User login
Diabetic amputations soared amid Italian pandemic lockdown
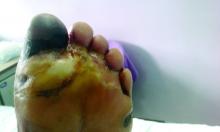
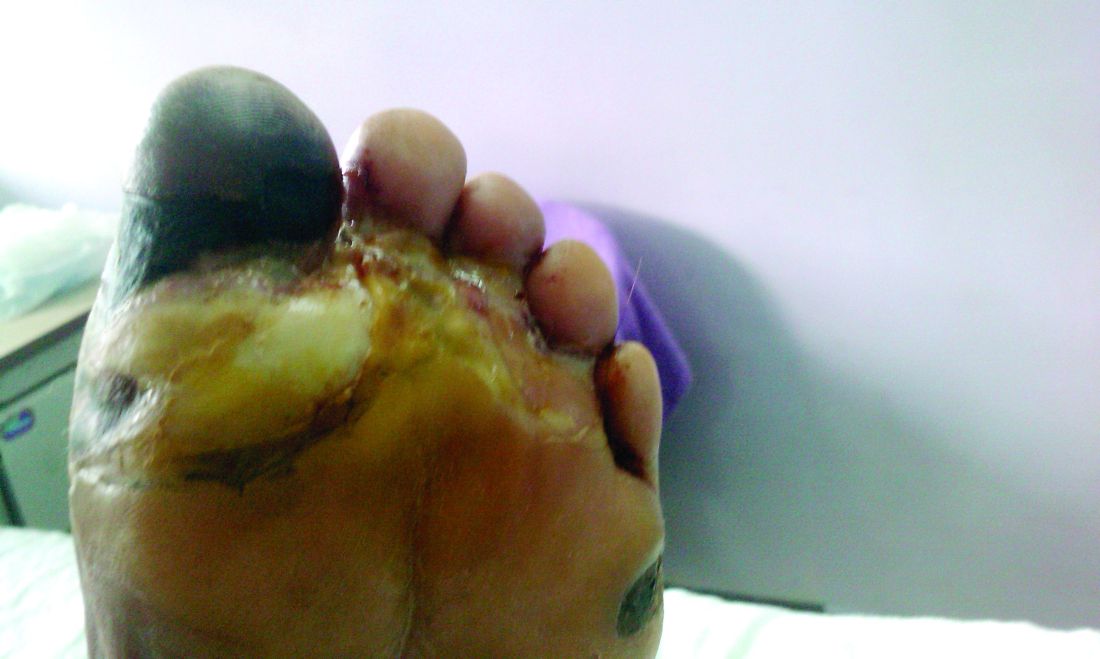
Amid a mandatory national lockdown, the rates of amputations skyrocketed at a hospital far from the hardest-hit region as many patients developed gangrene.
The findings offer critical lessons for the United States, said wound care specialist William H. Tettelbach, MD, of Western Peaks Specialty Hospital near Salt Lake City. “It’s become more obvious that outpatient wound care is a critical care need for the community because of the risk of ignoring these chronic wounds and letting them remain open. We cannot let these services be closed down like some were when the pandemic started.”
The study, led by Paola Caruso, MD, of the University of Campania Luigi Vanvitelli in Naples, appeared in Diabetes Care.
The researchers launched the study to understand how patients with diabetes and DFU fared during the height of the pandemic in Italy, where tens of thousands of people died, mainly in the northern region of the country. They focused on patients in the southern region who were admitted to the division of endocrinology and metabolic diseases at the Teaching Hospital at the University of Campania Luigi Vanvitelli.
The study compared 25 patients who were admitted from March 9 to May 18, 2020, with 38 patients who were admitted from a longer period between January and May 2019. The demographics of the groups are similar, with average ages in the early 60s and more men than women (21:4, respectively, in 2020 and 23:15, respectively, in 2019.)
The results reveal high numbers of emergent and serious cases in 2020. Compared with 2019, fewer were outpatients (16% vs. 45%, P = .028) and more were emergency patients (76% vs. 26%, P < .001).
Clinically, gangrene was much more common in the 2020 group, compared with the 2019 group (64% vs. 29%, P = .009), as was amputation (60% vs. 18%, P = .001).
The researchers determined that amputation was more than three times more likely in the 2020 versus the 2019 group (relative risk, 3.26; 95% confidence interval, 1.55-6.84) even though the 2019 period was longer. After adjustment for gender, the heightened risk in 2020 was 2.50 (95% CI, 1.18-5.29).
There was no statistically significant increase in the risk of revascularization.
“The COVID-19 lockdown may have had a detrimental impact on amputation risk because of the sudden interruption of DFU care and lower-limb preservation pathways, resulting in delayed diagnosis and treatment,” the researchers wrote. “DFU is often characterized by progressive clinical course, which can rapidly lead patients to critical worsening of their ulcers.”
They added that “the higher risk of amputation observed during COVID-19 lockdown confirms the need for proper and timely management of DFU patients to prevent dramatic outcomes responsible for a reduction of quality of life and increased morbidity and mortality.”
The study authors didn’t discuss why more patients seemed to have stayed home and not gotten proper care. It’s not clear if they were scared to get treatment or couldn’t obtain it because of the national shutdown.
Both have been factors affecting diabetic foot care in the United States during the pandemic, said Dr. Tettelbach. He called the study “timely and pertinent,” and said it highlights how wound care is “a critical need” that must remain available even when other medical services such as elective surgeries are shut down.
Infection-control protocols such as allowing patients to wait for appointments in their cars instead of waiting rooms will alleviate the fears of certain patients about seeking in-person care during the pandemic, he said. But some patients will be afraid to come in no matter what, he said, and home health may be the best solution for their care.
Several of the study authors reported various disclosures. Dr. Tettelbach reported no relevant disclosures.
SOURCE: Caruso P et al. Diabetes Care. 2020 Jul 23. doi:10.2337/dc20-1347.
Amid a mandatory national lockdown, the rates of amputations skyrocketed at a hospital far from the hardest-hit region as many patients developed gangrene.
The findings offer critical lessons for the United States, said wound care specialist William H. Tettelbach, MD, of Western Peaks Specialty Hospital near Salt Lake City. “It’s become more obvious that outpatient wound care is a critical care need for the community because of the risk of ignoring these chronic wounds and letting them remain open. We cannot let these services be closed down like some were when the pandemic started.”
The study, led by Paola Caruso, MD, of the University of Campania Luigi Vanvitelli in Naples, appeared in Diabetes Care.
The researchers launched the study to understand how patients with diabetes and DFU fared during the height of the pandemic in Italy, where tens of thousands of people died, mainly in the northern region of the country. They focused on patients in the southern region who were admitted to the division of endocrinology and metabolic diseases at the Teaching Hospital at the University of Campania Luigi Vanvitelli.
The study compared 25 patients who were admitted from March 9 to May 18, 2020, with 38 patients who were admitted from a longer period between January and May 2019. The demographics of the groups are similar, with average ages in the early 60s and more men than women (21:4, respectively, in 2020 and 23:15, respectively, in 2019.)
The results reveal high numbers of emergent and serious cases in 2020. Compared with 2019, fewer were outpatients (16% vs. 45%, P = .028) and more were emergency patients (76% vs. 26%, P < .001).
Clinically, gangrene was much more common in the 2020 group, compared with the 2019 group (64% vs. 29%, P = .009), as was amputation (60% vs. 18%, P = .001).
The researchers determined that amputation was more than three times more likely in the 2020 versus the 2019 group (relative risk, 3.26; 95% confidence interval, 1.55-6.84) even though the 2019 period was longer. After adjustment for gender, the heightened risk in 2020 was 2.50 (95% CI, 1.18-5.29).
There was no statistically significant increase in the risk of revascularization.
“The COVID-19 lockdown may have had a detrimental impact on amputation risk because of the sudden interruption of DFU care and lower-limb preservation pathways, resulting in delayed diagnosis and treatment,” the researchers wrote. “DFU is often characterized by progressive clinical course, which can rapidly lead patients to critical worsening of their ulcers.”
They added that “the higher risk of amputation observed during COVID-19 lockdown confirms the need for proper and timely management of DFU patients to prevent dramatic outcomes responsible for a reduction of quality of life and increased morbidity and mortality.”
The study authors didn’t discuss why more patients seemed to have stayed home and not gotten proper care. It’s not clear if they were scared to get treatment or couldn’t obtain it because of the national shutdown.
Both have been factors affecting diabetic foot care in the United States during the pandemic, said Dr. Tettelbach. He called the study “timely and pertinent,” and said it highlights how wound care is “a critical need” that must remain available even when other medical services such as elective surgeries are shut down.
Infection-control protocols such as allowing patients to wait for appointments in their cars instead of waiting rooms will alleviate the fears of certain patients about seeking in-person care during the pandemic, he said. But some patients will be afraid to come in no matter what, he said, and home health may be the best solution for their care.
Several of the study authors reported various disclosures. Dr. Tettelbach reported no relevant disclosures.
SOURCE: Caruso P et al. Diabetes Care. 2020 Jul 23. doi:10.2337/dc20-1347.
Amid a mandatory national lockdown, the rates of amputations skyrocketed at a hospital far from the hardest-hit region as many patients developed gangrene.
The findings offer critical lessons for the United States, said wound care specialist William H. Tettelbach, MD, of Western Peaks Specialty Hospital near Salt Lake City. “It’s become more obvious that outpatient wound care is a critical care need for the community because of the risk of ignoring these chronic wounds and letting them remain open. We cannot let these services be closed down like some were when the pandemic started.”
The study, led by Paola Caruso, MD, of the University of Campania Luigi Vanvitelli in Naples, appeared in Diabetes Care.
The researchers launched the study to understand how patients with diabetes and DFU fared during the height of the pandemic in Italy, where tens of thousands of people died, mainly in the northern region of the country. They focused on patients in the southern region who were admitted to the division of endocrinology and metabolic diseases at the Teaching Hospital at the University of Campania Luigi Vanvitelli.
The study compared 25 patients who were admitted from March 9 to May 18, 2020, with 38 patients who were admitted from a longer period between January and May 2019. The demographics of the groups are similar, with average ages in the early 60s and more men than women (21:4, respectively, in 2020 and 23:15, respectively, in 2019.)
The results reveal high numbers of emergent and serious cases in 2020. Compared with 2019, fewer were outpatients (16% vs. 45%, P = .028) and more were emergency patients (76% vs. 26%, P < .001).
Clinically, gangrene was much more common in the 2020 group, compared with the 2019 group (64% vs. 29%, P = .009), as was amputation (60% vs. 18%, P = .001).
The researchers determined that amputation was more than three times more likely in the 2020 versus the 2019 group (relative risk, 3.26; 95% confidence interval, 1.55-6.84) even though the 2019 period was longer. After adjustment for gender, the heightened risk in 2020 was 2.50 (95% CI, 1.18-5.29).
There was no statistically significant increase in the risk of revascularization.
“The COVID-19 lockdown may have had a detrimental impact on amputation risk because of the sudden interruption of DFU care and lower-limb preservation pathways, resulting in delayed diagnosis and treatment,” the researchers wrote. “DFU is often characterized by progressive clinical course, which can rapidly lead patients to critical worsening of their ulcers.”
They added that “the higher risk of amputation observed during COVID-19 lockdown confirms the need for proper and timely management of DFU patients to prevent dramatic outcomes responsible for a reduction of quality of life and increased morbidity and mortality.”
The study authors didn’t discuss why more patients seemed to have stayed home and not gotten proper care. It’s not clear if they were scared to get treatment or couldn’t obtain it because of the national shutdown.
Both have been factors affecting diabetic foot care in the United States during the pandemic, said Dr. Tettelbach. He called the study “timely and pertinent,” and said it highlights how wound care is “a critical need” that must remain available even when other medical services such as elective surgeries are shut down.
Infection-control protocols such as allowing patients to wait for appointments in their cars instead of waiting rooms will alleviate the fears of certain patients about seeking in-person care during the pandemic, he said. But some patients will be afraid to come in no matter what, he said, and home health may be the best solution for their care.
Several of the study authors reported various disclosures. Dr. Tettelbach reported no relevant disclosures.
SOURCE: Caruso P et al. Diabetes Care. 2020 Jul 23. doi:10.2337/dc20-1347.
FROM DIABETES CARE
Emicizumab performs well in surgical setting
PRAGUE – Emicizumab appears safe and effective for patients with hemophilia A undergoing surgical procedures, based on experience with a subpopulation of HAVEN 3 trial participants.
Out of 28 minor procedures performed without preventive factor VIII (FVIII), only 2 were associated with postoperative bleeds requiring treatment, reported lead author Elena Santagostino, MD, PhD, of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan, and her colleagues.
All events requiring bleeding treatment were associated with dental procedures, highlighting an area where clinicians and dentists may need to exercise caution. Still, overall results supported emicizumab in a surgical setting.
“There were no thrombotic complications or other unexpected events, including inhibitor development,” Dr. Santagostino said at the annual congress of the European Association for Haemophilia and Allied Disorders.
The findings were drawn from 30 patients who underwent 50 surgeries (46 minor, 4 major) during HAVEN 3, a previously reported phase 3 trial investigating the use of emicizumab, a humanized bispecific monoclonal antibody for patients with hemophilia A without inhibitors.
The minor surgeries included dental or orthopedic procedures, esophagogastroduodenoscopy, or colonoscopy. The four major procedures were all orthopedic (knee arthroscopic synovectomy, biceps femoris tear repair, total ankle arthroplasty, and total hip replacement). The investigators analyzed surgery-related bleeds and the nature of FVIII usage.
Preventive FVIII was used in 18 procedures; infusion duration was 24 hours or less in 14 procedures, between 25 hours and 48 hours in 2 procedures, and more than 72 hours in 2 procedures. The median cumulative preventive FVIII dose per procedure was 30 IU/kg.
Of the 46 minor procedures, 28 (61%) were performed without preventive FVIII, and 2 (7.1%) were associated with bleeding requiring treatment, both after dental procedures. Two other participants who received preventive FVIII also needed postoperative bleeding treatment. Of note, these events were also after dental procedures, meaning all four instances of bleeding requiring treatment during the trial were associated with dentistry.
“[I]n this experience, dental procedures were somewhat tricky because the bleeding complications were mainly there,” Dr. Santagostino said.
When asked by an audience member if this trend was unique to mucosal bleeding, Dr. Santagostino said it was too early to draw such a conclusion but offered some insight. “To control and prevent bleeding during a dental procedure is not trivial, because … sometimes if you stop factor VIII treatment quite early, you may have late bleeding, mainly due to local reasons, because … dental procedures are very heterogenous.”
Among three other participants who had postoperative bleeding but did not require treatment, two underwent dental procedures, further supporting this association. Although the study numbers are relatively small, the findings may at least support caution, if not preventive FVIII in the dental setting, Dr. Santagostino said.
The four major procedures – all orthopedic – were knee arthroscopic synovectomy, biceps femoris tear repair, total ankle arthroplasty, and total hip replacement. Along with preoperative preventive FVIII, three of four patients undergoing major surgery received preventive FVIII for 14-18 days postoperatively. Doses ranged from 99-522 IU/kg. No postoperative bleeds occurred in this subgroup.
Study funding was provided by F. Hoffmann–La Roche and Chugai Pharmaceutical. The investigators reported financial relationships with Bayer, Shire, Pfizer, Novo Nordisk, and others.
SOURCE: Santagostino E et al. EAHAD 2019, Abstract OR15.
PRAGUE – Emicizumab appears safe and effective for patients with hemophilia A undergoing surgical procedures, based on experience with a subpopulation of HAVEN 3 trial participants.
Out of 28 minor procedures performed without preventive factor VIII (FVIII), only 2 were associated with postoperative bleeds requiring treatment, reported lead author Elena Santagostino, MD, PhD, of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan, and her colleagues.
All events requiring bleeding treatment were associated with dental procedures, highlighting an area where clinicians and dentists may need to exercise caution. Still, overall results supported emicizumab in a surgical setting.
“There were no thrombotic complications or other unexpected events, including inhibitor development,” Dr. Santagostino said at the annual congress of the European Association for Haemophilia and Allied Disorders.
The findings were drawn from 30 patients who underwent 50 surgeries (46 minor, 4 major) during HAVEN 3, a previously reported phase 3 trial investigating the use of emicizumab, a humanized bispecific monoclonal antibody for patients with hemophilia A without inhibitors.
The minor surgeries included dental or orthopedic procedures, esophagogastroduodenoscopy, or colonoscopy. The four major procedures were all orthopedic (knee arthroscopic synovectomy, biceps femoris tear repair, total ankle arthroplasty, and total hip replacement). The investigators analyzed surgery-related bleeds and the nature of FVIII usage.
Preventive FVIII was used in 18 procedures; infusion duration was 24 hours or less in 14 procedures, between 25 hours and 48 hours in 2 procedures, and more than 72 hours in 2 procedures. The median cumulative preventive FVIII dose per procedure was 30 IU/kg.
Of the 46 minor procedures, 28 (61%) were performed without preventive FVIII, and 2 (7.1%) were associated with bleeding requiring treatment, both after dental procedures. Two other participants who received preventive FVIII also needed postoperative bleeding treatment. Of note, these events were also after dental procedures, meaning all four instances of bleeding requiring treatment during the trial were associated with dentistry.
“[I]n this experience, dental procedures were somewhat tricky because the bleeding complications were mainly there,” Dr. Santagostino said.
When asked by an audience member if this trend was unique to mucosal bleeding, Dr. Santagostino said it was too early to draw such a conclusion but offered some insight. “To control and prevent bleeding during a dental procedure is not trivial, because … sometimes if you stop factor VIII treatment quite early, you may have late bleeding, mainly due to local reasons, because … dental procedures are very heterogenous.”
Among three other participants who had postoperative bleeding but did not require treatment, two underwent dental procedures, further supporting this association. Although the study numbers are relatively small, the findings may at least support caution, if not preventive FVIII in the dental setting, Dr. Santagostino said.
The four major procedures – all orthopedic – were knee arthroscopic synovectomy, biceps femoris tear repair, total ankle arthroplasty, and total hip replacement. Along with preoperative preventive FVIII, three of four patients undergoing major surgery received preventive FVIII for 14-18 days postoperatively. Doses ranged from 99-522 IU/kg. No postoperative bleeds occurred in this subgroup.
Study funding was provided by F. Hoffmann–La Roche and Chugai Pharmaceutical. The investigators reported financial relationships with Bayer, Shire, Pfizer, Novo Nordisk, and others.
SOURCE: Santagostino E et al. EAHAD 2019, Abstract OR15.
PRAGUE – Emicizumab appears safe and effective for patients with hemophilia A undergoing surgical procedures, based on experience with a subpopulation of HAVEN 3 trial participants.
Out of 28 minor procedures performed without preventive factor VIII (FVIII), only 2 were associated with postoperative bleeds requiring treatment, reported lead author Elena Santagostino, MD, PhD, of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan, and her colleagues.
All events requiring bleeding treatment were associated with dental procedures, highlighting an area where clinicians and dentists may need to exercise caution. Still, overall results supported emicizumab in a surgical setting.
“There were no thrombotic complications or other unexpected events, including inhibitor development,” Dr. Santagostino said at the annual congress of the European Association for Haemophilia and Allied Disorders.
The findings were drawn from 30 patients who underwent 50 surgeries (46 minor, 4 major) during HAVEN 3, a previously reported phase 3 trial investigating the use of emicizumab, a humanized bispecific monoclonal antibody for patients with hemophilia A without inhibitors.
The minor surgeries included dental or orthopedic procedures, esophagogastroduodenoscopy, or colonoscopy. The four major procedures were all orthopedic (knee arthroscopic synovectomy, biceps femoris tear repair, total ankle arthroplasty, and total hip replacement). The investigators analyzed surgery-related bleeds and the nature of FVIII usage.
Preventive FVIII was used in 18 procedures; infusion duration was 24 hours or less in 14 procedures, between 25 hours and 48 hours in 2 procedures, and more than 72 hours in 2 procedures. The median cumulative preventive FVIII dose per procedure was 30 IU/kg.
Of the 46 minor procedures, 28 (61%) were performed without preventive FVIII, and 2 (7.1%) were associated with bleeding requiring treatment, both after dental procedures. Two other participants who received preventive FVIII also needed postoperative bleeding treatment. Of note, these events were also after dental procedures, meaning all four instances of bleeding requiring treatment during the trial were associated with dentistry.
“[I]n this experience, dental procedures were somewhat tricky because the bleeding complications were mainly there,” Dr. Santagostino said.
When asked by an audience member if this trend was unique to mucosal bleeding, Dr. Santagostino said it was too early to draw such a conclusion but offered some insight. “To control and prevent bleeding during a dental procedure is not trivial, because … sometimes if you stop factor VIII treatment quite early, you may have late bleeding, mainly due to local reasons, because … dental procedures are very heterogenous.”
Among three other participants who had postoperative bleeding but did not require treatment, two underwent dental procedures, further supporting this association. Although the study numbers are relatively small, the findings may at least support caution, if not preventive FVIII in the dental setting, Dr. Santagostino said.
The four major procedures – all orthopedic – were knee arthroscopic synovectomy, biceps femoris tear repair, total ankle arthroplasty, and total hip replacement. Along with preoperative preventive FVIII, three of four patients undergoing major surgery received preventive FVIII for 14-18 days postoperatively. Doses ranged from 99-522 IU/kg. No postoperative bleeds occurred in this subgroup.
Study funding was provided by F. Hoffmann–La Roche and Chugai Pharmaceutical. The investigators reported financial relationships with Bayer, Shire, Pfizer, Novo Nordisk, and others.
SOURCE: Santagostino E et al. EAHAD 2019, Abstract OR15.
REPORTING FROM EAHAD 2019
Fragility Fractures: Diagnosis and Treatment
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
TAKE-HOME POINTS
- 3 million people sustain fragility fractures annually, and nearly 30% die within a year of the fracture.
- The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.
- The World Health Organization has developed a set of T-core criteria to diagnose osteoporosis in postmenopausal women: a score >–1 is normal; <–1 but >–2.5 signifies osteopenia; <–2.5 denotes osteoporosis; and <–2.5 with fragility fracture indicates severe osteoporosis.
- The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children. The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender. Z scores <–2.0 indicate low BMD for chronological age. A Z score > –2.0 is considered within the expected range for age.
- After an initial fragility fracture, the risk for additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture.26
Foot and Ankle Injuries in Soccer
ABSTRACT
The ankle is one of the most commonly injured joints in soccer and represents a significant cost to the healthcare system. The ligaments that stabilize the ankle joint determine its biomechanics—alterations of which result from various soccer-related injuries. Acute sprains are among the most common injury in soccer players and are generally treated conservatively, with emphasis placed on secondary prevention to reduce the risk for future sprains and progression to chronic ankle instability. Repetitive ankle injuries in soccer players may cause chronic ankle instability, which includes both mechanical ligamentous laxity and functional changes. Chronic ankle pathology often requires surgery to repair ligamentous damage and remove soft-tissue or osseous impingement. Proper initial treatment, rehabilitation, and secondary prevention of ankle injuries can limit the amount of time lost from play and avoid negative long-term sequelae (eg, osteochondral lesions, arthritis). On the other hand, high ankle sprains portend a poorer prognosis and a longer recovery. These injuries will typically require surgical stabilization. Impingement-like syndromes of the ankle can undergo an initial trial of conservative treatment; when this fails, however, soccer players respond favorably to arthroscopic debridement of the lesions causing impingement. Finally, other pathologies (eg, stress fractures) are highly encouraged to be treated with surgical stabilization in elite soccer players.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
With roughly 200,000 professional and around 240 million amateur soccer players, soccer has been recognized as the most popular sport worldwide. Nevertheless, given its rising popularity in society, one must also consider the increasing incidence of injuries as a result. Elite soccer players sustain between 10 and 35 injuries per 1000 competitive playing hours.1 Approximately 80% are traumatic, and 20% are overuse injuries.2 Soccer injuries are more frequent with increasing age of the participants, whereas the incidence of injuries in preadolescent players is low. The incidence of injuries has been found to be higher during competition when compared with practice/training sessions, with some studies showing that 59% of injuries occurred during games.2 Amateur or recreational soccer players sustain fewer injuries than professional soccer players, as one would expect, given both the higher intensity of training and match schedule in professionals.
The ankle is one of the most commonly injured joints in soccer, with some studies suggesting it comprises one-fifth of all injuries sustained during soccer, which is only second to those of the knee.2 Ankle sprains specifically are quite a common occurrence in soccer.3-9 A recent study of an English premier league club showed that over a 4-season period, 20% of injuries were of the foot and ankle with a mean return to sport time of 54 days.10 Of all foot and ankle related injuries, ankle sprains are the most common, followed by bruises/contusions, and tendon lesions. Fractures are very rare (1%) in soccer, but when they do occur they impart a much more extended recovery. During the 2010 Fédération Internationale de Football Association (FIFA) World Cup, ankle sprains were among the most common injuries and approximately half lead to players missing training or competitive matches.5
ANATOMY
Knowledge of the biomechanics of both the foot and ankle joints is essential to understand soccer injuries. The ankle joint (talocrural articulation) consists of the distal ends of the tibia and fibula, which form the mortise, and the superior aspect of the talar dome.11 As a hinge joint, the ankle provides 20° of dorsiflexion and 50° of plantar flexion,12 with stability provided by the lateral, medial, and superior ligamentous complexes. The superior articular surface of the talus is narrower posteriorly, which creates a looser fit within the mortise during plantar flexion.11 This decreased stability could help explain why the most common injury in soccer involves a plantar flexion mechanism.13,14 Inferiorly, the talus articulates with the calcaneus to form the subtalar joint. It is at this site that the majority of both foot inversion and eversion occurs. The transverse tarsal joints (Chopart’s joints) separate the hindfoot from the midfoot. Movement of this joint depends on the relative alignment of its 2 articulations: the talonavicular and calcaneocuboid joints. During foot eversion, these 2 joints are parallel to each other allowing supple motion and aiding in shock absorption during the heel strike phase of the gait cycle. With foot inversion, the joints become nonparallel and thus lock the transverse tarsal joints providing a rigid lever needed for push-off.11,12
LATERAL LIGAMENTS
The ankle joint is stabilized laterally by a ligament complex consisting of 3 individual ligaments, all originating from the lateral malleolus: the anterior talofibular ligament (ATFL), the posterior talofibular ligament (PTFL), and the calcaneofibular ligament (CFL) (Figure 1).11,12,15 The ATFL is the primary restraint to inversion in plantar flexion, and it helps resist anterolateral translation of the talus in the mortise. However, it is the weakest and therefore the most frequently injured of the lateral ligaments. The PTFL plays only a supplementary role in ankle stability when the lateral ligament complex is intact. It is under the greatest strain in ankle dorsiflexion and acts to limit posterior talar displacement within the mortise as well as talar external rotation.13,16 The CFL is the primary restraint to inversion in the neutral or dorsiflexed position. It restrains subtalar inversion, thereby limiting talar tilt within the mortise.

DELTOID LIGAMENT
The deltoid ligament complex consists of 6 continuous adjacent superficial and deep ligaments that function synergistically to resist valgus and pronation forces, as well as external rotation of the talus in the mortise.11-13,17 The superficial layer crosses both ankle and subtalar joints. It originates from the anterior colliculus and fans out to insert into the navicular, neck of the talus, sustentaculum tali, and posteromedial talar tubercle. The tibiocalcaneal (sustentaculum tali) portion is the strongest component in the superficial layer and resists calcaneal eversion. The deep layer crosses the ankle joint only. It functions as the primary stabilizer of the medial ankle and prevents both lateral displacement and external rotation of the talus. It originates from the inferior and posterior aspects of the medial malleolus and inserts on the medial and posteromedial aspects of the talus.12,17,18
Continue to: SYNDESMOSIS
SYNDESMOSIS
The ankle syndesmosis, or inferior tibiofibular joint, is the distal articulation between the tibia and fibula. The syndesmosis contributes to ankle mortise integrity through its firm fixation of the lateral malleolus against the lateral surface of the talus. Ligaments comprising the ankle syndesmosis include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the inferior transverse ligament, and the interosseous ligament (IOL).12
ANKLE SPRAINS
Ankle sprains are the most common pathology encountered amongst soccer players, representing from one-half to two-thirds of all ankle related injuries. Most sprains occur outside of player contact.
LATERAL ANKLE SPRAINS AND INSTABILITY
Injury to the lateral ligaments of the ankle represents 77% to 91% of all ankle sprains in soccer.6,19 The greatest risk factor for an ankle sprain in a soccer player is a history of prior sprain.20 Other risk factors include increasing age, player-to-player contact, condition of the pitch, weight-bearing status of the injured limb at the time of injury, and joint instability or laxity.21,22
The evaluation of an ankle sprain to determine its severity is best done after the acute phase, approximately 4 to 7 days after the initial injury when both pain and swelling have subsided.23 The anterior drawer (ATFL instability) and talar tilt (CFL instability) tests are useful in evaluating ankle instability in the delayed or chronic setting; however, both have been shown to have limited sensitivity and significant variability amongst different examiners.24
Clinical examination will direct further diagnostic tests including X-rays, magnetic resonance imaging (MRI), and computed tomography (CT). The Ottawa ankle rules are generally helpful in determining whether plain X-rays are indicated in the acute setting.25,26 (Figure 2) According to these rules, ankle radiographs should be obtained if ankle pain is reported near the malleoli and 1 or more of the following is seen during examination: inability to bear weight immediately after injury and for 4 steps in the emergency department, and bony tenderness at the posterior edge or tip of the malleolus. Stress X-rays are generally not indicated in acute injuries but may be useful in chronic ankle instability cases.23

Continue to: Ankle sprains cover...
Ankle sprains cover a broad spectrum of injuries; therefore, a grading system was devised to aid in guiding treatment. Grade I (mild) sprains are those with minimal swelling and tenderness but have the ligaments still intact. Grade II (moderate) sprains occur when there are partial ligament tears associated with moderate pain, swelling, and tenderness. Finally, Grade III (severe) sprains are complete ligament tears with marked swelling, hemorrhage, tenderness, loss of function, and abnormal joint motion and instability.23, 24
Initial treatment for all ankle sprains is nonoperative and involves the RICE (rest, ice, compression, elevation) protocol with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) during the acute phase (first 4-5 days) with a short period (no >2 weeks) of immobilization.27 Most authors agree that early mobilization followed by phased rehabilitation is warranted to minimize time away from sports.28-31 Prolonged immobilization (>2 weeks) has detrimental effects and may lead to a longer return to play.28-31 The rehabilitation protocol is divided into stages: (1) pain and edema control, (2) range of motion (ROM) and strengthening exercises, (3) soccer specific functional training, and (4) prophylactic intervention with balance and proprioception exercises. Surgical intervention is rarely indicated for acute ankle sprains. There are exceptions, however, such as when ankle sprains are associated with other injuries that require acute intervention (eg, fracture, osteochondral lesion). Surgery is indicated in the setting of chronic, recurrent mechanical instability. Anatomical repairs (modified Brostrom) seem to produce better outcomes than non-anatomical reconstructions (eg, Chrisman-Snook). Surgical outcomes are good, and most athletes are able to return to their pre-injury level of function.32
In athletes, prevention of recurrent sprains is key. Braces may help prevent ankle sprains and bracing has been shown to be superior to taping, as tape loses its restrictive properties within 20 to 30 minutes of initiating activity.33,34 Application of an orthosis (lace-up ankle orthosis) has been shown to reduce the incidence of ankle re-injury in soccer players with previous ankle sprains. Several studies have found minimal, if any, effect of orthoses on athletic performance.20,35,36 Low-profile braces for soccer have been developed which allow for minimal disruption of the player’s boot and space proximally to insert the shin guard. Another essential component of prevention is prophylactic intervention with balance and proprioceptive exercises. A study looking at first division men’s league football (soccer) players in Iran showed a significant decrease in re-injury rates with proprioceptive training.37 In 2003, FIFA introduced a comprehensive warm-up program (FIFA 11+), which has since been shown in several studies to decrease the risk of injury in amateur soccer players.38-40
MEDIAL ANKLE SPRAINS AND INSTABILITY
Soccer places an unusually high demand on both the medial foot and ankle structures when compared with other sports. For instance, striking the ball requires the player to abduct and externally rotate the foot, which preloads medial structures.9 Hintermann18 looked at 54 cases of medial ankle instability and found that injury commonly occurred during landing on an uneven surface, which applies to soccer players when landing after heading the ball or jumping over a tackle. Pronation with eversion and extreme rotational injuries are well known to cause deltoid ligament injury. However, complete rupture of the deltoid ligament is rare and is more often associated with ankle fractures.41 Due to its close proximity and similarly shared function in medial plantar arch stabilization with the tibiospring and spring ligaments, posterior tibialis tendon dysfunction is also frequently seen in medial ankle instability.17 After an acute injury, patients can present with a medial ankle hematoma and pain along the deltoid ligament. Although chronic insufficiency is diagnosed based on the feeling of “giving way,” pain in the medial gutter of the ankle and a valgus and pronation deformity of the foot can be corrected by activating the peroneus tertius muscle. Arthroscopy is the most specific way to confirm clinically suspected instability of the medial ankle; however, MRI can demonstrate loss of organized medial fibers (Figures 3A, 3B).18 Primary surgical repair of deltoid ligament tears yield good to excellent results and should be considered in the soccer player to prevent problems associated with chronic non-repaired tears such as instability, osteoarthritis, and impingement syndromes.18 After surgical repair, players will undergo extensive physical therapy that progresses to sport-specific exercises with the ultimate goal of returning to competitive play around 4-6 months post-operatively.

HIGH ANKLE SPRAINS (SYNDESMOSIS)
High ankle sprains are much less common than low ankle sprains; however, when they do occur they portend a lengthier rehabilitation and a poorer prognosis, especially if undiagnosed. Lubberts and colleagues42 studied the epidemiology of isolated syndesmotic injuries in professional football players. They pooled data from 15 consecutive seasons of European professional football between 2001 and 2016. They examined a total of 3677 players from 61 teams across 17 countries. There were 1320 ankle ligament injuries registered during 15 seasons, of which 94 (7%) were isolated syndesmotic injuries. The incidence of these injuries increased annually between 2001 and 2016. Injuries were 74% contact-related, and isolated syndesmotic injuries were followed by a mean of a 39-day absence.42 Moreover, football players may have an increased risk of syndesmotic sprains due to foot planting and cutting action.41
Continue to: These injuriesa are typically...
These injuries are typically identified with pain over the AITFL and interosseous membrane. Physical examination tests that help identify syndesmotic injuries include the squeeze test, external rotation test, and crossed-leg test.41 The diagnosis can be made on plain X-ray when there is clear diastasis between the distal tibia and fibula. Two critical measurements on plain films are made 1 cm above the tibial plafond and are used to evaluate the integrity of the syndesmosis: tibiofibular clear space >6 mm, and tibiofibular overlap <1 mm, which indicate disruption of the syndesmosis.43 More subtle injuries can be diagnosed with better sensitivity and specificity using MRI, which can also reveal secondary findings such as bone bruises, ATFL injury, osteochondral lesions, and tibiofibular incongruity.44,45 Arthroscopy is an invaluable diagnostic tool for syndesmotic injuries with a characteristic triad finding of PITFL scarring, disrupted interosseous ligament, and posterolateral tibial plafond chondral damage.46
Classification of the ligaments involved can aid in the selection of appropriate treatment. Grade I injuries involve AITFL tears. Grade IIa injuries involve AITFL and IOL tears. Grade IIb injuries include AITFL, PITFL, and IOL tears. Grade III injuries involve injury to all 3 ligaments, as well as a fibular fracture. Conservative treatment is recommended for Grades I and IIa, while surgical intervention is necessary for Grades IIb and III (Figures 4A, 4B). Compared with other ankle sprains, syndesmotic injuries typically require a more prolonged recovery/rehabilitation. Some studies suggest that these injuries require twice as long to heal.47 Hopkinson and colleagues48 reported a mean recovery time of 55 days following syndesmotic injuries in cadets at the United States Military Academy at West Point. Some surgeons advocate surgical intervention in professional athletes with mild sprains to expedite return to play.49
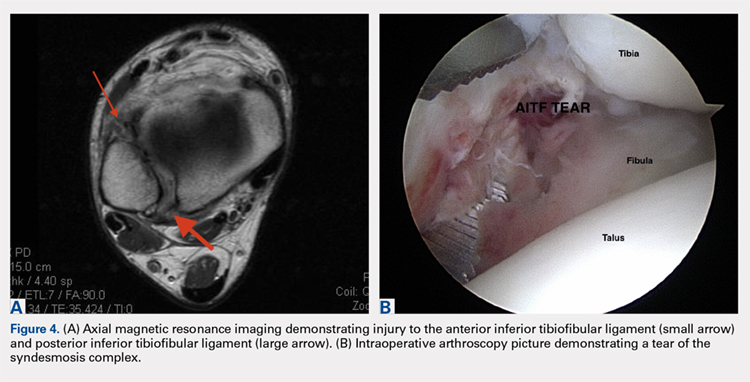
Surgery has been well established as necessary in more severe injuries where there is clear diastasis or instability of the syndesmosis. Traditionally, screws were used for surgical fixation; however, they often required a second surgery for subsequent removal. There is no general consensus on the optimal screw size, level of placement, or timing of removal.50,51 More recently, non-absorbable suture button fixation (eg, TightRope; Arthrex) has become more popular and provides certain advantages over screw fixation, such as avoiding the need for hardware removal. TightRope has been shown to provide more accurate stabilization of the syndesmosis as compared with screw fixation.52 Since malreduction is the most important indicator of poor long-term functional outcome, suture button fixation should be considered in the treatment of the football player.53 Finally, Colcuc and colleagues54 reported a lower complication rate and earlier return to sports in patients treated with knotless suture button devices compared with screw fixation.
OSTEOCHONDRAL LESIONS
Osteochondral lesions (OCLs) are cartilage-bone defects that are usually located in the talus. They can be caused by an acute traumatic event or repetitive microtrauma with no apparent history of trauma (eg, ankle instability). Acute OCLs can occur in soccer secondary to an ankle sprain or ankle fracture. Symptoms of OCLs include pain, swelling, and mechanical symptoms such as catching or locking, and on physical examination, one might see an effusion. The initial imaging modality of choice is radiographing; however, in ankle sprains with continued pain and swelling MRI may be indicated to rule out an underlying OCL. Missed acute lesions have a tendency not to heal and become chronic lesions, which can cause pain and playing disability. It is well established that chronic ankle instability is an important etiologic factor for OCLs. With the normal hydrostatic pressure within the ankle joint, synovial fluid gets pushed into cartilage/bone fissures, which can then lead to cystic degeneration of the subchondral bone.55-57
Surgical repair of an OCL is dependent on both the size and location of the lesion. Acute lesions can be managed by arthroscopic débridement, microfracture, or fixation of the lesion if enough bone remains attached to the chondral lesion. Return to play is based on development and maturation of fibrocartilage over the lesion (debridement/microfracture) or healing and incorporation of the new graft (chondral repair procedures). Meanwhile, chronic lesions can be managed in 1-stage (microfracture, osteochondral autograft transfer or 2-stage (autologous chondrocyte implantation [ACI]) procedures.56-57 Additional biologic healing augmentation with platelet-rich plasma has been described as well.58 Newer techniques in treating chronic talus OCLs, including ones that have failed to respond to bone marrow stimulation techniques, have been developed more recently such as the use of particulated juvenile articular cartilage allograft (DeNovo NT Natural Tissue Graft®; Zimmer Biomet).59 These newer techniques avoid the need for a 2-stage procedure, as is the case with ACI.
Continue to: Further studies are needed...
Further studies are needed to both investigate long-term outcomes and determine the superiority of the arthroscopic juvenile cartilage procedure compared with microfracture and other cartilage resurfacing procedures. When surgically treating OCLs, one must also restore normal ankle joint biomechanics for the lesion to heal. For instance, in the presence of ankle instability, ligament reconstruction must be performed. Also, one should also consider addressing any hindfoot malalignment with an osteotomy (calcaneus, supramalleolar). In a recent retrospective study, van Eekeren and colleagues60 showed that approximately 76% of patients were able to return to sports at long-term follow-up after arthroscopic débridement and bone marrow stimulation of talar OCLs. However, the activity level decreased at long-term follow-up and never attained the pre-injury level.60
ANKLE IMPINGEMENT
ANTERIOR ANKLE IMPINGEMENT (FOOTBALLER'S ANKLE)
Anterior ankle impingement is caused by anterior osteophytes on both the distal tibia and talar neck. It is thought to be related to repetitive microtrauma to the anteromedial aspect of the ankle from recurrent ball impact.61 It is very common amongst soccer players with some studies suggesting that 60% of soccer players have this syndrome. Ankle impingement is characterized by anterior pain with ankle dorsiflexion, decreased dorsiflexion, and swelling. It is primarily diagnosed with lateral ankle X-rays, which will show the osteophytes. An oblique anteromedial X-ray may increase detection of osteophytes (Figure 5). The early stages of anterior impingement can be treated successfully with injections and heel lifts. Treatment of lesions that fail to respond to conservative management involves arthroscopic or open excision of osteophytes. Most patients with no preexisting osteoarthritis treated arthroscopically will experience pain relief and return to full activity, though recurrent osteophyte formation has been noted at long-term follow-up.62
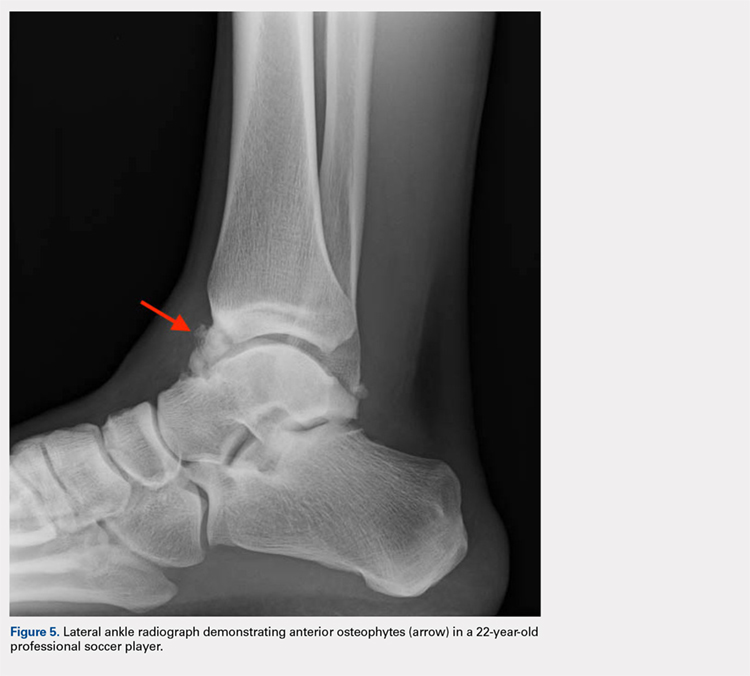
Anterior ankle impingement is most often caused by acute ankle sprains with an inversion type of mechanism.62 The subsequent reactive inflammation can cause fibrosis leading to distal fascicle enlargement of the AITFL. Impingement in the anterolateral gutter of this enlarged fascicle can also cause both chronic reactive synovitis and chondromalacia of the lateral talar dome.63 MRI can identify abnormal areas of pathology; however, 50% of cases are diagnosed based on clinical examination alone.63 Patients generally present with a history of anterolateral ankle pain and swelling with an occasional popping or snapping sensation.
Soccer players commonly develop anterior bony impingement due to repetitive loading of the anterior ankle from striking the ball. This repetition can lead to osteophyte formation of the anterior distal tibia and talar neck. After the osteophytes form, decreased dorsiflexion can occur due to a mechanical stop and inflammation of the interposed capsule.
The patient will exhibit tenderness to palpation along the anterolateral aspect of the ankle, with pain elicited at extreme passive dorsiflexion.62 Initially, an injection with local anesthetic and corticosteroid can serve both a diagnostic and therapeutic purpose; however, patients who fail conservative treatment can be treated with arthroscopy and resection of the involved scar tissue and osteophytes. The best results are seen in those patients with no concurrent intra-articular lesions or ankle osteoarthritis (Figure 5).62 When treated non-operatively, a player may return to play when pain resolves; however, if treated surgically with arthroscopic debridement/resection, a player must wait until his surgical scars have healed prior to attempting return to play.
Continue to: ANTEROMEDIAL ANKLE IMPINGEMENT
ANTEROMEDIAL ANKLE IMPINGEMENT
Anteromedial ankle impingement is a less common ankle impingement syndrome. It is associated with eversion injuries or following medial malleolar or talar fractures.64,65 Previous injury to the anterior tibiotalar fascicle of the deltoid complex leads to ligament thickening and subsequent impingement in the anteromedial corner of the talus. Adjacent fibrosis and synovitis are common consequences of impingement; however, osteophyte formation and chondral stripping along the anteromedial talus can also be seen. Patients typically complain of pain along the anteromedial joint line that is worse with activity, clicking or popping sensations, and painful, limited dorsiflexion. On examination, impingement can be detected through palpation over the anterior tibiotalar fascicle of the deltoid ligament and eversion or extreme passive dorsiflexion of the foot, all of which will elicit medial ankle tenderness.17,62 Initial treatment consists of rest, physical therapy, and NSAIDs. Refractory cases may be amenable to arthroscopic or open resection of the anterior tibiotalar fascicle with débridement of any adjacent synovitis and scar tissue.62
POSTERIOR ANKLE IMPINGEMENT
Posterior ankle impingement is often referred to as “os trigonum syndrome” since the posterior impingement is frequently associated with a prominent os trigonum. An os trigonum is an accessory ossicle representing the separated posterolateral tubercle of the talus. It is usually asymptomatic. However, in soccer players, pain can occur from impaction between the posterior tibial plafond and the os trigonum, or because of soft tissue compression between the 2 opposing osseous structures. The pain is due to repetitive microtrauma (ankle plantarflexion) or acute forced plantarflexion, which can present as an acute fracture of the os trigonum. Because soccer is a sport requiring both repetitive and extreme plantarflexion, it may predispose players to posterior ankle impingement (Figures 6A, 6B).62,66
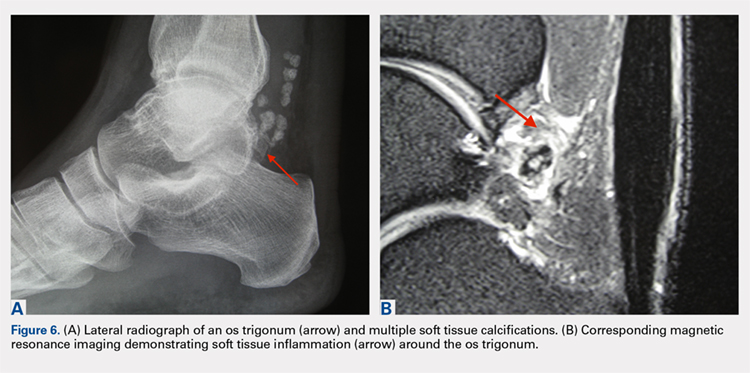
Clinically, it can be very difficult to detect and diagnose because the affected structures lie deep and it can coexist with other disease processes (eg, peroneal tendinopathy, Achilles tendinopathy).62,66 Patients will complain of chronic deep posterior ankle pain that is worse with push-off activities (eg, jumping). On examination, patients will exhibit pain with palpation over the posterolateral process and with the crunch test. Lateral radiograph with the foot in plantar flexion will show the os trigonum impinged between the posterior tibial malleolus and the calcaneal tuberosity. An MRI will demonstrate the os trigonum as well as any associated inflammation and edema, while it can also demonstrate coexisting pathologies.
Initial treatment consists of rest, NSAIDs, and taping to prevent plantar flexion. Ultrasound-guided cortisone injection of the capsule and posterior bursa can be both therapeutic and diagnostic. A posterior injection can be used to temporize the symptoms so that the soccer player can make it through the season.
Surgical excision is saved for refractory cases, and this can be done either through an open posterolateral approach or arthroscopic techniques. Recently, Georgiannos and Bisbinas67 showed in an athletic population that endoscopic excision had both a lower complication rate and a quicker return to sports compared with the traditional open approach. Carreira and colleagues68 conducted a retrospective case series of 20 patients (mostly competitive athletes). They found that posterior ankle arthroscopy to address posterior impingement allowed for the maintenance or restoration of anatomic ROM of the ankle and hindfoot, ability to return to at least the previous level of activity, and improvement in objective assessment of pain relief and a higher level of function parameters.68
Continue to: TENDON PATHOLOGY
TENDON PATHOLOGY
SUPERIOR PERONEAL RETINACULUM INJURY
The superior peroneal retinaculum (SPR) forms the roof of the superior peroneal tunnel. The tunnel contains the peroneus brevis and longus tendons and is bordered by the retromalleolar groove of the fibula and the lower aspect of the posterior intramuscular septum of the leg.69,70 The SPR originates from the posterolateral ridge of the fibula and inserts onto the lateral calcaneus, and it is the primary restraint of the peroneal tendons within the retromalleolar sulcus.
Injury to the retinaculum results from both ankle dorsiflexion and inversion, and forceful reflex contraction of the peroneal muscles, which causes subluxation or dislocation of the contained tendons.69 A high level of suspicion is required regarding these injuries since the mechanism of injury is similar to that of a simple lateral ankle sprain. In the setting of retrofibular pain, snapping or popping sensations around the lateral malleolus, or chronic ankle instability that worsens on uneven surfaces, one must consider an injury to the SPR.69 Radiographs are not always diagnostic; however, occasionally on an internal rotation view, one may see a cortical avulsion off the distal tip of the lateral malleolus (“fleck sign”) indicating a rim fracture from an SPR injury (Figure 7). MRI is the best imaging modality to assess the peroneal tendons, as well as an SPR injury. Recently, ultrasound has grown in popularity and may be more useful, since it allows for dynamic evaluation of subluxating/dislocating tendons.69

Conservative management is often associated with poor outcomes, and surgery is indicated for all acute and chronic dislocations in athletes.71 Anatomic reconstruction of the SPR is the preferred surgical method.72 Peroneus brevis debulking and fibular groove deepening may also augment the retinaculum repair.73 van Dijk and colleagues in their systematic review showed that patients treated with both groove deepening and SPR repair have higher rates of return to the sport than patients treated with SPR repair alone.74
STRESS FRACTURES
FIFTH METATARSAL
Fifth metatarsal stress fractures usually occur secondary to lateral overload or avulsion of the peroneus brevis. The fifth metatarsal base can be susceptible to injury in a cavovarus foot. Non-operative treatment typically requires a longer period of immobilization (boot or cast) and necessitates a longer period of non–weight-bearing (anywhere between 6-12 weeks). Therefore, surgery is typically recommended in athletes or in the setting of a recurrent base of the fifth metatarsal fracture to expedite healing and return to play. Return to play is still not recommended until there is evidence of radiographic healing of the fracture. There are certain distinctions with fifth metatarsal stress fractures regarding location and healing rates that need to be taken into account.75,76 In particular, zone 2 injuries (Jones fractures) represent a vascular watershed area, making these fractures prone to nonunion with nonunion rates as high as 15% to 30%. Occasionally, the cavovarus deformity will require correction as well as a reduction in the risk of recurrence or nonunion. Surgical fixation most commonly consists of a single screw placed in an antegrade fashion.77 One must pay attention to screw size since smaller diameter screws (<4.5 mm) are associated with delayed union or nonunion. Moreover, screws that are too long will straighten the curved metatarsal shaft and can lead to fracture distraction or malreduction (Figures 8A, 8B).77
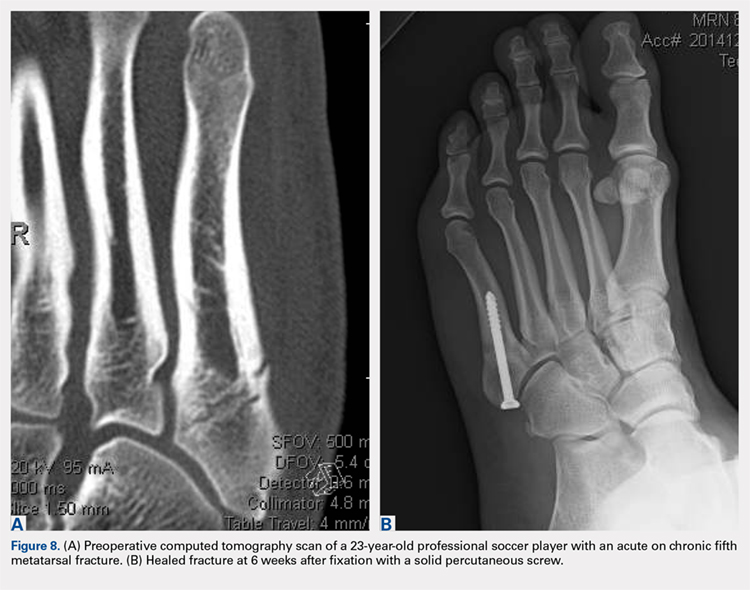
Patients have returned to competitive sports within 6 weeks; however, it should be noted that causes of failure were linked to early return and return to sports before a radiographic union can lead to failure of fixation. Ekstrand and van Dijk78 studied a large group of professional soccer players and found that out of 13,754 injuries, 0.5% (67) were fifth metatarsal fractures. Of note, they found that 45% of players had prodromal symptoms. Furthermore, after surgical treatment the fractures healed faster, compared with conservative treatment (75% vs 33%); however, there was no significant difference in lay-off days between both groups (80 vs 74 days).78 Matsuda and colleagues79 looked at 335 male collegiate soccer players, 29 of whom had a history of a fifth metatarsal stress fracture. They found that playing the midfield position and having an everted rearfoot and inverted forefoot alignment were associated with fifth metatarsal stress fractures.79 Saita and colleagues80 found that restricted hip internal rotation was associated with an increased risk of developing a Jones fracture in 162 professional football players. Finally, Fujitaka and colleagues81 looked at 273 male soccer players between 2005 and 2013. They found an association between weak toe-grip strength and fifth metatarsal fractures, suggesting that weak toe-grip may lead to an increase in the load applied onto the lateral side of the foot, resulting in a stress fracture.81
Continue to: NAVICULAR
NAVICULAR
Another common tarsal bone that sustains stress fractures is the navicular. It is not as common as calcaneal stress fractures in military recruits but can occur in the same type of population, as well as explosive athletics such as sprinters and soccer players. It commonly presents with an indistinct vague achy pain with activity that improves with rest, and pain at the dorsum of the midfoot or along the medial longitudinal arch with activity. It can easily go undiagnosed for quite some time given the difficulty in visualizing the navicular with plain radiographs. Clinically, it is difficult to make the diagnosis, and therefore advanced imaging is necessary when the injury is suspected. Both MRI and CT scans can be used to understand the extent of the injury (Figures 9A-9C). In non-displaced stress fractures, conservative non-operative treatment is the appropriate treatment modality with a brief period of immobilization and non–weight-bearing;82 however, operative treatment is also considered in elite athletes. In either case, return to play is discouraged until there is evidence of radiographic healing. When a displacement is noted, or there is a delay in diagnosis, then operative treatment is recommended.
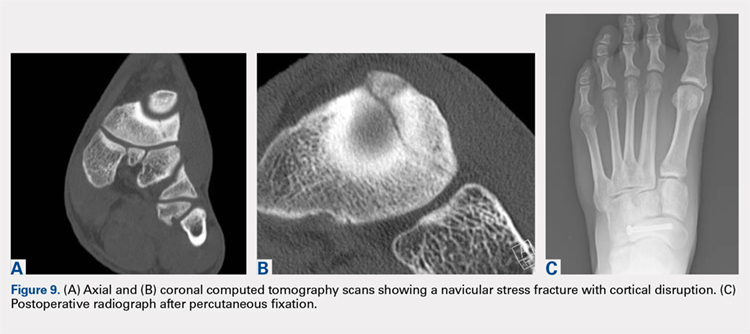
CONCLUSION
Ankle injuries are very common in soccer and can result in decreased performance or significant loss of playing time. Treatment of acute injury generally follows a conservative route, with surgical intervention reserved for severe ruptures or osteochondral fracture of the ankle joint. Chronic ankle pathology resulting in mechanical or functional instability generally requires surgery to repair ligamentous damage and restore normal ankle kinematics. It is critical for the soccer player to receive appropriate rehabilitation prior to returning to play in order to reduce the risk for reinjury and further chronic instability. Prevention and early intervention of ankle injuries is key in preventing the long-term sequelae of ankle injuries, such as arthritis, in former soccer players.
1. Dvorak J, Junge A. Football injuries and physical symptoms. A review of the literature. Am J Sports Med. 2000;28(5 Suppl):S3-S9.
2. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Am J Sports Med. 2000;28(5 Suppl):S58-S68.
3. Cloke DJ, Ansell P, Avery P, Deehan D. Ankle injuries in football academies: a three-centre prospective study. Br J Sports Med. 2011;45(9):702-708. doi:10.1136/bjsm.2009.067900.
4. Cloke DJ, Spencer S, Hodson A, Deehan D. The epidemiology of ankle injuries occurring in English Football Association academies. Br J Sports Med. 2009;43(14):1119-1125. doi:10.1136/bjsm.2008.052050.
5. Dvorak J, Junge A, Derman W, Schwellnus M. Injuries and illnesses of football players during the 2010 FIFA World Cup. Br J Sports Med. 2011;45(8):626-630. doi:10.1136/bjsm.2010.079905.
6. Ekstrand J, Gillquist J. Soccer injuries and their mechanisms: a prospective study. Med Sci Sports Exerc. 1983;15(3):267-270.
7. Fousekis K, Tsepis E, Vagenas G. Intrinsic risk factors of noncontact ankle sprains in soccer: a prospective study on 100 professional players. Am J Sports Med. 2012;40(8):1842-1850. doi:10.1177/0363546512449602.
8. Gaulrapp H, Becker A, Walther M, Hess H. Injuries in women’s soccer: a 1-year all players prospective field study of the women’s Bundesliga (German premiere league). Clin J Sports Med. 2010;20(4):264-271. doi:10.1097/JSM.0b013e3181e78e33.
9. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med. 2001;29(4):426-430. doi:10.1177/03635465010290040701.
10. Jain N, Murray D, Kemp S, Calder J. Frequency and trends in foot and ankle injuries within an English Premier League Football Club using a new impact factor of injury to identify a focus for injury prevention. Foot Ankle Surg. 2014;20(4):237-240. doi:10.1016/j.fas.2014.05.004.
11. Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, 2010:xxix, 1134.
12. Thompson JC, Netter FH. Netter’s Concise Orthopaedic Anatomy. 2nd ed. Philadelphia, PA: Saunders Elsevier, 2010:x, 404.
13. Giza E, Mandelbaum B. Chronic footballer’s ankle. In: Football Traumatology. Springer Milan, 2006:333-351.
14. Garrick JG. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am J Sports Med. 1977:5(6):241-242. doi:10.1177/036354657700500606.
15. Agur AMR, Grant JCB. Grant’s Atlas of Anatomy. 13th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2011.
16. Renstrom PA, Konradsen L. Ankle ligament injuries. Br J Sports Med. 1997;31(1):11-20.
17. Chhabra A, Subhawong TK, Carrino JA. MR imaging of deltoid ligament pathologic findings and associated impingement syndromes. Radiographics. 2010;30(3):751-761. doi:10.1148/rg.303095756.
18. Hintermann B. Medial ankle instability. Foot Ankle Clin. 2003;8(4):723-738.
19. Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football: an analysis of ankle sprains. Br J Sports Med. 2003;37(3):233-238.
20. Thacker SB, Stroup DF, Branche CM, Gilchrist J, Goodman RA, Weitman EA. The prevention of ankle sprains in sports. A systematic review of the literature. Am J Sports Med. 1999;27(6):753-760. doi:10.1177/03635465990270061201.
21. Giza E, Fuller C, Junge A, Dvorak J. Mechanisms of foot and ankle injuries in soccer. Am J Sports Med. 2003;31(4):550-554. doi:10.1177/03635465030310041201.
22. Tucker AM. Common soccer injuries. Diagnosis, treatment and rehabilitation. Sports Med. 1997;23(1):21-32.
23. Lynch SA, Renstrom PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. 1999;27(1):61-71.
24. Chan KW, Ding BC, Mroczek KJ. Acute and chronic lateral ankle instability in the athlete. Bull NYU Hosp Jt Dis. 2011;69(1):17-26.
25. Stiell IG, Greenberg GH, McKnight RD, Nair RC, McDowell I, Worthington JR. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21(4):384-390.
26. Bachmann LM, Kolb E, Koller MT, Steurer J, ter Riet G. Accuracy of Ottawa ankle rules to exclude fractures of the ankle and mid-foot: systematic review. BMJ. 2003;326(7386):417. doi:10.1136/bmj.326.7386.417.
27. Balduini FC, Vegso JJ, Torg JS, Torg E. Management and rehabilitation of ligamentous injuries to the ankle. Sports Med. 1987;4(5):364-380.
28. Kerkhoffs GM, Rowe BH, Assendelft WJ, Kelly KD, Struijs PA, van Dijk CN. Immobilisation for acute ankle sprain. A systematic review. Arch Orthop Trauma Surg. 2001;121(8):462-471.
29. Konradsen L, Holmer P, Sondergaard L. Early mobilizing treatment for grade III ankle ligament injuries. Foot Ankle. 1991;12(2):69-73.
30. Eiff MP, Smith AT, Smith GE. Early mobilization versus immobilization in the treatment of lateral ankle sprains. Am J Sports Med. 1994;22(1):83-88. doi:10.1177/036354659402200115.
31. Shrier I. Treatment of lateral collateral ligament sprains of the ankle: a critical appraisal of the literature. Clin J Sport Med. 1995;5(3):187-195.
32. DiGiovanni BF, Partal G, Baumhauer JF. Acute ankle injury and chronic lateral instability in the athlete. Clin Sports Med. 2004;23(1):1-19, v. doi:10.1016/S0278-5919(03)00095-4.
33. Alt W, Lohrer H, Gollhofer A. Functional properties of adhesive ankle taping: neuromuscular and mechanical effects before and after exercise. Foot Ankle Int. 1999;20(4):238-245. doi:10.1177/107110079902000406.
34. Garrick JG, Requa RK. Role of external support in the prevention of ankle sprains. Med Sci Sports. 1973;5(3):200-203.
35. Sharpe SR, Knapik J, Jones B. Ankle braces effectively reduce recurrence of ankle sprains in female soccer players. J Athl Train. 1997;32(1):21-24.
36. Surve I, Schwellnus MP, Noakes T, Lombard C. A fivefold reduction in the incidence of recurrent ankle sprains in soccer players using the Sport-Stirrup orthosis. Am J Sports Med. 1994;22(5):601-606. doi:10.1177/036354659402200506.
37. Mohammadi F. Comparison of 3 preventive methods to reduce the recurrence of ankle inversion sprains in male soccer players. Am J Sports Med. 2007;35(6):922-926. doi:10.1177/0363546507299259.
38. Steffen K, Meeuwisse WH, Romiti M, et al. Evaluation of how different implementation strategies of an injury prevention programme (FIFA 11+) impact team adherence and injury risk in Canadian female youth football players: a cluster-randomised trial. Br J Sports Med. 2013;47(8):480-487. doi:10.1136/bjsports-2012-091887.
39. Steffen K, Emery CA, Romiti M, et al. High adherence to a neuromuscular injury prevention programme (FIFA 11+) improves functional balance and reduces injury risk in Canadian youth female football players: a cluster randomised trial. Br J Sports Med. 2013;47(12):794-802. doi: 10.1136/bjsports-2012-091886.
40. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
41. Lin CF, Gross ML, Weinhold P. Ankle syndesmosis injuries: anatomy, biomechanics, mechanism of injury, and clinical guidelines for diagnosis and intervention. J Orthop Sports Phys Ther. 2006;36(6):372-384. doi:10.2519/jospt.2006.2195.
42. Lubberts B, D’Hooghe P, Bengtsson H, DiGiovanni CW, Calder J, Ekstrand J. Epidemiology and return to play following isolated syndesmotic injuries of the ankle: a prospective cohort study of 3677 male professional football players in the UEFA Elite Club Injury Study. Br J Sports Med. 2017. doi:10.1136/bjsports-2017-097710.
43. Harper MC, Keller TS. A radiographic evaluation of the tibiofibular syndesmosis. Foot Ankle. 1989;10(3):156-160.
44. Vogl TJ, Hochmuth K, Diebold T, et al. Magnetic resonance imaging in the diagnosis of acute injured distal tibiofibular syndesmosis. Invest Radiol. 1997;32(7):401-409.
45. Brown KW, Morrison WB, Schweitzer ME, Parellada JA, Nothnagel H. MRI findings associated with distal tibiofibular syndesmosis injury. AJR Am J Roentgenol. 2004;182(1):131-136. doi:10.2214/ajr.182.1.1820131.
46. Ogilvie-Harris DJ, Reed SC, Hedman TP. Disruption of the ankle syndesmosis: biomechanical study of the ligamentous restraints. Arthroscopy. 1994;10(5):558-560.
47. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298. doi:10.1177/036354659101900315.
48. Hopkinson WJ, St Pierre P, Ryan JB, Wheeler JH. Syndesmosis sprains of the ankle. Foot Ankle. 1990;10(6):325-330. doi:10.1177/107110079001000607.
49. Del Buono A, Florio A, Boccanera MS, Maffulli N. Syndesmosis injuries of the ankle. Curr Rev Musculoskelet Med. 2013;6(4):313-319. doi:10.1007/s12178-013-9183-x.
50. Dattani R, Patnaik S, Kantak A, Srikanth B, Selvan TP. Injuries to the tibiofibular syndesmosis. J Bone Joint Surg Br. 2008;90(4):405-410. doi:10.1302/0301-620X.90B4.19750.
51. Schepers T. To retain or remove the syndesmotic screw: a review of literature. Arch Orthop Trauma Surg. 2011;131(7):879-883. doi:10.1007/s00402-010-1225-x.
52. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835. doi:10.1177/0363546512461480.
53. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
54. Colcuc C, Blank M, Stein T, et al. Lower complication rate and faster return to sports in patients with acute syndesmotic rupture treated with a new knotless suture button device. Knee Surg Sports Traumatol Arthrosc. 2017. doi:10.1007/s00167-017-4820-4823.
55. Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec. 2014;7(5):414-422. doi:10.1177/1938640014543362.
56. Talusan PG, Milewski MD, Toy JO, Wall EJ. Osteochondritis dissecans of the talus: diagnosis and treatment in athletes. Clin Sports Med. 2014;33(2):267-284. doi:10.1016/j.csm.2014.01.003.
57. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045-1054. doi:10.2106/JBJS.L.00773.
58. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384-2389. doi:10.1007/s00167-013-2784-5.
59. Hatic SO, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3(6):361-364. doi:10.1177/1938640010388602.
60. van Eekeren IC, van Bergen CJ, Sierevelt IN, Reilingh ML, van Dijk CN. Return to sports after arthroscopic debridement and bone marrow stimulation of osteochondral talar defects: a 5- to 24-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1311-1315. doi:10.1007/s00167-016-3992-6.
61. Tol JL, Slim E, van Soest AJ, van Dijk CN. The relationship of the kicking action in soccer and anterior ankle impingement syndrome. A biomechanical analysis. Am J Sports Med. 2002;30(1):45-50. doi:10.1177/03635465020300012101.
62. Sanders TG, Rathur SK. Impingement syndromes of the ankle. Magn Reson Imaging Clin N Am. 2008;16(1):29-38. doi:10.1016/j.mric.2008.02.005.
63. Ogilvie-Harris DJ, Gilbart MK, Chorney K. Chronic pain following ankle sprains in athletes: the role of arthroscopic surgery. Arthroscopy. 1997;13(5):564-574.
64. Robinson P, White LM, Salonen D, Ogilvie-Harris D. Anteromedial impingement of the ankle: using MR arthrography to assess the anteromedial recess. AJR Am J Roentgenol. 2002;178(3):601-604. doi:10.2214/ajr.178.3.1780601.
65. Mosier-La Clair SM, Monroe MT, Manoli A. Medial impingement syndrome of the anterior tibiotalar fascicle of the deltoid ligament on the talus. Foot Ankle Int. 2000;21(5):385-391.
66. Maquirriain J. Posterior ankle impingement syndrome. J Am Acad Orthop Surg. 2005;13(6):365-371.
67. Georgiannos D, Bisbinas I. Endoscopic versus open excision of os trigonum for the treatment of posterior ankle impingement syndrome in an athletic population: a randomized controlled study with 5-year follow-up. Am J Sports Med. 2017;45(6):1388-1394. doi:10.1177/0363546516682498.
68. Carreira DS, Vora AM, Hearne KL, Kozy J. Outcome of arthroscopic treatment of posterior impingement of the ankle. Foot Ankle Int. 2016;37(4):394-400. doi:10.1177/1071100715620857.
69. Roth JA, Taylor WC, Whalen J. Peroneal tendon subluxation: the other lateral ankle injury. Br J Sports Med. 2010;44(14):1047-1053. doi:10.1136/bjsm.2008.057182.
70. Athavale SA, Swathi, Vangara SV. Anatomy of the superior peroneal tunnel. J Bone Joint Surg Am. 2011;93(6):564-571. doi:10.2106/JBJS.17.00836.
71. Porter D, McCarroll J, Knapp E, Torma J. Peroneal tendon subluxation in athletes: fibular groove deepening and retinacular reconstruction. Foot Ankle Int. 2005;26(6):436-441.
72. Ferran NA, Oliva F, Maffulli N. Recurrent subluxation of the peroneal tendons. Sports Med. 2006;36(10):839-846. doi:10.1053/j.jfas.2010.02.007.
73. Saxena A, Ewen B. Peroneal subluxation: surgical results in 31 athletic patients. J Foot Ankle Surg. 2010;49(3):238-241.
74. van Dijk PA, Gianakos AL, Kerkhoffs GM, Kennedy JG. Return to sports and clinical outcomes in patients treated for peroneal tendon dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1155-1164. doi:10.1007/s00167-015-3833-z.
75. Lee KT, Park YU, Young KW, Kim JS, Kim JB. The plantar gap: another prognostic factor for fifth metatarsal stress fracture. Am J Sports Med. 2011;39(10):2206-2211. doi:10.1177/0363546511414856.
76. Torg JS. Fractures of the base of the fifth metatarsal distal to the tuberosity. Orthopedics. 1990;13:731-737.
77. Smith TO, Clark A, Hing CB. Interventions for treating proximal fifth metatarsal fractures in adults: a meta-analysis of the current evidence-base. Foot Ankle Surg. 2011;17(4):300-307. doi:10.1016/j.fas.2010.12.005.
78. Ekstrand J, van Dijk CN. Fifth metatarsal fractures among male professional footballers: a potential career-ending disease. Br J Sports Med. 2013;47(12):754-758.
79. Matsuda S, Fukubayashi T, Hirose N. Characteristics of the foot static alignment and the plantar pressure associated with fifth metatarsal stress fracture history in male soccer players: a case-control study. Sports Med Open. 2017;3(1):27.
80. Saita Y, Nagao M, Kawasaki T, et al. Range limitation in hip internal rotation and fifth metatarsal stress fractures (Jones fracture) in professional football players. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):1943-1949. doi:10.1007/s00167-017-4552-4.
81. Fujitaka K, Taniguchi A, Isomoto S, et al. Pathogenesis of fifth metatarsal fractures in college soccer players. Orthop J Sports Med. 2015;18;3(9):2325967115603654.
82. Torg J, Moyer J, Gaughan J, Boden B. Management of tarsal navicular stress fractures: conservative versus surgical treatment: a meta-analysis. Am J Sports Med. 2010;38(5):1048-1053.
83. Haytmanek CT, Williams BT, James EW, et al. Radiographic identification of the primary lateral ankle structures. Am J Sports Med. 2015;43(1):79-87. doi:10.1177/0363546514553778.
ABSTRACT
The ankle is one of the most commonly injured joints in soccer and represents a significant cost to the healthcare system. The ligaments that stabilize the ankle joint determine its biomechanics—alterations of which result from various soccer-related injuries. Acute sprains are among the most common injury in soccer players and are generally treated conservatively, with emphasis placed on secondary prevention to reduce the risk for future sprains and progression to chronic ankle instability. Repetitive ankle injuries in soccer players may cause chronic ankle instability, which includes both mechanical ligamentous laxity and functional changes. Chronic ankle pathology often requires surgery to repair ligamentous damage and remove soft-tissue or osseous impingement. Proper initial treatment, rehabilitation, and secondary prevention of ankle injuries can limit the amount of time lost from play and avoid negative long-term sequelae (eg, osteochondral lesions, arthritis). On the other hand, high ankle sprains portend a poorer prognosis and a longer recovery. These injuries will typically require surgical stabilization. Impingement-like syndromes of the ankle can undergo an initial trial of conservative treatment; when this fails, however, soccer players respond favorably to arthroscopic debridement of the lesions causing impingement. Finally, other pathologies (eg, stress fractures) are highly encouraged to be treated with surgical stabilization in elite soccer players.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
With roughly 200,000 professional and around 240 million amateur soccer players, soccer has been recognized as the most popular sport worldwide. Nevertheless, given its rising popularity in society, one must also consider the increasing incidence of injuries as a result. Elite soccer players sustain between 10 and 35 injuries per 1000 competitive playing hours.1 Approximately 80% are traumatic, and 20% are overuse injuries.2 Soccer injuries are more frequent with increasing age of the participants, whereas the incidence of injuries in preadolescent players is low. The incidence of injuries has been found to be higher during competition when compared with practice/training sessions, with some studies showing that 59% of injuries occurred during games.2 Amateur or recreational soccer players sustain fewer injuries than professional soccer players, as one would expect, given both the higher intensity of training and match schedule in professionals.
The ankle is one of the most commonly injured joints in soccer, with some studies suggesting it comprises one-fifth of all injuries sustained during soccer, which is only second to those of the knee.2 Ankle sprains specifically are quite a common occurrence in soccer.3-9 A recent study of an English premier league club showed that over a 4-season period, 20% of injuries were of the foot and ankle with a mean return to sport time of 54 days.10 Of all foot and ankle related injuries, ankle sprains are the most common, followed by bruises/contusions, and tendon lesions. Fractures are very rare (1%) in soccer, but when they do occur they impart a much more extended recovery. During the 2010 Fédération Internationale de Football Association (FIFA) World Cup, ankle sprains were among the most common injuries and approximately half lead to players missing training or competitive matches.5
ANATOMY
Knowledge of the biomechanics of both the foot and ankle joints is essential to understand soccer injuries. The ankle joint (talocrural articulation) consists of the distal ends of the tibia and fibula, which form the mortise, and the superior aspect of the talar dome.11 As a hinge joint, the ankle provides 20° of dorsiflexion and 50° of plantar flexion,12 with stability provided by the lateral, medial, and superior ligamentous complexes. The superior articular surface of the talus is narrower posteriorly, which creates a looser fit within the mortise during plantar flexion.11 This decreased stability could help explain why the most common injury in soccer involves a plantar flexion mechanism.13,14 Inferiorly, the talus articulates with the calcaneus to form the subtalar joint. It is at this site that the majority of both foot inversion and eversion occurs. The transverse tarsal joints (Chopart’s joints) separate the hindfoot from the midfoot. Movement of this joint depends on the relative alignment of its 2 articulations: the talonavicular and calcaneocuboid joints. During foot eversion, these 2 joints are parallel to each other allowing supple motion and aiding in shock absorption during the heel strike phase of the gait cycle. With foot inversion, the joints become nonparallel and thus lock the transverse tarsal joints providing a rigid lever needed for push-off.11,12
LATERAL LIGAMENTS
The ankle joint is stabilized laterally by a ligament complex consisting of 3 individual ligaments, all originating from the lateral malleolus: the anterior talofibular ligament (ATFL), the posterior talofibular ligament (PTFL), and the calcaneofibular ligament (CFL) (Figure 1).11,12,15 The ATFL is the primary restraint to inversion in plantar flexion, and it helps resist anterolateral translation of the talus in the mortise. However, it is the weakest and therefore the most frequently injured of the lateral ligaments. The PTFL plays only a supplementary role in ankle stability when the lateral ligament complex is intact. It is under the greatest strain in ankle dorsiflexion and acts to limit posterior talar displacement within the mortise as well as talar external rotation.13,16 The CFL is the primary restraint to inversion in the neutral or dorsiflexed position. It restrains subtalar inversion, thereby limiting talar tilt within the mortise.

DELTOID LIGAMENT
The deltoid ligament complex consists of 6 continuous adjacent superficial and deep ligaments that function synergistically to resist valgus and pronation forces, as well as external rotation of the talus in the mortise.11-13,17 The superficial layer crosses both ankle and subtalar joints. It originates from the anterior colliculus and fans out to insert into the navicular, neck of the talus, sustentaculum tali, and posteromedial talar tubercle. The tibiocalcaneal (sustentaculum tali) portion is the strongest component in the superficial layer and resists calcaneal eversion. The deep layer crosses the ankle joint only. It functions as the primary stabilizer of the medial ankle and prevents both lateral displacement and external rotation of the talus. It originates from the inferior and posterior aspects of the medial malleolus and inserts on the medial and posteromedial aspects of the talus.12,17,18
Continue to: SYNDESMOSIS
SYNDESMOSIS
The ankle syndesmosis, or inferior tibiofibular joint, is the distal articulation between the tibia and fibula. The syndesmosis contributes to ankle mortise integrity through its firm fixation of the lateral malleolus against the lateral surface of the talus. Ligaments comprising the ankle syndesmosis include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the inferior transverse ligament, and the interosseous ligament (IOL).12
ANKLE SPRAINS
Ankle sprains are the most common pathology encountered amongst soccer players, representing from one-half to two-thirds of all ankle related injuries. Most sprains occur outside of player contact.
LATERAL ANKLE SPRAINS AND INSTABILITY
Injury to the lateral ligaments of the ankle represents 77% to 91% of all ankle sprains in soccer.6,19 The greatest risk factor for an ankle sprain in a soccer player is a history of prior sprain.20 Other risk factors include increasing age, player-to-player contact, condition of the pitch, weight-bearing status of the injured limb at the time of injury, and joint instability or laxity.21,22
The evaluation of an ankle sprain to determine its severity is best done after the acute phase, approximately 4 to 7 days after the initial injury when both pain and swelling have subsided.23 The anterior drawer (ATFL instability) and talar tilt (CFL instability) tests are useful in evaluating ankle instability in the delayed or chronic setting; however, both have been shown to have limited sensitivity and significant variability amongst different examiners.24
Clinical examination will direct further diagnostic tests including X-rays, magnetic resonance imaging (MRI), and computed tomography (CT). The Ottawa ankle rules are generally helpful in determining whether plain X-rays are indicated in the acute setting.25,26 (Figure 2) According to these rules, ankle radiographs should be obtained if ankle pain is reported near the malleoli and 1 or more of the following is seen during examination: inability to bear weight immediately after injury and for 4 steps in the emergency department, and bony tenderness at the posterior edge or tip of the malleolus. Stress X-rays are generally not indicated in acute injuries but may be useful in chronic ankle instability cases.23

Continue to: Ankle sprains cover...
Ankle sprains cover a broad spectrum of injuries; therefore, a grading system was devised to aid in guiding treatment. Grade I (mild) sprains are those with minimal swelling and tenderness but have the ligaments still intact. Grade II (moderate) sprains occur when there are partial ligament tears associated with moderate pain, swelling, and tenderness. Finally, Grade III (severe) sprains are complete ligament tears with marked swelling, hemorrhage, tenderness, loss of function, and abnormal joint motion and instability.23, 24
Initial treatment for all ankle sprains is nonoperative and involves the RICE (rest, ice, compression, elevation) protocol with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) during the acute phase (first 4-5 days) with a short period (no >2 weeks) of immobilization.27 Most authors agree that early mobilization followed by phased rehabilitation is warranted to minimize time away from sports.28-31 Prolonged immobilization (>2 weeks) has detrimental effects and may lead to a longer return to play.28-31 The rehabilitation protocol is divided into stages: (1) pain and edema control, (2) range of motion (ROM) and strengthening exercises, (3) soccer specific functional training, and (4) prophylactic intervention with balance and proprioception exercises. Surgical intervention is rarely indicated for acute ankle sprains. There are exceptions, however, such as when ankle sprains are associated with other injuries that require acute intervention (eg, fracture, osteochondral lesion). Surgery is indicated in the setting of chronic, recurrent mechanical instability. Anatomical repairs (modified Brostrom) seem to produce better outcomes than non-anatomical reconstructions (eg, Chrisman-Snook). Surgical outcomes are good, and most athletes are able to return to their pre-injury level of function.32
In athletes, prevention of recurrent sprains is key. Braces may help prevent ankle sprains and bracing has been shown to be superior to taping, as tape loses its restrictive properties within 20 to 30 minutes of initiating activity.33,34 Application of an orthosis (lace-up ankle orthosis) has been shown to reduce the incidence of ankle re-injury in soccer players with previous ankle sprains. Several studies have found minimal, if any, effect of orthoses on athletic performance.20,35,36 Low-profile braces for soccer have been developed which allow for minimal disruption of the player’s boot and space proximally to insert the shin guard. Another essential component of prevention is prophylactic intervention with balance and proprioceptive exercises. A study looking at first division men’s league football (soccer) players in Iran showed a significant decrease in re-injury rates with proprioceptive training.37 In 2003, FIFA introduced a comprehensive warm-up program (FIFA 11+), which has since been shown in several studies to decrease the risk of injury in amateur soccer players.38-40
MEDIAL ANKLE SPRAINS AND INSTABILITY
Soccer places an unusually high demand on both the medial foot and ankle structures when compared with other sports. For instance, striking the ball requires the player to abduct and externally rotate the foot, which preloads medial structures.9 Hintermann18 looked at 54 cases of medial ankle instability and found that injury commonly occurred during landing on an uneven surface, which applies to soccer players when landing after heading the ball or jumping over a tackle. Pronation with eversion and extreme rotational injuries are well known to cause deltoid ligament injury. However, complete rupture of the deltoid ligament is rare and is more often associated with ankle fractures.41 Due to its close proximity and similarly shared function in medial plantar arch stabilization with the tibiospring and spring ligaments, posterior tibialis tendon dysfunction is also frequently seen in medial ankle instability.17 After an acute injury, patients can present with a medial ankle hematoma and pain along the deltoid ligament. Although chronic insufficiency is diagnosed based on the feeling of “giving way,” pain in the medial gutter of the ankle and a valgus and pronation deformity of the foot can be corrected by activating the peroneus tertius muscle. Arthroscopy is the most specific way to confirm clinically suspected instability of the medial ankle; however, MRI can demonstrate loss of organized medial fibers (Figures 3A, 3B).18 Primary surgical repair of deltoid ligament tears yield good to excellent results and should be considered in the soccer player to prevent problems associated with chronic non-repaired tears such as instability, osteoarthritis, and impingement syndromes.18 After surgical repair, players will undergo extensive physical therapy that progresses to sport-specific exercises with the ultimate goal of returning to competitive play around 4-6 months post-operatively.

HIGH ANKLE SPRAINS (SYNDESMOSIS)
High ankle sprains are much less common than low ankle sprains; however, when they do occur they portend a lengthier rehabilitation and a poorer prognosis, especially if undiagnosed. Lubberts and colleagues42 studied the epidemiology of isolated syndesmotic injuries in professional football players. They pooled data from 15 consecutive seasons of European professional football between 2001 and 2016. They examined a total of 3677 players from 61 teams across 17 countries. There were 1320 ankle ligament injuries registered during 15 seasons, of which 94 (7%) were isolated syndesmotic injuries. The incidence of these injuries increased annually between 2001 and 2016. Injuries were 74% contact-related, and isolated syndesmotic injuries were followed by a mean of a 39-day absence.42 Moreover, football players may have an increased risk of syndesmotic sprains due to foot planting and cutting action.41
Continue to: These injuriesa are typically...
These injuries are typically identified with pain over the AITFL and interosseous membrane. Physical examination tests that help identify syndesmotic injuries include the squeeze test, external rotation test, and crossed-leg test.41 The diagnosis can be made on plain X-ray when there is clear diastasis between the distal tibia and fibula. Two critical measurements on plain films are made 1 cm above the tibial plafond and are used to evaluate the integrity of the syndesmosis: tibiofibular clear space >6 mm, and tibiofibular overlap <1 mm, which indicate disruption of the syndesmosis.43 More subtle injuries can be diagnosed with better sensitivity and specificity using MRI, which can also reveal secondary findings such as bone bruises, ATFL injury, osteochondral lesions, and tibiofibular incongruity.44,45 Arthroscopy is an invaluable diagnostic tool for syndesmotic injuries with a characteristic triad finding of PITFL scarring, disrupted interosseous ligament, and posterolateral tibial plafond chondral damage.46
Classification of the ligaments involved can aid in the selection of appropriate treatment. Grade I injuries involve AITFL tears. Grade IIa injuries involve AITFL and IOL tears. Grade IIb injuries include AITFL, PITFL, and IOL tears. Grade III injuries involve injury to all 3 ligaments, as well as a fibular fracture. Conservative treatment is recommended for Grades I and IIa, while surgical intervention is necessary for Grades IIb and III (Figures 4A, 4B). Compared with other ankle sprains, syndesmotic injuries typically require a more prolonged recovery/rehabilitation. Some studies suggest that these injuries require twice as long to heal.47 Hopkinson and colleagues48 reported a mean recovery time of 55 days following syndesmotic injuries in cadets at the United States Military Academy at West Point. Some surgeons advocate surgical intervention in professional athletes with mild sprains to expedite return to play.49

Surgery has been well established as necessary in more severe injuries where there is clear diastasis or instability of the syndesmosis. Traditionally, screws were used for surgical fixation; however, they often required a second surgery for subsequent removal. There is no general consensus on the optimal screw size, level of placement, or timing of removal.50,51 More recently, non-absorbable suture button fixation (eg, TightRope; Arthrex) has become more popular and provides certain advantages over screw fixation, such as avoiding the need for hardware removal. TightRope has been shown to provide more accurate stabilization of the syndesmosis as compared with screw fixation.52 Since malreduction is the most important indicator of poor long-term functional outcome, suture button fixation should be considered in the treatment of the football player.53 Finally, Colcuc and colleagues54 reported a lower complication rate and earlier return to sports in patients treated with knotless suture button devices compared with screw fixation.
OSTEOCHONDRAL LESIONS
Osteochondral lesions (OCLs) are cartilage-bone defects that are usually located in the talus. They can be caused by an acute traumatic event or repetitive microtrauma with no apparent history of trauma (eg, ankle instability). Acute OCLs can occur in soccer secondary to an ankle sprain or ankle fracture. Symptoms of OCLs include pain, swelling, and mechanical symptoms such as catching or locking, and on physical examination, one might see an effusion. The initial imaging modality of choice is radiographing; however, in ankle sprains with continued pain and swelling MRI may be indicated to rule out an underlying OCL. Missed acute lesions have a tendency not to heal and become chronic lesions, which can cause pain and playing disability. It is well established that chronic ankle instability is an important etiologic factor for OCLs. With the normal hydrostatic pressure within the ankle joint, synovial fluid gets pushed into cartilage/bone fissures, which can then lead to cystic degeneration of the subchondral bone.55-57
Surgical repair of an OCL is dependent on both the size and location of the lesion. Acute lesions can be managed by arthroscopic débridement, microfracture, or fixation of the lesion if enough bone remains attached to the chondral lesion. Return to play is based on development and maturation of fibrocartilage over the lesion (debridement/microfracture) or healing and incorporation of the new graft (chondral repair procedures). Meanwhile, chronic lesions can be managed in 1-stage (microfracture, osteochondral autograft transfer or 2-stage (autologous chondrocyte implantation [ACI]) procedures.56-57 Additional biologic healing augmentation with platelet-rich plasma has been described as well.58 Newer techniques in treating chronic talus OCLs, including ones that have failed to respond to bone marrow stimulation techniques, have been developed more recently such as the use of particulated juvenile articular cartilage allograft (DeNovo NT Natural Tissue Graft®; Zimmer Biomet).59 These newer techniques avoid the need for a 2-stage procedure, as is the case with ACI.
Continue to: Further studies are needed...
Further studies are needed to both investigate long-term outcomes and determine the superiority of the arthroscopic juvenile cartilage procedure compared with microfracture and other cartilage resurfacing procedures. When surgically treating OCLs, one must also restore normal ankle joint biomechanics for the lesion to heal. For instance, in the presence of ankle instability, ligament reconstruction must be performed. Also, one should also consider addressing any hindfoot malalignment with an osteotomy (calcaneus, supramalleolar). In a recent retrospective study, van Eekeren and colleagues60 showed that approximately 76% of patients were able to return to sports at long-term follow-up after arthroscopic débridement and bone marrow stimulation of talar OCLs. However, the activity level decreased at long-term follow-up and never attained the pre-injury level.60
ANKLE IMPINGEMENT
ANTERIOR ANKLE IMPINGEMENT (FOOTBALLER'S ANKLE)
Anterior ankle impingement is caused by anterior osteophytes on both the distal tibia and talar neck. It is thought to be related to repetitive microtrauma to the anteromedial aspect of the ankle from recurrent ball impact.61 It is very common amongst soccer players with some studies suggesting that 60% of soccer players have this syndrome. Ankle impingement is characterized by anterior pain with ankle dorsiflexion, decreased dorsiflexion, and swelling. It is primarily diagnosed with lateral ankle X-rays, which will show the osteophytes. An oblique anteromedial X-ray may increase detection of osteophytes (Figure 5). The early stages of anterior impingement can be treated successfully with injections and heel lifts. Treatment of lesions that fail to respond to conservative management involves arthroscopic or open excision of osteophytes. Most patients with no preexisting osteoarthritis treated arthroscopically will experience pain relief and return to full activity, though recurrent osteophyte formation has been noted at long-term follow-up.62

Anterior ankle impingement is most often caused by acute ankle sprains with an inversion type of mechanism.62 The subsequent reactive inflammation can cause fibrosis leading to distal fascicle enlargement of the AITFL. Impingement in the anterolateral gutter of this enlarged fascicle can also cause both chronic reactive synovitis and chondromalacia of the lateral talar dome.63 MRI can identify abnormal areas of pathology; however, 50% of cases are diagnosed based on clinical examination alone.63 Patients generally present with a history of anterolateral ankle pain and swelling with an occasional popping or snapping sensation.
Soccer players commonly develop anterior bony impingement due to repetitive loading of the anterior ankle from striking the ball. This repetition can lead to osteophyte formation of the anterior distal tibia and talar neck. After the osteophytes form, decreased dorsiflexion can occur due to a mechanical stop and inflammation of the interposed capsule.
The patient will exhibit tenderness to palpation along the anterolateral aspect of the ankle, with pain elicited at extreme passive dorsiflexion.62 Initially, an injection with local anesthetic and corticosteroid can serve both a diagnostic and therapeutic purpose; however, patients who fail conservative treatment can be treated with arthroscopy and resection of the involved scar tissue and osteophytes. The best results are seen in those patients with no concurrent intra-articular lesions or ankle osteoarthritis (Figure 5).62 When treated non-operatively, a player may return to play when pain resolves; however, if treated surgically with arthroscopic debridement/resection, a player must wait until his surgical scars have healed prior to attempting return to play.
Continue to: ANTEROMEDIAL ANKLE IMPINGEMENT
ANTEROMEDIAL ANKLE IMPINGEMENT
Anteromedial ankle impingement is a less common ankle impingement syndrome. It is associated with eversion injuries or following medial malleolar or talar fractures.64,65 Previous injury to the anterior tibiotalar fascicle of the deltoid complex leads to ligament thickening and subsequent impingement in the anteromedial corner of the talus. Adjacent fibrosis and synovitis are common consequences of impingement; however, osteophyte formation and chondral stripping along the anteromedial talus can also be seen. Patients typically complain of pain along the anteromedial joint line that is worse with activity, clicking or popping sensations, and painful, limited dorsiflexion. On examination, impingement can be detected through palpation over the anterior tibiotalar fascicle of the deltoid ligament and eversion or extreme passive dorsiflexion of the foot, all of which will elicit medial ankle tenderness.17,62 Initial treatment consists of rest, physical therapy, and NSAIDs. Refractory cases may be amenable to arthroscopic or open resection of the anterior tibiotalar fascicle with débridement of any adjacent synovitis and scar tissue.62
POSTERIOR ANKLE IMPINGEMENT
Posterior ankle impingement is often referred to as “os trigonum syndrome” since the posterior impingement is frequently associated with a prominent os trigonum. An os trigonum is an accessory ossicle representing the separated posterolateral tubercle of the talus. It is usually asymptomatic. However, in soccer players, pain can occur from impaction between the posterior tibial plafond and the os trigonum, or because of soft tissue compression between the 2 opposing osseous structures. The pain is due to repetitive microtrauma (ankle plantarflexion) or acute forced plantarflexion, which can present as an acute fracture of the os trigonum. Because soccer is a sport requiring both repetitive and extreme plantarflexion, it may predispose players to posterior ankle impingement (Figures 6A, 6B).62,66

Clinically, it can be very difficult to detect and diagnose because the affected structures lie deep and it can coexist with other disease processes (eg, peroneal tendinopathy, Achilles tendinopathy).62,66 Patients will complain of chronic deep posterior ankle pain that is worse with push-off activities (eg, jumping). On examination, patients will exhibit pain with palpation over the posterolateral process and with the crunch test. Lateral radiograph with the foot in plantar flexion will show the os trigonum impinged between the posterior tibial malleolus and the calcaneal tuberosity. An MRI will demonstrate the os trigonum as well as any associated inflammation and edema, while it can also demonstrate coexisting pathologies.
Initial treatment consists of rest, NSAIDs, and taping to prevent plantar flexion. Ultrasound-guided cortisone injection of the capsule and posterior bursa can be both therapeutic and diagnostic. A posterior injection can be used to temporize the symptoms so that the soccer player can make it through the season.
Surgical excision is saved for refractory cases, and this can be done either through an open posterolateral approach or arthroscopic techniques. Recently, Georgiannos and Bisbinas67 showed in an athletic population that endoscopic excision had both a lower complication rate and a quicker return to sports compared with the traditional open approach. Carreira and colleagues68 conducted a retrospective case series of 20 patients (mostly competitive athletes). They found that posterior ankle arthroscopy to address posterior impingement allowed for the maintenance or restoration of anatomic ROM of the ankle and hindfoot, ability to return to at least the previous level of activity, and improvement in objective assessment of pain relief and a higher level of function parameters.68
Continue to: TENDON PATHOLOGY
TENDON PATHOLOGY
SUPERIOR PERONEAL RETINACULUM INJURY
The superior peroneal retinaculum (SPR) forms the roof of the superior peroneal tunnel. The tunnel contains the peroneus brevis and longus tendons and is bordered by the retromalleolar groove of the fibula and the lower aspect of the posterior intramuscular septum of the leg.69,70 The SPR originates from the posterolateral ridge of the fibula and inserts onto the lateral calcaneus, and it is the primary restraint of the peroneal tendons within the retromalleolar sulcus.
Injury to the retinaculum results from both ankle dorsiflexion and inversion, and forceful reflex contraction of the peroneal muscles, which causes subluxation or dislocation of the contained tendons.69 A high level of suspicion is required regarding these injuries since the mechanism of injury is similar to that of a simple lateral ankle sprain. In the setting of retrofibular pain, snapping or popping sensations around the lateral malleolus, or chronic ankle instability that worsens on uneven surfaces, one must consider an injury to the SPR.69 Radiographs are not always diagnostic; however, occasionally on an internal rotation view, one may see a cortical avulsion off the distal tip of the lateral malleolus (“fleck sign”) indicating a rim fracture from an SPR injury (Figure 7). MRI is the best imaging modality to assess the peroneal tendons, as well as an SPR injury. Recently, ultrasound has grown in popularity and may be more useful, since it allows for dynamic evaluation of subluxating/dislocating tendons.69

Conservative management is often associated with poor outcomes, and surgery is indicated for all acute and chronic dislocations in athletes.71 Anatomic reconstruction of the SPR is the preferred surgical method.72 Peroneus brevis debulking and fibular groove deepening may also augment the retinaculum repair.73 van Dijk and colleagues in their systematic review showed that patients treated with both groove deepening and SPR repair have higher rates of return to the sport than patients treated with SPR repair alone.74
STRESS FRACTURES
FIFTH METATARSAL
Fifth metatarsal stress fractures usually occur secondary to lateral overload or avulsion of the peroneus brevis. The fifth metatarsal base can be susceptible to injury in a cavovarus foot. Non-operative treatment typically requires a longer period of immobilization (boot or cast) and necessitates a longer period of non–weight-bearing (anywhere between 6-12 weeks). Therefore, surgery is typically recommended in athletes or in the setting of a recurrent base of the fifth metatarsal fracture to expedite healing and return to play. Return to play is still not recommended until there is evidence of radiographic healing of the fracture. There are certain distinctions with fifth metatarsal stress fractures regarding location and healing rates that need to be taken into account.75,76 In particular, zone 2 injuries (Jones fractures) represent a vascular watershed area, making these fractures prone to nonunion with nonunion rates as high as 15% to 30%. Occasionally, the cavovarus deformity will require correction as well as a reduction in the risk of recurrence or nonunion. Surgical fixation most commonly consists of a single screw placed in an antegrade fashion.77 One must pay attention to screw size since smaller diameter screws (<4.5 mm) are associated with delayed union or nonunion. Moreover, screws that are too long will straighten the curved metatarsal shaft and can lead to fracture distraction or malreduction (Figures 8A, 8B).77

Patients have returned to competitive sports within 6 weeks; however, it should be noted that causes of failure were linked to early return and return to sports before a radiographic union can lead to failure of fixation. Ekstrand and van Dijk78 studied a large group of professional soccer players and found that out of 13,754 injuries, 0.5% (67) were fifth metatarsal fractures. Of note, they found that 45% of players had prodromal symptoms. Furthermore, after surgical treatment the fractures healed faster, compared with conservative treatment (75% vs 33%); however, there was no significant difference in lay-off days between both groups (80 vs 74 days).78 Matsuda and colleagues79 looked at 335 male collegiate soccer players, 29 of whom had a history of a fifth metatarsal stress fracture. They found that playing the midfield position and having an everted rearfoot and inverted forefoot alignment were associated with fifth metatarsal stress fractures.79 Saita and colleagues80 found that restricted hip internal rotation was associated with an increased risk of developing a Jones fracture in 162 professional football players. Finally, Fujitaka and colleagues81 looked at 273 male soccer players between 2005 and 2013. They found an association between weak toe-grip strength and fifth metatarsal fractures, suggesting that weak toe-grip may lead to an increase in the load applied onto the lateral side of the foot, resulting in a stress fracture.81
Continue to: NAVICULAR
NAVICULAR
Another common tarsal bone that sustains stress fractures is the navicular. It is not as common as calcaneal stress fractures in military recruits but can occur in the same type of population, as well as explosive athletics such as sprinters and soccer players. It commonly presents with an indistinct vague achy pain with activity that improves with rest, and pain at the dorsum of the midfoot or along the medial longitudinal arch with activity. It can easily go undiagnosed for quite some time given the difficulty in visualizing the navicular with plain radiographs. Clinically, it is difficult to make the diagnosis, and therefore advanced imaging is necessary when the injury is suspected. Both MRI and CT scans can be used to understand the extent of the injury (Figures 9A-9C). In non-displaced stress fractures, conservative non-operative treatment is the appropriate treatment modality with a brief period of immobilization and non–weight-bearing;82 however, operative treatment is also considered in elite athletes. In either case, return to play is discouraged until there is evidence of radiographic healing. When a displacement is noted, or there is a delay in diagnosis, then operative treatment is recommended.

CONCLUSION
Ankle injuries are very common in soccer and can result in decreased performance or significant loss of playing time. Treatment of acute injury generally follows a conservative route, with surgical intervention reserved for severe ruptures or osteochondral fracture of the ankle joint. Chronic ankle pathology resulting in mechanical or functional instability generally requires surgery to repair ligamentous damage and restore normal ankle kinematics. It is critical for the soccer player to receive appropriate rehabilitation prior to returning to play in order to reduce the risk for reinjury and further chronic instability. Prevention and early intervention of ankle injuries is key in preventing the long-term sequelae of ankle injuries, such as arthritis, in former soccer players.
ABSTRACT
The ankle is one of the most commonly injured joints in soccer and represents a significant cost to the healthcare system. The ligaments that stabilize the ankle joint determine its biomechanics—alterations of which result from various soccer-related injuries. Acute sprains are among the most common injury in soccer players and are generally treated conservatively, with emphasis placed on secondary prevention to reduce the risk for future sprains and progression to chronic ankle instability. Repetitive ankle injuries in soccer players may cause chronic ankle instability, which includes both mechanical ligamentous laxity and functional changes. Chronic ankle pathology often requires surgery to repair ligamentous damage and remove soft-tissue or osseous impingement. Proper initial treatment, rehabilitation, and secondary prevention of ankle injuries can limit the amount of time lost from play and avoid negative long-term sequelae (eg, osteochondral lesions, arthritis). On the other hand, high ankle sprains portend a poorer prognosis and a longer recovery. These injuries will typically require surgical stabilization. Impingement-like syndromes of the ankle can undergo an initial trial of conservative treatment; when this fails, however, soccer players respond favorably to arthroscopic debridement of the lesions causing impingement. Finally, other pathologies (eg, stress fractures) are highly encouraged to be treated with surgical stabilization in elite soccer players.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
With roughly 200,000 professional and around 240 million amateur soccer players, soccer has been recognized as the most popular sport worldwide. Nevertheless, given its rising popularity in society, one must also consider the increasing incidence of injuries as a result. Elite soccer players sustain between 10 and 35 injuries per 1000 competitive playing hours.1 Approximately 80% are traumatic, and 20% are overuse injuries.2 Soccer injuries are more frequent with increasing age of the participants, whereas the incidence of injuries in preadolescent players is low. The incidence of injuries has been found to be higher during competition when compared with practice/training sessions, with some studies showing that 59% of injuries occurred during games.2 Amateur or recreational soccer players sustain fewer injuries than professional soccer players, as one would expect, given both the higher intensity of training and match schedule in professionals.
The ankle is one of the most commonly injured joints in soccer, with some studies suggesting it comprises one-fifth of all injuries sustained during soccer, which is only second to those of the knee.2 Ankle sprains specifically are quite a common occurrence in soccer.3-9 A recent study of an English premier league club showed that over a 4-season period, 20% of injuries were of the foot and ankle with a mean return to sport time of 54 days.10 Of all foot and ankle related injuries, ankle sprains are the most common, followed by bruises/contusions, and tendon lesions. Fractures are very rare (1%) in soccer, but when they do occur they impart a much more extended recovery. During the 2010 Fédération Internationale de Football Association (FIFA) World Cup, ankle sprains were among the most common injuries and approximately half lead to players missing training or competitive matches.5
ANATOMY
Knowledge of the biomechanics of both the foot and ankle joints is essential to understand soccer injuries. The ankle joint (talocrural articulation) consists of the distal ends of the tibia and fibula, which form the mortise, and the superior aspect of the talar dome.11 As a hinge joint, the ankle provides 20° of dorsiflexion and 50° of plantar flexion,12 with stability provided by the lateral, medial, and superior ligamentous complexes. The superior articular surface of the talus is narrower posteriorly, which creates a looser fit within the mortise during plantar flexion.11 This decreased stability could help explain why the most common injury in soccer involves a plantar flexion mechanism.13,14 Inferiorly, the talus articulates with the calcaneus to form the subtalar joint. It is at this site that the majority of both foot inversion and eversion occurs. The transverse tarsal joints (Chopart’s joints) separate the hindfoot from the midfoot. Movement of this joint depends on the relative alignment of its 2 articulations: the talonavicular and calcaneocuboid joints. During foot eversion, these 2 joints are parallel to each other allowing supple motion and aiding in shock absorption during the heel strike phase of the gait cycle. With foot inversion, the joints become nonparallel and thus lock the transverse tarsal joints providing a rigid lever needed for push-off.11,12
LATERAL LIGAMENTS
The ankle joint is stabilized laterally by a ligament complex consisting of 3 individual ligaments, all originating from the lateral malleolus: the anterior talofibular ligament (ATFL), the posterior talofibular ligament (PTFL), and the calcaneofibular ligament (CFL) (Figure 1).11,12,15 The ATFL is the primary restraint to inversion in plantar flexion, and it helps resist anterolateral translation of the talus in the mortise. However, it is the weakest and therefore the most frequently injured of the lateral ligaments. The PTFL plays only a supplementary role in ankle stability when the lateral ligament complex is intact. It is under the greatest strain in ankle dorsiflexion and acts to limit posterior talar displacement within the mortise as well as talar external rotation.13,16 The CFL is the primary restraint to inversion in the neutral or dorsiflexed position. It restrains subtalar inversion, thereby limiting talar tilt within the mortise.

DELTOID LIGAMENT
The deltoid ligament complex consists of 6 continuous adjacent superficial and deep ligaments that function synergistically to resist valgus and pronation forces, as well as external rotation of the talus in the mortise.11-13,17 The superficial layer crosses both ankle and subtalar joints. It originates from the anterior colliculus and fans out to insert into the navicular, neck of the talus, sustentaculum tali, and posteromedial talar tubercle. The tibiocalcaneal (sustentaculum tali) portion is the strongest component in the superficial layer and resists calcaneal eversion. The deep layer crosses the ankle joint only. It functions as the primary stabilizer of the medial ankle and prevents both lateral displacement and external rotation of the talus. It originates from the inferior and posterior aspects of the medial malleolus and inserts on the medial and posteromedial aspects of the talus.12,17,18
Continue to: SYNDESMOSIS
SYNDESMOSIS
The ankle syndesmosis, or inferior tibiofibular joint, is the distal articulation between the tibia and fibula. The syndesmosis contributes to ankle mortise integrity through its firm fixation of the lateral malleolus against the lateral surface of the talus. Ligaments comprising the ankle syndesmosis include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the inferior transverse ligament, and the interosseous ligament (IOL).12
ANKLE SPRAINS
Ankle sprains are the most common pathology encountered amongst soccer players, representing from one-half to two-thirds of all ankle related injuries. Most sprains occur outside of player contact.
LATERAL ANKLE SPRAINS AND INSTABILITY
Injury to the lateral ligaments of the ankle represents 77% to 91% of all ankle sprains in soccer.6,19 The greatest risk factor for an ankle sprain in a soccer player is a history of prior sprain.20 Other risk factors include increasing age, player-to-player contact, condition of the pitch, weight-bearing status of the injured limb at the time of injury, and joint instability or laxity.21,22
The evaluation of an ankle sprain to determine its severity is best done after the acute phase, approximately 4 to 7 days after the initial injury when both pain and swelling have subsided.23 The anterior drawer (ATFL instability) and talar tilt (CFL instability) tests are useful in evaluating ankle instability in the delayed or chronic setting; however, both have been shown to have limited sensitivity and significant variability amongst different examiners.24
Clinical examination will direct further diagnostic tests including X-rays, magnetic resonance imaging (MRI), and computed tomography (CT). The Ottawa ankle rules are generally helpful in determining whether plain X-rays are indicated in the acute setting.25,26 (Figure 2) According to these rules, ankle radiographs should be obtained if ankle pain is reported near the malleoli and 1 or more of the following is seen during examination: inability to bear weight immediately after injury and for 4 steps in the emergency department, and bony tenderness at the posterior edge or tip of the malleolus. Stress X-rays are generally not indicated in acute injuries but may be useful in chronic ankle instability cases.23

Continue to: Ankle sprains cover...
Ankle sprains cover a broad spectrum of injuries; therefore, a grading system was devised to aid in guiding treatment. Grade I (mild) sprains are those with minimal swelling and tenderness but have the ligaments still intact. Grade II (moderate) sprains occur when there are partial ligament tears associated with moderate pain, swelling, and tenderness. Finally, Grade III (severe) sprains are complete ligament tears with marked swelling, hemorrhage, tenderness, loss of function, and abnormal joint motion and instability.23, 24
Initial treatment for all ankle sprains is nonoperative and involves the RICE (rest, ice, compression, elevation) protocol with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) during the acute phase (first 4-5 days) with a short period (no >2 weeks) of immobilization.27 Most authors agree that early mobilization followed by phased rehabilitation is warranted to minimize time away from sports.28-31 Prolonged immobilization (>2 weeks) has detrimental effects and may lead to a longer return to play.28-31 The rehabilitation protocol is divided into stages: (1) pain and edema control, (2) range of motion (ROM) and strengthening exercises, (3) soccer specific functional training, and (4) prophylactic intervention with balance and proprioception exercises. Surgical intervention is rarely indicated for acute ankle sprains. There are exceptions, however, such as when ankle sprains are associated with other injuries that require acute intervention (eg, fracture, osteochondral lesion). Surgery is indicated in the setting of chronic, recurrent mechanical instability. Anatomical repairs (modified Brostrom) seem to produce better outcomes than non-anatomical reconstructions (eg, Chrisman-Snook). Surgical outcomes are good, and most athletes are able to return to their pre-injury level of function.32
In athletes, prevention of recurrent sprains is key. Braces may help prevent ankle sprains and bracing has been shown to be superior to taping, as tape loses its restrictive properties within 20 to 30 minutes of initiating activity.33,34 Application of an orthosis (lace-up ankle orthosis) has been shown to reduce the incidence of ankle re-injury in soccer players with previous ankle sprains. Several studies have found minimal, if any, effect of orthoses on athletic performance.20,35,36 Low-profile braces for soccer have been developed which allow for minimal disruption of the player’s boot and space proximally to insert the shin guard. Another essential component of prevention is prophylactic intervention with balance and proprioceptive exercises. A study looking at first division men’s league football (soccer) players in Iran showed a significant decrease in re-injury rates with proprioceptive training.37 In 2003, FIFA introduced a comprehensive warm-up program (FIFA 11+), which has since been shown in several studies to decrease the risk of injury in amateur soccer players.38-40
MEDIAL ANKLE SPRAINS AND INSTABILITY
Soccer places an unusually high demand on both the medial foot and ankle structures when compared with other sports. For instance, striking the ball requires the player to abduct and externally rotate the foot, which preloads medial structures.9 Hintermann18 looked at 54 cases of medial ankle instability and found that injury commonly occurred during landing on an uneven surface, which applies to soccer players when landing after heading the ball or jumping over a tackle. Pronation with eversion and extreme rotational injuries are well known to cause deltoid ligament injury. However, complete rupture of the deltoid ligament is rare and is more often associated with ankle fractures.41 Due to its close proximity and similarly shared function in medial plantar arch stabilization with the tibiospring and spring ligaments, posterior tibialis tendon dysfunction is also frequently seen in medial ankle instability.17 After an acute injury, patients can present with a medial ankle hematoma and pain along the deltoid ligament. Although chronic insufficiency is diagnosed based on the feeling of “giving way,” pain in the medial gutter of the ankle and a valgus and pronation deformity of the foot can be corrected by activating the peroneus tertius muscle. Arthroscopy is the most specific way to confirm clinically suspected instability of the medial ankle; however, MRI can demonstrate loss of organized medial fibers (Figures 3A, 3B).18 Primary surgical repair of deltoid ligament tears yield good to excellent results and should be considered in the soccer player to prevent problems associated with chronic non-repaired tears such as instability, osteoarthritis, and impingement syndromes.18 After surgical repair, players will undergo extensive physical therapy that progresses to sport-specific exercises with the ultimate goal of returning to competitive play around 4-6 months post-operatively.

HIGH ANKLE SPRAINS (SYNDESMOSIS)
High ankle sprains are much less common than low ankle sprains; however, when they do occur they portend a lengthier rehabilitation and a poorer prognosis, especially if undiagnosed. Lubberts and colleagues42 studied the epidemiology of isolated syndesmotic injuries in professional football players. They pooled data from 15 consecutive seasons of European professional football between 2001 and 2016. They examined a total of 3677 players from 61 teams across 17 countries. There were 1320 ankle ligament injuries registered during 15 seasons, of which 94 (7%) were isolated syndesmotic injuries. The incidence of these injuries increased annually between 2001 and 2016. Injuries were 74% contact-related, and isolated syndesmotic injuries were followed by a mean of a 39-day absence.42 Moreover, football players may have an increased risk of syndesmotic sprains due to foot planting and cutting action.41
Continue to: These injuriesa are typically...
These injuries are typically identified with pain over the AITFL and interosseous membrane. Physical examination tests that help identify syndesmotic injuries include the squeeze test, external rotation test, and crossed-leg test.41 The diagnosis can be made on plain X-ray when there is clear diastasis between the distal tibia and fibula. Two critical measurements on plain films are made 1 cm above the tibial plafond and are used to evaluate the integrity of the syndesmosis: tibiofibular clear space >6 mm, and tibiofibular overlap <1 mm, which indicate disruption of the syndesmosis.43 More subtle injuries can be diagnosed with better sensitivity and specificity using MRI, which can also reveal secondary findings such as bone bruises, ATFL injury, osteochondral lesions, and tibiofibular incongruity.44,45 Arthroscopy is an invaluable diagnostic tool for syndesmotic injuries with a characteristic triad finding of PITFL scarring, disrupted interosseous ligament, and posterolateral tibial plafond chondral damage.46
Classification of the ligaments involved can aid in the selection of appropriate treatment. Grade I injuries involve AITFL tears. Grade IIa injuries involve AITFL and IOL tears. Grade IIb injuries include AITFL, PITFL, and IOL tears. Grade III injuries involve injury to all 3 ligaments, as well as a fibular fracture. Conservative treatment is recommended for Grades I and IIa, while surgical intervention is necessary for Grades IIb and III (Figures 4A, 4B). Compared with other ankle sprains, syndesmotic injuries typically require a more prolonged recovery/rehabilitation. Some studies suggest that these injuries require twice as long to heal.47 Hopkinson and colleagues48 reported a mean recovery time of 55 days following syndesmotic injuries in cadets at the United States Military Academy at West Point. Some surgeons advocate surgical intervention in professional athletes with mild sprains to expedite return to play.49

Surgery has been well established as necessary in more severe injuries where there is clear diastasis or instability of the syndesmosis. Traditionally, screws were used for surgical fixation; however, they often required a second surgery for subsequent removal. There is no general consensus on the optimal screw size, level of placement, or timing of removal.50,51 More recently, non-absorbable suture button fixation (eg, TightRope; Arthrex) has become more popular and provides certain advantages over screw fixation, such as avoiding the need for hardware removal. TightRope has been shown to provide more accurate stabilization of the syndesmosis as compared with screw fixation.52 Since malreduction is the most important indicator of poor long-term functional outcome, suture button fixation should be considered in the treatment of the football player.53 Finally, Colcuc and colleagues54 reported a lower complication rate and earlier return to sports in patients treated with knotless suture button devices compared with screw fixation.
OSTEOCHONDRAL LESIONS
Osteochondral lesions (OCLs) are cartilage-bone defects that are usually located in the talus. They can be caused by an acute traumatic event or repetitive microtrauma with no apparent history of trauma (eg, ankle instability). Acute OCLs can occur in soccer secondary to an ankle sprain or ankle fracture. Symptoms of OCLs include pain, swelling, and mechanical symptoms such as catching or locking, and on physical examination, one might see an effusion. The initial imaging modality of choice is radiographing; however, in ankle sprains with continued pain and swelling MRI may be indicated to rule out an underlying OCL. Missed acute lesions have a tendency not to heal and become chronic lesions, which can cause pain and playing disability. It is well established that chronic ankle instability is an important etiologic factor for OCLs. With the normal hydrostatic pressure within the ankle joint, synovial fluid gets pushed into cartilage/bone fissures, which can then lead to cystic degeneration of the subchondral bone.55-57
Surgical repair of an OCL is dependent on both the size and location of the lesion. Acute lesions can be managed by arthroscopic débridement, microfracture, or fixation of the lesion if enough bone remains attached to the chondral lesion. Return to play is based on development and maturation of fibrocartilage over the lesion (debridement/microfracture) or healing and incorporation of the new graft (chondral repair procedures). Meanwhile, chronic lesions can be managed in 1-stage (microfracture, osteochondral autograft transfer or 2-stage (autologous chondrocyte implantation [ACI]) procedures.56-57 Additional biologic healing augmentation with platelet-rich plasma has been described as well.58 Newer techniques in treating chronic talus OCLs, including ones that have failed to respond to bone marrow stimulation techniques, have been developed more recently such as the use of particulated juvenile articular cartilage allograft (DeNovo NT Natural Tissue Graft®; Zimmer Biomet).59 These newer techniques avoid the need for a 2-stage procedure, as is the case with ACI.
Continue to: Further studies are needed...
Further studies are needed to both investigate long-term outcomes and determine the superiority of the arthroscopic juvenile cartilage procedure compared with microfracture and other cartilage resurfacing procedures. When surgically treating OCLs, one must also restore normal ankle joint biomechanics for the lesion to heal. For instance, in the presence of ankle instability, ligament reconstruction must be performed. Also, one should also consider addressing any hindfoot malalignment with an osteotomy (calcaneus, supramalleolar). In a recent retrospective study, van Eekeren and colleagues60 showed that approximately 76% of patients were able to return to sports at long-term follow-up after arthroscopic débridement and bone marrow stimulation of talar OCLs. However, the activity level decreased at long-term follow-up and never attained the pre-injury level.60
ANKLE IMPINGEMENT
ANTERIOR ANKLE IMPINGEMENT (FOOTBALLER'S ANKLE)
Anterior ankle impingement is caused by anterior osteophytes on both the distal tibia and talar neck. It is thought to be related to repetitive microtrauma to the anteromedial aspect of the ankle from recurrent ball impact.61 It is very common amongst soccer players with some studies suggesting that 60% of soccer players have this syndrome. Ankle impingement is characterized by anterior pain with ankle dorsiflexion, decreased dorsiflexion, and swelling. It is primarily diagnosed with lateral ankle X-rays, which will show the osteophytes. An oblique anteromedial X-ray may increase detection of osteophytes (Figure 5). The early stages of anterior impingement can be treated successfully with injections and heel lifts. Treatment of lesions that fail to respond to conservative management involves arthroscopic or open excision of osteophytes. Most patients with no preexisting osteoarthritis treated arthroscopically will experience pain relief and return to full activity, though recurrent osteophyte formation has been noted at long-term follow-up.62

Anterior ankle impingement is most often caused by acute ankle sprains with an inversion type of mechanism.62 The subsequent reactive inflammation can cause fibrosis leading to distal fascicle enlargement of the AITFL. Impingement in the anterolateral gutter of this enlarged fascicle can also cause both chronic reactive synovitis and chondromalacia of the lateral talar dome.63 MRI can identify abnormal areas of pathology; however, 50% of cases are diagnosed based on clinical examination alone.63 Patients generally present with a history of anterolateral ankle pain and swelling with an occasional popping or snapping sensation.
Soccer players commonly develop anterior bony impingement due to repetitive loading of the anterior ankle from striking the ball. This repetition can lead to osteophyte formation of the anterior distal tibia and talar neck. After the osteophytes form, decreased dorsiflexion can occur due to a mechanical stop and inflammation of the interposed capsule.
The patient will exhibit tenderness to palpation along the anterolateral aspect of the ankle, with pain elicited at extreme passive dorsiflexion.62 Initially, an injection with local anesthetic and corticosteroid can serve both a diagnostic and therapeutic purpose; however, patients who fail conservative treatment can be treated with arthroscopy and resection of the involved scar tissue and osteophytes. The best results are seen in those patients with no concurrent intra-articular lesions or ankle osteoarthritis (Figure 5).62 When treated non-operatively, a player may return to play when pain resolves; however, if treated surgically with arthroscopic debridement/resection, a player must wait until his surgical scars have healed prior to attempting return to play.
Continue to: ANTEROMEDIAL ANKLE IMPINGEMENT
ANTEROMEDIAL ANKLE IMPINGEMENT
Anteromedial ankle impingement is a less common ankle impingement syndrome. It is associated with eversion injuries or following medial malleolar or talar fractures.64,65 Previous injury to the anterior tibiotalar fascicle of the deltoid complex leads to ligament thickening and subsequent impingement in the anteromedial corner of the talus. Adjacent fibrosis and synovitis are common consequences of impingement; however, osteophyte formation and chondral stripping along the anteromedial talus can also be seen. Patients typically complain of pain along the anteromedial joint line that is worse with activity, clicking or popping sensations, and painful, limited dorsiflexion. On examination, impingement can be detected through palpation over the anterior tibiotalar fascicle of the deltoid ligament and eversion or extreme passive dorsiflexion of the foot, all of which will elicit medial ankle tenderness.17,62 Initial treatment consists of rest, physical therapy, and NSAIDs. Refractory cases may be amenable to arthroscopic or open resection of the anterior tibiotalar fascicle with débridement of any adjacent synovitis and scar tissue.62
POSTERIOR ANKLE IMPINGEMENT
Posterior ankle impingement is often referred to as “os trigonum syndrome” since the posterior impingement is frequently associated with a prominent os trigonum. An os trigonum is an accessory ossicle representing the separated posterolateral tubercle of the talus. It is usually asymptomatic. However, in soccer players, pain can occur from impaction between the posterior tibial plafond and the os trigonum, or because of soft tissue compression between the 2 opposing osseous structures. The pain is due to repetitive microtrauma (ankle plantarflexion) or acute forced plantarflexion, which can present as an acute fracture of the os trigonum. Because soccer is a sport requiring both repetitive and extreme plantarflexion, it may predispose players to posterior ankle impingement (Figures 6A, 6B).62,66

Clinically, it can be very difficult to detect and diagnose because the affected structures lie deep and it can coexist with other disease processes (eg, peroneal tendinopathy, Achilles tendinopathy).62,66 Patients will complain of chronic deep posterior ankle pain that is worse with push-off activities (eg, jumping). On examination, patients will exhibit pain with palpation over the posterolateral process and with the crunch test. Lateral radiograph with the foot in plantar flexion will show the os trigonum impinged between the posterior tibial malleolus and the calcaneal tuberosity. An MRI will demonstrate the os trigonum as well as any associated inflammation and edema, while it can also demonstrate coexisting pathologies.
Initial treatment consists of rest, NSAIDs, and taping to prevent plantar flexion. Ultrasound-guided cortisone injection of the capsule and posterior bursa can be both therapeutic and diagnostic. A posterior injection can be used to temporize the symptoms so that the soccer player can make it through the season.
Surgical excision is saved for refractory cases, and this can be done either through an open posterolateral approach or arthroscopic techniques. Recently, Georgiannos and Bisbinas67 showed in an athletic population that endoscopic excision had both a lower complication rate and a quicker return to sports compared with the traditional open approach. Carreira and colleagues68 conducted a retrospective case series of 20 patients (mostly competitive athletes). They found that posterior ankle arthroscopy to address posterior impingement allowed for the maintenance or restoration of anatomic ROM of the ankle and hindfoot, ability to return to at least the previous level of activity, and improvement in objective assessment of pain relief and a higher level of function parameters.68
Continue to: TENDON PATHOLOGY
TENDON PATHOLOGY
SUPERIOR PERONEAL RETINACULUM INJURY
The superior peroneal retinaculum (SPR) forms the roof of the superior peroneal tunnel. The tunnel contains the peroneus brevis and longus tendons and is bordered by the retromalleolar groove of the fibula and the lower aspect of the posterior intramuscular septum of the leg.69,70 The SPR originates from the posterolateral ridge of the fibula and inserts onto the lateral calcaneus, and it is the primary restraint of the peroneal tendons within the retromalleolar sulcus.
Injury to the retinaculum results from both ankle dorsiflexion and inversion, and forceful reflex contraction of the peroneal muscles, which causes subluxation or dislocation of the contained tendons.69 A high level of suspicion is required regarding these injuries since the mechanism of injury is similar to that of a simple lateral ankle sprain. In the setting of retrofibular pain, snapping or popping sensations around the lateral malleolus, or chronic ankle instability that worsens on uneven surfaces, one must consider an injury to the SPR.69 Radiographs are not always diagnostic; however, occasionally on an internal rotation view, one may see a cortical avulsion off the distal tip of the lateral malleolus (“fleck sign”) indicating a rim fracture from an SPR injury (Figure 7). MRI is the best imaging modality to assess the peroneal tendons, as well as an SPR injury. Recently, ultrasound has grown in popularity and may be more useful, since it allows for dynamic evaluation of subluxating/dislocating tendons.69

Conservative management is often associated with poor outcomes, and surgery is indicated for all acute and chronic dislocations in athletes.71 Anatomic reconstruction of the SPR is the preferred surgical method.72 Peroneus brevis debulking and fibular groove deepening may also augment the retinaculum repair.73 van Dijk and colleagues in their systematic review showed that patients treated with both groove deepening and SPR repair have higher rates of return to the sport than patients treated with SPR repair alone.74
STRESS FRACTURES
FIFTH METATARSAL
Fifth metatarsal stress fractures usually occur secondary to lateral overload or avulsion of the peroneus brevis. The fifth metatarsal base can be susceptible to injury in a cavovarus foot. Non-operative treatment typically requires a longer period of immobilization (boot or cast) and necessitates a longer period of non–weight-bearing (anywhere between 6-12 weeks). Therefore, surgery is typically recommended in athletes or in the setting of a recurrent base of the fifth metatarsal fracture to expedite healing and return to play. Return to play is still not recommended until there is evidence of radiographic healing of the fracture. There are certain distinctions with fifth metatarsal stress fractures regarding location and healing rates that need to be taken into account.75,76 In particular, zone 2 injuries (Jones fractures) represent a vascular watershed area, making these fractures prone to nonunion with nonunion rates as high as 15% to 30%. Occasionally, the cavovarus deformity will require correction as well as a reduction in the risk of recurrence or nonunion. Surgical fixation most commonly consists of a single screw placed in an antegrade fashion.77 One must pay attention to screw size since smaller diameter screws (<4.5 mm) are associated with delayed union or nonunion. Moreover, screws that are too long will straighten the curved metatarsal shaft and can lead to fracture distraction or malreduction (Figures 8A, 8B).77

Patients have returned to competitive sports within 6 weeks; however, it should be noted that causes of failure were linked to early return and return to sports before a radiographic union can lead to failure of fixation. Ekstrand and van Dijk78 studied a large group of professional soccer players and found that out of 13,754 injuries, 0.5% (67) were fifth metatarsal fractures. Of note, they found that 45% of players had prodromal symptoms. Furthermore, after surgical treatment the fractures healed faster, compared with conservative treatment (75% vs 33%); however, there was no significant difference in lay-off days between both groups (80 vs 74 days).78 Matsuda and colleagues79 looked at 335 male collegiate soccer players, 29 of whom had a history of a fifth metatarsal stress fracture. They found that playing the midfield position and having an everted rearfoot and inverted forefoot alignment were associated with fifth metatarsal stress fractures.79 Saita and colleagues80 found that restricted hip internal rotation was associated with an increased risk of developing a Jones fracture in 162 professional football players. Finally, Fujitaka and colleagues81 looked at 273 male soccer players between 2005 and 2013. They found an association between weak toe-grip strength and fifth metatarsal fractures, suggesting that weak toe-grip may lead to an increase in the load applied onto the lateral side of the foot, resulting in a stress fracture.81
Continue to: NAVICULAR
NAVICULAR
Another common tarsal bone that sustains stress fractures is the navicular. It is not as common as calcaneal stress fractures in military recruits but can occur in the same type of population, as well as explosive athletics such as sprinters and soccer players. It commonly presents with an indistinct vague achy pain with activity that improves with rest, and pain at the dorsum of the midfoot or along the medial longitudinal arch with activity. It can easily go undiagnosed for quite some time given the difficulty in visualizing the navicular with plain radiographs. Clinically, it is difficult to make the diagnosis, and therefore advanced imaging is necessary when the injury is suspected. Both MRI and CT scans can be used to understand the extent of the injury (Figures 9A-9C). In non-displaced stress fractures, conservative non-operative treatment is the appropriate treatment modality with a brief period of immobilization and non–weight-bearing;82 however, operative treatment is also considered in elite athletes. In either case, return to play is discouraged until there is evidence of radiographic healing. When a displacement is noted, or there is a delay in diagnosis, then operative treatment is recommended.

CONCLUSION
Ankle injuries are very common in soccer and can result in decreased performance or significant loss of playing time. Treatment of acute injury generally follows a conservative route, with surgical intervention reserved for severe ruptures or osteochondral fracture of the ankle joint. Chronic ankle pathology resulting in mechanical or functional instability generally requires surgery to repair ligamentous damage and restore normal ankle kinematics. It is critical for the soccer player to receive appropriate rehabilitation prior to returning to play in order to reduce the risk for reinjury and further chronic instability. Prevention and early intervention of ankle injuries is key in preventing the long-term sequelae of ankle injuries, such as arthritis, in former soccer players.
1. Dvorak J, Junge A. Football injuries and physical symptoms. A review of the literature. Am J Sports Med. 2000;28(5 Suppl):S3-S9.
2. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Am J Sports Med. 2000;28(5 Suppl):S58-S68.
3. Cloke DJ, Ansell P, Avery P, Deehan D. Ankle injuries in football academies: a three-centre prospective study. Br J Sports Med. 2011;45(9):702-708. doi:10.1136/bjsm.2009.067900.
4. Cloke DJ, Spencer S, Hodson A, Deehan D. The epidemiology of ankle injuries occurring in English Football Association academies. Br J Sports Med. 2009;43(14):1119-1125. doi:10.1136/bjsm.2008.052050.
5. Dvorak J, Junge A, Derman W, Schwellnus M. Injuries and illnesses of football players during the 2010 FIFA World Cup. Br J Sports Med. 2011;45(8):626-630. doi:10.1136/bjsm.2010.079905.
6. Ekstrand J, Gillquist J. Soccer injuries and their mechanisms: a prospective study. Med Sci Sports Exerc. 1983;15(3):267-270.
7. Fousekis K, Tsepis E, Vagenas G. Intrinsic risk factors of noncontact ankle sprains in soccer: a prospective study on 100 professional players. Am J Sports Med. 2012;40(8):1842-1850. doi:10.1177/0363546512449602.
8. Gaulrapp H, Becker A, Walther M, Hess H. Injuries in women’s soccer: a 1-year all players prospective field study of the women’s Bundesliga (German premiere league). Clin J Sports Med. 2010;20(4):264-271. doi:10.1097/JSM.0b013e3181e78e33.
9. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med. 2001;29(4):426-430. doi:10.1177/03635465010290040701.
10. Jain N, Murray D, Kemp S, Calder J. Frequency and trends in foot and ankle injuries within an English Premier League Football Club using a new impact factor of injury to identify a focus for injury prevention. Foot Ankle Surg. 2014;20(4):237-240. doi:10.1016/j.fas.2014.05.004.
11. Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, 2010:xxix, 1134.
12. Thompson JC, Netter FH. Netter’s Concise Orthopaedic Anatomy. 2nd ed. Philadelphia, PA: Saunders Elsevier, 2010:x, 404.
13. Giza E, Mandelbaum B. Chronic footballer’s ankle. In: Football Traumatology. Springer Milan, 2006:333-351.
14. Garrick JG. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am J Sports Med. 1977:5(6):241-242. doi:10.1177/036354657700500606.
15. Agur AMR, Grant JCB. Grant’s Atlas of Anatomy. 13th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2011.
16. Renstrom PA, Konradsen L. Ankle ligament injuries. Br J Sports Med. 1997;31(1):11-20.
17. Chhabra A, Subhawong TK, Carrino JA. MR imaging of deltoid ligament pathologic findings and associated impingement syndromes. Radiographics. 2010;30(3):751-761. doi:10.1148/rg.303095756.
18. Hintermann B. Medial ankle instability. Foot Ankle Clin. 2003;8(4):723-738.
19. Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football: an analysis of ankle sprains. Br J Sports Med. 2003;37(3):233-238.
20. Thacker SB, Stroup DF, Branche CM, Gilchrist J, Goodman RA, Weitman EA. The prevention of ankle sprains in sports. A systematic review of the literature. Am J Sports Med. 1999;27(6):753-760. doi:10.1177/03635465990270061201.
21. Giza E, Fuller C, Junge A, Dvorak J. Mechanisms of foot and ankle injuries in soccer. Am J Sports Med. 2003;31(4):550-554. doi:10.1177/03635465030310041201.
22. Tucker AM. Common soccer injuries. Diagnosis, treatment and rehabilitation. Sports Med. 1997;23(1):21-32.
23. Lynch SA, Renstrom PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. 1999;27(1):61-71.
24. Chan KW, Ding BC, Mroczek KJ. Acute and chronic lateral ankle instability in the athlete. Bull NYU Hosp Jt Dis. 2011;69(1):17-26.
25. Stiell IG, Greenberg GH, McKnight RD, Nair RC, McDowell I, Worthington JR. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21(4):384-390.
26. Bachmann LM, Kolb E, Koller MT, Steurer J, ter Riet G. Accuracy of Ottawa ankle rules to exclude fractures of the ankle and mid-foot: systematic review. BMJ. 2003;326(7386):417. doi:10.1136/bmj.326.7386.417.
27. Balduini FC, Vegso JJ, Torg JS, Torg E. Management and rehabilitation of ligamentous injuries to the ankle. Sports Med. 1987;4(5):364-380.
28. Kerkhoffs GM, Rowe BH, Assendelft WJ, Kelly KD, Struijs PA, van Dijk CN. Immobilisation for acute ankle sprain. A systematic review. Arch Orthop Trauma Surg. 2001;121(8):462-471.
29. Konradsen L, Holmer P, Sondergaard L. Early mobilizing treatment for grade III ankle ligament injuries. Foot Ankle. 1991;12(2):69-73.
30. Eiff MP, Smith AT, Smith GE. Early mobilization versus immobilization in the treatment of lateral ankle sprains. Am J Sports Med. 1994;22(1):83-88. doi:10.1177/036354659402200115.
31. Shrier I. Treatment of lateral collateral ligament sprains of the ankle: a critical appraisal of the literature. Clin J Sport Med. 1995;5(3):187-195.
32. DiGiovanni BF, Partal G, Baumhauer JF. Acute ankle injury and chronic lateral instability in the athlete. Clin Sports Med. 2004;23(1):1-19, v. doi:10.1016/S0278-5919(03)00095-4.
33. Alt W, Lohrer H, Gollhofer A. Functional properties of adhesive ankle taping: neuromuscular and mechanical effects before and after exercise. Foot Ankle Int. 1999;20(4):238-245. doi:10.1177/107110079902000406.
34. Garrick JG, Requa RK. Role of external support in the prevention of ankle sprains. Med Sci Sports. 1973;5(3):200-203.
35. Sharpe SR, Knapik J, Jones B. Ankle braces effectively reduce recurrence of ankle sprains in female soccer players. J Athl Train. 1997;32(1):21-24.
36. Surve I, Schwellnus MP, Noakes T, Lombard C. A fivefold reduction in the incidence of recurrent ankle sprains in soccer players using the Sport-Stirrup orthosis. Am J Sports Med. 1994;22(5):601-606. doi:10.1177/036354659402200506.
37. Mohammadi F. Comparison of 3 preventive methods to reduce the recurrence of ankle inversion sprains in male soccer players. Am J Sports Med. 2007;35(6):922-926. doi:10.1177/0363546507299259.
38. Steffen K, Meeuwisse WH, Romiti M, et al. Evaluation of how different implementation strategies of an injury prevention programme (FIFA 11+) impact team adherence and injury risk in Canadian female youth football players: a cluster-randomised trial. Br J Sports Med. 2013;47(8):480-487. doi:10.1136/bjsports-2012-091887.
39. Steffen K, Emery CA, Romiti M, et al. High adherence to a neuromuscular injury prevention programme (FIFA 11+) improves functional balance and reduces injury risk in Canadian youth female football players: a cluster randomised trial. Br J Sports Med. 2013;47(12):794-802. doi: 10.1136/bjsports-2012-091886.
40. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
41. Lin CF, Gross ML, Weinhold P. Ankle syndesmosis injuries: anatomy, biomechanics, mechanism of injury, and clinical guidelines for diagnosis and intervention. J Orthop Sports Phys Ther. 2006;36(6):372-384. doi:10.2519/jospt.2006.2195.
42. Lubberts B, D’Hooghe P, Bengtsson H, DiGiovanni CW, Calder J, Ekstrand J. Epidemiology and return to play following isolated syndesmotic injuries of the ankle: a prospective cohort study of 3677 male professional football players in the UEFA Elite Club Injury Study. Br J Sports Med. 2017. doi:10.1136/bjsports-2017-097710.
43. Harper MC, Keller TS. A radiographic evaluation of the tibiofibular syndesmosis. Foot Ankle. 1989;10(3):156-160.
44. Vogl TJ, Hochmuth K, Diebold T, et al. Magnetic resonance imaging in the diagnosis of acute injured distal tibiofibular syndesmosis. Invest Radiol. 1997;32(7):401-409.
45. Brown KW, Morrison WB, Schweitzer ME, Parellada JA, Nothnagel H. MRI findings associated with distal tibiofibular syndesmosis injury. AJR Am J Roentgenol. 2004;182(1):131-136. doi:10.2214/ajr.182.1.1820131.
46. Ogilvie-Harris DJ, Reed SC, Hedman TP. Disruption of the ankle syndesmosis: biomechanical study of the ligamentous restraints. Arthroscopy. 1994;10(5):558-560.
47. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298. doi:10.1177/036354659101900315.
48. Hopkinson WJ, St Pierre P, Ryan JB, Wheeler JH. Syndesmosis sprains of the ankle. Foot Ankle. 1990;10(6):325-330. doi:10.1177/107110079001000607.
49. Del Buono A, Florio A, Boccanera MS, Maffulli N. Syndesmosis injuries of the ankle. Curr Rev Musculoskelet Med. 2013;6(4):313-319. doi:10.1007/s12178-013-9183-x.
50. Dattani R, Patnaik S, Kantak A, Srikanth B, Selvan TP. Injuries to the tibiofibular syndesmosis. J Bone Joint Surg Br. 2008;90(4):405-410. doi:10.1302/0301-620X.90B4.19750.
51. Schepers T. To retain or remove the syndesmotic screw: a review of literature. Arch Orthop Trauma Surg. 2011;131(7):879-883. doi:10.1007/s00402-010-1225-x.
52. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835. doi:10.1177/0363546512461480.
53. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
54. Colcuc C, Blank M, Stein T, et al. Lower complication rate and faster return to sports in patients with acute syndesmotic rupture treated with a new knotless suture button device. Knee Surg Sports Traumatol Arthrosc. 2017. doi:10.1007/s00167-017-4820-4823.
55. Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec. 2014;7(5):414-422. doi:10.1177/1938640014543362.
56. Talusan PG, Milewski MD, Toy JO, Wall EJ. Osteochondritis dissecans of the talus: diagnosis and treatment in athletes. Clin Sports Med. 2014;33(2):267-284. doi:10.1016/j.csm.2014.01.003.
57. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045-1054. doi:10.2106/JBJS.L.00773.
58. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384-2389. doi:10.1007/s00167-013-2784-5.
59. Hatic SO, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3(6):361-364. doi:10.1177/1938640010388602.
60. van Eekeren IC, van Bergen CJ, Sierevelt IN, Reilingh ML, van Dijk CN. Return to sports after arthroscopic debridement and bone marrow stimulation of osteochondral talar defects: a 5- to 24-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1311-1315. doi:10.1007/s00167-016-3992-6.
61. Tol JL, Slim E, van Soest AJ, van Dijk CN. The relationship of the kicking action in soccer and anterior ankle impingement syndrome. A biomechanical analysis. Am J Sports Med. 2002;30(1):45-50. doi:10.1177/03635465020300012101.
62. Sanders TG, Rathur SK. Impingement syndromes of the ankle. Magn Reson Imaging Clin N Am. 2008;16(1):29-38. doi:10.1016/j.mric.2008.02.005.
63. Ogilvie-Harris DJ, Gilbart MK, Chorney K. Chronic pain following ankle sprains in athletes: the role of arthroscopic surgery. Arthroscopy. 1997;13(5):564-574.
64. Robinson P, White LM, Salonen D, Ogilvie-Harris D. Anteromedial impingement of the ankle: using MR arthrography to assess the anteromedial recess. AJR Am J Roentgenol. 2002;178(3):601-604. doi:10.2214/ajr.178.3.1780601.
65. Mosier-La Clair SM, Monroe MT, Manoli A. Medial impingement syndrome of the anterior tibiotalar fascicle of the deltoid ligament on the talus. Foot Ankle Int. 2000;21(5):385-391.
66. Maquirriain J. Posterior ankle impingement syndrome. J Am Acad Orthop Surg. 2005;13(6):365-371.
67. Georgiannos D, Bisbinas I. Endoscopic versus open excision of os trigonum for the treatment of posterior ankle impingement syndrome in an athletic population: a randomized controlled study with 5-year follow-up. Am J Sports Med. 2017;45(6):1388-1394. doi:10.1177/0363546516682498.
68. Carreira DS, Vora AM, Hearne KL, Kozy J. Outcome of arthroscopic treatment of posterior impingement of the ankle. Foot Ankle Int. 2016;37(4):394-400. doi:10.1177/1071100715620857.
69. Roth JA, Taylor WC, Whalen J. Peroneal tendon subluxation: the other lateral ankle injury. Br J Sports Med. 2010;44(14):1047-1053. doi:10.1136/bjsm.2008.057182.
70. Athavale SA, Swathi, Vangara SV. Anatomy of the superior peroneal tunnel. J Bone Joint Surg Am. 2011;93(6):564-571. doi:10.2106/JBJS.17.00836.
71. Porter D, McCarroll J, Knapp E, Torma J. Peroneal tendon subluxation in athletes: fibular groove deepening and retinacular reconstruction. Foot Ankle Int. 2005;26(6):436-441.
72. Ferran NA, Oliva F, Maffulli N. Recurrent subluxation of the peroneal tendons. Sports Med. 2006;36(10):839-846. doi:10.1053/j.jfas.2010.02.007.
73. Saxena A, Ewen B. Peroneal subluxation: surgical results in 31 athletic patients. J Foot Ankle Surg. 2010;49(3):238-241.
74. van Dijk PA, Gianakos AL, Kerkhoffs GM, Kennedy JG. Return to sports and clinical outcomes in patients treated for peroneal tendon dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1155-1164. doi:10.1007/s00167-015-3833-z.
75. Lee KT, Park YU, Young KW, Kim JS, Kim JB. The plantar gap: another prognostic factor for fifth metatarsal stress fracture. Am J Sports Med. 2011;39(10):2206-2211. doi:10.1177/0363546511414856.
76. Torg JS. Fractures of the base of the fifth metatarsal distal to the tuberosity. Orthopedics. 1990;13:731-737.
77. Smith TO, Clark A, Hing CB. Interventions for treating proximal fifth metatarsal fractures in adults: a meta-analysis of the current evidence-base. Foot Ankle Surg. 2011;17(4):300-307. doi:10.1016/j.fas.2010.12.005.
78. Ekstrand J, van Dijk CN. Fifth metatarsal fractures among male professional footballers: a potential career-ending disease. Br J Sports Med. 2013;47(12):754-758.
79. Matsuda S, Fukubayashi T, Hirose N. Characteristics of the foot static alignment and the plantar pressure associated with fifth metatarsal stress fracture history in male soccer players: a case-control study. Sports Med Open. 2017;3(1):27.
80. Saita Y, Nagao M, Kawasaki T, et al. Range limitation in hip internal rotation and fifth metatarsal stress fractures (Jones fracture) in professional football players. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):1943-1949. doi:10.1007/s00167-017-4552-4.
81. Fujitaka K, Taniguchi A, Isomoto S, et al. Pathogenesis of fifth metatarsal fractures in college soccer players. Orthop J Sports Med. 2015;18;3(9):2325967115603654.
82. Torg J, Moyer J, Gaughan J, Boden B. Management of tarsal navicular stress fractures: conservative versus surgical treatment: a meta-analysis. Am J Sports Med. 2010;38(5):1048-1053.
83. Haytmanek CT, Williams BT, James EW, et al. Radiographic identification of the primary lateral ankle structures. Am J Sports Med. 2015;43(1):79-87. doi:10.1177/0363546514553778.
1. Dvorak J, Junge A. Football injuries and physical symptoms. A review of the literature. Am J Sports Med. 2000;28(5 Suppl):S3-S9.
2. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Am J Sports Med. 2000;28(5 Suppl):S58-S68.
3. Cloke DJ, Ansell P, Avery P, Deehan D. Ankle injuries in football academies: a three-centre prospective study. Br J Sports Med. 2011;45(9):702-708. doi:10.1136/bjsm.2009.067900.
4. Cloke DJ, Spencer S, Hodson A, Deehan D. The epidemiology of ankle injuries occurring in English Football Association academies. Br J Sports Med. 2009;43(14):1119-1125. doi:10.1136/bjsm.2008.052050.
5. Dvorak J, Junge A, Derman W, Schwellnus M. Injuries and illnesses of football players during the 2010 FIFA World Cup. Br J Sports Med. 2011;45(8):626-630. doi:10.1136/bjsm.2010.079905.
6. Ekstrand J, Gillquist J. Soccer injuries and their mechanisms: a prospective study. Med Sci Sports Exerc. 1983;15(3):267-270.
7. Fousekis K, Tsepis E, Vagenas G. Intrinsic risk factors of noncontact ankle sprains in soccer: a prospective study on 100 professional players. Am J Sports Med. 2012;40(8):1842-1850. doi:10.1177/0363546512449602.
8. Gaulrapp H, Becker A, Walther M, Hess H. Injuries in women’s soccer: a 1-year all players prospective field study of the women’s Bundesliga (German premiere league). Clin J Sports Med. 2010;20(4):264-271. doi:10.1097/JSM.0b013e3181e78e33.
9. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med. 2001;29(4):426-430. doi:10.1177/03635465010290040701.
10. Jain N, Murray D, Kemp S, Calder J. Frequency and trends in foot and ankle injuries within an English Premier League Football Club using a new impact factor of injury to identify a focus for injury prevention. Foot Ankle Surg. 2014;20(4):237-240. doi:10.1016/j.fas.2014.05.004.
11. Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, 2010:xxix, 1134.
12. Thompson JC, Netter FH. Netter’s Concise Orthopaedic Anatomy. 2nd ed. Philadelphia, PA: Saunders Elsevier, 2010:x, 404.
13. Giza E, Mandelbaum B. Chronic footballer’s ankle. In: Football Traumatology. Springer Milan, 2006:333-351.
14. Garrick JG. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am J Sports Med. 1977:5(6):241-242. doi:10.1177/036354657700500606.
15. Agur AMR, Grant JCB. Grant’s Atlas of Anatomy. 13th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2011.
16. Renstrom PA, Konradsen L. Ankle ligament injuries. Br J Sports Med. 1997;31(1):11-20.
17. Chhabra A, Subhawong TK, Carrino JA. MR imaging of deltoid ligament pathologic findings and associated impingement syndromes. Radiographics. 2010;30(3):751-761. doi:10.1148/rg.303095756.
18. Hintermann B. Medial ankle instability. Foot Ankle Clin. 2003;8(4):723-738.
19. Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football: an analysis of ankle sprains. Br J Sports Med. 2003;37(3):233-238.
20. Thacker SB, Stroup DF, Branche CM, Gilchrist J, Goodman RA, Weitman EA. The prevention of ankle sprains in sports. A systematic review of the literature. Am J Sports Med. 1999;27(6):753-760. doi:10.1177/03635465990270061201.
21. Giza E, Fuller C, Junge A, Dvorak J. Mechanisms of foot and ankle injuries in soccer. Am J Sports Med. 2003;31(4):550-554. doi:10.1177/03635465030310041201.
22. Tucker AM. Common soccer injuries. Diagnosis, treatment and rehabilitation. Sports Med. 1997;23(1):21-32.
23. Lynch SA, Renstrom PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. 1999;27(1):61-71.
24. Chan KW, Ding BC, Mroczek KJ. Acute and chronic lateral ankle instability in the athlete. Bull NYU Hosp Jt Dis. 2011;69(1):17-26.
25. Stiell IG, Greenberg GH, McKnight RD, Nair RC, McDowell I, Worthington JR. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21(4):384-390.
26. Bachmann LM, Kolb E, Koller MT, Steurer J, ter Riet G. Accuracy of Ottawa ankle rules to exclude fractures of the ankle and mid-foot: systematic review. BMJ. 2003;326(7386):417. doi:10.1136/bmj.326.7386.417.
27. Balduini FC, Vegso JJ, Torg JS, Torg E. Management and rehabilitation of ligamentous injuries to the ankle. Sports Med. 1987;4(5):364-380.
28. Kerkhoffs GM, Rowe BH, Assendelft WJ, Kelly KD, Struijs PA, van Dijk CN. Immobilisation for acute ankle sprain. A systematic review. Arch Orthop Trauma Surg. 2001;121(8):462-471.
29. Konradsen L, Holmer P, Sondergaard L. Early mobilizing treatment for grade III ankle ligament injuries. Foot Ankle. 1991;12(2):69-73.
30. Eiff MP, Smith AT, Smith GE. Early mobilization versus immobilization in the treatment of lateral ankle sprains. Am J Sports Med. 1994;22(1):83-88. doi:10.1177/036354659402200115.
31. Shrier I. Treatment of lateral collateral ligament sprains of the ankle: a critical appraisal of the literature. Clin J Sport Med. 1995;5(3):187-195.
32. DiGiovanni BF, Partal G, Baumhauer JF. Acute ankle injury and chronic lateral instability in the athlete. Clin Sports Med. 2004;23(1):1-19, v. doi:10.1016/S0278-5919(03)00095-4.
33. Alt W, Lohrer H, Gollhofer A. Functional properties of adhesive ankle taping: neuromuscular and mechanical effects before and after exercise. Foot Ankle Int. 1999;20(4):238-245. doi:10.1177/107110079902000406.
34. Garrick JG, Requa RK. Role of external support in the prevention of ankle sprains. Med Sci Sports. 1973;5(3):200-203.
35. Sharpe SR, Knapik J, Jones B. Ankle braces effectively reduce recurrence of ankle sprains in female soccer players. J Athl Train. 1997;32(1):21-24.
36. Surve I, Schwellnus MP, Noakes T, Lombard C. A fivefold reduction in the incidence of recurrent ankle sprains in soccer players using the Sport-Stirrup orthosis. Am J Sports Med. 1994;22(5):601-606. doi:10.1177/036354659402200506.
37. Mohammadi F. Comparison of 3 preventive methods to reduce the recurrence of ankle inversion sprains in male soccer players. Am J Sports Med. 2007;35(6):922-926. doi:10.1177/0363546507299259.
38. Steffen K, Meeuwisse WH, Romiti M, et al. Evaluation of how different implementation strategies of an injury prevention programme (FIFA 11+) impact team adherence and injury risk in Canadian female youth football players: a cluster-randomised trial. Br J Sports Med. 2013;47(8):480-487. doi:10.1136/bjsports-2012-091887.
39. Steffen K, Emery CA, Romiti M, et al. High adherence to a neuromuscular injury prevention programme (FIFA 11+) improves functional balance and reduces injury risk in Canadian youth female football players: a cluster randomised trial. Br J Sports Med. 2013;47(12):794-802. doi: 10.1136/bjsports-2012-091886.
40. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
41. Lin CF, Gross ML, Weinhold P. Ankle syndesmosis injuries: anatomy, biomechanics, mechanism of injury, and clinical guidelines for diagnosis and intervention. J Orthop Sports Phys Ther. 2006;36(6):372-384. doi:10.2519/jospt.2006.2195.
42. Lubberts B, D’Hooghe P, Bengtsson H, DiGiovanni CW, Calder J, Ekstrand J. Epidemiology and return to play following isolated syndesmotic injuries of the ankle: a prospective cohort study of 3677 male professional football players in the UEFA Elite Club Injury Study. Br J Sports Med. 2017. doi:10.1136/bjsports-2017-097710.
43. Harper MC, Keller TS. A radiographic evaluation of the tibiofibular syndesmosis. Foot Ankle. 1989;10(3):156-160.
44. Vogl TJ, Hochmuth K, Diebold T, et al. Magnetic resonance imaging in the diagnosis of acute injured distal tibiofibular syndesmosis. Invest Radiol. 1997;32(7):401-409.
45. Brown KW, Morrison WB, Schweitzer ME, Parellada JA, Nothnagel H. MRI findings associated with distal tibiofibular syndesmosis injury. AJR Am J Roentgenol. 2004;182(1):131-136. doi:10.2214/ajr.182.1.1820131.
46. Ogilvie-Harris DJ, Reed SC, Hedman TP. Disruption of the ankle syndesmosis: biomechanical study of the ligamentous restraints. Arthroscopy. 1994;10(5):558-560.
47. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298. doi:10.1177/036354659101900315.
48. Hopkinson WJ, St Pierre P, Ryan JB, Wheeler JH. Syndesmosis sprains of the ankle. Foot Ankle. 1990;10(6):325-330. doi:10.1177/107110079001000607.
49. Del Buono A, Florio A, Boccanera MS, Maffulli N. Syndesmosis injuries of the ankle. Curr Rev Musculoskelet Med. 2013;6(4):313-319. doi:10.1007/s12178-013-9183-x.
50. Dattani R, Patnaik S, Kantak A, Srikanth B, Selvan TP. Injuries to the tibiofibular syndesmosis. J Bone Joint Surg Br. 2008;90(4):405-410. doi:10.1302/0301-620X.90B4.19750.
51. Schepers T. To retain or remove the syndesmotic screw: a review of literature. Arch Orthop Trauma Surg. 2011;131(7):879-883. doi:10.1007/s00402-010-1225-x.
52. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835. doi:10.1177/0363546512461480.
53. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
54. Colcuc C, Blank M, Stein T, et al. Lower complication rate and faster return to sports in patients with acute syndesmotic rupture treated with a new knotless suture button device. Knee Surg Sports Traumatol Arthrosc. 2017. doi:10.1007/s00167-017-4820-4823.
55. Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec. 2014;7(5):414-422. doi:10.1177/1938640014543362.
56. Talusan PG, Milewski MD, Toy JO, Wall EJ. Osteochondritis dissecans of the talus: diagnosis and treatment in athletes. Clin Sports Med. 2014;33(2):267-284. doi:10.1016/j.csm.2014.01.003.
57. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045-1054. doi:10.2106/JBJS.L.00773.
58. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384-2389. doi:10.1007/s00167-013-2784-5.
59. Hatic SO, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3(6):361-364. doi:10.1177/1938640010388602.
60. van Eekeren IC, van Bergen CJ, Sierevelt IN, Reilingh ML, van Dijk CN. Return to sports after arthroscopic debridement and bone marrow stimulation of osteochondral talar defects: a 5- to 24-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1311-1315. doi:10.1007/s00167-016-3992-6.
61. Tol JL, Slim E, van Soest AJ, van Dijk CN. The relationship of the kicking action in soccer and anterior ankle impingement syndrome. A biomechanical analysis. Am J Sports Med. 2002;30(1):45-50. doi:10.1177/03635465020300012101.
62. Sanders TG, Rathur SK. Impingement syndromes of the ankle. Magn Reson Imaging Clin N Am. 2008;16(1):29-38. doi:10.1016/j.mric.2008.02.005.
63. Ogilvie-Harris DJ, Gilbart MK, Chorney K. Chronic pain following ankle sprains in athletes: the role of arthroscopic surgery. Arthroscopy. 1997;13(5):564-574.
64. Robinson P, White LM, Salonen D, Ogilvie-Harris D. Anteromedial impingement of the ankle: using MR arthrography to assess the anteromedial recess. AJR Am J Roentgenol. 2002;178(3):601-604. doi:10.2214/ajr.178.3.1780601.
65. Mosier-La Clair SM, Monroe MT, Manoli A. Medial impingement syndrome of the anterior tibiotalar fascicle of the deltoid ligament on the talus. Foot Ankle Int. 2000;21(5):385-391.
66. Maquirriain J. Posterior ankle impingement syndrome. J Am Acad Orthop Surg. 2005;13(6):365-371.
67. Georgiannos D, Bisbinas I. Endoscopic versus open excision of os trigonum for the treatment of posterior ankle impingement syndrome in an athletic population: a randomized controlled study with 5-year follow-up. Am J Sports Med. 2017;45(6):1388-1394. doi:10.1177/0363546516682498.
68. Carreira DS, Vora AM, Hearne KL, Kozy J. Outcome of arthroscopic treatment of posterior impingement of the ankle. Foot Ankle Int. 2016;37(4):394-400. doi:10.1177/1071100715620857.
69. Roth JA, Taylor WC, Whalen J. Peroneal tendon subluxation: the other lateral ankle injury. Br J Sports Med. 2010;44(14):1047-1053. doi:10.1136/bjsm.2008.057182.
70. Athavale SA, Swathi, Vangara SV. Anatomy of the superior peroneal tunnel. J Bone Joint Surg Am. 2011;93(6):564-571. doi:10.2106/JBJS.17.00836.
71. Porter D, McCarroll J, Knapp E, Torma J. Peroneal tendon subluxation in athletes: fibular groove deepening and retinacular reconstruction. Foot Ankle Int. 2005;26(6):436-441.
72. Ferran NA, Oliva F, Maffulli N. Recurrent subluxation of the peroneal tendons. Sports Med. 2006;36(10):839-846. doi:10.1053/j.jfas.2010.02.007.
73. Saxena A, Ewen B. Peroneal subluxation: surgical results in 31 athletic patients. J Foot Ankle Surg. 2010;49(3):238-241.
74. van Dijk PA, Gianakos AL, Kerkhoffs GM, Kennedy JG. Return to sports and clinical outcomes in patients treated for peroneal tendon dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1155-1164. doi:10.1007/s00167-015-3833-z.
75. Lee KT, Park YU, Young KW, Kim JS, Kim JB. The plantar gap: another prognostic factor for fifth metatarsal stress fracture. Am J Sports Med. 2011;39(10):2206-2211. doi:10.1177/0363546511414856.
76. Torg JS. Fractures of the base of the fifth metatarsal distal to the tuberosity. Orthopedics. 1990;13:731-737.
77. Smith TO, Clark A, Hing CB. Interventions for treating proximal fifth metatarsal fractures in adults: a meta-analysis of the current evidence-base. Foot Ankle Surg. 2011;17(4):300-307. doi:10.1016/j.fas.2010.12.005.
78. Ekstrand J, van Dijk CN. Fifth metatarsal fractures among male professional footballers: a potential career-ending disease. Br J Sports Med. 2013;47(12):754-758.
79. Matsuda S, Fukubayashi T, Hirose N. Characteristics of the foot static alignment and the plantar pressure associated with fifth metatarsal stress fracture history in male soccer players: a case-control study. Sports Med Open. 2017;3(1):27.
80. Saita Y, Nagao M, Kawasaki T, et al. Range limitation in hip internal rotation and fifth metatarsal stress fractures (Jones fracture) in professional football players. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):1943-1949. doi:10.1007/s00167-017-4552-4.
81. Fujitaka K, Taniguchi A, Isomoto S, et al. Pathogenesis of fifth metatarsal fractures in college soccer players. Orthop J Sports Med. 2015;18;3(9):2325967115603654.
82. Torg J, Moyer J, Gaughan J, Boden B. Management of tarsal navicular stress fractures: conservative versus surgical treatment: a meta-analysis. Am J Sports Med. 2010;38(5):1048-1053.
83. Haytmanek CT, Williams BT, James EW, et al. Radiographic identification of the primary lateral ankle structures. Am J Sports Med. 2015;43(1):79-87. doi:10.1177/0363546514553778.
TAKE-HOME POINTS
- Soccer injuries of the foot and ankle are becoming more prevalent due to the ever-growing popularity of the sport.
- Low ankle sprains represent the majority of foot and ankle–related injuries due to soccer and most can be treated non-operatively, with an early mobilization protocol followed by a phased rehabilitation.
- High ankle sprains are less common than low ankle sprains; however, they require a lengthier rehabilitation and most of the time are treated surgically.
- Impingement-like syndromes are common among soccer players and can be due to repetitive microtrauma from recurrent ball impact. Most of these syndromes respond favorably to non-operative modalities.
- Stress fractures of the foot, although less common, often require surgical stabilization in soccer players.
The In Vivo Impact of Leukocyte Injections on Normal Rat Achilles Tendons: Potential Detriment to Tendon Morphology, Cellularity, and Vascularity
ABSTRACT
In this study, we determine the in vivo effects of injecting sub-populations of leukocytes into normal rat Achilles tendons via a controlled laboratory study. Allogenic monocytes, granulocytes, or plasma were injected into 24 healthy rat Achilles tendons. Treated and contralateral un-treated control tendons then assessed for cellularity, histologic morphology, and vascularity after 7 and 14 days. Significant increases of 221% and 249% in cellularity (P = 0.014) were seen on day 14 within Achilles tendons injected with granulocytes as compared to plasma and monocytes, respectively. Also, significant improvement in morphology (P = 0.029) between days 7 and 14 was seen for the granulocyte injected Achilles tendons. Significant increases in cellularity after an injection of granulocytes, compared to monocytes and plasma, corresponds to a significant increase in inflammation within the tissue, suggesting that leukocyte-rich platelet-rich plasma (PRP) preparations are proinflammatory and potentially catabolic when injected into tendon tissue. The concentration and composition of white blood cells within PRP preparations is variable and needs to be better understood in order to optimize clinical utility of PRP injections.
Continue to: Tendinopathies are debilitating conditions...
Tendinopathies are debilitating conditions affecting patients worldwide every day. They arise most frequently from tendon overuse resulting in pathology.1 There are 2 major subtypes of tendinopathy: tendinosis and tendinitis. Tendinosis, the more common condition, is characterized by long-term, chronic degradation of tendon tissue resulting in fibrosis from infiltrating fibroblasts.2 Tendinitis, the less common condition, is characterized by an acute inflammatory response and inflammatory cell infiltrate.2 Both conditions are common, with Achilles tendinopathy affecting 11% of runners and lateral epicondylitis affecting 1% to 3% of the general population.3,4 Many sports-related overuse injuries, such as tendinopathies, go undiagnosed for extended periods of time because medical attention is avoided in order to prevent time loss from training or competing.5 These delays could be eliminated if a non-surgical option for treating tendon pathology was available.
Tendinopathies are believed to result from tendon overuse that causes micro-damage to collagen, as well as from significant changes in protein and enzyme composition within the tendon.6 The damage accumulates over time and eventually leads to chronic inflammation or fibrotic change within tendons, in both cases weakening the tendon and causing pain. Currently, accepted treatments for tendinopathies include: nonsteroidal anti-inflammatory drugs, physical therapy, ultrasound, laser-therapy, corticosteroids, glyceryl trinitrate patches, extracorporeal shock wave therapy, sclerotherapy, and surgery.7 Recently, platelet-rich plasma (PRP) therapy has emerged as a promising treatment for tendinopathies, as well as a variety of other orthopedic indications.
PRP consists of autologous blood from the patient, centrifuged to increase the amount of platelets in the sample above baseline, and subsequently injected around an affected tendon or joint.8 PRP is used to treat tendinopathy because it can supply injured tendons with blood components that aid in healing, which tendons do not receive due to poor vascularity.9 These components include growth factors, such as platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), endothelial growth factor, and leukocytes that can stimulate an inflammatory response within the injured tissue.10 The inflammatory response from the PRP induces a more robust reconstruction and revascularization of the injured tissue, stimulating proliferation, and remodeling.11,12However, significant variability exists within the platelets, leukocytes, and growth factors that comprise PRP. This is attributed to 3 major causes. First, current commercial preparations of PRP result in differing platelet concentrations, as well as leukocyte-rich and leukocyte-poor compositions.13,14 Variability in platelet concentrations results in unreliable amounts of growth factors, including cytokines, TGF-β, PDGF, VEGF and basic fibroblast growth factor in each preparation, while leukocyte levels affect inflammation, all leading to variable effects for each preparation.15,16Second, despite sex and age of the PRP donor not being significant factors influencing variation in growth factor concentrations, the existence of an unexplained variation in concentrations of growth factors between different donors has been observed.17 Third, the selection of activating agents, bovine thrombin or calcium chloride, and their application, whether to the elbow, shoulder, or knee, produces variability.18
While the effects of platelets and growth factors in PRP have been well studied, less is known about the effects of differing cell types. Recently it was reported that the concentrations of leukocytes directly affect the outcomes of PRP injections. McCarrel and colleagues19,20 found that as the number of leukocytes increased, there was a concomitant increase in the expression of inflammatory cytokines and catabolic activity. This effect may result in inferior healing of injured tissues and is attributed to the release of pro-inflammatory cytokines such as interleukin-1β from the leukocytes.21 There is also evidence that minimizing the catabolic effect of leukocytes may be just as important to tissue healing as the maximizing anabolic effect of platelets and growth factors.22
The use of PRP has been highly disputed in recent years due to conflicting reports of its success in treating orthopedic conditions. Numerous favorable studies have shown benefit for treating chronic and acute orthopedic injuries including; rotator cuff tear repair, chronic refractory patellar tendinopathy, and chronic lateral tendinosis/epicondylitis.23-26 Concurrently, articles demonstrating no significant effects from PRP have also been published. One study claiming that PRP injections did not improve outcomes of chronic Achilles tendinopathy did not differentiate whether patients had tendinosis or tendinitis, and did not consider leukocyte concentration in their PRP preparations27 Another study that determined PRP is not beneficial to the healing of ruptured Achilles tendons after surgical repair also failed to consider the concentration of leukocytes in their PRP preparations.28 One of the difficulties in comparing these studies is their heterogeneous nature. This arises from the use of different conditions in each study that makes the studies incomparable. Variations in PRP preparations lead to different concentrations of growth factors, platelets, and leukocyte concentrations. Additionally, tendinopathy models were not specified as tendinosis and tendonitis, and models or patients were not controlled for age, sex, or comorbidities. Given that leukocyte-rich and leukocyte-poor PRP preparations are currently widely used in clinical practice, the discovery of which type of preparation is indicated in which setting is paramount to evidence-based use of this treatment modality. Due to reports suggesting that leukocytes may be detrimental to tendon healing, determining which types of leukocytes are responsible for these effects is vital. As such, the purpose of this study is to determine the in vivo effects of sub-populations of leukocytes on normal rat tendons. This study design allowed us to isolate the effects of the injections to induce a response and remove confounding effects of normal healing response to a damaged tendon and effects from the injection itself. Our hypothesis was that the injection of leukocytes would cause an inflammatory response in rat tendons, leading to catabolic outcomes.
Continue to: METHODS...
METHODS
This was a prospective, in vivo, placebo controlled, randomized animal study. The University’s Institutional Animal Care and Use Committee approved all procedures prior to initiation. Twenty-four male Sprague-Dawley rats were randomized to 3 treatment groups (n = 8): monocytes; granulocytes, and; plasma, as a negative control.
Allogenic blood from 6 additional rats was collected into K2EDTA tubes via cardiac puncture. Allogenic, as opposed to autogenic, blood is commonly used in rat models because of low immunogenic response to blood from rats of the same strain and litter.29,30 The blood was then pooled and the red cells lysed by incubation with Red Blood Cell Lysis Buffer (Roche). The samples were then sorted into fractions containing monocytes and granulocytes using fluorescence activated cell sorting (FACS) using a FACSAria (BD Biosciences). Cells were sorted using Purified PE Mouse Anti-Rat CD11b/c antibodies (BD Pharmingen) specific to monocytes, granulocytes, macrophages, dendritic cells, and microglia, APC-Cy7 Mouse Anti-Rat CD45 antibodies (BD Pharmingen) specific to all hematopoietic cells except erythrocytes, and FITC Mouse Anti-Rat CD42d antibodies (BD Pharmingen) specific to megakaryocytes and platelets. 20 μL of 0.2 mg/mL CD11b/c, 20 μL of 0.2 mg/mL CD 45, and 10 μL of 0.5 mg/mL CD42d antibodies were added to 1 mL of condensed non-red cells collected from the 6 rats and incubated at room temperature in the dark for 15 minutes. A fraction containing only platelet-poor plasma was also collected. For all treatments the injection volume was 75 μL. Rats in the monocyte group were injected with 200,000 cells in platelet-poor plasma, those in the granulocyte group were injected with 900,000 cells in platelet-poor plasma, and rats in the plasma control group received only platelet-poor plasma. The cell concentrations were based on previous studies that documented these concentrations that are found in typical leukocyte-rich PRP preparations.13
The animals were anesthetized with isoflurane gas and then injected aseptically once into their right Achilles tendon. The left Achilles tendon was used as an un-injected control, giving a total of 48 total Achilles tendons studied. At days 7 and 14 post-injection, 4 rats from each group were euthanized and the Achilles tendons were harvested.
The tendons were fixed in neutral buffered formalin for 24 hours and then embedded in paraffin and sectioned sagittally at 12 μm. The tendons were then stained with hematoxylin and eosin (H&E) using standard histological protocols and examined by 3 individuals trained to assess cellularity and morphology. All samples were assigned unrecognizable numbers and randomized prior to examination by individuals. Cell counts were based on the number of nuclei present in 3 mid-tendon high-power fields (400x) per sample. Morphology was graded on a scale of 1 to 3, with 1 being a normal tendon and 3 having severe pathology with total loss of alignment and crimping on 3 low-power fields (100x) per sample (Figures 1A-1G).
Vascularity was assessed by immunohistochemical staining using Rabbit Polyclonal Anti-CD31 antibodies (Abcam), a marker for vascular endothelial cells, using a Vectastain ABC Kit (Vector Laboratories) system and the ImmPACT AEC Peroxidase (HRP) Substrate (Vector Laboratories). Following staining, automated image analysis was performed (Bioquant). Briefly, all areas that did not contain tendon were masked. CD31 positive areas were then quantified using global thresholding. Vascularity was then calculated as ratio of CD31 positive area to total tendon area. Analyses were performed on 3 mid-tendon medium-power (200x) fields per sample.
For cellularity and morphology, the results for the injected tendons were normalized to those of their contralateral untreated controls and reported as a percentage. Results for vascularity were compared directly between treated tendons. Differences were assessed between groups at each time-point using Independent Samples Median Tests. When significant differences were identified, pairwise comparisons were performed to identify the source of the differences. All analyses were conducted using SPSS (V22, SAS Institute) with significant differences determined for values of P < 0.05.
RESULTS
No significant differences in cellularity between groups were seen at day 7 (P = 0.368) (Figures 1A-1G). However, a significant difference in cellularity between groups was seen at day 14 (P = 0.014). Pairwise tests showed there to be a significant increase in the number of cells in the tendons treated with granulocytes from 221% and 249% in cellularity (P = 0.014) on day 14, as compared to both monocytes and plasma, respectively. Morphologically, no significant differences were seen between groups at either time-point (P = 0.091 for day 7 and P = 1.000 for day 14) (Figures 2A-2G). However, a significant improvement in morphology was observed from day 7 to day 14 in the granulocyte group from 60% to 165% (P = 0.029). Finally, no differences were seen in vascularity between treatment groups at either time-point (P = 0.368 for day 7 and P = 0.535 for day 14) (Figures 3A-3G).
Continue to: DISCUSSION...
DISCUSSION
Our hypothesis that the injection of leukocytes would cause an inflammatory response in rat tendons leading to catabolic outcomes was confirmed in the granulocyte group. It should be noted that prior to the catabolic outcome, there was a transient anabolic effect in the granulocyte group during the second week. Deterioration in morphology was observed in the tendons injected with granulocytes on day 7, which subsequently recovered in the following week. We found that injecting granulocytes into normal tendons resulted in an increase in inflammatory cellularity, when compared to monocytes and plasma injections.
Limitations inherent in this study are those similar to other in vivo studies. To begin with, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 Another limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the immunohistochemistry (IHC) and morphological data are clear, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. The increased cellularity could be due to the increased number of cells injected into the tendon; however, our conclusions are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32
In terms of morphology, we hypothesized that degenerative changes would be seen in the tendons that were injected with granulocytes due to the inflammatory action of these cells. As part of the granulocyte response, neutrophils release proteases and macrophages can stimulate collagen synthesis via fibroblasts, both causing change within the extracellular matrix.33,34 Indeed, we observed a significant change in tissue morphology in the granulocyte group over the course of 14 days. As the degenerative and regenerative effects of granulocytes take time to present, this is likely what we observed to occur between day 7 and 14 after treatment. These observations are also consistent with prior observations that leukocyte-rich PRP injections can be detrimental to tendon healing, but beneficial to tissue degeneration in the setting of chronic tendonitis.20
We hypothesized that the vascularity of the tendons would be similar in all preparations. This was based on previous studies demonstrating that the lack of platelets in the platelet-poor plasma fraction is sufficient to deplete VEGF, the angiogenic agent in PRP.35 In this study, there were no observable differences in vascularity of platelet-poor plasma, monocyte, and granulocyte injections. We attribute this to the lack of VEGF in any of these preparations. The aforementioned study also showed that the lack of platelets in injection was enough to prevent the angiogenic effect of this treatment.35
Continue to: The goal of this study was...
The goal of this study was to assess the morphology, cellularity, and vascularity of normal tendons after injections of different leukocyte populations. This is clinically important because of the potential to tailor future PRP injections on a patient-by-patient basis. In patients requiring an anabolic response, leukocyte-poor PRP may be the best option. In contrast, when patient pathology requires an inflammatory response to improve healing36 or breakdown fibrotic tissue, as seen in tendinosis, leukocyte-rich PRP may be warranted. Further, properly controlled clinical studies are needed to validate these recommendations.
Limitations inherent in this study are those similar to other in vivo studies. First, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 A second limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the IHC and morphological data show clear changes, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. The last limitation of this study is the lack of functional mechanical testing since, clinically, healing of the tendon is also related to the strength of the tendon. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. Moreover, our results are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32 It is interesting to note that the increase in inflammation does not lead to an increase in vascularity as could be expected.
CONCLUSION
We found that the injection of leukocytes into healthy rat Achilles tendons increases inflammation, as evidenced by increased cellularity and disrupted morphology, which suggests that leukocyte-rich PRP preparations may be contraindicated in settings of acute tendonitis. However, these preparations may be useful for a specific subset of tendinopathies, including chronic tendinosis.
1. Herring SA, Nilson KL. Introduction to overuse injuries. Clin Sports Med. 1987;6(2):225-239.
2. Bass E. Tendinopathy: why the difference between tendinitis and tendinosis matters. Int J Ther Massage Bodywork. 2012;5(1):14-17.
3. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
4. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
5. Bahr R. No injuries, but plenty of pain? On the methodology for recording overuse symptoms in sports. Br J Sports Med. 2009;43(13):966-972.
6. Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37(9):1855-1867.
7. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539-1554.
8. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602-608.
9. Smith JW. Blood Supply of Tendons. Am J Surg. 1965;109:272-276.
10. Wu PI, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825-853.
11. Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R. 2011;3(3):226-250.
12. Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S-32e-S.
13. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316.
14. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40(8):1742-1749.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271.
16. Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31(3):212-218.
17. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97-102.
18. Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21(4):344-352.
19. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27(8):1033-1042.
20. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94(19):e143(141-148).
21. Pillitteri D, Bassus S, Boller K, et al. Thrombin-induced interleukin 1beta synthesis in platelet suspensions: impact of contaminating leukocytes. Platelets. 2007;18(2):119-127.
22. Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42(1):42-49.
23. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
24. Maniscalco P, Gambera D, Lunati A, et al. The "Cascade" membrane: a new PRP device for tendon ruptures. Description and case report on rotator cuff tendon. Acta Biomed. 2008;79(3):223-226.
25. Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper's knee. Int Orthop. 2010;34(6):909-915.
26. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
27. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
28. Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39(1):38-47.
29. Welsh KI, Burgos H, Batchelor JR. The immune response to allogeneic rat platelets; Ag-B antigens in matrix form lacking Ia. Eur J Immunol. 1977;7(5):267-272.
30. Xue M, Del Bigio MR. Intracortical hemorrhage injury in rats : relationship between blood fractions and brain cell death. Stroke. 2000;31(7):1721-1727.
31. Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47-71.
32. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140.
33. Palmgren MS, deShazo RD, Carter RM, Zimny ML, Shah SV. Mechanisms of neutrophil damage to human alveolar extracellular matrix: the role of serine and metalloproteases. J Allergy Clin Immunol. 1992;89(4):905-915.
34. Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170(3):727-737.
35. Zhou Y, Zhang J, Wu H, Hogan MV, Wang JH. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173.
36. Su B, O'Connor JP. NSAID therapy effects on healing of bone, tendon, and the enthesis. J Appl Physiol (1985). 2013;115(6):892-899.
ABSTRACT
In this study, we determine the in vivo effects of injecting sub-populations of leukocytes into normal rat Achilles tendons via a controlled laboratory study. Allogenic monocytes, granulocytes, or plasma were injected into 24 healthy rat Achilles tendons. Treated and contralateral un-treated control tendons then assessed for cellularity, histologic morphology, and vascularity after 7 and 14 days. Significant increases of 221% and 249% in cellularity (P = 0.014) were seen on day 14 within Achilles tendons injected with granulocytes as compared to plasma and monocytes, respectively. Also, significant improvement in morphology (P = 0.029) between days 7 and 14 was seen for the granulocyte injected Achilles tendons. Significant increases in cellularity after an injection of granulocytes, compared to monocytes and plasma, corresponds to a significant increase in inflammation within the tissue, suggesting that leukocyte-rich platelet-rich plasma (PRP) preparations are proinflammatory and potentially catabolic when injected into tendon tissue. The concentration and composition of white blood cells within PRP preparations is variable and needs to be better understood in order to optimize clinical utility of PRP injections.
Continue to: Tendinopathies are debilitating conditions...
Tendinopathies are debilitating conditions affecting patients worldwide every day. They arise most frequently from tendon overuse resulting in pathology.1 There are 2 major subtypes of tendinopathy: tendinosis and tendinitis. Tendinosis, the more common condition, is characterized by long-term, chronic degradation of tendon tissue resulting in fibrosis from infiltrating fibroblasts.2 Tendinitis, the less common condition, is characterized by an acute inflammatory response and inflammatory cell infiltrate.2 Both conditions are common, with Achilles tendinopathy affecting 11% of runners and lateral epicondylitis affecting 1% to 3% of the general population.3,4 Many sports-related overuse injuries, such as tendinopathies, go undiagnosed for extended periods of time because medical attention is avoided in order to prevent time loss from training or competing.5 These delays could be eliminated if a non-surgical option for treating tendon pathology was available.
Tendinopathies are believed to result from tendon overuse that causes micro-damage to collagen, as well as from significant changes in protein and enzyme composition within the tendon.6 The damage accumulates over time and eventually leads to chronic inflammation or fibrotic change within tendons, in both cases weakening the tendon and causing pain. Currently, accepted treatments for tendinopathies include: nonsteroidal anti-inflammatory drugs, physical therapy, ultrasound, laser-therapy, corticosteroids, glyceryl trinitrate patches, extracorporeal shock wave therapy, sclerotherapy, and surgery.7 Recently, platelet-rich plasma (PRP) therapy has emerged as a promising treatment for tendinopathies, as well as a variety of other orthopedic indications.
PRP consists of autologous blood from the patient, centrifuged to increase the amount of platelets in the sample above baseline, and subsequently injected around an affected tendon or joint.8 PRP is used to treat tendinopathy because it can supply injured tendons with blood components that aid in healing, which tendons do not receive due to poor vascularity.9 These components include growth factors, such as platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), endothelial growth factor, and leukocytes that can stimulate an inflammatory response within the injured tissue.10 The inflammatory response from the PRP induces a more robust reconstruction and revascularization of the injured tissue, stimulating proliferation, and remodeling.11,12However, significant variability exists within the platelets, leukocytes, and growth factors that comprise PRP. This is attributed to 3 major causes. First, current commercial preparations of PRP result in differing platelet concentrations, as well as leukocyte-rich and leukocyte-poor compositions.13,14 Variability in platelet concentrations results in unreliable amounts of growth factors, including cytokines, TGF-β, PDGF, VEGF and basic fibroblast growth factor in each preparation, while leukocyte levels affect inflammation, all leading to variable effects for each preparation.15,16Second, despite sex and age of the PRP donor not being significant factors influencing variation in growth factor concentrations, the existence of an unexplained variation in concentrations of growth factors between different donors has been observed.17 Third, the selection of activating agents, bovine thrombin or calcium chloride, and their application, whether to the elbow, shoulder, or knee, produces variability.18
While the effects of platelets and growth factors in PRP have been well studied, less is known about the effects of differing cell types. Recently it was reported that the concentrations of leukocytes directly affect the outcomes of PRP injections. McCarrel and colleagues19,20 found that as the number of leukocytes increased, there was a concomitant increase in the expression of inflammatory cytokines and catabolic activity. This effect may result in inferior healing of injured tissues and is attributed to the release of pro-inflammatory cytokines such as interleukin-1β from the leukocytes.21 There is also evidence that minimizing the catabolic effect of leukocytes may be just as important to tissue healing as the maximizing anabolic effect of platelets and growth factors.22
The use of PRP has been highly disputed in recent years due to conflicting reports of its success in treating orthopedic conditions. Numerous favorable studies have shown benefit for treating chronic and acute orthopedic injuries including; rotator cuff tear repair, chronic refractory patellar tendinopathy, and chronic lateral tendinosis/epicondylitis.23-26 Concurrently, articles demonstrating no significant effects from PRP have also been published. One study claiming that PRP injections did not improve outcomes of chronic Achilles tendinopathy did not differentiate whether patients had tendinosis or tendinitis, and did not consider leukocyte concentration in their PRP preparations27 Another study that determined PRP is not beneficial to the healing of ruptured Achilles tendons after surgical repair also failed to consider the concentration of leukocytes in their PRP preparations.28 One of the difficulties in comparing these studies is their heterogeneous nature. This arises from the use of different conditions in each study that makes the studies incomparable. Variations in PRP preparations lead to different concentrations of growth factors, platelets, and leukocyte concentrations. Additionally, tendinopathy models were not specified as tendinosis and tendonitis, and models or patients were not controlled for age, sex, or comorbidities. Given that leukocyte-rich and leukocyte-poor PRP preparations are currently widely used in clinical practice, the discovery of which type of preparation is indicated in which setting is paramount to evidence-based use of this treatment modality. Due to reports suggesting that leukocytes may be detrimental to tendon healing, determining which types of leukocytes are responsible for these effects is vital. As such, the purpose of this study is to determine the in vivo effects of sub-populations of leukocytes on normal rat tendons. This study design allowed us to isolate the effects of the injections to induce a response and remove confounding effects of normal healing response to a damaged tendon and effects from the injection itself. Our hypothesis was that the injection of leukocytes would cause an inflammatory response in rat tendons, leading to catabolic outcomes.
Continue to: METHODS...
METHODS
This was a prospective, in vivo, placebo controlled, randomized animal study. The University’s Institutional Animal Care and Use Committee approved all procedures prior to initiation. Twenty-four male Sprague-Dawley rats were randomized to 3 treatment groups (n = 8): monocytes; granulocytes, and; plasma, as a negative control.
Allogenic blood from 6 additional rats was collected into K2EDTA tubes via cardiac puncture. Allogenic, as opposed to autogenic, blood is commonly used in rat models because of low immunogenic response to blood from rats of the same strain and litter.29,30 The blood was then pooled and the red cells lysed by incubation with Red Blood Cell Lysis Buffer (Roche). The samples were then sorted into fractions containing monocytes and granulocytes using fluorescence activated cell sorting (FACS) using a FACSAria (BD Biosciences). Cells were sorted using Purified PE Mouse Anti-Rat CD11b/c antibodies (BD Pharmingen) specific to monocytes, granulocytes, macrophages, dendritic cells, and microglia, APC-Cy7 Mouse Anti-Rat CD45 antibodies (BD Pharmingen) specific to all hematopoietic cells except erythrocytes, and FITC Mouse Anti-Rat CD42d antibodies (BD Pharmingen) specific to megakaryocytes and platelets. 20 μL of 0.2 mg/mL CD11b/c, 20 μL of 0.2 mg/mL CD 45, and 10 μL of 0.5 mg/mL CD42d antibodies were added to 1 mL of condensed non-red cells collected from the 6 rats and incubated at room temperature in the dark for 15 minutes. A fraction containing only platelet-poor plasma was also collected. For all treatments the injection volume was 75 μL. Rats in the monocyte group were injected with 200,000 cells in platelet-poor plasma, those in the granulocyte group were injected with 900,000 cells in platelet-poor plasma, and rats in the plasma control group received only platelet-poor plasma. The cell concentrations were based on previous studies that documented these concentrations that are found in typical leukocyte-rich PRP preparations.13
The animals were anesthetized with isoflurane gas and then injected aseptically once into their right Achilles tendon. The left Achilles tendon was used as an un-injected control, giving a total of 48 total Achilles tendons studied. At days 7 and 14 post-injection, 4 rats from each group were euthanized and the Achilles tendons were harvested.
The tendons were fixed in neutral buffered formalin for 24 hours and then embedded in paraffin and sectioned sagittally at 12 μm. The tendons were then stained with hematoxylin and eosin (H&E) using standard histological protocols and examined by 3 individuals trained to assess cellularity and morphology. All samples were assigned unrecognizable numbers and randomized prior to examination by individuals. Cell counts were based on the number of nuclei present in 3 mid-tendon high-power fields (400x) per sample. Morphology was graded on a scale of 1 to 3, with 1 being a normal tendon and 3 having severe pathology with total loss of alignment and crimping on 3 low-power fields (100x) per sample (Figures 1A-1G).
Vascularity was assessed by immunohistochemical staining using Rabbit Polyclonal Anti-CD31 antibodies (Abcam), a marker for vascular endothelial cells, using a Vectastain ABC Kit (Vector Laboratories) system and the ImmPACT AEC Peroxidase (HRP) Substrate (Vector Laboratories). Following staining, automated image analysis was performed (Bioquant). Briefly, all areas that did not contain tendon were masked. CD31 positive areas were then quantified using global thresholding. Vascularity was then calculated as ratio of CD31 positive area to total tendon area. Analyses were performed on 3 mid-tendon medium-power (200x) fields per sample.
For cellularity and morphology, the results for the injected tendons were normalized to those of their contralateral untreated controls and reported as a percentage. Results for vascularity were compared directly between treated tendons. Differences were assessed between groups at each time-point using Independent Samples Median Tests. When significant differences were identified, pairwise comparisons were performed to identify the source of the differences. All analyses were conducted using SPSS (V22, SAS Institute) with significant differences determined for values of P < 0.05.
RESULTS
No significant differences in cellularity between groups were seen at day 7 (P = 0.368) (Figures 1A-1G). However, a significant difference in cellularity between groups was seen at day 14 (P = 0.014). Pairwise tests showed there to be a significant increase in the number of cells in the tendons treated with granulocytes from 221% and 249% in cellularity (P = 0.014) on day 14, as compared to both monocytes and plasma, respectively. Morphologically, no significant differences were seen between groups at either time-point (P = 0.091 for day 7 and P = 1.000 for day 14) (Figures 2A-2G). However, a significant improvement in morphology was observed from day 7 to day 14 in the granulocyte group from 60% to 165% (P = 0.029). Finally, no differences were seen in vascularity between treatment groups at either time-point (P = 0.368 for day 7 and P = 0.535 for day 14) (Figures 3A-3G).
Continue to: DISCUSSION...
DISCUSSION
Our hypothesis that the injection of leukocytes would cause an inflammatory response in rat tendons leading to catabolic outcomes was confirmed in the granulocyte group. It should be noted that prior to the catabolic outcome, there was a transient anabolic effect in the granulocyte group during the second week. Deterioration in morphology was observed in the tendons injected with granulocytes on day 7, which subsequently recovered in the following week. We found that injecting granulocytes into normal tendons resulted in an increase in inflammatory cellularity, when compared to monocytes and plasma injections.
Limitations inherent in this study are those similar to other in vivo studies. To begin with, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 Another limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the immunohistochemistry (IHC) and morphological data are clear, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. The increased cellularity could be due to the increased number of cells injected into the tendon; however, our conclusions are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32
In terms of morphology, we hypothesized that degenerative changes would be seen in the tendons that were injected with granulocytes due to the inflammatory action of these cells. As part of the granulocyte response, neutrophils release proteases and macrophages can stimulate collagen synthesis via fibroblasts, both causing change within the extracellular matrix.33,34 Indeed, we observed a significant change in tissue morphology in the granulocyte group over the course of 14 days. As the degenerative and regenerative effects of granulocytes take time to present, this is likely what we observed to occur between day 7 and 14 after treatment. These observations are also consistent with prior observations that leukocyte-rich PRP injections can be detrimental to tendon healing, but beneficial to tissue degeneration in the setting of chronic tendonitis.20
We hypothesized that the vascularity of the tendons would be similar in all preparations. This was based on previous studies demonstrating that the lack of platelets in the platelet-poor plasma fraction is sufficient to deplete VEGF, the angiogenic agent in PRP.35 In this study, there were no observable differences in vascularity of platelet-poor plasma, monocyte, and granulocyte injections. We attribute this to the lack of VEGF in any of these preparations. The aforementioned study also showed that the lack of platelets in injection was enough to prevent the angiogenic effect of this treatment.35
Continue to: The goal of this study was...
The goal of this study was to assess the morphology, cellularity, and vascularity of normal tendons after injections of different leukocyte populations. This is clinically important because of the potential to tailor future PRP injections on a patient-by-patient basis. In patients requiring an anabolic response, leukocyte-poor PRP may be the best option. In contrast, when patient pathology requires an inflammatory response to improve healing36 or breakdown fibrotic tissue, as seen in tendinosis, leukocyte-rich PRP may be warranted. Further, properly controlled clinical studies are needed to validate these recommendations.
Limitations inherent in this study are those similar to other in vivo studies. First, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 A second limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the IHC and morphological data show clear changes, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. The last limitation of this study is the lack of functional mechanical testing since, clinically, healing of the tendon is also related to the strength of the tendon. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. Moreover, our results are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32 It is interesting to note that the increase in inflammation does not lead to an increase in vascularity as could be expected.
CONCLUSION
We found that the injection of leukocytes into healthy rat Achilles tendons increases inflammation, as evidenced by increased cellularity and disrupted morphology, which suggests that leukocyte-rich PRP preparations may be contraindicated in settings of acute tendonitis. However, these preparations may be useful for a specific subset of tendinopathies, including chronic tendinosis.
ABSTRACT
In this study, we determine the in vivo effects of injecting sub-populations of leukocytes into normal rat Achilles tendons via a controlled laboratory study. Allogenic monocytes, granulocytes, or plasma were injected into 24 healthy rat Achilles tendons. Treated and contralateral un-treated control tendons then assessed for cellularity, histologic morphology, and vascularity after 7 and 14 days. Significant increases of 221% and 249% in cellularity (P = 0.014) were seen on day 14 within Achilles tendons injected with granulocytes as compared to plasma and monocytes, respectively. Also, significant improvement in morphology (P = 0.029) between days 7 and 14 was seen for the granulocyte injected Achilles tendons. Significant increases in cellularity after an injection of granulocytes, compared to monocytes and plasma, corresponds to a significant increase in inflammation within the tissue, suggesting that leukocyte-rich platelet-rich plasma (PRP) preparations are proinflammatory and potentially catabolic when injected into tendon tissue. The concentration and composition of white blood cells within PRP preparations is variable and needs to be better understood in order to optimize clinical utility of PRP injections.
Continue to: Tendinopathies are debilitating conditions...
Tendinopathies are debilitating conditions affecting patients worldwide every day. They arise most frequently from tendon overuse resulting in pathology.1 There are 2 major subtypes of tendinopathy: tendinosis and tendinitis. Tendinosis, the more common condition, is characterized by long-term, chronic degradation of tendon tissue resulting in fibrosis from infiltrating fibroblasts.2 Tendinitis, the less common condition, is characterized by an acute inflammatory response and inflammatory cell infiltrate.2 Both conditions are common, with Achilles tendinopathy affecting 11% of runners and lateral epicondylitis affecting 1% to 3% of the general population.3,4 Many sports-related overuse injuries, such as tendinopathies, go undiagnosed for extended periods of time because medical attention is avoided in order to prevent time loss from training or competing.5 These delays could be eliminated if a non-surgical option for treating tendon pathology was available.
Tendinopathies are believed to result from tendon overuse that causes micro-damage to collagen, as well as from significant changes in protein and enzyme composition within the tendon.6 The damage accumulates over time and eventually leads to chronic inflammation or fibrotic change within tendons, in both cases weakening the tendon and causing pain. Currently, accepted treatments for tendinopathies include: nonsteroidal anti-inflammatory drugs, physical therapy, ultrasound, laser-therapy, corticosteroids, glyceryl trinitrate patches, extracorporeal shock wave therapy, sclerotherapy, and surgery.7 Recently, platelet-rich plasma (PRP) therapy has emerged as a promising treatment for tendinopathies, as well as a variety of other orthopedic indications.
PRP consists of autologous blood from the patient, centrifuged to increase the amount of platelets in the sample above baseline, and subsequently injected around an affected tendon or joint.8 PRP is used to treat tendinopathy because it can supply injured tendons with blood components that aid in healing, which tendons do not receive due to poor vascularity.9 These components include growth factors, such as platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), endothelial growth factor, and leukocytes that can stimulate an inflammatory response within the injured tissue.10 The inflammatory response from the PRP induces a more robust reconstruction and revascularization of the injured tissue, stimulating proliferation, and remodeling.11,12However, significant variability exists within the platelets, leukocytes, and growth factors that comprise PRP. This is attributed to 3 major causes. First, current commercial preparations of PRP result in differing platelet concentrations, as well as leukocyte-rich and leukocyte-poor compositions.13,14 Variability in platelet concentrations results in unreliable amounts of growth factors, including cytokines, TGF-β, PDGF, VEGF and basic fibroblast growth factor in each preparation, while leukocyte levels affect inflammation, all leading to variable effects for each preparation.15,16Second, despite sex and age of the PRP donor not being significant factors influencing variation in growth factor concentrations, the existence of an unexplained variation in concentrations of growth factors between different donors has been observed.17 Third, the selection of activating agents, bovine thrombin or calcium chloride, and their application, whether to the elbow, shoulder, or knee, produces variability.18
While the effects of platelets and growth factors in PRP have been well studied, less is known about the effects of differing cell types. Recently it was reported that the concentrations of leukocytes directly affect the outcomes of PRP injections. McCarrel and colleagues19,20 found that as the number of leukocytes increased, there was a concomitant increase in the expression of inflammatory cytokines and catabolic activity. This effect may result in inferior healing of injured tissues and is attributed to the release of pro-inflammatory cytokines such as interleukin-1β from the leukocytes.21 There is also evidence that minimizing the catabolic effect of leukocytes may be just as important to tissue healing as the maximizing anabolic effect of platelets and growth factors.22
The use of PRP has been highly disputed in recent years due to conflicting reports of its success in treating orthopedic conditions. Numerous favorable studies have shown benefit for treating chronic and acute orthopedic injuries including; rotator cuff tear repair, chronic refractory patellar tendinopathy, and chronic lateral tendinosis/epicondylitis.23-26 Concurrently, articles demonstrating no significant effects from PRP have also been published. One study claiming that PRP injections did not improve outcomes of chronic Achilles tendinopathy did not differentiate whether patients had tendinosis or tendinitis, and did not consider leukocyte concentration in their PRP preparations27 Another study that determined PRP is not beneficial to the healing of ruptured Achilles tendons after surgical repair also failed to consider the concentration of leukocytes in their PRP preparations.28 One of the difficulties in comparing these studies is their heterogeneous nature. This arises from the use of different conditions in each study that makes the studies incomparable. Variations in PRP preparations lead to different concentrations of growth factors, platelets, and leukocyte concentrations. Additionally, tendinopathy models were not specified as tendinosis and tendonitis, and models or patients were not controlled for age, sex, or comorbidities. Given that leukocyte-rich and leukocyte-poor PRP preparations are currently widely used in clinical practice, the discovery of which type of preparation is indicated in which setting is paramount to evidence-based use of this treatment modality. Due to reports suggesting that leukocytes may be detrimental to tendon healing, determining which types of leukocytes are responsible for these effects is vital. As such, the purpose of this study is to determine the in vivo effects of sub-populations of leukocytes on normal rat tendons. This study design allowed us to isolate the effects of the injections to induce a response and remove confounding effects of normal healing response to a damaged tendon and effects from the injection itself. Our hypothesis was that the injection of leukocytes would cause an inflammatory response in rat tendons, leading to catabolic outcomes.
Continue to: METHODS...
METHODS
This was a prospective, in vivo, placebo controlled, randomized animal study. The University’s Institutional Animal Care and Use Committee approved all procedures prior to initiation. Twenty-four male Sprague-Dawley rats were randomized to 3 treatment groups (n = 8): monocytes; granulocytes, and; plasma, as a negative control.
Allogenic blood from 6 additional rats was collected into K2EDTA tubes via cardiac puncture. Allogenic, as opposed to autogenic, blood is commonly used in rat models because of low immunogenic response to blood from rats of the same strain and litter.29,30 The blood was then pooled and the red cells lysed by incubation with Red Blood Cell Lysis Buffer (Roche). The samples were then sorted into fractions containing monocytes and granulocytes using fluorescence activated cell sorting (FACS) using a FACSAria (BD Biosciences). Cells were sorted using Purified PE Mouse Anti-Rat CD11b/c antibodies (BD Pharmingen) specific to monocytes, granulocytes, macrophages, dendritic cells, and microglia, APC-Cy7 Mouse Anti-Rat CD45 antibodies (BD Pharmingen) specific to all hematopoietic cells except erythrocytes, and FITC Mouse Anti-Rat CD42d antibodies (BD Pharmingen) specific to megakaryocytes and platelets. 20 μL of 0.2 mg/mL CD11b/c, 20 μL of 0.2 mg/mL CD 45, and 10 μL of 0.5 mg/mL CD42d antibodies were added to 1 mL of condensed non-red cells collected from the 6 rats and incubated at room temperature in the dark for 15 minutes. A fraction containing only platelet-poor plasma was also collected. For all treatments the injection volume was 75 μL. Rats in the monocyte group were injected with 200,000 cells in platelet-poor plasma, those in the granulocyte group were injected with 900,000 cells in platelet-poor plasma, and rats in the plasma control group received only platelet-poor plasma. The cell concentrations were based on previous studies that documented these concentrations that are found in typical leukocyte-rich PRP preparations.13
The animals were anesthetized with isoflurane gas and then injected aseptically once into their right Achilles tendon. The left Achilles tendon was used as an un-injected control, giving a total of 48 total Achilles tendons studied. At days 7 and 14 post-injection, 4 rats from each group were euthanized and the Achilles tendons were harvested.
The tendons were fixed in neutral buffered formalin for 24 hours and then embedded in paraffin and sectioned sagittally at 12 μm. The tendons were then stained with hematoxylin and eosin (H&E) using standard histological protocols and examined by 3 individuals trained to assess cellularity and morphology. All samples were assigned unrecognizable numbers and randomized prior to examination by individuals. Cell counts were based on the number of nuclei present in 3 mid-tendon high-power fields (400x) per sample. Morphology was graded on a scale of 1 to 3, with 1 being a normal tendon and 3 having severe pathology with total loss of alignment and crimping on 3 low-power fields (100x) per sample (Figures 1A-1G).
Vascularity was assessed by immunohistochemical staining using Rabbit Polyclonal Anti-CD31 antibodies (Abcam), a marker for vascular endothelial cells, using a Vectastain ABC Kit (Vector Laboratories) system and the ImmPACT AEC Peroxidase (HRP) Substrate (Vector Laboratories). Following staining, automated image analysis was performed (Bioquant). Briefly, all areas that did not contain tendon were masked. CD31 positive areas were then quantified using global thresholding. Vascularity was then calculated as ratio of CD31 positive area to total tendon area. Analyses were performed on 3 mid-tendon medium-power (200x) fields per sample.
For cellularity and morphology, the results for the injected tendons were normalized to those of their contralateral untreated controls and reported as a percentage. Results for vascularity were compared directly between treated tendons. Differences were assessed between groups at each time-point using Independent Samples Median Tests. When significant differences were identified, pairwise comparisons were performed to identify the source of the differences. All analyses were conducted using SPSS (V22, SAS Institute) with significant differences determined for values of P < 0.05.
RESULTS
No significant differences in cellularity between groups were seen at day 7 (P = 0.368) (Figures 1A-1G). However, a significant difference in cellularity between groups was seen at day 14 (P = 0.014). Pairwise tests showed there to be a significant increase in the number of cells in the tendons treated with granulocytes from 221% and 249% in cellularity (P = 0.014) on day 14, as compared to both monocytes and plasma, respectively. Morphologically, no significant differences were seen between groups at either time-point (P = 0.091 for day 7 and P = 1.000 for day 14) (Figures 2A-2G). However, a significant improvement in morphology was observed from day 7 to day 14 in the granulocyte group from 60% to 165% (P = 0.029). Finally, no differences were seen in vascularity between treatment groups at either time-point (P = 0.368 for day 7 and P = 0.535 for day 14) (Figures 3A-3G).
Continue to: DISCUSSION...
DISCUSSION
Our hypothesis that the injection of leukocytes would cause an inflammatory response in rat tendons leading to catabolic outcomes was confirmed in the granulocyte group. It should be noted that prior to the catabolic outcome, there was a transient anabolic effect in the granulocyte group during the second week. Deterioration in morphology was observed in the tendons injected with granulocytes on day 7, which subsequently recovered in the following week. We found that injecting granulocytes into normal tendons resulted in an increase in inflammatory cellularity, when compared to monocytes and plasma injections.
Limitations inherent in this study are those similar to other in vivo studies. To begin with, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 Another limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the immunohistochemistry (IHC) and morphological data are clear, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. The increased cellularity could be due to the increased number of cells injected into the tendon; however, our conclusions are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32
In terms of morphology, we hypothesized that degenerative changes would be seen in the tendons that were injected with granulocytes due to the inflammatory action of these cells. As part of the granulocyte response, neutrophils release proteases and macrophages can stimulate collagen synthesis via fibroblasts, both causing change within the extracellular matrix.33,34 Indeed, we observed a significant change in tissue morphology in the granulocyte group over the course of 14 days. As the degenerative and regenerative effects of granulocytes take time to present, this is likely what we observed to occur between day 7 and 14 after treatment. These observations are also consistent with prior observations that leukocyte-rich PRP injections can be detrimental to tendon healing, but beneficial to tissue degeneration in the setting of chronic tendonitis.20
We hypothesized that the vascularity of the tendons would be similar in all preparations. This was based on previous studies demonstrating that the lack of platelets in the platelet-poor plasma fraction is sufficient to deplete VEGF, the angiogenic agent in PRP.35 In this study, there were no observable differences in vascularity of platelet-poor plasma, monocyte, and granulocyte injections. We attribute this to the lack of VEGF in any of these preparations. The aforementioned study also showed that the lack of platelets in injection was enough to prevent the angiogenic effect of this treatment.35
Continue to: The goal of this study was...
The goal of this study was to assess the morphology, cellularity, and vascularity of normal tendons after injections of different leukocyte populations. This is clinically important because of the potential to tailor future PRP injections on a patient-by-patient basis. In patients requiring an anabolic response, leukocyte-poor PRP may be the best option. In contrast, when patient pathology requires an inflammatory response to improve healing36 or breakdown fibrotic tissue, as seen in tendinosis, leukocyte-rich PRP may be warranted. Further, properly controlled clinical studies are needed to validate these recommendations.
Limitations inherent in this study are those similar to other in vivo studies. First, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 A second limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the IHC and morphological data show clear changes, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. The last limitation of this study is the lack of functional mechanical testing since, clinically, healing of the tendon is also related to the strength of the tendon. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. Moreover, our results are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32 It is interesting to note that the increase in inflammation does not lead to an increase in vascularity as could be expected.
CONCLUSION
We found that the injection of leukocytes into healthy rat Achilles tendons increases inflammation, as evidenced by increased cellularity and disrupted morphology, which suggests that leukocyte-rich PRP preparations may be contraindicated in settings of acute tendonitis. However, these preparations may be useful for a specific subset of tendinopathies, including chronic tendinosis.
1. Herring SA, Nilson KL. Introduction to overuse injuries. Clin Sports Med. 1987;6(2):225-239.
2. Bass E. Tendinopathy: why the difference between tendinitis and tendinosis matters. Int J Ther Massage Bodywork. 2012;5(1):14-17.
3. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
4. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
5. Bahr R. No injuries, but plenty of pain? On the methodology for recording overuse symptoms in sports. Br J Sports Med. 2009;43(13):966-972.
6. Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37(9):1855-1867.
7. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539-1554.
8. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602-608.
9. Smith JW. Blood Supply of Tendons. Am J Surg. 1965;109:272-276.
10. Wu PI, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825-853.
11. Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R. 2011;3(3):226-250.
12. Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S-32e-S.
13. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316.
14. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40(8):1742-1749.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271.
16. Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31(3):212-218.
17. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97-102.
18. Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21(4):344-352.
19. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27(8):1033-1042.
20. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94(19):e143(141-148).
21. Pillitteri D, Bassus S, Boller K, et al. Thrombin-induced interleukin 1beta synthesis in platelet suspensions: impact of contaminating leukocytes. Platelets. 2007;18(2):119-127.
22. Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42(1):42-49.
23. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
24. Maniscalco P, Gambera D, Lunati A, et al. The "Cascade" membrane: a new PRP device for tendon ruptures. Description and case report on rotator cuff tendon. Acta Biomed. 2008;79(3):223-226.
25. Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper's knee. Int Orthop. 2010;34(6):909-915.
26. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
27. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
28. Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39(1):38-47.
29. Welsh KI, Burgos H, Batchelor JR. The immune response to allogeneic rat platelets; Ag-B antigens in matrix form lacking Ia. Eur J Immunol. 1977;7(5):267-272.
30. Xue M, Del Bigio MR. Intracortical hemorrhage injury in rats : relationship between blood fractions and brain cell death. Stroke. 2000;31(7):1721-1727.
31. Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47-71.
32. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140.
33. Palmgren MS, deShazo RD, Carter RM, Zimny ML, Shah SV. Mechanisms of neutrophil damage to human alveolar extracellular matrix: the role of serine and metalloproteases. J Allergy Clin Immunol. 1992;89(4):905-915.
34. Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170(3):727-737.
35. Zhou Y, Zhang J, Wu H, Hogan MV, Wang JH. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173.
36. Su B, O'Connor JP. NSAID therapy effects on healing of bone, tendon, and the enthesis. J Appl Physiol (1985). 2013;115(6):892-899.
1. Herring SA, Nilson KL. Introduction to overuse injuries. Clin Sports Med. 1987;6(2):225-239.
2. Bass E. Tendinopathy: why the difference between tendinitis and tendinosis matters. Int J Ther Massage Bodywork. 2012;5(1):14-17.
3. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
4. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
5. Bahr R. No injuries, but plenty of pain? On the methodology for recording overuse symptoms in sports. Br J Sports Med. 2009;43(13):966-972.
6. Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37(9):1855-1867.
7. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539-1554.
8. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602-608.
9. Smith JW. Blood Supply of Tendons. Am J Surg. 1965;109:272-276.
10. Wu PI, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825-853.
11. Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R. 2011;3(3):226-250.
12. Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S-32e-S.
13. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316.
14. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40(8):1742-1749.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271.
16. Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31(3):212-218.
17. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97-102.
18. Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21(4):344-352.
19. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27(8):1033-1042.
20. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94(19):e143(141-148).
21. Pillitteri D, Bassus S, Boller K, et al. Thrombin-induced interleukin 1beta synthesis in platelet suspensions: impact of contaminating leukocytes. Platelets. 2007;18(2):119-127.
22. Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42(1):42-49.
23. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
24. Maniscalco P, Gambera D, Lunati A, et al. The "Cascade" membrane: a new PRP device for tendon ruptures. Description and case report on rotator cuff tendon. Acta Biomed. 2008;79(3):223-226.
25. Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper's knee. Int Orthop. 2010;34(6):909-915.
26. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
27. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
28. Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39(1):38-47.
29. Welsh KI, Burgos H, Batchelor JR. The immune response to allogeneic rat platelets; Ag-B antigens in matrix form lacking Ia. Eur J Immunol. 1977;7(5):267-272.
30. Xue M, Del Bigio MR. Intracortical hemorrhage injury in rats : relationship between blood fractions and brain cell death. Stroke. 2000;31(7):1721-1727.
31. Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47-71.
32. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140.
33. Palmgren MS, deShazo RD, Carter RM, Zimny ML, Shah SV. Mechanisms of neutrophil damage to human alveolar extracellular matrix: the role of serine and metalloproteases. J Allergy Clin Immunol. 1992;89(4):905-915.
34. Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170(3):727-737.
35. Zhou Y, Zhang J, Wu H, Hogan MV, Wang JH. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173.
36. Su B, O'Connor JP. NSAID therapy effects on healing of bone, tendon, and the enthesis. J Appl Physiol (1985). 2013;115(6):892-899.
TAKE-HOME POINTS
- Injection of leukocytes into healthy rat Achilles tendons increases inflammation.
- Injection of leukocytes into healthy rat Achilles tendons does not affect vascularity.
- Leukocyte-rich PRP preparations may be contraindicated in settings of acute tendonitis.
- Leukocyte-rich PRP preparations may be useful for chronic tendinosis.
- The concentration and composition of white blood cells within PRP preparations is variable and needs to be better understood in order to optimize clinical utility of PRP injections.
Complex Ankle and Hindfoot Arthrodesis Using Circular External Fixation
ABSTRACT
Surgical reconstruction of the ankle and hindfoot in patients with diabetes, Charcot neuroarthropathy, osteomyelitis, deformity, and/or bone loss can be challenging and often results in amputation. In these patients, conventional internal fixation with plates, screws, and intramedullary nails is often not feasible because of ongoing infection or poor bone stock and soft tissue quality. The Ilizarov method of ankle and hindfoot arthrodesis is a well-established technique for limb reconstruction that uses circular external fixation to achieve solid bony fusion, optimal leg length, and eradication of infection in cases of complex pathology. This article discusses indications, contraindications, pearls, and pitfalls of performing ankle and hindfoot arthrodesis using the Ilizarov technique.
Continue to: Patients with complex ankle and hindfoot deformity...
Patients with complex ankle and hindfoot deformity present a unique challenge to both nonoperative management and surgical reconstruction. Nonoperative management focuses on wound care, bracing, and immobilization using ankle-foot orthoses, total contact casts, and Charcot restraint orthotic walker boots for external stabilization. Fusion using the Ilizarov technique with circular fixation is a salvage limb-preservation procedure that has shown good results in select patient populations.1-5 Indications include post-traumatic, degenerative, and rheumatoid arthritis, osteomyelitis, tumors, neuromuscular conditions, and salvage of failed ankle and hindfoot procedures.6-9 Relative contraindications include wet gangrene, severe limb ischemia, and soft tissue compromise requiring urgent amputation. In addition, circular frames are not recommended in patients who are unable to comply with postoperative restrictions, and pin and wire care for the duration of frame placement because of personal, psychological, or socioeconomic reasons.
The Ilizarov technique of ring fixation provides dynamic, modular, and rigid fixation in multiple planes to control shear, bending, and rotational forces, and allows for early weight-bearing and postoperative adjustments as needed.10,11 Percutaneously placed half-pins and wires allow for solid fixation in the setting of both poor bone and soft tissue quality, and fusion can be achieved in the presence of active infection in a 1-stage procedure. The goal of ankle and hindfoot fusion using the Ilizarov technique is to achieve an infection-free, stable, plantigrade foot with neutral ankle alignment to allow for patient ambulation and return to activities of daily living.
Nonunion rates with circular fixation are reported to be as high as 16% to 54%, due to medical comorbidities, such as smoking, peripheral vascular disease, and Charcot neuroarthropathy.1Charcot, in particular, is a risk factor for nonunion as patients lack protective sensation, and have a higher rate of wound dehiscence, noncompliance with weight-bearing precautions, pin site infections, and frame breakage. In these patients, tibiotalocalcaneal (TTC) arthrodesis is preferred over the isolated ankle, or subtalar fusion to both provide a stable platform for ambulation and reduce the incidence of adjacent joint breakdown. Common complications of the Ilizarov technique include pin site infections, wire breakage, talar necrosis, and tibial stress fractures after frame removal.1,2,6,11-13 Circular frames are typically maintained for 3 to 8 months, until solid fusion is achieved radiographically. Frames are removed in the operating room with the concurrent examination of the fusion sites under anesthesia followed by a period of protected weight-bearing in a cast or tall controlled ankle motion (CAM) boot.
This article reviews several technical details, tips, and tricks that can help improve the intraoperative and postoperative outcomes of combined ankle and hindfoot arthrodesis using the Ilizarov technique with circular external fixation.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
SETUP AND APPROACH
Patients are positioned supine with padding under the operative extremity to achieve neutral leg rotation (Figures 1A-1D). A thigh tourniquet is placed with the foot positioned at the end of the bed and on top of the radiolucent padding to avoid interference of the contralateral leg during lateral X-rays. After sterile prepping and draping, the extremity is exsanguinated above the level of an active infection, and the tourniquet inflated.
For isolated ankle arthrodesis, an anterior or lateral approach can be used, while for TTC arthrodesis, a lateral approach is required to access both the ankle and subtalar joints. A 10-cm longitudinal incision is made along the distal fibula, curving slightly and anteriorly along the distal extent of the incision. Dissection is continued down to bone using full thickness flaps, and the distal fibula is removed 2 to 3 cm above the ankle joint using a saw and osteotome (Figures 2A-2G). The distal fibula can be used subsequently as bone grafts depending on the quality of bone. The peroneal tendons are retracted posteriorly, and dissection is then continued to the posterior facet of the subtalar joint.
JOINT PREPARATION AND ALIGNMENT
Both the anterior and posterior neurovascular bundles are protected along the distal tibia with Hohmann retractors while a saw is used to create flat cuts across the tibial plafond and talus to allow apposition of flat, broad cancellous bony surfaces. Flat cuts followed by later joint compression will often shorten the limb by 2 to 3 cm. This leg length discrepancy can later be accommodated using a shoe lift, as needed. All retained hardware and/or infected and necrotic tissues in the ankle and hindfoot are removed using a rongeur and a pituitary rongeur.
The medial malleolus is osteotomized vertically using a direct medial incision and approach with full thickness flaps, and in line with the previous tibial plafond, is both cut and removed. The medial malleolus can also be used for bone grafts in fusion sites. A smooth-tip lamina spreader is placed in the subtalar joint for distraction and a curved osteotome, curettes, and a small rongeur are used to remove all remaining cartilage from the subtalar joint. Flat cuts in the subtalar joint can remove excessive bone, particularly from the inferior aspect of the talus. The subchondral bone is perforated using a 2.5- to 3.0-mm drill bit and a curved osteotome.
A bone graft from the distal fibula and medial malleolus, with or without the addition of allograft adjuvants, is placed evenly across the ankle and subtalar joints (Figures 3A-3E). At this point, the ankle and subtalar joints can be manipulated in multiple planes to achieve neutral coronal, sagittal, and axial alignment. With both the ankle and hindfoot held in a neutral position, multiple Steinman pins and K-wires in different orientations are inserted through the plantar aspect of the heel to hold the ankle and subtalar joints in place temporarily. Wires are cut short to prevent interference with subsequent foot olive wire placement through the frame.
Continue to: X-rays should be carefully checked...
X-rays should be carefully checked to ensure proper alignment. Wounds are gently irrigated, and vancomycin powder (2 g) can be placed within wounds for local antibiotic delivery. Lateral tissues are sharply debulked to allow for decreased tension on the incision, and small ulcers can be excised in their entirety. Wounds are closed in a layered fashion using 0-polydioxanone (PDS, Ethicon) suture for deep tissue, 2-0 PDS for subcutaneous tissue, and 2-0 nylon for skin closure. The tourniquet is deflated for the remainder of the case to reduce limb ischemia during frame placement.
CIRCULAR FRAME CONCEPTS AND PLACEMENT
The majority of circular frames for both ankle and hindfoot fusion have multiple ring sizes available in aluminum and radiolucent carbon fiber reinforced polymer (Hoffmann LRF, Stryker). Rings are available in full, open, segment, and both short- and long-foot options. Frames can be sterilized in a prebuilt 3 to 4 ring construct with 4 static or dynamic (telescopic) struts (100-277 mm). The most commonly used tibia and foot ring sizes are 155 cm, 180 cm, and 210 cm. Ring size should be able to accommodate posterior soft tissue swelling and avoid circumferential soft tissue abrasion against the rings. Anterior foot arches are used for increased construct stability and can be locked to the distal tibia ring for weight-bearing support. Wire and half-pin bolts, adaptors, and nuts are used to join each ring of the frame to the patient’s bone.
For TTC arthrodesis, 2 rings are typically used in the tibia, and 1 ring is used in the foot. For isolated ankle arthrodesis, an additional ring can be added with olive wires in the talus to permit compression only across the ankle joint. Multiple points of fixation are used in each ring in different planes to achieve both maximal stability and rotational control. If a single wire or half-pin becomes infected and requires removal, there are still multiple other points of fixation in the ring to maintain stability. Fixation within each ring should be off axis compared with the adjacent ring to both avoid stress risers and increase construct rigidity.
The prebuilt frame is checked on the back table to ensure proper orientation and component alignment. The frame is then placed over both the foot and ankle, and multiple stacks of towels are placed behind the heel, ankle, and calf to center the foot and ankle in the frame (Figures 4A-4F). At least 4 to 6 cm of space is needed in between the posterior soft tissues and each ring to accommodate postoperative swelling. On the lateral view, the foot ring should be in the mid-portion of the calcaneus. If there is a concern, particularly in Charcot patients, regarding early weight-bearing noncompliance, the foot ring can be placed flush with the plantar aspect of the foot, and olive wires can be inserted using longer adaptors. The frame should be checked from multiple viewpoints to ensure that both the foot and ankle are centered and in neutral rotation.
Continue to: TIBIA RING FIXATION...
TIBIA RING FIXATION
Tibia rings can be fixed using 2 to 3 half-pins (4-6 mm) alone or 2 half-pins in combination with a smooth wire. A small incision is made over the area of planned half-pin insertion, and the periosteum is cleared away using a hemostat. An adaptor sleeve is used, and the bone is drilled bicortically, followed by insertion of the half-pin. Hydroxyapatite-coated pins are used to improve the strength of the bone-pin interface and reduce the incidence of pin tract infections. Pins are inserted along both the anterior and medial aspects of the tibia, avoiding the thick lateral musculature. Care is taken to protect the medial neurovascular structures during pin placement following established Ilizarov safe zones.
After each pin is placed in the bone, the pin is secured to the adaptor that is then tightened to the ring. This process is repeated for both the proximal and distal tibia rings. Pins should be placed above and below each ring to avoid creating stress risers. During smooth wire placement, each wire is pushed by hand through the soft tissues and then drilled into the bone while the exposed segment is held with a damp sponge to reduce the incidence of thermal bone necrosis. Once the wire is drilled bicortically, a mallet is used to tap the wire through the remaining soft tissues to avoid wrapping them up in the wire. Each wire should be parallel to the ring to get an even line of compression.
Each wire is secured on 1 end and then tensioned to 130 kg using a hand tensioner. An additional tool can be placed in the wire adaptor to prevent the wire from bending during tensioning. If the wire is passing above or below the ring, longer wire adaptors should be used to build to the wire. The wire should never be bent toward the ring as this can increase the likelihood of improper pin tensioning and breakage. Wire placement should be avoided posteriorly as this can make it difficult to secure and/or tension wires, and also increases the risk of damage to posterior structures.
Ring fixation in the distal tibia near the plafond may require 1 half-pin and 2 wires to avoid damage to the tibialis anterior and posterior tibial tendons. In this case, smooth wires should be placed in a crossing pattern and tensioned simultaneously to avoid pulling the ankle away from the center of the frame. Wires should be bent and curved over each ring and then cut to facilitate subsequent removal.
FOOT RING FIXATION
In the foot, olive wires are used to increase fixation against bone. For each olive wire, a small incision is made to accommodate the diameter of the olive through the soft tissue. Similar to the distal tibia, 2 olive wires should be placed above and below the foot ring in a crossing pattern through the calcaneus (Figures 5A-5F). The axial view of the frame should be checked to ensure proper wire orientation. When using olive wires, it is essential to tension both at the same time to 90 kg, as the foot can be pulled medially or laterally in the frame if 1 wire is tensioned before the other.
Forefoot olive wires should also be placed in a crossing pattern, with 1 wire fixed through the first, second, and third metatarsals, and 1 wire through the fourth and fifth metatarsals. Additional forefoot olive wires can be placed if compression is needed across the midfoot or Chopart joints for fusion. Multiple X-rays should be checked to ensure that the calcaneus and forefoot olive wires are firmly fixed both in and against bone.
Continue to: JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS...
JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS
Once all rings are secured to the bone with half-pins and wires, the previously placed Steinman pins, and K-wires through the heel are removed. Both ankle and subtalar joint alignments are rechecked, and then axial compression is placed through the foot ring with the knee extended and the struts unlocked. Static or telescopic struts are used to achieve 8 to 10 mm of bony compression. X-rays are taken before and after to analyze final joint compression and alignment. Struts should be sequentially tightened (1/2 turn of a static strut) 1 at a time as final tightening of 1 strut alone can bind and interfere with both the compression and tightening of the remaining struts.
Once final compression is achieved, the struts are locked, and the front foot arch is closed anteriorly and connected to the distal tibia ring for increased stability (Figures 6A-6D). Each pin and wire is covered in a sterile dressing followed by gauze to allow for soft tissue padding. The entire frame is then overwrapped in bias stockinette rolls or ace wraps.
Walking attachments can be added immediately to the frame that allows for early weight-bearing. Rocker shoe attachments with a 15° anterior and posterior slope and rubber soles can help offload the ankle and subtalar joints, decrease pressure on heel strike, and reduce ankle motion during ambulation (Hoffmann LRF, Stryker).
POSTOPERATIVE PROTOCOL
Depending on individual characteristics, patients can be immediately weight-bearing in the circular frame. Patients with Charcot neuroarthropathy are recommended to remain non-weight-bearing for the first 2 months to reduce the likelihood of pin, wire, and frame breakage along with nonunion. Pin and wire site care and maintenance are initiated the day after surgery and continue on a daily basis for the duration of frame placement. Sutures are removed 4 to 5 weeks after surgery to ensure adequate wound healing. Serial X-rays are taken monthly to analyze fusion sites.
If pins or wires become infected, patients are placed on oral antibiotics, and both pins and wires can be removed or exchanged in the operating room. Once fusion is achieved in 3 to 8 months (Figures 7A-7C), the frame is removed in the operating room, and fusion sites are examined under dynamic fluoroscopy. If fusion is confirmed, patients are made weight-bearing as tolerated in a short-leg cast or tall CAM boot for 6 to 8 weeks, and then transitioned to an ankle brace in an accommodative shoe.
Continue to: DISCUSSION...
DISCUSSION
A key aspect of recovery after ankle and hindfoot fusion using the Ilizarov technique is balancing pin care, soft tissue swelling, and weight-bearing status. The average time patients will spend in the frame is approximately 25 to 28 weeks, but can range from 12 to 84 weeks.1,2Given the considerable variability in both soft tissue healing and bony union, patients should be extensively counseled before surgery to set expectations correctly and ensure that they have the necessary help and support to care for the frame during the treatment period. Patients should be followed closely during the first 6 weeks to ensure that pins and wires do not become infected or break, as both of these issues require immediate intervention.
In a review of 11 patients who underwent tibiocalcaneal arthrodesis using an Ilizarov external fixator for infected talar nonunions or extrusions, Rochman and colleagues8 reported an 81% rate of successful fusion with a final mean American Orthopaedic Foot and Ankle Society score of 65 (out of a maximum 86). Similar results were reported by Saltzman9 in a series of 8 patients with diffuse ankle osteomyelitis treated with resection of all infected tissue and hybrid-frame compression arthrodesis. All patients received 6 weeks of intravenous antibiotics, and frames were removed at 3 months, and walking casts were applied for 1 to 2 additional months. Ankle sepsis was eradicated in all patients, and 7/8 (87.5%) ankles successfully fused at an average of 13.5 weeks (range, 10-16 weeks). One limb required below-knee amputation at 5 weeks due to non-reconstructible vascular insufficiency. At an average of 3.4-year follow-up, none of the 7 fused ankles required further surgery.
Fragomen and colleagues1 retrospectively reviewed 101 patients who underwent complex ankle fusion using the Ilizarov technique and found that 76/91 (83.5%) patients achieved fusion at an average of 25 weeks (range, 10-65 weeks). Smoking was associated with a 54% rate of nonunion and 15/19 (79%) patients with Charcot neuroarthropathy achieved ankle fusion, but had a subsequent subtalar joint failure, thus highlighting the need for TTC arthrodesis in Charcot patients. Salem and colleagues2 reviewed 21 Ilizarov ankle fusions and reported that all patients achieved fusion at an average of 28 weeks (range, 12-84 weeks). Complications occurred in 11 patients, including 2 nonunions that healed after revision frame application and 4 pin tract infections.
CONCLUSION
Overall, the Ilizarov technique using circular external fixation is a powerful tool that can be used to treat a variety of disorders including complex foot and ankle deformity and infection. While case series generally show favorable outcomes, patients must be informed that this technique is a salvage procedure for limb preservation that requires meticulous operative technique, diligent postoperative care, and tight control of medical comorbidities, such as blood sugar levels in individuals with diabetes to achieve a successful outcome.
1. Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470(10):2864-2873. doi:10.1007/s11999-012-2470-9.
2. Salem KH, Kinzl L, Schmelz A. Ankle arthrodesis using Ilizarov ring fixators: a review of 22 cases. Foot Ankle Int. 2006;27(10):764-770. doi:10.1177/107110070602701002.
3. Cierny G 3rd, Cook WG, Mader JT. Ankle arthrodesis in the presence of ongoing sepsis. Indications, methods, and results. Orthop Clin North Am. 1989;20(4):709-721.
4. Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30(11):1065-1070. doi:10.3113/FAI.2009.1065.
5. Eylon S, Porat S, Bor N, Leibner ED. Outcome of Ilizarov ankle arthrodesis. Foot Ankle Int. 2007;28(8):873-879. doi:10.3113/FAI.2007.0873.
6. Kalish S, Fleming J, Weinstein R. External fixators for elective rearfoot and ankle arthrodesis. Techniques and indications. Clin Podiatr Med Surg. 2003;20(1):65-96, vi.
7. Kollig E, Esenwein SA, Muhr G, Kutscha-Lissberg F. Fusion of the septic ankle: experience with 15 cases using hybrid external fixation. J Trauma. 2003;55(4):685-691. doi:10.1097/01.TA.0000051933.83342.E4.
8. Rochman R, Jackson Hutson J, Alade O. Tibiocalcaneal arthrodesis using the Ilizarov technique in the presence of bone loss and infection of the talus. Foot Ankle Int. 2008;29(10):1001-1008. doi:10.3113/FAI.2008.1001.
9. Saltzman CL. Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J. 2005;2547-52.
10. Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007;3(1):13-29. doi:10.1007/s11420-006-9025-0.
11. Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;(303):217-225.
12. Jones CP, Youngblood CS, Waldrop N, Davis WH, Pinzur MS. Tibial Stress Fracture Secondary to Half-Pins in Circular Ring External Fixation for Charcot Foot. Foot Ankle Int. 2014;35(6):572-577. doi:10.1177/1071100714531229.
13. Kazmers NH, Fragomen AT, Rozbruch SR. Prevention of pin site infection in external fixation: a review of the literature. Strategies Trauma Limb Reconstr. 2016;11(2):75-85. doi:10.1007/s11751-016-0256-4.
ABSTRACT
Surgical reconstruction of the ankle and hindfoot in patients with diabetes, Charcot neuroarthropathy, osteomyelitis, deformity, and/or bone loss can be challenging and often results in amputation. In these patients, conventional internal fixation with plates, screws, and intramedullary nails is often not feasible because of ongoing infection or poor bone stock and soft tissue quality. The Ilizarov method of ankle and hindfoot arthrodesis is a well-established technique for limb reconstruction that uses circular external fixation to achieve solid bony fusion, optimal leg length, and eradication of infection in cases of complex pathology. This article discusses indications, contraindications, pearls, and pitfalls of performing ankle and hindfoot arthrodesis using the Ilizarov technique.
Continue to: Patients with complex ankle and hindfoot deformity...
Patients with complex ankle and hindfoot deformity present a unique challenge to both nonoperative management and surgical reconstruction. Nonoperative management focuses on wound care, bracing, and immobilization using ankle-foot orthoses, total contact casts, and Charcot restraint orthotic walker boots for external stabilization. Fusion using the Ilizarov technique with circular fixation is a salvage limb-preservation procedure that has shown good results in select patient populations.1-5 Indications include post-traumatic, degenerative, and rheumatoid arthritis, osteomyelitis, tumors, neuromuscular conditions, and salvage of failed ankle and hindfoot procedures.6-9 Relative contraindications include wet gangrene, severe limb ischemia, and soft tissue compromise requiring urgent amputation. In addition, circular frames are not recommended in patients who are unable to comply with postoperative restrictions, and pin and wire care for the duration of frame placement because of personal, psychological, or socioeconomic reasons.
The Ilizarov technique of ring fixation provides dynamic, modular, and rigid fixation in multiple planes to control shear, bending, and rotational forces, and allows for early weight-bearing and postoperative adjustments as needed.10,11 Percutaneously placed half-pins and wires allow for solid fixation in the setting of both poor bone and soft tissue quality, and fusion can be achieved in the presence of active infection in a 1-stage procedure. The goal of ankle and hindfoot fusion using the Ilizarov technique is to achieve an infection-free, stable, plantigrade foot with neutral ankle alignment to allow for patient ambulation and return to activities of daily living.
Nonunion rates with circular fixation are reported to be as high as 16% to 54%, due to medical comorbidities, such as smoking, peripheral vascular disease, and Charcot neuroarthropathy.1Charcot, in particular, is a risk factor for nonunion as patients lack protective sensation, and have a higher rate of wound dehiscence, noncompliance with weight-bearing precautions, pin site infections, and frame breakage. In these patients, tibiotalocalcaneal (TTC) arthrodesis is preferred over the isolated ankle, or subtalar fusion to both provide a stable platform for ambulation and reduce the incidence of adjacent joint breakdown. Common complications of the Ilizarov technique include pin site infections, wire breakage, talar necrosis, and tibial stress fractures after frame removal.1,2,6,11-13 Circular frames are typically maintained for 3 to 8 months, until solid fusion is achieved radiographically. Frames are removed in the operating room with the concurrent examination of the fusion sites under anesthesia followed by a period of protected weight-bearing in a cast or tall controlled ankle motion (CAM) boot.
This article reviews several technical details, tips, and tricks that can help improve the intraoperative and postoperative outcomes of combined ankle and hindfoot arthrodesis using the Ilizarov technique with circular external fixation.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
SETUP AND APPROACH
Patients are positioned supine with padding under the operative extremity to achieve neutral leg rotation (Figures 1A-1D). A thigh tourniquet is placed with the foot positioned at the end of the bed and on top of the radiolucent padding to avoid interference of the contralateral leg during lateral X-rays. After sterile prepping and draping, the extremity is exsanguinated above the level of an active infection, and the tourniquet inflated.
For isolated ankle arthrodesis, an anterior or lateral approach can be used, while for TTC arthrodesis, a lateral approach is required to access both the ankle and subtalar joints. A 10-cm longitudinal incision is made along the distal fibula, curving slightly and anteriorly along the distal extent of the incision. Dissection is continued down to bone using full thickness flaps, and the distal fibula is removed 2 to 3 cm above the ankle joint using a saw and osteotome (Figures 2A-2G). The distal fibula can be used subsequently as bone grafts depending on the quality of bone. The peroneal tendons are retracted posteriorly, and dissection is then continued to the posterior facet of the subtalar joint.
JOINT PREPARATION AND ALIGNMENT
Both the anterior and posterior neurovascular bundles are protected along the distal tibia with Hohmann retractors while a saw is used to create flat cuts across the tibial plafond and talus to allow apposition of flat, broad cancellous bony surfaces. Flat cuts followed by later joint compression will often shorten the limb by 2 to 3 cm. This leg length discrepancy can later be accommodated using a shoe lift, as needed. All retained hardware and/or infected and necrotic tissues in the ankle and hindfoot are removed using a rongeur and a pituitary rongeur.
The medial malleolus is osteotomized vertically using a direct medial incision and approach with full thickness flaps, and in line with the previous tibial plafond, is both cut and removed. The medial malleolus can also be used for bone grafts in fusion sites. A smooth-tip lamina spreader is placed in the subtalar joint for distraction and a curved osteotome, curettes, and a small rongeur are used to remove all remaining cartilage from the subtalar joint. Flat cuts in the subtalar joint can remove excessive bone, particularly from the inferior aspect of the talus. The subchondral bone is perforated using a 2.5- to 3.0-mm drill bit and a curved osteotome.
A bone graft from the distal fibula and medial malleolus, with or without the addition of allograft adjuvants, is placed evenly across the ankle and subtalar joints (Figures 3A-3E). At this point, the ankle and subtalar joints can be manipulated in multiple planes to achieve neutral coronal, sagittal, and axial alignment. With both the ankle and hindfoot held in a neutral position, multiple Steinman pins and K-wires in different orientations are inserted through the plantar aspect of the heel to hold the ankle and subtalar joints in place temporarily. Wires are cut short to prevent interference with subsequent foot olive wire placement through the frame.
Continue to: X-rays should be carefully checked...
X-rays should be carefully checked to ensure proper alignment. Wounds are gently irrigated, and vancomycin powder (2 g) can be placed within wounds for local antibiotic delivery. Lateral tissues are sharply debulked to allow for decreased tension on the incision, and small ulcers can be excised in their entirety. Wounds are closed in a layered fashion using 0-polydioxanone (PDS, Ethicon) suture for deep tissue, 2-0 PDS for subcutaneous tissue, and 2-0 nylon for skin closure. The tourniquet is deflated for the remainder of the case to reduce limb ischemia during frame placement.
CIRCULAR FRAME CONCEPTS AND PLACEMENT
The majority of circular frames for both ankle and hindfoot fusion have multiple ring sizes available in aluminum and radiolucent carbon fiber reinforced polymer (Hoffmann LRF, Stryker). Rings are available in full, open, segment, and both short- and long-foot options. Frames can be sterilized in a prebuilt 3 to 4 ring construct with 4 static or dynamic (telescopic) struts (100-277 mm). The most commonly used tibia and foot ring sizes are 155 cm, 180 cm, and 210 cm. Ring size should be able to accommodate posterior soft tissue swelling and avoid circumferential soft tissue abrasion against the rings. Anterior foot arches are used for increased construct stability and can be locked to the distal tibia ring for weight-bearing support. Wire and half-pin bolts, adaptors, and nuts are used to join each ring of the frame to the patient’s bone.
For TTC arthrodesis, 2 rings are typically used in the tibia, and 1 ring is used in the foot. For isolated ankle arthrodesis, an additional ring can be added with olive wires in the talus to permit compression only across the ankle joint. Multiple points of fixation are used in each ring in different planes to achieve both maximal stability and rotational control. If a single wire or half-pin becomes infected and requires removal, there are still multiple other points of fixation in the ring to maintain stability. Fixation within each ring should be off axis compared with the adjacent ring to both avoid stress risers and increase construct rigidity.
The prebuilt frame is checked on the back table to ensure proper orientation and component alignment. The frame is then placed over both the foot and ankle, and multiple stacks of towels are placed behind the heel, ankle, and calf to center the foot and ankle in the frame (Figures 4A-4F). At least 4 to 6 cm of space is needed in between the posterior soft tissues and each ring to accommodate postoperative swelling. On the lateral view, the foot ring should be in the mid-portion of the calcaneus. If there is a concern, particularly in Charcot patients, regarding early weight-bearing noncompliance, the foot ring can be placed flush with the plantar aspect of the foot, and olive wires can be inserted using longer adaptors. The frame should be checked from multiple viewpoints to ensure that both the foot and ankle are centered and in neutral rotation.
Continue to: TIBIA RING FIXATION...
TIBIA RING FIXATION
Tibia rings can be fixed using 2 to 3 half-pins (4-6 mm) alone or 2 half-pins in combination with a smooth wire. A small incision is made over the area of planned half-pin insertion, and the periosteum is cleared away using a hemostat. An adaptor sleeve is used, and the bone is drilled bicortically, followed by insertion of the half-pin. Hydroxyapatite-coated pins are used to improve the strength of the bone-pin interface and reduce the incidence of pin tract infections. Pins are inserted along both the anterior and medial aspects of the tibia, avoiding the thick lateral musculature. Care is taken to protect the medial neurovascular structures during pin placement following established Ilizarov safe zones.
After each pin is placed in the bone, the pin is secured to the adaptor that is then tightened to the ring. This process is repeated for both the proximal and distal tibia rings. Pins should be placed above and below each ring to avoid creating stress risers. During smooth wire placement, each wire is pushed by hand through the soft tissues and then drilled into the bone while the exposed segment is held with a damp sponge to reduce the incidence of thermal bone necrosis. Once the wire is drilled bicortically, a mallet is used to tap the wire through the remaining soft tissues to avoid wrapping them up in the wire. Each wire should be parallel to the ring to get an even line of compression.
Each wire is secured on 1 end and then tensioned to 130 kg using a hand tensioner. An additional tool can be placed in the wire adaptor to prevent the wire from bending during tensioning. If the wire is passing above or below the ring, longer wire adaptors should be used to build to the wire. The wire should never be bent toward the ring as this can increase the likelihood of improper pin tensioning and breakage. Wire placement should be avoided posteriorly as this can make it difficult to secure and/or tension wires, and also increases the risk of damage to posterior structures.
Ring fixation in the distal tibia near the plafond may require 1 half-pin and 2 wires to avoid damage to the tibialis anterior and posterior tibial tendons. In this case, smooth wires should be placed in a crossing pattern and tensioned simultaneously to avoid pulling the ankle away from the center of the frame. Wires should be bent and curved over each ring and then cut to facilitate subsequent removal.
FOOT RING FIXATION
In the foot, olive wires are used to increase fixation against bone. For each olive wire, a small incision is made to accommodate the diameter of the olive through the soft tissue. Similar to the distal tibia, 2 olive wires should be placed above and below the foot ring in a crossing pattern through the calcaneus (Figures 5A-5F). The axial view of the frame should be checked to ensure proper wire orientation. When using olive wires, it is essential to tension both at the same time to 90 kg, as the foot can be pulled medially or laterally in the frame if 1 wire is tensioned before the other.
Forefoot olive wires should also be placed in a crossing pattern, with 1 wire fixed through the first, second, and third metatarsals, and 1 wire through the fourth and fifth metatarsals. Additional forefoot olive wires can be placed if compression is needed across the midfoot or Chopart joints for fusion. Multiple X-rays should be checked to ensure that the calcaneus and forefoot olive wires are firmly fixed both in and against bone.
Continue to: JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS...
JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS
Once all rings are secured to the bone with half-pins and wires, the previously placed Steinman pins, and K-wires through the heel are removed. Both ankle and subtalar joint alignments are rechecked, and then axial compression is placed through the foot ring with the knee extended and the struts unlocked. Static or telescopic struts are used to achieve 8 to 10 mm of bony compression. X-rays are taken before and after to analyze final joint compression and alignment. Struts should be sequentially tightened (1/2 turn of a static strut) 1 at a time as final tightening of 1 strut alone can bind and interfere with both the compression and tightening of the remaining struts.
Once final compression is achieved, the struts are locked, and the front foot arch is closed anteriorly and connected to the distal tibia ring for increased stability (Figures 6A-6D). Each pin and wire is covered in a sterile dressing followed by gauze to allow for soft tissue padding. The entire frame is then overwrapped in bias stockinette rolls or ace wraps.
Walking attachments can be added immediately to the frame that allows for early weight-bearing. Rocker shoe attachments with a 15° anterior and posterior slope and rubber soles can help offload the ankle and subtalar joints, decrease pressure on heel strike, and reduce ankle motion during ambulation (Hoffmann LRF, Stryker).
POSTOPERATIVE PROTOCOL
Depending on individual characteristics, patients can be immediately weight-bearing in the circular frame. Patients with Charcot neuroarthropathy are recommended to remain non-weight-bearing for the first 2 months to reduce the likelihood of pin, wire, and frame breakage along with nonunion. Pin and wire site care and maintenance are initiated the day after surgery and continue on a daily basis for the duration of frame placement. Sutures are removed 4 to 5 weeks after surgery to ensure adequate wound healing. Serial X-rays are taken monthly to analyze fusion sites.
If pins or wires become infected, patients are placed on oral antibiotics, and both pins and wires can be removed or exchanged in the operating room. Once fusion is achieved in 3 to 8 months (Figures 7A-7C), the frame is removed in the operating room, and fusion sites are examined under dynamic fluoroscopy. If fusion is confirmed, patients are made weight-bearing as tolerated in a short-leg cast or tall CAM boot for 6 to 8 weeks, and then transitioned to an ankle brace in an accommodative shoe.
Continue to: DISCUSSION...
DISCUSSION
A key aspect of recovery after ankle and hindfoot fusion using the Ilizarov technique is balancing pin care, soft tissue swelling, and weight-bearing status. The average time patients will spend in the frame is approximately 25 to 28 weeks, but can range from 12 to 84 weeks.1,2Given the considerable variability in both soft tissue healing and bony union, patients should be extensively counseled before surgery to set expectations correctly and ensure that they have the necessary help and support to care for the frame during the treatment period. Patients should be followed closely during the first 6 weeks to ensure that pins and wires do not become infected or break, as both of these issues require immediate intervention.
In a review of 11 patients who underwent tibiocalcaneal arthrodesis using an Ilizarov external fixator for infected talar nonunions or extrusions, Rochman and colleagues8 reported an 81% rate of successful fusion with a final mean American Orthopaedic Foot and Ankle Society score of 65 (out of a maximum 86). Similar results were reported by Saltzman9 in a series of 8 patients with diffuse ankle osteomyelitis treated with resection of all infected tissue and hybrid-frame compression arthrodesis. All patients received 6 weeks of intravenous antibiotics, and frames were removed at 3 months, and walking casts were applied for 1 to 2 additional months. Ankle sepsis was eradicated in all patients, and 7/8 (87.5%) ankles successfully fused at an average of 13.5 weeks (range, 10-16 weeks). One limb required below-knee amputation at 5 weeks due to non-reconstructible vascular insufficiency. At an average of 3.4-year follow-up, none of the 7 fused ankles required further surgery.
Fragomen and colleagues1 retrospectively reviewed 101 patients who underwent complex ankle fusion using the Ilizarov technique and found that 76/91 (83.5%) patients achieved fusion at an average of 25 weeks (range, 10-65 weeks). Smoking was associated with a 54% rate of nonunion and 15/19 (79%) patients with Charcot neuroarthropathy achieved ankle fusion, but had a subsequent subtalar joint failure, thus highlighting the need for TTC arthrodesis in Charcot patients. Salem and colleagues2 reviewed 21 Ilizarov ankle fusions and reported that all patients achieved fusion at an average of 28 weeks (range, 12-84 weeks). Complications occurred in 11 patients, including 2 nonunions that healed after revision frame application and 4 pin tract infections.
CONCLUSION
Overall, the Ilizarov technique using circular external fixation is a powerful tool that can be used to treat a variety of disorders including complex foot and ankle deformity and infection. While case series generally show favorable outcomes, patients must be informed that this technique is a salvage procedure for limb preservation that requires meticulous operative technique, diligent postoperative care, and tight control of medical comorbidities, such as blood sugar levels in individuals with diabetes to achieve a successful outcome.
ABSTRACT
Surgical reconstruction of the ankle and hindfoot in patients with diabetes, Charcot neuroarthropathy, osteomyelitis, deformity, and/or bone loss can be challenging and often results in amputation. In these patients, conventional internal fixation with plates, screws, and intramedullary nails is often not feasible because of ongoing infection or poor bone stock and soft tissue quality. The Ilizarov method of ankle and hindfoot arthrodesis is a well-established technique for limb reconstruction that uses circular external fixation to achieve solid bony fusion, optimal leg length, and eradication of infection in cases of complex pathology. This article discusses indications, contraindications, pearls, and pitfalls of performing ankle and hindfoot arthrodesis using the Ilizarov technique.
Continue to: Patients with complex ankle and hindfoot deformity...
Patients with complex ankle and hindfoot deformity present a unique challenge to both nonoperative management and surgical reconstruction. Nonoperative management focuses on wound care, bracing, and immobilization using ankle-foot orthoses, total contact casts, and Charcot restraint orthotic walker boots for external stabilization. Fusion using the Ilizarov technique with circular fixation is a salvage limb-preservation procedure that has shown good results in select patient populations.1-5 Indications include post-traumatic, degenerative, and rheumatoid arthritis, osteomyelitis, tumors, neuromuscular conditions, and salvage of failed ankle and hindfoot procedures.6-9 Relative contraindications include wet gangrene, severe limb ischemia, and soft tissue compromise requiring urgent amputation. In addition, circular frames are not recommended in patients who are unable to comply with postoperative restrictions, and pin and wire care for the duration of frame placement because of personal, psychological, or socioeconomic reasons.
The Ilizarov technique of ring fixation provides dynamic, modular, and rigid fixation in multiple planes to control shear, bending, and rotational forces, and allows for early weight-bearing and postoperative adjustments as needed.10,11 Percutaneously placed half-pins and wires allow for solid fixation in the setting of both poor bone and soft tissue quality, and fusion can be achieved in the presence of active infection in a 1-stage procedure. The goal of ankle and hindfoot fusion using the Ilizarov technique is to achieve an infection-free, stable, plantigrade foot with neutral ankle alignment to allow for patient ambulation and return to activities of daily living.
Nonunion rates with circular fixation are reported to be as high as 16% to 54%, due to medical comorbidities, such as smoking, peripheral vascular disease, and Charcot neuroarthropathy.1Charcot, in particular, is a risk factor for nonunion as patients lack protective sensation, and have a higher rate of wound dehiscence, noncompliance with weight-bearing precautions, pin site infections, and frame breakage. In these patients, tibiotalocalcaneal (TTC) arthrodesis is preferred over the isolated ankle, or subtalar fusion to both provide a stable platform for ambulation and reduce the incidence of adjacent joint breakdown. Common complications of the Ilizarov technique include pin site infections, wire breakage, talar necrosis, and tibial stress fractures after frame removal.1,2,6,11-13 Circular frames are typically maintained for 3 to 8 months, until solid fusion is achieved radiographically. Frames are removed in the operating room with the concurrent examination of the fusion sites under anesthesia followed by a period of protected weight-bearing in a cast or tall controlled ankle motion (CAM) boot.
This article reviews several technical details, tips, and tricks that can help improve the intraoperative and postoperative outcomes of combined ankle and hindfoot arthrodesis using the Ilizarov technique with circular external fixation.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
SETUP AND APPROACH
Patients are positioned supine with padding under the operative extremity to achieve neutral leg rotation (Figures 1A-1D). A thigh tourniquet is placed with the foot positioned at the end of the bed and on top of the radiolucent padding to avoid interference of the contralateral leg during lateral X-rays. After sterile prepping and draping, the extremity is exsanguinated above the level of an active infection, and the tourniquet inflated.
For isolated ankle arthrodesis, an anterior or lateral approach can be used, while for TTC arthrodesis, a lateral approach is required to access both the ankle and subtalar joints. A 10-cm longitudinal incision is made along the distal fibula, curving slightly and anteriorly along the distal extent of the incision. Dissection is continued down to bone using full thickness flaps, and the distal fibula is removed 2 to 3 cm above the ankle joint using a saw and osteotome (Figures 2A-2G). The distal fibula can be used subsequently as bone grafts depending on the quality of bone. The peroneal tendons are retracted posteriorly, and dissection is then continued to the posterior facet of the subtalar joint.
JOINT PREPARATION AND ALIGNMENT
Both the anterior and posterior neurovascular bundles are protected along the distal tibia with Hohmann retractors while a saw is used to create flat cuts across the tibial plafond and talus to allow apposition of flat, broad cancellous bony surfaces. Flat cuts followed by later joint compression will often shorten the limb by 2 to 3 cm. This leg length discrepancy can later be accommodated using a shoe lift, as needed. All retained hardware and/or infected and necrotic tissues in the ankle and hindfoot are removed using a rongeur and a pituitary rongeur.
The medial malleolus is osteotomized vertically using a direct medial incision and approach with full thickness flaps, and in line with the previous tibial plafond, is both cut and removed. The medial malleolus can also be used for bone grafts in fusion sites. A smooth-tip lamina spreader is placed in the subtalar joint for distraction and a curved osteotome, curettes, and a small rongeur are used to remove all remaining cartilage from the subtalar joint. Flat cuts in the subtalar joint can remove excessive bone, particularly from the inferior aspect of the talus. The subchondral bone is perforated using a 2.5- to 3.0-mm drill bit and a curved osteotome.
A bone graft from the distal fibula and medial malleolus, with or without the addition of allograft adjuvants, is placed evenly across the ankle and subtalar joints (Figures 3A-3E). At this point, the ankle and subtalar joints can be manipulated in multiple planes to achieve neutral coronal, sagittal, and axial alignment. With both the ankle and hindfoot held in a neutral position, multiple Steinman pins and K-wires in different orientations are inserted through the plantar aspect of the heel to hold the ankle and subtalar joints in place temporarily. Wires are cut short to prevent interference with subsequent foot olive wire placement through the frame.
Continue to: X-rays should be carefully checked...
X-rays should be carefully checked to ensure proper alignment. Wounds are gently irrigated, and vancomycin powder (2 g) can be placed within wounds for local antibiotic delivery. Lateral tissues are sharply debulked to allow for decreased tension on the incision, and small ulcers can be excised in their entirety. Wounds are closed in a layered fashion using 0-polydioxanone (PDS, Ethicon) suture for deep tissue, 2-0 PDS for subcutaneous tissue, and 2-0 nylon for skin closure. The tourniquet is deflated for the remainder of the case to reduce limb ischemia during frame placement.
CIRCULAR FRAME CONCEPTS AND PLACEMENT
The majority of circular frames for both ankle and hindfoot fusion have multiple ring sizes available in aluminum and radiolucent carbon fiber reinforced polymer (Hoffmann LRF, Stryker). Rings are available in full, open, segment, and both short- and long-foot options. Frames can be sterilized in a prebuilt 3 to 4 ring construct with 4 static or dynamic (telescopic) struts (100-277 mm). The most commonly used tibia and foot ring sizes are 155 cm, 180 cm, and 210 cm. Ring size should be able to accommodate posterior soft tissue swelling and avoid circumferential soft tissue abrasion against the rings. Anterior foot arches are used for increased construct stability and can be locked to the distal tibia ring for weight-bearing support. Wire and half-pin bolts, adaptors, and nuts are used to join each ring of the frame to the patient’s bone.
For TTC arthrodesis, 2 rings are typically used in the tibia, and 1 ring is used in the foot. For isolated ankle arthrodesis, an additional ring can be added with olive wires in the talus to permit compression only across the ankle joint. Multiple points of fixation are used in each ring in different planes to achieve both maximal stability and rotational control. If a single wire or half-pin becomes infected and requires removal, there are still multiple other points of fixation in the ring to maintain stability. Fixation within each ring should be off axis compared with the adjacent ring to both avoid stress risers and increase construct rigidity.
The prebuilt frame is checked on the back table to ensure proper orientation and component alignment. The frame is then placed over both the foot and ankle, and multiple stacks of towels are placed behind the heel, ankle, and calf to center the foot and ankle in the frame (Figures 4A-4F). At least 4 to 6 cm of space is needed in between the posterior soft tissues and each ring to accommodate postoperative swelling. On the lateral view, the foot ring should be in the mid-portion of the calcaneus. If there is a concern, particularly in Charcot patients, regarding early weight-bearing noncompliance, the foot ring can be placed flush with the plantar aspect of the foot, and olive wires can be inserted using longer adaptors. The frame should be checked from multiple viewpoints to ensure that both the foot and ankle are centered and in neutral rotation.
Continue to: TIBIA RING FIXATION...
TIBIA RING FIXATION
Tibia rings can be fixed using 2 to 3 half-pins (4-6 mm) alone or 2 half-pins in combination with a smooth wire. A small incision is made over the area of planned half-pin insertion, and the periosteum is cleared away using a hemostat. An adaptor sleeve is used, and the bone is drilled bicortically, followed by insertion of the half-pin. Hydroxyapatite-coated pins are used to improve the strength of the bone-pin interface and reduce the incidence of pin tract infections. Pins are inserted along both the anterior and medial aspects of the tibia, avoiding the thick lateral musculature. Care is taken to protect the medial neurovascular structures during pin placement following established Ilizarov safe zones.
After each pin is placed in the bone, the pin is secured to the adaptor that is then tightened to the ring. This process is repeated for both the proximal and distal tibia rings. Pins should be placed above and below each ring to avoid creating stress risers. During smooth wire placement, each wire is pushed by hand through the soft tissues and then drilled into the bone while the exposed segment is held with a damp sponge to reduce the incidence of thermal bone necrosis. Once the wire is drilled bicortically, a mallet is used to tap the wire through the remaining soft tissues to avoid wrapping them up in the wire. Each wire should be parallel to the ring to get an even line of compression.
Each wire is secured on 1 end and then tensioned to 130 kg using a hand tensioner. An additional tool can be placed in the wire adaptor to prevent the wire from bending during tensioning. If the wire is passing above or below the ring, longer wire adaptors should be used to build to the wire. The wire should never be bent toward the ring as this can increase the likelihood of improper pin tensioning and breakage. Wire placement should be avoided posteriorly as this can make it difficult to secure and/or tension wires, and also increases the risk of damage to posterior structures.
Ring fixation in the distal tibia near the plafond may require 1 half-pin and 2 wires to avoid damage to the tibialis anterior and posterior tibial tendons. In this case, smooth wires should be placed in a crossing pattern and tensioned simultaneously to avoid pulling the ankle away from the center of the frame. Wires should be bent and curved over each ring and then cut to facilitate subsequent removal.
FOOT RING FIXATION
In the foot, olive wires are used to increase fixation against bone. For each olive wire, a small incision is made to accommodate the diameter of the olive through the soft tissue. Similar to the distal tibia, 2 olive wires should be placed above and below the foot ring in a crossing pattern through the calcaneus (Figures 5A-5F). The axial view of the frame should be checked to ensure proper wire orientation. When using olive wires, it is essential to tension both at the same time to 90 kg, as the foot can be pulled medially or laterally in the frame if 1 wire is tensioned before the other.
Forefoot olive wires should also be placed in a crossing pattern, with 1 wire fixed through the first, second, and third metatarsals, and 1 wire through the fourth and fifth metatarsals. Additional forefoot olive wires can be placed if compression is needed across the midfoot or Chopart joints for fusion. Multiple X-rays should be checked to ensure that the calcaneus and forefoot olive wires are firmly fixed both in and against bone.
Continue to: JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS...
JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS
Once all rings are secured to the bone with half-pins and wires, the previously placed Steinman pins, and K-wires through the heel are removed. Both ankle and subtalar joint alignments are rechecked, and then axial compression is placed through the foot ring with the knee extended and the struts unlocked. Static or telescopic struts are used to achieve 8 to 10 mm of bony compression. X-rays are taken before and after to analyze final joint compression and alignment. Struts should be sequentially tightened (1/2 turn of a static strut) 1 at a time as final tightening of 1 strut alone can bind and interfere with both the compression and tightening of the remaining struts.
Once final compression is achieved, the struts are locked, and the front foot arch is closed anteriorly and connected to the distal tibia ring for increased stability (Figures 6A-6D). Each pin and wire is covered in a sterile dressing followed by gauze to allow for soft tissue padding. The entire frame is then overwrapped in bias stockinette rolls or ace wraps.
Walking attachments can be added immediately to the frame that allows for early weight-bearing. Rocker shoe attachments with a 15° anterior and posterior slope and rubber soles can help offload the ankle and subtalar joints, decrease pressure on heel strike, and reduce ankle motion during ambulation (Hoffmann LRF, Stryker).
POSTOPERATIVE PROTOCOL
Depending on individual characteristics, patients can be immediately weight-bearing in the circular frame. Patients with Charcot neuroarthropathy are recommended to remain non-weight-bearing for the first 2 months to reduce the likelihood of pin, wire, and frame breakage along with nonunion. Pin and wire site care and maintenance are initiated the day after surgery and continue on a daily basis for the duration of frame placement. Sutures are removed 4 to 5 weeks after surgery to ensure adequate wound healing. Serial X-rays are taken monthly to analyze fusion sites.
If pins or wires become infected, patients are placed on oral antibiotics, and both pins and wires can be removed or exchanged in the operating room. Once fusion is achieved in 3 to 8 months (Figures 7A-7C), the frame is removed in the operating room, and fusion sites are examined under dynamic fluoroscopy. If fusion is confirmed, patients are made weight-bearing as tolerated in a short-leg cast or tall CAM boot for 6 to 8 weeks, and then transitioned to an ankle brace in an accommodative shoe.
Continue to: DISCUSSION...
DISCUSSION
A key aspect of recovery after ankle and hindfoot fusion using the Ilizarov technique is balancing pin care, soft tissue swelling, and weight-bearing status. The average time patients will spend in the frame is approximately 25 to 28 weeks, but can range from 12 to 84 weeks.1,2Given the considerable variability in both soft tissue healing and bony union, patients should be extensively counseled before surgery to set expectations correctly and ensure that they have the necessary help and support to care for the frame during the treatment period. Patients should be followed closely during the first 6 weeks to ensure that pins and wires do not become infected or break, as both of these issues require immediate intervention.
In a review of 11 patients who underwent tibiocalcaneal arthrodesis using an Ilizarov external fixator for infected talar nonunions or extrusions, Rochman and colleagues8 reported an 81% rate of successful fusion with a final mean American Orthopaedic Foot and Ankle Society score of 65 (out of a maximum 86). Similar results were reported by Saltzman9 in a series of 8 patients with diffuse ankle osteomyelitis treated with resection of all infected tissue and hybrid-frame compression arthrodesis. All patients received 6 weeks of intravenous antibiotics, and frames were removed at 3 months, and walking casts were applied for 1 to 2 additional months. Ankle sepsis was eradicated in all patients, and 7/8 (87.5%) ankles successfully fused at an average of 13.5 weeks (range, 10-16 weeks). One limb required below-knee amputation at 5 weeks due to non-reconstructible vascular insufficiency. At an average of 3.4-year follow-up, none of the 7 fused ankles required further surgery.
Fragomen and colleagues1 retrospectively reviewed 101 patients who underwent complex ankle fusion using the Ilizarov technique and found that 76/91 (83.5%) patients achieved fusion at an average of 25 weeks (range, 10-65 weeks). Smoking was associated with a 54% rate of nonunion and 15/19 (79%) patients with Charcot neuroarthropathy achieved ankle fusion, but had a subsequent subtalar joint failure, thus highlighting the need for TTC arthrodesis in Charcot patients. Salem and colleagues2 reviewed 21 Ilizarov ankle fusions and reported that all patients achieved fusion at an average of 28 weeks (range, 12-84 weeks). Complications occurred in 11 patients, including 2 nonunions that healed after revision frame application and 4 pin tract infections.
CONCLUSION
Overall, the Ilizarov technique using circular external fixation is a powerful tool that can be used to treat a variety of disorders including complex foot and ankle deformity and infection. While case series generally show favorable outcomes, patients must be informed that this technique is a salvage procedure for limb preservation that requires meticulous operative technique, diligent postoperative care, and tight control of medical comorbidities, such as blood sugar levels in individuals with diabetes to achieve a successful outcome.
1. Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470(10):2864-2873. doi:10.1007/s11999-012-2470-9.
2. Salem KH, Kinzl L, Schmelz A. Ankle arthrodesis using Ilizarov ring fixators: a review of 22 cases. Foot Ankle Int. 2006;27(10):764-770. doi:10.1177/107110070602701002.
3. Cierny G 3rd, Cook WG, Mader JT. Ankle arthrodesis in the presence of ongoing sepsis. Indications, methods, and results. Orthop Clin North Am. 1989;20(4):709-721.
4. Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30(11):1065-1070. doi:10.3113/FAI.2009.1065.
5. Eylon S, Porat S, Bor N, Leibner ED. Outcome of Ilizarov ankle arthrodesis. Foot Ankle Int. 2007;28(8):873-879. doi:10.3113/FAI.2007.0873.
6. Kalish S, Fleming J, Weinstein R. External fixators for elective rearfoot and ankle arthrodesis. Techniques and indications. Clin Podiatr Med Surg. 2003;20(1):65-96, vi.
7. Kollig E, Esenwein SA, Muhr G, Kutscha-Lissberg F. Fusion of the septic ankle: experience with 15 cases using hybrid external fixation. J Trauma. 2003;55(4):685-691. doi:10.1097/01.TA.0000051933.83342.E4.
8. Rochman R, Jackson Hutson J, Alade O. Tibiocalcaneal arthrodesis using the Ilizarov technique in the presence of bone loss and infection of the talus. Foot Ankle Int. 2008;29(10):1001-1008. doi:10.3113/FAI.2008.1001.
9. Saltzman CL. Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J. 2005;2547-52.
10. Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007;3(1):13-29. doi:10.1007/s11420-006-9025-0.
11. Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;(303):217-225.
12. Jones CP, Youngblood CS, Waldrop N, Davis WH, Pinzur MS. Tibial Stress Fracture Secondary to Half-Pins in Circular Ring External Fixation for Charcot Foot. Foot Ankle Int. 2014;35(6):572-577. doi:10.1177/1071100714531229.
13. Kazmers NH, Fragomen AT, Rozbruch SR. Prevention of pin site infection in external fixation: a review of the literature. Strategies Trauma Limb Reconstr. 2016;11(2):75-85. doi:10.1007/s11751-016-0256-4.
1. Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470(10):2864-2873. doi:10.1007/s11999-012-2470-9.
2. Salem KH, Kinzl L, Schmelz A. Ankle arthrodesis using Ilizarov ring fixators: a review of 22 cases. Foot Ankle Int. 2006;27(10):764-770. doi:10.1177/107110070602701002.
3. Cierny G 3rd, Cook WG, Mader JT. Ankle arthrodesis in the presence of ongoing sepsis. Indications, methods, and results. Orthop Clin North Am. 1989;20(4):709-721.
4. Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30(11):1065-1070. doi:10.3113/FAI.2009.1065.
5. Eylon S, Porat S, Bor N, Leibner ED. Outcome of Ilizarov ankle arthrodesis. Foot Ankle Int. 2007;28(8):873-879. doi:10.3113/FAI.2007.0873.
6. Kalish S, Fleming J, Weinstein R. External fixators for elective rearfoot and ankle arthrodesis. Techniques and indications. Clin Podiatr Med Surg. 2003;20(1):65-96, vi.
7. Kollig E, Esenwein SA, Muhr G, Kutscha-Lissberg F. Fusion of the septic ankle: experience with 15 cases using hybrid external fixation. J Trauma. 2003;55(4):685-691. doi:10.1097/01.TA.0000051933.83342.E4.
8. Rochman R, Jackson Hutson J, Alade O. Tibiocalcaneal arthrodesis using the Ilizarov technique in the presence of bone loss and infection of the talus. Foot Ankle Int. 2008;29(10):1001-1008. doi:10.3113/FAI.2008.1001.
9. Saltzman CL. Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J. 2005;2547-52.
10. Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007;3(1):13-29. doi:10.1007/s11420-006-9025-0.
11. Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;(303):217-225.
12. Jones CP, Youngblood CS, Waldrop N, Davis WH, Pinzur MS. Tibial Stress Fracture Secondary to Half-Pins in Circular Ring External Fixation for Charcot Foot. Foot Ankle Int. 2014;35(6):572-577. doi:10.1177/1071100714531229.
13. Kazmers NH, Fragomen AT, Rozbruch SR. Prevention of pin site infection in external fixation: a review of the literature. Strategies Trauma Limb Reconstr. 2016;11(2):75-85. doi:10.1007/s11751-016-0256-4.
TAKE-HOME POINTS
- Ankle and hindfoot fusion using circular external fixation is a useful surgical technique in patients with diabetes, Charcot, osteomyelitis, deformity, and/or bone and soft tissue compromise in order to obtain solid bony fusion, stable limb alignment, and eradication of infection in cases of complex pathology.
- Deformity correction with osteotomies and meticulous joint preparation is required in order to obtain broad, cancellous bony surfaces for fusion with neutral alignment. Autograft from the distal fibula and/or medial malleolus can be combined with bone allograft to assist with joint fusion.
- The ankle and hindfoot are provisionally pinned into neutral coronal and sagittal alignment through the plantar surface of the foot using large K-wires prior to placement of the lower leg in the center of a circular 3-ring compression frame. Typically, 2 to 3 points of fixation are used per ring with a combination of half-pins and smooth wires.
- Ring attachments are built up or down to the level of the half-pins and wires in order to prevent pins and wires from bending, breaking, or causing iatrogenic deformity during tensioning. Crossing olive wires are used in the midfoot and calcaneus with dual tensioning devices to ensure an even pull on both sides of the foot.
- Dynamic or static compression struts are used to obtain 8 to 10 mm of compression across the ankle and hindfoot, followed by addition of an anterior foot ring to increase construct rigidity. Daily pin care is started 3 to 4 days after surgery and patients are kept non-weight-bearing for approximately 2 months in the frame with a total frame period of 3 to 8 months depending on bony healing on X-ray.
The Effect of Insurance Type on Patient Access to Ankle Fracture Care Under the Affordable Care Act
ABSTRACT
The purpose of this study is to assess the effect of insurance type (Medicaid, Medicare, private insurance) on the ability for patients with operative ankle fractures to access orthopedic traumatologists. The research team called 245 board-certified orthopedic surgeons specializing in orthopedic trauma within 8 representative states. The caller requested an appointment for their fictitious mother in order to be evaluated for an ankle fracture which was previously evaluated by her primary care physician and believed to require surgery. Each office was called 3 times to assess the response for each insurance type. For each call, information was documented regarding whether the patient was able to receive an appointment and the barriers the patient confronted to receive an appointment. Overall, 35.7% of offices scheduled an appointment for a patient with Medicaid, in comparison to 81.4%and 88.6% for Medicare and BlueCross, respectively (P < .0001). Medicaid patients confronted more barriers for receiving appointments. There was no statistically significant difference in access for Medicaid patients in states that had expanded Medicaid eligibility vs states that had not expanded Medicaid. Medicaid reimbursement for open reduction and internal fixation of an ankle fracture did not significantly correlate with appointment success rates or wait times. Despite the passage of the Affordable Care Act, patients with Medicaid have reduced access to orthopedic surgeons and more complex barriers to receiving appointments. A more robust strategy for increasing care-access for patients with Medicaid would be more equitable.
Continue to: In 2010, the Patient Protection and Affordable Care Act...
In 2010, the Patient Protection and Affordable Care Act (PPACA) expanded the eligibility criteria for Medicaid to all individuals with an income up to 138% of the poverty level.1 A Supreme Court ruling stated that the decision to expand Medicaid was to be decided by individual states.2 Currently, 31 states have chosen to expand Medicaid eligibility to their residents.2 This expansion has allowed an additional 11.7 million people to enroll in Medicaid and the Children’s Health Insurance Program by May 2015.3-5
Even with the passage of the PPACA, Medicaid patients seeking specialty orthopedic care have experienced more barriers to accessing care than Medicare or commercially-insured patients.2,6-10 One major cited reason is Medicaid’s low reimbursement, which may discourage physicians from open panel participation in Medicaid.11,12
A common fundamental teaching for orthopedic traumatologists is the notion that they should be available to treat all injuries regardless of the patient’s ability to pay.13 This has resulted in both trauma centers and trauma surgeons becoming financially challenged due to the higher proportion of Medicaid and uninsured trauma patients and lower Medicaid reimbursement levels.14,15
This study focuses on the effect of different types of insurance (Medicaid, Medicare, or commercial insurance) on the ability of patients to obtain care for operative ankle fractures. The purpose of this study is to evaluate, in the context of the PPACA, patient access to orthopedic surgeons for operative ankle fractures based on insurance-type. We hypothesized that patients with Medicaid would face a greater volume of obstacles when seeking appointments for an ankle fracture, even after the PPACA.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
The study population included board-certified orthopedic surgeons who belonged to the Orthopaedic Trauma Association (OTA) from 8 representative states; 4 states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and 4 states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas). These states were selected due to their ability to represent diverse healthcare marketplaces throughout the country. Using the OTA website’s “Find a Surgeon” search tool,16 we created a list of surgeons for each state and matched each surgeon with a random number. The list of surgeons was ordered according to the value of the surgeon’s associated random number, and surgeons were called in ascending order. We excluded disconnected or inaccurate numbers from the calling list. Surgeons who did not manage ankle fractures were removed from the dataset. Approximately 30 orthopedic trauma surgeons per state were contacted.
Each office was called to make an appointment for the caller’s mother. Every surgeon’s office was specifically asked if the surgeon would accept the patient to be evaluated for an ankle fracture that occurred out-of-state. The caller had a standardized protocol to limit intra- and inter-office variations (Appendix). The scenario involved a request to be evaluated for an unstable ankle fracture, with the patient having Medicaid, Medicare, or BlueCross insurance. The scenario required 3 separate calls to the same surgeon in order to obtain data regarding each insurance-type. The calls were separated by at least 1 week to avoid caller recognition by the surgeon’s office.
Appendix
Scenario
1. Date of Birth: Medicaid–2/07/55; BlueCross PPO–2/09/55; Medicare–7/31/45.
2. Ankle fracture evaluated by primary care physician 1 or 2 days ago
3. Not seen previously by your clinic or hospital, she would be a new patient
4. Asked how early she could be scheduled for an appointment
5. Script:
“I’m calling for my mother who injured her ankle a few days ago. Her family doctor took an X-ray and believes she has a fracture and needs surgery. Is Dr. X accepting new patients for evaluation and treatment of ankle fractures?” If YES →
“I was wondering if you take Medicaid/Medicare/BlueCross plan?” If YES →
“When is your soonest available appointment?”
The date of each phone call and date of appointment, if provided, were recorded. If the office did not give an appointment, we asked for reasons why. If an appointment was denied for a patient with Medicaid, we asked for a referral to another office that accepted Medicaid. We considered barriers to obtaining an initial appointment, such as requiring a referral from a primary care physician (PCP), as an unsuccessful attempt at making an appointment. We determined the waiting period for an appointment by calculating the time between the date of the call and the date of the appointment. Appointments were not scheduled to ensure that actual patients were not disadvantaged. For both appointment success rates and waiting periods, we stratified the data into 2 groups: states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas).
We obtained Medicaid reimbursement rates for open reduction and internal fixation of an ankle fracture by querying each state’s reimbursement rate using Current Procedural Terminology code 27822.
Chi-square test or Fisher’s exact test was used to analyze acceptance rate differences based on the patient’s type of insurance. To compare the waiting periods for an appointment, we used an independent samples t-test after applying natural log-transformation, as the data was not normally distributed. We performed logistic regression analysis to detect whether reimbursement was a significant predictor of successfully making an appointment for patients, and a linear regression analysis was used to evaluate whether reimbursement predicted waiting periods. Unless otherwise stated, all statistical testing was performed two-tailed at an alpha-level of 0.05.
This study was approved by the Institutional Review Board of Yale University School of Medicine (HIC No. 1363).
Continue to: RESULTS...
RESULTS
In total, 350 offices were contacted across 8 states (4 states with and 4 states without expanded Medicaid eligibility) of which we identified 245 orthopedic surgeons who would surgically treat ankle fractures. The 245 surgeons’ offices were called 3 times for each separate insurance-type.
Table 1. Appointment Success Rate
| Medicaid | Medicare | Private |
All states |
|
| |
Yes (%) | 100 (35.7) | 228 (81.4) | 248 (88.6) |
No (%) | 180 (64.3) | 52 (18.60 | 32 (11.4) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 55 (39.6) | 116 (83.5) | 124 (89.2) |
No (%) | 84 (60.4) | 23 (16.5) | 15 (10.8) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 45 (31.9) | 112 (79.4) | 124 (87.9) |
No (%) | 96 (68.1) | 29 (20.6) | 17 (12.1) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross (Table 1). For states with expanded Medicaid eligibility, the success rate for obtaining an appointment was 39.6%, 83.5%, and 89.2% for Medicaid, Medicare, and BlueCross, respectively. For states without expanded Medicaid eligibility, the success rate for obtaining an appointment was 31.9% for Medicaid, 79.4% for Medicare, and 87.9% for BlueCross. In all cases, the success rate for obtaining an appointment was significantly lower for Medicaid, compared to Medicare (P < .0001) or BlueCross (P < .0001). Medicaid appointment success rate was 39.6% in expanded states vs 31.9% in non-expanded states, however, the difference was not statistically significant (Table 2).
Table 2. Medicaid Appointment Success Rate in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 55 (39.6) | 45 (31.9) | .181 |
No (%) | 84 (60.4) | 96 (68.1) |
|
In 43.7% of occasions, patients with Medicaid did not have their insurance accepted, compared to 7.3% for Medicare and 0% for BlueCross. The majority of offices which did not accept Medicaid were not able to refer patients to another surgeon who would accept Medicaid. The requirement to have a primary care referral was the second most common reason for Medicaid patients not obtaining an appointment. No Medicare (10.4% vs 0.0%, P < .0001) or BlueCross (10.4% vs 0.0%, P < .0001) patients experienced this requirement (Table 3). There was no difference found between the percent of Medicaid patients who were required to have referrals in states with and without expanded Medicaid eligibility (Table 4).
Table 3. Referral Rate
| Medicaid | Medicare | Private |
All states |
|
|
|
Yes (%) | 29 (10.4) | 0 (0) | 0 (0) |
No (%) | 251 (89.6) | 280 (100) | 280 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 12 (8.6) | 0 (0) | 0 (0) |
No (%) | 127 (91.4) | 139 (100) | 139 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 17 (12.1) | 0 (0) | 0 (0) |
No (%) | 124 (87.9) | 141 (100) | 141 (100) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
Table 4. Medicaid Referral Rates in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 12 (9.7) | 17 (14.0) | .35 |
No (%) | 127 (91.4) | 124 (87.9) |
|
Reimbursements for ankle fracture varied across states (Table 5). For Medicaid, Georgia paid the highest reimbursement ($1049.95) and Florida paid the lowest ($469.44). Logistic and linear regression analysis did not demonstrate a significant relationship between reimbursement and appointment success rate or waiting periods.
Table 5. Medicaid Reimbursements for Ankle Fracture Repair (CPT and HCPCS 27822) in 2014
State | Medicaid reimbursement |
Californiaa | $785.55 |
Texas | $678.95 |
Florida | $469.44 |
Ohioa | $617.08 |
New Yorka | $500.02 |
North Carolina | $621.63 |
Massachusettsa | $627.94 |
Georgia | $1,049.95 |
Average | $668.82 |
aStates with expanded Medicaid eligibility.
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
Waiting periods (Table 6) varied significantly by the type of insurance (7.3 days for Medicaid, 6.0 days for Medicare, and 6.0 days for BlueCross; P = .002). For states with expanded Medicaid eligibility, waiting periods varied significantly by insurance (7.7 days for Medicaid, 6.2 days for Medicare, P = .003; and 6.1 days for BlueCross, P = .01). Waiting periods did not vary significantly for states without expanded Medicaid. Additionally, waiting periods did not differ significantly when comparing between states with and without Medicaid expansion.
Table 6. Waiting Period (Days) by Insurance Type.
| Medicaid | Medicare | Private |
Comparison by Insurance Type |
|
|
|
All states |
|
|
|
Waiting period | 7.3 | 6.0 | 6.0 |
P-value |
| 0.002 | 0.002 |
States with expanded Medicaid eligibility |
|
|
|
Waiting period | 7.7 | 6.2 | 6.1 |
P-value |
| 0.003 | 0.01 |
States without expanded Medicaid eligibility |
|
|
|
Waiting period | 6.9 | 5.9 | 5.9 |
P-value |
| 0.15 | 0.15 |
Comparison by Medicaid Expansion |
|
|
|
States with expanded Medicaid eligibility | 7.7 | 6.2 | 6.1 |
States without expanded Medicaid eligibility | 6.9 | 5.9 | 5.9 |
P-value | 0.17 | 0.13 | 0.07 |
Continue to: DISCUSSION...
DISCUSSION
This study assessed how insurance type (Medicaid, Medicare, and BlueCross) affects patient access to orthopedic trauma surgeons in 8 geographically representative states. We selected unstable ankle fractures as they are basic fractures treated by nearly all trauma surgeons and should often be surgically treated to prevent serious long-term consequences. Our hypothesis stated that despite the passage of the PPACA, patients with Medicaid would have reduced access to care. As the PPACA has changed the healthcare marketplace by increasing the number of Medicaid enrollees, it is important to ensure that patient access to care improves.
This nationwide survey of orthopedic trauma surgeons demonstrates that Medicaid patients experience added barriers to care that ultimately results in lower rates of successfully obtaining care. This is consistent with other investigations which have assessed Medicaid patient healthcare access.6,8,10,17-19 This study did not demonstrate a statistically significant difference between Medicaid patients’ ability to obtain appointments in states with expanded Medicaid eligibility vs in states without expanded Medicaid eligibility (39.6% vs 31.9%, P < .18); this has been demonstrated in the literature.6
A barrier that was unique to Medicaid patients was the requirement to have a PCP referral (Table 3). A PCP referral was not a barrier to receiving an appointment for patients with Medicare or BlueCross. One reason to explain why Medicaid patients may be required to have PCP referrals is due to their increased medical complexity, extra documentation requirements, and low reimbursement.4 Patients who have obtained a PCP referral may be characterized as being more medically compliant.
It is important to note that the Medicaid policies for 4 states included in this study (Massachusetts, North Carolina, Texas, and New York) required a PCP referral in order to see a specialist. However, we found that many orthopedic trauma practices in these states scheduled appointments for Medicaid patients without a PCP referral, suggesting that the decision depended on individual policy. In addition, the majority of offices within these states cited that they simply did not accept Medicaid as an insurance policy, and not that they required a referral.
Our regression analysis did not find a significant relationship between being able to successfully obtain an appointment to be evaluated for an ankle fracture and reimbursement rates for Medicaid. Although studies have stressed the importance of Medicaid reimbursements on physician participation, this result is consistent with previous studies regarding carpal tunnel release and total ankle replacements.17,19 Long20 suggested that although reimbursements may help, additional strategies for promoting Medicaid acceptance may be needed, including: lowering the costs of participating in Medicaid by simplifying administrative processes, speeding up reimbursement, and reducing the costs associated with caring for those patients.
Continue to: Previous studies have demonstrated...
Previous studies have demonstrated that more physicians may accept Medicaid if reimbursements increased.4,12 Given the high percentage of trauma patients with Medicaid as their primary insurance or whom are emergently enrolled in Medicaid by hospital systems, it is concerning that the PPACA is reducing payments under the Medicare and Medicaid Disproportionate Share Hospital programs which provide hospitals for uncompensated care given to low-income and uninsured patients.21 Trauma centers generally operate at a deficit due to the higher proportion of Medicaid and uninsured patients.14 This is currently worsened by additional federal funding cuts for supporting trauma service’s humane mission.21
This study has several limitations. While the study evaluated access to care in 8 representative states, a thorough nationwide survey would be more representative. Some results may have become statistically significant if we had performed the study with a larger sample size. In addition, we were unable to control for many factors which could impact appointment wait times, such as physician call schedules and vacations. Socioeconomic factors can influence a patient’s ability to attend an appointment, such as transportation costs, time off from work, and childcare availability. In addition, this study did not assess access for the uninsured, who are predominantly the working poor who cannot afford health insurance, even with federal and state subsidies.
The authors apologize for inconveniencing these offices, however, data collection could not be achieved in a better manner. We hope that the value of this study compensates any inconvenience.
CONCLUSION
Overall, our results demonstrate that despite the ratification of the PPACA, Medicaid patients are confronted with more barriers to accessing care by comparison to patients with Medicare and BlueCross insurance. Medicaid patients have worse baseline health22 and are at an increased risk of complications. These disparities are thought to be due to decreased healthcare access,23,24 as well as socioeconomic challenges. Interventions, such as increasing Medicaid’s reimbursement levels, reducing burdensome administrative responsibilities, and establishing partnerships between trauma centers and trauma surgeons, may enable underinsured patients to be appropriately cared for.
This paper will be judged for the Resident Writer’s Award.
1. Blumenthal D, Collins SR. Health care coverage under the affordable care act--a progress report. N Engl J Med. 2014;371(3):275-281. doi:10.1056/NEJMhpr1405667.
2. Sommers BD. Health care reform's unfinished work--remaining barriers to coverage and access. N Engl J Med. 2015;373(25):2395-2397. doi:10.1056/NEJMp1509462.
3. US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicaid & CHIP: February 2015 monthly applications, eligibility determinations and enrollment report. https://www.medicaid.gov/medicaid/program-information/downloads/medicaid-and-chip-february-2015-application-eligibility-and-enrollment-data.pdf. Published May 1, 2015. Accessed May 2015.
4. Iglehart JK, Sommers BD. Medicaid at 50--from welfare program to nation's largest health insurer. N Engl J Med. 2015;372(22):2152-2159. doi:10.1056/NEJMhpr1500791.
5. Kaiser Family Foundation. Medicaid moving forward. http://kff.org/medicaid/fact-sheet/the-medicaid-program-at-a-glance-update/. Updated 2014. Accessed October 10, 2014.
6. Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the affordable care act. J Arthroplasty. 2015;30(9):1498-1501. doi:10.1016/j.arth.2015.03.015.
7. Draeger RW, Patterson BM, Olsson EC, Schaffer A, Patterson JM. The influence of patient insurance status on access to outpatient orthopedic care for flexor tendon lacerations. J Hand Surg Am. 2014;39(3):527-533. doi:10.1016/j.jhsa.2013.10.031.
8. Patterson BM, Spang JT, Draeger RW, Olsson EC, Creighton RA, Kamath GV. Access to outpatient care for adult rotator cuff patients with private insurance versus Medicaid in North Carolina. J Shoulder Elbow Surg. 2013;22(12):1623-1627. doi:10.1016/j.jse.2013.07.051.
9. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
10. Schwarzkopf R, Phan D, Hoang M, Ross S, Mukamel D. Do patients with income-based insurance have access to total joint arthroplasty? J Arthroplasty. 2014;29(6):1083-1086. doi:10.1016/j.arth.2013.11.022.
11. Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff (Millwood). 2012;31(8):1673-1679 doi:10.1377/hlthaff.2012.0294.
12. Perloff JD, Kletke P, Fossett JW. Which physicians limit their Medicaid participation, and why. Health Serv Res. 1995;30(1):7-26.
13. Althausen PL. Building a successful trauma practice in a community setting. J Orthop Trauma. 2011;25 Suppl 3:S113-S117. doi:10.1097/BOT.0b013e318237bcce.
14. Greenberg S, Mir HR, Jahangir AA, Mehta S, Sethi MK. Impacting policy change for orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S14-S16. doi:10.1097/BOT.0000000000000216.
15. Wiznia DH, Averbukh L, Kim CY, Goel A, Leslie MP. Motorcycle helmets: The economic burden of an incomplete helmet law to medical care in the state of Connecticut. Conn Med. 2015;79(8):453-459.
16. Orthopaedic Trauma Association. Find a surgeon. https://online.ota.org/otassa/otacenssafindasurgeon.query_page. Updated 2015. Accessed July, 2015.
17. Kim CY, Wiznia DH, Roth AS, Walls RJ, Pelker RR. Survey of patient insurance status on access to specialty foot and ankle care under the affordable care act. Foot Ankle Int. 2016;37(7):776-781. doi:1071100716642015.
18. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of Medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
19. Kim CY, Wiznia DH, Wang Y, et al. The effect of insurance type on patient access to carpal tunnel release under the affordable care act. J Hand Surg Am. 2016;41(4):503-509.e1. doi:S0363-5023(16)00104-0.
20. Long SK. Physicians may need more than higher reimbursements to expand Medicaid participation: findings from Washington state. Health Aff (Millwood). 2013;32(9):1560-1567. doi:10.1377/hlthaff.2012.1010.
21. Issar NM, Jahangir AA. The affordable care act and orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S5-S7. doi:10.1097/BOT.0000000000000211.
22. Hahn B, Flood AB. No insurance, public insurance, and private insurance: do these options contribute to differences in general health? J Health Care Poor Underserved. 1995;6(1):41-59.
23. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):9-14. doi:10.1016/j.arth.2008.05.010.
24. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi:10.1097/MLR.0000000000000177.
ABSTRACT
The purpose of this study is to assess the effect of insurance type (Medicaid, Medicare, private insurance) on the ability for patients with operative ankle fractures to access orthopedic traumatologists. The research team called 245 board-certified orthopedic surgeons specializing in orthopedic trauma within 8 representative states. The caller requested an appointment for their fictitious mother in order to be evaluated for an ankle fracture which was previously evaluated by her primary care physician and believed to require surgery. Each office was called 3 times to assess the response for each insurance type. For each call, information was documented regarding whether the patient was able to receive an appointment and the barriers the patient confronted to receive an appointment. Overall, 35.7% of offices scheduled an appointment for a patient with Medicaid, in comparison to 81.4%and 88.6% for Medicare and BlueCross, respectively (P < .0001). Medicaid patients confronted more barriers for receiving appointments. There was no statistically significant difference in access for Medicaid patients in states that had expanded Medicaid eligibility vs states that had not expanded Medicaid. Medicaid reimbursement for open reduction and internal fixation of an ankle fracture did not significantly correlate with appointment success rates or wait times. Despite the passage of the Affordable Care Act, patients with Medicaid have reduced access to orthopedic surgeons and more complex barriers to receiving appointments. A more robust strategy for increasing care-access for patients with Medicaid would be more equitable.
Continue to: In 2010, the Patient Protection and Affordable Care Act...
In 2010, the Patient Protection and Affordable Care Act (PPACA) expanded the eligibility criteria for Medicaid to all individuals with an income up to 138% of the poverty level.1 A Supreme Court ruling stated that the decision to expand Medicaid was to be decided by individual states.2 Currently, 31 states have chosen to expand Medicaid eligibility to their residents.2 This expansion has allowed an additional 11.7 million people to enroll in Medicaid and the Children’s Health Insurance Program by May 2015.3-5
Even with the passage of the PPACA, Medicaid patients seeking specialty orthopedic care have experienced more barriers to accessing care than Medicare or commercially-insured patients.2,6-10 One major cited reason is Medicaid’s low reimbursement, which may discourage physicians from open panel participation in Medicaid.11,12
A common fundamental teaching for orthopedic traumatologists is the notion that they should be available to treat all injuries regardless of the patient’s ability to pay.13 This has resulted in both trauma centers and trauma surgeons becoming financially challenged due to the higher proportion of Medicaid and uninsured trauma patients and lower Medicaid reimbursement levels.14,15
This study focuses on the effect of different types of insurance (Medicaid, Medicare, or commercial insurance) on the ability of patients to obtain care for operative ankle fractures. The purpose of this study is to evaluate, in the context of the PPACA, patient access to orthopedic surgeons for operative ankle fractures based on insurance-type. We hypothesized that patients with Medicaid would face a greater volume of obstacles when seeking appointments for an ankle fracture, even after the PPACA.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
The study population included board-certified orthopedic surgeons who belonged to the Orthopaedic Trauma Association (OTA) from 8 representative states; 4 states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and 4 states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas). These states were selected due to their ability to represent diverse healthcare marketplaces throughout the country. Using the OTA website’s “Find a Surgeon” search tool,16 we created a list of surgeons for each state and matched each surgeon with a random number. The list of surgeons was ordered according to the value of the surgeon’s associated random number, and surgeons were called in ascending order. We excluded disconnected or inaccurate numbers from the calling list. Surgeons who did not manage ankle fractures were removed from the dataset. Approximately 30 orthopedic trauma surgeons per state were contacted.
Each office was called to make an appointment for the caller’s mother. Every surgeon’s office was specifically asked if the surgeon would accept the patient to be evaluated for an ankle fracture that occurred out-of-state. The caller had a standardized protocol to limit intra- and inter-office variations (Appendix). The scenario involved a request to be evaluated for an unstable ankle fracture, with the patient having Medicaid, Medicare, or BlueCross insurance. The scenario required 3 separate calls to the same surgeon in order to obtain data regarding each insurance-type. The calls were separated by at least 1 week to avoid caller recognition by the surgeon’s office.
Appendix
Scenario
1. Date of Birth: Medicaid–2/07/55; BlueCross PPO–2/09/55; Medicare–7/31/45.
2. Ankle fracture evaluated by primary care physician 1 or 2 days ago
3. Not seen previously by your clinic or hospital, she would be a new patient
4. Asked how early she could be scheduled for an appointment
5. Script:
“I’m calling for my mother who injured her ankle a few days ago. Her family doctor took an X-ray and believes she has a fracture and needs surgery. Is Dr. X accepting new patients for evaluation and treatment of ankle fractures?” If YES →
“I was wondering if you take Medicaid/Medicare/BlueCross plan?” If YES →
“When is your soonest available appointment?”
The date of each phone call and date of appointment, if provided, were recorded. If the office did not give an appointment, we asked for reasons why. If an appointment was denied for a patient with Medicaid, we asked for a referral to another office that accepted Medicaid. We considered barriers to obtaining an initial appointment, such as requiring a referral from a primary care physician (PCP), as an unsuccessful attempt at making an appointment. We determined the waiting period for an appointment by calculating the time between the date of the call and the date of the appointment. Appointments were not scheduled to ensure that actual patients were not disadvantaged. For both appointment success rates and waiting periods, we stratified the data into 2 groups: states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas).
We obtained Medicaid reimbursement rates for open reduction and internal fixation of an ankle fracture by querying each state’s reimbursement rate using Current Procedural Terminology code 27822.
Chi-square test or Fisher’s exact test was used to analyze acceptance rate differences based on the patient’s type of insurance. To compare the waiting periods for an appointment, we used an independent samples t-test after applying natural log-transformation, as the data was not normally distributed. We performed logistic regression analysis to detect whether reimbursement was a significant predictor of successfully making an appointment for patients, and a linear regression analysis was used to evaluate whether reimbursement predicted waiting periods. Unless otherwise stated, all statistical testing was performed two-tailed at an alpha-level of 0.05.
This study was approved by the Institutional Review Board of Yale University School of Medicine (HIC No. 1363).
Continue to: RESULTS...
RESULTS
In total, 350 offices were contacted across 8 states (4 states with and 4 states without expanded Medicaid eligibility) of which we identified 245 orthopedic surgeons who would surgically treat ankle fractures. The 245 surgeons’ offices were called 3 times for each separate insurance-type.
Table 1. Appointment Success Rate
| Medicaid | Medicare | Private |
All states |
|
| |
Yes (%) | 100 (35.7) | 228 (81.4) | 248 (88.6) |
No (%) | 180 (64.3) | 52 (18.60 | 32 (11.4) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 55 (39.6) | 116 (83.5) | 124 (89.2) |
No (%) | 84 (60.4) | 23 (16.5) | 15 (10.8) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 45 (31.9) | 112 (79.4) | 124 (87.9) |
No (%) | 96 (68.1) | 29 (20.6) | 17 (12.1) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross (Table 1). For states with expanded Medicaid eligibility, the success rate for obtaining an appointment was 39.6%, 83.5%, and 89.2% for Medicaid, Medicare, and BlueCross, respectively. For states without expanded Medicaid eligibility, the success rate for obtaining an appointment was 31.9% for Medicaid, 79.4% for Medicare, and 87.9% for BlueCross. In all cases, the success rate for obtaining an appointment was significantly lower for Medicaid, compared to Medicare (P < .0001) or BlueCross (P < .0001). Medicaid appointment success rate was 39.6% in expanded states vs 31.9% in non-expanded states, however, the difference was not statistically significant (Table 2).
Table 2. Medicaid Appointment Success Rate in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 55 (39.6) | 45 (31.9) | .181 |
No (%) | 84 (60.4) | 96 (68.1) |
|
In 43.7% of occasions, patients with Medicaid did not have their insurance accepted, compared to 7.3% for Medicare and 0% for BlueCross. The majority of offices which did not accept Medicaid were not able to refer patients to another surgeon who would accept Medicaid. The requirement to have a primary care referral was the second most common reason for Medicaid patients not obtaining an appointment. No Medicare (10.4% vs 0.0%, P < .0001) or BlueCross (10.4% vs 0.0%, P < .0001) patients experienced this requirement (Table 3). There was no difference found between the percent of Medicaid patients who were required to have referrals in states with and without expanded Medicaid eligibility (Table 4).
Table 3. Referral Rate
| Medicaid | Medicare | Private |
All states |
|
|
|
Yes (%) | 29 (10.4) | 0 (0) | 0 (0) |
No (%) | 251 (89.6) | 280 (100) | 280 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 12 (8.6) | 0 (0) | 0 (0) |
No (%) | 127 (91.4) | 139 (100) | 139 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 17 (12.1) | 0 (0) | 0 (0) |
No (%) | 124 (87.9) | 141 (100) | 141 (100) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
Table 4. Medicaid Referral Rates in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 12 (9.7) | 17 (14.0) | .35 |
No (%) | 127 (91.4) | 124 (87.9) |
|
Reimbursements for ankle fracture varied across states (Table 5). For Medicaid, Georgia paid the highest reimbursement ($1049.95) and Florida paid the lowest ($469.44). Logistic and linear regression analysis did not demonstrate a significant relationship between reimbursement and appointment success rate or waiting periods.
Table 5. Medicaid Reimbursements for Ankle Fracture Repair (CPT and HCPCS 27822) in 2014
State | Medicaid reimbursement |
Californiaa | $785.55 |
Texas | $678.95 |
Florida | $469.44 |
Ohioa | $617.08 |
New Yorka | $500.02 |
North Carolina | $621.63 |
Massachusettsa | $627.94 |
Georgia | $1,049.95 |
Average | $668.82 |
aStates with expanded Medicaid eligibility.
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
Waiting periods (Table 6) varied significantly by the type of insurance (7.3 days for Medicaid, 6.0 days for Medicare, and 6.0 days for BlueCross; P = .002). For states with expanded Medicaid eligibility, waiting periods varied significantly by insurance (7.7 days for Medicaid, 6.2 days for Medicare, P = .003; and 6.1 days for BlueCross, P = .01). Waiting periods did not vary significantly for states without expanded Medicaid. Additionally, waiting periods did not differ significantly when comparing between states with and without Medicaid expansion.
Table 6. Waiting Period (Days) by Insurance Type.
| Medicaid | Medicare | Private |
Comparison by Insurance Type |
|
|
|
All states |
|
|
|
Waiting period | 7.3 | 6.0 | 6.0 |
P-value |
| 0.002 | 0.002 |
States with expanded Medicaid eligibility |
|
|
|
Waiting period | 7.7 | 6.2 | 6.1 |
P-value |
| 0.003 | 0.01 |
States without expanded Medicaid eligibility |
|
|
|
Waiting period | 6.9 | 5.9 | 5.9 |
P-value |
| 0.15 | 0.15 |
Comparison by Medicaid Expansion |
|
|
|
States with expanded Medicaid eligibility | 7.7 | 6.2 | 6.1 |
States without expanded Medicaid eligibility | 6.9 | 5.9 | 5.9 |
P-value | 0.17 | 0.13 | 0.07 |
Continue to: DISCUSSION...
DISCUSSION
This study assessed how insurance type (Medicaid, Medicare, and BlueCross) affects patient access to orthopedic trauma surgeons in 8 geographically representative states. We selected unstable ankle fractures as they are basic fractures treated by nearly all trauma surgeons and should often be surgically treated to prevent serious long-term consequences. Our hypothesis stated that despite the passage of the PPACA, patients with Medicaid would have reduced access to care. As the PPACA has changed the healthcare marketplace by increasing the number of Medicaid enrollees, it is important to ensure that patient access to care improves.
This nationwide survey of orthopedic trauma surgeons demonstrates that Medicaid patients experience added barriers to care that ultimately results in lower rates of successfully obtaining care. This is consistent with other investigations which have assessed Medicaid patient healthcare access.6,8,10,17-19 This study did not demonstrate a statistically significant difference between Medicaid patients’ ability to obtain appointments in states with expanded Medicaid eligibility vs in states without expanded Medicaid eligibility (39.6% vs 31.9%, P < .18); this has been demonstrated in the literature.6
A barrier that was unique to Medicaid patients was the requirement to have a PCP referral (Table 3). A PCP referral was not a barrier to receiving an appointment for patients with Medicare or BlueCross. One reason to explain why Medicaid patients may be required to have PCP referrals is due to their increased medical complexity, extra documentation requirements, and low reimbursement.4 Patients who have obtained a PCP referral may be characterized as being more medically compliant.
It is important to note that the Medicaid policies for 4 states included in this study (Massachusetts, North Carolina, Texas, and New York) required a PCP referral in order to see a specialist. However, we found that many orthopedic trauma practices in these states scheduled appointments for Medicaid patients without a PCP referral, suggesting that the decision depended on individual policy. In addition, the majority of offices within these states cited that they simply did not accept Medicaid as an insurance policy, and not that they required a referral.
Our regression analysis did not find a significant relationship between being able to successfully obtain an appointment to be evaluated for an ankle fracture and reimbursement rates for Medicaid. Although studies have stressed the importance of Medicaid reimbursements on physician participation, this result is consistent with previous studies regarding carpal tunnel release and total ankle replacements.17,19 Long20 suggested that although reimbursements may help, additional strategies for promoting Medicaid acceptance may be needed, including: lowering the costs of participating in Medicaid by simplifying administrative processes, speeding up reimbursement, and reducing the costs associated with caring for those patients.
Continue to: Previous studies have demonstrated...
Previous studies have demonstrated that more physicians may accept Medicaid if reimbursements increased.4,12 Given the high percentage of trauma patients with Medicaid as their primary insurance or whom are emergently enrolled in Medicaid by hospital systems, it is concerning that the PPACA is reducing payments under the Medicare and Medicaid Disproportionate Share Hospital programs which provide hospitals for uncompensated care given to low-income and uninsured patients.21 Trauma centers generally operate at a deficit due to the higher proportion of Medicaid and uninsured patients.14 This is currently worsened by additional federal funding cuts for supporting trauma service’s humane mission.21
This study has several limitations. While the study evaluated access to care in 8 representative states, a thorough nationwide survey would be more representative. Some results may have become statistically significant if we had performed the study with a larger sample size. In addition, we were unable to control for many factors which could impact appointment wait times, such as physician call schedules and vacations. Socioeconomic factors can influence a patient’s ability to attend an appointment, such as transportation costs, time off from work, and childcare availability. In addition, this study did not assess access for the uninsured, who are predominantly the working poor who cannot afford health insurance, even with federal and state subsidies.
The authors apologize for inconveniencing these offices, however, data collection could not be achieved in a better manner. We hope that the value of this study compensates any inconvenience.
CONCLUSION
Overall, our results demonstrate that despite the ratification of the PPACA, Medicaid patients are confronted with more barriers to accessing care by comparison to patients with Medicare and BlueCross insurance. Medicaid patients have worse baseline health22 and are at an increased risk of complications. These disparities are thought to be due to decreased healthcare access,23,24 as well as socioeconomic challenges. Interventions, such as increasing Medicaid’s reimbursement levels, reducing burdensome administrative responsibilities, and establishing partnerships between trauma centers and trauma surgeons, may enable underinsured patients to be appropriately cared for.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The purpose of this study is to assess the effect of insurance type (Medicaid, Medicare, private insurance) on the ability for patients with operative ankle fractures to access orthopedic traumatologists. The research team called 245 board-certified orthopedic surgeons specializing in orthopedic trauma within 8 representative states. The caller requested an appointment for their fictitious mother in order to be evaluated for an ankle fracture which was previously evaluated by her primary care physician and believed to require surgery. Each office was called 3 times to assess the response for each insurance type. For each call, information was documented regarding whether the patient was able to receive an appointment and the barriers the patient confronted to receive an appointment. Overall, 35.7% of offices scheduled an appointment for a patient with Medicaid, in comparison to 81.4%and 88.6% for Medicare and BlueCross, respectively (P < .0001). Medicaid patients confronted more barriers for receiving appointments. There was no statistically significant difference in access for Medicaid patients in states that had expanded Medicaid eligibility vs states that had not expanded Medicaid. Medicaid reimbursement for open reduction and internal fixation of an ankle fracture did not significantly correlate with appointment success rates or wait times. Despite the passage of the Affordable Care Act, patients with Medicaid have reduced access to orthopedic surgeons and more complex barriers to receiving appointments. A more robust strategy for increasing care-access for patients with Medicaid would be more equitable.
Continue to: In 2010, the Patient Protection and Affordable Care Act...
In 2010, the Patient Protection and Affordable Care Act (PPACA) expanded the eligibility criteria for Medicaid to all individuals with an income up to 138% of the poverty level.1 A Supreme Court ruling stated that the decision to expand Medicaid was to be decided by individual states.2 Currently, 31 states have chosen to expand Medicaid eligibility to their residents.2 This expansion has allowed an additional 11.7 million people to enroll in Medicaid and the Children’s Health Insurance Program by May 2015.3-5
Even with the passage of the PPACA, Medicaid patients seeking specialty orthopedic care have experienced more barriers to accessing care than Medicare or commercially-insured patients.2,6-10 One major cited reason is Medicaid’s low reimbursement, which may discourage physicians from open panel participation in Medicaid.11,12
A common fundamental teaching for orthopedic traumatologists is the notion that they should be available to treat all injuries regardless of the patient’s ability to pay.13 This has resulted in both trauma centers and trauma surgeons becoming financially challenged due to the higher proportion of Medicaid and uninsured trauma patients and lower Medicaid reimbursement levels.14,15
This study focuses on the effect of different types of insurance (Medicaid, Medicare, or commercial insurance) on the ability of patients to obtain care for operative ankle fractures. The purpose of this study is to evaluate, in the context of the PPACA, patient access to orthopedic surgeons for operative ankle fractures based on insurance-type. We hypothesized that patients with Medicaid would face a greater volume of obstacles when seeking appointments for an ankle fracture, even after the PPACA.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
The study population included board-certified orthopedic surgeons who belonged to the Orthopaedic Trauma Association (OTA) from 8 representative states; 4 states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and 4 states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas). These states were selected due to their ability to represent diverse healthcare marketplaces throughout the country. Using the OTA website’s “Find a Surgeon” search tool,16 we created a list of surgeons for each state and matched each surgeon with a random number. The list of surgeons was ordered according to the value of the surgeon’s associated random number, and surgeons were called in ascending order. We excluded disconnected or inaccurate numbers from the calling list. Surgeons who did not manage ankle fractures were removed from the dataset. Approximately 30 orthopedic trauma surgeons per state were contacted.
Each office was called to make an appointment for the caller’s mother. Every surgeon’s office was specifically asked if the surgeon would accept the patient to be evaluated for an ankle fracture that occurred out-of-state. The caller had a standardized protocol to limit intra- and inter-office variations (Appendix). The scenario involved a request to be evaluated for an unstable ankle fracture, with the patient having Medicaid, Medicare, or BlueCross insurance. The scenario required 3 separate calls to the same surgeon in order to obtain data regarding each insurance-type. The calls were separated by at least 1 week to avoid caller recognition by the surgeon’s office.
Appendix
Scenario
1. Date of Birth: Medicaid–2/07/55; BlueCross PPO–2/09/55; Medicare–7/31/45.
2. Ankle fracture evaluated by primary care physician 1 or 2 days ago
3. Not seen previously by your clinic or hospital, she would be a new patient
4. Asked how early she could be scheduled for an appointment
5. Script:
“I’m calling for my mother who injured her ankle a few days ago. Her family doctor took an X-ray and believes she has a fracture and needs surgery. Is Dr. X accepting new patients for evaluation and treatment of ankle fractures?” If YES →
“I was wondering if you take Medicaid/Medicare/BlueCross plan?” If YES →
“When is your soonest available appointment?”
The date of each phone call and date of appointment, if provided, were recorded. If the office did not give an appointment, we asked for reasons why. If an appointment was denied for a patient with Medicaid, we asked for a referral to another office that accepted Medicaid. We considered barriers to obtaining an initial appointment, such as requiring a referral from a primary care physician (PCP), as an unsuccessful attempt at making an appointment. We determined the waiting period for an appointment by calculating the time between the date of the call and the date of the appointment. Appointments were not scheduled to ensure that actual patients were not disadvantaged. For both appointment success rates and waiting periods, we stratified the data into 2 groups: states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas).
We obtained Medicaid reimbursement rates for open reduction and internal fixation of an ankle fracture by querying each state’s reimbursement rate using Current Procedural Terminology code 27822.
Chi-square test or Fisher’s exact test was used to analyze acceptance rate differences based on the patient’s type of insurance. To compare the waiting periods for an appointment, we used an independent samples t-test after applying natural log-transformation, as the data was not normally distributed. We performed logistic regression analysis to detect whether reimbursement was a significant predictor of successfully making an appointment for patients, and a linear regression analysis was used to evaluate whether reimbursement predicted waiting periods. Unless otherwise stated, all statistical testing was performed two-tailed at an alpha-level of 0.05.
This study was approved by the Institutional Review Board of Yale University School of Medicine (HIC No. 1363).
Continue to: RESULTS...
RESULTS
In total, 350 offices were contacted across 8 states (4 states with and 4 states without expanded Medicaid eligibility) of which we identified 245 orthopedic surgeons who would surgically treat ankle fractures. The 245 surgeons’ offices were called 3 times for each separate insurance-type.
Table 1. Appointment Success Rate
| Medicaid | Medicare | Private |
All states |
|
| |
Yes (%) | 100 (35.7) | 228 (81.4) | 248 (88.6) |
No (%) | 180 (64.3) | 52 (18.60 | 32 (11.4) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 55 (39.6) | 116 (83.5) | 124 (89.2) |
No (%) | 84 (60.4) | 23 (16.5) | 15 (10.8) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 45 (31.9) | 112 (79.4) | 124 (87.9) |
No (%) | 96 (68.1) | 29 (20.6) | 17 (12.1) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross (Table 1). For states with expanded Medicaid eligibility, the success rate for obtaining an appointment was 39.6%, 83.5%, and 89.2% for Medicaid, Medicare, and BlueCross, respectively. For states without expanded Medicaid eligibility, the success rate for obtaining an appointment was 31.9% for Medicaid, 79.4% for Medicare, and 87.9% for BlueCross. In all cases, the success rate for obtaining an appointment was significantly lower for Medicaid, compared to Medicare (P < .0001) or BlueCross (P < .0001). Medicaid appointment success rate was 39.6% in expanded states vs 31.9% in non-expanded states, however, the difference was not statistically significant (Table 2).
Table 2. Medicaid Appointment Success Rate in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 55 (39.6) | 45 (31.9) | .181 |
No (%) | 84 (60.4) | 96 (68.1) |
|
In 43.7% of occasions, patients with Medicaid did not have their insurance accepted, compared to 7.3% for Medicare and 0% for BlueCross. The majority of offices which did not accept Medicaid were not able to refer patients to another surgeon who would accept Medicaid. The requirement to have a primary care referral was the second most common reason for Medicaid patients not obtaining an appointment. No Medicare (10.4% vs 0.0%, P < .0001) or BlueCross (10.4% vs 0.0%, P < .0001) patients experienced this requirement (Table 3). There was no difference found between the percent of Medicaid patients who were required to have referrals in states with and without expanded Medicaid eligibility (Table 4).
Table 3. Referral Rate
| Medicaid | Medicare | Private |
All states |
|
|
|
Yes (%) | 29 (10.4) | 0 (0) | 0 (0) |
No (%) | 251 (89.6) | 280 (100) | 280 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 12 (8.6) | 0 (0) | 0 (0) |
No (%) | 127 (91.4) | 139 (100) | 139 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 17 (12.1) | 0 (0) | 0 (0) |
No (%) | 124 (87.9) | 141 (100) | 141 (100) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
Table 4. Medicaid Referral Rates in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 12 (9.7) | 17 (14.0) | .35 |
No (%) | 127 (91.4) | 124 (87.9) |
|
Reimbursements for ankle fracture varied across states (Table 5). For Medicaid, Georgia paid the highest reimbursement ($1049.95) and Florida paid the lowest ($469.44). Logistic and linear regression analysis did not demonstrate a significant relationship between reimbursement and appointment success rate or waiting periods.
Table 5. Medicaid Reimbursements for Ankle Fracture Repair (CPT and HCPCS 27822) in 2014
State | Medicaid reimbursement |
Californiaa | $785.55 |
Texas | $678.95 |
Florida | $469.44 |
Ohioa | $617.08 |
New Yorka | $500.02 |
North Carolina | $621.63 |
Massachusettsa | $627.94 |
Georgia | $1,049.95 |
Average | $668.82 |
aStates with expanded Medicaid eligibility.
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
Waiting periods (Table 6) varied significantly by the type of insurance (7.3 days for Medicaid, 6.0 days for Medicare, and 6.0 days for BlueCross; P = .002). For states with expanded Medicaid eligibility, waiting periods varied significantly by insurance (7.7 days for Medicaid, 6.2 days for Medicare, P = .003; and 6.1 days for BlueCross, P = .01). Waiting periods did not vary significantly for states without expanded Medicaid. Additionally, waiting periods did not differ significantly when comparing between states with and without Medicaid expansion.
Table 6. Waiting Period (Days) by Insurance Type.
| Medicaid | Medicare | Private |
Comparison by Insurance Type |
|
|
|
All states |
|
|
|
Waiting period | 7.3 | 6.0 | 6.0 |
P-value |
| 0.002 | 0.002 |
States with expanded Medicaid eligibility |
|
|
|
Waiting period | 7.7 | 6.2 | 6.1 |
P-value |
| 0.003 | 0.01 |
States without expanded Medicaid eligibility |
|
|
|
Waiting period | 6.9 | 5.9 | 5.9 |
P-value |
| 0.15 | 0.15 |
Comparison by Medicaid Expansion |
|
|
|
States with expanded Medicaid eligibility | 7.7 | 6.2 | 6.1 |
States without expanded Medicaid eligibility | 6.9 | 5.9 | 5.9 |
P-value | 0.17 | 0.13 | 0.07 |
Continue to: DISCUSSION...
DISCUSSION
This study assessed how insurance type (Medicaid, Medicare, and BlueCross) affects patient access to orthopedic trauma surgeons in 8 geographically representative states. We selected unstable ankle fractures as they are basic fractures treated by nearly all trauma surgeons and should often be surgically treated to prevent serious long-term consequences. Our hypothesis stated that despite the passage of the PPACA, patients with Medicaid would have reduced access to care. As the PPACA has changed the healthcare marketplace by increasing the number of Medicaid enrollees, it is important to ensure that patient access to care improves.
This nationwide survey of orthopedic trauma surgeons demonstrates that Medicaid patients experience added barriers to care that ultimately results in lower rates of successfully obtaining care. This is consistent with other investigations which have assessed Medicaid patient healthcare access.6,8,10,17-19 This study did not demonstrate a statistically significant difference between Medicaid patients’ ability to obtain appointments in states with expanded Medicaid eligibility vs in states without expanded Medicaid eligibility (39.6% vs 31.9%, P < .18); this has been demonstrated in the literature.6
A barrier that was unique to Medicaid patients was the requirement to have a PCP referral (Table 3). A PCP referral was not a barrier to receiving an appointment for patients with Medicare or BlueCross. One reason to explain why Medicaid patients may be required to have PCP referrals is due to their increased medical complexity, extra documentation requirements, and low reimbursement.4 Patients who have obtained a PCP referral may be characterized as being more medically compliant.
It is important to note that the Medicaid policies for 4 states included in this study (Massachusetts, North Carolina, Texas, and New York) required a PCP referral in order to see a specialist. However, we found that many orthopedic trauma practices in these states scheduled appointments for Medicaid patients without a PCP referral, suggesting that the decision depended on individual policy. In addition, the majority of offices within these states cited that they simply did not accept Medicaid as an insurance policy, and not that they required a referral.
Our regression analysis did not find a significant relationship between being able to successfully obtain an appointment to be evaluated for an ankle fracture and reimbursement rates for Medicaid. Although studies have stressed the importance of Medicaid reimbursements on physician participation, this result is consistent with previous studies regarding carpal tunnel release and total ankle replacements.17,19 Long20 suggested that although reimbursements may help, additional strategies for promoting Medicaid acceptance may be needed, including: lowering the costs of participating in Medicaid by simplifying administrative processes, speeding up reimbursement, and reducing the costs associated with caring for those patients.
Continue to: Previous studies have demonstrated...
Previous studies have demonstrated that more physicians may accept Medicaid if reimbursements increased.4,12 Given the high percentage of trauma patients with Medicaid as their primary insurance or whom are emergently enrolled in Medicaid by hospital systems, it is concerning that the PPACA is reducing payments under the Medicare and Medicaid Disproportionate Share Hospital programs which provide hospitals for uncompensated care given to low-income and uninsured patients.21 Trauma centers generally operate at a deficit due to the higher proportion of Medicaid and uninsured patients.14 This is currently worsened by additional federal funding cuts for supporting trauma service’s humane mission.21
This study has several limitations. While the study evaluated access to care in 8 representative states, a thorough nationwide survey would be more representative. Some results may have become statistically significant if we had performed the study with a larger sample size. In addition, we were unable to control for many factors which could impact appointment wait times, such as physician call schedules and vacations. Socioeconomic factors can influence a patient’s ability to attend an appointment, such as transportation costs, time off from work, and childcare availability. In addition, this study did not assess access for the uninsured, who are predominantly the working poor who cannot afford health insurance, even with federal and state subsidies.
The authors apologize for inconveniencing these offices, however, data collection could not be achieved in a better manner. We hope that the value of this study compensates any inconvenience.
CONCLUSION
Overall, our results demonstrate that despite the ratification of the PPACA, Medicaid patients are confronted with more barriers to accessing care by comparison to patients with Medicare and BlueCross insurance. Medicaid patients have worse baseline health22 and are at an increased risk of complications. These disparities are thought to be due to decreased healthcare access,23,24 as well as socioeconomic challenges. Interventions, such as increasing Medicaid’s reimbursement levels, reducing burdensome administrative responsibilities, and establishing partnerships between trauma centers and trauma surgeons, may enable underinsured patients to be appropriately cared for.
This paper will be judged for the Resident Writer’s Award.
1. Blumenthal D, Collins SR. Health care coverage under the affordable care act--a progress report. N Engl J Med. 2014;371(3):275-281. doi:10.1056/NEJMhpr1405667.
2. Sommers BD. Health care reform's unfinished work--remaining barriers to coverage and access. N Engl J Med. 2015;373(25):2395-2397. doi:10.1056/NEJMp1509462.
3. US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicaid & CHIP: February 2015 monthly applications, eligibility determinations and enrollment report. https://www.medicaid.gov/medicaid/program-information/downloads/medicaid-and-chip-february-2015-application-eligibility-and-enrollment-data.pdf. Published May 1, 2015. Accessed May 2015.
4. Iglehart JK, Sommers BD. Medicaid at 50--from welfare program to nation's largest health insurer. N Engl J Med. 2015;372(22):2152-2159. doi:10.1056/NEJMhpr1500791.
5. Kaiser Family Foundation. Medicaid moving forward. http://kff.org/medicaid/fact-sheet/the-medicaid-program-at-a-glance-update/. Updated 2014. Accessed October 10, 2014.
6. Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the affordable care act. J Arthroplasty. 2015;30(9):1498-1501. doi:10.1016/j.arth.2015.03.015.
7. Draeger RW, Patterson BM, Olsson EC, Schaffer A, Patterson JM. The influence of patient insurance status on access to outpatient orthopedic care for flexor tendon lacerations. J Hand Surg Am. 2014;39(3):527-533. doi:10.1016/j.jhsa.2013.10.031.
8. Patterson BM, Spang JT, Draeger RW, Olsson EC, Creighton RA, Kamath GV. Access to outpatient care for adult rotator cuff patients with private insurance versus Medicaid in North Carolina. J Shoulder Elbow Surg. 2013;22(12):1623-1627. doi:10.1016/j.jse.2013.07.051.
9. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
10. Schwarzkopf R, Phan D, Hoang M, Ross S, Mukamel D. Do patients with income-based insurance have access to total joint arthroplasty? J Arthroplasty. 2014;29(6):1083-1086. doi:10.1016/j.arth.2013.11.022.
11. Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff (Millwood). 2012;31(8):1673-1679 doi:10.1377/hlthaff.2012.0294.
12. Perloff JD, Kletke P, Fossett JW. Which physicians limit their Medicaid participation, and why. Health Serv Res. 1995;30(1):7-26.
13. Althausen PL. Building a successful trauma practice in a community setting. J Orthop Trauma. 2011;25 Suppl 3:S113-S117. doi:10.1097/BOT.0b013e318237bcce.
14. Greenberg S, Mir HR, Jahangir AA, Mehta S, Sethi MK. Impacting policy change for orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S14-S16. doi:10.1097/BOT.0000000000000216.
15. Wiznia DH, Averbukh L, Kim CY, Goel A, Leslie MP. Motorcycle helmets: The economic burden of an incomplete helmet law to medical care in the state of Connecticut. Conn Med. 2015;79(8):453-459.
16. Orthopaedic Trauma Association. Find a surgeon. https://online.ota.org/otassa/otacenssafindasurgeon.query_page. Updated 2015. Accessed July, 2015.
17. Kim CY, Wiznia DH, Roth AS, Walls RJ, Pelker RR. Survey of patient insurance status on access to specialty foot and ankle care under the affordable care act. Foot Ankle Int. 2016;37(7):776-781. doi:1071100716642015.
18. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of Medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
19. Kim CY, Wiznia DH, Wang Y, et al. The effect of insurance type on patient access to carpal tunnel release under the affordable care act. J Hand Surg Am. 2016;41(4):503-509.e1. doi:S0363-5023(16)00104-0.
20. Long SK. Physicians may need more than higher reimbursements to expand Medicaid participation: findings from Washington state. Health Aff (Millwood). 2013;32(9):1560-1567. doi:10.1377/hlthaff.2012.1010.
21. Issar NM, Jahangir AA. The affordable care act and orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S5-S7. doi:10.1097/BOT.0000000000000211.
22. Hahn B, Flood AB. No insurance, public insurance, and private insurance: do these options contribute to differences in general health? J Health Care Poor Underserved. 1995;6(1):41-59.
23. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):9-14. doi:10.1016/j.arth.2008.05.010.
24. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi:10.1097/MLR.0000000000000177.
1. Blumenthal D, Collins SR. Health care coverage under the affordable care act--a progress report. N Engl J Med. 2014;371(3):275-281. doi:10.1056/NEJMhpr1405667.
2. Sommers BD. Health care reform's unfinished work--remaining barriers to coverage and access. N Engl J Med. 2015;373(25):2395-2397. doi:10.1056/NEJMp1509462.
3. US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicaid & CHIP: February 2015 monthly applications, eligibility determinations and enrollment report. https://www.medicaid.gov/medicaid/program-information/downloads/medicaid-and-chip-february-2015-application-eligibility-and-enrollment-data.pdf. Published May 1, 2015. Accessed May 2015.
4. Iglehart JK, Sommers BD. Medicaid at 50--from welfare program to nation's largest health insurer. N Engl J Med. 2015;372(22):2152-2159. doi:10.1056/NEJMhpr1500791.
5. Kaiser Family Foundation. Medicaid moving forward. http://kff.org/medicaid/fact-sheet/the-medicaid-program-at-a-glance-update/. Updated 2014. Accessed October 10, 2014.
6. Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the affordable care act. J Arthroplasty. 2015;30(9):1498-1501. doi:10.1016/j.arth.2015.03.015.
7. Draeger RW, Patterson BM, Olsson EC, Schaffer A, Patterson JM. The influence of patient insurance status on access to outpatient orthopedic care for flexor tendon lacerations. J Hand Surg Am. 2014;39(3):527-533. doi:10.1016/j.jhsa.2013.10.031.
8. Patterson BM, Spang JT, Draeger RW, Olsson EC, Creighton RA, Kamath GV. Access to outpatient care for adult rotator cuff patients with private insurance versus Medicaid in North Carolina. J Shoulder Elbow Surg. 2013;22(12):1623-1627. doi:10.1016/j.jse.2013.07.051.
9. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
10. Schwarzkopf R, Phan D, Hoang M, Ross S, Mukamel D. Do patients with income-based insurance have access to total joint arthroplasty? J Arthroplasty. 2014;29(6):1083-1086. doi:10.1016/j.arth.2013.11.022.
11. Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff (Millwood). 2012;31(8):1673-1679 doi:10.1377/hlthaff.2012.0294.
12. Perloff JD, Kletke P, Fossett JW. Which physicians limit their Medicaid participation, and why. Health Serv Res. 1995;30(1):7-26.
13. Althausen PL. Building a successful trauma practice in a community setting. J Orthop Trauma. 2011;25 Suppl 3:S113-S117. doi:10.1097/BOT.0b013e318237bcce.
14. Greenberg S, Mir HR, Jahangir AA, Mehta S, Sethi MK. Impacting policy change for orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S14-S16. doi:10.1097/BOT.0000000000000216.
15. Wiznia DH, Averbukh L, Kim CY, Goel A, Leslie MP. Motorcycle helmets: The economic burden of an incomplete helmet law to medical care in the state of Connecticut. Conn Med. 2015;79(8):453-459.
16. Orthopaedic Trauma Association. Find a surgeon. https://online.ota.org/otassa/otacenssafindasurgeon.query_page. Updated 2015. Accessed July, 2015.
17. Kim CY, Wiznia DH, Roth AS, Walls RJ, Pelker RR. Survey of patient insurance status on access to specialty foot and ankle care under the affordable care act. Foot Ankle Int. 2016;37(7):776-781. doi:1071100716642015.
18. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of Medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
19. Kim CY, Wiznia DH, Wang Y, et al. The effect of insurance type on patient access to carpal tunnel release under the affordable care act. J Hand Surg Am. 2016;41(4):503-509.e1. doi:S0363-5023(16)00104-0.
20. Long SK. Physicians may need more than higher reimbursements to expand Medicaid participation: findings from Washington state. Health Aff (Millwood). 2013;32(9):1560-1567. doi:10.1377/hlthaff.2012.1010.
21. Issar NM, Jahangir AA. The affordable care act and orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S5-S7. doi:10.1097/BOT.0000000000000211.
22. Hahn B, Flood AB. No insurance, public insurance, and private insurance: do these options contribute to differences in general health? J Health Care Poor Underserved. 1995;6(1):41-59.
23. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):9-14. doi:10.1016/j.arth.2008.05.010.
24. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi:10.1097/MLR.0000000000000177.
TAKE-HOME POINTS
- One method in which the PPACA increased the number of individuals with health insurance coverage was by expanding Medicaid eligibility requirements.
- Despite this, Medicaid patients confronted more barriers to accessing care.
- The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross. Patients with Medicaid also confronted longer appointment wait times.
- The disparity in access for this operative trauma scenario suggests that patients with Medicaid are likely to be excluded from the practice of their choice and may need to make considerably more effort to secure an appointment.
- Ultimately, Medicaid patients may have access to care through federally funded community health centers and public and non-profit safety net hospitals, which generally care for more uninsured and Medicaid patient populations.
Headless Compression Screw Fixation of Vertical Medial Malleolus Fractures is Superior to Unicortical Screw Fixation
ABSTRACT
This study is the first biomechanical research of headless compression screws for fixation of vertical shear fractures of the medial malleolus, a promising alternative that potentially offers several advantages for fixation.
Vertical shear fractures were simulated by osteotomies in 20 synthetic distal tibiae. Models were randomly assigned to fixation with either 2 parallel cancellous screws or 2 parallel Acutrak 2 headless compression screws (Acumed). Specimens were subjected to offset axial loading to simulate supination-adduction loading and tracked using high-resolution video.
The headless compression screw construct was significantly stiffer (P < .0001) (360 ± 131 N/mm) than the partially threaded cancellous screws (180 ± 48 N/mm) and demonstrated a significantly increased (P < .0001) mean load to clinical failure (719 ± 91 N vs 343 ± 83 N). When specimens were displaced to 6 mm and allowed to relax, the headless compression screw constructs demonstrated an elastic recoil and were reduced to the pretesting fragment alignment, whereas the parallel cancellous screw constructs remained displaced.
Along with the headless design that may decrease soft tissue irritation, the increased stiffness and elastic recoil of the headless compression screw construct offers improved fixation of medial malleolus vertical shear fractures over the traditional methods.
Continue to: Headless compressions screws...
Headless compressions screws are cannulated tapered titanium screws with variable thread pitch angle, allowing a fully threaded screw to apply compression along its entire length. These screws have been most commonly used for scaphoid fractures1 but have also been studied in fractures of small bones, such as capitellum, midfoot, and talar neck,2-4 and arthrodesis in the foot, ankle, and hand.5-7 Headless compression screws have been found to produce equivalent fragment compression to partially threaded cancellous screws while allowing less fragment displacement.8,9 The lack of a head may decrease soft tissue irritation compared with the partially threaded cancellous screws. Finally, headless compression screws are independent of cortical integrity, as the entire length of the screw features a wide thread diameter to capture cancellous bone in the proximal fragment, unlike partially threaded cancellous screws, which only possess a thread purchase in the distal fragment and depend on an intact cortex.
Vertical shear fractures of the medial malleolus occur through the supination-adduction of the talus exerted onto the articular surface of the medial malleolus.10 Optimal fixation of these fractures must be sufficient to maintain stable anatomic reduction of the ankle joint articular surface, allowing early range of motion, maintaining congruency of the ankle joint, and decreasing the risk of future post-traumatic arthritis to maximize functional outcome.11
A wide variety of techniques are available for fixation of these fractures, including various configurations of cortical screws, cancellous screws, tension bands, and antiglide plates. Clinically, 2 parallel 4.0-mm partially threaded cancellous screws are most often used. Limited evidence indicates that headless compression screws may be a viable option for fixation of medial malleolus fractures. One case reports the use of a headless compression screw for a horizontal medial malleolar fracture,12 and a small retrospective case series that used headless compression screws for all medial malleolar fractures showed satisfactory outcomes, a high union rate, and low patient-reported pain.13
We evaluate the stiffness, force to 2-mm displacement of the joint surface, and elastic properties of these 2 different constructs in vertical medial malleolar fractures in synthetic distal tibiae. We hypothesize that the parallel headless compression screw fixation will be stiffer and require more force to 2-mm displacement than parallel unicortical cancellous screw fixation.
MATERIALS AND METHODS
Identical vertical osteotomies (17.5 mm) were made from the medial border of the medial malleolus using a custom jig in 20 left 4th-generation composite synthetic distal tibiae (Sawbones, Pacific Research Labs; Model No. 3401) to simulate an Orthopaedic Trauma Association type 44-A2.3 fracture. The tibiae were then cut 18 cm from the tibial plafond and randomized to 2 fixation groups (n = 10 specimens for each group): parallel unicortical screw fixation or parallel unicortical headless compression screw fixation (Figures 1A-1D). Custom polymethylmethacrylate jigs were used to reproducibly drill identical holes with a 3.2-mm drill for the parallel unicortical screw construct and the drill bits provided by the Acutrak 2 Headless Compression Screw System (Acumed). The parallel unicortical screw construct consisted of 2 parallel 4.0-mm-diameter, 40-mm partially threaded cancellous screws (Depuy Synthes), and the headless compression fixation construct consisted of 2 parallel 4.7-mm-diameter, 45-mm titanium Acutrak 2 screws parallel to each other in the transverse plane. The Acutrak screws were placed per manufacturer instructions by first drilling with the Acutrak 2-4.7 Long Drill bit (Acumed), followed by the Acutrak 2-4.7 Profile Drill bit for the near cortex.
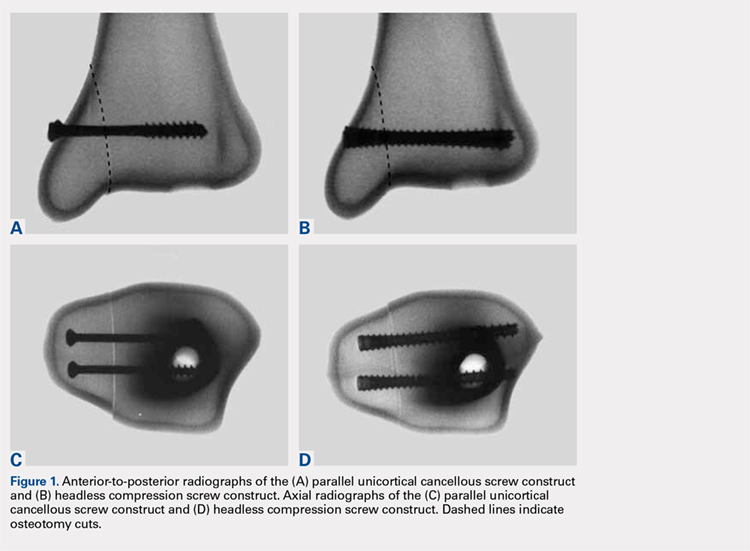
Continue to: Specimens...
Specimens were fixed to the base of a servohydraulic testing machine (Model 809, MTS Systems Corporation) with an axial-torsional load transducer (Model No. 662.20-01; Axial capacity of 250 kg, torsional capacity 2.88 kg-m; MTS Systems Corporation). The specimens were set in a vice tilted at 17° in the coronal plane to allow the MTS crosshead to apply an offset axial load simulating supination-adduction loading, which has been described previously (Figure 2).14,15 Load was applied to the inferolateral articular surface of the medial malleolus at 1 mm/s to a crosshead displacement of 6 mm and then cycled back to 0 mm. Load and axial displacement were measured at 60 Hz. The markers on the distal tibia and medial malleolus fracture fragment were tracked using high-resolution video (Fastcam PCI, Photron USA Inc). The motion of the video markers was determined using digitization and motion analysis software (Motus 9, Vicon).

Stiffness was calculated as the slope of the linear portion of the load-displacement curve over a range of 0.5 to 2.0 mm (Figure 3) and reported as mean (standard deviation). The force at 2 mm of fragment displacement was defined as a clinical failure.16,17 Student’s t test was used to determine the difference in construct stiffness and force for 2 mm displacement of the 2 groups. Significance was defined as P < .05. Institutional Review Board approval was not required for this study.
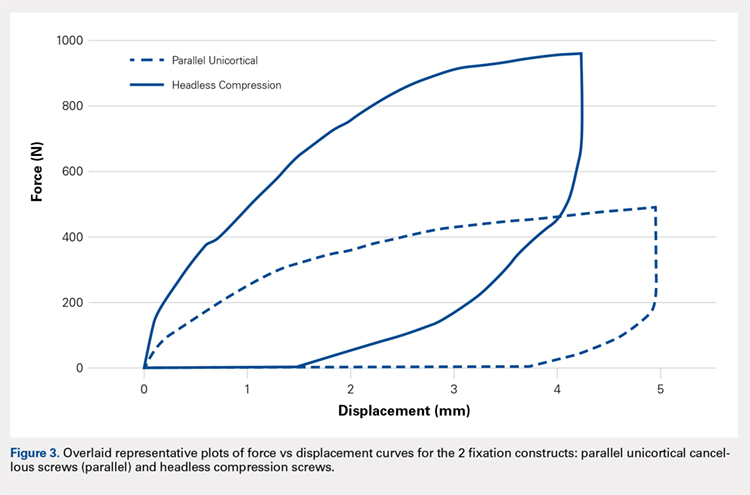
RESULTS
With offset axial testing to simulate supination-adduction force along with video motion analysis, the mean stiffness (± standard deviation) measured 180 ± 48 N/mm for the parallel unicortical screw fixation construct and 360 ± 131 N/mm for the headless compression screw fixation construct (Figure 4A). The headless compression screw fixation construct was over 2 times stiffer than the parallel unicortical construct during initial displacement of the fracture, indicating a statistically significant difference (P < .0001).
The mean force for 2 mm of fracture displacement, defined as clinical failure, reached 342 ± 83 N for the parallel unicortical screw fixation construct and 719 ± 91 N for the headless compression screw fixation construct (Figure 4B). The headless compression screw fixation construct resisted displacement significantly more (P = .0001) than the parallel unicortical screw construct, presenting a 100% increase.
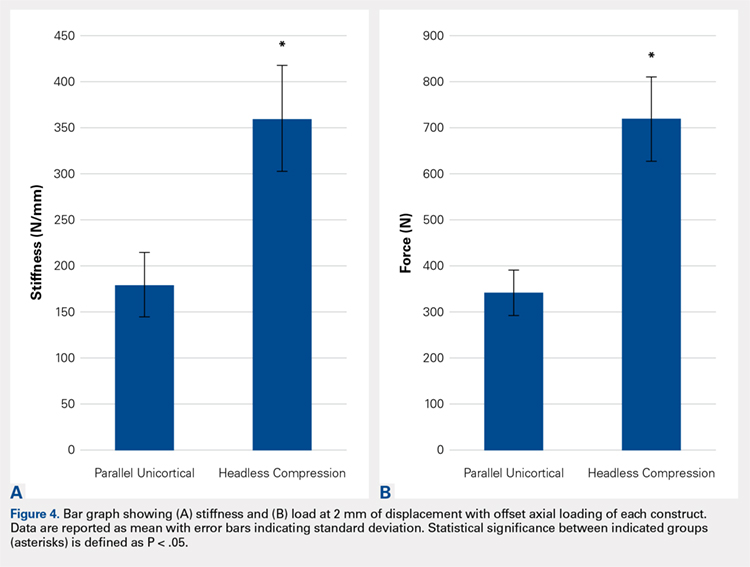
Upon cycling of the servohydraulic testing machine back to 0-mm displacement, the parallel unicortical construct demonstrated no elastic recoil, remaining displaced at 4 mm, whereas the headless compression screw construct rebounded to almost 0-mm displacement, which is well below the clinical definition of fixation failure of 2 mm (Figure 5).
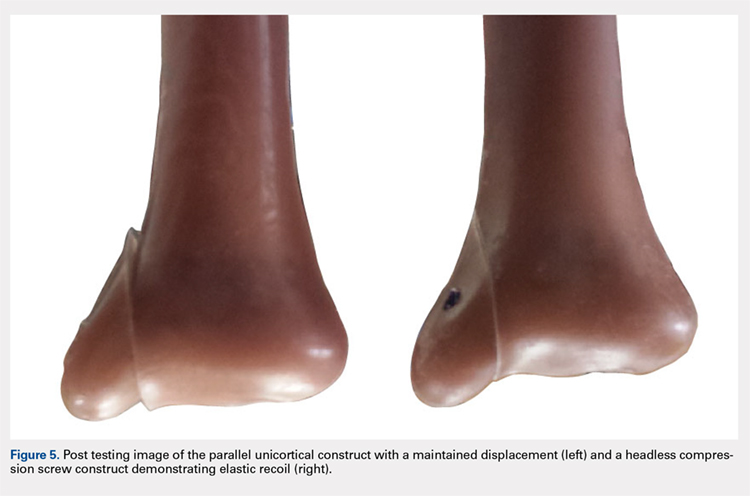
Continue to: Discussion...
DISCUSSION
When subjected to offset axial load, we observed that the headless compression screw construct exhibited significantly increased stiffness and load to 2 mm of displacement compared with a parallel unicortical screw construct. The headless compression screw also demonstrated elastic recoil to almost 0 mm of displacement, which is well below the 2-mm displacement.
We made reproducible fractures and fixation methods in synthetic distal tibiae, which feature less variability in size and quality than the cadaveric bone. Offset axial loading, rather than direct axial loading previously described by Amanatullah and colleagues,18 is the most physiologically relevant mode of force application to simulate the loading of the tauls onto the medial malleolus in the supination-adduction mechanism of injury.
The limitations of this study include the use of synthetic rather than cadaveric bone. Fourth-generation sawbones have been validated as possessing similar biomechanical properties as real bone.7,19 These results may also be inapplicable to osteoporotic bone, which would be significantly less dense than sawbones. This study is also an artificial situation designed to only test construct stiffness and load to clinical failure in a single mode of stress, offset axial loading and neglects other possible modes of force. This testing setup also disregards the structures surrounding the medial malleolus and tibia, including the talus, fibula, or soft tissue attachments, including the deltoid ligament and flexor retinaculum. These results are only relevant immediately after fixation and before bone healing occurs. We also tested the load to clinical failure rather than cyclic loading. Our testing more closely modeled a single traumatic force rather than the considerably smaller stresses that would be repeatedly exerted on the construct over several weeks after fixation in a clinical situation. This research is also not a clinical outcome study, rather, it suggests that headless compression screws are a viable, stronger, and possibly superior method for the initial fixation of vertical medial malleolar fractures.
As the load is offset axial, the larger thread purchase of the headless compression screws may lead to increased pullout strength, possibly increasing headless compression screw construct stiffness. Also, the variable diameter of headless compression screw, which reaches up to 4.7 mm, would increase the stiffness of the construct compared with the diameter of the cancellous screws. The elasticity of the headless compression construct may be because screws are made of titanium rather than stainless steel. Such property and given that the screws are cannulated rather than solid may also play a role, although several studies have shown variable results for cannulated vs solid screws of the same diameter.20,21 The elastic section modulus of both screws would have to be calculated to determine their exact effect on fixation.
CONCLUSION
The headless compression screw construct was found to be stiffer and features a higher load to clinical failure than a parallel unicortical cancellous screw construct for fixation of vertical medial malleolus fractures. Although significantly increased cost occurs with this construct, the headless design may decrease soft tissue irritation, and the elastic recoil of the construct after displacement may decrease clinical failure rates of this fixation method. This condition would eliminate the need for revision surgeries and thus be a cost effective alternative overall.
This paper will be judged for the Resident Writer’s Award.
- Fowler JR, Ilyas AM. Headless compression screw fixation of scaphoid fractures. Hand Clin. 2010;26(3):351-361, vi. doi:10.1016/j.hcl.2010.04.005.
- Karakasli A, Hapa O, Erduran M, Dincer C, Cecen B, Havitcioglu H. Mechanical comparison of headless screw fixation and locking plate fixation for talar neck fractures. J Foot Ankle Surg. 2015;54(5):905-909. doi:10.1053/j.jfas.2015.04.002.
- Elkowitz SJ, Polatsch DB, Egol KA, Kummer FJ, Koval KJ. Capitellum fractures: a biomechanical evaluation of three fixation methods. J Orthop Trauma. 2002;16(7):503-506. doi:10.1097/00005131-200208000-00009.
- Zhang H, Min L, Wang GL, et al. Primary open reduction and internal fixation with headless compression screws in the treatment of Chinese patients with acute Lisfranc joint injuries. J Trauma Acute Care Surg. 2012;72(5):1380-1385. doi:10.1097/TA.0b013e318246eabc.
- Lucas KJ, Morris RP, Buford WL Jr, Panchbhavi VK. Biomechanical comparison of first metatarsophalangeal joint arthrodeses using triple-threaded headless screws versus partially threaded lag screws. Foot Ankle Surg. 2014;20(2):144-148. doi:10.1016/j.fas.2014.02.009.
- Iwamoto T, Matsumura N, Sato K, Momohara S, Toyama Y, Nakamura T. An obliquely placed headless compression screw for distal interphalangeal joint arthrodesis. J Hand Surg. 2013;38(12):2360-2364. doi:10.1016/j.jhsa.2013.09.026.
- Odutola AA, Sheridan BD, Kelly AJ. Headless compression screw fixation prevents symptomatic metalwork in arthroscopic ankle arthrodesis. Foot Ankle Surg. 2012;18(2):111-113. doi:10.1016/j.fas.2011.03.013.
- Capelle JH, Couch CG, Wells KM, et al. Fixation strength of anteriorly inserted headless screws for talar neck fractures. Foot Ankle Int. 2013;34(7):1012-1016. doi:10.1177/1071100713479586.
- Wheeler DL, McLoughlin SW. Biomechanical assessment of compression screws. Clin Orthop Relat Res. 1998;350(350):237-245. doi:10.1097/00003086-199805000-00032.
- Rockwood CA, Green DP, Bucholz RW. Rockwood and Green's Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
- Simanski CJ, Maegele MG, Lefering R, et al. Functional treatment and early weightbearing after an ankle fracture: a prospective study. J Orthop Trauma. 2006;20(2):108-114. doi:10.1097/01.bot.0000197701.96954.8c.
- Reimer H, Kreibich M, Oettinger W. Extended uses for the Herbert/Whipple screw: six case reports out of 35 illustrating technique. J Orthop Trauma. 1996;10(1):7-14. doi:10.1097/00005131-199601000-00002.
- Barnes H, Cannada LK, Watson JT. A clinical evaluation of alternative fixation techniques for medial malleolus fractures. Injury. 2014;45(9):1365-1367. doi:10.1016/j.injury.2014.05.031.
- Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691. doi:10.1097/01.bot.0000247075.17548.3a.
- Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489. doi:10.1177/107110079401500905.
- Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357. doi:10.2106/00004623-197658030-00010.
- Thordarson DB, Motamed S, Hedman T, Ebramzadeh E, Bakshian S. The effect of fibular malreduction on contact pressures in an ankle fracture malunion model. J Bone Joint Surg Am. 1997;79(12):1809-1815. doi:10.2106/00004623-199712000-00006.
- Amanatullah DF, Khan SN, Curtiss S, Wolinsky PR. Effect of divergent screw fixation in vertical medial malleolus fractures. J Trauma Acute Care Surg. 2012;72(3):751-754. doi:10.1097/TA.0b013e31823b8b9f.
- Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284. doi:10.1016/j.jbiomech.2008.08.013.
- Brown GA, McCarthy T, Bourgeault CA, Callahan DJ. Mechanical performance of standard and cannulated 4.0-mm cancellous bone screws. J Orthop Res. 2000;18(2):307-312. doi:10.1002/jor.1100180220.
- Merk BR, Stern SH, Cordes S, Lautenschlager EP. A fatigue life analysis of small fragment screws. J Orthop Trauma. 2001;15(7):494-499. doi:10.1097/00005131-200109000-00006.
ABSTRACT
This study is the first biomechanical research of headless compression screws for fixation of vertical shear fractures of the medial malleolus, a promising alternative that potentially offers several advantages for fixation.
Vertical shear fractures were simulated by osteotomies in 20 synthetic distal tibiae. Models were randomly assigned to fixation with either 2 parallel cancellous screws or 2 parallel Acutrak 2 headless compression screws (Acumed). Specimens were subjected to offset axial loading to simulate supination-adduction loading and tracked using high-resolution video.
The headless compression screw construct was significantly stiffer (P < .0001) (360 ± 131 N/mm) than the partially threaded cancellous screws (180 ± 48 N/mm) and demonstrated a significantly increased (P < .0001) mean load to clinical failure (719 ± 91 N vs 343 ± 83 N). When specimens were displaced to 6 mm and allowed to relax, the headless compression screw constructs demonstrated an elastic recoil and were reduced to the pretesting fragment alignment, whereas the parallel cancellous screw constructs remained displaced.
Along with the headless design that may decrease soft tissue irritation, the increased stiffness and elastic recoil of the headless compression screw construct offers improved fixation of medial malleolus vertical shear fractures over the traditional methods.
Continue to: Headless compressions screws...
Headless compressions screws are cannulated tapered titanium screws with variable thread pitch angle, allowing a fully threaded screw to apply compression along its entire length. These screws have been most commonly used for scaphoid fractures1 but have also been studied in fractures of small bones, such as capitellum, midfoot, and talar neck,2-4 and arthrodesis in the foot, ankle, and hand.5-7 Headless compression screws have been found to produce equivalent fragment compression to partially threaded cancellous screws while allowing less fragment displacement.8,9 The lack of a head may decrease soft tissue irritation compared with the partially threaded cancellous screws. Finally, headless compression screws are independent of cortical integrity, as the entire length of the screw features a wide thread diameter to capture cancellous bone in the proximal fragment, unlike partially threaded cancellous screws, which only possess a thread purchase in the distal fragment and depend on an intact cortex.
Vertical shear fractures of the medial malleolus occur through the supination-adduction of the talus exerted onto the articular surface of the medial malleolus.10 Optimal fixation of these fractures must be sufficient to maintain stable anatomic reduction of the ankle joint articular surface, allowing early range of motion, maintaining congruency of the ankle joint, and decreasing the risk of future post-traumatic arthritis to maximize functional outcome.11
A wide variety of techniques are available for fixation of these fractures, including various configurations of cortical screws, cancellous screws, tension bands, and antiglide plates. Clinically, 2 parallel 4.0-mm partially threaded cancellous screws are most often used. Limited evidence indicates that headless compression screws may be a viable option for fixation of medial malleolus fractures. One case reports the use of a headless compression screw for a horizontal medial malleolar fracture,12 and a small retrospective case series that used headless compression screws for all medial malleolar fractures showed satisfactory outcomes, a high union rate, and low patient-reported pain.13
We evaluate the stiffness, force to 2-mm displacement of the joint surface, and elastic properties of these 2 different constructs in vertical medial malleolar fractures in synthetic distal tibiae. We hypothesize that the parallel headless compression screw fixation will be stiffer and require more force to 2-mm displacement than parallel unicortical cancellous screw fixation.
MATERIALS AND METHODS
Identical vertical osteotomies (17.5 mm) were made from the medial border of the medial malleolus using a custom jig in 20 left 4th-generation composite synthetic distal tibiae (Sawbones, Pacific Research Labs; Model No. 3401) to simulate an Orthopaedic Trauma Association type 44-A2.3 fracture. The tibiae were then cut 18 cm from the tibial plafond and randomized to 2 fixation groups (n = 10 specimens for each group): parallel unicortical screw fixation or parallel unicortical headless compression screw fixation (Figures 1A-1D). Custom polymethylmethacrylate jigs were used to reproducibly drill identical holes with a 3.2-mm drill for the parallel unicortical screw construct and the drill bits provided by the Acutrak 2 Headless Compression Screw System (Acumed). The parallel unicortical screw construct consisted of 2 parallel 4.0-mm-diameter, 40-mm partially threaded cancellous screws (Depuy Synthes), and the headless compression fixation construct consisted of 2 parallel 4.7-mm-diameter, 45-mm titanium Acutrak 2 screws parallel to each other in the transverse plane. The Acutrak screws were placed per manufacturer instructions by first drilling with the Acutrak 2-4.7 Long Drill bit (Acumed), followed by the Acutrak 2-4.7 Profile Drill bit for the near cortex.

Continue to: Specimens...
Specimens were fixed to the base of a servohydraulic testing machine (Model 809, MTS Systems Corporation) with an axial-torsional load transducer (Model No. 662.20-01; Axial capacity of 250 kg, torsional capacity 2.88 kg-m; MTS Systems Corporation). The specimens were set in a vice tilted at 17° in the coronal plane to allow the MTS crosshead to apply an offset axial load simulating supination-adduction loading, which has been described previously (Figure 2).14,15 Load was applied to the inferolateral articular surface of the medial malleolus at 1 mm/s to a crosshead displacement of 6 mm and then cycled back to 0 mm. Load and axial displacement were measured at 60 Hz. The markers on the distal tibia and medial malleolus fracture fragment were tracked using high-resolution video (Fastcam PCI, Photron USA Inc). The motion of the video markers was determined using digitization and motion analysis software (Motus 9, Vicon).

Stiffness was calculated as the slope of the linear portion of the load-displacement curve over a range of 0.5 to 2.0 mm (Figure 3) and reported as mean (standard deviation). The force at 2 mm of fragment displacement was defined as a clinical failure.16,17 Student’s t test was used to determine the difference in construct stiffness and force for 2 mm displacement of the 2 groups. Significance was defined as P < .05. Institutional Review Board approval was not required for this study.

RESULTS
With offset axial testing to simulate supination-adduction force along with video motion analysis, the mean stiffness (± standard deviation) measured 180 ± 48 N/mm for the parallel unicortical screw fixation construct and 360 ± 131 N/mm for the headless compression screw fixation construct (Figure 4A). The headless compression screw fixation construct was over 2 times stiffer than the parallel unicortical construct during initial displacement of the fracture, indicating a statistically significant difference (P < .0001).
The mean force for 2 mm of fracture displacement, defined as clinical failure, reached 342 ± 83 N for the parallel unicortical screw fixation construct and 719 ± 91 N for the headless compression screw fixation construct (Figure 4B). The headless compression screw fixation construct resisted displacement significantly more (P = .0001) than the parallel unicortical screw construct, presenting a 100% increase.

Upon cycling of the servohydraulic testing machine back to 0-mm displacement, the parallel unicortical construct demonstrated no elastic recoil, remaining displaced at 4 mm, whereas the headless compression screw construct rebounded to almost 0-mm displacement, which is well below the clinical definition of fixation failure of 2 mm (Figure 5).

Continue to: Discussion...
DISCUSSION
When subjected to offset axial load, we observed that the headless compression screw construct exhibited significantly increased stiffness and load to 2 mm of displacement compared with a parallel unicortical screw construct. The headless compression screw also demonstrated elastic recoil to almost 0 mm of displacement, which is well below the 2-mm displacement.
We made reproducible fractures and fixation methods in synthetic distal tibiae, which feature less variability in size and quality than the cadaveric bone. Offset axial loading, rather than direct axial loading previously described by Amanatullah and colleagues,18 is the most physiologically relevant mode of force application to simulate the loading of the tauls onto the medial malleolus in the supination-adduction mechanism of injury.
The limitations of this study include the use of synthetic rather than cadaveric bone. Fourth-generation sawbones have been validated as possessing similar biomechanical properties as real bone.7,19 These results may also be inapplicable to osteoporotic bone, which would be significantly less dense than sawbones. This study is also an artificial situation designed to only test construct stiffness and load to clinical failure in a single mode of stress, offset axial loading and neglects other possible modes of force. This testing setup also disregards the structures surrounding the medial malleolus and tibia, including the talus, fibula, or soft tissue attachments, including the deltoid ligament and flexor retinaculum. These results are only relevant immediately after fixation and before bone healing occurs. We also tested the load to clinical failure rather than cyclic loading. Our testing more closely modeled a single traumatic force rather than the considerably smaller stresses that would be repeatedly exerted on the construct over several weeks after fixation in a clinical situation. This research is also not a clinical outcome study, rather, it suggests that headless compression screws are a viable, stronger, and possibly superior method for the initial fixation of vertical medial malleolar fractures.
As the load is offset axial, the larger thread purchase of the headless compression screws may lead to increased pullout strength, possibly increasing headless compression screw construct stiffness. Also, the variable diameter of headless compression screw, which reaches up to 4.7 mm, would increase the stiffness of the construct compared with the diameter of the cancellous screws. The elasticity of the headless compression construct may be because screws are made of titanium rather than stainless steel. Such property and given that the screws are cannulated rather than solid may also play a role, although several studies have shown variable results for cannulated vs solid screws of the same diameter.20,21 The elastic section modulus of both screws would have to be calculated to determine their exact effect on fixation.
CONCLUSION
The headless compression screw construct was found to be stiffer and features a higher load to clinical failure than a parallel unicortical cancellous screw construct for fixation of vertical medial malleolus fractures. Although significantly increased cost occurs with this construct, the headless design may decrease soft tissue irritation, and the elastic recoil of the construct after displacement may decrease clinical failure rates of this fixation method. This condition would eliminate the need for revision surgeries and thus be a cost effective alternative overall.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
This study is the first biomechanical research of headless compression screws for fixation of vertical shear fractures of the medial malleolus, a promising alternative that potentially offers several advantages for fixation.
Vertical shear fractures were simulated by osteotomies in 20 synthetic distal tibiae. Models were randomly assigned to fixation with either 2 parallel cancellous screws or 2 parallel Acutrak 2 headless compression screws (Acumed). Specimens were subjected to offset axial loading to simulate supination-adduction loading and tracked using high-resolution video.
The headless compression screw construct was significantly stiffer (P < .0001) (360 ± 131 N/mm) than the partially threaded cancellous screws (180 ± 48 N/mm) and demonstrated a significantly increased (P < .0001) mean load to clinical failure (719 ± 91 N vs 343 ± 83 N). When specimens were displaced to 6 mm and allowed to relax, the headless compression screw constructs demonstrated an elastic recoil and were reduced to the pretesting fragment alignment, whereas the parallel cancellous screw constructs remained displaced.
Along with the headless design that may decrease soft tissue irritation, the increased stiffness and elastic recoil of the headless compression screw construct offers improved fixation of medial malleolus vertical shear fractures over the traditional methods.
Continue to: Headless compressions screws...
Headless compressions screws are cannulated tapered titanium screws with variable thread pitch angle, allowing a fully threaded screw to apply compression along its entire length. These screws have been most commonly used for scaphoid fractures1 but have also been studied in fractures of small bones, such as capitellum, midfoot, and talar neck,2-4 and arthrodesis in the foot, ankle, and hand.5-7 Headless compression screws have been found to produce equivalent fragment compression to partially threaded cancellous screws while allowing less fragment displacement.8,9 The lack of a head may decrease soft tissue irritation compared with the partially threaded cancellous screws. Finally, headless compression screws are independent of cortical integrity, as the entire length of the screw features a wide thread diameter to capture cancellous bone in the proximal fragment, unlike partially threaded cancellous screws, which only possess a thread purchase in the distal fragment and depend on an intact cortex.
Vertical shear fractures of the medial malleolus occur through the supination-adduction of the talus exerted onto the articular surface of the medial malleolus.10 Optimal fixation of these fractures must be sufficient to maintain stable anatomic reduction of the ankle joint articular surface, allowing early range of motion, maintaining congruency of the ankle joint, and decreasing the risk of future post-traumatic arthritis to maximize functional outcome.11
A wide variety of techniques are available for fixation of these fractures, including various configurations of cortical screws, cancellous screws, tension bands, and antiglide plates. Clinically, 2 parallel 4.0-mm partially threaded cancellous screws are most often used. Limited evidence indicates that headless compression screws may be a viable option for fixation of medial malleolus fractures. One case reports the use of a headless compression screw for a horizontal medial malleolar fracture,12 and a small retrospective case series that used headless compression screws for all medial malleolar fractures showed satisfactory outcomes, a high union rate, and low patient-reported pain.13
We evaluate the stiffness, force to 2-mm displacement of the joint surface, and elastic properties of these 2 different constructs in vertical medial malleolar fractures in synthetic distal tibiae. We hypothesize that the parallel headless compression screw fixation will be stiffer and require more force to 2-mm displacement than parallel unicortical cancellous screw fixation.
MATERIALS AND METHODS
Identical vertical osteotomies (17.5 mm) were made from the medial border of the medial malleolus using a custom jig in 20 left 4th-generation composite synthetic distal tibiae (Sawbones, Pacific Research Labs; Model No. 3401) to simulate an Orthopaedic Trauma Association type 44-A2.3 fracture. The tibiae were then cut 18 cm from the tibial plafond and randomized to 2 fixation groups (n = 10 specimens for each group): parallel unicortical screw fixation or parallel unicortical headless compression screw fixation (Figures 1A-1D). Custom polymethylmethacrylate jigs were used to reproducibly drill identical holes with a 3.2-mm drill for the parallel unicortical screw construct and the drill bits provided by the Acutrak 2 Headless Compression Screw System (Acumed). The parallel unicortical screw construct consisted of 2 parallel 4.0-mm-diameter, 40-mm partially threaded cancellous screws (Depuy Synthes), and the headless compression fixation construct consisted of 2 parallel 4.7-mm-diameter, 45-mm titanium Acutrak 2 screws parallel to each other in the transverse plane. The Acutrak screws were placed per manufacturer instructions by first drilling with the Acutrak 2-4.7 Long Drill bit (Acumed), followed by the Acutrak 2-4.7 Profile Drill bit for the near cortex.

Continue to: Specimens...
Specimens were fixed to the base of a servohydraulic testing machine (Model 809, MTS Systems Corporation) with an axial-torsional load transducer (Model No. 662.20-01; Axial capacity of 250 kg, torsional capacity 2.88 kg-m; MTS Systems Corporation). The specimens were set in a vice tilted at 17° in the coronal plane to allow the MTS crosshead to apply an offset axial load simulating supination-adduction loading, which has been described previously (Figure 2).14,15 Load was applied to the inferolateral articular surface of the medial malleolus at 1 mm/s to a crosshead displacement of 6 mm and then cycled back to 0 mm. Load and axial displacement were measured at 60 Hz. The markers on the distal tibia and medial malleolus fracture fragment were tracked using high-resolution video (Fastcam PCI, Photron USA Inc). The motion of the video markers was determined using digitization and motion analysis software (Motus 9, Vicon).

Stiffness was calculated as the slope of the linear portion of the load-displacement curve over a range of 0.5 to 2.0 mm (Figure 3) and reported as mean (standard deviation). The force at 2 mm of fragment displacement was defined as a clinical failure.16,17 Student’s t test was used to determine the difference in construct stiffness and force for 2 mm displacement of the 2 groups. Significance was defined as P < .05. Institutional Review Board approval was not required for this study.

RESULTS
With offset axial testing to simulate supination-adduction force along with video motion analysis, the mean stiffness (± standard deviation) measured 180 ± 48 N/mm for the parallel unicortical screw fixation construct and 360 ± 131 N/mm for the headless compression screw fixation construct (Figure 4A). The headless compression screw fixation construct was over 2 times stiffer than the parallel unicortical construct during initial displacement of the fracture, indicating a statistically significant difference (P < .0001).
The mean force for 2 mm of fracture displacement, defined as clinical failure, reached 342 ± 83 N for the parallel unicortical screw fixation construct and 719 ± 91 N for the headless compression screw fixation construct (Figure 4B). The headless compression screw fixation construct resisted displacement significantly more (P = .0001) than the parallel unicortical screw construct, presenting a 100% increase.

Upon cycling of the servohydraulic testing machine back to 0-mm displacement, the parallel unicortical construct demonstrated no elastic recoil, remaining displaced at 4 mm, whereas the headless compression screw construct rebounded to almost 0-mm displacement, which is well below the clinical definition of fixation failure of 2 mm (Figure 5).

Continue to: Discussion...
DISCUSSION
When subjected to offset axial load, we observed that the headless compression screw construct exhibited significantly increased stiffness and load to 2 mm of displacement compared with a parallel unicortical screw construct. The headless compression screw also demonstrated elastic recoil to almost 0 mm of displacement, which is well below the 2-mm displacement.
We made reproducible fractures and fixation methods in synthetic distal tibiae, which feature less variability in size and quality than the cadaveric bone. Offset axial loading, rather than direct axial loading previously described by Amanatullah and colleagues,18 is the most physiologically relevant mode of force application to simulate the loading of the tauls onto the medial malleolus in the supination-adduction mechanism of injury.
The limitations of this study include the use of synthetic rather than cadaveric bone. Fourth-generation sawbones have been validated as possessing similar biomechanical properties as real bone.7,19 These results may also be inapplicable to osteoporotic bone, which would be significantly less dense than sawbones. This study is also an artificial situation designed to only test construct stiffness and load to clinical failure in a single mode of stress, offset axial loading and neglects other possible modes of force. This testing setup also disregards the structures surrounding the medial malleolus and tibia, including the talus, fibula, or soft tissue attachments, including the deltoid ligament and flexor retinaculum. These results are only relevant immediately after fixation and before bone healing occurs. We also tested the load to clinical failure rather than cyclic loading. Our testing more closely modeled a single traumatic force rather than the considerably smaller stresses that would be repeatedly exerted on the construct over several weeks after fixation in a clinical situation. This research is also not a clinical outcome study, rather, it suggests that headless compression screws are a viable, stronger, and possibly superior method for the initial fixation of vertical medial malleolar fractures.
As the load is offset axial, the larger thread purchase of the headless compression screws may lead to increased pullout strength, possibly increasing headless compression screw construct stiffness. Also, the variable diameter of headless compression screw, which reaches up to 4.7 mm, would increase the stiffness of the construct compared with the diameter of the cancellous screws. The elasticity of the headless compression construct may be because screws are made of titanium rather than stainless steel. Such property and given that the screws are cannulated rather than solid may also play a role, although several studies have shown variable results for cannulated vs solid screws of the same diameter.20,21 The elastic section modulus of both screws would have to be calculated to determine their exact effect on fixation.
CONCLUSION
The headless compression screw construct was found to be stiffer and features a higher load to clinical failure than a parallel unicortical cancellous screw construct for fixation of vertical medial malleolus fractures. Although significantly increased cost occurs with this construct, the headless design may decrease soft tissue irritation, and the elastic recoil of the construct after displacement may decrease clinical failure rates of this fixation method. This condition would eliminate the need for revision surgeries and thus be a cost effective alternative overall.
This paper will be judged for the Resident Writer’s Award.
- Fowler JR, Ilyas AM. Headless compression screw fixation of scaphoid fractures. Hand Clin. 2010;26(3):351-361, vi. doi:10.1016/j.hcl.2010.04.005.
- Karakasli A, Hapa O, Erduran M, Dincer C, Cecen B, Havitcioglu H. Mechanical comparison of headless screw fixation and locking plate fixation for talar neck fractures. J Foot Ankle Surg. 2015;54(5):905-909. doi:10.1053/j.jfas.2015.04.002.
- Elkowitz SJ, Polatsch DB, Egol KA, Kummer FJ, Koval KJ. Capitellum fractures: a biomechanical evaluation of three fixation methods. J Orthop Trauma. 2002;16(7):503-506. doi:10.1097/00005131-200208000-00009.
- Zhang H, Min L, Wang GL, et al. Primary open reduction and internal fixation with headless compression screws in the treatment of Chinese patients with acute Lisfranc joint injuries. J Trauma Acute Care Surg. 2012;72(5):1380-1385. doi:10.1097/TA.0b013e318246eabc.
- Lucas KJ, Morris RP, Buford WL Jr, Panchbhavi VK. Biomechanical comparison of first metatarsophalangeal joint arthrodeses using triple-threaded headless screws versus partially threaded lag screws. Foot Ankle Surg. 2014;20(2):144-148. doi:10.1016/j.fas.2014.02.009.
- Iwamoto T, Matsumura N, Sato K, Momohara S, Toyama Y, Nakamura T. An obliquely placed headless compression screw for distal interphalangeal joint arthrodesis. J Hand Surg. 2013;38(12):2360-2364. doi:10.1016/j.jhsa.2013.09.026.
- Odutola AA, Sheridan BD, Kelly AJ. Headless compression screw fixation prevents symptomatic metalwork in arthroscopic ankle arthrodesis. Foot Ankle Surg. 2012;18(2):111-113. doi:10.1016/j.fas.2011.03.013.
- Capelle JH, Couch CG, Wells KM, et al. Fixation strength of anteriorly inserted headless screws for talar neck fractures. Foot Ankle Int. 2013;34(7):1012-1016. doi:10.1177/1071100713479586.
- Wheeler DL, McLoughlin SW. Biomechanical assessment of compression screws. Clin Orthop Relat Res. 1998;350(350):237-245. doi:10.1097/00003086-199805000-00032.
- Rockwood CA, Green DP, Bucholz RW. Rockwood and Green's Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
- Simanski CJ, Maegele MG, Lefering R, et al. Functional treatment and early weightbearing after an ankle fracture: a prospective study. J Orthop Trauma. 2006;20(2):108-114. doi:10.1097/01.bot.0000197701.96954.8c.
- Reimer H, Kreibich M, Oettinger W. Extended uses for the Herbert/Whipple screw: six case reports out of 35 illustrating technique. J Orthop Trauma. 1996;10(1):7-14. doi:10.1097/00005131-199601000-00002.
- Barnes H, Cannada LK, Watson JT. A clinical evaluation of alternative fixation techniques for medial malleolus fractures. Injury. 2014;45(9):1365-1367. doi:10.1016/j.injury.2014.05.031.
- Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691. doi:10.1097/01.bot.0000247075.17548.3a.
- Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489. doi:10.1177/107110079401500905.
- Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357. doi:10.2106/00004623-197658030-00010.
- Thordarson DB, Motamed S, Hedman T, Ebramzadeh E, Bakshian S. The effect of fibular malreduction on contact pressures in an ankle fracture malunion model. J Bone Joint Surg Am. 1997;79(12):1809-1815. doi:10.2106/00004623-199712000-00006.
- Amanatullah DF, Khan SN, Curtiss S, Wolinsky PR. Effect of divergent screw fixation in vertical medial malleolus fractures. J Trauma Acute Care Surg. 2012;72(3):751-754. doi:10.1097/TA.0b013e31823b8b9f.
- Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284. doi:10.1016/j.jbiomech.2008.08.013.
- Brown GA, McCarthy T, Bourgeault CA, Callahan DJ. Mechanical performance of standard and cannulated 4.0-mm cancellous bone screws. J Orthop Res. 2000;18(2):307-312. doi:10.1002/jor.1100180220.
- Merk BR, Stern SH, Cordes S, Lautenschlager EP. A fatigue life analysis of small fragment screws. J Orthop Trauma. 2001;15(7):494-499. doi:10.1097/00005131-200109000-00006.
- Fowler JR, Ilyas AM. Headless compression screw fixation of scaphoid fractures. Hand Clin. 2010;26(3):351-361, vi. doi:10.1016/j.hcl.2010.04.005.
- Karakasli A, Hapa O, Erduran M, Dincer C, Cecen B, Havitcioglu H. Mechanical comparison of headless screw fixation and locking plate fixation for talar neck fractures. J Foot Ankle Surg. 2015;54(5):905-909. doi:10.1053/j.jfas.2015.04.002.
- Elkowitz SJ, Polatsch DB, Egol KA, Kummer FJ, Koval KJ. Capitellum fractures: a biomechanical evaluation of three fixation methods. J Orthop Trauma. 2002;16(7):503-506. doi:10.1097/00005131-200208000-00009.
- Zhang H, Min L, Wang GL, et al. Primary open reduction and internal fixation with headless compression screws in the treatment of Chinese patients with acute Lisfranc joint injuries. J Trauma Acute Care Surg. 2012;72(5):1380-1385. doi:10.1097/TA.0b013e318246eabc.
- Lucas KJ, Morris RP, Buford WL Jr, Panchbhavi VK. Biomechanical comparison of first metatarsophalangeal joint arthrodeses using triple-threaded headless screws versus partially threaded lag screws. Foot Ankle Surg. 2014;20(2):144-148. doi:10.1016/j.fas.2014.02.009.
- Iwamoto T, Matsumura N, Sato K, Momohara S, Toyama Y, Nakamura T. An obliquely placed headless compression screw for distal interphalangeal joint arthrodesis. J Hand Surg. 2013;38(12):2360-2364. doi:10.1016/j.jhsa.2013.09.026.
- Odutola AA, Sheridan BD, Kelly AJ. Headless compression screw fixation prevents symptomatic metalwork in arthroscopic ankle arthrodesis. Foot Ankle Surg. 2012;18(2):111-113. doi:10.1016/j.fas.2011.03.013.
- Capelle JH, Couch CG, Wells KM, et al. Fixation strength of anteriorly inserted headless screws for talar neck fractures. Foot Ankle Int. 2013;34(7):1012-1016. doi:10.1177/1071100713479586.
- Wheeler DL, McLoughlin SW. Biomechanical assessment of compression screws. Clin Orthop Relat Res. 1998;350(350):237-245. doi:10.1097/00003086-199805000-00032.
- Rockwood CA, Green DP, Bucholz RW. Rockwood and Green's Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
- Simanski CJ, Maegele MG, Lefering R, et al. Functional treatment and early weightbearing after an ankle fracture: a prospective study. J Orthop Trauma. 2006;20(2):108-114. doi:10.1097/01.bot.0000197701.96954.8c.
- Reimer H, Kreibich M, Oettinger W. Extended uses for the Herbert/Whipple screw: six case reports out of 35 illustrating technique. J Orthop Trauma. 1996;10(1):7-14. doi:10.1097/00005131-199601000-00002.
- Barnes H, Cannada LK, Watson JT. A clinical evaluation of alternative fixation techniques for medial malleolus fractures. Injury. 2014;45(9):1365-1367. doi:10.1016/j.injury.2014.05.031.
- Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691. doi:10.1097/01.bot.0000247075.17548.3a.
- Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489. doi:10.1177/107110079401500905.
- Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357. doi:10.2106/00004623-197658030-00010.
- Thordarson DB, Motamed S, Hedman T, Ebramzadeh E, Bakshian S. The effect of fibular malreduction on contact pressures in an ankle fracture malunion model. J Bone Joint Surg Am. 1997;79(12):1809-1815. doi:10.2106/00004623-199712000-00006.
- Amanatullah DF, Khan SN, Curtiss S, Wolinsky PR. Effect of divergent screw fixation in vertical medial malleolus fractures. J Trauma Acute Care Surg. 2012;72(3):751-754. doi:10.1097/TA.0b013e31823b8b9f.
- Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284. doi:10.1016/j.jbiomech.2008.08.013.
- Brown GA, McCarthy T, Bourgeault CA, Callahan DJ. Mechanical performance of standard and cannulated 4.0-mm cancellous bone screws. J Orthop Res. 2000;18(2):307-312. doi:10.1002/jor.1100180220.
- Merk BR, Stern SH, Cordes S, Lautenschlager EP. A fatigue life analysis of small fragment screws. J Orthop Trauma. 2001;15(7):494-499. doi:10.1097/00005131-200109000-00006.
TAKE-HOME POINTS
- Optimal fixation of vertical sheer ankle fractures is unknown.
- Headless compression screws are stiffer than cancellous screws in offset axial load.
- Headless compression screws have a higher load to failure than cancellous screws.
- Headless compression screws may offer a soft tissue friendly fixation of method for vertical sheer ankle fractures.
- These findings may not apply when subject to cyclic loads or in osteoporotic bone.
Tranexamic Acid Reduces Perioperative Blood Loss and Hemarthrosis in Total Ankle Arthroplasty
ABSTRACT
Tranexamic acid (TXA) is an effective agent used for reducing perioperative blood loss and decreasing the potential for postoperative hemarthrosis. We hypothesized that patients who had received intraoperative TXA during total ankle arthroplasty (TAA) would have a reduction in postoperative drain output, thereby resulting in a reduced risk of postoperative hemarthrosis and lower wound complication rates.
A retrospective review was conducted on 50 consecutive patients, 25 receiving TXA (TXA-TAA) and 25 not receiving TXA (No TXA-TAA), who underwent an uncemented TAA between September 2011 and December 2015. Demographic characteristics, drain output, preoperative and postoperative hemoglobin levels, operative and postoperative course, and minor and major wound complications of the patients were reviewed.
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P < .0001). The overall wound complication rate in the No TXA-TAA group was higher (20%, 5/25) than that in the TXA-TAA group (8%, 2/25) (P = .114). The mean change in preoperative to postoperative hemoglobin level was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively, P = .01).
TXA is an effective hemostatic agent when used during TAA. TXA reduces perioperative blood loss, hemarthrosis, and the risk of wound complications.
Continue to: End-stage ankle arthritis...
End-stage ankle arthritis is a disabling condition that may lead to poor quality of life and difficulties with activities of daily living.1 The associated mental and physical disability has been demonstrated to be as severe as in end-stage hip arthrosis.2 Operative treatment for symptomatic end-stage ankle arthritis includes arthrodesis or total ankle arthroplasty (TAA) in those refractory to nonoperative treatment.3 Newer generation implants have made TAA a more attractive option for both the surgeon and the patient.
Over the past decade, the utility of TAA has increased and attention has turned toward the management of perioperative factors that would maximize patient satisfaction and decrease the length of stay and complication rates, as well as hospital costs.4 Comprehensive literature on total knee arthroplasty (TKA) and total hip arthroplasty (THA) has demonstrated that the management of perioperative blood loss, specifically postoperative hemarthrosis, is a modifiable factor affecting patient recovery, complication rates, and hospital costs.5-8 Drain output has been used as a direct measure of intra-articular blood accumulation.9 Decreased drain output implies decreased hemarthrosis, which could potentially alleviate the pressure on the wound and decrease wound complications.
One of the major strategies that has been recognized for reducing blood loss and decreasing the potential for postoperative hemarthrosis is the use of intravenous (IV) or topical tranexamic acid (TXA).10,11 TXA is a synthetic antifibrinolytic medication that has been extensively used throughout the medical field since the 1960s to help control the bleeding cascade. This medication stabilizes clot formation without inducing a pro-coaguable state.12 Intraoperative administration of TXA has been shown to reduce drain output and decrease transfusion requirements after TKA and THA without an associated increase in patient morbidity and mortality.6,11,13-15
Currently, there is a lack of studies evaluating the utility of TXA during TAA. We hypothesize that compared with patients who had not received TXA, those who had received intraoperative TXA during TAA would have a reduction in postoperative drain output and therefore decreased hemarthrosis, lower wound complication rate, and a diminished change in preoperative to postoperative hemoglobin levels, reflecting a reduction in perioperative blood loss.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University at Buffalo, State University of New York. A retrospective chart review was conducted on 50 consecutive patients who underwent an uncemented TAA with the Salto Talaris total ankle prosthesis (Tornier, Inc) between September 2011 and December 2015. All surgeries were performed at 1 institution by a single fellowship surgeon trained in foot and ankle surgery through the anterior approach where a midline incision was made over the ankle. The interval between the tibialis anterior tendon and the extensor hallucis longus tendon was used. We had incorporated intraoperative TXA into the TAA surgical protocol at our institution in January 2014. We evaluated the first 25 consecutive patients who underwent TAA after TXA use began (TXA-TAA) and another 25 consecutive patients who underwent TAA before the routine use of TXA (No TXA-TAA). Inclusion criteria were patients who presented with pain, decreased function, and radiographic parameters of end-stage tibiotalar arthritis due to degenerative arthritis, rheumatoid arthritis, or posttraumatic arthritis who subsequently underwent a TAA. Exclusion criteria were patients with a contraindication for IV TXA use, a preexisting coagulopathy, or where drain output was not recorded. Contraindications for IV TXA use included patients with impaired renal clearance, recent cardiac surgery, myocardial infarction, ischemic stroke, or venous thromboembolism (VTE). Seven patients were ultimately excluded from this study based on the inclusion and exclusion criteria, 3 patients from the TXA-TAA group and 4 patients from the No TXA-TAA group.
Continue to: Charts were reviewed for demographics...
Charts were reviewed for demographics, preoperative and postoperative hemoglobin levels, indications for surgery, surgical procedures, length of surgery, postoperative drain output, length of stay, postoperative pain visual analog scale (VAS) score, minor and major wound complications, and postoperative complications. Minor wound complications were defined as the anterior surgical incision that required local wound care in office or oral antibiotics without subsequent consequences. Major wound complications were defined as requiring surgical débridement and/or any additional treatment in the operating room.16 Postoperative complications other than wound complications were defined as those requiring a subsequent surgical intervention. Patient demographics and clinical and procedural characteristics of patients in both the TXA-TAA and the No TXA-TAA groups are outlined in Table 1. There were 14 males and 11 females in the TXA-TAA group and 16 males and 9 females in the No TXA-TAA group. The mean age was 65.8 ± 10.9 years in the TXA-TAA group and 66.9 ± 8.0 years in the No TXA-TAA group (P = .69). Mean body mass index (BMI) was 31.6 ± 6.3 in the TXA-TAA group and 29.4 ± 4.9 in the No TXA-TAA group (P = .18). The primary indication for TAA was degenerative osteoarthritis in 26 patients, posttraumatic arthritis in 21 patients, and rheumatoid arthritis in 3 patients. The most common associated procedure was Achilles tendon lengthening in both groups. The mean follow-up in the TXA-TAA group was 9.3 ± 5.8 months (range, 2.0-24.0 months). Postoperative complications due to TXA administration as described in previous literature were defined as VTE, myocardial infarction, or ischemic cerebral event. The TXA-TAA group received a standard 1 g dose of IV TXA 20 minutes prior to tourniquet inflation. A tourniquet was used intraoperatively on all patients included in this study. A postoperative 400-mL surgical drain (Hemovac, Zimmer Biomet) was placed in the ankle joint in all patients and subsequently discontinued on postoperative day 1. Recent literature has reported the minor wound complication rate associated with TAA to be as high as 25% and the major wound complication rate to be 8.5%.16 To assist in reducing the risk for wound complications, our protocol traditionally uses an intra-articular surgical drain to decrease any pressure on the wound from postoperative hemarthrosis.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
aP value was calculated from t-test continuous variables and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Total drain output was recorded in milliliters (mL) in all patients. The change between the preoperative hemoglobin level and the hemoglobin level on postoperative day 1 was calculated for each patient. The calculated blood loss was determined using Meunier’s equation, which estimates the total blood volume using Nadler’s formula and then uses preoperative hemoglobin and postoperative day 1 hemoglobin values to calculate blood loss.17,18 VAS scores (scale, 1-10) were obtained every 4 hours on postoperative day 1 according to the nursing protocol. The number 1 on the scale represents the least amount of pain, whereas 10 indicates the worst pain. The VAS scores were then averaged for each patient.
A power analysis using preliminary data determined that 15 patients were needed in each group to detect a 50% reduction in drain output at a power of 80% and a P value of 0.05. Descriptive statistics were used to analyze demographic data. We compared the demographic and clinical characteristics of patients in the TXA-TAA group with those of patients in the No TXA-TAA group using unpaired student t-tests for continuous variables and Chi-square or Fischer’s exact tests for categorical variables. Simple and adjusted linear regression analyses were used to examine the difference in drain output and blood loss between the 2 groups (TXA-TAA vs No TXA-TAA). Multivariate models were adjusted for age, BMI, and length of surgery. A P value <.05 was considered to be statistically significant. We performed all analyses using a statistical software package (SAS version 9.2, SAS Institute).
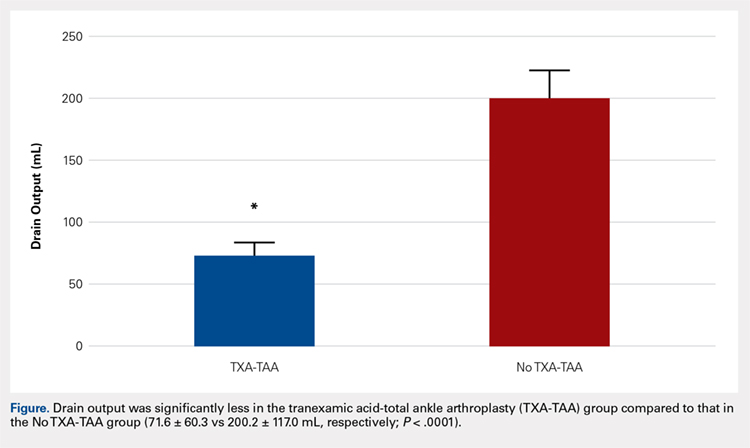
RESULTS
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P = .0001) (Figure). The clinical characteristics of the patients who underwent TAA with the use of TXA are outlined in Table 2. The mean change in preoperative to postoperative hemoglobin levels was significantly lower in the TXA-TAA group than in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively; P = .01). The calculated blood loss in patients in the TXA-TAA group was significantly lower than that in patients in the No TXA-TAA group (649.9 ± 332.7 vs 906.8 ± 287.4 mL, respectively; P = .01). No patient in either group received a blood transfusion. We did not observe a significant difference in the length of surgery between the TXA-TAA and the No TXA-TAA groups (112.8 ± 24.8 vs 108.6 ± 26.0 min, respectively; P = .57). The average American Society of Anesthesiologists’ (ASA) classification was similar between the groups (2.2 ± 0.6 and 2.2 ± 0.5, respectively; P = 1.00) as was the age-adjusted Charlson Comorbidity Index (2.8 ± 1.7 vs 2.9 ± 1.6, respectively; P = .93). Mean VAS scores on postoperative day 1 in the TXA-TAA and the No TXA-TAA group were 4.9 ± 1.7 and 5.3 ± 1.4, respectively (P = .71). The average length of stay in the TXA-TAA group was 1.6 ± 0.7 days vs 1.3 ± 0.6 days in the No TXA-TAA group (P = .23). Two patients in the TXA-TAA group had an extended hospital length of stay of 5 days due to discharge planning and social issues.
Table 2. Clinical Characteristics of Total Ankle Arthroplasty (TAA) Patients by Use of Tranexamic Acid (TXA), N = 50 | |||
|---|---|---|---|
| TXA use in TAA | P valuea | |
| Yes (n = 25 cases) | No (n = 25 controls) |
|
Clinical Characteristic |
|
|
|
Drain Output (ml), mean ± SD
| 71.6 ± 60.3 | 200.2 ± 117.0 | <0.0001 |
Preoperative to Postoperative Hgb Change (g/dL), mean ± SD
| 1.5 ± 0.6 | 2.0 ± 0.4 | 0.01 |
Blood Loss Calculated (ml), mean ± SD
| 649.9 ± 332.73 | 906.8 ± 287.4 | 0.01 |
Length of Surgery (min), mean ± SD
| 112.8 ± 24.8 | 108.6 ± 26.0 | 0.57 |
VAS scores on the POD (No.), mean ± SD
| 4.9 ± 1.7 | 5.3 ±1.4 | 0.71 |
LOS (day), mean ± SD
| 1.6 ± 0.7 | 1.3 ± 0.6 | 0.23 |
aP value was calculated from t-test for continuous variables, and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: LOS, length of stay; VAS, visual analog scale; POD, postoperative day.
Table 3. Linear Regression Analyses of Drain Output and Blood Loss using Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), Unadjusted and Adjusted Models for Length of Surgery, N = 50 | ||||
| TXA Use in TAA (Yes vs No) | |||
Drain Output (mL)
| Regression coefficient (β) | SE | Test statistics (t) | P valuea |
Unadjusted Model | -128.6 | 26.3 | -4.89 | < 0.0001 |
Adjusted for Age | -129.6 | 26.5 | -4.89 | <0.0001 |
Adjusted for BMI | -121.8 | 26.6 | -4.57 | <0.0001 |
Adjusted for Length of Surgery | -129.6 | 26.6 | -4.86 | <0.0001 |
Multivariable Modelb | -123.4 | 27.1 | -4.55 | <0.0001 |
Blood Loss (mL)
|
|
|
|
|
Unadjusted Model | -257.0 | 87.9 | -2.92 | 0.005 |
Adjusted for Age | -263.7 | 87.4 | -3.02 | 0.004 |
Adjusted for BMI | -268.7 | 90.2 | -2.98 | 0.005 |
Adjusted for Length of Surgery | -261.3 | 88.6 | -2.94 | 0.005 |
Multivariable Modelb | -275.6 | 90.7 | -3.04 | 0.004 |
aLinear regression was used to calculate the P value. bAdjusted for age, BMI and length of surgery.
Abbreviation: BMI, body mass index.
Table 4. Patient Wound Complication Categories by Use of Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), N = 50 | |||
|---|---|---|---|
| TXA Use in TAA | P valuea | |
Wound Complication | Yes (n = 25 cases) | No (n = 25 controls) | 0.114 |
None, n = 46 (86%) | 23 (40%) | 20 (46%) |
|
Minor, n = 6 (12%) | 2 (4%) | 4 (8%) |
|
Major, n = 1 (2%) | 0 (0%) | 1 (4%) |
|
aP value was calculated from Fisher’s Exact test (67% cells had count <5) test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
The crude linear regression model revealed a significant difference in drain output between the TXA-TAA and the No TXA-TAA groups (β = −128.6 ± 26.3, P < .0001) (Table 3). Further adjustment for age and length of surgery slightly strengthened the association (β = −129.6 ± 26.6, P < .0001). The nature of regression coefficient β showed that the mean estimate of drain output was 129.6 mL lower in the TXA-TAA group than that in the No TXA-TAA group. There was a significant difference in blood loss between the TXA-TAA and the No TXA-TAA groups in the crude linear regression model (β = −257.0 ± 87.9, P = .005). Additional adjustment for age, BMI, and length of surgery slightly strengthened the association (β = −275.6 ± 90.7, P = .004). The nature of regression coefficient β showed that the mean estimate of blood loss was 275.6 mL lower in the TXA-TAA group than in the No TXA-TAA group (Table 3).
Continue to: There was no statistically significant difference...
There was no statistically significant difference in wound complications between the TXA-TAA and the No TXA-TAA groups in this study population (P = .114). However, our results showed a higher overall wound complication rate in the No TXA-TAA group than in the TXA-TAA group (20% (5/25) vs 8% (2/25), respectively) (Table 4). In the No TXA-TAA group, there were 4 minor and 1 major wound complications. All 5 patients experiencing a postoperative wound complication required oral antibiotics for a minimum of 4 weeks and local wound care. One patient underwent a surgical débridement meeting the criteria for major wound complications. In the TXA-TAA group, there were 2 minor wound complications and no major wound complications. One patient was administered prophylactic oral antibiotics for 7 days with local wound care for blister formation without evidence of infection. The second patient experiencing a minor wound complication required 3 weeks of oral antibiotics and local wound care. No patients in either group had a deep infection requiring implant removal, IV antibiotics, or subsequent hospital admission. The surgical incisions in all patients healed after the aforementioned treatments with no persistent drainage or development of chronic wounds.
In the TXA-TAA group, there was 1 patient who sustained an intraoperative medial malleolus fracture. One patient developed an extensor hallucis longus contracture 5 months postoperatively that subsequently underwent release and lengthening. There was 1 patient in this group who sustained a distal tibia fracture 5 cm proximal to the prosthesis 3 months postoperatively after a mechanical fall. In the No TXA-TAA group, there were 2 patients who sustained intraoperative medial malleolus fractures. One patient underwent a revision of the tibial component 24 months postoperatively due to aseptic loosening. In addition, another patient in this group who sustained an Achilles tendon rupture 5 months postoperatively after a fall subsequently underwent repair with tibialis anterior tendon allograft.
There were no patients in either group who experienced any hospital readmissions in the acute follow-up period as defined by a 90-day period after discharge. There were no complications associated with TXA administration in either group.
DISCUSSION
Recent advances in total ankle prosthetic design coupled with increased survival and improved short- to midterm follow-up results make TAA an effective treatment option for end-stage ankle arthritis. Management of perioperative blood loss and reducing the potential for significant hemarthrosis and subsequent wound complications are important factors to consider for patients undergoing TAA. TXA administration is used in several centers as part of an intraoperative strategy to reduce blood loss and decrease intra-articular blood accumulation. To our knowledge, this is the first study to evaluate the management of blood loss and hemarthrosis using TXA during TAA.
IV and topical administrations of TXA have been demonstrated to be highly effective hemostatic agents in the perioperative period for TKA and THA.11 Recent literature has demonstrated a significant reduction in drain output and mean change in preoperative to postoperative hemoglobin levels in patients who received TXA compared to that in patients who did not receive TXA. The patients who did not receive TXA had more than twice as much drain output.5,10,14,19-21
Continue to: The ankle has a thin...
The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of the bone during TAA are not as extensive when compared with TKA and THA, but the intra-articular volume is smaller and the surrounding soft tissues may be less yielding when blood accumulation occurs.22 The vascular supply can be rich surrounding the ankle in the absence of arterial disease and is not as apt to tolerate dislocation and subluxation as in the case of THA or TKA.23 Shear forces can easily tear the branches of the anterior tibial artery that lie within the fascia that is continuous with the periosteum on the distal tibia.24 Reduction of hemarthrosis within the ankle joint may lead to a decrease in postoperative swelling, decreased pain, and increased range of motion due to the diminished potential for fibrosis. We also believe that there could be a reduced risk for wound complications. The current literature reports the rate of wound complications to be anywhere from 2% to 25%, with diabetes, inflammatory conditions, coronary artery disease, peripheral vascular disease, and smoking history >12-pack-years as risk factors.16,25,26 In this study, we observed a significant reduction in drain output and an overall reduced percentage of postoperative wound complications in patients who received TXA. These results demonstrate that TXA use decreases postoperative hemarthrosis.
TXA use in TKA and THA has been shown to decrease direct hospital costs and hospital length of stay.7,14,27 A recent study by Moskal and colleagues7 showed that topical TXA use has the potential to significantly decrease hospital man-hours for those patients undergoing TKA and achieve larger cost savings. Although there was no significant difference in the length of stay between the 2 groups, the average length of stay after TAA was shorter in both groups compared to the reported national average (1.49 vs 2.2 days, respectively).4 The administration of TXA in the appropriate patient has the potential to decrease hospital costs by controlling postoperative pain and swelling, allowing for earlier discharge. Long-term cost benefits could also include decreased infection rates and wound complications, and improved clinical outcomes because of improved range of motion and function scores.
The limitations of this study include the retrospective nature of its design and the relatively small sample size. The results showed nonstatistically significant differences in wound complications between the TXA-TAA and the No TXA-TAA groups, consistent with an insufficient sample size and thus inadequate power to detect the significant difference. However, this study clearly showed that the wound complication rates were higher in the No TXA-TAA group than in the TXA-TAA group, suggesting the importance of further similar studies using a larger sample size.
CONCLUSION
Current TAA offers a viable alternative to arthrodesis for end-stage ankle arthritis. TXA is an inexpensive and effective hemostatic agent used during TAA. If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management, decrease postoperative hemarthrosis, and help to reduce the risk of postoperative wound complications.
1. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
2. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi:10.2106/JBJS.F.01299.
3. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923-936.
4. Zhou H, Yakavonis M, Shaw JJ, Patel A, Li X. In-patient trends and complications after total ankle arthroplasty in the United States. Orthopedics. 2016:1-6. doi:10.3928/01477447-20151228-05.
5. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440.
6. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005-1015. doi:10.1302/0301-620X.96B8.33745.
7. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365-368. doi:10.1016/j.arth.2014.10.008.
8. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278. doi:10.2106/JBJS.L.01268.
9. Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442-1448. doi:10.1016/j.arth.2015.12.033.
10. Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):98-100. doi:10.1302/0301-620X.98B.36430.
11. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944. doi:10.2106/JBJS.N.00060.
12. Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71(1 Suppl):S9-14. doi:10.1097/TA.0b013e31822114af.
13. Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30(2):272-276. doi:10.1016/j.arth.2014.08.022.
14. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968. doi:10.2106/JBJS.L.00907.
15. Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/AIT.a2015.0011.
16. Raikin SM, Kane J, Ciminiello ME. Risk factors for incision-healing complications following total ankle arthroplasty. J Bone Joint Surg Am. 2010;92(12):2150-2155. doi:10.2106/JBJS.I.00870.
17. Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120-124. doi:10.1111/j.1423-0410.2008.01071.x.
18. Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735-739. doi:10.1007/s00264-013-1801-0
19. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124.
20. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi:10.1016/j.knee.2005.11.001.
21. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211.
22. Draeger RW, Singh B, Parekh SG. Quantifying normal ankle joint volume: An anatomic study. Indian J Orthop. 2009;43(1):72-75. doi:10.4103/0019-5413.45326.
23. Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int. 2004;25(4):195-207. doi:10.1177/107110070402500402.
24. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102(3):599-616; discussion 617-598. doi:10.1097/00006534-199809030-00001.
25. Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199-208. doi:10.1007/s11999-009-0987-3.
26. Noelle S, Egidy CC, Cross MB, Gebauer M, Klauser W. Complication rates after total ankle arthroplasty in one hundred consecutive prostheses. Int Orthop. 2013;37(9):1789-1794. doi:10.1007/s00264-013-1971-9.
27. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):74-77. doi:10.1016/j.arth.2013.06.037.
ABSTRACT
Tranexamic acid (TXA) is an effective agent used for reducing perioperative blood loss and decreasing the potential for postoperative hemarthrosis. We hypothesized that patients who had received intraoperative TXA during total ankle arthroplasty (TAA) would have a reduction in postoperative drain output, thereby resulting in a reduced risk of postoperative hemarthrosis and lower wound complication rates.
A retrospective review was conducted on 50 consecutive patients, 25 receiving TXA (TXA-TAA) and 25 not receiving TXA (No TXA-TAA), who underwent an uncemented TAA between September 2011 and December 2015. Demographic characteristics, drain output, preoperative and postoperative hemoglobin levels, operative and postoperative course, and minor and major wound complications of the patients were reviewed.
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P < .0001). The overall wound complication rate in the No TXA-TAA group was higher (20%, 5/25) than that in the TXA-TAA group (8%, 2/25) (P = .114). The mean change in preoperative to postoperative hemoglobin level was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively, P = .01).
TXA is an effective hemostatic agent when used during TAA. TXA reduces perioperative blood loss, hemarthrosis, and the risk of wound complications.
Continue to: End-stage ankle arthritis...
End-stage ankle arthritis is a disabling condition that may lead to poor quality of life and difficulties with activities of daily living.1 The associated mental and physical disability has been demonstrated to be as severe as in end-stage hip arthrosis.2 Operative treatment for symptomatic end-stage ankle arthritis includes arthrodesis or total ankle arthroplasty (TAA) in those refractory to nonoperative treatment.3 Newer generation implants have made TAA a more attractive option for both the surgeon and the patient.
Over the past decade, the utility of TAA has increased and attention has turned toward the management of perioperative factors that would maximize patient satisfaction and decrease the length of stay and complication rates, as well as hospital costs.4 Comprehensive literature on total knee arthroplasty (TKA) and total hip arthroplasty (THA) has demonstrated that the management of perioperative blood loss, specifically postoperative hemarthrosis, is a modifiable factor affecting patient recovery, complication rates, and hospital costs.5-8 Drain output has been used as a direct measure of intra-articular blood accumulation.9 Decreased drain output implies decreased hemarthrosis, which could potentially alleviate the pressure on the wound and decrease wound complications.
One of the major strategies that has been recognized for reducing blood loss and decreasing the potential for postoperative hemarthrosis is the use of intravenous (IV) or topical tranexamic acid (TXA).10,11 TXA is a synthetic antifibrinolytic medication that has been extensively used throughout the medical field since the 1960s to help control the bleeding cascade. This medication stabilizes clot formation without inducing a pro-coaguable state.12 Intraoperative administration of TXA has been shown to reduce drain output and decrease transfusion requirements after TKA and THA without an associated increase in patient morbidity and mortality.6,11,13-15
Currently, there is a lack of studies evaluating the utility of TXA during TAA. We hypothesize that compared with patients who had not received TXA, those who had received intraoperative TXA during TAA would have a reduction in postoperative drain output and therefore decreased hemarthrosis, lower wound complication rate, and a diminished change in preoperative to postoperative hemoglobin levels, reflecting a reduction in perioperative blood loss.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University at Buffalo, State University of New York. A retrospective chart review was conducted on 50 consecutive patients who underwent an uncemented TAA with the Salto Talaris total ankle prosthesis (Tornier, Inc) between September 2011 and December 2015. All surgeries were performed at 1 institution by a single fellowship surgeon trained in foot and ankle surgery through the anterior approach where a midline incision was made over the ankle. The interval between the tibialis anterior tendon and the extensor hallucis longus tendon was used. We had incorporated intraoperative TXA into the TAA surgical protocol at our institution in January 2014. We evaluated the first 25 consecutive patients who underwent TAA after TXA use began (TXA-TAA) and another 25 consecutive patients who underwent TAA before the routine use of TXA (No TXA-TAA). Inclusion criteria were patients who presented with pain, decreased function, and radiographic parameters of end-stage tibiotalar arthritis due to degenerative arthritis, rheumatoid arthritis, or posttraumatic arthritis who subsequently underwent a TAA. Exclusion criteria were patients with a contraindication for IV TXA use, a preexisting coagulopathy, or where drain output was not recorded. Contraindications for IV TXA use included patients with impaired renal clearance, recent cardiac surgery, myocardial infarction, ischemic stroke, or venous thromboembolism (VTE). Seven patients were ultimately excluded from this study based on the inclusion and exclusion criteria, 3 patients from the TXA-TAA group and 4 patients from the No TXA-TAA group.
Continue to: Charts were reviewed for demographics...
Charts were reviewed for demographics, preoperative and postoperative hemoglobin levels, indications for surgery, surgical procedures, length of surgery, postoperative drain output, length of stay, postoperative pain visual analog scale (VAS) score, minor and major wound complications, and postoperative complications. Minor wound complications were defined as the anterior surgical incision that required local wound care in office or oral antibiotics without subsequent consequences. Major wound complications were defined as requiring surgical débridement and/or any additional treatment in the operating room.16 Postoperative complications other than wound complications were defined as those requiring a subsequent surgical intervention. Patient demographics and clinical and procedural characteristics of patients in both the TXA-TAA and the No TXA-TAA groups are outlined in Table 1. There were 14 males and 11 females in the TXA-TAA group and 16 males and 9 females in the No TXA-TAA group. The mean age was 65.8 ± 10.9 years in the TXA-TAA group and 66.9 ± 8.0 years in the No TXA-TAA group (P = .69). Mean body mass index (BMI) was 31.6 ± 6.3 in the TXA-TAA group and 29.4 ± 4.9 in the No TXA-TAA group (P = .18). The primary indication for TAA was degenerative osteoarthritis in 26 patients, posttraumatic arthritis in 21 patients, and rheumatoid arthritis in 3 patients. The most common associated procedure was Achilles tendon lengthening in both groups. The mean follow-up in the TXA-TAA group was 9.3 ± 5.8 months (range, 2.0-24.0 months). Postoperative complications due to TXA administration as described in previous literature were defined as VTE, myocardial infarction, or ischemic cerebral event. The TXA-TAA group received a standard 1 g dose of IV TXA 20 minutes prior to tourniquet inflation. A tourniquet was used intraoperatively on all patients included in this study. A postoperative 400-mL surgical drain (Hemovac, Zimmer Biomet) was placed in the ankle joint in all patients and subsequently discontinued on postoperative day 1. Recent literature has reported the minor wound complication rate associated with TAA to be as high as 25% and the major wound complication rate to be 8.5%.16 To assist in reducing the risk for wound complications, our protocol traditionally uses an intra-articular surgical drain to decrease any pressure on the wound from postoperative hemarthrosis.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
aP value was calculated from t-test continuous variables and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Total drain output was recorded in milliliters (mL) in all patients. The change between the preoperative hemoglobin level and the hemoglobin level on postoperative day 1 was calculated for each patient. The calculated blood loss was determined using Meunier’s equation, which estimates the total blood volume using Nadler’s formula and then uses preoperative hemoglobin and postoperative day 1 hemoglobin values to calculate blood loss.17,18 VAS scores (scale, 1-10) were obtained every 4 hours on postoperative day 1 according to the nursing protocol. The number 1 on the scale represents the least amount of pain, whereas 10 indicates the worst pain. The VAS scores were then averaged for each patient.
A power analysis using preliminary data determined that 15 patients were needed in each group to detect a 50% reduction in drain output at a power of 80% and a P value of 0.05. Descriptive statistics were used to analyze demographic data. We compared the demographic and clinical characteristics of patients in the TXA-TAA group with those of patients in the No TXA-TAA group using unpaired student t-tests for continuous variables and Chi-square or Fischer’s exact tests for categorical variables. Simple and adjusted linear regression analyses were used to examine the difference in drain output and blood loss between the 2 groups (TXA-TAA vs No TXA-TAA). Multivariate models were adjusted for age, BMI, and length of surgery. A P value <.05 was considered to be statistically significant. We performed all analyses using a statistical software package (SAS version 9.2, SAS Institute).

RESULTS
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P = .0001) (Figure). The clinical characteristics of the patients who underwent TAA with the use of TXA are outlined in Table 2. The mean change in preoperative to postoperative hemoglobin levels was significantly lower in the TXA-TAA group than in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively; P = .01). The calculated blood loss in patients in the TXA-TAA group was significantly lower than that in patients in the No TXA-TAA group (649.9 ± 332.7 vs 906.8 ± 287.4 mL, respectively; P = .01). No patient in either group received a blood transfusion. We did not observe a significant difference in the length of surgery between the TXA-TAA and the No TXA-TAA groups (112.8 ± 24.8 vs 108.6 ± 26.0 min, respectively; P = .57). The average American Society of Anesthesiologists’ (ASA) classification was similar between the groups (2.2 ± 0.6 and 2.2 ± 0.5, respectively; P = 1.00) as was the age-adjusted Charlson Comorbidity Index (2.8 ± 1.7 vs 2.9 ± 1.6, respectively; P = .93). Mean VAS scores on postoperative day 1 in the TXA-TAA and the No TXA-TAA group were 4.9 ± 1.7 and 5.3 ± 1.4, respectively (P = .71). The average length of stay in the TXA-TAA group was 1.6 ± 0.7 days vs 1.3 ± 0.6 days in the No TXA-TAA group (P = .23). Two patients in the TXA-TAA group had an extended hospital length of stay of 5 days due to discharge planning and social issues.
Table 2. Clinical Characteristics of Total Ankle Arthroplasty (TAA) Patients by Use of Tranexamic Acid (TXA), N = 50 | |||
|---|---|---|---|
| TXA use in TAA | P valuea | |
| Yes (n = 25 cases) | No (n = 25 controls) |
|
Clinical Characteristic |
|
|
|
Drain Output (ml), mean ± SD
| 71.6 ± 60.3 | 200.2 ± 117.0 | <0.0001 |
Preoperative to Postoperative Hgb Change (g/dL), mean ± SD
| 1.5 ± 0.6 | 2.0 ± 0.4 | 0.01 |
Blood Loss Calculated (ml), mean ± SD
| 649.9 ± 332.73 | 906.8 ± 287.4 | 0.01 |
Length of Surgery (min), mean ± SD
| 112.8 ± 24.8 | 108.6 ± 26.0 | 0.57 |
VAS scores on the POD (No.), mean ± SD
| 4.9 ± 1.7 | 5.3 ±1.4 | 0.71 |
LOS (day), mean ± SD
| 1.6 ± 0.7 | 1.3 ± 0.6 | 0.23 |
aP value was calculated from t-test for continuous variables, and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: LOS, length of stay; VAS, visual analog scale; POD, postoperative day.
Table 3. Linear Regression Analyses of Drain Output and Blood Loss using Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), Unadjusted and Adjusted Models for Length of Surgery, N = 50 | ||||
| TXA Use in TAA (Yes vs No) | |||
Drain Output (mL)
| Regression coefficient (β) | SE | Test statistics (t) | P valuea |
Unadjusted Model | -128.6 | 26.3 | -4.89 | < 0.0001 |
Adjusted for Age | -129.6 | 26.5 | -4.89 | <0.0001 |
Adjusted for BMI | -121.8 | 26.6 | -4.57 | <0.0001 |
Adjusted for Length of Surgery | -129.6 | 26.6 | -4.86 | <0.0001 |
Multivariable Modelb | -123.4 | 27.1 | -4.55 | <0.0001 |
Blood Loss (mL)
|
|
|
|
|
Unadjusted Model | -257.0 | 87.9 | -2.92 | 0.005 |
Adjusted for Age | -263.7 | 87.4 | -3.02 | 0.004 |
Adjusted for BMI | -268.7 | 90.2 | -2.98 | 0.005 |
Adjusted for Length of Surgery | -261.3 | 88.6 | -2.94 | 0.005 |
Multivariable Modelb | -275.6 | 90.7 | -3.04 | 0.004 |
aLinear regression was used to calculate the P value. bAdjusted for age, BMI and length of surgery.
Abbreviation: BMI, body mass index.
Table 4. Patient Wound Complication Categories by Use of Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), N = 50 | |||
|---|---|---|---|
| TXA Use in TAA | P valuea | |
Wound Complication | Yes (n = 25 cases) | No (n = 25 controls) | 0.114 |
None, n = 46 (86%) | 23 (40%) | 20 (46%) |
|
Minor, n = 6 (12%) | 2 (4%) | 4 (8%) |
|
Major, n = 1 (2%) | 0 (0%) | 1 (4%) |
|
aP value was calculated from Fisher’s Exact test (67% cells had count <5) test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
The crude linear regression model revealed a significant difference in drain output between the TXA-TAA and the No TXA-TAA groups (β = −128.6 ± 26.3, P < .0001) (Table 3). Further adjustment for age and length of surgery slightly strengthened the association (β = −129.6 ± 26.6, P < .0001). The nature of regression coefficient β showed that the mean estimate of drain output was 129.6 mL lower in the TXA-TAA group than that in the No TXA-TAA group. There was a significant difference in blood loss between the TXA-TAA and the No TXA-TAA groups in the crude linear regression model (β = −257.0 ± 87.9, P = .005). Additional adjustment for age, BMI, and length of surgery slightly strengthened the association (β = −275.6 ± 90.7, P = .004). The nature of regression coefficient β showed that the mean estimate of blood loss was 275.6 mL lower in the TXA-TAA group than in the No TXA-TAA group (Table 3).
Continue to: There was no statistically significant difference...
There was no statistically significant difference in wound complications between the TXA-TAA and the No TXA-TAA groups in this study population (P = .114). However, our results showed a higher overall wound complication rate in the No TXA-TAA group than in the TXA-TAA group (20% (5/25) vs 8% (2/25), respectively) (Table 4). In the No TXA-TAA group, there were 4 minor and 1 major wound complications. All 5 patients experiencing a postoperative wound complication required oral antibiotics for a minimum of 4 weeks and local wound care. One patient underwent a surgical débridement meeting the criteria for major wound complications. In the TXA-TAA group, there were 2 minor wound complications and no major wound complications. One patient was administered prophylactic oral antibiotics for 7 days with local wound care for blister formation without evidence of infection. The second patient experiencing a minor wound complication required 3 weeks of oral antibiotics and local wound care. No patients in either group had a deep infection requiring implant removal, IV antibiotics, or subsequent hospital admission. The surgical incisions in all patients healed after the aforementioned treatments with no persistent drainage or development of chronic wounds.
In the TXA-TAA group, there was 1 patient who sustained an intraoperative medial malleolus fracture. One patient developed an extensor hallucis longus contracture 5 months postoperatively that subsequently underwent release and lengthening. There was 1 patient in this group who sustained a distal tibia fracture 5 cm proximal to the prosthesis 3 months postoperatively after a mechanical fall. In the No TXA-TAA group, there were 2 patients who sustained intraoperative medial malleolus fractures. One patient underwent a revision of the tibial component 24 months postoperatively due to aseptic loosening. In addition, another patient in this group who sustained an Achilles tendon rupture 5 months postoperatively after a fall subsequently underwent repair with tibialis anterior tendon allograft.
There were no patients in either group who experienced any hospital readmissions in the acute follow-up period as defined by a 90-day period after discharge. There were no complications associated with TXA administration in either group.
DISCUSSION
Recent advances in total ankle prosthetic design coupled with increased survival and improved short- to midterm follow-up results make TAA an effective treatment option for end-stage ankle arthritis. Management of perioperative blood loss and reducing the potential for significant hemarthrosis and subsequent wound complications are important factors to consider for patients undergoing TAA. TXA administration is used in several centers as part of an intraoperative strategy to reduce blood loss and decrease intra-articular blood accumulation. To our knowledge, this is the first study to evaluate the management of blood loss and hemarthrosis using TXA during TAA.
IV and topical administrations of TXA have been demonstrated to be highly effective hemostatic agents in the perioperative period for TKA and THA.11 Recent literature has demonstrated a significant reduction in drain output and mean change in preoperative to postoperative hemoglobin levels in patients who received TXA compared to that in patients who did not receive TXA. The patients who did not receive TXA had more than twice as much drain output.5,10,14,19-21
Continue to: The ankle has a thin...
The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of the bone during TAA are not as extensive when compared with TKA and THA, but the intra-articular volume is smaller and the surrounding soft tissues may be less yielding when blood accumulation occurs.22 The vascular supply can be rich surrounding the ankle in the absence of arterial disease and is not as apt to tolerate dislocation and subluxation as in the case of THA or TKA.23 Shear forces can easily tear the branches of the anterior tibial artery that lie within the fascia that is continuous with the periosteum on the distal tibia.24 Reduction of hemarthrosis within the ankle joint may lead to a decrease in postoperative swelling, decreased pain, and increased range of motion due to the diminished potential for fibrosis. We also believe that there could be a reduced risk for wound complications. The current literature reports the rate of wound complications to be anywhere from 2% to 25%, with diabetes, inflammatory conditions, coronary artery disease, peripheral vascular disease, and smoking history >12-pack-years as risk factors.16,25,26 In this study, we observed a significant reduction in drain output and an overall reduced percentage of postoperative wound complications in patients who received TXA. These results demonstrate that TXA use decreases postoperative hemarthrosis.
TXA use in TKA and THA has been shown to decrease direct hospital costs and hospital length of stay.7,14,27 A recent study by Moskal and colleagues7 showed that topical TXA use has the potential to significantly decrease hospital man-hours for those patients undergoing TKA and achieve larger cost savings. Although there was no significant difference in the length of stay between the 2 groups, the average length of stay after TAA was shorter in both groups compared to the reported national average (1.49 vs 2.2 days, respectively).4 The administration of TXA in the appropriate patient has the potential to decrease hospital costs by controlling postoperative pain and swelling, allowing for earlier discharge. Long-term cost benefits could also include decreased infection rates and wound complications, and improved clinical outcomes because of improved range of motion and function scores.
The limitations of this study include the retrospective nature of its design and the relatively small sample size. The results showed nonstatistically significant differences in wound complications between the TXA-TAA and the No TXA-TAA groups, consistent with an insufficient sample size and thus inadequate power to detect the significant difference. However, this study clearly showed that the wound complication rates were higher in the No TXA-TAA group than in the TXA-TAA group, suggesting the importance of further similar studies using a larger sample size.
CONCLUSION
Current TAA offers a viable alternative to arthrodesis for end-stage ankle arthritis. TXA is an inexpensive and effective hemostatic agent used during TAA. If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management, decrease postoperative hemarthrosis, and help to reduce the risk of postoperative wound complications.
ABSTRACT
Tranexamic acid (TXA) is an effective agent used for reducing perioperative blood loss and decreasing the potential for postoperative hemarthrosis. We hypothesized that patients who had received intraoperative TXA during total ankle arthroplasty (TAA) would have a reduction in postoperative drain output, thereby resulting in a reduced risk of postoperative hemarthrosis and lower wound complication rates.
A retrospective review was conducted on 50 consecutive patients, 25 receiving TXA (TXA-TAA) and 25 not receiving TXA (No TXA-TAA), who underwent an uncemented TAA between September 2011 and December 2015. Demographic characteristics, drain output, preoperative and postoperative hemoglobin levels, operative and postoperative course, and minor and major wound complications of the patients were reviewed.
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P < .0001). The overall wound complication rate in the No TXA-TAA group was higher (20%, 5/25) than that in the TXA-TAA group (8%, 2/25) (P = .114). The mean change in preoperative to postoperative hemoglobin level was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively, P = .01).
TXA is an effective hemostatic agent when used during TAA. TXA reduces perioperative blood loss, hemarthrosis, and the risk of wound complications.
Continue to: End-stage ankle arthritis...
End-stage ankle arthritis is a disabling condition that may lead to poor quality of life and difficulties with activities of daily living.1 The associated mental and physical disability has been demonstrated to be as severe as in end-stage hip arthrosis.2 Operative treatment for symptomatic end-stage ankle arthritis includes arthrodesis or total ankle arthroplasty (TAA) in those refractory to nonoperative treatment.3 Newer generation implants have made TAA a more attractive option for both the surgeon and the patient.
Over the past decade, the utility of TAA has increased and attention has turned toward the management of perioperative factors that would maximize patient satisfaction and decrease the length of stay and complication rates, as well as hospital costs.4 Comprehensive literature on total knee arthroplasty (TKA) and total hip arthroplasty (THA) has demonstrated that the management of perioperative blood loss, specifically postoperative hemarthrosis, is a modifiable factor affecting patient recovery, complication rates, and hospital costs.5-8 Drain output has been used as a direct measure of intra-articular blood accumulation.9 Decreased drain output implies decreased hemarthrosis, which could potentially alleviate the pressure on the wound and decrease wound complications.
One of the major strategies that has been recognized for reducing blood loss and decreasing the potential for postoperative hemarthrosis is the use of intravenous (IV) or topical tranexamic acid (TXA).10,11 TXA is a synthetic antifibrinolytic medication that has been extensively used throughout the medical field since the 1960s to help control the bleeding cascade. This medication stabilizes clot formation without inducing a pro-coaguable state.12 Intraoperative administration of TXA has been shown to reduce drain output and decrease transfusion requirements after TKA and THA without an associated increase in patient morbidity and mortality.6,11,13-15
Currently, there is a lack of studies evaluating the utility of TXA during TAA. We hypothesize that compared with patients who had not received TXA, those who had received intraoperative TXA during TAA would have a reduction in postoperative drain output and therefore decreased hemarthrosis, lower wound complication rate, and a diminished change in preoperative to postoperative hemoglobin levels, reflecting a reduction in perioperative blood loss.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University at Buffalo, State University of New York. A retrospective chart review was conducted on 50 consecutive patients who underwent an uncemented TAA with the Salto Talaris total ankle prosthesis (Tornier, Inc) between September 2011 and December 2015. All surgeries were performed at 1 institution by a single fellowship surgeon trained in foot and ankle surgery through the anterior approach where a midline incision was made over the ankle. The interval between the tibialis anterior tendon and the extensor hallucis longus tendon was used. We had incorporated intraoperative TXA into the TAA surgical protocol at our institution in January 2014. We evaluated the first 25 consecutive patients who underwent TAA after TXA use began (TXA-TAA) and another 25 consecutive patients who underwent TAA before the routine use of TXA (No TXA-TAA). Inclusion criteria were patients who presented with pain, decreased function, and radiographic parameters of end-stage tibiotalar arthritis due to degenerative arthritis, rheumatoid arthritis, or posttraumatic arthritis who subsequently underwent a TAA. Exclusion criteria were patients with a contraindication for IV TXA use, a preexisting coagulopathy, or where drain output was not recorded. Contraindications for IV TXA use included patients with impaired renal clearance, recent cardiac surgery, myocardial infarction, ischemic stroke, or venous thromboembolism (VTE). Seven patients were ultimately excluded from this study based on the inclusion and exclusion criteria, 3 patients from the TXA-TAA group and 4 patients from the No TXA-TAA group.
Continue to: Charts were reviewed for demographics...
Charts were reviewed for demographics, preoperative and postoperative hemoglobin levels, indications for surgery, surgical procedures, length of surgery, postoperative drain output, length of stay, postoperative pain visual analog scale (VAS) score, minor and major wound complications, and postoperative complications. Minor wound complications were defined as the anterior surgical incision that required local wound care in office or oral antibiotics without subsequent consequences. Major wound complications were defined as requiring surgical débridement and/or any additional treatment in the operating room.16 Postoperative complications other than wound complications were defined as those requiring a subsequent surgical intervention. Patient demographics and clinical and procedural characteristics of patients in both the TXA-TAA and the No TXA-TAA groups are outlined in Table 1. There were 14 males and 11 females in the TXA-TAA group and 16 males and 9 females in the No TXA-TAA group. The mean age was 65.8 ± 10.9 years in the TXA-TAA group and 66.9 ± 8.0 years in the No TXA-TAA group (P = .69). Mean body mass index (BMI) was 31.6 ± 6.3 in the TXA-TAA group and 29.4 ± 4.9 in the No TXA-TAA group (P = .18). The primary indication for TAA was degenerative osteoarthritis in 26 patients, posttraumatic arthritis in 21 patients, and rheumatoid arthritis in 3 patients. The most common associated procedure was Achilles tendon lengthening in both groups. The mean follow-up in the TXA-TAA group was 9.3 ± 5.8 months (range, 2.0-24.0 months). Postoperative complications due to TXA administration as described in previous literature were defined as VTE, myocardial infarction, or ischemic cerebral event. The TXA-TAA group received a standard 1 g dose of IV TXA 20 minutes prior to tourniquet inflation. A tourniquet was used intraoperatively on all patients included in this study. A postoperative 400-mL surgical drain (Hemovac, Zimmer Biomet) was placed in the ankle joint in all patients and subsequently discontinued on postoperative day 1. Recent literature has reported the minor wound complication rate associated with TAA to be as high as 25% and the major wound complication rate to be 8.5%.16 To assist in reducing the risk for wound complications, our protocol traditionally uses an intra-articular surgical drain to decrease any pressure on the wound from postoperative hemarthrosis.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
aP value was calculated from t-test continuous variables and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Total drain output was recorded in milliliters (mL) in all patients. The change between the preoperative hemoglobin level and the hemoglobin level on postoperative day 1 was calculated for each patient. The calculated blood loss was determined using Meunier’s equation, which estimates the total blood volume using Nadler’s formula and then uses preoperative hemoglobin and postoperative day 1 hemoglobin values to calculate blood loss.17,18 VAS scores (scale, 1-10) were obtained every 4 hours on postoperative day 1 according to the nursing protocol. The number 1 on the scale represents the least amount of pain, whereas 10 indicates the worst pain. The VAS scores were then averaged for each patient.
A power analysis using preliminary data determined that 15 patients were needed in each group to detect a 50% reduction in drain output at a power of 80% and a P value of 0.05. Descriptive statistics were used to analyze demographic data. We compared the demographic and clinical characteristics of patients in the TXA-TAA group with those of patients in the No TXA-TAA group using unpaired student t-tests for continuous variables and Chi-square or Fischer’s exact tests for categorical variables. Simple and adjusted linear regression analyses were used to examine the difference in drain output and blood loss between the 2 groups (TXA-TAA vs No TXA-TAA). Multivariate models were adjusted for age, BMI, and length of surgery. A P value <.05 was considered to be statistically significant. We performed all analyses using a statistical software package (SAS version 9.2, SAS Institute).

RESULTS
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P = .0001) (Figure). The clinical characteristics of the patients who underwent TAA with the use of TXA are outlined in Table 2. The mean change in preoperative to postoperative hemoglobin levels was significantly lower in the TXA-TAA group than in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively; P = .01). The calculated blood loss in patients in the TXA-TAA group was significantly lower than that in patients in the No TXA-TAA group (649.9 ± 332.7 vs 906.8 ± 287.4 mL, respectively; P = .01). No patient in either group received a blood transfusion. We did not observe a significant difference in the length of surgery between the TXA-TAA and the No TXA-TAA groups (112.8 ± 24.8 vs 108.6 ± 26.0 min, respectively; P = .57). The average American Society of Anesthesiologists’ (ASA) classification was similar between the groups (2.2 ± 0.6 and 2.2 ± 0.5, respectively; P = 1.00) as was the age-adjusted Charlson Comorbidity Index (2.8 ± 1.7 vs 2.9 ± 1.6, respectively; P = .93). Mean VAS scores on postoperative day 1 in the TXA-TAA and the No TXA-TAA group were 4.9 ± 1.7 and 5.3 ± 1.4, respectively (P = .71). The average length of stay in the TXA-TAA group was 1.6 ± 0.7 days vs 1.3 ± 0.6 days in the No TXA-TAA group (P = .23). Two patients in the TXA-TAA group had an extended hospital length of stay of 5 days due to discharge planning and social issues.
Table 2. Clinical Characteristics of Total Ankle Arthroplasty (TAA) Patients by Use of Tranexamic Acid (TXA), N = 50 | |||
|---|---|---|---|
| TXA use in TAA | P valuea | |
| Yes (n = 25 cases) | No (n = 25 controls) |
|
Clinical Characteristic |
|
|
|
Drain Output (ml), mean ± SD
| 71.6 ± 60.3 | 200.2 ± 117.0 | <0.0001 |
Preoperative to Postoperative Hgb Change (g/dL), mean ± SD
| 1.5 ± 0.6 | 2.0 ± 0.4 | 0.01 |
Blood Loss Calculated (ml), mean ± SD
| 649.9 ± 332.73 | 906.8 ± 287.4 | 0.01 |
Length of Surgery (min), mean ± SD
| 112.8 ± 24.8 | 108.6 ± 26.0 | 0.57 |
VAS scores on the POD (No.), mean ± SD
| 4.9 ± 1.7 | 5.3 ±1.4 | 0.71 |
LOS (day), mean ± SD
| 1.6 ± 0.7 | 1.3 ± 0.6 | 0.23 |
aP value was calculated from t-test for continuous variables, and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: LOS, length of stay; VAS, visual analog scale; POD, postoperative day.
Table 3. Linear Regression Analyses of Drain Output and Blood Loss using Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), Unadjusted and Adjusted Models for Length of Surgery, N = 50 | ||||
| TXA Use in TAA (Yes vs No) | |||
Drain Output (mL)
| Regression coefficient (β) | SE | Test statistics (t) | P valuea |
Unadjusted Model | -128.6 | 26.3 | -4.89 | < 0.0001 |
Adjusted for Age | -129.6 | 26.5 | -4.89 | <0.0001 |
Adjusted for BMI | -121.8 | 26.6 | -4.57 | <0.0001 |
Adjusted for Length of Surgery | -129.6 | 26.6 | -4.86 | <0.0001 |
Multivariable Modelb | -123.4 | 27.1 | -4.55 | <0.0001 |
Blood Loss (mL)
|
|
|
|
|
Unadjusted Model | -257.0 | 87.9 | -2.92 | 0.005 |
Adjusted for Age | -263.7 | 87.4 | -3.02 | 0.004 |
Adjusted for BMI | -268.7 | 90.2 | -2.98 | 0.005 |
Adjusted for Length of Surgery | -261.3 | 88.6 | -2.94 | 0.005 |
Multivariable Modelb | -275.6 | 90.7 | -3.04 | 0.004 |
aLinear regression was used to calculate the P value. bAdjusted for age, BMI and length of surgery.
Abbreviation: BMI, body mass index.
Table 4. Patient Wound Complication Categories by Use of Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), N = 50 | |||
|---|---|---|---|
| TXA Use in TAA | P valuea | |
Wound Complication | Yes (n = 25 cases) | No (n = 25 controls) | 0.114 |
None, n = 46 (86%) | 23 (40%) | 20 (46%) |
|
Minor, n = 6 (12%) | 2 (4%) | 4 (8%) |
|
Major, n = 1 (2%) | 0 (0%) | 1 (4%) |
|
aP value was calculated from Fisher’s Exact test (67% cells had count <5) test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
The crude linear regression model revealed a significant difference in drain output between the TXA-TAA and the No TXA-TAA groups (β = −128.6 ± 26.3, P < .0001) (Table 3). Further adjustment for age and length of surgery slightly strengthened the association (β = −129.6 ± 26.6, P < .0001). The nature of regression coefficient β showed that the mean estimate of drain output was 129.6 mL lower in the TXA-TAA group than that in the No TXA-TAA group. There was a significant difference in blood loss between the TXA-TAA and the No TXA-TAA groups in the crude linear regression model (β = −257.0 ± 87.9, P = .005). Additional adjustment for age, BMI, and length of surgery slightly strengthened the association (β = −275.6 ± 90.7, P = .004). The nature of regression coefficient β showed that the mean estimate of blood loss was 275.6 mL lower in the TXA-TAA group than in the No TXA-TAA group (Table 3).
Continue to: There was no statistically significant difference...
There was no statistically significant difference in wound complications between the TXA-TAA and the No TXA-TAA groups in this study population (P = .114). However, our results showed a higher overall wound complication rate in the No TXA-TAA group than in the TXA-TAA group (20% (5/25) vs 8% (2/25), respectively) (Table 4). In the No TXA-TAA group, there were 4 minor and 1 major wound complications. All 5 patients experiencing a postoperative wound complication required oral antibiotics for a minimum of 4 weeks and local wound care. One patient underwent a surgical débridement meeting the criteria for major wound complications. In the TXA-TAA group, there were 2 minor wound complications and no major wound complications. One patient was administered prophylactic oral antibiotics for 7 days with local wound care for blister formation without evidence of infection. The second patient experiencing a minor wound complication required 3 weeks of oral antibiotics and local wound care. No patients in either group had a deep infection requiring implant removal, IV antibiotics, or subsequent hospital admission. The surgical incisions in all patients healed after the aforementioned treatments with no persistent drainage or development of chronic wounds.
In the TXA-TAA group, there was 1 patient who sustained an intraoperative medial malleolus fracture. One patient developed an extensor hallucis longus contracture 5 months postoperatively that subsequently underwent release and lengthening. There was 1 patient in this group who sustained a distal tibia fracture 5 cm proximal to the prosthesis 3 months postoperatively after a mechanical fall. In the No TXA-TAA group, there were 2 patients who sustained intraoperative medial malleolus fractures. One patient underwent a revision of the tibial component 24 months postoperatively due to aseptic loosening. In addition, another patient in this group who sustained an Achilles tendon rupture 5 months postoperatively after a fall subsequently underwent repair with tibialis anterior tendon allograft.
There were no patients in either group who experienced any hospital readmissions in the acute follow-up period as defined by a 90-day period after discharge. There were no complications associated with TXA administration in either group.
DISCUSSION
Recent advances in total ankle prosthetic design coupled with increased survival and improved short- to midterm follow-up results make TAA an effective treatment option for end-stage ankle arthritis. Management of perioperative blood loss and reducing the potential for significant hemarthrosis and subsequent wound complications are important factors to consider for patients undergoing TAA. TXA administration is used in several centers as part of an intraoperative strategy to reduce blood loss and decrease intra-articular blood accumulation. To our knowledge, this is the first study to evaluate the management of blood loss and hemarthrosis using TXA during TAA.
IV and topical administrations of TXA have been demonstrated to be highly effective hemostatic agents in the perioperative period for TKA and THA.11 Recent literature has demonstrated a significant reduction in drain output and mean change in preoperative to postoperative hemoglobin levels in patients who received TXA compared to that in patients who did not receive TXA. The patients who did not receive TXA had more than twice as much drain output.5,10,14,19-21
Continue to: The ankle has a thin...
The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of the bone during TAA are not as extensive when compared with TKA and THA, but the intra-articular volume is smaller and the surrounding soft tissues may be less yielding when blood accumulation occurs.22 The vascular supply can be rich surrounding the ankle in the absence of arterial disease and is not as apt to tolerate dislocation and subluxation as in the case of THA or TKA.23 Shear forces can easily tear the branches of the anterior tibial artery that lie within the fascia that is continuous with the periosteum on the distal tibia.24 Reduction of hemarthrosis within the ankle joint may lead to a decrease in postoperative swelling, decreased pain, and increased range of motion due to the diminished potential for fibrosis. We also believe that there could be a reduced risk for wound complications. The current literature reports the rate of wound complications to be anywhere from 2% to 25%, with diabetes, inflammatory conditions, coronary artery disease, peripheral vascular disease, and smoking history >12-pack-years as risk factors.16,25,26 In this study, we observed a significant reduction in drain output and an overall reduced percentage of postoperative wound complications in patients who received TXA. These results demonstrate that TXA use decreases postoperative hemarthrosis.
TXA use in TKA and THA has been shown to decrease direct hospital costs and hospital length of stay.7,14,27 A recent study by Moskal and colleagues7 showed that topical TXA use has the potential to significantly decrease hospital man-hours for those patients undergoing TKA and achieve larger cost savings. Although there was no significant difference in the length of stay between the 2 groups, the average length of stay after TAA was shorter in both groups compared to the reported national average (1.49 vs 2.2 days, respectively).4 The administration of TXA in the appropriate patient has the potential to decrease hospital costs by controlling postoperative pain and swelling, allowing for earlier discharge. Long-term cost benefits could also include decreased infection rates and wound complications, and improved clinical outcomes because of improved range of motion and function scores.
The limitations of this study include the retrospective nature of its design and the relatively small sample size. The results showed nonstatistically significant differences in wound complications between the TXA-TAA and the No TXA-TAA groups, consistent with an insufficient sample size and thus inadequate power to detect the significant difference. However, this study clearly showed that the wound complication rates were higher in the No TXA-TAA group than in the TXA-TAA group, suggesting the importance of further similar studies using a larger sample size.
CONCLUSION
Current TAA offers a viable alternative to arthrodesis for end-stage ankle arthritis. TXA is an inexpensive and effective hemostatic agent used during TAA. If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management, decrease postoperative hemarthrosis, and help to reduce the risk of postoperative wound complications.
1. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
2. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi:10.2106/JBJS.F.01299.
3. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923-936.
4. Zhou H, Yakavonis M, Shaw JJ, Patel A, Li X. In-patient trends and complications after total ankle arthroplasty in the United States. Orthopedics. 2016:1-6. doi:10.3928/01477447-20151228-05.
5. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440.
6. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005-1015. doi:10.1302/0301-620X.96B8.33745.
7. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365-368. doi:10.1016/j.arth.2014.10.008.
8. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278. doi:10.2106/JBJS.L.01268.
9. Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442-1448. doi:10.1016/j.arth.2015.12.033.
10. Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):98-100. doi:10.1302/0301-620X.98B.36430.
11. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944. doi:10.2106/JBJS.N.00060.
12. Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71(1 Suppl):S9-14. doi:10.1097/TA.0b013e31822114af.
13. Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30(2):272-276. doi:10.1016/j.arth.2014.08.022.
14. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968. doi:10.2106/JBJS.L.00907.
15. Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/AIT.a2015.0011.
16. Raikin SM, Kane J, Ciminiello ME. Risk factors for incision-healing complications following total ankle arthroplasty. J Bone Joint Surg Am. 2010;92(12):2150-2155. doi:10.2106/JBJS.I.00870.
17. Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120-124. doi:10.1111/j.1423-0410.2008.01071.x.
18. Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735-739. doi:10.1007/s00264-013-1801-0
19. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124.
20. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi:10.1016/j.knee.2005.11.001.
21. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211.
22. Draeger RW, Singh B, Parekh SG. Quantifying normal ankle joint volume: An anatomic study. Indian J Orthop. 2009;43(1):72-75. doi:10.4103/0019-5413.45326.
23. Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int. 2004;25(4):195-207. doi:10.1177/107110070402500402.
24. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102(3):599-616; discussion 617-598. doi:10.1097/00006534-199809030-00001.
25. Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199-208. doi:10.1007/s11999-009-0987-3.
26. Noelle S, Egidy CC, Cross MB, Gebauer M, Klauser W. Complication rates after total ankle arthroplasty in one hundred consecutive prostheses. Int Orthop. 2013;37(9):1789-1794. doi:10.1007/s00264-013-1971-9.
27. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):74-77. doi:10.1016/j.arth.2013.06.037.
1. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
2. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi:10.2106/JBJS.F.01299.
3. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923-936.
4. Zhou H, Yakavonis M, Shaw JJ, Patel A, Li X. In-patient trends and complications after total ankle arthroplasty in the United States. Orthopedics. 2016:1-6. doi:10.3928/01477447-20151228-05.
5. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440.
6. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005-1015. doi:10.1302/0301-620X.96B8.33745.
7. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365-368. doi:10.1016/j.arth.2014.10.008.
8. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278. doi:10.2106/JBJS.L.01268.
9. Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442-1448. doi:10.1016/j.arth.2015.12.033.
10. Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):98-100. doi:10.1302/0301-620X.98B.36430.
11. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944. doi:10.2106/JBJS.N.00060.
12. Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71(1 Suppl):S9-14. doi:10.1097/TA.0b013e31822114af.
13. Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30(2):272-276. doi:10.1016/j.arth.2014.08.022.
14. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968. doi:10.2106/JBJS.L.00907.
15. Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/AIT.a2015.0011.
16. Raikin SM, Kane J, Ciminiello ME. Risk factors for incision-healing complications following total ankle arthroplasty. J Bone Joint Surg Am. 2010;92(12):2150-2155. doi:10.2106/JBJS.I.00870.
17. Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120-124. doi:10.1111/j.1423-0410.2008.01071.x.
18. Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735-739. doi:10.1007/s00264-013-1801-0
19. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124.
20. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi:10.1016/j.knee.2005.11.001.
21. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211.
22. Draeger RW, Singh B, Parekh SG. Quantifying normal ankle joint volume: An anatomic study. Indian J Orthop. 2009;43(1):72-75. doi:10.4103/0019-5413.45326.
23. Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int. 2004;25(4):195-207. doi:10.1177/107110070402500402.
24. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102(3):599-616; discussion 617-598. doi:10.1097/00006534-199809030-00001.
25. Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199-208. doi:10.1007/s11999-009-0987-3.
26. Noelle S, Egidy CC, Cross MB, Gebauer M, Klauser W. Complication rates after total ankle arthroplasty in one hundred consecutive prostheses. Int Orthop. 2013;37(9):1789-1794. doi:10.1007/s00264-013-1971-9.
27. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):74-77. doi:10.1016/j.arth.2013.06.037.
TAKE-HOME POINTS
- TXA is an inexpensive and effective hemostatic agent used during TAA.
- The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of bone during TAA are not as extensive when compared to TKA and THA, but the intra-articular volume is smaller and surrounding soft tissues may be less yielding when blood accumulation occurs.
- If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management during TAA.
- TXA decreases postoperative hemarthrosis and helps to reduce the risk of postoperative wound complications.
- The administration of TXA in the appropriate patient has the potential to decrease hospital cost by controlling postoperative pain and swelling allowing for earlier discharge.
Recurrence of Extranodal Natural Killer/T-cell Lymphoma Presenting as Tarsal Tunnel Syndrome
ABSTRACT
This case report is a rare form of lymphoma recurrence which presented as tarsal tunnel syndrome. The patient had been previously treated for the malignancy and was presumed to be in remission; however, standard radiology imaging protocols failed to include the distal extremities on these scans. The patient presented to the orthopedic clinic with tarsal tunnel symptoms and a mass in the tarsal tunnel. A complete evaluation resulted in a diagnosis of recurrence of the malignancy. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body fluorodeoxyglucose positron emission tomography computed tomography when evaluating for recurrence in patients.
Nasal-type, extranodal natural killer/T-cell lymphoma (ENKTL) is a rare form of non-Hodgkin lymphoma (NHL). Malignancies account for only 10% of NHL in Asian and South American populations. However, in Caucasians, it represents <1% of all cases. In addition, at 3:1 male to female ratio, the disease most commonly affects male patients who are 50 to 59 years old.1-3 The etiology of this malignancy is strongly related to prior infection with Epstein-Barr virus (EBV) as EBV-encoded early small ribonucleic acid on in situ hybridization of lymphoma cells is positive in 95% of cases.4-6
Typical sites of involvement include the nasal cavity, nasopharynx, and sinuses, causing patients to present with nasal obstruction, chronic sinusitis, or epistaxis. Additionally, ENKTL can occur primarily in the skin, gastrointestinal tract, spleen, and testis, whereas the bone marrow may be involved in 10% of cases. Although rare, unusual sites, including muscle, adrenals, and ovaries, have been published.7,8
Staging is best performed using the T-staging system, which accounts for the extent of local tumor involvement. Higher stages, such as T3 /T4, equate to locally advanced disease and imply a worse prognosis.9,10 Computed tomography (CT) and magnetic resonance imaging (MRI) help define local soft tissues and bony involvement. Furthermore, CT of the chest, abdomen, and pelvis as well as bone marrow biopsy are performed as part of the staging process. Lastly, fluorine-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) is often used to detect extranodal spread, define the extent of involvement, differentiate between lymphoma and inflammatory masses, and monitor for recurrence.11
Treatment for local ENKTL involves concurrent chemoradiotherapy followed by 3 cycles of etoposide, ifosfamide, cisplatin, and dexamethasone, which results in a complete response rate of 80%, and is the most favorable when comparing treatment modalities.12 Unfortunately, recurrence rates reach as high as 50%, whereas the 5-year survival rate is 59%.13,14 For recurrent or disseminated disease, high-dose chemotherapy and hematopoietic stem cell transplantation remain as alternative treatments for patients who have undergone 2 complete remissions and can be curative in some instances.13,15
Continue to: In summary, ENKTL is a rare form...
In summary, ENKTL is a rare form of NHL which classically presents in the nasal cavity; however, this type of lymphoma may present in a variety of extranodal sites.7,8 Despite the numerous published reports on ENKTL, no study has reported either primary or recurrent ENKTL in the feet or hands. To our knowledge, this is one of the first published cases of a patient who developed a rare and recurring ENKTL in the foot and ankle. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 59-year-old Caucasian woman was referred to the orthopedic foot and ankle clinic by her primary care physician for right medial ankle pain, skin ulceration, and numbness over the plantar aspect of her right foot. Upon questioning, the patient noted that the pain and numbness were present for almost 6 months. She denied trauma to the concerned area. Previously, the patient was observed and treated elsewhere for plantar fasciitis and was prescribed a brace before being immobilized in a controlled ankle motion (CAM) boot for 6 weeks. At follow-up with her outside provider, the patient had developed skin breakdown over the medial aspect of the right ankle, and this condition was presumed to be caused by the boot. After local wound care failed to improve her skin ulceration, she returned to her primary care physician, who ordered an MRI of the area and referred her to our specialty clinic.
Upon review, the patient’s past medical history included a diagnosis of nasal-type ENKTL. Her malignancy was treated with chemoradiotherapy 2 years prior to her consultation with the foot and ankle clinic.
The patient was noted by her medical oncologist and interventional radiologist to be in complete stage 4 remission since being treated. She underwent routine MRI and CT scans of the head and neck at 6-month intervals and FDG PET-CT scans at 3-month intervals, as per institutional protocol. The examinations showed no evidence of malignancy or metabolically active disease. The last imaging study occurred 2 months prior to admission to the foot and ankle clinic.
The patient consulted her medical oncologist 1 month prior to presenting to our clinic and was noted to exhibit an “excellent response to chemoradiotherapy” and “continues to remain disease free at 2 years.” She was instructed to continue routine follow-up. However, the office notes mentioned no ankle pain and non-healing wounds.
During physical examination, the patient presented an antalgic gait on the right side. Inspection demonstrated an increased circumference of the right ankle compared with the left, with a soft, palpable mass over the medial aspect of her right ankle. A 3 cm × 2 cm, grade 2 abrasion of the skin was observed over the medial mass just posterior to her medial malleolus. Range of motion was within normal limits. The patient exhibited a palpable posterior tibial artery pulse and full strength upon muscle testing of the lower extremities. She featured a positive Tinel’s sign and discomfort over the mass itself, with the pain radiating down to the plantar aspect of her foot and diffuse numbness over the plantar aspect of the foot.
Continue to: Review of her plain radiographs...
Review of her plain radiographs demonstrated no bony abnormalities, fractures, nor visible deformity (Figures 1A, 1B).
At presentation, our differential diagnosis included recurrence of the malignancy, secondary malignancy, infection, and inflammatory disease. After a lengthy discussion with the patient and consultation with our institution’s musculoskeletal oncologist, the decision was made to perform a right-ankle mass biopsy and marginal excision with wound irrigation and débridement and tarsal tunnel release.
The patient was placed in the supine position with standard prepping and draping. The medial eschar was excised in an elliptical fashion, and a curvilinear, longitudinal approach was performed within the compartment to access the mass along the posteromedial aspect of the ankle. Although no evidence of infection was observed, the tissue was thickened with areas of necrosis down to the flexor retinaculum. Once the flexor retinaculum was opened, a fibrous, plaque-like mass was observed, and it was encased with flexor tendons and neurovascular structures of the tarsal tunnel. After mass excision, a complete tarsal tunnel release was performed until the neurovascular bundle was free. Irrigation and débridement of the ulcer were performed along with complicated wound closure, and the patient was placed in a well-padded postoperative splint.
Pathology was finalized as a recurrent, EBV-positive, and nasal-type ENKTL. The patient underwent bone marrow biopsy, which yielded negative results. CT of the chest, abdomen, and pelvis were negative for the disease. FDG PET-CT, which included the extremities, was performed and demonstrated increased uptake in the right ankle, consistent with the malignancy (Figure 4).
DISCUSSION
ENKTL is an uncommon form of lymphoma and is exceedingly rare in Caucasian females.1-3 Although the patient’s primary occurrence was in the nasal cavity, recurrence in the foot and ankle must still be described.7,8 To our knowledge, this article is one of the first published cases of a patient who developed a rare-recurrence ENKTL about the foot and ankle. Occurrence in extremities is extremely rare that the staging protocol does not include FDG PET-CT of these areas. The patient’s “negative” scans led many providers to neglect the symptoms in her right ankle until the lesion had ulcerated through the skin. If one would have relied on imaging reports and outside records alone, the diagnosis would have been delayed longer or missed all together. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body FDG PET-CT when evaluating for recurrence in patients.
1. Quintanilla-Martinez L, Kremer M, Keller G, et al. p53 mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am J Pathol. 2001;159(6):2095-2105. doi:10.1016/S0002-9440(10)63061-1.
2. Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005;16(2):206-214. doi:10.1093/annonc/mdi037.
3. Armitage JO. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909-3918.
4. Medeiros LJ, Peiper SC, Elwood L, Yano T, Raffeld M, Jaffe ES. Angiocentric immunoproliferative lesions: a molecular analysis of eight cases. Hum Pathol. 1991;22(11):1150-1157. doi:10.1016/0046-8177(91)90269-U.
5. Ho FC, Srivastava G, Loke SL, et al. Presence of Epstein-Barr virus DNA in nasal lymphomas of B and ‘T’ cell type. Hematol Oncol. 1990;8(5):271-281. doi:10.1002/hon.2900080505.
6. Gelb AB, van de Rijn M, Regula DP Jr, et al. Epstein-Barr virus-associated natural killer-large granular lymphocyte leukemia. Hum Pathol. 1994;25(9):953-960. doi:10.1016/0046-8177(94)90018-3.
7. Petrella T, Delfau-Larue MH, Caillot D, et al. Nasopharyngeal lymphomas: further evidence for a natural killer cell origin. Hum Pathol. 1996;27(8):827-833. doi:10.1016/S0046-8177(96)90457-8.
8. Hasserjian RP, Harris NL. NK-cell lymphomas and leukemias: a spectrum of tumors with variable manifestations and immunophenotype. Am J Clin Pathol. 2007;127(6):860-868. doi:10.1309/2F39NX1AL3L54WU8.
9. Robbins KT, Fuller LM, Vlasak M. Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer. 1985;56(4):814-819. doi:10.1002/1097-0142(19850815)56.
10. Ooi GC, Chim CS, Liang R, Tsang KW, Kwong YL. Nasal T-cell/natural killer cell lymphoma: CT and MR imaging features of a new clinicopathologic entity. Am J Roentgenol. 2000;174(4):1141-1145. doi:10.2214/ajr.174.4.1741141.
11. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621. doi:10.1007/s00277-008-0494-8.
12. Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27(35):6027-6032. doi:10.1200/JCO.2009.23.8592.
13. Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005;19(12):2186-2194. doi:10.1038/sj.leu.2403955.
14. Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol. 2009;147(1):13-21. doi:10.1111/j.1365-2141.2009.07802.x.
15. Yokoyama H, Yamamoto J, Tohmiya Y, et al. Allogeneic hematopoietic stem cell transplant following chemotherapy containing l-asparaginase as a promising treatment for patients with relapsed or refractory extranodal natural killer/T cell lymphoma, nasal type. Leuk Lymphoma. 2010;51(8):1509-1512. doi:10.3109/10428194.2010.487958.
ABSTRACT
This case report is a rare form of lymphoma recurrence which presented as tarsal tunnel syndrome. The patient had been previously treated for the malignancy and was presumed to be in remission; however, standard radiology imaging protocols failed to include the distal extremities on these scans. The patient presented to the orthopedic clinic with tarsal tunnel symptoms and a mass in the tarsal tunnel. A complete evaluation resulted in a diagnosis of recurrence of the malignancy. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body fluorodeoxyglucose positron emission tomography computed tomography when evaluating for recurrence in patients.
Nasal-type, extranodal natural killer/T-cell lymphoma (ENKTL) is a rare form of non-Hodgkin lymphoma (NHL). Malignancies account for only 10% of NHL in Asian and South American populations. However, in Caucasians, it represents <1% of all cases. In addition, at 3:1 male to female ratio, the disease most commonly affects male patients who are 50 to 59 years old.1-3 The etiology of this malignancy is strongly related to prior infection with Epstein-Barr virus (EBV) as EBV-encoded early small ribonucleic acid on in situ hybridization of lymphoma cells is positive in 95% of cases.4-6
Typical sites of involvement include the nasal cavity, nasopharynx, and sinuses, causing patients to present with nasal obstruction, chronic sinusitis, or epistaxis. Additionally, ENKTL can occur primarily in the skin, gastrointestinal tract, spleen, and testis, whereas the bone marrow may be involved in 10% of cases. Although rare, unusual sites, including muscle, adrenals, and ovaries, have been published.7,8
Staging is best performed using the T-staging system, which accounts for the extent of local tumor involvement. Higher stages, such as T3 /T4, equate to locally advanced disease and imply a worse prognosis.9,10 Computed tomography (CT) and magnetic resonance imaging (MRI) help define local soft tissues and bony involvement. Furthermore, CT of the chest, abdomen, and pelvis as well as bone marrow biopsy are performed as part of the staging process. Lastly, fluorine-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) is often used to detect extranodal spread, define the extent of involvement, differentiate between lymphoma and inflammatory masses, and monitor for recurrence.11
Treatment for local ENKTL involves concurrent chemoradiotherapy followed by 3 cycles of etoposide, ifosfamide, cisplatin, and dexamethasone, which results in a complete response rate of 80%, and is the most favorable when comparing treatment modalities.12 Unfortunately, recurrence rates reach as high as 50%, whereas the 5-year survival rate is 59%.13,14 For recurrent or disseminated disease, high-dose chemotherapy and hematopoietic stem cell transplantation remain as alternative treatments for patients who have undergone 2 complete remissions and can be curative in some instances.13,15
Continue to: In summary, ENKTL is a rare form...
In summary, ENKTL is a rare form of NHL which classically presents in the nasal cavity; however, this type of lymphoma may present in a variety of extranodal sites.7,8 Despite the numerous published reports on ENKTL, no study has reported either primary or recurrent ENKTL in the feet or hands. To our knowledge, this is one of the first published cases of a patient who developed a rare and recurring ENKTL in the foot and ankle. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 59-year-old Caucasian woman was referred to the orthopedic foot and ankle clinic by her primary care physician for right medial ankle pain, skin ulceration, and numbness over the plantar aspect of her right foot. Upon questioning, the patient noted that the pain and numbness were present for almost 6 months. She denied trauma to the concerned area. Previously, the patient was observed and treated elsewhere for plantar fasciitis and was prescribed a brace before being immobilized in a controlled ankle motion (CAM) boot for 6 weeks. At follow-up with her outside provider, the patient had developed skin breakdown over the medial aspect of the right ankle, and this condition was presumed to be caused by the boot. After local wound care failed to improve her skin ulceration, she returned to her primary care physician, who ordered an MRI of the area and referred her to our specialty clinic.
Upon review, the patient’s past medical history included a diagnosis of nasal-type ENKTL. Her malignancy was treated with chemoradiotherapy 2 years prior to her consultation with the foot and ankle clinic.
The patient was noted by her medical oncologist and interventional radiologist to be in complete stage 4 remission since being treated. She underwent routine MRI and CT scans of the head and neck at 6-month intervals and FDG PET-CT scans at 3-month intervals, as per institutional protocol. The examinations showed no evidence of malignancy or metabolically active disease. The last imaging study occurred 2 months prior to admission to the foot and ankle clinic.
The patient consulted her medical oncologist 1 month prior to presenting to our clinic and was noted to exhibit an “excellent response to chemoradiotherapy” and “continues to remain disease free at 2 years.” She was instructed to continue routine follow-up. However, the office notes mentioned no ankle pain and non-healing wounds.
During physical examination, the patient presented an antalgic gait on the right side. Inspection demonstrated an increased circumference of the right ankle compared with the left, with a soft, palpable mass over the medial aspect of her right ankle. A 3 cm × 2 cm, grade 2 abrasion of the skin was observed over the medial mass just posterior to her medial malleolus. Range of motion was within normal limits. The patient exhibited a palpable posterior tibial artery pulse and full strength upon muscle testing of the lower extremities. She featured a positive Tinel’s sign and discomfort over the mass itself, with the pain radiating down to the plantar aspect of her foot and diffuse numbness over the plantar aspect of the foot.
Continue to: Review of her plain radiographs...
Review of her plain radiographs demonstrated no bony abnormalities, fractures, nor visible deformity (Figures 1A, 1B).
At presentation, our differential diagnosis included recurrence of the malignancy, secondary malignancy, infection, and inflammatory disease. After a lengthy discussion with the patient and consultation with our institution’s musculoskeletal oncologist, the decision was made to perform a right-ankle mass biopsy and marginal excision with wound irrigation and débridement and tarsal tunnel release.
The patient was placed in the supine position with standard prepping and draping. The medial eschar was excised in an elliptical fashion, and a curvilinear, longitudinal approach was performed within the compartment to access the mass along the posteromedial aspect of the ankle. Although no evidence of infection was observed, the tissue was thickened with areas of necrosis down to the flexor retinaculum. Once the flexor retinaculum was opened, a fibrous, plaque-like mass was observed, and it was encased with flexor tendons and neurovascular structures of the tarsal tunnel. After mass excision, a complete tarsal tunnel release was performed until the neurovascular bundle was free. Irrigation and débridement of the ulcer were performed along with complicated wound closure, and the patient was placed in a well-padded postoperative splint.
Pathology was finalized as a recurrent, EBV-positive, and nasal-type ENKTL. The patient underwent bone marrow biopsy, which yielded negative results. CT of the chest, abdomen, and pelvis were negative for the disease. FDG PET-CT, which included the extremities, was performed and demonstrated increased uptake in the right ankle, consistent with the malignancy (Figure 4).
DISCUSSION
ENKTL is an uncommon form of lymphoma and is exceedingly rare in Caucasian females.1-3 Although the patient’s primary occurrence was in the nasal cavity, recurrence in the foot and ankle must still be described.7,8 To our knowledge, this article is one of the first published cases of a patient who developed a rare-recurrence ENKTL about the foot and ankle. Occurrence in extremities is extremely rare that the staging protocol does not include FDG PET-CT of these areas. The patient’s “negative” scans led many providers to neglect the symptoms in her right ankle until the lesion had ulcerated through the skin. If one would have relied on imaging reports and outside records alone, the diagnosis would have been delayed longer or missed all together. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body FDG PET-CT when evaluating for recurrence in patients.
ABSTRACT
This case report is a rare form of lymphoma recurrence which presented as tarsal tunnel syndrome. The patient had been previously treated for the malignancy and was presumed to be in remission; however, standard radiology imaging protocols failed to include the distal extremities on these scans. The patient presented to the orthopedic clinic with tarsal tunnel symptoms and a mass in the tarsal tunnel. A complete evaluation resulted in a diagnosis of recurrence of the malignancy. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body fluorodeoxyglucose positron emission tomography computed tomography when evaluating for recurrence in patients.
Nasal-type, extranodal natural killer/T-cell lymphoma (ENKTL) is a rare form of non-Hodgkin lymphoma (NHL). Malignancies account for only 10% of NHL in Asian and South American populations. However, in Caucasians, it represents <1% of all cases. In addition, at 3:1 male to female ratio, the disease most commonly affects male patients who are 50 to 59 years old.1-3 The etiology of this malignancy is strongly related to prior infection with Epstein-Barr virus (EBV) as EBV-encoded early small ribonucleic acid on in situ hybridization of lymphoma cells is positive in 95% of cases.4-6
Typical sites of involvement include the nasal cavity, nasopharynx, and sinuses, causing patients to present with nasal obstruction, chronic sinusitis, or epistaxis. Additionally, ENKTL can occur primarily in the skin, gastrointestinal tract, spleen, and testis, whereas the bone marrow may be involved in 10% of cases. Although rare, unusual sites, including muscle, adrenals, and ovaries, have been published.7,8
Staging is best performed using the T-staging system, which accounts for the extent of local tumor involvement. Higher stages, such as T3 /T4, equate to locally advanced disease and imply a worse prognosis.9,10 Computed tomography (CT) and magnetic resonance imaging (MRI) help define local soft tissues and bony involvement. Furthermore, CT of the chest, abdomen, and pelvis as well as bone marrow biopsy are performed as part of the staging process. Lastly, fluorine-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) is often used to detect extranodal spread, define the extent of involvement, differentiate between lymphoma and inflammatory masses, and monitor for recurrence.11
Treatment for local ENKTL involves concurrent chemoradiotherapy followed by 3 cycles of etoposide, ifosfamide, cisplatin, and dexamethasone, which results in a complete response rate of 80%, and is the most favorable when comparing treatment modalities.12 Unfortunately, recurrence rates reach as high as 50%, whereas the 5-year survival rate is 59%.13,14 For recurrent or disseminated disease, high-dose chemotherapy and hematopoietic stem cell transplantation remain as alternative treatments for patients who have undergone 2 complete remissions and can be curative in some instances.13,15
Continue to: In summary, ENKTL is a rare form...
In summary, ENKTL is a rare form of NHL which classically presents in the nasal cavity; however, this type of lymphoma may present in a variety of extranodal sites.7,8 Despite the numerous published reports on ENKTL, no study has reported either primary or recurrent ENKTL in the feet or hands. To our knowledge, this is one of the first published cases of a patient who developed a rare and recurring ENKTL in the foot and ankle. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 59-year-old Caucasian woman was referred to the orthopedic foot and ankle clinic by her primary care physician for right medial ankle pain, skin ulceration, and numbness over the plantar aspect of her right foot. Upon questioning, the patient noted that the pain and numbness were present for almost 6 months. She denied trauma to the concerned area. Previously, the patient was observed and treated elsewhere for plantar fasciitis and was prescribed a brace before being immobilized in a controlled ankle motion (CAM) boot for 6 weeks. At follow-up with her outside provider, the patient had developed skin breakdown over the medial aspect of the right ankle, and this condition was presumed to be caused by the boot. After local wound care failed to improve her skin ulceration, she returned to her primary care physician, who ordered an MRI of the area and referred her to our specialty clinic.
Upon review, the patient’s past medical history included a diagnosis of nasal-type ENKTL. Her malignancy was treated with chemoradiotherapy 2 years prior to her consultation with the foot and ankle clinic.
The patient was noted by her medical oncologist and interventional radiologist to be in complete stage 4 remission since being treated. She underwent routine MRI and CT scans of the head and neck at 6-month intervals and FDG PET-CT scans at 3-month intervals, as per institutional protocol. The examinations showed no evidence of malignancy or metabolically active disease. The last imaging study occurred 2 months prior to admission to the foot and ankle clinic.
The patient consulted her medical oncologist 1 month prior to presenting to our clinic and was noted to exhibit an “excellent response to chemoradiotherapy” and “continues to remain disease free at 2 years.” She was instructed to continue routine follow-up. However, the office notes mentioned no ankle pain and non-healing wounds.
During physical examination, the patient presented an antalgic gait on the right side. Inspection demonstrated an increased circumference of the right ankle compared with the left, with a soft, palpable mass over the medial aspect of her right ankle. A 3 cm × 2 cm, grade 2 abrasion of the skin was observed over the medial mass just posterior to her medial malleolus. Range of motion was within normal limits. The patient exhibited a palpable posterior tibial artery pulse and full strength upon muscle testing of the lower extremities. She featured a positive Tinel’s sign and discomfort over the mass itself, with the pain radiating down to the plantar aspect of her foot and diffuse numbness over the plantar aspect of the foot.
Continue to: Review of her plain radiographs...
Review of her plain radiographs demonstrated no bony abnormalities, fractures, nor visible deformity (Figures 1A, 1B).
At presentation, our differential diagnosis included recurrence of the malignancy, secondary malignancy, infection, and inflammatory disease. After a lengthy discussion with the patient and consultation with our institution’s musculoskeletal oncologist, the decision was made to perform a right-ankle mass biopsy and marginal excision with wound irrigation and débridement and tarsal tunnel release.
The patient was placed in the supine position with standard prepping and draping. The medial eschar was excised in an elliptical fashion, and a curvilinear, longitudinal approach was performed within the compartment to access the mass along the posteromedial aspect of the ankle. Although no evidence of infection was observed, the tissue was thickened with areas of necrosis down to the flexor retinaculum. Once the flexor retinaculum was opened, a fibrous, plaque-like mass was observed, and it was encased with flexor tendons and neurovascular structures of the tarsal tunnel. After mass excision, a complete tarsal tunnel release was performed until the neurovascular bundle was free. Irrigation and débridement of the ulcer were performed along with complicated wound closure, and the patient was placed in a well-padded postoperative splint.
Pathology was finalized as a recurrent, EBV-positive, and nasal-type ENKTL. The patient underwent bone marrow biopsy, which yielded negative results. CT of the chest, abdomen, and pelvis were negative for the disease. FDG PET-CT, which included the extremities, was performed and demonstrated increased uptake in the right ankle, consistent with the malignancy (Figure 4).
DISCUSSION
ENKTL is an uncommon form of lymphoma and is exceedingly rare in Caucasian females.1-3 Although the patient’s primary occurrence was in the nasal cavity, recurrence in the foot and ankle must still be described.7,8 To our knowledge, this article is one of the first published cases of a patient who developed a rare-recurrence ENKTL about the foot and ankle. Occurrence in extremities is extremely rare that the staging protocol does not include FDG PET-CT of these areas. The patient’s “negative” scans led many providers to neglect the symptoms in her right ankle until the lesion had ulcerated through the skin. If one would have relied on imaging reports and outside records alone, the diagnosis would have been delayed longer or missed all together. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body FDG PET-CT when evaluating for recurrence in patients.
1. Quintanilla-Martinez L, Kremer M, Keller G, et al. p53 mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am J Pathol. 2001;159(6):2095-2105. doi:10.1016/S0002-9440(10)63061-1.
2. Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005;16(2):206-214. doi:10.1093/annonc/mdi037.
3. Armitage JO. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909-3918.
4. Medeiros LJ, Peiper SC, Elwood L, Yano T, Raffeld M, Jaffe ES. Angiocentric immunoproliferative lesions: a molecular analysis of eight cases. Hum Pathol. 1991;22(11):1150-1157. doi:10.1016/0046-8177(91)90269-U.
5. Ho FC, Srivastava G, Loke SL, et al. Presence of Epstein-Barr virus DNA in nasal lymphomas of B and ‘T’ cell type. Hematol Oncol. 1990;8(5):271-281. doi:10.1002/hon.2900080505.
6. Gelb AB, van de Rijn M, Regula DP Jr, et al. Epstein-Barr virus-associated natural killer-large granular lymphocyte leukemia. Hum Pathol. 1994;25(9):953-960. doi:10.1016/0046-8177(94)90018-3.
7. Petrella T, Delfau-Larue MH, Caillot D, et al. Nasopharyngeal lymphomas: further evidence for a natural killer cell origin. Hum Pathol. 1996;27(8):827-833. doi:10.1016/S0046-8177(96)90457-8.
8. Hasserjian RP, Harris NL. NK-cell lymphomas and leukemias: a spectrum of tumors with variable manifestations and immunophenotype. Am J Clin Pathol. 2007;127(6):860-868. doi:10.1309/2F39NX1AL3L54WU8.
9. Robbins KT, Fuller LM, Vlasak M. Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer. 1985;56(4):814-819. doi:10.1002/1097-0142(19850815)56.
10. Ooi GC, Chim CS, Liang R, Tsang KW, Kwong YL. Nasal T-cell/natural killer cell lymphoma: CT and MR imaging features of a new clinicopathologic entity. Am J Roentgenol. 2000;174(4):1141-1145. doi:10.2214/ajr.174.4.1741141.
11. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621. doi:10.1007/s00277-008-0494-8.
12. Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27(35):6027-6032. doi:10.1200/JCO.2009.23.8592.
13. Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005;19(12):2186-2194. doi:10.1038/sj.leu.2403955.
14. Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol. 2009;147(1):13-21. doi:10.1111/j.1365-2141.2009.07802.x.
15. Yokoyama H, Yamamoto J, Tohmiya Y, et al. Allogeneic hematopoietic stem cell transplant following chemotherapy containing l-asparaginase as a promising treatment for patients with relapsed or refractory extranodal natural killer/T cell lymphoma, nasal type. Leuk Lymphoma. 2010;51(8):1509-1512. doi:10.3109/10428194.2010.487958.
1. Quintanilla-Martinez L, Kremer M, Keller G, et al. p53 mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am J Pathol. 2001;159(6):2095-2105. doi:10.1016/S0002-9440(10)63061-1.
2. Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005;16(2):206-214. doi:10.1093/annonc/mdi037.
3. Armitage JO. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909-3918.
4. Medeiros LJ, Peiper SC, Elwood L, Yano T, Raffeld M, Jaffe ES. Angiocentric immunoproliferative lesions: a molecular analysis of eight cases. Hum Pathol. 1991;22(11):1150-1157. doi:10.1016/0046-8177(91)90269-U.
5. Ho FC, Srivastava G, Loke SL, et al. Presence of Epstein-Barr virus DNA in nasal lymphomas of B and ‘T’ cell type. Hematol Oncol. 1990;8(5):271-281. doi:10.1002/hon.2900080505.
6. Gelb AB, van de Rijn M, Regula DP Jr, et al. Epstein-Barr virus-associated natural killer-large granular lymphocyte leukemia. Hum Pathol. 1994;25(9):953-960. doi:10.1016/0046-8177(94)90018-3.
7. Petrella T, Delfau-Larue MH, Caillot D, et al. Nasopharyngeal lymphomas: further evidence for a natural killer cell origin. Hum Pathol. 1996;27(8):827-833. doi:10.1016/S0046-8177(96)90457-8.
8. Hasserjian RP, Harris NL. NK-cell lymphomas and leukemias: a spectrum of tumors with variable manifestations and immunophenotype. Am J Clin Pathol. 2007;127(6):860-868. doi:10.1309/2F39NX1AL3L54WU8.
9. Robbins KT, Fuller LM, Vlasak M. Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer. 1985;56(4):814-819. doi:10.1002/1097-0142(19850815)56.
10. Ooi GC, Chim CS, Liang R, Tsang KW, Kwong YL. Nasal T-cell/natural killer cell lymphoma: CT and MR imaging features of a new clinicopathologic entity. Am J Roentgenol. 2000;174(4):1141-1145. doi:10.2214/ajr.174.4.1741141.
11. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621. doi:10.1007/s00277-008-0494-8.
12. Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27(35):6027-6032. doi:10.1200/JCO.2009.23.8592.
13. Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005;19(12):2186-2194. doi:10.1038/sj.leu.2403955.
14. Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol. 2009;147(1):13-21. doi:10.1111/j.1365-2141.2009.07802.x.
15. Yokoyama H, Yamamoto J, Tohmiya Y, et al. Allogeneic hematopoietic stem cell transplant following chemotherapy containing l-asparaginase as a promising treatment for patients with relapsed or refractory extranodal natural killer/T cell lymphoma, nasal type. Leuk Lymphoma. 2010;51(8):1509-1512. doi:10.3109/10428194.2010.487958.
TAKE-HOME POINTS
- A thorough review of systems, physical examination, and personal review of a patient’s advanced imaging is critical to avoid missed diagnosis or delays in diagnosis.
- Any mass lesion encountered in clinical practice, no matter how benign appearing, should be presumed malignant until proven otherwise.
- Fluorine-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) should include whole-body scans when evaluating patients for recurrence of malignancy.