User login
Dexmedetomidine sublingual film for agitation
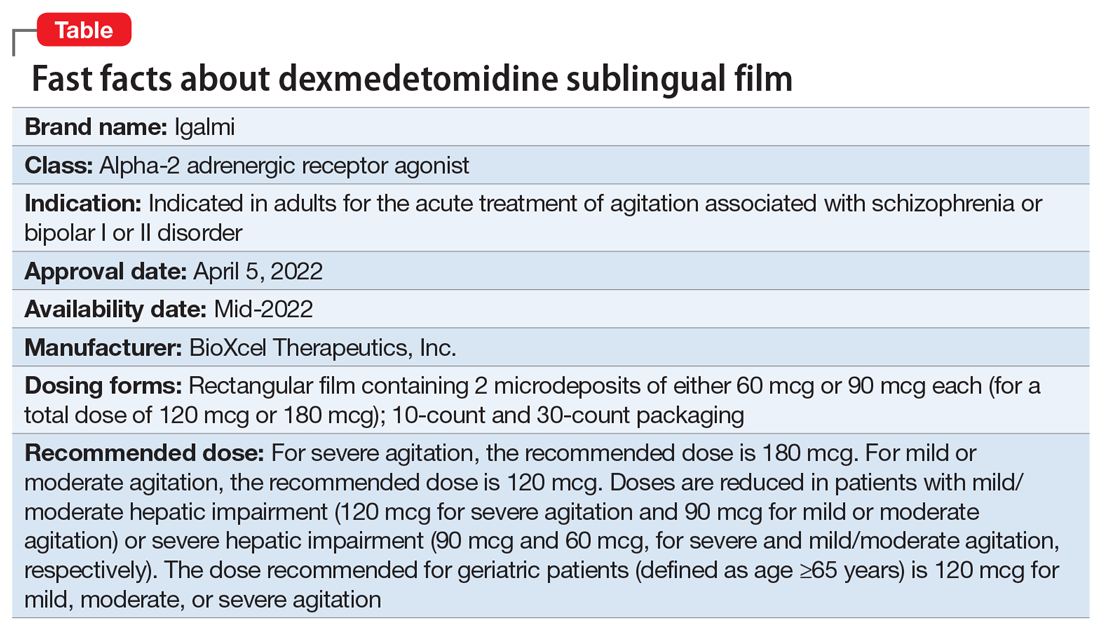
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2
Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2
Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2
Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
Antipsychotic safe, effective for resistant depression in phase 3 trial
, new results from a phase 3 study show.
Already approved by the U.S. Food and Drug Administration to treat adults with schizophrenia and manic, mixed, or depressive episodes of bipolar I disorder, cariprazine is under investigation as an add-on therapy for MDD.
“Even patients who appear to be nonresponsive to standard antidepressant drugs have a very good chance of responding” to cariprazine, lead study author Gary Sachs, MD, associate clinical professor of psychiatry at Massachusetts General Hospital, Boston, told this news organization.
He noted that cariprazine, which is a partial agonist at D2 and D3, as well as 5-HT1A, “is an entirely different class” of drugs.
“It’s worth understanding how to use drugs like cariprazine and expanding our nomenclature; instead of referring to these drugs as atypical antipsychotics, perhaps referring to them as atypical antidepressants makes more sense,” Dr. Sachs said.
The findings were presented at the annual meeting of the American Psychiatric Association.
More options critical
MDD is among the most common psychiatric disorders in the United States. In 2020, an estimated 21 million adults had at least one major depressive episode.
Previous research has shown almost half of patients with MDD do not experience satisfactory results from their current treatment regimen. Therefore, research on more options for patients is critical, Dr. Sachs said.
Results from a previously published placebo-controlled study showed adjunctive treatment with cariprazine at 2-mg to 4.5-mg per day doses was more effective than placebo in improving depressive symptoms in adults with MDD.
The new analysis included patients with MDD and an inadequate response to antidepressant therapy, including selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants. They were recruited from 116 centers in the United States and Europe.
Dr. Sachs noted that a nonresponse to an adequate dose of an antidepressant typically means having less than a 50% improvement over 6 weeks or more.
Researchers randomly assigned the patients to oral cariprazine 1.5 mg/day, cariprazine 3 mg/day, or placebo. All continued to take their antidepressant monotherapy.
The analysis included 757 mostly White participants (mean age, 44.8 years; 73.4% women). All had experienced depression for a “huge” part of their life (average, about 14 years), “not to mention their adult life,” said Dr. Sachs.
In addition, at the start of the study, the participants had been depressed for almost 8 months on average.
The primary endpoint was change at week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The mean baseline MADRS total score was 32.5.
Less is sometimes more
Results showed a significantly greater mean reduction in MADRS total score for cariprazine 1.5 mg/day vs. placebo at week 6 (P = .005). Significant differences from placebo were observed as early as week 2 and were maintained at week 4, as well as week 6.
“I can say with great confidence that the 1.5-mg dose met all the standards for efficacy,” Dr. Sachs said.
However, this was not the case for the 3-mg/day dose. Although there was a numerically greater reduction in MADRS total score for this dosage of the drug vs. placebo at week 6, the difference was not statistically significant (P = .07).
At week 6, more patients taking the active drug at 1.5 mg/day than placebo responded to treatment, defined as 50% or greater reduction in MADRS total score (44% vs. 34.9%, respectively; P < .05).
Researchers also assessed scores on the Clinical Global Impressions, finding significantly greater score improvement for both the 1.5-mg/day (P = .0026) and 3-mg/day (P =.0076) groups vs. the placebo group.
Improvement at week 6 in mean total score on the Hamilton Depression Rating Scale (HAM-17) reached nominal significance for cariprazine 1.5 mg/day vs. placebo – but not for 3 mg/day.
The results of this “high-quality” double-blind, randomized, controlled, parallel group study provide “what I regard as proven efficacy,” Dr. Sachs said.
He added that the investigational drug was also relatively safe. “The vast majority of patients tolerated it quite well,” he stressed. In addition, the drop-out rate because of adverse events was “quite low overall.”
The only adverse events (AEs) that occurred with the active treatment at a frequency of 5% or more and double that of placebo were akathisia and nausea. Changes in weight were relatively small, at less than 1 kg, in all treatment groups.
There was one serious AE in each active drug group, one of which was a kidney infection. There were two serious AEs reported in the placebo group, including one patient with multiple sclerosis. There were no deaths.
Dr. Sachs noted an advantage of cariprazine is its long half-life, which makes it more user-friendly because “it forgives you if you miss a dose or two.”
Drug manufacturer AbbVie’s supplemental New Drug Application for cariprazine is currently under review by the FDA for expanded use as adjunctive treatment of MDD. A decision by the agency is expected by the end of this year.
Another potential treatment option
Commenting on the findings, James Murrough, MD, PhD, associate professor of psychiatry and of neuroscience and director of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai, New York, said he welcomes research into additional treatments for MDD.
“Each medicine in a particular class has a unique pharmacology, so a larger number of medication options may help the clinician find a good match for a particular patient,” said Dr. Murrough, who was not involved with the research.
He noted cariprazine is “somewhat unique” among the dopamine modulators in “preferring interactions with the D3 receptor, one of many types of dopamine receptors.”
Although the study results showed cariprazine was effective in MDD, it “does not entirely break new ground” because previous research has already established the drug’s efficacy as adjunctive therapy for patients with depression not responding to a standard antidepressant, said Dr. Murrough.
He also noted that the lower dose, but not the higher dose, of the drug was found to be significantly beneficial for patients, compared with placebo.
“This is a good reminder that higher doses of a medication are not always better,” Dr. Murrough said.
The study was funded by AbbVie. Dr. Sachs is a full-time employee of Signant Health, which conducted the training and quality control for this study. Dr. Murrough has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new results from a phase 3 study show.
Already approved by the U.S. Food and Drug Administration to treat adults with schizophrenia and manic, mixed, or depressive episodes of bipolar I disorder, cariprazine is under investigation as an add-on therapy for MDD.
“Even patients who appear to be nonresponsive to standard antidepressant drugs have a very good chance of responding” to cariprazine, lead study author Gary Sachs, MD, associate clinical professor of psychiatry at Massachusetts General Hospital, Boston, told this news organization.
He noted that cariprazine, which is a partial agonist at D2 and D3, as well as 5-HT1A, “is an entirely different class” of drugs.
“It’s worth understanding how to use drugs like cariprazine and expanding our nomenclature; instead of referring to these drugs as atypical antipsychotics, perhaps referring to them as atypical antidepressants makes more sense,” Dr. Sachs said.
The findings were presented at the annual meeting of the American Psychiatric Association.
More options critical
MDD is among the most common psychiatric disorders in the United States. In 2020, an estimated 21 million adults had at least one major depressive episode.
Previous research has shown almost half of patients with MDD do not experience satisfactory results from their current treatment regimen. Therefore, research on more options for patients is critical, Dr. Sachs said.
Results from a previously published placebo-controlled study showed adjunctive treatment with cariprazine at 2-mg to 4.5-mg per day doses was more effective than placebo in improving depressive symptoms in adults with MDD.
The new analysis included patients with MDD and an inadequate response to antidepressant therapy, including selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants. They were recruited from 116 centers in the United States and Europe.
Dr. Sachs noted that a nonresponse to an adequate dose of an antidepressant typically means having less than a 50% improvement over 6 weeks or more.
Researchers randomly assigned the patients to oral cariprazine 1.5 mg/day, cariprazine 3 mg/day, or placebo. All continued to take their antidepressant monotherapy.
The analysis included 757 mostly White participants (mean age, 44.8 years; 73.4% women). All had experienced depression for a “huge” part of their life (average, about 14 years), “not to mention their adult life,” said Dr. Sachs.
In addition, at the start of the study, the participants had been depressed for almost 8 months on average.
The primary endpoint was change at week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The mean baseline MADRS total score was 32.5.
Less is sometimes more
Results showed a significantly greater mean reduction in MADRS total score for cariprazine 1.5 mg/day vs. placebo at week 6 (P = .005). Significant differences from placebo were observed as early as week 2 and were maintained at week 4, as well as week 6.
“I can say with great confidence that the 1.5-mg dose met all the standards for efficacy,” Dr. Sachs said.
However, this was not the case for the 3-mg/day dose. Although there was a numerically greater reduction in MADRS total score for this dosage of the drug vs. placebo at week 6, the difference was not statistically significant (P = .07).
At week 6, more patients taking the active drug at 1.5 mg/day than placebo responded to treatment, defined as 50% or greater reduction in MADRS total score (44% vs. 34.9%, respectively; P < .05).
Researchers also assessed scores on the Clinical Global Impressions, finding significantly greater score improvement for both the 1.5-mg/day (P = .0026) and 3-mg/day (P =.0076) groups vs. the placebo group.
Improvement at week 6 in mean total score on the Hamilton Depression Rating Scale (HAM-17) reached nominal significance for cariprazine 1.5 mg/day vs. placebo – but not for 3 mg/day.
The results of this “high-quality” double-blind, randomized, controlled, parallel group study provide “what I regard as proven efficacy,” Dr. Sachs said.
He added that the investigational drug was also relatively safe. “The vast majority of patients tolerated it quite well,” he stressed. In addition, the drop-out rate because of adverse events was “quite low overall.”
The only adverse events (AEs) that occurred with the active treatment at a frequency of 5% or more and double that of placebo were akathisia and nausea. Changes in weight were relatively small, at less than 1 kg, in all treatment groups.
There was one serious AE in each active drug group, one of which was a kidney infection. There were two serious AEs reported in the placebo group, including one patient with multiple sclerosis. There were no deaths.
Dr. Sachs noted an advantage of cariprazine is its long half-life, which makes it more user-friendly because “it forgives you if you miss a dose or two.”
Drug manufacturer AbbVie’s supplemental New Drug Application for cariprazine is currently under review by the FDA for expanded use as adjunctive treatment of MDD. A decision by the agency is expected by the end of this year.
Another potential treatment option
Commenting on the findings, James Murrough, MD, PhD, associate professor of psychiatry and of neuroscience and director of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai, New York, said he welcomes research into additional treatments for MDD.
“Each medicine in a particular class has a unique pharmacology, so a larger number of medication options may help the clinician find a good match for a particular patient,” said Dr. Murrough, who was not involved with the research.
He noted cariprazine is “somewhat unique” among the dopamine modulators in “preferring interactions with the D3 receptor, one of many types of dopamine receptors.”
Although the study results showed cariprazine was effective in MDD, it “does not entirely break new ground” because previous research has already established the drug’s efficacy as adjunctive therapy for patients with depression not responding to a standard antidepressant, said Dr. Murrough.
He also noted that the lower dose, but not the higher dose, of the drug was found to be significantly beneficial for patients, compared with placebo.
“This is a good reminder that higher doses of a medication are not always better,” Dr. Murrough said.
The study was funded by AbbVie. Dr. Sachs is a full-time employee of Signant Health, which conducted the training and quality control for this study. Dr. Murrough has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new results from a phase 3 study show.
Already approved by the U.S. Food and Drug Administration to treat adults with schizophrenia and manic, mixed, or depressive episodes of bipolar I disorder, cariprazine is under investigation as an add-on therapy for MDD.
“Even patients who appear to be nonresponsive to standard antidepressant drugs have a very good chance of responding” to cariprazine, lead study author Gary Sachs, MD, associate clinical professor of psychiatry at Massachusetts General Hospital, Boston, told this news organization.
He noted that cariprazine, which is a partial agonist at D2 and D3, as well as 5-HT1A, “is an entirely different class” of drugs.
“It’s worth understanding how to use drugs like cariprazine and expanding our nomenclature; instead of referring to these drugs as atypical antipsychotics, perhaps referring to them as atypical antidepressants makes more sense,” Dr. Sachs said.
The findings were presented at the annual meeting of the American Psychiatric Association.
More options critical
MDD is among the most common psychiatric disorders in the United States. In 2020, an estimated 21 million adults had at least one major depressive episode.
Previous research has shown almost half of patients with MDD do not experience satisfactory results from their current treatment regimen. Therefore, research on more options for patients is critical, Dr. Sachs said.
Results from a previously published placebo-controlled study showed adjunctive treatment with cariprazine at 2-mg to 4.5-mg per day doses was more effective than placebo in improving depressive symptoms in adults with MDD.
The new analysis included patients with MDD and an inadequate response to antidepressant therapy, including selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants. They were recruited from 116 centers in the United States and Europe.
Dr. Sachs noted that a nonresponse to an adequate dose of an antidepressant typically means having less than a 50% improvement over 6 weeks or more.
Researchers randomly assigned the patients to oral cariprazine 1.5 mg/day, cariprazine 3 mg/day, or placebo. All continued to take their antidepressant monotherapy.
The analysis included 757 mostly White participants (mean age, 44.8 years; 73.4% women). All had experienced depression for a “huge” part of their life (average, about 14 years), “not to mention their adult life,” said Dr. Sachs.
In addition, at the start of the study, the participants had been depressed for almost 8 months on average.
The primary endpoint was change at week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The mean baseline MADRS total score was 32.5.
Less is sometimes more
Results showed a significantly greater mean reduction in MADRS total score for cariprazine 1.5 mg/day vs. placebo at week 6 (P = .005). Significant differences from placebo were observed as early as week 2 and were maintained at week 4, as well as week 6.
“I can say with great confidence that the 1.5-mg dose met all the standards for efficacy,” Dr. Sachs said.
However, this was not the case for the 3-mg/day dose. Although there was a numerically greater reduction in MADRS total score for this dosage of the drug vs. placebo at week 6, the difference was not statistically significant (P = .07).
At week 6, more patients taking the active drug at 1.5 mg/day than placebo responded to treatment, defined as 50% or greater reduction in MADRS total score (44% vs. 34.9%, respectively; P < .05).
Researchers also assessed scores on the Clinical Global Impressions, finding significantly greater score improvement for both the 1.5-mg/day (P = .0026) and 3-mg/day (P =.0076) groups vs. the placebo group.
Improvement at week 6 in mean total score on the Hamilton Depression Rating Scale (HAM-17) reached nominal significance for cariprazine 1.5 mg/day vs. placebo – but not for 3 mg/day.
The results of this “high-quality” double-blind, randomized, controlled, parallel group study provide “what I regard as proven efficacy,” Dr. Sachs said.
He added that the investigational drug was also relatively safe. “The vast majority of patients tolerated it quite well,” he stressed. In addition, the drop-out rate because of adverse events was “quite low overall.”
The only adverse events (AEs) that occurred with the active treatment at a frequency of 5% or more and double that of placebo were akathisia and nausea. Changes in weight were relatively small, at less than 1 kg, in all treatment groups.
There was one serious AE in each active drug group, one of which was a kidney infection. There were two serious AEs reported in the placebo group, including one patient with multiple sclerosis. There were no deaths.
Dr. Sachs noted an advantage of cariprazine is its long half-life, which makes it more user-friendly because “it forgives you if you miss a dose or two.”
Drug manufacturer AbbVie’s supplemental New Drug Application for cariprazine is currently under review by the FDA for expanded use as adjunctive treatment of MDD. A decision by the agency is expected by the end of this year.
Another potential treatment option
Commenting on the findings, James Murrough, MD, PhD, associate professor of psychiatry and of neuroscience and director of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai, New York, said he welcomes research into additional treatments for MDD.
“Each medicine in a particular class has a unique pharmacology, so a larger number of medication options may help the clinician find a good match for a particular patient,” said Dr. Murrough, who was not involved with the research.
He noted cariprazine is “somewhat unique” among the dopamine modulators in “preferring interactions with the D3 receptor, one of many types of dopamine receptors.”
Although the study results showed cariprazine was effective in MDD, it “does not entirely break new ground” because previous research has already established the drug’s efficacy as adjunctive therapy for patients with depression not responding to a standard antidepressant, said Dr. Murrough.
He also noted that the lower dose, but not the higher dose, of the drug was found to be significantly beneficial for patients, compared with placebo.
“This is a good reminder that higher doses of a medication are not always better,” Dr. Murrough said.
The study was funded by AbbVie. Dr. Sachs is a full-time employee of Signant Health, which conducted the training and quality control for this study. Dr. Murrough has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM APA 2022
Lithium lowers osteoporosis risk in bipolar patients…and orthopedists take notice
NEW ORLEANS –
“Our findings emphasize that bone health should be a priority in the clinical management of bipolar disorder, and that the potential bone-protective effects of lithium should be subjected to further study – both in the context of osteoporosis and bipolar disorder,” said Soren D. Ostergaard, MD, PhD, the study’s first author and a professor in the psychosis research unit, Aarhus (Denmark) University Hospital – Psychiatry.
For the retrospective cohort study, presented at the annual meeting of the American Psychiatric Association, and also published recently in JAMA Psychiatry, the authors reviewed data on 22,912 patients treated for bipolar disorder in Denmark between 1996 and 2019, and compared each patient with 5 age- and sex-matched controls, amounting to 114,560 individuals in the general population.
Of the patients with bipolar disorder, 38.2% were treated with lithium, while 73.6% received an antipsychotic drug; 16.8% received valproate and 33.1% received lamotrigine.
With a median follow-up of 7.7 years, the incidence of osteoporosis per 1,000 person-years was 8.70 among patients with bipolar disorder, compared with an incidence of 7.84 among controls, (hazard rate ratio, 1.15).
The association of bipolar disorder with osteoporosis was notably more pronounced among males (HRR, 1.42) compared with females (HRR, 1.07).
Notably, those with bipolar disorder treated with lithium showed a significantly reduced risk of osteoporosis compared with patients not receiving lithium (HRR, 0.62), after adjustment for factors including age, sex, Charlson Comorbidity Index, use of systemic corticosteroids, use of sedative medication, and eating disorder diagnosis. No similar reductions in osteoporosis risk were observed among those treated with antipsychotics, valproate or lamotrigine.
Of note, the reduced risk of osteoporosis with lithium appeared after about year 2 of treatment (HR, 0.77) and remained steady at more than 4 years (HR, 0.76). A higher cumulative lithium dose was meanwhile associated with a greater decrease in the risk of osteoporosis (P < .001).
Results confirm prior research
The results are consistent with previous smaller studies indicating that people with bipolar disorders shown an increased risk of low bone density, osteopenia, and even fracture.
The higher risk of osteoporosis in bipolar disorder may be explained by lifestyle factors, Dr. Ostergaard noted in an interview.
“It could be the depressive and manic phases in bipolar disorder, but generally speaking, both phases can lead to an unhealthy lifestyle and that’s likely what drives the association between bipolar disorder and osteoporosis,” he said. “Increases in behaviors such as smoking and alcohol consumption may be factors as well. Similar findings are seen with depression.”
While more needs to be understood, Dr. Ostergaard speculated that higher rates of such behaviors in men with bipolar disorder may explain the higher osteoporosis risk observed in men.
In general, however, the increased risk underscores the importance of raising awareness of bone health among patients with bipolar disorder, the authors concluded.
“Specifically, guiding patients toward a lifestyle supporting bone health (no smoking, reduced alcohol consumption, healthy diet, and exercising) and monitoring bone density via dual-energy x-ray absorptiometry scans among those with additional risk factors seems warranted,” they wrote.
The implications of the lithium findings are trickier to determine, Dr. Ostergaard said.
“The evidence for lithium in bipolar disorder are well established, and our findings don’t really add to that,” he said. “The main thing is it suggests there might be some advantages of lithium that we’re not really aware of.”
Findings important for orthopedists
The unique properties observed with lithium have caught the attention of some in orthopedics, and researchers with the University of Toronto – having found intriguing bone healing with lithium in preclinical rodent studies – are currently conducting a first-of-its-kind multicenter, randomized, controlled clinical trial evaluating the potential effects of lithium in the healing of bone fractures.
Diane Nam, MD, of the division of orthopedic surgery, Sunnybrook Health Sciences Centre, Toronto, and lead investigator on the study, said in an interview that “I’m not surprised by [Dr. Ostergaard’s] paper because it’s consistent with what we have observed about the positive effects on bone healing.”
Dr. Nam and associates have already established administration parameters for their clinical study, determining that optimal effects in fracture healing appear to require that lithium treatment not begin at the time of fracture, but 2 weeks afterward, when new bone is ready to be laid down at the fracture site. In their trial, low daily doses of lithium (at 300 mg) are given only for a duration of 2 weeks.
“While our current trial is intended for a healthy, nonosteoporotic adult population, we have also demonstrated in our preclinical studies that lithium is just as effective in improving fracture healing in an osteoporotic model when the timing of administration is slightly delayed,” she said. “How this is relevant and translatable in patients with bipolar disorder requires further study.”
Dr. Nam said her research team thinks that “not only will the fracture heal faster, but it will heal reliably as delayed or impaired fracture healing remains a significant orthopedic problem.”
While details are not yet available, a preliminary analysis has shown results “going in a positive direction,” enough for the team to be granted funding for the multicenter trial.
Dr. Ostergaard and Dr. Nam reported no disclosures or conflicts.
NEW ORLEANS –
“Our findings emphasize that bone health should be a priority in the clinical management of bipolar disorder, and that the potential bone-protective effects of lithium should be subjected to further study – both in the context of osteoporosis and bipolar disorder,” said Soren D. Ostergaard, MD, PhD, the study’s first author and a professor in the psychosis research unit, Aarhus (Denmark) University Hospital – Psychiatry.
For the retrospective cohort study, presented at the annual meeting of the American Psychiatric Association, and also published recently in JAMA Psychiatry, the authors reviewed data on 22,912 patients treated for bipolar disorder in Denmark between 1996 and 2019, and compared each patient with 5 age- and sex-matched controls, amounting to 114,560 individuals in the general population.
Of the patients with bipolar disorder, 38.2% were treated with lithium, while 73.6% received an antipsychotic drug; 16.8% received valproate and 33.1% received lamotrigine.
With a median follow-up of 7.7 years, the incidence of osteoporosis per 1,000 person-years was 8.70 among patients with bipolar disorder, compared with an incidence of 7.84 among controls, (hazard rate ratio, 1.15).
The association of bipolar disorder with osteoporosis was notably more pronounced among males (HRR, 1.42) compared with females (HRR, 1.07).
Notably, those with bipolar disorder treated with lithium showed a significantly reduced risk of osteoporosis compared with patients not receiving lithium (HRR, 0.62), after adjustment for factors including age, sex, Charlson Comorbidity Index, use of systemic corticosteroids, use of sedative medication, and eating disorder diagnosis. No similar reductions in osteoporosis risk were observed among those treated with antipsychotics, valproate or lamotrigine.
Of note, the reduced risk of osteoporosis with lithium appeared after about year 2 of treatment (HR, 0.77) and remained steady at more than 4 years (HR, 0.76). A higher cumulative lithium dose was meanwhile associated with a greater decrease in the risk of osteoporosis (P < .001).
Results confirm prior research
The results are consistent with previous smaller studies indicating that people with bipolar disorders shown an increased risk of low bone density, osteopenia, and even fracture.
The higher risk of osteoporosis in bipolar disorder may be explained by lifestyle factors, Dr. Ostergaard noted in an interview.
“It could be the depressive and manic phases in bipolar disorder, but generally speaking, both phases can lead to an unhealthy lifestyle and that’s likely what drives the association between bipolar disorder and osteoporosis,” he said. “Increases in behaviors such as smoking and alcohol consumption may be factors as well. Similar findings are seen with depression.”
While more needs to be understood, Dr. Ostergaard speculated that higher rates of such behaviors in men with bipolar disorder may explain the higher osteoporosis risk observed in men.
In general, however, the increased risk underscores the importance of raising awareness of bone health among patients with bipolar disorder, the authors concluded.
“Specifically, guiding patients toward a lifestyle supporting bone health (no smoking, reduced alcohol consumption, healthy diet, and exercising) and monitoring bone density via dual-energy x-ray absorptiometry scans among those with additional risk factors seems warranted,” they wrote.
The implications of the lithium findings are trickier to determine, Dr. Ostergaard said.
“The evidence for lithium in bipolar disorder are well established, and our findings don’t really add to that,” he said. “The main thing is it suggests there might be some advantages of lithium that we’re not really aware of.”
Findings important for orthopedists
The unique properties observed with lithium have caught the attention of some in orthopedics, and researchers with the University of Toronto – having found intriguing bone healing with lithium in preclinical rodent studies – are currently conducting a first-of-its-kind multicenter, randomized, controlled clinical trial evaluating the potential effects of lithium in the healing of bone fractures.
Diane Nam, MD, of the division of orthopedic surgery, Sunnybrook Health Sciences Centre, Toronto, and lead investigator on the study, said in an interview that “I’m not surprised by [Dr. Ostergaard’s] paper because it’s consistent with what we have observed about the positive effects on bone healing.”
Dr. Nam and associates have already established administration parameters for their clinical study, determining that optimal effects in fracture healing appear to require that lithium treatment not begin at the time of fracture, but 2 weeks afterward, when new bone is ready to be laid down at the fracture site. In their trial, low daily doses of lithium (at 300 mg) are given only for a duration of 2 weeks.
“While our current trial is intended for a healthy, nonosteoporotic adult population, we have also demonstrated in our preclinical studies that lithium is just as effective in improving fracture healing in an osteoporotic model when the timing of administration is slightly delayed,” she said. “How this is relevant and translatable in patients with bipolar disorder requires further study.”
Dr. Nam said her research team thinks that “not only will the fracture heal faster, but it will heal reliably as delayed or impaired fracture healing remains a significant orthopedic problem.”
While details are not yet available, a preliminary analysis has shown results “going in a positive direction,” enough for the team to be granted funding for the multicenter trial.
Dr. Ostergaard and Dr. Nam reported no disclosures or conflicts.
NEW ORLEANS –
“Our findings emphasize that bone health should be a priority in the clinical management of bipolar disorder, and that the potential bone-protective effects of lithium should be subjected to further study – both in the context of osteoporosis and bipolar disorder,” said Soren D. Ostergaard, MD, PhD, the study’s first author and a professor in the psychosis research unit, Aarhus (Denmark) University Hospital – Psychiatry.
For the retrospective cohort study, presented at the annual meeting of the American Psychiatric Association, and also published recently in JAMA Psychiatry, the authors reviewed data on 22,912 patients treated for bipolar disorder in Denmark between 1996 and 2019, and compared each patient with 5 age- and sex-matched controls, amounting to 114,560 individuals in the general population.
Of the patients with bipolar disorder, 38.2% were treated with lithium, while 73.6% received an antipsychotic drug; 16.8% received valproate and 33.1% received lamotrigine.
With a median follow-up of 7.7 years, the incidence of osteoporosis per 1,000 person-years was 8.70 among patients with bipolar disorder, compared with an incidence of 7.84 among controls, (hazard rate ratio, 1.15).
The association of bipolar disorder with osteoporosis was notably more pronounced among males (HRR, 1.42) compared with females (HRR, 1.07).
Notably, those with bipolar disorder treated with lithium showed a significantly reduced risk of osteoporosis compared with patients not receiving lithium (HRR, 0.62), after adjustment for factors including age, sex, Charlson Comorbidity Index, use of systemic corticosteroids, use of sedative medication, and eating disorder diagnosis. No similar reductions in osteoporosis risk were observed among those treated with antipsychotics, valproate or lamotrigine.
Of note, the reduced risk of osteoporosis with lithium appeared after about year 2 of treatment (HR, 0.77) and remained steady at more than 4 years (HR, 0.76). A higher cumulative lithium dose was meanwhile associated with a greater decrease in the risk of osteoporosis (P < .001).
Results confirm prior research
The results are consistent with previous smaller studies indicating that people with bipolar disorders shown an increased risk of low bone density, osteopenia, and even fracture.
The higher risk of osteoporosis in bipolar disorder may be explained by lifestyle factors, Dr. Ostergaard noted in an interview.
“It could be the depressive and manic phases in bipolar disorder, but generally speaking, both phases can lead to an unhealthy lifestyle and that’s likely what drives the association between bipolar disorder and osteoporosis,” he said. “Increases in behaviors such as smoking and alcohol consumption may be factors as well. Similar findings are seen with depression.”
While more needs to be understood, Dr. Ostergaard speculated that higher rates of such behaviors in men with bipolar disorder may explain the higher osteoporosis risk observed in men.
In general, however, the increased risk underscores the importance of raising awareness of bone health among patients with bipolar disorder, the authors concluded.
“Specifically, guiding patients toward a lifestyle supporting bone health (no smoking, reduced alcohol consumption, healthy diet, and exercising) and monitoring bone density via dual-energy x-ray absorptiometry scans among those with additional risk factors seems warranted,” they wrote.
The implications of the lithium findings are trickier to determine, Dr. Ostergaard said.
“The evidence for lithium in bipolar disorder are well established, and our findings don’t really add to that,” he said. “The main thing is it suggests there might be some advantages of lithium that we’re not really aware of.”
Findings important for orthopedists
The unique properties observed with lithium have caught the attention of some in orthopedics, and researchers with the University of Toronto – having found intriguing bone healing with lithium in preclinical rodent studies – are currently conducting a first-of-its-kind multicenter, randomized, controlled clinical trial evaluating the potential effects of lithium in the healing of bone fractures.
Diane Nam, MD, of the division of orthopedic surgery, Sunnybrook Health Sciences Centre, Toronto, and lead investigator on the study, said in an interview that “I’m not surprised by [Dr. Ostergaard’s] paper because it’s consistent with what we have observed about the positive effects on bone healing.”
Dr. Nam and associates have already established administration parameters for their clinical study, determining that optimal effects in fracture healing appear to require that lithium treatment not begin at the time of fracture, but 2 weeks afterward, when new bone is ready to be laid down at the fracture site. In their trial, low daily doses of lithium (at 300 mg) are given only for a duration of 2 weeks.
“While our current trial is intended for a healthy, nonosteoporotic adult population, we have also demonstrated in our preclinical studies that lithium is just as effective in improving fracture healing in an osteoporotic model when the timing of administration is slightly delayed,” she said. “How this is relevant and translatable in patients with bipolar disorder requires further study.”
Dr. Nam said her research team thinks that “not only will the fracture heal faster, but it will heal reliably as delayed or impaired fracture healing remains a significant orthopedic problem.”
While details are not yet available, a preliminary analysis has shown results “going in a positive direction,” enough for the team to be granted funding for the multicenter trial.
Dr. Ostergaard and Dr. Nam reported no disclosures or conflicts.
AT APA 2022
Multiple mental health woes? Blame it on genetics
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE GENETICS
Neuropsychiatric risks of COVID-19: New data
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.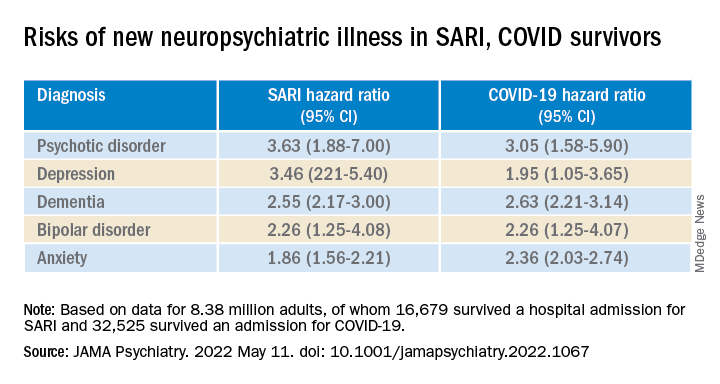
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
Do psychotropic meds raise or lower COVID risk in psych patients?
Investigators found that second-generation antipsychotics were associated with a 48% lower risk of COVID-19, while valproic acid was associated with a 39% increased risk of the disease.
“Exposures to several psychotropic medications were associated with risk of COVID-19 infection among inpatients with serious mental illness; decreased risk was observed with the use of second generation antipsychotics, with paliperidone use associated with the largest effect size. Valproic acid use was associated with an increased risk of infection,” the investigators, led by Katlyn Nemani, MD, at NYU Langone Medical Center, New York, write.
The study was published online in JAMA Network Open.
Vulnerable population
Patients with serious mental illness are particularly vulnerable to COVID-19. Several psychotropic medications have been identified as potential therapeutic agents to prevent or treat COVID-19, but they have not been systematically studied in this patient population.
The researchers analyzed data from 1,958 adults who were continuously hospitalized with serious mental illness from March 8 to July 1, 2020. The mean age was 51.4 years, and 1,442 (74%) were men.
A total of 969 patients (49.5%) had laboratory-confirmed COVID-19 while hospitalized, and 38 (3.9%) died – a mortality rate four times higher than estimates from the general population in New York during the same time frame, the researchers note.
“This finding is consistent with prior studies that have found increased rates of infection in congregate settings and increased mortality after infection among patients with serious mental illness,” the investigators write.
The use of second-generation antipsychotic medications, as a class, was associated with a lower likelihood of COVID-19 (odds ratio, 0.62; 95% confidence interval, 0.45-0.86), while the use of mood stabilizers was associated with increased likelihood of infection (OR, 1.23; 95% CI, 1.03-1.47).
In a multivariable model of individual medications, use of the long-acting atypical antipsychotic paliperidone was associated with a lower odds of infection (OR, 0.59; 95% CI, 0.41-0.84), and use of valproic acid was associated with increased odds of infection (OR, 1.39; 95% CI, 1.10-1.76).
Valproic acid downregulates angiotensin-converting enzyme 2 in endothelial cells, which may impair immune function and contribute to poor outcomes for patients with COVID-19, the researchers say.
The use of clozapine was associated with reduced odds of COVID-related death (unadjusted OR, 0.25; 95% CI, 0.10-0.62; fully adjusted OR, 0.43; 95% CI, 0.17-1.12).
“Although there have been concerns about clozapine use during the pandemic as a risk factor for pneumonia and potential toxic effects during acute infection, clozapine use was not associated with an increased risk of COVID-19 infection or death in the present study. In fact, unadjusted estimates suggested a significant protective association,” the investigators write.
However, they note, data on clozapine and COVID-19 have been mixed.
Two prior studies of health record data showed an increased risk of COVID-19 associated with clozapine treatment, while a study that was limited to inpatients found a lower risk of infection and a lower risk of symptomatic disease in association with clozapine use.
The researchers also found a lower mortality risk in patients taking antidepressants; there were no COVID-related deaths among patients taking escitalopram, venlafaxine, bupropion, or fluvoxamine.
Although the association was not statistically significant, this observation is in line with larger studies that showed reduced risk of adverse outcomes associated with antidepressant use, the researchers note.
A matter of debate
In an accompanying commentary, Benedetta Vai, PhD, and Mario Gennaro Mazza, MD, with IRCCS San Raffaele Scientific Institute, Milan, point out that the link between psychopharmacologic compounds, in particular antipsychotics, and severe COVID-19 outcomes remains “a matter of debate, with inconsistent findings between studies.”
They note further research is needed to determine whether the protective role of second-generation antipsychotics on risk of COVID-19 is mediated by an immune effect or by the direct antiviral properties of these molecules.
The study had no specific funding. Dr. Nemani, Dr. Vai, and Dr. Mazza have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that second-generation antipsychotics were associated with a 48% lower risk of COVID-19, while valproic acid was associated with a 39% increased risk of the disease.
“Exposures to several psychotropic medications were associated with risk of COVID-19 infection among inpatients with serious mental illness; decreased risk was observed with the use of second generation antipsychotics, with paliperidone use associated with the largest effect size. Valproic acid use was associated with an increased risk of infection,” the investigators, led by Katlyn Nemani, MD, at NYU Langone Medical Center, New York, write.
The study was published online in JAMA Network Open.
Vulnerable population
Patients with serious mental illness are particularly vulnerable to COVID-19. Several psychotropic medications have been identified as potential therapeutic agents to prevent or treat COVID-19, but they have not been systematically studied in this patient population.
The researchers analyzed data from 1,958 adults who were continuously hospitalized with serious mental illness from March 8 to July 1, 2020. The mean age was 51.4 years, and 1,442 (74%) were men.
A total of 969 patients (49.5%) had laboratory-confirmed COVID-19 while hospitalized, and 38 (3.9%) died – a mortality rate four times higher than estimates from the general population in New York during the same time frame, the researchers note.
“This finding is consistent with prior studies that have found increased rates of infection in congregate settings and increased mortality after infection among patients with serious mental illness,” the investigators write.
The use of second-generation antipsychotic medications, as a class, was associated with a lower likelihood of COVID-19 (odds ratio, 0.62; 95% confidence interval, 0.45-0.86), while the use of mood stabilizers was associated with increased likelihood of infection (OR, 1.23; 95% CI, 1.03-1.47).
In a multivariable model of individual medications, use of the long-acting atypical antipsychotic paliperidone was associated with a lower odds of infection (OR, 0.59; 95% CI, 0.41-0.84), and use of valproic acid was associated with increased odds of infection (OR, 1.39; 95% CI, 1.10-1.76).
Valproic acid downregulates angiotensin-converting enzyme 2 in endothelial cells, which may impair immune function and contribute to poor outcomes for patients with COVID-19, the researchers say.
The use of clozapine was associated with reduced odds of COVID-related death (unadjusted OR, 0.25; 95% CI, 0.10-0.62; fully adjusted OR, 0.43; 95% CI, 0.17-1.12).
“Although there have been concerns about clozapine use during the pandemic as a risk factor for pneumonia and potential toxic effects during acute infection, clozapine use was not associated with an increased risk of COVID-19 infection or death in the present study. In fact, unadjusted estimates suggested a significant protective association,” the investigators write.
However, they note, data on clozapine and COVID-19 have been mixed.
Two prior studies of health record data showed an increased risk of COVID-19 associated with clozapine treatment, while a study that was limited to inpatients found a lower risk of infection and a lower risk of symptomatic disease in association with clozapine use.
The researchers also found a lower mortality risk in patients taking antidepressants; there were no COVID-related deaths among patients taking escitalopram, venlafaxine, bupropion, or fluvoxamine.
Although the association was not statistically significant, this observation is in line with larger studies that showed reduced risk of adverse outcomes associated with antidepressant use, the researchers note.
A matter of debate
In an accompanying commentary, Benedetta Vai, PhD, and Mario Gennaro Mazza, MD, with IRCCS San Raffaele Scientific Institute, Milan, point out that the link between psychopharmacologic compounds, in particular antipsychotics, and severe COVID-19 outcomes remains “a matter of debate, with inconsistent findings between studies.”
They note further research is needed to determine whether the protective role of second-generation antipsychotics on risk of COVID-19 is mediated by an immune effect or by the direct antiviral properties of these molecules.
The study had no specific funding. Dr. Nemani, Dr. Vai, and Dr. Mazza have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that second-generation antipsychotics were associated with a 48% lower risk of COVID-19, while valproic acid was associated with a 39% increased risk of the disease.
“Exposures to several psychotropic medications were associated with risk of COVID-19 infection among inpatients with serious mental illness; decreased risk was observed with the use of second generation antipsychotics, with paliperidone use associated with the largest effect size. Valproic acid use was associated with an increased risk of infection,” the investigators, led by Katlyn Nemani, MD, at NYU Langone Medical Center, New York, write.
The study was published online in JAMA Network Open.
Vulnerable population
Patients with serious mental illness are particularly vulnerable to COVID-19. Several psychotropic medications have been identified as potential therapeutic agents to prevent or treat COVID-19, but they have not been systematically studied in this patient population.
The researchers analyzed data from 1,958 adults who were continuously hospitalized with serious mental illness from March 8 to July 1, 2020. The mean age was 51.4 years, and 1,442 (74%) were men.
A total of 969 patients (49.5%) had laboratory-confirmed COVID-19 while hospitalized, and 38 (3.9%) died – a mortality rate four times higher than estimates from the general population in New York during the same time frame, the researchers note.
“This finding is consistent with prior studies that have found increased rates of infection in congregate settings and increased mortality after infection among patients with serious mental illness,” the investigators write.
The use of second-generation antipsychotic medications, as a class, was associated with a lower likelihood of COVID-19 (odds ratio, 0.62; 95% confidence interval, 0.45-0.86), while the use of mood stabilizers was associated with increased likelihood of infection (OR, 1.23; 95% CI, 1.03-1.47).
In a multivariable model of individual medications, use of the long-acting atypical antipsychotic paliperidone was associated with a lower odds of infection (OR, 0.59; 95% CI, 0.41-0.84), and use of valproic acid was associated with increased odds of infection (OR, 1.39; 95% CI, 1.10-1.76).
Valproic acid downregulates angiotensin-converting enzyme 2 in endothelial cells, which may impair immune function and contribute to poor outcomes for patients with COVID-19, the researchers say.
The use of clozapine was associated with reduced odds of COVID-related death (unadjusted OR, 0.25; 95% CI, 0.10-0.62; fully adjusted OR, 0.43; 95% CI, 0.17-1.12).
“Although there have been concerns about clozapine use during the pandemic as a risk factor for pneumonia and potential toxic effects during acute infection, clozapine use was not associated with an increased risk of COVID-19 infection or death in the present study. In fact, unadjusted estimates suggested a significant protective association,” the investigators write.
However, they note, data on clozapine and COVID-19 have been mixed.
Two prior studies of health record data showed an increased risk of COVID-19 associated with clozapine treatment, while a study that was limited to inpatients found a lower risk of infection and a lower risk of symptomatic disease in association with clozapine use.
The researchers also found a lower mortality risk in patients taking antidepressants; there were no COVID-related deaths among patients taking escitalopram, venlafaxine, bupropion, or fluvoxamine.
Although the association was not statistically significant, this observation is in line with larger studies that showed reduced risk of adverse outcomes associated with antidepressant use, the researchers note.
A matter of debate
In an accompanying commentary, Benedetta Vai, PhD, and Mario Gennaro Mazza, MD, with IRCCS San Raffaele Scientific Institute, Milan, point out that the link between psychopharmacologic compounds, in particular antipsychotics, and severe COVID-19 outcomes remains “a matter of debate, with inconsistent findings between studies.”
They note further research is needed to determine whether the protective role of second-generation antipsychotics on risk of COVID-19 is mediated by an immune effect or by the direct antiviral properties of these molecules.
The study had no specific funding. Dr. Nemani, Dr. Vai, and Dr. Mazza have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Mood instability in childhood as a precursor to bipolar disorder
Mood instability, or sudden, unpredictable, and frequent shifts in emotional states, characterizes many types of psychiatric disorder, including attention-deficit/hyperactivity disorder (ADHD), personality disorders, depression, and posttraumatic stress disorder. To say that individuals with bipolar disorder (BD) have mood instability sounds like a tautology. Nonetheless, mood instability has particular relevance to BD: Many patients have irregular or labile moods even when they are between major episodes of mania and depression.1
Children of parents with BD who have high levels of mood instability are at particularly high risk for developing BD (types I or II) in late adolescence or early adulthood.2 The following case provides an illustration:
Patrick, age 14, entered treatment with diagnoses of ADHD and other specified bipolar disorder. His mother felt that his behavior resembled that of his father, who had been treated for manic episodes. During the COVID-19 pandemic, Patrick had become increasingly difficult at home, with significant oppositionality, impulsive behavior, and difficulty following through on school assignments or household tasks. His mother’s most significant complaints concerned Patrick’s sudden outbursts of anger and abrupt verbal abuse when she asked him to stop playing video games. When interrupted, he cursed loudly and sometimes turned violent; he had broken a window and a door at home and had on one occasion physically attacked his younger brother. Patrick agreed that he became angry at times, but felt that others provoked him. When queried about depression, he described anxiety and worry. He was unable to describe a particular trigger for his anxiety except for being interrupted in online games with his friends, which made him “feel like a total loser.”
His mother reported that Patrick had multiple 1- to 2-day intervals in which he became “really silly, laughing at nothing,” talking rapidly, jumping from one topic to another, and becoming annoyed when others didn’t share his enthusiasm. In these activated intervals, he slept little and seemed to be full of energy; his mother would hear him talking loudly into his phone throughout the night. During one such interval he had become verbally aggressive with a peer, which had ruined their friendship. Both Patrick and his mother reported that they had been fighting constantly and, in her words, “our house has become a war zone.”
In our recent article in the Journal of the American Academy of Child and Adolescent Psychiatry,3 my coauthors and I examined the association between parents’ ratings of mood instability and clinicians’ longitudinal ratings of symptoms and functioning among youth (ages 9-17 years) who were at high risk for BD. The participants met DSM-5 diagnostic criteria for major depressive disorder or other specified BD, defined as recurrent and brief periods of elevation and activation that did not meet syndromal mania or hypomania criteria. All participants had at least one first- or second-degree family member with a history of BD I or II. Following a period of evaluation, participants were randomly assigned to one of two 4-month psychological therapies: Family-focused therapy (12 sessions of psychoeducation, communication training, and problem-solving skills training) or enhanced usual care (6 sessions of family and individual psychoeducation and support). They also received pharmacological management from study-affiliated psychiatrists when warranted.
We measured mood instability at intake and every 4-6 months over an average of 2 years (range 0-255 weeks). We used a brief parent questionnaire – the Children’s Affective Lability Scale4 – which enables measurement of lability on the dimensions of elevation or activation (e.g., bursts of silliness or hilarity, excessive familiarity with others), irritability (e.g., temper outbursts), or anxious-depression (e.g., sudden bouts of crying).
Over the 1- to 4-year period of follow-up, mood instability was associated with poor prognosis indicators in high-risk youth: Being younger, having younger ages at first symptom onset, being diagnosed with other specified BD (vs. major depression), and having more complex patterns of comorbid disorders. Mood instability tracked closely with levels of mania, depression, and global functioning over the follow-up. There was a temporal pathway between a diagnosis of other specified bipolar disorder at intake and higher levels of mood instability at follow-up, which in turn predicted higher levels of parent/child conflict. High levels of mood lability may lead to isolation from peers and tension within family relationships, which may fuel further children’s expressions of frustration, rage, depression, or impulsive behavior.
Youth with higher levels of mood instability required more complex medication regimens over 1 year than did those with lower instability. There was an overall reduction in mood instability as children aged (or spent more time in treatment). Over the 1- to 4-year follow-up, family-focused therapy was associated with longer intervals prior to new mood episodes than was enhanced usual care, but reductions in mood instability were independent of the type of psychosocial treatment assigned to children.
The participants in this study could not be followed long enough to determine whether levels of mood instability were associated with the later development of syndromal BD. Other studies, however, have documented this relationship. Large-scale longitudinal studies of high-risk children find that measures of mood lability – along with early onset manic symptoms, depression, anxiety, and a family history of mania or hypomania – can be combined to calculate the risk that any individual child will develop BD I or II over the next 5-8 years.2,5
Clinicians should include measurement of the severity and psychosocial determinants of persistent mood shifts in youth under their care, particularly those with a family history of BD. Mood instability is associated with more severe symptom trajectories, more social isolation, and greater distress and conflict within the family. It may require a greater intensity of both pharmacological and psychosocial treatments to treat existing symptoms and functional impairments, and to prevent further mood deterioration.
Dr. Miklowitz is Distinguished Professor of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior. He is the author of “The Bipolar Disorder Survival Guide, 3rd Ed.” (New York: Guilford Press, 2019) and “Bipolar Disorder: A Family-Focused Treatment Approach, 2nd Ed” (New York: Guilford Press, 2010). He has no conflicts of interest to disclose. Contact Dr. Miklowitz at dmiklowitz@mednet.ucla.edu.
References
1. Bonsall MB, et al. Nonlinear time-series approaches in characterizing mood stability and mood instability in bipolar disorder. Proc Biol Sci. Mar 7 2012;279(1730):916-24. doi: 10.1098/rspb.2011.1246.
2. Hafeman DM, et al. Toward the definition of a bipolar prodrome: Dimensional predictors of bipolar spectrum disorders in at-risk youths. Am J Psychiatry. 2016;173(7):695-704. doi: 10.1176/appi.ajp.2015.15040414.
3. Miklowitz DJ, et al. Mood instability in youth at high risk for bipolar disorder. J Am Acad Child Adol Psychiatry. 2022 Mar 17;S0890-8567(22)00118-6. doi: 10.1016/j.jaac.2022.03.009.
4. Gerson AC, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. Dec 20 1996;65(3):189-98. doi: 10.1016/s0165-1781(96)02851-x.
5. Birmaher B, et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adol Psychiatry. 2018;57(10):755-63. doi: 10.1016/j.jaac.2018.05.023.
Mood instability, or sudden, unpredictable, and frequent shifts in emotional states, characterizes many types of psychiatric disorder, including attention-deficit/hyperactivity disorder (ADHD), personality disorders, depression, and posttraumatic stress disorder. To say that individuals with bipolar disorder (BD) have mood instability sounds like a tautology. Nonetheless, mood instability has particular relevance to BD: Many patients have irregular or labile moods even when they are between major episodes of mania and depression.1
Children of parents with BD who have high levels of mood instability are at particularly high risk for developing BD (types I or II) in late adolescence or early adulthood.2 The following case provides an illustration:
Patrick, age 14, entered treatment with diagnoses of ADHD and other specified bipolar disorder. His mother felt that his behavior resembled that of his father, who had been treated for manic episodes. During the COVID-19 pandemic, Patrick had become increasingly difficult at home, with significant oppositionality, impulsive behavior, and difficulty following through on school assignments or household tasks. His mother’s most significant complaints concerned Patrick’s sudden outbursts of anger and abrupt verbal abuse when she asked him to stop playing video games. When interrupted, he cursed loudly and sometimes turned violent; he had broken a window and a door at home and had on one occasion physically attacked his younger brother. Patrick agreed that he became angry at times, but felt that others provoked him. When queried about depression, he described anxiety and worry. He was unable to describe a particular trigger for his anxiety except for being interrupted in online games with his friends, which made him “feel like a total loser.”
His mother reported that Patrick had multiple 1- to 2-day intervals in which he became “really silly, laughing at nothing,” talking rapidly, jumping from one topic to another, and becoming annoyed when others didn’t share his enthusiasm. In these activated intervals, he slept little and seemed to be full of energy; his mother would hear him talking loudly into his phone throughout the night. During one such interval he had become verbally aggressive with a peer, which had ruined their friendship. Both Patrick and his mother reported that they had been fighting constantly and, in her words, “our house has become a war zone.”
In our recent article in the Journal of the American Academy of Child and Adolescent Psychiatry,3 my coauthors and I examined the association between parents’ ratings of mood instability and clinicians’ longitudinal ratings of symptoms and functioning among youth (ages 9-17 years) who were at high risk for BD. The participants met DSM-5 diagnostic criteria for major depressive disorder or other specified BD, defined as recurrent and brief periods of elevation and activation that did not meet syndromal mania or hypomania criteria. All participants had at least one first- or second-degree family member with a history of BD I or II. Following a period of evaluation, participants were randomly assigned to one of two 4-month psychological therapies: Family-focused therapy (12 sessions of psychoeducation, communication training, and problem-solving skills training) or enhanced usual care (6 sessions of family and individual psychoeducation and support). They also received pharmacological management from study-affiliated psychiatrists when warranted.
We measured mood instability at intake and every 4-6 months over an average of 2 years (range 0-255 weeks). We used a brief parent questionnaire – the Children’s Affective Lability Scale4 – which enables measurement of lability on the dimensions of elevation or activation (e.g., bursts of silliness or hilarity, excessive familiarity with others), irritability (e.g., temper outbursts), or anxious-depression (e.g., sudden bouts of crying).
Over the 1- to 4-year period of follow-up, mood instability was associated with poor prognosis indicators in high-risk youth: Being younger, having younger ages at first symptom onset, being diagnosed with other specified BD (vs. major depression), and having more complex patterns of comorbid disorders. Mood instability tracked closely with levels of mania, depression, and global functioning over the follow-up. There was a temporal pathway between a diagnosis of other specified bipolar disorder at intake and higher levels of mood instability at follow-up, which in turn predicted higher levels of parent/child conflict. High levels of mood lability may lead to isolation from peers and tension within family relationships, which may fuel further children’s expressions of frustration, rage, depression, or impulsive behavior.
Youth with higher levels of mood instability required more complex medication regimens over 1 year than did those with lower instability. There was an overall reduction in mood instability as children aged (or spent more time in treatment). Over the 1- to 4-year follow-up, family-focused therapy was associated with longer intervals prior to new mood episodes than was enhanced usual care, but reductions in mood instability were independent of the type of psychosocial treatment assigned to children.
The participants in this study could not be followed long enough to determine whether levels of mood instability were associated with the later development of syndromal BD. Other studies, however, have documented this relationship. Large-scale longitudinal studies of high-risk children find that measures of mood lability – along with early onset manic symptoms, depression, anxiety, and a family history of mania or hypomania – can be combined to calculate the risk that any individual child will develop BD I or II over the next 5-8 years.2,5
Clinicians should include measurement of the severity and psychosocial determinants of persistent mood shifts in youth under their care, particularly those with a family history of BD. Mood instability is associated with more severe symptom trajectories, more social isolation, and greater distress and conflict within the family. It may require a greater intensity of both pharmacological and psychosocial treatments to treat existing symptoms and functional impairments, and to prevent further mood deterioration.
Dr. Miklowitz is Distinguished Professor of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior. He is the author of “The Bipolar Disorder Survival Guide, 3rd Ed.” (New York: Guilford Press, 2019) and “Bipolar Disorder: A Family-Focused Treatment Approach, 2nd Ed” (New York: Guilford Press, 2010). He has no conflicts of interest to disclose. Contact Dr. Miklowitz at dmiklowitz@mednet.ucla.edu.
References
1. Bonsall MB, et al. Nonlinear time-series approaches in characterizing mood stability and mood instability in bipolar disorder. Proc Biol Sci. Mar 7 2012;279(1730):916-24. doi: 10.1098/rspb.2011.1246.
2. Hafeman DM, et al. Toward the definition of a bipolar prodrome: Dimensional predictors of bipolar spectrum disorders in at-risk youths. Am J Psychiatry. 2016;173(7):695-704. doi: 10.1176/appi.ajp.2015.15040414.
3. Miklowitz DJ, et al. Mood instability in youth at high risk for bipolar disorder. J Am Acad Child Adol Psychiatry. 2022 Mar 17;S0890-8567(22)00118-6. doi: 10.1016/j.jaac.2022.03.009.
4. Gerson AC, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. Dec 20 1996;65(3):189-98. doi: 10.1016/s0165-1781(96)02851-x.
5. Birmaher B, et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adol Psychiatry. 2018;57(10):755-63. doi: 10.1016/j.jaac.2018.05.023.
Mood instability, or sudden, unpredictable, and frequent shifts in emotional states, characterizes many types of psychiatric disorder, including attention-deficit/hyperactivity disorder (ADHD), personality disorders, depression, and posttraumatic stress disorder. To say that individuals with bipolar disorder (BD) have mood instability sounds like a tautology. Nonetheless, mood instability has particular relevance to BD: Many patients have irregular or labile moods even when they are between major episodes of mania and depression.1
Children of parents with BD who have high levels of mood instability are at particularly high risk for developing BD (types I or II) in late adolescence or early adulthood.2 The following case provides an illustration:
Patrick, age 14, entered treatment with diagnoses of ADHD and other specified bipolar disorder. His mother felt that his behavior resembled that of his father, who had been treated for manic episodes. During the COVID-19 pandemic, Patrick had become increasingly difficult at home, with significant oppositionality, impulsive behavior, and difficulty following through on school assignments or household tasks. His mother’s most significant complaints concerned Patrick’s sudden outbursts of anger and abrupt verbal abuse when she asked him to stop playing video games. When interrupted, he cursed loudly and sometimes turned violent; he had broken a window and a door at home and had on one occasion physically attacked his younger brother. Patrick agreed that he became angry at times, but felt that others provoked him. When queried about depression, he described anxiety and worry. He was unable to describe a particular trigger for his anxiety except for being interrupted in online games with his friends, which made him “feel like a total loser.”
His mother reported that Patrick had multiple 1- to 2-day intervals in which he became “really silly, laughing at nothing,” talking rapidly, jumping from one topic to another, and becoming annoyed when others didn’t share his enthusiasm. In these activated intervals, he slept little and seemed to be full of energy; his mother would hear him talking loudly into his phone throughout the night. During one such interval he had become verbally aggressive with a peer, which had ruined their friendship. Both Patrick and his mother reported that they had been fighting constantly and, in her words, “our house has become a war zone.”
In our recent article in the Journal of the American Academy of Child and Adolescent Psychiatry,3 my coauthors and I examined the association between parents’ ratings of mood instability and clinicians’ longitudinal ratings of symptoms and functioning among youth (ages 9-17 years) who were at high risk for BD. The participants met DSM-5 diagnostic criteria for major depressive disorder or other specified BD, defined as recurrent and brief periods of elevation and activation that did not meet syndromal mania or hypomania criteria. All participants had at least one first- or second-degree family member with a history of BD I or II. Following a period of evaluation, participants were randomly assigned to one of two 4-month psychological therapies: Family-focused therapy (12 sessions of psychoeducation, communication training, and problem-solving skills training) or enhanced usual care (6 sessions of family and individual psychoeducation and support). They also received pharmacological management from study-affiliated psychiatrists when warranted.
We measured mood instability at intake and every 4-6 months over an average of 2 years (range 0-255 weeks). We used a brief parent questionnaire – the Children’s Affective Lability Scale4 – which enables measurement of lability on the dimensions of elevation or activation (e.g., bursts of silliness or hilarity, excessive familiarity with others), irritability (e.g., temper outbursts), or anxious-depression (e.g., sudden bouts of crying).
Over the 1- to 4-year period of follow-up, mood instability was associated with poor prognosis indicators in high-risk youth: Being younger, having younger ages at first symptom onset, being diagnosed with other specified BD (vs. major depression), and having more complex patterns of comorbid disorders. Mood instability tracked closely with levels of mania, depression, and global functioning over the follow-up. There was a temporal pathway between a diagnosis of other specified bipolar disorder at intake and higher levels of mood instability at follow-up, which in turn predicted higher levels of parent/child conflict. High levels of mood lability may lead to isolation from peers and tension within family relationships, which may fuel further children’s expressions of frustration, rage, depression, or impulsive behavior.
Youth with higher levels of mood instability required more complex medication regimens over 1 year than did those with lower instability. There was an overall reduction in mood instability as children aged (or spent more time in treatment). Over the 1- to 4-year follow-up, family-focused therapy was associated with longer intervals prior to new mood episodes than was enhanced usual care, but reductions in mood instability were independent of the type of psychosocial treatment assigned to children.
The participants in this study could not be followed long enough to determine whether levels of mood instability were associated with the later development of syndromal BD. Other studies, however, have documented this relationship. Large-scale longitudinal studies of high-risk children find that measures of mood lability – along with early onset manic symptoms, depression, anxiety, and a family history of mania or hypomania – can be combined to calculate the risk that any individual child will develop BD I or II over the next 5-8 years.2,5
Clinicians should include measurement of the severity and psychosocial determinants of persistent mood shifts in youth under their care, particularly those with a family history of BD. Mood instability is associated with more severe symptom trajectories, more social isolation, and greater distress and conflict within the family. It may require a greater intensity of both pharmacological and psychosocial treatments to treat existing symptoms and functional impairments, and to prevent further mood deterioration.
Dr. Miklowitz is Distinguished Professor of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior. He is the author of “The Bipolar Disorder Survival Guide, 3rd Ed.” (New York: Guilford Press, 2019) and “Bipolar Disorder: A Family-Focused Treatment Approach, 2nd Ed” (New York: Guilford Press, 2010). He has no conflicts of interest to disclose. Contact Dr. Miklowitz at dmiklowitz@mednet.ucla.edu.
References
1. Bonsall MB, et al. Nonlinear time-series approaches in characterizing mood stability and mood instability in bipolar disorder. Proc Biol Sci. Mar 7 2012;279(1730):916-24. doi: 10.1098/rspb.2011.1246.
2. Hafeman DM, et al. Toward the definition of a bipolar prodrome: Dimensional predictors of bipolar spectrum disorders in at-risk youths. Am J Psychiatry. 2016;173(7):695-704. doi: 10.1176/appi.ajp.2015.15040414.
3. Miklowitz DJ, et al. Mood instability in youth at high risk for bipolar disorder. J Am Acad Child Adol Psychiatry. 2022 Mar 17;S0890-8567(22)00118-6. doi: 10.1016/j.jaac.2022.03.009.
4. Gerson AC, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. Dec 20 1996;65(3):189-98. doi: 10.1016/s0165-1781(96)02851-x.
5. Birmaher B, et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adol Psychiatry. 2018;57(10):755-63. doi: 10.1016/j.jaac.2018.05.023.
Higher ‘chemical restraint’ rates in Black psych patients in the ED
Black patients presenting with psychiatric disorders to hospital emergency departments across the United States have significantly higher rates of chemical restraint than their White counterparts, new research shows.
The investigators also found White patients were more likely to receive chemical sedation at hospitals with a higher proportion of Black patients – a finding that suggests hospital demographics influence practice patterns and that structural racism may be a root cause.
“There is a large disparity in the rates at which patients who presented to EDs nationally in the United States are restrained by race. You are 63% more likely, for the same set of chief complaints, to be chemically sedated if you are Black versus if you’re White,” senior investigator Ari Friedman, MD, PhD, an assistant professor of emergency medicine, and medical ethics and health policy, University of Pennsylvania, Philadelphia, told this news organization.
“The major mediator of that difference is the institution you are at – hospitals that primarily serve Black patients are more likely to chemically sedate their patients for these chief complaints – including White patients. So, it’s mediated by the practice pattern and environment,” Dr. Friedman added.
The study was published in the May issue of Annals of Epidemiology.
First large-scale study
Chemical sedation, also known as chemical restraint, is used to calm and help protect patients from harming themselves or others. Previous research on racial differences in the care of ED psychiatric patients with agitation suggests that there may be treatment disparities.
“Previous research from single institutions [has] shown that Black patients are more likely than White patients to be physically restrained, and this has been shown to be true among adult patients and pediatric patients,” lead author Utsha Khatri, MD, assistant professor of emergency medicine at the Icahn School of Medicine, New York, told this news organization.
Specifically, two single-institution studies within the last year revealed similar disparities, with higher rates of physical restraint for Black and Hispanic psychiatric patients in the ED. Another recent study showed an association with race, ethnicity, and pharmacological restraint use among pediatric patients presenting to the ED for mental health concerns.
“There has been work in psychiatry on disparities in this context, although there is less work in emergency departments,” said Dr. Friedman. “We looked across all U.S. EDs as opposed to within a single health system. The major trade-offs for us were that we weren’t able to observe restraint orders, which don’t find their way into national datasets, so we had to make some inferences based on the type of medications given.”
For the study the investigators analyzed data from 2008-2018 through the National Hospital Ambulatory Medical Survey (NHAMCS) database. They examined the association of race and the administration of chemical sedation, with either an antipsychotic or ketamine, in ED visits for psychiatric disorders. These were any visit where the reason for the visit was “symptoms referable to psychological and mental disorders.”
Of the 76.2 million total ED visits evaluated, the researchers found that Black patients presenting with a psychiatric disorder were significantly more likely to receive chemical sedation with antipsychotics or ketamine than White patients presenting with the same conditions (5.3% vs. 3.0%; P < .01). This difference remained significant when accounting for admission or transfer to psychiatric facilities.
Combatting the forces of racism
When researchers accounted for the percent of hospital population that was Black, they found that patient race no longer affected the likelihood of chemical restraint.
“We found the key source of this racial disparity in use of chemical sedation is accounted for by the fact that hospitals that treat a higher proportion of Black patients tend to use more sedation,” said Dr. Khatri.
“Our findings suggest that patients who present to hospitals that serve a patient population that is 60% Black would have [a] roughly 1.8 times likelihood of getting chemically sedated, compared with a hospital that serves a population that is 10% Black,” she added.
“When a hospital has fewer resources, they often don’t have the staff or time to de-escalate a patient in distress and can have to resort to chemical sedation more quickly than a hospital with ample staff and resources,” said Dr. Friedman in a release.
Dr. Khatri added that the study highlights the need to combat the forces of racism by focusing not just on provider bias but by addressing the “underlying structural issues that lead to Black patients getting worse care based on where they live.”
“Hospitals have unequal distribution of resources and quality, largely patterned on the racial makeup of their patients. Dedicated training and funding for de-escalation techniques as well as sufficient staffing and availability of outpatient mental health care may help keep both patients and staff safe by reducing the use of physical restraint and chemical sedation in appropriate circumstances,” said Dr. Khatri.
Dr. Friedman noted that there will always be a need for restraint use to facilitate rapid medical evaluation and stabilization of patients, but “we want to make it as humane, thoughtful, and rare as possible, and to have a large armamentarium of alternative strategies that can be equitably applied across emergency departments.”
Need for widespread, systemic change
Commenting on the findings, Regina James, MD, the American Psychiatric Association’s chief of Diversity and Health Equity and deputy medical director, said the large-scale study confirms the widespread existence of racial and ethnic disparities in patients with psychiatric disorders.
“This study and previous studies, not only in psychiatry but in other areas of medicine, all bring to light that there continues to be evidence of racial and ethnic disparities in health care, and this is consistent across a range of illnesses and health care services,” said Dr. James.
“It’s important that as we think about the solution, we also think about the etiology of the problem and the layers that have contributed to it – understanding, embracing, and recognizing that these differences didn’t just come up de novo. It’s policies, practices, and behaviors that got us to this point, and it’s going to be policies, practices, and behaviors that are going to move us away from this point,” noted Dr. James.
She added that future research should focus on further understanding which factors exacerbate agitation among patients and what resources directed at the hospital level, including de-escalation training, nursing staff, and waiting room crowding, may be effective at reducing the use of chemical sedation when clinically appropriate.
The authors and Dr. James report no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Black patients presenting with psychiatric disorders to hospital emergency departments across the United States have significantly higher rates of chemical restraint than their White counterparts, new research shows.
The investigators also found White patients were more likely to receive chemical sedation at hospitals with a higher proportion of Black patients – a finding that suggests hospital demographics influence practice patterns and that structural racism may be a root cause.
“There is a large disparity in the rates at which patients who presented to EDs nationally in the United States are restrained by race. You are 63% more likely, for the same set of chief complaints, to be chemically sedated if you are Black versus if you’re White,” senior investigator Ari Friedman, MD, PhD, an assistant professor of emergency medicine, and medical ethics and health policy, University of Pennsylvania, Philadelphia, told this news organization.
“The major mediator of that difference is the institution you are at – hospitals that primarily serve Black patients are more likely to chemically sedate their patients for these chief complaints – including White patients. So, it’s mediated by the practice pattern and environment,” Dr. Friedman added.
The study was published in the May issue of Annals of Epidemiology.
First large-scale study
Chemical sedation, also known as chemical restraint, is used to calm and help protect patients from harming themselves or others. Previous research on racial differences in the care of ED psychiatric patients with agitation suggests that there may be treatment disparities.
“Previous research from single institutions [has] shown that Black patients are more likely than White patients to be physically restrained, and this has been shown to be true among adult patients and pediatric patients,” lead author Utsha Khatri, MD, assistant professor of emergency medicine at the Icahn School of Medicine, New York, told this news organization.
Specifically, two single-institution studies within the last year revealed similar disparities, with higher rates of physical restraint for Black and Hispanic psychiatric patients in the ED. Another recent study showed an association with race, ethnicity, and pharmacological restraint use among pediatric patients presenting to the ED for mental health concerns.
“There has been work in psychiatry on disparities in this context, although there is less work in emergency departments,” said Dr. Friedman. “We looked across all U.S. EDs as opposed to within a single health system. The major trade-offs for us were that we weren’t able to observe restraint orders, which don’t find their way into national datasets, so we had to make some inferences based on the type of medications given.”
For the study the investigators analyzed data from 2008-2018 through the National Hospital Ambulatory Medical Survey (NHAMCS) database. They examined the association of race and the administration of chemical sedation, with either an antipsychotic or ketamine, in ED visits for psychiatric disorders. These were any visit where the reason for the visit was “symptoms referable to psychological and mental disorders.”
Of the 76.2 million total ED visits evaluated, the researchers found that Black patients presenting with a psychiatric disorder were significantly more likely to receive chemical sedation with antipsychotics or ketamine than White patients presenting with the same conditions (5.3% vs. 3.0%; P < .01). This difference remained significant when accounting for admission or transfer to psychiatric facilities.
Combatting the forces of racism
When researchers accounted for the percent of hospital population that was Black, they found that patient race no longer affected the likelihood of chemical restraint.
“We found the key source of this racial disparity in use of chemical sedation is accounted for by the fact that hospitals that treat a higher proportion of Black patients tend to use more sedation,” said Dr. Khatri.
“Our findings suggest that patients who present to hospitals that serve a patient population that is 60% Black would have [a] roughly 1.8 times likelihood of getting chemically sedated, compared with a hospital that serves a population that is 10% Black,” she added.
“When a hospital has fewer resources, they often don’t have the staff or time to de-escalate a patient in distress and can have to resort to chemical sedation more quickly than a hospital with ample staff and resources,” said Dr. Friedman in a release.
Dr. Khatri added that the study highlights the need to combat the forces of racism by focusing not just on provider bias but by addressing the “underlying structural issues that lead to Black patients getting worse care based on where they live.”
“Hospitals have unequal distribution of resources and quality, largely patterned on the racial makeup of their patients. Dedicated training and funding for de-escalation techniques as well as sufficient staffing and availability of outpatient mental health care may help keep both patients and staff safe by reducing the use of physical restraint and chemical sedation in appropriate circumstances,” said Dr. Khatri.
Dr. Friedman noted that there will always be a need for restraint use to facilitate rapid medical evaluation and stabilization of patients, but “we want to make it as humane, thoughtful, and rare as possible, and to have a large armamentarium of alternative strategies that can be equitably applied across emergency departments.”
Need for widespread, systemic change
Commenting on the findings, Regina James, MD, the American Psychiatric Association’s chief of Diversity and Health Equity and deputy medical director, said the large-scale study confirms the widespread existence of racial and ethnic disparities in patients with psychiatric disorders.
“This study and previous studies, not only in psychiatry but in other areas of medicine, all bring to light that there continues to be evidence of racial and ethnic disparities in health care, and this is consistent across a range of illnesses and health care services,” said Dr. James.
“It’s important that as we think about the solution, we also think about the etiology of the problem and the layers that have contributed to it – understanding, embracing, and recognizing that these differences didn’t just come up de novo. It’s policies, practices, and behaviors that got us to this point, and it’s going to be policies, practices, and behaviors that are going to move us away from this point,” noted Dr. James.
She added that future research should focus on further understanding which factors exacerbate agitation among patients and what resources directed at the hospital level, including de-escalation training, nursing staff, and waiting room crowding, may be effective at reducing the use of chemical sedation when clinically appropriate.
The authors and Dr. James report no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Black patients presenting with psychiatric disorders to hospital emergency departments across the United States have significantly higher rates of chemical restraint than their White counterparts, new research shows.
The investigators also found White patients were more likely to receive chemical sedation at hospitals with a higher proportion of Black patients – a finding that suggests hospital demographics influence practice patterns and that structural racism may be a root cause.
“There is a large disparity in the rates at which patients who presented to EDs nationally in the United States are restrained by race. You are 63% more likely, for the same set of chief complaints, to be chemically sedated if you are Black versus if you’re White,” senior investigator Ari Friedman, MD, PhD, an assistant professor of emergency medicine, and medical ethics and health policy, University of Pennsylvania, Philadelphia, told this news organization.
“The major mediator of that difference is the institution you are at – hospitals that primarily serve Black patients are more likely to chemically sedate their patients for these chief complaints – including White patients. So, it’s mediated by the practice pattern and environment,” Dr. Friedman added.
The study was published in the May issue of Annals of Epidemiology.
First large-scale study
Chemical sedation, also known as chemical restraint, is used to calm and help protect patients from harming themselves or others. Previous research on racial differences in the care of ED psychiatric patients with agitation suggests that there may be treatment disparities.
“Previous research from single institutions [has] shown that Black patients are more likely than White patients to be physically restrained, and this has been shown to be true among adult patients and pediatric patients,” lead author Utsha Khatri, MD, assistant professor of emergency medicine at the Icahn School of Medicine, New York, told this news organization.
Specifically, two single-institution studies within the last year revealed similar disparities, with higher rates of physical restraint for Black and Hispanic psychiatric patients in the ED. Another recent study showed an association with race, ethnicity, and pharmacological restraint use among pediatric patients presenting to the ED for mental health concerns.
“There has been work in psychiatry on disparities in this context, although there is less work in emergency departments,” said Dr. Friedman. “We looked across all U.S. EDs as opposed to within a single health system. The major trade-offs for us were that we weren’t able to observe restraint orders, which don’t find their way into national datasets, so we had to make some inferences based on the type of medications given.”
For the study the investigators analyzed data from 2008-2018 through the National Hospital Ambulatory Medical Survey (NHAMCS) database. They examined the association of race and the administration of chemical sedation, with either an antipsychotic or ketamine, in ED visits for psychiatric disorders. These were any visit where the reason for the visit was “symptoms referable to psychological and mental disorders.”
Of the 76.2 million total ED visits evaluated, the researchers found that Black patients presenting with a psychiatric disorder were significantly more likely to receive chemical sedation with antipsychotics or ketamine than White patients presenting with the same conditions (5.3% vs. 3.0%; P < .01). This difference remained significant when accounting for admission or transfer to psychiatric facilities.
Combatting the forces of racism
When researchers accounted for the percent of hospital population that was Black, they found that patient race no longer affected the likelihood of chemical restraint.
“We found the key source of this racial disparity in use of chemical sedation is accounted for by the fact that hospitals that treat a higher proportion of Black patients tend to use more sedation,” said Dr. Khatri.
“Our findings suggest that patients who present to hospitals that serve a patient population that is 60% Black would have [a] roughly 1.8 times likelihood of getting chemically sedated, compared with a hospital that serves a population that is 10% Black,” she added.
“When a hospital has fewer resources, they often don’t have the staff or time to de-escalate a patient in distress and can have to resort to chemical sedation more quickly than a hospital with ample staff and resources,” said Dr. Friedman in a release.
Dr. Khatri added that the study highlights the need to combat the forces of racism by focusing not just on provider bias but by addressing the “underlying structural issues that lead to Black patients getting worse care based on where they live.”
“Hospitals have unequal distribution of resources and quality, largely patterned on the racial makeup of their patients. Dedicated training and funding for de-escalation techniques as well as sufficient staffing and availability of outpatient mental health care may help keep both patients and staff safe by reducing the use of physical restraint and chemical sedation in appropriate circumstances,” said Dr. Khatri.
Dr. Friedman noted that there will always be a need for restraint use to facilitate rapid medical evaluation and stabilization of patients, but “we want to make it as humane, thoughtful, and rare as possible, and to have a large armamentarium of alternative strategies that can be equitably applied across emergency departments.”
Need for widespread, systemic change
Commenting on the findings, Regina James, MD, the American Psychiatric Association’s chief of Diversity and Health Equity and deputy medical director, said the large-scale study confirms the widespread existence of racial and ethnic disparities in patients with psychiatric disorders.
“This study and previous studies, not only in psychiatry but in other areas of medicine, all bring to light that there continues to be evidence of racial and ethnic disparities in health care, and this is consistent across a range of illnesses and health care services,” said Dr. James.
“It’s important that as we think about the solution, we also think about the etiology of the problem and the layers that have contributed to it – understanding, embracing, and recognizing that these differences didn’t just come up de novo. It’s policies, practices, and behaviors that got us to this point, and it’s going to be policies, practices, and behaviors that are going to move us away from this point,” noted Dr. James.
She added that future research should focus on further understanding which factors exacerbate agitation among patients and what resources directed at the hospital level, including de-escalation training, nursing staff, and waiting room crowding, may be effective at reducing the use of chemical sedation when clinically appropriate.
The authors and Dr. James report no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
The woman who kept passing out
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
Managing bipolar disorder in women who are pregnant
Psychiatrists who treat women of childbearing age should consider that those women may become pregnant, and that women with psychiatric illness are more likely to have unplanned pregnancies.1 Thus, thoughtful perinatal medication choices should begin before pregnancy. Pregnancy is a time of vulnerability to psychiatric illness for many reasons, including physiologic changes that can affect mental status; changes in medication efficacy; and numerous stressors, such as new responsibilities and limited sleep.1,2 For the treatment of pregnant—or potentially pregnant—patients, we recommend the following.
Do not panic! Knee-jerk medication changes in response to learning a patient is pregnant can lead to an exacerbation of psychiatric symptoms, as well as decrease trust in clinicians.2 Switching to a medication with a purportedly “safer” reproductive profile may worsen psychiatric illness, while also exposing the fetus to a medication of unknown benefit. 2
Recognize the risk of untreated or undertreated psychiatric illness, either of which has the potential to harm both the woman and her fetus. For example, a pregnant woman in a manic state may be more likely to engage in risky behaviors, such as drug use or risky sexual activity, which can lead to adverse fetal outcomes. They may also present with a higher risk of suicide. Compared to nonpregnant women, pregnant women for whom lithium was discontinued were equally likely to experience illness recurrence and significantly more likely to experience postpartum illness recurrence.3 In addition, the risk of recurrence was greater after rapid discontinuation compared with gradual discontinuation.3
Accurately communicate research findings. Pregnancy risk categories are no longer used. A nuanced interpretation of the potential adverse effects of a medication, such as malformations, impaired fetal growth, birth outcomes (such as preterm birth), and neurodevelopmental sequelae is necessary. Physicians must accurately convey information about risks to their patients, including both the absolute risk of an adverse event and the possible range of severity. For example, lithium use during pregnancy confers a higher relative risk of Ebstein’s anomaly (a cardiac defect).4 However, the absolute incidence of this risk remains low: 0.6% of lithium-exposed infants vs 0.18% among unexposed infants.4 Ebstein’s anomaly also varies significantly in severity—serious cases may require surgery, but less serious cases need only monitoring. A reliable database that compiles the latest evidence may help in staying abreast of the latest data.
Treat the psychiatric illness. Consider the optimal treatment for the psychiatric illness. Lithium remains the gold standard treatment for bipolar I disorder, regardless of reproductive status. Olanzapine and quetiapine are also commonly used and effective during pregnancy. This is an opportunity to conduct a detailed review of the patient’s previous medication regimens, including a review of medication trials and efficacy. Keep in mind that untreated bipolar disorder also carries an increased risk of adverse pregnancy outcomes.5
Consider pregnancy timing. Most organs form between weeks 3 to 8 of pregnancy. For example, if a medication potentially affects heart formation, but the patient is in the third trimester, explain to her that the heart has already been formed. Consider that medication may be required long-term and affect future pregnancies. Pregnant women require more frequent monitoring, because blood volume changes in pregnancy and postpartum can affect medication levels and efficacy. In addition, note whether a woman plans to breastfeed and be mindful of a medication’s profile in breastfeeding.
Ensure the patient can provide informed consent. Communicate your diagnostic formulation and treatment options. Consider involving the patient’s partner and/or support system in the discussion, if the patient consents. If a patient cannot provide informed consent, a surrogate decision-maker should be identified.6
Continue to: Collaborate with other clinicians
Collaborate with other clinicians, such as the patient’s OB/GYN and family medicine physician when possible. This will ensure that all clinicians are on the same page.
Plan for future pregnancies. Psychiatric medications can be long-term. Even patients who say they do not wish to become pregnant may someday become pregnant. Having discussions about medication choices, and their reproductive implications, prior to pregnancy allows patients to take an active role in their health.1,2
Consult a reproductive psychiatrist when indicated, and as early in the pregnancy as possible.
1. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
2. Friedman SH, Reed E. Treating psychosis in pregnant women: a measured approach. Current Psychiatry. 2021; 20(7):34-35.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157(2):179-184.
4. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
5. Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi:10.1136/bmj.e7085
6. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022:63(1);61-70.
Psychiatrists who treat women of childbearing age should consider that those women may become pregnant, and that women with psychiatric illness are more likely to have unplanned pregnancies.1 Thus, thoughtful perinatal medication choices should begin before pregnancy. Pregnancy is a time of vulnerability to psychiatric illness for many reasons, including physiologic changes that can affect mental status; changes in medication efficacy; and numerous stressors, such as new responsibilities and limited sleep.1,2 For the treatment of pregnant—or potentially pregnant—patients, we recommend the following.
Do not panic! Knee-jerk medication changes in response to learning a patient is pregnant can lead to an exacerbation of psychiatric symptoms, as well as decrease trust in clinicians.2 Switching to a medication with a purportedly “safer” reproductive profile may worsen psychiatric illness, while also exposing the fetus to a medication of unknown benefit. 2
Recognize the risk of untreated or undertreated psychiatric illness, either of which has the potential to harm both the woman and her fetus. For example, a pregnant woman in a manic state may be more likely to engage in risky behaviors, such as drug use or risky sexual activity, which can lead to adverse fetal outcomes. They may also present with a higher risk of suicide. Compared to nonpregnant women, pregnant women for whom lithium was discontinued were equally likely to experience illness recurrence and significantly more likely to experience postpartum illness recurrence.3 In addition, the risk of recurrence was greater after rapid discontinuation compared with gradual discontinuation.3
Accurately communicate research findings. Pregnancy risk categories are no longer used. A nuanced interpretation of the potential adverse effects of a medication, such as malformations, impaired fetal growth, birth outcomes (such as preterm birth), and neurodevelopmental sequelae is necessary. Physicians must accurately convey information about risks to their patients, including both the absolute risk of an adverse event and the possible range of severity. For example, lithium use during pregnancy confers a higher relative risk of Ebstein’s anomaly (a cardiac defect).4 However, the absolute incidence of this risk remains low: 0.6% of lithium-exposed infants vs 0.18% among unexposed infants.4 Ebstein’s anomaly also varies significantly in severity—serious cases may require surgery, but less serious cases need only monitoring. A reliable database that compiles the latest evidence may help in staying abreast of the latest data.
Treat the psychiatric illness. Consider the optimal treatment for the psychiatric illness. Lithium remains the gold standard treatment for bipolar I disorder, regardless of reproductive status. Olanzapine and quetiapine are also commonly used and effective during pregnancy. This is an opportunity to conduct a detailed review of the patient’s previous medication regimens, including a review of medication trials and efficacy. Keep in mind that untreated bipolar disorder also carries an increased risk of adverse pregnancy outcomes.5
Consider pregnancy timing. Most organs form between weeks 3 to 8 of pregnancy. For example, if a medication potentially affects heart formation, but the patient is in the third trimester, explain to her that the heart has already been formed. Consider that medication may be required long-term and affect future pregnancies. Pregnant women require more frequent monitoring, because blood volume changes in pregnancy and postpartum can affect medication levels and efficacy. In addition, note whether a woman plans to breastfeed and be mindful of a medication’s profile in breastfeeding.
Ensure the patient can provide informed consent. Communicate your diagnostic formulation and treatment options. Consider involving the patient’s partner and/or support system in the discussion, if the patient consents. If a patient cannot provide informed consent, a surrogate decision-maker should be identified.6
Continue to: Collaborate with other clinicians
Collaborate with other clinicians, such as the patient’s OB/GYN and family medicine physician when possible. This will ensure that all clinicians are on the same page.
Plan for future pregnancies. Psychiatric medications can be long-term. Even patients who say they do not wish to become pregnant may someday become pregnant. Having discussions about medication choices, and their reproductive implications, prior to pregnancy allows patients to take an active role in their health.1,2
Consult a reproductive psychiatrist when indicated, and as early in the pregnancy as possible.
Psychiatrists who treat women of childbearing age should consider that those women may become pregnant, and that women with psychiatric illness are more likely to have unplanned pregnancies.1 Thus, thoughtful perinatal medication choices should begin before pregnancy. Pregnancy is a time of vulnerability to psychiatric illness for many reasons, including physiologic changes that can affect mental status; changes in medication efficacy; and numerous stressors, such as new responsibilities and limited sleep.1,2 For the treatment of pregnant—or potentially pregnant—patients, we recommend the following.
Do not panic! Knee-jerk medication changes in response to learning a patient is pregnant can lead to an exacerbation of psychiatric symptoms, as well as decrease trust in clinicians.2 Switching to a medication with a purportedly “safer” reproductive profile may worsen psychiatric illness, while also exposing the fetus to a medication of unknown benefit. 2
Recognize the risk of untreated or undertreated psychiatric illness, either of which has the potential to harm both the woman and her fetus. For example, a pregnant woman in a manic state may be more likely to engage in risky behaviors, such as drug use or risky sexual activity, which can lead to adverse fetal outcomes. They may also present with a higher risk of suicide. Compared to nonpregnant women, pregnant women for whom lithium was discontinued were equally likely to experience illness recurrence and significantly more likely to experience postpartum illness recurrence.3 In addition, the risk of recurrence was greater after rapid discontinuation compared with gradual discontinuation.3
Accurately communicate research findings. Pregnancy risk categories are no longer used. A nuanced interpretation of the potential adverse effects of a medication, such as malformations, impaired fetal growth, birth outcomes (such as preterm birth), and neurodevelopmental sequelae is necessary. Physicians must accurately convey information about risks to their patients, including both the absolute risk of an adverse event and the possible range of severity. For example, lithium use during pregnancy confers a higher relative risk of Ebstein’s anomaly (a cardiac defect).4 However, the absolute incidence of this risk remains low: 0.6% of lithium-exposed infants vs 0.18% among unexposed infants.4 Ebstein’s anomaly also varies significantly in severity—serious cases may require surgery, but less serious cases need only monitoring. A reliable database that compiles the latest evidence may help in staying abreast of the latest data.
Treat the psychiatric illness. Consider the optimal treatment for the psychiatric illness. Lithium remains the gold standard treatment for bipolar I disorder, regardless of reproductive status. Olanzapine and quetiapine are also commonly used and effective during pregnancy. This is an opportunity to conduct a detailed review of the patient’s previous medication regimens, including a review of medication trials and efficacy. Keep in mind that untreated bipolar disorder also carries an increased risk of adverse pregnancy outcomes.5
Consider pregnancy timing. Most organs form between weeks 3 to 8 of pregnancy. For example, if a medication potentially affects heart formation, but the patient is in the third trimester, explain to her that the heart has already been formed. Consider that medication may be required long-term and affect future pregnancies. Pregnant women require more frequent monitoring, because blood volume changes in pregnancy and postpartum can affect medication levels and efficacy. In addition, note whether a woman plans to breastfeed and be mindful of a medication’s profile in breastfeeding.
Ensure the patient can provide informed consent. Communicate your diagnostic formulation and treatment options. Consider involving the patient’s partner and/or support system in the discussion, if the patient consents. If a patient cannot provide informed consent, a surrogate decision-maker should be identified.6
Continue to: Collaborate with other clinicians
Collaborate with other clinicians, such as the patient’s OB/GYN and family medicine physician when possible. This will ensure that all clinicians are on the same page.
Plan for future pregnancies. Psychiatric medications can be long-term. Even patients who say they do not wish to become pregnant may someday become pregnant. Having discussions about medication choices, and their reproductive implications, prior to pregnancy allows patients to take an active role in their health.1,2
Consult a reproductive psychiatrist when indicated, and as early in the pregnancy as possible.
1. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
2. Friedman SH, Reed E. Treating psychosis in pregnant women: a measured approach. Current Psychiatry. 2021; 20(7):34-35.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157(2):179-184.
4. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
5. Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi:10.1136/bmj.e7085
6. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022:63(1);61-70.
1. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
2. Friedman SH, Reed E. Treating psychosis in pregnant women: a measured approach. Current Psychiatry. 2021; 20(7):34-35.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157(2):179-184.
4. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
5. Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi:10.1136/bmj.e7085
6. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022:63(1);61-70.