User login
Point/Counterpoint: Is endograft PAA repair durable?
Endovascular repair is durable
Endovascular repair of popliteal artery aneurysms is vastly superior to all other previous techniques of popliteal aneurysm repair. Half of all popliteal artery aneurysms are bilateral, and 40% are associated with abdominal aortic aneurysm; 1%-2% of patients with abdominal aortic aneurysm have a popliteal aneurysm (ANZ J Surg. 2006 Oct;76[10]:912-5). Less than 0.01% of hospitalized patients have popliteal artery aneurysms, and men are 20 times more prone to them than women are.
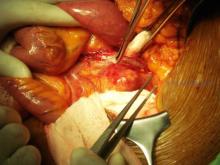
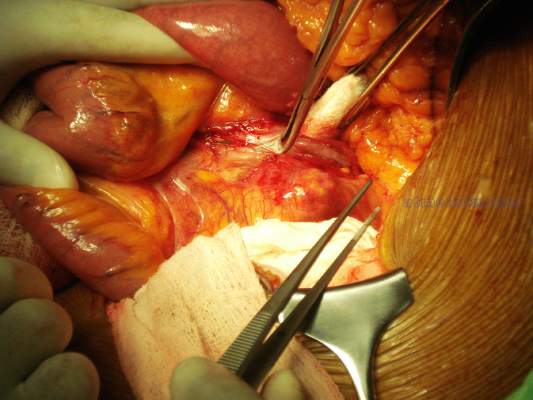
Traditional treatment involves either bypass with interval ligation or a direct posterior approach with an interposition graft, but surgery is not without its problems. I think of the retired anesthesiologist who came to me with a popliteal artery aneurysm (PAA) that his primary care doctor diagnosed. “I’m not having any damn femoral popliteal bypass operation,” he told me. “Every single one of those patients dies.”
Endograft repair is a technique that is reaching its prime, as a growing number of reports have shown – although none of these studies has large numbers because the volume just isn’t available. One recent paper compared 52 open and 23 endovascular PAA repairs (Ann Vasc Surg. 2016 Jan;30:253-7) and found both had similarly high rates of reintervention – 50% at 4 years. But it is noteworthy that the endovascular results improved with time.
A University of Pittsburgh study of 186 open and endovascular repairs found that patients with acute presentations of embolization or aneurysm thrombosis did better with open surgery. In addition, while open repair had superior patency initially after surgery, midterm secondary patency and amputation rates of open and endovascular repair were similar (J Vasc Surg. 2016 Jan;63[1]:70-6).
A Netherlands study of 72 PAA treated with endografting showed that 84% had primary patency at 1 year, and 74% had assisted primary patency at 3 years (Eur J Vasc Endovasc Surg. 2016 Jul;52[1]:99-104). Among these patients, 13 had late occlusions, 7 were converted to bypass, and 2 required thrombolysis; but none required limb amputation.
A meta-analysis of 540 patients found no statistically significant difference in outcomes between endovascular and open repair for PAA (Eur J Vasc Endovasc Surg. 2015 Sep;50(3):351-9). Another systematic review and meta-analysis of 14 studies and 514 patients also found no difference in pooled primary and secondary patency at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7).
There certainly are contradictory studies, such as one by Dr. Alik Farber’s group in Boston that showed open repair is superior to endovascular surgery (J Vasc Surg. 2015 Mar;61[3]:663-9); but retrospective database mining certainly has its limitations. Their retrospective study queried the Vascular Quality Initiative database and found that 95% of patients who had open elective popliteal aneurysm repair were free from major adverse limb events, vs. 80% for endovascular treatments.
The best outcomes of open repair happen with autologous vein, but there is precious little of that around now. Emergency patients would probably do better with open surgery, but in elective repair there is no clear differential data.
So, if that’s the case, I’m going to take the small incision.
Peter Rossi, MD, FACS, is an associate professor of surgery and radiology, and the clinical director of vascular surgery, at the Medical College of Wisconsin, Milwaukee. He is also on staff at Clement J. Zablocki Veterans Affairs Medical Center in Milwaukee. Dr. Rossi had no financial relationships to disclose.
Endovascular repair may not be durable
Debating the durability of elective endovascular repair of popliteal artery aneurysm raises a question: Who determines durability anyway?
Is it the patients who only want the Band-Aid and no incision? I don’t think so. Is it the interventionalist who only does endovascular repairs? I don’t think so. I’m sure it’s not the insurance companies, who only worry about cost containment, either.
So, who should determine durability of endovascular popliteal artery aneurysm (PAA) repair?
So, the question is, do we have such data?
There are multiple reports looking at how well open repair works. It has been done for decades. In 2008, a Veterans Affairs study of 583 open PAA repairs reported low death rates and excellent rates of limb salvage at 2 years, even in high-risk patients (J Vasc Surg. 2008 Oct;48[4]:845-51). Open surgical repair has excellent documented durability, and that is not the question at hand.
Endovascular repair has some presumed advantages. It’s less invasive and involves less postoperative pain and a quicker recovery. But it is not without problems – graft thrombosis and occlusion, endoleaks, distal limb ischemia, and stent fractures among them.
Surgery, to be clear, is not perfect, either. One of my patients who years ago presented with an occluded PAA underwent open bypass repair – but then went on later to have a pseudoaneurysm of the proximal anastomosis. I repaired this with an endograft, and he has done quite well. So, we all do endograft repairs, walk out, chest bump the Gore rep, and send the patient home that day.
Is it durable, though?
Most of the data on endovascular repair are from single-center studies dating back to 2003. There’s only one prospective trial comparing endovascular vs. open repair (J Vasc Surg. 2005 Aug;42[2]:185-93), but it was a single-center trial with a severe power limitation, because it involved only 30 patients. It found endovascular repair was comparable to open surgery. Also, I suspect a great deal of selection bias is involved in studies of endovascular repair.
A number of studies have found endovascular repair is not inferior to surgical repair. For example, a study by Dr. Audra Duncan, at Mayo Clinic, and her colleagues found that primary and secondary patency rates of elective and emergent stenting were excellent – but the study results only extended out to 2 years (J Vasc Surg. 2013 May;57[5]:1299-305). I don’t think we could hang our hat on that.
A Swedish study that compared open and endovascular surgery in 592 patients reported that endovascular repair has “significantly inferior results compared with open repair,” particularly in those who present with acute ischemia (Eur J Vasc Endovasc Surg. 2015 Sep;50[3]:342-50). A close look at the data shows that primary patency rates were 89% for open repair and 67.4% for stent graft.
Referencing the systematic review and meta-analysis that Dr. Rossi cited, the primary patency of endovascular repair was only 69% and the secondary patency rate was 77% at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7). As physicians, I submit that we can do better.
A Netherlands study investigated stent fractures, finding that 17% (13 out of 78 cases) had circumferential fractures (J Vasc Surg. 2010 Jun;51[6]:1413-8). This study only included circumferential stent fractures and excluded localized strut fractures. I think these studies show that endovascular repair is not always durable.
I want to remind you that we are vascular surgeons, so it is appropriate for us to embrace surgical bypass and its known durability, especially when the durability of endovascular repair is still not known.
Patrick Muck, MD, is chief of vascular surgery and director of vascular residency and fellowship at Good Samaritan Hospital, Cincinnati. He is also on staff at Bethesda North Hospital, Cincinnati, and is affiliated with TriHealth Heart Institute in southwestern Ohio. Dr. Muck had no financial relationships to disclose.
Endovascular repair is durable
Endovascular repair of popliteal artery aneurysms is vastly superior to all other previous techniques of popliteal aneurysm repair. Half of all popliteal artery aneurysms are bilateral, and 40% are associated with abdominal aortic aneurysm; 1%-2% of patients with abdominal aortic aneurysm have a popliteal aneurysm (ANZ J Surg. 2006 Oct;76[10]:912-5). Less than 0.01% of hospitalized patients have popliteal artery aneurysms, and men are 20 times more prone to them than women are.
Traditional treatment involves either bypass with interval ligation or a direct posterior approach with an interposition graft, but surgery is not without its problems. I think of the retired anesthesiologist who came to me with a popliteal artery aneurysm (PAA) that his primary care doctor diagnosed. “I’m not having any damn femoral popliteal bypass operation,” he told me. “Every single one of those patients dies.”
Endograft repair is a technique that is reaching its prime, as a growing number of reports have shown – although none of these studies has large numbers because the volume just isn’t available. One recent paper compared 52 open and 23 endovascular PAA repairs (Ann Vasc Surg. 2016 Jan;30:253-7) and found both had similarly high rates of reintervention – 50% at 4 years. But it is noteworthy that the endovascular results improved with time.
A University of Pittsburgh study of 186 open and endovascular repairs found that patients with acute presentations of embolization or aneurysm thrombosis did better with open surgery. In addition, while open repair had superior patency initially after surgery, midterm secondary patency and amputation rates of open and endovascular repair were similar (J Vasc Surg. 2016 Jan;63[1]:70-6).
A Netherlands study of 72 PAA treated with endografting showed that 84% had primary patency at 1 year, and 74% had assisted primary patency at 3 years (Eur J Vasc Endovasc Surg. 2016 Jul;52[1]:99-104). Among these patients, 13 had late occlusions, 7 were converted to bypass, and 2 required thrombolysis; but none required limb amputation.
A meta-analysis of 540 patients found no statistically significant difference in outcomes between endovascular and open repair for PAA (Eur J Vasc Endovasc Surg. 2015 Sep;50(3):351-9). Another systematic review and meta-analysis of 14 studies and 514 patients also found no difference in pooled primary and secondary patency at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7).
There certainly are contradictory studies, such as one by Dr. Alik Farber’s group in Boston that showed open repair is superior to endovascular surgery (J Vasc Surg. 2015 Mar;61[3]:663-9); but retrospective database mining certainly has its limitations. Their retrospective study queried the Vascular Quality Initiative database and found that 95% of patients who had open elective popliteal aneurysm repair were free from major adverse limb events, vs. 80% for endovascular treatments.
The best outcomes of open repair happen with autologous vein, but there is precious little of that around now. Emergency patients would probably do better with open surgery, but in elective repair there is no clear differential data.
So, if that’s the case, I’m going to take the small incision.
Peter Rossi, MD, FACS, is an associate professor of surgery and radiology, and the clinical director of vascular surgery, at the Medical College of Wisconsin, Milwaukee. He is also on staff at Clement J. Zablocki Veterans Affairs Medical Center in Milwaukee. Dr. Rossi had no financial relationships to disclose.
Endovascular repair may not be durable
Debating the durability of elective endovascular repair of popliteal artery aneurysm raises a question: Who determines durability anyway?
Is it the patients who only want the Band-Aid and no incision? I don’t think so. Is it the interventionalist who only does endovascular repairs? I don’t think so. I’m sure it’s not the insurance companies, who only worry about cost containment, either.
So, who should determine durability of endovascular popliteal artery aneurysm (PAA) repair?
So, the question is, do we have such data?
There are multiple reports looking at how well open repair works. It has been done for decades. In 2008, a Veterans Affairs study of 583 open PAA repairs reported low death rates and excellent rates of limb salvage at 2 years, even in high-risk patients (J Vasc Surg. 2008 Oct;48[4]:845-51). Open surgical repair has excellent documented durability, and that is not the question at hand.
Endovascular repair has some presumed advantages. It’s less invasive and involves less postoperative pain and a quicker recovery. But it is not without problems – graft thrombosis and occlusion, endoleaks, distal limb ischemia, and stent fractures among them.
Surgery, to be clear, is not perfect, either. One of my patients who years ago presented with an occluded PAA underwent open bypass repair – but then went on later to have a pseudoaneurysm of the proximal anastomosis. I repaired this with an endograft, and he has done quite well. So, we all do endograft repairs, walk out, chest bump the Gore rep, and send the patient home that day.
Is it durable, though?
Most of the data on endovascular repair are from single-center studies dating back to 2003. There’s only one prospective trial comparing endovascular vs. open repair (J Vasc Surg. 2005 Aug;42[2]:185-93), but it was a single-center trial with a severe power limitation, because it involved only 30 patients. It found endovascular repair was comparable to open surgery. Also, I suspect a great deal of selection bias is involved in studies of endovascular repair.
A number of studies have found endovascular repair is not inferior to surgical repair. For example, a study by Dr. Audra Duncan, at Mayo Clinic, and her colleagues found that primary and secondary patency rates of elective and emergent stenting were excellent – but the study results only extended out to 2 years (J Vasc Surg. 2013 May;57[5]:1299-305). I don’t think we could hang our hat on that.
A Swedish study that compared open and endovascular surgery in 592 patients reported that endovascular repair has “significantly inferior results compared with open repair,” particularly in those who present with acute ischemia (Eur J Vasc Endovasc Surg. 2015 Sep;50[3]:342-50). A close look at the data shows that primary patency rates were 89% for open repair and 67.4% for stent graft.
Referencing the systematic review and meta-analysis that Dr. Rossi cited, the primary patency of endovascular repair was only 69% and the secondary patency rate was 77% at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7). As physicians, I submit that we can do better.
A Netherlands study investigated stent fractures, finding that 17% (13 out of 78 cases) had circumferential fractures (J Vasc Surg. 2010 Jun;51[6]:1413-8). This study only included circumferential stent fractures and excluded localized strut fractures. I think these studies show that endovascular repair is not always durable.
I want to remind you that we are vascular surgeons, so it is appropriate for us to embrace surgical bypass and its known durability, especially when the durability of endovascular repair is still not known.
Patrick Muck, MD, is chief of vascular surgery and director of vascular residency and fellowship at Good Samaritan Hospital, Cincinnati. He is also on staff at Bethesda North Hospital, Cincinnati, and is affiliated with TriHealth Heart Institute in southwestern Ohio. Dr. Muck had no financial relationships to disclose.
Endovascular repair is durable
Endovascular repair of popliteal artery aneurysms is vastly superior to all other previous techniques of popliteal aneurysm repair. Half of all popliteal artery aneurysms are bilateral, and 40% are associated with abdominal aortic aneurysm; 1%-2% of patients with abdominal aortic aneurysm have a popliteal aneurysm (ANZ J Surg. 2006 Oct;76[10]:912-5). Less than 0.01% of hospitalized patients have popliteal artery aneurysms, and men are 20 times more prone to them than women are.
Traditional treatment involves either bypass with interval ligation or a direct posterior approach with an interposition graft, but surgery is not without its problems. I think of the retired anesthesiologist who came to me with a popliteal artery aneurysm (PAA) that his primary care doctor diagnosed. “I’m not having any damn femoral popliteal bypass operation,” he told me. “Every single one of those patients dies.”
Endograft repair is a technique that is reaching its prime, as a growing number of reports have shown – although none of these studies has large numbers because the volume just isn’t available. One recent paper compared 52 open and 23 endovascular PAA repairs (Ann Vasc Surg. 2016 Jan;30:253-7) and found both had similarly high rates of reintervention – 50% at 4 years. But it is noteworthy that the endovascular results improved with time.
A University of Pittsburgh study of 186 open and endovascular repairs found that patients with acute presentations of embolization or aneurysm thrombosis did better with open surgery. In addition, while open repair had superior patency initially after surgery, midterm secondary patency and amputation rates of open and endovascular repair were similar (J Vasc Surg. 2016 Jan;63[1]:70-6).
A Netherlands study of 72 PAA treated with endografting showed that 84% had primary patency at 1 year, and 74% had assisted primary patency at 3 years (Eur J Vasc Endovasc Surg. 2016 Jul;52[1]:99-104). Among these patients, 13 had late occlusions, 7 were converted to bypass, and 2 required thrombolysis; but none required limb amputation.
A meta-analysis of 540 patients found no statistically significant difference in outcomes between endovascular and open repair for PAA (Eur J Vasc Endovasc Surg. 2015 Sep;50(3):351-9). Another systematic review and meta-analysis of 14 studies and 514 patients also found no difference in pooled primary and secondary patency at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7).
There certainly are contradictory studies, such as one by Dr. Alik Farber’s group in Boston that showed open repair is superior to endovascular surgery (J Vasc Surg. 2015 Mar;61[3]:663-9); but retrospective database mining certainly has its limitations. Their retrospective study queried the Vascular Quality Initiative database and found that 95% of patients who had open elective popliteal aneurysm repair were free from major adverse limb events, vs. 80% for endovascular treatments.
The best outcomes of open repair happen with autologous vein, but there is precious little of that around now. Emergency patients would probably do better with open surgery, but in elective repair there is no clear differential data.
So, if that’s the case, I’m going to take the small incision.
Peter Rossi, MD, FACS, is an associate professor of surgery and radiology, and the clinical director of vascular surgery, at the Medical College of Wisconsin, Milwaukee. He is also on staff at Clement J. Zablocki Veterans Affairs Medical Center in Milwaukee. Dr. Rossi had no financial relationships to disclose.
Endovascular repair may not be durable
Debating the durability of elective endovascular repair of popliteal artery aneurysm raises a question: Who determines durability anyway?
Is it the patients who only want the Band-Aid and no incision? I don’t think so. Is it the interventionalist who only does endovascular repairs? I don’t think so. I’m sure it’s not the insurance companies, who only worry about cost containment, either.
So, who should determine durability of endovascular popliteal artery aneurysm (PAA) repair?
So, the question is, do we have such data?
There are multiple reports looking at how well open repair works. It has been done for decades. In 2008, a Veterans Affairs study of 583 open PAA repairs reported low death rates and excellent rates of limb salvage at 2 years, even in high-risk patients (J Vasc Surg. 2008 Oct;48[4]:845-51). Open surgical repair has excellent documented durability, and that is not the question at hand.
Endovascular repair has some presumed advantages. It’s less invasive and involves less postoperative pain and a quicker recovery. But it is not without problems – graft thrombosis and occlusion, endoleaks, distal limb ischemia, and stent fractures among them.
Surgery, to be clear, is not perfect, either. One of my patients who years ago presented with an occluded PAA underwent open bypass repair – but then went on later to have a pseudoaneurysm of the proximal anastomosis. I repaired this with an endograft, and he has done quite well. So, we all do endograft repairs, walk out, chest bump the Gore rep, and send the patient home that day.
Is it durable, though?
Most of the data on endovascular repair are from single-center studies dating back to 2003. There’s only one prospective trial comparing endovascular vs. open repair (J Vasc Surg. 2005 Aug;42[2]:185-93), but it was a single-center trial with a severe power limitation, because it involved only 30 patients. It found endovascular repair was comparable to open surgery. Also, I suspect a great deal of selection bias is involved in studies of endovascular repair.
A number of studies have found endovascular repair is not inferior to surgical repair. For example, a study by Dr. Audra Duncan, at Mayo Clinic, and her colleagues found that primary and secondary patency rates of elective and emergent stenting were excellent – but the study results only extended out to 2 years (J Vasc Surg. 2013 May;57[5]:1299-305). I don’t think we could hang our hat on that.
A Swedish study that compared open and endovascular surgery in 592 patients reported that endovascular repair has “significantly inferior results compared with open repair,” particularly in those who present with acute ischemia (Eur J Vasc Endovasc Surg. 2015 Sep;50[3]:342-50). A close look at the data shows that primary patency rates were 89% for open repair and 67.4% for stent graft.
Referencing the systematic review and meta-analysis that Dr. Rossi cited, the primary patency of endovascular repair was only 69% and the secondary patency rate was 77% at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7). As physicians, I submit that we can do better.
A Netherlands study investigated stent fractures, finding that 17% (13 out of 78 cases) had circumferential fractures (J Vasc Surg. 2010 Jun;51[6]:1413-8). This study only included circumferential stent fractures and excluded localized strut fractures. I think these studies show that endovascular repair is not always durable.
I want to remind you that we are vascular surgeons, so it is appropriate for us to embrace surgical bypass and its known durability, especially when the durability of endovascular repair is still not known.
Patrick Muck, MD, is chief of vascular surgery and director of vascular residency and fellowship at Good Samaritan Hospital, Cincinnati. He is also on staff at Bethesda North Hospital, Cincinnati, and is affiliated with TriHealth Heart Institute in southwestern Ohio. Dr. Muck had no financial relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Vascular surgeons underutilize palliative care planning
Investment in advanced palliative care planning has the potential to improve the quality of care for vascular surgery patients, according to investigators from Oregon Health and Science University, Portland.
Dale G. Wilson, MD, and his colleagues performed a retrospective review of electronic medical records for 111 patients, who died while on the vascular surgery service at the OHSU Hospital during 2005-2014.
Almost three-quarters (73%) of patients were transitioned to palliative care; of those, 14% presented with an advanced directive, and 28% received a palliative care consultation (JAMA Surg. 2017;152[2]:183-90. doi: 10.1001/jamasurg.2016.3970).
While palliative care services are increasing in hospitals, accounting for 4% of annual hospital admissions in 2012 according to the study, they are not implemented consistently. “Many teams from various specialties care for patients at end of life; however, we still do not know what prompts end-of-life discussions,” Dr. Wilson said. “There is still no consensus on when to involve palliative services in the care of critically ill patients.”
While the decision to advise a consultation is “variable and physician dependent,” the type of treatment required may help identify when consultations are appropriate.
Of the 14 patients who did not choose comfort care, 11 (79%) required CPR. Additionally, all had to be taken to the operating room and required mechanical ventilation.
Of 81 patients who chose palliative care, 31 did so despite potential medical options. These patients were older – average age, 77 years, as compared with 68 years for patients who did not choose comfort care – with 8 of the 31 (26%) presenting an advanced directive, compared with only 7 of 83 patients (8%) for those who did not receive palliative care.
Dr. Wilson and his colleagues found that patients who chose palliative care were more likely to have received a palliative care consultation, as well: 10 of 31 patients who chose comfort care received a consultation, as opposed to 1 of 83 who chose comfort care but did not receive a consultation.
The nature of the vascular surgery service calls for early efforts to gather information regarding patients’ views on end-of-life care, Dr. Wilson said, noting that 73% of patients studied were admitted emergently and 87% underwent surgery, leaving little time for patients to express their wishes.
“Because the events associated with withdrawal of care are often not anticipated, we argue that all vascular surgical patients should have an advance directive, and perhaps, those at particular high risk should have a preoperative palliative care consultation,” Dr. Wilson wrote.
Limitations to the study included the data abstraction, which was performed by a single unblinded physician. Researchers also gathered patients’ reasons for transitioning to comfort care retrospectively.
The low rate of palliative care consultations found in this study mirrors my own experience, as does the feeling of urgency to shed more light on the issue. The biggest hurdle surgeons face when it comes to palliative care consultations is that, in their minds, seeking these meetings is associated with immediate death care. Many surgeons are shy about bringing palliative care specialists on board because approaching families can be daunting.
Family members who do not know enough about comfort care can be upset by the idea. Addressing this misunderstanding is crucial. Consultations are not just conversations about hospice care but can be emotional and spiritual experiences that prepare both the family and the patient for alternative options when surgical intervention cannot guarantee a good quality of life. I would encourage surgeons to be more proactive and less defensive about comfort care . Luckily, understanding the importance of this issue among professionals is growing.
When I approach these situations, it’s important for me to have a full understanding of what families and patients usually expect. Decisions should not be based on how bad things are now but on the future. What was the patient’s last year like? What is the best-case scenario for moving forward on a proposed intervention? What will the patient’s quality of life be? Answering these questions helps the patient understand his or her situation, without diminishing a surgeon’s ability. If you are honest, the family will usually come to the conclusion that they do not want to subject the patient to ultimately unnecessary treatment.
Palliative care services help patients and their families deal with pain beyond the physical symptoms. Dealing with pain, depression, or delirium is only a part of comfort care – coping with a sense of hopelessness, family disruption, or feelings of guilt also can be a part and, significantly, a part that surgeons are not trained to diagnose or treat.
With more than 70 surgeons certified in hospice care and a growing number of fellowships in palliative care, I am extremely optimistic in the progress we have made and will continue to make.
Geoffrey Dunn, MD, FACS, is the medical director of the Palliative Care Consultation Service at UPMC Hamot Medical Center, Erie, Penn. He currently is Community Editor for the Pain and Palliative Care Community for the ACS’s web portal.
The low rate of palliative care consultations found in this study mirrors my own experience, as does the feeling of urgency to shed more light on the issue. The biggest hurdle surgeons face when it comes to palliative care consultations is that, in their minds, seeking these meetings is associated with immediate death care. Many surgeons are shy about bringing palliative care specialists on board because approaching families can be daunting.
Family members who do not know enough about comfort care can be upset by the idea. Addressing this misunderstanding is crucial. Consultations are not just conversations about hospice care but can be emotional and spiritual experiences that prepare both the family and the patient for alternative options when surgical intervention cannot guarantee a good quality of life. I would encourage surgeons to be more proactive and less defensive about comfort care . Luckily, understanding the importance of this issue among professionals is growing.
When I approach these situations, it’s important for me to have a full understanding of what families and patients usually expect. Decisions should not be based on how bad things are now but on the future. What was the patient’s last year like? What is the best-case scenario for moving forward on a proposed intervention? What will the patient’s quality of life be? Answering these questions helps the patient understand his or her situation, without diminishing a surgeon’s ability. If you are honest, the family will usually come to the conclusion that they do not want to subject the patient to ultimately unnecessary treatment.
Palliative care services help patients and their families deal with pain beyond the physical symptoms. Dealing with pain, depression, or delirium is only a part of comfort care – coping with a sense of hopelessness, family disruption, or feelings of guilt also can be a part and, significantly, a part that surgeons are not trained to diagnose or treat.
With more than 70 surgeons certified in hospice care and a growing number of fellowships in palliative care, I am extremely optimistic in the progress we have made and will continue to make.
Geoffrey Dunn, MD, FACS, is the medical director of the Palliative Care Consultation Service at UPMC Hamot Medical Center, Erie, Penn. He currently is Community Editor for the Pain and Palliative Care Community for the ACS’s web portal.
The low rate of palliative care consultations found in this study mirrors my own experience, as does the feeling of urgency to shed more light on the issue. The biggest hurdle surgeons face when it comes to palliative care consultations is that, in their minds, seeking these meetings is associated with immediate death care. Many surgeons are shy about bringing palliative care specialists on board because approaching families can be daunting.
Family members who do not know enough about comfort care can be upset by the idea. Addressing this misunderstanding is crucial. Consultations are not just conversations about hospice care but can be emotional and spiritual experiences that prepare both the family and the patient for alternative options when surgical intervention cannot guarantee a good quality of life. I would encourage surgeons to be more proactive and less defensive about comfort care . Luckily, understanding the importance of this issue among professionals is growing.
When I approach these situations, it’s important for me to have a full understanding of what families and patients usually expect. Decisions should not be based on how bad things are now but on the future. What was the patient’s last year like? What is the best-case scenario for moving forward on a proposed intervention? What will the patient’s quality of life be? Answering these questions helps the patient understand his or her situation, without diminishing a surgeon’s ability. If you are honest, the family will usually come to the conclusion that they do not want to subject the patient to ultimately unnecessary treatment.
Palliative care services help patients and their families deal with pain beyond the physical symptoms. Dealing with pain, depression, or delirium is only a part of comfort care – coping with a sense of hopelessness, family disruption, or feelings of guilt also can be a part and, significantly, a part that surgeons are not trained to diagnose or treat.
With more than 70 surgeons certified in hospice care and a growing number of fellowships in palliative care, I am extremely optimistic in the progress we have made and will continue to make.
Geoffrey Dunn, MD, FACS, is the medical director of the Palliative Care Consultation Service at UPMC Hamot Medical Center, Erie, Penn. He currently is Community Editor for the Pain and Palliative Care Community for the ACS’s web portal.
Investment in advanced palliative care planning has the potential to improve the quality of care for vascular surgery patients, according to investigators from Oregon Health and Science University, Portland.
Dale G. Wilson, MD, and his colleagues performed a retrospective review of electronic medical records for 111 patients, who died while on the vascular surgery service at the OHSU Hospital during 2005-2014.
Almost three-quarters (73%) of patients were transitioned to palliative care; of those, 14% presented with an advanced directive, and 28% received a palliative care consultation (JAMA Surg. 2017;152[2]:183-90. doi: 10.1001/jamasurg.2016.3970).
While palliative care services are increasing in hospitals, accounting for 4% of annual hospital admissions in 2012 according to the study, they are not implemented consistently. “Many teams from various specialties care for patients at end of life; however, we still do not know what prompts end-of-life discussions,” Dr. Wilson said. “There is still no consensus on when to involve palliative services in the care of critically ill patients.”
While the decision to advise a consultation is “variable and physician dependent,” the type of treatment required may help identify when consultations are appropriate.
Of the 14 patients who did not choose comfort care, 11 (79%) required CPR. Additionally, all had to be taken to the operating room and required mechanical ventilation.
Of 81 patients who chose palliative care, 31 did so despite potential medical options. These patients were older – average age, 77 years, as compared with 68 years for patients who did not choose comfort care – with 8 of the 31 (26%) presenting an advanced directive, compared with only 7 of 83 patients (8%) for those who did not receive palliative care.
Dr. Wilson and his colleagues found that patients who chose palliative care were more likely to have received a palliative care consultation, as well: 10 of 31 patients who chose comfort care received a consultation, as opposed to 1 of 83 who chose comfort care but did not receive a consultation.
The nature of the vascular surgery service calls for early efforts to gather information regarding patients’ views on end-of-life care, Dr. Wilson said, noting that 73% of patients studied were admitted emergently and 87% underwent surgery, leaving little time for patients to express their wishes.
“Because the events associated with withdrawal of care are often not anticipated, we argue that all vascular surgical patients should have an advance directive, and perhaps, those at particular high risk should have a preoperative palliative care consultation,” Dr. Wilson wrote.
Limitations to the study included the data abstraction, which was performed by a single unblinded physician. Researchers also gathered patients’ reasons for transitioning to comfort care retrospectively.
Investment in advanced palliative care planning has the potential to improve the quality of care for vascular surgery patients, according to investigators from Oregon Health and Science University, Portland.
Dale G. Wilson, MD, and his colleagues performed a retrospective review of electronic medical records for 111 patients, who died while on the vascular surgery service at the OHSU Hospital during 2005-2014.
Almost three-quarters (73%) of patients were transitioned to palliative care; of those, 14% presented with an advanced directive, and 28% received a palliative care consultation (JAMA Surg. 2017;152[2]:183-90. doi: 10.1001/jamasurg.2016.3970).
While palliative care services are increasing in hospitals, accounting for 4% of annual hospital admissions in 2012 according to the study, they are not implemented consistently. “Many teams from various specialties care for patients at end of life; however, we still do not know what prompts end-of-life discussions,” Dr. Wilson said. “There is still no consensus on when to involve palliative services in the care of critically ill patients.”
While the decision to advise a consultation is “variable and physician dependent,” the type of treatment required may help identify when consultations are appropriate.
Of the 14 patients who did not choose comfort care, 11 (79%) required CPR. Additionally, all had to be taken to the operating room and required mechanical ventilation.
Of 81 patients who chose palliative care, 31 did so despite potential medical options. These patients were older – average age, 77 years, as compared with 68 years for patients who did not choose comfort care – with 8 of the 31 (26%) presenting an advanced directive, compared with only 7 of 83 patients (8%) for those who did not receive palliative care.
Dr. Wilson and his colleagues found that patients who chose palliative care were more likely to have received a palliative care consultation, as well: 10 of 31 patients who chose comfort care received a consultation, as opposed to 1 of 83 who chose comfort care but did not receive a consultation.
The nature of the vascular surgery service calls for early efforts to gather information regarding patients’ views on end-of-life care, Dr. Wilson said, noting that 73% of patients studied were admitted emergently and 87% underwent surgery, leaving little time for patients to express their wishes.
“Because the events associated with withdrawal of care are often not anticipated, we argue that all vascular surgical patients should have an advance directive, and perhaps, those at particular high risk should have a preoperative palliative care consultation,” Dr. Wilson wrote.
Limitations to the study included the data abstraction, which was performed by a single unblinded physician. Researchers also gathered patients’ reasons for transitioning to comfort care retrospectively.
FROM JAMA SURGERY
Key clinical point:
Major finding: Of the 111 patients studied, 81 died on palliative care, but only 15 presented an advanced directive.
Data source: A retrospective cohort study of the records of patients aged 18-99 years who died in the vascular surgery service at Oregon Health and Science University Hospital from 2005-2014.
Disclosures: The authors reported no financial disclosures.
Hydrogel coils improve outcomes in medium-sized intracranial aneurysms
HOUSTON – Hydrogel coils significantly outperformed bare platinum coils in treating patients with medium-sized intracranial aneurysms, reducing the incidence of adverse outcomes by about 9% in an open-label, randomized trial.
Compared against the platinum coils, the self-expandable, hydrophilic coils scored significantly better on a composite endpoint of aneurysm recurrence at 18 months, re-treatment by 18 months, serious morbidity that prevented angiographic follow-up, and death, Christian Taschner, MD, said at the International Stroke Conference sponsored by the America Heart Association.
These are the company’s second-generation products, said Dr. Taschner, a neuroradiologist at University Hospital Freiburg (Germany). The initial hydrogel-coated coils were not well received because they were too stiff, he said in an interview.
The 18-month study comprised 513 patients and was conducted in 15 centers in France and 7 in Germany. Patients with medium-sized aneurysms (4-12 mm) were randomized to coiling with either the hydrogel coils or bare platinum coils and followed for 18 months. The cohort was stratified by rupture status in the analysis.
Angiographic images of the aneurysm were obtained before treatment, immediately after treatment, at 6 months, and at 18 months. All images were reviewed by an independent laboratory that was blinded to the treatment.
In addition to the primary composite outcome, the study assessed secondary endpoints of modified Rankin Scale (mRS) score at 18 months and coil packing density.
The final analysis included 484 patients. They were a mean of 52 years old, and about 70% were women. The aneurysms were ruptured at baseline in about 43% of cases. The mean aneurysm size was 7 mm, and the mean neck size, 3.5 mm. The dome-to-neck ratio was less than 1.5 in about a third of the group. Most lesions (89%) had anterior circulation.
Most patients needed some kind of adjunctive endovascular-assisting device during the procedure. These included balloon remodeling, which was necessary in about 50% of cases to help keep the coils from extruding into the parent vessel, and stents in 22%.
Intraoperative adverse events were uncommon in both groups. They were numerically, but not significantly, less common in the hydrogel group. These included thromboembolism (8 vs. 12), intraoperative rupture (3 vs. 7), parent vessel occlusion (1 vs. 3), and vessel perforation (one in each group). There were no vessel dissections.
By 18 months, 20% of the hydrogel group and 29% of the platinum coil group had experienced the composite primary endpoint. The 9% absolute difference was statistically significant. It was largely driven by the subcomponents of major recurrence (12% vs. 18%) and re-treatment (3% vs. 6%).
An mRS of 3-5 was used as the proxy for patients whose clinical status prevented them from having angiographic follow-up. That endpoint occurred in three patients in the hydrogel group but in none of the platinum coil group. Dr. Taschner said that difference was not statistically significant.
In an analysis that stratified by baseline rupture status and by aneurysm size, the hydrogel coils were largely superior to bare platinum. However, the hydrogel coils were not significantly more effective than the platinum coils for aneurysms 10 mm or larger, Dr. Taschner noted.
There was no significant difference in the secondary endpoint of mRS at 18 months. Most patients (85% vs. 86%) did well, with an mRS of 0. Scores of 1-2 were seen in 9% of each group. An mRS of 3-5 occurred in 3% of the hydrogel group and 1% of the platinum coil group. There were 17 deaths: 7 in the hydrogel group and 10 in the platinum coil group (3% vs. 4%). This was not a significant difference.
Patients whose aneurysms were unruptured at baseline did significantly better than did those with ruptured lesions, although the mRS scores did not vary significantly between treatment groups. Among those with intact lesions, 90% of the hydrogel and 94% of the platinum coil group achieved an mRS of 0, and 2% of each group died. Among those with ruptured lesions at baseline, 78% of the hydrogel group and 75% of the platinum coil group achieved an mRS of 0. Five patients in the hydrogel group and seven in the platinum coil group died.
On the technical outcome of coil volume, hydrogel did somewhat better (0.041 cm3 vs. 0.038 cm3). The mean packing density was significantly higher (39% vs. 31%).
Dr. Taschner said the study provides good support for using the hydrogel coils in medium-sized aneurysms, and that other recent data reaffirm this.
“A recent trial in Canada and the U.S. looked at hydrogel coils in aneurysms 12 mm or larger, with broad necks, and it failed to show a benefit of the hydrogel coils.”
Additionally, a study published in January found that hydrogel coils were no better than platinum coils in 250 patients with large or recurrent aneurysms (AJNR Am J Neuroradiol. 2017 Jan 12. doi: 10.3174/ajnr.A5101).
“I myself would use them in medium-sized aneurysms with an unfavorable dome-to-neck ratio, and in those that have a broad neck. In those instances, I do think that hydrogel coils have their place. I wouldn’t use them in really large aneurysms. I don’t think they are well suited for that. In those cases, I would probably use platinum coils in combination with a flow diverter. That really provides very stable aneurysm occlusion with acceptable complication rates.”
Dr. Taschner said that he received research support from MicroVention during the study.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
HOUSTON – Hydrogel coils significantly outperformed bare platinum coils in treating patients with medium-sized intracranial aneurysms, reducing the incidence of adverse outcomes by about 9% in an open-label, randomized trial.
Compared against the platinum coils, the self-expandable, hydrophilic coils scored significantly better on a composite endpoint of aneurysm recurrence at 18 months, re-treatment by 18 months, serious morbidity that prevented angiographic follow-up, and death, Christian Taschner, MD, said at the International Stroke Conference sponsored by the America Heart Association.
These are the company’s second-generation products, said Dr. Taschner, a neuroradiologist at University Hospital Freiburg (Germany). The initial hydrogel-coated coils were not well received because they were too stiff, he said in an interview.
The 18-month study comprised 513 patients and was conducted in 15 centers in France and 7 in Germany. Patients with medium-sized aneurysms (4-12 mm) were randomized to coiling with either the hydrogel coils or bare platinum coils and followed for 18 months. The cohort was stratified by rupture status in the analysis.
Angiographic images of the aneurysm were obtained before treatment, immediately after treatment, at 6 months, and at 18 months. All images were reviewed by an independent laboratory that was blinded to the treatment.
In addition to the primary composite outcome, the study assessed secondary endpoints of modified Rankin Scale (mRS) score at 18 months and coil packing density.
The final analysis included 484 patients. They were a mean of 52 years old, and about 70% were women. The aneurysms were ruptured at baseline in about 43% of cases. The mean aneurysm size was 7 mm, and the mean neck size, 3.5 mm. The dome-to-neck ratio was less than 1.5 in about a third of the group. Most lesions (89%) had anterior circulation.
Most patients needed some kind of adjunctive endovascular-assisting device during the procedure. These included balloon remodeling, which was necessary in about 50% of cases to help keep the coils from extruding into the parent vessel, and stents in 22%.
Intraoperative adverse events were uncommon in both groups. They were numerically, but not significantly, less common in the hydrogel group. These included thromboembolism (8 vs. 12), intraoperative rupture (3 vs. 7), parent vessel occlusion (1 vs. 3), and vessel perforation (one in each group). There were no vessel dissections.
By 18 months, 20% of the hydrogel group and 29% of the platinum coil group had experienced the composite primary endpoint. The 9% absolute difference was statistically significant. It was largely driven by the subcomponents of major recurrence (12% vs. 18%) and re-treatment (3% vs. 6%).
An mRS of 3-5 was used as the proxy for patients whose clinical status prevented them from having angiographic follow-up. That endpoint occurred in three patients in the hydrogel group but in none of the platinum coil group. Dr. Taschner said that difference was not statistically significant.
In an analysis that stratified by baseline rupture status and by aneurysm size, the hydrogel coils were largely superior to bare platinum. However, the hydrogel coils were not significantly more effective than the platinum coils for aneurysms 10 mm or larger, Dr. Taschner noted.
There was no significant difference in the secondary endpoint of mRS at 18 months. Most patients (85% vs. 86%) did well, with an mRS of 0. Scores of 1-2 were seen in 9% of each group. An mRS of 3-5 occurred in 3% of the hydrogel group and 1% of the platinum coil group. There were 17 deaths: 7 in the hydrogel group and 10 in the platinum coil group (3% vs. 4%). This was not a significant difference.
Patients whose aneurysms were unruptured at baseline did significantly better than did those with ruptured lesions, although the mRS scores did not vary significantly between treatment groups. Among those with intact lesions, 90% of the hydrogel and 94% of the platinum coil group achieved an mRS of 0, and 2% of each group died. Among those with ruptured lesions at baseline, 78% of the hydrogel group and 75% of the platinum coil group achieved an mRS of 0. Five patients in the hydrogel group and seven in the platinum coil group died.
On the technical outcome of coil volume, hydrogel did somewhat better (0.041 cm3 vs. 0.038 cm3). The mean packing density was significantly higher (39% vs. 31%).
Dr. Taschner said the study provides good support for using the hydrogel coils in medium-sized aneurysms, and that other recent data reaffirm this.
“A recent trial in Canada and the U.S. looked at hydrogel coils in aneurysms 12 mm or larger, with broad necks, and it failed to show a benefit of the hydrogel coils.”
Additionally, a study published in January found that hydrogel coils were no better than platinum coils in 250 patients with large or recurrent aneurysms (AJNR Am J Neuroradiol. 2017 Jan 12. doi: 10.3174/ajnr.A5101).
“I myself would use them in medium-sized aneurysms with an unfavorable dome-to-neck ratio, and in those that have a broad neck. In those instances, I do think that hydrogel coils have their place. I wouldn’t use them in really large aneurysms. I don’t think they are well suited for that. In those cases, I would probably use platinum coils in combination with a flow diverter. That really provides very stable aneurysm occlusion with acceptable complication rates.”
Dr. Taschner said that he received research support from MicroVention during the study.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
HOUSTON – Hydrogel coils significantly outperformed bare platinum coils in treating patients with medium-sized intracranial aneurysms, reducing the incidence of adverse outcomes by about 9% in an open-label, randomized trial.
Compared against the platinum coils, the self-expandable, hydrophilic coils scored significantly better on a composite endpoint of aneurysm recurrence at 18 months, re-treatment by 18 months, serious morbidity that prevented angiographic follow-up, and death, Christian Taschner, MD, said at the International Stroke Conference sponsored by the America Heart Association.
These are the company’s second-generation products, said Dr. Taschner, a neuroradiologist at University Hospital Freiburg (Germany). The initial hydrogel-coated coils were not well received because they were too stiff, he said in an interview.
The 18-month study comprised 513 patients and was conducted in 15 centers in France and 7 in Germany. Patients with medium-sized aneurysms (4-12 mm) were randomized to coiling with either the hydrogel coils or bare platinum coils and followed for 18 months. The cohort was stratified by rupture status in the analysis.
Angiographic images of the aneurysm were obtained before treatment, immediately after treatment, at 6 months, and at 18 months. All images were reviewed by an independent laboratory that was blinded to the treatment.
In addition to the primary composite outcome, the study assessed secondary endpoints of modified Rankin Scale (mRS) score at 18 months and coil packing density.
The final analysis included 484 patients. They were a mean of 52 years old, and about 70% were women. The aneurysms were ruptured at baseline in about 43% of cases. The mean aneurysm size was 7 mm, and the mean neck size, 3.5 mm. The dome-to-neck ratio was less than 1.5 in about a third of the group. Most lesions (89%) had anterior circulation.
Most patients needed some kind of adjunctive endovascular-assisting device during the procedure. These included balloon remodeling, which was necessary in about 50% of cases to help keep the coils from extruding into the parent vessel, and stents in 22%.
Intraoperative adverse events were uncommon in both groups. They were numerically, but not significantly, less common in the hydrogel group. These included thromboembolism (8 vs. 12), intraoperative rupture (3 vs. 7), parent vessel occlusion (1 vs. 3), and vessel perforation (one in each group). There were no vessel dissections.
By 18 months, 20% of the hydrogel group and 29% of the platinum coil group had experienced the composite primary endpoint. The 9% absolute difference was statistically significant. It was largely driven by the subcomponents of major recurrence (12% vs. 18%) and re-treatment (3% vs. 6%).
An mRS of 3-5 was used as the proxy for patients whose clinical status prevented them from having angiographic follow-up. That endpoint occurred in three patients in the hydrogel group but in none of the platinum coil group. Dr. Taschner said that difference was not statistically significant.
In an analysis that stratified by baseline rupture status and by aneurysm size, the hydrogel coils were largely superior to bare platinum. However, the hydrogel coils were not significantly more effective than the platinum coils for aneurysms 10 mm or larger, Dr. Taschner noted.
There was no significant difference in the secondary endpoint of mRS at 18 months. Most patients (85% vs. 86%) did well, with an mRS of 0. Scores of 1-2 were seen in 9% of each group. An mRS of 3-5 occurred in 3% of the hydrogel group and 1% of the platinum coil group. There were 17 deaths: 7 in the hydrogel group and 10 in the platinum coil group (3% vs. 4%). This was not a significant difference.
Patients whose aneurysms were unruptured at baseline did significantly better than did those with ruptured lesions, although the mRS scores did not vary significantly between treatment groups. Among those with intact lesions, 90% of the hydrogel and 94% of the platinum coil group achieved an mRS of 0, and 2% of each group died. Among those with ruptured lesions at baseline, 78% of the hydrogel group and 75% of the platinum coil group achieved an mRS of 0. Five patients in the hydrogel group and seven in the platinum coil group died.
On the technical outcome of coil volume, hydrogel did somewhat better (0.041 cm3 vs. 0.038 cm3). The mean packing density was significantly higher (39% vs. 31%).
Dr. Taschner said the study provides good support for using the hydrogel coils in medium-sized aneurysms, and that other recent data reaffirm this.
“A recent trial in Canada and the U.S. looked at hydrogel coils in aneurysms 12 mm or larger, with broad necks, and it failed to show a benefit of the hydrogel coils.”
Additionally, a study published in January found that hydrogel coils were no better than platinum coils in 250 patients with large or recurrent aneurysms (AJNR Am J Neuroradiol. 2017 Jan 12. doi: 10.3174/ajnr.A5101).
“I myself would use them in medium-sized aneurysms with an unfavorable dome-to-neck ratio, and in those that have a broad neck. In those instances, I do think that hydrogel coils have their place. I wouldn’t use them in really large aneurysms. I don’t think they are well suited for that. In those cases, I would probably use platinum coils in combination with a flow diverter. That really provides very stable aneurysm occlusion with acceptable complication rates.”
Dr. Taschner said that he received research support from MicroVention during the study.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: Compared with bare-metal coils, hydrogel coils reduced by 9% the incidence of a composite primary outcome of recurrence, re-treatment, morbidity, and death.
Data source: An 18-month, open-label, randomized study of 513 patients.
Disclosures: MicroVention sponsored the study. Dr. Taschner said that he received research support from the company during the study.
Abatacept efficacy differs in trials of giant cell and Takayasu’s arteritis
A pair of new studies offer mixed results regarding the use of the rheumatoid arthritis drug abatacept to treat two forms of large-vessel vasculitis: It appears to help patients with giant cell arteritis but not those with the rarer Takayasu’s arteritis.
“The results from the GCA [giant cell arteritis] study found that treatment with abatacept [Orencia] combined with prednisone resulted in a lower rate of relapse than treatment with prednisone alone,” said Carol A. Langford, MD, lead author of both studies, which were conducted by the Vasculitis Clinical Research Consortium. She is chair in rheumatic and immunologic diseases and director of the Center for Vasculitis Care and Research at the Cleveland Clinic.
Both studies appear online Jan. 30 in Arthritis & Rheumatology.
“GCA is the most common form of vasculitis with an estimated incidence of 19.8 per 100,000,” she said. “It occurs in people over the age of 50 with the average age of onset in the 70s.” Women are most affected by a 2:1 ratio.
She said the disease affects the cranium (causing headaches, scalp tenderness, and a risk of blindness) and causes signs of systemic inflammation.
“Almost one-third of patients with GCA can have large vessel involvement that specifically include thoracic aortic aneurysms and stenosis of the cervical and subclavian arteries,” she said. Fatal thoracic aneurysms are possible, she said, but “studies have shown that in GCA overall, while short-term mortality may be increased, long-term survival is similar to the age-matched general population.”
TAK is much rarer, she said, affecting 3-9 people per 1,000,000. “TAK has an average age of onset in the 20s with an even stronger female predisposition of up to 9:1.”
The condition affects the aorta, its main branches, and pulmonary arteries, she said, “Some of the more frequent vascular symptoms/signs can include extremity claudication, hypertension, chest pain, and features associated with cerebral hypoperfusion. TAK is associated with substantial morbidity which is influenced by a low rate of sustained remission in 28%-50% of patients. Up to 47% of patients experience permanent disability, which has a significant impact on this young population.”
The mortality from TAK is unclear, she said.
Glucocorticoids are the main treatment for both GCA and TAK, Dr. Langford said, but “while glucocorticoids effectively control disease, they do not prevent relapse and they are associated with significant toxicity.”
For GCA, methotrexate has shown a mild benefit at best, she said, while two studies show promise for tocilizumab (Actemra). As for TAK, she said doctors often turn to the use of immunosuppressants and tumor necrosis factor inhibitors, although their use is based on retrospective studies and small, open-label trials.
Dr. Langford and her colleagues launched the two randomized, double-blind, placebo-controlled, multicenter studies in parallel with the same protocols.
In the GCA trial (doi: 10.1002/art.40044), researchers enrolled 49 patients with newly diagnosed GCA or disease that had relapsed within the 2 prior months to prednisone 40-60 mg/day followed by a standardized tapering schedule plus abatacept 10 mg/kg IV on days 1, 15, 29, and week 8. At 12 weeks, 8 patients had withdrawn, relapsed, or were not in remission, and so 41 were randomized to receive placebo or monthly abatacept until they met criteria for early termination or 12 months had passed after the last patient was enrolled. At the time of the randomization at 12 weeks, all patients were taking prednisone 20 mg/day, which was tapered until discontinuation at week 28.
At 12 months, 48% of those who took abatacept survived without relapse, compared with 31% of those who took placebo (P = .049), and the median remission period was longer for abatacept (9.9 months) than placebo (3.9 months; P = .023).
“This difference between groups is clinically meaningful to patients with GCA, corresponding to a prolonged duration of remission during which time they are not exposed to glucocorticoids and their potential toxicities that can impact quality of life,” Dr. Langford said.
No patients died during the trial, and 23 serious adverse events were reported in 15 patients. The frequency and severity of adverse events and infections didn’t differ between the treatment and placebo groups.
The drug may work in GCA patients by blocking T-cell activation, Dr. Langford said. Physicians could consider the drug in clinical practice, she said, although more research is needed to understand the long-term effects of using the medication.
In the other study (doi: 10.1002/art.40037), researchers used the same protocol to treat 34 patients with TAK; 26 reached the 12-week midpoint and were randomized to placebo or continuing abatacept on a monthly basis.
At 12 months, the relapse-free rate was 22% for the abatacept group and 40% for the placebo group (P = .853). The median duration of remission was similar for the groups at 5.5 months for abatacept and 5.7 months for placebo (P = .125).
The researchers reported no difference in frequency or severity of adverse events such as infection.
Based on the study results, Dr. Langford said abatacept is not appropriate in clinical practice to treat TAK.
The National Institute of Arthritis and Musculoskeletal and Skin Diseases funded the studies, and Bristol-Myers Squibb, which markets abatacept, provided the drug. Dr. Langford disclosed receiving research grants from Bristol-Myers Squibb, Genentech, and GlaxoSmithKline.
A pair of new studies offer mixed results regarding the use of the rheumatoid arthritis drug abatacept to treat two forms of large-vessel vasculitis: It appears to help patients with giant cell arteritis but not those with the rarer Takayasu’s arteritis.
“The results from the GCA [giant cell arteritis] study found that treatment with abatacept [Orencia] combined with prednisone resulted in a lower rate of relapse than treatment with prednisone alone,” said Carol A. Langford, MD, lead author of both studies, which were conducted by the Vasculitis Clinical Research Consortium. She is chair in rheumatic and immunologic diseases and director of the Center for Vasculitis Care and Research at the Cleveland Clinic.
Both studies appear online Jan. 30 in Arthritis & Rheumatology.
“GCA is the most common form of vasculitis with an estimated incidence of 19.8 per 100,000,” she said. “It occurs in people over the age of 50 with the average age of onset in the 70s.” Women are most affected by a 2:1 ratio.
She said the disease affects the cranium (causing headaches, scalp tenderness, and a risk of blindness) and causes signs of systemic inflammation.
“Almost one-third of patients with GCA can have large vessel involvement that specifically include thoracic aortic aneurysms and stenosis of the cervical and subclavian arteries,” she said. Fatal thoracic aneurysms are possible, she said, but “studies have shown that in GCA overall, while short-term mortality may be increased, long-term survival is similar to the age-matched general population.”
TAK is much rarer, she said, affecting 3-9 people per 1,000,000. “TAK has an average age of onset in the 20s with an even stronger female predisposition of up to 9:1.”
The condition affects the aorta, its main branches, and pulmonary arteries, she said, “Some of the more frequent vascular symptoms/signs can include extremity claudication, hypertension, chest pain, and features associated with cerebral hypoperfusion. TAK is associated with substantial morbidity which is influenced by a low rate of sustained remission in 28%-50% of patients. Up to 47% of patients experience permanent disability, which has a significant impact on this young population.”
The mortality from TAK is unclear, she said.
Glucocorticoids are the main treatment for both GCA and TAK, Dr. Langford said, but “while glucocorticoids effectively control disease, they do not prevent relapse and they are associated with significant toxicity.”
For GCA, methotrexate has shown a mild benefit at best, she said, while two studies show promise for tocilizumab (Actemra). As for TAK, she said doctors often turn to the use of immunosuppressants and tumor necrosis factor inhibitors, although their use is based on retrospective studies and small, open-label trials.
Dr. Langford and her colleagues launched the two randomized, double-blind, placebo-controlled, multicenter studies in parallel with the same protocols.
In the GCA trial (doi: 10.1002/art.40044), researchers enrolled 49 patients with newly diagnosed GCA or disease that had relapsed within the 2 prior months to prednisone 40-60 mg/day followed by a standardized tapering schedule plus abatacept 10 mg/kg IV on days 1, 15, 29, and week 8. At 12 weeks, 8 patients had withdrawn, relapsed, or were not in remission, and so 41 were randomized to receive placebo or monthly abatacept until they met criteria for early termination or 12 months had passed after the last patient was enrolled. At the time of the randomization at 12 weeks, all patients were taking prednisone 20 mg/day, which was tapered until discontinuation at week 28.
At 12 months, 48% of those who took abatacept survived without relapse, compared with 31% of those who took placebo (P = .049), and the median remission period was longer for abatacept (9.9 months) than placebo (3.9 months; P = .023).
“This difference between groups is clinically meaningful to patients with GCA, corresponding to a prolonged duration of remission during which time they are not exposed to glucocorticoids and their potential toxicities that can impact quality of life,” Dr. Langford said.
No patients died during the trial, and 23 serious adverse events were reported in 15 patients. The frequency and severity of adverse events and infections didn’t differ between the treatment and placebo groups.
The drug may work in GCA patients by blocking T-cell activation, Dr. Langford said. Physicians could consider the drug in clinical practice, she said, although more research is needed to understand the long-term effects of using the medication.
In the other study (doi: 10.1002/art.40037), researchers used the same protocol to treat 34 patients with TAK; 26 reached the 12-week midpoint and were randomized to placebo or continuing abatacept on a monthly basis.
At 12 months, the relapse-free rate was 22% for the abatacept group and 40% for the placebo group (P = .853). The median duration of remission was similar for the groups at 5.5 months for abatacept and 5.7 months for placebo (P = .125).
The researchers reported no difference in frequency or severity of adverse events such as infection.
Based on the study results, Dr. Langford said abatacept is not appropriate in clinical practice to treat TAK.
The National Institute of Arthritis and Musculoskeletal and Skin Diseases funded the studies, and Bristol-Myers Squibb, which markets abatacept, provided the drug. Dr. Langford disclosed receiving research grants from Bristol-Myers Squibb, Genentech, and GlaxoSmithKline.
A pair of new studies offer mixed results regarding the use of the rheumatoid arthritis drug abatacept to treat two forms of large-vessel vasculitis: It appears to help patients with giant cell arteritis but not those with the rarer Takayasu’s arteritis.
“The results from the GCA [giant cell arteritis] study found that treatment with abatacept [Orencia] combined with prednisone resulted in a lower rate of relapse than treatment with prednisone alone,” said Carol A. Langford, MD, lead author of both studies, which were conducted by the Vasculitis Clinical Research Consortium. She is chair in rheumatic and immunologic diseases and director of the Center for Vasculitis Care and Research at the Cleveland Clinic.
Both studies appear online Jan. 30 in Arthritis & Rheumatology.
“GCA is the most common form of vasculitis with an estimated incidence of 19.8 per 100,000,” she said. “It occurs in people over the age of 50 with the average age of onset in the 70s.” Women are most affected by a 2:1 ratio.
She said the disease affects the cranium (causing headaches, scalp tenderness, and a risk of blindness) and causes signs of systemic inflammation.
“Almost one-third of patients with GCA can have large vessel involvement that specifically include thoracic aortic aneurysms and stenosis of the cervical and subclavian arteries,” she said. Fatal thoracic aneurysms are possible, she said, but “studies have shown that in GCA overall, while short-term mortality may be increased, long-term survival is similar to the age-matched general population.”
TAK is much rarer, she said, affecting 3-9 people per 1,000,000. “TAK has an average age of onset in the 20s with an even stronger female predisposition of up to 9:1.”
The condition affects the aorta, its main branches, and pulmonary arteries, she said, “Some of the more frequent vascular symptoms/signs can include extremity claudication, hypertension, chest pain, and features associated with cerebral hypoperfusion. TAK is associated with substantial morbidity which is influenced by a low rate of sustained remission in 28%-50% of patients. Up to 47% of patients experience permanent disability, which has a significant impact on this young population.”
The mortality from TAK is unclear, she said.
Glucocorticoids are the main treatment for both GCA and TAK, Dr. Langford said, but “while glucocorticoids effectively control disease, they do not prevent relapse and they are associated with significant toxicity.”
For GCA, methotrexate has shown a mild benefit at best, she said, while two studies show promise for tocilizumab (Actemra). As for TAK, she said doctors often turn to the use of immunosuppressants and tumor necrosis factor inhibitors, although their use is based on retrospective studies and small, open-label trials.
Dr. Langford and her colleagues launched the two randomized, double-blind, placebo-controlled, multicenter studies in parallel with the same protocols.
In the GCA trial (doi: 10.1002/art.40044), researchers enrolled 49 patients with newly diagnosed GCA or disease that had relapsed within the 2 prior months to prednisone 40-60 mg/day followed by a standardized tapering schedule plus abatacept 10 mg/kg IV on days 1, 15, 29, and week 8. At 12 weeks, 8 patients had withdrawn, relapsed, or were not in remission, and so 41 were randomized to receive placebo or monthly abatacept until they met criteria for early termination or 12 months had passed after the last patient was enrolled. At the time of the randomization at 12 weeks, all patients were taking prednisone 20 mg/day, which was tapered until discontinuation at week 28.
At 12 months, 48% of those who took abatacept survived without relapse, compared with 31% of those who took placebo (P = .049), and the median remission period was longer for abatacept (9.9 months) than placebo (3.9 months; P = .023).
“This difference between groups is clinically meaningful to patients with GCA, corresponding to a prolonged duration of remission during which time they are not exposed to glucocorticoids and their potential toxicities that can impact quality of life,” Dr. Langford said.
No patients died during the trial, and 23 serious adverse events were reported in 15 patients. The frequency and severity of adverse events and infections didn’t differ between the treatment and placebo groups.
The drug may work in GCA patients by blocking T-cell activation, Dr. Langford said. Physicians could consider the drug in clinical practice, she said, although more research is needed to understand the long-term effects of using the medication.
In the other study (doi: 10.1002/art.40037), researchers used the same protocol to treat 34 patients with TAK; 26 reached the 12-week midpoint and were randomized to placebo or continuing abatacept on a monthly basis.
At 12 months, the relapse-free rate was 22% for the abatacept group and 40% for the placebo group (P = .853). The median duration of remission was similar for the groups at 5.5 months for abatacept and 5.7 months for placebo (P = .125).
The researchers reported no difference in frequency or severity of adverse events such as infection.
Based on the study results, Dr. Langford said abatacept is not appropriate in clinical practice to treat TAK.
The National Institute of Arthritis and Musculoskeletal and Skin Diseases funded the studies, and Bristol-Myers Squibb, which markets abatacept, provided the drug. Dr. Langford disclosed receiving research grants from Bristol-Myers Squibb, Genentech, and GlaxoSmithKline.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: At 12 months, 48% of GCA patients who took abatacept survived without relapse, compared with 31% of those who took placebo (P = .049), and the median remission period was longer for abatacept (9.9 months) than placebo (3.9 months; P = .023) For TAK patients, the relapse-free rates were 22% for the abatacept group and 40% for the placebo group (P = .853). The median duration of remission was similar for the groups at 5.5 months for abatacept and 5.7 months for placebo (P = .125).
Data source: Two randomized, double-blinded, placebo-controlled, multicenter trials with identical protocols of prednisone plus abatacept 10 mg/kg IV on days 1, 15, 29, and week 8, then randomization to placebo or monthly abatacept until patients met criteria for early termination or 12 months passed after the last patient was enrolled.
Disclosures: The National Institute of Arthritis and Musculoskeletal and Skin Diseases funded the studies, and Bristol-Myers Squibb, which markets abatacept, provided the drug. Dr. Langford disclosed receiving research grants from Bristol-Myers Squibb, Genentech, and GlaxoSmithKline.
New data signal paradigm shift in FMD and arterial disease
CHICAGO – New data have shown that fibromuscular dysplasia is associated with high rates of dissection and/or aneurysm, and emerging recommendations call for routine imaging early on in the diagnosis of FMD to monitor for these vascular events, a researcher who developed those recommendations reported at a symposium on vascular surgery sponsored by Northwestern University.
“Given the very high rate of aneurysms in this population, it is now recommended that all patients with FMD should undergo at least one-time head-to pelvis imaging with CT angiography or MR angiography to screen for the presence of an aneurysm or to identify other areas of FMD involvement,” said Daniella Kadian-Dodov, MD, of Icahn School of Medicine at Mount Sinai in New York (J Am Coll Cardiol. 2016;68:176-85).
First described in 1938, FMD is a non-atherosclerotic, noninflammatory disease that had been thought to be a rare cause of renovascular hypertension with a classic “string-of-beads” appearance upon imaging, Dr. Kadian-Dodov noted. However, recent data from the Fibromuscular Dysplasia Society of America–sponsored U.S. registry has changed that thinking. “We now know it occurs more frequently in the carotid and renal arteries, although it has been observed in almost every artery,” she said. “The pathogenesis is still unknown but up to 10% of cases are familial.”
And manifestations of disease now extend beyond the “string-of-beads” appearance to include aneurysm, dissection, and arterial tortuosity, she said (Circulation. 2012;125:3182-90; Circulation. 2014;129:1048-78; J Am Coll Cardiol. 2016;68:176-85). The classification system for FMD has also undergone a recent change, according to Dr. Kadian-Dodov. “Traditionally, a histopathologic scheme was used to classify FMD,” she said. “Nowadays, fewer and fewer patients are undergoing surgical procedures, so the classification has changed to an angiographic system,” the most common of which is the American Heart Association system adopted in 2014 that distinguishes between multifocal, characterized by the classic “string-of-beads” appearance, and focal FMD with a single area of stenosis.
But the diagnosis of either variant of FMD is not exclusive. “Patients may have multiple areas of disease involvement and the same patient may have both focal and multifocal FMD findings,” Dr. Kadian-Dodov said.
The U.S. registry has helped clarify the thinking on FMD, Dr. Kadian-Dodov said. More than 1,400 patients are in the registry, 90% of whom are women with multifocal disease. The average age of onset of symptoms is 47 years, but 52 is the average age for diagnosis. “So these patients are experiencing several years delay to FMD diagnosis,” she said.
Manifestations depend on the vascular bed involved. “In the case of cervical artery FMD, headaches and pulsatile tinnitus are commonly reported, whereas with renal artery involvement hypertension is the most common symptom,” she said. A recent analysis showed 41.7% of the FMD population have either aneurysm and dissection or both (J Am Coll Cardiol. 2016;68:176-185).
But no specific guidelines for treatment of FMD yet exist, Dr. Kadian-Dodov said. “General guidelines should be applied for the management of dissection and aneurysm in patients with FMD,” she said. For patients with arterial dissection, that means conservative therapy comprising either anticoagulation or antiplatelet agents for 3-6 months followed by daily low-dose aspirin therapy. “Revascularization is rarely required for these patients,” she said. “Endovascular or surgical modalities should be reserved for those with continued ischemia despite conservative management or more complicated pseudoaneurysm formations.”
Daily aspirin therapy is likewise the recommendation for patients with cervical artery multifocal or focal FMD involvement without dissection or aneurysms. “We follow up with imaging every 6 months for 2 years,” Dr. Kadian-Dodov said. “If they’re stable, we switch over to annual surveillance; and if the patient has an aneurysm or dissection, that might alter the imaging and surveillance program.”
During angioplasty, determining the severity of stenosis upon visual inspection is difficult, especially in multifocal FMD. She advised measuring the gradient across the area of FMD involvement with a pressure wire to determine if angioplasty has adequately treated the lesion. “You should see obliteration of the gradient with successful treatment; you don’t have to target your therapy to a perfect angiographic result,” she said.
In patients with FMD and hypertension, she recommended renal artery angioplasty for hypertension of less than 5 years duration or in resistant or labile hypertension. “In this setting, stents are only reserved for complicated or refractory cases; angioplasty alone is sufficient,” Dr. Kadian-Dodov said. Cure rates decline with age, and hypertension in focal disease has a higher cure rate than does multifocal disease, she said (Hypertension. 2010;56:525-32).
Dr. Kadian-Dodov had no relevant financial relationships to disclose.
CHICAGO – New data have shown that fibromuscular dysplasia is associated with high rates of dissection and/or aneurysm, and emerging recommendations call for routine imaging early on in the diagnosis of FMD to monitor for these vascular events, a researcher who developed those recommendations reported at a symposium on vascular surgery sponsored by Northwestern University.
“Given the very high rate of aneurysms in this population, it is now recommended that all patients with FMD should undergo at least one-time head-to pelvis imaging with CT angiography or MR angiography to screen for the presence of an aneurysm or to identify other areas of FMD involvement,” said Daniella Kadian-Dodov, MD, of Icahn School of Medicine at Mount Sinai in New York (J Am Coll Cardiol. 2016;68:176-85).
First described in 1938, FMD is a non-atherosclerotic, noninflammatory disease that had been thought to be a rare cause of renovascular hypertension with a classic “string-of-beads” appearance upon imaging, Dr. Kadian-Dodov noted. However, recent data from the Fibromuscular Dysplasia Society of America–sponsored U.S. registry has changed that thinking. “We now know it occurs more frequently in the carotid and renal arteries, although it has been observed in almost every artery,” she said. “The pathogenesis is still unknown but up to 10% of cases are familial.”
And manifestations of disease now extend beyond the “string-of-beads” appearance to include aneurysm, dissection, and arterial tortuosity, she said (Circulation. 2012;125:3182-90; Circulation. 2014;129:1048-78; J Am Coll Cardiol. 2016;68:176-85). The classification system for FMD has also undergone a recent change, according to Dr. Kadian-Dodov. “Traditionally, a histopathologic scheme was used to classify FMD,” she said. “Nowadays, fewer and fewer patients are undergoing surgical procedures, so the classification has changed to an angiographic system,” the most common of which is the American Heart Association system adopted in 2014 that distinguishes between multifocal, characterized by the classic “string-of-beads” appearance, and focal FMD with a single area of stenosis.
But the diagnosis of either variant of FMD is not exclusive. “Patients may have multiple areas of disease involvement and the same patient may have both focal and multifocal FMD findings,” Dr. Kadian-Dodov said.
The U.S. registry has helped clarify the thinking on FMD, Dr. Kadian-Dodov said. More than 1,400 patients are in the registry, 90% of whom are women with multifocal disease. The average age of onset of symptoms is 47 years, but 52 is the average age for diagnosis. “So these patients are experiencing several years delay to FMD diagnosis,” she said.
Manifestations depend on the vascular bed involved. “In the case of cervical artery FMD, headaches and pulsatile tinnitus are commonly reported, whereas with renal artery involvement hypertension is the most common symptom,” she said. A recent analysis showed 41.7% of the FMD population have either aneurysm and dissection or both (J Am Coll Cardiol. 2016;68:176-185).
But no specific guidelines for treatment of FMD yet exist, Dr. Kadian-Dodov said. “General guidelines should be applied for the management of dissection and aneurysm in patients with FMD,” she said. For patients with arterial dissection, that means conservative therapy comprising either anticoagulation or antiplatelet agents for 3-6 months followed by daily low-dose aspirin therapy. “Revascularization is rarely required for these patients,” she said. “Endovascular or surgical modalities should be reserved for those with continued ischemia despite conservative management or more complicated pseudoaneurysm formations.”
Daily aspirin therapy is likewise the recommendation for patients with cervical artery multifocal or focal FMD involvement without dissection or aneurysms. “We follow up with imaging every 6 months for 2 years,” Dr. Kadian-Dodov said. “If they’re stable, we switch over to annual surveillance; and if the patient has an aneurysm or dissection, that might alter the imaging and surveillance program.”
During angioplasty, determining the severity of stenosis upon visual inspection is difficult, especially in multifocal FMD. She advised measuring the gradient across the area of FMD involvement with a pressure wire to determine if angioplasty has adequately treated the lesion. “You should see obliteration of the gradient with successful treatment; you don’t have to target your therapy to a perfect angiographic result,” she said.
In patients with FMD and hypertension, she recommended renal artery angioplasty for hypertension of less than 5 years duration or in resistant or labile hypertension. “In this setting, stents are only reserved for complicated or refractory cases; angioplasty alone is sufficient,” Dr. Kadian-Dodov said. Cure rates decline with age, and hypertension in focal disease has a higher cure rate than does multifocal disease, she said (Hypertension. 2010;56:525-32).
Dr. Kadian-Dodov had no relevant financial relationships to disclose.
CHICAGO – New data have shown that fibromuscular dysplasia is associated with high rates of dissection and/or aneurysm, and emerging recommendations call for routine imaging early on in the diagnosis of FMD to monitor for these vascular events, a researcher who developed those recommendations reported at a symposium on vascular surgery sponsored by Northwestern University.
“Given the very high rate of aneurysms in this population, it is now recommended that all patients with FMD should undergo at least one-time head-to pelvis imaging with CT angiography or MR angiography to screen for the presence of an aneurysm or to identify other areas of FMD involvement,” said Daniella Kadian-Dodov, MD, of Icahn School of Medicine at Mount Sinai in New York (J Am Coll Cardiol. 2016;68:176-85).
First described in 1938, FMD is a non-atherosclerotic, noninflammatory disease that had been thought to be a rare cause of renovascular hypertension with a classic “string-of-beads” appearance upon imaging, Dr. Kadian-Dodov noted. However, recent data from the Fibromuscular Dysplasia Society of America–sponsored U.S. registry has changed that thinking. “We now know it occurs more frequently in the carotid and renal arteries, although it has been observed in almost every artery,” she said. “The pathogenesis is still unknown but up to 10% of cases are familial.”
And manifestations of disease now extend beyond the “string-of-beads” appearance to include aneurysm, dissection, and arterial tortuosity, she said (Circulation. 2012;125:3182-90; Circulation. 2014;129:1048-78; J Am Coll Cardiol. 2016;68:176-85). The classification system for FMD has also undergone a recent change, according to Dr. Kadian-Dodov. “Traditionally, a histopathologic scheme was used to classify FMD,” she said. “Nowadays, fewer and fewer patients are undergoing surgical procedures, so the classification has changed to an angiographic system,” the most common of which is the American Heart Association system adopted in 2014 that distinguishes between multifocal, characterized by the classic “string-of-beads” appearance, and focal FMD with a single area of stenosis.
But the diagnosis of either variant of FMD is not exclusive. “Patients may have multiple areas of disease involvement and the same patient may have both focal and multifocal FMD findings,” Dr. Kadian-Dodov said.
The U.S. registry has helped clarify the thinking on FMD, Dr. Kadian-Dodov said. More than 1,400 patients are in the registry, 90% of whom are women with multifocal disease. The average age of onset of symptoms is 47 years, but 52 is the average age for diagnosis. “So these patients are experiencing several years delay to FMD diagnosis,” she said.
Manifestations depend on the vascular bed involved. “In the case of cervical artery FMD, headaches and pulsatile tinnitus are commonly reported, whereas with renal artery involvement hypertension is the most common symptom,” she said. A recent analysis showed 41.7% of the FMD population have either aneurysm and dissection or both (J Am Coll Cardiol. 2016;68:176-185).
But no specific guidelines for treatment of FMD yet exist, Dr. Kadian-Dodov said. “General guidelines should be applied for the management of dissection and aneurysm in patients with FMD,” she said. For patients with arterial dissection, that means conservative therapy comprising either anticoagulation or antiplatelet agents for 3-6 months followed by daily low-dose aspirin therapy. “Revascularization is rarely required for these patients,” she said. “Endovascular or surgical modalities should be reserved for those with continued ischemia despite conservative management or more complicated pseudoaneurysm formations.”
Daily aspirin therapy is likewise the recommendation for patients with cervical artery multifocal or focal FMD involvement without dissection or aneurysms. “We follow up with imaging every 6 months for 2 years,” Dr. Kadian-Dodov said. “If they’re stable, we switch over to annual surveillance; and if the patient has an aneurysm or dissection, that might alter the imaging and surveillance program.”
During angioplasty, determining the severity of stenosis upon visual inspection is difficult, especially in multifocal FMD. She advised measuring the gradient across the area of FMD involvement with a pressure wire to determine if angioplasty has adequately treated the lesion. “You should see obliteration of the gradient with successful treatment; you don’t have to target your therapy to a perfect angiographic result,” she said.
In patients with FMD and hypertension, she recommended renal artery angioplasty for hypertension of less than 5 years duration or in resistant or labile hypertension. “In this setting, stents are only reserved for complicated or refractory cases; angioplasty alone is sufficient,” Dr. Kadian-Dodov said. Cure rates decline with age, and hypertension in focal disease has a higher cure rate than does multifocal disease, she said (Hypertension. 2010;56:525-32).
Dr. Kadian-Dodov had no relevant financial relationships to disclose.
Key clinical point: Fibromuscular dysplasia was thought to be a rare cause of renovascular hypertension, but new data has challenged this thinking.
Major finding: FMD accounts for 15%-20% of patients with spontaneous carotid or vertebral artery dissection and 45%-86% of patients with spontaneous coronary artery dissection.
Data source: U.S. Registry for FMD maintained by the Fibromuscular Dystrophy Society of America.
Disclosures: Dr. Kadian-Dodov reported having no financial disclosures.
AAA screening showed no mortality reduction in new trial
In contrast to previous studies, screening for abdominal aortic aneurysms in older men does not appear to have a significant effect on overall mortality, according to a prospective, randomized study.
Mortality from ruptured AAA remains high in older men, which has prompted four previous large randomized trials to explore whether screening men aged 65 years and older might reduce mortality.
Writing in the October 31 online edition of JAMA Internal Medicine, the authors reported the long-term outcomes of an Australian population-based trial of screening for abdominal aortic aneurysms in 49,801 men aged 64-83 years, of whom 19 249 were invited to screening and 12,203 of those underwent screening (isrctn.org Identifier: ISRCTN16171472).
After a mean 12.8 years of follow-up, there was a non-significant 9% lower mortality in the invited screening group compared to the control group and a non-significant 8% lower mortality among men aged 65-74 years.
Overall, there were 90 deaths from ruptured AAA in the screening group and 98 in the control group (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6633).
The prevalence of abdominal aortic aneurysms with a diameter at or above 30 mm was 6.6% in men aged 65-74, and 0.4% for those with a diameter of 55 mm or above.
While the rate of ruptured abdominal aortic aneurysms was significantly lower in the invited group compared to the control group (72 vs. 99, P = .04), the 30-day mortality after surgery for rupture was higher in the invited group compared to the control group (61.5% vs. 43.2%).
Screening had no meaningful impact on the risk of all-cause, cardiovascular, and other mortality, but men who had smoked had a higher risk of rupture and of death from a rupture than those who had never smoked, regardless of screening status.
The rate of total elective operations was significantly higher in the invited group compared to controls (536 vs. 414, P < .001), mainly in the first year after screening.
The authors calculated that to prevent one death from a ruptured abdominal aortic aneurysm in five years, 4784 men aged 64-83 years or 3290 men aged 65-74 years would need to be invited for screening.
While the strength of the study was that it was truly population-based – using the electoral roll – the authors said the lack of a benefit from screening was likely due to the relatively low rate of rupture and death from AAA, as well as a high rate of elective surgery for this condition, in the control group.
The non-significant 8% reduction in mortality observed in the study was significantly less than the 42% and 66% reductions seen in previous trials with a similar length of follow-up.
The authors suggested this may also have been related to a lower fraction of invited men participating in screening, but pointed out that the incidence of AAA in men is declining.
“The reason for the decrease in incidence and prevalence is multifactorial but is probably driven by differences in rates of smoking and cessation because the relative risk for AAA events is 3- to 6-fold higher in smokers compared with non-smokers,” they wrote.
The authors said selective screening of smokers or ex-smokers may be more effective, but pointed out that this approach would miss around one-quarter of aneurysms. However they suggested more targeted screening may yet achieve a benefit.
“The small overall benefit of population-wide screening does not mean that finding AAAs in suitable older men is not worthwhile because deaths from AAAs in men who actually attended for screening were halved by early detection and successful treatment.”
The study was supported by the National Health and Medical Research Council Project. The authors reported that they had no conflicts of interest.
These new data will not change the finding of robust reduction in AAA-related mortality from screening seen in all previous meta-analyses. However, the most recently updated meta-analyses now reveal the small reduction in all-cause mortality with screening to be statistically significant.
So although the findings of the Western Australian trial remain negative and raise some concerns about screening, their aggregation with other studies does not change the overall conclusions that screening substantially reduced AAA-related mortality and also resulted in a statistically significant reduction in all-cause mortality. Restricting screening to men who have smoked (the strongest risk factor for AAA) further lowers cost and increases efficiency.
Frank A. Lederle, MD, is from the Center for Chronic Disease Outcomes Research at the Veterans Affairs Medical Center. These comments are taken from an accompanying editorial (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6663). No conflicts of interest were declared.
These new data will not change the finding of robust reduction in AAA-related mortality from screening seen in all previous meta-analyses. However, the most recently updated meta-analyses now reveal the small reduction in all-cause mortality with screening to be statistically significant.
So although the findings of the Western Australian trial remain negative and raise some concerns about screening, their aggregation with other studies does not change the overall conclusions that screening substantially reduced AAA-related mortality and also resulted in a statistically significant reduction in all-cause mortality. Restricting screening to men who have smoked (the strongest risk factor for AAA) further lowers cost and increases efficiency.
Frank A. Lederle, MD, is from the Center for Chronic Disease Outcomes Research at the Veterans Affairs Medical Center. These comments are taken from an accompanying editorial (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6663). No conflicts of interest were declared.
These new data will not change the finding of robust reduction in AAA-related mortality from screening seen in all previous meta-analyses. However, the most recently updated meta-analyses now reveal the small reduction in all-cause mortality with screening to be statistically significant.
So although the findings of the Western Australian trial remain negative and raise some concerns about screening, their aggregation with other studies does not change the overall conclusions that screening substantially reduced AAA-related mortality and also resulted in a statistically significant reduction in all-cause mortality. Restricting screening to men who have smoked (the strongest risk factor for AAA) further lowers cost and increases efficiency.
Frank A. Lederle, MD, is from the Center for Chronic Disease Outcomes Research at the Veterans Affairs Medical Center. These comments are taken from an accompanying editorial (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6663). No conflicts of interest were declared.
In contrast to previous studies, screening for abdominal aortic aneurysms in older men does not appear to have a significant effect on overall mortality, according to a prospective, randomized study.
Mortality from ruptured AAA remains high in older men, which has prompted four previous large randomized trials to explore whether screening men aged 65 years and older might reduce mortality.
Writing in the October 31 online edition of JAMA Internal Medicine, the authors reported the long-term outcomes of an Australian population-based trial of screening for abdominal aortic aneurysms in 49,801 men aged 64-83 years, of whom 19 249 were invited to screening and 12,203 of those underwent screening (isrctn.org Identifier: ISRCTN16171472).
After a mean 12.8 years of follow-up, there was a non-significant 9% lower mortality in the invited screening group compared to the control group and a non-significant 8% lower mortality among men aged 65-74 years.
Overall, there were 90 deaths from ruptured AAA in the screening group and 98 in the control group (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6633).
The prevalence of abdominal aortic aneurysms with a diameter at or above 30 mm was 6.6% in men aged 65-74, and 0.4% for those with a diameter of 55 mm or above.
While the rate of ruptured abdominal aortic aneurysms was significantly lower in the invited group compared to the control group (72 vs. 99, P = .04), the 30-day mortality after surgery for rupture was higher in the invited group compared to the control group (61.5% vs. 43.2%).
Screening had no meaningful impact on the risk of all-cause, cardiovascular, and other mortality, but men who had smoked had a higher risk of rupture and of death from a rupture than those who had never smoked, regardless of screening status.
The rate of total elective operations was significantly higher in the invited group compared to controls (536 vs. 414, P < .001), mainly in the first year after screening.
The authors calculated that to prevent one death from a ruptured abdominal aortic aneurysm in five years, 4784 men aged 64-83 years or 3290 men aged 65-74 years would need to be invited for screening.
While the strength of the study was that it was truly population-based – using the electoral roll – the authors said the lack of a benefit from screening was likely due to the relatively low rate of rupture and death from AAA, as well as a high rate of elective surgery for this condition, in the control group.
The non-significant 8% reduction in mortality observed in the study was significantly less than the 42% and 66% reductions seen in previous trials with a similar length of follow-up.
The authors suggested this may also have been related to a lower fraction of invited men participating in screening, but pointed out that the incidence of AAA in men is declining.
“The reason for the decrease in incidence and prevalence is multifactorial but is probably driven by differences in rates of smoking and cessation because the relative risk for AAA events is 3- to 6-fold higher in smokers compared with non-smokers,” they wrote.
The authors said selective screening of smokers or ex-smokers may be more effective, but pointed out that this approach would miss around one-quarter of aneurysms. However they suggested more targeted screening may yet achieve a benefit.
“The small overall benefit of population-wide screening does not mean that finding AAAs in suitable older men is not worthwhile because deaths from AAAs in men who actually attended for screening were halved by early detection and successful treatment.”
The study was supported by the National Health and Medical Research Council Project. The authors reported that they had no conflicts of interest.
In contrast to previous studies, screening for abdominal aortic aneurysms in older men does not appear to have a significant effect on overall mortality, according to a prospective, randomized study.
Mortality from ruptured AAA remains high in older men, which has prompted four previous large randomized trials to explore whether screening men aged 65 years and older might reduce mortality.
Writing in the October 31 online edition of JAMA Internal Medicine, the authors reported the long-term outcomes of an Australian population-based trial of screening for abdominal aortic aneurysms in 49,801 men aged 64-83 years, of whom 19 249 were invited to screening and 12,203 of those underwent screening (isrctn.org Identifier: ISRCTN16171472).
After a mean 12.8 years of follow-up, there was a non-significant 9% lower mortality in the invited screening group compared to the control group and a non-significant 8% lower mortality among men aged 65-74 years.
Overall, there were 90 deaths from ruptured AAA in the screening group and 98 in the control group (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6633).
The prevalence of abdominal aortic aneurysms with a diameter at or above 30 mm was 6.6% in men aged 65-74, and 0.4% for those with a diameter of 55 mm or above.
While the rate of ruptured abdominal aortic aneurysms was significantly lower in the invited group compared to the control group (72 vs. 99, P = .04), the 30-day mortality after surgery for rupture was higher in the invited group compared to the control group (61.5% vs. 43.2%).
Screening had no meaningful impact on the risk of all-cause, cardiovascular, and other mortality, but men who had smoked had a higher risk of rupture and of death from a rupture than those who had never smoked, regardless of screening status.
The rate of total elective operations was significantly higher in the invited group compared to controls (536 vs. 414, P < .001), mainly in the first year after screening.
The authors calculated that to prevent one death from a ruptured abdominal aortic aneurysm in five years, 4784 men aged 64-83 years or 3290 men aged 65-74 years would need to be invited for screening.
While the strength of the study was that it was truly population-based – using the electoral roll – the authors said the lack of a benefit from screening was likely due to the relatively low rate of rupture and death from AAA, as well as a high rate of elective surgery for this condition, in the control group.
The non-significant 8% reduction in mortality observed in the study was significantly less than the 42% and 66% reductions seen in previous trials with a similar length of follow-up.
The authors suggested this may also have been related to a lower fraction of invited men participating in screening, but pointed out that the incidence of AAA in men is declining.
“The reason for the decrease in incidence and prevalence is multifactorial but is probably driven by differences in rates of smoking and cessation because the relative risk for AAA events is 3- to 6-fold higher in smokers compared with non-smokers,” they wrote.
The authors said selective screening of smokers or ex-smokers may be more effective, but pointed out that this approach would miss around one-quarter of aneurysms. However they suggested more targeted screening may yet achieve a benefit.
“The small overall benefit of population-wide screening does not mean that finding AAAs in suitable older men is not worthwhile because deaths from AAAs in men who actually attended for screening were halved by early detection and successful treatment.”
The study was supported by the National Health and Medical Research Council Project. The authors reported that they had no conflicts of interest.
Key clinical point:
Major finding: Men invited to undergo screening for abdominal aortic aneurysms had a non-significant 9% lower mortality compared to a control group.
Data source: Prospective, population-based randomized controlled trial in 49,801 men aged 64-83 years.
Disclosures: The study was supported by the National Health and Medical Research Council Project. The authors reported that they had no conflicts of interest.
Midterm results of thoracic stenting for acute type B dissection promising
LAS VEGAS – Patients with acute, complicated type B aortic dissections are reported to have a greater than 50% likelihood of dying from their condition. Three-year results of the Valiant thoracic stent graft in the treatment of these dissections showed freedom from all-cause mortality of 79.4%, and a freedom from dissection-related mortality of 90%, according to Ali Azizzadeh, MD.
Dr. Azizzadeh presented the midterm results of the Medtronic Dissection US IDE trial of endovascular treatment with the Valiant Captivia thoracic stent graft (Medtronic) in acute, complicated type B aortic dissection patients at the 2016 Vascular Interventional Advances meeting.
One-year outcomes of the trial were reported last year in the Annals of Thoracic Surgery (2015 Sep;100:802-9).
Dr. Azizzadeh is a vascular surgeon at the Memorial Hermann Heart and Vascular Institute, Houston.
Between June 2010 and May 2012, 50 patients with acute, complicated type B aortic dissection were enrolled at 16 clinical sites in the United States in this multicenter, prospective, nonrandomized trial with a planned 5-year follow-up.
The primary safety endpoint was all-cause mortality within 30 days from the index procedure.
A total of 28 patients completed their 3-year follow-up. Through 3 years, there were no postindex ruptures or conversions to open surgical repair reported in the trial.
At 3 years, true lumen diameter over the stented region (or endograft segment) remained stable or increased in 92.3% of patients, according to Dr. Azizzadeh. False lumen diameter remained stable or decreased in 69.3% of patients, and the false lumen was partially or completely thrombosed in 75% of patients.
One death (from sepsis) occurred between years 2 and 3; and was adjudicated by the clinical events committee as unrelated to the device, the procedure, or the dissection.
Although these midterm results are encouraging, said Dr. Azizzadeh, longer-term outcomes are needed to assess the durability of the stent graft in this indication.
The trial was sponsored by Medtronic. Dr. Azizzadeh has consulted for and received research/trial funding from W.L. Gore & Associates and Medtronic.
LAS VEGAS – Patients with acute, complicated type B aortic dissections are reported to have a greater than 50% likelihood of dying from their condition. Three-year results of the Valiant thoracic stent graft in the treatment of these dissections showed freedom from all-cause mortality of 79.4%, and a freedom from dissection-related mortality of 90%, according to Ali Azizzadeh, MD.
Dr. Azizzadeh presented the midterm results of the Medtronic Dissection US IDE trial of endovascular treatment with the Valiant Captivia thoracic stent graft (Medtronic) in acute, complicated type B aortic dissection patients at the 2016 Vascular Interventional Advances meeting.
One-year outcomes of the trial were reported last year in the Annals of Thoracic Surgery (2015 Sep;100:802-9).
Dr. Azizzadeh is a vascular surgeon at the Memorial Hermann Heart and Vascular Institute, Houston.
Between June 2010 and May 2012, 50 patients with acute, complicated type B aortic dissection were enrolled at 16 clinical sites in the United States in this multicenter, prospective, nonrandomized trial with a planned 5-year follow-up.
The primary safety endpoint was all-cause mortality within 30 days from the index procedure.
A total of 28 patients completed their 3-year follow-up. Through 3 years, there were no postindex ruptures or conversions to open surgical repair reported in the trial.
At 3 years, true lumen diameter over the stented region (or endograft segment) remained stable or increased in 92.3% of patients, according to Dr. Azizzadeh. False lumen diameter remained stable or decreased in 69.3% of patients, and the false lumen was partially or completely thrombosed in 75% of patients.
One death (from sepsis) occurred between years 2 and 3; and was adjudicated by the clinical events committee as unrelated to the device, the procedure, or the dissection.
Although these midterm results are encouraging, said Dr. Azizzadeh, longer-term outcomes are needed to assess the durability of the stent graft in this indication.
The trial was sponsored by Medtronic. Dr. Azizzadeh has consulted for and received research/trial funding from W.L. Gore & Associates and Medtronic.
LAS VEGAS – Patients with acute, complicated type B aortic dissections are reported to have a greater than 50% likelihood of dying from their condition. Three-year results of the Valiant thoracic stent graft in the treatment of these dissections showed freedom from all-cause mortality of 79.4%, and a freedom from dissection-related mortality of 90%, according to Ali Azizzadeh, MD.
Dr. Azizzadeh presented the midterm results of the Medtronic Dissection US IDE trial of endovascular treatment with the Valiant Captivia thoracic stent graft (Medtronic) in acute, complicated type B aortic dissection patients at the 2016 Vascular Interventional Advances meeting.
One-year outcomes of the trial were reported last year in the Annals of Thoracic Surgery (2015 Sep;100:802-9).
Dr. Azizzadeh is a vascular surgeon at the Memorial Hermann Heart and Vascular Institute, Houston.
Between June 2010 and May 2012, 50 patients with acute, complicated type B aortic dissection were enrolled at 16 clinical sites in the United States in this multicenter, prospective, nonrandomized trial with a planned 5-year follow-up.
The primary safety endpoint was all-cause mortality within 30 days from the index procedure.
A total of 28 patients completed their 3-year follow-up. Through 3 years, there were no postindex ruptures or conversions to open surgical repair reported in the trial.
At 3 years, true lumen diameter over the stented region (or endograft segment) remained stable or increased in 92.3% of patients, according to Dr. Azizzadeh. False lumen diameter remained stable or decreased in 69.3% of patients, and the false lumen was partially or completely thrombosed in 75% of patients.
One death (from sepsis) occurred between years 2 and 3; and was adjudicated by the clinical events committee as unrelated to the device, the procedure, or the dissection.
Although these midterm results are encouraging, said Dr. Azizzadeh, longer-term outcomes are needed to assess the durability of the stent graft in this indication.
The trial was sponsored by Medtronic. Dr. Azizzadeh has consulted for and received research/trial funding from W.L. Gore & Associates and Medtronic.
AT VIVA16 LAS VEGAS
Key clinical point:
Major finding: Three-year results of the Valiant thoracic stent graft in the treatment of acute type B dissections showed freedom from all-cause mortality of 79.4%, and a freedom from dissection-related mortality of 90%.
Data source: Midterm results were presented from the multicenter, prospective, nonrandomized Medtronic Dissection US IDE trial.
Disclosures: The trial was sponsored by Medtronic. Dr. Azizzadeh has consulted for and received research/trial funding from W.L. Gore & Associates and Medtronic.
Fast-Track EVAR protocol shown safe, effective, and cheaper
LAS VEGAS – A ‘fast-track” stenting method for endovascular abdominal aortic aneurysm repair (EVAR) enabled patients to be treated safely and effectively without general anesthetic or ICU admission, and with next-day discharge, Zvonimir Krajcer, MD, said at the 2016 Vascular Interventional Advances meeting.
Dr. Krajcer discussed the final results of the Prospective LIFE Registry, which followed 250 patients treated with the Ovation Abdominal Stent Graft platform, who had suitable femoral arteries to allow the use of the Perclose ProGlide Suture-Mediated Closure (SMC) System.
For the Fast-Track patients, vascular access, stent graft delivery, and deployment were successful. The Fast-Track EVAR protocol was successfully completed in 216 (87%) of the patients. In comparing the Fast-Track cohort (n = 216) to the non–Fast-Track cohort (n = 34), procedure time was found to be 84 vs. 110 minutes, the use of general anesthesia was 0% vs. 18%, and the need for ICU stay was 0% vs. 32%. Hospital stay for the two groups was 1.2 vs. 1.9 days, respectively.
Quality of life score improvement from baseline to 30 days as assessed via the EQ-5D questionnaire, was significantly greater in the Fast-Track patients, compared with the EVAR controls, said Dr. Krajcer, an interventional cardiologist at Texas Heart Institute and St. Luke’s Hospital, Houston, and a clinical professor of medicine at Baylor College of Medicine.
To determine adverse events, patients were followed through 1 month after treatment. The researchers found no device- or procedure-related major adverse events, abdominal aortic aneurysm (AAA) ruptures, surgical conversions, or AAA-related secondary interventions. One patient in the fast-track group died from acute respiratory failure. Overall, for the Fast-Track and control groups, the freedom from type I/III endoleak was 99% and 100%, respectively.
Dr. Krajcer reported that the 30-day hospital readmission rate in the LIFE study was 1.6%, compared to 8% reported for EVAR from the American College of Surgeons National Surgical Quality Improvement Program.
The economic analysis was performed comparing the Fast-Track patients to a control group, which consisted of a database of 8,306 patients treated with elective infrarenal EVAR at 3,750 U.S. hospitals based on inpatient discharge between 2012 and 2015. The researchers calculated costs related to access, anesthesia, ICU stay, and hospital stay.
The Fast-Track protocol showed $21,000 in perioperative cost savings relative to standard EVAR, largely driven by differences in hospital stay costs, according to Dr. Krajcer.
“Fast-track EVAR using the Ovation Prime stent graft is safe and feasible and lowers perioperative costs,” said Dr. Krajcer. “Our results warrant the establishment of a Fast-Track EVAR protocol in experienced EVAR centers,” he concluded.
Dr. Krajcer had nothing to disclose.
LAS VEGAS – A ‘fast-track” stenting method for endovascular abdominal aortic aneurysm repair (EVAR) enabled patients to be treated safely and effectively without general anesthetic or ICU admission, and with next-day discharge, Zvonimir Krajcer, MD, said at the 2016 Vascular Interventional Advances meeting.
Dr. Krajcer discussed the final results of the Prospective LIFE Registry, which followed 250 patients treated with the Ovation Abdominal Stent Graft platform, who had suitable femoral arteries to allow the use of the Perclose ProGlide Suture-Mediated Closure (SMC) System.
For the Fast-Track patients, vascular access, stent graft delivery, and deployment were successful. The Fast-Track EVAR protocol was successfully completed in 216 (87%) of the patients. In comparing the Fast-Track cohort (n = 216) to the non–Fast-Track cohort (n = 34), procedure time was found to be 84 vs. 110 minutes, the use of general anesthesia was 0% vs. 18%, and the need for ICU stay was 0% vs. 32%. Hospital stay for the two groups was 1.2 vs. 1.9 days, respectively.
Quality of life score improvement from baseline to 30 days as assessed via the EQ-5D questionnaire, was significantly greater in the Fast-Track patients, compared with the EVAR controls, said Dr. Krajcer, an interventional cardiologist at Texas Heart Institute and St. Luke’s Hospital, Houston, and a clinical professor of medicine at Baylor College of Medicine.
To determine adverse events, patients were followed through 1 month after treatment. The researchers found no device- or procedure-related major adverse events, abdominal aortic aneurysm (AAA) ruptures, surgical conversions, or AAA-related secondary interventions. One patient in the fast-track group died from acute respiratory failure. Overall, for the Fast-Track and control groups, the freedom from type I/III endoleak was 99% and 100%, respectively.
Dr. Krajcer reported that the 30-day hospital readmission rate in the LIFE study was 1.6%, compared to 8% reported for EVAR from the American College of Surgeons National Surgical Quality Improvement Program.
The economic analysis was performed comparing the Fast-Track patients to a control group, which consisted of a database of 8,306 patients treated with elective infrarenal EVAR at 3,750 U.S. hospitals based on inpatient discharge between 2012 and 2015. The researchers calculated costs related to access, anesthesia, ICU stay, and hospital stay.
The Fast-Track protocol showed $21,000 in perioperative cost savings relative to standard EVAR, largely driven by differences in hospital stay costs, according to Dr. Krajcer.
“Fast-track EVAR using the Ovation Prime stent graft is safe and feasible and lowers perioperative costs,” said Dr. Krajcer. “Our results warrant the establishment of a Fast-Track EVAR protocol in experienced EVAR centers,” he concluded.
Dr. Krajcer had nothing to disclose.
LAS VEGAS – A ‘fast-track” stenting method for endovascular abdominal aortic aneurysm repair (EVAR) enabled patients to be treated safely and effectively without general anesthetic or ICU admission, and with next-day discharge, Zvonimir Krajcer, MD, said at the 2016 Vascular Interventional Advances meeting.
Dr. Krajcer discussed the final results of the Prospective LIFE Registry, which followed 250 patients treated with the Ovation Abdominal Stent Graft platform, who had suitable femoral arteries to allow the use of the Perclose ProGlide Suture-Mediated Closure (SMC) System.
For the Fast-Track patients, vascular access, stent graft delivery, and deployment were successful. The Fast-Track EVAR protocol was successfully completed in 216 (87%) of the patients. In comparing the Fast-Track cohort (n = 216) to the non–Fast-Track cohort (n = 34), procedure time was found to be 84 vs. 110 minutes, the use of general anesthesia was 0% vs. 18%, and the need for ICU stay was 0% vs. 32%. Hospital stay for the two groups was 1.2 vs. 1.9 days, respectively.
Quality of life score improvement from baseline to 30 days as assessed via the EQ-5D questionnaire, was significantly greater in the Fast-Track patients, compared with the EVAR controls, said Dr. Krajcer, an interventional cardiologist at Texas Heart Institute and St. Luke’s Hospital, Houston, and a clinical professor of medicine at Baylor College of Medicine.
To determine adverse events, patients were followed through 1 month after treatment. The researchers found no device- or procedure-related major adverse events, abdominal aortic aneurysm (AAA) ruptures, surgical conversions, or AAA-related secondary interventions. One patient in the fast-track group died from acute respiratory failure. Overall, for the Fast-Track and control groups, the freedom from type I/III endoleak was 99% and 100%, respectively.
Dr. Krajcer reported that the 30-day hospital readmission rate in the LIFE study was 1.6%, compared to 8% reported for EVAR from the American College of Surgeons National Surgical Quality Improvement Program.
The economic analysis was performed comparing the Fast-Track patients to a control group, which consisted of a database of 8,306 patients treated with elective infrarenal EVAR at 3,750 U.S. hospitals based on inpatient discharge between 2012 and 2015. The researchers calculated costs related to access, anesthesia, ICU stay, and hospital stay.
The Fast-Track protocol showed $21,000 in perioperative cost savings relative to standard EVAR, largely driven by differences in hospital stay costs, according to Dr. Krajcer.
“Fast-track EVAR using the Ovation Prime stent graft is safe and feasible and lowers perioperative costs,” said Dr. Krajcer. “Our results warrant the establishment of a Fast-Track EVAR protocol in experienced EVAR centers,” he concluded.
Dr. Krajcer had nothing to disclose.
AT VIVA16
Key clinical point:
Major finding: Quality of life score improvement from baseline to 30 days was significantly greater in the Fast-Track patients, compared with the EVAR controls.
Data source: The researchers assessed 250 patients in the Prospective LIFE Registry and compared results to more than 8,000 patients in an EVAR database as controls.
Disclosures: Dr. Krajcer had nothing to disclose.
NSQIP Study: Symptomatic AAAs have a twofold increased periop mortality risk over asymptomatic
A recent small study suggested that, in the age of endovascular aortic aneurysm repair (EVAR), the mortality rates between symptomatic and asymptomatic abdominal aortic aneurysm (AAA) repair have become similar, according to Peter A. Soden, MD, of Beth Deaconess Medical Center, Boston, and his colleagues. However, in their large database study, Dr. Soden and his colleagues found that outcomes for the repair of abdominal aortic aneurysms were increasingly worse from asymptomatic to symptomatic to ruptured AAA.
The researchers assessed all patients undergoing endovascular and open AAA repair in the 2011-2013 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data set, according to a report published in the August issue of the Journal of Vascular Surgery.
Symptomatic AAA was defined as lack of evidence of rupture but with the presence of abdominal or back pain or symptoms from local compression by the aneurysm causing early satiety, hydronephrosis, or deep vein thrombosis. Ruptured aneurysms were divided into two categories: hypotensive (defined as systolic blood pressure less than 90 mmm Hg or drop of greater than 40 mm HG from baseline) and nonhypotensive (J Vasc Surg. 2016;64:297-305).
There were numerous demographic and comorbidity differences between asymptomatic and symptomatic patients and between symptomatic and ruptured patients, with a general trend of increasing of commodities and factors influencing operative risk.
The final study included 5,502 patients undergoing repair of infrarenal (85%; 92% EVAR) or juxtarenal (15%;20% EVAR) aneurysms. These differences in the use of EVAR were statistically significant.
This population comprised 4,495 asymptomatic patients (82% EVAR), 455 symptomatic patients (69% EVAR), and 552 ruptured patients (52% EVAR).
The overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (5.1% vs. 1.9%; P less than .001).Similarly, for EVAR, the overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (3.8% vs. 1.4%; P less than .001). For open repair, there was no significant difference in mortality (7.7% vs. 4.3%) between symptomatic and asymptomatic patients, respectively.
Multivariate analysis showed that symptomatic patients had twice the operative mortality as asymptomatic patients (odds ratio, 2.1). A symptomatic aneurysm was also predictive of a major adverse event (OR, 1.5). Ruptured aneurysms had a significant nearly sevenfold increase in mortality risk vs. symptomatic aneurysms (OR, 6.5) and a fivefold increase of risk of a major adverse event (OR 5.1), with all ORs within their 95% confidence interval levels).
“In this large contemporary study of symptomatic AAA patients, in which the majority were treated with EVAR, we found that symptomatic patients have twice the perioperative mortality compared with asymptomatic patients. Despite this, we also find a reduction in perioperative mortality for symptomatic aneurysms compared with prior reports in which the majority were treated with open repair, and we believe this supports an EVAR-first approach for symptomatic aneurysms with suitable anatomy,” the researchers concluded.
The authors reported that they had no relevant disclosures.
A recent small study suggested that, in the age of endovascular aortic aneurysm repair (EVAR), the mortality rates between symptomatic and asymptomatic abdominal aortic aneurysm (AAA) repair have become similar, according to Peter A. Soden, MD, of Beth Deaconess Medical Center, Boston, and his colleagues. However, in their large database study, Dr. Soden and his colleagues found that outcomes for the repair of abdominal aortic aneurysms were increasingly worse from asymptomatic to symptomatic to ruptured AAA.
The researchers assessed all patients undergoing endovascular and open AAA repair in the 2011-2013 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data set, according to a report published in the August issue of the Journal of Vascular Surgery.
Symptomatic AAA was defined as lack of evidence of rupture but with the presence of abdominal or back pain or symptoms from local compression by the aneurysm causing early satiety, hydronephrosis, or deep vein thrombosis. Ruptured aneurysms were divided into two categories: hypotensive (defined as systolic blood pressure less than 90 mmm Hg or drop of greater than 40 mm HG from baseline) and nonhypotensive (J Vasc Surg. 2016;64:297-305).
There were numerous demographic and comorbidity differences between asymptomatic and symptomatic patients and between symptomatic and ruptured patients, with a general trend of increasing of commodities and factors influencing operative risk.
The final study included 5,502 patients undergoing repair of infrarenal (85%; 92% EVAR) or juxtarenal (15%;20% EVAR) aneurysms. These differences in the use of EVAR were statistically significant.
This population comprised 4,495 asymptomatic patients (82% EVAR), 455 symptomatic patients (69% EVAR), and 552 ruptured patients (52% EVAR).
The overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (5.1% vs. 1.9%; P less than .001).Similarly, for EVAR, the overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (3.8% vs. 1.4%; P less than .001). For open repair, there was no significant difference in mortality (7.7% vs. 4.3%) between symptomatic and asymptomatic patients, respectively.
Multivariate analysis showed that symptomatic patients had twice the operative mortality as asymptomatic patients (odds ratio, 2.1). A symptomatic aneurysm was also predictive of a major adverse event (OR, 1.5). Ruptured aneurysms had a significant nearly sevenfold increase in mortality risk vs. symptomatic aneurysms (OR, 6.5) and a fivefold increase of risk of a major adverse event (OR 5.1), with all ORs within their 95% confidence interval levels).
“In this large contemporary study of symptomatic AAA patients, in which the majority were treated with EVAR, we found that symptomatic patients have twice the perioperative mortality compared with asymptomatic patients. Despite this, we also find a reduction in perioperative mortality for symptomatic aneurysms compared with prior reports in which the majority were treated with open repair, and we believe this supports an EVAR-first approach for symptomatic aneurysms with suitable anatomy,” the researchers concluded.
The authors reported that they had no relevant disclosures.
A recent small study suggested that, in the age of endovascular aortic aneurysm repair (EVAR), the mortality rates between symptomatic and asymptomatic abdominal aortic aneurysm (AAA) repair have become similar, according to Peter A. Soden, MD, of Beth Deaconess Medical Center, Boston, and his colleagues. However, in their large database study, Dr. Soden and his colleagues found that outcomes for the repair of abdominal aortic aneurysms were increasingly worse from asymptomatic to symptomatic to ruptured AAA.
The researchers assessed all patients undergoing endovascular and open AAA repair in the 2011-2013 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data set, according to a report published in the August issue of the Journal of Vascular Surgery.
Symptomatic AAA was defined as lack of evidence of rupture but with the presence of abdominal or back pain or symptoms from local compression by the aneurysm causing early satiety, hydronephrosis, or deep vein thrombosis. Ruptured aneurysms were divided into two categories: hypotensive (defined as systolic blood pressure less than 90 mmm Hg or drop of greater than 40 mm HG from baseline) and nonhypotensive (J Vasc Surg. 2016;64:297-305).
There were numerous demographic and comorbidity differences between asymptomatic and symptomatic patients and between symptomatic and ruptured patients, with a general trend of increasing of commodities and factors influencing operative risk.
The final study included 5,502 patients undergoing repair of infrarenal (85%; 92% EVAR) or juxtarenal (15%;20% EVAR) aneurysms. These differences in the use of EVAR were statistically significant.
This population comprised 4,495 asymptomatic patients (82% EVAR), 455 symptomatic patients (69% EVAR), and 552 ruptured patients (52% EVAR).
The overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (5.1% vs. 1.9%; P less than .001).Similarly, for EVAR, the overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (3.8% vs. 1.4%; P less than .001). For open repair, there was no significant difference in mortality (7.7% vs. 4.3%) between symptomatic and asymptomatic patients, respectively.
Multivariate analysis showed that symptomatic patients had twice the operative mortality as asymptomatic patients (odds ratio, 2.1). A symptomatic aneurysm was also predictive of a major adverse event (OR, 1.5). Ruptured aneurysms had a significant nearly sevenfold increase in mortality risk vs. symptomatic aneurysms (OR, 6.5) and a fivefold increase of risk of a major adverse event (OR 5.1), with all ORs within their 95% confidence interval levels).
“In this large contemporary study of symptomatic AAA patients, in which the majority were treated with EVAR, we found that symptomatic patients have twice the perioperative mortality compared with asymptomatic patients. Despite this, we also find a reduction in perioperative mortality for symptomatic aneurysms compared with prior reports in which the majority were treated with open repair, and we believe this supports an EVAR-first approach for symptomatic aneurysms with suitable anatomy,” the researchers concluded.
The authors reported that they had no relevant disclosures.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point: Outcomes for repair of abdominal aortic aneurysm repair were increasingly worse from asymptomatic to symptomatic to ruptured AAA.
Major finding: Patients with symptomatic AAA had a twofold increased risk of perioperative mortality, compared with patients with asymptomatic AAA undergoing repair.
Data source: The study assessed all patients undergoing AAA repair in the 2011-2013 American College of Surgeons NSQIP data set.
Disclosures: The authors reported that they had no relevant disclosures.
Abdominal compartment syndrome – a common adverse event after rAAA repair
Abdominal compartment syndrome (ACS) was common after ruptured abdominal aortic aneurysm (AAA) repair, with a similar incidence for both open surgical and endovascular repair (EVAR), according to a report published in the European Journal of Vascular and Endovascular Surgery.
Samuel Ersryd, a doctoral student, and his colleagues at Uppsala (Sweden) University performed their study to determine the contemporary incidence, treatment, and outcomes of ACS after AAA repair.
The analysis included 6,634 patients in the Swedish vascular registry who were treated for abdominal aortic aneurysm repair at 31 institutions from May 2008 to December 2013. The mean patient age was 72.8 years, and 16.6% were women. There were 5,271 intact AAA (iAAA) repairs and 1,341 ruptured AAA (rAAA repairs). A total of 41.9% of iAAA repairs were open, as were 72.0% of the rAAA repairs (Eur J Vasc Endovasc Surg. 2016;52:158-65).The study found an incidence of ACS in the rAAA group of 6.8% after open surgery and 6.9% after EVAR.
The morbidity and mortality rates for iAAA and rAAA with ACS were “devastating” in both groups, according to the authors.
Mortality at 90 days for patients with ACS after rAAA was 58.7%, twice that of patients without ACS. In patients with iAAA repair with ACS, the 90-day mortality was 19.2%, six times higher than for those without ACS.
Prophylactic open abdomen treatment was performed in 10.7% of open-surgery patients.
The researchers found no differences in mortality among patients in either group that developed ACS, whether they were treated with decompression laparotomy or not.
Age, sex, and perioperative comorbidities were not associated with ACS, Mr. Ersryd and his associates said. Within the rAAA group, however, ACS was associated with the lowest measured preoperative blood pressure and with preoperative unconsciousness. In addition, ACS was more common in both the iAAA and rAAA groups after perioperative bleeding greater than 5 L, in the iAAA group after reimplantation of a renal artery, and in the rAAA group after the use of balloon occlusion after EVAR. In those patients operated on for iAAA, the risk of developing ACS was 8.1% in patients who had perioperative bleeding greater than 5 L, compared with only 0.8% if bleeding was less than 5 L (P less than .001).
“With such poor results among patients who developed ACS, prevention is the obvious key to success. Massive transfusion protocols and permissive hypotension in patients with ongoing bleeding are important, as well as being restrictive with crystalloids,” the authors said. In addition, they recommended a proactive strategy treating intra-abdominal hypertension with medical therapy, effective pain relief, and neuromuscular blockade as important preventive measures.
“ACS is associated with a devastating effect on outcome after surgery for both ruptured and intact AAA. There was no difference in outcome among those who developed ACS, depending on whether the primary treatment had been performed with an open or endovascular technique,” the researchers concluded.
The authors reported they had no conflicts of interest and the study was funded by the Swedish Research Council and Uppsala University.
On Twitter @VascularTweets
The authors of this paper provide compelling data demonstrating the seriousness of abdominal compartment syndrome developing following abdominal aortic surgery. Recognition of this complication is therefore mandatory and techniques to relieve it should be instituted immediately. In my practice, I have successfully used the Wittmann patch, but recently I switched to the VAC (vacuum-assisted closure) device. Following an endovascular approach for a ruptured AAA, patients may require concomitant exploration for retrograde bleeding sources, such as an inferior mesenteric artery or large lumbars that will continue to bleed unless ligated.
Dr. Russell Samson is the medical editor of Vascular Specialist.
The authors of this paper provide compelling data demonstrating the seriousness of abdominal compartment syndrome developing following abdominal aortic surgery. Recognition of this complication is therefore mandatory and techniques to relieve it should be instituted immediately. In my practice, I have successfully used the Wittmann patch, but recently I switched to the VAC (vacuum-assisted closure) device. Following an endovascular approach for a ruptured AAA, patients may require concomitant exploration for retrograde bleeding sources, such as an inferior mesenteric artery or large lumbars that will continue to bleed unless ligated.
Dr. Russell Samson is the medical editor of Vascular Specialist.
The authors of this paper provide compelling data demonstrating the seriousness of abdominal compartment syndrome developing following abdominal aortic surgery. Recognition of this complication is therefore mandatory and techniques to relieve it should be instituted immediately. In my practice, I have successfully used the Wittmann patch, but recently I switched to the VAC (vacuum-assisted closure) device. Following an endovascular approach for a ruptured AAA, patients may require concomitant exploration for retrograde bleeding sources, such as an inferior mesenteric artery or large lumbars that will continue to bleed unless ligated.
Dr. Russell Samson is the medical editor of Vascular Specialist.
Abdominal compartment syndrome (ACS) was common after ruptured abdominal aortic aneurysm (AAA) repair, with a similar incidence for both open surgical and endovascular repair (EVAR), according to a report published in the European Journal of Vascular and Endovascular Surgery.
Samuel Ersryd, a doctoral student, and his colleagues at Uppsala (Sweden) University performed their study to determine the contemporary incidence, treatment, and outcomes of ACS after AAA repair.
The analysis included 6,634 patients in the Swedish vascular registry who were treated for abdominal aortic aneurysm repair at 31 institutions from May 2008 to December 2013. The mean patient age was 72.8 years, and 16.6% were women. There were 5,271 intact AAA (iAAA) repairs and 1,341 ruptured AAA (rAAA repairs). A total of 41.9% of iAAA repairs were open, as were 72.0% of the rAAA repairs (Eur J Vasc Endovasc Surg. 2016;52:158-65).The study found an incidence of ACS in the rAAA group of 6.8% after open surgery and 6.9% after EVAR.
The morbidity and mortality rates for iAAA and rAAA with ACS were “devastating” in both groups, according to the authors.
Mortality at 90 days for patients with ACS after rAAA was 58.7%, twice that of patients without ACS. In patients with iAAA repair with ACS, the 90-day mortality was 19.2%, six times higher than for those without ACS.
Prophylactic open abdomen treatment was performed in 10.7% of open-surgery patients.
The researchers found no differences in mortality among patients in either group that developed ACS, whether they were treated with decompression laparotomy or not.
Age, sex, and perioperative comorbidities were not associated with ACS, Mr. Ersryd and his associates said. Within the rAAA group, however, ACS was associated with the lowest measured preoperative blood pressure and with preoperative unconsciousness. In addition, ACS was more common in both the iAAA and rAAA groups after perioperative bleeding greater than 5 L, in the iAAA group after reimplantation of a renal artery, and in the rAAA group after the use of balloon occlusion after EVAR. In those patients operated on for iAAA, the risk of developing ACS was 8.1% in patients who had perioperative bleeding greater than 5 L, compared with only 0.8% if bleeding was less than 5 L (P less than .001).
“With such poor results among patients who developed ACS, prevention is the obvious key to success. Massive transfusion protocols and permissive hypotension in patients with ongoing bleeding are important, as well as being restrictive with crystalloids,” the authors said. In addition, they recommended a proactive strategy treating intra-abdominal hypertension with medical therapy, effective pain relief, and neuromuscular blockade as important preventive measures.
“ACS is associated with a devastating effect on outcome after surgery for both ruptured and intact AAA. There was no difference in outcome among those who developed ACS, depending on whether the primary treatment had been performed with an open or endovascular technique,” the researchers concluded.
The authors reported they had no conflicts of interest and the study was funded by the Swedish Research Council and Uppsala University.
On Twitter @VascularTweets
Abdominal compartment syndrome (ACS) was common after ruptured abdominal aortic aneurysm (AAA) repair, with a similar incidence for both open surgical and endovascular repair (EVAR), according to a report published in the European Journal of Vascular and Endovascular Surgery.
Samuel Ersryd, a doctoral student, and his colleagues at Uppsala (Sweden) University performed their study to determine the contemporary incidence, treatment, and outcomes of ACS after AAA repair.
The analysis included 6,634 patients in the Swedish vascular registry who were treated for abdominal aortic aneurysm repair at 31 institutions from May 2008 to December 2013. The mean patient age was 72.8 years, and 16.6% were women. There were 5,271 intact AAA (iAAA) repairs and 1,341 ruptured AAA (rAAA repairs). A total of 41.9% of iAAA repairs were open, as were 72.0% of the rAAA repairs (Eur J Vasc Endovasc Surg. 2016;52:158-65).The study found an incidence of ACS in the rAAA group of 6.8% after open surgery and 6.9% after EVAR.
The morbidity and mortality rates for iAAA and rAAA with ACS were “devastating” in both groups, according to the authors.
Mortality at 90 days for patients with ACS after rAAA was 58.7%, twice that of patients without ACS. In patients with iAAA repair with ACS, the 90-day mortality was 19.2%, six times higher than for those without ACS.
Prophylactic open abdomen treatment was performed in 10.7% of open-surgery patients.
The researchers found no differences in mortality among patients in either group that developed ACS, whether they were treated with decompression laparotomy or not.
Age, sex, and perioperative comorbidities were not associated with ACS, Mr. Ersryd and his associates said. Within the rAAA group, however, ACS was associated with the lowest measured preoperative blood pressure and with preoperative unconsciousness. In addition, ACS was more common in both the iAAA and rAAA groups after perioperative bleeding greater than 5 L, in the iAAA group after reimplantation of a renal artery, and in the rAAA group after the use of balloon occlusion after EVAR. In those patients operated on for iAAA, the risk of developing ACS was 8.1% in patients who had perioperative bleeding greater than 5 L, compared with only 0.8% if bleeding was less than 5 L (P less than .001).
“With such poor results among patients who developed ACS, prevention is the obvious key to success. Massive transfusion protocols and permissive hypotension in patients with ongoing bleeding are important, as well as being restrictive with crystalloids,” the authors said. In addition, they recommended a proactive strategy treating intra-abdominal hypertension with medical therapy, effective pain relief, and neuromuscular blockade as important preventive measures.
“ACS is associated with a devastating effect on outcome after surgery for both ruptured and intact AAA. There was no difference in outcome among those who developed ACS, depending on whether the primary treatment had been performed with an open or endovascular technique,” the researchers concluded.
The authors reported they had no conflicts of interest and the study was funded by the Swedish Research Council and Uppsala University.
On Twitter @VascularTweets
FROM THE EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point: Abdominal compartment syndrome is a dangerous and frequent complication after treatment of ruptured abdominal aortic aneurysms.
Major finding: After ruptured AAA repair, ACS developed in 6.8% of patients with open repair and 6.9% of patients with EVAR.
Data source: Researchers performed a population-based study using the Swedish vascular registry and the Swedish national population registry.
Disclosures: The authors reported they had no conflicts of interest and the study was funded by the Swedish Research Council and Uppsala University.