User login
High antipsychotic switch rates suggest ‘suboptimal’ prescribing for first-episode psychosis
In a large-scale, real-world analysis of U.K. prescribing patterns, researchers found more than two-thirds of patients who received antipsychotics for FEP switched medication, and almost half switched drugs three times.
Although this is “one of the largest real-world studies examining antipsychotic treatment strategies,” it reflects findings from previous, smaller studies showing “antipsychotic switching in first episode psychosis is high,” said study investigator Aimee Brinn, Institute of Psychiatry, Psychology & Neuroscience at King’s College London.
This may reflect reports of poor efficacy and suggests that first-line prescribing is “suboptimal,” Ms. Brinn noted. In addition, olanzapine remains the most popular antipsychotic for prescribing despite recent guidelines indicating it is “not ideal ... due to its dangerous metabolic side effects,” she added.
The findings were presented at the Congress of the Schizophrenia International Research Society (SIRS) 2022.
Real-world data
The response to, and tolerability of, antipsychotics differs between patients with FEP; and prescribing patterns “reflect clinician and patient-led decisionmaking,” Ms. Brinn told meeting attendees.
Since randomized controlled trials “do not necessarily reflect prescribing practice in real-world clinical settings,” the researchers gathered data from a large mental health care electronic health record dataset.
The investigators examined records from the South London and Maudsley NHS Foundation Trust (SLaM), which has a catchment area of 1.2 million individuals across four boroughs of London. The group sees approximately 37,500 active patients per week.
The team used the Clinical Interactive Record Search tool to extract data on 2,309 adults with FEP who received care from a SLaM early intervention in psychosis service between April 1, 2008, and March 31, 2019.
They found that 12 different antipsychotics were prescribed as first-line treatment. The most common were olanzapine (43.9%), risperidone (24.7%), and aripiprazole (19.9%).
Results showed that over 81,969.5 person-years of follow-up, at a minimum of 24 months per patient, 68.8% had an antipsychotic switch. The most common first treatment switch, in 17.9% of patients, was from olanzapine to aripiprazole.
Of patients who switched to aripiprazole, 48.4% stayed on the drug, 26% switched back to olanzapine, and 25.6% received other treatment. Overall, 44.7% of patients switched medication at least three times.
Among patients with FEP who did not switch, 42.2% were prescribed olanzapine, 26.2% risperidone, 23.3% aripiprazole, 5.6% quetiapine, and 2.7% amisulpride.
During the post-presentation discussion, Ms. Brinn was asked whether the high rate of first-line olanzapine prescribing could be because patients started treatment as inpatients and were then switched once they were moved to community care.
“We found that a lot of patients would be prescribed olanzapine for around 7 days at the start of their prescription and then switch,” Ms. Brinn said, adding it is “likely” they started as inpatients. The investigators are currently examining the differences between inpatient and outpatient prescriptions to verify whether this is indeed the case, she added.
‘Pulling out the big guns too fast?’
Commenting on the findings, Thomas W. Sedlak, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, said the study raises a “number of questions.”
Both olanzapine and risperidone “tend to have higher treatment effect improvements than aripiprazole, so it’s curious that a switch to aripiprazole was common,” said Dr. Sedlak, who was not involved with the research.
“Are we pulling out the ‘big guns’ too fast, or inappropriately, especially as olanzapine and risperidone carry greater risk of weight gain?” he asked. In addition, “now that olanzapine is available with samidorphan to mitigate weight gain, will that shape future patterns, if it can be paid for?”
Dr. Sedlak noted it was unclear why olanzapine was chosen so often as first-line treatment in the study and agreed it is “possible that hospitalized patients had been prescribed a ‘stronger’ medication like olanzapine compared to never-hospitalized patients.”
He also underlined that it is “not clear if patients in this FEP program are representative of all FEP patients.”
“For instance, if the program is well known to inpatient hospital social workers, then the program might be disproportionately filled with patients who have had more severe symptoms,” Dr. Sedlak said.
The study was supported by Janssen-Cilag. The investigators and Dr. Sedlak have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large-scale, real-world analysis of U.K. prescribing patterns, researchers found more than two-thirds of patients who received antipsychotics for FEP switched medication, and almost half switched drugs three times.
Although this is “one of the largest real-world studies examining antipsychotic treatment strategies,” it reflects findings from previous, smaller studies showing “antipsychotic switching in first episode psychosis is high,” said study investigator Aimee Brinn, Institute of Psychiatry, Psychology & Neuroscience at King’s College London.
This may reflect reports of poor efficacy and suggests that first-line prescribing is “suboptimal,” Ms. Brinn noted. In addition, olanzapine remains the most popular antipsychotic for prescribing despite recent guidelines indicating it is “not ideal ... due to its dangerous metabolic side effects,” she added.
The findings were presented at the Congress of the Schizophrenia International Research Society (SIRS) 2022.
Real-world data
The response to, and tolerability of, antipsychotics differs between patients with FEP; and prescribing patterns “reflect clinician and patient-led decisionmaking,” Ms. Brinn told meeting attendees.
Since randomized controlled trials “do not necessarily reflect prescribing practice in real-world clinical settings,” the researchers gathered data from a large mental health care electronic health record dataset.
The investigators examined records from the South London and Maudsley NHS Foundation Trust (SLaM), which has a catchment area of 1.2 million individuals across four boroughs of London. The group sees approximately 37,500 active patients per week.
The team used the Clinical Interactive Record Search tool to extract data on 2,309 adults with FEP who received care from a SLaM early intervention in psychosis service between April 1, 2008, and March 31, 2019.
They found that 12 different antipsychotics were prescribed as first-line treatment. The most common were olanzapine (43.9%), risperidone (24.7%), and aripiprazole (19.9%).
Results showed that over 81,969.5 person-years of follow-up, at a minimum of 24 months per patient, 68.8% had an antipsychotic switch. The most common first treatment switch, in 17.9% of patients, was from olanzapine to aripiprazole.
Of patients who switched to aripiprazole, 48.4% stayed on the drug, 26% switched back to olanzapine, and 25.6% received other treatment. Overall, 44.7% of patients switched medication at least three times.
Among patients with FEP who did not switch, 42.2% were prescribed olanzapine, 26.2% risperidone, 23.3% aripiprazole, 5.6% quetiapine, and 2.7% amisulpride.
During the post-presentation discussion, Ms. Brinn was asked whether the high rate of first-line olanzapine prescribing could be because patients started treatment as inpatients and were then switched once they were moved to community care.
“We found that a lot of patients would be prescribed olanzapine for around 7 days at the start of their prescription and then switch,” Ms. Brinn said, adding it is “likely” they started as inpatients. The investigators are currently examining the differences between inpatient and outpatient prescriptions to verify whether this is indeed the case, she added.
‘Pulling out the big guns too fast?’
Commenting on the findings, Thomas W. Sedlak, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, said the study raises a “number of questions.”
Both olanzapine and risperidone “tend to have higher treatment effect improvements than aripiprazole, so it’s curious that a switch to aripiprazole was common,” said Dr. Sedlak, who was not involved with the research.
“Are we pulling out the ‘big guns’ too fast, or inappropriately, especially as olanzapine and risperidone carry greater risk of weight gain?” he asked. In addition, “now that olanzapine is available with samidorphan to mitigate weight gain, will that shape future patterns, if it can be paid for?”
Dr. Sedlak noted it was unclear why olanzapine was chosen so often as first-line treatment in the study and agreed it is “possible that hospitalized patients had been prescribed a ‘stronger’ medication like olanzapine compared to never-hospitalized patients.”
He also underlined that it is “not clear if patients in this FEP program are representative of all FEP patients.”
“For instance, if the program is well known to inpatient hospital social workers, then the program might be disproportionately filled with patients who have had more severe symptoms,” Dr. Sedlak said.
The study was supported by Janssen-Cilag. The investigators and Dr. Sedlak have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large-scale, real-world analysis of U.K. prescribing patterns, researchers found more than two-thirds of patients who received antipsychotics for FEP switched medication, and almost half switched drugs three times.
Although this is “one of the largest real-world studies examining antipsychotic treatment strategies,” it reflects findings from previous, smaller studies showing “antipsychotic switching in first episode psychosis is high,” said study investigator Aimee Brinn, Institute of Psychiatry, Psychology & Neuroscience at King’s College London.
This may reflect reports of poor efficacy and suggests that first-line prescribing is “suboptimal,” Ms. Brinn noted. In addition, olanzapine remains the most popular antipsychotic for prescribing despite recent guidelines indicating it is “not ideal ... due to its dangerous metabolic side effects,” she added.
The findings were presented at the Congress of the Schizophrenia International Research Society (SIRS) 2022.
Real-world data
The response to, and tolerability of, antipsychotics differs between patients with FEP; and prescribing patterns “reflect clinician and patient-led decisionmaking,” Ms. Brinn told meeting attendees.
Since randomized controlled trials “do not necessarily reflect prescribing practice in real-world clinical settings,” the researchers gathered data from a large mental health care electronic health record dataset.
The investigators examined records from the South London and Maudsley NHS Foundation Trust (SLaM), which has a catchment area of 1.2 million individuals across four boroughs of London. The group sees approximately 37,500 active patients per week.
The team used the Clinical Interactive Record Search tool to extract data on 2,309 adults with FEP who received care from a SLaM early intervention in psychosis service between April 1, 2008, and March 31, 2019.
They found that 12 different antipsychotics were prescribed as first-line treatment. The most common were olanzapine (43.9%), risperidone (24.7%), and aripiprazole (19.9%).
Results showed that over 81,969.5 person-years of follow-up, at a minimum of 24 months per patient, 68.8% had an antipsychotic switch. The most common first treatment switch, in 17.9% of patients, was from olanzapine to aripiprazole.
Of patients who switched to aripiprazole, 48.4% stayed on the drug, 26% switched back to olanzapine, and 25.6% received other treatment. Overall, 44.7% of patients switched medication at least three times.
Among patients with FEP who did not switch, 42.2% were prescribed olanzapine, 26.2% risperidone, 23.3% aripiprazole, 5.6% quetiapine, and 2.7% amisulpride.
During the post-presentation discussion, Ms. Brinn was asked whether the high rate of first-line olanzapine prescribing could be because patients started treatment as inpatients and were then switched once they were moved to community care.
“We found that a lot of patients would be prescribed olanzapine for around 7 days at the start of their prescription and then switch,” Ms. Brinn said, adding it is “likely” they started as inpatients. The investigators are currently examining the differences between inpatient and outpatient prescriptions to verify whether this is indeed the case, she added.
‘Pulling out the big guns too fast?’
Commenting on the findings, Thomas W. Sedlak, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, said the study raises a “number of questions.”
Both olanzapine and risperidone “tend to have higher treatment effect improvements than aripiprazole, so it’s curious that a switch to aripiprazole was common,” said Dr. Sedlak, who was not involved with the research.
“Are we pulling out the ‘big guns’ too fast, or inappropriately, especially as olanzapine and risperidone carry greater risk of weight gain?” he asked. In addition, “now that olanzapine is available with samidorphan to mitigate weight gain, will that shape future patterns, if it can be paid for?”
Dr. Sedlak noted it was unclear why olanzapine was chosen so often as first-line treatment in the study and agreed it is “possible that hospitalized patients had been prescribed a ‘stronger’ medication like olanzapine compared to never-hospitalized patients.”
He also underlined that it is “not clear if patients in this FEP program are representative of all FEP patients.”
“For instance, if the program is well known to inpatient hospital social workers, then the program might be disproportionately filled with patients who have had more severe symptoms,” Dr. Sedlak said.
The study was supported by Janssen-Cilag. The investigators and Dr. Sedlak have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SIRS 2022
New combination med for severe mental illness tied to less weight gain
, new research suggests. However, at least one expert says the weight difference between the two drugs is of “questionable clinical benefit.”
Last year, the Food and Drug Administration approved the drug for the treatment of adults with schizophrenia or bipolar I disorder, as a maintenance monotherapy or as either monotherapy or an adjunct to lithium or valproate for acute manic or mixed episodes.
In the ENLIGHTEN-Early trial, researchers examined weight-gain profiles of more than 400 patients with early schizophrenia, schizophreniform disorder, or bipolar I disorder.
Results showed those given combination treatment gained just over half the amount of weight as those given monotherapy. They were also 36% less likely to gain at least 10% of their body weight during the 12-week treatment period.
They indicate that the weight-mitigating effects shown with olanzapine plus samidorphan are “consistent, regardless of the stage of illness,” Dr. Kahn added.
He presented the findings at the annual congress of the Schizophrenia International Research Society.
Potential benefit
“Early intervention with antipsychotic treatment is critical in shaping the course of treatment and the disease trajectory,” coinvestigator Christine Graham, PhD, with Alkermes, which manufactures the drug, told this news organization.
Olanzapine is a “highly effective antipsychotic, but it’s really avoided a lot in this population,” Dr. Graham said. Therefore, patients “could really stand to benefit” from a combination that delivers the same amount of antipsychotic effect, but “reduces the propensity” for clinically significant weight gain, she added.
Dr. Kahn noted in his meeting presentation that antipsychotics are the “cornerstone” of the treatment of serious mental illness, but that “many are associated with concerning weight gain and cardiometabolic effects.”
While olanzapine is an effective medication, it has “one of the highest weight gain” profiles of the available antipsychotics and patients early on in their illness are “especially vulnerable,” Dr. Kahn said.
Previous studies have shown the combination of olanzapine plus samidorphan is similarly effective as olanzapine, but is associated with less weight gain.
To determine its impact in recent-onset illness, the current researchers screened patients with schizophrenia, schizophreniform disorder, or bipolar I disorder. The patients were aged 16-39 years and had an initial onset of active phase symptoms less than 4 years previously. They had less than 24 weeks’ cumulative lifetime exposure to antipsychotics.
Participants were randomly assigned to receive olanzapine plus samidorphan or olanzapine alone for 12 weeks, and then followed up for safety assessment for a further 4 weeks.
A total of 426 patients were recruited and 76.5% completed the study. The mean age was 25.8 years, 66.2% were men, 66.4% were White, and 28.2% were Black.
The mean body mass index at baseline was 23.69 kg/m2. The most common diagnosis among the participants was schizophrenia (62.9%) followed by bipolar I disorder (21.6%).
Less weight gain
Results of the 12-week study showed a significant difference in percent change in body weight from baseline between the two treatment groups, with a gain of 4.91% for the olanzapine plus samidorphan group vs. 6.77% for the olanzapine-alone group (between-group difference, 1.87%; P = .012).
Dr. Kahn noted this equates to an average weight gain of 2.8 kg (6.2 pounds) with olanzapine plus samidorphan and a gain of about 5 kg (11pounds) with olanzapine.
“It’s not a huge difference, but it’s certainly a significant one,” he said. “I also think it’s clinically important and significant.”
The reduction in weight gain compared with olanzapine was even maintained in patients assigned to olanzapine plus samidorphan who dropped out and did not complete the study, Dr. Kahn reported. “No one really had a weight gain,” he said.
In contrast, patients in the olanzapine groups who dropped out of the study had weight gain larger than their counterparts who stayed in it.
Further analysis showed the proportion of patients who gained 10% or more of their body weight by week 12 was 21.9% for those receiving olanzapine plus samidorphan vs. 30.4% for those receiving just olanzapine (odds ratio, 0.64; P = .075).
As expected, the improvement in Clinical Global Impression–Severity scale scores was almost identical between the olanzapine + samidorphan and olanzapine-only groups.
For safety, Dr. Kahn said the adverse event rates were “very, very similar” between the two treatment arms, which was a pattern that was repeated for serious AEs. This led him to note that “nothing out of the ordinary” was observed.
Clinical impact 'questionable'
Commenting on the study, Laura LaChance, MD, a psychiatrist at St. Mary’s Hospital Centre, McGill University, Montreal, said the actual amount of weight loss shown in the study “is of questionable clinical significance.”
On the other hand, Dr. LaChance said she has achieved “better results with metformin, which has a great safety profile and is cheap and widely available.
“Cost is always a concern in patients with psychotic disorders,” she concluded.
The study was funded by Alkermes. Dr. Kahn reported having relationships with Alkermes, Angelini, Janssen, Sunovion, Otsuka, Merck, Minerva Neuroscience, Roche, and Teva. Dr. Graham is an employee of Alkermes.
A version of this article first appeared on Medscape.com.
, new research suggests. However, at least one expert says the weight difference between the two drugs is of “questionable clinical benefit.”
Last year, the Food and Drug Administration approved the drug for the treatment of adults with schizophrenia or bipolar I disorder, as a maintenance monotherapy or as either monotherapy or an adjunct to lithium or valproate for acute manic or mixed episodes.
In the ENLIGHTEN-Early trial, researchers examined weight-gain profiles of more than 400 patients with early schizophrenia, schizophreniform disorder, or bipolar I disorder.
Results showed those given combination treatment gained just over half the amount of weight as those given monotherapy. They were also 36% less likely to gain at least 10% of their body weight during the 12-week treatment period.
They indicate that the weight-mitigating effects shown with olanzapine plus samidorphan are “consistent, regardless of the stage of illness,” Dr. Kahn added.
He presented the findings at the annual congress of the Schizophrenia International Research Society.
Potential benefit
“Early intervention with antipsychotic treatment is critical in shaping the course of treatment and the disease trajectory,” coinvestigator Christine Graham, PhD, with Alkermes, which manufactures the drug, told this news organization.
Olanzapine is a “highly effective antipsychotic, but it’s really avoided a lot in this population,” Dr. Graham said. Therefore, patients “could really stand to benefit” from a combination that delivers the same amount of antipsychotic effect, but “reduces the propensity” for clinically significant weight gain, she added.
Dr. Kahn noted in his meeting presentation that antipsychotics are the “cornerstone” of the treatment of serious mental illness, but that “many are associated with concerning weight gain and cardiometabolic effects.”
While olanzapine is an effective medication, it has “one of the highest weight gain” profiles of the available antipsychotics and patients early on in their illness are “especially vulnerable,” Dr. Kahn said.
Previous studies have shown the combination of olanzapine plus samidorphan is similarly effective as olanzapine, but is associated with less weight gain.
To determine its impact in recent-onset illness, the current researchers screened patients with schizophrenia, schizophreniform disorder, or bipolar I disorder. The patients were aged 16-39 years and had an initial onset of active phase symptoms less than 4 years previously. They had less than 24 weeks’ cumulative lifetime exposure to antipsychotics.
Participants were randomly assigned to receive olanzapine plus samidorphan or olanzapine alone for 12 weeks, and then followed up for safety assessment for a further 4 weeks.
A total of 426 patients were recruited and 76.5% completed the study. The mean age was 25.8 years, 66.2% were men, 66.4% were White, and 28.2% were Black.
The mean body mass index at baseline was 23.69 kg/m2. The most common diagnosis among the participants was schizophrenia (62.9%) followed by bipolar I disorder (21.6%).
Less weight gain
Results of the 12-week study showed a significant difference in percent change in body weight from baseline between the two treatment groups, with a gain of 4.91% for the olanzapine plus samidorphan group vs. 6.77% for the olanzapine-alone group (between-group difference, 1.87%; P = .012).
Dr. Kahn noted this equates to an average weight gain of 2.8 kg (6.2 pounds) with olanzapine plus samidorphan and a gain of about 5 kg (11pounds) with olanzapine.
“It’s not a huge difference, but it’s certainly a significant one,” he said. “I also think it’s clinically important and significant.”
The reduction in weight gain compared with olanzapine was even maintained in patients assigned to olanzapine plus samidorphan who dropped out and did not complete the study, Dr. Kahn reported. “No one really had a weight gain,” he said.
In contrast, patients in the olanzapine groups who dropped out of the study had weight gain larger than their counterparts who stayed in it.
Further analysis showed the proportion of patients who gained 10% or more of their body weight by week 12 was 21.9% for those receiving olanzapine plus samidorphan vs. 30.4% for those receiving just olanzapine (odds ratio, 0.64; P = .075).
As expected, the improvement in Clinical Global Impression–Severity scale scores was almost identical between the olanzapine + samidorphan and olanzapine-only groups.
For safety, Dr. Kahn said the adverse event rates were “very, very similar” between the two treatment arms, which was a pattern that was repeated for serious AEs. This led him to note that “nothing out of the ordinary” was observed.
Clinical impact 'questionable'
Commenting on the study, Laura LaChance, MD, a psychiatrist at St. Mary’s Hospital Centre, McGill University, Montreal, said the actual amount of weight loss shown in the study “is of questionable clinical significance.”
On the other hand, Dr. LaChance said she has achieved “better results with metformin, which has a great safety profile and is cheap and widely available.
“Cost is always a concern in patients with psychotic disorders,” she concluded.
The study was funded by Alkermes. Dr. Kahn reported having relationships with Alkermes, Angelini, Janssen, Sunovion, Otsuka, Merck, Minerva Neuroscience, Roche, and Teva. Dr. Graham is an employee of Alkermes.
A version of this article first appeared on Medscape.com.
, new research suggests. However, at least one expert says the weight difference between the two drugs is of “questionable clinical benefit.”
Last year, the Food and Drug Administration approved the drug for the treatment of adults with schizophrenia or bipolar I disorder, as a maintenance monotherapy or as either monotherapy or an adjunct to lithium or valproate for acute manic or mixed episodes.
In the ENLIGHTEN-Early trial, researchers examined weight-gain profiles of more than 400 patients with early schizophrenia, schizophreniform disorder, or bipolar I disorder.
Results showed those given combination treatment gained just over half the amount of weight as those given monotherapy. They were also 36% less likely to gain at least 10% of their body weight during the 12-week treatment period.
They indicate that the weight-mitigating effects shown with olanzapine plus samidorphan are “consistent, regardless of the stage of illness,” Dr. Kahn added.
He presented the findings at the annual congress of the Schizophrenia International Research Society.
Potential benefit
“Early intervention with antipsychotic treatment is critical in shaping the course of treatment and the disease trajectory,” coinvestigator Christine Graham, PhD, with Alkermes, which manufactures the drug, told this news organization.
Olanzapine is a “highly effective antipsychotic, but it’s really avoided a lot in this population,” Dr. Graham said. Therefore, patients “could really stand to benefit” from a combination that delivers the same amount of antipsychotic effect, but “reduces the propensity” for clinically significant weight gain, she added.
Dr. Kahn noted in his meeting presentation that antipsychotics are the “cornerstone” of the treatment of serious mental illness, but that “many are associated with concerning weight gain and cardiometabolic effects.”
While olanzapine is an effective medication, it has “one of the highest weight gain” profiles of the available antipsychotics and patients early on in their illness are “especially vulnerable,” Dr. Kahn said.
Previous studies have shown the combination of olanzapine plus samidorphan is similarly effective as olanzapine, but is associated with less weight gain.
To determine its impact in recent-onset illness, the current researchers screened patients with schizophrenia, schizophreniform disorder, or bipolar I disorder. The patients were aged 16-39 years and had an initial onset of active phase symptoms less than 4 years previously. They had less than 24 weeks’ cumulative lifetime exposure to antipsychotics.
Participants were randomly assigned to receive olanzapine plus samidorphan or olanzapine alone for 12 weeks, and then followed up for safety assessment for a further 4 weeks.
A total of 426 patients were recruited and 76.5% completed the study. The mean age was 25.8 years, 66.2% were men, 66.4% were White, and 28.2% were Black.
The mean body mass index at baseline was 23.69 kg/m2. The most common diagnosis among the participants was schizophrenia (62.9%) followed by bipolar I disorder (21.6%).
Less weight gain
Results of the 12-week study showed a significant difference in percent change in body weight from baseline between the two treatment groups, with a gain of 4.91% for the olanzapine plus samidorphan group vs. 6.77% for the olanzapine-alone group (between-group difference, 1.87%; P = .012).
Dr. Kahn noted this equates to an average weight gain of 2.8 kg (6.2 pounds) with olanzapine plus samidorphan and a gain of about 5 kg (11pounds) with olanzapine.
“It’s not a huge difference, but it’s certainly a significant one,” he said. “I also think it’s clinically important and significant.”
The reduction in weight gain compared with olanzapine was even maintained in patients assigned to olanzapine plus samidorphan who dropped out and did not complete the study, Dr. Kahn reported. “No one really had a weight gain,” he said.
In contrast, patients in the olanzapine groups who dropped out of the study had weight gain larger than their counterparts who stayed in it.
Further analysis showed the proportion of patients who gained 10% or more of their body weight by week 12 was 21.9% for those receiving olanzapine plus samidorphan vs. 30.4% for those receiving just olanzapine (odds ratio, 0.64; P = .075).
As expected, the improvement in Clinical Global Impression–Severity scale scores was almost identical between the olanzapine + samidorphan and olanzapine-only groups.
For safety, Dr. Kahn said the adverse event rates were “very, very similar” between the two treatment arms, which was a pattern that was repeated for serious AEs. This led him to note that “nothing out of the ordinary” was observed.
Clinical impact 'questionable'
Commenting on the study, Laura LaChance, MD, a psychiatrist at St. Mary’s Hospital Centre, McGill University, Montreal, said the actual amount of weight loss shown in the study “is of questionable clinical significance.”
On the other hand, Dr. LaChance said she has achieved “better results with metformin, which has a great safety profile and is cheap and widely available.
“Cost is always a concern in patients with psychotic disorders,” she concluded.
The study was funded by Alkermes. Dr. Kahn reported having relationships with Alkermes, Angelini, Janssen, Sunovion, Otsuka, Merck, Minerva Neuroscience, Roche, and Teva. Dr. Graham is an employee of Alkermes.
A version of this article first appeared on Medscape.com.
FROM SIRS 2022
FDA okays first sublingual med for agitation in serious mental illness
This is the first FDA-approved, orally dissolving, self-administered sublingual treatment for this indication. With a demonstrated onset of action as early as 20 minutes, it shows a high response rate in patients at both 120-mcg and 180-mcg doses.
An estimated 7.3 million individuals in the United States are diagnosed with schizophrenia or bipolar disorders, and up to one-quarter of them experience episodes of agitation that can occur 10-17 times annually. These episodes represent a significant burden for patients, caregivers, and the health care system.
“There are large numbers of patients who experience agitation associated with schizophrenia and bipolar disorders, and this condition has been a long-standing challenge for health care professionals to treat,” said John Krystal, MD, the Robert L. McNeil Jr. Professor of Translational Research and chair of the department of psychiatry at Yale University, New Haven, Conn.
“The approval of Igalmi, a self-administered film with a desirable onset of action, represents a milestone moment. It provides health care teams with an innovative tool to help control agitation. As clinicians, we welcome this much-needed new oral treatment option,” he added.
“Igalmi is the first new acute treatment for schizophrenia or bipolar disorder–associated agitation in nearly a decade and represents a differentiated approach to helping patients manage this difficult and debilitating symptom,” said Vimal Mehta, PhD, CEO of BioXcel Therapeutics.
The FDA approval of Igalmi is based on data from two pivotal randomized, double-blinded, placebo-controlled, parallel-group, phase 3 trials that evaluated Igalmi for the acute treatment of agitation associated with schizophrenia (SERENITY I) or bipolar I or II disorder (SERENITY II).
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were somnolence, paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. All adverse drug reactions were mild to moderate in severity. While Igalmi was not associated with any treatment-related serious adverse effects in phase 3 studies, it may cause notable side effects, including hypotension, orthostatic hypotension, bradycardia, QT interval prolongation, and somnolence.
As previously reported by this news organization, data from the phase 3 SERENITY II trial that evaluated Igalmi in bipolar disorders were published in JAMA.
A version of this article first appeared on Medscape.com.
This is the first FDA-approved, orally dissolving, self-administered sublingual treatment for this indication. With a demonstrated onset of action as early as 20 minutes, it shows a high response rate in patients at both 120-mcg and 180-mcg doses.
An estimated 7.3 million individuals in the United States are diagnosed with schizophrenia or bipolar disorders, and up to one-quarter of them experience episodes of agitation that can occur 10-17 times annually. These episodes represent a significant burden for patients, caregivers, and the health care system.
“There are large numbers of patients who experience agitation associated with schizophrenia and bipolar disorders, and this condition has been a long-standing challenge for health care professionals to treat,” said John Krystal, MD, the Robert L. McNeil Jr. Professor of Translational Research and chair of the department of psychiatry at Yale University, New Haven, Conn.
“The approval of Igalmi, a self-administered film with a desirable onset of action, represents a milestone moment. It provides health care teams with an innovative tool to help control agitation. As clinicians, we welcome this much-needed new oral treatment option,” he added.
“Igalmi is the first new acute treatment for schizophrenia or bipolar disorder–associated agitation in nearly a decade and represents a differentiated approach to helping patients manage this difficult and debilitating symptom,” said Vimal Mehta, PhD, CEO of BioXcel Therapeutics.
The FDA approval of Igalmi is based on data from two pivotal randomized, double-blinded, placebo-controlled, parallel-group, phase 3 trials that evaluated Igalmi for the acute treatment of agitation associated with schizophrenia (SERENITY I) or bipolar I or II disorder (SERENITY II).
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were somnolence, paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. All adverse drug reactions were mild to moderate in severity. While Igalmi was not associated with any treatment-related serious adverse effects in phase 3 studies, it may cause notable side effects, including hypotension, orthostatic hypotension, bradycardia, QT interval prolongation, and somnolence.
As previously reported by this news organization, data from the phase 3 SERENITY II trial that evaluated Igalmi in bipolar disorders were published in JAMA.
A version of this article first appeared on Medscape.com.
This is the first FDA-approved, orally dissolving, self-administered sublingual treatment for this indication. With a demonstrated onset of action as early as 20 minutes, it shows a high response rate in patients at both 120-mcg and 180-mcg doses.
An estimated 7.3 million individuals in the United States are diagnosed with schizophrenia or bipolar disorders, and up to one-quarter of them experience episodes of agitation that can occur 10-17 times annually. These episodes represent a significant burden for patients, caregivers, and the health care system.
“There are large numbers of patients who experience agitation associated with schizophrenia and bipolar disorders, and this condition has been a long-standing challenge for health care professionals to treat,” said John Krystal, MD, the Robert L. McNeil Jr. Professor of Translational Research and chair of the department of psychiatry at Yale University, New Haven, Conn.
“The approval of Igalmi, a self-administered film with a desirable onset of action, represents a milestone moment. It provides health care teams with an innovative tool to help control agitation. As clinicians, we welcome this much-needed new oral treatment option,” he added.
“Igalmi is the first new acute treatment for schizophrenia or bipolar disorder–associated agitation in nearly a decade and represents a differentiated approach to helping patients manage this difficult and debilitating symptom,” said Vimal Mehta, PhD, CEO of BioXcel Therapeutics.
The FDA approval of Igalmi is based on data from two pivotal randomized, double-blinded, placebo-controlled, parallel-group, phase 3 trials that evaluated Igalmi for the acute treatment of agitation associated with schizophrenia (SERENITY I) or bipolar I or II disorder (SERENITY II).
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were somnolence, paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. All adverse drug reactions were mild to moderate in severity. While Igalmi was not associated with any treatment-related serious adverse effects in phase 3 studies, it may cause notable side effects, including hypotension, orthostatic hypotension, bradycardia, QT interval prolongation, and somnolence.
As previously reported by this news organization, data from the phase 3 SERENITY II trial that evaluated Igalmi in bipolar disorders were published in JAMA.
A version of this article first appeared on Medscape.com.
The importance of treating insomnia in psychiatric illness
Data suggests this symptom, defined as chronic sleep onset and/or sleep continuity problems associated with impaired daytime functioning, is common in psychiatric illnesses, and can worsen their course.2
The incidence of psychiatric illness in patients with insomnia is estimated at near 50%, with the highest rates found in mood disorders such as depression and bipolar disorder, as well as anxiety disorders.3 In patients with diagnosed major depressive disorder, insomnia rates can approach 90%.4-6
Insomnia has been identified as a risk factor for development of mental illness, including doubling the risk of major depressive disorder and tripling the risk of any depressive or anxiety disorder.7,8 It can also significantly increase the risk of alcohol abuse and psychosis.8
Sleep disturbances can worsen symptoms of diagnosed mental illness, including substance abuse, mood and psychotic disorders.9-10 In one study, nearly 75% of patients with a diagnosis of schizophrenia or bipolar spectrum disorder had at least one type of sleep disturbance (insomnia, hypersomnia, or delayed sleep phase).10 This was almost twice the rate in healthy controls. Importantly, compared with well-rested subjects with mental illness in this study, sleep-disordered participants had higher rates of negative and depressive symptoms on the Positive and Negative Syndrome Scale, as well as significantly lower function via the global assessment of functioning.11,12
Additional data suggests simply being awake during the night (00:00-05:59) elevates risk of suicide. The mean incident rate of completed suicide in one study was a striking four times the rate noted during daytime hours (06:00-23:59 ) (P < .001).13
Although insomnia symptoms can resolve after relief from a particular life stressor, as many as half of patients with more severe symptoms develop a chronic course.14 This then leads to an extended use of many types of sedative-hypnotics designed and studied primarily for short-term use.15 In a survey reviewing national use of prescription drugs for insomnia, as many as 20% of individuals use a medication to target insomnia in a given month.16
Fortunately, despite the many challenges posed by COVID-19, particularly for those with psychiatric illness and limited access to care, telehealth has become more readily available. Additionally, digital versions of evidence-based treatments specifically for sleep problems, such as cognitive-behavioral therapy for insomnia (CBT-I), are regularly being developed.
The benefits of CBT-I have been demonstrated repeatedly and it is recommended as the first line treatment for insomnia by the Clinical Guidelines of the American Academy of Sleep Medicine, the Centers for Disease Control and Prevention, and the National Institutes of Health.17-21 Studies suggest benefits persist long-term, even after completing the therapy sessions, which differ in durability from medication choices.18
One group that may be particularly suited for treatment with CBT-I is women with insomnia during pregnancy or the postpartum period. In these women, options for treatment may be limited by risk of medication during breastfeeding, as well as difficulty traveling to a physician’s or therapist’s office to receive psychotherapy. However, two recent studies evaluated the use of digital CBT-I to treat insomnia during pregnancy and in the postpartum period, respectively.22-23
In both studies,the same group of women with insomnia diagnosed during pregnancy were given six weekly 20-minute sessions of digital CBT-I or standard treatment for insomnia, including medication and psychotherapy per their usual provider.
By study end, the pregnant women receiving the CBT-I intervention not only had significantly improved severity of insomnia, they also experienced improved depression and anxiety symptoms, and a decrease in the use of prescription or over-the-counter sleep aides, compared with the standard treatment group, lowering the fetal exposure to medication during pregnancy.22
In the more recent study, the same group was followed for 6 months post partum.23 Results were again notable, with the women who received CBT-I reporting significantly less insomnia, as well as significantly lower rates of probable major depression at 3 and 6 months (18% vs. 4%, 10% vs. 0%, respectively.) They also exhibited lower rates of moderate to severe anxiety (17% vs. 4%) at 3 months, compared with those receiving standard care. With as many as one in seven women suffering from postpartum depression, these findings represent a substantial public health benefit.
In summary, insomnia is a critical area of focus for any provider diagnosing and treating psychiatric illness. Attempts to optimize sleep, whether through CBT-I or other psychotherapy approaches, or evidence-based medications dosed for appropriate lengths and at safe doses, should be a part of most, if not all, clinical encounters.
Dr. Reid is a board-certified psychiatrist and award-winning medical educator with a private practice in Philadelphia, as well as a clinical faculty role at the University of Pennsylvania, also in Philadelphia. She attended medical school at Columbia University, New York, and completed her psychiatry residency at the University of California, Los Angeles. Dr. Reid is a regular contributor to Psychology Today with her blog, “Think Like a Shrink,” and writes and podcasts as The Reflective Doc.
References
1. Voitsidis P et al. Psychiatry Res. 2020 Jul;289:113076. doi: 10.1016/j.psychres.2020.113076.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, Va.: American Psychiatric Publishing, 2013.
3. Ford DE and Kamerow DB. JAMA. 1989;262(11):1479-84. doi: 10.1001/jama.1989.03430110069030.
4. Ohayon MM and Roth T. J Psychiatr Res. Jan-Feb 2003;37(1):9-15. doi: 10.1016/s0022-3956(02)00052-3.
5. Seow LSE et al. J Ment Health. 2016 Dec;25(6):492-9. doi: 10.3109/09638237.2015.1124390.
6. Thase ME. J Clin Psychiatry. 1999;60 Suppl 17:28-31; discussion 46-8.
7. Baglioni C et al. J Affect Disord. 2011 Dec;135(1-3):10-9. doi: 10.1016/j.jad.2011.01.011.
8. Hertenstein E et al. Sleep Med Rev. 2019 Feb;43:96-105. doi: 10.1016/j.smrv.2018.10.006.
9. Brower KJ et al. Medical Hypotheses. 2010;74(5):928-33. doi: 10.1016/j.mehy.2009.10.020.
10. Laskemoen JF et al. Compr Psychiatry. 2019 May;91:6-12. doi: 10.1016/j.comppsych.2019.02.006.
11. Kay SR et al. Schizophr Bull. 1987;13(2):261-76. doi: 10.1093/schbul/13.2.261.
12. Hall R. Psychosomatics. May-Jun 1995;36(3):267-75. doi: 10.1016/S0033-3182(95)71666-8.
13. Perlis ML et al. J Clin Psychiatry. 2016 Jun;77(6):e726-33. doi: 10.4088/JCP.15m10131.
14. Morin CM et al. Arch Intern Med. 2009 Mar 9. doi: 10.1001/archinternmed.2008.610.
15. Cheung J et al. Sleep Med Clin. 2019 Jun;14(2):253-65. doi: 10.1016/j.jsmc.2019.01.006.
16. Bertisch SM et al. Sleep. 2014 Feb 1. doi: 10.5665/sleep.3410.
17. Okajima I et al. Sleep Biol Rhythms. 2010 Nov 28. doi: 10.1111/j.1479-8425.2010.00481.x.
18. Trauer JM et al. Ann Intern Med. 2015 Aug 4. doi: 10.7326/M14-2841.
19. Edinger J et al. J Clin Sleep Med. 2021 Feb 1. doi: 10.5664/jcsm.8986.
20. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/sleep/for-clinicians.html.
21. National Institutes of Health. Sleep Health. https://www.nhlbi.nih.gov/health-topics/education-and-awareness/sleep-health.
22. Felder JN et al. JAMA Psychiatry. 2020;77(5):484-92. doi:10.1001/jamapsychiatry.2019.4491.
23. Felder JN et al. Sleep. 2022 Feb 14. doi: 10.1093/sleep/zsab280.
Data suggests this symptom, defined as chronic sleep onset and/or sleep continuity problems associated with impaired daytime functioning, is common in psychiatric illnesses, and can worsen their course.2
The incidence of psychiatric illness in patients with insomnia is estimated at near 50%, with the highest rates found in mood disorders such as depression and bipolar disorder, as well as anxiety disorders.3 In patients with diagnosed major depressive disorder, insomnia rates can approach 90%.4-6
Insomnia has been identified as a risk factor for development of mental illness, including doubling the risk of major depressive disorder and tripling the risk of any depressive or anxiety disorder.7,8 It can also significantly increase the risk of alcohol abuse and psychosis.8
Sleep disturbances can worsen symptoms of diagnosed mental illness, including substance abuse, mood and psychotic disorders.9-10 In one study, nearly 75% of patients with a diagnosis of schizophrenia or bipolar spectrum disorder had at least one type of sleep disturbance (insomnia, hypersomnia, or delayed sleep phase).10 This was almost twice the rate in healthy controls. Importantly, compared with well-rested subjects with mental illness in this study, sleep-disordered participants had higher rates of negative and depressive symptoms on the Positive and Negative Syndrome Scale, as well as significantly lower function via the global assessment of functioning.11,12
Additional data suggests simply being awake during the night (00:00-05:59) elevates risk of suicide. The mean incident rate of completed suicide in one study was a striking four times the rate noted during daytime hours (06:00-23:59 ) (P < .001).13
Although insomnia symptoms can resolve after relief from a particular life stressor, as many as half of patients with more severe symptoms develop a chronic course.14 This then leads to an extended use of many types of sedative-hypnotics designed and studied primarily for short-term use.15 In a survey reviewing national use of prescription drugs for insomnia, as many as 20% of individuals use a medication to target insomnia in a given month.16
Fortunately, despite the many challenges posed by COVID-19, particularly for those with psychiatric illness and limited access to care, telehealth has become more readily available. Additionally, digital versions of evidence-based treatments specifically for sleep problems, such as cognitive-behavioral therapy for insomnia (CBT-I), are regularly being developed.
The benefits of CBT-I have been demonstrated repeatedly and it is recommended as the first line treatment for insomnia by the Clinical Guidelines of the American Academy of Sleep Medicine, the Centers for Disease Control and Prevention, and the National Institutes of Health.17-21 Studies suggest benefits persist long-term, even after completing the therapy sessions, which differ in durability from medication choices.18
One group that may be particularly suited for treatment with CBT-I is women with insomnia during pregnancy or the postpartum period. In these women, options for treatment may be limited by risk of medication during breastfeeding, as well as difficulty traveling to a physician’s or therapist’s office to receive psychotherapy. However, two recent studies evaluated the use of digital CBT-I to treat insomnia during pregnancy and in the postpartum period, respectively.22-23
In both studies,the same group of women with insomnia diagnosed during pregnancy were given six weekly 20-minute sessions of digital CBT-I or standard treatment for insomnia, including medication and psychotherapy per their usual provider.
By study end, the pregnant women receiving the CBT-I intervention not only had significantly improved severity of insomnia, they also experienced improved depression and anxiety symptoms, and a decrease in the use of prescription or over-the-counter sleep aides, compared with the standard treatment group, lowering the fetal exposure to medication during pregnancy.22
In the more recent study, the same group was followed for 6 months post partum.23 Results were again notable, with the women who received CBT-I reporting significantly less insomnia, as well as significantly lower rates of probable major depression at 3 and 6 months (18% vs. 4%, 10% vs. 0%, respectively.) They also exhibited lower rates of moderate to severe anxiety (17% vs. 4%) at 3 months, compared with those receiving standard care. With as many as one in seven women suffering from postpartum depression, these findings represent a substantial public health benefit.
In summary, insomnia is a critical area of focus for any provider diagnosing and treating psychiatric illness. Attempts to optimize sleep, whether through CBT-I or other psychotherapy approaches, or evidence-based medications dosed for appropriate lengths and at safe doses, should be a part of most, if not all, clinical encounters.
Dr. Reid is a board-certified psychiatrist and award-winning medical educator with a private practice in Philadelphia, as well as a clinical faculty role at the University of Pennsylvania, also in Philadelphia. She attended medical school at Columbia University, New York, and completed her psychiatry residency at the University of California, Los Angeles. Dr. Reid is a regular contributor to Psychology Today with her blog, “Think Like a Shrink,” and writes and podcasts as The Reflective Doc.
References
1. Voitsidis P et al. Psychiatry Res. 2020 Jul;289:113076. doi: 10.1016/j.psychres.2020.113076.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, Va.: American Psychiatric Publishing, 2013.
3. Ford DE and Kamerow DB. JAMA. 1989;262(11):1479-84. doi: 10.1001/jama.1989.03430110069030.
4. Ohayon MM and Roth T. J Psychiatr Res. Jan-Feb 2003;37(1):9-15. doi: 10.1016/s0022-3956(02)00052-3.
5. Seow LSE et al. J Ment Health. 2016 Dec;25(6):492-9. doi: 10.3109/09638237.2015.1124390.
6. Thase ME. J Clin Psychiatry. 1999;60 Suppl 17:28-31; discussion 46-8.
7. Baglioni C et al. J Affect Disord. 2011 Dec;135(1-3):10-9. doi: 10.1016/j.jad.2011.01.011.
8. Hertenstein E et al. Sleep Med Rev. 2019 Feb;43:96-105. doi: 10.1016/j.smrv.2018.10.006.
9. Brower KJ et al. Medical Hypotheses. 2010;74(5):928-33. doi: 10.1016/j.mehy.2009.10.020.
10. Laskemoen JF et al. Compr Psychiatry. 2019 May;91:6-12. doi: 10.1016/j.comppsych.2019.02.006.
11. Kay SR et al. Schizophr Bull. 1987;13(2):261-76. doi: 10.1093/schbul/13.2.261.
12. Hall R. Psychosomatics. May-Jun 1995;36(3):267-75. doi: 10.1016/S0033-3182(95)71666-8.
13. Perlis ML et al. J Clin Psychiatry. 2016 Jun;77(6):e726-33. doi: 10.4088/JCP.15m10131.
14. Morin CM et al. Arch Intern Med. 2009 Mar 9. doi: 10.1001/archinternmed.2008.610.
15. Cheung J et al. Sleep Med Clin. 2019 Jun;14(2):253-65. doi: 10.1016/j.jsmc.2019.01.006.
16. Bertisch SM et al. Sleep. 2014 Feb 1. doi: 10.5665/sleep.3410.
17. Okajima I et al. Sleep Biol Rhythms. 2010 Nov 28. doi: 10.1111/j.1479-8425.2010.00481.x.
18. Trauer JM et al. Ann Intern Med. 2015 Aug 4. doi: 10.7326/M14-2841.
19. Edinger J et al. J Clin Sleep Med. 2021 Feb 1. doi: 10.5664/jcsm.8986.
20. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/sleep/for-clinicians.html.
21. National Institutes of Health. Sleep Health. https://www.nhlbi.nih.gov/health-topics/education-and-awareness/sleep-health.
22. Felder JN et al. JAMA Psychiatry. 2020;77(5):484-92. doi:10.1001/jamapsychiatry.2019.4491.
23. Felder JN et al. Sleep. 2022 Feb 14. doi: 10.1093/sleep/zsab280.
Data suggests this symptom, defined as chronic sleep onset and/or sleep continuity problems associated with impaired daytime functioning, is common in psychiatric illnesses, and can worsen their course.2
The incidence of psychiatric illness in patients with insomnia is estimated at near 50%, with the highest rates found in mood disorders such as depression and bipolar disorder, as well as anxiety disorders.3 In patients with diagnosed major depressive disorder, insomnia rates can approach 90%.4-6
Insomnia has been identified as a risk factor for development of mental illness, including doubling the risk of major depressive disorder and tripling the risk of any depressive or anxiety disorder.7,8 It can also significantly increase the risk of alcohol abuse and psychosis.8
Sleep disturbances can worsen symptoms of diagnosed mental illness, including substance abuse, mood and psychotic disorders.9-10 In one study, nearly 75% of patients with a diagnosis of schizophrenia or bipolar spectrum disorder had at least one type of sleep disturbance (insomnia, hypersomnia, or delayed sleep phase).10 This was almost twice the rate in healthy controls. Importantly, compared with well-rested subjects with mental illness in this study, sleep-disordered participants had higher rates of negative and depressive symptoms on the Positive and Negative Syndrome Scale, as well as significantly lower function via the global assessment of functioning.11,12
Additional data suggests simply being awake during the night (00:00-05:59) elevates risk of suicide. The mean incident rate of completed suicide in one study was a striking four times the rate noted during daytime hours (06:00-23:59 ) (P < .001).13
Although insomnia symptoms can resolve after relief from a particular life stressor, as many as half of patients with more severe symptoms develop a chronic course.14 This then leads to an extended use of many types of sedative-hypnotics designed and studied primarily for short-term use.15 In a survey reviewing national use of prescription drugs for insomnia, as many as 20% of individuals use a medication to target insomnia in a given month.16
Fortunately, despite the many challenges posed by COVID-19, particularly for those with psychiatric illness and limited access to care, telehealth has become more readily available. Additionally, digital versions of evidence-based treatments specifically for sleep problems, such as cognitive-behavioral therapy for insomnia (CBT-I), are regularly being developed.
The benefits of CBT-I have been demonstrated repeatedly and it is recommended as the first line treatment for insomnia by the Clinical Guidelines of the American Academy of Sleep Medicine, the Centers for Disease Control and Prevention, and the National Institutes of Health.17-21 Studies suggest benefits persist long-term, even after completing the therapy sessions, which differ in durability from medication choices.18
One group that may be particularly suited for treatment with CBT-I is women with insomnia during pregnancy or the postpartum period. In these women, options for treatment may be limited by risk of medication during breastfeeding, as well as difficulty traveling to a physician’s or therapist’s office to receive psychotherapy. However, two recent studies evaluated the use of digital CBT-I to treat insomnia during pregnancy and in the postpartum period, respectively.22-23
In both studies,the same group of women with insomnia diagnosed during pregnancy were given six weekly 20-minute sessions of digital CBT-I or standard treatment for insomnia, including medication and psychotherapy per their usual provider.
By study end, the pregnant women receiving the CBT-I intervention not only had significantly improved severity of insomnia, they also experienced improved depression and anxiety symptoms, and a decrease in the use of prescription or over-the-counter sleep aides, compared with the standard treatment group, lowering the fetal exposure to medication during pregnancy.22
In the more recent study, the same group was followed for 6 months post partum.23 Results were again notable, with the women who received CBT-I reporting significantly less insomnia, as well as significantly lower rates of probable major depression at 3 and 6 months (18% vs. 4%, 10% vs. 0%, respectively.) They also exhibited lower rates of moderate to severe anxiety (17% vs. 4%) at 3 months, compared with those receiving standard care. With as many as one in seven women suffering from postpartum depression, these findings represent a substantial public health benefit.
In summary, insomnia is a critical area of focus for any provider diagnosing and treating psychiatric illness. Attempts to optimize sleep, whether through CBT-I or other psychotherapy approaches, or evidence-based medications dosed for appropriate lengths and at safe doses, should be a part of most, if not all, clinical encounters.
Dr. Reid is a board-certified psychiatrist and award-winning medical educator with a private practice in Philadelphia, as well as a clinical faculty role at the University of Pennsylvania, also in Philadelphia. She attended medical school at Columbia University, New York, and completed her psychiatry residency at the University of California, Los Angeles. Dr. Reid is a regular contributor to Psychology Today with her blog, “Think Like a Shrink,” and writes and podcasts as The Reflective Doc.
References
1. Voitsidis P et al. Psychiatry Res. 2020 Jul;289:113076. doi: 10.1016/j.psychres.2020.113076.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, Va.: American Psychiatric Publishing, 2013.
3. Ford DE and Kamerow DB. JAMA. 1989;262(11):1479-84. doi: 10.1001/jama.1989.03430110069030.
4. Ohayon MM and Roth T. J Psychiatr Res. Jan-Feb 2003;37(1):9-15. doi: 10.1016/s0022-3956(02)00052-3.
5. Seow LSE et al. J Ment Health. 2016 Dec;25(6):492-9. doi: 10.3109/09638237.2015.1124390.
6. Thase ME. J Clin Psychiatry. 1999;60 Suppl 17:28-31; discussion 46-8.
7. Baglioni C et al. J Affect Disord. 2011 Dec;135(1-3):10-9. doi: 10.1016/j.jad.2011.01.011.
8. Hertenstein E et al. Sleep Med Rev. 2019 Feb;43:96-105. doi: 10.1016/j.smrv.2018.10.006.
9. Brower KJ et al. Medical Hypotheses. 2010;74(5):928-33. doi: 10.1016/j.mehy.2009.10.020.
10. Laskemoen JF et al. Compr Psychiatry. 2019 May;91:6-12. doi: 10.1016/j.comppsych.2019.02.006.
11. Kay SR et al. Schizophr Bull. 1987;13(2):261-76. doi: 10.1093/schbul/13.2.261.
12. Hall R. Psychosomatics. May-Jun 1995;36(3):267-75. doi: 10.1016/S0033-3182(95)71666-8.
13. Perlis ML et al. J Clin Psychiatry. 2016 Jun;77(6):e726-33. doi: 10.4088/JCP.15m10131.
14. Morin CM et al. Arch Intern Med. 2009 Mar 9. doi: 10.1001/archinternmed.2008.610.
15. Cheung J et al. Sleep Med Clin. 2019 Jun;14(2):253-65. doi: 10.1016/j.jsmc.2019.01.006.
16. Bertisch SM et al. Sleep. 2014 Feb 1. doi: 10.5665/sleep.3410.
17. Okajima I et al. Sleep Biol Rhythms. 2010 Nov 28. doi: 10.1111/j.1479-8425.2010.00481.x.
18. Trauer JM et al. Ann Intern Med. 2015 Aug 4. doi: 10.7326/M14-2841.
19. Edinger J et al. J Clin Sleep Med. 2021 Feb 1. doi: 10.5664/jcsm.8986.
20. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/sleep/for-clinicians.html.
21. National Institutes of Health. Sleep Health. https://www.nhlbi.nih.gov/health-topics/education-and-awareness/sleep-health.
22. Felder JN et al. JAMA Psychiatry. 2020;77(5):484-92. doi:10.1001/jamapsychiatry.2019.4491.
23. Felder JN et al. Sleep. 2022 Feb 14. doi: 10.1093/sleep/zsab280.
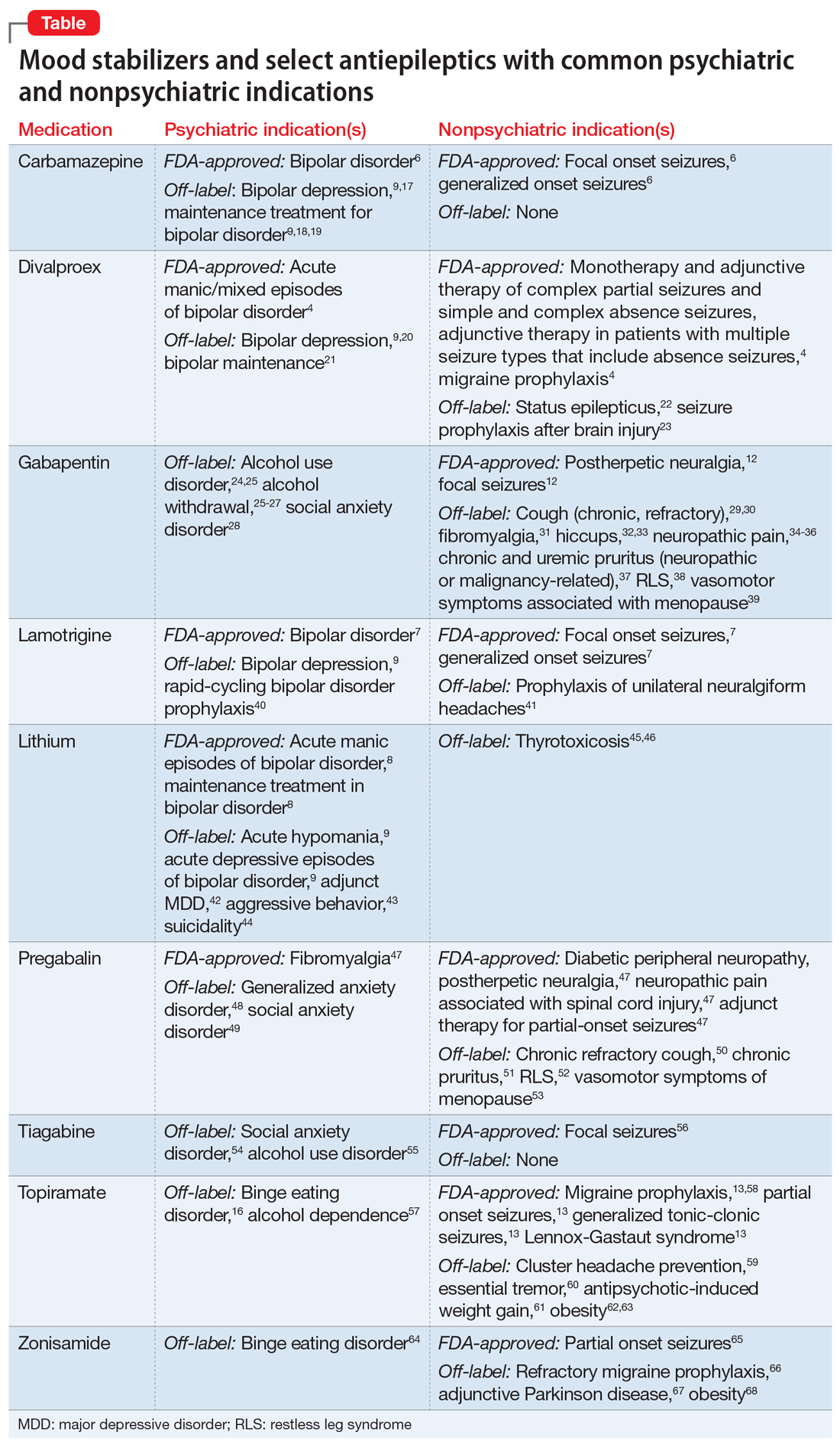
Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics
Mr. B, age 64, is being treated in the psychiatric clinic for generalized anxiety disorder. He also has a history of type 2 diabetes mellitus and osteoarthritis. His present medications include metformin 500 mg twice daily, escitalopram 20 mg/d, and a multivitamin.
Three months after a shingles outbreak on his left trunk, Mr. B develops a sharp, burning pain and hypersensitivity to light in the same area as the shingles flare-up. He is diagnosed with postherpetic neuralgia. Despite a 12-week trial of cognitive-behavioral therapy, Mr. B continues to report excessive worry, irritability, poor concentration, psychomotor restlessness, and poor sleep.
Contrasting with the serendipitous discovery of iproniazid and chlorpromazine leading to the development of the current spectrum of antidepressant and antipsychotic agents, discovery of the benefits various antiepileptic agents have in bipolar disorder has not led to a similar proliferation of medication development for bipolar mania or depression.1-3 Divalproex, one of the most commonly used antiepileptic drugs (AEDs) in psychiatry, was thought to be an inactive organic solvent until it was used in 1962 to test the anticonvulsant activity of other compounds. This led to the discovery and subsequent use of divalproex in patients with epilepsy, followed by FDA approval in bipolar disorder.4,5 Off-label use of many AEDs as mood-stabilizing agents in bipolar disorder led to the emergence of carbamazepine, divalproex, and lamotrigine, which joined lithium as classic mood-stabilizing agents.4,6-8 Amid varying definitions of “mood stabilizer,” many AEDs have failed to demonstrate mood-stabilizing effects in bipolar disorder and therefore should not all be considered mood stabilizers.9 Nonetheless, the dual use of a single AED for both psychiatric and nonpsychiatric indications can decrease polypharmacy and increase acceptability of medications in patients who have low insight into their illness.10,11
Because AEDs were originally purposed to treat neurologic disease, psychiatric indications must first be established before considering other indications. AEDs as a class have broad pharmacologic actions, but are generally CNS depressants, decreasing brain signaling through mechanisms such as ion channel antagonism (carbamazepine, gabapentin) or alterations to gamma-aminobutyric acid/glutamate signaling (divalproex, topiramate).4,6,12,13 Compared to antidepressants and antipsychotics, whose primary use for psychiatric conditions is firmly rooted in evidence, rational use of AEDs for psychiatric conditions and symptoms depends on the agent-specific efficacy. Patients with comorbid psychiatric and neurologic disorders are ideal candidates for dually indicated AEDs due to these agents’ class effects rooted in epilepsy. Due to the history of positive psychiatric benefits with AEDs, newer agents may be psychiatrically beneficial but will likely follow the discovery of these benefits in patients for whom epilepsy is the primary diagnosis.
Consider the limitations
Using AEDs to reduce polypharmacy should be done judiciously from a drug-drug interaction perspective, because certain AEDs (eg, carbamazepine, divalproex) can greatly influence the metabolism of other medications, which may defeat the best intentions of the original intervention.4,6
Several other limitations should be considered. This article does not include all AEDs, only those commonly used for psychiatric indications with known nonpsychiatric benefits. Some may worsen psychiatric conditions (such as rage and irritability in the case of levetiracetam), and all AEDs have an FDA warning regarding suicidal behaviors and ideation.14,15 Another important limitation is the potential for differential dosing across indications; tolerability concerns may limit adequate dosing across multiple uses. For example, topiramate’s migraine prophylaxis effect can be achieved at much lower doses than the patient-specific efficacy dosing seen in binge eating disorder, with higher doses increasing the propensity for adverse effects.13,16Dual-use AEDs should be considered wherever possible, but judicious review of evidence is necessary to appropriately adjudicate a specific patient’s risk vs benefit. The Table4,6-9,12,13,16-68 provides information on select AEDs with both psychiatric and nonpsychiatric indications, including both FDA-approved and common off-label uses. These indications are limited to adult use only.
CASE CONTINUED
After reviewing Mr. B’s medical history, the treating medical team decides to cross-taper escitalopram to duloxetine 30 mg twice daily. Though his pain lessens after several weeks, it persists enough to interfere with Mr. B’s daily life. In addition to duloxetine, he is started on pregabalin 50 mg 3 times a day. Mr. B’s pain decreases to a tolerable level, and he reports decreased worrying and restlessness, and improvements in concentration and sleep.
1. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. López-Mun
4. Depakote [package insert]. North Chicago, IL: AbbVie, Inc; 2021.
5. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16.
6. Tegretol and Tegretol-XR [package insert]. East Hanover, NJ: Pharmaceuticals Co.; 2020.
7. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
8. Lithobid [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc; 2009.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. National Alliance on Mental Illness. Anosognosia. Common with mental illness. Accessed March 3, 2022. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
11. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. NCHS Data Brief. 2019(347):1-8.
12. Neurontin [package insert]. New York, NY: Pfizer; 2017.
13. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
14. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81.
15. U.S. Food & Drug Administration. FDA Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality. 2008. Accessed March 3, 2022. https://www.fda.gov/files/drugs/published/Statistical-Review-and-Evaluation--Antiepileptic-Drugs-and-Suicidality.pdf
16. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
17. Zhang ZJ, Kang WH, Tan QR, et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: a double-blind, randomized, placebo-controlled study. J Psychiatr Res. 2007;41(3-4):360-369.
18. Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42 Suppl 1:2-10.
19. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
20. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85(3):259-266.
21. Gyulai L, Bowden CL, McElroy SL, et al. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology. 2003;28(7):1374-1382.
22. Limdi NA, Shimpi AV, Faught E, et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353-355.
23. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999; 91(4):593-600.
24. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90.
25. US Dept of Veterans Affairs, US Dept of Defense, The Management of Substance Use Disorders Work Group. VA/DoD clinical practice guideline for the management of substance use disorders. US Dept of Veterans Affairs/Dept of Defense; 2015. Accessed March 3, 2022. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
26. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
27. Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
28. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348.
29. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589.
30. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44.
31. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344.
32. Alonso-Navarro H, Rubio L, Jiménez-Jiménez FJ. Refractory hiccup: successful treatment with gabapentin. Clin Neuropharmacol. 2007;30(3):186-187.
33. Jatzko A, Stegmeier-Petroianu A, Petroianu GA. Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J Pain Symptom Manage. 2007;33(6):756-760.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
35. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938.
36. Yuan M, Zhou HY, Xiao ZL, et al. Efficacy and safety of gabapentin vs. carbamazepine in the treatment of trigeminal neuralgia: a meta-analysis. Pain Pract. 2016;16(8):1083-1091.
37. Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563-581.
38. Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-Foundation. Sleep Med. 2016;21:1-11.
39. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 update [published correction appears in Endocr Pract. 2017;23 (12):1488]. Endocr Pract. 2017;23(7):869-880.
40. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder: Lamictal 614 Study Group. J Clin Psychiatry. 2000;60(11):841-850.
41. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066-1077.
42. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640.
43. Craft M, Ismail IA, Krishnamurti D, et al. Lithium in the treatment of aggression in mentally handicapped patients: a double-blind trial. Br J Psychiatry. 1987;150:685-689.
44. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
45. Dickstein G, Shechner C, Adawi F, et al. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454-458.
46. Bogazzi F, Bartalena L, Brogioni S, et al. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84(2):499-503.
47. Lyrica [package insert]. New York, NY: Parke-Davis, Division of Pfizer Inc; 2020.
48. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241.
49. Pande AC, Feltner DE, Jefferson JW, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol. 2004;24(2):141-149.
50. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648.
51. Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619-625.e6.
52. Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
53. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1 [published correction appears in J Clin Oncol. 2010;28(10):1808]. J Clin Oncol. 2010;28(4):641-647.
54. Dunlop BW, Papp L, Garlow SJ, et al. Tiagabine for social anxiety disorder. Hum Psychopharmacol. 2007;22(4):241-244.
55. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375-1380.
56. Gabitril [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2015.
57. Johnson BA, Ait-Daoud N, Bowden C, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685.
58. Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010610.
59. Pascual J, Láinez MJ, Dodick D, et al. Antiepileptic drugs for the treatment of chronic and episodic cluster headache: a review. Headache. 2007;47(1):81-89.
60. Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672-677.
61. Ko YH, Joe SH, Jung IK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175.
62. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410.
63. Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007; 30(6):1480-1486.
64. McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
65. Zonegran [package insert]. Teaneck, NJ: Eisai Inc; 2006.
66. Drake ME Jr, Greathouse NI, Renner JB, et al. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278-280.
67. Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis. 2017;56(4):1229-1239.
68. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
Mr. B, age 64, is being treated in the psychiatric clinic for generalized anxiety disorder. He also has a history of type 2 diabetes mellitus and osteoarthritis. His present medications include metformin 500 mg twice daily, escitalopram 20 mg/d, and a multivitamin.
Three months after a shingles outbreak on his left trunk, Mr. B develops a sharp, burning pain and hypersensitivity to light in the same area as the shingles flare-up. He is diagnosed with postherpetic neuralgia. Despite a 12-week trial of cognitive-behavioral therapy, Mr. B continues to report excessive worry, irritability, poor concentration, psychomotor restlessness, and poor sleep.
Contrasting with the serendipitous discovery of iproniazid and chlorpromazine leading to the development of the current spectrum of antidepressant and antipsychotic agents, discovery of the benefits various antiepileptic agents have in bipolar disorder has not led to a similar proliferation of medication development for bipolar mania or depression.1-3 Divalproex, one of the most commonly used antiepileptic drugs (AEDs) in psychiatry, was thought to be an inactive organic solvent until it was used in 1962 to test the anticonvulsant activity of other compounds. This led to the discovery and subsequent use of divalproex in patients with epilepsy, followed by FDA approval in bipolar disorder.4,5 Off-label use of many AEDs as mood-stabilizing agents in bipolar disorder led to the emergence of carbamazepine, divalproex, and lamotrigine, which joined lithium as classic mood-stabilizing agents.4,6-8 Amid varying definitions of “mood stabilizer,” many AEDs have failed to demonstrate mood-stabilizing effects in bipolar disorder and therefore should not all be considered mood stabilizers.9 Nonetheless, the dual use of a single AED for both psychiatric and nonpsychiatric indications can decrease polypharmacy and increase acceptability of medications in patients who have low insight into their illness.10,11
Because AEDs were originally purposed to treat neurologic disease, psychiatric indications must first be established before considering other indications. AEDs as a class have broad pharmacologic actions, but are generally CNS depressants, decreasing brain signaling through mechanisms such as ion channel antagonism (carbamazepine, gabapentin) or alterations to gamma-aminobutyric acid/glutamate signaling (divalproex, topiramate).4,6,12,13 Compared to antidepressants and antipsychotics, whose primary use for psychiatric conditions is firmly rooted in evidence, rational use of AEDs for psychiatric conditions and symptoms depends on the agent-specific efficacy. Patients with comorbid psychiatric and neurologic disorders are ideal candidates for dually indicated AEDs due to these agents’ class effects rooted in epilepsy. Due to the history of positive psychiatric benefits with AEDs, newer agents may be psychiatrically beneficial but will likely follow the discovery of these benefits in patients for whom epilepsy is the primary diagnosis.
Consider the limitations
Using AEDs to reduce polypharmacy should be done judiciously from a drug-drug interaction perspective, because certain AEDs (eg, carbamazepine, divalproex) can greatly influence the metabolism of other medications, which may defeat the best intentions of the original intervention.4,6
Several other limitations should be considered. This article does not include all AEDs, only those commonly used for psychiatric indications with known nonpsychiatric benefits. Some may worsen psychiatric conditions (such as rage and irritability in the case of levetiracetam), and all AEDs have an FDA warning regarding suicidal behaviors and ideation.14,15 Another important limitation is the potential for differential dosing across indications; tolerability concerns may limit adequate dosing across multiple uses. For example, topiramate’s migraine prophylaxis effect can be achieved at much lower doses than the patient-specific efficacy dosing seen in binge eating disorder, with higher doses increasing the propensity for adverse effects.13,16Dual-use AEDs should be considered wherever possible, but judicious review of evidence is necessary to appropriately adjudicate a specific patient’s risk vs benefit. The Table4,6-9,12,13,16-68 provides information on select AEDs with both psychiatric and nonpsychiatric indications, including both FDA-approved and common off-label uses. These indications are limited to adult use only.
CASE CONTINUED
After reviewing Mr. B’s medical history, the treating medical team decides to cross-taper escitalopram to duloxetine 30 mg twice daily. Though his pain lessens after several weeks, it persists enough to interfere with Mr. B’s daily life. In addition to duloxetine, he is started on pregabalin 50 mg 3 times a day. Mr. B’s pain decreases to a tolerable level, and he reports decreased worrying and restlessness, and improvements in concentration and sleep.
Mr. B, age 64, is being treated in the psychiatric clinic for generalized anxiety disorder. He also has a history of type 2 diabetes mellitus and osteoarthritis. His present medications include metformin 500 mg twice daily, escitalopram 20 mg/d, and a multivitamin.
Three months after a shingles outbreak on his left trunk, Mr. B develops a sharp, burning pain and hypersensitivity to light in the same area as the shingles flare-up. He is diagnosed with postherpetic neuralgia. Despite a 12-week trial of cognitive-behavioral therapy, Mr. B continues to report excessive worry, irritability, poor concentration, psychomotor restlessness, and poor sleep.
Contrasting with the serendipitous discovery of iproniazid and chlorpromazine leading to the development of the current spectrum of antidepressant and antipsychotic agents, discovery of the benefits various antiepileptic agents have in bipolar disorder has not led to a similar proliferation of medication development for bipolar mania or depression.1-3 Divalproex, one of the most commonly used antiepileptic drugs (AEDs) in psychiatry, was thought to be an inactive organic solvent until it was used in 1962 to test the anticonvulsant activity of other compounds. This led to the discovery and subsequent use of divalproex in patients with epilepsy, followed by FDA approval in bipolar disorder.4,5 Off-label use of many AEDs as mood-stabilizing agents in bipolar disorder led to the emergence of carbamazepine, divalproex, and lamotrigine, which joined lithium as classic mood-stabilizing agents.4,6-8 Amid varying definitions of “mood stabilizer,” many AEDs have failed to demonstrate mood-stabilizing effects in bipolar disorder and therefore should not all be considered mood stabilizers.9 Nonetheless, the dual use of a single AED for both psychiatric and nonpsychiatric indications can decrease polypharmacy and increase acceptability of medications in patients who have low insight into their illness.10,11
Because AEDs were originally purposed to treat neurologic disease, psychiatric indications must first be established before considering other indications. AEDs as a class have broad pharmacologic actions, but are generally CNS depressants, decreasing brain signaling through mechanisms such as ion channel antagonism (carbamazepine, gabapentin) or alterations to gamma-aminobutyric acid/glutamate signaling (divalproex, topiramate).4,6,12,13 Compared to antidepressants and antipsychotics, whose primary use for psychiatric conditions is firmly rooted in evidence, rational use of AEDs for psychiatric conditions and symptoms depends on the agent-specific efficacy. Patients with comorbid psychiatric and neurologic disorders are ideal candidates for dually indicated AEDs due to these agents’ class effects rooted in epilepsy. Due to the history of positive psychiatric benefits with AEDs, newer agents may be psychiatrically beneficial but will likely follow the discovery of these benefits in patients for whom epilepsy is the primary diagnosis.
Consider the limitations
Using AEDs to reduce polypharmacy should be done judiciously from a drug-drug interaction perspective, because certain AEDs (eg, carbamazepine, divalproex) can greatly influence the metabolism of other medications, which may defeat the best intentions of the original intervention.4,6
Several other limitations should be considered. This article does not include all AEDs, only those commonly used for psychiatric indications with known nonpsychiatric benefits. Some may worsen psychiatric conditions (such as rage and irritability in the case of levetiracetam), and all AEDs have an FDA warning regarding suicidal behaviors and ideation.14,15 Another important limitation is the potential for differential dosing across indications; tolerability concerns may limit adequate dosing across multiple uses. For example, topiramate’s migraine prophylaxis effect can be achieved at much lower doses than the patient-specific efficacy dosing seen in binge eating disorder, with higher doses increasing the propensity for adverse effects.13,16Dual-use AEDs should be considered wherever possible, but judicious review of evidence is necessary to appropriately adjudicate a specific patient’s risk vs benefit. The Table4,6-9,12,13,16-68 provides information on select AEDs with both psychiatric and nonpsychiatric indications, including both FDA-approved and common off-label uses. These indications are limited to adult use only.
CASE CONTINUED
After reviewing Mr. B’s medical history, the treating medical team decides to cross-taper escitalopram to duloxetine 30 mg twice daily. Though his pain lessens after several weeks, it persists enough to interfere with Mr. B’s daily life. In addition to duloxetine, he is started on pregabalin 50 mg 3 times a day. Mr. B’s pain decreases to a tolerable level, and he reports decreased worrying and restlessness, and improvements in concentration and sleep.
1. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. López-Mun
4. Depakote [package insert]. North Chicago, IL: AbbVie, Inc; 2021.
5. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16.
6. Tegretol and Tegretol-XR [package insert]. East Hanover, NJ: Pharmaceuticals Co.; 2020.
7. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
8. Lithobid [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc; 2009.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. National Alliance on Mental Illness. Anosognosia. Common with mental illness. Accessed March 3, 2022. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
11. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. NCHS Data Brief. 2019(347):1-8.
12. Neurontin [package insert]. New York, NY: Pfizer; 2017.
13. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
14. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81.
15. U.S. Food & Drug Administration. FDA Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality. 2008. Accessed March 3, 2022. https://www.fda.gov/files/drugs/published/Statistical-Review-and-Evaluation--Antiepileptic-Drugs-and-Suicidality.pdf
16. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
17. Zhang ZJ, Kang WH, Tan QR, et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: a double-blind, randomized, placebo-controlled study. J Psychiatr Res. 2007;41(3-4):360-369.
18. Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42 Suppl 1:2-10.
19. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
20. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85(3):259-266.
21. Gyulai L, Bowden CL, McElroy SL, et al. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology. 2003;28(7):1374-1382.
22. Limdi NA, Shimpi AV, Faught E, et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353-355.
23. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999; 91(4):593-600.
24. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90.
25. US Dept of Veterans Affairs, US Dept of Defense, The Management of Substance Use Disorders Work Group. VA/DoD clinical practice guideline for the management of substance use disorders. US Dept of Veterans Affairs/Dept of Defense; 2015. Accessed March 3, 2022. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
26. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
27. Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
28. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348.
29. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589.
30. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44.
31. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344.
32. Alonso-Navarro H, Rubio L, Jiménez-Jiménez FJ. Refractory hiccup: successful treatment with gabapentin. Clin Neuropharmacol. 2007;30(3):186-187.
33. Jatzko A, Stegmeier-Petroianu A, Petroianu GA. Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J Pain Symptom Manage. 2007;33(6):756-760.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
35. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938.
36. Yuan M, Zhou HY, Xiao ZL, et al. Efficacy and safety of gabapentin vs. carbamazepine in the treatment of trigeminal neuralgia: a meta-analysis. Pain Pract. 2016;16(8):1083-1091.
37. Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563-581.
38. Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-Foundation. Sleep Med. 2016;21:1-11.
39. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 update [published correction appears in Endocr Pract. 2017;23 (12):1488]. Endocr Pract. 2017;23(7):869-880.
40. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder: Lamictal 614 Study Group. J Clin Psychiatry. 2000;60(11):841-850.
41. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066-1077.
42. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640.
43. Craft M, Ismail IA, Krishnamurti D, et al. Lithium in the treatment of aggression in mentally handicapped patients: a double-blind trial. Br J Psychiatry. 1987;150:685-689.
44. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
45. Dickstein G, Shechner C, Adawi F, et al. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454-458.
46. Bogazzi F, Bartalena L, Brogioni S, et al. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84(2):499-503.
47. Lyrica [package insert]. New York, NY: Parke-Davis, Division of Pfizer Inc; 2020.
48. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241.
49. Pande AC, Feltner DE, Jefferson JW, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol. 2004;24(2):141-149.
50. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648.
51. Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619-625.e6.
52. Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
53. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1 [published correction appears in J Clin Oncol. 2010;28(10):1808]. J Clin Oncol. 2010;28(4):641-647.
54. Dunlop BW, Papp L, Garlow SJ, et al. Tiagabine for social anxiety disorder. Hum Psychopharmacol. 2007;22(4):241-244.
55. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375-1380.
56. Gabitril [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2015.
57. Johnson BA, Ait-Daoud N, Bowden C, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685.
58. Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010610.
59. Pascual J, Láinez MJ, Dodick D, et al. Antiepileptic drugs for the treatment of chronic and episodic cluster headache: a review. Headache. 2007;47(1):81-89.
60. Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672-677.
61. Ko YH, Joe SH, Jung IK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175.
62. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410.
63. Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007; 30(6):1480-1486.
64. McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
65. Zonegran [package insert]. Teaneck, NJ: Eisai Inc; 2006.
66. Drake ME Jr, Greathouse NI, Renner JB, et al. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278-280.
67. Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis. 2017;56(4):1229-1239.
68. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
1. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. López-Mun
4. Depakote [package insert]. North Chicago, IL: AbbVie, Inc; 2021.
5. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16.
6. Tegretol and Tegretol-XR [package insert]. East Hanover, NJ: Pharmaceuticals Co.; 2020.
7. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
8. Lithobid [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc; 2009.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. National Alliance on Mental Illness. Anosognosia. Common with mental illness. Accessed March 3, 2022. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
11. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. NCHS Data Brief. 2019(347):1-8.
12. Neurontin [package insert]. New York, NY: Pfizer; 2017.
13. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
14. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81.
15. U.S. Food & Drug Administration. FDA Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality. 2008. Accessed March 3, 2022. https://www.fda.gov/files/drugs/published/Statistical-Review-and-Evaluation--Antiepileptic-Drugs-and-Suicidality.pdf
16. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
17. Zhang ZJ, Kang WH, Tan QR, et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: a double-blind, randomized, placebo-controlled study. J Psychiatr Res. 2007;41(3-4):360-369.
18. Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42 Suppl 1:2-10.
19. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
20. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85(3):259-266.
21. Gyulai L, Bowden CL, McElroy SL, et al. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology. 2003;28(7):1374-1382.
22. Limdi NA, Shimpi AV, Faught E, et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353-355.
23. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999; 91(4):593-600.
24. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90.
25. US Dept of Veterans Affairs, US Dept of Defense, The Management of Substance Use Disorders Work Group. VA/DoD clinical practice guideline for the management of substance use disorders. US Dept of Veterans Affairs/Dept of Defense; 2015. Accessed March 3, 2022. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
26. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
27. Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
28. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348.
29. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589.
30. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44.
31. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344.
32. Alonso-Navarro H, Rubio L, Jiménez-Jiménez FJ. Refractory hiccup: successful treatment with gabapentin. Clin Neuropharmacol. 2007;30(3):186-187.
33. Jatzko A, Stegmeier-Petroianu A, Petroianu GA. Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J Pain Symptom Manage. 2007;33(6):756-760.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
35. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938.
36. Yuan M, Zhou HY, Xiao ZL, et al. Efficacy and safety of gabapentin vs. carbamazepine in the treatment of trigeminal neuralgia: a meta-analysis. Pain Pract. 2016;16(8):1083-1091.
37. Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563-581.
38. Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-Foundation. Sleep Med. 2016;21:1-11.
39. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 update [published correction appears in Endocr Pract. 2017;23 (12):1488]. Endocr Pract. 2017;23(7):869-880.
40. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder: Lamictal 614 Study Group. J Clin Psychiatry. 2000;60(11):841-850.
41. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066-1077.
42. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640.
43. Craft M, Ismail IA, Krishnamurti D, et al. Lithium in the treatment of aggression in mentally handicapped patients: a double-blind trial. Br J Psychiatry. 1987;150:685-689.
44. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
45. Dickstein G, Shechner C, Adawi F, et al. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454-458.
46. Bogazzi F, Bartalena L, Brogioni S, et al. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84(2):499-503.
47. Lyrica [package insert]. New York, NY: Parke-Davis, Division of Pfizer Inc; 2020.
48. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241.
49. Pande AC, Feltner DE, Jefferson JW, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol. 2004;24(2):141-149.
50. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648.
51. Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619-625.e6.
52. Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
53. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1 [published correction appears in J Clin Oncol. 2010;28(10):1808]. J Clin Oncol. 2010;28(4):641-647.
54. Dunlop BW, Papp L, Garlow SJ, et al. Tiagabine for social anxiety disorder. Hum Psychopharmacol. 2007;22(4):241-244.
55. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375-1380.
56. Gabitril [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2015.
57. Johnson BA, Ait-Daoud N, Bowden C, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685.
58. Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010610.
59. Pascual J, Láinez MJ, Dodick D, et al. Antiepileptic drugs for the treatment of chronic and episodic cluster headache: a review. Headache. 2007;47(1):81-89.
60. Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672-677.
61. Ko YH, Joe SH, Jung IK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175.
62. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410.
63. Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007; 30(6):1480-1486.
64. McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
65. Zonegran [package insert]. Teaneck, NJ: Eisai Inc; 2006.
66. Drake ME Jr, Greathouse NI, Renner JB, et al. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278-280.
67. Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis. 2017;56(4):1229-1239.
68. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
Microdosing psychedelics: Untapped potential in psychiatry?
In her month-long memoir, A Really Good Day: How Microdosing Made a Mega Difference in My Mood, My Marriage, and My Life (Knopf, 2017), author Ayelet Waldman turns herself into a one-woman experiment.
Over a single month she takes one-tenth of a recreational dose of LSD every third day. She plots her emotions, her productivity, and her pain along the way. Ms. Waldman obtains the LSD in a single vial, enough for 10 doses, from a researcher, who is retiring. What she’s looking for, she tells the reader, is a really good day – something that has been elusive in her turbulent life.
Although psychedelics remain illegal for both recreational and therapeutic use, they are increasingly being studied at academic centers, and there is hope that they will offer something that our traditional medications might not. However, these are not “micro” doses, but full doses of psychedelic agents that induce clinically-monitored “trips” in order to treat conditions such as depression, anorexia nervosa, or for smoking cessation, to name just a few.
Yet
Because these drugs are illegal under most circumstances, many of the studies involve surveys of users in their natural environments who are already microdosing in an uncontrolled manner. In a 2019 study published in PLOS One, Vince Polito and Richard Stevenson, from Macquarie University, Sydney, gave daily surveys of psychological functioning to 98 microdosers over 6 weeks. Several participants were excluded for using doses that were too high or for concurrent use of other illicit substances.
Whereas the authors found that many people claimed to have positive experiences, there was an increase in neuroticism in some of the subjects. There was no control group and no uniformity to what the subject claimed to be ingesting with regard to dose, frequency, substance, or verification of the chemical content.
University of Chicago neuroscientist Harriet De Wit, PhD, leads one of the few laboratories that conducts controlled, double-blind studies looking at microdosing LSD.
“With microdosing there are expectations, and we don’t know if it’s the expectation or the agent that is making a difference,” she explained. And when asked who in her experience is experimenting with microdosing psychedelics, she expounded “Everybody under the sun!”
Dr. De Wit notes that people microdose to increase their creativity, productivity, focus, and energy, to heighten their spiritual awareness, improve empathy and social relational skills, and to improve their mood – all purported benefits of low-dose psychedelics.
Her group published a study in Addiction Biology, in which 39 subjects were administered low doses of LSD four times over 2 weeks. To address the issues of expectation, the subjects were not told they were participating in a study of hallucinogens specifically but were instead given a list of pharmaceuticals in different classes that they might be given. Microdoses of LSD did not improve either mood or performance, but they did appear to be safe, and they produced no adverse effects.
To date, studies on microdosing have looked at their effects on healthy populations, and the practice has been associated with “Silicon Valley techies” looking for performance enhancement. Ms. Waldman, however, is different.
She is open about her diagnosis of bipolar disorder, and her long history with therapy and medications. As she describes herself in the beginning of her book, she is emotionally uncomfortable, and both irritable and reactive to the point that her life is propelled by interpersonal chaos. In her uncontrolled ‘study,’ she is an N of 1, and she is pleased with the results. Microdosing, she believes, helped her become less irritable, more resilient, and in fact, have some very good days.
By the end of her memoir, she was looking for a way to continue microdosing but was unsure how to safely obtain more LSD and be certain of its purity. Her experience does raise the possibility that microdoses may have therapeutic benefits in people with certain psychiatric conditions, but this has yet to be studied.
J. Raymond DePaulo Jr., MD, is the chair of the National Network of Depression Centers and a distinguished service professor at Johns Hopkins Hospital, Baltimore. “Microdosing of psychedelics is very problematic for two equally serious reasons,” he cautioned. “There is no control over what it is that people are actually taking, it is completely unstudied scientifically, and there is no agreement on what a ‘micro’ dose is.”
He noted that one of his patients thought he was taking psilocybin. A chemical analysis was done that revealed the agent to contain a combination of THC, a stimulant, morphine, and fluoxetine. There wasn’t a trace of psilocybin. “Mislabeling is the rule, not the exception,” Dr. DePaulo has concluded.
He also believes the placebo effect has a powerful role with microdosing. “It’s not working because of what is in the pill, more likely it is working because of what is advertised to be in it.”
Ms. De Wit noted that when she started her study, she tried to find people who were elevated on measures of depression or anxiety, but she was not looking for a specific clinical population of patients with these clinical diagnoses. “We found a handful of people, and they improved, but so did those in the placebo group; they all got better.”
Psychedelic agents interact with antidepressants, so subjects in controlled studies need to go off their medications before enrolling – this is a limiting factor in studies of both macro- and microdosing. Ms. De Wit also notes that there are logistical and practical obstacles – it is difficult to get approval to use these agents, and the patients have to remain in the lab and be observed for several hours after they are administered, just as with standard doses.
As might be expected, data collection and anecdotal microdosing experiences are rampant on the internet. The social media forum Reddit alone boasts 192,000 members in its microdosing group, while Imperial College London invites microdosers to take part in surveys intended to add to the body of knowledge. But despite its popularity, there is little in the way of prospective, agent-verified, placebo-controlled research exploring whether or not microdosing is truly beneficial beyond just anecdotal evidence.
Perhaps microdosing is a fad, or perhaps it offers some benefits to some people. Given the current interest in the therapeutic uses of psychedelics, it would be useful to have controlled studies of lower doses that don’t carry the risk of “bad trips.”
Certainly, psychiatry could use more agents to address mental health issues, and society might benefit from the use of agents that are proven to be evidence-based options for improving creativity and productivity. Anything that has potential to reduce psychiatric suffering seems worthy of further study to delineate which populations could be helped or harmed.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
A version of this article first appeared on Medscape.com.
In her month-long memoir, A Really Good Day: How Microdosing Made a Mega Difference in My Mood, My Marriage, and My Life (Knopf, 2017), author Ayelet Waldman turns herself into a one-woman experiment.
Over a single month she takes one-tenth of a recreational dose of LSD every third day. She plots her emotions, her productivity, and her pain along the way. Ms. Waldman obtains the LSD in a single vial, enough for 10 doses, from a researcher, who is retiring. What she’s looking for, she tells the reader, is a really good day – something that has been elusive in her turbulent life.
Although psychedelics remain illegal for both recreational and therapeutic use, they are increasingly being studied at academic centers, and there is hope that they will offer something that our traditional medications might not. However, these are not “micro” doses, but full doses of psychedelic agents that induce clinically-monitored “trips” in order to treat conditions such as depression, anorexia nervosa, or for smoking cessation, to name just a few.
Yet
Because these drugs are illegal under most circumstances, many of the studies involve surveys of users in their natural environments who are already microdosing in an uncontrolled manner. In a 2019 study published in PLOS One, Vince Polito and Richard Stevenson, from Macquarie University, Sydney, gave daily surveys of psychological functioning to 98 microdosers over 6 weeks. Several participants were excluded for using doses that were too high or for concurrent use of other illicit substances.
Whereas the authors found that many people claimed to have positive experiences, there was an increase in neuroticism in some of the subjects. There was no control group and no uniformity to what the subject claimed to be ingesting with regard to dose, frequency, substance, or verification of the chemical content.
University of Chicago neuroscientist Harriet De Wit, PhD, leads one of the few laboratories that conducts controlled, double-blind studies looking at microdosing LSD.
“With microdosing there are expectations, and we don’t know if it’s the expectation or the agent that is making a difference,” she explained. And when asked who in her experience is experimenting with microdosing psychedelics, she expounded “Everybody under the sun!”
Dr. De Wit notes that people microdose to increase their creativity, productivity, focus, and energy, to heighten their spiritual awareness, improve empathy and social relational skills, and to improve their mood – all purported benefits of low-dose psychedelics.
Her group published a study in Addiction Biology, in which 39 subjects were administered low doses of LSD four times over 2 weeks. To address the issues of expectation, the subjects were not told they were participating in a study of hallucinogens specifically but were instead given a list of pharmaceuticals in different classes that they might be given. Microdoses of LSD did not improve either mood or performance, but they did appear to be safe, and they produced no adverse effects.
To date, studies on microdosing have looked at their effects on healthy populations, and the practice has been associated with “Silicon Valley techies” looking for performance enhancement. Ms. Waldman, however, is different.
She is open about her diagnosis of bipolar disorder, and her long history with therapy and medications. As she describes herself in the beginning of her book, she is emotionally uncomfortable, and both irritable and reactive to the point that her life is propelled by interpersonal chaos. In her uncontrolled ‘study,’ she is an N of 1, and she is pleased with the results. Microdosing, she believes, helped her become less irritable, more resilient, and in fact, have some very good days.
By the end of her memoir, she was looking for a way to continue microdosing but was unsure how to safely obtain more LSD and be certain of its purity. Her experience does raise the possibility that microdoses may have therapeutic benefits in people with certain psychiatric conditions, but this has yet to be studied.
J. Raymond DePaulo Jr., MD, is the chair of the National Network of Depression Centers and a distinguished service professor at Johns Hopkins Hospital, Baltimore. “Microdosing of psychedelics is very problematic for two equally serious reasons,” he cautioned. “There is no control over what it is that people are actually taking, it is completely unstudied scientifically, and there is no agreement on what a ‘micro’ dose is.”
He noted that one of his patients thought he was taking psilocybin. A chemical analysis was done that revealed the agent to contain a combination of THC, a stimulant, morphine, and fluoxetine. There wasn’t a trace of psilocybin. “Mislabeling is the rule, not the exception,” Dr. DePaulo has concluded.
He also believes the placebo effect has a powerful role with microdosing. “It’s not working because of what is in the pill, more likely it is working because of what is advertised to be in it.”
Ms. De Wit noted that when she started her study, she tried to find people who were elevated on measures of depression or anxiety, but she was not looking for a specific clinical population of patients with these clinical diagnoses. “We found a handful of people, and they improved, but so did those in the placebo group; they all got better.”
Psychedelic agents interact with antidepressants, so subjects in controlled studies need to go off their medications before enrolling – this is a limiting factor in studies of both macro- and microdosing. Ms. De Wit also notes that there are logistical and practical obstacles – it is difficult to get approval to use these agents, and the patients have to remain in the lab and be observed for several hours after they are administered, just as with standard doses.
As might be expected, data collection and anecdotal microdosing experiences are rampant on the internet. The social media forum Reddit alone boasts 192,000 members in its microdosing group, while Imperial College London invites microdosers to take part in surveys intended to add to the body of knowledge. But despite its popularity, there is little in the way of prospective, agent-verified, placebo-controlled research exploring whether or not microdosing is truly beneficial beyond just anecdotal evidence.
Perhaps microdosing is a fad, or perhaps it offers some benefits to some people. Given the current interest in the therapeutic uses of psychedelics, it would be useful to have controlled studies of lower doses that don’t carry the risk of “bad trips.”
Certainly, psychiatry could use more agents to address mental health issues, and society might benefit from the use of agents that are proven to be evidence-based options for improving creativity and productivity. Anything that has potential to reduce psychiatric suffering seems worthy of further study to delineate which populations could be helped or harmed.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
A version of this article first appeared on Medscape.com.
In her month-long memoir, A Really Good Day: How Microdosing Made a Mega Difference in My Mood, My Marriage, and My Life (Knopf, 2017), author Ayelet Waldman turns herself into a one-woman experiment.
Over a single month she takes one-tenth of a recreational dose of LSD every third day. She plots her emotions, her productivity, and her pain along the way. Ms. Waldman obtains the LSD in a single vial, enough for 10 doses, from a researcher, who is retiring. What she’s looking for, she tells the reader, is a really good day – something that has been elusive in her turbulent life.
Although psychedelics remain illegal for both recreational and therapeutic use, they are increasingly being studied at academic centers, and there is hope that they will offer something that our traditional medications might not. However, these are not “micro” doses, but full doses of psychedelic agents that induce clinically-monitored “trips” in order to treat conditions such as depression, anorexia nervosa, or for smoking cessation, to name just a few.
Yet
Because these drugs are illegal under most circumstances, many of the studies involve surveys of users in their natural environments who are already microdosing in an uncontrolled manner. In a 2019 study published in PLOS One, Vince Polito and Richard Stevenson, from Macquarie University, Sydney, gave daily surveys of psychological functioning to 98 microdosers over 6 weeks. Several participants were excluded for using doses that were too high or for concurrent use of other illicit substances.
Whereas the authors found that many people claimed to have positive experiences, there was an increase in neuroticism in some of the subjects. There was no control group and no uniformity to what the subject claimed to be ingesting with regard to dose, frequency, substance, or verification of the chemical content.
University of Chicago neuroscientist Harriet De Wit, PhD, leads one of the few laboratories that conducts controlled, double-blind studies looking at microdosing LSD.
“With microdosing there are expectations, and we don’t know if it’s the expectation or the agent that is making a difference,” she explained. And when asked who in her experience is experimenting with microdosing psychedelics, she expounded “Everybody under the sun!”
Dr. De Wit notes that people microdose to increase their creativity, productivity, focus, and energy, to heighten their spiritual awareness, improve empathy and social relational skills, and to improve their mood – all purported benefits of low-dose psychedelics.
Her group published a study in Addiction Biology, in which 39 subjects were administered low doses of LSD four times over 2 weeks. To address the issues of expectation, the subjects were not told they were participating in a study of hallucinogens specifically but were instead given a list of pharmaceuticals in different classes that they might be given. Microdoses of LSD did not improve either mood or performance, but they did appear to be safe, and they produced no adverse effects.
To date, studies on microdosing have looked at their effects on healthy populations, and the practice has been associated with “Silicon Valley techies” looking for performance enhancement. Ms. Waldman, however, is different.
She is open about her diagnosis of bipolar disorder, and her long history with therapy and medications. As she describes herself in the beginning of her book, she is emotionally uncomfortable, and both irritable and reactive to the point that her life is propelled by interpersonal chaos. In her uncontrolled ‘study,’ she is an N of 1, and she is pleased with the results. Microdosing, she believes, helped her become less irritable, more resilient, and in fact, have some very good days.
By the end of her memoir, she was looking for a way to continue microdosing but was unsure how to safely obtain more LSD and be certain of its purity. Her experience does raise the possibility that microdoses may have therapeutic benefits in people with certain psychiatric conditions, but this has yet to be studied.
J. Raymond DePaulo Jr., MD, is the chair of the National Network of Depression Centers and a distinguished service professor at Johns Hopkins Hospital, Baltimore. “Microdosing of psychedelics is very problematic for two equally serious reasons,” he cautioned. “There is no control over what it is that people are actually taking, it is completely unstudied scientifically, and there is no agreement on what a ‘micro’ dose is.”
He noted that one of his patients thought he was taking psilocybin. A chemical analysis was done that revealed the agent to contain a combination of THC, a stimulant, morphine, and fluoxetine. There wasn’t a trace of psilocybin. “Mislabeling is the rule, not the exception,” Dr. DePaulo has concluded.
He also believes the placebo effect has a powerful role with microdosing. “It’s not working because of what is in the pill, more likely it is working because of what is advertised to be in it.”
Ms. De Wit noted that when she started her study, she tried to find people who were elevated on measures of depression or anxiety, but she was not looking for a specific clinical population of patients with these clinical diagnoses. “We found a handful of people, and they improved, but so did those in the placebo group; they all got better.”
Psychedelic agents interact with antidepressants, so subjects in controlled studies need to go off their medications before enrolling – this is a limiting factor in studies of both macro- and microdosing. Ms. De Wit also notes that there are logistical and practical obstacles – it is difficult to get approval to use these agents, and the patients have to remain in the lab and be observed for several hours after they are administered, just as with standard doses.
As might be expected, data collection and anecdotal microdosing experiences are rampant on the internet. The social media forum Reddit alone boasts 192,000 members in its microdosing group, while Imperial College London invites microdosers to take part in surveys intended to add to the body of knowledge. But despite its popularity, there is little in the way of prospective, agent-verified, placebo-controlled research exploring whether or not microdosing is truly beneficial beyond just anecdotal evidence.
Perhaps microdosing is a fad, or perhaps it offers some benefits to some people. Given the current interest in the therapeutic uses of psychedelics, it would be useful to have controlled studies of lower doses that don’t carry the risk of “bad trips.”
Certainly, psychiatry could use more agents to address mental health issues, and society might benefit from the use of agents that are proven to be evidence-based options for improving creativity and productivity. Anything that has potential to reduce psychiatric suffering seems worthy of further study to delineate which populations could be helped or harmed.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
A version of this article first appeared on Medscape.com.
Psychotropic med use tied to ‘striking’ post-COVID dementia risk
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN MEDICINE
Common eye disorder in children tied to mental illness
Misaligned eyes in children are associated with an increased prevalence of mental illness, results of a large study suggest.
“Psychiatrists who have a patient with depression or anxiety and notice that patient also has strabismus might think about the link between those two conditions and refer that patient,” study investigator Stacy L. Pineles, MD, professor, department of ophthalmology, University of California, Los Angeles, told this news organization.
The study was published online March 10 in JAMA Ophthalmology.
A common condition
Strabismus, a condition in which the eyes don’t line up or are “crossed,” is one of the most common eye diseases in children, with some estimates suggesting it affects more than 1.5 million American youth.
Patients with strabismus have problems making eye contact and are affected socially and functionally, said Dr. Pineles. They’re often met with a negative bias, as shown by children’s responses to pictures of faces with and without strabismus, she said.
There is a signal from previous research suggesting that strabismus is linked to a higher risk of mental illness. However, most of these studies were small and had relatively homogenous populations, said Dr. Pineles.
The new study includes over 12 million children (mean age, 8.0 years) from a private health insurance claims database that represents diverse races and ethnicities as well as geographic regions across the United States.
The sample included 352,636 children with strabismus and 11,652,553 children with no diagnosed eye disease who served as controls. Most participants were White (51.6%), came from a family with an annual household income of $40,000 or more (51.0%), had point-of-service insurance (68.7%), and had at least one comorbid condition (64.5%).
The study evaluated five mental illness diagnoses. These included anxiety disorder, depressive disorder, substance use or addictive disorder, bipolar disorder, and schizophrenia.
Overall, children with strabismus had a higher prevalence of all these illnesses, with the exception of substance use disorder.
After adjusting for age, sex, race and ethnicity, census region, education level of caregiver, family net worth, and presence of at least one comorbid condition, the odds ratios among those with versus without strabismus were: 2.01 (95% confidence interval, 1.99-2.04; P < .001) for anxiety disorder, 1.83 (95% CI, 1.76-1.90; P < .001) for schizophrenia, 1.64 (95% CI, 1.59-1.70; P < .001) for bipolar disorder, and 1.61 (95% CI, 1.59-1.63; P < .001) for depressive disorder.
Substance use disorder had a negative unadjusted association with strabismus, but after adjustment for confounders, the association was not significant (OR, 0.99; 95% CI, 0.97-1.02; P = .48).
Dr. Pineles noted that the study participants, who were all under age 19, may be too young to have substance use disorders.
The results for substance use disorders provided something of an “internal control” and reaffirmed results for the other four conditions, said Dr. Pineles.
“When you do research on such a large database, you’re very likely to find significant associations; the dataset is so large that even very small differences become statistically significant. It was interesting that not everything gave us a positive association.”
Researchers divided the strabismus group into those with esotropia, where the eyes turn inward (52.2%), exotropia, where they turn outward (46.3%), and hypertropia, where one eye wanders upward (12.5%). Investigators found that all three conditions were associated with increased risk of anxiety disorder, depressive disorder, bipolar disorders, and schizophrenia.
Investigators note that rates in the current study were lower than in previous studies, which showed that children with congenital esotropia were 2.6 times more likely to receive a mental health diagnosis, and children with intermittent exotropia were 2.7 times more likely to receive a mental health diagnosis.
“It is probable that our study found a lower risk than these studies, because our study was cross-sectional and claims based, whereas these studies observed the children to early adulthood and were based on medical records,” the investigators note.
It’s impossible to determine from this study how strabismus is connected to mental illness. However, Dr. Pineles believes depression and anxiety might be tied to strabismus via teasing, which affects self-esteem, although genetics could also play a role. For conditions such as schizophrenia, a shared genetic link with strabismus might be more likely, she added.
“Schizophrenia is a pretty severe diagnosis, so just being teased or having poor self-esteem is probably not enough” to develop schizophrenia.
Based on her clinical experience, Dr. Pineles said that realigning the eyes of patients with milder forms of depression or anxiety “definitely anecdotally helps these patients a lot.”
Dr. Pineles and colleagues have another paper in press that examines mental illnesses and other serious eye disorders in children and shows similar findings, she said.
Implications for insurance coverage?
In an accompanying editorial, experts, led by S. Grace Prakalapakorn, MD, department of ophthalmology and pediatrics, Duke University Medical Center, Durham, N.C., noted the exclusion of children covered under government insurance or without insurance is an important study limitation, largely because socioeconomic status is a risk factor for poor mental health.
The editorialists point to studies showing that surgical correction of ocular misalignments may be associated with reduced anxiety and depression. However, health insurance coverage for such surgical correction “may not be available owing to a misconception that these conditions are ‘cosmetic’.”
Evidence of the broader association of strabismus with physical and mental health “may play an important role in shifting policy to promote insurance coverage for timely strabismus care,” they write.
As many mental health disorders begin in childhood or adolescence, “it is paramount to identify, address, and, if possible, prevent mental health disorders at a young age, because failure to intervene in a timely fashion can have lifelong health consequences,” say Dr. Prakalapakorn and colleagues.
With mental health conditions and disorders increasing worldwide, compounded by the stressors of the COVID-19 pandemic, additional studies are needed to explore the causal relationships between ocular and psychiatric phenomena, their treatment, and outcomes, they add.
The study was supported by a grant from the National Eye Institute and an unrestricted grant from Research to Prevent Blindness. Dr. Pineles has reported no relevant conflicts of interest. Commentary author Manpreet K. Singh, MD, has reported receiving research support from Stanford’s Maternal Child Health Research Institute and Stanford’s Department of Psychiatry and Behavioral Sciences, the National Institute of Mental Health, the National Institute on Aging, the Patient-Centered Outcomes Research Institute, Johnson & Johnson, Allergan, and the Brain and Behavior Research Foundation; serving on the advisory board for Sunovion and Skyland Trail; serving as a consultant for Johnson & Johnson; previously serving as a consultant for X, the moonshot factory, Alphabet, and Limbix Health; receiving honoraria from the American Academy of Child and Adolescent Psychiatry; and receiving royalties from American Psychiatric Association Publishing and Thrive Global. Commentary author Nathan Congdon, MD, has reported receiving personal fees from Belkin Vision outside the submitted work.
A version of this article first appeared on Medscape.com.
Misaligned eyes in children are associated with an increased prevalence of mental illness, results of a large study suggest.
“Psychiatrists who have a patient with depression or anxiety and notice that patient also has strabismus might think about the link between those two conditions and refer that patient,” study investigator Stacy L. Pineles, MD, professor, department of ophthalmology, University of California, Los Angeles, told this news organization.
The study was published online March 10 in JAMA Ophthalmology.
A common condition
Strabismus, a condition in which the eyes don’t line up or are “crossed,” is one of the most common eye diseases in children, with some estimates suggesting it affects more than 1.5 million American youth.
Patients with strabismus have problems making eye contact and are affected socially and functionally, said Dr. Pineles. They’re often met with a negative bias, as shown by children’s responses to pictures of faces with and without strabismus, she said.
There is a signal from previous research suggesting that strabismus is linked to a higher risk of mental illness. However, most of these studies were small and had relatively homogenous populations, said Dr. Pineles.
The new study includes over 12 million children (mean age, 8.0 years) from a private health insurance claims database that represents diverse races and ethnicities as well as geographic regions across the United States.
The sample included 352,636 children with strabismus and 11,652,553 children with no diagnosed eye disease who served as controls. Most participants were White (51.6%), came from a family with an annual household income of $40,000 or more (51.0%), had point-of-service insurance (68.7%), and had at least one comorbid condition (64.5%).
The study evaluated five mental illness diagnoses. These included anxiety disorder, depressive disorder, substance use or addictive disorder, bipolar disorder, and schizophrenia.
Overall, children with strabismus had a higher prevalence of all these illnesses, with the exception of substance use disorder.
After adjusting for age, sex, race and ethnicity, census region, education level of caregiver, family net worth, and presence of at least one comorbid condition, the odds ratios among those with versus without strabismus were: 2.01 (95% confidence interval, 1.99-2.04; P < .001) for anxiety disorder, 1.83 (95% CI, 1.76-1.90; P < .001) for schizophrenia, 1.64 (95% CI, 1.59-1.70; P < .001) for bipolar disorder, and 1.61 (95% CI, 1.59-1.63; P < .001) for depressive disorder.
Substance use disorder had a negative unadjusted association with strabismus, but after adjustment for confounders, the association was not significant (OR, 0.99; 95% CI, 0.97-1.02; P = .48).
Dr. Pineles noted that the study participants, who were all under age 19, may be too young to have substance use disorders.
The results for substance use disorders provided something of an “internal control” and reaffirmed results for the other four conditions, said Dr. Pineles.
“When you do research on such a large database, you’re very likely to find significant associations; the dataset is so large that even very small differences become statistically significant. It was interesting that not everything gave us a positive association.”
Researchers divided the strabismus group into those with esotropia, where the eyes turn inward (52.2%), exotropia, where they turn outward (46.3%), and hypertropia, where one eye wanders upward (12.5%). Investigators found that all three conditions were associated with increased risk of anxiety disorder, depressive disorder, bipolar disorders, and schizophrenia.
Investigators note that rates in the current study were lower than in previous studies, which showed that children with congenital esotropia were 2.6 times more likely to receive a mental health diagnosis, and children with intermittent exotropia were 2.7 times more likely to receive a mental health diagnosis.
“It is probable that our study found a lower risk than these studies, because our study was cross-sectional and claims based, whereas these studies observed the children to early adulthood and were based on medical records,” the investigators note.
It’s impossible to determine from this study how strabismus is connected to mental illness. However, Dr. Pineles believes depression and anxiety might be tied to strabismus via teasing, which affects self-esteem, although genetics could also play a role. For conditions such as schizophrenia, a shared genetic link with strabismus might be more likely, she added.
“Schizophrenia is a pretty severe diagnosis, so just being teased or having poor self-esteem is probably not enough” to develop schizophrenia.
Based on her clinical experience, Dr. Pineles said that realigning the eyes of patients with milder forms of depression or anxiety “definitely anecdotally helps these patients a lot.”
Dr. Pineles and colleagues have another paper in press that examines mental illnesses and other serious eye disorders in children and shows similar findings, she said.
Implications for insurance coverage?
In an accompanying editorial, experts, led by S. Grace Prakalapakorn, MD, department of ophthalmology and pediatrics, Duke University Medical Center, Durham, N.C., noted the exclusion of children covered under government insurance or without insurance is an important study limitation, largely because socioeconomic status is a risk factor for poor mental health.
The editorialists point to studies showing that surgical correction of ocular misalignments may be associated with reduced anxiety and depression. However, health insurance coverage for such surgical correction “may not be available owing to a misconception that these conditions are ‘cosmetic’.”
Evidence of the broader association of strabismus with physical and mental health “may play an important role in shifting policy to promote insurance coverage for timely strabismus care,” they write.
As many mental health disorders begin in childhood or adolescence, “it is paramount to identify, address, and, if possible, prevent mental health disorders at a young age, because failure to intervene in a timely fashion can have lifelong health consequences,” say Dr. Prakalapakorn and colleagues.
With mental health conditions and disorders increasing worldwide, compounded by the stressors of the COVID-19 pandemic, additional studies are needed to explore the causal relationships between ocular and psychiatric phenomena, their treatment, and outcomes, they add.
The study was supported by a grant from the National Eye Institute and an unrestricted grant from Research to Prevent Blindness. Dr. Pineles has reported no relevant conflicts of interest. Commentary author Manpreet K. Singh, MD, has reported receiving research support from Stanford’s Maternal Child Health Research Institute and Stanford’s Department of Psychiatry and Behavioral Sciences, the National Institute of Mental Health, the National Institute on Aging, the Patient-Centered Outcomes Research Institute, Johnson & Johnson, Allergan, and the Brain and Behavior Research Foundation; serving on the advisory board for Sunovion and Skyland Trail; serving as a consultant for Johnson & Johnson; previously serving as a consultant for X, the moonshot factory, Alphabet, and Limbix Health; receiving honoraria from the American Academy of Child and Adolescent Psychiatry; and receiving royalties from American Psychiatric Association Publishing and Thrive Global. Commentary author Nathan Congdon, MD, has reported receiving personal fees from Belkin Vision outside the submitted work.
A version of this article first appeared on Medscape.com.
Misaligned eyes in children are associated with an increased prevalence of mental illness, results of a large study suggest.
“Psychiatrists who have a patient with depression or anxiety and notice that patient also has strabismus might think about the link between those two conditions and refer that patient,” study investigator Stacy L. Pineles, MD, professor, department of ophthalmology, University of California, Los Angeles, told this news organization.
The study was published online March 10 in JAMA Ophthalmology.
A common condition
Strabismus, a condition in which the eyes don’t line up or are “crossed,” is one of the most common eye diseases in children, with some estimates suggesting it affects more than 1.5 million American youth.
Patients with strabismus have problems making eye contact and are affected socially and functionally, said Dr. Pineles. They’re often met with a negative bias, as shown by children’s responses to pictures of faces with and without strabismus, she said.
There is a signal from previous research suggesting that strabismus is linked to a higher risk of mental illness. However, most of these studies were small and had relatively homogenous populations, said Dr. Pineles.
The new study includes over 12 million children (mean age, 8.0 years) from a private health insurance claims database that represents diverse races and ethnicities as well as geographic regions across the United States.
The sample included 352,636 children with strabismus and 11,652,553 children with no diagnosed eye disease who served as controls. Most participants were White (51.6%), came from a family with an annual household income of $40,000 or more (51.0%), had point-of-service insurance (68.7%), and had at least one comorbid condition (64.5%).
The study evaluated five mental illness diagnoses. These included anxiety disorder, depressive disorder, substance use or addictive disorder, bipolar disorder, and schizophrenia.
Overall, children with strabismus had a higher prevalence of all these illnesses, with the exception of substance use disorder.
After adjusting for age, sex, race and ethnicity, census region, education level of caregiver, family net worth, and presence of at least one comorbid condition, the odds ratios among those with versus without strabismus were: 2.01 (95% confidence interval, 1.99-2.04; P < .001) for anxiety disorder, 1.83 (95% CI, 1.76-1.90; P < .001) for schizophrenia, 1.64 (95% CI, 1.59-1.70; P < .001) for bipolar disorder, and 1.61 (95% CI, 1.59-1.63; P < .001) for depressive disorder.
Substance use disorder had a negative unadjusted association with strabismus, but after adjustment for confounders, the association was not significant (OR, 0.99; 95% CI, 0.97-1.02; P = .48).
Dr. Pineles noted that the study participants, who were all under age 19, may be too young to have substance use disorders.
The results for substance use disorders provided something of an “internal control” and reaffirmed results for the other four conditions, said Dr. Pineles.
“When you do research on such a large database, you’re very likely to find significant associations; the dataset is so large that even very small differences become statistically significant. It was interesting that not everything gave us a positive association.”
Researchers divided the strabismus group into those with esotropia, where the eyes turn inward (52.2%), exotropia, where they turn outward (46.3%), and hypertropia, where one eye wanders upward (12.5%). Investigators found that all three conditions were associated with increased risk of anxiety disorder, depressive disorder, bipolar disorders, and schizophrenia.
Investigators note that rates in the current study were lower than in previous studies, which showed that children with congenital esotropia were 2.6 times more likely to receive a mental health diagnosis, and children with intermittent exotropia were 2.7 times more likely to receive a mental health diagnosis.
“It is probable that our study found a lower risk than these studies, because our study was cross-sectional and claims based, whereas these studies observed the children to early adulthood and were based on medical records,” the investigators note.
It’s impossible to determine from this study how strabismus is connected to mental illness. However, Dr. Pineles believes depression and anxiety might be tied to strabismus via teasing, which affects self-esteem, although genetics could also play a role. For conditions such as schizophrenia, a shared genetic link with strabismus might be more likely, she added.
“Schizophrenia is a pretty severe diagnosis, so just being teased or having poor self-esteem is probably not enough” to develop schizophrenia.
Based on her clinical experience, Dr. Pineles said that realigning the eyes of patients with milder forms of depression or anxiety “definitely anecdotally helps these patients a lot.”
Dr. Pineles and colleagues have another paper in press that examines mental illnesses and other serious eye disorders in children and shows similar findings, she said.
Implications for insurance coverage?
In an accompanying editorial, experts, led by S. Grace Prakalapakorn, MD, department of ophthalmology and pediatrics, Duke University Medical Center, Durham, N.C., noted the exclusion of children covered under government insurance or without insurance is an important study limitation, largely because socioeconomic status is a risk factor for poor mental health.
The editorialists point to studies showing that surgical correction of ocular misalignments may be associated with reduced anxiety and depression. However, health insurance coverage for such surgical correction “may not be available owing to a misconception that these conditions are ‘cosmetic’.”
Evidence of the broader association of strabismus with physical and mental health “may play an important role in shifting policy to promote insurance coverage for timely strabismus care,” they write.
As many mental health disorders begin in childhood or adolescence, “it is paramount to identify, address, and, if possible, prevent mental health disorders at a young age, because failure to intervene in a timely fashion can have lifelong health consequences,” say Dr. Prakalapakorn and colleagues.
With mental health conditions and disorders increasing worldwide, compounded by the stressors of the COVID-19 pandemic, additional studies are needed to explore the causal relationships between ocular and psychiatric phenomena, their treatment, and outcomes, they add.
The study was supported by a grant from the National Eye Institute and an unrestricted grant from Research to Prevent Blindness. Dr. Pineles has reported no relevant conflicts of interest. Commentary author Manpreet K. Singh, MD, has reported receiving research support from Stanford’s Maternal Child Health Research Institute and Stanford’s Department of Psychiatry and Behavioral Sciences, the National Institute of Mental Health, the National Institute on Aging, the Patient-Centered Outcomes Research Institute, Johnson & Johnson, Allergan, and the Brain and Behavior Research Foundation; serving on the advisory board for Sunovion and Skyland Trail; serving as a consultant for Johnson & Johnson; previously serving as a consultant for X, the moonshot factory, Alphabet, and Limbix Health; receiving honoraria from the American Academy of Child and Adolescent Psychiatry; and receiving royalties from American Psychiatric Association Publishing and Thrive Global. Commentary author Nathan Congdon, MD, has reported receiving personal fees from Belkin Vision outside the submitted work.
A version of this article first appeared on Medscape.com.
DSM-5 update: What’s new?
Ahead of its official release on March 18, the new Diagnostic and Statistical Manual of Mental Disorders, which is in the form of a textbook, is already drawing some criticism.
It also includes symptom codes for suicidal behavior and nonsuicidal self-injury, clarifying modifications to criteria sets for more than 70 disorders, including autism spectrum disorder; changes in terminology for gender dysphoria; and a comprehensive review of the impact of racism and discrimination on the diagnosis and manifestations of mental disorders.
The Text Revision is a compilation of iterative changes that have been made online on a rolling basis since the DSM-5 was first published in 2013.
“The goal of the Text Revision was to allow a thorough revision of the text, not the criteria,” Paul Appelbaum, MD, chair of the APA’s DSM steering committee, told this news organization.
For the Text Revision, some 200 experts across a variety of APA working groups recommended changes to the text based on a comprehensive literature review, said Appelbaum, who is the Elizabeth K. Dollard Professor of Psychiatry, Medicine and Law, and director of the division of law, ethics and psychiatry at Columbia University, New York.
However, there’s not a lot that’s new, in part, because there have been few therapeutic advances.
Money maker?
Allen Frances, MD, chair of the DSM-4 task force and professor and chair emeritus of psychiatry at Duke University, Durham, N.C., said the APA is publishing the Text Revision “just to make money. They’re very anxious to do anything that will increase sales and having a revision forces some people, especially in institutions, to buy the book, even though it may not have anything substantive to add to the original.”
Dr. Frances told this news organization that when the APA published the first DSM in the late 1970s, “it became an instantaneous best-seller, to everyone’s surprise.”
The APA would not comment on how many of the $170 (list price) volumes it sells or how much those sales contribute to its budget.
Dr. Appelbaum acknowledged, “at any point in time, the canonical version is the online version.” However, it’s clear from DSM-5 sales “that many people still value having a hard copy of the DSM available to them.”
Prolonged grief: Timely or overkill?
Persistent complex bereavement disorder (PCBD) was listed as a “condition for further study” in DSM-5. After a 2019 workshop aimed at getting consensus for diagnosis criteria, the APA board approved the new prolonged grief disorder in October 2020, and the APA assembly approved the new disorder in November 2020.
Given the 950,000 deaths from COVID-19 over the past 2 years, inclusion of prolonged grief disorder in the DSM-5 may arrive at just the right time.
The diagnostic criteria for PCBD include:
- The development of a persistent grief response (longer than a year for adults and 6 months for children and adolescents) characterized by one or both of the following symptoms, which have been present most days to a clinically significant degree, and have occurred nearly every day for at least the last month: intense yearning/longing for the deceased person; preoccupation with thoughts or memories of the deceased person.
- Since the death, at least three symptoms present most days to a clinically significant degree, and occurring nearly every day for at least the last month, including identity disruption, marked sense of disbelief about the death, avoidance of reminders that the person is dead, intense emotional pain related to the death, difficulty reintegrating into one’s relationships and activities after the death, emotional numbness, feeling that life is meaningless as a result of the death, and intense loneliness as a result of the death.
- The disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning.
- The duration and severity of the bereavement reaction clearly exceed expected social, cultural, or religious norms for the individual’s culture and context.
- The symptoms are not better explained by another mental disorder, such as major depressive disorder (MDD) or PTSD, and are not attributable to the physiological effects of a substance or another medical condition.
Dr. Frances said he believes creating a new diagnosis pathologizes grief. In DSM-3 and DSM-4, an exception was made under the diagnosis of MDD for individuals who had recently lost a loved one. “We wanted to have at least an opportunity for people to grieve without being stigmatized, mislabeled, and overtreated with medication.”
DSM-5 removed the bereavement exclusion. After 2 weeks, people who are grieving and have particular symptoms could receive a diagnosis of MDD, said Dr. Frances. He believes the exclusion should have been broadened to cover anyone experiencing a major loss – such as a job loss or divorce. If someone is having prolonged symptoms that interfere with functioning, they should get an MDD diagnosis.
The new disorder “doesn’t solve anything, it just adds to the confusion and stigmatization, and it’s part of a kind of creeping medical imperialization of everyday life, where everything has to have a mental disorder label,” Dr. Frances said.
However, Dr. Appelbaum countered that “the criteria for prolonged grief disorder are constructed in such a way as to make every effort to exclude people who are going through a normal grieving process.”
“Part of the purpose of the data analyses was to ensure the criteria that were adopted would, in fact, effectively distinguish between what anybody goes through, say when someone close to you dies, and this unusual prolonged grieving process without end that affects a much smaller number of people but which really can be crippling for them,” he added.
The Text Revision adds new symptom codes for suicidal behavior and nonsuicidal self-injury, which appear in the chapter, “Other Conditions That May Be a Focus of Clinical Attention,” said Dr. Appelbaum.
“Both suicidal behavior and nonsuicidal self-injury seem pretty persuasively to fall into that category – something a clinician would want to know about, pay attention to, and factor into treatment planning, although they are behaviors that cross many diagnostic categories,” he added.
Codes also provide a systematic way of ascertaining the incidence and prevalence of such behaviors, said Dr. Appelbaum.
Changes to gender terminology
The Text Revision also tweaks some terminology with respect to transgender individuals. The term “desired gender” is now “experienced gender”, the term “cross-sex medical procedure” is now “gender-affirming medical procedure”, and the terms “natal male/natal female” are now “individual assigned male/female at birth”.
Dr. Frances said that the existence of gender dysphoria as a diagnosis has been a matter of controversy ever since it was first included.
“The transgender community has had mixed feelings on whether there should be anything at all in the manual,” he said. On one hand is the argument that gender dysphoria should be removed because it’s not really a psychiatric issue.
“We seriously considered eliminating it altogether in DSM-4,” said Dr. Frances.
However, an argument in favor of keeping it was that if the diagnosis was removed, it would mean that people could not receive treatment. “There’s no right argument for this dilemma,” he said.
Dr. Frances, who has been a frequent critic of DSM-5, said he believes the manual continues to miss opportunities to tighten criteria for many diagnoses, including ADHD and autism spectrum disorder.
“There’s a consistent pattern of taking behaviors and symptoms of behaviors that are on the border with normality and expanding the definition of mental disorder and reducing the realm of normality,” he said.
That has consequences, Dr. Frances added. “When someone gets a diagnosis that they need to get, it’s the beginning of a much better future. When someone gets a diagnosis that’s a mislabel that they don’t need, it has all harms and no benefits. It’s stigmatizing, leads to too much treatment, the wrong treatment, and it’s much more harmful than helpful.”
A version of this article first appeared on Medscape.com.
Ahead of its official release on March 18, the new Diagnostic and Statistical Manual of Mental Disorders, which is in the form of a textbook, is already drawing some criticism.
It also includes symptom codes for suicidal behavior and nonsuicidal self-injury, clarifying modifications to criteria sets for more than 70 disorders, including autism spectrum disorder; changes in terminology for gender dysphoria; and a comprehensive review of the impact of racism and discrimination on the diagnosis and manifestations of mental disorders.
The Text Revision is a compilation of iterative changes that have been made online on a rolling basis since the DSM-5 was first published in 2013.
“The goal of the Text Revision was to allow a thorough revision of the text, not the criteria,” Paul Appelbaum, MD, chair of the APA’s DSM steering committee, told this news organization.
For the Text Revision, some 200 experts across a variety of APA working groups recommended changes to the text based on a comprehensive literature review, said Appelbaum, who is the Elizabeth K. Dollard Professor of Psychiatry, Medicine and Law, and director of the division of law, ethics and psychiatry at Columbia University, New York.
However, there’s not a lot that’s new, in part, because there have been few therapeutic advances.
Money maker?
Allen Frances, MD, chair of the DSM-4 task force and professor and chair emeritus of psychiatry at Duke University, Durham, N.C., said the APA is publishing the Text Revision “just to make money. They’re very anxious to do anything that will increase sales and having a revision forces some people, especially in institutions, to buy the book, even though it may not have anything substantive to add to the original.”
Dr. Frances told this news organization that when the APA published the first DSM in the late 1970s, “it became an instantaneous best-seller, to everyone’s surprise.”
The APA would not comment on how many of the $170 (list price) volumes it sells or how much those sales contribute to its budget.
Dr. Appelbaum acknowledged, “at any point in time, the canonical version is the online version.” However, it’s clear from DSM-5 sales “that many people still value having a hard copy of the DSM available to them.”
Prolonged grief: Timely or overkill?
Persistent complex bereavement disorder (PCBD) was listed as a “condition for further study” in DSM-5. After a 2019 workshop aimed at getting consensus for diagnosis criteria, the APA board approved the new prolonged grief disorder in October 2020, and the APA assembly approved the new disorder in November 2020.
Given the 950,000 deaths from COVID-19 over the past 2 years, inclusion of prolonged grief disorder in the DSM-5 may arrive at just the right time.
The diagnostic criteria for PCBD include:
- The development of a persistent grief response (longer than a year for adults and 6 months for children and adolescents) characterized by one or both of the following symptoms, which have been present most days to a clinically significant degree, and have occurred nearly every day for at least the last month: intense yearning/longing for the deceased person; preoccupation with thoughts or memories of the deceased person.
- Since the death, at least three symptoms present most days to a clinically significant degree, and occurring nearly every day for at least the last month, including identity disruption, marked sense of disbelief about the death, avoidance of reminders that the person is dead, intense emotional pain related to the death, difficulty reintegrating into one’s relationships and activities after the death, emotional numbness, feeling that life is meaningless as a result of the death, and intense loneliness as a result of the death.
- The disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning.
- The duration and severity of the bereavement reaction clearly exceed expected social, cultural, or religious norms for the individual’s culture and context.
- The symptoms are not better explained by another mental disorder, such as major depressive disorder (MDD) or PTSD, and are not attributable to the physiological effects of a substance or another medical condition.
Dr. Frances said he believes creating a new diagnosis pathologizes grief. In DSM-3 and DSM-4, an exception was made under the diagnosis of MDD for individuals who had recently lost a loved one. “We wanted to have at least an opportunity for people to grieve without being stigmatized, mislabeled, and overtreated with medication.”
DSM-5 removed the bereavement exclusion. After 2 weeks, people who are grieving and have particular symptoms could receive a diagnosis of MDD, said Dr. Frances. He believes the exclusion should have been broadened to cover anyone experiencing a major loss – such as a job loss or divorce. If someone is having prolonged symptoms that interfere with functioning, they should get an MDD diagnosis.
The new disorder “doesn’t solve anything, it just adds to the confusion and stigmatization, and it’s part of a kind of creeping medical imperialization of everyday life, where everything has to have a mental disorder label,” Dr. Frances said.
However, Dr. Appelbaum countered that “the criteria for prolonged grief disorder are constructed in such a way as to make every effort to exclude people who are going through a normal grieving process.”
“Part of the purpose of the data analyses was to ensure the criteria that were adopted would, in fact, effectively distinguish between what anybody goes through, say when someone close to you dies, and this unusual prolonged grieving process without end that affects a much smaller number of people but which really can be crippling for them,” he added.
The Text Revision adds new symptom codes for suicidal behavior and nonsuicidal self-injury, which appear in the chapter, “Other Conditions That May Be a Focus of Clinical Attention,” said Dr. Appelbaum.
“Both suicidal behavior and nonsuicidal self-injury seem pretty persuasively to fall into that category – something a clinician would want to know about, pay attention to, and factor into treatment planning, although they are behaviors that cross many diagnostic categories,” he added.
Codes also provide a systematic way of ascertaining the incidence and prevalence of such behaviors, said Dr. Appelbaum.
Changes to gender terminology
The Text Revision also tweaks some terminology with respect to transgender individuals. The term “desired gender” is now “experienced gender”, the term “cross-sex medical procedure” is now “gender-affirming medical procedure”, and the terms “natal male/natal female” are now “individual assigned male/female at birth”.
Dr. Frances said that the existence of gender dysphoria as a diagnosis has been a matter of controversy ever since it was first included.
“The transgender community has had mixed feelings on whether there should be anything at all in the manual,” he said. On one hand is the argument that gender dysphoria should be removed because it’s not really a psychiatric issue.
“We seriously considered eliminating it altogether in DSM-4,” said Dr. Frances.
However, an argument in favor of keeping it was that if the diagnosis was removed, it would mean that people could not receive treatment. “There’s no right argument for this dilemma,” he said.
Dr. Frances, who has been a frequent critic of DSM-5, said he believes the manual continues to miss opportunities to tighten criteria for many diagnoses, including ADHD and autism spectrum disorder.
“There’s a consistent pattern of taking behaviors and symptoms of behaviors that are on the border with normality and expanding the definition of mental disorder and reducing the realm of normality,” he said.
That has consequences, Dr. Frances added. “When someone gets a diagnosis that they need to get, it’s the beginning of a much better future. When someone gets a diagnosis that’s a mislabel that they don’t need, it has all harms and no benefits. It’s stigmatizing, leads to too much treatment, the wrong treatment, and it’s much more harmful than helpful.”
A version of this article first appeared on Medscape.com.
Ahead of its official release on March 18, the new Diagnostic and Statistical Manual of Mental Disorders, which is in the form of a textbook, is already drawing some criticism.
It also includes symptom codes for suicidal behavior and nonsuicidal self-injury, clarifying modifications to criteria sets for more than 70 disorders, including autism spectrum disorder; changes in terminology for gender dysphoria; and a comprehensive review of the impact of racism and discrimination on the diagnosis and manifestations of mental disorders.
The Text Revision is a compilation of iterative changes that have been made online on a rolling basis since the DSM-5 was first published in 2013.
“The goal of the Text Revision was to allow a thorough revision of the text, not the criteria,” Paul Appelbaum, MD, chair of the APA’s DSM steering committee, told this news organization.
For the Text Revision, some 200 experts across a variety of APA working groups recommended changes to the text based on a comprehensive literature review, said Appelbaum, who is the Elizabeth K. Dollard Professor of Psychiatry, Medicine and Law, and director of the division of law, ethics and psychiatry at Columbia University, New York.
However, there’s not a lot that’s new, in part, because there have been few therapeutic advances.
Money maker?
Allen Frances, MD, chair of the DSM-4 task force and professor and chair emeritus of psychiatry at Duke University, Durham, N.C., said the APA is publishing the Text Revision “just to make money. They’re very anxious to do anything that will increase sales and having a revision forces some people, especially in institutions, to buy the book, even though it may not have anything substantive to add to the original.”
Dr. Frances told this news organization that when the APA published the first DSM in the late 1970s, “it became an instantaneous best-seller, to everyone’s surprise.”
The APA would not comment on how many of the $170 (list price) volumes it sells or how much those sales contribute to its budget.
Dr. Appelbaum acknowledged, “at any point in time, the canonical version is the online version.” However, it’s clear from DSM-5 sales “that many people still value having a hard copy of the DSM available to them.”
Prolonged grief: Timely or overkill?
Persistent complex bereavement disorder (PCBD) was listed as a “condition for further study” in DSM-5. After a 2019 workshop aimed at getting consensus for diagnosis criteria, the APA board approved the new prolonged grief disorder in October 2020, and the APA assembly approved the new disorder in November 2020.
Given the 950,000 deaths from COVID-19 over the past 2 years, inclusion of prolonged grief disorder in the DSM-5 may arrive at just the right time.
The diagnostic criteria for PCBD include:
- The development of a persistent grief response (longer than a year for adults and 6 months for children and adolescents) characterized by one or both of the following symptoms, which have been present most days to a clinically significant degree, and have occurred nearly every day for at least the last month: intense yearning/longing for the deceased person; preoccupation with thoughts or memories of the deceased person.
- Since the death, at least three symptoms present most days to a clinically significant degree, and occurring nearly every day for at least the last month, including identity disruption, marked sense of disbelief about the death, avoidance of reminders that the person is dead, intense emotional pain related to the death, difficulty reintegrating into one’s relationships and activities after the death, emotional numbness, feeling that life is meaningless as a result of the death, and intense loneliness as a result of the death.
- The disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning.
- The duration and severity of the bereavement reaction clearly exceed expected social, cultural, or religious norms for the individual’s culture and context.
- The symptoms are not better explained by another mental disorder, such as major depressive disorder (MDD) or PTSD, and are not attributable to the physiological effects of a substance or another medical condition.
Dr. Frances said he believes creating a new diagnosis pathologizes grief. In DSM-3 and DSM-4, an exception was made under the diagnosis of MDD for individuals who had recently lost a loved one. “We wanted to have at least an opportunity for people to grieve without being stigmatized, mislabeled, and overtreated with medication.”
DSM-5 removed the bereavement exclusion. After 2 weeks, people who are grieving and have particular symptoms could receive a diagnosis of MDD, said Dr. Frances. He believes the exclusion should have been broadened to cover anyone experiencing a major loss – such as a job loss or divorce. If someone is having prolonged symptoms that interfere with functioning, they should get an MDD diagnosis.
The new disorder “doesn’t solve anything, it just adds to the confusion and stigmatization, and it’s part of a kind of creeping medical imperialization of everyday life, where everything has to have a mental disorder label,” Dr. Frances said.
However, Dr. Appelbaum countered that “the criteria for prolonged grief disorder are constructed in such a way as to make every effort to exclude people who are going through a normal grieving process.”
“Part of the purpose of the data analyses was to ensure the criteria that were adopted would, in fact, effectively distinguish between what anybody goes through, say when someone close to you dies, and this unusual prolonged grieving process without end that affects a much smaller number of people but which really can be crippling for them,” he added.
The Text Revision adds new symptom codes for suicidal behavior and nonsuicidal self-injury, which appear in the chapter, “Other Conditions That May Be a Focus of Clinical Attention,” said Dr. Appelbaum.
“Both suicidal behavior and nonsuicidal self-injury seem pretty persuasively to fall into that category – something a clinician would want to know about, pay attention to, and factor into treatment planning, although they are behaviors that cross many diagnostic categories,” he added.
Codes also provide a systematic way of ascertaining the incidence and prevalence of such behaviors, said Dr. Appelbaum.
Changes to gender terminology
The Text Revision also tweaks some terminology with respect to transgender individuals. The term “desired gender” is now “experienced gender”, the term “cross-sex medical procedure” is now “gender-affirming medical procedure”, and the terms “natal male/natal female” are now “individual assigned male/female at birth”.
Dr. Frances said that the existence of gender dysphoria as a diagnosis has been a matter of controversy ever since it was first included.
“The transgender community has had mixed feelings on whether there should be anything at all in the manual,” he said. On one hand is the argument that gender dysphoria should be removed because it’s not really a psychiatric issue.
“We seriously considered eliminating it altogether in DSM-4,” said Dr. Frances.
However, an argument in favor of keeping it was that if the diagnosis was removed, it would mean that people could not receive treatment. “There’s no right argument for this dilemma,” he said.
Dr. Frances, who has been a frequent critic of DSM-5, said he believes the manual continues to miss opportunities to tighten criteria for many diagnoses, including ADHD and autism spectrum disorder.
“There’s a consistent pattern of taking behaviors and symptoms of behaviors that are on the border with normality and expanding the definition of mental disorder and reducing the realm of normality,” he said.
That has consequences, Dr. Frances added. “When someone gets a diagnosis that they need to get, it’s the beginning of a much better future. When someone gets a diagnosis that’s a mislabel that they don’t need, it has all harms and no benefits. It’s stigmatizing, leads to too much treatment, the wrong treatment, and it’s much more harmful than helpful.”
A version of this article first appeared on Medscape.com.
Sublingual dexmedetomidine may rapidly calm bipolar agitation
The phase 3 SERENITY II trial included almost 400 adults with bipolar I or II disorder and acute agitation.
Results showed relief from acute agitation kicked in beginning at 20 minutes after administration of the treatment and continued to 120 minutes, principal investigator Sheldon H. Preskorn, MD, professor, department of psychiatry and behavioral sciences, University of Kansas, Wichita, and colleagues reported.
“Patients who are mild to moderately agitated in whom there is the potential for escalation to more severe agitation,” are good candidates for sublingual dexmedetomidine, Dr. Preskorn told this news organization.
He noted that, while “comparative claims require comparative studies,” a key advantage is that it “can be self-administered by patients reliably because of the adherence of the film to the mucosa.”
The findings were published online Feb. 22, 2022 in JAMA.
Tough-to-manage symptom
Preliminary results were presented at the 2021 annual meeting of the American Psychiatric Association and reported by this news organization at that time.
Agitation is a common and tough-to-manage symptom associated with multiple neuropsychiatric conditions, including bipolar disorder.
The phase 3 SERENITY II trial enrolled 380 adults (mean age, 45 years; 55% women) with bipolar I or II disorder and acute agitation in the emergency department.
All participants had a total score of 14 or greater on the five items of the Positive and Negative Syndrome Scale–Excited Component (PEC) scale at baseline and a score of 4 or greater on at least one PEC item.
Patients were randomly allocated to a single dose of sublingual dexmedetomidine (120 mcg or 180 mcg) or placebo. All but two patients completed the study.
Baseline agitation was mild to moderate, with an overall mean PEC total score of 18.
Rapid relief
Mean change from baseline in the PEC total score at 2 hours (primary endpoint) was –9 and –10.4 with the 120-mcg and 180-mcg doses of sublingual dexmedetomidine, respectively, versus –4.9 for placebo.
Least-square mean differences from placebo in the sublingual dexmedetomidine groups at 2 hours were statistically significant for both doses (both, P < .001 vs. placebo).
Statistically significant treatment effects were first evident 20 minutes after dosing for both of the dexmedetomidine doses versus placebo.
Patients in both active-treatment groups showed greater improvement in PEC total score than patients in the placebo group at all subsequent time points through 2 hours post dosing.
Sublingual dexmedetomidine was also associated with significant improvement on the secondary outcomes of Clinical Global Impressions–Improvement and Agitation-Calmness Evaluation Scale.
Adverse events occurred in 35.7% and 34.9% of patients taking 180 mcg and 120 mcg sublingual dexmedetomidine, respectively, compared with 17.5% of patients taking placebo. The most commonly reported adverse events were somnolence, dry mouth, hypotension, and dizziness. No treatment-related serious or severe adverse events were reported.
FDA action date: April 5
In an accompanying editorial, John K. Hsiao, MD, National Institutes of Health, Bethesda, Md., noted that an “out-of-control, agitated, possibly aggressive patient in a medical setting is a crisis demanding swift and safe resolution.
“Today, emergency departments now rival and perhaps surpass psychiatric units as settings where out-of-control, agitated patients must be managed,” Dr. Hsiao said.
The current study “provides evidence to support a novel, potentially important addition to the armamentarium for managing behavioral agitation,” he wrote.
BioXcel Therapeutics has submitted a new drug application to the Food and Drug Administration. The Prescription Drug User Fee Act target action date is April 5.
In a statement, Frank D. Yocca, PhD, chief scientific officer of BioXcel Therapeutics, said the company is looking forward to potential FDA approval of the treatment for agitation associated with bipolar disorders and schizophrenia.
“Building on the strength of these compelling data, we are also confidently progressing BXCL501 as a potential acute treatment for agitation associated with Alzheimer’s disease,” Dr. Yocca added.
The study was funded by BioXcel Therapeutics. Dr. Preskorn reported receiving consulting fees from BioXcel and receiving research grants from, serving as a consultant for, on advisory boards of, and on the speakers bureau of Alkermes, BioXcel Therapeutics, Eisai, Janssen, Novartis, Otsuka, Sunovion, and Usona Institute. Dr. Hsiao has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The phase 3 SERENITY II trial included almost 400 adults with bipolar I or II disorder and acute agitation.
Results showed relief from acute agitation kicked in beginning at 20 minutes after administration of the treatment and continued to 120 minutes, principal investigator Sheldon H. Preskorn, MD, professor, department of psychiatry and behavioral sciences, University of Kansas, Wichita, and colleagues reported.
“Patients who are mild to moderately agitated in whom there is the potential for escalation to more severe agitation,” are good candidates for sublingual dexmedetomidine, Dr. Preskorn told this news organization.
He noted that, while “comparative claims require comparative studies,” a key advantage is that it “can be self-administered by patients reliably because of the adherence of the film to the mucosa.”
The findings were published online Feb. 22, 2022 in JAMA.
Tough-to-manage symptom
Preliminary results were presented at the 2021 annual meeting of the American Psychiatric Association and reported by this news organization at that time.
Agitation is a common and tough-to-manage symptom associated with multiple neuropsychiatric conditions, including bipolar disorder.
The phase 3 SERENITY II trial enrolled 380 adults (mean age, 45 years; 55% women) with bipolar I or II disorder and acute agitation in the emergency department.
All participants had a total score of 14 or greater on the five items of the Positive and Negative Syndrome Scale–Excited Component (PEC) scale at baseline and a score of 4 or greater on at least one PEC item.
Patients were randomly allocated to a single dose of sublingual dexmedetomidine (120 mcg or 180 mcg) or placebo. All but two patients completed the study.
Baseline agitation was mild to moderate, with an overall mean PEC total score of 18.
Rapid relief
Mean change from baseline in the PEC total score at 2 hours (primary endpoint) was –9 and –10.4 with the 120-mcg and 180-mcg doses of sublingual dexmedetomidine, respectively, versus –4.9 for placebo.
Least-square mean differences from placebo in the sublingual dexmedetomidine groups at 2 hours were statistically significant for both doses (both, P < .001 vs. placebo).
Statistically significant treatment effects were first evident 20 minutes after dosing for both of the dexmedetomidine doses versus placebo.
Patients in both active-treatment groups showed greater improvement in PEC total score than patients in the placebo group at all subsequent time points through 2 hours post dosing.
Sublingual dexmedetomidine was also associated with significant improvement on the secondary outcomes of Clinical Global Impressions–Improvement and Agitation-Calmness Evaluation Scale.
Adverse events occurred in 35.7% and 34.9% of patients taking 180 mcg and 120 mcg sublingual dexmedetomidine, respectively, compared with 17.5% of patients taking placebo. The most commonly reported adverse events were somnolence, dry mouth, hypotension, and dizziness. No treatment-related serious or severe adverse events were reported.
FDA action date: April 5
In an accompanying editorial, John K. Hsiao, MD, National Institutes of Health, Bethesda, Md., noted that an “out-of-control, agitated, possibly aggressive patient in a medical setting is a crisis demanding swift and safe resolution.
“Today, emergency departments now rival and perhaps surpass psychiatric units as settings where out-of-control, agitated patients must be managed,” Dr. Hsiao said.
The current study “provides evidence to support a novel, potentially important addition to the armamentarium for managing behavioral agitation,” he wrote.
BioXcel Therapeutics has submitted a new drug application to the Food and Drug Administration. The Prescription Drug User Fee Act target action date is April 5.
In a statement, Frank D. Yocca, PhD, chief scientific officer of BioXcel Therapeutics, said the company is looking forward to potential FDA approval of the treatment for agitation associated with bipolar disorders and schizophrenia.
“Building on the strength of these compelling data, we are also confidently progressing BXCL501 as a potential acute treatment for agitation associated with Alzheimer’s disease,” Dr. Yocca added.
The study was funded by BioXcel Therapeutics. Dr. Preskorn reported receiving consulting fees from BioXcel and receiving research grants from, serving as a consultant for, on advisory boards of, and on the speakers bureau of Alkermes, BioXcel Therapeutics, Eisai, Janssen, Novartis, Otsuka, Sunovion, and Usona Institute. Dr. Hsiao has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The phase 3 SERENITY II trial included almost 400 adults with bipolar I or II disorder and acute agitation.
Results showed relief from acute agitation kicked in beginning at 20 minutes after administration of the treatment and continued to 120 minutes, principal investigator Sheldon H. Preskorn, MD, professor, department of psychiatry and behavioral sciences, University of Kansas, Wichita, and colleagues reported.
“Patients who are mild to moderately agitated in whom there is the potential for escalation to more severe agitation,” are good candidates for sublingual dexmedetomidine, Dr. Preskorn told this news organization.
He noted that, while “comparative claims require comparative studies,” a key advantage is that it “can be self-administered by patients reliably because of the adherence of the film to the mucosa.”
The findings were published online Feb. 22, 2022 in JAMA.
Tough-to-manage symptom
Preliminary results were presented at the 2021 annual meeting of the American Psychiatric Association and reported by this news organization at that time.
Agitation is a common and tough-to-manage symptom associated with multiple neuropsychiatric conditions, including bipolar disorder.
The phase 3 SERENITY II trial enrolled 380 adults (mean age, 45 years; 55% women) with bipolar I or II disorder and acute agitation in the emergency department.
All participants had a total score of 14 or greater on the five items of the Positive and Negative Syndrome Scale–Excited Component (PEC) scale at baseline and a score of 4 or greater on at least one PEC item.
Patients were randomly allocated to a single dose of sublingual dexmedetomidine (120 mcg or 180 mcg) or placebo. All but two patients completed the study.
Baseline agitation was mild to moderate, with an overall mean PEC total score of 18.
Rapid relief
Mean change from baseline in the PEC total score at 2 hours (primary endpoint) was –9 and –10.4 with the 120-mcg and 180-mcg doses of sublingual dexmedetomidine, respectively, versus –4.9 for placebo.
Least-square mean differences from placebo in the sublingual dexmedetomidine groups at 2 hours were statistically significant for both doses (both, P < .001 vs. placebo).
Statistically significant treatment effects were first evident 20 minutes after dosing for both of the dexmedetomidine doses versus placebo.
Patients in both active-treatment groups showed greater improvement in PEC total score than patients in the placebo group at all subsequent time points through 2 hours post dosing.
Sublingual dexmedetomidine was also associated with significant improvement on the secondary outcomes of Clinical Global Impressions–Improvement and Agitation-Calmness Evaluation Scale.
Adverse events occurred in 35.7% and 34.9% of patients taking 180 mcg and 120 mcg sublingual dexmedetomidine, respectively, compared with 17.5% of patients taking placebo. The most commonly reported adverse events were somnolence, dry mouth, hypotension, and dizziness. No treatment-related serious or severe adverse events were reported.
FDA action date: April 5
In an accompanying editorial, John K. Hsiao, MD, National Institutes of Health, Bethesda, Md., noted that an “out-of-control, agitated, possibly aggressive patient in a medical setting is a crisis demanding swift and safe resolution.
“Today, emergency departments now rival and perhaps surpass psychiatric units as settings where out-of-control, agitated patients must be managed,” Dr. Hsiao said.
The current study “provides evidence to support a novel, potentially important addition to the armamentarium for managing behavioral agitation,” he wrote.
BioXcel Therapeutics has submitted a new drug application to the Food and Drug Administration. The Prescription Drug User Fee Act target action date is April 5.
In a statement, Frank D. Yocca, PhD, chief scientific officer of BioXcel Therapeutics, said the company is looking forward to potential FDA approval of the treatment for agitation associated with bipolar disorders and schizophrenia.
“Building on the strength of these compelling data, we are also confidently progressing BXCL501 as a potential acute treatment for agitation associated with Alzheimer’s disease,” Dr. Yocca added.
The study was funded by BioXcel Therapeutics. Dr. Preskorn reported receiving consulting fees from BioXcel and receiving research grants from, serving as a consultant for, on advisory boards of, and on the speakers bureau of Alkermes, BioXcel Therapeutics, Eisai, Janssen, Novartis, Otsuka, Sunovion, and Usona Institute. Dr. Hsiao has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA