User login
Clinical factors drive hospitalization after self-harm
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRIC RESEARCH
Lithium-associated hypercalcemia: Monitoring and management
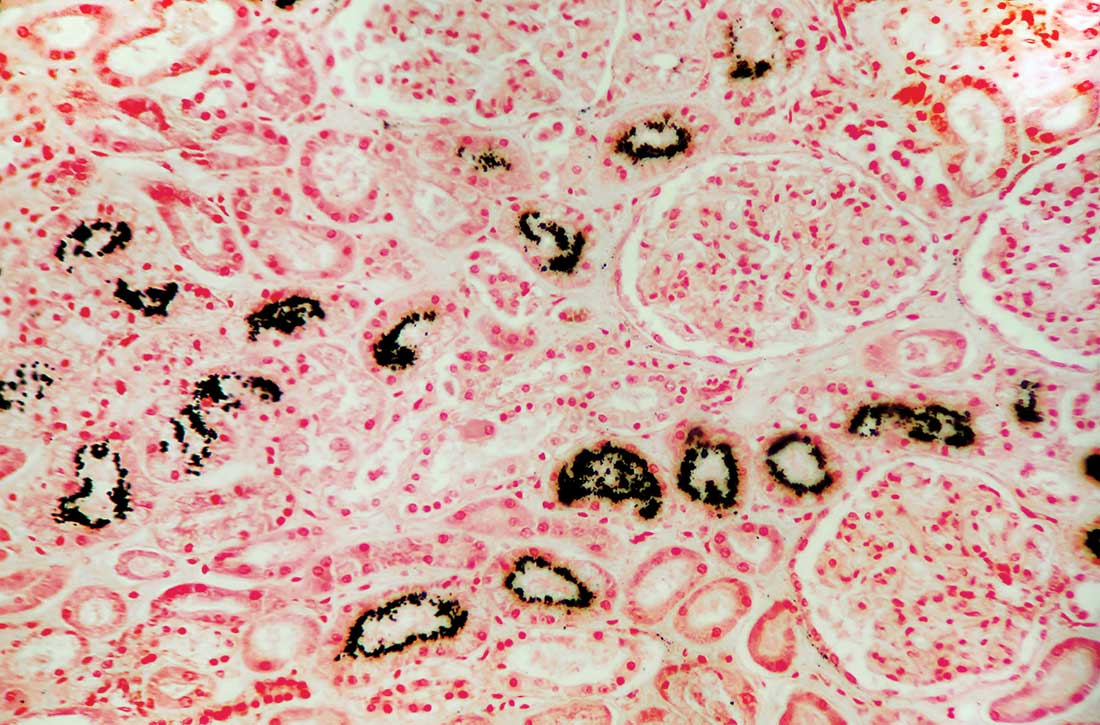
Hypercalcemia is a well-known but underrecognized adverse effect of lithium. Most patients with lithium-associated hypercalcemia (LAH) have either nonspecific symptoms (eg, persistent tiredness, constipation, polyuria, polydipsia) or no symptoms. Clinically, LAH differs from primary hyperparathyroidism, though the management protocol of these 2 conditions is almost the same. In this article, we discuss how lithium can affect calcium and parathyroid hormone (PTH) levels and how LAH and lithium-associated hyperparathyroidism (LAHP) differs from primary hyperparathyroidism. We also outline a suggested approach to monitoring and management.
An insidious problem
Due to the varying definitions and methods used to assess hypercalcemia, the reported prevalence of LAH varies from 4.3% to 80%.1 McKnight et al2 conducted a systematic review and meta-analysis of studies of the relationship between lithium and parathyroid function that included 14 case-control studies, 36 case reports, and 6 cross-sectional studies without a control group. They found that the levels of calcium and PTH were 10% higher in lithium-treated patients than in controls.2
Pathophysiology. Lithium is known to increase both calcium and PTH levels. PTH is responsible for calcium homeostasis. It is secreted in response to low calcium levels, which it increases by its action on bones, intestines, and kidneys. Vitamin D also plays a crucial role in calcium homeostasis. A deficiency of vitamin D triggers a compensatory increase in PTH to maintain calcium levels.3
Calcium and PTH levels increase soon after administration of lithium, but the rise is usually mild and insidious. In a small proportion of patients who receive long-term lithium treatment, calcium levels can exceed the normal range. Patients who develop LAH typically have serum calcium levels slightly above the normal range and PTH levels ranging from the higher side of the normal range to several times the upper limit of the normal range. Patients might also experience elevated PTH levels without any increase in calcium levels. Lithium can affect calcium and PTH levels in multiple ways. For instance, it increases the reabsorption of calcium in the kidney as well as the reset point of calcium-sensing receptors. Therefore, only higher levels of calcium can inhibit the release of PTH. Hence, in cases where the PTH level is within the normal range, it is generally higher than would be expected for a given serum calcium level. Lithium can also directly affect the parathyroid glands and can lead to either single-nodule or multimodule hyperplasia.4
Long-term lithium use can cause chronic kidney disease (CKD), which in turn leads to vitamin D deficiency and hyperparathyroidism. However, secondary hyperparathyroidism with CKD is usually seen in the more advanced stages of CKD, and is associated with low-to-normal calcium levels (as opposed to the high levels seen in LAH).3-5
Lithium-associated hyperparathyroidism
Primary hyperparathyroidism is the most common cause of hypercalcemia. Its prevalence ranges from 1 to 7 cases per 1,000 adults. The incidence of LAH/LAHP is 4- to 6-fold higher compared to the general population.6 Similar to LAH/LAHP, primary hyperparathyroidism is more common in older adults (age >60) and females. Hence, some researchers have suggested that lithium probably unmasks hyperparathyroidism in patients who are susceptible to primary hyperparathyroidism.3
Look for these clinical manifestations
Symptoms of primary hyperparathyroidism are related to high calcium and PTH levels. They are commonly described as “painful bones, renal stones, abdominal groans (due to hypercalcemia-induced ileus), and psychic moans (lethargy, poor concentration, depression).” Common adverse outcomes associated with primary hyperparathyroidism are renal stones, high risk of fracture, constipation, peptic ulcer, and pancreatitis.3,7
Continue: In contrast...
In contrast, LAHP is characterized by mild, intermittent, and/or persistent hypercalcemia and mildly increased PTH (Table 1).1,3,4 In some patients, it could improve without active intervention. Because lithium increases the absorption of urinary calcium, it is associated with hypocalciuria and a lower risk of renal stones. Additionally, lithium has osteoprotective effects and has not been associated with an increased risk of fracture. Some researchers have suggested that the presentation of LAHP is more like familial hypocalciuric hypercalcemia (FHC), which is also associated with hypocalciuria. FHC is a benign condition and does not require active intervention.3,4 Similar to those with FHC, many patients with LAHP may live with chronic asymptomatic hypercalcemia without any significant adverse outcome.
A suggested approach to monitoring
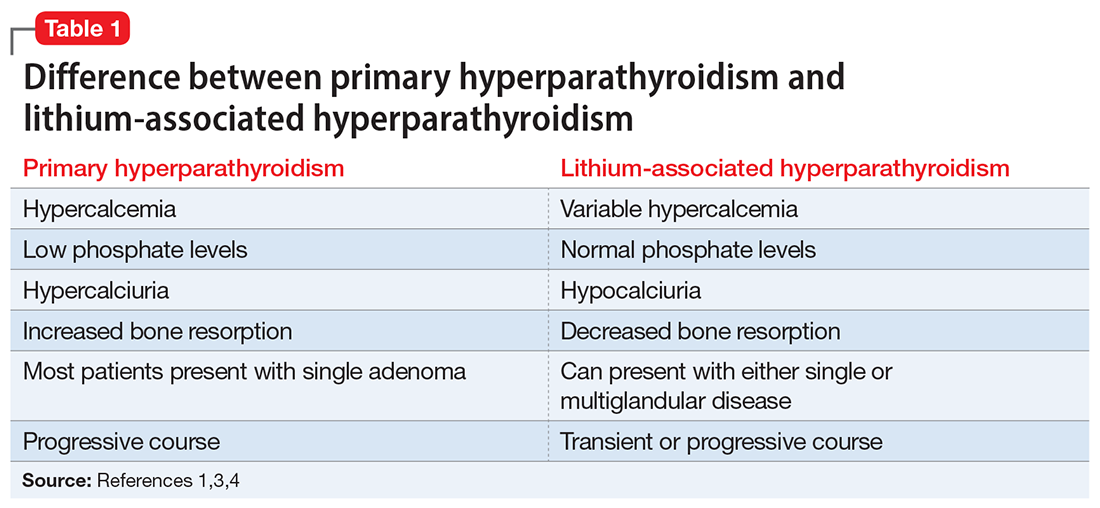
In most cases, LAH is an insidious adverse effect that is usually detected on blood tests after many years of lithium therapy.8 For patients starting lithium therapy, International Society of Bipolar Disorder guidelines recommend testing calcium levels at baseline, 6 months, and annually thereafter, or as clinically indicated, to detect and monitor hypercalcemia and hyperparathyroidism. However, these guidelines do not provide any recommendations regarding how to manage abnormal findings.9
Clinical laboratories report both total and adjusted calcium values. The adjusted calcium value takes into account albumin levels. This is a way to compensate for an abnormal concentration of albumin (establishing what a patient’s total calcium concentration would be if the albumin concentration was normal). Table 25 shows the categorization of adjusted calcium values.For patients receiving lithium, some researchers have suggested monitoring PTH as well as calcium.1
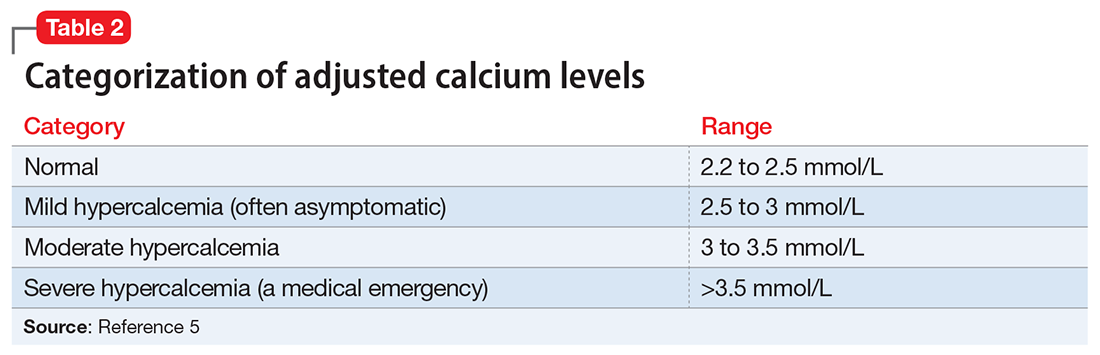
The Figure outlines our proposed approach to monitoring for LAH in patients receiving lithium. An isolated high value of calcium could be due to prolonged venous stasis if a tourniquet is used for phlebotomy. In such instances, the calcium level should be tested again without a tourniquet.10 If the repeat blood test shows elevated calcium levels, then both PTH and serum calcium should be tested.
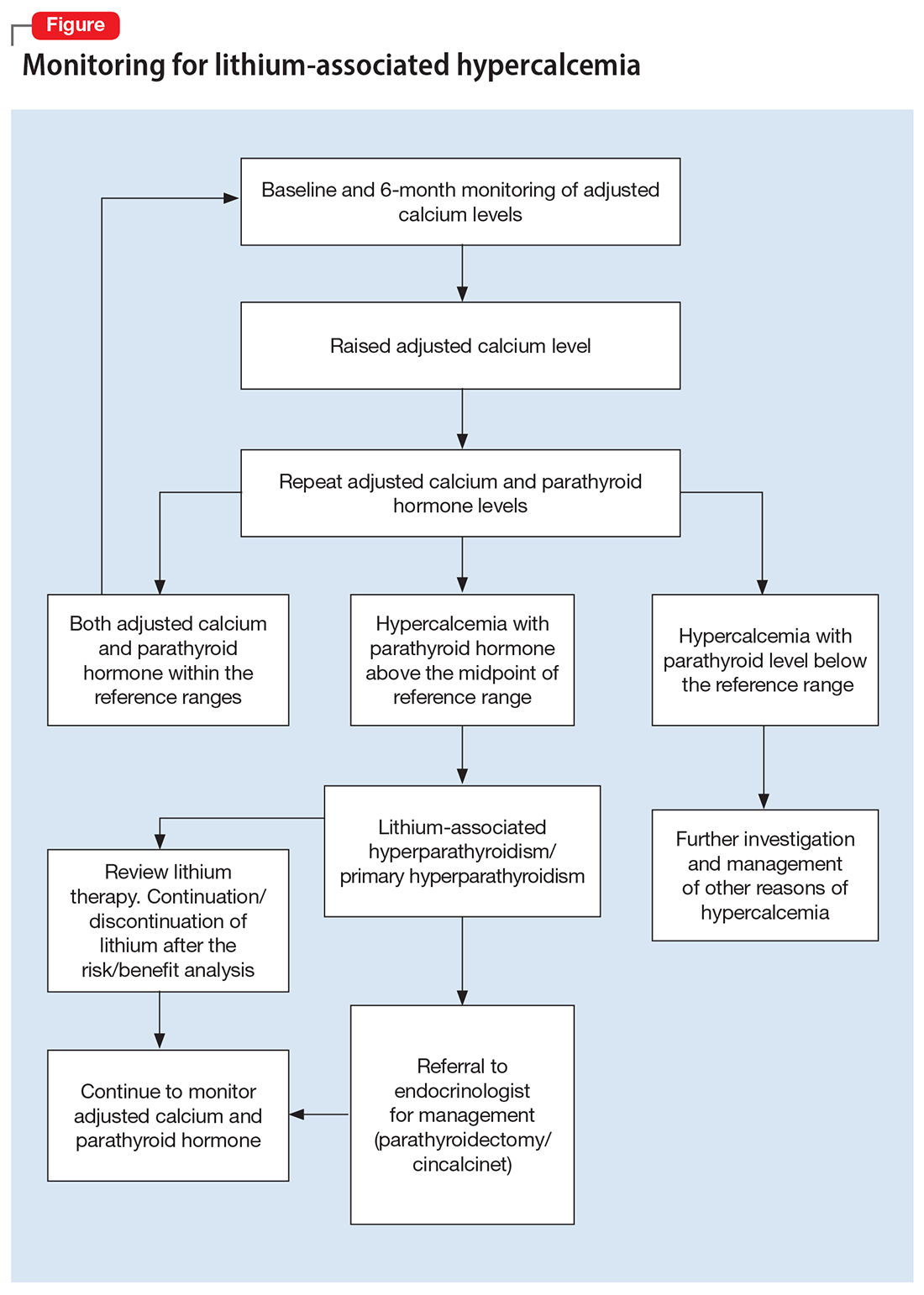
If the PTH level is higher than the midpoint of the reference range, LAH should be suspected, though sometimes hypercalcemia can present without raised PTH. LAH has also been reported to cause a transient increase in calcium levels. If hypercalcemia frequently recurs, PTH levels should be monitored. If PTH is suppressed, then the raised calcium levels are probably secondary to something other than lithium; common reasons for this include the use of vitamin D supplements or thiazide diuretics, or malignancies such as multiple myeloma.3,5,8
Continue to: Treatment
Treatment: Continue lithium?
There are several options for treating LAH. Lithium may be continued or discontinued following close monitoring of calcium and PTH levels, with or without active interventions such as surgery or pharmacotherapy, and as deemed appropriate after consultation with an endocrinologist. The decision should be informed by evaluating the risks and benefits to the patient’s physical and mental health. LAH can be reversed by discontinuing lithium, but this might not be the case in patients receiving long-term lithium therapy, especially if their elevated calcium levels are associated with parathyroid adenomas or hyperplasia. Hence, close monitoring of calcium and PTH is required even after discontinuing lithium.3,8
Surgical treatment. The primary treatment of LAH and primary hyperparathyroidism is parathyroidectomy. The possibility of recovery after parathyroidectomy for primary hyperparathyroidism is 60% to 80%, though a small proportion of patients might experience recurrence. This figure might be higher for LAH, because it is more likely to affect multiple glands.1,11 Other potential complications of parathyroidectomy are recurrent laryngeal nerve injury causing paralysis of vocal cords leading to hoarseness of voice, stridor, or aspiration, and local hematoma and hypocalcemia (requiring vitamin D and/or calcium supplements).12
Pharmacotherapy. Cinacalcet is a calcimimetic drug that decreases the reset point of the calcium-sensing receptor. It can be used if a patient is not suitable for or apprehensive about surgical intervention.1,8
Bottom Line
Calcium levels should be regularly monitored in patients receiving lithium. If calcium levels are persistently high, parathyroid hormone levels should also be measured. Management of lithium-associated hypercalcemia includes watchful waiting, discontinuing lithium, parathyroidectomy, and pharmacotherapy with cinacalcet.
Related Resources
- Laski M, Foreman R, Hancock H, et al. Lithium: an underutilized element. Current Psychiatry. 2021;20(12):27-30,34. doi:10.12788/cp.0193
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Cinacalcet • Sensipar
1. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg. 2018;42(2):415-424.
2. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
3. Shapiro HI, Davis KA. Hypercalcemia and “primary” hyperparathyroidism during lithium therapy. Am J Psychiatry. 2015;172(1):12-15.
4. Lerena VS, León NS, Sosa S, et al. Lithium and endocrine dysfunction. Medicina (B Aires). 2022;82(1):130-137.
5. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959-1966.
6. Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98(3):1122-1129.
7. Dandurand K, Ali DS, Khan AA. Primary hyperparathyroidism: a narrative review of diagnosis and medical management. J Clin Med. 2021;10(8):1604.
8. Mifsud S, Cilia K, Mifsud EL, et al. Lithium-associated hyperparathyroidism. Br J Hosp Med (Lond). 2020;81(11):1-9.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. Mieebi WM, Solomon AE, Wabote AP. The effect of tourniquet application on serum calcium and inorganic phosphorus determination. Journal of Health, Medicine and Nursing. 2019;65:51-54.
11. Awad SS, Miskulin J, Thompson N. Parathyroid adenomas versus four-gland hyperplasia as the cause of primary hyperparathyroidism in patients with prolonged lithium therapy. World J Surg. 2003;27(4):486-488.
12. Farndon JR. Postoperative complications of parathyroidectomy. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem-Oriented. Zuckschwerdt; 2001. Accessed October 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK6967
Hypercalcemia is a well-known but underrecognized adverse effect of lithium. Most patients with lithium-associated hypercalcemia (LAH) have either nonspecific symptoms (eg, persistent tiredness, constipation, polyuria, polydipsia) or no symptoms. Clinically, LAH differs from primary hyperparathyroidism, though the management protocol of these 2 conditions is almost the same. In this article, we discuss how lithium can affect calcium and parathyroid hormone (PTH) levels and how LAH and lithium-associated hyperparathyroidism (LAHP) differs from primary hyperparathyroidism. We also outline a suggested approach to monitoring and management.
An insidious problem
Due to the varying definitions and methods used to assess hypercalcemia, the reported prevalence of LAH varies from 4.3% to 80%.1 McKnight et al2 conducted a systematic review and meta-analysis of studies of the relationship between lithium and parathyroid function that included 14 case-control studies, 36 case reports, and 6 cross-sectional studies without a control group. They found that the levels of calcium and PTH were 10% higher in lithium-treated patients than in controls.2
Pathophysiology. Lithium is known to increase both calcium and PTH levels. PTH is responsible for calcium homeostasis. It is secreted in response to low calcium levels, which it increases by its action on bones, intestines, and kidneys. Vitamin D also plays a crucial role in calcium homeostasis. A deficiency of vitamin D triggers a compensatory increase in PTH to maintain calcium levels.3
Calcium and PTH levels increase soon after administration of lithium, but the rise is usually mild and insidious. In a small proportion of patients who receive long-term lithium treatment, calcium levels can exceed the normal range. Patients who develop LAH typically have serum calcium levels slightly above the normal range and PTH levels ranging from the higher side of the normal range to several times the upper limit of the normal range. Patients might also experience elevated PTH levels without any increase in calcium levels. Lithium can affect calcium and PTH levels in multiple ways. For instance, it increases the reabsorption of calcium in the kidney as well as the reset point of calcium-sensing receptors. Therefore, only higher levels of calcium can inhibit the release of PTH. Hence, in cases where the PTH level is within the normal range, it is generally higher than would be expected for a given serum calcium level. Lithium can also directly affect the parathyroid glands and can lead to either single-nodule or multimodule hyperplasia.4
Long-term lithium use can cause chronic kidney disease (CKD), which in turn leads to vitamin D deficiency and hyperparathyroidism. However, secondary hyperparathyroidism with CKD is usually seen in the more advanced stages of CKD, and is associated with low-to-normal calcium levels (as opposed to the high levels seen in LAH).3-5
Lithium-associated hyperparathyroidism
Primary hyperparathyroidism is the most common cause of hypercalcemia. Its prevalence ranges from 1 to 7 cases per 1,000 adults. The incidence of LAH/LAHP is 4- to 6-fold higher compared to the general population.6 Similar to LAH/LAHP, primary hyperparathyroidism is more common in older adults (age >60) and females. Hence, some researchers have suggested that lithium probably unmasks hyperparathyroidism in patients who are susceptible to primary hyperparathyroidism.3
Look for these clinical manifestations
Symptoms of primary hyperparathyroidism are related to high calcium and PTH levels. They are commonly described as “painful bones, renal stones, abdominal groans (due to hypercalcemia-induced ileus), and psychic moans (lethargy, poor concentration, depression).” Common adverse outcomes associated with primary hyperparathyroidism are renal stones, high risk of fracture, constipation, peptic ulcer, and pancreatitis.3,7
Continue: In contrast...
In contrast, LAHP is characterized by mild, intermittent, and/or persistent hypercalcemia and mildly increased PTH (Table 1).1,3,4 In some patients, it could improve without active intervention. Because lithium increases the absorption of urinary calcium, it is associated with hypocalciuria and a lower risk of renal stones. Additionally, lithium has osteoprotective effects and has not been associated with an increased risk of fracture. Some researchers have suggested that the presentation of LAHP is more like familial hypocalciuric hypercalcemia (FHC), which is also associated with hypocalciuria. FHC is a benign condition and does not require active intervention.3,4 Similar to those with FHC, many patients with LAHP may live with chronic asymptomatic hypercalcemia without any significant adverse outcome.

A suggested approach to monitoring
In most cases, LAH is an insidious adverse effect that is usually detected on blood tests after many years of lithium therapy.8 For patients starting lithium therapy, International Society of Bipolar Disorder guidelines recommend testing calcium levels at baseline, 6 months, and annually thereafter, or as clinically indicated, to detect and monitor hypercalcemia and hyperparathyroidism. However, these guidelines do not provide any recommendations regarding how to manage abnormal findings.9
Clinical laboratories report both total and adjusted calcium values. The adjusted calcium value takes into account albumin levels. This is a way to compensate for an abnormal concentration of albumin (establishing what a patient’s total calcium concentration would be if the albumin concentration was normal). Table 25 shows the categorization of adjusted calcium values.For patients receiving lithium, some researchers have suggested monitoring PTH as well as calcium.1

The Figure outlines our proposed approach to monitoring for LAH in patients receiving lithium. An isolated high value of calcium could be due to prolonged venous stasis if a tourniquet is used for phlebotomy. In such instances, the calcium level should be tested again without a tourniquet.10 If the repeat blood test shows elevated calcium levels, then both PTH and serum calcium should be tested.

If the PTH level is higher than the midpoint of the reference range, LAH should be suspected, though sometimes hypercalcemia can present without raised PTH. LAH has also been reported to cause a transient increase in calcium levels. If hypercalcemia frequently recurs, PTH levels should be monitored. If PTH is suppressed, then the raised calcium levels are probably secondary to something other than lithium; common reasons for this include the use of vitamin D supplements or thiazide diuretics, or malignancies such as multiple myeloma.3,5,8
Continue to: Treatment
Treatment: Continue lithium?
There are several options for treating LAH. Lithium may be continued or discontinued following close monitoring of calcium and PTH levels, with or without active interventions such as surgery or pharmacotherapy, and as deemed appropriate after consultation with an endocrinologist. The decision should be informed by evaluating the risks and benefits to the patient’s physical and mental health. LAH can be reversed by discontinuing lithium, but this might not be the case in patients receiving long-term lithium therapy, especially if their elevated calcium levels are associated with parathyroid adenomas or hyperplasia. Hence, close monitoring of calcium and PTH is required even after discontinuing lithium.3,8
Surgical treatment. The primary treatment of LAH and primary hyperparathyroidism is parathyroidectomy. The possibility of recovery after parathyroidectomy for primary hyperparathyroidism is 60% to 80%, though a small proportion of patients might experience recurrence. This figure might be higher for LAH, because it is more likely to affect multiple glands.1,11 Other potential complications of parathyroidectomy are recurrent laryngeal nerve injury causing paralysis of vocal cords leading to hoarseness of voice, stridor, or aspiration, and local hematoma and hypocalcemia (requiring vitamin D and/or calcium supplements).12
Pharmacotherapy. Cinacalcet is a calcimimetic drug that decreases the reset point of the calcium-sensing receptor. It can be used if a patient is not suitable for or apprehensive about surgical intervention.1,8
Bottom Line
Calcium levels should be regularly monitored in patients receiving lithium. If calcium levels are persistently high, parathyroid hormone levels should also be measured. Management of lithium-associated hypercalcemia includes watchful waiting, discontinuing lithium, parathyroidectomy, and pharmacotherapy with cinacalcet.
Related Resources
- Laski M, Foreman R, Hancock H, et al. Lithium: an underutilized element. Current Psychiatry. 2021;20(12):27-30,34. doi:10.12788/cp.0193
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Cinacalcet • Sensipar
Hypercalcemia is a well-known but underrecognized adverse effect of lithium. Most patients with lithium-associated hypercalcemia (LAH) have either nonspecific symptoms (eg, persistent tiredness, constipation, polyuria, polydipsia) or no symptoms. Clinically, LAH differs from primary hyperparathyroidism, though the management protocol of these 2 conditions is almost the same. In this article, we discuss how lithium can affect calcium and parathyroid hormone (PTH) levels and how LAH and lithium-associated hyperparathyroidism (LAHP) differs from primary hyperparathyroidism. We also outline a suggested approach to monitoring and management.
An insidious problem
Due to the varying definitions and methods used to assess hypercalcemia, the reported prevalence of LAH varies from 4.3% to 80%.1 McKnight et al2 conducted a systematic review and meta-analysis of studies of the relationship between lithium and parathyroid function that included 14 case-control studies, 36 case reports, and 6 cross-sectional studies without a control group. They found that the levels of calcium and PTH were 10% higher in lithium-treated patients than in controls.2
Pathophysiology. Lithium is known to increase both calcium and PTH levels. PTH is responsible for calcium homeostasis. It is secreted in response to low calcium levels, which it increases by its action on bones, intestines, and kidneys. Vitamin D also plays a crucial role in calcium homeostasis. A deficiency of vitamin D triggers a compensatory increase in PTH to maintain calcium levels.3
Calcium and PTH levels increase soon after administration of lithium, but the rise is usually mild and insidious. In a small proportion of patients who receive long-term lithium treatment, calcium levels can exceed the normal range. Patients who develop LAH typically have serum calcium levels slightly above the normal range and PTH levels ranging from the higher side of the normal range to several times the upper limit of the normal range. Patients might also experience elevated PTH levels without any increase in calcium levels. Lithium can affect calcium and PTH levels in multiple ways. For instance, it increases the reabsorption of calcium in the kidney as well as the reset point of calcium-sensing receptors. Therefore, only higher levels of calcium can inhibit the release of PTH. Hence, in cases where the PTH level is within the normal range, it is generally higher than would be expected for a given serum calcium level. Lithium can also directly affect the parathyroid glands and can lead to either single-nodule or multimodule hyperplasia.4
Long-term lithium use can cause chronic kidney disease (CKD), which in turn leads to vitamin D deficiency and hyperparathyroidism. However, secondary hyperparathyroidism with CKD is usually seen in the more advanced stages of CKD, and is associated with low-to-normal calcium levels (as opposed to the high levels seen in LAH).3-5
Lithium-associated hyperparathyroidism
Primary hyperparathyroidism is the most common cause of hypercalcemia. Its prevalence ranges from 1 to 7 cases per 1,000 adults. The incidence of LAH/LAHP is 4- to 6-fold higher compared to the general population.6 Similar to LAH/LAHP, primary hyperparathyroidism is more common in older adults (age >60) and females. Hence, some researchers have suggested that lithium probably unmasks hyperparathyroidism in patients who are susceptible to primary hyperparathyroidism.3
Look for these clinical manifestations
Symptoms of primary hyperparathyroidism are related to high calcium and PTH levels. They are commonly described as “painful bones, renal stones, abdominal groans (due to hypercalcemia-induced ileus), and psychic moans (lethargy, poor concentration, depression).” Common adverse outcomes associated with primary hyperparathyroidism are renal stones, high risk of fracture, constipation, peptic ulcer, and pancreatitis.3,7
Continue: In contrast...
In contrast, LAHP is characterized by mild, intermittent, and/or persistent hypercalcemia and mildly increased PTH (Table 1).1,3,4 In some patients, it could improve without active intervention. Because lithium increases the absorption of urinary calcium, it is associated with hypocalciuria and a lower risk of renal stones. Additionally, lithium has osteoprotective effects and has not been associated with an increased risk of fracture. Some researchers have suggested that the presentation of LAHP is more like familial hypocalciuric hypercalcemia (FHC), which is also associated with hypocalciuria. FHC is a benign condition and does not require active intervention.3,4 Similar to those with FHC, many patients with LAHP may live with chronic asymptomatic hypercalcemia without any significant adverse outcome.

A suggested approach to monitoring
In most cases, LAH is an insidious adverse effect that is usually detected on blood tests after many years of lithium therapy.8 For patients starting lithium therapy, International Society of Bipolar Disorder guidelines recommend testing calcium levels at baseline, 6 months, and annually thereafter, or as clinically indicated, to detect and monitor hypercalcemia and hyperparathyroidism. However, these guidelines do not provide any recommendations regarding how to manage abnormal findings.9
Clinical laboratories report both total and adjusted calcium values. The adjusted calcium value takes into account albumin levels. This is a way to compensate for an abnormal concentration of albumin (establishing what a patient’s total calcium concentration would be if the albumin concentration was normal). Table 25 shows the categorization of adjusted calcium values.For patients receiving lithium, some researchers have suggested monitoring PTH as well as calcium.1

The Figure outlines our proposed approach to monitoring for LAH in patients receiving lithium. An isolated high value of calcium could be due to prolonged venous stasis if a tourniquet is used for phlebotomy. In such instances, the calcium level should be tested again without a tourniquet.10 If the repeat blood test shows elevated calcium levels, then both PTH and serum calcium should be tested.

If the PTH level is higher than the midpoint of the reference range, LAH should be suspected, though sometimes hypercalcemia can present without raised PTH. LAH has also been reported to cause a transient increase in calcium levels. If hypercalcemia frequently recurs, PTH levels should be monitored. If PTH is suppressed, then the raised calcium levels are probably secondary to something other than lithium; common reasons for this include the use of vitamin D supplements or thiazide diuretics, or malignancies such as multiple myeloma.3,5,8
Continue to: Treatment
Treatment: Continue lithium?
There are several options for treating LAH. Lithium may be continued or discontinued following close monitoring of calcium and PTH levels, with or without active interventions such as surgery or pharmacotherapy, and as deemed appropriate after consultation with an endocrinologist. The decision should be informed by evaluating the risks and benefits to the patient’s physical and mental health. LAH can be reversed by discontinuing lithium, but this might not be the case in patients receiving long-term lithium therapy, especially if their elevated calcium levels are associated with parathyroid adenomas or hyperplasia. Hence, close monitoring of calcium and PTH is required even after discontinuing lithium.3,8
Surgical treatment. The primary treatment of LAH and primary hyperparathyroidism is parathyroidectomy. The possibility of recovery after parathyroidectomy for primary hyperparathyroidism is 60% to 80%, though a small proportion of patients might experience recurrence. This figure might be higher for LAH, because it is more likely to affect multiple glands.1,11 Other potential complications of parathyroidectomy are recurrent laryngeal nerve injury causing paralysis of vocal cords leading to hoarseness of voice, stridor, or aspiration, and local hematoma and hypocalcemia (requiring vitamin D and/or calcium supplements).12
Pharmacotherapy. Cinacalcet is a calcimimetic drug that decreases the reset point of the calcium-sensing receptor. It can be used if a patient is not suitable for or apprehensive about surgical intervention.1,8
Bottom Line
Calcium levels should be regularly monitored in patients receiving lithium. If calcium levels are persistently high, parathyroid hormone levels should also be measured. Management of lithium-associated hypercalcemia includes watchful waiting, discontinuing lithium, parathyroidectomy, and pharmacotherapy with cinacalcet.
Related Resources
- Laski M, Foreman R, Hancock H, et al. Lithium: an underutilized element. Current Psychiatry. 2021;20(12):27-30,34. doi:10.12788/cp.0193
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Cinacalcet • Sensipar
1. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg. 2018;42(2):415-424.
2. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
3. Shapiro HI, Davis KA. Hypercalcemia and “primary” hyperparathyroidism during lithium therapy. Am J Psychiatry. 2015;172(1):12-15.
4. Lerena VS, León NS, Sosa S, et al. Lithium and endocrine dysfunction. Medicina (B Aires). 2022;82(1):130-137.
5. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959-1966.
6. Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98(3):1122-1129.
7. Dandurand K, Ali DS, Khan AA. Primary hyperparathyroidism: a narrative review of diagnosis and medical management. J Clin Med. 2021;10(8):1604.
8. Mifsud S, Cilia K, Mifsud EL, et al. Lithium-associated hyperparathyroidism. Br J Hosp Med (Lond). 2020;81(11):1-9.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. Mieebi WM, Solomon AE, Wabote AP. The effect of tourniquet application on serum calcium and inorganic phosphorus determination. Journal of Health, Medicine and Nursing. 2019;65:51-54.
11. Awad SS, Miskulin J, Thompson N. Parathyroid adenomas versus four-gland hyperplasia as the cause of primary hyperparathyroidism in patients with prolonged lithium therapy. World J Surg. 2003;27(4):486-488.
12. Farndon JR. Postoperative complications of parathyroidectomy. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem-Oriented. Zuckschwerdt; 2001. Accessed October 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK6967
1. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg. 2018;42(2):415-424.
2. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
3. Shapiro HI, Davis KA. Hypercalcemia and “primary” hyperparathyroidism during lithium therapy. Am J Psychiatry. 2015;172(1):12-15.
4. Lerena VS, León NS, Sosa S, et al. Lithium and endocrine dysfunction. Medicina (B Aires). 2022;82(1):130-137.
5. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67(9):1959-1966.
6. Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98(3):1122-1129.
7. Dandurand K, Ali DS, Khan AA. Primary hyperparathyroidism: a narrative review of diagnosis and medical management. J Clin Med. 2021;10(8):1604.
8. Mifsud S, Cilia K, Mifsud EL, et al. Lithium-associated hyperparathyroidism. Br J Hosp Med (Lond). 2020;81(11):1-9.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. Mieebi WM, Solomon AE, Wabote AP. The effect of tourniquet application on serum calcium and inorganic phosphorus determination. Journal of Health, Medicine and Nursing. 2019;65:51-54.
11. Awad SS, Miskulin J, Thompson N. Parathyroid adenomas versus four-gland hyperplasia as the cause of primary hyperparathyroidism in patients with prolonged lithium therapy. World J Surg. 2003;27(4):486-488.
12. Farndon JR. Postoperative complications of parathyroidectomy. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem-Oriented. Zuckschwerdt; 2001. Accessed October 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK6967
Long-term behavioral follow-up of children exposed to mood stabilizers and antidepressants: A look forward
Much of the focus of reproductive psychiatry over the last 1 to 2 decades has been on issues regarding risk of fetal exposure to psychiatric medications in the context of the specific risk for teratogenesis or organ malformation. Concerns and questions are mostly focused on exposure to any number of medications that women take during the first trimester, as it is during that period that the major organs are formed.
More recently, there has been appropriate interest in the effect of fetal exposure to psychiatric medications with respect to risk for obstetrical and neonatal complications. This particularly has been the case with respect to antidepressants where fetal exposure to these medications, which while associated with symptoms of transient jitteriness and irritability about 20% of the time, have not been associated with symptoms requiring frank clinical intervention.
Concerning mood stabilizers, the risk for organ dysgenesis following fetal exposure to sodium valproate has been very well established, and we’ve known for over a decade about the adverse effects of fetal exposure to sodium valproate on behavioral outcomes (Lancet Neurol. 2013 Mar;12[3]:244-52). We also now have ample data on lamotrigine, one of the most widely used medicines by reproductive-age women for treatment of bipolar disorder that supports the absence of a risk of organ malformation in first-trimester exposure.
Most recently, in a study of 292 children of women with epilepsy, an evaluation of women being treated with more modern anticonvulsants such as lamotrigine and levetiracetam alone or as polytherapy was performed. The results showed no difference in language, motor, cognitive, social, emotional, and general adaptive functioning in children exposed to either lamotrigine or levetiracetam relative to unexposed children of women with epilepsy. However, the researchers found an increase in anti-epileptic drug plasma level appeared to be associated with decreased motor and sensory function. These are reassuring data that really confirm earlier work, which failed to reveal a signal of concern for lamotrigine and now provide some of the first data on levetiracetam, which is widely used by reproductive-age women with epilepsy (JAMA Neurol. 2021 Aug 1;78[8]:927-936). While one caveat of the study is a short follow-up of 2 years, the absence of a signal of concern is reassuring. With more and more data demonstrating bipolar disorder is an illness that requires chronic treatment for many people, and that discontinuation is associated with high risk for relapse, it is an advance in the field to have data on risk for teratogenesis and data on longer-term neurobehavioral outcomes.
There is vast information regarding reproductive safety, organ malformation, and acute neonatal outcomes for antidepressants. The last decade has brought interest in and analysis of specific reports of increased risk of both autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) following fetal exposure to antidepressants. What can be said based on reviews of pooled meta-analyses is that the risk for ASD and ADHD has been put to rest for most clinicians and patients (J Clin Psychiatry. 2020 May 26;81[3]:20f13463). With other neurodevelopmental disorders, results have been somewhat inconclusive. Over the last 5-10 years, there have been sporadic reports of concerns about problems in a specific domain of neurodevelopment in offspring of women who have used antidepressants during pregnancy, whether it be speech, language, or motor functioning, but no signal of concern has been consistent.
In a previous column, I addressed a Danish study that showed no increased risk of longer-term sequelae after fetal exposure to antidepressants. Now, a new study has examined 1.93 million pregnancies in the Medicaid Analytic eXtract and 1.25 million pregnancies in the IBM MarketScan Research Database with follow-up up to 14 years of age where the specific interval for fetal exposure was from gestational age of 19 weeks to delivery, as that is the period that corresponds most to synaptogenesis in the brain. The researchers examined a spectrum of neurodevelopmental disorders such as developmental speech issues, ADHD, ASD, dyslexia, and learning disorders, among others. They found a twofold increased risk for neurodevelopmental disorders in the unadjusted models that flattened to no finding when factoring in environmental and genetic risk variables, highlighting the importance of dealing appropriately with confounders when performing these analyses. Those confounders examined include the mother’s use of alcohol and tobacco, and her body mass index and overall general health (JAMA Intern Med. 2022;182[11]:1149-60).
Given the consistency of these results with earlier data, patients can be increasingly comfortable as they weigh the benefits and risks of antidepressant use during pregnancy, factoring in the risk of fetal exposure with added data on long-term neurobehavioral sequelae. With that said, we need to remember the importance of initiatives to address alcohol consumption, poor nutrition, tobacco use, elevated BMI, and general health during pregnancy. These are modifiable risks that we as clinicians should focus on in order to optimize outcomes during pregnancy.
We have come so far in knowledge about fetal exposure to antidepressants relative to other classes of medications women take during pregnancy, about which, frankly, we are still starved for data. As use of psychiatric medications during pregnancy continues to grow, we can rest a bit more comfortably. But we should also address some of the other behaviors that have adverse effects on maternal and child well-being.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Much of the focus of reproductive psychiatry over the last 1 to 2 decades has been on issues regarding risk of fetal exposure to psychiatric medications in the context of the specific risk for teratogenesis or organ malformation. Concerns and questions are mostly focused on exposure to any number of medications that women take during the first trimester, as it is during that period that the major organs are formed.
More recently, there has been appropriate interest in the effect of fetal exposure to psychiatric medications with respect to risk for obstetrical and neonatal complications. This particularly has been the case with respect to antidepressants where fetal exposure to these medications, which while associated with symptoms of transient jitteriness and irritability about 20% of the time, have not been associated with symptoms requiring frank clinical intervention.
Concerning mood stabilizers, the risk for organ dysgenesis following fetal exposure to sodium valproate has been very well established, and we’ve known for over a decade about the adverse effects of fetal exposure to sodium valproate on behavioral outcomes (Lancet Neurol. 2013 Mar;12[3]:244-52). We also now have ample data on lamotrigine, one of the most widely used medicines by reproductive-age women for treatment of bipolar disorder that supports the absence of a risk of organ malformation in first-trimester exposure.
Most recently, in a study of 292 children of women with epilepsy, an evaluation of women being treated with more modern anticonvulsants such as lamotrigine and levetiracetam alone or as polytherapy was performed. The results showed no difference in language, motor, cognitive, social, emotional, and general adaptive functioning in children exposed to either lamotrigine or levetiracetam relative to unexposed children of women with epilepsy. However, the researchers found an increase in anti-epileptic drug plasma level appeared to be associated with decreased motor and sensory function. These are reassuring data that really confirm earlier work, which failed to reveal a signal of concern for lamotrigine and now provide some of the first data on levetiracetam, which is widely used by reproductive-age women with epilepsy (JAMA Neurol. 2021 Aug 1;78[8]:927-936). While one caveat of the study is a short follow-up of 2 years, the absence of a signal of concern is reassuring. With more and more data demonstrating bipolar disorder is an illness that requires chronic treatment for many people, and that discontinuation is associated with high risk for relapse, it is an advance in the field to have data on risk for teratogenesis and data on longer-term neurobehavioral outcomes.
There is vast information regarding reproductive safety, organ malformation, and acute neonatal outcomes for antidepressants. The last decade has brought interest in and analysis of specific reports of increased risk of both autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) following fetal exposure to antidepressants. What can be said based on reviews of pooled meta-analyses is that the risk for ASD and ADHD has been put to rest for most clinicians and patients (J Clin Psychiatry. 2020 May 26;81[3]:20f13463). With other neurodevelopmental disorders, results have been somewhat inconclusive. Over the last 5-10 years, there have been sporadic reports of concerns about problems in a specific domain of neurodevelopment in offspring of women who have used antidepressants during pregnancy, whether it be speech, language, or motor functioning, but no signal of concern has been consistent.
In a previous column, I addressed a Danish study that showed no increased risk of longer-term sequelae after fetal exposure to antidepressants. Now, a new study has examined 1.93 million pregnancies in the Medicaid Analytic eXtract and 1.25 million pregnancies in the IBM MarketScan Research Database with follow-up up to 14 years of age where the specific interval for fetal exposure was from gestational age of 19 weeks to delivery, as that is the period that corresponds most to synaptogenesis in the brain. The researchers examined a spectrum of neurodevelopmental disorders such as developmental speech issues, ADHD, ASD, dyslexia, and learning disorders, among others. They found a twofold increased risk for neurodevelopmental disorders in the unadjusted models that flattened to no finding when factoring in environmental and genetic risk variables, highlighting the importance of dealing appropriately with confounders when performing these analyses. Those confounders examined include the mother’s use of alcohol and tobacco, and her body mass index and overall general health (JAMA Intern Med. 2022;182[11]:1149-60).
Given the consistency of these results with earlier data, patients can be increasingly comfortable as they weigh the benefits and risks of antidepressant use during pregnancy, factoring in the risk of fetal exposure with added data on long-term neurobehavioral sequelae. With that said, we need to remember the importance of initiatives to address alcohol consumption, poor nutrition, tobacco use, elevated BMI, and general health during pregnancy. These are modifiable risks that we as clinicians should focus on in order to optimize outcomes during pregnancy.
We have come so far in knowledge about fetal exposure to antidepressants relative to other classes of medications women take during pregnancy, about which, frankly, we are still starved for data. As use of psychiatric medications during pregnancy continues to grow, we can rest a bit more comfortably. But we should also address some of the other behaviors that have adverse effects on maternal and child well-being.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Much of the focus of reproductive psychiatry over the last 1 to 2 decades has been on issues regarding risk of fetal exposure to psychiatric medications in the context of the specific risk for teratogenesis or organ malformation. Concerns and questions are mostly focused on exposure to any number of medications that women take during the first trimester, as it is during that period that the major organs are formed.
More recently, there has been appropriate interest in the effect of fetal exposure to psychiatric medications with respect to risk for obstetrical and neonatal complications. This particularly has been the case with respect to antidepressants where fetal exposure to these medications, which while associated with symptoms of transient jitteriness and irritability about 20% of the time, have not been associated with symptoms requiring frank clinical intervention.
Concerning mood stabilizers, the risk for organ dysgenesis following fetal exposure to sodium valproate has been very well established, and we’ve known for over a decade about the adverse effects of fetal exposure to sodium valproate on behavioral outcomes (Lancet Neurol. 2013 Mar;12[3]:244-52). We also now have ample data on lamotrigine, one of the most widely used medicines by reproductive-age women for treatment of bipolar disorder that supports the absence of a risk of organ malformation in first-trimester exposure.
Most recently, in a study of 292 children of women with epilepsy, an evaluation of women being treated with more modern anticonvulsants such as lamotrigine and levetiracetam alone or as polytherapy was performed. The results showed no difference in language, motor, cognitive, social, emotional, and general adaptive functioning in children exposed to either lamotrigine or levetiracetam relative to unexposed children of women with epilepsy. However, the researchers found an increase in anti-epileptic drug plasma level appeared to be associated with decreased motor and sensory function. These are reassuring data that really confirm earlier work, which failed to reveal a signal of concern for lamotrigine and now provide some of the first data on levetiracetam, which is widely used by reproductive-age women with epilepsy (JAMA Neurol. 2021 Aug 1;78[8]:927-936). While one caveat of the study is a short follow-up of 2 years, the absence of a signal of concern is reassuring. With more and more data demonstrating bipolar disorder is an illness that requires chronic treatment for many people, and that discontinuation is associated with high risk for relapse, it is an advance in the field to have data on risk for teratogenesis and data on longer-term neurobehavioral outcomes.
There is vast information regarding reproductive safety, organ malformation, and acute neonatal outcomes for antidepressants. The last decade has brought interest in and analysis of specific reports of increased risk of both autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) following fetal exposure to antidepressants. What can be said based on reviews of pooled meta-analyses is that the risk for ASD and ADHD has been put to rest for most clinicians and patients (J Clin Psychiatry. 2020 May 26;81[3]:20f13463). With other neurodevelopmental disorders, results have been somewhat inconclusive. Over the last 5-10 years, there have been sporadic reports of concerns about problems in a specific domain of neurodevelopment in offspring of women who have used antidepressants during pregnancy, whether it be speech, language, or motor functioning, but no signal of concern has been consistent.
In a previous column, I addressed a Danish study that showed no increased risk of longer-term sequelae after fetal exposure to antidepressants. Now, a new study has examined 1.93 million pregnancies in the Medicaid Analytic eXtract and 1.25 million pregnancies in the IBM MarketScan Research Database with follow-up up to 14 years of age where the specific interval for fetal exposure was from gestational age of 19 weeks to delivery, as that is the period that corresponds most to synaptogenesis in the brain. The researchers examined a spectrum of neurodevelopmental disorders such as developmental speech issues, ADHD, ASD, dyslexia, and learning disorders, among others. They found a twofold increased risk for neurodevelopmental disorders in the unadjusted models that flattened to no finding when factoring in environmental and genetic risk variables, highlighting the importance of dealing appropriately with confounders when performing these analyses. Those confounders examined include the mother’s use of alcohol and tobacco, and her body mass index and overall general health (JAMA Intern Med. 2022;182[11]:1149-60).
Given the consistency of these results with earlier data, patients can be increasingly comfortable as they weigh the benefits and risks of antidepressant use during pregnancy, factoring in the risk of fetal exposure with added data on long-term neurobehavioral sequelae. With that said, we need to remember the importance of initiatives to address alcohol consumption, poor nutrition, tobacco use, elevated BMI, and general health during pregnancy. These are modifiable risks that we as clinicians should focus on in order to optimize outcomes during pregnancy.
We have come so far in knowledge about fetal exposure to antidepressants relative to other classes of medications women take during pregnancy, about which, frankly, we are still starved for data. As use of psychiatric medications during pregnancy continues to grow, we can rest a bit more comfortably. But we should also address some of the other behaviors that have adverse effects on maternal and child well-being.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
Machine learning identifies childhood characteristics that predict bipolar disorder
This is the first quantitative approach to predict bipolar disorder, offering sensitivity and specificity of 75% and 76%, respectively, reported lead author Mai Uchida, MD, director of the pediatric depression program at Massachusetts General Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, and colleagues. With further development, the model could be used to identify at-risk children via electronic medical records, enabling earlier monitoring and intervention.
“Although longitudinal studies have found the prognosis of early-onset mood disorders to be unfavorable, research has also shown there are effective treatments and therapies that could significantly alleviate the patients’ and their families’ struggles from the diagnoses,” the investigators wrote in the Journal of Psychiatric Research. “Thus, early identification of the risks and interventions for early symptoms of pediatric mood disorders is crucial.”
To this end, Dr. Uchida and colleagues teamed up with the Gabrieli Lab at MIT, who have published extensively in the realm of neurodevelopment. They sourced data from 492 children, 6-18 years at baseline, who were involved in two longitudinal case-control family studies focused on ADHD. Inputs included psychometric scales, structured diagnostic interviews, social and cognitive functioning assessments, and sociodemographic data.
At 10-year follow-up, 10% of these children had developed bipolar disorder, a notably higher rate than the 3%-4% prevalence in the general population.
“This is a population that’s overrepresented,” Dr. Uchida said in an interview.
She offered two primary reasons for this: First, the families involved in the study were probably willing to be followed for 10 years because they had ongoing concerns about their child’s mental health. Second, the studies enrolled children diagnosed with ADHD, a condition associated with increased risk of bipolar disorder.
Using machine learning algorithms that processed the baseline data while accounting for the skewed distribution, the investigators were able to predict which of the children in the population would go on to develop bipolar disorder. The final model offered a sensitivity of 75%, a specificity of 76%, and an area under the receiver operating characteristic curve of 75%.
“To the best of our knowledge, this represents the first study using machine-learning algorithms for this purpose in pediatric psychiatry,” the investigators wrote.
Integrating models into electronic medical records
In the future, this model, or one like it, could be incorporated into software that automatically analyzes electronic medical records and notifies physicians about high-risk patients, Dr. Uchida predicted.
“Not all patients would connect to intervention,” she said. “Maybe it just means that you invite them in for a visit, or you observe them a little bit more carefully. I think that’s where we are hoping that machine learning and medical practice will go.”
When asked about the potential bias posed by psychiatric evaluation, compared with something like blood work results, Dr. Uchida suggested that this subjectivity can be overcome.
“I’m not entirely bothered by that,” she said, offering a list of objective data points that could be harvested from records, such as number of referrals, medications, and hospitalizations. Narrative text in medical records could also be analyzed, she said, potentially detecting key words that are more often associated with high-risk patients.
“Risk prediction is never going to be 100% accurate,” Dr. Uchida said. “But I do think that there will be things [in electronic medical records] that could guide how worried we should be, or how quickly we should intervene.”
Opening doors to personalized care
Martin Gignac, MD, chief of psychiatry at Montreal Children’s Hospital and associate professor at McGill University, Montreal, said the present study offers further support for the existence of pediatric-onset bipolar disorder, which “remains controversial” despite “solid evidence.”
“I’m impressed that we have 10-year-long longitudinal follow-up studies that corroborate the importance of this disorder, and show strong predictors of who is at risk,” Dr. Gignac said in an interview. “Clinicians treating a pediatric population should be aware that some of those children with mental health problems might have severe mental health problems, and you have to have the appropriate tools to screen them.”
Advanced tools like the one developed by Dr. Uchida and colleagues should lead to more personalized care, he said.
“We’re going to be able to define what your individual risk is, and maybe most importantly, what you can do to prevent the development of certain disorders,” Dr. Gignac said. “Are there any risks that are dynamic in nature, and that we can act upon? Exposure to stress, for example.”
While more work is needed to bring machine learning into daily psychiatric practice, Dr. Gignac concluded on an optimistic note.
“These instruments should translate from research into clinical practice in order to make difference for the patients we care for,” he said. “This is the type of hope that I hold – that it’s going to be applicable in clinical practice, hopefully, in the near future.”
The investigators disclosed relationships with InCarda, Baylis Medical, Johnson & Johnson, and others. Dr. Gignac disclosed no relevant competing interests.
This is the first quantitative approach to predict bipolar disorder, offering sensitivity and specificity of 75% and 76%, respectively, reported lead author Mai Uchida, MD, director of the pediatric depression program at Massachusetts General Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, and colleagues. With further development, the model could be used to identify at-risk children via electronic medical records, enabling earlier monitoring and intervention.
“Although longitudinal studies have found the prognosis of early-onset mood disorders to be unfavorable, research has also shown there are effective treatments and therapies that could significantly alleviate the patients’ and their families’ struggles from the diagnoses,” the investigators wrote in the Journal of Psychiatric Research. “Thus, early identification of the risks and interventions for early symptoms of pediatric mood disorders is crucial.”
To this end, Dr. Uchida and colleagues teamed up with the Gabrieli Lab at MIT, who have published extensively in the realm of neurodevelopment. They sourced data from 492 children, 6-18 years at baseline, who were involved in two longitudinal case-control family studies focused on ADHD. Inputs included psychometric scales, structured diagnostic interviews, social and cognitive functioning assessments, and sociodemographic data.
At 10-year follow-up, 10% of these children had developed bipolar disorder, a notably higher rate than the 3%-4% prevalence in the general population.
“This is a population that’s overrepresented,” Dr. Uchida said in an interview.
She offered two primary reasons for this: First, the families involved in the study were probably willing to be followed for 10 years because they had ongoing concerns about their child’s mental health. Second, the studies enrolled children diagnosed with ADHD, a condition associated with increased risk of bipolar disorder.
Using machine learning algorithms that processed the baseline data while accounting for the skewed distribution, the investigators were able to predict which of the children in the population would go on to develop bipolar disorder. The final model offered a sensitivity of 75%, a specificity of 76%, and an area under the receiver operating characteristic curve of 75%.
“To the best of our knowledge, this represents the first study using machine-learning algorithms for this purpose in pediatric psychiatry,” the investigators wrote.
Integrating models into electronic medical records
In the future, this model, or one like it, could be incorporated into software that automatically analyzes electronic medical records and notifies physicians about high-risk patients, Dr. Uchida predicted.
“Not all patients would connect to intervention,” she said. “Maybe it just means that you invite them in for a visit, or you observe them a little bit more carefully. I think that’s where we are hoping that machine learning and medical practice will go.”
When asked about the potential bias posed by psychiatric evaluation, compared with something like blood work results, Dr. Uchida suggested that this subjectivity can be overcome.
“I’m not entirely bothered by that,” she said, offering a list of objective data points that could be harvested from records, such as number of referrals, medications, and hospitalizations. Narrative text in medical records could also be analyzed, she said, potentially detecting key words that are more often associated with high-risk patients.
“Risk prediction is never going to be 100% accurate,” Dr. Uchida said. “But I do think that there will be things [in electronic medical records] that could guide how worried we should be, or how quickly we should intervene.”
Opening doors to personalized care
Martin Gignac, MD, chief of psychiatry at Montreal Children’s Hospital and associate professor at McGill University, Montreal, said the present study offers further support for the existence of pediatric-onset bipolar disorder, which “remains controversial” despite “solid evidence.”
“I’m impressed that we have 10-year-long longitudinal follow-up studies that corroborate the importance of this disorder, and show strong predictors of who is at risk,” Dr. Gignac said in an interview. “Clinicians treating a pediatric population should be aware that some of those children with mental health problems might have severe mental health problems, and you have to have the appropriate tools to screen them.”
Advanced tools like the one developed by Dr. Uchida and colleagues should lead to more personalized care, he said.
“We’re going to be able to define what your individual risk is, and maybe most importantly, what you can do to prevent the development of certain disorders,” Dr. Gignac said. “Are there any risks that are dynamic in nature, and that we can act upon? Exposure to stress, for example.”
While more work is needed to bring machine learning into daily psychiatric practice, Dr. Gignac concluded on an optimistic note.
“These instruments should translate from research into clinical practice in order to make difference for the patients we care for,” he said. “This is the type of hope that I hold – that it’s going to be applicable in clinical practice, hopefully, in the near future.”
The investigators disclosed relationships with InCarda, Baylis Medical, Johnson & Johnson, and others. Dr. Gignac disclosed no relevant competing interests.
This is the first quantitative approach to predict bipolar disorder, offering sensitivity and specificity of 75% and 76%, respectively, reported lead author Mai Uchida, MD, director of the pediatric depression program at Massachusetts General Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, and colleagues. With further development, the model could be used to identify at-risk children via electronic medical records, enabling earlier monitoring and intervention.
“Although longitudinal studies have found the prognosis of early-onset mood disorders to be unfavorable, research has also shown there are effective treatments and therapies that could significantly alleviate the patients’ and their families’ struggles from the diagnoses,” the investigators wrote in the Journal of Psychiatric Research. “Thus, early identification of the risks and interventions for early symptoms of pediatric mood disorders is crucial.”
To this end, Dr. Uchida and colleagues teamed up with the Gabrieli Lab at MIT, who have published extensively in the realm of neurodevelopment. They sourced data from 492 children, 6-18 years at baseline, who were involved in two longitudinal case-control family studies focused on ADHD. Inputs included psychometric scales, structured diagnostic interviews, social and cognitive functioning assessments, and sociodemographic data.
At 10-year follow-up, 10% of these children had developed bipolar disorder, a notably higher rate than the 3%-4% prevalence in the general population.
“This is a population that’s overrepresented,” Dr. Uchida said in an interview.
She offered two primary reasons for this: First, the families involved in the study were probably willing to be followed for 10 years because they had ongoing concerns about their child’s mental health. Second, the studies enrolled children diagnosed with ADHD, a condition associated with increased risk of bipolar disorder.
Using machine learning algorithms that processed the baseline data while accounting for the skewed distribution, the investigators were able to predict which of the children in the population would go on to develop bipolar disorder. The final model offered a sensitivity of 75%, a specificity of 76%, and an area under the receiver operating characteristic curve of 75%.
“To the best of our knowledge, this represents the first study using machine-learning algorithms for this purpose in pediatric psychiatry,” the investigators wrote.
Integrating models into electronic medical records
In the future, this model, or one like it, could be incorporated into software that automatically analyzes electronic medical records and notifies physicians about high-risk patients, Dr. Uchida predicted.
“Not all patients would connect to intervention,” she said. “Maybe it just means that you invite them in for a visit, or you observe them a little bit more carefully. I think that’s where we are hoping that machine learning and medical practice will go.”
When asked about the potential bias posed by psychiatric evaluation, compared with something like blood work results, Dr. Uchida suggested that this subjectivity can be overcome.
“I’m not entirely bothered by that,” she said, offering a list of objective data points that could be harvested from records, such as number of referrals, medications, and hospitalizations. Narrative text in medical records could also be analyzed, she said, potentially detecting key words that are more often associated with high-risk patients.
“Risk prediction is never going to be 100% accurate,” Dr. Uchida said. “But I do think that there will be things [in electronic medical records] that could guide how worried we should be, or how quickly we should intervene.”
Opening doors to personalized care
Martin Gignac, MD, chief of psychiatry at Montreal Children’s Hospital and associate professor at McGill University, Montreal, said the present study offers further support for the existence of pediatric-onset bipolar disorder, which “remains controversial” despite “solid evidence.”
“I’m impressed that we have 10-year-long longitudinal follow-up studies that corroborate the importance of this disorder, and show strong predictors of who is at risk,” Dr. Gignac said in an interview. “Clinicians treating a pediatric population should be aware that some of those children with mental health problems might have severe mental health problems, and you have to have the appropriate tools to screen them.”
Advanced tools like the one developed by Dr. Uchida and colleagues should lead to more personalized care, he said.
“We’re going to be able to define what your individual risk is, and maybe most importantly, what you can do to prevent the development of certain disorders,” Dr. Gignac said. “Are there any risks that are dynamic in nature, and that we can act upon? Exposure to stress, for example.”
While more work is needed to bring machine learning into daily psychiatric practice, Dr. Gignac concluded on an optimistic note.
“These instruments should translate from research into clinical practice in order to make difference for the patients we care for,” he said. “This is the type of hope that I hold – that it’s going to be applicable in clinical practice, hopefully, in the near future.”
The investigators disclosed relationships with InCarda, Baylis Medical, Johnson & Johnson, and others. Dr. Gignac disclosed no relevant competing interests.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Lamotrigine for bipolar depression?
In reading Dr. Nasrallah's August 2022 editorial (“Reversing depression: A plethora of therapeutic strategies and mechanisms,”
Dr. Nasrallah responds
Thanks for your message. Lamotrigine is not FDA-approved for bipolar or unipolar depression, either as monotherapy or as an adjunctive therapy. It has never been approved for mania, either (no efficacy at all). Its only FDA-approved psychiatric indication is maintenance therapy after a patient with bipolar I disorder emerges from mania with the help of one of the antimanic drugs. Yet many clinicians may perceive lamotrigine as useful for bipolar depression because more than 20 years ago the manufacturer sponsored several small studies (not FDA trials). Two studies that showed efficacy were published, but 4 other studies that failed to show efficacy were not published. As a result, many clinicians got the false impression that lamotrigine is an effective antidepressant. I hope this explains why lamotrigine was not included in the list of antidepressants in my editorial.
In reading Dr. Nasrallah's August 2022 editorial (“Reversing depression: A plethora of therapeutic strategies and mechanisms,”
Dr. Nasrallah responds
Thanks for your message. Lamotrigine is not FDA-approved for bipolar or unipolar depression, either as monotherapy or as an adjunctive therapy. It has never been approved for mania, either (no efficacy at all). Its only FDA-approved psychiatric indication is maintenance therapy after a patient with bipolar I disorder emerges from mania with the help of one of the antimanic drugs. Yet many clinicians may perceive lamotrigine as useful for bipolar depression because more than 20 years ago the manufacturer sponsored several small studies (not FDA trials). Two studies that showed efficacy were published, but 4 other studies that failed to show efficacy were not published. As a result, many clinicians got the false impression that lamotrigine is an effective antidepressant. I hope this explains why lamotrigine was not included in the list of antidepressants in my editorial.
In reading Dr. Nasrallah's August 2022 editorial (“Reversing depression: A plethora of therapeutic strategies and mechanisms,”
Dr. Nasrallah responds
Thanks for your message. Lamotrigine is not FDA-approved for bipolar or unipolar depression, either as monotherapy or as an adjunctive therapy. It has never been approved for mania, either (no efficacy at all). Its only FDA-approved psychiatric indication is maintenance therapy after a patient with bipolar I disorder emerges from mania with the help of one of the antimanic drugs. Yet many clinicians may perceive lamotrigine as useful for bipolar depression because more than 20 years ago the manufacturer sponsored several small studies (not FDA trials). Two studies that showed efficacy were published, but 4 other studies that failed to show efficacy were not published. As a result, many clinicians got the false impression that lamotrigine is an effective antidepressant. I hope this explains why lamotrigine was not included in the list of antidepressants in my editorial.
Bipolar risk and parental age: What’s the relationship?
VIENNA –
Results from a meta-analysis of more than 210,000 patients with bipolar disorder and over 13 million healthy individuals showed that children of mothers younger than 20 years had a 23% increased risk for bipolar disorder vs. those whose parents were aged 25-29 years. For participants whose mothers were aged 35-39 years, there was a 10% increased risk for bipolar disorder, which rose to 20% if the mother was aged 40 or older.
Having a father younger than 20 years conferred a 29% increased risk for bipolar disorder, which was the same increase in risk found in individuals whose fathers were aged 45 years or older.
These findings, which are an update of data published in the journal European Pharmacology, were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
Fourteen studies included
Previous studies have suggested that parental age at birth is a risk factor for several psychiatric disorders in offspring, including bipolar disorder, and that advanced parental age, specifically, is associated with earlier onset schizophrenia.
To investigate further, the current researchers conducted a systematic review and meta-analysis, searching the PubMed/MEDLINE, EMBASE, Scopus, and PsychINFO databases for relevant studies published to Dec. 1, 2021.
From 712 studies initially identified, 16 met all the inclusion criteria and 14 were included in the quantitative analysis.
Five studies reported only paternal age and risk for bipolar disorder in their offspring, one included just maternal age, and eight reported both maternal and paternal age in relation to the risk for offspring bipolar disorder.
Individuals with a history of any psychiatric disorders were excluded, leaving a total of 13.4 million individuals without bipolar disorder and 217,089 who had received a diagnosis for the disorder.
The investigators also corrected for both socioeconomic status and, when assessing the impact of maternal or paternal age at birth, corrected for the age of the other parent. However, they were unable to correct for the number of children in a family.
Results after stratifying maternal and paternal age showed that, compared with those born to parents aged 25-29 years, there was an increased risk for bipolar disorder in the offspring of both fathers and mothers younger than 20 years of age, with adjusted odds ratios of 1.29 (95% confidence interval, 1.13-1.48) and 1.23 (95% CI, 1.14-1.33), respectively.
Compared with those aged 25-29 years, there was also an increased risk for bipolar disorder in children born to mothers aged 35-39 years (adjusted OR, 1.1; 95% CI, 1.01-1.19) and aged 40 or older (OR, 1.2; 95% CI, 1.02-1.40).
Among fathers, there was increased risk for offspring bipolar disorder in those aged 45 or older vs. those aged 25-29 years (adjusted OR, 1.29; 95% CI, 1.15-1.46).
Several hypotheses
There are several hypotheses that could explain the results, lead study author Giovanna Fico, MD, bipolar and depressive disorders unit, Hospital Clínic Barcelona, told this news organization.
In older age, it may be “more related to genetic or epigenetic modification, especially in fathers,” Dr. Fico said. “Some studies have shown that there are de novo mutations in the germ lines, which increase the risk of several diseases, including schizophrenia.”
In younger individuals, there could be a “mixed effect between sociocultural factors, such as substance abuse, low educational status,” and other issues, Dr. Fico noted.
Moreover, as bipolar disorder onset can be as late as 30 years of age, the younger group could include “undiagnosed patients with bipolar disorder, which would increase the risk” of the disease in their offspring, she added.
Dr. Fico noted the investigators are now planning on studying the impact of environmental factors such as pollution, climate change, and urbanization on risk for bipolar disorder, with the aim of being better able to inform parents or to develop prevention strategies.
Psychoeducation is “very common for infertility, birth defects, and Down syndrome, but it’s not so common for psychiatric disorders because we need more data. But I think it’s important that parents know they have an increased risk,” she said.
Nevertheless, “We must stress that this risk is moderate, and it must be kept in perspective,” Dr. Fico said in a news release.
‘Exciting’ questions raised
The study “raises several exciting research questions, including the possibility of early prevention and intervention,” Maj Vinberg, MD, PhD, clinical professor, department of clinical medicine, University of Copenhagen, said in the release.
She said she agrees there are likely to be different factors at play at different ages, with the risk for bipolar disorder associated with younger-age parenthood more likely to be related to socioeconomic status.
For older parents, “there has been a lot of speculation around the father’s age especially, which everybody thought didn’t matter,” said Dr. Vinberg, who was not involved with the research.
“But you might have some epigenetic changes as you grow older that might transfer into the next generation,” given that there is 20 years of additional exposure to potential epigenetic changes between a man aged 25 years and one aged 45 years, she noted.
Dr. Vinberg also highlighted that there could be cases of undiagnosed bipolar disorder among the younger parents, and she noted that “men with bipolar disorder tend to have more children,” particularly during manic phases.
She explained that if someone were to get divorced at 35 years of age, then have a new manic episode at 45 “and have a new wife and children, I don’t know whether it’s possible to correct for that.”
The research is supported by a fellowship from “la Caixa” Foundation. The investigators have reported no relevant financial relationships. Dr. Vinberg reported having relationships with Lundbeck and Janssen.
A version of this article first appeared on Medscape.com.
VIENNA –
Results from a meta-analysis of more than 210,000 patients with bipolar disorder and over 13 million healthy individuals showed that children of mothers younger than 20 years had a 23% increased risk for bipolar disorder vs. those whose parents were aged 25-29 years. For participants whose mothers were aged 35-39 years, there was a 10% increased risk for bipolar disorder, which rose to 20% if the mother was aged 40 or older.
Having a father younger than 20 years conferred a 29% increased risk for bipolar disorder, which was the same increase in risk found in individuals whose fathers were aged 45 years or older.
These findings, which are an update of data published in the journal European Pharmacology, were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
Fourteen studies included
Previous studies have suggested that parental age at birth is a risk factor for several psychiatric disorders in offspring, including bipolar disorder, and that advanced parental age, specifically, is associated with earlier onset schizophrenia.
To investigate further, the current researchers conducted a systematic review and meta-analysis, searching the PubMed/MEDLINE, EMBASE, Scopus, and PsychINFO databases for relevant studies published to Dec. 1, 2021.
From 712 studies initially identified, 16 met all the inclusion criteria and 14 were included in the quantitative analysis.
Five studies reported only paternal age and risk for bipolar disorder in their offspring, one included just maternal age, and eight reported both maternal and paternal age in relation to the risk for offspring bipolar disorder.
Individuals with a history of any psychiatric disorders were excluded, leaving a total of 13.4 million individuals without bipolar disorder and 217,089 who had received a diagnosis for the disorder.
The investigators also corrected for both socioeconomic status and, when assessing the impact of maternal or paternal age at birth, corrected for the age of the other parent. However, they were unable to correct for the number of children in a family.
Results after stratifying maternal and paternal age showed that, compared with those born to parents aged 25-29 years, there was an increased risk for bipolar disorder in the offspring of both fathers and mothers younger than 20 years of age, with adjusted odds ratios of 1.29 (95% confidence interval, 1.13-1.48) and 1.23 (95% CI, 1.14-1.33), respectively.
Compared with those aged 25-29 years, there was also an increased risk for bipolar disorder in children born to mothers aged 35-39 years (adjusted OR, 1.1; 95% CI, 1.01-1.19) and aged 40 or older (OR, 1.2; 95% CI, 1.02-1.40).
Among fathers, there was increased risk for offspring bipolar disorder in those aged 45 or older vs. those aged 25-29 years (adjusted OR, 1.29; 95% CI, 1.15-1.46).
Several hypotheses
There are several hypotheses that could explain the results, lead study author Giovanna Fico, MD, bipolar and depressive disorders unit, Hospital Clínic Barcelona, told this news organization.
In older age, it may be “more related to genetic or epigenetic modification, especially in fathers,” Dr. Fico said. “Some studies have shown that there are de novo mutations in the germ lines, which increase the risk of several diseases, including schizophrenia.”
In younger individuals, there could be a “mixed effect between sociocultural factors, such as substance abuse, low educational status,” and other issues, Dr. Fico noted.
Moreover, as bipolar disorder onset can be as late as 30 years of age, the younger group could include “undiagnosed patients with bipolar disorder, which would increase the risk” of the disease in their offspring, she added.
Dr. Fico noted the investigators are now planning on studying the impact of environmental factors such as pollution, climate change, and urbanization on risk for bipolar disorder, with the aim of being better able to inform parents or to develop prevention strategies.
Psychoeducation is “very common for infertility, birth defects, and Down syndrome, but it’s not so common for psychiatric disorders because we need more data. But I think it’s important that parents know they have an increased risk,” she said.
Nevertheless, “We must stress that this risk is moderate, and it must be kept in perspective,” Dr. Fico said in a news release.
‘Exciting’ questions raised
The study “raises several exciting research questions, including the possibility of early prevention and intervention,” Maj Vinberg, MD, PhD, clinical professor, department of clinical medicine, University of Copenhagen, said in the release.
She said she agrees there are likely to be different factors at play at different ages, with the risk for bipolar disorder associated with younger-age parenthood more likely to be related to socioeconomic status.
For older parents, “there has been a lot of speculation around the father’s age especially, which everybody thought didn’t matter,” said Dr. Vinberg, who was not involved with the research.
“But you might have some epigenetic changes as you grow older that might transfer into the next generation,” given that there is 20 years of additional exposure to potential epigenetic changes between a man aged 25 years and one aged 45 years, she noted.
Dr. Vinberg also highlighted that there could be cases of undiagnosed bipolar disorder among the younger parents, and she noted that “men with bipolar disorder tend to have more children,” particularly during manic phases.
She explained that if someone were to get divorced at 35 years of age, then have a new manic episode at 45 “and have a new wife and children, I don’t know whether it’s possible to correct for that.”
The research is supported by a fellowship from “la Caixa” Foundation. The investigators have reported no relevant financial relationships. Dr. Vinberg reported having relationships with Lundbeck and Janssen.
A version of this article first appeared on Medscape.com.
VIENNA –
Results from a meta-analysis of more than 210,000 patients with bipolar disorder and over 13 million healthy individuals showed that children of mothers younger than 20 years had a 23% increased risk for bipolar disorder vs. those whose parents were aged 25-29 years. For participants whose mothers were aged 35-39 years, there was a 10% increased risk for bipolar disorder, which rose to 20% if the mother was aged 40 or older.
Having a father younger than 20 years conferred a 29% increased risk for bipolar disorder, which was the same increase in risk found in individuals whose fathers were aged 45 years or older.
These findings, which are an update of data published in the journal European Pharmacology, were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
Fourteen studies included
Previous studies have suggested that parental age at birth is a risk factor for several psychiatric disorders in offspring, including bipolar disorder, and that advanced parental age, specifically, is associated with earlier onset schizophrenia.
To investigate further, the current researchers conducted a systematic review and meta-analysis, searching the PubMed/MEDLINE, EMBASE, Scopus, and PsychINFO databases for relevant studies published to Dec. 1, 2021.
From 712 studies initially identified, 16 met all the inclusion criteria and 14 were included in the quantitative analysis.
Five studies reported only paternal age and risk for bipolar disorder in their offspring, one included just maternal age, and eight reported both maternal and paternal age in relation to the risk for offspring bipolar disorder.
Individuals with a history of any psychiatric disorders were excluded, leaving a total of 13.4 million individuals without bipolar disorder and 217,089 who had received a diagnosis for the disorder.
The investigators also corrected for both socioeconomic status and, when assessing the impact of maternal or paternal age at birth, corrected for the age of the other parent. However, they were unable to correct for the number of children in a family.
Results after stratifying maternal and paternal age showed that, compared with those born to parents aged 25-29 years, there was an increased risk for bipolar disorder in the offspring of both fathers and mothers younger than 20 years of age, with adjusted odds ratios of 1.29 (95% confidence interval, 1.13-1.48) and 1.23 (95% CI, 1.14-1.33), respectively.
Compared with those aged 25-29 years, there was also an increased risk for bipolar disorder in children born to mothers aged 35-39 years (adjusted OR, 1.1; 95% CI, 1.01-1.19) and aged 40 or older (OR, 1.2; 95% CI, 1.02-1.40).
Among fathers, there was increased risk for offspring bipolar disorder in those aged 45 or older vs. those aged 25-29 years (adjusted OR, 1.29; 95% CI, 1.15-1.46).
Several hypotheses
There are several hypotheses that could explain the results, lead study author Giovanna Fico, MD, bipolar and depressive disorders unit, Hospital Clínic Barcelona, told this news organization.
In older age, it may be “more related to genetic or epigenetic modification, especially in fathers,” Dr. Fico said. “Some studies have shown that there are de novo mutations in the germ lines, which increase the risk of several diseases, including schizophrenia.”
In younger individuals, there could be a “mixed effect between sociocultural factors, such as substance abuse, low educational status,” and other issues, Dr. Fico noted.
Moreover, as bipolar disorder onset can be as late as 30 years of age, the younger group could include “undiagnosed patients with bipolar disorder, which would increase the risk” of the disease in their offspring, she added.
Dr. Fico noted the investigators are now planning on studying the impact of environmental factors such as pollution, climate change, and urbanization on risk for bipolar disorder, with the aim of being better able to inform parents or to develop prevention strategies.
Psychoeducation is “very common for infertility, birth defects, and Down syndrome, but it’s not so common for psychiatric disorders because we need more data. But I think it’s important that parents know they have an increased risk,” she said.
Nevertheless, “We must stress that this risk is moderate, and it must be kept in perspective,” Dr. Fico said in a news release.
‘Exciting’ questions raised
The study “raises several exciting research questions, including the possibility of early prevention and intervention,” Maj Vinberg, MD, PhD, clinical professor, department of clinical medicine, University of Copenhagen, said in the release.
She said she agrees there are likely to be different factors at play at different ages, with the risk for bipolar disorder associated with younger-age parenthood more likely to be related to socioeconomic status.
For older parents, “there has been a lot of speculation around the father’s age especially, which everybody thought didn’t matter,” said Dr. Vinberg, who was not involved with the research.
“But you might have some epigenetic changes as you grow older that might transfer into the next generation,” given that there is 20 years of additional exposure to potential epigenetic changes between a man aged 25 years and one aged 45 years, she noted.
Dr. Vinberg also highlighted that there could be cases of undiagnosed bipolar disorder among the younger parents, and she noted that “men with bipolar disorder tend to have more children,” particularly during manic phases.
She explained that if someone were to get divorced at 35 years of age, then have a new manic episode at 45 “and have a new wife and children, I don’t know whether it’s possible to correct for that.”
The research is supported by a fellowship from “la Caixa” Foundation. The investigators have reported no relevant financial relationships. Dr. Vinberg reported having relationships with Lundbeck and Janssen.
A version of this article first appeared on Medscape.com.
AT ECNP CONGRESS 2022
‘Disturbing’ lack of follow-up care after psychiatric crises
There is a concerning lack of follow-up care for young people who experience a mental health crisis, new research suggests.
The follow-up rate was less than 30% for those who had visited an ED.
The strongest predictor of follow-up was having received both primary and mental health care during the 6 months prior to using the acute service.
“For people discharging folks after a psychiatric crisis, whether it be in a hospital or emergency room setting, connecting them with their outpatient provider to ensure the transfer of care and continuity of care is vitally important to reduce risks for this population,” coinvestigator Brian Skehan, MD, PhD, assistant professor and psychiatrist, University of Massachusetts, Worcester, said during a press briefing.
If these discharged patients do not have a provider, “make sure they get one,” Lisa Dixon, MD, editor-in-chief of Psychiatric Services, added during the same briefing. “That’s the gift of life potentially for these young people.”
The findings were published online in Psychiatric Services.
Alarming trends
The alarming suicide trends among youths were exacerbated by the COVID-19 pandemic, Dr. Skehan noted.
He cited a 2021 study that showed more than 44% of high school students experienced persistent sadness or hopelessness over the previous year, 1 in 5 seriously considered suicide, and almost 1 in 10 actually attempted suicide.
“When we look at the number of young adults and adolescents struggling with behavioral health issues, the data trend is disturbing nationwide,” Dr. Skehan said.
The current study included participants aged 12-27 years who had private insurance. Many youth in this age category are experiencing significant changes, such as moving from high school to college and from pediatric providers to adult providers – and some “get lost in this transition,” said Dr. Skehan.
He noted many inpatient psychiatric units are not geared to young adults. “They may miss out on some aspects of inpatient care because it’s not geared to their developmental stage,” he said.
Assessing U.S. patient data in the IBM MarketScan commercial database (2013-2018), the researchers created two study samples: 95,153 inpatients and 108,576 patients who used the ED. All had an acute event stemming from a mental health condition.
The investigators explored the role of “established” outpatient care, defined as having had at least one visit with a provider of primary or mental health care in the 6 months prior to the acute psychiatric event.
Covariates included age at time of service (aged 12-17 years or 18-27 years), gender, health care plan type, psychiatric diagnosis, whether the acute event was self-harm or suicide related, and medical complexity.
Low follow-up rates
In the inpatient group, the average age was 18.9 years, the most common length of hospital stay was 4-6 days, and 1.5% left against medical advice. The most common primary diagnosis was major depression (53.7%), followed by bipolar disorder (22.3%). The least common disorders were PTSD, comorbid eating disorders, and disruptive disorders.
About one-third of participants had used both primary and mental health care during the 6 months before hospitalization, whereas 22.8% had no established outpatient care. Established care was most common among those with comorbid eating disorders and least common among those with psychotic disorders.
Results showed 42.7% of the hospitalized patients received follow up within 7 days and 67.4% received follow up within 30 days.
The strongest predictor of mental health follow-up care was established outpatient care. Compared with those who had no such care, those who had received both primary care and mental health care before the acute event had the highest odds of receiving follow-up (within 7 days, adjusted odds ratio, 2.81; 95% confidence interval, 2.68-2.94).
Older age and leaving against medical advice were associated with decreased likelihood of follow-up. Female sex, hospitalizations related to self-harm or suicidality, and longer length of stay were associated with increased likelihood of mental health follow-up care.
Compared with those hospitalized for major depression, those hospitalized for schizophrenia, bipolar disorder, PTSD, disruptive disorders, or comorbid substance use disorder were less likely to receive mental health follow-up. For example, only 23.7% of youth with comorbid substance use discharged from the hospital had follow-up within 7 days.
Similar patterns were observed for 30-day follow-up care.
‘Accessible and appealing’ options needed
In the ED-visit group, the average age was 19.5 years (58% female). Most (70.4%) had no chronic health conditions other than a psychiatric disorder. The primary diagnoses were anxiety disorders or phobias (44.1%) and major depression (23%).
One in four visits included a code for self-harm, suicidal ideation, or suicide attempt. And almost one third lacked established outpatient care before the ED visit.
Results showed 28.6% of the ED group received mental health care follow-up within 7 days and 46.4% received it within 30 days.
Again, the strongest predictor of mental health follow-up was prior outpatient care. For example, compared with participants with no established outpatient care, those with both primary care and mental health care were the most likely to receive follow-up within 7 days (aOR, 4.06; 95% CI, 3.72-4.42).
These numbers “are far from the goal of making sure everybody is getting follow-up care within 7 days of an acute psychiatric event,” Dr. Skehan said.
He stressed the need for “accessible and appealing options for youth.” These could include telehealth services, improved communication among health care providers in the ED, and reducing barriers to access follow-up care.
“This probably highlights the need to have more case management and referral services, and maybe make sure patients have a follow-up appointment before they leave the emergency room,” said Dr. Skehan. “This doesn’t necessarily guarantee they’ll get there but hopefully it makes it more likely they will have that access should they need it.”
The study was funded by grants from the National Institute of General Medical Sciences and the National Center for Advancing Translational Sciences, from the National Institutes of Health. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a concerning lack of follow-up care for young people who experience a mental health crisis, new research suggests.
The follow-up rate was less than 30% for those who had visited an ED.
The strongest predictor of follow-up was having received both primary and mental health care during the 6 months prior to using the acute service.
“For people discharging folks after a psychiatric crisis, whether it be in a hospital or emergency room setting, connecting them with their outpatient provider to ensure the transfer of care and continuity of care is vitally important to reduce risks for this population,” coinvestigator Brian Skehan, MD, PhD, assistant professor and psychiatrist, University of Massachusetts, Worcester, said during a press briefing.
If these discharged patients do not have a provider, “make sure they get one,” Lisa Dixon, MD, editor-in-chief of Psychiatric Services, added during the same briefing. “That’s the gift of life potentially for these young people.”
The findings were published online in Psychiatric Services.
Alarming trends
The alarming suicide trends among youths were exacerbated by the COVID-19 pandemic, Dr. Skehan noted.
He cited a 2021 study that showed more than 44% of high school students experienced persistent sadness or hopelessness over the previous year, 1 in 5 seriously considered suicide, and almost 1 in 10 actually attempted suicide.
“When we look at the number of young adults and adolescents struggling with behavioral health issues, the data trend is disturbing nationwide,” Dr. Skehan said.
The current study included participants aged 12-27 years who had private insurance. Many youth in this age category are experiencing significant changes, such as moving from high school to college and from pediatric providers to adult providers – and some “get lost in this transition,” said Dr. Skehan.
He noted many inpatient psychiatric units are not geared to young adults. “They may miss out on some aspects of inpatient care because it’s not geared to their developmental stage,” he said.
Assessing U.S. patient data in the IBM MarketScan commercial database (2013-2018), the researchers created two study samples: 95,153 inpatients and 108,576 patients who used the ED. All had an acute event stemming from a mental health condition.
The investigators explored the role of “established” outpatient care, defined as having had at least one visit with a provider of primary or mental health care in the 6 months prior to the acute psychiatric event.
Covariates included age at time of service (aged 12-17 years or 18-27 years), gender, health care plan type, psychiatric diagnosis, whether the acute event was self-harm or suicide related, and medical complexity.
Low follow-up rates
In the inpatient group, the average age was 18.9 years, the most common length of hospital stay was 4-6 days, and 1.5% left against medical advice. The most common primary diagnosis was major depression (53.7%), followed by bipolar disorder (22.3%). The least common disorders were PTSD, comorbid eating disorders, and disruptive disorders.
About one-third of participants had used both primary and mental health care during the 6 months before hospitalization, whereas 22.8% had no established outpatient care. Established care was most common among those with comorbid eating disorders and least common among those with psychotic disorders.
Results showed 42.7% of the hospitalized patients received follow up within 7 days and 67.4% received follow up within 30 days.
The strongest predictor of mental health follow-up care was established outpatient care. Compared with those who had no such care, those who had received both primary care and mental health care before the acute event had the highest odds of receiving follow-up (within 7 days, adjusted odds ratio, 2.81; 95% confidence interval, 2.68-2.94).
Older age and leaving against medical advice were associated with decreased likelihood of follow-up. Female sex, hospitalizations related to self-harm or suicidality, and longer length of stay were associated with increased likelihood of mental health follow-up care.
Compared with those hospitalized for major depression, those hospitalized for schizophrenia, bipolar disorder, PTSD, disruptive disorders, or comorbid substance use disorder were less likely to receive mental health follow-up. For example, only 23.7% of youth with comorbid substance use discharged from the hospital had follow-up within 7 days.
Similar patterns were observed for 30-day follow-up care.
‘Accessible and appealing’ options needed
In the ED-visit group, the average age was 19.5 years (58% female). Most (70.4%) had no chronic health conditions other than a psychiatric disorder. The primary diagnoses were anxiety disorders or phobias (44.1%) and major depression (23%).
One in four visits included a code for self-harm, suicidal ideation, or suicide attempt. And almost one third lacked established outpatient care before the ED visit.
Results showed 28.6% of the ED group received mental health care follow-up within 7 days and 46.4% received it within 30 days.
Again, the strongest predictor of mental health follow-up was prior outpatient care. For example, compared with participants with no established outpatient care, those with both primary care and mental health care were the most likely to receive follow-up within 7 days (aOR, 4.06; 95% CI, 3.72-4.42).
These numbers “are far from the goal of making sure everybody is getting follow-up care within 7 days of an acute psychiatric event,” Dr. Skehan said.
He stressed the need for “accessible and appealing options for youth.” These could include telehealth services, improved communication among health care providers in the ED, and reducing barriers to access follow-up care.
“This probably highlights the need to have more case management and referral services, and maybe make sure patients have a follow-up appointment before they leave the emergency room,” said Dr. Skehan. “This doesn’t necessarily guarantee they’ll get there but hopefully it makes it more likely they will have that access should they need it.”
The study was funded by grants from the National Institute of General Medical Sciences and the National Center for Advancing Translational Sciences, from the National Institutes of Health. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a concerning lack of follow-up care for young people who experience a mental health crisis, new research suggests.
The follow-up rate was less than 30% for those who had visited an ED.
The strongest predictor of follow-up was having received both primary and mental health care during the 6 months prior to using the acute service.
“For people discharging folks after a psychiatric crisis, whether it be in a hospital or emergency room setting, connecting them with their outpatient provider to ensure the transfer of care and continuity of care is vitally important to reduce risks for this population,” coinvestigator Brian Skehan, MD, PhD, assistant professor and psychiatrist, University of Massachusetts, Worcester, said during a press briefing.
If these discharged patients do not have a provider, “make sure they get one,” Lisa Dixon, MD, editor-in-chief of Psychiatric Services, added during the same briefing. “That’s the gift of life potentially for these young people.”
The findings were published online in Psychiatric Services.
Alarming trends
The alarming suicide trends among youths were exacerbated by the COVID-19 pandemic, Dr. Skehan noted.
He cited a 2021 study that showed more than 44% of high school students experienced persistent sadness or hopelessness over the previous year, 1 in 5 seriously considered suicide, and almost 1 in 10 actually attempted suicide.
“When we look at the number of young adults and adolescents struggling with behavioral health issues, the data trend is disturbing nationwide,” Dr. Skehan said.
The current study included participants aged 12-27 years who had private insurance. Many youth in this age category are experiencing significant changes, such as moving from high school to college and from pediatric providers to adult providers – and some “get lost in this transition,” said Dr. Skehan.
He noted many inpatient psychiatric units are not geared to young adults. “They may miss out on some aspects of inpatient care because it’s not geared to their developmental stage,” he said.
Assessing U.S. patient data in the IBM MarketScan commercial database (2013-2018), the researchers created two study samples: 95,153 inpatients and 108,576 patients who used the ED. All had an acute event stemming from a mental health condition.
The investigators explored the role of “established” outpatient care, defined as having had at least one visit with a provider of primary or mental health care in the 6 months prior to the acute psychiatric event.
Covariates included age at time of service (aged 12-17 years or 18-27 years), gender, health care plan type, psychiatric diagnosis, whether the acute event was self-harm or suicide related, and medical complexity.
Low follow-up rates
In the inpatient group, the average age was 18.9 years, the most common length of hospital stay was 4-6 days, and 1.5% left against medical advice. The most common primary diagnosis was major depression (53.7%), followed by bipolar disorder (22.3%). The least common disorders were PTSD, comorbid eating disorders, and disruptive disorders.
About one-third of participants had used both primary and mental health care during the 6 months before hospitalization, whereas 22.8% had no established outpatient care. Established care was most common among those with comorbid eating disorders and least common among those with psychotic disorders.
Results showed 42.7% of the hospitalized patients received follow up within 7 days and 67.4% received follow up within 30 days.
The strongest predictor of mental health follow-up care was established outpatient care. Compared with those who had no such care, those who had received both primary care and mental health care before the acute event had the highest odds of receiving follow-up (within 7 days, adjusted odds ratio, 2.81; 95% confidence interval, 2.68-2.94).
Older age and leaving against medical advice were associated with decreased likelihood of follow-up. Female sex, hospitalizations related to self-harm or suicidality, and longer length of stay were associated with increased likelihood of mental health follow-up care.
Compared with those hospitalized for major depression, those hospitalized for schizophrenia, bipolar disorder, PTSD, disruptive disorders, or comorbid substance use disorder were less likely to receive mental health follow-up. For example, only 23.7% of youth with comorbid substance use discharged from the hospital had follow-up within 7 days.
Similar patterns were observed for 30-day follow-up care.
‘Accessible and appealing’ options needed
In the ED-visit group, the average age was 19.5 years (58% female). Most (70.4%) had no chronic health conditions other than a psychiatric disorder. The primary diagnoses were anxiety disorders or phobias (44.1%) and major depression (23%).
One in four visits included a code for self-harm, suicidal ideation, or suicide attempt. And almost one third lacked established outpatient care before the ED visit.
Results showed 28.6% of the ED group received mental health care follow-up within 7 days and 46.4% received it within 30 days.
Again, the strongest predictor of mental health follow-up was prior outpatient care. For example, compared with participants with no established outpatient care, those with both primary care and mental health care were the most likely to receive follow-up within 7 days (aOR, 4.06; 95% CI, 3.72-4.42).
These numbers “are far from the goal of making sure everybody is getting follow-up care within 7 days of an acute psychiatric event,” Dr. Skehan said.
He stressed the need for “accessible and appealing options for youth.” These could include telehealth services, improved communication among health care providers in the ED, and reducing barriers to access follow-up care.
“This probably highlights the need to have more case management and referral services, and maybe make sure patients have a follow-up appointment before they leave the emergency room,” said Dr. Skehan. “This doesn’t necessarily guarantee they’ll get there but hopefully it makes it more likely they will have that access should they need it.”
The study was funded by grants from the National Institute of General Medical Sciences and the National Center for Advancing Translational Sciences, from the National Institutes of Health. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PSYCHIATRIC SERVICES
Clinical psychoeconomics: Accounting for money matters in psychiatric assessment and treatment
Despite money’s central role in our psychic lives, many trainees – and some seasoned practitioners – skirt around financial issues. Some clinicians confess that inquiring about patients’ finances feels “too personal.” They fear that asking about money could suggest that the clinician is primarily concerned with getting paid. Some clinicians feel that looking into patients’ finances might be unprofessional, outside one’s scope of practice. But it is not.
Trainees often receive little guidance concerning money matters in patients’ lives and treatments, considerations we have labeled clinical psychoeconomics. Considerable evidence suggests that financial concerns often provoke emotional distress and dysfunctional behaviors, and directly influence patient’s health care decisions. Financial issues also influence how clinicians view and react to patients.
We have recently reviewed (and illustrated through case vignettes) how money matters might impact psychiatric assessment, case formulation, treatment planning, and ongoing psychiatric treatments including psychotherapies.1 Consider how money affects people’s lives: Money helps people meet multiple practical, psychological, and social needs by enabling them to obtain food, clothing, shelter, other material goods, services, discretionary time, and opportunities. And money strongly influences relationships. Regardless of poverty or wealth, thoughts and behaviors connected to acquiring, possessing, and disposing of money, and feelings accompanying these processes such as greed, neediness, envy, pride, shame, guilt, and self-satisfaction often underly intrapsychic and interpersonal conflicts.
Individuals constantly engage in numerous simultaneous conscious, preconscious, and unconscious neuro-economic trade-offs that determine goals, efforts, and timing. Many are financially influenced. Money influences how virtually all patients seek, receive, and sustain their mental health care including psychotherapy.
Money problems can be associated with insecurity, impotence, feeling unloved, and lack of freedom or subjugation. Individuals may resent how they’re forced to acquire money, and feel shamed or morally injured by their jobs, financial dependence on other family members, public assistance, or their questionable ways of obtaining money.
Impoverished individuals may face choosing between food, housing, medications, and medical care. Domestically abused individuals may reluctantly remain with their abusers, risking physical harm or death rather than face destitution. Some families tolerate severely disabled individuals at home because they rely on their disability checks and caregiver payments. Suicides may turn on how individuals forecast financial repercussions affecting their families. Desires to avoid debt may lead to treatment avoidance.
Individuals with enough money to get by face daily financially related choices involving competing needs, desires, values, and loyalties. They may experience conflicts concerning spending on necessities vs. indulgences or spending on oneself vs. significant others.
Whereas some wealthy individuals may assume unwarranted airs of superiority and entitlement, others may feel guilty about wealth, or fearful that others like them only for their money. Individuals on the receiving end of wealth may feel emotionally and behaviorally manipulated by their benefactors.
Assessment
Assessments should consider how financial matters have shaped patients’ early psychological development as well their current lives. How do patients’ emotions, thoughts, and behaviors reflect money matters? What money-related pathologies are evident? What aspects of the patient’s “financial world” seem modifiable?
Financial questions should be posed colloquially. Screeners include: “Where do you live?”, “Who’s in the home?”, “How do you (all) manage financially?”, “What do you all do for a living?”, “How do you make ends meet?”, and “What financial problems are you facing?” Clinicians can quickly learn about patients’ financial self-sufficiencies, individuals for whom they bear financial responsibility, and others they rely on for support, for example, relatives. If patients avoid answering such questions forthrightly, particularly when financial arrangements are “complicated,” clinicians will want to revisit these issues later after establishing a firmer alliance but continue to wonder about the meaning of the patient’s reluctance.
When explicit, patients, families, and couples are fully aware of the conflicts but have difficulty resolving financial disputes. When conflicts are implicit, money problems may be unacknowledged, avoided, denied, or minimized. Conflicts concerning money are often transmitted trans-generationally.
Psychopathological conditions unequivocally linked to money include compulsive shopping, gambling disorders, miserly hoarding, impulse buying, and spending sprees during hypomanic and manic states. Mounting debts may create progressively insurmountable sources of distress. Money can be weaponized to sadistically create enticement, envy, or deprivation. Some monetarily antisocial individuals compromise interpersonal relationships as well as treatments. Individuals with alcohol/substance use disorders may spend so much on substances that little is left for necessities. Financially needy individuals may engage in morally questionable behaviors they might otherwise shun.
Case formulation and treatment planning
Incorporating money matters into case formulations entails demonstrating how financial concerns influenced maladaptive development and distort current attitudes, perceptions, and behaviors.
Concurrently, clinicians should acknowledge patients’ reality-based fiscal decisions, appreciating cultural and family value differences concerning how money should be acquired and spent. Since money often determines frequency and duration of treatment visits, clinicians are ethically obligated to discuss with patients what they might expect from different medications and psychotherapies, and their comparative costs.
Money matters’ impact on psychotherapies
Money matters often affect transference and countertransference reactions. Some reactions stem from how patients and clinicians compare their own financial situations with those of the other.
To help identify and ameliorate money-related countertransference responses, clinicians can reflect on questions such as: “How comfortable are you with people who are much poorer or richer than you are?” “How comfortable are you with impoverished individuals or with multimillionaires or their children?” And “why?” For trainees, all these reactions should be discussed in supervision.
Conclusions
To summarize, four clinical psychoeconomic issues should be routinely assessed and factored into psychiatric case formulations and treatment plans: how financial issues 1) have impacted patients’ psychological development; 2) impact patients’ current lives; 3) are likely to impact access, type, intensity, and duration of treatment visits; and 4) might provoke money-related transference and countertransference concerns.
In advising patients about treatment options, clinicians should discuss each treatment’s relative effectiveness and estimated costs of care. Patients’ decisions will likely be heavily influenced by financial considerations.
Dr. Yager is based in the department of psychiatry, University of Colorado at Denver, Aurora. Dr. Kay is based in the department of psychiatry, Wright State University, Dayton, Ohio. No external funds were received for this project, and the authors have no conflicts to disclose.
Reference
1. Yager J and Kay J. Money matters in psychiatric assessment, case formulation, treatment planning, and ongoing psychotherapy: Clinical psychoeconomics. J Nerv Ment Dis. 2022 Jun 10. doi: 10.1097/NMD.0000000000001552.
Despite money’s central role in our psychic lives, many trainees – and some seasoned practitioners – skirt around financial issues. Some clinicians confess that inquiring about patients’ finances feels “too personal.” They fear that asking about money could suggest that the clinician is primarily concerned with getting paid. Some clinicians feel that looking into patients’ finances might be unprofessional, outside one’s scope of practice. But it is not.
Trainees often receive little guidance concerning money matters in patients’ lives and treatments, considerations we have labeled clinical psychoeconomics. Considerable evidence suggests that financial concerns often provoke emotional distress and dysfunctional behaviors, and directly influence patient’s health care decisions. Financial issues also influence how clinicians view and react to patients.
We have recently reviewed (and illustrated through case vignettes) how money matters might impact psychiatric assessment, case formulation, treatment planning, and ongoing psychiatric treatments including psychotherapies.1 Consider how money affects people’s lives: Money helps people meet multiple practical, psychological, and social needs by enabling them to obtain food, clothing, shelter, other material goods, services, discretionary time, and opportunities. And money strongly influences relationships. Regardless of poverty or wealth, thoughts and behaviors connected to acquiring, possessing, and disposing of money, and feelings accompanying these processes such as greed, neediness, envy, pride, shame, guilt, and self-satisfaction often underly intrapsychic and interpersonal conflicts.
Individuals constantly engage in numerous simultaneous conscious, preconscious, and unconscious neuro-economic trade-offs that determine goals, efforts, and timing. Many are financially influenced. Money influences how virtually all patients seek, receive, and sustain their mental health care including psychotherapy.
Money problems can be associated with insecurity, impotence, feeling unloved, and lack of freedom or subjugation. Individuals may resent how they’re forced to acquire money, and feel shamed or morally injured by their jobs, financial dependence on other family members, public assistance, or their questionable ways of obtaining money.
Impoverished individuals may face choosing between food, housing, medications, and medical care. Domestically abused individuals may reluctantly remain with their abusers, risking physical harm or death rather than face destitution. Some families tolerate severely disabled individuals at home because they rely on their disability checks and caregiver payments. Suicides may turn on how individuals forecast financial repercussions affecting their families. Desires to avoid debt may lead to treatment avoidance.
Individuals with enough money to get by face daily financially related choices involving competing needs, desires, values, and loyalties. They may experience conflicts concerning spending on necessities vs. indulgences or spending on oneself vs. significant others.
Whereas some wealthy individuals may assume unwarranted airs of superiority and entitlement, others may feel guilty about wealth, or fearful that others like them only for their money. Individuals on the receiving end of wealth may feel emotionally and behaviorally manipulated by their benefactors.
Assessment
Assessments should consider how financial matters have shaped patients’ early psychological development as well their current lives. How do patients’ emotions, thoughts, and behaviors reflect money matters? What money-related pathologies are evident? What aspects of the patient’s “financial world” seem modifiable?
Financial questions should be posed colloquially. Screeners include: “Where do you live?”, “Who’s in the home?”, “How do you (all) manage financially?”, “What do you all do for a living?”, “How do you make ends meet?”, and “What financial problems are you facing?” Clinicians can quickly learn about patients’ financial self-sufficiencies, individuals for whom they bear financial responsibility, and others they rely on for support, for example, relatives. If patients avoid answering such questions forthrightly, particularly when financial arrangements are “complicated,” clinicians will want to revisit these issues later after establishing a firmer alliance but continue to wonder about the meaning of the patient’s reluctance.
When explicit, patients, families, and couples are fully aware of the conflicts but have difficulty resolving financial disputes. When conflicts are implicit, money problems may be unacknowledged, avoided, denied, or minimized. Conflicts concerning money are often transmitted trans-generationally.
Psychopathological conditions unequivocally linked to money include compulsive shopping, gambling disorders, miserly hoarding, impulse buying, and spending sprees during hypomanic and manic states. Mounting debts may create progressively insurmountable sources of distress. Money can be weaponized to sadistically create enticement, envy, or deprivation. Some monetarily antisocial individuals compromise interpersonal relationships as well as treatments. Individuals with alcohol/substance use disorders may spend so much on substances that little is left for necessities. Financially needy individuals may engage in morally questionable behaviors they might otherwise shun.
Case formulation and treatment planning
Incorporating money matters into case formulations entails demonstrating how financial concerns influenced maladaptive development and distort current attitudes, perceptions, and behaviors.
Concurrently, clinicians should acknowledge patients’ reality-based fiscal decisions, appreciating cultural and family value differences concerning how money should be acquired and spent. Since money often determines frequency and duration of treatment visits, clinicians are ethically obligated to discuss with patients what they might expect from different medications and psychotherapies, and their comparative costs.
Money matters’ impact on psychotherapies
Money matters often affect transference and countertransference reactions. Some reactions stem from how patients and clinicians compare their own financial situations with those of the other.
To help identify and ameliorate money-related countertransference responses, clinicians can reflect on questions such as: “How comfortable are you with people who are much poorer or richer than you are?” “How comfortable are you with impoverished individuals or with multimillionaires or their children?” And “why?” For trainees, all these reactions should be discussed in supervision.
Conclusions
To summarize, four clinical psychoeconomic issues should be routinely assessed and factored into psychiatric case formulations and treatment plans: how financial issues 1) have impacted patients’ psychological development; 2) impact patients’ current lives; 3) are likely to impact access, type, intensity, and duration of treatment visits; and 4) might provoke money-related transference and countertransference concerns.
In advising patients about treatment options, clinicians should discuss each treatment’s relative effectiveness and estimated costs of care. Patients’ decisions will likely be heavily influenced by financial considerations.
Dr. Yager is based in the department of psychiatry, University of Colorado at Denver, Aurora. Dr. Kay is based in the department of psychiatry, Wright State University, Dayton, Ohio. No external funds were received for this project, and the authors have no conflicts to disclose.
Reference
1. Yager J and Kay J. Money matters in psychiatric assessment, case formulation, treatment planning, and ongoing psychotherapy: Clinical psychoeconomics. J Nerv Ment Dis. 2022 Jun 10. doi: 10.1097/NMD.0000000000001552.
Despite money’s central role in our psychic lives, many trainees – and some seasoned practitioners – skirt around financial issues. Some clinicians confess that inquiring about patients’ finances feels “too personal.” They fear that asking about money could suggest that the clinician is primarily concerned with getting paid. Some clinicians feel that looking into patients’ finances might be unprofessional, outside one’s scope of practice. But it is not.
Trainees often receive little guidance concerning money matters in patients’ lives and treatments, considerations we have labeled clinical psychoeconomics. Considerable evidence suggests that financial concerns often provoke emotional distress and dysfunctional behaviors, and directly influence patient’s health care decisions. Financial issues also influence how clinicians view and react to patients.
We have recently reviewed (and illustrated through case vignettes) how money matters might impact psychiatric assessment, case formulation, treatment planning, and ongoing psychiatric treatments including psychotherapies.1 Consider how money affects people’s lives: Money helps people meet multiple practical, psychological, and social needs by enabling them to obtain food, clothing, shelter, other material goods, services, discretionary time, and opportunities. And money strongly influences relationships. Regardless of poverty or wealth, thoughts and behaviors connected to acquiring, possessing, and disposing of money, and feelings accompanying these processes such as greed, neediness, envy, pride, shame, guilt, and self-satisfaction often underly intrapsychic and interpersonal conflicts.
Individuals constantly engage in numerous simultaneous conscious, preconscious, and unconscious neuro-economic trade-offs that determine goals, efforts, and timing. Many are financially influenced. Money influences how virtually all patients seek, receive, and sustain their mental health care including psychotherapy.
Money problems can be associated with insecurity, impotence, feeling unloved, and lack of freedom or subjugation. Individuals may resent how they’re forced to acquire money, and feel shamed or morally injured by their jobs, financial dependence on other family members, public assistance, or their questionable ways of obtaining money.
Impoverished individuals may face choosing between food, housing, medications, and medical care. Domestically abused individuals may reluctantly remain with their abusers, risking physical harm or death rather than face destitution. Some families tolerate severely disabled individuals at home because they rely on their disability checks and caregiver payments. Suicides may turn on how individuals forecast financial repercussions affecting their families. Desires to avoid debt may lead to treatment avoidance.
Individuals with enough money to get by face daily financially related choices involving competing needs, desires, values, and loyalties. They may experience conflicts concerning spending on necessities vs. indulgences or spending on oneself vs. significant others.
Whereas some wealthy individuals may assume unwarranted airs of superiority and entitlement, others may feel guilty about wealth, or fearful that others like them only for their money. Individuals on the receiving end of wealth may feel emotionally and behaviorally manipulated by their benefactors.
Assessment
Assessments should consider how financial matters have shaped patients’ early psychological development as well their current lives. How do patients’ emotions, thoughts, and behaviors reflect money matters? What money-related pathologies are evident? What aspects of the patient’s “financial world” seem modifiable?
Financial questions should be posed colloquially. Screeners include: “Where do you live?”, “Who’s in the home?”, “How do you (all) manage financially?”, “What do you all do for a living?”, “How do you make ends meet?”, and “What financial problems are you facing?” Clinicians can quickly learn about patients’ financial self-sufficiencies, individuals for whom they bear financial responsibility, and others they rely on for support, for example, relatives. If patients avoid answering such questions forthrightly, particularly when financial arrangements are “complicated,” clinicians will want to revisit these issues later after establishing a firmer alliance but continue to wonder about the meaning of the patient’s reluctance.
When explicit, patients, families, and couples are fully aware of the conflicts but have difficulty resolving financial disputes. When conflicts are implicit, money problems may be unacknowledged, avoided, denied, or minimized. Conflicts concerning money are often transmitted trans-generationally.
Psychopathological conditions unequivocally linked to money include compulsive shopping, gambling disorders, miserly hoarding, impulse buying, and spending sprees during hypomanic and manic states. Mounting debts may create progressively insurmountable sources of distress. Money can be weaponized to sadistically create enticement, envy, or deprivation. Some monetarily antisocial individuals compromise interpersonal relationships as well as treatments. Individuals with alcohol/substance use disorders may spend so much on substances that little is left for necessities. Financially needy individuals may engage in morally questionable behaviors they might otherwise shun.
Case formulation and treatment planning
Incorporating money matters into case formulations entails demonstrating how financial concerns influenced maladaptive development and distort current attitudes, perceptions, and behaviors.
Concurrently, clinicians should acknowledge patients’ reality-based fiscal decisions, appreciating cultural and family value differences concerning how money should be acquired and spent. Since money often determines frequency and duration of treatment visits, clinicians are ethically obligated to discuss with patients what they might expect from different medications and psychotherapies, and their comparative costs.
Money matters’ impact on psychotherapies
Money matters often affect transference and countertransference reactions. Some reactions stem from how patients and clinicians compare their own financial situations with those of the other.
To help identify and ameliorate money-related countertransference responses, clinicians can reflect on questions such as: “How comfortable are you with people who are much poorer or richer than you are?” “How comfortable are you with impoverished individuals or with multimillionaires or their children?” And “why?” For trainees, all these reactions should be discussed in supervision.
Conclusions
To summarize, four clinical psychoeconomic issues should be routinely assessed and factored into psychiatric case formulations and treatment plans: how financial issues 1) have impacted patients’ psychological development; 2) impact patients’ current lives; 3) are likely to impact access, type, intensity, and duration of treatment visits; and 4) might provoke money-related transference and countertransference concerns.
In advising patients about treatment options, clinicians should discuss each treatment’s relative effectiveness and estimated costs of care. Patients’ decisions will likely be heavily influenced by financial considerations.
Dr. Yager is based in the department of psychiatry, University of Colorado at Denver, Aurora. Dr. Kay is based in the department of psychiatry, Wright State University, Dayton, Ohio. No external funds were received for this project, and the authors have no conflicts to disclose.
Reference
1. Yager J and Kay J. Money matters in psychiatric assessment, case formulation, treatment planning, and ongoing psychotherapy: Clinical psychoeconomics. J Nerv Ment Dis. 2022 Jun 10. doi: 10.1097/NMD.0000000000001552.
The hunt for N-acetylcysteine: Medicine or dietary supplement?
Medicine or dietary supplement? N-acetylcysteine (NAC) is marketed as both and in 2021, the supplement abruptly became difficult to find, causing distress to people who had been using it for a variety of conditions. The story behind its disappearance is one of a cat-and-mouse chase between manufacturers, advocacy agencies, and the Food and Drug Administration.
NAC is a medication that was approved by the FDA in 1963. It has two FDA-approved uses: To prevent hepatotoxicity after overdose with acetaminophen, administered intravenously or by mouth, and as a mucolytic agent – previously available as Mucomyst, now available only as a generic – given by inhaler or nebulizer for pulmonary illnesses. Since the 1990s, NAC has been labeled by manufacturers as a dietary supplement. It is a derivative of the amino acid L-cysteine and a precursor to glutathione, an antioxidant.
Studies have had small sample sizes, and the supplement has not been considered an “off label” use of an already available medication. People have purchased NAC from retail drug and big box stores, Amazon, and online companies. So what’s the problem?
In July 2020, the FDA issued a warning letter to Purple Biosciences LLC, because of claims on the company’s website that the product “Purple Tree,” sold by Amazon, could cure hangovers that result from alcohol intoxication. The letter discussed justification for why a hangover is a disease, and goes on to note:
Based on the product label on your website, it appears that you intend to market your Purple Tree® product, which contains N-acetyl-L-cysteine (NAC), as a dietary supplement. However, even if your product labeling did not have therapeutic claims that make your product an unapproved new drug, your product could not be a dietary supplement, because it does not meet the definition of dietary supplement ... products containing that article are outside the definition of a dietary supplement, unless before such approval that article was marketed as a dietary supplement or as a food. NAC was approved as a new drug under section 505 of the Act [21 U.S.C. § 355] on September 14, 1963.
The issue here is that because NAC was first approved as a drug in 1963, it cannot be marketed as a supplement. If it had been marketed as a supplement before it was approved as a drug by the FDA, then it could remain on the market. The fact that it had been sold as a supplement since the 1990s and could be classified as an “old dietary ingredient” according to the Dietary Supplement Health and Education Act of 1994 did not seem to matter. Following the warning letter, NAC was pulled from shelves and websites.
Citizen petitions allow people and organizations to request that the FDA change their policy. During summer 2021, there were two citizen petitions – one from the Council for Responsible Nutrition (CRN) and another from the Natural Products Association (NPA) – asking that the FDA look at policy around NAC. In response, in November 2021, the FDA put out a request for more information about how long NAC had been used and if there were safety concerns, to determine if making it a lawful supplement would be appropriate. CRN promptly released a response that they were “extremely dissatisfied” and felt this was an unnecessary tactic to delay a decision. Two members of Congress wrote letters to the FDA in support of leaving NAC as an available supplement. Congressman Jeff Duncan noted that there were over 1,170 products containing NAC.
The FDA continued to issue responses. On March 31, 2022, the agency formally denied the requests of the two citizen petitions, and 3 weeks later the Guidance for Industry: Policy Regarding N-acetyl-L-cysteine was released, saying that the FDA would “... exercise enforcement discretion with respect to the sale and distribution of certain products that contain NAC.” While NAC was still not considered a supplement, no safety issues had been identified to date. Thus, while the FDA investigation continues, the agency will essentially look the other way.
The agency will consider rulemaking to include NAC as a supplement, a process that may take years. In a notice of final guidance released in August 2022, the FDA reiterated, “unless we identify safety-related concerns during our ongoing review, FDA intends to exercise enforcement discretion until either of the following occurs: we complete notice-and-comment rulemaking to allow the use of NAC in or as a dietary supplement (should we move forward with such proceedings) or we deny the NPA citizen petition’s request for rulemaking.”
It’s a win for the consumer who wants the supplement, and a half-win for the supplement manufacturers and their advocacy organizations who would like NAC to be an official dietary supplement. But just to be clear, the issue is one of a technicality: If NAC had been marketed as a supplement before it was a drug, it would just be a dietary supplement without all the controversy and scrutiny. It was not pulled because of a clinical concern.
For now, NAC is again readily available.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She has disclosed no relevant financial relationships.
Medicine or dietary supplement? N-acetylcysteine (NAC) is marketed as both and in 2021, the supplement abruptly became difficult to find, causing distress to people who had been using it for a variety of conditions. The story behind its disappearance is one of a cat-and-mouse chase between manufacturers, advocacy agencies, and the Food and Drug Administration.
NAC is a medication that was approved by the FDA in 1963. It has two FDA-approved uses: To prevent hepatotoxicity after overdose with acetaminophen, administered intravenously or by mouth, and as a mucolytic agent – previously available as Mucomyst, now available only as a generic – given by inhaler or nebulizer for pulmonary illnesses. Since the 1990s, NAC has been labeled by manufacturers as a dietary supplement. It is a derivative of the amino acid L-cysteine and a precursor to glutathione, an antioxidant.
Studies have had small sample sizes, and the supplement has not been considered an “off label” use of an already available medication. People have purchased NAC from retail drug and big box stores, Amazon, and online companies. So what’s the problem?
In July 2020, the FDA issued a warning letter to Purple Biosciences LLC, because of claims on the company’s website that the product “Purple Tree,” sold by Amazon, could cure hangovers that result from alcohol intoxication. The letter discussed justification for why a hangover is a disease, and goes on to note:
Based on the product label on your website, it appears that you intend to market your Purple Tree® product, which contains N-acetyl-L-cysteine (NAC), as a dietary supplement. However, even if your product labeling did not have therapeutic claims that make your product an unapproved new drug, your product could not be a dietary supplement, because it does not meet the definition of dietary supplement ... products containing that article are outside the definition of a dietary supplement, unless before such approval that article was marketed as a dietary supplement or as a food. NAC was approved as a new drug under section 505 of the Act [21 U.S.C. § 355] on September 14, 1963.
The issue here is that because NAC was first approved as a drug in 1963, it cannot be marketed as a supplement. If it had been marketed as a supplement before it was approved as a drug by the FDA, then it could remain on the market. The fact that it had been sold as a supplement since the 1990s and could be classified as an “old dietary ingredient” according to the Dietary Supplement Health and Education Act of 1994 did not seem to matter. Following the warning letter, NAC was pulled from shelves and websites.
Citizen petitions allow people and organizations to request that the FDA change their policy. During summer 2021, there were two citizen petitions – one from the Council for Responsible Nutrition (CRN) and another from the Natural Products Association (NPA) – asking that the FDA look at policy around NAC. In response, in November 2021, the FDA put out a request for more information about how long NAC had been used and if there were safety concerns, to determine if making it a lawful supplement would be appropriate. CRN promptly released a response that they were “extremely dissatisfied” and felt this was an unnecessary tactic to delay a decision. Two members of Congress wrote letters to the FDA in support of leaving NAC as an available supplement. Congressman Jeff Duncan noted that there were over 1,170 products containing NAC.
The FDA continued to issue responses. On March 31, 2022, the agency formally denied the requests of the two citizen petitions, and 3 weeks later the Guidance for Industry: Policy Regarding N-acetyl-L-cysteine was released, saying that the FDA would “... exercise enforcement discretion with respect to the sale and distribution of certain products that contain NAC.” While NAC was still not considered a supplement, no safety issues had been identified to date. Thus, while the FDA investigation continues, the agency will essentially look the other way.
The agency will consider rulemaking to include NAC as a supplement, a process that may take years. In a notice of final guidance released in August 2022, the FDA reiterated, “unless we identify safety-related concerns during our ongoing review, FDA intends to exercise enforcement discretion until either of the following occurs: we complete notice-and-comment rulemaking to allow the use of NAC in or as a dietary supplement (should we move forward with such proceedings) or we deny the NPA citizen petition’s request for rulemaking.”
It’s a win for the consumer who wants the supplement, and a half-win for the supplement manufacturers and their advocacy organizations who would like NAC to be an official dietary supplement. But just to be clear, the issue is one of a technicality: If NAC had been marketed as a supplement before it was a drug, it would just be a dietary supplement without all the controversy and scrutiny. It was not pulled because of a clinical concern.
For now, NAC is again readily available.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She has disclosed no relevant financial relationships.
Medicine or dietary supplement? N-acetylcysteine (NAC) is marketed as both and in 2021, the supplement abruptly became difficult to find, causing distress to people who had been using it for a variety of conditions. The story behind its disappearance is one of a cat-and-mouse chase between manufacturers, advocacy agencies, and the Food and Drug Administration.
NAC is a medication that was approved by the FDA in 1963. It has two FDA-approved uses: To prevent hepatotoxicity after overdose with acetaminophen, administered intravenously or by mouth, and as a mucolytic agent – previously available as Mucomyst, now available only as a generic – given by inhaler or nebulizer for pulmonary illnesses. Since the 1990s, NAC has been labeled by manufacturers as a dietary supplement. It is a derivative of the amino acid L-cysteine and a precursor to glutathione, an antioxidant.
Studies have had small sample sizes, and the supplement has not been considered an “off label” use of an already available medication. People have purchased NAC from retail drug and big box stores, Amazon, and online companies. So what’s the problem?
In July 2020, the FDA issued a warning letter to Purple Biosciences LLC, because of claims on the company’s website that the product “Purple Tree,” sold by Amazon, could cure hangovers that result from alcohol intoxication. The letter discussed justification for why a hangover is a disease, and goes on to note:
Based on the product label on your website, it appears that you intend to market your Purple Tree® product, which contains N-acetyl-L-cysteine (NAC), as a dietary supplement. However, even if your product labeling did not have therapeutic claims that make your product an unapproved new drug, your product could not be a dietary supplement, because it does not meet the definition of dietary supplement ... products containing that article are outside the definition of a dietary supplement, unless before such approval that article was marketed as a dietary supplement or as a food. NAC was approved as a new drug under section 505 of the Act [21 U.S.C. § 355] on September 14, 1963.
The issue here is that because NAC was first approved as a drug in 1963, it cannot be marketed as a supplement. If it had been marketed as a supplement before it was approved as a drug by the FDA, then it could remain on the market. The fact that it had been sold as a supplement since the 1990s and could be classified as an “old dietary ingredient” according to the Dietary Supplement Health and Education Act of 1994 did not seem to matter. Following the warning letter, NAC was pulled from shelves and websites.
Citizen petitions allow people and organizations to request that the FDA change their policy. During summer 2021, there were two citizen petitions – one from the Council for Responsible Nutrition (CRN) and another from the Natural Products Association (NPA) – asking that the FDA look at policy around NAC. In response, in November 2021, the FDA put out a request for more information about how long NAC had been used and if there were safety concerns, to determine if making it a lawful supplement would be appropriate. CRN promptly released a response that they were “extremely dissatisfied” and felt this was an unnecessary tactic to delay a decision. Two members of Congress wrote letters to the FDA in support of leaving NAC as an available supplement. Congressman Jeff Duncan noted that there were over 1,170 products containing NAC.
The FDA continued to issue responses. On March 31, 2022, the agency formally denied the requests of the two citizen petitions, and 3 weeks later the Guidance for Industry: Policy Regarding N-acetyl-L-cysteine was released, saying that the FDA would “... exercise enforcement discretion with respect to the sale and distribution of certain products that contain NAC.” While NAC was still not considered a supplement, no safety issues had been identified to date. Thus, while the FDA investigation continues, the agency will essentially look the other way.
The agency will consider rulemaking to include NAC as a supplement, a process that may take years. In a notice of final guidance released in August 2022, the FDA reiterated, “unless we identify safety-related concerns during our ongoing review, FDA intends to exercise enforcement discretion until either of the following occurs: we complete notice-and-comment rulemaking to allow the use of NAC in or as a dietary supplement (should we move forward with such proceedings) or we deny the NPA citizen petition’s request for rulemaking.”
It’s a win for the consumer who wants the supplement, and a half-win for the supplement manufacturers and their advocacy organizations who would like NAC to be an official dietary supplement. But just to be clear, the issue is one of a technicality: If NAC had been marketed as a supplement before it was a drug, it would just be a dietary supplement without all the controversy and scrutiny. It was not pulled because of a clinical concern.
For now, NAC is again readily available.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She has disclosed no relevant financial relationships.
Lithium, valproate, and suicide risk: Analysis of 98,831 cases
The current academic psychiatry paradigm reinforces that lithium reduces suicide risk, more so than other medications, including valproate. However, data from multiple sources contradict this “evidence-based” belief.
Data do not support lithium’s supposed advantage
An 8-year prospective study in Sweden by Song et al1 tracked 51,535 patients with bipolar disorder from 2005 to 2013. In their conclusions, the authors of this study omitted some surprising numbers that contradict the dominant paradigm. There were 230 (1.089%) completed suicides in the lithium group (N = 21,129), 99 (1.177%) in the valproate group (N = 8,411), and 308 (1.195%) in the “other medication” group (N = 25,780). This difference of .088% is too small (95% CI, -.180% to .358%) to substantiate the purported advantage of lithium over valproate. More important is that in terms of suicide-related events, the medication group excluding lithium and valproate had 2,018 (7.8%) events vs lithium 2,142 (10.1%) and valproate 1,105 (13.1%). The difference of 2.3% is statistically significant (95% CI, 1.8% to 2.8%). These numbers reflect fewer suicide-related events with psychiatric medications other than lithium and valproate. Compounding the problem is a design flaw in which 3,785 patients were counted twice in the lithium and valproate groups (21,129 + 8,411 + 25,780 = 55,320, which is more than the 51,535 patients in the study). By falsely inflating the denominator (N) for the lithium and valproate groups, the respective published rates are deceptively lower than the actual rates. Song et al1 did not provide an adequate explanation for these findings and omitted them from their conclusions.
In Schatzberg’s Manual of Clinical Psychopharmacology, the authors cited Song et al1 but omitted these findings as well, and stated “lithium is clearly effective in preventing suicide attempts and completions in bipolar patients.”2 In Stahl’s Essential Psychopharmacology, the author wrote “lithium actually reduces suicide in patients with bipolar disorder.”3 In a review article,
In an overlapping period, National Poison Data System (NPDS) data of single substance exposures painted a different picture in the United States.6 During 2006-2013, the lithium group (N = 26,144) had 32 deaths (all causes) (.122%), and the valproate group (N = 25,630) had 16 deaths (.062%). During 2006-2020, the lithium group (N = 52,262) had 55 deaths (.105%), and the valproate group (N = 46,569) had 31 deaths (.067%). Clearly there is a major disconnect between lithium’s advertised ability to reduce suicide risk and the actual mortality rate, as evidenced by 98,831 cases reported to NPDS during 2006-2020. One would expect a lower rate in the lithium group, but data show it is higher than in the valproate group. This underscores the common fallacy of most lithium studies: each is based on a very small sample (N < 100), and the statistical inference about the entire population is tenuous. If lithium truly reduces suicide risk 5-fold, it would be seen in a sample of 98,831. The law of large numbers and central limit theorem state that as N increases, the variability of the rate progressively decreases. This can be easily demonstrated with computer simulation models and simple Python code, or on the average fuel economy display of most cars.
What about the relative lethality?
The APA Textbook of Suicide Risk Assessment and Management stated that it is important to consider the relative lethality (RL) of prescription medications.7 The RL equation (RL = 310x / LD50) represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person (x is the daily dose and LD50 is the rat oral lethal dose 50).8 Time series analysis shows that the lithium relative morbidity (RM) is consistently double that of valproate (Figure6). Regression models have shown high correlation and causality between RL and RM.9-11 It is surprising that valproate (RL = 1,666%) has a lower RM than lithium (RL = 1,063%). This paradox can be easily explained with clinical insight. The RL equation compares medications at the maximum daily dose, but in routine practice valproate is commonly prescribed at 1,000 mg/d (28% of the maximum 3,600 mg/d). Lithium is commonly prescribed at 1,200 mg/d (67% of the maximum 1,800 mg/d). Within these dosing parameters, the effective RL is valproate 463% and lithium 709%. The 2020 RM is valproate 22% and lithium 43%.12 The COVID-19 pandemic did not affect the predicted RM. Confirming these numbers, Song et al1 acknowledged “greater safety in case of overdose for valproate in clinical practice.” Baldessarini et al4 asserted “the fatality risk of lithium overdose is only moderate, and very similar to modern antidepressants and second-generation antipsychotics.”4 This claim is contradicted by the RL equation and regression models.7-11 Lithium’s RL is 19 times higher than that of fluoxetine, and 30 times higher than that of olanzapine.8 Lithium’s RM is nearly identical to amitriptyline (42%), vs fluoxetine (12%).12
Data-driven analysis shows that lithium has higher rates of morbidity and mortality than valproate, as evidenced by 98,831 NPDS cases during 2006-2020. These hard numbers speak for themselves and contradict the dominant paradigm, which proclaims lithium’s superiority in reducing suicide risk.
1. Song J, Sjölander A, Joas E, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017;174(8):795-802.
2. Schatzberg AF, DeBattista C. Schatzberg’s Manual of Clinical Psychopharmacology. 9th ed. American Psychiatric Association Publishing; 2019:335.
3. Stahl SM. Stahl’s Essential Psychopharmacology. 4th ed. Cambridge University Press; 2013:372.
4. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625-639.
5. Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056.
6. American Association of Poison Control Centers. Annual reports. Accessed August 25, 2022. https://aapcc.org/annual-reports
7. Gold LH, Frierson RL (eds). The American Psychiatric Association Publishing Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020:17-19.
8. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
9. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
10. Giurca D. Time series analysis of poison control data. Current Psychiatry. 2020;19(6):e5-e9.
11. Giurca D, Hodgman MJ. Relative lethality of hypertension drugs. J Med Toxicol. 2022;18(2):81. 2022 American College of Medical Toxicology Annual Scientific Meeting abstract 020.
12. Gummin DD, Mowry JB, Beuhler MD, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282-1501.
The current academic psychiatry paradigm reinforces that lithium reduces suicide risk, more so than other medications, including valproate. However, data from multiple sources contradict this “evidence-based” belief.
Data do not support lithium’s supposed advantage
An 8-year prospective study in Sweden by Song et al1 tracked 51,535 patients with bipolar disorder from 2005 to 2013. In their conclusions, the authors of this study omitted some surprising numbers that contradict the dominant paradigm. There were 230 (1.089%) completed suicides in the lithium group (N = 21,129), 99 (1.177%) in the valproate group (N = 8,411), and 308 (1.195%) in the “other medication” group (N = 25,780). This difference of .088% is too small (95% CI, -.180% to .358%) to substantiate the purported advantage of lithium over valproate. More important is that in terms of suicide-related events, the medication group excluding lithium and valproate had 2,018 (7.8%) events vs lithium 2,142 (10.1%) and valproate 1,105 (13.1%). The difference of 2.3% is statistically significant (95% CI, 1.8% to 2.8%). These numbers reflect fewer suicide-related events with psychiatric medications other than lithium and valproate. Compounding the problem is a design flaw in which 3,785 patients were counted twice in the lithium and valproate groups (21,129 + 8,411 + 25,780 = 55,320, which is more than the 51,535 patients in the study). By falsely inflating the denominator (N) for the lithium and valproate groups, the respective published rates are deceptively lower than the actual rates. Song et al1 did not provide an adequate explanation for these findings and omitted them from their conclusions.
In Schatzberg’s Manual of Clinical Psychopharmacology, the authors cited Song et al1 but omitted these findings as well, and stated “lithium is clearly effective in preventing suicide attempts and completions in bipolar patients.”2 In Stahl’s Essential Psychopharmacology, the author wrote “lithium actually reduces suicide in patients with bipolar disorder.”3 In a review article,
In an overlapping period, National Poison Data System (NPDS) data of single substance exposures painted a different picture in the United States.6 During 2006-2013, the lithium group (N = 26,144) had 32 deaths (all causes) (.122%), and the valproate group (N = 25,630) had 16 deaths (.062%). During 2006-2020, the lithium group (N = 52,262) had 55 deaths (.105%), and the valproate group (N = 46,569) had 31 deaths (.067%). Clearly there is a major disconnect between lithium’s advertised ability to reduce suicide risk and the actual mortality rate, as evidenced by 98,831 cases reported to NPDS during 2006-2020. One would expect a lower rate in the lithium group, but data show it is higher than in the valproate group. This underscores the common fallacy of most lithium studies: each is based on a very small sample (N < 100), and the statistical inference about the entire population is tenuous. If lithium truly reduces suicide risk 5-fold, it would be seen in a sample of 98,831. The law of large numbers and central limit theorem state that as N increases, the variability of the rate progressively decreases. This can be easily demonstrated with computer simulation models and simple Python code, or on the average fuel economy display of most cars.
What about the relative lethality?
The APA Textbook of Suicide Risk Assessment and Management stated that it is important to consider the relative lethality (RL) of prescription medications.7 The RL equation (RL = 310x / LD50) represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person (x is the daily dose and LD50 is the rat oral lethal dose 50).8 Time series analysis shows that the lithium relative morbidity (RM) is consistently double that of valproate (Figure6). Regression models have shown high correlation and causality between RL and RM.9-11 It is surprising that valproate (RL = 1,666%) has a lower RM than lithium (RL = 1,063%). This paradox can be easily explained with clinical insight. The RL equation compares medications at the maximum daily dose, but in routine practice valproate is commonly prescribed at 1,000 mg/d (28% of the maximum 3,600 mg/d). Lithium is commonly prescribed at 1,200 mg/d (67% of the maximum 1,800 mg/d). Within these dosing parameters, the effective RL is valproate 463% and lithium 709%. The 2020 RM is valproate 22% and lithium 43%.12 The COVID-19 pandemic did not affect the predicted RM. Confirming these numbers, Song et al1 acknowledged “greater safety in case of overdose for valproate in clinical practice.” Baldessarini et al4 asserted “the fatality risk of lithium overdose is only moderate, and very similar to modern antidepressants and second-generation antipsychotics.”4 This claim is contradicted by the RL equation and regression models.7-11 Lithium’s RL is 19 times higher than that of fluoxetine, and 30 times higher than that of olanzapine.8 Lithium’s RM is nearly identical to amitriptyline (42%), vs fluoxetine (12%).12

Data-driven analysis shows that lithium has higher rates of morbidity and mortality than valproate, as evidenced by 98,831 NPDS cases during 2006-2020. These hard numbers speak for themselves and contradict the dominant paradigm, which proclaims lithium’s superiority in reducing suicide risk.
The current academic psychiatry paradigm reinforces that lithium reduces suicide risk, more so than other medications, including valproate. However, data from multiple sources contradict this “evidence-based” belief.
Data do not support lithium’s supposed advantage
An 8-year prospective study in Sweden by Song et al1 tracked 51,535 patients with bipolar disorder from 2005 to 2013. In their conclusions, the authors of this study omitted some surprising numbers that contradict the dominant paradigm. There were 230 (1.089%) completed suicides in the lithium group (N = 21,129), 99 (1.177%) in the valproate group (N = 8,411), and 308 (1.195%) in the “other medication” group (N = 25,780). This difference of .088% is too small (95% CI, -.180% to .358%) to substantiate the purported advantage of lithium over valproate. More important is that in terms of suicide-related events, the medication group excluding lithium and valproate had 2,018 (7.8%) events vs lithium 2,142 (10.1%) and valproate 1,105 (13.1%). The difference of 2.3% is statistically significant (95% CI, 1.8% to 2.8%). These numbers reflect fewer suicide-related events with psychiatric medications other than lithium and valproate. Compounding the problem is a design flaw in which 3,785 patients were counted twice in the lithium and valproate groups (21,129 + 8,411 + 25,780 = 55,320, which is more than the 51,535 patients in the study). By falsely inflating the denominator (N) for the lithium and valproate groups, the respective published rates are deceptively lower than the actual rates. Song et al1 did not provide an adequate explanation for these findings and omitted them from their conclusions.
In Schatzberg’s Manual of Clinical Psychopharmacology, the authors cited Song et al1 but omitted these findings as well, and stated “lithium is clearly effective in preventing suicide attempts and completions in bipolar patients.”2 In Stahl’s Essential Psychopharmacology, the author wrote “lithium actually reduces suicide in patients with bipolar disorder.”3 In a review article,
In an overlapping period, National Poison Data System (NPDS) data of single substance exposures painted a different picture in the United States.6 During 2006-2013, the lithium group (N = 26,144) had 32 deaths (all causes) (.122%), and the valproate group (N = 25,630) had 16 deaths (.062%). During 2006-2020, the lithium group (N = 52,262) had 55 deaths (.105%), and the valproate group (N = 46,569) had 31 deaths (.067%). Clearly there is a major disconnect between lithium’s advertised ability to reduce suicide risk and the actual mortality rate, as evidenced by 98,831 cases reported to NPDS during 2006-2020. One would expect a lower rate in the lithium group, but data show it is higher than in the valproate group. This underscores the common fallacy of most lithium studies: each is based on a very small sample (N < 100), and the statistical inference about the entire population is tenuous. If lithium truly reduces suicide risk 5-fold, it would be seen in a sample of 98,831. The law of large numbers and central limit theorem state that as N increases, the variability of the rate progressively decreases. This can be easily demonstrated with computer simulation models and simple Python code, or on the average fuel economy display of most cars.
What about the relative lethality?
The APA Textbook of Suicide Risk Assessment and Management stated that it is important to consider the relative lethality (RL) of prescription medications.7 The RL equation (RL = 310x / LD50) represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person (x is the daily dose and LD50 is the rat oral lethal dose 50).8 Time series analysis shows that the lithium relative morbidity (RM) is consistently double that of valproate (Figure6). Regression models have shown high correlation and causality between RL and RM.9-11 It is surprising that valproate (RL = 1,666%) has a lower RM than lithium (RL = 1,063%). This paradox can be easily explained with clinical insight. The RL equation compares medications at the maximum daily dose, but in routine practice valproate is commonly prescribed at 1,000 mg/d (28% of the maximum 3,600 mg/d). Lithium is commonly prescribed at 1,200 mg/d (67% of the maximum 1,800 mg/d). Within these dosing parameters, the effective RL is valproate 463% and lithium 709%. The 2020 RM is valproate 22% and lithium 43%.12 The COVID-19 pandemic did not affect the predicted RM. Confirming these numbers, Song et al1 acknowledged “greater safety in case of overdose for valproate in clinical practice.” Baldessarini et al4 asserted “the fatality risk of lithium overdose is only moderate, and very similar to modern antidepressants and second-generation antipsychotics.”4 This claim is contradicted by the RL equation and regression models.7-11 Lithium’s RL is 19 times higher than that of fluoxetine, and 30 times higher than that of olanzapine.8 Lithium’s RM is nearly identical to amitriptyline (42%), vs fluoxetine (12%).12

Data-driven analysis shows that lithium has higher rates of morbidity and mortality than valproate, as evidenced by 98,831 NPDS cases during 2006-2020. These hard numbers speak for themselves and contradict the dominant paradigm, which proclaims lithium’s superiority in reducing suicide risk.
1. Song J, Sjölander A, Joas E, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017;174(8):795-802.
2. Schatzberg AF, DeBattista C. Schatzberg’s Manual of Clinical Psychopharmacology. 9th ed. American Psychiatric Association Publishing; 2019:335.
3. Stahl SM. Stahl’s Essential Psychopharmacology. 4th ed. Cambridge University Press; 2013:372.
4. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625-639.
5. Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056.
6. American Association of Poison Control Centers. Annual reports. Accessed August 25, 2022. https://aapcc.org/annual-reports
7. Gold LH, Frierson RL (eds). The American Psychiatric Association Publishing Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020:17-19.
8. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
9. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
10. Giurca D. Time series analysis of poison control data. Current Psychiatry. 2020;19(6):e5-e9.
11. Giurca D, Hodgman MJ. Relative lethality of hypertension drugs. J Med Toxicol. 2022;18(2):81. 2022 American College of Medical Toxicology Annual Scientific Meeting abstract 020.
12. Gummin DD, Mowry JB, Beuhler MD, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282-1501.
1. Song J, Sjölander A, Joas E, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017;174(8):795-802.
2. Schatzberg AF, DeBattista C. Schatzberg’s Manual of Clinical Psychopharmacology. 9th ed. American Psychiatric Association Publishing; 2019:335.
3. Stahl SM. Stahl’s Essential Psychopharmacology. 4th ed. Cambridge University Press; 2013:372.
4. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625-639.
5. Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056.
6. American Association of Poison Control Centers. Annual reports. Accessed August 25, 2022. https://aapcc.org/annual-reports
7. Gold LH, Frierson RL (eds). The American Psychiatric Association Publishing Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020:17-19.
8. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
9. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
10. Giurca D. Time series analysis of poison control data. Current Psychiatry. 2020;19(6):e5-e9.
11. Giurca D, Hodgman MJ. Relative lethality of hypertension drugs. J Med Toxicol. 2022;18(2):81. 2022 American College of Medical Toxicology Annual Scientific Meeting abstract 020.
12. Gummin DD, Mowry JB, Beuhler MD, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282-1501.