left untreated because of his ability to white-knuckle it through his many daily meetings and calls. A married father of three, he is a successful portfolio manager at a high-yield bond fund.
Adam tends to eat minimally during the day, thereby baffling his colleagues with the stark contrast between his minimal caloric intake and his large belly. However, when he returns from work late at night (kids safely tucked into bed), the floodgates open. He reports polishing off pints of ice cream, scarfing down bags of cookies, inhaling trays of brownies. No carbohydrate is off limits to him once he steps off the Metro North train and crosses the threshold from work to home.
Does Adam simply lack the desire or common-sense willpower to make the necessary changes in his lifestyle or is there something more complicated at play?
I would argue that Adam’s ADHD triggered a binge-eating disorder (BED) that festered unchecked over the past 20 years. Patients with BED typically eat massive quantities of food over short periods of time – often when they’re not even hungry. Adam admitted that he would generally continue to eat well after feeling stuffed to the brim.
The answer probably lies with dopamine, a neurotransmitter produced in the reward centers of the brain that regulates how people experience pleasure and control impulses. We believe that people with ADHD have low levels of dopamine (it’s actually a bit more complicated, but this is the general idea). These low levels of dopamine lead people to self-medicate with sugars, salt, and fats to increase dopamine levels.
Lisdexamfetamine (Vyvanse) is a Food and Drug Administration–approved treatment option for both ADHD and binge eating. It raises the levels of dopamine (as well as norepinephrine) in the brain’s reward center. Often, the strong urge to binge subsides rapidly once ADHD is properly treated.
Rather than starting Adam on a semaglutide or similar agent, I opted to start him on lisdexamfetamine. When I spoke to him 1 week later, he confided that the world suddenly shifted into focus, and he was able to plan his meals throughout the day and resist the urge to binge late at night.
I may eventually add a semaglutide-like medication if his weight loss plateaus, but for now, I will focus on raising his dopamine levels to tackle the underlying cause of his weight gain.
Dr. Messer is a clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In medical school, we were repeatedly advised that there is both a science and an art to the practice of medicine. In these days of doc-in-a-box online consultations for obesity, it’s tempting to think that there’s a one-size-fits-all purely scientific approach for these new weight loss medications. Yet, for every nine patients who lose weight seemingly effortlessly on this class of medication, there is always one whose body stubbornly refuses to submit.
Adam is a 58-year-old man who came to me recently because he was having difficulty losing weight. Over the past 20 years, he’d been steadily gaining weight and now, technically has morbid obesity (a term which should arguably be obsolete). His weight gain is complicated by high blood pressure, high cholesterol, and obstructive sleep apnea. His sleep apnea has caused such profound exhaustion that he no longer has the energy to work out. He also has significant ADHD, which has been left untreated because of his ability to white-knuckle it through his many daily meetings and calls. A married father of three, he is a successful portfolio manager at a high-yield bond fund.
Adam tends to eat minimally during the day, thereby baffling his colleagues with the stark contrast between his minimal caloric intake and his large belly. However, when he returns from work late at night (kids safely tucked into bed), the floodgates open. He reports polishing off pints of ice cream, scarfing down bags of cookies, inhaling trays of brownies. No carbohydrate is off limits to him once he steps off the Metro North train and crosses the threshold from work to home.
Does Adam simply lack the desire or common-sense willpower to make the necessary changes in his lifestyle or is there something more complicated at play?
I would argue that Adam’s ADHD triggered a binge-eating disorder (BED) that festered unchecked over the past 20 years. Patients with BED typically eat massive quantities of food over short periods of time – often when they’re not even hungry. Adam admitted that he would generally continue to eat well after feeling stuffed to the brim.
The answer probably lies with dopamine, a neurotransmitter produced in the reward centers of the brain that regulates how people experience pleasure and control impulses. We believe that people with ADHD have low levels of dopamine (it’s actually a bit more complicated, but this is the general idea). These low levels of dopamine lead people to self-medicate with sugars, salt, and fats to increase dopamine levels.
Lisdexamfetamine (Vyvanse) is a Food and Drug Administration–approved treatment option for both ADHD and binge eating. It raises the levels of dopamine (as well as norepinephrine) in the brain’s reward center. Often, the strong urge to binge subsides rapidly once ADHD is properly treated.
Rather than starting Adam on a semaglutide or similar agent, I opted to start him on lisdexamfetamine. When I spoke to him 1 week later, he confided that the world suddenly shifted into focus, and he was able to plan his meals throughout the day and resist the urge to binge late at night.
I may eventually add a semaglutide-like medication if his weight loss plateaus, but for now, I will focus on raising his dopamine levels to tackle the underlying cause of his weight gain.
Dr. Messer is a clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In medical school, we were repeatedly advised that there is both a science and an art to the practice of medicine. In these days of doc-in-a-box online consultations for obesity, it’s tempting to think that there’s a one-size-fits-all purely scientific approach for these new weight loss medications. Yet, for every nine patients who lose weight seemingly effortlessly on this class of medication, there is always one whose body stubbornly refuses to submit.
Adam is a 58-year-old man who came to me recently because he was having difficulty losing weight. Over the past 20 years, he’d been steadily gaining weight and now, technically has morbid obesity (a term which should arguably be obsolete). His weight gain is complicated by high blood pressure, high cholesterol, and obstructive sleep apnea. His sleep apnea has caused such profound exhaustion that he no longer has the energy to work out. He also has significant ADHD, which has been left untreated because of his ability to white-knuckle it through his many daily meetings and calls. A married father of three, he is a successful portfolio manager at a high-yield bond fund.
Adam tends to eat minimally during the day, thereby baffling his colleagues with the stark contrast between his minimal caloric intake and his large belly. However, when he returns from work late at night (kids safely tucked into bed), the floodgates open. He reports polishing off pints of ice cream, scarfing down bags of cookies, inhaling trays of brownies. No carbohydrate is off limits to him once he steps off the Metro North train and crosses the threshold from work to home.
Does Adam simply lack the desire or common-sense willpower to make the necessary changes in his lifestyle or is there something more complicated at play?
I would argue that Adam’s ADHD triggered a binge-eating disorder (BED) that festered unchecked over the past 20 years. Patients with BED typically eat massive quantities of food over short periods of time – often when they’re not even hungry. Adam admitted that he would generally continue to eat well after feeling stuffed to the brim.
The answer probably lies with dopamine, a neurotransmitter produced in the reward centers of the brain that regulates how people experience pleasure and control impulses. We believe that people with ADHD have low levels of dopamine (it’s actually a bit more complicated, but this is the general idea). These low levels of dopamine lead people to self-medicate with sugars, salt, and fats to increase dopamine levels.
Lisdexamfetamine (Vyvanse) is a Food and Drug Administration–approved treatment option for both ADHD and binge eating. It raises the levels of dopamine (as well as norepinephrine) in the brain’s reward center. Often, the strong urge to binge subsides rapidly once ADHD is properly treated.
Rather than starting Adam on a semaglutide or similar agent, I opted to start him on lisdexamfetamine. When I spoke to him 1 week later, he confided that the world suddenly shifted into focus, and he was able to plan his meals throughout the day and resist the urge to binge late at night.
I may eventually add a semaglutide-like medication if his weight loss plateaus, but for now, I will focus on raising his dopamine levels to tackle the underlying cause of his weight gain.
Dr. Messer is a clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
ADHD in older adults: A closer look
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).
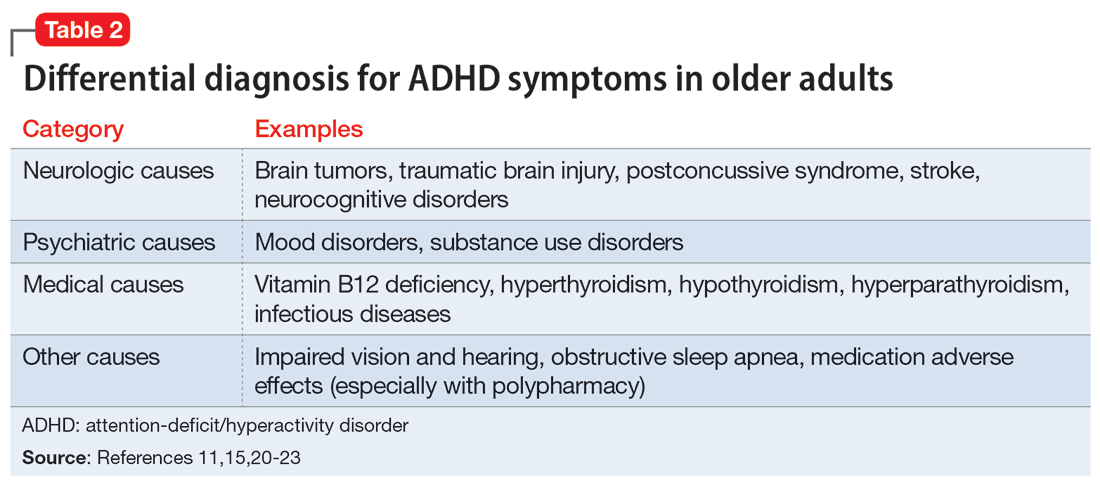
In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21
Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.
Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).

In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21

Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.

Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).

In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21

Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.

Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
More on prescribing controlled substances
I was disheartened with the June 2023 issue of
The benzodiazepine pharmacology discussed in this article is interesting, but it would be helpful if it had been integrated within a much more extensive discussion of careful prescribing practices. In 2020, the FDA updated the boxed warning to alert prescribers to the serious risks of abuse, addiction, physical dependence, and withdrawal reactions associated with benzodiazepines.2 I would hope that an article on benzodiazepines would provide more discussion and guidance surrounding these important issues.
The June 2023 issue also included “High-dose stimulants for adult ADHD” (p. 34-39, doi:10.12788/cp.0366). This article provided esoteric advice on managing stimulant therapy in the setting of Roux-en-Y gastric bypass surgery, yet I would regard stimulant misuse as a far more common and pressing issue.3,4 The recent Drug Enforcement Administration investigation of telehealth stimulant prescribing is a notable example of this problem.5
The patient discussed in this article was receiving large doses of stimulants for a purported case of refractory attention-deficit/hyperactivity disorder (ADHD). The article provided a sparse differential diagnosis for the patient’s intractable symptoms. While rapid metabolism may be an explanation, I would also like to know how the authors ruled out physiological dependence and/or addiction to a controlled substance. How was misuse excluded? Was urine drug testing (UDS) performed? UDS is highly irregular among prescribers,6 which suggests that practices for detecting covert substance abuse and stimulant misuse are inadequate. Wouldn’t such investigations be fundamental to ethical stimulant prescribing?
Jeff Sanders, MD, PhD
Atlanta, Georgia
References
1. Centers for Disease Control and Prevention. Trends in nonfatal and fatal overdoses involving benzodiazepines—38 states and the District of Columbia, 2019-2020. Accessed August 9, 2023. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034a2.htm
2. US Food & Drug Administration. FDA requiring boxed warning updated to improve safe use of benzodiazepine drug class. Accessed August 14, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requiring-boxed-warning-updated-improve-safe-use-benzodiazepine-drug-class
3. McCabe SE, Schulenberg JE, Wilens TE, et al. Prescription stimulant medical and nonmedical use among US secondary school students, 2005 to 2020. JAMA Netw Open. 2023;6(4):e238707. doi:10.1001/jamanetworkopen.2023.8707
4. US Food & Drug Administration. FDA updating warnings to improve safe use of prescription stimulants used to treat ADHD and other conditions. Accessed August 14, 2023. https://www.fda.gov/safety/medical-product-safety-information/fda-updating-warnings-improve-safe-use-prescription-stimulants-used-treat-adhd-and-other-conditions
5. Vaidya A. Report: telehealth company’s prescribing practices come under DEA scrutiny. September 16, 2022. Accessed August 9, 2023. https://mhealthintelligence.com/news/report-telehealth-company-dones-prescribing-practices-come-under-dea-scrutiny
6. Zionts A. Some ADHD patients are drug-tested often, while others are never asked. Kaiser Health News. March 25, 2023. Accessed August 9, 2023. https://www.nbcnews.com/news/amp/rcna76330
Continue to: Drs. Stimpfl and Strawn respond
Drs. Stimpfl and Strawn respond
We thank Dr. Sanders for highlighting the need for clinical equipoise in considering the risks and benefits of medications—something that is true for benzodiazepines, antipsychotics, antidepressants, and in fact all medications. He reminds us that the risks of misuse, dependence, and withdrawal associated with benzodiazepines led to a boxed warning in September 2020 and highlights recent trends of fatal and nonfatal benzodiazepine overdose, especially when combined with opiates.
Our article, which aimed to educate clinicians on benzodiazepine pharmacology and patient-specific factors influencing benzodiazepine selection and dosing, did not focus significantly on the risks associated with benzodiazepines. We do encourage careful and individualized benzodiazepine prescribing. However, we wish to remind our colleagues that benzodiazepines, while associated with risks, continue to have utility in acute and periprocedural settings, and remain an important treatment option for patients with panic disorder, generalized anxiety disorder (especially while waiting for other medications to take effect), catatonia, seizure disorders, and alcohol withdrawal.
We agree that patient-specific risk assessment is essential, as some patients benefit from benzodiazepines despite the risks. However, we also acknowledge that some individuals are at higher risk for adverse outcomes, including those with concurrent opiate use or who are prescribed other sedative-hypnotics; older adults and those with neurocognitive disorders; and patients susceptible to respiratory depression due to other medical reasons (eg, myasthenia gravis, sleep apnea, and chronic obstructive pulmonary disease). Further, we agree that benzodiazepine use during pregnancy is generally not advised due to the risks of neonatal hypotonia and neonatal withdrawal syndrome1 as well as a possible risk of cleft palate that has been reported in some studies.2 Finally, paradoxical reactions may be more common at the extremes of age and in patients with intellectual disability or personality disorders.3,4
Patient characteristics that have been associated with a higher risk of benzodiazepine use disorder include lower education/income, unemployment, having another substance use disorder, and severe psychopathology.5 In some studies, using benzodiazepines for prolonged periods at high doses as well as using those with a rapid onset of action was associated with an increased risk of benzodiazepine use disorder.5-7
Ultimately, we concur with Dr. Sanders on the perils of the “irresponsible use” of medication and emphasize the need for discernment when choosing treatments to avoid rashly discarding an effective remedy while attempting to mitigate all conceivable risks.
Julia Stimpfl, MD
Jeffrey R. Strawn, MD
Cincinnati, Ohio
References
1. McElhatton PR. The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol. 1994;8(6):461-475. doi:10.1016/0890-6238(94)90029-9
2. Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can. 2011;33(1):46-48. doi:10.1016/S1701-2163(16)34772-7 Erratum in: J Obstet Gynaecol Can. 2011;33(4):319.
3. Hakimi Y, Petitpain N, Pinzani V, et al. Paradoxical adverse drug reactions: descriptive analysis of French reports. Eur J Clin Pharmacol. 2020;76(8):1169-1174. doi:10.1007/s00228-020-02892-2
4. Paton C. Benzodiazepines and disinhibition: a review. Psychiatric Bulletin. 2002;26(12):460-462. doi:10.1192/pb.26.12.460
5. Fride Tvete I, Bjørner T, Skomedal T. Risk factors for excessive benzodiazepine use in a working age population: a nationwide 5-year survey in Norway. Scand J Prim Health Care. 2015;33(4):252-259. doi:10.3109/02813432.2015.1117282
6. Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66 Suppl 9:31-41.
7. Kan CC, Hilberink SR, Breteler MH. Determination of the main risk factors for benzodiazepine dependence using a multivariate and multidimensional approach. Compr Psychiatry. 2004;45(2):88-94. doi:10.1016/j.comppsych.2003.12.007
Continue to: Drs. Sarma and Grady respond
Drs. Sarma and Grady respond
Dr. Sanders’ letter highlights the potential caveats associated with prescribing controlled substances. We agree that our short case summary includes numerous interesting elements, each of which would be worthy of further exploration and discussion. Our choice was to highlight the patient history of bariatric surgery and use this as a springboard into a review of stimulants, including the newest formulations for ADHD. For more than 1 year, many generic stimulants have been in short supply, and patients and clinicians have been seeking other therapeutic options. Given this background and with newer, branded stimulant use becoming more commonplace, we believe our article was useful and timely.
Our original intent had been to include an example of a controlled substance agreement. Regrettably, there was simply not enough space for this document or the additional discussion that its inclusion would deem necessary. Nevertheless, had the May 2023 FDA requirement for manufacturers to update the labeling of prescription stimulants1 to clarify misuse and abuse been published before our article’s final revision, we would have mentioned it and provided the appropriate link.
Subbu J. Sarma, MD, FAPA
Kansas City, Missouri
Sarah E. Grady, PharmD, BCPS, BCPP
Des Moines, Iowa
References
1. US Food & Drug Administration. FDA requires updates to clarify labeling of prescription stimulants used to treat ADHD and other conditions. Accessed August 9, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-updates-clarify-labeling-prescription-stimulants-used-treat-adhd-and-other-conditions
I was disheartened with the June 2023 issue of
The benzodiazepine pharmacology discussed in this article is interesting, but it would be helpful if it had been integrated within a much more extensive discussion of careful prescribing practices. In 2020, the FDA updated the boxed warning to alert prescribers to the serious risks of abuse, addiction, physical dependence, and withdrawal reactions associated with benzodiazepines.2 I would hope that an article on benzodiazepines would provide more discussion and guidance surrounding these important issues.
The June 2023 issue also included “High-dose stimulants for adult ADHD” (p. 34-39, doi:10.12788/cp.0366). This article provided esoteric advice on managing stimulant therapy in the setting of Roux-en-Y gastric bypass surgery, yet I would regard stimulant misuse as a far more common and pressing issue.3,4 The recent Drug Enforcement Administration investigation of telehealth stimulant prescribing is a notable example of this problem.5
The patient discussed in this article was receiving large doses of stimulants for a purported case of refractory attention-deficit/hyperactivity disorder (ADHD). The article provided a sparse differential diagnosis for the patient’s intractable symptoms. While rapid metabolism may be an explanation, I would also like to know how the authors ruled out physiological dependence and/or addiction to a controlled substance. How was misuse excluded? Was urine drug testing (UDS) performed? UDS is highly irregular among prescribers,6 which suggests that practices for detecting covert substance abuse and stimulant misuse are inadequate. Wouldn’t such investigations be fundamental to ethical stimulant prescribing?
Jeff Sanders, MD, PhD
Atlanta, Georgia
References
1. Centers for Disease Control and Prevention. Trends in nonfatal and fatal overdoses involving benzodiazepines—38 states and the District of Columbia, 2019-2020. Accessed August 9, 2023. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034a2.htm
2. US Food & Drug Administration. FDA requiring boxed warning updated to improve safe use of benzodiazepine drug class. Accessed August 14, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requiring-boxed-warning-updated-improve-safe-use-benzodiazepine-drug-class
3. McCabe SE, Schulenberg JE, Wilens TE, et al. Prescription stimulant medical and nonmedical use among US secondary school students, 2005 to 2020. JAMA Netw Open. 2023;6(4):e238707. doi:10.1001/jamanetworkopen.2023.8707
4. US Food & Drug Administration. FDA updating warnings to improve safe use of prescription stimulants used to treat ADHD and other conditions. Accessed August 14, 2023. https://www.fda.gov/safety/medical-product-safety-information/fda-updating-warnings-improve-safe-use-prescription-stimulants-used-treat-adhd-and-other-conditions
5. Vaidya A. Report: telehealth company’s prescribing practices come under DEA scrutiny. September 16, 2022. Accessed August 9, 2023. https://mhealthintelligence.com/news/report-telehealth-company-dones-prescribing-practices-come-under-dea-scrutiny
6. Zionts A. Some ADHD patients are drug-tested often, while others are never asked. Kaiser Health News. March 25, 2023. Accessed August 9, 2023. https://www.nbcnews.com/news/amp/rcna76330
Continue to: Drs. Stimpfl and Strawn respond
Drs. Stimpfl and Strawn respond
We thank Dr. Sanders for highlighting the need for clinical equipoise in considering the risks and benefits of medications—something that is true for benzodiazepines, antipsychotics, antidepressants, and in fact all medications. He reminds us that the risks of misuse, dependence, and withdrawal associated with benzodiazepines led to a boxed warning in September 2020 and highlights recent trends of fatal and nonfatal benzodiazepine overdose, especially when combined with opiates.
Our article, which aimed to educate clinicians on benzodiazepine pharmacology and patient-specific factors influencing benzodiazepine selection and dosing, did not focus significantly on the risks associated with benzodiazepines. We do encourage careful and individualized benzodiazepine prescribing. However, we wish to remind our colleagues that benzodiazepines, while associated with risks, continue to have utility in acute and periprocedural settings, and remain an important treatment option for patients with panic disorder, generalized anxiety disorder (especially while waiting for other medications to take effect), catatonia, seizure disorders, and alcohol withdrawal.
We agree that patient-specific risk assessment is essential, as some patients benefit from benzodiazepines despite the risks. However, we also acknowledge that some individuals are at higher risk for adverse outcomes, including those with concurrent opiate use or who are prescribed other sedative-hypnotics; older adults and those with neurocognitive disorders; and patients susceptible to respiratory depression due to other medical reasons (eg, myasthenia gravis, sleep apnea, and chronic obstructive pulmonary disease). Further, we agree that benzodiazepine use during pregnancy is generally not advised due to the risks of neonatal hypotonia and neonatal withdrawal syndrome1 as well as a possible risk of cleft palate that has been reported in some studies.2 Finally, paradoxical reactions may be more common at the extremes of age and in patients with intellectual disability or personality disorders.3,4
Patient characteristics that have been associated with a higher risk of benzodiazepine use disorder include lower education/income, unemployment, having another substance use disorder, and severe psychopathology.5 In some studies, using benzodiazepines for prolonged periods at high doses as well as using those with a rapid onset of action was associated with an increased risk of benzodiazepine use disorder.5-7
Ultimately, we concur with Dr. Sanders on the perils of the “irresponsible use” of medication and emphasize the need for discernment when choosing treatments to avoid rashly discarding an effective remedy while attempting to mitigate all conceivable risks.
Julia Stimpfl, MD
Jeffrey R. Strawn, MD
Cincinnati, Ohio
References
1. McElhatton PR. The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol. 1994;8(6):461-475. doi:10.1016/0890-6238(94)90029-9
2. Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can. 2011;33(1):46-48. doi:10.1016/S1701-2163(16)34772-7 Erratum in: J Obstet Gynaecol Can. 2011;33(4):319.
3. Hakimi Y, Petitpain N, Pinzani V, et al. Paradoxical adverse drug reactions: descriptive analysis of French reports. Eur J Clin Pharmacol. 2020;76(8):1169-1174. doi:10.1007/s00228-020-02892-2
4. Paton C. Benzodiazepines and disinhibition: a review. Psychiatric Bulletin. 2002;26(12):460-462. doi:10.1192/pb.26.12.460
5. Fride Tvete I, Bjørner T, Skomedal T. Risk factors for excessive benzodiazepine use in a working age population: a nationwide 5-year survey in Norway. Scand J Prim Health Care. 2015;33(4):252-259. doi:10.3109/02813432.2015.1117282
6. Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66 Suppl 9:31-41.
7. Kan CC, Hilberink SR, Breteler MH. Determination of the main risk factors for benzodiazepine dependence using a multivariate and multidimensional approach. Compr Psychiatry. 2004;45(2):88-94. doi:10.1016/j.comppsych.2003.12.007
Continue to: Drs. Sarma and Grady respond
Drs. Sarma and Grady respond
Dr. Sanders’ letter highlights the potential caveats associated with prescribing controlled substances. We agree that our short case summary includes numerous interesting elements, each of which would be worthy of further exploration and discussion. Our choice was to highlight the patient history of bariatric surgery and use this as a springboard into a review of stimulants, including the newest formulations for ADHD. For more than 1 year, many generic stimulants have been in short supply, and patients and clinicians have been seeking other therapeutic options. Given this background and with newer, branded stimulant use becoming more commonplace, we believe our article was useful and timely.
Our original intent had been to include an example of a controlled substance agreement. Regrettably, there was simply not enough space for this document or the additional discussion that its inclusion would deem necessary. Nevertheless, had the May 2023 FDA requirement for manufacturers to update the labeling of prescription stimulants1 to clarify misuse and abuse been published before our article’s final revision, we would have mentioned it and provided the appropriate link.
Subbu J. Sarma, MD, FAPA
Kansas City, Missouri
Sarah E. Grady, PharmD, BCPS, BCPP
Des Moines, Iowa
References
1. US Food & Drug Administration. FDA requires updates to clarify labeling of prescription stimulants used to treat ADHD and other conditions. Accessed August 9, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-updates-clarify-labeling-prescription-stimulants-used-treat-adhd-and-other-conditions
I was disheartened with the June 2023 issue of
The benzodiazepine pharmacology discussed in this article is interesting, but it would be helpful if it had been integrated within a much more extensive discussion of careful prescribing practices. In 2020, the FDA updated the boxed warning to alert prescribers to the serious risks of abuse, addiction, physical dependence, and withdrawal reactions associated with benzodiazepines.2 I would hope that an article on benzodiazepines would provide more discussion and guidance surrounding these important issues.
The June 2023 issue also included “High-dose stimulants for adult ADHD” (p. 34-39, doi:10.12788/cp.0366). This article provided esoteric advice on managing stimulant therapy in the setting of Roux-en-Y gastric bypass surgery, yet I would regard stimulant misuse as a far more common and pressing issue.3,4 The recent Drug Enforcement Administration investigation of telehealth stimulant prescribing is a notable example of this problem.5
The patient discussed in this article was receiving large doses of stimulants for a purported case of refractory attention-deficit/hyperactivity disorder (ADHD). The article provided a sparse differential diagnosis for the patient’s intractable symptoms. While rapid metabolism may be an explanation, I would also like to know how the authors ruled out physiological dependence and/or addiction to a controlled substance. How was misuse excluded? Was urine drug testing (UDS) performed? UDS is highly irregular among prescribers,6 which suggests that practices for detecting covert substance abuse and stimulant misuse are inadequate. Wouldn’t such investigations be fundamental to ethical stimulant prescribing?
Jeff Sanders, MD, PhD
Atlanta, Georgia
References
1. Centers for Disease Control and Prevention. Trends in nonfatal and fatal overdoses involving benzodiazepines—38 states and the District of Columbia, 2019-2020. Accessed August 9, 2023. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034a2.htm
2. US Food & Drug Administration. FDA requiring boxed warning updated to improve safe use of benzodiazepine drug class. Accessed August 14, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requiring-boxed-warning-updated-improve-safe-use-benzodiazepine-drug-class
3. McCabe SE, Schulenberg JE, Wilens TE, et al. Prescription stimulant medical and nonmedical use among US secondary school students, 2005 to 2020. JAMA Netw Open. 2023;6(4):e238707. doi:10.1001/jamanetworkopen.2023.8707
4. US Food & Drug Administration. FDA updating warnings to improve safe use of prescription stimulants used to treat ADHD and other conditions. Accessed August 14, 2023. https://www.fda.gov/safety/medical-product-safety-information/fda-updating-warnings-improve-safe-use-prescription-stimulants-used-treat-adhd-and-other-conditions
5. Vaidya A. Report: telehealth company’s prescribing practices come under DEA scrutiny. September 16, 2022. Accessed August 9, 2023. https://mhealthintelligence.com/news/report-telehealth-company-dones-prescribing-practices-come-under-dea-scrutiny
6. Zionts A. Some ADHD patients are drug-tested often, while others are never asked. Kaiser Health News. March 25, 2023. Accessed August 9, 2023. https://www.nbcnews.com/news/amp/rcna76330
Continue to: Drs. Stimpfl and Strawn respond
Drs. Stimpfl and Strawn respond
We thank Dr. Sanders for highlighting the need for clinical equipoise in considering the risks and benefits of medications—something that is true for benzodiazepines, antipsychotics, antidepressants, and in fact all medications. He reminds us that the risks of misuse, dependence, and withdrawal associated with benzodiazepines led to a boxed warning in September 2020 and highlights recent trends of fatal and nonfatal benzodiazepine overdose, especially when combined with opiates.
Our article, which aimed to educate clinicians on benzodiazepine pharmacology and patient-specific factors influencing benzodiazepine selection and dosing, did not focus significantly on the risks associated with benzodiazepines. We do encourage careful and individualized benzodiazepine prescribing. However, we wish to remind our colleagues that benzodiazepines, while associated with risks, continue to have utility in acute and periprocedural settings, and remain an important treatment option for patients with panic disorder, generalized anxiety disorder (especially while waiting for other medications to take effect), catatonia, seizure disorders, and alcohol withdrawal.
We agree that patient-specific risk assessment is essential, as some patients benefit from benzodiazepines despite the risks. However, we also acknowledge that some individuals are at higher risk for adverse outcomes, including those with concurrent opiate use or who are prescribed other sedative-hypnotics; older adults and those with neurocognitive disorders; and patients susceptible to respiratory depression due to other medical reasons (eg, myasthenia gravis, sleep apnea, and chronic obstructive pulmonary disease). Further, we agree that benzodiazepine use during pregnancy is generally not advised due to the risks of neonatal hypotonia and neonatal withdrawal syndrome1 as well as a possible risk of cleft palate that has been reported in some studies.2 Finally, paradoxical reactions may be more common at the extremes of age and in patients with intellectual disability or personality disorders.3,4
Patient characteristics that have been associated with a higher risk of benzodiazepine use disorder include lower education/income, unemployment, having another substance use disorder, and severe psychopathology.5 In some studies, using benzodiazepines for prolonged periods at high doses as well as using those with a rapid onset of action was associated with an increased risk of benzodiazepine use disorder.5-7
Ultimately, we concur with Dr. Sanders on the perils of the “irresponsible use” of medication and emphasize the need for discernment when choosing treatments to avoid rashly discarding an effective remedy while attempting to mitigate all conceivable risks.
Julia Stimpfl, MD
Jeffrey R. Strawn, MD
Cincinnati, Ohio
References
1. McElhatton PR. The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol. 1994;8(6):461-475. doi:10.1016/0890-6238(94)90029-9
2. Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can. 2011;33(1):46-48. doi:10.1016/S1701-2163(16)34772-7 Erratum in: J Obstet Gynaecol Can. 2011;33(4):319.
3. Hakimi Y, Petitpain N, Pinzani V, et al. Paradoxical adverse drug reactions: descriptive analysis of French reports. Eur J Clin Pharmacol. 2020;76(8):1169-1174. doi:10.1007/s00228-020-02892-2
4. Paton C. Benzodiazepines and disinhibition: a review. Psychiatric Bulletin. 2002;26(12):460-462. doi:10.1192/pb.26.12.460
5. Fride Tvete I, Bjørner T, Skomedal T. Risk factors for excessive benzodiazepine use in a working age population: a nationwide 5-year survey in Norway. Scand J Prim Health Care. 2015;33(4):252-259. doi:10.3109/02813432.2015.1117282
6. Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66 Suppl 9:31-41.
7. Kan CC, Hilberink SR, Breteler MH. Determination of the main risk factors for benzodiazepine dependence using a multivariate and multidimensional approach. Compr Psychiatry. 2004;45(2):88-94. doi:10.1016/j.comppsych.2003.12.007
Continue to: Drs. Sarma and Grady respond
Drs. Sarma and Grady respond
Dr. Sanders’ letter highlights the potential caveats associated with prescribing controlled substances. We agree that our short case summary includes numerous interesting elements, each of which would be worthy of further exploration and discussion. Our choice was to highlight the patient history of bariatric surgery and use this as a springboard into a review of stimulants, including the newest formulations for ADHD. For more than 1 year, many generic stimulants have been in short supply, and patients and clinicians have been seeking other therapeutic options. Given this background and with newer, branded stimulant use becoming more commonplace, we believe our article was useful and timely.
Our original intent had been to include an example of a controlled substance agreement. Regrettably, there was simply not enough space for this document or the additional discussion that its inclusion would deem necessary. Nevertheless, had the May 2023 FDA requirement for manufacturers to update the labeling of prescription stimulants1 to clarify misuse and abuse been published before our article’s final revision, we would have mentioned it and provided the appropriate link.
Subbu J. Sarma, MD, FAPA
Kansas City, Missouri
Sarah E. Grady, PharmD, BCPS, BCPP
Des Moines, Iowa
References
1. US Food & Drug Administration. FDA requires updates to clarify labeling of prescription stimulants used to treat ADHD and other conditions. Accessed August 9, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-updates-clarify-labeling-prescription-stimulants-used-treat-adhd-and-other-conditions
ADHD meds cut hospitalization risk in borderline personality disorder patients
Although most patients with borderline personality disorder (BPD) receive psychopharmacological treatment, clinical guidance and outcomes data for specific medication use in these patients are lacking, wrote Johannes Lieslehto, MD, PhD, of the University of Eastern Finland, Niuvankuja, and colleagues.
In a study published in Acta Psychiatrica Scandinavica , the researchers – using national databases in Sweden – identified 17,532 adults with BPD who were treated with medications between 2006 and 2018.
Medications included benzodiazepines, antipsychotics, and antidepressants, as well as medications often used for ADHD: clozapine, lisdexamphetamine, bupropion, and methylphenidate. The mean age of the study population was 29.8 years and 2,649 were men.
The primary outcomes were psychiatric hospitalization (which served as an indication of treatment failure), all-cause hospitalization, or death.
Overall, treatment with benzodiazepines, antipsychotics, and antidepressants was associated with increased risk of psychiatric rehospitalization, with hazard ratios of 1.38, 1.19, and 1.18, respectively, and with increased risk of all-cause hospitalization or death (HR 1.37, HR 1.21, HR 1.17, respectively).
By contrast, treatment with ADHD medication was associated with decreased risk of psychiatric hospitalization (HR = 0.88), as well as a decreased risk of all-cause hospitalization or death (HR = 0.86).
Specifically, clozapine, lisdexamphetamine, bupropion, and methylphenidate were associated with decreased risk of psychiatric rehospitalization, with hazard ratios of 0.54, 0.79, 0.84, and 0.90, respectively.
Treatment with mood stabilizers had no significant impact on outcomes.
BPD patients treated with ADHD medications also may exhibit ADHD symptoms, the researchers wrote in their discussion. However, “Although BPD and ADHD partially overlap in symptoms such as impulsivity and emotion dysregulation, previous efforts to investigate the efficacy of ADHD medication treatment in BPD are scarce,” and randomized, controlled trials are needed to determine whether these medications should be given to BPD patients without comorbid ADHD symptoms, they said.
The findings were limited by several factors including the lack of clinical parameters on symptom severity, quality of life, and level of function, and premature prescribing of medication (protopathic bias) may have affected the results, the researchers noted.
The results were strengthened by the large sample size and long follow-up, which increases the generalizability to real-world patients, and suggest that many pharmacological treatments for BPD may not improve outcomes, the researchers said. However, “even in the presence of possible protopathic bias, treatment with lisdexamphetamine, bupropion, methylphenidate, and clozapine was associated with improved outcomes, encouraging further research on these treatments,” they said.
The study was supported by the Finnish Ministry of Social Affairs and Health and the Academy of Finland. Dr. Lieslehto had no financial conflicts to disclose.
Although most patients with borderline personality disorder (BPD) receive psychopharmacological treatment, clinical guidance and outcomes data for specific medication use in these patients are lacking, wrote Johannes Lieslehto, MD, PhD, of the University of Eastern Finland, Niuvankuja, and colleagues.
In a study published in Acta Psychiatrica Scandinavica , the researchers – using national databases in Sweden – identified 17,532 adults with BPD who were treated with medications between 2006 and 2018.
Medications included benzodiazepines, antipsychotics, and antidepressants, as well as medications often used for ADHD: clozapine, lisdexamphetamine, bupropion, and methylphenidate. The mean age of the study population was 29.8 years and 2,649 were men.
The primary outcomes were psychiatric hospitalization (which served as an indication of treatment failure), all-cause hospitalization, or death.
Overall, treatment with benzodiazepines, antipsychotics, and antidepressants was associated with increased risk of psychiatric rehospitalization, with hazard ratios of 1.38, 1.19, and 1.18, respectively, and with increased risk of all-cause hospitalization or death (HR 1.37, HR 1.21, HR 1.17, respectively).
By contrast, treatment with ADHD medication was associated with decreased risk of psychiatric hospitalization (HR = 0.88), as well as a decreased risk of all-cause hospitalization or death (HR = 0.86).
Specifically, clozapine, lisdexamphetamine, bupropion, and methylphenidate were associated with decreased risk of psychiatric rehospitalization, with hazard ratios of 0.54, 0.79, 0.84, and 0.90, respectively.
Treatment with mood stabilizers had no significant impact on outcomes.
BPD patients treated with ADHD medications also may exhibit ADHD symptoms, the researchers wrote in their discussion. However, “Although BPD and ADHD partially overlap in symptoms such as impulsivity and emotion dysregulation, previous efforts to investigate the efficacy of ADHD medication treatment in BPD are scarce,” and randomized, controlled trials are needed to determine whether these medications should be given to BPD patients without comorbid ADHD symptoms, they said.
The findings were limited by several factors including the lack of clinical parameters on symptom severity, quality of life, and level of function, and premature prescribing of medication (protopathic bias) may have affected the results, the researchers noted.
The results were strengthened by the large sample size and long follow-up, which increases the generalizability to real-world patients, and suggest that many pharmacological treatments for BPD may not improve outcomes, the researchers said. However, “even in the presence of possible protopathic bias, treatment with lisdexamphetamine, bupropion, methylphenidate, and clozapine was associated with improved outcomes, encouraging further research on these treatments,” they said.
The study was supported by the Finnish Ministry of Social Affairs and Health and the Academy of Finland. Dr. Lieslehto had no financial conflicts to disclose.
Although most patients with borderline personality disorder (BPD) receive psychopharmacological treatment, clinical guidance and outcomes data for specific medication use in these patients are lacking, wrote Johannes Lieslehto, MD, PhD, of the University of Eastern Finland, Niuvankuja, and colleagues.
In a study published in Acta Psychiatrica Scandinavica , the researchers – using national databases in Sweden – identified 17,532 adults with BPD who were treated with medications between 2006 and 2018.
Medications included benzodiazepines, antipsychotics, and antidepressants, as well as medications often used for ADHD: clozapine, lisdexamphetamine, bupropion, and methylphenidate. The mean age of the study population was 29.8 years and 2,649 were men.
The primary outcomes were psychiatric hospitalization (which served as an indication of treatment failure), all-cause hospitalization, or death.
Overall, treatment with benzodiazepines, antipsychotics, and antidepressants was associated with increased risk of psychiatric rehospitalization, with hazard ratios of 1.38, 1.19, and 1.18, respectively, and with increased risk of all-cause hospitalization or death (HR 1.37, HR 1.21, HR 1.17, respectively).
By contrast, treatment with ADHD medication was associated with decreased risk of psychiatric hospitalization (HR = 0.88), as well as a decreased risk of all-cause hospitalization or death (HR = 0.86).
Specifically, clozapine, lisdexamphetamine, bupropion, and methylphenidate were associated with decreased risk of psychiatric rehospitalization, with hazard ratios of 0.54, 0.79, 0.84, and 0.90, respectively.
Treatment with mood stabilizers had no significant impact on outcomes.
BPD patients treated with ADHD medications also may exhibit ADHD symptoms, the researchers wrote in their discussion. However, “Although BPD and ADHD partially overlap in symptoms such as impulsivity and emotion dysregulation, previous efforts to investigate the efficacy of ADHD medication treatment in BPD are scarce,” and randomized, controlled trials are needed to determine whether these medications should be given to BPD patients without comorbid ADHD symptoms, they said.
The findings were limited by several factors including the lack of clinical parameters on symptom severity, quality of life, and level of function, and premature prescribing of medication (protopathic bias) may have affected the results, the researchers noted.
The results were strengthened by the large sample size and long follow-up, which increases the generalizability to real-world patients, and suggest that many pharmacological treatments for BPD may not improve outcomes, the researchers said. However, “even in the presence of possible protopathic bias, treatment with lisdexamphetamine, bupropion, methylphenidate, and clozapine was associated with improved outcomes, encouraging further research on these treatments,” they said.
The study was supported by the Finnish Ministry of Social Affairs and Health and the Academy of Finland. Dr. Lieslehto had no financial conflicts to disclose.
FROM ACTA PSYCHIATRICA SCANDINAVICA
Brain volume patterns vary across psychiatric disorders
A large brain imaging study of adults with six different psychiatric illnesses shows that heterogeneity in regional gray matter volume deviations is a general feature of psychiatric illness, but that these regionally heterogeneous areas are often embedded within common functional circuits and networks.
study investigator Ashlea Segal said in an email.
The findings also suggest that it’s “unlikely that a single cause or mechanism of a given disorder exists, and that a ‘one-size-fits-all’ approach to treatment is likely only appropriate for a small subset of individuals. In fact, one size doesn’t fit all. It probably doesn’t even fit most,” said Ms. Segal, a PhD candidate with the Turner Institute for Brain and Mental Health’s Neural Systems and Behaviour Lab at Monash University in Melbourne.
“Focusing on brain alterations at an individual level allows us to develop more personally tailored treatments,” Ms. Segal added.
Regional heterogeneity, the authors write, “thus offers a plausible explanation for the well-described clinical heterogeneity observed in psychiatric disorders, while circuit- and network-level aggregation of deviations is a putative neural substrate for phenotypic similarities between patients assigned the same diagnosis.”
The study was published online in Nature Neuroscience
Beyond group averages
For decades, researchers have mapped brain areas showing reduced gray matter volume (GMV) in people diagnosed with a variety of mental illnesses, but these maps have only been generated at the level of group averages, Ms. Segal explained.
“This means that we understand how the brains of people with, say, schizophrenia, differ from those without schizophrenia on average, but we can’t really say much about individual people,” Ms. Segal said.
For their study, the researchers used new statistical techniques developed by Andre Marquand, PhD, who co-led the project, to characterize the heterogeneity of GMV differences in 1,294 individuals diagnosed with one of six psychiatric conditions and 1,465 matched controls. Dr. Marquand is affiliated with the Donders Institute for Brain, Cognition, and Behavior in Nijmegen, the Netherlands.
These techniques “allow us to benchmark the size of over 1,000 different brain regions in any given person relative to what we should expect to see in the general population. In this way, we can identify, for any person, brain regions showing unusually small or large volumes, given that person’s age and sex,” Ms. Segal told this news organization.
The clinical sample included 202 individuals with autism spectrum disorder, 153 with attention-deficit/hyperactivity disorder (ADHD), 228 with bipolar disorder, 161 with major depressive disorder, 167 with obsessive-compulsive disorder, and 383 individuals with schizophrenia.
Confirming earlier findings, those with psychiatric illness showed more GMV deviations than healthy controls, the researchers found.
However, at the individual level, deviations from population expectations for regional gray matter volumes were “highly heterogeneous,” affecting the same area in less than 7% of people with the same diagnosis, they note. “This result means that it is difficult to pinpoint treatment targets or causal mechanisms by focusing on group averages alone,” Alex Fornito, PhD, of Monash University, who led the research team, said in a statement.
“It may also explain why people with the same diagnosis show wide variability in their symptom profiles and treatment outcomes,” Dr. Fornito added.
Yet, despite considerable heterogeneity at the regional level across different diagnoses, these deviations were embedded within common functional circuits and networks in up to 56% of cases.
The salience-ventral attention network, for example, which plays a central role in cognitive control, interoceptive awareness, and switching between internally and externally focused attention, was implicated across diagnoses, with other neural networks selectively involved in depression, bipolar disorder, schizophrenia, and ADHD.
The researchers say the approach they developed opens new opportunities for mapping brain changes in mental illness.
“The framework we have developed allows us to understand the diversity of brain changes in people with mental illness at different levels, from individual regions through to more widespread brain circuits and networks, offering a deeper insight into how the brain is affected in individual people,” Dr. Fornito said in a statement.
The study had no commercial funding. Ms. Segal, Dr. Fornito, and Dr. Marquand report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large brain imaging study of adults with six different psychiatric illnesses shows that heterogeneity in regional gray matter volume deviations is a general feature of psychiatric illness, but that these regionally heterogeneous areas are often embedded within common functional circuits and networks.
study investigator Ashlea Segal said in an email.
The findings also suggest that it’s “unlikely that a single cause or mechanism of a given disorder exists, and that a ‘one-size-fits-all’ approach to treatment is likely only appropriate for a small subset of individuals. In fact, one size doesn’t fit all. It probably doesn’t even fit most,” said Ms. Segal, a PhD candidate with the Turner Institute for Brain and Mental Health’s Neural Systems and Behaviour Lab at Monash University in Melbourne.
“Focusing on brain alterations at an individual level allows us to develop more personally tailored treatments,” Ms. Segal added.
Regional heterogeneity, the authors write, “thus offers a plausible explanation for the well-described clinical heterogeneity observed in psychiatric disorders, while circuit- and network-level aggregation of deviations is a putative neural substrate for phenotypic similarities between patients assigned the same diagnosis.”
The study was published online in Nature Neuroscience
Beyond group averages
For decades, researchers have mapped brain areas showing reduced gray matter volume (GMV) in people diagnosed with a variety of mental illnesses, but these maps have only been generated at the level of group averages, Ms. Segal explained.
“This means that we understand how the brains of people with, say, schizophrenia, differ from those without schizophrenia on average, but we can’t really say much about individual people,” Ms. Segal said.
For their study, the researchers used new statistical techniques developed by Andre Marquand, PhD, who co-led the project, to characterize the heterogeneity of GMV differences in 1,294 individuals diagnosed with one of six psychiatric conditions and 1,465 matched controls. Dr. Marquand is affiliated with the Donders Institute for Brain, Cognition, and Behavior in Nijmegen, the Netherlands.
These techniques “allow us to benchmark the size of over 1,000 different brain regions in any given person relative to what we should expect to see in the general population. In this way, we can identify, for any person, brain regions showing unusually small or large volumes, given that person’s age and sex,” Ms. Segal told this news organization.
The clinical sample included 202 individuals with autism spectrum disorder, 153 with attention-deficit/hyperactivity disorder (ADHD), 228 with bipolar disorder, 161 with major depressive disorder, 167 with obsessive-compulsive disorder, and 383 individuals with schizophrenia.
Confirming earlier findings, those with psychiatric illness showed more GMV deviations than healthy controls, the researchers found.
However, at the individual level, deviations from population expectations for regional gray matter volumes were “highly heterogeneous,” affecting the same area in less than 7% of people with the same diagnosis, they note. “This result means that it is difficult to pinpoint treatment targets or causal mechanisms by focusing on group averages alone,” Alex Fornito, PhD, of Monash University, who led the research team, said in a statement.
“It may also explain why people with the same diagnosis show wide variability in their symptom profiles and treatment outcomes,” Dr. Fornito added.
Yet, despite considerable heterogeneity at the regional level across different diagnoses, these deviations were embedded within common functional circuits and networks in up to 56% of cases.
The salience-ventral attention network, for example, which plays a central role in cognitive control, interoceptive awareness, and switching between internally and externally focused attention, was implicated across diagnoses, with other neural networks selectively involved in depression, bipolar disorder, schizophrenia, and ADHD.
The researchers say the approach they developed opens new opportunities for mapping brain changes in mental illness.
“The framework we have developed allows us to understand the diversity of brain changes in people with mental illness at different levels, from individual regions through to more widespread brain circuits and networks, offering a deeper insight into how the brain is affected in individual people,” Dr. Fornito said in a statement.
The study had no commercial funding. Ms. Segal, Dr. Fornito, and Dr. Marquand report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large brain imaging study of adults with six different psychiatric illnesses shows that heterogeneity in regional gray matter volume deviations is a general feature of psychiatric illness, but that these regionally heterogeneous areas are often embedded within common functional circuits and networks.
study investigator Ashlea Segal said in an email.
The findings also suggest that it’s “unlikely that a single cause or mechanism of a given disorder exists, and that a ‘one-size-fits-all’ approach to treatment is likely only appropriate for a small subset of individuals. In fact, one size doesn’t fit all. It probably doesn’t even fit most,” said Ms. Segal, a PhD candidate with the Turner Institute for Brain and Mental Health’s Neural Systems and Behaviour Lab at Monash University in Melbourne.
“Focusing on brain alterations at an individual level allows us to develop more personally tailored treatments,” Ms. Segal added.
Regional heterogeneity, the authors write, “thus offers a plausible explanation for the well-described clinical heterogeneity observed in psychiatric disorders, while circuit- and network-level aggregation of deviations is a putative neural substrate for phenotypic similarities between patients assigned the same diagnosis.”
The study was published online in Nature Neuroscience
Beyond group averages
For decades, researchers have mapped brain areas showing reduced gray matter volume (GMV) in people diagnosed with a variety of mental illnesses, but these maps have only been generated at the level of group averages, Ms. Segal explained.
“This means that we understand how the brains of people with, say, schizophrenia, differ from those without schizophrenia on average, but we can’t really say much about individual people,” Ms. Segal said.
For their study, the researchers used new statistical techniques developed by Andre Marquand, PhD, who co-led the project, to characterize the heterogeneity of GMV differences in 1,294 individuals diagnosed with one of six psychiatric conditions and 1,465 matched controls. Dr. Marquand is affiliated with the Donders Institute for Brain, Cognition, and Behavior in Nijmegen, the Netherlands.
These techniques “allow us to benchmark the size of over 1,000 different brain regions in any given person relative to what we should expect to see in the general population. In this way, we can identify, for any person, brain regions showing unusually small or large volumes, given that person’s age and sex,” Ms. Segal told this news organization.
The clinical sample included 202 individuals with autism spectrum disorder, 153 with attention-deficit/hyperactivity disorder (ADHD), 228 with bipolar disorder, 161 with major depressive disorder, 167 with obsessive-compulsive disorder, and 383 individuals with schizophrenia.
Confirming earlier findings, those with psychiatric illness showed more GMV deviations than healthy controls, the researchers found.
However, at the individual level, deviations from population expectations for regional gray matter volumes were “highly heterogeneous,” affecting the same area in less than 7% of people with the same diagnosis, they note. “This result means that it is difficult to pinpoint treatment targets or causal mechanisms by focusing on group averages alone,” Alex Fornito, PhD, of Monash University, who led the research team, said in a statement.
“It may also explain why people with the same diagnosis show wide variability in their symptom profiles and treatment outcomes,” Dr. Fornito added.
Yet, despite considerable heterogeneity at the regional level across different diagnoses, these deviations were embedded within common functional circuits and networks in up to 56% of cases.
The salience-ventral attention network, for example, which plays a central role in cognitive control, interoceptive awareness, and switching between internally and externally focused attention, was implicated across diagnoses, with other neural networks selectively involved in depression, bipolar disorder, schizophrenia, and ADHD.
The researchers say the approach they developed opens new opportunities for mapping brain changes in mental illness.
“The framework we have developed allows us to understand the diversity of brain changes in people with mental illness at different levels, from individual regions through to more widespread brain circuits and networks, offering a deeper insight into how the brain is affected in individual people,” Dr. Fornito said in a statement.
The study had no commercial funding. Ms. Segal, Dr. Fornito, and Dr. Marquand report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE NEUROSCIENCE
Nutritional psychiatry: Does it exist?
Matt was diagnosed with ADHD combined type when he was 6 years old. Given his age, the family was reluctant to try medications, but after a couple years of parenting classes and reward charts, the parents requested a stimulant. He had significant improvement in focus and impulsivity but also reduced appetite. Now at age 13, irritability and depressive symptoms have been increasing for 9 months. Skeptical of adding another medication, his parents ask whether nutrition might be an alternative tool to treat his symptoms?
What a healthy diet even consists of seems to be a moving target over decades and years. Trendy research, supplements, and dietary approaches proliferate alongside appealing theories of action. In the end, weighing which intervention is effective for which disorder and at what cost becomes murky.
Yet several fundamental principles seem clear and consistent over time and across studies.
Starting early
There is reliable evidence that in the perinatal environment, nutrition sets the stage for many aspects of healthy development. These effects are likely mediated variously through the hypothalamic-pituitary-adrenal axis, the trillions of gut bacteria that make up the microbiome, gene-environment interactions, and more. Maternal malnutrition and stress prenatally puts infants at risk for not only poor birth outcomes but also psychiatric challenges throughout childhood, such as ADHD, anxiety, depression, and autism.1
Intervening in the perinatal period has long-term benefits. A first step includes assessing food security, beginning with consistent access to nutritious food. It is important to inquire about the role of food and nutrition in the family’s history and culture, as well as identifying resources to support access to affordable nutrition. This can be paired with parenting interventions, such as family meals without screens. This may require scaffolding positive conversations in high-conflict family settings (see The Family Dinner Project).
Healthy diets promote mental health
If food security is achieved, what is next? Clinicians can inquire about the who, what, where, when, and why of nutrition to learn about a family’s eating habits.2 While randomized controlled data is very limited, both cross-sectional and longitudinal studies show that healthy diets in youth correlate with mental health – more healthy foods reducing internalizing and externalizing disorders, and more typical Western diets increasing the risk. On average, dietary interventions include higher levels of fruits and vegetables, fish, and nuts, and lower levels of processed foods.2 There is not evidence that restrictive diets or fasting is appropriate or safe for youth. Additionally, involving children in getting, growing, or preparing food with gradually increasing autonomy fosters self-confidence and skill development.
In those struggling with restrictive eating disorders, food is medicine – helping those with restrictive diets to develop more balanced and adequate intake for metabolic needs. Outside of diagnosable eating disorders, weight or body mass index is less of a goal or marker when it comes to mental health. Instead, look for participation in enjoyable activities, opportunities to move and rest, and a body image that supports self-care and self-confidence (see the National Institutes of Health’s We Can! Program). Creating dissonance with cultural ideals of appearance centered on thinness can prevent future eating disorders.3
Nutraceutical options
Outside of eating disorders, specific foods and plants with health or medicinal properties – variously called nutraceuticals, phytoceuticals, or micronutrients – have emerging evidence in mental health. A 2022 expert academic consensus panel reviewed the literature to create clinical guidelines in this area.4 For major depression, adding omega-3 fatty acids to standard antidepressant treatment or standalone St. John’s wort have adequate evidence to recommend, while adjunctive probiotics, zinc, saffron, and curcumin have sufficient though less robust evidence. S-adenosyl methionine, vitamin D, and methyfolate showed only weak evidence for depression, while vitamin C, magnesium, creatine, N-acetylcysteine, folate, and monotherapy omega-3s do not have sufficient evidence to be recommended. For ADHD there was weak support for vitamin D, but no clear evidence for omega-3s, zinc, gingko, or acetyl L-carnitine. For anxiety, there is moderate evidence for ashwagandha and lavender in adults. A child psychiatry review suggests also trying chamomile for generalized anxiety based on the evidence in young adults, and underscores some data for N-acetylcysteine for OCD in particular.5
Many of these nutraceuticals exhibit small or moderate effects in a limited number of trials, with generally much less data for youth, compared with adults. While the same could be said for many on- and off-label uses of psychiatric medications for kids, clinicians would be wise to consider these highly specific nutritional interventions as items on the menu of treatment options rather than stand-alone treatments.
Revisitng the case study
Reflecting on Matt’s care, his pediatrician first assessed his dietary patterns, noting late-night eating and caffeine use with minimal hydration or fiber across the day. Recommendations for keeping fruit and vegetable snacks easily accessible as well as carrying a water flask are well received. They also discuss adding omega-3 fatty acids and probiotics with his morning stimulant while he awaits a referral for cognitive-behavioral therapy in order to address his depressive symptoms and minimize medication needs.
Beyond addressing food security and balanced family meals, specific interventions may be appropriate as initial treatment adjuncts for mild and some moderate mental illness. For more intense moderate to severe illness, nutritional psychiatry may be considered in combination with treatments with stronger evidence. At a community level, clinicians can help advocate for universal school meal programs to address food security, and so-called salad bar interventions to increase fruit/vegetable uptake among school-age children.
Dr. Rosenfeld is associate professor of psychiatry and pediatrics at University of Vermont and the Vermont Center for Children, Youth, and Families, both in Burlington. He has no disclosures.
References
1 Vohr BR et al. Pediatrics. 2017;139:S38-49.
2. Hosker DK et al. Child Adol Psychiatr Clin N Am. 2019;28(2):171-93.
3. Stice E et al. Int J Eat Disord. 2013;46(5):478-85.
4. Sarris J et al. World J Biol Psychiatry. 2022;23(6):424-55.
5. Simkin DR et al. Child Adolesc Psychiatric Clin N Am. 2023;32:193-216.
Matt was diagnosed with ADHD combined type when he was 6 years old. Given his age, the family was reluctant to try medications, but after a couple years of parenting classes and reward charts, the parents requested a stimulant. He had significant improvement in focus and impulsivity but also reduced appetite. Now at age 13, irritability and depressive symptoms have been increasing for 9 months. Skeptical of adding another medication, his parents ask whether nutrition might be an alternative tool to treat his symptoms?
What a healthy diet even consists of seems to be a moving target over decades and years. Trendy research, supplements, and dietary approaches proliferate alongside appealing theories of action. In the end, weighing which intervention is effective for which disorder and at what cost becomes murky.
Yet several fundamental principles seem clear and consistent over time and across studies.
Starting early
There is reliable evidence that in the perinatal environment, nutrition sets the stage for many aspects of healthy development. These effects are likely mediated variously through the hypothalamic-pituitary-adrenal axis, the trillions of gut bacteria that make up the microbiome, gene-environment interactions, and more. Maternal malnutrition and stress prenatally puts infants at risk for not only poor birth outcomes but also psychiatric challenges throughout childhood, such as ADHD, anxiety, depression, and autism.1
Intervening in the perinatal period has long-term benefits. A first step includes assessing food security, beginning with consistent access to nutritious food. It is important to inquire about the role of food and nutrition in the family’s history and culture, as well as identifying resources to support access to affordable nutrition. This can be paired with parenting interventions, such as family meals without screens. This may require scaffolding positive conversations in high-conflict family settings (see The Family Dinner Project).
Healthy diets promote mental health
If food security is achieved, what is next? Clinicians can inquire about the who, what, where, when, and why of nutrition to learn about a family’s eating habits.2 While randomized controlled data is very limited, both cross-sectional and longitudinal studies show that healthy diets in youth correlate with mental health – more healthy foods reducing internalizing and externalizing disorders, and more typical Western diets increasing the risk. On average, dietary interventions include higher levels of fruits and vegetables, fish, and nuts, and lower levels of processed foods.2 There is not evidence that restrictive diets or fasting is appropriate or safe for youth. Additionally, involving children in getting, growing, or preparing food with gradually increasing autonomy fosters self-confidence and skill development.
In those struggling with restrictive eating disorders, food is medicine – helping those with restrictive diets to develop more balanced and adequate intake for metabolic needs. Outside of diagnosable eating disorders, weight or body mass index is less of a goal or marker when it comes to mental health. Instead, look for participation in enjoyable activities, opportunities to move and rest, and a body image that supports self-care and self-confidence (see the National Institutes of Health’s We Can! Program). Creating dissonance with cultural ideals of appearance centered on thinness can prevent future eating disorders.3
Nutraceutical options
Outside of eating disorders, specific foods and plants with health or medicinal properties – variously called nutraceuticals, phytoceuticals, or micronutrients – have emerging evidence in mental health. A 2022 expert academic consensus panel reviewed the literature to create clinical guidelines in this area.4 For major depression, adding omega-3 fatty acids to standard antidepressant treatment or standalone St. John’s wort have adequate evidence to recommend, while adjunctive probiotics, zinc, saffron, and curcumin have sufficient though less robust evidence. S-adenosyl methionine, vitamin D, and methyfolate showed only weak evidence for depression, while vitamin C, magnesium, creatine, N-acetylcysteine, folate, and monotherapy omega-3s do not have sufficient evidence to be recommended. For ADHD there was weak support for vitamin D, but no clear evidence for omega-3s, zinc, gingko, or acetyl L-carnitine. For anxiety, there is moderate evidence for ashwagandha and lavender in adults. A child psychiatry review suggests also trying chamomile for generalized anxiety based on the evidence in young adults, and underscores some data for N-acetylcysteine for OCD in particular.5
Many of these nutraceuticals exhibit small or moderate effects in a limited number of trials, with generally much less data for youth, compared with adults. While the same could be said for many on- and off-label uses of psychiatric medications for kids, clinicians would be wise to consider these highly specific nutritional interventions as items on the menu of treatment options rather than stand-alone treatments.
Revisitng the case study
Reflecting on Matt’s care, his pediatrician first assessed his dietary patterns, noting late-night eating and caffeine use with minimal hydration or fiber across the day. Recommendations for keeping fruit and vegetable snacks easily accessible as well as carrying a water flask are well received. They also discuss adding omega-3 fatty acids and probiotics with his morning stimulant while he awaits a referral for cognitive-behavioral therapy in order to address his depressive symptoms and minimize medication needs.
Beyond addressing food security and balanced family meals, specific interventions may be appropriate as initial treatment adjuncts for mild and some moderate mental illness. For more intense moderate to severe illness, nutritional psychiatry may be considered in combination with treatments with stronger evidence. At a community level, clinicians can help advocate for universal school meal programs to address food security, and so-called salad bar interventions to increase fruit/vegetable uptake among school-age children.
Dr. Rosenfeld is associate professor of psychiatry and pediatrics at University of Vermont and the Vermont Center for Children, Youth, and Families, both in Burlington. He has no disclosures.
References
1 Vohr BR et al. Pediatrics. 2017;139:S38-49.
2. Hosker DK et al. Child Adol Psychiatr Clin N Am. 2019;28(2):171-93.
3. Stice E et al. Int J Eat Disord. 2013;46(5):478-85.
4. Sarris J et al. World J Biol Psychiatry. 2022;23(6):424-55.
5. Simkin DR et al. Child Adolesc Psychiatric Clin N Am. 2023;32:193-216.
Matt was diagnosed with ADHD combined type when he was 6 years old. Given his age, the family was reluctant to try medications, but after a couple years of parenting classes and reward charts, the parents requested a stimulant. He had significant improvement in focus and impulsivity but also reduced appetite. Now at age 13, irritability and depressive symptoms have been increasing for 9 months. Skeptical of adding another medication, his parents ask whether nutrition might be an alternative tool to treat his symptoms?
What a healthy diet even consists of seems to be a moving target over decades and years. Trendy research, supplements, and dietary approaches proliferate alongside appealing theories of action. In the end, weighing which intervention is effective for which disorder and at what cost becomes murky.
Yet several fundamental principles seem clear and consistent over time and across studies.
Starting early
There is reliable evidence that in the perinatal environment, nutrition sets the stage for many aspects of healthy development. These effects are likely mediated variously through the hypothalamic-pituitary-adrenal axis, the trillions of gut bacteria that make up the microbiome, gene-environment interactions, and more. Maternal malnutrition and stress prenatally puts infants at risk for not only poor birth outcomes but also psychiatric challenges throughout childhood, such as ADHD, anxiety, depression, and autism.1
Intervening in the perinatal period has long-term benefits. A first step includes assessing food security, beginning with consistent access to nutritious food. It is important to inquire about the role of food and nutrition in the family’s history and culture, as well as identifying resources to support access to affordable nutrition. This can be paired with parenting interventions, such as family meals without screens. This may require scaffolding positive conversations in high-conflict family settings (see The Family Dinner Project).
Healthy diets promote mental health
If food security is achieved, what is next? Clinicians can inquire about the who, what, where, when, and why of nutrition to learn about a family’s eating habits.2 While randomized controlled data is very limited, both cross-sectional and longitudinal studies show that healthy diets in youth correlate with mental health – more healthy foods reducing internalizing and externalizing disorders, and more typical Western diets increasing the risk. On average, dietary interventions include higher levels of fruits and vegetables, fish, and nuts, and lower levels of processed foods.2 There is not evidence that restrictive diets or fasting is appropriate or safe for youth. Additionally, involving children in getting, growing, or preparing food with gradually increasing autonomy fosters self-confidence and skill development.
In those struggling with restrictive eating disorders, food is medicine – helping those with restrictive diets to develop more balanced and adequate intake for metabolic needs. Outside of diagnosable eating disorders, weight or body mass index is less of a goal or marker when it comes to mental health. Instead, look for participation in enjoyable activities, opportunities to move and rest, and a body image that supports self-care and self-confidence (see the National Institutes of Health’s We Can! Program). Creating dissonance with cultural ideals of appearance centered on thinness can prevent future eating disorders.3
Nutraceutical options
Outside of eating disorders, specific foods and plants with health or medicinal properties – variously called nutraceuticals, phytoceuticals, or micronutrients – have emerging evidence in mental health. A 2022 expert academic consensus panel reviewed the literature to create clinical guidelines in this area.4 For major depression, adding omega-3 fatty acids to standard antidepressant treatment or standalone St. John’s wort have adequate evidence to recommend, while adjunctive probiotics, zinc, saffron, and curcumin have sufficient though less robust evidence. S-adenosyl methionine, vitamin D, and methyfolate showed only weak evidence for depression, while vitamin C, magnesium, creatine, N-acetylcysteine, folate, and monotherapy omega-3s do not have sufficient evidence to be recommended. For ADHD there was weak support for vitamin D, but no clear evidence for omega-3s, zinc, gingko, or acetyl L-carnitine. For anxiety, there is moderate evidence for ashwagandha and lavender in adults. A child psychiatry review suggests also trying chamomile for generalized anxiety based on the evidence in young adults, and underscores some data for N-acetylcysteine for OCD in particular.5
Many of these nutraceuticals exhibit small or moderate effects in a limited number of trials, with generally much less data for youth, compared with adults. While the same could be said for many on- and off-label uses of psychiatric medications for kids, clinicians would be wise to consider these highly specific nutritional interventions as items on the menu of treatment options rather than stand-alone treatments.
Revisitng the case study
Reflecting on Matt’s care, his pediatrician first assessed his dietary patterns, noting late-night eating and caffeine use with minimal hydration or fiber across the day. Recommendations for keeping fruit and vegetable snacks easily accessible as well as carrying a water flask are well received. They also discuss adding omega-3 fatty acids and probiotics with his morning stimulant while he awaits a referral for cognitive-behavioral therapy in order to address his depressive symptoms and minimize medication needs.
Beyond addressing food security and balanced family meals, specific interventions may be appropriate as initial treatment adjuncts for mild and some moderate mental illness. For more intense moderate to severe illness, nutritional psychiatry may be considered in combination with treatments with stronger evidence. At a community level, clinicians can help advocate for universal school meal programs to address food security, and so-called salad bar interventions to increase fruit/vegetable uptake among school-age children.
Dr. Rosenfeld is associate professor of psychiatry and pediatrics at University of Vermont and the Vermont Center for Children, Youth, and Families, both in Burlington. He has no disclosures.
References
1 Vohr BR et al. Pediatrics. 2017;139:S38-49.
2. Hosker DK et al. Child Adol Psychiatr Clin N Am. 2019;28(2):171-93.
3. Stice E et al. Int J Eat Disord. 2013;46(5):478-85.
4. Sarris J et al. World J Biol Psychiatry. 2022;23(6):424-55.
5. Simkin DR et al. Child Adolesc Psychiatric Clin N Am. 2023;32:193-216.
Reassuring data on stimulants for ADHD in kids and later substance abuse
“Throughout rigorous analyses, and after accounting for more than 70 variables in this longitudinal sample of children with ADHD taking stimulants, we did not find an association with later substance use,” lead investigator Brooke Molina, PhD, director of the youth and family research program at the University of Pittsburgh, said in an interview.
The findings were published online in JAMA Psychiatry.
Protective effect?
Owing to symptoms of impulsivity inherent to ADHD, the disorder itself carries a risk for elevated substance use, the investigators note.
They speculate that this may be why some previous research suggests prescription stimulants reduce the risk of subsequent substance use disorder. However, other studies have found no such protective link.
To shed more light on the issue, the investigators used data from the Multimodal Treatment Study of ADHD, a multicenter, 14-month randomized clinical trial of medication and behavioral therapy for children with ADHD. However, for the purposes of the present study, investigators focused only on stimulant use in children.
At the time of recruitment, the children were aged 7-9 and had been diagnosed with ADHD between 1994 and 1996.
Investigators assessed the participants prior to randomization, at months 3 and 9, and at the end of treatment. They were then followed for 16 years and were assessed at years 2, 3, 6, 8, 10, 12, 14, and 16 until a mean age of 25.
During 12-, 14-, and 16-year follow-up, participants completed a questionnaire on their use of alcohol, marijuana, cigarettes, and several illicit and prescription drugs.
Investigators collected information on participants’ stimulant treatment via the Services for Children and Adolescents Parent Interview until they reached age 18. After that, participants reported their own stimulant treatment.
A total of 579 participants were included in the analysis. Of these, 61% were White, 20% were Black, and 8% were Hispanic.
Decline in stimulant use over time
The analysis showed that stimulant use declined “precipitously” over time – from 60% at the 2- and 3-year assessments to an average of 7% during early adulthood.
The investigators also found that for some participants, substance use increased steadily through adolescence and remained stable through early adulthood. For instance, 36.5% of the adolescents in the total cohort reported smoking tobacco daily, and 29.6% reported using marijuana every week.
In addition, approximately 21% of the participants indulged in heavy drinking at least once a week, and 6% reported “other” substance use, which included sedative misuse, heroin, inhalants, hallucinogens, or other substances taken to “get high.”
After accounting for developmental trends in substance use in the sample through adolescence into early adulthood with several rigorous statistical models, the researchers found no association between current or prior stimulant treatment and cigarette, marijuana, alcohol, or other substance use, with one exception.
While cumulative stimulant treatment was associated with increased heavy drinking, the effect size of this association was small. Each additional year of cumulative stimulant use was estimated to increase participants’ likelihood of any binge drinking/drunkenness vs. none in the past year by 4% (95% confidence interval, 0.01-0.08; P =.03).
When the investigators used a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence that current (B range, –0.62-0.34) or prior stimulant treatment (B range, –0.06-0.70) or their interaction (B range, –0.49-0.86) was associated with substance use in adulthood.
Dr. Molina noted that although participants were recruited from multiple sites, the sample may not be generalizable because children and parents who present for an intensive treatment study such as this are not necessarily representative of the general ADHD population.
Reassuring findings
In a comment, Julie Schweitzer, PhD, professor of psychiatry and behavioral sciences at the University of California, Davis, said she hopes the study findings will quell the stigma surrounding stimulant use by children with ADHD.
“Parents’ fears that stimulant use will lead to a substance use disorder inhibits them from bringing their children for an ADHD evaluation, thus reducing the likelihood that they will receive timely treatment,” Dr. Schweitzer said.
“While stimulant medication is the first-line treatment most often recommended for most persons with ADHD, by not following through on evaluations, parents also miss the opportunity to learn about nonpharmacological strategies that might also be helpful to help cope with ADHD symptoms and its potential co-occurring challenges,” she added.
Dr. Schweitzer also noted that many parents hope their children will outgrow the symptoms without realizing that by not obtaining an evaluation and treatment for their child, there is an associated cost, including less than optimal academic performance, social relationships, and emotional health.
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and under a National Institute on Drug Abuse contract followed by a data analysis grant. Dr. Molina reported grants from the NIMH and the National Institute on Drug Abuse during the conduct of the study.
A version of this article first appeared on Medscape.com.
“Throughout rigorous analyses, and after accounting for more than 70 variables in this longitudinal sample of children with ADHD taking stimulants, we did not find an association with later substance use,” lead investigator Brooke Molina, PhD, director of the youth and family research program at the University of Pittsburgh, said in an interview.
The findings were published online in JAMA Psychiatry.
Protective effect?
Owing to symptoms of impulsivity inherent to ADHD, the disorder itself carries a risk for elevated substance use, the investigators note.
They speculate that this may be why some previous research suggests prescription stimulants reduce the risk of subsequent substance use disorder. However, other studies have found no such protective link.
To shed more light on the issue, the investigators used data from the Multimodal Treatment Study of ADHD, a multicenter, 14-month randomized clinical trial of medication and behavioral therapy for children with ADHD. However, for the purposes of the present study, investigators focused only on stimulant use in children.
At the time of recruitment, the children were aged 7-9 and had been diagnosed with ADHD between 1994 and 1996.
Investigators assessed the participants prior to randomization, at months 3 and 9, and at the end of treatment. They were then followed for 16 years and were assessed at years 2, 3, 6, 8, 10, 12, 14, and 16 until a mean age of 25.
During 12-, 14-, and 16-year follow-up, participants completed a questionnaire on their use of alcohol, marijuana, cigarettes, and several illicit and prescription drugs.
Investigators collected information on participants’ stimulant treatment via the Services for Children and Adolescents Parent Interview until they reached age 18. After that, participants reported their own stimulant treatment.
A total of 579 participants were included in the analysis. Of these, 61% were White, 20% were Black, and 8% were Hispanic.
Decline in stimulant use over time
The analysis showed that stimulant use declined “precipitously” over time – from 60% at the 2- and 3-year assessments to an average of 7% during early adulthood.
The investigators also found that for some participants, substance use increased steadily through adolescence and remained stable through early adulthood. For instance, 36.5% of the adolescents in the total cohort reported smoking tobacco daily, and 29.6% reported using marijuana every week.
In addition, approximately 21% of the participants indulged in heavy drinking at least once a week, and 6% reported “other” substance use, which included sedative misuse, heroin, inhalants, hallucinogens, or other substances taken to “get high.”
After accounting for developmental trends in substance use in the sample through adolescence into early adulthood with several rigorous statistical models, the researchers found no association between current or prior stimulant treatment and cigarette, marijuana, alcohol, or other substance use, with one exception.
While cumulative stimulant treatment was associated with increased heavy drinking, the effect size of this association was small. Each additional year of cumulative stimulant use was estimated to increase participants’ likelihood of any binge drinking/drunkenness vs. none in the past year by 4% (95% confidence interval, 0.01-0.08; P =.03).
When the investigators used a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence that current (B range, –0.62-0.34) or prior stimulant treatment (B range, –0.06-0.70) or their interaction (B range, –0.49-0.86) was associated with substance use in adulthood.
Dr. Molina noted that although participants were recruited from multiple sites, the sample may not be generalizable because children and parents who present for an intensive treatment study such as this are not necessarily representative of the general ADHD population.
Reassuring findings
In a comment, Julie Schweitzer, PhD, professor of psychiatry and behavioral sciences at the University of California, Davis, said she hopes the study findings will quell the stigma surrounding stimulant use by children with ADHD.
“Parents’ fears that stimulant use will lead to a substance use disorder inhibits them from bringing their children for an ADHD evaluation, thus reducing the likelihood that they will receive timely treatment,” Dr. Schweitzer said.
“While stimulant medication is the first-line treatment most often recommended for most persons with ADHD, by not following through on evaluations, parents also miss the opportunity to learn about nonpharmacological strategies that might also be helpful to help cope with ADHD symptoms and its potential co-occurring challenges,” she added.
Dr. Schweitzer also noted that many parents hope their children will outgrow the symptoms without realizing that by not obtaining an evaluation and treatment for their child, there is an associated cost, including less than optimal academic performance, social relationships, and emotional health.
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and under a National Institute on Drug Abuse contract followed by a data analysis grant. Dr. Molina reported grants from the NIMH and the National Institute on Drug Abuse during the conduct of the study.
A version of this article first appeared on Medscape.com.
“Throughout rigorous analyses, and after accounting for more than 70 variables in this longitudinal sample of children with ADHD taking stimulants, we did not find an association with later substance use,” lead investigator Brooke Molina, PhD, director of the youth and family research program at the University of Pittsburgh, said in an interview.
The findings were published online in JAMA Psychiatry.
Protective effect?
Owing to symptoms of impulsivity inherent to ADHD, the disorder itself carries a risk for elevated substance use, the investigators note.
They speculate that this may be why some previous research suggests prescription stimulants reduce the risk of subsequent substance use disorder. However, other studies have found no such protective link.
To shed more light on the issue, the investigators used data from the Multimodal Treatment Study of ADHD, a multicenter, 14-month randomized clinical trial of medication and behavioral therapy for children with ADHD. However, for the purposes of the present study, investigators focused only on stimulant use in children.
At the time of recruitment, the children were aged 7-9 and had been diagnosed with ADHD between 1994 and 1996.
Investigators assessed the participants prior to randomization, at months 3 and 9, and at the end of treatment. They were then followed for 16 years and were assessed at years 2, 3, 6, 8, 10, 12, 14, and 16 until a mean age of 25.
During 12-, 14-, and 16-year follow-up, participants completed a questionnaire on their use of alcohol, marijuana, cigarettes, and several illicit and prescription drugs.
Investigators collected information on participants’ stimulant treatment via the Services for Children and Adolescents Parent Interview until they reached age 18. After that, participants reported their own stimulant treatment.
A total of 579 participants were included in the analysis. Of these, 61% were White, 20% were Black, and 8% were Hispanic.
Decline in stimulant use over time
The analysis showed that stimulant use declined “precipitously” over time – from 60% at the 2- and 3-year assessments to an average of 7% during early adulthood.
The investigators also found that for some participants, substance use increased steadily through adolescence and remained stable through early adulthood. For instance, 36.5% of the adolescents in the total cohort reported smoking tobacco daily, and 29.6% reported using marijuana every week.
In addition, approximately 21% of the participants indulged in heavy drinking at least once a week, and 6% reported “other” substance use, which included sedative misuse, heroin, inhalants, hallucinogens, or other substances taken to “get high.”
After accounting for developmental trends in substance use in the sample through adolescence into early adulthood with several rigorous statistical models, the researchers found no association between current or prior stimulant treatment and cigarette, marijuana, alcohol, or other substance use, with one exception.
While cumulative stimulant treatment was associated with increased heavy drinking, the effect size of this association was small. Each additional year of cumulative stimulant use was estimated to increase participants’ likelihood of any binge drinking/drunkenness vs. none in the past year by 4% (95% confidence interval, 0.01-0.08; P =.03).
When the investigators used a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence that current (B range, –0.62-0.34) or prior stimulant treatment (B range, –0.06-0.70) or their interaction (B range, –0.49-0.86) was associated with substance use in adulthood.
Dr. Molina noted that although participants were recruited from multiple sites, the sample may not be generalizable because children and parents who present for an intensive treatment study such as this are not necessarily representative of the general ADHD population.
Reassuring findings
In a comment, Julie Schweitzer, PhD, professor of psychiatry and behavioral sciences at the University of California, Davis, said she hopes the study findings will quell the stigma surrounding stimulant use by children with ADHD.
“Parents’ fears that stimulant use will lead to a substance use disorder inhibits them from bringing their children for an ADHD evaluation, thus reducing the likelihood that they will receive timely treatment,” Dr. Schweitzer said.
“While stimulant medication is the first-line treatment most often recommended for most persons with ADHD, by not following through on evaluations, parents also miss the opportunity to learn about nonpharmacological strategies that might also be helpful to help cope with ADHD symptoms and its potential co-occurring challenges,” she added.
Dr. Schweitzer also noted that many parents hope their children will outgrow the symptoms without realizing that by not obtaining an evaluation and treatment for their child, there is an associated cost, including less than optimal academic performance, social relationships, and emotional health.
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and under a National Institute on Drug Abuse contract followed by a data analysis grant. Dr. Molina reported grants from the NIMH and the National Institute on Drug Abuse during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Nonstimulants: A better option for ADHD?
.
Investigators studied patients who started out taking atomoxetine and, after a washout period, initiated treatment with viloxazine. Participants’ ADHD symptoms were assessed prior to initiation of each treatment and after 4 weeks.
Children and adults showed significantly larger improvement in inattentiveness and hyperactivity/impulsivity when taking viloxazine vs. atomoxetine, with almost all patients preferring the former to the latter, according to results of the study.
In addition, close to one half of the study participants were taking a prior stimulant, and 85% were able to taper off stimulant treatment. Viloxazine’s effects were more rapid than were those of atomoxetine.
“It is timely to have a rapidly acting, and highly effective nonstimulant option across the full spectrum of ADHD symptoms, for both children and adults, in light of recent stimulant shortages and the new [Food and Drug Administration] boxed warnings regarding increased mortality associated with overuse of stimulants” study investigator Maxwell Z. Price, a medical student at Hackensack Meridian School of Medicine, Nutley, N.J., said in an interview.
Nonstimulant treatment options
Study coauthor Richard L. Price, MD, noted that the study was conducted to find a more acceptable alternative to psychostimulant treatments for ADHD, which are currently considered the “gold standard.”
Although they are effective, said Dr. Price, they are fraught with adverse effects, including appetite suppression, insomnia, exacerbation of mood disorders, anxiety, tics, or misuse.
Atomoxetine, a nonstimulant option, has been around for a few decades and is often used in combination with a stimulant medication. However, he said, the drug has a mild effect, requires frequent dosage adjustment, takes a long time to work, and people have “soured” on its utility, Dr. Price added.
Like atomoxetine, viloxazine is a selective norepinephrine inhibitor that has been used an antidepressant in Europe for 30 years. It was recently reformulated as an extended-release medication and approved by the FDA for pediatric and adult ADHD.
However, unlike atomoxetine, viloxazine is associated with increased prefrontal cortex 5-hydroxytrytamine, norepinephrine, and dopamine levels in vivo.
There have been no head-to-head trials comparing the two agents. However, even in head-to-head ADHD medication trials, the agents that are under investigation are typically compared in matched patients. The current investigators wanted to compare the two agents in the same patients whose insurers mandate a trial of generic atomoxetine prior to covering branded viloxazine.
“We wanted to find out whether patients taking atomoxetine for ADHD combined type would experience improvement in ADHD symptoms following voluntary, open-label switch to viloxazine,” said Dr. Price.
The researchers studied 50 patients who presented with ADHD combined type and had no other psychiatric, medical, or substance-related comorbidities or prior exposure to atomoxetine or viloxazine.
The study included 35 children (mean age, 11.9 ± 2.9 years; 94.3% male) and 15 adults (mean age, 29.3 ± 9.0 years; 73.3% male). Of these, 42.9% and 73.3%, respectively, were taking concurrent stimulants.
Patients received mean doses of atomoxetine once daily followed by viloxazine once daily after a 5-day washout period between the two drugs. Participants were seen weekly for titration and monitoring.
At baseline, the pediatric ADHD–Rating Scale 5 (ADHD-RS-5) and the Adult Investigator Symptoms Rating Scale (AISRS) were completed, then again after 4 weeks of treatment with atomoxetine (or upon earlier response or discontinuation due to side effects, whichever came first), and 5 days after discontinuing atomoxetine, which “reestablished the baseline score.” The same protocol was then repeated with viloxazine.
‘Paradigm shift’
At baseline, the total ADHD-RS-5 mean score was 40.3 ± 10.3. Improvements at 4 weeks were greater in viloxazine vs atomoxetine, with scores of 13.9 ± 10.2 vs 33.1 ± 12.1, respectively (t = -10.12, P < .00001). In inattention and hyperactivity/impulsivity, the t values were –8.57 and –9.87, respectively (both P values < .0001).
Similarly, from the baseline total, AISRS mean score of 37.3 ± 11.8, improvements were greater on viloxazine vs. atomoxetine, with scores of 11.9 ± 9.4 vs. 28.8 ± 14.9, respectively (t = −4.18, P = .0009 overall; for inattention, t = −3.50, P > .004 and for hyperactivity/impulsivity, t = 3.90, P > .002).
By 2 weeks, 86% of patients taking viloxazine reported a positive response vs. 14% when taking atomoxetine.
Side effects were lower in viloxazine vs. atomoxetine, with 36% of patients discontinuing treatment with atomoxetine because of side effects that included gastrointestinal upset, irritability, fatigue, and insomnia vs. 4% who discontinued viloxazine because of fatigue.
Almost all participants (96%) preferred viloxazine over atomoxetine and 85% were able to taper off stimulant treatment following stabilization on viloxazine.
“These were not small differences,” said Dr. Richard L. Price. “These were clinically and statistically meaningful differences.”
The findings could represent “a paradigm shift for the field” because “we always think of starting ADHD treatment with stimulants, but perhaps treatment with viloxazine could help patients to avoid stimulants entirely,” he suggested.
Real-world study
Commenting for this article, Greg Mattingly, MD, associate clinical professor, Washington University, St. Louis, called it “a timely addition to the clinical literature where for the first time ever we have two nonstimulant options approved for adults with ADHD.”
This real-world clinic study “yields many answers,” said Dr. Mattingly, president-elect of the American Professional Society of ADHD and Related Disorders (APSARD), who was not involved with the study.
“Simply put, this real-world study of 50 clinic patients found that viloxazine ER had faster onset, was significantly more effective, and was preferred by 96% of patients as compared to atomoxetine,” he said.
“Another intriguing part of the study that will be of high interest to both patients and providers was that, of those initially treated concurrently with stimulant and viloxazine ER, 85% were able to discontinue their stimulant medication,” Dr. Mattingly added.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The open access fee was funded by the investigators. Dr. Maxwell Z. Price certifies that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. Dr. Richard L. Price has received honoraria from AbbVie, Alkermes, Idorsia, Intra-Cellular Therapies, Janssen, Jazz, Lundbeck, Neuronetics, Otsuka, and Supernus. Dr. Mattingly reports financial disclosures with various pharmaceutical companies, which are listed in full in the paper.
A version of this article first appeared on Medscape.com.
.
Investigators studied patients who started out taking atomoxetine and, after a washout period, initiated treatment with viloxazine. Participants’ ADHD symptoms were assessed prior to initiation of each treatment and after 4 weeks.
Children and adults showed significantly larger improvement in inattentiveness and hyperactivity/impulsivity when taking viloxazine vs. atomoxetine, with almost all patients preferring the former to the latter, according to results of the study.
In addition, close to one half of the study participants were taking a prior stimulant, and 85% were able to taper off stimulant treatment. Viloxazine’s effects were more rapid than were those of atomoxetine.
“It is timely to have a rapidly acting, and highly effective nonstimulant option across the full spectrum of ADHD symptoms, for both children and adults, in light of recent stimulant shortages and the new [Food and Drug Administration] boxed warnings regarding increased mortality associated with overuse of stimulants” study investigator Maxwell Z. Price, a medical student at Hackensack Meridian School of Medicine, Nutley, N.J., said in an interview.
Nonstimulant treatment options
Study coauthor Richard L. Price, MD, noted that the study was conducted to find a more acceptable alternative to psychostimulant treatments for ADHD, which are currently considered the “gold standard.”
Although they are effective, said Dr. Price, they are fraught with adverse effects, including appetite suppression, insomnia, exacerbation of mood disorders, anxiety, tics, or misuse.
Atomoxetine, a nonstimulant option, has been around for a few decades and is often used in combination with a stimulant medication. However, he said, the drug has a mild effect, requires frequent dosage adjustment, takes a long time to work, and people have “soured” on its utility, Dr. Price added.
Like atomoxetine, viloxazine is a selective norepinephrine inhibitor that has been used an antidepressant in Europe for 30 years. It was recently reformulated as an extended-release medication and approved by the FDA for pediatric and adult ADHD.
However, unlike atomoxetine, viloxazine is associated with increased prefrontal cortex 5-hydroxytrytamine, norepinephrine, and dopamine levels in vivo.
There have been no head-to-head trials comparing the two agents. However, even in head-to-head ADHD medication trials, the agents that are under investigation are typically compared in matched patients. The current investigators wanted to compare the two agents in the same patients whose insurers mandate a trial of generic atomoxetine prior to covering branded viloxazine.
“We wanted to find out whether patients taking atomoxetine for ADHD combined type would experience improvement in ADHD symptoms following voluntary, open-label switch to viloxazine,” said Dr. Price.
The researchers studied 50 patients who presented with ADHD combined type and had no other psychiatric, medical, or substance-related comorbidities or prior exposure to atomoxetine or viloxazine.
The study included 35 children (mean age, 11.9 ± 2.9 years; 94.3% male) and 15 adults (mean age, 29.3 ± 9.0 years; 73.3% male). Of these, 42.9% and 73.3%, respectively, were taking concurrent stimulants.
Patients received mean doses of atomoxetine once daily followed by viloxazine once daily after a 5-day washout period between the two drugs. Participants were seen weekly for titration and monitoring.
At baseline, the pediatric ADHD–Rating Scale 5 (ADHD-RS-5) and the Adult Investigator Symptoms Rating Scale (AISRS) were completed, then again after 4 weeks of treatment with atomoxetine (or upon earlier response or discontinuation due to side effects, whichever came first), and 5 days after discontinuing atomoxetine, which “reestablished the baseline score.” The same protocol was then repeated with viloxazine.
‘Paradigm shift’
At baseline, the total ADHD-RS-5 mean score was 40.3 ± 10.3. Improvements at 4 weeks were greater in viloxazine vs atomoxetine, with scores of 13.9 ± 10.2 vs 33.1 ± 12.1, respectively (t = -10.12, P < .00001). In inattention and hyperactivity/impulsivity, the t values were –8.57 and –9.87, respectively (both P values < .0001).
Similarly, from the baseline total, AISRS mean score of 37.3 ± 11.8, improvements were greater on viloxazine vs. atomoxetine, with scores of 11.9 ± 9.4 vs. 28.8 ± 14.9, respectively (t = −4.18, P = .0009 overall; for inattention, t = −3.50, P > .004 and for hyperactivity/impulsivity, t = 3.90, P > .002).
By 2 weeks, 86% of patients taking viloxazine reported a positive response vs. 14% when taking atomoxetine.
Side effects were lower in viloxazine vs. atomoxetine, with 36% of patients discontinuing treatment with atomoxetine because of side effects that included gastrointestinal upset, irritability, fatigue, and insomnia vs. 4% who discontinued viloxazine because of fatigue.
Almost all participants (96%) preferred viloxazine over atomoxetine and 85% were able to taper off stimulant treatment following stabilization on viloxazine.
“These were not small differences,” said Dr. Richard L. Price. “These were clinically and statistically meaningful differences.”
The findings could represent “a paradigm shift for the field” because “we always think of starting ADHD treatment with stimulants, but perhaps treatment with viloxazine could help patients to avoid stimulants entirely,” he suggested.
Real-world study
Commenting for this article, Greg Mattingly, MD, associate clinical professor, Washington University, St. Louis, called it “a timely addition to the clinical literature where for the first time ever we have two nonstimulant options approved for adults with ADHD.”
This real-world clinic study “yields many answers,” said Dr. Mattingly, president-elect of the American Professional Society of ADHD and Related Disorders (APSARD), who was not involved with the study.
“Simply put, this real-world study of 50 clinic patients found that viloxazine ER had faster onset, was significantly more effective, and was preferred by 96% of patients as compared to atomoxetine,” he said.
“Another intriguing part of the study that will be of high interest to both patients and providers was that, of those initially treated concurrently with stimulant and viloxazine ER, 85% were able to discontinue their stimulant medication,” Dr. Mattingly added.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The open access fee was funded by the investigators. Dr. Maxwell Z. Price certifies that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. Dr. Richard L. Price has received honoraria from AbbVie, Alkermes, Idorsia, Intra-Cellular Therapies, Janssen, Jazz, Lundbeck, Neuronetics, Otsuka, and Supernus. Dr. Mattingly reports financial disclosures with various pharmaceutical companies, which are listed in full in the paper.
A version of this article first appeared on Medscape.com.
.
Investigators studied patients who started out taking atomoxetine and, after a washout period, initiated treatment with viloxazine. Participants’ ADHD symptoms were assessed prior to initiation of each treatment and after 4 weeks.
Children and adults showed significantly larger improvement in inattentiveness and hyperactivity/impulsivity when taking viloxazine vs. atomoxetine, with almost all patients preferring the former to the latter, according to results of the study.
In addition, close to one half of the study participants were taking a prior stimulant, and 85% were able to taper off stimulant treatment. Viloxazine’s effects were more rapid than were those of atomoxetine.
“It is timely to have a rapidly acting, and highly effective nonstimulant option across the full spectrum of ADHD symptoms, for both children and adults, in light of recent stimulant shortages and the new [Food and Drug Administration] boxed warnings regarding increased mortality associated with overuse of stimulants” study investigator Maxwell Z. Price, a medical student at Hackensack Meridian School of Medicine, Nutley, N.J., said in an interview.
Nonstimulant treatment options
Study coauthor Richard L. Price, MD, noted that the study was conducted to find a more acceptable alternative to psychostimulant treatments for ADHD, which are currently considered the “gold standard.”
Although they are effective, said Dr. Price, they are fraught with adverse effects, including appetite suppression, insomnia, exacerbation of mood disorders, anxiety, tics, or misuse.
Atomoxetine, a nonstimulant option, has been around for a few decades and is often used in combination with a stimulant medication. However, he said, the drug has a mild effect, requires frequent dosage adjustment, takes a long time to work, and people have “soured” on its utility, Dr. Price added.
Like atomoxetine, viloxazine is a selective norepinephrine inhibitor that has been used an antidepressant in Europe for 30 years. It was recently reformulated as an extended-release medication and approved by the FDA for pediatric and adult ADHD.
However, unlike atomoxetine, viloxazine is associated with increased prefrontal cortex 5-hydroxytrytamine, norepinephrine, and dopamine levels in vivo.
There have been no head-to-head trials comparing the two agents. However, even in head-to-head ADHD medication trials, the agents that are under investigation are typically compared in matched patients. The current investigators wanted to compare the two agents in the same patients whose insurers mandate a trial of generic atomoxetine prior to covering branded viloxazine.
“We wanted to find out whether patients taking atomoxetine for ADHD combined type would experience improvement in ADHD symptoms following voluntary, open-label switch to viloxazine,” said Dr. Price.
The researchers studied 50 patients who presented with ADHD combined type and had no other psychiatric, medical, or substance-related comorbidities or prior exposure to atomoxetine or viloxazine.
The study included 35 children (mean age, 11.9 ± 2.9 years; 94.3% male) and 15 adults (mean age, 29.3 ± 9.0 years; 73.3% male). Of these, 42.9% and 73.3%, respectively, were taking concurrent stimulants.
Patients received mean doses of atomoxetine once daily followed by viloxazine once daily after a 5-day washout period between the two drugs. Participants were seen weekly for titration and monitoring.
At baseline, the pediatric ADHD–Rating Scale 5 (ADHD-RS-5) and the Adult Investigator Symptoms Rating Scale (AISRS) were completed, then again after 4 weeks of treatment with atomoxetine (or upon earlier response or discontinuation due to side effects, whichever came first), and 5 days after discontinuing atomoxetine, which “reestablished the baseline score.” The same protocol was then repeated with viloxazine.
‘Paradigm shift’
At baseline, the total ADHD-RS-5 mean score was 40.3 ± 10.3. Improvements at 4 weeks were greater in viloxazine vs atomoxetine, with scores of 13.9 ± 10.2 vs 33.1 ± 12.1, respectively (t = -10.12, P < .00001). In inattention and hyperactivity/impulsivity, the t values were –8.57 and –9.87, respectively (both P values < .0001).
Similarly, from the baseline total, AISRS mean score of 37.3 ± 11.8, improvements were greater on viloxazine vs. atomoxetine, with scores of 11.9 ± 9.4 vs. 28.8 ± 14.9, respectively (t = −4.18, P = .0009 overall; for inattention, t = −3.50, P > .004 and for hyperactivity/impulsivity, t = 3.90, P > .002).
By 2 weeks, 86% of patients taking viloxazine reported a positive response vs. 14% when taking atomoxetine.
Side effects were lower in viloxazine vs. atomoxetine, with 36% of patients discontinuing treatment with atomoxetine because of side effects that included gastrointestinal upset, irritability, fatigue, and insomnia vs. 4% who discontinued viloxazine because of fatigue.
Almost all participants (96%) preferred viloxazine over atomoxetine and 85% were able to taper off stimulant treatment following stabilization on viloxazine.
“These were not small differences,” said Dr. Richard L. Price. “These were clinically and statistically meaningful differences.”
The findings could represent “a paradigm shift for the field” because “we always think of starting ADHD treatment with stimulants, but perhaps treatment with viloxazine could help patients to avoid stimulants entirely,” he suggested.
Real-world study
Commenting for this article, Greg Mattingly, MD, associate clinical professor, Washington University, St. Louis, called it “a timely addition to the clinical literature where for the first time ever we have two nonstimulant options approved for adults with ADHD.”
This real-world clinic study “yields many answers,” said Dr. Mattingly, president-elect of the American Professional Society of ADHD and Related Disorders (APSARD), who was not involved with the study.
“Simply put, this real-world study of 50 clinic patients found that viloxazine ER had faster onset, was significantly more effective, and was preferred by 96% of patients as compared to atomoxetine,” he said.
“Another intriguing part of the study that will be of high interest to both patients and providers was that, of those initially treated concurrently with stimulant and viloxazine ER, 85% were able to discontinue their stimulant medication,” Dr. Mattingly added.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The open access fee was funded by the investigators. Dr. Maxwell Z. Price certifies that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. Dr. Richard L. Price has received honoraria from AbbVie, Alkermes, Idorsia, Intra-Cellular Therapies, Janssen, Jazz, Lundbeck, Neuronetics, Otsuka, and Supernus. Dr. Mattingly reports financial disclosures with various pharmaceutical companies, which are listed in full in the paper.
A version of this article first appeared on Medscape.com.
FROM CNS DRUGS